
International Programme on Chemical Safety (WHO/ILO/UNEP)
Evaluation
Antidotes for Poisoning by Organophosphorus Pesticides
Completed May 2004
T.C. Marrs MD DSc FRCPath MRCP
Chief Toxicologist
Food Standards Agency
125 Kingsway
London WC2B 6NH
UK
The views herein are those of the author, and cannot be taken to represent those of any UK Government Department or Agency.
Edited by: Nicola Bates, MSc, MA: National Poisons Information Service (London Centre), UK
Peer-reviewed by: Dr M Eddleston1, Dr A Dawson2 and Professor M Balali Mood3
©World Health Organization 2004
All rights reserved. Publications of the World Health Organization can be obtained from Marketing and Dissemination, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 2476; fax: +41 22 791 4857; email: ). Requests for permission to reproduce or translate WHO publications — whether for sale or for noncommercial distribution — should be addressed to Publications, at the above address (fax: +41 22 791 4806; email: permissions@who.int).
The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement.
The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters.
The World Health Organization does not warrant that the information contained in this publication is complete and correct and shall not be liable for any damages incurred as a result of its use.
The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals.
|
5.3 Methods for analysis of the antidote in biological fluids |
|
8.1.1.1 Studies involving OP compounds other than nerve agents |
|
8.1.2.1 Studies involving OP compounds other than nerve agents |
Diazepam and certain other anticonvulsants were studied initially in the treatment of organophosphate-induced convulsions on a purely symptomatic basis. Although convulsions are relatively uncommon (e.g. Hayes et al., 1978) in most series of organophosphate (OP) intoxications, they may have long-term neurological sequelae. Consequently, the use of diazepam is an important part of the treatment regimen of severe OP poisoning to prevent, or reduce the duration of convulsions that might be expected to cause structural damage to the central nervous system (CNS). The usefulness of diazepam in treating convulsions in OP poisoning has been accepted generally (Stocker, 1982; IPCS, 1986; Lund & Monteagudo, 1986; Eddleston et al., 2002). It is now considered that the indications for diazepam in the management of OP poisoning are somewhat wider, than solely for convulsive states; diazepam is also used for sedation in patients with anxiety (Karalliedde & Senanaykae, 1989; Eddleston personal communication, 2003), and for the amelioration of muscle fasciculation (Vale & Scott, 1974). Although the latter is a less serious clinical feature, it is often a subjectively unpleasant aspect of organophosphorus insecticide poisoning. It has also been suggested that diazepam may be neuroprotective even in the absence of apparent convulsions and may protect against organophosphate-induced central respiratory depression (Dickson et al., 2003).
Diazepam is indicated in poisoning by organophosphorus insecticides where convulsions or pronounced muscle fasciculation are present. If artificial ventilatory support is indicated in an organophosphate-poisoned patient without fasciculation or convulsions, diazepam is the drug of choice for sedation.
Diazepam has also been used in combination with atropine in treating carbamate poisoning, for example, from carbamate drugs, such as physostigmine (Niemegers et al., 1982; Klemm, 1983) and carbamate pesticides, such as carbofuran (Poirier et al., 1987). The only conclusion to be drawn from these reports is that the use of diazepam can be associated with a favourable outcome.
|
International non-proprietary name: |
Diazepam |
|
Synonyms: |
Methyl diazepinone, diacepin |
|
IUPAC name: |
7-chloro-1,3-dihydro-1-methyl-5-phenyl-2H-1,4-benzodiazepin-2-one |
|
CAS number: |
439-14-5 |
|
Chemical formula: |
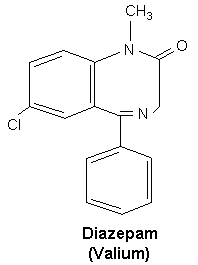
C16H13ClN2O (Figure 1) |

Fig 1 chemical structure of diazepam
Molecular mass: 284.74
Conversion table:
|
1 mmol |
= |
285 mg |
|
1 g |
= |
3.51 mmol |
|
µmol/L |
= |
3.51 × mg/L |
|
mg/L |
= |
0.285 × µmol/L |
Manufacturers:
The major manufacturer of diazepam (ValiumR) worldwide is Hoffmann-La Roche, Basle, Switzerland. Other brand names containing diazepam includes Alboral, Aliseum, Alupam, Aneural, Antenex, Ansilive, Ansiolin, Anxicalm, Apaurin, Apozepam, Arzipam, Assival, Atensine, Atilen, Benzopin, Benzyme, Betapam, Bialtepam, Bialzepam, Calmociteno, Calmpose, Ceregulart, Complutine, Diaceplex, Dialar, Diapam, Diapanil, Diapine, Diaquel, Diatrex, Diazelong, Diazemuls, Diaz, Diazep, Dienpax, Dipam, Disopam, Dizan, Doval, D-Pam, Drenidan, Ducene, Eridan, Evacalm, Farmin, Faustan, Freudal, Gewacalm, Gobanal, Hexalid, Imepas, Kiatrium, Kratium, Lamra, Laxyl, Lembrol, Levium, Medipam, Metamidol, Micronoan, Morosan, Nerolyd, Neurolytril, Noan, Novazam, Novo-Dipam, Onapan, Ortopsique, Pacitran, Pacium, Pax, Paxate, Paxel, Pazolini, Podium, Prizem, Propam, Psychopax, Rayne, Relanium, Relasan, Relazepam, Rimapam, Sedapam, Sediver, Seduxen, Seredyn, Setonil, Sico Relax, Sipam, Somaplus, Stesolid, Stesolin, Tandial, Tensium, Tranimul, Tranquase, Tranquirit, Tranquo-Tablinen, Umbrium, Unisedil, Valaxona, Valenium, Valiquid, Valrelease, Vanzor, Vatran, Vival, Vivol, Zepan, Zeprat, Zopam.
|
Melting point: |
131-135°C |
|
Physical state: |
Diazepam is a white or yellowish crystalline material. It is tasteless but has a bitter after-taste. |
|
Solubility: |
Soluble in water (1:333), ethanol (1:25), and chloroform (1:2) |
|
pKa: |
3.4 |
|
pH: |
The standard intravenous solution (5 mg/mL) has a pH of 6.2-6.9 (McEvoy, 2003). |
Pharmaceutical incompatibilities: Diazepam should not be mixed with other drugs in the same infusion solution or in the same syringe (Roche, 1988a) as there are numerous incompatibilities (Trissel, 2003).
A method for the synthesis of diazepam has been described (Sternbach et al., 1961). Benzoyl chloride reacts with p-chloroaniline to produce 2-amino-5-chlorobenzophenone. This is converted to the oxime with hydroxylamine. After cyclization with chloroacetyl chloride and ring enlargement with alkali treatment, 7-chloro-1,3-dihydro-5-phenyl-2H-1,4-benzodiazepin-2-one-4-oxide is reduced and methylated to diazepam.
See section 4.1.
Diazepam for injection (British Pharmacopoeia 1988) is a solution of diazepam in a suitable solvent, it is available as 2 mL ampoules, 10 mL vials and disposable syringes containing 5 mg diazepam/mL. The Roche products contain diazepam dissolved in 40% propylene glycol, with 10% ethanol, 5% sodium benzoate and benzoic acid and 1.5% benzyl alcohol. This formulation produces a greenish-yellow solution (Roche, 1988b).
For pharmaceutical quality control purposes the procedure described in the British Pharmacopoeia is satisfactory. This depends on the light absorption at 230-333 nm of a 0.0005% solution in 0.05M methanolic sulphuric acid. There are two absorbance maxima, 242 and 285 nm: the A(1%, 1cm) at 242 nm is approximately 1020. In the range 325-400 nm there is one absorption maximum at 366 nm: the A(1%, 1cm) is 140-155 (British Pharmacopoeia, 1988).
A solution of 10 mg diazepam in 3 mL sulphuric acid shows a green-yellow fluorescence under ultraviolet light (365 nm) (British Pharmacopoeia, 1988). Other methods of identification have also been described (e.g. Abdel-Hamid et al., 1984).
A high performance liquid chromatography (HPLC) method can be used to determine diazepam concentrations in blood. Extraction is with ethyl acetate/methylcyclohexane. The mobile phase is a mixture of methanol and Sorensen's buffer pH 7.8, used at a flow rate of 1.00 mL/minute and the column is u-Bondipak C18. A proprietary internal standard is used (Roche, 1989). This method is similar to that described by Klockowski & Levy (1987), where carbamazepine was used as the internal standard. An absorbance detector set at 254 nm was used and the method also measures the pharmacologically active metabolites (desmethyldiazepam, oxazepam) as well as the parent compound. An alternative HPLC method was described by Mura et al. (1987).
Various methods have been described for the detection of organophosphates in biological fluids but measurement of red blood cell (or plasma) acetylcholinesterase is more commonly used for diagnosis, and often to determine the severity of poisoning.
Diazepam should be protected from light. Roche gives a shelf life of vials of three years and recommends storage at a maximum of 30 °C (Roche, 1988a).
The injectable solution retains potency for three months at room temperature when packed in tubex cartridges (Levin et al., 1973). Diazepam may be absorbed onto plastic surfaces.
Diazepam is a benzodiazepine central nervous system depressant and anxiolytic. Its main use is in anxiety states and it is an effective sedative. The drug has muscle relaxant properties (Diamantis & Kletzkin, 1966) and has minor effects on the cardiovascular system (Baldessarini, 1980). Long-term use can result in habituation and a withdrawal syndrome, which may include convulsions. Diazepam is effective in treating convulsions from a variety of causes (Pieri et al., 1981); however tolerance to the anticonvulsant effects of benzodiazepines can develop, and consequently, benzodiazepines are used rarely in the long-term management of epilepsy (Sellström, 1992).
The main site of action of diazepam, as with other benzodiazepines, is at the gamma-aminobutyric acid (GABA) receptor. The GABAA receptor is a ligand-gated chloride ion channel and part of a superfamily of receptors which also includes the nicotinic acetylcholine receptor and the glycine receptor (Ortells & Lunt, 1995). GABA is the major inhibitory neurotransmitter in the mammalian central nervous system. Benzodiazepines including diazepam alter GABA binding at the GABAA receptor in an allosteric fashion but these drugs do not directly activate the receptors (Charney et al., 2001), which may account in part for their safety. Therefore the benzodiazepines may be envisaged as potentiating the effects of GABA receptors in the central nervous system (Costa & Guidotti, 1979). Thus, the benzodiazepines potentiate the endogenous control of the central nervous system against hyper-excitation (Sellström, 1992) and inhibit calcium channels (Rampe et al., 1987). The discovery of the specific benzodiazepine receptors in the central nervous system has also resulted in the development of an effective specific antagonist (flumazenil) (see Volume I of this series). The GABAA receptor is composed of five subunits and a number of these have been cloned. It is probable that the majority of receptors are composed of alpha, beta and gamma subunits but other subunits also exist (delta, epsilon, pi). It has been suggested that the various actions of the benzodiazepines may be mediated by different GABAA receptor subtypes (see reviews by Johnston, 1996 and Sieghart & Sperk, 2002).
Diazepam was one of the earliest anxiolytics; therefore, the number of reported animal studies is very large. Only the studies investigating its use in the context of OP poisoning are summarized below. It should be noted that in most studies the organophosphate compounds used were substances not used as pesticides (e.g. diisopropyl phosphorofluoridate and nerve agents, including soman, tabun, sarin, VX). In addition, in some cases antidotal therapy was given as a pretreatment prior to organophosphate exposure. Consequently, the results of these studies have limited clinical relevance.
In the context of organophosphorus insecticide poisoning, most experimental work on the efficacy of diazepam has been carried out using treatment combinations of atropine, oximes and diazepam. Most frequently, the mode of administration has been by the intravenous route. Furthermore, a considerable proportion of the experimental work has been designed to evaluate the use of diazepam in nerve agent poisoning. Some studies have been performed in which carbamate pretreatment has also been applied. Some of the studies involving nerve agent poisoning are discussed here as these chemicals are very similar to organophosphorus insecticides.
Most studies involving use of diazepam, when used in combination with other antidotes, in the management of poisoning with both nerve agents and other OP compounds have demonstrated a beneficial effect of diazepam administration.
Rump & Grudzinska (1974) showed that diazepam, as an adjunct treatment to atropine and obidoxime, considerably raised the LD50 of diisopropyl phosphorofluoridate in rats. Also in rats, Bokonjić et al. (1987) showed that atropine and diazepam decreased the acute lethal toxicity of quinalphos 3.3-fold. Addition of the oxime HI-6 to the therapeutic combination further increased protection. In the same species, Rump et al. (1976) found atropine and diazepam had a greater beneficial effect upon the LD50 of diisopropyl phosphorofluoridate than atropine alone, when given two hours after exposure. Krutak-Krol & Domino (1985) showed that pretreatment with atropine and diazepam in the rat reduced the acute lethal toxicity of paraoxon compared to atropine alone. Furthermore, in buffalo calves, a combination of atropine and diazepam was reported to be more effective than atropine alone; this combination was as effective as the combination of 2-PAM (pralidoxime chloride) and atropine in preventing death (Gupta, 1984). Kassa & Bajgar (1994) reported that in the rat, treatment with atropine, obidoxime and diazepam practically eliminated the stressogenic effects of dichlorvos (as determined by measurement of corticosterone and tyrosine aminotransferase concentrations).
Not all studies, however, combining diazepam and an oxime and/or atropine have shown a beneficial action on lethality. For example, Kleinrok & Jagiełło-Wojtowicz (1977) found no effect on the LD50 of diisopropyl phosphorofluoridate in mice pretreated with diazepam and given obidoxime or obidoxime in combination with atropine.
Lipp (1972, 1973) showed that a combination of diazepam and atropine in monkeys exposed to soman was more effective than atropine alone in abolishing seizure activity and preventing death. In rabbits poisoned with soman Johnson & Lowndes (1974) found that pre-treatment with diazepam and atropine increased the antidotal activity of atropine, while Bošković (1981) found that atropine and diazepam trebled survival time. Similarly, a combination of atropine and diazepam, after carbamate pretreatment of guinea pigs poisoned with tabun, sarin, VX and soman also increased the LD50 (Inns & Leadbeater, 1983).
In addition to its action in the central nervous system, diazepam also abolishes the effect of soman in producing repetitive electrical activity in the rat anterior tibial and phrenic nerves (Johnson & Lowndes, 1974).
The efficacy of diazepam alone in reducing lethality in organophosphate poisoned individuals is doubtful.
Diazepam alone had no significant effect on the LD50, when administered to rats 30 seconds after injection with diisopropyl phosphorofluoridate (Rump & Grudzinska, 1974) or when given to mice as a pretreatment for the same compound (Kleinrok & Jagiełło-Wojtowicz, 1977). Furthermore, Krutak-Krol & Domino (1985), who pretreated rats with diazepam and/or atropine before exposure to paraoxon, found that atropine alone reduced lethality but did not prevent convulsions, whilst the reverse was true with diazepam.
Tonkopii et al. (1978) found that the toxicity of ethyl-p-nitrophenyl ethylphosphonate (Armin) and paraoxon was unchanged when diazepam was used to treat mice poisoned with these two substances. Matsubara & Horikoshi (1983) found that diazepam, given immediately after oral administration of fenitrothion to rats, marginally worsened the outcome of poisoning.
In buffalo calves (Gupta, 1984) given sublethal oral doses of malathion, diazepam or 2-PAM given alone appeared to increase lethality (survival ratios of 0/12 and 0/9, respectively; 3/3 in controls). It is, however, difficult to interpret the clinical relevance of these data in large animals not given any supportive therapy.
A study in dichlorvos-poisoned rats found that pretreatment with diazepam or atropine significantly improved survival. This study supported the hypothesis that OP-induced respiratory depression is due to paradoxical over-stimulation of the CNS respiratory centres (Dickson et al., 2003).
In rats, diazepam has been shown to be effective in the management of soman-induced convulsions (Lundy et al., 1978; Churchill et al., 1987).
Doebler et al. (1985) showed that diazepam pretreatment almost completely prevented soman-induced RNA depletion in rats, but did not result in any change in mean time of death or in 24-hour survival. These data were interpreted as indicative of a beneficial activity of diazepam against soman-induced central effects, including convulsions, but that convulsions were not associated with the lethal activity of soman. Moreover, diazepam may exacerbate respiratory depression in soman-poisoned rabbits (Johnson & Wilcox, 1975). In contrast to these reports, a marginal benefit, as measured by alleviation of repetitive electrical activity near the motor endplate, was seen after pretreatment with diazepam alone in soman-poisoned rabbits (Johnson & Lowndes, 1974).
Martin et al. (1985) showed that histological changes produced in the central nervous systems of rats by injection of just sublethal doses of soman could be prevented with diazepam, injected 10 minutes prior to dosing. This suggests that sequelae commonly associated with convulsions might be preventable with diazepam administration.
A number of studies have been performed, which are relevant to the question of whether diazepam has any action in organophosphate poisoning other than as an anticonvulsant. These studies have concentrated on various aspects of the cholinergic system, as well as the GABAergic system and cGMP concentrations. Very large doses of diazepam (20 mg/kg), increased the acetylcholine content of mouse brain (Tonkopii et al., 1978). In the corpus striatum and hippocampus of rats exposed to sarin and soman, diazepam decreased the magnitude of the elevations in choline concentrations, but not those of acetylcholine (Flynn & Wecker, 1986). On the basis of studies on the acetylcholine synthetic system of the mouse brain, Lundgren et al. (1987) suggested that in addition to observed effects on acetylcholine turnover, diazepam might have an effect on choline transport across the blood-brain barrier. Whether diazepam-induced effects on the GABAergic system are responsible for anticonvulsant activity in soman poisoning is unresolved (Lundy et al., 1978). An effect on soman-induced elevations in central nervous cGMP concentrations has been hypothesized as a mechanism of action of the benzodiazepines (Lundy & Magor, 1978; Bošković, 1981).
Numerous studies in experimental animals have shown that absorption of diazepam following oral administration is almost complete (Mandelli et al., 1978; Roche 1988b). In the present context, however, only intravenous administration is of relevance.
In all species examined, Klotz et al. (1976a) found that more than 85% of the plasma diazepam was protein bound; a similar high degree of protein binding was observed with the metabolite desmethyldiazepam (Table 1).
Table 1. Plasma protein binding of diazepam & desmethyldiazepam (% bound). Data from Klotz et al. (1976a).
|
Species |
Sex |
Diazepam |
Desmethyldiazepam |
|
Human |
Male |
96.8 |
96.6 |
|
Dog |
Not stated |
96.0 |
95.6 |
|
Rabbit |
Not stated |
89.9 |
94.7 |
|
Guinea Pig |
Not stated |
91.3 |
78.6 |
|
Rat |
Not stated |
86.3 |
90.5 |
The distribution and elimination of diazepam was studied in dogs, rabbits, guinea pigs and rats, as well as humans, by Klotz et al. (1976a,b). A two-compartment open model for elimination was probably appropriate in the experimental species, as well as humans. However, major pharmacokinetic differences greatly detract from the value of the animal data (Table 2). In the presence of adequate data on human volunteers (see below), this does not greatly matter.
Table 2. Pharmacokinetics of diazepam in various species. Data from Klotz et al. (1976a). Figures for pharmacokinetic variables are mean ± SD.
|
Species |
Sex |
T2alpha |
T2beta |
Volume of distribution (L/kg) |
|
Human |
Male |
0.96 ± 0.34 |
32.9 ± 8.8 |
0.89 ± 0.18 |
|
Dog |
Not stated |
0.46 ± 0.10 |
7.6 ± 1.2 |
5.6 ± 1.3 |
|
Rabbit |
Not stated |
0.30 ± 0.11 |
2.7 ± 0.3 |
5.5 ± 1.5 |
|
Guinea Pig |
Not stated |
0.30 |
2.4 |
2.5 |
|
Rat |
Not stated |
0.29 ± 0.11 |
1.1 ± 0.2 |
4.5 ± 0.6 |
In cats, diazepam is rapidly distributed in the grey matter and more slowly accumulated by the white matter; the effect of repeated dosing suggests that the latter acts as a deep compartment (Morselli et al., 1973). Since diazepam is lipophilic it is likely to be distributed to adipose tissue and possibly released upon fasting.
The main metabolic pathway is demethylation and hydroxylation to active metabolites (desmethyldiazepam & oxazepam) in animals and humans. Biliary excretion of diazepam metabolites does not seem to be important in rats (Klotz et al., 1975; 1976a), but it may be of more significance in mice (van der Kleijn et al., 1971).
Very extensive toxicological studies have been carried out on diazepam. Many of the data obtained are only relevant to its use as an anxiolytic over long periods, but some long-term toxicological studies are discussed briefly below.
The acute toxicity of diazepam is a result of its central nervous depressant effect (Table 3). In these animal studies few clinical signs have been observed other than somnolence preceding death. The drug is notably more toxic by the intravenous route than by other modes of administration.
Table 3. Acute lethality data for diazepam: LD50 for several species by a variety of routes.
|
Species |
Sex |
Route |
LD50 mg/kg (range) |
Reference |
|
Mice |
male |
i.v. |
25 ± 1.1 |
Pieri et al. (1981) |
|
male |
332 (313-353) |
Scrollini et al. (1975) |
||
|
male |
i.p. |
300 |
Gogerty et al. (1977) |
|
|
mixed |
i.p. |
121 (82.5-178) |
Kulkarni et al. (1982) |
|
|
male |
p.o. |
970 ± 66 |
Pieri et al. (1981) |
|
|
male |
p.o. |
686 (642-732) |
Scrollini et al. (1975) |
|
|
Rat |
male |
i.p. |
663 (622-707) |
Scrollini et al. (1975) |
|
male |
p.o |
2075 (1760-2445) |
Scrollini et al. (1975) |
|
|
Rabbit |
not stated |
i.v. |
8.8 ± 1.6 |
Diamantis & Kletzkin (1966) |
|
male |
p.o. |
454 (366-562) |
Scrollini et al. (1975) |
A number of repeated dose studies have been carried out. In general, toxic effects have not been remarkable. In a three-month study in rats (50, 100 or 200 mg/kg/day orally) and a six-month study in dogs (20 mg/kg/day orally), some increase in liver size was seen, together with a rise in blood cholesterol; in dogs an elevation of plasma alanine aminotransferase activity was also observed (Scrollini et al., 1975). The clinical significance of these data is unclear. In view of findings with other benzodiazepines, carcinogenicity studies on diazepam are of interest. De la Iglesia et al. (1981) found no increase of tumour frequency after feeding diazepam, 75 mg/kg/day to rats and mice for 104 and 80 weeks, respectively. In this study, diazepam was being used as a reference material in a carcinogenesis bioassay of prazepam. A metabolite of diazepam, oxazepam, when added to the diet, is reported to have produced liver tumours in mice (Fox & Lahcen, 1974; IARC, 1996).
A large amount of work has been carried out on the possible adverse effects of diazepam on the fetus. Earlier work, for example that of Scrollini et al., (1975) in rats, rabbits and dogs (see previous section), failed to suggest an adverse effect from dosing during pregnancy. However, Ryan & Pappas (1986) showed that in the rat, prenatal exposure to diazepam (1.0 or 5.0 mg/kg s.c.) caused fetal toxicity and long-term neurobehavioural alterations in the progeny. Long-term changes were also observed in the behaviour of cats born to mothers treated with diazepam during pregnancy (average dose of 0.4 mg/kg/day, i.m. between days 20 and 53). Deficits in the number of benzodiazepine receptors were also seen in the central nervous system (Livezey et al., 1986). Developmental neurotoxicity was observed in rats exposed perinatally to diazepam. The drug (2.0 mg/kg/day) was given to the mothers from gestational day 14 to 20 or from the 1st to the 21st day of lactation (Silva & Palermo-Neto, 1999). Martire et al. (2002) reported that in rats administered diazepam (1.25 mg/kg s.c.) on days 14-20 of gestation, there was functional alteration of the hippocampal GABAA receptors in the offspring. Other observations have included alteration to the hypothalamic-pituitary-thyroid axis in rats administered 0.2 or 2 mg/kg s.c. on days 15-21 of gestation (Fujii et al., 1983).
There is a report of diazepam being positive in the Salmonella typhimurium tester strain TA100 in the Ames test (Batzinger et al., 1978). Diazepam was genotoxic in mouse bone marrow micronucleus tests in vivo (Das & Kar, 1986; Leal Garza et al., 1998), but little or no effect was seen in an assay for chromosomal aberrations, performed in Chinese hamster cells in vitro (Matsuoka et al., 1979).
Studies in healthy volunteers have elucidated the pharmacokinetics of diazepam in humans. Qualitatively, the pharmacokinetics of diazepam in humans and in experimental animals appears similar, but quantitatively there are considerable differences (Table 2).
In organophosphate poisoning diazepam is usually given intravenously. Blood concentrations of 400 and 1,200 µg/L were found 15 minutes after intravenous bolus doses of 10 and 20 mg, respectively, in 9 volunteers aged 19-35 (body weight not stated) (Hillestad et al., 1974).
Absorption following oral administration is almost complete with bioavailability close to 1 (Mandelli et al., 1978). Absorption is relatively poor after intramuscular injection; plasma concentrations attained are equal to only 60% of those reached after the same oral dose (Hillestad et al., 1974). Nevertheless, intramuscular diazepam was used in the organophosphate-poisoned patient described by Vale & Scott (1974).
In human volunteers, the plasma protein binding of diazepam was greater than 95% (Klotz et al., 1976a; Mandelli et al., 1978). The concentration in the cerebrospinal fluid (CSF) seems to correspond to the plasma free fraction (Kanto et al., 1975). The volume of distribution of diazepam has been estimated to be 0.95-2 L/kg (Mandelli et al., 1978).
A two-compartment open model is usually used to describe elimination kinetics of diazepam and a plasma clearance of 26-35 mL/min after a single intravenous dose has been reported (Klotz et al., 1975; Andreasen et al., 1976; Klotz et al., 1976a). The half-life for diazepam in the beta-phase is 1-2 days with a longer half-life for the active metabolite desmethyldiazepam (Klotz et al., 1976a; Mandelli et al., 1978). The half-life of diazepam appears to increase with age (Klotz et al., 1975) and in individuals with liver damage (Andreasen et al., 1976). Elimination is slower after subchronic dosing (Klotz et al., 1976b). Although there is some correlation between response to diazepam and plasma concentrations after single doses (Booker & Celesia, 1973), this disappears with repeated dosing (Mandelli et al., 1978). Moreover, there is evidence that the disposition of diazepam is affected by chronic dosing and by plasma desmethyldiazepam concentrations (Klotz et al., 1976b).
There is some evidence for species differences in biliary excretion (see above), but studies by Klotz et al. (1975, 1976a,b) suggest that biliary excretion of diazepam is unimportant in humans. Urinary excretion of diazepam is mainly in the form of sulphate and glucuronide conjugates (Mandelli et al., 1978).
Clinical trials of diazepam used either alone or in combination with other therapy in the management of organophosphorus insecticide poisoning are not available. There are no randomised clinical trials comparing the use of diazepam or other benzodiazepines with a placebo in the management of OP poisoning, and it would be considered clinically unethical to undertake such a study in patients with convulsions (Eddleston et al., 2002).
Studies have shown that an anticonvulsant effect of diazepam is obtained at plasma concentrations of 400-500 µg/L (1390-1740 nmol/L) or above, corresponding to a single intravenous dose of 10-20 mg in an adult (Hvidberg & Dam, 1976). A single intravenous dose of 20 mg would produce a concentration above 400 µg/L for just over 2 hours, based on the data of Hillestad et al. (1974).
Patients with severe organophosphorus insecticide poisoning are generally treated with atropine and an oxime; diazepam was used only as an adjunct to antidotal therapy although its use has become more widespread (Karalliedde & Szinicz, 2001). In the case reports cited below, diazepam was administered if convulsions or muscle fasciculation were present. The only conclusion that can be derived from these reports is that the use of diazepam in these cases was associated with cessation of convulsions and/or muscle fasciculation and a favourable outcome.
Some series reporting the use of diazepam in organophosphate poisoning have been very large (Yacoub et al., 1981; Kušić et al., 1991). In the latter st
udy, which was to evaluate the efficacy of the oxime HI-6 in OP poisoning, diazepam (total doses varied from 30 mg to 230 mg) was part of the standard therapy and used as an anticonvulsant or to reduce the central effects of atropine. In a review of 908 cases of OP poisoning (79% of the suicide cases involved parathion) anticonvulsants were used in 99 patients. The drugs used were diazepam, phenobarbital and chlormethiazole; diazepam was used most frequently but no assessment of its efficacy was made (Yacoub et al., 1981).Diazepam has been used in dimethoate intoxication (Marti et al., 1985; LeBlanc et al., 1986). The patient of Marti et al. (1985) was atropinized, while in the latter case, the patient was treated with both 2-PAM and atropine (a total of 30g was given). Diazepam was given, together with phenytoin and phenobarbital, to control seizures. In a case reported by de Kort et al. (1988) diazepam 20 mg, together with phenytoin 375 mg and phenobarbital by infusion was used to combat convulsions induced by poisoning with an unknown organophosphate. Atropine and obidoxime were also given to this patient; nevertheless a cardiac arrest could not be reversed. Another case, where the patient had fasciculations, was described in the same report. Treatment was primarily with N-methylatropine nitrate and obidoxime, but 60 mg/day of diazepam was administered "for sedation" and the patient recovered. The apparently successful use of diazepam in combination with the oxime HI-6 in a series of organophosphate-poisoned patients has been described (Kušić et al., 1991).
A number of case reports describe the use of diazepam primarily for the management of muscle fasciculation. In one of the earliest case reports of the use of diazepam in organophosphorus insecticide poisoning (Barckow et al. 1969), three victims of parathion exposure were treated with a number of drugs including atropine and obidoxime; two patients received diazepam for muscle fasciculation. Likewise, Vale & Scott (1974) used diazepam to suppress the muscle fasciculation induced by occupational poisoning with demeton-S-methyl. Jovanović et al. (1990) found diazepam effective in suppressing psychomotor agitation produced by poisoning with a dimethoate formulation. A curiosity in the literature is an attempted suicide with an organophosphorus insecticide (fenthion) in combination with diazepam (Merrill & Mihm, 1982). In this case, although the patient showed muscle fasciculation, she did not convulse. Since the subsequent course was prolonged and indicative of severe intoxication, the absence of convulsions may have been due to the co-ingestion of diazepam.
In the absence of convulsions, diazepam administration at doses of 5-10 mg intravenously in adults has also been recommended in cases of organophosphate poisoning accompanied by anxiety and restlessness (Johnson & Vale, 1992) or muscle fasciculation. If large doses of diazepam are required to suppress seizure activity, barbiturates should be considered as an alternative.
The most frequent adverse effects of diazepam are drowsiness, muscle weakness and ataxia. Less frequently reported effects include dysarthria, dizziness, confusion, visual disturbances, urinary retention or incontinence and amnesia. Some patients may develop paradoxical excitation with aggression and disinhibition. Respiratory depression and hypotension may occur with high doses, particularly when given parentally (Sweetman, 2002). Pain and thrombophlebitis may occur with some intravenous formulations.
Animal studies demonstrate that diazepam prevents and treats convulsions produced by organophosphates and may prevent the late effects produced by damage to the central nervous system resulting from convulsions. Clinically, there is evidence from case reports that diazepam is effective in managing muscle fasciculation and convulsions in organophosphate poisoning.
There are no data, either experimental or clinical, demonstrating any positive effect of diazepam alone on outcome in organophosphate poisoning. However, in animals experimentally poisoned with organophosphates combined treatment with atropine and diazepam significantly reduced lethality compared to atropine treatment alone, indicating a clear positive effect.
Whether the GABAergic effects of the benzodiazepines have any relation to their effect in organophosphate poisoning remains unproven.
In one study of large animals diazepam given alone increased lethality. This outcome, however, was probably related to a combination of diazepam-induced respiratory depression and organophosphate-induced bronchial hypersecretion, without adequate supportive therapy and other antidotes.
Based on the summary above, diazepam should be given to any organophosphate-poisoned patient with convulsions. In a potentially severe exposure, prophylactic use of diazepam may be beneficial. Diazepam is also useful for sedation in patients with anxiety and has been used to reduce muscle fasciculations. It should be noted that diazepam does not replace the use of atropine or an oxime in the management of organophosphate poisoning.
If artificial ventilatory support is indicated in a patient, even without fasciculation or convulsions, it seems justified to use diazepam for sedation.
There is no basis for a specific dosage regimen for diazepam controlling muscle fasciculation or convulsions in organophosphate poisoning. Therefore, the standard intravenous dose used for convulsions from other causes is recommended: 10-20 mg i.v. slowly in adults and 300-400 µ g/kg i.v. in children, repeated as necessary.
Diazepam is an adjunct to supportive therapy and the specific antidotes, atropine and an oxime. If for some reason, convulsions cannot be controlled by diazepam, a barbiturate (such as phentobarbital) or phenytoin should be considered.
The routine use of diazepam in organophosphate poisoning, though based theoretically upon the GABAergic properties of the drug, is controversial and not recommended.
Diazepam should not be considered as an ‘antidote’ to organophosphate poisoning because its effects are non-specific.
Although the place of diazepam in organophosphate-induced convulsions is well established, the use of this drug in other aspects of organophosphate poisoning, for example to control muscle fasciculation, needs further investigation in an animal model, with particular respect to the prevention of myopathic changes. There are no randomised clinical trials comparing the use of diazepam with a placebo in the management of OP poisoning, and it would be considered clinically unethical to undertake such a study in patients with convulsions.
No significant adverse effects have been related to the recommended use of diazepam in organophosphate poisoning. Of the well-recognised side-effects of diazepam, respiratory depression is probably the most important, particularly in patients with bronchial hypersecretion and/or impending muscle weakness.
Diazepam has been used for many years and there appears to be no restrictions to its use in organophosphate poisoning. In the elderly and in patients with respiratory problems, however, equipment for ventilatory support should be readily available if diazepam is to be used.
Diazepam is indicated in poisoning by organophosphorus insecticides where convulsions or pronounced muscle fasciculation are present.
If artificial ventilatory support is indicated in an organophosphate-poisoned patient without fasciculation or convulsions, diazepam is the drug of choice for sedation.
Where convulsions are present, the standard intravenous dosage regimen for treatment of convulsions is used:
|
Adults: |
10-20 mg i.v. at a rate of 5 mg/minute, repeated after 30-60 minutes if required. A slow i.v. infusion providing up to 3 mg/kg over 24 hours may be given (Sweetman, 2002). |
|
Children: |
300-400 µg/kg i.v. or by infusion at a rate of 100 µg/kg/hour to a maximum of 400 µg/kg/hour (RCPCH, 2003). |
|
Elderly: |
half the adult dose |
These doses may be repeated as necessary, or followed by slow intravenous infusion. No maximum dose can be recommended as the severity of convulsions may vary. However, if more than 3-5 mg/kg in adults is needed over 24 hours, other possible causes for the refractory convulsions should be investigated e.g. insufficient atropine/oxime therapy, inadequate oxygenation or ventilatory support.
Diazepam should not be given to patients with uncorrected impaired ventilation unless hypoxia is due to severe convulsions. Diazepam may also aggravate hypotension in critically ill patients.
Diazepam potentiates the sedative effects of other sedatives/hypnotics, especially alcohol.
Diazepam should not be mixed with other drugs in the same infusion solution or in the same syringe as there are numerous incompatibilities.
Adverse effects are usually mild and consist of dizziness, sedation, respiratory depression and hypotension. However, with the intravenous doses needed in some organophosphate-poisoned patients, respiratory depression may be significant. Intravenous administration of diazepam may cause thrombophlebitis unless special formulations, an emulsion such as DiazemulsR, rather than a solution, are given.
The use of diazepam in pregnancy is not contraindicated. Acute organophosphate poisoning represents the major risk to the fetus and should be managed as normal, including the use of diazepam. Breast feeding should not be performed because many organophosphate insecticides are highly lipid soluble; in addition diazepam is also lipid-soluble and is excreted in breast milk.
Vials stored below 30°C have a shelf life of 3 years.
Abdel-Hamid ME, Abdel-Khalek MM, & Mahrous MS (1984) Application of difference and derivative ultraviolet spectrometry for assay of some benzodiazepines. Anal Lett, 17: 1353-1371.
Andreasen PB, Hendel J, Greisen G, & Hvidberg EF (1976) Pharmacokinetics of diazepam in disordered liver function. Eur J Clin Pharmacol, 10: 115-120.
Baldessarini RJ (1980) Drugs and the treatment of psychiatric disorders. In: Gilman AG, Goodman LS, Gilman A eds. Goodman and Gilman's the pharmacological basis of therapeutics. New York, Macmillan, pp 391-447.
Barckow D, Neuhaus G, & Erdmann WD (1969) [Treatment of parathion (E 605) poisoning with the cholinesterase-reactivating substance obidoxime (Toxogonin)]. (German) Arch Toxikol, 24: 133-146.
Batzinger RP, Ou SY, & Bueding E (1978) Antimutagenic effects of 2(3)-tert-butyl-4-hydroxyanisole and of antimicrobial agents. Cancer Res, 38: 4478-4485.
Bokonjić D, Jovanović D, Jokanović M, & Maksimović M (1987) Protective effects of oximes HI-6 and PAM-2 applied by osmotic minipumps in quinalphos-poisoned rats. Arch Int Pharmacodyn Ther,
288: 309-318.Booker HE & Celesia GG (1973) Serum concentrations of diazepam in subjects with epilepsy. Arch Neurol, 29: 191-194.
Bošković B (1981) The treatment of soman poisoning and its perspectives. Fund Appl Toxicol,
1: 203-213.British Pharmacopoeia (1988) London, Her Majesty's Stationary Office, pp 183-184.
Churchill L, Padzernik TL, Cross RS, Giesler MP, Nelson SR, & Samson FE (1987) Cholinergic systems influence local cerebral glucose use in specific anatomical areas: diisopropyl phosphorofluoridate versus soman. Neuroscience, 20: 329-340.
Costa E & Guidotti A (1979) Molecular mechanisms in the receptor actions of benzodiazepines. Ann Rev Pharmacol Toxicol, 19: 531-545.
Das RK & Kar RN (1986) Genotoxic effects of three benzodiazepine tranquilizers in mouse bone marrow as revealed by the micronucleus test. Caryologia, 39: 193-198.
de Kort WM, Kiestra SH, & Sangster B (1988) The use of atropine and oximes in organophosphate intoxications: a modified approach. Clin Toxicol, 26: 199-208.
de la Iglesia FA, Basoum N, Gough A, Mitchell L, Martin RA, di Fonzo C, & McGuire EJ (1981) Carcinogenesis bioassay of prazepam (Verstran) in rats and mice. Toxicol Appl Pharmacol, 57: 39-54.
Diamantis W & Kletzkin M (1966) Evaluation of muscle relaxant drugs by head-drop and by decerebrate rigidity. Int J Neuropharmacol, 5: 305-310.
Dickson EW, Bird SB, Gaspari RJ, Barnett KA, & Ferris CF (2003) Diazepam inhibits organophosphate-induced central respiratory depression. Acad Emerg Med, 10: 1303-1306.
Doebler JA, Wall TJ, Martin LJ, Shih T-MA, & Anthony A (1985) Effects of diazepam on soman-induced brain neuronal RNA depletion and lethality in rats. Life Sci, 36: 1107-1115.
Eddleston M, Singh S, & Buckley N (2002) Acute organophosphate poisoning. Clin Evid, 9: 0-2.
Flynn CJ & Wecker L (1986) Elevated choline levels in brain. A noncholinergic component of organophosphate toxicity. Biochem Pharmacol, 35: 3115-3122.
Fox KA & Lahcen RB (1974) Liver-cell adenomas and peliosis hepatitis in mice associated with oxazepam. Res Commin Chem Pathol Pharmacol, 8: 481-488.
Fujii T, Yamamoto N, & Fuchino K (1983) Functional alterations in the hypothalamic-pituitary-thyroid axis in rats exposed prenatally to diazepam. Toxicol Lett, 16: 131-137.
Gogerty JH, Griot RG, Habeck D, Iorio LC, & Houlihan WJ (1977) Synthesis and central nervous system evaluation of some 6-alkoxy-3H-1,4-benzodiazepin-2(IH)-ones. J Med Chem, 20: 952-956.
Gupta RC (1984) Acute malathion toxicosis and related enzymatic alterations in Bubulus bubalis: antidotal treatment with atropine, 2-PAM, and diazepam. J Toxicol Environ Health, 14: 291-303.
Hayes MMM, van der Westhuizen NG, & Gelfand N (1978) Organophosphate poisoning in Rhodesia. A study of the clinical features and management of 105 patients. S Afr Med J, 54: 230-234.
Hillestad L, Hansen T, Melsom H, & Drivenes A (1974) Diazepam metabolism in normal man 1. Serum concentrations and clinical effects after intravenous, intramuscular, and oral administration. Clin Pharmacol Ther, 16: 479-484.
Hvidberg EF & Dam M (1976) Clinical pharmacokinetics of anticonvulsants. Clin Pharmacokinet, 1: 161-188.
IARC (1996) IARC monographs on the evaluation of carcinogenic risks of chemicals to man. Vol 66, Some pharmaceutical drugs. Lyons, International Agency for Research on Cancer, p 115.
Inns RH & Leadbeater L (1983) The efficacy of bispyridinium derivatives in the treatment of organophosphate poisoning in the guinea-pig. J Pharm Pharmacol, 35: 427-433.
IPCS (1986) Environmental Health Criteria 63, Organophosphorus insecticides: a general introduction. Geneva, World Health Organization.
Johnson DD & Lowndes HE (1974) Reduction by diazepam of repetitive electrical activity and toxicity resulting from soman. Eur J Pharmacol, 28: 245-250.
Johnson DD & Wilcox WC (1975) Studies on the mechanism of the protective and antidotal actions of diazepam in organophosphate poisoning. Eur J Pharmacol, 34: 127-132.
Johnson MK & Vale JA (1992) Clinical management of acute organophosphate poisoning: an overview. In: Clinical and experimental toxicology of organophosphates & carbamates. In: Ballantyne B & Marrs TC eds. Oxford, Butterworth-Heinemann, pp 528-535.
Johnston GAR (1996) GABAA receptor pharmacology. Pharmacol Ther, 69: 173-198.
Jovanović D, Randjelović S, & Joksović DC (1990) A case of unusual suicidal poisoning by the organophosphorus insecticide, dimethoate. Hum Exp Toxicol,
9: 49-51.Kanto J, Kangas L, & Siirtola T (1975) Cerebrospinal-fluid concentrations of diazepam and its metabolites in man. Acta Pharmacol Toxicol (Copenh), 36: 328-334.
Karalliedde L & Senanyake N (1989) Organophosphate insecticide poisoning. Br J Anaesth, 63: 736-750.
Karalliedde L & Szinicz L (2001) Management of organophosphorus compound poisoning. In: Karalliedde L, Feldman S, Henry J, Marrs T eds. Organophosphates and Health. London, Imperial College Press, pp 257-293.
Kassa J & Bajgar J (1994) Treatment of stressogenic effect of dichlorvos. Sb Ved Pr Lek Fak Karlovy Univerzity Hradci Králove, 37: 29-32.
Kleinrok Z & Jagiełło-Wojtowicz E (1977) [The influence of some anticonvulsant drugs on the acute toxicity of disopropylfluorophosphate in mice] (Polish). Ann Univers Mariae Curie-Skłodowska (Lublin),
34: 127-132.Klemm WR (1983) Efficacy and toxicity of drug combinations in treatment of physostigmine toxicosis. Toxicology, 27: 41-53.
Klotz U, Avant GR, Hoyumpa A, Schenker S, & Wilkinson GR (1975) The effects of age and liver disease on the disposition and elimination of diazepam in adult man. J Clin Invest, 55: 347-359.
Klotz U, Antonin KH, & Bieck PR (1976a) Pharmacokinetics and plasma binding of diazepam in man, dog, rabbit, guinea pig and rat. J Pharmacol Exp Ther, 199: 67-73.
Klotz U, Antonin KH, & Bieck PR (1976b) Comparison of the pharmacokinetics of diazepam after single and subchronic doses. Eur J Clin Pharmacol, 10: 121-126.
Klockowski PM & Levy G (1987) Simultaneous determination of diazepam and its active metabolites in rat serum, brain and cerebrospinal fluid by high-performance liquid chromatography. J Chromatogr, 422: 334-339.
Krutak-Krol H & Domino EF (1985) Comparative effects of diazepam and midazolam on paraoxon toxicity in rats. Toxicol Appl Pharmacol, 81: 545- 550.
Kulkarni YD, Sharma VL, Dua PR, & Shanker G (1982) Substituted benzophenones as possible anticonvulsants and tranquillizers. Indian J Pharm Sci, 44: 1-4.
Kušić R, Jovanović D, Randjelović S, Joksović D, Todorović V, Bošković B, Jokanović M, & Vojvodić V (1991). HI-6 in man: efficacy of the oxime in poisoning by organophospho
rous insecticides. Hum Exp Toxicol, 10: 113-118.Leal Garza CH, Valenciano Cedillo GG, Rojas Alvarad MA, & Cortes Gutierrez EI (1998) Mutagenic activity of diazepam evaluated by in vivo cytogenetic tests. Arch Med Res, 29: 285-289.
LeBlanc FN, Benson BE, & Gilg AD (1986) A severe organophosphate poisoning requiring the use of an atropine drip. Clin Toxicol, 24: 69-76.
Levin HJ, Fieber RA, & Levi RS (1973) Stability data for Tubex filled by hospital pharmacists. Hosp Pharm, 8: 310-311.
Lipp JA (1972) Effect of diazepam upon soman-induced seizure activity and convulsions. Electroenceph Clin Neurophys, 32: 557-560.
Lipp JA (1973) Effect of benzodiazepine derivatives on soman-induced seizure activity and convulsions in the monkey. Arch Int Pharmacodyn Ther, 202: 244-251.
Livezey GT, Marczynski TJ, & Isaac L (1986) Enduring effects of prenatal diazepam on the behavior, EEG, and brain receptors of the adult cat progeny. Neurotoxicology, 7: 319-334.
Lund C & Monteagudo FSE (1986) Therapeutic protocol No 1. Early management of organophosphate poisoning. S Afr Med J, 69: 6.
Lundgren G, Nordgren I, Karlen B, & Jacobsson G (1987) Effects of diazepam on blood choline and acetylcholine turnover in brain of mice. Pharmacol Toxicol, 60: 96-99.
Lundy PM, Magor G, & Shaw RK (1978) Gamma aminobutyric acid metabolism in different areas of rat brain at the onset of soman-induced convulsions. Arch Int Pharmacodyn Ther, 234: 64-73.
Lundy PM & Magor G (1978) Cyclic GMP concentrations in cerebellum following organophosphate administration. J Pharm Pharmacol, 30: 251-252.
Mandelli M, Tognoni G, & Garattini S (1978) Clinical pharmacokinetics of diazepam. Clin Pharmacokinet, 3: 72-91.
Marti J, Reverte C, Roig M, Pascual I, & Prats J (1985) [Use of diazepam in organophosphate insecticide poisoning] (Spanish). An Esp Pediatr, 23: 299-301.
Martin LJ, Doebler JA, Shih T-M, & Anthony A (1985) Protective effect of diazepam pretreatment on soman-induced brain lesion formation. Brain Res, 325: 287-289.
Martire M, Altobelli D, Cannizzaro C, Maurizi S, & Prezi P (2002). Prenatal diazepam exposure functionally alters the GABA(A) receptor that modulates (3H) noradrenaline release from rat hippocampal synaptosomes. Dev Neurosci, 24: 71-78.
Matsubara T & Horikoshi I (1983) Possible antidotes in severe intoxication with fenitrothion in rats. J Pharmacobiodyn, 6: 699-707.
Matsuoka A, Hayashi M, & Ishadate M (1979) Chromosomal aberration tests on 29 chemicals combined with S9 mix in vitro. Mutat Res, 66: 277-290.
McEvoy GK ed (2003) American hospital formulary service drug information. Bethesda, American Society of Health-System Pharmacists, pp 2364-2366.
Merrill DG & Mihm FG (1982) Prolonged toxicity of organophosphate poisoning. Crit Care Med, 10: 550-551.
Morselli PL, Cassano GB, Placidi GF, Muscattola GB, & Rizzo M (1973) Kinetics of the distribution of 14C-diazepam and its metabolites in various areas of cat brain. In: Garattini, S. Mussini L & Randall LO eds. The benzodiazepines. New York, Raven Press, pp 259-270.
Mura P, Piriou A, Fraillon P, Papet Y, & Reiss D (1987) Screening procedure for benzodiazepines in biological fluids by high-performance liquid chromatography using a rapid-scanning multichannel detector. J Chromatogr, 416: 303-310.
Niemegeers CJE, Awouters F, Lenaerts FM, Vermeire J, & Janssen PAJ (1982) Prevention of physostigmine-induced lethality in rats. A pharmacological analysis. Arch Int Pharmacodyn Ther, 259: 153-165.
Ortells MO & Lunt GG (1995). Evolutionary history of the ligand-gated ion-channel superfamily of receptors. Trends Neurosci, 18, 121-127.
Pieri L, Schaffner R, Scherschlicht R, Polc P, & Sepinwall J (1981) Pharmacology of midazolam. Arzneimittelforschung, 31: 2180-2201.
Poirier T, Farnos JM, Charpy E, Meyer G, & Martignon M (1987) Intoxication aigüe par carbamate anticholinésterasique. Place des curares acétylcholino-compétitifs. Ann Franc Anesth Réanim, 6: 457-459.
Rampe D, Ferrante J, & Triggle DJ (1987) The actions of diazepam and diphenylhydantoin on fast and slow Ca2+ uptake in the guinea pig cerebral cortex synaptosome. Can J Physiol Pharmacol, 68: 538-543.
RCPCH. (2003) Medicines for children, 2nd edition. London, Royal College of Paediatrics and Child Health Publications.
Roche (1988a) Valium Roche Ampoules Data Sheet, Roche Products Ltd., Welwyn Garden City, England.
Roche (1988b) Valium Roche Tablets, Syrup & Suppositories Data Sheet. Roche Products Ltd., Welwyn Garden City, England.
Roche (1989) Letter, Medical Dept. Roche Products Ltd., Welwyn Garden City, England.
Rump S & Grudzinska E (1974) Investigations on the effects of diazepam in acute experimental intoxications with fluostigmine. Arch Toxikol, 31: 223-232.
Rump S, Faff J, Szymanska T, Bak W, & Borkowska G (1976) Efficacy of repeated pharmacotherapy in experimental acute poisonings with fluostigmine. Arch Toxicol, 35: 275-280.
Ryan CL & Pappas BA (1986) Intrauterine diazepam exposures: effects on physical and neurobehavioral development in the rat. Neurobehav Toxicol Teratol, 8: 279-286.
Scrollini F, Caliari S, Romano A, & Torchio P (1975) Toxicological and pharmacological investigations of pinazepam (7-chloro-1-propargyl-5-phenyl-3H-1,4-benzodiazepin-2-one): a new psychotherapeutic agent. Arzneimittelforschung, 25: 934-940.
Sellström A (1992) Anticonvulsants in anticholinesterase poisoning. In: Ballantyne B & Marrs TC eds. Clinical & experimental toxicology of organophosphates & carbamates. Oxford-Baltimore, Butterworth-Heinemann, pp 578-586.
Sieghart W & Sperk G (2002). Subunit composition, distribution and function of GABAA receptor subtypes. Curr Top Medicinal Chem, 2: 795-816.
Silva FR & Palermo-Neto J (1999) Developmental, neuro and immunotoxic effect of perinatal diazepam treatment in rats. Immunopharmacol Immunotoxicol, 21: 247-265.
Sternbach LH, Reeder E, Keller O, & Metlesics W (1961) Quinazolines and 1,4-benzodiazepines III substituted 2-amino-5-phenyl-3H-1,4-benzodiazepine 4-oxides. J Org Chem, 26: 4488-4497.
Stocker WG (1982) [Acute poisoning with crop protectors] (German). Intensivmedizin, 19: 255-258.
Sweetman SC (ed) 2002 Martindale. The complete drug reference, 33rd edition. London, Pharmaceutical Press, pp 674-682.
Tonkopii VD, Sofronov GA, Aleksandriiskaya IE, & Brestkina LM (1978) [Mechanism of the effect of diazepam on acetylcholine concentration in mouse brain] (Russian). Biull Eksp Biol Med, 86: 38-40.
Trissel LA (2003) Handbook of injectable drugs, 12th edition. Bethesda, American Society of Health-System Pharmacists, pp 421-430.
Vale JA & Scott GW (1974) Organophosphorus poisoning. Guy's Hosp Rep, 123: 13-25.
van der Kleijn E, van Rossum JM, Muskens ETJM, & Rijntjes NVM (1971) Pharmacokinetics of diazepam in dogs, mice and humans. Acta Pharmacol Toxicol, 29: 109-127.
Yacoub M, Skouri H, Amamou M, & Besencenot F (1981) Intoxications aigües par les insecticides organo-phosphoré. J Toxicol Med, 1: 165-188.
ENDNOTES:
See Also: Toxicological Abbreviations