
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Concise International Chemical Assessment Document 11
1,1,1,2-Tetrafluorethane
First draft prepared by
Mrs P. Barker and Mr R. Cary, Health and Safety Executive, Liverpool,
United Kingdom,
and
Dr S. Dobson, Institute of Terrestrial Ecology, Huntingdon, United
Kingdom
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization Geneva, 1998
The International Programme on Chemical Safety (IPCS),
established in 1980, is a joint venture of the United Nations
Environment Programme (UNEP), the International Labour Organisation
(ILO), and the World Health Organization (WHO). The overall objectives
of the IPCS are to establish the scientific basis for assessment of
the risk to human health and the environment from exposure to
chemicals, through international peer review processes, as a
prerequisite for the promotion of chemical safety, and to provide
technical assistance in strengthening national capacities for the
sound management of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization, the United Nations
Institute for Training and Research, and the Organisation for Economic
Co-operation and Development (Participating Organizations), following
recommendations made by the 1992 UN Conference on Environment and
Development to strengthen cooperation and increase coordination in the
field of chemical safety. The purpose of the IOMC is to promote
coordination of the policies and activities pursued by the
Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing in Publication Data
1,1,1,2-Tetrafluoroethane.
(Concise international chemical assessment document ; 11)
1.Hydrocarbons, Fluorinated - adverse effects
2.Hydrocarbons, Fluorinated - toxicity
3.Occupational exposure 4.Dose-response relationship, Drug
I.International Programme on Chemical Safety
II.Series
ISBN 92 4 153011 1 (NLM Classification: QD 341.H9)
ISSN 1020-6167
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) Copyright World Health Organization 1998
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city, or area
or of its authorities, or concerning the delimitation of its frontiers
or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
The Federal Ministry for the Environment, Nature Conservation and
Nuclear Safety, Germany, provided financial support for the printing
of this publication.
TABLE OF CONTENTS
FOREWORD
1. EXECUTIVE SUMMARY
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
3. ANALYTICAL METHODS
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1. Environmental levels
6.2. Human exposure
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposure
8.2. Irritation and sensitization
8.3. Short-term exposure
8.4. Long-term exposure
8.4.1. Subchronic exposure
8.4.2. Chronic exposure and carcinogenicity
8.5. Genotoxicity and related end-points
8.6. Reproductive and developmental toxicity
8.7. Immunological and neurological effects
9. EFFECTS ON HUMANS
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
11. EFFECTS EVALUATION
11.1. Evaluation of health effects
11.1.1. Hazard identification and dose-response assessment
11.1.2. Criteria for setting guidance values for 1,1,1,2-tetrafluoroethane
11.1.3. Sample risk characterization
11.2. Evaluation of environmental effects
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
13.1. Human health hazards
13.2. Advice to physicians
13.3. Spillage
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
INTERNATIONAL CHEMICAL SAFETY CARD
REFERENCES
APPENDIX 1 - SOURCE DOCUMENT
APPENDIX 2 - CICAD PEER REVIEW
APPENDIX 3 - CICAD FINAL REVIEW BOARD
RÉSUMÉ D'ORIENTATION
RESUMEN DE ORIENTACION
FOREWORD
Concise International Chemical Assessment Documents (CICADs) are
the latest in a family of publications from the International
Programme on Chemical Safety (IPCS) - a cooperative programme of the
World Health Organization (WHO), the International Labour Organisation
(ILO), and the United Nations Environment Programme (UNEP). CICADs
join the Environmental Health Criteria documents (EHCs) as
authoritative documents on the risk assessment of chemicals.
CICADs are concise documents that provide summaries of the
relevant scientific information concerning the potential effects of
chemicals upon human health and/or the environment. They are based on
selected national or regional evaluation documents or on existing
EHCs. Before acceptance for publication as CICADs by IPCS, these
documents undergo extensive peer review by internationally selected
experts to ensure their completeness, accuracy in the way in which the
original data are represented, and the validity of the conclusions
drawn.
The primary objective of CICADs is characterization of hazard and
dose-response from exposure to a chemical. CICADs are not a summary of
all available data on a particular chemical; rather, they include only
that information considered critical for characterization of the risk
posed by the chemical. The critical studies are, however, presented in
sufficient detail to support the conclusions drawn. For additional
information, the reader should consult the identified source documents
upon which the CICAD has been based.
Risks to human health and the environment will vary considerably
depending upon the type and extent of exposure. Responsible
authorities are strongly encouraged to characterize risk on the basis
of locally measured or predicted exposure scenarios. To assist the
reader, examples of exposure estimation and risk characterization are
provided in CICADs, whenever possible. These examples cannot be
considered as representing all possible exposure situations, but are
provided as guidance only. The reader is referred to EHC 1701 for
advice on the derivation of health-based guidance values.
1 International Programme on Chemical Safety (1994)
Assessing human health risks of chemicals: derivation of guidance
values for health-based exposure limits. Geneva World Health
Organization (Environmental Health Criteria 170)
While every effort is made to ensure that CICADs represent the
current status of knowledge, new information is being developed
constantly. Unless otherwise stated, CICADs are based on a search of
the scientific literature to the date shown in the executive summary.
In the event that a reader becomes aware of new information that would
change the conclusions drawn in a CICAD, the reader is requested to
contact IPCS to inform it of the new information.
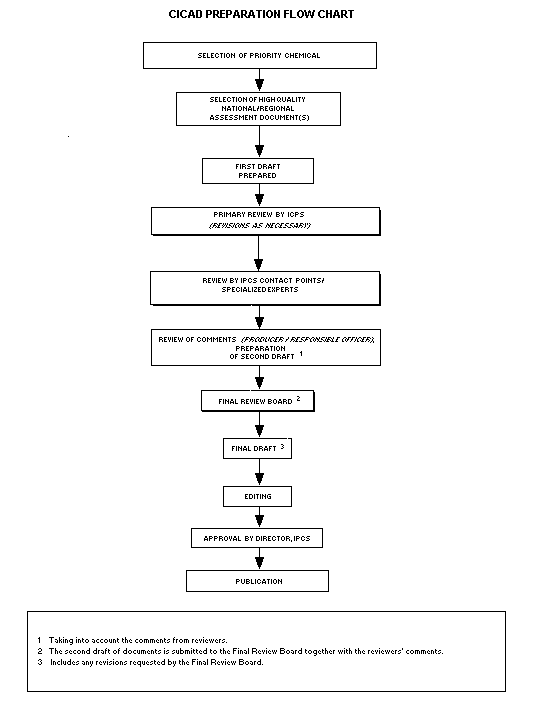
Procedures
The flow chart shows the procedures followed to produce a CICAD.
These procedures are designed to take advantage of the expertise that
exists around the world - expertise that is required to produce the
high quality evaluations of toxicological, exposure, and other data
that are necessary for assessing risks to human health and/or the
environment.
The first draft is based on an existing national, regional, or
international review. Authors of the first draft are usually, but not
necessarily, from the institution that developed the original review.
A standard outline has been developed to encourage consistency in
form. The first draft undergoes primary review by IPCS to ensure that
it meets the specified criteria for CICADs.
The second stage involves international peer review by scientists
known for their particular expertise and by scientists selected from
an international roster compiled by IPCS through recommendations from
IPCS national Contact Points and from IPCS Participating Institutions.
Adequate time is allowed for the selected experts to undertake a
thorough review. Authors are required to take reviewers' comments into
account and revise their draft, if necessary. The resulting second
draft is submitted to a Final Review Board together with the
reviewers' comments.
The CICAD Final Review Board has several important functions.
- to ensure that each CICAD has been subjected to an appropriate
and thorough peer review;
- to verify that the peer reviewers' comments have been addressed
appropriately;
- to provide guidance to those responsible for the preparation of
CICADs on how to resolve any remaining issues if, in the opinion
of the Board, the author has not adequately addressed all
comments of the reviewers; and
- to approve CICADs as international assessments.
Board members serve in their personal capacity, not as representatives
of any organization, government, or industry. They are selected
because of their expertise in human and environmental toxicology or
because of their experience in the regulation of chemicals. Boards are
chosen according to the range of expertise required for a meeting and
the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
 1. EXECUTIVE SUMMARY
This CICAD on 1,1,1,2-tetrafluoroethane was based on a review of
human health concerns (primarily occupational) prepared by the United
Kingdom Health and Safety Executive in 1995 (Standring el al., 1995).
Additional information on effects on human health and the environment
was identified in ECETOC (1995). Data identified up to December 1994
were covered by these reviews. Additional data identified after these
reviews were published have been incorporated as appropriate.
Information on the nature of the peer review and availability of the
source document is presented in Appendix 1. Information on the peer
review of this CICAD is presented in Appendix 2. This CICAD was
approved as an international assessment at a meeting of the Final
Review Board, held in Berlin, Germany, on 26-28 November 1997.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 1281) for
1,1,1,2-tetrafluoroethane, produced by the International Programme on
Chemical Safety (IPCS, 1998), has also been reproduced in this
document.
1,1,1,2-Tetrafluoroethane (CAS no. 811-97-2) is a gaseous
fluorocarbon that is manufactured by the reaction of hydrogen fluoride
with trichloroethylene in a closed system. It is used primarily as a
refrigerant for "high-temperature" refrigeration, such as domestic
refrigerators and automobile air conditioners. Other potential uses
include application in plastic foam blowing, as a solvent for special
cleaning applications, as an aerosol propellant for medical inhalers,
and as a fire extinguishant in place of halons.
Little information was identified on exposure of the general
public or workers to 1,1,1,2-tetrafluoroethane. During its manufacture
in the United Kingdom, employee exposure to the chemical was very low,
with no measured concentrations above 7 ppm (29.2 mg/m3). There are
no exposure measurements from its use in the manufacturing industry
and no data on the exposure of field servicing personnel. The
situation in the workplace in the United Kingdom and analogous data
from a single study of exposure to dichlorotrifluoroethane (HCFC 123)
would suggest that exposure to 1,1,1,2-tetrafluoroethane in the
workplace is normally low (i.e., below 10 ppm [41.7 mg/m3]), with
occasional short-term peak exposures of up to several hundred parts
per million
Information on the effects of 1,1,1,2-tetrafluoroethane on humans
is limited to one report; most available data on the toxicological
effects of 1,1,1,2-tetrafluoroethane have been derived from studies
conducted with laboratory animals. 1,1,1,2-tetrafluoroethane exhibits
relatively low toxicity. A reduction in maternal body weight gain in
rabbits exposed to 40 000 ppm (166 800 mg/m3)
1,1,1,2-tetrafluoroethane and signs of delayed fetal development in
rats following exposure of the dams to 50 000 ppm (208 500 mg/m3)
1,1, 1,2-tetrafluoroethane have been noted in developmental toxicity
studies. In other toxicological investigations, adverse health effects
have not been observed following exposure to concentrations up to
10 000 ppm (41 700 mg/m3). The weight of evidence for carcinogenicity
is limited to an increased incidence of Leydig cell adenomas following
exposure to 50 000 ppm (208 500 mg/m3), and 1,1,1,2-tetrafluoroethane
has not been found to be genotoxic in studies conducted to date.
The low toxicity of 1,1,1,2-tetrafluoroethane to the few aquatic
organisms tested as well as its high volatility indicate negligible
risk to aquatic organisms.
Atmospheric effects have been assessed by modelling. Recent
observations have shown a rapid increase in atmospheric concentrations
of 1,1,1,2-tetrafluoroethane, mainly as a result of emissions over the
past decade. Modelling indicates insignificant ozone depiction
potential, a significant global warming potential, and negligible
acidification potential.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
1,1,1,2-Tetrafluoroethane (CAS no. 811-97-2; C2H2F4;
1,2,2,2-tetrafluoroethane, HFC 134a, HFA 134a, HCFC 134a) is a gaseous
fluorocarbon with a faint ether-like odour. It is soluble in alcohols,
esters, and chlorinated solvents, but it is only slightly soluble in
water. It has a boiling point of -26°C and a vapour pressure of 630
kPa at 25°C. Additional properties are presented in the International
Chemical Safety Card reproduced in this document. The conversion for
1,1,1,2-tetrafluoroethane is 1 ppm=4.17 mg/m3 (at 25°C). The
structural formula for 1,1,1,2-tetrafluoroethane is:
F H
' '
F - C - C - F
' '
F H
3. ANALYTICAL METHODS
An unpublished method based on a Health and Safety Executive
(1995) procedure has been used to monitor exposure to
1,1,1,2-tetrafluoroethane. The sample is collected diffusively onto
Spherocarb and analysed by thermal desorption into a gas chromatograph
fitted with a flame ionization detector (FID). The diffusive uptake
rate is reported as 1.2 ng/ppm per minute, and the method has been
validated down to 0.1 ppm for exposure periods of 30-480 min.1 Pumped
sampling onto Anasorb CMS followed by solvent desorption and analysis
with a gas chromatograph fitted with an FID has also been validated
(Griffiths, 1998), Both the Miran infrared monitor (Quantitech Ltd)2
and the Innova 1312 photoacoustic monitor (CBISS)3 can be used to
measure airborne concentrations of 1,1,1,2-tetrafluoroethane to
sub-ppm concentrations.
There are no published methods for the biological monitoring of
occupational exposure to 1,1,1,2-tetrafluoroethane. However, by
analogy with other haloalkanes, it may be possible to develop
biological monitoring methods based on the analysis of
1,1,1,2-tetrafluoroethane in the breathing zone or urine (Woollen et
al., 1990, 1992). In addition, a study of its use in medical inhalers
revealed that 1,1,1,2-tetrafluoroethane can be measured in blood
samples; sampling at 2 min indicated 1,1,1,2-tetrafluoroethane levels
of 200-700 ng/ml, with a substantial reduction by 12 min (Donnell et
al., 1995).
1 Personal communication, ICI Laboratories, The Heath, Runcorn, UK.
2 Unit 3, Old Wolverton Road, Old Wolverton, Milton Keynes, UK
MK12 5NP.
3 5-11 Coronation Drive, Bromborough, Wirral, UK L62 3LF.
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
1,1,1,2-Tetrafluoroethane is manufactured by the reaction of
hydrogen fluoride with trichloroethylene in a closed system. It is
available as a liquefied gas and is supplied in a variety of
pressurized containers. 1,1,1,2-tetrafluoroethane is used primarily as
a refrigerant for "high-temperature" refrigeration, such as domestic
refrigerators and automobile air conditioners. Other potential uses
include application in plastic foam blowing, as a solvent for special
cleaning applications, as an aerosol propellant for medical inhalers,
and as a fire extinguishant in place of halons.
Between 1990 and 1995, the estimated global production of
1,1,1,2-tetrafluoroethane for dispersive use increased from 0.2 to 73.8
kilotonnes per year; over this same period, the estimated global
release of this chemical increased from 0.1 to 20.3 kilotonnes per
year (AFEAS, 1996).
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
1,1,1,2-Tetrafluoroethane is expected to partition almost
exclusively to the atmosphere. Aqueous discharges would be expected to
volatilize, with half-lives of days to a few weeks. It is not expected
that 1,1,1,2-tetrafluoroethane will accumulate in biota (log Kow
approx. 1.06) or adsorb to soil or sediment (log Koc approx. 1.5).
The atmospheric equilibrium concentration in cloud water has been
estimated at less than 0.2 ppt by weight based on predicted
atmospheric concentrations of 100-200 ppt by volume (0.4-0.8 µg/m3)
for the year 2020 (McCulloch, 1993). The long atmospheric half-life
will result in more or less uniform distribution in the atmosphere on
a global scale (Franklin, 1993),
The overall estimated lifetime of 1,1,1,2-tetrafluoroethane in
the troposphere is 14.6 years (IPCC, 1995); degradation is initiated
by hydroxyl (OH) radicals. It is theoretically possible that
1,1,1,2-tetrafluoroethane could contribute to ozone depletion by means
of CF3Ox, radicals arising from the atmospheric degradation of
tetrafluoroethane; however, this contribution has been estimated to be
insignificant in recent studies (Ko et al., l994; Ravishankara et al.,
1994).
1 1,1,2-Tetrafluroethane's global warming potential over a
100-year time horizon (relative to carbon dioxide) has been estimated
at 1300, compared with 3800 for CFC-11 and 8100 for CFC-12, for which
1,1,1,2-tetrafluoroethane is the main substitute (IPCC, 1995).
Franklin (1993) has estimated that 1,1,1,2-tetrafluoroethane will
reach an atmospheric background concentration of 100 ppt by volume
(0.4 µg/m3) by 2010-2020 and will then be responsible for only about
0.3% of the radiative forcing due to all anthropogenic greenhouse
gases present in the atmosphere.
Hydroxyl radicals break down 1,1,1,2-tetrafluoroethane to form
the CF3CHFO radical, which reacts with oxygen to generate
trifluoroacetyl fluoride (CF3COF) or undergoes cleavage to give
formyl fluoride (HCOF) and the CF3 radical, which is ultimately
converted to carbonyl fluoride (COF2) and hydrogen fluoride (HF).
Modelling studies predict that 40% of 1,1,1,2-tetrafluoroethane
breakdown will proceed via the former route and 60% by the latter
route (Franklin, 1993). Recent research suggests that the yield of
trifluoroacetyl fluoride is in the range of 7-20% rather than 40%, as
was previously assumed (Wallington et al., 1996).
The principal fate of the acid fluorides (CF3COF, HCOF, and
COF2) will be uptake by cloud water and hydrolysis to trifluoroacetic
acid, formic acid, carbon dioxide, and hydrogen fluoride. Dry
deposition to ocean or land surfaces may occur to a limited extent and
will be followed by hydrolysis (AFEAS, 1992, 1993).
The contribution of degradation products to environmental
fluorides and acidity of rainwater is expected to be negligible (WMO,
1989; Franklin, 1993).
There are no known natural sources of trifluoroacetic acid.
However, recent work (Frank et al., 1996) has measured trifluoroacetic
acid in rainwater and surface waters in Europe and Israel at levels
too high to be explained by the atmospheric degradation of
1,1,1,2-tetrafluoroethane and other chlorofluorocarbon substitutes.
The origin of this trifluoroacetic acid is currently unexplained, and
a natural source cannot be ruled out. Using the same assumptions for
emission and atmospheric degradation as above (Franklin, 1993),
deposition of trifluoroacetic acid in rainwater would be 45 kilotonnes
per year (in the years 2010-2020), with an average concentration in
precipitation globally at 0.1 µg/litre. Trifluoroacetic acid will
partition into the aqueous environment; assuming accumulation in the
upper levels of seawater, an increased concentration of 1.5 ng/litre
would be expected for each 100 kilotonnes of 1,1,1,2-tetrafluoroethane
degraded.
Laboratory tests have demonstrated no appreciable degradation of
1,1,1,2-tetrafluoroethane in activated sludge (Tobeta, 1989) or by the
methanotropic bacterium Methylosimus trichosporium (DeFlaun et al.,
1992). Trifluoroacetic acid can be degraded under anoxic conditions to
trifluoromethane, inorganic fluoride, methane, and carbon dioxide
(Visscher et al., 1994).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
In 1995, the average atmospheric concentration of
1,1,1,2-tetrafluoroethane was about 2 ppt (8.3 ng/m3);
there had been a significant increase in concentration measured
throughout 1994 and 1995, rising from about 0.3 ppt (1.3 ng/M3) in
early 1994 to a range of 1.2-3.4 ppt (5.0-14.2 ng/m3) in late 1995
(Montzka et al., 1996). Measurements were taken on land in Canada, the
continental United States, Hawaii, American Samoa, and Tasmania and at
sea in the Pacific and Atlantic oceans.
6.2 Human exposure
Information on potential exposure of the general public to
1,1,1,2-tetrafluoroethane was not identified, and there are limited
data concerning occupational exposure. During its manufacture in the
United Kingdom in a modern plant, employee exposure was very low, with
no measured concentrations above 7 ppm (29.2 mg/m3) (Standring et
al., 1995). There are no exposure measurements from its use in the
manufacturing industry and no data on the exposure of field servicing
personnel. At the time of review, there was only one manufacturer in
the United Kingdom, although other production facilities are envisaged
to come on stream in the near future. The situation in the United
Kingdom and analogous data from a single study of exposure to
dichlorotrifluoroethane (HCFC 123) (Standring et al., 1995) would
suggest that exposure is normally low (i.e., below 10 ppm [41.7
mg/m3], 12-hour time-weighted average), with occasional short-term
peak exposures of up to several hundred parts per million (H. Sibley,
undated).
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND
HUMANS
The elimination and distribution of a single breath inhalation of
[18F] 1,1,1,2-tetrafluoroethane were measured in a small group of
volunteers using whole body gamma-counting (Pike et al., 1995).
Distribution was extensive, and elimination was rapid and essentially
complete within 6 h (half-life approx. 1.5-4 h). Elimination of
radioactivity in the urine was observed in some but not all subjects,
and there was no evidence of accumulation.
A group of four volunteers received 16 breath inhalations of 1,
1,1,2-tetrafluoroethane (total dose 1200 mg) over a 10-min period in
an investigation of its use as a propellant in medical devices (Monte
et al., 1994). Urine samples were collected over a 24-h period and
analysed for trifluoroacetic acid using 19F nuclear magnetic
resonance spectroscopy (detection limit 10 ng/ml). The amounts of
trifluoroacetic acid measured in urine ranged from undetectable to
0.0004% of the administered 1,1,1,2-tetrafluoroethane. There were no
1,1,1,2-Tetrafluoroethane other fluorinated products detected in urine
using this technique.
In two inhalation studies conducted with rats,
1,1,1,2-tetrafluoroethane was poorly absorbed (Ellis el al., 1991,
1993), and elimination was rapid and achieved mainly by exhalation of
unchanged 1,1,1,2-tetrafluoroethane (Finch et al., 1995). Very little
metabolism occurred, with the main metabolite being carbon dioxide;
trifluoroacetic acid was identified in urine. There was no significant
accumulation of absorbed 1,1,1,2-tetrafluoroethane in specific
tissues.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
1,1,1,2-Tetrafluoroethane has low acute toxicity. An approximate
4-h lethal concentration of 567 000 ppm (2.36 × 106 mg/m3) has been
reported for rats; no effects were observed at 81 000 ppm (337 770
mg/m3) (Kennedy, 1979a, as cited in ECETOC, 1995). At concentrations
in excess of 200 000 ppm (834 000 mg/m3), exposure to
1,1,1,2-tetrafluoroethane depressed the central nervous system of
rats.1 Anaesthetic effects have also been observed in other species.
Cardiac sensitization (an increased sensitivity of the heart) to
exogenous adrenalin has been observed in dogs exposed to
1,1,1,2-tetrafluoroethane, with a no-observed-effect level (NOEL) of
40 000 ppm (166 800 mg/m3) (Hardy el al., 1991).
1 Programme for Alternative Fluorocarbon Testing, presentation at
Toulouse International Toxicology Forum, September 1989; meeting notes
presented as a personal communication to P Standring, 1993.
8.2 Irritation and sensitization
Studies on irritation or sensitization were not available.
8.3 Short-term exposure
Information from typical short-term repeated exposure toxicity
studies was not identified.
8.4 Long-term exposure
8.4.1 Subchronic exposure
No significant exposure-related toxicological effects were
observed in an inhalation study in which groups of male and female
rats were exposed for 13 weeks to 1,1,1,2-tetrafluoroethane at
concentrations up to 50 000 ppm (208 500 mg/m3) (Hext, 1989; Collins
el al., 1995),
8.4.2 Chronic exposure and carcinogenicity
In a study conducted according to a contemporary protocol, groups
of 85 male and 85 female Wistar-derived Alderley Park rats were
exposed (whole-body) to 0 (air only), 2500, 10 000, or 50 000 ppm (0,
10 425, 41 700, or 208 500 mg/m3) 1,1,1,2-tetrafluoroethane, 6 h/day,
5 days/week, for 2 years (Hext & Parr-Dobrzanski, 1993; Collins el
al., 1995). Mortality rates were low and similar in the control and
exposed groups. No exposure-related pathological findings were
recorded at interim sacrifice (52 weeks). At termination, the only
exposure-related pathological findings were increased incidences of
Leydig (interstitial) cell hyperplasia and benign Leydig cell adenomas
in the testes. The microscopic findings in the testes occurred mainly
in animals surviving to the end of the study. In the control, 2500,
10 000, and 50 000 ppm (0, 10 425, 41 700, or 208 500 mg/m3) groups,
the incidence of Leydig cell hyperplasia was 27/85, 25/79, 31/85, and
40/85 (32, 32, 36, and 47%), respectively; the incidence of Leydig
cell adenoma was 9/85, 7/79, 12/85, and 23/85 (11, 9, 14, and 27%),
respectively. Hyperplasia was observed in most animals with such
tumours. At 50 000 ppm (208 500 mg/m3) 1,1,1,2-tetrafluoroethane, the
incidence of Leydig cell adenoma was significantly (p < 0.05)
increased above the controls. The incidence of Leydig cell adenomas
and hyperplasia at 10 000 ppm (41 700 mg/m3) was within the
historical control levels observed at this laboratory; from 1985 to
1995, the background incidence of this tumour ranged between 4 and
19%. The no-observed-adverse-effect level (NOAEL) in this study is
considered to be 10 000 ppm (41 700 mg/m3).
Other studies were less rigorously performed, but no
exposure-related neoplastic or non-neoplastic effects were observed in
2-year inhalation studies (1 -h daily nose-only exposure) at
concentrations up to 50 000 ppm (208 500 mg/m3) in rats and up to
75 000 ppm (312 750 mg/m3) in mice (Alexander el al., 1995a) or in
a similarly designed 1 - year study in which dogs were exposed to
120 000 ppm (500 400 mg/m3) 1,1,1,2-tetrafluoroethane (Alexander el
al., 1995b).
8.5 Genotoxicity and related end-points
The genotoxic potential of 1,1,1,2-tetrafluoroethane has been
investigated in several well-conducted studies (bacterial mutagenicity
[Ames] test, an in vitro mammalian cell cytogenetics study, an
in vivo chromosomal aberration assay, a micronucleus study, an
in vivo unscheduled DNA synthesis assay, and a dominant lethal
study). 1,1,1,2-Tetrafluoroethane was not genotoxic in any of the
tests (Anderson & Richardson, 1979; Hodge et al., 1979; Longstaff et
al., 1984; Müller & Hofmann, 1989; Callander & Priestley, 1990;
Mackay, 1990; Trueman, 1990; Collins et al., 1995).
8.6 Reproductive and developmental toxicity
No exposure-related effects were observed in a standard fertility
study in which groups of rats were exposed to 0, 2500, 10 000, or
50 000 ppm (0, 10 425, 41 700, or 208 500 mg/m3)
1,1,1,2-tetrafluoroethane, 1 h/day during gametogenesis, mating, and
post-mating (Alexander et al., 1996). The results from a dominant
lethal study revealed no effect on fertility in male rats (Hodge et
al., 1979). In a standard developmental toxicity study in rats,
delayed fetal development (a statistically significant reduction in
mean fetal weight, delayed ossification of digits) was observed when
the dams were exposed to 50 000 ppm (208 500 mg/m3)
1,1,1,2-tetrafluoroethane; no significant exposure-related effects
were observed at 10 000 ppm (41 700 mg/m3) (Hodge et al., 1980). No
other exposure-related developmental effects were observed in rats at
levels up to 40 000 ppm (166 800 mg/m3) 1,1,1,2-tetrafluoroethane, a
concentration causing decreased maternal body weight gain in rabbits
(Wickramaratne, 1989; Collins et al., 1995). In the study with
rabbits, there was a 30% reduction during exposure with subsequent
recovery, resulting in a net reduction in body weight of 3% compared
with controls.
8.7 Immunological and neurological effects
Based upon the available evidence, specific immunological or
neurological effects associated with long-term exposure to
1,1,1,2-tetrafluoroethane were not identified.
9. EFFECTS ON HUMANS
Limited data are available from an investigation into the use of
1,1,1,2-tetrafluoroethane as a propellant in a metered-dose inhaler
(Donnell et al., 1995). Volunteers received up to 16 breath
inhalations of 1,1,1,2-tetrafluoroethane within about 10 min.
Investigations included blood pressure and heart rhythm, limited blood
biochemistry, and pulmonary function tests; no abnormalities were
observed, and there were no clinical signs of toxicity.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
1,1,1,2-Tetrafluoroethane has no significant effect on the growth
of the bacterium Pseudomonas putida (6-h EC50 >730 mg/litre)
(Coleman & Thompson, 1990). Acute toxicity to freshwater organisms is
low ( Daphnia magna, 48-h EC50 980 mg/litre; rainbow trout
Oncorhynchus mykiss, 96-h LC50 450 mg/litre) (Stewart & Thompson,
1990; Thompson, 1990). The high aqueous concentrations used in these
studies can only be maintained artificially. In the environment, there
would be rapid partitioning to the air compartment from the aqueous
phase; the high concentrations used in the studies could be reached
only if the atmosphere above the water were entirely
1,1,1,2-tetrafluoroethane. Additional data on toxicity of
1,1,1,2-tetrafluoroethane to aquatic or terrestrial organisms were not
identified.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Information on the effects of 1,1,1,2-tetrafluoroethane on humans
is limited to one report; most available data on the toxicological
effects of 1,1,1,2-tetrafluoroethane have been derived from studies
conducted with laboratory animals. 1,1,1,2-Tetrafluoroethane exhibits
relatively low toxicity. This chemical is a gas, appears to be
essentially non-reactive, and is unlikely to be either an irritant or
a sensitizer, although appropriate studies were not identified. A
reduction in maternal body weight gain in rabbits exposed to 40 000
ppm (166 800 mg/m3) 1,1,1,2-tetrafluoroethane and signs of delayed
fetal development in rats following exposure of the dams to 50 000 ppm
(208 500 mg/m3) 1,1,1,2-tetrafluoroethane have been noted in
developmental toxicity studies. In other toxicological investigations,
adverse health effects have not been observed following exposure to
concentrations up to 10 000 ppm (41 700 mg/m3)
1,1,1,2-tetrafluoroethane.
The weight of evidence for carcinogenicity of
1,1,1,2-tetrafluoroethane is limited. A statistically significant,
exposure-related increase in the incidence of benign Leydig cell
adenomas was observed in Wistar-derived rats exposed to a very high
concentration (50 000 ppm [208 500 mg/m3]) of
1,1,1,2-tetrafluoroethane for 2 years. However, the spontaneous
incidence of these tumours is high in this and other strains of rats,
and 1,1, 1,2-tetrafluoroethane has not been found to be genotoxic in
studies conducted to date.
11.1.2 Criteria for setting guidance values for
1,1,1,2-tetrafluoroethane
Based upon the available data, no adverse effects have been
observed in laboratory animals exposed to 10 000 ppm (41 700 mg/m3)
1,1,1,2-tetrafluoroethane. This value can therefore serve as a basis
for comparison with estimated exposure for risk characterization,
either with application of appropriate uncertainty factors or
directly. Examples of both approaches are presented in section 11.1.3.
11.1.3 Sample risk characterization
The scenario chosen as an example is the occupational environment
within the United Kingdom, where, under the current conditions of use,
anticipated occupational exposure (8- or 12-h time-weighted average)
to 1,1,1,2-tetrafluoroethane is in the vicinity of 10 ppm (41.7
mg/m3), with occasional short-term peak exposures of up to several
hundred parts per million. These concentrations are 1-3 orders of
magnitude less than the NOAEL of 10 000 ppm (41 700 mg/m3) derived
from toxicological studies conducted with laboratory animals. However,
data on exposure in occupational circumstances within the United
Kingdom are limited, and it is difficult to anticipate exposure
conditions for other countries.
A health-based occupational exposure limit for
1,1,1,2-tetrafluoroethane of 1000 ppm (4170 mg/m3) (8-h time-weighted
average) has been established within the United Kingdom. This equates
to division of the NOAEL of 10 000 ppm (41 700 mg/m3) by an
uncertainty factor of 10.
11.2 Evaluation of environmental effects
The low toxicity of 1,1,1,2-tetrafluoroethane to the few aquatic
organisms tested as well as its high volatility indicate negligible
risk to aquatic organisms.
Atmospheric effects have been assessed by modelling. Recent
observations have shown a rapid increase in atmospheric concentrations
of 1,1,1,2-tetrafluoroethane, mainly as a result of emissions over the
past decade. Modelling indicates insignificant ozone depletion
potential, a significant global warming potential, and negligible
acidification potential.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Previous evaluations of 1,1,1,2-tetrafluoroethane by
international bodies were not identified. Information on international
hazard classification and labelling is included in the International
Chemical Safety Card reproduced in this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventative and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 1281) reproduced in this
document.
13.1 Human health hazards
1,1,1,2-Tetrafluoroethane is essentially non-toxic and flammable.
There is a possibility of frostbite if the liquefied gas is released
rapidly.
13.2 Advice to physicians
Symptomatic treatment and supportive therapy should be provided
as indicated. Adrenaline and similar sympathomimetic drugs should be
avoided following exposure, as cardiac arrhythmia may result, with
possible subsequent cardiac arrest.
13.3 Spillage
In the event of spillage of 1,1,1,2-tetrafluoroethane, emergency
crews should wear proper personal protection, including respiratory
protection. Because the vapour is heavier than air, it may accumulate
in lower spaces, causing a deficiency of oxygen. The oxygen content of
the air should always be checked before the affected area is entered.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards
can be found in the International Register of Potentially Toxic
Chemicals (IRPTC), available from UNEP Chemicals (IRPTC), Geneva.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
1,1,1,2-TETRAFLUOROETHANE ICSC: 1281
26.03.1998
CAS# 811-97-2 HFC 134a
RTECS# K18842500 (cylinder)
UN# 3159 C2H2F4
Molecular mass: 102.03
TYPES OF HAZARD / ACUTE HAZARDS / PREVENTION FIRST AID / FIRE FIGHTING
EXPOSURE SYMPTOMS
FIRE Not combustible. Gives off irritating NO open flames. NO contact with In case of fire in the surroundings;
or toxic fumes (or gases) in a fire. hot surfaces. all extinguishing agents allowed.
EXPLOSION In case of fire: keep cylinder cool
by spraying with water.
EXPOSURE
Inhalation Dizziness. Drowsiness. Dullness. Local exhaust or breathing Fresh air, rest. Refer for medical
protection attention.
Skin ON CONTACT WITH LIQUID: Cold-insulating gloves. ON FROSTBITE rinse with plenty of
FROSTBITE. water, do NOT remove clothes.
Eyes Safety goggles.
Ingestion
(continued)
SPILLAGE DISPOSAL PACKAGING & LABELLING
NEVER direct water jet on liquid. Do NOT let this chemical enter the Symbol
environment Chemical protection suit including self-contained breathing R:
apparatus. S:
UN Hazard Class: 2.2
UN Subsidiary Risks:
UN Pack Group:
EMERGENCY RESPONSE STORAGE
Transport Emergency Card TEC (R)-20G39 Fireproof. Keep in a well-ventilated room.
IMPORTANT DATA
PHYSICAL STATE; APPEARANCE: ROUTES OF EXPOSURE:
COMPRESSED LIQUEFIED GAS, WITH CHARACTERISTIC ODOUR. The substance can be absorbed into the body by inhalation.
CHEMICAL DANGERS: INHALATION RISK:
On contact with hot surfaces or flames this substance A harmful concentration of this gas in the air will be reached
decomposes forming toxic and corrosive fumes. very quickly on loss of containment.
OCCUPATIONAL EXPOSURE LIMITS: EFFECTS OF SHORT-TERM EXPOSURE:
TLV not established Rapid evaporation of the liquid may cause frostbite. The
substance may cause effects on the central nervous system and
cardiovascular system, resulting in cardiac disorders.
(continued)
PHYSICAL PROPERTIES
Boiling point: -26°C
Melting point: -101°C
Solubility in water none
Vapour pressure, kPa at 25°C: 630
Relative vapour density (air = 1): 3.5
0ctanolwater partition coefficient as log Pow: 1.06
ENVIRONMENTAL DATA
Avoid release to the environment in circumstances different to normal use.
NOTES
Do NOT use in the vicinity of a fire or a hot surface, or during welding. Turn leaking cylinder with the leak up to prevent escape of
gas in liquid state.
ADDITIONAL INFORMATION
LEGAL NOTICE Neither the CEC or the IPCS nor any person acting on behalf of the CEC or the IPCS is responsible
for the use which might be made of this information.
REFERENCES
AFEAS (1992) Proceedings of the AFEAS workshop: Atmospheric wet and
dry deposition of carbonyl and haloacetyl halides. Brussels, 22
September 1992. Washington, DC, Alternative Fluorocarbons
Environmental Acceptability Study.
AFEAS (1993) Proceedings of the STEP-HALOCSIDE/AFEAS workshop on
kinetics and mechanisms for the reactions of halogenated organic
compounds in the troposphere. Dublin, 23-25 March 1993. Washington,
DC, Alternative Fluorocarbons Environmental Acceptability Study.
AFEAS (1996) Production, sales and atmospheric emissions of
fluorocarbons through 1995. Washington, DC, Alternative Fluorocarbons
Environmental Acceptability Study.
Alexander D, Libretto S, Chevalier H-J, Imamura T, Pappritz G, Wilson
J (1995a) HFA-134a (1,1,1,2-tetrafluoroethane): lack of oncogenicity
in rodents after inhalation. Human and experimental toxicology, 14
706-714.
Alexander D, Mortimer E, Dines G, Libretto S, Mallett D (1995b) One
year study in dogs of the toxicity of HFA-134a by inhalation.
inhalation toxicology, 7: 1153-1162.
Alexander D, Libretto S, Adams M, Hughes E, Bannerman M (1996)
HFA-134a (1,1,1,2-tetrafluoroethane): effects of inhalation exposure
upon reproductive performance, development and maturation of rats.
Human and experimental toxicology, 15: 508-517.
Anderson D, Richardson C (1979) Arcton 134a: a cytogenetic study in
the rat. Alderley Park, Cheshire, ICI Central Toxicology Laboratory
(Report No CTL/P/444, unpublished).
Callander R, Priestley K (1990) HFC 134a - An evaluation using the
Salmonella mutagenicity assay. Alderley Park, Cheshire, ICI Central
Toxicology Laboratory (Report No. CTL/P/2422, unpublished).
Coleman C, Thompson R (1990) HFC 134a. Determination of the acute
toxicity to Pseudomonas putida. Alderley Park, Cheshire, ICI Central
Toxicology Laboratory (Report No. BL3980/B, unpublished).
Collins M, Rusch G, Sato F, Hext P, Millischer R (1995) 1,1,1,2-
Tetrafluoroethane repeat exposure inhalation toxicity in the rat,
developmental toxicity in the rabbit and genotoxicity in vitro and
in vivo. Fundamental and applied toxicology, 25: 271-280
DeFlaun M, Ensley B, Steffan R (1992) Biological oxidation of
hydrochlorfluorocarbons (HCFC's) by a methanotrophic bacterium.
Bio/Technology, 10(12): 1576-1578.
Donnell D Harrison L, Ward S, Klinger N, Ekholm B, Cooper K, Porietis
I, McEwen J (1995) Acute safety of the CFC-free propellant HFA-134a
from a pressurized metered dose inhaler. Journal of clinical
pharmacology, 48: 473-477.
ECETOC (1995) 1,1,1,2-Tetrafluoroethane (HCF-134a), Brussels European
Centre for Ecotoxicology and Toxicology of Chemicals (Joint Assessment
of Commodity Chemicals No. 31).
Ellis M, Gowans L, Green T (1991) Hydrofluorocarbon 134a:
Pharmacokinetics and metabolism in rats following a single exposure by
inhalation Alderley Park, Cheshire, ICI Central Toxicology Laboratory
(Report No CTL/R/1090, unpublished).
Ellis M, Gowans L, Green T. Tanner R (1993) Metabolic fate and
disposition of 1,1,1,2-tetrafluoroethane (HCF 134a) in rat following a
single exposure by inhalation. Xenobiotica, 23(7) 719-729.
Finch J, Dadey E, Smith S, Harrison L, Digenis G (1995) Dynamic
monitoring of total-body absorption by 19F NMR spectroscopy: one hour
ventilation of HFA 134a in male and female rats, Magnetic resonance in
medicine, 33: 409-413.
Frank H, Klein A, Renschen D (1996) Environmental trifluoroacetate.
Nature, 382 34.
Franklin J (1993) The atmospheric degradation and impact of
1,1,1,2-tetrafluoroethane (Hydrofluorocarbon 134a), Chemosphere,
27(8): 1565-1601.
Griffiths JR (1998) Measurement of airborne 1,1,1,2-tetrafluoroethane.
Health and Safety Executive (IR/L/SP/97/08)
Hardy C, Sharman I, Clark G (1991) Assessment of cardiac sensitisation
potential in dogs. Comparison of HFA 134a and A12. Alderley Park,
Cheshire, ICI Central Toxicology Laboratory (Report No. CTL/C/2521,
unpublished).
Health and Safety Executive (1995) Methods for determination of
hazardous substances: MDHS 72. Volatile organic chemicals in air.
London, HMSO.
Hext P (1989) HFC 134a: 90-day inhalation toxicity study in the rat.
Alderley, Park, Cheshire, ICI Central Toxicology Laboratory (Report
No. CTL/P/2466, unpublished).
Hext P, Parr-Dobrzanski R (1993) HFC 134a: 2-year inhalation toxicity
study in the rat. Alderley Park, Cheshire, ICI Central Toxicology
Laboratory (Report No. CTL/P/3841, unpublished).
Hodge M, Anderson D, Bennett I, Weight T (1979) Arcton 134a: Dominant
lethal study in the mouse. Alderley Park, Cheshire, ICI Central
Toxicology Laboratory (Report No. CTL/R/437, unpublished).
Hodge M, Kilmartin M, Riley R, Weight T, Wilson J (1980) Arcton 134a:
Teratogenicity study in the rat, Alderley Park, Cheshire, ICI Central
Toxicology Laboratory (Report No. CTL/P/417, unpublished).
IPCC (1995) Climate change 1995. The science of climate change.
Contribution of group 1 to the Second Assessment Report of the
Intergovernmental Panel on Climate Change. Geneva, World
Meteorological Organization/United Nations Environment Programme.
IPCS (1998) International Chemical Safety Card -
1,1,1,2-Tetrafluoroethane Geneva, World Health Organization,
International Programme on Chemical Safety (No. 1281).
Ko M, Sze N, Rodriguez J, Weisenstein D, Heisey C, Wayne R, Biggs P,
Canosa-Mas C, Sidebottom H, Treacy J (1994) CF3 chemistry: Potential
implications for stratospheric ozone. Geophysics research letters,
21(2): 101-104.
Longstaff E, Robinson M, Bradbrook C, Styles J, Purchase I (1984)
Genotoxicity and carcinogenicity of fluorocarbons assessment by
short-term in vitro tests and chronic exposure to rats. Toxicology
and applied pharmacology, 72: 15-31
Mackay J (1990) HFC 134a An evaluation in the in vitro cytogenetic
assay in human lymphocytes, Alderley Park, Cheshire, 1Ci Central
Toxicology Laboratory (Report No CTUP/2977, unpublished).
McCulloch A (1993) [German Federal Government Responsibility for the
future - ways to sustainable management of substances and waste flows.
Intermediate report of the Enquete Commission "Protection of man and
the environment. Valuation criteria and perspectives for
environmentally friendly substance cycling in industrialised society"
to the 12th German Bundestag.] Bonn, Bundesrepublik Deutschland
Economica Verlag (in German).
Monte S, Ismail I, Mallett D, Matthews C, Tanner R (1994) The minimal
metabolism of inhaled 1,1,1,2-tetrafluoroethane to trifluoroacetic
acid in man as determined by high sensitivity nuclear magnetic
resonance spectroscopy of urine samples. Journal of pharmacology and
biomedical analysis, 12(12): 1489,-1493.
Montzka S, Butler J, Myers R, Thompson T, Swanson T, Clarke A, Lock L,
Elkins J (1996) Decline in the tropospheric abundance of halogen from
halocarbons: implications for stratospheric ozone depletion. Science,
272: 1318-1322.
Müller W, Hofmann T (1989) CFC 134a micronucleus test in male and
female NMRI mice after inhalation. Frankfurt, Hoechst Laboratories
(Report No. 89.0115, unpublished).
Pike V, Aigbirhio F, Freemantle C, Page B, Rhodes C, Waters S, Jones
T, Olsson P, Ventresca G, Tanner R, Hayes M, Hughes J (1995)
Disposition of inhaled 1,1,1,2-tetrafluoroethane (HFA 134a) in healthy
subjects and in patients with chronic airflow limitation. Drug
metabolism and disposition, 23(8): 832-839
Ravishankara A. Turnipseed A, Jensen N, Barone S, Mills M, Howard C,
Solomon S (1994) Do hydrofluorocarbons deplete stratospheric ozone?
Science, 263: 71-75.
Sibley H (undated) A study for determining refrigerant exposure levels
while servicing an HCFC-123 centrifugal chiller. Syracuse, NY, Carrier
Corporation (internal paper).
Standring P, Maidiment S, Ogunbiyi A, Groves J. Cocker J (1995)
1,1,1,2-Tetrafluoroethane; Criteria document for an occupational
exposure limit. Sudbury, Suffolk, Health and Safety Executive, HSE
Books (ISBN 0 7176 0947 2).
Stewart K, Thompson R (1990) HFC 134a: Determination of the acute
toxicity to Daphnia magna. Alderley Park, Cheshire, ICI Central
Toxicology Laboratory (Report No BL3908/B, unpublished).
Thompson R (1990) HFC 134a: Determination off the acute toxicity to
rainbow trout (Salmo gairdneri). Alderley Park, Cheshire, ICI Central
Toxicology Laboratory (Report No. BL4035/B, unpublished).
Tobeta V (1989) Test of biodegradability of HFC 134a by microorganisms
Fukuoka, Kurume Research Laboratories (Report No. 11598, unpublished)
Trueman R (1990) HFC 134a: Assessment for the induction of unscheduled
DNA syntheses in rat hepatocytes in vivo, Alderley Park, Cheshire, ICI
Central Toxicology Laboratory (Report No. CTL/P/2550, unpublished).
Visseher PT, Culbertson CW, Oremland RS (1994) Degradation of
trifluoroacetate in oxic and anoxic sediments Nature, 369(6483):
729-731.
Wallington T, Hurley M, Fracheboud J, Orlando J, Tyndall G, Sehested
J, Mogelberg T, Nielsen O (1996) Role of excited CF3CFHO radicals in
the atmospheric chemistry of HCF-134a. Journal of physical chemistry,
100: 18116-18122.
Wickramaratne G (1989) HFC 134a: Teratogenicity inhalation study in
the rabbit. Alderley Park, Cheshire, ICI Central Toxicology Laboratory
(Report No CTL/P/2504, unpublished).
WMO (1989) Scientific assessment of stratospheric ozone: 1989. Geneva,
World Meteorological Organization (Global Ozone Research and
Monitoring Project, Report No 20).
Woollen B, Guest E. Howe W, Marsh J, Wilson H, Auton T, Blaine P
(1990) Human inhalation pharmacokinetics of 1,1,2-trichloro-
1,2,2-trifluoroethane(FC113). International archives of occupational
and environmental health, 64(5): 383-387.
Woollen B, Marsh J, Mahler J, Auton T, Makepeace D, Cocker J. Blaine P
(1992) Human inhalation pharmacokinetics of chlorodifluoromethane
(HFA22) International archives of occupational and environmental
health, 64(5): 383-387.
APPENDIX 1 - SOURCE DOCUMENT
Standring et al. (1995)
The draft report entitled 1,1,1,2-Tetrafluoroethane, Criteria
document for an occupational exposure limit (prepared by P. Standring,
S. Maidment, A. Ogunbiyi, J. Groves, and J. Cocker) was initially
reviewed internally by a group of approximately 10 Health and Safety
Executive experts (mainly toxicologists, but also experts in other
relevant disciplines, such as epidemiology and occupational hygiene).
The toxicology section of the amended draft was then reviewed by
toxicologists from the United Kingdom Department of Health.
Subsequently, the entire criteria document was reviewed by a
tripartite advisory committee to the United Kingdom Health and Safety
Commission, the Working Group for the Assessment of Toxic Chemicals
(WATCH). This committee is composed of experts in toxicology and
occupational health and hygiene from industry, trade unions, and
academia.
Members of the WATCH committee at the time of the peer review
were Mr S. Bailey, Independent Consultant; Professor J, Bridges,
University of Surrey; Dr I. Guest, Chemical Industries Association; Dr
A. Hay, Trade Unions Congress; Dr L. Levy, Institute of Occupational
Hygiene, Birmingham; Dr M. Molyneux, Chemical Industries Association;
Mr A. Moses, Chemical Industries Association; Dr R. Owen, Trade Unions
Congress; and Mr J. Sanderson, Independent Consultant.
APPENDIX 2 - CICAD PEER REVIEW
The draft CICAD on 1,1,1,2-tetrafluoroethane was sent for review
to institutions and organizations identified by IPCS after contact
with IPCS national Contact Points and Participating Institutions, as
well as to identified experts. Comments were received from:
Department of Health, London, United Kingdom
Department of Public Health, Albert Szent-Gyorgyi University
Medical School, Szeged, Hungary
European Centre for Ecotoxicology and Toxicology of Chemicals
(ECETOC), Brussels, Belgium
Health Canada, Ottawa, Canada
International Agency for Research on Cancer, Lyon, France
Ministry of Health and Welfare, International Affairs Division,
Government of Japan, Tokyo, Japan
National Institute for Working Life, Solna, Sweden
National Institute of Occupational Health, Budapest, Hungary
United States Department of Health and Human Services (National
Institute of Environmental Health Sciences)
United States Environmental Protection Agency (Office of
Pollution Prevention and Toxics, National Center for
Environmental Assessment, Office of Research and Development;
Office of Drinking Water)
APPENDIX 3 - CICAD FINAL REVIEW BOARD
Berlin, Germany, 26-28 November 1997
Members
Dr H. Ahlers, Education and Information Division, National Institute
for Occupational Safety and Health, Cincinnati, OH, USA
Mr R. Cary, Health Directorate, Health and Safety Executive, Bootle,
United Kingdom
Dr S. Dobson, Institute of Terrestrial Ecology, Huntingdon, United
Kingdom
Dr R.F Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany (Chairperson)
Mr J.R. Hickman, Health Protection Branch, Health Canada, Ottawa
Ontario, Canada
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany
Ms M.E. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada (Rapporteur)
Dr K. Paksy, Department of Reproductive Toxicology, National Institute
of Occupational Health, Budapest, Hungary
Mr V. Quarg, Ministry for the Environment, Nature Conservation &
Nuclear Safety, Bonn, Germany
Mr D. Renshaw, Department of Health, London, United Kingdom
Dr. J. Sekizawa, Division of Chemo-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan
Prof. S. Soliman, Department of Pesticide Chemistry, Alexandria
University, Alexandria, Egypt (Vice-Chairperson)
Dr M. Wallen National Chemicals Inspectorate (KEMI), Solna, Sweden
Ms D Willcocks, Chemical Assessment Division, Worksafe Australia,
Camperdown, Australia
Dr M. Williams-Johnson, Division of Toxicology, Agency for Toxic
Substances and Disease Registry, Atlanta, GA, USA
Dr K. Ziegler-Skylakakis, Senatskommission der Deutschen
Forschungsgemeinschaft zuer Pruefung gesundheitsschaedlicher
Arbeitisstoffe, GSF-Institut fuer Toxikologie, Neutherberg,
Oberschleissheim, Germany
Observers
Mrs B. Dinham,1 The Pesticide Trust, London, United Kingdom
Dr R. Ebert, KSU Ps-Toxicology, Huels AG, Mart, Germany (representing
ECETOC, the European Centre for Ecotoxicology and Toxicology of
Chemicals)
Mr R Green,1 International Federation of Chemical, Energy, Mine and
General Workers' Unions, Brussels, Belgium
Dr B. Hansen,1 European Chemicals Bureau, European Commission, Ispra,
Italy
Dr J. Heuer, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Mr T. Jacob,1 DuPont, Washington, DC, USA
Ms L. Onyon, Environment Directorate, Organisation for Economic
Co-operation and Development, Paris, France
Dr H.J. Weideli, Ciba Speciality Chemicals Inc., Basel, Switzerland
(representing CEFIC, the European Chemical Industry Council)
Secretariat
Dr M. Baril, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr R.G. Liteplo, Health Canada, Ottawa, Ontario, Canada
Ms L. Regis, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Mr A, Strawson, Health and Safety Executive, London, United Kingdom
Dr P. Toft, Associate Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
1 Invited but unable to attend.
RÉSUMÉ D'ORIENTATION
Ce CICAD relatif au 1,1,1,2-tétrafluoréthane est fondé sur une
étude des problèmes de santé (notamment dans le domaine de l'hygiène
du travail) réalisée par le Health and Safety Executive du Royaume-Uni
en 1995 (Standring et al., 1995). Des informations complémentaires
concernant les effets de cette substance sur la santé humaine et
l'environnement ont été identifiées dans l'étude ECETOC (1995). Ces
deux études prennent en compte les données antérieures à décembre
1994. Les données postérieures à leur publication ont été incorporées
dans le présent document. Les informations relatives à l'examen du
document initial par les pairs et à sa disponibilité figurent à
l'appendice 1. Les renseignements concernant l'examen du CICAD par les
pairs font l'objet de l'appendice 2. Ce CICAD a été approuvé entant
qu'évaluation internationale lors d'une réunion du Comité d'évaluation
finale qui s'est tenue à Berlin (Allemagne) du 26 au 28 novembre 1997.
La liste des participants à cette réunion figure à l'appendice 3. La
fiche d'information sur la sécurité chimique du
1,1,1,2-tétrafluoréthane (ICSC 128 1), établie parle Programme
international sur la Sécurité chimique (IPCS, 1998), est également
reproduite dans le présent document.
Le 1,1,1,2-tétratluoréthane (CAS N° 811-97-2) est un
fluorocarbure gazeux obtenu par réaction du fluorure d'hydrogène sur
le trichloréthylène en vase clos. Il est utilisé principalement comme
réfrigérant dans des appareils fonctionnant à température relativement
élevée, comme les réfrigérateurs domestiques et les climatiseurs
d'automobiles. Il peut également être employé dans la fabrication de
mousses plastiques, comme solvant pour certaines opérations de
nettoyage, comme propulseur d'aérosols pour inhalateurs médicaux, et
comme produit extincteur à la place des halons.
On dispose de peu d'informations sur l'exposition du grand public
ou des travailleurs au 1,1,1,2-tétrafluoréthane, Le personnel employé
à sa fabrication au Royaume-Uni a été soumis à une très faible
exposition, les concentrations ne dépassant jamais 7 ppm (29,2
mg/m3). L'exposition résultant de son utilisation dans l'industrie
manufacturière n'a pas été mesurée et l'on ne dispose pas de données
concernant l'exposition des personnels appelés à l'utiliser sur le
terrain. Compte tenu de la situation sur les lieux de travail au
Royaume-Uni, et par analogie avec les données obtenues lors d'une
étude de l'exposition au dichlorotrifluoréthane (HCFC 123), il semble
que la concentration de 1,1,1,2-tétratluoréthane sur les lieux de
travail soit normalement faible (inférieure à 10 ppm, soit 41,7
mg/m3), avec parfois des pies d'exposition de courte durée atteignant
quelques centaines de parties par million.
Les renseignements concernant les effets du
1,1,1,2-tétrafluoréthane sur l'homme se limitent à une étude; la plupart
des données disponibles sur les effets toxicologiques de cette
substance ont été obtenues à partir d'expériences chez l'animal. Le
1,1,1,2-tétrafluoréthane a une toxicité relativement faible. Des
études visant à évaluer sa toxicité pour le développement font état
d'un ralentissement du gain pondéral chez des lapines exposées à
40 000 ppm (166 800 mg/m3) et d'un retard de développement des foetus
chez des rates exposées à 50 000 ppm (208 500 mg/m3). D'autres études
toxicologiques ne signalent aucun effet défavorable chez des animaux
exposés à des concentrations allant jusqu'à 10 000 ppm (41 700
mg/m3). Les indices de cancérogénicité se limitent à une incidence
accrue des adénomes des cellules de Leydig après exposition à 50 000
ppm (208 500 mg/m3); d'autre part, les études menées jusqu'à présent
ne révèlent aucun signe de génotoxicité,
La faible toxicité du 1,1,1,2-tétrafluoréthane pour les quelques
organismes aquatiques sur lesquels il a été testé et sa grande
volatilité donnent à penser qu'il constitue un risque négligeable pour
ces organismes.
Les effets du 1,1,1,2-tétrafluoréthane sur l'atmosphère ont été
évalués à l'aide de modèles. Des observations récentes ont révélé une
augmentation rapide de sa concentration atmosphérique, résultant
principalement des émissions qui ont eu lieu au cours de la dernière
décennie. Les résultats de la modélisation montrent que le
1,1,1,2-tétrafluoréthane présente un risque insignifiant en ce qui
concerne la destruction de l'ozone, un risque significatif pour ce qui
est du réchauffement mondial et un risque négligeable d'acidification.
RESUMEN DE ORIENTACION
Este CICAD (resumen de evaluación internacional de sustancias
químicas) sobre el 1,1,1,2-tetrafluoroetano está basado en un estudio
sobre su posible incidencia (fundamentalmente ocupacional) en la salud
humana preparado por la Dirección de Salud y Seguridad del Reino Unido
en 1995 (Standring et al., 1995), En el ECETOC (1995) se halló más
información sobre los efectos en la salud humana y en el medio. Los
datos manejados en esos dos estudios abarcan hasta diciembre de 1994.
También se ha incluido cuando procedia información adicional hallada
tras la publicación de esos estudios. En el apéndice 1 se informa
sobre la naturaleza del examen colegiado y la disponibilidad del
documento de base, y en el apéndice 2 se facilita información sobre el
examen colegiado del presente resumen. Este CICAD fue aprobado como
resumen de evaluación internacional en una reunión de la Junta de
Revisión Final celebrada en Berlín (Alemania) los días 26 a 28 de
noviembre de 1997. La lista de los participantes en la reunión de la
Junta de Revisión Final figura en el apéndice 3. En este documento se
reproduce también la ficha internacional de seguridad química (ICSC
1281) para el 1,1,1,2-tetrafluoroetano, preparada por el Programa
Internacional de Seguridad de las Sustancias Químicas (IPCS, 1998).
El 1,1,1,2-tetrafluoroetano (CAS n° 811-97-2) es un fluorocarburo
gaseoso que se fabrica haciendo reaccionar el ácido fluorhídrico y el
tricloroetileno en un sistema cerrado. Se usa fundamentalmente como
refrigerante para el enfriamiento de "alta temperatura," por ejemplo
en los frigoríficos domésticos y en los sistemas de aire acondicionado
de los automóviles. Otros usos posibles son su empleo en espumación,
como disolvente en aplicaciones de limpieza especiales, como propulsor
de aerosoles para inhaladores médicos y como extintor de incendios en
lugar de los halones.
La información hallada sobre la exposición del público general o
los trabajadores al 1,1,1,2-tetrafluoroetano es escasa. Durante su
fabricación en el Reino Unido, la exposición de los empleados a ese
producto fue muy baja, y las concentraciones medidas no superaron en
ningún caso las 7 ppm (29,2 mg/m3). No se dispone de datos sobre la
exposición asociada a su uso en la industria fabril, ni sobre la
exposición del personal de servicios sobre el terreno. La situación en
los lugares de trabajo en el Reino Unido y otros datos análogos de un
solo estudio de exposición al diclorotrifluoroetano (HCFC 123) parecen
indicar que la exposición al 1,1,1,2-tetrafluoroetano en el lugar de
trabajo es normalmente baja (es decir, inferior a 10 ppm [41,7
mg/m3]), registrándose ocasionalmente exposiciones máximas breves de
hasta varios cientos de partes por millón.
Sólo hay un informe que trate de los efectos del
1,1,1,2-tetrafluoroetano en el ser humano; la mayor parte de los datos
disponibles sobre sus efectos toxicológicos proceden de estudios
realizados en animales de laboratorio. El 1,1,1,2-tetrafluoroetano
tiene una toxicidad relativamente baja. Los estudios sobre su
toxicidad en el desarrollo han mostrado una reducción del aumento del
peso corporal materno en conejos expuestos a 40 000 ppm (166 800
mg/m3) de 1,1,1,2-tetrafluoroetano, así como signos de retraso del
desarrollo fetal en ratas tras la exposición de las madres a
concentraciones de 50 000 ppm (208 500 mg/m3). En otras
investigaciones toxicológicas no se han observado efectos adversos
para la salud tras la exposición a concentraciones de hasta 10 000 ppm
(41 700 mg/m3). Los indicios de carcinogenicidad se limitan a un
aumento de la incidencia de adenomas de las células de Leydig tras la
exposición a 50 000 ppm (208 500 mg/m3), y no se han detectado
efectos genotóxicos en los estudios realizados hasta la fecha.
La baja toxicidad del 1,1,1,2-tetrafluoroetano para los escasos
organismos acuáticos analizados, así como su elevada volatilidad,
indican que el riesgo es insignificante para los organismos acuáticos.
Se han elaborado modelos para evaluar los efectos atmosféricos.
Observaciones recientes han puesto de manifiesto un rápido incremento
de las concentraciones atmosféricas de 1,1,1,2-tetrafluoroetano,
principalmente como resultado de las emisiones realizadas a lo largo
del último decenio. La modelización ha revelado una desdeñable
contribución potencial al agotamiento del ozono, una considerable
capacidad de contribución al calentamiento mundial y un potencial de
acidificación despreciable.
1. EXECUTIVE SUMMARY
This CICAD on 1,1,1,2-tetrafluoroethane was based on a review of
human health concerns (primarily occupational) prepared by the United
Kingdom Health and Safety Executive in 1995 (Standring el al., 1995).
Additional information on effects on human health and the environment
was identified in ECETOC (1995). Data identified up to December 1994
were covered by these reviews. Additional data identified after these
reviews were published have been incorporated as appropriate.
Information on the nature of the peer review and availability of the
source document is presented in Appendix 1. Information on the peer
review of this CICAD is presented in Appendix 2. This CICAD was
approved as an international assessment at a meeting of the Final
Review Board, held in Berlin, Germany, on 26-28 November 1997.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 1281) for
1,1,1,2-tetrafluoroethane, produced by the International Programme on
Chemical Safety (IPCS, 1998), has also been reproduced in this
document.
1,1,1,2-Tetrafluoroethane (CAS no. 811-97-2) is a gaseous
fluorocarbon that is manufactured by the reaction of hydrogen fluoride
with trichloroethylene in a closed system. It is used primarily as a
refrigerant for "high-temperature" refrigeration, such as domestic
refrigerators and automobile air conditioners. Other potential uses
include application in plastic foam blowing, as a solvent for special
cleaning applications, as an aerosol propellant for medical inhalers,
and as a fire extinguishant in place of halons.
Little information was identified on exposure of the general
public or workers to 1,1,1,2-tetrafluoroethane. During its manufacture
in the United Kingdom, employee exposure to the chemical was very low,
with no measured concentrations above 7 ppm (29.2 mg/m3). There are
no exposure measurements from its use in the manufacturing industry
and no data on the exposure of field servicing personnel. The
situation in the workplace in the United Kingdom and analogous data
from a single study of exposure to dichlorotrifluoroethane (HCFC 123)
would suggest that exposure to 1,1,1,2-tetrafluoroethane in the
workplace is normally low (i.e., below 10 ppm [41.7 mg/m3]), with
occasional short-term peak exposures of up to several hundred parts
per million
Information on the effects of 1,1,1,2-tetrafluoroethane on humans
is limited to one report; most available data on the toxicological
effects of 1,1,1,2-tetrafluoroethane have been derived from studies
conducted with laboratory animals. 1,1,1,2-tetrafluoroethane exhibits
relatively low toxicity. A reduction in maternal body weight gain in
rabbits exposed to 40 000 ppm (166 800 mg/m3)
1,1,1,2-tetrafluoroethane and signs of delayed fetal development in
rats following exposure of the dams to 50 000 ppm (208 500 mg/m3)
1,1, 1,2-tetrafluoroethane have been noted in developmental toxicity
studies. In other toxicological investigations, adverse health effects
have not been observed following exposure to concentrations up to
10 000 ppm (41 700 mg/m3). The weight of evidence for carcinogenicity
is limited to an increased incidence of Leydig cell adenomas following
exposure to 50 000 ppm (208 500 mg/m3), and 1,1,1,2-tetrafluoroethane
has not been found to be genotoxic in studies conducted to date.
The low toxicity of 1,1,1,2-tetrafluoroethane to the few aquatic
organisms tested as well as its high volatility indicate negligible
risk to aquatic organisms.
Atmospheric effects have been assessed by modelling. Recent
observations have shown a rapid increase in atmospheric concentrations
of 1,1,1,2-tetrafluoroethane, mainly as a result of emissions over the
past decade. Modelling indicates insignificant ozone depiction
potential, a significant global warming potential, and negligible
acidification potential.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
1,1,1,2-Tetrafluoroethane (CAS no. 811-97-2; C2H2F4;
1,2,2,2-tetrafluoroethane, HFC 134a, HFA 134a, HCFC 134a) is a gaseous
fluorocarbon with a faint ether-like odour. It is soluble in alcohols,
esters, and chlorinated solvents, but it is only slightly soluble in
water. It has a boiling point of -26°C and a vapour pressure of 630
kPa at 25°C. Additional properties are presented in the International
Chemical Safety Card reproduced in this document. The conversion for
1,1,1,2-tetrafluoroethane is 1 ppm=4.17 mg/m3 (at 25°C). The
structural formula for 1,1,1,2-tetrafluoroethane is:
F H
' '
F - C - C - F
' '
F H
3. ANALYTICAL METHODS
An unpublished method based on a Health and Safety Executive
(1995) procedure has been used to monitor exposure to
1,1,1,2-tetrafluoroethane. The sample is collected diffusively onto
Spherocarb and analysed by thermal desorption into a gas chromatograph
fitted with a flame ionization detector (FID). The diffusive uptake
rate is reported as 1.2 ng/ppm per minute, and the method has been
validated down to 0.1 ppm for exposure periods of 30-480 min.1 Pumped
sampling onto Anasorb CMS followed by solvent desorption and analysis
with a gas chromatograph fitted with an FID has also been validated
(Griffiths, 1998), Both the Miran infrared monitor (Quantitech Ltd)2
and the Innova 1312 photoacoustic monitor (CBISS)3 can be used to
measure airborne concentrations of 1,1,1,2-tetrafluoroethane to
sub-ppm concentrations.
There are no published methods for the biological monitoring of
occupational exposure to 1,1,1,2-tetrafluoroethane. However, by
analogy with other haloalkanes, it may be possible to develop
biological monitoring methods based on the analysis of
1,1,1,2-tetrafluoroethane in the breathing zone or urine (Woollen et
al., 1990, 1992). In addition, a study of its use in medical inhalers
revealed that 1,1,1,2-tetrafluoroethane can be measured in blood
samples; sampling at 2 min indicated 1,1,1,2-tetrafluoroethane levels
of 200-700 ng/ml, with a substantial reduction by 12 min (Donnell et
al., 1995).
1 Personal communication, ICI Laboratories, The Heath, Runcorn, UK.
2 Unit 3, Old Wolverton Road, Old Wolverton, Milton Keynes, UK
MK12 5NP.
3 5-11 Coronation Drive, Bromborough, Wirral, UK L62 3LF.
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
1,1,1,2-Tetrafluoroethane is manufactured by the reaction of
hydrogen fluoride with trichloroethylene in a closed system. It is
available as a liquefied gas and is supplied in a variety of
pressurized containers. 1,1,1,2-tetrafluoroethane is used primarily as
a refrigerant for "high-temperature" refrigeration, such as domestic
refrigerators and automobile air conditioners. Other potential uses
include application in plastic foam blowing, as a solvent for special
cleaning applications, as an aerosol propellant for medical inhalers,
and as a fire extinguishant in place of halons.
Between 1990 and 1995, the estimated global production of
1,1,1,2-tetrafluoroethane for dispersive use increased from 0.2 to 73.8
kilotonnes per year; over this same period, the estimated global
release of this chemical increased from 0.1 to 20.3 kilotonnes per
year (AFEAS, 1996).
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
1,1,1,2-Tetrafluoroethane is expected to partition almost
exclusively to the atmosphere. Aqueous discharges would be expected to
volatilize, with half-lives of days to a few weeks. It is not expected
that 1,1,1,2-tetrafluoroethane will accumulate in biota (log Kow
approx. 1.06) or adsorb to soil or sediment (log Koc approx. 1.5).
The atmospheric equilibrium concentration in cloud water has been
estimated at less than 0.2 ppt by weight based on predicted
atmospheric concentrations of 100-200 ppt by volume (0.4-0.8 µg/m3)
for the year 2020 (McCulloch, 1993). The long atmospheric half-life
will result in more or less uniform distribution in the atmosphere on
a global scale (Franklin, 1993),
The overall estimated lifetime of 1,1,1,2-tetrafluoroethane in
the troposphere is 14.6 years (IPCC, 1995); degradation is initiated
by hydroxyl (OH) radicals. It is theoretically possible that
1,1,1,2-tetrafluoroethane could contribute to ozone depletion by means
of CF3Ox, radicals arising from the atmospheric degradation of
tetrafluoroethane; however, this contribution has been estimated to be
insignificant in recent studies (Ko et al., l994; Ravishankara et al.,
1994).
1 1,1,2-Tetrafluroethane's global warming potential over a
100-year time horizon (relative to carbon dioxide) has been estimated
at 1300, compared with 3800 for CFC-11 and 8100 for CFC-12, for which
1,1,1,2-tetrafluoroethane is the main substitute (IPCC, 1995).
Franklin (1993) has estimated that 1,1,1,2-tetrafluoroethane will
reach an atmospheric background concentration of 100 ppt by volume
(0.4 µg/m3) by 2010-2020 and will then be responsible for only about
0.3% of the radiative forcing due to all anthropogenic greenhouse
gases present in the atmosphere.
Hydroxyl radicals break down 1,1,1,2-tetrafluoroethane to form
the CF3CHFO radical, which reacts with oxygen to generate
trifluoroacetyl fluoride (CF3COF) or undergoes cleavage to give
formyl fluoride (HCOF) and the CF3 radical, which is ultimately
converted to carbonyl fluoride (COF2) and hydrogen fluoride (HF).
Modelling studies predict that 40% of 1,1,1,2-tetrafluoroethane
breakdown will proceed via the former route and 60% by the latter
route (Franklin, 1993). Recent research suggests that the yield of
trifluoroacetyl fluoride is in the range of 7-20% rather than 40%, as
was previously assumed (Wallington et al., 1996).
The principal fate of the acid fluorides (CF3COF, HCOF, and
COF2) will be uptake by cloud water and hydrolysis to trifluoroacetic
acid, formic acid, carbon dioxide, and hydrogen fluoride. Dry
deposition to ocean or land surfaces may occur to a limited extent and
will be followed by hydrolysis (AFEAS, 1992, 1993).
The contribution of degradation products to environmental
fluorides and acidity of rainwater is expected to be negligible (WMO,
1989; Franklin, 1993).
There are no known natural sources of trifluoroacetic acid.
However, recent work (Frank et al., 1996) has measured trifluoroacetic
acid in rainwater and surface waters in Europe and Israel at levels
too high to be explained by the atmospheric degradation of
1,1,1,2-tetrafluoroethane and other chlorofluorocarbon substitutes.
The origin of this trifluoroacetic acid is currently unexplained, and
a natural source cannot be ruled out. Using the same assumptions for
emission and atmospheric degradation as above (Franklin, 1993),
deposition of trifluoroacetic acid in rainwater would be 45 kilotonnes
per year (in the years 2010-2020), with an average concentration in
precipitation globally at 0.1 µg/litre. Trifluoroacetic acid will
partition into the aqueous environment; assuming accumulation in the
upper levels of seawater, an increased concentration of 1.5 ng/litre
would be expected for each 100 kilotonnes of 1,1,1,2-tetrafluoroethane
degraded.
Laboratory tests have demonstrated no appreciable degradation of
1,1,1,2-tetrafluoroethane in activated sludge (Tobeta, 1989) or by the
methanotropic bacterium Methylosimus trichosporium (DeFlaun et al.,
1992). Trifluoroacetic acid can be degraded under anoxic conditions to
trifluoromethane, inorganic fluoride, methane, and carbon dioxide
(Visscher et al., 1994).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
In 1995, the average atmospheric concentration of
1,1,1,2-tetrafluoroethane was about 2 ppt (8.3 ng/m3);
there had been a significant increase in concentration measured
throughout 1994 and 1995, rising from about 0.3 ppt (1.3 ng/M3) in
early 1994 to a range of 1.2-3.4 ppt (5.0-14.2 ng/m3) in late 1995
(Montzka et al., 1996). Measurements were taken on land in Canada, the
continental United States, Hawaii, American Samoa, and Tasmania and at
sea in the Pacific and Atlantic oceans.
6.2 Human exposure
Information on potential exposure of the general public to
1,1,1,2-tetrafluoroethane was not identified, and there are limited
data concerning occupational exposure. During its manufacture in the
United Kingdom in a modern plant, employee exposure was very low, with
no measured concentrations above 7 ppm (29.2 mg/m3) (Standring et
al., 1995). There are no exposure measurements from its use in the
manufacturing industry and no data on the exposure of field servicing
personnel. At the time of review, there was only one manufacturer in
the United Kingdom, although other production facilities are envisaged
to come on stream in the near future. The situation in the United
Kingdom and analogous data from a single study of exposure to
dichlorotrifluoroethane (HCFC 123) (Standring et al., 1995) would
suggest that exposure is normally low (i.e., below 10 ppm [41.7
mg/m3], 12-hour time-weighted average), with occasional short-term
peak exposures of up to several hundred parts per million (H. Sibley,
undated).
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND
HUMANS
The elimination and distribution of a single breath inhalation of
[18F] 1,1,1,2-tetrafluoroethane were measured in a small group of
volunteers using whole body gamma-counting (Pike et al., 1995).
Distribution was extensive, and elimination was rapid and essentially
complete within 6 h (half-life approx. 1.5-4 h). Elimination of
radioactivity in the urine was observed in some but not all subjects,
and there was no evidence of accumulation.
A group of four volunteers received 16 breath inhalations of 1,
1,1,2-tetrafluoroethane (total dose 1200 mg) over a 10-min period in
an investigation of its use as a propellant in medical devices (Monte
et al., 1994). Urine samples were collected over a 24-h period and
analysed for trifluoroacetic acid using 19F nuclear magnetic
resonance spectroscopy (detection limit 10 ng/ml). The amounts of
trifluoroacetic acid measured in urine ranged from undetectable to
0.0004% of the administered 1,1,1,2-tetrafluoroethane. There were no
1,1,1,2-Tetrafluoroethane other fluorinated products detected in urine
using this technique.
In two inhalation studies conducted with rats,
1,1,1,2-tetrafluoroethane was poorly absorbed (Ellis el al., 1991,
1993), and elimination was rapid and achieved mainly by exhalation of
unchanged 1,1,1,2-tetrafluoroethane (Finch et al., 1995). Very little
metabolism occurred, with the main metabolite being carbon dioxide;
trifluoroacetic acid was identified in urine. There was no significant
accumulation of absorbed 1,1,1,2-tetrafluoroethane in specific
tissues.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
1,1,1,2-Tetrafluoroethane has low acute toxicity. An approximate
4-h lethal concentration of 567 000 ppm (2.36 × 106 mg/m3) has been
reported for rats; no effects were observed at 81 000 ppm (337 770
mg/m3) (Kennedy, 1979a, as cited in ECETOC, 1995). At concentrations
in excess of 200 000 ppm (834 000 mg/m3), exposure to
1,1,1,2-tetrafluoroethane depressed the central nervous system of
rats.1 Anaesthetic effects have also been observed in other species.
Cardiac sensitization (an increased sensitivity of the heart) to
exogenous adrenalin has been observed in dogs exposed to
1,1,1,2-tetrafluoroethane, with a no-observed-effect level (NOEL) of
40 000 ppm (166 800 mg/m3) (Hardy el al., 1991).
1 Programme for Alternative Fluorocarbon Testing, presentation at
Toulouse International Toxicology Forum, September 1989; meeting notes
presented as a personal communication to P Standring, 1993.
8.2 Irritation and sensitization
Studies on irritation or sensitization were not available.
8.3 Short-term exposure
Information from typical short-term repeated exposure toxicity
studies was not identified.
8.4 Long-term exposure
8.4.1 Subchronic exposure
No significant exposure-related toxicological effects were
observed in an inhalation study in which groups of male and female
rats were exposed for 13 weeks to 1,1,1,2-tetrafluoroethane at
concentrations up to 50 000 ppm (208 500 mg/m3) (Hext, 1989; Collins
el al., 1995),
8.4.2 Chronic exposure and carcinogenicity
In a study conducted according to a contemporary protocol, groups
of 85 male and 85 female Wistar-derived Alderley Park rats were
exposed (whole-body) to 0 (air only), 2500, 10 000, or 50 000 ppm (0,
10 425, 41 700, or 208 500 mg/m3) 1,1,1,2-tetrafluoroethane, 6 h/day,
5 days/week, for 2 years (Hext & Parr-Dobrzanski, 1993; Collins el
al., 1995). Mortality rates were low and similar in the control and
exposed groups. No exposure-related pathological findings were
recorded at interim sacrifice (52 weeks). At termination, the only
exposure-related pathological findings were increased incidences of
Leydig (interstitial) cell hyperplasia and benign Leydig cell adenomas
in the testes. The microscopic findings in the testes occurred mainly
in animals surviving to the end of the study. In the control, 2500,
10 000, and 50 000 ppm (0, 10 425, 41 700, or 208 500 mg/m3) groups,
the incidence of Leydig cell hyperplasia was 27/85, 25/79, 31/85, and
40/85 (32, 32, 36, and 47%), respectively; the incidence of Leydig
cell adenoma was 9/85, 7/79, 12/85, and 23/85 (11, 9, 14, and 27%),
respectively. Hyperplasia was observed in most animals with such
tumours. At 50 000 ppm (208 500 mg/m3) 1,1,1,2-tetrafluoroethane, the
incidence of Leydig cell adenoma was significantly (p < 0.05)
increased above the controls. The incidence of Leydig cell adenomas
and hyperplasia at 10 000 ppm (41 700 mg/m3) was within the
historical control levels observed at this laboratory; from 1985 to
1995, the background incidence of this tumour ranged between 4 and
19%. The no-observed-adverse-effect level (NOAEL) in this study is
considered to be 10 000 ppm (41 700 mg/m3).
Other studies were less rigorously performed, but no
exposure-related neoplastic or non-neoplastic effects were observed in
2-year inhalation studies (1 -h daily nose-only exposure) at
concentrations up to 50 000 ppm (208 500 mg/m3) in rats and up to
75 000 ppm (312 750 mg/m3) in mice (Alexander el al., 1995a) or in
a similarly designed 1 - year study in which dogs were exposed to
120 000 ppm (500 400 mg/m3) 1,1,1,2-tetrafluoroethane (Alexander el
al., 1995b).
8.5 Genotoxicity and related end-points
The genotoxic potential of 1,1,1,2-tetrafluoroethane has been
investigated in several well-conducted studies (bacterial mutagenicity
[Ames] test, an in vitro mammalian cell cytogenetics study, an
in vivo chromosomal aberration assay, a micronucleus study, an
in vivo unscheduled DNA synthesis assay, and a dominant lethal
study). 1,1,1,2-Tetrafluoroethane was not genotoxic in any of the
tests (Anderson & Richardson, 1979; Hodge et al., 1979; Longstaff et
al., 1984; Müller & Hofmann, 1989; Callander & Priestley, 1990;
Mackay, 1990; Trueman, 1990; Collins et al., 1995).
8.6 Reproductive and developmental toxicity
No exposure-related effects were observed in a standard fertility
study in which groups of rats were exposed to 0, 2500, 10 000, or
50 000 ppm (0, 10 425, 41 700, or 208 500 mg/m3)
1,1,1,2-tetrafluoroethane, 1 h/day during gametogenesis, mating, and
post-mating (Alexander et al., 1996). The results from a dominant
lethal study revealed no effect on fertility in male rats (Hodge et
al., 1979). In a standard developmental toxicity study in rats,
delayed fetal development (a statistically significant reduction in
mean fetal weight, delayed ossification of digits) was observed when
the dams were exposed to 50 000 ppm (208 500 mg/m3)
1,1,1,2-tetrafluoroethane; no significant exposure-related effects
were observed at 10 000 ppm (41 700 mg/m3) (Hodge et al., 1980). No
other exposure-related developmental effects were observed in rats at
levels up to 40 000 ppm (166 800 mg/m3) 1,1,1,2-tetrafluoroethane, a
concentration causing decreased maternal body weight gain in rabbits
(Wickramaratne, 1989; Collins et al., 1995). In the study with
rabbits, there was a 30% reduction during exposure with subsequent
recovery, resulting in a net reduction in body weight of 3% compared
with controls.
8.7 Immunological and neurological effects
Based upon the available evidence, specific immunological or
neurological effects associated with long-term exposure to
1,1,1,2-tetrafluoroethane were not identified.
9. EFFECTS ON HUMANS
Limited data are available from an investigation into the use of
1,1,1,2-tetrafluoroethane as a propellant in a metered-dose inhaler
(Donnell et al., 1995). Volunteers received up to 16 breath
inhalations of 1,1,1,2-tetrafluoroethane within about 10 min.
Investigations included blood pressure and heart rhythm, limited blood
biochemistry, and pulmonary function tests; no abnormalities were
observed, and there were no clinical signs of toxicity.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
1,1,1,2-Tetrafluoroethane has no significant effect on the growth
of the bacterium Pseudomonas putida (6-h EC50 >730 mg/litre)
(Coleman & Thompson, 1990). Acute toxicity to freshwater organisms is
low ( Daphnia magna, 48-h EC50 980 mg/litre; rainbow trout
Oncorhynchus mykiss, 96-h LC50 450 mg/litre) (Stewart & Thompson,
1990; Thompson, 1990). The high aqueous concentrations used in these
studies can only be maintained artificially. In the environment, there
would be rapid partitioning to the air compartment from the aqueous
phase; the high concentrations used in the studies could be reached
only if the atmosphere above the water were entirely
1,1,1,2-tetrafluoroethane. Additional data on toxicity of
1,1,1,2-tetrafluoroethane to aquatic or terrestrial organisms were not
identified.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Information on the effects of 1,1,1,2-tetrafluoroethane on humans
is limited to one report; most available data on the toxicological
effects of 1,1,1,2-tetrafluoroethane have been derived from studies
conducted with laboratory animals. 1,1,1,2-Tetrafluoroethane exhibits
relatively low toxicity. This chemical is a gas, appears to be
essentially non-reactive, and is unlikely to be either an irritant or
a sensitizer, although appropriate studies were not identified. A
reduction in maternal body weight gain in rabbits exposed to 40 000
ppm (166 800 mg/m3) 1,1,1,2-tetrafluoroethane and signs of delayed
fetal development in rats following exposure of the dams to 50 000 ppm
(208 500 mg/m3) 1,1,1,2-tetrafluoroethane have been noted in
developmental toxicity studies. In other toxicological investigations,
adverse health effects have not been observed following exposure to
concentrations up to 10 000 ppm (41 700 mg/m3)
1,1,1,2-tetrafluoroethane.
The weight of evidence for carcinogenicity of
1,1,1,2-tetrafluoroethane is limited. A statistically significant,
exposure-related increase in the incidence of benign Leydig cell
adenomas was observed in Wistar-derived rats exposed to a very high
concentration (50 000 ppm [208 500 mg/m3]) of
1,1,1,2-tetrafluoroethane for 2 years. However, the spontaneous
incidence of these tumours is high in this and other strains of rats,
and 1,1, 1,2-tetrafluoroethane has not been found to be genotoxic in
studies conducted to date.
11.1.2 Criteria for setting guidance values for
1,1,1,2-tetrafluoroethane
Based upon the available data, no adverse effects have been
observed in laboratory animals exposed to 10 000 ppm (41 700 mg/m3)
1,1,1,2-tetrafluoroethane. This value can therefore serve as a basis
for comparison with estimated exposure for risk characterization,
either with application of appropriate uncertainty factors or
directly. Examples of both approaches are presented in section 11.1.3.
11.1.3 Sample risk characterization
The scenario chosen as an example is the occupational environment
within the United Kingdom, where, under the current conditions of use,
anticipated occupational exposure (8- or 12-h time-weighted average)
to 1,1,1,2-tetrafluoroethane is in the vicinity of 10 ppm (41.7
mg/m3), with occasional short-term peak exposures of up to several
hundred parts per million. These concentrations are 1-3 orders of
magnitude less than the NOAEL of 10 000 ppm (41 700 mg/m3) derived
from toxicological studies conducted with laboratory animals. However,
data on exposure in occupational circumstances within the United
Kingdom are limited, and it is difficult to anticipate exposure
conditions for other countries.
A health-based occupational exposure limit for
1,1,1,2-tetrafluoroethane of 1000 ppm (4170 mg/m3) (8-h time-weighted
average) has been established within the United Kingdom. This equates
to division of the NOAEL of 10 000 ppm (41 700 mg/m3) by an
uncertainty factor of 10.
11.2 Evaluation of environmental effects
The low toxicity of 1,1,1,2-tetrafluoroethane to the few aquatic
organisms tested as well as its high volatility indicate negligible
risk to aquatic organisms.
Atmospheric effects have been assessed by modelling. Recent
observations have shown a rapid increase in atmospheric concentrations
of 1,1,1,2-tetrafluoroethane, mainly as a result of emissions over the
past decade. Modelling indicates insignificant ozone depletion
potential, a significant global warming potential, and negligible
acidification potential.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Previous evaluations of 1,1,1,2-tetrafluoroethane by
international bodies were not identified. Information on international
hazard classification and labelling is included in the International
Chemical Safety Card reproduced in this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventative and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 1281) reproduced in this
document.
13.1 Human health hazards
1,1,1,2-Tetrafluoroethane is essentially non-toxic and flammable.
There is a possibility of frostbite if the liquefied gas is released
rapidly.
13.2 Advice to physicians
Symptomatic treatment and supportive therapy should be provided
as indicated. Adrenaline and similar sympathomimetic drugs should be
avoided following exposure, as cardiac arrhythmia may result, with
possible subsequent cardiac arrest.
13.3 Spillage
In the event of spillage of 1,1,1,2-tetrafluoroethane, emergency
crews should wear proper personal protection, including respiratory
protection. Because the vapour is heavier than air, it may accumulate
in lower spaces, causing a deficiency of oxygen. The oxygen content of
the air should always be checked before the affected area is entered.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards
can be found in the International Register of Potentially Toxic
Chemicals (IRPTC), available from UNEP Chemicals (IRPTC), Geneva.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
1,1,1,2-TETRAFLUOROETHANE ICSC: 1281
26.03.1998
CAS# 811-97-2 HFC 134a
RTECS# K18842500 (cylinder)
UN# 3159 C2H2F4
Molecular mass: 102.03
TYPES OF HAZARD / ACUTE HAZARDS / PREVENTION FIRST AID / FIRE FIGHTING
EXPOSURE SYMPTOMS
FIRE Not combustible. Gives off irritating NO open flames. NO contact with In case of fire in the surroundings;
or toxic fumes (or gases) in a fire. hot surfaces. all extinguishing agents allowed.
EXPLOSION In case of fire: keep cylinder cool
by spraying with water.
EXPOSURE
Inhalation Dizziness. Drowsiness. Dullness. Local exhaust or breathing Fresh air, rest. Refer for medical
protection attention.
Skin ON CONTACT WITH LIQUID: Cold-insulating gloves. ON FROSTBITE rinse with plenty of
FROSTBITE. water, do NOT remove clothes.
Eyes Safety goggles.
Ingestion
(continued)
SPILLAGE DISPOSAL PACKAGING & LABELLING
NEVER direct water jet on liquid. Do NOT let this chemical enter the Symbol
environment Chemical protection suit including self-contained breathing R:
apparatus. S:
UN Hazard Class: 2.2
UN Subsidiary Risks:
UN Pack Group:
EMERGENCY RESPONSE STORAGE
Transport Emergency Card TEC (R)-20G39 Fireproof. Keep in a well-ventilated room.
IMPORTANT DATA
PHYSICAL STATE; APPEARANCE: ROUTES OF EXPOSURE:
COMPRESSED LIQUEFIED GAS, WITH CHARACTERISTIC ODOUR. The substance can be absorbed into the body by inhalation.
CHEMICAL DANGERS: INHALATION RISK:
On contact with hot surfaces or flames this substance A harmful concentration of this gas in the air will be reached
decomposes forming toxic and corrosive fumes. very quickly on loss of containment.
OCCUPATIONAL EXPOSURE LIMITS: EFFECTS OF SHORT-TERM EXPOSURE:
TLV not established Rapid evaporation of the liquid may cause frostbite. The
substance may cause effects on the central nervous system and
cardiovascular system, resulting in cardiac disorders.
(continued)
PHYSICAL PROPERTIES
Boiling point: -26°C
Melting point: -101°C
Solubility in water none
Vapour pressure, kPa at 25°C: 630
Relative vapour density (air = 1): 3.5
0ctanolwater partition coefficient as log Pow: 1.06
ENVIRONMENTAL DATA
Avoid release to the environment in circumstances different to normal use.
NOTES
Do NOT use in the vicinity of a fire or a hot surface, or during welding. Turn leaking cylinder with the leak up to prevent escape of
gas in liquid state.
ADDITIONAL INFORMATION
LEGAL NOTICE Neither the CEC or the IPCS nor any person acting on behalf of the CEC or the IPCS is responsible
for the use which might be made of this information.
REFERENCES
AFEAS (1992) Proceedings of the AFEAS workshop: Atmospheric wet and
dry deposition of carbonyl and haloacetyl halides. Brussels, 22
September 1992. Washington, DC, Alternative Fluorocarbons
Environmental Acceptability Study.
AFEAS (1993) Proceedings of the STEP-HALOCSIDE/AFEAS workshop on
kinetics and mechanisms for the reactions of halogenated organic
compounds in the troposphere. Dublin, 23-25 March 1993. Washington,
DC, Alternative Fluorocarbons Environmental Acceptability Study.
AFEAS (1996) Production, sales and atmospheric emissions of
fluorocarbons through 1995. Washington, DC, Alternative Fluorocarbons
Environmental Acceptability Study.
Alexander D, Libretto S, Chevalier H-J, Imamura T, Pappritz G, Wilson
J (1995a) HFA-134a (1,1,1,2-tetrafluoroethane): lack of oncogenicity
in rodents after inhalation. Human and experimental toxicology, 14
706-714.
Alexander D, Mortimer E, Dines G, Libretto S, Mallett D (1995b) One
year study in dogs of the toxicity of HFA-134a by inhalation.
inhalation toxicology, 7: 1153-1162.
Alexander D, Libretto S, Adams M, Hughes E, Bannerman M (1996)
HFA-134a (1,1,1,2-tetrafluoroethane): effects of inhalation exposure
upon reproductive performance, development and maturation of rats.
Human and experimental toxicology, 15: 508-517.
Anderson D, Richardson C (1979) Arcton 134a: a cytogenetic study in
the rat. Alderley Park, Cheshire, ICI Central Toxicology Laboratory
(Report No CTL/P/444, unpublished).
Callander R, Priestley K (1990) HFC 134a - An evaluation using the
Salmonella mutagenicity assay. Alderley Park, Cheshire, ICI Central
Toxicology Laboratory (Report No. CTL/P/2422, unpublished).
Coleman C, Thompson R (1990) HFC 134a. Determination of the acute
toxicity to Pseudomonas putida. Alderley Park, Cheshire, ICI Central
Toxicology Laboratory (Report No. BL3980/B, unpublished).
Collins M, Rusch G, Sato F, Hext P, Millischer R (1995) 1,1,1,2-
Tetrafluoroethane repeat exposure inhalation toxicity in the rat,
developmental toxicity in the rabbit and genotoxicity in vitro and
in vivo. Fundamental and applied toxicology, 25: 271-280
DeFlaun M, Ensley B, Steffan R (1992) Biological oxidation of
hydrochlorfluorocarbons (HCFC's) by a methanotrophic bacterium.
Bio/Technology, 10(12): 1576-1578.
Donnell D Harrison L, Ward S, Klinger N, Ekholm B, Cooper K, Porietis
I, McEwen J (1995) Acute safety of the CFC-free propellant HFA-134a
from a pressurized metered dose inhaler. Journal of clinical
pharmacology, 48: 473-477.
ECETOC (1995) 1,1,1,2-Tetrafluoroethane (HCF-134a), Brussels European
Centre for Ecotoxicology and Toxicology of Chemicals (Joint Assessment
of Commodity Chemicals No. 31).
Ellis M, Gowans L, Green T (1991) Hydrofluorocarbon 134a:
Pharmacokinetics and metabolism in rats following a single exposure by
inhalation Alderley Park, Cheshire, ICI Central Toxicology Laboratory
(Report No CTL/R/1090, unpublished).
Ellis M, Gowans L, Green T. Tanner R (1993) Metabolic fate and
disposition of 1,1,1,2-tetrafluoroethane (HCF 134a) in rat following a
single exposure by inhalation. Xenobiotica, 23(7) 719-729.
Finch J, Dadey E, Smith S, Harrison L, Digenis G (1995) Dynamic
monitoring of total-body absorption by 19F NMR spectroscopy: one hour
ventilation of HFA 134a in male and female rats, Magnetic resonance in
medicine, 33: 409-413.
Frank H, Klein A, Renschen D (1996) Environmental trifluoroacetate.
Nature, 382 34.
Franklin J (1993) The atmospheric degradation and impact of
1,1,1,2-tetrafluoroethane (Hydrofluorocarbon 134a), Chemosphere,
27(8): 1565-1601.
Griffiths JR (1998) Measurement of airborne 1,1,1,2-tetrafluoroethane.
Health and Safety Executive (IR/L/SP/97/08)
Hardy C, Sharman I, Clark G (1991) Assessment of cardiac sensitisation
potential in dogs. Comparison of HFA 134a and A12. Alderley Park,
Cheshire, ICI Central Toxicology Laboratory (Report No. CTL/C/2521,
unpublished).
Health and Safety Executive (1995) Methods for determination of
hazardous substances: MDHS 72. Volatile organic chemicals in air.
London, HMSO.
Hext P (1989) HFC 134a: 90-day inhalation toxicity study in the rat.
Alderley, Park, Cheshire, ICI Central Toxicology Laboratory (Report
No. CTL/P/2466, unpublished).
Hext P, Parr-Dobrzanski R (1993) HFC 134a: 2-year inhalation toxicity
study in the rat. Alderley Park, Cheshire, ICI Central Toxicology
Laboratory (Report No. CTL/P/3841, unpublished).
Hodge M, Anderson D, Bennett I, Weight T (1979) Arcton 134a: Dominant
lethal study in the mouse. Alderley Park, Cheshire, ICI Central
Toxicology Laboratory (Report No. CTL/R/437, unpublished).
Hodge M, Kilmartin M, Riley R, Weight T, Wilson J (1980) Arcton 134a:
Teratogenicity study in the rat, Alderley Park, Cheshire, ICI Central
Toxicology Laboratory (Report No. CTL/P/417, unpublished).
IPCC (1995) Climate change 1995. The science of climate change.
Contribution of group 1 to the Second Assessment Report of the
Intergovernmental Panel on Climate Change. Geneva, World
Meteorological Organization/United Nations Environment Programme.
IPCS (1998) International Chemical Safety Card -
1,1,1,2-Tetrafluoroethane Geneva, World Health Organization,
International Programme on Chemical Safety (No. 1281).
Ko M, Sze N, Rodriguez J, Weisenstein D, Heisey C, Wayne R, Biggs P,
Canosa-Mas C, Sidebottom H, Treacy J (1994) CF3 chemistry: Potential
implications for stratospheric ozone. Geophysics research letters,
21(2): 101-104.
Longstaff E, Robinson M, Bradbrook C, Styles J, Purchase I (1984)
Genotoxicity and carcinogenicity of fluorocarbons assessment by
short-term in vitro tests and chronic exposure to rats. Toxicology
and applied pharmacology, 72: 15-31
Mackay J (1990) HFC 134a An evaluation in the in vitro cytogenetic
assay in human lymphocytes, Alderley Park, Cheshire, 1Ci Central
Toxicology Laboratory (Report No CTUP/2977, unpublished).
McCulloch A (1993) [German Federal Government Responsibility for the
future - ways to sustainable management of substances and waste flows.
Intermediate report of the Enquete Commission "Protection of man and
the environment. Valuation criteria and perspectives for
environmentally friendly substance cycling in industrialised society"
to the 12th German Bundestag.] Bonn, Bundesrepublik Deutschland
Economica Verlag (in German).
Monte S, Ismail I, Mallett D, Matthews C, Tanner R (1994) The minimal
metabolism of inhaled 1,1,1,2-tetrafluoroethane to trifluoroacetic
acid in man as determined by high sensitivity nuclear magnetic
resonance spectroscopy of urine samples. Journal of pharmacology and
biomedical analysis, 12(12): 1489,-1493.
Montzka S, Butler J, Myers R, Thompson T, Swanson T, Clarke A, Lock L,
Elkins J (1996) Decline in the tropospheric abundance of halogen from
halocarbons: implications for stratospheric ozone depletion. Science,
272: 1318-1322.
Müller W, Hofmann T (1989) CFC 134a micronucleus test in male and
female NMRI mice after inhalation. Frankfurt, Hoechst Laboratories
(Report No. 89.0115, unpublished).
Pike V, Aigbirhio F, Freemantle C, Page B, Rhodes C, Waters S, Jones
T, Olsson P, Ventresca G, Tanner R, Hayes M, Hughes J (1995)
Disposition of inhaled 1,1,1,2-tetrafluoroethane (HFA 134a) in healthy
subjects and in patients with chronic airflow limitation. Drug
metabolism and disposition, 23(8): 832-839
Ravishankara A. Turnipseed A, Jensen N, Barone S, Mills M, Howard C,
Solomon S (1994) Do hydrofluorocarbons deplete stratospheric ozone?
Science, 263: 71-75.
Sibley H (undated) A study for determining refrigerant exposure levels
while servicing an HCFC-123 centrifugal chiller. Syracuse, NY, Carrier
Corporation (internal paper).
Standring P, Maidiment S, Ogunbiyi A, Groves J. Cocker J (1995)
1,1,1,2-Tetrafluoroethane; Criteria document for an occupational
exposure limit. Sudbury, Suffolk, Health and Safety Executive, HSE
Books (ISBN 0 7176 0947 2).
Stewart K, Thompson R (1990) HFC 134a: Determination of the acute
toxicity to Daphnia magna. Alderley Park, Cheshire, ICI Central
Toxicology Laboratory (Report No BL3908/B, unpublished).
Thompson R (1990) HFC 134a: Determination off the acute toxicity to
rainbow trout (Salmo gairdneri). Alderley Park, Cheshire, ICI Central
Toxicology Laboratory (Report No. BL4035/B, unpublished).
Tobeta V (1989) Test of biodegradability of HFC 134a by microorganisms
Fukuoka, Kurume Research Laboratories (Report No. 11598, unpublished)
Trueman R (1990) HFC 134a: Assessment for the induction of unscheduled
DNA syntheses in rat hepatocytes in vivo, Alderley Park, Cheshire, ICI
Central Toxicology Laboratory (Report No. CTL/P/2550, unpublished).
Visseher PT, Culbertson CW, Oremland RS (1994) Degradation of
trifluoroacetate in oxic and anoxic sediments Nature, 369(6483):
729-731.
Wallington T, Hurley M, Fracheboud J, Orlando J, Tyndall G, Sehested
J, Mogelberg T, Nielsen O (1996) Role of excited CF3CFHO radicals in
the atmospheric chemistry of HCF-134a. Journal of physical chemistry,
100: 18116-18122.
Wickramaratne G (1989) HFC 134a: Teratogenicity inhalation study in
the rabbit. Alderley Park, Cheshire, ICI Central Toxicology Laboratory
(Report No CTL/P/2504, unpublished).
WMO (1989) Scientific assessment of stratospheric ozone: 1989. Geneva,
World Meteorological Organization (Global Ozone Research and
Monitoring Project, Report No 20).
Woollen B, Guest E. Howe W, Marsh J, Wilson H, Auton T, Blaine P
(1990) Human inhalation pharmacokinetics of 1,1,2-trichloro-
1,2,2-trifluoroethane(FC113). International archives of occupational
and environmental health, 64(5): 383-387.
Woollen B, Marsh J, Mahler J, Auton T, Makepeace D, Cocker J. Blaine P
(1992) Human inhalation pharmacokinetics of chlorodifluoromethane
(HFA22) International archives of occupational and environmental
health, 64(5): 383-387.
APPENDIX 1 - SOURCE DOCUMENT
Standring et al. (1995)
The draft report entitled 1,1,1,2-Tetrafluoroethane, Criteria
document for an occupational exposure limit (prepared by P. Standring,
S. Maidment, A. Ogunbiyi, J. Groves, and J. Cocker) was initially
reviewed internally by a group of approximately 10 Health and Safety
Executive experts (mainly toxicologists, but also experts in other
relevant disciplines, such as epidemiology and occupational hygiene).
The toxicology section of the amended draft was then reviewed by
toxicologists from the United Kingdom Department of Health.
Subsequently, the entire criteria document was reviewed by a
tripartite advisory committee to the United Kingdom Health and Safety
Commission, the Working Group for the Assessment of Toxic Chemicals
(WATCH). This committee is composed of experts in toxicology and
occupational health and hygiene from industry, trade unions, and
academia.
Members of the WATCH committee at the time of the peer review
were Mr S. Bailey, Independent Consultant; Professor J, Bridges,
University of Surrey; Dr I. Guest, Chemical Industries Association; Dr
A. Hay, Trade Unions Congress; Dr L. Levy, Institute of Occupational
Hygiene, Birmingham; Dr M. Molyneux, Chemical Industries Association;
Mr A. Moses, Chemical Industries Association; Dr R. Owen, Trade Unions
Congress; and Mr J. Sanderson, Independent Consultant.
APPENDIX 2 - CICAD PEER REVIEW
The draft CICAD on 1,1,1,2-tetrafluoroethane was sent for review
to institutions and organizations identified by IPCS after contact
with IPCS national Contact Points and Participating Institutions, as
well as to identified experts. Comments were received from:
Department of Health, London, United Kingdom
Department of Public Health, Albert Szent-Gyorgyi University
Medical School, Szeged, Hungary
European Centre for Ecotoxicology and Toxicology of Chemicals
(ECETOC), Brussels, Belgium
Health Canada, Ottawa, Canada
International Agency for Research on Cancer, Lyon, France
Ministry of Health and Welfare, International Affairs Division,
Government of Japan, Tokyo, Japan
National Institute for Working Life, Solna, Sweden
National Institute of Occupational Health, Budapest, Hungary
United States Department of Health and Human Services (National
Institute of Environmental Health Sciences)
United States Environmental Protection Agency (Office of
Pollution Prevention and Toxics, National Center for
Environmental Assessment, Office of Research and Development;
Office of Drinking Water)
APPENDIX 3 - CICAD FINAL REVIEW BOARD
Berlin, Germany, 26-28 November 1997
Members
Dr H. Ahlers, Education and Information Division, National Institute
for Occupational Safety and Health, Cincinnati, OH, USA
Mr R. Cary, Health Directorate, Health and Safety Executive, Bootle,
United Kingdom
Dr S. Dobson, Institute of Terrestrial Ecology, Huntingdon, United
Kingdom
Dr R.F Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany (Chairperson)
Mr J.R. Hickman, Health Protection Branch, Health Canada, Ottawa
Ontario, Canada
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany
Ms M.E. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada (Rapporteur)
Dr K. Paksy, Department of Reproductive Toxicology, National Institute
of Occupational Health, Budapest, Hungary
Mr V. Quarg, Ministry for the Environment, Nature Conservation &
Nuclear Safety, Bonn, Germany
Mr D. Renshaw, Department of Health, London, United Kingdom
Dr. J. Sekizawa, Division of Chemo-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan
Prof. S. Soliman, Department of Pesticide Chemistry, Alexandria
University, Alexandria, Egypt (Vice-Chairperson)
Dr M. Wallen National Chemicals Inspectorate (KEMI), Solna, Sweden
Ms D Willcocks, Chemical Assessment Division, Worksafe Australia,
Camperdown, Australia
Dr M. Williams-Johnson, Division of Toxicology, Agency for Toxic
Substances and Disease Registry, Atlanta, GA, USA
Dr K. Ziegler-Skylakakis, Senatskommission der Deutschen
Forschungsgemeinschaft zuer Pruefung gesundheitsschaedlicher
Arbeitisstoffe, GSF-Institut fuer Toxikologie, Neutherberg,
Oberschleissheim, Germany
Observers
Mrs B. Dinham,1 The Pesticide Trust, London, United Kingdom
Dr R. Ebert, KSU Ps-Toxicology, Huels AG, Mart, Germany (representing
ECETOC, the European Centre for Ecotoxicology and Toxicology of
Chemicals)
Mr R Green,1 International Federation of Chemical, Energy, Mine and
General Workers' Unions, Brussels, Belgium
Dr B. Hansen,1 European Chemicals Bureau, European Commission, Ispra,
Italy
Dr J. Heuer, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Mr T. Jacob,1 DuPont, Washington, DC, USA
Ms L. Onyon, Environment Directorate, Organisation for Economic
Co-operation and Development, Paris, France
Dr H.J. Weideli, Ciba Speciality Chemicals Inc., Basel, Switzerland
(representing CEFIC, the European Chemical Industry Council)
Secretariat
Dr M. Baril, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr R.G. Liteplo, Health Canada, Ottawa, Ontario, Canada
Ms L. Regis, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Mr A, Strawson, Health and Safety Executive, London, United Kingdom
Dr P. Toft, Associate Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
1 Invited but unable to attend.
RÉSUMÉ D'ORIENTATION
Ce CICAD relatif au 1,1,1,2-tétrafluoréthane est fondé sur une
étude des problèmes de santé (notamment dans le domaine de l'hygiène
du travail) réalisée par le Health and Safety Executive du Royaume-Uni
en 1995 (Standring et al., 1995). Des informations complémentaires
concernant les effets de cette substance sur la santé humaine et
l'environnement ont été identifiées dans l'étude ECETOC (1995). Ces
deux études prennent en compte les données antérieures à décembre
1994. Les données postérieures à leur publication ont été incorporées
dans le présent document. Les informations relatives à l'examen du
document initial par les pairs et à sa disponibilité figurent à
l'appendice 1. Les renseignements concernant l'examen du CICAD par les
pairs font l'objet de l'appendice 2. Ce CICAD a été approuvé entant
qu'évaluation internationale lors d'une réunion du Comité d'évaluation
finale qui s'est tenue à Berlin (Allemagne) du 26 au 28 novembre 1997.
La liste des participants à cette réunion figure à l'appendice 3. La
fiche d'information sur la sécurité chimique du
1,1,1,2-tétrafluoréthane (ICSC 128 1), établie parle Programme
international sur la Sécurité chimique (IPCS, 1998), est également
reproduite dans le présent document.
Le 1,1,1,2-tétratluoréthane (CAS N° 811-97-2) est un
fluorocarbure gazeux obtenu par réaction du fluorure d'hydrogène sur
le trichloréthylène en vase clos. Il est utilisé principalement comme
réfrigérant dans des appareils fonctionnant à température relativement
élevée, comme les réfrigérateurs domestiques et les climatiseurs
d'automobiles. Il peut également être employé dans la fabrication de
mousses plastiques, comme solvant pour certaines opérations de
nettoyage, comme propulseur d'aérosols pour inhalateurs médicaux, et
comme produit extincteur à la place des halons.
On dispose de peu d'informations sur l'exposition du grand public
ou des travailleurs au 1,1,1,2-tétrafluoréthane, Le personnel employé
à sa fabrication au Royaume-Uni a été soumis à une très faible
exposition, les concentrations ne dépassant jamais 7 ppm (29,2
mg/m3). L'exposition résultant de son utilisation dans l'industrie
manufacturière n'a pas été mesurée et l'on ne dispose pas de données
concernant l'exposition des personnels appelés à l'utiliser sur le
terrain. Compte tenu de la situation sur les lieux de travail au
Royaume-Uni, et par analogie avec les données obtenues lors d'une
étude de l'exposition au dichlorotrifluoréthane (HCFC 123), il semble
que la concentration de 1,1,1,2-tétratluoréthane sur les lieux de
travail soit normalement faible (inférieure à 10 ppm, soit 41,7
mg/m3), avec parfois des pies d'exposition de courte durée atteignant
quelques centaines de parties par million.
Les renseignements concernant les effets du
1,1,1,2-tétrafluoréthane sur l'homme se limitent à une étude; la plupart
des données disponibles sur les effets toxicologiques de cette
substance ont été obtenues à partir d'expériences chez l'animal. Le
1,1,1,2-tétrafluoréthane a une toxicité relativement faible. Des
études visant à évaluer sa toxicité pour le développement font état
d'un ralentissement du gain pondéral chez des lapines exposées à
40 000 ppm (166 800 mg/m3) et d'un retard de développement des foetus
chez des rates exposées à 50 000 ppm (208 500 mg/m3). D'autres études
toxicologiques ne signalent aucun effet défavorable chez des animaux
exposés à des concentrations allant jusqu'à 10 000 ppm (41 700
mg/m3). Les indices de cancérogénicité se limitent à une incidence
accrue des adénomes des cellules de Leydig après exposition à 50 000
ppm (208 500 mg/m3); d'autre part, les études menées jusqu'à présent
ne révèlent aucun signe de génotoxicité,
La faible toxicité du 1,1,1,2-tétrafluoréthane pour les quelques
organismes aquatiques sur lesquels il a été testé et sa grande
volatilité donnent à penser qu'il constitue un risque négligeable pour
ces organismes.
Les effets du 1,1,1,2-tétrafluoréthane sur l'atmosphère ont été
évalués à l'aide de modèles. Des observations récentes ont révélé une
augmentation rapide de sa concentration atmosphérique, résultant
principalement des émissions qui ont eu lieu au cours de la dernière
décennie. Les résultats de la modélisation montrent que le
1,1,1,2-tétrafluoréthane présente un risque insignifiant en ce qui
concerne la destruction de l'ozone, un risque significatif pour ce qui
est du réchauffement mondial et un risque négligeable d'acidification.
RESUMEN DE ORIENTACION
Este CICAD (resumen de evaluación internacional de sustancias
químicas) sobre el 1,1,1,2-tetrafluoroetano está basado en un estudio
sobre su posible incidencia (fundamentalmente ocupacional) en la salud
humana preparado por la Dirección de Salud y Seguridad del Reino Unido
en 1995 (Standring et al., 1995), En el ECETOC (1995) se halló más
información sobre los efectos en la salud humana y en el medio. Los
datos manejados en esos dos estudios abarcan hasta diciembre de 1994.
También se ha incluido cuando procedia información adicional hallada
tras la publicación de esos estudios. En el apéndice 1 se informa
sobre la naturaleza del examen colegiado y la disponibilidad del
documento de base, y en el apéndice 2 se facilita información sobre el
examen colegiado del presente resumen. Este CICAD fue aprobado como
resumen de evaluación internacional en una reunión de la Junta de
Revisión Final celebrada en Berlín (Alemania) los días 26 a 28 de
noviembre de 1997. La lista de los participantes en la reunión de la
Junta de Revisión Final figura en el apéndice 3. En este documento se
reproduce también la ficha internacional de seguridad química (ICSC
1281) para el 1,1,1,2-tetrafluoroetano, preparada por el Programa
Internacional de Seguridad de las Sustancias Químicas (IPCS, 1998).
El 1,1,1,2-tetrafluoroetano (CAS n° 811-97-2) es un fluorocarburo
gaseoso que se fabrica haciendo reaccionar el ácido fluorhídrico y el
tricloroetileno en un sistema cerrado. Se usa fundamentalmente como
refrigerante para el enfriamiento de "alta temperatura," por ejemplo
en los frigoríficos domésticos y en los sistemas de aire acondicionado
de los automóviles. Otros usos posibles son su empleo en espumación,
como disolvente en aplicaciones de limpieza especiales, como propulsor
de aerosoles para inhaladores médicos y como extintor de incendios en
lugar de los halones.
La información hallada sobre la exposición del público general o
los trabajadores al 1,1,1,2-tetrafluoroetano es escasa. Durante su
fabricación en el Reino Unido, la exposición de los empleados a ese
producto fue muy baja, y las concentraciones medidas no superaron en
ningún caso las 7 ppm (29,2 mg/m3). No se dispone de datos sobre la
exposición asociada a su uso en la industria fabril, ni sobre la
exposición del personal de servicios sobre el terreno. La situación en
los lugares de trabajo en el Reino Unido y otros datos análogos de un
solo estudio de exposición al diclorotrifluoroetano (HCFC 123) parecen
indicar que la exposición al 1,1,1,2-tetrafluoroetano en el lugar de
trabajo es normalmente baja (es decir, inferior a 10 ppm [41,7
mg/m3]), registrándose ocasionalmente exposiciones máximas breves de
hasta varios cientos de partes por millón.
Sólo hay un informe que trate de los efectos del
1,1,1,2-tetrafluoroetano en el ser humano; la mayor parte de los datos
disponibles sobre sus efectos toxicológicos proceden de estudios
realizados en animales de laboratorio. El 1,1,1,2-tetrafluoroetano
tiene una toxicidad relativamente baja. Los estudios sobre su
toxicidad en el desarrollo han mostrado una reducción del aumento del
peso corporal materno en conejos expuestos a 40 000 ppm (166 800
mg/m3) de 1,1,1,2-tetrafluoroetano, así como signos de retraso del
desarrollo fetal en ratas tras la exposición de las madres a
concentraciones de 50 000 ppm (208 500 mg/m3). En otras
investigaciones toxicológicas no se han observado efectos adversos
para la salud tras la exposición a concentraciones de hasta 10 000 ppm
(41 700 mg/m3). Los indicios de carcinogenicidad se limitan a un
aumento de la incidencia de adenomas de las células de Leydig tras la
exposición a 50 000 ppm (208 500 mg/m3), y no se han detectado
efectos genotóxicos en los estudios realizados hasta la fecha.
La baja toxicidad del 1,1,1,2-tetrafluoroetano para los escasos
organismos acuáticos analizados, así como su elevada volatilidad,
indican que el riesgo es insignificante para los organismos acuáticos.
Se han elaborado modelos para evaluar los efectos atmosféricos.
Observaciones recientes han puesto de manifiesto un rápido incremento
de las concentraciones atmosféricas de 1,1,1,2-tetrafluoroetano,
principalmente como resultado de las emisiones realizadas a lo largo
del último decenio. La modelización ha revelado una desdeñable
contribución potencial al agotamiento del ozono, una considerable
capacidad de contribución al calentamiento mundial y un potencial de
acidificación despreciable.
 1. EXECUTIVE SUMMARY
This CICAD on 1,1,1,2-tetrafluoroethane was based on a review of
human health concerns (primarily occupational) prepared by the United
Kingdom Health and Safety Executive in 1995 (Standring el al., 1995).
Additional information on effects on human health and the environment
was identified in ECETOC (1995). Data identified up to December 1994
were covered by these reviews. Additional data identified after these
reviews were published have been incorporated as appropriate.
Information on the nature of the peer review and availability of the
source document is presented in Appendix 1. Information on the peer
review of this CICAD is presented in Appendix 2. This CICAD was
approved as an international assessment at a meeting of the Final
Review Board, held in Berlin, Germany, on 26-28 November 1997.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 1281) for
1,1,1,2-tetrafluoroethane, produced by the International Programme on
Chemical Safety (IPCS, 1998), has also been reproduced in this
document.
1,1,1,2-Tetrafluoroethane (CAS no.
1. EXECUTIVE SUMMARY
This CICAD on 1,1,1,2-tetrafluoroethane was based on a review of
human health concerns (primarily occupational) prepared by the United
Kingdom Health and Safety Executive in 1995 (Standring el al., 1995).
Additional information on effects on human health and the environment
was identified in ECETOC (1995). Data identified up to December 1994
were covered by these reviews. Additional data identified after these
reviews were published have been incorporated as appropriate.
Information on the nature of the peer review and availability of the
source document is presented in Appendix 1. Information on the peer
review of this CICAD is presented in Appendix 2. This CICAD was
approved as an international assessment at a meeting of the Final
Review Board, held in Berlin, Germany, on 26-28 November 1997.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 1281) for
1,1,1,2-tetrafluoroethane, produced by the International Programme on
Chemical Safety (IPCS, 1998), has also been reproduced in this
document.
1,1,1,2-Tetrafluoroethane (CAS no. 