
Concise International Chemical Assessment Document 6
BIPHENYL
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by Dr A. Boehncke, Dr G. Koennecker, Dr I.
Mangelsdorf, and Dr A. Wibbertmann, Fraunhofer Institute for
Toxicology and Aerosol Research, Hanover, Germany
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization Geneva, 1999
The International Programme on Chemical Safety (IPCS),
established in 1980, is a joint venture of the United Nations
Environment Programme (UNEP), the International Labour Organisation
(ILO), and the World Health Organization (WHO). The overall objectives
of the IPCS are to establish the scientific basis for assessment of
the risk to human health and the environment from exposure to
chemicals, through international peer review processes, as a
prerequisite for the promotion of chemical safety, and to provide
technical assistance in strengthening national capacities for the
sound management of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization, the United Nations
Institute for Training and Research, and the Organisation for Economic
Co-operation and Development (Participating Organizations), following
recommendations made by the 1992 UN Conference on Environment and
Development to strengthen cooperation and increase coordination in the
field of chemical safety. The purpose of the IOMC is to promote
coordination of the policies and activities pursued by the
Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing-in-Publication Data
Biphenyl.
(Concise international chemical assessment document ; 6)
1. Biphenyl compounds - adverse effects
2. Biphenyl compounds - toxicity
3. Environmental exposure
I. International Programme on Chemical Safety
II. Series
ISBN 92 4 153006 5 (NLM classification: WA 240)
ISSN 1020-6167
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1999
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city, or area
or of its authorities, or concerning the delimitation of its frontiers
or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
The Federal Ministry for the Environment, Nature Conservation and
Nuclear Safety, Germany, provided financial support for the printing
of this publication.
TABLE OF CONTENTS
FOREWORD
1. EXECUTIVE SUMMARY
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
3. ANALYTICAL METHODS
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1. Environmental levels
6.2. Human exposure
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposure
8.2. Irritation and sensitization
8.3. Short-term exposure
8.3.1. Oral exposure
8.3.2. Inhalation exposure
8.3.3. Dermal exposure
8.4. Long-term exposure
8.4.1. Subchronic exposure
8.4.1.1 Oral exposure
8.4.1.2 Inhalation exposure
8.4.2. Chronic exposure and carcinogenicity
8.4.2.1 Tumour promotion
8.5. Genotoxicity and related end-points
8.6. Reproductive and developmental toxicity
8.7. Immunological and neurological effects
9. EFFECTS ON HUMANS
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1. Aquatic environment
10.2. Terrestrial environment
11. EFFECTS EVALUATION
11.1. Evaluation of health effects
11.1.1. Hazard identification and dose-response assessment
11.1.2. Criteria for setting guidance values for biphenyl
11.1.3. Sample risk characterization
11.2. Evaluation of environmental effects
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
13.1. Advice to physicians
13.2. Health surveillance advice
13.3. Spillage
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
INTERNATIONAL CHEMICAL SAFETY CARD
REFERENCES
APPENDIX 1 -- SOURCE DOCUMENTS
APPENDIX 2 -- CICAD PEER REVIEW
APPENDIX 3 -- CICAD FINAL REVIEW BOARD (Brussels, Belgium)
APPENDIX 4 -- SPECIALIZED CICAD PEER REVIEW
APPENDIX 5 -- CICAD FINAL REVIEW BOARD (Washington, DC)
RÉSUMÉ D'ORIENTATION
RESUMEN DE ORIENTACION
FOREWORD
Concise International Chemical Assessment Documents (CICADs) are
the latest in a family of publications from the International
Programme on Chemical Safety (IPCS) -- a cooperative programme of the
World Health Organization (WHO), the International Labour Organisation
(ILO), and the United Nations Environment Programme (UNEP). CICADs
join the Environmental Health Criteria documents (EHCs) as
authoritative documents on the risk assessment of chemicals.
CICADs are concise documents that provide summaries of the
relevant scientific information concerning the potential effects of
chemicals upon human health and/or the environment. They are based on
selected national or regional evaluation documents or on existing
EHCs. Before acceptance for publication as CICADs by IPCS, these
documents undergo extensive peer review by internationally selected
experts to ensure their completeness, accuracy in the way in which the
original data are represented, and the validity of the conclusions
drawn.
The primary objective of CICADs is characterization of hazard and
dose-response from exposure to a chemical. CICADs are not a summary of
all available data on a particular chemical; rather, they include only
that information considered critical for characterization of the risk
posed by the chemical. The critical studies are, however, presented in
sufficient detail to support the conclusions drawn. For additional
information, the reader should consult the identified source documents
upon which the CICAD has been based.
Risks to human health and the environment will vary considerably
depending upon the type and extent of exposure. Responsible
authorities are strongly encouraged to characterize risk on the basis
of locally measured or predicted exposure scenarios. To assist the
reader, examples of exposure estimation and risk characterization are
provided in CICADs, whenever possible. These examples cannot be
considered as representing all possible exposure situations, but are
provided as guidance only. The reader is referred to EHC 1701 for
advice on the derivation of health-based guidance values.
While every effort is made to ensure that CICADs represent the
current status of knowledge, new information is being developed
constantly. Unless otherwise stated, CICADs are based on a search of
the scientific literature to the date shown in the executive summary.
In the event that a reader becomes aware of new information that would
change the conclusions drawn in a CICAD, the reader is requested to
contact IPCS to inform it of the new information.
1 International Programme on Chemical Safety (1994) Assessing
human health risks of chemicals: derivation of guidance values
for health-based exposure limits. Geneva, World Health Organization
(Environmental Health Criteria 170).
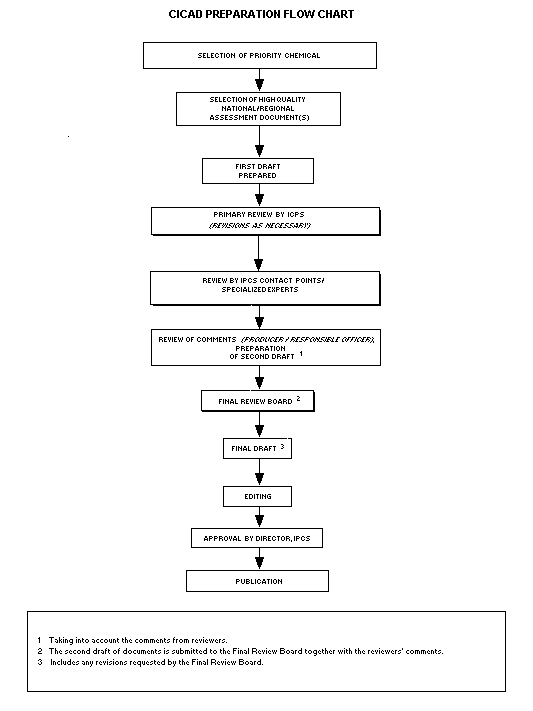
Procedures
The flow chart shows the procedures followed to produce a CICAD.
These procedures are designed to take advantage of the expertise that
exists around the world -- expertise that is required to produce the
high-quality evaluations of toxicological, exposure, and other data
that are necessary for assessing risks to human health and/or the
environment.
The first draft is based on an existing national, regional, or
international review. Authors of the first draft are usually, but not
necessarily, from the institution that developed the original review.
A standard outline has been developed to encourage consistency in
form. The first draft undergoes primary review by IPCS to ensure that
it meets the specified criteria for CICADs.
The second stage involves international peer review by scientists
known for their particular expertise and by scientists selected from
an international roster compiled by IPCS through recommendations from
IPCS national Contact Points and from IPCS Participating Institutions.
Adequate time is allowed for the selected experts to undertake a
thorough review. Authors are required to take reviewers' comments into
account and revise their draft, if necessary. The resulting second
draft is submitted to a Final Review Board together with the
reviewers' comments.
The CICAD Final Review Board has several important functions:
- to ensure that each CICAD has been subjected to an appropriate
and thorough peer review;
- to verify that the peer reviewers' comments have been addressed
appropriately;
- to provide guidance to those responsible for the preparation of
CICADs on how to resolve any remaining issues if, in the opinion
of the Board, the author has not adequately addressed all
comments of the reviewers; and
- to approve CICADs as international assessments.
Board members serve in their personal capacity, not as representatives
of any organization, government, or industry. They are selected
because of their expertise in human and environmental toxicology or
because of their experience in the regulation of chemicals. Boards are
chosen according to the range of expertise required for a meeting and
the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
 1. EXECUTIVE SUMMARY
This CICAD on biphenyl was prepared by the Fraunhofer Institute
for Toxicology and Aerosol Research, Hanover, Germany, based
principally on a review prepared by the German Advisory Committee on
Existing Chemicals of Environmental Relevance (BUA, 1990) as well as a
supplementary report (BUA, 1994) to assess the potential effects of
biphenyl on humans and the environment. The source documents and a
description of their review processes are presented in Appendix 1. A
comprehensive literature search of several online databases to June
1996 was also conducted to identify any additional data. Additional
information identified during review by contact points and
consideration by the Final Review Board (Brussels, Belgium) has also
been incorporated into this CICAD. Information on the peer review of
this CICAD is presented in Appendix 2. This CICAD was given
provisional approval as an international assessment at a meeting of
the Final Review Board held in Brussels, Belgium, on 18-20 November
1996. Participants at the Brussels Final Review Board meeting are
listed in Appendix 3. Following the incorporation of data related to
the carcinogenicity of this substance from a recently completed 2-year
carcinogenicity bioassay, the revised document was subjected to two
rounds of written peer review. In addition, that part of the document
dealing specifically with the human health assessment (i.e. section
11.1) was reviewed at a meeting held at the National Center for
Environmental Assessment, US Environmental Protection Agency, in
Washington, DC (USA), on 7 December 1998. Individuals contributing to
the additional specialized reviews of this CICAD are listed in
Appendix 4. This CICAD was approved as an international assessment at
a meeting of the Final Review Board held in Washington, DC (USA), on
8-11 December 1998. Participants at the Washington Final Review Board
meeting are listed in Appendix 5. The International Chemical Safety
Card (ICSC 0106) produced by the International Programme on Chemical
Safety (IPCS, 1993) has also been reproduced in this document.
Biphenyl (CAS No. 92-52-4), an aromatic hydrocarbon, is a
colourless solid at room temperature. It is used as an intermediate in
the production of a variety of compounds (e.g. emulsifiers, optical
brighteners, crop protection products, plastics), as a heat transfer
medium in heating fluids, as a dyestuff carrier for textiles and
copying paper, as a solvent in pharmaceutical production, and in the
preservation of citrus fruits.
Biphenyl occurs naturally in coal tar, crude oil, and natural
gas. Anthropogenic sources of environmental exposure include
production and processing plants, citrus fruit or wood preserving
facilities, and municipal waste disposal sites. Biphenyl is formed
during the incomplete combustion of mineral oil and coal and is
present in the exhaust gases of vehicle traffic and in exhaust air
from residential and industrial heating devices. In ambient air,
typical concentrations of biphenyl range from 1 to 100 ng/m3. Levels
in indoor air are higher (100-1000 ng/m3), likely as a result of
cigarette smoke and emissions from heating devices or nearby garages.
In measurements conducted in the 1970s, levels of biphenyl in
tap-water were usually below 5 ng/litre. More recent data were not
identified. In surface waters, concentrations are typically below 500
ng/litre. In sediment, soil, and biota, biphenyl was measured only in
the direct vicinity of industrial plants and waste dumps.
Biphenyl volatilizes from aqueous solution and has a low water
solubility. The main degradation pathway in the troposphere is the
reaction with hydroxyl radicals, for which a mean half-life of
approximately 2 days has been calculated. The substance is not
expected to hydrolyse under environmental conditions. It is
biodegradable under aerobic conditions. Based upon available data,
biphenyl should be almost immobile in soil; the probability of
infiltration into groundwater is low. In the food-chain important to
humans, bioaccumulation can take place, specifically in plants;
however, based upon the potential bioaccumulation of biphenyl,
biomagnification of biphenyl in higher trophic levels of the aquatic
or terrestrial food-chain is expected to be of minor importance.
Biphenyl is well absorbed through the gastrointestinal tract and
presumably also via lung and skin. In those species examined, the
metabolites of biphenyl, mainly 4-hydroxybiphenyl, are excreted
rapidly and almost exclusively in the urine. The acute oral toxicity
of biphenyl is moderate. It is non-irritating to skin and only
slightly irritating to the eyes. There is no evidence of dermal
sensitization. Toxicity studies with biphenyl after repeated exposure
by inhalation are not adequate to establish a no-observed-effect level
(NOEL) with confidence. Subchronic exposure by inhalation caused
bronchopulmonary changes, whereas long-term toxicity studies following
inhalation exposure were not identified in the literature.
In toxicological studies in which rodents have been administered
diets containing biphenyl for various periods of time, effects on the
urinary system have often been reported. A marked increase in the
incidence of morphological (i.e. formation of calculi) and
histopathological (e.g. hyperplasia, desquamation) effects has been
observed within the urinary tract of male rats administered diets
containing more than 2500 mg biphenyl/kg. An increase in the
occurrence of calculi and squamous metaplasia within the urinary
bladder of female rats has also been observed, but at a lower
incidence than in males. In male mice, only 1 of 10 animals given a
diet containing 10 000 mg biphenyl/kg (1500 mg/kg body weight per day)
for 32 weeks developed simple hyperplasia and papillary or nodular
dysplasia of the urinary bladder. Effects on blood chemistry and
haematological parameters have also been observed in animals
administered biphenyl orally; these effects occur in male and female
rats and mice at intakes lower than those associated with the
development of effects in the urinary bladder of male rats
administered biphenyl. For non-neoplastic effects, the
lowest-observed-effect level (LOEL) was 38 mg/kg body weight per day,
based upon the development of alterations in haematological parameters
(i.e. decreased haemoglobin concentration and haematocrit) in rats fed
diets containing 0, 500, 1500, or 4500 mg biphenyl/kg (reported
intakes of 0, 38, 113, or 338 mg/kg body weight per day) for 2 years.
In vitro studies with bacteria have provided no evidence of
mutagenic potential for biphenyl; with Saccharomyces cerevisiae D7,
gene mutation and mitotic recombination were observed with or without
metabolic activation. However, genetic toxicology testing in mammalian
cells has produced positive results in the presence of metabolic
activation and negative results in the absence of metabolic
activation. In one inadequately documented and inadequately performed
in vivo study, biphenyl did not induce chromosomal aber rations in
the bone marrow of rats after exposure by inhalation. The results of
in vitro studies indicate that biphenyl has mutagenic potential; in
the absence of reassurance from reliable results from in vivo tests,
it is assumed that exposure to biphenyl may be associated with a
mutagenic risk.
In female mice, there were slight increases in the incidences of
benign and malignant liver tumours in animals receiving biphenyl in
the diet for 2 years. An increased incidence of bladder tumours was
observed in male rats, but not in female rats or male and female mice,
administered diets containing high levels of biphenyl. There have been
suggestions that the formation of such bladder tumours may be linked
to the regenerative hyperplasia of the urinary epithelium, caused by
the abrasion and damage to the urothelium that are produced by calculi
formed within the urinary tract only at very high levels of exposure;
it has also been suggested that, because of anatomical and
physiological differences, the sex- and species-specific development
of bladder tumours in male rats receiving high doses of biphenyl might
not be strictly relevant to humans exposed to lower levels. However,
1) observations of an increased incidence of histopathological effects
and the formation of calculi within the urinary bladder, in the
absence of bladder tumours, in female rats administered biphenyl for 2
years, 2) a lack of data identifying a direct association between
calculi formation, regenerative hyperplasia of the urothelium, and the
development of bladder tumours within individual male animals, and 3)
the potential genotoxicity of biphenyl could suggest that the
development of bladder tumours in the male rats may not have been
entirely due to effects associated with the formation of calculi
within the urinary bladder. This observation, as well as evidence of
hepatocarcinogenicity in female mice, raises some concerns with
respect to the potential carcinogenicity of biphenyl.
Available data on the reproductive toxicity of biphenyl are
limited. Apart from results of a three-generation study in rats, in
which adverse effects (decreased fertility, litter size, growth rate)
were noted, there was no evidence that biphenyl induced reproductive
or developmental effects.
Exposure to high levels of biphenyl vapours or dust at the
workplace results in irritation of the eyes and inflammation of the
respiratory tract. Long-term exposure for several years to high
biphenyl concentrations (up to 128 mg/m3) caused damage to the liver
and persistent neuronal changes; direct skin contact may have played a
part, in addition to uptake through the respiratory tract.
The available data on biphenyl levels in the general environment
are limited, and the information is insufficient to permit a
reasonable estimation of the intake of biphenyl. Inhalation (from
polluted air and from the use of biphenyl-containing creosotes in wood
preservation) and ingestion (from biphenyl's use as a preservative for
citrus fruits) are possible sources of exposure.
Biphenyl has weak bactericidal and fungistatic properties. In
toxicity studies on aquatic organisms of four trophic levels, the
lowest no-observed-effect concentration (NOEC) reported for the most
sensitive species ( Daphnia magna) in chronic tests was 0.17
mg/litre. A predicted no-effect concentration (PNEC) of 1.7 µg/litre
can be calculated by applying an assessment factor of 10, considering
ecotoxicological and environmental fate characteristics of the
substance. Valid data on toxic effects on terrestrial organisms and
ecosystems, which could be used for risk assessment, are not
available.
Owing to the lack of data from other parts of the world, a sample
risk assessment was performed for Germany. From concentrations
measured in German rivers in the 1990s, the ratio of the predicted
environmental concentration (PEC) to PNEC is calculated to be
<0.29. A PEC/PNEC ratio of <1 indicates that a significant risk
for the environment is not to be expected.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
Biphenyl (CAS No. 92-52-4; C12H10; 1,1'-biphenyl, diphenyl,
phenylbenzene) has the following structural formula:
1. EXECUTIVE SUMMARY
This CICAD on biphenyl was prepared by the Fraunhofer Institute
for Toxicology and Aerosol Research, Hanover, Germany, based
principally on a review prepared by the German Advisory Committee on
Existing Chemicals of Environmental Relevance (BUA, 1990) as well as a
supplementary report (BUA, 1994) to assess the potential effects of
biphenyl on humans and the environment. The source documents and a
description of their review processes are presented in Appendix 1. A
comprehensive literature search of several online databases to June
1996 was also conducted to identify any additional data. Additional
information identified during review by contact points and
consideration by the Final Review Board (Brussels, Belgium) has also
been incorporated into this CICAD. Information on the peer review of
this CICAD is presented in Appendix 2. This CICAD was given
provisional approval as an international assessment at a meeting of
the Final Review Board held in Brussels, Belgium, on 18-20 November
1996. Participants at the Brussels Final Review Board meeting are
listed in Appendix 3. Following the incorporation of data related to
the carcinogenicity of this substance from a recently completed 2-year
carcinogenicity bioassay, the revised document was subjected to two
rounds of written peer review. In addition, that part of the document
dealing specifically with the human health assessment (i.e. section
11.1) was reviewed at a meeting held at the National Center for
Environmental Assessment, US Environmental Protection Agency, in
Washington, DC (USA), on 7 December 1998. Individuals contributing to
the additional specialized reviews of this CICAD are listed in
Appendix 4. This CICAD was approved as an international assessment at
a meeting of the Final Review Board held in Washington, DC (USA), on
8-11 December 1998. Participants at the Washington Final Review Board
meeting are listed in Appendix 5. The International Chemical Safety
Card (ICSC 0106) produced by the International Programme on Chemical
Safety (IPCS, 1993) has also been reproduced in this document.
Biphenyl (CAS No. 92-52-4), an aromatic hydrocarbon, is a
colourless solid at room temperature. It is used as an intermediate in
the production of a variety of compounds (e.g. emulsifiers, optical
brighteners, crop protection products, plastics), as a heat transfer
medium in heating fluids, as a dyestuff carrier for textiles and
copying paper, as a solvent in pharmaceutical production, and in the
preservation of citrus fruits.
Biphenyl occurs naturally in coal tar, crude oil, and natural
gas. Anthropogenic sources of environmental exposure include
production and processing plants, citrus fruit or wood preserving
facilities, and municipal waste disposal sites. Biphenyl is formed
during the incomplete combustion of mineral oil and coal and is
present in the exhaust gases of vehicle traffic and in exhaust air
from residential and industrial heating devices. In ambient air,
typical concentrations of biphenyl range from 1 to 100 ng/m3. Levels
in indoor air are higher (100-1000 ng/m3), likely as a result of
cigarette smoke and emissions from heating devices or nearby garages.
In measurements conducted in the 1970s, levels of biphenyl in
tap-water were usually below 5 ng/litre. More recent data were not
identified. In surface waters, concentrations are typically below 500
ng/litre. In sediment, soil, and biota, biphenyl was measured only in
the direct vicinity of industrial plants and waste dumps.
Biphenyl volatilizes from aqueous solution and has a low water
solubility. The main degradation pathway in the troposphere is the
reaction with hydroxyl radicals, for which a mean half-life of
approximately 2 days has been calculated. The substance is not
expected to hydrolyse under environmental conditions. It is
biodegradable under aerobic conditions. Based upon available data,
biphenyl should be almost immobile in soil; the probability of
infiltration into groundwater is low. In the food-chain important to
humans, bioaccumulation can take place, specifically in plants;
however, based upon the potential bioaccumulation of biphenyl,
biomagnification of biphenyl in higher trophic levels of the aquatic
or terrestrial food-chain is expected to be of minor importance.
Biphenyl is well absorbed through the gastrointestinal tract and
presumably also via lung and skin. In those species examined, the
metabolites of biphenyl, mainly 4-hydroxybiphenyl, are excreted
rapidly and almost exclusively in the urine. The acute oral toxicity
of biphenyl is moderate. It is non-irritating to skin and only
slightly irritating to the eyes. There is no evidence of dermal
sensitization. Toxicity studies with biphenyl after repeated exposure
by inhalation are not adequate to establish a no-observed-effect level
(NOEL) with confidence. Subchronic exposure by inhalation caused
bronchopulmonary changes, whereas long-term toxicity studies following
inhalation exposure were not identified in the literature.
In toxicological studies in which rodents have been administered
diets containing biphenyl for various periods of time, effects on the
urinary system have often been reported. A marked increase in the
incidence of morphological (i.e. formation of calculi) and
histopathological (e.g. hyperplasia, desquamation) effects has been
observed within the urinary tract of male rats administered diets
containing more than 2500 mg biphenyl/kg. An increase in the
occurrence of calculi and squamous metaplasia within the urinary
bladder of female rats has also been observed, but at a lower
incidence than in males. In male mice, only 1 of 10 animals given a
diet containing 10 000 mg biphenyl/kg (1500 mg/kg body weight per day)
for 32 weeks developed simple hyperplasia and papillary or nodular
dysplasia of the urinary bladder. Effects on blood chemistry and
haematological parameters have also been observed in animals
administered biphenyl orally; these effects occur in male and female
rats and mice at intakes lower than those associated with the
development of effects in the urinary bladder of male rats
administered biphenyl. For non-neoplastic effects, the
lowest-observed-effect level (LOEL) was 38 mg/kg body weight per day,
based upon the development of alterations in haematological parameters
(i.e. decreased haemoglobin concentration and haematocrit) in rats fed
diets containing 0, 500, 1500, or 4500 mg biphenyl/kg (reported
intakes of 0, 38, 113, or 338 mg/kg body weight per day) for 2 years.
In vitro studies with bacteria have provided no evidence of
mutagenic potential for biphenyl; with Saccharomyces cerevisiae D7,
gene mutation and mitotic recombination were observed with or without
metabolic activation. However, genetic toxicology testing in mammalian
cells has produced positive results in the presence of metabolic
activation and negative results in the absence of metabolic
activation. In one inadequately documented and inadequately performed
in vivo study, biphenyl did not induce chromosomal aber rations in
the bone marrow of rats after exposure by inhalation. The results of
in vitro studies indicate that biphenyl has mutagenic potential; in
the absence of reassurance from reliable results from in vivo tests,
it is assumed that exposure to biphenyl may be associated with a
mutagenic risk.
In female mice, there were slight increases in the incidences of
benign and malignant liver tumours in animals receiving biphenyl in
the diet for 2 years. An increased incidence of bladder tumours was
observed in male rats, but not in female rats or male and female mice,
administered diets containing high levels of biphenyl. There have been
suggestions that the formation of such bladder tumours may be linked
to the regenerative hyperplasia of the urinary epithelium, caused by
the abrasion and damage to the urothelium that are produced by calculi
formed within the urinary tract only at very high levels of exposure;
it has also been suggested that, because of anatomical and
physiological differences, the sex- and species-specific development
of bladder tumours in male rats receiving high doses of biphenyl might
not be strictly relevant to humans exposed to lower levels. However,
1) observations of an increased incidence of histopathological effects
and the formation of calculi within the urinary bladder, in the
absence of bladder tumours, in female rats administered biphenyl for 2
years, 2) a lack of data identifying a direct association between
calculi formation, regenerative hyperplasia of the urothelium, and the
development of bladder tumours within individual male animals, and 3)
the potential genotoxicity of biphenyl could suggest that the
development of bladder tumours in the male rats may not have been
entirely due to effects associated with the formation of calculi
within the urinary bladder. This observation, as well as evidence of
hepatocarcinogenicity in female mice, raises some concerns with
respect to the potential carcinogenicity of biphenyl.
Available data on the reproductive toxicity of biphenyl are
limited. Apart from results of a three-generation study in rats, in
which adverse effects (decreased fertility, litter size, growth rate)
were noted, there was no evidence that biphenyl induced reproductive
or developmental effects.
Exposure to high levels of biphenyl vapours or dust at the
workplace results in irritation of the eyes and inflammation of the
respiratory tract. Long-term exposure for several years to high
biphenyl concentrations (up to 128 mg/m3) caused damage to the liver
and persistent neuronal changes; direct skin contact may have played a
part, in addition to uptake through the respiratory tract.
The available data on biphenyl levels in the general environment
are limited, and the information is insufficient to permit a
reasonable estimation of the intake of biphenyl. Inhalation (from
polluted air and from the use of biphenyl-containing creosotes in wood
preservation) and ingestion (from biphenyl's use as a preservative for
citrus fruits) are possible sources of exposure.
Biphenyl has weak bactericidal and fungistatic properties. In
toxicity studies on aquatic organisms of four trophic levels, the
lowest no-observed-effect concentration (NOEC) reported for the most
sensitive species ( Daphnia magna) in chronic tests was 0.17
mg/litre. A predicted no-effect concentration (PNEC) of 1.7 µg/litre
can be calculated by applying an assessment factor of 10, considering
ecotoxicological and environmental fate characteristics of the
substance. Valid data on toxic effects on terrestrial organisms and
ecosystems, which could be used for risk assessment, are not
available.
Owing to the lack of data from other parts of the world, a sample
risk assessment was performed for Germany. From concentrations
measured in German rivers in the 1990s, the ratio of the predicted
environmental concentration (PEC) to PNEC is calculated to be
<0.29. A PEC/PNEC ratio of <1 indicates that a significant risk
for the environment is not to be expected.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
Biphenyl (CAS No. 92-52-4; C12H10; 1,1'-biphenyl, diphenyl,
phenylbenzene) has the following structural formula:
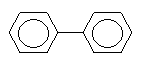 The chemical is an aromatic hydrocarbon with a peculiar, strong
odour similar to that of geraniums (BUA, 1990). At room temperature,
the substance is a colourless solid (melting point 62.2°C). Because of
its significant vapour pressure (4 Pa at 20°C) and low water
solubility (4.45 mg/litre at 20°C), biphenyl shows considerable
volatility from aqueous solutions (BUA, 1990). Its measured
n-octanol/water partition coefficient (log Kow) is between 3.88
and 4.04. The commercial product from the only German producer has a
biphenyl content of 99.85%; named impurities are terphenyl (0.15%),
sulfur (10-20 mg/kg), and benzene (1 mg/kg) (BUA, 1990). Additional
physical/chemical properties are presented in the International
Chemical Safety Card reproduced in this document.
3. ANALYTICAL METHODS
Biphenyl is usually measured in environmental media by capillary
gas chromatography in combination with flame ionization or mass
spectrometric detection (Malins et al., 1985; BUA, 1990; Hawthorne et
al., 1992; Anklam & Mueller, 1994; Karanassios et al., 1994; Otson et
al., 1994; Kostiainen, 1995). For aquatic samples, methods involving
high-performance liquid chromatography with ultraviolet detection at
254 nm have also been described. The following enrichment techniques
are used: solid-phase adsorption (Tenax material or XAD resins) with
thermal desorption or liquid extraction for air samples, solid-phase
adsorption (XAD resins) or liquid/liquid extraction (diethylether) for
water samples, and liquid extraction (dichloromethane/methanol,
ethanol) for soil and biotic samples. The detection limits range from
0.1 to 5 ng/m3 for air and from 0.01 to 8000 ng/litre for water. For
sediment, a detection limit of 2 µg/kg dry weight has been reported.
Detection limits between 12 and 87 µg/kg have been reported for levels
of biphenyl in fish (Malins et al., 1985).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Biphenyl occurs in varying concentrations in coal tar, crude oil
(up to 0.4 mg/g oil), and natural gas (3-42 µg/m3) (BUA, 1990).
Because of its natural occurrence in coal tar and crude oil, biphenyl
has also been detected in products derived from these substances. The
biphenyl content in coal tar-derived creosotes ranges between 0.2 and
1.6% (IARC, 1985; ATSDR, 1990; Collin & Hoeke, 1995). In the aromatic
fraction of an unused lubricating oil sample, biphenyl was measured at
a concentration of 1.5 mg/kg (Paschke et al., 1992).
Biphenyl is also produced from anthropogenic sources. In 1984,
the estimated production capacities of biphenyl in the Federal
Republic of Germany, in Western Europe, and throughout the world were
approximately >6000, 30 000, and 80 000 t, respectively. In 1989,
2000-2500 t of biphenyl were produced in Germany, of which about 900 t
were exported and 200-300 t sold domestically (BUA, 1990).
Approximately 5000 t of biphenyl were manufactured in each of 1992 and
1993 in Japan (Anon., 1994-95).
Until the early 1970s, biphenyl was used principally as an
intermediate in the production of polychlorinated biphenyls (PCBs). As
the production, processing, distribution, and use of PCB compounds are
now prohibited or restricted in many countries (e.g. USA, Germany,
United Kingdom) (IRPTC, 1994), it is likely that previous production
levels were much higher than those today. In 1984, the estimated use
patterns of biphenyl worldwide were, as a final product: 35% in heat
transfer medium in heating fluids, 20% as a dyestuff carrier for
textiles, 5% as a solvent in pharmaceutical production, 5% as a
dyestuff carrier for copying paper, and 5% as a preservative for
citrus fruits; patterns of biphenyl use as an intermediate were: 10%
each in emulsifiers and optical brighteners, 5% for crop protection
products, and 5% for precursors and auxiliaries for plastics (BUA,
1990). At present in Germany, the use of biphenyl as a dyestuff
carrier in textiles is minor.1
Although emissions from exhaust gases during production and
processing of biphenyl and during the use of biphenyl-containing
creosotes for wood preservation may be significant, available data
were not identified. Biphenyl is also released into the atmosphere
during the incomplete combustion of fossil fuels in motor vehicles
(post-catalyst emission factor of 3.5 µg/km; Siegl & Chladek, 1992),
residential heating (1.24 mg biphenyl/kg burnt coal; Engewald et al.,
1993), and coal-burning power plants (with concentrations in flue gas
up to 30 µg/m3; Yao & Xu, 1991; Wienecke et al., 1992) or foundries
(qualitatively detected; Deusen & Kleinermanns, 1993).
1 Personal communication from Verband d. Textilhilfsmittel-,
Lederhilfsmittel-, Gerbstoff- und Waschrohstoff-Industrie e.V.,
Frankfurt/M., Germany, to Bayer AG, 1996.
In 1990, biphenyl was not detected in the effluent of the sewage
plant of the German producer (detection limit 5 µg/litre; weekly
sampling). Based on a biphenyl concentration of <5 µg/litre, a
maximum annual release of 274 kg biphenyl can be calculated for this
plant. No data are available from other producing or processing
facilities. Biphenyl may also be released to surface water through the
processing of coal tar (e.g. coking plants) and mineral oils (e.g.
processing residual oils from pyrolysis in refineries) (BUA, 1990).1
Based upon levels of biphenyl measured in sediment samples during the
1970s, this chemical has been released from various industrial
wastewater outlets associated with a melting plant for iron alloys, a
chemical factory, and an oil storage depot. Data were not available on
releases of biphenyl from textile industries where the chemical is
used as a dyestuff carrier, from wood preservation plants that use
creosotes containing biphenyl, or from treated citrus fruits and their
packaging materials contained in domestic waste sites. Concentrations
of biphenyl between 0.016 µg/kg and 1730 mg/kg have been measured in
sewage sludge, which may be applied to arable land (BUA, 1990).
1 Personal communication from Bayer AG to the GDCh- Advisory
Committee on Existing Chemicals of Environmental Relevance, 1997.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Based upon a Level II fugacity model (Mackay et al., 1992), the
atmosphere appears to be the main target compartment (>90%) for
biphenyl. Biphenyl's Henry's law constant indicates that it
volatilizes from aqueous solutions. The calculated half-life for the
photooxidative degradation of biphenyl by hydroxyl radicals in air is
about 2 days (Atkinson & Aschmann, 1985; BUA, 1990; Atkinson & Arey,
1994). Reactions with tropospheric ozone and nitrate are expected to
be of minor importance, with calculated half-lives of >80 days and
>105 days, respectively. Data on the photodegradation of biphenyl in
water are not available. Biphenyl is not expected to hydrolyse under
environmental conditions.
In one reported standard test with activated sludge, according to
Guideline 301C of the Organisation for Economic Co-operation and
Development (OECD), a biochemical oxygen demand of 66% after 14 days'
incubation was reported when non-adapted inoculum was used (CITI,
1992). Microbial populations from natural waters mineralize biphenyl;
100% degradation was observed after 4 days' incubation of biphenyl in
river water (BUA, 1990). Numerous microorganisms isolated from soil,
sediment, surface water, and groundwater samples have been shown to
metabolize biphenyl (BUA, 1990; Bevinakatti & Ninnekar, 1992; Kiyohara
et al., 1992; Nielsen & Christensen, 1994). Investigations on the
anaerobic biodegradation of biphenyl were not identified.
Soil sorption coefficients ( Koc) based upon laboratory
experiments and calculated values range from 1100 to 18 000 (BUA,
1990; Kishi & Hasimoto, 1991); from numerous literature data, Jeng et
al. (1992) calculated a mean value of 4230. On the basis of these
data, it is expected that biphenyl will be almost immobile in soil,
with a low probability of groundwater infiltration. Volatilization
from soil surfaces is significantly lower than that from aqueous
media; however, no significant geoaccumulation of biphenyl is expected
under aerobic conditions owing to its degradation by microbial
organisms.
In static tests on the bioaccumulation of biphenyl in activated
sludge conducted with several aquatic species (yeast, algae, molluscs,
daphnia, and freshwater fish), bioconcentration factors (BCFs) ranging
between 57 for the marine mussel Mytilus edulis (calculated on wet
weight) (Donkin et al., 1991) and 540 for the green alga Chlorella
vulgaris (based on dry weight) (Kotzias et al., 1980; Freitag et
al., 1982) have been reported. With the exception of two tests, there
was no information on whether equilibrium was reached before the
determination of the BCF. The depuration of accumulated biphenyl was
determined in one study. After a 24-h incubation in a static test
system, the BCF at equilibrium (based on wet weight) for Daphnia
magna was 473; depuration was dependent upon the temperature of the
water. Twenty-four hours after being transferred to pure water, 53% of
the accumulated biphenyl was depurated at 2-3°C, whereas 88% was
depurated at 22°C (Zhang et al., 1983). Although the available data
indicate a potential for bioaccumulation, evaporation, adsorption to
soil/sediment, and degradation are expected to reduce the
bioavailability of biphenyl. Therefore, bioaccumulation of the
chemical should be of minor importance for aquatic organisms.
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
In 1985, concentrations of biphenyl in the air of an
industrialized city in Finland ranged from 1.7 to 26.2 ng/m3 (BUA,
1990). Similar concentrations were measured during the winter of
1988-89 in two US cities (12-119 ng/m3; 30 ng/m3 mean value;
Hawthorne et al., 1992) and in 1992 in two Greek cities (all data
below the detection limit of 5 ng/m3; Karanassios et al., 1994).
The concentrations of biphenyl in the German parts of the Rhine
River declined from a maximum of 1000 ng/litre in the 1970s (BUA,
1990) to levels below 500 ng/litre (the detection limit) in 1993-1995
(LWA, 1993-94).1 Although levels in most of the German tributaries
have remained below the detection limit (500 ng/litre), peak
concentrations of 560 and 1600 ng/litre were reported in 1993 and
1994, respectively, for the highly polluted Emscher tributary (LWA,
1993-94); these elevated levels were attributed to the coking plants
in this area.2 In the USA, similar data for one river have been
reported (Hites, 1973). In some German estuaries, biphenyl
concentrations were significantly lower (1-5 ng/litre) (BUA, 1990).
During 1986-1989, biphenyl was detected at concentrations between
10 and 100 ng/litre in samples of groundwater obtained near Zagreb, in
the former Yugoslavia, in the vicinity of a municipal landfill site.
In a test well sunk 720 m from a heavily polluted river, the levels
were about 10-fold lower (Ahel, 1991). Concentrations of biphenyl
between 2 and 17 ng/litre have been measured in samples of snow and
rain collected in Switzerland (BUA, 1990).
In river and estuarine sediments, biphenyl was detected at very
high concentrations in the direct vicinity of industrial plants or
waste dumps; concentrations ranged between 0.1 and 8 mg/kg, depending
on the source. The highest concentrations were found near waste dump
sites in the USA (Malins et al., 1985; BUA, 1990). Information on the
occurrence of biphenyl in soils with no direct pollution was not
identified. A maximum biphenyl concentration of 13 µg/kg was found in
soil samples collected near a pit for wastewater from oil production
in New Mexico, USA (BUA, 1990).
Biphenyl has been found in fish collected from water contaminated
with mineral oil; concentrations in liver samples were <25 µg/kg dry
weight (i.e. below the detection limit) and 13 620 µg/kg fresh weight
(Paasivirta et al., 1982; Malins et al., 1985).
1 Personal communication from the Northrhine-Westfalian
Environmental Protection Agency to Bayer AG, 1996.
2 Personal communication from Bayer AG to the GDCh- Advisory
Committee on Existing Chemicals of Environmental Relevance, 1997.
6.2 Human exposure
The levels of biphenyl in indoor air may depend mainly on smoking
habits (BUA, 1990). Concentrations of biphenyl are also elevated in
homes having a source of oil and exhaust fumes nearby (e.g. gasoline
station, parking garage) (Otson et al., 1994; Kostiainen, 1995).
Monitoring data from Finnish ( n = 50) (Kostiainen, 1995) and
Canadian ( n = 757) homes (Otson et al., 1994) have revealed average
biphenyl concentrations in the range of 160-1000 ng/m3, with a
maximum concentration of 4700 ng/m3 (Kostiainen, 1995). Biphenyl has
been detected in floor dust collected from five of nine investigated
Danish city halls (Wilkins et al., 1993). Assuming that 20 h are spent
indoors (exposure to 160-1000 ng/m3) and 4 h are spent outdoors
(exposure to 30 ng/m3) each day and that the inhalation volume and
weight of the average adult are 22 m3/day and 64 kg, respectively,
the intake of biphenyl from air can be calculated from these data to
range from 45 to 300 ng/kg body weight per day.
There is only limited intake of biphenyl from drinking-water.
Although more recent data were not identified, concentrations of
biphenyl in tap-water in various countries, such as Finland, Norway,
Sweden, the USA, and Canada, were low (i.e. 0.1-5 ng/litre) in the
1970s (BUA, 1990). Based upon these data and assuming that the average
adult (weighing 64 kg) consumes 2 litres of drinking-water per day,
the estimated intake of biphenyl from drinking-water may range from
0.003 to 0.16 ng/kg body weight per day.
Food may contain biphenyl as a result of its use as a fungistatic
agent for citrus fruits. In older studies with citrus fruits to which
a biphenyl-containing wax had been applied, peels of treated fruits
were found to contain up to 220 mg biphenyl/kg (whole fruit) and the
pulp up to 3.9 mg/kg (whole fruit) (BUA, 1990); however, these levels
were determined with a rather unspecific spectrophotometric method,
which may have overestimated the biphenyl content. In a more reliable
study with 6 grapefruits, 8 oranges, and 10 lemons obtained from
Japanese markets, levels of biphenyl ranged from 17 to 123 mg/kg in
the peel and from <0.01 (the detection limit) to 0.18 mg/kg (average
0.06 mg/kg pulp) in the edible parts of the fruit. In samples of lemon
tea, levels of biphenyl were similar to those in the edible parts of
the fruit (Isshiki et al., 1982). In a survey of foodstuffs conducted
in Italy between 1988 and 1995, which included more than 70 types of
food, biphenyl was detected in only one peach sample (detection limit
not given) (Di Muccio et al., 1995). Based upon the average content of
biphenyl in the pulp of citrus fruits of about 0.06 mg/kg (calculated
from the concentrations measured by Isshiki et al., 1982), the intake
from the consumption of the pulp of one biphenyl-preserved citrus
fruit weighing about 120-400 g is calculated to be about 113-375 ng/kg
body weight. An average intake of 64 ng biphenyl/kg body weight per
day from food was calculated based upon the consumption of foodstuffs
in Finland (Penttilae & Siivinen, 1996). The general population may
also be exposed to biphenyl through contact with consumer products,
such as creosote-preserved wood, textiles, copying papers, or
pharmaceuticals, although quantitative data were not identified.
The intake of biphenyl from the occupational environment may
occur via inhalation and dermal contact. Concentrations of biphenyl in
the air of one facility producing biphenyl-impregnated paper were
reported to range from 4.4 to 128 mg/m3 in 1959 and from 0.6 to 123
mg/m3 in 1970 (Haekkinen et al., 1973). In a nylon production
facility in which workers were exposed to a eutectic mixture of 26.5%
(w/w) biphenyl and 73.5% (w/w) biphenyl ether, time-weighted average
concentrations of biphenyl were reported to range from 0.24 to 1.28
mg/m3 (Dorgelo et al., 1985). Coke oven emissions to indoor air at a
coking plant in Australia contained 0.2-0.5% biphenyl (Kirton et al.,
1991). In a Polish bituminous pulp production facility, reported
time-weighted average concentrations (1988-89) of biphenyl ranged from
0.5 to 0.6 µg/m3 (Baranski, 1991). In a study of two Canadian
pilot-scale coal liquefaction facilities, the levels of biphenyl were
below the detection limit of 60 µg/m3 (Leach et al., 1987).
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS
AND HUMANS
Studies providing quantitative information on the absorption or
distribution of biphenyl in humans were not identified. In various
species of experimental animals, the absorption of biphenyl following
oral exposure has been inferred by the detection of metabolites in the
urine and faeces. Biphenyl is oxidized by multifunctional oxygenases,
irrespective of the route of exposure. Mono-, di-, and trihydroxy
metabolites of biphenyl have been identified in the urine of exposed
species. 4-Hydroxybiphenyl has been observed as the major metabolite
in rats, rabbits, guinea-pigs, and pigs; minor metabolites include
mono-, di-, and trihydroxymethoxy biphenyls, dihydrodiols, and
hydroxydihydrodiols. The phenolic compounds are conjugated with
sulfate or glucuronic acid. The formation of mercapturic acid as well
as evidence of a metabolic pathway involving opening of the benzene
ring have also been described (BUA, 1990). There is no evidence for
enterohepatic circulation in different species (Meyer & Scheline,
1976; Meyer et al., 1976a; Meyer, 1977). On the basis of in vitro
studies, the metabolism of biphenyl is not restricted to the liver
(Bend et al., 1972; Hook et al., 1972; Matsubara et al., 1974; Prough
& Burke, 1975; Hook & Bend, 1976; Powis et al., 1987). Based upon
total cytochrome P-450 content, biphenyl-4-hydroxylase activity was
higher in pulmonary microsomes than in liver microsomes (Hook et al.,
1972; Hook & Bend, 1976). A time-dependent covalent binding of
[14C]bi phenyl to mouse hepatic microsomal proteins has been observed
after metabolic activation (Tanaka et al., 1993).
In rats, rabbits, and pigs, most biphenyl metabolites are
excreted in the urine (BUA, 1990). Following an oral dose of 100 mg
[14C]biphenyl/kg body weight, rats excreted 82% of the administered
radioactivity (76% in urine) within 24 h. The recovery rate after 4
days was 92%, with 7% of the administered radioactivity detected in
the faeces and traces in the exhaled air. Eight days after
administration, radioactivity in the tissues was 0.6% of the applied
dose (Meyer et al., 1976b). In none of the species examined was
unmetabolized biphenyl found in the urine (BUA, 1990).
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
The acute oral toxicity of biphenyl is moderate. The LD50 for
rats and mice is >1900 mg/kg body weight (BUA, 1990). Acute effects
include polyuria, accelerated breathing, lacrimation, anorexia, weight
loss, muscular weakness and coma, fatty liver cell degeneration,
severe nephrotic lesions (often manifested as acute and subacute
glomerulotubular nephritis), degenerative myocardial lesions,
pulmonary hyperaemia, and, occasionally, alveolar oedema. Interstitial
and lobular pneumonitis have been observed in animals that survived
for a few days.
The LC50 (4-h) for the mouse is >43 ppm (275 mg/m3). During
exposure, hyperactivity and mild respiratory discomfort have been
observed; however, these effects were not evident at the end of a
14-day recovery period. Gross pathological examination performed on
surviving animals revealed slight lung congestion (Sun Co. Inc.,
1977a). In an inhalation study conducted with Sprague-Dawley rats, no
effects were observed after a 6-h exposure to 0.8 or 3 ppm (5.1 or
19.2 mg/m3) biphenyl at temperatures of approximately 26°C or 32°C,
respectively (Monsanto Co., 1959). No treatment-related alterations in
appearance, demeanour, food consumption, or survival were observed
following a 7-h inhalation exposure of rats to 3 g biphenyl/m3
(nominal concentration); no detailed information on the methods or
particle size of the substance were presented (Dow Chemical Co.,
1974).
Information on the acute toxicity of biphenyl associated with
dermal exposure was not identified.
8.2 Irritation and sensitization
Biphenyl is non-irritating to both intact and scarified rabbit
skin. The substance was slightly irritating when applied to rabbits'
eyes in an eye irritation test (BUA, 1990).
In a guinea-pig maximization test conducted according to OECD
Guideline 406, there was reportedly no evidence of a skin sensitizing
potential for biphenyl (Dreist & Kolb, 1993).
8.3 Short-term exposure
8.3.1 Oral exposure
In the studies cited below, the histopathological examination was
focused on the urinary system. In a study in which male and female
Wistar rats were fed 0, 50, 150, 300, or 450 mg biphenyl/kg body
weight per day via the diet for 21 days, increased relative kidney
weights and polycystic renal changes (with increased urine volume and
specific gravity) were observed at doses of 50 and 150 mg/kg body
weight per day, respectively. An increase in urine volume, specific
gravity, and absolute kidney weight as well as polycystic renal
changes were also observed in rats administered 500 or 1000 mg
biphenyl/kg body weight per day in the diet for 14 days (Sœndergaard &
Blom, 1979).
When male albino rats were administered diets containing 0, 1000,
2500, 5000, or 10 000 mg biphenyl/kg (estimated intakes of 0, 75, 188,
375, or 750 mg/kg body weight per day) for 26 days, there was a
dose-dependent (at doses of >188 mg/kg body weight per day)
intensification of cloudiness in the urine, associated with an
increase in precipitate formation in samples, which had been cooled or
treated with sulfosalicylic acid. The precipitate consisted of free
4-hydroxybiphenyl and its glucuronide. These effects were largely
reversible during a 28-day recovery period (Booth et al., 1956, 1961).
8.3.2 Inhalation exposure
No evidence of histopathological changes in the lung, trachea,
liver, kidney, or spleen were observed in male and female mice exposed
by inhalation to 25 or 55 ppm (about 160 or 350 mg/m3) biphenyl for 7
h/day, 5 days/week, for 2 weeks (Sun Co. Inc., 1977b).
8.3.3 Dermal exposure
In rabbits, the repeated dermal application (20 in total) of
purified biphenyl (0.5 g/kg body weight) for 2 h/day, 5 days/week,
produced decreased body weight; one animal died after 8 applications.
A decrease in body weight was also observed after repeated dermal
application of technical biphenyl. In both studies, no effect upon the
skin was observed. Histopathological examination revealed minimal
changes in the heart, liver, kidneys, and, in some cases, spleen (no
further details provided) (Deichmann et al., 1947). No adverse effects
were observed when biphenyl was applied (8 h/day, 5 days/week, for a
total of 30 applications) to the intact and abraded skin of rabbits at
doses of 600 or 2000 mg/kg body weight per day (no further information
provided) (Newell, 1953).
8.4 Long-term exposure
8.4.1 Subchronic exposure
8.4.1.1 Oral exposure
In all of the studies cited below, the histopathological
examination was focused on the urinary system. A summary of the
effects related to this specific end-point (changes in kidneys,
ureter, and urinary bladder) for both subchronic and chronic exposures
is provided in Table 1.
Table 1: Summary of the effects of repeated biphenyl administration via diet on urine status, urinary bladder, and kidneys.
Species/strain/ Application/duration NO(A)EL
sex (mg/kg) Urine status Urinary bladder Kidneys Reference
rat/F344/male 0 or 5000 mg/kg <5000 crystals with simple hyperplasia not examined Shibata et al.
8 weeks microcalculi in and increased (1989a)
urinary sediment DNA synthesis
in the urothelium
rat/F344/male 0 or 5000 mg/kg <5000 microcalculi simple hyperplasia, increase in absolute/relative Shibata et al.
up to 24 weeks papillary or kidney weights; no (1989b)
nodular hyperplasia morphological changes of
of the urothelium the renal papillae or
after >16 weeks pelvis; no changes in DNA
synthesis in the renal
papillae and pelvis; focal
calcification of the renal
medulla
rat/albino/male 0, 1000, 2500, or 1000 >2500 mg/kg: not examined 2500 mg/kg: morphological Booth et al.
and female 5000 mg/kg increase in changes (tubular dilation) (1961)
up to 24 weeksa volume, turbidity, after >60 days
and precipitate 5000 mg/kg: morphological
after >30 days; changes (tubular dilation)
urinary sediment: after >30 days; effects
4-hydroxybiphenyl not completely reversible
and its
glucuronide;
effects reversible
rat/F344/male 0 or 5000 mg/kg <5000 increase in pH increased incidence not given Kurata et al.
32 weeks value and Na+ of calculi; (1986)
concentration; histopathology:
urinary sediment: no effects
4-hydroxybiphenyl
Table 1 (Cont'd.)
Species/strain/ Application/duration NO(A)EL
sex (mg/kg) Urine status Urinary bladder Kidneys Reference
rat/Wistar/male 0, 1250, or 1250 not given 5000 mg/kg: 5000 mg/kg: increased Shiraiwa et al.
5000 mg/kg increased incidence incidence of calculi (1989)
36 weeks of calculi in ureter and increase in
and bladder relative kidney weights
rat/Wistar/male 0, 2500, or <2500 haematuria at >2500 mg/kg: >2500 mg/kg: increased Takita (1983);
and female 5000 mg/kg >2500 mg/kg increased incidence incidence of calculi Shiraiwa et al.
75 weeks after >16 weeks of calculi (composed (composed of protein) (1989)
of magnesium after >16 weeks;
ammonium phosphate) kidneys with stones:
after >16 weeks in obstructive
ureter (females) pyelonephritis,
5000 mg/kg:increased tubular atrophy,
incidence of calculi fibrosis
in ureter and 5000 mg/kg: increase
bladder after >16 in relative kidney
weeks; bladder with weights in females
calculi: simple or
diffuse hyperplasia
and papillomatosis
of the urothelium
rat/Wistar/male 0, 630, or 1250 mg/kg 1250 not given no urolithiasis not given Takita (1983)
and female 104 weeks (no further
information given)
rat/F344/DuCrj/ 0, 500, 1500, or <500b >500 mg/kg: 4500 mg/kg: increase >1500 mg/kg: increase Japan Bioassay
male and female 4500 mg/kg decrease in in pH (males and in hyperplasia of Research Center
104 weeks protein (males) females) 4500 mg/kg: the renal pelvis in (1996)
>1500 mg/kg: increase in calculi females
decrease in and hyperplasia 4500 mg/kg: increase
protein (females) of the urothelium in calculi (males >
Table 1 (Cont'd.)
Species/strain/ Application/duration NO(A)EL
sex (mg/kg) Urine status Urinary bladder Kidneys Reference
(males >> females); females) and
increased tumour hyperplasia of the
incidence in males renal pelvis (males)
mouse/B6C3F1/ 0 or 10 000 mg/kg 10 000 decreased Na+ no changes in DNA not given Tamano et al.
male 8 weeks concentration synthesis of the (1993)
at week 4 urothelium
mouse/B6C3F1/ 0 or 10 000 mg/kg <10 000 small amounts of simple hyperplasia interstitial nephritis Tamano et al.
male 32 weeks greyish white and papillary or in 5/10 (1993)
and finely nodular dysplasia
granular material in 1/10 mice
mouse/Crj:BDF1/ 0, 667, 2000, or 667 6000 mg/kg: no changes >2000 mg/kg: mineralization Japan Bioassay
male and female 6000 mg/kg decrease in of the medulla in females Research Center
104 weeks protein and 6000 mg/kg: desquamation (1996)
ketone body and mineralization of the
pelvis in males
a Plus a post-treatment observation period of 9 weeks.
b Effects were observed at the lowest dose tested.
Effects on the urinary tract (i.e. an increased number of
microcalculi in urinary sediment, simple hyperplasia of the bladder
epithelium) were observed in male F344 rats administered 5000 mg
biphenyl/kg (estimated intake 252 mg/kg body weight per day) in the
diet for 8 weeks. Morphological changes and the increased synthesis of
DNA detected in the bladder epithelium after 4 weeks were attributed
to constant irritation by the microcalculi (Shibata et al., 1989a).
In male B6C3F1 mice, no changes in the urinary pH or in levels
of DNA synthesis in the urinary bladder were noted after
administration of 0 or 10 000 mg biphenyl/kg in the diet (0 or 1500
mg/kg body weight per day) over a period of 8 weeks. The urinary Na+
concentration was significantly lower in mice administered 1500 mg/kg
body weight per day compared with controls only at week 4 (Tamano et
al., 1993).
In a range-finding study in which male and female Wistar rats
were administered diets containing biphenyl at 0, 1250, 2500, 5000, 10
000, or 20 000 mg/kg (estimated intakes of 0, 94, 188, 375, 750, or
1500 mg/kg body weight per day) for 10 weeks, dose-dependent effects
(i.e. reduction in weight gain; increased serum activities of
aspartate transaminase, alanine transaminase, and lactate
dehydrogenase; and an increase in blood urea nitrogen) were observed
at all doses (Takita, 1983).
In male F344 rats (20 per group) administered 0 or 5000 mg
biphenyl/kg in the diet (0 or 375 mg/kg body weight per day) for 24
weeks (5 rats per group were sacrificed after 4, 8, 16, or 24 weeks),
reduced body weight gain and an increase in absolute/relative kidney
weights without differences in feed intake were observed in the
biphenyl-exposed rats. The analysis of the urine showed that
administration of biphenyl was associated with the formation of
microcalculi. The histopathological examination of the renal papillae
or pelvis performed after 4, 8, 16, and 24 weeks revealed no
morphological changes; the rate of DNA synthesis in the renal papillae
and pelvis determined after 4 weeks of treatment was unchanged. In
most of the treated animals, a focal calcification of the renal
medulla (no further information given) was noted. The examination of
the epithelium of the urinary bladder revealed a simple hyperplasia in
5/5 rats after 16 and 24 weeks and papillary or nodular hyperplasia in
3/5 rats at week 16 and in 5/5 rats at week 24; no data on controls
were presented (Shibata et al., 1989b).
In albino rats (42 animals of each sex per group) given 0, 1000,
2500, or 5000 mg biphenyl/kg in the diet (0, 75, 188, or 375 mg/kg
body weight per day) for up to 24 weeks, the urine analysis and
histopathological investigations of the kidneys during the study (days
30, 60, and 120) showed no apparent effects at a dose level of 75
mg/kg body weight (Booth et al., 1961). In some animals, early
indications of a kidney-damaging effect were observed at doses of 188
and 375 mg/kg body weight at 30 days -- polyuria, increasing
cloudiness of the urine, and initial morphological changes (tubular
dilation) in the kidney. These effects increased in frequency and
degree as the administrations progressed. The urinary sediment
consisted of 4-hydroxybiphenyl and its glucuronide. During a
subsequent 60-day observation period, the urinary status normalized;
however, changes in kidneys were not completely reversible (a few
dilated tubules and scars remained) (Booth et al., 1961).
In male F344 rats (25 per group) administered 0 or 5000 mg
biphenyl/kg in the diet (0 or 375 mg/kg body weight per day) for 32
weeks, a reduction in body weight gain but no clinical signs of
poisoning were observed in dosed animals. In urine, an increase in pH
value and Na+ concentration was noted. At terminal necropsy, calculi
were found in the urinary bladder. The urinary sediment consisted
mainly of 4-hydroxybiphenyl. No associated histopathology was detected
upon microscopic examination of the urinary bladder (Kurata et al.,
1986).
When groups ( n = 25) of male Wistar rats were provided diets
containing 0, 1250, or 5000 mg biphenyl/kg (0, 94, or 375 mg/kg body
weight per day) for 36 weeks, a reduction in body weight, increased
relative kidney weights, and an increase in the number of animals with
stones in the kidneys (0/25, 0/25, and 4/25, respectively), ureter
(0/25, 0/25, and 1/25, respectively), and urinary bladder (0/25, 0/25,
and 3/25, respectively) were noted in the high-dose group (Shiraiwa et
al., 1989).
8.4.1.2 Inhalation exposure
Exposure of groups ( n = 50) of male and female CD-1 mice to 25
or 50 ppm (160 or 320 mg/m3; analytical concentrations) biphenyl for
7 h/day, 5 days/week, for 13 weeks produced hyperaemia and focal
haemorrhage in the lung and an increase in hyperplasia of the tracheal
epithelium. As these effects were also observed in some unexposed
controls, they were attributed to the method of aerosol generation
(i.e. inhalation of hot air) (Sun Co. Inc., 1977c).
Marked species differences were observed in a study in which
rabbits, rats, and mice were exposed by inhalation to biphenyl in the
form of dust (50% biphenyl on zeolite) at 5, 40, or 300 mg/m3, 7
h/day, 5 days/week, for up to 13 weeks. No adverse effects were
observed in rabbits. Rats exposed to 40 or 300 mg biphenyl/m3
exhibited increased mortality and irritation of the mucous membranes;
no effects were observed following exposure to 5 mg/m3. Mice were the
most sensitive species. Exposure to 5 mg biphenyl/m3 (the only
concentration tested) resulted in slightly increased mortality, with
all mice exhibiting irritation of the upper respiratory tract (no
further information available). Necropsy of dead rats and mice
revealed mainly inflammatory bronchopulmonary changes. No information
on control animals or particle size was provided (Deichmann et al.,
1947).
8.4.2 Chronic exposure and carcinogenicity
In older studies, which were not further taken into consideration
because of the small number of animals used, limited documentation,
and/or limited exposures, no increase in tumour incidence was observed
in rats and mice when biphenyl was administered orally (at doses up to
750 mg/kg body weight per day for 2 years) (Ambrose et al., 1960;
Innes et al., 1969) or following a single subcutaneous injection of
46.4 mg biphenyl/kg body weight, in which the animals were examined
macroscopically and microscopically after an 18-month observation
period (NTIS, 1968).
In Wistar rats (50 animals of each sex per group) given 0, 2500,
or 5000 mg biphenyl/kg in the diet (0, 188, or 375 mg/kg body weight
per day) for 75 weeks, dose-dependent effects (i.e. reduction in
weight gain; alterations in serum activities of aspartate transaminase
[increased and decreased], alanine transaminase [increased], and
lactate dehydrogenase [increased]; and an increase in blood urea
nitrogen) and a dose-dependent increase in stones of the kidney
(females: 0/43, 1/43, 18/39; males: 0/44, 6/46, 15/47) and ureter
(females: 0/43, 1/43, 2/39; males: 0/44, 0/46, 2/47), accompanied by
haematuria as early as 16 weeks after initiation of exposure, were
seen at >188 mg/kg body weight. At 375 mg/kg body weight, the
relative kidney weights were significantly increased in females, and
an increase in stones of the urinary bladder was seen in males and
females (females: 0/43, 0/43, 6/39; males: 0/44, 0/46, 13/47). The
histopathological examination of the urinary bladder with stones
revealed simple or diffuse hyperplasia and papillomatosis of the
epithelium, but no cancerous changes. The overall tumour incidence was
not increased compared with controls. Kidneys with stones exhibited
obstructive pyelonephritis, tubular atrophy, and fibrosis. Kidney
stones were composed of protein, whereas urinary stones were composed
of magnesium ammonium phosphate (Takita, 1983; Shiraiwa et al., 1989).
No urolithiasis and no increased overall tumour incidence
compared with controls were observed in Wistar rats (50 animals of
each sex per group) provided with diets containing 0, 630, or 1250 mg
biphenyl/kg (0, 47, or 94 mg/kg body weight per day) for 104 weeks.
Dose-dependent effects (i.e. reduction in weight gain; alterations in
serum activities of aspartate transaminase [decreased], alanine
transaminase [increased and decreased], and lactate dehydrogenase
[increased and decreased]) were noted at both doses; 47 mg/kg body
weight is considered the lowest-observed-(adverse-)effect level, or
LO(A)EL (Takita, 1983).
In a study with F344/DuCrj rats performed according to standard
protocols and described in sufficient detail, a significant increase
in neoplastic and non-neoplastic lesions of the urinary bladder and a
significant increase in calculi within the urinary bladder were
observed in high-dose males, after the animals had been given diets
containing 0, 500, 1500, or 4500 mg biphenyl/kg (0, 38, 113, or 338
mg/kg body weight per day) for 104 weeks. In males and females, a
dose-dependent increase in hyperplasia of the epithelium of the renal
pelvis was seen. The results of the histopathological findings in the
urinary bladder and kidneys are summarized in Table 2. Other findings
included increased serum enzyme levels (alkaline phosphatase,
aspartate transaminase, and alanine transaminase) and an increased
urea nitrogen level in low-dose males and mid-dose females, which
became more pronounced with increasing doses. In mid- and high-dose
females and high-dose males, haematological effects (i.e. reduced
haemoglobin concentration and haematocrit) were noted (Japan Bioassay
Research Center, 1996). From this study, a LOEL of 38 mg/kg body
weight was derived.
In a study in which groups of male and female Crj:BDF1 mice were
given diets containing 0, 667, 2000, or 6000 mg biphenyl/kg (0, 100,
300, or 900 mg/kg body weight per day) for 104 weeks, a slight
increase in liver tumours (hepatocellular adenomas and carcinomas) and
basophilic cell foci of the liver was observed in the females at doses
of 300 and 900 mg/kg body weight per day; however, the effects were
not concentration dependent (Japan Bioassay Research Center, 1996)
(see Table 3). In the male and female mice, degenerative changes of
the respiratory epithelium of the nasal cavity and nasopharynx were
observed at doses of >100 mg/kg body weight per day or >300
mg/kg body weight per day, respectively. Other findings included
variations in serum enzyme levels (increase in alkaline phosphatase,
aspartate transaminase, and alanine transaminase) and an increased
urea nitrogen level in the low-dose males and females, which became
more pronounced with increasing doses. In female mice receiving
>300 mg biphenyl/ kg body weight per day and in the high-dose
males, degenerative changes in the kidney (increased mineralization of
the inner stripe of the outer medulla, increase in desquamation of the
epithelium of the renal pelvis) were also observed. Body weight gain
and food consumption were reduced in the high-dose animals (Japan
Bioassay Research Center, 1996).
Information on the toxicological effects of biphenyl following
chronic inhalation or dermal exposure were not identified.
8.4.2.1 Tumour promotion
A number of studies have investigated the tumour-promoting
potential of biphenyl. Kurata et al. (1986) considered biphenyl a
tumour promoter, based upon the results of a study in which groups of
male F344 rats were administered drinking-water containing 0.05%
N-butyl- N-(4-hydroxybutyl)nitrosamine (BBN) (as an initiator) for
4 weeks, followed by 0.5% biphenyl in the diet (estimated intake of
approximately 375 mg/kg body weight per day) for a further 32 weeks,
after which time the animals were examined histopathologically. The
results are provided in Table 4. The effects of biphenyl were
attributed to the increased Na+ content in the urine of exposed
rats, as well as to crystals containing mainly 4-hydroxybiphenyl
detected in the urine.
In a study in which male Wistar rats were administered 0.1%
N-ethyl- N-hydroxyethylnitrosamine in the diet for 2 weeks,
followed by 0, 0.125, or 0.5% biphenyl in the diet for 34 weeks,
exposure to biphenyl had no effect upon the incidence of dysplastic
foci and renal cell tumours induced by N-ethyl- N-hydroxyethylnitro
samine; however, an increase in stones of the kidneys, ureter, and
bladder was observed in rats administered 0.5% biphenyl with or
without N-ethyl- N-hydroxyethyl nitrosamine (Shiraiwa et al.,
1989).
Tamano et al. (1993) maintained male B6C3F1 mice on
drinking-water containing 0.05% BBN supplement for 4 weeks. After the
BBN pre-treatment, the animals received diets with 10 000 mg
biphenyl/kg (1500 mg/kg body weight per day) for 32 weeks. At the end
of the study, the urinary bladder and kidneys were examined
histopathologically. The results are provided in Table 5. In mice
exposed to BBN and biphenyl, the average food consumption and the
final body weight were reduced, whereas the relative weight of the
urinary bladder was increased. The induction of simple hyperplasia and
papillary or nodular dysplasia of the urinary bladder in 1/10 mice
treated with biphenyl only was associated with urolithic residues
(small amounts of greyish white and finely granular material). In mice
treated with biphenyl with or without BBN pre-treatment, the incidence
of interstitial nephritis in the kidneys was 65 and 50%, respectively.
In an early study in which albino mice "initiated" with a single
dermal application of 9,10-dimethyl-1,2-benzanthracene (0.3% in
benzene) received dermal applications of biphenyl (20% in benzene)
twice weekly for 15 weeks, no skin papillomas or carcinomas were
observed in either the vehicle controls or exposed mice 16 weeks after
application of the initiator (Boutwell & Bosch, 1959).
Table 2: Data from Japanese cancer bioassay with male and female rats fed biphenyl in the diet for 2 years.a
Male rats Female rats
0 500 1500 4500 0 500 1500 4500
End-point mg/kg mg/kg mg/kg mg/kg mg/kg mg/kg mg/kg mg/kg
survival rate 37/50 41/50 38/50 31/50 44/50 38/50 44/50 37/50
urinary bladder: neoplastic lesions
transitional cell papilloma 0 0 0 10/50 0 0 0 0
transitional cell carcinoma 0 0 0 24/50 0 0 0 0
(p=0.0001)b
squamous cell papilloma 0 0 0 1/50 0 0 0 0
squamous cell carcinoma 0 0 0 1/50 0 0 0 0
urinary bladder: non-neoplastic lesions (transitional epithelium)
simple hyperplasia 0 0 0 12/50 0 0 1/50 1/50
nodular hyperplasia 0 0 0 40/50 1/50 0 0 5/50
papillary hyperplasia 0 0 0 17/50 0 0 0 4/50
basal cell hyperplasia 0 0 0 27/50 0 0 0 4/50
squamous cell hyperplasia 0 0 0 13/50 0 0 0 1/50
squamous cell metaplasia 0 0 0 19/50 0 0 0 4/50
inflammatory polyps 0 0 0 10/50 0 0 0 0
calculi 0 0 0 43/50 0 0 0 8/50
kidney
mineralization -- papilla 9/50 9/50 14/50 23/50 2/50 6/50 3/50 13/50
mineralization -- pelvis 9/50 6/50 10/50 18/50 12/50 12/50 18/50 27/50
calculi 0/50 0/50 0/50 13/50 0/50 0/50 0/50 3/50
desquamation -- pelvis 1/50 0/50 0/50 11/50 0/50 0/50 0/50 2/50
simple hyperplasia of the 6/50 8/50 5/50 19/50 3/50 5/50 12/50 25/50
transitional epithelium
nodular hyperplasia of the 0/50 1/50 1/50 21/50 0/50 0/50 1/50 12/50
transitional epithelium
ureter dilatation 0/50 0/50 1/50 14/50 0/50 0/50 0/50 6/50
a From Japan Bioassay Research Center (1996).
b Fisher Exact Test.
Table 3: Data from Japanese cancer bioassay with male and female mice fed biphenyl in the diet for 2 years.a
Male mice Female mice
0 667 2000 6000 0 667 2000 6000
End-point mg/kg mg/kg mg/kg mg/kg mg/kg mg/kg mg/kg mg/kg
survival rate 35/50 41/50 41/50 39/50 31/50 22/50 25/50 32/49
liver: neoplastic lesions
hepatocellular carcinoma 8/50 8/49 5/50 4/50 1/50 5/50 7/50 5/49
(p=0.121)b (p=0.043)b
(p=0.1163)b
hepatocellular adenoma 8/50 6/49 7/50 3/50 2/50 3/50 12/50 10/49
(p=0.4909)b (p=0.016)b
(p=0.0251)b
historical control data: historical control data:
carcinoma: 171/700 with a range of 1/50 - 19/50 carcinoma: 15/699 with a range of 0/50 - 2/50
adenoma: 119/700 with a range of 2/50 - 15/50 adenoma: 33/699 with a range of 1/50 - 5/50
basophilic cell foci 0/50 6/49 1/50 2/50 1/50 1/50 12/50 6/49
a From Japan Bioassay Research Center (1996).
b Fisher Exact Test.
Table 4: Results of the tumour-promoting potential (urinary bladder) of
biphenyl in male rats.a
Hyperplasia Papilloma Carcinoma
n (%) n (%) n (%)
biphenyl only
(21 rats) 0 0 0 0 0 0
BBNb only
(24 rats) 6 -25 3 -12 0 0
BBN and biphenyl
(18 rats) 17 -94 15 -83 11 -61
a Taken from Kurata et al. (1986).
b BBN = N-butyl-N-(4-hydroxybutyl)nitrosamine.
Table 5: Results of the tumour-promoting potential (urinary bladder) of
biphenyl in male mice.a
Papillary or
Hyperplasia nodular dysplasia Carcinoma
n (%) n (%) n (%)
biphenyl only
(10 mice) 1 -10 1 -10 0 0
BBNb only
(20 mice) 12 -60 2 -10 0 0
BBN and biphenyl
(20 mice) 14 -70 1 -5 0 0
a Taken from Tamano et al. (1993).
b BBN = N-butyl-N-(4-hydroxybutyl)nitrosamine.
8.5 Genotoxicity and related end-points
The results of the in vitro tests with biphenyl are summarized
in Table 6. In vitro studies with bacteria have yielded no evidence
of mutagenic potential for biphenyl, whereas gene mutation and mitotic
recombination were observed with Saccharomyces cerevisiae D7 in the
presence or absence of metabolic activation. However, no gene
conversion was observed with S. cerevisiae D3. Testing in mammalian
cells has produced positive results for gene mutations and
clastogenicity in the presence of metabolic activation and negative
results in the absence of metabolic activation.
In an in vivo rat cytogenetic assay of bone marrow cells, the
incidence of chromosomal aberrations was not increased (no further
information available) (Kawachi et al., 1980). The exposure by
inhalation of male Sprague-Dawley rats to 64 or 320 mg biphenyl/m3
(as dust aerosol) for 7 h/day, 5 days/week, for 30 days (20 exposures
in total) reportedly did not increase the frequency of chromosomal
aberrations in the bone marrow (Dow Chemical Co., 1976). However,
owing to insufficient documentation (i.e. no data available concerning
dust particle size distribution or cell harvesting times) and the low
number of metaphase cells examined (i.e. 50 cells per animal), the
validity of this study cannot be ascertained.
The results of in vitro studies indicate that biphenyl has
mutagenic potential; in the absence of reassurance from reliable
results from in vivo tests, it is assumed that exposure to biphenyl
may be associated with a mutagenic risk.
8.6 Reproductive and developmental toxicity
Available data on the reproductive or developmental toxicity of
biphenyl are limited. In Wistar rats administered (by gavage) 0, 125,
250, 500, or 1000 mg biphenyl/kg body weight (in corn oil) on days
6-15 of gestation, no maternal toxicity occurred at doses of <500
mg/kg body weight. In the highest dose group, 5 of 20 rats died. Also
at 1000 mg/kg body weight, reduced fetal weight and an increased
number of dead and resorbed fetuses were noted; however, these values
were statistically not significantly different from those of control
animals. At doses of >500 mg/kg body weight, there were
non-significant increases in the incidence of fetuses with missing or
non-ossified sternebrae (Khera et al., 1979).
Table 6: Results of in vitro genotoxicity studies on biphenyl.
Resultsa
Species Concentration Without With
(test system) End-point range MA MA References
Salmonella typhimurium Reverse 0-5000 µg/plate - - Cline & McMahon (1977); Purchase et al.
TA92, TA94, TA97, TA97a, mutations (1978); Kawachi et al. (1980); NTP (1980);
TA98, TA100, TA102, Bronzetti et al. (1981); Probst et al. (1981);
TA1532, TA1535, TA1537, Waters et al. (1982); Haworth et al. (1983);
TA1538, TA2636 Pagano et al. (1983, 1988); Ishidate et al.
(1984); Fujita et al. (1985); Brams et al.
(1987); Bos et al. (1988); Glatt et al. (1992)
Escherichia coli WP2, WP2 Gene mutations 0.1-1000 µg/ml - - Cline & McMahon (1977); Probst et al. (1981);
uvrA- Waters et al. (1982)
E. coli PQ37 DNA damage 2.4-154 µg/ml - - Brams et al. (1987)
Bacillus subtilis rec assay DNA damage no data - 0 Kawachi et al. (1980)
Saccharomyces cerevisiae Gene mutation/ <154 µg/ml + + Pagano et al. (1983)
D7 gene conversion
S. cerevisiae D3 Gene conversion no data - - Waters et al. (1982); Zimmermann et al. (1984)
Chinese hamster cells Gene mutation 0-100 µg/ml - + Glatt et al. (1992)
(V79)
L5178Y TK+/- cells Gene mutation 0-61 µg/ml - (+) Wangenheim & Bolcsfoldi (1988)
(mouse lymphoma assay)
Chinese hamster cells Chromosomal 0-125 µg/ml - 0 Ishidate & Odashima (1977); Kawachi et al.
(CHL) aberration (1980); Sofuni et al. (1985)
Table 6 (Cont'd.)
Resultsa
Species Concentration Without With
(test system) End-point range MA MA References
Chinese hamster cells Chromosomal 0-20 µg/ml - + Sofuni et al. (1985)
(CHL) aberration
Chinese hamster cells Chromosomal 15.4-154 µg/ml - 0 Abe & Sasaki (1977)
(DON) aberration
Rat hepatocytes Unscheduled 0.002-154 µg/ml 0 - Williams (1978); Brouns et al. (1979);
DNA synthesis Probst et al. (1981)
Chinese hamster cells Sister chromatid no data - 0 Kawachi et al. (1980)
(CHL) exchange
Chinese hamster cells Sister chromatid 15.4-154 µg/ml - 0 Abe & Sasaki (1977)
(DON) exchange
L5178Y cells (alkaline DNA damage 0-231 µg/ml - + Garberg et al. (1988)
unwinding assay)
human lung fibroblasts Unscheduled no data - - Waters et al. (1982)
(WI-38 cells) DNA synthesis
human fibroblasts ("nick DNA damage 15.4 µg/ml - 0 Snyder & Matheson (1985)
translation assay")
a -, negative; +, positive; (+) weakly positive; 0, not tested; MA, metabolic activation.
Compared with unexposed controls, litter size (the only parameter
examined) was not affected in a study in which small numbers of male
and female rats were administered diets containing 0.1 or 0.5%
biphenyl (estimated intakes of 75 and 375 mg/kg body weight per day)
before mating and throughout gestation (Ambrose et al., 1960). In an
unpublished three-generation study, dietary biphenyl concentrations of
100 or 1000 mg/kg (estimated intakes of approximately 7.5 or 75 mg
biphenyl/kg body weight per day) had no effect on reproduction in
rats; after intake of 10 000 mg/kg (estimated intake of 750 mg/kg body
weight per day), decreased fertility, litter size, and growth rate
were noted (no further information available) (Stanford Research
Institute, undated). Histopathological changes within the male and
female reproductive systems were not observed in rats or mice
administered biphenyl at 500-4500 mg/kg in the diet for 2 years (Japan
Bioassay Research Center, 1996).
8.7 Immunological and neurological effects
Data on immunological and neurological effects of biphenyl in
laboratory animals were not identified.
9. EFFECTS ON HUMANS
Information on effects on human health resulting from exposure to
biphenyl are limited to case reports; epidemiological studies were not
identified. In one report, a single application of 0.5 ml of a 4%
biphenyl solution (no further details provided) to the skin of the
lower arm of two test subjects caused no apparent irritation
(Macintosh, 1945). Biphenyl did not cause skin irritation in a study
in which a solution of 23% biphenyl in oil was applied to the forearm
three times per week for 8 weeks (Selle, 1952). In one human volunteer
orally given 35 mg biphenyl per week for 13 weeks, no adverse effects
were reported (Farkas, 1939). Cases of headache, nausea, and
inflammation of the respiratory tract have been reported in workers
exposed to biphenyl vapours (and other substances) during paper
production (Weil et al., 1965). Liver damage and effects upon the
central and peripheral nervous systems were attributed to long-term
exposure to high concentrations of biphenyl (see section 6.2) in a
case-study of workers engaged in the production of
biphenyl-impregnated paper (Haekkinen et al., 1973). Chronic
persistent hepatitis in a woman who had worked for 25 years in a
citrus packing plant in which biphenyl-impregnated paper was used was
attributed to the absorption of biphenyl through the skin and
digestive tract (Carella & Bettolo, 1994).
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1 Aquatic environment
The acute toxicity of biphenyl in aquatic organisms has been
investigated in microorganisms, plants, invertebrates, and
vertebrates; however, in several studies cited in the main source
document (BUA, 1990), information on whether results were based on
nominal or effective concentrations was either lacking or not clearly
stated. For biphenyl, nominal concentrations may not correspond to the
effective concentrations, as the water solubility of biphenyl is low
(4.45 mg/litre at 20°C) (test results with higher nominal effect
concentrations have not been reported here) and its volatility
significant. In tests with open systems and longer test durations,
results based on nominal concentrations may lead to an underestimation
of toxic effects because of evaporation of the chemical; therefore,
emphasis has been placed on studies with lowest reported effect levels
in which loss of the test substance was minimized.
Several studies were conducted on the toxic effects of biphenyl
on mixed microbial cultures as well as on single bacterial species.
The most sensitive species was Photobacterium phosphoreum, with a
30-min EC50 of 1.9 mg/litre in a bioluminescence inhibition test
(BUA, 1990). For Colpidium campylum, one of three different species
of ciliata tested, the minimal active concentration of biphenyl for
the inhibition of cell proliferation after a 43-h exposure was 5.6
mg/litre (nominal concentration; test substance predissolved in
acetone) (Dive et al., 1980). In studies on the reduction of
photosynthesis, 3-h EC50 values of 3.86 and 1.28 mg/litre were deter
mined with the unicellular green algae Chlorella vulgaris and
Chlamydomonas angulosa, respectively (BUA, 1990).
Forty-eight-hour LC50 values from acute toxicity tests with
Daphnia magna were in the range of 1.1-4.7 mg/litre when experiments
were conducted in more or less tightly closed test vessels. From a
test in a closed system with continuous flow, a 48-h LC50 of 0.36
mg/litre and a NOEC of 0.04 mg/litre were reported. In a reproduction
test with Daphnia magna in the same closed continuous-flow system,
the NOEC after 21 days' incubation was 0.17 mg/litre (LC50 = 0.23
mg/litre) (Gersich et al., 1989). In studies on the inhibition of food
intake by the marine mussel Mytilus edulis in a static system with
loosely covered (not airtight) test vessels, a 40-min EC50 of 0.3 mg
biphenyl/litre was reported (Donkin et al., 1991). From static tests
on the toxicity of biphenyl on freshwater fish, 96-h LC50 values in
the range of 1.5-4.7 mg/litre have been reported, with rainbow trout
(Oncorhynchus mykiss) and fathead minnow (Pimephales promelas) the
most sensitive species (BUA, 1990).
10.2 Terrestrial environment
Biphenyl has weak bactericidal and fungistatic properties. In
studies with numerous mould fungus species, biphenyl (applied as
vapour or imbedded in solid media) caused a reversible inhibition
(50-100%) of cellular proliferation. Suppression of sporogenesis and,
in Penicillium digitatum and Diplodia natalensis, the occurrence
of biphenyl-resistant mutants were observed. Yeast species showed
little or no inhibition of cell proliferation following exposure to
biphenyl (BUA, 1990).
In a plant growth inhibition test conducted according to OECD
Guideline 208, a nominal NOEC of 100 mg/kg dry soil was reported for
sorghum (Sorghum bicolor) and soya bean (Glycine max); a NOEC of
>1000 mg biphenyl/kg was reported for the sunflower (Helianthus
annuus) (Windeatt et al., 1991). Biphenyl mixed into soil inhibited
growth of lettuce (Lactuca sativa) seedlings with a 7-day EC50 of
54 mg/kg (nominal concentration). At the end of incubation, the
concentration of test substance in the soil had decreased to <10% of
the initial level (Hulzebos et al., 1993).
Data on toxic effects of biphenyl on terrestrial animals and
ecosystems were not identified.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
There are limited data on the effects of biphenyl exposure on
humans. The assessment of potential health hazards has relied
primarily on studies conducted in laboratory animals. The acute oral
toxicity of biphenyl is moderate. The chemical is not irritating to
the skin and is slightly irritating to the eyes, and there is no
evidence of skin sensitizing potential.
Aside from differences in mortality among laboratory species
exposed subchronically to biphenyl-containing dusts, the available
data on effects associated with the inhalation of biphenyl are
insufficient to form the basis of an assessment of potential health
hazards associated with long-term airborne exposure to this chemical.
In toxicological studies in which rodents have been administered
diets containing biphenyl for various periods of time, effects on the
urinary system have often been reported. A marked increase in the
incidence of morphological (i.e. formation of calculi) and/or
histopathological (e.g. hyperplasia, desquamation) effects has been
observed within the urinary tract of male rats administered diets
containing more than 2500 mg biphenyl/kg for periods ranging from 32
to 104 weeks (Takita, 1983; Kurata et al., 1986; Shiraiwa et al.,
1989; Japan Bioassay Research Center, 1996). An increase in the
occurrence of calculi within the urinary bladder has also been
observed in female rats, but at a lower incidence than in males
(Takita, 1983; Shiraiwa et al., 1989; Japan Bioassay Research Center,
1996). Similarly, in a long-term dietary study (Japan Bioassay
Research Center, 1996), increased squamous metaplasia within the
urinary transitional epithelium was also observed in female rats;
again, however, the incidence was lower than that observed in males.
In male mice, only 1 of 10 animals given a diet containing 10 000 mg
biphenyl/kg (1500 mg/kg body weight per day) for 32 weeks developed
simple hyperplasia and papillary or nodular dysplasia of the urinary
bladder (Tamano et al., 1993). Effects on blood chemistry and
haematological parameters have also been observed in animals
administered biphenyl orally; these effects occurred in male and
female rats and mice at intakes lower than those associated with the
development of effects in the urinary bladder of male rats
administered biphenyl (Takita, 1983; Shiraiwa et al., 1989; Japan
Bioassay Research Center, 1996). For non-neoplastic effects, the LOEL
was 38 mg/kg body weight per day based upon the development of
alterations in haematological parameters (i.e. decreased haemoglobin
concentration and haematocrit) in rats fed diets containing 0, 500,
1500, or 4500 mg biphenyl/kg (reported intakes of 0, 38, 113, or 338
mg/kg body weight per day) for 2 years (Japan Bioassay Research
Center, 1996).
Although limited, the available information indicates that
biphenyl has no reproductive or developmental effects at doses lower
than those associated with the development of adverse effects in the
parental generation.
In one study, an increased incidence of benign and malignant
tumours within the urinary bladder was observed in male F344/DuCrj
rats administered diets containing high levels (i.e. not less than
4500 mg/kg) of biphenyl for 2 years. Tumour incidence was not
increased in female rats or in male or female Crj:BDF1 mice (Japan
Bioassay Research Center, 1996). In female mice, there were slight
increases in the incidences of benign and malignant liver tumours in
animals receiving biphenyl in the diet; however, the results were not
dose dependent over the entire range of concentrations tested. In
other studies, biphenyl exhibited tumour-promoting activity with
respect to the development of bladder neoplasms in male rats (Kurata
et al., 1986) but not in male mice (Tamano et al., 1993).
The mechanism by which high doses of biphenyl induce bladder
tumours specifically in male rats has not been fully elucidated. There
have been suggestions that for some non-genotoxic chemicals, the
formation of such tumours is linked to the regenerative hyperplasia of
the urinary epithelium, caused by the abrasion and damage to the
urothelium that are produced by calculi formed within the urinary
tract only at very high levels of exposure (Cohen, 1995). Owing to
anatomical and physiological differences, male rats are considered to
be more susceptible than female rats, mice, and humans to the
development of bladder tumours via such a mechanism; it has therefore
been suggested that the sex- and species-specific development of
bladder tumours in male rats receiving high doses of biphenyl might
not be strictly relevant to humans exposed to lower levels. However,
the incidence of histopathological effects and calculi formation
within the urinary bladder also increased in female rats administered
biphenyl for 2 years, in the absence of bladder tumours (Japan
Bioassay Research Center, 1996). Moreover, data identifying a direct
association between calculi formation, regenerative hyperplasia of the
urothelium, and the development of bladder tumours within individual
male animals are not available. Biphenyl has been reported to be
mutagenic and clastogenic in some, but not all, in vitro assays and
to bind covalently to macromolecules (i.e. proteins) following
metabolic activation; the available data have been considered
inadequate to assess its genotoxicity in vivo. These considerations,
coupled with the structural relationship of 4-hydroxybiphenyl, a major
metabolite of biphenyl, to the potent human bladder carcinogen
4-aminobiphenyl and the lack of critical information related to the
mechanism by which these bladder tumours arise in male rats, could
suggest that the development of bladder tumours in the male rats may
not have been entirely due to effects associated with the formation of
calculi within the urinary bladder. This observation, as well as the
slightly increased incidence of liver tumours in female mice, raises
some concerns with respect to the potential carcinogenicity of
biphenyl to humans.
11.1.2 Criteria for setting guidance values for
biphenyl
A provisional tolerable daily intake (TDI), based upon the
development of effects in the blood of rats administered diets
containing biphenyl for 2 years (Japan Bioassay Research Center,
1996), can be derived as follows:
TDI = 38 mg/kg body weight per day
1000
= 38 µg/kg body weight per day
where:
* 38 mg/kg body weight per day is the lowest LOEL for the
development of alterations in blood chemistry in rats provided
diets containing biphenyl for 2 years. This intake is lower than
intakes associated with the development of haematological
effects, calculi, and histopathological changes in the bladder
and/or kidneys in rats and the development of haematological and
histopathological effects in mice administered biphenyl in the
diet for 2 years.
* 1000 is the uncertainty factor (×10 for interspecies variation;
×10 for intraspecies variation; ×10 for extrapolation from a LOEL
to a NOEL).
However, based upon the currently available data, there is some
uncertainty surrounding the health-protective nature of the value,
owing to lack of critical information concerning the mechanism by
which biphenyl induces bladder tumours in male rats, the slight
increase in hepatocellular tumours in female mice, and the potential
genotoxicity of this substance. Because of this uncertainty, the TDI
is designated provisional.
Available data were inadequate to serve as a basis for the
derivation of guidance values for biphenyl in air.
11.1.3 Sample risk characterization
For the general population, there is insufficient information
available concerning the intake of biphenyl from the general
environment; therefore, comparisons with the provisional TDI are
provided merely as an example. From limited information on the content
of biphenyl in citrus fruits, the estimated daily intake is <375 ng
biphenyl/kg body weight from each individual fruit consumed, while the
estimated daily intake from tap-water is <0.16 ng/kg body weight.
Assuming that a person consumes one citrus fruit per day, the
estimated intake of biphenyl from drinking-water and citrus fruit
consumption is approximately two orders of magnitude lower than the
provisional TDI based upon the development of effects in the blood.
From a study on workers exposed in a plant producing
biphenyl-impregnated paper, it can be derived that exposure to high
biphenyl concentrations up to 128 mg/m3 pose a hazard. Neurotoxic
effects and liver damage have been observed.
Additional information is required to better define any potential
carcinogenic risks that might be associated with exposure to biphenyl.
11.2 Evaluation of environmental effects
The atmosphere is likely the main target compartment for
biphenyl; however, the chemical has been detected in all environmental
compartments as a result of its release from vehicle emissions,
heating, smoking, and industrial activities. Water, soil, and biota
appear to be affected mainly in the direct vicinity of industrial
activities. The decline in the levels of biphenyl in surface waters
may be due to restrictions on the production and use of PCBs. There
are no data available on the release of biphenyl into environmental
compartments from industrial use, from the application of
biphenyl-contaminated sewage sludge to arable land, from
volatilization during the storage of treated fruits, and from leaching
from preserved citrus fruits and their packaging materials in domestic
waste dumps. Only estimated values are available for the
photooxidation of biphenyl in the atmosphere, which is probably the
most important degradation process in this compartment.
Biomagnification of biphenyl in higher trophic levels of the
aquatic or terrestrial food-chain, based upon the potential of
biphenyl to bioaccumulate, is expected to be of minor importance. For
the aquatic compartment, a significant elimination of biphenyl can be
expected as a result of evaporation and degradation. Furthermore,
rapid metabolism of biphenyl in laboratory animals and low acute
toxicity after ingestion have been reported.
Data on effect concentrations for terrestrial organisms and
exposure levels in air and soil are insufficient for risk
characterization for terrestrial organisms. A sample risk
characterization with respect to the aquatic environment can be
performed (based upon data derived from Germany) by calculating the
ratio between a (local or regional) PEC (based on measured or model
concentrations) and a PNEC (EC, 1996).
The annual average concentration of biphenyl (<0.5 µg/litre)
measured in the 1990s in the Rhine River and its tributaries was used
as a PECmeasured. A PEClocal (water), based upon data derived from the
effluent of the wastewater treatment plant of a German producer, may
be calculated as follows:
PEClocal (water) = Ceffluent
(1 + Kp (susp) × Csusp × 10-6) × D
= <0.0064 µg/litre
where:
* Ceffluent is <5 µg/litre, the concentration of biphenyl in
wastewater in 1990 (BUA, 1990);
* Kp (susp) = 423, the suspended matter/water adsorption
coefficient, estimated from the mean soil sorption coefficient
( Koc) of 4230 ( Kp (susp) = Koc × 0.1, where 0.1 is the
fraction of organic carbon in suspended matter, foc(susp));
* Csusp = 15 mg/litre (default value; EC, 1996), the
concentration of suspended matter in the river; and
* D = 781, the dilution factor for river flow, calculated from
sewage treatment plant and river flow data (EC, 1996).1
A PNEC for surface waters may be calculated by dividing the
lowest valid LC50 or NOEC by an appropriate assessment factor (equal
to a safety factor):
PNEC = 170 µg/litre
10
= 1.7 µg/litre
where:
* 170 µg/litre is the lowest NOEC from a chronic study with
Daphnia magna; and
1 Personal communication from Verband d. Textilhilfsmittel-,
Lederhilfsmittel-, Gerbstoff- und Waschrohstoff-Industrie e.V.,
Frankfurt/M., Germany, to Bayer AG, 1996.
* 10 is the assessment factor. For biphenyl, based upon data for
four trophic levels, including one long-term NOEC, the
recommended assessment factor would be 100 (EC, 1996). However,
because of its significant volatility and degradation, and as
effect concentrations from a wide selection of species covering
different taxonomic groups are reported, an assessment factor of
10 is used.
Therefore, based upon the annual average concentrations of
biphenyl measured in the Rhine River and in industrial wastewater
effluent in Germany, the PECmeasured/PNEC and PEClocal (water)/PNEC
ratios are <0.29 and <0.04, respectively. In some jurisdictions
(EC, 1996), chemicals with a PEC/PNEC ratio of <1 may not require
implementation of risk reduction measures beyond those already in
place.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The Joint FAO/WHO Meeting on Pesticide Residues has allocated an
acceptable daily intake of biphenyl of 0-0.125 mg/kg body weight
(JMPR, 1967).
Information on international hazard classification and labelling
is included in the International Chemical Safety Card reproduced in
this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventative and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0106) reproduced in this
document.
13.1 Advice to physicians
Treatment is symptomatic and supportive following intoxication.
The clinical picture is characterized by central and peripheral nerve
damage and liver injury in human poisoning. There is some evidence to
indicate that electroencephalographic abnormalities could persist up
to 1 or 2 years after exposure.
13.2 Health surveillance advice
Objective change in electroencephalogram and electroneuromyogram
could be an indication of biphenyl poisoning in exposed individuals.
Monitoring of liver function could also be integrated into a medical
surveillance programme.
13.3 Spillage
In the event of spillage, measures should be under taken to
prevent biphenyl from reaching drains and watercourses, because of its
toxicity to aquatic organisms.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards
can be found in the International Register of Potentially Toxic
Chemicals (IRPTC), available from UNEP Chemicals (IRPTC), Geneva.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
BIPHENYL ICSC: 0106
April 1994
CAS # 92-52-4 Diphenyl
RTECS # DU8050000 Phenylbenzene
EC # 601-042-00-8 Dibenzene
C12H10/C6H5C6H5
Molecular mass: 154.2
TYPES OF HAZARD/ ACUTE HAZARDS/ PREVENTION FIRST AID/FIRE FIGHTING
EXPOSURE SYMPTOMS
FIRE Combustible NO open flames Water spray, powder, AFFF, foam,
carbon dioxide
EXPLOSION Finely dispersed particles Prevent deposition of dust, closed
form explosive mixtures in air. system, dust explosion-proof
electrical equipment and lighting.
Prevent build-up of electrostatic
charges (e.g., by grounding)
EXPOSURE PREVENT DISPERSION OF DUST!
Inhalation Cough, Nausea, Vomiting Avoid inhalation of fine dust Fresh air, rest. Refer for medical
and mist. attention.
Skin Protective gloves. Remove contaminated clothes. Rinse
and then wash skin with water and
soap.
Eyes Redness. Pain Safety goggles, or eye protection First rinse with plenty of water for
in combination with breathing several minutes (remove contact lenses
protection if powder. if easily possible), then take to a
doctor.
TYPES OF HAZARD/ ACUTE HAZARDS/ PREVENTION FIRST AID/FIRE FIGHTING
EXPOSURE SYMPTOMS
Ingestion (further see Inhalation) Do not eat, drink, or smoke Rinse mouth. Refer for medical
during work. Wash hands before attention.
eating.
SPILLAGE DISPOSAL PACKAGING & LABELLING
Sweep spilled substance into sealable containers; if appropriate, moisten Do not transport with food and feedstuffs.
first to prevent dusting. Carefully collect remainder, then remove to safe EU Classification
place. (Extra personal protection: A/P2 filter respirator for organic vapour Symbol: Xi
and harmful dust). R: 36/37/38
S: (2-)23
UN Classification
EMERGENCY RESPONSE STORAGE
NFPA Code: H 2; F1; R O; Separated from food and feedstuffs and oxidants.
IMPORTANT DATA
PHYSICAL STATE; APPEARANCE: ROUTES OF EXPOSURE:
WHITE CRYSTALS OR FLAKES, WITH CHARACTERISTIC ODOUR The substance can be absorbed into the body by inhalation and by ingestion.
PHYSICAL DANGERS: INHALATION RISK:
Dust explosion possible if in powder or granular form, A harmful contamination of the air can be reached rather quickly on
mixed with air. evaporation of this substance at 20°C.
CHEMICAL DANGERS: EFFECTS OF SHORT-TERM EXPOSURE:
Reacts with oxidants. The substance irritates the eyes and the respiratory tract.
OCCUPATIONAL EXPOSURE LIMITS: EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
TLV: 0.2 ppm; 1.3 mg/m3 (ACGIH 1993-1994) The substance may have effects on the liver and nervous system, resulting
impaired functions.
PHYSICAL PROPERTIES
Boiling point 256°C Flash point: 113°C c.c.
Melting point 70°C Auto-ignition temperature: 540°C
Relative density (water = 1): 1.04 Explosive limits, vol% in air: 0.6 (at 111°C) - 5.8 (at 166°C)
Solubility in water: none Octanol/water partition coefficient as log Pow: 3.16/4.09
Vapour pressure, Pa at 71°C: 133
Relative vapour density (air = 1): 5.3
Relative density of the vapour/air-mixture
at 20°C (air = 1): 1.0
ENVIRONMENTAL DATA
The substance may be hazardous to the environment, special attention should be given to water. In the food chain important to humans,
bioaccumulation takes place, specifically in plants. It is strongly advised not to let the chemical enter into the environment because it
persists in the environment.
NOTES
Do NOT take working clothes home. Dowtherm A is a trade name.
ADDITIONAL INFORMATION
LEGAL NOTICE: Neither the CEC or the IPCS nor any person acting on behalf of the CEC or the IPCS is responsible for the use which
might be made of this information.
REFERENCES
Abe S, Sasaki M (1977) Chromosome aberrations and sister chromatid
exchanges in Chinese hamster cells exposed to various chemicals.
Journal of the National Cancer Institute, 58:1635-1641.
Ahel M (1991) Infiltration of organic pollutants into groundwater:
field studies in the alluvial aquifer of the Sava river. Bulletin
of environmental contamination and toxicology, 47:586-593.
Ambrose AM, Booth AN, DeEds F, Cox AJ (1960) A toxicological study of
biphenyl, a citrus fungistat. Food research, 25:328-336.
Anklam E, Mueller A (1994) A simple method of sample preparation for
analysis of biphenyl residues in citrus fruit peels by gas
chromatography. Zeitschrift fuer Lebensmittel Untersuchung und
Forschung, 198:329-330.
Anon. (1994-95) The Chemical Daily, pp. 679-680 (no further
information available).
Atkinson R, Arey J (1994) Atmospheric chemistry of gas-phase
polycyclic aromatic hydrocarbons: formation of atmospheric mutagens.
Environmental health perspectives, 102 (Suppl. 4):117-126.
Atkinson R, Aschmann SM (1985) Rate constants for the gas-phase
reaction of hydroxyl radicals with biphenyl and the
monochlorobiphenyls at 294 ± 1 K. Environmental science and
technology, 19:462-464.
ATSDR (1990) Toxicological profile for creosote. Atlanta, GA, US
Department of Health and Human Services, Public Health Service, Agency
for Toxic Substances and Disease Registry (Report No. TP-90-09).
Baranski Z (1991) Evaluation of occupational exposure to aromatic
polycyclic hydrocarbons in bituminous mastics plants. Roczniki
Panstwowego Zakladu Higieny, 42:223-237.
Bend JR, Hook GER, Easterling RE, Gram TE, Fouts JR (1972) A
comparative study of the hepatic and pulmonary microsomal
mixed-function oxidase systems in the rabbit. Journal of
pharmacology and experimental therapeutics, 183:206-217.
Bevinakatti BG, Ninnekar HZ (1992) Degradation of biphenyl by a
Micrococcus species. Applied microbiology and biotechnology,
38:273-275.
Booth AN, Ambrose AM, DeEds F (1956) Reversible nephrotoxic effects of
biphenyl. Federation proceedings, 15:403 (Abstract 1313).
Booth AN, Ambrose AM, DeEds F, Cox AJ (1961) The reversible
nephrotoxic effects of biphenyl. Toxicology and applied
pharmacology, 3:560-567.
Bos RP, Theuws JLG, Jongeneelen FJ, Henderson PT (1988) Mutagenicity
of bi-, tri- and tetra-cyclic aromatic hydrocarbons in the
"taped-plate assay" and in the conventional Salmonella mutagenicity
assay. Mutation research, 204:203-206.
Boutwell RK, Bosch DK (1959) The tumor-promoting action of phenol and
related compounds for mouse skin. Cancer research, 19:413-424.
Brams A, Buchet JP, Crutzen-Fayt MC, de Meester C, Lauwerys R, Leonard
A (1987) A comparative study, with 40 chemicals, of the efficiency of
the Salmonella assay and the SOS chromotest (kit procedure).
Toxicology letters, 38:123-133.
Bronzetti G, Esposito A, Pagano G, Quinto I (1981) A comparative study
on the toxicity and mutagenicity of biphenyl (BP) and diphenyl ether
(DPE) in sea urchin, S. typhimurium and S. cerevisiae. Mutation
research, 85:233.
Brouns RE, Poot M, de Vrind R, van Hoek-Kon T, Henderson PT (1979)
Measurement of DNA-excision repair in suspensions of freshly isolated
rat hepatocytes after exposure to some carcinogenic compounds. Its
possible use in carcinogenicity screening. Mutation research,
64:425-432.
BUA (1990) BUA-Stoffbericht Biphenyl (1,1'-Biphenyl). Berater
gremium fuer Umweltrelevante Altstoffe. Weinheim, VCH VerlagsGmbH
(Report No. 50; July 1990).
BUA (1994) BUA-Stoffbericht 133 (Ergaenzungsberichte II). Berater
gremium fuer Umweltrelevante Altstoffe. Weinheim, VCH VerlagsGmbH.
Carella G, Bettolo PM (1994) Reversible hepatotoxic effects of
diphenyl: report of a case and a review of the literature. Journal
of occupational medicine, 36:575-576.
CITI (1992) Biodegradation and bioaccumulation. Data of existing
chemicals based on the CSCL Japan. Tokyo, Japan Chemical Industry
Ecology-Toxicology & Information Center, Chemicals Inspection &
Testing Institute, p. 4-1.
Cline JC, McMahon RE (1977) Detection of chemical mutagens. Use of
concentration gradient plates in a high capacity screen. Research
communications in chemical pathology and pharmacology, 16:523-533.
Cohen SM (1995) Human relevance of animal carcinogenicity studies.
Regulatory toxicology and pharmacology, 21:75-80.
Collin G, Hoeke H (1995) Tar and pitch. In: Elvers B, Hawkins S,
Russes W, eds. Ullmann's encyclopedia of industrial chemistry.
Vol. A26. 5th ed. Weinheim, VCH Verlagsgesellschaft, pp. 91-127.
Deichmann WB, Kitzmiller KV, Dierker M, Witherup S (1947) Observations
on the effects of diphenyl, o- and p-aminodiphenyl, o- and
p-nitrodiphenyl and dihydroxyoctachlorodiphenyl upon experimental
animals. Journal of industrial hygiene and toxicology, 29:1-13.
Deusen C, Kleinermanns K (1993) Identification of exhaust air
components with thermal inertization of dusts from cold setting
moulding materials. Giessereiforschung, 45:147-150.
Di Muccio A, Barbini DA, De Merulis G, Vergori L, Girolimetti S,
Sernicola L, Dommarco R (1995) Rapporto sulle revisioni di analisi
per residui di antiparassitari: 1988-1995. Rome, Istituto Superiore
di Sanita.
Dive D, Leclerc H, Persoone G (1980) Pesticide toxicity on the ciliate
protozoan Colpidium campylum: possible consequences of the effect of
pesticides in the aquatic environment. Ecotoxicology and
environmental safety, 4:129-133.
Donkin P, Widdows J, Evans SV, Brinsley MD (1991) QSARs for the
sublethal responses of marine mussels (Mytilus edulis). Science of
the total environment, 109(100):461-476.
Dorgelo FO, Verver G, Wieling G, Topp RJ, Boleij JSM, Pal TM (1985)
Urinary hydroxydiphenyl excretion of workers occupationally exposed to
a mixture of diphenyl and diphenylether (Dowtherm A). International
archives of occupational and environmental health, 56:129-134.
Dow Chemical Co. (1974) Acute inhalation toxicity and industrial
handling hazards of biphenyl heated to 85°C (EPA Document I.D.:
878213725, received 1983) [cited in BUA, 1994].
Dow Chemical Co. (1976) Cytogenetic effects of diphenyl-99 on rat
bone marrow cells (EPA Document I.D.: 878213726, received 1983)
[cited in BUA, 1994].
Dreist M, Kolb J (1993) Untersuchungen auf hautsensibilisierende
Wirkung am Meerschweinchen (Maximierungstest nach Magnusson und
Kligman) (Bericht Nr. 22057 vom 19.02.1993) [cited in BUA, 1994].
EC (1996) Technical guidance documents in support of the
Commission Directive 93/67/EEC on risk assessment for new notified
substances and the Commission Regulation (EC) 1488/94 on risk
assessment for existing substances. Ispra, European Chemicals
Bureau.
Engewald W, Knobloch T, Efer J (1993) Fluechtige organische
Verbindungen in Emissionen aus dem Hausbrandvon Braunkohle.
Umweltwissenschaften Schadstoff Forschung, 6:303-308.
Farkas A (1939) Hadar, 12:227 (no further information available)
[cited in JMPR, 1967].
Freitag D, Geyer H, Kraus A, Viswanathan R, Kotzias D, Attar A, Klein
W, Korte F (1982) Ecotoxicological profile analysis -- VII. Screening
chemicals for their environmental behavior by comparative evaluation.
Ecotoxicology and environmental safety, 6:60-81.
Fujita H, Kojima A, Sasaki M, Hiraga K (1985) Mutagenicity test of
antioxidants and fungicides with Salmonella typhimurium TA97a,
TA102. Kenkyu Nenpo-Tokyo-toritsu Eisei Kenkyusho, 36:413-417.
Garberg P, Akerblom E-L, Bolcsfoldi G (1988) Evaluation of a
genotoxicity test measuring DNA-strand breaks in mouse lymphoma cells
by alkaline unwinding and hydroxyapatite elution. Mutation
research, 203:155-176.
Gersich FM, Bartlett EA, Murphy PG, Milazzo DP (1989) Chronic toxicity
of biphenyl to Daphnia magna Straus. Bulletin of environmental
contamination and toxicology, 43:355-362.
Glatt H, Anklam E, Robertson LW (1992) Biphenyl and fluorinated
derivatives: liver enzyme-mediated mutagenicity detected in
Salmonella typhimurium and Chinese hamster V79 cells. Mutation
research, 281:151-156.
Haekkinen I, Siltanen E, Hernberg S, Seppaelaeinen AM, Karli P,
Vikkula E (1973) Diphenyl poisoning in fruit paper production.
Archives of environmental health, 26:70-74.
Haworth S, Lawlor T, Mortelmans K, Speck W, Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals.
Environmental mutagenesis, 5 (Suppl. 1):3-142.
Hawthorne SB, Miller DJ, Langenfeld JJ, Krieger MS (1992) PM-10
high-volume collection and quantitation of semi- and nonvolatile
phenols, methoxylated phenols, alkanes, and polycyclic aromatic
hydrocarbons from winter urban air and their relationship to wood
smoke emissions. Environmental science and technology, 26:2251-2262.
Hites RA (1973) Analysis of trace organic compounds in New England
rivers. Journal of chromatographic science, 11:570-574.
Hook GER, Bend JR (1976) Pulmonary metabolism of xenobiotics. Life
sciences, 18:279-290.
Hook GER, Bend JR, Hoel D, Fouts JR, Gram TE (1972) Preparation of
lung microsomes and a comparison of the distribution of enzymes
between subcellular fractions of rabbit lung and liver. Journal
of pharmacology and experimental therapeutics, 182:474-490.
Hulzebos EM, Adema DMM, Dirven-van Breemen EM, Henzen L, van Dis HA,
Herbold HA, Hoekstra JA, Baerselman R, van Gestel CAM (1993)
Phytotoxicity with Lactuca sativa in soil and nutrient solution.
Environmental toxicology and chemistry, 12:1079-1094.
IARC (1985) Polynuclear aromatic compounds. Part 4. Bitumens,
coal-tars and derived products, shale-oils and soots. Lyon,
International Agency for Research on Cancer, pp. 83-159 (IARC
Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to
Humans, Vol. 35).
Innes JRM, Ulland BM, Valerio MG, Petrucelli L, Fishbein L, Hart ER,
Pallotta AJ, Bates RR, Falk HL, Gart JJ, Klein M, Mitchell I, Peters J
(1969) Bioassay of pesticides and industrial chemicals for
tumorigenicity in mice: a preliminary note. Journal of the National
Cancer Institute, 42:1101-1114.
IPCS (1993) International Chemical Safety Card -- Biphenyl. Geneva,
World Health Organization, International Programme on Chemical Safety
(ICSC 0106).
IRPTC (1994) IRPTC legal and treatment and disposal methods for
waste chemical files: Biphenyl (CAS-No. 92-52-4) and
polychlorinated biphenyls (CAS-No. 1336-36-3). Geneva, United
Nations Environment Programme, International Register of Potentially
Toxic Chemicals.
Ishidate M, Odashima S (1977) Chromosome tests with 134 compounds on
Chinese hamster cells in vitro -- a screening for chemical
carcinogens. Mutation research, 48:337-354.
Ishidate M, Sofuni T, Yoshikawa K, Hayashi M, Nohmi T, Sawada M,
Matsuoka A (1984) Primary mutagenicity screening of food additives
currently used in Japan. Food and chemical toxicology, 22:623-636.
Isshiki K, Tsumura S, Watanabe T (1982) Post-harvest fungicides in
edible parts of citrus fruits. Agricultural and biological
chemistry, 46:993-999.
Japan Bioassay Research Center (1996) Two year feeding study of
biphenyl in rats and mice. Tokyo, National Institute of Health
Sciences (unpublished report).
Jeng CY, Chen DH, Yaws DL (1992) Data compilation for soil sorption
coefficient. Pollution engineering, 15:54-60.
JMPR (1967) 1967 evaluations of some pesticide residues in food.
Joint meeting of the FAO working party of experts and the WHO expert
committee on pesticide residues, Rome, 4-11 December 1967. Rome, World
Health Organization.
Karanassios IG, Georakilas V, Lahaniatis ES, Pilidis GA (1994)
Determination of airborne aromatic and polyaromatic hydrocarbons in
two towns of north-western Greece. Fresenius environmental
bulletin, 3:511-516.
Kawachi T, Yahagi T, Kada T, Tazima Y, Ishidate M, Sasaki M, Sugiyama
T (1980) Cooperative programme on short-term assays for
carcinogenicity in Japan. In: Montesano R, Bartsch H, Tomatis L, eds.
Molecular and cellular aspects of carcinogen screening tests. Lyon,
International Agency for Research on Cancer, pp. 323-330 (IARC
Scientific Publications No. 27).
Khera KS, Whalen C, Angers G, Trivett G (1979) Assessment of the
teratogenic potential of piperonyl butoxide, biphenyl, and phosalone
in the rat. Toxicology and applied pharmacology, 47:353-358.
Kirton P, Ellis J, Crisp PT (1991) The analysis of organic matter in
coke oven emissions. Fuel, 70:1383-1389.
Kishi H, Hasimoto Y (1991) Contribution of soil constituents to the
adsorption of chemicals. In: Arend F, Hinsenveld M, Van den Brink WJ,
eds. Altlastensanierung 90, 3rd KfK/TNO Kongress. Bundesministerium
Forschung Technologie, pp. 387-393.
Kiyohara H, Takizawa N, Nagao K (1992) Natural distribution of
bacteria metabolizing many kinds of polycyclic aromatic hydrocarbons.
Journal of fermentation and bioengineering, 74:49-51.
Kostiainen R (1995) Volatile organic compounds in the indoor air of
normal and sick houses. Atmospheric environment, 29:693-702.
Kotzias D, Geyer H, Viswanathan R, Kraus A, Freitag D, Klein W, Korte
F (1980) An approach to the ecotoxicological evaluation of
environmental chemicals. In: Kovatsis A, ed. Toxicological aspects.
9th International Congress of the European Association of Poison
Control Centers and the European Meeting of the International
Association of Forensic Toxicologists, Thessaloniki, 24-27 August
1980, pp. 257-268.
Kurata Y, Asamoto M, Hagiwara A, Masui T, Fukushima S (1986) Promoting
effects of various agents in rat urinary bladder carcinogenesis
initiated by N-butyl- N(4-hydroxybutyl)nitrosamine. Cancer
letters, 32:125-135.
Leach JM, Otson R, Armstrong V (1987) Airborne contaminants in two
small Canadian coal liquefaction pilot plants. American Industrial
Hygiene Association Journal, 48:693-697.
LWA (1993-94) Gewaesserguetebericht '93/'94. Nordrhein-Westfalen,
Landesamt fuer Wasser und Abfall.
Macintosh FC (1945) The toxicity of diphenyl and o-phenyl-phenol.
Analyst, 70:334-335.
Mackay D, Shiu WY, Ma KC (1992) Illustrated handbook of
physical-chemical properties and environmental fate for organic
chemicals. Vol. II. Polynuclear aromatic hydrocarbons,
polychlorinated dioxins, and dibenzofurans. Boca Raton, FL, Lewis
Publishers.
Malins DC, Krahn MM, Brown DW, Rhodes LD, Myers MS, McCain BB, Chan S
(1985) Toxic chemicals in marine sediment and biota from Mukilteo,
Washington: Relationships with hepatic neoplasms and other hepatic
lesions in English sole (Parophrys vetulus). Journal of the
National Cancer Institute, 74:487-494.
Matsubara T, Prough RA, Burke MD, Estabrook RW (1974) The preparation
of microsomal fractions of rodent respiratory tract and their
characterization. Cancer research, 34:2196-2203.
Meyer T (1977) The metabolism of biphenyl. IV. Phenolic metabolites in
the guinea pig and the rabbit. Acta Pharmacologica et Toxicologica,
40:193-200.
Meyer T, Scheline RR (1976) The metabolism of biphenyl. II. Phenolic
metobolites in the rat. Acta Pharmacologica et Toxicologica,
39:419-432.
Meyer T, Larsen JC, Hansen EV, Scheline RR (1976a) The metabolism of
biphenyl. III. Phenolic metobolites in the pig. Acta Pharmacologica
et Toxicologica, 39:433-441.
Meyer T, Aarbakke J, Scheline RR (1976b) The metabolism of biphenyl.
I. Metabolic disposition of 14C-biphenyl in the rat. Acta
Pharmacologica et Toxicologica, 39:412-418.
Monsanto Co. (1959) Animal inhalation study on biphenyl at 80°F and
100°F (EPA Document I.D.: 878213571, received 1983) [cited in BUA,
1994].
Newell GW (1953) A toxicological study of diphenyl in citrus wraps
(Stanford Research Institute Report No. B 326) [cited in Monsanto Co.
(1996) Toxicological data on biphenyl].
Nielsen PH, Christensen TH (1994) Variability of biological
degradation of aromatic hydrocarbons in an aerobic aquifer determined
by laboratory batch experiments. Journal of contaminant hydrology,
15:305-320.
NTIS (1968) Evaluation of carcinogenic, teratogenic and mutagenic
activities of selected pesticides and industrial chemicals (National
Technical Information Service PB-223159, August 1968) [cited in BUA,
1990].
NTP (1980) Annual plan for fiscal year 1981. Research Triangle Park,
NC, US Department of Health and Human Services, National Toxicology
Program, p. 32.
Otson R, Fellin R, Tran Q (1994) VOCs in representative Canadian
residences. Atmospheric environment, 28:3563-3569.
Paasivirta J, Kaeaeriaeinen H, Lahtiperae M, Pellinen J, Sinkkonen S
(1982) Oil residues in Baltic sediment, mussel and fish. II. Study of
the Finnish Archipelago 1979-81. Chemosphere, 11:811-821.
Pagano G, Esposito A, Giordano GG, Vamvakinos E, Quinto I, Bronzetti
G, Bauer C, Corsi C, Nieri R, Ciajolo A (1983) Genotoxicity and
teratogenicity of diphenyl and diphenyl ether: a study of sea urchins,
yeast, and Salmonella typhimurium. Teratogenesis, carcinogenesis,
and mutagenesis, 3:377-393.
Pagano G, Cipollaro M, Corsale G, Della Morte R, Esposito A, Giordano
GG, Micallo G, Quinto I, Staiano N (1988) Comparative toxicity of
diphenyl, diphenyl ester, and some of their hydroxy derivatives.
Médecine Biologie Environnement, 16:291-297.
Paschke A, Herbel W, Steinhart H (1992) Determination of mono- to
tetracyclic aromatic hydrocarbons in lubricating oil. Journal of
high resolution chromatography, 15:827-833.
Penttilae P-L, Siivinen K (1996) Control and intake of pesticide
residues during 1981-1993 in Finland. Food additives and
contaminants, 13:609-622.
Powis G, Moore DJ, Wilke TJ, Santone KS (1987) A high-performance
liquid chromatography assay for measuring integrated biphenyl
metabolism by intact cells: its use with rat liver; human liver and
kidney. Analytical biochemistry, 167:191-198.
Probst GS, McMahon RE, Hill LE, Thompson CZ, Epp JK, Neal SB (1981)
Chemically-induced unscheduled DNA synthesis in primary rat hepatocyte
cultures: a comparison with bacterial mutagenicity using 218
compounds. Environmental mutagenesis, 3:11-32.
Prough RA, Burke MD (1975) The role of NADPH-cytochrome c reductase in
microsomal hydroxylation reactions. Archives of biochemistry and
biophysics, 170:160-168.
Purchase IFH, Longstaff E, Ashby J, Styles JA, Anderson D, Lefevre PA,
Westwood FR (1978) An evaluation of 6 short-term tests for detecting
organic chemical carcinogens. British journal of cancer, 37:873-959.
Selle WA (1952) Progress reports on the effects of topical
application of diphenyl (November 6, 1952, July 23, 1953, February
23, 1954) [cited in Verwendung von Diphenyl, Orthophenylphenol
und dessen Natriumverbindung zur Oberflaechenbehandlung von
Zitrusfruechten. Bundesgesundheitsblatt 11 (dated 26 May 1967), pp.
168-173].
Shibata MA, Yamada M, Tanaka H, Kagawa M, Fukushima S (1989a) Changes
in urine composition, bladder epithelial morphology, and DNA synthesis
in male F344 rats in response to ingestion of bladder tumor promoters.
Toxicology and applied pharmacology, 99:37-49.
Shibata MA, Tanaka H, Yamada M, Tamano S, Fukushima S (1989b)
Proliferative response of renal pelvic epithelium in rats to oral
administration of ortho-phenylphenol sodium ortho-phenylphenate and
diphenyl. Cancer letters, 48:19-28.
Shiraiwa K, Takita M, Tsutsumi M, Kinugasa T, Denda A, Takahashi S,
Konishi Y (1989) Diphenyl induces urolithiasis but does not possess
the ability to promote carcinogenesis by
N-ethyl- N-hydroxyethylnitrosamine in kidneys of rats. Journal
of toxicologic pathology, 2:41-48.
Siegl WO, Chladek E (1992) Measurement of gas-phase polycyclic
aromatic hydrocarbons (PAH) in gasoline vehicle exhaust. American
Chemical Society Division of Petroleum Chemistry, 4:1499-1504.
Snyder RD, Matheson DW (1985) Nick translation -- a new assay for
monitoring DNA damage and repair in cultured human fibroblasts.
Environmental mutagenesis, 7:267-279.
Sofuni T, Hayashi M, Matsuoka A, Sawada M, Hatanaka M, Ishidate M
(1985) Mutagenicity tests on organic chemical contaminants in city
water and related compounds. II. Chromosome aberration tests in
cultured mammalian cells. Eisei Shikensho Hokoku, 103:64-75.
Sondergaard D, Blom L (1979) Polycystic changes in rat kidney induced
by biphenyl fed in different diets. Archives for toxicology, Suppl.
2:499-502.
Stanford Research Institute (undated) Final report -- a
toxicological study of diphenyl in citrus wraps. Menlo Park, CA
[cited in US EPA (1984) Health and environmental effects profile
for 1,1'-biphenyl. Cincinnati, OH, US Environmental Protection
Agency].
Sun Co. Inc. (1977a) Acute inhalation toxicity of biphenyl -- with
cover letter (EPA Document I.D.: 878213530, received 1983) [cited in
BUA, 1994].
Sun Co. Inc. (1977b) Subacute inhalation toxicity of biphenyl (EPA
Document I.D.: 878213531, received 1983) [cited in BUA, 1994].
Sun Co. Inc. (1977c) 90-day inhalation toxicity study of biphenyl
(99+% purity) in CD mice (EPA Document I.D.: 878213532, received
1983) [cited in BUA, 1994].
Takita M (1983) Urolithiasis induced by oral administration of
diphenyl in rats. Journal of the Nara (Medical University) Medical
Association, 34:565-584.
Tamano S, Asakawa E, Boomyaphiphat P, Masui T, Fukushima S (1993) Lack
of promotion of N-butyl- N-(4-hydroxybutyl)nitrosamine-initiated
urinary bladder carcinogenesis in mice by rat cancer promotors.
Teratogenesis, carcinogenesis, and mutagenesis, 13:89-96.
Tanaka A, Morimoto K, Yamaha T (1993) Small scale synthesis of labeled
diphenyl and its binding to mouse liver microsomes. Radio isotopes,
42:564-568.
Wangenheim J, Bolcsfoldi G (1988) Mouse lymphoma L5178Y thymidine
kinase locus assay of 50 compounds. Mutagenesis, 3:193-205.
Waters MD, Sandhu SS, Simmon VF, Mortelmans KE, Mitchell AD, Jorgenson
TA, Jones DCL, Valencia R, Garrett NE (1982) Study of pesticide
genotoxicity. Basic life sciences, 21:275-326.
Weil E, Kusterer L, Brogard MH (1965) Archives des Maladies
Professionnelles, 26:405 [cited in Henschler D (1979)
Toxikologischarbeitsmedizinische Begruendungen von MAK-Werten.
Weinheim, VCH VerlagsGmbH].
Wienecke J, Kruse H, Wassermann O (1992) Organic compounds in the flue
gas from a power station with circulating fluid bed combustion of
coal. Chemosphere, 25:1889-1895.
Wilkins CK, Wolkoff P, Gyntelberg F, Skov P, Valbjoern O (1993)
Characterization of office dust by VOCs and TVOC release --
identification of potential irritant VOCs by partial least squares
analysis. Indoor air, 3:283-290.
Williams GM (1978) Further improvements in the hepatocyte primary
culture DNA repair test for carcinogens: Detection of carcinogenic
biphenyl derivatives. Cancer letters, 4:69-75.
Windeatt AJ, Tapp JF, Stanley RD (1991) The use of soil-based tests
based on the OECD guidelines. In: Gorsuch JW, Lower WR, Wang W, Lewis
MA, eds. Plants for toxicity assessment. Vol. 2. Philadelphia, PA,
American Society for Testing and Materials, pp. 29-40 (ASTM Special
Technical Publication 1115).
Yao W, Xu X (1991) Determination of pollutants emitted from burning
coal and of their mutagenicities. Analytical science, 7:1381-1384.
Zhang Y, Rott B, Freitag D (1983) Accumulation and elimination of
14-C-PCB's by Daphnia magna Straus 1820. Chemosphere,
12:1645-1651.
Zimmermann FK, von Borstel RC, von Halle ES, Parry JM, Siebert D,
Zetterberg G, Barale R, Loprieno N (1984) Testing of chemicals for
genetic activity with Saccharomyces cerevisiae: a report of the U.S.
Environmental Protection Agency Gene-Tox Program. Mutation
research, 133:199-244.
APPENDIX 1 -- SOURCE DOCUMENTS
BUA-Stoffbericht Biphenyl (1,1'-Biphenyl). Beratergremium fuer
Umweltrelevante Altstoffe (Report No. 50; July 1990). VCH VerlagsGmbH,
Weinheim
BUA-Stoffbericht 133 (Ergaenzungsberichte II). Beratergremium fuer
Umweltrelevante Altstoffe (1994). VCH VerlagsGmbH, Weinheim
For the BUA review process, the company that is in charge of
writing the report (usually the largest producer in Germany) prepares
a draft report using literature from an extensive literature search as
well as internal company studies. This draft is subject to a peer
review during several readings of a working group consisting of
representatives from government agencies, the scientific community,
and industry.
The English translation of BUA Report No. 50 (BUA Report Biphenyl
(1,1'-Biphenyl). GDCh-Advisory Committee on Existing Chemicals of
Environmental Relevance (July 1990). VCH VerlagsGmbH, Weinheim) was
released in 1992. The English translation of its Supplementary Report
No. 133 is in preparation.
APPENDIX 2 -- CICAD PEER REVIEW
The draft CICAD on biphenyl was sent for review to institutions
and organizations identified by IPCS after contact with IPCS national
Contact Points and Participating Institutions, as well as to
identified experts. Comments were received from:
Bayer AG, Leverkusen, Germany
Department of Health, London, United Kingdom
Department of Public Health, Albert Szent-Gyorgyi University Medical
School, Szeged, Hungary
Environmental Health Directorate, Health Canada, Ottawa, Canada
International Agency for Research on Cancer, Lyon, France
Ministry of Health and Welfare, International Affairs Division,
Government of Japan, Tokyo, Japan
Monsanto Europe SA, Louvain-La-Neuve, Belgium
National Institute for Working Life, Solna, Sweden
National Institute of Occupational Health, Budapest, Hungary
United States Department of Health and Human Services (National
Institute of Environmental Health Sciences)
United States Environmental Protection Agency (Office of Pollution
Prevention and Toxics; Office of Drinking Water)
APPENDIX 3 -- CICAD FINAL REVIEW BOARD (Brussels, Belgium)
Brussels, Belgium, 18-20 November 1996
Members
Dr A. Aitio, Institute of Occupational Health, Helsinki, Finland
Dr K. Bentley, Director, Environment Policy Section, Commonwealth
Department of Human Services and Health, Canberra, Australia
Mr R. Cary, Toxicology and Existing Substances Regulation Unit, Health
and Safety Executive, Merseyside, United Kingdom
Dr J. de Fouw, National Institute of Public Health and Environmental
Protection, Bilthoven, The Netherlands
Dr C. DeRosa, Director, Division of Toxicology, Agency for Toxic
Substances and Disease Registry, Atlanta, GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr W. Farland, Director, National Center for Environmental Assessment,
Office of Research and Development, US Environmental Protection
Agency, Washington, DC, USA (Chairperson)
Dr T.I. Fortoul, Depto. Biologia Celular y Tisular, National
University of Mexico and Environmental Health Directorate of the
Health Ministry, Mexico D.F., Mexico
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Mr J.R. Hickman, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Dr T. Lakhanisky, Head, Division of Toxicology, Institute of Hygiene
and Epidemiology, Brussels, Belgium (Vice-Chairperson)
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany
Ms M.E. Meek, Head, Priority Substances Section, Environmental Health
Directorate, Health Canada, Ottawa, Ontario, Canada
Dr K. Paksy, National Institute of Occupational Health, Budapest,
Hungary
Mr D. Renshaw, Department of Health, London, United Kingdom
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan
Dr H. Sterzl-Eckert, GSF-Forschungszentrum für Umwelt und Gesundheit
GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Professor S. Tarkowski, Department of Environmental Health Hazards,
The Nofer Institute of Occupational Medicine, Lodz, Poland
Dr M. Wallen, National Chemicals Inspectorate (KEMI), Solna, Sweden
Observers
Professor F.M.C. Carpanini,1 Director, Centre for Ecotoxicology and
Toxicology of Chemicals (ECETOC), Brussels, Belgium
Mr R. Haigh,1 Head of Unit, Health and Safety Directorate, European
Commission, Luxembourg
Mr B.U. Hildebrandt, Federal Ministry for the Environment, Nature
Conservation and Nuclear Safety, Bonn, Germany
Mr P. Hurst,1 Chemical and Consumer Policy Officer, Conservation
Policy Division, World Wide Fund for Nature, Gland, Switzerland
Dr A. Lombard (CEFIC representative), ELF-ATOCHEM, Paris, France
Dr P. McCutcheon,1 Environment, Consumer Protection and Nuclear
Safety, European Commission, Brussels, Belgium
Dr R. Montaigne, Counsellor, Technical Affairs Department, European
Chemical Industry Council (CEFIC), Brussels, Belgium
Dr M. Pemberton, ICI Acrylics, Lancashire, United Kingdom
Dr A. Smith, Environment Division, Organisation for Economic
Co-operation and Development, Paris, France
Secretariat
Dr M. Baril, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr L. Harrison, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M. Mercier, Director, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr P. Toft, Associate Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
1 Invited but unable to attend.
APPENDIX 4 -- SPECIALIZED CICAD PEER REVIEW
Individuals contributing written comments to the specialized
review of this CICAD were:
Dr S. Cohen, University of Nebraska Medical Center, Omaha, USA
Ms M.E. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Canada
Mr D. Renshaw, Department of Health, London, United Kingdom
Dr J. Rice, Unit of Carcinogen Identification and Evaluation,
International Agency for Research on Cancer, Lyon, France
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan
Dr D. Singh, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Individuals participating in the review of the human health
assessment (section 11.1) were:
Dr A. Chiu, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr C. Hiremath, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr R.G. Liteplo, Environmental Health Directorate, Health Canada,
Ottawa, Canada
Dr D. Singh, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
APPENDIX 5 -- CICAD FINAL REVIEW BOARD (Washington, DC)
Washington, DC (USA), 8-11 December 1998
Members
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
Mr R. Cary, Toxicology Unit, Health Directorate, Health and Safety
Executive, Bootle, Merseyside, United Kingdom
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr O. Faroon, Agency for Toxic Substances and Disease Registry,
Centers for Disease Control and Prevention, Atlanta, GA, USA
Dr G. Foureman, National Center for Environmental Assessment, US
Environmental Protection Agency, Research Triangle Park, NC, USA
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany
Dr A. Nishikawa, Division of Pathology, National Institute of Health
Sciences, Tokyo, Japan
Dr E.V. Ohanian, Office of Water/Office of Science and Technology, US
Environmental Protection Agency, Washington, DC, USA
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan
Professor P. Yao, Institute of Occupational Medicine, Chinese Academy
of Preventive Medicine, Beijing, People's Republic of China
Observers
Dr K. Austin, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr I. Daly (ICCA representative), Regulatory and Technical Associates,
Lebanon, PA, USA
Ms K.L. Lang (CEFIC representative), Shell International, London,
United Kingdom
Ms K. Roberts (ICCA representative), Chemical Self-funded Technical
Advocacy and Research (CHEMSTAR),
Chemical Manufacturers Association, Arlington, VA, USA
Dr W. Snellings (ICCA representative), Union Carbide Corporation,
Danbury, CN, USA
Dr M. Sweeney, Document Development Branch, National Institute for
Occupational Safety and Health, Cincinnati, OH, USA
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Secretariat
Dr A. Aitio,1 International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
1 Invited but unable to attend.
Dr M. Baril, Institut de recherche en santé et en sécurité du travail
du Québec, Montreal, Quebec, Canada
Dr H. Galal-Gorchev, Chevy Chase, MD, USA
Ms M. Godden, Health and Safety Executive, Bootle, Merseyside, United
Kingdom
Dr R.G. Liteplo, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Ms L. Regis, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Mr A. Strawson, Health and Safety Executive, London, United Kingdom
Dr P. Toft, Associate Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
Ce CICAD relatif au biphényle a été préparé par l'Institut
Fraunhofer de Toxicologie et de Recherche sur les Aérosols de Hanovre
(Allemagne), principalement sur la base d'une étude préparée par le
Comité consultatif allemand sur les substances chimiques importantes
pour l'environnement (BUA, 1990) et d'un rapport complémentaire (BUA,
1994) afin d'évaluer les effets potentiels du biphényle sur l'Homme et
l'environnement. On trouvera à l'appendice 1 des indications sur les
sources documentaires utilisées ainsi que sur leur mode de
dépouillement. Une étude bibliographique approfondie a été menée sur
plusieurs bases de données jusqu'en juin 1996 à la recherche de
données supplémentaires. Ce CICAD contient également les
renseignements complémentaires recueillis par les points de contact et
le Comité d'évaluation finale (Bruxelles, Belgique) lors du
dépouillement des documents. Les renseignements concernant l'examen du
CICAD par les pairs font l'objet de l'appendice 2. Ce CICAD a été
approuvé en tant qu'évaluation internationale lors d'une réunion du
Comité d'évaluation finale qui s'est tenue à Bruxelles (Belgique) du
18 au 20 novembre 1996. La liste des participants à cette réunion
figure à l'appendice 3. Après avoir été révisé pour y incorporer les
résultats d'une étude de cancérogénicité de 2 ans récemment achevée,
le document a été soumis à des pairs pour deux séries d'évaluations
écrites. En outre, la partie du document spécialement consacrée à
l'évaluation des effets sur la santé humaine (à savoir la section
11.1), a été examinée lors d'une réunion qui s'est tenue le 7 décembre
1998 à Washington (Etats-Unis), dans les locaux du National Center for
Environmental Assessment, US Environmental Protection Agency. La liste
des personnes qui ont participé aux évaluations spécialisées du
présent CICAD figure à l'appendice 4. Ce CICAD a été approuvé en tant
qu'évaluation internationale lors de la réunion du Comité d'évaluation
finale qui a eu lieu à Washington du 8 au 11 décembre 1998. La liste
des participants à cette réunion figure à l'appendice 5. La fiche
d'information internationale sur la sécurité chimique (ICSC 0106)
établie par le Programme international sur la sécurité chimique (IPCS,
1993) est également reproduite dans ce document.
Le biphényle (CAS N° 92-52-4) est un hydro carbure aromatique qui
se présente à la température ambiante sous la forme d'un solide
cristallin incolore. Il est utilisé comme intermédiaire dans la
production de divers composés (par exemple, émulsifiants, azurants
optiques, produits pour la protection des cultures, matières
plastiques), comme fluide caloporteur, comme support de colorants pour
textiles et papier à photocopie, comme solvant dans l'industrie
pharmaceutique ou encore pour la conservation des agrumes.
Le biphényle existe à l'état naturel dans le goudron de houille,
le pétrole brut et le gaz naturel. Parmi les sources artificielles de
pollution par le biphényle figurent les unités qui produisent ou
utilisent ce composé, les installations où l'on traite des agrumes ou
des bois ou encore les décharges municipales. Il se forme lors de la
combustion incomplète des huiles minérales et de la houille et on en
trouve dans les gaz d'échappement des véhicules à moteur et dans l'air
libéré dans l'atmosphère par les appareils de chauffage des
installations industrielles ou des immeubles résidentiels. Des valeurs
de 1 à 100 ng/m3 sont caractéristiques de la concentration dans l'air
ambiant. A l'intérieur des bâtiments, elles sont plus élevées
(100-1000 ng/m3), probablement par suite de la présence de fumée de
cigarette, d'émissions provenant des appareils de chauffage et de
garages situés dans le voisinage. Des mesures effectuées dans les
années 70 ont donné des valeurs généralement inférieures à 5 ng/litre
pour l'eau du robinet. On n'a pas connaissance de valeurs plus
récentes. Dans les eaux superficielles, la concentration usuelle est
inférieure à 500 ng/litre. Dans les sédiments, le sol et les êtres
vivants, on n'a procédé à des dosages de biphényle qu'à proximité
immédiate d'installations industrielles et de décharges.
Peu soluble dans l'eau, le biphényle s'évapore de ses solutions
aqueuses. Sa principale voie de décomposition troposphérique est due à
l'action des radicaux hydroxyles et la demi-vie calculée dans ces
conditions est de l'ordre de 2 jours en moyenne. Le biphényle ne
devrait pas s'hydrolyser dans les conditions qui règnent dans
l'environnement. Il est biodégradable en aérobiose. D'après les
données disponibles, il devrait être quasiment immobile dans le sol;
sa probabilité d'infiltration dans les eaux souterraines est faible.
Dans la chaîne alimentaire qui joue un rôle important pour
l'alimentation humaine, il peut y avoir accumulation du biphényle,
notamment dans les plantes. Toutefois, compte tenu de son potentiel de
bioaccumulation, il ne semble pas qu'il puisse subir une
bioamplification importante aux niveaux trophiques supérieurs de la
chaîne alimentaire aquatique ou terrestre.
Le biphényle est bien absorbé dans les voies digestives et
probablement aussi par la voie pulmonaire et percutanée. Chez les
espèces étudiées, les métabolites du biphényle, principalement le
4-hydroxybiphényle, sont rapidement excrétés et presque exclusivement
par la voie urinaire. Le biphényle est modérément toxique par voie
buccale. Il n'est pas irritant pour la peau et n'irrite que très
légèrement la muqueuse oculaire. Rien n'indique qu'il puisse provoquer
une sensibilisation cutanée. Les études toxicologiques au cours
desquels on a soumis les animaux de laboratoire à des expositions
répétées au biphényle, ne permettent pas d'établir la valeur de la
dose sans effet observable (NOEL) avec un bon niveau de confiance. Une
exposition subchronique par la voie respiratoire a provoqué
l'apparition d'anomalies bronchopulmonaires, mais la littérature ne
mentionne pas d'études toxicologiques de longue durée comportant une
exposition par cette voie.
Les études toxicologiques comportant l'administration, pendant
diverses périodes, de nourriture additionnée de biphényle à des
rongeurs, font souvent mention d'effets aux niveau des voies
urinaires. Chez des rats mâles ayant reçu une alimentation contenant
plus de 2500 mg de biphényle par kg, on a observé une augmentation
sensible dans l'incidence des effets morphologiques (par ex. formation
de calculs) et histopathologiques (par ex. hyperplasie, desquamation)
au niveau des voies urinaires. On a également observé une augmentation
des calculs et des cas de métaplasie spino-cellulaire vésicale chez
des rattes, mais dans une proportion moindre que chez les mâles. Chez
des souris mâles soumises pendant 32 semaines à un régime alimentaire
comportant 10 000 mg de biphényle par kg (1500 mg par kg de poids
corporel), on a observé une simple hyperplasie et une dysplasie
papillomateuse ou nodulaire de la vessie, mais chez seulement un
animal sur dix. Sur des animaux à qui on avait fait ingérer du
biphényle, on a également observé des effets hématologiques
(biochimiques et autres); ces effets peuvent être constatés chez des
rats ou des souris mâles et femelles à des doses inférieures à celles
qui produisent des effets sur la vessie des rats mâles. En ce qui
concerne les effets non néoplasiques, la dose la plus faible
produisant un effet observable (LOEL) se situait à 38 mg/kg p.c. par
jour, le critère retenu étant l'apparition d'anomalies hématologiques
(diminution du taux d'hémoglobine et de l'hématocrite) chez des rats
ayant reçu pendant 2 ans une nourriture contenant respectivement 0,
500, 1500 ou 4500 mg/kg de biphényle (les doses indiquées étant égales
à 0, 38, 113 ou 338 mg/kg p.c.).
Les études in vitro sur des bactéries n'ont pas mis en évidence
d'activité mutagène. On a observé des mutations et des recombinaisons
mitotiques chez Saccharomyces cerevisiae D7 avec ou sans activation
métabolique. Les tests de génotoxicité sur cellules mammaliennes ont
toutefois donné des résultats positifs en présence d'activation
métabolique et des résultats négatifs en l'absence d'une telle
activation. Une étude in vivo insuffisamment documentée et dont
l'exécution laisse à désirer, n'a pas permis de constater la présence
d'aberrations chromosomiques dans la moelle osseuse de rats exposés à
du biphényle par la voie respiratoire. Les résultats d'autres études
in vitro indiquent que le biphényle possède un pouvoir mutagène;
dans ces conditions et devant le manque de fiabilité des résultats
fournis par les études in vivo, on estime que le biphényle comporte
un risque mutagène.
Chez des souris femelles, on a constaté une légère augmentation
de l'incidence des tumeurs bénignes ou malignes du foie lorsque les
animaux avaient reçu pendant 2 ans du biphényle mêlé à leur
nourriture. Une augmentation de l'incidence des tumeurs vésicales a
été observée chez des rats mâles, mais ni chez les femelles ni chez
des souris des deux sexes, après administration d'une nourriture
contenant une grande quantité de biphényle. On a avancé que ces
tumeurs pouvaient être dues à l'hyperplasie régénérative de
l'épithélium vésical provoquée par l'abrasion et les lésions que
produisent les calculs, lesquels ne se forment que si l'exposition au
biphényle est suffisamment intense. On pense également qu'en raison de
différences anatomiques et physiologiques, ces tumeurs vésicales
observées à forte doses chez des rats mâles et qui présentent une
spécificité de sexe et d'espèce, n'ont guère de signification pour des
sujets humains exposés à des doses plus faibles. Quoi qu'il en soit,
1) l'observation d'un accroissement de l'incidence des effets
histopathologiques et la formation de calculs au niveau de la vessie,
en l'absence de tumeurs vésicales , chez des rattes ayant reçu du
biphényle pendant 2 ans, 2) l'absence de données permettant de mettre
en évidence une association directe entre cette lithiase vésicale,
l'hyperplasie régénérative de l'urothélium et l'apparition de tumeurs
vésicales chez les rats mâles et 3) la génotoxicité potentielle du
biphényle, incitent à penser que la formation de tumeurs vésicales
chez les rats mâles n'est peut-être pas entièrement due à l'action des
calculs. Cette observation, de même que les signes d'hépatotoxicité
relevés chez les souris femelles, font craindre que le biphényle ne
comporte un risque de cancérogénicité.
Les données relatives à la toxicité génésique du biphényle sont
limitées. A part une étude portant sur trois générations de rats, qui
a mis en évidence des effets indésirables (réduction de la fécondité,
de la taille des portées et du taux de croissance), rien ne prouve que
le biphényle puisse avoir des effets sur la reproduction ou le
développement.
L'exposition, sur les lieux de travail, à des vapeurs ou à des
poussières à forte teneur en biphényle entraîne une irritation des
yeux et une inflammation des voies respiratoires. Une exposition
pendant plusieurs années à de fortes concentrations de biphényle
(jusqu'à 128 mg/m3) a provoqué des lésions hépatiques et les
anomalies neuronales permanentes; il est possible que dans ce cas,
l'effet du contact cutané avec le composé se soit ajouté à celui de
son absorption par la voie respiratoire.
On ne possède qu'un nombre limité de données sur la concentration
du biphényle dans l'environnement et les renseignements qu'on peut en
tirer sont insuffisants pour permettre une estimation raisonnable de
la dose de composé absorbée. Il peut y avoir exposition par inhalation
(inhalation d'air pollué et utilisation de créosote contenant du
biphényle pour la préservation du bois) ou par ingestion (lorsque le
biphényle est utilisé comme conservateur d'agrumes).
Le biphényle est faiblement bactéricide et fongistatique. Lors
d'études toxicologiques sur des organismes aquatiques représentant
quatre niveaux trophiques, la concentration la plus faible sans effet
observable (NOEC) pour l'espèce la plus sensible (Daphnia magna) a
été trouvée égale 0,17 mg/litre lors de tests de longue durée. Le
calcul donne une concentration sans effet prévisible (PNEC) de 1,7
µg/litre en utilisant un facteur d'évaluation de 10, compte tenu des
caractéristiques écotoxicologiques et du devenir environnemental du
composé. On ne dispose pas, au sujet des effets toxiques du biphényle
sur les organismes terrestres et les écosystèmes, de données valables
permettant une évaluation du risque.
En raison de l'absence de données émanant d'autres régions du
monde, l'évaluation du risque qui a été effectuée concerne
spécifiquement l'Allemagne. A partir des concentrations mesurées dans
les cours d'eau allemands au cours des années 90, on a obtenu un
rapport de la concentration environnementale prévue (PEC) à la
concentration sans effet prévisible (PNEC) qui est égal à <0,29. Un
rapport PEC/PNEC <1 signifie qu'il n'y a vraisemblablement pas de
risque important pour l'environnement.
RESUMEN DE ORIENTACION
El presente CICAD, preparado por el Instituto Fraunhofer de
Toxicología y de Investigación sobre los Aerosoles de Hannover,
Alemania, se basa fundamentalmente en un examen preparado por el
Comité Consultivo Alemán sobre las Sustancias Químicas Importantes
para el Medio Ambiente (BUA, 1990), así como en un informe
complementario (BUA, 1994) para evaluar los efectos potenciales del
bifenilo en el ser humano y en el medio ambiente. En el apéndice 1
figuran los documentos originales y una descripción de sus procesos de
examen. Se realizó asimismo una búsqueda bibliografía amplia en varias
bases de datos en línea en junio de 1996 para identificar datos
adicionales. También se ha incorporado a este CICAD nueva información
adicional obtenida durante el examen efectuado por los servicios de
información y por la Junta de Evaluación Final (Bruselas, Bélgica). En
el apéndice 2 se presenta información relativa al examen colegiado de
este CICAD. La Junta de Evaluación Final lo aprobó provisionalmente
como evaluación internacional en una reunión celebrada en Bruselas
(Bélgica) los días 18-20 de noviembre de 1996. En el apéndice 3 figura
la lista de participantes en dicha reunión. Tras la incorporación de
los datos relativos a la carcinogenicidad de esta sustancia obtenidos
de una biovaloración de la carcinogenicidad de dos años de duración
completada recientemente, el documento revisado se sometió a dos
rondas de examen colegiado por escrito. Además, la parte del documento
en la que se aborda específicamente la evaluación de la salud humana
(es decir, la sección 11.1) se examinó en una reunión celebrada en el
Centro Nacional para la Evaluación del Medio Ambiente, Organismo para
la Protección del Medio Ambiente de los Estados Unidos, en Washington,
DC (Estados Unidos) el 7 de diciembre de 1998. El apéndice 4 contiene
la lista de quienes contribuyeron a los exámenes especializados
adicionales del presente CICAD. Este CICAD se aprobó como evaluación
internacional en una reunión de la Junta de Evaluación Final celebrada
en Washington, DC (Estados Unidos), los días 8-11 de diciembre de
1998. En el apéndice 5 figura la lista de participantes en esta
reunión. La Ficha internacional de seguridad química (ICSC 0106),
preparada por el Programa Internacional de Seguridad de las Sustancias
Químicas (IPCS, 1993), también se reproduce en el presente documento.
El bifenilo (CAS N° 92-52-4), un hidrocarburo aromático, es una
sustancia sólida incolora a temperatura ambiente. Se utiliza como
intermediario en la producción de diversos compuestos (por ejemplo,
emulsionantes, abrillantadores ópticos, productos para la protección
de los cultivos, plásticos), como medio de transferencia de calor en
fluidos térmicos, como colorante de textiles y papel para copias, como
disolvente en la producción farmacéutica y en la conservación de
cítricos.
El bifenilo se encuentra en la naturaleza en el alquitrán
mineral, el petróleo bruto y el gas natural. Las fuentes
antropogénicas de exposición en el medio ambiente incluyen las plantas
de producción y elaboración, las instalaciones de conservación de
cítricos o de madera y los vertederos municipales. Se forma bifenilo
durante la combustión incompleta del aceite mineral y el carbón y está
presente en los gases de escape de los vehículos y de las calderas de
calefacción de las viviendas y las industrias. En la atmósfera, las
concentraciones habituales de bifenilo oscilan entre 1 y 100 ng/m3;
en el aire de espacios cerrados los niveles son superiores (100-1000
ng/m3), probablemente debido al humo de los cigarrillos y a las
emisiones de las calderas de calefacción o de garajes cercanos. En las
mediciones realizadas en los años setenta, las concentraciones de
bifenilo en el agua de grifo eran normalmente inferiores a 5 ng/litro.
No se conocen datos más recientes. En el agua superficial, las
concentraciones suelen ser inferiores a 500 ng/litro. En los
sedimentos, el suelo y la biota, sólo se midió el bifenilo en las
cercanías de instalaciones industriales y vertederos.
El bifenilo se volatiliza de la solución acuosa y tiene una
solubilidad baja en agua. La principal vía de degradación en la
troposfera es la reacción con radicales hidroxilo, para la que se ha
calculado una semivida de unos dos días. No cabe prever que la
sustancia se hidrolice en las condiciones del medio ambiente. Es
biodegradable en condiciones aerobias. Según los datos disponibles, el
bifenilo debería mantenerse casi inmóvil en el suelo; la probabilidad
de infiltración hacia el agua freática es baja. En la cadena
alimentaria importante para el ser humano se puede producir
bioacumulación, especialmente en las plantas; sin embargo, teniendo en
cuenta la posible bioacumulación del bifenilo, cabe prever que su
bioamplificación en los niveles tróficos superiores de la cadena
alimentaria acuática o terrestre será de escasa importancia.
El bifenilo se absorbe bien a través del tracto gastrointestinal
y posiblemente también por vía pulmonar y cutánea. En las especies
examinadas, los metabolitos del bifenilo, sobre todo el
4-hidroxibifenilo, se excretan con rapidez y casi exclusivamente en la
orina. La toxicidad aguda del bifenilo por vía oral es moderada. No es
irritante de la piel y sólo ligeramente de los ojos. No hay pruebas de
sensibilización cutánea. Los estudios de toxicidad con bifenilo tras
la exposición repetida por inhalación no son adecuados para establecer
con confianza una concentración sin efectos observados (NOEL). La
exposición subcrónica por inhalación produjo cambios broncopulmonares,
pero no se han encontrado en la bibliografía estudios de toxicidad
prolongados tras la exposición por inhalación.
En estudios toxicológicos realizados con roedores que recibieron
alimentos con bifenilo durante diversos períodos de tiempo, se han
notificado con frecuencia efectos en el sistema urinario. Se ha
observado un aumento acentuado en la incidencia de efectos
morfológicos (es decir, formación de cálculos) e histopatológicos (por
ejemplo, hiperplasia, descamación) en el tracto urinario de ratas
macho a las que se suministraron alimentos con concentraciones de
bifenilo superiores a 2500 mg/kg. Se observó asimismo un aumento en la
aparición de cálculos y metaplasia escamosa en la vejiga urinaria de
ratas hembras, pero con una incidencia inferior a la registrada en los
machos. En ratones macho, sólo uno de 10 animales que recibieron
alimentos con 10 000 mg de bifenilo/kg (1500 mg/kg de peso corporal al
día) durante 32 semanas presentó hiperplasia simple y displasia
papilar o nodular en la vejiga urinaria. En animales que recibieron
bifenilo por vía oral se detectaron asimismo efectos en la química
sanguínea y los parámetros hematológicos; estos efectos se producen en
ratas y ratones machos y hembras con ingestas inferiores a las
asociadas con la aparición de efectos en la vejiga urinaria de las
ratas machos tratadas con bifenilo. Para los efectos no neoplásicos,
la concentración más baja de efectos observados (LOEL) fue de 38 mg/kg
de peso corporal al día, basada en la aparición de alteraciones en los
parámetros hematológicos (es decir, disminución de la concentración de
hemoglobina y del valor hematócrito) en ratas a las que se
suministraron alimentos que contenían 0, 500, 1500 ó 4500 mg de
bifenilo/kg (ingestas notificadas de 0, 38, 113 ó 338 mg/kg de peso
corporal al día) durante dos años.
En estudios in vitro con bacterias no se han obtenido pruebas
de potencial mutagénico del bifenilo; con Saccharomyces cerevisiae
D7, se observaron mutaciones genéticas y recombinación mitótica con
activación metabólica y sin ella. Sin embargo, en pruebas de
toxicología genética en células de mamíferos se obtuvieron resultados
positivos con activación metabólica y negativos en su ausencia. En un
estudio in vivo insuficientemente documentado y realizado de manera
inadecuada, el bifenilo no indujo aberraciones cromosómicas en la
médula ósea de ratas tras la exposición por inhalación. Los resultados
de los estudios in vitro ponen de manifiesto que el bifenilo tiene
potencial mutagénico; sin la garantía de resultados fidedignos de
pruebas in vivo, se supone que la exposición al bifenillo se puede
asociar con un riesgo mutagénico.
En ratones hembra, se produjo un ligero aumento de la incidencia
de tumores hepáticos benignos y malignos en animales que recibieron
bifenilo con los alimentos durante dos años. Se observó un aumento de
la incidencia de tumores de vejiga en ratas machos, pero no en las
ratas hembras o los ratones machos y hembras, que recibieron alimentos
con concentraciones elevadas de bifenilo. Se ha indicado que la
formación de dichos tumores de vejiga podría estar vinculada a la
hiperplasia regenerativa del epitelio urinario, debida a la abrasión y
las lesiones del urotelio producidas por los cálculos formados en el
tracto urinario sólo con niveles muy altos de exposición; se ha
señalado asimismo que, debido a las diferencias anatómicas y
fisiológicas, la aparición de tumores de vejiga específicos del sexo y
la especie en ratas macho que recibieron dosis elevadas de bifenilo
tal vez no sea pertinente para las personas expuestas a niveles más
bajos. Sin embargo, 1) las observaciones de una mayor incidencia de
efectos histopatológicos y la formación de cálculos en la vejiga
urinaria, en ausencia de tumores de vejiga, en ratas hembras tratadas
con bifenilo durante dos años, 2) la falta de datos que establezcan
una asociación directa entre la formación de cálculos, la hiperplasia
regenerativa del urotelio y la aparición de tumores de vejiga en
determinados animales machos, y 3) la genotoxicidad del bifenilo,
podrían indicar que la aparición de tumores de vejiga en ratas macho
tal vez no se deba completamente a los efectos asociados a la
formación de cálculos en la vejiga urinaria. Esta observación, así
como la prueba de hepatocarcinogenicidad en ratones hembras, despierta
alguna preocupación con respecto a la carcinogenicidad potencial del
bifenilo.
Los datos disponibles sobre la toxicidad reproductiva del
bifenilo son limitados. Si se exceptúan los resultados obtenidos en un
estudio de tres generaciones en ratas, en el que se observaron efectos
adversos (disminución de la fecundidad, del tamaño de la camada y de
la tasa de crecimiento), no se encontraron pruebas de que el bifenilo
indujera efectos reproductivos o en el desarrollo.
La exposición a concentraciones elevadas de vapores o polvo de
bifenilo en el lugar de trabajo produce irritación de los ojos e
inflamación del tracto respiratorio. La exposición prolongada durante
varios años a concentraciones elevadas de bifenilo (hasta 128 mg/m3)
provo có lesiones hepáticas y cambios neuronales persistentes; el
contacto directo con la piel puede haber influido, además de la
absorción a través del tracto respiratorio.
Los datos disponibles sobre las concentraciones de bifenilo en el
medio ambiente general son limitados y la información es insuficiente
para permitir una estimación razonable de su ingesta. La inhalación
(del aire contaminado y por el uso de creosotas con bifenilos en la
conservación de la madera) y la ingestión (debido a su uso como
conservante de cítricos) pueden ser fuentes de exposición.
El bifenilo tiene propiedades bactericidas y fungicidas débiles.
En estudios de toxicidad sobre organismos acuáticos de cuatro niveles
tróficos, la concentración más baja sin efectos observados (NOEC)
notificada para la especie más sensible (Daphnia magna) en pruebas
crónicas fue de 0,17 mg/litro. Se puede calcular una concentración sin
efectos prevista (PNEC) de 1,7 µg/litro mediante la aplicación de un
factor de evaluación de 10, teniendo en cuenta las características de
ecotoxicidad y destino en el medio ambiente de la sustancia. No se
dispone de datos válidos sobre efectos tóxicos en organismos y
ecosistemas terrestres, que se podrían utilizar para la evaluación del
riesgo.
Debido a la falta de datos de otras partes del mundo, se realizó
en Alemania una evaluación del riesgo de muestra. A partir de las
concentraciones medias en los ríos alemanes en los años noventa, la
relación entre la concentración prevista en el medio ambiente (PEC) y
la PNEC se calcula que es <0,29. Una razón PEC/PNEC <1 indica que
no cabe prever un riesgo importante para el medio ambiente.
The chemical is an aromatic hydrocarbon with a peculiar, strong
odour similar to that of geraniums (BUA, 1990). At room temperature,
the substance is a colourless solid (melting point 62.2°C). Because of
its significant vapour pressure (4 Pa at 20°C) and low water
solubility (4.45 mg/litre at 20°C), biphenyl shows considerable
volatility from aqueous solutions (BUA, 1990). Its measured
n-octanol/water partition coefficient (log Kow) is between 3.88
and 4.04. The commercial product from the only German producer has a
biphenyl content of 99.85%; named impurities are terphenyl (0.15%),
sulfur (10-20 mg/kg), and benzene (1 mg/kg) (BUA, 1990). Additional
physical/chemical properties are presented in the International
Chemical Safety Card reproduced in this document.
3. ANALYTICAL METHODS
Biphenyl is usually measured in environmental media by capillary
gas chromatography in combination with flame ionization or mass
spectrometric detection (Malins et al., 1985; BUA, 1990; Hawthorne et
al., 1992; Anklam & Mueller, 1994; Karanassios et al., 1994; Otson et
al., 1994; Kostiainen, 1995). For aquatic samples, methods involving
high-performance liquid chromatography with ultraviolet detection at
254 nm have also been described. The following enrichment techniques
are used: solid-phase adsorption (Tenax material or XAD resins) with
thermal desorption or liquid extraction for air samples, solid-phase
adsorption (XAD resins) or liquid/liquid extraction (diethylether) for
water samples, and liquid extraction (dichloromethane/methanol,
ethanol) for soil and biotic samples. The detection limits range from
0.1 to 5 ng/m3 for air and from 0.01 to 8000 ng/litre for water. For
sediment, a detection limit of 2 µg/kg dry weight has been reported.
Detection limits between 12 and 87 µg/kg have been reported for levels
of biphenyl in fish (Malins et al., 1985).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Biphenyl occurs in varying concentrations in coal tar, crude oil
(up to 0.4 mg/g oil), and natural gas (3-42 µg/m3) (BUA, 1990).
Because of its natural occurrence in coal tar and crude oil, biphenyl
has also been detected in products derived from these substances. The
biphenyl content in coal tar-derived creosotes ranges between 0.2 and
1.6% (IARC, 1985; ATSDR, 1990; Collin & Hoeke, 1995). In the aromatic
fraction of an unused lubricating oil sample, biphenyl was measured at
a concentration of 1.5 mg/kg (Paschke et al., 1992).
Biphenyl is also produced from anthropogenic sources. In 1984,
the estimated production capacities of biphenyl in the Federal
Republic of Germany, in Western Europe, and throughout the world were
approximately >6000, 30 000, and 80 000 t, respectively. In 1989,
2000-2500 t of biphenyl were produced in Germany, of which about 900 t
were exported and 200-300 t sold domestically (BUA, 1990).
Approximately 5000 t of biphenyl were manufactured in each of 1992 and
1993 in Japan (Anon., 1994-95).
Until the early 1970s, biphenyl was used principally as an
intermediate in the production of polychlorinated biphenyls (PCBs). As
the production, processing, distribution, and use of PCB compounds are
now prohibited or restricted in many countries (e.g. USA, Germany,
United Kingdom) (IRPTC, 1994), it is likely that previous production
levels were much higher than those today. In 1984, the estimated use
patterns of biphenyl worldwide were, as a final product: 35% in heat
transfer medium in heating fluids, 20% as a dyestuff carrier for
textiles, 5% as a solvent in pharmaceutical production, 5% as a
dyestuff carrier for copying paper, and 5% as a preservative for
citrus fruits; patterns of biphenyl use as an intermediate were: 10%
each in emulsifiers and optical brighteners, 5% for crop protection
products, and 5% for precursors and auxiliaries for plastics (BUA,
1990). At present in Germany, the use of biphenyl as a dyestuff
carrier in textiles is minor.1
Although emissions from exhaust gases during production and
processing of biphenyl and during the use of biphenyl-containing
creosotes for wood preservation may be significant, available data
were not identified. Biphenyl is also released into the atmosphere
during the incomplete combustion of fossil fuels in motor vehicles
(post-catalyst emission factor of 3.5 µg/km; Siegl & Chladek, 1992),
residential heating (1.24 mg biphenyl/kg burnt coal; Engewald et al.,
1993), and coal-burning power plants (with concentrations in flue gas
up to 30 µg/m3; Yao & Xu, 1991; Wienecke et al., 1992) or foundries
(qualitatively detected; Deusen & Kleinermanns, 1993).
1 Personal communication from Verband d. Textilhilfsmittel-,
Lederhilfsmittel-, Gerbstoff- und Waschrohstoff-Industrie e.V.,
Frankfurt/M., Germany, to Bayer AG, 1996.
In 1990, biphenyl was not detected in the effluent of the sewage
plant of the German producer (detection limit 5 µg/litre; weekly
sampling). Based on a biphenyl concentration of <5 µg/litre, a
maximum annual release of 274 kg biphenyl can be calculated for this
plant. No data are available from other producing or processing
facilities. Biphenyl may also be released to surface water through the
processing of coal tar (e.g. coking plants) and mineral oils (e.g.
processing residual oils from pyrolysis in refineries) (BUA, 1990).1
Based upon levels of biphenyl measured in sediment samples during the
1970s, this chemical has been released from various industrial
wastewater outlets associated with a melting plant for iron alloys, a
chemical factory, and an oil storage depot. Data were not available on
releases of biphenyl from textile industries where the chemical is
used as a dyestuff carrier, from wood preservation plants that use
creosotes containing biphenyl, or from treated citrus fruits and their
packaging materials contained in domestic waste sites. Concentrations
of biphenyl between 0.016 µg/kg and 1730 mg/kg have been measured in
sewage sludge, which may be applied to arable land (BUA, 1990).
1 Personal communication from Bayer AG to the GDCh- Advisory
Committee on Existing Chemicals of Environmental Relevance, 1997.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Based upon a Level II fugacity model (Mackay et al., 1992), the
atmosphere appears to be the main target compartment (>90%) for
biphenyl. Biphenyl's Henry's law constant indicates that it
volatilizes from aqueous solutions. The calculated half-life for the
photooxidative degradation of biphenyl by hydroxyl radicals in air is
about 2 days (Atkinson & Aschmann, 1985; BUA, 1990; Atkinson & Arey,
1994). Reactions with tropospheric ozone and nitrate are expected to
be of minor importance, with calculated half-lives of >80 days and
>105 days, respectively. Data on the photodegradation of biphenyl in
water are not available. Biphenyl is not expected to hydrolyse under
environmental conditions.
In one reported standard test with activated sludge, according to
Guideline 301C of the Organisation for Economic Co-operation and
Development (OECD), a biochemical oxygen demand of 66% after 14 days'
incubation was reported when non-adapted inoculum was used (CITI,
1992). Microbial populations from natural waters mineralize biphenyl;
100% degradation was observed after 4 days' incubation of biphenyl in
river water (BUA, 1990). Numerous microorganisms isolated from soil,
sediment, surface water, and groundwater samples have been shown to
metabolize biphenyl (BUA, 1990; Bevinakatti & Ninnekar, 1992; Kiyohara
et al., 1992; Nielsen & Christensen, 1994). Investigations on the
anaerobic biodegradation of biphenyl were not identified.
Soil sorption coefficients ( Koc) based upon laboratory
experiments and calculated values range from 1100 to 18 000 (BUA,
1990; Kishi & Hasimoto, 1991); from numerous literature data, Jeng et
al. (1992) calculated a mean value of 4230. On the basis of these
data, it is expected that biphenyl will be almost immobile in soil,
with a low probability of groundwater infiltration. Volatilization
from soil surfaces is significantly lower than that from aqueous
media; however, no significant geoaccumulation of biphenyl is expected
under aerobic conditions owing to its degradation by microbial
organisms.
In static tests on the bioaccumulation of biphenyl in activated
sludge conducted with several aquatic species (yeast, algae, molluscs,
daphnia, and freshwater fish), bioconcentration factors (BCFs) ranging
between 57 for the marine mussel Mytilus edulis (calculated on wet
weight) (Donkin et al., 1991) and 540 for the green alga Chlorella
vulgaris (based on dry weight) (Kotzias et al., 1980; Freitag et
al., 1982) have been reported. With the exception of two tests, there
was no information on whether equilibrium was reached before the
determination of the BCF. The depuration of accumulated biphenyl was
determined in one study. After a 24-h incubation in a static test
system, the BCF at equilibrium (based on wet weight) for Daphnia
magna was 473; depuration was dependent upon the temperature of the
water. Twenty-four hours after being transferred to pure water, 53% of
the accumulated biphenyl was depurated at 2-3°C, whereas 88% was
depurated at 22°C (Zhang et al., 1983). Although the available data
indicate a potential for bioaccumulation, evaporation, adsorption to
soil/sediment, and degradation are expected to reduce the
bioavailability of biphenyl. Therefore, bioaccumulation of the
chemical should be of minor importance for aquatic organisms.
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
In 1985, concentrations of biphenyl in the air of an
industrialized city in Finland ranged from 1.7 to 26.2 ng/m3 (BUA,
1990). Similar concentrations were measured during the winter of
1988-89 in two US cities (12-119 ng/m3; 30 ng/m3 mean value;
Hawthorne et al., 1992) and in 1992 in two Greek cities (all data
below the detection limit of 5 ng/m3; Karanassios et al., 1994).
The concentrations of biphenyl in the German parts of the Rhine
River declined from a maximum of 1000 ng/litre in the 1970s (BUA,
1990) to levels below 500 ng/litre (the detection limit) in 1993-1995
(LWA, 1993-94).1 Although levels in most of the German tributaries
have remained below the detection limit (500 ng/litre), peak
concentrations of 560 and 1600 ng/litre were reported in 1993 and
1994, respectively, for the highly polluted Emscher tributary (LWA,
1993-94); these elevated levels were attributed to the coking plants
in this area.2 In the USA, similar data for one river have been
reported (Hites, 1973). In some German estuaries, biphenyl
concentrations were significantly lower (1-5 ng/litre) (BUA, 1990).
During 1986-1989, biphenyl was detected at concentrations between
10 and 100 ng/litre in samples of groundwater obtained near Zagreb, in
the former Yugoslavia, in the vicinity of a municipal landfill site.
In a test well sunk 720 m from a heavily polluted river, the levels
were about 10-fold lower (Ahel, 1991). Concentrations of biphenyl
between 2 and 17 ng/litre have been measured in samples of snow and
rain collected in Switzerland (BUA, 1990).
In river and estuarine sediments, biphenyl was detected at very
high concentrations in the direct vicinity of industrial plants or
waste dumps; concentrations ranged between 0.1 and 8 mg/kg, depending
on the source. The highest concentrations were found near waste dump
sites in the USA (Malins et al., 1985; BUA, 1990). Information on the
occurrence of biphenyl in soils with no direct pollution was not
identified. A maximum biphenyl concentration of 13 µg/kg was found in
soil samples collected near a pit for wastewater from oil production
in New Mexico, USA (BUA, 1990).
Biphenyl has been found in fish collected from water contaminated
with mineral oil; concentrations in liver samples were <25 µg/kg dry
weight (i.e. below the detection limit) and 13 620 µg/kg fresh weight
(Paasivirta et al., 1982; Malins et al., 1985).
1 Personal communication from the Northrhine-Westfalian
Environmental Protection Agency to Bayer AG, 1996.
2 Personal communication from Bayer AG to the GDCh- Advisory
Committee on Existing Chemicals of Environmental Relevance, 1997.
6.2 Human exposure
The levels of biphenyl in indoor air may depend mainly on smoking
habits (BUA, 1990). Concentrations of biphenyl are also elevated in
homes having a source of oil and exhaust fumes nearby (e.g. gasoline
station, parking garage) (Otson et al., 1994; Kostiainen, 1995).
Monitoring data from Finnish ( n = 50) (Kostiainen, 1995) and
Canadian ( n = 757) homes (Otson et al., 1994) have revealed average
biphenyl concentrations in the range of 160-1000 ng/m3, with a
maximum concentration of 4700 ng/m3 (Kostiainen, 1995). Biphenyl has
been detected in floor dust collected from five of nine investigated
Danish city halls (Wilkins et al., 1993). Assuming that 20 h are spent
indoors (exposure to 160-1000 ng/m3) and 4 h are spent outdoors
(exposure to 30 ng/m3) each day and that the inhalation volume and
weight of the average adult are 22 m3/day and 64 kg, respectively,
the intake of biphenyl from air can be calculated from these data to
range from 45 to 300 ng/kg body weight per day.
There is only limited intake of biphenyl from drinking-water.
Although more recent data were not identified, concentrations of
biphenyl in tap-water in various countries, such as Finland, Norway,
Sweden, the USA, and Canada, were low (i.e. 0.1-5 ng/litre) in the
1970s (BUA, 1990). Based upon these data and assuming that the average
adult (weighing 64 kg) consumes 2 litres of drinking-water per day,
the estimated intake of biphenyl from drinking-water may range from
0.003 to 0.16 ng/kg body weight per day.
Food may contain biphenyl as a result of its use as a fungistatic
agent for citrus fruits. In older studies with citrus fruits to which
a biphenyl-containing wax had been applied, peels of treated fruits
were found to contain up to 220 mg biphenyl/kg (whole fruit) and the
pulp up to 3.9 mg/kg (whole fruit) (BUA, 1990); however, these levels
were determined with a rather unspecific spectrophotometric method,
which may have overestimated the biphenyl content. In a more reliable
study with 6 grapefruits, 8 oranges, and 10 lemons obtained from
Japanese markets, levels of biphenyl ranged from 17 to 123 mg/kg in
the peel and from <0.01 (the detection limit) to 0.18 mg/kg (average
0.06 mg/kg pulp) in the edible parts of the fruit. In samples of lemon
tea, levels of biphenyl were similar to those in the edible parts of
the fruit (Isshiki et al., 1982). In a survey of foodstuffs conducted
in Italy between 1988 and 1995, which included more than 70 types of
food, biphenyl was detected in only one peach sample (detection limit
not given) (Di Muccio et al., 1995). Based upon the average content of
biphenyl in the pulp of citrus fruits of about 0.06 mg/kg (calculated
from the concentrations measured by Isshiki et al., 1982), the intake
from the consumption of the pulp of one biphenyl-preserved citrus
fruit weighing about 120-400 g is calculated to be about 113-375 ng/kg
body weight. An average intake of 64 ng biphenyl/kg body weight per
day from food was calculated based upon the consumption of foodstuffs
in Finland (Penttilae & Siivinen, 1996). The general population may
also be exposed to biphenyl through contact with consumer products,
such as creosote-preserved wood, textiles, copying papers, or
pharmaceuticals, although quantitative data were not identified.
The intake of biphenyl from the occupational environment may
occur via inhalation and dermal contact. Concentrations of biphenyl in
the air of one facility producing biphenyl-impregnated paper were
reported to range from 4.4 to 128 mg/m3 in 1959 and from 0.6 to 123
mg/m3 in 1970 (Haekkinen et al., 1973). In a nylon production
facility in which workers were exposed to a eutectic mixture of 26.5%
(w/w) biphenyl and 73.5% (w/w) biphenyl ether, time-weighted average
concentrations of biphenyl were reported to range from 0.24 to 1.28
mg/m3 (Dorgelo et al., 1985). Coke oven emissions to indoor air at a
coking plant in Australia contained 0.2-0.5% biphenyl (Kirton et al.,
1991). In a Polish bituminous pulp production facility, reported
time-weighted average concentrations (1988-89) of biphenyl ranged from
0.5 to 0.6 µg/m3 (Baranski, 1991). In a study of two Canadian
pilot-scale coal liquefaction facilities, the levels of biphenyl were
below the detection limit of 60 µg/m3 (Leach et al., 1987).
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS
AND HUMANS
Studies providing quantitative information on the absorption or
distribution of biphenyl in humans were not identified. In various
species of experimental animals, the absorption of biphenyl following
oral exposure has been inferred by the detection of metabolites in the
urine and faeces. Biphenyl is oxidized by multifunctional oxygenases,
irrespective of the route of exposure. Mono-, di-, and trihydroxy
metabolites of biphenyl have been identified in the urine of exposed
species. 4-Hydroxybiphenyl has been observed as the major metabolite
in rats, rabbits, guinea-pigs, and pigs; minor metabolites include
mono-, di-, and trihydroxymethoxy biphenyls, dihydrodiols, and
hydroxydihydrodiols. The phenolic compounds are conjugated with
sulfate or glucuronic acid. The formation of mercapturic acid as well
as evidence of a metabolic pathway involving opening of the benzene
ring have also been described (BUA, 1990). There is no evidence for
enterohepatic circulation in different species (Meyer & Scheline,
1976; Meyer et al., 1976a; Meyer, 1977). On the basis of in vitro
studies, the metabolism of biphenyl is not restricted to the liver
(Bend et al., 1972; Hook et al., 1972; Matsubara et al., 1974; Prough
& Burke, 1975; Hook & Bend, 1976; Powis et al., 1987). Based upon
total cytochrome P-450 content, biphenyl-4-hydroxylase activity was
higher in pulmonary microsomes than in liver microsomes (Hook et al.,
1972; Hook & Bend, 1976). A time-dependent covalent binding of
[14C]bi phenyl to mouse hepatic microsomal proteins has been observed
after metabolic activation (Tanaka et al., 1993).
In rats, rabbits, and pigs, most biphenyl metabolites are
excreted in the urine (BUA, 1990). Following an oral dose of 100 mg
[14C]biphenyl/kg body weight, rats excreted 82% of the administered
radioactivity (76% in urine) within 24 h. The recovery rate after 4
days was 92%, with 7% of the administered radioactivity detected in
the faeces and traces in the exhaled air. Eight days after
administration, radioactivity in the tissues was 0.6% of the applied
dose (Meyer et al., 1976b). In none of the species examined was
unmetabolized biphenyl found in the urine (BUA, 1990).
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
The acute oral toxicity of biphenyl is moderate. The LD50 for
rats and mice is >1900 mg/kg body weight (BUA, 1990). Acute effects
include polyuria, accelerated breathing, lacrimation, anorexia, weight
loss, muscular weakness and coma, fatty liver cell degeneration,
severe nephrotic lesions (often manifested as acute and subacute
glomerulotubular nephritis), degenerative myocardial lesions,
pulmonary hyperaemia, and, occasionally, alveolar oedema. Interstitial
and lobular pneumonitis have been observed in animals that survived
for a few days.
The LC50 (4-h) for the mouse is >43 ppm (275 mg/m3). During
exposure, hyperactivity and mild respiratory discomfort have been
observed; however, these effects were not evident at the end of a
14-day recovery period. Gross pathological examination performed on
surviving animals revealed slight lung congestion (Sun Co. Inc.,
1977a). In an inhalation study conducted with Sprague-Dawley rats, no
effects were observed after a 6-h exposure to 0.8 or 3 ppm (5.1 or
19.2 mg/m3) biphenyl at temperatures of approximately 26°C or 32°C,
respectively (Monsanto Co., 1959). No treatment-related alterations in
appearance, demeanour, food consumption, or survival were observed
following a 7-h inhalation exposure of rats to 3 g biphenyl/m3
(nominal concentration); no detailed information on the methods or
particle size of the substance were presented (Dow Chemical Co.,
1974).
Information on the acute toxicity of biphenyl associated with
dermal exposure was not identified.
8.2 Irritation and sensitization
Biphenyl is non-irritating to both intact and scarified rabbit
skin. The substance was slightly irritating when applied to rabbits'
eyes in an eye irritation test (BUA, 1990).
In a guinea-pig maximization test conducted according to OECD
Guideline 406, there was reportedly no evidence of a skin sensitizing
potential for biphenyl (Dreist & Kolb, 1993).
8.3 Short-term exposure
8.3.1 Oral exposure
In the studies cited below, the histopathological examination was
focused on the urinary system. In a study in which male and female
Wistar rats were fed 0, 50, 150, 300, or 450 mg biphenyl/kg body
weight per day via the diet for 21 days, increased relative kidney
weights and polycystic renal changes (with increased urine volume and
specific gravity) were observed at doses of 50 and 150 mg/kg body
weight per day, respectively. An increase in urine volume, specific
gravity, and absolute kidney weight as well as polycystic renal
changes were also observed in rats administered 500 or 1000 mg
biphenyl/kg body weight per day in the diet for 14 days (Sœndergaard &
Blom, 1979).
When male albino rats were administered diets containing 0, 1000,
2500, 5000, or 10 000 mg biphenyl/kg (estimated intakes of 0, 75, 188,
375, or 750 mg/kg body weight per day) for 26 days, there was a
dose-dependent (at doses of >188 mg/kg body weight per day)
intensification of cloudiness in the urine, associated with an
increase in precipitate formation in samples, which had been cooled or
treated with sulfosalicylic acid. The precipitate consisted of free
4-hydroxybiphenyl and its glucuronide. These effects were largely
reversible during a 28-day recovery period (Booth et al., 1956, 1961).
8.3.2 Inhalation exposure
No evidence of histopathological changes in the lung, trachea,
liver, kidney, or spleen were observed in male and female mice exposed
by inhalation to 25 or 55 ppm (about 160 or 350 mg/m3) biphenyl for 7
h/day, 5 days/week, for 2 weeks (Sun Co. Inc., 1977b).
8.3.3 Dermal exposure
In rabbits, the repeated dermal application (20 in total) of
purified biphenyl (0.5 g/kg body weight) for 2 h/day, 5 days/week,
produced decreased body weight; one animal died after 8 applications.
A decrease in body weight was also observed after repeated dermal
application of technical biphenyl. In both studies, no effect upon the
skin was observed. Histopathological examination revealed minimal
changes in the heart, liver, kidneys, and, in some cases, spleen (no
further details provided) (Deichmann et al., 1947). No adverse effects
were observed when biphenyl was applied (8 h/day, 5 days/week, for a
total of 30 applications) to the intact and abraded skin of rabbits at
doses of 600 or 2000 mg/kg body weight per day (no further information
provided) (Newell, 1953).
8.4 Long-term exposure
8.4.1 Subchronic exposure
8.4.1.1 Oral exposure
In all of the studies cited below, the histopathological
examination was focused on the urinary system. A summary of the
effects related to this specific end-point (changes in kidneys,
ureter, and urinary bladder) for both subchronic and chronic exposures
is provided in Table 1.
Table 1: Summary of the effects of repeated biphenyl administration via diet on urine status, urinary bladder, and kidneys.
Species/strain/ Application/duration NO(A)EL
sex (mg/kg) Urine status Urinary bladder Kidneys Reference
rat/F344/male 0 or 5000 mg/kg <5000 crystals with simple hyperplasia not examined Shibata et al.
8 weeks microcalculi in and increased (1989a)
urinary sediment DNA synthesis
in the urothelium
rat/F344/male 0 or 5000 mg/kg <5000 microcalculi simple hyperplasia, increase in absolute/relative Shibata et al.
up to 24 weeks papillary or kidney weights; no (1989b)
nodular hyperplasia morphological changes of
of the urothelium the renal papillae or
after >16 weeks pelvis; no changes in DNA
synthesis in the renal
papillae and pelvis; focal
calcification of the renal
medulla
rat/albino/male 0, 1000, 2500, or 1000 >2500 mg/kg: not examined 2500 mg/kg: morphological Booth et al.
and female 5000 mg/kg increase in changes (tubular dilation) (1961)
up to 24 weeksa volume, turbidity, after >60 days
and precipitate 5000 mg/kg: morphological
after >30 days; changes (tubular dilation)
urinary sediment: after >30 days; effects
4-hydroxybiphenyl not completely reversible
and its
glucuronide;
effects reversible
rat/F344/male 0 or 5000 mg/kg <5000 increase in pH increased incidence not given Kurata et al.
32 weeks value and Na+ of calculi; (1986)
concentration; histopathology:
urinary sediment: no effects
4-hydroxybiphenyl
Table 1 (Cont'd.)
Species/strain/ Application/duration NO(A)EL
sex (mg/kg) Urine status Urinary bladder Kidneys Reference
rat/Wistar/male 0, 1250, or 1250 not given 5000 mg/kg: 5000 mg/kg: increased Shiraiwa et al.
5000 mg/kg increased incidence incidence of calculi (1989)
36 weeks of calculi in ureter and increase in
and bladder relative kidney weights
rat/Wistar/male 0, 2500, or <2500 haematuria at >2500 mg/kg: >2500 mg/kg: increased Takita (1983);
and female 5000 mg/kg >2500 mg/kg increased incidence incidence of calculi Shiraiwa et al.
75 weeks after >16 weeks of calculi (composed (composed of protein) (1989)
of magnesium after >16 weeks;
ammonium phosphate) kidneys with stones:
after >16 weeks in obstructive
ureter (females) pyelonephritis,
5000 mg/kg:increased tubular atrophy,
incidence of calculi fibrosis
in ureter and 5000 mg/kg: increase
bladder after >16 in relative kidney
weeks; bladder with weights in females
calculi: simple or
diffuse hyperplasia
and papillomatosis
of the urothelium
rat/Wistar/male 0, 630, or 1250 mg/kg 1250 not given no urolithiasis not given Takita (1983)
and female 104 weeks (no further
information given)
rat/F344/DuCrj/ 0, 500, 1500, or <500b >500 mg/kg: 4500 mg/kg: increase >1500 mg/kg: increase Japan Bioassay
male and female 4500 mg/kg decrease in in pH (males and in hyperplasia of Research Center
104 weeks protein (males) females) 4500 mg/kg: the renal pelvis in (1996)
>1500 mg/kg: increase in calculi females
decrease in and hyperplasia 4500 mg/kg: increase
protein (females) of the urothelium in calculi (males >
Table 1 (Cont'd.)
Species/strain/ Application/duration NO(A)EL
sex (mg/kg) Urine status Urinary bladder Kidneys Reference
(males >> females); females) and
increased tumour hyperplasia of the
incidence in males renal pelvis (males)
mouse/B6C3F1/ 0 or 10 000 mg/kg 10 000 decreased Na+ no changes in DNA not given Tamano et al.
male 8 weeks concentration synthesis of the (1993)
at week 4 urothelium
mouse/B6C3F1/ 0 or 10 000 mg/kg <10 000 small amounts of simple hyperplasia interstitial nephritis Tamano et al.
male 32 weeks greyish white and papillary or in 5/10 (1993)
and finely nodular dysplasia
granular material in 1/10 mice
mouse/Crj:BDF1/ 0, 667, 2000, or 667 6000 mg/kg: no changes >2000 mg/kg: mineralization Japan Bioassay
male and female 6000 mg/kg decrease in of the medulla in females Research Center
104 weeks protein and 6000 mg/kg: desquamation (1996)
ketone body and mineralization of the
pelvis in males
a Plus a post-treatment observation period of 9 weeks.
b Effects were observed at the lowest dose tested.
Effects on the urinary tract (i.e. an increased number of
microcalculi in urinary sediment, simple hyperplasia of the bladder
epithelium) were observed in male F344 rats administered 5000 mg
biphenyl/kg (estimated intake 252 mg/kg body weight per day) in the
diet for 8 weeks. Morphological changes and the increased synthesis of
DNA detected in the bladder epithelium after 4 weeks were attributed
to constant irritation by the microcalculi (Shibata et al., 1989a).
In male B6C3F1 mice, no changes in the urinary pH or in levels
of DNA synthesis in the urinary bladder were noted after
administration of 0 or 10 000 mg biphenyl/kg in the diet (0 or 1500
mg/kg body weight per day) over a period of 8 weeks. The urinary Na+
concentration was significantly lower in mice administered 1500 mg/kg
body weight per day compared with controls only at week 4 (Tamano et
al., 1993).
In a range-finding study in which male and female Wistar rats
were administered diets containing biphenyl at 0, 1250, 2500, 5000, 10
000, or 20 000 mg/kg (estimated intakes of 0, 94, 188, 375, 750, or
1500 mg/kg body weight per day) for 10 weeks, dose-dependent effects
(i.e. reduction in weight gain; increased serum activities of
aspartate transaminase, alanine transaminase, and lactate
dehydrogenase; and an increase in blood urea nitrogen) were observed
at all doses (Takita, 1983).
In male F344 rats (20 per group) administered 0 or 5000 mg
biphenyl/kg in the diet (0 or 375 mg/kg body weight per day) for 24
weeks (5 rats per group were sacrificed after 4, 8, 16, or 24 weeks),
reduced body weight gain and an increase in absolute/relative kidney
weights without differences in feed intake were observed in the
biphenyl-exposed rats. The analysis of the urine showed that
administration of biphenyl was associated with the formation of
microcalculi. The histopathological examination of the renal papillae
or pelvis performed after 4, 8, 16, and 24 weeks revealed no
morphological changes; the rate of DNA synthesis in the renal papillae
and pelvis determined after 4 weeks of treatment was unchanged. In
most of the treated animals, a focal calcification of the renal
medulla (no further information given) was noted. The examination of
the epithelium of the urinary bladder revealed a simple hyperplasia in
5/5 rats after 16 and 24 weeks and papillary or nodular hyperplasia in
3/5 rats at week 16 and in 5/5 rats at week 24; no data on controls
were presented (Shibata et al., 1989b).
In albino rats (42 animals of each sex per group) given 0, 1000,
2500, or 5000 mg biphenyl/kg in the diet (0, 75, 188, or 375 mg/kg
body weight per day) for up to 24 weeks, the urine analysis and
histopathological investigations of the kidneys during the study (days
30, 60, and 120) showed no apparent effects at a dose level of 75
mg/kg body weight (Booth et al., 1961). In some animals, early
indications of a kidney-damaging effect were observed at doses of 188
and 375 mg/kg body weight at 30 days -- polyuria, increasing
cloudiness of the urine, and initial morphological changes (tubular
dilation) in the kidney. These effects increased in frequency and
degree as the administrations progressed. The urinary sediment
consisted of 4-hydroxybiphenyl and its glucuronide. During a
subsequent 60-day observation period, the urinary status normalized;
however, changes in kidneys were not completely reversible (a few
dilated tubules and scars remained) (Booth et al., 1961).
In male F344 rats (25 per group) administered 0 or 5000 mg
biphenyl/kg in the diet (0 or 375 mg/kg body weight per day) for 32
weeks, a reduction in body weight gain but no clinical signs of
poisoning were observed in dosed animals. In urine, an increase in pH
value and Na+ concentration was noted. At terminal necropsy, calculi
were found in the urinary bladder. The urinary sediment consisted
mainly of 4-hydroxybiphenyl. No associated histopathology was detected
upon microscopic examination of the urinary bladder (Kurata et al.,
1986).
When groups ( n = 25) of male Wistar rats were provided diets
containing 0, 1250, or 5000 mg biphenyl/kg (0, 94, or 375 mg/kg body
weight per day) for 36 weeks, a reduction in body weight, increased
relative kidney weights, and an increase in the number of animals with
stones in the kidneys (0/25, 0/25, and 4/25, respectively), ureter
(0/25, 0/25, and 1/25, respectively), and urinary bladder (0/25, 0/25,
and 3/25, respectively) were noted in the high-dose group (Shiraiwa et
al., 1989).
8.4.1.2 Inhalation exposure
Exposure of groups ( n = 50) of male and female CD-1 mice to 25
or 50 ppm (160 or 320 mg/m3; analytical concentrations) biphenyl for
7 h/day, 5 days/week, for 13 weeks produced hyperaemia and focal
haemorrhage in the lung and an increase in hyperplasia of the tracheal
epithelium. As these effects were also observed in some unexposed
controls, they were attributed to the method of aerosol generation
(i.e. inhalation of hot air) (Sun Co. Inc., 1977c).
Marked species differences were observed in a study in which
rabbits, rats, and mice were exposed by inhalation to biphenyl in the
form of dust (50% biphenyl on zeolite) at 5, 40, or 300 mg/m3, 7
h/day, 5 days/week, for up to 13 weeks. No adverse effects were
observed in rabbits. Rats exposed to 40 or 300 mg biphenyl/m3
exhibited increased mortality and irritation of the mucous membranes;
no effects were observed following exposure to 5 mg/m3. Mice were the
most sensitive species. Exposure to 5 mg biphenyl/m3 (the only
concentration tested) resulted in slightly increased mortality, with
all mice exhibiting irritation of the upper respiratory tract (no
further information available). Necropsy of dead rats and mice
revealed mainly inflammatory bronchopulmonary changes. No information
on control animals or particle size was provided (Deichmann et al.,
1947).
8.4.2 Chronic exposure and carcinogenicity
In older studies, which were not further taken into consideration
because of the small number of animals used, limited documentation,
and/or limited exposures, no increase in tumour incidence was observed
in rats and mice when biphenyl was administered orally (at doses up to
750 mg/kg body weight per day for 2 years) (Ambrose et al., 1960;
Innes et al., 1969) or following a single subcutaneous injection of
46.4 mg biphenyl/kg body weight, in which the animals were examined
macroscopically and microscopically after an 18-month observation
period (NTIS, 1968).
In Wistar rats (50 animals of each sex per group) given 0, 2500,
or 5000 mg biphenyl/kg in the diet (0, 188, or 375 mg/kg body weight
per day) for 75 weeks, dose-dependent effects (i.e. reduction in
weight gain; alterations in serum activities of aspartate transaminase
[increased and decreased], alanine transaminase [increased], and
lactate dehydrogenase [increased]; and an increase in blood urea
nitrogen) and a dose-dependent increase in stones of the kidney
(females: 0/43, 1/43, 18/39; males: 0/44, 6/46, 15/47) and ureter
(females: 0/43, 1/43, 2/39; males: 0/44, 0/46, 2/47), accompanied by
haematuria as early as 16 weeks after initiation of exposure, were
seen at >188 mg/kg body weight. At 375 mg/kg body weight, the
relative kidney weights were significantly increased in females, and
an increase in stones of the urinary bladder was seen in males and
females (females: 0/43, 0/43, 6/39; males: 0/44, 0/46, 13/47). The
histopathological examination of the urinary bladder with stones
revealed simple or diffuse hyperplasia and papillomatosis of the
epithelium, but no cancerous changes. The overall tumour incidence was
not increased compared with controls. Kidneys with stones exhibited
obstructive pyelonephritis, tubular atrophy, and fibrosis. Kidney
stones were composed of protein, whereas urinary stones were composed
of magnesium ammonium phosphate (Takita, 1983; Shiraiwa et al., 1989).
No urolithiasis and no increased overall tumour incidence
compared with controls were observed in Wistar rats (50 animals of
each sex per group) provided with diets containing 0, 630, or 1250 mg
biphenyl/kg (0, 47, or 94 mg/kg body weight per day) for 104 weeks.
Dose-dependent effects (i.e. reduction in weight gain; alterations in
serum activities of aspartate transaminase [decreased], alanine
transaminase [increased and decreased], and lactate dehydrogenase
[increased and decreased]) were noted at both doses; 47 mg/kg body
weight is considered the lowest-observed-(adverse-)effect level, or
LO(A)EL (Takita, 1983).
In a study with F344/DuCrj rats performed according to standard
protocols and described in sufficient detail, a significant increase
in neoplastic and non-neoplastic lesions of the urinary bladder and a
significant increase in calculi within the urinary bladder were
observed in high-dose males, after the animals had been given diets
containing 0, 500, 1500, or 4500 mg biphenyl/kg (0, 38, 113, or 338
mg/kg body weight per day) for 104 weeks. In males and females, a
dose-dependent increase in hyperplasia of the epithelium of the renal
pelvis was seen. The results of the histopathological findings in the
urinary bladder and kidneys are summarized in Table 2. Other findings
included increased serum enzyme levels (alkaline phosphatase,
aspartate transaminase, and alanine transaminase) and an increased
urea nitrogen level in low-dose males and mid-dose females, which
became more pronounced with increasing doses. In mid- and high-dose
females and high-dose males, haematological effects (i.e. reduced
haemoglobin concentration and haematocrit) were noted (Japan Bioassay
Research Center, 1996). From this study, a LOEL of 38 mg/kg body
weight was derived.
In a study in which groups of male and female Crj:BDF1 mice were
given diets containing 0, 667, 2000, or 6000 mg biphenyl/kg (0, 100,
300, or 900 mg/kg body weight per day) for 104 weeks, a slight
increase in liver tumours (hepatocellular adenomas and carcinomas) and
basophilic cell foci of the liver was observed in the females at doses
of 300 and 900 mg/kg body weight per day; however, the effects were
not concentration dependent (Japan Bioassay Research Center, 1996)
(see Table 3). In the male and female mice, degenerative changes of
the respiratory epithelium of the nasal cavity and nasopharynx were
observed at doses of >100 mg/kg body weight per day or >300
mg/kg body weight per day, respectively. Other findings included
variations in serum enzyme levels (increase in alkaline phosphatase,
aspartate transaminase, and alanine transaminase) and an increased
urea nitrogen level in the low-dose males and females, which became
more pronounced with increasing doses. In female mice receiving
>300 mg biphenyl/ kg body weight per day and in the high-dose
males, degenerative changes in the kidney (increased mineralization of
the inner stripe of the outer medulla, increase in desquamation of the
epithelium of the renal pelvis) were also observed. Body weight gain
and food consumption were reduced in the high-dose animals (Japan
Bioassay Research Center, 1996).
Information on the toxicological effects of biphenyl following
chronic inhalation or dermal exposure were not identified.
8.4.2.1 Tumour promotion
A number of studies have investigated the tumour-promoting
potential of biphenyl. Kurata et al. (1986) considered biphenyl a
tumour promoter, based upon the results of a study in which groups of
male F344 rats were administered drinking-water containing 0.05%
N-butyl- N-(4-hydroxybutyl)nitrosamine (BBN) (as an initiator) for
4 weeks, followed by 0.5% biphenyl in the diet (estimated intake of
approximately 375 mg/kg body weight per day) for a further 32 weeks,
after which time the animals were examined histopathologically. The
results are provided in Table 4. The effects of biphenyl were
attributed to the increased Na+ content in the urine of exposed
rats, as well as to crystals containing mainly 4-hydroxybiphenyl
detected in the urine.
In a study in which male Wistar rats were administered 0.1%
N-ethyl- N-hydroxyethylnitrosamine in the diet for 2 weeks,
followed by 0, 0.125, or 0.5% biphenyl in the diet for 34 weeks,
exposure to biphenyl had no effect upon the incidence of dysplastic
foci and renal cell tumours induced by N-ethyl- N-hydroxyethylnitro
samine; however, an increase in stones of the kidneys, ureter, and
bladder was observed in rats administered 0.5% biphenyl with or
without N-ethyl- N-hydroxyethyl nitrosamine (Shiraiwa et al.,
1989).
Tamano et al. (1993) maintained male B6C3F1 mice on
drinking-water containing 0.05% BBN supplement for 4 weeks. After the
BBN pre-treatment, the animals received diets with 10 000 mg
biphenyl/kg (1500 mg/kg body weight per day) for 32 weeks. At the end
of the study, the urinary bladder and kidneys were examined
histopathologically. The results are provided in Table 5. In mice
exposed to BBN and biphenyl, the average food consumption and the
final body weight were reduced, whereas the relative weight of the
urinary bladder was increased. The induction of simple hyperplasia and
papillary or nodular dysplasia of the urinary bladder in 1/10 mice
treated with biphenyl only was associated with urolithic residues
(small amounts of greyish white and finely granular material). In mice
treated with biphenyl with or without BBN pre-treatment, the incidence
of interstitial nephritis in the kidneys was 65 and 50%, respectively.
In an early study in which albino mice "initiated" with a single
dermal application of 9,10-dimethyl-1,2-benzanthracene (0.3% in
benzene) received dermal applications of biphenyl (20% in benzene)
twice weekly for 15 weeks, no skin papillomas or carcinomas were
observed in either the vehicle controls or exposed mice 16 weeks after
application of the initiator (Boutwell & Bosch, 1959).
Table 2: Data from Japanese cancer bioassay with male and female rats fed biphenyl in the diet for 2 years.a
Male rats Female rats
0 500 1500 4500 0 500 1500 4500
End-point mg/kg mg/kg mg/kg mg/kg mg/kg mg/kg mg/kg mg/kg
survival rate 37/50 41/50 38/50 31/50 44/50 38/50 44/50 37/50
urinary bladder: neoplastic lesions
transitional cell papilloma 0 0 0 10/50 0 0 0 0
transitional cell carcinoma 0 0 0 24/50 0 0 0 0
(p=0.0001)b
squamous cell papilloma 0 0 0 1/50 0 0 0 0
squamous cell carcinoma 0 0 0 1/50 0 0 0 0
urinary bladder: non-neoplastic lesions (transitional epithelium)
simple hyperplasia 0 0 0 12/50 0 0 1/50 1/50
nodular hyperplasia 0 0 0 40/50 1/50 0 0 5/50
papillary hyperplasia 0 0 0 17/50 0 0 0 4/50
basal cell hyperplasia 0 0 0 27/50 0 0 0 4/50
squamous cell hyperplasia 0 0 0 13/50 0 0 0 1/50
squamous cell metaplasia 0 0 0 19/50 0 0 0 4/50
inflammatory polyps 0 0 0 10/50 0 0 0 0
calculi 0 0 0 43/50 0 0 0 8/50
kidney
mineralization -- papilla 9/50 9/50 14/50 23/50 2/50 6/50 3/50 13/50
mineralization -- pelvis 9/50 6/50 10/50 18/50 12/50 12/50 18/50 27/50
calculi 0/50 0/50 0/50 13/50 0/50 0/50 0/50 3/50
desquamation -- pelvis 1/50 0/50 0/50 11/50 0/50 0/50 0/50 2/50
simple hyperplasia of the 6/50 8/50 5/50 19/50 3/50 5/50 12/50 25/50
transitional epithelium
nodular hyperplasia of the 0/50 1/50 1/50 21/50 0/50 0/50 1/50 12/50
transitional epithelium
ureter dilatation 0/50 0/50 1/50 14/50 0/50 0/50 0/50 6/50
a From Japan Bioassay Research Center (1996).
b Fisher Exact Test.
Table 3: Data from Japanese cancer bioassay with male and female mice fed biphenyl in the diet for 2 years.a
Male mice Female mice
0 667 2000 6000 0 667 2000 6000
End-point mg/kg mg/kg mg/kg mg/kg mg/kg mg/kg mg/kg mg/kg
survival rate 35/50 41/50 41/50 39/50 31/50 22/50 25/50 32/49
liver: neoplastic lesions
hepatocellular carcinoma 8/50 8/49 5/50 4/50 1/50 5/50 7/50 5/49
(p=0.121)b (p=0.043)b
(p=0.1163)b
hepatocellular adenoma 8/50 6/49 7/50 3/50 2/50 3/50 12/50 10/49
(p=0.4909)b (p=0.016)b
(p=0.0251)b
historical control data: historical control data:
carcinoma: 171/700 with a range of 1/50 - 19/50 carcinoma: 15/699 with a range of 0/50 - 2/50
adenoma: 119/700 with a range of 2/50 - 15/50 adenoma: 33/699 with a range of 1/50 - 5/50
basophilic cell foci 0/50 6/49 1/50 2/50 1/50 1/50 12/50 6/49
a From Japan Bioassay Research Center (1996).
b Fisher Exact Test.
Table 4: Results of the tumour-promoting potential (urinary bladder) of
biphenyl in male rats.a
Hyperplasia Papilloma Carcinoma
n (%) n (%) n (%)
biphenyl only
(21 rats) 0 0 0 0 0 0
BBNb only
(24 rats) 6 -25 3 -12 0 0
BBN and biphenyl
(18 rats) 17 -94 15 -83 11 -61
a Taken from Kurata et al. (1986).
b BBN = N-butyl-N-(4-hydroxybutyl)nitrosamine.
Table 5: Results of the tumour-promoting potential (urinary bladder) of
biphenyl in male mice.a
Papillary or
Hyperplasia nodular dysplasia Carcinoma
n (%) n (%) n (%)
biphenyl only
(10 mice) 1 -10 1 -10 0 0
BBNb only
(20 mice) 12 -60 2 -10 0 0
BBN and biphenyl
(20 mice) 14 -70 1 -5 0 0
a Taken from Tamano et al. (1993).
b BBN = N-butyl-N-(4-hydroxybutyl)nitrosamine.
8.5 Genotoxicity and related end-points
The results of the in vitro tests with biphenyl are summarized
in Table 6. In vitro studies with bacteria have yielded no evidence
of mutagenic potential for biphenyl, whereas gene mutation and mitotic
recombination were observed with Saccharomyces cerevisiae D7 in the
presence or absence of metabolic activation. However, no gene
conversion was observed with S. cerevisiae D3. Testing in mammalian
cells has produced positive results for gene mutations and
clastogenicity in the presence of metabolic activation and negative
results in the absence of metabolic activation.
In an in vivo rat cytogenetic assay of bone marrow cells, the
incidence of chromosomal aberrations was not increased (no further
information available) (Kawachi et al., 1980). The exposure by
inhalation of male Sprague-Dawley rats to 64 or 320 mg biphenyl/m3
(as dust aerosol) for 7 h/day, 5 days/week, for 30 days (20 exposures
in total) reportedly did not increase the frequency of chromosomal
aberrations in the bone marrow (Dow Chemical Co., 1976). However,
owing to insufficient documentation (i.e. no data available concerning
dust particle size distribution or cell harvesting times) and the low
number of metaphase cells examined (i.e. 50 cells per animal), the
validity of this study cannot be ascertained.
The results of in vitro studies indicate that biphenyl has
mutagenic potential; in the absence of reassurance from reliable
results from in vivo tests, it is assumed that exposure to biphenyl
may be associated with a mutagenic risk.
8.6 Reproductive and developmental toxicity
Available data on the reproductive or developmental toxicity of
biphenyl are limited. In Wistar rats administered (by gavage) 0, 125,
250, 500, or 1000 mg biphenyl/kg body weight (in corn oil) on days
6-15 of gestation, no maternal toxicity occurred at doses of <500
mg/kg body weight. In the highest dose group, 5 of 20 rats died. Also
at 1000 mg/kg body weight, reduced fetal weight and an increased
number of dead and resorbed fetuses were noted; however, these values
were statistically not significantly different from those of control
animals. At doses of >500 mg/kg body weight, there were
non-significant increases in the incidence of fetuses with missing or
non-ossified sternebrae (Khera et al., 1979).
Table 6: Results of in vitro genotoxicity studies on biphenyl.
Resultsa
Species Concentration Without With
(test system) End-point range MA MA References
Salmonella typhimurium Reverse 0-5000 µg/plate - - Cline & McMahon (1977); Purchase et al.
TA92, TA94, TA97, TA97a, mutations (1978); Kawachi et al. (1980); NTP (1980);
TA98, TA100, TA102, Bronzetti et al. (1981); Probst et al. (1981);
TA1532, TA1535, TA1537, Waters et al. (1982); Haworth et al. (1983);
TA1538, TA2636 Pagano et al. (1983, 1988); Ishidate et al.
(1984); Fujita et al. (1985); Brams et al.
(1987); Bos et al. (1988); Glatt et al. (1992)
Escherichia coli WP2, WP2 Gene mutations 0.1-1000 µg/ml - - Cline & McMahon (1977); Probst et al. (1981);
uvrA- Waters et al. (1982)
E. coli PQ37 DNA damage 2.4-154 µg/ml - - Brams et al. (1987)
Bacillus subtilis rec assay DNA damage no data - 0 Kawachi et al. (1980)
Saccharomyces cerevisiae Gene mutation/ <154 µg/ml + + Pagano et al. (1983)
D7 gene conversion
S. cerevisiae D3 Gene conversion no data - - Waters et al. (1982); Zimmermann et al. (1984)
Chinese hamster cells Gene mutation 0-100 µg/ml - + Glatt et al. (1992)
(V79)
L5178Y TK+/- cells Gene mutation 0-61 µg/ml - (+) Wangenheim & Bolcsfoldi (1988)
(mouse lymphoma assay)
Chinese hamster cells Chromosomal 0-125 µg/ml - 0 Ishidate & Odashima (1977); Kawachi et al.
(CHL) aberration (1980); Sofuni et al. (1985)
Table 6 (Cont'd.)
Resultsa
Species Concentration Without With
(test system) End-point range MA MA References
Chinese hamster cells Chromosomal 0-20 µg/ml - + Sofuni et al. (1985)
(CHL) aberration
Chinese hamster cells Chromosomal 15.4-154 µg/ml - 0 Abe & Sasaki (1977)
(DON) aberration
Rat hepatocytes Unscheduled 0.002-154 µg/ml 0 - Williams (1978); Brouns et al. (1979);
DNA synthesis Probst et al. (1981)
Chinese hamster cells Sister chromatid no data - 0 Kawachi et al. (1980)
(CHL) exchange
Chinese hamster cells Sister chromatid 15.4-154 µg/ml - 0 Abe & Sasaki (1977)
(DON) exchange
L5178Y cells (alkaline DNA damage 0-231 µg/ml - + Garberg et al. (1988)
unwinding assay)
human lung fibroblasts Unscheduled no data - - Waters et al. (1982)
(WI-38 cells) DNA synthesis
human fibroblasts ("nick DNA damage 15.4 µg/ml - 0 Snyder & Matheson (1985)
translation assay")
a -, negative; +, positive; (+) weakly positive; 0, not tested; MA, metabolic activation.
Compared with unexposed controls, litter size (the only parameter
examined) was not affected in a study in which small numbers of male
and female rats were administered diets containing 0.1 or 0.5%
biphenyl (estimated intakes of 75 and 375 mg/kg body weight per day)
before mating and throughout gestation (Ambrose et al., 1960). In an
unpublished three-generation study, dietary biphenyl concentrations of
100 or 1000 mg/kg (estimated intakes of approximately 7.5 or 75 mg
biphenyl/kg body weight per day) had no effect on reproduction in
rats; after intake of 10 000 mg/kg (estimated intake of 750 mg/kg body
weight per day), decreased fertility, litter size, and growth rate
were noted (no further information available) (Stanford Research
Institute, undated). Histopathological changes within the male and
female reproductive systems were not observed in rats or mice
administered biphenyl at 500-4500 mg/kg in the diet for 2 years (Japan
Bioassay Research Center, 1996).
8.7 Immunological and neurological effects
Data on immunological and neurological effects of biphenyl in
laboratory animals were not identified.
9. EFFECTS ON HUMANS
Information on effects on human health resulting from exposure to
biphenyl are limited to case reports; epidemiological studies were not
identified. In one report, a single application of 0.5 ml of a 4%
biphenyl solution (no further details provided) to the skin of the
lower arm of two test subjects caused no apparent irritation
(Macintosh, 1945). Biphenyl did not cause skin irritation in a study
in which a solution of 23% biphenyl in oil was applied to the forearm
three times per week for 8 weeks (Selle, 1952). In one human volunteer
orally given 35 mg biphenyl per week for 13 weeks, no adverse effects
were reported (Farkas, 1939). Cases of headache, nausea, and
inflammation of the respiratory tract have been reported in workers
exposed to biphenyl vapours (and other substances) during paper
production (Weil et al., 1965). Liver damage and effects upon the
central and peripheral nervous systems were attributed to long-term
exposure to high concentrations of biphenyl (see section 6.2) in a
case-study of workers engaged in the production of
biphenyl-impregnated paper (Haekkinen et al., 1973). Chronic
persistent hepatitis in a woman who had worked for 25 years in a
citrus packing plant in which biphenyl-impregnated paper was used was
attributed to the absorption of biphenyl through the skin and
digestive tract (Carella & Bettolo, 1994).
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1 Aquatic environment
The acute toxicity of biphenyl in aquatic organisms has been
investigated in microorganisms, plants, invertebrates, and
vertebrates; however, in several studies cited in the main source
document (BUA, 1990), information on whether results were based on
nominal or effective concentrations was either lacking or not clearly
stated. For biphenyl, nominal concentrations may not correspond to the
effective concentrations, as the water solubility of biphenyl is low
(4.45 mg/litre at 20°C) (test results with higher nominal effect
concentrations have not been reported here) and its volatility
significant. In tests with open systems and longer test durations,
results based on nominal concentrations may lead to an underestimation
of toxic effects because of evaporation of the chemical; therefore,
emphasis has been placed on studies with lowest reported effect levels
in which loss of the test substance was minimized.
Several studies were conducted on the toxic effects of biphenyl
on mixed microbial cultures as well as on single bacterial species.
The most sensitive species was Photobacterium phosphoreum, with a
30-min EC50 of 1.9 mg/litre in a bioluminescence inhibition test
(BUA, 1990). For Colpidium campylum, one of three different species
of ciliata tested, the minimal active concentration of biphenyl for
the inhibition of cell proliferation after a 43-h exposure was 5.6
mg/litre (nominal concentration; test substance predissolved in
acetone) (Dive et al., 1980). In studies on the reduction of
photosynthesis, 3-h EC50 values of 3.86 and 1.28 mg/litre were deter
mined with the unicellular green algae Chlorella vulgaris and
Chlamydomonas angulosa, respectively (BUA, 1990).
Forty-eight-hour LC50 values from acute toxicity tests with
Daphnia magna were in the range of 1.1-4.7 mg/litre when experiments
were conducted in more or less tightly closed test vessels. From a
test in a closed system with continuous flow, a 48-h LC50 of 0.36
mg/litre and a NOEC of 0.04 mg/litre were reported. In a reproduction
test with Daphnia magna in the same closed continuous-flow system,
the NOEC after 21 days' incubation was 0.17 mg/litre (LC50 = 0.23
mg/litre) (Gersich et al., 1989). In studies on the inhibition of food
intake by the marine mussel Mytilus edulis in a static system with
loosely covered (not airtight) test vessels, a 40-min EC50 of 0.3 mg
biphenyl/litre was reported (Donkin et al., 1991). From static tests
on the toxicity of biphenyl on freshwater fish, 96-h LC50 values in
the range of 1.5-4.7 mg/litre have been reported, with rainbow trout
(Oncorhynchus mykiss) and fathead minnow (Pimephales promelas) the
most sensitive species (BUA, 1990).
10.2 Terrestrial environment
Biphenyl has weak bactericidal and fungistatic properties. In
studies with numerous mould fungus species, biphenyl (applied as
vapour or imbedded in solid media) caused a reversible inhibition
(50-100%) of cellular proliferation. Suppression of sporogenesis and,
in Penicillium digitatum and Diplodia natalensis, the occurrence
of biphenyl-resistant mutants were observed. Yeast species showed
little or no inhibition of cell proliferation following exposure to
biphenyl (BUA, 1990).
In a plant growth inhibition test conducted according to OECD
Guideline 208, a nominal NOEC of 100 mg/kg dry soil was reported for
sorghum (Sorghum bicolor) and soya bean (Glycine max); a NOEC of
>1000 mg biphenyl/kg was reported for the sunflower (Helianthus
annuus) (Windeatt et al., 1991). Biphenyl mixed into soil inhibited
growth of lettuce (Lactuca sativa) seedlings with a 7-day EC50 of
54 mg/kg (nominal concentration). At the end of incubation, the
concentration of test substance in the soil had decreased to <10% of
the initial level (Hulzebos et al., 1993).
Data on toxic effects of biphenyl on terrestrial animals and
ecosystems were not identified.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
There are limited data on the effects of biphenyl exposure on
humans. The assessment of potential health hazards has relied
primarily on studies conducted in laboratory animals. The acute oral
toxicity of biphenyl is moderate. The chemical is not irritating to
the skin and is slightly irritating to the eyes, and there is no
evidence of skin sensitizing potential.
Aside from differences in mortality among laboratory species
exposed subchronically to biphenyl-containing dusts, the available
data on effects associated with the inhalation of biphenyl are
insufficient to form the basis of an assessment of potential health
hazards associated with long-term airborne exposure to this chemical.
In toxicological studies in which rodents have been administered
diets containing biphenyl for various periods of time, effects on the
urinary system have often been reported. A marked increase in the
incidence of morphological (i.e. formation of calculi) and/or
histopathological (e.g. hyperplasia, desquamation) effects has been
observed within the urinary tract of male rats administered diets
containing more than 2500 mg biphenyl/kg for periods ranging from 32
to 104 weeks (Takita, 1983; Kurata et al., 1986; Shiraiwa et al.,
1989; Japan Bioassay Research Center, 1996). An increase in the
occurrence of calculi within the urinary bladder has also been
observed in female rats, but at a lower incidence than in males
(Takita, 1983; Shiraiwa et al., 1989; Japan Bioassay Research Center,
1996). Similarly, in a long-term dietary study (Japan Bioassay
Research Center, 1996), increased squamous metaplasia within the
urinary transitional epithelium was also observed in female rats;
again, however, the incidence was lower than that observed in males.
In male mice, only 1 of 10 animals given a diet containing 10 000 mg
biphenyl/kg (1500 mg/kg body weight per day) for 32 weeks developed
simple hyperplasia and papillary or nodular dysplasia of the urinary
bladder (Tamano et al., 1993). Effects on blood chemistry and
haematological parameters have also been observed in animals
administered biphenyl orally; these effects occurred in male and
female rats and mice at intakes lower than those associated with the
development of effects in the urinary bladder of male rats
administered biphenyl (Takita, 1983; Shiraiwa et al., 1989; Japan
Bioassay Research Center, 1996). For non-neoplastic effects, the LOEL
was 38 mg/kg body weight per day based upon the development of
alterations in haematological parameters (i.e. decreased haemoglobin
concentration and haematocrit) in rats fed diets containing 0, 500,
1500, or 4500 mg biphenyl/kg (reported intakes of 0, 38, 113, or 338
mg/kg body weight per day) for 2 years (Japan Bioassay Research
Center, 1996).
Although limited, the available information indicates that
biphenyl has no reproductive or developmental effects at doses lower
than those associated with the development of adverse effects in the
parental generation.
In one study, an increased incidence of benign and malignant
tumours within the urinary bladder was observed in male F344/DuCrj
rats administered diets containing high levels (i.e. not less than
4500 mg/kg) of biphenyl for 2 years. Tumour incidence was not
increased in female rats or in male or female Crj:BDF1 mice (Japan
Bioassay Research Center, 1996). In female mice, there were slight
increases in the incidences of benign and malignant liver tumours in
animals receiving biphenyl in the diet; however, the results were not
dose dependent over the entire range of concentrations tested. In
other studies, biphenyl exhibited tumour-promoting activity with
respect to the development of bladder neoplasms in male rats (Kurata
et al., 1986) but not in male mice (Tamano et al., 1993).
The mechanism by which high doses of biphenyl induce bladder
tumours specifically in male rats has not been fully elucidated. There
have been suggestions that for some non-genotoxic chemicals, the
formation of such tumours is linked to the regenerative hyperplasia of
the urinary epithelium, caused by the abrasion and damage to the
urothelium that are produced by calculi formed within the urinary
tract only at very high levels of exposure (Cohen, 1995). Owing to
anatomical and physiological differences, male rats are considered to
be more susceptible than female rats, mice, and humans to the
development of bladder tumours via such a mechanism; it has therefore
been suggested that the sex- and species-specific development of
bladder tumours in male rats receiving high doses of biphenyl might
not be strictly relevant to humans exposed to lower levels. However,
the incidence of histopathological effects and calculi formation
within the urinary bladder also increased in female rats administered
biphenyl for 2 years, in the absence of bladder tumours (Japan
Bioassay Research Center, 1996). Moreover, data identifying a direct
association between calculi formation, regenerative hyperplasia of the
urothelium, and the development of bladder tumours within individual
male animals are not available. Biphenyl has been reported to be
mutagenic and clastogenic in some, but not all, in vitro assays and
to bind covalently to macromolecules (i.e. proteins) following
metabolic activation; the available data have been considered
inadequate to assess its genotoxicity in vivo. These considerations,
coupled with the structural relationship of 4-hydroxybiphenyl, a major
metabolite of biphenyl, to the potent human bladder carcinogen
4-aminobiphenyl and the lack of critical information related to the
mechanism by which these bladder tumours arise in male rats, could
suggest that the development of bladder tumours in the male rats may
not have been entirely due to effects associated with the formation of
calculi within the urinary bladder. This observation, as well as the
slightly increased incidence of liver tumours in female mice, raises
some concerns with respect to the potential carcinogenicity of
biphenyl to humans.
11.1.2 Criteria for setting guidance values for
biphenyl
A provisional tolerable daily intake (TDI), based upon the
development of effects in the blood of rats administered diets
containing biphenyl for 2 years (Japan Bioassay Research Center,
1996), can be derived as follows:
TDI = 38 mg/kg body weight per day
1000
= 38 µg/kg body weight per day
where:
* 38 mg/kg body weight per day is the lowest LOEL for the
development of alterations in blood chemistry in rats provided
diets containing biphenyl for 2 years. This intake is lower than
intakes associated with the development of haematological
effects, calculi, and histopathological changes in the bladder
and/or kidneys in rats and the development of haematological and
histopathological effects in mice administered biphenyl in the
diet for 2 years.
* 1000 is the uncertainty factor (×10 for interspecies variation;
×10 for intraspecies variation; ×10 for extrapolation from a LOEL
to a NOEL).
However, based upon the currently available data, there is some
uncertainty surrounding the health-protective nature of the value,
owing to lack of critical information concerning the mechanism by
which biphenyl induces bladder tumours in male rats, the slight
increase in hepatocellular tumours in female mice, and the potential
genotoxicity of this substance. Because of this uncertainty, the TDI
is designated provisional.
Available data were inadequate to serve as a basis for the
derivation of guidance values for biphenyl in air.
11.1.3 Sample risk characterization
For the general population, there is insufficient information
available concerning the intake of biphenyl from the general
environment; therefore, comparisons with the provisional TDI are
provided merely as an example. From limited information on the content
of biphenyl in citrus fruits, the estimated daily intake is <375 ng
biphenyl/kg body weight from each individual fruit consumed, while the
estimated daily intake from tap-water is <0.16 ng/kg body weight.
Assuming that a person consumes one citrus fruit per day, the
estimated intake of biphenyl from drinking-water and citrus fruit
consumption is approximately two orders of magnitude lower than the
provisional TDI based upon the development of effects in the blood.
From a study on workers exposed in a plant producing
biphenyl-impregnated paper, it can be derived that exposure to high
biphenyl concentrations up to 128 mg/m3 pose a hazard. Neurotoxic
effects and liver damage have been observed.
Additional information is required to better define any potential
carcinogenic risks that might be associated with exposure to biphenyl.
11.2 Evaluation of environmental effects
The atmosphere is likely the main target compartment for
biphenyl; however, the chemical has been detected in all environmental
compartments as a result of its release from vehicle emissions,
heating, smoking, and industrial activities. Water, soil, and biota
appear to be affected mainly in the direct vicinity of industrial
activities. The decline in the levels of biphenyl in surface waters
may be due to restrictions on the production and use of PCBs. There
are no data available on the release of biphenyl into environmental
compartments from industrial use, from the application of
biphenyl-contaminated sewage sludge to arable land, from
volatilization during the storage of treated fruits, and from leaching
from preserved citrus fruits and their packaging materials in domestic
waste dumps. Only estimated values are available for the
photooxidation of biphenyl in the atmosphere, which is probably the
most important degradation process in this compartment.
Biomagnification of biphenyl in higher trophic levels of the
aquatic or terrestrial food-chain, based upon the potential of
biphenyl to bioaccumulate, is expected to be of minor importance. For
the aquatic compartment, a significant elimination of biphenyl can be
expected as a result of evaporation and degradation. Furthermore,
rapid metabolism of biphenyl in laboratory animals and low acute
toxicity after ingestion have been reported.
Data on effect concentrations for terrestrial organisms and
exposure levels in air and soil are insufficient for risk
characterization for terrestrial organisms. A sample risk
characterization with respect to the aquatic environment can be
performed (based upon data derived from Germany) by calculating the
ratio between a (local or regional) PEC (based on measured or model
concentrations) and a PNEC (EC, 1996).
The annual average concentration of biphenyl (<0.5 µg/litre)
measured in the 1990s in the Rhine River and its tributaries was used
as a PECmeasured. A PEClocal (water), based upon data derived from the
effluent of the wastewater treatment plant of a German producer, may
be calculated as follows:
PEClocal (water) = Ceffluent
(1 + Kp (susp) × Csusp × 10-6) × D
= <0.0064 µg/litre
where:
* Ceffluent is <5 µg/litre, the concentration of biphenyl in
wastewater in 1990 (BUA, 1990);
* Kp (susp) = 423, the suspended matter/water adsorption
coefficient, estimated from the mean soil sorption coefficient
( Koc) of 4230 ( Kp (susp) = Koc × 0.1, where 0.1 is the
fraction of organic carbon in suspended matter, foc(susp));
* Csusp = 15 mg/litre (default value; EC, 1996), the
concentration of suspended matter in the river; and
* D = 781, the dilution factor for river flow, calculated from
sewage treatment plant and river flow data (EC, 1996).1
A PNEC for surface waters may be calculated by dividing the
lowest valid LC50 or NOEC by an appropriate assessment factor (equal
to a safety factor):
PNEC = 170 µg/litre
10
= 1.7 µg/litre
where:
* 170 µg/litre is the lowest NOEC from a chronic study with
Daphnia magna; and
1 Personal communication from Verband d. Textilhilfsmittel-,
Lederhilfsmittel-, Gerbstoff- und Waschrohstoff-Industrie e.V.,
Frankfurt/M., Germany, to Bayer AG, 1996.
* 10 is the assessment factor. For biphenyl, based upon data for
four trophic levels, including one long-term NOEC, the
recommended assessment factor would be 100 (EC, 1996). However,
because of its significant volatility and degradation, and as
effect concentrations from a wide selection of species covering
different taxonomic groups are reported, an assessment factor of
10 is used.
Therefore, based upon the annual average concentrations of
biphenyl measured in the Rhine River and in industrial wastewater
effluent in Germany, the PECmeasured/PNEC and PEClocal (water)/PNEC
ratios are <0.29 and <0.04, respectively. In some jurisdictions
(EC, 1996), chemicals with a PEC/PNEC ratio of <1 may not require
implementation of risk reduction measures beyond those already in
place.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The Joint FAO/WHO Meeting on Pesticide Residues has allocated an
acceptable daily intake of biphenyl of 0-0.125 mg/kg body weight
(JMPR, 1967).
Information on international hazard classification and labelling
is included in the International Chemical Safety Card reproduced in
this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventative and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0106) reproduced in this
document.
13.1 Advice to physicians
Treatment is symptomatic and supportive following intoxication.
The clinical picture is characterized by central and peripheral nerve
damage and liver injury in human poisoning. There is some evidence to
indicate that electroencephalographic abnormalities could persist up
to 1 or 2 years after exposure.
13.2 Health surveillance advice
Objective change in electroencephalogram and electroneuromyogram
could be an indication of biphenyl poisoning in exposed individuals.
Monitoring of liver function could also be integrated into a medical
surveillance programme.
13.3 Spillage
In the event of spillage, measures should be under taken to
prevent biphenyl from reaching drains and watercourses, because of its
toxicity to aquatic organisms.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards
can be found in the International Register of Potentially Toxic
Chemicals (IRPTC), available from UNEP Chemicals (IRPTC), Geneva.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
BIPHENYL ICSC: 0106
April 1994
CAS # 92-52-4 Diphenyl
RTECS # DU8050000 Phenylbenzene
EC # 601-042-00-8 Dibenzene
C12H10/C6H5C6H5
Molecular mass: 154.2
TYPES OF HAZARD/ ACUTE HAZARDS/ PREVENTION FIRST AID/FIRE FIGHTING
EXPOSURE SYMPTOMS
FIRE Combustible NO open flames Water spray, powder, AFFF, foam,
carbon dioxide
EXPLOSION Finely dispersed particles Prevent deposition of dust, closed
form explosive mixtures in air. system, dust explosion-proof
electrical equipment and lighting.
Prevent build-up of electrostatic
charges (e.g., by grounding)
EXPOSURE PREVENT DISPERSION OF DUST!
Inhalation Cough, Nausea, Vomiting Avoid inhalation of fine dust Fresh air, rest. Refer for medical
and mist. attention.
Skin Protective gloves. Remove contaminated clothes. Rinse
and then wash skin with water and
soap.
Eyes Redness. Pain Safety goggles, or eye protection First rinse with plenty of water for
in combination with breathing several minutes (remove contact lenses
protection if powder. if easily possible), then take to a
doctor.
TYPES OF HAZARD/ ACUTE HAZARDS/ PREVENTION FIRST AID/FIRE FIGHTING
EXPOSURE SYMPTOMS
Ingestion (further see Inhalation) Do not eat, drink, or smoke Rinse mouth. Refer for medical
during work. Wash hands before attention.
eating.
SPILLAGE DISPOSAL PACKAGING & LABELLING
Sweep spilled substance into sealable containers; if appropriate, moisten Do not transport with food and feedstuffs.
first to prevent dusting. Carefully collect remainder, then remove to safe EU Classification
place. (Extra personal protection: A/P2 filter respirator for organic vapour Symbol: Xi
and harmful dust). R: 36/37/38
S: (2-)23
UN Classification
EMERGENCY RESPONSE STORAGE
NFPA Code: H 2; F1; R O; Separated from food and feedstuffs and oxidants.
IMPORTANT DATA
PHYSICAL STATE; APPEARANCE: ROUTES OF EXPOSURE:
WHITE CRYSTALS OR FLAKES, WITH CHARACTERISTIC ODOUR The substance can be absorbed into the body by inhalation and by ingestion.
PHYSICAL DANGERS: INHALATION RISK:
Dust explosion possible if in powder or granular form, A harmful contamination of the air can be reached rather quickly on
mixed with air. evaporation of this substance at 20°C.
CHEMICAL DANGERS: EFFECTS OF SHORT-TERM EXPOSURE:
Reacts with oxidants. The substance irritates the eyes and the respiratory tract.
OCCUPATIONAL EXPOSURE LIMITS: EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
TLV: 0.2 ppm; 1.3 mg/m3 (ACGIH 1993-1994) The substance may have effects on the liver and nervous system, resulting
impaired functions.
PHYSICAL PROPERTIES
Boiling point 256°C Flash point: 113°C c.c.
Melting point 70°C Auto-ignition temperature: 540°C
Relative density (water = 1): 1.04 Explosive limits, vol% in air: 0.6 (at 111°C) - 5.8 (at 166°C)
Solubility in water: none Octanol/water partition coefficient as log Pow: 3.16/4.09
Vapour pressure, Pa at 71°C: 133
Relative vapour density (air = 1): 5.3
Relative density of the vapour/air-mixture
at 20°C (air = 1): 1.0
ENVIRONMENTAL DATA
The substance may be hazardous to the environment, special attention should be given to water. In the food chain important to humans,
bioaccumulation takes place, specifically in plants. It is strongly advised not to let the chemical enter into the environment because it
persists in the environment.
NOTES
Do NOT take working clothes home. Dowtherm A is a trade name.
ADDITIONAL INFORMATION
LEGAL NOTICE: Neither the CEC or the IPCS nor any person acting on behalf of the CEC or the IPCS is responsible for the use which
might be made of this information.
REFERENCES
Abe S, Sasaki M (1977) Chromosome aberrations and sister chromatid
exchanges in Chinese hamster cells exposed to various chemicals.
Journal of the National Cancer Institute, 58:1635-1641.
Ahel M (1991) Infiltration of organic pollutants into groundwater:
field studies in the alluvial aquifer of the Sava river. Bulletin
of environmental contamination and toxicology, 47:586-593.
Ambrose AM, Booth AN, DeEds F, Cox AJ (1960) A toxicological study of
biphenyl, a citrus fungistat. Food research, 25:328-336.
Anklam E, Mueller A (1994) A simple method of sample preparation for
analysis of biphenyl residues in citrus fruit peels by gas
chromatography. Zeitschrift fuer Lebensmittel Untersuchung und
Forschung, 198:329-330.
Anon. (1994-95) The Chemical Daily, pp. 679-680 (no further
information available).
Atkinson R, Arey J (1994) Atmospheric chemistry of gas-phase
polycyclic aromatic hydrocarbons: formation of atmospheric mutagens.
Environmental health perspectives, 102 (Suppl. 4):117-126.
Atkinson R, Aschmann SM (1985) Rate constants for the gas-phase
reaction of hydroxyl radicals with biphenyl and the
monochlorobiphenyls at 294 ± 1 K. Environmental science and
technology, 19:462-464.
ATSDR (1990) Toxicological profile for creosote. Atlanta, GA, US
Department of Health and Human Services, Public Health Service, Agency
for Toxic Substances and Disease Registry (Report No. TP-90-09).
Baranski Z (1991) Evaluation of occupational exposure to aromatic
polycyclic hydrocarbons in bituminous mastics plants. Roczniki
Panstwowego Zakladu Higieny, 42:223-237.
Bend JR, Hook GER, Easterling RE, Gram TE, Fouts JR (1972) A
comparative study of the hepatic and pulmonary microsomal
mixed-function oxidase systems in the rabbit. Journal of
pharmacology and experimental therapeutics, 183:206-217.
Bevinakatti BG, Ninnekar HZ (1992) Degradation of biphenyl by a
Micrococcus species. Applied microbiology and biotechnology,
38:273-275.
Booth AN, Ambrose AM, DeEds F (1956) Reversible nephrotoxic effects of
biphenyl. Federation proceedings, 15:403 (Abstract 1313).
Booth AN, Ambrose AM, DeEds F, Cox AJ (1961) The reversible
nephrotoxic effects of biphenyl. Toxicology and applied
pharmacology, 3:560-567.
Bos RP, Theuws JLG, Jongeneelen FJ, Henderson PT (1988) Mutagenicity
of bi-, tri- and tetra-cyclic aromatic hydrocarbons in the
"taped-plate assay" and in the conventional Salmonella mutagenicity
assay. Mutation research, 204:203-206.
Boutwell RK, Bosch DK (1959) The tumor-promoting action of phenol and
related compounds for mouse skin. Cancer research, 19:413-424.
Brams A, Buchet JP, Crutzen-Fayt MC, de Meester C, Lauwerys R, Leonard
A (1987) A comparative study, with 40 chemicals, of the efficiency of
the Salmonella assay and the SOS chromotest (kit procedure).
Toxicology letters, 38:123-133.
Bronzetti G, Esposito A, Pagano G, Quinto I (1981) A comparative study
on the toxicity and mutagenicity of biphenyl (BP) and diphenyl ether
(DPE) in sea urchin, S. typhimurium and S. cerevisiae. Mutation
research, 85:233.
Brouns RE, Poot M, de Vrind R, van Hoek-Kon T, Henderson PT (1979)
Measurement of DNA-excision repair in suspensions of freshly isolated
rat hepatocytes after exposure to some carcinogenic compounds. Its
possible use in carcinogenicity screening. Mutation research,
64:425-432.
BUA (1990) BUA-Stoffbericht Biphenyl (1,1'-Biphenyl). Berater
gremium fuer Umweltrelevante Altstoffe. Weinheim, VCH VerlagsGmbH
(Report No. 50; July 1990).
BUA (1994) BUA-Stoffbericht 133 (Ergaenzungsberichte II). Berater
gremium fuer Umweltrelevante Altstoffe. Weinheim, VCH VerlagsGmbH.
Carella G, Bettolo PM (1994) Reversible hepatotoxic effects of
diphenyl: report of a case and a review of the literature. Journal
of occupational medicine, 36:575-576.
CITI (1992) Biodegradation and bioaccumulation. Data of existing
chemicals based on the CSCL Japan. Tokyo, Japan Chemical Industry
Ecology-Toxicology & Information Center, Chemicals Inspection &
Testing Institute, p. 4-1.
Cline JC, McMahon RE (1977) Detection of chemical mutagens. Use of
concentration gradient plates in a high capacity screen. Research
communications in chemical pathology and pharmacology, 16:523-533.
Cohen SM (1995) Human relevance of animal carcinogenicity studies.
Regulatory toxicology and pharmacology, 21:75-80.
Collin G, Hoeke H (1995) Tar and pitch. In: Elvers B, Hawkins S,
Russes W, eds. Ullmann's encyclopedia of industrial chemistry.
Vol. A26. 5th ed. Weinheim, VCH Verlagsgesellschaft, pp. 91-127.
Deichmann WB, Kitzmiller KV, Dierker M, Witherup S (1947) Observations
on the effects of diphenyl, o- and p-aminodiphenyl, o- and
p-nitrodiphenyl and dihydroxyoctachlorodiphenyl upon experimental
animals. Journal of industrial hygiene and toxicology, 29:1-13.
Deusen C, Kleinermanns K (1993) Identification of exhaust air
components with thermal inertization of dusts from cold setting
moulding materials. Giessereiforschung, 45:147-150.
Di Muccio A, Barbini DA, De Merulis G, Vergori L, Girolimetti S,
Sernicola L, Dommarco R (1995) Rapporto sulle revisioni di analisi
per residui di antiparassitari: 1988-1995. Rome, Istituto Superiore
di Sanita.
Dive D, Leclerc H, Persoone G (1980) Pesticide toxicity on the ciliate
protozoan Colpidium campylum: possible consequences of the effect of
pesticides in the aquatic environment. Ecotoxicology and
environmental safety, 4:129-133.
Donkin P, Widdows J, Evans SV, Brinsley MD (1991) QSARs for the
sublethal responses of marine mussels (Mytilus edulis). Science of
the total environment, 109(100):461-476.
Dorgelo FO, Verver G, Wieling G, Topp RJ, Boleij JSM, Pal TM (1985)
Urinary hydroxydiphenyl excretion of workers occupationally exposed to
a mixture of diphenyl and diphenylether (Dowtherm A). International
archives of occupational and environmental health, 56:129-134.
Dow Chemical Co. (1974) Acute inhalation toxicity and industrial
handling hazards of biphenyl heated to 85°C (EPA Document I.D.:
878213725, received 1983) [cited in BUA, 1994].
Dow Chemical Co. (1976) Cytogenetic effects of diphenyl-99 on rat
bone marrow cells (EPA Document I.D.: 878213726, received 1983)
[cited in BUA, 1994].
Dreist M, Kolb J (1993) Untersuchungen auf hautsensibilisierende
Wirkung am Meerschweinchen (Maximierungstest nach Magnusson und
Kligman) (Bericht Nr. 22057 vom 19.02.1993) [cited in BUA, 1994].
EC (1996) Technical guidance documents in support of the
Commission Directive 93/67/EEC on risk assessment for new notified
substances and the Commission Regulation (EC) 1488/94 on risk
assessment for existing substances. Ispra, European Chemicals
Bureau.
Engewald W, Knobloch T, Efer J (1993) Fluechtige organische
Verbindungen in Emissionen aus dem Hausbrandvon Braunkohle.
Umweltwissenschaften Schadstoff Forschung, 6:303-308.
Farkas A (1939) Hadar, 12:227 (no further information available)
[cited in JMPR, 1967].
Freitag D, Geyer H, Kraus A, Viswanathan R, Kotzias D, Attar A, Klein
W, Korte F (1982) Ecotoxicological profile analysis -- VII. Screening
chemicals for their environmental behavior by comparative evaluation.
Ecotoxicology and environmental safety, 6:60-81.
Fujita H, Kojima A, Sasaki M, Hiraga K (1985) Mutagenicity test of
antioxidants and fungicides with Salmonella typhimurium TA97a,
TA102. Kenkyu Nenpo-Tokyo-toritsu Eisei Kenkyusho, 36:413-417.
Garberg P, Akerblom E-L, Bolcsfoldi G (1988) Evaluation of a
genotoxicity test measuring DNA-strand breaks in mouse lymphoma cells
by alkaline unwinding and hydroxyapatite elution. Mutation
research, 203:155-176.
Gersich FM, Bartlett EA, Murphy PG, Milazzo DP (1989) Chronic toxicity
of biphenyl to Daphnia magna Straus. Bulletin of environmental
contamination and toxicology, 43:355-362.
Glatt H, Anklam E, Robertson LW (1992) Biphenyl and fluorinated
derivatives: liver enzyme-mediated mutagenicity detected in
Salmonella typhimurium and Chinese hamster V79 cells. Mutation
research, 281:151-156.
Haekkinen I, Siltanen E, Hernberg S, Seppaelaeinen AM, Karli P,
Vikkula E (1973) Diphenyl poisoning in fruit paper production.
Archives of environmental health, 26:70-74.
Haworth S, Lawlor T, Mortelmans K, Speck W, Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals.
Environmental mutagenesis, 5 (Suppl. 1):3-142.
Hawthorne SB, Miller DJ, Langenfeld JJ, Krieger MS (1992) PM-10
high-volume collection and quantitation of semi- and nonvolatile
phenols, methoxylated phenols, alkanes, and polycyclic aromatic
hydrocarbons from winter urban air and their relationship to wood
smoke emissions. Environmental science and technology, 26:2251-2262.
Hites RA (1973) Analysis of trace organic compounds in New England
rivers. Journal of chromatographic science, 11:570-574.
Hook GER, Bend JR (1976) Pulmonary metabolism of xenobiotics. Life
sciences, 18:279-290.
Hook GER, Bend JR, Hoel D, Fouts JR, Gram TE (1972) Preparation of
lung microsomes and a comparison of the distribution of enzymes
between subcellular fractions of rabbit lung and liver. Journal
of pharmacology and experimental therapeutics, 182:474-490.
Hulzebos EM, Adema DMM, Dirven-van Breemen EM, Henzen L, van Dis HA,
Herbold HA, Hoekstra JA, Baerselman R, van Gestel CAM (1993)
Phytotoxicity with Lactuca sativa in soil and nutrient solution.
Environmental toxicology and chemistry, 12:1079-1094.
IARC (1985) Polynuclear aromatic compounds. Part 4. Bitumens,
coal-tars and derived products, shale-oils and soots. Lyon,
International Agency for Research on Cancer, pp. 83-159 (IARC
Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to
Humans, Vol. 35).
Innes JRM, Ulland BM, Valerio MG, Petrucelli L, Fishbein L, Hart ER,
Pallotta AJ, Bates RR, Falk HL, Gart JJ, Klein M, Mitchell I, Peters J
(1969) Bioassay of pesticides and industrial chemicals for
tumorigenicity in mice: a preliminary note. Journal of the National
Cancer Institute, 42:1101-1114.
IPCS (1993) International Chemical Safety Card -- Biphenyl. Geneva,
World Health Organization, International Programme on Chemical Safety
(ICSC 0106).
IRPTC (1994) IRPTC legal and treatment and disposal methods for
waste chemical files: Biphenyl (CAS-No. 92-52-4) and
polychlorinated biphenyls (CAS-No. 1336-36-3). Geneva, United
Nations Environment Programme, International Register of Potentially
Toxic Chemicals.
Ishidate M, Odashima S (1977) Chromosome tests with 134 compounds on
Chinese hamster cells in vitro -- a screening for chemical
carcinogens. Mutation research, 48:337-354.
Ishidate M, Sofuni T, Yoshikawa K, Hayashi M, Nohmi T, Sawada M,
Matsuoka A (1984) Primary mutagenicity screening of food additives
currently used in Japan. Food and chemical toxicology, 22:623-636.
Isshiki K, Tsumura S, Watanabe T (1982) Post-harvest fungicides in
edible parts of citrus fruits. Agricultural and biological
chemistry, 46:993-999.
Japan Bioassay Research Center (1996) Two year feeding study of
biphenyl in rats and mice. Tokyo, National Institute of Health
Sciences (unpublished report).
Jeng CY, Chen DH, Yaws DL (1992) Data compilation for soil sorption
coefficient. Pollution engineering, 15:54-60.
JMPR (1967) 1967 evaluations of some pesticide residues in food.
Joint meeting of the FAO working party of experts and the WHO expert
committee on pesticide residues, Rome, 4-11 December 1967. Rome, World
Health Organization.
Karanassios IG, Georakilas V, Lahaniatis ES, Pilidis GA (1994)
Determination of airborne aromatic and polyaromatic hydrocarbons in
two towns of north-western Greece. Fresenius environmental
bulletin, 3:511-516.
Kawachi T, Yahagi T, Kada T, Tazima Y, Ishidate M, Sasaki M, Sugiyama
T (1980) Cooperative programme on short-term assays for
carcinogenicity in Japan. In: Montesano R, Bartsch H, Tomatis L, eds.
Molecular and cellular aspects of carcinogen screening tests. Lyon,
International Agency for Research on Cancer, pp. 323-330 (IARC
Scientific Publications No. 27).
Khera KS, Whalen C, Angers G, Trivett G (1979) Assessment of the
teratogenic potential of piperonyl butoxide, biphenyl, and phosalone
in the rat. Toxicology and applied pharmacology, 47:353-358.
Kirton P, Ellis J, Crisp PT (1991) The analysis of organic matter in
coke oven emissions. Fuel, 70:1383-1389.
Kishi H, Hasimoto Y (1991) Contribution of soil constituents to the
adsorption of chemicals. In: Arend F, Hinsenveld M, Van den Brink WJ,
eds. Altlastensanierung 90, 3rd KfK/TNO Kongress. Bundesministerium
Forschung Technologie, pp. 387-393.
Kiyohara H, Takizawa N, Nagao K (1992) Natural distribution of
bacteria metabolizing many kinds of polycyclic aromatic hydrocarbons.
Journal of fermentation and bioengineering, 74:49-51.
Kostiainen R (1995) Volatile organic compounds in the indoor air of
normal and sick houses. Atmospheric environment, 29:693-702.
Kotzias D, Geyer H, Viswanathan R, Kraus A, Freitag D, Klein W, Korte
F (1980) An approach to the ecotoxicological evaluation of
environmental chemicals. In: Kovatsis A, ed. Toxicological aspects.
9th International Congress of the European Association of Poison
Control Centers and the European Meeting of the International
Association of Forensic Toxicologists, Thessaloniki, 24-27 August
1980, pp. 257-268.
Kurata Y, Asamoto M, Hagiwara A, Masui T, Fukushima S (1986) Promoting
effects of various agents in rat urinary bladder carcinogenesis
initiated by N-butyl- N(4-hydroxybutyl)nitrosamine. Cancer
letters, 32:125-135.
Leach JM, Otson R, Armstrong V (1987) Airborne contaminants in two
small Canadian coal liquefaction pilot plants. American Industrial
Hygiene Association Journal, 48:693-697.
LWA (1993-94) Gewaesserguetebericht '93/'94. Nordrhein-Westfalen,
Landesamt fuer Wasser und Abfall.
Macintosh FC (1945) The toxicity of diphenyl and o-phenyl-phenol.
Analyst, 70:334-335.
Mackay D, Shiu WY, Ma KC (1992) Illustrated handbook of
physical-chemical properties and environmental fate for organic
chemicals. Vol. II. Polynuclear aromatic hydrocarbons,
polychlorinated dioxins, and dibenzofurans. Boca Raton, FL, Lewis
Publishers.
Malins DC, Krahn MM, Brown DW, Rhodes LD, Myers MS, McCain BB, Chan S
(1985) Toxic chemicals in marine sediment and biota from Mukilteo,
Washington: Relationships with hepatic neoplasms and other hepatic
lesions in English sole (Parophrys vetulus). Journal of the
National Cancer Institute, 74:487-494.
Matsubara T, Prough RA, Burke MD, Estabrook RW (1974) The preparation
of microsomal fractions of rodent respiratory tract and their
characterization. Cancer research, 34:2196-2203.
Meyer T (1977) The metabolism of biphenyl. IV. Phenolic metabolites in
the guinea pig and the rabbit. Acta Pharmacologica et Toxicologica,
40:193-200.
Meyer T, Scheline RR (1976) The metabolism of biphenyl. II. Phenolic
metobolites in the rat. Acta Pharmacologica et Toxicologica,
39:419-432.
Meyer T, Larsen JC, Hansen EV, Scheline RR (1976a) The metabolism of
biphenyl. III. Phenolic metobolites in the pig. Acta Pharmacologica
et Toxicologica, 39:433-441.
Meyer T, Aarbakke J, Scheline RR (1976b) The metabolism of biphenyl.
I. Metabolic disposition of 14C-biphenyl in the rat. Acta
Pharmacologica et Toxicologica, 39:412-418.
Monsanto Co. (1959) Animal inhalation study on biphenyl at 80°F and
100°F (EPA Document I.D.: 878213571, received 1983) [cited in BUA,
1994].
Newell GW (1953) A toxicological study of diphenyl in citrus wraps
(Stanford Research Institute Report No. B 326) [cited in Monsanto Co.
(1996) Toxicological data on biphenyl].
Nielsen PH, Christensen TH (1994) Variability of biological
degradation of aromatic hydrocarbons in an aerobic aquifer determined
by laboratory batch experiments. Journal of contaminant hydrology,
15:305-320.
NTIS (1968) Evaluation of carcinogenic, teratogenic and mutagenic
activities of selected pesticides and industrial chemicals (National
Technical Information Service PB-223159, August 1968) [cited in BUA,
1990].
NTP (1980) Annual plan for fiscal year 1981. Research Triangle Park,
NC, US Department of Health and Human Services, National Toxicology
Program, p. 32.
Otson R, Fellin R, Tran Q (1994) VOCs in representative Canadian
residences. Atmospheric environment, 28:3563-3569.
Paasivirta J, Kaeaeriaeinen H, Lahtiperae M, Pellinen J, Sinkkonen S
(1982) Oil residues in Baltic sediment, mussel and fish. II. Study of
the Finnish Archipelago 1979-81. Chemosphere, 11:811-821.
Pagano G, Esposito A, Giordano GG, Vamvakinos E, Quinto I, Bronzetti
G, Bauer C, Corsi C, Nieri R, Ciajolo A (1983) Genotoxicity and
teratogenicity of diphenyl and diphenyl ether: a study of sea urchins,
yeast, and Salmonella typhimurium. Teratogenesis, carcinogenesis,
and mutagenesis, 3:377-393.
Pagano G, Cipollaro M, Corsale G, Della Morte R, Esposito A, Giordano
GG, Micallo G, Quinto I, Staiano N (1988) Comparative toxicity of
diphenyl, diphenyl ester, and some of their hydroxy derivatives.
Médecine Biologie Environnement, 16:291-297.
Paschke A, Herbel W, Steinhart H (1992) Determination of mono- to
tetracyclic aromatic hydrocarbons in lubricating oil. Journal of
high resolution chromatography, 15:827-833.
Penttilae P-L, Siivinen K (1996) Control and intake of pesticide
residues during 1981-1993 in Finland. Food additives and
contaminants, 13:609-622.
Powis G, Moore DJ, Wilke TJ, Santone KS (1987) A high-performance
liquid chromatography assay for measuring integrated biphenyl
metabolism by intact cells: its use with rat liver; human liver and
kidney. Analytical biochemistry, 167:191-198.
Probst GS, McMahon RE, Hill LE, Thompson CZ, Epp JK, Neal SB (1981)
Chemically-induced unscheduled DNA synthesis in primary rat hepatocyte
cultures: a comparison with bacterial mutagenicity using 218
compounds. Environmental mutagenesis, 3:11-32.
Prough RA, Burke MD (1975) The role of NADPH-cytochrome c reductase in
microsomal hydroxylation reactions. Archives of biochemistry and
biophysics, 170:160-168.
Purchase IFH, Longstaff E, Ashby J, Styles JA, Anderson D, Lefevre PA,
Westwood FR (1978) An evaluation of 6 short-term tests for detecting
organic chemical carcinogens. British journal of cancer, 37:873-959.
Selle WA (1952) Progress reports on the effects of topical
application of diphenyl (November 6, 1952, July 23, 1953, February
23, 1954) [cited in Verwendung von Diphenyl, Orthophenylphenol
und dessen Natriumverbindung zur Oberflaechenbehandlung von
Zitrusfruechten. Bundesgesundheitsblatt 11 (dated 26 May 1967), pp.
168-173].
Shibata MA, Yamada M, Tanaka H, Kagawa M, Fukushima S (1989a) Changes
in urine composition, bladder epithelial morphology, and DNA synthesis
in male F344 rats in response to ingestion of bladder tumor promoters.
Toxicology and applied pharmacology, 99:37-49.
Shibata MA, Tanaka H, Yamada M, Tamano S, Fukushima S (1989b)
Proliferative response of renal pelvic epithelium in rats to oral
administration of ortho-phenylphenol sodium ortho-phenylphenate and
diphenyl. Cancer letters, 48:19-28.
Shiraiwa K, Takita M, Tsutsumi M, Kinugasa T, Denda A, Takahashi S,
Konishi Y (1989) Diphenyl induces urolithiasis but does not possess
the ability to promote carcinogenesis by
N-ethyl- N-hydroxyethylnitrosamine in kidneys of rats. Journal
of toxicologic pathology, 2:41-48.
Siegl WO, Chladek E (1992) Measurement of gas-phase polycyclic
aromatic hydrocarbons (PAH) in gasoline vehicle exhaust. American
Chemical Society Division of Petroleum Chemistry, 4:1499-1504.
Snyder RD, Matheson DW (1985) Nick translation -- a new assay for
monitoring DNA damage and repair in cultured human fibroblasts.
Environmental mutagenesis, 7:267-279.
Sofuni T, Hayashi M, Matsuoka A, Sawada M, Hatanaka M, Ishidate M
(1985) Mutagenicity tests on organic chemical contaminants in city
water and related compounds. II. Chromosome aberration tests in
cultured mammalian cells. Eisei Shikensho Hokoku, 103:64-75.
Sondergaard D, Blom L (1979) Polycystic changes in rat kidney induced
by biphenyl fed in different diets. Archives for toxicology, Suppl.
2:499-502.
Stanford Research Institute (undated) Final report -- a
toxicological study of diphenyl in citrus wraps. Menlo Park, CA
[cited in US EPA (1984) Health and environmental effects profile
for 1,1'-biphenyl. Cincinnati, OH, US Environmental Protection
Agency].
Sun Co. Inc. (1977a) Acute inhalation toxicity of biphenyl -- with
cover letter (EPA Document I.D.: 878213530, received 1983) [cited in
BUA, 1994].
Sun Co. Inc. (1977b) Subacute inhalation toxicity of biphenyl (EPA
Document I.D.: 878213531, received 1983) [cited in BUA, 1994].
Sun Co. Inc. (1977c) 90-day inhalation toxicity study of biphenyl
(99+% purity) in CD mice (EPA Document I.D.: 878213532, received
1983) [cited in BUA, 1994].
Takita M (1983) Urolithiasis induced by oral administration of
diphenyl in rats. Journal of the Nara (Medical University) Medical
Association, 34:565-584.
Tamano S, Asakawa E, Boomyaphiphat P, Masui T, Fukushima S (1993) Lack
of promotion of N-butyl- N-(4-hydroxybutyl)nitrosamine-initiated
urinary bladder carcinogenesis in mice by rat cancer promotors.
Teratogenesis, carcinogenesis, and mutagenesis, 13:89-96.
Tanaka A, Morimoto K, Yamaha T (1993) Small scale synthesis of labeled
diphenyl and its binding to mouse liver microsomes. Radio isotopes,
42:564-568.
Wangenheim J, Bolcsfoldi G (1988) Mouse lymphoma L5178Y thymidine
kinase locus assay of 50 compounds. Mutagenesis, 3:193-205.
Waters MD, Sandhu SS, Simmon VF, Mortelmans KE, Mitchell AD, Jorgenson
TA, Jones DCL, Valencia R, Garrett NE (1982) Study of pesticide
genotoxicity. Basic life sciences, 21:275-326.
Weil E, Kusterer L, Brogard MH (1965) Archives des Maladies
Professionnelles, 26:405 [cited in Henschler D (1979)
Toxikologischarbeitsmedizinische Begruendungen von MAK-Werten.
Weinheim, VCH VerlagsGmbH].
Wienecke J, Kruse H, Wassermann O (1992) Organic compounds in the flue
gas from a power station with circulating fluid bed combustion of
coal. Chemosphere, 25:1889-1895.
Wilkins CK, Wolkoff P, Gyntelberg F, Skov P, Valbjoern O (1993)
Characterization of office dust by VOCs and TVOC release --
identification of potential irritant VOCs by partial least squares
analysis. Indoor air, 3:283-290.
Williams GM (1978) Further improvements in the hepatocyte primary
culture DNA repair test for carcinogens: Detection of carcinogenic
biphenyl derivatives. Cancer letters, 4:69-75.
Windeatt AJ, Tapp JF, Stanley RD (1991) The use of soil-based tests
based on the OECD guidelines. In: Gorsuch JW, Lower WR, Wang W, Lewis
MA, eds. Plants for toxicity assessment. Vol. 2. Philadelphia, PA,
American Society for Testing and Materials, pp. 29-40 (ASTM Special
Technical Publication 1115).
Yao W, Xu X (1991) Determination of pollutants emitted from burning
coal and of their mutagenicities. Analytical science, 7:1381-1384.
Zhang Y, Rott B, Freitag D (1983) Accumulation and elimination of
14-C-PCB's by Daphnia magna Straus 1820. Chemosphere,
12:1645-1651.
Zimmermann FK, von Borstel RC, von Halle ES, Parry JM, Siebert D,
Zetterberg G, Barale R, Loprieno N (1984) Testing of chemicals for
genetic activity with Saccharomyces cerevisiae: a report of the U.S.
Environmental Protection Agency Gene-Tox Program. Mutation
research, 133:199-244.
APPENDIX 1 -- SOURCE DOCUMENTS
BUA-Stoffbericht Biphenyl (1,1'-Biphenyl). Beratergremium fuer
Umweltrelevante Altstoffe (Report No. 50; July 1990). VCH VerlagsGmbH,
Weinheim
BUA-Stoffbericht 133 (Ergaenzungsberichte II). Beratergremium fuer
Umweltrelevante Altstoffe (1994). VCH VerlagsGmbH, Weinheim
For the BUA review process, the company that is in charge of
writing the report (usually the largest producer in Germany) prepares
a draft report using literature from an extensive literature search as
well as internal company studies. This draft is subject to a peer
review during several readings of a working group consisting of
representatives from government agencies, the scientific community,
and industry.
The English translation of BUA Report No. 50 (BUA Report Biphenyl
(1,1'-Biphenyl). GDCh-Advisory Committee on Existing Chemicals of
Environmental Relevance (July 1990). VCH VerlagsGmbH, Weinheim) was
released in 1992. The English translation of its Supplementary Report
No. 133 is in preparation.
APPENDIX 2 -- CICAD PEER REVIEW
The draft CICAD on biphenyl was sent for review to institutions
and organizations identified by IPCS after contact with IPCS national
Contact Points and Participating Institutions, as well as to
identified experts. Comments were received from:
Bayer AG, Leverkusen, Germany
Department of Health, London, United Kingdom
Department of Public Health, Albert Szent-Gyorgyi University Medical
School, Szeged, Hungary
Environmental Health Directorate, Health Canada, Ottawa, Canada
International Agency for Research on Cancer, Lyon, France
Ministry of Health and Welfare, International Affairs Division,
Government of Japan, Tokyo, Japan
Monsanto Europe SA, Louvain-La-Neuve, Belgium
National Institute for Working Life, Solna, Sweden
National Institute of Occupational Health, Budapest, Hungary
United States Department of Health and Human Services (National
Institute of Environmental Health Sciences)
United States Environmental Protection Agency (Office of Pollution
Prevention and Toxics; Office of Drinking Water)
APPENDIX 3 -- CICAD FINAL REVIEW BOARD (Brussels, Belgium)
Brussels, Belgium, 18-20 November 1996
Members
Dr A. Aitio, Institute of Occupational Health, Helsinki, Finland
Dr K. Bentley, Director, Environment Policy Section, Commonwealth
Department of Human Services and Health, Canberra, Australia
Mr R. Cary, Toxicology and Existing Substances Regulation Unit, Health
and Safety Executive, Merseyside, United Kingdom
Dr J. de Fouw, National Institute of Public Health and Environmental
Protection, Bilthoven, The Netherlands
Dr C. DeRosa, Director, Division of Toxicology, Agency for Toxic
Substances and Disease Registry, Atlanta, GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr W. Farland, Director, National Center for Environmental Assessment,
Office of Research and Development, US Environmental Protection
Agency, Washington, DC, USA (Chairperson)
Dr T.I. Fortoul, Depto. Biologia Celular y Tisular, National
University of Mexico and Environmental Health Directorate of the
Health Ministry, Mexico D.F., Mexico
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Mr J.R. Hickman, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Dr T. Lakhanisky, Head, Division of Toxicology, Institute of Hygiene
and Epidemiology, Brussels, Belgium (Vice-Chairperson)
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany
Ms M.E. Meek, Head, Priority Substances Section, Environmental Health
Directorate, Health Canada, Ottawa, Ontario, Canada
Dr K. Paksy, National Institute of Occupational Health, Budapest,
Hungary
Mr D. Renshaw, Department of Health, London, United Kingdom
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan
Dr H. Sterzl-Eckert, GSF-Forschungszentrum für Umwelt und Gesundheit
GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Professor S. Tarkowski, Department of Environmental Health Hazards,
The Nofer Institute of Occupational Medicine, Lodz, Poland
Dr M. Wallen, National Chemicals Inspectorate (KEMI), Solna, Sweden
Observers
Professor F.M.C. Carpanini,1 Director, Centre for Ecotoxicology and
Toxicology of Chemicals (ECETOC), Brussels, Belgium
Mr R. Haigh,1 Head of Unit, Health and Safety Directorate, European
Commission, Luxembourg
Mr B.U. Hildebrandt, Federal Ministry for the Environment, Nature
Conservation and Nuclear Safety, Bonn, Germany
Mr P. Hurst,1 Chemical and Consumer Policy Officer, Conservation
Policy Division, World Wide Fund for Nature, Gland, Switzerland
Dr A. Lombard (CEFIC representative), ELF-ATOCHEM, Paris, France
Dr P. McCutcheon,1 Environment, Consumer Protection and Nuclear
Safety, European Commission, Brussels, Belgium
Dr R. Montaigne, Counsellor, Technical Affairs Department, European
Chemical Industry Council (CEFIC), Brussels, Belgium
Dr M. Pemberton, ICI Acrylics, Lancashire, United Kingdom
Dr A. Smith, Environment Division, Organisation for Economic
Co-operation and Development, Paris, France
Secretariat
Dr M. Baril, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr L. Harrison, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M. Mercier, Director, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr P. Toft, Associate Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
1 Invited but unable to attend.
APPENDIX 4 -- SPECIALIZED CICAD PEER REVIEW
Individuals contributing written comments to the specialized
review of this CICAD were:
Dr S. Cohen, University of Nebraska Medical Center, Omaha, USA
Ms M.E. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Canada
Mr D. Renshaw, Department of Health, London, United Kingdom
Dr J. Rice, Unit of Carcinogen Identification and Evaluation,
International Agency for Research on Cancer, Lyon, France
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan
Dr D. Singh, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Individuals participating in the review of the human health
assessment (section 11.1) were:
Dr A. Chiu, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr C. Hiremath, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr R.G. Liteplo, Environmental Health Directorate, Health Canada,
Ottawa, Canada
Dr D. Singh, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
APPENDIX 5 -- CICAD FINAL REVIEW BOARD (Washington, DC)
Washington, DC (USA), 8-11 December 1998
Members
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
Mr R. Cary, Toxicology Unit, Health Directorate, Health and Safety
Executive, Bootle, Merseyside, United Kingdom
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr O. Faroon, Agency for Toxic Substances and Disease Registry,
Centers for Disease Control and Prevention, Atlanta, GA, USA
Dr G. Foureman, National Center for Environmental Assessment, US
Environmental Protection Agency, Research Triangle Park, NC, USA
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany
Dr A. Nishikawa, Division of Pathology, National Institute of Health
Sciences, Tokyo, Japan
Dr E.V. Ohanian, Office of Water/Office of Science and Technology, US
Environmental Protection Agency, Washington, DC, USA
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan
Professor P. Yao, Institute of Occupational Medicine, Chinese Academy
of Preventive Medicine, Beijing, People's Republic of China
Observers
Dr K. Austin, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr I. Daly (ICCA representative), Regulatory and Technical Associates,
Lebanon, PA, USA
Ms K.L. Lang (CEFIC representative), Shell International, London,
United Kingdom
Ms K. Roberts (ICCA representative), Chemical Self-funded Technical
Advocacy and Research (CHEMSTAR),
Chemical Manufacturers Association, Arlington, VA, USA
Dr W. Snellings (ICCA representative), Union Carbide Corporation,
Danbury, CN, USA
Dr M. Sweeney, Document Development Branch, National Institute for
Occupational Safety and Health, Cincinnati, OH, USA
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Secretariat
Dr A. Aitio,1 International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
1 Invited but unable to attend.
Dr M. Baril, Institut de recherche en santé et en sécurité du travail
du Québec, Montreal, Quebec, Canada
Dr H. Galal-Gorchev, Chevy Chase, MD, USA
Ms M. Godden, Health and Safety Executive, Bootle, Merseyside, United
Kingdom
Dr R.G. Liteplo, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Ms L. Regis, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Mr A. Strawson, Health and Safety Executive, London, United Kingdom
Dr P. Toft, Associate Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
Ce CICAD relatif au biphényle a été préparé par l'Institut
Fraunhofer de Toxicologie et de Recherche sur les Aérosols de Hanovre
(Allemagne), principalement sur la base d'une étude préparée par le
Comité consultatif allemand sur les substances chimiques importantes
pour l'environnement (BUA, 1990) et d'un rapport complémentaire (BUA,
1994) afin d'évaluer les effets potentiels du biphényle sur l'Homme et
l'environnement. On trouvera à l'appendice 1 des indications sur les
sources documentaires utilisées ainsi que sur leur mode de
dépouillement. Une étude bibliographique approfondie a été menée sur
plusieurs bases de données jusqu'en juin 1996 à la recherche de
données supplémentaires. Ce CICAD contient également les
renseignements complémentaires recueillis par les points de contact et
le Comité d'évaluation finale (Bruxelles, Belgique) lors du
dépouillement des documents. Les renseignements concernant l'examen du
CICAD par les pairs font l'objet de l'appendice 2. Ce CICAD a été
approuvé en tant qu'évaluation internationale lors d'une réunion du
Comité d'évaluation finale qui s'est tenue à Bruxelles (Belgique) du
18 au 20 novembre 1996. La liste des participants à cette réunion
figure à l'appendice 3. Après avoir été révisé pour y incorporer les
résultats d'une étude de cancérogénicité de 2 ans récemment achevée,
le document a été soumis à des pairs pour deux séries d'évaluations
écrites. En outre, la partie du document spécialement consacrée à
l'évaluation des effets sur la santé humaine (à savoir la section
11.1), a été examinée lors d'une réunion qui s'est tenue le 7 décembre
1998 à Washington (Etats-Unis), dans les locaux du National Center for
Environmental Assessment, US Environmental Protection Agency. La liste
des personnes qui ont participé aux évaluations spécialisées du
présent CICAD figure à l'appendice 4. Ce CICAD a été approuvé en tant
qu'évaluation internationale lors de la réunion du Comité d'évaluation
finale qui a eu lieu à Washington du 8 au 11 décembre 1998. La liste
des participants à cette réunion figure à l'appendice 5. La fiche
d'information internationale sur la sécurité chimique (ICSC 0106)
établie par le Programme international sur la sécurité chimique (IPCS,
1993) est également reproduite dans ce document.
Le biphényle (CAS N° 92-52-4) est un hydro carbure aromatique qui
se présente à la température ambiante sous la forme d'un solide
cristallin incolore. Il est utilisé comme intermédiaire dans la
production de divers composés (par exemple, émulsifiants, azurants
optiques, produits pour la protection des cultures, matières
plastiques), comme fluide caloporteur, comme support de colorants pour
textiles et papier à photocopie, comme solvant dans l'industrie
pharmaceutique ou encore pour la conservation des agrumes.
Le biphényle existe à l'état naturel dans le goudron de houille,
le pétrole brut et le gaz naturel. Parmi les sources artificielles de
pollution par le biphényle figurent les unités qui produisent ou
utilisent ce composé, les installations où l'on traite des agrumes ou
des bois ou encore les décharges municipales. Il se forme lors de la
combustion incomplète des huiles minérales et de la houille et on en
trouve dans les gaz d'échappement des véhicules à moteur et dans l'air
libéré dans l'atmosphère par les appareils de chauffage des
installations industrielles ou des immeubles résidentiels. Des valeurs
de 1 à 100 ng/m3 sont caractéristiques de la concentration dans l'air
ambiant. A l'intérieur des bâtiments, elles sont plus élevées
(100-1000 ng/m3), probablement par suite de la présence de fumée de
cigarette, d'émissions provenant des appareils de chauffage et de
garages situés dans le voisinage. Des mesures effectuées dans les
années 70 ont donné des valeurs généralement inférieures à 5 ng/litre
pour l'eau du robinet. On n'a pas connaissance de valeurs plus
récentes. Dans les eaux superficielles, la concentration usuelle est
inférieure à 500 ng/litre. Dans les sédiments, le sol et les êtres
vivants, on n'a procédé à des dosages de biphényle qu'à proximité
immédiate d'installations industrielles et de décharges.
Peu soluble dans l'eau, le biphényle s'évapore de ses solutions
aqueuses. Sa principale voie de décomposition troposphérique est due à
l'action des radicaux hydroxyles et la demi-vie calculée dans ces
conditions est de l'ordre de 2 jours en moyenne. Le biphényle ne
devrait pas s'hydrolyser dans les conditions qui règnent dans
l'environnement. Il est biodégradable en aérobiose. D'après les
données disponibles, il devrait être quasiment immobile dans le sol;
sa probabilité d'infiltration dans les eaux souterraines est faible.
Dans la chaîne alimentaire qui joue un rôle important pour
l'alimentation humaine, il peut y avoir accumulation du biphényle,
notamment dans les plantes. Toutefois, compte tenu de son potentiel de
bioaccumulation, il ne semble pas qu'il puisse subir une
bioamplification importante aux niveaux trophiques supérieurs de la
chaîne alimentaire aquatique ou terrestre.
Le biphényle est bien absorbé dans les voies digestives et
probablement aussi par la voie pulmonaire et percutanée. Chez les
espèces étudiées, les métabolites du biphényle, principalement le
4-hydroxybiphényle, sont rapidement excrétés et presque exclusivement
par la voie urinaire. Le biphényle est modérément toxique par voie
buccale. Il n'est pas irritant pour la peau et n'irrite que très
légèrement la muqueuse oculaire. Rien n'indique qu'il puisse provoquer
une sensibilisation cutanée. Les études toxicologiques au cours
desquels on a soumis les animaux de laboratoire à des expositions
répétées au biphényle, ne permettent pas d'établir la valeur de la
dose sans effet observable (NOEL) avec un bon niveau de confiance. Une
exposition subchronique par la voie respiratoire a provoqué
l'apparition d'anomalies bronchopulmonaires, mais la littérature ne
mentionne pas d'études toxicologiques de longue durée comportant une
exposition par cette voie.
Les études toxicologiques comportant l'administration, pendant
diverses périodes, de nourriture additionnée de biphényle à des
rongeurs, font souvent mention d'effets aux niveau des voies
urinaires. Chez des rats mâles ayant reçu une alimentation contenant
plus de 2500 mg de biphényle par kg, on a observé une augmentation
sensible dans l'incidence des effets morphologiques (par ex. formation
de calculs) et histopathologiques (par ex. hyperplasie, desquamation)
au niveau des voies urinaires. On a également observé une augmentation
des calculs et des cas de métaplasie spino-cellulaire vésicale chez
des rattes, mais dans une proportion moindre que chez les mâles. Chez
des souris mâles soumises pendant 32 semaines à un régime alimentaire
comportant 10 000 mg de biphényle par kg (1500 mg par kg de poids
corporel), on a observé une simple hyperplasie et une dysplasie
papillomateuse ou nodulaire de la vessie, mais chez seulement un
animal sur dix. Sur des animaux à qui on avait fait ingérer du
biphényle, on a également observé des effets hématologiques
(biochimiques et autres); ces effets peuvent être constatés chez des
rats ou des souris mâles et femelles à des doses inférieures à celles
qui produisent des effets sur la vessie des rats mâles. En ce qui
concerne les effets non néoplasiques, la dose la plus faible
produisant un effet observable (LOEL) se situait à 38 mg/kg p.c. par
jour, le critère retenu étant l'apparition d'anomalies hématologiques
(diminution du taux d'hémoglobine et de l'hématocrite) chez des rats
ayant reçu pendant 2 ans une nourriture contenant respectivement 0,
500, 1500 ou 4500 mg/kg de biphényle (les doses indiquées étant égales
à 0, 38, 113 ou 338 mg/kg p.c.).
Les études in vitro sur des bactéries n'ont pas mis en évidence
d'activité mutagène. On a observé des mutations et des recombinaisons
mitotiques chez Saccharomyces cerevisiae D7 avec ou sans activation
métabolique. Les tests de génotoxicité sur cellules mammaliennes ont
toutefois donné des résultats positifs en présence d'activation
métabolique et des résultats négatifs en l'absence d'une telle
activation. Une étude in vivo insuffisamment documentée et dont
l'exécution laisse à désirer, n'a pas permis de constater la présence
d'aberrations chromosomiques dans la moelle osseuse de rats exposés à
du biphényle par la voie respiratoire. Les résultats d'autres études
in vitro indiquent que le biphényle possède un pouvoir mutagène;
dans ces conditions et devant le manque de fiabilité des résultats
fournis par les études in vivo, on estime que le biphényle comporte
un risque mutagène.
Chez des souris femelles, on a constaté une légère augmentation
de l'incidence des tumeurs bénignes ou malignes du foie lorsque les
animaux avaient reçu pendant 2 ans du biphényle mêlé à leur
nourriture. Une augmentation de l'incidence des tumeurs vésicales a
été observée chez des rats mâles, mais ni chez les femelles ni chez
des souris des deux sexes, après administration d'une nourriture
contenant une grande quantité de biphényle. On a avancé que ces
tumeurs pouvaient être dues à l'hyperplasie régénérative de
l'épithélium vésical provoquée par l'abrasion et les lésions que
produisent les calculs, lesquels ne se forment que si l'exposition au
biphényle est suffisamment intense. On pense également qu'en raison de
différences anatomiques et physiologiques, ces tumeurs vésicales
observées à forte doses chez des rats mâles et qui présentent une
spécificité de sexe et d'espèce, n'ont guère de signification pour des
sujets humains exposés à des doses plus faibles. Quoi qu'il en soit,
1) l'observation d'un accroissement de l'incidence des effets
histopathologiques et la formation de calculs au niveau de la vessie,
en l'absence de tumeurs vésicales , chez des rattes ayant reçu du
biphényle pendant 2 ans, 2) l'absence de données permettant de mettre
en évidence une association directe entre cette lithiase vésicale,
l'hyperplasie régénérative de l'urothélium et l'apparition de tumeurs
vésicales chez les rats mâles et 3) la génotoxicité potentielle du
biphényle, incitent à penser que la formation de tumeurs vésicales
chez les rats mâles n'est peut-être pas entièrement due à l'action des
calculs. Cette observation, de même que les signes d'hépatotoxicité
relevés chez les souris femelles, font craindre que le biphényle ne
comporte un risque de cancérogénicité.
Les données relatives à la toxicité génésique du biphényle sont
limitées. A part une étude portant sur trois générations de rats, qui
a mis en évidence des effets indésirables (réduction de la fécondité,
de la taille des portées et du taux de croissance), rien ne prouve que
le biphényle puisse avoir des effets sur la reproduction ou le
développement.
L'exposition, sur les lieux de travail, à des vapeurs ou à des
poussières à forte teneur en biphényle entraîne une irritation des
yeux et une inflammation des voies respiratoires. Une exposition
pendant plusieurs années à de fortes concentrations de biphényle
(jusqu'à 128 mg/m3) a provoqué des lésions hépatiques et les
anomalies neuronales permanentes; il est possible que dans ce cas,
l'effet du contact cutané avec le composé se soit ajouté à celui de
son absorption par la voie respiratoire.
On ne possède qu'un nombre limité de données sur la concentration
du biphényle dans l'environnement et les renseignements qu'on peut en
tirer sont insuffisants pour permettre une estimation raisonnable de
la dose de composé absorbée. Il peut y avoir exposition par inhalation
(inhalation d'air pollué et utilisation de créosote contenant du
biphényle pour la préservation du bois) ou par ingestion (lorsque le
biphényle est utilisé comme conservateur d'agrumes).
Le biphényle est faiblement bactéricide et fongistatique. Lors
d'études toxicologiques sur des organismes aquatiques représentant
quatre niveaux trophiques, la concentration la plus faible sans effet
observable (NOEC) pour l'espèce la plus sensible (Daphnia magna) a
été trouvée égale 0,17 mg/litre lors de tests de longue durée. Le
calcul donne une concentration sans effet prévisible (PNEC) de 1,7
µg/litre en utilisant un facteur d'évaluation de 10, compte tenu des
caractéristiques écotoxicologiques et du devenir environnemental du
composé. On ne dispose pas, au sujet des effets toxiques du biphényle
sur les organismes terrestres et les écosystèmes, de données valables
permettant une évaluation du risque.
En raison de l'absence de données émanant d'autres régions du
monde, l'évaluation du risque qui a été effectuée concerne
spécifiquement l'Allemagne. A partir des concentrations mesurées dans
les cours d'eau allemands au cours des années 90, on a obtenu un
rapport de la concentration environnementale prévue (PEC) à la
concentration sans effet prévisible (PNEC) qui est égal à <0,29. Un
rapport PEC/PNEC <1 signifie qu'il n'y a vraisemblablement pas de
risque important pour l'environnement.
RESUMEN DE ORIENTACION
El presente CICAD, preparado por el Instituto Fraunhofer de
Toxicología y de Investigación sobre los Aerosoles de Hannover,
Alemania, se basa fundamentalmente en un examen preparado por el
Comité Consultivo Alemán sobre las Sustancias Químicas Importantes
para el Medio Ambiente (BUA, 1990), así como en un informe
complementario (BUA, 1994) para evaluar los efectos potenciales del
bifenilo en el ser humano y en el medio ambiente. En el apéndice 1
figuran los documentos originales y una descripción de sus procesos de
examen. Se realizó asimismo una búsqueda bibliografía amplia en varias
bases de datos en línea en junio de 1996 para identificar datos
adicionales. También se ha incorporado a este CICAD nueva información
adicional obtenida durante el examen efectuado por los servicios de
información y por la Junta de Evaluación Final (Bruselas, Bélgica). En
el apéndice 2 se presenta información relativa al examen colegiado de
este CICAD. La Junta de Evaluación Final lo aprobó provisionalmente
como evaluación internacional en una reunión celebrada en Bruselas
(Bélgica) los días 18-20 de noviembre de 1996. En el apéndice 3 figura
la lista de participantes en dicha reunión. Tras la incorporación de
los datos relativos a la carcinogenicidad de esta sustancia obtenidos
de una biovaloración de la carcinogenicidad de dos años de duración
completada recientemente, el documento revisado se sometió a dos
rondas de examen colegiado por escrito. Además, la parte del documento
en la que se aborda específicamente la evaluación de la salud humana
(es decir, la sección 11.1) se examinó en una reunión celebrada en el
Centro Nacional para la Evaluación del Medio Ambiente, Organismo para
la Protección del Medio Ambiente de los Estados Unidos, en Washington,
DC (Estados Unidos) el 7 de diciembre de 1998. El apéndice 4 contiene
la lista de quienes contribuyeron a los exámenes especializados
adicionales del presente CICAD. Este CICAD se aprobó como evaluación
internacional en una reunión de la Junta de Evaluación Final celebrada
en Washington, DC (Estados Unidos), los días 8-11 de diciembre de
1998. En el apéndice 5 figura la lista de participantes en esta
reunión. La Ficha internacional de seguridad química (ICSC 0106),
preparada por el Programa Internacional de Seguridad de las Sustancias
Químicas (IPCS, 1993), también se reproduce en el presente documento.
El bifenilo (CAS N° 92-52-4), un hidrocarburo aromático, es una
sustancia sólida incolora a temperatura ambiente. Se utiliza como
intermediario en la producción de diversos compuestos (por ejemplo,
emulsionantes, abrillantadores ópticos, productos para la protección
de los cultivos, plásticos), como medio de transferencia de calor en
fluidos térmicos, como colorante de textiles y papel para copias, como
disolvente en la producción farmacéutica y en la conservación de
cítricos.
El bifenilo se encuentra en la naturaleza en el alquitrán
mineral, el petróleo bruto y el gas natural. Las fuentes
antropogénicas de exposición en el medio ambiente incluyen las plantas
de producción y elaboración, las instalaciones de conservación de
cítricos o de madera y los vertederos municipales. Se forma bifenilo
durante la combustión incompleta del aceite mineral y el carbón y está
presente en los gases de escape de los vehículos y de las calderas de
calefacción de las viviendas y las industrias. En la atmósfera, las
concentraciones habituales de bifenilo oscilan entre 1 y 100 ng/m3;
en el aire de espacios cerrados los niveles son superiores (100-1000
ng/m3), probablemente debido al humo de los cigarrillos y a las
emisiones de las calderas de calefacción o de garajes cercanos. En las
mediciones realizadas en los años setenta, las concentraciones de
bifenilo en el agua de grifo eran normalmente inferiores a 5 ng/litro.
No se conocen datos más recientes. En el agua superficial, las
concentraciones suelen ser inferiores a 500 ng/litro. En los
sedimentos, el suelo y la biota, sólo se midió el bifenilo en las
cercanías de instalaciones industriales y vertederos.
El bifenilo se volatiliza de la solución acuosa y tiene una
solubilidad baja en agua. La principal vía de degradación en la
troposfera es la reacción con radicales hidroxilo, para la que se ha
calculado una semivida de unos dos días. No cabe prever que la
sustancia se hidrolice en las condiciones del medio ambiente. Es
biodegradable en condiciones aerobias. Según los datos disponibles, el
bifenilo debería mantenerse casi inmóvil en el suelo; la probabilidad
de infiltración hacia el agua freática es baja. En la cadena
alimentaria importante para el ser humano se puede producir
bioacumulación, especialmente en las plantas; sin embargo, teniendo en
cuenta la posible bioacumulación del bifenilo, cabe prever que su
bioamplificación en los niveles tróficos superiores de la cadena
alimentaria acuática o terrestre será de escasa importancia.
El bifenilo se absorbe bien a través del tracto gastrointestinal
y posiblemente también por vía pulmonar y cutánea. En las especies
examinadas, los metabolitos del bifenilo, sobre todo el
4-hidroxibifenilo, se excretan con rapidez y casi exclusivamente en la
orina. La toxicidad aguda del bifenilo por vía oral es moderada. No es
irritante de la piel y sólo ligeramente de los ojos. No hay pruebas de
sensibilización cutánea. Los estudios de toxicidad con bifenilo tras
la exposición repetida por inhalación no son adecuados para establecer
con confianza una concentración sin efectos observados (NOEL). La
exposición subcrónica por inhalación produjo cambios broncopulmonares,
pero no se han encontrado en la bibliografía estudios de toxicidad
prolongados tras la exposición por inhalación.
En estudios toxicológicos realizados con roedores que recibieron
alimentos con bifenilo durante diversos períodos de tiempo, se han
notificado con frecuencia efectos en el sistema urinario. Se ha
observado un aumento acentuado en la incidencia de efectos
morfológicos (es decir, formación de cálculos) e histopatológicos (por
ejemplo, hiperplasia, descamación) en el tracto urinario de ratas
macho a las que se suministraron alimentos con concentraciones de
bifenilo superiores a 2500 mg/kg. Se observó asimismo un aumento en la
aparición de cálculos y metaplasia escamosa en la vejiga urinaria de
ratas hembras, pero con una incidencia inferior a la registrada en los
machos. En ratones macho, sólo uno de 10 animales que recibieron
alimentos con 10 000 mg de bifenilo/kg (1500 mg/kg de peso corporal al
día) durante 32 semanas presentó hiperplasia simple y displasia
papilar o nodular en la vejiga urinaria. En animales que recibieron
bifenilo por vía oral se detectaron asimismo efectos en la química
sanguínea y los parámetros hematológicos; estos efectos se producen en
ratas y ratones machos y hembras con ingestas inferiores a las
asociadas con la aparición de efectos en la vejiga urinaria de las
ratas machos tratadas con bifenilo. Para los efectos no neoplásicos,
la concentración más baja de efectos observados (LOEL) fue de 38 mg/kg
de peso corporal al día, basada en la aparición de alteraciones en los
parámetros hematológicos (es decir, disminución de la concentración de
hemoglobina y del valor hematócrito) en ratas a las que se
suministraron alimentos que contenían 0, 500, 1500 ó 4500 mg de
bifenilo/kg (ingestas notificadas de 0, 38, 113 ó 338 mg/kg de peso
corporal al día) durante dos años.
En estudios in vitro con bacterias no se han obtenido pruebas
de potencial mutagénico del bifenilo; con Saccharomyces cerevisiae
D7, se observaron mutaciones genéticas y recombinación mitótica con
activación metabólica y sin ella. Sin embargo, en pruebas de
toxicología genética en células de mamíferos se obtuvieron resultados
positivos con activación metabólica y negativos en su ausencia. En un
estudio in vivo insuficientemente documentado y realizado de manera
inadecuada, el bifenilo no indujo aberraciones cromosómicas en la
médula ósea de ratas tras la exposición por inhalación. Los resultados
de los estudios in vitro ponen de manifiesto que el bifenilo tiene
potencial mutagénico; sin la garantía de resultados fidedignos de
pruebas in vivo, se supone que la exposición al bifenillo se puede
asociar con un riesgo mutagénico.
En ratones hembra, se produjo un ligero aumento de la incidencia
de tumores hepáticos benignos y malignos en animales que recibieron
bifenilo con los alimentos durante dos años. Se observó un aumento de
la incidencia de tumores de vejiga en ratas machos, pero no en las
ratas hembras o los ratones machos y hembras, que recibieron alimentos
con concentraciones elevadas de bifenilo. Se ha indicado que la
formación de dichos tumores de vejiga podría estar vinculada a la
hiperplasia regenerativa del epitelio urinario, debida a la abrasión y
las lesiones del urotelio producidas por los cálculos formados en el
tracto urinario sólo con niveles muy altos de exposición; se ha
señalado asimismo que, debido a las diferencias anatómicas y
fisiológicas, la aparición de tumores de vejiga específicos del sexo y
la especie en ratas macho que recibieron dosis elevadas de bifenilo
tal vez no sea pertinente para las personas expuestas a niveles más
bajos. Sin embargo, 1) las observaciones de una mayor incidencia de
efectos histopatológicos y la formación de cálculos en la vejiga
urinaria, en ausencia de tumores de vejiga, en ratas hembras tratadas
con bifenilo durante dos años, 2) la falta de datos que establezcan
una asociación directa entre la formación de cálculos, la hiperplasia
regenerativa del urotelio y la aparición de tumores de vejiga en
determinados animales machos, y 3) la genotoxicidad del bifenilo,
podrían indicar que la aparición de tumores de vejiga en ratas macho
tal vez no se deba completamente a los efectos asociados a la
formación de cálculos en la vejiga urinaria. Esta observación, así
como la prueba de hepatocarcinogenicidad en ratones hembras, despierta
alguna preocupación con respecto a la carcinogenicidad potencial del
bifenilo.
Los datos disponibles sobre la toxicidad reproductiva del
bifenilo son limitados. Si se exceptúan los resultados obtenidos en un
estudio de tres generaciones en ratas, en el que se observaron efectos
adversos (disminución de la fecundidad, del tamaño de la camada y de
la tasa de crecimiento), no se encontraron pruebas de que el bifenilo
indujera efectos reproductivos o en el desarrollo.
La exposición a concentraciones elevadas de vapores o polvo de
bifenilo en el lugar de trabajo produce irritación de los ojos e
inflamación del tracto respiratorio. La exposición prolongada durante
varios años a concentraciones elevadas de bifenilo (hasta 128 mg/m3)
provo có lesiones hepáticas y cambios neuronales persistentes; el
contacto directo con la piel puede haber influido, además de la
absorción a través del tracto respiratorio.
Los datos disponibles sobre las concentraciones de bifenilo en el
medio ambiente general son limitados y la información es insuficiente
para permitir una estimación razonable de su ingesta. La inhalación
(del aire contaminado y por el uso de creosotas con bifenilos en la
conservación de la madera) y la ingestión (debido a su uso como
conservante de cítricos) pueden ser fuentes de exposición.
El bifenilo tiene propiedades bactericidas y fungicidas débiles.
En estudios de toxicidad sobre organismos acuáticos de cuatro niveles
tróficos, la concentración más baja sin efectos observados (NOEC)
notificada para la especie más sensible (Daphnia magna) en pruebas
crónicas fue de 0,17 mg/litro. Se puede calcular una concentración sin
efectos prevista (PNEC) de 1,7 µg/litro mediante la aplicación de un
factor de evaluación de 10, teniendo en cuenta las características de
ecotoxicidad y destino en el medio ambiente de la sustancia. No se
dispone de datos válidos sobre efectos tóxicos en organismos y
ecosistemas terrestres, que se podrían utilizar para la evaluación del
riesgo.
Debido a la falta de datos de otras partes del mundo, se realizó
en Alemania una evaluación del riesgo de muestra. A partir de las
concentraciones medias en los ríos alemanes en los años noventa, la
relación entre la concentración prevista en el medio ambiente (PEC) y
la PNEC se calcula que es <0,29. Una razón PEC/PNEC <1 indica que
no cabe prever un riesgo importante para el medio ambiente.
See Also:
Toxicological Abbreviations
Biphenyl (ICSC)
BIPHENYL (JECFA Evaluation)
 1. EXECUTIVE SUMMARY
This CICAD on biphenyl was prepared by the Fraunhofer Institute
for Toxicology and Aerosol Research, Hanover, Germany, based
principally on a review prepared by the German Advisory Committee on
Existing Chemicals of Environmental Relevance (BUA, 1990) as well as a
supplementary report (BUA, 1994) to assess the potential effects of
biphenyl on humans and the environment. The source documents and a
description of their review processes are presented in Appendix 1. A
comprehensive literature search of several online databases to June
1996 was also conducted to identify any additional data. Additional
information identified during review by contact points and
consideration by the Final Review Board (Brussels, Belgium) has also
been incorporated into this CICAD. Information on the peer review of
this CICAD is presented in Appendix 2. This CICAD was given
provisional approval as an international assessment at a meeting of
the Final Review Board held in Brussels, Belgium, on 18-20 November
1996. Participants at the Brussels Final Review Board meeting are
listed in Appendix 3. Following the incorporation of data related to
the carcinogenicity of this substance from a recently completed 2-year
carcinogenicity bioassay, the revised document was subjected to two
rounds of written peer review. In addition, that part of the document
dealing specifically with the human health assessment (i.e. section
11.1) was reviewed at a meeting held at the National Center for
Environmental Assessment, US Environmental Protection Agency, in
Washington, DC (USA), on 7 December 1998. Individuals contributing to
the additional specialized reviews of this CICAD are listed in
Appendix 4. This CICAD was approved as an international assessment at
a meeting of the Final Review Board held in Washington, DC (USA), on
8-11 December 1998. Participants at the Washington Final Review Board
meeting are listed in Appendix 5. The International Chemical Safety
Card (ICSC 0106) produced by the International Programme on Chemical
Safety (IPCS, 1993) has also been reproduced in this document.
Biphenyl (CAS No.
1. EXECUTIVE SUMMARY
This CICAD on biphenyl was prepared by the Fraunhofer Institute
for Toxicology and Aerosol Research, Hanover, Germany, based
principally on a review prepared by the German Advisory Committee on
Existing Chemicals of Environmental Relevance (BUA, 1990) as well as a
supplementary report (BUA, 1994) to assess the potential effects of
biphenyl on humans and the environment. The source documents and a
description of their review processes are presented in Appendix 1. A
comprehensive literature search of several online databases to June
1996 was also conducted to identify any additional data. Additional
information identified during review by contact points and
consideration by the Final Review Board (Brussels, Belgium) has also
been incorporated into this CICAD. Information on the peer review of
this CICAD is presented in Appendix 2. This CICAD was given
provisional approval as an international assessment at a meeting of
the Final Review Board held in Brussels, Belgium, on 18-20 November
1996. Participants at the Brussels Final Review Board meeting are
listed in Appendix 3. Following the incorporation of data related to
the carcinogenicity of this substance from a recently completed 2-year
carcinogenicity bioassay, the revised document was subjected to two
rounds of written peer review. In addition, that part of the document
dealing specifically with the human health assessment (i.e. section
11.1) was reviewed at a meeting held at the National Center for
Environmental Assessment, US Environmental Protection Agency, in
Washington, DC (USA), on 7 December 1998. Individuals contributing to
the additional specialized reviews of this CICAD are listed in
Appendix 4. This CICAD was approved as an international assessment at
a meeting of the Final Review Board held in Washington, DC (USA), on
8-11 December 1998. Participants at the Washington Final Review Board
meeting are listed in Appendix 5. The International Chemical Safety
Card (ICSC 0106) produced by the International Programme on Chemical
Safety (IPCS, 1993) has also been reproduced in this document.
Biphenyl (CAS No.  The chemical is an aromatic hydrocarbon with a peculiar, strong
odour similar to that of geraniums (BUA, 1990). At room temperature,
the substance is a colourless solid (melting point 62.2°C). Because of
its significant vapour pressure (4 Pa at 20°C) and low water
solubility (4.45 mg/litre at 20°C), biphenyl shows considerable
volatility from aqueous solutions (BUA, 1990). Its measured
n-octanol/water partition coefficient (log Kow) is between 3.88
and 4.04. The commercial product from the only German producer has a
biphenyl content of 99.85%; named impurities are terphenyl (0.15%),
sulfur (10-20 mg/kg), and benzene (1 mg/kg) (BUA, 1990). Additional
physical/chemical properties are presented in the International
Chemical Safety Card reproduced in this document.
3. ANALYTICAL METHODS
Biphenyl is usually measured in environmental media by capillary
gas chromatography in combination with flame ionization or mass
spectrometric detection (Malins et al., 1985; BUA, 1990; Hawthorne et
al., 1992; Anklam & Mueller, 1994; Karanassios et al., 1994; Otson et
al., 1994; Kostiainen, 1995). For aquatic samples, methods involving
high-performance liquid chromatography with ultraviolet detection at
254 nm have also been described. The following enrichment techniques
are used: solid-phase adsorption (Tenax material or XAD resins) with
thermal desorption or liquid extraction for air samples, solid-phase
adsorption (XAD resins) or liquid/liquid extraction (diethylether) for
water samples, and liquid extraction (dichloromethane/methanol,
ethanol) for soil and biotic samples. The detection limits range from
0.1 to 5 ng/m3 for air and from 0.01 to 8000 ng/litre for water. For
sediment, a detection limit of 2 µg/kg dry weight has been reported.
Detection limits between 12 and 87 µg/kg have been reported for levels
of biphenyl in fish (Malins et al., 1985).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Biphenyl occurs in varying concentrations in coal tar, crude oil
(up to 0.4 mg/g oil), and natural gas (3-42 µg/m3) (BUA, 1990).
Because of its natural occurrence in coal tar and crude oil, biphenyl
has also been detected in products derived from these substances. The
biphenyl content in coal tar-derived creosotes ranges between 0.2 and
1.6% (IARC, 1985; ATSDR, 1990; Collin & Hoeke, 1995). In the aromatic
fraction of an unused lubricating oil sample, biphenyl was measured at
a concentration of 1.5 mg/kg (Paschke et al., 1992).
Biphenyl is also produced from anthropogenic sources. In 1984,
the estimated production capacities of biphenyl in the Federal
Republic of Germany, in Western Europe, and throughout the world were
approximately >6000, 30 000, and 80 000 t, respectively. In 1989,
2000-2500 t of biphenyl were produced in Germany, of which about 900 t
were exported and 200-300 t sold domestically (BUA, 1990).
Approximately 5000 t of biphenyl were manufactured in each of 1992 and
1993 in Japan (Anon., 1994-95).
Until the early 1970s, biphenyl was used principally as an
intermediate in the production of polychlorinated biphenyls (PCBs). As
the production, processing, distribution, and use of PCB compounds are
now prohibited or restricted in many countries (e.g. USA, Germany,
United Kingdom) (IRPTC, 1994), it is likely that previous production
levels were much higher than those today. In 1984, the estimated use
patterns of biphenyl worldwide were, as a final product: 35% in heat
transfer medium in heating fluids, 20% as a dyestuff carrier for
textiles, 5% as a solvent in pharmaceutical production, 5% as a
dyestuff carrier for copying paper, and 5% as a preservative for
citrus fruits; patterns of biphenyl use as an intermediate were: 10%
each in emulsifiers and optical brighteners, 5% for crop protection
products, and 5% for precursors and auxiliaries for plastics (BUA,
1990). At present in Germany, the use of biphenyl as a dyestuff
carrier in textiles is minor.1
Although emissions from exhaust gases during production and
processing of biphenyl and during the use of biphenyl-containing
creosotes for wood preservation may be significant, available data
were not identified. Biphenyl is also released into the atmosphere
during the incomplete combustion of fossil fuels in motor vehicles
(post-catalyst emission factor of 3.5 µg/km; Siegl & Chladek, 1992),
residential heating (1.24 mg biphenyl/kg burnt coal; Engewald et al.,
1993), and coal-burning power plants (with concentrations in flue gas
up to 30 µg/m3; Yao & Xu, 1991; Wienecke et al., 1992) or foundries
(qualitatively detected; Deusen & Kleinermanns, 1993).
1 Personal communication from Verband d. Textilhilfsmittel-,
Lederhilfsmittel-, Gerbstoff- und Waschrohstoff-Industrie e.V.,
Frankfurt/M., Germany, to Bayer AG, 1996.
In 1990, biphenyl was not detected in the effluent of the sewage
plant of the German producer (detection limit 5 µg/litre; weekly
sampling). Based on a biphenyl concentration of <5 µg/litre, a
maximum annual release of 274 kg biphenyl can be calculated for this
plant. No data are available from other producing or processing
facilities. Biphenyl may also be released to surface water through the
processing of coal tar (e.g. coking plants) and mineral oils (e.g.
processing residual oils from pyrolysis in refineries) (BUA, 1990).1
Based upon levels of biphenyl measured in sediment samples during the
1970s, this chemical has been released from various industrial
wastewater outlets associated with a melting plant for iron alloys, a
chemical factory, and an oil storage depot. Data were not available on
releases of biphenyl from textile industries where the chemical is
used as a dyestuff carrier, from wood preservation plants that use
creosotes containing biphenyl, or from treated citrus fruits and their
packaging materials contained in domestic waste sites. Concentrations
of biphenyl between 0.016 µg/kg and 1730 mg/kg have been measured in
sewage sludge, which may be applied to arable land (BUA, 1990).
1 Personal communication from Bayer AG to the GDCh- Advisory
Committee on Existing Chemicals of Environmental Relevance, 1997.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Based upon a Level II fugacity model (Mackay et al., 1992), the
atmosphere appears to be the main target compartment (>90%) for
biphenyl. Biphenyl's Henry's law constant indicates that it
volatilizes from aqueous solutions. The calculated half-life for the
photooxidative degradation of biphenyl by hydroxyl radicals in air is
about 2 days (Atkinson & Aschmann, 1985; BUA, 1990; Atkinson & Arey,
1994). Reactions with tropospheric ozone and nitrate are expected to
be of minor importance, with calculated half-lives of >80 days and
>105 days, respectively. Data on the photodegradation of biphenyl in
water are not available. Biphenyl is not expected to hydrolyse under
environmental conditions.
In one reported standard test with activated sludge, according to
Guideline 301C of the Organisation for Economic Co-operation and
Development (OECD), a biochemical oxygen demand of 66% after 14 days'
incubation was reported when non-adapted inoculum was used (CITI,
1992). Microbial populations from natural waters mineralize biphenyl;
100% degradation was observed after 4 days' incubation of biphenyl in
river water (BUA, 1990). Numerous microorganisms isolated from soil,
sediment, surface water, and groundwater samples have been shown to
metabolize biphenyl (BUA, 1990; Bevinakatti & Ninnekar, 1992; Kiyohara
et al., 1992; Nielsen & Christensen, 1994). Investigations on the
anaerobic biodegradation of biphenyl were not identified.
Soil sorption coefficients ( Koc) based upon laboratory
experiments and calculated values range from 1100 to 18 000 (BUA,
1990; Kishi & Hasimoto, 1991); from numerous literature data, Jeng et
al. (1992) calculated a mean value of 4230. On the basis of these
data, it is expected that biphenyl will be almost immobile in soil,
with a low probability of groundwater infiltration. Volatilization
from soil surfaces is significantly lower than that from aqueous
media; however, no significant geoaccumulation of biphenyl is expected
under aerobic conditions owing to its degradation by microbial
organisms.
In static tests on the bioaccumulation of biphenyl in activated
sludge conducted with several aquatic species (yeast, algae, molluscs,
daphnia, and freshwater fish), bioconcentration factors (BCFs) ranging
between 57 for the marine mussel Mytilus edulis (calculated on wet
weight) (Donkin et al., 1991) and 540 for the green alga Chlorella
vulgaris (based on dry weight) (Kotzias et al., 1980; Freitag et
al., 1982) have been reported. With the exception of two tests, there
was no information on whether equilibrium was reached before the
determination of the BCF. The depuration of accumulated biphenyl was
determined in one study. After a 24-h incubation in a static test
system, the BCF at equilibrium (based on wet weight) for Daphnia
magna was 473; depuration was dependent upon the temperature of the
water. Twenty-four hours after being transferred to pure water, 53% of
the accumulated biphenyl was depurated at 2-3°C, whereas 88% was
depurated at 22°C (Zhang et al., 1983). Although the available data
indicate a potential for bioaccumulation, evaporation, adsorption to
soil/sediment, and degradation are expected to reduce the
bioavailability of biphenyl. Therefore, bioaccumulation of the
chemical should be of minor importance for aquatic organisms.
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
In 1985, concentrations of biphenyl in the air of an
industrialized city in Finland ranged from 1.7 to 26.2 ng/m3 (BUA,
1990). Similar concentrations were measured during the winter of
1988-89 in two US cities (12-119 ng/m3; 30 ng/m3 mean value;
Hawthorne et al., 1992) and in 1992 in two Greek cities (all data
below the detection limit of 5 ng/m3; Karanassios et al., 1994).
The concentrations of biphenyl in the German parts of the Rhine
River declined from a maximum of 1000 ng/litre in the 1970s (BUA,
1990) to levels below 500 ng/litre (the detection limit) in 1993-1995
(LWA, 1993-94).1 Although levels in most of the German tributaries
have remained below the detection limit (500 ng/litre), peak
concentrations of 560 and 1600 ng/litre were reported in 1993 and
1994, respectively, for the highly polluted Emscher tributary (LWA,
1993-94); these elevated levels were attributed to the coking plants
in this area.2 In the USA, similar data for one river have been
reported (Hites, 1973). In some German estuaries, biphenyl
concentrations were significantly lower (1-5 ng/litre) (BUA, 1990).
During 1986-1989, biphenyl was detected at concentrations between
10 and 100 ng/litre in samples of groundwater obtained near Zagreb, in
the former Yugoslavia, in the vicinity of a municipal landfill site.
In a test well sunk 720 m from a heavily polluted river, the levels
were about 10-fold lower (Ahel, 1991). Concentrations of biphenyl
between 2 and 17 ng/litre have been measured in samples of snow and
rain collected in Switzerland (BUA, 1990).
In river and estuarine sediments, biphenyl was detected at very
high concentrations in the direct vicinity of industrial plants or
waste dumps; concentrations ranged between 0.1 and 8 mg/kg, depending
on the source. The highest concentrations were found near waste dump
sites in the USA (Malins et al., 1985; BUA, 1990). Information on the
occurrence of biphenyl in soils with no direct pollution was not
identified. A maximum biphenyl concentration of 13 µg/kg was found in
soil samples collected near a pit for wastewater from oil production
in New Mexico, USA (BUA, 1990).
Biphenyl has been found in fish collected from water contaminated
with mineral oil; concentrations in liver samples were <25 µg/kg dry
weight (i.e. below the detection limit) and 13 620 µg/kg fresh weight
(Paasivirta et al., 1982; Malins et al., 1985).
1 Personal communication from the Northrhine-Westfalian
Environmental Protection Agency to Bayer AG, 1996.
2 Personal communication from Bayer AG to the GDCh- Advisory
Committee on Existing Chemicals of Environmental Relevance, 1997.
6.2 Human exposure
The levels of biphenyl in indoor air may depend mainly on smoking
habits (BUA, 1990). Concentrations of biphenyl are also elevated in
homes having a source of oil and exhaust fumes nearby (e.g. gasoline
station, parking garage) (Otson et al., 1994; Kostiainen, 1995).
Monitoring data from Finnish ( n = 50) (Kostiainen, 1995) and
Canadian ( n = 757) homes (Otson et al., 1994) have revealed average
biphenyl concentrations in the range of 160-1000 ng/m3, with a
maximum concentration of 4700 ng/m3 (Kostiainen, 1995). Biphenyl has
been detected in floor dust collected from five of nine investigated
Danish city halls (Wilkins et al., 1993). Assuming that 20 h are spent
indoors (exposure to 160-1000 ng/m3) and 4 h are spent outdoors
(exposure to 30 ng/m3) each day and that the inhalation volume and
weight of the average adult are 22 m3/day and 64 kg, respectively,
the intake of biphenyl from air can be calculated from these data to
range from 45 to 300 ng/kg body weight per day.
There is only limited intake of biphenyl from drinking-water.
Although more recent data were not identified, concentrations of
biphenyl in tap-water in various countries, such as Finland, Norway,
Sweden, the USA, and Canada, were low (i.e. 0.1-5 ng/litre) in the
1970s (BUA, 1990). Based upon these data and assuming that the average
adult (weighing 64 kg) consumes 2 litres of drinking-water per day,
the estimated intake of biphenyl from drinking-water may range from
0.003 to 0.16 ng/kg body weight per day.
Food may contain biphenyl as a result of its use as a fungistatic
agent for citrus fruits. In older studies with citrus fruits to which
a biphenyl-containing wax had been applied, peels of treated fruits
were found to contain up to 220 mg biphenyl/kg (whole fruit) and the
pulp up to 3.9 mg/kg (whole fruit) (BUA, 1990); however, these levels
were determined with a rather unspecific spectrophotometric method,
which may have overestimated the biphenyl content. In a more reliable
study with 6 grapefruits, 8 oranges, and 10 lemons obtained from
Japanese markets, levels of biphenyl ranged from 17 to 123 mg/kg in
the peel and from <0.01 (the detection limit) to 0.18 mg/kg (average
0.06 mg/kg pulp) in the edible parts of the fruit. In samples of lemon
tea, levels of biphenyl were similar to those in the edible parts of
the fruit (Isshiki et al., 1982). In a survey of foodstuffs conducted
in Italy between 1988 and 1995, which included more than 70 types of
food, biphenyl was detected in only one peach sample (detection limit
not given) (Di Muccio et al., 1995). Based upon the average content of
biphenyl in the pulp of citrus fruits of about 0.06 mg/kg (calculated
from the concentrations measured by Isshiki et al., 1982), the intake
from the consumption of the pulp of one biphenyl-preserved citrus
fruit weighing about 120-400 g is calculated to be about 113-375 ng/kg
body weight. An average intake of 64 ng biphenyl/kg body weight per
day from food was calculated based upon the consumption of foodstuffs
in Finland (Penttilae & Siivinen, 1996). The general population may
also be exposed to biphenyl through contact with consumer products,
such as creosote-preserved wood, textiles, copying papers, or
pharmaceuticals, although quantitative data were not identified.
The intake of biphenyl from the occupational environment may
occur via inhalation and dermal contact. Concentrations of biphenyl in
the air of one facility producing biphenyl-impregnated paper were
reported to range from 4.4 to 128 mg/m3 in 1959 and from 0.6 to 123
mg/m3 in 1970 (Haekkinen et al., 1973). In a nylon production
facility in which workers were exposed to a eutectic mixture of 26.5%
(w/w) biphenyl and 73.5% (w/w) biphenyl ether, time-weighted average
concentrations of biphenyl were reported to range from 0.24 to 1.28
mg/m3 (Dorgelo et al., 1985). Coke oven emissions to indoor air at a
coking plant in Australia contained 0.2-0.5% biphenyl (Kirton et al.,
1991). In a Polish bituminous pulp production facility, reported
time-weighted average concentrations (1988-89) of biphenyl ranged from
0.5 to 0.6 µg/m3 (Baranski, 1991). In a study of two Canadian
pilot-scale coal liquefaction facilities, the levels of biphenyl were
below the detection limit of 60 µg/m3 (Leach et al., 1987).
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS
AND HUMANS
Studies providing quantitative information on the absorption or
distribution of biphenyl in humans were not identified. In various
species of experimental animals, the absorption of biphenyl following
oral exposure has been inferred by the detection of metabolites in the
urine and faeces. Biphenyl is oxidized by multifunctional oxygenases,
irrespective of the route of exposure. Mono-, di-, and trihydroxy
metabolites of biphenyl have been identified in the urine of exposed
species. 4-Hydroxybiphenyl has been observed as the major metabolite
in rats, rabbits, guinea-pigs, and pigs; minor metabolites include
mono-, di-, and trihydroxymethoxy biphenyls, dihydrodiols, and
hydroxydihydrodiols. The phenolic compounds are conjugated with
sulfate or glucuronic acid. The formation of mercapturic acid as well
as evidence of a metabolic pathway involving opening of the benzene
ring have also been described (BUA, 1990). There is no evidence for
enterohepatic circulation in different species (Meyer & Scheline,
1976; Meyer et al., 1976a; Meyer, 1977). On the basis of in vitro
studies, the metabolism of biphenyl is not restricted to the liver
(Bend et al., 1972; Hook et al., 1972; Matsubara et al., 1974; Prough
& Burke, 1975; Hook & Bend, 1976; Powis et al., 1987). Based upon
total cytochrome P-450 content, biphenyl-4-hydroxylase activity was
higher in pulmonary microsomes than in liver microsomes (Hook et al.,
1972; Hook & Bend, 1976). A time-dependent covalent binding of
[14C]bi phenyl to mouse hepatic microsomal proteins has been observed
after metabolic activation (Tanaka et al., 1993).
In rats, rabbits, and pigs, most biphenyl metabolites are
excreted in the urine (BUA, 1990). Following an oral dose of 100 mg
[14C]biphenyl/kg body weight, rats excreted 82% of the administered
radioactivity (76% in urine) within 24 h. The recovery rate after 4
days was 92%, with 7% of the administered radioactivity detected in
the faeces and traces in the exhaled air. Eight days after
administration, radioactivity in the tissues was 0.6% of the applied
dose (Meyer et al., 1976b). In none of the species examined was
unmetabolized biphenyl found in the urine (BUA, 1990).
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
The acute oral toxicity of biphenyl is moderate. The LD50 for
rats and mice is >1900 mg/kg body weight (BUA, 1990). Acute effects
include polyuria, accelerated breathing, lacrimation, anorexia, weight
loss, muscular weakness and coma, fatty liver cell degeneration,
severe nephrotic lesions (often manifested as acute and subacute
glomerulotubular nephritis), degenerative myocardial lesions,
pulmonary hyperaemia, and, occasionally, alveolar oedema. Interstitial
and lobular pneumonitis have been observed in animals that survived
for a few days.
The LC50 (4-h) for the mouse is >43 ppm (275 mg/m3). During
exposure, hyperactivity and mild respiratory discomfort have been
observed; however, these effects were not evident at the end of a
14-day recovery period. Gross pathological examination performed on
surviving animals revealed slight lung congestion (Sun Co. Inc.,
1977a). In an inhalation study conducted with Sprague-Dawley rats, no
effects were observed after a 6-h exposure to 0.8 or 3 ppm (5.1 or
19.2 mg/m3) biphenyl at temperatures of approximately 26°C or 32°C,
respectively (Monsanto Co., 1959). No treatment-related alterations in
appearance, demeanour, food consumption, or survival were observed
following a 7-h inhalation exposure of rats to 3 g biphenyl/m3
(nominal concentration); no detailed information on the methods or
particle size of the substance were presented (Dow Chemical Co.,
1974).
Information on the acute toxicity of biphenyl associated with
dermal exposure was not identified.
8.2 Irritation and sensitization
Biphenyl is non-irritating to both intact and scarified rabbit
skin. The substance was slightly irritating when applied to rabbits'
eyes in an eye irritation test (BUA, 1990).
In a guinea-pig maximization test conducted according to OECD
Guideline 406, there was reportedly no evidence of a skin sensitizing
potential for biphenyl (Dreist & Kolb, 1993).
8.3 Short-term exposure
8.3.1 Oral exposure
In the studies cited below, the histopathological examination was
focused on the urinary system. In a study in which male and female
Wistar rats were fed 0, 50, 150, 300, or 450 mg biphenyl/kg body
weight per day via the diet for 21 days, increased relative kidney
weights and polycystic renal changes (with increased urine volume and
specific gravity) were observed at doses of 50 and 150 mg/kg body
weight per day, respectively. An increase in urine volume, specific
gravity, and absolute kidney weight as well as polycystic renal
changes were also observed in rats administered 500 or 1000 mg
biphenyl/kg body weight per day in the diet for 14 days (Sœndergaard &
Blom, 1979).
When male albino rats were administered diets containing 0, 1000,
2500, 5000, or 10 000 mg biphenyl/kg (estimated intakes of 0, 75, 188,
375, or 750 mg/kg body weight per day) for 26 days, there was a
dose-dependent (at doses of >188 mg/kg body weight per day)
intensification of cloudiness in the urine, associated with an
increase in precipitate formation in samples, which had been cooled or
treated with sulfosalicylic acid. The precipitate consisted of free
4-hydroxybiphenyl and its glucuronide. These effects were largely
reversible during a 28-day recovery period (Booth et al., 1956, 1961).
8.3.2 Inhalation exposure
No evidence of histopathological changes in the lung, trachea,
liver, kidney, or spleen were observed in male and female mice exposed
by inhalation to 25 or 55 ppm (about 160 or 350 mg/m3) biphenyl for 7
h/day, 5 days/week, for 2 weeks (Sun Co. Inc., 1977b).
8.3.3 Dermal exposure
In rabbits, the repeated dermal application (20 in total) of
purified biphenyl (0.5 g/kg body weight) for 2 h/day, 5 days/week,
produced decreased body weight; one animal died after 8 applications.
A decrease in body weight was also observed after repeated dermal
application of technical biphenyl. In both studies, no effect upon the
skin was observed. Histopathological examination revealed minimal
changes in the heart, liver, kidneys, and, in some cases, spleen (no
further details provided) (Deichmann et al., 1947). No adverse effects
were observed when biphenyl was applied (8 h/day, 5 days/week, for a
total of 30 applications) to the intact and abraded skin of rabbits at
doses of 600 or 2000 mg/kg body weight per day (no further information
provided) (Newell, 1953).
8.4 Long-term exposure
8.4.1 Subchronic exposure
8.4.1.1 Oral exposure
In all of the studies cited below, the histopathological
examination was focused on the urinary system. A summary of the
effects related to this specific end-point (changes in kidneys,
ureter, and urinary bladder) for both subchronic and chronic exposures
is provided in Table 1.
Table 1: Summary of the effects of repeated biphenyl administration via diet on urine status, urinary bladder, and kidneys.
Species/strain/ Application/duration NO(A)EL
sex (mg/kg) Urine status Urinary bladder Kidneys Reference
rat/F344/male 0 or 5000 mg/kg <5000 crystals with simple hyperplasia not examined Shibata et al.
8 weeks microcalculi in and increased (1989a)
urinary sediment DNA synthesis
in the urothelium
rat/F344/male 0 or 5000 mg/kg <5000 microcalculi simple hyperplasia, increase in absolute/relative Shibata et al.
up to 24 weeks papillary or kidney weights; no (1989b)
nodular hyperplasia morphological changes of
of the urothelium the renal papillae or
after >16 weeks pelvis; no changes in DNA
synthesis in the renal
papillae and pelvis; focal
calcification of the renal
medulla
rat/albino/male 0, 1000, 2500, or 1000 >2500 mg/kg: not examined 2500 mg/kg: morphological Booth et al.
and female 5000 mg/kg increase in changes (tubular dilation) (1961)
up to 24 weeksa volume, turbidity, after >60 days
and precipitate 5000 mg/kg: morphological
after >30 days; changes (tubular dilation)
urinary sediment: after >30 days; effects
4-hydroxybiphenyl not completely reversible
and its
glucuronide;
effects reversible
rat/F344/male 0 or 5000 mg/kg <5000 increase in pH increased incidence not given Kurata et al.
32 weeks value and Na+ of calculi; (1986)
concentration; histopathology:
urinary sediment: no effects
4-hydroxybiphenyl
Table 1 (Cont'd.)
Species/strain/ Application/duration NO(A)EL
sex (mg/kg) Urine status Urinary bladder Kidneys Reference
rat/Wistar/male 0, 1250, or 1250 not given 5000 mg/kg: 5000 mg/kg: increased Shiraiwa et al.
5000 mg/kg increased incidence incidence of calculi (1989)
36 weeks of calculi in ureter and increase in
and bladder relative kidney weights
rat/Wistar/male 0, 2500, or <2500 haematuria at >2500 mg/kg: >2500 mg/kg: increased Takita (1983);
and female 5000 mg/kg >2500 mg/kg increased incidence incidence of calculi Shiraiwa et al.
75 weeks after >16 weeks of calculi (composed (composed of protein) (1989)
of magnesium after >16 weeks;
ammonium phosphate) kidneys with stones:
after >16 weeks in obstructive
ureter (females) pyelonephritis,
5000 mg/kg:increased tubular atrophy,
incidence of calculi fibrosis
in ureter and 5000 mg/kg: increase
bladder after >16 in relative kidney
weeks; bladder with weights in females
calculi: simple or
diffuse hyperplasia
and papillomatosis
of the urothelium
rat/Wistar/male 0, 630, or 1250 mg/kg 1250 not given no urolithiasis not given Takita (1983)
and female 104 weeks (no further
information given)
rat/F344/DuCrj/ 0, 500, 1500, or <500b >500 mg/kg: 4500 mg/kg: increase >1500 mg/kg: increase Japan Bioassay
male and female 4500 mg/kg decrease in in pH (males and in hyperplasia of Research Center
104 weeks protein (males) females) 4500 mg/kg: the renal pelvis in (1996)
>1500 mg/kg: increase in calculi females
decrease in and hyperplasia 4500 mg/kg: increase
protein (females) of the urothelium in calculi (males >
Table 1 (Cont'd.)
Species/strain/ Application/duration NO(A)EL
sex (mg/kg) Urine status Urinary bladder Kidneys Reference
(males >> females); females) and
increased tumour hyperplasia of the
incidence in males renal pelvis (males)
mouse/B6C3F1/ 0 or 10 000 mg/kg 10 000 decreased Na+ no changes in DNA not given Tamano et al.
male 8 weeks concentration synthesis of the (1993)
at week 4 urothelium
mouse/B6C3F1/ 0 or 10 000 mg/kg <10 000 small amounts of simple hyperplasia interstitial nephritis Tamano et al.
male 32 weeks greyish white and papillary or in 5/10 (1993)
and finely nodular dysplasia
granular material in 1/10 mice
mouse/Crj:BDF1/ 0, 667, 2000, or 667 6000 mg/kg: no changes >2000 mg/kg: mineralization Japan Bioassay
male and female 6000 mg/kg decrease in of the medulla in females Research Center
104 weeks protein and 6000 mg/kg: desquamation (1996)
ketone body and mineralization of the
pelvis in males
a Plus a post-treatment observation period of 9 weeks.
b Effects were observed at the lowest dose tested.
Effects on the urinary tract (i.e. an increased number of
microcalculi in urinary sediment, simple hyperplasia of the bladder
epithelium) were observed in male F344 rats administered 5000 mg
biphenyl/kg (estimated intake 252 mg/kg body weight per day) in the
diet for 8 weeks. Morphological changes and the increased synthesis of
DNA detected in the bladder epithelium after 4 weeks were attributed
to constant irritation by the microcalculi (Shibata et al., 1989a).
In male B6C3F1 mice, no changes in the urinary pH or in levels
of DNA synthesis in the urinary bladder were noted after
administration of 0 or 10 000 mg biphenyl/kg in the diet (0 or 1500
mg/kg body weight per day) over a period of 8 weeks. The urinary Na+
concentration was significantly lower in mice administered 1500 mg/kg
body weight per day compared with controls only at week 4 (Tamano et
al., 1993).
In a range-finding study in which male and female Wistar rats
were administered diets containing biphenyl at 0, 1250, 2500, 5000, 10
000, or 20 000 mg/kg (estimated intakes of 0, 94, 188, 375, 750, or
1500 mg/kg body weight per day) for 10 weeks, dose-dependent effects
(i.e. reduction in weight gain; increased serum activities of
aspartate transaminase, alanine transaminase, and lactate
dehydrogenase; and an increase in blood urea nitrogen) were observed
at all doses (Takita, 1983).
In male F344 rats (20 per group) administered 0 or 5000 mg
biphenyl/kg in the diet (0 or 375 mg/kg body weight per day) for 24
weeks (5 rats per group were sacrificed after 4, 8, 16, or 24 weeks),
reduced body weight gain and an increase in absolute/relative kidney
weights without differences in feed intake were observed in the
biphenyl-exposed rats. The analysis of the urine showed that
administration of biphenyl was associated with the formation of
microcalculi. The histopathological examination of the renal papillae
or pelvis performed after 4, 8, 16, and 24 weeks revealed no
morphological changes; the rate of DNA synthesis in the renal papillae
and pelvis determined after 4 weeks of treatment was unchanged. In
most of the treated animals, a focal calcification of the renal
medulla (no further information given) was noted. The examination of
the epithelium of the urinary bladder revealed a simple hyperplasia in
5/5 rats after 16 and 24 weeks and papillary or nodular hyperplasia in
3/5 rats at week 16 and in 5/5 rats at week 24; no data on controls
were presented (Shibata et al., 1989b).
In albino rats (42 animals of each sex per group) given 0, 1000,
2500, or 5000 mg biphenyl/kg in the diet (0, 75, 188, or 375 mg/kg
body weight per day) for up to 24 weeks, the urine analysis and
histopathological investigations of the kidneys during the study (days
30, 60, and 120) showed no apparent effects at a dose level of 75
mg/kg body weight (Booth et al., 1961). In some animals, early
indications of a kidney-damaging effect were observed at doses of 188
and 375 mg/kg body weight at 30 days -- polyuria, increasing
cloudiness of the urine, and initial morphological changes (tubular
dilation) in the kidney. These effects increased in frequency and
degree as the administrations progressed. The urinary sediment
consisted of 4-hydroxybiphenyl and its glucuronide. During a
subsequent 60-day observation period, the urinary status normalized;
however, changes in kidneys were not completely reversible (a few
dilated tubules and scars remained) (Booth et al., 1961).
In male F344 rats (25 per group) administered 0 or 5000 mg
biphenyl/kg in the diet (0 or 375 mg/kg body weight per day) for 32
weeks, a reduction in body weight gain but no clinical signs of
poisoning were observed in dosed animals. In urine, an increase in pH
value and Na+ concentration was noted. At terminal necropsy, calculi
were found in the urinary bladder. The urinary sediment consisted
mainly of 4-hydroxybiphenyl. No associated histopathology was detected
upon microscopic examination of the urinary bladder (Kurata et al.,
1986).
When groups ( n = 25) of male Wistar rats were provided diets
containing 0, 1250, or 5000 mg biphenyl/kg (0, 94, or 375 mg/kg body
weight per day) for 36 weeks, a reduction in body weight, increased
relative kidney weights, and an increase in the number of animals with
stones in the kidneys (0/25, 0/25, and 4/25, respectively), ureter
(0/25, 0/25, and 1/25, respectively), and urinary bladder (0/25, 0/25,
and 3/25, respectively) were noted in the high-dose group (Shiraiwa et
al., 1989).
8.4.1.2 Inhalation exposure
Exposure of groups ( n = 50) of male and female CD-1 mice to 25
or 50 ppm (160 or 320 mg/m3; analytical concentrations) biphenyl for
7 h/day, 5 days/week, for 13 weeks produced hyperaemia and focal
haemorrhage in the lung and an increase in hyperplasia of the tracheal
epithelium. As these effects were also observed in some unexposed
controls, they were attributed to the method of aerosol generation
(i.e. inhalation of hot air) (Sun Co. Inc., 1977c).
Marked species differences were observed in a study in which
rabbits, rats, and mice were exposed by inhalation to biphenyl in the
form of dust (50% biphenyl on zeolite) at 5, 40, or 300 mg/m3, 7
h/day, 5 days/week, for up to 13 weeks. No adverse effects were
observed in rabbits. Rats exposed to 40 or 300 mg biphenyl/m3
exhibited increased mortality and irritation of the mucous membranes;
no effects were observed following exposure to 5 mg/m3. Mice were the
most sensitive species. Exposure to 5 mg biphenyl/m3 (the only
concentration tested) resulted in slightly increased mortality, with
all mice exhibiting irritation of the upper respiratory tract (no
further information available). Necropsy of dead rats and mice
revealed mainly inflammatory bronchopulmonary changes. No information
on control animals or particle size was provided (Deichmann et al.,
1947).
8.4.2 Chronic exposure and carcinogenicity
In older studies, which were not further taken into consideration
because of the small number of animals used, limited documentation,
and/or limited exposures, no increase in tumour incidence was observed
in rats and mice when biphenyl was administered orally (at doses up to
750 mg/kg body weight per day for 2 years) (Ambrose et al., 1960;
Innes et al., 1969) or following a single subcutaneous injection of
46.4 mg biphenyl/kg body weight, in which the animals were examined
macroscopically and microscopically after an 18-month observation
period (NTIS, 1968).
In Wistar rats (50 animals of each sex per group) given 0, 2500,
or 5000 mg biphenyl/kg in the diet (0, 188, or 375 mg/kg body weight
per day) for 75 weeks, dose-dependent effects (i.e. reduction in
weight gain; alterations in serum activities of aspartate transaminase
[increased and decreased], alanine transaminase [increased], and
lactate dehydrogenase [increased]; and an increase in blood urea
nitrogen) and a dose-dependent increase in stones of the kidney
(females: 0/43, 1/43, 18/39; males: 0/44, 6/46, 15/47) and ureter
(females: 0/43, 1/43, 2/39; males: 0/44, 0/46, 2/47), accompanied by
haematuria as early as 16 weeks after initiation of exposure, were
seen at >188 mg/kg body weight. At 375 mg/kg body weight, the
relative kidney weights were significantly increased in females, and
an increase in stones of the urinary bladder was seen in males and
females (females: 0/43, 0/43, 6/39; males: 0/44, 0/46, 13/47). The
histopathological examination of the urinary bladder with stones
revealed simple or diffuse hyperplasia and papillomatosis of the
epithelium, but no cancerous changes. The overall tumour incidence was
not increased compared with controls. Kidneys with stones exhibited
obstructive pyelonephritis, tubular atrophy, and fibrosis. Kidney
stones were composed of protein, whereas urinary stones were composed
of magnesium ammonium phosphate (Takita, 1983; Shiraiwa et al., 1989).
No urolithiasis and no increased overall tumour incidence
compared with controls were observed in Wistar rats (50 animals of
each sex per group) provided with diets containing 0, 630, or 1250 mg
biphenyl/kg (0, 47, or 94 mg/kg body weight per day) for 104 weeks.
Dose-dependent effects (i.e. reduction in weight gain; alterations in
serum activities of aspartate transaminase [decreased], alanine
transaminase [increased and decreased], and lactate dehydrogenase
[increased and decreased]) were noted at both doses; 47 mg/kg body
weight is considered the lowest-observed-(adverse-)effect level, or
LO(A)EL (Takita, 1983).
In a study with F344/DuCrj rats performed according to standard
protocols and described in sufficient detail, a significant increase
in neoplastic and non-neoplastic lesions of the urinary bladder and a
significant increase in calculi within the urinary bladder were
observed in high-dose males, after the animals had been given diets
containing 0, 500, 1500, or 4500 mg biphenyl/kg (0, 38, 113, or 338
mg/kg body weight per day) for 104 weeks. In males and females, a
dose-dependent increase in hyperplasia of the epithelium of the renal
pelvis was seen. The results of the histopathological findings in the
urinary bladder and kidneys are summarized in Table 2. Other findings
included increased serum enzyme levels (alkaline phosphatase,
aspartate transaminase, and alanine transaminase) and an increased
urea nitrogen level in low-dose males and mid-dose females, which
became more pronounced with increasing doses. In mid- and high-dose
females and high-dose males, haematological effects (i.e. reduced
haemoglobin concentration and haematocrit) were noted (Japan Bioassay
Research Center, 1996). From this study, a LOEL of 38 mg/kg body
weight was derived.
In a study in which groups of male and female Crj:BDF1 mice were
given diets containing 0, 667, 2000, or 6000 mg biphenyl/kg (0, 100,
300, or 900 mg/kg body weight per day) for 104 weeks, a slight
increase in liver tumours (hepatocellular adenomas and carcinomas) and
basophilic cell foci of the liver was observed in the females at doses
of 300 and 900 mg/kg body weight per day; however, the effects were
not concentration dependent (Japan Bioassay Research Center, 1996)
(see Table 3). In the male and female mice, degenerative changes of
the respiratory epithelium of the nasal cavity and nasopharynx were
observed at doses of >100 mg/kg body weight per day or >300
mg/kg body weight per day, respectively. Other findings included
variations in serum enzyme levels (increase in alkaline phosphatase,
aspartate transaminase, and alanine transaminase) and an increased
urea nitrogen level in the low-dose males and females, which became
more pronounced with increasing doses. In female mice receiving
>300 mg biphenyl/ kg body weight per day and in the high-dose
males, degenerative changes in the kidney (increased mineralization of
the inner stripe of the outer medulla, increase in desquamation of the
epithelium of the renal pelvis) were also observed. Body weight gain
and food consumption were reduced in the high-dose animals (Japan
Bioassay Research Center, 1996).
Information on the toxicological effects of biphenyl following
chronic inhalation or dermal exposure were not identified.
8.4.2.1 Tumour promotion
A number of studies have investigated the tumour-promoting
potential of biphenyl. Kurata et al. (1986) considered biphenyl a
tumour promoter, based upon the results of a study in which groups of
male F344 rats were administered drinking-water containing 0.05%
N-butyl- N-(4-hydroxybutyl)nitrosamine (BBN) (as an initiator) for
4 weeks, followed by 0.5% biphenyl in the diet (estimated intake of
approximately 375 mg/kg body weight per day) for a further 32 weeks,
after which time the animals were examined histopathologically. The
results are provided in Table 4. The effects of biphenyl were
attributed to the increased Na+ content in the urine of exposed
rats, as well as to crystals containing mainly 4-hydroxybiphenyl
detected in the urine.
In a study in which male Wistar rats were administered 0.1%
N-ethyl- N-hydroxyethylnitrosamine in the diet for 2 weeks,
followed by 0, 0.125, or 0.5% biphenyl in the diet for 34 weeks,
exposure to biphenyl had no effect upon the incidence of dysplastic
foci and renal cell tumours induced by N-ethyl- N-hydroxyethylnitro
samine; however, an increase in stones of the kidneys, ureter, and
bladder was observed in rats administered 0.5% biphenyl with or
without N-ethyl- N-hydroxyethyl nitrosamine (Shiraiwa et al.,
1989).
Tamano et al. (1993) maintained male B6C3F1 mice on
drinking-water containing 0.05% BBN supplement for 4 weeks. After the
BBN pre-treatment, the animals received diets with 10 000 mg
biphenyl/kg (1500 mg/kg body weight per day) for 32 weeks. At the end
of the study, the urinary bladder and kidneys were examined
histopathologically. The results are provided in Table 5. In mice
exposed to BBN and biphenyl, the average food consumption and the
final body weight were reduced, whereas the relative weight of the
urinary bladder was increased. The induction of simple hyperplasia and
papillary or nodular dysplasia of the urinary bladder in 1/10 mice
treated with biphenyl only was associated with urolithic residues
(small amounts of greyish white and finely granular material). In mice
treated with biphenyl with or without BBN pre-treatment, the incidence
of interstitial nephritis in the kidneys was 65 and 50%, respectively.
In an early study in which albino mice "initiated" with a single
dermal application of 9,10-dimethyl-1,2-benzanthracene (0.3% in
benzene) received dermal applications of biphenyl (20% in benzene)
twice weekly for 15 weeks, no skin papillomas or carcinomas were
observed in either the vehicle controls or exposed mice 16 weeks after
application of the initiator (Boutwell & Bosch, 1959).
Table 2: Data from Japanese cancer bioassay with male and female rats fed biphenyl in the diet for 2 years.a
Male rats Female rats
0 500 1500 4500 0 500 1500 4500
End-point mg/kg mg/kg mg/kg mg/kg mg/kg mg/kg mg/kg mg/kg
survival rate 37/50 41/50 38/50 31/50 44/50 38/50 44/50 37/50
urinary bladder: neoplastic lesions
transitional cell papilloma 0 0 0 10/50 0 0 0 0
transitional cell carcinoma 0 0 0 24/50 0 0 0 0
(p=0.0001)b
squamous cell papilloma 0 0 0 1/50 0 0 0 0
squamous cell carcinoma 0 0 0 1/50 0 0 0 0
urinary bladder: non-neoplastic lesions (transitional epithelium)
simple hyperplasia 0 0 0 12/50 0 0 1/50 1/50
nodular hyperplasia 0 0 0 40/50 1/50 0 0 5/50
papillary hyperplasia 0 0 0 17/50 0 0 0 4/50
basal cell hyperplasia 0 0 0 27/50 0 0 0 4/50
squamous cell hyperplasia 0 0 0 13/50 0 0 0 1/50
squamous cell metaplasia 0 0 0 19/50 0 0 0 4/50
inflammatory polyps 0 0 0 10/50 0 0 0 0
calculi 0 0 0 43/50 0 0 0 8/50
kidney
mineralization -- papilla 9/50 9/50 14/50 23/50 2/50 6/50 3/50 13/50
mineralization -- pelvis 9/50 6/50 10/50 18/50 12/50 12/50 18/50 27/50
calculi 0/50 0/50 0/50 13/50 0/50 0/50 0/50 3/50
desquamation -- pelvis 1/50 0/50 0/50 11/50 0/50 0/50 0/50 2/50
simple hyperplasia of the 6/50 8/50 5/50 19/50 3/50 5/50 12/50 25/50
transitional epithelium
nodular hyperplasia of the 0/50 1/50 1/50 21/50 0/50 0/50 1/50 12/50
transitional epithelium
ureter dilatation 0/50 0/50 1/50 14/50 0/50 0/50 0/50 6/50
a From Japan Bioassay Research Center (1996).
b Fisher Exact Test.
Table 3: Data from Japanese cancer bioassay with male and female mice fed biphenyl in the diet for 2 years.a
Male mice Female mice
0 667 2000 6000 0 667 2000 6000
End-point mg/kg mg/kg mg/kg mg/kg mg/kg mg/kg mg/kg mg/kg
survival rate 35/50 41/50 41/50 39/50 31/50 22/50 25/50 32/49
liver: neoplastic lesions
hepatocellular carcinoma 8/50 8/49 5/50 4/50 1/50 5/50 7/50 5/49
(p=0.121)b (p=0.043)b
(p=0.1163)b
hepatocellular adenoma 8/50 6/49 7/50 3/50 2/50 3/50 12/50 10/49
(p=0.4909)b (p=0.016)b
(p=0.0251)b
historical control data: historical control data:
carcinoma: 171/700 with a range of 1/50 - 19/50 carcinoma: 15/699 with a range of 0/50 - 2/50
adenoma: 119/700 with a range of 2/50 - 15/50 adenoma: 33/699 with a range of 1/50 - 5/50
basophilic cell foci 0/50 6/49 1/50 2/50 1/50 1/50 12/50 6/49
a From Japan Bioassay Research Center (1996).
b Fisher Exact Test.
Table 4: Results of the tumour-promoting potential (urinary bladder) of
biphenyl in male rats.a
Hyperplasia Papilloma Carcinoma
n (%) n (%) n (%)
biphenyl only
(21 rats) 0 0 0 0 0 0
BBNb only
(24 rats) 6 -25 3 -12 0 0
BBN and biphenyl
(18 rats) 17 -94 15 -83 11 -61
a Taken from Kurata et al. (1986).
b BBN = N-butyl-N-(4-hydroxybutyl)nitrosamine.
Table 5: Results of the tumour-promoting potential (urinary bladder) of
biphenyl in male mice.a
Papillary or
Hyperplasia nodular dysplasia Carcinoma
n (%) n (%) n (%)
biphenyl only
(10 mice) 1 -10 1 -10 0 0
BBNb only
(20 mice) 12 -60 2 -10 0 0
BBN and biphenyl
(20 mice) 14 -70 1 -5 0 0
a Taken from Tamano et al. (1993).
b BBN = N-butyl-N-(4-hydroxybutyl)nitrosamine.
8.5 Genotoxicity and related end-points
The results of the in vitro tests with biphenyl are summarized
in Table 6. In vitro studies with bacteria have yielded no evidence
of mutagenic potential for biphenyl, whereas gene mutation and mitotic
recombination were observed with Saccharomyces cerevisiae D7 in the
presence or absence of metabolic activation. However, no gene
conversion was observed with S. cerevisiae D3. Testing in mammalian
cells has produced positive results for gene mutations and
clastogenicity in the presence of metabolic activation and negative
results in the absence of metabolic activation.
In an in vivo rat cytogenetic assay of bone marrow cells, the
incidence of chromosomal aberrations was not increased (no further
information available) (Kawachi et al., 1980). The exposure by
inhalation of male Sprague-Dawley rats to 64 or 320 mg biphenyl/m3
(as dust aerosol) for 7 h/day, 5 days/week, for 30 days (20 exposures
in total) reportedly did not increase the frequency of chromosomal
aberrations in the bone marrow (Dow Chemical Co., 1976). However,
owing to insufficient documentation (i.e. no data available concerning
dust particle size distribution or cell harvesting times) and the low
number of metaphase cells examined (i.e. 50 cells per animal), the
validity of this study cannot be ascertained.
The results of in vitro studies indicate that biphenyl has
mutagenic potential; in the absence of reassurance from reliable
results from in vivo tests, it is assumed that exposure to biphenyl
may be associated with a mutagenic risk.
8.6 Reproductive and developmental toxicity
Available data on the reproductive or developmental toxicity of
biphenyl are limited. In Wistar rats administered (by gavage) 0, 125,
250, 500, or 1000 mg biphenyl/kg body weight (in corn oil) on days
6-15 of gestation, no maternal toxicity occurred at doses of <500
mg/kg body weight. In the highest dose group, 5 of 20 rats died. Also
at 1000 mg/kg body weight, reduced fetal weight and an increased
number of dead and resorbed fetuses were noted; however, these values
were statistically not significantly different from those of control
animals. At doses of >500 mg/kg body weight, there were
non-significant increases in the incidence of fetuses with missing or
non-ossified sternebrae (Khera et al., 1979).
Table 6: Results of in vitro genotoxicity studies on biphenyl.
Resultsa
Species Concentration Without With
(test system) End-point range MA MA References
Salmonella typhimurium Reverse 0-5000 µg/plate - - Cline & McMahon (1977); Purchase et al.
TA92, TA94, TA97, TA97a, mutations (1978); Kawachi et al. (1980); NTP (1980);
TA98, TA100, TA102, Bronzetti et al. (1981); Probst et al. (1981);
TA1532, TA1535, TA1537, Waters et al. (1982); Haworth et al. (1983);
TA1538, TA2636 Pagano et al. (1983, 1988); Ishidate et al.
(1984); Fujita et al. (1985); Brams et al.
(1987); Bos et al. (1988); Glatt et al. (1992)
Escherichia coli WP2, WP2 Gene mutations 0.1-1000 µg/ml - - Cline & McMahon (1977); Probst et al. (1981);
uvrA- Waters et al. (1982)
E. coli PQ37 DNA damage 2.4-154 µg/ml - - Brams et al. (1987)
Bacillus subtilis rec assay DNA damage no data - 0 Kawachi et al. (1980)
Saccharomyces cerevisiae Gene mutation/ <154 µg/ml + + Pagano et al. (1983)
D7 gene conversion
S. cerevisiae D3 Gene conversion no data - - Waters et al. (1982); Zimmermann et al. (1984)
Chinese hamster cells Gene mutation 0-100 µg/ml - + Glatt et al. (1992)
(V79)
L5178Y TK+/- cells Gene mutation 0-61 µg/ml - (+) Wangenheim & Bolcsfoldi (1988)
(mouse lymphoma assay)
Chinese hamster cells Chromosomal 0-125 µg/ml - 0 Ishidate & Odashima (1977); Kawachi et al.
(CHL) aberration (1980); Sofuni et al. (1985)
Table 6 (Cont'd.)
Resultsa
Species Concentration Without With
(test system) End-point range MA MA References
Chinese hamster cells Chromosomal 0-20 µg/ml - + Sofuni et al. (1985)
(CHL) aberration
Chinese hamster cells Chromosomal 15.4-154 µg/ml - 0 Abe & Sasaki (1977)
(DON) aberration
Rat hepatocytes Unscheduled 0.002-154 µg/ml 0 - Williams (1978); Brouns et al. (1979);
DNA synthesis Probst et al. (1981)
Chinese hamster cells Sister chromatid no data - 0 Kawachi et al. (1980)
(CHL) exchange
Chinese hamster cells Sister chromatid 15.4-154 µg/ml - 0 Abe & Sasaki (1977)
(DON) exchange
L5178Y cells (alkaline DNA damage 0-231 µg/ml - + Garberg et al. (1988)
unwinding assay)
human lung fibroblasts Unscheduled no data - - Waters et al. (1982)
(WI-38 cells) DNA synthesis
human fibroblasts ("nick DNA damage 15.4 µg/ml - 0 Snyder & Matheson (1985)
translation assay")
a -, negative; +, positive; (+) weakly positive; 0, not tested; MA, metabolic activation.
Compared with unexposed controls, litter size (the only parameter
examined) was not affected in a study in which small numbers of male
and female rats were administered diets containing 0.1 or 0.5%
biphenyl (estimated intakes of 75 and 375 mg/kg body weight per day)
before mating and throughout gestation (Ambrose et al., 1960). In an
unpublished three-generation study, dietary biphenyl concentrations of
100 or 1000 mg/kg (estimated intakes of approximately 7.5 or 75 mg
biphenyl/kg body weight per day) had no effect on reproduction in
rats; after intake of 10 000 mg/kg (estimated intake of 750 mg/kg body
weight per day), decreased fertility, litter size, and growth rate
were noted (no further information available) (Stanford Research
Institute, undated). Histopathological changes within the male and
female reproductive systems were not observed in rats or mice
administered biphenyl at 500-4500 mg/kg in the diet for 2 years (Japan
Bioassay Research Center, 1996).
8.7 Immunological and neurological effects
Data on immunological and neurological effects of biphenyl in
laboratory animals were not identified.
9. EFFECTS ON HUMANS
Information on effects on human health resulting from exposure to
biphenyl are limited to case reports; epidemiological studies were not
identified. In one report, a single application of 0.5 ml of a 4%
biphenyl solution (no further details provided) to the skin of the
lower arm of two test subjects caused no apparent irritation
(Macintosh, 1945). Biphenyl did not cause skin irritation in a study
in which a solution of 23% biphenyl in oil was applied to the forearm
three times per week for 8 weeks (Selle, 1952). In one human volunteer
orally given 35 mg biphenyl per week for 13 weeks, no adverse effects
were reported (Farkas, 1939). Cases of headache, nausea, and
inflammation of the respiratory tract have been reported in workers
exposed to biphenyl vapours (and other substances) during paper
production (Weil et al., 1965). Liver damage and effects upon the
central and peripheral nervous systems were attributed to long-term
exposure to high concentrations of biphenyl (see section 6.2) in a
case-study of workers engaged in the production of
biphenyl-impregnated paper (Haekkinen et al., 1973). Chronic
persistent hepatitis in a woman who had worked for 25 years in a
citrus packing plant in which biphenyl-impregnated paper was used was
attributed to the absorption of biphenyl through the skin and
digestive tract (Carella & Bettolo, 1994).
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1 Aquatic environment
The acute toxicity of biphenyl in aquatic organisms has been
investigated in microorganisms, plants, invertebrates, and
vertebrates; however, in several studies cited in the main source
document (BUA, 1990), information on whether results were based on
nominal or effective concentrations was either lacking or not clearly
stated. For biphenyl, nominal concentrations may not correspond to the
effective concentrations, as the water solubility of biphenyl is low
(4.45 mg/litre at 20°C) (test results with higher nominal effect
concentrations have not been reported here) and its volatility
significant. In tests with open systems and longer test durations,
results based on nominal concentrations may lead to an underestimation
of toxic effects because of evaporation of the chemical; therefore,
emphasis has been placed on studies with lowest reported effect levels
in which loss of the test substance was minimized.
Several studies were conducted on the toxic effects of biphenyl
on mixed microbial cultures as well as on single bacterial species.
The most sensitive species was Photobacterium phosphoreum, with a
30-min EC50 of 1.9 mg/litre in a bioluminescence inhibition test
(BUA, 1990). For Colpidium campylum, one of three different species
of ciliata tested, the minimal active concentration of biphenyl for
the inhibition of cell proliferation after a 43-h exposure was 5.6
mg/litre (nominal concentration; test substance predissolved in
acetone) (Dive et al., 1980). In studies on the reduction of
photosynthesis, 3-h EC50 values of 3.86 and 1.28 mg/litre were deter
mined with the unicellular green algae Chlorella vulgaris and
Chlamydomonas angulosa, respectively (BUA, 1990).
Forty-eight-hour LC50 values from acute toxicity tests with
Daphnia magna were in the range of 1.1-4.7 mg/litre when experiments
were conducted in more or less tightly closed test vessels. From a
test in a closed system with continuous flow, a 48-h LC50 of 0.36
mg/litre and a NOEC of 0.04 mg/litre were reported. In a reproduction
test with Daphnia magna in the same closed continuous-flow system,
the NOEC after 21 days' incubation was 0.17 mg/litre (LC50 = 0.23
mg/litre) (Gersich et al., 1989). In studies on the inhibition of food
intake by the marine mussel Mytilus edulis in a static system with
loosely covered (not airtight) test vessels, a 40-min EC50 of 0.3 mg
biphenyl/litre was reported (Donkin et al., 1991). From static tests
on the toxicity of biphenyl on freshwater fish, 96-h LC50 values in
the range of 1.5-4.7 mg/litre have been reported, with rainbow trout
(Oncorhynchus mykiss) and fathead minnow (Pimephales promelas) the
most sensitive species (BUA, 1990).
10.2 Terrestrial environment
Biphenyl has weak bactericidal and fungistatic properties. In
studies with numerous mould fungus species, biphenyl (applied as
vapour or imbedded in solid media) caused a reversible inhibition
(50-100%) of cellular proliferation. Suppression of sporogenesis and,
in Penicillium digitatum and Diplodia natalensis, the occurrence
of biphenyl-resistant mutants were observed. Yeast species showed
little or no inhibition of cell proliferation following exposure to
biphenyl (BUA, 1990).
In a plant growth inhibition test conducted according to OECD
Guideline 208, a nominal NOEC of 100 mg/kg dry soil was reported for
sorghum (Sorghum bicolor) and soya bean (Glycine max); a NOEC of
>1000 mg biphenyl/kg was reported for the sunflower (Helianthus
annuus) (Windeatt et al., 1991). Biphenyl mixed into soil inhibited
growth of lettuce (Lactuca sativa) seedlings with a 7-day EC50 of
54 mg/kg (nominal concentration). At the end of incubation, the
concentration of test substance in the soil had decreased to <10% of
the initial level (Hulzebos et al., 1993).
Data on toxic effects of biphenyl on terrestrial animals and
ecosystems were not identified.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
There are limited data on the effects of biphenyl exposure on
humans. The assessment of potential health hazards has relied
primarily on studies conducted in laboratory animals. The acute oral
toxicity of biphenyl is moderate. The chemical is not irritating to
the skin and is slightly irritating to the eyes, and there is no
evidence of skin sensitizing potential.
Aside from differences in mortality among laboratory species
exposed subchronically to biphenyl-containing dusts, the available
data on effects associated with the inhalation of biphenyl are
insufficient to form the basis of an assessment of potential health
hazards associated with long-term airborne exposure to this chemical.
In toxicological studies in which rodents have been administered
diets containing biphenyl for various periods of time, effects on the
urinary system have often been reported. A marked increase in the
incidence of morphological (i.e. formation of calculi) and/or
histopathological (e.g. hyperplasia, desquamation) effects has been
observed within the urinary tract of male rats administered diets
containing more than 2500 mg biphenyl/kg for periods ranging from 32
to 104 weeks (Takita, 1983; Kurata et al., 1986; Shiraiwa et al.,
1989; Japan Bioassay Research Center, 1996). An increase in the
occurrence of calculi within the urinary bladder has also been
observed in female rats, but at a lower incidence than in males
(Takita, 1983; Shiraiwa et al., 1989; Japan Bioassay Research Center,
1996). Similarly, in a long-term dietary study (Japan Bioassay
Research Center, 1996), increased squamous metaplasia within the
urinary transitional epithelium was also observed in female rats;
again, however, the incidence was lower than that observed in males.
In male mice, only 1 of 10 animals given a diet containing 10 000 mg
biphenyl/kg (1500 mg/kg body weight per day) for 32 weeks developed
simple hyperplasia and papillary or nodular dysplasia of the urinary
bladder (Tamano et al., 1993). Effects on blood chemistry and
haematological parameters have also been observed in animals
administered biphenyl orally; these effects occurred in male and
female rats and mice at intakes lower than those associated with the
development of effects in the urinary bladder of male rats
administered biphenyl (Takita, 1983; Shiraiwa et al., 1989; Japan
Bioassay Research Center, 1996). For non-neoplastic effects, the LOEL
was 38 mg/kg body weight per day based upon the development of
alterations in haematological parameters (i.e. decreased haemoglobin
concentration and haematocrit) in rats fed diets containing 0, 500,
1500, or 4500 mg biphenyl/kg (reported intakes of 0, 38, 113, or 338
mg/kg body weight per day) for 2 years (Japan Bioassay Research
Center, 1996).
Although limited, the available information indicates that
biphenyl has no reproductive or developmental effects at doses lower
than those associated with the development of adverse effects in the
parental generation.
In one study, an increased incidence of benign and malignant
tumours within the urinary bladder was observed in male F344/DuCrj
rats administered diets containing high levels (i.e. not less than
4500 mg/kg) of biphenyl for 2 years. Tumour incidence was not
increased in female rats or in male or female Crj:BDF1 mice (Japan
Bioassay Research Center, 1996). In female mice, there were slight
increases in the incidences of benign and malignant liver tumours in
animals receiving biphenyl in the diet; however, the results were not
dose dependent over the entire range of concentrations tested. In
other studies, biphenyl exhibited tumour-promoting activity with
respect to the development of bladder neoplasms in male rats (Kurata
et al., 1986) but not in male mice (Tamano et al., 1993).
The mechanism by which high doses of biphenyl induce bladder
tumours specifically in male rats has not been fully elucidated. There
have been suggestions that for some non-genotoxic chemicals, the
formation of such tumours is linked to the regenerative hyperplasia of
the urinary epithelium, caused by the abrasion and damage to the
urothelium that are produced by calculi formed within the urinary
tract only at very high levels of exposure (Cohen, 1995). Owing to
anatomical and physiological differences, male rats are considered to
be more susceptible than female rats, mice, and humans to the
development of bladder tumours via such a mechanism; it has therefore
been suggested that the sex- and species-specific development of
bladder tumours in male rats receiving high doses of biphenyl might
not be strictly relevant to humans exposed to lower levels. However,
the incidence of histopathological effects and calculi formation
within the urinary bladder also increased in female rats administered
biphenyl for 2 years, in the absence of bladder tumours (Japan
Bioassay Research Center, 1996). Moreover, data identifying a direct
association between calculi formation, regenerative hyperplasia of the
urothelium, and the development of bladder tumours within individual
male animals are not available. Biphenyl has been reported to be
mutagenic and clastogenic in some, but not all, in vitro assays and
to bind covalently to macromolecules (i.e. proteins) following
metabolic activation; the available data have been considered
inadequate to assess its genotoxicity in vivo. These considerations,
coupled with the structural relationship of 4-hydroxybiphenyl, a major
metabolite of biphenyl, to the potent human bladder carcinogen
4-aminobiphenyl and the lack of critical information related to the
mechanism by which these bladder tumours arise in male rats, could
suggest that the development of bladder tumours in the male rats may
not have been entirely due to effects associated with the formation of
calculi within the urinary bladder. This observation, as well as the
slightly increased incidence of liver tumours in female mice, raises
some concerns with respect to the potential carcinogenicity of
biphenyl to humans.
11.1.2 Criteria for setting guidance values for
biphenyl
A provisional tolerable daily intake (TDI), based upon the
development of effects in the blood of rats administered diets
containing biphenyl for 2 years (Japan Bioassay Research Center,
1996), can be derived as follows:
TDI = 38 mg/kg body weight per day
1000
= 38 µg/kg body weight per day
where:
* 38 mg/kg body weight per day is the lowest LOEL for the
development of alterations in blood chemistry in rats provided
diets containing biphenyl for 2 years. This intake is lower than
intakes associated with the development of haematological
effects, calculi, and histopathological changes in the bladder
and/or kidneys in rats and the development of haematological and
histopathological effects in mice administered biphenyl in the
diet for 2 years.
* 1000 is the uncertainty factor (×10 for interspecies variation;
×10 for intraspecies variation; ×10 for extrapolation from a LOEL
to a NOEL).
However, based upon the currently available data, there is some
uncertainty surrounding the health-protective nature of the value,
owing to lack of critical information concerning the mechanism by
which biphenyl induces bladder tumours in male rats, the slight
increase in hepatocellular tumours in female mice, and the potential
genotoxicity of this substance. Because of this uncertainty, the TDI
is designated provisional.
Available data were inadequate to serve as a basis for the
derivation of guidance values for biphenyl in air.
11.1.3 Sample risk characterization
For the general population, there is insufficient information
available concerning the intake of biphenyl from the general
environment; therefore, comparisons with the provisional TDI are
provided merely as an example. From limited information on the content
of biphenyl in citrus fruits, the estimated daily intake is <375 ng
biphenyl/kg body weight from each individual fruit consumed, while the
estimated daily intake from tap-water is <0.16 ng/kg body weight.
Assuming that a person consumes one citrus fruit per day, the
estimated intake of biphenyl from drinking-water and citrus fruit
consumption is approximately two orders of magnitude lower than the
provisional TDI based upon the development of effects in the blood.
From a study on workers exposed in a plant producing
biphenyl-impregnated paper, it can be derived that exposure to high
biphenyl concentrations up to 128 mg/m3 pose a hazard. Neurotoxic
effects and liver damage have been observed.
Additional information is required to better define any potential
carcinogenic risks that might be associated with exposure to biphenyl.
11.2 Evaluation of environmental effects
The atmosphere is likely the main target compartment for
biphenyl; however, the chemical has been detected in all environmental
compartments as a result of its release from vehicle emissions,
heating, smoking, and industrial activities. Water, soil, and biota
appear to be affected mainly in the direct vicinity of industrial
activities. The decline in the levels of biphenyl in surface waters
may be due to restrictions on the production and use of PCBs. There
are no data available on the release of biphenyl into environmental
compartments from industrial use, from the application of
biphenyl-contaminated sewage sludge to arable land, from
volatilization during the storage of treated fruits, and from leaching
from preserved citrus fruits and their packaging materials in domestic
waste dumps. Only estimated values are available for the
photooxidation of biphenyl in the atmosphere, which is probably the
most important degradation process in this compartment.
Biomagnification of biphenyl in higher trophic levels of the
aquatic or terrestrial food-chain, based upon the potential of
biphenyl to bioaccumulate, is expected to be of minor importance. For
the aquatic compartment, a significant elimination of biphenyl can be
expected as a result of evaporation and degradation. Furthermore,
rapid metabolism of biphenyl in laboratory animals and low acute
toxicity after ingestion have been reported.
Data on effect concentrations for terrestrial organisms and
exposure levels in air and soil are insufficient for risk
characterization for terrestrial organisms. A sample risk
characterization with respect to the aquatic environment can be
performed (based upon data derived from Germany) by calculating the
ratio between a (local or regional) PEC (based on measured or model
concentrations) and a PNEC (EC, 1996).
The annual average concentration of biphenyl (<0.5 µg/litre)
measured in the 1990s in the Rhine River and its tributaries was used
as a PECmeasured. A PEClocal (water), based upon data derived from the
effluent of the wastewater treatment plant of a German producer, may
be calculated as follows:
PEClocal (water) = Ceffluent
(1 + Kp (susp) × Csusp × 10-6) × D
= <0.0064 µg/litre
where:
* Ceffluent is <5 µg/litre, the concentration of biphenyl in
wastewater in 1990 (BUA, 1990);
* Kp (susp) = 423, the suspended matter/water adsorption
coefficient, estimated from the mean soil sorption coefficient
( Koc) of 4230 ( Kp (susp) = Koc × 0.1, where 0.1 is the
fraction of organic carbon in suspended matter, foc(susp));
* Csusp = 15 mg/litre (default value; EC, 1996), the
concentration of suspended matter in the river; and
* D = 781, the dilution factor for river flow, calculated from
sewage treatment plant and river flow data (EC, 1996).1
A PNEC for surface waters may be calculated by dividing the
lowest valid LC50 or NOEC by an appropriate assessment factor (equal
to a safety factor):
PNEC = 170 µg/litre
10
= 1.7 µg/litre
where:
* 170 µg/litre is the lowest NOEC from a chronic study with
Daphnia magna; and
1 Personal communication from Verband d. Textilhilfsmittel-,
Lederhilfsmittel-, Gerbstoff- und Waschrohstoff-Industrie e.V.,
Frankfurt/M., Germany, to Bayer AG, 1996.
* 10 is the assessment factor. For biphenyl, based upon data for
four trophic levels, including one long-term NOEC, the
recommended assessment factor would be 100 (EC, 1996). However,
because of its significant volatility and degradation, and as
effect concentrations from a wide selection of species covering
different taxonomic groups are reported, an assessment factor of
10 is used.
Therefore, based upon the annual average concentrations of
biphenyl measured in the Rhine River and in industrial wastewater
effluent in Germany, the PECmeasured/PNEC and PEClocal (water)/PNEC
ratios are <0.29 and <0.04, respectively. In some jurisdictions
(EC, 1996), chemicals with a PEC/PNEC ratio of <1 may not require
implementation of risk reduction measures beyond those already in
place.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The Joint FAO/WHO Meeting on Pesticide Residues has allocated an
acceptable daily intake of biphenyl of 0-0.125 mg/kg body weight
(JMPR, 1967).
Information on international hazard classification and labelling
is included in the International Chemical Safety Card reproduced in
this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventative and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0106) reproduced in this
document.
13.1 Advice to physicians
Treatment is symptomatic and supportive following intoxication.
The clinical picture is characterized by central and peripheral nerve
damage and liver injury in human poisoning. There is some evidence to
indicate that electroencephalographic abnormalities could persist up
to 1 or 2 years after exposure.
13.2 Health surveillance advice
Objective change in electroencephalogram and electroneuromyogram
could be an indication of biphenyl poisoning in exposed individuals.
Monitoring of liver function could also be integrated into a medical
surveillance programme.
13.3 Spillage
In the event of spillage, measures should be under taken to
prevent biphenyl from reaching drains and watercourses, because of its
toxicity to aquatic organisms.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards
can be found in the International Register of Potentially Toxic
Chemicals (IRPTC), available from UNEP Chemicals (IRPTC), Geneva.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
BIPHENYL ICSC: 0106
April 1994
CAS #
The chemical is an aromatic hydrocarbon with a peculiar, strong
odour similar to that of geraniums (BUA, 1990). At room temperature,
the substance is a colourless solid (melting point 62.2°C). Because of
its significant vapour pressure (4 Pa at 20°C) and low water
solubility (4.45 mg/litre at 20°C), biphenyl shows considerable
volatility from aqueous solutions (BUA, 1990). Its measured
n-octanol/water partition coefficient (log Kow) is between 3.88
and 4.04. The commercial product from the only German producer has a
biphenyl content of 99.85%; named impurities are terphenyl (0.15%),
sulfur (10-20 mg/kg), and benzene (1 mg/kg) (BUA, 1990). Additional
physical/chemical properties are presented in the International
Chemical Safety Card reproduced in this document.
3. ANALYTICAL METHODS
Biphenyl is usually measured in environmental media by capillary
gas chromatography in combination with flame ionization or mass
spectrometric detection (Malins et al., 1985; BUA, 1990; Hawthorne et
al., 1992; Anklam & Mueller, 1994; Karanassios et al., 1994; Otson et
al., 1994; Kostiainen, 1995). For aquatic samples, methods involving
high-performance liquid chromatography with ultraviolet detection at
254 nm have also been described. The following enrichment techniques
are used: solid-phase adsorption (Tenax material or XAD resins) with
thermal desorption or liquid extraction for air samples, solid-phase
adsorption (XAD resins) or liquid/liquid extraction (diethylether) for
water samples, and liquid extraction (dichloromethane/methanol,
ethanol) for soil and biotic samples. The detection limits range from
0.1 to 5 ng/m3 for air and from 0.01 to 8000 ng/litre for water. For
sediment, a detection limit of 2 µg/kg dry weight has been reported.
Detection limits between 12 and 87 µg/kg have been reported for levels
of biphenyl in fish (Malins et al., 1985).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Biphenyl occurs in varying concentrations in coal tar, crude oil
(up to 0.4 mg/g oil), and natural gas (3-42 µg/m3) (BUA, 1990).
Because of its natural occurrence in coal tar and crude oil, biphenyl
has also been detected in products derived from these substances. The
biphenyl content in coal tar-derived creosotes ranges between 0.2 and
1.6% (IARC, 1985; ATSDR, 1990; Collin & Hoeke, 1995). In the aromatic
fraction of an unused lubricating oil sample, biphenyl was measured at
a concentration of 1.5 mg/kg (Paschke et al., 1992).
Biphenyl is also produced from anthropogenic sources. In 1984,
the estimated production capacities of biphenyl in the Federal
Republic of Germany, in Western Europe, and throughout the world were
approximately >6000, 30 000, and 80 000 t, respectively. In 1989,
2000-2500 t of biphenyl were produced in Germany, of which about 900 t
were exported and 200-300 t sold domestically (BUA, 1990).
Approximately 5000 t of biphenyl were manufactured in each of 1992 and
1993 in Japan (Anon., 1994-95).
Until the early 1970s, biphenyl was used principally as an
intermediate in the production of polychlorinated biphenyls (PCBs). As
the production, processing, distribution, and use of PCB compounds are
now prohibited or restricted in many countries (e.g. USA, Germany,
United Kingdom) (IRPTC, 1994), it is likely that previous production
levels were much higher than those today. In 1984, the estimated use
patterns of biphenyl worldwide were, as a final product: 35% in heat
transfer medium in heating fluids, 20% as a dyestuff carrier for
textiles, 5% as a solvent in pharmaceutical production, 5% as a
dyestuff carrier for copying paper, and 5% as a preservative for
citrus fruits; patterns of biphenyl use as an intermediate were: 10%
each in emulsifiers and optical brighteners, 5% for crop protection
products, and 5% for precursors and auxiliaries for plastics (BUA,
1990). At present in Germany, the use of biphenyl as a dyestuff
carrier in textiles is minor.1
Although emissions from exhaust gases during production and
processing of biphenyl and during the use of biphenyl-containing
creosotes for wood preservation may be significant, available data
were not identified. Biphenyl is also released into the atmosphere
during the incomplete combustion of fossil fuels in motor vehicles
(post-catalyst emission factor of 3.5 µg/km; Siegl & Chladek, 1992),
residential heating (1.24 mg biphenyl/kg burnt coal; Engewald et al.,
1993), and coal-burning power plants (with concentrations in flue gas
up to 30 µg/m3; Yao & Xu, 1991; Wienecke et al., 1992) or foundries
(qualitatively detected; Deusen & Kleinermanns, 1993).
1 Personal communication from Verband d. Textilhilfsmittel-,
Lederhilfsmittel-, Gerbstoff- und Waschrohstoff-Industrie e.V.,
Frankfurt/M., Germany, to Bayer AG, 1996.
In 1990, biphenyl was not detected in the effluent of the sewage
plant of the German producer (detection limit 5 µg/litre; weekly
sampling). Based on a biphenyl concentration of <5 µg/litre, a
maximum annual release of 274 kg biphenyl can be calculated for this
plant. No data are available from other producing or processing
facilities. Biphenyl may also be released to surface water through the
processing of coal tar (e.g. coking plants) and mineral oils (e.g.
processing residual oils from pyrolysis in refineries) (BUA, 1990).1
Based upon levels of biphenyl measured in sediment samples during the
1970s, this chemical has been released from various industrial
wastewater outlets associated with a melting plant for iron alloys, a
chemical factory, and an oil storage depot. Data were not available on
releases of biphenyl from textile industries where the chemical is
used as a dyestuff carrier, from wood preservation plants that use
creosotes containing biphenyl, or from treated citrus fruits and their
packaging materials contained in domestic waste sites. Concentrations
of biphenyl between 0.016 µg/kg and 1730 mg/kg have been measured in
sewage sludge, which may be applied to arable land (BUA, 1990).
1 Personal communication from Bayer AG to the GDCh- Advisory
Committee on Existing Chemicals of Environmental Relevance, 1997.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Based upon a Level II fugacity model (Mackay et al., 1992), the
atmosphere appears to be the main target compartment (>90%) for
biphenyl. Biphenyl's Henry's law constant indicates that it
volatilizes from aqueous solutions. The calculated half-life for the
photooxidative degradation of biphenyl by hydroxyl radicals in air is
about 2 days (Atkinson & Aschmann, 1985; BUA, 1990; Atkinson & Arey,
1994). Reactions with tropospheric ozone and nitrate are expected to
be of minor importance, with calculated half-lives of >80 days and
>105 days, respectively. Data on the photodegradation of biphenyl in
water are not available. Biphenyl is not expected to hydrolyse under
environmental conditions.
In one reported standard test with activated sludge, according to
Guideline 301C of the Organisation for Economic Co-operation and
Development (OECD), a biochemical oxygen demand of 66% after 14 days'
incubation was reported when non-adapted inoculum was used (CITI,
1992). Microbial populations from natural waters mineralize biphenyl;
100% degradation was observed after 4 days' incubation of biphenyl in
river water (BUA, 1990). Numerous microorganisms isolated from soil,
sediment, surface water, and groundwater samples have been shown to
metabolize biphenyl (BUA, 1990; Bevinakatti & Ninnekar, 1992; Kiyohara
et al., 1992; Nielsen & Christensen, 1994). Investigations on the
anaerobic biodegradation of biphenyl were not identified.
Soil sorption coefficients ( Koc) based upon laboratory
experiments and calculated values range from 1100 to 18 000 (BUA,
1990; Kishi & Hasimoto, 1991); from numerous literature data, Jeng et
al. (1992) calculated a mean value of 4230. On the basis of these
data, it is expected that biphenyl will be almost immobile in soil,
with a low probability of groundwater infiltration. Volatilization
from soil surfaces is significantly lower than that from aqueous
media; however, no significant geoaccumulation of biphenyl is expected
under aerobic conditions owing to its degradation by microbial
organisms.
In static tests on the bioaccumulation of biphenyl in activated
sludge conducted with several aquatic species (yeast, algae, molluscs,
daphnia, and freshwater fish), bioconcentration factors (BCFs) ranging
between 57 for the marine mussel Mytilus edulis (calculated on wet
weight) (Donkin et al., 1991) and 540 for the green alga Chlorella
vulgaris (based on dry weight) (Kotzias et al., 1980; Freitag et
al., 1982) have been reported. With the exception of two tests, there
was no information on whether equilibrium was reached before the
determination of the BCF. The depuration of accumulated biphenyl was
determined in one study. After a 24-h incubation in a static test
system, the BCF at equilibrium (based on wet weight) for Daphnia
magna was 473; depuration was dependent upon the temperature of the
water. Twenty-four hours after being transferred to pure water, 53% of
the accumulated biphenyl was depurated at 2-3°C, whereas 88% was
depurated at 22°C (Zhang et al., 1983). Although the available data
indicate a potential for bioaccumulation, evaporation, adsorption to
soil/sediment, and degradation are expected to reduce the
bioavailability of biphenyl. Therefore, bioaccumulation of the
chemical should be of minor importance for aquatic organisms.
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
In 1985, concentrations of biphenyl in the air of an
industrialized city in Finland ranged from 1.7 to 26.2 ng/m3 (BUA,
1990). Similar concentrations were measured during the winter of
1988-89 in two US cities (12-119 ng/m3; 30 ng/m3 mean value;
Hawthorne et al., 1992) and in 1992 in two Greek cities (all data
below the detection limit of 5 ng/m3; Karanassios et al., 1994).
The concentrations of biphenyl in the German parts of the Rhine
River declined from a maximum of 1000 ng/litre in the 1970s (BUA,
1990) to levels below 500 ng/litre (the detection limit) in 1993-1995
(LWA, 1993-94).1 Although levels in most of the German tributaries
have remained below the detection limit (500 ng/litre), peak
concentrations of 560 and 1600 ng/litre were reported in 1993 and
1994, respectively, for the highly polluted Emscher tributary (LWA,
1993-94); these elevated levels were attributed to the coking plants
in this area.2 In the USA, similar data for one river have been
reported (Hites, 1973). In some German estuaries, biphenyl
concentrations were significantly lower (1-5 ng/litre) (BUA, 1990).
During 1986-1989, biphenyl was detected at concentrations between
10 and 100 ng/litre in samples of groundwater obtained near Zagreb, in
the former Yugoslavia, in the vicinity of a municipal landfill site.
In a test well sunk 720 m from a heavily polluted river, the levels
were about 10-fold lower (Ahel, 1991). Concentrations of biphenyl
between 2 and 17 ng/litre have been measured in samples of snow and
rain collected in Switzerland (BUA, 1990).
In river and estuarine sediments, biphenyl was detected at very
high concentrations in the direct vicinity of industrial plants or
waste dumps; concentrations ranged between 0.1 and 8 mg/kg, depending
on the source. The highest concentrations were found near waste dump
sites in the USA (Malins et al., 1985; BUA, 1990). Information on the
occurrence of biphenyl in soils with no direct pollution was not
identified. A maximum biphenyl concentration of 13 µg/kg was found in
soil samples collected near a pit for wastewater from oil production
in New Mexico, USA (BUA, 1990).
Biphenyl has been found in fish collected from water contaminated
with mineral oil; concentrations in liver samples were <25 µg/kg dry
weight (i.e. below the detection limit) and 13 620 µg/kg fresh weight
(Paasivirta et al., 1982; Malins et al., 1985).
1 Personal communication from the Northrhine-Westfalian
Environmental Protection Agency to Bayer AG, 1996.
2 Personal communication from Bayer AG to the GDCh- Advisory
Committee on Existing Chemicals of Environmental Relevance, 1997.
6.2 Human exposure
The levels of biphenyl in indoor air may depend mainly on smoking
habits (BUA, 1990). Concentrations of biphenyl are also elevated in
homes having a source of oil and exhaust fumes nearby (e.g. gasoline
station, parking garage) (Otson et al., 1994; Kostiainen, 1995).
Monitoring data from Finnish ( n = 50) (Kostiainen, 1995) and
Canadian ( n = 757) homes (Otson et al., 1994) have revealed average
biphenyl concentrations in the range of 160-1000 ng/m3, with a
maximum concentration of 4700 ng/m3 (Kostiainen, 1995). Biphenyl has
been detected in floor dust collected from five of nine investigated
Danish city halls (Wilkins et al., 1993). Assuming that 20 h are spent
indoors (exposure to 160-1000 ng/m3) and 4 h are spent outdoors
(exposure to 30 ng/m3) each day and that the inhalation volume and
weight of the average adult are 22 m3/day and 64 kg, respectively,
the intake of biphenyl from air can be calculated from these data to
range from 45 to 300 ng/kg body weight per day.
There is only limited intake of biphenyl from drinking-water.
Although more recent data were not identified, concentrations of
biphenyl in tap-water in various countries, such as Finland, Norway,
Sweden, the USA, and Canada, were low (i.e. 0.1-5 ng/litre) in the
1970s (BUA, 1990). Based upon these data and assuming that the average
adult (weighing 64 kg) consumes 2 litres of drinking-water per day,
the estimated intake of biphenyl from drinking-water may range from
0.003 to 0.16 ng/kg body weight per day.
Food may contain biphenyl as a result of its use as a fungistatic
agent for citrus fruits. In older studies with citrus fruits to which
a biphenyl-containing wax had been applied, peels of treated fruits
were found to contain up to 220 mg biphenyl/kg (whole fruit) and the
pulp up to 3.9 mg/kg (whole fruit) (BUA, 1990); however, these levels
were determined with a rather unspecific spectrophotometric method,
which may have overestimated the biphenyl content. In a more reliable
study with 6 grapefruits, 8 oranges, and 10 lemons obtained from
Japanese markets, levels of biphenyl ranged from 17 to 123 mg/kg in
the peel and from <0.01 (the detection limit) to 0.18 mg/kg (average
0.06 mg/kg pulp) in the edible parts of the fruit. In samples of lemon
tea, levels of biphenyl were similar to those in the edible parts of
the fruit (Isshiki et al., 1982). In a survey of foodstuffs conducted
in Italy between 1988 and 1995, which included more than 70 types of
food, biphenyl was detected in only one peach sample (detection limit
not given) (Di Muccio et al., 1995). Based upon the average content of
biphenyl in the pulp of citrus fruits of about 0.06 mg/kg (calculated
from the concentrations measured by Isshiki et al., 1982), the intake
from the consumption of the pulp of one biphenyl-preserved citrus
fruit weighing about 120-400 g is calculated to be about 113-375 ng/kg
body weight. An average intake of 64 ng biphenyl/kg body weight per
day from food was calculated based upon the consumption of foodstuffs
in Finland (Penttilae & Siivinen, 1996). The general population may
also be exposed to biphenyl through contact with consumer products,
such as creosote-preserved wood, textiles, copying papers, or
pharmaceuticals, although quantitative data were not identified.
The intake of biphenyl from the occupational environment may
occur via inhalation and dermal contact. Concentrations of biphenyl in
the air of one facility producing biphenyl-impregnated paper were
reported to range from 4.4 to 128 mg/m3 in 1959 and from 0.6 to 123
mg/m3 in 1970 (Haekkinen et al., 1973). In a nylon production
facility in which workers were exposed to a eutectic mixture of 26.5%
(w/w) biphenyl and 73.5% (w/w) biphenyl ether, time-weighted average
concentrations of biphenyl were reported to range from 0.24 to 1.28
mg/m3 (Dorgelo et al., 1985). Coke oven emissions to indoor air at a
coking plant in Australia contained 0.2-0.5% biphenyl (Kirton et al.,
1991). In a Polish bituminous pulp production facility, reported
time-weighted average concentrations (1988-89) of biphenyl ranged from
0.5 to 0.6 µg/m3 (Baranski, 1991). In a study of two Canadian
pilot-scale coal liquefaction facilities, the levels of biphenyl were
below the detection limit of 60 µg/m3 (Leach et al., 1987).
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS
AND HUMANS
Studies providing quantitative information on the absorption or
distribution of biphenyl in humans were not identified. In various
species of experimental animals, the absorption of biphenyl following
oral exposure has been inferred by the detection of metabolites in the
urine and faeces. Biphenyl is oxidized by multifunctional oxygenases,
irrespective of the route of exposure. Mono-, di-, and trihydroxy
metabolites of biphenyl have been identified in the urine of exposed
species. 4-Hydroxybiphenyl has been observed as the major metabolite
in rats, rabbits, guinea-pigs, and pigs; minor metabolites include
mono-, di-, and trihydroxymethoxy biphenyls, dihydrodiols, and
hydroxydihydrodiols. The phenolic compounds are conjugated with
sulfate or glucuronic acid. The formation of mercapturic acid as well
as evidence of a metabolic pathway involving opening of the benzene
ring have also been described (BUA, 1990). There is no evidence for
enterohepatic circulation in different species (Meyer & Scheline,
1976; Meyer et al., 1976a; Meyer, 1977). On the basis of in vitro
studies, the metabolism of biphenyl is not restricted to the liver
(Bend et al., 1972; Hook et al., 1972; Matsubara et al., 1974; Prough
& Burke, 1975; Hook & Bend, 1976; Powis et al., 1987). Based upon
total cytochrome P-450 content, biphenyl-4-hydroxylase activity was
higher in pulmonary microsomes than in liver microsomes (Hook et al.,
1972; Hook & Bend, 1976). A time-dependent covalent binding of
[14C]bi phenyl to mouse hepatic microsomal proteins has been observed
after metabolic activation (Tanaka et al., 1993).
In rats, rabbits, and pigs, most biphenyl metabolites are
excreted in the urine (BUA, 1990). Following an oral dose of 100 mg
[14C]biphenyl/kg body weight, rats excreted 82% of the administered
radioactivity (76% in urine) within 24 h. The recovery rate after 4
days was 92%, with 7% of the administered radioactivity detected in
the faeces and traces in the exhaled air. Eight days after
administration, radioactivity in the tissues was 0.6% of the applied
dose (Meyer et al., 1976b). In none of the species examined was
unmetabolized biphenyl found in the urine (BUA, 1990).
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
The acute oral toxicity of biphenyl is moderate. The LD50 for
rats and mice is >1900 mg/kg body weight (BUA, 1990). Acute effects
include polyuria, accelerated breathing, lacrimation, anorexia, weight
loss, muscular weakness and coma, fatty liver cell degeneration,
severe nephrotic lesions (often manifested as acute and subacute
glomerulotubular nephritis), degenerative myocardial lesions,
pulmonary hyperaemia, and, occasionally, alveolar oedema. Interstitial
and lobular pneumonitis have been observed in animals that survived
for a few days.
The LC50 (4-h) for the mouse is >43 ppm (275 mg/m3). During
exposure, hyperactivity and mild respiratory discomfort have been
observed; however, these effects were not evident at the end of a
14-day recovery period. Gross pathological examination performed on
surviving animals revealed slight lung congestion (Sun Co. Inc.,
1977a). In an inhalation study conducted with Sprague-Dawley rats, no
effects were observed after a 6-h exposure to 0.8 or 3 ppm (5.1 or
19.2 mg/m3) biphenyl at temperatures of approximately 26°C or 32°C,
respectively (Monsanto Co., 1959). No treatment-related alterations in
appearance, demeanour, food consumption, or survival were observed
following a 7-h inhalation exposure of rats to 3 g biphenyl/m3
(nominal concentration); no detailed information on the methods or
particle size of the substance were presented (Dow Chemical Co.,
1974).
Information on the acute toxicity of biphenyl associated with
dermal exposure was not identified.
8.2 Irritation and sensitization
Biphenyl is non-irritating to both intact and scarified rabbit
skin. The substance was slightly irritating when applied to rabbits'
eyes in an eye irritation test (BUA, 1990).
In a guinea-pig maximization test conducted according to OECD
Guideline 406, there was reportedly no evidence of a skin sensitizing
potential for biphenyl (Dreist & Kolb, 1993).
8.3 Short-term exposure
8.3.1 Oral exposure
In the studies cited below, the histopathological examination was
focused on the urinary system. In a study in which male and female
Wistar rats were fed 0, 50, 150, 300, or 450 mg biphenyl/kg body
weight per day via the diet for 21 days, increased relative kidney
weights and polycystic renal changes (with increased urine volume and
specific gravity) were observed at doses of 50 and 150 mg/kg body
weight per day, respectively. An increase in urine volume, specific
gravity, and absolute kidney weight as well as polycystic renal
changes were also observed in rats administered 500 or 1000 mg
biphenyl/kg body weight per day in the diet for 14 days (Sœndergaard &
Blom, 1979).
When male albino rats were administered diets containing 0, 1000,
2500, 5000, or 10 000 mg biphenyl/kg (estimated intakes of 0, 75, 188,
375, or 750 mg/kg body weight per day) for 26 days, there was a
dose-dependent (at doses of >188 mg/kg body weight per day)
intensification of cloudiness in the urine, associated with an
increase in precipitate formation in samples, which had been cooled or
treated with sulfosalicylic acid. The precipitate consisted of free
4-hydroxybiphenyl and its glucuronide. These effects were largely
reversible during a 28-day recovery period (Booth et al., 1956, 1961).
8.3.2 Inhalation exposure
No evidence of histopathological changes in the lung, trachea,
liver, kidney, or spleen were observed in male and female mice exposed
by inhalation to 25 or 55 ppm (about 160 or 350 mg/m3) biphenyl for 7
h/day, 5 days/week, for 2 weeks (Sun Co. Inc., 1977b).
8.3.3 Dermal exposure
In rabbits, the repeated dermal application (20 in total) of
purified biphenyl (0.5 g/kg body weight) for 2 h/day, 5 days/week,
produced decreased body weight; one animal died after 8 applications.
A decrease in body weight was also observed after repeated dermal
application of technical biphenyl. In both studies, no effect upon the
skin was observed. Histopathological examination revealed minimal
changes in the heart, liver, kidneys, and, in some cases, spleen (no
further details provided) (Deichmann et al., 1947). No adverse effects
were observed when biphenyl was applied (8 h/day, 5 days/week, for a
total of 30 applications) to the intact and abraded skin of rabbits at
doses of 600 or 2000 mg/kg body weight per day (no further information
provided) (Newell, 1953).
8.4 Long-term exposure
8.4.1 Subchronic exposure
8.4.1.1 Oral exposure
In all of the studies cited below, the histopathological
examination was focused on the urinary system. A summary of the
effects related to this specific end-point (changes in kidneys,
ureter, and urinary bladder) for both subchronic and chronic exposures
is provided in Table 1.
Table 1: Summary of the effects of repeated biphenyl administration via diet on urine status, urinary bladder, and kidneys.
Species/strain/ Application/duration NO(A)EL
sex (mg/kg) Urine status Urinary bladder Kidneys Reference
rat/F344/male 0 or 5000 mg/kg <5000 crystals with simple hyperplasia not examined Shibata et al.
8 weeks microcalculi in and increased (1989a)
urinary sediment DNA synthesis
in the urothelium
rat/F344/male 0 or 5000 mg/kg <5000 microcalculi simple hyperplasia, increase in absolute/relative Shibata et al.
up to 24 weeks papillary or kidney weights; no (1989b)
nodular hyperplasia morphological changes of
of the urothelium the renal papillae or
after >16 weeks pelvis; no changes in DNA
synthesis in the renal
papillae and pelvis; focal
calcification of the renal
medulla
rat/albino/male 0, 1000, 2500, or 1000 >2500 mg/kg: not examined 2500 mg/kg: morphological Booth et al.
and female 5000 mg/kg increase in changes (tubular dilation) (1961)
up to 24 weeksa volume, turbidity, after >60 days
and precipitate 5000 mg/kg: morphological
after >30 days; changes (tubular dilation)
urinary sediment: after >30 days; effects
4-hydroxybiphenyl not completely reversible
and its
glucuronide;
effects reversible
rat/F344/male 0 or 5000 mg/kg <5000 increase in pH increased incidence not given Kurata et al.
32 weeks value and Na+ of calculi; (1986)
concentration; histopathology:
urinary sediment: no effects
4-hydroxybiphenyl
Table 1 (Cont'd.)
Species/strain/ Application/duration NO(A)EL
sex (mg/kg) Urine status Urinary bladder Kidneys Reference
rat/Wistar/male 0, 1250, or 1250 not given 5000 mg/kg: 5000 mg/kg: increased Shiraiwa et al.
5000 mg/kg increased incidence incidence of calculi (1989)
36 weeks of calculi in ureter and increase in
and bladder relative kidney weights
rat/Wistar/male 0, 2500, or <2500 haematuria at >2500 mg/kg: >2500 mg/kg: increased Takita (1983);
and female 5000 mg/kg >2500 mg/kg increased incidence incidence of calculi Shiraiwa et al.
75 weeks after >16 weeks of calculi (composed (composed of protein) (1989)
of magnesium after >16 weeks;
ammonium phosphate) kidneys with stones:
after >16 weeks in obstructive
ureter (females) pyelonephritis,
5000 mg/kg:increased tubular atrophy,
incidence of calculi fibrosis
in ureter and 5000 mg/kg: increase
bladder after >16 in relative kidney
weeks; bladder with weights in females
calculi: simple or
diffuse hyperplasia
and papillomatosis
of the urothelium
rat/Wistar/male 0, 630, or 1250 mg/kg 1250 not given no urolithiasis not given Takita (1983)
and female 104 weeks (no further
information given)
rat/F344/DuCrj/ 0, 500, 1500, or <500b >500 mg/kg: 4500 mg/kg: increase >1500 mg/kg: increase Japan Bioassay
male and female 4500 mg/kg decrease in in pH (males and in hyperplasia of Research Center
104 weeks protein (males) females) 4500 mg/kg: the renal pelvis in (1996)
>1500 mg/kg: increase in calculi females
decrease in and hyperplasia 4500 mg/kg: increase
protein (females) of the urothelium in calculi (males >
Table 1 (Cont'd.)
Species/strain/ Application/duration NO(A)EL
sex (mg/kg) Urine status Urinary bladder Kidneys Reference
(males >> females); females) and
increased tumour hyperplasia of the
incidence in males renal pelvis (males)
mouse/B6C3F1/ 0 or 10 000 mg/kg 10 000 decreased Na+ no changes in DNA not given Tamano et al.
male 8 weeks concentration synthesis of the (1993)
at week 4 urothelium
mouse/B6C3F1/ 0 or 10 000 mg/kg <10 000 small amounts of simple hyperplasia interstitial nephritis Tamano et al.
male 32 weeks greyish white and papillary or in 5/10 (1993)
and finely nodular dysplasia
granular material in 1/10 mice
mouse/Crj:BDF1/ 0, 667, 2000, or 667 6000 mg/kg: no changes >2000 mg/kg: mineralization Japan Bioassay
male and female 6000 mg/kg decrease in of the medulla in females Research Center
104 weeks protein and 6000 mg/kg: desquamation (1996)
ketone body and mineralization of the
pelvis in males
a Plus a post-treatment observation period of 9 weeks.
b Effects were observed at the lowest dose tested.
Effects on the urinary tract (i.e. an increased number of
microcalculi in urinary sediment, simple hyperplasia of the bladder
epithelium) were observed in male F344 rats administered 5000 mg
biphenyl/kg (estimated intake 252 mg/kg body weight per day) in the
diet for 8 weeks. Morphological changes and the increased synthesis of
DNA detected in the bladder epithelium after 4 weeks were attributed
to constant irritation by the microcalculi (Shibata et al., 1989a).
In male B6C3F1 mice, no changes in the urinary pH or in levels
of DNA synthesis in the urinary bladder were noted after
administration of 0 or 10 000 mg biphenyl/kg in the diet (0 or 1500
mg/kg body weight per day) over a period of 8 weeks. The urinary Na+
concentration was significantly lower in mice administered 1500 mg/kg
body weight per day compared with controls only at week 4 (Tamano et
al., 1993).
In a range-finding study in which male and female Wistar rats
were administered diets containing biphenyl at 0, 1250, 2500, 5000, 10
000, or 20 000 mg/kg (estimated intakes of 0, 94, 188, 375, 750, or
1500 mg/kg body weight per day) for 10 weeks, dose-dependent effects
(i.e. reduction in weight gain; increased serum activities of
aspartate transaminase, alanine transaminase, and lactate
dehydrogenase; and an increase in blood urea nitrogen) were observed
at all doses (Takita, 1983).
In male F344 rats (20 per group) administered 0 or 5000 mg
biphenyl/kg in the diet (0 or 375 mg/kg body weight per day) for 24
weeks (5 rats per group were sacrificed after 4, 8, 16, or 24 weeks),
reduced body weight gain and an increase in absolute/relative kidney
weights without differences in feed intake were observed in the
biphenyl-exposed rats. The analysis of the urine showed that
administration of biphenyl was associated with the formation of
microcalculi. The histopathological examination of the renal papillae
or pelvis performed after 4, 8, 16, and 24 weeks revealed no
morphological changes; the rate of DNA synthesis in the renal papillae
and pelvis determined after 4 weeks of treatment was unchanged. In
most of the treated animals, a focal calcification of the renal
medulla (no further information given) was noted. The examination of
the epithelium of the urinary bladder revealed a simple hyperplasia in
5/5 rats after 16 and 24 weeks and papillary or nodular hyperplasia in
3/5 rats at week 16 and in 5/5 rats at week 24; no data on controls
were presented (Shibata et al., 1989b).
In albino rats (42 animals of each sex per group) given 0, 1000,
2500, or 5000 mg biphenyl/kg in the diet (0, 75, 188, or 375 mg/kg
body weight per day) for up to 24 weeks, the urine analysis and
histopathological investigations of the kidneys during the study (days
30, 60, and 120) showed no apparent effects at a dose level of 75
mg/kg body weight (Booth et al., 1961). In some animals, early
indications of a kidney-damaging effect were observed at doses of 188
and 375 mg/kg body weight at 30 days -- polyuria, increasing
cloudiness of the urine, and initial morphological changes (tubular
dilation) in the kidney. These effects increased in frequency and
degree as the administrations progressed. The urinary sediment
consisted of 4-hydroxybiphenyl and its glucuronide. During a
subsequent 60-day observation period, the urinary status normalized;
however, changes in kidneys were not completely reversible (a few
dilated tubules and scars remained) (Booth et al., 1961).
In male F344 rats (25 per group) administered 0 or 5000 mg
biphenyl/kg in the diet (0 or 375 mg/kg body weight per day) for 32
weeks, a reduction in body weight gain but no clinical signs of
poisoning were observed in dosed animals. In urine, an increase in pH
value and Na+ concentration was noted. At terminal necropsy, calculi
were found in the urinary bladder. The urinary sediment consisted
mainly of 4-hydroxybiphenyl. No associated histopathology was detected
upon microscopic examination of the urinary bladder (Kurata et al.,
1986).
When groups ( n = 25) of male Wistar rats were provided diets
containing 0, 1250, or 5000 mg biphenyl/kg (0, 94, or 375 mg/kg body
weight per day) for 36 weeks, a reduction in body weight, increased
relative kidney weights, and an increase in the number of animals with
stones in the kidneys (0/25, 0/25, and 4/25, respectively), ureter
(0/25, 0/25, and 1/25, respectively), and urinary bladder (0/25, 0/25,
and 3/25, respectively) were noted in the high-dose group (Shiraiwa et
al., 1989).
8.4.1.2 Inhalation exposure
Exposure of groups ( n = 50) of male and female CD-1 mice to 25
or 50 ppm (160 or 320 mg/m3; analytical concentrations) biphenyl for
7 h/day, 5 days/week, for 13 weeks produced hyperaemia and focal
haemorrhage in the lung and an increase in hyperplasia of the tracheal
epithelium. As these effects were also observed in some unexposed
controls, they were attributed to the method of aerosol generation
(i.e. inhalation of hot air) (Sun Co. Inc., 1977c).
Marked species differences were observed in a study in which
rabbits, rats, and mice were exposed by inhalation to biphenyl in the
form of dust (50% biphenyl on zeolite) at 5, 40, or 300 mg/m3, 7
h/day, 5 days/week, for up to 13 weeks. No adverse effects were
observed in rabbits. Rats exposed to 40 or 300 mg biphenyl/m3
exhibited increased mortality and irritation of the mucous membranes;
no effects were observed following exposure to 5 mg/m3. Mice were the
most sensitive species. Exposure to 5 mg biphenyl/m3 (the only
concentration tested) resulted in slightly increased mortality, with
all mice exhibiting irritation of the upper respiratory tract (no
further information available). Necropsy of dead rats and mice
revealed mainly inflammatory bronchopulmonary changes. No information
on control animals or particle size was provided (Deichmann et al.,
1947).
8.4.2 Chronic exposure and carcinogenicity
In older studies, which were not further taken into consideration
because of the small number of animals used, limited documentation,
and/or limited exposures, no increase in tumour incidence was observed
in rats and mice when biphenyl was administered orally (at doses up to
750 mg/kg body weight per day for 2 years) (Ambrose et al., 1960;
Innes et al., 1969) or following a single subcutaneous injection of
46.4 mg biphenyl/kg body weight, in which the animals were examined
macroscopically and microscopically after an 18-month observation
period (NTIS, 1968).
In Wistar rats (50 animals of each sex per group) given 0, 2500,
or 5000 mg biphenyl/kg in the diet (0, 188, or 375 mg/kg body weight
per day) for 75 weeks, dose-dependent effects (i.e. reduction in
weight gain; alterations in serum activities of aspartate transaminase
[increased and decreased], alanine transaminase [increased], and
lactate dehydrogenase [increased]; and an increase in blood urea
nitrogen) and a dose-dependent increase in stones of the kidney
(females: 0/43, 1/43, 18/39; males: 0/44, 6/46, 15/47) and ureter
(females: 0/43, 1/43, 2/39; males: 0/44, 0/46, 2/47), accompanied by
haematuria as early as 16 weeks after initiation of exposure, were
seen at >188 mg/kg body weight. At 375 mg/kg body weight, the
relative kidney weights were significantly increased in females, and
an increase in stones of the urinary bladder was seen in males and
females (females: 0/43, 0/43, 6/39; males: 0/44, 0/46, 13/47). The
histopathological examination of the urinary bladder with stones
revealed simple or diffuse hyperplasia and papillomatosis of the
epithelium, but no cancerous changes. The overall tumour incidence was
not increased compared with controls. Kidneys with stones exhibited
obstructive pyelonephritis, tubular atrophy, and fibrosis. Kidney
stones were composed of protein, whereas urinary stones were composed
of magnesium ammonium phosphate (Takita, 1983; Shiraiwa et al., 1989).
No urolithiasis and no increased overall tumour incidence
compared with controls were observed in Wistar rats (50 animals of
each sex per group) provided with diets containing 0, 630, or 1250 mg
biphenyl/kg (0, 47, or 94 mg/kg body weight per day) for 104 weeks.
Dose-dependent effects (i.e. reduction in weight gain; alterations in
serum activities of aspartate transaminase [decreased], alanine
transaminase [increased and decreased], and lactate dehydrogenase
[increased and decreased]) were noted at both doses; 47 mg/kg body
weight is considered the lowest-observed-(adverse-)effect level, or
LO(A)EL (Takita, 1983).
In a study with F344/DuCrj rats performed according to standard
protocols and described in sufficient detail, a significant increase
in neoplastic and non-neoplastic lesions of the urinary bladder and a
significant increase in calculi within the urinary bladder were
observed in high-dose males, after the animals had been given diets
containing 0, 500, 1500, or 4500 mg biphenyl/kg (0, 38, 113, or 338
mg/kg body weight per day) for 104 weeks. In males and females, a
dose-dependent increase in hyperplasia of the epithelium of the renal
pelvis was seen. The results of the histopathological findings in the
urinary bladder and kidneys are summarized in Table 2. Other findings
included increased serum enzyme levels (alkaline phosphatase,
aspartate transaminase, and alanine transaminase) and an increased
urea nitrogen level in low-dose males and mid-dose females, which
became more pronounced with increasing doses. In mid- and high-dose
females and high-dose males, haematological effects (i.e. reduced
haemoglobin concentration and haematocrit) were noted (Japan Bioassay
Research Center, 1996). From this study, a LOEL of 38 mg/kg body
weight was derived.
In a study in which groups of male and female Crj:BDF1 mice were
given diets containing 0, 667, 2000, or 6000 mg biphenyl/kg (0, 100,
300, or 900 mg/kg body weight per day) for 104 weeks, a slight
increase in liver tumours (hepatocellular adenomas and carcinomas) and
basophilic cell foci of the liver was observed in the females at doses
of 300 and 900 mg/kg body weight per day; however, the effects were
not concentration dependent (Japan Bioassay Research Center, 1996)
(see Table 3). In the male and female mice, degenerative changes of
the respiratory epithelium of the nasal cavity and nasopharynx were
observed at doses of >100 mg/kg body weight per day or >300
mg/kg body weight per day, respectively. Other findings included
variations in serum enzyme levels (increase in alkaline phosphatase,
aspartate transaminase, and alanine transaminase) and an increased
urea nitrogen level in the low-dose males and females, which became
more pronounced with increasing doses. In female mice receiving
>300 mg biphenyl/ kg body weight per day and in the high-dose
males, degenerative changes in the kidney (increased mineralization of
the inner stripe of the outer medulla, increase in desquamation of the
epithelium of the renal pelvis) were also observed. Body weight gain
and food consumption were reduced in the high-dose animals (Japan
Bioassay Research Center, 1996).
Information on the toxicological effects of biphenyl following
chronic inhalation or dermal exposure were not identified.
8.4.2.1 Tumour promotion
A number of studies have investigated the tumour-promoting
potential of biphenyl. Kurata et al. (1986) considered biphenyl a
tumour promoter, based upon the results of a study in which groups of
male F344 rats were administered drinking-water containing 0.05%
N-butyl- N-(4-hydroxybutyl)nitrosamine (BBN) (as an initiator) for
4 weeks, followed by 0.5% biphenyl in the diet (estimated intake of
approximately 375 mg/kg body weight per day) for a further 32 weeks,
after which time the animals were examined histopathologically. The
results are provided in Table 4. The effects of biphenyl were
attributed to the increased Na+ content in the urine of exposed
rats, as well as to crystals containing mainly 4-hydroxybiphenyl
detected in the urine.
In a study in which male Wistar rats were administered 0.1%
N-ethyl- N-hydroxyethylnitrosamine in the diet for 2 weeks,
followed by 0, 0.125, or 0.5% biphenyl in the diet for 34 weeks,
exposure to biphenyl had no effect upon the incidence of dysplastic
foci and renal cell tumours induced by N-ethyl- N-hydroxyethylnitro
samine; however, an increase in stones of the kidneys, ureter, and
bladder was observed in rats administered 0.5% biphenyl with or
without N-ethyl- N-hydroxyethyl nitrosamine (Shiraiwa et al.,
1989).
Tamano et al. (1993) maintained male B6C3F1 mice on
drinking-water containing 0.05% BBN supplement for 4 weeks. After the
BBN pre-treatment, the animals received diets with 10 000 mg
biphenyl/kg (1500 mg/kg body weight per day) for 32 weeks. At the end
of the study, the urinary bladder and kidneys were examined
histopathologically. The results are provided in Table 5. In mice
exposed to BBN and biphenyl, the average food consumption and the
final body weight were reduced, whereas the relative weight of the
urinary bladder was increased. The induction of simple hyperplasia and
papillary or nodular dysplasia of the urinary bladder in 1/10 mice
treated with biphenyl only was associated with urolithic residues
(small amounts of greyish white and finely granular material). In mice
treated with biphenyl with or without BBN pre-treatment, the incidence
of interstitial nephritis in the kidneys was 65 and 50%, respectively.
In an early study in which albino mice "initiated" with a single
dermal application of 9,10-dimethyl-1,2-benzanthracene (0.3% in
benzene) received dermal applications of biphenyl (20% in benzene)
twice weekly for 15 weeks, no skin papillomas or carcinomas were
observed in either the vehicle controls or exposed mice 16 weeks after
application of the initiator (Boutwell & Bosch, 1959).
Table 2: Data from Japanese cancer bioassay with male and female rats fed biphenyl in the diet for 2 years.a
Male rats Female rats
0 500 1500 4500 0 500 1500 4500
End-point mg/kg mg/kg mg/kg mg/kg mg/kg mg/kg mg/kg mg/kg
survival rate 37/50 41/50 38/50 31/50 44/50 38/50 44/50 37/50
urinary bladder: neoplastic lesions
transitional cell papilloma 0 0 0 10/50 0 0 0 0
transitional cell carcinoma 0 0 0 24/50 0 0 0 0
(p=0.0001)b
squamous cell papilloma 0 0 0 1/50 0 0 0 0
squamous cell carcinoma 0 0 0 1/50 0 0 0 0
urinary bladder: non-neoplastic lesions (transitional epithelium)
simple hyperplasia 0 0 0 12/50 0 0 1/50 1/50
nodular hyperplasia 0 0 0 40/50 1/50 0 0 5/50
papillary hyperplasia 0 0 0 17/50 0 0 0 4/50
basal cell hyperplasia 0 0 0 27/50 0 0 0 4/50
squamous cell hyperplasia 0 0 0 13/50 0 0 0 1/50
squamous cell metaplasia 0 0 0 19/50 0 0 0 4/50
inflammatory polyps 0 0 0 10/50 0 0 0 0
calculi 0 0 0 43/50 0 0 0 8/50
kidney
mineralization -- papilla 9/50 9/50 14/50 23/50 2/50 6/50 3/50 13/50
mineralization -- pelvis 9/50 6/50 10/50 18/50 12/50 12/50 18/50 27/50
calculi 0/50 0/50 0/50 13/50 0/50 0/50 0/50 3/50
desquamation -- pelvis 1/50 0/50 0/50 11/50 0/50 0/50 0/50 2/50
simple hyperplasia of the 6/50 8/50 5/50 19/50 3/50 5/50 12/50 25/50
transitional epithelium
nodular hyperplasia of the 0/50 1/50 1/50 21/50 0/50 0/50 1/50 12/50
transitional epithelium
ureter dilatation 0/50 0/50 1/50 14/50 0/50 0/50 0/50 6/50
a From Japan Bioassay Research Center (1996).
b Fisher Exact Test.
Table 3: Data from Japanese cancer bioassay with male and female mice fed biphenyl in the diet for 2 years.a
Male mice Female mice
0 667 2000 6000 0 667 2000 6000
End-point mg/kg mg/kg mg/kg mg/kg mg/kg mg/kg mg/kg mg/kg
survival rate 35/50 41/50 41/50 39/50 31/50 22/50 25/50 32/49
liver: neoplastic lesions
hepatocellular carcinoma 8/50 8/49 5/50 4/50 1/50 5/50 7/50 5/49
(p=0.121)b (p=0.043)b
(p=0.1163)b
hepatocellular adenoma 8/50 6/49 7/50 3/50 2/50 3/50 12/50 10/49
(p=0.4909)b (p=0.016)b
(p=0.0251)b
historical control data: historical control data:
carcinoma: 171/700 with a range of 1/50 - 19/50 carcinoma: 15/699 with a range of 0/50 - 2/50
adenoma: 119/700 with a range of 2/50 - 15/50 adenoma: 33/699 with a range of 1/50 - 5/50
basophilic cell foci 0/50 6/49 1/50 2/50 1/50 1/50 12/50 6/49
a From Japan Bioassay Research Center (1996).
b Fisher Exact Test.
Table 4: Results of the tumour-promoting potential (urinary bladder) of
biphenyl in male rats.a
Hyperplasia Papilloma Carcinoma
n (%) n (%) n (%)
biphenyl only
(21 rats) 0 0 0 0 0 0
BBNb only
(24 rats) 6 -25 3 -12 0 0
BBN and biphenyl
(18 rats) 17 -94 15 -83 11 -61
a Taken from Kurata et al. (1986).
b BBN = N-butyl-N-(4-hydroxybutyl)nitrosamine.
Table 5: Results of the tumour-promoting potential (urinary bladder) of
biphenyl in male mice.a
Papillary or
Hyperplasia nodular dysplasia Carcinoma
n (%) n (%) n (%)
biphenyl only
(10 mice) 1 -10 1 -10 0 0
BBNb only
(20 mice) 12 -60 2 -10 0 0
BBN and biphenyl
(20 mice) 14 -70 1 -5 0 0
a Taken from Tamano et al. (1993).
b BBN = N-butyl-N-(4-hydroxybutyl)nitrosamine.
8.5 Genotoxicity and related end-points
The results of the in vitro tests with biphenyl are summarized
in Table 6. In vitro studies with bacteria have yielded no evidence
of mutagenic potential for biphenyl, whereas gene mutation and mitotic
recombination were observed with Saccharomyces cerevisiae D7 in the
presence or absence of metabolic activation. However, no gene
conversion was observed with S. cerevisiae D3. Testing in mammalian
cells has produced positive results for gene mutations and
clastogenicity in the presence of metabolic activation and negative
results in the absence of metabolic activation.
In an in vivo rat cytogenetic assay of bone marrow cells, the
incidence of chromosomal aberrations was not increased (no further
information available) (Kawachi et al., 1980). The exposure by
inhalation of male Sprague-Dawley rats to 64 or 320 mg biphenyl/m3
(as dust aerosol) for 7 h/day, 5 days/week, for 30 days (20 exposures
in total) reportedly did not increase the frequency of chromosomal
aberrations in the bone marrow (Dow Chemical Co., 1976). However,
owing to insufficient documentation (i.e. no data available concerning
dust particle size distribution or cell harvesting times) and the low
number of metaphase cells examined (i.e. 50 cells per animal), the
validity of this study cannot be ascertained.
The results of in vitro studies indicate that biphenyl has
mutagenic potential; in the absence of reassurance from reliable
results from in vivo tests, it is assumed that exposure to biphenyl
may be associated with a mutagenic risk.
8.6 Reproductive and developmental toxicity
Available data on the reproductive or developmental toxicity of
biphenyl are limited. In Wistar rats administered (by gavage) 0, 125,
250, 500, or 1000 mg biphenyl/kg body weight (in corn oil) on days
6-15 of gestation, no maternal toxicity occurred at doses of <500
mg/kg body weight. In the highest dose group, 5 of 20 rats died. Also
at 1000 mg/kg body weight, reduced fetal weight and an increased
number of dead and resorbed fetuses were noted; however, these values
were statistically not significantly different from those of control
animals. At doses of >500 mg/kg body weight, there were
non-significant increases in the incidence of fetuses with missing or
non-ossified sternebrae (Khera et al., 1979).
Table 6: Results of in vitro genotoxicity studies on biphenyl.
Resultsa
Species Concentration Without With
(test system) End-point range MA MA References
Salmonella typhimurium Reverse 0-5000 µg/plate - - Cline & McMahon (1977); Purchase et al.
TA92, TA94, TA97, TA97a, mutations (1978); Kawachi et al. (1980); NTP (1980);
TA98, TA100, TA102, Bronzetti et al. (1981); Probst et al. (1981);
TA1532, TA1535, TA1537, Waters et al. (1982); Haworth et al. (1983);
TA1538, TA2636 Pagano et al. (1983, 1988); Ishidate et al.
(1984); Fujita et al. (1985); Brams et al.
(1987); Bos et al. (1988); Glatt et al. (1992)
Escherichia coli WP2, WP2 Gene mutations 0.1-1000 µg/ml - - Cline & McMahon (1977); Probst et al. (1981);
uvrA- Waters et al. (1982)
E. coli PQ37 DNA damage 2.4-154 µg/ml - - Brams et al. (1987)
Bacillus subtilis rec assay DNA damage no data - 0 Kawachi et al. (1980)
Saccharomyces cerevisiae Gene mutation/ <154 µg/ml + + Pagano et al. (1983)
D7 gene conversion
S. cerevisiae D3 Gene conversion no data - - Waters et al. (1982); Zimmermann et al. (1984)
Chinese hamster cells Gene mutation 0-100 µg/ml - + Glatt et al. (1992)
(V79)
L5178Y TK+/- cells Gene mutation 0-61 µg/ml - (+) Wangenheim & Bolcsfoldi (1988)
(mouse lymphoma assay)
Chinese hamster cells Chromosomal 0-125 µg/ml - 0 Ishidate & Odashima (1977); Kawachi et al.
(CHL) aberration (1980); Sofuni et al. (1985)
Table 6 (Cont'd.)
Resultsa
Species Concentration Without With
(test system) End-point range MA MA References
Chinese hamster cells Chromosomal 0-20 µg/ml - + Sofuni et al. (1985)
(CHL) aberration
Chinese hamster cells Chromosomal 15.4-154 µg/ml - 0 Abe & Sasaki (1977)
(DON) aberration
Rat hepatocytes Unscheduled 0.002-154 µg/ml 0 - Williams (1978); Brouns et al. (1979);
DNA synthesis Probst et al. (1981)
Chinese hamster cells Sister chromatid no data - 0 Kawachi et al. (1980)
(CHL) exchange
Chinese hamster cells Sister chromatid 15.4-154 µg/ml - 0 Abe & Sasaki (1977)
(DON) exchange
L5178Y cells (alkaline DNA damage 0-231 µg/ml - + Garberg et al. (1988)
unwinding assay)
human lung fibroblasts Unscheduled no data - - Waters et al. (1982)
(WI-38 cells) DNA synthesis
human fibroblasts ("nick DNA damage 15.4 µg/ml - 0 Snyder & Matheson (1985)
translation assay")
a -, negative; +, positive; (+) weakly positive; 0, not tested; MA, metabolic activation.
Compared with unexposed controls, litter size (the only parameter
examined) was not affected in a study in which small numbers of male
and female rats were administered diets containing 0.1 or 0.5%
biphenyl (estimated intakes of 75 and 375 mg/kg body weight per day)
before mating and throughout gestation (Ambrose et al., 1960). In an
unpublished three-generation study, dietary biphenyl concentrations of
100 or 1000 mg/kg (estimated intakes of approximately 7.5 or 75 mg
biphenyl/kg body weight per day) had no effect on reproduction in
rats; after intake of 10 000 mg/kg (estimated intake of 750 mg/kg body
weight per day), decreased fertility, litter size, and growth rate
were noted (no further information available) (Stanford Research
Institute, undated). Histopathological changes within the male and
female reproductive systems were not observed in rats or mice
administered biphenyl at 500-4500 mg/kg in the diet for 2 years (Japan
Bioassay Research Center, 1996).
8.7 Immunological and neurological effects
Data on immunological and neurological effects of biphenyl in
laboratory animals were not identified.
9. EFFECTS ON HUMANS
Information on effects on human health resulting from exposure to
biphenyl are limited to case reports; epidemiological studies were not
identified. In one report, a single application of 0.5 ml of a 4%
biphenyl solution (no further details provided) to the skin of the
lower arm of two test subjects caused no apparent irritation
(Macintosh, 1945). Biphenyl did not cause skin irritation in a study
in which a solution of 23% biphenyl in oil was applied to the forearm
three times per week for 8 weeks (Selle, 1952). In one human volunteer
orally given 35 mg biphenyl per week for 13 weeks, no adverse effects
were reported (Farkas, 1939). Cases of headache, nausea, and
inflammation of the respiratory tract have been reported in workers
exposed to biphenyl vapours (and other substances) during paper
production (Weil et al., 1965). Liver damage and effects upon the
central and peripheral nervous systems were attributed to long-term
exposure to high concentrations of biphenyl (see section 6.2) in a
case-study of workers engaged in the production of
biphenyl-impregnated paper (Haekkinen et al., 1973). Chronic
persistent hepatitis in a woman who had worked for 25 years in a
citrus packing plant in which biphenyl-impregnated paper was used was
attributed to the absorption of biphenyl through the skin and
digestive tract (Carella & Bettolo, 1994).
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1 Aquatic environment
The acute toxicity of biphenyl in aquatic organisms has been
investigated in microorganisms, plants, invertebrates, and
vertebrates; however, in several studies cited in the main source
document (BUA, 1990), information on whether results were based on
nominal or effective concentrations was either lacking or not clearly
stated. For biphenyl, nominal concentrations may not correspond to the
effective concentrations, as the water solubility of biphenyl is low
(4.45 mg/litre at 20°C) (test results with higher nominal effect
concentrations have not been reported here) and its volatility
significant. In tests with open systems and longer test durations,
results based on nominal concentrations may lead to an underestimation
of toxic effects because of evaporation of the chemical; therefore,
emphasis has been placed on studies with lowest reported effect levels
in which loss of the test substance was minimized.
Several studies were conducted on the toxic effects of biphenyl
on mixed microbial cultures as well as on single bacterial species.
The most sensitive species was Photobacterium phosphoreum, with a
30-min EC50 of 1.9 mg/litre in a bioluminescence inhibition test
(BUA, 1990). For Colpidium campylum, one of three different species
of ciliata tested, the minimal active concentration of biphenyl for
the inhibition of cell proliferation after a 43-h exposure was 5.6
mg/litre (nominal concentration; test substance predissolved in
acetone) (Dive et al., 1980). In studies on the reduction of
photosynthesis, 3-h EC50 values of 3.86 and 1.28 mg/litre were deter
mined with the unicellular green algae Chlorella vulgaris and
Chlamydomonas angulosa, respectively (BUA, 1990).
Forty-eight-hour LC50 values from acute toxicity tests with
Daphnia magna were in the range of 1.1-4.7 mg/litre when experiments
were conducted in more or less tightly closed test vessels. From a
test in a closed system with continuous flow, a 48-h LC50 of 0.36
mg/litre and a NOEC of 0.04 mg/litre were reported. In a reproduction
test with Daphnia magna in the same closed continuous-flow system,
the NOEC after 21 days' incubation was 0.17 mg/litre (LC50 = 0.23
mg/litre) (Gersich et al., 1989). In studies on the inhibition of food
intake by the marine mussel Mytilus edulis in a static system with
loosely covered (not airtight) test vessels, a 40-min EC50 of 0.3 mg
biphenyl/litre was reported (Donkin et al., 1991). From static tests
on the toxicity of biphenyl on freshwater fish, 96-h LC50 values in
the range of 1.5-4.7 mg/litre have been reported, with rainbow trout
(Oncorhynchus mykiss) and fathead minnow (Pimephales promelas) the
most sensitive species (BUA, 1990).
10.2 Terrestrial environment
Biphenyl has weak bactericidal and fungistatic properties. In
studies with numerous mould fungus species, biphenyl (applied as
vapour or imbedded in solid media) caused a reversible inhibition
(50-100%) of cellular proliferation. Suppression of sporogenesis and,
in Penicillium digitatum and Diplodia natalensis, the occurrence
of biphenyl-resistant mutants were observed. Yeast species showed
little or no inhibition of cell proliferation following exposure to
biphenyl (BUA, 1990).
In a plant growth inhibition test conducted according to OECD
Guideline 208, a nominal NOEC of 100 mg/kg dry soil was reported for
sorghum (Sorghum bicolor) and soya bean (Glycine max); a NOEC of
>1000 mg biphenyl/kg was reported for the sunflower (Helianthus
annuus) (Windeatt et al., 1991). Biphenyl mixed into soil inhibited
growth of lettuce (Lactuca sativa) seedlings with a 7-day EC50 of
54 mg/kg (nominal concentration). At the end of incubation, the
concentration of test substance in the soil had decreased to <10% of
the initial level (Hulzebos et al., 1993).
Data on toxic effects of biphenyl on terrestrial animals and
ecosystems were not identified.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
There are limited data on the effects of biphenyl exposure on
humans. The assessment of potential health hazards has relied
primarily on studies conducted in laboratory animals. The acute oral
toxicity of biphenyl is moderate. The chemical is not irritating to
the skin and is slightly irritating to the eyes, and there is no
evidence of skin sensitizing potential.
Aside from differences in mortality among laboratory species
exposed subchronically to biphenyl-containing dusts, the available
data on effects associated with the inhalation of biphenyl are
insufficient to form the basis of an assessment of potential health
hazards associated with long-term airborne exposure to this chemical.
In toxicological studies in which rodents have been administered
diets containing biphenyl for various periods of time, effects on the
urinary system have often been reported. A marked increase in the
incidence of morphological (i.e. formation of calculi) and/or
histopathological (e.g. hyperplasia, desquamation) effects has been
observed within the urinary tract of male rats administered diets
containing more than 2500 mg biphenyl/kg for periods ranging from 32
to 104 weeks (Takita, 1983; Kurata et al., 1986; Shiraiwa et al.,
1989; Japan Bioassay Research Center, 1996). An increase in the
occurrence of calculi within the urinary bladder has also been
observed in female rats, but at a lower incidence than in males
(Takita, 1983; Shiraiwa et al., 1989; Japan Bioassay Research Center,
1996). Similarly, in a long-term dietary study (Japan Bioassay
Research Center, 1996), increased squamous metaplasia within the
urinary transitional epithelium was also observed in female rats;
again, however, the incidence was lower than that observed in males.
In male mice, only 1 of 10 animals given a diet containing 10 000 mg
biphenyl/kg (1500 mg/kg body weight per day) for 32 weeks developed
simple hyperplasia and papillary or nodular dysplasia of the urinary
bladder (Tamano et al., 1993). Effects on blood chemistry and
haematological parameters have also been observed in animals
administered biphenyl orally; these effects occurred in male and
female rats and mice at intakes lower than those associated with the
development of effects in the urinary bladder of male rats
administered biphenyl (Takita, 1983; Shiraiwa et al., 1989; Japan
Bioassay Research Center, 1996). For non-neoplastic effects, the LOEL
was 38 mg/kg body weight per day based upon the development of
alterations in haematological parameters (i.e. decreased haemoglobin
concentration and haematocrit) in rats fed diets containing 0, 500,
1500, or 4500 mg biphenyl/kg (reported intakes of 0, 38, 113, or 338
mg/kg body weight per day) for 2 years (Japan Bioassay Research
Center, 1996).
Although limited, the available information indicates that
biphenyl has no reproductive or developmental effects at doses lower
than those associated with the development of adverse effects in the
parental generation.
In one study, an increased incidence of benign and malignant
tumours within the urinary bladder was observed in male F344/DuCrj
rats administered diets containing high levels (i.e. not less than
4500 mg/kg) of biphenyl for 2 years. Tumour incidence was not
increased in female rats or in male or female Crj:BDF1 mice (Japan
Bioassay Research Center, 1996). In female mice, there were slight
increases in the incidences of benign and malignant liver tumours in
animals receiving biphenyl in the diet; however, the results were not
dose dependent over the entire range of concentrations tested. In
other studies, biphenyl exhibited tumour-promoting activity with
respect to the development of bladder neoplasms in male rats (Kurata
et al., 1986) but not in male mice (Tamano et al., 1993).
The mechanism by which high doses of biphenyl induce bladder
tumours specifically in male rats has not been fully elucidated. There
have been suggestions that for some non-genotoxic chemicals, the
formation of such tumours is linked to the regenerative hyperplasia of
the urinary epithelium, caused by the abrasion and damage to the
urothelium that are produced by calculi formed within the urinary
tract only at very high levels of exposure (Cohen, 1995). Owing to
anatomical and physiological differences, male rats are considered to
be more susceptible than female rats, mice, and humans to the
development of bladder tumours via such a mechanism; it has therefore
been suggested that the sex- and species-specific development of
bladder tumours in male rats receiving high doses of biphenyl might
not be strictly relevant to humans exposed to lower levels. However,
the incidence of histopathological effects and calculi formation
within the urinary bladder also increased in female rats administered
biphenyl for 2 years, in the absence of bladder tumours (Japan
Bioassay Research Center, 1996). Moreover, data identifying a direct
association between calculi formation, regenerative hyperplasia of the
urothelium, and the development of bladder tumours within individual
male animals are not available. Biphenyl has been reported to be
mutagenic and clastogenic in some, but not all, in vitro assays and
to bind covalently to macromolecules (i.e. proteins) following
metabolic activation; the available data have been considered
inadequate to assess its genotoxicity in vivo. These considerations,
coupled with the structural relationship of 4-hydroxybiphenyl, a major
metabolite of biphenyl, to the potent human bladder carcinogen
4-aminobiphenyl and the lack of critical information related to the
mechanism by which these bladder tumours arise in male rats, could
suggest that the development of bladder tumours in the male rats may
not have been entirely due to effects associated with the formation of
calculi within the urinary bladder. This observation, as well as the
slightly increased incidence of liver tumours in female mice, raises
some concerns with respect to the potential carcinogenicity of
biphenyl to humans.
11.1.2 Criteria for setting guidance values for
biphenyl
A provisional tolerable daily intake (TDI), based upon the
development of effects in the blood of rats administered diets
containing biphenyl for 2 years (Japan Bioassay Research Center,
1996), can be derived as follows:
TDI = 38 mg/kg body weight per day
1000
= 38 µg/kg body weight per day
where:
* 38 mg/kg body weight per day is the lowest LOEL for the
development of alterations in blood chemistry in rats provided
diets containing biphenyl for 2 years. This intake is lower than
intakes associated with the development of haematological
effects, calculi, and histopathological changes in the bladder
and/or kidneys in rats and the development of haematological and
histopathological effects in mice administered biphenyl in the
diet for 2 years.
* 1000 is the uncertainty factor (×10 for interspecies variation;
×10 for intraspecies variation; ×10 for extrapolation from a LOEL
to a NOEL).
However, based upon the currently available data, there is some
uncertainty surrounding the health-protective nature of the value,
owing to lack of critical information concerning the mechanism by
which biphenyl induces bladder tumours in male rats, the slight
increase in hepatocellular tumours in female mice, and the potential
genotoxicity of this substance. Because of this uncertainty, the TDI
is designated provisional.
Available data were inadequate to serve as a basis for the
derivation of guidance values for biphenyl in air.
11.1.3 Sample risk characterization
For the general population, there is insufficient information
available concerning the intake of biphenyl from the general
environment; therefore, comparisons with the provisional TDI are
provided merely as an example. From limited information on the content
of biphenyl in citrus fruits, the estimated daily intake is <375 ng
biphenyl/kg body weight from each individual fruit consumed, while the
estimated daily intake from tap-water is <0.16 ng/kg body weight.
Assuming that a person consumes one citrus fruit per day, the
estimated intake of biphenyl from drinking-water and citrus fruit
consumption is approximately two orders of magnitude lower than the
provisional TDI based upon the development of effects in the blood.
From a study on workers exposed in a plant producing
biphenyl-impregnated paper, it can be derived that exposure to high
biphenyl concentrations up to 128 mg/m3 pose a hazard. Neurotoxic
effects and liver damage have been observed.
Additional information is required to better define any potential
carcinogenic risks that might be associated with exposure to biphenyl.
11.2 Evaluation of environmental effects
The atmosphere is likely the main target compartment for
biphenyl; however, the chemical has been detected in all environmental
compartments as a result of its release from vehicle emissions,
heating, smoking, and industrial activities. Water, soil, and biota
appear to be affected mainly in the direct vicinity of industrial
activities. The decline in the levels of biphenyl in surface waters
may be due to restrictions on the production and use of PCBs. There
are no data available on the release of biphenyl into environmental
compartments from industrial use, from the application of
biphenyl-contaminated sewage sludge to arable land, from
volatilization during the storage of treated fruits, and from leaching
from preserved citrus fruits and their packaging materials in domestic
waste dumps. Only estimated values are available for the
photooxidation of biphenyl in the atmosphere, which is probably the
most important degradation process in this compartment.
Biomagnification of biphenyl in higher trophic levels of the
aquatic or terrestrial food-chain, based upon the potential of
biphenyl to bioaccumulate, is expected to be of minor importance. For
the aquatic compartment, a significant elimination of biphenyl can be
expected as a result of evaporation and degradation. Furthermore,
rapid metabolism of biphenyl in laboratory animals and low acute
toxicity after ingestion have been reported.
Data on effect concentrations for terrestrial organisms and
exposure levels in air and soil are insufficient for risk
characterization for terrestrial organisms. A sample risk
characterization with respect to the aquatic environment can be
performed (based upon data derived from Germany) by calculating the
ratio between a (local or regional) PEC (based on measured or model
concentrations) and a PNEC (EC, 1996).
The annual average concentration of biphenyl (<0.5 µg/litre)
measured in the 1990s in the Rhine River and its tributaries was used
as a PECmeasured. A PEClocal (water), based upon data derived from the
effluent of the wastewater treatment plant of a German producer, may
be calculated as follows:
PEClocal (water) = Ceffluent
(1 + Kp (susp) × Csusp × 10-6) × D
= <0.0064 µg/litre
where:
* Ceffluent is <5 µg/litre, the concentration of biphenyl in
wastewater in 1990 (BUA, 1990);
* Kp (susp) = 423, the suspended matter/water adsorption
coefficient, estimated from the mean soil sorption coefficient
( Koc) of 4230 ( Kp (susp) = Koc × 0.1, where 0.1 is the
fraction of organic carbon in suspended matter, foc(susp));
* Csusp = 15 mg/litre (default value; EC, 1996), the
concentration of suspended matter in the river; and
* D = 781, the dilution factor for river flow, calculated from
sewage treatment plant and river flow data (EC, 1996).1
A PNEC for surface waters may be calculated by dividing the
lowest valid LC50 or NOEC by an appropriate assessment factor (equal
to a safety factor):
PNEC = 170 µg/litre
10
= 1.7 µg/litre
where:
* 170 µg/litre is the lowest NOEC from a chronic study with
Daphnia magna; and
1 Personal communication from Verband d. Textilhilfsmittel-,
Lederhilfsmittel-, Gerbstoff- und Waschrohstoff-Industrie e.V.,
Frankfurt/M., Germany, to Bayer AG, 1996.
* 10 is the assessment factor. For biphenyl, based upon data for
four trophic levels, including one long-term NOEC, the
recommended assessment factor would be 100 (EC, 1996). However,
because of its significant volatility and degradation, and as
effect concentrations from a wide selection of species covering
different taxonomic groups are reported, an assessment factor of
10 is used.
Therefore, based upon the annual average concentrations of
biphenyl measured in the Rhine River and in industrial wastewater
effluent in Germany, the PECmeasured/PNEC and PEClocal (water)/PNEC
ratios are <0.29 and <0.04, respectively. In some jurisdictions
(EC, 1996), chemicals with a PEC/PNEC ratio of <1 may not require
implementation of risk reduction measures beyond those already in
place.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The Joint FAO/WHO Meeting on Pesticide Residues has allocated an
acceptable daily intake of biphenyl of 0-0.125 mg/kg body weight
(JMPR, 1967).
Information on international hazard classification and labelling
is included in the International Chemical Safety Card reproduced in
this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventative and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0106) reproduced in this
document.
13.1 Advice to physicians
Treatment is symptomatic and supportive following intoxication.
The clinical picture is characterized by central and peripheral nerve
damage and liver injury in human poisoning. There is some evidence to
indicate that electroencephalographic abnormalities could persist up
to 1 or 2 years after exposure.
13.2 Health surveillance advice
Objective change in electroencephalogram and electroneuromyogram
could be an indication of biphenyl poisoning in exposed individuals.
Monitoring of liver function could also be integrated into a medical
surveillance programme.
13.3 Spillage
In the event of spillage, measures should be under taken to
prevent biphenyl from reaching drains and watercourses, because of its
toxicity to aquatic organisms.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards
can be found in the International Register of Potentially Toxic
Chemicals (IRPTC), available from UNEP Chemicals (IRPTC), Geneva.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
BIPHENYL ICSC: 0106
April 1994
CAS # 