
PARAQUAT
EXPLANATION
Paraquat was evaluated for acceptable daily intake by the Joint
Meetings in 1970, 1972, 1976, 1982, and 1985 (Annex 1, FAO/WHO, 1971a,
1973a, 1977a, 1983a, and 1986a). A toxicological monograph was
published after the 1970 Meeting (Annex 1, FAO/WHO, 1971b) and
monograph addenda were published after the Meetings in 1972, 1976, and
1982 (Annex 1, FAO/WHO, 1973b, 1977b, and 1983b). In 1970 the Meeting
estimated an ADI of 0.001 mg/kg b.w. (as paraquat dichloride). The
1982 Joint Meeting noted that the higher ADI established by the 1972
Meeting (0.002 mg/kg b.w. as paraquat dichloride) was based on
long-term studies conducted by Industrial Bio-Test Laboratories (IBT),
for which no replacement studies, validations, or additional data had
been submitted. Considering the evidence available, the 1982 Meeting
recommended that a reduced ADI (0.001 mg/kg b.w. as paraquat
dichloride) be retained on a temporary basis, pending receipt of
further data.
Data were submitted to the 1985 Meeting which met the 1982
request. These data were reviewed by the 1985 Meeting, but logistical
difficulties precluded their full evaluation, especially in the light
of the considerable amount of information previously evaluated by the
Joint Meeting. The 1985 Joint Meeting was aware that the 2-year study
in rats that was submitted had been considered by 1 national authority
to indicate a possible oncogenic potential in the rat. The Meeting
also noted differing interpretations of the observed lesions by
different pathologists. The Meeting therefore recommended that a
complete evaluation of all valid data available should be undertaken
by the 1986 Joint Meeting. In addition, it requested submission of
full discriptions of the lung lesions seen in the new long-term rat
study and of historical control data on all lung lesions in the strain
of rats utilized in the study in the laboratory in which it was
conducted. The Joint Meeting extended the existing temporary ADI until
1986.
This monograph incorporates the relevant studies summarized in
earlier monographs and monograph addenda, the studies submitted for
consideration by the 1985 Joint Meeting, and the studies required by
the 1985 Joint Meeting, all of which were reviewed by the 1986
Meeting.
IDENTITY AND PROPERTIES
CHEMICAL NAMES 1,1'-dimethyl-4,4'-bipyridylium ion
1,1'-dimethyl-4,4'-bipyridinium ion
1,1'-dimethyl-4,4'-dipyridylium ion
N,N'-dimethyl-gamma,gamma-dipyridylium ion
Present as the dichloride.
SYNONYMS Methyl viologen, PP-148, Gramoxone, Gramoxone S,
Gramoxone ZU, Dextrone X, Esgram, Dexuron,
Tota-Col, Gramuron, Simpar, Toxer Total, PP-910,
Para-Col, Pathclear, Gramonol, Cleansweep,
Terraklene, Actar, Priglone, Preeglone, Mofisal,
Sweep, Crisquat, Herboxone, Pillarquat,
Pillarxone, Duanti, Dukatalon, Frankol Prompt,
Gramazin, Gramixel, Katalon, Ortho Paraquat CL,
Ortho Spot Weed & Grass Killer, Orvar, Paradi,
Seythe, Spray Seed, Tryquat, Weedrite, Crisquat,
Goldquat-276, Paraquat CL.
STRUCTURAL FORMULA
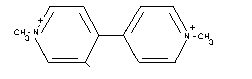 EMPIRICAL FORMULA
[C12H14N2]2+
MOLECULAR WEIGHTS
186.2 (ion)
257.2 (dichloride)
PHYSICAL STATE* Colorless crystalline solid.
MELTING POINT Decomposes at about 300°C.
VAPOUR PRESSURE Not measurable.
SOLUBILITY Very soluble in water, slightly soluble in lower
alcohols, insoluble in hydrocarbons.
STABILITY Stable in acid or neutral solutions, unstable in
alkaline solutions. Inactivated by inert clays,
anionic surfactants, and ultraviolet light.
OTHER PROPERTIES Solutions of paraquat become intensely purple on
reduction, due to the formation of a water
soluble, relatively stable free radical, which
absorbs at 400 nm. The unreduced form absorbs at
258 nm. The extinction coefficients of the reduced
and the oxidized paraquat at these absorption
maxima are xi mM400 = 46.0 and xi mM258 =
53.6, respectively (Autor, 1977). Vigorous
reduction gives tetrahydro derivatives and
ultimately the fully saturated base. The redox
potential (-446 mV) is independent of pH.
Concentrated aqueous solutions of paraquat are
corrosive to metal.
FORMULATIONS These include aqueous concentrates (100 - 240 g/l)
and water-soluble granules (24 g/kg) of paraquat
dichloride.
COMBINATIONS These include mixtures of paraquat with diquat
(e.g., Weedol), diuron (e.g., Dexuron),
monolinuron (e.g., Gramonol), and simazine
(e.g., Terraklene).
* All chemical properties are for the dichloride.
ANALYTICAL METHODS These include spectrophotometric, gas chromato-
graphic and radioimmunoassay methods. They
have been extensively reviewed by WHO (1984).
EVALUATION FOR ACCEPTABLE INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution and excretion
The absorption, distribution, and excretion of paraquat in
experimental animals have been reviewed by WHO (1984).
Following oral single-dose administration of 4 - 6 mg/kg b.w.
14C-paraquat dichloride to rats, 99 - 102% of the administered dose
was found in the faeces (93 - 96%) and in the urine (6%) within 3
days. This information, together with the absence of significant
biliary excretion, provided evidence that paraquat is poorly absorbed
from the gut (Daniel & Gage, 1966).
The low rate of paraquat absorption by the gut was confirmed in
experiments in which rats, guinea pigs, and monkeys, orally
administered with LD50 doses of 14C-paraquat, had low peak serum
concentrations (2.1 - 4.8 mg/litre). The radioactivity levels reached
a maximum 30 - 60 minutes after administration and then remained
relatively constant for 32 hours (Litchfield et al., 1973; Conning
et al., 1969).
A dose of 126 mg/kg b.w. paraquat resulted in a maximum rat serum
level of 4.8 mg/litre (Murray & Gibson, 1974).
In fasting dogs, low oral doses of paraquat were rapidly but
incompletely absorbed, the peak plasma concentration being attained 75
minutes after dosing. After an oral dose of 0.12 mg/kg b.w., 46 - 66%
was absorbed in 6 hours. After doses of 2 and 5 mg/kg b.w., only 22 -
38% and 25 - 28% of the doses were absorbed, respectively (Bennett
et al., 1976).
Dose-dependent data from dogs and whole-body autoradiography seem
to suggest that absorption is facilitated in the small intestine
(WHO, 1984).
The pulmonary absorption of 14C-paraquat after an intratracheal
injection of 1.86 nmol/lung was investigated in the isolated perfused
rat lung. The efflux of 14C-paraquat was diphasic, with a
rapid-phase half-life of 2.65 minutes and a slow-phase half-life of
356 minutes. It was suggested that the slow phase represented a
storage pool, possibly responsible for the pulmonary toxicity of
paraquat (Charles et al., 1978).
Various doses of 3H-paraquat (1 pg - 10 µg) in 0.1 ml saline
were introduced directly into the left bronchus of rats. Fifteen
minutes after instilling 10 ng of 3H-paraquat, 90% of the ion could
be accounted for in the tissues and urine, 50% being present in the
lung. With doses at or greater than 10 µg, pathological changes were
seen in the lung that were similar to those seen after systemic
poisoning (Wyatt et al., 1981).
Paraquat absorption through animal and human skin has been
studied using an in vitro technique. Human skin was shown to be
impermeable to paraquat, having a very low permeability constant of
0.73. Furthermore, human skin was found to be at least 40 times less
permeable than that of the animals tested, including rats, rabbits,
and guinea pigs (Walker et al., 1983).
Observations of dose-related dermal toxicity in experimental
animals and human percutaneous poisoning suggest that paraquat
absorption is markedly increased in damaged or occluded skin
(WHO, 1984).
High concentrations and retention of paraquat were found in lung
tissue, relative to other tissues, following oral, i.v., i.p., s.c.,
and intrabronchial routes of administration in rats, guinea pigs,
rabbits, and monkeys (Sharp et al., 1972; Ilett et al., 1974;
Murray & Gibson, 1974; Maling et al., 1978; Kurisaki & Sato, 1979;
Waddell & Marlowe, 1980; Wyatt et al., 1981. Some of these data are
summarized in Tables 1 and 2.
An association between paraquat concentrations in the lung and
degree of toxicity or lung injury has been reported (Sharp et al.,
1972; Ilett et al., 1974; Waddell & Marlowe, 1980; Wyatt et al.,
1981).
In 1 study toxic doses of 14C-paraquat were administered orally
and i.v. to rats. Paraquat concentrations in the whole blood were
similar to those in the plasma. The distribution of the herbicide in
various tissues was then followed for up to 10 days. The initial and
secondary half-lives of paraquat in plasma following i.v.
administration were 23 minutes and 56 hours, respectively. The
concentration in the kidney, lung, and muscle declined at the same
rate as in the plasma initially, but the rapid phase in the lung ended
after 20 minutes (compared with 1 - 4 hours in other organs), after
which it declined, with a half-life of 50 hours. The lung had the
greatest retention and consequently contained the highest
concentration 4 hours after dosing. Four to 10 days after dosing, the
paraquat concentration in the lung was 30 - 80 times higher than in
the plasma (Sharp et al., 1972).
Table 1. Paraquat distribution in tissues*
Route Dose Species Time Tissue Concentration Reference
of after
entry treatment
Intrabronchial 10 ng rat 60 min plasma 0.0092 g/l Wyatt et al.,
lung 5.2 ng 1981
kidney 0.052 ng
liver not measured
heart not measured
brain not measured
I.v. 20 mg/kg rat 24 h plasma 0.07 mg/l Sharp et al.,
lung 6.00 mg/kg 1972
kidney 1.45 mg/kg
liver 0.48 mg/kg
heart 1.20 mg/kg
brain not measured
I.v. 20 mg/kg rat 24 h plasma not measured Ilett et al.,
lung 11.36 mol/kg 1974
kidney 1.93 mol/kg
liver 0.90 mol/kg
heart 1.13 mol/kg
brain 0.87 mol/kg
* From WHO, 1984
Table 1. (cont'd).
Route Dose Species Time Tissue Concentration Reference
of after
entry treatment
I.v. cont'd. 20 mg/kg rabbit 24 h plasma 0.28 mol/l
lung 7.90 mol/kg
kidney 5.25 mol/kg
liver 1.59 mol/kg
heart 1.52 mol/kg
brain 0.49 mol/kg
I.p. 15 mg/kg rat 24 h plasma 0.32 mol/kg Maling et al.,
lung 26.28 mol/kg 1978
kidney 10.40 mol/kg
liver 5.04 mol/kg
heart 4.59 mol/kg
brain 1.22 mol/kg
Oral 126 mg/kg rat 16 h plasma 0.9 mg/l Murray &
lung 5.0 mg/kg Gibson, 1974
kidney 7.0 mg/kg
liver 2.1 mg/kg
heart 2.7 mg/kg
brain not measured
22 mg/kg guinea 16 h plasma 0.03 mg/l
pig lung 1.29 mg/kg
kidney 1.99 mg/kg
liver 0.08 mg/kg
heart 0.31 mg/kg
brain not measured
Table 2. Paraquat distribution in tissues (in mg/kg (mean) tissue)*
Route Dose Species Time Lung Kidney Liver Heart Plasma Reference
of mg/kg after
entry body dosing
weight
Oral 126 rat 1 h 3.3 27.5 2.0 1.8 4.7 Murray & Gibson,
4 h 3.7 4.5 4.4 0.9 0.8 1974
32 h 13.6 9.4 5.7 2.8 1.1
64 h 1.7 1.0 7.7 0.2 0.1
I.v. 20 rat 1 h 9.0 25.0 5.0 - 6.0 Sharp et al.,
4 h 8.0 6.0 2.0 - 0.3 1972
24 h 6.0 1.0 0.4 - 0.07
2 d 4.0 0.8 0.3 - 0.05
* From WHO, 1984
The high lung tissue concentrations of paraquat were confirmed in
another study in rats and rabbits after i.v. injection of 20 mg
14C-paraquat/kg b.w. Although the herbicide showed a selective
localization in the rabbit lung, the concentration decreased far more
rapidly in the rabbit lung than in the rat lung. The rabbit, unlike
the rat, did not show any histological or biochemical signs of lung
damage. No preferential subcellular localization of paraquat was found
in the lungs of either species. No evidence of covalent binding of
paraquat in lung tissue was found. After thorough washing of tissue
precipitate with dilute trichloracetic acid, only insignificant
amounts of 14C-paraquat were detected in protein from the brain,
heart, kidney, liver, lung, and plasma (Ilett et al., 1974).
Autoradiographic studies using 14C-paraquat have been carried
out on mice and rats. Paraquat was observed in nearly all organs 10
minutes after i.v. injection of 20 mg/kg b.w. (Litchfield et al.,
1973).
Autoradiographic results similar to those above were obtained in
mice after i.v. injection of 288 - 338 g/kg b.w. of 3H-paraquat
dichloride. Cellular resolution autoradiography showed that paraquat
was confined almost entirely to cells having the distribution of
alveolar Type II cells. The authors suggested that it was unlikely
that the radioactivity was bound to cellular constituents. The Type II
cells were found to be susceptible to the toxicity of paraquat
(Waddell & Marlowe, 1980; Kimbrough & Gaines, 1970).
No paraquat was detected in the kidney, brain, liver, or lungs
when administered in the diet to rats at a concentration of 50 ppm for
a period of 8 weeks. At 120 ppm it was found at low concentrations in
the lung, kidney, gastrointestinal system, and brain. When
administered at 250 ppm, it was detected in the tissues within 2
weeks. No sex differences or any clear pattern of accumulation were
noted throughout the 8-week study. Within 1 week of return to a normal
diet, no paraquat was detected in any tissue examined. Histological
changes were observed in all lungs of animals fed paraquat at 250 ppm
in the diet (Litchfield, et al., 1973).
Rose et al. (1974) demonstrated an energy-dependent
accumulation of paraquat in slices of rat lung that obeyed saturation
kinetics. The same investigators later examined the ability of
paraquat to accumulate in tissue slices from other organs in vitro.
The uptake of the herbicide in brain, adrenal gland, and kidney slices
was less than 10% of that observed in lung slices. The authors
established the uptake of paraquat by the lung in various species
(rat, rabbit, dog, monkey, and man). The human lung accumulated
paraquat as readily as that of the rat. Indeed, the kinetics (Vmax
and Km) of the process were found to be very similar in the 2
species. Moreover, there was a relationship between the concentration
of paraquat in the different lung areas and the development of
microscopic lung lesions (Rose et al., 1976a; Rose & Smith, 1977).
It has been demonstrated that the rate of paraquat efflux from
lung tissue is less than its rate of accumulation in lung slices.
Efflux from lung slices, prepared from rats dosed i.v. with the
herbicide, was found to be biphasic. There was a fast component
(half-life of 20 minutes), followed by a first-order slow component
characterised by a half-life of 17 hours. The half-life in vitro was
similar to that seen in vivo following i.v. administration to rats
(Smith et al., 1981). These results are partially consistent with
those obtained by Charles et al. (1978) in the isolated perfused rat
lung.
A biphasic elimination of paraquat from the plasma of rats after
i.v. injection has been reported. The initial rapid phase had a 20 -
30 minute half-life, and the slower phase a half-life of 56 hours
(Sharp et al., 1972).
Prolonged paraquat disappearance from serum following a rapid
initial decline was also found after oral administration to rats,
guinea pigs, and monkeys. Both the urinary and faecal routes were
important in all species studied. In rats 32 hours after dosing, 52%
of the administered paraquat was found in the gastrointestinal tract
and 17 and 14% were excreted in the faeces and urine, respectively. No
radioactivity was found in the expired air. The paraquat in the faeces
was due primarily to elimination of unabsorbed paraquat. The prolonged
elimination of paraquat in all animals tested indicated retention of
the herbicide in the body (Murray & Gibson, 1974).
Following i.v. administration of paraquat to rats, 75 - 79% of
the dose was excreted in the urine within 6 hours. In this study, the
plasma disappearance of 5 mg/kg paraquat was fitted to a 3-compartment
model. Total body clearance was estimated to be 8.39 ± 0.54 ml/kg
/minute. The relatively high concentration of paraquat found in the
duodenal and jejunal walls suggested biliary secretion of the
herbicide. The authors' hypothesis was later supported by the
observation of radioactivity in the intestines of mice injected i.v.
with 14C-paraquat in whole-body autoradiographic studies (Maling
et al., 1978; Waddell & Marlowe, 1980).
The dog was used as a model to evaluate the influence of
paraquat-induced renal failure on the kinetics of paraquat
elimination. After i.v. injection of a trace dose of 14C-paraquat
(30 - 50 g/kg b.w.), the kinetics of distribution was described by a
3-compartment model. To obtain a good fit of the curve, it was
necessary to sample the central (plasma) compartment for at least
24 hours after dosing. Simulation of paraquat levels in the peripheral
compartments suggested the existence of a compartment with rapid
uptake and removal (kidney) and another with slow uptake (lung). The
renal clearance of paraquat approximated total body clearance,
indicating that paraquat elimination occurs through renal excretion.
The urinary excretion rate of an i.v. dose was rapid, approximately 80
- 90% of the dose being eliminated during the first 6 hours.
Intravenous injection of a large toxic dose of paraquat (20 mg/kg
b.w.), however, brought about a marked decrease in renal clearance,
from 73 ml/minute to 18 ml/minute after 2.5 hours and 2 ml/minute
after 6 hours. These data suggest that kidney damage could contribute
to paraquat accumulation in the lung (Hawksworth et al., 1981).
Metabolism
Rats, dogs, and guinea pigs
After oral administration of 14C-paraquat to rats, dogs, and
guinea pigs, most of the radioactivity was excreted in 4 days, mainly
in the faeces as unchanged paraquat. The remaining label was present
in urine, which contained 12% (rats), 45% (dogs), and 9% (guinea pigs)
of the dose administered. Paraquat was the main radioactive component
of rat and dog urine, with monquat and the dipyridone of paraquat
accounting for 0.4%, 0.3%, and 0.1% of the administered dose in rat
urine, and 0.4%, 0.5%, and 0% of the dose in dog urine. After s.c.
administration of 14C-paraquat to rats, over 90% of the administered
radioactivity was excreted in the urine in 4 days. While the excretion
produce was mainly paraquat, chromatography indicated that monoquat
(1.9%), paraquat monopyridone (3.2%), and paraquat dipyridone (1.1%)
were also present. Although traces of monoquat and paraquat
monopyridone were also found in rat faeces, there was no evidence of
extensive metabolism of paraquat by the gut microflora. Intestinal
bacteria from rat caecal contents did not degrade paraquat in vitro
to any measurable extent (Annex 1, FAO/WHO, 1977b).
These conclusions were in contrast with the results of other
studies previously evaluated which indicated that when paraquat
(50 mg/kg b.w. of 14C-labelled dichloride salt) was given to rats,
25% of the radioactivity excreted in the faeces could be attributed to
products of metabolism by gut microflora. Examination of extracts
indicated the presence of only 1 metabolite in addition to paraquat.
Thirty percent of the paraquat was broken down when incubated
anaerobically with rat caecal contents; the metabolites were not
identified. Urine from rats injected i.p. with 14C-methyl-labelled
paraquat contained 87% of the administered radioactivity in 24 hours,
which was entirely unchanged paraquat (Plant Protection Ltd, 1972).
Hens
When a single oral dose of 14C-methyl-labelled paraquat was
administered to hens, all of the dose was recovered quantitatively in
the faeces within 3 days. At least 98% of the recovered radioactivity
was unchanged paraquat. Analysis of the tissues of hens after about 3
weeks of dosing with 14C-paraquat (6 ppm in the total diet)
indicated that it did not accumulate in the hens (Hemingway & Oliver,
1974).
Continuous dosing of hens with radiolabelled paraquat for up to
22 days, at rates up to 30 ppm in the diet, resulted in total
radioactive residues in the eggs of up to approximately 0.05 mg/kg
paraquat ion equivalent. At least 80% of the radioactivity was due to
unchanged paraquat. The residue was almost entirely in the yolk rather
than in the albumin (Hemingway & Oliver, 1974; Hendley et al.,
1976a).
Pigs
Pigs excreted an oral dose of paraquat principally in the faeces
as unchanged paraquat. Two pigs were dosed with 14C-labelled
paraquat for 7 consecutive days at a rate equivalent to 50 ppm in
the diet. One was dosed with 14C-methyl- and the second with
14C-ring-labelled paraquat. The pigs were sacrificed 2 hours after
receiving the final dose. By this time 69 - 73% of the administered
residue had been recovered in the faeces and approximately 3% had been
recovered in the urine. More than 90% of the radioactivity in the
faeces was present as unchanged paraquat. Total radioactive residues
in the tissues were low. More than 90% of these residues were due to
unchanged paraquat, except in liver, where approximately 70% was due
to unchanged paraquat and 4 - 7% was due to monoquat ion (Leahey
et al., 1976; Spinks et al., 1976).
Goats
14C-ring-labelled paraquat was administered to a goat in
mid-lactation twice daily for 7 days at a dose equivalent to 100 ppm
in the diet. Total radioactive residues in the milk were less than
0.01 mg/kg paraquat ion equivalent; 76% was unchanged paraquat. Total
radioactive residues were 0.74, 0.56, and 0.1 mg/kg in kidney, liver,
and muscle, respectively. There was no significant metabolism of
paraquat, except in the liver, where 50% of the residue was paraquat
and about 5% was each of the metabolites monoquat ion and monopyridone
ion (Hendley et al., 1976b).
Sheep
A dose of 14C-methyl-labelled paraquat administered to a sheep
via a rumen fistula was recovered quantitatively within 10 days.
Approximately 4% of the dose was excreted in the urine and the
remainder in the faeces. More than 95% of the radioactivity in urine
and faeces was present as unchanged paraquat. Small amounts of
monoquat ion (1%) and monopyridone ion (2.3%) were also detected
(Hemingway et al., 1972).
When injected s.c., paraquat was also excreted rapidly in the
urine (over 80% of the dose), 69% within the first day after
treatment. Unchanged paraquat accounted for most (90%) of the
radioactivity; the monopyridone derivative was present as 2 - 3% of
the dose and monoquat was a trace metabolite. This pattern of
metabolism was virtually identical to that seen in the urine following
dosing via the rumen (Hemingway et al., 1972).
Cows
When cows were given single oral doses of 14C-methyl paraquat
at 8 mg/kg, 96% of the radioactivity was recovered in the faeces
during the following 9 days; 0.7% was recovered in the urine.
Unchanged paraquat accounted for most of the radioactivity in the
faeces (96%) and urine (62 - 90%), but traces of the monoquat ion and
monopyridone ion were also detected in the urine. Only 0.003 - 0.004%
of the radioactivity was recovered in milk; the maximum radioactive
residue (0.005 mg/kg, paraquat ion equivalent) was observed on the day
after dosing. About 15% of this radioactivity was present as unchanged
paraquat. Monoquat ion and monopyridone ion (3 - 25%) were also found
in the milk. The radioactivity not identified as paraquat, monoquat,
or monopyridone was incorporated into natural constituents of milk
resulting from the anabolism of the radioactive methyl group cleaved
from paraquat (Hemingway et al., 1974).
Cows were fed for 3 months diets containing 24, 80, or 170 ppm
paraquat ion (equivalent to 0.8, 2.5, or 5.5 mg/kg b.w./day). The
paraquat was present as a residue in dried grass obtained from a
pasture that had been sprayed with Gramoxone and subsequently
weathered. The diet was accepted satisfactorily and no toxicological
effects were observed during the trial. Pathological examination of
tissues from animals slaughtered within 24 hours of the end of the
feeding trial showed no toxic effects attributable to paraquat. The
tissue residues, including muscle and liver, determined in cows at the
2 higher dose rates, varied between 0.01 and 0.09 mg/kg except in the
kidney, where 0.21 - 0.31 mg/kg was found. These fell to low
(0.04 mg/kg in the kidney) or non-detectable levels in an animal fed
the high-paraquat diet for 30 days and then maintained on an untreated
diet for 12 days before slaughter. Very low residues of paraquat were
present in milk samples taken weekly during the trial (121 samples
ranging from 0.0001 - 0.0006 mg/kg; 1 sample = 0.001 mg/kg)
(Edwards et al., 1974).
Effects on enzymes and other biochemical parameters
Several reviews or monographs have summarised the biochemical
mechanism of paraquat toxicity in plants (Calderbank, 1968), bacteria
(Fridovich & Hassan, 1979), and animals (Bus et al., 1976; Autor,
1977; Smith et al., 1979; Smith, 1985). The mechanism of the toxic
action of paraquat has also been extensively reviewed by WHO (1984).
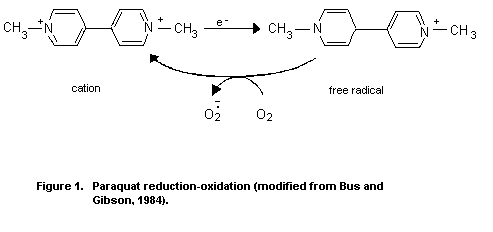
Paraquat has long been known to participate in cyclic
reduction-oxidation reactions in biological systems. The compound
readily undergoes a single electron reduction in tissues, forming a
free radical. In an aerobic environment, the free radical is
immediately oxidised by molecular oxygen, generating the superoxide
anion radical. The reoxidized paraquat is capable of accepting another
electron and continuing the electron transfer reactions in a catalytic
manner (Figure 1).
EMPIRICAL FORMULA
[C12H14N2]2+
MOLECULAR WEIGHTS
186.2 (ion)
257.2 (dichloride)
PHYSICAL STATE* Colorless crystalline solid.
MELTING POINT Decomposes at about 300°C.
VAPOUR PRESSURE Not measurable.
SOLUBILITY Very soluble in water, slightly soluble in lower
alcohols, insoluble in hydrocarbons.
STABILITY Stable in acid or neutral solutions, unstable in
alkaline solutions. Inactivated by inert clays,
anionic surfactants, and ultraviolet light.
OTHER PROPERTIES Solutions of paraquat become intensely purple on
reduction, due to the formation of a water
soluble, relatively stable free radical, which
absorbs at 400 nm. The unreduced form absorbs at
258 nm. The extinction coefficients of the reduced
and the oxidized paraquat at these absorption
maxima are xi mM400 = 46.0 and xi mM258 =
53.6, respectively (Autor, 1977). Vigorous
reduction gives tetrahydro derivatives and
ultimately the fully saturated base. The redox
potential (-446 mV) is independent of pH.
Concentrated aqueous solutions of paraquat are
corrosive to metal.
FORMULATIONS These include aqueous concentrates (100 - 240 g/l)
and water-soluble granules (24 g/kg) of paraquat
dichloride.
COMBINATIONS These include mixtures of paraquat with diquat
(e.g., Weedol), diuron (e.g., Dexuron),
monolinuron (e.g., Gramonol), and simazine
(e.g., Terraklene).
* All chemical properties are for the dichloride.
ANALYTICAL METHODS These include spectrophotometric, gas chromato-
graphic and radioimmunoassay methods. They
have been extensively reviewed by WHO (1984).
EVALUATION FOR ACCEPTABLE INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution and excretion
The absorption, distribution, and excretion of paraquat in
experimental animals have been reviewed by WHO (1984).
Following oral single-dose administration of 4 - 6 mg/kg b.w.
14C-paraquat dichloride to rats, 99 - 102% of the administered dose
was found in the faeces (93 - 96%) and in the urine (6%) within 3
days. This information, together with the absence of significant
biliary excretion, provided evidence that paraquat is poorly absorbed
from the gut (Daniel & Gage, 1966).
The low rate of paraquat absorption by the gut was confirmed in
experiments in which rats, guinea pigs, and monkeys, orally
administered with LD50 doses of 14C-paraquat, had low peak serum
concentrations (2.1 - 4.8 mg/litre). The radioactivity levels reached
a maximum 30 - 60 minutes after administration and then remained
relatively constant for 32 hours (Litchfield et al., 1973; Conning
et al., 1969).
A dose of 126 mg/kg b.w. paraquat resulted in a maximum rat serum
level of 4.8 mg/litre (Murray & Gibson, 1974).
In fasting dogs, low oral doses of paraquat were rapidly but
incompletely absorbed, the peak plasma concentration being attained 75
minutes after dosing. After an oral dose of 0.12 mg/kg b.w., 46 - 66%
was absorbed in 6 hours. After doses of 2 and 5 mg/kg b.w., only 22 -
38% and 25 - 28% of the doses were absorbed, respectively (Bennett
et al., 1976).
Dose-dependent data from dogs and whole-body autoradiography seem
to suggest that absorption is facilitated in the small intestine
(WHO, 1984).
The pulmonary absorption of 14C-paraquat after an intratracheal
injection of 1.86 nmol/lung was investigated in the isolated perfused
rat lung. The efflux of 14C-paraquat was diphasic, with a
rapid-phase half-life of 2.65 minutes and a slow-phase half-life of
356 minutes. It was suggested that the slow phase represented a
storage pool, possibly responsible for the pulmonary toxicity of
paraquat (Charles et al., 1978).
Various doses of 3H-paraquat (1 pg - 10 µg) in 0.1 ml saline
were introduced directly into the left bronchus of rats. Fifteen
minutes after instilling 10 ng of 3H-paraquat, 90% of the ion could
be accounted for in the tissues and urine, 50% being present in the
lung. With doses at or greater than 10 µg, pathological changes were
seen in the lung that were similar to those seen after systemic
poisoning (Wyatt et al., 1981).
Paraquat absorption through animal and human skin has been
studied using an in vitro technique. Human skin was shown to be
impermeable to paraquat, having a very low permeability constant of
0.73. Furthermore, human skin was found to be at least 40 times less
permeable than that of the animals tested, including rats, rabbits,
and guinea pigs (Walker et al., 1983).
Observations of dose-related dermal toxicity in experimental
animals and human percutaneous poisoning suggest that paraquat
absorption is markedly increased in damaged or occluded skin
(WHO, 1984).
High concentrations and retention of paraquat were found in lung
tissue, relative to other tissues, following oral, i.v., i.p., s.c.,
and intrabronchial routes of administration in rats, guinea pigs,
rabbits, and monkeys (Sharp et al., 1972; Ilett et al., 1974;
Murray & Gibson, 1974; Maling et al., 1978; Kurisaki & Sato, 1979;
Waddell & Marlowe, 1980; Wyatt et al., 1981. Some of these data are
summarized in Tables 1 and 2.
An association between paraquat concentrations in the lung and
degree of toxicity or lung injury has been reported (Sharp et al.,
1972; Ilett et al., 1974; Waddell & Marlowe, 1980; Wyatt et al.,
1981).
In 1 study toxic doses of 14C-paraquat were administered orally
and i.v. to rats. Paraquat concentrations in the whole blood were
similar to those in the plasma. The distribution of the herbicide in
various tissues was then followed for up to 10 days. The initial and
secondary half-lives of paraquat in plasma following i.v.
administration were 23 minutes and 56 hours, respectively. The
concentration in the kidney, lung, and muscle declined at the same
rate as in the plasma initially, but the rapid phase in the lung ended
after 20 minutes (compared with 1 - 4 hours in other organs), after
which it declined, with a half-life of 50 hours. The lung had the
greatest retention and consequently contained the highest
concentration 4 hours after dosing. Four to 10 days after dosing, the
paraquat concentration in the lung was 30 - 80 times higher than in
the plasma (Sharp et al., 1972).
Table 1. Paraquat distribution in tissues*
Route Dose Species Time Tissue Concentration Reference
of after
entry treatment
Intrabronchial 10 ng rat 60 min plasma 0.0092 g/l Wyatt et al.,
lung 5.2 ng 1981
kidney 0.052 ng
liver not measured
heart not measured
brain not measured
I.v. 20 mg/kg rat 24 h plasma 0.07 mg/l Sharp et al.,
lung 6.00 mg/kg 1972
kidney 1.45 mg/kg
liver 0.48 mg/kg
heart 1.20 mg/kg
brain not measured
I.v. 20 mg/kg rat 24 h plasma not measured Ilett et al.,
lung 11.36 mol/kg 1974
kidney 1.93 mol/kg
liver 0.90 mol/kg
heart 1.13 mol/kg
brain 0.87 mol/kg
* From WHO, 1984
Table 1. (cont'd).
Route Dose Species Time Tissue Concentration Reference
of after
entry treatment
I.v. cont'd. 20 mg/kg rabbit 24 h plasma 0.28 mol/l
lung 7.90 mol/kg
kidney 5.25 mol/kg
liver 1.59 mol/kg
heart 1.52 mol/kg
brain 0.49 mol/kg
I.p. 15 mg/kg rat 24 h plasma 0.32 mol/kg Maling et al.,
lung 26.28 mol/kg 1978
kidney 10.40 mol/kg
liver 5.04 mol/kg
heart 4.59 mol/kg
brain 1.22 mol/kg
Oral 126 mg/kg rat 16 h plasma 0.9 mg/l Murray &
lung 5.0 mg/kg Gibson, 1974
kidney 7.0 mg/kg
liver 2.1 mg/kg
heart 2.7 mg/kg
brain not measured
22 mg/kg guinea 16 h plasma 0.03 mg/l
pig lung 1.29 mg/kg
kidney 1.99 mg/kg
liver 0.08 mg/kg
heart 0.31 mg/kg
brain not measured
Table 2. Paraquat distribution in tissues (in mg/kg (mean) tissue)*
Route Dose Species Time Lung Kidney Liver Heart Plasma Reference
of mg/kg after
entry body dosing
weight
Oral 126 rat 1 h 3.3 27.5 2.0 1.8 4.7 Murray & Gibson,
4 h 3.7 4.5 4.4 0.9 0.8 1974
32 h 13.6 9.4 5.7 2.8 1.1
64 h 1.7 1.0 7.7 0.2 0.1
I.v. 20 rat 1 h 9.0 25.0 5.0 - 6.0 Sharp et al.,
4 h 8.0 6.0 2.0 - 0.3 1972
24 h 6.0 1.0 0.4 - 0.07
2 d 4.0 0.8 0.3 - 0.05
* From WHO, 1984
The high lung tissue concentrations of paraquat were confirmed in
another study in rats and rabbits after i.v. injection of 20 mg
14C-paraquat/kg b.w. Although the herbicide showed a selective
localization in the rabbit lung, the concentration decreased far more
rapidly in the rabbit lung than in the rat lung. The rabbit, unlike
the rat, did not show any histological or biochemical signs of lung
damage. No preferential subcellular localization of paraquat was found
in the lungs of either species. No evidence of covalent binding of
paraquat in lung tissue was found. After thorough washing of tissue
precipitate with dilute trichloracetic acid, only insignificant
amounts of 14C-paraquat were detected in protein from the brain,
heart, kidney, liver, lung, and plasma (Ilett et al., 1974).
Autoradiographic studies using 14C-paraquat have been carried
out on mice and rats. Paraquat was observed in nearly all organs 10
minutes after i.v. injection of 20 mg/kg b.w. (Litchfield et al.,
1973).
Autoradiographic results similar to those above were obtained in
mice after i.v. injection of 288 - 338 g/kg b.w. of 3H-paraquat
dichloride. Cellular resolution autoradiography showed that paraquat
was confined almost entirely to cells having the distribution of
alveolar Type II cells. The authors suggested that it was unlikely
that the radioactivity was bound to cellular constituents. The Type II
cells were found to be susceptible to the toxicity of paraquat
(Waddell & Marlowe, 1980; Kimbrough & Gaines, 1970).
No paraquat was detected in the kidney, brain, liver, or lungs
when administered in the diet to rats at a concentration of 50 ppm for
a period of 8 weeks. At 120 ppm it was found at low concentrations in
the lung, kidney, gastrointestinal system, and brain. When
administered at 250 ppm, it was detected in the tissues within 2
weeks. No sex differences or any clear pattern of accumulation were
noted throughout the 8-week study. Within 1 week of return to a normal
diet, no paraquat was detected in any tissue examined. Histological
changes were observed in all lungs of animals fed paraquat at 250 ppm
in the diet (Litchfield, et al., 1973).
Rose et al. (1974) demonstrated an energy-dependent
accumulation of paraquat in slices of rat lung that obeyed saturation
kinetics. The same investigators later examined the ability of
paraquat to accumulate in tissue slices from other organs in vitro.
The uptake of the herbicide in brain, adrenal gland, and kidney slices
was less than 10% of that observed in lung slices. The authors
established the uptake of paraquat by the lung in various species
(rat, rabbit, dog, monkey, and man). The human lung accumulated
paraquat as readily as that of the rat. Indeed, the kinetics (Vmax
and Km) of the process were found to be very similar in the 2
species. Moreover, there was a relationship between the concentration
of paraquat in the different lung areas and the development of
microscopic lung lesions (Rose et al., 1976a; Rose & Smith, 1977).
It has been demonstrated that the rate of paraquat efflux from
lung tissue is less than its rate of accumulation in lung slices.
Efflux from lung slices, prepared from rats dosed i.v. with the
herbicide, was found to be biphasic. There was a fast component
(half-life of 20 minutes), followed by a first-order slow component
characterised by a half-life of 17 hours. The half-life in vitro was
similar to that seen in vivo following i.v. administration to rats
(Smith et al., 1981). These results are partially consistent with
those obtained by Charles et al. (1978) in the isolated perfused rat
lung.
A biphasic elimination of paraquat from the plasma of rats after
i.v. injection has been reported. The initial rapid phase had a 20 -
30 minute half-life, and the slower phase a half-life of 56 hours
(Sharp et al., 1972).
Prolonged paraquat disappearance from serum following a rapid
initial decline was also found after oral administration to rats,
guinea pigs, and monkeys. Both the urinary and faecal routes were
important in all species studied. In rats 32 hours after dosing, 52%
of the administered paraquat was found in the gastrointestinal tract
and 17 and 14% were excreted in the faeces and urine, respectively. No
radioactivity was found in the expired air. The paraquat in the faeces
was due primarily to elimination of unabsorbed paraquat. The prolonged
elimination of paraquat in all animals tested indicated retention of
the herbicide in the body (Murray & Gibson, 1974).
Following i.v. administration of paraquat to rats, 75 - 79% of
the dose was excreted in the urine within 6 hours. In this study, the
plasma disappearance of 5 mg/kg paraquat was fitted to a 3-compartment
model. Total body clearance was estimated to be 8.39 ± 0.54 ml/kg
/minute. The relatively high concentration of paraquat found in the
duodenal and jejunal walls suggested biliary secretion of the
herbicide. The authors' hypothesis was later supported by the
observation of radioactivity in the intestines of mice injected i.v.
with 14C-paraquat in whole-body autoradiographic studies (Maling
et al., 1978; Waddell & Marlowe, 1980).
The dog was used as a model to evaluate the influence of
paraquat-induced renal failure on the kinetics of paraquat
elimination. After i.v. injection of a trace dose of 14C-paraquat
(30 - 50 g/kg b.w.), the kinetics of distribution was described by a
3-compartment model. To obtain a good fit of the curve, it was
necessary to sample the central (plasma) compartment for at least
24 hours after dosing. Simulation of paraquat levels in the peripheral
compartments suggested the existence of a compartment with rapid
uptake and removal (kidney) and another with slow uptake (lung). The
renal clearance of paraquat approximated total body clearance,
indicating that paraquat elimination occurs through renal excretion.
The urinary excretion rate of an i.v. dose was rapid, approximately 80
- 90% of the dose being eliminated during the first 6 hours.
Intravenous injection of a large toxic dose of paraquat (20 mg/kg
b.w.), however, brought about a marked decrease in renal clearance,
from 73 ml/minute to 18 ml/minute after 2.5 hours and 2 ml/minute
after 6 hours. These data suggest that kidney damage could contribute
to paraquat accumulation in the lung (Hawksworth et al., 1981).
Metabolism
Rats, dogs, and guinea pigs
After oral administration of 14C-paraquat to rats, dogs, and
guinea pigs, most of the radioactivity was excreted in 4 days, mainly
in the faeces as unchanged paraquat. The remaining label was present
in urine, which contained 12% (rats), 45% (dogs), and 9% (guinea pigs)
of the dose administered. Paraquat was the main radioactive component
of rat and dog urine, with monquat and the dipyridone of paraquat
accounting for 0.4%, 0.3%, and 0.1% of the administered dose in rat
urine, and 0.4%, 0.5%, and 0% of the dose in dog urine. After s.c.
administration of 14C-paraquat to rats, over 90% of the administered
radioactivity was excreted in the urine in 4 days. While the excretion
produce was mainly paraquat, chromatography indicated that monoquat
(1.9%), paraquat monopyridone (3.2%), and paraquat dipyridone (1.1%)
were also present. Although traces of monoquat and paraquat
monopyridone were also found in rat faeces, there was no evidence of
extensive metabolism of paraquat by the gut microflora. Intestinal
bacteria from rat caecal contents did not degrade paraquat in vitro
to any measurable extent (Annex 1, FAO/WHO, 1977b).
These conclusions were in contrast with the results of other
studies previously evaluated which indicated that when paraquat
(50 mg/kg b.w. of 14C-labelled dichloride salt) was given to rats,
25% of the radioactivity excreted in the faeces could be attributed to
products of metabolism by gut microflora. Examination of extracts
indicated the presence of only 1 metabolite in addition to paraquat.
Thirty percent of the paraquat was broken down when incubated
anaerobically with rat caecal contents; the metabolites were not
identified. Urine from rats injected i.p. with 14C-methyl-labelled
paraquat contained 87% of the administered radioactivity in 24 hours,
which was entirely unchanged paraquat (Plant Protection Ltd, 1972).
Hens
When a single oral dose of 14C-methyl-labelled paraquat was
administered to hens, all of the dose was recovered quantitatively in
the faeces within 3 days. At least 98% of the recovered radioactivity
was unchanged paraquat. Analysis of the tissues of hens after about 3
weeks of dosing with 14C-paraquat (6 ppm in the total diet)
indicated that it did not accumulate in the hens (Hemingway & Oliver,
1974).
Continuous dosing of hens with radiolabelled paraquat for up to
22 days, at rates up to 30 ppm in the diet, resulted in total
radioactive residues in the eggs of up to approximately 0.05 mg/kg
paraquat ion equivalent. At least 80% of the radioactivity was due to
unchanged paraquat. The residue was almost entirely in the yolk rather
than in the albumin (Hemingway & Oliver, 1974; Hendley et al.,
1976a).
Pigs
Pigs excreted an oral dose of paraquat principally in the faeces
as unchanged paraquat. Two pigs were dosed with 14C-labelled
paraquat for 7 consecutive days at a rate equivalent to 50 ppm in
the diet. One was dosed with 14C-methyl- and the second with
14C-ring-labelled paraquat. The pigs were sacrificed 2 hours after
receiving the final dose. By this time 69 - 73% of the administered
residue had been recovered in the faeces and approximately 3% had been
recovered in the urine. More than 90% of the radioactivity in the
faeces was present as unchanged paraquat. Total radioactive residues
in the tissues were low. More than 90% of these residues were due to
unchanged paraquat, except in liver, where approximately 70% was due
to unchanged paraquat and 4 - 7% was due to monoquat ion (Leahey
et al., 1976; Spinks et al., 1976).
Goats
14C-ring-labelled paraquat was administered to a goat in
mid-lactation twice daily for 7 days at a dose equivalent to 100 ppm
in the diet. Total radioactive residues in the milk were less than
0.01 mg/kg paraquat ion equivalent; 76% was unchanged paraquat. Total
radioactive residues were 0.74, 0.56, and 0.1 mg/kg in kidney, liver,
and muscle, respectively. There was no significant metabolism of
paraquat, except in the liver, where 50% of the residue was paraquat
and about 5% was each of the metabolites monoquat ion and monopyridone
ion (Hendley et al., 1976b).
Sheep
A dose of 14C-methyl-labelled paraquat administered to a sheep
via a rumen fistula was recovered quantitatively within 10 days.
Approximately 4% of the dose was excreted in the urine and the
remainder in the faeces. More than 95% of the radioactivity in urine
and faeces was present as unchanged paraquat. Small amounts of
monoquat ion (1%) and monopyridone ion (2.3%) were also detected
(Hemingway et al., 1972).
When injected s.c., paraquat was also excreted rapidly in the
urine (over 80% of the dose), 69% within the first day after
treatment. Unchanged paraquat accounted for most (90%) of the
radioactivity; the monopyridone derivative was present as 2 - 3% of
the dose and monoquat was a trace metabolite. This pattern of
metabolism was virtually identical to that seen in the urine following
dosing via the rumen (Hemingway et al., 1972).
Cows
When cows were given single oral doses of 14C-methyl paraquat
at 8 mg/kg, 96% of the radioactivity was recovered in the faeces
during the following 9 days; 0.7% was recovered in the urine.
Unchanged paraquat accounted for most of the radioactivity in the
faeces (96%) and urine (62 - 90%), but traces of the monoquat ion and
monopyridone ion were also detected in the urine. Only 0.003 - 0.004%
of the radioactivity was recovered in milk; the maximum radioactive
residue (0.005 mg/kg, paraquat ion equivalent) was observed on the day
after dosing. About 15% of this radioactivity was present as unchanged
paraquat. Monoquat ion and monopyridone ion (3 - 25%) were also found
in the milk. The radioactivity not identified as paraquat, monoquat,
or monopyridone was incorporated into natural constituents of milk
resulting from the anabolism of the radioactive methyl group cleaved
from paraquat (Hemingway et al., 1974).
Cows were fed for 3 months diets containing 24, 80, or 170 ppm
paraquat ion (equivalent to 0.8, 2.5, or 5.5 mg/kg b.w./day). The
paraquat was present as a residue in dried grass obtained from a
pasture that had been sprayed with Gramoxone and subsequently
weathered. The diet was accepted satisfactorily and no toxicological
effects were observed during the trial. Pathological examination of
tissues from animals slaughtered within 24 hours of the end of the
feeding trial showed no toxic effects attributable to paraquat. The
tissue residues, including muscle and liver, determined in cows at the
2 higher dose rates, varied between 0.01 and 0.09 mg/kg except in the
kidney, where 0.21 - 0.31 mg/kg was found. These fell to low
(0.04 mg/kg in the kidney) or non-detectable levels in an animal fed
the high-paraquat diet for 30 days and then maintained on an untreated
diet for 12 days before slaughter. Very low residues of paraquat were
present in milk samples taken weekly during the trial (121 samples
ranging from 0.0001 - 0.0006 mg/kg; 1 sample = 0.001 mg/kg)
(Edwards et al., 1974).
Effects on enzymes and other biochemical parameters
Several reviews or monographs have summarised the biochemical
mechanism of paraquat toxicity in plants (Calderbank, 1968), bacteria
(Fridovich & Hassan, 1979), and animals (Bus et al., 1976; Autor,
1977; Smith et al., 1979; Smith, 1985). The mechanism of the toxic
action of paraquat has also been extensively reviewed by WHO (1984).
Paraquat has long been known to participate in cyclic
reduction-oxidation reactions in biological systems. The compound
readily undergoes a single electron reduction in tissues, forming a
free radical. In an aerobic environment, the free radical is
immediately oxidised by molecular oxygen, generating the superoxide
anion radical. The reoxidized paraquat is capable of accepting another
electron and continuing the electron transfer reactions in a catalytic
manner (Figure 1).
 Research into the mechanism of paraquat toxicity has identified
at least 2 partially toxic consequences of the redox cycling reaction:
a) generation of the superoxide anion radical, and b) oxidation of
cellular NADPH, which is the major source of reducing equivalents for
the intracellular reduction of paraquat. Generation of the superoxide
anion radical can lead to the formation of more toxic forms of reduced
oxygen, hydrogen peroxide (H2O2), and hydroxyl radicals. Hydroxyl
radicals have been implicated in the initiation of membrane damage by
lipid peroxidation, depolymerization of hyaluronic acid, inactivation
of proteins, and damage to DNA. Depletion of NADPH, on the other hand,
may disrupt important NADPH-requiring biochemical processes such as
fatty acid synthesis (Hassan & Fridovich, 1980; Smith et al., 1979).
The importance of molecular oxygen and the potential role of
superoxide anion radical generation in mediating paraquat toxicity
have been implicated in studies on plants, bacteria, and in vitro
and in vivo mammalian systems. In cultures of E. coli, Hassan &
Fridovich (1977, 1978, & 1979) demonstrated that paraquat stimulated
cyanide-resistant respiration, which could be almost entirely
accounted for by the NADPH-dependent formation of the superoxide anion
radical.
The possibility that formation of the superoxide anion radical
might be responsible for the toxicity of paraquat in bacteria is
supported by observations that bacteria containing elevated activities
of superoxide dismutase, an enzyme that detoxifies the superoxide
anion radical, were resistant to paraquat toxicity (Hassan &
Fridovich, 1977, 1978; Moody & Hassan, 1982).
In vitro studies on lung and liver preparations from various
animal species have supported the hypothesis that paraquat redox
cycling and associated superoxide anion radical and H202
generation also occur in mammalian systems (Gage, 1968; Ilett
et al., 1974; Montgomery, 1976, 1977; Steffen & Netter, 1979;
Talcott et al., 1979).
Bus et al. (1974) reported that the single electron reduction
of paraquat in mammalian systems was catalysed by microsmal cytochrome
P-450 reductase and NADPH. The observation that the in vivo toxicity
of paraquat in animals is markedly potentiated by exposure to elevated
oxygen tensions further supports the potential role for molecular
oxygen in mediating toxicity (Fisher et al., 1973; Autor, 1974;
Bus & Gibson, 1975; Witschi et al., 1977; Kehrer et al., 1979;
Keeling et al., 1981; Selman et al., 1985).
The results of in vivo studies conducted by Bus et al. (1974)
suggest that stimulation of lipid peroxidation, which is dependent on
paraquat redox cycling and associated superoxide anion radical
generation, might be an important toxic mechanism in mammalian
systems. Consistent with this hypothesis, animals fed diets deficient
in selenium or vitamin E in order to diminish cellular antioxidant
defenses were significantly more sensitive to paraquat toxicity than
control animals (Bus et al., 1975a; Omaye et al., 1978). Moreover,
selenium deficiency potentiated paraquat-induced lipid peroxidation in
isolated perfused rat lung (Glass et al., 1985). In contrast to
these studies, a number of studies have shown that paraquat inhibited
in vitro microsomal lipid peroxidation (Ilett et al., 1974;
Montgomery & Niewoehner, 1979; Steffen & Netter, 1979; Kornburst &
Mavis, 1980). Subsequent studies have indicated, however, that
paraquat would stimulate microsomal lipid peroxidation when an
adequate supply of electrons (NADPH) and in vitro oxygen tension
were maintained (Trush et al., 1981, 1982).
Despite the evidence described above, the hypothesis that lipid
peroxidation is the underlying toxic mechanism functioning in vivo
has not been conclusively demonstrated. Direct quantification of
paraquat-induced lipid peroxidation damage in vivo by analysis of
tissue malonadialdehyde levels or ethane exhalation, both markers of
peroxidation injury, has been largely unsuccessful (Reddy et al.,
1977; Shu et al., 1979; Steffen et al., 1980), although
significant increases of serum malondialdehyde levels have been
recently reported in patients with paraquat poisoning (Yasaka
et al., 1986). Furthermore, attempts to counteract paraquat
toxicity by administration of various antioxidants have also been
unsuccessful (Fairshter, 1981).
Superoxide radicals generated in paraquat redox cycling may
induce biochemical changes other than the initiation of the
peroxidation reaction. Ross et al. (1979) demonstrated that paraquat
increased DNA strand breaks in cultured mouse lymphoblasts. Paraquat
was also reported to induce a superoxide-dependent stimulation of
guanylate cyclase activity in rat liver (Vesely et al., 1979) and
guinea pig lung (Giri & Krishna, 1980). These investigators postulated
that increased cyclic-GMP might stimulate the pulmonary fibroproli-
ferative changes characteristic of paraquat toxicity. In other
studies, paraquat has also been found to increase collagen synthesis
in the rat lung (Greenberg et al., 1978; Thompson & Patrick, 1978;
Hussain & Bhatnagar, 1979).
Redox cycling of paraquat has also been proposed to lead to
increased oxidation of cellular NADPH (Brigelius et al., 1981;
Keeling et al., 1982). The activity of pentose shunt enzymes in the
lung rapidly increased in rats treated with paraquat, which suggested
an increased demand for NADPH (Fisher et al., 1975; Rose et al.,
1976b). The observation that paraquat decreased fatty acid synthesis
in lung slices (Smith et al., 1979) further supported this
hypothesis, since fatty acid synthesis requires NADPH. Direct analysis
of NADPH in the lung has long confirmed that paraquat treatment
decreases the NADPH content in rat lung (Witschi et al., 1977;
Smith et al., 1979). More recently, both oxygen consumption and
NADPH oxidation in lung microsomes were found to be significantly and
specifically stimulated by the addition of paraquat (Rossouw et al.,
1984). The above observations led Smith et al. (1979) to propose
that oxidation of NADPH might interrupt not only vital physiological
processes, such as fatty acid synthesis, but also may render tissues
more susceptible to lipid peroxidation by decreasing the equivalents
(NADPH) necessary for functioning of the antioxidant enzyme
glutathione peroxidase (Figure 2). Indeed, a significant increase
(589%) in lung-oxidized glultathione (GSSG) content was found over
control levels after perfusion of isolated rabbit lung with a 0.4 mM
paraquat solution. This effect was significantly increased (225%) by
hyperoxia (Dunbar et al., 1984).
Toxicological studies
Special studies on carcinogenicity
Mice
Groups of 60 male and 60 female Alderly Park SPF mice were fed
diets containing 0 (2 groups), 12.5, 37.5, or 100/125 ppm paraquat
cation for 97 - 99 weeks. The initial top dose of 100 ppm was
increased to 125 ppm at week 36 in order to evoke a toxic effect. The
study was terminated at weeks 97 - 99 when 80% mortality was reached
in a female control group and was approaching 80% overall. Clinical
observations, body-weight gain, food consumption, and urinary paraquat
were measured throughout the study. Histopathological examination of
approximately 40 tissues was performed on animals killed or dying
during the study and at termination. Further groups of 10 males and 10
females were fed the same dose levels as above for 52 weeks for
measurement of paraquat concentrations in the kidney, lung, and plasma
at termination.
Mortality ranged from 32 - 55% at 80 weeks and from 58 - 87% at
termination and was higher in the 37.5 ppm and 125 ppm groups than in
the combined controls. Effects due to treatment were renal lesions in
both sexes at 100/125 ppm and, in males, at 37.5 ppm; lung lesions in
both sexes at 100/125 ppm; and decreased food consumption and
body-weight gain and increased mortality in females at 100/125 ppm.
Histopathologically, the treatment-related renal lesions were manifest
as mild dilatation and degenerative changes in the tubules. The
incidences of tubular degeneration (with and without dilatation) in
male mice dying during the study were 31/48 at 100/125 ppm, 15/47 at
37.5 ppm, 9/45 at 12.5 ppm, and 8/45 and 3/35 in controls. The
paraquat-induced lung lesions noted at 100/125 ppm included focal
pneumonitis/alveolitis and hypercellularity of the alveolar walls.
Statistically-significant increases in the incidence of fatty changes
of the liver were reported at 37.5 and 100/125 ppm in males, when
compared to controls. Other hepatic changes were noted, with a
significantly-higher incidence in treated compared to control mice.
These changes, however, were not considered by the authors of the
study to be treatment-related. There were no effects observed at
12.5 ppm. Histopathological examination showed no clear evidence of
treatment-related neoplastic changes in these mice. The incidence of
pulmonary tumours in both males (7/24) and females (8/20) in the
100/125 ppm dose group dying from 79 - 98 weeks was somewhat higher
than in controls (5/37 in males and 6/39 in females). However, the
incidences of pulmonary tumours in the animals of the same groups
surviving to termination were lower than in controls.
The authors of the study concluded that paraquat was not
oncogenic to the mouse. Based on the renal lesions, the no-effect
level of paraquat cation for Alderley Park SPF mice in this study was
12.5 ppm, equal to 1.4 mg/kg b.w./day in males and 37.5 ppm, equal to
4.3 mg/kg b.w./day in females.
Rats
Groups of 80 male and 80 female Fisher SPF rats were maintained
on diets containing 0, 7.2, 22, 72, or 217 ppm paraquat cation for 104
weeks. Eight rats/sex/group were sacrificed after urinalysis at 26,
52, and 78 weeks and were subjected to haematological examination. All
surviving animals were sacrificed at 104 weeks and, among these, 10
rats/sex/group were subjected to haematological and biochemical
examination. All animals, including those killed on schedule and those
found moribund and killed during the study, were autopsied and
subjected to gross necropsy and histopathological examination of
approximately 30 tissues.
Mortality was increased in female rats of the 217 ppm group from
week 66 to week 74 when compared with that of other groups, including
controls. Both male and female rats at the 217 ppm dietary level
showed a marked statistically-significant reduction in body-weight
gain when compared to control groups. Food consumption, efficiency of
food utilisation, and water consumption were also statistically-
significantly lower in these rats when compared to control animals.
Haematological examination showed a statistically-significant
reduction in total white cell count in male rats of the 217 ppm group,
when compared to controls, at 26, 52, and 78 weeks, but not at 104
weeks. This change was not considered by the authors of the study to
be attributable to the administration of paraquat. Biochemical
examination indicated a statistically-significant reduction in
globulin in male rats of the 217 ppm group at 26, 78, and 104 weeks
when compared to controls. Clinical observations, RBC counts,
haemoglobin, mean red-cell volume (MCV), mean cell hamoglobin (MCH),
mean cell haemoglobin concentration (MCHC), platelet counts,
differential WBC counts, plasma alkaline phosphatase, lactic acid
dehydrogenase, blood urea nitrogen, glucose, total cholesterol, GOT,
GPT, total and direct bilirubin, GGPT, calcium, total protein,
albumin, and urinalysis indicated no significant effects attributable
to the administration of paraquat at any dose levels.
Throughout the entire administration period, a statistically-
significant reduction was found in the absolute weights of various
organs of male and female rats of the 217 ppm group at interim
sacrifices. This change was considered by the authors of the study
to be related to the reduction in body weight observed in these
animals. Histological examination of the lung at termination showed a
marked, treatment-related, statistically-significant increase in the
incidence of proliferation of interalveolar septum cells and of
hyperplasia of alveolar epithelium in both male and female rats at 217
ppm and in male rats at 72 ppm, when compared to controls. There was
a marked, statistically-significant increase in the incidence of
cataract in male and female rats of the 217 ppm group killed or found
dead after week 79. This treatment-related change was reported to be
the same microscopically as that observed in the tissues collected
from those control rats which had spontaneous, age-related cataracts.
Male rats of the 217 ppm group also showed a statistically-significant
increase in the incidence of local atrophy of renal tubules when
compared to controls. Females of the same dietary group had a
statistically-significant increase in the overall incidence of
diffusive fatty changes of the liver and pulmonary fibrosis when
compared to controls. Kidney and liver lesions were not considered by
the authors of the study to be attributable to the administration of
paraquat. A significant increase (details of statistical analysis were
not available) in the incidence of pulmonary adenoma (7/80) was found
in female rats of the 217 ppm group when compared to controls (1/80).
There was no significant increase in the incidence of lung adenoma in
male rats, but a few of them had lung adenocarcinoma (1 in each of the
22 and 72 ppm groups, 3 in the 217 ppm group, and none in the
controls). The authors noted that, although the historical incidence
of pulmonary adenoma in rats of this strain is reportedly rather low
(about 2%), 6/80 (7.5%) of the control rats developed pulmonary
adenoma in a 24-month chronic toxicity study carried out separately in
their laboratory. Based on these considerations, the authors of the
study concluded that the incidence of pulmonary adenoma found in the
present paraquat study in female rats in the 217 ppm group did not
exceed the background incidence of pulmonary adenoma in rats of this
strain. On the basis of the lung and eye lesions the no-effect level
of paraquat cation determined in this study for Fisher SPF rats after
104-week treatment was 22 ppm, equal to 0.77 mg/kg b.w./day in male
rats and 72 ppm, equal to 3.12 mg/kg b.w./day in female rats
(Yoshida et al., 1982).
Research into the mechanism of paraquat toxicity has identified
at least 2 partially toxic consequences of the redox cycling reaction:
a) generation of the superoxide anion radical, and b) oxidation of
cellular NADPH, which is the major source of reducing equivalents for
the intracellular reduction of paraquat. Generation of the superoxide
anion radical can lead to the formation of more toxic forms of reduced
oxygen, hydrogen peroxide (H2O2), and hydroxyl radicals. Hydroxyl
radicals have been implicated in the initiation of membrane damage by
lipid peroxidation, depolymerization of hyaluronic acid, inactivation
of proteins, and damage to DNA. Depletion of NADPH, on the other hand,
may disrupt important NADPH-requiring biochemical processes such as
fatty acid synthesis (Hassan & Fridovich, 1980; Smith et al., 1979).
The importance of molecular oxygen and the potential role of
superoxide anion radical generation in mediating paraquat toxicity
have been implicated in studies on plants, bacteria, and in vitro
and in vivo mammalian systems. In cultures of E. coli, Hassan &
Fridovich (1977, 1978, & 1979) demonstrated that paraquat stimulated
cyanide-resistant respiration, which could be almost entirely
accounted for by the NADPH-dependent formation of the superoxide anion
radical.
The possibility that formation of the superoxide anion radical
might be responsible for the toxicity of paraquat in bacteria is
supported by observations that bacteria containing elevated activities
of superoxide dismutase, an enzyme that detoxifies the superoxide
anion radical, were resistant to paraquat toxicity (Hassan &
Fridovich, 1977, 1978; Moody & Hassan, 1982).
In vitro studies on lung and liver preparations from various
animal species have supported the hypothesis that paraquat redox
cycling and associated superoxide anion radical and H202
generation also occur in mammalian systems (Gage, 1968; Ilett
et al., 1974; Montgomery, 1976, 1977; Steffen & Netter, 1979;
Talcott et al., 1979).
Bus et al. (1974) reported that the single electron reduction
of paraquat in mammalian systems was catalysed by microsmal cytochrome
P-450 reductase and NADPH. The observation that the in vivo toxicity
of paraquat in animals is markedly potentiated by exposure to elevated
oxygen tensions further supports the potential role for molecular
oxygen in mediating toxicity (Fisher et al., 1973; Autor, 1974;
Bus & Gibson, 1975; Witschi et al., 1977; Kehrer et al., 1979;
Keeling et al., 1981; Selman et al., 1985).
The results of in vivo studies conducted by Bus et al. (1974)
suggest that stimulation of lipid peroxidation, which is dependent on
paraquat redox cycling and associated superoxide anion radical
generation, might be an important toxic mechanism in mammalian
systems. Consistent with this hypothesis, animals fed diets deficient
in selenium or vitamin E in order to diminish cellular antioxidant
defenses were significantly more sensitive to paraquat toxicity than
control animals (Bus et al., 1975a; Omaye et al., 1978). Moreover,
selenium deficiency potentiated paraquat-induced lipid peroxidation in
isolated perfused rat lung (Glass et al., 1985). In contrast to
these studies, a number of studies have shown that paraquat inhibited
in vitro microsomal lipid peroxidation (Ilett et al., 1974;
Montgomery & Niewoehner, 1979; Steffen & Netter, 1979; Kornburst &
Mavis, 1980). Subsequent studies have indicated, however, that
paraquat would stimulate microsomal lipid peroxidation when an
adequate supply of electrons (NADPH) and in vitro oxygen tension
were maintained (Trush et al., 1981, 1982).
Despite the evidence described above, the hypothesis that lipid
peroxidation is the underlying toxic mechanism functioning in vivo
has not been conclusively demonstrated. Direct quantification of
paraquat-induced lipid peroxidation damage in vivo by analysis of
tissue malonadialdehyde levels or ethane exhalation, both markers of
peroxidation injury, has been largely unsuccessful (Reddy et al.,
1977; Shu et al., 1979; Steffen et al., 1980), although
significant increases of serum malondialdehyde levels have been
recently reported in patients with paraquat poisoning (Yasaka
et al., 1986). Furthermore, attempts to counteract paraquat
toxicity by administration of various antioxidants have also been
unsuccessful (Fairshter, 1981).
Superoxide radicals generated in paraquat redox cycling may
induce biochemical changes other than the initiation of the
peroxidation reaction. Ross et al. (1979) demonstrated that paraquat
increased DNA strand breaks in cultured mouse lymphoblasts. Paraquat
was also reported to induce a superoxide-dependent stimulation of
guanylate cyclase activity in rat liver (Vesely et al., 1979) and
guinea pig lung (Giri & Krishna, 1980). These investigators postulated
that increased cyclic-GMP might stimulate the pulmonary fibroproli-
ferative changes characteristic of paraquat toxicity. In other
studies, paraquat has also been found to increase collagen synthesis
in the rat lung (Greenberg et al., 1978; Thompson & Patrick, 1978;
Hussain & Bhatnagar, 1979).
Redox cycling of paraquat has also been proposed to lead to
increased oxidation of cellular NADPH (Brigelius et al., 1981;
Keeling et al., 1982). The activity of pentose shunt enzymes in the
lung rapidly increased in rats treated with paraquat, which suggested
an increased demand for NADPH (Fisher et al., 1975; Rose et al.,
1976b). The observation that paraquat decreased fatty acid synthesis
in lung slices (Smith et al., 1979) further supported this
hypothesis, since fatty acid synthesis requires NADPH. Direct analysis
of NADPH in the lung has long confirmed that paraquat treatment
decreases the NADPH content in rat lung (Witschi et al., 1977;
Smith et al., 1979). More recently, both oxygen consumption and
NADPH oxidation in lung microsomes were found to be significantly and
specifically stimulated by the addition of paraquat (Rossouw et al.,
1984). The above observations led Smith et al. (1979) to propose
that oxidation of NADPH might interrupt not only vital physiological
processes, such as fatty acid synthesis, but also may render tissues
more susceptible to lipid peroxidation by decreasing the equivalents
(NADPH) necessary for functioning of the antioxidant enzyme
glutathione peroxidase (Figure 2). Indeed, a significant increase
(589%) in lung-oxidized glultathione (GSSG) content was found over
control levels after perfusion of isolated rabbit lung with a 0.4 mM
paraquat solution. This effect was significantly increased (225%) by
hyperoxia (Dunbar et al., 1984).
Toxicological studies
Special studies on carcinogenicity
Mice
Groups of 60 male and 60 female Alderly Park SPF mice were fed
diets containing 0 (2 groups), 12.5, 37.5, or 100/125 ppm paraquat
cation for 97 - 99 weeks. The initial top dose of 100 ppm was
increased to 125 ppm at week 36 in order to evoke a toxic effect. The
study was terminated at weeks 97 - 99 when 80% mortality was reached
in a female control group and was approaching 80% overall. Clinical
observations, body-weight gain, food consumption, and urinary paraquat
were measured throughout the study. Histopathological examination of
approximately 40 tissues was performed on animals killed or dying
during the study and at termination. Further groups of 10 males and 10
females were fed the same dose levels as above for 52 weeks for
measurement of paraquat concentrations in the kidney, lung, and plasma
at termination.
Mortality ranged from 32 - 55% at 80 weeks and from 58 - 87% at
termination and was higher in the 37.5 ppm and 125 ppm groups than in
the combined controls. Effects due to treatment were renal lesions in
both sexes at 100/125 ppm and, in males, at 37.5 ppm; lung lesions in
both sexes at 100/125 ppm; and decreased food consumption and
body-weight gain and increased mortality in females at 100/125 ppm.
Histopathologically, the treatment-related renal lesions were manifest
as mild dilatation and degenerative changes in the tubules. The
incidences of tubular degeneration (with and without dilatation) in
male mice dying during the study were 31/48 at 100/125 ppm, 15/47 at
37.5 ppm, 9/45 at 12.5 ppm, and 8/45 and 3/35 in controls. The
paraquat-induced lung lesions noted at 100/125 ppm included focal
pneumonitis/alveolitis and hypercellularity of the alveolar walls.
Statistically-significant increases in the incidence of fatty changes
of the liver were reported at 37.5 and 100/125 ppm in males, when
compared to controls. Other hepatic changes were noted, with a
significantly-higher incidence in treated compared to control mice.
These changes, however, were not considered by the authors of the
study to be treatment-related. There were no effects observed at
12.5 ppm. Histopathological examination showed no clear evidence of
treatment-related neoplastic changes in these mice. The incidence of
pulmonary tumours in both males (7/24) and females (8/20) in the
100/125 ppm dose group dying from 79 - 98 weeks was somewhat higher
than in controls (5/37 in males and 6/39 in females). However, the
incidences of pulmonary tumours in the animals of the same groups
surviving to termination were lower than in controls.
The authors of the study concluded that paraquat was not
oncogenic to the mouse. Based on the renal lesions, the no-effect
level of paraquat cation for Alderley Park SPF mice in this study was
12.5 ppm, equal to 1.4 mg/kg b.w./day in males and 37.5 ppm, equal to
4.3 mg/kg b.w./day in females.
Rats
Groups of 80 male and 80 female Fisher SPF rats were maintained
on diets containing 0, 7.2, 22, 72, or 217 ppm paraquat cation for 104
weeks. Eight rats/sex/group were sacrificed after urinalysis at 26,
52, and 78 weeks and were subjected to haematological examination. All
surviving animals were sacrificed at 104 weeks and, among these, 10
rats/sex/group were subjected to haematological and biochemical
examination. All animals, including those killed on schedule and those
found moribund and killed during the study, were autopsied and
subjected to gross necropsy and histopathological examination of
approximately 30 tissues.
Mortality was increased in female rats of the 217 ppm group from
week 66 to week 74 when compared with that of other groups, including
controls. Both male and female rats at the 217 ppm dietary level
showed a marked statistically-significant reduction in body-weight
gain when compared to control groups. Food consumption, efficiency of
food utilisation, and water consumption were also statistically-
significantly lower in these rats when compared to control animals.
Haematological examination showed a statistically-significant
reduction in total white cell count in male rats of the 217 ppm group,
when compared to controls, at 26, 52, and 78 weeks, but not at 104
weeks. This change was not considered by the authors of the study to
be attributable to the administration of paraquat. Biochemical
examination indicated a statistically-significant reduction in
globulin in male rats of the 217 ppm group at 26, 78, and 104 weeks
when compared to controls. Clinical observations, RBC counts,
haemoglobin, mean red-cell volume (MCV), mean cell hamoglobin (MCH),
mean cell haemoglobin concentration (MCHC), platelet counts,
differential WBC counts, plasma alkaline phosphatase, lactic acid
dehydrogenase, blood urea nitrogen, glucose, total cholesterol, GOT,
GPT, total and direct bilirubin, GGPT, calcium, total protein,
albumin, and urinalysis indicated no significant effects attributable
to the administration of paraquat at any dose levels.
Throughout the entire administration period, a statistically-
significant reduction was found in the absolute weights of various
organs of male and female rats of the 217 ppm group at interim
sacrifices. This change was considered by the authors of the study
to be related to the reduction in body weight observed in these
animals. Histological examination of the lung at termination showed a
marked, treatment-related, statistically-significant increase in the
incidence of proliferation of interalveolar septum cells and of
hyperplasia of alveolar epithelium in both male and female rats at 217
ppm and in male rats at 72 ppm, when compared to controls. There was
a marked, statistically-significant increase in the incidence of
cataract in male and female rats of the 217 ppm group killed or found
dead after week 79. This treatment-related change was reported to be
the same microscopically as that observed in the tissues collected
from those control rats which had spontaneous, age-related cataracts.
Male rats of the 217 ppm group also showed a statistically-significant
increase in the incidence of local atrophy of renal tubules when
compared to controls. Females of the same dietary group had a
statistically-significant increase in the overall incidence of
diffusive fatty changes of the liver and pulmonary fibrosis when
compared to controls. Kidney and liver lesions were not considered by
the authors of the study to be attributable to the administration of
paraquat. A significant increase (details of statistical analysis were
not available) in the incidence of pulmonary adenoma (7/80) was found
in female rats of the 217 ppm group when compared to controls (1/80).
There was no significant increase in the incidence of lung adenoma in
male rats, but a few of them had lung adenocarcinoma (1 in each of the
22 and 72 ppm groups, 3 in the 217 ppm group, and none in the
controls). The authors noted that, although the historical incidence
of pulmonary adenoma in rats of this strain is reportedly rather low
(about 2%), 6/80 (7.5%) of the control rats developed pulmonary
adenoma in a 24-month chronic toxicity study carried out separately in
their laboratory. Based on these considerations, the authors of the
study concluded that the incidence of pulmonary adenoma found in the
present paraquat study in female rats in the 217 ppm group did not
exceed the background incidence of pulmonary adenoma in rats of this
strain. On the basis of the lung and eye lesions the no-effect level
of paraquat cation determined in this study for Fisher SPF rats after
104-week treatment was 22 ppm, equal to 0.77 mg/kg b.w./day in male
rats and 72 ppm, equal to 3.12 mg/kg b.w./day in female rats
(Yoshida et al., 1982).
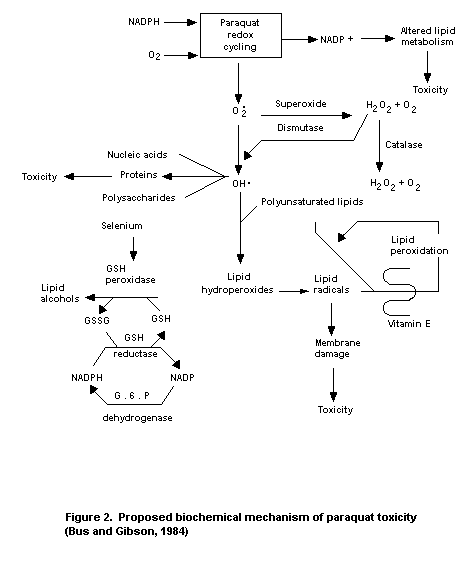 Historical control incidence data of neoplasia in F-344 rats in
the laboratory in which the preceding study was conducted were made
available and are summarized in Table 3.
Table 3. Spontaneous lung tumours observed in F-344 rats at the
Institute of Environmental Toxicology from 1980 to 19831
No. of tumour-bearing animals (%)2
Males Females
Adenoma 40 (4.2%) 21 (2.2%)
Adenocarcinoma 5 (0.5%) 1 (0.1%)
Bronchial gland adenoma 1 (0.1%) -
Total 46 (4.8%) 22 (2.3%)
1 From Maita, 1986
2 These data were taken from 12 studies that included 960 male and
959 female F-344/DuCrj rats.
Groups of 60 male and 60 female Fischer 344 rats were maintained
on diets containing paraquat cation at 0 (2 groups), 25, 75, or
150 ppm for at least 113 weeks (males) or 122 weeks (females). Further
groups of 10 rats/sex/group received the same diets for 1 year. All
animals were studied for mortality, food and water consumption, and
body weight, and were subjected to periodical ophthalmoscopic and
haematological examinations throughout the study.
The distribution of mortality was unaffected by treatment. There
was approximately 50% mortality in all groups at the end of the study.
At 150 ppm, statistically-significant reductions in body-weight gain,
food consumption, and efficiency of food utilisation in both sexes
were observed. There was a statistically-significant depression of
body-weight gain in the first year of the study in males receiving
75 ppm paraquat. Water consumption was not significantly affected at
any dietary level tested. Paraquat accelerated, in a dosage-dependent
manner, the onset and progression of cataract changes, ranging from
minor opacity to total cataract in both males and females.
Treatment-related ocular lesions were first seen at 52 weeks.
Thereafter, ophthalmoscopy revealed a statistically-significant
dosage-related increase in the incidence, progression, and severity of
lenticular cataract in the 150 ppm group and, toward the end of the
study (103 weeks), in the 75 ppm group. There was evidence of
paraquat-dependent ocular effects in all treatment groups of both
sexes at termination. A statistically-significant higher incidence of
secondary eye lesions was found at termination in females receiving 75
or 150 ppm paraquat when compared to controls. Haematological
investigation (RBC counts, total and differential leucocyte counts,
haemoglobin, haematocrit, mean cell volume, mean cell haemoglobin
concentration, platelet and reticulocyte counts, and prothrombin and
partial thromboplastin times) and blood biochemistry (urea, glucose,
ALT, and AST) indicated no significant effects attributable to
paraquat administration. Urinalysis did not reveal any treatment-
related changes. Reductions in liver and testicular weights were noted
at termination in the 150 ppm dietary group.
Macroscopic examination at necropsy revealed a treatment-related
increase in the incidence of focal subpleural changes in animals
killed at termination in all dietary groups. This effect was most
marked in females receiving 75 ppm and in both sexes receiving 150 ppm
paraquat. Microscopic examination of lung tissues indicated that
treatment with paraquat at 150 ppm, in both sexes, and possibly at
75 ppm in males, was associated with proliferative lesions of the
alveolar epithelium. These lesions were not easily classified into
non-neoplastic or neoplastic, nor into adenoma or carcinoma. This
study provided strong evidence for the induction by paraquat of a
proliferative lesion of the alveolar epithelium and some controversial
evidence for the induction of lung adenomas in female Fischer 344
rats. There was no treatment-related increase in the incidence of lung
adenocarcinoma at any dose level in either sex. At 25 ppm, significant
increases in the incidence of proliferative lung lesions, compared to
the controls, were not observed. Microscopic examination of the eyes
confirmed a dose-related effect of paraquat on the onset and
progression of cataract lesions normally present in both male and
female F-344 rats. Slight dilation of the fourth ventricle of the
brain was evident in females receiving 150 or 75 ppm paraquat, but not
in males at these dosages or in either sex at 25 ppm. A statistically-
significant increase in the incidence of apparent degeneration of
occasional/several sciatic nerve fibers was noted in decedent males
receiving 75 or 150 ppm paraquat. Both hydrocephalus and nervous
tissue changes were considered by the authors of the study possibly
to be associated with paraquat treatment. Pathology summaries
indicate that atrophy of the testes was recorded in the high-dietary
group (5/33) but not in controls at termination, and moderate lymphoid
hyperplasia was observed in the respiratory epithelium of males
receiving 75 and 150 ppm paraquat and dying between 52 weeks and
termination.
The authors of the study concluded that "a wide range of tumour
types was observed in treated and control animals, and there was no
evidence that treatment with paraquat resulted in a marked change in
the group distribution of any of these tumours". A no-effect level for
paraquat was not found in this study due to the higher incidence of
cataract observed in animals of the 25 ppm group when compared to
controls. Paraquat accelerated, in a dosage-dependent manner, the
onset and progression of cataract change in F-334 rats. The authors
considered 25 ppm to be near the no-effect level for this change at
the end of the study (Ashby et al., 1983; Busey, 1986; Ishmael &
Godley, 1983).
Data on the historical incidence of lung neoplastic lesions in
F-344 rats from 7 studies performed in the laboratory in which the
preceding study was conducted were made available and are summarised
in Table 4.
Table 4. Spontaneous lung tumours observed in F-344 rats at Life
Science Research1
No. of tumour-bearing animals (%)2
Males Females
Adenoma 6/357 (1.7%) 4/363 (1.1%)
Carcinoma 3/357 (0.8%) 0/363 (0%)
Total pulmonary tumours 9/357 (2.5%) 4/363 (1.1%)
1 From Ashby et al., 1983
2 The range (% incidence) of adenoma was 0 - 4.4% in males and
0 - 4.0% in females; the range of carcinoma was 0 - 4.0% in
males.
Special studies on embryotoxicity and teratogenicity
Mice
The teratogenicity and fetal toxicity of paraquat were examined
after oral (20 mg/kg b.w./day) or i.p. (1.67 or 3.35 mg/kg b.w./day)
administration of paraquat to pregnant mice during the period of
organogenesis (days 8 - 16 of gestation). The oral dose (which was
equal to 1/10 of the oral LD50/day) did not produce significant
maternal toxicity, but at the higher of the 2 i.p. doses (which was
equal to 1/10 of the i.p. LD50/day) a significant maternal mortality
(5/7) and a statistically-significant increase of resorption rate,
when compared to controls, were observed. Paraquat did not
significantly increase the incidence of gross, soft-tissue, or
skeletal abnormalities. At the lower i.p. dose and after oral
administration of paraquat, there was a slight but non-significant
increase in the number of fetuses with absent or non-ossified
sternebrae. However, a significant difference was observed in the
incidence of abnormal sternebrae between the 2 control groups (6.9 ±
3.2% in the i.p. control group and 13.2 ± 5.8% in the oral control
group). The authors of the study concluded that the potential for
paraquat as a teratogen appeared to be minimal (Bus et al., 1975b).
The same authors examined the effects of paraquat dichloride on
the development of Swiss-Webster mice when administered in the
drinking water at concentrations of 0, 50, or 100 ppm. Paraquat was
given to pregnant mice from day 8 of gestation and administration was
continued to the newborns until 42 days after birth, when both the
control and paraquat-treated mice were sacrificed and subjected to
histopathological examination of the lungs, liver, and kidneys. A
significant increase in postnatal mortality in mice receiving 100 ppm
paraquat was observed. Histopathological examination of the lungs of
these mice showed extensive alveolar consolidation and collapse, and
areas of thickening of intra-alveolar septa. No significant
pathological changes were seen in the lungs of the 50 ppm or control
mice, nor in the liver or kidneys of mice of any treatment group.
There were no treatment-related effects on the number of live fetuses
nor on postnatal growth rate at either treatment level (Bus & Gibson,
1975).
Four groups of at least 20 pregnant SPF Alderley Park mice were
given orally 0, 1, 5, or 10 mg/kg b.w./day of paraquat cation during
days 6 to 15 of pregnancy, inclusive. On day 18 the animals were
killed, their uteri were examined, and the fetuses were removed,
weighed, sexed, and observed for gross abnormalities. There was some
evidence of maternal toxicity in the form of slight reductions in
body-weight gain at 5 and 10 mg/kg b.w./day, although only that of the
middle-dose group was statistically significant. There were no
clinical signs nor pathological changes in maternal animals
attributable to paraquat administration. Water and food consumption
were not quantified in this study. Numbers of implantations, viable
fetuses and resorptions, sex ratios, and fetal and litter weights
showed no significant differences between treated and control groups.
There were no increases in fetal external or soft-tissue abnormalities
which could be associated with paraquat treatment. There were
occasional statistically-significant differences in ossification of
individual bones between treated and control groups, but no
dose-related trend indicating either retardation of ossification or
increased abnormalities was observed. The authors of the study
concluded that paraquat was not teratogenic and had no significant
influence on embryonic or fetal development of the mouse at levels up
to and including 10 mg/kg b.w./day (Hodge et al., 1978a).
Rats
The teratogenic effects of paraquat were studied in 4 groups of
at least 20 pregnant SPF Alderley Park rats after oral administration
of 0, 1, 5, or 10 mg/kg b.w./day of paraquat cation during days 6 to
15 of pregnancy, inclusive. On day 21 the animals were killed. There
were clear clinical signs of maternal toxicity at 5 and 10 mg/kg
b.w./day. Apparently, 6 rats at the highest-dose level and 2 at the
middle-dose level died or became moribund during the experiment.
Histological changes found in the lungs and kidneys of the animals
receiving 10 mg/kg b.w./day paraquat which died or became moribund
were those known to be associated with oral paraquat poisoning. Slight
fetotoxicity was seen at 5 and 10 mg/kg b.w./day, as shown by a
statistically-significant reduction in fetal weight and retardation in
ossification, and by a decrease in the number of viable fetuses per
number of implants. According to the authors of the study, these
effects were probably associated with maternal toxicity. There were no
effects on embryonic or fetal survival and increases in fetal
abnormalities were not observed. The authors of the study concluded
that paraquat was not teratogenic when administered orally to rats,
even when there was clear evidence of maternal toxicity. However, it
did cause slight fetotoxicity at the 2 highest-dose levels (Hodge
et al., 1978b).
Special studies on eye irritation
The effects of paraquat on the eye have been reviewed by WHO
(1984). The instillation of diluted paraquat (up to 500 g/litre) in
rabbits' eyes induced inflammation within 24 hours, and this continued
for 96 hours (Clark et al., 1966). In another experiment, 62.5, 125,
250, 500, or 1000 g/litre of paraquat was introduced into the eyes of
rabbits. Concentrations of 62.5 and 125 g/litre caused severe
conjunctival reactions; higher levels (250 - 500 g/litre) provoked
ititis and pannus, while at the 500 g/litre concentration corneal
opacification, iritis, and conjunctivitis occurred. All rabbits
receiving 0.2 ml of paraquat at 1000 g/litre in 1 eye or 0.2 ml of
500 g/litre paraquat in both eyes died within 6 days of application
(Sinow & Wei, 1973).
Special studies on mutagenicity
In a review of published mutagenicity data (WHO, 1984) it was
noted that paraquat had been found to have minimal to no genotoxic
activity when evaluated in a variety of in vitro and in vivo test
systems. In in vitro studies producing weakly positive results,
paraquat genotoxicity was accompanied by high cytotoxicity (Moody &
Hassan, 1982; Parry, 1973 & 1977; Tweats, 1975; Benigni et al.,
1979; Bignami & Grebelli, 1979). Moody and Hassan (1982) have shown
that the mutagenicity of paraquat in bacterial test systems
(Salmonella typhimurium TA98 and TA100) was mediated by the
formation of superoxide. More recently, paraquat was found to induce
superoxide dismutase, chromosomal aberrations, and sister-chromatid
exchange in Chinese hamster fibroblasts, suggesting that superoxide
production is responsible for the chromosomal damage (Nicotera
et al., 1985). Other investigators (Anderson et al., 1972; Levin
et al., 1982) have not found mutagenic activity in bacterial test
systems. Furthermore, paraquat was not mutagenic when evaluated in
human leukocytes nor in in vivo cytogenic tests on mouse bone marrow
(Selypes & Paldy, 1978) or in dominant lethal tests on mice
(Pasi et al., 1974; Anderson et al., 1976).
A set of recently completed studies indicate that in most tests
paraquat was not mutagenic (see Table 5). Clastogenic potential has
been shown in vitro at very high concentration levels which were
themselves cytotoxic. Paraquat was not found to be mutagenic
in vivo.
Special studies on reproduction
Rats
In a 3-generation study, groups of 15 male and 30 female (F0
parents) weanling Alderley Park SPF rats were fed diets containing
0, 25, 75, or 150 ppm paraquat cation. After 12 weeks, animals were
mated to produce the first (F1a) litter and subsequently re-mated to
produce a second (F1b) litter. The breeding programme was repeated
twice with F1 parents selected from the F1b offspring and F2
parents selected from the F2b offspring. Test diets were fed
continuously throughout the study.
There were no adverse effects on parental body weights or food
consumption, and no treatment-related changes were found in the
reproductive performance (male and female fertility, live-born and
survival indexes, and litter size) or in the reproductive tract of
parents or offspring. Development of the reproductive tract in all
treated offspring was substantially comparable to that in controls.
The mild atrophy of seminal tubules found in a few of the treated
males of the F2b offspring at termination was considered by the
authors of the study to have no toxicological significance. Lung
changes due to paraquat administration occurred mainly in females
receiving 150 ppm paraquat. One pulmonary adenoma was found in 1
female receiving 150 ppm paraquat. Death resulting from severe, acute,
or sub-acute lung damage was confined to females with litters of
weaning age, and to 3 F0 females which died during the first 2 weeks
of the study. There were dose-related increases in the incidence and
Table 5. Mutagenicity assays on paraquat
Test system Test object Concentration Purity Results Reference
used (%)
Ames test1 S. typhimurium 0.12, 0.6, 2.9, 99 Negative Anderson, 1977
TA98 14, 72, 361,
TA100 & 1807 µg/plate
TA1535 (dissolved in
TA1538 H2O)
Ames test1 S. typhimurium 0.4, 0.7, 3.6, 100 Negative, growth Shirasu et al., 1978
TA98 7, 36, 72, & inhibition at 72
TA100 360 µg/plate at 72 & 360
TA1535 (dissolved in µg/plate2
TA1538 H2O)
Host-mediated S. typhimurium 3.6 & 14 mg/kg 100 negative2 Shirasu et al., 1978
assay G 46 (host: male (2 equal doses
ICR mice) orally)
Rec-assay B. subtilis 14 - 361 100 negative2 Shirasu et al., 1978
µg/disk
Mouse L 5178 Y 23, 45, 90, 180, 99 ? Clay & Thomas, 1985
lymphoma mouse & 361 µg/ml
test1 lymphoma
cells
Table 5. (cont'd).
Test system Test object Concentration Purity Results Reference
used (%)
Clastogenic Human 90, 903, & 1807 99.6 positive at 2 Sheldon et al., 1985a
potential lymphocytes µg/ml highest levels
test1 in vitro (also cytotoxic)
Mouse Male & female 51.7 & 82.8 99.4 negative Sheldon et al., 1985b
micronucleus C 57/BL/6J/ mg/kg (single
test Alpk mice dose orally)
In vitro Chinese 0.9, 1.8, 9, 18, 99.4 positive (reduced Howard et al., 1985
sister hamster lung 90 & 177 µg/ml with metabolic
chromatid fibroblasts activation)
exchange1
Unscheduled Hepatocyte 1 nM - 10 mM 99.6 negative Trueman et al., 1985
DNA cultures (0.19 ng/ml -
synthesis from male 1.86 mg/ml)
Alderley Park
albino rats
1 Both with and without metabolic activation
2 Positive control compounds gave positive responses
severity of focal alveolar histiocytosis in the lungs of male and
female parents receiving 75 and 150 ppm paraquat. A mild perivascular
inflammation was observed in the lungs of F1b pups receiving 150 ppm
paraquat. No changes due to paraquat were seen in animals receiving
25 ppm paraquat. The authors concluded that paraquat had no effect on
reproductive performance or development of the reproductive organs of
Alderley Park rats when administered at dietary levels up to 150 ppm
over 3 generations (Lindsay et al., 1982).
Groups of 12 male and 24 female rats were fed diets containing
0, 30, or 100 ppm paraquat ion from 35 days of age. Three generations
bred from these animals received the same diets during the whole
period under test. Two litters were bred from each generation, and the
effects on growth, food intake, fertility, fecundity, neonatal
morbidity, and mortality were noted. No evidence was seen of damage to
germ-cell production or of structural or functional damage in the
animals. In this study, pregnant and young animals did not appear to
be more vulnerable to paraquat than did adults. However, the incidence
of renal hydropic degeneration in 3 - 4 week-old offspring was
slightly increased in the 100 ppm group (Fletcher et al., 1972).
In a 3-generation study with 2 litters per generation, groups of
30 male and 30 female Sprague-Dawley rats (F0 parental generation)
were given diets containing 0, 72, 145, or 290 ppm paraquat cation
from 5 weeks of age (13 weeks prior to mating to obtain the first
litter, F1a) until the end of the second lactation (lactation of
F1b litters). In the second generation (F1b), 30 males and 30
females per group were treated from immediately after weaning until
the end of the second lactation (lactation of F2b litters). In the
third generation (F2b), the same number of rats were treated
immediately after weaning for at least 13 weeks. In a teratology study
sub-group 5 pregnant females of the parental generation (F0) and 10
pregnant females of the second generation (F1b) were killed on day
20 of pregnancy and examined macroscopically. Fetuses were examined
for number, sex, weight, external and internal abnormalities, and
progress of ossification. Another subgroup was used as a postnatal
investigation group, where natural parturition of pregnant females was
permitted to occur. In this group the duration of gestation,
parturition conditions, the number of live and still-born pups, sex,
and external abnormalities were recorded. Live pups were investigated
until weaning. Five male and 5 female rats per group of the first
(F0) and second (F1b) generations and 10 male and 10 female rats
per group of the F2b litters were subjected to histopathological
examination of approximately 25 tissues.
In the parental generation there were significant increases in
mortality and clinical signs attributable to paraquat (asthmatoid
wheezing) in several rats of the 290 ppm group of each generation from
the early stage of the dosing period. There were 29 treatment-related
deaths/moribund animals, all from the 290 ppm dietary level (5 among
F1b animals and 24 among F2b animals), but only 4 deaths among
control rats (1 among F0 female rats and 2 among F1b female rats
at parturition and 1 among F2b rats during the dosing period).
Histopathological examination of these dead or moribund F2b rats
showed, in some cases, hyperplasia of the alveolar epithelium and, in
most cases, diffuse thickening and fibrosis of the alveolar walls.
There was a decrease in body-weight gain in both male and female rats
of the F0 and F2b generations at 290 ppm during the early stage of
the dosing period. Body-weight gain was also reduced in F1b females
at 290 ppm during the gestation and lactation periods. Reductions in
food consumption and efficiency of food utilisation were seen in F0
and F2b females. There was a significant decrease in water
consumption in F0 and F1b females during lactation. No effect of
the compound was observed on the reproductive performance of parental
rats. Macroscopic examination revealed an apparently higher incidence
of white spots in the lungs of both male and female rats of the
290 ppm group in all 3 generations. Treatment-related changes of the
lung were confirmed by histopathology in rats of each generation.
These lesions were dose-dependent and included zonal thickening and
fibrosis of the alveolar walls, zonal atelectasis, and accumulation of
foam cells. There were no treatment-related changes in organ weights.
A treatment-related statistically-significant reduction in the
lactation index was found in F1a and F1b litters of the 290 ppm
group. A statistically-significant reduction in the lactation index
was also observed in F2a litters of the 290 ppm group, but it was
not clear to the authors of the report whether this change was
attributable to treatment. There were no statistically-significant
differences in lactation index between other treatment groups and
controls nor in the number of still-births and live births, sex
ratios, or viability indexes in any of the treated groups of both
generations, when compared to controls. The prolonged duration of
gestation in F0 rats of the parental group at 145 and 290 ppm was
considered by the authors of the report to be accidental. The
teratology phase showed a statistically-significant delay in
ossification in F1b fetuses from F0 parents treated with 290 ppm
paraquat and in F2b fetuses from F1b parents of all treated
groups. It was not clear to the authors of the report whether the
retarded growth was due to the treatment. There was a treatment-
related statistically-significant higher incidence of female
pups with retarded opening of the vagina in both F1b and F2b
litters at 290 ppm. There was a statistically-significant decrease in
body weight in male, but not in female, fetuses at 72 and 290 ppm.
There were no statistically-significant differences between fetuses
from treated rats and control fetuses in the number of corpora lutea
or implantations, percentage of implantations, number of dead or live
fetuses, sex ratios, or placental weights. No external or internal
malformations were detected in fetuses of any treatment group.
The authors of the report concluded that there was no evidence
suggesting that paraquat was teratogenic and that the only treatment-
related change which was enhanced by treatment of successive
generations of Sprague-Dawley rats with paraquat was "an increase in
death" at 290 ppm. A no-effect level for paraquat was not found in
this study due to delayed ossification in F2b fetuses in all treated
groups (Suzuki et al., 1983).
Rabbits
Forty rabbits received paraquat i.p. at total dosages of 2 to
100 mg/kg b.w. in 1 to 5 separate administrations. Multinuclear giant
cells were found in testicular tubules of 7/20 rabbits receiving
50 mg/kg b.w. or more paraquat (Butler & Kleinerman, 1971). However,
it has been reported that, when paraquat was orally administered at
4 mg/kg b.w. to male rats for 60 days and testes were examined, there
were no significant deviations in the spermatozoa count or motility,
nor were there any biochemical changes in the several enzymes of
testes homogenates. The histoenzyme activity of lactate dehydrogenase,
succinate dehydrogenase, DPN-diaphorase, alkaline phosphatase, and
acid phosphatase in the treated animals did not differ from those of
the controls, nor did quantitative and qualitative histological
examination of the testicular tubule cells reveal any abnormalities
(WHO, 1984).
Special studies on skin irritation
The effects of paraquat on the skin have been reviewed by WHO
(1984). Paraquat can provoke local irritation of the skin and eyes.
Clark et al. (1966) found skin irritation in rabbits only when
paraquat was applied beneath occlusive dressings in aqueous solutions
(total doses 1.56, 5.0, and 6.25 mg ion/kg b.w.). In mice and rats,
the application of solutions of 5 - 20g paraquat/litre in single and
21-day repeated dermal toxicity tests provoked dose-related toxic
dermatitis with erythema, oedema, desquamation, and necrosis (Bainova,
1969). Doses from 1.56 to 50 mg/kg, in repeated 20-day studies using
the occlusive technique (McElligott, 1972), resulted in local erythema
and scab formation. The histological changes consisted of
parakeratosis and occasional intra-epidermal pustules. A delayed
skin-irritant action of the herbicide was reported by Fodri et al.
(1977) in guinea pig studies.
Acute toxicity
The LD50 and LC50 values for paraquat in various species are
given in Table 6.
Table 6. Acute toxicity of paraquat in various species
LD50 LC50
Species Route Sex (mg/kg b.w.) (mg/l) Reference
Mouse oral M 260 -- Shirasu & Takahashi, 1977
F 210 --
i.p. M & F 29-30 -- Shirasu & Takahashi, 1977
Bus et al., 1975a
i.v. -- 50 -- Ecker et al., 1975
s.c. M 30 -- Shirasu & Takahashi, 1977
F 27 --
dermal -- 62 -- Bainova, 1971
Rat oral M 161 -- Shirasu & Takahashi, 1977
F 187 --
M 110 -- Kimbrough & Gaines, 1970
F 100 --
-- 126 -- Murray & Gibson, 1972
-- 200 -- Howe & Wright, 1965
i.p. M 18 -- Shirasu & Takahashi, 1977
F 19 --
F 19 -- Clark et al., 1966
s.c. M 19 -- Shirasu & Takahashi, 1977
F 23 --
-- 22 -- Makovskii, 1972
dermal M 90 -- Kimbrough & Gaines, 1970
F 80 --
-- 350 -- Makovskii, 1972
Table 6. (cont'd).
LD50 LC50
Species Route Sex (mg/kg b.w.) (mg/l) Reference
inhalation M & F -- 10 Bainova & Vulcheva, 1972
-- -- 1 Gage, 1968
-- -- 6 Makovskii, 1972
Guinea oral M 30 -- Clark et al., 1966
pig
-- 40-80 -- Howe & Wright, 1965
-- 22 -- Murray & Gibson, 1972
-- 42 -- Makovskii, 1972
i.p. F 3 -- Clark et al., 1966
s.c. -- 5 -- Makovskii, 1972
dermal -- 319 -- Makovskii, 1972
inhalation -- -- 4 Makovskii, 1972
Cat oral F 35 -- Clark et al., 1966
-- 40-50 -- Howe & Wright, 1965
Hen oral -- 300-380 -- Howe & Wright, 1965
-- 262 -- Clark et al., 1966
Turkey oral -- 250-280 -- Smalley, 1973
i.p. -- 100 -- Smalley, 1973
i.v. -- 20 -- Smalley, 1973
dermal -- 375 -- Smalley, 1973
Monkey oral -- 50 -- Murray & Gibson, 1972
Sheep oral -- 50-75 -- Howe & Wright, 1965
Cow oral -- 50-75 -- Howe & Wright, 1965
Following a single high dose of paraquat to animals, the earliest
ultrastructural changes were observed in the Type I alveolar
epithelial cells, approximately 4 - 6 hours after treatment, and were
usually characterised by cellular and mitochondrial swelling,
increased numbers of mitochondria, and the appearance of dark granules
in the cytoplasm. When a high dose was given (equal to approximately
the LD50 or greater), the lesions in the Type I cells often
progressed to the point of complete cellular disintegration, leaving
areas of exposed basement membrane (Kimbrough & Gaines, 1970;
Smith et al., 1973; Smith & Heath, 1974; Vijeyaratnam & Corrin,
1971; Klika et al., 1980). In contrast to the effects on Type I
pneumocytes, however, the capillary endothelial cells were remarkably
resistant to the toxic effects of paraquat (Sykes et al., 1977).
Ultrastructural lesions in the alveolar Type II pneumocytes were
also observed shortly after single-dose paraquat exposure, although,
generally, these lesions were not apparent until after the first
lesions were seen in the Type I cells (Kimbrough & Gaines, 1970).
Swollen mitochondria and damage to the lamellar bodies usually
occurred between 8 and 24 hours after a high dose of paraquat
(Robertson, 1973; Robertson et al., 1976). Progressive deterioration
of the Type II cells continued, resulting in completely denuded
alveolar basement membranes and debris-filled alveolar spaces
(Vijeyaratnam & Corrin, 1971). Infiltration and proliferation of
fibroblasts may produce fibrosis that obliterated the alveolar
structure (Smith & Heath, 1974).
Vijeyaratnam & Corrin (1971) observed that less-severely affected
parts of the lung appeared to undergo epithelial regeneration 7 - 14
days after a single dose of paraquat. Electron microscopic examination
revealed the alveoli to be lined with cuboidal epithelial cells that
closely resembled Type II pneumocytes, except for a general lack of
lamellar bodies. Similar phenomena have also been noted by other
investigators who administered paraquat in the diet (Kimbrough &
Linder, 1973) or as repetitive i.p. administrations (Smith et al.,
1974). Thus, in animals where the paraquat dose was sufficient to kill
only the Type I pneumocytes, the surviving Type II cells repaired the
damaged epithelium by proliferating and subsequently differentiating
into Type I epithelial cells. Inhaled paraquat in aerosol produced
initial necrosis of the epithelia and Type II pbeumocyte hyperplasia,
fibroblast proliferation, and increased synthesis of collagen in mice
(Popenoe, 1979).
The pathogenesis of the paraquat lung lesion has been well
characterised, and has been reviewed by Smith & Heath (1976). The
acute pulmonary toxicity of paraquat in animals has been described as
occuring in 2 phases. In the initial "destructive" phase, alveolar
epithelial cells were extensively damaged and their subsequent
disintegration often resulted in a completely denuded alveolar
basement membrane. Pulmonary oedema was also a characteristic of the
destructive phase, and was frequently of sufficient severity to result
in the death of the animals. Animals surviving the initial destructive
phase, which occurred in the first 1 - 4 days after acute paraquat
overexposure, progressed to what has been termed the "proliferative"
phase. In this phase, the lung was infiltrated with prolifroblastic
cells that rapidly differentiated into fibroblasts which, in some
cases, progressed to fibrosis. The histopathological outcome of the
second phase may be influenced by the treatment regimen, however.
Administration of repeated low doses of paraquat, which less-severely
damaged the alveolar epithelial cells, was also able to induce a
hyperplasia of the Type II cells. This response may represent an
attempt by the lung to repair the damaged epithelium (WHO, 1984).
When rabbits were injected i.p. with total doses of paraquat from
2 - 100 mg/kg b.w., thymic atrophy was observed, but most lungs showed
only occasional and small histological deviations that were poorly
correlated with the clinical signs of paraquat intoxication. These
results confirmed the resistance of the rabbit to paraquat-induced
lung lesions (Butler & Kleinerman, 1971).
According to Murray & Gibson (1972) and Hundsdorfer & Rose
(1980), guinea pigs treated with paraquat either orally or s.c. did
not develop the same type of progressive pulmonary fibrosis as
paraquat-intoxicated rats. In hamsters, a single administration of
paraquat did not induce lung damage, but prolonged exposure resulted
in lung fibrosis (Butler, 1975).
In conclusion, from lung toxicity studies, a characteristic
dose-related pulmonary fibrosis can be induced in rats, mice, dogs,
and monkeys, but not in rabbits, guinea pigs, or hamsters.
In paraquat toxicity, kidney damage often precedes signs of
respiratory distress (Clark et al., 1966; Butler & Kleinerman, 1971;
Murray & Gibson, 1972). Paraquat is excreted primarily via the urine
and the concentrations of the herbicide in the kidneys are relatively
high (see Table 1). Gross pathological and histological examination of
paraquat-poisoned rats, guinea pigs, rabbits, and dogs revealed
vacuolation of the convoluted renal tubules and proximal tubular
necrosis (Murray & Gibson, 1972). The nephrotoxicity caused by
paraquat is pronounced and appears to be restricted to the proximal
nephron (Ecker et al., 1975; Gibson & Cagen, 1977; Lock & Ishmael,
1979; Purser & Rose, 1979). The degeneration of the proximal tubular
cells has also been confirmed by electron-optical studies (Fowler &
Brooks, 1971; Marek et al., 1981).
In contrast with lung and kidney damage, liver damage in
experimental animals has not been severe and serum enzyme activities
(SGOT, SGPT, LAP) only increased when large amounts of paraquat were
given (Girl et al., 1979). Recently, electron microscopic
examination of the liver of paraquat-treated rats showed early,
localised changes (degranualtion of the RER, proliferation of the SER,
and mitochondrial swelling) in hepatocytes within 2 layers around the
central vein (Matsumori et al., 1984).
Short-term studies
Mice
Groups of 20 male and 20 female ICR-CRJ SPF mice were maintained
on diets containing 0, 7.2, 22, 72, or 217 ppm paraquat cation for 13
weeks. At the 217 ppm dietary level 2 female mice died from pulmonary
damage. Both males and females in this group showed significantly
reduced body-weight gain and a slight reduction in efficiency of food
utilisation. Food intake and water intake were not affected. No
abnormalities considered related to paraquat treatment were seen
during haematological, blood biochemistry, or urine analysis. A few
statistically-significant changes in absolute and relative organ
weights were seen at termination, mainly in males and females of the
217 ppm group. However, only an increase in lung weight of females in
the 217 ppm group was reported by the authors of the study to coincide
with histopathological changes of the same organ, namely eosinophilic
swelling of the alveolar epithelium walls which was observed in both
sexes at this dietary level. The no-effect level in this study with
respect to pulmonary damage and other parameters was 72 ppm, equal to
12 (males) and 14 (females) mg/kg b.w./day (Malta et al., 1980a).
Rats
Groups of 20 male and 20 female Fischer 344 rats were maintained
on diets containing 0, 7.2, 22, 72, or 217 ppm paraquat cation for 13
weeks. During the study there were no deaths. Body-weight gain was
reduced markedly in both sexes at the 217 ppm dietary level, at which
level food consumption, efficiency of food utilization, and water
consumption were also reduced. Histopathological examination revealed
swelling of alveolar epithelium cells in males and increased deposits
of brown pigment in the spleen of females at the 217 ppm dietary
inclusing level. No abnormalities considered attributable to paraquat
administration were observed during heamatological, blood
biochemistry, urine, organ weight, or gross necropsy investigations.
The no-effect level in this study with respect to lung lesions and
other parameters was 72 ppm, equal to 6.5 (males) and 7.1 (females)
mg/kg b.w./day (Malta et al., 1980b).
Dogs
Groups of beagle dogs, 3 males and 3 females per group, received
diets containing 0, 7, 20, 60, or 120 ppm paraquat cation for 13
weeks. Two males and 2 females in the 120 ppm group showed marked
paraquat toxicity and were killed in extremis between days 16 and
23, having shown marked dyspnoea and body-weight loss. Both surviving
dogs at 120 ppm also showed body-weight loss. A slight overall
reduction in body-weight gain among the females of the other treatment
groups was not considered by the authors of the study to be
treatment-related. Lung weights were increased in all animals in the
120 ppm group and in 2 animals from the 60 ppm group. All other organ
weights were in the normal range. Distinct gross and histological
treatment-related lung lesions were seen in all dogs in the 60 and
120 ppm groups. Minor renal lesions (swelling of the cortical tubules)
were also found histologically in a few of these animals. There were
no discernible gross or histological treatment-related pulmonary
lesions in the dogs of the 7 or 20 ppm groups. The focal pulmonary
lesions in these animals were of a type and incidence similar to those
found in the controls. Microscopic examination of 34 other tissues
from each animal showed no treatment-related changes. There were no
treatment-related effects on food intake except for 1 surviving
high-dose female which showed a loss of appetite from week 8 onward.
There were no distinct treatment-related changes in any of the
haematological (RBC counts, mean cell volume, haemoglobin, total and
differential white cell counts, platelets, prothrombin, and partial
thromboplastin time), biochemical (glucose, cholesterol, blood urea
nitrogen, bilirubin, total and partial protein, GOT, GPT, ALP, and
CPK), or urinary parameters examined. Slight haemoconcentration was
seen in 1 high-dose dog at termination. The no-effect level in this
study after administration of paraquat for 13 weeks to beagle dogs, on
the basis of lung and kidney lesions, was considered to be 20 ppm,
equal to 0.57 mg/kg b.w./day (Sheppard, 1981).
Long-term studies
See also under "Special studies on carcinogenicity".
Mice
Groups of 60 male and 60 female JCL:ICR mice were maintained on
diets containing 0, 1.4, 7.2, 22, or 72 ppm paraquat cation for 104
weeks and then killed and examined. Further groups of 10 males and 10
females receiving the same diets were sacrificed after 26 weeks or 52
weeks of treatment. Animals in each group, including the control
groups, showed approximately 60 - 70% mortality at the end of the
study. Haematological changes, attributed by the authors of the study
to the administration of paraquat, included reduced erythrocytes,
hematocrit, and haemoglobin at the 72 ppm level in both sexes. Total
serum protein was decreased and blood glucose increased at the 72 ppm
dietary level in both sexes. Various rumours, mostly malignant, were
observed in all groups in this study, the main types being lung
adenocarcinoma in males and leukaemia in females. However, no tumour
type showed a higher incidence in the treated groups than in controls
and no correlations between tumour incidence and concentration of the
test substance were observed. No effects attributable to paraquat were
observed in absolute or relative organ weights, urinalysis,
body-weight gain, food consumption, efficiency of food utilization, or
water intake. Based on the haematological and blood biochemistry
changes observed, the no-effect level for paraquat after 104 weeks of
administration to JCL:ICR mice in this study was 22 ppm (as paraquat
cation), equal to 2.8 mg/kg b.w./day (Toyoshima et al., 1982a).
Rats
Groups of 50 male and 50 female JCL:Wistar rats were maintained
on diets containing 0, 4.3, 22, 72, or 217 ppm paraquat cation for 104
weeks and then killed and examined. Further groups of 6 males and 6
females receiving the same diets were sacrificed after 26 or 52 weeks
of treatment. There was 38 - 66% mortality in all groups at the end of
the study. The distribution of mortality and of abnormalities were not
significantly affected by treatment. Females in the 217 ppm group
showed a transient tendency to lower body-weight gain, compared to
controls, at weeks 34, 42 - 48, and 54. Food consumption, efficiency
of food utilization, and water consumption were not affected by
paraquat administration. Haematological changes attributed by the
authors of the study to paraquat administration at the 217 ppm level
included reduced erythrocytes and haemoglobin in both sexes and
decreased haematocrits and increased reticulocytes in males after 26
weeks. Total serum protein was slightly but constantly decreased at
the 217 ppm level in both sexes.
During histopathological examination of approximately 20 tissues,
various rumours were observed in this study in all groups, the main
types being benign pituitary tumours in males and benign mammary
rumours in females. However, none of the tumours were present at a
significantly-higher incidence in the treated groups than in controls.
Body weight, food consumption, food efficiency, water intake,
leucocyte counts, platelet counts, prothrombin time, GOT, GPT,
alkaline phosphatase activity, blood glucose, blood urea nitrogen,
cholesterol, Na+, K+, C1-, creatine, and brain, serum, and
corpuscular cholinesterase activities, as well as ophthamological
examination indicated no significant effects attributable to paraquat
at any dose level. Based on the haematological and blood biochemistry
changes the no-effect level of paraquat after 104 weeks of
administration to JCL:Wister rats in this study was 72 ppm (as
paraquat cation) equal to 3.0 (males) and 3.7 (females) mg/kg b.w./day
(Toyoshima et al., 1982b).
Dogs
Groups of 6 male and 6 female beagle dogs received diets
containing 0, 15, 30, or 50 ppm paraquat cation for 1 year. During the
study there were no deaths. No effects due to paraquat were observed
on body weight. The reduced food consumption of 1 male and 1 female
dog, both in the 50 ppm group, was considered by the authors of the
study to be treatment-related. There was clinical evidence of
respiratory dysfunction (hyperpnoea) in some dogs fed 50 ppm paraquat.
Mean lung weights of male and female dogs fed 50 ppm paraquat were 35
and 60% higher than those of controls, respectively. Histopathological
examination of the lungs showed a statistically-significant increase
in the incidence of chronic pneumonitis in both sexes at the 30 and
50 ppm dietary levels when compared to controls. This lesion consisted
of interstitial fibrosis, alveolar epithelialization, and mononuclear
cell infiltration. No other toxicologically-significant treatment-
related effects were seen during clinical observations, haemotological
or biochemical investigations, or during gross and microscopic
examination of approximately 40 tissues from each animal at
termination. On the basis of the pulmonary changes, the authors of
this study concluded that the dietary no-effect level for paraquat in
dogs over 1 year of treatment was 15 ppm, equal to 0.45 (males) and
0.48 (females) mg/kg b.w./day (Kalinowski et al., 1983).
Observations in humans
Information on the effects of paraquat in humans has been
obtained from occupational exposure studies (epidemiological data and
case reports), descriptions of accidental or suicidal poisonings, and
volunteer studies. These data have been extensively reviewed by WHO
(1984).
In 1965, a study was carried out on a team of 6 sprayers, and in
1967 on 4 teams in Malaysian rubber plantations, to estimate the
efficacy of individual protective measures. The operators used a spray
dilution containing paraquat at 0.5 g/litre for 12 weeks. Attention
was paid to personal hygiene. Each man was given a thorough physical
examination, and urine samples were taken before spraying began and at
weekly intervals throughout the study. Chest X-rays were taken before
the study started and at the end of the 6th and 12th weeks. In the 2
studies, a total of 528 urine samples were examined. Paraquat was
found on 131 occasions (78/134 and 53/394 in the 2 studies,
respectively), the maximum concentration detected being 0.32 mg/litre
in the first study and 0.15 mg/litre in the second. Average urine
levels of paraquat of 0.04 mg/litre were found in the 1965 study and
of 0.006 mg/litre in the 1967 study. After spraying ceased, these
levels declined steadily to become undetectable within a week, with 1
exception. Both trials showed that about half of the men had suffered
mild irritation of the skin and eyes, but had recovered rapidly with
treatment. Two cases of scrotal dermatitis occurred in workers wearing
trousers that were continuously soaked by the spray solution. There
were also 2 cases of epistaxis. All chest radiographs were normal
(Swan, 1969).
Studies performed over a period of several years on 296 Trinidad
sugar estate workers drew attention to nail damage that ranged in
severity from localized discoloration to nail loss following gross
contamination with paraquat at 1 - 2 g/litre. The typical distribution
of the lesions, affecting the index, middle, and ring fingers of the
working hand, suggested that they had occurred through leakage from
the knapsack sprayer and inadequate personal hygiene. Apart from 2
cases of contact dermatitis of the hands, no skin, eye, or nose
irritation was reported, nor were there any systemic effects (Hearn &
Keir, 1971).
Similar data were obtained on several groups of workers spraying
paraquat as an herbicide and dessicant in cotton fields during the hot
season. These workers were exposed to paraquat aerosol concentrations
of 0.13 - 0.55 mg/m3 air. Dermal exposure was low, not more than
0.05 - 0.08 mg paraquat on the hands and face. There were no
complaints, nor did the clinical and laboratory examinations of the
workers demonstrate any significant deviations from the matched
control groups (Makovskii, 1972).
In the USA, the exposure of field workers operating
tractor-mounted spray equipment in orchards was determined. About
4.6 litres of paraquat liquid concentrate (291 g/litre) was used in
935 litres of water per hour. In addition, exposure from yard and
garden applications were studied in volunteers using pressurized hand
dispensers containing paraquat solution (4.4 g/litre). Dermal
contamination was measured by adsorbent cellulose pads attached to the
worker's body or clothing, and by hand-rinsing in water in a
polyethylene bag. Special filter pads were used in the filter
cartridges of the respirators worn by the subjects under study. In
all, 230 dermal and respiratory exposure pads, 95 samples of
hand-rinse water, and 130 urine samples, collected during and
following spraying, were analysed, which involved 35 different
paraquat application situations. The exposure of field workers was
found to range from about 0.40 mg/hour (dermal) to less than
0.001 mg/hour (inhalation). As for individuals spraying yards or
gardens, exposure ranged from 0.29 mg/hour (dermal) to less than
0.001 mg/hour (inhalation). In almost all cases dermal exposure
affected the hands. The respiratory paraquat values were generally
below the sensitivity levels of the analytical method. No detectable
paraquat concentrations were found in the urine samples (lower limit
0.02 mg/litre). This study confirmed the general safety of paraquat
under correct conditions of use (Staiff et al., 1975).
The potential long-term hazard associated with the use of
paraquat has been studied by comparing the health conditions of 27
sprayers who had been exposed to paraquat for many months per year for
an average of 5.3 years with those of 2 unexposed control groups
consisting of 24 general workers and 23 factory workers. The workers
were given full clinical examinations; lung, liver and kidney function
tests were also carried out. There were a few skin lesions resulting
from poor spraying techniques and 1 case of eye injury. There were no
significant differences between exposed and control groups in any
health parameters measured, which led the authors to suggest that the
long-term use of paraquat is not associated with harmful effects on
health (Howard et al., 1981).
To evaluate the effects of protective equipment on occupational
human exposure to paraquat, a paraquat formulation (240 g/litre)
diluted 300 times by volume with water was sprayed for 2 hours on
weedy ground. During the spraying operations, the concentrations of
paraquat aerosol were 11 - 33 µg/m3 air. The total dermal exposure
was about 0.22 mg. No irritation of the eyes or the skin was reported.
The urine of the workers who wore gauze masks contained significant
amounts of paraquat 24 hours after spraying. The urine of the workers
who had worn a high-performance mask did not contain detectable levels
of paraquat. The authors discussed the need for protective equipment
to decrease skin contact with paraquat and to avoid aerosol inhalation
(Kawai & Yoshida, 1981).
Quantitative estimates of dermal and respiratory exposure of 26
plantation workers in Malaysia have shown a mean dermal dose of
1.1 mg/kg b.w./hour. The highest individual total exposure was
equivalent to 2.8 mg/kg b.w./hour; the mean respiratory exposure was
0.24 - 0.97 g/paraquat/m3 air, which is 1% or less of a TLV of
0.1 mg/m3 for respirable paraquat. Urine levels of paraquat were
generally below 0.05 mg/litre (Chester & Woollen, 1982).
A study was carried out on a group of 14 sprayers in Thailand
using conventional high-volume knapsack sprayers and low-volume
spinning-disc applicators with paraquat ion concentrations of
1.5 g/litre and 20 g/litre, respectively. Irritation of unprotected
skin was found, and this was severe (caustic burns on the feet) in
workers using high spray conentrations and spinning-disc applicators.
Urinary paraquat levels ranging from 0.73 - 10.2 mg/litre after 14
days of spraying were detected in unprotected men using both
concentrations, and there was evidence that urinary levels of paraquat
increased as the trial progressed. No evidence of systemic toxicity
was discovered among the sprayers undergoing clinical and radiographic
examination 1 week after spraying ended. The author concluded that
spray concentrations in hand-held equipment should not exceed 5 g
paraquat ion/litre (Howard, 1982).
After tomato spraying in the USA, the total body exposure to
paraquat was determined to be 169 mg/hour. The use of enclosed tractor
cabs or a high-clearance tractor reduced total body exposures to
27 mg/hour or 18 mg/hour, respectively. The authors reported that the
total body exposure of tractor sprayers working in 2 citrus locations
was proportional to the tank concentrations (paraquat dilutions of
0.7 g/litre and 1.1 g/litre were applied). Exposure levels of 13 and
28 mg/hour were found for workers using the lower and high
concentrations, respectively. In all situations studied, the
respiratory exposure was consistently a small fraction (< 0.1%) of
the total body exposure, which was primarily through the skin
(Wojeck et al., 1983).
Two groups of workers exposed to paraquat formulations were
examined. The first group of 18 workers, in England, consisted of
subjects exposed to dust and liquid paraquat formulations during a
37.5-hour working week, the mean length of exposure being 5 years. The
second group also consisted of 18 males, from Malaysia, exposed to
liquid concentrate formulations during a 42-hour working week, the
mean length of exposure being 2.3 years. Partly protective clothing
was worn. However, in Malaysia, no gloves, rubber aprons, or goggles
were used. The medical records and the dermatological examinations
revealed acute skin rashes, nail damage, epistaxis, blepharitis, and
delayed wound healing in 12 - 66% of these workers. Delayed caustic
effects were often found among the Malaysian formulation workers,
where low levels of safety and hygiene were apparent. Clinical
examination did not reveal any evidence of chronic contact dermatitis,
hyperkeratosis, or eczematous lesions (Howard, 1979).
Some studies designed to estimate dermal and inhalation exposure
to paraquat are summarized in Table 7. From the data reported it can
be seen that:
(a) the main route of exposure of agricultural workers to
paraquat is via the skin; respiratory exposure is
negligible.
(b) The worst case of exposure (of those examined) was via
knapsack spraying).
From 1956 - 1973, no deaths attributable to paraquat were
registered among agricultural workers in the USA, but in 1974 4 fatal
cases were associated with this herbicide. However, it is not clear
whether they were accidental, suicidal, or occupational (Hayes &
Vaughan, 1977).
Fitzgerald et al. (1978a) summarized the clinical findings and
pathological details concerning 13 accidents involving paraquat among
agricultural workers, 6 of which were fatal. In 5 of these cases,
swallowing was involved. Of the 6 fatalities studied, 3 swallowed
Gramoxone (a 20% solution of paraquat dichloride in water) after
sucking the outlet of a sprayer. In 1 non-fatal case, the man had
sucked out a nozzle containing diluted paraquat, while in another case
the man who had blown into the jet, to clear it, escaped with only
minor signs of poisoning. The use of a leaking sprayer by another
worker with severe extensive dermatitis probably resulted in fatal
absorption of paraquat through the damaged skin.
Table 7. Comparison of dermal and inhalation exposure to paraquat resulting from
various methods of application1.
Dermal Respiratory
Method of application exposure exposure Reference
(mg/hour) (mg/hour)
Hand-held knapsack 66 (0.45 - 1.3) × 10-3 Chester & Woollen,
(12.1 - 169.8) 1982
Vehicle mounted 0.4 (0 - 2) × 10-3 Staiff et al., 1975
(0.1 - 3.4)
Aerial Chester & Ward, 1981
Flagman 0.1 - 2.4 (0 - 47) × 10-3
Pilot 0.5 - 0.1 (0 - 0.6) × 10-3
Mixer/loader 0.18 (1.3 - 1.5) × 10-3
1 From WHO, 1984
Several other cases of fatal poisoning resulting from dermal
absorption of paraquat have been reported. Jaros (1978) has described
how the use of concentrated solutions of paraquat (50 g/litre instead
of 5 g/litre), with an old leaking knapsack sprayer, resulted in
paraquat contamination of the neck, back, and legs of a worker. After
4 hours of work, the worker complained of a burning sensation on the
neck and scrotum. On admission to a hospital 6 days later, cough and
respiratory difficulties were recorded. Three days later the patient
died of renal and respiratory failure.
Severe skin damage, followed by death due to respiratory
insufficiency, occurred in a woman 8 weeks after initial contact with
paraquat. The toxic dermatitis started with scratches on the arms and
legs from the branches of fruit trees. The patient had often failed to
wear protective clothing or to shower after spraying. During the 4
weeks preceding her first admission to the hospital, she developed
ulcers and respiratory complaints combined with anorexia. Damaged and
broken skin was thus exposed to paraquat. A chest X-ray and needle
biopsy of the lung revealed pulmonary lesions. Seventeen days after
discharge from hospital, without a specific diagnosis, she was
re-admitted, and died 2 weeks later with progressive lung, hepatic,
and renal dysfunction (Newhouse et al., 1978).
The clinical and pathomorphological investigation of a patient
who died of hypoxia after repeated dermal exposure to paraquat
(28 g/litre) and diquat (29 g/litre) in a water-oil dilution has been
described recently. The worker had used a leaking sprayer. A
characteristic ulcer developed at the site of paraquat contact. There
was also lung damage (Levin et al., 1979).
Another reported fatal case of dermal poisoning with paraquat
occurred after prolonged contact with a concentrated formulation
following spillage from a bottle in the back trouser pocket
(Waight & Wheather, 1979).
Wohlfahrt (1982) discussed the factors related to severe paraquat
poisoning due to dermal absorption in tropical agriculture. Three
fatal incidents followed skin contamination; 1 victim used paraquat to
treat scabies infestation, and 1 used it to treat lice. In all cases,
the skin was blistered and ulcerated. The patients died of progressive
respiratory failure 4 - 7 days after the accidents. In 1 of these
cases, the presence of mouth and throat ulceration strongly suggested
that ingestion might also have occurred (Davies, 1982).
Local skin and nail effects of paraquat have been reviewed by WHO
(1984). Brief contact with liquid formulations, as well as repeated
exposure to dilute solutions, produced skin irritation, desquamation,
and, finally, necrosis at the site of contact (Ongom et al., 1974;
Binns, 1976; Newhouse et al., 1978; Waight & Wheather, 1979;
Levin et al., 1979, Horiuchi et al., 1980). Harmful dermal effects
have been reported among sprayers who worked without protective
clothes and with naked feet (Howard, 1982). The blistering and
ulceration of the skin were due to excessive contact and inadequate
hygiene. Horiuchi and Ando (1980) carried out patch testing on 60
patients with contact dermatitis due to Gramoxone. In 8 patients
(13.3%) positive allergic reactions were established. In another
survey with 52 persons, a positive photo-patch response was reported
in 11 patients. Nail damage may also occur after frequent exposure to
paraquat concentrations during the formulation of the herbicide or the
preparation of the working dilution (Howard, 1979).
There have been some reports (Malone et al., 1971; Mircev,
1976; Bismuth et al., 1982) of adverse effects as a result of
inhalation exposure to paraquat. However, inhalation of droplets in
normal paraquat spraying does not appear to represent a significant
health hazard (Howard, 1980), and the effects of occupational
inhalation have usually been limited to nose bleeds and nasal and
throat irritation (Swan, 1969; Howard, 1979).
Ocular damage may result from splashes of concentrated paraquat
that come into contact with the eye. Apart from irritation of the eye
and blepharitis, a week later more serious ocular damage may occur,
such as destruction of the bulbar and tarsal conjunctiva and of the
corneal epithelium. Anterior uveitis, conjunctival necrosis,
progressive keratitis with gross corneal opacity, and decreased visual
acuity may also occur (WHO, 1984).
It has been noted that when the recommended dilution rates were
correctly used, systemic effects of oral, inhalation, or dermal
exposure to paraquat have not been observed. Skin and eye irritation
have occurred only when protective measures were disregarded
(WHO, 1984).
In a volunteer study the percutaneous absorption of
14C-paraquat through the legs, hands, and forearms of 6 human
subjects was studied. The total dose absorbed in 5 days after a single
application of 0.64 mg paraquat dichloride was very low (0.3%) at all
sites of application (Wester et al., 1984).
A large number of cases of accidental or suicidal poisoning have
been reported since 1966, the earlier cases being mostly accidental
which apparently resulted from the habit of decanting the liquid
formulations into small unmarked or incorrectly labelled containers
such as beer, wine, or soft-drink bottles. An increasing ratio of
suicidal to accidental poisonings has been noted in recent years
(Fitzgerald et al., 1978b; Bramley & Hart, 1983). This change from
accidental to suicidal poisoning was considered to be reflected by
enhanced percentages of fatal cases, shorter survival times, and
higher tissue and body fluid levels (WHO, 1984). The vast majority of
cases of paraquat poisoning have been due to ingestion. A few cases of
fatal or non-fatal poisonings have been reported following either skin
contamination (McDonagh & Martin, 1970; Kimura et al., 1980) or skin
application in order to kill body lice (Ongom et al., 1974;
Binns, 1976). Symptoms of poisoning depend on the dose absorbed.
It is difficult to estimate the dose absorbed from case histories
since in many cases the patients spat out part of the paraquat
concentrate or vomited profusely after swallowing the herbicide. Some
patients have survived after apparently ingesting 10 - 20 g paraquat,
whereas some died after taking as little as 2.5 g paraquat. The
probability of survival after paraquat poisoning can be predicted from
plasma paraquat concentration at a given time after ingestion
(Proudfoot et al., 1979; Hart et al., 1984). The minimum lethal
dose of paraquat for human beings has been estimated to be about
35 mg/kg b.w. (Pederson et al., 1981; Bismuth et al., 1982). The
common factor of most, if not all, cases of paraquat poisoning is
damage to the lung. Cases of fatal paraquat poisoning have been
divided into 2 broad categories:
a) Cases of acute fulminant poisoning due to massive amounts of
paraquat absorbed, resulting in death within 1 to 5 days
from ingestion. Death is due to multi-organ failure
associated with damage to the lungs, kidneys, liver, brain,
and adrenals.
b) Cases of poisoning due to ingestion or absorption of smaller
doses of paraquat resulting in death 6 to several days
later. In these cases death is due primarily to lung and
kidney damage.
COMMENTS
A small amount of paraquat is rapidly absorbed by the gut of
rats, guinea pigs, dogs, monkeys, and man, most of the oral dose being
excreted as unchanged paraquat. Following administration by different
routes to animals, paraquat is rapidly distributed in most tissues,
the highest concentrations being found in the lungs and kidneys. The
compound accumulates slowly in the lung owing to uptake by an
energy-dependent process which is also responsible for the uptake of
putrescine. Saturation kinetics for the uptake of paraquat by the lung
have been shown to be similar in rats and man. Excretion of absorbed
paraquat is biphasic, owing to lung accumulation, and occurs largely
in the urine as unchanged paraquat, but also to a limited extent in
the bile. Biotransformation of absorbed paraquat is, in general,
remarkably poor in all species studied (rats, guinea pigs, dogs, hens,
pigs, goats, and sheep) although there is some controversy as to the
possibility and extent of its metabolism by the gut microflora.
Metabolism occurs via demethylation (monomethyl dipyridone ion) or
oxidation (paraquat pyridone ion and paraquat dipyridone ion).
The mechanism of paraquat toxicity has been investigated
extensively, but it has not yet been elucidated completely. The
available evidence indicates that paraquat toxicity is due to the
ability of the compound to undergo redox cycling in biological
systems, resulting in the production of superoxide anion radicals and
in the oxidation of cellular NADPH. These effects may lead to the
formation of other highly toxic oxygen species and to depletion of
important defense mechanisms, both events which are potentially
capable of switching on further pathological processes, resulting in
damage to Type I and Type II pneumocytes.
Two teratogenicity studies, 1 in mice and 1 in rats, showed
paraquat to be non-teratogenic at doses up to 10 mg/kg b.w./day.
Slight maternal toxicity was observed in mice and high maternal
toxicity and some fetal toxicity were observed in rats at 5 and
10 mg/kg b.w./day paraquat ion. In a teratology sub-group in a
3-generation reproduction study in rats, paraquat was not teratogenic,
but delays in ossification were found at all 3 dose levels tested
(72, 145, and 290 ppm paraquat cation).
In previous in vitro studies both positive and negative results
were obtained, with mutagenicity usually associated with high
cytotoxicity. Recent studies have shown paraquat to be non-mutagenic,
both in the presence and absence of metabolic activation, when tested
by the Ames test, mouse micronucleus test, unscheduled DNA synthesis
assay, rec-assay, and host-mediated assay. A mouse lymphoma cell test
was inconclusive. The herbicide was clastogenic to human lymphocytes
in vitro at cytotoxic doses and induced sister chromatid exchange in
Chinese hamster lung fibroblasts.
In 2 rat 3-generation reproduction studies paraquat had no
effects on the reproductive performance or development of the
reproductive organs of rats. In 1 of these studies a reduction of
lactation index was observed at 290 ppm paraquat cation.
The acute oral toxicity of paraquat is higher in guinea pigs,
monkeys, cattle, and man than in rats and birds. Confidence limits of
LD50 values are small. There are no significant differences between
the 2 sexes in the oral, s.c., or i.p. LD50 values of paraquat in
rats or mice. Paraquat induces characteristic dose-related fibrotic
changes in the lungs of mice, rats, dogs, and monkeys, but not in
rabbits, guinea pigs, or hamsters. The acute pulmonary toxicity of
paraquat in rats and man is biphasic. In the early, destructive phase,
the alveolar epithelial cells are extensively damaged. Death may occur
within a few days due to pulmonary oedema. In the later, proliferative
phase, fibroblasts and collagen accumulate in the lungs of surviving
animals and humans, possibly resulting in fibrosis.
In a short-term study in mice, treatment-related lung, body
weight, and organ-weight changes were found. Another short-term study
in rats indicated that paraquat was responsible for lung lesions and
other minor changes. In a short-term study in dogs using 3
animals/sex/group, paraquat-dependent lung and kidney lesions were
observed.
In a 1-year feeding study in dogs, paraquat caused lung changes
at the 2 highest dietary levels; the no-effect level in this study was
15 ppm (as paraquat cation), equal to 0.45 mg/kg b.w./day in males and
0.48 mg/kg b.w./day in females.
In 2 long-term feeding studies, 1 in mice and 1 in rats,
haematological and blood biochemical changes were observed in both
species. These changes have not been reported previously and were
considered to be of little toxicological significance.
A lifetime feeding study in mice showed no oncogenic potential
for paraquat, although a slightly higher incidence of lung tumours was
noted in animals of the high-dose group dying between 79 and 98 weeks
of treatment when compared with control mice. Based on renal lesions
observed in males, the no-effect level in this study was 12.5 ppm
(as paraquat cation), equal to 1.4 mg/kg b.w./day.
A long-term feeding study in F344 rats indicated that paraquat
administration for 104 weeks at 217 ppm was responsible for a
significant increase in the incidence of pulmonary adenomas in
females. This incidence (8.7%) was significantly higher than that
observed in historical control animals of the same laboratory (2.2%).
Paraquat was also cataractogenic in both male and female rats. Based
on the lung and eye changes the no-effect level in this study was
22 ppm (as paraquat cation), equal to 0.77 mg/kg b.w./day.
In another long-term study in F344 rats paraquat induced
proliferative benign lung lesions in females in the high-dose group,
which were considered neoplastic by 1 pathologist and non-neoplastic
by 2 others (the total incidence of pulmonary adenoma in females
ranged from 0/70 to 8/70). The reasons for the discrepancy were
unclear, owing to the lack in most cases of adequately detailed
histopathological descriptions of the lung lesions. The historical
incidence of lung adenoma in control female F344 rats in the
laboratory in which the study was conducted was reported to be 1.1%,
and the incidences reported in the literature are 1.4% for females and
1.9% or 0% for males. The results of these studies indicate a weak,
organ-specific, sex-related potential of paraquat to produce benign
proliferative changes in the lungs of female F344 rats.
The following observations were considered by the Joint Meeting:
a) paraquat was shown to be non-mutagenic in most in vitro
and, apparently, all in vivo tests;
b) the lung is the target organ for both acute and chronic
paraquat toxicity in rats;
c) only female F344 rats, but not male F344 rats, developed
treatment-related benign neoplastic lesions;
d) proliferative changes were observed in rats and not in mice;
and
e) paraquat selectively interferes with the uptake of
polyamines by pneumocytes.
Polyamines are endogenous substrates playing an important role in
cell division and growth. These observations suggest that the
potential of paraquat to induce lung cell proliferation may be
modulated by species-, sex-, and organ-dependent factors, such as
hormonal activity, cell growth, defense and repair mechanisms, etc. No
lung changes were observed at 24 ppm in any of the dose groups, and
significant increases in the incidence of lung adenocarcinomas were
not observed in any of the treated groups when compared to controls in
these studies.
Observations in man, including reports on accidental or suicidal
poisonings, confirm the type of acute and subacute toxicity observed
in some experimental animals. Death occurred after oral and, in some
cases, dermal absorption of high doses of paraquat. The organs
primarily involved are the lung and kidney and, to a lower extent, the
intestinal tract, liver, pancreas, adrenals, and CNS. The minimal
lethal dose of paraquat in man is estimated to be about 35 mg/kg b.w.
Skin, nail, and eye lesions were found in subjects with prolonged
occupational exposure to paraquat. Significant paraquat concentrations
(up to 10 mg/1) were detected in the urine of workers using high spray
concentrations, but not in protected workers. The use of protective
measures has proved to be effective in preventing lesions due to skin
contact.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
Mice: 13 ppm (as paraquat cation), corresponding to 17 ppm
(as paraquat dichloride) in the diet, equal to
1.9 mg/kg b.w./day for males and 38 ppm (as paraquat
cation, corresponding to 52 ppm (as paraquat
dichloride), equal to 5.9 mg/kg b.w./day for females.
Rats: a) 22 ppm (as paraquat cation), corresponding to 30 ppm
(as paraquat dichloride) in the diet, equal to
1.1 mg/kg b.w./day for males and 1.2 mg/kg b.w./day for
females (cataract).
b) 25 ppm (as paraquat cation), corresponding to 35 ppm
(as paraquat dichloride) in the diet, equal to
1.4 mg/kg b.w./day for males and 1.7 mg/kg b.w./day for
females (proliferative lung changes).
Dogs: 15 ppm (as paraquat cation), corresponding to 20 ppm
(as paraquat dichloride) in the diet, equal to
0.62 mg/kg b.w./day for males and 0.66 mg/kg b.w./day
for females.
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.004 mg/kg b.w. as paraquat cation (0 - 0.006 mg/kg b.w.
expressed as paraquat dichloride).
STUDIES WHICH WILL PROVIDE INFORMATION VALUABLE FOR THE CONTINUED
EVALUATION OF THE COMPOUND
1. Further observations in humans.
2. Studies on the mechanism of paraquat-induced proliferative
lung changes.
REFERENCES
Anderson, K.J., Leighty, E.G., & Takahashi, M.T. Evaluation of
1972 herbicides for possible mutagenic protperties.
J. Agric. Food Chem., 20: 649 - 656.
Anderson, D., McGregor, D.B., & Purchase, I.F.H. Dominant lethal
1976 studies with diquat and paraquat in male CD-1 mice.
Mutat. Res., 40: 349 - 358.
Anderson, D. Paraquat: Estimation of mutagenic potential in the
1977 Salmonella typhimurium mutagenicity assay. Unpublished
Report No. CTL/P/243 from ICI Central Toxicology Laboratory.
Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Ashby, R., Hepworth, P.L., Whinney, A.K., Brown, P.M., Whitney, J.C.,
1983 & Finn, J.P. Paraquat: combined toxicity and carcinogenicity
study in rats. Unpublished report No. 82/ILY217/328 from
Life Science Research, Stock, Essex, UK. Submitted to WHO by
Imperial Chemical Industries PLC, Haslemere, UK.
Autor, A.P. Reduction of paraquat toxicity by superoxide dismutase.
1974 Life Sci., 14: 1309 - 1319.
Autor, A. P., ed. Biochemical mechanisms of paraquat toxicity. New
1977 York, Academic Press.
Bainova, A. Experimental assessment of the effect of dipyridylium
1969 herbicides on the skin. Letopisi HEI, 9: 25 - 30
(in Bulgarian).
Bainova, A. Determination of the zones of acute and chronic inhalatory
1971 toxicity after exposure to dipyridylium herbicides Gramoxone
and Reglone. Letopisi HEI, 28: 59 - 62 (in Bulgarian).
Bainova, A. & Vulcheva, V. Experimental substantiation of Gramoxone
1972 MAC in working areas. In: Works of Research Institute of
Hygiene & Labour Protection. Sofia, Medizine i Fizkultuna,
Vol. 23: 71 - 77.
Benigni, R., Bignami, M., Carera, A., Conti, G., Conti, R., Grebelli,
1979 R.A., Dogliotti, E., Gualandi, G., Novelleto, A., & Ortalia,
V.A. Mutational studies with diquat and paraquat in vitro.
Mutat. Res., 68: 183 - 193.
Bennett, P.N., Davies, D.S., & Hawksworth, G.M. In-vivo absorption
1976 studies with paraquat and diquat in the dog. Proceedings of
the British Pharmaceutical Society, 15 - 16 July, England,
284 pp.
Bignami, M. & Grebelli, R.A. A simplified method for the induction of
1979 8-azaguanine resistance in S. typhimurium. Toxicol.
Lett., 3: 169 - 175.
Binns, C.W. A deadly cure for lice. Papua New Guinea Med. J.,
1976 19: 105 107.
Bismuth, C., Garnier, R., Dally, S., Fournier, P.E., & Scherrmann,
1982 J.M. Prognosis and treatment of paraquat poisoning. A review
of 28 cases. J. Toxicol. Clin. Toxicol., 19: 461 - 474.
Bramley, A. & Hart, T.B. Paraquat poisoning in the United Kingdom.
1983 Hum. Toxicol., 2: 417.
Brigelius, R., Hashem, A., & Lengfelder, E. Paraquat-induced
1981 alterations of phospholipids and GSSG release in the
isolated perfused rat liver and the effect of SOD-active
copper complexes. Biochem. Pharmacol., 30: 349 - 354.
Bus, J.S., Aust, S.D., & Gibson, J.E. Superoxide and singlet oxygen
1974 catalysed lipid peroxidation as a possible mechanism for
paraquat toxicity. Biochem. Biophys. Res. Commun.,
58; 749 - 755.
Bus, J.S. & Gibson, J.E. Postnatal toxicity of chronically
1975 administered paraquat in mice and interactions with oxygen
and bromobenzene. Toxicol. Appl. Pharmacol.,
33: 461 - 470.
Bus, J.S., Aust, S.D., & Gibson, J.E. Lipid peroxidation as a possible
1975a mechanism for paraquat toxicity. Res. Commun. Chem.
Pathol. Pharmacol., 11: 31 - 38.
Bus, J.S., Preachie, M.M., Cagen, S.Z., Posner, H.S., Eliason, B.C.,
1975b Sharp, C.W., & Gibson, J.E. Fetal toxicity and distribution
of paraquat and diquat in mice and rats. Toxicol. Appl.
Pharmacol., 33: 450 - 460.
Bus, J.S., Cagen, S.Z., Olgaard, M., & Gibson, J.E. A mechanism of
1976 paraquat toxicity in mice and rats. Toxicol. Appl.
Pharmacol., 35: 501 - 513.
Bus, J.S. & Gibson, J.E. Paraquat: model for oxidant-initiated
1984 toxicity. Environ. Health Perspect., 55: 37 - 46.
Busey, W.M. An independent pathology review of the lung slides from a
1986 rat chronic toxicity/carcinogenicity study with paraquat.
Unpublished report from ICI Plant Protection, Ltd. Submitted
to WHO by Imperial Chemical Industries PLC, Haslemere,
Surrey, UK.
Butler, C. & Kleinerman, J. Paraquat in the rabbit. Br. J. Ind. Med.,
1971 28: 67 -71.
Butler, C. Pulmonary interstitial fibrosis from paraquat in the
1975 hamster. Arch. Pathol., 99: 503 - 507.
Calderbank, A. The bipyridylium herbicides. Effects on man. In
1968 Metcalf, R.L, ed. Advances in Pest Control Research. New
York, Interscience Publishers, Vol. 8; 224 pp.
Charles, J.M., Abou-Donia, M.B., & Menzel, D.B. Absorption of paraquat
1978 from the airways of perfused rat lung. Toxicology,
8: 59 - 67.
Chester, G. & Ward, R.J. Paraquat - occupational exposure and drift
1981 hazard evaluation during aerial application to cotton in
California, USA. Unpublished Report No. CTL/P/581 from ICI
Central Toxicology Laboratory. Submitted to WHO by Imperial
Chemical Industries PLC, Haslemere, Surrey, UK.
Chester, G. & Woollen, B.H. Studies of the occupational exposure of
1982 Malaysian plantation workers to paraquat.
Br. J. Ind. Med., 38: 23 - 33.
Clark, D.G., McElligott, T.F., & Hurst, E.W. The toxicity of paraquat.
1966 Br. J. Ind. Med., 23: 126 - 133.
Clay, P. & Thomas, M. Paraquat dichloride (technical liquor);
1985 assessment of mutagenic potential using L5178Y mouse
lymphoma cells. Unpublished Report No. CTL/P/1398 from ICI
Central Toxicology Laboratory. Submitted to WHO by Imperial
Chemical Industries PLC, Haslemere, Surrey, UK.
Conning, D.M., Fletcher, K., & Swan, A.A.B. Paraquat and related
1969 bipyridyliums. Br. Med. Bull., 25: 245 - 249.
Daniel, J.M. & Gage, J.C. Absorption and excretion of diquat and
1966 paraquat in rats. Br. J. Ind. Med., 23: 133 - 136.
Davies, R.E. Skin absorption of paraquat. Med. J. Aust., 7: 222.
1982
Dunbar, J.R., DeLucia, A.J., & Bryant, L.R. Glutathione status of
1984 isolated rabbit lungs. Effects of nitrofurantoin and
paraquat perfusion with normoxic and hyperoxic ventilation.
Biochem. Pharmacol., 33: 1343 - 1348.
Ecker, J.L., Hook, J.B., & Gibson, J.E. Nephrotoxicity of paraquat in
1975 mice. Toxicol. Appl. Pharmacol., 34: 178 - 186.
Edwards, M.J., Hemingway, R.J., Kinch, D.A., and Slape, P. Paraquat:
1974 residue and toxicology trial with cows fed a treated grass.
Unpublished report No. AR2465A(R) from ICI Plant Protection,
Ltd. Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Fairshter, R.D. Paraquat toxicity and lipid peroxidation. Arch.
1981 Intern. Med., 141: 1121 - 1123.
Fisher, H.K., Clements, J.A., & Wright, R.R. Enhancement of oxygen
1973 toxicity by the herbicide paraquat. Ann. Rev.
Respir. Dis., 107: 246 - 252.
Fisher, H.K., Clements, J.A. Tierney, D.F., & Wright, R.R. Pulmonary
1975 effects of paraquat in the first day after injection.
Am. J. Physiol., 228: 1217 - 1223.
Fitzgerald, G.R., Barniville, G., Flanagan, M., Silke, B., Carmody,
1978a M., & O'Dwyer, W.F. The changing pattern of paraquat
poisoning; an epidemiologic study. J. Irish Med. Assoc.,
71; 103 - 108.
Fitzgerald, G.R., Barniville, G., Black, J., Silke, B., Carmody, M., &
1978b O'Dwyer, W.F. Paraquat poisoning in agricultural workers.
J. Irish Med. Assoc., 71; 149 - 152.
Fletcher, K., Herring, C.M., & Robinson, V.M. Paraquat three-
1972 generation reproduction study in rats. Unpublished report
from ICI Industrial Hygiene Research Laboratories. Submitted
to WHO by Imperial Chemical Industries PLC, Haslemere,
Surrey, UK.
Fodri, Z., Sipos, K., & Berencsi, G. Study of the irritation and
1977 allergic effects of Gramoxone in guinea-pig.
Egeszsegtudomouny, 21: 244 - 249 (in Hungarian).
Fowler, B.A. & Brooks, R.E. Effects of the herbicide paraquat on the
1971 ultrastructure of mouse kidney. Am. J. Pathol.,
63: 505 - 512.
Fridovich, I. & Hassan, H.M. Paraquat and the exacerbation of oxygen
1979 toxicity. Trends Biochem. Sci., 4: 113 - 115.
Gage, J.C. The action of paraquat and diquat on the respiration of
1968 liver cell fractions. Biochem. J., 109: 757 - 761.
Gibson, J.E. & Cagen, S.Z. Paraquat-induced functional changes in
1977 kidney and liver. In: Autor, A.P., ed. Biochemical
Mechanisms of Paraquat Toxicity. New York Academic Press,
pp. 117 - 136.
Giri, S.N., Curry, D.L., Hollinger, M.A., & Freywald, N. Effect of
1979 paraquat on plasma enzymes, insulin, glucose and liver
glycogen in the rat. Environ. Res., 20; 300 - 308.
Giri, S.N. & Krishna, G.A. The effect of paraquat on guanylate cyclase
1980 activity in relation to morphological changes in guinea-pig
lungs. Lung, 157: 127 - 134.
Glass, M., Sutherland, M.W., Forman, H.J., & Fisher, A.B. Selenium
1985 deficiency potentiates paraquat-induced lipid peroxidation
in isolated perfused rat lung. J. Appl. Physiol.,
59: 619 - 622.
Greenberg, D.B., Lyons, A., & Last, J.A. Paraquat-induced changes in
1978 the rat of collagen biosynthesis by rat lung.
J. Lab. Clin. Med., 92: 1033 - 1042.
Hart, T.B., Nevitt, A., & Whitehead, A. A new statistical approach to
1984 the prognostic significance of plasma paraquat
concentrations. Lancet, November 24: 1222 - 1223.
Hassan, H.M. & Fridovich, I. Regulation of synthesis of superoxide
1977 dismutase in Escherichia coli. J. Biol. Chem.,
253: 7667 - 7672.
Hassan, H.M. & Fridovich, I. Superoxide radical and the enhancement of
1978 oxygen toxicity of paraquat in Escherichia coli.
J. Biol. Chem., 253: 8143 - 8148.
Hassan, H.M. & Fridovich, I. Paraquat and Escherichia coli. Mechanism
1979 of production of extracellular superoxide radical.
J. Biol. Chem., 254: 10846 - 10852.
Hassan, H.M. & Fridovich, I. Superoxide dismutases: detoxication by a
1980 free radical. In: Jakoby, W.B., ed. Enzymatic basis of
detoxication. New York, Academic Press, Vol. 1: 311 - 322.
Hawksworth, G.M., Bennett, P.N., & Davies, D.S. Kinetics of paraquat
1981 elimination in the dog. Toxicol. Appl. Pharmacol.,
57: 139 - 145.
Hayes, W.J. & Vaughn, W.K. Mortality from pesticides in the United
1977 States in 1973 and 1974. Toxicol. Appl. Pharmacol.,
42: 235 - 252.
Hearn, C.E.D. & Keir, W. Nail damage in spray operators exposed to
1971 paraquat. Br. J. Ind. Med., 28: 399 - 403.
Hemingway, R.J., Leahey, J.P., Griggs, R.E., & Davis, J.A. Paraquat
1972 metabolism in sheep. Unpublished report No. AR2359A from ICI
Plant Protection Division. Submitted to WHO by Imperial
Chemical Industries PLC, Haslemere, Surrey, UK.
Hemingway, R.J., Leahey, J.P., Griggs, R.E., & Davis, J.A. Paraquat
1974 metabolism in ruminants. 3rd International Congress of
Pesticide Chemistry, Helsinki.
Hemingway, R.J. & Oliver, C. Paraquat: a study of the metabolism and
1974 residues in hens and their eggs. Unpublished report No.
AR251A from ICI Plant Protection Division. Submitted to WHO
by Imperial Chemical Industries PLC, Haslemere, Surrey, UK.
Hendley, P., Leahey, J.P., & Spinks, C.A. Paraquat: Metabolism and
1976a residues in hens. Unpublished report No. AR2676A from ICI
Plant Protection Division. Submitted to WHO by Imperial
Chemical Industries PLC, Haslemere, Surrey, UK.
Hendley, P. Leahey, J.P., Spinks, C.A., Neale, D., & Carpenter, P.K.
1976b Paraquat metabolism and residues in goats. Unpublished
report No. AR2680A from ICI Plant Protection Division.
Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Hodge, M.C.E., Palmer, S, Weight, T.M., & Wilson, J. Paraquat
1978a dichloride: teratogenicity study in the mouse. Unpublished
report No. CTL/P/364 from ICI Central Toxicology Laboratory.
Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Hodge, M.C.E., Palmer, S., Weight, T.M., & Wilson, J. Paraquat
1978b dichloride: teratogenicity study in the rat. Unpublished
report No. CTL/P/365 from ICI Central Toxicology Laboratory.
Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Horiuchi, N. & Ando, S. Contact dermatitis due to pesticides for
1980 agricultural use. Nippon Hifuka Gakkai Zasshi, 90: 289
(in Japanese).
Horiuchi, N., Ando, S., & Kambe, Y. Dermatitis due to pesticides for
1980 agricultural use. Nippon Hifuka Gakkai Zasshi, 90: 27
(in Japanese).
Howard, J.K. A clinical survey of paraquat formulation workers. Br. J.
1979 Ind. Med., 36: 220 - 223.
Howard, J.K. Paraquat: a review of worker exposure in normal usage.
1980 J. Soc. Occup. Med., 30; 6 - 11.
Howard, J.K., Sahopathy, N.N., & Whitehead, P.A. A study of the health
1981 of Malaysian plantation workers with particular reference to
paraquat spraymen. Br. J. Ind. Med., 38: 110 - 116.
Howard, J.K. Paraquat spraying. Comparative risks from high and low
1982 volume application methods. In: Proceedings of the 10th
Asian Conference on Occupational Health, Singapore,
pp. 1 - 7.
Howard, C.A., Wildgoose, J., Clay, P., & Richardson, C.R. Paraquat
1985 dichloride: an in vitro sister chromatid exchange study in
Chinese hamster lung fibroblasts. Unpublished Report
No. CTL/P/1392 from ICI Central Toxicology Laboratory.
Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Howe, D.J.T. & Wright, N. The toxicity of paraquat and diquat. In:
1965 Proceedings of the 18th New Zealand Weed and Pest Control
Conference, pp. 105 - 114.
Hundsdorfer, S. & Rose, I. Lung changes produced by paraquat:
1980 respiratory distress model in animals. Ergeb. Exp. Med.,
35: 603 - 690 (in German).
Hussain, M.Z. & Bhatnagar, R.S. Involvement of superoxide in the
1979 paraquat-induced enhancement of lung collagen synthesis in
organ culture. Biochem. Biophys. Res. Commun.,
89: 71 - 76.
Ilett, K.I., Stripp, B., Menard, R.H., Reid, W.D., & Gillette, J.R.
1974 Studies on the mechanism of the lung toxicity of paraquat.
Comparison of tissue distribution and some biochemical
parameters in rats and rabbits. Toxicol. Appl. Pharmacol,
28: 216 - 226.
Ishmael, J. & Godley, M.J. Paraquat:. lifetime feeding study in rats.
1983 Histopathological examination of lungs. Unpublished Report
No. CTL/P/738 from ICI Central Toxicology Laboratory.
Submitted to WHO by imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Jaros, F. Acute percutaneous paraquat poisoning. Lancet 1: 275.
1978
Kalinowski, A.E., Doe, J.E., Chart, I.S., Gore, C.W., Godley, M.J.,
1983 Hollis, K., Robinson, M., & Woollen, B.H. Paraquat: One year
feeding study in dogs. Unpublished Report No. CTL/P/734 from
ICI Central Toxicology Laboratory. Submitted to WHO by
Imperial Chemical Industries PLC, Haslemere, Surrey, UK.
Kawai, M. & Yoshida, M. Exposure of spray operators to paraquat.
1981 Nippon Doshu Eisei Zasshi, 28: 353 - 359 (in Japanese).
Keeling, P.L., Pratt, I.S., Aldridge, W.N., & Smith, L.L. The
1981 enhancement of paraquat toxicity in rats by 85% oxygen:
lethality and cell-specific lung damage.
Br. J. Exp. Pathol., 62: 643 - 654.
Keeling, P.L., Smith, L.L., & Aldridge, W.N. The formation of mixed
1982 disulfides in rat lung following paraquat administration.
Biochem. Biophys. Acta, 716: 249 - 257.
Kehrer, J.P., Haschek, W.M., & Witschi, H. The influence of hyperoxia
1979 on the acute toxicity of paraquat and diquat. Drug Chem.
Toxicol., 2: 397 - 408.
Kimbrough, R.D. & Gaines, T.B. Toxicity of paraquat in rats and its
1970 effect on rat lungs. Toxicol. Appl. Pharmacol.,
17: 679 - 690.
Kimbrough, R.D. & Linder, E.W. The ultrastructure of the paraquat lung
1973 lesion in the rat. Environ. Res., 6: 265 - 273.
Kimura, M., Suzuki, E., & Ohnishi, H. A case of autopsy of an infant
1980 intoxicated by paraquat dichloride. Shoni Ka Rinsho,
33: 732 - 734 (in Japanese).
Klika, E., Kunc, L., Antalikova, K., & Kuncova, M. The morphology of
1980 the lung in the albino rat after paraquat administration.
Folia Morphol., 28: 188 - 191.
Kornburst, D.J. & Mavis, R.D. The effect of paraquat on microsomal
1980 lipid peroxidation in vitro and in vivo.
Toxicol. Appl. Pharmacol., 53: 323 - 332.
Kurisaki, E. & Sato, H. Toxicological studies on herbicides.
1979 Intracorporeal distribution of paraquat dichloride and
diquat dibromide in rats. Nippon Hoigaki Zasshi,
33: 656 (in Japanese).
Leahey, J.P., Hendley, P., & Spinks, C.A. Paraquat: Metabolism and
1976 residues in pigs using 14C-methyl labelled paraquat.
Unpublished report No. AR2694A from ICI Plant Protection
Division. Submitted to WHO by Imperial Chemical Industries
PLC, Haslemere, Surrey, UK.
Levin, P.J., Klaff, L.J., Rose, A.G., & Ferguson, A.D. Pulmonary
1979 effects of contact exposure to paraquat: a clinical and
experimental study. Thorax, 34: 150 - 160.
Levin, D.E., Hollstein, M., Christman, M.F., Schwiers, E.A., & Ames,
1982 B.N. A new Salmonella tester strain (TA 102) with AT base
pairs at the site of mutation detects oxidative mutagens.
Proc. Natl. Acad. Sci. USA, 79: 7445 - 7449.
Lindsay, S., Banham, P.B., Godley, M.J., Moreland, S., Wickramaratne,
1982 G.A., & Woollen, B.H. Paraquat: multigeneration reproduction
study in rats - three generations. Unpublished Report
No. CTL/P/719 from ICI Central Toxicology Laboratory.
Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Litchfield, M.H., Daniel, J.W., & Longshaw, S. The tissue distribution
1973 of the bipyridylium herbicides diquat and paraquat in rats
and mice. Toxicology, 1; 155 - 165.
Lock, E.A. & Ishmael, J. The acute toxic effects of paraquat and
1979 diquat on the rat kidney. Toxicol. Appl. Pharmacol.,
50: 67 - 76.
Maita, K., Saito, T., Tsuda, S., & Shirasu, Y. AT-5: sub-acute
1980a toxicity study in mouse. Unpublished report from the
Institute of Environmental Toxicology. Submitted to WHO by
Imperial Chemical Industries PLC, Haslemere, Surrey, UK.
Maita, K., Saito, T., Tsuda, S., & Shirasu, Y. AT-5: sub-acute
1980b toxicity study in rat. Unpublished report from the Institute
of Environmental Toxicology. Submitted to WHO by Imperial
Chemical Industries PLC, Haslemere, Surrey, UK.
Maita, K. Historical control incidence data in F344 rats. Unpublished
1986 report from Institute of Environmental Toxicology. Submitted
to WHO by Otsuka Chemical Co. Ltd., Osaka, Japan.
Makovskii, V.N. Toxicological and hygiene studies of bipyridylium
1972 herbicides diquat and paraquat. Referat, Vinnize, USSR
pp 1 - 23 (PhD degree thesis) (in Russian).
Maling, H.M., Saul, W., Williams, M.A., Brown, E.A.B., & Gillette,
1978 J.R. Reduced body clearance as the major mechanism of the
potentiation by ß-adrenergic agonists of paraquat lethality
in rats. Toxicol. Appl. Pharmacol., 43: 57 - 72.
Malone, J.D.G., Carmoday, M., & Keogh, B. Paraquat poisoning. A review
1971 of nineteen cases. J. Irish Med. Assoc., 64: 59 - 68.
Marek, J., Velenska, Z., & Haskovcova, I. Kidney changes in acute
1981 phase of paraquat intoxication in rats. Sb. Lek.,
83: 100 - 104 (in Czech.).
Matsumori, H., Matsumoto, T., & Ishikawa, H. Acute toxic effects of
1984 paraquat on ultrastructure of rat liver. Acta Pathol. Jpn,
34: 507-518.
McDonagh, B.J. & Martin, J. Paraquat poisoning in children. Arch. Dis.
1970 Child., 45: 425 - 427.
McElligot, T.F. The dermal toxicity of paraquat. Differences due to
1972 techniques of application. Toxicol. Appl. Pharmacol.,
21: 361 - 368.
Mircev, N. Acute intoxication with Gramoxone (paraquat). Vatreshni
1976 Bolesti, 16: 99 - 101 (in Bulgarian).
Montgomery, M.R. Interaction of paraquat with the pulmonary microsomal
1976 fatty acid desaturase system. Toxicol. Appl. Pharmacol.,
36: 543 - 554.
Montgomery, M.R. Paraquat toxicity and pulmonary superoxide dismutase;
1977 an enzymic deficiency of lung microsomes. Res. Commun.
Chem. Pathol. Pharmacol., 16: 155 - 158.
Montgomery, M.R. & Niewoehner, D.E. Oxidant-induced alterations in
1979 pulmonary microsomal mixed-function oxidation. Acute effects
of paraquat and ozone. J. Environ. Sci. Health,
13: 205 - 219.
Moody, C.S. & Hassan, H.M. Mutagenicity of oxygen free radicals.
1982 Proc. Natl. Acad. Sci., 79: 2855 - 2859.
Murray, R.E. & Gibson, J.E. A comparative study of paraquat
1972 intoxication in rats, guinea-pigs and monkeys.
Exp. Mol. Pathol., 17: 317 - 325.
Murray, R.E. & Gibson, J.E. Paraquat disposition in rats, guinea-pigs
1974 and monkeys. Toxicol. Appl. Pharmacol., 27: 283 - 291.
Newhouse, M., McEvoy, D., & Rosenthal, D. Percutaneous paraquat
1978 absorption. Arch. Dermatol., 114: 1516 - 1519.
Nicotera, T.M., Block, A.W., Gibas, Z., & Sandberg, A.A. Induction of
1985 superoxide dismutase, chromosomal aberrations and
sister-chromatid exchanges in Chinese hamster fibroblasts.
Mutat. Res., 151: 263 - 268.
Omaye, S.T., Reddy, K.A., & Cross, C.E. Enhanced lung toxicity of
1978 paraquat in selenium-deficient rats. Toxicol. Appl.
Pharmacol., 43: 237 - 247.
Ongom, V.L., Owor, R., & Tonusange, E.T. Paraquat (Gramoxone) used as
1974 a pediculocide. In: Bagshore, A.F., ed. The uses and abuses
of drugs and chemicals in tropical Africa. Nairobi, East
Africa Literature Bureau, pp. 229 - 233.
Parry, J.M. The induction of gene conversion in yeast by herbicide
1973 preparations. Mutat. Res., 21: 83 - 91.
Parry, J.M. The use of yeast for the detection of environmental
1977 mutagens using a fluctuation test. Mutat. Res.,
46: 165 - 176.
Pasi, A., Embree, J.W., Eisenlord, G.H., & Hine, C.H. Assessment of
1974 the mutagenic properties of diquat and paraquat in the
murine dominant lethal test. Mutat. Res., 26: 171 -175.
Pedersen, G., Pedersen, A., Gregersen, M., & Kampe, B. Paraquat
1981 poisoning. Ugeskr. Laeg., 143: 1202 - 1206 (in Danish).
Plant Protection Ltd. Data to support a revised acceptable daily
1972 intake and tolerance levels for paraquat. Unpublished report
from Plant Protection Ltd.
Popenoe, D. Effects of paraquat aerosol on mouse lung. Arch. Pathol.
1979 Lab. Med., 103: 331 - 334.
Proudfoot, A.T., Steward, M.S., Levitt, T., a Widdop, B. Paraquat
1979 poisoning: significance of plasma-paraquat concentrations.
Lancet, 2: 330-332.
Purser, D.A. & Rose, M.S. The toxicity and renal handling of paraquat
1979 in cynomolgus monkeys. Toxicology, 15: 31 - 41.
Reddy, K.A., Litov, R.E., & Omaye, S.T. Effect of pre-treatment with
1977 anti-inflammatory agents on paraquat toxicity in the rat.
Res. Commun. Chem. Pathol. Pharmacol., 17: 87 - 100.
Robertson, B. Paraquat poisoning as an experimental model of the
1973 idiopathic respiratory distress syndrome.
Bull. Phys. Pathol. Res., 9: 1433 - 1452.
Robertson, B., Grossmann, G., & Ivemark, B. The alveolar lining layer
1976 in experimental paraquat poisoning. Acta. Pathol.
Microbiol. Scand., Section A., 84: 40 - 46.
Rose, M.S., Smith, L.L., & Wyatt, I. Evidence of energy-dependent
1974 accumulation of paraquat in rat lung. Nature (Lond.),
252: 314 - 315.
Rose, M.S., Lock, E.A., & Smith, L.L. Biochem. Pharmacol.,
1976a 25: 419 - 423.
Rose, M.S., Smith, L.L., & Wyatt, I. The relevance of pentosephosphate
1976b pathway stimulation in rat lung to the mechanism of paraquat
toxicity. Biochem. Pharmacol., 25: 1763 - 1767.
Rose, M.S. & Smith, L.L. The relevance of paraquat accumulation by
1977 tissues. In: Autor, A.P., ed., Biochemical Mechanisms of
Paraquat Toxicity. New York, Academic Press, pp. 71 - 91.
Ross, W.E., Block, E.R., & Chang, R.Y. Paraquat-induced DNA damage in
1979 mammalian cells. Biochem. Biophys. Res. Commun.,
91: 1302 - 1308.
Rossouw, D.J., Chase, C.C., & Engelbrecht, F.M. The effect of paraquat
1984 on the in vitro activity of cytosol, mitochondrial, and
microsomal enzyme systems. S. Afr. Med. J., 65; 555 - 563.
Selman, M. Montano, M., Montfort, I., & Perez-Tamayo, R. The duration
1985 of the pulmonary paraquat toxicity-enhancement of O2 in
the rat. Exp. Mol. Pathol., 43: 388 - 396.
Selypes, A. & Paldy, A. The examination of the mutagenic effect of two
1978 pesticides: Krezonit E and Gramoxone. Proc. Hung. Ann.
Meet. Biochem., 18: 77- 78.
Sharp, C.W., Ottolenghi, A., & Posner, H.S. Correlation of paraquat
1972 toxicity with tissue concentration and weight loss of the
rat. Toxicol. Appl. Pharmacol., 22: 241 - 251.
Sheldon, T., Howard, C.A., Wildgoose, J., & Richardson, C.R. Paraquat
1985a dichloride: a cytogenetic study in human lymphocytes
in vitro. Unpublished Report No. CTL/P/1357 from ICI
Central Toxicology Laboratory. Submitted to WHO by Imperial
Chemical Industries PLC, Haslemere, Surrey, UK.
Sheldon, T., Richardson, C.R., Shaw, J., & Barber, G. An evaluation of
1985b paraquat dichloride (technical) in the mouse micronucleus
test. Unpublished Report No. CTL/P/1369 from ICI Central
Toxicology Laboratory. Submitted to WHO by Imperial Chemical
Industries PLC, Haslemere, Surrey, UK.
Sheppard, D.B. Paraquat thirteen week (dietary administration)
1981 toxicity study in beagles. Unpublished Report
No. 2481-72/111A from Hazelton Laboratories Europe Ltd.
Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, UK.
Shirasu, Y. & Takahashi, K. Acute toxicity of AT-5 in rat and mouse.
1977 Unpublished report from the Institute of Environmental
Toxicology. Submitted to WHO by Imperial Chemical Industries
PLC, Haslemere, Surrey, UK.
Shirasu, Y., Moriya, M., & Ohta, T. Mutagenicity testing on paraquat
1978 in microbial systems. Unpublished report from the Institute
of Environmental Toxicology. Submitted to WHO by Imperial
Chemical Industries PLC, Haslemere, Surrey, UK.
Shu, H., Talcott, R.E., Rice, S.A., & Wei, E.T. Lipid peroxidation and
1979 paraquat toxicity. Biochem. Pharmacol., 28: 327 - 331.
Sinow, J. & Wei, E. Ocular toxicity of paraquat. Bull. Environ.
1973 Contam. Toxicol., 9: 163 - 168.
Smalley, H.E. Toxicity and hazard of the herbicide paraquat in
1973 turkeys. Poultry Sci., 52: 1625 - 1629.
Smith, P., Heath, D., & Ray, J.M. The pathogenesis and structure of
1973 paraquat-induced pulmonary fibrosis in rats. J. Pathol.,
114: 57 - 67.
Smith, P. & Heath, D. The ultrastructure and time sequence of the
1974 early stages of paraquat lung in rats. J. Pathol.,
114: 177 - 184.
Smith, L.L., Wright, A., Wyatt, I., & Rose, M.S. Effective treatment
1974 for paraquat poisoning in rats and its relevance to
treatment of paraquat poisoning in man. Br. Med. J.,
4: 569 - 571.
Smith, P. & Heath, D. Paraquat. Clin. Rev. Toxicol., 4: 411 - 445.
1976
Smith, L.L., Rose, M.S., & Wyatt, I. The pathology and biochemistry of
1979 paraquat. In: Oxygen Free Radicals and Tissue Damage.
Amsterdam, North Holland, Excerpta Medica, pp. 321 - 341
(CIBA Foundatin Series 65 - new series).
Smith, L.L., Wyatt, I., & Rose, M.S. Factors affecting the efflux of
1981 paraquat from rat lung slices. Toxicology, 19: 197 - 207.
Smith, L.L. Paraquat toxicity. Phil. Trans. R. Soc. Lond. B.,
1985 311: 647 - 657.
Sotheran, M., Banham, P.B., Godley, M.J., Lindsay, S., Pratt, I.,
1981 Taylor, K., & Woollen, B.H. Paraquat; lifetime feeding study
in the mouse. Unpublished Report No. CTL/P/556 from ICI
Central Toxicology Laboratory. Submitted to WHO by Imperial
Chemical Industries PLC, Haslemere, Surrey, UK.
Spinks, C.A., Hendley, P., Leahey, J.P., & Carpenter, P.K. Paraquat:
1976 Metabolism and residues in pigs using 14C-ring labelled
paraquat. Unpublished report No. 2692A from ICI Plant
Protection Division. Submitted to WHO by Imperial Chemical
Industries PLC, Haslemere, Surrey, UK.
Staiff, D.C., Comer, S.W., Armstrong, J.F., & Wolfe, H.R. Exposure to
1975 the herbicide paraquat. Bull. Environ. Contam. Toxicol.,
14: 334 - 340.
Steffen, C. & Netter, K.J. On the mechanism of paraquat action on
1979 microsomal oxygen reduction and its relation to lipid
peroxidation. Toxicol. Appl. Pharmacol., 47: 593 - 602.
Steffen, C., Muliawan, H., & Kappus, H. Lack of in vivo lipid
1980 peroxidation in experimental paraquat poisoning.
Arch. Pharmacol., 310: 241 - 243.
Suzuki, T., Igarashi, N., & Tamura, K. Paraquat D.C.; Multiple
1983 generation study in rats. Unpublished Report No. R-015 from
Bozo Research Centre Co. Ltd. Submitted to WHO by Otsuka
Chemical Co., Osaka, Japan.
Swan, A.A.B. Exposure of spray operators to paraquat.
1969 Br. J. Ind. Med., 26: 665 - 671.
Sykes, B.J., Purchase, I.F.H., & Smith, L.L. Pulmonary ultrastructure
1977 after oral and intravenous dosage of paraquat to rats.
J. Pathol., 121: 233 - 241.
Talcott, R.E., Shu, H., & Wei, E.T. Dissociation of microsomal oxygen
1979 reduction and lipid peroxidation with the electron
acceptors, paraquat and menadione. Biochem. Pharmacol.,
26: 665 - 671.
Thompson, W.D. & Patrick, R.S. Collagen propyl hydroxylase levels in
1978 experimental paraquat poisoning. Br. J. Exp. Pathol.,
59: 288 - 291.
Toyoshima, S., Sato, R., Kashima, M., Sato, H., Sato, H., Sato, H.,
1982a Motoyama, M., Ichiba, J., & Kimura, R. AT-5: chronic
toxicity study result - 104 week dosing study in mouse.
Unpublished report from Nippon Experimental Medical Research
Co. Ltd. Submitted to WHO by Imperial Chemical Industries
PLC, Haslemere, Surrey, UK.
Toyoshima, S., Sato, R., Kashima, M., Sato, H., Sato, H., Sato, H.,
1982b Motoyama, M., Ishidawa, A., Ichiba, J. & Kimura, R. AT-5:
chronic toxicity study result - 104 week dosing study in
rat. Unpublished report from Nippon Experimental Medical
Research Co. Ltd. Submitted to WHO by Imperial Chemical
Industries PLC, Haslemere, Surrey, UK.
Trueman, R.W., Ashby, J., & Burlinson, B. Paraquat dichloride:
1985 assessment for the induction of unscheduled DNA synthesis in
primary rat hepatocyte cultures. Unpublished Report
No. CTL/P/1339 from ICI Central Toxicology Laboratory.
Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Trush, M.A., Mimnaugh, E.G., Ginsburg, E., & Gram, T.E. In vitro
1981 stimulation by paraquat of reactive oxygen-mediated lipid
peroxidation in rat lung microsomes. Toxicol. Appl.
Pharmacol., 60: 279 - 286.
Trush, M.A., Mimnaugh, E.G., Ginsburg, E., & Gram, T.E. Studies on the
1982 in vitro interaction of mitomycin C, nitrofurantoin, and
paraquat with pulmonary microsomes. Simulation of reactive
oxygen-dependent lipid peroxidation. Biochem. Pharmacol.,
31: 3335 - 3346.
Tweats, D.J. The effect of R (drug resistance) factors on the
1975 detection of mutagens by E. coli K 12. Brussels, Belgium,
Annual Euratom Report, Biological Sciences, 316 pp.
Veseley, D.L., Watson, B., & Levey, G.A. Activation of liver guanylate
1979 cyclase by paraquat: possible role of superoxide anion.
J. Pharmacol. Exp. Ther., 209: 162 - 164.
Vijeyaratnam, G.S. & Corrin, B. Experimental paraquat poisoning: A
1971 histological and electron-optical study of the changes in
the lung. J. Pathol., 103: 123 - 129.
Waddell, W.J. & Marlowe, C. Tissue and cellular disposition of
1980 paraquat in mice. Toxicol. Appl. Pharmacol.,
56: 127 - 140.
Waight, J.J.J. & Wheather, R.H. Fatal percutaneous paraquat poisoning.
1979 J. Am. Med. Assoc., 242: 472.
Walker, M., Dugard, P.H., & Scott, R.C. Absorption through human
1983 laboratory animal skins: in vitro comparison.
Acta Pharm. Suec., 20: 207 - 213.
Wester, R.C., Maibach, H.I., Bucks, D.A.W., & Aufrere, M.B. In vivo
1984 percutaneous absorption o[ paraquat from hand, leg, and
forearm of humans. J. Toxicol. Environ. Health,
14: 759 - 762.
WHO. Paraquat and diquat. WHO Environmental Health Criteria, No. 39.
1984 International Programme on Chemical Safety, World Health
Organization, Geneva.
Witschi, H.P., Kacew, S., Hirai, K., & Cote, M.G. In vivo oxidation of
1977 reduced nicotinamide-adenine dinucleotide phosphate by
paraquat and diquat in rat lung. Chem. Biol. Interactions,
19: 143 - 160.
Wohlfart, D.J. Fatal paraquat poisoning after skin absorption. Med. J.
1982 Aust., 1: 512 - 513.
Wojeck, G.A., Price, J.F., Nigg, A.N., & Stamper, J.H. Worker exposure
1983 to paraquat and diquat. Arch. Environ. Contam. Toxicol.,
12: 65 - 70.
Wyatt, I., Doss, A.W., Zavala, D.C., & Smith, L.L. Intrabronchial
1981 instillation of paraquat in rats: lung morphology and
retention study. Br. J. Ind. Med., 38: 42 - 48.
Yasaka, T., Okudaira, K., Fujito, H., Matsumoto, J., Ohya, I., &
1986 Miyamoto, Y. Further studies of lipid peroxidation in human
paraquat poisoning. Arch. Int. Med., 146: 681 - 685.
Yoshida, A., Maita, K., Tsuda, S., Saito, T., & Shirasu, Y. Paraquat
1982 D.C.: chronic toxicity - rats: 24 month study. Unpublished
report from the Institute of Environmental Toxicology,
Japan. Submitted to WHO by Otsuka Chemical Co., Osaka,
Japan.
Historical control incidence data of neoplasia in F-344 rats in
the laboratory in which the preceding study was conducted were made
available and are summarized in Table 3.
Table 3. Spontaneous lung tumours observed in F-344 rats at the
Institute of Environmental Toxicology from 1980 to 19831
No. of tumour-bearing animals (%)2
Males Females
Adenoma 40 (4.2%) 21 (2.2%)
Adenocarcinoma 5 (0.5%) 1 (0.1%)
Bronchial gland adenoma 1 (0.1%) -
Total 46 (4.8%) 22 (2.3%)
1 From Maita, 1986
2 These data were taken from 12 studies that included 960 male and
959 female F-344/DuCrj rats.
Groups of 60 male and 60 female Fischer 344 rats were maintained
on diets containing paraquat cation at 0 (2 groups), 25, 75, or
150 ppm for at least 113 weeks (males) or 122 weeks (females). Further
groups of 10 rats/sex/group received the same diets for 1 year. All
animals were studied for mortality, food and water consumption, and
body weight, and were subjected to periodical ophthalmoscopic and
haematological examinations throughout the study.
The distribution of mortality was unaffected by treatment. There
was approximately 50% mortality in all groups at the end of the study.
At 150 ppm, statistically-significant reductions in body-weight gain,
food consumption, and efficiency of food utilisation in both sexes
were observed. There was a statistically-significant depression of
body-weight gain in the first year of the study in males receiving
75 ppm paraquat. Water consumption was not significantly affected at
any dietary level tested. Paraquat accelerated, in a dosage-dependent
manner, the onset and progression of cataract changes, ranging from
minor opacity to total cataract in both males and females.
Treatment-related ocular lesions were first seen at 52 weeks.
Thereafter, ophthalmoscopy revealed a statistically-significant
dosage-related increase in the incidence, progression, and severity of
lenticular cataract in the 150 ppm group and, toward the end of the
study (103 weeks), in the 75 ppm group. There was evidence of
paraquat-dependent ocular effects in all treatment groups of both
sexes at termination. A statistically-significant higher incidence of
secondary eye lesions was found at termination in females receiving 75
or 150 ppm paraquat when compared to controls. Haematological
investigation (RBC counts, total and differential leucocyte counts,
haemoglobin, haematocrit, mean cell volume, mean cell haemoglobin
concentration, platelet and reticulocyte counts, and prothrombin and
partial thromboplastin times) and blood biochemistry (urea, glucose,
ALT, and AST) indicated no significant effects attributable to
paraquat administration. Urinalysis did not reveal any treatment-
related changes. Reductions in liver and testicular weights were noted
at termination in the 150 ppm dietary group.
Macroscopic examination at necropsy revealed a treatment-related
increase in the incidence of focal subpleural changes in animals
killed at termination in all dietary groups. This effect was most
marked in females receiving 75 ppm and in both sexes receiving 150 ppm
paraquat. Microscopic examination of lung tissues indicated that
treatment with paraquat at 150 ppm, in both sexes, and possibly at
75 ppm in males, was associated with proliferative lesions of the
alveolar epithelium. These lesions were not easily classified into
non-neoplastic or neoplastic, nor into adenoma or carcinoma. This
study provided strong evidence for the induction by paraquat of a
proliferative lesion of the alveolar epithelium and some controversial
evidence for the induction of lung adenomas in female Fischer 344
rats. There was no treatment-related increase in the incidence of lung
adenocarcinoma at any dose level in either sex. At 25 ppm, significant
increases in the incidence of proliferative lung lesions, compared to
the controls, were not observed. Microscopic examination of the eyes
confirmed a dose-related effect of paraquat on the onset and
progression of cataract lesions normally present in both male and
female F-344 rats. Slight dilation of the fourth ventricle of the
brain was evident in females receiving 150 or 75 ppm paraquat, but not
in males at these dosages or in either sex at 25 ppm. A statistically-
significant increase in the incidence of apparent degeneration of
occasional/several sciatic nerve fibers was noted in decedent males
receiving 75 or 150 ppm paraquat. Both hydrocephalus and nervous
tissue changes were considered by the authors of the study possibly
to be associated with paraquat treatment. Pathology summaries
indicate that atrophy of the testes was recorded in the high-dietary
group (5/33) but not in controls at termination, and moderate lymphoid
hyperplasia was observed in the respiratory epithelium of males
receiving 75 and 150 ppm paraquat and dying between 52 weeks and
termination.
The authors of the study concluded that "a wide range of tumour
types was observed in treated and control animals, and there was no
evidence that treatment with paraquat resulted in a marked change in
the group distribution of any of these tumours". A no-effect level for
paraquat was not found in this study due to the higher incidence of
cataract observed in animals of the 25 ppm group when compared to
controls. Paraquat accelerated, in a dosage-dependent manner, the
onset and progression of cataract change in F-334 rats. The authors
considered 25 ppm to be near the no-effect level for this change at
the end of the study (Ashby et al., 1983; Busey, 1986; Ishmael &
Godley, 1983).
Data on the historical incidence of lung neoplastic lesions in
F-344 rats from 7 studies performed in the laboratory in which the
preceding study was conducted were made available and are summarised
in Table 4.
Table 4. Spontaneous lung tumours observed in F-344 rats at Life
Science Research1
No. of tumour-bearing animals (%)2
Males Females
Adenoma 6/357 (1.7%) 4/363 (1.1%)
Carcinoma 3/357 (0.8%) 0/363 (0%)
Total pulmonary tumours 9/357 (2.5%) 4/363 (1.1%)
1 From Ashby et al., 1983
2 The range (% incidence) of adenoma was 0 - 4.4% in males and
0 - 4.0% in females; the range of carcinoma was 0 - 4.0% in
males.
Special studies on embryotoxicity and teratogenicity
Mice
The teratogenicity and fetal toxicity of paraquat were examined
after oral (20 mg/kg b.w./day) or i.p. (1.67 or 3.35 mg/kg b.w./day)
administration of paraquat to pregnant mice during the period of
organogenesis (days 8 - 16 of gestation). The oral dose (which was
equal to 1/10 of the oral LD50/day) did not produce significant
maternal toxicity, but at the higher of the 2 i.p. doses (which was
equal to 1/10 of the i.p. LD50/day) a significant maternal mortality
(5/7) and a statistically-significant increase of resorption rate,
when compared to controls, were observed. Paraquat did not
significantly increase the incidence of gross, soft-tissue, or
skeletal abnormalities. At the lower i.p. dose and after oral
administration of paraquat, there was a slight but non-significant
increase in the number of fetuses with absent or non-ossified
sternebrae. However, a significant difference was observed in the
incidence of abnormal sternebrae between the 2 control groups (6.9 ±
3.2% in the i.p. control group and 13.2 ± 5.8% in the oral control
group). The authors of the study concluded that the potential for
paraquat as a teratogen appeared to be minimal (Bus et al., 1975b).
The same authors examined the effects of paraquat dichloride on
the development of Swiss-Webster mice when administered in the
drinking water at concentrations of 0, 50, or 100 ppm. Paraquat was
given to pregnant mice from day 8 of gestation and administration was
continued to the newborns until 42 days after birth, when both the
control and paraquat-treated mice were sacrificed and subjected to
histopathological examination of the lungs, liver, and kidneys. A
significant increase in postnatal mortality in mice receiving 100 ppm
paraquat was observed. Histopathological examination of the lungs of
these mice showed extensive alveolar consolidation and collapse, and
areas of thickening of intra-alveolar septa. No significant
pathological changes were seen in the lungs of the 50 ppm or control
mice, nor in the liver or kidneys of mice of any treatment group.
There were no treatment-related effects on the number of live fetuses
nor on postnatal growth rate at either treatment level (Bus & Gibson,
1975).
Four groups of at least 20 pregnant SPF Alderley Park mice were
given orally 0, 1, 5, or 10 mg/kg b.w./day of paraquat cation during
days 6 to 15 of pregnancy, inclusive. On day 18 the animals were
killed, their uteri were examined, and the fetuses were removed,
weighed, sexed, and observed for gross abnormalities. There was some
evidence of maternal toxicity in the form of slight reductions in
body-weight gain at 5 and 10 mg/kg b.w./day, although only that of the
middle-dose group was statistically significant. There were no
clinical signs nor pathological changes in maternal animals
attributable to paraquat administration. Water and food consumption
were not quantified in this study. Numbers of implantations, viable
fetuses and resorptions, sex ratios, and fetal and litter weights
showed no significant differences between treated and control groups.
There were no increases in fetal external or soft-tissue abnormalities
which could be associated with paraquat treatment. There were
occasional statistically-significant differences in ossification of
individual bones between treated and control groups, but no
dose-related trend indicating either retardation of ossification or
increased abnormalities was observed. The authors of the study
concluded that paraquat was not teratogenic and had no significant
influence on embryonic or fetal development of the mouse at levels up
to and including 10 mg/kg b.w./day (Hodge et al., 1978a).
Rats
The teratogenic effects of paraquat were studied in 4 groups of
at least 20 pregnant SPF Alderley Park rats after oral administration
of 0, 1, 5, or 10 mg/kg b.w./day of paraquat cation during days 6 to
15 of pregnancy, inclusive. On day 21 the animals were killed. There
were clear clinical signs of maternal toxicity at 5 and 10 mg/kg
b.w./day. Apparently, 6 rats at the highest-dose level and 2 at the
middle-dose level died or became moribund during the experiment.
Histological changes found in the lungs and kidneys of the animals
receiving 10 mg/kg b.w./day paraquat which died or became moribund
were those known to be associated with oral paraquat poisoning. Slight
fetotoxicity was seen at 5 and 10 mg/kg b.w./day, as shown by a
statistically-significant reduction in fetal weight and retardation in
ossification, and by a decrease in the number of viable fetuses per
number of implants. According to the authors of the study, these
effects were probably associated with maternal toxicity. There were no
effects on embryonic or fetal survival and increases in fetal
abnormalities were not observed. The authors of the study concluded
that paraquat was not teratogenic when administered orally to rats,
even when there was clear evidence of maternal toxicity. However, it
did cause slight fetotoxicity at the 2 highest-dose levels (Hodge
et al., 1978b).
Special studies on eye irritation
The effects of paraquat on the eye have been reviewed by WHO
(1984). The instillation of diluted paraquat (up to 500 g/litre) in
rabbits' eyes induced inflammation within 24 hours, and this continued
for 96 hours (Clark et al., 1966). In another experiment, 62.5, 125,
250, 500, or 1000 g/litre of paraquat was introduced into the eyes of
rabbits. Concentrations of 62.5 and 125 g/litre caused severe
conjunctival reactions; higher levels (250 - 500 g/litre) provoked
ititis and pannus, while at the 500 g/litre concentration corneal
opacification, iritis, and conjunctivitis occurred. All rabbits
receiving 0.2 ml of paraquat at 1000 g/litre in 1 eye or 0.2 ml of
500 g/litre paraquat in both eyes died within 6 days of application
(Sinow & Wei, 1973).
Special studies on mutagenicity
In a review of published mutagenicity data (WHO, 1984) it was
noted that paraquat had been found to have minimal to no genotoxic
activity when evaluated in a variety of in vitro and in vivo test
systems. In in vitro studies producing weakly positive results,
paraquat genotoxicity was accompanied by high cytotoxicity (Moody &
Hassan, 1982; Parry, 1973 & 1977; Tweats, 1975; Benigni et al.,
1979; Bignami & Grebelli, 1979). Moody and Hassan (1982) have shown
that the mutagenicity of paraquat in bacterial test systems
(Salmonella typhimurium TA98 and TA100) was mediated by the
formation of superoxide. More recently, paraquat was found to induce
superoxide dismutase, chromosomal aberrations, and sister-chromatid
exchange in Chinese hamster fibroblasts, suggesting that superoxide
production is responsible for the chromosomal damage (Nicotera
et al., 1985). Other investigators (Anderson et al., 1972; Levin
et al., 1982) have not found mutagenic activity in bacterial test
systems. Furthermore, paraquat was not mutagenic when evaluated in
human leukocytes nor in in vivo cytogenic tests on mouse bone marrow
(Selypes & Paldy, 1978) or in dominant lethal tests on mice
(Pasi et al., 1974; Anderson et al., 1976).
A set of recently completed studies indicate that in most tests
paraquat was not mutagenic (see Table 5). Clastogenic potential has
been shown in vitro at very high concentration levels which were
themselves cytotoxic. Paraquat was not found to be mutagenic
in vivo.
Special studies on reproduction
Rats
In a 3-generation study, groups of 15 male and 30 female (F0
parents) weanling Alderley Park SPF rats were fed diets containing
0, 25, 75, or 150 ppm paraquat cation. After 12 weeks, animals were
mated to produce the first (F1a) litter and subsequently re-mated to
produce a second (F1b) litter. The breeding programme was repeated
twice with F1 parents selected from the F1b offspring and F2
parents selected from the F2b offspring. Test diets were fed
continuously throughout the study.
There were no adverse effects on parental body weights or food
consumption, and no treatment-related changes were found in the
reproductive performance (male and female fertility, live-born and
survival indexes, and litter size) or in the reproductive tract of
parents or offspring. Development of the reproductive tract in all
treated offspring was substantially comparable to that in controls.
The mild atrophy of seminal tubules found in a few of the treated
males of the F2b offspring at termination was considered by the
authors of the study to have no toxicological significance. Lung
changes due to paraquat administration occurred mainly in females
receiving 150 ppm paraquat. One pulmonary adenoma was found in 1
female receiving 150 ppm paraquat. Death resulting from severe, acute,
or sub-acute lung damage was confined to females with litters of
weaning age, and to 3 F0 females which died during the first 2 weeks
of the study. There were dose-related increases in the incidence and
Table 5. Mutagenicity assays on paraquat
Test system Test object Concentration Purity Results Reference
used (%)
Ames test1 S. typhimurium 0.12, 0.6, 2.9, 99 Negative Anderson, 1977
TA98 14, 72, 361,
TA100 & 1807 µg/plate
TA1535 (dissolved in
TA1538 H2O)
Ames test1 S. typhimurium 0.4, 0.7, 3.6, 100 Negative, growth Shirasu et al., 1978
TA98 7, 36, 72, & inhibition at 72
TA100 360 µg/plate at 72 & 360
TA1535 (dissolved in µg/plate2
TA1538 H2O)
Host-mediated S. typhimurium 3.6 & 14 mg/kg 100 negative2 Shirasu et al., 1978
assay G 46 (host: male (2 equal doses
ICR mice) orally)
Rec-assay B. subtilis 14 - 361 100 negative2 Shirasu et al., 1978
µg/disk
Mouse L 5178 Y 23, 45, 90, 180, 99 ? Clay & Thomas, 1985
lymphoma mouse & 361 µg/ml
test1 lymphoma
cells
Table 5. (cont'd).
Test system Test object Concentration Purity Results Reference
used (%)
Clastogenic Human 90, 903, & 1807 99.6 positive at 2 Sheldon et al., 1985a
potential lymphocytes µg/ml highest levels
test1 in vitro (also cytotoxic)
Mouse Male & female 51.7 & 82.8 99.4 negative Sheldon et al., 1985b
micronucleus C 57/BL/6J/ mg/kg (single
test Alpk mice dose orally)
In vitro Chinese 0.9, 1.8, 9, 18, 99.4 positive (reduced Howard et al., 1985
sister hamster lung 90 & 177 µg/ml with metabolic
chromatid fibroblasts activation)
exchange1
Unscheduled Hepatocyte 1 nM - 10 mM 99.6 negative Trueman et al., 1985
DNA cultures (0.19 ng/ml -
synthesis from male 1.86 mg/ml)
Alderley Park
albino rats
1 Both with and without metabolic activation
2 Positive control compounds gave positive responses
severity of focal alveolar histiocytosis in the lungs of male and
female parents receiving 75 and 150 ppm paraquat. A mild perivascular
inflammation was observed in the lungs of F1b pups receiving 150 ppm
paraquat. No changes due to paraquat were seen in animals receiving
25 ppm paraquat. The authors concluded that paraquat had no effect on
reproductive performance or development of the reproductive organs of
Alderley Park rats when administered at dietary levels up to 150 ppm
over 3 generations (Lindsay et al., 1982).
Groups of 12 male and 24 female rats were fed diets containing
0, 30, or 100 ppm paraquat ion from 35 days of age. Three generations
bred from these animals received the same diets during the whole
period under test. Two litters were bred from each generation, and the
effects on growth, food intake, fertility, fecundity, neonatal
morbidity, and mortality were noted. No evidence was seen of damage to
germ-cell production or of structural or functional damage in the
animals. In this study, pregnant and young animals did not appear to
be more vulnerable to paraquat than did adults. However, the incidence
of renal hydropic degeneration in 3 - 4 week-old offspring was
slightly increased in the 100 ppm group (Fletcher et al., 1972).
In a 3-generation study with 2 litters per generation, groups of
30 male and 30 female Sprague-Dawley rats (F0 parental generation)
were given diets containing 0, 72, 145, or 290 ppm paraquat cation
from 5 weeks of age (13 weeks prior to mating to obtain the first
litter, F1a) until the end of the second lactation (lactation of
F1b litters). In the second generation (F1b), 30 males and 30
females per group were treated from immediately after weaning until
the end of the second lactation (lactation of F2b litters). In the
third generation (F2b), the same number of rats were treated
immediately after weaning for at least 13 weeks. In a teratology study
sub-group 5 pregnant females of the parental generation (F0) and 10
pregnant females of the second generation (F1b) were killed on day
20 of pregnancy and examined macroscopically. Fetuses were examined
for number, sex, weight, external and internal abnormalities, and
progress of ossification. Another subgroup was used as a postnatal
investigation group, where natural parturition of pregnant females was
permitted to occur. In this group the duration of gestation,
parturition conditions, the number of live and still-born pups, sex,
and external abnormalities were recorded. Live pups were investigated
until weaning. Five male and 5 female rats per group of the first
(F0) and second (F1b) generations and 10 male and 10 female rats
per group of the F2b litters were subjected to histopathological
examination of approximately 25 tissues.
In the parental generation there were significant increases in
mortality and clinical signs attributable to paraquat (asthmatoid
wheezing) in several rats of the 290 ppm group of each generation from
the early stage of the dosing period. There were 29 treatment-related
deaths/moribund animals, all from the 290 ppm dietary level (5 among
F1b animals and 24 among F2b animals), but only 4 deaths among
control rats (1 among F0 female rats and 2 among F1b female rats
at parturition and 1 among F2b rats during the dosing period).
Histopathological examination of these dead or moribund F2b rats
showed, in some cases, hyperplasia of the alveolar epithelium and, in
most cases, diffuse thickening and fibrosis of the alveolar walls.
There was a decrease in body-weight gain in both male and female rats
of the F0 and F2b generations at 290 ppm during the early stage of
the dosing period. Body-weight gain was also reduced in F1b females
at 290 ppm during the gestation and lactation periods. Reductions in
food consumption and efficiency of food utilisation were seen in F0
and F2b females. There was a significant decrease in water
consumption in F0 and F1b females during lactation. No effect of
the compound was observed on the reproductive performance of parental
rats. Macroscopic examination revealed an apparently higher incidence
of white spots in the lungs of both male and female rats of the
290 ppm group in all 3 generations. Treatment-related changes of the
lung were confirmed by histopathology in rats of each generation.
These lesions were dose-dependent and included zonal thickening and
fibrosis of the alveolar walls, zonal atelectasis, and accumulation of
foam cells. There were no treatment-related changes in organ weights.
A treatment-related statistically-significant reduction in the
lactation index was found in F1a and F1b litters of the 290 ppm
group. A statistically-significant reduction in the lactation index
was also observed in F2a litters of the 290 ppm group, but it was
not clear to the authors of the report whether this change was
attributable to treatment. There were no statistically-significant
differences in lactation index between other treatment groups and
controls nor in the number of still-births and live births, sex
ratios, or viability indexes in any of the treated groups of both
generations, when compared to controls. The prolonged duration of
gestation in F0 rats of the parental group at 145 and 290 ppm was
considered by the authors of the report to be accidental. The
teratology phase showed a statistically-significant delay in
ossification in F1b fetuses from F0 parents treated with 290 ppm
paraquat and in F2b fetuses from F1b parents of all treated
groups. It was not clear to the authors of the report whether the
retarded growth was due to the treatment. There was a treatment-
related statistically-significant higher incidence of female
pups with retarded opening of the vagina in both F1b and F2b
litters at 290 ppm. There was a statistically-significant decrease in
body weight in male, but not in female, fetuses at 72 and 290 ppm.
There were no statistically-significant differences between fetuses
from treated rats and control fetuses in the number of corpora lutea
or implantations, percentage of implantations, number of dead or live
fetuses, sex ratios, or placental weights. No external or internal
malformations were detected in fetuses of any treatment group.
The authors of the report concluded that there was no evidence
suggesting that paraquat was teratogenic and that the only treatment-
related change which was enhanced by treatment of successive
generations of Sprague-Dawley rats with paraquat was "an increase in
death" at 290 ppm. A no-effect level for paraquat was not found in
this study due to delayed ossification in F2b fetuses in all treated
groups (Suzuki et al., 1983).
Rabbits
Forty rabbits received paraquat i.p. at total dosages of 2 to
100 mg/kg b.w. in 1 to 5 separate administrations. Multinuclear giant
cells were found in testicular tubules of 7/20 rabbits receiving
50 mg/kg b.w. or more paraquat (Butler & Kleinerman, 1971). However,
it has been reported that, when paraquat was orally administered at
4 mg/kg b.w. to male rats for 60 days and testes were examined, there
were no significant deviations in the spermatozoa count or motility,
nor were there any biochemical changes in the several enzymes of
testes homogenates. The histoenzyme activity of lactate dehydrogenase,
succinate dehydrogenase, DPN-diaphorase, alkaline phosphatase, and
acid phosphatase in the treated animals did not differ from those of
the controls, nor did quantitative and qualitative histological
examination of the testicular tubule cells reveal any abnormalities
(WHO, 1984).
Special studies on skin irritation
The effects of paraquat on the skin have been reviewed by WHO
(1984). Paraquat can provoke local irritation of the skin and eyes.
Clark et al. (1966) found skin irritation in rabbits only when
paraquat was applied beneath occlusive dressings in aqueous solutions
(total doses 1.56, 5.0, and 6.25 mg ion/kg b.w.). In mice and rats,
the application of solutions of 5 - 20g paraquat/litre in single and
21-day repeated dermal toxicity tests provoked dose-related toxic
dermatitis with erythema, oedema, desquamation, and necrosis (Bainova,
1969). Doses from 1.56 to 50 mg/kg, in repeated 20-day studies using
the occlusive technique (McElligott, 1972), resulted in local erythema
and scab formation. The histological changes consisted of
parakeratosis and occasional intra-epidermal pustules. A delayed
skin-irritant action of the herbicide was reported by Fodri et al.
(1977) in guinea pig studies.
Acute toxicity
The LD50 and LC50 values for paraquat in various species are
given in Table 6.
Table 6. Acute toxicity of paraquat in various species
LD50 LC50
Species Route Sex (mg/kg b.w.) (mg/l) Reference
Mouse oral M 260 -- Shirasu & Takahashi, 1977
F 210 --
i.p. M & F 29-30 -- Shirasu & Takahashi, 1977
Bus et al., 1975a
i.v. -- 50 -- Ecker et al., 1975
s.c. M 30 -- Shirasu & Takahashi, 1977
F 27 --
dermal -- 62 -- Bainova, 1971
Rat oral M 161 -- Shirasu & Takahashi, 1977
F 187 --
M 110 -- Kimbrough & Gaines, 1970
F 100 --
-- 126 -- Murray & Gibson, 1972
-- 200 -- Howe & Wright, 1965
i.p. M 18 -- Shirasu & Takahashi, 1977
F 19 --
F 19 -- Clark et al., 1966
s.c. M 19 -- Shirasu & Takahashi, 1977
F 23 --
-- 22 -- Makovskii, 1972
dermal M 90 -- Kimbrough & Gaines, 1970
F 80 --
-- 350 -- Makovskii, 1972
Table 6. (cont'd).
LD50 LC50
Species Route Sex (mg/kg b.w.) (mg/l) Reference
inhalation M & F -- 10 Bainova & Vulcheva, 1972
-- -- 1 Gage, 1968
-- -- 6 Makovskii, 1972
Guinea oral M 30 -- Clark et al., 1966
pig
-- 40-80 -- Howe & Wright, 1965
-- 22 -- Murray & Gibson, 1972
-- 42 -- Makovskii, 1972
i.p. F 3 -- Clark et al., 1966
s.c. -- 5 -- Makovskii, 1972
dermal -- 319 -- Makovskii, 1972
inhalation -- -- 4 Makovskii, 1972
Cat oral F 35 -- Clark et al., 1966
-- 40-50 -- Howe & Wright, 1965
Hen oral -- 300-380 -- Howe & Wright, 1965
-- 262 -- Clark et al., 1966
Turkey oral -- 250-280 -- Smalley, 1973
i.p. -- 100 -- Smalley, 1973
i.v. -- 20 -- Smalley, 1973
dermal -- 375 -- Smalley, 1973
Monkey oral -- 50 -- Murray & Gibson, 1972
Sheep oral -- 50-75 -- Howe & Wright, 1965
Cow oral -- 50-75 -- Howe & Wright, 1965
Following a single high dose of paraquat to animals, the earliest
ultrastructural changes were observed in the Type I alveolar
epithelial cells, approximately 4 - 6 hours after treatment, and were
usually characterised by cellular and mitochondrial swelling,
increased numbers of mitochondria, and the appearance of dark granules
in the cytoplasm. When a high dose was given (equal to approximately
the LD50 or greater), the lesions in the Type I cells often
progressed to the point of complete cellular disintegration, leaving
areas of exposed basement membrane (Kimbrough & Gaines, 1970;
Smith et al., 1973; Smith & Heath, 1974; Vijeyaratnam & Corrin,
1971; Klika et al., 1980). In contrast to the effects on Type I
pneumocytes, however, the capillary endothelial cells were remarkably
resistant to the toxic effects of paraquat (Sykes et al., 1977).
Ultrastructural lesions in the alveolar Type II pneumocytes were
also observed shortly after single-dose paraquat exposure, although,
generally, these lesions were not apparent until after the first
lesions were seen in the Type I cells (Kimbrough & Gaines, 1970).
Swollen mitochondria and damage to the lamellar bodies usually
occurred between 8 and 24 hours after a high dose of paraquat
(Robertson, 1973; Robertson et al., 1976). Progressive deterioration
of the Type II cells continued, resulting in completely denuded
alveolar basement membranes and debris-filled alveolar spaces
(Vijeyaratnam & Corrin, 1971). Infiltration and proliferation of
fibroblasts may produce fibrosis that obliterated the alveolar
structure (Smith & Heath, 1974).
Vijeyaratnam & Corrin (1971) observed that less-severely affected
parts of the lung appeared to undergo epithelial regeneration 7 - 14
days after a single dose of paraquat. Electron microscopic examination
revealed the alveoli to be lined with cuboidal epithelial cells that
closely resembled Type II pneumocytes, except for a general lack of
lamellar bodies. Similar phenomena have also been noted by other
investigators who administered paraquat in the diet (Kimbrough &
Linder, 1973) or as repetitive i.p. administrations (Smith et al.,
1974). Thus, in animals where the paraquat dose was sufficient to kill
only the Type I pneumocytes, the surviving Type II cells repaired the
damaged epithelium by proliferating and subsequently differentiating
into Type I epithelial cells. Inhaled paraquat in aerosol produced
initial necrosis of the epithelia and Type II pbeumocyte hyperplasia,
fibroblast proliferation, and increased synthesis of collagen in mice
(Popenoe, 1979).
The pathogenesis of the paraquat lung lesion has been well
characterised, and has been reviewed by Smith & Heath (1976). The
acute pulmonary toxicity of paraquat in animals has been described as
occuring in 2 phases. In the initial "destructive" phase, alveolar
epithelial cells were extensively damaged and their subsequent
disintegration often resulted in a completely denuded alveolar
basement membrane. Pulmonary oedema was also a characteristic of the
destructive phase, and was frequently of sufficient severity to result
in the death of the animals. Animals surviving the initial destructive
phase, which occurred in the first 1 - 4 days after acute paraquat
overexposure, progressed to what has been termed the "proliferative"
phase. In this phase, the lung was infiltrated with prolifroblastic
cells that rapidly differentiated into fibroblasts which, in some
cases, progressed to fibrosis. The histopathological outcome of the
second phase may be influenced by the treatment regimen, however.
Administration of repeated low doses of paraquat, which less-severely
damaged the alveolar epithelial cells, was also able to induce a
hyperplasia of the Type II cells. This response may represent an
attempt by the lung to repair the damaged epithelium (WHO, 1984).
When rabbits were injected i.p. with total doses of paraquat from
2 - 100 mg/kg b.w., thymic atrophy was observed, but most lungs showed
only occasional and small histological deviations that were poorly
correlated with the clinical signs of paraquat intoxication. These
results confirmed the resistance of the rabbit to paraquat-induced
lung lesions (Butler & Kleinerman, 1971).
According to Murray & Gibson (1972) and Hundsdorfer & Rose
(1980), guinea pigs treated with paraquat either orally or s.c. did
not develop the same type of progressive pulmonary fibrosis as
paraquat-intoxicated rats. In hamsters, a single administration of
paraquat did not induce lung damage, but prolonged exposure resulted
in lung fibrosis (Butler, 1975).
In conclusion, from lung toxicity studies, a characteristic
dose-related pulmonary fibrosis can be induced in rats, mice, dogs,
and monkeys, but not in rabbits, guinea pigs, or hamsters.
In paraquat toxicity, kidney damage often precedes signs of
respiratory distress (Clark et al., 1966; Butler & Kleinerman, 1971;
Murray & Gibson, 1972). Paraquat is excreted primarily via the urine
and the concentrations of the herbicide in the kidneys are relatively
high (see Table 1). Gross pathological and histological examination of
paraquat-poisoned rats, guinea pigs, rabbits, and dogs revealed
vacuolation of the convoluted renal tubules and proximal tubular
necrosis (Murray & Gibson, 1972). The nephrotoxicity caused by
paraquat is pronounced and appears to be restricted to the proximal
nephron (Ecker et al., 1975; Gibson & Cagen, 1977; Lock & Ishmael,
1979; Purser & Rose, 1979). The degeneration of the proximal tubular
cells has also been confirmed by electron-optical studies (Fowler &
Brooks, 1971; Marek et al., 1981).
In contrast with lung and kidney damage, liver damage in
experimental animals has not been severe and serum enzyme activities
(SGOT, SGPT, LAP) only increased when large amounts of paraquat were
given (Girl et al., 1979). Recently, electron microscopic
examination of the liver of paraquat-treated rats showed early,
localised changes (degranualtion of the RER, proliferation of the SER,
and mitochondrial swelling) in hepatocytes within 2 layers around the
central vein (Matsumori et al., 1984).
Short-term studies
Mice
Groups of 20 male and 20 female ICR-CRJ SPF mice were maintained
on diets containing 0, 7.2, 22, 72, or 217 ppm paraquat cation for 13
weeks. At the 217 ppm dietary level 2 female mice died from pulmonary
damage. Both males and females in this group showed significantly
reduced body-weight gain and a slight reduction in efficiency of food
utilisation. Food intake and water intake were not affected. No
abnormalities considered related to paraquat treatment were seen
during haematological, blood biochemistry, or urine analysis. A few
statistically-significant changes in absolute and relative organ
weights were seen at termination, mainly in males and females of the
217 ppm group. However, only an increase in lung weight of females in
the 217 ppm group was reported by the authors of the study to coincide
with histopathological changes of the same organ, namely eosinophilic
swelling of the alveolar epithelium walls which was observed in both
sexes at this dietary level. The no-effect level in this study with
respect to pulmonary damage and other parameters was 72 ppm, equal to
12 (males) and 14 (females) mg/kg b.w./day (Malta et al., 1980a).
Rats
Groups of 20 male and 20 female Fischer 344 rats were maintained
on diets containing 0, 7.2, 22, 72, or 217 ppm paraquat cation for 13
weeks. During the study there were no deaths. Body-weight gain was
reduced markedly in both sexes at the 217 ppm dietary level, at which
level food consumption, efficiency of food utilization, and water
consumption were also reduced. Histopathological examination revealed
swelling of alveolar epithelium cells in males and increased deposits
of brown pigment in the spleen of females at the 217 ppm dietary
inclusing level. No abnormalities considered attributable to paraquat
administration were observed during heamatological, blood
biochemistry, urine, organ weight, or gross necropsy investigations.
The no-effect level in this study with respect to lung lesions and
other parameters was 72 ppm, equal to 6.5 (males) and 7.1 (females)
mg/kg b.w./day (Malta et al., 1980b).
Dogs
Groups of beagle dogs, 3 males and 3 females per group, received
diets containing 0, 7, 20, 60, or 120 ppm paraquat cation for 13
weeks. Two males and 2 females in the 120 ppm group showed marked
paraquat toxicity and were killed in extremis between days 16 and
23, having shown marked dyspnoea and body-weight loss. Both surviving
dogs at 120 ppm also showed body-weight loss. A slight overall
reduction in body-weight gain among the females of the other treatment
groups was not considered by the authors of the study to be
treatment-related. Lung weights were increased in all animals in the
120 ppm group and in 2 animals from the 60 ppm group. All other organ
weights were in the normal range. Distinct gross and histological
treatment-related lung lesions were seen in all dogs in the 60 and
120 ppm groups. Minor renal lesions (swelling of the cortical tubules)
were also found histologically in a few of these animals. There were
no discernible gross or histological treatment-related pulmonary
lesions in the dogs of the 7 or 20 ppm groups. The focal pulmonary
lesions in these animals were of a type and incidence similar to those
found in the controls. Microscopic examination of 34 other tissues
from each animal showed no treatment-related changes. There were no
treatment-related effects on food intake except for 1 surviving
high-dose female which showed a loss of appetite from week 8 onward.
There were no distinct treatment-related changes in any of the
haematological (RBC counts, mean cell volume, haemoglobin, total and
differential white cell counts, platelets, prothrombin, and partial
thromboplastin time), biochemical (glucose, cholesterol, blood urea
nitrogen, bilirubin, total and partial protein, GOT, GPT, ALP, and
CPK), or urinary parameters examined. Slight haemoconcentration was
seen in 1 high-dose dog at termination. The no-effect level in this
study after administration of paraquat for 13 weeks to beagle dogs, on
the basis of lung and kidney lesions, was considered to be 20 ppm,
equal to 0.57 mg/kg b.w./day (Sheppard, 1981).
Long-term studies
See also under "Special studies on carcinogenicity".
Mice
Groups of 60 male and 60 female JCL:ICR mice were maintained on
diets containing 0, 1.4, 7.2, 22, or 72 ppm paraquat cation for 104
weeks and then killed and examined. Further groups of 10 males and 10
females receiving the same diets were sacrificed after 26 weeks or 52
weeks of treatment. Animals in each group, including the control
groups, showed approximately 60 - 70% mortality at the end of the
study. Haematological changes, attributed by the authors of the study
to the administration of paraquat, included reduced erythrocytes,
hematocrit, and haemoglobin at the 72 ppm level in both sexes. Total
serum protein was decreased and blood glucose increased at the 72 ppm
dietary level in both sexes. Various rumours, mostly malignant, were
observed in all groups in this study, the main types being lung
adenocarcinoma in males and leukaemia in females. However, no tumour
type showed a higher incidence in the treated groups than in controls
and no correlations between tumour incidence and concentration of the
test substance were observed. No effects attributable to paraquat were
observed in absolute or relative organ weights, urinalysis,
body-weight gain, food consumption, efficiency of food utilization, or
water intake. Based on the haematological and blood biochemistry
changes observed, the no-effect level for paraquat after 104 weeks of
administration to JCL:ICR mice in this study was 22 ppm (as paraquat
cation), equal to 2.8 mg/kg b.w./day (Toyoshima et al., 1982a).
Rats
Groups of 50 male and 50 female JCL:Wistar rats were maintained
on diets containing 0, 4.3, 22, 72, or 217 ppm paraquat cation for 104
weeks and then killed and examined. Further groups of 6 males and 6
females receiving the same diets were sacrificed after 26 or 52 weeks
of treatment. There was 38 - 66% mortality in all groups at the end of
the study. The distribution of mortality and of abnormalities were not
significantly affected by treatment. Females in the 217 ppm group
showed a transient tendency to lower body-weight gain, compared to
controls, at weeks 34, 42 - 48, and 54. Food consumption, efficiency
of food utilization, and water consumption were not affected by
paraquat administration. Haematological changes attributed by the
authors of the study to paraquat administration at the 217 ppm level
included reduced erythrocytes and haemoglobin in both sexes and
decreased haematocrits and increased reticulocytes in males after 26
weeks. Total serum protein was slightly but constantly decreased at
the 217 ppm level in both sexes.
During histopathological examination of approximately 20 tissues,
various rumours were observed in this study in all groups, the main
types being benign pituitary tumours in males and benign mammary
rumours in females. However, none of the tumours were present at a
significantly-higher incidence in the treated groups than in controls.
Body weight, food consumption, food efficiency, water intake,
leucocyte counts, platelet counts, prothrombin time, GOT, GPT,
alkaline phosphatase activity, blood glucose, blood urea nitrogen,
cholesterol, Na+, K+, C1-, creatine, and brain, serum, and
corpuscular cholinesterase activities, as well as ophthamological
examination indicated no significant effects attributable to paraquat
at any dose level. Based on the haematological and blood biochemistry
changes the no-effect level of paraquat after 104 weeks of
administration to JCL:Wister rats in this study was 72 ppm (as
paraquat cation) equal to 3.0 (males) and 3.7 (females) mg/kg b.w./day
(Toyoshima et al., 1982b).
Dogs
Groups of 6 male and 6 female beagle dogs received diets
containing 0, 15, 30, or 50 ppm paraquat cation for 1 year. During the
study there were no deaths. No effects due to paraquat were observed
on body weight. The reduced food consumption of 1 male and 1 female
dog, both in the 50 ppm group, was considered by the authors of the
study to be treatment-related. There was clinical evidence of
respiratory dysfunction (hyperpnoea) in some dogs fed 50 ppm paraquat.
Mean lung weights of male and female dogs fed 50 ppm paraquat were 35
and 60% higher than those of controls, respectively. Histopathological
examination of the lungs showed a statistically-significant increase
in the incidence of chronic pneumonitis in both sexes at the 30 and
50 ppm dietary levels when compared to controls. This lesion consisted
of interstitial fibrosis, alveolar epithelialization, and mononuclear
cell infiltration. No other toxicologically-significant treatment-
related effects were seen during clinical observations, haemotological
or biochemical investigations, or during gross and microscopic
examination of approximately 40 tissues from each animal at
termination. On the basis of the pulmonary changes, the authors of
this study concluded that the dietary no-effect level for paraquat in
dogs over 1 year of treatment was 15 ppm, equal to 0.45 (males) and
0.48 (females) mg/kg b.w./day (Kalinowski et al., 1983).
Observations in humans
Information on the effects of paraquat in humans has been
obtained from occupational exposure studies (epidemiological data and
case reports), descriptions of accidental or suicidal poisonings, and
volunteer studies. These data have been extensively reviewed by WHO
(1984).
In 1965, a study was carried out on a team of 6 sprayers, and in
1967 on 4 teams in Malaysian rubber plantations, to estimate the
efficacy of individual protective measures. The operators used a spray
dilution containing paraquat at 0.5 g/litre for 12 weeks. Attention
was paid to personal hygiene. Each man was given a thorough physical
examination, and urine samples were taken before spraying began and at
weekly intervals throughout the study. Chest X-rays were taken before
the study started and at the end of the 6th and 12th weeks. In the 2
studies, a total of 528 urine samples were examined. Paraquat was
found on 131 occasions (78/134 and 53/394 in the 2 studies,
respectively), the maximum concentration detected being 0.32 mg/litre
in the first study and 0.15 mg/litre in the second. Average urine
levels of paraquat of 0.04 mg/litre were found in the 1965 study and
of 0.006 mg/litre in the 1967 study. After spraying ceased, these
levels declined steadily to become undetectable within a week, with 1
exception. Both trials showed that about half of the men had suffered
mild irritation of the skin and eyes, but had recovered rapidly with
treatment. Two cases of scrotal dermatitis occurred in workers wearing
trousers that were continuously soaked by the spray solution. There
were also 2 cases of epistaxis. All chest radiographs were normal
(Swan, 1969).
Studies performed over a period of several years on 296 Trinidad
sugar estate workers drew attention to nail damage that ranged in
severity from localized discoloration to nail loss following gross
contamination with paraquat at 1 - 2 g/litre. The typical distribution
of the lesions, affecting the index, middle, and ring fingers of the
working hand, suggested that they had occurred through leakage from
the knapsack sprayer and inadequate personal hygiene. Apart from 2
cases of contact dermatitis of the hands, no skin, eye, or nose
irritation was reported, nor were there any systemic effects (Hearn &
Keir, 1971).
Similar data were obtained on several groups of workers spraying
paraquat as an herbicide and dessicant in cotton fields during the hot
season. These workers were exposed to paraquat aerosol concentrations
of 0.13 - 0.55 mg/m3 air. Dermal exposure was low, not more than
0.05 - 0.08 mg paraquat on the hands and face. There were no
complaints, nor did the clinical and laboratory examinations of the
workers demonstrate any significant deviations from the matched
control groups (Makovskii, 1972).
In the USA, the exposure of field workers operating
tractor-mounted spray equipment in orchards was determined. About
4.6 litres of paraquat liquid concentrate (291 g/litre) was used in
935 litres of water per hour. In addition, exposure from yard and
garden applications were studied in volunteers using pressurized hand
dispensers containing paraquat solution (4.4 g/litre). Dermal
contamination was measured by adsorbent cellulose pads attached to the
worker's body or clothing, and by hand-rinsing in water in a
polyethylene bag. Special filter pads were used in the filter
cartridges of the respirators worn by the subjects under study. In
all, 230 dermal and respiratory exposure pads, 95 samples of
hand-rinse water, and 130 urine samples, collected during and
following spraying, were analysed, which involved 35 different
paraquat application situations. The exposure of field workers was
found to range from about 0.40 mg/hour (dermal) to less than
0.001 mg/hour (inhalation). As for individuals spraying yards or
gardens, exposure ranged from 0.29 mg/hour (dermal) to less than
0.001 mg/hour (inhalation). In almost all cases dermal exposure
affected the hands. The respiratory paraquat values were generally
below the sensitivity levels of the analytical method. No detectable
paraquat concentrations were found in the urine samples (lower limit
0.02 mg/litre). This study confirmed the general safety of paraquat
under correct conditions of use (Staiff et al., 1975).
The potential long-term hazard associated with the use of
paraquat has been studied by comparing the health conditions of 27
sprayers who had been exposed to paraquat for many months per year for
an average of 5.3 years with those of 2 unexposed control groups
consisting of 24 general workers and 23 factory workers. The workers
were given full clinical examinations; lung, liver and kidney function
tests were also carried out. There were a few skin lesions resulting
from poor spraying techniques and 1 case of eye injury. There were no
significant differences between exposed and control groups in any
health parameters measured, which led the authors to suggest that the
long-term use of paraquat is not associated with harmful effects on
health (Howard et al., 1981).
To evaluate the effects of protective equipment on occupational
human exposure to paraquat, a paraquat formulation (240 g/litre)
diluted 300 times by volume with water was sprayed for 2 hours on
weedy ground. During the spraying operations, the concentrations of
paraquat aerosol were 11 - 33 µg/m3 air. The total dermal exposure
was about 0.22 mg. No irritation of the eyes or the skin was reported.
The urine of the workers who wore gauze masks contained significant
amounts of paraquat 24 hours after spraying. The urine of the workers
who had worn a high-performance mask did not contain detectable levels
of paraquat. The authors discussed the need for protective equipment
to decrease skin contact with paraquat and to avoid aerosol inhalation
(Kawai & Yoshida, 1981).
Quantitative estimates of dermal and respiratory exposure of 26
plantation workers in Malaysia have shown a mean dermal dose of
1.1 mg/kg b.w./hour. The highest individual total exposure was
equivalent to 2.8 mg/kg b.w./hour; the mean respiratory exposure was
0.24 - 0.97 g/paraquat/m3 air, which is 1% or less of a TLV of
0.1 mg/m3 for respirable paraquat. Urine levels of paraquat were
generally below 0.05 mg/litre (Chester & Woollen, 1982).
A study was carried out on a group of 14 sprayers in Thailand
using conventional high-volume knapsack sprayers and low-volume
spinning-disc applicators with paraquat ion concentrations of
1.5 g/litre and 20 g/litre, respectively. Irritation of unprotected
skin was found, and this was severe (caustic burns on the feet) in
workers using high spray conentrations and spinning-disc applicators.
Urinary paraquat levels ranging from 0.73 - 10.2 mg/litre after 14
days of spraying were detected in unprotected men using both
concentrations, and there was evidence that urinary levels of paraquat
increased as the trial progressed. No evidence of systemic toxicity
was discovered among the sprayers undergoing clinical and radiographic
examination 1 week after spraying ended. The author concluded that
spray concentrations in hand-held equipment should not exceed 5 g
paraquat ion/litre (Howard, 1982).
After tomato spraying in the USA, the total body exposure to
paraquat was determined to be 169 mg/hour. The use of enclosed tractor
cabs or a high-clearance tractor reduced total body exposures to
27 mg/hour or 18 mg/hour, respectively. The authors reported that the
total body exposure of tractor sprayers working in 2 citrus locations
was proportional to the tank concentrations (paraquat dilutions of
0.7 g/litre and 1.1 g/litre were applied). Exposure levels of 13 and
28 mg/hour were found for workers using the lower and high
concentrations, respectively. In all situations studied, the
respiratory exposure was consistently a small fraction (< 0.1%) of
the total body exposure, which was primarily through the skin
(Wojeck et al., 1983).
Two groups of workers exposed to paraquat formulations were
examined. The first group of 18 workers, in England, consisted of
subjects exposed to dust and liquid paraquat formulations during a
37.5-hour working week, the mean length of exposure being 5 years. The
second group also consisted of 18 males, from Malaysia, exposed to
liquid concentrate formulations during a 42-hour working week, the
mean length of exposure being 2.3 years. Partly protective clothing
was worn. However, in Malaysia, no gloves, rubber aprons, or goggles
were used. The medical records and the dermatological examinations
revealed acute skin rashes, nail damage, epistaxis, blepharitis, and
delayed wound healing in 12 - 66% of these workers. Delayed caustic
effects were often found among the Malaysian formulation workers,
where low levels of safety and hygiene were apparent. Clinical
examination did not reveal any evidence of chronic contact dermatitis,
hyperkeratosis, or eczematous lesions (Howard, 1979).
Some studies designed to estimate dermal and inhalation exposure
to paraquat are summarized in Table 7. From the data reported it can
be seen that:
(a) the main route of exposure of agricultural workers to
paraquat is via the skin; respiratory exposure is
negligible.
(b) The worst case of exposure (of those examined) was via
knapsack spraying).
From 1956 - 1973, no deaths attributable to paraquat were
registered among agricultural workers in the USA, but in 1974 4 fatal
cases were associated with this herbicide. However, it is not clear
whether they were accidental, suicidal, or occupational (Hayes &
Vaughan, 1977).
Fitzgerald et al. (1978a) summarized the clinical findings and
pathological details concerning 13 accidents involving paraquat among
agricultural workers, 6 of which were fatal. In 5 of these cases,
swallowing was involved. Of the 6 fatalities studied, 3 swallowed
Gramoxone (a 20% solution of paraquat dichloride in water) after
sucking the outlet of a sprayer. In 1 non-fatal case, the man had
sucked out a nozzle containing diluted paraquat, while in another case
the man who had blown into the jet, to clear it, escaped with only
minor signs of poisoning. The use of a leaking sprayer by another
worker with severe extensive dermatitis probably resulted in fatal
absorption of paraquat through the damaged skin.
Table 7. Comparison of dermal and inhalation exposure to paraquat resulting from
various methods of application1.
Dermal Respiratory
Method of application exposure exposure Reference
(mg/hour) (mg/hour)
Hand-held knapsack 66 (0.45 - 1.3) × 10-3 Chester & Woollen,
(12.1 - 169.8) 1982
Vehicle mounted 0.4 (0 - 2) × 10-3 Staiff et al., 1975
(0.1 - 3.4)
Aerial Chester & Ward, 1981
Flagman 0.1 - 2.4 (0 - 47) × 10-3
Pilot 0.5 - 0.1 (0 - 0.6) × 10-3
Mixer/loader 0.18 (1.3 - 1.5) × 10-3
1 From WHO, 1984
Several other cases of fatal poisoning resulting from dermal
absorption of paraquat have been reported. Jaros (1978) has described
how the use of concentrated solutions of paraquat (50 g/litre instead
of 5 g/litre), with an old leaking knapsack sprayer, resulted in
paraquat contamination of the neck, back, and legs of a worker. After
4 hours of work, the worker complained of a burning sensation on the
neck and scrotum. On admission to a hospital 6 days later, cough and
respiratory difficulties were recorded. Three days later the patient
died of renal and respiratory failure.
Severe skin damage, followed by death due to respiratory
insufficiency, occurred in a woman 8 weeks after initial contact with
paraquat. The toxic dermatitis started with scratches on the arms and
legs from the branches of fruit trees. The patient had often failed to
wear protective clothing or to shower after spraying. During the 4
weeks preceding her first admission to the hospital, she developed
ulcers and respiratory complaints combined with anorexia. Damaged and
broken skin was thus exposed to paraquat. A chest X-ray and needle
biopsy of the lung revealed pulmonary lesions. Seventeen days after
discharge from hospital, without a specific diagnosis, she was
re-admitted, and died 2 weeks later with progressive lung, hepatic,
and renal dysfunction (Newhouse et al., 1978).
The clinical and pathomorphological investigation of a patient
who died of hypoxia after repeated dermal exposure to paraquat
(28 g/litre) and diquat (29 g/litre) in a water-oil dilution has been
described recently. The worker had used a leaking sprayer. A
characteristic ulcer developed at the site of paraquat contact. There
was also lung damage (Levin et al., 1979).
Another reported fatal case of dermal poisoning with paraquat
occurred after prolonged contact with a concentrated formulation
following spillage from a bottle in the back trouser pocket
(Waight & Wheather, 1979).
Wohlfahrt (1982) discussed the factors related to severe paraquat
poisoning due to dermal absorption in tropical agriculture. Three
fatal incidents followed skin contamination; 1 victim used paraquat to
treat scabies infestation, and 1 used it to treat lice. In all cases,
the skin was blistered and ulcerated. The patients died of progressive
respiratory failure 4 - 7 days after the accidents. In 1 of these
cases, the presence of mouth and throat ulceration strongly suggested
that ingestion might also have occurred (Davies, 1982).
Local skin and nail effects of paraquat have been reviewed by WHO
(1984). Brief contact with liquid formulations, as well as repeated
exposure to dilute solutions, produced skin irritation, desquamation,
and, finally, necrosis at the site of contact (Ongom et al., 1974;
Binns, 1976; Newhouse et al., 1978; Waight & Wheather, 1979;
Levin et al., 1979, Horiuchi et al., 1980). Harmful dermal effects
have been reported among sprayers who worked without protective
clothes and with naked feet (Howard, 1982). The blistering and
ulceration of the skin were due to excessive contact and inadequate
hygiene. Horiuchi and Ando (1980) carried out patch testing on 60
patients with contact dermatitis due to Gramoxone. In 8 patients
(13.3%) positive allergic reactions were established. In another
survey with 52 persons, a positive photo-patch response was reported
in 11 patients. Nail damage may also occur after frequent exposure to
paraquat concentrations during the formulation of the herbicide or the
preparation of the working dilution (Howard, 1979).
There have been some reports (Malone et al., 1971; Mircev,
1976; Bismuth et al., 1982) of adverse effects as a result of
inhalation exposure to paraquat. However, inhalation of droplets in
normal paraquat spraying does not appear to represent a significant
health hazard (Howard, 1980), and the effects of occupational
inhalation have usually been limited to nose bleeds and nasal and
throat irritation (Swan, 1969; Howard, 1979).
Ocular damage may result from splashes of concentrated paraquat
that come into contact with the eye. Apart from irritation of the eye
and blepharitis, a week later more serious ocular damage may occur,
such as destruction of the bulbar and tarsal conjunctiva and of the
corneal epithelium. Anterior uveitis, conjunctival necrosis,
progressive keratitis with gross corneal opacity, and decreased visual
acuity may also occur (WHO, 1984).
It has been noted that when the recommended dilution rates were
correctly used, systemic effects of oral, inhalation, or dermal
exposure to paraquat have not been observed. Skin and eye irritation
have occurred only when protective measures were disregarded
(WHO, 1984).
In a volunteer study the percutaneous absorption of
14C-paraquat through the legs, hands, and forearms of 6 human
subjects was studied. The total dose absorbed in 5 days after a single
application of 0.64 mg paraquat dichloride was very low (0.3%) at all
sites of application (Wester et al., 1984).
A large number of cases of accidental or suicidal poisoning have
been reported since 1966, the earlier cases being mostly accidental
which apparently resulted from the habit of decanting the liquid
formulations into small unmarked or incorrectly labelled containers
such as beer, wine, or soft-drink bottles. An increasing ratio of
suicidal to accidental poisonings has been noted in recent years
(Fitzgerald et al., 1978b; Bramley & Hart, 1983). This change from
accidental to suicidal poisoning was considered to be reflected by
enhanced percentages of fatal cases, shorter survival times, and
higher tissue and body fluid levels (WHO, 1984). The vast majority of
cases of paraquat poisoning have been due to ingestion. A few cases of
fatal or non-fatal poisonings have been reported following either skin
contamination (McDonagh & Martin, 1970; Kimura et al., 1980) or skin
application in order to kill body lice (Ongom et al., 1974;
Binns, 1976). Symptoms of poisoning depend on the dose absorbed.
It is difficult to estimate the dose absorbed from case histories
since in many cases the patients spat out part of the paraquat
concentrate or vomited profusely after swallowing the herbicide. Some
patients have survived after apparently ingesting 10 - 20 g paraquat,
whereas some died after taking as little as 2.5 g paraquat. The
probability of survival after paraquat poisoning can be predicted from
plasma paraquat concentration at a given time after ingestion
(Proudfoot et al., 1979; Hart et al., 1984). The minimum lethal
dose of paraquat for human beings has been estimated to be about
35 mg/kg b.w. (Pederson et al., 1981; Bismuth et al., 1982). The
common factor of most, if not all, cases of paraquat poisoning is
damage to the lung. Cases of fatal paraquat poisoning have been
divided into 2 broad categories:
a) Cases of acute fulminant poisoning due to massive amounts of
paraquat absorbed, resulting in death within 1 to 5 days
from ingestion. Death is due to multi-organ failure
associated with damage to the lungs, kidneys, liver, brain,
and adrenals.
b) Cases of poisoning due to ingestion or absorption of smaller
doses of paraquat resulting in death 6 to several days
later. In these cases death is due primarily to lung and
kidney damage.
COMMENTS
A small amount of paraquat is rapidly absorbed by the gut of
rats, guinea pigs, dogs, monkeys, and man, most of the oral dose being
excreted as unchanged paraquat. Following administration by different
routes to animals, paraquat is rapidly distributed in most tissues,
the highest concentrations being found in the lungs and kidneys. The
compound accumulates slowly in the lung owing to uptake by an
energy-dependent process which is also responsible for the uptake of
putrescine. Saturation kinetics for the uptake of paraquat by the lung
have been shown to be similar in rats and man. Excretion of absorbed
paraquat is biphasic, owing to lung accumulation, and occurs largely
in the urine as unchanged paraquat, but also to a limited extent in
the bile. Biotransformation of absorbed paraquat is, in general,
remarkably poor in all species studied (rats, guinea pigs, dogs, hens,
pigs, goats, and sheep) although there is some controversy as to the
possibility and extent of its metabolism by the gut microflora.
Metabolism occurs via demethylation (monomethyl dipyridone ion) or
oxidation (paraquat pyridone ion and paraquat dipyridone ion).
The mechanism of paraquat toxicity has been investigated
extensively, but it has not yet been elucidated completely. The
available evidence indicates that paraquat toxicity is due to the
ability of the compound to undergo redox cycling in biological
systems, resulting in the production of superoxide anion radicals and
in the oxidation of cellular NADPH. These effects may lead to the
formation of other highly toxic oxygen species and to depletion of
important defense mechanisms, both events which are potentially
capable of switching on further pathological processes, resulting in
damage to Type I and Type II pneumocytes.
Two teratogenicity studies, 1 in mice and 1 in rats, showed
paraquat to be non-teratogenic at doses up to 10 mg/kg b.w./day.
Slight maternal toxicity was observed in mice and high maternal
toxicity and some fetal toxicity were observed in rats at 5 and
10 mg/kg b.w./day paraquat ion. In a teratology sub-group in a
3-generation reproduction study in rats, paraquat was not teratogenic,
but delays in ossification were found at all 3 dose levels tested
(72, 145, and 290 ppm paraquat cation).
In previous in vitro studies both positive and negative results
were obtained, with mutagenicity usually associated with high
cytotoxicity. Recent studies have shown paraquat to be non-mutagenic,
both in the presence and absence of metabolic activation, when tested
by the Ames test, mouse micronucleus test, unscheduled DNA synthesis
assay, rec-assay, and host-mediated assay. A mouse lymphoma cell test
was inconclusive. The herbicide was clastogenic to human lymphocytes
in vitro at cytotoxic doses and induced sister chromatid exchange in
Chinese hamster lung fibroblasts.
In 2 rat 3-generation reproduction studies paraquat had no
effects on the reproductive performance or development of the
reproductive organs of rats. In 1 of these studies a reduction of
lactation index was observed at 290 ppm paraquat cation.
The acute oral toxicity of paraquat is higher in guinea pigs,
monkeys, cattle, and man than in rats and birds. Confidence limits of
LD50 values are small. There are no significant differences between
the 2 sexes in the oral, s.c., or i.p. LD50 values of paraquat in
rats or mice. Paraquat induces characteristic dose-related fibrotic
changes in the lungs of mice, rats, dogs, and monkeys, but not in
rabbits, guinea pigs, or hamsters. The acute pulmonary toxicity of
paraquat in rats and man is biphasic. In the early, destructive phase,
the alveolar epithelial cells are extensively damaged. Death may occur
within a few days due to pulmonary oedema. In the later, proliferative
phase, fibroblasts and collagen accumulate in the lungs of surviving
animals and humans, possibly resulting in fibrosis.
In a short-term study in mice, treatment-related lung, body
weight, and organ-weight changes were found. Another short-term study
in rats indicated that paraquat was responsible for lung lesions and
other minor changes. In a short-term study in dogs using 3
animals/sex/group, paraquat-dependent lung and kidney lesions were
observed.
In a 1-year feeding study in dogs, paraquat caused lung changes
at the 2 highest dietary levels; the no-effect level in this study was
15 ppm (as paraquat cation), equal to 0.45 mg/kg b.w./day in males and
0.48 mg/kg b.w./day in females.
In 2 long-term feeding studies, 1 in mice and 1 in rats,
haematological and blood biochemical changes were observed in both
species. These changes have not been reported previously and were
considered to be of little toxicological significance.
A lifetime feeding study in mice showed no oncogenic potential
for paraquat, although a slightly higher incidence of lung tumours was
noted in animals of the high-dose group dying between 79 and 98 weeks
of treatment when compared with control mice. Based on renal lesions
observed in males, the no-effect level in this study was 12.5 ppm
(as paraquat cation), equal to 1.4 mg/kg b.w./day.
A long-term feeding study in F344 rats indicated that paraquat
administration for 104 weeks at 217 ppm was responsible for a
significant increase in the incidence of pulmonary adenomas in
females. This incidence (8.7%) was significantly higher than that
observed in historical control animals of the same laboratory (2.2%).
Paraquat was also cataractogenic in both male and female rats. Based
on the lung and eye changes the no-effect level in this study was
22 ppm (as paraquat cation), equal to 0.77 mg/kg b.w./day.
In another long-term study in F344 rats paraquat induced
proliferative benign lung lesions in females in the high-dose group,
which were considered neoplastic by 1 pathologist and non-neoplastic
by 2 others (the total incidence of pulmonary adenoma in females
ranged from 0/70 to 8/70). The reasons for the discrepancy were
unclear, owing to the lack in most cases of adequately detailed
histopathological descriptions of the lung lesions. The historical
incidence of lung adenoma in control female F344 rats in the
laboratory in which the study was conducted was reported to be 1.1%,
and the incidences reported in the literature are 1.4% for females and
1.9% or 0% for males. The results of these studies indicate a weak,
organ-specific, sex-related potential of paraquat to produce benign
proliferative changes in the lungs of female F344 rats.
The following observations were considered by the Joint Meeting:
a) paraquat was shown to be non-mutagenic in most in vitro
and, apparently, all in vivo tests;
b) the lung is the target organ for both acute and chronic
paraquat toxicity in rats;
c) only female F344 rats, but not male F344 rats, developed
treatment-related benign neoplastic lesions;
d) proliferative changes were observed in rats and not in mice;
and
e) paraquat selectively interferes with the uptake of
polyamines by pneumocytes.
Polyamines are endogenous substrates playing an important role in
cell division and growth. These observations suggest that the
potential of paraquat to induce lung cell proliferation may be
modulated by species-, sex-, and organ-dependent factors, such as
hormonal activity, cell growth, defense and repair mechanisms, etc. No
lung changes were observed at 24 ppm in any of the dose groups, and
significant increases in the incidence of lung adenocarcinomas were
not observed in any of the treated groups when compared to controls in
these studies.
Observations in man, including reports on accidental or suicidal
poisonings, confirm the type of acute and subacute toxicity observed
in some experimental animals. Death occurred after oral and, in some
cases, dermal absorption of high doses of paraquat. The organs
primarily involved are the lung and kidney and, to a lower extent, the
intestinal tract, liver, pancreas, adrenals, and CNS. The minimal
lethal dose of paraquat in man is estimated to be about 35 mg/kg b.w.
Skin, nail, and eye lesions were found in subjects with prolonged
occupational exposure to paraquat. Significant paraquat concentrations
(up to 10 mg/1) were detected in the urine of workers using high spray
concentrations, but not in protected workers. The use of protective
measures has proved to be effective in preventing lesions due to skin
contact.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
Mice: 13 ppm (as paraquat cation), corresponding to 17 ppm
(as paraquat dichloride) in the diet, equal to
1.9 mg/kg b.w./day for males and 38 ppm (as paraquat
cation, corresponding to 52 ppm (as paraquat
dichloride), equal to 5.9 mg/kg b.w./day for females.
Rats: a) 22 ppm (as paraquat cation), corresponding to 30 ppm
(as paraquat dichloride) in the diet, equal to
1.1 mg/kg b.w./day for males and 1.2 mg/kg b.w./day for
females (cataract).
b) 25 ppm (as paraquat cation), corresponding to 35 ppm
(as paraquat dichloride) in the diet, equal to
1.4 mg/kg b.w./day for males and 1.7 mg/kg b.w./day for
females (proliferative lung changes).
Dogs: 15 ppm (as paraquat cation), corresponding to 20 ppm
(as paraquat dichloride) in the diet, equal to
0.62 mg/kg b.w./day for males and 0.66 mg/kg b.w./day
for females.
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.004 mg/kg b.w. as paraquat cation (0 - 0.006 mg/kg b.w.
expressed as paraquat dichloride).
STUDIES WHICH WILL PROVIDE INFORMATION VALUABLE FOR THE CONTINUED
EVALUATION OF THE COMPOUND
1. Further observations in humans.
2. Studies on the mechanism of paraquat-induced proliferative
lung changes.
REFERENCES
Anderson, K.J., Leighty, E.G., & Takahashi, M.T. Evaluation of
1972 herbicides for possible mutagenic protperties.
J. Agric. Food Chem., 20: 649 - 656.
Anderson, D., McGregor, D.B., & Purchase, I.F.H. Dominant lethal
1976 studies with diquat and paraquat in male CD-1 mice.
Mutat. Res., 40: 349 - 358.
Anderson, D. Paraquat: Estimation of mutagenic potential in the
1977 Salmonella typhimurium mutagenicity assay. Unpublished
Report No. CTL/P/243 from ICI Central Toxicology Laboratory.
Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Ashby, R., Hepworth, P.L., Whinney, A.K., Brown, P.M., Whitney, J.C.,
1983 & Finn, J.P. Paraquat: combined toxicity and carcinogenicity
study in rats. Unpublished report No. 82/ILY217/328 from
Life Science Research, Stock, Essex, UK. Submitted to WHO by
Imperial Chemical Industries PLC, Haslemere, UK.
Autor, A.P. Reduction of paraquat toxicity by superoxide dismutase.
1974 Life Sci., 14: 1309 - 1319.
Autor, A. P., ed. Biochemical mechanisms of paraquat toxicity. New
1977 York, Academic Press.
Bainova, A. Experimental assessment of the effect of dipyridylium
1969 herbicides on the skin. Letopisi HEI, 9: 25 - 30
(in Bulgarian).
Bainova, A. Determination of the zones of acute and chronic inhalatory
1971 toxicity after exposure to dipyridylium herbicides Gramoxone
and Reglone. Letopisi HEI, 28: 59 - 62 (in Bulgarian).
Bainova, A. & Vulcheva, V. Experimental substantiation of Gramoxone
1972 MAC in working areas. In: Works of Research Institute of
Hygiene & Labour Protection. Sofia, Medizine i Fizkultuna,
Vol. 23: 71 - 77.
Benigni, R., Bignami, M., Carera, A., Conti, G., Conti, R., Grebelli,
1979 R.A., Dogliotti, E., Gualandi, G., Novelleto, A., & Ortalia,
V.A. Mutational studies with diquat and paraquat in vitro.
Mutat. Res., 68: 183 - 193.
Bennett, P.N., Davies, D.S., & Hawksworth, G.M. In-vivo absorption
1976 studies with paraquat and diquat in the dog. Proceedings of
the British Pharmaceutical Society, 15 - 16 July, England,
284 pp.
Bignami, M. & Grebelli, R.A. A simplified method for the induction of
1979 8-azaguanine resistance in S. typhimurium. Toxicol.
Lett., 3: 169 - 175.
Binns, C.W. A deadly cure for lice. Papua New Guinea Med. J.,
1976 19: 105 107.
Bismuth, C., Garnier, R., Dally, S., Fournier, P.E., & Scherrmann,
1982 J.M. Prognosis and treatment of paraquat poisoning. A review
of 28 cases. J. Toxicol. Clin. Toxicol., 19: 461 - 474.
Bramley, A. & Hart, T.B. Paraquat poisoning in the United Kingdom.
1983 Hum. Toxicol., 2: 417.
Brigelius, R., Hashem, A., & Lengfelder, E. Paraquat-induced
1981 alterations of phospholipids and GSSG release in the
isolated perfused rat liver and the effect of SOD-active
copper complexes. Biochem. Pharmacol., 30: 349 - 354.
Bus, J.S., Aust, S.D., & Gibson, J.E. Superoxide and singlet oxygen
1974 catalysed lipid peroxidation as a possible mechanism for
paraquat toxicity. Biochem. Biophys. Res. Commun.,
58; 749 - 755.
Bus, J.S. & Gibson, J.E. Postnatal toxicity of chronically
1975 administered paraquat in mice and interactions with oxygen
and bromobenzene. Toxicol. Appl. Pharmacol.,
33: 461 - 470.
Bus, J.S., Aust, S.D., & Gibson, J.E. Lipid peroxidation as a possible
1975a mechanism for paraquat toxicity. Res. Commun. Chem.
Pathol. Pharmacol., 11: 31 - 38.
Bus, J.S., Preachie, M.M., Cagen, S.Z., Posner, H.S., Eliason, B.C.,
1975b Sharp, C.W., & Gibson, J.E. Fetal toxicity and distribution
of paraquat and diquat in mice and rats. Toxicol. Appl.
Pharmacol., 33: 450 - 460.
Bus, J.S., Cagen, S.Z., Olgaard, M., & Gibson, J.E. A mechanism of
1976 paraquat toxicity in mice and rats. Toxicol. Appl.
Pharmacol., 35: 501 - 513.
Bus, J.S. & Gibson, J.E. Paraquat: model for oxidant-initiated
1984 toxicity. Environ. Health Perspect., 55: 37 - 46.
Busey, W.M. An independent pathology review of the lung slides from a
1986 rat chronic toxicity/carcinogenicity study with paraquat.
Unpublished report from ICI Plant Protection, Ltd. Submitted
to WHO by Imperial Chemical Industries PLC, Haslemere,
Surrey, UK.
Butler, C. & Kleinerman, J. Paraquat in the rabbit. Br. J. Ind. Med.,
1971 28: 67 -71.
Butler, C. Pulmonary interstitial fibrosis from paraquat in the
1975 hamster. Arch. Pathol., 99: 503 - 507.
Calderbank, A. The bipyridylium herbicides. Effects on man. In
1968 Metcalf, R.L, ed. Advances in Pest Control Research. New
York, Interscience Publishers, Vol. 8; 224 pp.
Charles, J.M., Abou-Donia, M.B., & Menzel, D.B. Absorption of paraquat
1978 from the airways of perfused rat lung. Toxicology,
8: 59 - 67.
Chester, G. & Ward, R.J. Paraquat - occupational exposure and drift
1981 hazard evaluation during aerial application to cotton in
California, USA. Unpublished Report No. CTL/P/581 from ICI
Central Toxicology Laboratory. Submitted to WHO by Imperial
Chemical Industries PLC, Haslemere, Surrey, UK.
Chester, G. & Woollen, B.H. Studies of the occupational exposure of
1982 Malaysian plantation workers to paraquat.
Br. J. Ind. Med., 38: 23 - 33.
Clark, D.G., McElligott, T.F., & Hurst, E.W. The toxicity of paraquat.
1966 Br. J. Ind. Med., 23: 126 - 133.
Clay, P. & Thomas, M. Paraquat dichloride (technical liquor);
1985 assessment of mutagenic potential using L5178Y mouse
lymphoma cells. Unpublished Report No. CTL/P/1398 from ICI
Central Toxicology Laboratory. Submitted to WHO by Imperial
Chemical Industries PLC, Haslemere, Surrey, UK.
Conning, D.M., Fletcher, K., & Swan, A.A.B. Paraquat and related
1969 bipyridyliums. Br. Med. Bull., 25: 245 - 249.
Daniel, J.M. & Gage, J.C. Absorption and excretion of diquat and
1966 paraquat in rats. Br. J. Ind. Med., 23: 133 - 136.
Davies, R.E. Skin absorption of paraquat. Med. J. Aust., 7: 222.
1982
Dunbar, J.R., DeLucia, A.J., & Bryant, L.R. Glutathione status of
1984 isolated rabbit lungs. Effects of nitrofurantoin and
paraquat perfusion with normoxic and hyperoxic ventilation.
Biochem. Pharmacol., 33: 1343 - 1348.
Ecker, J.L., Hook, J.B., & Gibson, J.E. Nephrotoxicity of paraquat in
1975 mice. Toxicol. Appl. Pharmacol., 34: 178 - 186.
Edwards, M.J., Hemingway, R.J., Kinch, D.A., and Slape, P. Paraquat:
1974 residue and toxicology trial with cows fed a treated grass.
Unpublished report No. AR2465A(R) from ICI Plant Protection,
Ltd. Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Fairshter, R.D. Paraquat toxicity and lipid peroxidation. Arch.
1981 Intern. Med., 141: 1121 - 1123.
Fisher, H.K., Clements, J.A., & Wright, R.R. Enhancement of oxygen
1973 toxicity by the herbicide paraquat. Ann. Rev.
Respir. Dis., 107: 246 - 252.
Fisher, H.K., Clements, J.A. Tierney, D.F., & Wright, R.R. Pulmonary
1975 effects of paraquat in the first day after injection.
Am. J. Physiol., 228: 1217 - 1223.
Fitzgerald, G.R., Barniville, G., Flanagan, M., Silke, B., Carmody,
1978a M., & O'Dwyer, W.F. The changing pattern of paraquat
poisoning; an epidemiologic study. J. Irish Med. Assoc.,
71; 103 - 108.
Fitzgerald, G.R., Barniville, G., Black, J., Silke, B., Carmody, M., &
1978b O'Dwyer, W.F. Paraquat poisoning in agricultural workers.
J. Irish Med. Assoc., 71; 149 - 152.
Fletcher, K., Herring, C.M., & Robinson, V.M. Paraquat three-
1972 generation reproduction study in rats. Unpublished report
from ICI Industrial Hygiene Research Laboratories. Submitted
to WHO by Imperial Chemical Industries PLC, Haslemere,
Surrey, UK.
Fodri, Z., Sipos, K., & Berencsi, G. Study of the irritation and
1977 allergic effects of Gramoxone in guinea-pig.
Egeszsegtudomouny, 21: 244 - 249 (in Hungarian).
Fowler, B.A. & Brooks, R.E. Effects of the herbicide paraquat on the
1971 ultrastructure of mouse kidney. Am. J. Pathol.,
63: 505 - 512.
Fridovich, I. & Hassan, H.M. Paraquat and the exacerbation of oxygen
1979 toxicity. Trends Biochem. Sci., 4: 113 - 115.
Gage, J.C. The action of paraquat and diquat on the respiration of
1968 liver cell fractions. Biochem. J., 109: 757 - 761.
Gibson, J.E. & Cagen, S.Z. Paraquat-induced functional changes in
1977 kidney and liver. In: Autor, A.P., ed. Biochemical
Mechanisms of Paraquat Toxicity. New York Academic Press,
pp. 117 - 136.
Giri, S.N., Curry, D.L., Hollinger, M.A., & Freywald, N. Effect of
1979 paraquat on plasma enzymes, insulin, glucose and liver
glycogen in the rat. Environ. Res., 20; 300 - 308.
Giri, S.N. & Krishna, G.A. The effect of paraquat on guanylate cyclase
1980 activity in relation to morphological changes in guinea-pig
lungs. Lung, 157: 127 - 134.
Glass, M., Sutherland, M.W., Forman, H.J., & Fisher, A.B. Selenium
1985 deficiency potentiates paraquat-induced lipid peroxidation
in isolated perfused rat lung. J. Appl. Physiol.,
59: 619 - 622.
Greenberg, D.B., Lyons, A., & Last, J.A. Paraquat-induced changes in
1978 the rat of collagen biosynthesis by rat lung.
J. Lab. Clin. Med., 92: 1033 - 1042.
Hart, T.B., Nevitt, A., & Whitehead, A. A new statistical approach to
1984 the prognostic significance of plasma paraquat
concentrations. Lancet, November 24: 1222 - 1223.
Hassan, H.M. & Fridovich, I. Regulation of synthesis of superoxide
1977 dismutase in Escherichia coli. J. Biol. Chem.,
253: 7667 - 7672.
Hassan, H.M. & Fridovich, I. Superoxide radical and the enhancement of
1978 oxygen toxicity of paraquat in Escherichia coli.
J. Biol. Chem., 253: 8143 - 8148.
Hassan, H.M. & Fridovich, I. Paraquat and Escherichia coli. Mechanism
1979 of production of extracellular superoxide radical.
J. Biol. Chem., 254: 10846 - 10852.
Hassan, H.M. & Fridovich, I. Superoxide dismutases: detoxication by a
1980 free radical. In: Jakoby, W.B., ed. Enzymatic basis of
detoxication. New York, Academic Press, Vol. 1: 311 - 322.
Hawksworth, G.M., Bennett, P.N., & Davies, D.S. Kinetics of paraquat
1981 elimination in the dog. Toxicol. Appl. Pharmacol.,
57: 139 - 145.
Hayes, W.J. & Vaughn, W.K. Mortality from pesticides in the United
1977 States in 1973 and 1974. Toxicol. Appl. Pharmacol.,
42: 235 - 252.
Hearn, C.E.D. & Keir, W. Nail damage in spray operators exposed to
1971 paraquat. Br. J. Ind. Med., 28: 399 - 403.
Hemingway, R.J., Leahey, J.P., Griggs, R.E., & Davis, J.A. Paraquat
1972 metabolism in sheep. Unpublished report No. AR2359A from ICI
Plant Protection Division. Submitted to WHO by Imperial
Chemical Industries PLC, Haslemere, Surrey, UK.
Hemingway, R.J., Leahey, J.P., Griggs, R.E., & Davis, J.A. Paraquat
1974 metabolism in ruminants. 3rd International Congress of
Pesticide Chemistry, Helsinki.
Hemingway, R.J. & Oliver, C. Paraquat: a study of the metabolism and
1974 residues in hens and their eggs. Unpublished report No.
AR251A from ICI Plant Protection Division. Submitted to WHO
by Imperial Chemical Industries PLC, Haslemere, Surrey, UK.
Hendley, P., Leahey, J.P., & Spinks, C.A. Paraquat: Metabolism and
1976a residues in hens. Unpublished report No. AR2676A from ICI
Plant Protection Division. Submitted to WHO by Imperial
Chemical Industries PLC, Haslemere, Surrey, UK.
Hendley, P. Leahey, J.P., Spinks, C.A., Neale, D., & Carpenter, P.K.
1976b Paraquat metabolism and residues in goats. Unpublished
report No. AR2680A from ICI Plant Protection Division.
Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Hodge, M.C.E., Palmer, S, Weight, T.M., & Wilson, J. Paraquat
1978a dichloride: teratogenicity study in the mouse. Unpublished
report No. CTL/P/364 from ICI Central Toxicology Laboratory.
Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Hodge, M.C.E., Palmer, S., Weight, T.M., & Wilson, J. Paraquat
1978b dichloride: teratogenicity study in the rat. Unpublished
report No. CTL/P/365 from ICI Central Toxicology Laboratory.
Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Horiuchi, N. & Ando, S. Contact dermatitis due to pesticides for
1980 agricultural use. Nippon Hifuka Gakkai Zasshi, 90: 289
(in Japanese).
Horiuchi, N., Ando, S., & Kambe, Y. Dermatitis due to pesticides for
1980 agricultural use. Nippon Hifuka Gakkai Zasshi, 90: 27
(in Japanese).
Howard, J.K. A clinical survey of paraquat formulation workers. Br. J.
1979 Ind. Med., 36: 220 - 223.
Howard, J.K. Paraquat: a review of worker exposure in normal usage.
1980 J. Soc. Occup. Med., 30; 6 - 11.
Howard, J.K., Sahopathy, N.N., & Whitehead, P.A. A study of the health
1981 of Malaysian plantation workers with particular reference to
paraquat spraymen. Br. J. Ind. Med., 38: 110 - 116.
Howard, J.K. Paraquat spraying. Comparative risks from high and low
1982 volume application methods. In: Proceedings of the 10th
Asian Conference on Occupational Health, Singapore,
pp. 1 - 7.
Howard, C.A., Wildgoose, J., Clay, P., & Richardson, C.R. Paraquat
1985 dichloride: an in vitro sister chromatid exchange study in
Chinese hamster lung fibroblasts. Unpublished Report
No. CTL/P/1392 from ICI Central Toxicology Laboratory.
Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Howe, D.J.T. & Wright, N. The toxicity of paraquat and diquat. In:
1965 Proceedings of the 18th New Zealand Weed and Pest Control
Conference, pp. 105 - 114.
Hundsdorfer, S. & Rose, I. Lung changes produced by paraquat:
1980 respiratory distress model in animals. Ergeb. Exp. Med.,
35: 603 - 690 (in German).
Hussain, M.Z. & Bhatnagar, R.S. Involvement of superoxide in the
1979 paraquat-induced enhancement of lung collagen synthesis in
organ culture. Biochem. Biophys. Res. Commun.,
89: 71 - 76.
Ilett, K.I., Stripp, B., Menard, R.H., Reid, W.D., & Gillette, J.R.
1974 Studies on the mechanism of the lung toxicity of paraquat.
Comparison of tissue distribution and some biochemical
parameters in rats and rabbits. Toxicol. Appl. Pharmacol,
28: 216 - 226.
Ishmael, J. & Godley, M.J. Paraquat:. lifetime feeding study in rats.
1983 Histopathological examination of lungs. Unpublished Report
No. CTL/P/738 from ICI Central Toxicology Laboratory.
Submitted to WHO by imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Jaros, F. Acute percutaneous paraquat poisoning. Lancet 1: 275.
1978
Kalinowski, A.E., Doe, J.E., Chart, I.S., Gore, C.W., Godley, M.J.,
1983 Hollis, K., Robinson, M., & Woollen, B.H. Paraquat: One year
feeding study in dogs. Unpublished Report No. CTL/P/734 from
ICI Central Toxicology Laboratory. Submitted to WHO by
Imperial Chemical Industries PLC, Haslemere, Surrey, UK.
Kawai, M. & Yoshida, M. Exposure of spray operators to paraquat.
1981 Nippon Doshu Eisei Zasshi, 28: 353 - 359 (in Japanese).
Keeling, P.L., Pratt, I.S., Aldridge, W.N., & Smith, L.L. The
1981 enhancement of paraquat toxicity in rats by 85% oxygen:
lethality and cell-specific lung damage.
Br. J. Exp. Pathol., 62: 643 - 654.
Keeling, P.L., Smith, L.L., & Aldridge, W.N. The formation of mixed
1982 disulfides in rat lung following paraquat administration.
Biochem. Biophys. Acta, 716: 249 - 257.
Kehrer, J.P., Haschek, W.M., & Witschi, H. The influence of hyperoxia
1979 on the acute toxicity of paraquat and diquat. Drug Chem.
Toxicol., 2: 397 - 408.
Kimbrough, R.D. & Gaines, T.B. Toxicity of paraquat in rats and its
1970 effect on rat lungs. Toxicol. Appl. Pharmacol.,
17: 679 - 690.
Kimbrough, R.D. & Linder, E.W. The ultrastructure of the paraquat lung
1973 lesion in the rat. Environ. Res., 6: 265 - 273.
Kimura, M., Suzuki, E., & Ohnishi, H. A case of autopsy of an infant
1980 intoxicated by paraquat dichloride. Shoni Ka Rinsho,
33: 732 - 734 (in Japanese).
Klika, E., Kunc, L., Antalikova, K., & Kuncova, M. The morphology of
1980 the lung in the albino rat after paraquat administration.
Folia Morphol., 28: 188 - 191.
Kornburst, D.J. & Mavis, R.D. The effect of paraquat on microsomal
1980 lipid peroxidation in vitro and in vivo.
Toxicol. Appl. Pharmacol., 53: 323 - 332.
Kurisaki, E. & Sato, H. Toxicological studies on herbicides.
1979 Intracorporeal distribution of paraquat dichloride and
diquat dibromide in rats. Nippon Hoigaki Zasshi,
33: 656 (in Japanese).
Leahey, J.P., Hendley, P., & Spinks, C.A. Paraquat: Metabolism and
1976 residues in pigs using 14C-methyl labelled paraquat.
Unpublished report No. AR2694A from ICI Plant Protection
Division. Submitted to WHO by Imperial Chemical Industries
PLC, Haslemere, Surrey, UK.
Levin, P.J., Klaff, L.J., Rose, A.G., & Ferguson, A.D. Pulmonary
1979 effects of contact exposure to paraquat: a clinical and
experimental study. Thorax, 34: 150 - 160.
Levin, D.E., Hollstein, M., Christman, M.F., Schwiers, E.A., & Ames,
1982 B.N. A new Salmonella tester strain (TA 102) with AT base
pairs at the site of mutation detects oxidative mutagens.
Proc. Natl. Acad. Sci. USA, 79: 7445 - 7449.
Lindsay, S., Banham, P.B., Godley, M.J., Moreland, S., Wickramaratne,
1982 G.A., & Woollen, B.H. Paraquat: multigeneration reproduction
study in rats - three generations. Unpublished Report
No. CTL/P/719 from ICI Central Toxicology Laboratory.
Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Litchfield, M.H., Daniel, J.W., & Longshaw, S. The tissue distribution
1973 of the bipyridylium herbicides diquat and paraquat in rats
and mice. Toxicology, 1; 155 - 165.
Lock, E.A. & Ishmael, J. The acute toxic effects of paraquat and
1979 diquat on the rat kidney. Toxicol. Appl. Pharmacol.,
50: 67 - 76.
Maita, K., Saito, T., Tsuda, S., & Shirasu, Y. AT-5: sub-acute
1980a toxicity study in mouse. Unpublished report from the
Institute of Environmental Toxicology. Submitted to WHO by
Imperial Chemical Industries PLC, Haslemere, Surrey, UK.
Maita, K., Saito, T., Tsuda, S., & Shirasu, Y. AT-5: sub-acute
1980b toxicity study in rat. Unpublished report from the Institute
of Environmental Toxicology. Submitted to WHO by Imperial
Chemical Industries PLC, Haslemere, Surrey, UK.
Maita, K. Historical control incidence data in F344 rats. Unpublished
1986 report from Institute of Environmental Toxicology. Submitted
to WHO by Otsuka Chemical Co. Ltd., Osaka, Japan.
Makovskii, V.N. Toxicological and hygiene studies of bipyridylium
1972 herbicides diquat and paraquat. Referat, Vinnize, USSR
pp 1 - 23 (PhD degree thesis) (in Russian).
Maling, H.M., Saul, W., Williams, M.A., Brown, E.A.B., & Gillette,
1978 J.R. Reduced body clearance as the major mechanism of the
potentiation by ß-adrenergic agonists of paraquat lethality
in rats. Toxicol. Appl. Pharmacol., 43: 57 - 72.
Malone, J.D.G., Carmoday, M., & Keogh, B. Paraquat poisoning. A review
1971 of nineteen cases. J. Irish Med. Assoc., 64: 59 - 68.
Marek, J., Velenska, Z., & Haskovcova, I. Kidney changes in acute
1981 phase of paraquat intoxication in rats. Sb. Lek.,
83: 100 - 104 (in Czech.).
Matsumori, H., Matsumoto, T., & Ishikawa, H. Acute toxic effects of
1984 paraquat on ultrastructure of rat liver. Acta Pathol. Jpn,
34: 507-518.
McDonagh, B.J. & Martin, J. Paraquat poisoning in children. Arch. Dis.
1970 Child., 45: 425 - 427.
McElligot, T.F. The dermal toxicity of paraquat. Differences due to
1972 techniques of application. Toxicol. Appl. Pharmacol.,
21: 361 - 368.
Mircev, N. Acute intoxication with Gramoxone (paraquat). Vatreshni
1976 Bolesti, 16: 99 - 101 (in Bulgarian).
Montgomery, M.R. Interaction of paraquat with the pulmonary microsomal
1976 fatty acid desaturase system. Toxicol. Appl. Pharmacol.,
36: 543 - 554.
Montgomery, M.R. Paraquat toxicity and pulmonary superoxide dismutase;
1977 an enzymic deficiency of lung microsomes. Res. Commun.
Chem. Pathol. Pharmacol., 16: 155 - 158.
Montgomery, M.R. & Niewoehner, D.E. Oxidant-induced alterations in
1979 pulmonary microsomal mixed-function oxidation. Acute effects
of paraquat and ozone. J. Environ. Sci. Health,
13: 205 - 219.
Moody, C.S. & Hassan, H.M. Mutagenicity of oxygen free radicals.
1982 Proc. Natl. Acad. Sci., 79: 2855 - 2859.
Murray, R.E. & Gibson, J.E. A comparative study of paraquat
1972 intoxication in rats, guinea-pigs and monkeys.
Exp. Mol. Pathol., 17: 317 - 325.
Murray, R.E. & Gibson, J.E. Paraquat disposition in rats, guinea-pigs
1974 and monkeys. Toxicol. Appl. Pharmacol., 27: 283 - 291.
Newhouse, M., McEvoy, D., & Rosenthal, D. Percutaneous paraquat
1978 absorption. Arch. Dermatol., 114: 1516 - 1519.
Nicotera, T.M., Block, A.W., Gibas, Z., & Sandberg, A.A. Induction of
1985 superoxide dismutase, chromosomal aberrations and
sister-chromatid exchanges in Chinese hamster fibroblasts.
Mutat. Res., 151: 263 - 268.
Omaye, S.T., Reddy, K.A., & Cross, C.E. Enhanced lung toxicity of
1978 paraquat in selenium-deficient rats. Toxicol. Appl.
Pharmacol., 43: 237 - 247.
Ongom, V.L., Owor, R., & Tonusange, E.T. Paraquat (Gramoxone) used as
1974 a pediculocide. In: Bagshore, A.F., ed. The uses and abuses
of drugs and chemicals in tropical Africa. Nairobi, East
Africa Literature Bureau, pp. 229 - 233.
Parry, J.M. The induction of gene conversion in yeast by herbicide
1973 preparations. Mutat. Res., 21: 83 - 91.
Parry, J.M. The use of yeast for the detection of environmental
1977 mutagens using a fluctuation test. Mutat. Res.,
46: 165 - 176.
Pasi, A., Embree, J.W., Eisenlord, G.H., & Hine, C.H. Assessment of
1974 the mutagenic properties of diquat and paraquat in the
murine dominant lethal test. Mutat. Res., 26: 171 -175.
Pedersen, G., Pedersen, A., Gregersen, M., & Kampe, B. Paraquat
1981 poisoning. Ugeskr. Laeg., 143: 1202 - 1206 (in Danish).
Plant Protection Ltd. Data to support a revised acceptable daily
1972 intake and tolerance levels for paraquat. Unpublished report
from Plant Protection Ltd.
Popenoe, D. Effects of paraquat aerosol on mouse lung. Arch. Pathol.
1979 Lab. Med., 103: 331 - 334.
Proudfoot, A.T., Steward, M.S., Levitt, T., a Widdop, B. Paraquat
1979 poisoning: significance of plasma-paraquat concentrations.
Lancet, 2: 330-332.
Purser, D.A. & Rose, M.S. The toxicity and renal handling of paraquat
1979 in cynomolgus monkeys. Toxicology, 15: 31 - 41.
Reddy, K.A., Litov, R.E., & Omaye, S.T. Effect of pre-treatment with
1977 anti-inflammatory agents on paraquat toxicity in the rat.
Res. Commun. Chem. Pathol. Pharmacol., 17: 87 - 100.
Robertson, B. Paraquat poisoning as an experimental model of the
1973 idiopathic respiratory distress syndrome.
Bull. Phys. Pathol. Res., 9: 1433 - 1452.
Robertson, B., Grossmann, G., & Ivemark, B. The alveolar lining layer
1976 in experimental paraquat poisoning. Acta. Pathol.
Microbiol. Scand., Section A., 84: 40 - 46.
Rose, M.S., Smith, L.L., & Wyatt, I. Evidence of energy-dependent
1974 accumulation of paraquat in rat lung. Nature (Lond.),
252: 314 - 315.
Rose, M.S., Lock, E.A., & Smith, L.L. Biochem. Pharmacol.,
1976a 25: 419 - 423.
Rose, M.S., Smith, L.L., & Wyatt, I. The relevance of pentosephosphate
1976b pathway stimulation in rat lung to the mechanism of paraquat
toxicity. Biochem. Pharmacol., 25: 1763 - 1767.
Rose, M.S. & Smith, L.L. The relevance of paraquat accumulation by
1977 tissues. In: Autor, A.P., ed., Biochemical Mechanisms of
Paraquat Toxicity. New York, Academic Press, pp. 71 - 91.
Ross, W.E., Block, E.R., & Chang, R.Y. Paraquat-induced DNA damage in
1979 mammalian cells. Biochem. Biophys. Res. Commun.,
91: 1302 - 1308.
Rossouw, D.J., Chase, C.C., & Engelbrecht, F.M. The effect of paraquat
1984 on the in vitro activity of cytosol, mitochondrial, and
microsomal enzyme systems. S. Afr. Med. J., 65; 555 - 563.
Selman, M. Montano, M., Montfort, I., & Perez-Tamayo, R. The duration
1985 of the pulmonary paraquat toxicity-enhancement of O2 in
the rat. Exp. Mol. Pathol., 43: 388 - 396.
Selypes, A. & Paldy, A. The examination of the mutagenic effect of two
1978 pesticides: Krezonit E and Gramoxone. Proc. Hung. Ann.
Meet. Biochem., 18: 77- 78.
Sharp, C.W., Ottolenghi, A., & Posner, H.S. Correlation of paraquat
1972 toxicity with tissue concentration and weight loss of the
rat. Toxicol. Appl. Pharmacol., 22: 241 - 251.
Sheldon, T., Howard, C.A., Wildgoose, J., & Richardson, C.R. Paraquat
1985a dichloride: a cytogenetic study in human lymphocytes
in vitro. Unpublished Report No. CTL/P/1357 from ICI
Central Toxicology Laboratory. Submitted to WHO by Imperial
Chemical Industries PLC, Haslemere, Surrey, UK.
Sheldon, T., Richardson, C.R., Shaw, J., & Barber, G. An evaluation of
1985b paraquat dichloride (technical) in the mouse micronucleus
test. Unpublished Report No. CTL/P/1369 from ICI Central
Toxicology Laboratory. Submitted to WHO by Imperial Chemical
Industries PLC, Haslemere, Surrey, UK.
Sheppard, D.B. Paraquat thirteen week (dietary administration)
1981 toxicity study in beagles. Unpublished Report
No. 2481-72/111A from Hazelton Laboratories Europe Ltd.
Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, UK.
Shirasu, Y. & Takahashi, K. Acute toxicity of AT-5 in rat and mouse.
1977 Unpublished report from the Institute of Environmental
Toxicology. Submitted to WHO by Imperial Chemical Industries
PLC, Haslemere, Surrey, UK.
Shirasu, Y., Moriya, M., & Ohta, T. Mutagenicity testing on paraquat
1978 in microbial systems. Unpublished report from the Institute
of Environmental Toxicology. Submitted to WHO by Imperial
Chemical Industries PLC, Haslemere, Surrey, UK.
Shu, H., Talcott, R.E., Rice, S.A., & Wei, E.T. Lipid peroxidation and
1979 paraquat toxicity. Biochem. Pharmacol., 28: 327 - 331.
Sinow, J. & Wei, E. Ocular toxicity of paraquat. Bull. Environ.
1973 Contam. Toxicol., 9: 163 - 168.
Smalley, H.E. Toxicity and hazard of the herbicide paraquat in
1973 turkeys. Poultry Sci., 52: 1625 - 1629.
Smith, P., Heath, D., & Ray, J.M. The pathogenesis and structure of
1973 paraquat-induced pulmonary fibrosis in rats. J. Pathol.,
114: 57 - 67.
Smith, P. & Heath, D. The ultrastructure and time sequence of the
1974 early stages of paraquat lung in rats. J. Pathol.,
114: 177 - 184.
Smith, L.L., Wright, A., Wyatt, I., & Rose, M.S. Effective treatment
1974 for paraquat poisoning in rats and its relevance to
treatment of paraquat poisoning in man. Br. Med. J.,
4: 569 - 571.
Smith, P. & Heath, D. Paraquat. Clin. Rev. Toxicol., 4: 411 - 445.
1976
Smith, L.L., Rose, M.S., & Wyatt, I. The pathology and biochemistry of
1979 paraquat. In: Oxygen Free Radicals and Tissue Damage.
Amsterdam, North Holland, Excerpta Medica, pp. 321 - 341
(CIBA Foundatin Series 65 - new series).
Smith, L.L., Wyatt, I., & Rose, M.S. Factors affecting the efflux of
1981 paraquat from rat lung slices. Toxicology, 19: 197 - 207.
Smith, L.L. Paraquat toxicity. Phil. Trans. R. Soc. Lond. B.,
1985 311: 647 - 657.
Sotheran, M., Banham, P.B., Godley, M.J., Lindsay, S., Pratt, I.,
1981 Taylor, K., & Woollen, B.H. Paraquat; lifetime feeding study
in the mouse. Unpublished Report No. CTL/P/556 from ICI
Central Toxicology Laboratory. Submitted to WHO by Imperial
Chemical Industries PLC, Haslemere, Surrey, UK.
Spinks, C.A., Hendley, P., Leahey, J.P., & Carpenter, P.K. Paraquat:
1976 Metabolism and residues in pigs using 14C-ring labelled
paraquat. Unpublished report No. 2692A from ICI Plant
Protection Division. Submitted to WHO by Imperial Chemical
Industries PLC, Haslemere, Surrey, UK.
Staiff, D.C., Comer, S.W., Armstrong, J.F., & Wolfe, H.R. Exposure to
1975 the herbicide paraquat. Bull. Environ. Contam. Toxicol.,
14: 334 - 340.
Steffen, C. & Netter, K.J. On the mechanism of paraquat action on
1979 microsomal oxygen reduction and its relation to lipid
peroxidation. Toxicol. Appl. Pharmacol., 47: 593 - 602.
Steffen, C., Muliawan, H., & Kappus, H. Lack of in vivo lipid
1980 peroxidation in experimental paraquat poisoning.
Arch. Pharmacol., 310: 241 - 243.
Suzuki, T., Igarashi, N., & Tamura, K. Paraquat D.C.; Multiple
1983 generation study in rats. Unpublished Report No. R-015 from
Bozo Research Centre Co. Ltd. Submitted to WHO by Otsuka
Chemical Co., Osaka, Japan.
Swan, A.A.B. Exposure of spray operators to paraquat.
1969 Br. J. Ind. Med., 26: 665 - 671.
Sykes, B.J., Purchase, I.F.H., & Smith, L.L. Pulmonary ultrastructure
1977 after oral and intravenous dosage of paraquat to rats.
J. Pathol., 121: 233 - 241.
Talcott, R.E., Shu, H., & Wei, E.T. Dissociation of microsomal oxygen
1979 reduction and lipid peroxidation with the electron
acceptors, paraquat and menadione. Biochem. Pharmacol.,
26: 665 - 671.
Thompson, W.D. & Patrick, R.S. Collagen propyl hydroxylase levels in
1978 experimental paraquat poisoning. Br. J. Exp. Pathol.,
59: 288 - 291.
Toyoshima, S., Sato, R., Kashima, M., Sato, H., Sato, H., Sato, H.,
1982a Motoyama, M., Ichiba, J., & Kimura, R. AT-5: chronic
toxicity study result - 104 week dosing study in mouse.
Unpublished report from Nippon Experimental Medical Research
Co. Ltd. Submitted to WHO by Imperial Chemical Industries
PLC, Haslemere, Surrey, UK.
Toyoshima, S., Sato, R., Kashima, M., Sato, H., Sato, H., Sato, H.,
1982b Motoyama, M., Ishidawa, A., Ichiba, J. & Kimura, R. AT-5:
chronic toxicity study result - 104 week dosing study in
rat. Unpublished report from Nippon Experimental Medical
Research Co. Ltd. Submitted to WHO by Imperial Chemical
Industries PLC, Haslemere, Surrey, UK.
Trueman, R.W., Ashby, J., & Burlinson, B. Paraquat dichloride:
1985 assessment for the induction of unscheduled DNA synthesis in
primary rat hepatocyte cultures. Unpublished Report
No. CTL/P/1339 from ICI Central Toxicology Laboratory.
Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Trush, M.A., Mimnaugh, E.G., Ginsburg, E., & Gram, T.E. In vitro
1981 stimulation by paraquat of reactive oxygen-mediated lipid
peroxidation in rat lung microsomes. Toxicol. Appl.
Pharmacol., 60: 279 - 286.
Trush, M.A., Mimnaugh, E.G., Ginsburg, E., & Gram, T.E. Studies on the
1982 in vitro interaction of mitomycin C, nitrofurantoin, and
paraquat with pulmonary microsomes. Simulation of reactive
oxygen-dependent lipid peroxidation. Biochem. Pharmacol.,
31: 3335 - 3346.
Tweats, D.J. The effect of R (drug resistance) factors on the
1975 detection of mutagens by E. coli K 12. Brussels, Belgium,
Annual Euratom Report, Biological Sciences, 316 pp.
Veseley, D.L., Watson, B., & Levey, G.A. Activation of liver guanylate
1979 cyclase by paraquat: possible role of superoxide anion.
J. Pharmacol. Exp. Ther., 209: 162 - 164.
Vijeyaratnam, G.S. & Corrin, B. Experimental paraquat poisoning: A
1971 histological and electron-optical study of the changes in
the lung. J. Pathol., 103: 123 - 129.
Waddell, W.J. & Marlowe, C. Tissue and cellular disposition of
1980 paraquat in mice. Toxicol. Appl. Pharmacol.,
56: 127 - 140.
Waight, J.J.J. & Wheather, R.H. Fatal percutaneous paraquat poisoning.
1979 J. Am. Med. Assoc., 242: 472.
Walker, M., Dugard, P.H., & Scott, R.C. Absorption through human
1983 laboratory animal skins: in vitro comparison.
Acta Pharm. Suec., 20: 207 - 213.
Wester, R.C., Maibach, H.I., Bucks, D.A.W., & Aufrere, M.B. In vivo
1984 percutaneous absorption o[ paraquat from hand, leg, and
forearm of humans. J. Toxicol. Environ. Health,
14: 759 - 762.
WHO. Paraquat and diquat. WHO Environmental Health Criteria, No. 39.
1984 International Programme on Chemical Safety, World Health
Organization, Geneva.
Witschi, H.P., Kacew, S., Hirai, K., & Cote, M.G. In vivo oxidation of
1977 reduced nicotinamide-adenine dinucleotide phosphate by
paraquat and diquat in rat lung. Chem. Biol. Interactions,
19: 143 - 160.
Wohlfart, D.J. Fatal paraquat poisoning after skin absorption. Med. J.
1982 Aust., 1: 512 - 513.
Wojeck, G.A., Price, J.F., Nigg, A.N., & Stamper, J.H. Worker exposure
1983 to paraquat and diquat. Arch. Environ. Contam. Toxicol.,
12: 65 - 70.
Wyatt, I., Doss, A.W., Zavala, D.C., & Smith, L.L. Intrabronchial
1981 instillation of paraquat in rats: lung morphology and
retention study. Br. J. Ind. Med., 38: 42 - 48.
Yasaka, T., Okudaira, K., Fujito, H., Matsumoto, J., Ohya, I., &
1986 Miyamoto, Y. Further studies of lipid peroxidation in human
paraquat poisoning. Arch. Int. Med., 146: 681 - 685.
Yoshida, A., Maita, K., Tsuda, S., Saito, T., & Shirasu, Y. Paraquat
1982 D.C.: chronic toxicity - rats: 24 month study. Unpublished
report from the Institute of Environmental Toxicology,
Japan. Submitted to WHO by Otsuka Chemical Co., Osaka,
Japan.
 EMPIRICAL FORMULA
[C12H14N2]2+
MOLECULAR WEIGHTS
186.2 (ion)
257.2 (dichloride)
PHYSICAL STATE* Colorless crystalline solid.
MELTING POINT Decomposes at about 300°C.
VAPOUR PRESSURE Not measurable.
SOLUBILITY Very soluble in water, slightly soluble in lower
alcohols, insoluble in hydrocarbons.
STABILITY Stable in acid or neutral solutions, unstable in
alkaline solutions. Inactivated by inert clays,
anionic surfactants, and ultraviolet light.
OTHER PROPERTIES Solutions of paraquat become intensely purple on
reduction, due to the formation of a water
soluble, relatively stable free radical, which
absorbs at 400 nm. The unreduced form absorbs at
258 nm. The extinction coefficients of the reduced
and the oxidized paraquat at these absorption
maxima are xi mM400 = 46.0 and xi mM258 =
53.6, respectively (Autor, 1977). Vigorous
reduction gives tetrahydro derivatives and
ultimately the fully saturated base. The redox
potential (-446 mV) is independent of pH.
Concentrated aqueous solutions of paraquat are
corrosive to metal.
FORMULATIONS These include aqueous concentrates (100 - 240 g/l)
and water-soluble granules (24 g/kg) of paraquat
dichloride.
COMBINATIONS These include mixtures of paraquat with diquat
(e.g., Weedol), diuron (e.g., Dexuron),
monolinuron (e.g., Gramonol), and simazine
(e.g., Terraklene).
* All chemical properties are for the dichloride.
ANALYTICAL METHODS These include spectrophotometric, gas chromato-
graphic and radioimmunoassay methods. They
have been extensively reviewed by WHO (1984).
EVALUATION FOR ACCEPTABLE INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution and excretion
The absorption, distribution, and excretion of paraquat in
experimental animals have been reviewed by WHO (1984).
Following oral single-dose administration of 4 - 6 mg/kg b.w.
14C-paraquat dichloride to rats, 99 - 102% of the administered dose
was found in the faeces (93 - 96%) and in the urine (6%) within 3
days. This information, together with the absence of significant
biliary excretion, provided evidence that paraquat is poorly absorbed
from the gut (Daniel & Gage, 1966).
The low rate of paraquat absorption by the gut was confirmed in
experiments in which rats, guinea pigs, and monkeys, orally
administered with LD50 doses of 14C-paraquat, had low peak serum
concentrations (2.1 - 4.8 mg/litre). The radioactivity levels reached
a maximum 30 - 60 minutes after administration and then remained
relatively constant for 32 hours (Litchfield et al., 1973; Conning
et al., 1969).
A dose of 126 mg/kg b.w. paraquat resulted in a maximum rat serum
level of 4.8 mg/litre (Murray & Gibson, 1974).
In fasting dogs, low oral doses of paraquat were rapidly but
incompletely absorbed, the peak plasma concentration being attained 75
minutes after dosing. After an oral dose of 0.12 mg/kg b.w., 46 - 66%
was absorbed in 6 hours. After doses of 2 and 5 mg/kg b.w., only 22 -
38% and 25 - 28% of the doses were absorbed, respectively (Bennett
et al., 1976).
Dose-dependent data from dogs and whole-body autoradiography seem
to suggest that absorption is facilitated in the small intestine
(WHO, 1984).
The pulmonary absorption of 14C-paraquat after an intratracheal
injection of 1.86 nmol/lung was investigated in the isolated perfused
rat lung. The efflux of 14C-paraquat was diphasic, with a
rapid-phase half-life of 2.65 minutes and a slow-phase half-life of
356 minutes. It was suggested that the slow phase represented a
storage pool, possibly responsible for the pulmonary toxicity of
paraquat (Charles et al., 1978).
Various doses of 3H-paraquat (1 pg - 10 µg) in 0.1 ml saline
were introduced directly into the left bronchus of rats. Fifteen
minutes after instilling 10 ng of 3H-paraquat, 90% of the ion could
be accounted for in the tissues and urine, 50% being present in the
lung. With doses at or greater than 10 µg, pathological changes were
seen in the lung that were similar to those seen after systemic
poisoning (Wyatt et al., 1981).
Paraquat absorption through animal and human skin has been
studied using an in vitro technique. Human skin was shown to be
impermeable to paraquat, having a very low permeability constant of
0.73. Furthermore, human skin was found to be at least 40 times less
permeable than that of the animals tested, including rats, rabbits,
and guinea pigs (Walker et al., 1983).
Observations of dose-related dermal toxicity in experimental
animals and human percutaneous poisoning suggest that paraquat
absorption is markedly increased in damaged or occluded skin
(WHO, 1984).
High concentrations and retention of paraquat were found in lung
tissue, relative to other tissues, following oral, i.v., i.p., s.c.,
and intrabronchial routes of administration in rats, guinea pigs,
rabbits, and monkeys (Sharp et al., 1972; Ilett et al., 1974;
Murray & Gibson, 1974; Maling et al., 1978; Kurisaki & Sato, 1979;
Waddell & Marlowe, 1980; Wyatt et al., 1981. Some of these data are
summarized in Tables 1 and 2.
An association between paraquat concentrations in the lung and
degree of toxicity or lung injury has been reported (Sharp et al.,
1972; Ilett et al., 1974; Waddell & Marlowe, 1980; Wyatt et al.,
1981).
In 1 study toxic doses of 14C-paraquat were administered orally
and i.v. to rats. Paraquat concentrations in the whole blood were
similar to those in the plasma. The distribution of the herbicide in
various tissues was then followed for up to 10 days. The initial and
secondary half-lives of paraquat in plasma following i.v.
administration were 23 minutes and 56 hours, respectively. The
concentration in the kidney, lung, and muscle declined at the same
rate as in the plasma initially, but the rapid phase in the lung ended
after 20 minutes (compared with 1 - 4 hours in other organs), after
which it declined, with a half-life of 50 hours. The lung had the
greatest retention and consequently contained the highest
concentration 4 hours after dosing. Four to 10 days after dosing, the
paraquat concentration in the lung was 30 - 80 times higher than in
the plasma (Sharp et al., 1972).
Table 1. Paraquat distribution in tissues*
Route Dose Species Time Tissue Concentration Reference
of after
entry treatment
Intrabronchial 10 ng rat 60 min plasma 0.0092 g/l Wyatt et al.,
lung 5.2 ng 1981
kidney 0.052 ng
liver not measured
heart not measured
brain not measured
I.v. 20 mg/kg rat 24 h plasma 0.07 mg/l Sharp et al.,
lung 6.00 mg/kg 1972
kidney 1.45 mg/kg
liver 0.48 mg/kg
heart 1.20 mg/kg
brain not measured
I.v. 20 mg/kg rat 24 h plasma not measured Ilett et al.,
lung 11.36 mol/kg 1974
kidney 1.93 mol/kg
liver 0.90 mol/kg
heart 1.13 mol/kg
brain 0.87 mol/kg
* From WHO, 1984
Table 1. (cont'd).
Route Dose Species Time Tissue Concentration Reference
of after
entry treatment
I.v. cont'd. 20 mg/kg rabbit 24 h plasma 0.28 mol/l
lung 7.90 mol/kg
kidney 5.25 mol/kg
liver 1.59 mol/kg
heart 1.52 mol/kg
brain 0.49 mol/kg
I.p. 15 mg/kg rat 24 h plasma 0.32 mol/kg Maling et al.,
lung 26.28 mol/kg 1978
kidney 10.40 mol/kg
liver 5.04 mol/kg
heart 4.59 mol/kg
brain 1.22 mol/kg
Oral 126 mg/kg rat 16 h plasma 0.9 mg/l Murray &
lung 5.0 mg/kg Gibson, 1974
kidney 7.0 mg/kg
liver 2.1 mg/kg
heart 2.7 mg/kg
brain not measured
22 mg/kg guinea 16 h plasma 0.03 mg/l
pig lung 1.29 mg/kg
kidney 1.99 mg/kg
liver 0.08 mg/kg
heart 0.31 mg/kg
brain not measured
Table 2. Paraquat distribution in tissues (in mg/kg (mean) tissue)*
Route Dose Species Time Lung Kidney Liver Heart Plasma Reference
of mg/kg after
entry body dosing
weight
Oral 126 rat 1 h 3.3 27.5 2.0 1.8 4.7 Murray & Gibson,
4 h 3.7 4.5 4.4 0.9 0.8 1974
32 h 13.6 9.4 5.7 2.8 1.1
64 h 1.7 1.0 7.7 0.2 0.1
I.v. 20 rat 1 h 9.0 25.0 5.0 - 6.0 Sharp et al.,
4 h 8.0 6.0 2.0 - 0.3 1972
24 h 6.0 1.0 0.4 - 0.07
2 d 4.0 0.8 0.3 - 0.05
* From WHO, 1984
The high lung tissue concentrations of paraquat were confirmed in
another study in rats and rabbits after i.v. injection of 20 mg
14C-paraquat/kg b.w. Although the herbicide showed a selective
localization in the rabbit lung, the concentration decreased far more
rapidly in the rabbit lung than in the rat lung. The rabbit, unlike
the rat, did not show any histological or biochemical signs of lung
damage. No preferential subcellular localization of paraquat was found
in the lungs of either species. No evidence of covalent binding of
paraquat in lung tissue was found. After thorough washing of tissue
precipitate with dilute trichloracetic acid, only insignificant
amounts of 14C-paraquat were detected in protein from the brain,
heart, kidney, liver, lung, and plasma (Ilett et al., 1974).
Autoradiographic studies using 14C-paraquat have been carried
out on mice and rats. Paraquat was observed in nearly all organs 10
minutes after i.v. injection of 20 mg/kg b.w. (Litchfield et al.,
1973).
Autoradiographic results similar to those above were obtained in
mice after i.v. injection of 288 - 338 g/kg b.w. of 3H-paraquat
dichloride. Cellular resolution autoradiography showed that paraquat
was confined almost entirely to cells having the distribution of
alveolar Type II cells. The authors suggested that it was unlikely
that the radioactivity was bound to cellular constituents. The Type II
cells were found to be susceptible to the toxicity of paraquat
(Waddell & Marlowe, 1980; Kimbrough & Gaines, 1970).
No paraquat was detected in the kidney, brain, liver, or lungs
when administered in the diet to rats at a concentration of 50 ppm for
a period of 8 weeks. At 120 ppm it was found at low concentrations in
the lung, kidney, gastrointestinal system, and brain. When
administered at 250 ppm, it was detected in the tissues within 2
weeks. No sex differences or any clear pattern of accumulation were
noted throughout the 8-week study. Within 1 week of return to a normal
diet, no paraquat was detected in any tissue examined. Histological
changes were observed in all lungs of animals fed paraquat at 250 ppm
in the diet (Litchfield, et al., 1973).
Rose et al. (1974) demonstrated an energy-dependent
accumulation of paraquat in slices of rat lung that obeyed saturation
kinetics. The same investigators later examined the ability of
paraquat to accumulate in tissue slices from other organs in vitro.
The uptake of the herbicide in brain, adrenal gland, and kidney slices
was less than 10% of that observed in lung slices. The authors
established the uptake of paraquat by the lung in various species
(rat, rabbit, dog, monkey, and man). The human lung accumulated
paraquat as readily as that of the rat. Indeed, the kinetics (Vmax
and Km) of the process were found to be very similar in the 2
species. Moreover, there was a relationship between the concentration
of paraquat in the different lung areas and the development of
microscopic lung lesions (Rose et al., 1976a; Rose & Smith, 1977).
It has been demonstrated that the rate of paraquat efflux from
lung tissue is less than its rate of accumulation in lung slices.
Efflux from lung slices, prepared from rats dosed i.v. with the
herbicide, was found to be biphasic. There was a fast component
(half-life of 20 minutes), followed by a first-order slow component
characterised by a half-life of 17 hours. The half-life in vitro was
similar to that seen in vivo following i.v. administration to rats
(Smith et al., 1981). These results are partially consistent with
those obtained by Charles et al. (1978) in the isolated perfused rat
lung.
A biphasic elimination of paraquat from the plasma of rats after
i.v. injection has been reported. The initial rapid phase had a 20 -
30 minute half-life, and the slower phase a half-life of 56 hours
(Sharp et al., 1972).
Prolonged paraquat disappearance from serum following a rapid
initial decline was also found after oral administration to rats,
guinea pigs, and monkeys. Both the urinary and faecal routes were
important in all species studied. In rats 32 hours after dosing, 52%
of the administered paraquat was found in the gastrointestinal tract
and 17 and 14% were excreted in the faeces and urine, respectively. No
radioactivity was found in the expired air. The paraquat in the faeces
was due primarily to elimination of unabsorbed paraquat. The prolonged
elimination of paraquat in all animals tested indicated retention of
the herbicide in the body (Murray & Gibson, 1974).
Following i.v. administration of paraquat to rats, 75 - 79% of
the dose was excreted in the urine within 6 hours. In this study, the
plasma disappearance of 5 mg/kg paraquat was fitted to a 3-compartment
model. Total body clearance was estimated to be 8.39 ± 0.54 ml/kg
/minute. The relatively high concentration of paraquat found in the
duodenal and jejunal walls suggested biliary secretion of the
herbicide. The authors' hypothesis was later supported by the
observation of radioactivity in the intestines of mice injected i.v.
with 14C-paraquat in whole-body autoradiographic studies (Maling
et al., 1978; Waddell & Marlowe, 1980).
The dog was used as a model to evaluate the influence of
paraquat-induced renal failure on the kinetics of paraquat
elimination. After i.v. injection of a trace dose of 14C-paraquat
(30 - 50 g/kg b.w.), the kinetics of distribution was described by a
3-compartment model. To obtain a good fit of the curve, it was
necessary to sample the central (plasma) compartment for at least
24 hours after dosing. Simulation of paraquat levels in the peripheral
compartments suggested the existence of a compartment with rapid
uptake and removal (kidney) and another with slow uptake (lung). The
renal clearance of paraquat approximated total body clearance,
indicating that paraquat elimination occurs through renal excretion.
The urinary excretion rate of an i.v. dose was rapid, approximately 80
- 90% of the dose being eliminated during the first 6 hours.
Intravenous injection of a large toxic dose of paraquat (20 mg/kg
b.w.), however, brought about a marked decrease in renal clearance,
from 73 ml/minute to 18 ml/minute after 2.5 hours and 2 ml/minute
after 6 hours. These data suggest that kidney damage could contribute
to paraquat accumulation in the lung (Hawksworth et al., 1981).
Metabolism
Rats, dogs, and guinea pigs
After oral administration of 14C-paraquat to rats, dogs, and
guinea pigs, most of the radioactivity was excreted in 4 days, mainly
in the faeces as unchanged paraquat. The remaining label was present
in urine, which contained 12% (rats), 45% (dogs), and 9% (guinea pigs)
of the dose administered. Paraquat was the main radioactive component
of rat and dog urine, with monquat and the dipyridone of paraquat
accounting for 0.4%, 0.3%, and 0.1% of the administered dose in rat
urine, and 0.4%, 0.5%, and 0% of the dose in dog urine. After s.c.
administration of 14C-paraquat to rats, over 90% of the administered
radioactivity was excreted in the urine in 4 days. While the excretion
produce was mainly paraquat, chromatography indicated that monoquat
(1.9%), paraquat monopyridone (3.2%), and paraquat dipyridone (1.1%)
were also present. Although traces of monoquat and paraquat
monopyridone were also found in rat faeces, there was no evidence of
extensive metabolism of paraquat by the gut microflora. Intestinal
bacteria from rat caecal contents did not degrade paraquat in vitro
to any measurable extent (Annex 1, FAO/WHO, 1977b).
These conclusions were in contrast with the results of other
studies previously evaluated which indicated that when paraquat
(50 mg/kg b.w. of 14C-labelled dichloride salt) was given to rats,
25% of the radioactivity excreted in the faeces could be attributed to
products of metabolism by gut microflora. Examination of extracts
indicated the presence of only 1 metabolite in addition to paraquat.
Thirty percent of the paraquat was broken down when incubated
anaerobically with rat caecal contents; the metabolites were not
identified. Urine from rats injected i.p. with 14C-methyl-labelled
paraquat contained 87% of the administered radioactivity in 24 hours,
which was entirely unchanged paraquat (Plant Protection Ltd, 1972).
Hens
When a single oral dose of 14C-methyl-labelled paraquat was
administered to hens, all of the dose was recovered quantitatively in
the faeces within 3 days. At least 98% of the recovered radioactivity
was unchanged paraquat. Analysis of the tissues of hens after about 3
weeks of dosing with 14C-paraquat (6 ppm in the total diet)
indicated that it did not accumulate in the hens (Hemingway & Oliver,
1974).
Continuous dosing of hens with radiolabelled paraquat for up to
22 days, at rates up to 30 ppm in the diet, resulted in total
radioactive residues in the eggs of up to approximately 0.05 mg/kg
paraquat ion equivalent. At least 80% of the radioactivity was due to
unchanged paraquat. The residue was almost entirely in the yolk rather
than in the albumin (Hemingway & Oliver, 1974; Hendley et al.,
1976a).
Pigs
Pigs excreted an oral dose of paraquat principally in the faeces
as unchanged paraquat. Two pigs were dosed with 14C-labelled
paraquat for 7 consecutive days at a rate equivalent to 50 ppm in
the diet. One was dosed with 14C-methyl- and the second with
14C-ring-labelled paraquat. The pigs were sacrificed 2 hours after
receiving the final dose. By this time 69 - 73% of the administered
residue had been recovered in the faeces and approximately 3% had been
recovered in the urine. More than 90% of the radioactivity in the
faeces was present as unchanged paraquat. Total radioactive residues
in the tissues were low. More than 90% of these residues were due to
unchanged paraquat, except in liver, where approximately 70% was due
to unchanged paraquat and 4 - 7% was due to monoquat ion (Leahey
et al., 1976; Spinks et al., 1976).
Goats
14C-ring-labelled paraquat was administered to a goat in
mid-lactation twice daily for 7 days at a dose equivalent to 100 ppm
in the diet. Total radioactive residues in the milk were less than
0.01 mg/kg paraquat ion equivalent; 76% was unchanged paraquat. Total
radioactive residues were 0.74, 0.56, and 0.1 mg/kg in kidney, liver,
and muscle, respectively. There was no significant metabolism of
paraquat, except in the liver, where 50% of the residue was paraquat
and about 5% was each of the metabolites monoquat ion and monopyridone
ion (Hendley et al., 1976b).
Sheep
A dose of 14C-methyl-labelled paraquat administered to a sheep
via a rumen fistula was recovered quantitatively within 10 days.
Approximately 4% of the dose was excreted in the urine and the
remainder in the faeces. More than 95% of the radioactivity in urine
and faeces was present as unchanged paraquat. Small amounts of
monoquat ion (1%) and monopyridone ion (2.3%) were also detected
(Hemingway et al., 1972).
When injected s.c., paraquat was also excreted rapidly in the
urine (over 80% of the dose), 69% within the first day after
treatment. Unchanged paraquat accounted for most (90%) of the
radioactivity; the monopyridone derivative was present as 2 - 3% of
the dose and monoquat was a trace metabolite. This pattern of
metabolism was virtually identical to that seen in the urine following
dosing via the rumen (Hemingway et al., 1972).
Cows
When cows were given single oral doses of 14C-methyl paraquat
at 8 mg/kg, 96% of the radioactivity was recovered in the faeces
during the following 9 days; 0.7% was recovered in the urine.
Unchanged paraquat accounted for most of the radioactivity in the
faeces (96%) and urine (62 - 90%), but traces of the monoquat ion and
monopyridone ion were also detected in the urine. Only 0.003 - 0.004%
of the radioactivity was recovered in milk; the maximum radioactive
residue (0.005 mg/kg, paraquat ion equivalent) was observed on the day
after dosing. About 15% of this radioactivity was present as unchanged
paraquat. Monoquat ion and monopyridone ion (3 - 25%) were also found
in the milk. The radioactivity not identified as paraquat, monoquat,
or monopyridone was incorporated into natural constituents of milk
resulting from the anabolism of the radioactive methyl group cleaved
from paraquat (Hemingway et al., 1974).
Cows were fed for 3 months diets containing 24, 80, or 170 ppm
paraquat ion (equivalent to 0.8, 2.5, or 5.5 mg/kg b.w./day). The
paraquat was present as a residue in dried grass obtained from a
pasture that had been sprayed with Gramoxone and subsequently
weathered. The diet was accepted satisfactorily and no toxicological
effects were observed during the trial. Pathological examination of
tissues from animals slaughtered within 24 hours of the end of the
feeding trial showed no toxic effects attributable to paraquat. The
tissue residues, including muscle and liver, determined in cows at the
2 higher dose rates, varied between 0.01 and 0.09 mg/kg except in the
kidney, where 0.21 - 0.31 mg/kg was found. These fell to low
(0.04 mg/kg in the kidney) or non-detectable levels in an animal fed
the high-paraquat diet for 30 days and then maintained on an untreated
diet for 12 days before slaughter. Very low residues of paraquat were
present in milk samples taken weekly during the trial (121 samples
ranging from 0.0001 - 0.0006 mg/kg; 1 sample = 0.001 mg/kg)
(Edwards et al., 1974).
Effects on enzymes and other biochemical parameters
Several reviews or monographs have summarised the biochemical
mechanism of paraquat toxicity in plants (Calderbank, 1968), bacteria
(Fridovich & Hassan, 1979), and animals (Bus et al., 1976; Autor,
1977; Smith et al., 1979; Smith, 1985). The mechanism of the toxic
action of paraquat has also been extensively reviewed by WHO (1984).
Paraquat has long been known to participate in cyclic
reduction-oxidation reactions in biological systems. The compound
readily undergoes a single electron reduction in tissues, forming a
free radical. In an aerobic environment, the free radical is
immediately oxidised by molecular oxygen, generating the superoxide
anion radical. The reoxidized paraquat is capable of accepting another
electron and continuing the electron transfer reactions in a catalytic
manner (Figure 1).
EMPIRICAL FORMULA
[C12H14N2]2+
MOLECULAR WEIGHTS
186.2 (ion)
257.2 (dichloride)
PHYSICAL STATE* Colorless crystalline solid.
MELTING POINT Decomposes at about 300°C.
VAPOUR PRESSURE Not measurable.
SOLUBILITY Very soluble in water, slightly soluble in lower
alcohols, insoluble in hydrocarbons.
STABILITY Stable in acid or neutral solutions, unstable in
alkaline solutions. Inactivated by inert clays,
anionic surfactants, and ultraviolet light.
OTHER PROPERTIES Solutions of paraquat become intensely purple on
reduction, due to the formation of a water
soluble, relatively stable free radical, which
absorbs at 400 nm. The unreduced form absorbs at
258 nm. The extinction coefficients of the reduced
and the oxidized paraquat at these absorption
maxima are xi mM400 = 46.0 and xi mM258 =
53.6, respectively (Autor, 1977). Vigorous
reduction gives tetrahydro derivatives and
ultimately the fully saturated base. The redox
potential (-446 mV) is independent of pH.
Concentrated aqueous solutions of paraquat are
corrosive to metal.
FORMULATIONS These include aqueous concentrates (100 - 240 g/l)
and water-soluble granules (24 g/kg) of paraquat
dichloride.
COMBINATIONS These include mixtures of paraquat with diquat
(e.g., Weedol), diuron (e.g., Dexuron),
monolinuron (e.g., Gramonol), and simazine
(e.g., Terraklene).
* All chemical properties are for the dichloride.
ANALYTICAL METHODS These include spectrophotometric, gas chromato-
graphic and radioimmunoassay methods. They
have been extensively reviewed by WHO (1984).
EVALUATION FOR ACCEPTABLE INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution and excretion
The absorption, distribution, and excretion of paraquat in
experimental animals have been reviewed by WHO (1984).
Following oral single-dose administration of 4 - 6 mg/kg b.w.
14C-paraquat dichloride to rats, 99 - 102% of the administered dose
was found in the faeces (93 - 96%) and in the urine (6%) within 3
days. This information, together with the absence of significant
biliary excretion, provided evidence that paraquat is poorly absorbed
from the gut (Daniel & Gage, 1966).
The low rate of paraquat absorption by the gut was confirmed in
experiments in which rats, guinea pigs, and monkeys, orally
administered with LD50 doses of 14C-paraquat, had low peak serum
concentrations (2.1 - 4.8 mg/litre). The radioactivity levels reached
a maximum 30 - 60 minutes after administration and then remained
relatively constant for 32 hours (Litchfield et al., 1973; Conning
et al., 1969).
A dose of 126 mg/kg b.w. paraquat resulted in a maximum rat serum
level of 4.8 mg/litre (Murray & Gibson, 1974).
In fasting dogs, low oral doses of paraquat were rapidly but
incompletely absorbed, the peak plasma concentration being attained 75
minutes after dosing. After an oral dose of 0.12 mg/kg b.w., 46 - 66%
was absorbed in 6 hours. After doses of 2 and 5 mg/kg b.w., only 22 -
38% and 25 - 28% of the doses were absorbed, respectively (Bennett
et al., 1976).
Dose-dependent data from dogs and whole-body autoradiography seem
to suggest that absorption is facilitated in the small intestine
(WHO, 1984).
The pulmonary absorption of 14C-paraquat after an intratracheal
injection of 1.86 nmol/lung was investigated in the isolated perfused
rat lung. The efflux of 14C-paraquat was diphasic, with a
rapid-phase half-life of 2.65 minutes and a slow-phase half-life of
356 minutes. It was suggested that the slow phase represented a
storage pool, possibly responsible for the pulmonary toxicity of
paraquat (Charles et al., 1978).
Various doses of 3H-paraquat (1 pg - 10 µg) in 0.1 ml saline
were introduced directly into the left bronchus of rats. Fifteen
minutes after instilling 10 ng of 3H-paraquat, 90% of the ion could
be accounted for in the tissues and urine, 50% being present in the
lung. With doses at or greater than 10 µg, pathological changes were
seen in the lung that were similar to those seen after systemic
poisoning (Wyatt et al., 1981).
Paraquat absorption through animal and human skin has been
studied using an in vitro technique. Human skin was shown to be
impermeable to paraquat, having a very low permeability constant of
0.73. Furthermore, human skin was found to be at least 40 times less
permeable than that of the animals tested, including rats, rabbits,
and guinea pigs (Walker et al., 1983).
Observations of dose-related dermal toxicity in experimental
animals and human percutaneous poisoning suggest that paraquat
absorption is markedly increased in damaged or occluded skin
(WHO, 1984).
High concentrations and retention of paraquat were found in lung
tissue, relative to other tissues, following oral, i.v., i.p., s.c.,
and intrabronchial routes of administration in rats, guinea pigs,
rabbits, and monkeys (Sharp et al., 1972; Ilett et al., 1974;
Murray & Gibson, 1974; Maling et al., 1978; Kurisaki & Sato, 1979;
Waddell & Marlowe, 1980; Wyatt et al., 1981. Some of these data are
summarized in Tables 1 and 2.
An association between paraquat concentrations in the lung and
degree of toxicity or lung injury has been reported (Sharp et al.,
1972; Ilett et al., 1974; Waddell & Marlowe, 1980; Wyatt et al.,
1981).
In 1 study toxic doses of 14C-paraquat were administered orally
and i.v. to rats. Paraquat concentrations in the whole blood were
similar to those in the plasma. The distribution of the herbicide in
various tissues was then followed for up to 10 days. The initial and
secondary half-lives of paraquat in plasma following i.v.
administration were 23 minutes and 56 hours, respectively. The
concentration in the kidney, lung, and muscle declined at the same
rate as in the plasma initially, but the rapid phase in the lung ended
after 20 minutes (compared with 1 - 4 hours in other organs), after
which it declined, with a half-life of 50 hours. The lung had the
greatest retention and consequently contained the highest
concentration 4 hours after dosing. Four to 10 days after dosing, the
paraquat concentration in the lung was 30 - 80 times higher than in
the plasma (Sharp et al., 1972).
Table 1. Paraquat distribution in tissues*
Route Dose Species Time Tissue Concentration Reference
of after
entry treatment
Intrabronchial 10 ng rat 60 min plasma 0.0092 g/l Wyatt et al.,
lung 5.2 ng 1981
kidney 0.052 ng
liver not measured
heart not measured
brain not measured
I.v. 20 mg/kg rat 24 h plasma 0.07 mg/l Sharp et al.,
lung 6.00 mg/kg 1972
kidney 1.45 mg/kg
liver 0.48 mg/kg
heart 1.20 mg/kg
brain not measured
I.v. 20 mg/kg rat 24 h plasma not measured Ilett et al.,
lung 11.36 mol/kg 1974
kidney 1.93 mol/kg
liver 0.90 mol/kg
heart 1.13 mol/kg
brain 0.87 mol/kg
* From WHO, 1984
Table 1. (cont'd).
Route Dose Species Time Tissue Concentration Reference
of after
entry treatment
I.v. cont'd. 20 mg/kg rabbit 24 h plasma 0.28 mol/l
lung 7.90 mol/kg
kidney 5.25 mol/kg
liver 1.59 mol/kg
heart 1.52 mol/kg
brain 0.49 mol/kg
I.p. 15 mg/kg rat 24 h plasma 0.32 mol/kg Maling et al.,
lung 26.28 mol/kg 1978
kidney 10.40 mol/kg
liver 5.04 mol/kg
heart 4.59 mol/kg
brain 1.22 mol/kg
Oral 126 mg/kg rat 16 h plasma 0.9 mg/l Murray &
lung 5.0 mg/kg Gibson, 1974
kidney 7.0 mg/kg
liver 2.1 mg/kg
heart 2.7 mg/kg
brain not measured
22 mg/kg guinea 16 h plasma 0.03 mg/l
pig lung 1.29 mg/kg
kidney 1.99 mg/kg
liver 0.08 mg/kg
heart 0.31 mg/kg
brain not measured
Table 2. Paraquat distribution in tissues (in mg/kg (mean) tissue)*
Route Dose Species Time Lung Kidney Liver Heart Plasma Reference
of mg/kg after
entry body dosing
weight
Oral 126 rat 1 h 3.3 27.5 2.0 1.8 4.7 Murray & Gibson,
4 h 3.7 4.5 4.4 0.9 0.8 1974
32 h 13.6 9.4 5.7 2.8 1.1
64 h 1.7 1.0 7.7 0.2 0.1
I.v. 20 rat 1 h 9.0 25.0 5.0 - 6.0 Sharp et al.,
4 h 8.0 6.0 2.0 - 0.3 1972
24 h 6.0 1.0 0.4 - 0.07
2 d 4.0 0.8 0.3 - 0.05
* From WHO, 1984
The high lung tissue concentrations of paraquat were confirmed in
another study in rats and rabbits after i.v. injection of 20 mg
14C-paraquat/kg b.w. Although the herbicide showed a selective
localization in the rabbit lung, the concentration decreased far more
rapidly in the rabbit lung than in the rat lung. The rabbit, unlike
the rat, did not show any histological or biochemical signs of lung
damage. No preferential subcellular localization of paraquat was found
in the lungs of either species. No evidence of covalent binding of
paraquat in lung tissue was found. After thorough washing of tissue
precipitate with dilute trichloracetic acid, only insignificant
amounts of 14C-paraquat were detected in protein from the brain,
heart, kidney, liver, lung, and plasma (Ilett et al., 1974).
Autoradiographic studies using 14C-paraquat have been carried
out on mice and rats. Paraquat was observed in nearly all organs 10
minutes after i.v. injection of 20 mg/kg b.w. (Litchfield et al.,
1973).
Autoradiographic results similar to those above were obtained in
mice after i.v. injection of 288 - 338 g/kg b.w. of 3H-paraquat
dichloride. Cellular resolution autoradiography showed that paraquat
was confined almost entirely to cells having the distribution of
alveolar Type II cells. The authors suggested that it was unlikely
that the radioactivity was bound to cellular constituents. The Type II
cells were found to be susceptible to the toxicity of paraquat
(Waddell & Marlowe, 1980; Kimbrough & Gaines, 1970).
No paraquat was detected in the kidney, brain, liver, or lungs
when administered in the diet to rats at a concentration of 50 ppm for
a period of 8 weeks. At 120 ppm it was found at low concentrations in
the lung, kidney, gastrointestinal system, and brain. When
administered at 250 ppm, it was detected in the tissues within 2
weeks. No sex differences or any clear pattern of accumulation were
noted throughout the 8-week study. Within 1 week of return to a normal
diet, no paraquat was detected in any tissue examined. Histological
changes were observed in all lungs of animals fed paraquat at 250 ppm
in the diet (Litchfield, et al., 1973).
Rose et al. (1974) demonstrated an energy-dependent
accumulation of paraquat in slices of rat lung that obeyed saturation
kinetics. The same investigators later examined the ability of
paraquat to accumulate in tissue slices from other organs in vitro.
The uptake of the herbicide in brain, adrenal gland, and kidney slices
was less than 10% of that observed in lung slices. The authors
established the uptake of paraquat by the lung in various species
(rat, rabbit, dog, monkey, and man). The human lung accumulated
paraquat as readily as that of the rat. Indeed, the kinetics (Vmax
and Km) of the process were found to be very similar in the 2
species. Moreover, there was a relationship between the concentration
of paraquat in the different lung areas and the development of
microscopic lung lesions (Rose et al., 1976a; Rose & Smith, 1977).
It has been demonstrated that the rate of paraquat efflux from
lung tissue is less than its rate of accumulation in lung slices.
Efflux from lung slices, prepared from rats dosed i.v. with the
herbicide, was found to be biphasic. There was a fast component
(half-life of 20 minutes), followed by a first-order slow component
characterised by a half-life of 17 hours. The half-life in vitro was
similar to that seen in vivo following i.v. administration to rats
(Smith et al., 1981). These results are partially consistent with
those obtained by Charles et al. (1978) in the isolated perfused rat
lung.
A biphasic elimination of paraquat from the plasma of rats after
i.v. injection has been reported. The initial rapid phase had a 20 -
30 minute half-life, and the slower phase a half-life of 56 hours
(Sharp et al., 1972).
Prolonged paraquat disappearance from serum following a rapid
initial decline was also found after oral administration to rats,
guinea pigs, and monkeys. Both the urinary and faecal routes were
important in all species studied. In rats 32 hours after dosing, 52%
of the administered paraquat was found in the gastrointestinal tract
and 17 and 14% were excreted in the faeces and urine, respectively. No
radioactivity was found in the expired air. The paraquat in the faeces
was due primarily to elimination of unabsorbed paraquat. The prolonged
elimination of paraquat in all animals tested indicated retention of
the herbicide in the body (Murray & Gibson, 1974).
Following i.v. administration of paraquat to rats, 75 - 79% of
the dose was excreted in the urine within 6 hours. In this study, the
plasma disappearance of 5 mg/kg paraquat was fitted to a 3-compartment
model. Total body clearance was estimated to be 8.39 ± 0.54 ml/kg
/minute. The relatively high concentration of paraquat found in the
duodenal and jejunal walls suggested biliary secretion of the
herbicide. The authors' hypothesis was later supported by the
observation of radioactivity in the intestines of mice injected i.v.
with 14C-paraquat in whole-body autoradiographic studies (Maling
et al., 1978; Waddell & Marlowe, 1980).
The dog was used as a model to evaluate the influence of
paraquat-induced renal failure on the kinetics of paraquat
elimination. After i.v. injection of a trace dose of 14C-paraquat
(30 - 50 g/kg b.w.), the kinetics of distribution was described by a
3-compartment model. To obtain a good fit of the curve, it was
necessary to sample the central (plasma) compartment for at least
24 hours after dosing. Simulation of paraquat levels in the peripheral
compartments suggested the existence of a compartment with rapid
uptake and removal (kidney) and another with slow uptake (lung). The
renal clearance of paraquat approximated total body clearance,
indicating that paraquat elimination occurs through renal excretion.
The urinary excretion rate of an i.v. dose was rapid, approximately 80
- 90% of the dose being eliminated during the first 6 hours.
Intravenous injection of a large toxic dose of paraquat (20 mg/kg
b.w.), however, brought about a marked decrease in renal clearance,
from 73 ml/minute to 18 ml/minute after 2.5 hours and 2 ml/minute
after 6 hours. These data suggest that kidney damage could contribute
to paraquat accumulation in the lung (Hawksworth et al., 1981).
Metabolism
Rats, dogs, and guinea pigs
After oral administration of 14C-paraquat to rats, dogs, and
guinea pigs, most of the radioactivity was excreted in 4 days, mainly
in the faeces as unchanged paraquat. The remaining label was present
in urine, which contained 12% (rats), 45% (dogs), and 9% (guinea pigs)
of the dose administered. Paraquat was the main radioactive component
of rat and dog urine, with monquat and the dipyridone of paraquat
accounting for 0.4%, 0.3%, and 0.1% of the administered dose in rat
urine, and 0.4%, 0.5%, and 0% of the dose in dog urine. After s.c.
administration of 14C-paraquat to rats, over 90% of the administered
radioactivity was excreted in the urine in 4 days. While the excretion
produce was mainly paraquat, chromatography indicated that monoquat
(1.9%), paraquat monopyridone (3.2%), and paraquat dipyridone (1.1%)
were also present. Although traces of monoquat and paraquat
monopyridone were also found in rat faeces, there was no evidence of
extensive metabolism of paraquat by the gut microflora. Intestinal
bacteria from rat caecal contents did not degrade paraquat in vitro
to any measurable extent (Annex 1, FAO/WHO, 1977b).
These conclusions were in contrast with the results of other
studies previously evaluated which indicated that when paraquat
(50 mg/kg b.w. of 14C-labelled dichloride salt) was given to rats,
25% of the radioactivity excreted in the faeces could be attributed to
products of metabolism by gut microflora. Examination of extracts
indicated the presence of only 1 metabolite in addition to paraquat.
Thirty percent of the paraquat was broken down when incubated
anaerobically with rat caecal contents; the metabolites were not
identified. Urine from rats injected i.p. with 14C-methyl-labelled
paraquat contained 87% of the administered radioactivity in 24 hours,
which was entirely unchanged paraquat (Plant Protection Ltd, 1972).
Hens
When a single oral dose of 14C-methyl-labelled paraquat was
administered to hens, all of the dose was recovered quantitatively in
the faeces within 3 days. At least 98% of the recovered radioactivity
was unchanged paraquat. Analysis of the tissues of hens after about 3
weeks of dosing with 14C-paraquat (6 ppm in the total diet)
indicated that it did not accumulate in the hens (Hemingway & Oliver,
1974).
Continuous dosing of hens with radiolabelled paraquat for up to
22 days, at rates up to 30 ppm in the diet, resulted in total
radioactive residues in the eggs of up to approximately 0.05 mg/kg
paraquat ion equivalent. At least 80% of the radioactivity was due to
unchanged paraquat. The residue was almost entirely in the yolk rather
than in the albumin (Hemingway & Oliver, 1974; Hendley et al.,
1976a).
Pigs
Pigs excreted an oral dose of paraquat principally in the faeces
as unchanged paraquat. Two pigs were dosed with 14C-labelled
paraquat for 7 consecutive days at a rate equivalent to 50 ppm in
the diet. One was dosed with 14C-methyl- and the second with
14C-ring-labelled paraquat. The pigs were sacrificed 2 hours after
receiving the final dose. By this time 69 - 73% of the administered
residue had been recovered in the faeces and approximately 3% had been
recovered in the urine. More than 90% of the radioactivity in the
faeces was present as unchanged paraquat. Total radioactive residues
in the tissues were low. More than 90% of these residues were due to
unchanged paraquat, except in liver, where approximately 70% was due
to unchanged paraquat and 4 - 7% was due to monoquat ion (Leahey
et al., 1976; Spinks et al., 1976).
Goats
14C-ring-labelled paraquat was administered to a goat in
mid-lactation twice daily for 7 days at a dose equivalent to 100 ppm
in the diet. Total radioactive residues in the milk were less than
0.01 mg/kg paraquat ion equivalent; 76% was unchanged paraquat. Total
radioactive residues were 0.74, 0.56, and 0.1 mg/kg in kidney, liver,
and muscle, respectively. There was no significant metabolism of
paraquat, except in the liver, where 50% of the residue was paraquat
and about 5% was each of the metabolites monoquat ion and monopyridone
ion (Hendley et al., 1976b).
Sheep
A dose of 14C-methyl-labelled paraquat administered to a sheep
via a rumen fistula was recovered quantitatively within 10 days.
Approximately 4% of the dose was excreted in the urine and the
remainder in the faeces. More than 95% of the radioactivity in urine
and faeces was present as unchanged paraquat. Small amounts of
monoquat ion (1%) and monopyridone ion (2.3%) were also detected
(Hemingway et al., 1972).
When injected s.c., paraquat was also excreted rapidly in the
urine (over 80% of the dose), 69% within the first day after
treatment. Unchanged paraquat accounted for most (90%) of the
radioactivity; the monopyridone derivative was present as 2 - 3% of
the dose and monoquat was a trace metabolite. This pattern of
metabolism was virtually identical to that seen in the urine following
dosing via the rumen (Hemingway et al., 1972).
Cows
When cows were given single oral doses of 14C-methyl paraquat
at 8 mg/kg, 96% of the radioactivity was recovered in the faeces
during the following 9 days; 0.7% was recovered in the urine.
Unchanged paraquat accounted for most of the radioactivity in the
faeces (96%) and urine (62 - 90%), but traces of the monoquat ion and
monopyridone ion were also detected in the urine. Only 0.003 - 0.004%
of the radioactivity was recovered in milk; the maximum radioactive
residue (0.005 mg/kg, paraquat ion equivalent) was observed on the day
after dosing. About 15% of this radioactivity was present as unchanged
paraquat. Monoquat ion and monopyridone ion (3 - 25%) were also found
in the milk. The radioactivity not identified as paraquat, monoquat,
or monopyridone was incorporated into natural constituents of milk
resulting from the anabolism of the radioactive methyl group cleaved
from paraquat (Hemingway et al., 1974).
Cows were fed for 3 months diets containing 24, 80, or 170 ppm
paraquat ion (equivalent to 0.8, 2.5, or 5.5 mg/kg b.w./day). The
paraquat was present as a residue in dried grass obtained from a
pasture that had been sprayed with Gramoxone and subsequently
weathered. The diet was accepted satisfactorily and no toxicological
effects were observed during the trial. Pathological examination of
tissues from animals slaughtered within 24 hours of the end of the
feeding trial showed no toxic effects attributable to paraquat. The
tissue residues, including muscle and liver, determined in cows at the
2 higher dose rates, varied between 0.01 and 0.09 mg/kg except in the
kidney, where 0.21 - 0.31 mg/kg was found. These fell to low
(0.04 mg/kg in the kidney) or non-detectable levels in an animal fed
the high-paraquat diet for 30 days and then maintained on an untreated
diet for 12 days before slaughter. Very low residues of paraquat were
present in milk samples taken weekly during the trial (121 samples
ranging from 0.0001 - 0.0006 mg/kg; 1 sample = 0.001 mg/kg)
(Edwards et al., 1974).
Effects on enzymes and other biochemical parameters
Several reviews or monographs have summarised the biochemical
mechanism of paraquat toxicity in plants (Calderbank, 1968), bacteria
(Fridovich & Hassan, 1979), and animals (Bus et al., 1976; Autor,
1977; Smith et al., 1979; Smith, 1985). The mechanism of the toxic
action of paraquat has also been extensively reviewed by WHO (1984).
Paraquat has long been known to participate in cyclic
reduction-oxidation reactions in biological systems. The compound
readily undergoes a single electron reduction in tissues, forming a
free radical. In an aerobic environment, the free radical is
immediately oxidised by molecular oxygen, generating the superoxide
anion radical. The reoxidized paraquat is capable of accepting another
electron and continuing the electron transfer reactions in a catalytic
manner (Figure 1).
 Research into the mechanism of paraquat toxicity has identified
at least 2 partially toxic consequences of the redox cycling reaction:
a) generation of the superoxide anion radical, and b) oxidation of
cellular NADPH, which is the major source of reducing equivalents for
the intracellular reduction of paraquat. Generation of the superoxide
anion radical can lead to the formation of more toxic forms of reduced
oxygen, hydrogen peroxide (H2O2), and hydroxyl radicals. Hydroxyl
radicals have been implicated in the initiation of membrane damage by
lipid peroxidation, depolymerization of hyaluronic acid, inactivation
of proteins, and damage to DNA. Depletion of NADPH, on the other hand,
may disrupt important NADPH-requiring biochemical processes such as
fatty acid synthesis (Hassan & Fridovich, 1980; Smith et al., 1979).
The importance of molecular oxygen and the potential role of
superoxide anion radical generation in mediating paraquat toxicity
have been implicated in studies on plants, bacteria, and in vitro
and in vivo mammalian systems. In cultures of E. coli, Hassan &
Fridovich (1977, 1978, & 1979) demonstrated that paraquat stimulated
cyanide-resistant respiration, which could be almost entirely
accounted for by the NADPH-dependent formation of the superoxide anion
radical.
The possibility that formation of the superoxide anion radical
might be responsible for the toxicity of paraquat in bacteria is
supported by observations that bacteria containing elevated activities
of superoxide dismutase, an enzyme that detoxifies the superoxide
anion radical, were resistant to paraquat toxicity (Hassan &
Fridovich, 1977, 1978; Moody & Hassan, 1982).
In vitro studies on lung and liver preparations from various
animal species have supported the hypothesis that paraquat redox
cycling and associated superoxide anion radical and H202
generation also occur in mammalian systems (Gage, 1968; Ilett
et al., 1974; Montgomery, 1976, 1977; Steffen & Netter, 1979;
Talcott et al., 1979).
Bus et al. (1974) reported that the single electron reduction
of paraquat in mammalian systems was catalysed by microsmal cytochrome
P-450 reductase and NADPH. The observation that the in vivo toxicity
of paraquat in animals is markedly potentiated by exposure to elevated
oxygen tensions further supports the potential role for molecular
oxygen in mediating toxicity (Fisher et al., 1973; Autor, 1974;
Bus & Gibson, 1975; Witschi et al., 1977; Kehrer et al., 1979;
Keeling et al., 1981; Selman et al., 1985).
The results of in vivo studies conducted by Bus et al. (1974)
suggest that stimulation of lipid peroxidation, which is dependent on
paraquat redox cycling and associated superoxide anion radical
generation, might be an important toxic mechanism in mammalian
systems. Consistent with this hypothesis, animals fed diets deficient
in selenium or vitamin E in order to diminish cellular antioxidant
defenses were significantly more sensitive to paraquat toxicity than
control animals (Bus et al., 1975a; Omaye et al., 1978). Moreover,
selenium deficiency potentiated paraquat-induced lipid peroxidation in
isolated perfused rat lung (Glass et al., 1985). In contrast to
these studies, a number of studies have shown that paraquat inhibited
in vitro microsomal lipid peroxidation (Ilett et al., 1974;
Montgomery & Niewoehner, 1979; Steffen & Netter, 1979; Kornburst &
Mavis, 1980). Subsequent studies have indicated, however, that
paraquat would stimulate microsomal lipid peroxidation when an
adequate supply of electrons (NADPH) and in vitro oxygen tension
were maintained (Trush et al., 1981, 1982).
Despite the evidence described above, the hypothesis that lipid
peroxidation is the underlying toxic mechanism functioning in vivo
has not been conclusively demonstrated. Direct quantification of
paraquat-induced lipid peroxidation damage in vivo by analysis of
tissue malonadialdehyde levels or ethane exhalation, both markers of
peroxidation injury, has been largely unsuccessful (Reddy et al.,
1977; Shu et al., 1979; Steffen et al., 1980), although
significant increases of serum malondialdehyde levels have been
recently reported in patients with paraquat poisoning (Yasaka
et al., 1986). Furthermore, attempts to counteract paraquat
toxicity by administration of various antioxidants have also been
unsuccessful (Fairshter, 1981).
Superoxide radicals generated in paraquat redox cycling may
induce biochemical changes other than the initiation of the
peroxidation reaction. Ross et al. (1979) demonstrated that paraquat
increased DNA strand breaks in cultured mouse lymphoblasts. Paraquat
was also reported to induce a superoxide-dependent stimulation of
guanylate cyclase activity in rat liver (Vesely et al., 1979) and
guinea pig lung (Giri & Krishna, 1980). These investigators postulated
that increased cyclic-GMP might stimulate the pulmonary fibroproli-
ferative changes characteristic of paraquat toxicity. In other
studies, paraquat has also been found to increase collagen synthesis
in the rat lung (Greenberg et al., 1978; Thompson & Patrick, 1978;
Hussain & Bhatnagar, 1979).
Redox cycling of paraquat has also been proposed to lead to
increased oxidation of cellular NADPH (Brigelius et al., 1981;
Keeling et al., 1982). The activity of pentose shunt enzymes in the
lung rapidly increased in rats treated with paraquat, which suggested
an increased demand for NADPH (Fisher et al., 1975; Rose et al.,
1976b). The observation that paraquat decreased fatty acid synthesis
in lung slices (Smith et al., 1979) further supported this
hypothesis, since fatty acid synthesis requires NADPH. Direct analysis
of NADPH in the lung has long confirmed that paraquat treatment
decreases the NADPH content in rat lung (Witschi et al., 1977;
Smith et al., 1979). More recently, both oxygen consumption and
NADPH oxidation in lung microsomes were found to be significantly and
specifically stimulated by the addition of paraquat (Rossouw et al.,
1984). The above observations led Smith et al. (1979) to propose
that oxidation of NADPH might interrupt not only vital physiological
processes, such as fatty acid synthesis, but also may render tissues
more susceptible to lipid peroxidation by decreasing the equivalents
(NADPH) necessary for functioning of the antioxidant enzyme
glutathione peroxidase (Figure 2). Indeed, a significant increase
(589%) in lung-oxidized glultathione (GSSG) content was found over
control levels after perfusion of isolated rabbit lung with a 0.4 mM
paraquat solution. This effect was significantly increased (225%) by
hyperoxia (Dunbar et al., 1984).
Toxicological studies
Special studies on carcinogenicity
Mice
Groups of 60 male and 60 female Alderly Park SPF mice were fed
diets containing 0 (2 groups), 12.5, 37.5, or 100/125 ppm paraquat
cation for 97 - 99 weeks. The initial top dose of 100 ppm was
increased to 125 ppm at week 36 in order to evoke a toxic effect. The
study was terminated at weeks 97 - 99 when 80% mortality was reached
in a female control group and was approaching 80% overall. Clinical
observations, body-weight gain, food consumption, and urinary paraquat
were measured throughout the study. Histopathological examination of
approximately 40 tissues was performed on animals killed or dying
during the study and at termination. Further groups of 10 males and 10
females were fed the same dose levels as above for 52 weeks for
measurement of paraquat concentrations in the kidney, lung, and plasma
at termination.
Mortality ranged from 32 - 55% at 80 weeks and from 58 - 87% at
termination and was higher in the 37.5 ppm and 125 ppm groups than in
the combined controls. Effects due to treatment were renal lesions in
both sexes at 100/125 ppm and, in males, at 37.5 ppm; lung lesions in
both sexes at 100/125 ppm; and decreased food consumption and
body-weight gain and increased mortality in females at 100/125 ppm.
Histopathologically, the treatment-related renal lesions were manifest
as mild dilatation and degenerative changes in the tubules. The
incidences of tubular degeneration (with and without dilatation) in
male mice dying during the study were 31/48 at 100/125 ppm, 15/47 at
37.5 ppm, 9/45 at 12.5 ppm, and 8/45 and 3/35 in controls. The
paraquat-induced lung lesions noted at 100/125 ppm included focal
pneumonitis/alveolitis and hypercellularity of the alveolar walls.
Statistically-significant increases in the incidence of fatty changes
of the liver were reported at 37.5 and 100/125 ppm in males, when
compared to controls. Other hepatic changes were noted, with a
significantly-higher incidence in treated compared to control mice.
These changes, however, were not considered by the authors of the
study to be treatment-related. There were no effects observed at
12.5 ppm. Histopathological examination showed no clear evidence of
treatment-related neoplastic changes in these mice. The incidence of
pulmonary tumours in both males (7/24) and females (8/20) in the
100/125 ppm dose group dying from 79 - 98 weeks was somewhat higher
than in controls (5/37 in males and 6/39 in females). However, the
incidences of pulmonary tumours in the animals of the same groups
surviving to termination were lower than in controls.
The authors of the study concluded that paraquat was not
oncogenic to the mouse. Based on the renal lesions, the no-effect
level of paraquat cation for Alderley Park SPF mice in this study was
12.5 ppm, equal to 1.4 mg/kg b.w./day in males and 37.5 ppm, equal to
4.3 mg/kg b.w./day in females.
Rats
Groups of 80 male and 80 female Fisher SPF rats were maintained
on diets containing 0, 7.2, 22, 72, or 217 ppm paraquat cation for 104
weeks. Eight rats/sex/group were sacrificed after urinalysis at 26,
52, and 78 weeks and were subjected to haematological examination. All
surviving animals were sacrificed at 104 weeks and, among these, 10
rats/sex/group were subjected to haematological and biochemical
examination. All animals, including those killed on schedule and those
found moribund and killed during the study, were autopsied and
subjected to gross necropsy and histopathological examination of
approximately 30 tissues.
Mortality was increased in female rats of the 217 ppm group from
week 66 to week 74 when compared with that of other groups, including
controls. Both male and female rats at the 217 ppm dietary level
showed a marked statistically-significant reduction in body-weight
gain when compared to control groups. Food consumption, efficiency of
food utilisation, and water consumption were also statistically-
significantly lower in these rats when compared to control animals.
Haematological examination showed a statistically-significant
reduction in total white cell count in male rats of the 217 ppm group,
when compared to controls, at 26, 52, and 78 weeks, but not at 104
weeks. This change was not considered by the authors of the study to
be attributable to the administration of paraquat. Biochemical
examination indicated a statistically-significant reduction in
globulin in male rats of the 217 ppm group at 26, 78, and 104 weeks
when compared to controls. Clinical observations, RBC counts,
haemoglobin, mean red-cell volume (MCV), mean cell hamoglobin (MCH),
mean cell haemoglobin concentration (MCHC), platelet counts,
differential WBC counts, plasma alkaline phosphatase, lactic acid
dehydrogenase, blood urea nitrogen, glucose, total cholesterol, GOT,
GPT, total and direct bilirubin, GGPT, calcium, total protein,
albumin, and urinalysis indicated no significant effects attributable
to the administration of paraquat at any dose levels.
Throughout the entire administration period, a statistically-
significant reduction was found in the absolute weights of various
organs of male and female rats of the 217 ppm group at interim
sacrifices. This change was considered by the authors of the study
to be related to the reduction in body weight observed in these
animals. Histological examination of the lung at termination showed a
marked, treatment-related, statistically-significant increase in the
incidence of proliferation of interalveolar septum cells and of
hyperplasia of alveolar epithelium in both male and female rats at 217
ppm and in male rats at 72 ppm, when compared to controls. There was
a marked, statistically-significant increase in the incidence of
cataract in male and female rats of the 217 ppm group killed or found
dead after week 79. This treatment-related change was reported to be
the same microscopically as that observed in the tissues collected
from those control rats which had spontaneous, age-related cataracts.
Male rats of the 217 ppm group also showed a statistically-significant
increase in the incidence of local atrophy of renal tubules when
compared to controls. Females of the same dietary group had a
statistically-significant increase in the overall incidence of
diffusive fatty changes of the liver and pulmonary fibrosis when
compared to controls. Kidney and liver lesions were not considered by
the authors of the study to be attributable to the administration of
paraquat. A significant increase (details of statistical analysis were
not available) in the incidence of pulmonary adenoma (7/80) was found
in female rats of the 217 ppm group when compared to controls (1/80).
There was no significant increase in the incidence of lung adenoma in
male rats, but a few of them had lung adenocarcinoma (1 in each of the
22 and 72 ppm groups, 3 in the 217 ppm group, and none in the
controls). The authors noted that, although the historical incidence
of pulmonary adenoma in rats of this strain is reportedly rather low
(about 2%), 6/80 (7.5%) of the control rats developed pulmonary
adenoma in a 24-month chronic toxicity study carried out separately in
their laboratory. Based on these considerations, the authors of the
study concluded that the incidence of pulmonary adenoma found in the
present paraquat study in female rats in the 217 ppm group did not
exceed the background incidence of pulmonary adenoma in rats of this
strain. On the basis of the lung and eye lesions the no-effect level
of paraquat cation determined in this study for Fisher SPF rats after
104-week treatment was 22 ppm, equal to 0.77 mg/kg b.w./day in male
rats and 72 ppm, equal to 3.12 mg/kg b.w./day in female rats
(Yoshida et al., 1982).
Research into the mechanism of paraquat toxicity has identified
at least 2 partially toxic consequences of the redox cycling reaction:
a) generation of the superoxide anion radical, and b) oxidation of
cellular NADPH, which is the major source of reducing equivalents for
the intracellular reduction of paraquat. Generation of the superoxide
anion radical can lead to the formation of more toxic forms of reduced
oxygen, hydrogen peroxide (H2O2), and hydroxyl radicals. Hydroxyl
radicals have been implicated in the initiation of membrane damage by
lipid peroxidation, depolymerization of hyaluronic acid, inactivation
of proteins, and damage to DNA. Depletion of NADPH, on the other hand,
may disrupt important NADPH-requiring biochemical processes such as
fatty acid synthesis (Hassan & Fridovich, 1980; Smith et al., 1979).
The importance of molecular oxygen and the potential role of
superoxide anion radical generation in mediating paraquat toxicity
have been implicated in studies on plants, bacteria, and in vitro
and in vivo mammalian systems. In cultures of E. coli, Hassan &
Fridovich (1977, 1978, & 1979) demonstrated that paraquat stimulated
cyanide-resistant respiration, which could be almost entirely
accounted for by the NADPH-dependent formation of the superoxide anion
radical.
The possibility that formation of the superoxide anion radical
might be responsible for the toxicity of paraquat in bacteria is
supported by observations that bacteria containing elevated activities
of superoxide dismutase, an enzyme that detoxifies the superoxide
anion radical, were resistant to paraquat toxicity (Hassan &
Fridovich, 1977, 1978; Moody & Hassan, 1982).
In vitro studies on lung and liver preparations from various
animal species have supported the hypothesis that paraquat redox
cycling and associated superoxide anion radical and H202
generation also occur in mammalian systems (Gage, 1968; Ilett
et al., 1974; Montgomery, 1976, 1977; Steffen & Netter, 1979;
Talcott et al., 1979).
Bus et al. (1974) reported that the single electron reduction
of paraquat in mammalian systems was catalysed by microsmal cytochrome
P-450 reductase and NADPH. The observation that the in vivo toxicity
of paraquat in animals is markedly potentiated by exposure to elevated
oxygen tensions further supports the potential role for molecular
oxygen in mediating toxicity (Fisher et al., 1973; Autor, 1974;
Bus & Gibson, 1975; Witschi et al., 1977; Kehrer et al., 1979;
Keeling et al., 1981; Selman et al., 1985).
The results of in vivo studies conducted by Bus et al. (1974)
suggest that stimulation of lipid peroxidation, which is dependent on
paraquat redox cycling and associated superoxide anion radical
generation, might be an important toxic mechanism in mammalian
systems. Consistent with this hypothesis, animals fed diets deficient
in selenium or vitamin E in order to diminish cellular antioxidant
defenses were significantly more sensitive to paraquat toxicity than
control animals (Bus et al., 1975a; Omaye et al., 1978). Moreover,
selenium deficiency potentiated paraquat-induced lipid peroxidation in
isolated perfused rat lung (Glass et al., 1985). In contrast to
these studies, a number of studies have shown that paraquat inhibited
in vitro microsomal lipid peroxidation (Ilett et al., 1974;
Montgomery & Niewoehner, 1979; Steffen & Netter, 1979; Kornburst &
Mavis, 1980). Subsequent studies have indicated, however, that
paraquat would stimulate microsomal lipid peroxidation when an
adequate supply of electrons (NADPH) and in vitro oxygen tension
were maintained (Trush et al., 1981, 1982).
Despite the evidence described above, the hypothesis that lipid
peroxidation is the underlying toxic mechanism functioning in vivo
has not been conclusively demonstrated. Direct quantification of
paraquat-induced lipid peroxidation damage in vivo by analysis of
tissue malonadialdehyde levels or ethane exhalation, both markers of
peroxidation injury, has been largely unsuccessful (Reddy et al.,
1977; Shu et al., 1979; Steffen et al., 1980), although
significant increases of serum malondialdehyde levels have been
recently reported in patients with paraquat poisoning (Yasaka
et al., 1986). Furthermore, attempts to counteract paraquat
toxicity by administration of various antioxidants have also been
unsuccessful (Fairshter, 1981).
Superoxide radicals generated in paraquat redox cycling may
induce biochemical changes other than the initiation of the
peroxidation reaction. Ross et al. (1979) demonstrated that paraquat
increased DNA strand breaks in cultured mouse lymphoblasts. Paraquat
was also reported to induce a superoxide-dependent stimulation of
guanylate cyclase activity in rat liver (Vesely et al., 1979) and
guinea pig lung (Giri & Krishna, 1980). These investigators postulated
that increased cyclic-GMP might stimulate the pulmonary fibroproli-
ferative changes characteristic of paraquat toxicity. In other
studies, paraquat has also been found to increase collagen synthesis
in the rat lung (Greenberg et al., 1978; Thompson & Patrick, 1978;
Hussain & Bhatnagar, 1979).
Redox cycling of paraquat has also been proposed to lead to
increased oxidation of cellular NADPH (Brigelius et al., 1981;
Keeling et al., 1982). The activity of pentose shunt enzymes in the
lung rapidly increased in rats treated with paraquat, which suggested
an increased demand for NADPH (Fisher et al., 1975; Rose et al.,
1976b). The observation that paraquat decreased fatty acid synthesis
in lung slices (Smith et al., 1979) further supported this
hypothesis, since fatty acid synthesis requires NADPH. Direct analysis
of NADPH in the lung has long confirmed that paraquat treatment
decreases the NADPH content in rat lung (Witschi et al., 1977;
Smith et al., 1979). More recently, both oxygen consumption and
NADPH oxidation in lung microsomes were found to be significantly and
specifically stimulated by the addition of paraquat (Rossouw et al.,
1984). The above observations led Smith et al. (1979) to propose
that oxidation of NADPH might interrupt not only vital physiological
processes, such as fatty acid synthesis, but also may render tissues
more susceptible to lipid peroxidation by decreasing the equivalents
(NADPH) necessary for functioning of the antioxidant enzyme
glutathione peroxidase (Figure 2). Indeed, a significant increase
(589%) in lung-oxidized glultathione (GSSG) content was found over
control levels after perfusion of isolated rabbit lung with a 0.4 mM
paraquat solution. This effect was significantly increased (225%) by
hyperoxia (Dunbar et al., 1984).
Toxicological studies
Special studies on carcinogenicity
Mice
Groups of 60 male and 60 female Alderly Park SPF mice were fed
diets containing 0 (2 groups), 12.5, 37.5, or 100/125 ppm paraquat
cation for 97 - 99 weeks. The initial top dose of 100 ppm was
increased to 125 ppm at week 36 in order to evoke a toxic effect. The
study was terminated at weeks 97 - 99 when 80% mortality was reached
in a female control group and was approaching 80% overall. Clinical
observations, body-weight gain, food consumption, and urinary paraquat
were measured throughout the study. Histopathological examination of
approximately 40 tissues was performed on animals killed or dying
during the study and at termination. Further groups of 10 males and 10
females were fed the same dose levels as above for 52 weeks for
measurement of paraquat concentrations in the kidney, lung, and plasma
at termination.
Mortality ranged from 32 - 55% at 80 weeks and from 58 - 87% at
termination and was higher in the 37.5 ppm and 125 ppm groups than in
the combined controls. Effects due to treatment were renal lesions in
both sexes at 100/125 ppm and, in males, at 37.5 ppm; lung lesions in
both sexes at 100/125 ppm; and decreased food consumption and
body-weight gain and increased mortality in females at 100/125 ppm.
Histopathologically, the treatment-related renal lesions were manifest
as mild dilatation and degenerative changes in the tubules. The
incidences of tubular degeneration (with and without dilatation) in
male mice dying during the study were 31/48 at 100/125 ppm, 15/47 at
37.5 ppm, 9/45 at 12.5 ppm, and 8/45 and 3/35 in controls. The
paraquat-induced lung lesions noted at 100/125 ppm included focal
pneumonitis/alveolitis and hypercellularity of the alveolar walls.
Statistically-significant increases in the incidence of fatty changes
of the liver were reported at 37.5 and 100/125 ppm in males, when
compared to controls. Other hepatic changes were noted, with a
significantly-higher incidence in treated compared to control mice.
These changes, however, were not considered by the authors of the
study to be treatment-related. There were no effects observed at
12.5 ppm. Histopathological examination showed no clear evidence of
treatment-related neoplastic changes in these mice. The incidence of
pulmonary tumours in both males (7/24) and females (8/20) in the
100/125 ppm dose group dying from 79 - 98 weeks was somewhat higher
than in controls (5/37 in males and 6/39 in females). However, the
incidences of pulmonary tumours in the animals of the same groups
surviving to termination were lower than in controls.
The authors of the study concluded that paraquat was not
oncogenic to the mouse. Based on the renal lesions, the no-effect
level of paraquat cation for Alderley Park SPF mice in this study was
12.5 ppm, equal to 1.4 mg/kg b.w./day in males and 37.5 ppm, equal to
4.3 mg/kg b.w./day in females.
Rats
Groups of 80 male and 80 female Fisher SPF rats were maintained
on diets containing 0, 7.2, 22, 72, or 217 ppm paraquat cation for 104
weeks. Eight rats/sex/group were sacrificed after urinalysis at 26,
52, and 78 weeks and were subjected to haematological examination. All
surviving animals were sacrificed at 104 weeks and, among these, 10
rats/sex/group were subjected to haematological and biochemical
examination. All animals, including those killed on schedule and those
found moribund and killed during the study, were autopsied and
subjected to gross necropsy and histopathological examination of
approximately 30 tissues.
Mortality was increased in female rats of the 217 ppm group from
week 66 to week 74 when compared with that of other groups, including
controls. Both male and female rats at the 217 ppm dietary level
showed a marked statistically-significant reduction in body-weight
gain when compared to control groups. Food consumption, efficiency of
food utilisation, and water consumption were also statistically-
significantly lower in these rats when compared to control animals.
Haematological examination showed a statistically-significant
reduction in total white cell count in male rats of the 217 ppm group,
when compared to controls, at 26, 52, and 78 weeks, but not at 104
weeks. This change was not considered by the authors of the study to
be attributable to the administration of paraquat. Biochemical
examination indicated a statistically-significant reduction in
globulin in male rats of the 217 ppm group at 26, 78, and 104 weeks
when compared to controls. Clinical observations, RBC counts,
haemoglobin, mean red-cell volume (MCV), mean cell hamoglobin (MCH),
mean cell haemoglobin concentration (MCHC), platelet counts,
differential WBC counts, plasma alkaline phosphatase, lactic acid
dehydrogenase, blood urea nitrogen, glucose, total cholesterol, GOT,
GPT, total and direct bilirubin, GGPT, calcium, total protein,
albumin, and urinalysis indicated no significant effects attributable
to the administration of paraquat at any dose levels.
Throughout the entire administration period, a statistically-
significant reduction was found in the absolute weights of various
organs of male and female rats of the 217 ppm group at interim
sacrifices. This change was considered by the authors of the study
to be related to the reduction in body weight observed in these
animals. Histological examination of the lung at termination showed a
marked, treatment-related, statistically-significant increase in the
incidence of proliferation of interalveolar septum cells and of
hyperplasia of alveolar epithelium in both male and female rats at 217
ppm and in male rats at 72 ppm, when compared to controls. There was
a marked, statistically-significant increase in the incidence of
cataract in male and female rats of the 217 ppm group killed or found
dead after week 79. This treatment-related change was reported to be
the same microscopically as that observed in the tissues collected
from those control rats which had spontaneous, age-related cataracts.
Male rats of the 217 ppm group also showed a statistically-significant
increase in the incidence of local atrophy of renal tubules when
compared to controls. Females of the same dietary group had a
statistically-significant increase in the overall incidence of
diffusive fatty changes of the liver and pulmonary fibrosis when
compared to controls. Kidney and liver lesions were not considered by
the authors of the study to be attributable to the administration of
paraquat. A significant increase (details of statistical analysis were
not available) in the incidence of pulmonary adenoma (7/80) was found
in female rats of the 217 ppm group when compared to controls (1/80).
There was no significant increase in the incidence of lung adenoma in
male rats, but a few of them had lung adenocarcinoma (1 in each of the
22 and 72 ppm groups, 3 in the 217 ppm group, and none in the
controls). The authors noted that, although the historical incidence
of pulmonary adenoma in rats of this strain is reportedly rather low
(about 2%), 6/80 (7.5%) of the control rats developed pulmonary
adenoma in a 24-month chronic toxicity study carried out separately in
their laboratory. Based on these considerations, the authors of the
study concluded that the incidence of pulmonary adenoma found in the
present paraquat study in female rats in the 217 ppm group did not
exceed the background incidence of pulmonary adenoma in rats of this
strain. On the basis of the lung and eye lesions the no-effect level
of paraquat cation determined in this study for Fisher SPF rats after
104-week treatment was 22 ppm, equal to 0.77 mg/kg b.w./day in male
rats and 72 ppm, equal to 3.12 mg/kg b.w./day in female rats
(Yoshida et al., 1982).
 Historical control incidence data of neoplasia in F-344 rats in
the laboratory in which the preceding study was conducted were made
available and are summarized in Table 3.
Table 3. Spontaneous lung tumours observed in F-344 rats at the
Institute of Environmental Toxicology from 1980 to 19831
No. of tumour-bearing animals (%)2
Males Females
Adenoma 40 (4.2%) 21 (2.2%)
Adenocarcinoma 5 (0.5%) 1 (0.1%)
Bronchial gland adenoma 1 (0.1%) -
Total 46 (4.8%) 22 (2.3%)
1 From Maita, 1986
2 These data were taken from 12 studies that included 960 male and
959 female F-344/DuCrj rats.
Groups of 60 male and 60 female Fischer 344 rats were maintained
on diets containing paraquat cation at 0 (2 groups), 25, 75, or
150 ppm for at least 113 weeks (males) or 122 weeks (females). Further
groups of 10 rats/sex/group received the same diets for 1 year. All
animals were studied for mortality, food and water consumption, and
body weight, and were subjected to periodical ophthalmoscopic and
haematological examinations throughout the study.
The distribution of mortality was unaffected by treatment. There
was approximately 50% mortality in all groups at the end of the study.
At 150 ppm, statistically-significant reductions in body-weight gain,
food consumption, and efficiency of food utilisation in both sexes
were observed. There was a statistically-significant depression of
body-weight gain in the first year of the study in males receiving
75 ppm paraquat. Water consumption was not significantly affected at
any dietary level tested. Paraquat accelerated, in a dosage-dependent
manner, the onset and progression of cataract changes, ranging from
minor opacity to total cataract in both males and females.
Treatment-related ocular lesions were first seen at 52 weeks.
Thereafter, ophthalmoscopy revealed a statistically-significant
dosage-related increase in the incidence, progression, and severity of
lenticular cataract in the 150 ppm group and, toward the end of the
study (103 weeks), in the 75 ppm group. There was evidence of
paraquat-dependent ocular effects in all treatment groups of both
sexes at termination. A statistically-significant higher incidence of
secondary eye lesions was found at termination in females receiving 75
or 150 ppm paraquat when compared to controls. Haematological
investigation (RBC counts, total and differential leucocyte counts,
haemoglobin, haematocrit, mean cell volume, mean cell haemoglobin
concentration, platelet and reticulocyte counts, and prothrombin and
partial thromboplastin times) and blood biochemistry (urea, glucose,
ALT, and AST) indicated no significant effects attributable to
paraquat administration. Urinalysis did not reveal any treatment-
related changes. Reductions in liver and testicular weights were noted
at termination in the 150 ppm dietary group.
Macroscopic examination at necropsy revealed a treatment-related
increase in the incidence of focal subpleural changes in animals
killed at termination in all dietary groups. This effect was most
marked in females receiving 75 ppm and in both sexes receiving 150 ppm
paraquat. Microscopic examination of lung tissues indicated that
treatment with paraquat at 150 ppm, in both sexes, and possibly at
75 ppm in males, was associated with proliferative lesions of the
alveolar epithelium. These lesions were not easily classified into
non-neoplastic or neoplastic, nor into adenoma or carcinoma. This
study provided strong evidence for the induction by paraquat of a
proliferative lesion of the alveolar epithelium and some controversial
evidence for the induction of lung adenomas in female Fischer 344
rats. There was no treatment-related increase in the incidence of lung
adenocarcinoma at any dose level in either sex. At 25 ppm, significant
increases in the incidence of proliferative lung lesions, compared to
the controls, were not observed. Microscopic examination of the eyes
confirmed a dose-related effect of paraquat on the onset and
progression of cataract lesions normally present in both male and
female F-344 rats. Slight dilation of the fourth ventricle of the
brain was evident in females receiving 150 or 75 ppm paraquat, but not
in males at these dosages or in either sex at 25 ppm. A statistically-
significant increase in the incidence of apparent degeneration of
occasional/several sciatic nerve fibers was noted in decedent males
receiving 75 or 150 ppm paraquat. Both hydrocephalus and nervous
tissue changes were considered by the authors of the study possibly
to be associated with paraquat treatment. Pathology summaries
indicate that atrophy of the testes was recorded in the high-dietary
group (5/33) but not in controls at termination, and moderate lymphoid
hyperplasia was observed in the respiratory epithelium of males
receiving 75 and 150 ppm paraquat and dying between 52 weeks and
termination.
The authors of the study concluded that "a wide range of tumour
types was observed in treated and control animals, and there was no
evidence that treatment with paraquat resulted in a marked change in
the group distribution of any of these tumours". A no-effect level for
paraquat was not found in this study due to the higher incidence of
cataract observed in animals of the 25 ppm group when compared to
controls. Paraquat accelerated, in a dosage-dependent manner, the
onset and progression of cataract change in F-334 rats. The authors
considered 25 ppm to be near the no-effect level for this change at
the end of the study (Ashby et al., 1983; Busey, 1986; Ishmael &
Godley, 1983).
Data on the historical incidence of lung neoplastic lesions in
F-344 rats from 7 studies performed in the laboratory in which the
preceding study was conducted were made available and are summarised
in Table 4.
Table 4. Spontaneous lung tumours observed in F-344 rats at Life
Science Research1
No. of tumour-bearing animals (%)2
Males Females
Adenoma 6/357 (1.7%) 4/363 (1.1%)
Carcinoma 3/357 (0.8%) 0/363 (0%)
Total pulmonary tumours 9/357 (2.5%) 4/363 (1.1%)
1 From Ashby et al., 1983
2 The range (% incidence) of adenoma was 0 - 4.4% in males and
0 - 4.0% in females; the range of carcinoma was 0 - 4.0% in
males.
Special studies on embryotoxicity and teratogenicity
Mice
The teratogenicity and fetal toxicity of paraquat were examined
after oral (20 mg/kg b.w./day) or i.p. (1.67 or 3.35 mg/kg b.w./day)
administration of paraquat to pregnant mice during the period of
organogenesis (days 8 - 16 of gestation). The oral dose (which was
equal to 1/10 of the oral LD50/day) did not produce significant
maternal toxicity, but at the higher of the 2 i.p. doses (which was
equal to 1/10 of the i.p. LD50/day) a significant maternal mortality
(5/7) and a statistically-significant increase of resorption rate,
when compared to controls, were observed. Paraquat did not
significantly increase the incidence of gross, soft-tissue, or
skeletal abnormalities. At the lower i.p. dose and after oral
administration of paraquat, there was a slight but non-significant
increase in the number of fetuses with absent or non-ossified
sternebrae. However, a significant difference was observed in the
incidence of abnormal sternebrae between the 2 control groups (6.9 ±
3.2% in the i.p. control group and 13.2 ± 5.8% in the oral control
group). The authors of the study concluded that the potential for
paraquat as a teratogen appeared to be minimal (Bus et al., 1975b).
The same authors examined the effects of paraquat dichloride on
the development of Swiss-Webster mice when administered in the
drinking water at concentrations of 0, 50, or 100 ppm. Paraquat was
given to pregnant mice from day 8 of gestation and administration was
continued to the newborns until 42 days after birth, when both the
control and paraquat-treated mice were sacrificed and subjected to
histopathological examination of the lungs, liver, and kidneys. A
significant increase in postnatal mortality in mice receiving 100 ppm
paraquat was observed. Histopathological examination of the lungs of
these mice showed extensive alveolar consolidation and collapse, and
areas of thickening of intra-alveolar septa. No significant
pathological changes were seen in the lungs of the 50 ppm or control
mice, nor in the liver or kidneys of mice of any treatment group.
There were no treatment-related effects on the number of live fetuses
nor on postnatal growth rate at either treatment level (Bus & Gibson,
1975).
Four groups of at least 20 pregnant SPF Alderley Park mice were
given orally 0, 1, 5, or 10 mg/kg b.w./day of paraquat cation during
days 6 to 15 of pregnancy, inclusive. On day 18 the animals were
killed, their uteri were examined, and the fetuses were removed,
weighed, sexed, and observed for gross abnormalities. There was some
evidence of maternal toxicity in the form of slight reductions in
body-weight gain at 5 and 10 mg/kg b.w./day, although only that of the
middle-dose group was statistically significant. There were no
clinical signs nor pathological changes in maternal animals
attributable to paraquat administration. Water and food consumption
were not quantified in this study. Numbers of implantations, viable
fetuses and resorptions, sex ratios, and fetal and litter weights
showed no significant differences between treated and control groups.
There were no increases in fetal external or soft-tissue abnormalities
which could be associated with paraquat treatment. There were
occasional statistically-significant differences in ossification of
individual bones between treated and control groups, but no
dose-related trend indicating either retardation of ossification or
increased abnormalities was observed. The authors of the study
concluded that paraquat was not teratogenic and had no significant
influence on embryonic or fetal development of the mouse at levels up
to and including 10 mg/kg b.w./day (Hodge et al., 1978a).
Rats
The teratogenic effects of paraquat were studied in 4 groups of
at least 20 pregnant SPF Alderley Park rats after oral administration
of 0, 1, 5, or 10 mg/kg b.w./day of paraquat cation during days 6 to
15 of pregnancy, inclusive. On day 21 the animals were killed. There
were clear clinical signs of maternal toxicity at 5 and 10 mg/kg
b.w./day. Apparently, 6 rats at the highest-dose level and 2 at the
middle-dose level died or became moribund during the experiment.
Histological changes found in the lungs and kidneys of the animals
receiving 10 mg/kg b.w./day paraquat which died or became moribund
were those known to be associated with oral paraquat poisoning. Slight
fetotoxicity was seen at 5 and 10 mg/kg b.w./day, as shown by a
statistically-significant reduction in fetal weight and retardation in
ossification, and by a decrease in the number of viable fetuses per
number of implants. According to the authors of the study, these
effects were probably associated with maternal toxicity. There were no
effects on embryonic or fetal survival and increases in fetal
abnormalities were not observed. The authors of the study concluded
that paraquat was not teratogenic when administered orally to rats,
even when there was clear evidence of maternal toxicity. However, it
did cause slight fetotoxicity at the 2 highest-dose levels (Hodge
et al., 1978b).
Special studies on eye irritation
The effects of paraquat on the eye have been reviewed by WHO
(1984). The instillation of diluted paraquat (up to 500 g/litre) in
rabbits' eyes induced inflammation within 24 hours, and this continued
for 96 hours (Clark et al., 1966). In another experiment, 62.5, 125,
250, 500, or 1000 g/litre of paraquat was introduced into the eyes of
rabbits. Concentrations of 62.5 and 125 g/litre caused severe
conjunctival reactions; higher levels (250 - 500 g/litre) provoked
ititis and pannus, while at the 500 g/litre concentration corneal
opacification, iritis, and conjunctivitis occurred. All rabbits
receiving 0.2 ml of paraquat at 1000 g/litre in 1 eye or 0.2 ml of
500 g/litre paraquat in both eyes died within 6 days of application
(Sinow & Wei, 1973).
Special studies on mutagenicity
In a review of published mutagenicity data (WHO, 1984) it was
noted that paraquat had been found to have minimal to no genotoxic
activity when evaluated in a variety of in vitro and in vivo test
systems. In in vitro studies producing weakly positive results,
paraquat genotoxicity was accompanied by high cytotoxicity (Moody &
Hassan, 1982; Parry, 1973 & 1977; Tweats, 1975; Benigni et al.,
1979; Bignami & Grebelli, 1979). Moody and Hassan (1982) have shown
that the mutagenicity of paraquat in bacterial test systems
(Salmonella typhimurium TA98 and TA100) was mediated by the
formation of superoxide. More recently, paraquat was found to induce
superoxide dismutase, chromosomal aberrations, and sister-chromatid
exchange in Chinese hamster fibroblasts, suggesting that superoxide
production is responsible for the chromosomal damage (Nicotera
et al., 1985). Other investigators (Anderson et al., 1972; Levin
et al., 1982) have not found mutagenic activity in bacterial test
systems. Furthermore, paraquat was not mutagenic when evaluated in
human leukocytes nor in in vivo cytogenic tests on mouse bone marrow
(Selypes & Paldy, 1978) or in dominant lethal tests on mice
(Pasi et al., 1974; Anderson et al., 1976).
A set of recently completed studies indicate that in most tests
paraquat was not mutagenic (see Table 5). Clastogenic potential has
been shown in vitro at very high concentration levels which were
themselves cytotoxic. Paraquat was not found to be mutagenic
in vivo.
Special studies on reproduction
Rats
In a 3-generation study, groups of 15 male and 30 female (F0
parents) weanling Alderley Park SPF rats were fed diets containing
0, 25, 75, or 150 ppm paraquat cation. After 12 weeks, animals were
mated to produce the first (F1a) litter and subsequently re-mated to
produce a second (F1b) litter. The breeding programme was repeated
twice with F1 parents selected from the F1b offspring and F2
parents selected from the F2b offspring. Test diets were fed
continuously throughout the study.
There were no adverse effects on parental body weights or food
consumption, and no treatment-related changes were found in the
reproductive performance (male and female fertility, live-born and
survival indexes, and litter size) or in the reproductive tract of
parents or offspring. Development of the reproductive tract in all
treated offspring was substantially comparable to that in controls.
The mild atrophy of seminal tubules found in a few of the treated
males of the F2b offspring at termination was considered by the
authors of the study to have no toxicological significance. Lung
changes due to paraquat administration occurred mainly in females
receiving 150 ppm paraquat. One pulmonary adenoma was found in 1
female receiving 150 ppm paraquat. Death resulting from severe, acute,
or sub-acute lung damage was confined to females with litters of
weaning age, and to 3 F0 females which died during the first 2 weeks
of the study. There were dose-related increases in the incidence and
Table 5. Mutagenicity assays on paraquat
Test system Test object Concentration Purity Results Reference
used (%)
Ames test1 S. typhimurium 0.12, 0.6, 2.9, 99 Negative Anderson, 1977
TA98 14, 72, 361,
TA100 & 1807 µg/plate
TA1535 (dissolved in
TA1538 H2O)
Ames test1 S. typhimurium 0.4, 0.7, 3.6, 100 Negative, growth Shirasu et al., 1978
TA98 7, 36, 72, & inhibition at 72
TA100 360 µg/plate at 72 & 360
TA1535 (dissolved in µg/plate2
TA1538 H2O)
Host-mediated S. typhimurium 3.6 & 14 mg/kg 100 negative2 Shirasu et al., 1978
assay G 46 (host: male (2 equal doses
ICR mice) orally)
Rec-assay B. subtilis 14 - 361 100 negative2 Shirasu et al., 1978
µg/disk
Mouse L 5178 Y 23, 45, 90, 180, 99 ? Clay & Thomas, 1985
lymphoma mouse & 361 µg/ml
test1 lymphoma
cells
Table 5. (cont'd).
Test system Test object Concentration Purity Results Reference
used (%)
Clastogenic Human 90, 903, & 1807 99.6 positive at 2 Sheldon et al., 1985a
potential lymphocytes µg/ml highest levels
test1 in vitro (also cytotoxic)
Mouse Male & female 51.7 & 82.8 99.4 negative Sheldon et al., 1985b
micronucleus C 57/BL/6J/ mg/kg (single
test Alpk mice dose orally)
In vitro Chinese 0.9, 1.8, 9, 18, 99.4 positive (reduced Howard et al., 1985
sister hamster lung 90 & 177 µg/ml with metabolic
chromatid fibroblasts activation)
exchange1
Unscheduled Hepatocyte 1 nM - 10 mM 99.6 negative Trueman et al., 1985
DNA cultures (0.19 ng/ml -
synthesis from male 1.86 mg/ml)
Alderley Park
albino rats
1 Both with and without metabolic activation
2 Positive control compounds gave positive responses
severity of focal alveolar histiocytosis in the lungs of male and
female parents receiving 75 and 150 ppm paraquat. A mild perivascular
inflammation was observed in the lungs of F1b pups receiving 150 ppm
paraquat. No changes due to paraquat were seen in animals receiving
25 ppm paraquat. The authors concluded that paraquat had no effect on
reproductive performance or development of the reproductive organs of
Alderley Park rats when administered at dietary levels up to 150 ppm
over 3 generations (Lindsay et al., 1982).
Groups of 12 male and 24 female rats were fed diets containing
0, 30, or 100 ppm paraquat ion from 35 days of age. Three generations
bred from these animals received the same diets during the whole
period under test. Two litters were bred from each generation, and the
effects on growth, food intake, fertility, fecundity, neonatal
morbidity, and mortality were noted. No evidence was seen of damage to
germ-cell production or of structural or functional damage in the
animals. In this study, pregnant and young animals did not appear to
be more vulnerable to paraquat than did adults. However, the incidence
of renal hydropic degeneration in 3 - 4 week-old offspring was
slightly increased in the 100 ppm group (Fletcher et al., 1972).
In a 3-generation study with 2 litters per generation, groups of
30 male and 30 female Sprague-Dawley rats (F0 parental generation)
were given diets containing 0, 72, 145, or 290 ppm paraquat cation
from 5 weeks of age (13 weeks prior to mating to obtain the first
litter, F1a) until the end of the second lactation (lactation of
F1b litters). In the second generation (F1b), 30 males and 30
females per group were treated from immediately after weaning until
the end of the second lactation (lactation of F2b litters). In the
third generation (F2b), the same number of rats were treated
immediately after weaning for at least 13 weeks. In a teratology study
sub-group 5 pregnant females of the parental generation (F0) and 10
pregnant females of the second generation (F1b) were killed on day
20 of pregnancy and examined macroscopically. Fetuses were examined
for number, sex, weight, external and internal abnormalities, and
progress of ossification. Another subgroup was used as a postnatal
investigation group, where natural parturition of pregnant females was
permitted to occur. In this group the duration of gestation,
parturition conditions, the number of live and still-born pups, sex,
and external abnormalities were recorded. Live pups were investigated
until weaning. Five male and 5 female rats per group of the first
(F0) and second (F1b) generations and 10 male and 10 female rats
per group of the F2b litters were subjected to histopathological
examination of approximately 25 tissues.
In the parental generation there were significant increases in
mortality and clinical signs attributable to paraquat (asthmatoid
wheezing) in several rats of the 290 ppm group of each generation from
the early stage of the dosing period. There were 29 treatment-related
deaths/moribund animals, all from the 290 ppm dietary level (5 among
F1b animals and 24 among F2b animals), but only 4 deaths among
control rats (1 among F0 female rats and 2 among F1b female rats
at parturition and 1 among F2b rats during the dosing period).
Histopathological examination of these dead or moribund F2b rats
showed, in some cases, hyperplasia of the alveolar epithelium and, in
most cases, diffuse thickening and fibrosis of the alveolar walls.
There was a decrease in body-weight gain in both male and female rats
of the F0 and F2b generations at 290 ppm during the early stage of
the dosing period. Body-weight gain was also reduced in F1b females
at 290 ppm during the gestation and lactation periods. Reductions in
food consumption and efficiency of food utilisation were seen in F0
and F2b females. There was a significant decrease in water
consumption in F0 and F1b females during lactation. No effect of
the compound was observed on the reproductive performance of parental
rats. Macroscopic examination revealed an apparently higher incidence
of white spots in the lungs of both male and female rats of the
290 ppm group in all 3 generations. Treatment-related changes of the
lung were confirmed by histopathology in rats of each generation.
These lesions were dose-dependent and included zonal thickening and
fibrosis of the alveolar walls, zonal atelectasis, and accumulation of
foam cells. There were no treatment-related changes in organ weights.
A treatment-related statistically-significant reduction in the
lactation index was found in F1a and F1b litters of the 290 ppm
group. A statistically-significant reduction in the lactation index
was also observed in F2a litters of the 290 ppm group, but it was
not clear to the authors of the report whether this change was
attributable to treatment. There were no statistically-significant
differences in lactation index between other treatment groups and
controls nor in the number of still-births and live births, sex
ratios, or viability indexes in any of the treated groups of both
generations, when compared to controls. The prolonged duration of
gestation in F0 rats of the parental group at 145 and 290 ppm was
considered by the authors of the report to be accidental. The
teratology phase showed a statistically-significant delay in
ossification in F1b fetuses from F0 parents treated with 290 ppm
paraquat and in F2b fetuses from F1b parents of all treated
groups. It was not clear to the authors of the report whether the
retarded growth was due to the treatment. There was a treatment-
related statistically-significant higher incidence of female
pups with retarded opening of the vagina in both F1b and F2b
litters at 290 ppm. There was a statistically-significant decrease in
body weight in male, but not in female, fetuses at 72 and 290 ppm.
There were no statistically-significant differences between fetuses
from treated rats and control fetuses in the number of corpora lutea
or implantations, percentage of implantations, number of dead or live
fetuses, sex ratios, or placental weights. No external or internal
malformations were detected in fetuses of any treatment group.
The authors of the report concluded that there was no evidence
suggesting that paraquat was teratogenic and that the only treatment-
related change which was enhanced by treatment of successive
generations of Sprague-Dawley rats with paraquat was "an increase in
death" at 290 ppm. A no-effect level for paraquat was not found in
this study due to delayed ossification in F2b fetuses in all treated
groups (Suzuki et al., 1983).
Rabbits
Forty rabbits received paraquat i.p. at total dosages of 2 to
100 mg/kg b.w. in 1 to 5 separate administrations. Multinuclear giant
cells were found in testicular tubules of 7/20 rabbits receiving
50 mg/kg b.w. or more paraquat (Butler & Kleinerman, 1971). However,
it has been reported that, when paraquat was orally administered at
4 mg/kg b.w. to male rats for 60 days and testes were examined, there
were no significant deviations in the spermatozoa count or motility,
nor were there any biochemical changes in the several enzymes of
testes homogenates. The histoenzyme activity of lactate dehydrogenase,
succinate dehydrogenase, DPN-diaphorase, alkaline phosphatase, and
acid phosphatase in the treated animals did not differ from those of
the controls, nor did quantitative and qualitative histological
examination of the testicular tubule cells reveal any abnormalities
(WHO, 1984).
Special studies on skin irritation
The effects of paraquat on the skin have been reviewed by WHO
(1984). Paraquat can provoke local irritation of the skin and eyes.
Clark et al. (1966) found skin irritation in rabbits only when
paraquat was applied beneath occlusive dressings in aqueous solutions
(total doses 1.56, 5.0, and 6.25 mg ion/kg b.w.). In mice and rats,
the application of solutions of 5 - 20g paraquat/litre in single and
21-day repeated dermal toxicity tests provoked dose-related toxic
dermatitis with erythema, oedema, desquamation, and necrosis (Bainova,
1969). Doses from 1.56 to 50 mg/kg, in repeated 20-day studies using
the occlusive technique (McElligott, 1972), resulted in local erythema
and scab formation. The histological changes consisted of
parakeratosis and occasional intra-epidermal pustules. A delayed
skin-irritant action of the herbicide was reported by Fodri et al.
(1977) in guinea pig studies.
Acute toxicity
The LD50 and LC50 values for paraquat in various species are
given in Table 6.
Table 6. Acute toxicity of paraquat in various species
LD50 LC50
Species Route Sex (mg/kg b.w.) (mg/l) Reference
Mouse oral M 260 -- Shirasu & Takahashi, 1977
F 210 --
i.p. M & F 29-30 -- Shirasu & Takahashi, 1977
Bus et al., 1975a
i.v. -- 50 -- Ecker et al., 1975
s.c. M 30 -- Shirasu & Takahashi, 1977
F 27 --
dermal -- 62 -- Bainova, 1971
Rat oral M 161 -- Shirasu & Takahashi, 1977
F 187 --
M 110 -- Kimbrough & Gaines, 1970
F 100 --
-- 126 -- Murray & Gibson, 1972
-- 200 -- Howe & Wright, 1965
i.p. M 18 -- Shirasu & Takahashi, 1977
F 19 --
F 19 -- Clark et al., 1966
s.c. M 19 -- Shirasu & Takahashi, 1977
F 23 --
-- 22 -- Makovskii, 1972
dermal M 90 -- Kimbrough & Gaines, 1970
F 80 --
-- 350 -- Makovskii, 1972
Table 6. (cont'd).
LD50 LC50
Species Route Sex (mg/kg b.w.) (mg/l) Reference
inhalation M & F -- 10 Bainova & Vulcheva, 1972
-- -- 1 Gage, 1968
-- -- 6 Makovskii, 1972
Guinea oral M 30 -- Clark et al., 1966
pig
-- 40-80 -- Howe & Wright, 1965
-- 22 -- Murray & Gibson, 1972
-- 42 -- Makovskii, 1972
i.p. F 3 -- Clark et al., 1966
s.c. -- 5 -- Makovskii, 1972
dermal -- 319 -- Makovskii, 1972
inhalation -- -- 4 Makovskii, 1972
Cat oral F 35 -- Clark et al., 1966
-- 40-50 -- Howe & Wright, 1965
Hen oral -- 300-380 -- Howe & Wright, 1965
-- 262 -- Clark et al., 1966
Turkey oral -- 250-280 -- Smalley, 1973
i.p. -- 100 -- Smalley, 1973
i.v. -- 20 -- Smalley, 1973
dermal -- 375 -- Smalley, 1973
Monkey oral -- 50 -- Murray & Gibson, 1972
Sheep oral -- 50-75 -- Howe & Wright, 1965
Cow oral -- 50-75 -- Howe & Wright, 1965
Following a single high dose of paraquat to animals, the earliest
ultrastructural changes were observed in the Type I alveolar
epithelial cells, approximately 4 - 6 hours after treatment, and were
usually characterised by cellular and mitochondrial swelling,
increased numbers of mitochondria, and the appearance of dark granules
in the cytoplasm. When a high dose was given (equal to approximately
the LD50 or greater), the lesions in the Type I cells often
progressed to the point of complete cellular disintegration, leaving
areas of exposed basement membrane (Kimbrough & Gaines, 1970;
Smith et al., 1973; Smith & Heath, 1974; Vijeyaratnam & Corrin,
1971; Klika et al., 1980). In contrast to the effects on Type I
pneumocytes, however, the capillary endothelial cells were remarkably
resistant to the toxic effects of paraquat (Sykes et al., 1977).
Ultrastructural lesions in the alveolar Type II pneumocytes were
also observed shortly after single-dose paraquat exposure, although,
generally, these lesions were not apparent until after the first
lesions were seen in the Type I cells (Kimbrough & Gaines, 1970).
Swollen mitochondria and damage to the lamellar bodies usually
occurred between 8 and 24 hours after a high dose of paraquat
(Robertson, 1973; Robertson et al., 1976). Progressive deterioration
of the Type II cells continued, resulting in completely denuded
alveolar basement membranes and debris-filled alveolar spaces
(Vijeyaratnam & Corrin, 1971). Infiltration and proliferation of
fibroblasts may produce fibrosis that obliterated the alveolar
structure (Smith & Heath, 1974).
Vijeyaratnam & Corrin (1971) observed that less-severely affected
parts of the lung appeared to undergo epithelial regeneration 7 - 14
days after a single dose of paraquat. Electron microscopic examination
revealed the alveoli to be lined with cuboidal epithelial cells that
closely resembled Type II pneumocytes, except for a general lack of
lamellar bodies. Similar phenomena have also been noted by other
investigators who administered paraquat in the diet (Kimbrough &
Linder, 1973) or as repetitive i.p. administrations (Smith et al.,
1974). Thus, in animals where the paraquat dose was sufficient to kill
only the Type I pneumocytes, the surviving Type II cells repaired the
damaged epithelium by proliferating and subsequently differentiating
into Type I epithelial cells. Inhaled paraquat in aerosol produced
initial necrosis of the epithelia and Type II pbeumocyte hyperplasia,
fibroblast proliferation, and increased synthesis of collagen in mice
(Popenoe, 1979).
The pathogenesis of the paraquat lung lesion has been well
characterised, and has been reviewed by Smith & Heath (1976). The
acute pulmonary toxicity of paraquat in animals has been described as
occuring in 2 phases. In the initial "destructive" phase, alveolar
epithelial cells were extensively damaged and their subsequent
disintegration often resulted in a completely denuded alveolar
basement membrane. Pulmonary oedema was also a characteristic of the
destructive phase, and was frequently of sufficient severity to result
in the death of the animals. Animals surviving the initial destructive
phase, which occurred in the first 1 - 4 days after acute paraquat
overexposure, progressed to what has been termed the "proliferative"
phase. In this phase, the lung was infiltrated with prolifroblastic
cells that rapidly differentiated into fibroblasts which, in some
cases, progressed to fibrosis. The histopathological outcome of the
second phase may be influenced by the treatment regimen, however.
Administration of repeated low doses of paraquat, which less-severely
damaged the alveolar epithelial cells, was also able to induce a
hyperplasia of the Type II cells. This response may represent an
attempt by the lung to repair the damaged epithelium (WHO, 1984).
When rabbits were injected i.p. with total doses of paraquat from
2 - 100 mg/kg b.w., thymic atrophy was observed, but most lungs showed
only occasional and small histological deviations that were poorly
correlated with the clinical signs of paraquat intoxication. These
results confirmed the resistance of the rabbit to paraquat-induced
lung lesions (Butler & Kleinerman, 1971).
According to Murray & Gibson (1972) and Hundsdorfer & Rose
(1980), guinea pigs treated with paraquat either orally or s.c. did
not develop the same type of progressive pulmonary fibrosis as
paraquat-intoxicated rats. In hamsters, a single administration of
paraquat did not induce lung damage, but prolonged exposure resulted
in lung fibrosis (Butler, 1975).
In conclusion, from lung toxicity studies, a characteristic
dose-related pulmonary fibrosis can be induced in rats, mice, dogs,
and monkeys, but not in rabbits, guinea pigs, or hamsters.
In paraquat toxicity, kidney damage often precedes signs of
respiratory distress (Clark et al., 1966; Butler & Kleinerman, 1971;
Murray & Gibson, 1972). Paraquat is excreted primarily via the urine
and the concentrations of the herbicide in the kidneys are relatively
high (see Table 1). Gross pathological and histological examination of
paraquat-poisoned rats, guinea pigs, rabbits, and dogs revealed
vacuolation of the convoluted renal tubules and proximal tubular
necrosis (Murray & Gibson, 1972). The nephrotoxicity caused by
paraquat is pronounced and appears to be restricted to the proximal
nephron (Ecker et al., 1975; Gibson & Cagen, 1977; Lock & Ishmael,
1979; Purser & Rose, 1979). The degeneration of the proximal tubular
cells has also been confirmed by electron-optical studies (Fowler &
Brooks, 1971; Marek et al., 1981).
In contrast with lung and kidney damage, liver damage in
experimental animals has not been severe and serum enzyme activities
(SGOT, SGPT, LAP) only increased when large amounts of paraquat were
given (Girl et al., 1979). Recently, electron microscopic
examination of the liver of paraquat-treated rats showed early,
localised changes (degranualtion of the RER, proliferation of the SER,
and mitochondrial swelling) in hepatocytes within 2 layers around the
central vein (Matsumori et al., 1984).
Short-term studies
Mice
Groups of 20 male and 20 female ICR-CRJ SPF mice were maintained
on diets containing 0, 7.2, 22, 72, or 217 ppm paraquat cation for 13
weeks. At the 217 ppm dietary level 2 female mice died from pulmonary
damage. Both males and females in this group showed significantly
reduced body-weight gain and a slight reduction in efficiency of food
utilisation. Food intake and water intake were not affected. No
abnormalities considered related to paraquat treatment were seen
during haematological, blood biochemistry, or urine analysis. A few
statistically-significant changes in absolute and relative organ
weights were seen at termination, mainly in males and females of the
217 ppm group. However, only an increase in lung weight of females in
the 217 ppm group was reported by the authors of the study to coincide
with histopathological changes of the same organ, namely eosinophilic
swelling of the alveolar epithelium walls which was observed in both
sexes at this dietary level. The no-effect level in this study with
respect to pulmonary damage and other parameters was 72 ppm, equal to
12 (males) and 14 (females) mg/kg b.w./day (Malta et al., 1980a).
Rats
Groups of 20 male and 20 female Fischer 344 rats were maintained
on diets containing 0, 7.2, 22, 72, or 217 ppm paraquat cation for 13
weeks. During the study there were no deaths. Body-weight gain was
reduced markedly in both sexes at the 217 ppm dietary level, at which
level food consumption, efficiency of food utilization, and water
consumption were also reduced. Histopathological examination revealed
swelling of alveolar epithelium cells in males and increased deposits
of brown pigment in the spleen of females at the 217 ppm dietary
inclusing level. No abnormalities considered attributable to paraquat
administration were observed during heamatological, blood
biochemistry, urine, organ weight, or gross necropsy investigations.
The no-effect level in this study with respect to lung lesions and
other parameters was 72 ppm, equal to 6.5 (males) and 7.1 (females)
mg/kg b.w./day (Malta et al., 1980b).
Dogs
Groups of beagle dogs, 3 males and 3 females per group, received
diets containing 0, 7, 20, 60, or 120 ppm paraquat cation for 13
weeks. Two males and 2 females in the 120 ppm group showed marked
paraquat toxicity and were killed in extremis between days 16 and
23, having shown marked dyspnoea and body-weight loss. Both surviving
dogs at 120 ppm also showed body-weight loss. A slight overall
reduction in body-weight gain among the females of the other treatment
groups was not considered by the authors of the study to be
treatment-related. Lung weights were increased in all animals in the
120 ppm group and in 2 animals from the 60 ppm group. All other organ
weights were in the normal range. Distinct gross and histological
treatment-related lung lesions were seen in all dogs in the 60 and
120 ppm groups. Minor renal lesions (swelling of the cortical tubules)
were also found histologically in a few of these animals. There were
no discernible gross or histological treatment-related pulmonary
lesions in the dogs of the 7 or 20 ppm groups. The focal pulmonary
lesions in these animals were of a type and incidence similar to those
found in the controls. Microscopic examination of 34 other tissues
from each animal showed no treatment-related changes. There were no
treatment-related effects on food intake except for 1 surviving
high-dose female which showed a loss of appetite from week 8 onward.
There were no distinct treatment-related changes in any of the
haematological (RBC counts, mean cell volume, haemoglobin, total and
differential white cell counts, platelets, prothrombin, and partial
thromboplastin time), biochemical (glucose, cholesterol, blood urea
nitrogen, bilirubin, total and partial protein, GOT, GPT, ALP, and
CPK), or urinary parameters examined. Slight haemoconcentration was
seen in 1 high-dose dog at termination. The no-effect level in this
study after administration of paraquat for 13 weeks to beagle dogs, on
the basis of lung and kidney lesions, was considered to be 20 ppm,
equal to 0.57 mg/kg b.w./day (Sheppard, 1981).
Long-term studies
See also under "Special studies on carcinogenicity".
Mice
Groups of 60 male and 60 female JCL:ICR mice were maintained on
diets containing 0, 1.4, 7.2, 22, or 72 ppm paraquat cation for 104
weeks and then killed and examined. Further groups of 10 males and 10
females receiving the same diets were sacrificed after 26 weeks or 52
weeks of treatment. Animals in each group, including the control
groups, showed approximately 60 - 70% mortality at the end of the
study. Haematological changes, attributed by the authors of the study
to the administration of paraquat, included reduced erythrocytes,
hematocrit, and haemoglobin at the 72 ppm level in both sexes. Total
serum protein was decreased and blood glucose increased at the 72 ppm
dietary level in both sexes. Various rumours, mostly malignant, were
observed in all groups in this study, the main types being lung
adenocarcinoma in males and leukaemia in females. However, no tumour
type showed a higher incidence in the treated groups than in controls
and no correlations between tumour incidence and concentration of the
test substance were observed. No effects attributable to paraquat were
observed in absolute or relative organ weights, urinalysis,
body-weight gain, food consumption, efficiency of food utilization, or
water intake. Based on the haematological and blood biochemistry
changes observed, the no-effect level for paraquat after 104 weeks of
administration to JCL:ICR mice in this study was 22 ppm (as paraquat
cation), equal to 2.8 mg/kg b.w./day (Toyoshima et al., 1982a).
Rats
Groups of 50 male and 50 female JCL:Wistar rats were maintained
on diets containing 0, 4.3, 22, 72, or 217 ppm paraquat cation for 104
weeks and then killed and examined. Further groups of 6 males and 6
females receiving the same diets were sacrificed after 26 or 52 weeks
of treatment. There was 38 - 66% mortality in all groups at the end of
the study. The distribution of mortality and of abnormalities were not
significantly affected by treatment. Females in the 217 ppm group
showed a transient tendency to lower body-weight gain, compared to
controls, at weeks 34, 42 - 48, and 54. Food consumption, efficiency
of food utilization, and water consumption were not affected by
paraquat administration. Haematological changes attributed by the
authors of the study to paraquat administration at the 217 ppm level
included reduced erythrocytes and haemoglobin in both sexes and
decreased haematocrits and increased reticulocytes in males after 26
weeks. Total serum protein was slightly but constantly decreased at
the 217 ppm level in both sexes.
During histopathological examination of approximately 20 tissues,
various rumours were observed in this study in all groups, the main
types being benign pituitary tumours in males and benign mammary
rumours in females. However, none of the tumours were present at a
significantly-higher incidence in the treated groups than in controls.
Body weight, food consumption, food efficiency, water intake,
leucocyte counts, platelet counts, prothrombin time, GOT, GPT,
alkaline phosphatase activity, blood glucose, blood urea nitrogen,
cholesterol, Na+, K+, C1-, creatine, and brain, serum, and
corpuscular cholinesterase activities, as well as ophthamological
examination indicated no significant effects attributable to paraquat
at any dose level. Based on the haematological and blood biochemistry
changes the no-effect level of paraquat after 104 weeks of
administration to JCL:Wister rats in this study was 72 ppm (as
paraquat cation) equal to 3.0 (males) and 3.7 (females) mg/kg b.w./day
(Toyoshima et al., 1982b).
Dogs
Groups of 6 male and 6 female beagle dogs received diets
containing 0, 15, 30, or 50 ppm paraquat cation for 1 year. During the
study there were no deaths. No effects due to paraquat were observed
on body weight. The reduced food consumption of 1 male and 1 female
dog, both in the 50 ppm group, was considered by the authors of the
study to be treatment-related. There was clinical evidence of
respiratory dysfunction (hyperpnoea) in some dogs fed 50 ppm paraquat.
Mean lung weights of male and female dogs fed 50 ppm paraquat were 35
and 60% higher than those of controls, respectively. Histopathological
examination of the lungs showed a statistically-significant increase
in the incidence of chronic pneumonitis in both sexes at the 30 and
50 ppm dietary levels when compared to controls. This lesion consisted
of interstitial fibrosis, alveolar epithelialization, and mononuclear
cell infiltration. No other toxicologically-significant treatment-
related effects were seen during clinical observations, haemotological
or biochemical investigations, or during gross and microscopic
examination of approximately 40 tissues from each animal at
termination. On the basis of the pulmonary changes, the authors of
this study concluded that the dietary no-effect level for paraquat in
dogs over 1 year of treatment was 15 ppm, equal to 0.45 (males) and
0.48 (females) mg/kg b.w./day (Kalinowski et al., 1983).
Observations in humans
Information on the effects of paraquat in humans has been
obtained from occupational exposure studies (epidemiological data and
case reports), descriptions of accidental or suicidal poisonings, and
volunteer studies. These data have been extensively reviewed by WHO
(1984).
In 1965, a study was carried out on a team of 6 sprayers, and in
1967 on 4 teams in Malaysian rubber plantations, to estimate the
efficacy of individual protective measures. The operators used a spray
dilution containing paraquat at 0.5 g/litre for 12 weeks. Attention
was paid to personal hygiene. Each man was given a thorough physical
examination, and urine samples were taken before spraying began and at
weekly intervals throughout the study. Chest X-rays were taken before
the study started and at the end of the 6th and 12th weeks. In the 2
studies, a total of 528 urine samples were examined. Paraquat was
found on 131 occasions (78/134 and 53/394 in the 2 studies,
respectively), the maximum concentration detected being 0.32 mg/litre
in the first study and 0.15 mg/litre in the second. Average urine
levels of paraquat of 0.04 mg/litre were found in the 1965 study and
of 0.006 mg/litre in the 1967 study. After spraying ceased, these
levels declined steadily to become undetectable within a week, with 1
exception. Both trials showed that about half of the men had suffered
mild irritation of the skin and eyes, but had recovered rapidly with
treatment. Two cases of scrotal dermatitis occurred in workers wearing
trousers that were continuously soaked by the spray solution. There
were also 2 cases of epistaxis. All chest radiographs were normal
(Swan, 1969).
Studies performed over a period of several years on 296 Trinidad
sugar estate workers drew attention to nail damage that ranged in
severity from localized discoloration to nail loss following gross
contamination with paraquat at 1 - 2 g/litre. The typical distribution
of the lesions, affecting the index, middle, and ring fingers of the
working hand, suggested that they had occurred through leakage from
the knapsack sprayer and inadequate personal hygiene. Apart from 2
cases of contact dermatitis of the hands, no skin, eye, or nose
irritation was reported, nor were there any systemic effects (Hearn &
Keir, 1971).
Similar data were obtained on several groups of workers spraying
paraquat as an herbicide and dessicant in cotton fields during the hot
season. These workers were exposed to paraquat aerosol concentrations
of 0.13 - 0.55 mg/m3 air. Dermal exposure was low, not more than
0.05 - 0.08 mg paraquat on the hands and face. There were no
complaints, nor did the clinical and laboratory examinations of the
workers demonstrate any significant deviations from the matched
control groups (Makovskii, 1972).
In the USA, the exposure of field workers operating
tractor-mounted spray equipment in orchards was determined. About
4.6 litres of paraquat liquid concentrate (291 g/litre) was used in
935 litres of water per hour. In addition, exposure from yard and
garden applications were studied in volunteers using pressurized hand
dispensers containing paraquat solution (4.4 g/litre). Dermal
contamination was measured by adsorbent cellulose pads attached to the
worker's body or clothing, and by hand-rinsing in water in a
polyethylene bag. Special filter pads were used in the filter
cartridges of the respirators worn by the subjects under study. In
all, 230 dermal and respiratory exposure pads, 95 samples of
hand-rinse water, and 130 urine samples, collected during and
following spraying, were analysed, which involved 35 different
paraquat application situations. The exposure of field workers was
found to range from about 0.40 mg/hour (dermal) to less than
0.001 mg/hour (inhalation). As for individuals spraying yards or
gardens, exposure ranged from 0.29 mg/hour (dermal) to less than
0.001 mg/hour (inhalation). In almost all cases dermal exposure
affected the hands. The respiratory paraquat values were generally
below the sensitivity levels of the analytical method. No detectable
paraquat concentrations were found in the urine samples (lower limit
0.02 mg/litre). This study confirmed the general safety of paraquat
under correct conditions of use (Staiff et al., 1975).
The potential long-term hazard associated with the use of
paraquat has been studied by comparing the health conditions of 27
sprayers who had been exposed to paraquat for many months per year for
an average of 5.3 years with those of 2 unexposed control groups
consisting of 24 general workers and 23 factory workers. The workers
were given full clinical examinations; lung, liver and kidney function
tests were also carried out. There were a few skin lesions resulting
from poor spraying techniques and 1 case of eye injury. There were no
significant differences between exposed and control groups in any
health parameters measured, which led the authors to suggest that the
long-term use of paraquat is not associated with harmful effects on
health (Howard et al., 1981).
To evaluate the effects of protective equipment on occupational
human exposure to paraquat, a paraquat formulation (240 g/litre)
diluted 300 times by volume with water was sprayed for 2 hours on
weedy ground. During the spraying operations, the concentrations of
paraquat aerosol were 11 - 33 µg/m3 air. The total dermal exposure
was about 0.22 mg. No irritation of the eyes or the skin was reported.
The urine of the workers who wore gauze masks contained significant
amounts of paraquat 24 hours after spraying. The urine of the workers
who had worn a high-performance mask did not contain detectable levels
of paraquat. The authors discussed the need for protective equipment
to decrease skin contact with paraquat and to avoid aerosol inhalation
(Kawai & Yoshida, 1981).
Quantitative estimates of dermal and respiratory exposure of 26
plantation workers in Malaysia have shown a mean dermal dose of
1.1 mg/kg b.w./hour. The highest individual total exposure was
equivalent to 2.8 mg/kg b.w./hour; the mean respiratory exposure was
0.24 - 0.97 g/paraquat/m3 air, which is 1% or less of a TLV of
0.1 mg/m3 for respirable paraquat. Urine levels of paraquat were
generally below 0.05 mg/litre (Chester & Woollen, 1982).
A study was carried out on a group of 14 sprayers in Thailand
using conventional high-volume knapsack sprayers and low-volume
spinning-disc applicators with paraquat ion concentrations of
1.5 g/litre and 20 g/litre, respectively. Irritation of unprotected
skin was found, and this was severe (caustic burns on the feet) in
workers using high spray conentrations and spinning-disc applicators.
Urinary paraquat levels ranging from 0.73 - 10.2 mg/litre after 14
days of spraying were detected in unprotected men using both
concentrations, and there was evidence that urinary levels of paraquat
increased as the trial progressed. No evidence of systemic toxicity
was discovered among the sprayers undergoing clinical and radiographic
examination 1 week after spraying ended. The author concluded that
spray concentrations in hand-held equipment should not exceed 5 g
paraquat ion/litre (Howard, 1982).
After tomato spraying in the USA, the total body exposure to
paraquat was determined to be 169 mg/hour. The use of enclosed tractor
cabs or a high-clearance tractor reduced total body exposures to
27 mg/hour or 18 mg/hour, respectively. The authors reported that the
total body exposure of tractor sprayers working in 2 citrus locations
was proportional to the tank concentrations (paraquat dilutions of
0.7 g/litre and 1.1 g/litre were applied). Exposure levels of 13 and
28 mg/hour were found for workers using the lower and high
concentrations, respectively. In all situations studied, the
respiratory exposure was consistently a small fraction (< 0.1%) of
the total body exposure, which was primarily through the skin
(Wojeck et al., 1983).
Two groups of workers exposed to paraquat formulations were
examined. The first group of 18 workers, in England, consisted of
subjects exposed to dust and liquid paraquat formulations during a
37.5-hour working week, the mean length of exposure being 5 years. The
second group also consisted of 18 males, from Malaysia, exposed to
liquid concentrate formulations during a 42-hour working week, the
mean length of exposure being 2.3 years. Partly protective clothing
was worn. However, in Malaysia, no gloves, rubber aprons, or goggles
were used. The medical records and the dermatological examinations
revealed acute skin rashes, nail damage, epistaxis, blepharitis, and
delayed wound healing in 12 - 66% of these workers. Delayed caustic
effects were often found among the Malaysian formulation workers,
where low levels of safety and hygiene were apparent. Clinical
examination did not reveal any evidence of chronic contact dermatitis,
hyperkeratosis, or eczematous lesions (Howard, 1979).
Some studies designed to estimate dermal and inhalation exposure
to paraquat are summarized in Table 7. From the data reported it can
be seen that:
(a) the main route of exposure of agricultural workers to
paraquat is via the skin; respiratory exposure is
negligible.
(b) The worst case of exposure (of those examined) was via
knapsack spraying).
From 1956 - 1973, no deaths attributable to paraquat were
registered among agricultural workers in the USA, but in 1974 4 fatal
cases were associated with this herbicide. However, it is not clear
whether they were accidental, suicidal, or occupational (Hayes &
Vaughan, 1977).
Fitzgerald et al. (1978a) summarized the clinical findings and
pathological details concerning 13 accidents involving paraquat among
agricultural workers, 6 of which were fatal. In 5 of these cases,
swallowing was involved. Of the 6 fatalities studied, 3 swallowed
Gramoxone (a 20% solution of paraquat dichloride in water) after
sucking the outlet of a sprayer. In 1 non-fatal case, the man had
sucked out a nozzle containing diluted paraquat, while in another case
the man who had blown into the jet, to clear it, escaped with only
minor signs of poisoning. The use of a leaking sprayer by another
worker with severe extensive dermatitis probably resulted in fatal
absorption of paraquat through the damaged skin.
Table 7. Comparison of dermal and inhalation exposure to paraquat resulting from
various methods of application1.
Dermal Respiratory
Method of application exposure exposure Reference
(mg/hour) (mg/hour)
Hand-held knapsack 66 (0.45 - 1.3) × 10-3 Chester & Woollen,
(12.1 - 169.8) 1982
Vehicle mounted 0.4 (0 - 2) × 10-3 Staiff et al., 1975
(0.1 - 3.4)
Aerial Chester & Ward, 1981
Flagman 0.1 - 2.4 (0 - 47) × 10-3
Pilot 0.5 - 0.1 (0 - 0.6) × 10-3
Mixer/loader 0.18 (1.3 - 1.5) × 10-3
1 From WHO, 1984
Several other cases of fatal poisoning resulting from dermal
absorption of paraquat have been reported. Jaros (1978) has described
how the use of concentrated solutions of paraquat (50 g/litre instead
of 5 g/litre), with an old leaking knapsack sprayer, resulted in
paraquat contamination of the neck, back, and legs of a worker. After
4 hours of work, the worker complained of a burning sensation on the
neck and scrotum. On admission to a hospital 6 days later, cough and
respiratory difficulties were recorded. Three days later the patient
died of renal and respiratory failure.
Severe skin damage, followed by death due to respiratory
insufficiency, occurred in a woman 8 weeks after initial contact with
paraquat. The toxic dermatitis started with scratches on the arms and
legs from the branches of fruit trees. The patient had often failed to
wear protective clothing or to shower after spraying. During the 4
weeks preceding her first admission to the hospital, she developed
ulcers and respiratory complaints combined with anorexia. Damaged and
broken skin was thus exposed to paraquat. A chest X-ray and needle
biopsy of the lung revealed pulmonary lesions. Seventeen days after
discharge from hospital, without a specific diagnosis, she was
re-admitted, and died 2 weeks later with progressive lung, hepatic,
and renal dysfunction (Newhouse et al., 1978).
The clinical and pathomorphological investigation of a patient
who died of hypoxia after repeated dermal exposure to paraquat
(28 g/litre) and diquat (29 g/litre) in a water-oil dilution has been
described recently. The worker had used a leaking sprayer. A
characteristic ulcer developed at the site of paraquat contact. There
was also lung damage (Levin et al., 1979).
Another reported fatal case of dermal poisoning with paraquat
occurred after prolonged contact with a concentrated formulation
following spillage from a bottle in the back trouser pocket
(Waight & Wheather, 1979).
Wohlfahrt (1982) discussed the factors related to severe paraquat
poisoning due to dermal absorption in tropical agriculture. Three
fatal incidents followed skin contamination; 1 victim used paraquat to
treat scabies infestation, and 1 used it to treat lice. In all cases,
the skin was blistered and ulcerated. The patients died of progressive
respiratory failure 4 - 7 days after the accidents. In 1 of these
cases, the presence of mouth and throat ulceration strongly suggested
that ingestion might also have occurred (Davies, 1982).
Local skin and nail effects of paraquat have been reviewed by WHO
(1984). Brief contact with liquid formulations, as well as repeated
exposure to dilute solutions, produced skin irritation, desquamation,
and, finally, necrosis at the site of contact (Ongom et al., 1974;
Binns, 1976; Newhouse et al., 1978; Waight & Wheather, 1979;
Levin et al., 1979, Horiuchi et al., 1980). Harmful dermal effects
have been reported among sprayers who worked without protective
clothes and with naked feet (Howard, 1982). The blistering and
ulceration of the skin were due to excessive contact and inadequate
hygiene. Horiuchi and Ando (1980) carried out patch testing on 60
patients with contact dermatitis due to Gramoxone. In 8 patients
(13.3%) positive allergic reactions were established. In another
survey with 52 persons, a positive photo-patch response was reported
in 11 patients. Nail damage may also occur after frequent exposure to
paraquat concentrations during the formulation of the herbicide or the
preparation of the working dilution (Howard, 1979).
There have been some reports (Malone et al., 1971; Mircev,
1976; Bismuth et al., 1982) of adverse effects as a result of
inhalation exposure to paraquat. However, inhalation of droplets in
normal paraquat spraying does not appear to represent a significant
health hazard (Howard, 1980), and the effects of occupational
inhalation have usually been limited to nose bleeds and nasal and
throat irritation (Swan, 1969; Howard, 1979).
Ocular damage may result from splashes of concentrated paraquat
that come into contact with the eye. Apart from irritation of the eye
and blepharitis, a week later more serious ocular damage may occur,
such as destruction of the bulbar and tarsal conjunctiva and of the
corneal epithelium. Anterior uveitis, conjunctival necrosis,
progressive keratitis with gross corneal opacity, and decreased visual
acuity may also occur (WHO, 1984).
It has been noted that when the recommended dilution rates were
correctly used, systemic effects of oral, inhalation, or dermal
exposure to paraquat have not been observed. Skin and eye irritation
have occurred only when protective measures were disregarded
(WHO, 1984).
In a volunteer study the percutaneous absorption of
14C-paraquat through the legs, hands, and forearms of 6 human
subjects was studied. The total dose absorbed in 5 days after a single
application of 0.64 mg paraquat dichloride was very low (0.3%) at all
sites of application (Wester et al., 1984).
A large number of cases of accidental or suicidal poisoning have
been reported since 1966, the earlier cases being mostly accidental
which apparently resulted from the habit of decanting the liquid
formulations into small unmarked or incorrectly labelled containers
such as beer, wine, or soft-drink bottles. An increasing ratio of
suicidal to accidental poisonings has been noted in recent years
(Fitzgerald et al., 1978b; Bramley & Hart, 1983). This change from
accidental to suicidal poisoning was considered to be reflected by
enhanced percentages of fatal cases, shorter survival times, and
higher tissue and body fluid levels (WHO, 1984). The vast majority of
cases of paraquat poisoning have been due to ingestion. A few cases of
fatal or non-fatal poisonings have been reported following either skin
contamination (McDonagh & Martin, 1970; Kimura et al., 1980) or skin
application in order to kill body lice (Ongom et al., 1974;
Binns, 1976). Symptoms of poisoning depend on the dose absorbed.
It is difficult to estimate the dose absorbed from case histories
since in many cases the patients spat out part of the paraquat
concentrate or vomited profusely after swallowing the herbicide. Some
patients have survived after apparently ingesting 10 - 20 g paraquat,
whereas some died after taking as little as 2.5 g paraquat. The
probability of survival after paraquat poisoning can be predicted from
plasma paraquat concentration at a given time after ingestion
(Proudfoot et al., 1979; Hart et al., 1984). The minimum lethal
dose of paraquat for human beings has been estimated to be about
35 mg/kg b.w. (Pederson et al., 1981; Bismuth et al., 1982). The
common factor of most, if not all, cases of paraquat poisoning is
damage to the lung. Cases of fatal paraquat poisoning have been
divided into 2 broad categories:
a) Cases of acute fulminant poisoning due to massive amounts of
paraquat absorbed, resulting in death within 1 to 5 days
from ingestion. Death is due to multi-organ failure
associated with damage to the lungs, kidneys, liver, brain,
and adrenals.
b) Cases of poisoning due to ingestion or absorption of smaller
doses of paraquat resulting in death 6 to several days
later. In these cases death is due primarily to lung and
kidney damage.
COMMENTS
A small amount of paraquat is rapidly absorbed by the gut of
rats, guinea pigs, dogs, monkeys, and man, most of the oral dose being
excreted as unchanged paraquat. Following administration by different
routes to animals, paraquat is rapidly distributed in most tissues,
the highest concentrations being found in the lungs and kidneys. The
compound accumulates slowly in the lung owing to uptake by an
energy-dependent process which is also responsible for the uptake of
putrescine. Saturation kinetics for the uptake of paraquat by the lung
have been shown to be similar in rats and man. Excretion of absorbed
paraquat is biphasic, owing to lung accumulation, and occurs largely
in the urine as unchanged paraquat, but also to a limited extent in
the bile. Biotransformation of absorbed paraquat is, in general,
remarkably poor in all species studied (rats, guinea pigs, dogs, hens,
pigs, goats, and sheep) although there is some controversy as to the
possibility and extent of its metabolism by the gut microflora.
Metabolism occurs via demethylation (monomethyl dipyridone ion) or
oxidation (paraquat pyridone ion and paraquat dipyridone ion).
The mechanism of paraquat toxicity has been investigated
extensively, but it has not yet been elucidated completely. The
available evidence indicates that paraquat toxicity is due to the
ability of the compound to undergo redox cycling in biological
systems, resulting in the production of superoxide anion radicals and
in the oxidation of cellular NADPH. These effects may lead to the
formation of other highly toxic oxygen species and to depletion of
important defense mechanisms, both events which are potentially
capable of switching on further pathological processes, resulting in
damage to Type I and Type II pneumocytes.
Two teratogenicity studies, 1 in mice and 1 in rats, showed
paraquat to be non-teratogenic at doses up to 10 mg/kg b.w./day.
Slight maternal toxicity was observed in mice and high maternal
toxicity and some fetal toxicity were observed in rats at 5 and
10 mg/kg b.w./day paraquat ion. In a teratology sub-group in a
3-generation reproduction study in rats, paraquat was not teratogenic,
but delays in ossification were found at all 3 dose levels tested
(72, 145, and 290 ppm paraquat cation).
In previous in vitro studies both positive and negative results
were obtained, with mutagenicity usually associated with high
cytotoxicity. Recent studies have shown paraquat to be non-mutagenic,
both in the presence and absence of metabolic activation, when tested
by the Ames test, mouse micronucleus test, unscheduled DNA synthesis
assay, rec-assay, and host-mediated assay. A mouse lymphoma cell test
was inconclusive. The herbicide was clastogenic to human lymphocytes
in vitro at cytotoxic doses and induced sister chromatid exchange in
Chinese hamster lung fibroblasts.
In 2 rat 3-generation reproduction studies paraquat had no
effects on the reproductive performance or development of the
reproductive organs of rats. In 1 of these studies a reduction of
lactation index was observed at 290 ppm paraquat cation.
The acute oral toxicity of paraquat is higher in guinea pigs,
monkeys, cattle, and man than in rats and birds. Confidence limits of
LD50 values are small. There are no significant differences between
the 2 sexes in the oral, s.c., or i.p. LD50 values of paraquat in
rats or mice. Paraquat induces characteristic dose-related fibrotic
changes in the lungs of mice, rats, dogs, and monkeys, but not in
rabbits, guinea pigs, or hamsters. The acute pulmonary toxicity of
paraquat in rats and man is biphasic. In the early, destructive phase,
the alveolar epithelial cells are extensively damaged. Death may occur
within a few days due to pulmonary oedema. In the later, proliferative
phase, fibroblasts and collagen accumulate in the lungs of surviving
animals and humans, possibly resulting in fibrosis.
In a short-term study in mice, treatment-related lung, body
weight, and organ-weight changes were found. Another short-term study
in rats indicated that paraquat was responsible for lung lesions and
other minor changes. In a short-term study in dogs using 3
animals/sex/group, paraquat-dependent lung and kidney lesions were
observed.
In a 1-year feeding study in dogs, paraquat caused lung changes
at the 2 highest dietary levels; the no-effect level in this study was
15 ppm (as paraquat cation), equal to 0.45 mg/kg b.w./day in males and
0.48 mg/kg b.w./day in females.
In 2 long-term feeding studies, 1 in mice and 1 in rats,
haematological and blood biochemical changes were observed in both
species. These changes have not been reported previously and were
considered to be of little toxicological significance.
A lifetime feeding study in mice showed no oncogenic potential
for paraquat, although a slightly higher incidence of lung tumours was
noted in animals of the high-dose group dying between 79 and 98 weeks
of treatment when compared with control mice. Based on renal lesions
observed in males, the no-effect level in this study was 12.5 ppm
(as paraquat cation), equal to 1.4 mg/kg b.w./day.
A long-term feeding study in F344 rats indicated that paraquat
administration for 104 weeks at 217 ppm was responsible for a
significant increase in the incidence of pulmonary adenomas in
females. This incidence (8.7%) was significantly higher than that
observed in historical control animals of the same laboratory (2.2%).
Paraquat was also cataractogenic in both male and female rats. Based
on the lung and eye changes the no-effect level in this study was
22 ppm (as paraquat cation), equal to 0.77 mg/kg b.w./day.
In another long-term study in F344 rats paraquat induced
proliferative benign lung lesions in females in the high-dose group,
which were considered neoplastic by 1 pathologist and non-neoplastic
by 2 others (the total incidence of pulmonary adenoma in females
ranged from 0/70 to 8/70). The reasons for the discrepancy were
unclear, owing to the lack in most cases of adequately detailed
histopathological descriptions of the lung lesions. The historical
incidence of lung adenoma in control female F344 rats in the
laboratory in which the study was conducted was reported to be 1.1%,
and the incidences reported in the literature are 1.4% for females and
1.9% or 0% for males. The results of these studies indicate a weak,
organ-specific, sex-related potential of paraquat to produce benign
proliferative changes in the lungs of female F344 rats.
The following observations were considered by the Joint Meeting:
a) paraquat was shown to be non-mutagenic in most in vitro
and, apparently, all in vivo tests;
b) the lung is the target organ for both acute and chronic
paraquat toxicity in rats;
c) only female F344 rats, but not male F344 rats, developed
treatment-related benign neoplastic lesions;
d) proliferative changes were observed in rats and not in mice;
and
e) paraquat selectively interferes with the uptake of
polyamines by pneumocytes.
Polyamines are endogenous substrates playing an important role in
cell division and growth. These observations suggest that the
potential of paraquat to induce lung cell proliferation may be
modulated by species-, sex-, and organ-dependent factors, such as
hormonal activity, cell growth, defense and repair mechanisms, etc. No
lung changes were observed at 24 ppm in any of the dose groups, and
significant increases in the incidence of lung adenocarcinomas were
not observed in any of the treated groups when compared to controls in
these studies.
Observations in man, including reports on accidental or suicidal
poisonings, confirm the type of acute and subacute toxicity observed
in some experimental animals. Death occurred after oral and, in some
cases, dermal absorption of high doses of paraquat. The organs
primarily involved are the lung and kidney and, to a lower extent, the
intestinal tract, liver, pancreas, adrenals, and CNS. The minimal
lethal dose of paraquat in man is estimated to be about 35 mg/kg b.w.
Skin, nail, and eye lesions were found in subjects with prolonged
occupational exposure to paraquat. Significant paraquat concentrations
(up to 10 mg/1) were detected in the urine of workers using high spray
concentrations, but not in protected workers. The use of protective
measures has proved to be effective in preventing lesions due to skin
contact.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
Mice: 13 ppm (as paraquat cation), corresponding to 17 ppm
(as paraquat dichloride) in the diet, equal to
1.9 mg/kg b.w./day for males and 38 ppm (as paraquat
cation, corresponding to 52 ppm (as paraquat
dichloride), equal to 5.9 mg/kg b.w./day for females.
Rats: a) 22 ppm (as paraquat cation), corresponding to 30 ppm
(as paraquat dichloride) in the diet, equal to
1.1 mg/kg b.w./day for males and 1.2 mg/kg b.w./day for
females (cataract).
b) 25 ppm (as paraquat cation), corresponding to 35 ppm
(as paraquat dichloride) in the diet, equal to
1.4 mg/kg b.w./day for males and 1.7 mg/kg b.w./day for
females (proliferative lung changes).
Dogs: 15 ppm (as paraquat cation), corresponding to 20 ppm
(as paraquat dichloride) in the diet, equal to
0.62 mg/kg b.w./day for males and 0.66 mg/kg b.w./day
for females.
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.004 mg/kg b.w. as paraquat cation (0 - 0.006 mg/kg b.w.
expressed as paraquat dichloride).
STUDIES WHICH WILL PROVIDE INFORMATION VALUABLE FOR THE CONTINUED
EVALUATION OF THE COMPOUND
1. Further observations in humans.
2. Studies on the mechanism of paraquat-induced proliferative
lung changes.
REFERENCES
Anderson, K.J., Leighty, E.G., & Takahashi, M.T. Evaluation of
1972 herbicides for possible mutagenic protperties.
J. Agric. Food Chem., 20: 649 - 656.
Anderson, D., McGregor, D.B., & Purchase, I.F.H. Dominant lethal
1976 studies with diquat and paraquat in male CD-1 mice.
Mutat. Res., 40: 349 - 358.
Anderson, D. Paraquat: Estimation of mutagenic potential in the
1977 Salmonella typhimurium mutagenicity assay. Unpublished
Report No. CTL/P/243 from ICI Central Toxicology Laboratory.
Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Ashby, R., Hepworth, P.L., Whinney, A.K., Brown, P.M., Whitney, J.C.,
1983 & Finn, J.P. Paraquat: combined toxicity and carcinogenicity
study in rats. Unpublished report No. 82/ILY217/328 from
Life Science Research, Stock, Essex, UK. Submitted to WHO by
Imperial Chemical Industries PLC, Haslemere, UK.
Autor, A.P. Reduction of paraquat toxicity by superoxide dismutase.
1974 Life Sci., 14: 1309 - 1319.
Autor, A. P., ed. Biochemical mechanisms of paraquat toxicity. New
1977 York, Academic Press.
Bainova, A. Experimental assessment of the effect of dipyridylium
1969 herbicides on the skin. Letopisi HEI, 9: 25 - 30
(in Bulgarian).
Bainova, A. Determination of the zones of acute and chronic inhalatory
1971 toxicity after exposure to dipyridylium herbicides Gramoxone
and Reglone. Letopisi HEI, 28: 59 - 62 (in Bulgarian).
Bainova, A. & Vulcheva, V. Experimental substantiation of Gramoxone
1972 MAC in working areas. In: Works of Research Institute of
Hygiene & Labour Protection. Sofia, Medizine i Fizkultuna,
Vol. 23: 71 - 77.
Benigni, R., Bignami, M., Carera, A., Conti, G., Conti, R., Grebelli,
1979 R.A., Dogliotti, E., Gualandi, G., Novelleto, A., & Ortalia,
V.A. Mutational studies with diquat and paraquat in vitro.
Mutat. Res., 68: 183 - 193.
Bennett, P.N., Davies, D.S., & Hawksworth, G.M. In-vivo absorption
1976 studies with paraquat and diquat in the dog. Proceedings of
the British Pharmaceutical Society, 15 - 16 July, England,
284 pp.
Bignami, M. & Grebelli, R.A. A simplified method for the induction of
1979 8-azaguanine resistance in S. typhimurium. Toxicol.
Lett., 3: 169 - 175.
Binns, C.W. A deadly cure for lice. Papua New Guinea Med. J.,
1976 19: 105 107.
Bismuth, C., Garnier, R., Dally, S., Fournier, P.E., & Scherrmann,
1982 J.M. Prognosis and treatment of paraquat poisoning. A review
of 28 cases. J. Toxicol. Clin. Toxicol., 19: 461 - 474.
Bramley, A. & Hart, T.B. Paraquat poisoning in the United Kingdom.
1983 Hum. Toxicol., 2: 417.
Brigelius, R., Hashem, A., & Lengfelder, E. Paraquat-induced
1981 alterations of phospholipids and GSSG release in the
isolated perfused rat liver and the effect of SOD-active
copper complexes. Biochem. Pharmacol., 30: 349 - 354.
Bus, J.S., Aust, S.D., & Gibson, J.E. Superoxide and singlet oxygen
1974 catalysed lipid peroxidation as a possible mechanism for
paraquat toxicity. Biochem. Biophys. Res. Commun.,
58; 749 - 755.
Bus, J.S. & Gibson, J.E. Postnatal toxicity of chronically
1975 administered paraquat in mice and interactions with oxygen
and bromobenzene. Toxicol. Appl. Pharmacol.,
33: 461 - 470.
Bus, J.S., Aust, S.D., & Gibson, J.E. Lipid peroxidation as a possible
1975a mechanism for paraquat toxicity. Res. Commun. Chem.
Pathol. Pharmacol., 11: 31 - 38.
Bus, J.S., Preachie, M.M., Cagen, S.Z., Posner, H.S., Eliason, B.C.,
1975b Sharp, C.W., & Gibson, J.E. Fetal toxicity and distribution
of paraquat and diquat in mice and rats. Toxicol. Appl.
Pharmacol., 33: 450 - 460.
Bus, J.S., Cagen, S.Z., Olgaard, M., & Gibson, J.E. A mechanism of
1976 paraquat toxicity in mice and rats. Toxicol. Appl.
Pharmacol., 35: 501 - 513.
Bus, J.S. & Gibson, J.E. Paraquat: model for oxidant-initiated
1984 toxicity. Environ. Health Perspect., 55: 37 - 46.
Busey, W.M. An independent pathology review of the lung slides from a
1986 rat chronic toxicity/carcinogenicity study with paraquat.
Unpublished report from ICI Plant Protection, Ltd. Submitted
to WHO by Imperial Chemical Industries PLC, Haslemere,
Surrey, UK.
Butler, C. & Kleinerman, J. Paraquat in the rabbit. Br. J. Ind. Med.,
1971 28: 67 -71.
Butler, C. Pulmonary interstitial fibrosis from paraquat in the
1975 hamster. Arch. Pathol., 99: 503 - 507.
Calderbank, A. The bipyridylium herbicides. Effects on man. In
1968 Metcalf, R.L, ed. Advances in Pest Control Research. New
York, Interscience Publishers, Vol. 8; 224 pp.
Charles, J.M., Abou-Donia, M.B., & Menzel, D.B. Absorption of paraquat
1978 from the airways of perfused rat lung. Toxicology,
8: 59 - 67.
Chester, G. & Ward, R.J. Paraquat - occupational exposure and drift
1981 hazard evaluation during aerial application to cotton in
California, USA. Unpublished Report No. CTL/P/581 from ICI
Central Toxicology Laboratory. Submitted to WHO by Imperial
Chemical Industries PLC, Haslemere, Surrey, UK.
Chester, G. & Woollen, B.H. Studies of the occupational exposure of
1982 Malaysian plantation workers to paraquat.
Br. J. Ind. Med., 38: 23 - 33.
Clark, D.G., McElligott, T.F., & Hurst, E.W. The toxicity of paraquat.
1966 Br. J. Ind. Med., 23: 126 - 133.
Clay, P. & Thomas, M. Paraquat dichloride (technical liquor);
1985 assessment of mutagenic potential using L5178Y mouse
lymphoma cells. Unpublished Report No. CTL/P/1398 from ICI
Central Toxicology Laboratory. Submitted to WHO by Imperial
Chemical Industries PLC, Haslemere, Surrey, UK.
Conning, D.M., Fletcher, K., & Swan, A.A.B. Paraquat and related
1969 bipyridyliums. Br. Med. Bull., 25: 245 - 249.
Daniel, J.M. & Gage, J.C. Absorption and excretion of diquat and
1966 paraquat in rats. Br. J. Ind. Med., 23: 133 - 136.
Davies, R.E. Skin absorption of paraquat. Med. J. Aust., 7: 222.
1982
Dunbar, J.R., DeLucia, A.J., & Bryant, L.R. Glutathione status of
1984 isolated rabbit lungs. Effects of nitrofurantoin and
paraquat perfusion with normoxic and hyperoxic ventilation.
Biochem. Pharmacol., 33: 1343 - 1348.
Ecker, J.L., Hook, J.B., & Gibson, J.E. Nephrotoxicity of paraquat in
1975 mice. Toxicol. Appl. Pharmacol., 34: 178 - 186.
Edwards, M.J., Hemingway, R.J., Kinch, D.A., and Slape, P. Paraquat:
1974 residue and toxicology trial with cows fed a treated grass.
Unpublished report No. AR2465A(R) from ICI Plant Protection,
Ltd. Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Fairshter, R.D. Paraquat toxicity and lipid peroxidation. Arch.
1981 Intern. Med., 141: 1121 - 1123.
Fisher, H.K., Clements, J.A., & Wright, R.R. Enhancement of oxygen
1973 toxicity by the herbicide paraquat. Ann. Rev.
Respir. Dis., 107: 246 - 252.
Fisher, H.K., Clements, J.A. Tierney, D.F., & Wright, R.R. Pulmonary
1975 effects of paraquat in the first day after injection.
Am. J. Physiol., 228: 1217 - 1223.
Fitzgerald, G.R., Barniville, G., Flanagan, M., Silke, B., Carmody,
1978a M., & O'Dwyer, W.F. The changing pattern of paraquat
poisoning; an epidemiologic study. J. Irish Med. Assoc.,
71; 103 - 108.
Fitzgerald, G.R., Barniville, G., Black, J., Silke, B., Carmody, M., &
1978b O'Dwyer, W.F. Paraquat poisoning in agricultural workers.
J. Irish Med. Assoc., 71; 149 - 152.
Fletcher, K., Herring, C.M., & Robinson, V.M. Paraquat three-
1972 generation reproduction study in rats. Unpublished report
from ICI Industrial Hygiene Research Laboratories. Submitted
to WHO by Imperial Chemical Industries PLC, Haslemere,
Surrey, UK.
Fodri, Z., Sipos, K., & Berencsi, G. Study of the irritation and
1977 allergic effects of Gramoxone in guinea-pig.
Egeszsegtudomouny, 21: 244 - 249 (in Hungarian).
Fowler, B.A. & Brooks, R.E. Effects of the herbicide paraquat on the
1971 ultrastructure of mouse kidney. Am. J. Pathol.,
63: 505 - 512.
Fridovich, I. & Hassan, H.M. Paraquat and the exacerbation of oxygen
1979 toxicity. Trends Biochem. Sci., 4: 113 - 115.
Gage, J.C. The action of paraquat and diquat on the respiration of
1968 liver cell fractions. Biochem. J., 109: 757 - 761.
Gibson, J.E. & Cagen, S.Z. Paraquat-induced functional changes in
1977 kidney and liver. In: Autor, A.P., ed. Biochemical
Mechanisms of Paraquat Toxicity. New York Academic Press,
pp. 117 - 136.
Giri, S.N., Curry, D.L., Hollinger, M.A., & Freywald, N. Effect of
1979 paraquat on plasma enzymes, insulin, glucose and liver
glycogen in the rat. Environ. Res., 20; 300 - 308.
Giri, S.N. & Krishna, G.A. The effect of paraquat on guanylate cyclase
1980 activity in relation to morphological changes in guinea-pig
lungs. Lung, 157: 127 - 134.
Glass, M., Sutherland, M.W., Forman, H.J., & Fisher, A.B. Selenium
1985 deficiency potentiates paraquat-induced lipid peroxidation
in isolated perfused rat lung. J. Appl. Physiol.,
59: 619 - 622.
Greenberg, D.B., Lyons, A., & Last, J.A. Paraquat-induced changes in
1978 the rat of collagen biosynthesis by rat lung.
J. Lab. Clin. Med., 92: 1033 - 1042.
Hart, T.B., Nevitt, A., & Whitehead, A. A new statistical approach to
1984 the prognostic significance of plasma paraquat
concentrations. Lancet, November 24: 1222 - 1223.
Hassan, H.M. & Fridovich, I. Regulation of synthesis of superoxide
1977 dismutase in Escherichia coli. J. Biol. Chem.,
253: 7667 - 7672.
Hassan, H.M. & Fridovich, I. Superoxide radical and the enhancement of
1978 oxygen toxicity of paraquat in Escherichia coli.
J. Biol. Chem., 253: 8143 - 8148.
Hassan, H.M. & Fridovich, I. Paraquat and Escherichia coli. Mechanism
1979 of production of extracellular superoxide radical.
J. Biol. Chem., 254: 10846 - 10852.
Hassan, H.M. & Fridovich, I. Superoxide dismutases: detoxication by a
1980 free radical. In: Jakoby, W.B., ed. Enzymatic basis of
detoxication. New York, Academic Press, Vol. 1: 311 - 322.
Hawksworth, G.M., Bennett, P.N., & Davies, D.S. Kinetics of paraquat
1981 elimination in the dog. Toxicol. Appl. Pharmacol.,
57: 139 - 145.
Hayes, W.J. & Vaughn, W.K. Mortality from pesticides in the United
1977 States in 1973 and 1974. Toxicol. Appl. Pharmacol.,
42: 235 - 252.
Hearn, C.E.D. & Keir, W. Nail damage in spray operators exposed to
1971 paraquat. Br. J. Ind. Med., 28: 399 - 403.
Hemingway, R.J., Leahey, J.P., Griggs, R.E., & Davis, J.A. Paraquat
1972 metabolism in sheep. Unpublished report No. AR2359A from ICI
Plant Protection Division. Submitted to WHO by Imperial
Chemical Industries PLC, Haslemere, Surrey, UK.
Hemingway, R.J., Leahey, J.P., Griggs, R.E., & Davis, J.A. Paraquat
1974 metabolism in ruminants. 3rd International Congress of
Pesticide Chemistry, Helsinki.
Hemingway, R.J. & Oliver, C. Paraquat: a study of the metabolism and
1974 residues in hens and their eggs. Unpublished report No.
AR251A from ICI Plant Protection Division. Submitted to WHO
by Imperial Chemical Industries PLC, Haslemere, Surrey, UK.
Hendley, P., Leahey, J.P., & Spinks, C.A. Paraquat: Metabolism and
1976a residues in hens. Unpublished report No. AR2676A from ICI
Plant Protection Division. Submitted to WHO by Imperial
Chemical Industries PLC, Haslemere, Surrey, UK.
Hendley, P. Leahey, J.P., Spinks, C.A., Neale, D., & Carpenter, P.K.
1976b Paraquat metabolism and residues in goats. Unpublished
report No. AR2680A from ICI Plant Protection Division.
Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Hodge, M.C.E., Palmer, S, Weight, T.M., & Wilson, J. Paraquat
1978a dichloride: teratogenicity study in the mouse. Unpublished
report No. CTL/P/364 from ICI Central Toxicology Laboratory.
Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Hodge, M.C.E., Palmer, S., Weight, T.M., & Wilson, J. Paraquat
1978b dichloride: teratogenicity study in the rat. Unpublished
report No. CTL/P/365 from ICI Central Toxicology Laboratory.
Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Horiuchi, N. & Ando, S. Contact dermatitis due to pesticides for
1980 agricultural use. Nippon Hifuka Gakkai Zasshi, 90: 289
(in Japanese).
Horiuchi, N., Ando, S., & Kambe, Y. Dermatitis due to pesticides for
1980 agricultural use. Nippon Hifuka Gakkai Zasshi, 90: 27
(in Japanese).
Howard, J.K. A clinical survey of paraquat formulation workers. Br. J.
1979 Ind. Med., 36: 220 - 223.
Howard, J.K. Paraquat: a review of worker exposure in normal usage.
1980 J. Soc. Occup. Med., 30; 6 - 11.
Howard, J.K., Sahopathy, N.N., & Whitehead, P.A. A study of the health
1981 of Malaysian plantation workers with particular reference to
paraquat spraymen. Br. J. Ind. Med., 38: 110 - 116.
Howard, J.K. Paraquat spraying. Comparative risks from high and low
1982 volume application methods. In: Proceedings of the 10th
Asian Conference on Occupational Health, Singapore,
pp. 1 - 7.
Howard, C.A., Wildgoose, J., Clay, P., & Richardson, C.R. Paraquat
1985 dichloride: an in vitro sister chromatid exchange study in
Chinese hamster lung fibroblasts. Unpublished Report
No. CTL/P/1392 from ICI Central Toxicology Laboratory.
Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Howe, D.J.T. & Wright, N. The toxicity of paraquat and diquat. In:
1965 Proceedings of the 18th New Zealand Weed and Pest Control
Conference, pp. 105 - 114.
Hundsdorfer, S. & Rose, I. Lung changes produced by paraquat:
1980 respiratory distress model in animals. Ergeb. Exp. Med.,
35: 603 - 690 (in German).
Hussain, M.Z. & Bhatnagar, R.S. Involvement of superoxide in the
1979 paraquat-induced enhancement of lung collagen synthesis in
organ culture. Biochem. Biophys. Res. Commun.,
89: 71 - 76.
Ilett, K.I., Stripp, B., Menard, R.H., Reid, W.D., & Gillette, J.R.
1974 Studies on the mechanism of the lung toxicity of paraquat.
Comparison of tissue distribution and some biochemical
parameters in rats and rabbits. Toxicol. Appl. Pharmacol,
28: 216 - 226.
Ishmael, J. & Godley, M.J. Paraquat:. lifetime feeding study in rats.
1983 Histopathological examination of lungs. Unpublished Report
No. CTL/P/738 from ICI Central Toxicology Laboratory.
Submitted to WHO by imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Jaros, F. Acute percutaneous paraquat poisoning. Lancet 1: 275.
1978
Kalinowski, A.E., Doe, J.E., Chart, I.S., Gore, C.W., Godley, M.J.,
1983 Hollis, K., Robinson, M., & Woollen, B.H. Paraquat: One year
feeding study in dogs. Unpublished Report No. CTL/P/734 from
ICI Central Toxicology Laboratory. Submitted to WHO by
Imperial Chemical Industries PLC, Haslemere, Surrey, UK.
Kawai, M. & Yoshida, M. Exposure of spray operators to paraquat.
1981 Nippon Doshu Eisei Zasshi, 28: 353 - 359 (in Japanese).
Keeling, P.L., Pratt, I.S., Aldridge, W.N., & Smith, L.L. The
1981 enhancement of paraquat toxicity in rats by 85% oxygen:
lethality and cell-specific lung damage.
Br. J. Exp. Pathol., 62: 643 - 654.
Keeling, P.L., Smith, L.L., & Aldridge, W.N. The formation of mixed
1982 disulfides in rat lung following paraquat administration.
Biochem. Biophys. Acta, 716: 249 - 257.
Kehrer, J.P., Haschek, W.M., & Witschi, H. The influence of hyperoxia
1979 on the acute toxicity of paraquat and diquat. Drug Chem.
Toxicol., 2: 397 - 408.
Kimbrough, R.D. & Gaines, T.B. Toxicity of paraquat in rats and its
1970 effect on rat lungs. Toxicol. Appl. Pharmacol.,
17: 679 - 690.
Kimbrough, R.D. & Linder, E.W. The ultrastructure of the paraquat lung
1973 lesion in the rat. Environ. Res., 6: 265 - 273.
Kimura, M., Suzuki, E., & Ohnishi, H. A case of autopsy of an infant
1980 intoxicated by paraquat dichloride. Shoni Ka Rinsho,
33: 732 - 734 (in Japanese).
Klika, E., Kunc, L., Antalikova, K., & Kuncova, M. The morphology of
1980 the lung in the albino rat after paraquat administration.
Folia Morphol., 28: 188 - 191.
Kornburst, D.J. & Mavis, R.D. The effect of paraquat on microsomal
1980 lipid peroxidation in vitro and in vivo.
Toxicol. Appl. Pharmacol., 53: 323 - 332.
Kurisaki, E. & Sato, H. Toxicological studies on herbicides.
1979 Intracorporeal distribution of paraquat dichloride and
diquat dibromide in rats. Nippon Hoigaki Zasshi,
33: 656 (in Japanese).
Leahey, J.P., Hendley, P., & Spinks, C.A. Paraquat: Metabolism and
1976 residues in pigs using 14C-methyl labelled paraquat.
Unpublished report No. AR2694A from ICI Plant Protection
Division. Submitted to WHO by Imperial Chemical Industries
PLC, Haslemere, Surrey, UK.
Levin, P.J., Klaff, L.J., Rose, A.G., & Ferguson, A.D. Pulmonary
1979 effects of contact exposure to paraquat: a clinical and
experimental study. Thorax, 34: 150 - 160.
Levin, D.E., Hollstein, M., Christman, M.F., Schwiers, E.A., & Ames,
1982 B.N. A new Salmonella tester strain (TA 102) with AT base
pairs at the site of mutation detects oxidative mutagens.
Proc. Natl. Acad. Sci. USA, 79: 7445 - 7449.
Lindsay, S., Banham, P.B., Godley, M.J., Moreland, S., Wickramaratne,
1982 G.A., & Woollen, B.H. Paraquat: multigeneration reproduction
study in rats - three generations. Unpublished Report
No. CTL/P/719 from ICI Central Toxicology Laboratory.
Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Litchfield, M.H., Daniel, J.W., & Longshaw, S. The tissue distribution
1973 of the bipyridylium herbicides diquat and paraquat in rats
and mice. Toxicology, 1; 155 - 165.
Lock, E.A. & Ishmael, J. The acute toxic effects of paraquat and
1979 diquat on the rat kidney. Toxicol. Appl. Pharmacol.,
50: 67 - 76.
Maita, K., Saito, T., Tsuda, S., & Shirasu, Y. AT-5: sub-acute
1980a toxicity study in mouse. Unpublished report from the
Institute of Environmental Toxicology. Submitted to WHO by
Imperial Chemical Industries PLC, Haslemere, Surrey, UK.
Maita, K., Saito, T., Tsuda, S., & Shirasu, Y. AT-5: sub-acute
1980b toxicity study in rat. Unpublished report from the Institute
of Environmental Toxicology. Submitted to WHO by Imperial
Chemical Industries PLC, Haslemere, Surrey, UK.
Maita, K. Historical control incidence data in F344 rats. Unpublished
1986 report from Institute of Environmental Toxicology. Submitted
to WHO by Otsuka Chemical Co. Ltd., Osaka, Japan.
Makovskii, V.N. Toxicological and hygiene studies of bipyridylium
1972 herbicides diquat and paraquat. Referat, Vinnize, USSR
pp 1 - 23 (PhD degree thesis) (in Russian).
Maling, H.M., Saul, W., Williams, M.A., Brown, E.A.B., & Gillette,
1978 J.R. Reduced body clearance as the major mechanism of the
potentiation by ß-adrenergic agonists of paraquat lethality
in rats. Toxicol. Appl. Pharmacol., 43: 57 - 72.
Malone, J.D.G., Carmoday, M., & Keogh, B. Paraquat poisoning. A review
1971 of nineteen cases. J. Irish Med. Assoc., 64: 59 - 68.
Marek, J., Velenska, Z., & Haskovcova, I. Kidney changes in acute
1981 phase of paraquat intoxication in rats. Sb. Lek.,
83: 100 - 104 (in Czech.).
Matsumori, H., Matsumoto, T., & Ishikawa, H. Acute toxic effects of
1984 paraquat on ultrastructure of rat liver. Acta Pathol. Jpn,
34: 507-518.
McDonagh, B.J. & Martin, J. Paraquat poisoning in children. Arch. Dis.
1970 Child., 45: 425 - 427.
McElligot, T.F. The dermal toxicity of paraquat. Differences due to
1972 techniques of application. Toxicol. Appl. Pharmacol.,
21: 361 - 368.
Mircev, N. Acute intoxication with Gramoxone (paraquat). Vatreshni
1976 Bolesti, 16: 99 - 101 (in Bulgarian).
Montgomery, M.R. Interaction of paraquat with the pulmonary microsomal
1976 fatty acid desaturase system. Toxicol. Appl. Pharmacol.,
36: 543 - 554.
Montgomery, M.R. Paraquat toxicity and pulmonary superoxide dismutase;
1977 an enzymic deficiency of lung microsomes. Res. Commun.
Chem. Pathol. Pharmacol., 16: 155 - 158.
Montgomery, M.R. & Niewoehner, D.E. Oxidant-induced alterations in
1979 pulmonary microsomal mixed-function oxidation. Acute effects
of paraquat and ozone. J. Environ. Sci. Health,
13: 205 - 219.
Moody, C.S. & Hassan, H.M. Mutagenicity of oxygen free radicals.
1982 Proc. Natl. Acad. Sci., 79: 2855 - 2859.
Murray, R.E. & Gibson, J.E. A comparative study of paraquat
1972 intoxication in rats, guinea-pigs and monkeys.
Exp. Mol. Pathol., 17: 317 - 325.
Murray, R.E. & Gibson, J.E. Paraquat disposition in rats, guinea-pigs
1974 and monkeys. Toxicol. Appl. Pharmacol., 27: 283 - 291.
Newhouse, M., McEvoy, D., & Rosenthal, D. Percutaneous paraquat
1978 absorption. Arch. Dermatol., 114: 1516 - 1519.
Nicotera, T.M., Block, A.W., Gibas, Z., & Sandberg, A.A. Induction of
1985 superoxide dismutase, chromosomal aberrations and
sister-chromatid exchanges in Chinese hamster fibroblasts.
Mutat. Res., 151: 263 - 268.
Omaye, S.T., Reddy, K.A., & Cross, C.E. Enhanced lung toxicity of
1978 paraquat in selenium-deficient rats. Toxicol. Appl.
Pharmacol., 43: 237 - 247.
Ongom, V.L., Owor, R., & Tonusange, E.T. Paraquat (Gramoxone) used as
1974 a pediculocide. In: Bagshore, A.F., ed. The uses and abuses
of drugs and chemicals in tropical Africa. Nairobi, East
Africa Literature Bureau, pp. 229 - 233.
Parry, J.M. The induction of gene conversion in yeast by herbicide
1973 preparations. Mutat. Res., 21: 83 - 91.
Parry, J.M. The use of yeast for the detection of environmental
1977 mutagens using a fluctuation test. Mutat. Res.,
46: 165 - 176.
Pasi, A., Embree, J.W., Eisenlord, G.H., & Hine, C.H. Assessment of
1974 the mutagenic properties of diquat and paraquat in the
murine dominant lethal test. Mutat. Res., 26: 171 -175.
Pedersen, G., Pedersen, A., Gregersen, M., & Kampe, B. Paraquat
1981 poisoning. Ugeskr. Laeg., 143: 1202 - 1206 (in Danish).
Plant Protection Ltd. Data to support a revised acceptable daily
1972 intake and tolerance levels for paraquat. Unpublished report
from Plant Protection Ltd.
Popenoe, D. Effects of paraquat aerosol on mouse lung. Arch. Pathol.
1979 Lab. Med., 103: 331 - 334.
Proudfoot, A.T., Steward, M.S., Levitt, T., a Widdop, B. Paraquat
1979 poisoning: significance of plasma-paraquat concentrations.
Lancet, 2: 330-332.
Purser, D.A. & Rose, M.S. The toxicity and renal handling of paraquat
1979 in cynomolgus monkeys. Toxicology, 15: 31 - 41.
Reddy, K.A., Litov, R.E., & Omaye, S.T. Effect of pre-treatment with
1977 anti-inflammatory agents on paraquat toxicity in the rat.
Res. Commun. Chem. Pathol. Pharmacol., 17: 87 - 100.
Robertson, B. Paraquat poisoning as an experimental model of the
1973 idiopathic respiratory distress syndrome.
Bull. Phys. Pathol. Res., 9: 1433 - 1452.
Robertson, B., Grossmann, G., & Ivemark, B. The alveolar lining layer
1976 in experimental paraquat poisoning. Acta. Pathol.
Microbiol. Scand., Section A., 84: 40 - 46.
Rose, M.S., Smith, L.L., & Wyatt, I. Evidence of energy-dependent
1974 accumulation of paraquat in rat lung. Nature (Lond.),
252: 314 - 315.
Rose, M.S., Lock, E.A., & Smith, L.L. Biochem. Pharmacol.,
1976a 25: 419 - 423.
Rose, M.S., Smith, L.L., & Wyatt, I. The relevance of pentosephosphate
1976b pathway stimulation in rat lung to the mechanism of paraquat
toxicity. Biochem. Pharmacol., 25: 1763 - 1767.
Rose, M.S. & Smith, L.L. The relevance of paraquat accumulation by
1977 tissues. In: Autor, A.P., ed., Biochemical Mechanisms of
Paraquat Toxicity. New York, Academic Press, pp. 71 - 91.
Ross, W.E., Block, E.R., & Chang, R.Y. Paraquat-induced DNA damage in
1979 mammalian cells. Biochem. Biophys. Res. Commun.,
91: 1302 - 1308.
Rossouw, D.J., Chase, C.C., & Engelbrecht, F.M. The effect of paraquat
1984 on the in vitro activity of cytosol, mitochondrial, and
microsomal enzyme systems. S. Afr. Med. J., 65; 555 - 563.
Selman, M. Montano, M., Montfort, I., & Perez-Tamayo, R. The duration
1985 of the pulmonary paraquat toxicity-enhancement of O2 in
the rat. Exp. Mol. Pathol., 43: 388 - 396.
Selypes, A. & Paldy, A. The examination of the mutagenic effect of two
1978 pesticides: Krezonit E and Gramoxone. Proc. Hung. Ann.
Meet. Biochem., 18: 77- 78.
Sharp, C.W., Ottolenghi, A., & Posner, H.S. Correlation of paraquat
1972 toxicity with tissue concentration and weight loss of the
rat. Toxicol. Appl. Pharmacol., 22: 241 - 251.
Sheldon, T., Howard, C.A., Wildgoose, J., & Richardson, C.R. Paraquat
1985a dichloride: a cytogenetic study in human lymphocytes
in vitro. Unpublished Report No. CTL/P/1357 from ICI
Central Toxicology Laboratory. Submitted to WHO by Imperial
Chemical Industries PLC, Haslemere, Surrey, UK.
Sheldon, T., Richardson, C.R., Shaw, J., & Barber, G. An evaluation of
1985b paraquat dichloride (technical) in the mouse micronucleus
test. Unpublished Report No. CTL/P/1369 from ICI Central
Toxicology Laboratory. Submitted to WHO by Imperial Chemical
Industries PLC, Haslemere, Surrey, UK.
Sheppard, D.B. Paraquat thirteen week (dietary administration)
1981 toxicity study in beagles. Unpublished Report
No. 2481-72/111A from Hazelton Laboratories Europe Ltd.
Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, UK.
Shirasu, Y. & Takahashi, K. Acute toxicity of AT-5 in rat and mouse.
1977 Unpublished report from the Institute of Environmental
Toxicology. Submitted to WHO by Imperial Chemical Industries
PLC, Haslemere, Surrey, UK.
Shirasu, Y., Moriya, M., & Ohta, T. Mutagenicity testing on paraquat
1978 in microbial systems. Unpublished report from the Institute
of Environmental Toxicology. Submitted to WHO by Imperial
Chemical Industries PLC, Haslemere, Surrey, UK.
Shu, H., Talcott, R.E., Rice, S.A., & Wei, E.T. Lipid peroxidation and
1979 paraquat toxicity. Biochem. Pharmacol., 28: 327 - 331.
Sinow, J. & Wei, E. Ocular toxicity of paraquat. Bull. Environ.
1973 Contam. Toxicol., 9: 163 - 168.
Smalley, H.E. Toxicity and hazard of the herbicide paraquat in
1973 turkeys. Poultry Sci., 52: 1625 - 1629.
Smith, P., Heath, D., & Ray, J.M. The pathogenesis and structure of
1973 paraquat-induced pulmonary fibrosis in rats. J. Pathol.,
114: 57 - 67.
Smith, P. & Heath, D. The ultrastructure and time sequence of the
1974 early stages of paraquat lung in rats. J. Pathol.,
114: 177 - 184.
Smith, L.L., Wright, A., Wyatt, I., & Rose, M.S. Effective treatment
1974 for paraquat poisoning in rats and its relevance to
treatment of paraquat poisoning in man. Br. Med. J.,
4: 569 - 571.
Smith, P. & Heath, D. Paraquat. Clin. Rev. Toxicol., 4: 411 - 445.
1976
Smith, L.L., Rose, M.S., & Wyatt, I. The pathology and biochemistry of
1979 paraquat. In: Oxygen Free Radicals and Tissue Damage.
Amsterdam, North Holland, Excerpta Medica, pp. 321 - 341
(CIBA Foundatin Series 65 - new series).
Smith, L.L., Wyatt, I., & Rose, M.S. Factors affecting the efflux of
1981 paraquat from rat lung slices. Toxicology, 19: 197 - 207.
Smith, L.L. Paraquat toxicity. Phil. Trans. R. Soc. Lond. B.,
1985 311: 647 - 657.
Sotheran, M., Banham, P.B., Godley, M.J., Lindsay, S., Pratt, I.,
1981 Taylor, K., & Woollen, B.H. Paraquat; lifetime feeding study
in the mouse. Unpublished Report No. CTL/P/556 from ICI
Central Toxicology Laboratory. Submitted to WHO by Imperial
Chemical Industries PLC, Haslemere, Surrey, UK.
Spinks, C.A., Hendley, P., Leahey, J.P., & Carpenter, P.K. Paraquat:
1976 Metabolism and residues in pigs using 14C-ring labelled
paraquat. Unpublished report No. 2692A from ICI Plant
Protection Division. Submitted to WHO by Imperial Chemical
Industries PLC, Haslemere, Surrey, UK.
Staiff, D.C., Comer, S.W., Armstrong, J.F., & Wolfe, H.R. Exposure to
1975 the herbicide paraquat. Bull. Environ. Contam. Toxicol.,
14: 334 - 340.
Steffen, C. & Netter, K.J. On the mechanism of paraquat action on
1979 microsomal oxygen reduction and its relation to lipid
peroxidation. Toxicol. Appl. Pharmacol., 47: 593 - 602.
Steffen, C., Muliawan, H., & Kappus, H. Lack of in vivo lipid
1980 peroxidation in experimental paraquat poisoning.
Arch. Pharmacol., 310: 241 - 243.
Suzuki, T., Igarashi, N., & Tamura, K. Paraquat D.C.; Multiple
1983 generation study in rats. Unpublished Report No. R-015 from
Bozo Research Centre Co. Ltd. Submitted to WHO by Otsuka
Chemical Co., Osaka, Japan.
Swan, A.A.B. Exposure of spray operators to paraquat.
1969 Br. J. Ind. Med., 26: 665 - 671.
Sykes, B.J., Purchase, I.F.H., & Smith, L.L. Pulmonary ultrastructure
1977 after oral and intravenous dosage of paraquat to rats.
J. Pathol., 121: 233 - 241.
Talcott, R.E., Shu, H., & Wei, E.T. Dissociation of microsomal oxygen
1979 reduction and lipid peroxidation with the electron
acceptors, paraquat and menadione. Biochem. Pharmacol.,
26: 665 - 671.
Thompson, W.D. & Patrick, R.S. Collagen propyl hydroxylase levels in
1978 experimental paraquat poisoning. Br. J. Exp. Pathol.,
59: 288 - 291.
Toyoshima, S., Sato, R., Kashima, M., Sato, H., Sato, H., Sato, H.,
1982a Motoyama, M., Ichiba, J., & Kimura, R. AT-5: chronic
toxicity study result - 104 week dosing study in mouse.
Unpublished report from Nippon Experimental Medical Research
Co. Ltd. Submitted to WHO by Imperial Chemical Industries
PLC, Haslemere, Surrey, UK.
Toyoshima, S., Sato, R., Kashima, M., Sato, H., Sato, H., Sato, H.,
1982b Motoyama, M., Ishidawa, A., Ichiba, J. & Kimura, R. AT-5:
chronic toxicity study result - 104 week dosing study in
rat. Unpublished report from Nippon Experimental Medical
Research Co. Ltd. Submitted to WHO by Imperial Chemical
Industries PLC, Haslemere, Surrey, UK.
Trueman, R.W., Ashby, J., & Burlinson, B. Paraquat dichloride:
1985 assessment for the induction of unscheduled DNA synthesis in
primary rat hepatocyte cultures. Unpublished Report
No. CTL/P/1339 from ICI Central Toxicology Laboratory.
Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Trush, M.A., Mimnaugh, E.G., Ginsburg, E., & Gram, T.E. In vitro
1981 stimulation by paraquat of reactive oxygen-mediated lipid
peroxidation in rat lung microsomes. Toxicol. Appl.
Pharmacol., 60: 279 - 286.
Trush, M.A., Mimnaugh, E.G., Ginsburg, E., & Gram, T.E. Studies on the
1982 in vitro interaction of mitomycin C, nitrofurantoin, and
paraquat with pulmonary microsomes. Simulation of reactive
oxygen-dependent lipid peroxidation. Biochem. Pharmacol.,
31: 3335 - 3346.
Tweats, D.J. The effect of R (drug resistance) factors on the
1975 detection of mutagens by E. coli K 12. Brussels, Belgium,
Annual Euratom Report, Biological Sciences, 316 pp.
Veseley, D.L., Watson, B., & Levey, G.A. Activation of liver guanylate
1979 cyclase by paraquat: possible role of superoxide anion.
J. Pharmacol. Exp. Ther., 209: 162 - 164.
Vijeyaratnam, G.S. & Corrin, B. Experimental paraquat poisoning: A
1971 histological and electron-optical study of the changes in
the lung. J. Pathol., 103: 123 - 129.
Waddell, W.J. & Marlowe, C. Tissue and cellular disposition of
1980 paraquat in mice. Toxicol. Appl. Pharmacol.,
56: 127 - 140.
Waight, J.J.J. & Wheather, R.H. Fatal percutaneous paraquat poisoning.
1979 J. Am. Med. Assoc., 242: 472.
Walker, M., Dugard, P.H., & Scott, R.C. Absorption through human
1983 laboratory animal skins: in vitro comparison.
Acta Pharm. Suec., 20: 207 - 213.
Wester, R.C., Maibach, H.I., Bucks, D.A.W., & Aufrere, M.B. In vivo
1984 percutaneous absorption o[ paraquat from hand, leg, and
forearm of humans. J. Toxicol. Environ. Health,
14: 759 - 762.
WHO. Paraquat and diquat. WHO Environmental Health Criteria, No. 39.
1984 International Programme on Chemical Safety, World Health
Organization, Geneva.
Witschi, H.P., Kacew, S., Hirai, K., & Cote, M.G. In vivo oxidation of
1977 reduced nicotinamide-adenine dinucleotide phosphate by
paraquat and diquat in rat lung. Chem. Biol. Interactions,
19: 143 - 160.
Wohlfart, D.J. Fatal paraquat poisoning after skin absorption. Med. J.
1982 Aust., 1: 512 - 513.
Wojeck, G.A., Price, J.F., Nigg, A.N., & Stamper, J.H. Worker exposure
1983 to paraquat and diquat. Arch. Environ. Contam. Toxicol.,
12: 65 - 70.
Wyatt, I., Doss, A.W., Zavala, D.C., & Smith, L.L. Intrabronchial
1981 instillation of paraquat in rats: lung morphology and
retention study. Br. J. Ind. Med., 38: 42 - 48.
Yasaka, T., Okudaira, K., Fujito, H., Matsumoto, J., Ohya, I., &
1986 Miyamoto, Y. Further studies of lipid peroxidation in human
paraquat poisoning. Arch. Int. Med., 146: 681 - 685.
Yoshida, A., Maita, K., Tsuda, S., Saito, T., & Shirasu, Y. Paraquat
1982 D.C.: chronic toxicity - rats: 24 month study. Unpublished
report from the Institute of Environmental Toxicology,
Japan. Submitted to WHO by Otsuka Chemical Co., Osaka,
Japan.
Historical control incidence data of neoplasia in F-344 rats in
the laboratory in which the preceding study was conducted were made
available and are summarized in Table 3.
Table 3. Spontaneous lung tumours observed in F-344 rats at the
Institute of Environmental Toxicology from 1980 to 19831
No. of tumour-bearing animals (%)2
Males Females
Adenoma 40 (4.2%) 21 (2.2%)
Adenocarcinoma 5 (0.5%) 1 (0.1%)
Bronchial gland adenoma 1 (0.1%) -
Total 46 (4.8%) 22 (2.3%)
1 From Maita, 1986
2 These data were taken from 12 studies that included 960 male and
959 female F-344/DuCrj rats.
Groups of 60 male and 60 female Fischer 344 rats were maintained
on diets containing paraquat cation at 0 (2 groups), 25, 75, or
150 ppm for at least 113 weeks (males) or 122 weeks (females). Further
groups of 10 rats/sex/group received the same diets for 1 year. All
animals were studied for mortality, food and water consumption, and
body weight, and were subjected to periodical ophthalmoscopic and
haematological examinations throughout the study.
The distribution of mortality was unaffected by treatment. There
was approximately 50% mortality in all groups at the end of the study.
At 150 ppm, statistically-significant reductions in body-weight gain,
food consumption, and efficiency of food utilisation in both sexes
were observed. There was a statistically-significant depression of
body-weight gain in the first year of the study in males receiving
75 ppm paraquat. Water consumption was not significantly affected at
any dietary level tested. Paraquat accelerated, in a dosage-dependent
manner, the onset and progression of cataract changes, ranging from
minor opacity to total cataract in both males and females.
Treatment-related ocular lesions were first seen at 52 weeks.
Thereafter, ophthalmoscopy revealed a statistically-significant
dosage-related increase in the incidence, progression, and severity of
lenticular cataract in the 150 ppm group and, toward the end of the
study (103 weeks), in the 75 ppm group. There was evidence of
paraquat-dependent ocular effects in all treatment groups of both
sexes at termination. A statistically-significant higher incidence of
secondary eye lesions was found at termination in females receiving 75
or 150 ppm paraquat when compared to controls. Haematological
investigation (RBC counts, total and differential leucocyte counts,
haemoglobin, haematocrit, mean cell volume, mean cell haemoglobin
concentration, platelet and reticulocyte counts, and prothrombin and
partial thromboplastin times) and blood biochemistry (urea, glucose,
ALT, and AST) indicated no significant effects attributable to
paraquat administration. Urinalysis did not reveal any treatment-
related changes. Reductions in liver and testicular weights were noted
at termination in the 150 ppm dietary group.
Macroscopic examination at necropsy revealed a treatment-related
increase in the incidence of focal subpleural changes in animals
killed at termination in all dietary groups. This effect was most
marked in females receiving 75 ppm and in both sexes receiving 150 ppm
paraquat. Microscopic examination of lung tissues indicated that
treatment with paraquat at 150 ppm, in both sexes, and possibly at
75 ppm in males, was associated with proliferative lesions of the
alveolar epithelium. These lesions were not easily classified into
non-neoplastic or neoplastic, nor into adenoma or carcinoma. This
study provided strong evidence for the induction by paraquat of a
proliferative lesion of the alveolar epithelium and some controversial
evidence for the induction of lung adenomas in female Fischer 344
rats. There was no treatment-related increase in the incidence of lung
adenocarcinoma at any dose level in either sex. At 25 ppm, significant
increases in the incidence of proliferative lung lesions, compared to
the controls, were not observed. Microscopic examination of the eyes
confirmed a dose-related effect of paraquat on the onset and
progression of cataract lesions normally present in both male and
female F-344 rats. Slight dilation of the fourth ventricle of the
brain was evident in females receiving 150 or 75 ppm paraquat, but not
in males at these dosages or in either sex at 25 ppm. A statistically-
significant increase in the incidence of apparent degeneration of
occasional/several sciatic nerve fibers was noted in decedent males
receiving 75 or 150 ppm paraquat. Both hydrocephalus and nervous
tissue changes were considered by the authors of the study possibly
to be associated with paraquat treatment. Pathology summaries
indicate that atrophy of the testes was recorded in the high-dietary
group (5/33) but not in controls at termination, and moderate lymphoid
hyperplasia was observed in the respiratory epithelium of males
receiving 75 and 150 ppm paraquat and dying between 52 weeks and
termination.
The authors of the study concluded that "a wide range of tumour
types was observed in treated and control animals, and there was no
evidence that treatment with paraquat resulted in a marked change in
the group distribution of any of these tumours". A no-effect level for
paraquat was not found in this study due to the higher incidence of
cataract observed in animals of the 25 ppm group when compared to
controls. Paraquat accelerated, in a dosage-dependent manner, the
onset and progression of cataract change in F-334 rats. The authors
considered 25 ppm to be near the no-effect level for this change at
the end of the study (Ashby et al., 1983; Busey, 1986; Ishmael &
Godley, 1983).
Data on the historical incidence of lung neoplastic lesions in
F-344 rats from 7 studies performed in the laboratory in which the
preceding study was conducted were made available and are summarised
in Table 4.
Table 4. Spontaneous lung tumours observed in F-344 rats at Life
Science Research1
No. of tumour-bearing animals (%)2
Males Females
Adenoma 6/357 (1.7%) 4/363 (1.1%)
Carcinoma 3/357 (0.8%) 0/363 (0%)
Total pulmonary tumours 9/357 (2.5%) 4/363 (1.1%)
1 From Ashby et al., 1983
2 The range (% incidence) of adenoma was 0 - 4.4% in males and
0 - 4.0% in females; the range of carcinoma was 0 - 4.0% in
males.
Special studies on embryotoxicity and teratogenicity
Mice
The teratogenicity and fetal toxicity of paraquat were examined
after oral (20 mg/kg b.w./day) or i.p. (1.67 or 3.35 mg/kg b.w./day)
administration of paraquat to pregnant mice during the period of
organogenesis (days 8 - 16 of gestation). The oral dose (which was
equal to 1/10 of the oral LD50/day) did not produce significant
maternal toxicity, but at the higher of the 2 i.p. doses (which was
equal to 1/10 of the i.p. LD50/day) a significant maternal mortality
(5/7) and a statistically-significant increase of resorption rate,
when compared to controls, were observed. Paraquat did not
significantly increase the incidence of gross, soft-tissue, or
skeletal abnormalities. At the lower i.p. dose and after oral
administration of paraquat, there was a slight but non-significant
increase in the number of fetuses with absent or non-ossified
sternebrae. However, a significant difference was observed in the
incidence of abnormal sternebrae between the 2 control groups (6.9 ±
3.2% in the i.p. control group and 13.2 ± 5.8% in the oral control
group). The authors of the study concluded that the potential for
paraquat as a teratogen appeared to be minimal (Bus et al., 1975b).
The same authors examined the effects of paraquat dichloride on
the development of Swiss-Webster mice when administered in the
drinking water at concentrations of 0, 50, or 100 ppm. Paraquat was
given to pregnant mice from day 8 of gestation and administration was
continued to the newborns until 42 days after birth, when both the
control and paraquat-treated mice were sacrificed and subjected to
histopathological examination of the lungs, liver, and kidneys. A
significant increase in postnatal mortality in mice receiving 100 ppm
paraquat was observed. Histopathological examination of the lungs of
these mice showed extensive alveolar consolidation and collapse, and
areas of thickening of intra-alveolar septa. No significant
pathological changes were seen in the lungs of the 50 ppm or control
mice, nor in the liver or kidneys of mice of any treatment group.
There were no treatment-related effects on the number of live fetuses
nor on postnatal growth rate at either treatment level (Bus & Gibson,
1975).
Four groups of at least 20 pregnant SPF Alderley Park mice were
given orally 0, 1, 5, or 10 mg/kg b.w./day of paraquat cation during
days 6 to 15 of pregnancy, inclusive. On day 18 the animals were
killed, their uteri were examined, and the fetuses were removed,
weighed, sexed, and observed for gross abnormalities. There was some
evidence of maternal toxicity in the form of slight reductions in
body-weight gain at 5 and 10 mg/kg b.w./day, although only that of the
middle-dose group was statistically significant. There were no
clinical signs nor pathological changes in maternal animals
attributable to paraquat administration. Water and food consumption
were not quantified in this study. Numbers of implantations, viable
fetuses and resorptions, sex ratios, and fetal and litter weights
showed no significant differences between treated and control groups.
There were no increases in fetal external or soft-tissue abnormalities
which could be associated with paraquat treatment. There were
occasional statistically-significant differences in ossification of
individual bones between treated and control groups, but no
dose-related trend indicating either retardation of ossification or
increased abnormalities was observed. The authors of the study
concluded that paraquat was not teratogenic and had no significant
influence on embryonic or fetal development of the mouse at levels up
to and including 10 mg/kg b.w./day (Hodge et al., 1978a).
Rats
The teratogenic effects of paraquat were studied in 4 groups of
at least 20 pregnant SPF Alderley Park rats after oral administration
of 0, 1, 5, or 10 mg/kg b.w./day of paraquat cation during days 6 to
15 of pregnancy, inclusive. On day 21 the animals were killed. There
were clear clinical signs of maternal toxicity at 5 and 10 mg/kg
b.w./day. Apparently, 6 rats at the highest-dose level and 2 at the
middle-dose level died or became moribund during the experiment.
Histological changes found in the lungs and kidneys of the animals
receiving 10 mg/kg b.w./day paraquat which died or became moribund
were those known to be associated with oral paraquat poisoning. Slight
fetotoxicity was seen at 5 and 10 mg/kg b.w./day, as shown by a
statistically-significant reduction in fetal weight and retardation in
ossification, and by a decrease in the number of viable fetuses per
number of implants. According to the authors of the study, these
effects were probably associated with maternal toxicity. There were no
effects on embryonic or fetal survival and increases in fetal
abnormalities were not observed. The authors of the study concluded
that paraquat was not teratogenic when administered orally to rats,
even when there was clear evidence of maternal toxicity. However, it
did cause slight fetotoxicity at the 2 highest-dose levels (Hodge
et al., 1978b).
Special studies on eye irritation
The effects of paraquat on the eye have been reviewed by WHO
(1984). The instillation of diluted paraquat (up to 500 g/litre) in
rabbits' eyes induced inflammation within 24 hours, and this continued
for 96 hours (Clark et al., 1966). In another experiment, 62.5, 125,
250, 500, or 1000 g/litre of paraquat was introduced into the eyes of
rabbits. Concentrations of 62.5 and 125 g/litre caused severe
conjunctival reactions; higher levels (250 - 500 g/litre) provoked
ititis and pannus, while at the 500 g/litre concentration corneal
opacification, iritis, and conjunctivitis occurred. All rabbits
receiving 0.2 ml of paraquat at 1000 g/litre in 1 eye or 0.2 ml of
500 g/litre paraquat in both eyes died within 6 days of application
(Sinow & Wei, 1973).
Special studies on mutagenicity
In a review of published mutagenicity data (WHO, 1984) it was
noted that paraquat had been found to have minimal to no genotoxic
activity when evaluated in a variety of in vitro and in vivo test
systems. In in vitro studies producing weakly positive results,
paraquat genotoxicity was accompanied by high cytotoxicity (Moody &
Hassan, 1982; Parry, 1973 & 1977; Tweats, 1975; Benigni et al.,
1979; Bignami & Grebelli, 1979). Moody and Hassan (1982) have shown
that the mutagenicity of paraquat in bacterial test systems
(Salmonella typhimurium TA98 and TA100) was mediated by the
formation of superoxide. More recently, paraquat was found to induce
superoxide dismutase, chromosomal aberrations, and sister-chromatid
exchange in Chinese hamster fibroblasts, suggesting that superoxide
production is responsible for the chromosomal damage (Nicotera
et al., 1985). Other investigators (Anderson et al., 1972; Levin
et al., 1982) have not found mutagenic activity in bacterial test
systems. Furthermore, paraquat was not mutagenic when evaluated in
human leukocytes nor in in vivo cytogenic tests on mouse bone marrow
(Selypes & Paldy, 1978) or in dominant lethal tests on mice
(Pasi et al., 1974; Anderson et al., 1976).
A set of recently completed studies indicate that in most tests
paraquat was not mutagenic (see Table 5). Clastogenic potential has
been shown in vitro at very high concentration levels which were
themselves cytotoxic. Paraquat was not found to be mutagenic
in vivo.
Special studies on reproduction
Rats
In a 3-generation study, groups of 15 male and 30 female (F0
parents) weanling Alderley Park SPF rats were fed diets containing
0, 25, 75, or 150 ppm paraquat cation. After 12 weeks, animals were
mated to produce the first (F1a) litter and subsequently re-mated to
produce a second (F1b) litter. The breeding programme was repeated
twice with F1 parents selected from the F1b offspring and F2
parents selected from the F2b offspring. Test diets were fed
continuously throughout the study.
There were no adverse effects on parental body weights or food
consumption, and no treatment-related changes were found in the
reproductive performance (male and female fertility, live-born and
survival indexes, and litter size) or in the reproductive tract of
parents or offspring. Development of the reproductive tract in all
treated offspring was substantially comparable to that in controls.
The mild atrophy of seminal tubules found in a few of the treated
males of the F2b offspring at termination was considered by the
authors of the study to have no toxicological significance. Lung
changes due to paraquat administration occurred mainly in females
receiving 150 ppm paraquat. One pulmonary adenoma was found in 1
female receiving 150 ppm paraquat. Death resulting from severe, acute,
or sub-acute lung damage was confined to females with litters of
weaning age, and to 3 F0 females which died during the first 2 weeks
of the study. There were dose-related increases in the incidence and
Table 5. Mutagenicity assays on paraquat
Test system Test object Concentration Purity Results Reference
used (%)
Ames test1 S. typhimurium 0.12, 0.6, 2.9, 99 Negative Anderson, 1977
TA98 14, 72, 361,
TA100 & 1807 µg/plate
TA1535 (dissolved in
TA1538 H2O)
Ames test1 S. typhimurium 0.4, 0.7, 3.6, 100 Negative, growth Shirasu et al., 1978
TA98 7, 36, 72, & inhibition at 72
TA100 360 µg/plate at 72 & 360
TA1535 (dissolved in µg/plate2
TA1538 H2O)
Host-mediated S. typhimurium 3.6 & 14 mg/kg 100 negative2 Shirasu et al., 1978
assay G 46 (host: male (2 equal doses
ICR mice) orally)
Rec-assay B. subtilis 14 - 361 100 negative2 Shirasu et al., 1978
µg/disk
Mouse L 5178 Y 23, 45, 90, 180, 99 ? Clay & Thomas, 1985
lymphoma mouse & 361 µg/ml
test1 lymphoma
cells
Table 5. (cont'd).
Test system Test object Concentration Purity Results Reference
used (%)
Clastogenic Human 90, 903, & 1807 99.6 positive at 2 Sheldon et al., 1985a
potential lymphocytes µg/ml highest levels
test1 in vitro (also cytotoxic)
Mouse Male & female 51.7 & 82.8 99.4 negative Sheldon et al., 1985b
micronucleus C 57/BL/6J/ mg/kg (single
test Alpk mice dose orally)
In vitro Chinese 0.9, 1.8, 9, 18, 99.4 positive (reduced Howard et al., 1985
sister hamster lung 90 & 177 µg/ml with metabolic
chromatid fibroblasts activation)
exchange1
Unscheduled Hepatocyte 1 nM - 10 mM 99.6 negative Trueman et al., 1985
DNA cultures (0.19 ng/ml -
synthesis from male 1.86 mg/ml)
Alderley Park
albino rats
1 Both with and without metabolic activation
2 Positive control compounds gave positive responses
severity of focal alveolar histiocytosis in the lungs of male and
female parents receiving 75 and 150 ppm paraquat. A mild perivascular
inflammation was observed in the lungs of F1b pups receiving 150 ppm
paraquat. No changes due to paraquat were seen in animals receiving
25 ppm paraquat. The authors concluded that paraquat had no effect on
reproductive performance or development of the reproductive organs of
Alderley Park rats when administered at dietary levels up to 150 ppm
over 3 generations (Lindsay et al., 1982).
Groups of 12 male and 24 female rats were fed diets containing
0, 30, or 100 ppm paraquat ion from 35 days of age. Three generations
bred from these animals received the same diets during the whole
period under test. Two litters were bred from each generation, and the
effects on growth, food intake, fertility, fecundity, neonatal
morbidity, and mortality were noted. No evidence was seen of damage to
germ-cell production or of structural or functional damage in the
animals. In this study, pregnant and young animals did not appear to
be more vulnerable to paraquat than did adults. However, the incidence
of renal hydropic degeneration in 3 - 4 week-old offspring was
slightly increased in the 100 ppm group (Fletcher et al., 1972).
In a 3-generation study with 2 litters per generation, groups of
30 male and 30 female Sprague-Dawley rats (F0 parental generation)
were given diets containing 0, 72, 145, or 290 ppm paraquat cation
from 5 weeks of age (13 weeks prior to mating to obtain the first
litter, F1a) until the end of the second lactation (lactation of
F1b litters). In the second generation (F1b), 30 males and 30
females per group were treated from immediately after weaning until
the end of the second lactation (lactation of F2b litters). In the
third generation (F2b), the same number of rats were treated
immediately after weaning for at least 13 weeks. In a teratology study
sub-group 5 pregnant females of the parental generation (F0) and 10
pregnant females of the second generation (F1b) were killed on day
20 of pregnancy and examined macroscopically. Fetuses were examined
for number, sex, weight, external and internal abnormalities, and
progress of ossification. Another subgroup was used as a postnatal
investigation group, where natural parturition of pregnant females was
permitted to occur. In this group the duration of gestation,
parturition conditions, the number of live and still-born pups, sex,
and external abnormalities were recorded. Live pups were investigated
until weaning. Five male and 5 female rats per group of the first
(F0) and second (F1b) generations and 10 male and 10 female rats
per group of the F2b litters were subjected to histopathological
examination of approximately 25 tissues.
In the parental generation there were significant increases in
mortality and clinical signs attributable to paraquat (asthmatoid
wheezing) in several rats of the 290 ppm group of each generation from
the early stage of the dosing period. There were 29 treatment-related
deaths/moribund animals, all from the 290 ppm dietary level (5 among
F1b animals and 24 among F2b animals), but only 4 deaths among
control rats (1 among F0 female rats and 2 among F1b female rats
at parturition and 1 among F2b rats during the dosing period).
Histopathological examination of these dead or moribund F2b rats
showed, in some cases, hyperplasia of the alveolar epithelium and, in
most cases, diffuse thickening and fibrosis of the alveolar walls.
There was a decrease in body-weight gain in both male and female rats
of the F0 and F2b generations at 290 ppm during the early stage of
the dosing period. Body-weight gain was also reduced in F1b females
at 290 ppm during the gestation and lactation periods. Reductions in
food consumption and efficiency of food utilisation were seen in F0
and F2b females. There was a significant decrease in water
consumption in F0 and F1b females during lactation. No effect of
the compound was observed on the reproductive performance of parental
rats. Macroscopic examination revealed an apparently higher incidence
of white spots in the lungs of both male and female rats of the
290 ppm group in all 3 generations. Treatment-related changes of the
lung were confirmed by histopathology in rats of each generation.
These lesions were dose-dependent and included zonal thickening and
fibrosis of the alveolar walls, zonal atelectasis, and accumulation of
foam cells. There were no treatment-related changes in organ weights.
A treatment-related statistically-significant reduction in the
lactation index was found in F1a and F1b litters of the 290 ppm
group. A statistically-significant reduction in the lactation index
was also observed in F2a litters of the 290 ppm group, but it was
not clear to the authors of the report whether this change was
attributable to treatment. There were no statistically-significant
differences in lactation index between other treatment groups and
controls nor in the number of still-births and live births, sex
ratios, or viability indexes in any of the treated groups of both
generations, when compared to controls. The prolonged duration of
gestation in F0 rats of the parental group at 145 and 290 ppm was
considered by the authors of the report to be accidental. The
teratology phase showed a statistically-significant delay in
ossification in F1b fetuses from F0 parents treated with 290 ppm
paraquat and in F2b fetuses from F1b parents of all treated
groups. It was not clear to the authors of the report whether the
retarded growth was due to the treatment. There was a treatment-
related statistically-significant higher incidence of female
pups with retarded opening of the vagina in both F1b and F2b
litters at 290 ppm. There was a statistically-significant decrease in
body weight in male, but not in female, fetuses at 72 and 290 ppm.
There were no statistically-significant differences between fetuses
from treated rats and control fetuses in the number of corpora lutea
or implantations, percentage of implantations, number of dead or live
fetuses, sex ratios, or placental weights. No external or internal
malformations were detected in fetuses of any treatment group.
The authors of the report concluded that there was no evidence
suggesting that paraquat was teratogenic and that the only treatment-
related change which was enhanced by treatment of successive
generations of Sprague-Dawley rats with paraquat was "an increase in
death" at 290 ppm. A no-effect level for paraquat was not found in
this study due to delayed ossification in F2b fetuses in all treated
groups (Suzuki et al., 1983).
Rabbits
Forty rabbits received paraquat i.p. at total dosages of 2 to
100 mg/kg b.w. in 1 to 5 separate administrations. Multinuclear giant
cells were found in testicular tubules of 7/20 rabbits receiving
50 mg/kg b.w. or more paraquat (Butler & Kleinerman, 1971). However,
it has been reported that, when paraquat was orally administered at
4 mg/kg b.w. to male rats for 60 days and testes were examined, there
were no significant deviations in the spermatozoa count or motility,
nor were there any biochemical changes in the several enzymes of
testes homogenates. The histoenzyme activity of lactate dehydrogenase,
succinate dehydrogenase, DPN-diaphorase, alkaline phosphatase, and
acid phosphatase in the treated animals did not differ from those of
the controls, nor did quantitative and qualitative histological
examination of the testicular tubule cells reveal any abnormalities
(WHO, 1984).
Special studies on skin irritation
The effects of paraquat on the skin have been reviewed by WHO
(1984). Paraquat can provoke local irritation of the skin and eyes.
Clark et al. (1966) found skin irritation in rabbits only when
paraquat was applied beneath occlusive dressings in aqueous solutions
(total doses 1.56, 5.0, and 6.25 mg ion/kg b.w.). In mice and rats,
the application of solutions of 5 - 20g paraquat/litre in single and
21-day repeated dermal toxicity tests provoked dose-related toxic
dermatitis with erythema, oedema, desquamation, and necrosis (Bainova,
1969). Doses from 1.56 to 50 mg/kg, in repeated 20-day studies using
the occlusive technique (McElligott, 1972), resulted in local erythema
and scab formation. The histological changes consisted of
parakeratosis and occasional intra-epidermal pustules. A delayed
skin-irritant action of the herbicide was reported by Fodri et al.
(1977) in guinea pig studies.
Acute toxicity
The LD50 and LC50 values for paraquat in various species are
given in Table 6.
Table 6. Acute toxicity of paraquat in various species
LD50 LC50
Species Route Sex (mg/kg b.w.) (mg/l) Reference
Mouse oral M 260 -- Shirasu & Takahashi, 1977
F 210 --
i.p. M & F 29-30 -- Shirasu & Takahashi, 1977
Bus et al., 1975a
i.v. -- 50 -- Ecker et al., 1975
s.c. M 30 -- Shirasu & Takahashi, 1977
F 27 --
dermal -- 62 -- Bainova, 1971
Rat oral M 161 -- Shirasu & Takahashi, 1977
F 187 --
M 110 -- Kimbrough & Gaines, 1970
F 100 --
-- 126 -- Murray & Gibson, 1972
-- 200 -- Howe & Wright, 1965
i.p. M 18 -- Shirasu & Takahashi, 1977
F 19 --
F 19 -- Clark et al., 1966
s.c. M 19 -- Shirasu & Takahashi, 1977
F 23 --
-- 22 -- Makovskii, 1972
dermal M 90 -- Kimbrough & Gaines, 1970
F 80 --
-- 350 -- Makovskii, 1972
Table 6. (cont'd).
LD50 LC50
Species Route Sex (mg/kg b.w.) (mg/l) Reference
inhalation M & F -- 10 Bainova & Vulcheva, 1972
-- -- 1 Gage, 1968
-- -- 6 Makovskii, 1972
Guinea oral M 30 -- Clark et al., 1966
pig
-- 40-80 -- Howe & Wright, 1965
-- 22 -- Murray & Gibson, 1972
-- 42 -- Makovskii, 1972
i.p. F 3 -- Clark et al., 1966
s.c. -- 5 -- Makovskii, 1972
dermal -- 319 -- Makovskii, 1972
inhalation -- -- 4 Makovskii, 1972
Cat oral F 35 -- Clark et al., 1966
-- 40-50 -- Howe & Wright, 1965
Hen oral -- 300-380 -- Howe & Wright, 1965
-- 262 -- Clark et al., 1966
Turkey oral -- 250-280 -- Smalley, 1973
i.p. -- 100 -- Smalley, 1973
i.v. -- 20 -- Smalley, 1973
dermal -- 375 -- Smalley, 1973
Monkey oral -- 50 -- Murray & Gibson, 1972
Sheep oral -- 50-75 -- Howe & Wright, 1965
Cow oral -- 50-75 -- Howe & Wright, 1965
Following a single high dose of paraquat to animals, the earliest
ultrastructural changes were observed in the Type I alveolar
epithelial cells, approximately 4 - 6 hours after treatment, and were
usually characterised by cellular and mitochondrial swelling,
increased numbers of mitochondria, and the appearance of dark granules
in the cytoplasm. When a high dose was given (equal to approximately
the LD50 or greater), the lesions in the Type I cells often
progressed to the point of complete cellular disintegration, leaving
areas of exposed basement membrane (Kimbrough & Gaines, 1970;
Smith et al., 1973; Smith & Heath, 1974; Vijeyaratnam & Corrin,
1971; Klika et al., 1980). In contrast to the effects on Type I
pneumocytes, however, the capillary endothelial cells were remarkably
resistant to the toxic effects of paraquat (Sykes et al., 1977).
Ultrastructural lesions in the alveolar Type II pneumocytes were
also observed shortly after single-dose paraquat exposure, although,
generally, these lesions were not apparent until after the first
lesions were seen in the Type I cells (Kimbrough & Gaines, 1970).
Swollen mitochondria and damage to the lamellar bodies usually
occurred between 8 and 24 hours after a high dose of paraquat
(Robertson, 1973; Robertson et al., 1976). Progressive deterioration
of the Type II cells continued, resulting in completely denuded
alveolar basement membranes and debris-filled alveolar spaces
(Vijeyaratnam & Corrin, 1971). Infiltration and proliferation of
fibroblasts may produce fibrosis that obliterated the alveolar
structure (Smith & Heath, 1974).
Vijeyaratnam & Corrin (1971) observed that less-severely affected
parts of the lung appeared to undergo epithelial regeneration 7 - 14
days after a single dose of paraquat. Electron microscopic examination
revealed the alveoli to be lined with cuboidal epithelial cells that
closely resembled Type II pneumocytes, except for a general lack of
lamellar bodies. Similar phenomena have also been noted by other
investigators who administered paraquat in the diet (Kimbrough &
Linder, 1973) or as repetitive i.p. administrations (Smith et al.,
1974). Thus, in animals where the paraquat dose was sufficient to kill
only the Type I pneumocytes, the surviving Type II cells repaired the
damaged epithelium by proliferating and subsequently differentiating
into Type I epithelial cells. Inhaled paraquat in aerosol produced
initial necrosis of the epithelia and Type II pbeumocyte hyperplasia,
fibroblast proliferation, and increased synthesis of collagen in mice
(Popenoe, 1979).
The pathogenesis of the paraquat lung lesion has been well
characterised, and has been reviewed by Smith & Heath (1976). The
acute pulmonary toxicity of paraquat in animals has been described as
occuring in 2 phases. In the initial "destructive" phase, alveolar
epithelial cells were extensively damaged and their subsequent
disintegration often resulted in a completely denuded alveolar
basement membrane. Pulmonary oedema was also a characteristic of the
destructive phase, and was frequently of sufficient severity to result
in the death of the animals. Animals surviving the initial destructive
phase, which occurred in the first 1 - 4 days after acute paraquat
overexposure, progressed to what has been termed the "proliferative"
phase. In this phase, the lung was infiltrated with prolifroblastic
cells that rapidly differentiated into fibroblasts which, in some
cases, progressed to fibrosis. The histopathological outcome of the
second phase may be influenced by the treatment regimen, however.
Administration of repeated low doses of paraquat, which less-severely
damaged the alveolar epithelial cells, was also able to induce a
hyperplasia of the Type II cells. This response may represent an
attempt by the lung to repair the damaged epithelium (WHO, 1984).
When rabbits were injected i.p. with total doses of paraquat from
2 - 100 mg/kg b.w., thymic atrophy was observed, but most lungs showed
only occasional and small histological deviations that were poorly
correlated with the clinical signs of paraquat intoxication. These
results confirmed the resistance of the rabbit to paraquat-induced
lung lesions (Butler & Kleinerman, 1971).
According to Murray & Gibson (1972) and Hundsdorfer & Rose
(1980), guinea pigs treated with paraquat either orally or s.c. did
not develop the same type of progressive pulmonary fibrosis as
paraquat-intoxicated rats. In hamsters, a single administration of
paraquat did not induce lung damage, but prolonged exposure resulted
in lung fibrosis (Butler, 1975).
In conclusion, from lung toxicity studies, a characteristic
dose-related pulmonary fibrosis can be induced in rats, mice, dogs,
and monkeys, but not in rabbits, guinea pigs, or hamsters.
In paraquat toxicity, kidney damage often precedes signs of
respiratory distress (Clark et al., 1966; Butler & Kleinerman, 1971;
Murray & Gibson, 1972). Paraquat is excreted primarily via the urine
and the concentrations of the herbicide in the kidneys are relatively
high (see Table 1). Gross pathological and histological examination of
paraquat-poisoned rats, guinea pigs, rabbits, and dogs revealed
vacuolation of the convoluted renal tubules and proximal tubular
necrosis (Murray & Gibson, 1972). The nephrotoxicity caused by
paraquat is pronounced and appears to be restricted to the proximal
nephron (Ecker et al., 1975; Gibson & Cagen, 1977; Lock & Ishmael,
1979; Purser & Rose, 1979). The degeneration of the proximal tubular
cells has also been confirmed by electron-optical studies (Fowler &
Brooks, 1971; Marek et al., 1981).
In contrast with lung and kidney damage, liver damage in
experimental animals has not been severe and serum enzyme activities
(SGOT, SGPT, LAP) only increased when large amounts of paraquat were
given (Girl et al., 1979). Recently, electron microscopic
examination of the liver of paraquat-treated rats showed early,
localised changes (degranualtion of the RER, proliferation of the SER,
and mitochondrial swelling) in hepatocytes within 2 layers around the
central vein (Matsumori et al., 1984).
Short-term studies
Mice
Groups of 20 male and 20 female ICR-CRJ SPF mice were maintained
on diets containing 0, 7.2, 22, 72, or 217 ppm paraquat cation for 13
weeks. At the 217 ppm dietary level 2 female mice died from pulmonary
damage. Both males and females in this group showed significantly
reduced body-weight gain and a slight reduction in efficiency of food
utilisation. Food intake and water intake were not affected. No
abnormalities considered related to paraquat treatment were seen
during haematological, blood biochemistry, or urine analysis. A few
statistically-significant changes in absolute and relative organ
weights were seen at termination, mainly in males and females of the
217 ppm group. However, only an increase in lung weight of females in
the 217 ppm group was reported by the authors of the study to coincide
with histopathological changes of the same organ, namely eosinophilic
swelling of the alveolar epithelium walls which was observed in both
sexes at this dietary level. The no-effect level in this study with
respect to pulmonary damage and other parameters was 72 ppm, equal to
12 (males) and 14 (females) mg/kg b.w./day (Malta et al., 1980a).
Rats
Groups of 20 male and 20 female Fischer 344 rats were maintained
on diets containing 0, 7.2, 22, 72, or 217 ppm paraquat cation for 13
weeks. During the study there were no deaths. Body-weight gain was
reduced markedly in both sexes at the 217 ppm dietary level, at which
level food consumption, efficiency of food utilization, and water
consumption were also reduced. Histopathological examination revealed
swelling of alveolar epithelium cells in males and increased deposits
of brown pigment in the spleen of females at the 217 ppm dietary
inclusing level. No abnormalities considered attributable to paraquat
administration were observed during heamatological, blood
biochemistry, urine, organ weight, or gross necropsy investigations.
The no-effect level in this study with respect to lung lesions and
other parameters was 72 ppm, equal to 6.5 (males) and 7.1 (females)
mg/kg b.w./day (Malta et al., 1980b).
Dogs
Groups of beagle dogs, 3 males and 3 females per group, received
diets containing 0, 7, 20, 60, or 120 ppm paraquat cation for 13
weeks. Two males and 2 females in the 120 ppm group showed marked
paraquat toxicity and were killed in extremis between days 16 and
23, having shown marked dyspnoea and body-weight loss. Both surviving
dogs at 120 ppm also showed body-weight loss. A slight overall
reduction in body-weight gain among the females of the other treatment
groups was not considered by the authors of the study to be
treatment-related. Lung weights were increased in all animals in the
120 ppm group and in 2 animals from the 60 ppm group. All other organ
weights were in the normal range. Distinct gross and histological
treatment-related lung lesions were seen in all dogs in the 60 and
120 ppm groups. Minor renal lesions (swelling of the cortical tubules)
were also found histologically in a few of these animals. There were
no discernible gross or histological treatment-related pulmonary
lesions in the dogs of the 7 or 20 ppm groups. The focal pulmonary
lesions in these animals were of a type and incidence similar to those
found in the controls. Microscopic examination of 34 other tissues
from each animal showed no treatment-related changes. There were no
treatment-related effects on food intake except for 1 surviving
high-dose female which showed a loss of appetite from week 8 onward.
There were no distinct treatment-related changes in any of the
haematological (RBC counts, mean cell volume, haemoglobin, total and
differential white cell counts, platelets, prothrombin, and partial
thromboplastin time), biochemical (glucose, cholesterol, blood urea
nitrogen, bilirubin, total and partial protein, GOT, GPT, ALP, and
CPK), or urinary parameters examined. Slight haemoconcentration was
seen in 1 high-dose dog at termination. The no-effect level in this
study after administration of paraquat for 13 weeks to beagle dogs, on
the basis of lung and kidney lesions, was considered to be 20 ppm,
equal to 0.57 mg/kg b.w./day (Sheppard, 1981).
Long-term studies
See also under "Special studies on carcinogenicity".
Mice
Groups of 60 male and 60 female JCL:ICR mice were maintained on
diets containing 0, 1.4, 7.2, 22, or 72 ppm paraquat cation for 104
weeks and then killed and examined. Further groups of 10 males and 10
females receiving the same diets were sacrificed after 26 weeks or 52
weeks of treatment. Animals in each group, including the control
groups, showed approximately 60 - 70% mortality at the end of the
study. Haematological changes, attributed by the authors of the study
to the administration of paraquat, included reduced erythrocytes,
hematocrit, and haemoglobin at the 72 ppm level in both sexes. Total
serum protein was decreased and blood glucose increased at the 72 ppm
dietary level in both sexes. Various rumours, mostly malignant, were
observed in all groups in this study, the main types being lung
adenocarcinoma in males and leukaemia in females. However, no tumour
type showed a higher incidence in the treated groups than in controls
and no correlations between tumour incidence and concentration of the
test substance were observed. No effects attributable to paraquat were
observed in absolute or relative organ weights, urinalysis,
body-weight gain, food consumption, efficiency of food utilization, or
water intake. Based on the haematological and blood biochemistry
changes observed, the no-effect level for paraquat after 104 weeks of
administration to JCL:ICR mice in this study was 22 ppm (as paraquat
cation), equal to 2.8 mg/kg b.w./day (Toyoshima et al., 1982a).
Rats
Groups of 50 male and 50 female JCL:Wistar rats were maintained
on diets containing 0, 4.3, 22, 72, or 217 ppm paraquat cation for 104
weeks and then killed and examined. Further groups of 6 males and 6
females receiving the same diets were sacrificed after 26 or 52 weeks
of treatment. There was 38 - 66% mortality in all groups at the end of
the study. The distribution of mortality and of abnormalities were not
significantly affected by treatment. Females in the 217 ppm group
showed a transient tendency to lower body-weight gain, compared to
controls, at weeks 34, 42 - 48, and 54. Food consumption, efficiency
of food utilization, and water consumption were not affected by
paraquat administration. Haematological changes attributed by the
authors of the study to paraquat administration at the 217 ppm level
included reduced erythrocytes and haemoglobin in both sexes and
decreased haematocrits and increased reticulocytes in males after 26
weeks. Total serum protein was slightly but constantly decreased at
the 217 ppm level in both sexes.
During histopathological examination of approximately 20 tissues,
various rumours were observed in this study in all groups, the main
types being benign pituitary tumours in males and benign mammary
rumours in females. However, none of the tumours were present at a
significantly-higher incidence in the treated groups than in controls.
Body weight, food consumption, food efficiency, water intake,
leucocyte counts, platelet counts, prothrombin time, GOT, GPT,
alkaline phosphatase activity, blood glucose, blood urea nitrogen,
cholesterol, Na+, K+, C1-, creatine, and brain, serum, and
corpuscular cholinesterase activities, as well as ophthamological
examination indicated no significant effects attributable to paraquat
at any dose level. Based on the haematological and blood biochemistry
changes the no-effect level of paraquat after 104 weeks of
administration to JCL:Wister rats in this study was 72 ppm (as
paraquat cation) equal to 3.0 (males) and 3.7 (females) mg/kg b.w./day
(Toyoshima et al., 1982b).
Dogs
Groups of 6 male and 6 female beagle dogs received diets
containing 0, 15, 30, or 50 ppm paraquat cation for 1 year. During the
study there were no deaths. No effects due to paraquat were observed
on body weight. The reduced food consumption of 1 male and 1 female
dog, both in the 50 ppm group, was considered by the authors of the
study to be treatment-related. There was clinical evidence of
respiratory dysfunction (hyperpnoea) in some dogs fed 50 ppm paraquat.
Mean lung weights of male and female dogs fed 50 ppm paraquat were 35
and 60% higher than those of controls, respectively. Histopathological
examination of the lungs showed a statistically-significant increase
in the incidence of chronic pneumonitis in both sexes at the 30 and
50 ppm dietary levels when compared to controls. This lesion consisted
of interstitial fibrosis, alveolar epithelialization, and mononuclear
cell infiltration. No other toxicologically-significant treatment-
related effects were seen during clinical observations, haemotological
or biochemical investigations, or during gross and microscopic
examination of approximately 40 tissues from each animal at
termination. On the basis of the pulmonary changes, the authors of
this study concluded that the dietary no-effect level for paraquat in
dogs over 1 year of treatment was 15 ppm, equal to 0.45 (males) and
0.48 (females) mg/kg b.w./day (Kalinowski et al., 1983).
Observations in humans
Information on the effects of paraquat in humans has been
obtained from occupational exposure studies (epidemiological data and
case reports), descriptions of accidental or suicidal poisonings, and
volunteer studies. These data have been extensively reviewed by WHO
(1984).
In 1965, a study was carried out on a team of 6 sprayers, and in
1967 on 4 teams in Malaysian rubber plantations, to estimate the
efficacy of individual protective measures. The operators used a spray
dilution containing paraquat at 0.5 g/litre for 12 weeks. Attention
was paid to personal hygiene. Each man was given a thorough physical
examination, and urine samples were taken before spraying began and at
weekly intervals throughout the study. Chest X-rays were taken before
the study started and at the end of the 6th and 12th weeks. In the 2
studies, a total of 528 urine samples were examined. Paraquat was
found on 131 occasions (78/134 and 53/394 in the 2 studies,
respectively), the maximum concentration detected being 0.32 mg/litre
in the first study and 0.15 mg/litre in the second. Average urine
levels of paraquat of 0.04 mg/litre were found in the 1965 study and
of 0.006 mg/litre in the 1967 study. After spraying ceased, these
levels declined steadily to become undetectable within a week, with 1
exception. Both trials showed that about half of the men had suffered
mild irritation of the skin and eyes, but had recovered rapidly with
treatment. Two cases of scrotal dermatitis occurred in workers wearing
trousers that were continuously soaked by the spray solution. There
were also 2 cases of epistaxis. All chest radiographs were normal
(Swan, 1969).
Studies performed over a period of several years on 296 Trinidad
sugar estate workers drew attention to nail damage that ranged in
severity from localized discoloration to nail loss following gross
contamination with paraquat at 1 - 2 g/litre. The typical distribution
of the lesions, affecting the index, middle, and ring fingers of the
working hand, suggested that they had occurred through leakage from
the knapsack sprayer and inadequate personal hygiene. Apart from 2
cases of contact dermatitis of the hands, no skin, eye, or nose
irritation was reported, nor were there any systemic effects (Hearn &
Keir, 1971).
Similar data were obtained on several groups of workers spraying
paraquat as an herbicide and dessicant in cotton fields during the hot
season. These workers were exposed to paraquat aerosol concentrations
of 0.13 - 0.55 mg/m3 air. Dermal exposure was low, not more than
0.05 - 0.08 mg paraquat on the hands and face. There were no
complaints, nor did the clinical and laboratory examinations of the
workers demonstrate any significant deviations from the matched
control groups (Makovskii, 1972).
In the USA, the exposure of field workers operating
tractor-mounted spray equipment in orchards was determined. About
4.6 litres of paraquat liquid concentrate (291 g/litre) was used in
935 litres of water per hour. In addition, exposure from yard and
garden applications were studied in volunteers using pressurized hand
dispensers containing paraquat solution (4.4 g/litre). Dermal
contamination was measured by adsorbent cellulose pads attached to the
worker's body or clothing, and by hand-rinsing in water in a
polyethylene bag. Special filter pads were used in the filter
cartridges of the respirators worn by the subjects under study. In
all, 230 dermal and respiratory exposure pads, 95 samples of
hand-rinse water, and 130 urine samples, collected during and
following spraying, were analysed, which involved 35 different
paraquat application situations. The exposure of field workers was
found to range from about 0.40 mg/hour (dermal) to less than
0.001 mg/hour (inhalation). As for individuals spraying yards or
gardens, exposure ranged from 0.29 mg/hour (dermal) to less than
0.001 mg/hour (inhalation). In almost all cases dermal exposure
affected the hands. The respiratory paraquat values were generally
below the sensitivity levels of the analytical method. No detectable
paraquat concentrations were found in the urine samples (lower limit
0.02 mg/litre). This study confirmed the general safety of paraquat
under correct conditions of use (Staiff et al., 1975).
The potential long-term hazard associated with the use of
paraquat has been studied by comparing the health conditions of 27
sprayers who had been exposed to paraquat for many months per year for
an average of 5.3 years with those of 2 unexposed control groups
consisting of 24 general workers and 23 factory workers. The workers
were given full clinical examinations; lung, liver and kidney function
tests were also carried out. There were a few skin lesions resulting
from poor spraying techniques and 1 case of eye injury. There were no
significant differences between exposed and control groups in any
health parameters measured, which led the authors to suggest that the
long-term use of paraquat is not associated with harmful effects on
health (Howard et al., 1981).
To evaluate the effects of protective equipment on occupational
human exposure to paraquat, a paraquat formulation (240 g/litre)
diluted 300 times by volume with water was sprayed for 2 hours on
weedy ground. During the spraying operations, the concentrations of
paraquat aerosol were 11 - 33 µg/m3 air. The total dermal exposure
was about 0.22 mg. No irritation of the eyes or the skin was reported.
The urine of the workers who wore gauze masks contained significant
amounts of paraquat 24 hours after spraying. The urine of the workers
who had worn a high-performance mask did not contain detectable levels
of paraquat. The authors discussed the need for protective equipment
to decrease skin contact with paraquat and to avoid aerosol inhalation
(Kawai & Yoshida, 1981).
Quantitative estimates of dermal and respiratory exposure of 26
plantation workers in Malaysia have shown a mean dermal dose of
1.1 mg/kg b.w./hour. The highest individual total exposure was
equivalent to 2.8 mg/kg b.w./hour; the mean respiratory exposure was
0.24 - 0.97 g/paraquat/m3 air, which is 1% or less of a TLV of
0.1 mg/m3 for respirable paraquat. Urine levels of paraquat were
generally below 0.05 mg/litre (Chester & Woollen, 1982).
A study was carried out on a group of 14 sprayers in Thailand
using conventional high-volume knapsack sprayers and low-volume
spinning-disc applicators with paraquat ion concentrations of
1.5 g/litre and 20 g/litre, respectively. Irritation of unprotected
skin was found, and this was severe (caustic burns on the feet) in
workers using high spray conentrations and spinning-disc applicators.
Urinary paraquat levels ranging from 0.73 - 10.2 mg/litre after 14
days of spraying were detected in unprotected men using both
concentrations, and there was evidence that urinary levels of paraquat
increased as the trial progressed. No evidence of systemic toxicity
was discovered among the sprayers undergoing clinical and radiographic
examination 1 week after spraying ended. The author concluded that
spray concentrations in hand-held equipment should not exceed 5 g
paraquat ion/litre (Howard, 1982).
After tomato spraying in the USA, the total body exposure to
paraquat was determined to be 169 mg/hour. The use of enclosed tractor
cabs or a high-clearance tractor reduced total body exposures to
27 mg/hour or 18 mg/hour, respectively. The authors reported that the
total body exposure of tractor sprayers working in 2 citrus locations
was proportional to the tank concentrations (paraquat dilutions of
0.7 g/litre and 1.1 g/litre were applied). Exposure levels of 13 and
28 mg/hour were found for workers using the lower and high
concentrations, respectively. In all situations studied, the
respiratory exposure was consistently a small fraction (< 0.1%) of
the total body exposure, which was primarily through the skin
(Wojeck et al., 1983).
Two groups of workers exposed to paraquat formulations were
examined. The first group of 18 workers, in England, consisted of
subjects exposed to dust and liquid paraquat formulations during a
37.5-hour working week, the mean length of exposure being 5 years. The
second group also consisted of 18 males, from Malaysia, exposed to
liquid concentrate formulations during a 42-hour working week, the
mean length of exposure being 2.3 years. Partly protective clothing
was worn. However, in Malaysia, no gloves, rubber aprons, or goggles
were used. The medical records and the dermatological examinations
revealed acute skin rashes, nail damage, epistaxis, blepharitis, and
delayed wound healing in 12 - 66% of these workers. Delayed caustic
effects were often found among the Malaysian formulation workers,
where low levels of safety and hygiene were apparent. Clinical
examination did not reveal any evidence of chronic contact dermatitis,
hyperkeratosis, or eczematous lesions (Howard, 1979).
Some studies designed to estimate dermal and inhalation exposure
to paraquat are summarized in Table 7. From the data reported it can
be seen that:
(a) the main route of exposure of agricultural workers to
paraquat is via the skin; respiratory exposure is
negligible.
(b) The worst case of exposure (of those examined) was via
knapsack spraying).
From 1956 - 1973, no deaths attributable to paraquat were
registered among agricultural workers in the USA, but in 1974 4 fatal
cases were associated with this herbicide. However, it is not clear
whether they were accidental, suicidal, or occupational (Hayes &
Vaughan, 1977).
Fitzgerald et al. (1978a) summarized the clinical findings and
pathological details concerning 13 accidents involving paraquat among
agricultural workers, 6 of which were fatal. In 5 of these cases,
swallowing was involved. Of the 6 fatalities studied, 3 swallowed
Gramoxone (a 20% solution of paraquat dichloride in water) after
sucking the outlet of a sprayer. In 1 non-fatal case, the man had
sucked out a nozzle containing diluted paraquat, while in another case
the man who had blown into the jet, to clear it, escaped with only
minor signs of poisoning. The use of a leaking sprayer by another
worker with severe extensive dermatitis probably resulted in fatal
absorption of paraquat through the damaged skin.
Table 7. Comparison of dermal and inhalation exposure to paraquat resulting from
various methods of application1.
Dermal Respiratory
Method of application exposure exposure Reference
(mg/hour) (mg/hour)
Hand-held knapsack 66 (0.45 - 1.3) × 10-3 Chester & Woollen,
(12.1 - 169.8) 1982
Vehicle mounted 0.4 (0 - 2) × 10-3 Staiff et al., 1975
(0.1 - 3.4)
Aerial Chester & Ward, 1981
Flagman 0.1 - 2.4 (0 - 47) × 10-3
Pilot 0.5 - 0.1 (0 - 0.6) × 10-3
Mixer/loader 0.18 (1.3 - 1.5) × 10-3
1 From WHO, 1984
Several other cases of fatal poisoning resulting from dermal
absorption of paraquat have been reported. Jaros (1978) has described
how the use of concentrated solutions of paraquat (50 g/litre instead
of 5 g/litre), with an old leaking knapsack sprayer, resulted in
paraquat contamination of the neck, back, and legs of a worker. After
4 hours of work, the worker complained of a burning sensation on the
neck and scrotum. On admission to a hospital 6 days later, cough and
respiratory difficulties were recorded. Three days later the patient
died of renal and respiratory failure.
Severe skin damage, followed by death due to respiratory
insufficiency, occurred in a woman 8 weeks after initial contact with
paraquat. The toxic dermatitis started with scratches on the arms and
legs from the branches of fruit trees. The patient had often failed to
wear protective clothing or to shower after spraying. During the 4
weeks preceding her first admission to the hospital, she developed
ulcers and respiratory complaints combined with anorexia. Damaged and
broken skin was thus exposed to paraquat. A chest X-ray and needle
biopsy of the lung revealed pulmonary lesions. Seventeen days after
discharge from hospital, without a specific diagnosis, she was
re-admitted, and died 2 weeks later with progressive lung, hepatic,
and renal dysfunction (Newhouse et al., 1978).
The clinical and pathomorphological investigation of a patient
who died of hypoxia after repeated dermal exposure to paraquat
(28 g/litre) and diquat (29 g/litre) in a water-oil dilution has been
described recently. The worker had used a leaking sprayer. A
characteristic ulcer developed at the site of paraquat contact. There
was also lung damage (Levin et al., 1979).
Another reported fatal case of dermal poisoning with paraquat
occurred after prolonged contact with a concentrated formulation
following spillage from a bottle in the back trouser pocket
(Waight & Wheather, 1979).
Wohlfahrt (1982) discussed the factors related to severe paraquat
poisoning due to dermal absorption in tropical agriculture. Three
fatal incidents followed skin contamination; 1 victim used paraquat to
treat scabies infestation, and 1 used it to treat lice. In all cases,
the skin was blistered and ulcerated. The patients died of progressive
respiratory failure 4 - 7 days after the accidents. In 1 of these
cases, the presence of mouth and throat ulceration strongly suggested
that ingestion might also have occurred (Davies, 1982).
Local skin and nail effects of paraquat have been reviewed by WHO
(1984). Brief contact with liquid formulations, as well as repeated
exposure to dilute solutions, produced skin irritation, desquamation,
and, finally, necrosis at the site of contact (Ongom et al., 1974;
Binns, 1976; Newhouse et al., 1978; Waight & Wheather, 1979;
Levin et al., 1979, Horiuchi et al., 1980). Harmful dermal effects
have been reported among sprayers who worked without protective
clothes and with naked feet (Howard, 1982). The blistering and
ulceration of the skin were due to excessive contact and inadequate
hygiene. Horiuchi and Ando (1980) carried out patch testing on 60
patients with contact dermatitis due to Gramoxone. In 8 patients
(13.3%) positive allergic reactions were established. In another
survey with 52 persons, a positive photo-patch response was reported
in 11 patients. Nail damage may also occur after frequent exposure to
paraquat concentrations during the formulation of the herbicide or the
preparation of the working dilution (Howard, 1979).
There have been some reports (Malone et al., 1971; Mircev,
1976; Bismuth et al., 1982) of adverse effects as a result of
inhalation exposure to paraquat. However, inhalation of droplets in
normal paraquat spraying does not appear to represent a significant
health hazard (Howard, 1980), and the effects of occupational
inhalation have usually been limited to nose bleeds and nasal and
throat irritation (Swan, 1969; Howard, 1979).
Ocular damage may result from splashes of concentrated paraquat
that come into contact with the eye. Apart from irritation of the eye
and blepharitis, a week later more serious ocular damage may occur,
such as destruction of the bulbar and tarsal conjunctiva and of the
corneal epithelium. Anterior uveitis, conjunctival necrosis,
progressive keratitis with gross corneal opacity, and decreased visual
acuity may also occur (WHO, 1984).
It has been noted that when the recommended dilution rates were
correctly used, systemic effects of oral, inhalation, or dermal
exposure to paraquat have not been observed. Skin and eye irritation
have occurred only when protective measures were disregarded
(WHO, 1984).
In a volunteer study the percutaneous absorption of
14C-paraquat through the legs, hands, and forearms of 6 human
subjects was studied. The total dose absorbed in 5 days after a single
application of 0.64 mg paraquat dichloride was very low (0.3%) at all
sites of application (Wester et al., 1984).
A large number of cases of accidental or suicidal poisoning have
been reported since 1966, the earlier cases being mostly accidental
which apparently resulted from the habit of decanting the liquid
formulations into small unmarked or incorrectly labelled containers
such as beer, wine, or soft-drink bottles. An increasing ratio of
suicidal to accidental poisonings has been noted in recent years
(Fitzgerald et al., 1978b; Bramley & Hart, 1983). This change from
accidental to suicidal poisoning was considered to be reflected by
enhanced percentages of fatal cases, shorter survival times, and
higher tissue and body fluid levels (WHO, 1984). The vast majority of
cases of paraquat poisoning have been due to ingestion. A few cases of
fatal or non-fatal poisonings have been reported following either skin
contamination (McDonagh & Martin, 1970; Kimura et al., 1980) or skin
application in order to kill body lice (Ongom et al., 1974;
Binns, 1976). Symptoms of poisoning depend on the dose absorbed.
It is difficult to estimate the dose absorbed from case histories
since in many cases the patients spat out part of the paraquat
concentrate or vomited profusely after swallowing the herbicide. Some
patients have survived after apparently ingesting 10 - 20 g paraquat,
whereas some died after taking as little as 2.5 g paraquat. The
probability of survival after paraquat poisoning can be predicted from
plasma paraquat concentration at a given time after ingestion
(Proudfoot et al., 1979; Hart et al., 1984). The minimum lethal
dose of paraquat for human beings has been estimated to be about
35 mg/kg b.w. (Pederson et al., 1981; Bismuth et al., 1982). The
common factor of most, if not all, cases of paraquat poisoning is
damage to the lung. Cases of fatal paraquat poisoning have been
divided into 2 broad categories:
a) Cases of acute fulminant poisoning due to massive amounts of
paraquat absorbed, resulting in death within 1 to 5 days
from ingestion. Death is due to multi-organ failure
associated with damage to the lungs, kidneys, liver, brain,
and adrenals.
b) Cases of poisoning due to ingestion or absorption of smaller
doses of paraquat resulting in death 6 to several days
later. In these cases death is due primarily to lung and
kidney damage.
COMMENTS
A small amount of paraquat is rapidly absorbed by the gut of
rats, guinea pigs, dogs, monkeys, and man, most of the oral dose being
excreted as unchanged paraquat. Following administration by different
routes to animals, paraquat is rapidly distributed in most tissues,
the highest concentrations being found in the lungs and kidneys. The
compound accumulates slowly in the lung owing to uptake by an
energy-dependent process which is also responsible for the uptake of
putrescine. Saturation kinetics for the uptake of paraquat by the lung
have been shown to be similar in rats and man. Excretion of absorbed
paraquat is biphasic, owing to lung accumulation, and occurs largely
in the urine as unchanged paraquat, but also to a limited extent in
the bile. Biotransformation of absorbed paraquat is, in general,
remarkably poor in all species studied (rats, guinea pigs, dogs, hens,
pigs, goats, and sheep) although there is some controversy as to the
possibility and extent of its metabolism by the gut microflora.
Metabolism occurs via demethylation (monomethyl dipyridone ion) or
oxidation (paraquat pyridone ion and paraquat dipyridone ion).
The mechanism of paraquat toxicity has been investigated
extensively, but it has not yet been elucidated completely. The
available evidence indicates that paraquat toxicity is due to the
ability of the compound to undergo redox cycling in biological
systems, resulting in the production of superoxide anion radicals and
in the oxidation of cellular NADPH. These effects may lead to the
formation of other highly toxic oxygen species and to depletion of
important defense mechanisms, both events which are potentially
capable of switching on further pathological processes, resulting in
damage to Type I and Type II pneumocytes.
Two teratogenicity studies, 1 in mice and 1 in rats, showed
paraquat to be non-teratogenic at doses up to 10 mg/kg b.w./day.
Slight maternal toxicity was observed in mice and high maternal
toxicity and some fetal toxicity were observed in rats at 5 and
10 mg/kg b.w./day paraquat ion. In a teratology sub-group in a
3-generation reproduction study in rats, paraquat was not teratogenic,
but delays in ossification were found at all 3 dose levels tested
(72, 145, and 290 ppm paraquat cation).
In previous in vitro studies both positive and negative results
were obtained, with mutagenicity usually associated with high
cytotoxicity. Recent studies have shown paraquat to be non-mutagenic,
both in the presence and absence of metabolic activation, when tested
by the Ames test, mouse micronucleus test, unscheduled DNA synthesis
assay, rec-assay, and host-mediated assay. A mouse lymphoma cell test
was inconclusive. The herbicide was clastogenic to human lymphocytes
in vitro at cytotoxic doses and induced sister chromatid exchange in
Chinese hamster lung fibroblasts.
In 2 rat 3-generation reproduction studies paraquat had no
effects on the reproductive performance or development of the
reproductive organs of rats. In 1 of these studies a reduction of
lactation index was observed at 290 ppm paraquat cation.
The acute oral toxicity of paraquat is higher in guinea pigs,
monkeys, cattle, and man than in rats and birds. Confidence limits of
LD50 values are small. There are no significant differences between
the 2 sexes in the oral, s.c., or i.p. LD50 values of paraquat in
rats or mice. Paraquat induces characteristic dose-related fibrotic
changes in the lungs of mice, rats, dogs, and monkeys, but not in
rabbits, guinea pigs, or hamsters. The acute pulmonary toxicity of
paraquat in rats and man is biphasic. In the early, destructive phase,
the alveolar epithelial cells are extensively damaged. Death may occur
within a few days due to pulmonary oedema. In the later, proliferative
phase, fibroblasts and collagen accumulate in the lungs of surviving
animals and humans, possibly resulting in fibrosis.
In a short-term study in mice, treatment-related lung, body
weight, and organ-weight changes were found. Another short-term study
in rats indicated that paraquat was responsible for lung lesions and
other minor changes. In a short-term study in dogs using 3
animals/sex/group, paraquat-dependent lung and kidney lesions were
observed.
In a 1-year feeding study in dogs, paraquat caused lung changes
at the 2 highest dietary levels; the no-effect level in this study was
15 ppm (as paraquat cation), equal to 0.45 mg/kg b.w./day in males and
0.48 mg/kg b.w./day in females.
In 2 long-term feeding studies, 1 in mice and 1 in rats,
haematological and blood biochemical changes were observed in both
species. These changes have not been reported previously and were
considered to be of little toxicological significance.
A lifetime feeding study in mice showed no oncogenic potential
for paraquat, although a slightly higher incidence of lung tumours was
noted in animals of the high-dose group dying between 79 and 98 weeks
of treatment when compared with control mice. Based on renal lesions
observed in males, the no-effect level in this study was 12.5 ppm
(as paraquat cation), equal to 1.4 mg/kg b.w./day.
A long-term feeding study in F344 rats indicated that paraquat
administration for 104 weeks at 217 ppm was responsible for a
significant increase in the incidence of pulmonary adenomas in
females. This incidence (8.7%) was significantly higher than that
observed in historical control animals of the same laboratory (2.2%).
Paraquat was also cataractogenic in both male and female rats. Based
on the lung and eye changes the no-effect level in this study was
22 ppm (as paraquat cation), equal to 0.77 mg/kg b.w./day.
In another long-term study in F344 rats paraquat induced
proliferative benign lung lesions in females in the high-dose group,
which were considered neoplastic by 1 pathologist and non-neoplastic
by 2 others (the total incidence of pulmonary adenoma in females
ranged from 0/70 to 8/70). The reasons for the discrepancy were
unclear, owing to the lack in most cases of adequately detailed
histopathological descriptions of the lung lesions. The historical
incidence of lung adenoma in control female F344 rats in the
laboratory in which the study was conducted was reported to be 1.1%,
and the incidences reported in the literature are 1.4% for females and
1.9% or 0% for males. The results of these studies indicate a weak,
organ-specific, sex-related potential of paraquat to produce benign
proliferative changes in the lungs of female F344 rats.
The following observations were considered by the Joint Meeting:
a) paraquat was shown to be non-mutagenic in most in vitro
and, apparently, all in vivo tests;
b) the lung is the target organ for both acute and chronic
paraquat toxicity in rats;
c) only female F344 rats, but not male F344 rats, developed
treatment-related benign neoplastic lesions;
d) proliferative changes were observed in rats and not in mice;
and
e) paraquat selectively interferes with the uptake of
polyamines by pneumocytes.
Polyamines are endogenous substrates playing an important role in
cell division and growth. These observations suggest that the
potential of paraquat to induce lung cell proliferation may be
modulated by species-, sex-, and organ-dependent factors, such as
hormonal activity, cell growth, defense and repair mechanisms, etc. No
lung changes were observed at 24 ppm in any of the dose groups, and
significant increases in the incidence of lung adenocarcinomas were
not observed in any of the treated groups when compared to controls in
these studies.
Observations in man, including reports on accidental or suicidal
poisonings, confirm the type of acute and subacute toxicity observed
in some experimental animals. Death occurred after oral and, in some
cases, dermal absorption of high doses of paraquat. The organs
primarily involved are the lung and kidney and, to a lower extent, the
intestinal tract, liver, pancreas, adrenals, and CNS. The minimal
lethal dose of paraquat in man is estimated to be about 35 mg/kg b.w.
Skin, nail, and eye lesions were found in subjects with prolonged
occupational exposure to paraquat. Significant paraquat concentrations
(up to 10 mg/1) were detected in the urine of workers using high spray
concentrations, but not in protected workers. The use of protective
measures has proved to be effective in preventing lesions due to skin
contact.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
Mice: 13 ppm (as paraquat cation), corresponding to 17 ppm
(as paraquat dichloride) in the diet, equal to
1.9 mg/kg b.w./day for males and 38 ppm (as paraquat
cation, corresponding to 52 ppm (as paraquat
dichloride), equal to 5.9 mg/kg b.w./day for females.
Rats: a) 22 ppm (as paraquat cation), corresponding to 30 ppm
(as paraquat dichloride) in the diet, equal to
1.1 mg/kg b.w./day for males and 1.2 mg/kg b.w./day for
females (cataract).
b) 25 ppm (as paraquat cation), corresponding to 35 ppm
(as paraquat dichloride) in the diet, equal to
1.4 mg/kg b.w./day for males and 1.7 mg/kg b.w./day for
females (proliferative lung changes).
Dogs: 15 ppm (as paraquat cation), corresponding to 20 ppm
(as paraquat dichloride) in the diet, equal to
0.62 mg/kg b.w./day for males and 0.66 mg/kg b.w./day
for females.
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.004 mg/kg b.w. as paraquat cation (0 - 0.006 mg/kg b.w.
expressed as paraquat dichloride).
STUDIES WHICH WILL PROVIDE INFORMATION VALUABLE FOR THE CONTINUED
EVALUATION OF THE COMPOUND
1. Further observations in humans.
2. Studies on the mechanism of paraquat-induced proliferative
lung changes.
REFERENCES
Anderson, K.J., Leighty, E.G., & Takahashi, M.T. Evaluation of
1972 herbicides for possible mutagenic protperties.
J. Agric. Food Chem., 20: 649 - 656.
Anderson, D., McGregor, D.B., & Purchase, I.F.H. Dominant lethal
1976 studies with diquat and paraquat in male CD-1 mice.
Mutat. Res., 40: 349 - 358.
Anderson, D. Paraquat: Estimation of mutagenic potential in the
1977 Salmonella typhimurium mutagenicity assay. Unpublished
Report No. CTL/P/243 from ICI Central Toxicology Laboratory.
Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Ashby, R., Hepworth, P.L., Whinney, A.K., Brown, P.M., Whitney, J.C.,
1983 & Finn, J.P. Paraquat: combined toxicity and carcinogenicity
study in rats. Unpublished report No. 82/ILY217/328 from
Life Science Research, Stock, Essex, UK. Submitted to WHO by
Imperial Chemical Industries PLC, Haslemere, UK.
Autor, A.P. Reduction of paraquat toxicity by superoxide dismutase.
1974 Life Sci., 14: 1309 - 1319.
Autor, A. P., ed. Biochemical mechanisms of paraquat toxicity. New
1977 York, Academic Press.
Bainova, A. Experimental assessment of the effect of dipyridylium
1969 herbicides on the skin. Letopisi HEI, 9: 25 - 30
(in Bulgarian).
Bainova, A. Determination of the zones of acute and chronic inhalatory
1971 toxicity after exposure to dipyridylium herbicides Gramoxone
and Reglone. Letopisi HEI, 28: 59 - 62 (in Bulgarian).
Bainova, A. & Vulcheva, V. Experimental substantiation of Gramoxone
1972 MAC in working areas. In: Works of Research Institute of
Hygiene & Labour Protection. Sofia, Medizine i Fizkultuna,
Vol. 23: 71 - 77.
Benigni, R., Bignami, M., Carera, A., Conti, G., Conti, R., Grebelli,
1979 R.A., Dogliotti, E., Gualandi, G., Novelleto, A., & Ortalia,
V.A. Mutational studies with diquat and paraquat in vitro.
Mutat. Res., 68: 183 - 193.
Bennett, P.N., Davies, D.S., & Hawksworth, G.M. In-vivo absorption
1976 studies with paraquat and diquat in the dog. Proceedings of
the British Pharmaceutical Society, 15 - 16 July, England,
284 pp.
Bignami, M. & Grebelli, R.A. A simplified method for the induction of
1979 8-azaguanine resistance in S. typhimurium. Toxicol.
Lett., 3: 169 - 175.
Binns, C.W. A deadly cure for lice. Papua New Guinea Med. J.,
1976 19: 105 107.
Bismuth, C., Garnier, R., Dally, S., Fournier, P.E., & Scherrmann,
1982 J.M. Prognosis and treatment of paraquat poisoning. A review
of 28 cases. J. Toxicol. Clin. Toxicol., 19: 461 - 474.
Bramley, A. & Hart, T.B. Paraquat poisoning in the United Kingdom.
1983 Hum. Toxicol., 2: 417.
Brigelius, R., Hashem, A., & Lengfelder, E. Paraquat-induced
1981 alterations of phospholipids and GSSG release in the
isolated perfused rat liver and the effect of SOD-active
copper complexes. Biochem. Pharmacol., 30: 349 - 354.
Bus, J.S., Aust, S.D., & Gibson, J.E. Superoxide and singlet oxygen
1974 catalysed lipid peroxidation as a possible mechanism for
paraquat toxicity. Biochem. Biophys. Res. Commun.,
58; 749 - 755.
Bus, J.S. & Gibson, J.E. Postnatal toxicity of chronically
1975 administered paraquat in mice and interactions with oxygen
and bromobenzene. Toxicol. Appl. Pharmacol.,
33: 461 - 470.
Bus, J.S., Aust, S.D., & Gibson, J.E. Lipid peroxidation as a possible
1975a mechanism for paraquat toxicity. Res. Commun. Chem.
Pathol. Pharmacol., 11: 31 - 38.
Bus, J.S., Preachie, M.M., Cagen, S.Z., Posner, H.S., Eliason, B.C.,
1975b Sharp, C.W., & Gibson, J.E. Fetal toxicity and distribution
of paraquat and diquat in mice and rats. Toxicol. Appl.
Pharmacol., 33: 450 - 460.
Bus, J.S., Cagen, S.Z., Olgaard, M., & Gibson, J.E. A mechanism of
1976 paraquat toxicity in mice and rats. Toxicol. Appl.
Pharmacol., 35: 501 - 513.
Bus, J.S. & Gibson, J.E. Paraquat: model for oxidant-initiated
1984 toxicity. Environ. Health Perspect., 55: 37 - 46.
Busey, W.M. An independent pathology review of the lung slides from a
1986 rat chronic toxicity/carcinogenicity study with paraquat.
Unpublished report from ICI Plant Protection, Ltd. Submitted
to WHO by Imperial Chemical Industries PLC, Haslemere,
Surrey, UK.
Butler, C. & Kleinerman, J. Paraquat in the rabbit. Br. J. Ind. Med.,
1971 28: 67 -71.
Butler, C. Pulmonary interstitial fibrosis from paraquat in the
1975 hamster. Arch. Pathol., 99: 503 - 507.
Calderbank, A. The bipyridylium herbicides. Effects on man. In
1968 Metcalf, R.L, ed. Advances in Pest Control Research. New
York, Interscience Publishers, Vol. 8; 224 pp.
Charles, J.M., Abou-Donia, M.B., & Menzel, D.B. Absorption of paraquat
1978 from the airways of perfused rat lung. Toxicology,
8: 59 - 67.
Chester, G. & Ward, R.J. Paraquat - occupational exposure and drift
1981 hazard evaluation during aerial application to cotton in
California, USA. Unpublished Report No. CTL/P/581 from ICI
Central Toxicology Laboratory. Submitted to WHO by Imperial
Chemical Industries PLC, Haslemere, Surrey, UK.
Chester, G. & Woollen, B.H. Studies of the occupational exposure of
1982 Malaysian plantation workers to paraquat.
Br. J. Ind. Med., 38: 23 - 33.
Clark, D.G., McElligott, T.F., & Hurst, E.W. The toxicity of paraquat.
1966 Br. J. Ind. Med., 23: 126 - 133.
Clay, P. & Thomas, M. Paraquat dichloride (technical liquor);
1985 assessment of mutagenic potential using L5178Y mouse
lymphoma cells. Unpublished Report No. CTL/P/1398 from ICI
Central Toxicology Laboratory. Submitted to WHO by Imperial
Chemical Industries PLC, Haslemere, Surrey, UK.
Conning, D.M., Fletcher, K., & Swan, A.A.B. Paraquat and related
1969 bipyridyliums. Br. Med. Bull., 25: 245 - 249.
Daniel, J.M. & Gage, J.C. Absorption and excretion of diquat and
1966 paraquat in rats. Br. J. Ind. Med., 23: 133 - 136.
Davies, R.E. Skin absorption of paraquat. Med. J. Aust., 7: 222.
1982
Dunbar, J.R., DeLucia, A.J., & Bryant, L.R. Glutathione status of
1984 isolated rabbit lungs. Effects of nitrofurantoin and
paraquat perfusion with normoxic and hyperoxic ventilation.
Biochem. Pharmacol., 33: 1343 - 1348.
Ecker, J.L., Hook, J.B., & Gibson, J.E. Nephrotoxicity of paraquat in
1975 mice. Toxicol. Appl. Pharmacol., 34: 178 - 186.
Edwards, M.J., Hemingway, R.J., Kinch, D.A., and Slape, P. Paraquat:
1974 residue and toxicology trial with cows fed a treated grass.
Unpublished report No. AR2465A(R) from ICI Plant Protection,
Ltd. Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Fairshter, R.D. Paraquat toxicity and lipid peroxidation. Arch.
1981 Intern. Med., 141: 1121 - 1123.
Fisher, H.K., Clements, J.A., & Wright, R.R. Enhancement of oxygen
1973 toxicity by the herbicide paraquat. Ann. Rev.
Respir. Dis., 107: 246 - 252.
Fisher, H.K., Clements, J.A. Tierney, D.F., & Wright, R.R. Pulmonary
1975 effects of paraquat in the first day after injection.
Am. J. Physiol., 228: 1217 - 1223.
Fitzgerald, G.R., Barniville, G., Flanagan, M., Silke, B., Carmody,
1978a M., & O'Dwyer, W.F. The changing pattern of paraquat
poisoning; an epidemiologic study. J. Irish Med. Assoc.,
71; 103 - 108.
Fitzgerald, G.R., Barniville, G., Black, J., Silke, B., Carmody, M., &
1978b O'Dwyer, W.F. Paraquat poisoning in agricultural workers.
J. Irish Med. Assoc., 71; 149 - 152.
Fletcher, K., Herring, C.M., & Robinson, V.M. Paraquat three-
1972 generation reproduction study in rats. Unpublished report
from ICI Industrial Hygiene Research Laboratories. Submitted
to WHO by Imperial Chemical Industries PLC, Haslemere,
Surrey, UK.
Fodri, Z., Sipos, K., & Berencsi, G. Study of the irritation and
1977 allergic effects of Gramoxone in guinea-pig.
Egeszsegtudomouny, 21: 244 - 249 (in Hungarian).
Fowler, B.A. & Brooks, R.E. Effects of the herbicide paraquat on the
1971 ultrastructure of mouse kidney. Am. J. Pathol.,
63: 505 - 512.
Fridovich, I. & Hassan, H.M. Paraquat and the exacerbation of oxygen
1979 toxicity. Trends Biochem. Sci., 4: 113 - 115.
Gage, J.C. The action of paraquat and diquat on the respiration of
1968 liver cell fractions. Biochem. J., 109: 757 - 761.
Gibson, J.E. & Cagen, S.Z. Paraquat-induced functional changes in
1977 kidney and liver. In: Autor, A.P., ed. Biochemical
Mechanisms of Paraquat Toxicity. New York Academic Press,
pp. 117 - 136.
Giri, S.N., Curry, D.L., Hollinger, M.A., & Freywald, N. Effect of
1979 paraquat on plasma enzymes, insulin, glucose and liver
glycogen in the rat. Environ. Res., 20; 300 - 308.
Giri, S.N. & Krishna, G.A. The effect of paraquat on guanylate cyclase
1980 activity in relation to morphological changes in guinea-pig
lungs. Lung, 157: 127 - 134.
Glass, M., Sutherland, M.W., Forman, H.J., & Fisher, A.B. Selenium
1985 deficiency potentiates paraquat-induced lipid peroxidation
in isolated perfused rat lung. J. Appl. Physiol.,
59: 619 - 622.
Greenberg, D.B., Lyons, A., & Last, J.A. Paraquat-induced changes in
1978 the rat of collagen biosynthesis by rat lung.
J. Lab. Clin. Med., 92: 1033 - 1042.
Hart, T.B., Nevitt, A., & Whitehead, A. A new statistical approach to
1984 the prognostic significance of plasma paraquat
concentrations. Lancet, November 24: 1222 - 1223.
Hassan, H.M. & Fridovich, I. Regulation of synthesis of superoxide
1977 dismutase in Escherichia coli. J. Biol. Chem.,
253: 7667 - 7672.
Hassan, H.M. & Fridovich, I. Superoxide radical and the enhancement of
1978 oxygen toxicity of paraquat in Escherichia coli.
J. Biol. Chem., 253: 8143 - 8148.
Hassan, H.M. & Fridovich, I. Paraquat and Escherichia coli. Mechanism
1979 of production of extracellular superoxide radical.
J. Biol. Chem., 254: 10846 - 10852.
Hassan, H.M. & Fridovich, I. Superoxide dismutases: detoxication by a
1980 free radical. In: Jakoby, W.B., ed. Enzymatic basis of
detoxication. New York, Academic Press, Vol. 1: 311 - 322.
Hawksworth, G.M., Bennett, P.N., & Davies, D.S. Kinetics of paraquat
1981 elimination in the dog. Toxicol. Appl. Pharmacol.,
57: 139 - 145.
Hayes, W.J. & Vaughn, W.K. Mortality from pesticides in the United
1977 States in 1973 and 1974. Toxicol. Appl. Pharmacol.,
42: 235 - 252.
Hearn, C.E.D. & Keir, W. Nail damage in spray operators exposed to
1971 paraquat. Br. J. Ind. Med., 28: 399 - 403.
Hemingway, R.J., Leahey, J.P., Griggs, R.E., & Davis, J.A. Paraquat
1972 metabolism in sheep. Unpublished report No. AR2359A from ICI
Plant Protection Division. Submitted to WHO by Imperial
Chemical Industries PLC, Haslemere, Surrey, UK.
Hemingway, R.J., Leahey, J.P., Griggs, R.E., & Davis, J.A. Paraquat
1974 metabolism in ruminants. 3rd International Congress of
Pesticide Chemistry, Helsinki.
Hemingway, R.J. & Oliver, C. Paraquat: a study of the metabolism and
1974 residues in hens and their eggs. Unpublished report No.
AR251A from ICI Plant Protection Division. Submitted to WHO
by Imperial Chemical Industries PLC, Haslemere, Surrey, UK.
Hendley, P., Leahey, J.P., & Spinks, C.A. Paraquat: Metabolism and
1976a residues in hens. Unpublished report No. AR2676A from ICI
Plant Protection Division. Submitted to WHO by Imperial
Chemical Industries PLC, Haslemere, Surrey, UK.
Hendley, P. Leahey, J.P., Spinks, C.A., Neale, D., & Carpenter, P.K.
1976b Paraquat metabolism and residues in goats. Unpublished
report No. AR2680A from ICI Plant Protection Division.
Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Hodge, M.C.E., Palmer, S, Weight, T.M., & Wilson, J. Paraquat
1978a dichloride: teratogenicity study in the mouse. Unpublished
report No. CTL/P/364 from ICI Central Toxicology Laboratory.
Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Hodge, M.C.E., Palmer, S., Weight, T.M., & Wilson, J. Paraquat
1978b dichloride: teratogenicity study in the rat. Unpublished
report No. CTL/P/365 from ICI Central Toxicology Laboratory.
Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Horiuchi, N. & Ando, S. Contact dermatitis due to pesticides for
1980 agricultural use. Nippon Hifuka Gakkai Zasshi, 90: 289
(in Japanese).
Horiuchi, N., Ando, S., & Kambe, Y. Dermatitis due to pesticides for
1980 agricultural use. Nippon Hifuka Gakkai Zasshi, 90: 27
(in Japanese).
Howard, J.K. A clinical survey of paraquat formulation workers. Br. J.
1979 Ind. Med., 36: 220 - 223.
Howard, J.K. Paraquat: a review of worker exposure in normal usage.
1980 J. Soc. Occup. Med., 30; 6 - 11.
Howard, J.K., Sahopathy, N.N., & Whitehead, P.A. A study of the health
1981 of Malaysian plantation workers with particular reference to
paraquat spraymen. Br. J. Ind. Med., 38: 110 - 116.
Howard, J.K. Paraquat spraying. Comparative risks from high and low
1982 volume application methods. In: Proceedings of the 10th
Asian Conference on Occupational Health, Singapore,
pp. 1 - 7.
Howard, C.A., Wildgoose, J., Clay, P., & Richardson, C.R. Paraquat
1985 dichloride: an in vitro sister chromatid exchange study in
Chinese hamster lung fibroblasts. Unpublished Report
No. CTL/P/1392 from ICI Central Toxicology Laboratory.
Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Howe, D.J.T. & Wright, N. The toxicity of paraquat and diquat. In:
1965 Proceedings of the 18th New Zealand Weed and Pest Control
Conference, pp. 105 - 114.
Hundsdorfer, S. & Rose, I. Lung changes produced by paraquat:
1980 respiratory distress model in animals. Ergeb. Exp. Med.,
35: 603 - 690 (in German).
Hussain, M.Z. & Bhatnagar, R.S. Involvement of superoxide in the
1979 paraquat-induced enhancement of lung collagen synthesis in
organ culture. Biochem. Biophys. Res. Commun.,
89: 71 - 76.
Ilett, K.I., Stripp, B., Menard, R.H., Reid, W.D., & Gillette, J.R.
1974 Studies on the mechanism of the lung toxicity of paraquat.
Comparison of tissue distribution and some biochemical
parameters in rats and rabbits. Toxicol. Appl. Pharmacol,
28: 216 - 226.
Ishmael, J. & Godley, M.J. Paraquat:. lifetime feeding study in rats.
1983 Histopathological examination of lungs. Unpublished Report
No. CTL/P/738 from ICI Central Toxicology Laboratory.
Submitted to WHO by imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Jaros, F. Acute percutaneous paraquat poisoning. Lancet 1: 275.
1978
Kalinowski, A.E., Doe, J.E., Chart, I.S., Gore, C.W., Godley, M.J.,
1983 Hollis, K., Robinson, M., & Woollen, B.H. Paraquat: One year
feeding study in dogs. Unpublished Report No. CTL/P/734 from
ICI Central Toxicology Laboratory. Submitted to WHO by
Imperial Chemical Industries PLC, Haslemere, Surrey, UK.
Kawai, M. & Yoshida, M. Exposure of spray operators to paraquat.
1981 Nippon Doshu Eisei Zasshi, 28: 353 - 359 (in Japanese).
Keeling, P.L., Pratt, I.S., Aldridge, W.N., & Smith, L.L. The
1981 enhancement of paraquat toxicity in rats by 85% oxygen:
lethality and cell-specific lung damage.
Br. J. Exp. Pathol., 62: 643 - 654.
Keeling, P.L., Smith, L.L., & Aldridge, W.N. The formation of mixed
1982 disulfides in rat lung following paraquat administration.
Biochem. Biophys. Acta, 716: 249 - 257.
Kehrer, J.P., Haschek, W.M., & Witschi, H. The influence of hyperoxia
1979 on the acute toxicity of paraquat and diquat. Drug Chem.
Toxicol., 2: 397 - 408.
Kimbrough, R.D. & Gaines, T.B. Toxicity of paraquat in rats and its
1970 effect on rat lungs. Toxicol. Appl. Pharmacol.,
17: 679 - 690.
Kimbrough, R.D. & Linder, E.W. The ultrastructure of the paraquat lung
1973 lesion in the rat. Environ. Res., 6: 265 - 273.
Kimura, M., Suzuki, E., & Ohnishi, H. A case of autopsy of an infant
1980 intoxicated by paraquat dichloride. Shoni Ka Rinsho,
33: 732 - 734 (in Japanese).
Klika, E., Kunc, L., Antalikova, K., & Kuncova, M. The morphology of
1980 the lung in the albino rat after paraquat administration.
Folia Morphol., 28: 188 - 191.
Kornburst, D.J. & Mavis, R.D. The effect of paraquat on microsomal
1980 lipid peroxidation in vitro and in vivo.
Toxicol. Appl. Pharmacol., 53: 323 - 332.
Kurisaki, E. & Sato, H. Toxicological studies on herbicides.
1979 Intracorporeal distribution of paraquat dichloride and
diquat dibromide in rats. Nippon Hoigaki Zasshi,
33: 656 (in Japanese).
Leahey, J.P., Hendley, P., & Spinks, C.A. Paraquat: Metabolism and
1976 residues in pigs using 14C-methyl labelled paraquat.
Unpublished report No. AR2694A from ICI Plant Protection
Division. Submitted to WHO by Imperial Chemical Industries
PLC, Haslemere, Surrey, UK.
Levin, P.J., Klaff, L.J., Rose, A.G., & Ferguson, A.D. Pulmonary
1979 effects of contact exposure to paraquat: a clinical and
experimental study. Thorax, 34: 150 - 160.
Levin, D.E., Hollstein, M., Christman, M.F., Schwiers, E.A., & Ames,
1982 B.N. A new Salmonella tester strain (TA 102) with AT base
pairs at the site of mutation detects oxidative mutagens.
Proc. Natl. Acad. Sci. USA, 79: 7445 - 7449.
Lindsay, S., Banham, P.B., Godley, M.J., Moreland, S., Wickramaratne,
1982 G.A., & Woollen, B.H. Paraquat: multigeneration reproduction
study in rats - three generations. Unpublished Report
No. CTL/P/719 from ICI Central Toxicology Laboratory.
Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Litchfield, M.H., Daniel, J.W., & Longshaw, S. The tissue distribution
1973 of the bipyridylium herbicides diquat and paraquat in rats
and mice. Toxicology, 1; 155 - 165.
Lock, E.A. & Ishmael, J. The acute toxic effects of paraquat and
1979 diquat on the rat kidney. Toxicol. Appl. Pharmacol.,
50: 67 - 76.
Maita, K., Saito, T., Tsuda, S., & Shirasu, Y. AT-5: sub-acute
1980a toxicity study in mouse. Unpublished report from the
Institute of Environmental Toxicology. Submitted to WHO by
Imperial Chemical Industries PLC, Haslemere, Surrey, UK.
Maita, K., Saito, T., Tsuda, S., & Shirasu, Y. AT-5: sub-acute
1980b toxicity study in rat. Unpublished report from the Institute
of Environmental Toxicology. Submitted to WHO by Imperial
Chemical Industries PLC, Haslemere, Surrey, UK.
Maita, K. Historical control incidence data in F344 rats. Unpublished
1986 report from Institute of Environmental Toxicology. Submitted
to WHO by Otsuka Chemical Co. Ltd., Osaka, Japan.
Makovskii, V.N. Toxicological and hygiene studies of bipyridylium
1972 herbicides diquat and paraquat. Referat, Vinnize, USSR
pp 1 - 23 (PhD degree thesis) (in Russian).
Maling, H.M., Saul, W., Williams, M.A., Brown, E.A.B., & Gillette,
1978 J.R. Reduced body clearance as the major mechanism of the
potentiation by ß-adrenergic agonists of paraquat lethality
in rats. Toxicol. Appl. Pharmacol., 43: 57 - 72.
Malone, J.D.G., Carmoday, M., & Keogh, B. Paraquat poisoning. A review
1971 of nineteen cases. J. Irish Med. Assoc., 64: 59 - 68.
Marek, J., Velenska, Z., & Haskovcova, I. Kidney changes in acute
1981 phase of paraquat intoxication in rats. Sb. Lek.,
83: 100 - 104 (in Czech.).
Matsumori, H., Matsumoto, T., & Ishikawa, H. Acute toxic effects of
1984 paraquat on ultrastructure of rat liver. Acta Pathol. Jpn,
34: 507-518.
McDonagh, B.J. & Martin, J. Paraquat poisoning in children. Arch. Dis.
1970 Child., 45: 425 - 427.
McElligot, T.F. The dermal toxicity of paraquat. Differences due to
1972 techniques of application. Toxicol. Appl. Pharmacol.,
21: 361 - 368.
Mircev, N. Acute intoxication with Gramoxone (paraquat). Vatreshni
1976 Bolesti, 16: 99 - 101 (in Bulgarian).
Montgomery, M.R. Interaction of paraquat with the pulmonary microsomal
1976 fatty acid desaturase system. Toxicol. Appl. Pharmacol.,
36: 543 - 554.
Montgomery, M.R. Paraquat toxicity and pulmonary superoxide dismutase;
1977 an enzymic deficiency of lung microsomes. Res. Commun.
Chem. Pathol. Pharmacol., 16: 155 - 158.
Montgomery, M.R. & Niewoehner, D.E. Oxidant-induced alterations in
1979 pulmonary microsomal mixed-function oxidation. Acute effects
of paraquat and ozone. J. Environ. Sci. Health,
13: 205 - 219.
Moody, C.S. & Hassan, H.M. Mutagenicity of oxygen free radicals.
1982 Proc. Natl. Acad. Sci., 79: 2855 - 2859.
Murray, R.E. & Gibson, J.E. A comparative study of paraquat
1972 intoxication in rats, guinea-pigs and monkeys.
Exp. Mol. Pathol., 17: 317 - 325.
Murray, R.E. & Gibson, J.E. Paraquat disposition in rats, guinea-pigs
1974 and monkeys. Toxicol. Appl. Pharmacol., 27: 283 - 291.
Newhouse, M., McEvoy, D., & Rosenthal, D. Percutaneous paraquat
1978 absorption. Arch. Dermatol., 114: 1516 - 1519.
Nicotera, T.M., Block, A.W., Gibas, Z., & Sandberg, A.A. Induction of
1985 superoxide dismutase, chromosomal aberrations and
sister-chromatid exchanges in Chinese hamster fibroblasts.
Mutat. Res., 151: 263 - 268.
Omaye, S.T., Reddy, K.A., & Cross, C.E. Enhanced lung toxicity of
1978 paraquat in selenium-deficient rats. Toxicol. Appl.
Pharmacol., 43: 237 - 247.
Ongom, V.L., Owor, R., & Tonusange, E.T. Paraquat (Gramoxone) used as
1974 a pediculocide. In: Bagshore, A.F., ed. The uses and abuses
of drugs and chemicals in tropical Africa. Nairobi, East
Africa Literature Bureau, pp. 229 - 233.
Parry, J.M. The induction of gene conversion in yeast by herbicide
1973 preparations. Mutat. Res., 21: 83 - 91.
Parry, J.M. The use of yeast for the detection of environmental
1977 mutagens using a fluctuation test. Mutat. Res.,
46: 165 - 176.
Pasi, A., Embree, J.W., Eisenlord, G.H., & Hine, C.H. Assessment of
1974 the mutagenic properties of diquat and paraquat in the
murine dominant lethal test. Mutat. Res., 26: 171 -175.
Pedersen, G., Pedersen, A., Gregersen, M., & Kampe, B. Paraquat
1981 poisoning. Ugeskr. Laeg., 143: 1202 - 1206 (in Danish).
Plant Protection Ltd. Data to support a revised acceptable daily
1972 intake and tolerance levels for paraquat. Unpublished report
from Plant Protection Ltd.
Popenoe, D. Effects of paraquat aerosol on mouse lung. Arch. Pathol.
1979 Lab. Med., 103: 331 - 334.
Proudfoot, A.T., Steward, M.S., Levitt, T., a Widdop, B. Paraquat
1979 poisoning: significance of plasma-paraquat concentrations.
Lancet, 2: 330-332.
Purser, D.A. & Rose, M.S. The toxicity and renal handling of paraquat
1979 in cynomolgus monkeys. Toxicology, 15: 31 - 41.
Reddy, K.A., Litov, R.E., & Omaye, S.T. Effect of pre-treatment with
1977 anti-inflammatory agents on paraquat toxicity in the rat.
Res. Commun. Chem. Pathol. Pharmacol., 17: 87 - 100.
Robertson, B. Paraquat poisoning as an experimental model of the
1973 idiopathic respiratory distress syndrome.
Bull. Phys. Pathol. Res., 9: 1433 - 1452.
Robertson, B., Grossmann, G., & Ivemark, B. The alveolar lining layer
1976 in experimental paraquat poisoning. Acta. Pathol.
Microbiol. Scand., Section A., 84: 40 - 46.
Rose, M.S., Smith, L.L., & Wyatt, I. Evidence of energy-dependent
1974 accumulation of paraquat in rat lung. Nature (Lond.),
252: 314 - 315.
Rose, M.S., Lock, E.A., & Smith, L.L. Biochem. Pharmacol.,
1976a 25: 419 - 423.
Rose, M.S., Smith, L.L., & Wyatt, I. The relevance of pentosephosphate
1976b pathway stimulation in rat lung to the mechanism of paraquat
toxicity. Biochem. Pharmacol., 25: 1763 - 1767.
Rose, M.S. & Smith, L.L. The relevance of paraquat accumulation by
1977 tissues. In: Autor, A.P., ed., Biochemical Mechanisms of
Paraquat Toxicity. New York, Academic Press, pp. 71 - 91.
Ross, W.E., Block, E.R., & Chang, R.Y. Paraquat-induced DNA damage in
1979 mammalian cells. Biochem. Biophys. Res. Commun.,
91: 1302 - 1308.
Rossouw, D.J., Chase, C.C., & Engelbrecht, F.M. The effect of paraquat
1984 on the in vitro activity of cytosol, mitochondrial, and
microsomal enzyme systems. S. Afr. Med. J., 65; 555 - 563.
Selman, M. Montano, M., Montfort, I., & Perez-Tamayo, R. The duration
1985 of the pulmonary paraquat toxicity-enhancement of O2 in
the rat. Exp. Mol. Pathol., 43: 388 - 396.
Selypes, A. & Paldy, A. The examination of the mutagenic effect of two
1978 pesticides: Krezonit E and Gramoxone. Proc. Hung. Ann.
Meet. Biochem., 18: 77- 78.
Sharp, C.W., Ottolenghi, A., & Posner, H.S. Correlation of paraquat
1972 toxicity with tissue concentration and weight loss of the
rat. Toxicol. Appl. Pharmacol., 22: 241 - 251.
Sheldon, T., Howard, C.A., Wildgoose, J., & Richardson, C.R. Paraquat
1985a dichloride: a cytogenetic study in human lymphocytes
in vitro. Unpublished Report No. CTL/P/1357 from ICI
Central Toxicology Laboratory. Submitted to WHO by Imperial
Chemical Industries PLC, Haslemere, Surrey, UK.
Sheldon, T., Richardson, C.R., Shaw, J., & Barber, G. An evaluation of
1985b paraquat dichloride (technical) in the mouse micronucleus
test. Unpublished Report No. CTL/P/1369 from ICI Central
Toxicology Laboratory. Submitted to WHO by Imperial Chemical
Industries PLC, Haslemere, Surrey, UK.
Sheppard, D.B. Paraquat thirteen week (dietary administration)
1981 toxicity study in beagles. Unpublished Report
No. 2481-72/111A from Hazelton Laboratories Europe Ltd.
Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, UK.
Shirasu, Y. & Takahashi, K. Acute toxicity of AT-5 in rat and mouse.
1977 Unpublished report from the Institute of Environmental
Toxicology. Submitted to WHO by Imperial Chemical Industries
PLC, Haslemere, Surrey, UK.
Shirasu, Y., Moriya, M., & Ohta, T. Mutagenicity testing on paraquat
1978 in microbial systems. Unpublished report from the Institute
of Environmental Toxicology. Submitted to WHO by Imperial
Chemical Industries PLC, Haslemere, Surrey, UK.
Shu, H., Talcott, R.E., Rice, S.A., & Wei, E.T. Lipid peroxidation and
1979 paraquat toxicity. Biochem. Pharmacol., 28: 327 - 331.
Sinow, J. & Wei, E. Ocular toxicity of paraquat. Bull. Environ.
1973 Contam. Toxicol., 9: 163 - 168.
Smalley, H.E. Toxicity and hazard of the herbicide paraquat in
1973 turkeys. Poultry Sci., 52: 1625 - 1629.
Smith, P., Heath, D., & Ray, J.M. The pathogenesis and structure of
1973 paraquat-induced pulmonary fibrosis in rats. J. Pathol.,
114: 57 - 67.
Smith, P. & Heath, D. The ultrastructure and time sequence of the
1974 early stages of paraquat lung in rats. J. Pathol.,
114: 177 - 184.
Smith, L.L., Wright, A., Wyatt, I., & Rose, M.S. Effective treatment
1974 for paraquat poisoning in rats and its relevance to
treatment of paraquat poisoning in man. Br. Med. J.,
4: 569 - 571.
Smith, P. & Heath, D. Paraquat. Clin. Rev. Toxicol., 4: 411 - 445.
1976
Smith, L.L., Rose, M.S., & Wyatt, I. The pathology and biochemistry of
1979 paraquat. In: Oxygen Free Radicals and Tissue Damage.
Amsterdam, North Holland, Excerpta Medica, pp. 321 - 341
(CIBA Foundatin Series 65 - new series).
Smith, L.L., Wyatt, I., & Rose, M.S. Factors affecting the efflux of
1981 paraquat from rat lung slices. Toxicology, 19: 197 - 207.
Smith, L.L. Paraquat toxicity. Phil. Trans. R. Soc. Lond. B.,
1985 311: 647 - 657.
Sotheran, M., Banham, P.B., Godley, M.J., Lindsay, S., Pratt, I.,
1981 Taylor, K., & Woollen, B.H. Paraquat; lifetime feeding study
in the mouse. Unpublished Report No. CTL/P/556 from ICI
Central Toxicology Laboratory. Submitted to WHO by Imperial
Chemical Industries PLC, Haslemere, Surrey, UK.
Spinks, C.A., Hendley, P., Leahey, J.P., & Carpenter, P.K. Paraquat:
1976 Metabolism and residues in pigs using 14C-ring labelled
paraquat. Unpublished report No. 2692A from ICI Plant
Protection Division. Submitted to WHO by Imperial Chemical
Industries PLC, Haslemere, Surrey, UK.
Staiff, D.C., Comer, S.W., Armstrong, J.F., & Wolfe, H.R. Exposure to
1975 the herbicide paraquat. Bull. Environ. Contam. Toxicol.,
14: 334 - 340.
Steffen, C. & Netter, K.J. On the mechanism of paraquat action on
1979 microsomal oxygen reduction and its relation to lipid
peroxidation. Toxicol. Appl. Pharmacol., 47: 593 - 602.
Steffen, C., Muliawan, H., & Kappus, H. Lack of in vivo lipid
1980 peroxidation in experimental paraquat poisoning.
Arch. Pharmacol., 310: 241 - 243.
Suzuki, T., Igarashi, N., & Tamura, K. Paraquat D.C.; Multiple
1983 generation study in rats. Unpublished Report No. R-015 from
Bozo Research Centre Co. Ltd. Submitted to WHO by Otsuka
Chemical Co., Osaka, Japan.
Swan, A.A.B. Exposure of spray operators to paraquat.
1969 Br. J. Ind. Med., 26: 665 - 671.
Sykes, B.J., Purchase, I.F.H., & Smith, L.L. Pulmonary ultrastructure
1977 after oral and intravenous dosage of paraquat to rats.
J. Pathol., 121: 233 - 241.
Talcott, R.E., Shu, H., & Wei, E.T. Dissociation of microsomal oxygen
1979 reduction and lipid peroxidation with the electron
acceptors, paraquat and menadione. Biochem. Pharmacol.,
26: 665 - 671.
Thompson, W.D. & Patrick, R.S. Collagen propyl hydroxylase levels in
1978 experimental paraquat poisoning. Br. J. Exp. Pathol.,
59: 288 - 291.
Toyoshima, S., Sato, R., Kashima, M., Sato, H., Sato, H., Sato, H.,
1982a Motoyama, M., Ichiba, J., & Kimura, R. AT-5: chronic
toxicity study result - 104 week dosing study in mouse.
Unpublished report from Nippon Experimental Medical Research
Co. Ltd. Submitted to WHO by Imperial Chemical Industries
PLC, Haslemere, Surrey, UK.
Toyoshima, S., Sato, R., Kashima, M., Sato, H., Sato, H., Sato, H.,
1982b Motoyama, M., Ishidawa, A., Ichiba, J. & Kimura, R. AT-5:
chronic toxicity study result - 104 week dosing study in
rat. Unpublished report from Nippon Experimental Medical
Research Co. Ltd. Submitted to WHO by Imperial Chemical
Industries PLC, Haslemere, Surrey, UK.
Trueman, R.W., Ashby, J., & Burlinson, B. Paraquat dichloride:
1985 assessment for the induction of unscheduled DNA synthesis in
primary rat hepatocyte cultures. Unpublished Report
No. CTL/P/1339 from ICI Central Toxicology Laboratory.
Submitted to WHO by Imperial Chemical Industries PLC,
Haslemere, Surrey, UK.
Trush, M.A., Mimnaugh, E.G., Ginsburg, E., & Gram, T.E. In vitro
1981 stimulation by paraquat of reactive oxygen-mediated lipid
peroxidation in rat lung microsomes. Toxicol. Appl.
Pharmacol., 60: 279 - 286.
Trush, M.A., Mimnaugh, E.G., Ginsburg, E., & Gram, T.E. Studies on the
1982 in vitro interaction of mitomycin C, nitrofurantoin, and
paraquat with pulmonary microsomes. Simulation of reactive
oxygen-dependent lipid peroxidation. Biochem. Pharmacol.,
31: 3335 - 3346.
Tweats, D.J. The effect of R (drug resistance) factors on the
1975 detection of mutagens by E. coli K 12. Brussels, Belgium,
Annual Euratom Report, Biological Sciences, 316 pp.
Veseley, D.L., Watson, B., & Levey, G.A. Activation of liver guanylate
1979 cyclase by paraquat: possible role of superoxide anion.
J. Pharmacol. Exp. Ther., 209: 162 - 164.
Vijeyaratnam, G.S. & Corrin, B. Experimental paraquat poisoning: A
1971 histological and electron-optical study of the changes in
the lung. J. Pathol., 103: 123 - 129.
Waddell, W.J. & Marlowe, C. Tissue and cellular disposition of
1980 paraquat in mice. Toxicol. Appl. Pharmacol.,
56: 127 - 140.
Waight, J.J.J. & Wheather, R.H. Fatal percutaneous paraquat poisoning.
1979 J. Am. Med. Assoc., 242: 472.
Walker, M., Dugard, P.H., & Scott, R.C. Absorption through human
1983 laboratory animal skins: in vitro comparison.
Acta Pharm. Suec., 20: 207 - 213.
Wester, R.C., Maibach, H.I., Bucks, D.A.W., & Aufrere, M.B. In vivo
1984 percutaneous absorption o[ paraquat from hand, leg, and
forearm of humans. J. Toxicol. Environ. Health,
14: 759 - 762.
WHO. Paraquat and diquat. WHO Environmental Health Criteria, No. 39.
1984 International Programme on Chemical Safety, World Health
Organization, Geneva.
Witschi, H.P., Kacew, S., Hirai, K., & Cote, M.G. In vivo oxidation of
1977 reduced nicotinamide-adenine dinucleotide phosphate by
paraquat and diquat in rat lung. Chem. Biol. Interactions,
19: 143 - 160.
Wohlfart, D.J. Fatal paraquat poisoning after skin absorption. Med. J.
1982 Aust., 1: 512 - 513.
Wojeck, G.A., Price, J.F., Nigg, A.N., & Stamper, J.H. Worker exposure
1983 to paraquat and diquat. Arch. Environ. Contam. Toxicol.,
12: 65 - 70.
Wyatt, I., Doss, A.W., Zavala, D.C., & Smith, L.L. Intrabronchial
1981 instillation of paraquat in rats: lung morphology and
retention study. Br. J. Ind. Med., 38: 42 - 48.
Yasaka, T., Okudaira, K., Fujito, H., Matsumoto, J., Ohya, I., &
1986 Miyamoto, Y. Further studies of lipid peroxidation in human
paraquat poisoning. Arch. Int. Med., 146: 681 - 685.
Yoshida, A., Maita, K., Tsuda, S., Saito, T., & Shirasu, Y. Paraquat
1982 D.C.: chronic toxicity - rats: 24 month study. Unpublished
report from the Institute of Environmental Toxicology,
Japan. Submitted to WHO by Otsuka Chemical Co., Osaka,
Japan.
