
Concise International Chemical Assessment Document 12
MANGANESE AND ITS COMPOUNDS
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Concise International Chemical Assessment Document 12
MANGANESE AND ITS COMPOUNDS
First draft prepared by Dr Mildred Williams-Johnson, Division of
Toxicology, Agency for Toxic Substances and Disease Registry, Atlanta,
Georgia, USA
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 1999
The International Programme on Chemical Safety (IPCS),
established in 1980, is a joint venture of the United Nations
Environment Programme (UNEP), the International Labour Organisation
(ILO), and the World Health Organization (WHO). The overall objectives
of the IPCS are to establish the scientific basis for assessment of
the risk to human health and the environment from exposure to
chemicals, through international peer review processes, as a
prerequisite for the promotion of chemical safety, and to provide
technical assistance in strengthening national capacities for the
sound management of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization, the United Nations
Institute for Training and Research, and the Organisation for Economic
Co-operation and Development (Participating Organizations), following
recommendations made by the 1992 UN Conference on Environment and
Development to strengthen cooperation and increase coordination in the
field of chemical safety. The purpose of the IOMC is to promote
coordination of the policies and activities pursued by the
Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing-in-Publication Data
Manganese and its compounds.
(Concise international chemical assessment document ; 12)
1.Manganese - adverse effects 2.Manganese - toxicity
3.Environmental exposure 4.Maximum permissible exposure level
I.International Programme on Chemical Safety II.Series
ISBN 92 4 153012 X (NLM classification: QV 290)
ISSN 1020-6167
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1999
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city, or area
or of its authorities, or concerning the delimitation of its frontiers
or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
TABLE OF CONTENTS
FOREWORD
1. EXECUTIVE SUMMARY
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
3. ANALYTICAL METHODS
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1. Environmental levels
6.2. Human exposure
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposure
8.2. Irritation and sensitization
8.3. Short-term exposure
8.4. Long-term exposure
8.4.1. Subchronic exposure
8.4.2. Chronic exposure and carcinogenicity
8.5. Genotoxicity and related end-points
8.6. Reproductive and developmental toxicity
8.7. Immunological and neurological effects
9. EFFECTS ON HUMANS
9.1. Case reports
9.2. Epidemiological studies
10. EFFECTS EVALUATION
10.1. Evaluation of health effects
10.1.1. Hazard identification and dose-response assessment
10.1.2. Criteria for setting guidance values for manganese
10.1.3. Sample risk characterization
11. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
12. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
12.1. Human health hazards
12.2. Advice to physicians
12.3. Health surveillance programme
13. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
INTERNATIONAL CHEMICAL SAFETY CARD
REFERENCES
APPENDIX 1 -- SOURCE DOCUMENTS
APPENDIX 2 -- CICAD PEER REVIEW
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
APPENDIX 4 -- ADDITIONAL APPROACHES FOR GUIDANCE VALUE
DEVELOPMENT
RÉSUMÉ D'ORIENTATION
RESUMEN DE ORIENTACION
FOREWORD
Concise International Chemical Assessment Documents (CICADs) are
the latest in a family of publications from the International
Programme on Chemical Safety (IPCS) -- a cooperative programme of the
World Health Organization (WHO), the International Labour Organisation
(ILO), and the United Nations Environment Programme (UNEP). CICADs
join the Environmental Health Criteria documents (EHCs) as
authoritative documents on the risk assessment of chemicals.
CICADs are concise documents that provide summaries of the
relevant scientific information concerning the potential effects of
chemicals upon human health and/or the environment. They are based on
selected national or regional evaluation documents or on existing
EHCs. Before acceptance for publication as CICADs by IPCS, these
documents undergo extensive peer review by internationally selected
experts to ensure their completeness, accuracy in the way in which the
original data are represented, and the validity of the conclusions
drawn.
The primary objective of CICADs is characterization of hazard and
dose-response from exposure to a chemical. CICADs are not a summary of
all available data on a particular chemical; rather, they include only
that information considered critical for characterization of the risk
posed by the chemical. The critical studies are, however, presented in
sufficient detail to support the conclusions drawn. For additional
information, the reader should consult the identified source documents
upon which the CICAD has been based.
Risks to human health and the environment will vary considerably
depending upon the type and extent of exposure. Responsible
authorities are strongly encouraged to characterize risk on the basis
of locally measured or predicted exposure scenarios. To assist the
reader, examples of exposure estimation and risk characterization are
provided in CICADs, whenever possible. These examples cannot be
considered as representing all possible exposure situations, but are
provided as guidance only. The reader is referred to EHC 1701 for
advice on the derivation of health-based guidance values.
While every effort is made to ensure that CICADs represent the
current status of knowledge, new information is being developed
constantly. Unless otherwise stated, CICADs are based on a search of
the scientific literature to the date shown in the executive summary.
In the event that a reader becomes aware of new information that would
change the conclusions drawn in a CICAD, the reader is requested to
contact IPCS to inform it of the new information.
1 International Programme on Chemical Safety (1994) Assessing
human health risks of chemicals: deriviation of guidance values for
health-based exposure limits. Geneva, World Health Organization
(Environmental Health Criteria 170).
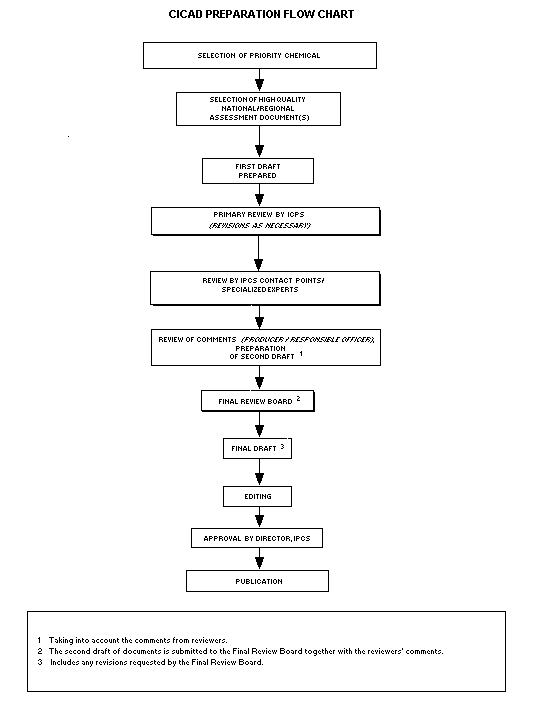
Procedures
The flow chart shows the procedures followed to produce a CICAD.
These procedures are designed to take advantage of the expertise that
exists around the world -- expertise that is required to produce the
high-quality evaluations of toxicological, exposure, and other data
that are necessary for assessing risks to human health and/or the
environment.
The first draft is based on an existing national, regional, or
international review. Authors of the first draft are usually, but not
necessarily, from the institution that developed the original review.
A standard outline has been developed to encourage consistency in
form. The first draft undergoes primary review by IPCS to ensure that
it meets the specified criteria for CICADs.
The second stage involves international peer review by scientists
known for their particular expertise and by scientists selected from
an international roster compiled by IPCS through recommendations from
IPCS national Contact Points and from IPCS Participating Institutions.
Adequate time is allowed for the selected experts to undertake a
thorough review. Authors are required to take reviewers' comments into
account and revise their draft, if necessary. The resulting second
draft is submitted to a Final Review Board together with the
reviewers' comments.
The CICAD Final Review Board has several important functions:
- to ensure that each CICAD has been subjected to an appropriate
and thorough peer review;
- to verify that the peer reviewers' comments have been addressed
appropriately;
- to provide guidance to those responsible for the preparation of
CICADs on how to resolve any remaining issues if, in the opinion
of the Board, the author has not adequately addressed all
comments of the reviewers; and
- to approve CICADs as international assessments.
Board members serve in their personal capacity, not as representatives
of any organization, government, or industry. They are selected
because of their expertise in human and environmental toxicology or
because of their experience in the regulation of chemicals. Boards are
chosen according to the range of expertise required for a meeting and
the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
 1. EXECUTIVE SUMMARY
This CICAD on manganese and its compounds was based principally
on the report entitled Toxicological profile for manganese (update),
draft for public comment, prepared by the Agency for Toxic
Substances and Disease Registry, US Department of Health and Human
Services (ATSDR, 1996). Information contained in the Hazardous
Substances Data Bank, developed and maintained by the National Library
of Medicine, US Department of Health and Human Services, was also used
(HSDB, 1998). Data identified as of November 1998 were considered in
these source documents. Additional data came from other references,
such as assessments prepared by the US Environmental Protection Agency
(EPA) and the World Health Organization (WHO), as well as a variety of
reports in the literature. The source documents used to develop this
CICAD do not cover the effects of manganese on the ecological
environment. No other sources (documents developed by a national
organization and subject to rigorous scientific review) on this topic
were identified. Therefore, this CICAD addresses environmental levels
as a source of human exposure only. No attempt has been made in this
document to assess effects on organisms in the environment.
Information on the availability of the source documents is presented
in Appendix 1. Information on the peer review of this CICAD is
presented in Appendix 2. This CICAD was approved as an international
assessment at a meeting of the Final Review Board, held in Berlin,
Germany, on 26-28 November 1997. Participants at the Final Review
Board meeting are presented in Appendix 3. The International Chemical
Safety Card (ICSC 0174) for manganese, produced by the International
Programme on Chemical Safety (IPCS, 1993), has also been reproduced in
this document.
Manganese (Mn) is a naturally occurring element that is found in
rock, soil, water, and food. Thus, all humans are exposed to
manganese, and it is a normal component of the human body. Food is
usually the most important route of exposure for humans. Estimated
Safe and Adequate Daily Intakes of 1-5 mg manganese have been
established for children 1 year of age and older through to adults;
these levels generally parallel amounts of the compound delivered via
the diet.
Manganese is released to air mainly as particulate matter, and
the fate and transport of the particles depend on their size and
density and on wind speed and direction. Some manganese compounds are
readily soluble in water, so significant exposures can also occur by
ingestion of contaminated drinking-water. Manganese in surface water
can oxidize or adsorb to sediment particles and settle to the bottom.
Manganese in soil can migrate as particulate matter to air or water,
or soluble manganese compounds can be leached from the soil.
Above-average exposures to manganese are most likely to occur in
people who work at or live near a factory or other site where
significant amounts of manganese dust are released into the air. In
some regions, the general population can be exposed to manganese
released into air by the combustion of unleaded gasoline containing
the organomanganese compound methylcyclopentadienyl manganese
tricarbonyl (MMT) as an antiknock ingredient. Some people can be
exposed to excess manganese in drinking-water -- for example, when
manganese from batteries or pesticides leaches into well-water.
Children can be exposed to excess manganese in soils through
hand-to-mouth behaviour.
In humans, manganese is an essential nutrient that plays a role
in bone mineralization, protein and energy metabolism, metabolic
regulation, cellular protection from damaging free radical species,
and the formation of glycosaminoglycans. However, exposure to high
levels via inhalation or ingestion can cause adverse health effects.
Given comparable doses, more manganese reaches the brain following
inhalation than following ingestion, and most health effects are
associated with chronic inhalation exposure. Little is known about the
relative toxicity of different manganese compounds. However, available
evidence indicates that various manganese compounds can induce
neurological effects; these effects have been observed following
chronic (365 days or more) inhalation exposures in humans and
intermediate (15-364 days) and chronic oral exposures in animals.
In general, the available data indicate that exposure to excess
manganese for 14 days or less (acute duration) or up to a year
(intermediate duration) has an effect on the respiratory system and
the nervous system, with little to no effect on other organ systems.
Acute inhalation exposure to high concentrations of manganese dusts
(specifically manganese dioxide [MnO2] and manganese tetroxide
[Mn3O4]) can cause an inflammatory response in the lung, which, over
time, can result in impaired lung function. Lung toxicity is
manifested as an increased susceptibility to infections such as
bronchitis and can result in manganic pneumonia. Pneumonia has also
been observed following acute inhalation exposures to particulates
containing other metals. Thus, this effect might be characteristic of
inhalable particulate matter and might not depend solely on the
manganese content of the particle.
There are a few reports suggesting that intermediate inhalation
exposure to manganese compounds produces effects on the central
nervous system, but reliable estimates of exposure levels are not
available. Inhalation studies in animals resulted in biochemical,
respiratory, and neurobehavioural effects. However, a threshold for
these effects has not been identified, because the exposure levels
associated with these effects range over an order of magnitude.
In chronic inhalation exposure to manganese, the main organ
systems affected are the lungs, nervous system, and reproductive
system, although effects on other organ systems have also been
observed. A recurring manganic pneumonia and acute respiratory effects
have been associated with chronic inhalation exposures to manganese.
Effects on the nervous system include neurological and
neuropsychiatric symptoms that can culminate in a Parkinsonism-like
disease known as manganism; evidence suggests that laboratory animals,
especially rodents, are not as sensitive as humans, and possibly other
primates, to the neurological effects of inhalation exposure to
manganese. Reproductive effects of chronic inhalation exposure to
manganese include decreased libido, impotence, and decreased fertility
in men; information is not available on reproductive effects in women.
Studies in animals indicate that manganese can cause direct damage to
the testes and late resorptions. Data from animal studies on the
effects of inhaled manganese on the immunological system and the
developing fetus are too limited to make firm conclusions on the
significance of these effects for humans.
Information on the carcinogenic potential of manganese is
limited, and the results are difficult to interpret with certainty. In
rats, chronic oral studies with manganese sulfate (MnSO4) showed a
small increase in the incidence of pancreatic tumours in males and a
small increase in pituitary adenomas in females. In other studies with
manganese sulfate, no evidence for cancer was noted in rats and a
marginally increased incidence of thyroid gland follicular cell
adenomas was observed in mice. The results of in vitro studies show
that at least some chemical forms of manganese have mutagenic
potential. However, as the results of in vivo studies in mammals are
inconsistent, no overall conclusion can be made about the possible
genotoxic hazard to humans from exposure to manganese compounds.
Large oral doses of concentrated manganese salts given by gavage
can cause death in animals, but oral exposures via food or water have
not been found to cause significant toxicity over acute or short-term
exposures. Similarly, parenteral administration of manganese salts can
cause developmental toxicity, but effects were not found with oral
exposure. Intermediate-duration oral exposure of humans to manganese
has been reported to cause neurotoxicity in two cases, but the data
are too limited to define the threshold or to judge if these effects
were due entirely to the manganese exposure. Some data on neurological
or other health effects in humans from chronic oral intake of
manganese exist, but these studies are limited by uncertainties in the
exposure routes and total exposure levels as well as by the existence
of other confounding factors. The studies in humans and animals do not
provide sufficient information to determine dose levels or effects of
concern following chronic oral exposure. Thus, the available evidence
for adverse effects associated with chronic ingestion of excess
manganese is suggestive but inconclusive.
The dermal route does not appear to be of significant concern and
has not been investigated to any extent. Available information is
limited to reports on the corrosive effects of potassium permanganate
(KMnO4) and case reports of effects from dermal absorption of organic
manganese compounds such as MMT.
From these data, it is clear that adverse neurological and
respiratory effects from manganese exposure can occur in occupational
settings. Limited evidence also suggests that adverse neurological
effects can be associated with ingestion of excess manganese in
environmental settings. As a result of predisposing factors, certain
individuals might be more susceptible to adverse effects from exposure
to excess manganese. These might include people with lung disease,
people who are exposed to other lung irritants, neonates, older
people, individuals with iron deficiency, or people with liver
disease.
There are several approaches to the development of a guidance
value for manganese in air. A recently developed guidance value of
0.15 µg manganese/m3 is highlighted here as one possible example;
some additional approaches are also presented.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
Table 1 lists common synonyms and other relevant information on
the chemical identity and properties of manganese and several of its
most important compounds. Manganese is a naturally occurring element
that is found in rock, soil, water, and food. Manganese can exist in a
number of oxidation states. Manganese and its compounds can exist as
solids in the soil and as solutes or small particles in water. Most
manganese salts are readily soluble in water, with only the phosphate
and the carbonate having low solubilities. The manganese oxides
(manganese dioxide and manganese tetroxide) are poorly soluble in
water. Manganese can also be present in small dust-like particles in
the air. Additional physical/chemical properties are presented in the
International Chemical Safety Card (ICSC 0174) reproduced in this
document.
Table 1: Chemical identity of manganese and its compounds.a
Manganese Manganous Manganese Manganese Manganese potassium
chloride sulfate (II, III) oxide dioxide permanganate
Synonyms Elemental Manganese Manganous Trimanganese Manganese Permanganic acid;
manganese chlorideb; sulfate; tetroxide; peroxide; potassium saltc;
colloidal manganese sulfuric acid mangano-manganic manganese chameleon material
manganese; dichloride manganese oxidec; binoxide;
cutavalb manganese manganese
tetroxide black;
battery
manganese
Chemical Mn MnCl2 MnSO4 Mn3O4 MnO2 KMnO4
formula
CAS 7439-96-5 7773-01-5 7785-87-7 1317-35-7 1313-13-9 7722-64-7
Number
Molecular 54.94c 125.85c 151.00c 228.81d 86.94c 158.04c
weight
Colour Grey-whited Pinkd Pale rose-red Blackd Black Purple
Physical Solid Solid Solid Solid Solid Solid
state
Melting 1244 °Cd 650 °C 700 °C 1564 °C 535 °Cd <240 °C
point (decomposes)
Boiling 1962 °Cd 1190 °Cd Decomposes No data No data No data
point at 850 °C
Table 1 (continued)
Manganese Manganous Manganese Manganese Manganese potassium
chloride sulfate (II, III) oxide dioxide permanganate
Solubility Dissolves in Very soluble Soluble in Insoluble Soluble in Soluble in
dilute mineral in water; water and in water; hydrochloric water, acetone
acidsd; soluble in alcohol soluble in acid; insoluble and sulfuric
decomposes alcohol hydrochloric in water acid
in water acid
a Adapted from ATSDR (1996). All information obtained from Sax & Lewis (1987), except where noted.
b HSDB (1998).
c Windholz (1983).
d Lide (1993).
Table 1a
Methylcyclo- Manganese Mancozebb
pentadienyl- ethylene-bis-
manganese dithiocarbamate
tricarbonyla
Synonyms MMTc; Trimangol 80; manebd Dithane M-45
methyl-cymantrene; ethylene-bis[dithiocarbamic manganese
Antiknock-33; acid], manganous salt; ethylenebis
manganese Dithane (dithiocarbamate)
tricarbonyl (polymeric);
methylcyclopentadienyl Manzate; Manzeb;
Zimaneb
Chemical C9H7MnO3 C4H6MnN2S4 C4H6MnN2S4.
formula C4H6N2S4Zn
CAS 12108-13-3 12427-38-2 12427-38-2
Number
Molecular 218.10 265.31 541.03
weight
Colour Dark orange-rede Yellow-brown Greyish-yellow
Physical Liquide Powder Powder
state
Melting No data Decomposes on Decomposes
point heating without melting
Boiling 232.8 °Ce No data No data
point
Table 1a (continued)
Methylcyclo- Manganese Mancozebb
pentadienyl- ethylene-bis-
manganese dithiocarbamate
tricarbonyla
Solubility Practically insoluble Slightly soluble Practically
in water (70 ppm at in water; soluble in insoluble in water
25 °C); completely chloroform as well as most organic
soluble in hydrocarbons solvents
CAS = Chemical Abstracts Service
a NTP (1999).
b Hamilton (1995).
c Zayed et al. (1994).
d Ferraz et al. (1988).
e Verschueren (1983).
3. ANALYTICAL METHODS
Atomic absorption spectrophotometric analysis is the most widely
used method for determining manganese in biological materials and
environmental samples. Fluorimetric, colorimetric, neutron activation
analysis, and plasma atomic emission techniques are also recommended
for measuring manganese in such samples. Most of these methods require
wet digestion, derivatization, and/or extraction before detection. In
most cases, distinguishing between different oxidation states of
manganese is impossible, so total manganese is measured.
The detection limits of these methods range from <0.01 to 0.2
µg/g for biological tissues and fluids, from 5 to 10 µg/m3 for air,
and from 0.01 to 50 µg/litre for water (Kucera et al., 1986; Abbasi,
1988; Lavi et al., 1989; Mori et al., 1989; Chin et al., 1992; ATSDR,
1996). Determination of manganese levels in soil, sludge, or other
solid wastes requires an acid extraction/digestion step before
analysis. The details vary with the specific characteristics of the
sample, but treatment usually involves heating in nitric acid,
oxidation with hydrogen peroxide, and filtration and/or centrifugation
to remove insoluble matter (ATSDR, 1996).
A nuclear magnetic resonance method (Kellar & Foster, 1991) and a
method using on-line concentration analysis (Resing & Mottl, 1992)
were used to determine both free and complexed manganese ions in
aqueous media. The latter method was highly sensitive, with a
detection limit of 36 pmol/litre (1.98 ng/litre when concentrating 15
ml of seawater).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Manganese is ubiquitous in the environment. It comprises about
0.1% of the earth's crust (NAS, 1973; Graedel, 1978). Because
manganese occurs in soil, air, water, and food, all humans are exposed
to it. Manganese is a normal component of the human body, and food is
usually the most important route of exposure for humans. Manganese
does not occur naturally as a base metal but is a component of more
than 100 minerals, including various sulfides, oxides, carbonates,
silicates, phosphates, and borates (NAS, 1973). The most commonly
occurring manganese-bearing minerals include pyrolusite (manganese
dioxide), rhodocrosite (manganese carbonate), and rhodonite (manganese
silicate) (NAS, 1973; Windholz, 1983; US EPA, 1984; HSDB, 1998).
The manganese content in ore produced worldwide was estimated to
be 8.8 million tonnes in 1986. Production levels of manganese ore and
its total manganese metal content remained nearly the same through
1990 (US Department of the Interior, 1993). Levels of ore produced
worldwide in 1995, 1996, and 1997 declined slightly, with total
manganese metal content declining proportionately to 8.0, 8.1, and 7.7
million tonnes, respectively (US Department of the Interior, 1996,
1998). Although modern steelmaking technologies call for lower unit
consumption of manganese, worldwide demand for steel is projected to
increase moderately in the future, particularly in developing
countries (US Department of the Interior, 1995, 1998). Although
manganese usage in other industries is increasing, this will have
minor overall effect on manganese demand, and future trends for
manganese are still expected to increase with demands for steel (EM,
1993; US Department of the Interior, 1995, 1998). The demand for
manganese in other industries (e.g., dry-cell battery manufacturing)
might also increase, but the overall effect of these other uses on
global trends in manganese production and use is minor (US Department
of the Interior, 1995, 1998).
Manganese compounds are produced from manganese ores or from
manganese metal. The organomanganese compound MMT, an antiknock
additive in unleaded gasoline, is produced by the reaction of
manganese chloride (MnCl2), cyclopentadiene, and carbon monoxide in
the presence of manganese carbonyl (NAS, 1973; US EPA, 1984; Sax &
Lewis, 1987; HSDB, 1998). Metallic manganese (ferromanganese) is used
principally in steel production along with cast iron and superalloys
to improve hardness, stiffness, and strength (NAS, 1973; US EPA, 1984;
HSDB, 1998). Manganese compounds have a variety of uses. Manganese
dioxide is commonly used in the production of dry-cell batteries,
matches, fireworks, porcelain and glass-bonding materials, and
amethyst glass; it is also used as the starting material for the
production of other manganese compounds (NAS, 1973; Venugopal &
Luckey, 1978; US EPA, 1984). Manganese chloride is used as a precursor
for other manganese compounds, as a catalyst in the chlorination of
organic compounds, in animal feed to supply essential trace minerals,
and in dry-cell batteries (US EPA, 1984; HSDB, 1998). Manganese
sulfate is used primarily as a fertilizer and as a livestock
supplement; it is also used in some glazes, varnishes, ceramics, and
fungicides (Windholz, 1983; US EPA, 1984; HSDB, 1998). Manganese
ethylene-bis-dithiocarbamate (maneb) is widely applied to edible crops
as a fungicide and is therefore a potential source of manganese in
soil and in food crops (Ferraz et al., 1988; Ruijten et al., 1994).
Potassium permanganate is used as an oxidizing agent; as a
disinfectant; as an antialgal agent; for metal cleaning, tanning, and
bleaching; as a purifier in water and waste treatment plants; and as a
preservative for fresh flowers and fruits (HSDB, 1998).
The main sources of manganese releases to the air are industrial
emissions, combustion of fossil fuels, and re-entrainment of
manganese-containing soils (Lioy, 1983; US EPA, 1983, 1984, 1985a,
1985b). Manganese can also be released to the air during other
anthropogenic processes, such as welding and fungicide application
(Ferraz et al., 1988; MAK, 1994; Ruijten et al., 1994). Total
emissions to air from anthropogenic sources in the USA were estimated
to be 16 400 t in 1978, with about 80% (13 200 t) from industrial
facilities and 20% (3200 t) from fossil fuel combustion (US EPA,
1983). Air emissions by US industrial sources reported for 1987
totalled 1200 t (TRI87, 1989). In 1991, air emissions from facilities
in the USA ranged from 0 to 74 t, with several US states reporting no
emissions (TRI91, 1993). Air erosion of dusts and soils is also an
important atmospheric source of manganese, but no quantitative
estimates of manganese release to air from this source were identified
(US EPA, 1984). Volcanic eruptions can also release manganese to the
atmosphere (Schroeder et al., 1987).
In some countries, combustion of gasoline containing MMT
contributes approximately 8% to levels of manganese tetroxide in urban
air (Loranger & Zayed, 1995). MMT was used as a gasoline additive in
the USA for a number of years, resulting in manganese emissions.
Analysis of manganese levels in the air indicated that vehicular
emissions contributed an average of 13 ng manganese/m3 in southern
California, whereas vehicular emissions were only about 3 ng/m3 in
central and northern California (Davis et al., 1988). A ban on MMT use
as a fuel additive was imposed for a period of time, then lifted by
the US EPA in 1995.
In Canada, MMT use as a fuel additive has gradually increased
since 1976. Manganese emissions from gasoline combustion rose sharply
from 1976 through the early 1980s, reaching an estimated 200.2 t by
1985 (Jacques, 1984). In 1990, lead was completely replaced by MMT in
gasoline in Canada (Loranger & Zayed, 1994). MMT use peaked in 1989 at
over 400 t, which was more than twice the usage in 1983 and 1.5 times
the usage in 1986. MMT use declined to about 300 t by 1992, owing to
reductions in its concentration in gasoline. However, ambient
monitoring data for manganese in Canadian cities without industrial
sources for the 1989-1992 period did not reflect this peak in MMT use.
Air manganese levels (PM2.5, or particulate matter with an aerodynamic
diameter less than or equal to 2.5 µm) remained constant at 0.11-0.013
µg/m3 for small cities and 0.020-0.025 µg/m3 for large cities
(Health Canada, 1994; Egyed & Wood, 1996). Manganese emission levels
can vary depending on the concentration of MMT in gasoline and
gasoline usage patterns. One study reported a correlation between
atmospheric manganese concentrations in 1990 air samples and traffic
density in Montreal (Loranger et al., 1994). However, a later study by
these investigators reported that atmospheric manganese concentrations
in Montreal decreased in 1991 and 1992 despite an estimated 100%
increase in manganese emission rates from MMT in gasoline (Loranger &
Zayed, 1994). Another study suggested that the high manganese levels
in Montreal were, in part, due to the presence of a silico- and
ferro-manganese facility that ceased operation in 1991 (Egyed & Wood,
1996).
Manganese can be released to water by discharge from industrial
facilities or as leachate from landfills and soil (US EPA, 1979, 1984;
Francis & White, 1987; TRI91, 1993). In the USA, reported industrial
discharges in 1991 ranged from 0 to 17.2 t for surface water, from 0
to 57.3 t for transfers to public sewage, and from 0 to 0.114 t for
underground injection (TRI91, 1993). An estimated total of 58.6 t, or
1% of the total environmental release of manganese in the USA, was
discharged to water in 1991 (TRI91, 1993).
Land disposal of manganese-containing wastes is the principal
source of manganese releases to soil. In 1991, reported industrial
releases to land in the USA ranged from 0 to 1000 t. More than 50% of
the total environmental release of manganese (3753 t) was to land
(TRI91, 1993).
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Elemental manganese and inorganic manganese compounds have
negligible vapour pressures but can exist in air as suspended
particulate matter derived from industrial emissions or the erosion of
soils. Manganese-containing particles are removed from the atmosphere
mainly by gravitational settling or by rain (US EPA, 1984).
Soil particulate matter containing manganese can be transported
in air. The fate and transport of manganese in air are largely
determined by the size and density of the particle and wind speed and
direction. An estimated 80% of the manganese in suspended particulate
matter is associated with particles with a Mass Median Equivalent
Diameter (MMED) of <5 µm, and 50% of this manganese is estimated to
be associated with particles that are <2 µm in MMED. (Whether these
data are for particles in urban or rural areas is unclear. However, it
is known that the size of manganese particles in the air tends to vary
by source; small particles dominate around ferromanganese and dry-cell
battery plants, whereas large particles tend to predominate near
mining operations [WHO, 1999].) Based on these data, manganese's small
particle size is within the respirable range, and widespread airborne
distribution would be expected (WHO, 1981). Very little information is
available on atmospheric reactions of manganese (US EPA, 1984).
Although manganese can react with sulfur dioxide and nitrogen dioxide,
the occurrence of such reactions in the atmosphere has not been
demonstrated.
The transport and partitioning of manganese in water are
controlled by the solubility of the specific manganese compound
present. In most waters (pH 4-7), Mn(II) predominates and is
associated principally with carbonate, which has relatively low
solubility (US EPA, 1984; Schaanning et al., 1988). The solubility of
Mn(II) can be controlled by manganese oxide equilibria (Ponnamperuma
et al., 1969), with manganese being converted to other oxidation
states (Rai et al., 1986). In extremely reduced water, the fate of
manganese tends to be controlled by the formation of the poorly
soluble sulfide (US EPA, 1984). In groundwater with low oxygen levels,
Mn(IV) can be reduced both chemically and bacterially to the Mn(II)
oxidation state (Jaudon et al., 1989). MMT has been found to be
persistent in natural aquatic and soil environments in the absence of
sunlight, with a tendency to sorb to soil and sediment particles
(Garrison et al., 1995). In the presence of light, photodegradation of
MMT is rapid, with identified products including a manganese carbonyl
that readily oxidizes to manganese tetroxide (Garrison et al., 1995).
Manganese is often transported in rivers adsorbed to suspended
sediments. Most of the manganese from industrial sources found in a
South American river was bound to suspended particles (Malm et al.,
1988). The tendency of soluble manganese compounds to adsorb to soils
and sediments can be highly variable, depending mainly on the cation
exchange capacity and the organic composition of the soil (Hemstock &
Low, 1953; Schnitzer, 1969; McBride, 1979; Curtin et al., 1980; Baes &
Sharp, 1983; Kabata-Pendias & Pendias, 1984). The oxidation state of
manganese in soils and sediments can be altered by microbial activity
(Geering et al., 1969; Francis, 1985).
Manganese in water can be significantly bioconcentrated at lower
trophic levels. Bioconcentration factors (BCFs) of 10 000-20 000 for
marine and freshwater plants, 2500-6300 for phytoplankton, 300-5500
for marine algae, 800-830 for intertidal mussels, and 35-930 for fish
have been estimated (Folsom et al., 1963; Thompson et al., 1972). The
high reported BCFs probably reflect the essentiality of manganese for
a wide variety of organisms; specific uptake mechanisms exist for
essential elements.
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
Concentrations of manganese in seawater reportedly range from 0.4
to 10 µg/litre (US EPA, 1984). In the North Sea, the northeast
Atlantic Ocean, the English Channel, and the Indian Ocean, manganese
content was reported to range from 0.03 to 4.0 µg/litre. Levels found
in coastal waters of the Irish Sea and in the North Sea off the coast
of the United Kingdom ranged from 0.2 to 25.5 µg/litre (Alessio &
Lucchini, 1996). In a number of cases, higher levels in water (in
excess of 1000 µg/litre) have been detected at US hazardous waste
sites, suggesting that, in some instances, wastes from industrial
sources can lead to significant contamination of water (ATSDR, 1996).
In a 1974-1981 survey of 286 US river water samples,
concentrations of dissolved manganese ranged from less than 11
µg/litre (25th percentile) to more than 51 µg/litre (75th percentile)
(Smith et al., 1987), with a median of 24 µg/litre. Mean groundwater
concentrations were 20 and 90 µg/litre from two geological zones in
California (Deverel & Millard, 1988). The surface waters of Welsh
rivers were reported to contain from 0.8 to 28 µg manganese/litre.
Concentrations of manganese ranged from 1 to 530 µg/litre in 37 rivers
in the United Kingdom and in the Rhine and the Maas and their
tributaries (Alessio & Lucchini, 1996).
Concentrations of manganese in surface water are usually reported
as dissolved manganese. Total manganese might be a better indicator,
because manganese adsorbed to suspended solids can exceed dissolved
manganese in many systems, and the bioavailability of manganese in
this form has not been established (NAS, 1977; US EPA, 1984).
Natural ("background") levels of manganese in soil range from 40
to 900 mg/kg, with an estimated mean of 330 mg/kg (Cooper, 1984; US
EPA, 1985a; Schroeder et al., 1987; Eckel & Langley, 1988; Rope et
al., 1988). Accumulation of manganese in soil usually occurs in the
subsoil and not on the soil surface (WHO, 1981).
According to a National Research Council of Canada report (Stokes
et al., 1988), manganese concentrations in air tend to be lowest in
remote locations (about 0.5-14 ng/m3 on average), higher in rural
areas (40 ng/m3 on average), and still higher in urban areas (about
65-166 ng/m3 on average) (see Table 2). Similar concentrations have
been reported elsewhere, leading to the conclusion that annual
manganese concentrations average 10-30 ng/m3 in areas far from known
sources and 10-70 ng/m3 in urban and rural areas without major point
sources of manganese (WHO, 1999). Manganese concentrations in air tend
to be highest in source-dominated areas (e.g., those with foundries),
where values can reach 8000 ng/m3 (US EPA, 1984; Stokes et al.,
1988). Annual averages of manganese concentrations in air near
foundries may rise to 200-300 ng/m3 and to over 500 ng/m3 in air
near ferro- and silicomanganese industries (WHO, 1999).
Table 2: Average levels of manganese in air.
a) Atmospheric air (worldwide)a:
Type of location Average
concentration (ng/m3) Range(ng/m3)
Remote
Continental 3.4 <0.18-9.30
Oceanic 14.2 0.02-79
Polar 0.5 0.01-1.5
Rural 40 6.5-199
Urban
Canada 65 20.0-270
USA 93 5.0-390
Europe 166 23.0-850
Other 149 10.0-590
b) US ambient airb:
Type of
location Concentration (ng/m3)
1953-1957 1965-1967 1982
Nonurban 60 12 5
Urban 110 73 33
Source dominated No data 250-8300 130-140
a Adapted from Stokes et al. (1988).
b Adapted from US EPA (1984).
Manganese concentrations in air have been measured in many
specific locations. In the Vancouver, Canada, area, for example,
annual geometric mean concentrations of manganese ranged from <10 to
30 ng/m3 in 1984 (Stokes et al., 1988). Over the period of 1981-1992,
Loranger & Zayed (1994) found average manganese concentrations in
Montreal, Canada, of 20 and 60 ng/m3 in areas of low and high traffic
density, respectively. More recently, Loranger & Zayed (1997) found
the average concentration of total manganese in an urban site in
Montreal to be 27 ng/m3. In selected periods in the 1970s, annual
mean concentrations of manganese were reported to range from 3 to 16
ng/m3 in two German cities, from 42 to 455 ng/m3 in Belgium, and
from 20 to 800 ng/m3 in Japanese cities (WHO, 1999).
As Table 2 shows, manganese concentrations in air in the USA have
decreased over the past three decades (Kleinman et al., 1980; US EPA,
1984), a trend believed to be due primarily to the installation of
industrial emission controls (US EPA, 1984, 1985b). In Ontario,
Canada, as well, annual average manganese concentrations in air have
decreased along with total suspended particulate levels (Stokes et
al., 1988).
6.2 Human exposure
The most significant source of manganese exposure for the general
population is food (Table 3). A summary of mean manganese
concentrations in 234 foods analysed by the US Food and Drug
Administration is presented in Table 4. Although wide ranges of
manganese concentrations in foods have been reported, the highest
manganese concentrations are found in nuts (up to 47 µg/g) and grains
(up to 41 µg/g). Lower levels are found in milk products (0.02-0.49),
meat, poultry, fish, and eggs (0.10-3.99 µg/g), and fruits (0.20-10.38
µg/g). Tea and leafy green vegetables have also been found to be
dietary sources of manganese (Davis et al., 1992). The US
concentrations given in Table 4 are generally similar to
concentrations reported from other countries. For example, during a
1992 survey conducted by Canada's Department of Fisheries and Oceans,
manganese was detected in muscle samples from bluefin tuna ( Thunnus
thynnus) (Hellou et al., 1992); concentrations in 14 samples ranged
from 0.16 to 0.31 µg/g dry weight, with a mean of 0.22 µg/g.
Although manganese is considered an essential element, a
Recommended Daily Allowance (RDA) has not been established in the USA
because of insufficient data (NRC, 1989). However, the Food and
Nutrition Board of the US National Research Council establishes
Estimated Safe and Adequate Daily Dietary Intake (ESADDI) levels when
data are insufficient to establish an RDA. These levels generally
parallel amounts of the compound usually delivered via the diet,
although some individuals consume greater or smaller amounts. The
ESADDI levels for manganese are 0.3-0.6 mg/day for infants up to 6
months old, 0.6-1.0 mg/day for infants 6 months to 1 year old, 1.0-1.5
mg/day for children 1-3 years old, 1.0-2.0 mg/day for children 4-10
years old, and 2.0-5.0 mg/day for people over 10 years old (NRC,
1989).
Table 3 presents an example of manganese intake from foodstuffs
based on estimated dietary patterns in the USA. Manganese intake among
individuals varies greatly, however, depending upon dietary habits.
For example, an average cup of tea contains 0.4-1.3 mg manganese, so
individuals consuming three cups of tea per day can receive negligible
amounts of manganese or up to 4 mg daily from this source alone
(Schroeder et al., 1966; Pennington et al., 1986). Thus, some persons
consume more or less than the estimated daily intakes noted above
(NAS, 1980; Pennington et al., 1986; Davis et al., 1992). Indeed,
estimates of daily intake for adults in the USA range from 2.0 to
8.8 mg (NAS, 1977; Patterson et al., 1984; US EPA, 1984; WHO, 1984;
Pennington et al., 1986).
Although gastrointestinal absorption of manganese is only 3-5%
(Mena et al., 1969; Davidsson et al., 1988) (see section 7), food is
not only the largest source of manganese exposure in the general
population, but also the primary source of absorbed manganese
(Table 3). The bioavailability of manganese from vegetable sources is
substantially decreased by dietary components such as fibre and
phytates (US EPA, 1993). Individuals with iron deficiency exhibit
increased rates of manganese absorption (Mena et al., 1969, 1974).
In 1962, the public drinking-water supplies in 100 large cities
in the USA were surveyed, and 97% contained less than 100 µg
manganese/litre (Durfor & Becker, 1964). A 1969 survey of 969 systems
reported that 91% contained less than 50 µg/litre, with a mean
concentration of 22 µg/litre (ATSDR, 1996). In the Federal Republic of
Germany, mean concentrations of manganese in drinking-water were
reported to range from 1 to 63 µg/litre (Alessio & Lucchini, 1996).
Certain groups are more highly exposed to manganese than the
general population. Infants given prepared infant foods and formulas,
for example, may be more highly exposed to manganese than adults in
the general population. Collipp et al. (1983) reported that
concentrations of manganese in infant formulas range from 34 to
1000 µg/litre, compared with concentrations of 10 µg/litre in human
milk and 30 µg/litre in cow's milk; Lavi et al. (1989) found an even
lower concentration of manganese in market milk (16 + 2 µg/litre),
suggesting that the difference between formula and milk could be even
greater in some regions. Because of the high manganese levels in
prepared infant foods and formulas, some infants might ingest more
than the ESADDI for their age group (Pennington et al., 1986; NRC,
1989).
In addition, people living in the vicinity of ferromanganese or
iron and steel manufacturing facilities, coal-fired power plants, or
hazardous waste sites can be exposed to elevated manganese particulate
matter in air, although this exposure is likely to be much lower than
in the workplace. Loranger & Zayed (1997) estimated average exposure
doses of respirable manganese and total manganese in an urban site
(botanical gardens) in Montreal, Canada, to be 0.005 and 0.008 µg/kg
Table 3: Summary of typical human exposure to manganese.a
Parameter Exposure medium
Water Air Food
Typical concentration 4 µg/litre 0.023 µg/m3 1.28 µg/calorie
in medium
Assumed daily 2 litres 20 m3 3000 calories
intake of medium
by 70-kg adult
Estimated average 8 µg 0.46 µgb 3800 µg
daily intake by
70-kg adult
Assumed 0.03c 1c 0.03d
absorption fraction
Approximate 0.24 µg 0.46 µg 114 µg
absorbed dose
a Adapted from US EPA (1984).
b Assumes 100% deposition in the lungs.
c No data; assumed value.
d Davidsson et al. (1988).
Table 4: Manganese concentrations in selected foods.a
Type of food Range of mean concentrations
(ppm; µg/g or mg/litre)
Nuts and nut products 18.21-46.83
Grains and grain products 0.42-40.70
Legumes 2.24-6.73
Fruits 0.20-10.38
Fruit juices and drinks 0.05-11.47
Vegetables and vegetable products 0.42-6.64
Desserts 0.04-7.98
Infant foods 0.17-4.83
Meat, poultry, fish, and eggs 0.10-3.99
Mixed dishes 0.69-2.98
Condiments, fats, and sweeteners 0.04-1.45
Beverages (including tea) 0.00-2.09
Soups 0.19-0.65
Milk and milk products 0.02-0.49
a Adapted from Pennington et al. (1986).
body weight per day (0.35 and 0.56 µg/day for a 70-kg person),
respectively. Similarly, the daily intake of manganese in the air by
the general US population was estimated to be less than 2 µg (WHO,
1981). According to a study by Pellizari et al. (1992) and subsequent
analyses by the US EPA (1994a, 1994b), measurements of personal
exposure levels in an urban area in the USA (Riverside, California) in
1990 indicated that about half the population had 24-h personal
exposures to PM10 (particulate matter with an aerodynamic diameter
less than or equal to 10 µm) manganese above 0.035 µg/m3 (0.7 µg/day,
assuming a ventilation rate of 20 m3/day), while the highest 1% of
the population had exposures above 0.223 µg/m3 (4.46 µg/day). By
contrast, intakes in areas of the USA with ferro- or silicomanganese
industries were as high as 10 µg/day, with 24-h peak values exceeding
100 µg/day (WHO, 1981).
People living in regions of natural manganese ore deposits or
where manganese-containing materials (e.g., pesticides, batteries) are
used or disposed of can also be exposed to elevated levels of
manganese in soil or water. For example, Kawamura et al. (1941)
reported on six Japanese families exposed to high levels (at least
14 mg/litre) of manganese in their drinking-water; the contamination
was believed to result from manganese that leached from batteries
buried near the well. Children are especially likely to receive
elevated doses from manganese-containing soils because they have a
higher intake of soil (mainly through hand-to-mouth contact) than
adults (Calabrese et al., 1989). Organomanganese compounds such as MMT
can be absorbed through the skin (Tanaka, 1994).
In the workplace, exposure to manganese is most likely to occur
by inhalation of manganese fumes or manganese-containing dusts. These
dusts can contain various manganese oxides as well as manganese in the
oxides of other elements, such as potassium permanganate, manganese
ferric oxide (MnFe2O4), and manganese silicate (MnSiO3) (Pflaumbaum
et al., 1990). Exposure is a concern mainly in the ferromanganese,
iron and steel, dry-cell battery, and welding industries (WHO, 1986).
Exposure can also occur during manganese mining and ore processing,
and dermal exposure and inhalation can occur during the application of
manganese-containing fungicides.
Manganese air concentrations of 1.5-450 mg/m3 have been reported
in US manganese mines (US EPA, 1984), 0.30-20 mg/m3 in ferroalloy
production facilities (Saric et al., 1977), 0.02-5 mg/m3 in German
foundries (Coenen et al., 1989), 1-4 mg/m3 during welding with
electrodes (Sjögren et al., 1990), up to 14 mg/m3 during welding with
welding wire (Pflaumbaum et al., 1990), and 3-18 mg/m3 in a dry-cell
battery facility (Emara et al., 1971). Many of the more recent studies
on occupational exposures to manganese have recorded average exposure
levels of 1 mg manganese/m3 or less in the workplace (Roels et al.,
1987, 1992; Mergler et al., 1994; Lucchini et al., 1995). Thus, for
workers in industries using manganese, the major route of exposure
might be inhalation from workplace air rather than ingestion of food.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
Manganese absorption occurs primarily from the gastrointestinal
tract after ingestion and from the alveolar lining after inhalation of
manganese-containing dust or fumes. Several studies in animals
indicate that key determinants of absorption are the absorption
pathway and the specific compound in which manganese is present (Smith
et al., 1995; Roels et al., 1997). Roels et al. (1997) studied
manganese levels in the blood and brain tissue of rats exposed to
repeated doses of manganese chloride or manganese dioxide administered
by oral gavage, intraperitoneal injection, or intratracheal
instillation. Manganese chloride was readily absorbed after
administration by each of these routes and distributed in brain tissue
to varying degrees. Manganese dioxide, on the other hand, was
significantly absorbed and distributed in the brain to varying degrees
when administered by intraperitoneal injection and intratracheal
instillation, but not when administered orally. Higher levels of
manganese in tissue were found after administering manganese chloride
by intratracheal instillation compared with manganese dioxide. The
authors concluded that the route of exposure might be a critical
determinant of how absorbed manganese is distributed in the brain. In
addition, when manganese dioxide was administered by either
intratracheal instillation or oral gavage, manganese levels in the
blood rose and fell more slowly than when manganese chloride was
given, indicating a marked difference in the absorption kinetics of
these two manganese compounds. The finding that the body handles
manganese dioxide more slowly than manganese chloride suggests that
manganese dioxide might remain in the body longer, contributing longer
to body burden, albeit at much lower levels. Whether this is true and
whether this indicates greater toxicological risk in cases of
prolonged low-level exposure to manganese dioxide are unclear.
A second study also found that route of exposure affects
absorption of manganese. Tjälve et al. (1996) found that intranasal
instillation of manganese (Mn2+) in rats resulted in initial uptake
of manganese in the olfactory bulbs of the brain, whereas
intraperitoneal administration resulted in low uptake in the olfactory
bulbs. The authors suggested that olfactory neurons might serve as a
pathway for manganese uptake and distribution to the brain (bypassing
the blood-brain barrier) during intranasal exposure.
Another key determinant of absorption appears to be dietary iron
intake, with low iron levels leading to increased manganese absorption
(Mena et al., 1969). In addition, several studies in animals indicate
that gastrointestinal absorption of manganese might vary with age
(Rehnberg et al., 1980, 1981).
The amount of manganese absorbed across the gastrointestinal
tract in humans varies, but typically averages about 3-5% (Mena
et al., 1969; Davidsson et al., 1988). Particles that are deposited in
the lower airways are probably absorbed, whereas particles deposited
in the upper airways are generally swallowed via mucociliary
clearance; thus, they can be absorbed from the gastrointestinal tract
as well.
Regardless of manganese intake, adult humans generally maintain
stable tissue levels of manganese through a homeostatic mechanism
regulating the excretion of excess manganese (US EPA, 1984). The major
route of manganese excretion is via the bile, although some excretion
occurs in urine, milk, and sweat (US EPA, 1993).
Limited data suggest that manganese can undergo changes in
oxidation state within the body. Support for this hypothesis comes
from the observation that the oxidation state of the manganese ion in
several enzymes appears to be Mn(III) (Utter, 1976; Leach & Lilburn,
1978), whereas most manganese intake from the environment is as Mn(II)
or Mn(IV). The rate and extent of manganese reduction/oxidation
reactions might be important determinants of manganese retention in
the body.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
Lung inflammation has been reported following single inhalation
exposures to 2.8-43 mg/m3 for manganese dioxide or manganese
tetroxide particulates in rodent species (Bergstrom, 1977; Adkins et
al., 1980; Shiotsuka, 1984). It is important to note that an
inflammatory response of this type is not unique to
manganese-containing particles, but is characteristic of nearly all
inhalable particulate matter (US EPA, 1985b). Thus, it might not be
manganese alone that causes the inflammatory response from single
exposures, but possibly the particulate matter itself.
Following single oral exposures, LD50s ranged from 275 to 804
mg/kg body weight per day for manganese chloride in different rat
strains (Holbrook et al., 1975; Kostial et al., 1989; Singh &
Junnarkar, 1991). Reported LD50s from single exposures to manganese
sulfate and manganese acetate in rats were 782 and 1082 mg/kg body
weight per day, respectively (Smyth et al., 1969; Singh & Junnarkar,
1991).
8.2 Irritation and sensitization
Little information is available on the irritant and contact
sensitivity properties of manganese compounds. Manganese salts failed
to induce lymph node cell proliferation in the murine local lymph node
assay, a predictive test for the detection of contact allergens
(Ikarashi et al., 1992). The manganese-containing fungicide maneb has
been reported to be a sensitizer in animal tests, but little
information exists on whether this effect occurs in humans (Thomas et
al., 1990). Contact sensitization in humans has been reported in one
study (see section 9.2).
8.3 Short-term exposure
Results from studies of short-term exposures in experimental
animals indicate that the lungs and nervous system are the major
target organs following the inhalation of manganese compounds. For
example, Maigetter et al. (1976) found increased susceptibility to
pneumonia in mice exposed via inhalation to 69 mg manganese/m3 as
manganese dioxide for 3 h/day for 1-4 days. Effects on the nervous
system associated with short-term exposure to manganese compounds are
presented in section 8.7.
8.4 Long-term exposure
8.4.1 Subchronic exposure
Results from studies of subchronic exposures in experimental
animals also indicate that the lungs and nervous system are the major
target organs following the inhalation of manganese compounds. Signs
of lung inflammation have been reported in rhesus monkeys exposed via
inhalation to 0.7 mg manganese/m3 as manganese dioxide for 22 h/day
over 10 months (Suzuki et al., 1978). Effects on the nervous system
associated with subchronic exposure to manganese compounds are
presented in section 8.7.
Systemic effects reported following subchronic oral exposures to
manganese compounds include changes in blood cell counts (leukocytes,
erythrocytes, neutrophils), reduced liver weight, and decreased body
weight (Gray & Laskey, 1980; Komura & Sakamoto, 1991; NTP, 1993). In
mice fed 284 mg manganese/kg body weight per day for 100 days, for
example, red blood cell count was decreased by manganese acetate and
manganese chloride; white blood cell count was decreased by manganese
acetate, manganese chloride, and manganese dioxide; and haematocrit
was decreased by manganese carbonate (MnCO3) (Komura & Sakamoto,
1991).
8.4.2 Chronic exposure and carcinogenicity
Available data from animal studies involving oral exposure to
manganese as well as from epidemiological studies involving inhalation
exposure to manganese suggest that similar chronic toxicities (i.e.,
neurological effects) occur regardless of the valence state of the
inorganic manganese compounds (e.g., manganese dioxide, manganese
tetroxide). In experimental animals, the nervous system is the major
organ affected following long-term oral and inhalation exposure to
manganese. These data are described in more detail in section 8.7. Few
chronic inhalation exposure studies in animals are available, and
these studies reported effects in the nervous system. Significant
effects in other organ systems following long-term exposure to
manganese have not been reported. Available data from animal studies
suggest that it is unlikely that other significant effects result from
long-term oral exposure to manganese (NTP, 1993).
Information on the carcinogenic potential of manganese is
limited, and the results are difficult to interpret with certainty.
For example, male rats exposed to up to 331 mg manganese/kg body
weight per day (as manganese sulfate) for 2 years had an increased
incidence of pancreatic cell adenomas (3/50, 4/51, and 2/51 in the
low, mid, and high dose groups); this type of tumour was noted in only
one female in the mid dose group. The investigators indicated that
these lesions, although low in incidence, were "a concern" and
attributed to manganese treatment because pancreatic cell hyperplasia
was observed in all treatment groups, although neither hyperplasia nor
adenomas were observed in controls of either sex (Hejtmancik et al.,
1987a). On the other hand, a small increase in the incidence of
pituitary adenomas was noted in female mice at 905 mg manganese/kg
body weight per day (as manganese sulfate), but not in males at 722 mg
manganese/kg body weight per day. The incidence was considered
equivocal because lesions had been observed in previous studies as
well as in historical controls (Hejtmancik et al., 1987b). In a 2-year
study, no evidence of cancer was noted in male and female F344 rats
given 20-200 and 23-232 mg manganese sulfate/kg body weight per day,
respectively, via feed (NTP, 1993). A marginally increased incidence
of thyroid gland follicular cell adenomas was observed in male and
female B6C3F1 mice given 52-585 and 65-731 mg manganese sulfate/kg
body weight per day, respectively, in the feed for 2 years (NTP,
1993). Intraperitoneal injection of mice with manganese sulfate
(20 weeks) led to an increased incidence of lung tumours (Stoner
et al., 1976), but intramuscular injection of rats and mice with
manganese or manganese dioxide did not result in tumours (Furst,
1978). Firm conclusions on the carcinogenic potential of manganese
cannot be made based on the equivocal carcinogenicity data reported
for rodents and the paucity of evidence from other species.
8.5 Genotoxicity and related end-points
Manganese sulfate was not mutagenic to Salmonella typhimurium
strains TA97, TA98, TA100, TA1535, or TA1537 in either the presence or
absence of S9 from Aroclor 1254-induced liver from rats or Syrian
hamsters in studies performed at two different laboratories
(Mortelmans et al., 1986), but it was reported elsewhere to be
genotoxic to strain TA97 (Pagano & Zeiger, 1992). Manganese chloride
was not mutagenic in S. typhimurium strains TA98, TA100, and TA1535,
but it was mutagenic in TA1537, and conflicting results were obtained
for TA102 (Wong, 1988; De Méo et al., 1991). A fungal gene conversion/
reverse mutation assay in Saccharomyces cerevisiae strain D7
indicated that manganese sulfate was mutagenic (Singh, 1984).
Manganese chloride produced gene mutations in vitro in a mouse
lymphoma assay (Oberly et al., 1982). It also caused DNA damage in
human lymphocytes when tested in vitro using the single-cell gel
assay technique in the absence of metabolic activation, but it caused
no DNA damage when S9 was present (De Méo et al., 1991). The results
of an in vitro assay using Chinese hamster ovary (CHO) cells showed
that manganese sulfate induced sister chromatid exchange in both the
presence and absence of S9 from Aroclor 1254-induced rat liver
(Galloway et al., 1987). In a separate assay, manganese sulfate also
induced chromosomal aberrations in CHO cells in the absence of S9 but
not in its presence (Galloway et al., 1987). In contrast, manganese
chloride was not clastogenic when tested in vitro in the absence of
metabolic activation using FM3A cells (Umeda & Nishimura, 1979),
although it did cause chromosomal aberrations in the root tips of
Vicia faba (Glass, 1955, 1956). Potassium permanganate caused
chromosomal aberrations in FM3A cells (Umeda & Nishimura, 1979) but
not in a primary culture of cells from Syrian hamster embryos (Tsuda &
Kato, 1977) when tested in the absence of metabolic activation.
Magnesium chloride caused cell transformation in Syrian hamster embryo
cells (Casto et al., 1979).
Manganese chloride did not produce somatic mutations in
Drosophila melanogaster fruit flies (Rasmuson, 1985). Manganese
sulfate did not induce sex-linked recessive lethal mutations in the
germ cells of male D. melanogaster (Valencia et al., 1985).
In vivo assays in mice showed that oral doses of manganese
sulfate or potassium permanganate caused micronuclei and chromosomal
aberrations in bone marrow (Joardar & Sharma, 1990). In contrast, oral
doses of manganese chloride did not cause chromosomal aberrations in
the bone marrow or spermatogonia of rats (Dikshith & Chandra, 1978).
The results of in vitro studies show that at least some
chemical forms of manganese have mutagenic potential. However, as the
results of in vivo studies in mammals are inconsistent, no overall
conclusion can be made about the possible genotoxic hazard to humans
from exposure to manganese compounds.
8.6 Reproductive and developmental toxicity
Considerable information is available on the reproductive and
developmental effects of manganese in animals. Mice exposed
subcutaneously to 0, 2, 4, 8, or 16 mg manganese chloride
tetrahydrate/kg body weight per day on gestation days 6-15 showed no
treatment-related effects on the number of total implants, early
resorptions, dead fetuses, or sex ratio. However, a significant
increase in the number of late resorptions was found in the 4, 8, and
16 mg/kg body weight per day groups. Significant maternal toxicity was
associated with the 8 and 16 mg/kg body weight per day groups (Sánchez
et al., 1993). A single intratracheal dose of 160 mg manganese/kg (as
manganese dioxide) in rabbits caused slow degenerative changes in the
seminiferous tubules and led to sterility (Seth et al., 1973; Chandra
et al., 1975). Abnormal sperm morphology was observed in mice treated
with 23-198 mg manganese/kg body weight per day as potassium
permanganate or manganese sulfate by gavage in water for up to 3 weeks
(Joardar & Sharma, 1990). No gross or histopathological lesions or
organ weight changes were observed in the reproductive organs of
rodents exposed to 1300 mg manganese/kg body weight per day for 14
days or fed up to 1950 mg manganese/kg body weight per day for 13
weeks (NTP, 1993). From the available evidence, no firm conclusions on
effects in male reproductive organs can be made, and reproductive
performance was not evaluated in many of these studies.
A slight decrease in pregnancy rate was observed in female rats
exposed to 130 mg manganese/kg body weight per day as manganese
tetroxide in the diet for 90-100 days before breeding (Laskey et al.,
1982). Female reproductive parameters such as litter size, ovulations,
resorptions, or fetal weights were not affected in rats consuming
excess manganese as manganese tetroxide in feed or water (Laskey et
al., 1982; Kontur & Fechter, 1985), except at concentrations so high
(1240 mg/kg body weight per day) that water intake by the dams was
severely reduced. In mice, inhalation exposure of females to 85 mg
manganese/m3 (as manganese dioxide) for 16 weeks prior to conception
and 17 days after conception led to a decrease in average pup weight
at birth and decreased activity levels (Lown et al., 1984). Webster &
Valois (1987) found that intraperitoneal injection of pregnant mice
with 12.5 mg manganese/kg body weight (as manganese sulfate) on days
8-10 of gestation resulted in exencephaly and embryolethality.
Finally, manganese chloride administered by gavage at doses of 0, 25,
50, or 75 mg/kg body weight per day caused major dose-dependent
abnormalities in the fetuses when administered to gestating rats for
the duration of gestation, but did not cause major abnormalities in
the fetuses when administered to pregnant rabbits during the period of
organogenesis (Szakmáry et al., 1995).
In a rat teratology study, intravenous injection of 20 µmol
manganese chloride/kg body weight (1.1 mg manganese/kg body weight) on
days 6-17 of pregnancy induced mild skeletal malformations in the
fetuses; the no-observed-adverse-effect level (NOAEL) was 0.28 mg
manganese/kg body weight (Treinen et al., 1995). Similar effects were
observed in another study (Grant & Ege, 1995) when administration was
by injection, but not when manganese was administered by gavage at
400 µmol manganese chloride/kg body weight (22 mg manganese/kg body
weight). These results suggest that parenteral administration has a
much greater potential for developmental toxicity than oral exposure.
In rabbits exposed to manganese by intratracheal instillation, a
single dose of 160 mg manganese/kg body weight (as manganese dioxide)
resulted in a slow degeneration of the seminiferous tubules over a
period of 1-8 months. This was associated with loss of spermatogenesis
and complete infertility (Seth et al., 1973; Chandra et al., 1975).
Similar degenerative changes in testes have been observed in rats and
mice following intraperitoneal injection of manganese sulfate (Singh
et al., 1974; Chandra et al., 1975) and in rabbits following
intravenous injection of manganese chloride (Imam & Chandra, 1975).
8.7 Immunological and neurological effects
As with exposure to other airborne particulate matter, an
increased susceptibility to infection has been observed in mice and
guinea-pigs exposed to manganese via inhalation for a short period
(Maigetter et al., 1976; Adkins et al., 1980). Altered blood levels of
leukocytes, lymphocytes, and neutrophils have been observed in rats
and mice that ingested manganese in the feed for short-term (33 mg/kg
body weight per day for 14 days) or subchronic (284 mg/kg body weight
per day for 100 days) durations (Komura & Sakamoto, 1991; NTP, 1993).
However, it is unknown if these changes are associated with any
significant impairment of the immune system.
No evidence of neurological effects was seen in rhesus monkeys
(0.01-1.1 mg manganese tetroxide/m3) or macaque monkeys (20-40 mg
manganese chloride/m3) exposed to manganese via inhalation over
subchronic and chronic periods (Ulrich et al., 1979). However,
intravenous administration of 5-40 mg manganese/kg (as manganese
chloride) to cebus monkeys did result in movement tremors accompanied
by increased manganese in the globus pallidus and substantia nigra
regions of the brain (Newland & Weiss, 1992). Decreased levels of
dopamine were found in several regions of the brain (caudate and
globus pallidus) in rhesus monkeys exposed to 30 mg manganese/m3 (as
manganese dioxide) via inhalation for 2 years (Bird et al., 1984).
A decrease in pup retrieval behaviour was observed in maternal
mice exposed to 61 mg manganese/m3 (as manganese dioxide) via
inhalation for 18 weeks (Lown et al., 1984). In another study,
Morganti et al. (1985) observed moderate changes in open-field
behaviour in mice exposed to 72 mg manganese/m3 (as manganese
dioxide) for 18 weeks.
In general, effects from inhalation exposure to manganese in
experimental animals occur at levels higher (30-70 mg manganese/m3)
than those at which effects have been reported in humans (0.14-1 mg
total manganese dust/m3 for preclinical neurological alterations and
2-22 mg total manganese dust/m3 for overt neurological disease). This
evidence suggests that laboratory animals, especially rodents, might
not be as sensitive as humans, and possibly other primates, to the
neurological effects of inhalation exposure to manganese.
There are substantial data on neurological effects in animals
following ingestion of manganese. In one study, decreases in
spontaneous activity, alertness, touch response, muscle tone, and
respiration were observed in mice dosed once by oral gavage with 58 mg
manganese/kg body weight (as manganese chloride) (Singh & Junnarkar,
1991). Rats developed a rigid and unsteady gait after 2-3 weeks of
exposure to a higher level (150 mg/kg body weight per day) of
manganese chloride (Kristensson et al., 1986).
Mice ingesting food containing manganese chloride, manganese
acetate, manganese carbonate, or manganese dioxide (284 mg/kg body
weight per day) for 100 days or manganese tetroxide (137 mg/kg body
weight per day) for 90 days showed significantly decreased motor
activity (Gray & Laskey, 1980; Komura & Sakamoto, 1991). Two of the
third-generation mice exhibited staggered gait and histochemical
changes after drinking water containing manganese chloride (10.6 mg/kg
body weight per day) over three generations (Ishizuka et al., 1991).
Conversely, rats showed increased activity and aggression when exposed
to 140 mg manganese chloride/kg body weight per day in drinking-water
for 4 weeks (Chandra, 1983) and just increased activity when exposed
to 40 mg manganese chloride/kg body weight per day for 65 weeks
(Nachtman et al., 1986).
Numerous studies have reported alterations in brain
neurotransmitter levels and function, brain histochemistry, or
neuronal enzyme function. These neurochemical changes have been
observed in rats and mice following ingestion of manganese (as
manganese chloride) administered via the feed, drinking-water, or
gavage (in water) at doses ranging from 1 to 2270 mg manganese/kg body
weight over intermediate exposure periods (i.e., 14-364 days)
(Bonilla, 1978; Chandra & Shukla, 1978; Deskin et al., 1980; Gianutsos
& Murray, 1982; Chandra, 1983; Bonilla & Prasad, 1984; Ali et al.,
1985; Eriksson et al., 1987; Subhash & Padmashree, 1991). Similar
alterations were reported after chronic exposures (>365 days) to 275
mg manganese dioxide/kg body weight in the feed of mice (Komura &
Sakamoto, 1992) or 40 mg manganese chloride/kg body weight in
drinking-water of rats (Lai et al., 1984).
Neurochemical alterations have also been reported in rats
following intraperitoneal injection of manganese at doses ranging from
2.2 to 4.4 mg manganese chloride/kg body weight over intermediate
exposure periods (Sitaramayya et al., 1974; Shukla et al., 1980; Seth
et al., 1981). Decreased neurotransmitter receptor binding was
observed in macaca monkeys following subcutaneous injection of
manganese dioxide at 38 mg/kg body weight for 26 months (Eriksson
et al., 1992). Changes in region-specific neuronal populations were
reported in rats receiving manganese chloride from their
drinking-water for either 4 or 8 weeks (Sarhan et al., 1986). The
actual manganese dose administered over the total experimental period
was not reported by the authors. However, daily intakes of at least
10.7 mg manganese/kg body weight are estimated based on initial
average body weight and water intake reported in the study.
Neurobiochemical changes have been detected in neonate rats at
doses similar to or slightly above dietary levels (1-10 mg
manganese/kg body weight per day for 24-60 days, as manganese
chloride) (Chandra & Shukla, 1978; Deskin et al., 1980), which could
indicate that young animals may be more susceptible to manganese than
adults. Oner & Senturk (1995) demonstrated that manganese induces
learning deficits in rats dosed with 357 µg manganese/kg body weight
for 15 or 30 days; these effects were reversible.
9. EFFECTS ON HUMANS
A requirement for manganese in humans was determined based on
symptoms observed in a subject inadvertently fed a diet deficient in
manganese for 3.5 months (Doisy, 1972). It has been determined that
manganese is needed for the functioning of key enzymes that play a
role in cellular protection from damaging free radical species,
maintenance of healthy skin, and synthesis of cholesterol
(Freeland-Graves et al., 1987; Friedman et al., 1987). Based upon
case-studies in people with low blood manganese and known requirements
in animals, it is thought that manganese may also play a role in bone
mineralization, metabolism of proteins, lipids, and carbohydrates,
energy production, metabolic regulation, and nervous system
functioning (Schroeder et al., 1966; Freeland-Graves et al., 1987;
Hurley & Keen, 1987; Freeland-Graves & Llanes, 1994; Wedler, 1994).
However, the link between inadequate manganese nutrition and its role
in these body functions in humans requires further investigation.
Manganism is a progressive, disabling neurological syndrome that
typically begins with relatively mild symptoms and evolves to include
dull affect, altered gait, fine tremor, and sometimes psychiatric
disturbances. Because some of these symptoms resemble those of
Parkinson's disease, many investigators have used terms such as
"Parkinsonism-like disease" and "manganese-induced Parkinsonism" to
describe symptoms observed with manganese poisoning. Although symptoms
of manganism resemble those of Parkinson's disease, significant
differences have been noted. In terms of clinical presentation,
Barbeau (1984) noted that the hypokinesia and tremor present in
patients suffering from manganism differed from those seen in
Parkinson's disease. Drawing from the literature, Calne et al. (1994)
noted other features that can also distinguish manganism from
Parkinson's disease; psychiatric disturbances early in the disease (in
some cases), the "cock walk" (see below), a propensity to fall
backward when displaced, less frequent resting tremor, more frequent
dystonia, and failure to respond to dopaminomimetics (at least in the
late stages of the disease) were characteristic of manganism. Beuter
et al. (1994) showed that 10 manganese-exposed workers (average
exposure of 13.9 years; average blood manganese level of 1.06 µg/dl)
and 11 patients with Parkinsonism were significantly different from
the controls ( n = 11) in functional asymmetries between right and
left hand. Therefore, use of terms such as "Parkinsonism-like disease"
and "manganese-induced Parkinsonism" are somewhat misleading.
Nonetheless, the use of these terms may help health providers and
health surveillance workers recognize the effects of manganese
poisoning when encountering it for the first time in occupational or
environmental settings. These terms appear in the discussion below
when they were used by study authors in their reports (shown in
italics). The term "manganism" is used as well.
Long-term exposures to manganese in occupational settings can
result in a progressive neurological dysfunction, which can produce a
disabling syndrome referred to as manganism. Mergler & Baldwin (1997)
have described this disease progression as a "slow deterioration of
well-being which can be initially detected as early neurofunctional
alterations... [among exposed groups], later on, as sub-clinical signs
in individuals... and finally as a full blown neurological disease
-- manganism." Progression along this continuum is thought to be a
function of the dose and duration of exposure, as well as individual
susceptibilities. In general, the clinical effects of high-level
inhalation exposure to manganese do not become apparent until exposure
has occurred for several years, but some individuals begin to show
signs of neurological alterations after as little as 1-3 months of
exposure (Rodier, 1955).
Pathological findings in manganism and Parkinson's disease also
differ. In humans with chronic manganese poisoning, lesions are more
diffuse, found mainly in the pallidum, the caudate nucleus, the
putamen, and even the cortex. In people with Parkinson's disease,
lesions are found in the substantia nigra and other pigmented areas of
the brain (Barbeau, 1984). Moreover, Lewy bodies are usually not found
in substantia nigra in cases of manganism, but are almost always found
in cases of Parkinson's disease (Calne et al., 1994). Magnetic
resonance imaging of the brain reveals accumulation of manganese in
cases of manganism, but little or no changes in people with
Parkinson's disease; fluorodopa positron emission tomography scans are
normal in cases of manganism, but abnormal in people with Parkinson's
disease (Calne et al., 1994).
The first signs of manganism are usually subjective and
non-specific, often involving generalized feelings of weakness,
heaviness or stiffness of the legs, anorexia, muscle pain,
nervousness, irritability, and headache (Rodier, 1955; Whitlock
et al., 1966; Mena et al., 1967; Tanaka & Lieben, 1969; Sjögren et
al., 1996). These signs are frequently accompanied by apathy and
dullness, along with impotence and loss of libido; especially in the
case of miners, more extreme manifestations of psychomotor excitement,
such as aggressive or destructive behaviour, emotional lability, and
bizarre compulsive activities, are also associated with the first
stages of manganism (Rodier, 1955; Schuler et al., 1957; Mena et al.,
1967; Emara et al., 1971; Abdel-Hamid et al., 1990; Wennberg et al.,
1991; Chu et al., 1995).
More specific clinical signs of basal ganglia dysfunction
characterize the next stage and can include a slow or halting speech
without tone or inflection, a dull and emotionless facial expression,
slow and clumsy movement of the limbs or altered gait, late motor
deficits, and fine tremor (Rodier, 1955; Schuler et al., 1957; Mena
et al., 1967; Tanaka & Lieben, 1969; Smyth et al., 1973; Yamada et
al., 1986; Ky et al., 1992; Wennberg et al., 1992; Hochberg et al.,
1996; Mergler & Baldwin, 1997).
As the disease progresses, walking becomes difficult and a
characteristic staggering gait develops, the "cock walk," in which
patients strut on their toes, with elbows flexed and the spine erect
(Calne et al., 1994). Muscles become hypertonic, and voluntary
movements can be accompanied by fine tremor (Chu et al., 1995; Mergler
& Baldwin, 1997). In some cases, psychological disturbances (manganese
mania, manganese psychosis) precede or accompany the final stages of
disease (Rodier, 1955; Mena et al., 1967; Cook et al., 1974; Mergler &
Baldwin, 1997). Few data are available regarding the reversibility of
these effects; they are thought to be largely irreversible. Some
evidence indicates that recovery can occur when exposure ceases (Smyth
et al., 1973). Manganism has been documented in welders and in workers
exposed to high levels of manganese dust or fumes in mines or
foundries.
The studies cited above describe overt manganism resulting from
long-term inhalation exposures to 2-22 mg total manganese dust/m3
(Schuler et al., 1957; Whitlock et al., 1966; Tanaka & Lieben, 1969;
Cook et al., 1974; Saric et al., 1977; Huang et al., 1989). Evidence
from recent occupational exposure studies (described below) suggests
that early or preclinical signs of neurological effects can occur in
generally asymptomatic workers exposed to much lower levels of
manganese (about 0.14-1 mg total manganese dust/m3) for several years
(Roels et al., 1987, 1992; Iregren, 1990; Chia et al., 1993; Mergler
et al., 1994; Lucchini et al., 1995). However, the reported values are
only estimates of actual exposure levels. Often, time-weighted
averages of workplace exposures are reported, and dose-response
relationships cannot be determined. In addition, exposures are
generally reported as total manganese dust or the respirable fraction
of total dust, which can be defined differently across studies (e.g.,
PM5 [particulate matter with an aerodynamic diameter less than or
equal to 5 µm] or PM10).
9.1 Case reports
Whitlock et al. (1966) reported a case-study of two workers
exposed to manganese-containing fumes (3.5 mg manganese/m3 average;
no data on exact compounds) from an electric arc used to cut and
cleave manganese castings. Symptoms of ataxia, weakness, and decreased
mental ability developed about 9-12 months following exposure. These
symptoms improved after the patients were treated with
ethylenediamine-tetraacetic acid (EDTA). Rosenstock et al. (1971)
reported a case of a male who developed classic symptoms of manganism
after 14 months of exposure to manganese (dose unknown) from the fumes
and dust of a steel foundry. After being unable to work for 3 years,
the patient was treated with 6-12 g levodopa/day, with the largest
dose providing improvement in facial expression, speech, and muscle
tone. Six men exposed to manganese (22 mg manganese/m3) for an
unspecified period at an ore crushing plant developed signs including
somnolence, abnormal gait, slurred speech, ataxia, masklike faces, and
bradykinesia. Treatment with 8 g levodopa/day did not alleviate the
neurological effects observed in these workers (Cook et al., 1974).
An outbreak of a disease with manganism-like symptoms was
reported in a group of six Japanese families (about 25 people) exposed
to high levels of manganese in their drinking-water (Kawamura et al.,
1941). Symptoms included a masklike face, muscle rigidity and tremors,
and mental disturbance. Five people, all elderly, were severely
affected (2 died), 2 were moderately affected, 8 were mildly affected,
and 10 (all children or young adults) were not affected. These effects
were postulated to be due to the contamination of well-water with
manganese (14 mg/litre) that leached from batteries buried near the
well. Manganese concentrations decreased over time, so the original
level of manganese was probably higher than 14 mg/litre. This case has
been interpreted as an indication that the elderly may be more
sensitive than younger people to the toxic effects of manganese (Davis
& Elias, 1996).
A man noticed weakness and impaired mental capacity after
mistakenly ingesting low doses of potassium permanganate (1.8 mg/kg)
instead of potassium iodide for several weeks to treat lung congestion
(Holzgraefe et al., 1986). Although exposure was stopped after
4 weeks, a syndrome similar to Parkinson's disease developed after
about 9 months. In another case, five patients given manganese
parenterally for an average of 6 years showed early neurological
symptoms of poisoning, while four others, exposed for an average of 4
years, did not (Mirowitz et al., 1991). In a child, accidental
ingestion of potassium permanganate (174 mg/kg) resulted in severe
local corrosion of the mouth, oesophagus, and stomach, but there was
no evidence of systemic toxicity (Southwood et al., 1987).
There are few reports regarding dermal exposure to manganese in
humans. In most cases, manganese uptake across intact skin is expected
to be very limited. However, effects and elevated urinary manganese
levels were observed in a man burned with a hot acid solution
containing 6% manganese (Laitung & Mercer, 1983). There are also
reports of workers experiencing effects from dermal exposure to
organic manganese compounds. Headache and paraesthesia were among the
symptoms reported in workers exposed dermally to MMT after a spill
(doses unknown; Tanaka, 1994). Two young Brazilian agricultural
workers developed Parkinsonian syndrome (Ferraz et al., 1988) and a
37-year-old Italian man developed Parkinsonism (Meco et al., 1994)
after chronic dermal and inhalation exposure to the fungicide maneb.
9.2 Epidemiological studies
The lungs, nervous system, and reproductive system are the main
organs affected following inhalation exposures to manganese, although
other effects have also been observed. For example, in a study of 126
enamellers and 64 decorators from five factories in the ceramics
industry, Motolese et al. (1993) found that 48 workers were sensitized
to at least one substance; positive sensitization test results with
manganese dioxide were found in only 2 of the workers, however. The
remainder of this section focuses on the effects more commonly
reported in epidemiological studies -- lung, nervous system, and
reproductive system effects.
Inhalation of particulate manganese compounds such as manganese
dioxide and manganese tetroxide leads to an inflammatory response in
human lungs. Symptoms and signs of lung irritation and injury can
include cough, bronchitis, pneumonitis, and reductions in lung
function (Lloyd Davies, 1946; Roels et al., 1987; Abdel-Hamid et al.,
1990; Akbar-Khanzadeh, 1993).
Pneumonia has been reported to result from both acute and
long-term inhalation exposure to manganese dioxide dusts (Lloyd
Davies, 1946; Tanaka, 1994). These effects have been noted mainly in
people exposed to manganese dust under occupational conditions,
although respiratory effects have also occurred in residential
populations (WHO, 1987). A higher incidence of pneumonia and a higher
rate of deaths from pneumonia compared with the general population
were observed among residents exposed to manganese dust from a
ferromanganese factory as well as among the factory workers
(WHO, 1987; Tanaka, 1994). However, a threshold level for respiratory
effects has not been established. The increased susceptibility to
respiratory infection might be secondary to the lung irritation and
inflammation caused by inhaled particulate matter rather than caused
by the manganese alone. It is likely that the inflammatory response
begins shortly after exposure and continues for the duration of the
exposure.
Although available studies are not adequate to define the
dose-response curve or determine whether there is a threshold for
neurotoxicity, the lowest level of exposure to manganese dust at which
neurological effects occur was reported by Iregren (1990) and Wennberg
et al. (1991). These investigators compared 30 male workers exposed to
manganese for 1-35 years during employment at two Swedish foundries
with an unexposed control group of 60 workers (matched by age, type of
work, and geographical area) using eight tests from the Swedish
Performance Evaluation System and two additional manual tests. The
mean and median levels of manganese in the foundry air were measured
at 0.25 and 0.14 mg/m3, respectively, and available data indicated
that these levels had been consistent over the past 17-18 years. The
exposed workers exhibited significantly inferior performance in simple
reaction time, digit span, and finger tapping. When a secondary match
was performed, with scores on verbal tests used as an additional
matching criterion (which reduced the size of the reference group to
30), the same test differences remained, although the difference was
not significant for the digit span test. Although the subjects did not
exhibit the signs of clinical manganism described above, these changes
were indicators of manganese-induced neurological effects (Iregren,
1990; Wennberg et al., 1991).
The study results reported by Iregren (1990) and Wennberg et al.
(1991) are supported by evidence presented by Roels et al. (1987,
1992) and Chia et al. (1993, 1995). Roels et al. (1992) detected early
neurological effects in male workers at an alkaline battery plant
exposed to manganese dusts (manganese dioxide). Compared with 101 male
workers without industrial exposure, the 92 exposed workers showed
significantly poorer eye-hand coordination, hand steadiness, and
visual reaction time. A Lifetime Integrated Exposure, for both
respirable and total manganese dust, was estimated for each of the
exposed workers (expressed as exposure in mg manganese/m3 multiplied
by the number of years of exposure, or mg/m3 × year). Based on an
analysis of the data by a logistic regression model, it was suggested
that there was an increased risk of peripheral tremor at a Lifetime
Integrated Exposure level of 3.575 mg/m3 × year total manganese dust
or 0.73 mg/m3 × year respirable (PM5) dust; dividing by an exposure
duration of 5.3 years, these values are equivalent to 0.67 mg/m3 and
0.14 mg/m3 for total manganese dust and respirable manganese dust,
respectively. This total manganese dust exposure level (0.67 mg/m3)
is slightly higher than the median found to be associated with effects
in the 1990 Iregren and the 1991 Wennberg et al. studies (0.14
mg/m3). The Lifetime Integrated Exposure at which an increased risk
of abnormal neurofunction occurs is based on exposures in an
occupational setting and might be biased because of the "healthy
worker effect" (i.e., the most susceptible individuals were not
incorporated into the study).
The Chia et al. (1993) study also reported neurological deficits
in an occupational cohort of 17 manganese "baggers" in Singapore who
were administered the WHO Neurobehavioural Core Test Battery, as well
as several supplementary tests and a subjective questionnaire (with
questions on 37 symptoms related to the nervous system) taken from the
Operational Guide to the Neurobehavioural Core Test Battery. The
exposed workers had significantly poorer motor speed, visual-motor
coordination, visual scanning, visual-motor and response speed, and
visual-motor coordination and steadiness than a control group. Twenty
of the 37 symptoms in the questionnaire were also reported more
frequently by the exposed workers than by the control group, although
the differences were significant only for insomnia and profuse
sweating. The mean manganese level in air (from 1981 to 1991) in the
factories was reported to be 1.59 µg/litre (1.59 mg/m3; 8-hour
time-weighted average). Chia et al. (1995) conducted another study
with a larger group of exposed workers (32 subjects exposed to the
same mean level of manganese in air reported above), focusing in more
detail on postural stability; the exposed workers exhibited
significantly poorer postural stability compared with a control group.
A study by Mergler et al. (1994) also supports the findings of
Iregren (1990), Wennberg et al. (1991), Roels et al. (1987, 1992), and
Chia et al. (1993, 1995). This epidemiological study included 74 male
workers from a ferromanganese and silicomanganese alloy factory,
matched with 74 referents from a pool of 145 non-occupationally
exposed men residing in the vicinity. Environmental levels of total
manganese dust at the factory were measured at 0.014-11.48 mg/m3
(median 0.151 mg/m3; mean 1.186 mg/m3), whereas manganese levels in
respirable dust (PM10 samples) ranged from 0.001 to 1.27 mg/m3
(median 0.032 mg/m3; mean 0.122 mg/m3). The authors noted that
exposures at the factory were known to have been much higher in the
recent past. The mean duration of exposure was 16.7 years. The
manganese-exposed workers showed decreased performance on tests of
motor function, and they exhibited lower levels of cognitive
flexibility, difficulty in set shifting, and lower olfactory
perception thresholds. This is the first study to report the latter
effect (lower olfactory perception threshold). The workers also
displayed significantly greater anger, tension, fatigue, and confusion
as determined by the Profile of Mood States test.
A study by Lucchini et al. (1995) also found evidence of
neurobehavioural effects at exposure levels comparable to those
reported above. During a period of forced cessation from work, 58
clinically asymptomatic workers exposed to manganese dust for periods
of 1-28 years (mean 13 years) were tested for simple reaction time,
finger tapping, digit span, additions, symbol digit, and shapes
comparison. Geometric mean concentrations of manganese in total dust
were measured in different work areas and ranged from 70-1590 µg/m3
(10 years before the study was undertaken) to 27-270 µg/m3 (at the
time of the study). A Cumulative Exposure Index was calculated for
each subject. It took into account the type of job(s) the subject
performed at the plant, the average annual airborne manganese
concentration in respirable dust characteristic of the job(s), and the
duration of employment in the job(s). The authors found correlations
between the Cumulative Exposure Index and performance on the finger
tapping, symbol digit, digit span, and additions tests; higher indices
were associated with poorer performance. In addition, the authors
found correlations between manganese levels in blood and urine of the
workers and performance (the higher the blood and urine levels, the
poorer the performance) when the levels were measured after exposure
ended. This study is significant in that it is the first to
demonstrate an association between biomarkers of exposure/body burden
and the occurrence of neurological effects.
Impotence and loss of libido are common symptoms in male workers
afflicted with clinically identifiable signs of manganism attributed
to occupational exposure to manganese for 1-21 years (Rodier, 1955;
Schuler et al., 1957; Mena et al., 1967; Emara et al., 1971). These
effects could lead to reduced reproductive success in men. Impaired
fertility (measured as a decreased number of children per married
couple) has been observed in male workers exposed for 1-19 years to
manganese dust at levels (0.97 mg/m3) that did not produce frank
manganism (Lauwerys et al., 1985). In another study, Gennart et al.
(1992) did not find an effect of manganese exposure (0.71 mg/m3 for
6.2 years on average) on fertility. Impaired sexual function in men
might be one of the earliest clinical manifestations of manganism;
however, because dose-response information is unavailable, it is not
possible to define a threshold for this effect. No information was
found regarding reproductive effects in women.
Although most effects have been seen following chronic inhalation
exposure to manganese in occupational settings, some epidemiological
studies have reported adverse effects from ingestion of excess
manganese in the environment. A manganism-like neurological syndrome
was observed in an aboriginal population living on an island near
Australia where environmental levels of manganese are high (Kilburn,
1987). Exposure levels were not provided, but the authors noted that
manganese intake could occur not only through the oral route (food,
water, soil), but also by inhaling manganese-containing dusts in the
air (Cawte et al., 1987). Although manganese exposure was probably an
etiologic factor, genetic factors, dietary deficiencies in
antioxidants and calcium, and excess alcohol consumption could also
have contributed to the neurological effects (Cawte et al., 1989).
More recently, Kondakis et al. (1989) reported that chronic
intake of drinking-water containing elevated levels of manganese
(1.8-2.3 mg/litre) led to an increased prevalence of neurological
signs in elderly residents (average age 67 years) of two small towns
in Greece. The effects were compared with those in similarly aged
residents in two other communities where manganese levels were within
ambient range (0.004 and 0.0015 mg/litre). The findings suggested that
above-average oral exposure to manganese might be of health concern.
However, although the comparison populations were reportedly very
similar to each other, differences in age, occupational exposures, or
general health status could have accounted for the small differences
observed. Similarly, Goldsmith et al. (1990) investigated a cluster of
Parkinson's disease in southern Israel. The authors suggested that
excess levels of aluminum, iron, and manganese in the drinking-water
and the use of agricultural chemicals, including maneb and paraquat,
in the area were common environmental factors that may have
contributed to the observed cluster. However, the observed symptoms
could not be conclusively attributed to manganese poisoning alone. By
contrast, a recent study by Vieregge et al. (1995) on the neurological
impacts of chronic oral intake of manganese in well-water found no
significant differences between exposed and control populations in
northern Germany. A group of 41 subjects exposed to 0.300-160 mg
manganese/litre in well-water was compared with a control group of 71
subjects (matched for age, sex, nutritional habits, and drug intake)
exposed to a maximum manganese concentration in well-water of 0.050
mg/litre. Neurological assessments revealed no significant difference
between the two groups. Although the effects reported by Kondakis et
al. (1989) and Goldsmith et al. (1990) are consistent with the known
toxicological effects of manganese, the findings are inconclusive and
are contradicted by the results of Vieregge et al. (1995). As a
result, no firm conclusions on manganese-induced neurological effects
in humans from chronic oral intake of manganese in drinking-water can
be made at this time.
One report partially attributed neurological effects to chronic
oral intake of manganese in food. Iwami et al. (1994), studying metal
content in food and drinking-water in an area with a high rate of
motor neuron disease (as determined from death certificates) compared
with control areas, concluded that a high manganese content in food
and a low magnesium content in drinking-water together explained the
high incidence of motor neuron disease. The manganese content per
1800-kcal diet averaged 6.20 mg for local rice eaters and 3.83-4.67 mg
in the control areas.
Several studies have reported an association between chronic
exposure to maneb and neurological symptoms, but the effects could not
be conclusively attributed to maneb alone. Ruijten et al. (1994)
investigated the effects of chronic exposure to mixed pesticides
(including zineb and maneb) on peripheral and autonomic nerve function
using a previously exposed group of 131 Dutch bulb farmers and a
control group of 67. The findings suggested exposure-related decreases
in both autonomic and peripheral nerve function. Ferraz et al. (1988)
reported the results of a questionnaire and neurological examination
administered to 50 rural workers in Brazil who had had close contact
with maneb (preparation and/or fumigation) for at least 6 months.
Compared with a control group, the exposed group had a significantly
higher prevalence of plastic rigidity with cogwheel phenomenon
(neurological examination), as well as headache, fatigue, nervousness,
memory complaints, and sleepiness (questionnaire). In both studies,
however, the subjects were exposed to other substances, so the effects
could not be definitively attributed to maneb. Meco et al. (1994)
reported that Parkinsonism developed in a patient 2 years after
chronic exposure to maneb had been discontinued. Initial symptoms
observed were generalized bradykinesia, rigidity, and mild tremor
associated with paraesthesias in the right leg, which subsequently
spread to the right arm. Over a 3-year period, the tremor worsened and
spread to the left limbs as well. Exposure levels were not defined in
these studies.
10. EFFECTS EVALUATION
10.1 Evaluation of health effects
10.1.1 Hazard identification and dose-response assessment
Manganism, manganic pneumonia, and male reproductive effects
(decreased libido, impotence, and decreased fertility) have been
documented following chronic inhalation of manganese-containing
respirable dusts in occupational settings (Rodier, 1955; Schuler et
al., 1957; Mena et al., 1967; Emara et al., 1971; Lauwerys et al.,
1985). More recent reports have shown subclinical changes in
neurological performance at low occupational exposure levels (Roels et
al., 1987, 1992; Iregren, 1990; Wennberg et al., 1991; Mergler et al.,
1994; Lucchini et al., 1995); it should be noted that even these low
occupational exposure levels were at least three orders of magnitude
higher than manganese levels in areas without industrial sources of
manganese. A dose-response curve has not been well defined, but early
signs of nervous system toxicity and overt manganism have been
observed after inhalation exposure to total manganese dust levels that
range from 0.14 to 1 mg/m3 for the former and from 2 to 22 mg/m3
for the latter. These neurological effects have been observed
following exposure durations that span from 1 to 35 years (Schuler et
al., 1957; Whitlock et al., 1966; Tanaka & Lieben, 1969; Cook et al.,
1974; Saric et al., 1977; Roels et al., 1987, 1992; Iregren, 1990;
Wennberg et al., 1991; Chia et al., 1993, 1995; Mergler et al., 1994;
Lucchini et al., 1995). Estimated levels of inhalation exposure to
manganese compounds have been reported as manganese in either total
dust particles or the respirable fraction, based on particle size.
Although inconclusive, limited case reports and epidemiological
studies report neurological effects associated with ingesting water
(or other media) containing elevated manganese (Kawamura et al., 1941;
Kilburn, 1987; Kondakis et al., 1989; Goldsmith et al., 1990; Iwami et
al., 1994). Reports on neurological effects following exposure to
pesticides containing manganese are similarly inconclusive (Ferraz et
al., 1988; Ruijten et al., 1994).
Some evidence suggests that the elderly might be more sensitive
than younger people to manganese (Davis & Elias, 1996). In addition,
owing to various predisposing factors, certain other individuals might
be more susceptible to adverse effects from exposure to excess
manganese. These might include people with lung disease, people who
are exposed to other lung irritants, neonates, individuals with iron
deficiency, and people with liver disease.
Available data suggest that neurological effects can occur
following chronic inhalation exposures in humans and intermediate and
chronic oral exposures in animals to different manganese compounds.
Manganese-induced neurological effects have been reported at lower
airborne manganese concentrations in humans than in animals (Bird et
al., 1984; Newland & Weiss, 1992). These data suggest that animal
models, particularly rodent species, might be less useful for defining
quantitative dose-response relationships, but helpful in elucidating
the mechanism(s) for these effects. The basis for the difference in
susceptibility across species is not yet understood and may be related
to possible differences in the sensitivity of test methods used to
detect neurobehavioural effects in animals compared with methods used
to detect neurobehavioural effects in humans.
Little is known about the relative toxicity of different
manganese compounds. Inhaled manganese compounds tend to produce more
severe toxicity than ingested manganese compounds. This is probably
attributable to the difference in route-specific uptake of manganese
from the lung (often assumed at 100%) compared with the
gastrointestinal tract (3-5%). Studies have shown that a greater
proportion of a manganese dose appears in the blood and brain of rats
exposed via inhalation or intranasal instillation than when the same
dose is given orally (Tjälve et al., 1996; Roels et al., 1997).
10.1.2 Criteria for setting guidance values for manganese
There are several approaches to the development of a guidance
value for manganese in air. A recently developed guidance value of
0.15 µg manganese/m3 (WHO, 1999) is highlighted here as one example;
other approaches are outlined in Appendix 4. The WHO (1999) guidance
value was derived from the results of the study by Roels et al.
(1992), which examined neurobehavioural end-points in 92 male workers
exposed to manganese dioxide dust at an alkaline battery plant and 101
male workers without industrial manganese exposure. The
manganese-exposed workers exhibited significantly poorer eye-hand
coordination, hand steadiness, and visual reaction time. Sufficient
data on participants' exposure levels and test performance were
provided to enable development of a dose-response relationship and
calculation of a benchmark dose. The lower 95% confidence limit of the
benchmark dose (30 µg/m3 for the 5% effect level) was used as an
estimate of a NOAEL for neurological effects. The guidance value for
manganese in air (WHO, 1999) was then derived as follows:
Guidance value = (30 µg/m3 ‰ 50) × (5/7) × (8/24)
= 0.15 µg/m3 (rounded value)
where:
* 30 µg/m3 is the estimated NOAEL for neurological effects,
calculated based on a benchmark dose analysis of results from a
quality epidemiological study of workers exposed to manganese;
* 5/7 and 8/24 are factors used to convert intermittent exposure
(5 days/week, 8 h/day) to continuous exposure; and
* 50 is the uncertainty factor (×10 for interindividual variation;
×5 for the potential for developmental effects in younger
children). The uncertainty factor for developmental effects in
younger children was obtained by analogy with lead, where
neurobehavioural effects were found in younger children at blood
lead levels five times lower than in adults; this finding was
considered to be supported by evidence from studies in animals
(WHO, 1999).
In considering development of a guidance value for oral intake of
manganese, it must be noted that there is wide variability in human
intake of manganese (from all sources) and that manganese is an
essential nutrient for humans and animals. Daily manganese intake from
food is estimated to be about 2-9 mg for adults, with an absorbed
amount of about 100-450 µg/day based upon 5% gastrointestinal
absorption (WHO, 1981). Some studies have reported that neurological
effects may be related to ingestion of manganese in non-worker
populations. However, these reports provide little information on the
levels of ingested manganese that were associated with these effects.
Although neurological effects might be a potential concern for people
working or living at or near sites where ingestion or inhalation of
high levels of manganese can occur (see section 9.2), no firm
conclusion on a guidance value level for oral intake of manganese
other than estimated daily intake levels is considered possible.
10.1.3 Sample risk characterization
A theoretical estimate of inhalation exposures for the general
population is presented based on available monitoring data on levels
of manganese in air.
Table 2 shows estimates of the average levels of manganese in
ambient air in remote, rural, and urban areas around the world (US
EPA, 1984; Stokes et al., 1988). Using these data and a daily
inhalation volume of 20 m3 for a 70-kg adult, the average estimated
daily intake of manganese from air in rural areas would be 0.8 µg/day,
and this might increase to 1.3, 1.9, and 3.3 µg/day in urban areas in
Canada, the USA, and Europe, respectively. In source-dominated areas
with manganese-emitting industries or major foundry facilities, intake
from air might rise to 4-6 µg/day (WHO, 1999). These estimates are
based on the assumptions that there is 100% absorption of inhaled
manganese and that an individual lives or works in these environments
for a complete 24-h period. Thus, these exposure estimates could be
further adjusted to reflect that fraction of a 24-h period during
which a person is actually in any of these areas. For example, a
person working for 8 h/day in an urban area of Canada, the USA, or
Europe would be exposed to an adjusted estimate of 0.43-1.1 µg
manganese during the workday (1.3-3.3 µg/day - 8/24). Consequently,
for persons living or working in rural or urban environments,
estimates of inhalation exposure would fall below or in the range of
the reported guidance values for inhaled manganese. Appendix 4
includes examples of other guideline values reported for inhaled
manganese. These values range from 0.8 µg/day (0.04 µg/m3 × 20
m3/day = 0.8 µg/day) up to an estimate of 3.0 µg inhaled
manganese/day based on the guidance value described in section 10.1.2
(0.15 µg/m3 × 20 m3/day = 3.0 µg/day). It should be noted that the
studies used in the risk assessment of inhaled manganese are
occupational studies of adult male workers, and there is uncertainty
about extrapolating the risk to women and children.
11. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
WHO (1981, 1986, 1987, 1999) has previously evaluated manganese,
concluding that chronic manganese poisoning is a hazard in
occupational settings. However, little information was available to
assess the potential health risks in community exposure scenarios.
WHO has established a provisional guideline value of 0.5 mg/litre
for manganese in drinking-water based on health (WHO, 1993), an annual
air quality guideline of 0.15 µg/m3 (WHO, 1999), and a workplace
exposure limit in air of 0.3 mg/m3 for respirable particles
containing manganese (WHO, 1984, 1986, 1987, 1999).
Information on international hazard classification and labelling
is included in the International Chemical Safety Card reproduced in
this document.
12. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventative and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0174) reproduced in this
document.
12.1 Human health hazards
Following long-term or repeated exposure to manganese, humans may
present neurological and neuropsychiatric disorders known under the
term manganism.
12.2 Advice to physicians
The psychiatric symptoms of manganese poisoning are transient.
However, the neurological damage might be irreversible, although some
patients have experienced partial regression of their symptoms after
early removal from exposure (Mena et al., 1974).
Consequently, it is very important to remove from further
exposure patients who present preclinical neurological disturbances or
symptoms of manganism. Chelation therapy with, for example, EDTA might
be effective in removing manganese from blood and tissues, but it has
no permanent effect on symptomatic patients in the late stages of
manganism (Bismuth et al., 1987; Dreisbach & Robertson, 1987;
Ellenhorn & Barceloux, 1988; Tomes, 1997). Normal manganese levels are
2-8 µg/dl in blood and 0.1-0.8 µg/dl in urine. Plasma and urine
manganese levels do not seem to correlate well with the severity of
symptoms.
12.3 Health surveillance programme
The health surveillance programme of people exposed to manganese
needs to include elements such as central nervous system disturbances
(asthenia, anorexia, sleep problems, irritability, diminished libido).
13. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards
can be found in the International Register of Potentially Toxic
Chemicals (IRPTC), available from UNEP Chemicals (IRPTC), Geneva.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
MANGANESE ICSC:0174
March 1995
CAS# 7439-96-5
RTECS# 00927500
(powder)
Mn
Atomic mass: 54.9
TYPES OF HAZARD/ ACUTE HAZARDS/ PREVENTION FIRST AID/
EXPOSURE SYMPTOMS FIRE FIGHTING
FIRE Combustible No open Dry sand,
flames special powder
EXPLOSION Finely dispersed Prevent deposition
particles form of dust, closed
explosive mixtures system, dust
in air explosion-proof
electrical equipment
and lighting.
EXPOSURE PREVENT DISPERSION
OF DUST! AVOID
EXPOSURE OF (PREGNANT)
WOMEN!
Inhalation Cough, shortness Local exhaust or Fresh air, rest.
of breath. breathing protection. Refer for medical
attention.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
Skin
Eyes Safety goggles or First rinse with
eye protection in plenty of water
combination with for several
breathing protection minutes (remove
if powder. contact lenses if
easily possible),
then take to a
doctor.
Ingestion Abdominal pain. Do not eat, drink, Rinse mouth.
Nausea. or smoke during Refer for medical
work. Wash hands attention.
before eating.
SPILLAGE DISPOSAL PACKAGING & LABELLING
Sweep spilled substance into containers. EU Classification
Carefully collect remainder, then Symbol:
remove to safe place (extra personal UN Classification
protection: P2 filter respirator
for harmful particles).
EMERGENCY RESPONSE STORAGE
Separated from acids. Dry
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
IMPORTANT DATA
PHYSICAL STATE; APPEARANCE ROUTES OF EXPOSURE:
GRAY-WHITE POWDER The substance can be absorbed into the
the body by inhalation of its aerosol or
fumes, and by ingestion.
PHYSICAL DANGERS: INHALATION RISK:
Dust explosion possible if in powder Evaporation at 20°C is negligible;
or granular form, mixed with air. a harmful concentration of airborne
particles can, however, be reached
quickly when dispersed.
CHEMICAL DANGERS: EFFECTS OF SHORT TERM EXPOSURE:
Upon heating, toxic fumes are formed. Inhalation of dust may cause bronchitis
Reacts violently with concentrated and pneumonia. The effects may be
hydrogen peroxide. Reacts slowly with delayed.
water more rapidly with steam and
acids to produce flammable gas
(hydrogen see - ICSC # 0001) causing
fire and explosion hazard. Burns in
nitrogen oxide above 200°C.
OCCASIONAL EXPOSURE LIMITS: EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
TLV: ppm; 0.2 mg/m3 (ACGIH 1996). The substance may have effects on the
MAK: ppm; 0.5 mg/m3 (1994). lungs and nervous system, resulting in
bronchitis, pneumonia, neurologic and
neuropsychiatric disorders (manganism).
Animal tests show that this substance
possibly causes toxic effects upon
human reproduction.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
PHYSICAL PROPERTIES
Boiling point: 1962 °C
Melting point: 1244 °C
Relative density (water = 1): 7.2-7.4
Solubility in water: none
ENVIRONMENTAL DATA
NOTES
Depending on the degree of exposure, periodic medical examination is indicated.
The recommendations on this Card also apply to ferro manganese.
ADDITIONAL INFORMATION
LEGAL NOTICE Neither the CEC nor the IPCS nor any person acting on behalf of
the CEC or the IPCS is responsible for the use which might be
made of this information.
REFERENCES
Abbasi SA (1988) Synergistic extraction and atomic absorption
spectrometric determination of manganese in environmental samples
using N- p-aminophenyl-2-furyl-acrylohydroxamic acid and
trioctylmethyl ammonium cation. Analytical letters,
21(10):1935-1944.
Abdel-Hamid MM, El-Desoky SA, Magdi SM (1990) Estimation of manganese
in blood between exposed workers to different concentrations at
industrial units. Egyptian journal of pharmaceutical sciences,
31(1-4):143-150.
Adkins B, Luginbuhl HG, Miller JF, Gardner DE (1980) Increased
pulmonary susceptibility to streptococcal infection following
inhalation of manganese oxide. Environmental research, 23:110-120.
Akbar-Khanzadeh F (1993) Short-term respiratory function changes in
relation to workshift welding fume exposures. International archives
of occupational and environmental health, 64(6):393-397.
Alessio L, Lucchini R (1996) Manganese and manganese compounds. In:
Argentesi F, Roi R, Sevilla Marcos JM, eds. Data profiles for
selected chemicals and pharmaceuticals 3, 4, 5. Ispra, Italy,
European Commission, Joint Research Commission.
Ali MM, Murthy RC, Mandal SK, Chandra SV (1985) Effect of low protein
diet on manganese neurotoxicity. III. Brain neurotransmitter levels.
Neurobehavioral toxicology and teratology, 7:427-431.
ATSDR (1996) Toxicological profile for manganese (update). Draft
for public comment. Atlanta, GA, US Department of Health and Human
Services, Public Health Service, Agency for Toxic Substances and
Disease Registry.
Baes CF III, Sharp RD (1983) A proposal for estimation of soil
leaching and leaching constants for use in assessment models.
Journal of environmental quality, 12:17-28.
Barbeau A (1984) Manganese and extrapyramidal disorders.
Neurotoxicology, 5(2):13-36.
Bergstrom R (1977) Acute pulmonary toxicity of manganese dioxide.
Scandinavian journal of work and environmental health, 3 (Suppl.
1):1-40.
Beuter A, Mergler D, de Geoffroy A, Belanger S, Carriere L, Varghese
L, Skeekumar J, Gauthier S (1994) Diadochokinesimetry: a study of
patients with Parkinson's Disease and manganese-exposed workers.
Neurotoxicology, 15(3):655-664.
Bird ED, Anton AH, Bullock B (1984) The effect of manganese inhalation
on basal ganglia dopamine concentrations in rhesus monkey.
Neurotoxicology, 5:59-65.
Bismuth C, Baud F, Conso F, Fréjaville JP, Garnier R (1987)
Toxicologie clinique. Médecines-Sciences, Flammarion, p. 509.
Bonilla E (1978) Increased GABA content in caudate nucleus of rats
after chronic manganese chloride administration. Journal of
neurochemistry, 31:551-552.
Bonilla E, Prasad AL (1984) Effects of chronic manganese intake on the
levels of biogenic amines in rat brain regions. Neurobehavioral
toxicology and teratology, 6:341-344.
Calabrese EJ, Barnes R, Stanek EJ, Pastides H, Gilbekt CE, Veneman P,
Xioaru W, Lasztity A, Kostecki PT (1989) How much soil do young
children ingest: an epidemiologic study. Regulatory toxicology and
pharmacology, 10:123-137.
Calne DB, Chu N-S, Huang C-C, Lu C-S, Olanow W (1994) Manganese and
idiopathic parkinsonism: Similarities and differences. Neurology,
44:1583-1586.
Casto BC, Meyers J, DiPaulo JA (1979) Enhancement of viral
transformation for evaluation of the carcinogenic or mutagenic
potential of inorganic metal salts. Cancer research, 39:193-198.
Cawte J, Hams G, Kilburn C (1987) Manganism in a neurological ethnic
complex in northern Australia [letter]. Lancet, (May 30):1257.
Cawte J, Kilburn C, Florence M (1989) Motor neurone disease of the
Western Pacific: do the foci extend to Australia? Neurotoxicology,
10:263-270.
Chandra SV (1983) Psychiatric illness due to manganese poisoning.
Acta Psychiatrica Scandinavica, 67 (Suppl. 303):49-54.
Chandra SV, Shukla GS (1978) Manganese encephalopathy in growing rats.
Environmental research, 15:28-37.
Chandra SV, Saxena DK, Hasan MZ (1975) Effect of zinc on manganese
induced testicular injury in rats. Industrial health, 13:51-56.
Chia S-E, Foo S-C, Gan S-L, Jeyaratnam J (1993) Neurobehavioral
functions among workers exposed to manganese ore. Scandinavian
journal of work and environmental health, 19:264-270.
Chia S-E, Gan S-L, Chua L-H, Foo S-C, Jeyaratnam J (1995) Postural
stability among manganese exposed workers. Neurotoxicology,
16(3):519-526.
Chin CS, Johnson KS, Coale KH (1992) Spectrophotometric determination
of dissolved manganese in natural waters with
1-(2-pyridylazo)-2-naphthol: application to analysis in situ in
hydrothermal plumes. Marine chemistry, 37(1-2):65-82.
Chu N, Hochberg F, Calne D, Olanow C (1995) Neurotoxicology of
manganese. In: Chang L, Dyyer R, eds. Handbook of neurotoxicology of
manganese. New York, NY, Marcel Dekker, Inc., pp. 91-103.
Coenen W, Lambert J, Schenk H (1989) Gefahrstoffe an gie
bereiarbeitsplatzen bericht. Sankt Augustin,
Berufsgenossenschaftliches Institut fur Arbeitssicherheit (BIA).
Collipp PJ, Chen SY, Maitinsky S (1983) Manganese in infant formulas
and learning disability. Annals of nutrition and metabolism,
27:488-494.
Cook DG, Fahn S, Brait KA (1974) Chronic manganese intoxication.
Archives of neurology, 30:59-64.
Cooper WC (1984) The health implications of increased manganese in the
environment resulting from the combustion of fuel additives: a review
of the literature. Journal of toxicology and environmental health,
14:23-46.
Curtin D, Ryan J, Chaudhary RA (1980) Manganese adsorption and
desorption in calcareous Lebanese soils. Journal of the Soil Science
Society of America, 44:947-950.
Davidsson L, Cederblad A, Hagebo E, Lonnerdal B, Sandström B (1988)
Intrinsic and extrinsic labeling for studies of manganese absorption
in humans. Journal of nutrition, 118:1517-1524.
Davis CD, Malecki EA, Greger JL (1992) Interactions among dietary
manganese, heme iron and non-heme iron in women. American journal of
clinical nutrition, 56:926-932.
Davis DW, Hsiao K, Ingels R, Shiklya J (1988) Origins of manganese in
air particulates in California. Journal of the Air Pollution Control
Association, 38:1152-1157.
Davis JM (1998) Methylcyclopentadienyl manganese tricarbonyl (MMT):
health risk uncertainties and research directions. Environmental
health perspectives, 106 (Suppl. 1):191-201.
Davis JM, Elias RW (1996) Risk assessment of metals. In: Chang LW, ed.
Toxicology of metals. Boca Raton, FL, CRC Lewis Publishers.
De Méo M, Laget M, Castegnaro M, Dumenil G (1991) Genotoxic activity
of potassium permanganate in acidic solutions. Mutation research,
260(3):295-306.
Deskin R, Bursian SJ, Edens FW (1980) Neurochemical alterations
induced by manganese chloride in neonatal rats. Neurotoxicology,
2:65-73.
Deverel SJ, Millard SP (1988) Distribution and mobility of selenium
and other trace elements in shallow groundwater of the western San
Joaquin Valley, CA. Environmental science and technology,
22:697-702.
Dikshith TS, Chandra SV (1978) Cytological studies in albino rats
after oral administration of manganese chloride. Bulletin of
environmental contamination and toxicology, 19:741-746.
Doisy EA (1972) Effects of deficiency of manganese upon plasma levels
of clotting proteins and cholesterol in man. In: Hoekstra WG, Suttie
JW, Gantner ME, eds. Trace element metabolism in animals. Vol. 2.
Baltimore, MD, University Park Press, pp. 668-670.
Dreisbach RH, Robertson WO (1987) Handbook of poisoning: prevention,
diagnosis & treatment, 12th ed. Norwalk, CT, Appleton & Lange, 589
pp.
Durfor CN, Becker E (1964) Public water supplies of the 100 largest
cities in the United States, 1962. Washington, DC, US Department of
the Interior, Geological Survey (Water-Supply Paper 1812).
Eckel WP, Langley WD (1988) A background-based ranking technique for
assessment of elemental enrichment in soils at hazardous waste sites.
In: Superfund '88: Proceedings of the 9th National Conference, 28-30
November 1988, Washington, DC. Silver Spring, MD, The Institute, pp.
282-286.
Egyed M, Wood GC (1996) Risk assessment for combustion products of the
gasoline additive MMT in Canada. Science of the total environment,
189/190:11-20.
Ellenhorn MJ, Barceloux DG (1988) Ellenhorn's medical toxicology:
diagnosis and treatment of human poisoning. New York, NY, Elsevier,
1512 pp.
EM (1993) The economics of manganese, 7th ed. London, Roskill
Information Services, Ltd.
Emara AM, El-Ghawabi HS, Madkour IO, El-Samara GH (1971) Chronic
manganese poisoning in the dry battery industry. British journal of
industrial medicine, 28:78-82.
Eriksson H, Lenngren S, Heilbronn E (1987) Effect of long-term
administration of manganese on biogenic amine levels in discrete
striatal regions of rat brain. Archives of toxicology, 59:426-431.
Eriksson H, Gillberg P, Aquilonius S, Hedstrom KG, Heilbronn E (1992)
Receptor alterations in manganese intoxicated monkeys. Archives of
toxicology, 66:359-364.
Ferraz HB, Bertolucci PHF, Pereira JS, Lima JGC, Andrade LAF (1988)
Chronic exposure to the fungicide maneb may produce symptoms and signs
of CNS manganese intoxication. Neurology, 38(4):550-553.
Folsom TR, Young DR, Johnson JN, Pillai KC (1963) Manganese-54 and
zinc-65 in coastal organisms of California. Nature, 200:327-329.
Francis AJ (1985) Anaerobic microbial dissolution of toxic metals in
subsurface environments. Upton, NY, Brookhaven National Laboratory
(BNL-36571).
Francis CW, White GH (1987) Leaching of toxic metals from incinerator
ashes. Journal of the Water Pollution Control Federation,
59:979-986.
Freeland-Graves J, Llanes C (1994) Models to study manganese
deficiency. In: Klimis-Tavantzis DJ, ed. Manganese in health and
disease. Boca Raton, FL, CRC Press, pp. 59-86.
Freeland-Graves JH, Bales CW, Behmardi F (1987) Manganese requirements
of humans. In: Kies C, ed. Nutritional bioavailability of manganese.
Washington, DC, American Chemical Society.
Friedman BJ, Freeland-Graves JH, Bales CW, Behmardi F, Shorey-Kutschke
RL, Willis RA, Crosby JB, Trickett PC, Houston SD (1987) Manganese
balance and clinical observations in young men fed a
manganese-deficient diet. Journal of nutrition, 117:133-143.
Furst A (1978) Tumorigenic effect of an organomanganese compound on
F344 rats and Swiss albino mice [brief communication]. Journal of
the National Cancer Institute, 60:1171-1173.
Galloway SM, Armstrong MJ, Reuben C, Colman S, Brown B, Cannon C,
Bloom AD, Nakamura F, Ahmed M, Duk S, Rimpo J, Margolin BH, Resnick
MA, Anderson B, Zeiger E (1987) Chromosome aberrations and sister
chromatid exchanges in Chinese hamster ovary cells: Evaluations of 108
chemicals. Environmental and molecular mutagenesis, 10 (Suppl.
10):1-175.
Garrison AW, Cipollone MG, Wolfe NL, Swank RR (1995) Environmental
fate of methylcyclopentadienyl manganese tricarbonyl. Environmental
toxicology and chemistry, 14(11):1859-1864.
Geering HR, Hodgson JF, Sdano C (1969) Micronutrient cation complexes
in soil solution. IV. The chemical state of manganese in soil
solution. Soil Science Society of America, proceedings, 33:81-85.
Gennart J-P, Buchet J-P, Roels H, Ghyselen P, Ceulemans E, Lauwerys R
(1992) Fertility of male workers exposed to cadmium, lead, or
manganese. American journal of epidemiology, 135(2):1208-1219.
Gianutsos G, Murray MT (1982) Alterations in brain dopamine and GABA
following inorganic or organic manganese administration.
Neurotoxicology, 3:75-81.
Glass E (1955) Untersuchungen uber die einwirkung von
schwermetallsalzen auf dir wurzelspitzenmitose von Vicia faba.
Zeitschrift fuer Botanik, 43:359-403.
Glass E (1956) Untersuchungen uber die einwirkung von
schwermetallsalzen auf dir wurzelspitzenmitose von Vicia faba.
Zeitschrift fuer Botanik, 44:1-58.
Goldsmith J, Herishanu Y, Abarbanel J, Weinbaum Z (1990) Clustering of
Parkinson's disease points to environmental etiology. Archives of
environmental health, 45:88-94.
Graedel TE (1978) Inorganic elements, hydrides, oxides, and
carbonates. In: Chemical compounds in the atmosphere. New York, NY,
Academic Press, pp. 35-41, 44-49.
Grant D, Ege T (1995) Teratogenicity in the rat after repeated
intravenous injection of manganese, either as a complex (mangafodipir
trisodium, MNDPDP) or as the inorganic chloride. Toxicology,
15(1):160.
Gray EL, Laskey JW (1980) Multivariate analysis of the effects of
manganese on the reproductive physiology and behavior of the male
house mouse. Journal of toxicology and environmental health,
6:861-867.
Hamilton K (1995) The Australian directory of registered pesticides
and their uses. Brisbane, Australia, University of Queensland,
Centre for Pesticide Application and Safety.
Health Canada (1994) Canadian Environmental Protection Act. Human
health risk assessment for priority substances. Ottawa, ON, Minister
of Supply and Services Canada.
Hejtmancik M, Peters AC, Toft JD, Basaran A, Sheridan A, Athey P
(1987a) The chronic study of manganese sulfate monohydrate (CAS No.
10034-96-5) in F344 rats. Report to National Toxicology Program,
Research Triangle Park, NC, by Battelle's Columbus Laboratories,
Columbus, OH.
Hejtmancik M, Peters AC, Toft JD, Dersing R, Basaran A, Sheridan A,
Athey P (1987b) The chronic study of manganese sulfate monohydrate
(CAS No. 10034-96-5) in B6C3F1 mice. Report to National Toxicology
Program, Research Triangle Park, NC, by Battelle's Columbus
Laboratories, Columbus, OH.
Hellou J, Fancey LL, Payne JF (1992) Concentrations of twenty-four
elements in bluefin tuna, Thunnus thynnus, from the Northwest
Atlantic. Chemosphere, 24(2):211-218.
Hemstock GA, Low PF (1953) Mechanisms responsible for retention of
manganese in the colloidal fraction of soil. Soil science,
76:331-343.
Hochberg F, Miller G, Valenzuela R, McNelis S, Crump KS, Covington T,
Valdivia G, Hochberg B, Trustman JW (1996) Late motor deficits of
Chilean manganese miners: a blinded control study. Neurology,
47:788-795.
Holbrook DJ Jr, Washington ME, Leake HB, Brubaker PE (1975) Studies on
the evaluation of the toxicity of various salts of lead, manganese,
platinum, and palladium. Environmental health perspectives,
10:95-101.
Holzgraefe M, Poser W, Kijewski H, Beuche W (1986) Chronic enteral
poisoning caused by potassium permanganate: a case report. Journal
of toxicology and clinical toxicology, 24:235-244.
HSDB (1998) Hazardous substances data bank. Bethesda, MD, National
Institutes of Health, National Library of Medicine.
Huang CC, Chu NS, Lu CS, Wang JD, Tsai JL, Tzeng JL, Wolters EC, Calne
DB (1989) Chronic manganese intoxication. Archives of neurology,
46:1104-1106.
Hurley LS, Keen CL (1987) Manganese. In: Mertz W, ed. Trace elements
in human and animal nutrition, 5th ed. San Diego, CA, Academic
Press, Inc.
Ikarashi Y, Tsuchiya T, Nakamura A (1992) Detection of contact
sensitivity of metal salts using the murine local lymph node assay.
Toxicology letters, 62(10):53-61.
Imam Z, Chandra VS (1975) Histochemical alterations in rabbit testis
produced by manganese chloride. Toxicology and applied pharmacology,
32:534-544.
IPCS (1993) International Chemical Safety Card -- Manganese. Geneva,
World Health Organization, International Programme on Chemical Safety
(ICSC 0174).
Iregren A (1990) Psychological test performance in foundry workers
exposed to low levels of manganese. Neurotoxicology and teratology,
12:673-675.
Ishizuka H, Nishida M, Kawada J (1991) Changes in stainability
observed by light microscopy in the brains of ataxial mice subjected
to three generations of manganese administration. Biochemistry
international, 25(4):677-687.
Iwami O, Watanabe T, Moon C-S, Nakatsuka H, Ikeda M (1994) Motor
neuron disease on the Kii Peninsula of Japan: Excess manganese intake
from food coupled with low magnesium in drinking water as a risk
factor. Science of the total environment, 149:121-135.
Jacques AP (1984) National inventory of sources and emissions of
manganese -- 1984. Ottawa, ON, Environment Canada, Conservation and
Protection, Environmental Protection.
Jaudon P, Massiani C, Galea J, Rey J, Vacelet E (1989) Groundwater
pollution by manganese. Manganese speciation: application to the
selection and discussion of an in situ groundwater treatment.
Science of the total environment, 84:169-183.
Joardar M, Sharma A (1990) Comparison of clastogenicity of inorganic
Mn administered in cationic and anionic forms in vivo. Mutation
research, 240(3):159-163.
Kabata-Pendias A, Pendias H (1984) Trace elements in soils and
plants. Boca Raton, FL, CRC Press, Inc.
Kawamura R, Ikuta H, Fukuzumi S, Yamada R, Tsubaki S (1941)
Intoxication by manganese in well water. Kitasato archives of
experimental medicine, 18:145-171.
Kellar KE, Foster N (1991) Determination of the relative amounts of
free and complexed manganese ions in aqueous solution by nuclear
magnetic resonance. Analytical chemistry, 63(24):2919-2924.
Kilburn CJ (1987) Manganese, malformations and motor disorders:
findings in a manganese-exposed population. Neurotoxicology,
8:421-429.
Kleinman MT, Pasternack BS, Eisenbud M, Kneip TJ (1980) Identifying
and estimating the relative importance of airborne particulates.
Environmental science and technology, 14:62-65.
Komura J, Sakamoto M (1991) Short-term oral administration of several
manganese compounds in mice: physiological and behavioral alterations
caused by different forms of manganese. Bulletin of environmental
contamination and toxicology, 46(6):921-928.
Komura J, Sakamoto M (1992) Effects of manganese forms on biogenic
amines in the brain and behavioral alterations in the mouse: long-term
oral administration of several manganese compounds. Environmental
research, 57(1):34-44.
Kondakis XG, Makris N, Leotsinidis M, Prinou M, Papapetropoulos T
(1989) Possible health effects of high manganese concentration in
drinking water. Archives of environmental health, 44:175-178.
Kontur PJ, Fechter LD (1985) Brain manganese, catecholamine turnover,
and the development of startle in rats prenatally exposed to
manganese. Teratology, 32:1-11.
Kostial K, Blanusa M, Maljkovic T, Kello D, Rabar I, Stara JF (1989)
Effect of a metal mixture in diet on the toxicokinetics and toxicity
of cadmium, mercury and manganese in rats. Toxicology and industrial
health, 5(5):685-698.
Kristensson K, Eriksson H, Lundh B, Plantin L-O, Wachtmeister L, El
Azazi M, Morath C, Heilbronn E (1986) Effects of manganese chloride on
the rat developing nervous system. Acta Pharmacologica et
Toxicologica, 59:345-348.
Kucera J, Soukal L, Faltejsek J (1986) Low level determination of
manganese in biological reference materials by neutron activation
analysis. Journal of radioanalytical nuclear chemistry,
107(6):361-369.
Ky S, Deng H, Xie P, Hu W (1992) A reprot of two chronic cases of
serious manganese poisoning treated with sodium para-aminosalicylic
acid. British journal of industrial medicine, 49:66-69.
Lai JC, Leung TK, Lim L (1984) Differences in the neurotoxic effects
of manganese during development and aging: some observations on brain
regional neurotransmitter and non-neurotransmitter metabolism in a
developmental rat model of chronic manganese encephalopathy.
Neurotoxicology, 5:37-47.
Laitung JK, Mercer DM (1983) Manganese absorption through a burn.
Burns, including thermal injuries, 10:145-146.
Laskey JW, Rehnberg GL, Hein JF (1982) Effects of chronic manganese
(Mn3O4) exposure on selected reproductive parameters in rats.
Journal of toxicology and environmental health, 9:677-687.
Lauwerys R, Roels H, Genet P, Toussaint G, Bouchaerta A, Cooman S
(1985) Fertility of male workers exposed to mercury vapor or to
manganese dust: a questionnaire study. American journal of
industrial medicine, 7:171-176.
Lavi N, Lux F, Al-Fassi ZB (1989) Determination of magnesium,
aluminum, phosphorus, copper and manganese in biological fluids by
neutron activation analysis. Journal of radioanalytical nuclear
chemistry, 129(1):93-102.
Leach RM, Lilburn SM (1978) Manganese metabolism and its function.
World review of nutrition and diet, 32:123-134.
Lide DR (1993) CRC handbook of chemistry and physics. Boca Raton, FL,
Chemical Rubber Publishing Company, pp. 4, 34-35, 113.
Lioy PJ (1983) Air pollution emission profiles of toxic and trace
elements from energy related sources: status and needs.
Neurotoxicology, 4(3):103-112.
Lloyd Davies TA (1946) Manganese pneumonitis. British journal of
industrial medicine, 3:111-135.
Loranger S, Zayed J (1994) Manganese and lead concentrations in
ambient air and emission rates from unleaded and leaded gasoline
between 1981 and 1992 in Canada: a comparative study. Atmospheric
environment, 28(9):1645-1651.
Loranger S, Zayed J (1995) Contribution of methylcyclopentadienyl
manganese tricarbonyl (MMT) to atmospheric Mn concentrations near
expressway: dispersion modeling estimations. Atmospheric
environment, 29(5):591-599.
Loranger S, Zayed J (1997) Environmental contamination and human
exposure to airborne total and respirable manganese in Montreal. Air
and waste management, 47:983-989.
Loranger S, Zayed J, Forget E (1994) Manganese contamination in
Montreal in relation with traffic density. Water, air and soil
pollution, 74:385-396.
Lown BA, Morganti JB, D'Agostino R, Stineman CH, Massaro EJ (1984)
Effects on the postnatal development of the mouse of preconception,
postconception and/or suckling exposure to manganese via maternal
inhalation exposure to MnO2 dust. Neurotoxicology, 5:119-129.
Lucchini R, Selis L, Folli D, Apostoli P, Mutti A, Vanoni O, Iregren
A, Alessio L (1995) Neurobehavioral effects of manganese in workers
from a ferroalloy plant after temporary cessation of exposure.
Scandinavian journal of work and environmental health, 21:143-149.
Maigetter RZ, Ehrlich R, Fenters JD, Gardner DE (1976) Potentiating
effects of manganese dioxide on experimental respiratory infections.
Environmental research, 11:386-391.
MAK (1994) Manganese and its inorganic compounds. Weinheim, Germany,
MAK - Deutsche Forschungsgemeinschaft, VCH, mbh D-69451 (English
translation).
Malm O, Pfeiffer WC, Fiszman M, Azcue JM (1988) Transport and
availability of heavy metals in the Paraiba do Sul-Guandu River
system, Rio de Janeiro State, Brazil. Science of the total
environment, 75:201-209.
McBride MB (1979) Chemisorption and precipitation of Mn2+ at CaCO3
surfaces. Journal of the Soil Science Society of America,
43:693-698.
Meco G, Bonifati V, Vanacore N, Fabrizio E (1994) Parkinsonism after
chronic exposure to the fungicide maneb (manganese
ethylene-bis-dithiocarbamate). Scandinavian journal of work and
environmental health, 20(4):301-305.
Mena I, Marin O, Fuenzalida S, Cotzias GC (1967) Chronic manganese
poisoning: clinical picture and manganese turnover. Neurology,
17:128-136.
Mena I, Horiuchi K, Burke K, Cotzias GC (1969) Chronic manganese
poisoning: individual susceptibility and absorption of iron.
Neurology, 19:1000-1006.
Mena I, Horiuchi K, Lopez G (1974) Factors enhancing entrance of
manganese into the brain: iron deficiency and age. Journal of
nuclear medicine, 15:516.
Mergler D, Baldwin M (1997) Early manifestations of manganese
neurotoxicity in humans: an update. Environmental research,
78(1-2):92-100.
Mergler D, Huel G, Bowler R, Iregren A, Bélanger S, Baldwin M, Tardif
R, Smargiassi A, Martin L (1994) Nervous system dysfunction among
workers with long-term exposure to manganese. Environmental
research, 64(2):151-180.
Mirowitz SA, Westrich TJ, Hirsch JD (1991) Hyperintense basal ganglia
on T1-weighted MR images in patients receiving parenteral nutrition.
Radiology, 181:117-120.
Morganti JB, Lown BA, Stineman CH, D'Agostino RB, Massaro EJ (1985)
Uptake, distribution and behavioral effects of inhalation exposure to
manganese (MnO2) in the adult mouse. Neurotoxicology, 6:1-15.
Mori I, Fujita Y, Ikuta K, Nakahashi Y, Kato K (1989) [Highly
sensitive and selective fluorimetric methods for the determination of
iron (III) and manganese (II) using fluorescein-hydrogen
peroxide-triethylenetetramine and fluorescein-hydrogen
peroxide-triethylenetetramine/iron, respectively.] Fresenius
Zeitschrift fuer Analytische Chemie, 334(3):252-255 (in German).
Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B, Zeiger E (1986)
Salmonella mutagenicity tests: II. Results from testing of 270
chemicals. Environmental mutagenesis, 8:1-26.
Motolese A, Truzzi M, Giannini A, Seidenari S (1993) Contact
dermatitis and contact sensitization among enamellers and decorators
in the ceramics industry. Contact dermatitis, 28(2):59-62.
Nachtman JP, Tubben RE, Commissaris RL (1986) Behavioral effects of
chronic manganese administration in rats: locomotor activity studies.
Neurobehavioral toxicology and teratology, 8:711-715.
NAS (1973) Medical and biological effects of environmental
pollutants: manganese. Washington, DC, National Academy of Sciences,
National Academy Press.
NAS (1977) Drinking water and health. Washington, DC, National
Academy of Sciences, National Academy Press, pp. 214-215, 265-270,
311-312.
NAS (1980) Drinking water and health. Vol. 3. Washington, DC,
National Academy of Sciences, National Academy Press, pp. 331-337.
Newland MC, Weiss B (1992) Persistent effects of manganese on
effortful responding and their relationship to manganese accumulation
in the primate globus pallidus. Toxicology and applied pharmacology,
113(1):87-97.
NRC (1989) Recommended dietary allowances, 10th ed. Washington, DC,
National Research Council, pp. 231-235.
NTP (1993) Toxicology and carcinogenesis studies of manganese (II)
sulfate monohydrate in F344/N rats and B6C3F1 mice. Research
Triangle Park, NC, US Department of Health and Human Services,
National Toxicology Program (NTP TR 428).
NTP (1999) NTP chemical health and safety data. On-line retrieval
(25 May 1999) from the National Toxicology Program, US Department of
Health and Human Services, website:
http://ntp-server.niehs.nih.gov/Main_Pages/Chem-HS.html.
Oberly TJ, Piper CE, McDonald DS (1982) Mutagenicity of metal salts in
the L5178Y mouse lymphoma assay. Journal of toxicology and
environmental health, 9:367-376.
Oner G, Senturk UK (1995) Reversibility of manganese-induced learning
defect in rats. Food and chemical toxicology, 33(7):559-563.
Pagano DA, Zeiger E (1992) Conditions for detecting the mutagenicity
of divalent metals in Salmonella typhimurium. Environmental and
molecular mutagenesis, 19(2):139-146.
Patterson KY, Holbrook JF, Bodner JE, Kelsay JL, Smith JC, Veillon C
(1984) Zinc, copper, and manganese intake and balance for adults
consuming self-selected diets. American journal of clinical
nutrition, 40:1397-1403.
Pellizari ED, Thomas KW, Clayton CA, Whitmore RW, Shores RC, Zelon HS,
Perritt RL (1992) Particle total exposure assessment methodology
(PTEAM): Riverside, California pilot study. Vol. I [final report].
Research Triangle Park, NC, US Environmental Protection Agency,
Atmospheric Research and Exposure Assessment Laboratory (EPA Report
No. EPA/600/R-93/050; available from NTIS, Springfield, VA:
PB93-166957/XAB).
Pennington JAT, Young BE, Wilson DB, Johnson RD, Vanderveen JE (1986)
Mineral content of foods and total diets: The selected minerals in
foods survey, 1982 to 1984. Journal of the American Medical
Association, 86:876-891.
Pflaumbaum W, Blome H, Heidermanns G (1990) [Presence of manganese and
manganese compounds at work-places.] Staub-Reinhalt Luft,
50(9):307-310 (in German).
Ponnamperuma FN, Loy TA, Tianco EM (1969) Redox equilibria in flooded
soils. II. The manganese oxide systems. Soil science, 108:48-57.
Rai D, Zachara JM, Schwab AP, Schmidt RL, Girvin DC, Rogers JE (1986)
Manganese. In: Chemical attenuation rates, coefficients, and
constants in leachate migration. Vol. 1. A critical review. Report
to Electric Power Research Institute, Palo Alto, CA, by Battelle,
Pacific Northwest Laboratories, Richland, WA, pp. 15-1-15-4.
Rasmuson A (1985) Mutagenic effects of some water-soluble metal
compounds in a somatic eye-color test system in Drosophila
melanogaster. Mutation research, 157:157-162.
Rehnberg LG, Hein FJ, Carter DS, Linko RS, Laskey JW (1980) Chronic
manganese oxide administration to preweanling rats: manganese
accumulation and distribution. Journal of toxicology and
environmental health, 6:217-226.
Rehnberg LG, Hein FJ, Carter DS, Linko RS, Laskey JW (1981) Chronic
ingestion of Mn3O4 by young rats: tissue accumulation, distribution,
and depletion. Journal of toxicology and environmental health,
7:263-272.
Resing JA, Mottl MJ (1992) Determination of manganese in seawater
using flow injection analysis with on-line preconcentration and
spectrophotometric detection. Analytical chemistry,
64(22):2682-2687.
Rodier J (1955) Manganese poisoning in Moroccan miners. British
journal of industrial medicine, 12:21-35.
Roels H, Lauwerys R, Buchet JP, Genet P, Sarhan MJ, Hanotiau I, deFays
M, Bernard A, Stanescu D (1987) Epidemiological survey among workers
exposed to manganese: effects on lung, central nervous system, and
some biological indices. American journal of industrial medicine,
11:307-327 [Erratum (1987), American journal of industrial medicine,
12:119-120].
Roels HA, Ghyselen P, Buchet JP, Ceulemans E, Lauwerys RR (1992)
Assessment of the permissible exposure level to manganese in workers
exposed to manganese dioxide dust. British journal of industrial
medicine, 49(1):25-34.
Roels H, Meiers G, Delos M, Ortega P, Lauwerys R, Buchet JP, Lison D
(1997) Influence of the route of administration and the chemical form
(MnCl2, MnO2) on the absorption and cerebral distribution of
manganese in rats. Archives of toxicology, 71:223-230.
Rope SK, Arthur WJ, Craig TH, Craig EH (1988) Nutrient and trace
elements in soil and desert vegetation of southern Idaho.
Environmental monitoring and assessment, 10:1-24.
Rosenstock HA, Simons GD, Meyer SJ (1971) Chronic manganism:
neurologic and laboratory studies during treatment with levodopa.
Journal of the American Medical Association, 217:1354-1358.
Ruijten MWMM, Sall HJA, Verberk MM, Smink M (1994) Effect of chronic
mixed pesticide exposure on peripheral and autonomic nerve function.
Archives of environmental health, 49(3):188-195.
Sánchez DJ, Domingo JL, Llobet JM, Keen CL (1993) Maternal and
developmental toxicity of manganese in the mouse. Toxicology
letters, 69:45-52.
Sarhan MJ, Roels H, Lauwerys R (1986) Influence of manganese on the
gastrointestinal absorption of cadmium in rats. Journal of applied
toxicology, 6(5):313-316.
Saric M, Markicevic A, Hrustic O (1977) Occupational exposure to
manganese. British journal of industrial medicine, 34:114-118.
Sax NI, Lewis RJ Sr (1987) Hawley's condensed chemical dictionary,
11th ed. New York, NY, Van Nostrand Reinhold Company, pp. 727-731.
Schaanning M, Naes K, Egeberg PK, Bome F (1988) Cycling of manganese
in the permanently anoxic Drammens fjord. Marine chemistry,
23:365-382.
Schnitzer M (1969) Reactions between fulvic acid, a soil humic
compound and inorganic soil constituents. Soil Science Society of
America, proceedings, 33:75-80.
Schroeder HA, Balassa JJ, Tipton IH (1966) Essential trace metals in
man: manganese. A study in homeostasis. Journal of chronic diseases,
19:545-571.
Schroeder WH, Dobson M, Kane DM (1987) Toxic trace elements associated
with airborne particulate matter: a review. Journal of the Air
Pollution Control Association, 37:1267-1285.
Schuler P, Oyanguren H, Maturana V, Valenzuela A, Cruz E, Plaza V,
Schmidt E, Haddad R (1957) Manganese poisoning: environmental and
medical study at a Chilean mine. Industrial medicine and surgery,
26:167-173.
Seth PK, Nagar N, Husain R, Chandra SV (1973) Effects of manganese on
rabbit testes. Environmental physiology and biochemistry, 3:263-267.
Seth PK, Hong JS, Kilts CD, Bondy SC (1981) Alteration of cerebral
neurotransmitter receptor function by exposure of rats to manganese.
Toxicology letters, 9(3):247-254.
Shiotsuka RN (1984) Inhalation toxicity of manganese dioxide and a
magnesium oxide-manganese dioxide mixture. Report to US Army Medical
Research and Developmental Command, Fort Detrick, Frederick, MD, by
Inhalation Toxicology Facility, Medical Department, Brookhaven
National Laboratory, Uptown, NY (NTIS No. ADA-148868).
Shukla GS, Dubey MP, Chandra SV (1980) Manganese-induced biochemical
changes in growing versus adult rats. Archives of environmental
contamination and toxicology, 9(4):383-391.
Singh I (1984) Induction of gene conversion and reverse mutation by
manganese sulphate and nickel sulphate in Saccharomyces cerevisiae.
Mutation research, 137:47-49.
Singh J, Husain R, Tandon SK, Chandra SV (1974) Biochemical and
histopathological alterations in early manganese toxicity in rats.
Environmental physiology and biochemistry, 4:16-23.
Singh PP, Junnarkar AY (1991) Behavioral and toxic profile of some
essential trace metal salts in mice and rats. Indian journal of
physiological pharmacology, 23(3):153-159.
Sitaramayya A, Nagar N, Chandra SV (1974) Effect of manganese on
enzymes in rat brain. Acta pharmacology and toxicology, 35:185-190.
Sjögren B, Gustavsson P, Hogstedt C (1990) Neuropsychiatric symptoms
among welders exposed to neurotoxic metals. British journal of
industrial medicine, 47:704-707.
Sjögren B, Iregren A, Frech W, Hagman M, Johansson L, Tesarz M,
Wennberg A (1996) Effects on the nervous system among welders exposed
to aluminum and manganese. Occupational and environmental medicine,
53:32-40.
Smith MO, Sherman IL, Miller LC, Robbins KR, Halley JT (1995) Relative
biological availability of manganese from manganese proteinate,
manganese sulfate, and manganese monoxide in broilers reared at
elevated temperatures. Poultry science, 74(4):702-707.
Smith RA, Alexander RB, Wolman MG (1987) Water-quality trends in the
nation's rivers. Science, 235:1607-1615.
Smyth HF, Carpenter CP, Weil CS, Pozzani UC, Striegel JA, Nycum JS
(1969) Range-finding toxicity data. List VII. American Industrial
Hygiene Association journal, 30:470-476.
Smyth LT, Ruhf RC, Whitman NE, Dugan T (1973) Clinical manganism and
exposure to manganese in the production and processing of
ferromanganese alloy. Journal of occupational medicine, 15:101-109.
Southwood T, Lamb CM, Freeman J (1987) Ingestion of potassium
permanganate crystals by a 3-yr-old boy. Medical journal of
Australia, 146:639-640.
Stokes PM, Campbell PGC, Schroeder WH, Trick C, France RL, Puckett KJ,
LaZerte B, Speyer M, Hanna JE, Donaldson J (1988) Manganese in the
Canadian environment. Ottawa, ON, National Research Council of
Canada, Associate Committee on Scientific Criteria for Environmental
Quality, pp. 30-32 (NRCC No. 26193).
Stoner GD, Shimkin MB, Troxell MC, Thompson TL, Terry LS (1976) Test
for carcinogenicity of metallic compounds by the pulmonary tumor
response in strain A mice. Cancer research, 36:1744-1747.
Subhash MN, Padmashree TS (1991) Effect of manganese on biogenic amine
metabolism in regions of the rat brain. Food and chemical
toxicology, 29(8):579-582.
Suzuki Y, Fujii N, Yano H, Ohkita T, Ichikawa A, Nishiyama K (1978)
Effects of the inhalation of manganese dioxide dust on monkey lungs.
Tokushima journal of experimental medicine, 25:119-125.
Szakmáry E, Ungváry G, Hudák A, Naray M, Tatrai E, Szeberenyi S, Varga
B, Morvai V (1995) Developmental effect of manganese in rat and
rabbit. Central European journal of occupational and environmental
medicine, 1(2):149-159.
Tanaka S (1994) Manganese and its compounds. In: Zenz C, Dickerson OB,
Horvath EP, eds. Occupational medicine, 3rd ed. St. Louis, MO,
Mosby, pp. 542-548.
Tanaka S, Lieben J (1969) Manganese poisoning and exposure in
Pennsylvania. Archives of environmental health, 19:674-684.
Thomas PT, Basse WW, Kerkuliet NI, Luster MI, Munson AE, Murray M,
Roberts D, Robinson M, Silkworth J, Sjoblad R, Smialowicz R (1990)
Immunologic effects of pesticides. In: Balars SR, Wilkinson CF, eds.
The effects of pesticides on human health. Vol. 18. New York, NY,
Princeton Scientific Publishers Inc., pp. 261-295.
Thompson SE, Burton CA, Quinn DJ, Ng YC (1972) Concentration factors
of chemical elements in edible aquatic organisms. Livermore, CA,
University of California, Lawrence Livermore Laboratory, Bio-Medical
Division.
Tjälve H, Henriksson J, Tullkvisi J, Larsson BS, Lindquist NG (1996)
Uptake of manganese and cadmium from nasal mucosa into the central
nervous system via olfactory pathways in rats. Pharmacology and
toxicology, 79:347-356.
Tomes R (1997) Medical management database: Manganese. CD-ROM,
Micromedex, Inc., June.
Treinen KA, Gray TJB, Blazak WF (1995) Developmental toxicity of
mangafodipir trisodium and manganese chloride in Sprague-Dawley rats.
Teratology, 52:109-115.
TRI87 (1989) Toxic chemical release inventory. Washington, DC, US
Environmental Protection Agency, Office of Toxic Substances.
TRI91 (1993) Toxic chemical release inventory. Washington, DC, US
Environmental Protection Agency, Office of Toxic Substances.
Tsuda H, Kato K (1977) Chromosomal aberrations and morphological
transformation in hamster embryonic cells treated with potassium
dichromate in vitro. Mutation research, 46:87-94.
Ulrich CE, Rinehart W, Brandt M (1979) Evaluation of the chronic
inhalation toxicity of a manganese oxide aerosol. III -- Pulmonary
function, electromyograms, limb tremor, and tissue manganese data.
American Industrial Hygiene Association journal, 40:349-353.
Umeda M, Nishimura M (1979) Inducibility of chromosomal aberrations by
metal compounds in cultured mammalian cells. Mutation research,
67:221-229.
US Department of the Interior (1993) Manganese statistical
compendium. Washington, DC, Bureau of Mines.
US Department of the Interior (1995) Mineral industry surveys.
Manganese. Washington, DC, Bureau of Mines and the US Geological
Survey.
US Department of the Interior (1996) Mineral commodity summary
(annual): Manganese. Washington, DC, Bureau of Mines and the US
Geological Survey.
US Department of the Interior (1998) Mineral industry surveys.
Manganese. Washington, DC, Bureau of Mines and the US Geological
Survey.
US EPA (1979) Sources of toxic pollutants found in influents to
sewage treatment plants. VI. Integrated interpresentation.
Washington, DC, US Environmental Protection Agency, Office of Water
Planning and Standards (EPA 440/4-008; NTIS No. PB81-219685).
US EPA (1983) Human exposure to atmospheric concentrations of
selected chemicals. Vol. II. Report to US Environmental Protection
Agency, Office of Air Quality Planning and Standards, Research
Triangle Park, NC, by Systems Applications, Inc., San Rafael, CA (NTIS
PB83-265249).
US EPA (1984) Health assessment document for manganese. Final draft.
Cincinnati, OH, US Environmental Protection Agency, Office of Research
and Development (EPA-600/8-83-013F).
US EPA (1985a) Locating and emitting air emissions from sources of
manganese. Research Triangle Park, NC, US Environmental Protection
Agency, Office of Air Quality Planning and Standards
(EPA-450/4-84-007h).
US EPA (1985b) Decision not to regulate manganese under the Clean Air
Act. US Environmental Protection Agency. Federal register,
50:32627-32628.
US EPA (1993) Drinking water criteria document for manganese.
Cincinnati, OH, US Environmental Protection Agency, Office of Health
and Environmental Assessment (EPA/ECAO-CIN-D008).
US EPA (1994a) ORD's response to public comments on health and
exposure issues in relation to the waiver petition of Ethyl
Corporation to add methylcyclopentadienyl manganese tricarbonyl
(MMT) to unleaded gasoline. Review draft. Washington, DC, US
Environmental Protection Agency.
US EPA (1994b) Reevaluation of inhalation health risks associated
with methylcyclopentadienyl manganese tricarbonyl (MMT) in gasoline.
Research Triangle Park, NC, US Environmental Protection Agency, Office
of Research and Development (EPA-600/R-94/062).
US EPA (1994c) Reevaluation of inhalation health risks associated
with methylcyclopentadienyl manganese tricarbonyl (MMT) in gasoline.
Appendix C: Manganese inhalation reference concentration. Research
Triangle Park, NC, US Environmental Protection Agency, Office of
Research and Development (EPA-600/R-94/062).
Utter MF (1976) The biochemistry of manganese. Medical clinics of
North America, 60:713-727.
Valencia R, Mason JM, Woodruff RC, Zimmering S (1985) Chemical
mutagenesis testing in Drosophila. III. Results of 48 coded
compounds tested for the National Toxicology Program. Environmental
mutagenesis, 7:325-348.
Venugopal B, Luckey TD (1978) Toxicity of group VII metals.
In: Metal toxicity in mammals. 2. Chemical toxicity of metals and
metalloids. New York, NY, Plenum Press, pp. 262-268.
Verschueren K (1983) Handbook of environmental data on organic
chemicals, 2nd ed. New York, NY, Van Nostrand Reinhold, pp. 806,
844.
Vieregge P, Heinzow B, Korf G, Teichert HM, Schleifenbaum P, Mosinger
HU (1995) Long-term exposure to manganese in rural well water has no
neurological effects. Canadian journal of neurological sciences,
22(4):286-289.
Webster WS, Valois AA (1987) Reproductive toxicology of manganese in
rodents, including exposure during the postnatal period.
Neurotoxicology, 8:437-444.
Wedler F (1994) Biochemical and nutritional role of manganese: an
overview. In: Klimis-Tavantzis DJ, ed. Manganese in health and
disease. Boca Raton, FL, CRC Press, pp. 1-37.
Wennberg A, Iregren A, Struwe G, Cizinsky G, Hagman M, Johansson L
(1991) Manganese exposure in steel smelters a health hazard to the
nervous system. Scandinavian journal of work and environmental
health, 17:255-262.
Wennberg A, Hagman M, Johansson L (1992) Preclinical
neurophysiological signs of parkinsonism in occupational manganese
exposure. Neurotoxicology, 13(1):271-274.
Whitlock CM, Amuso SJ, Bittenbender JB (1966). Chronic neurological
disease in two manganese steel workers. American Industrial Hygiene
Association journal, 27:454-459.
WHO (1981) Environmental health criteria 17: Manganese. Geneva,
World Health Organization.
WHO (1984) Guidelines for drinking water quality. Vol. 1.
Recommendations. Geneva, World Health Organization, pp. 7, 52, 79,
82.
WHO (1986) Diseases caused by manganese and its toxic compounds.
Early detection of occupational diseases. Geneva, World Health
Organization, pp. 69-73.
WHO (1987) Manganese. In: Air quality guidelines for Europe.
Copenhagen, World Health Organization Regional Office for Europe, pp.
262-271 (European Series No. 23).
WHO (1993) Guidelines for drinking-water quality, 2nd ed. Vol. 1.
Recommendations. Geneva, World Health Organization.
WHO (1999) WHO air quality guidelines for Europe, 2nd ed.
Copenhagen, World Health Organization Regional Office for Europe (in
press).
Windholz M, ed. (1983) The Merck index: An encyclopedia of
chemicals, drugs and biologicals, 10th ed. Rahway, NJ, Merck and
Company, Inc., pp. 816-818.
Wong PK (1988) Mutagenicity of heavy metals. Bulletin of
environmental contamination and toxicology, 40:597-603.
Yamada M, Ohno S, Okayasu I, Okeda R, Hatakeyama S, Watanabe H, Ushio
K, Tsukagoshi H (1986) Chronic manganese poisoning: a
neuropathological study with determination of manganese distribution
in the brain. Acta neuropathologica (Berlin), 70:273-278.
Zayed J, Gérin G, Loranger S, Sierra P, Bégin D, Kennedy G (1994)
Occupational and environmental exposure of garage workers and taxi
drivers to airborne manganese arising from the use of
methylcyclopentadienyl manganese tricarbonyl in unleaded gasoline.
American Industrial Hygiene Association journal, 55:53-58.
APPENDIX 1 -- SOURCE DOCUMENTS
Agency for Toxic Substances and Disease Registry (1996)
The Toxicological profile for manganese (update) (ATSDR, 1996)
was prepared by the Agency for Toxic Substances and Disease Registry
(ATSDR) through a contract with the Research Triangle Institute. The
updated profile was published as a draft for public comment in
February 1998. Copies of the profile can be obtained from:
Division of Toxicology
Agency for Toxic Substances and Disease Registry
Public Health Service
US Department of Health and Human Services
1600 Clifton Road NE, Mailstop E-29
Atlanta, Georgia 30333
USA
Dr M. Williams-Johnson, Division of Toxicology, ATSDR, and Dr
S.G. Donkin, Dr S.W. Rhodes, and L. Kolb, Sciences International,
Inc., Alexandria, Virginia, contributed to the development of the
toxicological profile as chemical manager and authors. The profile has
undergone three ATSDR internal reviews, including Green Border Review
to assure consistency with ATSDR policy, a Health Effects Review, and
a Minimal Risk Level Review. An external peer review panel was
assembled for the update profile for manganese. The panel consisted of
the following members: Dr J Greger, University of Wisconsin, Madison,
Wisconsin; Dr D.J. Hodgson, University of Wyoming, Laramie, Wyoming;
and Dr C. Newland, Auburn University, Auburn, Alabama. These experts
collectively have knowledge of manganese's physical and chemical
properties, toxicokinetics, key health end-points, mechanisms of
action, human and animal exposure, and quantification of risk to
humans. All reviewers were selected in conformity with the conditions
for peer review specified in Section 104(i)(13) of the US
Comprehensive Environmental Response, Compensation, and Liability
Act, as amended.
Scientists from ATSDR reviewed the peer reviewers' comments and
determined which comments were to be included in the profile. A
listing of the peer reviewers' comments not incorporated in the
profile, with a brief explanation of the rationale for their
exclusion, exists as part of the administrative record for this
compound. A list of databases reviewed and a list of unpublished
documents cited are also included in the administrative record. The
citation of the peer review panel should not be understood to imply
its approval of the profile's final content.
Hazardous Substances Data Bank (1998)
Copies of the information on manganese stored in the Hazardous
Substances Data Bank (HSDB) can be obtained from:
Specialized Information Services
National Library of Medicine
National Institutes of Health
Public Health Service
US Department of Health and Human Services
8600 Rockville Pike
Bethesda, Maryland 20894
USA
HSDB is peer reviewed by a scientific review panel composed of
expert toxicologists and other scientists.
APPENDIX 2 -- CICAD PEER REVIEW
The draft CICAD on manganese and its compounds was sent for
review to institutions and organizations identified by IPCS after
contact with IPCS national Contact Points and Participating
Institutions, as well as to identified experts. Comments were received
from:
BHP Minerals, San Francisco, USA
Department of Health, London, United Kingdom
Department of Toxicology and Chemistry, National Institute for
Working Life, Solna, Sweden
Environment Canada, Ottawa, Canada
Health Canada, Ottawa, Canada
Institute of Occupational Health, Helsinki, Finland
National Chemicals Inspectorate, Solna, Sweden
National Institute for Environmental Health and Safety,
Washington, DC, USA
National Institute for Occupational Safety and Health, Atlanta,
USA
National Institute for Public Health, Prague, Czech Republic
National Institute of Occupational Health, Budapest, Hungary
Public Health Centre of the Capital City, Prague, Czech Republic
United States Environmental Protection Agency, Washington, DC,
USA
Université de Montréal, Montreal, Canada
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
Berlin, Germany, 26-28 November 1997
Members
Dr H. Ahlers, Education and Information Division, National Institute
for Occupational Safety and Health, Cincinnati, OH, USA
Mr R. Cary, Health Directorate, Health and Safety Executive, Bootle,
United Kingdom
Dr S. Dobson, Institute of Terrestrial Ecology, Huntingdon, United
Kingdom
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany (Chairperson)
Mr J.R. Hickman, Health Protection Branch, Health Canada, Ottawa,
Ontario, Canada
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany
Ms M.E. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada (Rapporteur)
Dr K. Paksy, Department of Reproductive Toxicology, National Institute
of Occupational Health, Budapest, Hungary
Mr V. Quarg, Ministry for the Environment, Nature Conservation &
Nuclear Safety, Bonn, Germany
Mr D. Renshaw, Department of Health, London, United Kingdom
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan
Prof. S. Soliman, Department of Pesticide Chemistry, Alexandria
University, Alexandria, Egypt (Vice-Chairperson)
Dr M. Wallen, National Chemicals Inspectorate (KEMI), Solna, Sweden
Ms D. Willcocks, Chemical Assessment Division, Worksafe Australia,
Camperdown, Australia
Dr M. Williams-Johnson, Division of Toxicology, Agency for Toxic
Substances and Disease Registry, Atlanta, GA, USA
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Observers
Mrs B. Dinham1, The Pesticide Trust, London, United Kingdom
Dr R. Ebert, KSU Ps-Toxicology, Huels AG, Marl, Germany (representing
ECETOC, the European Centre for Ecotoxicology and Toxicology of
Chemicals)
Mr R. Green,1 International Federation of Chemical, Energy, Mine and
General Workers' Unions, Brussels, Belgium
Dr B. Hansen,1 European Chemicals Bureau, European Commission, Ispra,
Italy
Dr J. Heuer, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Mr T. Jacob,1 DuPont, Washington, DC, USA
Ms L. Onyon, Environment Directorate, Organisation for Economic
Co-operation and Development, Paris, France
Dr H.J. Weideli, Ciba Speciality Chemicals Inc., Basel, Switzerland
(representing CEFIC, the European Chemical Industry Council)
Secretariat
Dr M. Baril, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr R.G. Liteplo, Health Canada, Ottawa, Ontario, Canada
Ms L. Regis, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Mr A. Strawson, Health and Safety Executive, London, United Kingdom
Dr P. Toft, Associate Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
1 Invited but unable to attend.
APPENDIX 4 -- ADDITIONAL APPROACHES FOR GUIDANCE VALUE DEVELOPMENT
An additional approach in deriving an inhalation guidance value
for manganese could involve dividing the exposed workers from the
Roels et al. (1992) study into four quartiles to estimate a
dose-response relationship and using the average cumulative exposure
and average exposure duration for the lowest quartile of workers to
calculate a NOAEL of 32 µg/m3 (adjusted for continuous exposure),
which could be divided by an uncertainty factor of 300 (10 to account
for human variability, 10 to account for less than lifetime exposure,
and 3 to account for other weaknesses in the study and overall
database -- i.e., statistical weakness from only 23 people in each
quartile, exposure to a manganese oxide other than manganese
tetroxide, and a lack of reproductive data), yielding a guidance value
of 0.1 µg/m3 (Egyed & Wood, 1996).
A guidance value might also be derived by using the geometric
mean integrated respirable dust concentration reported in the Roels et
al. (1992) study and adjusting for exposure duration to calculate an
exposure-adjusted lowest-observed-adverse-effect level (LOAEL) of 50
µg/m3, which could be divided by an uncertainty factor of 1000 (10 to
protect sensitive individuals, 10 to account for using a LOAEL instead
of a NOAEL, and 10 to account for database limitations
-- extrapolation from subchronic to chronic exposure, inadequate
reproductive and developmental data, and unknown differences in the
toxicity of different forms of manganese), yielding a guidance value
of 0.05 µg/m3 (US EPA, 1994b, 1994c; Davis, 1998). Other possible
guideline values, calculated using other methods (e.g., benchmark,
Bayesian, etc.), would range from 0.09 to 0.2 µg/m3 (US EPA, 1994a,
1994b, 1994c; Davis, 1998).
Alternatively, an inhalation guidance value could be derived
based upon a LOAEL of 140 µg total manganese dust/m3 reported in the
study by Iregren (1990), divided by an uncertainty/modifying factor of
900 (10 to account for use of a LOAEL instead of a NOAEL; 10 to
account for human variability; and two modifying factors -- 3 to
account for effects from cumulative exposure to manganese, and 3 to
account for potential differences in toxicity from different forms of
manganese) and adjusted for continuous rather than intermittent
exposure, yielding a provisional guidance value of 0.04 µg/m3 (ATSDR,
1996).
RÉSUMÉ D'ORIENTATION
Ce CICAD relatif au manganèse et à ses dérivés repose
essentiellement sur un rapport intitulé Toxicological profile for
manganese (update), draft for public comment et rédigé par l'Agency
for Toxic Substances and Disease Registry, US Department of Health and
Human Services (ATSDR, 1996). On a également utilisé les informations
contenues dans la Banque de données pour les substances dangereuses,
une banque de données constituée et gérée par la National Library of
Medicine, US Department of Health and Human Services (HSDB, 1998). Les
dernières données sur lesquels s'appuient ces documents de base
remontent à novembre 1998. Enfin, des données complémentaires ont été
tirées des évaluations publiées par l'US Environmental Protection
Agency (EPA) et l'Organisation mondiale de la Santé (OMS) ainsi que de
divers autres documents. Les documents de base utilisés pour la
rédaction de ce CICAD ne prennent pas en compte les effets du
manganèse sur les écosystèmes. Aucune autre source documentaire
(c'est-à-dire des documents rédigés par des organismes internationaux
et soumis à une contrôle scientifique rigoureux) n'a pu être trouvée.
Ce CICAD ne concerne donc que les effets que la présence de manganèse
dans l'environnement peut avoir sur la santé humaine. On n'a pas
cherché à déterminer les effets exercés sur les autres êtres vivants
dans leur milieu naturel. On trouvera à l'appendice 1 des indications
sur les sources documentaires utilisées. Les renseignements concernant
l'examen du CICAD par des pairs font l'objet de l'appendice 2. Ce
CICAD a été approuvé en tant qu'évaluation internationale lors d'une
réunion du Comité d'évaluation finale qui s'est tenue à Berlin
(Allemagne) du 26 au 28 novembre 1997. La liste des participants à
cette réunion figure à l'appendice 3. La fiche d'information
internationale sur la sécurité chimique (ICSC No 0174) établie par le
Programme international sur la Sécurité chimique (IPCS, 1993) est
également reproduite dans ce document.
Le manganèse (Mn) est un élément présent à l'état naturel dans
les roches, le sol, l'eau et les aliments. Tous les êtres humains sont
donc exposés au manganèse et ce dernier est un constituant naturel de
l'organisme. La voie d'exposition la plus importante pour l'Homme est
habituellement la voie alimentaire. Les doses journalières considérées
comme suffisantes et sans danger vont de 1 à 5 mg de manganèse pour
les enfants de 1 an et plus et les adultes. Elles correspondent
généralement à l'apport d'origine alimentaire.
Le manganèse est libéré dans l'atmosphère principalement sous la
forme de particules dont la destinée et le transport dépendent de leur
taille et de leur densité ainsi que de la vitesse et de la direction
du vent. Certains dérivés du manganèse sont très solubles dans l'eau,
de sorte qu'on s'expose facilement à en ingérer une quantité
importante en buvant de l'eau contaminée. Le manganèse présent dans
l'eau peut subir une oxydation, s'adsorber sur les particules en
suspension et sédimenter ensuite. Dans le sol, le manganèse peut
migrer sous forme de particules dans l'air ou dans l'eau ou encore,
s'il est présent sous la forme de composés solubles, en être éliminé
par lessivage.
C'est chez les personnes employées dans des ateliers libérant des
poussières à forte teneur en manganèse, ou qui vivent dans leur
voisinage, que l'exposition au manganèse a le plus de chances d'être
supérieure à la moyenne. Dans certaines régions, la population peut
être exposée au manganèse libéré dans l'atmosphère par la combustion
d'essence sans plomb additionnée d'un antidétonnant organomanganique,
le méthylcyclopentadiénylmanganèse-tricarbonyle (MMT). Certaines
personnes peuvent absorber une quantité excessive de manganèse en
consommant l'eau de puits contaminés par du manganése provenant de
piles ou de pesticides. De même les enfants peuvent se contaminer en
portant à leur bouche de la terre contenant du manganèse en excès.
Le manganèse est un nutriment essentiel pour l'Homme. Il
intervient dans la minéralisation des os, dans le métabolisme
énergétique et dans celui des protéines, dans la régulation du
métabolisme, dans la protection des cellules contre les radicaux
libres et dans la formation de glycosaminoglycanes. L'inhalation ou
l'ingestion de quantités excessives de manganèse peut toutefois avoir
des effets indésirables. A dose administrée équivalente, on retrouve
davantage de manganèse dans l'encéphale après inhalation qu'après
ingestion et la plupart des effets qu'il provoque sont liés à une
exposition chronique par la voie respiratoire. On sait peu de chose de
la toxicité relative des divers composés du manganèse. Toutefois, les
données disponibles indiquent que plusieurs d'entre eux sont capables
de provoquer des effets neurologiques; on a observé ces effets chez
l'Homme après exposition chronique (365 jours ou davantage) par la
voie respiratoire ainsi que chez l'animal, après exposition de durée
intermédiaire (15-364 jours) ou exposition chronique par voie buccale.
D'une manière générale, on constate, à la lumière des données
disponibles, qu'une exposition à des concentrations excessives de
manganèse pendant deux semaines ou moins (exposition brève) ou une
période pouvant aller jusqu'à un an (exposition de durée
intermédiaire) exerce un effet sur les voies respiratoires et sur le
système nerveux, mais peu ou pas d'effets sur les autres organes. Une
intoxication aiguë par inhalation de poussières à forte teneur en
manganèse (en particulier sous forme de dioxyde MnO2 ou de tétroxyde
Mn3O4) peut provoquer une réaction inflammatoire au niveau
pulmonaire qui, avec le temps, peut aboutir à une détérioration de la
capacité fonctionnelle du poumon. Cette toxicité pulmonaire se
manifeste sous la forme d'une sensibilité accrue aux maladies
infectieuses - bronchites par exemple - et peut évoluer vers une
pneumopathie fibreuse. On a également observé une pneumopathie après
une brève inhalation de particules contenant d'autres métaux. Cet
effet pourrait donc être caractéristique d'une exposition à des
particules par la voie respiratoire et ne pas être uniquement lié à la
teneur de ces particules en manganèse.
Il existe quelques documents selon lesquels une exposition de
durée intermédiaire à des dérivés du manganèse est susceptible
d'exercer des effets sur le système nerveux central, mais on ne
dispose pas d'estimation fiable du niveau d'exposition nécessaire pour
produire de tels effets. Les études d'inhalation effectuées sur des
animaux ont révélé l'existence d'effets biochimiques, respiratoires et
neurocomportementaux. On n'a cependant pas déterminé quel était le
seuil d'apparition de ces effets car le niveau d'exposition nécessaire
à leur manifestation varie dans la proportion de 1 à 10.
En cas d'exposition chronique par la voie respiratoire, les
principaux organes-cibles sont les poumons, le système nerveux et les
gonades, encore que l'on ait également observé des effets au niveau
d'autres organes. On a constaté des cas de pneumopathie fibreuse
récidivante et des effets respiratoires aigus à la suite d'une
exposition chronique au manganèse. Les effets sur le système nerveux
se traduisent notamment par des troubles neurologiques et
neuropsychiatriques pouvant aboutir à une pathologie de type
parkinsonien connue sous le nom de manganisme. L'expérience incite à
penser que les animaux de laboratoire, et notamment les rongeurs, ne
sont pas aussi sensibles que l'Homme aux effets neurologiques provoqué
par l'inhalation de manganèse. Au nombre des effets sur la fonction de
reproduction figurent une réduction de la libido, l'impuissance et une
diminution de la fécondité chez les sujets de sexe masculin. On ne
dispose d'aucune information concernant les effets qui s'exerceraient
sur la fonction génitale de la femme. L'expérimentation animale
indique que le manganèse peut provoquer des lésions testiculaires et
des résorptions tardives. Les données tirées de l'expérimentation
animale et relatives aux effets de l'inhalation de manganèse sur le
système immunitaire ou sur le développement foetal sont trop limitées
pour que l'on puisse se prononcer véritablement au sujet de la
signification de ces effets pour l'Homme.
On ne dispose que de données limitées sur le pouvoir cancérogène
du manganèse et les résultats expérimentaux sont difficiles à
interpréter de façon catégorique. L'administration chronique de
sulfate de manganèse (MnSO4) à des rats par la voie buccale a
provoqué une légère augmentation des tumeurs du pancréas chez les
mâles et un nombre un peu plus élevé d'adénomes hypophysaires chez les
femelles. D'autres études portant sur le même composé n'ont pas mis de
cancers en évidence chez le rat et une augmentation marginale de
l'incidence des tumeurs affectant les cellules folliculaires de la
thyroïde a été relevée chez des souris. D'après des études in vitro,
il existe, chez certains composés tout au moins, un pouvoir mutagène.
Quoi qu'il en soit, les études in vivo chez les mammifères donnent
des résultats irréguliers et aucune conclusion générale ne peut en
être tirée en ce qui concerne le risque génotoxique que pourrait
comporter une exposition aux dérivés du manganèse.
Ingérés à forte dose par gavage, des sels concentrés de manganèse
peuvent entraîner la mort des animaux, mais une exposition de brève
durée par suite de la consommation d'aliments ou d'eau contaminés par
du manganèse ne semble pas entraîner d'intoxication importante. De
même, l'administration de sels de manganèse par la voie parentérale
peut avoir un effet toxique sur le développement, mais ces effets ne
s'observent pas après ingestion. On a décrit deux cas de neurotoxicité
après ingestion, pendant une période de durée intermédiaire, de
dérivés du manganèse par des sujets humains, mais les données sont
trop limitées pour que l'on puisse définir le seuil de toxicité ou
savoir si ces effets étaient imputables en totalité au manganèse. On
possède quelques données sur les effets neurologiques ou autres,
consécutifs, chez l'Homme, à l'ingestion prolongée de manganèse, mais
les études dont elles sont tirées pêchent par l'incertitude qui
entoure les voies d'exposition, les doses totales et l'existence
d'autres facteurs de confusion. Les données fournies par les études
sur l'Homme et l'animal sont insuffisantes pour permettre de
déterminer les doses ou les effets à prendre en considération dans le
cas d'une exposition de longue durée par la voie digestive. Les
éléments dont on dispose au sujet des effets indésirables d'une
ingestion prolongée de quantités excessives de manganèse sont
évocateurs mais non concluants.
La voie percutanée ne semble pas devoir être prise sérieusement
en considération et n'a d'ailleurs guère été étudiée. Les données dont
on dispose se limitent à l'effet corrosif du permanganate de potassium
(KMnO4) ou à des cas d'absorption percutanée de dérivés organiques du
manganèse comme le MMT.
A la lumière de ces données, il apparaît clairement qu'une
exposition au manganèse sur les lieux de travail peut entraîner des
effets neurologiques ou respiratoires. D'autres données, plus
limitées, incitent également à penser que l'ingestion de manganèse en
quantités excessives du fait de sa présence dans l'environnement peut
aussi avoir des effets neurologiques indésirables. Certains individus
peuvent être porteurs de facteurs qui les prédisposent à être plus
vunérables aux effets indésirables d'une exposition à des quantités
excessives de manganèse. Il peut s'agir notamment de malades atteints
de pneumopathies, de personnes exposées à d'autres irritants
pulmonaires, de nouveau-nés, de personnes âgées, de sujets souffrant
de carence martiale ou encore d'hépatiques.
Il y a plusieurs manières de parvenir à une valeur-guide pour le
manganèse présent dans l'air. On peut citer ici à titre d'exemple le
chiffre de 0,15 µg de manganèse par m3 qui a été récemment proposé;
on trouvera également d'autres méthodes envisageables pour obtenir ces
valeurs-guides.
RESUMEN DE ORIENTACION
Este CICAD sobre el manganeso y sus compuestos se basa
fundamentalmente en el informe titulado Perfil toxicológico del
manganeso (actualización), proyecto de información pública,
preparado por la Agencia para el Registro de Sustancias Tóxicas y
Enfermedades, Departamento de Salud y Servicios Sociales de los
Estados Unidos (ATSDR, 1996). Se utilizó asimismo la información
contenida en el Banco de Datos de Sustancias Peligrosas, servicio que
ha creado y mantiene la Biblioteca Nacional de Medicina del
Departamento de Salud y Servicios Sociales de los Estados Unidos
(HSDB, 1998). Se examinaron los datos identificados en estos
documentos originales hasta noviembre 1998. Hay información adicional
procedente de otras referencias, como las evaluaciones preparadas por
la Agencia para la Protección del Medio Ambiente de los Estados Unidos
(EPA) y la Organización Mundial de la Salud (OMS), así como de
diversos informes publicados. Los documentos originales utilizados en
la preparación del presente CICAD no comprenden los efectos del
manganeso en el medio ambiente. No se identificaron otras fuentes
(documentos preparados por una organización nacional y sujetos a un
examen científico riguroso) sobre este tema. Por consiguiente, en este
CICAD se abordan sólo los niveles en el medio ambiente como fuente de
exposición humana. Tampoco se ha intentado evaluar en el presente
documento los efectos sobre los organismos en el medio ambiente. La
información sobre la disponibilidad de los documentos originales
figura en el apéndice 1. La información acerca del examen colegiado de
este CICAD se presenta en el apéndice 2. Su aprobación como evaluación
internacional se realizó en una reunión de la Junta de Evaluación
Final, celebrada en Berlín, Alemania, los días 26-28 de noviembre de
1997. La lista de participantes en esta reunión figura en el apéndice
3. La Ficha internacional de seguridad química (ICSC 0174) para el
manganeso, preparada por el Programa Internacional de Seguridad de las
Sustancias Químicas (IPCS, 1993), también se reproduce en el presente
documento.
El manganeso (Mn) es un elemento natural del medio ambiente, que
se encuentra en las rocas, el suelo, el agua y los alimentos. Así
pues, todas las personas están expuestas al manganeso y es un
componente normal del organismo. La vía de exposición más importante
para el ser humano suelen ser los alimentos. Se ha establecido una
ingesta diaria inocua y suficiente de 1-5 mg de manganeso para niños
de un año y mayores hasta la edad adulta; estos niveles generalmente
se corresponden con las cantidades del compuesto que se reciben a
través de los alimentos.
El manganeso se libera en el aire fundamentalmente como materia
particulada, y el destino final y el transporte de las partículas
dependen de su tamaño y densidad y de la velocidad y la dirección del
viento. Algunos compuestos de manganeso son muy solubles en agua, de
manera que se puede producir una exposición importante por el consumo
de agua de bebida contaminada. El manganeso del agua superficial se
puede oxidar o adsorber en las partículas del sedimento y depositarse
en el fondo. El del suelo puede pasar como materia particulada al aire
o al agua, y los compuestos solubles de manganeso pueden sufrir un
proceso de lixiviación a partir del suelo.
Las exposiciones al manganeso mencionadas más arriba son más
probables en el caso de las personas que trabajan o viven cerca de
fábricas o de otros lugares donde se liberan cantidades significativas
de polvo de manganeso en el aire. En algunas regiones, la población
general puede estar expuesta al manganeso liberado en el aire por la
combustión de la gasolina sin plomo que contiene el compuesto orgánico
de manganeso, metilciclopentadienilo-manganeso tricarbonilo (MMT) como
ingrediente antidetonante. Algunas personas pueden estar expuestas a
un exceso de manganeso en el agua potable, por ejemplo cuando se
filtra al agua de los pozos manganeso de las baterías o de
plaguicidas. Los niños pueden estar expuestos a un exceso de manganeso
en el suelo por la costumbre de llevarse las manos a la boca.
El manganeso es un nutriente esencial del ser humano que
desempeña una función en la mineralización de los huesos, en el
metabolismo proteico y energético, en la regulación metabólica, en la
protección de las células del efecto perjudicial de sustancias con
radicales libres nocivos y en la formación de glucosaminoglucanos. Sin
embargo, la exposición a niveles elevados mediante inhalación o
ingestión puede producir efectos adversos en la salud. Para dosis
comparables, llega al cerebro más manganeso después de la inhalación
que de la ingestión, y la mayor parte de los efectos en la salud están
asociados con la exposición crónica por inhalación. Es poco lo que se
sabe acerca de la toxicidad relativa de los distintos compuestos de
manganeso. Sin embargo, hay pruebas que ponen de manifiesto que
diversos compuestos de manganeso pueden inducir efectos neurológicos;
estos efectos se han observado tras una exposición crónica por
inhalación (365 días o más) en el ser humano y una exposición oral
intermedia (15-364 días) y crónica en animales.
En general, los datos disponibles indican que la exposición a un
exceso de manganeso durante 14 días o menos (aguda) o hasta un año
(intermedia) tiene efectos en el sistema respiratorio y en el sistema
nervioso, con un efecto escaso o nulo en otros órganos. La exposición
aguda por inhalación a concentraciones elevadas de polvo de manganeso
(en concreto de dióxido de manganeso [MnO2] y de tetróxido de
manganeso [Mn3O4]) puede provocar una respuesta inflamatoria en el
pulmón, que con el tiempo puede dar lugar a un trastorno de la función
pulmonar. La toxicidad pulmonar se manifiesta en forma de una mayor
susceptibilidad a infecciones como la bronquitis y puede producir
neumonía mangánica. Se ha observado también neumonía tras exposiciones
agudas por inhalación a partículas que contenían otros metales. Así
pues, este efecto podría ser característico de la materia particulada
inhalable y no depender solamente del contenido en manganeso de las
partículas.
Hay un pequeño número de informes que parecen indicar que en la
exposición intermedia a compuestos de manganeso por inhalación se
producen efectos en el sistema nervioso central, pero no se dispone de
estimaciones fidedignas sobre los niveles de exposición. Los estudios
de inhalación en animales pusieron de manifiesto efectos bioquímicos,
respiratorios y en el neurocomportamiento. Sin embargo, no se ha
determinado un umbral para estos efectos, porque los niveles de
exposición asociados con ellos varían en más de un orden de magnitud.
En la exposición crónica al manganeso por inhalación, los
principales órganos afectados son los pulmones, el sistema nervioso y
el sistema reproductor, aunque también se han observado efectos en
otros sistemas de órganos. Este tipo de exposición se ha asociado con
una neumonía mangánica recurrente y con efectos respiratorios agudos.
Los efectos en el sistema nervioso incluyen síntomas neurológicos y
neuropsiquiátricos que pueden culminar en una enfermedad semejante a
la de Parkinson, conocida como manganismo; hay pruebas que indican que
los animales de laboratorio, especialmente los roedores, no son tan
sensibles como el ser humano, y posiblemente otros primates, a los
efectos neurológicos provocados por la exposición al manganeso por
inhalación. Los efectos reproductivos de la exposición crónica por
inhalación son una reducción de la libido, impotencia y menor
fecundidad en los hombres; no se dispone de información acerca de los
efectos reproductivos en las mujeres. Los estudios realizados en
animales indican que el manganeso puede producir daños directos en los
testículos y resorciones tardías. Los datos obtenidos de estudios
realizados con animales sobre los efectos del manganeso inhalado en el
sistema inmunitario y en el desarrollo del feto son demasiado
limitados para sacar conclusiones sobre la importancia de estos
efectos en el ser humano.
La información sobre el potencial carcinogénico del manganeso es
limitada y los resultados son difíciles de interpretar con certeza.
Los estudios de exposición crónica por vía oral realizados con sulfato
de manganeso (MnSO4) en ratas pusieron de manifiesto un pequeño
aumento en la incidencia de tumores pancreáticos en los machos y un
ligero incremento de los adenomas de hipófisis en las hembras. En
otros estudios realizados con sulfato de manganeso, no se observaron
signos de cáncer en ratas y en ratones se detectó un aumento marginal
de la incidencia de adenomas de las células foliculares de la glándula
tiroides. Los resultados de los estudios in vitro ponen de
manifiesto que por lo menos algunas formas químicas del manganeso
tienen potencial mutagénico. Sin embargo, debido a las discrepancias
entre los resultados de los estudios in vivo realizados en
mamíferos, no se puede llegar a una conclusión general acerca del
posible peligro genotóxico para el ser humano como consecuencia de la
exposición a compuestos de manganeso.
La administración de dosis elevadas de sales concentradas de
manganeso por vía oral mediante sonda puede provocar la muerte de los
animales, pero no se ha observado que la exposición oral a través de
los alimentos o del agua produzca una toxicidad significativa durante
una exposición aguda o breve. Igualmente, la administración parenteral
de sales de manganeso puede producir toxicidad en el desarrollo, pero
no se encontraron efectos tras las exposición por vía oral. Se ha
notificado que la exposición oral de duración intermedia del ser
humano al manganeso produjo neurotoxicidad en dos casos, pero los
datos son demasiado limitados para definir el umbral o juzgar si estos
efectos se debieron completamente a la exposición al manganeso.
Existen algunos datos acerca de los efectos neurológicos o de otro
tipo para la salud de las personas debidos a la exposición oral
crónica al manganeso, pero estos estudios están limitados por la
incertidumbre en relación con las vías de exposición y los niveles de
exposición total, así como por la existencia de otros factores de
confusión. Los estudios en el ser humano y en animales no proporcionan
información suficiente para determinar las dosis o los efectos que
despiertan preocupación tras la exposición crónica por vía oral. Así
pues, las pruebas disponibles para los efectos adversos asociados con
la ingesta crónica de un exceso de manganeso son indicativas, pero no
concluyentes.
La vía cutánea no parece revestir especial importancia y no se ha
investigado en absoluto. Los datos disponibles se limitan a informes
sobre los efectos corrosivos del permanganato potásico (KMnO4) y a
informes de casos de efectos debidos a la absorción cutánea de
compuestos orgánicos del manganeso, como el MMT.
Según estos datos, es evidente que la exposición al manganeso
puede producir efectos neurológicos y respiratorios adversos en el
ámbito ocupacional. También hay pruebas limitadas que indican que los
efectos neurológicos adversos pueden estar asociados con la ingesta de
un exceso de manganeso en las condiciones del medio ambiente. Como
consecuencia de los factores de predisposición, determinadas personas
podrían ser más susceptibles a los efectos adversos de la exposición a
un exceso de manganeso. Entre ellas podrían estar las afectadas por
enfermedades pulmonares, las expuestas a otras sustancias irritantes
de los pulmones, los recién nacidos, los ancianos, y las personas con
déficit de hierro o con enfermedades hepáticas.
Existen varios métodos para la obtención de un valor guía para el
manganeso en el aire. Recientemente se ha obtenido un valor guía de
0,15 µg de manganeso/m3, que se destaca aquí como un posible ejemplo;
también se han presentado algunos métodos adicionales.
1. EXECUTIVE SUMMARY
This CICAD on manganese and its compounds was based principally
on the report entitled Toxicological profile for manganese (update),
draft for public comment, prepared by the Agency for Toxic
Substances and Disease Registry, US Department of Health and Human
Services (ATSDR, 1996). Information contained in the Hazardous
Substances Data Bank, developed and maintained by the National Library
of Medicine, US Department of Health and Human Services, was also used
(HSDB, 1998). Data identified as of November 1998 were considered in
these source documents. Additional data came from other references,
such as assessments prepared by the US Environmental Protection Agency
(EPA) and the World Health Organization (WHO), as well as a variety of
reports in the literature. The source documents used to develop this
CICAD do not cover the effects of manganese on the ecological
environment. No other sources (documents developed by a national
organization and subject to rigorous scientific review) on this topic
were identified. Therefore, this CICAD addresses environmental levels
as a source of human exposure only. No attempt has been made in this
document to assess effects on organisms in the environment.
Information on the availability of the source documents is presented
in Appendix 1. Information on the peer review of this CICAD is
presented in Appendix 2. This CICAD was approved as an international
assessment at a meeting of the Final Review Board, held in Berlin,
Germany, on 26-28 November 1997. Participants at the Final Review
Board meeting are presented in Appendix 3. The International Chemical
Safety Card (ICSC 0174) for manganese, produced by the International
Programme on Chemical Safety (IPCS, 1993), has also been reproduced in
this document.
Manganese (Mn) is a naturally occurring element that is found in
rock, soil, water, and food. Thus, all humans are exposed to
manganese, and it is a normal component of the human body. Food is
usually the most important route of exposure for humans. Estimated
Safe and Adequate Daily Intakes of 1-5 mg manganese have been
established for children 1 year of age and older through to adults;
these levels generally parallel amounts of the compound delivered via
the diet.
Manganese is released to air mainly as particulate matter, and
the fate and transport of the particles depend on their size and
density and on wind speed and direction. Some manganese compounds are
readily soluble in water, so significant exposures can also occur by
ingestion of contaminated drinking-water. Manganese in surface water
can oxidize or adsorb to sediment particles and settle to the bottom.
Manganese in soil can migrate as particulate matter to air or water,
or soluble manganese compounds can be leached from the soil.
Above-average exposures to manganese are most likely to occur in
people who work at or live near a factory or other site where
significant amounts of manganese dust are released into the air. In
some regions, the general population can be exposed to manganese
released into air by the combustion of unleaded gasoline containing
the organomanganese compound methylcyclopentadienyl manganese
tricarbonyl (MMT) as an antiknock ingredient. Some people can be
exposed to excess manganese in drinking-water -- for example, when
manganese from batteries or pesticides leaches into well-water.
Children can be exposed to excess manganese in soils through
hand-to-mouth behaviour.
In humans, manganese is an essential nutrient that plays a role
in bone mineralization, protein and energy metabolism, metabolic
regulation, cellular protection from damaging free radical species,
and the formation of glycosaminoglycans. However, exposure to high
levels via inhalation or ingestion can cause adverse health effects.
Given comparable doses, more manganese reaches the brain following
inhalation than following ingestion, and most health effects are
associated with chronic inhalation exposure. Little is known about the
relative toxicity of different manganese compounds. However, available
evidence indicates that various manganese compounds can induce
neurological effects; these effects have been observed following
chronic (365 days or more) inhalation exposures in humans and
intermediate (15-364 days) and chronic oral exposures in animals.
In general, the available data indicate that exposure to excess
manganese for 14 days or less (acute duration) or up to a year
(intermediate duration) has an effect on the respiratory system and
the nervous system, with little to no effect on other organ systems.
Acute inhalation exposure to high concentrations of manganese dusts
(specifically manganese dioxide [MnO2] and manganese tetroxide
[Mn3O4]) can cause an inflammatory response in the lung, which, over
time, can result in impaired lung function. Lung toxicity is
manifested as an increased susceptibility to infections such as
bronchitis and can result in manganic pneumonia. Pneumonia has also
been observed following acute inhalation exposures to particulates
containing other metals. Thus, this effect might be characteristic of
inhalable particulate matter and might not depend solely on the
manganese content of the particle.
There are a few reports suggesting that intermediate inhalation
exposure to manganese compounds produces effects on the central
nervous system, but reliable estimates of exposure levels are not
available. Inhalation studies in animals resulted in biochemical,
respiratory, and neurobehavioural effects. However, a threshold for
these effects has not been identified, because the exposure levels
associated with these effects range over an order of magnitude.
In chronic inhalation exposure to manganese, the main organ
systems affected are the lungs, nervous system, and reproductive
system, although effects on other organ systems have also been
observed. A recurring manganic pneumonia and acute respiratory effects
have been associated with chronic inhalation exposures to manganese.
Effects on the nervous system include neurological and
neuropsychiatric symptoms that can culminate in a Parkinsonism-like
disease known as manganism; evidence suggests that laboratory animals,
especially rodents, are not as sensitive as humans, and possibly other
primates, to the neurological effects of inhalation exposure to
manganese. Reproductive effects of chronic inhalation exposure to
manganese include decreased libido, impotence, and decreased fertility
in men; information is not available on reproductive effects in women.
Studies in animals indicate that manganese can cause direct damage to
the testes and late resorptions. Data from animal studies on the
effects of inhaled manganese on the immunological system and the
developing fetus are too limited to make firm conclusions on the
significance of these effects for humans.
Information on the carcinogenic potential of manganese is
limited, and the results are difficult to interpret with certainty. In
rats, chronic oral studies with manganese sulfate (MnSO4) showed a
small increase in the incidence of pancreatic tumours in males and a
small increase in pituitary adenomas in females. In other studies with
manganese sulfate, no evidence for cancer was noted in rats and a
marginally increased incidence of thyroid gland follicular cell
adenomas was observed in mice. The results of in vitro studies show
that at least some chemical forms of manganese have mutagenic
potential. However, as the results of in vivo studies in mammals are
inconsistent, no overall conclusion can be made about the possible
genotoxic hazard to humans from exposure to manganese compounds.
Large oral doses of concentrated manganese salts given by gavage
can cause death in animals, but oral exposures via food or water have
not been found to cause significant toxicity over acute or short-term
exposures. Similarly, parenteral administration of manganese salts can
cause developmental toxicity, but effects were not found with oral
exposure. Intermediate-duration oral exposure of humans to manganese
has been reported to cause neurotoxicity in two cases, but the data
are too limited to define the threshold or to judge if these effects
were due entirely to the manganese exposure. Some data on neurological
or other health effects in humans from chronic oral intake of
manganese exist, but these studies are limited by uncertainties in the
exposure routes and total exposure levels as well as by the existence
of other confounding factors. The studies in humans and animals do not
provide sufficient information to determine dose levels or effects of
concern following chronic oral exposure. Thus, the available evidence
for adverse effects associated with chronic ingestion of excess
manganese is suggestive but inconclusive.
The dermal route does not appear to be of significant concern and
has not been investigated to any extent. Available information is
limited to reports on the corrosive effects of potassium permanganate
(KMnO4) and case reports of effects from dermal absorption of organic
manganese compounds such as MMT.
From these data, it is clear that adverse neurological and
respiratory effects from manganese exposure can occur in occupational
settings. Limited evidence also suggests that adverse neurological
effects can be associated with ingestion of excess manganese in
environmental settings. As a result of predisposing factors, certain
individuals might be more susceptible to adverse effects from exposure
to excess manganese. These might include people with lung disease,
people who are exposed to other lung irritants, neonates, older
people, individuals with iron deficiency, or people with liver
disease.
There are several approaches to the development of a guidance
value for manganese in air. A recently developed guidance value of
0.15 µg manganese/m3 is highlighted here as one possible example;
some additional approaches are also presented.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
Table 1 lists common synonyms and other relevant information on
the chemical identity and properties of manganese and several of its
most important compounds. Manganese is a naturally occurring element
that is found in rock, soil, water, and food. Manganese can exist in a
number of oxidation states. Manganese and its compounds can exist as
solids in the soil and as solutes or small particles in water. Most
manganese salts are readily soluble in water, with only the phosphate
and the carbonate having low solubilities. The manganese oxides
(manganese dioxide and manganese tetroxide) are poorly soluble in
water. Manganese can also be present in small dust-like particles in
the air. Additional physical/chemical properties are presented in the
International Chemical Safety Card (ICSC 0174) reproduced in this
document.
Table 1: Chemical identity of manganese and its compounds.a
Manganese Manganous Manganese Manganese Manganese potassium
chloride sulfate (II, III) oxide dioxide permanganate
Synonyms Elemental Manganese Manganous Trimanganese Manganese Permanganic acid;
manganese chlorideb; sulfate; tetroxide; peroxide; potassium saltc;
colloidal manganese sulfuric acid mangano-manganic manganese chameleon material
manganese; dichloride manganese oxidec; binoxide;
cutavalb manganese manganese
tetroxide black;
battery
manganese
Chemical Mn MnCl2 MnSO4 Mn3O4 MnO2 KMnO4
formula
CAS 7439-96-5 7773-01-5 7785-87-7 1317-35-7 1313-13-9 7722-64-7
Number
Molecular 54.94c 125.85c 151.00c 228.81d 86.94c 158.04c
weight
Colour Grey-whited Pinkd Pale rose-red Blackd Black Purple
Physical Solid Solid Solid Solid Solid Solid
state
Melting 1244 °Cd 650 °C 700 °C 1564 °C 535 °Cd <240 °C
point (decomposes)
Boiling 1962 °Cd 1190 °Cd Decomposes No data No data No data
point at 850 °C
Table 1 (continued)
Manganese Manganous Manganese Manganese Manganese potassium
chloride sulfate (II, III) oxide dioxide permanganate
Solubility Dissolves in Very soluble Soluble in Insoluble Soluble in Soluble in
dilute mineral in water; water and in water; hydrochloric water, acetone
acidsd; soluble in alcohol soluble in acid; insoluble and sulfuric
decomposes alcohol hydrochloric in water acid
in water acid
a Adapted from ATSDR (1996). All information obtained from Sax & Lewis (1987), except where noted.
b HSDB (1998).
c Windholz (1983).
d Lide (1993).
Table 1a
Methylcyclo- Manganese Mancozebb
pentadienyl- ethylene-bis-
manganese dithiocarbamate
tricarbonyla
Synonyms MMTc; Trimangol 80; manebd Dithane M-45
methyl-cymantrene; ethylene-bis[dithiocarbamic manganese
Antiknock-33; acid], manganous salt; ethylenebis
manganese Dithane (dithiocarbamate)
tricarbonyl (polymeric);
methylcyclopentadienyl Manzate; Manzeb;
Zimaneb
Chemical C9H7MnO3 C4H6MnN2S4 C4H6MnN2S4.
formula C4H6N2S4Zn
CAS 12108-13-3 12427-38-2 12427-38-2
Number
Molecular 218.10 265.31 541.03
weight
Colour Dark orange-rede Yellow-brown Greyish-yellow
Physical Liquide Powder Powder
state
Melting No data Decomposes on Decomposes
point heating without melting
Boiling 232.8 °Ce No data No data
point
Table 1a (continued)
Methylcyclo- Manganese Mancozebb
pentadienyl- ethylene-bis-
manganese dithiocarbamate
tricarbonyla
Solubility Practically insoluble Slightly soluble Practically
in water (70 ppm at in water; soluble in insoluble in water
25 °C); completely chloroform as well as most organic
soluble in hydrocarbons solvents
CAS = Chemical Abstracts Service
a NTP (1999).
b Hamilton (1995).
c Zayed et al. (1994).
d Ferraz et al. (1988).
e Verschueren (1983).
3. ANALYTICAL METHODS
Atomic absorption spectrophotometric analysis is the most widely
used method for determining manganese in biological materials and
environmental samples. Fluorimetric, colorimetric, neutron activation
analysis, and plasma atomic emission techniques are also recommended
for measuring manganese in such samples. Most of these methods require
wet digestion, derivatization, and/or extraction before detection. In
most cases, distinguishing between different oxidation states of
manganese is impossible, so total manganese is measured.
The detection limits of these methods range from <0.01 to 0.2
µg/g for biological tissues and fluids, from 5 to 10 µg/m3 for air,
and from 0.01 to 50 µg/litre for water (Kucera et al., 1986; Abbasi,
1988; Lavi et al., 1989; Mori et al., 1989; Chin et al., 1992; ATSDR,
1996). Determination of manganese levels in soil, sludge, or other
solid wastes requires an acid extraction/digestion step before
analysis. The details vary with the specific characteristics of the
sample, but treatment usually involves heating in nitric acid,
oxidation with hydrogen peroxide, and filtration and/or centrifugation
to remove insoluble matter (ATSDR, 1996).
A nuclear magnetic resonance method (Kellar & Foster, 1991) and a
method using on-line concentration analysis (Resing & Mottl, 1992)
were used to determine both free and complexed manganese ions in
aqueous media. The latter method was highly sensitive, with a
detection limit of 36 pmol/litre (1.98 ng/litre when concentrating 15
ml of seawater).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Manganese is ubiquitous in the environment. It comprises about
0.1% of the earth's crust (NAS, 1973; Graedel, 1978). Because
manganese occurs in soil, air, water, and food, all humans are exposed
to it. Manganese is a normal component of the human body, and food is
usually the most important route of exposure for humans. Manganese
does not occur naturally as a base metal but is a component of more
than 100 minerals, including various sulfides, oxides, carbonates,
silicates, phosphates, and borates (NAS, 1973). The most commonly
occurring manganese-bearing minerals include pyrolusite (manganese
dioxide), rhodocrosite (manganese carbonate), and rhodonite (manganese
silicate) (NAS, 1973; Windholz, 1983; US EPA, 1984; HSDB, 1998).
The manganese content in ore produced worldwide was estimated to
be 8.8 million tonnes in 1986. Production levels of manganese ore and
its total manganese metal content remained nearly the same through
1990 (US Department of the Interior, 1993). Levels of ore produced
worldwide in 1995, 1996, and 1997 declined slightly, with total
manganese metal content declining proportionately to 8.0, 8.1, and 7.7
million tonnes, respectively (US Department of the Interior, 1996,
1998). Although modern steelmaking technologies call for lower unit
consumption of manganese, worldwide demand for steel is projected to
increase moderately in the future, particularly in developing
countries (US Department of the Interior, 1995, 1998). Although
manganese usage in other industries is increasing, this will have
minor overall effect on manganese demand, and future trends for
manganese are still expected to increase with demands for steel (EM,
1993; US Department of the Interior, 1995, 1998). The demand for
manganese in other industries (e.g., dry-cell battery manufacturing)
might also increase, but the overall effect of these other uses on
global trends in manganese production and use is minor (US Department
of the Interior, 1995, 1998).
Manganese compounds are produced from manganese ores or from
manganese metal. The organomanganese compound MMT, an antiknock
additive in unleaded gasoline, is produced by the reaction of
manganese chloride (MnCl2), cyclopentadiene, and carbon monoxide in
the presence of manganese carbonyl (NAS, 1973; US EPA, 1984; Sax &
Lewis, 1987; HSDB, 1998). Metallic manganese (ferromanganese) is used
principally in steel production along with cast iron and superalloys
to improve hardness, stiffness, and strength (NAS, 1973; US EPA, 1984;
HSDB, 1998). Manganese compounds have a variety of uses. Manganese
dioxide is commonly used in the production of dry-cell batteries,
matches, fireworks, porcelain and glass-bonding materials, and
amethyst glass; it is also used as the starting material for the
production of other manganese compounds (NAS, 1973; Venugopal &
Luckey, 1978; US EPA, 1984). Manganese chloride is used as a precursor
for other manganese compounds, as a catalyst in the chlorination of
organic compounds, in animal feed to supply essential trace minerals,
and in dry-cell batteries (US EPA, 1984; HSDB, 1998). Manganese
sulfate is used primarily as a fertilizer and as a livestock
supplement; it is also used in some glazes, varnishes, ceramics, and
fungicides (Windholz, 1983; US EPA, 1984; HSDB, 1998). Manganese
ethylene-bis-dithiocarbamate (maneb) is widely applied to edible crops
as a fungicide and is therefore a potential source of manganese in
soil and in food crops (Ferraz et al., 1988; Ruijten et al., 1994).
Potassium permanganate is used as an oxidizing agent; as a
disinfectant; as an antialgal agent; for metal cleaning, tanning, and
bleaching; as a purifier in water and waste treatment plants; and as a
preservative for fresh flowers and fruits (HSDB, 1998).
The main sources of manganese releases to the air are industrial
emissions, combustion of fossil fuels, and re-entrainment of
manganese-containing soils (Lioy, 1983; US EPA, 1983, 1984, 1985a,
1985b). Manganese can also be released to the air during other
anthropogenic processes, such as welding and fungicide application
(Ferraz et al., 1988; MAK, 1994; Ruijten et al., 1994). Total
emissions to air from anthropogenic sources in the USA were estimated
to be 16 400 t in 1978, with about 80% (13 200 t) from industrial
facilities and 20% (3200 t) from fossil fuel combustion (US EPA,
1983). Air emissions by US industrial sources reported for 1987
totalled 1200 t (TRI87, 1989). In 1991, air emissions from facilities
in the USA ranged from 0 to 74 t, with several US states reporting no
emissions (TRI91, 1993). Air erosion of dusts and soils is also an
important atmospheric source of manganese, but no quantitative
estimates of manganese release to air from this source were identified
(US EPA, 1984). Volcanic eruptions can also release manganese to the
atmosphere (Schroeder et al., 1987).
In some countries, combustion of gasoline containing MMT
contributes approximately 8% to levels of manganese tetroxide in urban
air (Loranger & Zayed, 1995). MMT was used as a gasoline additive in
the USA for a number of years, resulting in manganese emissions.
Analysis of manganese levels in the air indicated that vehicular
emissions contributed an average of 13 ng manganese/m3 in southern
California, whereas vehicular emissions were only about 3 ng/m3 in
central and northern California (Davis et al., 1988). A ban on MMT use
as a fuel additive was imposed for a period of time, then lifted by
the US EPA in 1995.
In Canada, MMT use as a fuel additive has gradually increased
since 1976. Manganese emissions from gasoline combustion rose sharply
from 1976 through the early 1980s, reaching an estimated 200.2 t by
1985 (Jacques, 1984). In 1990, lead was completely replaced by MMT in
gasoline in Canada (Loranger & Zayed, 1994). MMT use peaked in 1989 at
over 400 t, which was more than twice the usage in 1983 and 1.5 times
the usage in 1986. MMT use declined to about 300 t by 1992, owing to
reductions in its concentration in gasoline. However, ambient
monitoring data for manganese in Canadian cities without industrial
sources for the 1989-1992 period did not reflect this peak in MMT use.
Air manganese levels (PM2.5, or particulate matter with an aerodynamic
diameter less than or equal to 2.5 µm) remained constant at 0.11-0.013
µg/m3 for small cities and 0.020-0.025 µg/m3 for large cities
(Health Canada, 1994; Egyed & Wood, 1996). Manganese emission levels
can vary depending on the concentration of MMT in gasoline and
gasoline usage patterns. One study reported a correlation between
atmospheric manganese concentrations in 1990 air samples and traffic
density in Montreal (Loranger et al., 1994). However, a later study by
these investigators reported that atmospheric manganese concentrations
in Montreal decreased in 1991 and 1992 despite an estimated 100%
increase in manganese emission rates from MMT in gasoline (Loranger &
Zayed, 1994). Another study suggested that the high manganese levels
in Montreal were, in part, due to the presence of a silico- and
ferro-manganese facility that ceased operation in 1991 (Egyed & Wood,
1996).
Manganese can be released to water by discharge from industrial
facilities or as leachate from landfills and soil (US EPA, 1979, 1984;
Francis & White, 1987; TRI91, 1993). In the USA, reported industrial
discharges in 1991 ranged from 0 to 17.2 t for surface water, from 0
to 57.3 t for transfers to public sewage, and from 0 to 0.114 t for
underground injection (TRI91, 1993). An estimated total of 58.6 t, or
1% of the total environmental release of manganese in the USA, was
discharged to water in 1991 (TRI91, 1993).
Land disposal of manganese-containing wastes is the principal
source of manganese releases to soil. In 1991, reported industrial
releases to land in the USA ranged from 0 to 1000 t. More than 50% of
the total environmental release of manganese (3753 t) was to land
(TRI91, 1993).
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Elemental manganese and inorganic manganese compounds have
negligible vapour pressures but can exist in air as suspended
particulate matter derived from industrial emissions or the erosion of
soils. Manganese-containing particles are removed from the atmosphere
mainly by gravitational settling or by rain (US EPA, 1984).
Soil particulate matter containing manganese can be transported
in air. The fate and transport of manganese in air are largely
determined by the size and density of the particle and wind speed and
direction. An estimated 80% of the manganese in suspended particulate
matter is associated with particles with a Mass Median Equivalent
Diameter (MMED) of <5 µm, and 50% of this manganese is estimated to
be associated with particles that are <2 µm in MMED. (Whether these
data are for particles in urban or rural areas is unclear. However, it
is known that the size of manganese particles in the air tends to vary
by source; small particles dominate around ferromanganese and dry-cell
battery plants, whereas large particles tend to predominate near
mining operations [WHO, 1999].) Based on these data, manganese's small
particle size is within the respirable range, and widespread airborne
distribution would be expected (WHO, 1981). Very little information is
available on atmospheric reactions of manganese (US EPA, 1984).
Although manganese can react with sulfur dioxide and nitrogen dioxide,
the occurrence of such reactions in the atmosphere has not been
demonstrated.
The transport and partitioning of manganese in water are
controlled by the solubility of the specific manganese compound
present. In most waters (pH 4-7), Mn(II) predominates and is
associated principally with carbonate, which has relatively low
solubility (US EPA, 1984; Schaanning et al., 1988). The solubility of
Mn(II) can be controlled by manganese oxide equilibria (Ponnamperuma
et al., 1969), with manganese being converted to other oxidation
states (Rai et al., 1986). In extremely reduced water, the fate of
manganese tends to be controlled by the formation of the poorly
soluble sulfide (US EPA, 1984). In groundwater with low oxygen levels,
Mn(IV) can be reduced both chemically and bacterially to the Mn(II)
oxidation state (Jaudon et al., 1989). MMT has been found to be
persistent in natural aquatic and soil environments in the absence of
sunlight, with a tendency to sorb to soil and sediment particles
(Garrison et al., 1995). In the presence of light, photodegradation of
MMT is rapid, with identified products including a manganese carbonyl
that readily oxidizes to manganese tetroxide (Garrison et al., 1995).
Manganese is often transported in rivers adsorbed to suspended
sediments. Most of the manganese from industrial sources found in a
South American river was bound to suspended particles (Malm et al.,
1988). The tendency of soluble manganese compounds to adsorb to soils
and sediments can be highly variable, depending mainly on the cation
exchange capacity and the organic composition of the soil (Hemstock &
Low, 1953; Schnitzer, 1969; McBride, 1979; Curtin et al., 1980; Baes &
Sharp, 1983; Kabata-Pendias & Pendias, 1984). The oxidation state of
manganese in soils and sediments can be altered by microbial activity
(Geering et al., 1969; Francis, 1985).
Manganese in water can be significantly bioconcentrated at lower
trophic levels. Bioconcentration factors (BCFs) of 10 000-20 000 for
marine and freshwater plants, 2500-6300 for phytoplankton, 300-5500
for marine algae, 800-830 for intertidal mussels, and 35-930 for fish
have been estimated (Folsom et al., 1963; Thompson et al., 1972). The
high reported BCFs probably reflect the essentiality of manganese for
a wide variety of organisms; specific uptake mechanisms exist for
essential elements.
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
Concentrations of manganese in seawater reportedly range from 0.4
to 10 µg/litre (US EPA, 1984). In the North Sea, the northeast
Atlantic Ocean, the English Channel, and the Indian Ocean, manganese
content was reported to range from 0.03 to 4.0 µg/litre. Levels found
in coastal waters of the Irish Sea and in the North Sea off the coast
of the United Kingdom ranged from 0.2 to 25.5 µg/litre (Alessio &
Lucchini, 1996). In a number of cases, higher levels in water (in
excess of 1000 µg/litre) have been detected at US hazardous waste
sites, suggesting that, in some instances, wastes from industrial
sources can lead to significant contamination of water (ATSDR, 1996).
In a 1974-1981 survey of 286 US river water samples,
concentrations of dissolved manganese ranged from less than 11
µg/litre (25th percentile) to more than 51 µg/litre (75th percentile)
(Smith et al., 1987), with a median of 24 µg/litre. Mean groundwater
concentrations were 20 and 90 µg/litre from two geological zones in
California (Deverel & Millard, 1988). The surface waters of Welsh
rivers were reported to contain from 0.8 to 28 µg manganese/litre.
Concentrations of manganese ranged from 1 to 530 µg/litre in 37 rivers
in the United Kingdom and in the Rhine and the Maas and their
tributaries (Alessio & Lucchini, 1996).
Concentrations of manganese in surface water are usually reported
as dissolved manganese. Total manganese might be a better indicator,
because manganese adsorbed to suspended solids can exceed dissolved
manganese in many systems, and the bioavailability of manganese in
this form has not been established (NAS, 1977; US EPA, 1984).
Natural ("background") levels of manganese in soil range from 40
to 900 mg/kg, with an estimated mean of 330 mg/kg (Cooper, 1984; US
EPA, 1985a; Schroeder et al., 1987; Eckel & Langley, 1988; Rope et
al., 1988). Accumulation of manganese in soil usually occurs in the
subsoil and not on the soil surface (WHO, 1981).
According to a National Research Council of Canada report (Stokes
et al., 1988), manganese concentrations in air tend to be lowest in
remote locations (about 0.5-14 ng/m3 on average), higher in rural
areas (40 ng/m3 on average), and still higher in urban areas (about
65-166 ng/m3 on average) (see Table 2). Similar concentrations have
been reported elsewhere, leading to the conclusion that annual
manganese concentrations average 10-30 ng/m3 in areas far from known
sources and 10-70 ng/m3 in urban and rural areas without major point
sources of manganese (WHO, 1999). Manganese concentrations in air tend
to be highest in source-dominated areas (e.g., those with foundries),
where values can reach 8000 ng/m3 (US EPA, 1984; Stokes et al.,
1988). Annual averages of manganese concentrations in air near
foundries may rise to 200-300 ng/m3 and to over 500 ng/m3 in air
near ferro- and silicomanganese industries (WHO, 1999).
Table 2: Average levels of manganese in air.
a) Atmospheric air (worldwide)a:
Type of location Average
concentration (ng/m3) Range(ng/m3)
Remote
Continental 3.4 <0.18-9.30
Oceanic 14.2 0.02-79
Polar 0.5 0.01-1.5
Rural 40 6.5-199
Urban
Canada 65 20.0-270
USA 93 5.0-390
Europe 166 23.0-850
Other 149 10.0-590
b) US ambient airb:
Type of
location Concentration (ng/m3)
1953-1957 1965-1967 1982
Nonurban 60 12 5
Urban 110 73 33
Source dominated No data 250-8300 130-140
a Adapted from Stokes et al. (1988).
b Adapted from US EPA (1984).
Manganese concentrations in air have been measured in many
specific locations. In the Vancouver, Canada, area, for example,
annual geometric mean concentrations of manganese ranged from <10 to
30 ng/m3 in 1984 (Stokes et al., 1988). Over the period of 1981-1992,
Loranger & Zayed (1994) found average manganese concentrations in
Montreal, Canada, of 20 and 60 ng/m3 in areas of low and high traffic
density, respectively. More recently, Loranger & Zayed (1997) found
the average concentration of total manganese in an urban site in
Montreal to be 27 ng/m3. In selected periods in the 1970s, annual
mean concentrations of manganese were reported to range from 3 to 16
ng/m3 in two German cities, from 42 to 455 ng/m3 in Belgium, and
from 20 to 800 ng/m3 in Japanese cities (WHO, 1999).
As Table 2 shows, manganese concentrations in air in the USA have
decreased over the past three decades (Kleinman et al., 1980; US EPA,
1984), a trend believed to be due primarily to the installation of
industrial emission controls (US EPA, 1984, 1985b). In Ontario,
Canada, as well, annual average manganese concentrations in air have
decreased along with total suspended particulate levels (Stokes et
al., 1988).
6.2 Human exposure
The most significant source of manganese exposure for the general
population is food (Table 3). A summary of mean manganese
concentrations in 234 foods analysed by the US Food and Drug
Administration is presented in Table 4. Although wide ranges of
manganese concentrations in foods have been reported, the highest
manganese concentrations are found in nuts (up to 47 µg/g) and grains
(up to 41 µg/g). Lower levels are found in milk products (0.02-0.49),
meat, poultry, fish, and eggs (0.10-3.99 µg/g), and fruits (0.20-10.38
µg/g). Tea and leafy green vegetables have also been found to be
dietary sources of manganese (Davis et al., 1992). The US
concentrations given in Table 4 are generally similar to
concentrations reported from other countries. For example, during a
1992 survey conducted by Canada's Department of Fisheries and Oceans,
manganese was detected in muscle samples from bluefin tuna ( Thunnus
thynnus) (Hellou et al., 1992); concentrations in 14 samples ranged
from 0.16 to 0.31 µg/g dry weight, with a mean of 0.22 µg/g.
Although manganese is considered an essential element, a
Recommended Daily Allowance (RDA) has not been established in the USA
because of insufficient data (NRC, 1989). However, the Food and
Nutrition Board of the US National Research Council establishes
Estimated Safe and Adequate Daily Dietary Intake (ESADDI) levels when
data are insufficient to establish an RDA. These levels generally
parallel amounts of the compound usually delivered via the diet,
although some individuals consume greater or smaller amounts. The
ESADDI levels for manganese are 0.3-0.6 mg/day for infants up to 6
months old, 0.6-1.0 mg/day for infants 6 months to 1 year old, 1.0-1.5
mg/day for children 1-3 years old, 1.0-2.0 mg/day for children 4-10
years old, and 2.0-5.0 mg/day for people over 10 years old (NRC,
1989).
Table 3 presents an example of manganese intake from foodstuffs
based on estimated dietary patterns in the USA. Manganese intake among
individuals varies greatly, however, depending upon dietary habits.
For example, an average cup of tea contains 0.4-1.3 mg manganese, so
individuals consuming three cups of tea per day can receive negligible
amounts of manganese or up to 4 mg daily from this source alone
(Schroeder et al., 1966; Pennington et al., 1986). Thus, some persons
consume more or less than the estimated daily intakes noted above
(NAS, 1980; Pennington et al., 1986; Davis et al., 1992). Indeed,
estimates of daily intake for adults in the USA range from 2.0 to
8.8 mg (NAS, 1977; Patterson et al., 1984; US EPA, 1984; WHO, 1984;
Pennington et al., 1986).
Although gastrointestinal absorption of manganese is only 3-5%
(Mena et al., 1969; Davidsson et al., 1988) (see section 7), food is
not only the largest source of manganese exposure in the general
population, but also the primary source of absorbed manganese
(Table 3). The bioavailability of manganese from vegetable sources is
substantially decreased by dietary components such as fibre and
phytates (US EPA, 1993). Individuals with iron deficiency exhibit
increased rates of manganese absorption (Mena et al., 1969, 1974).
In 1962, the public drinking-water supplies in 100 large cities
in the USA were surveyed, and 97% contained less than 100 µg
manganese/litre (Durfor & Becker, 1964). A 1969 survey of 969 systems
reported that 91% contained less than 50 µg/litre, with a mean
concentration of 22 µg/litre (ATSDR, 1996). In the Federal Republic of
Germany, mean concentrations of manganese in drinking-water were
reported to range from 1 to 63 µg/litre (Alessio & Lucchini, 1996).
Certain groups are more highly exposed to manganese than the
general population. Infants given prepared infant foods and formulas,
for example, may be more highly exposed to manganese than adults in
the general population. Collipp et al. (1983) reported that
concentrations of manganese in infant formulas range from 34 to
1000 µg/litre, compared with concentrations of 10 µg/litre in human
milk and 30 µg/litre in cow's milk; Lavi et al. (1989) found an even
lower concentration of manganese in market milk (16 + 2 µg/litre),
suggesting that the difference between formula and milk could be even
greater in some regions. Because of the high manganese levels in
prepared infant foods and formulas, some infants might ingest more
than the ESADDI for their age group (Pennington et al., 1986; NRC,
1989).
In addition, people living in the vicinity of ferromanganese or
iron and steel manufacturing facilities, coal-fired power plants, or
hazardous waste sites can be exposed to elevated manganese particulate
matter in air, although this exposure is likely to be much lower than
in the workplace. Loranger & Zayed (1997) estimated average exposure
doses of respirable manganese and total manganese in an urban site
(botanical gardens) in Montreal, Canada, to be 0.005 and 0.008 µg/kg
Table 3: Summary of typical human exposure to manganese.a
Parameter Exposure medium
Water Air Food
Typical concentration 4 µg/litre 0.023 µg/m3 1.28 µg/calorie
in medium
Assumed daily 2 litres 20 m3 3000 calories
intake of medium
by 70-kg adult
Estimated average 8 µg 0.46 µgb 3800 µg
daily intake by
70-kg adult
Assumed 0.03c 1c 0.03d
absorption fraction
Approximate 0.24 µg 0.46 µg 114 µg
absorbed dose
a Adapted from US EPA (1984).
b Assumes 100% deposition in the lungs.
c No data; assumed value.
d Davidsson et al. (1988).
Table 4: Manganese concentrations in selected foods.a
Type of food Range of mean concentrations
(ppm; µg/g or mg/litre)
Nuts and nut products 18.21-46.83
Grains and grain products 0.42-40.70
Legumes 2.24-6.73
Fruits 0.20-10.38
Fruit juices and drinks 0.05-11.47
Vegetables and vegetable products 0.42-6.64
Desserts 0.04-7.98
Infant foods 0.17-4.83
Meat, poultry, fish, and eggs 0.10-3.99
Mixed dishes 0.69-2.98
Condiments, fats, and sweeteners 0.04-1.45
Beverages (including tea) 0.00-2.09
Soups 0.19-0.65
Milk and milk products 0.02-0.49
a Adapted from Pennington et al. (1986).
body weight per day (0.35 and 0.56 µg/day for a 70-kg person),
respectively. Similarly, the daily intake of manganese in the air by
the general US population was estimated to be less than 2 µg (WHO,
1981). According to a study by Pellizari et al. (1992) and subsequent
analyses by the US EPA (1994a, 1994b), measurements of personal
exposure levels in an urban area in the USA (Riverside, California) in
1990 indicated that about half the population had 24-h personal
exposures to PM10 (particulate matter with an aerodynamic diameter
less than or equal to 10 µm) manganese above 0.035 µg/m3 (0.7 µg/day,
assuming a ventilation rate of 20 m3/day), while the highest 1% of
the population had exposures above 0.223 µg/m3 (4.46 µg/day). By
contrast, intakes in areas of the USA with ferro- or silicomanganese
industries were as high as 10 µg/day, with 24-h peak values exceeding
100 µg/day (WHO, 1981).
People living in regions of natural manganese ore deposits or
where manganese-containing materials (e.g., pesticides, batteries) are
used or disposed of can also be exposed to elevated levels of
manganese in soil or water. For example, Kawamura et al. (1941)
reported on six Japanese families exposed to high levels (at least
14 mg/litre) of manganese in their drinking-water; the contamination
was believed to result from manganese that leached from batteries
buried near the well. Children are especially likely to receive
elevated doses from manganese-containing soils because they have a
higher intake of soil (mainly through hand-to-mouth contact) than
adults (Calabrese et al., 1989). Organomanganese compounds such as MMT
can be absorbed through the skin (Tanaka, 1994).
In the workplace, exposure to manganese is most likely to occur
by inhalation of manganese fumes or manganese-containing dusts. These
dusts can contain various manganese oxides as well as manganese in the
oxides of other elements, such as potassium permanganate, manganese
ferric oxide (MnFe2O4), and manganese silicate (MnSiO3) (Pflaumbaum
et al., 1990). Exposure is a concern mainly in the ferromanganese,
iron and steel, dry-cell battery, and welding industries (WHO, 1986).
Exposure can also occur during manganese mining and ore processing,
and dermal exposure and inhalation can occur during the application of
manganese-containing fungicides.
Manganese air concentrations of 1.5-450 mg/m3 have been reported
in US manganese mines (US EPA, 1984), 0.30-20 mg/m3 in ferroalloy
production facilities (Saric et al., 1977), 0.02-5 mg/m3 in German
foundries (Coenen et al., 1989), 1-4 mg/m3 during welding with
electrodes (Sjögren et al., 1990), up to 14 mg/m3 during welding with
welding wire (Pflaumbaum et al., 1990), and 3-18 mg/m3 in a dry-cell
battery facility (Emara et al., 1971). Many of the more recent studies
on occupational exposures to manganese have recorded average exposure
levels of 1 mg manganese/m3 or less in the workplace (Roels et al.,
1987, 1992; Mergler et al., 1994; Lucchini et al., 1995). Thus, for
workers in industries using manganese, the major route of exposure
might be inhalation from workplace air rather than ingestion of food.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
Manganese absorption occurs primarily from the gastrointestinal
tract after ingestion and from the alveolar lining after inhalation of
manganese-containing dust or fumes. Several studies in animals
indicate that key determinants of absorption are the absorption
pathway and the specific compound in which manganese is present (Smith
et al., 1995; Roels et al., 1997). Roels et al. (1997) studied
manganese levels in the blood and brain tissue of rats exposed to
repeated doses of manganese chloride or manganese dioxide administered
by oral gavage, intraperitoneal injection, or intratracheal
instillation. Manganese chloride was readily absorbed after
administration by each of these routes and distributed in brain tissue
to varying degrees. Manganese dioxide, on the other hand, was
significantly absorbed and distributed in the brain to varying degrees
when administered by intraperitoneal injection and intratracheal
instillation, but not when administered orally. Higher levels of
manganese in tissue were found after administering manganese chloride
by intratracheal instillation compared with manganese dioxide. The
authors concluded that the route of exposure might be a critical
determinant of how absorbed manganese is distributed in the brain. In
addition, when manganese dioxide was administered by either
intratracheal instillation or oral gavage, manganese levels in the
blood rose and fell more slowly than when manganese chloride was
given, indicating a marked difference in the absorption kinetics of
these two manganese compounds. The finding that the body handles
manganese dioxide more slowly than manganese chloride suggests that
manganese dioxide might remain in the body longer, contributing longer
to body burden, albeit at much lower levels. Whether this is true and
whether this indicates greater toxicological risk in cases of
prolonged low-level exposure to manganese dioxide are unclear.
A second study also found that route of exposure affects
absorption of manganese. Tjälve et al. (1996) found that intranasal
instillation of manganese (Mn2+) in rats resulted in initial uptake
of manganese in the olfactory bulbs of the brain, whereas
intraperitoneal administration resulted in low uptake in the olfactory
bulbs. The authors suggested that olfactory neurons might serve as a
pathway for manganese uptake and distribution to the brain (bypassing
the blood-brain barrier) during intranasal exposure.
Another key determinant of absorption appears to be dietary iron
intake, with low iron levels leading to increased manganese absorption
(Mena et al., 1969). In addition, several studies in animals indicate
that gastrointestinal absorption of manganese might vary with age
(Rehnberg et al., 1980, 1981).
The amount of manganese absorbed across the gastrointestinal
tract in humans varies, but typically averages about 3-5% (Mena
et al., 1969; Davidsson et al., 1988). Particles that are deposited in
the lower airways are probably absorbed, whereas particles deposited
in the upper airways are generally swallowed via mucociliary
clearance; thus, they can be absorbed from the gastrointestinal tract
as well.
Regardless of manganese intake, adult humans generally maintain
stable tissue levels of manganese through a homeostatic mechanism
regulating the excretion of excess manganese (US EPA, 1984). The major
route of manganese excretion is via the bile, although some excretion
occurs in urine, milk, and sweat (US EPA, 1993).
Limited data suggest that manganese can undergo changes in
oxidation state within the body. Support for this hypothesis comes
from the observation that the oxidation state of the manganese ion in
several enzymes appears to be Mn(III) (Utter, 1976; Leach & Lilburn,
1978), whereas most manganese intake from the environment is as Mn(II)
or Mn(IV). The rate and extent of manganese reduction/oxidation
reactions might be important determinants of manganese retention in
the body.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
Lung inflammation has been reported following single inhalation
exposures to 2.8-43 mg/m3 for manganese dioxide or manganese
tetroxide particulates in rodent species (Bergstrom, 1977; Adkins et
al., 1980; Shiotsuka, 1984). It is important to note that an
inflammatory response of this type is not unique to
manganese-containing particles, but is characteristic of nearly all
inhalable particulate matter (US EPA, 1985b). Thus, it might not be
manganese alone that causes the inflammatory response from single
exposures, but possibly the particulate matter itself.
Following single oral exposures, LD50s ranged from 275 to 804
mg/kg body weight per day for manganese chloride in different rat
strains (Holbrook et al., 1975; Kostial et al., 1989; Singh &
Junnarkar, 1991). Reported LD50s from single exposures to manganese
sulfate and manganese acetate in rats were 782 and 1082 mg/kg body
weight per day, respectively (Smyth et al., 1969; Singh & Junnarkar,
1991).
8.2 Irritation and sensitization
Little information is available on the irritant and contact
sensitivity properties of manganese compounds. Manganese salts failed
to induce lymph node cell proliferation in the murine local lymph node
assay, a predictive test for the detection of contact allergens
(Ikarashi et al., 1992). The manganese-containing fungicide maneb has
been reported to be a sensitizer in animal tests, but little
information exists on whether this effect occurs in humans (Thomas et
al., 1990). Contact sensitization in humans has been reported in one
study (see section 9.2).
8.3 Short-term exposure
Results from studies of short-term exposures in experimental
animals indicate that the lungs and nervous system are the major
target organs following the inhalation of manganese compounds. For
example, Maigetter et al. (1976) found increased susceptibility to
pneumonia in mice exposed via inhalation to 69 mg manganese/m3 as
manganese dioxide for 3 h/day for 1-4 days. Effects on the nervous
system associated with short-term exposure to manganese compounds are
presented in section 8.7.
8.4 Long-term exposure
8.4.1 Subchronic exposure
Results from studies of subchronic exposures in experimental
animals also indicate that the lungs and nervous system are the major
target organs following the inhalation of manganese compounds. Signs
of lung inflammation have been reported in rhesus monkeys exposed via
inhalation to 0.7 mg manganese/m3 as manganese dioxide for 22 h/day
over 10 months (Suzuki et al., 1978). Effects on the nervous system
associated with subchronic exposure to manganese compounds are
presented in section 8.7.
Systemic effects reported following subchronic oral exposures to
manganese compounds include changes in blood cell counts (leukocytes,
erythrocytes, neutrophils), reduced liver weight, and decreased body
weight (Gray & Laskey, 1980; Komura & Sakamoto, 1991; NTP, 1993). In
mice fed 284 mg manganese/kg body weight per day for 100 days, for
example, red blood cell count was decreased by manganese acetate and
manganese chloride; white blood cell count was decreased by manganese
acetate, manganese chloride, and manganese dioxide; and haematocrit
was decreased by manganese carbonate (MnCO3) (Komura & Sakamoto,
1991).
8.4.2 Chronic exposure and carcinogenicity
Available data from animal studies involving oral exposure to
manganese as well as from epidemiological studies involving inhalation
exposure to manganese suggest that similar chronic toxicities (i.e.,
neurological effects) occur regardless of the valence state of the
inorganic manganese compounds (e.g., manganese dioxide, manganese
tetroxide). In experimental animals, the nervous system is the major
organ affected following long-term oral and inhalation exposure to
manganese. These data are described in more detail in section 8.7. Few
chronic inhalation exposure studies in animals are available, and
these studies reported effects in the nervous system. Significant
effects in other organ systems following long-term exposure to
manganese have not been reported. Available data from animal studies
suggest that it is unlikely that other significant effects result from
long-term oral exposure to manganese (NTP, 1993).
Information on the carcinogenic potential of manganese is
limited, and the results are difficult to interpret with certainty.
For example, male rats exposed to up to 331 mg manganese/kg body
weight per day (as manganese sulfate) for 2 years had an increased
incidence of pancreatic cell adenomas (3/50, 4/51, and 2/51 in the
low, mid, and high dose groups); this type of tumour was noted in only
one female in the mid dose group. The investigators indicated that
these lesions, although low in incidence, were "a concern" and
attributed to manganese treatment because pancreatic cell hyperplasia
was observed in all treatment groups, although neither hyperplasia nor
adenomas were observed in controls of either sex (Hejtmancik et al.,
1987a). On the other hand, a small increase in the incidence of
pituitary adenomas was noted in female mice at 905 mg manganese/kg
body weight per day (as manganese sulfate), but not in males at 722 mg
manganese/kg body weight per day. The incidence was considered
equivocal because lesions had been observed in previous studies as
well as in historical controls (Hejtmancik et al., 1987b). In a 2-year
study, no evidence of cancer was noted in male and female F344 rats
given 20-200 and 23-232 mg manganese sulfate/kg body weight per day,
respectively, via feed (NTP, 1993). A marginally increased incidence
of thyroid gland follicular cell adenomas was observed in male and
female B6C3F1 mice given 52-585 and 65-731 mg manganese sulfate/kg
body weight per day, respectively, in the feed for 2 years (NTP,
1993). Intraperitoneal injection of mice with manganese sulfate
(20 weeks) led to an increased incidence of lung tumours (Stoner
et al., 1976), but intramuscular injection of rats and mice with
manganese or manganese dioxide did not result in tumours (Furst,
1978). Firm conclusions on the carcinogenic potential of manganese
cannot be made based on the equivocal carcinogenicity data reported
for rodents and the paucity of evidence from other species.
8.5 Genotoxicity and related end-points
Manganese sulfate was not mutagenic to Salmonella typhimurium
strains TA97, TA98, TA100, TA1535, or TA1537 in either the presence or
absence of S9 from Aroclor 1254-induced liver from rats or Syrian
hamsters in studies performed at two different laboratories
(Mortelmans et al., 1986), but it was reported elsewhere to be
genotoxic to strain TA97 (Pagano & Zeiger, 1992). Manganese chloride
was not mutagenic in S. typhimurium strains TA98, TA100, and TA1535,
but it was mutagenic in TA1537, and conflicting results were obtained
for TA102 (Wong, 1988; De Méo et al., 1991). A fungal gene conversion/
reverse mutation assay in Saccharomyces cerevisiae strain D7
indicated that manganese sulfate was mutagenic (Singh, 1984).
Manganese chloride produced gene mutations in vitro in a mouse
lymphoma assay (Oberly et al., 1982). It also caused DNA damage in
human lymphocytes when tested in vitro using the single-cell gel
assay technique in the absence of metabolic activation, but it caused
no DNA damage when S9 was present (De Méo et al., 1991). The results
of an in vitro assay using Chinese hamster ovary (CHO) cells showed
that manganese sulfate induced sister chromatid exchange in both the
presence and absence of S9 from Aroclor 1254-induced rat liver
(Galloway et al., 1987). In a separate assay, manganese sulfate also
induced chromosomal aberrations in CHO cells in the absence of S9 but
not in its presence (Galloway et al., 1987). In contrast, manganese
chloride was not clastogenic when tested in vitro in the absence of
metabolic activation using FM3A cells (Umeda & Nishimura, 1979),
although it did cause chromosomal aberrations in the root tips of
Vicia faba (Glass, 1955, 1956). Potassium permanganate caused
chromosomal aberrations in FM3A cells (Umeda & Nishimura, 1979) but
not in a primary culture of cells from Syrian hamster embryos (Tsuda &
Kato, 1977) when tested in the absence of metabolic activation.
Magnesium chloride caused cell transformation in Syrian hamster embryo
cells (Casto et al., 1979).
Manganese chloride did not produce somatic mutations in
Drosophila melanogaster fruit flies (Rasmuson, 1985). Manganese
sulfate did not induce sex-linked recessive lethal mutations in the
germ cells of male D. melanogaster (Valencia et al., 1985).
In vivo assays in mice showed that oral doses of manganese
sulfate or potassium permanganate caused micronuclei and chromosomal
aberrations in bone marrow (Joardar & Sharma, 1990). In contrast, oral
doses of manganese chloride did not cause chromosomal aberrations in
the bone marrow or spermatogonia of rats (Dikshith & Chandra, 1978).
The results of in vitro studies show that at least some
chemical forms of manganese have mutagenic potential. However, as the
results of in vivo studies in mammals are inconsistent, no overall
conclusion can be made about the possible genotoxic hazard to humans
from exposure to manganese compounds.
8.6 Reproductive and developmental toxicity
Considerable information is available on the reproductive and
developmental effects of manganese in animals. Mice exposed
subcutaneously to 0, 2, 4, 8, or 16 mg manganese chloride
tetrahydrate/kg body weight per day on gestation days 6-15 showed no
treatment-related effects on the number of total implants, early
resorptions, dead fetuses, or sex ratio. However, a significant
increase in the number of late resorptions was found in the 4, 8, and
16 mg/kg body weight per day groups. Significant maternal toxicity was
associated with the 8 and 16 mg/kg body weight per day groups (Sánchez
et al., 1993). A single intratracheal dose of 160 mg manganese/kg (as
manganese dioxide) in rabbits caused slow degenerative changes in the
seminiferous tubules and led to sterility (Seth et al., 1973; Chandra
et al., 1975). Abnormal sperm morphology was observed in mice treated
with 23-198 mg manganese/kg body weight per day as potassium
permanganate or manganese sulfate by gavage in water for up to 3 weeks
(Joardar & Sharma, 1990). No gross or histopathological lesions or
organ weight changes were observed in the reproductive organs of
rodents exposed to 1300 mg manganese/kg body weight per day for 14
days or fed up to 1950 mg manganese/kg body weight per day for 13
weeks (NTP, 1993). From the available evidence, no firm conclusions on
effects in male reproductive organs can be made, and reproductive
performance was not evaluated in many of these studies.
A slight decrease in pregnancy rate was observed in female rats
exposed to 130 mg manganese/kg body weight per day as manganese
tetroxide in the diet for 90-100 days before breeding (Laskey et al.,
1982). Female reproductive parameters such as litter size, ovulations,
resorptions, or fetal weights were not affected in rats consuming
excess manganese as manganese tetroxide in feed or water (Laskey et
al., 1982; Kontur & Fechter, 1985), except at concentrations so high
(1240 mg/kg body weight per day) that water intake by the dams was
severely reduced. In mice, inhalation exposure of females to 85 mg
manganese/m3 (as manganese dioxide) for 16 weeks prior to conception
and 17 days after conception led to a decrease in average pup weight
at birth and decreased activity levels (Lown et al., 1984). Webster &
Valois (1987) found that intraperitoneal injection of pregnant mice
with 12.5 mg manganese/kg body weight (as manganese sulfate) on days
8-10 of gestation resulted in exencephaly and embryolethality.
Finally, manganese chloride administered by gavage at doses of 0, 25,
50, or 75 mg/kg body weight per day caused major dose-dependent
abnormalities in the fetuses when administered to gestating rats for
the duration of gestation, but did not cause major abnormalities in
the fetuses when administered to pregnant rabbits during the period of
organogenesis (Szakmáry et al., 1995).
In a rat teratology study, intravenous injection of 20 µmol
manganese chloride/kg body weight (1.1 mg manganese/kg body weight) on
days 6-17 of pregnancy induced mild skeletal malformations in the
fetuses; the no-observed-adverse-effect level (NOAEL) was 0.28 mg
manganese/kg body weight (Treinen et al., 1995). Similar effects were
observed in another study (Grant & Ege, 1995) when administration was
by injection, but not when manganese was administered by gavage at
400 µmol manganese chloride/kg body weight (22 mg manganese/kg body
weight). These results suggest that parenteral administration has a
much greater potential for developmental toxicity than oral exposure.
In rabbits exposed to manganese by intratracheal instillation, a
single dose of 160 mg manganese/kg body weight (as manganese dioxide)
resulted in a slow degeneration of the seminiferous tubules over a
period of 1-8 months. This was associated with loss of spermatogenesis
and complete infertility (Seth et al., 1973; Chandra et al., 1975).
Similar degenerative changes in testes have been observed in rats and
mice following intraperitoneal injection of manganese sulfate (Singh
et al., 1974; Chandra et al., 1975) and in rabbits following
intravenous injection of manganese chloride (Imam & Chandra, 1975).
8.7 Immunological and neurological effects
As with exposure to other airborne particulate matter, an
increased susceptibility to infection has been observed in mice and
guinea-pigs exposed to manganese via inhalation for a short period
(Maigetter et al., 1976; Adkins et al., 1980). Altered blood levels of
leukocytes, lymphocytes, and neutrophils have been observed in rats
and mice that ingested manganese in the feed for short-term (33 mg/kg
body weight per day for 14 days) or subchronic (284 mg/kg body weight
per day for 100 days) durations (Komura & Sakamoto, 1991; NTP, 1993).
However, it is unknown if these changes are associated with any
significant impairment of the immune system.
No evidence of neurological effects was seen in rhesus monkeys
(0.01-1.1 mg manganese tetroxide/m3) or macaque monkeys (20-40 mg
manganese chloride/m3) exposed to manganese via inhalation over
subchronic and chronic periods (Ulrich et al., 1979). However,
intravenous administration of 5-40 mg manganese/kg (as manganese
chloride) to cebus monkeys did result in movement tremors accompanied
by increased manganese in the globus pallidus and substantia nigra
regions of the brain (Newland & Weiss, 1992). Decreased levels of
dopamine were found in several regions of the brain (caudate and
globus pallidus) in rhesus monkeys exposed to 30 mg manganese/m3 (as
manganese dioxide) via inhalation for 2 years (Bird et al., 1984).
A decrease in pup retrieval behaviour was observed in maternal
mice exposed to 61 mg manganese/m3 (as manganese dioxide) via
inhalation for 18 weeks (Lown et al., 1984). In another study,
Morganti et al. (1985) observed moderate changes in open-field
behaviour in mice exposed to 72 mg manganese/m3 (as manganese
dioxide) for 18 weeks.
In general, effects from inhalation exposure to manganese in
experimental animals occur at levels higher (30-70 mg manganese/m3)
than those at which effects have been reported in humans (0.14-1 mg
total manganese dust/m3 for preclinical neurological alterations and
2-22 mg total manganese dust/m3 for overt neurological disease). This
evidence suggests that laboratory animals, especially rodents, might
not be as sensitive as humans, and possibly other primates, to the
neurological effects of inhalation exposure to manganese.
There are substantial data on neurological effects in animals
following ingestion of manganese. In one study, decreases in
spontaneous activity, alertness, touch response, muscle tone, and
respiration were observed in mice dosed once by oral gavage with 58 mg
manganese/kg body weight (as manganese chloride) (Singh & Junnarkar,
1991). Rats developed a rigid and unsteady gait after 2-3 weeks of
exposure to a higher level (150 mg/kg body weight per day) of
manganese chloride (Kristensson et al., 1986).
Mice ingesting food containing manganese chloride, manganese
acetate, manganese carbonate, or manganese dioxide (284 mg/kg body
weight per day) for 100 days or manganese tetroxide (137 mg/kg body
weight per day) for 90 days showed significantly decreased motor
activity (Gray & Laskey, 1980; Komura & Sakamoto, 1991). Two of the
third-generation mice exhibited staggered gait and histochemical
changes after drinking water containing manganese chloride (10.6 mg/kg
body weight per day) over three generations (Ishizuka et al., 1991).
Conversely, rats showed increased activity and aggression when exposed
to 140 mg manganese chloride/kg body weight per day in drinking-water
for 4 weeks (Chandra, 1983) and just increased activity when exposed
to 40 mg manganese chloride/kg body weight per day for 65 weeks
(Nachtman et al., 1986).
Numerous studies have reported alterations in brain
neurotransmitter levels and function, brain histochemistry, or
neuronal enzyme function. These neurochemical changes have been
observed in rats and mice following ingestion of manganese (as
manganese chloride) administered via the feed, drinking-water, or
gavage (in water) at doses ranging from 1 to 2270 mg manganese/kg body
weight over intermediate exposure periods (i.e., 14-364 days)
(Bonilla, 1978; Chandra & Shukla, 1978; Deskin et al., 1980; Gianutsos
& Murray, 1982; Chandra, 1983; Bonilla & Prasad, 1984; Ali et al.,
1985; Eriksson et al., 1987; Subhash & Padmashree, 1991). Similar
alterations were reported after chronic exposures (>365 days) to 275
mg manganese dioxide/kg body weight in the feed of mice (Komura &
Sakamoto, 1992) or 40 mg manganese chloride/kg body weight in
drinking-water of rats (Lai et al., 1984).
Neurochemical alterations have also been reported in rats
following intraperitoneal injection of manganese at doses ranging from
2.2 to 4.4 mg manganese chloride/kg body weight over intermediate
exposure periods (Sitaramayya et al., 1974; Shukla et al., 1980; Seth
et al., 1981). Decreased neurotransmitter receptor binding was
observed in macaca monkeys following subcutaneous injection of
manganese dioxide at 38 mg/kg body weight for 26 months (Eriksson
et al., 1992). Changes in region-specific neuronal populations were
reported in rats receiving manganese chloride from their
drinking-water for either 4 or 8 weeks (Sarhan et al., 1986). The
actual manganese dose administered over the total experimental period
was not reported by the authors. However, daily intakes of at least
10.7 mg manganese/kg body weight are estimated based on initial
average body weight and water intake reported in the study.
Neurobiochemical changes have been detected in neonate rats at
doses similar to or slightly above dietary levels (1-10 mg
manganese/kg body weight per day for 24-60 days, as manganese
chloride) (Chandra & Shukla, 1978; Deskin et al., 1980), which could
indicate that young animals may be more susceptible to manganese than
adults. Oner & Senturk (1995) demonstrated that manganese induces
learning deficits in rats dosed with 357 µg manganese/kg body weight
for 15 or 30 days; these effects were reversible.
9. EFFECTS ON HUMANS
A requirement for manganese in humans was determined based on
symptoms observed in a subject inadvertently fed a diet deficient in
manganese for 3.5 months (Doisy, 1972). It has been determined that
manganese is needed for the functioning of key enzymes that play a
role in cellular protection from damaging free radical species,
maintenance of healthy skin, and synthesis of cholesterol
(Freeland-Graves et al., 1987; Friedman et al., 1987). Based upon
case-studies in people with low blood manganese and known requirements
in animals, it is thought that manganese may also play a role in bone
mineralization, metabolism of proteins, lipids, and carbohydrates,
energy production, metabolic regulation, and nervous system
functioning (Schroeder et al., 1966; Freeland-Graves et al., 1987;
Hurley & Keen, 1987; Freeland-Graves & Llanes, 1994; Wedler, 1994).
However, the link between inadequate manganese nutrition and its role
in these body functions in humans requires further investigation.
Manganism is a progressive, disabling neurological syndrome that
typically begins with relatively mild symptoms and evolves to include
dull affect, altered gait, fine tremor, and sometimes psychiatric
disturbances. Because some of these symptoms resemble those of
Parkinson's disease, many investigators have used terms such as
"Parkinsonism-like disease" and "manganese-induced Parkinsonism" to
describe symptoms observed with manganese poisoning. Although symptoms
of manganism resemble those of Parkinson's disease, significant
differences have been noted. In terms of clinical presentation,
Barbeau (1984) noted that the hypokinesia and tremor present in
patients suffering from manganism differed from those seen in
Parkinson's disease. Drawing from the literature, Calne et al. (1994)
noted other features that can also distinguish manganism from
Parkinson's disease; psychiatric disturbances early in the disease (in
some cases), the "cock walk" (see below), a propensity to fall
backward when displaced, less frequent resting tremor, more frequent
dystonia, and failure to respond to dopaminomimetics (at least in the
late stages of the disease) were characteristic of manganism. Beuter
et al. (1994) showed that 10 manganese-exposed workers (average
exposure of 13.9 years; average blood manganese level of 1.06 µg/dl)
and 11 patients with Parkinsonism were significantly different from
the controls ( n = 11) in functional asymmetries between right and
left hand. Therefore, use of terms such as "Parkinsonism-like disease"
and "manganese-induced Parkinsonism" are somewhat misleading.
Nonetheless, the use of these terms may help health providers and
health surveillance workers recognize the effects of manganese
poisoning when encountering it for the first time in occupational or
environmental settings. These terms appear in the discussion below
when they were used by study authors in their reports (shown in
italics). The term "manganism" is used as well.
Long-term exposures to manganese in occupational settings can
result in a progressive neurological dysfunction, which can produce a
disabling syndrome referred to as manganism. Mergler & Baldwin (1997)
have described this disease progression as a "slow deterioration of
well-being which can be initially detected as early neurofunctional
alterations... [among exposed groups], later on, as sub-clinical signs
in individuals... and finally as a full blown neurological disease
-- manganism." Progression along this continuum is thought to be a
function of the dose and duration of exposure, as well as individual
susceptibilities. In general, the clinical effects of high-level
inhalation exposure to manganese do not become apparent until exposure
has occurred for several years, but some individuals begin to show
signs of neurological alterations after as little as 1-3 months of
exposure (Rodier, 1955).
Pathological findings in manganism and Parkinson's disease also
differ. In humans with chronic manganese poisoning, lesions are more
diffuse, found mainly in the pallidum, the caudate nucleus, the
putamen, and even the cortex. In people with Parkinson's disease,
lesions are found in the substantia nigra and other pigmented areas of
the brain (Barbeau, 1984). Moreover, Lewy bodies are usually not found
in substantia nigra in cases of manganism, but are almost always found
in cases of Parkinson's disease (Calne et al., 1994). Magnetic
resonance imaging of the brain reveals accumulation of manganese in
cases of manganism, but little or no changes in people with
Parkinson's disease; fluorodopa positron emission tomography scans are
normal in cases of manganism, but abnormal in people with Parkinson's
disease (Calne et al., 1994).
The first signs of manganism are usually subjective and
non-specific, often involving generalized feelings of weakness,
heaviness or stiffness of the legs, anorexia, muscle pain,
nervousness, irritability, and headache (Rodier, 1955; Whitlock
et al., 1966; Mena et al., 1967; Tanaka & Lieben, 1969; Sjögren et
al., 1996). These signs are frequently accompanied by apathy and
dullness, along with impotence and loss of libido; especially in the
case of miners, more extreme manifestations of psychomotor excitement,
such as aggressive or destructive behaviour, emotional lability, and
bizarre compulsive activities, are also associated with the first
stages of manganism (Rodier, 1955; Schuler et al., 1957; Mena et al.,
1967; Emara et al., 1971; Abdel-Hamid et al., 1990; Wennberg et al.,
1991; Chu et al., 1995).
More specific clinical signs of basal ganglia dysfunction
characterize the next stage and can include a slow or halting speech
without tone or inflection, a dull and emotionless facial expression,
slow and clumsy movement of the limbs or altered gait, late motor
deficits, and fine tremor (Rodier, 1955; Schuler et al., 1957; Mena
et al., 1967; Tanaka & Lieben, 1969; Smyth et al., 1973; Yamada et
al., 1986; Ky et al., 1992; Wennberg et al., 1992; Hochberg et al.,
1996; Mergler & Baldwin, 1997).
As the disease progresses, walking becomes difficult and a
characteristic staggering gait develops, the "cock walk," in which
patients strut on their toes, with elbows flexed and the spine erect
(Calne et al., 1994). Muscles become hypertonic, and voluntary
movements can be accompanied by fine tremor (Chu et al., 1995; Mergler
& Baldwin, 1997). In some cases, psychological disturbances (manganese
mania, manganese psychosis) precede or accompany the final stages of
disease (Rodier, 1955; Mena et al., 1967; Cook et al., 1974; Mergler &
Baldwin, 1997). Few data are available regarding the reversibility of
these effects; they are thought to be largely irreversible. Some
evidence indicates that recovery can occur when exposure ceases (Smyth
et al., 1973). Manganism has been documented in welders and in workers
exposed to high levels of manganese dust or fumes in mines or
foundries.
The studies cited above describe overt manganism resulting from
long-term inhalation exposures to 2-22 mg total manganese dust/m3
(Schuler et al., 1957; Whitlock et al., 1966; Tanaka & Lieben, 1969;
Cook et al., 1974; Saric et al., 1977; Huang et al., 1989). Evidence
from recent occupational exposure studies (described below) suggests
that early or preclinical signs of neurological effects can occur in
generally asymptomatic workers exposed to much lower levels of
manganese (about 0.14-1 mg total manganese dust/m3) for several years
(Roels et al., 1987, 1992; Iregren, 1990; Chia et al., 1993; Mergler
et al., 1994; Lucchini et al., 1995). However, the reported values are
only estimates of actual exposure levels. Often, time-weighted
averages of workplace exposures are reported, and dose-response
relationships cannot be determined. In addition, exposures are
generally reported as total manganese dust or the respirable fraction
of total dust, which can be defined differently across studies (e.g.,
PM5 [particulate matter with an aerodynamic diameter less than or
equal to 5 µm] or PM10).
9.1 Case reports
Whitlock et al. (1966) reported a case-study of two workers
exposed to manganese-containing fumes (3.5 mg manganese/m3 average;
no data on exact compounds) from an electric arc used to cut and
cleave manganese castings. Symptoms of ataxia, weakness, and decreased
mental ability developed about 9-12 months following exposure. These
symptoms improved after the patients were treated with
ethylenediamine-tetraacetic acid (EDTA). Rosenstock et al. (1971)
reported a case of a male who developed classic symptoms of manganism
after 14 months of exposure to manganese (dose unknown) from the fumes
and dust of a steel foundry. After being unable to work for 3 years,
the patient was treated with 6-12 g levodopa/day, with the largest
dose providing improvement in facial expression, speech, and muscle
tone. Six men exposed to manganese (22 mg manganese/m3) for an
unspecified period at an ore crushing plant developed signs including
somnolence, abnormal gait, slurred speech, ataxia, masklike faces, and
bradykinesia. Treatment with 8 g levodopa/day did not alleviate the
neurological effects observed in these workers (Cook et al., 1974).
An outbreak of a disease with manganism-like symptoms was
reported in a group of six Japanese families (about 25 people) exposed
to high levels of manganese in their drinking-water (Kawamura et al.,
1941). Symptoms included a masklike face, muscle rigidity and tremors,
and mental disturbance. Five people, all elderly, were severely
affected (2 died), 2 were moderately affected, 8 were mildly affected,
and 10 (all children or young adults) were not affected. These effects
were postulated to be due to the contamination of well-water with
manganese (14 mg/litre) that leached from batteries buried near the
well. Manganese concentrations decreased over time, so the original
level of manganese was probably higher than 14 mg/litre. This case has
been interpreted as an indication that the elderly may be more
sensitive than younger people to the toxic effects of manganese (Davis
& Elias, 1996).
A man noticed weakness and impaired mental capacity after
mistakenly ingesting low doses of potassium permanganate (1.8 mg/kg)
instead of potassium iodide for several weeks to treat lung congestion
(Holzgraefe et al., 1986). Although exposure was stopped after
4 weeks, a syndrome similar to Parkinson's disease developed after
about 9 months. In another case, five patients given manganese
parenterally for an average of 6 years showed early neurological
symptoms of poisoning, while four others, exposed for an average of 4
years, did not (Mirowitz et al., 1991). In a child, accidental
ingestion of potassium permanganate (174 mg/kg) resulted in severe
local corrosion of the mouth, oesophagus, and stomach, but there was
no evidence of systemic toxicity (Southwood et al., 1987).
There are few reports regarding dermal exposure to manganese in
humans. In most cases, manganese uptake across intact skin is expected
to be very limited. However, effects and elevated urinary manganese
levels were observed in a man burned with a hot acid solution
containing 6% manganese (Laitung & Mercer, 1983). There are also
reports of workers experiencing effects from dermal exposure to
organic manganese compounds. Headache and paraesthesia were among the
symptoms reported in workers exposed dermally to MMT after a spill
(doses unknown; Tanaka, 1994). Two young Brazilian agricultural
workers developed Parkinsonian syndrome (Ferraz et al., 1988) and a
37-year-old Italian man developed Parkinsonism (Meco et al., 1994)
after chronic dermal and inhalation exposure to the fungicide maneb.
9.2 Epidemiological studies
The lungs, nervous system, and reproductive system are the main
organs affected following inhalation exposures to manganese, although
other effects have also been observed. For example, in a study of 126
enamellers and 64 decorators from five factories in the ceramics
industry, Motolese et al. (1993) found that 48 workers were sensitized
to at least one substance; positive sensitization test results with
manganese dioxide were found in only 2 of the workers, however. The
remainder of this section focuses on the effects more commonly
reported in epidemiological studies -- lung, nervous system, and
reproductive system effects.
Inhalation of particulate manganese compounds such as manganese
dioxide and manganese tetroxide leads to an inflammatory response in
human lungs. Symptoms and signs of lung irritation and injury can
include cough, bronchitis, pneumonitis, and reductions in lung
function (Lloyd Davies, 1946; Roels et al., 1987; Abdel-Hamid et al.,
1990; Akbar-Khanzadeh, 1993).
Pneumonia has been reported to result from both acute and
long-term inhalation exposure to manganese dioxide dusts (Lloyd
Davies, 1946; Tanaka, 1994). These effects have been noted mainly in
people exposed to manganese dust under occupational conditions,
although respiratory effects have also occurred in residential
populations (WHO, 1987). A higher incidence of pneumonia and a higher
rate of deaths from pneumonia compared with the general population
were observed among residents exposed to manganese dust from a
ferromanganese factory as well as among the factory workers
(WHO, 1987; Tanaka, 1994). However, a threshold level for respiratory
effects has not been established. The increased susceptibility to
respiratory infection might be secondary to the lung irritation and
inflammation caused by inhaled particulate matter rather than caused
by the manganese alone. It is likely that the inflammatory response
begins shortly after exposure and continues for the duration of the
exposure.
Although available studies are not adequate to define the
dose-response curve or determine whether there is a threshold for
neurotoxicity, the lowest level of exposure to manganese dust at which
neurological effects occur was reported by Iregren (1990) and Wennberg
et al. (1991). These investigators compared 30 male workers exposed to
manganese for 1-35 years during employment at two Swedish foundries
with an unexposed control group of 60 workers (matched by age, type of
work, and geographical area) using eight tests from the Swedish
Performance Evaluation System and two additional manual tests. The
mean and median levels of manganese in the foundry air were measured
at 0.25 and 0.14 mg/m3, respectively, and available data indicated
that these levels had been consistent over the past 17-18 years. The
exposed workers exhibited significantly inferior performance in simple
reaction time, digit span, and finger tapping. When a secondary match
was performed, with scores on verbal tests used as an additional
matching criterion (which reduced the size of the reference group to
30), the same test differences remained, although the difference was
not significant for the digit span test. Although the subjects did not
exhibit the signs of clinical manganism described above, these changes
were indicators of manganese-induced neurological effects (Iregren,
1990; Wennberg et al., 1991).
The study results reported by Iregren (1990) and Wennberg et al.
(1991) are supported by evidence presented by Roels et al. (1987,
1992) and Chia et al. (1993, 1995). Roels et al. (1992) detected early
neurological effects in male workers at an alkaline battery plant
exposed to manganese dusts (manganese dioxide). Compared with 101 male
workers without industrial exposure, the 92 exposed workers showed
significantly poorer eye-hand coordination, hand steadiness, and
visual reaction time. A Lifetime Integrated Exposure, for both
respirable and total manganese dust, was estimated for each of the
exposed workers (expressed as exposure in mg manganese/m3 multiplied
by the number of years of exposure, or mg/m3 × year). Based on an
analysis of the data by a logistic regression model, it was suggested
that there was an increased risk of peripheral tremor at a Lifetime
Integrated Exposure level of 3.575 mg/m3 × year total manganese dust
or 0.73 mg/m3 × year respirable (PM5) dust; dividing by an exposure
duration of 5.3 years, these values are equivalent to 0.67 mg/m3 and
0.14 mg/m3 for total manganese dust and respirable manganese dust,
respectively. This total manganese dust exposure level (0.67 mg/m3)
is slightly higher than the median found to be associated with effects
in the 1990 Iregren and the 1991 Wennberg et al. studies (0.14
mg/m3). The Lifetime Integrated Exposure at which an increased risk
of abnormal neurofunction occurs is based on exposures in an
occupational setting and might be biased because of the "healthy
worker effect" (i.e., the most susceptible individuals were not
incorporated into the study).
The Chia et al. (1993) study also reported neurological deficits
in an occupational cohort of 17 manganese "baggers" in Singapore who
were administered the WHO Neurobehavioural Core Test Battery, as well
as several supplementary tests and a subjective questionnaire (with
questions on 37 symptoms related to the nervous system) taken from the
Operational Guide to the Neurobehavioural Core Test Battery. The
exposed workers had significantly poorer motor speed, visual-motor
coordination, visual scanning, visual-motor and response speed, and
visual-motor coordination and steadiness than a control group. Twenty
of the 37 symptoms in the questionnaire were also reported more
frequently by the exposed workers than by the control group, although
the differences were significant only for insomnia and profuse
sweating. The mean manganese level in air (from 1981 to 1991) in the
factories was reported to be 1.59 µg/litre (1.59 mg/m3; 8-hour
time-weighted average). Chia et al. (1995) conducted another study
with a larger group of exposed workers (32 subjects exposed to the
same mean level of manganese in air reported above), focusing in more
detail on postural stability; the exposed workers exhibited
significantly poorer postural stability compared with a control group.
A study by Mergler et al. (1994) also supports the findings of
Iregren (1990), Wennberg et al. (1991), Roels et al. (1987, 1992), and
Chia et al. (1993, 1995). This epidemiological study included 74 male
workers from a ferromanganese and silicomanganese alloy factory,
matched with 74 referents from a pool of 145 non-occupationally
exposed men residing in the vicinity. Environmental levels of total
manganese dust at the factory were measured at 0.014-11.48 mg/m3
(median 0.151 mg/m3; mean 1.186 mg/m3), whereas manganese levels in
respirable dust (PM10 samples) ranged from 0.001 to 1.27 mg/m3
(median 0.032 mg/m3; mean 0.122 mg/m3). The authors noted that
exposures at the factory were known to have been much higher in the
recent past. The mean duration of exposure was 16.7 years. The
manganese-exposed workers showed decreased performance on tests of
motor function, and they exhibited lower levels of cognitive
flexibility, difficulty in set shifting, and lower olfactory
perception thresholds. This is the first study to report the latter
effect (lower olfactory perception threshold). The workers also
displayed significantly greater anger, tension, fatigue, and confusion
as determined by the Profile of Mood States test.
A study by Lucchini et al. (1995) also found evidence of
neurobehavioural effects at exposure levels comparable to those
reported above. During a period of forced cessation from work, 58
clinically asymptomatic workers exposed to manganese dust for periods
of 1-28 years (mean 13 years) were tested for simple reaction time,
finger tapping, digit span, additions, symbol digit, and shapes
comparison. Geometric mean concentrations of manganese in total dust
were measured in different work areas and ranged from 70-1590 µg/m3
(10 years before the study was undertaken) to 27-270 µg/m3 (at the
time of the study). A Cumulative Exposure Index was calculated for
each subject. It took into account the type of job(s) the subject
performed at the plant, the average annual airborne manganese
concentration in respirable dust characteristic of the job(s), and the
duration of employment in the job(s). The authors found correlations
between the Cumulative Exposure Index and performance on the finger
tapping, symbol digit, digit span, and additions tests; higher indices
were associated with poorer performance. In addition, the authors
found correlations between manganese levels in blood and urine of the
workers and performance (the higher the blood and urine levels, the
poorer the performance) when the levels were measured after exposure
ended. This study is significant in that it is the first to
demonstrate an association between biomarkers of exposure/body burden
and the occurrence of neurological effects.
Impotence and loss of libido are common symptoms in male workers
afflicted with clinically identifiable signs of manganism attributed
to occupational exposure to manganese for 1-21 years (Rodier, 1955;
Schuler et al., 1957; Mena et al., 1967; Emara et al., 1971). These
effects could lead to reduced reproductive success in men. Impaired
fertility (measured as a decreased number of children per married
couple) has been observed in male workers exposed for 1-19 years to
manganese dust at levels (0.97 mg/m3) that did not produce frank
manganism (Lauwerys et al., 1985). In another study, Gennart et al.
(1992) did not find an effect of manganese exposure (0.71 mg/m3 for
6.2 years on average) on fertility. Impaired sexual function in men
might be one of the earliest clinical manifestations of manganism;
however, because dose-response information is unavailable, it is not
possible to define a threshold for this effect. No information was
found regarding reproductive effects in women.
Although most effects have been seen following chronic inhalation
exposure to manganese in occupational settings, some epidemiological
studies have reported adverse effects from ingestion of excess
manganese in the environment. A manganism-like neurological syndrome
was observed in an aboriginal population living on an island near
Australia where environmental levels of manganese are high (Kilburn,
1987). Exposure levels were not provided, but the authors noted that
manganese intake could occur not only through the oral route (food,
water, soil), but also by inhaling manganese-containing dusts in the
air (Cawte et al., 1987). Although manganese exposure was probably an
etiologic factor, genetic factors, dietary deficiencies in
antioxidants and calcium, and excess alcohol consumption could also
have contributed to the neurological effects (Cawte et al., 1989).
More recently, Kondakis et al. (1989) reported that chronic
intake of drinking-water containing elevated levels of manganese
(1.8-2.3 mg/litre) led to an increased prevalence of neurological
signs in elderly residents (average age 67 years) of two small towns
in Greece. The effects were compared with those in similarly aged
residents in two other communities where manganese levels were within
ambient range (0.004 and 0.0015 mg/litre). The findings suggested that
above-average oral exposure to manganese might be of health concern.
However, although the comparison populations were reportedly very
similar to each other, differences in age, occupational exposures, or
general health status could have accounted for the small differences
observed. Similarly, Goldsmith et al. (1990) investigated a cluster of
Parkinson's disease in southern Israel. The authors suggested that
excess levels of aluminum, iron, and manganese in the drinking-water
and the use of agricultural chemicals, including maneb and paraquat,
in the area were common environmental factors that may have
contributed to the observed cluster. However, the observed symptoms
could not be conclusively attributed to manganese poisoning alone. By
contrast, a recent study by Vieregge et al. (1995) on the neurological
impacts of chronic oral intake of manganese in well-water found no
significant differences between exposed and control populations in
northern Germany. A group of 41 subjects exposed to 0.300-160 mg
manganese/litre in well-water was compared with a control group of 71
subjects (matched for age, sex, nutritional habits, and drug intake)
exposed to a maximum manganese concentration in well-water of 0.050
mg/litre. Neurological assessments revealed no significant difference
between the two groups. Although the effects reported by Kondakis et
al. (1989) and Goldsmith et al. (1990) are consistent with the known
toxicological effects of manganese, the findings are inconclusive and
are contradicted by the results of Vieregge et al. (1995). As a
result, no firm conclusions on manganese-induced neurological effects
in humans from chronic oral intake of manganese in drinking-water can
be made at this time.
One report partially attributed neurological effects to chronic
oral intake of manganese in food. Iwami et al. (1994), studying metal
content in food and drinking-water in an area with a high rate of
motor neuron disease (as determined from death certificates) compared
with control areas, concluded that a high manganese content in food
and a low magnesium content in drinking-water together explained the
high incidence of motor neuron disease. The manganese content per
1800-kcal diet averaged 6.20 mg for local rice eaters and 3.83-4.67 mg
in the control areas.
Several studies have reported an association between chronic
exposure to maneb and neurological symptoms, but the effects could not
be conclusively attributed to maneb alone. Ruijten et al. (1994)
investigated the effects of chronic exposure to mixed pesticides
(including zineb and maneb) on peripheral and autonomic nerve function
using a previously exposed group of 131 Dutch bulb farmers and a
control group of 67. The findings suggested exposure-related decreases
in both autonomic and peripheral nerve function. Ferraz et al. (1988)
reported the results of a questionnaire and neurological examination
administered to 50 rural workers in Brazil who had had close contact
with maneb (preparation and/or fumigation) for at least 6 months.
Compared with a control group, the exposed group had a significantly
higher prevalence of plastic rigidity with cogwheel phenomenon
(neurological examination), as well as headache, fatigue, nervousness,
memory complaints, and sleepiness (questionnaire). In both studies,
however, the subjects were exposed to other substances, so the effects
could not be definitively attributed to maneb. Meco et al. (1994)
reported that Parkinsonism developed in a patient 2 years after
chronic exposure to maneb had been discontinued. Initial symptoms
observed were generalized bradykinesia, rigidity, and mild tremor
associated with paraesthesias in the right leg, which subsequently
spread to the right arm. Over a 3-year period, the tremor worsened and
spread to the left limbs as well. Exposure levels were not defined in
these studies.
10. EFFECTS EVALUATION
10.1 Evaluation of health effects
10.1.1 Hazard identification and dose-response assessment
Manganism, manganic pneumonia, and male reproductive effects
(decreased libido, impotence, and decreased fertility) have been
documented following chronic inhalation of manganese-containing
respirable dusts in occupational settings (Rodier, 1955; Schuler et
al., 1957; Mena et al., 1967; Emara et al., 1971; Lauwerys et al.,
1985). More recent reports have shown subclinical changes in
neurological performance at low occupational exposure levels (Roels et
al., 1987, 1992; Iregren, 1990; Wennberg et al., 1991; Mergler et al.,
1994; Lucchini et al., 1995); it should be noted that even these low
occupational exposure levels were at least three orders of magnitude
higher than manganese levels in areas without industrial sources of
manganese. A dose-response curve has not been well defined, but early
signs of nervous system toxicity and overt manganism have been
observed after inhalation exposure to total manganese dust levels that
range from 0.14 to 1 mg/m3 for the former and from 2 to 22 mg/m3
for the latter. These neurological effects have been observed
following exposure durations that span from 1 to 35 years (Schuler et
al., 1957; Whitlock et al., 1966; Tanaka & Lieben, 1969; Cook et al.,
1974; Saric et al., 1977; Roels et al., 1987, 1992; Iregren, 1990;
Wennberg et al., 1991; Chia et al., 1993, 1995; Mergler et al., 1994;
Lucchini et al., 1995). Estimated levels of inhalation exposure to
manganese compounds have been reported as manganese in either total
dust particles or the respirable fraction, based on particle size.
Although inconclusive, limited case reports and epidemiological
studies report neurological effects associated with ingesting water
(or other media) containing elevated manganese (Kawamura et al., 1941;
Kilburn, 1987; Kondakis et al., 1989; Goldsmith et al., 1990; Iwami et
al., 1994). Reports on neurological effects following exposure to
pesticides containing manganese are similarly inconclusive (Ferraz et
al., 1988; Ruijten et al., 1994).
Some evidence suggests that the elderly might be more sensitive
than younger people to manganese (Davis & Elias, 1996). In addition,
owing to various predisposing factors, certain other individuals might
be more susceptible to adverse effects from exposure to excess
manganese. These might include people with lung disease, people who
are exposed to other lung irritants, neonates, individuals with iron
deficiency, and people with liver disease.
Available data suggest that neurological effects can occur
following chronic inhalation exposures in humans and intermediate and
chronic oral exposures in animals to different manganese compounds.
Manganese-induced neurological effects have been reported at lower
airborne manganese concentrations in humans than in animals (Bird et
al., 1984; Newland & Weiss, 1992). These data suggest that animal
models, particularly rodent species, might be less useful for defining
quantitative dose-response relationships, but helpful in elucidating
the mechanism(s) for these effects. The basis for the difference in
susceptibility across species is not yet understood and may be related
to possible differences in the sensitivity of test methods used to
detect neurobehavioural effects in animals compared with methods used
to detect neurobehavioural effects in humans.
Little is known about the relative toxicity of different
manganese compounds. Inhaled manganese compounds tend to produce more
severe toxicity than ingested manganese compounds. This is probably
attributable to the difference in route-specific uptake of manganese
from the lung (often assumed at 100%) compared with the
gastrointestinal tract (3-5%). Studies have shown that a greater
proportion of a manganese dose appears in the blood and brain of rats
exposed via inhalation or intranasal instillation than when the same
dose is given orally (Tjälve et al., 1996; Roels et al., 1997).
10.1.2 Criteria for setting guidance values for manganese
There are several approaches to the development of a guidance
value for manganese in air. A recently developed guidance value of
0.15 µg manganese/m3 (WHO, 1999) is highlighted here as one example;
other approaches are outlined in Appendix 4. The WHO (1999) guidance
value was derived from the results of the study by Roels et al.
(1992), which examined neurobehavioural end-points in 92 male workers
exposed to manganese dioxide dust at an alkaline battery plant and 101
male workers without industrial manganese exposure. The
manganese-exposed workers exhibited significantly poorer eye-hand
coordination, hand steadiness, and visual reaction time. Sufficient
data on participants' exposure levels and test performance were
provided to enable development of a dose-response relationship and
calculation of a benchmark dose. The lower 95% confidence limit of the
benchmark dose (30 µg/m3 for the 5% effect level) was used as an
estimate of a NOAEL for neurological effects. The guidance value for
manganese in air (WHO, 1999) was then derived as follows:
Guidance value = (30 µg/m3 ‰ 50) × (5/7) × (8/24)
= 0.15 µg/m3 (rounded value)
where:
* 30 µg/m3 is the estimated NOAEL for neurological effects,
calculated based on a benchmark dose analysis of results from a
quality epidemiological study of workers exposed to manganese;
* 5/7 and 8/24 are factors used to convert intermittent exposure
(5 days/week, 8 h/day) to continuous exposure; and
* 50 is the uncertainty factor (×10 for interindividual variation;
×5 for the potential for developmental effects in younger
children). The uncertainty factor for developmental effects in
younger children was obtained by analogy with lead, where
neurobehavioural effects were found in younger children at blood
lead levels five times lower than in adults; this finding was
considered to be supported by evidence from studies in animals
(WHO, 1999).
In considering development of a guidance value for oral intake of
manganese, it must be noted that there is wide variability in human
intake of manganese (from all sources) and that manganese is an
essential nutrient for humans and animals. Daily manganese intake from
food is estimated to be about 2-9 mg for adults, with an absorbed
amount of about 100-450 µg/day based upon 5% gastrointestinal
absorption (WHO, 1981). Some studies have reported that neurological
effects may be related to ingestion of manganese in non-worker
populations. However, these reports provide little information on the
levels of ingested manganese that were associated with these effects.
Although neurological effects might be a potential concern for people
working or living at or near sites where ingestion or inhalation of
high levels of manganese can occur (see section 9.2), no firm
conclusion on a guidance value level for oral intake of manganese
other than estimated daily intake levels is considered possible.
10.1.3 Sample risk characterization
A theoretical estimate of inhalation exposures for the general
population is presented based on available monitoring data on levels
of manganese in air.
Table 2 shows estimates of the average levels of manganese in
ambient air in remote, rural, and urban areas around the world (US
EPA, 1984; Stokes et al., 1988). Using these data and a daily
inhalation volume of 20 m3 for a 70-kg adult, the average estimated
daily intake of manganese from air in rural areas would be 0.8 µg/day,
and this might increase to 1.3, 1.9, and 3.3 µg/day in urban areas in
Canada, the USA, and Europe, respectively. In source-dominated areas
with manganese-emitting industries or major foundry facilities, intake
from air might rise to 4-6 µg/day (WHO, 1999). These estimates are
based on the assumptions that there is 100% absorption of inhaled
manganese and that an individual lives or works in these environments
for a complete 24-h period. Thus, these exposure estimates could be
further adjusted to reflect that fraction of a 24-h period during
which a person is actually in any of these areas. For example, a
person working for 8 h/day in an urban area of Canada, the USA, or
Europe would be exposed to an adjusted estimate of 0.43-1.1 µg
manganese during the workday (1.3-3.3 µg/day - 8/24). Consequently,
for persons living or working in rural or urban environments,
estimates of inhalation exposure would fall below or in the range of
the reported guidance values for inhaled manganese. Appendix 4
includes examples of other guideline values reported for inhaled
manganese. These values range from 0.8 µg/day (0.04 µg/m3 × 20
m3/day = 0.8 µg/day) up to an estimate of 3.0 µg inhaled
manganese/day based on the guidance value described in section 10.1.2
(0.15 µg/m3 × 20 m3/day = 3.0 µg/day). It should be noted that the
studies used in the risk assessment of inhaled manganese are
occupational studies of adult male workers, and there is uncertainty
about extrapolating the risk to women and children.
11. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
WHO (1981, 1986, 1987, 1999) has previously evaluated manganese,
concluding that chronic manganese poisoning is a hazard in
occupational settings. However, little information was available to
assess the potential health risks in community exposure scenarios.
WHO has established a provisional guideline value of 0.5 mg/litre
for manganese in drinking-water based on health (WHO, 1993), an annual
air quality guideline of 0.15 µg/m3 (WHO, 1999), and a workplace
exposure limit in air of 0.3 mg/m3 for respirable particles
containing manganese (WHO, 1984, 1986, 1987, 1999).
Information on international hazard classification and labelling
is included in the International Chemical Safety Card reproduced in
this document.
12. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventative and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0174) reproduced in this
document.
12.1 Human health hazards
Following long-term or repeated exposure to manganese, humans may
present neurological and neuropsychiatric disorders known under the
term manganism.
12.2 Advice to physicians
The psychiatric symptoms of manganese poisoning are transient.
However, the neurological damage might be irreversible, although some
patients have experienced partial regression of their symptoms after
early removal from exposure (Mena et al., 1974).
Consequently, it is very important to remove from further
exposure patients who present preclinical neurological disturbances or
symptoms of manganism. Chelation therapy with, for example, EDTA might
be effective in removing manganese from blood and tissues, but it has
no permanent effect on symptomatic patients in the late stages of
manganism (Bismuth et al., 1987; Dreisbach & Robertson, 1987;
Ellenhorn & Barceloux, 1988; Tomes, 1997). Normal manganese levels are
2-8 µg/dl in blood and 0.1-0.8 µg/dl in urine. Plasma and urine
manganese levels do not seem to correlate well with the severity of
symptoms.
12.3 Health surveillance programme
The health surveillance programme of people exposed to manganese
needs to include elements such as central nervous system disturbances
(asthenia, anorexia, sleep problems, irritability, diminished libido).
13. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards
can be found in the International Register of Potentially Toxic
Chemicals (IRPTC), available from UNEP Chemicals (IRPTC), Geneva.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
MANGANESE ICSC:0174
March 1995
CAS# 7439-96-5
RTECS# 00927500
(powder)
Mn
Atomic mass: 54.9
TYPES OF HAZARD/ ACUTE HAZARDS/ PREVENTION FIRST AID/
EXPOSURE SYMPTOMS FIRE FIGHTING
FIRE Combustible No open Dry sand,
flames special powder
EXPLOSION Finely dispersed Prevent deposition
particles form of dust, closed
explosive mixtures system, dust
in air explosion-proof
electrical equipment
and lighting.
EXPOSURE PREVENT DISPERSION
OF DUST! AVOID
EXPOSURE OF (PREGNANT)
WOMEN!
Inhalation Cough, shortness Local exhaust or Fresh air, rest.
of breath. breathing protection. Refer for medical
attention.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
Skin
Eyes Safety goggles or First rinse with
eye protection in plenty of water
combination with for several
breathing protection minutes (remove
if powder. contact lenses if
easily possible),
then take to a
doctor.
Ingestion Abdominal pain. Do not eat, drink, Rinse mouth.
Nausea. or smoke during Refer for medical
work. Wash hands attention.
before eating.
SPILLAGE DISPOSAL PACKAGING & LABELLING
Sweep spilled substance into containers. EU Classification
Carefully collect remainder, then Symbol:
remove to safe place (extra personal UN Classification
protection: P2 filter respirator
for harmful particles).
EMERGENCY RESPONSE STORAGE
Separated from acids. Dry
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
IMPORTANT DATA
PHYSICAL STATE; APPEARANCE ROUTES OF EXPOSURE:
GRAY-WHITE POWDER The substance can be absorbed into the
the body by inhalation of its aerosol or
fumes, and by ingestion.
PHYSICAL DANGERS: INHALATION RISK:
Dust explosion possible if in powder Evaporation at 20°C is negligible;
or granular form, mixed with air. a harmful concentration of airborne
particles can, however, be reached
quickly when dispersed.
CHEMICAL DANGERS: EFFECTS OF SHORT TERM EXPOSURE:
Upon heating, toxic fumes are formed. Inhalation of dust may cause bronchitis
Reacts violently with concentrated and pneumonia. The effects may be
hydrogen peroxide. Reacts slowly with delayed.
water more rapidly with steam and
acids to produce flammable gas
(hydrogen see - ICSC # 0001) causing
fire and explosion hazard. Burns in
nitrogen oxide above 200°C.
OCCASIONAL EXPOSURE LIMITS: EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
TLV: ppm; 0.2 mg/m3 (ACGIH 1996). The substance may have effects on the
MAK: ppm; 0.5 mg/m3 (1994). lungs and nervous system, resulting in
bronchitis, pneumonia, neurologic and
neuropsychiatric disorders (manganism).
Animal tests show that this substance
possibly causes toxic effects upon
human reproduction.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
PHYSICAL PROPERTIES
Boiling point: 1962 °C
Melting point: 1244 °C
Relative density (water = 1): 7.2-7.4
Solubility in water: none
ENVIRONMENTAL DATA
NOTES
Depending on the degree of exposure, periodic medical examination is indicated.
The recommendations on this Card also apply to ferro manganese.
ADDITIONAL INFORMATION
LEGAL NOTICE Neither the CEC nor the IPCS nor any person acting on behalf of
the CEC or the IPCS is responsible for the use which might be
made of this information.
REFERENCES
Abbasi SA (1988) Synergistic extraction and atomic absorption
spectrometric determination of manganese in environmental samples
using N- p-aminophenyl-2-furyl-acrylohydroxamic acid and
trioctylmethyl ammonium cation. Analytical letters,
21(10):1935-1944.
Abdel-Hamid MM, El-Desoky SA, Magdi SM (1990) Estimation of manganese
in blood between exposed workers to different concentrations at
industrial units. Egyptian journal of pharmaceutical sciences,
31(1-4):143-150.
Adkins B, Luginbuhl HG, Miller JF, Gardner DE (1980) Increased
pulmonary susceptibility to streptococcal infection following
inhalation of manganese oxide. Environmental research, 23:110-120.
Akbar-Khanzadeh F (1993) Short-term respiratory function changes in
relation to workshift welding fume exposures. International archives
of occupational and environmental health, 64(6):393-397.
Alessio L, Lucchini R (1996) Manganese and manganese compounds. In:
Argentesi F, Roi R, Sevilla Marcos JM, eds. Data profiles for
selected chemicals and pharmaceuticals 3, 4, 5. Ispra, Italy,
European Commission, Joint Research Commission.
Ali MM, Murthy RC, Mandal SK, Chandra SV (1985) Effect of low protein
diet on manganese neurotoxicity. III. Brain neurotransmitter levels.
Neurobehavioral toxicology and teratology, 7:427-431.
ATSDR (1996) Toxicological profile for manganese (update). Draft
for public comment. Atlanta, GA, US Department of Health and Human
Services, Public Health Service, Agency for Toxic Substances and
Disease Registry.
Baes CF III, Sharp RD (1983) A proposal for estimation of soil
leaching and leaching constants for use in assessment models.
Journal of environmental quality, 12:17-28.
Barbeau A (1984) Manganese and extrapyramidal disorders.
Neurotoxicology, 5(2):13-36.
Bergstrom R (1977) Acute pulmonary toxicity of manganese dioxide.
Scandinavian journal of work and environmental health, 3 (Suppl.
1):1-40.
Beuter A, Mergler D, de Geoffroy A, Belanger S, Carriere L, Varghese
L, Skeekumar J, Gauthier S (1994) Diadochokinesimetry: a study of
patients with Parkinson's Disease and manganese-exposed workers.
Neurotoxicology, 15(3):655-664.
Bird ED, Anton AH, Bullock B (1984) The effect of manganese inhalation
on basal ganglia dopamine concentrations in rhesus monkey.
Neurotoxicology, 5:59-65.
Bismuth C, Baud F, Conso F, Fréjaville JP, Garnier R (1987)
Toxicologie clinique. Médecines-Sciences, Flammarion, p. 509.
Bonilla E (1978) Increased GABA content in caudate nucleus of rats
after chronic manganese chloride administration. Journal of
neurochemistry, 31:551-552.
Bonilla E, Prasad AL (1984) Effects of chronic manganese intake on the
levels of biogenic amines in rat brain regions. Neurobehavioral
toxicology and teratology, 6:341-344.
Calabrese EJ, Barnes R, Stanek EJ, Pastides H, Gilbekt CE, Veneman P,
Xioaru W, Lasztity A, Kostecki PT (1989) How much soil do young
children ingest: an epidemiologic study. Regulatory toxicology and
pharmacology, 10:123-137.
Calne DB, Chu N-S, Huang C-C, Lu C-S, Olanow W (1994) Manganese and
idiopathic parkinsonism: Similarities and differences. Neurology,
44:1583-1586.
Casto BC, Meyers J, DiPaulo JA (1979) Enhancement of viral
transformation for evaluation of the carcinogenic or mutagenic
potential of inorganic metal salts. Cancer research, 39:193-198.
Cawte J, Hams G, Kilburn C (1987) Manganism in a neurological ethnic
complex in northern Australia [letter]. Lancet, (May 30):1257.
Cawte J, Kilburn C, Florence M (1989) Motor neurone disease of the
Western Pacific: do the foci extend to Australia? Neurotoxicology,
10:263-270.
Chandra SV (1983) Psychiatric illness due to manganese poisoning.
Acta Psychiatrica Scandinavica, 67 (Suppl. 303):49-54.
Chandra SV, Shukla GS (1978) Manganese encephalopathy in growing rats.
Environmental research, 15:28-37.
Chandra SV, Saxena DK, Hasan MZ (1975) Effect of zinc on manganese
induced testicular injury in rats. Industrial health, 13:51-56.
Chia S-E, Foo S-C, Gan S-L, Jeyaratnam J (1993) Neurobehavioral
functions among workers exposed to manganese ore. Scandinavian
journal of work and environmental health, 19:264-270.
Chia S-E, Gan S-L, Chua L-H, Foo S-C, Jeyaratnam J (1995) Postural
stability among manganese exposed workers. Neurotoxicology,
16(3):519-526.
Chin CS, Johnson KS, Coale KH (1992) Spectrophotometric determination
of dissolved manganese in natural waters with
1-(2-pyridylazo)-2-naphthol: application to analysis in situ in
hydrothermal plumes. Marine chemistry, 37(1-2):65-82.
Chu N, Hochberg F, Calne D, Olanow C (1995) Neurotoxicology of
manganese. In: Chang L, Dyyer R, eds. Handbook of neurotoxicology of
manganese. New York, NY, Marcel Dekker, Inc., pp. 91-103.
Coenen W, Lambert J, Schenk H (1989) Gefahrstoffe an gie
bereiarbeitsplatzen bericht. Sankt Augustin,
Berufsgenossenschaftliches Institut fur Arbeitssicherheit (BIA).
Collipp PJ, Chen SY, Maitinsky S (1983) Manganese in infant formulas
and learning disability. Annals of nutrition and metabolism,
27:488-494.
Cook DG, Fahn S, Brait KA (1974) Chronic manganese intoxication.
Archives of neurology, 30:59-64.
Cooper WC (1984) The health implications of increased manganese in the
environment resulting from the combustion of fuel additives: a review
of the literature. Journal of toxicology and environmental health,
14:23-46.
Curtin D, Ryan J, Chaudhary RA (1980) Manganese adsorption and
desorption in calcareous Lebanese soils. Journal of the Soil Science
Society of America, 44:947-950.
Davidsson L, Cederblad A, Hagebo E, Lonnerdal B, Sandström B (1988)
Intrinsic and extrinsic labeling for studies of manganese absorption
in humans. Journal of nutrition, 118:1517-1524.
Davis CD, Malecki EA, Greger JL (1992) Interactions among dietary
manganese, heme iron and non-heme iron in women. American journal of
clinical nutrition, 56:926-932.
Davis DW, Hsiao K, Ingels R, Shiklya J (1988) Origins of manganese in
air particulates in California. Journal of the Air Pollution Control
Association, 38:1152-1157.
Davis JM (1998) Methylcyclopentadienyl manganese tricarbonyl (MMT):
health risk uncertainties and research directions. Environmental
health perspectives, 106 (Suppl. 1):191-201.
Davis JM, Elias RW (1996) Risk assessment of metals. In: Chang LW, ed.
Toxicology of metals. Boca Raton, FL, CRC Lewis Publishers.
De Méo M, Laget M, Castegnaro M, Dumenil G (1991) Genotoxic activity
of potassium permanganate in acidic solutions. Mutation research,
260(3):295-306.
Deskin R, Bursian SJ, Edens FW (1980) Neurochemical alterations
induced by manganese chloride in neonatal rats. Neurotoxicology,
2:65-73.
Deverel SJ, Millard SP (1988) Distribution and mobility of selenium
and other trace elements in shallow groundwater of the western San
Joaquin Valley, CA. Environmental science and technology,
22:697-702.
Dikshith TS, Chandra SV (1978) Cytological studies in albino rats
after oral administration of manganese chloride. Bulletin of
environmental contamination and toxicology, 19:741-746.
Doisy EA (1972) Effects of deficiency of manganese upon plasma levels
of clotting proteins and cholesterol in man. In: Hoekstra WG, Suttie
JW, Gantner ME, eds. Trace element metabolism in animals. Vol. 2.
Baltimore, MD, University Park Press, pp. 668-670.
Dreisbach RH, Robertson WO (1987) Handbook of poisoning: prevention,
diagnosis & treatment, 12th ed. Norwalk, CT, Appleton & Lange, 589
pp.
Durfor CN, Becker E (1964) Public water supplies of the 100 largest
cities in the United States, 1962. Washington, DC, US Department of
the Interior, Geological Survey (Water-Supply Paper 1812).
Eckel WP, Langley WD (1988) A background-based ranking technique for
assessment of elemental enrichment in soils at hazardous waste sites.
In: Superfund '88: Proceedings of the 9th National Conference, 28-30
November 1988, Washington, DC. Silver Spring, MD, The Institute, pp.
282-286.
Egyed M, Wood GC (1996) Risk assessment for combustion products of the
gasoline additive MMT in Canada. Science of the total environment,
189/190:11-20.
Ellenhorn MJ, Barceloux DG (1988) Ellenhorn's medical toxicology:
diagnosis and treatment of human poisoning. New York, NY, Elsevier,
1512 pp.
EM (1993) The economics of manganese, 7th ed. London, Roskill
Information Services, Ltd.
Emara AM, El-Ghawabi HS, Madkour IO, El-Samara GH (1971) Chronic
manganese poisoning in the dry battery industry. British journal of
industrial medicine, 28:78-82.
Eriksson H, Lenngren S, Heilbronn E (1987) Effect of long-term
administration of manganese on biogenic amine levels in discrete
striatal regions of rat brain. Archives of toxicology, 59:426-431.
Eriksson H, Gillberg P, Aquilonius S, Hedstrom KG, Heilbronn E (1992)
Receptor alterations in manganese intoxicated monkeys. Archives of
toxicology, 66:359-364.
Ferraz HB, Bertolucci PHF, Pereira JS, Lima JGC, Andrade LAF (1988)
Chronic exposure to the fungicide maneb may produce symptoms and signs
of CNS manganese intoxication. Neurology, 38(4):550-553.
Folsom TR, Young DR, Johnson JN, Pillai KC (1963) Manganese-54 and
zinc-65 in coastal organisms of California. Nature, 200:327-329.
Francis AJ (1985) Anaerobic microbial dissolution of toxic metals in
subsurface environments. Upton, NY, Brookhaven National Laboratory
(BNL-36571).
Francis CW, White GH (1987) Leaching of toxic metals from incinerator
ashes. Journal of the Water Pollution Control Federation,
59:979-986.
Freeland-Graves J, Llanes C (1994) Models to study manganese
deficiency. In: Klimis-Tavantzis DJ, ed. Manganese in health and
disease. Boca Raton, FL, CRC Press, pp. 59-86.
Freeland-Graves JH, Bales CW, Behmardi F (1987) Manganese requirements
of humans. In: Kies C, ed. Nutritional bioavailability of manganese.
Washington, DC, American Chemical Society.
Friedman BJ, Freeland-Graves JH, Bales CW, Behmardi F, Shorey-Kutschke
RL, Willis RA, Crosby JB, Trickett PC, Houston SD (1987) Manganese
balance and clinical observations in young men fed a
manganese-deficient diet. Journal of nutrition, 117:133-143.
Furst A (1978) Tumorigenic effect of an organomanganese compound on
F344 rats and Swiss albino mice [brief communication]. Journal of
the National Cancer Institute, 60:1171-1173.
Galloway SM, Armstrong MJ, Reuben C, Colman S, Brown B, Cannon C,
Bloom AD, Nakamura F, Ahmed M, Duk S, Rimpo J, Margolin BH, Resnick
MA, Anderson B, Zeiger E (1987) Chromosome aberrations and sister
chromatid exchanges in Chinese hamster ovary cells: Evaluations of 108
chemicals. Environmental and molecular mutagenesis, 10 (Suppl.
10):1-175.
Garrison AW, Cipollone MG, Wolfe NL, Swank RR (1995) Environmental
fate of methylcyclopentadienyl manganese tricarbonyl. Environmental
toxicology and chemistry, 14(11):1859-1864.
Geering HR, Hodgson JF, Sdano C (1969) Micronutrient cation complexes
in soil solution. IV. The chemical state of manganese in soil
solution. Soil Science Society of America, proceedings, 33:81-85.
Gennart J-P, Buchet J-P, Roels H, Ghyselen P, Ceulemans E, Lauwerys R
(1992) Fertility of male workers exposed to cadmium, lead, or
manganese. American journal of epidemiology, 135(2):1208-1219.
Gianutsos G, Murray MT (1982) Alterations in brain dopamine and GABA
following inorganic or organic manganese administration.
Neurotoxicology, 3:75-81.
Glass E (1955) Untersuchungen uber die einwirkung von
schwermetallsalzen auf dir wurzelspitzenmitose von Vicia faba.
Zeitschrift fuer Botanik, 43:359-403.
Glass E (1956) Untersuchungen uber die einwirkung von
schwermetallsalzen auf dir wurzelspitzenmitose von Vicia faba.
Zeitschrift fuer Botanik, 44:1-58.
Goldsmith J, Herishanu Y, Abarbanel J, Weinbaum Z (1990) Clustering of
Parkinson's disease points to environmental etiology. Archives of
environmental health, 45:88-94.
Graedel TE (1978) Inorganic elements, hydrides, oxides, and
carbonates. In: Chemical compounds in the atmosphere. New York, NY,
Academic Press, pp. 35-41, 44-49.
Grant D, Ege T (1995) Teratogenicity in the rat after repeated
intravenous injection of manganese, either as a complex (mangafodipir
trisodium, MNDPDP) or as the inorganic chloride. Toxicology,
15(1):160.
Gray EL, Laskey JW (1980) Multivariate analysis of the effects of
manganese on the reproductive physiology and behavior of the male
house mouse. Journal of toxicology and environmental health,
6:861-867.
Hamilton K (1995) The Australian directory of registered pesticides
and their uses. Brisbane, Australia, University of Queensland,
Centre for Pesticide Application and Safety.
Health Canada (1994) Canadian Environmental Protection Act. Human
health risk assessment for priority substances. Ottawa, ON, Minister
of Supply and Services Canada.
Hejtmancik M, Peters AC, Toft JD, Basaran A, Sheridan A, Athey P
(1987a) The chronic study of manganese sulfate monohydrate (CAS No.
10034-96-5) in F344 rats. Report to National Toxicology Program,
Research Triangle Park, NC, by Battelle's Columbus Laboratories,
Columbus, OH.
Hejtmancik M, Peters AC, Toft JD, Dersing R, Basaran A, Sheridan A,
Athey P (1987b) The chronic study of manganese sulfate monohydrate
(CAS No. 10034-96-5) in B6C3F1 mice. Report to National Toxicology
Program, Research Triangle Park, NC, by Battelle's Columbus
Laboratories, Columbus, OH.
Hellou J, Fancey LL, Payne JF (1992) Concentrations of twenty-four
elements in bluefin tuna, Thunnus thynnus, from the Northwest
Atlantic. Chemosphere, 24(2):211-218.
Hemstock GA, Low PF (1953) Mechanisms responsible for retention of
manganese in the colloidal fraction of soil. Soil science,
76:331-343.
Hochberg F, Miller G, Valenzuela R, McNelis S, Crump KS, Covington T,
Valdivia G, Hochberg B, Trustman JW (1996) Late motor deficits of
Chilean manganese miners: a blinded control study. Neurology,
47:788-795.
Holbrook DJ Jr, Washington ME, Leake HB, Brubaker PE (1975) Studies on
the evaluation of the toxicity of various salts of lead, manganese,
platinum, and palladium. Environmental health perspectives,
10:95-101.
Holzgraefe M, Poser W, Kijewski H, Beuche W (1986) Chronic enteral
poisoning caused by potassium permanganate: a case report. Journal
of toxicology and clinical toxicology, 24:235-244.
HSDB (1998) Hazardous substances data bank. Bethesda, MD, National
Institutes of Health, National Library of Medicine.
Huang CC, Chu NS, Lu CS, Wang JD, Tsai JL, Tzeng JL, Wolters EC, Calne
DB (1989) Chronic manganese intoxication. Archives of neurology,
46:1104-1106.
Hurley LS, Keen CL (1987) Manganese. In: Mertz W, ed. Trace elements
in human and animal nutrition, 5th ed. San Diego, CA, Academic
Press, Inc.
Ikarashi Y, Tsuchiya T, Nakamura A (1992) Detection of contact
sensitivity of metal salts using the murine local lymph node assay.
Toxicology letters, 62(10):53-61.
Imam Z, Chandra VS (1975) Histochemical alterations in rabbit testis
produced by manganese chloride. Toxicology and applied pharmacology,
32:534-544.
IPCS (1993) International Chemical Safety Card -- Manganese. Geneva,
World Health Organization, International Programme on Chemical Safety
(ICSC 0174).
Iregren A (1990) Psychological test performance in foundry workers
exposed to low levels of manganese. Neurotoxicology and teratology,
12:673-675.
Ishizuka H, Nishida M, Kawada J (1991) Changes in stainability
observed by light microscopy in the brains of ataxial mice subjected
to three generations of manganese administration. Biochemistry
international, 25(4):677-687.
Iwami O, Watanabe T, Moon C-S, Nakatsuka H, Ikeda M (1994) Motor
neuron disease on the Kii Peninsula of Japan: Excess manganese intake
from food coupled with low magnesium in drinking water as a risk
factor. Science of the total environment, 149:121-135.
Jacques AP (1984) National inventory of sources and emissions of
manganese -- 1984. Ottawa, ON, Environment Canada, Conservation and
Protection, Environmental Protection.
Jaudon P, Massiani C, Galea J, Rey J, Vacelet E (1989) Groundwater
pollution by manganese. Manganese speciation: application to the
selection and discussion of an in situ groundwater treatment.
Science of the total environment, 84:169-183.
Joardar M, Sharma A (1990) Comparison of clastogenicity of inorganic
Mn administered in cationic and anionic forms in vivo. Mutation
research, 240(3):159-163.
Kabata-Pendias A, Pendias H (1984) Trace elements in soils and
plants. Boca Raton, FL, CRC Press, Inc.
Kawamura R, Ikuta H, Fukuzumi S, Yamada R, Tsubaki S (1941)
Intoxication by manganese in well water. Kitasato archives of
experimental medicine, 18:145-171.
Kellar KE, Foster N (1991) Determination of the relative amounts of
free and complexed manganese ions in aqueous solution by nuclear
magnetic resonance. Analytical chemistry, 63(24):2919-2924.
Kilburn CJ (1987) Manganese, malformations and motor disorders:
findings in a manganese-exposed population. Neurotoxicology,
8:421-429.
Kleinman MT, Pasternack BS, Eisenbud M, Kneip TJ (1980) Identifying
and estimating the relative importance of airborne particulates.
Environmental science and technology, 14:62-65.
Komura J, Sakamoto M (1991) Short-term oral administration of several
manganese compounds in mice: physiological and behavioral alterations
caused by different forms of manganese. Bulletin of environmental
contamination and toxicology, 46(6):921-928.
Komura J, Sakamoto M (1992) Effects of manganese forms on biogenic
amines in the brain and behavioral alterations in the mouse: long-term
oral administration of several manganese compounds. Environmental
research, 57(1):34-44.
Kondakis XG, Makris N, Leotsinidis M, Prinou M, Papapetropoulos T
(1989) Possible health effects of high manganese concentration in
drinking water. Archives of environmental health, 44:175-178.
Kontur PJ, Fechter LD (1985) Brain manganese, catecholamine turnover,
and the development of startle in rats prenatally exposed to
manganese. Teratology, 32:1-11.
Kostial K, Blanusa M, Maljkovic T, Kello D, Rabar I, Stara JF (1989)
Effect of a metal mixture in diet on the toxicokinetics and toxicity
of cadmium, mercury and manganese in rats. Toxicology and industrial
health, 5(5):685-698.
Kristensson K, Eriksson H, Lundh B, Plantin L-O, Wachtmeister L, El
Azazi M, Morath C, Heilbronn E (1986) Effects of manganese chloride on
the rat developing nervous system. Acta Pharmacologica et
Toxicologica, 59:345-348.
Kucera J, Soukal L, Faltejsek J (1986) Low level determination of
manganese in biological reference materials by neutron activation
analysis. Journal of radioanalytical nuclear chemistry,
107(6):361-369.
Ky S, Deng H, Xie P, Hu W (1992) A reprot of two chronic cases of
serious manganese poisoning treated with sodium para-aminosalicylic
acid. British journal of industrial medicine, 49:66-69.
Lai JC, Leung TK, Lim L (1984) Differences in the neurotoxic effects
of manganese during development and aging: some observations on brain
regional neurotransmitter and non-neurotransmitter metabolism in a
developmental rat model of chronic manganese encephalopathy.
Neurotoxicology, 5:37-47.
Laitung JK, Mercer DM (1983) Manganese absorption through a burn.
Burns, including thermal injuries, 10:145-146.
Laskey JW, Rehnberg GL, Hein JF (1982) Effects of chronic manganese
(Mn3O4) exposure on selected reproductive parameters in rats.
Journal of toxicology and environmental health, 9:677-687.
Lauwerys R, Roels H, Genet P, Toussaint G, Bouchaerta A, Cooman S
(1985) Fertility of male workers exposed to mercury vapor or to
manganese dust: a questionnaire study. American journal of
industrial medicine, 7:171-176.
Lavi N, Lux F, Al-Fassi ZB (1989) Determination of magnesium,
aluminum, phosphorus, copper and manganese in biological fluids by
neutron activation analysis. Journal of radioanalytical nuclear
chemistry, 129(1):93-102.
Leach RM, Lilburn SM (1978) Manganese metabolism and its function.
World review of nutrition and diet, 32:123-134.
Lide DR (1993) CRC handbook of chemistry and physics. Boca Raton, FL,
Chemical Rubber Publishing Company, pp. 4, 34-35, 113.
Lioy PJ (1983) Air pollution emission profiles of toxic and trace
elements from energy related sources: status and needs.
Neurotoxicology, 4(3):103-112.
Lloyd Davies TA (1946) Manganese pneumonitis. British journal of
industrial medicine, 3:111-135.
Loranger S, Zayed J (1994) Manganese and lead concentrations in
ambient air and emission rates from unleaded and leaded gasoline
between 1981 and 1992 in Canada: a comparative study. Atmospheric
environment, 28(9):1645-1651.
Loranger S, Zayed J (1995) Contribution of methylcyclopentadienyl
manganese tricarbonyl (MMT) to atmospheric Mn concentrations near
expressway: dispersion modeling estimations. Atmospheric
environment, 29(5):591-599.
Loranger S, Zayed J (1997) Environmental contamination and human
exposure to airborne total and respirable manganese in Montreal. Air
and waste management, 47:983-989.
Loranger S, Zayed J, Forget E (1994) Manganese contamination in
Montreal in relation with traffic density. Water, air and soil
pollution, 74:385-396.
Lown BA, Morganti JB, D'Agostino R, Stineman CH, Massaro EJ (1984)
Effects on the postnatal development of the mouse of preconception,
postconception and/or suckling exposure to manganese via maternal
inhalation exposure to MnO2 dust. Neurotoxicology, 5:119-129.
Lucchini R, Selis L, Folli D, Apostoli P, Mutti A, Vanoni O, Iregren
A, Alessio L (1995) Neurobehavioral effects of manganese in workers
from a ferroalloy plant after temporary cessation of exposure.
Scandinavian journal of work and environmental health, 21:143-149.
Maigetter RZ, Ehrlich R, Fenters JD, Gardner DE (1976) Potentiating
effects of manganese dioxide on experimental respiratory infections.
Environmental research, 11:386-391.
MAK (1994) Manganese and its inorganic compounds. Weinheim, Germany,
MAK - Deutsche Forschungsgemeinschaft, VCH, mbh D-69451 (English
translation).
Malm O, Pfeiffer WC, Fiszman M, Azcue JM (1988) Transport and
availability of heavy metals in the Paraiba do Sul-Guandu River
system, Rio de Janeiro State, Brazil. Science of the total
environment, 75:201-209.
McBride MB (1979) Chemisorption and precipitation of Mn2+ at CaCO3
surfaces. Journal of the Soil Science Society of America,
43:693-698.
Meco G, Bonifati V, Vanacore N, Fabrizio E (1994) Parkinsonism after
chronic exposure to the fungicide maneb (manganese
ethylene-bis-dithiocarbamate). Scandinavian journal of work and
environmental health, 20(4):301-305.
Mena I, Marin O, Fuenzalida S, Cotzias GC (1967) Chronic manganese
poisoning: clinical picture and manganese turnover. Neurology,
17:128-136.
Mena I, Horiuchi K, Burke K, Cotzias GC (1969) Chronic manganese
poisoning: individual susceptibility and absorption of iron.
Neurology, 19:1000-1006.
Mena I, Horiuchi K, Lopez G (1974) Factors enhancing entrance of
manganese into the brain: iron deficiency and age. Journal of
nuclear medicine, 15:516.
Mergler D, Baldwin M (1997) Early manifestations of manganese
neurotoxicity in humans: an update. Environmental research,
78(1-2):92-100.
Mergler D, Huel G, Bowler R, Iregren A, Bélanger S, Baldwin M, Tardif
R, Smargiassi A, Martin L (1994) Nervous system dysfunction among
workers with long-term exposure to manganese. Environmental
research, 64(2):151-180.
Mirowitz SA, Westrich TJ, Hirsch JD (1991) Hyperintense basal ganglia
on T1-weighted MR images in patients receiving parenteral nutrition.
Radiology, 181:117-120.
Morganti JB, Lown BA, Stineman CH, D'Agostino RB, Massaro EJ (1985)
Uptake, distribution and behavioral effects of inhalation exposure to
manganese (MnO2) in the adult mouse. Neurotoxicology, 6:1-15.
Mori I, Fujita Y, Ikuta K, Nakahashi Y, Kato K (1989) [Highly
sensitive and selective fluorimetric methods for the determination of
iron (III) and manganese (II) using fluorescein-hydrogen
peroxide-triethylenetetramine and fluorescein-hydrogen
peroxide-triethylenetetramine/iron, respectively.] Fresenius
Zeitschrift fuer Analytische Chemie, 334(3):252-255 (in German).
Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B, Zeiger E (1986)
Salmonella mutagenicity tests: II. Results from testing of 270
chemicals. Environmental mutagenesis, 8:1-26.
Motolese A, Truzzi M, Giannini A, Seidenari S (1993) Contact
dermatitis and contact sensitization among enamellers and decorators
in the ceramics industry. Contact dermatitis, 28(2):59-62.
Nachtman JP, Tubben RE, Commissaris RL (1986) Behavioral effects of
chronic manganese administration in rats: locomotor activity studies.
Neurobehavioral toxicology and teratology, 8:711-715.
NAS (1973) Medical and biological effects of environmental
pollutants: manganese. Washington, DC, National Academy of Sciences,
National Academy Press.
NAS (1977) Drinking water and health. Washington, DC, National
Academy of Sciences, National Academy Press, pp. 214-215, 265-270,
311-312.
NAS (1980) Drinking water and health. Vol. 3. Washington, DC,
National Academy of Sciences, National Academy Press, pp. 331-337.
Newland MC, Weiss B (1992) Persistent effects of manganese on
effortful responding and their relationship to manganese accumulation
in the primate globus pallidus. Toxicology and applied pharmacology,
113(1):87-97.
NRC (1989) Recommended dietary allowances, 10th ed. Washington, DC,
National Research Council, pp. 231-235.
NTP (1993) Toxicology and carcinogenesis studies of manganese (II)
sulfate monohydrate in F344/N rats and B6C3F1 mice. Research
Triangle Park, NC, US Department of Health and Human Services,
National Toxicology Program (NTP TR 428).
NTP (1999) NTP chemical health and safety data. On-line retrieval
(25 May 1999) from the National Toxicology Program, US Department of
Health and Human Services, website:
http://ntp-server.niehs.nih.gov/Main_Pages/Chem-HS.html.
Oberly TJ, Piper CE, McDonald DS (1982) Mutagenicity of metal salts in
the L5178Y mouse lymphoma assay. Journal of toxicology and
environmental health, 9:367-376.
Oner G, Senturk UK (1995) Reversibility of manganese-induced learning
defect in rats. Food and chemical toxicology, 33(7):559-563.
Pagano DA, Zeiger E (1992) Conditions for detecting the mutagenicity
of divalent metals in Salmonella typhimurium. Environmental and
molecular mutagenesis, 19(2):139-146.
Patterson KY, Holbrook JF, Bodner JE, Kelsay JL, Smith JC, Veillon C
(1984) Zinc, copper, and manganese intake and balance for adults
consuming self-selected diets. American journal of clinical
nutrition, 40:1397-1403.
Pellizari ED, Thomas KW, Clayton CA, Whitmore RW, Shores RC, Zelon HS,
Perritt RL (1992) Particle total exposure assessment methodology
(PTEAM): Riverside, California pilot study. Vol. I [final report].
Research Triangle Park, NC, US Environmental Protection Agency,
Atmospheric Research and Exposure Assessment Laboratory (EPA Report
No. EPA/600/R-93/050; available from NTIS, Springfield, VA:
PB93-166957/XAB).
Pennington JAT, Young BE, Wilson DB, Johnson RD, Vanderveen JE (1986)
Mineral content of foods and total diets: The selected minerals in
foods survey, 1982 to 1984. Journal of the American Medical
Association, 86:876-891.
Pflaumbaum W, Blome H, Heidermanns G (1990) [Presence of manganese and
manganese compounds at work-places.] Staub-Reinhalt Luft,
50(9):307-310 (in German).
Ponnamperuma FN, Loy TA, Tianco EM (1969) Redox equilibria in flooded
soils. II. The manganese oxide systems. Soil science, 108:48-57.
Rai D, Zachara JM, Schwab AP, Schmidt RL, Girvin DC, Rogers JE (1986)
Manganese. In: Chemical attenuation rates, coefficients, and
constants in leachate migration. Vol. 1. A critical review. Report
to Electric Power Research Institute, Palo Alto, CA, by Battelle,
Pacific Northwest Laboratories, Richland, WA, pp. 15-1-15-4.
Rasmuson A (1985) Mutagenic effects of some water-soluble metal
compounds in a somatic eye-color test system in Drosophila
melanogaster. Mutation research, 157:157-162.
Rehnberg LG, Hein FJ, Carter DS, Linko RS, Laskey JW (1980) Chronic
manganese oxide administration to preweanling rats: manganese
accumulation and distribution. Journal of toxicology and
environmental health, 6:217-226.
Rehnberg LG, Hein FJ, Carter DS, Linko RS, Laskey JW (1981) Chronic
ingestion of Mn3O4 by young rats: tissue accumulation, distribution,
and depletion. Journal of toxicology and environmental health,
7:263-272.
Resing JA, Mottl MJ (1992) Determination of manganese in seawater
using flow injection analysis with on-line preconcentration and
spectrophotometric detection. Analytical chemistry,
64(22):2682-2687.
Rodier J (1955) Manganese poisoning in Moroccan miners. British
journal of industrial medicine, 12:21-35.
Roels H, Lauwerys R, Buchet JP, Genet P, Sarhan MJ, Hanotiau I, deFays
M, Bernard A, Stanescu D (1987) Epidemiological survey among workers
exposed to manganese: effects on lung, central nervous system, and
some biological indices. American journal of industrial medicine,
11:307-327 [Erratum (1987), American journal of industrial medicine,
12:119-120].
Roels HA, Ghyselen P, Buchet JP, Ceulemans E, Lauwerys RR (1992)
Assessment of the permissible exposure level to manganese in workers
exposed to manganese dioxide dust. British journal of industrial
medicine, 49(1):25-34.
Roels H, Meiers G, Delos M, Ortega P, Lauwerys R, Buchet JP, Lison D
(1997) Influence of the route of administration and the chemical form
(MnCl2, MnO2) on the absorption and cerebral distribution of
manganese in rats. Archives of toxicology, 71:223-230.
Rope SK, Arthur WJ, Craig TH, Craig EH (1988) Nutrient and trace
elements in soil and desert vegetation of southern Idaho.
Environmental monitoring and assessment, 10:1-24.
Rosenstock HA, Simons GD, Meyer SJ (1971) Chronic manganism:
neurologic and laboratory studies during treatment with levodopa.
Journal of the American Medical Association, 217:1354-1358.
Ruijten MWMM, Sall HJA, Verberk MM, Smink M (1994) Effect of chronic
mixed pesticide exposure on peripheral and autonomic nerve function.
Archives of environmental health, 49(3):188-195.
Sánchez DJ, Domingo JL, Llobet JM, Keen CL (1993) Maternal and
developmental toxicity of manganese in the mouse. Toxicology
letters, 69:45-52.
Sarhan MJ, Roels H, Lauwerys R (1986) Influence of manganese on the
gastrointestinal absorption of cadmium in rats. Journal of applied
toxicology, 6(5):313-316.
Saric M, Markicevic A, Hrustic O (1977) Occupational exposure to
manganese. British journal of industrial medicine, 34:114-118.
Sax NI, Lewis RJ Sr (1987) Hawley's condensed chemical dictionary,
11th ed. New York, NY, Van Nostrand Reinhold Company, pp. 727-731.
Schaanning M, Naes K, Egeberg PK, Bome F (1988) Cycling of manganese
in the permanently anoxic Drammens fjord. Marine chemistry,
23:365-382.
Schnitzer M (1969) Reactions between fulvic acid, a soil humic
compound and inorganic soil constituents. Soil Science Society of
America, proceedings, 33:75-80.
Schroeder HA, Balassa JJ, Tipton IH (1966) Essential trace metals in
man: manganese. A study in homeostasis. Journal of chronic diseases,
19:545-571.
Schroeder WH, Dobson M, Kane DM (1987) Toxic trace elements associated
with airborne particulate matter: a review. Journal of the Air
Pollution Control Association, 37:1267-1285.
Schuler P, Oyanguren H, Maturana V, Valenzuela A, Cruz E, Plaza V,
Schmidt E, Haddad R (1957) Manganese poisoning: environmental and
medical study at a Chilean mine. Industrial medicine and surgery,
26:167-173.
Seth PK, Nagar N, Husain R, Chandra SV (1973) Effects of manganese on
rabbit testes. Environmental physiology and biochemistry, 3:263-267.
Seth PK, Hong JS, Kilts CD, Bondy SC (1981) Alteration of cerebral
neurotransmitter receptor function by exposure of rats to manganese.
Toxicology letters, 9(3):247-254.
Shiotsuka RN (1984) Inhalation toxicity of manganese dioxide and a
magnesium oxide-manganese dioxide mixture. Report to US Army Medical
Research and Developmental Command, Fort Detrick, Frederick, MD, by
Inhalation Toxicology Facility, Medical Department, Brookhaven
National Laboratory, Uptown, NY (NTIS No. ADA-148868).
Shukla GS, Dubey MP, Chandra SV (1980) Manganese-induced biochemical
changes in growing versus adult rats. Archives of environmental
contamination and toxicology, 9(4):383-391.
Singh I (1984) Induction of gene conversion and reverse mutation by
manganese sulphate and nickel sulphate in Saccharomyces cerevisiae.
Mutation research, 137:47-49.
Singh J, Husain R, Tandon SK, Chandra SV (1974) Biochemical and
histopathological alterations in early manganese toxicity in rats.
Environmental physiology and biochemistry, 4:16-23.
Singh PP, Junnarkar AY (1991) Behavioral and toxic profile of some
essential trace metal salts in mice and rats. Indian journal of
physiological pharmacology, 23(3):153-159.
Sitaramayya A, Nagar N, Chandra SV (1974) Effect of manganese on
enzymes in rat brain. Acta pharmacology and toxicology, 35:185-190.
Sjögren B, Gustavsson P, Hogstedt C (1990) Neuropsychiatric symptoms
among welders exposed to neurotoxic metals. British journal of
industrial medicine, 47:704-707.
Sjögren B, Iregren A, Frech W, Hagman M, Johansson L, Tesarz M,
Wennberg A (1996) Effects on the nervous system among welders exposed
to aluminum and manganese. Occupational and environmental medicine,
53:32-40.
Smith MO, Sherman IL, Miller LC, Robbins KR, Halley JT (1995) Relative
biological availability of manganese from manganese proteinate,
manganese sulfate, and manganese monoxide in broilers reared at
elevated temperatures. Poultry science, 74(4):702-707.
Smith RA, Alexander RB, Wolman MG (1987) Water-quality trends in the
nation's rivers. Science, 235:1607-1615.
Smyth HF, Carpenter CP, Weil CS, Pozzani UC, Striegel JA, Nycum JS
(1969) Range-finding toxicity data. List VII. American Industrial
Hygiene Association journal, 30:470-476.
Smyth LT, Ruhf RC, Whitman NE, Dugan T (1973) Clinical manganism and
exposure to manganese in the production and processing of
ferromanganese alloy. Journal of occupational medicine, 15:101-109.
Southwood T, Lamb CM, Freeman J (1987) Ingestion of potassium
permanganate crystals by a 3-yr-old boy. Medical journal of
Australia, 146:639-640.
Stokes PM, Campbell PGC, Schroeder WH, Trick C, France RL, Puckett KJ,
LaZerte B, Speyer M, Hanna JE, Donaldson J (1988) Manganese in the
Canadian environment. Ottawa, ON, National Research Council of
Canada, Associate Committee on Scientific Criteria for Environmental
Quality, pp. 30-32 (NRCC No. 26193).
Stoner GD, Shimkin MB, Troxell MC, Thompson TL, Terry LS (1976) Test
for carcinogenicity of metallic compounds by the pulmonary tumor
response in strain A mice. Cancer research, 36:1744-1747.
Subhash MN, Padmashree TS (1991) Effect of manganese on biogenic amine
metabolism in regions of the rat brain. Food and chemical
toxicology, 29(8):579-582.
Suzuki Y, Fujii N, Yano H, Ohkita T, Ichikawa A, Nishiyama K (1978)
Effects of the inhalation of manganese dioxide dust on monkey lungs.
Tokushima journal of experimental medicine, 25:119-125.
Szakmáry E, Ungváry G, Hudák A, Naray M, Tatrai E, Szeberenyi S, Varga
B, Morvai V (1995) Developmental effect of manganese in rat and
rabbit. Central European journal of occupational and environmental
medicine, 1(2):149-159.
Tanaka S (1994) Manganese and its compounds. In: Zenz C, Dickerson OB,
Horvath EP, eds. Occupational medicine, 3rd ed. St. Louis, MO,
Mosby, pp. 542-548.
Tanaka S, Lieben J (1969) Manganese poisoning and exposure in
Pennsylvania. Archives of environmental health, 19:674-684.
Thomas PT, Basse WW, Kerkuliet NI, Luster MI, Munson AE, Murray M,
Roberts D, Robinson M, Silkworth J, Sjoblad R, Smialowicz R (1990)
Immunologic effects of pesticides. In: Balars SR, Wilkinson CF, eds.
The effects of pesticides on human health. Vol. 18. New York, NY,
Princeton Scientific Publishers Inc., pp. 261-295.
Thompson SE, Burton CA, Quinn DJ, Ng YC (1972) Concentration factors
of chemical elements in edible aquatic organisms. Livermore, CA,
University of California, Lawrence Livermore Laboratory, Bio-Medical
Division.
Tjälve H, Henriksson J, Tullkvisi J, Larsson BS, Lindquist NG (1996)
Uptake of manganese and cadmium from nasal mucosa into the central
nervous system via olfactory pathways in rats. Pharmacology and
toxicology, 79:347-356.
Tomes R (1997) Medical management database: Manganese. CD-ROM,
Micromedex, Inc., June.
Treinen KA, Gray TJB, Blazak WF (1995) Developmental toxicity of
mangafodipir trisodium and manganese chloride in Sprague-Dawley rats.
Teratology, 52:109-115.
TRI87 (1989) Toxic chemical release inventory. Washington, DC, US
Environmental Protection Agency, Office of Toxic Substances.
TRI91 (1993) Toxic chemical release inventory. Washington, DC, US
Environmental Protection Agency, Office of Toxic Substances.
Tsuda H, Kato K (1977) Chromosomal aberrations and morphological
transformation in hamster embryonic cells treated with potassium
dichromate in vitro. Mutation research, 46:87-94.
Ulrich CE, Rinehart W, Brandt M (1979) Evaluation of the chronic
inhalation toxicity of a manganese oxide aerosol. III -- Pulmonary
function, electromyograms, limb tremor, and tissue manganese data.
American Industrial Hygiene Association journal, 40:349-353.
Umeda M, Nishimura M (1979) Inducibility of chromosomal aberrations by
metal compounds in cultured mammalian cells. Mutation research,
67:221-229.
US Department of the Interior (1993) Manganese statistical
compendium. Washington, DC, Bureau of Mines.
US Department of the Interior (1995) Mineral industry surveys.
Manganese. Washington, DC, Bureau of Mines and the US Geological
Survey.
US Department of the Interior (1996) Mineral commodity summary
(annual): Manganese. Washington, DC, Bureau of Mines and the US
Geological Survey.
US Department of the Interior (1998) Mineral industry surveys.
Manganese. Washington, DC, Bureau of Mines and the US Geological
Survey.
US EPA (1979) Sources of toxic pollutants found in influents to
sewage treatment plants. VI. Integrated interpresentation.
Washington, DC, US Environmental Protection Agency, Office of Water
Planning and Standards (EPA 440/4-008; NTIS No. PB81-219685).
US EPA (1983) Human exposure to atmospheric concentrations of
selected chemicals. Vol. II. Report to US Environmental Protection
Agency, Office of Air Quality Planning and Standards, Research
Triangle Park, NC, by Systems Applications, Inc., San Rafael, CA (NTIS
PB83-265249).
US EPA (1984) Health assessment document for manganese. Final draft.
Cincinnati, OH, US Environmental Protection Agency, Office of Research
and Development (EPA-600/8-83-013F).
US EPA (1985a) Locating and emitting air emissions from sources of
manganese. Research Triangle Park, NC, US Environmental Protection
Agency, Office of Air Quality Planning and Standards
(EPA-450/4-84-007h).
US EPA (1985b) Decision not to regulate manganese under the Clean Air
Act. US Environmental Protection Agency. Federal register,
50:32627-32628.
US EPA (1993) Drinking water criteria document for manganese.
Cincinnati, OH, US Environmental Protection Agency, Office of Health
and Environmental Assessment (EPA/ECAO-CIN-D008).
US EPA (1994a) ORD's response to public comments on health and
exposure issues in relation to the waiver petition of Ethyl
Corporation to add methylcyclopentadienyl manganese tricarbonyl
(MMT) to unleaded gasoline. Review draft. Washington, DC, US
Environmental Protection Agency.
US EPA (1994b) Reevaluation of inhalation health risks associated
with methylcyclopentadienyl manganese tricarbonyl (MMT) in gasoline.
Research Triangle Park, NC, US Environmental Protection Agency, Office
of Research and Development (EPA-600/R-94/062).
US EPA (1994c) Reevaluation of inhalation health risks associated
with methylcyclopentadienyl manganese tricarbonyl (MMT) in gasoline.
Appendix C: Manganese inhalation reference concentration. Research
Triangle Park, NC, US Environmental Protection Agency, Office of
Research and Development (EPA-600/R-94/062).
Utter MF (1976) The biochemistry of manganese. Medical clinics of
North America, 60:713-727.
Valencia R, Mason JM, Woodruff RC, Zimmering S (1985) Chemical
mutagenesis testing in Drosophila. III. Results of 48 coded
compounds tested for the National Toxicology Program. Environmental
mutagenesis, 7:325-348.
Venugopal B, Luckey TD (1978) Toxicity of group VII metals.
In: Metal toxicity in mammals. 2. Chemical toxicity of metals and
metalloids. New York, NY, Plenum Press, pp. 262-268.
Verschueren K (1983) Handbook of environmental data on organic
chemicals, 2nd ed. New York, NY, Van Nostrand Reinhold, pp. 806,
844.
Vieregge P, Heinzow B, Korf G, Teichert HM, Schleifenbaum P, Mosinger
HU (1995) Long-term exposure to manganese in rural well water has no
neurological effects. Canadian journal of neurological sciences,
22(4):286-289.
Webster WS, Valois AA (1987) Reproductive toxicology of manganese in
rodents, including exposure during the postnatal period.
Neurotoxicology, 8:437-444.
Wedler F (1994) Biochemical and nutritional role of manganese: an
overview. In: Klimis-Tavantzis DJ, ed. Manganese in health and
disease. Boca Raton, FL, CRC Press, pp. 1-37.
Wennberg A, Iregren A, Struwe G, Cizinsky G, Hagman M, Johansson L
(1991) Manganese exposure in steel smelters a health hazard to the
nervous system. Scandinavian journal of work and environmental
health, 17:255-262.
Wennberg A, Hagman M, Johansson L (1992) Preclinical
neurophysiological signs of parkinsonism in occupational manganese
exposure. Neurotoxicology, 13(1):271-274.
Whitlock CM, Amuso SJ, Bittenbender JB (1966). Chronic neurological
disease in two manganese steel workers. American Industrial Hygiene
Association journal, 27:454-459.
WHO (1981) Environmental health criteria 17: Manganese. Geneva,
World Health Organization.
WHO (1984) Guidelines for drinking water quality. Vol. 1.
Recommendations. Geneva, World Health Organization, pp. 7, 52, 79,
82.
WHO (1986) Diseases caused by manganese and its toxic compounds.
Early detection of occupational diseases. Geneva, World Health
Organization, pp. 69-73.
WHO (1987) Manganese. In: Air quality guidelines for Europe.
Copenhagen, World Health Organization Regional Office for Europe, pp.
262-271 (European Series No. 23).
WHO (1993) Guidelines for drinking-water quality, 2nd ed. Vol. 1.
Recommendations. Geneva, World Health Organization.
WHO (1999) WHO air quality guidelines for Europe, 2nd ed.
Copenhagen, World Health Organization Regional Office for Europe (in
press).
Windholz M, ed. (1983) The Merck index: An encyclopedia of
chemicals, drugs and biologicals, 10th ed. Rahway, NJ, Merck and
Company, Inc., pp. 816-818.
Wong PK (1988) Mutagenicity of heavy metals. Bulletin of
environmental contamination and toxicology, 40:597-603.
Yamada M, Ohno S, Okayasu I, Okeda R, Hatakeyama S, Watanabe H, Ushio
K, Tsukagoshi H (1986) Chronic manganese poisoning: a
neuropathological study with determination of manganese distribution
in the brain. Acta neuropathologica (Berlin), 70:273-278.
Zayed J, Gérin G, Loranger S, Sierra P, Bégin D, Kennedy G (1994)
Occupational and environmental exposure of garage workers and taxi
drivers to airborne manganese arising from the use of
methylcyclopentadienyl manganese tricarbonyl in unleaded gasoline.
American Industrial Hygiene Association journal, 55:53-58.
APPENDIX 1 -- SOURCE DOCUMENTS
Agency for Toxic Substances and Disease Registry (1996)
The Toxicological profile for manganese (update) (ATSDR, 1996)
was prepared by the Agency for Toxic Substances and Disease Registry
(ATSDR) through a contract with the Research Triangle Institute. The
updated profile was published as a draft for public comment in
February 1998. Copies of the profile can be obtained from:
Division of Toxicology
Agency for Toxic Substances and Disease Registry
Public Health Service
US Department of Health and Human Services
1600 Clifton Road NE, Mailstop E-29
Atlanta, Georgia 30333
USA
Dr M. Williams-Johnson, Division of Toxicology, ATSDR, and Dr
S.G. Donkin, Dr S.W. Rhodes, and L. Kolb, Sciences International,
Inc., Alexandria, Virginia, contributed to the development of the
toxicological profile as chemical manager and authors. The profile has
undergone three ATSDR internal reviews, including Green Border Review
to assure consistency with ATSDR policy, a Health Effects Review, and
a Minimal Risk Level Review. An external peer review panel was
assembled for the update profile for manganese. The panel consisted of
the following members: Dr J Greger, University of Wisconsin, Madison,
Wisconsin; Dr D.J. Hodgson, University of Wyoming, Laramie, Wyoming;
and Dr C. Newland, Auburn University, Auburn, Alabama. These experts
collectively have knowledge of manganese's physical and chemical
properties, toxicokinetics, key health end-points, mechanisms of
action, human and animal exposure, and quantification of risk to
humans. All reviewers were selected in conformity with the conditions
for peer review specified in Section 104(i)(13) of the US
Comprehensive Environmental Response, Compensation, and Liability
Act, as amended.
Scientists from ATSDR reviewed the peer reviewers' comments and
determined which comments were to be included in the profile. A
listing of the peer reviewers' comments not incorporated in the
profile, with a brief explanation of the rationale for their
exclusion, exists as part of the administrative record for this
compound. A list of databases reviewed and a list of unpublished
documents cited are also included in the administrative record. The
citation of the peer review panel should not be understood to imply
its approval of the profile's final content.
Hazardous Substances Data Bank (1998)
Copies of the information on manganese stored in the Hazardous
Substances Data Bank (HSDB) can be obtained from:
Specialized Information Services
National Library of Medicine
National Institutes of Health
Public Health Service
US Department of Health and Human Services
8600 Rockville Pike
Bethesda, Maryland 20894
USA
HSDB is peer reviewed by a scientific review panel composed of
expert toxicologists and other scientists.
APPENDIX 2 -- CICAD PEER REVIEW
The draft CICAD on manganese and its compounds was sent for
review to institutions and organizations identified by IPCS after
contact with IPCS national Contact Points and Participating
Institutions, as well as to identified experts. Comments were received
from:
BHP Minerals, San Francisco, USA
Department of Health, London, United Kingdom
Department of Toxicology and Chemistry, National Institute for
Working Life, Solna, Sweden
Environment Canada, Ottawa, Canada
Health Canada, Ottawa, Canada
Institute of Occupational Health, Helsinki, Finland
National Chemicals Inspectorate, Solna, Sweden
National Institute for Environmental Health and Safety,
Washington, DC, USA
National Institute for Occupational Safety and Health, Atlanta,
USA
National Institute for Public Health, Prague, Czech Republic
National Institute of Occupational Health, Budapest, Hungary
Public Health Centre of the Capital City, Prague, Czech Republic
United States Environmental Protection Agency, Washington, DC,
USA
Université de Montréal, Montreal, Canada
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
Berlin, Germany, 26-28 November 1997
Members
Dr H. Ahlers, Education and Information Division, National Institute
for Occupational Safety and Health, Cincinnati, OH, USA
Mr R. Cary, Health Directorate, Health and Safety Executive, Bootle,
United Kingdom
Dr S. Dobson, Institute of Terrestrial Ecology, Huntingdon, United
Kingdom
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany (Chairperson)
Mr J.R. Hickman, Health Protection Branch, Health Canada, Ottawa,
Ontario, Canada
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany
Ms M.E. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada (Rapporteur)
Dr K. Paksy, Department of Reproductive Toxicology, National Institute
of Occupational Health, Budapest, Hungary
Mr V. Quarg, Ministry for the Environment, Nature Conservation &
Nuclear Safety, Bonn, Germany
Mr D. Renshaw, Department of Health, London, United Kingdom
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan
Prof. S. Soliman, Department of Pesticide Chemistry, Alexandria
University, Alexandria, Egypt (Vice-Chairperson)
Dr M. Wallen, National Chemicals Inspectorate (KEMI), Solna, Sweden
Ms D. Willcocks, Chemical Assessment Division, Worksafe Australia,
Camperdown, Australia
Dr M. Williams-Johnson, Division of Toxicology, Agency for Toxic
Substances and Disease Registry, Atlanta, GA, USA
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Observers
Mrs B. Dinham1, The Pesticide Trust, London, United Kingdom
Dr R. Ebert, KSU Ps-Toxicology, Huels AG, Marl, Germany (representing
ECETOC, the European Centre for Ecotoxicology and Toxicology of
Chemicals)
Mr R. Green,1 International Federation of Chemical, Energy, Mine and
General Workers' Unions, Brussels, Belgium
Dr B. Hansen,1 European Chemicals Bureau, European Commission, Ispra,
Italy
Dr J. Heuer, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Mr T. Jacob,1 DuPont, Washington, DC, USA
Ms L. Onyon, Environment Directorate, Organisation for Economic
Co-operation and Development, Paris, France
Dr H.J. Weideli, Ciba Speciality Chemicals Inc., Basel, Switzerland
(representing CEFIC, the European Chemical Industry Council)
Secretariat
Dr M. Baril, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr R.G. Liteplo, Health Canada, Ottawa, Ontario, Canada
Ms L. Regis, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Mr A. Strawson, Health and Safety Executive, London, United Kingdom
Dr P. Toft, Associate Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
1 Invited but unable to attend.
APPENDIX 4 -- ADDITIONAL APPROACHES FOR GUIDANCE VALUE DEVELOPMENT
An additional approach in deriving an inhalation guidance value
for manganese could involve dividing the exposed workers from the
Roels et al. (1992) study into four quartiles to estimate a
dose-response relationship and using the average cumulative exposure
and average exposure duration for the lowest quartile of workers to
calculate a NOAEL of 32 µg/m3 (adjusted for continuous exposure),
which could be divided by an uncertainty factor of 300 (10 to account
for human variability, 10 to account for less than lifetime exposure,
and 3 to account for other weaknesses in the study and overall
database -- i.e., statistical weakness from only 23 people in each
quartile, exposure to a manganese oxide other than manganese
tetroxide, and a lack of reproductive data), yielding a guidance value
of 0.1 µg/m3 (Egyed & Wood, 1996).
A guidance value might also be derived by using the geometric
mean integrated respirable dust concentration reported in the Roels et
al. (1992) study and adjusting for exposure duration to calculate an
exposure-adjusted lowest-observed-adverse-effect level (LOAEL) of 50
µg/m3, which could be divided by an uncertainty factor of 1000 (10 to
protect sensitive individuals, 10 to account for using a LOAEL instead
of a NOAEL, and 10 to account for database limitations
-- extrapolation from subchronic to chronic exposure, inadequate
reproductive and developmental data, and unknown differences in the
toxicity of different forms of manganese), yielding a guidance value
of 0.05 µg/m3 (US EPA, 1994b, 1994c; Davis, 1998). Other possible
guideline values, calculated using other methods (e.g., benchmark,
Bayesian, etc.), would range from 0.09 to 0.2 µg/m3 (US EPA, 1994a,
1994b, 1994c; Davis, 1998).
Alternatively, an inhalation guidance value could be derived
based upon a LOAEL of 140 µg total manganese dust/m3 reported in the
study by Iregren (1990), divided by an uncertainty/modifying factor of
900 (10 to account for use of a LOAEL instead of a NOAEL; 10 to
account for human variability; and two modifying factors -- 3 to
account for effects from cumulative exposure to manganese, and 3 to
account for potential differences in toxicity from different forms of
manganese) and adjusted for continuous rather than intermittent
exposure, yielding a provisional guidance value of 0.04 µg/m3 (ATSDR,
1996).
RÉSUMÉ D'ORIENTATION
Ce CICAD relatif au manganèse et à ses dérivés repose
essentiellement sur un rapport intitulé Toxicological profile for
manganese (update), draft for public comment et rédigé par l'Agency
for Toxic Substances and Disease Registry, US Department of Health and
Human Services (ATSDR, 1996). On a également utilisé les informations
contenues dans la Banque de données pour les substances dangereuses,
une banque de données constituée et gérée par la National Library of
Medicine, US Department of Health and Human Services (HSDB, 1998). Les
dernières données sur lesquels s'appuient ces documents de base
remontent à novembre 1998. Enfin, des données complémentaires ont été
tirées des évaluations publiées par l'US Environmental Protection
Agency (EPA) et l'Organisation mondiale de la Santé (OMS) ainsi que de
divers autres documents. Les documents de base utilisés pour la
rédaction de ce CICAD ne prennent pas en compte les effets du
manganèse sur les écosystèmes. Aucune autre source documentaire
(c'est-à-dire des documents rédigés par des organismes internationaux
et soumis à une contrôle scientifique rigoureux) n'a pu être trouvée.
Ce CICAD ne concerne donc que les effets que la présence de manganèse
dans l'environnement peut avoir sur la santé humaine. On n'a pas
cherché à déterminer les effets exercés sur les autres êtres vivants
dans leur milieu naturel. On trouvera à l'appendice 1 des indications
sur les sources documentaires utilisées. Les renseignements concernant
l'examen du CICAD par des pairs font l'objet de l'appendice 2. Ce
CICAD a été approuvé en tant qu'évaluation internationale lors d'une
réunion du Comité d'évaluation finale qui s'est tenue à Berlin
(Allemagne) du 26 au 28 novembre 1997. La liste des participants à
cette réunion figure à l'appendice 3. La fiche d'information
internationale sur la sécurité chimique (ICSC No 0174) établie par le
Programme international sur la Sécurité chimique (IPCS, 1993) est
également reproduite dans ce document.
Le manganèse (Mn) est un élément présent à l'état naturel dans
les roches, le sol, l'eau et les aliments. Tous les êtres humains sont
donc exposés au manganèse et ce dernier est un constituant naturel de
l'organisme. La voie d'exposition la plus importante pour l'Homme est
habituellement la voie alimentaire. Les doses journalières considérées
comme suffisantes et sans danger vont de 1 à 5 mg de manganèse pour
les enfants de 1 an et plus et les adultes. Elles correspondent
généralement à l'apport d'origine alimentaire.
Le manganèse est libéré dans l'atmosphère principalement sous la
forme de particules dont la destinée et le transport dépendent de leur
taille et de leur densité ainsi que de la vitesse et de la direction
du vent. Certains dérivés du manganèse sont très solubles dans l'eau,
de sorte qu'on s'expose facilement à en ingérer une quantité
importante en buvant de l'eau contaminée. Le manganèse présent dans
l'eau peut subir une oxydation, s'adsorber sur les particules en
suspension et sédimenter ensuite. Dans le sol, le manganèse peut
migrer sous forme de particules dans l'air ou dans l'eau ou encore,
s'il est présent sous la forme de composés solubles, en être éliminé
par lessivage.
C'est chez les personnes employées dans des ateliers libérant des
poussières à forte teneur en manganèse, ou qui vivent dans leur
voisinage, que l'exposition au manganèse a le plus de chances d'être
supérieure à la moyenne. Dans certaines régions, la population peut
être exposée au manganèse libéré dans l'atmosphère par la combustion
d'essence sans plomb additionnée d'un antidétonnant organomanganique,
le méthylcyclopentadiénylmanganèse-tricarbonyle (MMT). Certaines
personnes peuvent absorber une quantité excessive de manganèse en
consommant l'eau de puits contaminés par du manganése provenant de
piles ou de pesticides. De même les enfants peuvent se contaminer en
portant à leur bouche de la terre contenant du manganèse en excès.
Le manganèse est un nutriment essentiel pour l'Homme. Il
intervient dans la minéralisation des os, dans le métabolisme
énergétique et dans celui des protéines, dans la régulation du
métabolisme, dans la protection des cellules contre les radicaux
libres et dans la formation de glycosaminoglycanes. L'inhalation ou
l'ingestion de quantités excessives de manganèse peut toutefois avoir
des effets indésirables. A dose administrée équivalente, on retrouve
davantage de manganèse dans l'encéphale après inhalation qu'après
ingestion et la plupart des effets qu'il provoque sont liés à une
exposition chronique par la voie respiratoire. On sait peu de chose de
la toxicité relative des divers composés du manganèse. Toutefois, les
données disponibles indiquent que plusieurs d'entre eux sont capables
de provoquer des effets neurologiques; on a observé ces effets chez
l'Homme après exposition chronique (365 jours ou davantage) par la
voie respiratoire ainsi que chez l'animal, après exposition de durée
intermédiaire (15-364 jours) ou exposition chronique par voie buccale.
D'une manière générale, on constate, à la lumière des données
disponibles, qu'une exposition à des concentrations excessives de
manganèse pendant deux semaines ou moins (exposition brève) ou une
période pouvant aller jusqu'à un an (exposition de durée
intermédiaire) exerce un effet sur les voies respiratoires et sur le
système nerveux, mais peu ou pas d'effets sur les autres organes. Une
intoxication aiguë par inhalation de poussières à forte teneur en
manganèse (en particulier sous forme de dioxyde MnO2 ou de tétroxyde
Mn3O4) peut provoquer une réaction inflammatoire au niveau
pulmonaire qui, avec le temps, peut aboutir à une détérioration de la
capacité fonctionnelle du poumon. Cette toxicité pulmonaire se
manifeste sous la forme d'une sensibilité accrue aux maladies
infectieuses - bronchites par exemple - et peut évoluer vers une
pneumopathie fibreuse. On a également observé une pneumopathie après
une brève inhalation de particules contenant d'autres métaux. Cet
effet pourrait donc être caractéristique d'une exposition à des
particules par la voie respiratoire et ne pas être uniquement lié à la
teneur de ces particules en manganèse.
Il existe quelques documents selon lesquels une exposition de
durée intermédiaire à des dérivés du manganèse est susceptible
d'exercer des effets sur le système nerveux central, mais on ne
dispose pas d'estimation fiable du niveau d'exposition nécessaire pour
produire de tels effets. Les études d'inhalation effectuées sur des
animaux ont révélé l'existence d'effets biochimiques, respiratoires et
neurocomportementaux. On n'a cependant pas déterminé quel était le
seuil d'apparition de ces effets car le niveau d'exposition nécessaire
à leur manifestation varie dans la proportion de 1 à 10.
En cas d'exposition chronique par la voie respiratoire, les
principaux organes-cibles sont les poumons, le système nerveux et les
gonades, encore que l'on ait également observé des effets au niveau
d'autres organes. On a constaté des cas de pneumopathie fibreuse
récidivante et des effets respiratoires aigus à la suite d'une
exposition chronique au manganèse. Les effets sur le système nerveux
se traduisent notamment par des troubles neurologiques et
neuropsychiatriques pouvant aboutir à une pathologie de type
parkinsonien connue sous le nom de manganisme. L'expérience incite à
penser que les animaux de laboratoire, et notamment les rongeurs, ne
sont pas aussi sensibles que l'Homme aux effets neurologiques provoqué
par l'inhalation de manganèse. Au nombre des effets sur la fonction de
reproduction figurent une réduction de la libido, l'impuissance et une
diminution de la fécondité chez les sujets de sexe masculin. On ne
dispose d'aucune information concernant les effets qui s'exerceraient
sur la fonction génitale de la femme. L'expérimentation animale
indique que le manganèse peut provoquer des lésions testiculaires et
des résorptions tardives. Les données tirées de l'expérimentation
animale et relatives aux effets de l'inhalation de manganèse sur le
système immunitaire ou sur le développement foetal sont trop limitées
pour que l'on puisse se prononcer véritablement au sujet de la
signification de ces effets pour l'Homme.
On ne dispose que de données limitées sur le pouvoir cancérogène
du manganèse et les résultats expérimentaux sont difficiles à
interpréter de façon catégorique. L'administration chronique de
sulfate de manganèse (MnSO4) à des rats par la voie buccale a
provoqué une légère augmentation des tumeurs du pancréas chez les
mâles et un nombre un peu plus élevé d'adénomes hypophysaires chez les
femelles. D'autres études portant sur le même composé n'ont pas mis de
cancers en évidence chez le rat et une augmentation marginale de
l'incidence des tumeurs affectant les cellules folliculaires de la
thyroïde a été relevée chez des souris. D'après des études in vitro,
il existe, chez certains composés tout au moins, un pouvoir mutagène.
Quoi qu'il en soit, les études in vivo chez les mammifères donnent
des résultats irréguliers et aucune conclusion générale ne peut en
être tirée en ce qui concerne le risque génotoxique que pourrait
comporter une exposition aux dérivés du manganèse.
Ingérés à forte dose par gavage, des sels concentrés de manganèse
peuvent entraîner la mort des animaux, mais une exposition de brève
durée par suite de la consommation d'aliments ou d'eau contaminés par
du manganèse ne semble pas entraîner d'intoxication importante. De
même, l'administration de sels de manganèse par la voie parentérale
peut avoir un effet toxique sur le développement, mais ces effets ne
s'observent pas après ingestion. On a décrit deux cas de neurotoxicité
après ingestion, pendant une période de durée intermédiaire, de
dérivés du manganèse par des sujets humains, mais les données sont
trop limitées pour que l'on puisse définir le seuil de toxicité ou
savoir si ces effets étaient imputables en totalité au manganèse. On
possède quelques données sur les effets neurologiques ou autres,
consécutifs, chez l'Homme, à l'ingestion prolongée de manganèse, mais
les études dont elles sont tirées pêchent par l'incertitude qui
entoure les voies d'exposition, les doses totales et l'existence
d'autres facteurs de confusion. Les données fournies par les études
sur l'Homme et l'animal sont insuffisantes pour permettre de
déterminer les doses ou les effets à prendre en considération dans le
cas d'une exposition de longue durée par la voie digestive. Les
éléments dont on dispose au sujet des effets indésirables d'une
ingestion prolongée de quantités excessives de manganèse sont
évocateurs mais non concluants.
La voie percutanée ne semble pas devoir être prise sérieusement
en considération et n'a d'ailleurs guère été étudiée. Les données dont
on dispose se limitent à l'effet corrosif du permanganate de potassium
(KMnO4) ou à des cas d'absorption percutanée de dérivés organiques du
manganèse comme le MMT.
A la lumière de ces données, il apparaît clairement qu'une
exposition au manganèse sur les lieux de travail peut entraîner des
effets neurologiques ou respiratoires. D'autres données, plus
limitées, incitent également à penser que l'ingestion de manganèse en
quantités excessives du fait de sa présence dans l'environnement peut
aussi avoir des effets neurologiques indésirables. Certains individus
peuvent être porteurs de facteurs qui les prédisposent à être plus
vunérables aux effets indésirables d'une exposition à des quantités
excessives de manganèse. Il peut s'agir notamment de malades atteints
de pneumopathies, de personnes exposées à d'autres irritants
pulmonaires, de nouveau-nés, de personnes âgées, de sujets souffrant
de carence martiale ou encore d'hépatiques.
Il y a plusieurs manières de parvenir à une valeur-guide pour le
manganèse présent dans l'air. On peut citer ici à titre d'exemple le
chiffre de 0,15 µg de manganèse par m3 qui a été récemment proposé;
on trouvera également d'autres méthodes envisageables pour obtenir ces
valeurs-guides.
RESUMEN DE ORIENTACION
Este CICAD sobre el manganeso y sus compuestos se basa
fundamentalmente en el informe titulado Perfil toxicológico del
manganeso (actualización), proyecto de información pública,
preparado por la Agencia para el Registro de Sustancias Tóxicas y
Enfermedades, Departamento de Salud y Servicios Sociales de los
Estados Unidos (ATSDR, 1996). Se utilizó asimismo la información
contenida en el Banco de Datos de Sustancias Peligrosas, servicio que
ha creado y mantiene la Biblioteca Nacional de Medicina del
Departamento de Salud y Servicios Sociales de los Estados Unidos
(HSDB, 1998). Se examinaron los datos identificados en estos
documentos originales hasta noviembre 1998. Hay información adicional
procedente de otras referencias, como las evaluaciones preparadas por
la Agencia para la Protección del Medio Ambiente de los Estados Unidos
(EPA) y la Organización Mundial de la Salud (OMS), así como de
diversos informes publicados. Los documentos originales utilizados en
la preparación del presente CICAD no comprenden los efectos del
manganeso en el medio ambiente. No se identificaron otras fuentes
(documentos preparados por una organización nacional y sujetos a un
examen científico riguroso) sobre este tema. Por consiguiente, en este
CICAD se abordan sólo los niveles en el medio ambiente como fuente de
exposición humana. Tampoco se ha intentado evaluar en el presente
documento los efectos sobre los organismos en el medio ambiente. La
información sobre la disponibilidad de los documentos originales
figura en el apéndice 1. La información acerca del examen colegiado de
este CICAD se presenta en el apéndice 2. Su aprobación como evaluación
internacional se realizó en una reunión de la Junta de Evaluación
Final, celebrada en Berlín, Alemania, los días 26-28 de noviembre de
1997. La lista de participantes en esta reunión figura en el apéndice
3. La Ficha internacional de seguridad química (ICSC 0174) para el
manganeso, preparada por el Programa Internacional de Seguridad de las
Sustancias Químicas (IPCS, 1993), también se reproduce en el presente
documento.
El manganeso (Mn) es un elemento natural del medio ambiente, que
se encuentra en las rocas, el suelo, el agua y los alimentos. Así
pues, todas las personas están expuestas al manganeso y es un
componente normal del organismo. La vía de exposición más importante
para el ser humano suelen ser los alimentos. Se ha establecido una
ingesta diaria inocua y suficiente de 1-5 mg de manganeso para niños
de un año y mayores hasta la edad adulta; estos niveles generalmente
se corresponden con las cantidades del compuesto que se reciben a
través de los alimentos.
El manganeso se libera en el aire fundamentalmente como materia
particulada, y el destino final y el transporte de las partículas
dependen de su tamaño y densidad y de la velocidad y la dirección del
viento. Algunos compuestos de manganeso son muy solubles en agua, de
manera que se puede producir una exposición importante por el consumo
de agua de bebida contaminada. El manganeso del agua superficial se
puede oxidar o adsorber en las partículas del sedimento y depositarse
en el fondo. El del suelo puede pasar como materia particulada al aire
o al agua, y los compuestos solubles de manganeso pueden sufrir un
proceso de lixiviación a partir del suelo.
Las exposiciones al manganeso mencionadas más arriba son más
probables en el caso de las personas que trabajan o viven cerca de
fábricas o de otros lugares donde se liberan cantidades significativas
de polvo de manganeso en el aire. En algunas regiones, la población
general puede estar expuesta al manganeso liberado en el aire por la
combustión de la gasolina sin plomo que contiene el compuesto orgánico
de manganeso, metilciclopentadienilo-manganeso tricarbonilo (MMT) como
ingrediente antidetonante. Algunas personas pueden estar expuestas a
un exceso de manganeso en el agua potable, por ejemplo cuando se
filtra al agua de los pozos manganeso de las baterías o de
plaguicidas. Los niños pueden estar expuestos a un exceso de manganeso
en el suelo por la costumbre de llevarse las manos a la boca.
El manganeso es un nutriente esencial del ser humano que
desempeña una función en la mineralización de los huesos, en el
metabolismo proteico y energético, en la regulación metabólica, en la
protección de las células del efecto perjudicial de sustancias con
radicales libres nocivos y en la formación de glucosaminoglucanos. Sin
embargo, la exposición a niveles elevados mediante inhalación o
ingestión puede producir efectos adversos en la salud. Para dosis
comparables, llega al cerebro más manganeso después de la inhalación
que de la ingestión, y la mayor parte de los efectos en la salud están
asociados con la exposición crónica por inhalación. Es poco lo que se
sabe acerca de la toxicidad relativa de los distintos compuestos de
manganeso. Sin embargo, hay pruebas que ponen de manifiesto que
diversos compuestos de manganeso pueden inducir efectos neurológicos;
estos efectos se han observado tras una exposición crónica por
inhalación (365 días o más) en el ser humano y una exposición oral
intermedia (15-364 días) y crónica en animales.
En general, los datos disponibles indican que la exposición a un
exceso de manganeso durante 14 días o menos (aguda) o hasta un año
(intermedia) tiene efectos en el sistema respiratorio y en el sistema
nervioso, con un efecto escaso o nulo en otros órganos. La exposición
aguda por inhalación a concentraciones elevadas de polvo de manganeso
(en concreto de dióxido de manganeso [MnO2] y de tetróxido de
manganeso [Mn3O4]) puede provocar una respuesta inflamatoria en el
pulmón, que con el tiempo puede dar lugar a un trastorno de la función
pulmonar. La toxicidad pulmonar se manifiesta en forma de una mayor
susceptibilidad a infecciones como la bronquitis y puede producir
neumonía mangánica. Se ha observado también neumonía tras exposiciones
agudas por inhalación a partículas que contenían otros metales. Así
pues, este efecto podría ser característico de la materia particulada
inhalable y no depender solamente del contenido en manganeso de las
partículas.
Hay un pequeño número de informes que parecen indicar que en la
exposición intermedia a compuestos de manganeso por inhalación se
producen efectos en el sistema nervioso central, pero no se dispone de
estimaciones fidedignas sobre los niveles de exposición. Los estudios
de inhalación en animales pusieron de manifiesto efectos bioquímicos,
respiratorios y en el neurocomportamiento. Sin embargo, no se ha
determinado un umbral para estos efectos, porque los niveles de
exposición asociados con ellos varían en más de un orden de magnitud.
En la exposición crónica al manganeso por inhalación, los
principales órganos afectados son los pulmones, el sistema nervioso y
el sistema reproductor, aunque también se han observado efectos en
otros sistemas de órganos. Este tipo de exposición se ha asociado con
una neumonía mangánica recurrente y con efectos respiratorios agudos.
Los efectos en el sistema nervioso incluyen síntomas neurológicos y
neuropsiquiátricos que pueden culminar en una enfermedad semejante a
la de Parkinson, conocida como manganismo; hay pruebas que indican que
los animales de laboratorio, especialmente los roedores, no son tan
sensibles como el ser humano, y posiblemente otros primates, a los
efectos neurológicos provocados por la exposición al manganeso por
inhalación. Los efectos reproductivos de la exposición crónica por
inhalación son una reducción de la libido, impotencia y menor
fecundidad en los hombres; no se dispone de información acerca de los
efectos reproductivos en las mujeres. Los estudios realizados en
animales indican que el manganeso puede producir daños directos en los
testículos y resorciones tardías. Los datos obtenidos de estudios
realizados con animales sobre los efectos del manganeso inhalado en el
sistema inmunitario y en el desarrollo del feto son demasiado
limitados para sacar conclusiones sobre la importancia de estos
efectos en el ser humano.
La información sobre el potencial carcinogénico del manganeso es
limitada y los resultados son difíciles de interpretar con certeza.
Los estudios de exposición crónica por vía oral realizados con sulfato
de manganeso (MnSO4) en ratas pusieron de manifiesto un pequeño
aumento en la incidencia de tumores pancreáticos en los machos y un
ligero incremento de los adenomas de hipófisis en las hembras. En
otros estudios realizados con sulfato de manganeso, no se observaron
signos de cáncer en ratas y en ratones se detectó un aumento marginal
de la incidencia de adenomas de las células foliculares de la glándula
tiroides. Los resultados de los estudios in vitro ponen de
manifiesto que por lo menos algunas formas químicas del manganeso
tienen potencial mutagénico. Sin embargo, debido a las discrepancias
entre los resultados de los estudios in vivo realizados en
mamíferos, no se puede llegar a una conclusión general acerca del
posible peligro genotóxico para el ser humano como consecuencia de la
exposición a compuestos de manganeso.
La administración de dosis elevadas de sales concentradas de
manganeso por vía oral mediante sonda puede provocar la muerte de los
animales, pero no se ha observado que la exposición oral a través de
los alimentos o del agua produzca una toxicidad significativa durante
una exposición aguda o breve. Igualmente, la administración parenteral
de sales de manganeso puede producir toxicidad en el desarrollo, pero
no se encontraron efectos tras las exposición por vía oral. Se ha
notificado que la exposición oral de duración intermedia del ser
humano al manganeso produjo neurotoxicidad en dos casos, pero los
datos son demasiado limitados para definir el umbral o juzgar si estos
efectos se debieron completamente a la exposición al manganeso.
Existen algunos datos acerca de los efectos neurológicos o de otro
tipo para la salud de las personas debidos a la exposición oral
crónica al manganeso, pero estos estudios están limitados por la
incertidumbre en relación con las vías de exposición y los niveles de
exposición total, así como por la existencia de otros factores de
confusión. Los estudios en el ser humano y en animales no proporcionan
información suficiente para determinar las dosis o los efectos que
despiertan preocupación tras la exposición crónica por vía oral. Así
pues, las pruebas disponibles para los efectos adversos asociados con
la ingesta crónica de un exceso de manganeso son indicativas, pero no
concluyentes.
La vía cutánea no parece revestir especial importancia y no se ha
investigado en absoluto. Los datos disponibles se limitan a informes
sobre los efectos corrosivos del permanganato potásico (KMnO4) y a
informes de casos de efectos debidos a la absorción cutánea de
compuestos orgánicos del manganeso, como el MMT.
Según estos datos, es evidente que la exposición al manganeso
puede producir efectos neurológicos y respiratorios adversos en el
ámbito ocupacional. También hay pruebas limitadas que indican que los
efectos neurológicos adversos pueden estar asociados con la ingesta de
un exceso de manganeso en las condiciones del medio ambiente. Como
consecuencia de los factores de predisposición, determinadas personas
podrían ser más susceptibles a los efectos adversos de la exposición a
un exceso de manganeso. Entre ellas podrían estar las afectadas por
enfermedades pulmonares, las expuestas a otras sustancias irritantes
de los pulmones, los recién nacidos, los ancianos, y las personas con
déficit de hierro o con enfermedades hepáticas.
Existen varios métodos para la obtención de un valor guía para el
manganeso en el aire. Recientemente se ha obtenido un valor guía de
0,15 µg de manganeso/m3, que se destaca aquí como un posible ejemplo;
también se han presentado algunos métodos adicionales.
 1. EXECUTIVE SUMMARY
This CICAD on manganese and its compounds was based principally
on the report entitled Toxicological profile for manganese (update),
draft for public comment, prepared by the Agency for Toxic
Substances and Disease Registry, US Department of Health and Human
Services (ATSDR, 1996). Information contained in the Hazardous
Substances Data Bank, developed and maintained by the National Library
of Medicine, US Department of Health and Human Services, was also used
(HSDB, 1998). Data identified as of November 1998 were considered in
these source documents. Additional data came from other references,
such as assessments prepared by the US Environmental Protection Agency
(EPA) and the World Health Organization (WHO), as well as a variety of
reports in the literature. The source documents used to develop this
CICAD do not cover the effects of manganese on the ecological
environment. No other sources (documents developed by a national
organization and subject to rigorous scientific review) on this topic
were identified. Therefore, this CICAD addresses environmental levels
as a source of human exposure only. No attempt has been made in this
document to assess effects on organisms in the environment.
Information on the availability of the source documents is presented
in Appendix 1. Information on the peer review of this CICAD is
presented in Appendix 2. This CICAD was approved as an international
assessment at a meeting of the Final Review Board, held in Berlin,
Germany, on 26-28 November 1997. Participants at the Final Review
Board meeting are presented in Appendix 3. The International Chemical
Safety Card (ICSC 0174) for manganese, produced by the International
Programme on Chemical Safety (IPCS, 1993), has also been reproduced in
this document.
Manganese (Mn) is a naturally occurring element that is found in
rock, soil, water, and food. Thus, all humans are exposed to
manganese, and it is a normal component of the human body. Food is
usually the most important route of exposure for humans. Estimated
Safe and Adequate Daily Intakes of 1-5 mg manganese have been
established for children 1 year of age and older through to adults;
these levels generally parallel amounts of the compound delivered via
the diet.
Manganese is released to air mainly as particulate matter, and
the fate and transport of the particles depend on their size and
density and on wind speed and direction. Some manganese compounds are
readily soluble in water, so significant exposures can also occur by
ingestion of contaminated drinking-water. Manganese in surface water
can oxidize or adsorb to sediment particles and settle to the bottom.
Manganese in soil can migrate as particulate matter to air or water,
or soluble manganese compounds can be leached from the soil.
Above-average exposures to manganese are most likely to occur in
people who work at or live near a factory or other site where
significant amounts of manganese dust are released into the air. In
some regions, the general population can be exposed to manganese
released into air by the combustion of unleaded gasoline containing
the organomanganese compound methylcyclopentadienyl manganese
tricarbonyl (MMT) as an antiknock ingredient. Some people can be
exposed to excess manganese in drinking-water -- for example, when
manganese from batteries or pesticides leaches into well-water.
Children can be exposed to excess manganese in soils through
hand-to-mouth behaviour.
In humans, manganese is an essential nutrient that plays a role
in bone mineralization, protein and energy metabolism, metabolic
regulation, cellular protection from damaging free radical species,
and the formation of glycosaminoglycans. However, exposure to high
levels via inhalation or ingestion can cause adverse health effects.
Given comparable doses, more manganese reaches the brain following
inhalation than following ingestion, and most health effects are
associated with chronic inhalation exposure. Little is known about the
relative toxicity of different manganese compounds. However, available
evidence indicates that various manganese compounds can induce
neurological effects; these effects have been observed following
chronic (365 days or more) inhalation exposures in humans and
intermediate (15-364 days) and chronic oral exposures in animals.
In general, the available data indicate that exposure to excess
manganese for 14 days or less (acute duration) or up to a year
(intermediate duration) has an effect on the respiratory system and
the nervous system, with little to no effect on other organ systems.
Acute inhalation exposure to high concentrations of manganese dusts
(specifically manganese dioxide [MnO2] and manganese tetroxide
[Mn3O4]) can cause an inflammatory response in the lung, which, over
time, can result in impaired lung function. Lung toxicity is
manifested as an increased susceptibility to infections such as
bronchitis and can result in manganic pneumonia. Pneumonia has also
been observed following acute inhalation exposures to particulates
containing other metals. Thus, this effect might be characteristic of
inhalable particulate matter and might not depend solely on the
manganese content of the particle.
There are a few reports suggesting that intermediate inhalation
exposure to manganese compounds produces effects on the central
nervous system, but reliable estimates of exposure levels are not
available. Inhalation studies in animals resulted in biochemical,
respiratory, and neurobehavioural effects. However, a threshold for
these effects has not been identified, because the exposure levels
associated with these effects range over an order of magnitude.
In chronic inhalation exposure to manganese, the main organ
systems affected are the lungs, nervous system, and reproductive
system, although effects on other organ systems have also been
observed. A recurring manganic pneumonia and acute respiratory effects
have been associated with chronic inhalation exposures to manganese.
Effects on the nervous system include neurological and
neuropsychiatric symptoms that can culminate in a Parkinsonism-like
disease known as manganism; evidence suggests that laboratory animals,
especially rodents, are not as sensitive as humans, and possibly other
primates, to the neurological effects of inhalation exposure to
manganese. Reproductive effects of chronic inhalation exposure to
manganese include decreased libido, impotence, and decreased fertility
in men; information is not available on reproductive effects in women.
Studies in animals indicate that manganese can cause direct damage to
the testes and late resorptions. Data from animal studies on the
effects of inhaled manganese on the immunological system and the
developing fetus are too limited to make firm conclusions on the
significance of these effects for humans.
Information on the carcinogenic potential of manganese is
limited, and the results are difficult to interpret with certainty. In
rats, chronic oral studies with manganese sulfate (MnSO4) showed a
small increase in the incidence of pancreatic tumours in males and a
small increase in pituitary adenomas in females. In other studies with
manganese sulfate, no evidence for cancer was noted in rats and a
marginally increased incidence of thyroid gland follicular cell
adenomas was observed in mice. The results of in vitro studies show
that at least some chemical forms of manganese have mutagenic
potential. However, as the results of in vivo studies in mammals are
inconsistent, no overall conclusion can be made about the possible
genotoxic hazard to humans from exposure to manganese compounds.
Large oral doses of concentrated manganese salts given by gavage
can cause death in animals, but oral exposures via food or water have
not been found to cause significant toxicity over acute or short-term
exposures. Similarly, parenteral administration of manganese salts can
cause developmental toxicity, but effects were not found with oral
exposure. Intermediate-duration oral exposure of humans to manganese
has been reported to cause neurotoxicity in two cases, but the data
are too limited to define the threshold or to judge if these effects
were due entirely to the manganese exposure. Some data on neurological
or other health effects in humans from chronic oral intake of
manganese exist, but these studies are limited by uncertainties in the
exposure routes and total exposure levels as well as by the existence
of other confounding factors. The studies in humans and animals do not
provide sufficient information to determine dose levels or effects of
concern following chronic oral exposure. Thus, the available evidence
for adverse effects associated with chronic ingestion of excess
manganese is suggestive but inconclusive.
The dermal route does not appear to be of significant concern and
has not been investigated to any extent. Available information is
limited to reports on the corrosive effects of potassium permanganate
(KMnO4) and case reports of effects from dermal absorption of organic
manganese compounds such as MMT.
From these data, it is clear that adverse neurological and
respiratory effects from manganese exposure can occur in occupational
settings. Limited evidence also suggests that adverse neurological
effects can be associated with ingestion of excess manganese in
environmental settings. As a result of predisposing factors, certain
individuals might be more susceptible to adverse effects from exposure
to excess manganese. These might include people with lung disease,
people who are exposed to other lung irritants, neonates, older
people, individuals with iron deficiency, or people with liver
disease.
There are several approaches to the development of a guidance
value for manganese in air. A recently developed guidance value of
0.15 µg manganese/m3 is highlighted here as one possible example;
some additional approaches are also presented.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
Table 1 lists common synonyms and other relevant information on
the chemical identity and properties of manganese and several of its
most important compounds. Manganese is a naturally occurring element
that is found in rock, soil, water, and food. Manganese can exist in a
number of oxidation states. Manganese and its compounds can exist as
solids in the soil and as solutes or small particles in water. Most
manganese salts are readily soluble in water, with only the phosphate
and the carbonate having low solubilities. The manganese oxides
(manganese dioxide and manganese tetroxide) are poorly soluble in
water. Manganese can also be present in small dust-like particles in
the air. Additional physical/chemical properties are presented in the
International Chemical Safety Card (ICSC 0174) reproduced in this
document.
Table 1: Chemical identity of manganese and its compounds.a
Manganese Manganous Manganese Manganese Manganese potassium
chloride sulfate (II, III) oxide dioxide permanganate
Synonyms Elemental Manganese Manganous Trimanganese Manganese Permanganic acid;
manganese chlorideb; sulfate; tetroxide; peroxide; potassium saltc;
colloidal manganese sulfuric acid mangano-manganic manganese chameleon material
manganese; dichloride manganese oxidec; binoxide;
cutavalb manganese manganese
tetroxide black;
battery
manganese
Chemical Mn MnCl2 MnSO4 Mn3O4 MnO2 KMnO4
formula
CAS
1. EXECUTIVE SUMMARY
This CICAD on manganese and its compounds was based principally
on the report entitled Toxicological profile for manganese (update),
draft for public comment, prepared by the Agency for Toxic
Substances and Disease Registry, US Department of Health and Human
Services (ATSDR, 1996). Information contained in the Hazardous
Substances Data Bank, developed and maintained by the National Library
of Medicine, US Department of Health and Human Services, was also used
(HSDB, 1998). Data identified as of November 1998 were considered in
these source documents. Additional data came from other references,
such as assessments prepared by the US Environmental Protection Agency
(EPA) and the World Health Organization (WHO), as well as a variety of
reports in the literature. The source documents used to develop this
CICAD do not cover the effects of manganese on the ecological
environment. No other sources (documents developed by a national
organization and subject to rigorous scientific review) on this topic
were identified. Therefore, this CICAD addresses environmental levels
as a source of human exposure only. No attempt has been made in this
document to assess effects on organisms in the environment.
Information on the availability of the source documents is presented
in Appendix 1. Information on the peer review of this CICAD is
presented in Appendix 2. This CICAD was approved as an international
assessment at a meeting of the Final Review Board, held in Berlin,
Germany, on 26-28 November 1997. Participants at the Final Review
Board meeting are presented in Appendix 3. The International Chemical
Safety Card (ICSC 0174) for manganese, produced by the International
Programme on Chemical Safety (IPCS, 1993), has also been reproduced in
this document.
Manganese (Mn) is a naturally occurring element that is found in
rock, soil, water, and food. Thus, all humans are exposed to
manganese, and it is a normal component of the human body. Food is
usually the most important route of exposure for humans. Estimated
Safe and Adequate Daily Intakes of 1-5 mg manganese have been
established for children 1 year of age and older through to adults;
these levels generally parallel amounts of the compound delivered via
the diet.
Manganese is released to air mainly as particulate matter, and
the fate and transport of the particles depend on their size and
density and on wind speed and direction. Some manganese compounds are
readily soluble in water, so significant exposures can also occur by
ingestion of contaminated drinking-water. Manganese in surface water
can oxidize or adsorb to sediment particles and settle to the bottom.
Manganese in soil can migrate as particulate matter to air or water,
or soluble manganese compounds can be leached from the soil.
Above-average exposures to manganese are most likely to occur in
people who work at or live near a factory or other site where
significant amounts of manganese dust are released into the air. In
some regions, the general population can be exposed to manganese
released into air by the combustion of unleaded gasoline containing
the organomanganese compound methylcyclopentadienyl manganese
tricarbonyl (MMT) as an antiknock ingredient. Some people can be
exposed to excess manganese in drinking-water -- for example, when
manganese from batteries or pesticides leaches into well-water.
Children can be exposed to excess manganese in soils through
hand-to-mouth behaviour.
In humans, manganese is an essential nutrient that plays a role
in bone mineralization, protein and energy metabolism, metabolic
regulation, cellular protection from damaging free radical species,
and the formation of glycosaminoglycans. However, exposure to high
levels via inhalation or ingestion can cause adverse health effects.
Given comparable doses, more manganese reaches the brain following
inhalation than following ingestion, and most health effects are
associated with chronic inhalation exposure. Little is known about the
relative toxicity of different manganese compounds. However, available
evidence indicates that various manganese compounds can induce
neurological effects; these effects have been observed following
chronic (365 days or more) inhalation exposures in humans and
intermediate (15-364 days) and chronic oral exposures in animals.
In general, the available data indicate that exposure to excess
manganese for 14 days or less (acute duration) or up to a year
(intermediate duration) has an effect on the respiratory system and
the nervous system, with little to no effect on other organ systems.
Acute inhalation exposure to high concentrations of manganese dusts
(specifically manganese dioxide [MnO2] and manganese tetroxide
[Mn3O4]) can cause an inflammatory response in the lung, which, over
time, can result in impaired lung function. Lung toxicity is
manifested as an increased susceptibility to infections such as
bronchitis and can result in manganic pneumonia. Pneumonia has also
been observed following acute inhalation exposures to particulates
containing other metals. Thus, this effect might be characteristic of
inhalable particulate matter and might not depend solely on the
manganese content of the particle.
There are a few reports suggesting that intermediate inhalation
exposure to manganese compounds produces effects on the central
nervous system, but reliable estimates of exposure levels are not
available. Inhalation studies in animals resulted in biochemical,
respiratory, and neurobehavioural effects. However, a threshold for
these effects has not been identified, because the exposure levels
associated with these effects range over an order of magnitude.
In chronic inhalation exposure to manganese, the main organ
systems affected are the lungs, nervous system, and reproductive
system, although effects on other organ systems have also been
observed. A recurring manganic pneumonia and acute respiratory effects
have been associated with chronic inhalation exposures to manganese.
Effects on the nervous system include neurological and
neuropsychiatric symptoms that can culminate in a Parkinsonism-like
disease known as manganism; evidence suggests that laboratory animals,
especially rodents, are not as sensitive as humans, and possibly other
primates, to the neurological effects of inhalation exposure to
manganese. Reproductive effects of chronic inhalation exposure to
manganese include decreased libido, impotence, and decreased fertility
in men; information is not available on reproductive effects in women.
Studies in animals indicate that manganese can cause direct damage to
the testes and late resorptions. Data from animal studies on the
effects of inhaled manganese on the immunological system and the
developing fetus are too limited to make firm conclusions on the
significance of these effects for humans.
Information on the carcinogenic potential of manganese is
limited, and the results are difficult to interpret with certainty. In
rats, chronic oral studies with manganese sulfate (MnSO4) showed a
small increase in the incidence of pancreatic tumours in males and a
small increase in pituitary adenomas in females. In other studies with
manganese sulfate, no evidence for cancer was noted in rats and a
marginally increased incidence of thyroid gland follicular cell
adenomas was observed in mice. The results of in vitro studies show
that at least some chemical forms of manganese have mutagenic
potential. However, as the results of in vivo studies in mammals are
inconsistent, no overall conclusion can be made about the possible
genotoxic hazard to humans from exposure to manganese compounds.
Large oral doses of concentrated manganese salts given by gavage
can cause death in animals, but oral exposures via food or water have
not been found to cause significant toxicity over acute or short-term
exposures. Similarly, parenteral administration of manganese salts can
cause developmental toxicity, but effects were not found with oral
exposure. Intermediate-duration oral exposure of humans to manganese
has been reported to cause neurotoxicity in two cases, but the data
are too limited to define the threshold or to judge if these effects
were due entirely to the manganese exposure. Some data on neurological
or other health effects in humans from chronic oral intake of
manganese exist, but these studies are limited by uncertainties in the
exposure routes and total exposure levels as well as by the existence
of other confounding factors. The studies in humans and animals do not
provide sufficient information to determine dose levels or effects of
concern following chronic oral exposure. Thus, the available evidence
for adverse effects associated with chronic ingestion of excess
manganese is suggestive but inconclusive.
The dermal route does not appear to be of significant concern and
has not been investigated to any extent. Available information is
limited to reports on the corrosive effects of potassium permanganate
(KMnO4) and case reports of effects from dermal absorption of organic
manganese compounds such as MMT.
From these data, it is clear that adverse neurological and
respiratory effects from manganese exposure can occur in occupational
settings. Limited evidence also suggests that adverse neurological
effects can be associated with ingestion of excess manganese in
environmental settings. As a result of predisposing factors, certain
individuals might be more susceptible to adverse effects from exposure
to excess manganese. These might include people with lung disease,
people who are exposed to other lung irritants, neonates, older
people, individuals with iron deficiency, or people with liver
disease.
There are several approaches to the development of a guidance
value for manganese in air. A recently developed guidance value of
0.15 µg manganese/m3 is highlighted here as one possible example;
some additional approaches are also presented.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
Table 1 lists common synonyms and other relevant information on
the chemical identity and properties of manganese and several of its
most important compounds. Manganese is a naturally occurring element
that is found in rock, soil, water, and food. Manganese can exist in a
number of oxidation states. Manganese and its compounds can exist as
solids in the soil and as solutes or small particles in water. Most
manganese salts are readily soluble in water, with only the phosphate
and the carbonate having low solubilities. The manganese oxides
(manganese dioxide and manganese tetroxide) are poorly soluble in
water. Manganese can also be present in small dust-like particles in
the air. Additional physical/chemical properties are presented in the
International Chemical Safety Card (ICSC 0174) reproduced in this
document.
Table 1: Chemical identity of manganese and its compounds.a
Manganese Manganous Manganese Manganese Manganese potassium
chloride sulfate (II, III) oxide dioxide permanganate
Synonyms Elemental Manganese Manganous Trimanganese Manganese Permanganic acid;
manganese chlorideb; sulfate; tetroxide; peroxide; potassium saltc;
colloidal manganese sulfuric acid mangano-manganic manganese chameleon material
manganese; dichloride manganese oxidec; binoxide;
cutavalb manganese manganese
tetroxide black;
battery
manganese
Chemical Mn MnCl2 MnSO4 Mn3O4 MnO2 KMnO4
formula
CAS 