
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
CONCISE INTERNATIONAL CHEMICAL ASSESSMENT DOCUMENT NO. 4
METHYL METHACRYLATE
INTER-ORGANIZATION PROGRAMME FOR THE SOUND MANAGEMENT OF CHEMICALS
A cooperative agreement among UNEP, ILO, FAO, WHO, UNIDO, UNITAR and
OECD
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by Ms W. Dormer, Ms R. Gomes, and Ms M.E. Meek,
Environmental Health Directorate,
Health Canada
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization Geneva, 1998
The International Programme on Chemical Safety (IPCS),
established in 1980, is a joint venture of the United Nations
Environment Programme (UNEP), the International Labour Organisation
(ILO), and the World Health Organization (WHO). The overall
objectives of the IPCS are to establish the scientific basis for
assessment of the risk to human health and the environment from
exposure to chemicals, through international peer review processes, as
a prerequisite for the promotion of chemical safety, and to provide
technical assistance in strengthening national capacities for the
sound management of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization, and the Organisation for
Economic Co-operation and Development (Participating Organizations),
following recommendations made by the 1992 UN Conference on
Environment and Development to strengthen cooperation and increase
coordination in the field of chemical safety. The purpose of the IOMC
is to promote coordination of the policies and activities pursued by
the Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing in Publication Data
Methyl methacrylate.
(Concise international chemical assessment document ; 4)
1.Methacrylates - toxicity 2.Environmental exposure
3.Occupational exposure I.International Programme on Chemical
Safety II.Series
ISBN 92 4 153004 9 (NLM Classification: QV 50)
ISSN 1020-6167
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1998
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city, or area
or of its authorities, or concerning the delimitation of its frontiers
or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
TABLE OF CONTENTS
FOREWORD
1. EXECUTIVE SUMMARY
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
3. ANALYTICAL METHODS
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1. Environmental levels
6.2. Human exposure
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposure
8.2. Irritation and sensitization
8.3. Short-term exposure
8.4. Long-term exposure
8.4.1. Subchronic exposure
8.4.2. Chronic exposure and carcinogenicity
8.5. Genotoxicity and related end-points
8.6. Reproductive and developmental toxicity
8.7. Immunological and neurological effects
9. EFFECTS ON HUMANS
9.1. Case reports
9.2. Epidemiological studies
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1. Aquatic environment
10.2. Terrestrial environment
11. EFFECTS EVALUATION
11.1. Evaluation of health effects
11.1.1. Hazard identification and dose-response assessment
11.1.2. Criteria for setting guidance values for methyl methacrylate
11.1.3. Sample risk characterization
11.2. Evaluation of environmental effects
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
13.1. Human health hazards
13.2. Advice to physicians
13.3. Health surveillance advice
13.4. Explosion and fire hazards
13.4.1. Explosion hazards
13.4.2. Fire hazards
13.4.3. Fire-extinguishing agents
13.5. Storage
13.6. Transport
13.7. Spillage
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
INTERNATIONAL CHEMICAL SAFETY CARD
REFERENCES
APPENDIX 1 - SOURCE DOCUMENTS
APPENDIX 2 - CICAD PEER REVIEW
APPENDIX 3 - CICAD FINAL REVIEW BOARD
RÉSUMÉ D'ORIENTATION
RESUMEN DE ORIENTACION
FOREWORD
Concise International Chemical Assessment Documents (CICADs) are
the latest in a family of publications from the International
Programme on Chemical Safety (IPCS) - a cooperative programme of the
World Health Organization (WHO), the International Labour Organisation
(ILO), and the United Nations Environment Programme (UNEP). CICADs
join the Environmental Health Criteria documents (EHCs) as
authoritative documents on the risk assessment of chemicals.
CICADs are concise documents that provide summaries of the
relevant scientific information concerning the potential effects of
chemicals upon human health and/or the environment. They are based on
selected national or regional evaluation documents or on existing
EHCs. Before acceptance for publication as CICADs by IPCS, these
documents have undergone extensive peer review by internationally
selected experts to ensure their completeness, accuracy in the way in
which the original data are represented, and the validity of the
conclusions drawn.
The primary objective of CICADs is characterization of hazard and
dose-response from exposure to a chemical. CICADs are not a summary
of all available data on a particular chemical; rather, they include
only that information considered critical for characterization of the
risk posed by the chemical. The critical studies are, however,
presented in sufficient detail to support the conclusions drawn. For
additional information, the reader should consult the identified
source documents upon which the CICAD has been based.
Risks to human health and the environment will vary considerably
depending upon the type and extent of exposure. Responsible
authorities are strongly encouraged to characterize risk on the basis
of locally measured or predicted exposure scenarios. To assist the
reader, examples of exposure estimation and risk characterization are
provided in CICADs, whenever possible. These examples cannot be
considered as representing all possible exposure situations, but are
provided as guidance only. The reader is referred to EHC 1701 for
advice on the derivation of health-based guidance values.
1 International Programme on Chemical Safety (1994) Assessing
human health risks of chemicals: derivation of guidance values
for health-based exposure limits. Geneva, World Health Organization
(Environmental Health Criteria 170).
While every effort is made to ensure that CICADs represent the
current status of knowledge, new information is being developed
constantly. Unless otherwise stated, CICADs are based on a search of
the scientific literature to the date shown in the executive summary.
In the event that a reader becomes aware of new information that would
change the conclusions drawn in a CICAD, the reader is requested to
contact the IPCS to inform it of the new information.
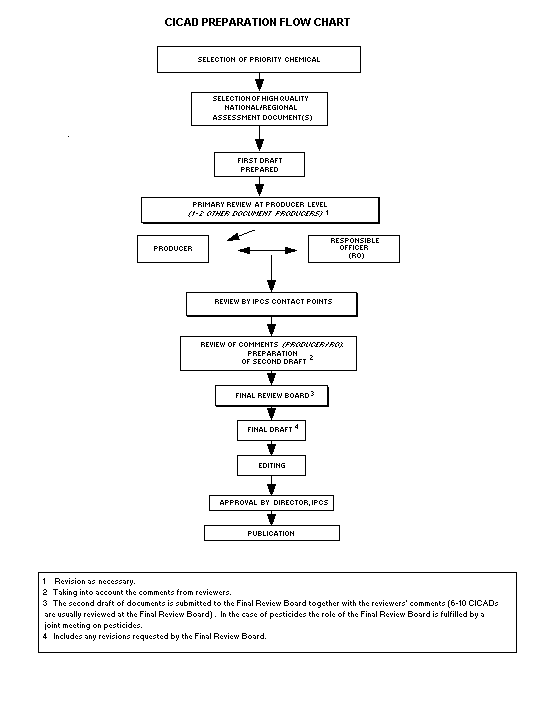
Procedures
The flow chart shows the procedures followed to produce a CICAD.
These procedures are designed to take advantage of the expertise that
exists around the world - expertise that is required to produce the
high-quality evaluations of toxicological, exposure, and other data
that are necessary for assessing risks to human health and/or the
environment.
The first draft is based on an existing national, regional, or
international review. Authors of the first draft are usually, but not
necessarily, from the institution that developed the original review.
A standard outline has been developed to encourage consistency in
form. The first draft undergoes primary review by IPCS and one or
more experienced authors of criteria documents to ensure that it meets
the specified criteria for CICADs.
The second stage involves international peer review by scientists
known for their particular expertise and by scientists selected from
an international roster compiled by IPCS through recommendations from
IPCS national Contact Points and from IPCS Participating Institutions.
Adequate time is allowed for the selected experts to undertake a
thorough review. Authors are required to take reviewers' comments
into account and revise their draft, if necessary. The resulting
second draft is submitted to a Final Review Board together with the
reviewers' comments.
The CICAD Final Review Board has several important functions:
- to ensure that each CICAD has been subjected to an appropriate
and thorough peer review;
- to verify that the peer reviewers' comments have been addressed
appropriately;
- to provide guidance to those responsible for the preparation of
CICADs on how to resolve any remaining issues if, in the opinion
of the Board, the author has not adequately addressed all
comments of the reviewers; and
- to approve CICADs as international assessments.
Board members serve in their personal capacity, not as representatives
of any organization, government, or industry. They are selected
because of their expertise in human and environmental toxicology or
because of their experience in the regulation of chemicals. Boards
are chosen according to the range of expertise required for a meeting
and the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
 1. EXECUTIVE SUMMARY
This CICAD on methyl methacrylate was prepared by the
Environmental Health Directorate of Health Canada and was based
principally on a Government of Canada (1993) review to assess the
potential effects on human health of indirect exposure to methyl
methacrylate in the general environment as well as the chemical's
environmental effects and an International Agency for Research on
Cancer review (IARC, 1994) concerning primarily hazard identification
for carcinogenicity. Data identified as of March 1992 were considered
in the Government of Canada (1993) review and were subsequently
updated, based on a comprehensive literature search conducted in
September 1995 of on-line databases and the International Register of
Potentially Toxic Chemicals. Information on the nature of peer review
and the availability of the Government of Canada (1993) and IARC
(1994) reviews is presented in Appendix 1. During the peer review
phase for this CICAD, additional draft reviews of the United Kingdom
Health and Safety Executive (Cary et al., 1995) and the European Union
(Draft Assessment on Methyl Methacrylate) and published reviews of
ECETOC (1995) and the Finnish Advisory Board of Chemicals (1992) were
considered, primarily for identification of relevant additional
information for review. Additional information identified during
review by Contact Points and consideration by the Final Review Board
has also been incorporated. Information on the peer review of this
CICAD is presented in Appendix 2. This CICAD was approved for
publication at a meeting of the Final Review Board, held in Brussels,
Belgium, on 18-20 November 1996. Participants at the Final Review
Board meeting are listed in Appendix 3. The International Chemical
Safety Card for methyl methacrylate (ICSC 0300), produced by the
International Programme on Chemical Safety (IPCS, 1993), has also been
reproduced in this document.
Methyl methacrylate (CAS no. 80-62-6) is a volatile synthetic
chemical that is used principally in the production of cast acrylic
sheet, acrylic emulsions, and moulding and extrusion resins. Polymers
and copolymers of methyl methacrylate are also used in waterborne,
solvent, and undissolved surface coatings, adhesives, sealants,
leather and paper coatings, inks, floor polishes, textile finishes,
dental prostheses, surgical bone cements, and leaded acrylic radiation
shields and in the preparation of synthetic fingernails and orthotic
shoe inserts. The majority of methyl methacrylate is predicted to be
emitted to air, with very small amounts being released into water and
soil. The persistence of methyl methacrylate in the atmosphere is
short, and the chemical is not considered to contribute directly to
depletion of the ozone layer. Methyl methacrylate is not expected to
bioconcentrate in the environment, and inhalation from air is likely
the primary route of human exposure.
Methyl methacrylate is rapidly absorbed and distributed following
inhalation or oral administration to experimental animals. Data on
absorption following dermal exposure are limited. In both
experimental animals and humans, methyl methacrylate is rapidly
metabolized to methacrylic acid. Following inhalation, 16-20% of the
chemical is deposited in the upper respiratory tract of rats, where it
is primarily metabolized by local tissue esterases.
The acute toxicity of methyl methacrylate is low. Irritation of
the skin, eye, and nasal cavity has been observed in rodents and
rabbits exposed to relatively high concentrations of methyl
methacrylate. The chemical is a mild skin sensitizer in animals. The
effect observed most frequently at lowest concentration after repeated
inhalation exposure to methyl methacrylate is irritation of the nasal
cavity. Effects on the kidney and liver at higher concentrations have
also been reported. The lowest reported effect level for inhalation
was 410 mg/m3 in rats exposed to methyl methacrylate for 2 years
(based upon inflammatory degeneration of the nasal epithelium); the
no-observed-effect level (NOEL) in this investigation was
approximately 100 mg/m3.
In a well conducted study in rats, there were no developmental
effects, although there were decreases in maternal body weight
following inhalation of concentrations up to 8315 mg/m3. Other
available data on developmental toxicity are restricted to results of
limited early or poorly documented studies in which fetotoxic effects
were observed at concentrations that (where reported) were toxic to
the mothers. Available data on reproductive effects of methyl
methacrylate are limited. There was no reduction in fertility in a
dominant lethal assay in mice exposed to methyl methacrylate
concentrations up to 36 900 mg/m3 and no adverse effects on
reproductive organs in repeated-dose studies conducted to date.
Available data on the neurotoxicity of methyl methacrylate are
limited; impairment of locomotor activity and learning and behavioural
and biochemical effects on the brain were observed in rats exposed
orally to 500 mg/kg body weight per day for 21 days.
Methyl methacrylate was not carcinogenic in an extensive, well
documented 2-year bioassay in rats and mice exposed by inhalation and
in additional chronic inhalation studies in rats and hamsters.
Although not mutagenic in vitro in bacterial systems, methyl
methacrylate has been mutagenic and clastogenic in mammalian cells
in vitro. In in vivo studies (primarily by the inhalation route)
in which there has been clear evidence of toxicity within the target
tissue, there has been limited evidence of the genotoxicity of methyl
methacrylate.
Methyl methacrylate is a mild skin irritant in humans and has
the potential to induce skin sensitization in susceptible individuals.
Although occupational asthma associated with methyl methacrylate has
also been reported, there is no conclusive evidence that methyl
methacrylate is a respiratory sensitizer. As a whole, the available
epidemiological studies do not provide strong or consistent evidence
of a carcinogenic effect of methyl methacrylate on any target organ in
humans, nor can it be inferred with any degree of confidence that the
possibility of an excess risk has been disproved.
The toxicity of methyl methacrylate to aquatic organisms is low.
Although no chronic studies on aquatic organisms were identified,
acute tests have been conducted on fish, Daphnia magna, and algae.
The most sensitive effect was the onset of inhibition of cell
multiplication by the green alga Scenedesmus quadricauda at 37
mg/litre following 8 days of exposure. The lowest reported 24-hour
EC50 for immobilization in Daphnia is 720 mg/litre. The 96-hour
LC50 in juvenile bluegill sunfish (Lepomis macrochirus) under
flow-through conditions was 191 mg/litre, whereas LC50 values for
durations of 1-24 hours ranged from 420 to 356 mg/litre, respectively.
The 96-hour LC50 for rainbow trout (Oncorhynchus mykiss) under
flow-through conditions was >79 mg/litre, the highest concentration
tested. Sublethal/behavioural responses were noted among the fish at
40 mg/litre.
The available studies in humans are considered inadequate as the
principal basis for derivation of a guidance value; therefore, in
order to provide guidance, a tolerable concentration has been
established on the basis of inflammatory degeneration of the nasal
epithelium of rats exposed to methyl methacrylate at a concentration
of 410 mg/m3 for 2 years. The NOEL in this investigation was
approximately 100 mg/m3. Data available to serve as a basis for
estimation of indirect exposure in the general environment or consumer
exposure are extremely limited. The derived (likely conservative)
tolerable concentration of approximately 0.2 mg/m3 is many orders of
magnitude higher than the sample predicted concentrations of methyl
methacrylate in ambient air of the general environment. Inhalation
exposure predicted from the use of dispersion and oil-based paints
containing methyl methacrylate may be up to an order of magnitude
higher than the tolerable intake associated with exposure at the level
of the tolerable concentration, although it has been reported that in
some countries these products are not supplied to the general public.
Information on use patterns of these products in other countries was
not identified. Based on a chronic study by the oral route of
administration, a tolerable daily intake (TDI) of 1.2 mg/kg body
weight per day has been derived.
Although available data on the environmental effects of methyl
methacrylate are limited and predicted values in various media are
highly uncertain, a wide margin exists between observed effect levels
and uncertain predicted environmental concentrations of methyl
methacrylate.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
Methyl methacrylate (CAS no. 80-62-6) is a colourless, volatile
liquid with an acrid fruity odour. It has a relatively high vapour
pressure (4 kPa at 20°C), moderate water solubility (15.8 g/litre),
and a low log octanol/water partition coefficient ( Kow = 1.38)
(Government of Canada, 1993). The empirical formula for methyl
methacrylate is C5H8O2. The structural formula for methyl
methacrylate is given below. Additional physical/chemical properties
are presented in the International Chemical Safety Card reproduced in
this document.
O
"
H2C = C - C - O - CH3
'
CH3
The purity of commercial methyl methacrylate is typically 99.9%.
It contains traces of acidity as methacrylic acid (0.003% max.;
specification, 0.005% max.) and water (0.03% max.; specification,
0.05% max.). Inhibitors added for storage and transportation are
usually 2-100 ppm methyl ether of hydroquinone and 25-100 ppm
hydroquinone, although other phenolic inhibitors, such as dimethyl
tert-butylphenol, may also be used (IARC, 1994; M. Pemberton,
personal communication, 1996).
3. ANALYTICAL METHODS
Methods commonly used for the analysis of acrylic compounds
include gas chromatography (GC), mass spectrometry (MS), GC/MS,
nuclear magnetic resonance, and infrared spectroscopy (Government of
Canada, 1993). Methyl methacrylate can be determined in air by gas
chromatography with flame ionization detection; the sample is adsorbed
on fused silica (XAD-2 resin) or charcoal coated with
4- tert-butylcatechol and desorbed with carbon disulfide or toluene.
The estimated limit of detection for this method is 0.01 mg per
sample. A detection limit of 0.8 mg/m3 is obtained with a method
involving desorption with 5% isopropanol in carbon disulfide from
charcoal (IARC, 1994).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Methyl methacrylate is not known to occur naturally (IARC, 1994).
It is used principally in the production of cast acrylic sheet,
acrylic emulsions, and moulding and extrusion resins (IARC, 1994).
Polymers and copolymers of methyl methacrylate are used in waterborne,
solvent, and undissolved surface coatings (exterior latex paint based
on emulsions containing methyl methacrylate is the surface coating in
which it is used most widely). Solvent reducible polymers containing
methyl methacrylate are used for industrial finishes, metal and foil
coatings, and a variety of overlays for special purposes. Solvent and
emulsion polymers containing methyl methacrylate are also used in
adhesives, sealants, leather and paper coatings, inks, floor polishes,
and textile finishes (IARC, 1994). Methyl methacrylate and polymers
of methyl methacrylate are also used for dental prostheses, surgical
bone cements, and leaded acrylic radiation shields and in the
preparation of synthetic fingernails and orthotic shoe inserts (IARC,
1994).
Global production of methyl methacrylate was estimated to be 1.4
million tonnes in 1988 (IARC, 1994). In the USA and Japan, production
of methyl methacrylate ranged from 380 000 to 536 000 t and from
384 000 to 403 000 t, respectively, between 1990 and 1992 (IARC,
1994). Total production volume within the European Union was
447 000 t in 1993 (CEFIC, 1994).
Methyl methacrylate can enter the environment during its
transport, bulk storage, and use. Based on data from the US Toxic
Chemical Release Inventory, emissions to air, water, and soil from
industries in the USA are estimated to be about 0.46% of
production.1 Most of the released methyl methacrylate (i.e. 98%) is
estimated to be emitted to air, with very small amounts being released
into water and soil. Data on emissions of methyl methacrylate in
other countries have not been identified. Assuming a production in
the USA in 1992 of approximately 500 000 t (IARC, 1994), approximately
2300 t are estimated to have been released to the environment.
1 Source: Toxic Chemical Release Inventory (TRI), databank produced
by the National Library of Medicine and the US Environmental
Protection Agency (1989).
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
As methyl methacrylate is highly reactive with hydroxyl radicals,
its estimated half-life in the troposphere is short: from <5 hours in
summer to a few days in winter at a latitude such as that of Toronto,
Canada. The reported photooxidation half-life of methyl methacrylate
is 1.1-9.7 hours. Methyl methacrylate is readily polymerized by light
and heat but is not expected to photolyze (Government of Canada,
1993).
In neutral or acidic aquatic environments, hydrolysis of methyl
methacrylate is not significant. Based upon its measured second-order
hydrolysis rate constant of 200 (mol/litre)-1 h-1 at 25°C, the
hydrolysis half-life of methyl methacrylate is estimated to be 3.9
years at pH 7 and 14.4 days at pH 9 (Howard, 1989).
No data were identified on the rate of volatilization of methyl
methacrylate; however, the half-life for evaporation from a river 1 m
deep with a 1 m/s current and 3 m/s wind has been calculated as 6.3
hours. Evaporation of methyl methacrylate from soil is expected to be
rapid, owing to its high vapour pressure and weak adsorption to soil.
A Level I fugacity model in an evaluative environment predicts
the following equilibrium partitioning of methyl methacrylate: air,
86.6%; water, 13.1%; and soil/sediment, <0.4% (Mackay et al., 1995).
Biodegradation contributes significantly to removal of methyl
methacrylate from the environment. The aqueous aerobic degradation
half-life is estimated to be 1-4 weeks, and the anaerobic degradation
half-life is estimated to be 4-16 weeks (Howard, 1989).
Although no studies have been conducted to measure
bioconcentration factors for methyl methacrylate, a bioconcentration
factor of 3 has been estimated from the log Kow; based on this
value, methyl methacrylate is not expected to bioconcentrate or
biomagnify in food-chains (Government of Canada, 1993).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
In an analysis of 204 samples of water collected from 14 heavily
industrialized river basins in the USA (Ewing & Chian, 1977), methyl
methacrylate was detected (detection limit 1.0 µg/litre) only once at
a concentration of 10 µg/litre in final tap-water after chlorination
in Chicago, Illinois, in 1976. No additional information was
provided. Methyl methacrylate was not detected in 24 water samples
(limit of determination 0.005-1 µg/litre) or in 24 sediment samples
(limit of determination 0.000 11-0.01 µg/g dry weight) taken in Japan
in eight locations (harbour or estuarine areas) in 1979 (no further
information provided) (S. Tsuda, personal communication, 1996).
Methyl methacrylate was not detected (detection limit 0.01 µg/g wet
weight) in 30 samples of (edible) shellfish collected from various
locations in Atlantic Canada (Environment Canada, 1989). Methyl
methacrylate may be present in food as a result of migration of the
monomer from food containers made from polymethyl methacrylate (IARC,
1994); for example, concentrations ranged from 180 to 275 ppb (ng/g)
in maple syrup that had been packaged in plastic containers
(Hollifield et al., 1980). The migration of methyl methacrylate from
commercial plastic wrap into 20% ethanol at 25°C was 1 ppm in 1 day
and 10 ppm in 90 days. Migration into water and acetic acid was not
detected (detection limit 0.05 ppm) (Inoue et al., 1981).
In view of the limited available monitoring data, estimates of
the fate and concentrations of methyl methacrylate in the Canadian
environment were generated by a Level III fugacity model (Mackay &
Paterson, 1981, 1982, 1991; Mackay et al., 1985) developed for
southern Ontario, incorporating data on the physical and chemical
properties of the chemical (Government of Canada, 1993),
transformation half-lives (Howard et al., 1991), and proportion of
production in the USA emitted to environmental media (see section 4)
applied to the volume imported into Canada. Methyl methacrylate is
not produced in Canada; approximately 22 000 t are imported (CPI,
1989). The model assumed emissions of 95% to air, 4.5% to water, and
0.5% to soil. The estimated relative proportions of methyl
methacrylate predicted for air, water, soil, and sediment at steady
state were 26.6%, 60.8%, 12.6%, and 0.03%, respectively. The amount
of methyl methacrylate estimated to partition to fish was negligible.
The relatively longer half-life for methyl methacrylate in water
compared with air accounts for the higher estimated relative
proportion predicted for the water compartment. Although such models
are useful primarily for identification of the relative proportions of
exposure from various media rather than for quantitative estimates of
concentrations, the latter are presented here primarily as a baseline
for comparison with measured concentrations. It should also be noted
that such predicted values will vary in different countries depending
upon production and releases of methyl methacrylate. The average
concentrations estimated on the basis of the model were 2.44 × 10-4
µg/m3 in air, 0.13 ng/litre in surface water, 1.2 × 10-6 µg/g in
soil, 8.7 × 10-8 µg/g in sediment, and 1.5 × 10-7 µg/g in fish
(Government of Canada, 1993).
6.2 Human exposure
Examples of estimated indirect exposure in the general
environment and during use of consumer products are presented here.
Levels determined in various occupational settings are also
summarized. Estimates of indirect exposure in the general environment
are based in Canada owing to the availability of relevant data for
input; however, predicted levels will vary considerably as a function
of production and use patterns in various countries. Consumer
exposure estimates are based on data on the percent composition of
methyl methacrylate in products provided by European manufacturers.
Levels in occupational environments are those reported from various
countries. Countries are strongly encouraged, however, to estimate
exposure on the basis of local data, possibly in a manner similar to
that outlined here.
Adequate data on measured concentrations of methyl methacrylate
in air, drinking-water, foodstuffs, and soil have not been identified;
indeed, they are limited to non-detectable values in a limited number
of small surveys. Although predicted concentrations in environmental
media based on fugacity modelling are uncertain, they are helpful in
estimating proportions of exposure from various media. Based on a
daily inhalation volume for adults of 22 m3, a mean body weight for
males and females of 64 kg, and a predicted concentration (by fugacity
modelling; see section 6.1) of methyl methacrylate in ambient air in
Canada of 2.44 × 10-4 µg/m3, the estimated intake of methyl
methacrylate from air for the general population represents
approximately 97% of the total intake from air, drinking-water, fish,
and soil. Based on a daily volume of water consumption for adults of
1.4 litres, a mean body weight of 64 kg, and a predicted concentration
of methyl methacrylate in surface water in Canada of 0.13 ng/litre
(see section 6.1), the estimated intake of methyl methacrylate from
drinking-water for the general population represents approximately
3.3% of total intake. Available data were inadequate to estimate the
intake of methyl methacrylate from food, with the exception of intake
from fish. Based on a daily amount of fish ingested for adults of 23
g/day, a mean body weight for adults of 64 kg, and the predicted
concentration of methyl methacrylate in fish in Canada of 1.5 × 10-7
µg/g (see section 6.1), the estimated intake of methyl methacrylate
from fish represents 0.06% of total intake. Based on a daily amount
of soil ingested for adults of 20 mg, a mean body weight for adults of
64 kg, and a predicted concentration of methyl methacrylate in soil in
Canada of 1.2 × 10-6 µg/g (see section 6.1), the estimated intake of
methyl methacrylate from soil, as a proportion of total intake, is
negligible (0.0004%). Therefore, based on predicted concentrations in
the Canadian environment, the overwhelmingly principal source of
indirect exposure to methyl methacrylate for most of the general
population is air.
Inhalation exposure to methyl methacrylate from the use of
consumer products containing methyl methacrylate (e.g. dispersion
paints and oil-based paints) was modelled using the US EPA Screening
Consumers Inhalation Exposure Software (SCIES) computer model. All
scenarios were based on the assumption that the percent composition of
methyl methacrylate-based polymers in formulations of dispersion
paints, varnishes, or lacquers is 15%, although residual monomer
content is much less (European Union Draft Assessment on Methyl
Methacrylate), and that 100% is absorbed. Although it has been
reported that in some countries these products are not supplied to the
general public, information on use patterns of these products in other
countries was not available.
For the use of dispersion paints, the standard default values of
the SCIES model were assumed for the following parameters: frequency
of use, six events per year; mass of product, 13.6 kg; room size, 40
m3; duration of use, 4.9 hours; house air exchange rate, 0.2 room air
exchanges per hour; and user inhalation rate, 1.3 m3/hour. The
vapour pressure of methyl methacrylate was considered to be 38.4 torr
(5.12 kPa) (Howard, 1989). Resulting estimated consumer exposure from
inhalation was in the range of 10-100 mg/kg body weight per day.
However, as the residual methyl methacrylate monomer content in
dispersion paints is specified to be 0.1% (ECETOC, 1995), consumer
exposure to methyl methacrylate would fall within the range of 10-100
µg/kg body weight per day.
For the estimation of consumer exposure from the use of oil-based
(solvent-based) paints, the standard default values of the SCIES model
were assumed as above, with the exception of the following parameters,
for which default values were: mass of product, 6.71 kg; and duration
of use, 3.2 hours. The vapour pressure of methyl methacrylate and
absorption were the same as those for the scenario mentioned above.
The resulting estimated consumer exposure from inhalation was again in
the range of 10-100 mg/kg body weight per day. However, as the
residual methyl methacrylate monomer content in solvent-based paints
is assumed to be 1.5% by the producer (European Union Draft Assessment
on Methyl Methacrylate), consumer exposure to methyl methacrylate
would fall within the range of 100-1000 µg/kg body weight per day.
Occupations in which there is potential exposure to methyl
methacrylate include those in the medical, dental, and beauty
professions, such as chemical process operators, surgeons and surgical
assistants, operating room nurses, dental technicians and hygienists,
and beauty technicians applying synthetic fingernails (IARC, 1994).
Exposure to methyl methacrylate in the workplace could be
substantially greater than that in the general environment. Based on
experience in the United Kingdom, for example, long-term personal
exposures during monomer production average about 2 ppm (8.2 mg/m3)
and are less than 60 ppm (246 mg/m3) (Cary et al., 1995). In open
system industries such as cast sheet production, long-term exposures
are higher, averaging 22.2 ppm (91 mg/m3) and ranging from 0.5 to 165
ppm (2-677 mg/m3). For various end uses of methyl methacrylate,
including aerospace manufacture, plastics processing, and artificial
teeth production, the mean long-term value for personal exposure was
13.4 ppm (55 mg/m3), with a range of 0.8-109 ppm (3.3-447 mg/m3).
In medical and dental applications, peak concentrations up to 374 ppm
(1533 mg/m3) have been recorded, although short-term
time-weighted-average exposures are likely to be less than 100 ppm
(410 mg/m3).
Mean levels (time period often unspecified) of methyl
methacrylate in the air of various chemical manufacturing and
processing plants (located in Europe, the USA, Canada, Russia, Japan,
and China) vary widely, ranging from not detectable (detection limit
not reported) to 1500 mg/m3 (CEFIC, 1993; Mizunuma et al., 1993;
IARC, 1994; M. Baril, personal communication, 1996). Peak values as
high as 7900 mg/m3 have been reported for some manufacturing
facilities (M. Baril, personal communication, 1996). Mean
concentrations of methyl methacrylate in the air of dental clinics and
dental laboratories (in the USA, Norway, Denmark, and the United
Kingdom) have ranged from not detectable (detection limit not
reported) to 273 mg/m3 during denture prosthesis manufacture and
repair (IARC, 1994). Mean concentrations of methyl methacrylate in
the air of beauty salons (in the USA) have ranged from 21.7 to 87.5
mg/m3 during the application of artificial fingernails (IARC, 1994).
It should be noted that in some cases these values reflect
shorter-term peak exposures rather than time-weighted averages.
Elevated levels (above 1500 mg/m3) during floor coating with methyl
methacrylate-containing resins have been reported, although these
levels were measured during activities that normally do not cover a
full shift; hence, time-weighted-average concentrations would be
less.1
1 Source: Excerpts from the (1995) BIA file provided by BG Chemie
containing measurement data of occupational exposures to methyl
methacrylate in industry and trade. Communication to Bundesinstitut
für Gesundheitlichen Verbraucherschutz und Veterinarmedizin (BgVV).
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS
AND HUMANS
Methyl methacrylate is rapidly absorbed and distributed following
inhalation or oral administration to rats. On the basis of available
data, methyl methacrylate appears to be rapidly metabolized to
methacrylic acid, which is subsequently converted to carbon dioxide
via the tricarboxylic acid cycle in both experimental animals and
humans. Adequate studies on the dermal absorption of methyl
methacrylate were not identified. Methyl methacrylate is rapidly
eliminated, primarily via the lungs in expired air. After oral or
intravenous administration to rats, approximately 65% of the dose was
exhaled in the expired air as 14CO2 within 2 hours (Bratt & Hathway,
1977). Lesser amounts are eliminated in the urine, and an even
smaller fraction in the faeces. Owing to its rapid metabolism and
excretion, there appears to be little potential for accumulation of
methyl methacrylate within tissues (Government of Canada, 1993;
ECETOC, 1995).
Deposition in the surgically isolated upper respiratory tract of
urethane-anaesthetized male F344 rats exposed to methyl methacrylate
at 90, 437, or 2262 mg/m3 under cyclic flow conditions was 16-20%
(Morris & Frederick, 1995). Deposition was 3% less on average in the
unidirectional flow groups than in the cyclic flow groups. Deposition
was less efficient at the high than at the low and middle
concentrations, although the mechanism is unknown. (The deposition
efficiency of inhaled methacrylic acid under similar conditions was
much greater, averaging 95% under unidirectional flow.) Pretreatment
with a carboxylesterase inhibitor (bis-nitrophenylphosphate) decreased
uptake of methyl methacrylate by one-third, suggesting that methyl
methacrylate is hydrolysed by carboxylesterase in nasal tissues and
that such metabolism serves to enhance its deposition efficiency.
Methyl methacrylate decreased nasal non-protein content by
approximately 25% at the highest concentration, but not at lower
concentrations. Nasal non-protein content was not decreased by
exposure to methacrylic acid even at delivered dose rates twofold more
than that for methyl methacrylate, suggesting that this effect is
attributable to the ester itself and not to the acid metabolite
(Morris & Frederick, 1995).
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
The acute toxicity of methyl methacrylate is consistently low,
although unconfirmed effects on the lungs were reported at relatively
low concentrations in one study of poor design (Raje et al., 1985).
The 4-hour LC50s for methyl methacrylate in rats ranged from 3750 to
7093 ppm (15 375-29 080 mg/m3). The oral LD50s ranged from 5.0
ml/kg body weight (4.7 g/kg body weight) in dogs to 10.0 ml/kg body
weight (9.44 g/kg body weight) in rats (Government of Canada, 1993).
8.2 Irritation and sensitization
Irritation of the skin, eye, and mucosa of the respiratory tract
has been observed in rodents and rabbits exposed to relatively high
concentrations of methyl methacrylate (dermal application of
approximately 2-38 g/kg body weight; inhalation of 100-17 600 ppm
[410-72 160 mg/m3]; or instillation of approximately 0.1 ml into the
cornea) (Spealman et al., 1945; Castellino & Colicchio, 1969; Rohm &
Haas, 1982; Raje et al., 1985; Kanerva & Verkkala, 1986; NTP, 1986;
Ouyang et al., 1990).
The weight of evidence is that methyl methacrylate is a skin
sensitizer in animals (Cary et al., 1995; ECETOC, 1995).
8.3 Short-term exposure
Death, decreases in body weight, changes in respiration rate,
increases in level of blood urea nitrogen, and pulmonary damage were
observed after exposure to high concentrations in short-term
repeated-dose studies in rats and mice in which inhaled concentrations
of methyl methacrylate ranged up to 5000 ppm (20 500 mg/m3)
(Government of Canada, 1993). Cardiovascular effects (irregular ECG,
changes in blood pressure) were also observed in rats exposed to
undocumented concentrations of vaporized methyl methacrylate for 20
minutes/day for 21 or 42 days in a limited study (Blanchet et al.,
1982).
In short-term studies, mice were more susceptible than rats, with
effects on the respiratory tract (redness and swelling of the nasal
region) observed after exposure to 500 ppm (2050 mg/m3; the lowest
tested concentration in the study) for 10 days (NTP, 1986). No
systemic histopathological effects were observed after inhalation of
concentrations up to 5000 ppm (20 500 mg/m3).
8.4 Long-term exposure
The protocols and results of available long-term studies on
methyl methacrylate are summarized in Table 1.
Table 1: Summary of effect levels in long-term studies.
Species Study design Effects Effect levels Comments Reference
INHALATION
Rats, Exposed to 0 or 116 ppm Rats exposed for 3 months lacked Effects at 116 One dose group only Tansy et al.,
Sprague-Dawley, (476 mg/m3) methyl methacrylate, visceral and subcutaneous fat ppm (476 1976
50 males per 8 hours/day, for 5 days/week. deposits, had significantly lower mg/m3)
group Approximately half of the rats body, lung, and spleen weights,
in each group were sacrificed and had significantly higher mean
after 3 months; blood and serum alkaline phosphatase
tissue samples were taken. The concentration. Rats exposed for
remainder of the rats were 6 months had less subcutaneous
exposed for 6 months. fat, significantly lower mean body
weights, popliteal fat pad
weights, and mean intestinal transit
time, and significantly higher mean
alkaline phosphatase and inorganic
phosphate concentrations compared
with controls.
Rats, Exposure to 0 or 116 ppm Exposed rats had significantly Effects at 116 One dose group only Tansy et al.,
Sprague-Dawley, (476 mg/m3) methyl methacrylate, lower total bilirubin and higher ppm (476 1980a
23 males per 5 days/week, averaging 7 total cholesterol levels; possible mg/m3)
group hours/day, for 542 hours (3 liver damage in the exposed group,
months). Excretion studies in but details not reported.
nine rats from each group;
histopathological examinations
of heart, lung, kidneys, spleen,
stomach, small bowel, liver,
and adrenal.
Table 1 (continued)
Species Study design Effects Effect levels Comments Reference
Rats, Exposure to 0 or 116 ppm Mild lung damage in some of the Effects at 116 One dose group only; Tansy et al.,
Sprague-Dawley, (476 mg/m3) methyl methacrylate, rats exposed for 3 and 6 months ppm (476 mg/m3) statistical 1980b
23 males per 7 hours/day, 5 days/week, for and the sham-exposed controls. significance not
group for 3 3 or 6 months. Histopathological Rats exposed for 6 months had reported; similar
months and examinations of heart, lung, damaged tracheal mucosa. The effects in
unspecified kidneys, spleen, stomach, small epithelium was denuded of cilia, sham-exposed controls
number for bowel, liver, and adrenal. and the cellular covering of
6 months microvilli was reduced in rats
exposed for 3 months.
Rats, F344, Exposure to 0, 63, 125, 250, Some clinical signs and one death NOEL = 1000 Rohm & Haas,
10 per sex 500, or 1000 ppm (0, 258, 512, each in groups exposed to 63 ppm ppm (4100 1977
per group 1025, 2050, or 4100 mg/m3) and controls, but not mg/m3)
methyl methacrylate, 6 hrs/day, dose-related.
for 65 days. Complete gross
pathological and histopathological
examinations.
Rats, F344/N, Inhalation of 0, 63, 125, 250, No methyl methacrylate-related NOEL = 1000 NTP, 1986
10 per sex 500, or 1000 ppm (0, 258, 512, effects. ppm (4100
per group 1025, 2050, or 4100 mg/m3) mg/m3)
methyl methacrylate, 6 hours/day,
5 days/week, for 14 weeks (65
exposures). Histological
examinations were conducted of
an unspecified range of tissues
from all high-dose and control
rats, those that died before
the end of the study, and some
of the rats from the other
groups.
Table 1 (continued)
Species Study design Effects Effect levels Comments Reference
Rats, F344/N, Exposure to 0, 500, 1000, 2000, At 1000 ppm, a low incidence of LOEL = 1000 NTP, 1986
10 per sex 3000, or 5000 ppm (0, 2050, mild effects on the brain and ppm (4100
per group 4100, 8200, 12 300, or 20 500 nasal turbinates in females was mg/m3)
mg/m3) methyl methacrylate, 6 observed. At 2000-5000 ppm,
hours/day, 5 days/week, for 14 death, effects on body weight, NOEL = 500
weeks (65 exposures). and lesions of nasal turbinates ppm (2050
Histological examinations were and brain were observed; changes mg/m3)
performed on the controls, the in spleen were observed at 3000
two highest dose groups, and ppm and above. Also, follicular
rats that died before the end atrophy of the spleen in 4/10
of the study. Tissues from the males, bone marrow atrophy in
nasal turbinates, larynx, 8/10 males (5000 ppm exposure
trachea, lungs, and brain for group), and cerebellar congestion
all rats exposed at 1000 ppm and penducle haemorrhage in the
and survivors of the 2000 ppm females exposed to 3000 and 5000
groups were also examined ppm that died early. At 5000 ppm,
histopathologically. listlessness, nasal and serous
ocular discharge, and prostration
during the first 2 days, nasal
cavity inflammation with necrosis
and loss of epithelium, follicular
atrophy of the spleen, and bone
marrow atrophy in the males.
Cerebellar congestion and
penducle haemorrhage in the
early-death females exposed to
3000 and 5000 ppm, and malacia and
gliosis in 5/9 females exposed to
2000 ppm and 1/8 females exposed
to 1000 ppm.
Table 1 (continued)
Species Study design Effects Effect levels Comments Reference
Rats, albino Exposure to 0, 25, 100, or 400 Decreased body weights; slight NOEL = 25 ppm Rohm & Haas,
F344, 70 per ppm (0, 102.5, 410, or 1640 increase in the incidence of mild (102.5 mg/m3) 1979a; Lomax,
sex per group mg/m3) methyl methacrylate, 6 rhinitis in the nasal mucosal 1992; Lomax et
hours/day, 5 days/week, for up lining of the turbinates. al., 1997
to 104 weeks. Histopathological
examination of a wide range of The re-examination revealed that LOEL =
tissues from controls and rats exposed to 100 or 400 ppm 100 ppm (410
high-dose groups, as well as methyl methacrylate had mg/m3)
selected tissues from other exposure-related and
dose groups (ovaries or testes concentration-dependent
and nasal turbinates). microscopic changes in the
olfactory epithelium lining
the dorsal meatus in the anterior
A re-examination of the nasal region of the nasal cavity.
tissues from the rats of the The microscopic changes consisted
Rohm & Haas (1979a) study was of degeneration/atrophy of the
conducted. The review consisted olfactory epithelium and
of microscopic examination of underlying Bowman's glands,
nasal tissue from at least 10% hyperplasia of basal (reserve)
of randomly selected rats from cells, replacement of olfactory
each group, and the slides epithelium by ciliated
evaluated included the original (respiratory-like) epithelium,
study slides plus slides from and inflammation of the mucosa
tissue sections taken deeper and/or submucosa. The squamous
into the block. epithelium of the nasal cavity
was not affected. The lesions
tended to be bilateral in
distribution in rats exposed to
both 100 and 400 ppm methyl
methacrylate. A small nasal
polypoid adenoma was observed in
one male from both the 100 and
400 ppm exposure groups.
Table 1 (continued)
Species Study design Effects Effect levels Comments Reference
Rats, F344/N, Rats exposed to methyl Inflammation and degeneration of LOEL = 250 NTP, 1986; Chan
50 per sex methacrylate at 0, 2050, or the olfactory epithelium ppm (1025 et al., 1988
per group 4100 mg/m3 (males) or 0, 1025, (accompanied by variable atrophy mg/m3)
or 2050 mg/m3 (females), 6 of the nerve bundles in the
hours/day, 5 days/week, for submucosa and, in the most
102 weeks. Histological severely affected areas,
examination of a comprehensive replacement of sensory
range of tissues. neuroepithelial cells with
respiratory epithelium) and
minimal increases in the numbers
of alveolar macrophages in the
nasal cavity at all dose levels.
The incidence of focal or
multifocal fibrosis of the lung
was increased in the females
exposed to 2050 mg/m3.
Rats, Fischer Exposure to 0, 25, 100, or 400 Mild rhinitis was observed Abstract only Smith et al.,
344, male and ppm (0, 102.5, 410, or 1640 (dose level not specified). 1979
female (number mg/m3) methyl methacrylate, 6
not specified) hours/day, 5 days/week, for 24
months. Evaluation of haemograms,
clinical chemistries, and urine,
as well as gross histopathological
examination.
Table 1 (continued)
Species Study design Effects Effect levels Comments Reference
Mice, B6C3F1, Exposure to 0, 63, 125, 250, Final mean body weight of the NOEL = 500 NTP, 1986
10 per sex 500, or 1000 ppm (0, 258, 512, highest-dose males was 7% lower ppm (2050
per group 1025, 2050, or 4100 mg/m3) than controls. mg/m3)
methyl methacrylate, 6 hours/day,
5 days/week, for 14 weeks (64 LOEL = 1000
exposures). Histological ppm (4100
examination of an unspecified mg/m3)
range of tissues in all mice of
the highest-dose and control
groups, all animals that died
before the end of the study, and
some mice in the other groups.
Mice, B6C3F1, Exposure to 0, 63, 125, 250, Some clinical signs and one NOEL = 250 Rohm & Haas,
10 per sex 500, or 1000 ppm (0, 258, 512, death in the group exposed to ppm (1025 1977
per group 1025, 2050, or 4100 mg/m3) 500 ppm, but not dose-related. mg/m3)
methyl methacrylate, 6 Body weights of males receiving
hours/day, for 64 days. Complete the two highest doses were LOEL = 500
gross pathological and significantly decreased during ppm (2050
histopathological examinations. weeks 11-13 (500 ppm) and weeks mg/m3)
6, 11, and 12 (1000 ppm). In
female mice, the total body
weight changes were statistically
significantly lower in animals
exposed to 500 ppm but not to 1000
ppm.
Table 1 (continued)
Species Study design Effects Effect levels Comments Reference
Mice, B6C3F1, Exposure to 0, 500, 1000, 2000, The final mean body weights of LOEL = 500 NTP, 1986
10 per sex 3000, or 5000 ppm (0, 2050, all groups of exposed mice were ppm (2050
per group 4100, 8200, 12 300, or 20 500 lower than controls. Deaths at mg/m3)
mg/m3) methyl methacrylate, 6 2000 ppm and above. Renal
hours/day, 5 days/week, for 14 cortical necrosis, cortical
weeks. Histological examinations tubular degeneration and/or focal
of tissues from the major organs mineralization, nasal cavity
of all mice in the highest-dose inflammation with necrosis, and
and control groups and mice that loss of olfactory epithelium at
died before the end of the 2000-5000 ppm in males and extensive
study, of the lung and nasal liver necrosis in males exposed
turbinates of the males and the to 5000 ppm. Inflammation of the
nasal membranes of all females nasal turbinates in females
in the 2000 and 3000 ppm groups, exposed to 2000 ppm and above.
and of the liver of the males Metaplasia of the nasal epithelium
in the 2000 ppm group. At 1000 in all exposed mice.
ppm, the nasal turbinates from
both sexes and brain from the
males were also histologically
examined.
Mice, B6C3F1, Exposure to 0, 2050, or 4100 Decrease in mean body weights; LOEL = 500 NTP, 1986; Chan
50 per sex mg/m3 methyl methacrylate, 6 localized histopathological ppm (2050 et al., 1988
per group hours/day, 5 days/week, for effects (inflammation and mg/m3)
102 weeks. Histological degeneration of the olfactory
examination of a comprehensive epithelium) in the nasal
range of tissues. epithelium.
Table 1 (continued)
Species Study design Effects Effect levels Comments Reference
Golden Exposure to 0, 25, 100, or 400 Decreased body weights; increased LOEL = 400 Rohm & Haas,
hamsters, 56 ppm (0, 102.5, 410, or 1640 mortality. ppm (1640 1979b
per sex per mg/m3) methyl methacrylate, 6 mg/m3)
group hours/day, 5 days/week, for 18
months. Haematological analysis NOEL = 100
and gross and microscopic ppm (410
examination of a comprehensive mg/m3)
range of tissues.
Golden Exposure to 0, 25, 100, or 400 No exposure-related toxic NOEL = 400 Abstract only Smith et al.,
hamsters, ppm (0, 102.5, 410, or 1640 effects were observed. ppm (1640 1979
male and mg/m3) methyl methacrylate, 6 mg/m3)
female (number hours/day, 5 days/week, for 18
not specified) months. Evaluation of haemograms,
clinical chemistries, and urine,
as well as gross
histopathological examination.
Dogs, beagles, Exposure to 0, 100, or 400 ppm No significant differences in NOEL = 400 Tansy & Drees,
6 per group, (0, 410, or 1640 mg/m3) methyl systolic and diastolic blood ppm (1640 1979
sex methacrylate vapour, 6 pressure, ECG, heart and mg/m3)
unspecified hours/day, 5 days/week, for 3 respiratory rates, haematology,
months. Each dog had an external clinical chemistries, and
iliac artery catheter. Two dogs urinalysis; histopathological
from each group sacrificed at examination of the major organs
the end of the 3-month period; was unremarkable.
remaining dogs observed for
another month.
Table 1 (continued)
Species Study design Effects Effect levels Comments Reference
Dogs, Exposure to 0, 100, or 400 ppm No exposure-related toxic NOEL = 400 Abstract only Smith et al.,
beagles, male (0, 410, or 1640 mg/m3) methyl effects were observed. ppm (1640 1979
(number not methacrylate vapour, 6 mg/m3)
specified) hours/day, 5 days/week, for 3
months. Gross and
histopathological evaluations
in addition to evaluation of
haemograms, clinical chemistries
and urine, ECGs, and blood
pressure.
INGESTION
Rats (sex and Ingestion of 0, 1, 3, or 5 Rats in mid-dose group did not NOAEL = 3 Small group sizes; Deichmann-Gruebler
strain ml/kg body weight (0, 0.9, 2.8, gain as much weight as those in ml/kg body histopathological & Read, undated
unspecified, or 4.7 mg/kg body weight) low-dose group; animals in weight (2832 examination
groups of 5) orally by gavage, every second high-dose group died before the mg/kg body unspecified
day for 70 days. Urine samples 4th treatment. All high-dose rats weight)
from rats of all groups were had distended bladders filled
periodically collected and with blood; a moderate degree of
examined for blood. cellular degeneration in the
Histopathological examinations liver, but without necrosis or
unspecified. fibrosis; renal effects
(haemorrhages in the tubules,
marked hyperaemia, and
degeneration of the tubular
epithelium).
Table 1 (continued)
Species Study design Effects Effect levels Comments Reference
Rats, Wistar, Ingestion of 0, 6, 60, or 2000 Increase in relative kidney NOEL = 60 ppm Borzelleca et
25 per sex ppm (mg/litre) (equivalent to weight in females only. (5 mg/kg body al., 1964
per group 0, 0.4, 4, and 121 mg/kg body weight per day)
weight per day for males; and
0, 0.5, 5, and 146 mg/kg body NOAEL = 2000
weight per day for females) ppm (146 mg/kg
methyl methacrylate in body weight per
drinking-water for 2 years. day)
(Groups received 6 and 60 ppm
for 5 months, then the
concentrations were increased
to 7 and 70 ppm for the remainder
of the 2 years.)
Histopathological examination of
a wide range of tissues from
mid- and high-dose groups.
Limited haematological and urine
analyses conducted.
Dogs, Ingestion of 0, 10, 100, or No treatment-related effects. NOEL = 1500 Extremely small Borzelleca et
beagles, 2 1000 ppm (mg/kg) methyl ppm (38 mg/kg number of animals al., 1964
per sex per methacrylate in corn oil in body weight
group the diet (high dose gradually per day)
increased to 1500 ppm
[equivalent to about 38 mg/kg
body weight per day] at week
9) for 2 years.
Histopathological examination
of a wide range of tissues.
Limited haematological and
urine analyses conducted.
8.4.1 Subchronic exposure
In most subchronic studies conducted to date, rats and mice have
been exposed to methyl methacrylate by inhalation. Effects observed
most commonly in these investigations were decreases in body weight
gain and irritation of the skin, nasal cavity, and eye at high
concentrations (generally >500 ppm [2050 mg/m3]) (Rohm & Haas,
1977; NTP, 1986). At higher concentrations, other effects, such as
renal cortical necrosis and tubular degeneration (rats and mice) and
hepatic necrosis (mice), have also been reported (Tansy et al., 1980a;
NTP, 1986; Deichmann-Gruebler & Read, undated).
On the basis of decreases in final mean body weight and squamous
metaplasia at the site of entry (i.e. nasal epithelium), the lowest
reported NOEL and lowest-observed-effect level (LOEL) in a subchronic
inhalation bioassay in which several concentration levels were
administered were 250 and 500 ppm (1025 and 2050 mg/m3),
respectively, in mice exposed to methyl methacrylate for 64 days or 14
weeks (Rohm & Haas, 1977; NTP, 1986). Except for effects at the site
of entry, histopathological changes have not been observed in the two
most extensive subchronic bioassays in rats exposed to methyl
methacrylate for 65 days or 14 weeks, at concentrations up to 1000 ppm
(4100 mg/m3) (Rohm & Haas, 1977; NTP, 1986).
In less extensive and less well documented studies conducted by
Tansy et al. (1976, 1980a,b), effects on the trachea and some
indications of liver damage in rats were observed at the only tested
concentration of 116 ppm (476 mg/m3), administered for 7 hours/day
for 3 or 6 months, although the statistical significance of the
pulmonary changes was not specified, and similar effects were observed
in some of the sham-exposed control animals. In a supplementary
study, there was weak evidence of an effect on liver function
(barbiturate sleeping time) in male rats administered "intermittent
daily exposures" of 100 ppm (410 mg/m3) methyl methacrylate for a
total of 160 hours (Tansy et al., 1980b). Initial reports of reduced
fat deposits after exposure for 3 months were not confirmed in later
studies of similar protocol by the same investigators (Tansy et al.,
1980a,b).
8.4.2 Chronic exposure and carcinogenicity
In the few studies identified in which the chronic toxicity and
carcinogenicity of methyl methacrylate were investigated, the observed
effects were, in general, similar to those reported in short-term and
subchronic studies and included inflammation and epithelial
hyperplasia of the nasal cavity and degeneration of the olfactory
sensory epithelium. Based on the results of a well documented
inhalation study in F344/N rats and B6C3F1 mice reported by the NTP
(1986) and Chan et al. (1988), there was no evidence of
carcinogenicity of methyl methacrylate for groups of 50 male F344/N
rats and 50 male and 50 female B6C3F1 mice exposed to 500 or 1000 ppm
(2050 or 4100 mg/m3) and groups of 50 female rats exposed to 250 or
500 ppm (1025 or 2050 mg/m3) for 2 years. Based on inflammation and
degeneration of the olfactory epithelium in the nasal cavity
(accompanied by variable atrophy of the nerve bundles in the submucosa
and, in the most severely affected areas, replacement of sensory
neuroepithelial cells with respiratory epithelium) and minimal
increases in the numbers of alveolar macrophages in the nasal cavity
at all dose levels, the LOEL in rats was considered to be 250 ppm
(1025 mg/m3). In mice, the LOEL was considered to be 500 ppm (2050
mg/m3) on the basis of lower mean body weights in exposed animals and
localized histopathological effects at the site of entry (including
inflammation and degeneration of the olfactory epithelium).
In earlier studies conducted for Rohm & Haas (1979a,b), no
treatment-related increases in tumour incidence occurred in either
groups of 56 male and 56 female golden hamsters or groups of 70 male
and 70 female albino F344 rats exposed to 0, 25, 100, or 400 ppm (0,
102.5, 410, or 1640 mg/m3) methyl methacrylate 6 hours/day, 5
days/week, for 18 months and 2 years, respectively. At the highest
concentration, body weight decreased significantly in both species,
mortality increased in hamsters, and the incidence of mild rhinitis in
the nasal mucosa increased slightly in rats.
A histopathological review of the nasal tissues from the rats in
the above-mentioned Rohm & Haas (1979a) study was commissioned by the
US Methacrylate Producers Association (Lomax, 1992; Lomax et al.,
1997). The review consisted of microscopic examination of nasal
tissue from at least 10% of randomly selected rats from each group,
and the slides evaluated included the original study slides plus
slides from tissue sections taken deeper into the block. The tissues
from male and female rats that had been exposed to 25 ppm (102.5
mg/m3) methyl methacrylate for 2 years were morphologically similar
to those of controls. Rats exposed to 100 or 400 ppm (410 or 1640
mg/m3) methyl methacrylate had exposure-related and concentration-
dependent microscopic changes in the olfactory epithelium lining the
dorsal meatus in the anterior region of the nasal cavity. The
microscopic changes consisted of degeneration/atrophy of the olfactory
epithelium and underlying Bowman's glands, hyperplasia of basal
(reserve) cells, replacement of olfactory epithelium by ciliated
(respiratory-like) epithelium, and inflammation of the mucosa and/or
submucosa (Lomax et al., 1997). Changes in the respiratory epithelium
were observed only at the high concentration (400 ppm [1640 mg/m3])
and were limited to hyperplasia of the submucosal gland and/or goblet
cells in the anterior region of the nasal cavity. The squamous
epithelium of the nasal cavity was not affected. The lesions tended
to be bilateral in distribution in rats exposed to both 100 and 400
ppm (410 and 1640 mg/m3) methyl methacrylate. A small nasal polypoid
adenoma was observed in one male from both the 100 and 400 ppm (410
and 1640 mg/m3) exposure groups. Based on this re-examination, the
NOEL and LOEL are considered to be 25 ppm (102.5 mg/m3) and 100 ppm
(410 mg/m3), respectively.
Data available on the effects of methyl methacrylate following
ingestion are limited. In an early study (Borzelleca et al., 1964) in
which organ to body weight ratios were determined and
histopathological examination of a wide range of tissues as well as
limited haematological and urine analyses were conducted, the relative
kidney weight was increased in a small group of female rats (n = 25)
exposed to 2000 ppm (mg/litre) methyl methacrylate in drinking-water
for 2 years. This effect was not observed in the males, and
histopathological examination revealed no damage. The authors also
reported a decrease in fluid consumption in rats exposed to 2000 ppm.
The no-observed-adverse-effect level (NOAEL) was therefore considered
to be 2000 ppm (equivalent to a dose of about 146 mg/kg body weight
per day for females and 121 mg/kg body weight per day for males, based
on intake and body weight data presented by the authors). There were
no treatmentrelated effects, based upon gross or histopathological
examination, in extremely small groups of beagle dogs (n = 2)
exposed to concentrations of up to 1500 ppm (mg/kg) methyl
methacrylate (equivalent to a dose of about 38 mg/kg body weight per
day) in their feed for 2 years (Borzelleca et al., 1964).
8.5 Genotoxicity and related end-points
Results of available genotoxicity studies on methyl methacrylate
are summarized in Table 2. In a number of well conducted in vitro
studies with precautions taken to limit evaporation, methyl
methacrylate was not mutagenic in Salmonella typhimurium with or
without metabolic activation. In a single study (Poss et al., 1979),
results were positive at clearly cytotoxic concentrations in the
presence of metabolic activation in a poorly validated forward
mutation assay in S. typhimurium TM677; results were negative in the
absence of metabolic activation.
Methyl methacrylate has been mutagenic and clastogenic in
mammalian cells in culture. It induced gene mutation in mouse
lymphoma L5178Y cells without metabolic activation in five
investigations and was positive with metabolic activation in all of
the three investigations in which it was examined. Results for
chromosomal aberrations and micronucleus formation were also positive
in this cell line without metabolic activation at concentrations at
which there was poor cell survival (Doerr et al., 1989). An increase
in chromosomal aberrations and sister chromatid exchanges in Chinese
hamster ovary cells has also been observed in the presence and absence
of metabolic activation in assays conducted in two laboratories (NTP,
1986; Anderson et al., 1990).
In in vivo studies conducted to date, there has been limited
evidence of genotoxicity. In an early study in which rats were
exposed to methyl methacrylate as either a single 2-hour exposure or
for 5 hours/day for 5 days at concentrations up to 9000 ppm (36 900
mg/m3), there were small but significant increases in chromosomal
aberrations in bone marrow cells from rats exposed to the highest
concentration in the multiple-exposure study (Anderson & Richardson,
1976). Although of questionable biological significance, small
increases in gaps were also noted at the two highest concentrations.
In a follow-up study with a larger number of intermediate dose levels,
there were significant increases in chromosomal aberrations following
both single and repeated exposures (Anderson et al., 1979); although
there was no clear dose-response, the pattern of effect may have been
attributable to chemically induced cell cycle delay (Anderson et al.,
1979). The maximum concentration tested in the follow-up study (1000
ppm [4100 mg/m3]) caused significant reductions in the mitotic
activity in the bone marrow of all exposed animals. Results were
negative in a well conducted dominant lethal assay in which mice were
exposed to concentrations of methyl methacrylate up to 9000 ppm
(36 900 mg/m3) 6 hours/day for 5 days (Anderson & Hodge, 1976).
No significant increase in the incidence of micronuclei was
observed in the bone marrow of mice following a single administration
of methyl methacrylate by gavage at doses up to 4.52 g/kg body weight
or in an additional investigation with one dose group that was exposed
to 1.13 g/kg body weight per day for 4 days; however, cells were
harvested at one time point (24 hours) only, and there was no evidence
of toxicity in the target tissue (Hachitani et al., 1981). Negative
results of an additional in vivo micronucleus assay in mice do not
contribute to an assessment of the weight of evidence of genotoxicity
owing to inadequate dose levels (Jensen et al., 1991). Available data
in the published accounts were inadequate to allow the assessment of
the mixed results of two additional studies in which chromosomal
aberrations in bone marrow cells of rats were examined following
intraperitoneal administration of methyl methacrylate (Fedyukovich et
al., 1988; Fedyukovich & Egorova, 1991).
Although not mutagenic in bacterial systems in vitro, methyl
methacrylate has induced mutation and chromosomal aberrations in
mammalian cells in vitro. In in vivo inhalation studies in which
there has been clear evidence of toxicity within the target tissue,
there has been limited evidence of genotoxicity of methyl
methacrylate.
8.6 Reproductive and developmental toxicity
In a well conducted study in Crl:CDBR rats, there was no
embryotoxicity or fetotoxicity and no increase in the incidence of
malformations or variations following exposure for 6 hours/day on days
6-15 of gestation to concentrations of methyl methacrylate that ranged
from 99 to 2028 ppm (406-8315 mg/m3; NOEL = 8315 mg/m3). However,
there were treatment-related effects on maternal body weight at all
concentrations (Solomon et al., 1993). In an earlier study in which
pregnant ICR mice were exposed to 1330 ppm (5450 mg/m3) methyl
methacrylate for 2 hours twice daily during days 6-15 of pregnancy,
there were no developmental effects. Maternal toxicity was not
addressed in the report (McLaughlin et al., 1978).
Table 2: Genetic effects (adapted from IARC, 1994).
Resultsb
Without With
exogenous exogenous
Dosea metabolic metabolic
Test system End-point (LED/HID) system system Reference
Salmonella typhimurium TM677 Forward mutation 5000 - + Poss et al., 1979
Salmonella typhimurium TA100 Reverse mutation 500 - - Lijinsky & Andrews, 1980
5000 - - Hachitani et al., 1981
2300 - - Waegemaekers & Bensink, 1984
5000 - - Zeiger et al., 1987
25 mg/plate - - Schweikl et al., 1994
Salmonella typhimurium TA1535 Reverse mutation 500 - - Lijinsky & Andrews, 1980
2300 - - Hachitani et al., 1981
5000 - - Waegemaekers & Bensink, 1984
1700 - - Zeiger et al., 1987
Salmonella typhimurium TA1537 Reverse mutation 500 - - Lijinsky & Andrews, 1980
2300 - - Hachitani et al., 1981
5000 - - Waegemaekers & Bensink, 1984
5000 - - Zeiger et al., 1987
Salmonella typhimurium TA1538 Reverse mutation 500 - - Lijinsky & Andrews, 1980
2300 - - Hachitani et al., 1981
5000 - - Waegemaekers & Bensink, 1984
Salmonella typhimurium TA98 Reverse mutation 500 - - Lijinsky & Andrews, 1980
2300 - - Hachitani et al., 1981
5000 - - Waegemaekers & Bensink, 1984
5000 - - Zeiger et al., 1987
25 mg/plate - - Schweikl et al., 1994
Table 2 (continued)
Resultsb
Without With
exogenous exogenous
Dose metabolic metabolic
Test system End-point (LED/HID) system system Reference
Salmonella typhimurium TA97 Reverse mutation 1700 - - Zeiger et al., 1987
Salmonella typhimurium TA97a 25 mg/plate - - Schweikl et al., 1994
Salmonella typhimurium TA102 25 mg/plate - - Schweikl et al., 1994
Salmonella typhimurium TA104 25 mg/plate - - Schweikl et al., 1994
Mouse lymphoma L5178Y cells Gene mutation (tk locus) 2200 + 0 Doerr et al., 1989
in vitro 2000 + 0 Moore et al., 1988
250 + Myhr et al., 1990
500 + Myhr et al., 1990
500 + + Dearfield et al., 1991
117.5 (0.125 µl/ml) + + NTP, 1986
Mouse lymphoma L5178Y cells Micronucleus formation 2200 (+) 0 Doerr et al., 1989
in vitro
Chinese hamster ovary cells Sister chromatid exchange 16 + + Anderson et al., 1990
in vitro 750 + NTP, 1986
500 + NTP, 1986
Chinese hamster ovary cells Chromosomal aberrations 1600 + (+) Anderson et al., 1990
in vitro 5000 +c NTP, 1986
1600 +c NTP, 1986
Mouse lymphoma L5178Y cells Chromosomal aberrations 2200 (+) 0 Doerr et al., 1989
in vitro
Human lymphocytes in vitro Sister chromatid exchange 0.1 ? 0 Cannas et al., 1987
Table 2 (continued)
Resultsb
Without With
exogenous exogenous
Dose metabolic metabolic
Test system End-point (LED/HID) system system Reference
Mouse bone marrow cells Micronucleus formation <4.52 g/kg body - Hachitani et al., 1981
in vivo weight x 1 p.o.d
1.13 g/kg body - Hachitani et al., 1981
weight x 4 p.o.d
Rat bone marrow cells in vivo Chromosomal aberrations 36 900 mg/m3, - Anderson & Richardson, 1976
2 hour x 1 inhal.
36 900 mg/m3, + Anderson & Richardson, 1976
5 hours/day,
5 days inhal.
4100 mg/m3, 2 hour Equivocal Anderson et al., 1979
x 1 inhal.
4100 mg/m3,
5 hours/day, Equivocal Anderson et al., 1979
5 days inhal.
Male mice in vivo Dominant lethal assay <36 900 mg/m3, - Anderson & Hodge, 1976
6 hours/day,
5 days inhal.
Table 2 (continued)
a In vitro tests, µg/ml; in vivo tests, mg/kg body weight; LED = lowest effective dose; HID = highest ineffective dose.
b +, positive; (+), weak positive; -, negative; 0, not tested; ?, inconclusive (variable response within several experiments
within an adequate study). Negative results of an additional in vivo micronucleus assay in mice do not contribute to an assessment
of the weight of evidence of genotoxicity owing to inadequate dose levels (Jensen et al., 1991). Available data in the published
accounts were inadequate to permit an assessment of the mixed results of two additional studies in which chromosomal aberrations
in bone marrow cells of rats were examined following intraperitoneal administration (Fedyukovich et al., 1988; Fedyukovich &
Egorova, 1991).
c 5% of cells affected without exogenous metabolic system; 30% of cells affected with exogenous metabolic system.
d No toxicity in target tissue. p.o. = per os.
In a study reported only in the form of an abstract, a number of
effects, including intrauterine deaths, an increase in the number of
fetuses with vascular pathology, and an increase in the frequency of
"functional immaturity," were observed in the offspring of rat dams
exposed to concentrations of methyl methacrylate as low as 0.01 mg/m3
(Farmakovskaya & Tikhomirov, 1993). The information presented in the
published account of this study is inadequate to permit assessment of
the protocol and results.
In early studies, developmental effects, including decreases in
fetal weights, embryo-fetal deaths, and skeletal abnormalities, were
observed in rats following inhalation of concentrations of methyl
methacrylate that were toxic to the dams (Hodge & Palmer, 1977;
Nicholas et al., 1979). Similar effects were reported in studies in
mice in which maternal toxicity was not addressed (Tansy, 1975) and in
studies in rats in which the protocol and results were not well
documented (Luo et al., 1986).
Data on reproductive effects are limited to a dominant lethal
assay and examination of gonads in repeated-dose toxicity studies.
There was no reduction in fertility as measured by the number and
percentage of successful matings each week or the percentage of female
mice that become pregnant in a dominant lethal assay in mice exposed
to 100, 1000, or 9000 ppm (410, 4100, or 36 900 mg/m3) methyl
methacrylate by inhalation for 6 hours/day for 5 days (Anderson &
Hodge, 1976).
Adverse effects on the reproductive organs of experimental
animals have not been observed in repeated-dose studies in animals
exposed to methyl methacrylate (see sections 8.3 and 8.4).
8.7 Immunological and neurological effects
In a study in which the leukocyte migration inhibition method was
employed to determine if methyl methacrylate was potentially a
causative agent in denture stomatitis, three groups of five albino
rabbits of both sexes were injected intramuscularly with 1 ml of
methyl methacrylate on days 1, 5, and 14 (Zafiropoulos et al., 1985).
On the 36th day, blood was drawn to test the inhibition of leukocyte
migration. The results indicated that methyl methacrylate was a
specific antigen that was capable of inducing cellular immune
reaction.
Methyl methacrylate markedly impaired locomotor activity and
learning while significantly increasing aggressive behaviour in male
rats orally administered the chemical at 500 mg/kg body weight for 21
days (Husain et al., 1985). There was an overall increase in levels
of biogenic amine in the pons-medulla and hippocampus. Levels of
noradrenaline in the cerebral cortex and 5-hydroxytryptamine in the
mid-brain and the hypothalamus were increased, whereas there was a
slight decrease in dopamine levels in the corpus striatum (Husain et
al., 1985). In a separate study under the same experimental
conditions, a significant increase in cholesterol (26%) and
triglycerides (65%) and a slight decrease in the total phospholipid
content of the sciatic nerve were noted (Husain et al., 1989).
In a study investigating the neurotoxic effects of acrylamide, no
evidence of neurotoxicity (evaluated as observation of ataxia) or
enhancement of acrylamide neuropathy was observed in male rats fed a
diet containing 18 800 ppm (mg/kg) methyl methacrylate for 5 weeks
(the intake of methyl methacrylate was estimated to be 410 mg/day)
(Edwards, 1975). Other limited studies that have been identified do
not contribute to our understanding of the neurotoxicity of methyl
methacrylate (Innes & Tansy, 1981; Wynkoop et al., 1982; Kanerva &
Verkkala, 1986).
9. EFFECTS ON HUMANS
Data on effects of methyl methacrylate on humans are informative
primarily with respect to irritation and sensitization (for exposure
both dermally and by inhalation), respiratory effects, and
carcinogenicity; however, in cross-sectional epidemiological studies
conducted to date, effects on the nervous (Seppalainen & Rajaniemi,
1984; Schwartz et al., 1989) and cardiac (Cromer & Kronoveter, 1976;
NIOSH, 1976) systems have also been examined.
Hypotension, changes in pulse rate, and cardiac arrest have been
reported following bone replacement surgery with polymethyl
methacrylate cemented prostheses; however, the significance of these
observations with respect to methyl methacrylate exposure is
questionable owing to lack of correlation between peak plasma
concentrations of methyl methacrylate and reported effects and the
absence of similar effects in younger patients (Government of Canada,
1993; Cary et al., 1995; ECETOC, 1995).
9.1 Case reports
There are reports of skin irritation and sensitization in human
volunteers and in patients suspected of occupational sensitization to
acrylates from exposure to dental materials or anaerobic sealants
(Spealman et al., 1945; Estlander et al., 1984; Kassis et al., 1984;
Rajaniemi & Tola, 1985; Conde-Salazar et al., 1988; Kanerva et al.,
1988, 1989; Farli et al., 1990; Guerra et al., 1993). Occupational
asthma associated with methyl methacrylate has also been reported
(Lozewicz et al., 1985; Pickering et al., 1986, 1993); however, there
is no conclusive evidence that methyl methacrylate is a respiratory
sensitizer, and the possibility of a non-specific response due to
respiratory tract irritation cannot be excluded.
9.2 Epidemiological studies
Protocols and results of cross-sectional studies in which
respiratory effects of methyl methacrylate have been investigated in
occupationally exposed populations are presented in Table 3. For
example, in a study in which smoking was taken into account, an
increase in the prevalence of chronic cough (as evaluated by
questionnaire) was observed in a small group of workers (n = 40)
exposed exclusively to methyl methacrylate for at least 5 years in two
factories (mean atmospheric levels of methyl methacrylate in the two
factories were 18.5 and 21.6 ppm [75.8 and 88.6 mg/m3]) compared with
controls engaged in similar job categories, but without exposure to
methyl methacrylate (Marez et al., 1993). Spirometric values did not
differ before the work shift, but two of nine parameters decreased
during the work shift. Information concerning exposure to other
respiratory irritants was not provided; although increased cough and
mild airway resistance correlated with exposure to methyl
methacrylate, peak versus mean exposures were not examined. In other
studies in which there was some quantitative information on exposure,
results have varied, with effects on respiratory function being
observed in some cases at mean concentrations as low as 11 mg/m3
(Jedrychowski, 1982) and no effects in other investigations at
time-weighted-average concentrations up to 40-50 ppm (164-205 mg/m3)
(Cromer & Kronoveter, 1976; NIOSH, 1976; Röhm, 1994). It is
difficult, however, to draw meaningful conclusions concerning levels
of exposure that induced effects in these studies, as there was little
attempt to assess mean versus peak exposures. Moreover,
interpretation of several of the investigations is complicated by
concomitant exposure of the examined populations to other substances.
In other investigations reported to date, quantitative data on
exposure of workers to methyl methacrylate were not included (Andrews
et al., 1979; Schwartz et al., 1989). An additional cross-sectional
study of the prevalence of disorders of smell in methyl
methacrylate-exposed workers is under way (A. Muttray, personal
communication, 1997).
Table 3: Cross-sectional epidemiological studies - respiratory effects
Protocol Results Reference
Study population composed of 40 workers from two factories who An increase in the prevalence of chronic cough Marez et al., 1993
were exposed to methyl methacrylate for >5 years and 45 controls observed in exposed workers compared with controls
engaged in similar job categories but without exposure to methyl (p = 0.04). This difference remained significant
methacrylate. Mean atmospheric concentrations of methyl after adjustment for smoking (p = 0.03). Airway
methacrylate at the two factories were 18.5 ppm (75.9 mg/m3) resistance increased during the 8-hour work shift
(range 9-32 ppm [36.9-131.2 mg/m3]) and 21.6 ppm in workers exposed to methyl methacrylate (as
(88.6 mg/m3) (range 11.9-38.5 ppm [48.8-157.9 mg/m3]). measured by MEF50 [p = 0.04] and MEF50/MEF
Smoking history and information on the presence of respiratory [p = 0.0)). The obstruction was mild, and forced
symptoms were gathered by means of a questionnaire. Respiratory expiratory volume in one second (FEV,) did not
measurements (maximum expiratory flow volume [MEFV], decrease during the work shift.
forced vital capacity [FVC], forced expiratory volume [FEV])
were performed by means of a spirometer: one before the
working shift, and the second in the last 2 hours of the 8-hour
shift.
Ninety-one exposed and 43 non-exposed workers were evaluated at No significant differences were observed for Cromer & Kronoveter,
five plants manufacturing polymethyl methacrylate sheets. For respiratory function, chronic liver and 1976
exposed workers, 8-hour time-weighted-average concentrations of gastrointestinal effects, skin and allergic
methyl methacrylate were between 4 and 49 ppm (16.4-200.9 problems, blood pressure and pulse rate, white
mg/m3). Evaluation of chronic effects was conducted through an blood cell count, and haemoglobin values. The
extensive questionnaire, a comparison of mean blood pressure only parameters for which effects were observed
values with predicted values from the 1971-1972 US National were serum glucose, blood urea nitrogen, cholesterol,
Health Survey, and results of pulmonary function tests, albumin, and total bilirubin values, although the
haemoglobin and white blood cell counts, urinalysis, and blood implication of these effects remains unclear.
chemistry. Although not statistically significant, the data also
"suggested possible alterations in skin and nervous
system symptomatology, urinalysis findings, and
serum triglycerides."
Employees of the Rohm & Haas Co. (which manufactures acrylic Upon cross-sectional analysis, when the age, ethnic Schwartz et al., 1989
acid, acrylates, and methacrylates) - 618 males and 113 females group, and smoking status were considered, the mean
(mean age 42.9 years), out of the total number of 909 short- and UPSIT scores in the four exposure groups did not
long-term employees - were asked to complete a University of differ. For the "no significant chemical exposures,"
Pennsylvania Smell Identification Test (UPSIT) and "exposure to other chemicals," "exposure to low
questionnaires on job histories as well as personal and medical levels of acrylate/methacrylate," and "exposure to
information. Employees were grouped into four exposure higher levels of acrylate/methacrylate" groups, the
categories: no significant chemical exposures (n = 319), exposure scores were 37.8, 37.4, 37.0, and 37.6, respectively.
to other chemicals (n = 193), exposure to low levels of Based on logistic regression analysis, adjusting for
acrylate/methacrylate (n = 164), and exposure to higher levels of multiple confounders, in the nested case-control
acrylate/methacrylate (n = 55). In a nested case-control study, 77 study, the odds ratios for the association of UPSIT
workers who scored below the 10th percentile in their age group score with exposure to methyl methacrylate for all
on the UPSIT were matched with controls (scored at or above the workers was 2.8 (95% CI 1.1-7.0) and for those who
50th percentile). Exposure was classified in terms of whether never smoked was 13.5 (95% CI 2.1-87.6); the crude
workers had been exposed to methyl methacrylate for at least 6 odds ratios were 2.0 and 6.0, respectively. There
weeks, the total time of employment at the plant, and a cumulative was a dose-response relationship between olfactory
exposure score - a semi-quantitative index of lifetime exposure dysfunction and the cumulative exposure. The odds
to the acrylates - for each worker. ratios increased with the cumulative exposure scores,
except for a decrease in the highest exposure
category. The olfactory dysfunction may be
reversible, as the odds ratios decreased with the
length of time since the last exposure.
Four hundred and fifty-four males from a plant (Plant A) There was a non-significantly lower occurrence of Jedrychowski, 1982
producing styrene and methyl methacrylate were compared with bronchitis and/or asthma in the exposed (17.8%)
683 males from a plant producing carbon derivatives who served compared with the control (19.5%) group. There was
as controls (jobs were similar in both plants, but there was no no significant difference in the incidence of
exposure to styrene or methyl methacrylate in the latter plant). chronic chest symptoms between the two groups.
Standardized interviews on chest symptoms, measured heights, However, the frequency of lung obstruction was over
lung function tests, and examinations for chronic bronchitis and twice as high in the exposed workers (45.4% vs
asthmatic syndrome were conducted. The workers were divided 18.0%); this percentage was higher for smokers than
into the following groups: non-smokers, ex-smokers, and current for non-smokers (20.9% vs 13.6%). Within the
smokers. Styrene and methyl methacrylate concentrations were exposed group, the occurrence of lung obstruction
determined in 18 workplaces in Plant A. For methyl methacrylate, in smokers and in non-smokers did not differ
the mean concentration in Plant A was 11 mg/m3. significantly. Fifty-six per cent of the controls and
76% of the exposed workers with lung obstruction
did not have any chronic chest symptoms. The lung
function of the exposed group was significantly
poorer than that of the controls; the effects were
slightly worse among smokers in both groups. The
relative risk of lung obstruction (compared with
non-exposed ex- and non-smokers) was 1.7 for non-
exposed smokers, 4.7 for exposed ex- and
non-smokers, and 5.5 for exposed smokers.
Five hundred and two dental students (who handled methyl In exposed students, 6% reported respiratory Andrews et al., 1979
methacrylate in their laboratories) completed self-administered symptoms associated with exposure to methyl
multiple-choice questionnaires concerning their past histories methacrylate (88% had histories of asthma or
and any symptoms (not specified) associated with activities in the allergic rhinitis), and 5% when using high-speed
lab. Spirometric tests were performed before and after exposure to drills. Among the 77 students who underwent
unreported amounts of methyl methacrylate for 77 students who spirometric tests, there was no significant change
had allergic rhinitis, smoked, or had symptoms upon usual in symptoms or spirometry.
exposure.
A study of 91 exposed and 43 non-exposed workers from five Some significant differences in terms of coughing NIOSH, 1976
methyl methacrylate cast sheet manufacturing plants in the USA. and expectoration, but these were likely due to
The survey included a medical questionnaire, measurement of differences in smoking habits. When smoking
clinical symptoms, blood pressure, and pulse rate, testing of histories were taken into consideration, there was
pulmonary function and blood chemistry, urinalysis, and white no significant change in pulmonary function among
blood cell counts. Based on 8-hour time-weighted-average the exposure groups. No significant differences in
exposures to methyl methacrylate, workers were divided into five blood pressure or in white blood cell count were
categories: <5 ppm (20.5 mg/m3) (n = 13), 5-25 ppm (20.5-102.5 found. There were several significant differences
mg/m3) (n = 20), 25-50 ppm (102.5-205 mg/m3) (n = 33), no in the blood chemistry tests of the "no current
current exposure but past exposure >1 year (n = 25), and the exposure" group, but this was likely due to the
control group with no exposure (n = 43). The ages and smoking fact that they were significantly older than the
histories of exposure groups were not matched very well because controls.
of the low number of volunteers.
A cross-sectional study involving 211 workers at a polymethyl There were no significant respiratory effects Röhm, 1994
methacrylate sheet producing factory in Germany. The study associated with exposure in any of the groups.
report period was 1991-1993. Working areas were classified into There were some observations of eye and respiratory
the following exposure ranges: 3-10 ppm (12.3-41 mg/m3), 10-20 tract irritation, which were reported to be
ppm (41-82 mg/m3), 20-30 ppm (82-123 mg/m3), and 30-40 ppm transient and were limited to short-term exposures
(123-164 mg/m3) (8-hour time-weighted averages; ranges (5-15 minutes in duration) at concentrations
represent geometric means). The numbers of persons in each exceeding 100 ppm (410 mg/m3).
exposure group were 7, 128, 20, and 56, respectively. The
examination of the workers consisted of a self-administered
questionnaire (concerning lifestyle, occupation, and medical
history, with emphasis on complaints of nose, throat, and
respiratory system failures and allergic reactions, including
skin and asthmatic reactions) as well as a visual examination
of the nasal cavity.
Owing to an excess of mortality from colon cancer observed in
early investigations in exposed workers, several historical cohort
studies have been conducted to examine the mortality rate from cancer
of the colon or rectum among male workers employed at two plastics
manufacturing plants in Bristol, Pennsylvania, and Knoxville,
Tennessee (DeFonso & Maher, 1981, 1986; Maher & DeFonso, 1987a,b;
Walker et al., 1991). An additional cohort study of workers at a
small number of polymethyl methacrylate sheet production factories in
the United Kingdom has also been identified (Tomenson & Bonner, 1994;
Cary et al., 1995); however, documentation available at this time is
inadequate for evaluation. In the most recent and extensive follow-up
by Walker et al. (1991) in the above-mentioned plastics manufacturing
plants, data were reanalysed as a function of the period of employment
of the workers. In this investigation, the two cohorts were composed
of 10 482 men who had worked during the period 1933-1982 in the
Bristol plant and 3381 men hired between 1943 and 1982 in the
Knoxville plant. The population of workers at the Bristol plant was
further divided into an early cohort (men employed at some time
between 1933 and 1945, inclusive) and late cohort (1946-1982,
inclusive). The early cohort worked in conditions that are thought to
have involved high exposures to the vapour phase of ethyl acrylate and
methyl methacrylate monomer, as well as to a variety of volatile
by-products of the ethyl acrylate/methyl methacrylate polymerization
process.
In the two cohorts with later dates of first hire (Knoxville and
late Bristol), there was no excess mortality due to cancer of the
colon or rectum. In the early (Bristol) cohort, there was an apparent
excess of deaths due to colon cancer (38 cases observed overall in
those persons who accumulated a dose of >0 units, compared with an
expected number of 25.4). Although the highest risk was in the
subgroup of workers with the highest cumulative exposure, there was no
trend of increasing risk with increasing exposure after allowing for a
long latency period. There was no systematic pattern of excess risk
of cancer at any other site. For respiratory cancer, however, there
was a significantly high standardized mortality ratio (of 1.44) in the
Knoxville cohort, with no excess in either of the Bristol cohorts.
Owing to the large number of statistical estimates in this study and
the absence of a clear dose-response trend, the association of methyl
methacrylate with respiratory cancer is unclear. The apparent excess
may have been due to statistical fluctuation or to confounding by
other occupational exposures in the environment at the time.
Collins et al. (1989) reported a limited study of a much smaller
cohort of workers exposed for considerably shorter periods to methyl
methacrylate at two plants that either manufactured methyl
methacrylate or used methyl methacrylate in other product manufacture
between 1951 and 1983. There was no excess mortality for any type of
cancer examined (Collins et al., 1989). There was a very weak
indication of an excess of rectal cancer (two cases in exposed workers
with the expected number much less than 1.0) and weak to moderate
indication of an excess risk of lung cancer (odds ratios of 4-5) in a
population-based study of a small number of workers exposed to methyl
methacrylate in Montreal, Canada (Siemiatycki, 1991).
Identified studies on the potential genotoxicity of methyl
methacrylate in occupationally exposed populations contribute limited
information. There was no increase in the number of sister chromatid
exchanges in the peripheral lymphocytes of 31 male workers
occupationally exposed to methyl methacrylate (mean value per 8 hours
ranged from 0.70 to 21.6 ppm [2.9-88.6 mg/m3]) in four factories
compared with that of 31 unexposed male workers of similar mean age
and smoking habits (Marez et al., 1991). The distribution frequency
of sister chromatid exchange, however, was significantly higher in the
group exposed to methyl methacrylate at peak concentrations ranging
from 114 to 400 ppm (467-1640 mg/m3), although the number of
individuals in this exposure subgroup was small (n = 6). Similarly,
no increase in the frequency of chromosomal aberrations was observed
in the peripheral lymphocytes of 38 male workers who were engaged in
organic glass production (polymethyl methacrylate plates) and exposed
to 8-hour, time-weighted-average concentrations of methyl methacrylate
of 0.9-71.9 ppm (3.7-295 mg/m3) (Seiji et al., 1994). The frequency
of sister chromatid exchange was higher in the exposed group than in
controls; however, this was considered to be due to a higher age
distribution in the exposed workers.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1 Aquatic environment
Bailey et al. (1985) studied the toxicity of methyl methacrylate
in juvenile bluegill sunfish at 22°C under static and flow-through
conditions of various durations (1-96 hours). The 96-hour LC50 under
flow-through conditions was 191 mg/litre, whereas LC50 values for
durations of 1-24 hours ranged from 420 to 356 mg/litre, respectively.
The 96-hour LC50 for rainbow trout under flow-through conditions was
>79 mg/litre, the highest concentration tested.
Sublethal/behavioural responses were noted among the fish in the 40
and 79 mg/litre concentration groups (Bowman, 1990).
The 24-hour EC50 for immobilization of Daphnia magna was 720
mg/litre, with extrapolated EC0 and EC100 values of 502 and 1042
mg/litre, respectively (Bringmann & Kuhn, 1982). The 24-hour LC50
was 1760 mg/litre, with extrapolated values for the LC0 and LC100 of
875 and 2500 mg/litre, respectively (Bringmann & Kuhn, 1977). The
threshold for onset of inhibition of cell multiplication was 447
mg/litre for the flagellate protozoan Entosiphon sulcatum after 72
hours of exposure (Bringmann, 1978). These studies were done in
static, open systems, with only nominal concentrations reported.
Thresholds for onset of inhibition of cell multiplication
following 8 days of exposure to methyl methacrylate were 120 mg/litre
for the blue-green alga Microcystis aeruginosa and 37 mg/litre for
the green alga Scenedesmus quadricauda at pH 7 (Bringmann & Kuhn,
1976, 1978a,b). The 96-hour LC50 for Selenastrum capricornutum was
170 mg/litre, with a NOEL of 100 mg/litre (Forbis, 1990). No studies
were identified on the effects of methyl methacrylate on higher
aquatic plants.
10.2 Terrestrial environment
Data on the toxicity of methyl methacrylate to terrestrial
animals are limited to a single study on soil microflora in which no
effects of biological significance were observed (Hossack & Thomas,
1992).
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Data on effects of methyl methacrylate in humans are informative
primarily with respect to irritation and sensitization (for exposure
both dermally and by inhalation) and carcinogenicity. Although there
are some quantitative data on exposure to methyl methacrylate in
available cross-sectional investigations of other end-points (NIOSH,
1976; Jedrychowski, 1982; Marez et al., 1993), they are considered
inadequate as the principal basis for hazard identification and
dose-response assessment owing to limitations of design and the
potential role of confounding factors. Data on hazard identification
and dose-response assessment for effects other than
irritation/sensitization and carcinogenicity are derived primarily,
therefore, from investigations in experimental animals.
The acute toxicity of methyl methacrylate is low. Irritation of
the skin, eye, and nasal cavity has been observed in rodents and
rabbits exposed to relatively high concentrations of methyl
methacrylate. This substance is a mild skin sensitizer in animals.
Methyl methacrylate is a mild skin irritant in humans and has the
potential to induce skin sensitization in susceptible individuals.
Although occupational asthma associated with methyl methacrylate has
also been reported, there is no conclusive evidence that methyl
methacrylate is a respiratory sensitizer.
The effect observed most frequently at lowest concentration after
repeated inhalation exposure of experimental animals to methyl
methacrylate is irritation of the nasal cavity. Effects on the kidney
and liver at higher concentrations have also been reported.
Limited available data indicate that methyl methacrylate is
unlikely to induce fetotoxic effects in the absence of maternal
toxicity. There has been no evidence of reproductive toxicity, based
on limited available data (a dominant lethal assay in mice and
examination of the gonads in repeated-dose toxicity studies). Based
on limited available data, neurological effects have been observed
following ingestion of doses greater than those that induce minimal
renal effects.
As a whole, the available epidemiological studies do not provide
strong or consistent evidence of a carcinogenic effect of methyl
methacrylate on any target organ in humans, nor can it be inferred
with any degree of confidence that the possibility of an excess risk
has been disproved. Methyl methacrylate has not been carcinogenic in
an extensive, well documented 2-year bioassay in rats and mice exposed
by inhalation and in additional chronic inhalation studies in rats and
hamsters. Although not mutagenic in vitro in bacterial systems,
methyl methacrylate has been mutagenic and clastogenic in mammalian
cells in vitro. In in vivo studies (primarily by the inhalation
route) in which there has been clear evidence of toxicity within the
target tissue, there has been limited evidence of genotoxicity of
methyl methacrylate. On the basis of these observations, methyl
methacrylate is considered unclassifiable with respect to
carcinogenicity in humans.
Owing to the limitations of the available studies in humans on
effects associated with longer-term exposure to methyl methacrylate,
it is necessary to rely primarily on information obtained from the
studies in animals for determination of critical effect levels. The
lowest reported effect level for inhalation was 100 ppm (410 mg/m3)
in rats exposed to methyl methacrylate for 2 years (based upon
inflammatory degeneration of the nasal epithelium); the NOEL in this
investigation was 25 ppm (102.5 mg/m3) (Rohm & Haas, 1979a; Lomax,
1992; Lomax et al., 1997).
11.1.2 Criteria for setting guidance values for methyl methacrylate
The following quantitative guidance is provided as an example of
a possible basis for derivation of limits of exposure and judgement of
the quality of environmental media by relevant authorities. As methyl
methacrylate is considered to be "unclassifiable with respect to
carcinogenicity in humans," guidance values are derived on the basis
of a lowest-observed-(adverse)-effect level [LO(A)EL] or a
no-observed-(adverse)-effect level [NO(A)EL] for non-neoplastic
effects. For methyl methacrylate, the route of exposure most relevant
to the general population is likely inhalation.
The value considered most appropriate as a basis for development
of a tolerable concentration in air is the NOEL of 25 ppm (102.5
mg/m3) in rats exposed to methyl methacrylate for 2 years (Rohm &
Haas, 1979a; Lomax, 1992; Lomax et al., 1997). Effects at the next
higher concentration were degenerative changes in the olfactory
epithelium.
There is some debate currently about extrapolation of data on
nasal irritation of the olfactory epithelium observed in rodents in
risk assessment for humans. There are significant morphological
differences between species in the structure of the nasal cavity,
which result in differences in concentrations of inhaled materials at
the nasal tissue. These are reflected in differences in surface area
normalized to minute ventilation, being fivefold greater in rodents
than in humans (DeSesso, 1993). A much greater percentage of the
nasal cavity is lined by olfactory epithelium in rats than in humans.
In addition, rodents are obligate nose breathers, whereas humans can
also breathe through their mouths, which is expected to reduce
exposure of the nasal epithelium for much of the population. There
are also differing nasal flow patterns, with the greater airflow
across the human olfactory epithelium during the expiratory phase when
the vapour concentration would be considerably reduced as a result of
absorption in the lower respiratory tract.
The pattern of the critical effects of inhalation of methyl
methacrylate in animal studies (i.e. the olfactory epithelium being
affected at lowest concentration) is consistent with toxicity
resulting from metabolism of the inhaled material in the olfactory
tissue by carboxylic esterases to methacrylic acid. Data on species
differences in olfactory tissue carboxylesterase activity have not
been identified; however, based on limited data from human tissue
samples that may not have been morphologically normal taken at polyp
biopsy, the activity of alpha-naphthylbutyrate carboxylesterase in
human nasal respiratory tissue is less than that in the rat (Mattes &
Mattes, 1992).
Although it is possible that humans may be less sensitive than
rodents to lesions of the nasal epithelium caused by methyl
methacrylate, currently available data are inadequate to account
quantitatively for potential interspecies variation in sensitivity.
However, studies that are currently under way may shed some additional
light on this aspect (T. Green, personal communication, 1997; P.J.
Pinto, personal communication, 1997). Therefore, on the basis of the
available data, a tolerable concentration (TC) has been derived on the
basis of a commonly adopted default value of 10-fold for interspecies
variation as follows:
TC = (102.5 mg/m3/100) × (6/24) × (5/7)
= 0.2 mg/m3 (rounded to one significant figure)
where:
* 102.5 mg/m3 (25 ppm) is the lowest NOEL reported in inhalation
bioassays of adequate quality in animal species (rats) conducted
to date (exposure-related and concentration-dependent microscopic
changes [degeneration/atrophy of the olfactory epithelium and
underlying Bowman's glands, hyperplasia of basal (reserve) cells,
replacement of olfactory epithelium by ciliated
(respiratory-like) epithelium, and inflammation of the mucosa
and/or submucosa] were observed in anterior portions of the nasal
cavity in rats exposed to the next higher concentration) (Rohm &
Haas, 1979a; Lomax, 1992; Lomax et al., 1997);
* 6/24 and 5/7 are the conversion of intermittent exposure of rats
(i.e. 6 hours/day, 5 days/week) to continuous exposure of humans;
this is appropriate in view of data that suggest that continuous
exposure to methyl methacrylate could result in effects at
concentrations below the NOAEL for intermittent exposure (Lomax
et al., 1994); no scaling factor for inhalation volume to body
weight was used, as effects at the next higher dose level are
limited to the site of entry; and
* 100 is the uncertainty factor (×10 for intraspecies variation;
×10 for interspecies variation).
Based on the results of limited available cross-sectional studies
on respiratory effects in human populations, this value is likely to
be protective.
A TDI can be derived for the oral route of exposure based on a
2-year drinking-water study in rats in which the NOAEL in females was
considered to be 146 mg/kg body weight per day; the NOEL in males was
121 mg/kg body weight per day, the highest dose level tested
(Borzelleca et al., 1964). Incorporating an uncertainty factor of 100
(×10 for intraspecies variation; ×10 for interspecies variation), the
TDI would be 1.2 mg/kg body weight per day.
11.1.3 Sample risk characterization
The extremely limited nature of the available data as a basis for
estimation of exposure should be borne in mind in interpreting the
comparisons presented here for predicted indirect population exposure
in the general environment and estimated exposure from the use of
consumer products containing methyl methacrylate. Moreover, the
sample exposure estimates presented in section 6.2 will vary
considerably as a function of production and use patterns and control
measures in various countries.
Based upon the sample predicted concentration (based on fugacity
modelling) of methyl methacrylate in air of 2.44 × 10-4 µg/m3,
presented in section 6.1, levels of methyl methacrylate in ambient air
are many orders of magnitude less than the calculated tolerable
concentration of 200 µg/m3. Estimated intakes associated with
inhalation exposure (predicted by computer modelling) during the use
of consumer products containing methyl methacrylate, such as
dispersion paints (estimated to be in the 10-100 µg/kg body weight per
day range) and oil-based paints (predicted exposure in the 100-1000
µg/kg body weight per day range) (see section 6.2), may be up to an
order of magnitude higher than the tolerable intake associated with
exposure at the level of the tolerable concentration. Although it has
been reported that in some countries these products are not supplied
to the general public, information on use patterns of these products
in other countries was not available.
With respect to occupational exposure, mean levels of methyl
methacrylate in the air of production and manufacturing facilities and
dental facilities range up to several hundred mg/m3, whereas levels
in beauty salons are generally less than 100 mg/m3 (IARC, 1994).
Elevated levels (greater than 1500 mg/m3) during floor coating with
methyl methacrylate-containing resins have been reported, although
time-weighted-average concentrations would be less.
11.2 Evaluation of environmental effects
Because of its release principally in emissions from industrial
sources and its relatively high volatility, the atmosphere is the
predominant environmental sink for methyl methacrylate. It is highly
reactive with hydroxyl radicals; thus, its lifetime in the atmosphere
is short. Substances whose atmospheric half-lives do not exceed 1
year are not considered to contribute to global warming; therefore,
methyl methacrylate is not considered to be a greenhouse gas, nor
would it contribute directly to depletion of the ozone layer. Methyl
methacrylate is not expected to bioconcentrate in the environment.
Terrestrial organisms will have the greatest potential for
exposure to methyl methacrylate in ambient air. However, as no field
or laboratory studies on birds, terrestrial invertebrates, or
terrestrial plants were identified, the toxicity of methyl
methacrylate to these organisms could not be assessed. Chronic
studies on laboratory mammals are available, however, as well as data
on levels of exposure of aquatic-based mammals to methyl methacrylate,
thus permitting the comparison between effects and environmental
exposure for these organisms. The mink was chosen as the model
species, with aquatic organisms comprising up to 100% of its diet.
Based on the concentrations of methyl methacrylate in air, water, and
fish predicted by fugacity modelling and assuming daily consumption
rates for mink of 0.55 m3 of air, 0.1 litre of water, and 158 g of
fish, the estimated total daily intake of methyl methacrylate by mink
in southern Ontario, Canada, is 0.17 ng/kg body weight per day, with
approximately 80% of the exposure being attributable to inhalation
(Government of Canada, 1993). The lowest NOEL observed in chronic
inhalation studies in laboratory animals is 102.5 mg/m3, based on
exposure-related and concentration-dependent microscopic changes in
anterior portions of the nasal cavity (degeneration/atrophy of the
olfactory epithelium and underlying Bowman's glands, hyperplasia of
basal cells, replacement of olfactory epithelium by respiratory
epithelium, and inflammation of the mucosa and/or submucosa) (Rohm &
Haas, 1979a; Lomax, 1992; Lomax et al., 1997). Using a factor of 10
to account for interspecies variability in sensitivity, the NOEL is
108 times higher than levels predicted to occur in the environment in
Canada (i.e. 0.24 ng/m3).
No chronic studies on aquatic organisms were identified; however,
acute tests have been conducted on fish, Daphnia magna, and algae.
The most sensitive effect was the onset of inhibition of cell
multiplication by the green alga Scenedesmus quadricauda at 37
mg/litre following 8 days of exposure. This is similar to the
concentration (i.e. 40 mg/litre) at which sublethal/ behavioural
responses were noted in rainbow trout following 96 hours of exposure.
Using a factor of 20 to convert from an acute to a chronic end-point
and another factor of 10 to account for interspecies variability in
sensitivity, the estimated effects threshold is approximately
106-fold higher than the concentration predicted to occur in surface
water in Canada (i.e. 0.13 ng/litre).
Therefore, although available data on the environmental effects
of methyl methacrylate are limited and predicted concentrations in
various media are highly uncertain, a wide margin exists between
observed effect levels and uncertain predicted environmental
concentrations of methyl methacrylate. As such, the concentrations of
methyl methacrylate predicted to be in the environment are unlikely to
pose a risk to aquatic or terrestrial organisms.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer (IARC, 1994) has
classified methyl methacrylate in Group 3 (not classifiable as to its
carcinogenicity to humans) based on inadequate evidence for
carcinogenicity in humans and evidence suggesting a lack of
carcinogenicity in experimental animals.
Information on international hazard classification and labelling
is included in the International Chemical Safety Card reproduced in
this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards together with preventative and protective
measures and first aid recommendations are presented in the
International Chemical Safety Card (ICSC 0300) reproduced in this
document.
13.1 Human health hazards
Methyl methacrylate is highly flammable. After long-term or
repeated exposure, it may cause skin sensitization and asthma and may
have effects on the nervous system.
13.2 Advice to physicians
In the event of poisoning, the treatment is supportive. Owing to
reports of systemic vasodilation and transient hypotension in patients
following use of methyl methacrylate as a bone cement in total hip
replacement procedures, monitoring for hypotension and respiratory
depression is recommended. Because a stabilizer or inhibitor is
always a part of the formulation, toxicological properties may be
different.
13.3 Health surveillance advice
Periodic medical examination of the area of the skin exposed to
methyl methacrylate, tests to determine any disturbances of the
nervous system, and surveillance of the respiratory system should be
included in the health surveillance programme.
13.4 Explosion and fire hazards
13.4.1 Explosion hazards
Methyl methacrylate is explosive in the form of vapour when
exposed to heat, sparks, or flame. Vapours may travel to a source of
ignition and flash back. Methyl methacrylate may undergo spontaneous,
explosive polymerization. It reacts in air to form a heat-sensitive
explosive product. Containers of methyl methacrylate may explode in
the heat of a fire. Runoff to sewers may create a fire or explosion
hazard.
13.4.2 Fire hazards
Methyl methacrylate is highly flammable material. When heated to
decomposition, methyl methacrylate emits acrid smoke and irritating
fumes.
13.4.3 Fire-extinguishing agents
Water may not be effective, except to absorb heat, keep
containers cool, and protect exposed materials.
13.5 Storage
Store in a cool, dry, well ventilated area, out of direct
sunlight. Store away from heat and ignition sources and incompatible
materials, such as flammable/combustible materials, materials that
support combustion (oxidizing materials), and corrosive materials
(strong acids or bases). If storing small quantities under
refrigeration, use an approved, explosion-proof refrigerator. Methyl
methacrylate monomer should not be stored for longer than 1 year.
13.6 Transport
Methyl methacrylate cannot be carried on passenger or cargo
aircraft.
13.7 Spillage
Methyl methacrylate is highly flammable. In the event of
spillage, eliminate all sources of ignition in the vicinity. Because
the chemical is absorbed through the skin, do not touch or walk
through the spilled material without proper equipment. To avoid the
flammability hazard, remove clothing immediately if wet or
contaminated, and use non-sparking tools to clean up. Do not let the
chemical enter drains or watercourses.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards is
available from the International Register of Potentially Toxic
Chemicals (IRPTC) legal file.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
METHYL METHACRYLATE MONOMER, INHIBITED ICSC:0300
METHYL METHACRYLATE MONOMER, INHIBITED
Methacrylic acid methyl ester
Methyl 2-methylpropenoate
CH2C(CH3)COOCH3
Molecular mass: 100.1
CAS # 80-62-6
RTECS # 0Z5075000
ICSC # 0300
UN # 1247
EC # 607-035-00-6
TYPES OF ACUTE HAZARDS/ PREVENTION FIRST AID/
HAZARD/ SYMPTOMS FIRE FIGHTING
EXPOSURE
FIRE Highly flammable. No open flames, NO sparks, Foam, powder, carbon dioxide.
and NO smoking.
EXPLOSION Vapour/air mixtures are Closed system, ventilation, In case of fire: keep drums,
explosive. explosion-proof electrical etc., cool by spraying with
equipment and lighting. Do water.
NOT use compressed air for
filling, discharging, or
handling.
EXPOSURE PREVENT GENERATION OF MISTS!
* INHALATION Cough. Drowsiness. Headache. Ventilation, local exhaust, Fresh air, rest. Refer for
Shortness of breath. Sore or breathing protection. medical attention.
throat. Unconsciousness.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
TYPES OF ACUTE HAZARDS/ PREVENTION FIRST AID/
HAZARD/ SYMPTOMS FIRE FIGHTING
EXPOSURE
* SKIN Redness. Protective gloves. Protective Remove contaminated clothes.
clothing. Rinse and then wash skin with
water and soap.
* EYES Redness. Pain. Safety goggles or eye First rinse with plenty of
protection in combination water for several minutes
with breathing protection. (remove contact lenses if
easily possible), then take
to a doctor.
* INGESTION Nausea. Vomiting. Do not eat, drink, or smoke Rinse mouth. Refer for
during work. medical attention.
SPILLAGE DISPOSAL STORAGE PACKAGING & LABELLING
Collect leaking and spilled liquid in Fireproof. Separated from strong F symbol
sealable containers as far as oxidants, strong bases, strong acids. Xi symbol
possible. Absorb remaining liquid in Cool. Keep in the dark. Keep in a
sand or inert absorbent and remove to well-ventilated room. Store only if R: 11-36/37/38-43
safe place. Do NOT wash away into stabilized. S: (2-)9-16-29-33
sewer.
Note: D
UN Hazard Class: 3
UN Packing Group: II
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
IMPORTANT DATA PHYSICAL STATE; APPEARANCE: EFFECTS OF SHORT-TERM EXPOSURE:
COLOURLESS LIQUID, WITH CHARACTERISTIC ODOUR. A harmful contamination of the air can be
reached rather quickly on evaporation of this
substance at 20°C.
PHYSICAL DANGERS: EFFECTS OF SHORT-TERM EXPOSURE:
The vapour is heavier than air and may travel The substance irritates the eyes, the skin and
along the ground; distant ignition possible. the respiratory tract.
The vapour mixes well with air, explosive
mixtures are easily formed. Vapours are not
inhibited, they may polymerize and block the
vents.
CHEMICAL DANGERS: EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
The substance may polymerize due to warming or Repeated or prolonged contact may cause skin
due to heating under the influence of light, sensitization. Repeated or prolonged
polymerization catalysts and strong oxidants inhalation exposure may cause asthma. The
with fire or explosion hazard. Reacts with substance may have effects on the central
strong acids, strong bases and oxidants. nervous system and the peripheral nervous
system.
OCCUPATIONAL EXPOSURE LIMITS (OELs):
TLV: 100 ppm; 410 mg/m3 (as TWA)
(ACGIH 1994-1995).
MAK: 50 ppm; 210 mg/m3; I, A II (1993).
ROUTES OF EXPOSURE:
The substance can be absorbed into the body by
inhalation of its vapour, through the skin and
by ingestion.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
PHYSICAL Boiling point: 100-101°C Relative density of the vapour/
PROPERTIES Melting -48°C air-mixture at 20°C (air = 1): 1.1
Relative density (water = 1): 0.94 Flash point: 10°C o.c.
Solubility in water, g/100 ml Auto-ignition temperature: 421°C
at 20°C 1.6 Explosive limits, vol% in air: 1.7-12.5
Vapour pressure, kPa at 20°C: 3.9 Octanol/water partition coefficient
Relative vapour density (air = 1): 4.16 as log Pow: 1.38
Vapour pressure, kPa at 20°C: 0.647
Vapour pressure, Pa at 25°C: 780
ENVIRONMENTAL This substance may be hazardous to the environment; special attention should be given to water.
DATA
NOTES
Usually contains hydroquinone, hydroquinone methyl ether and dimethyl t-butylphenol as inhibitors of polymerization. An added
stabilizer or inhibitor can influence the toxicological properties of this substance, consult an expert.
ICSC: 0300 1.1 Transport Emergency Card: TEC (R)-196
NFPA Code: H2; F3; R2
REFERENCES
Anderson BE, Zeiger E, Shelby MD, Resnick MA, Gulate DK, Ivett JL,
Loveday KS (1990) Chromosome aberration and sister chromatid exchange
test results with 42 chemicals. Environmental and molecular
mutagenesis, 16 (Suppl. 18):55-137 [cited in IARC, 1994].
Anderson D, Hodge MCE (1976) Methyl methacrylate monomer: dominant
lethal study in the mouse. Macclesfield, Cheshire, ICI (Report No.
CTL/P/295).
Anderson D, Richardson CR (1976) Methyl methacrylate monomer:
cytogenetic study in the rat. Macclesfield, Cheshire, ICI (Report
No. CTL/P/292).
Anderson D, Richardson CR, Weight TM (1979). Methyl methacrylate
monomer: a second cytogenetic study in the rat. Macclesfield,
Cheshire, ICI (Report No. CTL/P/449).
Andrews CP, Smith JD, Johanson WG Jr (1979) Pulmonary effects of
methyl methacrylate vapor exposure in dental students. Clinical
research, 27:759A (abstract).
Bailey HD, Liu DHW, Javitz HA (1985) Time/toxicity relationships in
short-term static, dynamic and plug-flow bioassays. In: Bahner RC,
Hansen DJ, eds. Aquatic toxicology and hazard assessment: eighth
symposium. Philadelphia, PA, American Society for Testing and
Materials, pp. 193-212 (ASTM Special Technical Publication 891).
Blanchet LJ, Bowman BC, McReynolds HD (1982) Effects of methyl
methacrylate monomer vapors on respiration and circulation in
unanesthetized rats. Journal of prosthetic dentistry, 48:344-348.
Borzelleca JF, Larson PS, Hennigar GR, Hluf EG, Crawford EM, Blackwell
Smith R (1964) Studies on the chronic oral toxicity of monomeric ethyl
acrylate and methyl methacrylate. Toxicology and applied
pharmacology, 6:29-36.
Bowman JH (1990) Acute flow-through toxicity of methyl methacrylate
to rainbow trout. Analytical Bio-Chemistry Laboratories Inc. (Report
No. 37327) [cited in Clary JJ (1991) Methyl methacrylate: a
toxicity review. Prepared by Bio Risk. New York, NY, US Methacrylate
Producers Association].
Bratt H, Hathway DE (1977) Fate of methyl methacrylate in rats.
British journal of cancer, 36:114-119.
Bringmann VG (1978) Bestimmung der biologischen Schadwirkung
wassergefahrdender Stoffe gegen Protozoen. Zeitschrift für
Wasser-und Abwasser-Forschung, 6:210-215.
Bringmann VG, Kuhn R (1976) Vergleichende Befunde der Schadwirkung
wassergefahrdender Stoffe gegen Bakterien (Pseudomonas putida) und
Blaualgen (Microcystis aeruginosa). Gwf-wasser/Abwasser,
117:410-413.
Bringmann VG, Kuhn R (1977) Befunde der Schadwirkung
wassergefahrdender Stoffe gegen Daphnia magna. Zeitschrift für
Wasser-und Abwasser-Forschung, 5:161-166.
Bringmann VG, Kuhn R (1978a) Testing of substances for their toxicity
threshold: model organisms Microcystis (Diplocystis) aeruginosa and
Scenedesmus quadricauda. Mitteilungen Internationale Vereinigung
für Theoretische und Angewandte Limnologie, 21:275-284.
Bringmann VG, Kuhn R (1978b) Grenzwerte der Schadwirkung
wassergefahrdender Stoffe gegen Blaualgen (Microcystis aeruginosa)
und Grunalgen (Scenedesmus quadricauda) im Zellvermehrungshemmtest.
Vom Wasser, 50:45-60.
Bringmann VG, Kuhn R (1982) Ergebnisse der Schadwirkung
wassergefahrdender Stoffe gegen Daphnia magna in einem
weiterentwickelten standardisierten Testverfahren. Zeitschrift für
Wasser-und Abwasser-Forschung, 15:1-6.
Cannas M, Bigatti P, Rossi E, Rossi P (1987) In vitro research on
the possibility of chromosomal damage caused by polymethyl
methacrylate in orthopaedics. Italian journal of orthopaedics and
traumatology, 13:387-391 [cited in IARC, 1994].
Cary R, Morris L, Cocker J, Groves J, Ogunbiyi A (1995) Methyl
methacrylate, criteria for an occupational exposure limit. London,
UK Health and Safety Executive.
Castellino N, Colicchio G (1969) [Experimental research on the acute
toxicity of methyl methacrylate.] Folia Medica, 52:337-347 (in
Italian) [cited in ECETOC, 1995].
CEFIC (1993) Questionnaire on exposure data, methyl methacrylate
(MMA). Data from ELF-ATOCHEM, ICI, Repsol, and Röhm. Brussels,
CEFIC, Methacrylates Toxicology Committee.
CEFIC (1994) MMA - Quarterly statistics on sales; effective
capacity, production, captive use. Brussels, CEFIC, Methyl
Methacrylate Sector Group.
Chan PC, Eustis SL, Huff JE, Haseman JK, Ragan H (1988) Two-year
inhalation carcinogenesis studies of methyl methacrylate in rats and
mice: Inflammation and degeneration of nasal epithelium.
Toxicology, 52:237-252.
Collins JJ, Page LC, Caporossi JC, Utidjian HM, Saipher JN (1989)
Mortality patterns among men exposed to methyl methacrylate.
Journal of occupational medicine, 31:41-46.
Conde-Salazar L, Guimaraens D, Romero L (1988) Occupational allergic
contact dermatitis from anaerobic acrylic sealants. Contact
dermatitis, 18(3):129-132 [cited in Cary et al., 1995].
CPI (1989) CPI product profiles: Methyl methacrylate. Don Mills,
Ontario, Canadian Process Industries, Corpus Information Services.
Cromer J, Kronoveter K (1976) A study of methyl methacrylate
exposures and employee health. Cincinnati, OH, US Department of
Health, Education and Welfare, National Institute for Occupational
Safety and Health (DHEW (NIOSH) Publication No. 77-119; NTIS
PB-27489).
Dearfield KL, Harrington-Brock K, Doerr CL, Rabinowitz JR, Moore MM
(1991) Genotoxicity in mouse lymphoma cells of chemicals capable of
Michael addition. Mutagenesis, 6:519-525 [cited in IARC, 1994].
DeFonso LR, Maher KV (1981) Texas Plant mortality study
(1948-1978). Report prepared for Rohm and Haas Company.
DeFonso LR, Maher KV (1986) A matched case-control study nested
within an historical cohort study of acrylate/methacrylate workers.
Report prepared for Rohm and Haas Company for the US Environmental
Protection Agency's TSCA Section 8(d) submission.
Deichmann-Gruebler A, Read RT (undated) In: Submission to US
Environmental Protection Agency under TSCA Section 8(d) by E.I. duPont
Company, 1989 (NTIS/OTS 0520934).
DeSesso JM (1993) The relevance to humans of animal models for
inhalation studies of cancer in the nose and upper airways.
Quality assurance: good practice, regulation and law, 2:213-231.
Doerr CL, Harrington-Brock K, Moore MM (1989) Micronucleus, chromosome
aberration, and small-colony TK mutant analysis to quantitate
chromosomal damage in L51784 mouse lymphoma cells. Mutation
research, 222:191-203.
ECETOC (1995) Methyl methacrylate - CAS No. 80-62-6. Brussels,
European Centre for Ecotoxicology and Toxicology of Chemicals, 167 pp.
(Joint Assessment of Commodity Chemicals No. 30).
Edwards PM (1975) Neurotoxicity of acrylamide and its analogues and
effects of these analogues and other agents on acrylamide neuropathy.
British journal of industrial medicine, 32:31-38.
Environment Canada (1989) Analysis of shellfish for organic and
inorganic contaminants. Zenon Environmental Inc., March.
Estlander T, Rajaniemi R, Jolanki R (1984) Hand dermatitis in dental
technicians. Contact dermatitis, 10(4):201-205 [cited in Cary et
al., 1995].
Ewing BB, Chian ESK (1977) Monitoring to detect previously
unrecognized pollutants in surface waters. US Environmental
Protection Agency (USEPA/560/6-77/015A).
Farli M, Gasperini M, Francalanci S, Gola M, Sertoli A (1990)
Occupational contact dermatitis in two dental technicians. Contact
dermatitis, 22(5):282-287.
Farmakovskaya TB, Tikhomirov YP (1993) The influence of
methylmethacrylate and butylmethacrylate on the reproductive function
of animals during round-the-clock inhalation. Reproductive
toxicology, 7(5):520-521.
Fedyukovich LV, Egorova AB (1991) [Genotoxic effect of acrylates.]
Gigiena i Sanitariya, 12:62-64 (in Russian) [cited in IARC, 1994].
Fedyukovich L, Kotlovskii Y, Sviderskaya L, Borisov Y (1988)
[Mutagenic and cytotoxic effect of acrylates.] Genetika,
24:1132-1134 (in Russian) [cited in ECETOC, 1995].
Finnish Advisory Board of Chemicals (1992) Acrylate compounds: Uses
and evaluation of health effects. Helsinki.
Forbis DA (1990) Acute toxicity of methyl methacrylate to
Selenastrum capricornutum printz. Analytical Bio-Chemistry
Laboratories Inc. (Report No. 37329) [cited in Clary JJ (1991) Methyl
methacrylate: a toxicity review. Prepared by Bio Risk. New York, NY,
US Methacrylate Producers Association].
Government of Canada (1993) Canadian Environmental Protection Act.
Priority Substances List assessment report for methyl methacrylate.
Prepared by Health Canada and Environment Canada. Ottawa, Ontario,
Canada Communication Group Publishing (ISBN 0-662-20418-2).
Guerra L, Vincenzi C, Peluso AM, Tosti A (1993) Prevalence and sources
of occupational contact sensitization to acrylates in Italy.
Contact dermatitis, 28:101-103 [cited in ECETOC, 1995].
Hachitani N, Taketani A, Takizawa Y (1981) [Mutagenicity study on
environmental substances. 3. Ames test and mouse bone marrow
micronucleus test on acrylic resin monomer and other additives.]
Nippon Koshu Eisei Zasshi, 29:236-239 (in Japanese).
Hodge MCE, Palmer S (1977) Methylmethacrylate monomer
teratogenicity studies in the rat. Submission by Rohm and Haas
Company to the US Environmental Protection Agency under TSCA Section
8(d), July 1979.
Hollifield H, Breder C, Dennison J, Roach J, Adams W (1980)
Container-derived contamination of maple syrup with methyl
methacrylate, toluene and styrene as determined by headspace
gas-liquid chromatography. Journal of the Association of Official
Analytical Chemists, 63:173-177.
Hossack DJN, Thomas FJ (1992) Methyl methacrylate: Effects on soil
carbon cycle (respiration). Prepared by Huntingdon Research Centre,
Huntingdon, England. Washington, DC, US Methacrylate Producers
Association [cited in ECETOC, 1995].
Howard P (1989) Handbook of environmental fate and exposure data
for organic chemicals. Vol. 1. Chelsea, MI, Lewis Publishers Inc.,
pp. 402-407.
Howard P, Boethling RS, Jarvis WF, Meyland WM, Michalenko EM (1991)
Handbook of environmental degradation rates. Chelsea, MI, Lewis
Publishers Inc.
Husain R, Srivastava SP, Seth PK (1985) Methyl methacrylate induced
behavioural and neurochemical changes in rats. Archives of
toxicology, 58:33-36.
Husain R, Khan S, Husain I, Seth PK, Pandya KP (1989) Effect of methyl
methacrylate on selected lipids in rat brain and sciatic nerve.
Industrial health, 27:121-124.
IARC (1994) Some industrial chemicals. Lyon, International Agency
for Research on Cancer, pp. 445-474 (IARC Monographs on the Evaluation
of the Carcinogenic Risk of Chemicals to Humans, Vol. 60).
Innes D, Tansy MF (1981) Central nervous system effects of methyl
methacrylate vapours. Neurotoxicology, 2:515-522.
Inoue T, Tatsuno T, Tanimura A (1981) Hygienic chemical studies on
plastics. III. Migration test of methyl methacrylate and plastic
additives from polymethyl methacrylate. Bulletin. National
Institute of Hygienic Sciences (Tokyo), 99:144-147.
IPCS (1993) International Chemical Safety Card - Methyl
methacrylate. Geneva, World Health Organization, International
Programme on Chemical Safety (No. 0300).
Jedrychowski W (1982) Styrene and methyl methacrylate in the
industrial environment as a risk factor of chronic obstructive lung
disease. International archives of occupational and environmental
health, 51:151-157.
Jensen JS, Sylvest A, Trap B, Jensen JC (1991) Genotoxicity of acrylic
bone cements. Pharmacology and toxicology, 69:386-389.
Kanerva L, Verkkala E (1986) Electron microscopy and
immunohistochemistry of toxic and allergic effects of methyl
methacrylate on the skin. Archives of toxicology, Suppl. 9:456-459.
Kanerva L, Estlander T, Jolanki R (1988) Sensitization to patch test
acrylates. Contact dermatitis, 18:10-15 [cited in ECETOC, 1995].
Kanerva L, Estlander T, Jolanki R (1989) Allergic contact dermatitis
from dental composite resins due to aromatic and aliphatic epoxy
acrylates. Contact dermatitis, 20(3):201-211 [cited in Cary et al.,
1995].
Kassis V, Vedel P, Darre E (1984) Contact dermatitis due to methyl
methacrylate. Contact dermatitis, 11(1):26-28.
Lijinsky W, Andrews AW (1980) Mutagenicity of vinyl compounds in
Salmonella typhimurium. Teratogenesis, carcinogenesis, and
mutagenesis, 1:259-267.
Lomax LG (1992) Histopathologic evaluation of nasal cavities from
Fischer 344 rats exposed to methyl methacrylate vapor for two
years. Spring House, PA, Rohm and Haas Company, Toxicology
Department (Project No. 3302.5E, finalized 7 May 1992).
Lomax LG, Brown DW, Frederick CB (1994) Regional histopathology of
the mouse nasal cavity following two weeks of exposure to acrylic
acid for either 6 or 22 hours per day. Spring House, PA, Rohm and
Haas Company.
Lomax LG, Krivanek ND, Frame SR (1997) Chronic inhalation toxicity and
oncogenicity of methyl methacrylate in rats and hamsters. Food and
chemical toxicology, 35:393-407.
Lozewicz S, Davison A, Hopkirk A, Burge P, Boldy D, Riordan J,
McGivern DV, Platts B, Davies D, Taylor A (1985) Occupational asthma
due to methyl methacrylate and cyanoacrylates. Thorax,
40(11):836-839.
Luo SQ, Gang BQ, Sun SZ (1986) Study on embryotoxicity and
fetotoxicity in rats by maternal inhalation of low level methyl
methacrylate. Toxicology letters, 31:80 (abstract no. P3-29).
Mackay D, Paterson S (1981) Calculating fugacity. Environmental
science and technology, 15:1006-1014.
Mackay D, Paterson S (1982) Fugacity revisited. Environmental
science and technology, 16:654-660.
Mackay D, Paterson S (1991) Evaluating the regional multimedia fate of
organic chemicals: a Level III fugacity model. Environmental
science and technology, 25:427.
Mackay D, Paterson S, Cheung B, Neely W (1985) Evaluating the
environmental behaviour of chemicals with a Level III fugacity model.
Chemosphere, 14:335-374.
Mackay D, Shiu WY, Ma KC (1995) Illustrated handbook of
physical-chemical properties and environmental fate for organic
chemicals. Vol. IV. Boca Raton, FL, CRC Press, Inc./Lewis
Publishers.
Maher K, DeFonso LR (1987a) Mortality study of Bristol Plant
employees hired 1946-1982. Draft report prepared for Rohm and Haas
Company.
Maher K, DeFonso LR (1987b) Mortality study of Knoxville Plant
employees (1943-1982). Report prepared for Rohm and Haas Company.
Marez T, Shirali P, Hildebrand HF, Haguenoer JM (1991) Increased
frequency of sister chromatid exchange in workers exposed to high
doses of methylmethacrylate. Mutagenesis, 6:127-129.
Marez T, Edmé JL, Boulenguez C, Shirali P, Haguenoer JM (1993)
Bronchial symptoms and respiratory function in workers exposed to
methylmethacrylate. British journal of industrial medicine,
50:894-897.
Mattes PM, Mattes WB (1992) alpha-Naphthyl butyrate carboxylesterase
activity in human and rat nasal tissue. Toxicology and applied
pharmacology, 114:71-76.
McLaughlin RE, Reger SJ, Barkalow JA, Allen MJ, Diffazio CA (1978)
Methyl methacrylate: A study of teratogenicity and fetal toxicity of
the vapor in the mouse. Journal of bone and joint surgery,
60A:355-358.
Mizunuma K, Kawai T, Yasagui T, Horiguichi S, Takeda S, Miyashita K,
Taniuchi T, Moon C-S, Ikeda M (1993) Biological monitoring and
possible health effects in workers occupationally exposed to methyl
methacrylate. Archives of occupational and environmental health,
65:227-232.
Moore MM, Amanda A, Doerr CL, Brock KH, Dearfield KL (1988)
Genotoxicity of acrylic acid, methyl acrylate, ethyl acrylate, methyl
methacrylate and ethyl methacrylate in L5178Y mouse lymphoma cells.
Environmental and molecular mutagenesis, 11:49-63.
Morris JB, Frederick CB (1995) Upper respiratory tract uptake of
acrylate ester and acid vapours. Inhalation toxicology, 7:557-574.
Myhr B, McGregor D, Bowers L, Riach C, Brown AG, Edwards I, McBride D,
Martin R, Caspary WJ (1990) L5178Y mouse lymphoma cell mutation assay
results with 41 compounds. Environmental and molecular mutagenesis,
16 (Suppl. 18):138-167 [cited in IARC, 1994].
Nicholas CA, Lawrence WH, Autian J (1979) Embryotoxicity and
genotoxicity from maternal inhalation of methyl methacrylate monomer
in rats. Toxicology and applied pharmacology, 50:451-458.
NIOSH (1976) A study of methyl methacrylate exposures and employee
health. Cincinnati, OH, US Department of Health, Education and
Welfare, National Institute for Occupational Safety and Health
(Publication No. 77-119).
NTP (1986) NTP technical report on the toxicology and
carcinogenesis studies of methyl methacrylate (CAS No. 80-62-6) in
F344/N rats and B6C3F1 mice (inhalation studies). Research Triangle
Park, NC, US Department of Health and Human Services, National
Institutes of Health, National Toxicology Program, 202 pp. (NTP
TR314).
Ouyang G, Shi T, Fan Z, Zhang B, Yu T, Hao A, Tang G (1990) Acute
toxicity and toxicokinetics of methyl methacrylate. Chinese
chemical abstracts, 112:133952q.
Pickering C, Bainbridge D, Birtwistle I, Griffiths D (1986)
Occupational asthma due to methyl methacrylate in an orthopaedic
theatre sister. British medical journal, 292:1362-1363.
Pickering CAC, Niven R, Simpson J (1993) A study of the prevalence
of occupational asthma at the ICI Acrylics site at Darwen,
Lancashire. Lancashire, ICI Acrylics.
Poss R, Thilly WG, Kaden DA (1979) Methylmethacrylate is a mutagen for
Salmonella typhimurium. Journal of bone and joint surgery,
61-A:1203-1207 [cited in IARC, 1994].
Rajaniemi R, Tola S (1985) Subjective symptoms among dental
technicians exposed to the monomer methyl methacrylate.
Scandinavian journal of work, environment & health, 11:281-286.
Raje RR, Ahmad S, Weisbroth SH (1985) Methyl methacrylate: tissue
distribution and pulmonary damage in rats following acute inhalation.
Research communications in chemical pathology and pharmacology,
50:151-154.
Röhm (1994) Medical examination of workers in acrylic sheet
production exposed to methyl methacrylate. Pausch, Höffer, Claus,
Lehr, Jacobi, 15.03.1994. Darmstadt, Röhm [cited in ECETOC, 1995].
Rohm and Haas (1977) Subchronic vapor inhalation study with methyl
methacrylate (C50680) in F344 rats and B6C3F1 mice. Report to
Tracor Jitco, Inc., submitted by IBT Laboratories Inc.
Rohm and Haas (1979a) A two-year vapor inhalation safety evaluation
study in rats. Methyl methacrylate. Final report. Submitted by
Hazleton Laboratories America Inc., 217 pp.
Rohm and Haas (1979b) 18-month vapor inhalation safety evaluation
study in hamsters. Methyl methacrylate vapor. Final report.
Submitted by Hazleton Laboratories America Inc., 85 pp. (Project No.
417-354).
Rohm and Haas (1982) Acute oral LD50 range finding rat, acute
dermal LD50 range finding rabbit, acute skin irritation range
finding rabbit 4-hr contact, acute eye irritation range finding
rabbit. Test substance methyl methacrylate - 10 ppm Topanol A [cited
in ECETOC, 1995].
Schwartz BS, Doty RL, Monroe C, Frye R, Baker S (1989) Olfactory
function in chemical workers exposed to acrylate and methacrylate
vapours. American journal of public health, 79:613-618.
Schweikl H, Schmalz G, Bey B (1994) Mutagenicity of dentin bonding
agents. Journal of biomedical materials research, 28:1061-1067.
Seiji K, Inoue O, Kawai T, Mizunuma K, Yasugi T, Moon C, Takeda S,
Ikeda M (1994) Absence of mutagenicity in peripheral lymphocytes of
workers occupationally exposed to methyl methacrylate. Industrial
health, 32:97-105.
Seppalainen AM, Rajaniemi TC (1984) Local neurotoxicity of methyl
methacrylate among dental technicians. American journal of
industrial medicine, 5:471-478.
Siemiatycki J (1991) Risk factors for cancer in the workplace. Boca
Raton, FL, CRC Press, 310 pp.
Smith JM, Cruzan G, Drees JA, Tansy MF, Coate WB, Reno FE (1979)
Methyl methacrylate: subchronic, chronic and oncogenic inhalation
safety evaluation studies. Toxicology and applied pharmacology,
48:A30.
Solomon HM, McLaughlin JE, Swenson RE, Hagan JV, Wanner FJ, O'Hara GP,
Krivanek ND (1993) Methyl methacrylate: inhalation developmental
toxicity study in rats. Teratology, 48:115-125.
Spealman CR, Main RJ, Haag HB, Larson PS (1945) Monomeric methyl
methacrylate - Studies on toxicity. Industrial medicine, 14:292-298.
Tansy MF (1975) Progress report on teratology studies of mice
exposed to methyl methacrylate monomer vapour. Submitted to Rohm and
Haas Company, 5 pp.
Tansy MF, Drees JA (1979) Methyl methacrylate, three month
subchronic vapour inhalation safety evaluation study, beagle dogs.
Prepared for Rohm and Haas Company, 239 pp.
Tansy MF, Kendall FM, Benhayem S, Hohenleitner FJ, Landin WE, Gold M
(1976) Chronic biological effects of methyl methacrylate vapor. 1.
Body and tissue weights, blood chemistries, and intestinal transit in
the rat. Environmental research, 11:66-77.
Tansy MF, Hohenleitner FJ, Landin WE, Kendall FM (1980a) Chronic
biological effects of methyl methacrylate vapor. II. Body and tissue
weights, blood chemistries and gross metabolic performance in the rat.
Environmental research, 21:108-116.
Tansy M, Hohenleitner F, White D, Oberly R, Landin W, Kendall F
(1980b) Chronic biological effects of methyl methacrylate vapour. III.
Histopathology, blood chemistries and hepatic and ciliary function in
the rat. Environmental research, 21:117-125.
Tomenson JA, Bonner SM (1994) A cohort study of employees in
Perspex plants. Northwich, Cheshire, ICI Epidemiology Unit, 15
December.
Waegemaekers THJM, Bensink MPM (1984) Nonmutagenicity of 27 aliphatic
acrylate esters in the Salmonella microsome test. Mutation
research, 137:95-102.
Walker AM, Cohen AJ, Loughlin JE, Rothman KJ, DeFonso LR (1991)
Mortality from cancer of the colon or rectum among workers exposed to
ethyl acrylate and methyl methacrylate. Scandinavian journal of
work, environment & health, 17:7-19.
Wynkoop JR, Miller RA, Cheong V, Lorton L (1982) Levels of neuroactive
substances following exposure to methyl methacrylate monomer.
Journal of dental research, 61:202 (abstract no. 213).
Zafiropoulos GG, Apostolopoulos AX, Patramani I (1985) Study of the
antigenic properties of methyl methacrylate using the
leukocyte-migration inhibition test. Dental materials, 1:200-204.
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K, Speck W
(1987) Salmonella mutagenicity tests: III. Results from the testing
of 255 chemicals. Environmental mutagenesis, 9 (Suppl. 9):1-110
[cited in IARC, 1994].
APPENDIX 1 - SOURCE DOCUMENTS
Government of Canada (1993)
Copies of the Canadian Environmental Protection Act Priority
Substances List Assessment Report (Government of Canada, 1993) and
unpublished Supporting Documentation for methyl methacrylate may be
obtained from the:
Commercial Chemicals Branch
Environment Canada
14th Floor, Place Vincent Massey
351 St. Joseph Blvd.
Hull, Quebec
Canada K1A 0H3
Environmental Health Centre
Health Canada
Address Locator: 0801A
Tunney's Pasture
Ottawa, Ontario
Canada K1A 0L2
Initial drafts of the Assessment Report and unpublished
Supporting Documentation for methyl methacrylate were prepared by
staff of Health Canada and Environment Canada. The human health-
related sections of this document were reviewed externally by Dr J.
Siemiatycki (University of Quebec), Dr N. Krivanek (E.I. duPont de
Nemours) (Supporting Documentation only), and the Information
Department of BIBRA Toxicology International. These sections were
approved by the Standards and Guidelines Rulings Committee of the
Bureau of Chemical Hazards of Health Canada. The environmental
sections were reviewed externally by Dr N. Bunce (University of
Waterloo) and Dr N. Krivanek (E.I. duPont de Nemours).
IARC (1994)
Copies of Some industrial chemicals (IARC Monographs on the
Evaluation of Carcinogenic Risks to Humans, Vol. 60) (IARC, 1994) may
be obtained from the:
International Agency for Research on Cancer
150 cours Albert Thomas
69372 Lyon Cedex 08
France
The members of the Working Group on the Evaluation of
Carcinogenic Risks to Humans of Some Industrial Chemicals (including
methyl methacrylate), which met in Lyon on 15-22 February 1994, were:
P.A. Bertazzi, Institute of Occupational Health, Clinica del
Lavoro "Luigi Devoto," University of Milan, via S. Barnaba 8,
20122 Milan, Italy
C.J. Calleman, School of Public Health and Community Medicine,
Department of Environmental Health, SC-34, University of
Washington, Seattle, WA 98195, USA
D. Coggon, MRC Environmental Epidemiology Unit, Southampton
General Hospital, Southampton, SO9 4XY, United Kingdom
T.A. Dragani, Division of Experimental Oncology A, National
Institute for the Study and Treatment of Tumours, via Venezian 1,
20133 Milan, Italy
M.R. Elwell, Toxicology Research and Testing Program, National
Institute of Environmental Health Sciences, PO Box 12233,
Research Triangle Park, NC 27709, USA
H.J. Evans, MRC Human Genetic Unit, Western General Hospital,
Crewe Road, Edinburgh EH4 2XU, United Kingdom (Chairperson)
J.G. Filser, GSF Institute of Toxicology, Neuherberg, PO Box
1129, 85758 Oberschleissheim, Germany
M. Gérin, University of Montréal, Department of Occupational and
Environmental Health, Faculty of Medicine, CP 6128, Station A,
Montréal, Québec, Canada H3C 3J7
K. Hemminki, Centre for Nutrition and Toxicology, Karolinska
Institute, Novum, 141 57 Huddinge, Sweden
C. Hogstedt, National Institute of Occupational Health, 171 84
Solna, Sweden
M. Kirsch-Volders, Laboratorium Antropogenetica, Free University
of Brussels, Pleinlaan 2, 1050 Brussels, Belgium
W. Lutz, Institute of Toxicology, Swiss Federal Institute of
Technology, Schorenstrasse 16, 8603 Schwerzenbach, Switzerland
S.S. Olin, International Life Sciences Institute, Risk Science
Institute, 1126 Sixteenth Street NW, Washington, DC 20036, USA
A. Pintér, "Johan Béla" National Institute of Hygiene, Gyáli ut.
2-6, 1966 Budapest, Hungary
P. Schulte, Screening and Notification Section, National
Institute for Occupational Safety and Health, Robert A Taft
Laboratories, 4676 Columbia Parkway, R-42, Cincinnati, OH
45226-1998, USA
T. Sofuni, Division of Genetics and Mutagenesis, Biological
Safety Research Centre, National Institute of Hygienic Sciences,
1-18-1 Kamiyoga, Setagaya-ku, Tokyo 158, Japan
M. Sorsa, Institute of Occupational Health, Topeliuksenkatu 41 a
A, 00250 Helsinki, Finland (Vice-Chairperson)
F.M. Sullivan, Division of Pharmacology and Toxicology, UMDS, St.
Thomas's Hospital, Lambeth Palace Road, London SE17EH, United
Kingdom
V.S. Turusov, Cancer Research Centre, Russian Academy of Medical
Sciences, Kashirskoye Shosse 24, 115478 Moscow, Russia
M.A. Waters, National Institute for Occupational Safety and
Health, 4676 Columbia Parkway, R-14, Cincinnati, OH 45226-1998,
USA
M.D. Waters, International Programs, MD-51A, Health Effects
Research Laboratory, US Environmental Protection Agency, Research
Triangle Park, NC 27711, USA
APPENDIX 2 - CICAD PEER REVIEW
The draft CICAD on methyl methacrylate was sent for review to
institutions and organizations identified by IPCS after contact with
IPCS national Contact Points and Participating Institutions, as well
as to identified experts. Comments were received from:
Bundesinstitut für Gesundheitlichen Verbraucherschutz und
Veterinarmedizin, Berlin, Germany
CEFIC, Brussels, Belgium
Department of Health, London, United Kingdom
Department of Public Health, Albert Szent-Gyorgyi University
Medical School, Szeged, Hungary
Direccion General de Salud Ambiental, Subsecretario de Regulacion
y Fomento Sanitario, San Luis Potosi, Mexico
ECETOC, Brussels, Belgium
Guy's & St. Thomas' Hospital Trust, Medical Toxicology Unit,
London, United Kingdom
International Agency for Research on Cancer, Lyon, France
Ministry of Health, National Centre of Hygiene, Medical Ecology
and Nutrition, Sofia, Bulgaria
Ministry of Health and Welfare, International Affairs Division,
Government of Japan, Tokyo, Japan
National Institute for Working Life, Solna, Sweden
National Institute of Public Health, Oslo, Norway
Russian Register of Potentially Hazardous Chemical and Biological
Substances, Moscow, Russia
United States Department of Health and Human Services (National
Institute of Environmental Health Sciences)
United States Environmental Protection Agency (Office of
Pollution Prevention and Toxics; National Center for
Environmental Assessment, Office of Research and Development;
Office of Drinking Water)
APPENDIX 3 - CICAD FINAL REVIEW BOARD
Brussels, Belgium, 18-20 November 1996
Members
Dr A. Aitio, Institute of Occupational Health, Helsinki, Finland
Dr K. Bentley, Director, Environment Policy Section, Commonwealth
Department of Human Services and Health, Canberra, Australia
Mr R. Cary, Toxicology and Existing Substances Regulation Unit, Health
and Safety Executive, Merseyside, United Kingdom
Dr J. de Fouw, National Institute of Public Health and Environmental
Protection, Bilthoven, The Netherlands
Dr C. DeRosa, Director, Division of Toxicology, Agency for Toxic
Substances and Disease Registry, Atlanta, GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr W. Farland, Director, National Center for Environmental Assessment,
Office of Research and Development, US Environmental Protection
Agency, Washington, DC, USA (Chairperson)
Dr T.I. Fortoul, Depto. Biologia Celular y Tisular, National
University of Mexico and Environmental Health Directorate of the
Health Ministry, Mexico D.F., Mexico
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Mr J.R. Hickman, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Dr T. Lakhanisky, Head, Division of Toxicology, Institute of Hygiene
and Epidemiology, Brussels, Belgium (Vice-Chairperson)
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Sciences, Hanover,
Germany
Ms E. Meek, Head, Priority Substances Section, Environmental Health
Directorate, Health Canada, Ottawa, Ontario, Canada
Dr K. Paksy, National Institute of Occupational Health, Budapest,
Hungary
Mr D. Renshaw, Department of Health, London, United Kingdom
Dr J. Sekizawa, Division of Chemo-Bio Informatics, National Institute
of Hygienic Sciences, Tokyo, Japan
Dr H. Sterzl-Eckert, GSF-Forschungszentrum für Umwelt und Gesundheit
GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Professor S. Tarkowski, Department of Environmental Health Hazards,
The Nofer Institute of Occupational Medicine, Lodz, Poland
Dr M. Wallen, National Chemicals Inspectorate (KEMI), Solna, Sweden
Observers
Professor F.M.C. Carpanini,1 Director, Centre for Ecotoxicology and
Toxicology of Chemicals (ECETOC), Brussels, Belgium
Mr R. Haigh,1 Head of Unit, Health and Safety Directorate, European
Commission, Luxembourg
Mr B.U. Hildebrandt, Federal Ministry for the Environment, Nature
Conservation and Nuclear Safety, Bonn, Germany
Mr P. Hurst,1 Chemical and Consumer Policy Officer, Conservation
Policy Division, World Wide Fund for Nature, Gland, Switzerland
Dr A. Lombard (Representative of CEFIC), ELF-ATOCHEM, Paris, France
Dr P. McCutcheon,1 Environment, Consumer Protection and Nuclear
Safety, European Commission, Brussels, Belgium
Dr R. Montaigne, Counsellor, Technical Affairs Department, European
Chemical Industry Council (CEFIC), Brussels, Belgium
Dr M. Pemberton, ICI Acrylics, Lancashire, United Kingdom
Dr A. Smith, Organisation for Economic Co-operation and Development,
Environment Division, Paris, France
1 Invited but unable to attend.
Secretariat
Dr M. Baril, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr L. Harrison, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M. Mercier, Director, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr P. Toft, Associate Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
La Direction de l'Hygiène du Milieu de Santé Canada a rédigé ce
CICAD (document international succinct sur l'évaluation des risques
chimiques) relatif au méthacrylate de méthyle en s'inspirant
principalement d'une étude menée par le Gouvernement du Canada (1993)
pour évaluer les effets potentiels d'une exposition indirecte au
méthacrylate de méthyle dans l'environnement général et les effets de
cette substance sur l'environnement, ainsi que d'une étude du Centre
international de Recherche sur le Cancer (CIRC, 1994), visant
principalement à déterminer les risques de cancérogénicité. L'étude
du Gouvernement du Canada (1993) a pris en compte les données qui
étaient disponibles en mars 1992; ces données ont été mises à jour
ultérieurement à la suite d'une recherche bibliographique approfondie
menée en septembre 1995 dans des bases de données en ligne et dans le
Registre international des substances chimiques potentiellement
toxiques. Des informations concernant la nature de l'évaluation par
les pairs et la disponibilité des études du Gouvernement du Canada
(1993) et du CIRC (1994) figurent à l'appendice 1. Au cours de la
phase d'évaluation par les pairs du présent CICAD, d'autres travaux
ont été pris en compte, à savoir des projets d'études du United
Kingdom Health Safety Executive (Cary et al., 1995) et de l'Union
européenne (Draft Assessment on Methyl Methacrylate), ainsi que des
études publiées par l'ECETOC (1995) et le Bureau consultatif
finlandais des substances chimiques (1992), principalement en vue de
rechercher des informations supplémentaires pertinentes. Des
informations supplémentaires identifiées lors des examens pratiqués
par les correspondants et par le Comité d'évaluation finale ont
également été incorporées. L'appendice 2 contient des informations
sur le processus d'évaluation par les pairs du présent CICAD. Ce
CICAD a été approuvé pour publication à une réunion du Comité
d'évaluation finale qui s'est tenue à Bruxelles (Belgique) du 18 au
20 novembre 1996. La liste des participants à cette réunion figure à
l'appendice 3. La fiche d'information sur la sécurité chimique du
méthacrylate de méthyle (ICSC 0300), préparée par le Programme
international sur la Sécurité chimique (IPCS, 1993), est également
reproduite dans le présent document.
Le méthacrylate de méthyle (CAS N° 80-62-6) est un produit
chimique de synthèse volatil, utilisé principalement dans la
production de feuilles acryliques moulées, d'émulsions acryliques et
de résines pour moulage et extrusion. Des polymères et copolymères de
méthacrylate de méthyle entrent dans la composition de nombreux
produits: revêtements de surfaces, adhésifs, produits d'étanchéité,
enduits pour cuirs et papiers, encres, encaustiques, apprêts pour
textiles, prothèses dentaires, ciments pour chirurgie osseuse, écrans
antiradiations au plomb, ongles artificiels, semelles orthopédiques,
etc. Selon les calculs, la plus grande partie du méthacrylate de
méthyle devrait être émis dans l'atmosphère, les quantités émises dans
l'eau et le sol étant minimes. Le méthacrylate de méthyle ne persiste
pas longtemps dans l'atmosphère et il est admis qu'il ne contribue pas
directement à la destruction de la couche d'ozone. Il ne devrait pas
subir de bioconcentration dans l'environnement et l'inhalation est
probablement la principale voie d'exposition pour l'homme.
Le méthacrylate de méthyle est rapidement absorbé et distribué
dans l'organisme après inhalation ou administration par voie orale aux
animaux d'expérience. Les données concernant l'absorption après
exposition cutanée sont limitées. Le méthacrylate de méthyle est
rapidement métabolisé en acide méthacrylique, aussi bien chez l'animal
que chez l'homme. Chez le rat, 16 à 20 % du produit inhalé se dépose
dans les voies respiratoires supérieures, où il est surtout métabolisé
par les estérases tissulaires locales.
La toxicité aiguë du méthacrylate de méthyle est faible. On a
observé une irritation de la peau, des yeux et des fosses nasales chez
des rongeurs et des lapins exposés à des concentrations relativement
élevées. Il se révèle légèrement sensibilisant pour la peau des
animaux. L'effet le plus souvent observé après inhalation répétée de
faibles doses est une irritation des fosses nasales. Des effets ont
également été signalés sur les reins et le foie à des concentrations
plus élevées. La plus faible concentration suivie d'effet
(dégénérescence inflammatoire de l'épithélium nasal) a été de
410 mg/m3 chez des rats exposés pendant 2 ans; dans cette étude, la
dose sans effet observé (NOEL) a été évaluée à 100 mg/m3 environ.
Une étude de qualité menée sur des rats n'a révélé aucun effet
sur le développement, malgré une diminution du poids des mères après
inhalation de concentrations allant jusqu'à 8315 mg/m3. Les seules
autres données disponibles en ce qui concerne les effets sur le
développement sont les résultats d'études limitées, anciennes ou peu
documentées, faisant état d'une foetotoxicité à des concentrations qui,
lorsqu'elles étaient mentionnées, étaient toxiques pour la mère. Les
données relatives aux effets du méthacrylate de méthyle sur la
reproduction sont limitées. Il n'y a pas eu de baisse de la fécondité
dans un test de létalité dominante chez des souris exposées à des
concentrations atteignant 36 900 mg/m3 et aucun effet indésirable n'a
été observé sur les organes de la reproduction dans les études menées
jusqu'ici avec des doses répétées. Les données disponibles sur la
neurotoxicité du méthacrylate de méthyle sont également limitées; on a
observé une dégradation de l'activité locomotrice, de la capacité
d'apprentissage et du comportement, ainsi que des effets biochimiques
sur le cerveau de rats exposés par voie orale à une dose de 500 mg/kg
de poids corporel par jour pendant 21 jours.
Le méthacrylate de méthyle ne s'est pas révélé cancérogène dans
une étude approfondie et bien documentée de 2 ans au cours de laquelle
des rats et des souris ont été exposés par inhalation, ni dans une
autre étude d'inhalation chronique menée sur des rats et des hamsters.
In vitro, le méthacrylate de méthyle n'est pas mutagène dans les
systèmes bactériens, mais il s'est révélé mutagène et clastogène dans
des cellules mammaliennes. Dans les études in vivo (principalement
les études d'inhalation) qui ont clairement démontré la toxicité du
méthacrylate de méthyle pour les tissus cibles, on trouve également
quelques indices de génotoxicité.
Le méthacrylate de méthyle est légèrement irritant pour la peau
de l'homme et il peut induire une sensibilisation cutanée chez des
individus prédisposés. Bien que l'on ait également signalé des cas
d'asthme professionnel liés au méthacrylate de méthyle, il n'est pas
prouvé de façon concluante que cette substance soit un sensibilisant
des voies respiratoires. Dans l'ensemble, les études épidémiologiques
disponibles n'apportent pas de preuve convaincante d'un effet
cancérogène chez l'homme, mais on ne peut pas en déduire non plus avec
quelque certitude que l'exposition au méthacrylate de méthyle
n'entraîne aucun risque supplémentaire.
Le méthacrylate de méthyle est peu toxique pour les organismes
aquatiques. Aucune étude de toxicité chronique portant sur ce type
d'organismes n'a été retrouvée, mais des épreuves de toxicité aiguë
ont été effectuées sur des poissons, sur Daphnia magna et sur des
algues. L'effet le plus sensible a été l'inhibition de la
multiplication cellulaire chez l'algue verte Scenedesmus
quadricauda après 8 jours d'exposition à la concentration de
37 mg/litre. La valeur la plus faible de CE50 à 24 heures pour
l'immobilisation des Daphnia est de 720 mg/litre. La CL50 à
96 heures pour le poisson Lepomis macrochirus, en conditions de
renouvellement continu, était de 191 mg/litre, tandis que les CL50
pour des durées de 1 à 24 heures allaient de 420 à 356 mg/litre. La
CL50 à 96 heures pour la truite arc-en-ciel (Oncorhynchus mykiss)
en conditions de renouvellement continu était supérieure à la plus
forte concentration testée, soit 79 mg/litre. Des réactions
sublétales/comportementales ont été notées chez des poissons à
40 mg/litre.
Les données disponibles chez l'homme sont jugées insuffisantes
pour servir de base principale au calcul d'une valeur guide; on a donc
établi une concentration tolérable sur la base de la dégénérescence
inflammatoire de l'épithélium nasal observée chez des rats exposés
pendant 2 ans à une concentration de 410 mg/m3. Dans cette étude, la
NOEL était d'approximativement 100 mg/m3. Les données pouvant servir
à évaluer l'exposition indirecte dans l'environnement général ou
l'exposition des consommateurs sont extrêmement limitées. La
concentration tolérable calculée (probablement avec une certaine marge
de sécurité), qui est d'environ 0,2 mg/m3, est supérieure de
plusieurs ordres de grandeur aux quelques prédictions qui ont été
faites des concentrations atmosphériques de méthacrylate de méthyle
dans l'environnement général. Le niveau d'exposition par inhalation
auquel on peut s'attendre du fait de l'utilisation de peintures
dispersables ou de peintures à l'huile contenant du méthacrylate de
méthyle est peut-être supérieur d'un ordre de grandeur à la dose
qu'entraînerait une exposition à la concentration tolérable, encore
que dans certains pays ces produits ne soient pas destinés au grand
public. Aucun renseignement n'a été trouvé sur leurs conditions
d'utilisation dans d'autres pays. Une dose journalière tolérable
(DJT) de 1,2 mg/kg de poids corporel par jour a été calculée sur la
base d'une étude de toxicité chronique par voie orale.
Bien que les données disponibles au sujet des effets du
méthacrylate de méthyle sur l'environnement soient limitées et que les
concentrations prévues dans divers milieux soient très incertaines, il
existe une marge considérable entre les concentrations suivies
d'effets avérés et les concentrations prévues de façon approximative
dans l'environnement.
RESUMEN DE ORIENTACION
Esta reseña de la evaluación química internacional del
metacrilato de metilo fue preparada por la Dirección de Higiene del
Medio de Health Canada y se basó principalmente en un examen realizado
por el Gobierno del Canadá (1993) para evaluar los efectos potenciales
sobre la salud humana de la exposición indirecta a dicha sustancia en
el medio ambiente general, así como sus efectos ambientales, y en un
examen del Centro Internacional de Investigaciones sobre el Cáncer
(CIIC, 1994) centrado principalmente en la identificación de riesgos
de carcinogenicidad. En el examen del Gobierno del Canadá (1993) se
tuvieron presentes los datos obtenidos en marzo de 1992, actualizados
posteriormente sobre la base de una amplia búsqueda bibliográfica
realizada en septiembre de 1995 en las bases de datos en línea y el
Registro Internacional de Productos Químicos Potencialmente Tóxicos.
En el apéndice 1 se presenta información sobre la naturaleza de la
revisión científica y sobre los exámenes del Gobierno del Canadá
(1993) y del CIIC (1994). Durante la fase de revisión científica de
esta reseña se tomaron en consideración otros exámenes provisionales
de la Dirección de Salud y Seguridad del Reino Unido (Cary et al.,
1995) y de la Unión Europea (Evaluación Provisional del Metacrilato de
Metilo), así como exámenes ya publicados del ECETOC (1995) y de la
Junta Consultiva Finlandesa de Sustancias Químicas (1992), con miras
principalmente a obtener información adicional pertinente para la
revisión. También se ha incorporado la información adicional
identificada durante la revisión efectuada por los puntos de contacto
y el examen realizado por el Comité de Revisión Final. La información
relativa a la revisión científica de la presente reseña figura en el
apéndice 2. La publicación de esta reseña fue aprobada en una reunión
del Comité de Revisión Final celebrada en Bruselas (Bélgica) del 18 al
20 de noviembre de 1996. La lista de participantes en la reunión del
Comité de Revisión Final figura en el apéndice 3. También se ha
reproducido en este documento la ficha internacional de seguridad
química para el metacrilato de metilo (ICSC 0300), emitida por el
Programa Internacional de Seguridad de las Sustancias Químicas (IPCS,
1993).
El metacrilato de metilo (N° CAS 80-62-6) es una sustancia
química sintética y volátil que se utiliza principalmente en la
producción de hojas acrílicas fraguadas, emulsiones acrílicas y
resinas moldeadas y extruidas. Los polímeros y copolímeros de
metacrilato de metilo también se utilizan en los revestimientos de
exterior con base acuosa, con base disolvente y sin diluir, en
adhesivos, selladores, revestimientos de cuero y de papel, tintas,
ceras para suelos, aprestos textiles, prótesis dentales, cementos
quirúrgicos fosfatados y pantallas acrílicas emplomadas contra la
radiación, así como en la preparación de uñas sintéticas y separadores
ortéticos para zapatos. La mayor parte de las emisiones del
metacrilato de metilo se producen en el aire, liberándose en muy
pequeñas cantidades en el agua y en el suelo. Su persistencia en la
atmósfera es corta y no se considera que contribuya directamente al
agotamiento de la capa de ozono. No parece que se bioconcentre en el
medio ambiente y su inhalación con el aire probablemente sea la
principal vía de exposición humana.
El metacrilato de metilo tiene una absorción y distribución
rápidas en animales de experimentación, tras su inhalación o
administración por vía oral. Los datos de que se dispone sobre su
absorción tras una exposición cutánea son limitados. Tanto en
animales de experimentación como en seres humanos, el metacrilato de
metilo se metaboliza rápidamente en ácido metacrílico. Tras su
inhalación, el 16-20% de la sustancia química se deposita en las vías
respiratorias altas de las ratas, donde principalmente es metabolizada
por las esterasas del tejido local.
El metacrilato de metilo tiene una toxicidad aguda baja. En los
roedores y conejos expuestos a concentraciones relativamente altas se
han observado irritaciones cutáneas, oculares y de la cavidad nasal.
Esta sustancia química es un sensibilizador cutáneo ligero en los
animales. El efecto observado con mayor frecuencia tras una
exposición repetida por inhalación en su concentración más baja es la
irritación de la cavidad nasal. También se han señalado efectos en el
riñón y el hígado con concentraciones más altas. El nivel mínimo de
inhalación con efectos comunicados fue de 410 mg/m3 en las ratas
expuestas a metacrilato de metilo durante dos años (basado en la
degeneración inflamatoria del epitelio nasal); el nivel sin efectos
observados (NOEL) en esta investigación fue de aproximadamente 100
mg/m3.
En un estudio bien llevado sobre ratas no se observaron efectos
en el desarrollo, aunque se produjeron disminuciones en el peso
corporal materno tras la inhalación de concentraciones de hasta 8315
mg/m3. Los demás datos de que se dispone sobre la toxicidad para el
desarrollo se limitan a los resultados de un número reducido de
estudios precoces o mal documentados en los que se observaron efectos
fetotóxicos con concentraciones (cuando se comunicaron) que resultaron
tóxicas para las madres. Se dispone de pocos datos sobre los efectos
del metacrilato de metilo en la reproducción. No hubo disminución de
la fecundidad en una valoración de dominancia letal en ratones
expuestos a concentraciones de hasta 36 900 mg/m3 ni se observaron
efectos negativos sobre los órganos reproductores en los estudios de
administración repetida realizados hasta la fecha. Se dispone de
pocos datos sobre la neurotoxicidad del metacrilato de metilo; se
observaron una disminución de la actividad locomotora y efectos a
nivel de aprendizaje, de comportamiento y bioquímico en el cerebro, en
ratas expuestas por vía oral a 500 mg/kg de peso corporal al día
durante 21 días.
El metacrilato de metilo no resultó carcinógeno en una amplia
biovaloración bien documentada de dos años realizada en ratas y
ratones expuestos por inhalación, ni en otros estudios de inhalación
crónica realizados en ratas y hámsters. Aunque no es mutagénico en
los sistemas bacterianos in vitro, ha resultado mutagénico y
clastogénico en las células de mamíferos in vitro. En los estudios
in vivo (principalmente por inhalación) en los que ha habido pruebas
claras de toxicidad en el tejido diana, ha habido pocos indicios de la
genotoxicidad del metacrilato de metilo.
En los seres humanos, el metacrilato de metilo es un irritante
cutáneo ligero y puede inducir sensibilización cutánea en las personas
con predisposición. Aunque también se han señalado casos de asma
profesional asociados a dicha sustancia, no existen pruebas
concluyentes de que el metacrilato de metilo sea un sensibilizador
respiratorio. En general, los estudios epidemiológicos disponibles no
ofrecen indicios firmes ni sistemáticos de un efecto carcinogénico del
metacrilato de metilo en ningún órgano diana en los seres humanos,
pero tampoco se puede inferir con algún grado de confianza que se haya
descartado la posibilidad de un exceso de riesgo.
El metacrilato de metilo tiene una toxicidad baja en organismos
acuáticos. Aunque no se identificaron estudios de toxicidad crónica
en organismos acuáticos, se han realizado pruebas de toxicidad aguda
en Daphnia magna y en algas. El efecto más sensible fue el comienzo
de la inhibición de la multiplicación celular en el alga verde
Scenedesmus quadricauda, con niveles de 37 mg/litro tras ocho días
de exposición. La CE50 de inmovilización más baja notificada a las
24 horas para Daphnia es de 720 mg/litro. La CL50 a las 96 horas
en juveniles de Lepomis macrochirus en condiciones de flujo continuo
fue de 191 mg/litro, mientras que los valores de la CL50 para
periodos de 1 a 24 horas oscilaron entre 420 y 356 mg/litro,
respectivamente. La CL50 a las 96 horas para la trucha arco iris
(Oncorhynchus mykiss) en condiciones de flujo continuo fue >79
mg/litro, la concentración más alta probada. Se observaron respuestas
subletales/comportamentales en los peces, con niveles de 40 mg/litro.
Los estudios de que se dispone en seres humanos se consideran
inadecuados como base principal para establecer un valor orientativo;
por consiguiente, a modo de orientación, se ha establecido una
concentración tolerable sobre la base de la degeneración inflamatoria
del epitelio nasal de las ratas expuestas a concentraciones de 410
mg/m3 durante dos años. La concentración sin efectos observados en
esta investigación fue de aproximadamente 100 mg/m3. Los datos de
que se dispone para establecer una base para la estimación de la
exposición indirecta en el medio ambiente general y de la exposición
en los usuarios son muy limitados. La concentración tolerable
obtenida (probablemente moderada) de aproximadamente 0,2 mg/m3 es
muchos órdenes de magnitud más elevada que las concentraciones
previstas de la muestra de metacrilato de metilo en el aire ambiente
del medio ambiente general. La exposición por inhalación prevista por
el uso de pinturas de dispersión y al aceite que contienen metacrilato
de metilo puede ascender a un orden de magnitud superior a la ingesta
tolerable asociada con la exposición al nivel de concentración
tolerable, si bien se ha notificado que en algunos países esos
productos no se suministran al público en general. No se obtuvo
información sobre las modalidades de utilización de estos productos en
otros países. Sobre la base de un estudio de toxicidad crónica
realizado por vía oral, se ha determinado una ingesta diaria tolerable
(IDT) de 1,2 mg/kg de peso corporal al día.
Si bien los datos de que se dispone sobre los efectos ambientales
del metacrilato de metilo son limitados y los valores pronosticados en
distintos medios son muy inciertos, existe un amplio margen entre los
niveles con efectos observados y las concentraciones ambientales de
pronóstico incierto.
1. EXECUTIVE SUMMARY
This CICAD on methyl methacrylate was prepared by the
Environmental Health Directorate of Health Canada and was based
principally on a Government of Canada (1993) review to assess the
potential effects on human health of indirect exposure to methyl
methacrylate in the general environment as well as the chemical's
environmental effects and an International Agency for Research on
Cancer review (IARC, 1994) concerning primarily hazard identification
for carcinogenicity. Data identified as of March 1992 were considered
in the Government of Canada (1993) review and were subsequently
updated, based on a comprehensive literature search conducted in
September 1995 of on-line databases and the International Register of
Potentially Toxic Chemicals. Information on the nature of peer review
and the availability of the Government of Canada (1993) and IARC
(1994) reviews is presented in Appendix 1. During the peer review
phase for this CICAD, additional draft reviews of the United Kingdom
Health and Safety Executive (Cary et al., 1995) and the European Union
(Draft Assessment on Methyl Methacrylate) and published reviews of
ECETOC (1995) and the Finnish Advisory Board of Chemicals (1992) were
considered, primarily for identification of relevant additional
information for review. Additional information identified during
review by Contact Points and consideration by the Final Review Board
has also been incorporated. Information on the peer review of this
CICAD is presented in Appendix 2. This CICAD was approved for
publication at a meeting of the Final Review Board, held in Brussels,
Belgium, on 18-20 November 1996. Participants at the Final Review
Board meeting are listed in Appendix 3. The International Chemical
Safety Card for methyl methacrylate (ICSC 0300), produced by the
International Programme on Chemical Safety (IPCS, 1993), has also been
reproduced in this document.
Methyl methacrylate (CAS no. 80-62-6) is a volatile synthetic
chemical that is used principally in the production of cast acrylic
sheet, acrylic emulsions, and moulding and extrusion resins. Polymers
and copolymers of methyl methacrylate are also used in waterborne,
solvent, and undissolved surface coatings, adhesives, sealants,
leather and paper coatings, inks, floor polishes, textile finishes,
dental prostheses, surgical bone cements, and leaded acrylic radiation
shields and in the preparation of synthetic fingernails and orthotic
shoe inserts. The majority of methyl methacrylate is predicted to be
emitted to air, with very small amounts being released into water and
soil. The persistence of methyl methacrylate in the atmosphere is
short, and the chemical is not considered to contribute directly to
depletion of the ozone layer. Methyl methacrylate is not expected to
bioconcentrate in the environment, and inhalation from air is likely
the primary route of human exposure.
Methyl methacrylate is rapidly absorbed and distributed following
inhalation or oral administration to experimental animals. Data on
absorption following dermal exposure are limited. In both
experimental animals and humans, methyl methacrylate is rapidly
metabolized to methacrylic acid. Following inhalation, 16-20% of the
chemical is deposited in the upper respiratory tract of rats, where it
is primarily metabolized by local tissue esterases.
The acute toxicity of methyl methacrylate is low. Irritation of
the skin, eye, and nasal cavity has been observed in rodents and
rabbits exposed to relatively high concentrations of methyl
methacrylate. The chemical is a mild skin sensitizer in animals. The
effect observed most frequently at lowest concentration after repeated
inhalation exposure to methyl methacrylate is irritation of the nasal
cavity. Effects on the kidney and liver at higher concentrations have
also been reported. The lowest reported effect level for inhalation
was 410 mg/m3 in rats exposed to methyl methacrylate for 2 years
(based upon inflammatory degeneration of the nasal epithelium); the
no-observed-effect level (NOEL) in this investigation was
approximately 100 mg/m3.
In a well conducted study in rats, there were no developmental
effects, although there were decreases in maternal body weight
following inhalation of concentrations up to 8315 mg/m3. Other
available data on developmental toxicity are restricted to results of
limited early or poorly documented studies in which fetotoxic effects
were observed at concentrations that (where reported) were toxic to
the mothers. Available data on reproductive effects of methyl
methacrylate are limited. There was no reduction in fertility in a
dominant lethal assay in mice exposed to methyl methacrylate
concentrations up to 36 900 mg/m3 and no adverse effects on
reproductive organs in repeated-dose studies conducted to date.
Available data on the neurotoxicity of methyl methacrylate are
limited; impairment of locomotor activity and learning and behavioural
and biochemical effects on the brain were observed in rats exposed
orally to 500 mg/kg body weight per day for 21 days.
Methyl methacrylate was not carcinogenic in an extensive, well
documented 2-year bioassay in rats and mice exposed by inhalation and
in additional chronic inhalation studies in rats and hamsters.
Although not mutagenic in vitro in bacterial systems, methyl
methacrylate has been mutagenic and clastogenic in mammalian cells
in vitro. In in vivo studies (primarily by the inhalation route)
in which there has been clear evidence of toxicity within the target
tissue, there has been limited evidence of the genotoxicity of methyl
methacrylate.
Methyl methacrylate is a mild skin irritant in humans and has
the potential to induce skin sensitization in susceptible individuals.
Although occupational asthma associated with methyl methacrylate has
also been reported, there is no conclusive evidence that methyl
methacrylate is a respiratory sensitizer. As a whole, the available
epidemiological studies do not provide strong or consistent evidence
of a carcinogenic effect of methyl methacrylate on any target organ in
humans, nor can it be inferred with any degree of confidence that the
possibility of an excess risk has been disproved.
The toxicity of methyl methacrylate to aquatic organisms is low.
Although no chronic studies on aquatic organisms were identified,
acute tests have been conducted on fish, Daphnia magna, and algae.
The most sensitive effect was the onset of inhibition of cell
multiplication by the green alga Scenedesmus quadricauda at 37
mg/litre following 8 days of exposure. The lowest reported 24-hour
EC50 for immobilization in Daphnia is 720 mg/litre. The 96-hour
LC50 in juvenile bluegill sunfish (Lepomis macrochirus) under
flow-through conditions was 191 mg/litre, whereas LC50 values for
durations of 1-24 hours ranged from 420 to 356 mg/litre, respectively.
The 96-hour LC50 for rainbow trout (Oncorhynchus mykiss) under
flow-through conditions was >79 mg/litre, the highest concentration
tested. Sublethal/behavioural responses were noted among the fish at
40 mg/litre.
The available studies in humans are considered inadequate as the
principal basis for derivation of a guidance value; therefore, in
order to provide guidance, a tolerable concentration has been
established on the basis of inflammatory degeneration of the nasal
epithelium of rats exposed to methyl methacrylate at a concentration
of 410 mg/m3 for 2 years. The NOEL in this investigation was
approximately 100 mg/m3. Data available to serve as a basis for
estimation of indirect exposure in the general environment or consumer
exposure are extremely limited. The derived (likely conservative)
tolerable concentration of approximately 0.2 mg/m3 is many orders of
magnitude higher than the sample predicted concentrations of methyl
methacrylate in ambient air of the general environment. Inhalation
exposure predicted from the use of dispersion and oil-based paints
containing methyl methacrylate may be up to an order of magnitude
higher than the tolerable intake associated with exposure at the level
of the tolerable concentration, although it has been reported that in
some countries these products are not supplied to the general public.
Information on use patterns of these products in other countries was
not identified. Based on a chronic study by the oral route of
administration, a tolerable daily intake (TDI) of 1.2 mg/kg body
weight per day has been derived.
Although available data on the environmental effects of methyl
methacrylate are limited and predicted values in various media are
highly uncertain, a wide margin exists between observed effect levels
and uncertain predicted environmental concentrations of methyl
methacrylate.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
Methyl methacrylate (CAS no. 80-62-6) is a colourless, volatile
liquid with an acrid fruity odour. It has a relatively high vapour
pressure (4 kPa at 20°C), moderate water solubility (15.8 g/litre),
and a low log octanol/water partition coefficient ( Kow = 1.38)
(Government of Canada, 1993). The empirical formula for methyl
methacrylate is C5H8O2. The structural formula for methyl
methacrylate is given below. Additional physical/chemical properties
are presented in the International Chemical Safety Card reproduced in
this document.
O
"
H2C = C - C - O - CH3
'
CH3
The purity of commercial methyl methacrylate is typically 99.9%.
It contains traces of acidity as methacrylic acid (0.003% max.;
specification, 0.005% max.) and water (0.03% max.; specification,
0.05% max.). Inhibitors added for storage and transportation are
usually 2-100 ppm methyl ether of hydroquinone and 25-100 ppm
hydroquinone, although other phenolic inhibitors, such as dimethyl
tert-butylphenol, may also be used (IARC, 1994; M. Pemberton,
personal communication, 1996).
3. ANALYTICAL METHODS
Methods commonly used for the analysis of acrylic compounds
include gas chromatography (GC), mass spectrometry (MS), GC/MS,
nuclear magnetic resonance, and infrared spectroscopy (Government of
Canada, 1993). Methyl methacrylate can be determined in air by gas
chromatography with flame ionization detection; the sample is adsorbed
on fused silica (XAD-2 resin) or charcoal coated with
4- tert-butylcatechol and desorbed with carbon disulfide or toluene.
The estimated limit of detection for this method is 0.01 mg per
sample. A detection limit of 0.8 mg/m3 is obtained with a method
involving desorption with 5% isopropanol in carbon disulfide from
charcoal (IARC, 1994).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Methyl methacrylate is not known to occur naturally (IARC, 1994).
It is used principally in the production of cast acrylic sheet,
acrylic emulsions, and moulding and extrusion resins (IARC, 1994).
Polymers and copolymers of methyl methacrylate are used in waterborne,
solvent, and undissolved surface coatings (exterior latex paint based
on emulsions containing methyl methacrylate is the surface coating in
which it is used most widely). Solvent reducible polymers containing
methyl methacrylate are used for industrial finishes, metal and foil
coatings, and a variety of overlays for special purposes. Solvent and
emulsion polymers containing methyl methacrylate are also used in
adhesives, sealants, leather and paper coatings, inks, floor polishes,
and textile finishes (IARC, 1994). Methyl methacrylate and polymers
of methyl methacrylate are also used for dental prostheses, surgical
bone cements, and leaded acrylic radiation shields and in the
preparation of synthetic fingernails and orthotic shoe inserts (IARC,
1994).
Global production of methyl methacrylate was estimated to be 1.4
million tonnes in 1988 (IARC, 1994). In the USA and Japan, production
of methyl methacrylate ranged from 380 000 to 536 000 t and from
384 000 to 403 000 t, respectively, between 1990 and 1992 (IARC,
1994). Total production volume within the European Union was
447 000 t in 1993 (CEFIC, 1994).
Methyl methacrylate can enter the environment during its
transport, bulk storage, and use. Based on data from the US Toxic
Chemical Release Inventory, emissions to air, water, and soil from
industries in the USA are estimated to be about 0.46% of
production.1 Most of the released methyl methacrylate (i.e. 98%) is
estimated to be emitted to air, with very small amounts being released
into water and soil. Data on emissions of methyl methacrylate in
other countries have not been identified. Assuming a production in
the USA in 1992 of approximately 500 000 t (IARC, 1994), approximately
2300 t are estimated to have been released to the environment.
1 Source: Toxic Chemical Release Inventory (TRI), databank produced
by the National Library of Medicine and the US Environmental
Protection Agency (1989).
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
As methyl methacrylate is highly reactive with hydroxyl radicals,
its estimated half-life in the troposphere is short: from <5 hours in
summer to a few days in winter at a latitude such as that of Toronto,
Canada. The reported photooxidation half-life of methyl methacrylate
is 1.1-9.7 hours. Methyl methacrylate is readily polymerized by light
and heat but is not expected to photolyze (Government of Canada,
1993).
In neutral or acidic aquatic environments, hydrolysis of methyl
methacrylate is not significant. Based upon its measured second-order
hydrolysis rate constant of 200 (mol/litre)-1 h-1 at 25°C, the
hydrolysis half-life of methyl methacrylate is estimated to be 3.9
years at pH 7 and 14.4 days at pH 9 (Howard, 1989).
No data were identified on the rate of volatilization of methyl
methacrylate; however, the half-life for evaporation from a river 1 m
deep with a 1 m/s current and 3 m/s wind has been calculated as 6.3
hours. Evaporation of methyl methacrylate from soil is expected to be
rapid, owing to its high vapour pressure and weak adsorption to soil.
A Level I fugacity model in an evaluative environment predicts
the following equilibrium partitioning of methyl methacrylate: air,
86.6%; water, 13.1%; and soil/sediment, <0.4% (Mackay et al., 1995).
Biodegradation contributes significantly to removal of methyl
methacrylate from the environment. The aqueous aerobic degradation
half-life is estimated to be 1-4 weeks, and the anaerobic degradation
half-life is estimated to be 4-16 weeks (Howard, 1989).
Although no studies have been conducted to measure
bioconcentration factors for methyl methacrylate, a bioconcentration
factor of 3 has been estimated from the log Kow; based on this
value, methyl methacrylate is not expected to bioconcentrate or
biomagnify in food-chains (Government of Canada, 1993).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
In an analysis of 204 samples of water collected from 14 heavily
industrialized river basins in the USA (Ewing & Chian, 1977), methyl
methacrylate was detected (detection limit 1.0 µg/litre) only once at
a concentration of 10 µg/litre in final tap-water after chlorination
in Chicago, Illinois, in 1976. No additional information was
provided. Methyl methacrylate was not detected in 24 water samples
(limit of determination 0.005-1 µg/litre) or in 24 sediment samples
(limit of determination 0.000 11-0.01 µg/g dry weight) taken in Japan
in eight locations (harbour or estuarine areas) in 1979 (no further
information provided) (S. Tsuda, personal communication, 1996).
Methyl methacrylate was not detected (detection limit 0.01 µg/g wet
weight) in 30 samples of (edible) shellfish collected from various
locations in Atlantic Canada (Environment Canada, 1989). Methyl
methacrylate may be present in food as a result of migration of the
monomer from food containers made from polymethyl methacrylate (IARC,
1994); for example, concentrations ranged from 180 to 275 ppb (ng/g)
in maple syrup that had been packaged in plastic containers
(Hollifield et al., 1980). The migration of methyl methacrylate from
commercial plastic wrap into 20% ethanol at 25°C was 1 ppm in 1 day
and 10 ppm in 90 days. Migration into water and acetic acid was not
detected (detection limit 0.05 ppm) (Inoue et al., 1981).
In view of the limited available monitoring data, estimates of
the fate and concentrations of methyl methacrylate in the Canadian
environment were generated by a Level III fugacity model (Mackay &
Paterson, 1981, 1982, 1991; Mackay et al., 1985) developed for
southern Ontario, incorporating data on the physical and chemical
properties of the chemical (Government of Canada, 1993),
transformation half-lives (Howard et al., 1991), and proportion of
production in the USA emitted to environmental media (see section 4)
applied to the volume imported into Canada. Methyl methacrylate is
not produced in Canada; approximately 22 000 t are imported (CPI,
1989). The model assumed emissions of 95% to air, 4.5% to water, and
0.5% to soil. The estimated relative proportions of methyl
methacrylate predicted for air, water, soil, and sediment at steady
state were 26.6%, 60.8%, 12.6%, and 0.03%, respectively. The amount
of methyl methacrylate estimated to partition to fish was negligible.
The relatively longer half-life for methyl methacrylate in water
compared with air accounts for the higher estimated relative
proportion predicted for the water compartment. Although such models
are useful primarily for identification of the relative proportions of
exposure from various media rather than for quantitative estimates of
concentrations, the latter are presented here primarily as a baseline
for comparison with measured concentrations. It should also be noted
that such predicted values will vary in different countries depending
upon production and releases of methyl methacrylate. The average
concentrations estimated on the basis of the model were 2.44 × 10-4
µg/m3 in air, 0.13 ng/litre in surface water, 1.2 × 10-6 µg/g in
soil, 8.7 × 10-8 µg/g in sediment, and 1.5 × 10-7 µg/g in fish
(Government of Canada, 1993).
6.2 Human exposure
Examples of estimated indirect exposure in the general
environment and during use of consumer products are presented here.
Levels determined in various occupational settings are also
summarized. Estimates of indirect exposure in the general environment
are based in Canada owing to the availability of relevant data for
input; however, predicted levels will vary considerably as a function
of production and use patterns in various countries. Consumer
exposure estimates are based on data on the percent composition of
methyl methacrylate in products provided by European manufacturers.
Levels in occupational environments are those reported from various
countries. Countries are strongly encouraged, however, to estimate
exposure on the basis of local data, possibly in a manner similar to
that outlined here.
Adequate data on measured concentrations of methyl methacrylate
in air, drinking-water, foodstuffs, and soil have not been identified;
indeed, they are limited to non-detectable values in a limited number
of small surveys. Although predicted concentrations in environmental
media based on fugacity modelling are uncertain, they are helpful in
estimating proportions of exposure from various media. Based on a
daily inhalation volume for adults of 22 m3, a mean body weight for
males and females of 64 kg, and a predicted concentration (by fugacity
modelling; see section 6.1) of methyl methacrylate in ambient air in
Canada of 2.44 × 10-4 µg/m3, the estimated intake of methyl
methacrylate from air for the general population represents
approximately 97% of the total intake from air, drinking-water, fish,
and soil. Based on a daily volume of water consumption for adults of
1.4 litres, a mean body weight of 64 kg, and a predicted concentration
of methyl methacrylate in surface water in Canada of 0.13 ng/litre
(see section 6.1), the estimated intake of methyl methacrylate from
drinking-water for the general population represents approximately
3.3% of total intake. Available data were inadequate to estimate the
intake of methyl methacrylate from food, with the exception of intake
from fish. Based on a daily amount of fish ingested for adults of 23
g/day, a mean body weight for adults of 64 kg, and the predicted
concentration of methyl methacrylate in fish in Canada of 1.5 × 10-7
µg/g (see section 6.1), the estimated intake of methyl methacrylate
from fish represents 0.06% of total intake. Based on a daily amount
of soil ingested for adults of 20 mg, a mean body weight for adults of
64 kg, and a predicted concentration of methyl methacrylate in soil in
Canada of 1.2 × 10-6 µg/g (see section 6.1), the estimated intake of
methyl methacrylate from soil, as a proportion of total intake, is
negligible (0.0004%). Therefore, based on predicted concentrations in
the Canadian environment, the overwhelmingly principal source of
indirect exposure to methyl methacrylate for most of the general
population is air.
Inhalation exposure to methyl methacrylate from the use of
consumer products containing methyl methacrylate (e.g. dispersion
paints and oil-based paints) was modelled using the US EPA Screening
Consumers Inhalation Exposure Software (SCIES) computer model. All
scenarios were based on the assumption that the percent composition of
methyl methacrylate-based polymers in formulations of dispersion
paints, varnishes, or lacquers is 15%, although residual monomer
content is much less (European Union Draft Assessment on Methyl
Methacrylate), and that 100% is absorbed. Although it has been
reported that in some countries these products are not supplied to the
general public, information on use patterns of these products in other
countries was not available.
For the use of dispersion paints, the standard default values of
the SCIES model were assumed for the following parameters: frequency
of use, six events per year; mass of product, 13.6 kg; room size, 40
m3; duration of use, 4.9 hours; house air exchange rate, 0.2 room air
exchanges per hour; and user inhalation rate, 1.3 m3/hour. The
vapour pressure of methyl methacrylate was considered to be 38.4 torr
(5.12 kPa) (Howard, 1989). Resulting estimated consumer exposure from
inhalation was in the range of 10-100 mg/kg body weight per day.
However, as the residual methyl methacrylate monomer content in
dispersion paints is specified to be 0.1% (ECETOC, 1995), consumer
exposure to methyl methacrylate would fall within the range of 10-100
µg/kg body weight per day.
For the estimation of consumer exposure from the use of oil-based
(solvent-based) paints, the standard default values of the SCIES model
were assumed as above, with the exception of the following parameters,
for which default values were: mass of product, 6.71 kg; and duration
of use, 3.2 hours. The vapour pressure of methyl methacrylate and
absorption were the same as those for the scenario mentioned above.
The resulting estimated consumer exposure from inhalation was again in
the range of 10-100 mg/kg body weight per day. However, as the
residual methyl methacrylate monomer content in solvent-based paints
is assumed to be 1.5% by the producer (European Union Draft Assessment
on Methyl Methacrylate), consumer exposure to methyl methacrylate
would fall within the range of 100-1000 µg/kg body weight per day.
Occupations in which there is potential exposure to methyl
methacrylate include those in the medical, dental, and beauty
professions, such as chemical process operators, surgeons and surgical
assistants, operating room nurses, dental technicians and hygienists,
and beauty technicians applying synthetic fingernails (IARC, 1994).
Exposure to methyl methacrylate in the workplace could be
substantially greater than that in the general environment. Based on
experience in the United Kingdom, for example, long-term personal
exposures during monomer production average about 2 ppm (8.2 mg/m3)
and are less than 60 ppm (246 mg/m3) (Cary et al., 1995). In open
system industries such as cast sheet production, long-term exposures
are higher, averaging 22.2 ppm (91 mg/m3) and ranging from 0.5 to 165
ppm (2-677 mg/m3). For various end uses of methyl methacrylate,
including aerospace manufacture, plastics processing, and artificial
teeth production, the mean long-term value for personal exposure was
13.4 ppm (55 mg/m3), with a range of 0.8-109 ppm (3.3-447 mg/m3).
In medical and dental applications, peak concentrations up to 374 ppm
(1533 mg/m3) have been recorded, although short-term
time-weighted-average exposures are likely to be less than 100 ppm
(410 mg/m3).
Mean levels (time period often unspecified) of methyl
methacrylate in the air of various chemical manufacturing and
processing plants (located in Europe, the USA, Canada, Russia, Japan,
and China) vary widely, ranging from not detectable (detection limit
not reported) to 1500 mg/m3 (CEFIC, 1993; Mizunuma et al., 1993;
IARC, 1994; M. Baril, personal communication, 1996). Peak values as
high as 7900 mg/m3 have been reported for some manufacturing
facilities (M. Baril, personal communication, 1996). Mean
concentrations of methyl methacrylate in the air of dental clinics and
dental laboratories (in the USA, Norway, Denmark, and the United
Kingdom) have ranged from not detectable (detection limit not
reported) to 273 mg/m3 during denture prosthesis manufacture and
repair (IARC, 1994). Mean concentrations of methyl methacrylate in
the air of beauty salons (in the USA) have ranged from 21.7 to 87.5
mg/m3 during the application of artificial fingernails (IARC, 1994).
It should be noted that in some cases these values reflect
shorter-term peak exposures rather than time-weighted averages.
Elevated levels (above 1500 mg/m3) during floor coating with methyl
methacrylate-containing resins have been reported, although these
levels were measured during activities that normally do not cover a
full shift; hence, time-weighted-average concentrations would be
less.1
1 Source: Excerpts from the (1995) BIA file provided by BG Chemie
containing measurement data of occupational exposures to methyl
methacrylate in industry and trade. Communication to Bundesinstitut
für Gesundheitlichen Verbraucherschutz und Veterinarmedizin (BgVV).
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS
AND HUMANS
Methyl methacrylate is rapidly absorbed and distributed following
inhalation or oral administration to rats. On the basis of available
data, methyl methacrylate appears to be rapidly metabolized to
methacrylic acid, which is subsequently converted to carbon dioxide
via the tricarboxylic acid cycle in both experimental animals and
humans. Adequate studies on the dermal absorption of methyl
methacrylate were not identified. Methyl methacrylate is rapidly
eliminated, primarily via the lungs in expired air. After oral or
intravenous administration to rats, approximately 65% of the dose was
exhaled in the expired air as 14CO2 within 2 hours (Bratt & Hathway,
1977). Lesser amounts are eliminated in the urine, and an even
smaller fraction in the faeces. Owing to its rapid metabolism and
excretion, there appears to be little potential for accumulation of
methyl methacrylate within tissues (Government of Canada, 1993;
ECETOC, 1995).
Deposition in the surgically isolated upper respiratory tract of
urethane-anaesthetized male F344 rats exposed to methyl methacrylate
at 90, 437, or 2262 mg/m3 under cyclic flow conditions was 16-20%
(Morris & Frederick, 1995). Deposition was 3% less on average in the
unidirectional flow groups than in the cyclic flow groups. Deposition
was less efficient at the high than at the low and middle
concentrations, although the mechanism is unknown. (The deposition
efficiency of inhaled methacrylic acid under similar conditions was
much greater, averaging 95% under unidirectional flow.) Pretreatment
with a carboxylesterase inhibitor (bis-nitrophenylphosphate) decreased
uptake of methyl methacrylate by one-third, suggesting that methyl
methacrylate is hydrolysed by carboxylesterase in nasal tissues and
that such metabolism serves to enhance its deposition efficiency.
Methyl methacrylate decreased nasal non-protein content by
approximately 25% at the highest concentration, but not at lower
concentrations. Nasal non-protein content was not decreased by
exposure to methacrylic acid even at delivered dose rates twofold more
than that for methyl methacrylate, suggesting that this effect is
attributable to the ester itself and not to the acid metabolite
(Morris & Frederick, 1995).
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
The acute toxicity of methyl methacrylate is consistently low,
although unconfirmed effects on the lungs were reported at relatively
low concentrations in one study of poor design (Raje et al., 1985).
The 4-hour LC50s for methyl methacrylate in rats ranged from 3750 to
7093 ppm (15 375-29 080 mg/m3). The oral LD50s ranged from 5.0
ml/kg body weight (4.7 g/kg body weight) in dogs to 10.0 ml/kg body
weight (9.44 g/kg body weight) in rats (Government of Canada, 1993).
8.2 Irritation and sensitization
Irritation of the skin, eye, and mucosa of the respiratory tract
has been observed in rodents and rabbits exposed to relatively high
concentrations of methyl methacrylate (dermal application of
approximately 2-38 g/kg body weight; inhalation of 100-17 600 ppm
[410-72 160 mg/m3]; or instillation of approximately 0.1 ml into the
cornea) (Spealman et al., 1945; Castellino & Colicchio, 1969; Rohm &
Haas, 1982; Raje et al., 1985; Kanerva & Verkkala, 1986; NTP, 1986;
Ouyang et al., 1990).
The weight of evidence is that methyl methacrylate is a skin
sensitizer in animals (Cary et al., 1995; ECETOC, 1995).
8.3 Short-term exposure
Death, decreases in body weight, changes in respiration rate,
increases in level of blood urea nitrogen, and pulmonary damage were
observed after exposure to high concentrations in short-term
repeated-dose studies in rats and mice in which inhaled concentrations
of methyl methacrylate ranged up to 5000 ppm (20 500 mg/m3)
(Government of Canada, 1993). Cardiovascular effects (irregular ECG,
changes in blood pressure) were also observed in rats exposed to
undocumented concentrations of vaporized methyl methacrylate for 20
minutes/day for 21 or 42 days in a limited study (Blanchet et al.,
1982).
In short-term studies, mice were more susceptible than rats, with
effects on the respiratory tract (redness and swelling of the nasal
region) observed after exposure to 500 ppm (2050 mg/m3; the lowest
tested concentration in the study) for 10 days (NTP, 1986). No
systemic histopathological effects were observed after inhalation of
concentrations up to 5000 ppm (20 500 mg/m3).
8.4 Long-term exposure
The protocols and results of available long-term studies on
methyl methacrylate are summarized in Table 1.
Table 1: Summary of effect levels in long-term studies.
Species Study design Effects Effect levels Comments Reference
INHALATION
Rats, Exposed to 0 or 116 ppm Rats exposed for 3 months lacked Effects at 116 One dose group only Tansy et al.,
Sprague-Dawley, (476 mg/m3) methyl methacrylate, visceral and subcutaneous fat ppm (476 1976
50 males per 8 hours/day, for 5 days/week. deposits, had significantly lower mg/m3)
group Approximately half of the rats body, lung, and spleen weights,
in each group were sacrificed and had significantly higher mean
after 3 months; blood and serum alkaline phosphatase
tissue samples were taken. The concentration. Rats exposed for
remainder of the rats were 6 months had less subcutaneous
exposed for 6 months. fat, significantly lower mean body
weights, popliteal fat pad
weights, and mean intestinal transit
time, and significantly higher mean
alkaline phosphatase and inorganic
phosphate concentrations compared
with controls.
Rats, Exposure to 0 or 116 ppm Exposed rats had significantly Effects at 116 One dose group only Tansy et al.,
Sprague-Dawley, (476 mg/m3) methyl methacrylate, lower total bilirubin and higher ppm (476 1980a
23 males per 5 days/week, averaging 7 total cholesterol levels; possible mg/m3)
group hours/day, for 542 hours (3 liver damage in the exposed group,
months). Excretion studies in but details not reported.
nine rats from each group;
histopathological examinations
of heart, lung, kidneys, spleen,
stomach, small bowel, liver,
and adrenal.
Table 1 (continued)
Species Study design Effects Effect levels Comments Reference
Rats, Exposure to 0 or 116 ppm Mild lung damage in some of the Effects at 116 One dose group only; Tansy et al.,
Sprague-Dawley, (476 mg/m3) methyl methacrylate, rats exposed for 3 and 6 months ppm (476 mg/m3) statistical 1980b
23 males per 7 hours/day, 5 days/week, for and the sham-exposed controls. significance not
group for 3 3 or 6 months. Histopathological Rats exposed for 6 months had reported; similar
months and examinations of heart, lung, damaged tracheal mucosa. The effects in
unspecified kidneys, spleen, stomach, small epithelium was denuded of cilia, sham-exposed controls
number for bowel, liver, and adrenal. and the cellular covering of
6 months microvilli was reduced in rats
exposed for 3 months.
Rats, F344, Exposure to 0, 63, 125, 250, Some clinical signs and one death NOEL = 1000 Rohm & Haas,
10 per sex 500, or 1000 ppm (0, 258, 512, each in groups exposed to 63 ppm ppm (4100 1977
per group 1025, 2050, or 4100 mg/m3) and controls, but not mg/m3)
methyl methacrylate, 6 hrs/day, dose-related.
for 65 days. Complete gross
pathological and histopathological
examinations.
Rats, F344/N, Inhalation of 0, 63, 125, 250, No methyl methacrylate-related NOEL = 1000 NTP, 1986
10 per sex 500, or 1000 ppm (0, 258, 512, effects. ppm (4100
per group 1025, 2050, or 4100 mg/m3) mg/m3)
methyl methacrylate, 6 hours/day,
5 days/week, for 14 weeks (65
exposures). Histological
examinations were conducted of
an unspecified range of tissues
from all high-dose and control
rats, those that died before
the end of the study, and some
of the rats from the other
groups.
Table 1 (continued)
Species Study design Effects Effect levels Comments Reference
Rats, F344/N, Exposure to 0, 500, 1000, 2000, At 1000 ppm, a low incidence of LOEL = 1000 NTP, 1986
10 per sex 3000, or 5000 ppm (0, 2050, mild effects on the brain and ppm (4100
per group 4100, 8200, 12 300, or 20 500 nasal turbinates in females was mg/m3)
mg/m3) methyl methacrylate, 6 observed. At 2000-5000 ppm,
hours/day, 5 days/week, for 14 death, effects on body weight, NOEL = 500
weeks (65 exposures). and lesions of nasal turbinates ppm (2050
Histological examinations were and brain were observed; changes mg/m3)
performed on the controls, the in spleen were observed at 3000
two highest dose groups, and ppm and above. Also, follicular
rats that died before the end atrophy of the spleen in 4/10
of the study. Tissues from the males, bone marrow atrophy in
nasal turbinates, larynx, 8/10 males (5000 ppm exposure
trachea, lungs, and brain for group), and cerebellar congestion
all rats exposed at 1000 ppm and penducle haemorrhage in the
and survivors of the 2000 ppm females exposed to 3000 and 5000
groups were also examined ppm that died early. At 5000 ppm,
histopathologically. listlessness, nasal and serous
ocular discharge, and prostration
during the first 2 days, nasal
cavity inflammation with necrosis
and loss of epithelium, follicular
atrophy of the spleen, and bone
marrow atrophy in the males.
Cerebellar congestion and
penducle haemorrhage in the
early-death females exposed to
3000 and 5000 ppm, and malacia and
gliosis in 5/9 females exposed to
2000 ppm and 1/8 females exposed
to 1000 ppm.
Table 1 (continued)
Species Study design Effects Effect levels Comments Reference
Rats, albino Exposure to 0, 25, 100, or 400 Decreased body weights; slight NOEL = 25 ppm Rohm & Haas,
F344, 70 per ppm (0, 102.5, 410, or 1640 increase in the incidence of mild (102.5 mg/m3) 1979a; Lomax,
sex per group mg/m3) methyl methacrylate, 6 rhinitis in the nasal mucosal 1992; Lomax et
hours/day, 5 days/week, for up lining of the turbinates. al., 1997
to 104 weeks. Histopathological
examination of a wide range of The re-examination revealed that LOEL =
tissues from controls and rats exposed to 100 or 400 ppm 100 ppm (410
high-dose groups, as well as methyl methacrylate had mg/m3)
selected tissues from other exposure-related and
dose groups (ovaries or testes concentration-dependent
and nasal turbinates). microscopic changes in the
olfactory epithelium lining
the dorsal meatus in the anterior
A re-examination of the nasal region of the nasal cavity.
tissues from the rats of the The microscopic changes consisted
Rohm & Haas (1979a) study was of degeneration/atrophy of the
conducted. The review consisted olfactory epithelium and
of microscopic examination of underlying Bowman's glands,
nasal tissue from at least 10% hyperplasia of basal (reserve)
of randomly selected rats from cells, replacement of olfactory
each group, and the slides epithelium by ciliated
evaluated included the original (respiratory-like) epithelium,
study slides plus slides from and inflammation of the mucosa
tissue sections taken deeper and/or submucosa. The squamous
into the block. epithelium of the nasal cavity
was not affected. The lesions
tended to be bilateral in
distribution in rats exposed to
both 100 and 400 ppm methyl
methacrylate. A small nasal
polypoid adenoma was observed in
one male from both the 100 and
400 ppm exposure groups.
Table 1 (continued)
Species Study design Effects Effect levels Comments Reference
Rats, F344/N, Rats exposed to methyl Inflammation and degeneration of LOEL = 250 NTP, 1986; Chan
50 per sex methacrylate at 0, 2050, or the olfactory epithelium ppm (1025 et al., 1988
per group 4100 mg/m3 (males) or 0, 1025, (accompanied by variable atrophy mg/m3)
or 2050 mg/m3 (females), 6 of the nerve bundles in the
hours/day, 5 days/week, for submucosa and, in the most
102 weeks. Histological severely affected areas,
examination of a comprehensive replacement of sensory
range of tissues. neuroepithelial cells with
respiratory epithelium) and
minimal increases in the numbers
of alveolar macrophages in the
nasal cavity at all dose levels.
The incidence of focal or
multifocal fibrosis of the lung
was increased in the females
exposed to 2050 mg/m3.
Rats, Fischer Exposure to 0, 25, 100, or 400 Mild rhinitis was observed Abstract only Smith et al.,
344, male and ppm (0, 102.5, 410, or 1640 (dose level not specified). 1979
female (number mg/m3) methyl methacrylate, 6
not specified) hours/day, 5 days/week, for 24
months. Evaluation of haemograms,
clinical chemistries, and urine,
as well as gross histopathological
examination.
Table 1 (continued)
Species Study design Effects Effect levels Comments Reference
Mice, B6C3F1, Exposure to 0, 63, 125, 250, Final mean body weight of the NOEL = 500 NTP, 1986
10 per sex 500, or 1000 ppm (0, 258, 512, highest-dose males was 7% lower ppm (2050
per group 1025, 2050, or 4100 mg/m3) than controls. mg/m3)
methyl methacrylate, 6 hours/day,
5 days/week, for 14 weeks (64 LOEL = 1000
exposures). Histological ppm (4100
examination of an unspecified mg/m3)
range of tissues in all mice of
the highest-dose and control
groups, all animals that died
before the end of the study, and
some mice in the other groups.
Mice, B6C3F1, Exposure to 0, 63, 125, 250, Some clinical signs and one NOEL = 250 Rohm & Haas,
10 per sex 500, or 1000 ppm (0, 258, 512, death in the group exposed to ppm (1025 1977
per group 1025, 2050, or 4100 mg/m3) 500 ppm, but not dose-related. mg/m3)
methyl methacrylate, 6 Body weights of males receiving
hours/day, for 64 days. Complete the two highest doses were LOEL = 500
gross pathological and significantly decreased during ppm (2050
histopathological examinations. weeks 11-13 (500 ppm) and weeks mg/m3)
6, 11, and 12 (1000 ppm). In
female mice, the total body
weight changes were statistically
significantly lower in animals
exposed to 500 ppm but not to 1000
ppm.
Table 1 (continued)
Species Study design Effects Effect levels Comments Reference
Mice, B6C3F1, Exposure to 0, 500, 1000, 2000, The final mean body weights of LOEL = 500 NTP, 1986
10 per sex 3000, or 5000 ppm (0, 2050, all groups of exposed mice were ppm (2050
per group 4100, 8200, 12 300, or 20 500 lower than controls. Deaths at mg/m3)
mg/m3) methyl methacrylate, 6 2000 ppm and above. Renal
hours/day, 5 days/week, for 14 cortical necrosis, cortical
weeks. Histological examinations tubular degeneration and/or focal
of tissues from the major organs mineralization, nasal cavity
of all mice in the highest-dose inflammation with necrosis, and
and control groups and mice that loss of olfactory epithelium at
died before the end of the 2000-5000 ppm in males and extensive
study, of the lung and nasal liver necrosis in males exposed
turbinates of the males and the to 5000 ppm. Inflammation of the
nasal membranes of all females nasal turbinates in females
in the 2000 and 3000 ppm groups, exposed to 2000 ppm and above.
and of the liver of the males Metaplasia of the nasal epithelium
in the 2000 ppm group. At 1000 in all exposed mice.
ppm, the nasal turbinates from
both sexes and brain from the
males were also histologically
examined.
Mice, B6C3F1, Exposure to 0, 2050, or 4100 Decrease in mean body weights; LOEL = 500 NTP, 1986; Chan
50 per sex mg/m3 methyl methacrylate, 6 localized histopathological ppm (2050 et al., 1988
per group hours/day, 5 days/week, for effects (inflammation and mg/m3)
102 weeks. Histological degeneration of the olfactory
examination of a comprehensive epithelium) in the nasal
range of tissues. epithelium.
Table 1 (continued)
Species Study design Effects Effect levels Comments Reference
Golden Exposure to 0, 25, 100, or 400 Decreased body weights; increased LOEL = 400 Rohm & Haas,
hamsters, 56 ppm (0, 102.5, 410, or 1640 mortality. ppm (1640 1979b
per sex per mg/m3) methyl methacrylate, 6 mg/m3)
group hours/day, 5 days/week, for 18
months. Haematological analysis NOEL = 100
and gross and microscopic ppm (410
examination of a comprehensive mg/m3)
range of tissues.
Golden Exposure to 0, 25, 100, or 400 No exposure-related toxic NOEL = 400 Abstract only Smith et al.,
hamsters, ppm (0, 102.5, 410, or 1640 effects were observed. ppm (1640 1979
male and mg/m3) methyl methacrylate, 6 mg/m3)
female (number hours/day, 5 days/week, for 18
not specified) months. Evaluation of haemograms,
clinical chemistries, and urine,
as well as gross
histopathological examination.
Dogs, beagles, Exposure to 0, 100, or 400 ppm No significant differences in NOEL = 400 Tansy & Drees,
6 per group, (0, 410, or 1640 mg/m3) methyl systolic and diastolic blood ppm (1640 1979
sex methacrylate vapour, 6 pressure, ECG, heart and mg/m3)
unspecified hours/day, 5 days/week, for 3 respiratory rates, haematology,
months. Each dog had an external clinical chemistries, and
iliac artery catheter. Two dogs urinalysis; histopathological
from each group sacrificed at examination of the major organs
the end of the 3-month period; was unremarkable.
remaining dogs observed for
another month.
Table 1 (continued)
Species Study design Effects Effect levels Comments Reference
Dogs, Exposure to 0, 100, or 400 ppm No exposure-related toxic NOEL = 400 Abstract only Smith et al.,
beagles, male (0, 410, or 1640 mg/m3) methyl effects were observed. ppm (1640 1979
(number not methacrylate vapour, 6 mg/m3)
specified) hours/day, 5 days/week, for 3
months. Gross and
histopathological evaluations
in addition to evaluation of
haemograms, clinical chemistries
and urine, ECGs, and blood
pressure.
INGESTION
Rats (sex and Ingestion of 0, 1, 3, or 5 Rats in mid-dose group did not NOAEL = 3 Small group sizes; Deichmann-Gruebler
strain ml/kg body weight (0, 0.9, 2.8, gain as much weight as those in ml/kg body histopathological & Read, undated
unspecified, or 4.7 mg/kg body weight) low-dose group; animals in weight (2832 examination
groups of 5) orally by gavage, every second high-dose group died before the mg/kg body unspecified
day for 70 days. Urine samples 4th treatment. All high-dose rats weight)
from rats of all groups were had distended bladders filled
periodically collected and with blood; a moderate degree of
examined for blood. cellular degeneration in the
Histopathological examinations liver, but without necrosis or
unspecified. fibrosis; renal effects
(haemorrhages in the tubules,
marked hyperaemia, and
degeneration of the tubular
epithelium).
Table 1 (continued)
Species Study design Effects Effect levels Comments Reference
Rats, Wistar, Ingestion of 0, 6, 60, or 2000 Increase in relative kidney NOEL = 60 ppm Borzelleca et
25 per sex ppm (mg/litre) (equivalent to weight in females only. (5 mg/kg body al., 1964
per group 0, 0.4, 4, and 121 mg/kg body weight per day)
weight per day for males; and
0, 0.5, 5, and 146 mg/kg body NOAEL = 2000
weight per day for females) ppm (146 mg/kg
methyl methacrylate in body weight per
drinking-water for 2 years. day)
(Groups received 6 and 60 ppm
for 5 months, then the
concentrations were increased
to 7 and 70 ppm for the remainder
of the 2 years.)
Histopathological examination of
a wide range of tissues from
mid- and high-dose groups.
Limited haematological and urine
analyses conducted.
Dogs, Ingestion of 0, 10, 100, or No treatment-related effects. NOEL = 1500 Extremely small Borzelleca et
beagles, 2 1000 ppm (mg/kg) methyl ppm (38 mg/kg number of animals al., 1964
per sex per methacrylate in corn oil in body weight
group the diet (high dose gradually per day)
increased to 1500 ppm
[equivalent to about 38 mg/kg
body weight per day] at week
9) for 2 years.
Histopathological examination
of a wide range of tissues.
Limited haematological and
urine analyses conducted.
8.4.1 Subchronic exposure
In most subchronic studies conducted to date, rats and mice have
been exposed to methyl methacrylate by inhalation. Effects observed
most commonly in these investigations were decreases in body weight
gain and irritation of the skin, nasal cavity, and eye at high
concentrations (generally >500 ppm [2050 mg/m3]) (Rohm & Haas,
1977; NTP, 1986). At higher concentrations, other effects, such as
renal cortical necrosis and tubular degeneration (rats and mice) and
hepatic necrosis (mice), have also been reported (Tansy et al., 1980a;
NTP, 1986; Deichmann-Gruebler & Read, undated).
On the basis of decreases in final mean body weight and squamous
metaplasia at the site of entry (i.e. nasal epithelium), the lowest
reported NOEL and lowest-observed-effect level (LOEL) in a subchronic
inhalation bioassay in which several concentration levels were
administered were 250 and 500 ppm (1025 and 2050 mg/m3),
respectively, in mice exposed to methyl methacrylate for 64 days or 14
weeks (Rohm & Haas, 1977; NTP, 1986). Except for effects at the site
of entry, histopathological changes have not been observed in the two
most extensive subchronic bioassays in rats exposed to methyl
methacrylate for 65 days or 14 weeks, at concentrations up to 1000 ppm
(4100 mg/m3) (Rohm & Haas, 1977; NTP, 1986).
In less extensive and less well documented studies conducted by
Tansy et al. (1976, 1980a,b), effects on the trachea and some
indications of liver damage in rats were observed at the only tested
concentration of 116 ppm (476 mg/m3), administered for 7 hours/day
for 3 or 6 months, although the statistical significance of the
pulmonary changes was not specified, and similar effects were observed
in some of the sham-exposed control animals. In a supplementary
study, there was weak evidence of an effect on liver function
(barbiturate sleeping time) in male rats administered "intermittent
daily exposures" of 100 ppm (410 mg/m3) methyl methacrylate for a
total of 160 hours (Tansy et al., 1980b). Initial reports of reduced
fat deposits after exposure for 3 months were not confirmed in later
studies of similar protocol by the same investigators (Tansy et al.,
1980a,b).
8.4.2 Chronic exposure and carcinogenicity
In the few studies identified in which the chronic toxicity and
carcinogenicity of methyl methacrylate were investigated, the observed
effects were, in general, similar to those reported in short-term and
subchronic studies and included inflammation and epithelial
hyperplasia of the nasal cavity and degeneration of the olfactory
sensory epithelium. Based on the results of a well documented
inhalation study in F344/N rats and B6C3F1 mice reported by the NTP
(1986) and Chan et al. (1988), there was no evidence of
carcinogenicity of methyl methacrylate for groups of 50 male F344/N
rats and 50 male and 50 female B6C3F1 mice exposed to 500 or 1000 ppm
(2050 or 4100 mg/m3) and groups of 50 female rats exposed to 250 or
500 ppm (1025 or 2050 mg/m3) for 2 years. Based on inflammation and
degeneration of the olfactory epithelium in the nasal cavity
(accompanied by variable atrophy of the nerve bundles in the submucosa
and, in the most severely affected areas, replacement of sensory
neuroepithelial cells with respiratory epithelium) and minimal
increases in the numbers of alveolar macrophages in the nasal cavity
at all dose levels, the LOEL in rats was considered to be 250 ppm
(1025 mg/m3). In mice, the LOEL was considered to be 500 ppm (2050
mg/m3) on the basis of lower mean body weights in exposed animals and
localized histopathological effects at the site of entry (including
inflammation and degeneration of the olfactory epithelium).
In earlier studies conducted for Rohm & Haas (1979a,b), no
treatment-related increases in tumour incidence occurred in either
groups of 56 male and 56 female golden hamsters or groups of 70 male
and 70 female albino F344 rats exposed to 0, 25, 100, or 400 ppm (0,
102.5, 410, or 1640 mg/m3) methyl methacrylate 6 hours/day, 5
days/week, for 18 months and 2 years, respectively. At the highest
concentration, body weight decreased significantly in both species,
mortality increased in hamsters, and the incidence of mild rhinitis in
the nasal mucosa increased slightly in rats.
A histopathological review of the nasal tissues from the rats in
the above-mentioned Rohm & Haas (1979a) study was commissioned by the
US Methacrylate Producers Association (Lomax, 1992; Lomax et al.,
1997). The review consisted of microscopic examination of nasal
tissue from at least 10% of randomly selected rats from each group,
and the slides evaluated included the original study slides plus
slides from tissue sections taken deeper into the block. The tissues
from male and female rats that had been exposed to 25 ppm (102.5
mg/m3) methyl methacrylate for 2 years were morphologically similar
to those of controls. Rats exposed to 100 or 400 ppm (410 or 1640
mg/m3) methyl methacrylate had exposure-related and concentration-
dependent microscopic changes in the olfactory epithelium lining the
dorsal meatus in the anterior region of the nasal cavity. The
microscopic changes consisted of degeneration/atrophy of the olfactory
epithelium and underlying Bowman's glands, hyperplasia of basal
(reserve) cells, replacement of olfactory epithelium by ciliated
(respiratory-like) epithelium, and inflammation of the mucosa and/or
submucosa (Lomax et al., 1997). Changes in the respiratory epithelium
were observed only at the high concentration (400 ppm [1640 mg/m3])
and were limited to hyperplasia of the submucosal gland and/or goblet
cells in the anterior region of the nasal cavity. The squamous
epithelium of the nasal cavity was not affected. The lesions tended
to be bilateral in distribution in rats exposed to both 100 and 400
ppm (410 and 1640 mg/m3) methyl methacrylate. A small nasal polypoid
adenoma was observed in one male from both the 100 and 400 ppm (410
and 1640 mg/m3) exposure groups. Based on this re-examination, the
NOEL and LOEL are considered to be 25 ppm (102.5 mg/m3) and 100 ppm
(410 mg/m3), respectively.
Data available on the effects of methyl methacrylate following
ingestion are limited. In an early study (Borzelleca et al., 1964) in
which organ to body weight ratios were determined and
histopathological examination of a wide range of tissues as well as
limited haematological and urine analyses were conducted, the relative
kidney weight was increased in a small group of female rats (n = 25)
exposed to 2000 ppm (mg/litre) methyl methacrylate in drinking-water
for 2 years. This effect was not observed in the males, and
histopathological examination revealed no damage. The authors also
reported a decrease in fluid consumption in rats exposed to 2000 ppm.
The no-observed-adverse-effect level (NOAEL) was therefore considered
to be 2000 ppm (equivalent to a dose of about 146 mg/kg body weight
per day for females and 121 mg/kg body weight per day for males, based
on intake and body weight data presented by the authors). There were
no treatmentrelated effects, based upon gross or histopathological
examination, in extremely small groups of beagle dogs (n = 2)
exposed to concentrations of up to 1500 ppm (mg/kg) methyl
methacrylate (equivalent to a dose of about 38 mg/kg body weight per
day) in their feed for 2 years (Borzelleca et al., 1964).
8.5 Genotoxicity and related end-points
Results of available genotoxicity studies on methyl methacrylate
are summarized in Table 2. In a number of well conducted in vitro
studies with precautions taken to limit evaporation, methyl
methacrylate was not mutagenic in Salmonella typhimurium with or
without metabolic activation. In a single study (Poss et al., 1979),
results were positive at clearly cytotoxic concentrations in the
presence of metabolic activation in a poorly validated forward
mutation assay in S. typhimurium TM677; results were negative in the
absence of metabolic activation.
Methyl methacrylate has been mutagenic and clastogenic in
mammalian cells in culture. It induced gene mutation in mouse
lymphoma L5178Y cells without metabolic activation in five
investigations and was positive with metabolic activation in all of
the three investigations in which it was examined. Results for
chromosomal aberrations and micronucleus formation were also positive
in this cell line without metabolic activation at concentrations at
which there was poor cell survival (Doerr et al., 1989). An increase
in chromosomal aberrations and sister chromatid exchanges in Chinese
hamster ovary cells has also been observed in the presence and absence
of metabolic activation in assays conducted in two laboratories (NTP,
1986; Anderson et al., 1990).
In in vivo studies conducted to date, there has been limited
evidence of genotoxicity. In an early study in which rats were
exposed to methyl methacrylate as either a single 2-hour exposure or
for 5 hours/day for 5 days at concentrations up to 9000 ppm (36 900
mg/m3), there were small but significant increases in chromosomal
aberrations in bone marrow cells from rats exposed to the highest
concentration in the multiple-exposure study (Anderson & Richardson,
1976). Although of questionable biological significance, small
increases in gaps were also noted at the two highest concentrations.
In a follow-up study with a larger number of intermediate dose levels,
there were significant increases in chromosomal aberrations following
both single and repeated exposures (Anderson et al., 1979); although
there was no clear dose-response, the pattern of effect may have been
attributable to chemically induced cell cycle delay (Anderson et al.,
1979). The maximum concentration tested in the follow-up study (1000
ppm [4100 mg/m3]) caused significant reductions in the mitotic
activity in the bone marrow of all exposed animals. Results were
negative in a well conducted dominant lethal assay in which mice were
exposed to concentrations of methyl methacrylate up to 9000 ppm
(36 900 mg/m3) 6 hours/day for 5 days (Anderson & Hodge, 1976).
No significant increase in the incidence of micronuclei was
observed in the bone marrow of mice following a single administration
of methyl methacrylate by gavage at doses up to 4.52 g/kg body weight
or in an additional investigation with one dose group that was exposed
to 1.13 g/kg body weight per day for 4 days; however, cells were
harvested at one time point (24 hours) only, and there was no evidence
of toxicity in the target tissue (Hachitani et al., 1981). Negative
results of an additional in vivo micronucleus assay in mice do not
contribute to an assessment of the weight of evidence of genotoxicity
owing to inadequate dose levels (Jensen et al., 1991). Available data
in the published accounts were inadequate to allow the assessment of
the mixed results of two additional studies in which chromosomal
aberrations in bone marrow cells of rats were examined following
intraperitoneal administration of methyl methacrylate (Fedyukovich et
al., 1988; Fedyukovich & Egorova, 1991).
Although not mutagenic in bacterial systems in vitro, methyl
methacrylate has induced mutation and chromosomal aberrations in
mammalian cells in vitro. In in vivo inhalation studies in which
there has been clear evidence of toxicity within the target tissue,
there has been limited evidence of genotoxicity of methyl
methacrylate.
8.6 Reproductive and developmental toxicity
In a well conducted study in Crl:CDBR rats, there was no
embryotoxicity or fetotoxicity and no increase in the incidence of
malformations or variations following exposure for 6 hours/day on days
6-15 of gestation to concentrations of methyl methacrylate that ranged
from 99 to 2028 ppm (406-8315 mg/m3; NOEL = 8315 mg/m3). However,
there were treatment-related effects on maternal body weight at all
concentrations (Solomon et al., 1993). In an earlier study in which
pregnant ICR mice were exposed to 1330 ppm (5450 mg/m3) methyl
methacrylate for 2 hours twice daily during days 6-15 of pregnancy,
there were no developmental effects. Maternal toxicity was not
addressed in the report (McLaughlin et al., 1978).
Table 2: Genetic effects (adapted from IARC, 1994).
Resultsb
Without With
exogenous exogenous
Dosea metabolic metabolic
Test system End-point (LED/HID) system system Reference
Salmonella typhimurium TM677 Forward mutation 5000 - + Poss et al., 1979
Salmonella typhimurium TA100 Reverse mutation 500 - - Lijinsky & Andrews, 1980
5000 - - Hachitani et al., 1981
2300 - - Waegemaekers & Bensink, 1984
5000 - - Zeiger et al., 1987
25 mg/plate - - Schweikl et al., 1994
Salmonella typhimurium TA1535 Reverse mutation 500 - - Lijinsky & Andrews, 1980
2300 - - Hachitani et al., 1981
5000 - - Waegemaekers & Bensink, 1984
1700 - - Zeiger et al., 1987
Salmonella typhimurium TA1537 Reverse mutation 500 - - Lijinsky & Andrews, 1980
2300 - - Hachitani et al., 1981
5000 - - Waegemaekers & Bensink, 1984
5000 - - Zeiger et al., 1987
Salmonella typhimurium TA1538 Reverse mutation 500 - - Lijinsky & Andrews, 1980
2300 - - Hachitani et al., 1981
5000 - - Waegemaekers & Bensink, 1984
Salmonella typhimurium TA98 Reverse mutation 500 - - Lijinsky & Andrews, 1980
2300 - - Hachitani et al., 1981
5000 - - Waegemaekers & Bensink, 1984
5000 - - Zeiger et al., 1987
25 mg/plate - - Schweikl et al., 1994
Table 2 (continued)
Resultsb
Without With
exogenous exogenous
Dose metabolic metabolic
Test system End-point (LED/HID) system system Reference
Salmonella typhimurium TA97 Reverse mutation 1700 - - Zeiger et al., 1987
Salmonella typhimurium TA97a 25 mg/plate - - Schweikl et al., 1994
Salmonella typhimurium TA102 25 mg/plate - - Schweikl et al., 1994
Salmonella typhimurium TA104 25 mg/plate - - Schweikl et al., 1994
Mouse lymphoma L5178Y cells Gene mutation (tk locus) 2200 + 0 Doerr et al., 1989
in vitro 2000 + 0 Moore et al., 1988
250 + Myhr et al., 1990
500 + Myhr et al., 1990
500 + + Dearfield et al., 1991
117.5 (0.125 µl/ml) + + NTP, 1986
Mouse lymphoma L5178Y cells Micronucleus formation 2200 (+) 0 Doerr et al., 1989
in vitro
Chinese hamster ovary cells Sister chromatid exchange 16 + + Anderson et al., 1990
in vitro 750 + NTP, 1986
500 + NTP, 1986
Chinese hamster ovary cells Chromosomal aberrations 1600 + (+) Anderson et al., 1990
in vitro 5000 +c NTP, 1986
1600 +c NTP, 1986
Mouse lymphoma L5178Y cells Chromosomal aberrations 2200 (+) 0 Doerr et al., 1989
in vitro
Human lymphocytes in vitro Sister chromatid exchange 0.1 ? 0 Cannas et al., 1987
Table 2 (continued)
Resultsb
Without With
exogenous exogenous
Dose metabolic metabolic
Test system End-point (LED/HID) system system Reference
Mouse bone marrow cells Micronucleus formation <4.52 g/kg body - Hachitani et al., 1981
in vivo weight x 1 p.o.d
1.13 g/kg body - Hachitani et al., 1981
weight x 4 p.o.d
Rat bone marrow cells in vivo Chromosomal aberrations 36 900 mg/m3, - Anderson & Richardson, 1976
2 hour x 1 inhal.
36 900 mg/m3, + Anderson & Richardson, 1976
5 hours/day,
5 days inhal.
4100 mg/m3, 2 hour Equivocal Anderson et al., 1979
x 1 inhal.
4100 mg/m3,
5 hours/day, Equivocal Anderson et al., 1979
5 days inhal.
Male mice in vivo Dominant lethal assay <36 900 mg/m3, - Anderson & Hodge, 1976
6 hours/day,
5 days inhal.
Table 2 (continued)
a In vitro tests, µg/ml; in vivo tests, mg/kg body weight; LED = lowest effective dose; HID = highest ineffective dose.
b +, positive; (+), weak positive; -, negative; 0, not tested; ?, inconclusive (variable response within several experiments
within an adequate study). Negative results of an additional in vivo micronucleus assay in mice do not contribute to an assessment
of the weight of evidence of genotoxicity owing to inadequate dose levels (Jensen et al., 1991). Available data in the published
accounts were inadequate to permit an assessment of the mixed results of two additional studies in which chromosomal aberrations
in bone marrow cells of rats were examined following intraperitoneal administration (Fedyukovich et al., 1988; Fedyukovich &
Egorova, 1991).
c 5% of cells affected without exogenous metabolic system; 30% of cells affected with exogenous metabolic system.
d No toxicity in target tissue. p.o. = per os.
In a study reported only in the form of an abstract, a number of
effects, including intrauterine deaths, an increase in the number of
fetuses with vascular pathology, and an increase in the frequency of
"functional immaturity," were observed in the offspring of rat dams
exposed to concentrations of methyl methacrylate as low as 0.01 mg/m3
(Farmakovskaya & Tikhomirov, 1993). The information presented in the
published account of this study is inadequate to permit assessment of
the protocol and results.
In early studies, developmental effects, including decreases in
fetal weights, embryo-fetal deaths, and skeletal abnormalities, were
observed in rats following inhalation of concentrations of methyl
methacrylate that were toxic to the dams (Hodge & Palmer, 1977;
Nicholas et al., 1979). Similar effects were reported in studies in
mice in which maternal toxicity was not addressed (Tansy, 1975) and in
studies in rats in which the protocol and results were not well
documented (Luo et al., 1986).
Data on reproductive effects are limited to a dominant lethal
assay and examination of gonads in repeated-dose toxicity studies.
There was no reduction in fertility as measured by the number and
percentage of successful matings each week or the percentage of female
mice that become pregnant in a dominant lethal assay in mice exposed
to 100, 1000, or 9000 ppm (410, 4100, or 36 900 mg/m3) methyl
methacrylate by inhalation for 6 hours/day for 5 days (Anderson &
Hodge, 1976).
Adverse effects on the reproductive organs of experimental
animals have not been observed in repeated-dose studies in animals
exposed to methyl methacrylate (see sections 8.3 and 8.4).
8.7 Immunological and neurological effects
In a study in which the leukocyte migration inhibition method was
employed to determine if methyl methacrylate was potentially a
causative agent in denture stomatitis, three groups of five albino
rabbits of both sexes were injected intramuscularly with 1 ml of
methyl methacrylate on days 1, 5, and 14 (Zafiropoulos et al., 1985).
On the 36th day, blood was drawn to test the inhibition of leukocyte
migration. The results indicated that methyl methacrylate was a
specific antigen that was capable of inducing cellular immune
reaction.
Methyl methacrylate markedly impaired locomotor activity and
learning while significantly increasing aggressive behaviour in male
rats orally administered the chemical at 500 mg/kg body weight for 21
days (Husain et al., 1985). There was an overall increase in levels
of biogenic amine in the pons-medulla and hippocampus. Levels of
noradrenaline in the cerebral cortex and 5-hydroxytryptamine in the
mid-brain and the hypothalamus were increased, whereas there was a
slight decrease in dopamine levels in the corpus striatum (Husain et
al., 1985). In a separate study under the same experimental
conditions, a significant increase in cholesterol (26%) and
triglycerides (65%) and a slight decrease in the total phospholipid
content of the sciatic nerve were noted (Husain et al., 1989).
In a study investigating the neurotoxic effects of acrylamide, no
evidence of neurotoxicity (evaluated as observation of ataxia) or
enhancement of acrylamide neuropathy was observed in male rats fed a
diet containing 18 800 ppm (mg/kg) methyl methacrylate for 5 weeks
(the intake of methyl methacrylate was estimated to be 410 mg/day)
(Edwards, 1975). Other limited studies that have been identified do
not contribute to our understanding of the neurotoxicity of methyl
methacrylate (Innes & Tansy, 1981; Wynkoop et al., 1982; Kanerva &
Verkkala, 1986).
9. EFFECTS ON HUMANS
Data on effects of methyl methacrylate on humans are informative
primarily with respect to irritation and sensitization (for exposure
both dermally and by inhalation), respiratory effects, and
carcinogenicity; however, in cross-sectional epidemiological studies
conducted to date, effects on the nervous (Seppalainen & Rajaniemi,
1984; Schwartz et al., 1989) and cardiac (Cromer & Kronoveter, 1976;
NIOSH, 1976) systems have also been examined.
Hypotension, changes in pulse rate, and cardiac arrest have been
reported following bone replacement surgery with polymethyl
methacrylate cemented prostheses; however, the significance of these
observations with respect to methyl methacrylate exposure is
questionable owing to lack of correlation between peak plasma
concentrations of methyl methacrylate and reported effects and the
absence of similar effects in younger patients (Government of Canada,
1993; Cary et al., 1995; ECETOC, 1995).
9.1 Case reports
There are reports of skin irritation and sensitization in human
volunteers and in patients suspected of occupational sensitization to
acrylates from exposure to dental materials or anaerobic sealants
(Spealman et al., 1945; Estlander et al., 1984; Kassis et al., 1984;
Rajaniemi & Tola, 1985; Conde-Salazar et al., 1988; Kanerva et al.,
1988, 1989; Farli et al., 1990; Guerra et al., 1993). Occupational
asthma associated with methyl methacrylate has also been reported
(Lozewicz et al., 1985; Pickering et al., 1986, 1993); however, there
is no conclusive evidence that methyl methacrylate is a respiratory
sensitizer, and the possibility of a non-specific response due to
respiratory tract irritation cannot be excluded.
9.2 Epidemiological studies
Protocols and results of cross-sectional studies in which
respiratory effects of methyl methacrylate have been investigated in
occupationally exposed populations are presented in Table 3. For
example, in a study in which smoking was taken into account, an
increase in the prevalence of chronic cough (as evaluated by
questionnaire) was observed in a small group of workers (n = 40)
exposed exclusively to methyl methacrylate for at least 5 years in two
factories (mean atmospheric levels of methyl methacrylate in the two
factories were 18.5 and 21.6 ppm [75.8 and 88.6 mg/m3]) compared with
controls engaged in similar job categories, but without exposure to
methyl methacrylate (Marez et al., 1993). Spirometric values did not
differ before the work shift, but two of nine parameters decreased
during the work shift. Information concerning exposure to other
respiratory irritants was not provided; although increased cough and
mild airway resistance correlated with exposure to methyl
methacrylate, peak versus mean exposures were not examined. In other
studies in which there was some quantitative information on exposure,
results have varied, with effects on respiratory function being
observed in some cases at mean concentrations as low as 11 mg/m3
(Jedrychowski, 1982) and no effects in other investigations at
time-weighted-average concentrations up to 40-50 ppm (164-205 mg/m3)
(Cromer & Kronoveter, 1976; NIOSH, 1976; Röhm, 1994). It is
difficult, however, to draw meaningful conclusions concerning levels
of exposure that induced effects in these studies, as there was little
attempt to assess mean versus peak exposures. Moreover,
interpretation of several of the investigations is complicated by
concomitant exposure of the examined populations to other substances.
In other investigations reported to date, quantitative data on
exposure of workers to methyl methacrylate were not included (Andrews
et al., 1979; Schwartz et al., 1989). An additional cross-sectional
study of the prevalence of disorders of smell in methyl
methacrylate-exposed workers is under way (A. Muttray, personal
communication, 1997).
Table 3: Cross-sectional epidemiological studies - respiratory effects
Protocol Results Reference
Study population composed of 40 workers from two factories who An increase in the prevalence of chronic cough Marez et al., 1993
were exposed to methyl methacrylate for >5 years and 45 controls observed in exposed workers compared with controls
engaged in similar job categories but without exposure to methyl (p = 0.04). This difference remained significant
methacrylate. Mean atmospheric concentrations of methyl after adjustment for smoking (p = 0.03). Airway
methacrylate at the two factories were 18.5 ppm (75.9 mg/m3) resistance increased during the 8-hour work shift
(range 9-32 ppm [36.9-131.2 mg/m3]) and 21.6 ppm in workers exposed to methyl methacrylate (as
(88.6 mg/m3) (range 11.9-38.5 ppm [48.8-157.9 mg/m3]). measured by MEF50 [p = 0.04] and MEF50/MEF
Smoking history and information on the presence of respiratory [p = 0.0)). The obstruction was mild, and forced
symptoms were gathered by means of a questionnaire. Respiratory expiratory volume in one second (FEV,) did not
measurements (maximum expiratory flow volume [MEFV], decrease during the work shift.
forced vital capacity [FVC], forced expiratory volume [FEV])
were performed by means of a spirometer: one before the
working shift, and the second in the last 2 hours of the 8-hour
shift.
Ninety-one exposed and 43 non-exposed workers were evaluated at No significant differences were observed for Cromer & Kronoveter,
five plants manufacturing polymethyl methacrylate sheets. For respiratory function, chronic liver and 1976
exposed workers, 8-hour time-weighted-average concentrations of gastrointestinal effects, skin and allergic
methyl methacrylate were between 4 and 49 ppm (16.4-200.9 problems, blood pressure and pulse rate, white
mg/m3). Evaluation of chronic effects was conducted through an blood cell count, and haemoglobin values. The
extensive questionnaire, a comparison of mean blood pressure only parameters for which effects were observed
values with predicted values from the 1971-1972 US National were serum glucose, blood urea nitrogen, cholesterol,
Health Survey, and results of pulmonary function tests, albumin, and total bilirubin values, although the
haemoglobin and white blood cell counts, urinalysis, and blood implication of these effects remains unclear.
chemistry. Although not statistically significant, the data also
"suggested possible alterations in skin and nervous
system symptomatology, urinalysis findings, and
serum triglycerides."
Employees of the Rohm & Haas Co. (which manufactures acrylic Upon cross-sectional analysis, when the age, ethnic Schwartz et al., 1989
acid, acrylates, and methacrylates) - 618 males and 113 females group, and smoking status were considered, the mean
(mean age 42.9 years), out of the total number of 909 short- and UPSIT scores in the four exposure groups did not
long-term employees - were asked to complete a University of differ. For the "no significant chemical exposures,"
Pennsylvania Smell Identification Test (UPSIT) and "exposure to other chemicals," "exposure to low
questionnaires on job histories as well as personal and medical levels of acrylate/methacrylate," and "exposure to
information. Employees were grouped into four exposure higher levels of acrylate/methacrylate" groups, the
categories: no significant chemical exposures (n = 319), exposure scores were 37.8, 37.4, 37.0, and 37.6, respectively.
to other chemicals (n = 193), exposure to low levels of Based on logistic regression analysis, adjusting for
acrylate/methacrylate (n = 164), and exposure to higher levels of multiple confounders, in the nested case-control
acrylate/methacrylate (n = 55). In a nested case-control study, 77 study, the odds ratios for the association of UPSIT
workers who scored below the 10th percentile in their age group score with exposure to methyl methacrylate for all
on the UPSIT were matched with controls (scored at or above the workers was 2.8 (95% CI 1.1-7.0) and for those who
50th percentile). Exposure was classified in terms of whether never smoked was 13.5 (95% CI 2.1-87.6); the crude
workers had been exposed to methyl methacrylate for at least 6 odds ratios were 2.0 and 6.0, respectively. There
weeks, the total time of employment at the plant, and a cumulative was a dose-response relationship between olfactory
exposure score - a semi-quantitative index of lifetime exposure dysfunction and the cumulative exposure. The odds
to the acrylates - for each worker. ratios increased with the cumulative exposure scores,
except for a decrease in the highest exposure
category. The olfactory dysfunction may be
reversible, as the odds ratios decreased with the
length of time since the last exposure.
Four hundred and fifty-four males from a plant (Plant A) There was a non-significantly lower occurrence of Jedrychowski, 1982
producing styrene and methyl methacrylate were compared with bronchitis and/or asthma in the exposed (17.8%)
683 males from a plant producing carbon derivatives who served compared with the control (19.5%) group. There was
as controls (jobs were similar in both plants, but there was no no significant difference in the incidence of
exposure to styrene or methyl methacrylate in the latter plant). chronic chest symptoms between the two groups.
Standardized interviews on chest symptoms, measured heights, However, the frequency of lung obstruction was over
lung function tests, and examinations for chronic bronchitis and twice as high in the exposed workers (45.4% vs
asthmatic syndrome were conducted. The workers were divided 18.0%); this percentage was higher for smokers than
into the following groups: non-smokers, ex-smokers, and current for non-smokers (20.9% vs 13.6%). Within the
smokers. Styrene and methyl methacrylate concentrations were exposed group, the occurrence of lung obstruction
determined in 18 workplaces in Plant A. For methyl methacrylate, in smokers and in non-smokers did not differ
the mean concentration in Plant A was 11 mg/m3. significantly. Fifty-six per cent of the controls and
76% of the exposed workers with lung obstruction
did not have any chronic chest symptoms. The lung
function of the exposed group was significantly
poorer than that of the controls; the effects were
slightly worse among smokers in both groups. The
relative risk of lung obstruction (compared with
non-exposed ex- and non-smokers) was 1.7 for non-
exposed smokers, 4.7 for exposed ex- and
non-smokers, and 5.5 for exposed smokers.
Five hundred and two dental students (who handled methyl In exposed students, 6% reported respiratory Andrews et al., 1979
methacrylate in their laboratories) completed self-administered symptoms associated with exposure to methyl
multiple-choice questionnaires concerning their past histories methacrylate (88% had histories of asthma or
and any symptoms (not specified) associated with activities in the allergic rhinitis), and 5% when using high-speed
lab. Spirometric tests were performed before and after exposure to drills. Among the 77 students who underwent
unreported amounts of methyl methacrylate for 77 students who spirometric tests, there was no significant change
had allergic rhinitis, smoked, or had symptoms upon usual in symptoms or spirometry.
exposure.
A study of 91 exposed and 43 non-exposed workers from five Some significant differences in terms of coughing NIOSH, 1976
methyl methacrylate cast sheet manufacturing plants in the USA. and expectoration, but these were likely due to
The survey included a medical questionnaire, measurement of differences in smoking habits. When smoking
clinical symptoms, blood pressure, and pulse rate, testing of histories were taken into consideration, there was
pulmonary function and blood chemistry, urinalysis, and white no significant change in pulmonary function among
blood cell counts. Based on 8-hour time-weighted-average the exposure groups. No significant differences in
exposures to methyl methacrylate, workers were divided into five blood pressure or in white blood cell count were
categories: <5 ppm (20.5 mg/m3) (n = 13), 5-25 ppm (20.5-102.5 found. There were several significant differences
mg/m3) (n = 20), 25-50 ppm (102.5-205 mg/m3) (n = 33), no in the blood chemistry tests of the "no current
current exposure but past exposure >1 year (n = 25), and the exposure" group, but this was likely due to the
control group with no exposure (n = 43). The ages and smoking fact that they were significantly older than the
histories of exposure groups were not matched very well because controls.
of the low number of volunteers.
A cross-sectional study involving 211 workers at a polymethyl There were no significant respiratory effects Röhm, 1994
methacrylate sheet producing factory in Germany. The study associated with exposure in any of the groups.
report period was 1991-1993. Working areas were classified into There were some observations of eye and respiratory
the following exposure ranges: 3-10 ppm (12.3-41 mg/m3), 10-20 tract irritation, which were reported to be
ppm (41-82 mg/m3), 20-30 ppm (82-123 mg/m3), and 30-40 ppm transient and were limited to short-term exposures
(123-164 mg/m3) (8-hour time-weighted averages; ranges (5-15 minutes in duration) at concentrations
represent geometric means). The numbers of persons in each exceeding 100 ppm (410 mg/m3).
exposure group were 7, 128, 20, and 56, respectively. The
examination of the workers consisted of a self-administered
questionnaire (concerning lifestyle, occupation, and medical
history, with emphasis on complaints of nose, throat, and
respiratory system failures and allergic reactions, including
skin and asthmatic reactions) as well as a visual examination
of the nasal cavity.
Owing to an excess of mortality from colon cancer observed in
early investigations in exposed workers, several historical cohort
studies have been conducted to examine the mortality rate from cancer
of the colon or rectum among male workers employed at two plastics
manufacturing plants in Bristol, Pennsylvania, and Knoxville,
Tennessee (DeFonso & Maher, 1981, 1986; Maher & DeFonso, 1987a,b;
Walker et al., 1991). An additional cohort study of workers at a
small number of polymethyl methacrylate sheet production factories in
the United Kingdom has also been identified (Tomenson & Bonner, 1994;
Cary et al., 1995); however, documentation available at this time is
inadequate for evaluation. In the most recent and extensive follow-up
by Walker et al. (1991) in the above-mentioned plastics manufacturing
plants, data were reanalysed as a function of the period of employment
of the workers. In this investigation, the two cohorts were composed
of 10 482 men who had worked during the period 1933-1982 in the
Bristol plant and 3381 men hired between 1943 and 1982 in the
Knoxville plant. The population of workers at the Bristol plant was
further divided into an early cohort (men employed at some time
between 1933 and 1945, inclusive) and late cohort (1946-1982,
inclusive). The early cohort worked in conditions that are thought to
have involved high exposures to the vapour phase of ethyl acrylate and
methyl methacrylate monomer, as well as to a variety of volatile
by-products of the ethyl acrylate/methyl methacrylate polymerization
process.
In the two cohorts with later dates of first hire (Knoxville and
late Bristol), there was no excess mortality due to cancer of the
colon or rectum. In the early (Bristol) cohort, there was an apparent
excess of deaths due to colon cancer (38 cases observed overall in
those persons who accumulated a dose of >0 units, compared with an
expected number of 25.4). Although the highest risk was in the
subgroup of workers with the highest cumulative exposure, there was no
trend of increasing risk with increasing exposure after allowing for a
long latency period. There was no systematic pattern of excess risk
of cancer at any other site. For respiratory cancer, however, there
was a significantly high standardized mortality ratio (of 1.44) in the
Knoxville cohort, with no excess in either of the Bristol cohorts.
Owing to the large number of statistical estimates in this study and
the absence of a clear dose-response trend, the association of methyl
methacrylate with respiratory cancer is unclear. The apparent excess
may have been due to statistical fluctuation or to confounding by
other occupational exposures in the environment at the time.
Collins et al. (1989) reported a limited study of a much smaller
cohort of workers exposed for considerably shorter periods to methyl
methacrylate at two plants that either manufactured methyl
methacrylate or used methyl methacrylate in other product manufacture
between 1951 and 1983. There was no excess mortality for any type of
cancer examined (Collins et al., 1989). There was a very weak
indication of an excess of rectal cancer (two cases in exposed workers
with the expected number much less than 1.0) and weak to moderate
indication of an excess risk of lung cancer (odds ratios of 4-5) in a
population-based study of a small number of workers exposed to methyl
methacrylate in Montreal, Canada (Siemiatycki, 1991).
Identified studies on the potential genotoxicity of methyl
methacrylate in occupationally exposed populations contribute limited
information. There was no increase in the number of sister chromatid
exchanges in the peripheral lymphocytes of 31 male workers
occupationally exposed to methyl methacrylate (mean value per 8 hours
ranged from 0.70 to 21.6 ppm [2.9-88.6 mg/m3]) in four factories
compared with that of 31 unexposed male workers of similar mean age
and smoking habits (Marez et al., 1991). The distribution frequency
of sister chromatid exchange, however, was significantly higher in the
group exposed to methyl methacrylate at peak concentrations ranging
from 114 to 400 ppm (467-1640 mg/m3), although the number of
individuals in this exposure subgroup was small (n = 6). Similarly,
no increase in the frequency of chromosomal aberrations was observed
in the peripheral lymphocytes of 38 male workers who were engaged in
organic glass production (polymethyl methacrylate plates) and exposed
to 8-hour, time-weighted-average concentrations of methyl methacrylate
of 0.9-71.9 ppm (3.7-295 mg/m3) (Seiji et al., 1994). The frequency
of sister chromatid exchange was higher in the exposed group than in
controls; however, this was considered to be due to a higher age
distribution in the exposed workers.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1 Aquatic environment
Bailey et al. (1985) studied the toxicity of methyl methacrylate
in juvenile bluegill sunfish at 22°C under static and flow-through
conditions of various durations (1-96 hours). The 96-hour LC50 under
flow-through conditions was 191 mg/litre, whereas LC50 values for
durations of 1-24 hours ranged from 420 to 356 mg/litre, respectively.
The 96-hour LC50 for rainbow trout under flow-through conditions was
>79 mg/litre, the highest concentration tested.
Sublethal/behavioural responses were noted among the fish in the 40
and 79 mg/litre concentration groups (Bowman, 1990).
The 24-hour EC50 for immobilization of Daphnia magna was 720
mg/litre, with extrapolated EC0 and EC100 values of 502 and 1042
mg/litre, respectively (Bringmann & Kuhn, 1982). The 24-hour LC50
was 1760 mg/litre, with extrapolated values for the LC0 and LC100 of
875 and 2500 mg/litre, respectively (Bringmann & Kuhn, 1977). The
threshold for onset of inhibition of cell multiplication was 447
mg/litre for the flagellate protozoan Entosiphon sulcatum after 72
hours of exposure (Bringmann, 1978). These studies were done in
static, open systems, with only nominal concentrations reported.
Thresholds for onset of inhibition of cell multiplication
following 8 days of exposure to methyl methacrylate were 120 mg/litre
for the blue-green alga Microcystis aeruginosa and 37 mg/litre for
the green alga Scenedesmus quadricauda at pH 7 (Bringmann & Kuhn,
1976, 1978a,b). The 96-hour LC50 for Selenastrum capricornutum was
170 mg/litre, with a NOEL of 100 mg/litre (Forbis, 1990). No studies
were identified on the effects of methyl methacrylate on higher
aquatic plants.
10.2 Terrestrial environment
Data on the toxicity of methyl methacrylate to terrestrial
animals are limited to a single study on soil microflora in which no
effects of biological significance were observed (Hossack & Thomas,
1992).
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Data on effects of methyl methacrylate in humans are informative
primarily with respect to irritation and sensitization (for exposure
both dermally and by inhalation) and carcinogenicity. Although there
are some quantitative data on exposure to methyl methacrylate in
available cross-sectional investigations of other end-points (NIOSH,
1976; Jedrychowski, 1982; Marez et al., 1993), they are considered
inadequate as the principal basis for hazard identification and
dose-response assessment owing to limitations of design and the
potential role of confounding factors. Data on hazard identification
and dose-response assessment for effects other than
irritation/sensitization and carcinogenicity are derived primarily,
therefore, from investigations in experimental animals.
The acute toxicity of methyl methacrylate is low. Irritation of
the skin, eye, and nasal cavity has been observed in rodents and
rabbits exposed to relatively high concentrations of methyl
methacrylate. This substance is a mild skin sensitizer in animals.
Methyl methacrylate is a mild skin irritant in humans and has the
potential to induce skin sensitization in susceptible individuals.
Although occupational asthma associated with methyl methacrylate has
also been reported, there is no conclusive evidence that methyl
methacrylate is a respiratory sensitizer.
The effect observed most frequently at lowest concentration after
repeated inhalation exposure of experimental animals to methyl
methacrylate is irritation of the nasal cavity. Effects on the kidney
and liver at higher concentrations have also been reported.
Limited available data indicate that methyl methacrylate is
unlikely to induce fetotoxic effects in the absence of maternal
toxicity. There has been no evidence of reproductive toxicity, based
on limited available data (a dominant lethal assay in mice and
examination of the gonads in repeated-dose toxicity studies). Based
on limited available data, neurological effects have been observed
following ingestion of doses greater than those that induce minimal
renal effects.
As a whole, the available epidemiological studies do not provide
strong or consistent evidence of a carcinogenic effect of methyl
methacrylate on any target organ in humans, nor can it be inferred
with any degree of confidence that the possibility of an excess risk
has been disproved. Methyl methacrylate has not been carcinogenic in
an extensive, well documented 2-year bioassay in rats and mice exposed
by inhalation and in additional chronic inhalation studies in rats and
hamsters. Although not mutagenic in vitro in bacterial systems,
methyl methacrylate has been mutagenic and clastogenic in mammalian
cells in vitro. In in vivo studies (primarily by the inhalation
route) in which there has been clear evidence of toxicity within the
target tissue, there has been limited evidence of genotoxicity of
methyl methacrylate. On the basis of these observations, methyl
methacrylate is considered unclassifiable with respect to
carcinogenicity in humans.
Owing to the limitations of the available studies in humans on
effects associated with longer-term exposure to methyl methacrylate,
it is necessary to rely primarily on information obtained from the
studies in animals for determination of critical effect levels. The
lowest reported effect level for inhalation was 100 ppm (410 mg/m3)
in rats exposed to methyl methacrylate for 2 years (based upon
inflammatory degeneration of the nasal epithelium); the NOEL in this
investigation was 25 ppm (102.5 mg/m3) (Rohm & Haas, 1979a; Lomax,
1992; Lomax et al., 1997).
11.1.2 Criteria for setting guidance values for methyl methacrylate
The following quantitative guidance is provided as an example of
a possible basis for derivation of limits of exposure and judgement of
the quality of environmental media by relevant authorities. As methyl
methacrylate is considered to be "unclassifiable with respect to
carcinogenicity in humans," guidance values are derived on the basis
of a lowest-observed-(adverse)-effect level [LO(A)EL] or a
no-observed-(adverse)-effect level [NO(A)EL] for non-neoplastic
effects. For methyl methacrylate, the route of exposure most relevant
to the general population is likely inhalation.
The value considered most appropriate as a basis for development
of a tolerable concentration in air is the NOEL of 25 ppm (102.5
mg/m3) in rats exposed to methyl methacrylate for 2 years (Rohm &
Haas, 1979a; Lomax, 1992; Lomax et al., 1997). Effects at the next
higher concentration were degenerative changes in the olfactory
epithelium.
There is some debate currently about extrapolation of data on
nasal irritation of the olfactory epithelium observed in rodents in
risk assessment for humans. There are significant morphological
differences between species in the structure of the nasal cavity,
which result in differences in concentrations of inhaled materials at
the nasal tissue. These are reflected in differences in surface area
normalized to minute ventilation, being fivefold greater in rodents
than in humans (DeSesso, 1993). A much greater percentage of the
nasal cavity is lined by olfactory epithelium in rats than in humans.
In addition, rodents are obligate nose breathers, whereas humans can
also breathe through their mouths, which is expected to reduce
exposure of the nasal epithelium for much of the population. There
are also differing nasal flow patterns, with the greater airflow
across the human olfactory epithelium during the expiratory phase when
the vapour concentration would be considerably reduced as a result of
absorption in the lower respiratory tract.
The pattern of the critical effects of inhalation of methyl
methacrylate in animal studies (i.e. the olfactory epithelium being
affected at lowest concentration) is consistent with toxicity
resulting from metabolism of the inhaled material in the olfactory
tissue by carboxylic esterases to methacrylic acid. Data on species
differences in olfactory tissue carboxylesterase activity have not
been identified; however, based on limited data from human tissue
samples that may not have been morphologically normal taken at polyp
biopsy, the activity of alpha-naphthylbutyrate carboxylesterase in
human nasal respiratory tissue is less than that in the rat (Mattes &
Mattes, 1992).
Although it is possible that humans may be less sensitive than
rodents to lesions of the nasal epithelium caused by methyl
methacrylate, currently available data are inadequate to account
quantitatively for potential interspecies variation in sensitivity.
However, studies that are currently under way may shed some additional
light on this aspect (T. Green, personal communication, 1997; P.J.
Pinto, personal communication, 1997). Therefore, on the basis of the
available data, a tolerable concentration (TC) has been derived on the
basis of a commonly adopted default value of 10-fold for interspecies
variation as follows:
TC = (102.5 mg/m3/100) × (6/24) × (5/7)
= 0.2 mg/m3 (rounded to one significant figure)
where:
* 102.5 mg/m3 (25 ppm) is the lowest NOEL reported in inhalation
bioassays of adequate quality in animal species (rats) conducted
to date (exposure-related and concentration-dependent microscopic
changes [degeneration/atrophy of the olfactory epithelium and
underlying Bowman's glands, hyperplasia of basal (reserve) cells,
replacement of olfactory epithelium by ciliated
(respiratory-like) epithelium, and inflammation of the mucosa
and/or submucosa] were observed in anterior portions of the nasal
cavity in rats exposed to the next higher concentration) (Rohm &
Haas, 1979a; Lomax, 1992; Lomax et al., 1997);
* 6/24 and 5/7 are the conversion of intermittent exposure of rats
(i.e. 6 hours/day, 5 days/week) to continuous exposure of humans;
this is appropriate in view of data that suggest that continuous
exposure to methyl methacrylate could result in effects at
concentrations below the NOAEL for intermittent exposure (Lomax
et al., 1994); no scaling factor for inhalation volume to body
weight was used, as effects at the next higher dose level are
limited to the site of entry; and
* 100 is the uncertainty factor (×10 for intraspecies variation;
×10 for interspecies variation).
Based on the results of limited available cross-sectional studies
on respiratory effects in human populations, this value is likely to
be protective.
A TDI can be derived for the oral route of exposure based on a
2-year drinking-water study in rats in which the NOAEL in females was
considered to be 146 mg/kg body weight per day; the NOEL in males was
121 mg/kg body weight per day, the highest dose level tested
(Borzelleca et al., 1964). Incorporating an uncertainty factor of 100
(×10 for intraspecies variation; ×10 for interspecies variation), the
TDI would be 1.2 mg/kg body weight per day.
11.1.3 Sample risk characterization
The extremely limited nature of the available data as a basis for
estimation of exposure should be borne in mind in interpreting the
comparisons presented here for predicted indirect population exposure
in the general environment and estimated exposure from the use of
consumer products containing methyl methacrylate. Moreover, the
sample exposure estimates presented in section 6.2 will vary
considerably as a function of production and use patterns and control
measures in various countries.
Based upon the sample predicted concentration (based on fugacity
modelling) of methyl methacrylate in air of 2.44 × 10-4 µg/m3,
presented in section 6.1, levels of methyl methacrylate in ambient air
are many orders of magnitude less than the calculated tolerable
concentration of 200 µg/m3. Estimated intakes associated with
inhalation exposure (predicted by computer modelling) during the use
of consumer products containing methyl methacrylate, such as
dispersion paints (estimated to be in the 10-100 µg/kg body weight per
day range) and oil-based paints (predicted exposure in the 100-1000
µg/kg body weight per day range) (see section 6.2), may be up to an
order of magnitude higher than the tolerable intake associated with
exposure at the level of the tolerable concentration. Although it has
been reported that in some countries these products are not supplied
to the general public, information on use patterns of these products
in other countries was not available.
With respect to occupational exposure, mean levels of methyl
methacrylate in the air of production and manufacturing facilities and
dental facilities range up to several hundred mg/m3, whereas levels
in beauty salons are generally less than 100 mg/m3 (IARC, 1994).
Elevated levels (greater than 1500 mg/m3) during floor coating with
methyl methacrylate-containing resins have been reported, although
time-weighted-average concentrations would be less.
11.2 Evaluation of environmental effects
Because of its release principally in emissions from industrial
sources and its relatively high volatility, the atmosphere is the
predominant environmental sink for methyl methacrylate. It is highly
reactive with hydroxyl radicals; thus, its lifetime in the atmosphere
is short. Substances whose atmospheric half-lives do not exceed 1
year are not considered to contribute to global warming; therefore,
methyl methacrylate is not considered to be a greenhouse gas, nor
would it contribute directly to depletion of the ozone layer. Methyl
methacrylate is not expected to bioconcentrate in the environment.
Terrestrial organisms will have the greatest potential for
exposure to methyl methacrylate in ambient air. However, as no field
or laboratory studies on birds, terrestrial invertebrates, or
terrestrial plants were identified, the toxicity of methyl
methacrylate to these organisms could not be assessed. Chronic
studies on laboratory mammals are available, however, as well as data
on levels of exposure of aquatic-based mammals to methyl methacrylate,
thus permitting the comparison between effects and environmental
exposure for these organisms. The mink was chosen as the model
species, with aquatic organisms comprising up to 100% of its diet.
Based on the concentrations of methyl methacrylate in air, water, and
fish predicted by fugacity modelling and assuming daily consumption
rates for mink of 0.55 m3 of air, 0.1 litre of water, and 158 g of
fish, the estimated total daily intake of methyl methacrylate by mink
in southern Ontario, Canada, is 0.17 ng/kg body weight per day, with
approximately 80% of the exposure being attributable to inhalation
(Government of Canada, 1993). The lowest NOEL observed in chronic
inhalation studies in laboratory animals is 102.5 mg/m3, based on
exposure-related and concentration-dependent microscopic changes in
anterior portions of the nasal cavity (degeneration/atrophy of the
olfactory epithelium and underlying Bowman's glands, hyperplasia of
basal cells, replacement of olfactory epithelium by respiratory
epithelium, and inflammation of the mucosa and/or submucosa) (Rohm &
Haas, 1979a; Lomax, 1992; Lomax et al., 1997). Using a factor of 10
to account for interspecies variability in sensitivity, the NOEL is
108 times higher than levels predicted to occur in the environment in
Canada (i.e. 0.24 ng/m3).
No chronic studies on aquatic organisms were identified; however,
acute tests have been conducted on fish, Daphnia magna, and algae.
The most sensitive effect was the onset of inhibition of cell
multiplication by the green alga Scenedesmus quadricauda at 37
mg/litre following 8 days of exposure. This is similar to the
concentration (i.e. 40 mg/litre) at which sublethal/ behavioural
responses were noted in rainbow trout following 96 hours of exposure.
Using a factor of 20 to convert from an acute to a chronic end-point
and another factor of 10 to account for interspecies variability in
sensitivity, the estimated effects threshold is approximately
106-fold higher than the concentration predicted to occur in surface
water in Canada (i.e. 0.13 ng/litre).
Therefore, although available data on the environmental effects
of methyl methacrylate are limited and predicted concentrations in
various media are highly uncertain, a wide margin exists between
observed effect levels and uncertain predicted environmental
concentrations of methyl methacrylate. As such, the concentrations of
methyl methacrylate predicted to be in the environment are unlikely to
pose a risk to aquatic or terrestrial organisms.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer (IARC, 1994) has
classified methyl methacrylate in Group 3 (not classifiable as to its
carcinogenicity to humans) based on inadequate evidence for
carcinogenicity in humans and evidence suggesting a lack of
carcinogenicity in experimental animals.
Information on international hazard classification and labelling
is included in the International Chemical Safety Card reproduced in
this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards together with preventative and protective
measures and first aid recommendations are presented in the
International Chemical Safety Card (ICSC 0300) reproduced in this
document.
13.1 Human health hazards
Methyl methacrylate is highly flammable. After long-term or
repeated exposure, it may cause skin sensitization and asthma and may
have effects on the nervous system.
13.2 Advice to physicians
In the event of poisoning, the treatment is supportive. Owing to
reports of systemic vasodilation and transient hypotension in patients
following use of methyl methacrylate as a bone cement in total hip
replacement procedures, monitoring for hypotension and respiratory
depression is recommended. Because a stabilizer or inhibitor is
always a part of the formulation, toxicological properties may be
different.
13.3 Health surveillance advice
Periodic medical examination of the area of the skin exposed to
methyl methacrylate, tests to determine any disturbances of the
nervous system, and surveillance of the respiratory system should be
included in the health surveillance programme.
13.4 Explosion and fire hazards
13.4.1 Explosion hazards
Methyl methacrylate is explosive in the form of vapour when
exposed to heat, sparks, or flame. Vapours may travel to a source of
ignition and flash back. Methyl methacrylate may undergo spontaneous,
explosive polymerization. It reacts in air to form a heat-sensitive
explosive product. Containers of methyl methacrylate may explode in
the heat of a fire. Runoff to sewers may create a fire or explosion
hazard.
13.4.2 Fire hazards
Methyl methacrylate is highly flammable material. When heated to
decomposition, methyl methacrylate emits acrid smoke and irritating
fumes.
13.4.3 Fire-extinguishing agents
Water may not be effective, except to absorb heat, keep
containers cool, and protect exposed materials.
13.5 Storage
Store in a cool, dry, well ventilated area, out of direct
sunlight. Store away from heat and ignition sources and incompatible
materials, such as flammable/combustible materials, materials that
support combustion (oxidizing materials), and corrosive materials
(strong acids or bases). If storing small quantities under
refrigeration, use an approved, explosion-proof refrigerator. Methyl
methacrylate monomer should not be stored for longer than 1 year.
13.6 Transport
Methyl methacrylate cannot be carried on passenger or cargo
aircraft.
13.7 Spillage
Methyl methacrylate is highly flammable. In the event of
spillage, eliminate all sources of ignition in the vicinity. Because
the chemical is absorbed through the skin, do not touch or walk
through the spilled material without proper equipment. To avoid the
flammability hazard, remove clothing immediately if wet or
contaminated, and use non-sparking tools to clean up. Do not let the
chemical enter drains or watercourses.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards is
available from the International Register of Potentially Toxic
Chemicals (IRPTC) legal file.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
METHYL METHACRYLATE MONOMER, INHIBITED ICSC:0300
METHYL METHACRYLATE MONOMER, INHIBITED
Methacrylic acid methyl ester
Methyl 2-methylpropenoate
CH2C(CH3)COOCH3
Molecular mass: 100.1
CAS # 80-62-6
RTECS # 0Z5075000
ICSC # 0300
UN # 1247
EC # 607-035-00-6
TYPES OF ACUTE HAZARDS/ PREVENTION FIRST AID/
HAZARD/ SYMPTOMS FIRE FIGHTING
EXPOSURE
FIRE Highly flammable. No open flames, NO sparks, Foam, powder, carbon dioxide.
and NO smoking.
EXPLOSION Vapour/air mixtures are Closed system, ventilation, In case of fire: keep drums,
explosive. explosion-proof electrical etc., cool by spraying with
equipment and lighting. Do water.
NOT use compressed air for
filling, discharging, or
handling.
EXPOSURE PREVENT GENERATION OF MISTS!
* INHALATION Cough. Drowsiness. Headache. Ventilation, local exhaust, Fresh air, rest. Refer for
Shortness of breath. Sore or breathing protection. medical attention.
throat. Unconsciousness.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
TYPES OF ACUTE HAZARDS/ PREVENTION FIRST AID/
HAZARD/ SYMPTOMS FIRE FIGHTING
EXPOSURE
* SKIN Redness. Protective gloves. Protective Remove contaminated clothes.
clothing. Rinse and then wash skin with
water and soap.
* EYES Redness. Pain. Safety goggles or eye First rinse with plenty of
protection in combination water for several minutes
with breathing protection. (remove contact lenses if
easily possible), then take
to a doctor.
* INGESTION Nausea. Vomiting. Do not eat, drink, or smoke Rinse mouth. Refer for
during work. medical attention.
SPILLAGE DISPOSAL STORAGE PACKAGING & LABELLING
Collect leaking and spilled liquid in Fireproof. Separated from strong F symbol
sealable containers as far as oxidants, strong bases, strong acids. Xi symbol
possible. Absorb remaining liquid in Cool. Keep in the dark. Keep in a
sand or inert absorbent and remove to well-ventilated room. Store only if R: 11-36/37/38-43
safe place. Do NOT wash away into stabilized. S: (2-)9-16-29-33
sewer.
Note: D
UN Hazard Class: 3
UN Packing Group: II
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
IMPORTANT DATA PHYSICAL STATE; APPEARANCE: EFFECTS OF SHORT-TERM EXPOSURE:
COLOURLESS LIQUID, WITH CHARACTERISTIC ODOUR. A harmful contamination of the air can be
reached rather quickly on evaporation of this
substance at 20°C.
PHYSICAL DANGERS: EFFECTS OF SHORT-TERM EXPOSURE:
The vapour is heavier than air and may travel The substance irritates the eyes, the skin and
along the ground; distant ignition possible. the respiratory tract.
The vapour mixes well with air, explosive
mixtures are easily formed. Vapours are not
inhibited, they may polymerize and block the
vents.
CHEMICAL DANGERS: EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
The substance may polymerize due to warming or Repeated or prolonged contact may cause skin
due to heating under the influence of light, sensitization. Repeated or prolonged
polymerization catalysts and strong oxidants inhalation exposure may cause asthma. The
with fire or explosion hazard. Reacts with substance may have effects on the central
strong acids, strong bases and oxidants. nervous system and the peripheral nervous
system.
OCCUPATIONAL EXPOSURE LIMITS (OELs):
TLV: 100 ppm; 410 mg/m3 (as TWA)
(ACGIH 1994-1995).
MAK: 50 ppm; 210 mg/m3; I, A II (1993).
ROUTES OF EXPOSURE:
The substance can be absorbed into the body by
inhalation of its vapour, through the skin and
by ingestion.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
PHYSICAL Boiling point: 100-101°C Relative density of the vapour/
PROPERTIES Melting -48°C air-mixture at 20°C (air = 1): 1.1
Relative density (water = 1): 0.94 Flash point: 10°C o.c.
Solubility in water, g/100 ml Auto-ignition temperature: 421°C
at 20°C 1.6 Explosive limits, vol% in air: 1.7-12.5
Vapour pressure, kPa at 20°C: 3.9 Octanol/water partition coefficient
Relative vapour density (air = 1): 4.16 as log Pow: 1.38
Vapour pressure, kPa at 20°C: 0.647
Vapour pressure, Pa at 25°C: 780
ENVIRONMENTAL This substance may be hazardous to the environment; special attention should be given to water.
DATA
NOTES
Usually contains hydroquinone, hydroquinone methyl ether and dimethyl t-butylphenol as inhibitors of polymerization. An added
stabilizer or inhibitor can influence the toxicological properties of this substance, consult an expert.
ICSC: 0300 1.1 Transport Emergency Card: TEC (R)-196
NFPA Code: H2; F3; R2
REFERENCES
Anderson BE, Zeiger E, Shelby MD, Resnick MA, Gulate DK, Ivett JL,
Loveday KS (1990) Chromosome aberration and sister chromatid exchange
test results with 42 chemicals. Environmental and molecular
mutagenesis, 16 (Suppl. 18):55-137 [cited in IARC, 1994].
Anderson D, Hodge MCE (1976) Methyl methacrylate monomer: dominant
lethal study in the mouse. Macclesfield, Cheshire, ICI (Report No.
CTL/P/295).
Anderson D, Richardson CR (1976) Methyl methacrylate monomer:
cytogenetic study in the rat. Macclesfield, Cheshire, ICI (Report
No. CTL/P/292).
Anderson D, Richardson CR, Weight TM (1979). Methyl methacrylate
monomer: a second cytogenetic study in the rat. Macclesfield,
Cheshire, ICI (Report No. CTL/P/449).
Andrews CP, Smith JD, Johanson WG Jr (1979) Pulmonary effects of
methyl methacrylate vapor exposure in dental students. Clinical
research, 27:759A (abstract).
Bailey HD, Liu DHW, Javitz HA (1985) Time/toxicity relationships in
short-term static, dynamic and plug-flow bioassays. In: Bahner RC,
Hansen DJ, eds. Aquatic toxicology and hazard assessment: eighth
symposium. Philadelphia, PA, American Society for Testing and
Materials, pp. 193-212 (ASTM Special Technical Publication 891).
Blanchet LJ, Bowman BC, McReynolds HD (1982) Effects of methyl
methacrylate monomer vapors on respiration and circulation in
unanesthetized rats. Journal of prosthetic dentistry, 48:344-348.
Borzelleca JF, Larson PS, Hennigar GR, Hluf EG, Crawford EM, Blackwell
Smith R (1964) Studies on the chronic oral toxicity of monomeric ethyl
acrylate and methyl methacrylate. Toxicology and applied
pharmacology, 6:29-36.
Bowman JH (1990) Acute flow-through toxicity of methyl methacrylate
to rainbow trout. Analytical Bio-Chemistry Laboratories Inc. (Report
No. 37327) [cited in Clary JJ (1991) Methyl methacrylate: a
toxicity review. Prepared by Bio Risk. New York, NY, US Methacrylate
Producers Association].
Bratt H, Hathway DE (1977) Fate of methyl methacrylate in rats.
British journal of cancer, 36:114-119.
Bringmann VG (1978) Bestimmung der biologischen Schadwirkung
wassergefahrdender Stoffe gegen Protozoen. Zeitschrift für
Wasser-und Abwasser-Forschung, 6:210-215.
Bringmann VG, Kuhn R (1976) Vergleichende Befunde der Schadwirkung
wassergefahrdender Stoffe gegen Bakterien (Pseudomonas putida) und
Blaualgen (Microcystis aeruginosa). Gwf-wasser/Abwasser,
117:410-413.
Bringmann VG, Kuhn R (1977) Befunde der Schadwirkung
wassergefahrdender Stoffe gegen Daphnia magna. Zeitschrift für
Wasser-und Abwasser-Forschung, 5:161-166.
Bringmann VG, Kuhn R (1978a) Testing of substances for their toxicity
threshold: model organisms Microcystis (Diplocystis) aeruginosa and
Scenedesmus quadricauda. Mitteilungen Internationale Vereinigung
für Theoretische und Angewandte Limnologie, 21:275-284.
Bringmann VG, Kuhn R (1978b) Grenzwerte der Schadwirkung
wassergefahrdender Stoffe gegen Blaualgen (Microcystis aeruginosa)
und Grunalgen (Scenedesmus quadricauda) im Zellvermehrungshemmtest.
Vom Wasser, 50:45-60.
Bringmann VG, Kuhn R (1982) Ergebnisse der Schadwirkung
wassergefahrdender Stoffe gegen Daphnia magna in einem
weiterentwickelten standardisierten Testverfahren. Zeitschrift für
Wasser-und Abwasser-Forschung, 15:1-6.
Cannas M, Bigatti P, Rossi E, Rossi P (1987) In vitro research on
the possibility of chromosomal damage caused by polymethyl
methacrylate in orthopaedics. Italian journal of orthopaedics and
traumatology, 13:387-391 [cited in IARC, 1994].
Cary R, Morris L, Cocker J, Groves J, Ogunbiyi A (1995) Methyl
methacrylate, criteria for an occupational exposure limit. London,
UK Health and Safety Executive.
Castellino N, Colicchio G (1969) [Experimental research on the acute
toxicity of methyl methacrylate.] Folia Medica, 52:337-347 (in
Italian) [cited in ECETOC, 1995].
CEFIC (1993) Questionnaire on exposure data, methyl methacrylate
(MMA). Data from ELF-ATOCHEM, ICI, Repsol, and Röhm. Brussels,
CEFIC, Methacrylates Toxicology Committee.
CEFIC (1994) MMA - Quarterly statistics on sales; effective
capacity, production, captive use. Brussels, CEFIC, Methyl
Methacrylate Sector Group.
Chan PC, Eustis SL, Huff JE, Haseman JK, Ragan H (1988) Two-year
inhalation carcinogenesis studies of methyl methacrylate in rats and
mice: Inflammation and degeneration of nasal epithelium.
Toxicology, 52:237-252.
Collins JJ, Page LC, Caporossi JC, Utidjian HM, Saipher JN (1989)
Mortality patterns among men exposed to methyl methacrylate.
Journal of occupational medicine, 31:41-46.
Conde-Salazar L, Guimaraens D, Romero L (1988) Occupational allergic
contact dermatitis from anaerobic acrylic sealants. Contact
dermatitis, 18(3):129-132 [cited in Cary et al., 1995].
CPI (1989) CPI product profiles: Methyl methacrylate. Don Mills,
Ontario, Canadian Process Industries, Corpus Information Services.
Cromer J, Kronoveter K (1976) A study of methyl methacrylate
exposures and employee health. Cincinnati, OH, US Department of
Health, Education and Welfare, National Institute for Occupational
Safety and Health (DHEW (NIOSH) Publication No. 77-119; NTIS
PB-27489).
Dearfield KL, Harrington-Brock K, Doerr CL, Rabinowitz JR, Moore MM
(1991) Genotoxicity in mouse lymphoma cells of chemicals capable of
Michael addition. Mutagenesis, 6:519-525 [cited in IARC, 1994].
DeFonso LR, Maher KV (1981) Texas Plant mortality study
(1948-1978). Report prepared for Rohm and Haas Company.
DeFonso LR, Maher KV (1986) A matched case-control study nested
within an historical cohort study of acrylate/methacrylate workers.
Report prepared for Rohm and Haas Company for the US Environmental
Protection Agency's TSCA Section 8(d) submission.
Deichmann-Gruebler A, Read RT (undated) In: Submission to US
Environmental Protection Agency under TSCA Section 8(d) by E.I. duPont
Company, 1989 (NTIS/OTS 0520934).
DeSesso JM (1993) The relevance to humans of animal models for
inhalation studies of cancer in the nose and upper airways.
Quality assurance: good practice, regulation and law, 2:213-231.
Doerr CL, Harrington-Brock K, Moore MM (1989) Micronucleus, chromosome
aberration, and small-colony TK mutant analysis to quantitate
chromosomal damage in L51784 mouse lymphoma cells. Mutation
research, 222:191-203.
ECETOC (1995) Methyl methacrylate - CAS No. 80-62-6. Brussels,
European Centre for Ecotoxicology and Toxicology of Chemicals, 167 pp.
(Joint Assessment of Commodity Chemicals No. 30).
Edwards PM (1975) Neurotoxicity of acrylamide and its analogues and
effects of these analogues and other agents on acrylamide neuropathy.
British journal of industrial medicine, 32:31-38.
Environment Canada (1989) Analysis of shellfish for organic and
inorganic contaminants. Zenon Environmental Inc., March.
Estlander T, Rajaniemi R, Jolanki R (1984) Hand dermatitis in dental
technicians. Contact dermatitis, 10(4):201-205 [cited in Cary et
al., 1995].
Ewing BB, Chian ESK (1977) Monitoring to detect previously
unrecognized pollutants in surface waters. US Environmental
Protection Agency (USEPA/560/6-77/015A).
Farli M, Gasperini M, Francalanci S, Gola M, Sertoli A (1990)
Occupational contact dermatitis in two dental technicians. Contact
dermatitis, 22(5):282-287.
Farmakovskaya TB, Tikhomirov YP (1993) The influence of
methylmethacrylate and butylmethacrylate on the reproductive function
of animals during round-the-clock inhalation. Reproductive
toxicology, 7(5):520-521.
Fedyukovich LV, Egorova AB (1991) [Genotoxic effect of acrylates.]
Gigiena i Sanitariya, 12:62-64 (in Russian) [cited in IARC, 1994].
Fedyukovich L, Kotlovskii Y, Sviderskaya L, Borisov Y (1988)
[Mutagenic and cytotoxic effect of acrylates.] Genetika,
24:1132-1134 (in Russian) [cited in ECETOC, 1995].
Finnish Advisory Board of Chemicals (1992) Acrylate compounds: Uses
and evaluation of health effects. Helsinki.
Forbis DA (1990) Acute toxicity of methyl methacrylate to
Selenastrum capricornutum printz. Analytical Bio-Chemistry
Laboratories Inc. (Report No. 37329) [cited in Clary JJ (1991) Methyl
methacrylate: a toxicity review. Prepared by Bio Risk. New York, NY,
US Methacrylate Producers Association].
Government of Canada (1993) Canadian Environmental Protection Act.
Priority Substances List assessment report for methyl methacrylate.
Prepared by Health Canada and Environment Canada. Ottawa, Ontario,
Canada Communication Group Publishing (ISBN 0-662-20418-2).
Guerra L, Vincenzi C, Peluso AM, Tosti A (1993) Prevalence and sources
of occupational contact sensitization to acrylates in Italy.
Contact dermatitis, 28:101-103 [cited in ECETOC, 1995].
Hachitani N, Taketani A, Takizawa Y (1981) [Mutagenicity study on
environmental substances. 3. Ames test and mouse bone marrow
micronucleus test on acrylic resin monomer and other additives.]
Nippon Koshu Eisei Zasshi, 29:236-239 (in Japanese).
Hodge MCE, Palmer S (1977) Methylmethacrylate monomer
teratogenicity studies in the rat. Submission by Rohm and Haas
Company to the US Environmental Protection Agency under TSCA Section
8(d), July 1979.
Hollifield H, Breder C, Dennison J, Roach J, Adams W (1980)
Container-derived contamination of maple syrup with methyl
methacrylate, toluene and styrene as determined by headspace
gas-liquid chromatography. Journal of the Association of Official
Analytical Chemists, 63:173-177.
Hossack DJN, Thomas FJ (1992) Methyl methacrylate: Effects on soil
carbon cycle (respiration). Prepared by Huntingdon Research Centre,
Huntingdon, England. Washington, DC, US Methacrylate Producers
Association [cited in ECETOC, 1995].
Howard P (1989) Handbook of environmental fate and exposure data
for organic chemicals. Vol. 1. Chelsea, MI, Lewis Publishers Inc.,
pp. 402-407.
Howard P, Boethling RS, Jarvis WF, Meyland WM, Michalenko EM (1991)
Handbook of environmental degradation rates. Chelsea, MI, Lewis
Publishers Inc.
Husain R, Srivastava SP, Seth PK (1985) Methyl methacrylate induced
behavioural and neurochemical changes in rats. Archives of
toxicology, 58:33-36.
Husain R, Khan S, Husain I, Seth PK, Pandya KP (1989) Effect of methyl
methacrylate on selected lipids in rat brain and sciatic nerve.
Industrial health, 27:121-124.
IARC (1994) Some industrial chemicals. Lyon, International Agency
for Research on Cancer, pp. 445-474 (IARC Monographs on the Evaluation
of the Carcinogenic Risk of Chemicals to Humans, Vol. 60).
Innes D, Tansy MF (1981) Central nervous system effects of methyl
methacrylate vapours. Neurotoxicology, 2:515-522.
Inoue T, Tatsuno T, Tanimura A (1981) Hygienic chemical studies on
plastics. III. Migration test of methyl methacrylate and plastic
additives from polymethyl methacrylate. Bulletin. National
Institute of Hygienic Sciences (Tokyo), 99:144-147.
IPCS (1993) International Chemical Safety Card - Methyl
methacrylate. Geneva, World Health Organization, International
Programme on Chemical Safety (No. 0300).
Jedrychowski W (1982) Styrene and methyl methacrylate in the
industrial environment as a risk factor of chronic obstructive lung
disease. International archives of occupational and environmental
health, 51:151-157.
Jensen JS, Sylvest A, Trap B, Jensen JC (1991) Genotoxicity of acrylic
bone cements. Pharmacology and toxicology, 69:386-389.
Kanerva L, Verkkala E (1986) Electron microscopy and
immunohistochemistry of toxic and allergic effects of methyl
methacrylate on the skin. Archives of toxicology, Suppl. 9:456-459.
Kanerva L, Estlander T, Jolanki R (1988) Sensitization to patch test
acrylates. Contact dermatitis, 18:10-15 [cited in ECETOC, 1995].
Kanerva L, Estlander T, Jolanki R (1989) Allergic contact dermatitis
from dental composite resins due to aromatic and aliphatic epoxy
acrylates. Contact dermatitis, 20(3):201-211 [cited in Cary et al.,
1995].
Kassis V, Vedel P, Darre E (1984) Contact dermatitis due to methyl
methacrylate. Contact dermatitis, 11(1):26-28.
Lijinsky W, Andrews AW (1980) Mutagenicity of vinyl compounds in
Salmonella typhimurium. Teratogenesis, carcinogenesis, and
mutagenesis, 1:259-267.
Lomax LG (1992) Histopathologic evaluation of nasal cavities from
Fischer 344 rats exposed to methyl methacrylate vapor for two
years. Spring House, PA, Rohm and Haas Company, Toxicology
Department (Project No. 3302.5E, finalized 7 May 1992).
Lomax LG, Brown DW, Frederick CB (1994) Regional histopathology of
the mouse nasal cavity following two weeks of exposure to acrylic
acid for either 6 or 22 hours per day. Spring House, PA, Rohm and
Haas Company.
Lomax LG, Krivanek ND, Frame SR (1997) Chronic inhalation toxicity and
oncogenicity of methyl methacrylate in rats and hamsters. Food and
chemical toxicology, 35:393-407.
Lozewicz S, Davison A, Hopkirk A, Burge P, Boldy D, Riordan J,
McGivern DV, Platts B, Davies D, Taylor A (1985) Occupational asthma
due to methyl methacrylate and cyanoacrylates. Thorax,
40(11):836-839.
Luo SQ, Gang BQ, Sun SZ (1986) Study on embryotoxicity and
fetotoxicity in rats by maternal inhalation of low level methyl
methacrylate. Toxicology letters, 31:80 (abstract no. P3-29).
Mackay D, Paterson S (1981) Calculating fugacity. Environmental
science and technology, 15:1006-1014.
Mackay D, Paterson S (1982) Fugacity revisited. Environmental
science and technology, 16:654-660.
Mackay D, Paterson S (1991) Evaluating the regional multimedia fate of
organic chemicals: a Level III fugacity model. Environmental
science and technology, 25:427.
Mackay D, Paterson S, Cheung B, Neely W (1985) Evaluating the
environmental behaviour of chemicals with a Level III fugacity model.
Chemosphere, 14:335-374.
Mackay D, Shiu WY, Ma KC (1995) Illustrated handbook of
physical-chemical properties and environmental fate for organic
chemicals. Vol. IV. Boca Raton, FL, CRC Press, Inc./Lewis
Publishers.
Maher K, DeFonso LR (1987a) Mortality study of Bristol Plant
employees hired 1946-1982. Draft report prepared for Rohm and Haas
Company.
Maher K, DeFonso LR (1987b) Mortality study of Knoxville Plant
employees (1943-1982). Report prepared for Rohm and Haas Company.
Marez T, Shirali P, Hildebrand HF, Haguenoer JM (1991) Increased
frequency of sister chromatid exchange in workers exposed to high
doses of methylmethacrylate. Mutagenesis, 6:127-129.
Marez T, Edmé JL, Boulenguez C, Shirali P, Haguenoer JM (1993)
Bronchial symptoms and respiratory function in workers exposed to
methylmethacrylate. British journal of industrial medicine,
50:894-897.
Mattes PM, Mattes WB (1992) alpha-Naphthyl butyrate carboxylesterase
activity in human and rat nasal tissue. Toxicology and applied
pharmacology, 114:71-76.
McLaughlin RE, Reger SJ, Barkalow JA, Allen MJ, Diffazio CA (1978)
Methyl methacrylate: A study of teratogenicity and fetal toxicity of
the vapor in the mouse. Journal of bone and joint surgery,
60A:355-358.
Mizunuma K, Kawai T, Yasagui T, Horiguichi S, Takeda S, Miyashita K,
Taniuchi T, Moon C-S, Ikeda M (1993) Biological monitoring and
possible health effects in workers occupationally exposed to methyl
methacrylate. Archives of occupational and environmental health,
65:227-232.
Moore MM, Amanda A, Doerr CL, Brock KH, Dearfield KL (1988)
Genotoxicity of acrylic acid, methyl acrylate, ethyl acrylate, methyl
methacrylate and ethyl methacrylate in L5178Y mouse lymphoma cells.
Environmental and molecular mutagenesis, 11:49-63.
Morris JB, Frederick CB (1995) Upper respiratory tract uptake of
acrylate ester and acid vapours. Inhalation toxicology, 7:557-574.
Myhr B, McGregor D, Bowers L, Riach C, Brown AG, Edwards I, McBride D,
Martin R, Caspary WJ (1990) L5178Y mouse lymphoma cell mutation assay
results with 41 compounds. Environmental and molecular mutagenesis,
16 (Suppl. 18):138-167 [cited in IARC, 1994].
Nicholas CA, Lawrence WH, Autian J (1979) Embryotoxicity and
genotoxicity from maternal inhalation of methyl methacrylate monomer
in rats. Toxicology and applied pharmacology, 50:451-458.
NIOSH (1976) A study of methyl methacrylate exposures and employee
health. Cincinnati, OH, US Department of Health, Education and
Welfare, National Institute for Occupational Safety and Health
(Publication No. 77-119).
NTP (1986) NTP technical report on the toxicology and
carcinogenesis studies of methyl methacrylate (CAS No. 80-62-6) in
F344/N rats and B6C3F1 mice (inhalation studies). Research Triangle
Park, NC, US Department of Health and Human Services, National
Institutes of Health, National Toxicology Program, 202 pp. (NTP
TR314).
Ouyang G, Shi T, Fan Z, Zhang B, Yu T, Hao A, Tang G (1990) Acute
toxicity and toxicokinetics of methyl methacrylate. Chinese
chemical abstracts, 112:133952q.
Pickering C, Bainbridge D, Birtwistle I, Griffiths D (1986)
Occupational asthma due to methyl methacrylate in an orthopaedic
theatre sister. British medical journal, 292:1362-1363.
Pickering CAC, Niven R, Simpson J (1993) A study of the prevalence
of occupational asthma at the ICI Acrylics site at Darwen,
Lancashire. Lancashire, ICI Acrylics.
Poss R, Thilly WG, Kaden DA (1979) Methylmethacrylate is a mutagen for
Salmonella typhimurium. Journal of bone and joint surgery,
61-A:1203-1207 [cited in IARC, 1994].
Rajaniemi R, Tola S (1985) Subjective symptoms among dental
technicians exposed to the monomer methyl methacrylate.
Scandinavian journal of work, environment & health, 11:281-286.
Raje RR, Ahmad S, Weisbroth SH (1985) Methyl methacrylate: tissue
distribution and pulmonary damage in rats following acute inhalation.
Research communications in chemical pathology and pharmacology,
50:151-154.
Röhm (1994) Medical examination of workers in acrylic sheet
production exposed to methyl methacrylate. Pausch, Höffer, Claus,
Lehr, Jacobi, 15.03.1994. Darmstadt, Röhm [cited in ECETOC, 1995].
Rohm and Haas (1977) Subchronic vapor inhalation study with methyl
methacrylate (C50680) in F344 rats and B6C3F1 mice. Report to
Tracor Jitco, Inc., submitted by IBT Laboratories Inc.
Rohm and Haas (1979a) A two-year vapor inhalation safety evaluation
study in rats. Methyl methacrylate. Final report. Submitted by
Hazleton Laboratories America Inc., 217 pp.
Rohm and Haas (1979b) 18-month vapor inhalation safety evaluation
study in hamsters. Methyl methacrylate vapor. Final report.
Submitted by Hazleton Laboratories America Inc., 85 pp. (Project No.
417-354).
Rohm and Haas (1982) Acute oral LD50 range finding rat, acute
dermal LD50 range finding rabbit, acute skin irritation range
finding rabbit 4-hr contact, acute eye irritation range finding
rabbit. Test substance methyl methacrylate - 10 ppm Topanol A [cited
in ECETOC, 1995].
Schwartz BS, Doty RL, Monroe C, Frye R, Baker S (1989) Olfactory
function in chemical workers exposed to acrylate and methacrylate
vapours. American journal of public health, 79:613-618.
Schweikl H, Schmalz G, Bey B (1994) Mutagenicity of dentin bonding
agents. Journal of biomedical materials research, 28:1061-1067.
Seiji K, Inoue O, Kawai T, Mizunuma K, Yasugi T, Moon C, Takeda S,
Ikeda M (1994) Absence of mutagenicity in peripheral lymphocytes of
workers occupationally exposed to methyl methacrylate. Industrial
health, 32:97-105.
Seppalainen AM, Rajaniemi TC (1984) Local neurotoxicity of methyl
methacrylate among dental technicians. American journal of
industrial medicine, 5:471-478.
Siemiatycki J (1991) Risk factors for cancer in the workplace. Boca
Raton, FL, CRC Press, 310 pp.
Smith JM, Cruzan G, Drees JA, Tansy MF, Coate WB, Reno FE (1979)
Methyl methacrylate: subchronic, chronic and oncogenic inhalation
safety evaluation studies. Toxicology and applied pharmacology,
48:A30.
Solomon HM, McLaughlin JE, Swenson RE, Hagan JV, Wanner FJ, O'Hara GP,
Krivanek ND (1993) Methyl methacrylate: inhalation developmental
toxicity study in rats. Teratology, 48:115-125.
Spealman CR, Main RJ, Haag HB, Larson PS (1945) Monomeric methyl
methacrylate - Studies on toxicity. Industrial medicine, 14:292-298.
Tansy MF (1975) Progress report on teratology studies of mice
exposed to methyl methacrylate monomer vapour. Submitted to Rohm and
Haas Company, 5 pp.
Tansy MF, Drees JA (1979) Methyl methacrylate, three month
subchronic vapour inhalation safety evaluation study, beagle dogs.
Prepared for Rohm and Haas Company, 239 pp.
Tansy MF, Kendall FM, Benhayem S, Hohenleitner FJ, Landin WE, Gold M
(1976) Chronic biological effects of methyl methacrylate vapor. 1.
Body and tissue weights, blood chemistries, and intestinal transit in
the rat. Environmental research, 11:66-77.
Tansy MF, Hohenleitner FJ, Landin WE, Kendall FM (1980a) Chronic
biological effects of methyl methacrylate vapor. II. Body and tissue
weights, blood chemistries and gross metabolic performance in the rat.
Environmental research, 21:108-116.
Tansy M, Hohenleitner F, White D, Oberly R, Landin W, Kendall F
(1980b) Chronic biological effects of methyl methacrylate vapour. III.
Histopathology, blood chemistries and hepatic and ciliary function in
the rat. Environmental research, 21:117-125.
Tomenson JA, Bonner SM (1994) A cohort study of employees in
Perspex plants. Northwich, Cheshire, ICI Epidemiology Unit, 15
December.
Waegemaekers THJM, Bensink MPM (1984) Nonmutagenicity of 27 aliphatic
acrylate esters in the Salmonella microsome test. Mutation
research, 137:95-102.
Walker AM, Cohen AJ, Loughlin JE, Rothman KJ, DeFonso LR (1991)
Mortality from cancer of the colon or rectum among workers exposed to
ethyl acrylate and methyl methacrylate. Scandinavian journal of
work, environment & health, 17:7-19.
Wynkoop JR, Miller RA, Cheong V, Lorton L (1982) Levels of neuroactive
substances following exposure to methyl methacrylate monomer.
Journal of dental research, 61:202 (abstract no. 213).
Zafiropoulos GG, Apostolopoulos AX, Patramani I (1985) Study of the
antigenic properties of methyl methacrylate using the
leukocyte-migration inhibition test. Dental materials, 1:200-204.
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K, Speck W
(1987) Salmonella mutagenicity tests: III. Results from the testing
of 255 chemicals. Environmental mutagenesis, 9 (Suppl. 9):1-110
[cited in IARC, 1994].
APPENDIX 1 - SOURCE DOCUMENTS
Government of Canada (1993)
Copies of the Canadian Environmental Protection Act Priority
Substances List Assessment Report (Government of Canada, 1993) and
unpublished Supporting Documentation for methyl methacrylate may be
obtained from the:
Commercial Chemicals Branch
Environment Canada
14th Floor, Place Vincent Massey
351 St. Joseph Blvd.
Hull, Quebec
Canada K1A 0H3
Environmental Health Centre
Health Canada
Address Locator: 0801A
Tunney's Pasture
Ottawa, Ontario
Canada K1A 0L2
Initial drafts of the Assessment Report and unpublished
Supporting Documentation for methyl methacrylate were prepared by
staff of Health Canada and Environment Canada. The human health-
related sections of this document were reviewed externally by Dr J.
Siemiatycki (University of Quebec), Dr N. Krivanek (E.I. duPont de
Nemours) (Supporting Documentation only), and the Information
Department of BIBRA Toxicology International. These sections were
approved by the Standards and Guidelines Rulings Committee of the
Bureau of Chemical Hazards of Health Canada. The environmental
sections were reviewed externally by Dr N. Bunce (University of
Waterloo) and Dr N. Krivanek (E.I. duPont de Nemours).
IARC (1994)
Copies of Some industrial chemicals (IARC Monographs on the
Evaluation of Carcinogenic Risks to Humans, Vol. 60) (IARC, 1994) may
be obtained from the:
International Agency for Research on Cancer
150 cours Albert Thomas
69372 Lyon Cedex 08
France
The members of the Working Group on the Evaluation of
Carcinogenic Risks to Humans of Some Industrial Chemicals (including
methyl methacrylate), which met in Lyon on 15-22 February 1994, were:
P.A. Bertazzi, Institute of Occupational Health, Clinica del
Lavoro "Luigi Devoto," University of Milan, via S. Barnaba 8,
20122 Milan, Italy
C.J. Calleman, School of Public Health and Community Medicine,
Department of Environmental Health, SC-34, University of
Washington, Seattle, WA 98195, USA
D. Coggon, MRC Environmental Epidemiology Unit, Southampton
General Hospital, Southampton, SO9 4XY, United Kingdom
T.A. Dragani, Division of Experimental Oncology A, National
Institute for the Study and Treatment of Tumours, via Venezian 1,
20133 Milan, Italy
M.R. Elwell, Toxicology Research and Testing Program, National
Institute of Environmental Health Sciences, PO Box 12233,
Research Triangle Park, NC 27709, USA
H.J. Evans, MRC Human Genetic Unit, Western General Hospital,
Crewe Road, Edinburgh EH4 2XU, United Kingdom (Chairperson)
J.G. Filser, GSF Institute of Toxicology, Neuherberg, PO Box
1129, 85758 Oberschleissheim, Germany
M. Gérin, University of Montréal, Department of Occupational and
Environmental Health, Faculty of Medicine, CP 6128, Station A,
Montréal, Québec, Canada H3C 3J7
K. Hemminki, Centre for Nutrition and Toxicology, Karolinska
Institute, Novum, 141 57 Huddinge, Sweden
C. Hogstedt, National Institute of Occupational Health, 171 84
Solna, Sweden
M. Kirsch-Volders, Laboratorium Antropogenetica, Free University
of Brussels, Pleinlaan 2, 1050 Brussels, Belgium
W. Lutz, Institute of Toxicology, Swiss Federal Institute of
Technology, Schorenstrasse 16, 8603 Schwerzenbach, Switzerland
S.S. Olin, International Life Sciences Institute, Risk Science
Institute, 1126 Sixteenth Street NW, Washington, DC 20036, USA
A. Pintér, "Johan Béla" National Institute of Hygiene, Gyáli ut.
2-6, 1966 Budapest, Hungary
P. Schulte, Screening and Notification Section, National
Institute for Occupational Safety and Health, Robert A Taft
Laboratories, 4676 Columbia Parkway, R-42, Cincinnati, OH
45226-1998, USA
T. Sofuni, Division of Genetics and Mutagenesis, Biological
Safety Research Centre, National Institute of Hygienic Sciences,
1-18-1 Kamiyoga, Setagaya-ku, Tokyo 158, Japan
M. Sorsa, Institute of Occupational Health, Topeliuksenkatu 41 a
A, 00250 Helsinki, Finland (Vice-Chairperson)
F.M. Sullivan, Division of Pharmacology and Toxicology, UMDS, St.
Thomas's Hospital, Lambeth Palace Road, London SE17EH, United
Kingdom
V.S. Turusov, Cancer Research Centre, Russian Academy of Medical
Sciences, Kashirskoye Shosse 24, 115478 Moscow, Russia
M.A. Waters, National Institute for Occupational Safety and
Health, 4676 Columbia Parkway, R-14, Cincinnati, OH 45226-1998,
USA
M.D. Waters, International Programs, MD-51A, Health Effects
Research Laboratory, US Environmental Protection Agency, Research
Triangle Park, NC 27711, USA
APPENDIX 2 - CICAD PEER REVIEW
The draft CICAD on methyl methacrylate was sent for review to
institutions and organizations identified by IPCS after contact with
IPCS national Contact Points and Participating Institutions, as well
as to identified experts. Comments were received from:
Bundesinstitut für Gesundheitlichen Verbraucherschutz und
Veterinarmedizin, Berlin, Germany
CEFIC, Brussels, Belgium
Department of Health, London, United Kingdom
Department of Public Health, Albert Szent-Gyorgyi University
Medical School, Szeged, Hungary
Direccion General de Salud Ambiental, Subsecretario de Regulacion
y Fomento Sanitario, San Luis Potosi, Mexico
ECETOC, Brussels, Belgium
Guy's & St. Thomas' Hospital Trust, Medical Toxicology Unit,
London, United Kingdom
International Agency for Research on Cancer, Lyon, France
Ministry of Health, National Centre of Hygiene, Medical Ecology
and Nutrition, Sofia, Bulgaria
Ministry of Health and Welfare, International Affairs Division,
Government of Japan, Tokyo, Japan
National Institute for Working Life, Solna, Sweden
National Institute of Public Health, Oslo, Norway
Russian Register of Potentially Hazardous Chemical and Biological
Substances, Moscow, Russia
United States Department of Health and Human Services (National
Institute of Environmental Health Sciences)
United States Environmental Protection Agency (Office of
Pollution Prevention and Toxics; National Center for
Environmental Assessment, Office of Research and Development;
Office of Drinking Water)
APPENDIX 3 - CICAD FINAL REVIEW BOARD
Brussels, Belgium, 18-20 November 1996
Members
Dr A. Aitio, Institute of Occupational Health, Helsinki, Finland
Dr K. Bentley, Director, Environment Policy Section, Commonwealth
Department of Human Services and Health, Canberra, Australia
Mr R. Cary, Toxicology and Existing Substances Regulation Unit, Health
and Safety Executive, Merseyside, United Kingdom
Dr J. de Fouw, National Institute of Public Health and Environmental
Protection, Bilthoven, The Netherlands
Dr C. DeRosa, Director, Division of Toxicology, Agency for Toxic
Substances and Disease Registry, Atlanta, GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr W. Farland, Director, National Center for Environmental Assessment,
Office of Research and Development, US Environmental Protection
Agency, Washington, DC, USA (Chairperson)
Dr T.I. Fortoul, Depto. Biologia Celular y Tisular, National
University of Mexico and Environmental Health Directorate of the
Health Ministry, Mexico D.F., Mexico
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Mr J.R. Hickman, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Dr T. Lakhanisky, Head, Division of Toxicology, Institute of Hygiene
and Epidemiology, Brussels, Belgium (Vice-Chairperson)
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Sciences, Hanover,
Germany
Ms E. Meek, Head, Priority Substances Section, Environmental Health
Directorate, Health Canada, Ottawa, Ontario, Canada
Dr K. Paksy, National Institute of Occupational Health, Budapest,
Hungary
Mr D. Renshaw, Department of Health, London, United Kingdom
Dr J. Sekizawa, Division of Chemo-Bio Informatics, National Institute
of Hygienic Sciences, Tokyo, Japan
Dr H. Sterzl-Eckert, GSF-Forschungszentrum für Umwelt und Gesundheit
GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Professor S. Tarkowski, Department of Environmental Health Hazards,
The Nofer Institute of Occupational Medicine, Lodz, Poland
Dr M. Wallen, National Chemicals Inspectorate (KEMI), Solna, Sweden
Observers
Professor F.M.C. Carpanini,1 Director, Centre for Ecotoxicology and
Toxicology of Chemicals (ECETOC), Brussels, Belgium
Mr R. Haigh,1 Head of Unit, Health and Safety Directorate, European
Commission, Luxembourg
Mr B.U. Hildebrandt, Federal Ministry for the Environment, Nature
Conservation and Nuclear Safety, Bonn, Germany
Mr P. Hurst,1 Chemical and Consumer Policy Officer, Conservation
Policy Division, World Wide Fund for Nature, Gland, Switzerland
Dr A. Lombard (Representative of CEFIC), ELF-ATOCHEM, Paris, France
Dr P. McCutcheon,1 Environment, Consumer Protection and Nuclear
Safety, European Commission, Brussels, Belgium
Dr R. Montaigne, Counsellor, Technical Affairs Department, European
Chemical Industry Council (CEFIC), Brussels, Belgium
Dr M. Pemberton, ICI Acrylics, Lancashire, United Kingdom
Dr A. Smith, Organisation for Economic Co-operation and Development,
Environment Division, Paris, France
1 Invited but unable to attend.
Secretariat
Dr M. Baril, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr L. Harrison, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M. Mercier, Director, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr P. Toft, Associate Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
La Direction de l'Hygiène du Milieu de Santé Canada a rédigé ce
CICAD (document international succinct sur l'évaluation des risques
chimiques) relatif au méthacrylate de méthyle en s'inspirant
principalement d'une étude menée par le Gouvernement du Canada (1993)
pour évaluer les effets potentiels d'une exposition indirecte au
méthacrylate de méthyle dans l'environnement général et les effets de
cette substance sur l'environnement, ainsi que d'une étude du Centre
international de Recherche sur le Cancer (CIRC, 1994), visant
principalement à déterminer les risques de cancérogénicité. L'étude
du Gouvernement du Canada (1993) a pris en compte les données qui
étaient disponibles en mars 1992; ces données ont été mises à jour
ultérieurement à la suite d'une recherche bibliographique approfondie
menée en septembre 1995 dans des bases de données en ligne et dans le
Registre international des substances chimiques potentiellement
toxiques. Des informations concernant la nature de l'évaluation par
les pairs et la disponibilité des études du Gouvernement du Canada
(1993) et du CIRC (1994) figurent à l'appendice 1. Au cours de la
phase d'évaluation par les pairs du présent CICAD, d'autres travaux
ont été pris en compte, à savoir des projets d'études du United
Kingdom Health Safety Executive (Cary et al., 1995) et de l'Union
européenne (Draft Assessment on Methyl Methacrylate), ainsi que des
études publiées par l'ECETOC (1995) et le Bureau consultatif
finlandais des substances chimiques (1992), principalement en vue de
rechercher des informations supplémentaires pertinentes. Des
informations supplémentaires identifiées lors des examens pratiqués
par les correspondants et par le Comité d'évaluation finale ont
également été incorporées. L'appendice 2 contient des informations
sur le processus d'évaluation par les pairs du présent CICAD. Ce
CICAD a été approuvé pour publication à une réunion du Comité
d'évaluation finale qui s'est tenue à Bruxelles (Belgique) du 18 au
20 novembre 1996. La liste des participants à cette réunion figure à
l'appendice 3. La fiche d'information sur la sécurité chimique du
méthacrylate de méthyle (ICSC 0300), préparée par le Programme
international sur la Sécurité chimique (IPCS, 1993), est également
reproduite dans le présent document.
Le méthacrylate de méthyle (CAS N° 80-62-6) est un produit
chimique de synthèse volatil, utilisé principalement dans la
production de feuilles acryliques moulées, d'émulsions acryliques et
de résines pour moulage et extrusion. Des polymères et copolymères de
méthacrylate de méthyle entrent dans la composition de nombreux
produits: revêtements de surfaces, adhésifs, produits d'étanchéité,
enduits pour cuirs et papiers, encres, encaustiques, apprêts pour
textiles, prothèses dentaires, ciments pour chirurgie osseuse, écrans
antiradiations au plomb, ongles artificiels, semelles orthopédiques,
etc. Selon les calculs, la plus grande partie du méthacrylate de
méthyle devrait être émis dans l'atmosphère, les quantités émises dans
l'eau et le sol étant minimes. Le méthacrylate de méthyle ne persiste
pas longtemps dans l'atmosphère et il est admis qu'il ne contribue pas
directement à la destruction de la couche d'ozone. Il ne devrait pas
subir de bioconcentration dans l'environnement et l'inhalation est
probablement la principale voie d'exposition pour l'homme.
Le méthacrylate de méthyle est rapidement absorbé et distribué
dans l'organisme après inhalation ou administration par voie orale aux
animaux d'expérience. Les données concernant l'absorption après
exposition cutanée sont limitées. Le méthacrylate de méthyle est
rapidement métabolisé en acide méthacrylique, aussi bien chez l'animal
que chez l'homme. Chez le rat, 16 à 20 % du produit inhalé se dépose
dans les voies respiratoires supérieures, où il est surtout métabolisé
par les estérases tissulaires locales.
La toxicité aiguë du méthacrylate de méthyle est faible. On a
observé une irritation de la peau, des yeux et des fosses nasales chez
des rongeurs et des lapins exposés à des concentrations relativement
élevées. Il se révèle légèrement sensibilisant pour la peau des
animaux. L'effet le plus souvent observé après inhalation répétée de
faibles doses est une irritation des fosses nasales. Des effets ont
également été signalés sur les reins et le foie à des concentrations
plus élevées. La plus faible concentration suivie d'effet
(dégénérescence inflammatoire de l'épithélium nasal) a été de
410 mg/m3 chez des rats exposés pendant 2 ans; dans cette étude, la
dose sans effet observé (NOEL) a été évaluée à 100 mg/m3 environ.
Une étude de qualité menée sur des rats n'a révélé aucun effet
sur le développement, malgré une diminution du poids des mères après
inhalation de concentrations allant jusqu'à 8315 mg/m3. Les seules
autres données disponibles en ce qui concerne les effets sur le
développement sont les résultats d'études limitées, anciennes ou peu
documentées, faisant état d'une foetotoxicité à des concentrations qui,
lorsqu'elles étaient mentionnées, étaient toxiques pour la mère. Les
données relatives aux effets du méthacrylate de méthyle sur la
reproduction sont limitées. Il n'y a pas eu de baisse de la fécondité
dans un test de létalité dominante chez des souris exposées à des
concentrations atteignant 36 900 mg/m3 et aucun effet indésirable n'a
été observé sur les organes de la reproduction dans les études menées
jusqu'ici avec des doses répétées. Les données disponibles sur la
neurotoxicité du méthacrylate de méthyle sont également limitées; on a
observé une dégradation de l'activité locomotrice, de la capacité
d'apprentissage et du comportement, ainsi que des effets biochimiques
sur le cerveau de rats exposés par voie orale à une dose de 500 mg/kg
de poids corporel par jour pendant 21 jours.
Le méthacrylate de méthyle ne s'est pas révélé cancérogène dans
une étude approfondie et bien documentée de 2 ans au cours de laquelle
des rats et des souris ont été exposés par inhalation, ni dans une
autre étude d'inhalation chronique menée sur des rats et des hamsters.
In vitro, le méthacrylate de méthyle n'est pas mutagène dans les
systèmes bactériens, mais il s'est révélé mutagène et clastogène dans
des cellules mammaliennes. Dans les études in vivo (principalement
les études d'inhalation) qui ont clairement démontré la toxicité du
méthacrylate de méthyle pour les tissus cibles, on trouve également
quelques indices de génotoxicité.
Le méthacrylate de méthyle est légèrement irritant pour la peau
de l'homme et il peut induire une sensibilisation cutanée chez des
individus prédisposés. Bien que l'on ait également signalé des cas
d'asthme professionnel liés au méthacrylate de méthyle, il n'est pas
prouvé de façon concluante que cette substance soit un sensibilisant
des voies respiratoires. Dans l'ensemble, les études épidémiologiques
disponibles n'apportent pas de preuve convaincante d'un effet
cancérogène chez l'homme, mais on ne peut pas en déduire non plus avec
quelque certitude que l'exposition au méthacrylate de méthyle
n'entraîne aucun risque supplémentaire.
Le méthacrylate de méthyle est peu toxique pour les organismes
aquatiques. Aucune étude de toxicité chronique portant sur ce type
d'organismes n'a été retrouvée, mais des épreuves de toxicité aiguë
ont été effectuées sur des poissons, sur Daphnia magna et sur des
algues. L'effet le plus sensible a été l'inhibition de la
multiplication cellulaire chez l'algue verte Scenedesmus
quadricauda après 8 jours d'exposition à la concentration de
37 mg/litre. La valeur la plus faible de CE50 à 24 heures pour
l'immobilisation des Daphnia est de 720 mg/litre. La CL50 à
96 heures pour le poisson Lepomis macrochirus, en conditions de
renouvellement continu, était de 191 mg/litre, tandis que les CL50
pour des durées de 1 à 24 heures allaient de 420 à 356 mg/litre. La
CL50 à 96 heures pour la truite arc-en-ciel (Oncorhynchus mykiss)
en conditions de renouvellement continu était supérieure à la plus
forte concentration testée, soit 79 mg/litre. Des réactions
sublétales/comportementales ont été notées chez des poissons à
40 mg/litre.
Les données disponibles chez l'homme sont jugées insuffisantes
pour servir de base principale au calcul d'une valeur guide; on a donc
établi une concentration tolérable sur la base de la dégénérescence
inflammatoire de l'épithélium nasal observée chez des rats exposés
pendant 2 ans à une concentration de 410 mg/m3. Dans cette étude, la
NOEL était d'approximativement 100 mg/m3. Les données pouvant servir
à évaluer l'exposition indirecte dans l'environnement général ou
l'exposition des consommateurs sont extrêmement limitées. La
concentration tolérable calculée (probablement avec une certaine marge
de sécurité), qui est d'environ 0,2 mg/m3, est supérieure de
plusieurs ordres de grandeur aux quelques prédictions qui ont été
faites des concentrations atmosphériques de méthacrylate de méthyle
dans l'environnement général. Le niveau d'exposition par inhalation
auquel on peut s'attendre du fait de l'utilisation de peintures
dispersables ou de peintures à l'huile contenant du méthacrylate de
méthyle est peut-être supérieur d'un ordre de grandeur à la dose
qu'entraînerait une exposition à la concentration tolérable, encore
que dans certains pays ces produits ne soient pas destinés au grand
public. Aucun renseignement n'a été trouvé sur leurs conditions
d'utilisation dans d'autres pays. Une dose journalière tolérable
(DJT) de 1,2 mg/kg de poids corporel par jour a été calculée sur la
base d'une étude de toxicité chronique par voie orale.
Bien que les données disponibles au sujet des effets du
méthacrylate de méthyle sur l'environnement soient limitées et que les
concentrations prévues dans divers milieux soient très incertaines, il
existe une marge considérable entre les concentrations suivies
d'effets avérés et les concentrations prévues de façon approximative
dans l'environnement.
RESUMEN DE ORIENTACION
Esta reseña de la evaluación química internacional del
metacrilato de metilo fue preparada por la Dirección de Higiene del
Medio de Health Canada y se basó principalmente en un examen realizado
por el Gobierno del Canadá (1993) para evaluar los efectos potenciales
sobre la salud humana de la exposición indirecta a dicha sustancia en
el medio ambiente general, así como sus efectos ambientales, y en un
examen del Centro Internacional de Investigaciones sobre el Cáncer
(CIIC, 1994) centrado principalmente en la identificación de riesgos
de carcinogenicidad. En el examen del Gobierno del Canadá (1993) se
tuvieron presentes los datos obtenidos en marzo de 1992, actualizados
posteriormente sobre la base de una amplia búsqueda bibliográfica
realizada en septiembre de 1995 en las bases de datos en línea y el
Registro Internacional de Productos Químicos Potencialmente Tóxicos.
En el apéndice 1 se presenta información sobre la naturaleza de la
revisión científica y sobre los exámenes del Gobierno del Canadá
(1993) y del CIIC (1994). Durante la fase de revisión científica de
esta reseña se tomaron en consideración otros exámenes provisionales
de la Dirección de Salud y Seguridad del Reino Unido (Cary et al.,
1995) y de la Unión Europea (Evaluación Provisional del Metacrilato de
Metilo), así como exámenes ya publicados del ECETOC (1995) y de la
Junta Consultiva Finlandesa de Sustancias Químicas (1992), con miras
principalmente a obtener información adicional pertinente para la
revisión. También se ha incorporado la información adicional
identificada durante la revisión efectuada por los puntos de contacto
y el examen realizado por el Comité de Revisión Final. La información
relativa a la revisión científica de la presente reseña figura en el
apéndice 2. La publicación de esta reseña fue aprobada en una reunión
del Comité de Revisión Final celebrada en Bruselas (Bélgica) del 18 al
20 de noviembre de 1996. La lista de participantes en la reunión del
Comité de Revisión Final figura en el apéndice 3. También se ha
reproducido en este documento la ficha internacional de seguridad
química para el metacrilato de metilo (ICSC 0300), emitida por el
Programa Internacional de Seguridad de las Sustancias Químicas (IPCS,
1993).
El metacrilato de metilo (N° CAS 80-62-6) es una sustancia
química sintética y volátil que se utiliza principalmente en la
producción de hojas acrílicas fraguadas, emulsiones acrílicas y
resinas moldeadas y extruidas. Los polímeros y copolímeros de
metacrilato de metilo también se utilizan en los revestimientos de
exterior con base acuosa, con base disolvente y sin diluir, en
adhesivos, selladores, revestimientos de cuero y de papel, tintas,
ceras para suelos, aprestos textiles, prótesis dentales, cementos
quirúrgicos fosfatados y pantallas acrílicas emplomadas contra la
radiación, así como en la preparación de uñas sintéticas y separadores
ortéticos para zapatos. La mayor parte de las emisiones del
metacrilato de metilo se producen en el aire, liberándose en muy
pequeñas cantidades en el agua y en el suelo. Su persistencia en la
atmósfera es corta y no se considera que contribuya directamente al
agotamiento de la capa de ozono. No parece que se bioconcentre en el
medio ambiente y su inhalación con el aire probablemente sea la
principal vía de exposición humana.
El metacrilato de metilo tiene una absorción y distribución
rápidas en animales de experimentación, tras su inhalación o
administración por vía oral. Los datos de que se dispone sobre su
absorción tras una exposición cutánea son limitados. Tanto en
animales de experimentación como en seres humanos, el metacrilato de
metilo se metaboliza rápidamente en ácido metacrílico. Tras su
inhalación, el 16-20% de la sustancia química se deposita en las vías
respiratorias altas de las ratas, donde principalmente es metabolizada
por las esterasas del tejido local.
El metacrilato de metilo tiene una toxicidad aguda baja. En los
roedores y conejos expuestos a concentraciones relativamente altas se
han observado irritaciones cutáneas, oculares y de la cavidad nasal.
Esta sustancia química es un sensibilizador cutáneo ligero en los
animales. El efecto observado con mayor frecuencia tras una
exposición repetida por inhalación en su concentración más baja es la
irritación de la cavidad nasal. También se han señalado efectos en el
riñón y el hígado con concentraciones más altas. El nivel mínimo de
inhalación con efectos comunicados fue de 410 mg/m3 en las ratas
expuestas a metacrilato de metilo durante dos años (basado en la
degeneración inflamatoria del epitelio nasal); el nivel sin efectos
observados (NOEL) en esta investigación fue de aproximadamente 100
mg/m3.
En un estudio bien llevado sobre ratas no se observaron efectos
en el desarrollo, aunque se produjeron disminuciones en el peso
corporal materno tras la inhalación de concentraciones de hasta 8315
mg/m3. Los demás datos de que se dispone sobre la toxicidad para el
desarrollo se limitan a los resultados de un número reducido de
estudios precoces o mal documentados en los que se observaron efectos
fetotóxicos con concentraciones (cuando se comunicaron) que resultaron
tóxicas para las madres. Se dispone de pocos datos sobre los efectos
del metacrilato de metilo en la reproducción. No hubo disminución de
la fecundidad en una valoración de dominancia letal en ratones
expuestos a concentraciones de hasta 36 900 mg/m3 ni se observaron
efectos negativos sobre los órganos reproductores en los estudios de
administración repetida realizados hasta la fecha. Se dispone de
pocos datos sobre la neurotoxicidad del metacrilato de metilo; se
observaron una disminución de la actividad locomotora y efectos a
nivel de aprendizaje, de comportamiento y bioquímico en el cerebro, en
ratas expuestas por vía oral a 500 mg/kg de peso corporal al día
durante 21 días.
El metacrilato de metilo no resultó carcinógeno en una amplia
biovaloración bien documentada de dos años realizada en ratas y
ratones expuestos por inhalación, ni en otros estudios de inhalación
crónica realizados en ratas y hámsters. Aunque no es mutagénico en
los sistemas bacterianos in vitro, ha resultado mutagénico y
clastogénico en las células de mamíferos in vitro. En los estudios
in vivo (principalmente por inhalación) en los que ha habido pruebas
claras de toxicidad en el tejido diana, ha habido pocos indicios de la
genotoxicidad del metacrilato de metilo.
En los seres humanos, el metacrilato de metilo es un irritante
cutáneo ligero y puede inducir sensibilización cutánea en las personas
con predisposición. Aunque también se han señalado casos de asma
profesional asociados a dicha sustancia, no existen pruebas
concluyentes de que el metacrilato de metilo sea un sensibilizador
respiratorio. En general, los estudios epidemiológicos disponibles no
ofrecen indicios firmes ni sistemáticos de un efecto carcinogénico del
metacrilato de metilo en ningún órgano diana en los seres humanos,
pero tampoco se puede inferir con algún grado de confianza que se haya
descartado la posibilidad de un exceso de riesgo.
El metacrilato de metilo tiene una toxicidad baja en organismos
acuáticos. Aunque no se identificaron estudios de toxicidad crónica
en organismos acuáticos, se han realizado pruebas de toxicidad aguda
en Daphnia magna y en algas. El efecto más sensible fue el comienzo
de la inhibición de la multiplicación celular en el alga verde
Scenedesmus quadricauda, con niveles de 37 mg/litro tras ocho días
de exposición. La CE50 de inmovilización más baja notificada a las
24 horas para Daphnia es de 720 mg/litro. La CL50 a las 96 horas
en juveniles de Lepomis macrochirus en condiciones de flujo continuo
fue de 191 mg/litro, mientras que los valores de la CL50 para
periodos de 1 a 24 horas oscilaron entre 420 y 356 mg/litro,
respectivamente. La CL50 a las 96 horas para la trucha arco iris
(Oncorhynchus mykiss) en condiciones de flujo continuo fue >79
mg/litro, la concentración más alta probada. Se observaron respuestas
subletales/comportamentales en los peces, con niveles de 40 mg/litro.
Los estudios de que se dispone en seres humanos se consideran
inadecuados como base principal para establecer un valor orientativo;
por consiguiente, a modo de orientación, se ha establecido una
concentración tolerable sobre la base de la degeneración inflamatoria
del epitelio nasal de las ratas expuestas a concentraciones de 410
mg/m3 durante dos años. La concentración sin efectos observados en
esta investigación fue de aproximadamente 100 mg/m3. Los datos de
que se dispone para establecer una base para la estimación de la
exposición indirecta en el medio ambiente general y de la exposición
en los usuarios son muy limitados. La concentración tolerable
obtenida (probablemente moderada) de aproximadamente 0,2 mg/m3 es
muchos órdenes de magnitud más elevada que las concentraciones
previstas de la muestra de metacrilato de metilo en el aire ambiente
del medio ambiente general. La exposición por inhalación prevista por
el uso de pinturas de dispersión y al aceite que contienen metacrilato
de metilo puede ascender a un orden de magnitud superior a la ingesta
tolerable asociada con la exposición al nivel de concentración
tolerable, si bien se ha notificado que en algunos países esos
productos no se suministran al público en general. No se obtuvo
información sobre las modalidades de utilización de estos productos en
otros países. Sobre la base de un estudio de toxicidad crónica
realizado por vía oral, se ha determinado una ingesta diaria tolerable
(IDT) de 1,2 mg/kg de peso corporal al día.
Si bien los datos de que se dispone sobre los efectos ambientales
del metacrilato de metilo son limitados y los valores pronosticados en
distintos medios son muy inciertos, existe un amplio margen entre los
niveles con efectos observados y las concentraciones ambientales de
pronóstico incierto.
See Also:
Toxicological Abbreviations
Methyl methacrylate (ICSC)
Methyl Methacrylate (IARC Summary & Evaluation, Volume 60, 1994)
 1. EXECUTIVE SUMMARY
This CICAD on methyl methacrylate was prepared by the
Environmental Health Directorate of Health Canada and was based
principally on a Government of Canada (1993) review to assess the
potential effects on human health of indirect exposure to methyl
methacrylate in the general environment as well as the chemical's
environmental effects and an International Agency for Research on
Cancer review (IARC, 1994) concerning primarily hazard identification
for carcinogenicity. Data identified as of March 1992 were considered
in the Government of Canada (1993) review and were subsequently
updated, based on a comprehensive literature search conducted in
September 1995 of on-line databases and the International Register of
Potentially Toxic Chemicals. Information on the nature of peer review
and the availability of the Government of Canada (1993) and IARC
(1994) reviews is presented in Appendix 1. During the peer review
phase for this CICAD, additional draft reviews of the United Kingdom
Health and Safety Executive (Cary et al., 1995) and the European Union
(Draft Assessment on Methyl Methacrylate) and published reviews of
ECETOC (1995) and the Finnish Advisory Board of Chemicals (1992) were
considered, primarily for identification of relevant additional
information for review. Additional information identified during
review by Contact Points and consideration by the Final Review Board
has also been incorporated. Information on the peer review of this
CICAD is presented in Appendix 2. This CICAD was approved for
publication at a meeting of the Final Review Board, held in Brussels,
Belgium, on 18-20 November 1996. Participants at the Final Review
Board meeting are listed in Appendix 3. The International Chemical
Safety Card for methyl methacrylate (ICSC 0300), produced by the
International Programme on Chemical Safety (IPCS, 1993), has also been
reproduced in this document.
Methyl methacrylate (CAS no.
1. EXECUTIVE SUMMARY
This CICAD on methyl methacrylate was prepared by the
Environmental Health Directorate of Health Canada and was based
principally on a Government of Canada (1993) review to assess the
potential effects on human health of indirect exposure to methyl
methacrylate in the general environment as well as the chemical's
environmental effects and an International Agency for Research on
Cancer review (IARC, 1994) concerning primarily hazard identification
for carcinogenicity. Data identified as of March 1992 were considered
in the Government of Canada (1993) review and were subsequently
updated, based on a comprehensive literature search conducted in
September 1995 of on-line databases and the International Register of
Potentially Toxic Chemicals. Information on the nature of peer review
and the availability of the Government of Canada (1993) and IARC
(1994) reviews is presented in Appendix 1. During the peer review
phase for this CICAD, additional draft reviews of the United Kingdom
Health and Safety Executive (Cary et al., 1995) and the European Union
(Draft Assessment on Methyl Methacrylate) and published reviews of
ECETOC (1995) and the Finnish Advisory Board of Chemicals (1992) were
considered, primarily for identification of relevant additional
information for review. Additional information identified during
review by Contact Points and consideration by the Final Review Board
has also been incorporated. Information on the peer review of this
CICAD is presented in Appendix 2. This CICAD was approved for
publication at a meeting of the Final Review Board, held in Brussels,
Belgium, on 18-20 November 1996. Participants at the Final Review
Board meeting are listed in Appendix 3. The International Chemical
Safety Card for methyl methacrylate (ICSC 0300), produced by the
International Programme on Chemical Safety (IPCS, 1993), has also been
reproduced in this document.
Methyl methacrylate (CAS no. 