
Concise International Chemical Assessment Document 17
BUTYL BENZYL PHTHALATE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Concise International Chemical Assessment Document 17
BUTYL BENZYL PHTHALATE
First draft prepared by Ms M.E. Meek, Environmental Health
Directorate, Health Canada
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 1999
The International Programme on Chemical Safety (IPCS),
established in 1980, is a joint venture of the United Nations
Environment Programme (UNEP), the International Labour Organisation
(ILO), and the World Health Organization (WHO). The overall objectives
of the IPCS are to establish the scientific basis for assessment of
the risk to human health and the environment from exposure to
chemicals, through international peer review processes, as a
prerequisite for the promotion of chemical safety, and to provide
technical assistance in strengthening national capacities for the
sound management of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization, the United Nations
Institute for Training and Research, and the Organisation for Economic
Co-operation and Development (Participating Organizations), following
recommendations made by the 1992 UN Conference on Environment and
Development to strengthen cooperation and increase coordination in the
field of chemical safety. The purpose of the IOMC is to promote
coordination of the policies and activities pursued by the
Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing-in-Publication Data
Butyl benzyl phthalate.
(Concise international chemical assessment document ; 17)
1.Phthalic acids 2.Environmental exposure 3.Risk assessment
I.International Programme on Chemical Safety II.Series
ISBN 92 4 153017 0 (NLM classification: QV 612)
ISSN 1020-6167
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1999
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city, or area
or of its authorities, or concerning the delimitation of its frontiers
or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
TABLE OF CONTENTS
FOREWORD
1. EXECUTIVE SUMMARY
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
3. ANALYTICAL METHODS
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1. Environmental levels
6.2. Human exposure
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposure
8.2. Irritation and sensitization
8.3. Short-term exposure
8.4. Long-term exposure
8.4.1. Subchronic exposure
8.4.2. Chronic exposure and carcinogenicity
8.5. Genotoxicity and related end-points
8.6. Reproductive and developmental toxicity
8.7. Peroxisomal proliferation
8.8. Immunological and neurological effects
9. EFFECTS ON HUMANS
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1. Aquatic environment
10.1.1. Pelagic organisms
10.1.2. Benthic organisms
10.2. Terrestrial environment
11. EFFECTS EVALUATION
11.1. Evaluation of health effects
11.1.1. Hazard identification and dose-response assessment
11.1.2. Criteria for setting guidance values for BBP
11.1.3. Sample risk characterization
11.2. Evaluation of environmental effects
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
13.1. Human health hazards
13.2. Advice to physicians
13.3. Spillage
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
INTERNATIONAL CHEMICAL SAFETY CARD
REFERENCES
APPENDIX 1 -- SOURCE DOCUMENTS
APPENDIX 2 -- CICAD PEER REVIEW
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
RÉSUMÉ D'ORIENTATION
RESUMEN DE ORIENTACION
FOREWORD
Concise International Chemical Assessment Documents (CICADs) are
the latest in a family of publications from the International
Programme on Chemical Safety (IPCS) -- a cooperative programme of the
World Health Organization (WHO), the International Labour Organisation
(ILO), and the United Nations Environment Programme (UNEP). CICADs
join the Environmental Health Criteria documents (EHCs) as
authoritative documents on the risk assessment of chemicals.
CICADs are concise documents that provide summaries of the
relevant scientific information concerning the potential effects of
chemicals upon human health and/or the environment. They are based on
selected national or regional evaluation documents or on existing
EHCs. Before acceptance for publication as CICADs by IPCS, these
documents undergo extensive peer review by internationally selected
experts to ensure their completeness, accuracy in the way in which the
original data are represented, and the validity of the conclusions
drawn.
The primary objective of CICADs is characterization of hazard and
dose-response from exposure to a chemical. CICADs are not a summary of
all available data on a particular chemical; rather, they include only
that information considered critical for characterization of the risk
posed by the chemical. The critical studies are, however, presented in
sufficient detail to support the conclusions drawn. For additional
information, the reader should consult the identified source documents
upon which the CICAD has been based.
Risks to human health and the environment will vary considerably
depending upon the type and extent of exposure. Responsible
authorities are strongly encouraged to characterize risk on the basis
of locally measured or predicted exposure scenarios. To assist the
reader, examples of exposure estimation and risk characterization are
provided in CICADs, whenever possible. These examples cannot be
considered as representing all possible exposure situations, but are
provided as guidance only. The reader is referred to EHC 1701 for
advice on the derivation of health-based guidance values.
While every effort is made to ensure that CICADs represent the
current status of knowledge, new information is being developed
constantly. Unless otherwise stated, CICADs are based on a search of
the scientific literature to the date shown in the executive summary.
In the event that a reader becomes aware of new information that would
change the conclusions drawn in a CICAD, the reader is requested to
contact IPCS to inform it of the new information.
1 International Programme on Chemical Safety (1994)
Assessing human health risks of chemicals: deriviation of
guidance values for health-based exposure limits: Geneva,
World Health Organization (Environmental Health Criteria 170).
Procedures
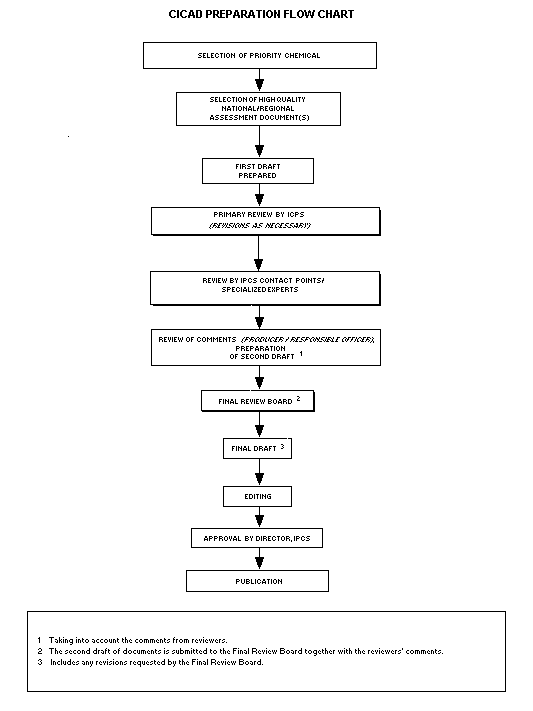
The flow chart shows the procedures followed to produce a CICAD.
These procedures are designed to take advantage of the expertise that
exists around the world -- expertise that is required to produce the
high-quality evaluations of toxicological, exposure, and other data
that are necessary for assessing risks to human health and/or the
environment.
The first draft is based on an existing national, regional, or
international review. Authors of the first draft are usually, but not
necessarily, from the institution that developed the original review.
A standard outline has been developed to encourage consistency in
form. The first draft undergoes primary review by IPCS to ensure that
it meets the specified criteria for CICADs.
The second stage involves international peer review by scientists
known for their particular expertise and by scientists selected from
an international roster compiled by IPCS through recommendations from
IPCS national Contact Points and from IPCS Participating Institutions.
Adequate time is allowed for the selected experts to undertake a
thorough review. Authors are required to take reviewers' comments into
account and revise their draft, if necessary. The resulting second
draft is submitted to a Final Review Board together with the
reviewers' comments.
The CICAD Final Review Board has several important functions:
- to ensure that each CICAD has been subjected to an appropriate
and thorough peer review;
- to verify that the peer reviewers' comments have been addressed
appropriately;
- to provide guidance to those responsible for the preparation of
CICADs on how to resolve any remaining issues if, in the opinion
of the Board, the author has not adequately addressed all
comments of the reviewers; and
- to approve CICADs as international assessments.
Board members serve in their personal capacity, not as representatives
of any organization, government, or industry. They are selected
because of their expertise in human and environmental toxicology or
because of their experience in the regulation of chemicals. Boards are
chosen according to the range of expertise required for a meeting and
the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
 1. EXECUTIVE SUMMARY
This CICAD on butyl benzyl phthalate was prepared jointly by the
Environmental Health Directorate of Health Canada and the Commercial
Chemicals Evaluation Division of Environment Canada based on
documentation prepared concurrently as part of the Priority Substances
Program under the Canadian Environmental Protection Act (CEPA). The
objective of assessments on Priority Substances under CEPA is
to assess potential effects of indirect exposure in the general
environment on human health as well as environmental effects. Data
identified as of the end of April 1998 were considered in these
reviews. Information on the nature of the peer review and availability
of the source document is presented in Appendix 1. Information on the
peer review of this CICAD is presented in Appendix 2. This CICAD was
approved as an international assessment at a meeting of the Final
Review Board, held in Tokyo, Japan, on 30 June - 2 July 1998.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 0834) for butyl benzyl
phthalate, produced by the International Programme on Chemical Safety
(IPCS, 1993), has also been reproduced in this document.
Butyl benzyl phthalate (CAS No. 85-68-7), or BBP, is a clear,
oily liquid that is used as a plasticizer mainly in polyvinyl chloride
(PVC) for vinyl floor tile, vinyl foams, and carpet backing and to a
minor extent also in cellulose plastics and polyurethane. Most
environmental release is to the air. Once in the environment, BBP
partitions to the atmosphere, soil, surface water, sediments, and
biota and has been detected in each of these compartments.
BBP is removed from the atmosphere by photooxidation and by
rainwater, with a half-life of a few hours to a few days. BBP is not
persistent in water, sediments, or soil under aerobic conditions, with
a half-life of a few days. Under anaerobic conditions, BBP is more
persistent, with a half-life of a few months. BBP is readily
metabolized by vertebrates and invertebrates. Reported
bioconcentration factors (BCFs) are less than 1000 based on total
residues and well under 100 based on intact BBP residues.
Available data in humans are inadequate to serve as a basis for
assessment of the effects of long-term exposure to BBP in human
populations.
The acute toxicity of BBP is relatively low, with oral LD50
values in rats being greater than 2 g/kg body weight. Target organs
following acute exposure include the haematological and central
nervous systems.
Available data are inadequate to assess the irritant and
sensitizing effects of BBP in animal species.
The repeated-dose toxicity of BBP has been well investigated in
recent studies, primarily in the rat, in which dose-response was well
characterized. Effects observed consistently have been decreases in
body weight gain (often accompanied by decreases in food consumption)
and increases in organ to body weight ratios, particularly for the
kidney and liver. Histopathological effects on the pancreas and kidney
and haematological effects have also been observed. At higher doses,
degenerative effects on the testes and, occasionally,
histopathological effects on the liver have been reported. In
specialized investigations, peroxisomal proliferation in the liver has
been observed, although potency in this regard was less than that for
other phthalates, such as bis(2-ethylhexyl) phthalate (DEHP).
The chronic toxicity and carcinogenicity of BBP have been
investigated in US National Toxicology Program (NTP) bioassays in rats
(including standard and feed-restricted protocols) and mice. It was
concluded that there was "some evidence" of carcinogenicity in male
rats, based on an increased incidence of pancreatic tumours, and
equivocal evidence in female rats, based on marginal increases in
pancreatic and bladder tumours. Dietary restriction prevented full
expression of the pancreatic tumours and delayed appearance of the
bladder tumours. There was no evidence of carcinogenicity in mice.
The weight of evidence of the genotoxicity of BBP is clearly
negative. However, available data are inadequate to conclude
unequivocally that BBP is not clastogenic, although in identified
studies it has induced, at most, weak activity of a magnitude
consistent with secondary effects on DNA.
Therefore, BBP has induced an increase in pancreatic tumours
primarily in one sex of one species, the full expression of which was
prevented in a dietary restriction protocol, and a marginal increase
in bladder tumours in the other sex, which was delayed upon dietary
restriction. The weight of evidence of genotoxicity is negative, and,
although weak clastogenic potential cannot be ruled out, available
data are consistent with the compound not interacting directly with
DNA. On this basis, BBP can be considered, at most, possibly
carcinogenic to humans, likely inducing tumours through a
non-genotoxic (although unknown) mechanism.
In a range of studies, including those designed to investigate
the reproductive effects of BBP on the testes and endocrine hormones
of male rats, a modified mating protocol conducted by the NTP, and a
one-generation study, adverse effects on the testes and, consequently,
fertility have generally been observed only at doses higher than those
that induce effects on other organs (such as the kidney and liver),
although decreases in sperm counts have been observed at doses similar
to those that induce effects in the kidney and liver. This is
consistent with the results of repeated-dose toxicity studies.
Reductions in testes weight and daily sperm production in
offspring were reported at a relatively low level in rats exposed in
utero and during lactation in a study in which dose-response was not
investigated. However, such effects were not observed in a recent
study of similar, but not identical, design in another strain of rats
in which only increases in absolute and relative liver weights were
observed at postnatal day 90. Additional investigation of potential
effects on the reproductive systems of male and female animals exposed
in utero and during lactation in studies designed to address
dose-response is desirable and is under way.
Although BBP has been estrogenic in human breast cell cancer
lines in vitro, results in yeast cells have been mixed. Neither BBP
nor its principal metabolites have been uterotrophic in vivo in rats
or mice. Although available data do not support the conclusion that
BBP is estrogenic, other potential endocrine-mediated effects such as
anti-androgenic activity associated with dibutyl phthalate (DBP) are
not precluded.
There is considerable emphasis currently on development of more
sensitive frameworks for testing and assessment of
endocrine-disrupting substances; compounds such as phthalates are
likely early candidates for additional testing.
In several well-conducted studies in rats and mice, BBP has
induced marked developmental effects, but only at dose levels that
induce significant maternal toxicity.
Although the potential neurotoxicity of BBP has not been well
investigated, histopathological effects on the central and peripheral
nervous systems have not been observed following short-term exposure
to relatively high dietary concentrations. Available data are
inadequate to assess the potential immunotoxicity of BBP.
A sample tolerable daily intake (TDI) of 1300 µg/kg body weight
per day has been derived for BBP. It is based upon the lower 95%
confidence limit for the benchmark dose associated with a 5% increase
in the incidence of pancreatic lesions in male rats in an oral
subchronic bioassay divided by an uncertainty factor of 100 (10 for
interspecies variation and 10 for intraspecies variation). Based upon
concentrations in various environmental media, it appears (from sample
estimates) that food contributes all of the estimated intake, which is
considered, for the general population, to range from 2 to 6 µg/kg
body weight per day. These estimates are 200-650 times less than the
TDI. Data were inadequate to estimate exposure in the occupational
environment or from consumer products.
A range of toxicity tests with aquatic organisms has indicated
that adverse effects occur at exposure concentrations equal to or
greater than 100 µg/litre. As concentrations in surface waters are
generally less than 1 µg/litre, it is likely that BBP poses low risk
to aquatic organisms.
No information about the effects of BBP on sediment-dwelling
organisms, soil invertebrates, terrestrial plants, or birds has been
identified on which to base an estimate of risk to these organisms.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
The physical and chemical properties of BBP have been summarized
by Skinner (1992). BBP (CAS No. 85-68-7) is an aromatic ester that
conforms to the formula C19H20O4. Synonyms include
1,2-benzenedicarboxylic acid, butyl phenylmethyl ester, and benzyl
n-butyl phthalate. BBP is a clear, oily liquid at room temperature
with a molecular weight of 312.4 g/mol. Reported log octanol/water
partition coefficients (log Kow) range from 3.6 to 5.8; 4.91 is a
measured value, whereas the 4.77 provided in the International
Chemical Safety Card is an estimated value. Additional physical/
chemical properties are presented in the International Chemical Safety
Card reproduced in this document.
3. ANALYTICAL METHODS
Analytical methods have been reviewed by Skinner (1992). BBP may
be analysed by gas chromatography/mass spectrometry and by
high-performance liquid chromatography. It is determined in water by
an enrichment procedure using sequential reverse osmosis, followed by
extraction and analysis by gas chromatography/mass spectrometry, or
after adsorption onto Tenax material and thermal desorption to a fused
silica capillary gas chromatography column under whole-column
cryotrapping conditions (Pankow et al., 1988). In air, BBP has been
determined by liquid chromatographic separation using a florisil
adsorbent and 10% 2-propanol in hexane as the eluent followed by gas
chromatographic analysis with detection by 63Ni electron capture
(Stein et al., 1987). BBP can be determined in mixtures of
semivolatile organic pollutants using gas chromatography/Fourier
transform infrared spectroscopy with wall-coated open tubular
capillary columns. Combining this technique with gas
chromatography/mass spectrometry allows for better and faster
identification of the components of complex mixtures of environmental
pollutants.
In reports of analyses of environmental media for BBP, detection
limits were 1 µg/litre for samples of drinking-water (G. Halina,
personal communication, 1994; method not specified) and 0.2 mg/kg dry
weight for soil (gas chromatography/mass spectrometry; Webber & Wang,
1995). Detection limits for analyses (by gas chromatography/flame
ionization detection) of table-ready foods were 0.5 µg/g (butter), 0.2
µg/g (vegetables and fruits), and 0.1 µg/g (meat and fish) (Page &
Lacroix, 1995). The detection limits varied with the fat content of
the food and food matrix interferences.
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
BBP is manufactured by the reaction of the monobutyl ester of
phthalic acid with benzyl chloride (Skinner, 1992). In the USA,
Monsanto Company is the sole manufacturer of BBP (Anon., 1996). BBP is
used mainly as a plasticizer in PVC for vinyl floor tile, vinyl foams,
and carpet backing. Other polymers plasticized with BBP include
cellulose plastics, polyvinyl acetate, polysulfides, and polyurethane.
Consumption of BBP in Europe is approximately 18 000-45 000 tonnes per
year (Harris et al., 1997).
BBP is released from facilities that manufacture the substance or
blend it with PVC (Howard, 1990). Releases may also occur through
diffusion of BBP from PVC products.
Total on-site environmental releases of BBP reported to the
Canadian National Pollutant Release Inventory by 11 facilities using
BBP amounted to 3.7 tonnes in 1994, all to the atmosphere. Total
transfers of BBP for off-site disposal were much higher, amounting to
33.3 tonnes in 1994, with 25.1 tonnes going to incinerators and the
remainder, 8.2 tonnes, to landfill. A reported total of 3.7 tonnes of
BBP was sent for recovery in 1994, 2.3 tonnes for energy recovery and
1.4 tonnes for recovery, reuse, or recycling (NPRI, 1996).
In the USA, it was estimated that manufacturing facilities
released approximately 176 tonnes to the environment in 1993, with
about 99% released to the atmosphere (TRI93, 1995).
BBP may be released to air through automobile emissions and from
combustion of refuse (Graedel et al., 1986). It has also been detected
in stack emissions from hazardous waste combustion facilities and from
coal-fired power plants in the USA (Oppelt, 1987). Reasonable
worst-case emissions of BBP from incinerators, boilers, and industrial
furnaces burning such wastes were predicted to be 3 µg/m3 waste gas
(Dempsey & Oppelt, 1993). In a study of four US coal-fired utility
boiler plants, the emission rates for BBP in flue gases ranged from
210 to 3400 mg/h (Haile et al., 1984). BBP was identified, but not
quantified, in extracts of municipal incinerator fly ash from the
Netherlands, but it was not detected in extracts from Japan or Ontario
(Eiceman et al., 1979).
In leachate from municipal landfills in the USA, BBP was
detected, but not quantified (Brown & Donnelly, 1988). BBP has also
been detected (detection limits not reported) in groundwater at
disposal sites in the USA (Plumb, 1991). BBP was also detected in 2 of
44 groundwater samples at a Superfund site in Michigan, USA, at
estimated concentrations of 0.6 and 1.0 µg/litre (US EPA, 1996).
In Canada, BBP has been detected in storm sewer effluents at
concentrations up to 50 µg/litre (Hargesheimer & Lewis, 1987) and in
effluents from municipal sewage treatment plants and industrial plants
at concentrations up to 25 µg/litre (Munro et al., 1985; SIGMA, 1985;
OMOE, 1988, 1990, 1991).1 BBP has also been detected in sludges from
Canadian sewage treatment plants at concentrations up to 914 498 ng/g
dry weight (OMOE, 1988).
BBP can be emitted from products containing the substance. For
example, BBP has been detected in emissions from carpets (Bayer &
Papanicolopoulos, 1990), PVC floorings (Bremer et al., 1993), and
vinyl wall coverings (Etkin, 1995), although quantitative data were
not identified. It is also a component of some consumer products, such
as nail polish (Martin, 1996). The possibility that toys made of
plastic might contain BBP is currently being investigated, although
quantitative data are not yet available.
1 Additional data from ENVIRODAT, Surveys and Information Systems
Branch, Environment Canada, 1993.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Fugacity modelling was based upon the assumption of continuous
emissions of 1000 kg/h to air, water, or soil (DMER & AEL, 1996). Most
environmental releases of BBP are to the atmosphere. The Level III
calculations of the EQC fugacity model predict that when BBP is
emitted into air, approximately 72% is found in soil, 22% in air, 4%
in water, and 2% in sediment. When BBP is emitted into water, 65% is
found in water, while about 35% partitions to sediment and a very
small fraction to soil. When BBP is released to soil, more than 99% is
found in the soil. Values for input parameters were as follows:
molecular weight, 312.4 g/mol; vapour pressure, 0.001 15 Pa; water
solubility, 2.69 mg/litre; melting point, -35°C; and log Kow, 4.9.
Average degrading reaction half-lives were assumed to be 55 h in air,
170 h in water, 550 h in soil, and 1700 h in sediment (Mackay et al.,
1995). The calculated organic carbon/water partition coefficient (log
Koc) is 4.51 (based on the correlation Koc = 0.41 Kow), and
the Henry's law constant is 0.133 Pa.m3/mol at 25°C.
Photooxidation is the most important process for the breakdown of
BBP in the atmosphere (Atkinson, 1987). Howard et al. (1991) estimated
a half-life in air of 6-60 h for BBP based on photooxidation rates.
BBP is also readily removed from air by rain (Ligocki et al., 1985a).
BBP is readily biodegraded in aerobic surface water, with a
half-life of about 1-7 days (Saeger & Tucker, 1976; Gledhill et al.,
1980; Howard et al., 1991; Adams & Saeger, 1993). Biodegradation is
considerably slower in cold water, as BBP was almost completely
biodegraded after 7 days in Rhine River water at 20°C but was not
biodegraded in the same water after 10 days at 4°C (Ritsema et al.,
1989). BBP is expected to adsorb to suspended matter, sediments, and
biota.
Biodegradation is the most important degradation pathway in
sediments (Gledhill et al., 1980; Adams & Saeger, 1993). In a river
water/sediment microcosm, the degradation pathway appeared to be
BBP -> monobutyl/monobenzyl phthalate -> phthalic acid ->
4,5-dihydroxyphthalic acid -> oxalic acid -> formic acid -> carbon
dioxide (Adams et al., 1986, 1989; Adams & Saeger, 1993). The
half-life for complete mineralization of BBP in this study was 13 days
(Adams & Saeger, 1993). BBP can also be biodegraded in sediment under
anaerobic conditions (Shelton & Tiedje, 1984; Painter & Jones, 1990;
Ejlertsson et al., 1996), with an estimated half-life of about 1 day
to 6 months (Howard et al., 1991).
Biodegradation of BBP occurs readily in aerobic soils, with a
half-life of about 1-7 days at room temperature (Howard et al., 1991).
It is also biodegraded in anaerobic soils. For the removal of BBP in a
silt loam, Kincannon & Lin (1985) determined a half-life of 59.2 days.
BBP sorbs to soil, so soil leaching should not be significant (Zurmhhl
et al., 1991).
With reported log Kow values ranging from 3.6 to 5.8, BBP
would appear to have a high potential for bioaccumulation. However,
reported BCFs in oysters, microorganisms, and several species of fish
are less than 1000, because BBP is readily metabolized, with a
depuration half-life of less than 2 days (Barrows et al., 1980; Veith
et al., 1980). The highest reported BCF was 776 for bluegill
( Lepomis macrochirus) (Veith et al., 1980).
Based on physical/chemical properties of BBP, Wild & Jones (1992)
predicted that retention of the substance by root surfaces of plants
would be high, but that subsequent uptake by plants would be low. This
prediction was confirmed by Müller and Kördel (1993), who demonstrated
that plants grown on phthalate-enriched soil did not take up BBP from
the soil through the roots. However, plants exposed to
phthalate-treated dust did take up BBP through leaf cuticles
(quantitative data not available).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
In air samples from Greater Vancouver, British Columbia, Canada,
BBP was detected at concentrations ranging from 0.38 to 1.78 ng/m3
(W. Belzer, personal communication, 1997). Concentrations of BBP in
air up to 9.6 ng/m3 (in the aerosol phase at Portland, Oregon, USA)
have been reported (Ligocki et al., 1985a,b). BBP has been identified
in ambient air in Barcelona, Spain; concentrations of 1.0 and 8.0
ng/m3 in winter and 0.25 and 2.0 ng/m3 in summer, associated with
coarse (>7.2 µm) and fine (<0.5 µm) aerosol fractions, respectively,
have been reported (Aceves & Grimalt, 1993).
In Canadian surface waters, BBP was detected at concentrations up
to 1 µg/litre.1 Gledhill et al. (1980) reported a concentration of
2.4 µg/litre in the Mississippi River south of St. Louis, Missouri,
USA. In central Italy, BBP was detected at concentrations up to
6.6 µg/litre in Lake Scandarello (Vitali et al., 1997). In water
samples collected from the Rhine River and its tributaries, levels
ranged up to 5.2 µg/litre (ECPI, 1996). In samples of inflow and
outflow from sewage treatment plants in Sweden and Norway,
concentrations of BBP up to 2.4 µg/litre and 0.58 µg/litre,
respectively, were reported (ECPI, 1996; NIWR, 1996).
BBP has been reported in marine sediments from British Columbia,
Canada, at concentrations up to 370 ng/g dry weight (Axys Analytical
Services Limited, 1992; D. Goyette, personal communication, 1993).
Outside Canada, the highest reported concentration of BBP in sediments
was 3800 ng/g dry weight, in sediments from the Lower Passaic River,
Newark, New Jersey, USA, adjacent to combined sewer overflow outfalls
(Iannuzzi et al., 1997).
In limited surveys of soils from agricultural and typical urban
residential and parkland locations in Canada, concentrations of BBP
were less than 0.3 µg/g (Golder Associates, 1987; Webber & Wang,
1995).
At a lime disposal area of a refinery in Regina, BBP
concentrations in soil of 0.15 and 0.55 µg/g were reported.2 In soil
in the neighbourhood of three phthalate-emitting plants in Germany
from 1986 to 1989, the highest concentration in an individual sample
was 100 µg/kg; the highest mean value for a single site was 30 µg/kg
(Müller & Kördel, 1993).
1 Data from ENVIRODAT, Surveys and Information Systems Branch,
Environment Canada, 1993.
2 Letter from D. Fast, Saskatchewan Department of Environment and
Public Safety, to Senes Consultants Limited, Richmond Hill, Ontario,
1989.
In Canadian biota, BBP has been detected at concentrations up to
1470 ng/g wet weight (in butter sole, Isopsetta [ Pleuronectes]
isolepis, from Boundary Bay, British Columbia; Swain & Walton,
1990). BBP was detected in US biota at 3% of 182 STORET stations, with
a median concentration of <2500 ng/g (Staples et al., 1985).
6.2 Human exposure
In 125 homes in California, USA, two 12-h indoor air samples were
collected during daytime and overnight periods. In indoor air, median
daytime and nighttime concentrations were 34 and 35 ng/m3,
respectively. In a subset of 65 homes, outdoor air samples were also
collected. In outdoor air, the median (for both daytime and nighttime
sampling) was below the method quantifiable limit of 5.1 ng/m3; the
90th percentiles were 5.3 and 6.7 ng/m3 for daytime and nighttime
sampling, respectively (California Environmental Protection Agency,
1992). In an early study, BBP concentrations of 1 and 20 ng/m3 were
reported in office air at two locations in the USA, although the
compound was not detected (detection limit not reported) in ambient
air (Weschler, 1984).
In surveys of drinking-water primarily from surface water
supplies conducted at over 300 sites in two provinces in Canada
between 1985 and 1994, BBP was detected in only one sample in 1991
(2.8 µg/litre; limits of detection 1-3 µg/litre) (D. Spink, personal
communication, 1986; G. Halina, personal communication, 1994; A.
Riopel, unpublished data, 1994, 1996).
Of approximately 100 foodstuffs (generally single composite
samples from four supermarkets) purchased in Ontario, Canada, in 1985
and 1988 in a total diet study, BBP was detected only in yoghurt
(0.6 µg/g), cheddar cheese (1.6 µg/g), butter (0.64 µg/g), and
crackers (0.48 µg/g) (detection limits ranged from 0.005 to 0.5 µg/g;
Page & Lacroix, 1995).
In foods purchased at retail stores in the United Kingdom and
stored in their packaging until their "best before" date, BBP was not
detected in chocolate or sugar confectioneries, although it was
detected in baked savouries (1.5 mg/kg), meat pies (4.8 mg/kg), and
sandwiches (14 mg/kg) (MAFF, 1987). In stored samples of composite
fatty foods in a total diet study in the United Kingdom, BBP was
detected in carcass meat (0.09 mg/kg), poultry (0.03 mg/kg), eggs
(0.09 mg/kg), and milk (0.002 mg/kg) (MAFF, 1996a). Concentrations in
59 individual samples of 15 different brands of infant formula from
retail outlets in five towns across the United Kingdom ranged from
<0.004 to 0.25 mg/kg (MAFF, 1996b).
An example of indirect exposure in the general environment is
presented here. Exposure of the general population to BBP in
environmental media may be estimated based upon concentrations
determined in various media and reference values for body weight and
consumption patterns. Owing to the availability of relevant data,
exposure has been estimated based primarily upon data from Canada.
However, countries are encouraged to estimate total exposure on the
basis of national data, possibly in a manner similar to that outlined
here. Indeed, estimates based on the data on concentrations in
foodstuffs determined in the United Kingdom presented above would be
higher than those provided as examples here.
Although concentrations of BBP in air (both ambient and indoor),
drinking-water, and soil have been reported, they are so low that
intakes from these routes are essentially negligible. Estimates of
exposure for the general population are based almost entirely upon the
estimates for intake from food. The estimates presented here are based
upon identified concentrations for foodstuffs in Canada, as well as
assumed concentrations of zero or method detection limits for foods in
which BBP was not identified (minimum and maximum estimates,
respectively). Adults are assumed to breathe 15.8 m3 of air per day
(Allan, 1995), weigh 70 kg, drink 1.4 litres of water per day, ingest
20 mg soil per day, and consume, on a daily basis, 13.61 g butter,
3.81 g processed cheddar cheese, 1.54 g yoghurt, 22.73 g fresh pork,
and 3.45 g crackers (Health Canada, 1994). Estimated intake for adults
is 2 µg/kg body weight per day; intake values for infants and children
are up to threefold higher. Data are inadequate to estimate intake in
breast-fed infants.
Identified data on concentrations of BBP in the occupational
environment are inadequate as a basis for estimation of exposure.
Similarly, data are inadequate for estimation of exposure from
consumer products, although it should be noted that inclusion of
information on levels in indoor air in the estimates of exposure for
the general population presented here should account at least
partially for exposure from consumer products.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND
HUMANS
No data were identified concerning the absorption, metabolism, or
elimination of BBP in humans.
Based upon a limited number of studies (Erickson, 1965; Kluwe,
1984; Eigenberg et al., 1986; Mikuriya et al., 1988; Elsisi et al.,
1989) conducted principally in rats following oral administration, BBP
is readily hydrolysed in the gastrointestinal tract and the liver to
the corresponding monobutyl or benzyl ester. These phthalate
monoesters are then rapidly eliminated (90% in 24 h) in the excreta in
ratios of approximately 80% in urine and 20% in faeces, although
results of one study indicate that the fraction eliminated in faeces
increases at higher doses (of approximately 2 g/kg body weight)
(Eigenberg et al., 1986). The monobutyl ester is generally present in
highest amounts; for example, the ratio of monobutyl to monobenzyl
phthalate in rats in one study was 5:3 (Mikuriya et al., 1988).
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
The acute toxicity of BBP is relatively low. Reported oral LD50
values for rats range from 2 to 20 g/kg body weight (NTP, 1982;
Hammond et al., 1987); a dermal LD50 of 6.7 g/kg in both rats and
mice has been reported (Statsek, 1974). Clinical signs at or near
lethal doses following oral exposure of rats included weight loss,
apathy, and leukocytosis. Histological examination revealed toxic
splenitis and degenerative lesions of the central nervous system with
congestive encephalopathy, myelin degeneration, and glial
proliferation.
8.2 Irritation and sensitization
No tests have been conducted according to validated international
protocols.
Exposure to BBP did not cause immediate or delayed
hypersensitivity in mice in a series of studies following
administration of an initiating dose either intraperitoneally or by
application to the abdomen or footpad and a challenging dose to the
dorsal side of the ear up to 15 days later. Similarly, there was no
immediate or delayed hypersensitivity in guinea-pigs initiated on the
footpad and receiving a challenge dose on shaved abdominal skin. BBP
did not form a detectable amount of hapten-protein complex when
assayed with bovine serum albumin, although results were equivocal
following intradermal initiation and challenge (24 h later) of mice
with serum from BBP-exposed (intraperitoneally) mice (Little & Little,
1983).
Data reported in accounts of early studies on the irritancy of
BBP, the results of which were inconsistent, are inadequate for
evaluation (Dueva & Aldyreva, 1969; Hammond et al., 1987). Calley et
al. (1966) injected BBP intradermally into the backs of rabbits,
followed by trypan blue into the ear vein. Moderate irritation was
indicated by extravasated trypan blue at the site of administration.
8.3 Short-term exposure
In short-term investigations by the oral route (excluding those
addressing specifically reproductive effects or peroxisomal
proliferation, which are addressed elsewhere) for which the range of
end-points examined was often limited, consistent effects on body
weight gain in rats were observed at doses of approximately 1000 mg/kg
body weight per day and above, sometimes accompanied by a decrease in
food consumption (NTP, 1982; Hammond et al., 1987). Although some
effects on body weight gain were observed at lower doses, the pattern
was inconsistent. In one study, minimal testicular changes were
observed in one of six male rats at 480 mg/kg body weight per day
(Bibra, 1978; Hammond et al., 19871). In general, though, testicular
effects were observed only at much higher doses (i.e., atrophy at 1600
mg/kg body weight per day; Hammond et al., 1987). In a 6-week
investigation, there were no adverse histopathological effects on the
nervous system of rats exposed to 3000 mg/kg body weight per day,
although reversible clinical signs were observed (Robinson, 1991).
In an inhalation study in rats, there were no effects upon
haematology, urinalysis, blood chemistry, or histopathology following
exposure for 4 weeks to 144 mg/m3 (Hammond et al., 1987). At 526
mg/m3, effects included reduced body weight gain and reduced serum
glucose. In a similar study, exposure to 2100 mg/m3 resulted in death
of some animals; effects after 4 weeks included reduced body weight
gain and atrophy of the spleen and testes (Hammond et al., 1987).
8.4 Long-term exposure
Detailed descriptions of the protocol and effect levels are
presented here for critical studies only. Experimental details and
effect levels for all key subchronic and chronic studies via ingestion
are provided in Table 1.
8.4.1 Subchronic exposure
The protocol of an early study in Charles River rats included
examination of body weight, clinical signs, organ weight, and limited
histopathology of liver, spleen, kidney, adrenal, stomach, and small
and large intestine (Hazleton Laboratories, 1958). The only effect
observed was a decrease in body weight gain in males at the highest
dose (1253 mg/kg body weight per day).
In a range-finding NTP subchronic (13-week) dietary bioassay in
F344 rats, the only adverse effects observed upon examination of body
weight and clinical signs and histopathological examination of control
and high-dose animals were depressed weight gain and testicular
degeneration (nature of degeneration not specified) at the highest
dose (1250 mg/kg body weight per day) (NTP, 1982).
Sprague-Dawley rats were administered BBP in the diet for 3
months at dose levels of 0, 188, 375, 750, 1125, or 1500 mg/kg body
weight per day in males and females (Hammond et al., 1987). End-points
examined included body weight gain, haematology, urinalysis, and
histopathology (control and high-dose groups only). No
compound-related lesions were observed at necropsy or upon
histopathological examination. In females, the increase in liver to
body weight ratio was significant at 750 mg/kg body weight per day and
higher; in males, the increase was significant at 1125 mg/kg body
1 Full study reports for several of the investigations described
therein were available to the authors.
weight per day and higher. No change occurred in kidney to body weight
ratio in females, but there was a significant increase in males at 750
mg/kg body weight per day and higher.
A subchronic dietary study was also conducted in Wistar rats
(Monsanto Company, 1980a; Hammond et al., 1987) at dose levels of 0,
151, 381, or 960 mg/kg body weight per day in males and 0, 171, 422,
or 1069 mg/kg body weight per day in females for 3 months. Intake of
BBP, based on body weight and food consumption, was calculated at
4-day intervals throughout the study. Observations included slight
anaemia in males at the highest dose and decreased urinary pH in males
at the mid and high doses. At the highest dose, no reduction in food
consumption was apparent, suggesting that the reduced body weight gain
in those groups may have been compound related. Liver to body weight
ratio was significantly increased at all dose levels in females and at
the highest dose in males. A significant increase in kidney to body
weight ratio occurred in a dose-related manner in both sexes at the
mid and high doses. The caecum to body weight ratio was unaffected in
males but increased at all dose levels in females in a dose-related
manner. Gross pathological lesions were limited to increased incidence
of red spots on the liver of mid- and high-dose males.
Histopathological lesions of the pancreas were observed in males at
the mid and high doses and included islet enlargement with cell
vacuolization and peri-islet congestion. The liver of high-dose males
had small areas of cellular necrosis. No histopathological lesions
were described for females. The lowest-observed-adverse-effect level
(LOAEL) is 381 mg/kg body weight per day, based upon histopathological
effects in the pancreas in males. The lowest-observed-effect level
(LOEL) in females is 171 mg/kg body weight per day, based upon
increases in organ to body weight ratio at all doses for the liver and
caecum (the no-observed-effect level, or NOEL, in males is 151 mg/kg
body weight per day).
In a 6-month dietary study in male F344 rats (NTP, 1997a),
effects on haematological parameters were reported at 550 mg/kg body
weight per day. Only transitory changes in haematological parameters
were reported at 180 mg/kg body weight per day.
In a 3-month dietary study in dogs (Hammond et al., 1987),
decreases in body weight gain were associated with decreases in food
consumption at the highest dose (1852 and 1973 mg/kg body weight per
day for males and females, respectively).
In a 90-day study in mice, there were decreases in body weight
gain at 208 mg/kg body weight per day and greater in males, although
no histopathological effects were observed and food consumption was
not reported (NTP, 1982). End-points included clinical observations,
body weight, and histopathology (control and high dose).
One subchronic inhalation bioassay was identified, in which
groups of 25 male or 25 female Sprague-Dawley rats were exposed to
concentrations of 0, 51, 218, or 789 mg/m3 for 6 h/day, 5 days/week,
for a total of 59 exposures. End-points examined were limited to organ
weight changes and histopathological examination of control and
high-dose groups (Monsanto Company, 1982a; Hammond et al., 1987). A
LOEL of 218 mg/m3 was reported for male rats, based upon increases in
kidney weight, measured at interim sacrifice only, although no
dose-related histopathological changes were observed in any group. The
NOEL was 51 mg/m3.
8.4.2 Chronic exposure and carcinogenicity
A carcinogenicity bioassay was conducted by the NTP (1982) in
F344 rats. Fifty rats per sex per group were administered BBP via the
diet, at levels of 0, 6000, or 12 000 ppm (0, 300, and 600 mg/kg body
weight per day,1 respectively). Females were exposed for 103 weeks.
Because of poor survival, all males were sacrificed at weeks 29-30;
this part of the study was later repeated (NTP, 1997a).
Only females were examined histopathologically. The incidence of
mononuclear cell leukaemias was increased in the high-dose group
( P = 0.011); the trend was significant ( P = 0.006). (Incidences
for the control, low-dose, and high-dose groups were 7/49, 7/49, and
18/50, respectively.) The incidence in the high-dose group and the
overall trend remained significant ( P = 0.008 and P = 0.019,
respectively) when compared with historical control data. The NTP
concluded that BBP was "probably carcinogenic for female F344/N rats,
causing an increased incidence of mononuclear cell leukemias" (NTP,
1982).
However, these results were not repeated in the 2-year dietary
study in F344/N rats recently completed by the NTP (1997a). The
average daily doses (reported by the authors) were 0, 120, 240, or 500
mg/kg body weight per day for males and 0, 300, 600, or 1200 mg/kg
body weight per day for females. The protocol included periodic
haematological evaluation and hormonal assays and a 15-month interim
sacrifice.
There were no differences in survival between exposed groups and
their controls. A mild decrease in triiodothyronine concentration in
the high-dose females at 6 and 15 months and at termination was
considered to be related to a non-thyroidal disorder. Changes in
haematological parameters were sporadic and minor. In this bioassay,
there was no increase in the incidence of mononuclear cell leukaemia
in female rats, as was reported in the earlier bioassay (NTP, 1982),
although the level of exposure (600 mg/kg body weight per day) at
which the incidence was observed in the early bioassay was common to
both studies.
1 Conversion factor; 1 ppm in food = 0.05 mg/kg body weight per day
(Health Canada, 1994).
Table 1: Effect levels in subchronic, chronic, reproductive, and developmental studies by the ingestion route.
Study protocol Effect levela Critical end-point/comments Reference
Subchronic exposure
Charles River rats LOAEL (males) = 1253 mg/kg Limited range of end-points, Hazleton
(10/sex/group) body weight per day including body weight, food Laboratories, 1958
90 days NOEL (females) = 1270 mg/kg consumption, organ weight, and
median intake body weight per day histopathological examination of
in diet: (highest doses administered) only seven organs/tissues,
males: 0, 447, or excluding the testes.
1253 mg/kg body weight
females: 0, 462, or
1270 mg/kg body weight
F344/N rats LOAEL (males) = 1250 mg/kg body Histopathological degeneration of NTP, 1982
(10/sex/group) weight per day the testes and depressed weight
13 weeks NOEL = 625 mg/kg body weight gain. End-points examined were
approximate intake in per day restricted to clinical
diet: 0, 80, 155, 315, observations, body weight gain,
625, or 1250 mg/kg body and histopathological observation
weight per day of control and high-dose animals.
Sprague-Dawley rats LOEL (females) = 750 mg/kg Significant increases in liver to Hammond et al., 1987
(10/sex/group) body weight per day body weight ratio (females) and
3 months NOEL = 375 mg/kg body kidney to body weight ratio
approximate intake in weight per day (males). No histopathological
diet: 0, 188, 375, 750, changes.
1125, or 1500 mg/kg
body weight per day
Wistar rats LOAEL (males) (pancreas) = 381 Histopathological changes in Monsanto Company,
(27-45/sex/group) mg/kg body weight per day the pancreas in males from 1980a;
(15-27 exposed for LOEL (females) = 171 mg/kg body the two highest dose groups. Hammond et al.,
entire period) weight per day (lowest dose Increases in organ to body 1987
3 months administered) weight ratio at all doses
Table 1 (continued)
Study protocol Effect levela Critical end-point/comments Reference
approximate intake for the liver (females) and
in diet: caecum (females). No
males: 0, 151, 381, histopathological effects in either
or 960 mg/kg body of these organs at higher doses in
weight per day the same sex.
females: 0, 171, 422,
or 1069 mg/kg body
weight per day
Male F344 rats LOEL = 550 mg/kg body weight Effects on haematological parameters NTP, 1997a
(15/group) 26 weeks per day (significant increase in mean cell
approximate intake NOAEL = 180 mg/kg body weight haemoglobin and mean cell
in diet for four per day haemoglobin concentration) and
lowest doses: 0, 30, increased relative liver weight.
60, 180, or 550 mg/kg Transitory changes only in
body weight per day haematological parameters at the
lower dose (NOAEL).
Beagle dogs (3 males NOAEL (males) = 1852 mg/kg Decreases in body weight gain at Hammond et al., 1987
and 3 females) body weight per day the highest doses associated with
3 months NOAEL (females) = 1973 mg/kg decreases in food consumption.
approximate intake in body weight per day (Body weight increased but remained
diet or by capsule (highest doses administered) depressed in relation to controls
(high-dose group): during 2 months of administration
males: 0, 400, 1000, by capsule.)
or 1852 mg/kg body
weight per day
females: 0, 700, 1270,
or 1973 mg/kg body
weight per day
Table 1 (continued)
Study protocol Effect levela Critical end-point/comments Reference
B6C3F1 mice LOEL (males) = 208 mg/kg Decreases in body weight (of NTP, 1982
(10/sex/group) body weight per day unspecified statistical
13 weeks LOEL (females) = 1625 mg/kg significance), but no
approximate intake body weight per day histopathological effects;
in diet: 0, 208, 403, food consumption was not
819, 1625, or 3250 reported. End-points examined
mg/kg body weight restricted to body weight gain,
per day clinical observations, and
histopathology in control
and high-dose groups.
Chronic exposure
F344/N rats LOEL (females) = 300 mg/kg Increased nephropathy (the latter NTP, 1997a
(60/sex/group) body weight per day observed at all dose levels
2 years LOEL (males) = 120 mg/kg in females). Relative kidney weight
approximate intake in body weight per day increased at all doses in males at
diet: interim sacrifice (not determined
males: 0, 120, 240, at terminal sacrifice). At high
or 500 mg/kg body dose, increase in severity of renal
weight per day tubular pigmentation in both sexes.
females: 0, 300, 600,
or 1200 mg/kg body
weight per day
B6C3F1 mice LOEL = 780 mg/kg Decrease in body weight gain NTP, 1982
(50/sex/group) body weight per day (unspecified statistical
103 weeks significance). End-points
approximate intake examined restricted to clinical
in diet: 0, 780, or signs, body weight, and
1560 mg/kg body weight histopathology.
per day
Table 1 (continued)
Study protocol Effect levela Critical end-point/comments Reference
Reproductive/developmental studies
RIVM-bred WU rats LOAEL = 1000 mg/kg body weight At top dose, reduction in body weight Piersma et al.,
(10/sex/group) per day gain, fluctuation in food consumption, 1995
14 days prior to and NOAEL = 500 mg/kg body weight reduction in weight of testis and
throughout mating per day epididymis, and increase in testicular
(OECD 421 - combined degeneration (males). At top dose,
reproductive/developmental decrease in body weight gain and
toxicity screening protocol) effects on food consumption; adverse
gavage in corn oil: 0, 250, effects on reproductive indices
500, or 1000 mg/kg body (females). The only effect observed at
weight per day 500 mg/kg body weight per day was a
transient decrease (day 1) in
pup weight.
Male F344 rats LOAEL = 312.5 mg/kg body weight Dose-related increases in relative Kluwe et al., 1984;
(10/group) per day weights of kidney and liver at all Agarwal et al., 1985
14 days doses; increase in absolute kidney
approximate intake in diet: weight at two lowest doses, and
0, 312.5, 625, 1250, or decrease in absolute kidney weight
2500 mg/kg body weight at two highest doses. Proximal
per day tubular regeneration and
histopathological changes in the
thymus were also observed at all
dose levels; however, latter was
minimal and not considered dose
related. Histopathological effects
on the liver, testes, epididymis,
seminal vesicles, and prostate were
observed only at higher concentrations.
Table 1 (continued)
Study protocol Effect levela Critical end-point/comments Reference
Male F344/N rats NOEL = 20 mg/kg body weight Significant dose-related decrease in NTP, 1997a
(15/group) per day epididymal spermatozoal concentration
10 weeks prior to mating LOAEL = 200 mg/kg body weight at the two highest dose levels.
(modified mating protocol) per day Histopathological evidence of
approximate intake in diet: (it should be noted that hypospermia and a decrease in fertility
0, 20, 200, or 2200 mg/kg dose spacing was poor in index were observed at the highest dose
body weight per day the study) only (2200 mg/kg body weight per day).
Wistar rats (12 males, Reproductive effects: No effects on reproductive performance
24 females per group) NOAEL (males) = 418 mg/kg body and development of offspring.
one-generation reproductive weight per day TNO Biotechnology and
study approximate intake NOAEL (females) = 446 mg/kg body Chemistry Institute,
in diet: weight per day 1993
males: 0, 108, 206, or (highest doses administered)
418 mg/kg body weight per day
females: 0, 106, 217, or Parental effects: At top dose, significant increase in
446 mg/kg body weight NOEL (males) = 418 mg/kg body relative weight of livers and
per day weight per day decrease in food consumption
NOAEL (females) = 217 mg/kg and body weight (females).
body weight per day
LOEL (females) = 446 mg/kg
body weight per day
Examination of effects on Reduction in testes weight Dose-response was not investigated. Sharpe et al., 1995
the testes of offspring of and daily sperm production;
female Wistar rats (number effects were not replicated
unspecified) administered in the study of similar design
0 or 1000 µg BBP/litre in in another strain of rats
drinking-water (estimated by Ashby et al. (1997a)
to be approximately 126-366
µg/kg body weight per day)
for 2 weeks prior to mating,
throughout mating and
gestation, and until 22
days after giving birth
Table 1 (continued)
Study protocol Effect levela Critical end-point/comments Reference
Examination of effects on Reversible increase in absolute and Dose-response was not investigated. Ashby et al., 1997a
reproductive systems of relative liver weight at postnatal
male and female offspring day 90 in male offspring
of female Alpk:APfSD rats
(n = 19) exposed during
gestation and lactation
to 0 or 1000 µg BBP/litre
in drinking-water (estimated
to be 183 µg/kg body weight
per day)
Sprague-Dawley rats NOAEL (maternal and offspring) An increase in the percentage of NTP, 1989; Price et
(30 females/group) = 420 mg/kg body weight per day fetuses with variations per litter. al., 1990
gestational days 6-15 LOAEL (significant maternal and Maternal toxicity was evident at the
approximate intake in diet: minimal developmental effects) mid and high dose levels (decreased
0, 420, 1100, or 1640 mg/kg = 1100 mg/kg body weight per day maternal weight gain, increased
body weight per day relative liver weight, increased food
and water consumption).
Swiss albino mice (30 NOAEL (maternal and Increased percentage of late fetal NTP, 1990; Price et
females/group) gestational developmental) = 182 mg/kg body deaths per litter and non-live al., 1990
days 6-15 weight per day implants per litter, decreased number
approximate intake in diet: LOAEL (maternal and developmental) of live fetuses per litter, increased
0, 182, 910, or 2330 mg/kg = 910 mg/kg body weight per day percentage of litters with malformed
body weight per day fetuses, and an increased percentage
of malformed fetuses per litter.
Decreased maternal weight gain at two
highest doses; increased relative
kidney and liver weight in mothers
at top dose.
Table 1 (continued)
Study protocol Effect levela Critical end-point/comments Reference
Wistar rats (15-19 LOAEL (maternal) = 654 mg/kg Significant dose-related reduction Ema et al., 1990
females/group) body weight per day in maternal body weight gain and
gestational days 0-20 NOEL (embryo/fetal toxicity) reduced food consumption at three
approximate intake in = 654 mg/kg body weight per day highest doses (significant only at
diet: 180, 375, 654, Significant reduction in the two highest doses when adjusted
or 974 mg/kg body number of live fetuses per for gravid uterus). Additional
weight per day litter at 375 and 654 mg/kg studies conducted by these
body weight per day investigators in which effects
(administration on days 0-20 in offspring were examined
in study of unconventional design) following administration of
(maternal LOEL) doses greater than 500 mg/kg
NOEL (reproduction) = 185 mg/kg body weight per day either in
body weight per day the diet or by gavage during
various periods of gestation are
not additionally informative with
respect to effect levels.
Peroxisomal proliferation
F344 rats (5/sex/group) LOEL (males) = 639 mg/kg Increase in relative liver BIBRA, 1985
21 days body weight per day and kidney weights in males
approximate intake LOEL (females) = 679 mg/kg and females; increase in
in diet: body weight per day cyanide-insensitive
males: 0, 639, 1277, or (lowest doses administered) palmitoyl-CoA oxidation;
2450 mg/kg body weight increase in lauric acid
per day 11- and 12-hydroxylase activity
females: 0, 679, 1346, in males.
or 2628 mg/kg body weight
per day
Table 1 (continued)
Study protocol Effect levela Critical end-point/comments Reference
female F344/N rats (5 or 10) LOEL = 300 mg/kg body weight Increase in peroxisomal NTP, 1997a
1 or 12 months in the diet per day proliferation (carnitine
approximate intake in diet: (lowest dose administered) acetyl transferase activity).
300, 600, or 1200 mg/kg body
weight per day
a NOEL = no-observed-effect level; NOAEL = no-observed-adverse-effect level; LOEL = lowest-observed-effect level;
LOAEL = lowest-observed-adverse-effect level.
At the 15-month interim sacrifice, the absolute weight of the
right kidney in the females at 600 mg/kg body weight per day and the
relative weight in all exposed males were significantly greater than
in controls. The severity of renal tubule pigmentation in high-dose
males and females was greater than in controls, both at 15 months and
at 2 years. The incidence of mineralization in kidney was
significantly less than in controls in low- and high-dose females at 2
years; severity decreased in all groups of exposed females. The
incidence of nephropathy was significantly increased in all groups of
exposed females (34/50, 47/50, 43/50, and 45/50 in control, 300, 600,
and 1200 mg/kg body weight per day groups, respectively) (see Table 3
in section 11.1.2). The incidence of transitional cell hyperplasia
(0/50, 3/50, 7/50, and 4/50 in control, 300, 600, and 1200 mg/kg body
weight per day groups, respectively) was significantly increased at
600 mg/kg body weight per day.
At final necropsy, the incidences of pancreatic acinar cell
adenoma (3/50, 2/49, 3/50, and 10/50 in control, 120, 240, and 500
mg/kg body weight per day groups, respectively) and pancreatic acinar
cell adenoma or carcinoma (combined) (3/50, 2/49, 3/50, and 11/50 in
control, 120, 240, and 500 mg/kg body weight per day groups,
respectively) in the high-dose males were significantly greater than
in the controls and exceeded those in the ranges of historical
controls from NTP 2-year feeding studies. One carcinoma was observed
in a high-dose male; this neoplasm had never been observed in the
historical controls. The incidence of focal hyperplasia of the
pancreatic acinar cell in the high-dose males was also significantly
greater than in the controls (4/50, 0/49, 9/50, and 12/50 in control,
120, 240, and 500 mg/kg body weight per day groups, respectively). Two
pancreatic acinar cell adenomas were observed in the high-dose
females.
The incidences of transitional cell papilloma of the urinary
bladder in female rats at 2 years were 1/50, 0/50, 0/50, and 2/50 in
control, 300, 600, and 1200 mg/kg body weight per day groups,
respectively.
The authors concluded that there was "some evidence of
carcinogenic activity" in male rats, based upon the increased
incidences of pancreatic acinar cell adenoma and of acinar cell
adenoma or carcinoma (combined). There was "equivocal evidence of
carcinogenic activity" in female rats, based upon the marginally
increased incidences of pancreatic acinar cell adenoma and of
transitional cell papilloma of the urinary bladder.
The NTP (1997b) has released a technical report of a study that
compared outcomes when chemicals were evaluated under typical NTP
bioassay conditions as well as under protocols employing dietary
restriction. The experiments were designed to evaluate the effect of
dietary restriction on the sensitivity of bioassays towards
chemical-induced chronic toxicity and carcinogenicity and to evaluate
the effect of weight-matched control groups on the sensitivity of the
bioassays. BBP was included in the protocol; the results were
summarized as follows:
"Butyl benzyl phthalate caused an increased incidence of pancreatic
acinar cell neoplasms in ad libitum-fed male rats relative to ad
libitum-fed and weight-matched controls. This change did not occur
in rats in the restricted feed protocol after 2 years ... Butyl benzyl
phthalate also caused an increased incidence of urinary bladder
neoplasms in female rats in the 32-month restricted feed protocol. The
incidences of urinary bladder neoplasms were not significantly
increased in female rats in any of the 2-year protocols, suggesting
that the length of the study, and not body weight, was the primary
factor in the detection of this carcinogenic response."
Fifty B6C3F1 mice per sex per group were exposed to 0, 6000, or
12 000 ppm BBP (0, 780, or 1560 mg/kg body weight per day1) via the
diet for 103 weeks (NTP, 1982). Approximately 35 tissues were examined
histopathologically. The only compound-related sign of exposure was a
dose-related decrease (statistical significance not specified) in body
weight in both sexes. Survival was not affected, and there was no
increased incidence of any neoplasm that was compound related. As
well, non-neoplastic changes were all within the normal limits of
incidence for B6C3F1 mice. The NTP concluded that, under the
conditions of the bioassay, BBP "was not carcinogenic for B6C3F1 mice
of either sex."
8.5 Genotoxicity and related end-points
In the (few) published reports of Ames assays with BBP, results
have been negative (Litton Bionetics Inc., 1976; Rubin et al., 1979;
Kozumbo et al., 1982; Zeiger et al., 1982, 1985). Negative results
have also been reported for mouse lymphoma assays (Litton Bionetics
Inc., 1977; Hazleton Biotechnologies Company, 1986), although
equivocal findings have also been published (Myhr et al., 1986; Myhr &
Caspary, 1991). In an assay for in vitro transformation of
Balb/c-3T3 cells (Litton Bionetics Inc., 1985), results were negative.
In an assay for chromosomal aberrations and sister chromatid exchanges
in Chinese hamster ovary cells (Galloway et al., 1987), there was
slight evidence for a trend in one sister chromatid exchange test
without activation, but no convincing evidence for positive results
for sister chromatid exchanges or aberrations.
1 Conversion factor: 1 ppm in food = 0.13 mg/kg body weight per day
(Health Canada, 1994).
The results from the mouse lymphoma (Myhr et al., 1986; Myhr &
Caspary, 1991) and chromosomal aberration (Galloway et al., 1987)
assays are equivocal. For the mouse lymphoma assay, the NTP concluded
that "Increases in mutant colonies were observed in the absence of S9
in cultures treated with concentrations that produced precipitation,
but such responses were not considered valid by experimental quality
control parameters." However, it is difficult to dismiss the observed
dose-response in several studies as spurious, although the repeat
tests were negative, particularly in view of inconsistencies of
results of the latter. In repeat studies ( n = 5) in the absence of
S9, there was limited evidence of activity in only one case; however,
although BBP was positive at 80 nl/ml in the second trial, it was
toxic at concentrations above 30 nl/ml in the third. The
inconsistently observed increase in small colony mutants and percent
damaged Chinese hamster ovary cells may be indicative of weak
clastogenic activity, which warrants proper confirmation in
well-conducted assays.
A negative response was reported for an assay for the induction
of sex-linked recessive lethals in Drosophila melanogaster (Valencia
et al., 1985). Recently, the NTP (1997a) published summary results of
mouse bone marrow tests for sister chromatid exchanges and induction
of chromosomal aberrations; responses were weak, and the sister
chromatid exchange test was not repeated. Both of these responses,
although statistically significant, were small and indicative of only
weak clastogenic activity. Ashby et al. (1997a) reported negative
results in a micronucleus assay in rats.
8.6 Reproductive and developmental toxicity
Detailed descriptions of the protocol and effect levels are
presented here for critical studies only. Experimental details and
effect levels for all key reproductive and developmental studies via
ingestion are provided in Table 1.
With respect to reproductive effects, in repeated-dose toxicity
studies by the oral route, decreases in the weight of the testes and
histopathological effects in the testes have been observed, although
only at doses greater than those that induce other effects, such as
variations in organ to body weight ratios for the kidney and liver or
histopathological effects in the pancreas or kidney. With the
exception of a short-term gavage study in which minimal
histopathological effects in the testes of rats were observed at 480
mg/kg body weight per day in one of six animals (control data not
presented, and no statistical analysis) (Hammond et al., 1987),
testicular atrophy or degeneration has been observed only in rats only
at doses exceeding 1250 mg/kg body weight per day (NTP, 1982, 1997a;
Hammond et al., 1987).
In a combined reproductive/developmental screening protocol, at
1000 mg/kg body weight per day there was a decrease in body weight
gain, fluctuation in food consumption, reduction in the weight of
testis and epididymis, and increase in testicular degeneration in
males. In females at this dose, there was a decrease in body weight
gain, effects on food consumption, and adverse effects on reproductive
indices. With the exception of a transient decrease in pup weight,
there were no effects on the parental generation or offspring at
500 mg/kg body weight per day (Piersma et al., 1995).
Reproductive effects of BBP in male Fischer 344 rats have been
investigated by the NTP (Kluwe et al., 1984; Agarwal et al., 1985).
Groups of 10 males were administered 0, 0.625, 1.25, 2.5, or 5.0% (0,
312.5, 625, 1250, or 2500 mg/kg body weight per day1) in the diet
for 14 days. The protocol included measurement of endocrine hormones
and histopathological examination of brain, liver, kidney, spleen,
thyroid, thymus, pituitary, testes, epididymis, prostate, seminal
vesicles, and mesenteric lymph nodes. Bone marrow was also examined.
No deaths occurred during the study. Body weight was reduced in
the two highest dose groups. Food consumption was consistently reduced
in the highest dose group throughout the experiment. Absolute weights
of testis, epididymis, prostate, and seminal vesicles were
significantly reduced at the two highest dose levels in a dose-related
manner and were accompanied by "generalized histological atrophy."
Statistical analyses were presented for histopathological changes in
testis (aspermatogenesis/seminiferous tubular atrophy), seminal
vesicles (atrophy), and prostate (atrophy); significant changes were
consistently observed at the two highest doses. The authors noted a
"clear relationship" between dose and severity of morphological
changes in testis, seminal vesicles, and prostate; the changes
occurred only at the two highest dose levels. Similarly, effects on
the epididymis were observed in only the two highest dose groups.
Absolute weight of liver was increased at the two lowest doses
and decreased at the highest dose. The relative weight was increased
at all levels of exposure, in a dose-related manner. Histopathological
changes (mild multifocal chronic hepatitis) were described only for
the highest dose. Absolute weight of kidney was also increased at the
two lowest doses and decreased at the two highest. The relative weight
was increased at all levels of exposure, in a dose-related manner.
Proximal tubular regeneration was observed at all dose levels. Thymic
weight was reduced at the two highest doses in a dose-related manner.
Although histopathological changes were described for all dose groups,
atrophy was observed only in the highest dose group. There were no
effects upon absolute or relative pituitary weight, nor were
morphological changes observed in thyroid, pituitary, spleen, or lymph
nodes. Statistical analyses were not presented for histopathological
observation of these organs.
1 Conversion factor: 1 ppm in food = 0.05 mg/kg body weight per day
(Health Canada, 1994).
Plasma testosterone was decreased at the highest dose.
Follicle-stimulating hormone was increased at the two highest doses in
a dose-related manner. Luteinizing hormone was increased at the lowest
dose and at the two highest doses; there was a limited number of
samples at the high dose. No effects were observed upon such
haematological parameters as red blood cell count, packed cell volume,
haemoglobin, mean corpuscular volume, or white blood cell count. There
was no significant effect upon blood clotting ability, as measured by
prothrombin time. Bone marrow cell count was reduced at the two
highest doses.
At the lowest dose (312.5 mg/kg body weight per day), there was a
significant increase in both the absolute and relative weights of both
liver and kidney. There was proximal tubular regeneration at all
levels of exposure. Focal thymic medullary haemorrhage (minimal
severity) was observed in a small number of animals in all BBP-exposed
groups, but the incidences were not dose related. Based upon these
observations, the LOAEL is 312.5 mg/kg body weight per day for effects
on the liver and kidney.
Male F344/N rats (15 per group) were administered BBP via the
diet for 10 weeks, then each mated to 2 unexposed females (NTP,
1997a). Dietary concentrations were relatively widely spaced at 0,
300, 2800, or 25 000 ppm, which were reported by the authors to be
equivalent to 0, 20, 200, or 2200 mg/kg body weight per day. The final
body weight and body weight gain of the high-dose group were
significantly lower than in the controls. Minimal changes in
haematological parameters were observed in the high-dose group. Both
the absolute and relative weights of prostate and testis were
significantly decreased in the high-dose group (2200 mg/kg body weight
per day). (Other lower organ weights in this group were attributed to
the lower mean body weight.) Other effects observed at the high dose
included degeneration of the seminiferous tubular germinal epithelium
and significantly reduced weight of right cauda, right epididymis, and
right testis. Epididymal spermatozoal concentrations were
significantly reduced in a dose-related manner at the two highest
doses. However, histopathological evidence of hypospermia and a
decrease in fertility index were observed only at the highest dose.
Ten of 30 females mated to high-dose males were found to be sperm
positive, but none was pregnant at necropsy. Fertility indices were
significantly lower at the high dose. At the lower two doses, there
were no exposure-related effects observed on maternal body weight,
maternal clinical observations, or litter data. A NOEL of 20 mg/kg
body weight per day can be designated, based upon a significant and
dose-related decrease in epididymal spermatozoal concentration at the
two highest doses and associated effects on fertility at the highest
dose (LOAEL = 200 mg/kg body weight per day).
Concentrations of BBP of 0.2, 0.4, and 0.8% (108, 206, and 418
mg/kg body weight per day for males; 106, 217, and 446 mg/kg body
weight per day for females) were administered in the diet to males for
10 weeks and to females for 2 weeks premating. Two litters were
produced, and no adverse effects were observed on fertility,
pregnancy, or offspring development (TNO Biotechnology and Chemistry
Institute, 1993).
Sharpe et al. (1995) administered a single dose level of BBP via
drinking-water to pregnant Wistar rats, to determine the effects of
gestational and lactational exposure upon male offspring. Dams were
exposed for 2 weeks prior to mating and throughout gestation until
weaning. This procedure was then repeated on the same dams, and
observations were also carried out on the second litters. Based upon
measurement of drinking-water consumption in six animals, intake of
BBP was estimated to range from 126 to 366 µg/kg body weight per day,
from postnatal days 1-2 to postnatal days 20-21, respectively. There
was a significant reduction in daily sperm production in the
BBP-exposed animals examined at 90-95 days. Sperm production in the
positive control group, which received 100 µg diethylstilbestrol
(DES)/litre in drinking-water, was also reduced ( P < 0.01); the
negative control group (which received 1000 µg octylphenol
polyethoxylate/litre) was not evaluated. The authors questioned the
relevance of the effects to humans on the basis that this would
require detailed dose-response data and measurement of the actual
levels of the administered chemical in the male rats. Moreover, these
results vary from those reported by Sharpe et al. (1995) in an
investigation of effects on male offspring.
Ashby et al. (1997a) exposed Alpk:APfSD rats during gestation
and lactation to 1000 µg BBP/litre in drinking-water or 50 µg
DES/litre in drinking-water (positive control). Negative controls
received 100 µl ethanol/litre in drinking-water. Glass drinking-water
bottles were used in the experiment, as the authors had determined
that 60% of BBP was adsorbed onto plastic drinking-water bottles
within 24 h (Ashby et al., 1997b). The authors reported that the
overall exposures were 183 µg BBP/kg body weight per day and 8.6 µg
DES/kg body weight per day. There were no effects upon weight of right
testis after decapsulation, total sperm count (right testis), sperm
count per gram of right testis, or total sperm count in right cauda on
either postnatal day 90 or postnatal day 137. The authors noted the
contrast between these results and those reported by Sharpe et al.
(1995).1
1 It is noted that TNO Nutrition and Food Research Institute (1997)
is conducting an experiment in which the protocol was designed "to
investigate the reproducibility of, and expand on, the findings of
Sharpe et al. ... related to the development of the reproductive
system in Wistar rats exposed in utero and during lactation to
butyl benzyl phthalate in drinking water." Data from this study have
not yet been published.
It should be noted that there were significant differences
between these studies with respect to exposure of the dams. In the
Sharpe et al. (1995) study, the dams were exposed for 2 weeks prior to
mating, throughout gestation and weaning, and subsequently for another
2 weeks prior to mating, during gestation, and during lactation. In
the study by Ashby et al. (1997a), dams were exposed only during
gestation and lactation.
Although BBP has been estrogenic in human breast cancer cells
in vitro (Jobling et al., 1995; Soto et al., 1995; Meek et al.,
1996), results in yeast have been both positive (Coldham et al., 1997;
Harris et al., 1997) and negative, the latter for both BBP and its
principal metabolites (Gaido et al., 1997); it should be noted,
however, that administered concentrations were unclear in two of the
studies (Coldham et al., 1997; Harris et al., 1997). However, neither
BBP nor its metabolites monobutyl benzyl phthalate and monobenzyl
phthalate have been uterotrophic in vivo in rats (Monsanto Europe
SA, 1995a, 1996a) or in rats and mice (Monsanto Europe SA, 1995b,
1996b), respectively. There was no estrogenic effect in an acute in
vivo assay (Milligan et al., 1998) in mice (stimulation of increased
uterine vascular permeability).
The developmental toxicity of BBP following dietary
administration has been well investigated by the NTP in studies in
both rats and mice (NTP, 1989, 1990; Price et al., 1990) and in a
series of investigations in rats by Ema et al. (1990, 1991a,b,c, 1993,
1994, 1995) following both dietary and gavage administration. In
general, developmental effects of BBP have been observed only at dose
levels that induced significant maternal toxicity; in pair feeding
studies, however, malformations observed at high doses were not fully
attributable to maternal toxicity (Ema et al., 1992). In a
well-conducted NTP (1989) study in rats, at 1100 mg/kg body weight per
day there were significant effects in the mothers but minimal effects
in the offspring. At the highest dose (1640 mg/kg body weight per
day), there was an increased incidence of rudimentary extra lumbar
ribs. Results of studies by Ema and colleagues in which BBP was
administered in the diet to rats for various periods, including the
full 21 days of gestation, were similar. Although the
no-observed-adverse-effect levels (NOAELs) for both maternal and
developmental toxicity were less in the NTP (1990) study in mice (182
mg/kg body weight per day), this was primarily a function of wide dose
spacing, with maternal (decreased weight gain) and developmental
effects being observed at 910 mg/kg body weight per day. At both 910
and 2330 mg/kg body weight per day, there was a significant increase
in the percentage of fetuses malformed per litter. Ema et al. (1998)
observed a significant decrease in uterine decidual growth at 750
mg/kg body weight and higher following administration on days 0-8 to
pseudopregnant rats. Functional effects have not been investigated in
available studies.
Metabolites of BBP have induced effects on the testes similar to
those of BBP, with the monobutyl ester being more potent in this
regard (Mikuriya et al., 1988). Similarly, profiles of effects (e.g.,
fusion of sternebrae, cleft palate) in the offspring observed at
maternally toxic doses of the metabolites of BBP are similar to those
induced by BBP itself, with effects being observed at lower doses of
monobenzyl than of monobutyl phthalate (see, for example, Ema et al.,
1996a,b,c).
In a recent multigeneration study by continuous breeding in rats
exposed to DBP for which the sole metabolite is monobutyl phthalate,
testicular effects observed in the F1 generation were attributed to
impairment of normal androgen signalling in the fetus, although
available data were considered insufficient to conclude that the
monoester was responsible (Foster, 1997). Multigeneration studies for
BBP have not been identified.
8.7 Peroxisomal proliferation
BIBRA (1985) investigated peroxisomal proliferation of BBP and
reported an increase in relative liver and relative kidney weights, an
increase in cyanide-insensitive palmitoyl-CoA oxidation, and an
increase in lauric acid 11- and 12-hydroxylase activity in male F344
rats at 639 mg/kg body weight per day. In female rats, an increase in
relative liver and relative kidney weights was reported at 679 mg/kg
body weight per day. These were the lowest levels of exposure. The NTP
(1997a) reported an increase in peroxisomal proliferation in female
F344/N rats after exposure to 300 mg/kg body weight per day for either
1 or 12 months. In a comparative study, whereas DEHP induced a "very
marked" increase in peroxisomal proliferation, that for BBP was
considered "moderate" (Barber et al., 1987).
8.8 Immunological and neurological effects
Data additional to those presented in sections 8.2 and 8.3
relevant to assessment of the potential immunotoxicity and
neurotoxicity of BBP were not identified.
9. EFFECTS ON HUMANS
Although in an early study (Mallette & von Haam, 1952) BBP was
reported to have a moderately irritating effect upon 15-30 volunteers,
Hammond et al. (1987) observed neither primary irritation nor
sensitization reactions in a patch test with 200 volunteers. Other
identified data in humans relevant to the assessment of the potential
adverse effects of BBP are restricted to limited studies of
respiratory/neurological effects or cancer in populations of workers
generally exposed to mixtures of plasticizers, of which BBP was a
minor component (Nielsen et al., 1985; Hagmar et al., 1990).
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1 Aquatic environment
10.1.1 Pelagic organisms
Data on acute toxicity are available for approximately two dozen
species, including microorganisms, algae, invertebrates, and fish
(Table 2). The lowest reported acute toxicity value was a 96-h LC50
of 510 µg/litre for the shiner perch ( Cymatogaster aggregata) in a
flow-through study using measured concentrations (Ozretich et al.,
1983). Values of the LC50 for most other fish species exceeded 1000
µg/litre. The most sensitive invertebrate species in acute toxicity
tests was the mysid shrimp ( Mysidopsis bahia), with a 96-h LC50 of
900 µg/litre in a static bioassay using nominal concentrations
(Gledhill et al., 1980). Reported LC50 values for other invertebrates
exceeded 1000 µg/litre.
Data on chronic toxicity are available for about a dozen species,
including algae, invertebrates, and fish. The lowest reported chronic
toxicity value was a 96-h EC50 of 110 µg/litre, reported for the
green alga Selenastrum, based on chlorophyll a measurements and
cell number reductions using nominal concentrations (Suggatt & Foote,
1981). The most sensitive invertebrate species in chronic toxicity
tests was the mysid shrimp ( Mysidopsis bahia), with a 28-day
lowest-observed-effect concentration (LOEC) of 170 µg/litre, based on
reproduction and growth in a flow-through study using measured
concentrations (Springborn Bionomics, 1986a). The most sensitive fish
species in chronic toxicity tests was the fathead minnow ( Pimephales
promelas), with a 30-day LOEC of 360 µg/litre based on hatching of
eggs and survival and growth of larvae using measured concentrations
(LeBlanc, 1984).
Table 2: Toxicity to aquatic organisms.
Organism Effect Reference
Acute toxicity
mixed microbial cultures 8% inhibition of oxygen consumption Volskay & Grady, 1988;
at solubility limit (2900 µg/litre) Volskay et al., 1990
with Organisation for Economic
Co-operation and Development (OECD)
Method 209 and Respiration Inhibition
Kinetic Analysis (RIKA)a Screening Test
selected pure bacterial cultures little or no inhibition of growth in Painter & Jones, 1990
cultures amended with 625-625 000
µg/litre
unacclimated wastewater treatment 8.4% chemical oxygen demand (COD) removal Adams
plant sludge inhibition after 3 h at 3000 µg/litre, & Bianchini-Akbeg,
no effect on nitrification of ammonia 1989
Photobacterium phosphoreum 5-min Apparent Effects Thresholdb (AET), Tetra Tech Inc., 1986;
Puget Sound (reduced sediment Barrick et al., 1988
luminescence), 63 ng/g dry weight
sediment
Photobacterium phosphoreum 5-min, Puget Sound (reduced sediment
luminescence), 4900 ng/g organic carbon Barrick et al., 1988
variety of algae, invertebrates, and fish acute toxicity = 500-5000 µg/litre TOXNETc
Hydra littoralis 96-h EC50 = 1100 µg/litre (mortality Monsanto Company, 1986a
and presence of "tulip" stage)
polychaetes (Nereis/Neanthes virens) 96-h LC50 > 3000 µg/litre Monsanto Company, 1986b
Table 2 (continued)
Organism Effect Reference
oyster (Crassostrea gigas) AET, Puget Sound (increased Tetra Tech Inc., 1986;
abnormalities), >470 ng/g dry weight Barrick et al., 1988
sediment
oyster (Crassostrea gigas) AET, Puget Sound (increased Barrick et al., 1988
abnormalities), >9200 ng/g organic carbon
oyster (Crassostrea virginica) 96-h EC50 (shell deposition) Monsanto Company, 1986c
= 1300 µg/litre
amphipod (Rhepoxynius abronius) AET, Puget Sound (increased mortality), Tetra Tech Inc., 1986
>470 ng/g dry weight sediment
amphipod (Rhepoxynius abronius) AET, Puget Sound (increased mortality), Barrick et al., 1988
900 ng/g dry weight sediment
amphipod (Rhepoxynius abronius) AET, Puget Sound (increased mortality), Barrick et al., 1988
42 000 ng/g organic carbon
daphnids 48-h LC50 = 1000-3700 µg/litre Nabholz, 1987
Daphnia magna 24-h LC50 > 460 000 µg/litre LeBlanc, 1980
Daphnia magna 24-h EC50 = 3800 µg/litre Adams & Heidolph, 1985
Daphnia magna 48-h EC50 > 960 µg/litre Adams et al., 1995
Daphnia magna 48-h EC50 > 1400 µg/litre CMA, 1984
Daphnia magna 48-h LC50 = 1800 µg/litre Zeigenfuss et al., 1986
Daphnia magna 48-h EC50 = 1800 µg/litre Adams & Heidolph, 1985
Daphnia magna 48-h EC50 = 3700 µg/litre Gledhill et al., 1980
Table 2 (continued)
Organism Effect Reference
Daphnia magna 48-h LC50 = 92 000 µg/litre LeBlanc, 1980
Daphnia magna 48-h LC50 = 1600 µg/litre (no food) to Barera & Adams, 1983
>10 000 µg/litre (2000 µg/litre algae
or 30 000 µg/litre
trout chow/alfalfa yeast as food)
Daphnia magna 48-h LC50 = 1000 µg/litre (no organic Barera & Adams, 1983
solvent) to 2200 µg/litre (triethylene
glycol solvent)
Daphnia magna 2-, 7-, 14-, and 21-day EC50 > 760 Adams & Heidolph, 1985
µg/litre (flow-through); 21-day EC50
= 680 µg/litre (static renewal)
Daphnia magna 48-h LC50 = 3700 µg/litre (no fulvic acid) Monsanto Company, 1978
48-h LC50 = 2430 µg/litre (250 mg natural
fulvic acid/litre)
48-h LC50 = 1910 µg/litre (250 mg
purchased fulvic acid/litre)
Mysid shrimp (Mysidopsis bahia) 48-h LC50 = 1700 µg/litre CMA, 1984
Mysid shrimp (Mysidopsis bahia) 96-h LC50 = 900 µg/litre Gledhill et al., 1980
Mysid shrimp (Mysidopsis bahia) 96-h LC50 > 740 µg/litre Monsanto Company, 1988
(estimate = 1100 µg/litre)
Mysid shrimp (Mysidopsis bahia) 96-h LC50 = 9630 µg/litre Suggatt & Foote, 1981
Grass shrimp (Paleomonetes vulgaris) 96-h LC50 > 2700 µg/litre Monsanto Company, 1986d
Pink shrimp (Penaeus duorarum) 96-h LC50 > 3400 µg/litre Springborn Bionomics, 1986b
Table 2 (continued)
Organism Effect Reference
Crayfish (Procambarus sp.) 96-h LC50 > 2400 µg/litre Monsanto Company, 1986e
Chironomus tentans 48-h LC50 = 1600 µg/litre Zeigenfuss et al., 1986
Chironomus tentans 48-h LC50 = 1640 µg/litre Monsanto Company, 1982b
Chironomus tentans 48-h LC50 = 3600 µg/litre Monsanto Company, 1981a
Paratanytarsus dissimilis 48-h LC50 > 3600 µg/litre Monsanto Company, 1981a
Midge (Paratanytarsus parthenogenetica) 48-h LC50 = 7200 µg/litre Monsanto Company, 1981b;
CMA, 1984
Midge (Paratanytarsus parthenogenetica) 48-h LC50 = 13 400 µg/litre Monsanto Company, 1981c
Midge (Paratanytarsus parthenogenetica) 96-h LC50 > 3600 µg/litre Adams et al., 1995
Mayfly (Hexagenia sp.) 96-h LC50 = 1100 µg/litre Monsanto Company, 1986f
Fathead minnow (Pimephales promelas) 96-h LC50 = 2100 µg/litre (hardness Gledhill et al., 1980
40 000 µg calcium carbonate/litre)
Fathead minnow (Pimephales promelas) 96-h LC50 = 5300 µg/litre (hardness Gledhill et al., 1980
160 000 µg calcium carbonate/litre)
Fathead minnow (Pimephales promelas) 96-h LC50 > 780 µg/litre (static test) Adams et al., 1995
Fathead minnow (Pimephales promelas) 96-h LC50 = 1500 µg/litre CMA, 1984; Adams et al., 1995
(flow-through test)
Fathead minnow (Pimephales promelas) 96-h LC50 > 1600 µg/litre (static test) CMA, 1984
Fathead minnow (Pimephales promelas) 96-h LC50 = 2320 µg/litre Gledhill et al., 1980
Fathead minnow (Pimephales promelas) 14-day LC50 = 2250 µg/litre Gledhill et al., 1980
Table 2 (continued)
Organism Effect Reference
Bluegill (Lepomis macrochirus) 24-h LC50 = 62 000 µg/litre Buccafusco et al., 1981
Bluegill (Lepomis macrochirus) 96-h LC50 = 43 000 µg/litre Buccafusco et al., 1981
Bluegill (Lepomis macrochirus) 96-h LC50 = 1700 µg/litre Gledhill et al., 1980;
CMA, 1984
Rainbow trout (Oncorhynchus mykiss) 96-h LC50 = 3300 µg/litre Gledhill et al., 1980
Rainbow trout (Oncorhynchus mykiss) 96-h LC50 = 820 µg/litre (flow-through test) CMA, 1984; Adams et al., 1995
Sheepshead minnow (Cyprinodon variegatus) 48-h LC50 = 3300 µg/litre AQUIREd
Sheepshead minnow (Cyprinodon variegatus) 96-h LC50 > 680 µg/litre (static test) CMA, 1984; Adams et al., 1995
Sheepshead minnow (Cyprinodon variegatus) 96-h LC50 = 3000 µg/litre Gledhill et al., 1980
Sheepshead minnow (Cyprinodon variegatus) 96-h LC50 = 440 000 µg/litre Heitmuller et al., 1981
Shiner perch (Cymatogaster aggregata) 96-h LC50 = 510 µg/litre Ozretich et al., 1983
English sole (Parophrys vetulus) 96-h LC50 = 660 µg/litre (static Randall et al., 1983
replenish), 550 µg/litre (flow-through)
Chronic toxicity
Algae 96-h EC50 = 200 µg/litre CMA, 1984
Anacystis 96-h EC50 = 1000 µg/litre (cell count) AQUIREd
Microcystis 96-h EC50 = 1 000 000 µg/litre (cell count) Gledhill et al., 1980
Dunaliella 96-h EC50 = 1000 µg/litre (cell count) Gledhill et al., 1980
Navicula 96-h EC50 = 600 µg/litre (cell count) Gledhill et al., 1980
Table 2 (continued)
Organism Effect Reference
Skeletonema 96-h EC50 = 600 µg/litre (cell count) Gledhill et al., 1980
Skeletonema EC50 = 190 µg/litre (cell count) Suggatt & Foote, 1981
Skeletonema EC50 = 170 µg/litre (chlorophyll a) Suggatt & Foote, 1981
Selenastrum 96-h EC50= 400 µg/litre (cell count) Gledhill et al., 1980
Selenastrum 96-h EC50= 110 µg/litre (chlorophyll a) Suggatt & Foote, 1981
Selenastrum 96-h EC50 = 130 µg/litre (cell count) Suggatt & Foote, 1981
Selenastrum 96-h EC50 = 600 µg/litre (cell count) AQUIREd
Selenastrum capricornutum 96-h EC50 = 210 µg/litre (cell count) Adams et al., 1995
Selenastrum capricornutum 96-h EC50 = 520 µg/litre (red blood cells, Tucker et al., 1985
reduced dry weight)
Selenastrum capricornutum 5-day EC50 = 720 µg/litre (cell count) Monsanto Company, 1980b
Selenastrum capricornutum 14-day EC50 = 520 µg/litre (cell count) Monsanto Company, 1980b
Daphnids 21-day NOEC = 440-630 µg/litre Nabholz, 1987
Daphnia and fathead minnow (Pimephales promelas) chronic toxicity = 100-800 µg/litre TOXNETc
Daphnia magna 21-day LOEC = 350 µg/litre; NOEC = 220
µg/litre (reproduction) (chronic renewal) Monsanto Company, 1982c
Daphnia magna 21-day LOEC = 760 µg/litre; NOEC = 260 Adams & Heidolph, 1985
µg/litre (reproduction) (flow-through)
Table 2 (continued)
Organism Effect Reference
Daphnia magna 21-day LOEC = 700 µg/litre; NOEC = 350 Adams & Heidolph, 1985
µg/litre (growth, survival, and
reproduction) (static)
Daphnia magna 14-day (Springborn = 21-day) LOEC = 1400 CMA, 1984; Springborn Bionomics,
µg/litre; NOEC = 280 µg/litre (survival 1984; Rhodes et al., 1995
and reproduction)
Daphnia magna 42-day LOEC = 760 µg/litre; NOEC = Gledhill et al., 1980
260 µg/litre (decreased reproduction,
both generations; decreased survival in
second generation)
Mysid shrimp (Mysidopsis bahia) 28-day LOEC = 170 µg/litre; NOEC = Springborn Bionomics, 1986a
75 µg/litre (reproduction and growth)
Fathead minnow (Pimephales promelas) 30-day LOEC = 360 µg/litre; NOEC = LeBlanc, 1984
140 µg/litre (decreased growth,
embryo-larvae study)
Fathead minnow (Pimephales promelas) maximum acceptable toxicant concentration Sun et al., 1995
(MATC) > 360 µg/litre; chronic LC01 =
547 µg/litre (estimated)
Fathead minnow (Pimephales promelas) 30-day mean chronic value = 220 µg/litre Pickering, 1983
Bluegill (Lepomis macrochirus) NOEC = 380 µg/litre Verschueren, 1983
Table 2 (continued)
Organism Effect Reference
Rainbow trout (Oncorhynchus mykiss) 109-day NOEC > 200 µg/litre (hatchability, Monsanto Company, 1986g;
growth, survival) Rhodes et al., 1995
English sole (Parophrys vetulus) sublethal effects at all exposures, TOXNETc
lowest = 100 µg/litre
a According to Volskay et al. (1990).
b The concentration above which statistically significant adverse effects are always expected relative
to appropriate reference conditions.
c Toxicology Data Network, National Library of Medicine, US Department of Health and Human Services,
Bethesda, MD.
d Aquatic Information Retrieval Database, US Environmental Protection Agency.
10.1.2 Benthic organisms
There were no acute or chronic toxicity studies identified for
BBP in sediments.
Tetra Tech Inc. (1986) calculated a sediment quality value1 of
55 000 ng BBP/g dry weight for sediment containing 1% organic carbon
using the equilibrium partitioning approach. The assumption behind
this approach is that non-polar organic compounds partition to the
organic carbon fraction of the sediments to varying degrees depending
upon their organic carbon/water partition coefficients (Di Toro et
al., 1991).
10.2 Terrestrial environment
No studies on the effects of BBP on wild mammals were identified.
Information about the effects of BBP on laboratory mammals is
presented in section 8.
No studies on the effects of BBP on plants were identified.
1 Sediment quality values represent concentrations of chemicals in
sediments that are expected to be associated with adverse biological
effects based either on field evidence or on theoretical predictions
(Tetra Tech Inc., 1986).
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Following oral administration to rats, BBP is readily hydrolysed
in the gastrointestinal tract and the liver to phthalate monoesters
(monobutyl and monobenzyl phthalate), which are rapidly eliminated,
predominantly in urine.
Available data in humans are inadequate to serve as a basis for
assessment of the effects of long-term exposure to BBP in human
populations. The remainder of this section, therefore, addresses
effects in experimental animals.
The acute toxicity of BBP is relatively low, with oral LD50
values in rats being greater than 2 g/kg body weight. Target organs
following acute exposure include the haematological and central
nervous systems.
Available data are inadequate to assess the irritant or
sensitizing effects of BBP in animal species.
The repeated-dose toxicity of BBP has been well investigated in
recent studies, primarily in the rat, in which dose-response was well
characterized. Effects observed consistently have been decreases in
body weight gain (often accompanied by decreases in food consumption)
and increases in organ to body weight ratios, particularly for the
kidney and liver. In addition, histopathological effects on the
pancreas and kidney and haematological effects have also been
observed. At higher doses, degenerative effects on the testes and,
occasionally, histopathological effects on the liver have been
reported. In specialized investigations, peroxisomal proliferation in
the liver has been observed, although potency in this regard was less
than that for other phthalates, such as DEHP.
The chronic toxicity and carcinogenicity of BBP have been
investigated in NTP bioassays in rats (including standard and
feed-restricted protocols) and mice. An increase in mononuclear cell
leukaemias observed in female F344 rats was not confirmed in a repeat
study. It was concluded that there was "some evidence" of
carcinogenicity in male rats, based on an increased incidence of
pancreatic tumours, and equivocal evidence in female rats, based on
marginal increases in pancreatic and bladder tumours. Dietary
restriction prevented full expression of the pancreatic tumours and
delayed appearance of the bladder tumours. There was no evidence of
carcinogenicity in mice.
The weight of evidence of the genotoxicity of BBP is clearly
negative. However, available data are inadequate to conclude
unequivocally that BBP is not clastogenic, although in identified
studies it has induced, at most, weak activity of a magnitude
consistent with secondary effects on DNA.
Therefore, BBP has induced an increase in pancreatic tumours
primarily in one sex of one species, the full expression of which was
prevented in a dietary restriction protocol, and a marginal increase
in bladder tumours in the other sex, which was delayed upon dietary
restriction. Available data are consistent with the compound not
interacting directly with DNA. On this basis, BBP can be considered,
at most, possibly carcinogenic to humans, likely inducing tumours
through a non-genotoxic (although unknown) mechanism.
In a range of studies, including those designed to investigate
the reproductive effects of BBP on the testes and endocrine hormones
of male rats, a modified mating protocol conducted by the NTP, and a
one-generation study, adverse effects on the testes and, consequently,
fertility have generally been observed only at doses higher than those
that induce effects on other organs (such as the kidney and liver),
although decreases in sperm counts have been observed at doses similar
to those that induce effects in the kidney and liver. This is
consistent with the results of repeated-dose toxicity studies.
Reductions in testes weight and daily sperm production in
offspring were reported at a relatively low level in rats exposed in
utero and during lactation in a study in which dose-response was not
investigated. However, such effects were not observed in a recent
study in another strain of rats in which only increases in absolute
and relative liver weights were observed at postnatal day 90.
Additional investigation of potential effects on the reproductive
systems of male and female animals exposed in utero and during
lactation in studies designed to address dose-response is desirable
and under way.
Although BBP has been estrogenic in human breast cell cancer
lines in vitro, results in yeast cells have been mixed. Neither BBP
nor its principal metabolites have been uterotrophic in vivo in rats
or mice. Although available data do not support the conclusion that
BBP is estrogenic, other potential endocrine-mediated effects such as
anti-androgenic activity associated with DBP are not precluded.
There is considerable emphasis currently on development of more
sensitive frameworks for testing and assessment of
endocrine-disrupting substances; compounds such as phthalates are
likely early candidates for additional testing.
In several well-conducted studies in rats and mice, BBP has
induced marked developmental effects, but only at dose levels that
induce significant maternal toxicity.
Although the potential neurotoxicity of BBP has not been well
investigated, histopathological effects on the central and peripheral
nervous systems have not been observed following short-term exposure
to relatively high dietary concentrations. Available data are
inadequate to assess the potential immunotoxicity of BBP.
Effect levels in available studies for the oral route are
summarized in Table 1. Based on consideration of the complete database
on repeated-dose toxicity by the oral route (including subchronic,
chronic, and reproductive/developmental studies), effects that occur
at lowest concentrations in rats are increases in organ to body weight
ratios, primarily for the liver and kidney, and histopathological
effects on the pancreas and kidney at dose levels in the range of 120
to just greater than 300 mg/kg body weight per day. Specifically,
these include increases in ratios of liver to body weight and
pancreatic lesions in the Wistar rat observed in a 90-day study
(Hammond et al., 1987), increases in (absolute and relative) kidney
weight and relative liver weight and proximal tubular regeneration in
a 2-week reproductive study (Agarwal et al., 1985), and increased
relative kidney weight at interim (15 month; not determined at
termination) sacrifice in male F344 rats in the 2-year NTP bioassay
(NTP, 1997a). Although nephropathy was also increased at all doses
(300 mg/kg body weight per day and higher) in the kidney of female
F344 rats in the 2-year NTP bioassay (NTP, 1997a), incidence was high
in all groups, with no evidence of dose-response (incidence or
severity) and no increase in severity between the interim and final
sacrifices.
Increases in hepatic peroxisomal proliferation in F344 rats also
occur at doses similar to those at which the effects mentioned above
have been observed following exposure for 1 or 12 months (NTP, 1997a).
Decreases in body weight in mice (of unspecified statistical
significance) have also been observed in this dose range in a 90-day
study, although food consumption was not reported (NTP, 1982).
Decreases in epididymal spermatozoal concentrations have also been
reported at these levels, although without accompanying
histopathological effects on the testes or adverse impact on fertility
(NTP, 1997a).
11.1.2 Criteria for setting guidance values for BBP
The following guidance is provided as a possible basis for
derivation of limits of exposure and judgement of the quality of
environmental media by relevant authorities.
Benchmark doses have been developed for histopathological lesions
in the pancreas of male Wistar rats in the 90-day study (Hammond et
al., 1987) and renal lesions in male F344 rats in the 2-week
reproductive study conducted by the NTP (Agarwal et al., 1985).
Primarily for purposes of comparison, a benchmark dose for renal
lesions in female F344/N rats in the 2-year carcinogenesis bioassays
conducted by the NTP is also presented (NTP, 1997a). Information on
the incidence of these lesions, resulting benchmark doses calculated
using the THRESH program (Howe, 1995), and associated parameter
estimates and statistics of fit are presented in Table 3. Each
benchmark dose is based upon a 5% effect level; 95% lower confidence
limits are also presented.
Histopathological effects have not been associated with increases
in organ to body weight ratios in the same sex except at much higher
doses, nor have decreases in epididymal spermatozoal concentrations at
lowest doses been accompanied by histopathological effects and adverse
impact on fertility. Owing principally to these considerations and
less to the inadequacy of current statistical techniques to adequately
model continuous data for these end-points and for peroxisomal
proliferation, benchmark doses for these end-points have not been
developed; for completeness, however, relevant effect levels are
included in Table 3 for comparison.
The fit of the model was best for pancreatic lesions in male
Wistar rats in the subchronic study by Hammond et al. (1987)
( P = 0.98), adequate for proximal tubular regeneration in the kidney
of male rats in the 2-week reproductive protocol (Agarwal et al.,
1985) ( P = 0.39), and inadequate for nephropathy in female rats in
the 2-year bioassay (NTP, 1997a) ( P = 0.03). Inadequate fit for the
latter is attributable to high incidence in all dose groups and little
evidence of dose-response. On this basis and consideration of the fact
that pancreatic lesions and tumours were also observed in the NTP
2-year bioassay in males of another strain of rats, the pancreatic
lesions in the Hammond et al. (1987) study have been selected as the
point of departure for development of a tolerable intake.
For comparison, a benchmark dose calculated using the THRESH
program (Howe, 1995) and associated parameter estimates and statistics
of fit for pancreatic focal hyperplasia (acinus) in male F344 rats in
the 2-year NTP bioassay are presented in Table 4. Although the
benchmark dose and associated lower 95% confidence limit are slightly
less than those calculated on the basis of the Hammond et al. (1987)
study in Wistar rats, it should be noted that the distinction between
hyperplasia and adenomas in the carcinogenesis bioassay was not
readily apparent. A tumorigenic dose (TD05) calculated on the basis
of multistage modelling (Global 82) of the incidence of pancreatic
adenomas or carcinomas (acinus) in male rats in the NTP bioassay is
also presented in Table 4 and, as would be expected, is greater than
the benchmark dose for hyperplasia. Data presented in the published
account were insufficient to develop a benchmark dose on the basis of
hyperplasia and adenomas combined.
Table 3: Benchmark doses for non-neoplastic effects.
Study Data for calculating Parameter estimates
benchmark dosea
(reference) Effect levels Dose Response Benchmark dose Goodness of fit
Subchronic dietary LOAEL = 381 mg/kg body Males: Lesions in 5% dose: 167 mg/kg Chi-square goodness
study weight per day (based pancreas: body weight per day of fit: 9.3 × 10-4
Wistar rats, upon histopathological
27-45/group lesions in males in control 0/27 (0%) 95% lower confidence Degrees of freedom:
3-month duration pancreas at two highest 151 mg/kg body 0/14 (0%) limit: 132 mg/kg body 1
doses) (males) weight per day 8/15 (53%) weight per day P-value: 0.98
381 mg/kg body 13/14 (93%)
weight per day
960 mg/kg body
weight per day
(Monsanto Company, LOEL = 171 mg/kg body
1980a; Hammond et al., weight per day (based
1987) on increases in organ
to body weight ratios
at all doses for the
kidney, liver, and
caecum) (females)
Reproductive study LOAEL = 312.5 mg/kg Males: Kidney,
F344 male rats, body weight per day proximal
10/group (based upon significant control tubular 5% dose: 228 mg/kg Chi-square goodness
14-day dietary increase in the relative regeneration: body weight per day of fit: 3.01
administration weight of liver and both 312.5 mg/kg body 0/10
the absolute and weight per day 2/10
(Kluwe et al., 1984; relative weights of 625 mg/kg body 2/10 95% lower confidence Degrees of freedom: 3
Agarwal et al., 1985) kidney and proximal weight per day 4/10 limit:
tubular regeneration 1250 mg/kg body 3/10 117 mg/kg body weight P-value: 0.39
at all levels of weight per day per day
exposure) 2500 mg/kg body
weight per day
Table 3 (continued)
Study Data for calculating Parameter estimates
benchmark dosea
(reference) Effect levels Dose Response Benchmark dose Goodness of fit
Carcinogenicity LOEL = 120 mg/kg body Females: 2-year sacrifice; 5% dose: 50 mg/kg body Chi-square goodness
bioassay weight per day (based kidney weight per day of fit: 7.09
F344/N rats, on increased relative nephropathy:
60/sex/group kidney weight in males
Dietary at interim sacrifice)
administration (not determined at
for 2 years terminal sacrifice)
(NTP, 1997a) Increase in renal control 34/50 95% lower confidence Degrees of freedom: 2
nephropathy in females 300 mg/kg body 47/50 (P < 0.01) limit: 28 mg/kg body
at all doses (300 mg/kg weight per day 43/50 (P < 0.05) weight per day P-value: 2.9 × 10-2
body weight and above); 600 mg/kg body 45/50 (P < 0.01)
however, unacceptable weight per day
goodness of fit for 1200 mg/kg body
benchmark dose weight per day
F344/N female LOEL = 300 mg/kg body
rats, 5/group weight per day (based
Dietary administration on increase in
for 1 or 12 months peroxisomal
proliferation)
(NTP, 1997a)
Table 3 (continued)
Study Data for calculating Parameter estimates
benchmark dosea
(reference) Effect levels Dose Response Benchmark dose Goodness of fit
F344 males, 15/group LOAEL = 200 mg/kg body
Modified mating weight per day (decrease
protocol in epididymal
spermatozoal
concentration without
histopathological
evidence of hypospermia
(NTP, 1997a) or decrease in fertility)
(It should be noted that
the dose levels in this
protocol increase by a
factor of 10.)
a Benchmark doses were calculated with the THRESH program (Howe, 1995). The approach to the use of benchmark doses in risk
assessment is described by US EPA (1995).
The TDI is developed, therefore, as follows:
TDI = 132 mg/kg body weight per day
100
= 1.3 mg/kg body weight per day
where:
132 mg/kg body weight per day is the lower 95% confidence limit for
the benchmark dose (167 mg/kg body weight per day) associated with a
5% increase in the incidence of pancreatic lesions in male Wistar rats
in the subchronic study of Hammond et al. (1987). It is noted that
increased excretion in faeces at higher doses observed in one study
might impact on the dose-response curve and resulting benchmark dose,
although it was not possible to address this quantitatively.
100 is the uncertainty factor (×10 for intraspecies variation; ×10 for
interspecies variation). An additional factor for extrapolation from
subchronic to chronic has not been incorporated as, on the basis of a
fairly robust database, there is no indication that effect levels are
lower in chronic studies than in investigations of shorter duration;
moreover, the compound is rapidly eliminated. Also, the incidence of
pancreatic lesions in the Wistar rat in the subchronic study on which
the benchmark dose is based is higher than that observed in the F344/N
rat in the 2-year carcinogenesis bioassay. Available data were
considered insufficient to replace default values for toxicokinetic
and toxicodynamic components of interspecies and intraspecies
variation with data-derived values.
This TDI is similar to values that could be developed based on
the LOELs for the continuous end-points such as peroxisomal
proliferation and increases in organ to body weight ratios included in
Table 1.
Data on the toxicity of BBP following repeated exposure by
inhalation are limited, with information relevant to characterization
of exposure-response being confined to results of two short-term and
one subchronic study in rats, with the range of end-points examined in
the latter investigation being more limited (Hammond et al., 1987). In
the short-term study with lowest concentrations, effects on body
weight gain and serum glucose were observed at 526 mg/m3; there were
no effects on haematology, blood chemistry, urinalysis, organ weights,
or histopathology at 144 mg/m3. Increases in organ weights were
observed at 218 mg/m3 in the subchronic study, although there were no
histopathological effects at the highest dose (789 mg/m3); the NOEL
was 51 mg/m3. Although the database for inhalation is somewhat
limited, it is of interest to note that the NOELs for effects by this
route are similar to those for ingestion. For example, the NOEL in the
investigation in which the range of end-points examined was more
extensive (144 mg/m3) is equivalent to a dose approximately threefold
less than the point of departure (i.e., the 95% lower confidence
limit) for the TDI presented above.1
11.1.3 Sample risk characterization
Based upon a sample estimate (section 6.2), intake of BBP for the
general population ranges from 2 µg/kg body weight per day in adults
to 6 µg/kg body weight per day in children. Food is by far the
greatest source, contributing essentially all of the intake.
The maximum and minimum estimates of total daily intake are 200
and 650 times less, respectively, than the TDI derived above for the
general population.
Identified data were inadequate to provide sample estimates of
exposure to BBP in the occupational environment or from consumer
products and hence sample risk characterizations for these scenarios.
It should be noted, though, that the inclusion of concentrations in
indoor air in the estimates of exposure for the general population
should account, at least to some extent, for exposure from consumer
products.
11.2 Evaluation of environmental effects
BBP may be released to the environment from a number of
industrial and municipal sources. Most releases are reported to be to
the atmosphere, but BBP is also released to the aquatic environment
from industrial and municipal liquid effluents.
Once in the environment, BBP partitions to soil, surface water,
sediments, and biota, and the substance has been detected in each of
these compartments. Likely sinks are soil and sediment.
BBP is removed from the atmosphere by photooxidation and by
rainwater, with a half-life of a few hours to a few days. BBP is not
persistent in water, sediments, or soil under aerobic conditions, with
a half-life of a few days. Under anaerobic conditions, BBP is more
persistent, with a half-life of a few months. BBP is readily
metabolized by vertebrates and invertebrates. Reported BCFs are less
than 1000, based on total residues, and well under 100, based on
intact BBP residues.
1 Based on the following conversion: 1 mg/m3 in air = 0.31 mg/kg
body weight per day ingested in rats (Health Canada, 1994).
Table 4: Benchmark dose for pancreatic hyperplasia and tumorigenic dose (NTP 2-year bioassay).
Study Data for calculating benchmark dose Parameter estimates
(reference) Effect levels Dose Response Benchmark dose Goodness of fit
Carcinogenicity Pancreas, acinus, Males: Incidence: 5% dose: 130 mg/kg P-value for lack of
bioassay focal hyperplasia body weight per day fit: 0.916
F344/N rats control 4/50
Dietary 120 mg/kg body 7/49 95% lower confidence
administration weight per day 9/50 limit: 73 mg/kg body
for 2 years 240 mg/kg body weight 12/50 weight per day
per day (P < 0.05)
(NTP, 1997a) 500 mg/kg body weight
per day
Carcinogenicity Pancreas, acinus, Males: Incidence: TD05: 320 mg/kg body P-value for lack of
bioassay adenoma, or carcinoma weight per day fit: 0.854
F344/N rats control 3/50
Dietary 120 mg/kg body weight 2/49 95% lower confidence
administration per day 3/50 limit:
for 2 years 240 mg/kg body weight 11/50 (P = 0.014) 160 mg/kg body weight
per day per day
(NTP, 1997a) 500 mg/kg body weight
per day
P = 0.003
for trend
In acute toxicity tests on approximately two dozen species and
chronic tests on about a dozen species, adverse effects occur at
exposure concentrations equal to or greater than 100 µg/litre.
Although higher concentrations have sometimes been reported,
concentrations in surface waters are generally less than 1 µg/litre.
Therefore, it is likely that BBP poses low risk to aquatic organisms.
No information about the effects of BBP on sediment-dwelling
organisms, soil invertebrates, terrestrial plants, or birds has been
identified on which to base an estimate of risk to these organisms.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
IARC (1987) has classified BBP in Group 3: "the agent is not
classifiable as to its carcinogenicity to humans." There were no
adequate data for humans, and evidence in animals was inadequate.
Information on international hazard classification and labelling
is included in the International Chemical Safety Card reproduced in
this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventive and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0834) reproduced in this
document.
13.1 Human health hazards
BBP has the potential to adversely affect reproductive function,
although effects on kidney, liver, and pancreas are noted at generally
lower doses.
13.2 Advice to physicians
In case of poisoning, treatment is supportive.
13.3 Spillage
In the event of spillage, measures should be undertaken to
prevent BBP from reaching drains or watercourses because of its
toxicity to aquatic organisms.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards
may be obtained from UNEP Chemicals (IRPTC), Geneva. The reader should
be aware that regulatory decisions about chemicals taken in a certain
country can be fully understood only in the framework of the
legislation of that country. The regulations and guidelines of all
countries are subject to change and should always be verified with
appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
BUTYL BENZYL PHTHALATE ICSC: 0834
March 1998
CAS # 85-68-7 Benzyl Butyl phthalate
RTECS # TH9990000 1,2-Benzenedicarboxylic acid, butyl phenymethyl ester
BBP
1,2-C6H4(COOCH2C6H5)(COOC4H9)/C19H20O4
Molecular Mass: 312.4
TYPES OF HAZARD/ ACUTE HAZARDS/ PREVENTION FIRST AID/
EXPOSURE SYMPTOMS FIRE FIGHTING
FIRE Combustible. Gives off NO open flames. Water spray, powder,
irritating or toxic fumes carbon dioxide.
(or gases) in a fire.
EXPLOSION
EXPOSURE
Inhalation Cough. Sore throat. Ventilation, local exhaust, Fresh air, rest.
or breathing protection.
Skin Redness. Protective gloves. Remove contaminated
clothes. Rinse and then
wash skin with water and
soap.
Eyes Redness. Safety spectacles. First rinse with plenty
of water for several
minutes (remove contact
lenses if easily possible),
then take to a doctor.
Ingestion Do not eat, drink, or smoke Rinse mouth. Rest.
during work.
SPILLAGE DISPOSAL PACKAGING & LABELLING
Collect leaking and spilled liquid in sealable metal Marine pollutant.
containers as far as possible. Absorb remaining liquid EU Classification
in sand or inert absorbent and remove to safe place. Symbol:
Do NOT let this chemical enter the environment. (Extra UN Classification
personal protection: A/P2 filter respirator for organic
vapour and harmful dust).
EMERGENCY RESPONSE STORAGE
NFPA Code: H1; F1; R0; Separated from strong oxidants.
IMPORTANT DATA
PHYSICAL STATE; APPEARANCE: ROUTES OF EXPOSURE:
COLOURLESS OILY LIQUID The substance can be absorbed into the body
by inhalation of its vapour.
CHEMICAL DANGERS: INHALATION RISK:
The substance decomposes on heating producing toxic No indication can be given about the rate in
fumes (phthalic anhydride). Reacts with oxidants. which a harmful concentration in the air is
reached on evaporation of this substance
at 20°C.
OCCUPATIONAL EXPOSURE LIMITS: EFFECTS OF SHORT-TERM EXPOSURE:
TLV not established. The substance irritates the eyes, the skin
and the respiratory tract.
EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
The substance may have effects on the liver and
kidneys, resulting in impaired functions.
PHYSICAL PROPERTIES
Boiling point: 370°C
Melting point: -35°C
Relative density (water = 1): 1.1
Solubility in water: none
Vapour pressure, Pa at 20°C: <0.1
Relative vapour density (air = 1): 10.8
Flash point: 199°C
Octanol/water partition
coefficient as log Pow: 4.77
ENVIRONMENTAL DATA
The substance is very toxic to aquatic organisms. In the food chain important to humans,
bioaccumulation takes place, specifically in fish.
NOTES
Saniticizer 160, Sicol 160, Unimoll BB and Palatinol BB are trade names. Also consult
ICSC #0271 Di(2-ethylhexyl)phthalate.
ADDITIONAL INFORMATION
LEGAL NOTICE: Neither the CEC nor the IPCS nor any person acting on behalf of the CEC
or the IPCS is responsible for the use which might be made of this
information.
REFERENCES
Aceves M, Grimalt JO (1993) Large and small particle size screening of
organic compounds in urban air. Atmospheric environment,
27B(2):251-263.
Adams WJ, Bianchini-Akbeg M (1989) Determination of inhibition of
nitrification and COD removal in an activated sludge waste treatment
plant by butylbenzyl phthalate, CAS #85687. St. Louis, MO, Monsanto
Company (Report No. ESC 98-49).
Adams WJ, Heidolph BB (1985) Short-cut chronic toxicity estimates
using Daphnia magna. In: Cardwell RD, Purdy R, Bahner RC, eds.
Aquatic toxicology and hazard assessment: seventh symposium.
Philadelphia, PA, American Society for Testing and Materials, pp.
87-103 (ASTM STP 854).
Adams WJ, Saeger VW (1993) Utility of laboratory microcosms for
predicting the environmental fate of chemicals: a comparison of two
microcosm designs with butyl benzyl phthalate. In: Gorsuch JW, Dwyer
FJ, Ingersoll CG, La Point TW, eds. Environmental toxicology and
risk assessment. Vol. 2. Philadelphia, PA, American Society for
Testing and Materials, pp. 103-119 (ASTM STP 1216).
Adams WJ, Renaudette WJ, Doi JD, Stepro MG, Tucker MW (1986)
Experimental freshwater microcosm biodegradability study of butyl
benzyl phthalate. St. Louis, MO, Monsanto Company, 104 pp. (Report
No. ESC-EAG-86-01).
Adams WJ, Renaudette WJ, Doi JD, Stepro MG, Tucker MW, Kimerle RA,
Franklin BB, Nabholz JV (1989) Experimental freshwater microcosm
biodegradability study of butyl benzyl phthalate. In: Suter GW, II,
Lewis MA, eds. Aquatic toxicology and environmental fate. Vol. 11.
Philadelphia, PA, American Society for Testing and Materials, pp.
19-40 (ASTM STP 1007).
Adams WJ, Biddinger GR, Robillard KA, Gorsuch JW (1995) A summary of
the acute toxicity of 14 phthalate esters to representative aquatic
organisms. Environmental toxicology and chemistry, 14:1569-1574.
Agarwal DK, Maronpot RR, Lamb JC, Kluwe WM (1985) Adverse effects of
butyl benzyl phthalate on the reproductive and hematopoietic systems
of male rats. Toxicology, 35(3):189-206.
Allan M (1995) Probabilistic assessment of 24-hour breathing rates.
Unpublished report prepared by Cornerstone Engineering and Consulting
Inc. for Environmental Health Directorate, Health Canada, October
1995.
Anon. (1996) Reporting relief denied by Massachusetts due to possible
health threat from plasticizer. Chemical regulation reporter,
20(22):766.
Ashby J, Tinwell H, Lefevre PA, Odum J, Paton D, Millward SW,
Tittensor S, Brooks AN (1997a) Normal sexual development of rats
exposed to butyl benzyl phthalate from conception to weaning.
Regulatory toxicology and pharmacology, 26:102-118.
Ashby J, Odum J, Tinwell H, Lefevre PA (1997b) Assessing the risks of
adverse endocrine-mediated effects: where to from here? Regulatory
toxicology and pharmacology, 26:80-93.
Atkinson R (1987) Structure-activity relationship for the estimation
of rate constants for the gas-phase reactions of OH radicals with
organic compounds. International journal of chemical kinetics,
19:799-828.
Axys Analytical Services Limited (1992) Phthalate esters analysis
report. Report prepared for Environment Canada, Conservation and
Protection (Axys File: AS1171).
Barber ED, Astill BD, Moran EJ, Schneider BF, Gray TJB, Lake BG, Evans
JG (1987) Peroxisome induction studies on seven phthalate esters.
Toxicology and industrial health, 3(2):7-24.
Barera Y, Adams WJ (1983) Resolving some practical questions about
Daphnia acute toxicity tests. In: Bishop WE, Cardwell RD, Heidolph
BB, eds. Aquatic toxicology and hazard assessment: sixth symposium.
Philadelphia, PA, American Society for Testing and Materials, pp.
509-518 (ASTM STP 802).
Barrick R, Becker S, Brown L, Beller H, Pastorok R (1988) Sediment
quality values refinement: Vol. 1. 1988 update and evaluation of
Puget Sound AET. Final report. Bellevue, WA, PTI Environmental
Services (NTIS PB89-200398).
Barrows ME, Petrocelli SR, Macek KJ (1980) Bioconcentration and
elimination of selected water pollutants by bluegill sunfish
( Lepomis macrochirus). In: Haque R, ed. Dynamics, exposure, and
hazard assessment of toxic chemicals. Ann Arbor, MI, Ann Arbor
Science Publishers Inc., pp. 379-392.
Bayer CW, Papanicolopoulos CD (1990) Exposure assessments to volatile
organic compound emissions from textile products. In: Indoor air
'90. Proceedings of the 5th international conference on indoor air
quality and climate. Vol. 3. Toronto, Ontario, 29 July - 3 August
1990, pp. 725-730.
BIBRA (1978) Studies on the metabolism and biological effects of
n -butyl benzyl phthalate in the rat. Surrey, British Industrial
Biological Research Association, 44 pp. (BIBRA Report No. 232/78).
BIBRA (1985) A 21-day feeding study of butyl benzyl phthalate to
rats: Effects on the liver and liver lipids. Report of the British
Industrial Biological Research Association to the Chemical
Manufacturers Association, Washington, DC (Project No. 3.0495.1;
Report No. 0495/1/84; CMA Reference PE 28.0-BT-BIB; US Environmental
Protection Agency Document No. 40+8626201; Office of Toxic Substances
Fiche No. OTS050943).
Bremer J, Witte E, Schneider D (1993) Measurement and characterisation
of emissions from PVC materials for indoor use. In: Indoor air '93.
Proceedings of the 6th international conference on indoor air
quality and climate. Vol. 2. Chemicals in indoor air, material
emissions. Helsinki, 4-8 July 1993, pp. 419-424.
Brown KW, Donnelly KC (1988) An estimation of the risk associated with
the organic constituents of hazardous and municipal waste landfill
leachates. Hazardous waste & hazardous materials, 5:1-30.
Buccafusco RJ, Ells SJ, LeBlanc GA (1981) Acute toxicity of priority
pollutants to bluegill ( Lepomis macrochirus). Bulletin of
environmental contamination and toxicology, 26:446-452.
California Environmental Protection Agency (1992) PTEAM: Monitoring
of phthalates and PAHs in indoor and outdoor air samples in
Riverside, California. Final report, Vol. II. Prepared under
contract by Research Triangle Institute, Research Triangle Park, NC,
for Air Resources Board, Research Division (Contract No. A933-144).
Calley D, Autian J, Guess WL (1966) Toxicology of a series of
phthalate esters. Journal of pharmacy science, 55(2):158-162.
CMA (1984) Generation of environmental fate and effects data base on
14 phthalate esters. Summary report -- Environmental studies
-- Phase I. December 15, 1984. Washington, DC, Chemical
Manufacturers Association, Phthalate Esters Program Panel.
Coldham NG, Dave M, Sivapathasundaram S, McDonnell DP, Connor C, Sauer
MJ (1997) Evaluation of a recombinant yeast cell estrogen screening
assay. Environmental health perspectives, 105(7):734-742.
Dempsey CR, Oppelt ET (1993) Incineration of hazardous waste: a
critical review update. Air & Waste, 43:25-73.
Di Toro DM, Zarba CS, Hansen DJ, Berry WJ, Swartz RC, Cowan CE, Pavlou
SP, Allen HE, Thomas NA, Paquin PR (1991) Technical basis for
establishing sediment quality criteria for nonionic organic chemicals
using equilibrium partitioning. Environmental toxicology and
chemistry, 10:1541-1583.
DMER, AEL (1996) Pathways analysis of butyl benzyl phthalate for the
second Priority Substances List using fugacity modelling. Report
prepared for Chemicals Evaluation Division, Commercial Chemicals
Evaluation Branch, Environment Canada, by Don Mackay Environmental
Research, Peterborough, Ontario, and Angus Environmental Limited, Don
Mills, Ontario. March 1996.
Dueva LA, Aldyreva MV (1969) Experimental assessment of sensitizing
and irritating action of phthalate plasticizers. Gigiena Truda i
Professional'nye Zabolevaniya, 13(10):7-19 (in Russian with English
abstract).
ECPI (1996) Phthalates in the aquatic environment. Brussels,
European Council for Plasticisers and Intermediates.
Eiceman GA, Clement RE, Karasek FW (1979) Analysis of fly ash from
municipal incinerators for trace organic compounds. Analytical
chemistry, 51:2343-2350.
Eigenberg DA, Bozigian HP, Carter DE, Sipes IG (1986) Distribution,
excretion, and metabolism of butylbenzyl phthalate in the rat.
Journal of toxicology and environmental health, 17(4):445-456.
Ejlertsson J, Johansson E, Karlsson A, Meyerson U, Svensson BH (1996)
Anaerobic degradation of xenobiotics by organisms from municipal solid
waste under landfilling conditions. Antonie van Leeuwenhoek,
69:67-74.
Elsisi AE, Carter DE, Sipes IG (1989) Dermal absorption of phthalate
diesters in rats. Fundamental and applied toxicology, 12(1):70-77.
Ema M, Murai T, Itami T, Kawasaki H (1990) Evaluation of the
teratogenic potential of the plasticizer butyl benzyl phthalate in
rats. Journal of applied toxicology, 10(5):339-343.
Ema M, Itami T, Kawasaki H (1991a) Embryolethality of butyl benzyl
phthalate in rats [abstract]. The FASEB journal, 5(5):A1237.
Ema M, Itami T, Kawasaki H (1991b) Evaluation of the embryolethality
of butyl benzyl phthalate by conventional and pair-feeding studies in
rats. Journal of applied toxicology, 11(1):39-42.
Ema M, Itami T, Kawasaki H (1991c) Teratogenicity of butyl benzyl
phthalate in rats [abstract]. Teratology, 44(6):16B.
Ema M, Itami T, Kawasaki H (1992) Effect of period of exposure on the
developmental toxicity of butyl benzyl phthalate in rats. Journal of
applied toxicology, 12(1):57-61.
Ema M, Itami T, Kawasaki H (1993) Teratogenic phase specificity of
butyl benzyl phthalate in rats. Toxicology, 79(1):11-19.
Ema M, Kurosaka R, Amano H, Ogawa Y (1994) Embryolethality of butyl
benzyl phthalate during early pregnancy in rats. Reproductive
toxicology, 8(3):231-236.
Ema M, Kurosaka R, Amano H, Ogawa Y (1995) Comparative developmental
toxicity of n-butyl benzyl phthalate and di- n-butyl phthalate in
rats. Archives of environmental contamination and toxicology,
28(2):223-228.
Ema M, Harazono A, Miyawaki E, Ogawa Y (1996a) Developmental toxicity
of mono- n-benzyl phthalate, one of the major metabolites of the
plasticizer n-butyl benzyl phthalate, in rats. Toxicology letters,
86(1):19-25.
Ema M, Harazono A, Miyawaki E, Ogawa Y (1996b) Characterization of
developmental toxicity of mono- n-benzyl phthalate in rats.
Reproductive toxicology, 10(5):365-372.
Ema M, Kurosaka R, Harazono A, Amano H, Ogawa Y (1996c) Phase
specificity of developmental toxicity after oral administration of
mono- n-butyl phthalate in rats. Archives of environmental
contamination and toxicology, 31(2):170-176.
Ema M, Miyawaki E, Kawashima K (1998) Reproductive effects of butyl
benzyl phthalate in pregnant and pseudopregnant rats. Reproductive
toxicology, 12(2):127-132.
Erickson NG (1965) The metabolism of diphenyl phthalate and
butylbenzyl phthalate in the beagle dog. Dissertation abstracts,
26(5):3014-3015.
Etkin DS (1995) Chemical contaminants and their health effects. Indoor
air quality in schools. In: Indoor air quality update: A guide to
the practical control of indoor air problems. Arlington, MA, Cutter
Information Corporation, pp. 27-45.
Foster PMD (1997) Assessing the effects of chemicals on male
reproduction: lessons learned from di- n-butyl phthalate. CIIT
activities, 17(9):1-9.
Gaido KW, Leonard LS, Lovell S, Gould JC, Babai D, Portier CJ,
McDonnell DP (1997) Evaluation of chemicals with endocrine modulating
activity in a yeast-based steroid hormone receptor gene transcription
assay. Toxicology and applied pharmacology, 143(1):205-212.
Galloway SM, Armstrong MJ, Reuben C, Colman S, Brown B, Cannon C,
Bloom AD, Nakamura F, Ahmed M, Duk S, Rimpo J, Margolin BH, Resnick
MA, Anderson B, Zeiger E (1987) Chromosome aberrations and sister
chromatid exchanges in Chinese hamster ovary cells: evaluations of 108
chemicals. Environmental and molecular mutagenesis, 10 (Suppl.
10):1-175.
Gledhill WE, Kaley RG, Adams WJ, Hicks O, Michael PR, Saeger VW (1980)
An environmental safety assessment of butyl benzyl phthalate.
Environmental science and technology, 14:301-305.
Golder Associates (1987) Testing of specific organic compounds in
soils in urban areas. Port Credit and Oakville/Burlington, Ontario.
Draft working paper to Shell Canada Limited and Texaco Canada Limited,
Mississauga, Ontario.
Government of Canada (in press) Canadian Environmental Protection Act
Priority Substances List assessment report -- Butyl benzyl
phthalate. Priority Substances Program, Ottawa, Canada.
Graedel TE, Hawkins DT, Claxton LD (1986) Atmospheric chemical
compounds: Sources, occurrence, and bioassay. New York, NY, Academic
Press, Inc., Harcourt Brace Jovanovich Publishers.
Hagmar L, Akesson B, Nielsen J, Andersson C, Linden K, Attewell R,
Moller T (1990) Mortality and cancer morbidity in workers exposed to
low levels of vinyl chloride monomer at a polyvinyl choride processing
plant. American journal of industrial medicine, 17(5):553-565.
Haile CL, Stanley JS, Magin AM, Northcutt RV, Redford DP (1984)
Emissions of organic pollutants from coal-fired utility boiler plants.
In: Identification and analysis of organic pollutants in air.
Conference proceedings. Boston, MA, Lawrence H. Butterworth, pp.
443-458.
Hammond BG, Levinskas GJ, Robinson EC, Johannsen FR (1987) A review of
the subchronic toxicity of butyl benzyl phthalate. Toxicology and
industrial health, 3(2):79-98.
Hargesheimer EE, Lewis CM (1987) Comparative source water quality
monitoring at two surface reservoirs. In: Proceedings of the
American Water Works Association annual conference. Kansas City, MO,
14-18 June 1987, pp. 153-175.
Harris CA, Henttu P, Parker MG, Sumpter JP (1997) The estrogenic
activity of phthalate esters in vitro. Environmental health
perspectives, 105(8):802-811.
Hazleton Biotechnologies Company (1986) Mutagenicity of 1D in a
mouse lymphoma mutation assay. Final report. Submitted by Chemical
Manufacturers Association, Washington, DC, to Office of Toxic
Substances, US Environmental Protection Agency (Document
Identification No. 40-8626225; Microfiche No. OTS0510527).
Hazleton Laboratories (1958) Final report. Subacute feeding
-- Albino rats. Sponsored by Monsanto Chemical Company. Office of
Toxic Substances, US Environmental Protection Agency (Document No.
878213590; Microfiche No. 206416).
Health Canada (1994) Human health risk assessment for Priority
Substances. Canada Communications Group, Ottawa, Ontario.
Heitmuller PT, Hollister TA, Parrish PR (1981) Acute toxicity of 54
industrial chemicals to sheepshead minnows ( Cyprinodon variegatus).
Bulletin of environmental contamination and toxicology, 27:596-604.
Howard PH (1990) Handbook of environmental fate and exposure data
for organic chemicals. Vol. 1. Large production and priority
pollutants. Chelsea, MI, Lewis Publishers Inc.
Howard PH, Boethling RS, Jarvis WF, Meylan WM, Michalenko EM (1991)
Handbook of environmental degradation rates. Chelsea, MI, Lewis
Publishers Inc.
Howe RB (1995) THRESH: A computer program to compute a reference
dose from quantal animal toxicity data using the benchmark dose
method. Ruston, LA, ICF Kaiser Engineers, Inc.
Iannuzzi TJ, Huntley SL, Schmidt CW, Finley BL, McNutt RP, Burton SJ
(1997) Combined sewer overflows (CSOs) as sources of sediment
contamination in the lower Passaic River, New Jersey. I. Priority
pollutants and inorganic chemicals. Chemosphere, 34:213-231.
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs volumes 1 to 42. Lyon, International Agency for
Research on Cancer (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Supplement No. 7).
IPCS (1993) International Chemical Safety Card -- Butyl benzyl
phthalate. Geneva, World Health Organization, International
Programme on Chemical Safety (ICSC 0834).
Jobling S, Reynolds T, White R, Parker MG, Sumpter JP (1995) A variety
of environmentally persistent chemicals, including some phthalate
plasticizers, are weakly estrogenic. Environmental health
perspectives, 103:582-588.
Kincannon DF, Lin YS (1985) Microbial degradation of hazardous wastes
by land treatment. In: Proceedings of the 40th industrial waste
conference, Purdue University, West Lafayette, Indiana. Boston, MA,
Butterworths, pp. 607-619.
Kluwe W (1984) Comprehensive evaluation of the biological effects of
phthalate esters. Presented at the International Conference on
Phthalic Acid Esters, University of Surrey, Guildford, England [cited
in Woodward K (1988) Phthalate esters: Toxicity and metabolism. Vol.
1. Boca Raton, FL, CRC Press].
Kluwe WM, Maronpot RR, Lamb JC, Agarwal DK (1984) Adverse effects of
butyl benzyl phthalate on bone marrow and the male reproductive system
[abstract]. The Toxicologist, 4:136.
Kozumbo WJ, Kroll R, Rubin RJ (1982) Assessment of the mutagenicity of
phthalate esters. Environmental health perspectives, 45:103-109.
LeBlanc GA (1980) Acute toxicity of priority pollutants to water flea
( Daphnia magna). Bulletin of environmental contamination and
toxicology, 24:684-691.
LeBlanc GA (1984) Comparative structure-toxicity relationships between
acute and chronic effects to aquatic organisms. In: Kaiser KLE, ed.
QSAR in environmental toxicology. Proceedings of the Workshop on
Quantitative Structure-Activity Relationships (QSAR) in Environmental
Toxicology, held at McMaster University, Hamilton, Ontario, 16-18
August 1983. Boston, MA, D. Reidel Publishing Company, pp. 235-260.
Ligocki MP, Leuenberger C, Pankow JF (1985a) Trace organic compounds
in rain -- II. Gas scavenging of neutral organic compounds.
Atmospheric environment, 19:1609-1617.
Ligocki MP, Leuenberger C, Pankow JF (1985b) Trace organic compounds
in rain -- III. Particle scavenging of neutral organic compounds.
Atmospheric environment, 19:1619-1626.
Little KD, Little JR (1983) Investigation of Santicizer 160 (benzyl
butyl phthalate) as a potential allergen. St. Louis, MO, Washington
University School of Medicine. Report to Monsanto Company (Project No.
JH-81-302).
Litton Bionetics Inc. (1976) Mutagenicity evaluation of BIO-76-17
Santicizer 160. NB 259784. Final report. Submitted by Monsanto
Company, St. Louis, MO, to Office of Toxic Substances, US
Environmental Protection Agency (Document Identification No.
87-7800282; Microfiche No. OTS200290).
Litton Bionetics Inc. (1977) Mutagenicity evaluation of BIO-76-243
CP731 (Santicizer 160) in the mouse lymphoma assay. Final report.
Submitted by Monsanto Company, St. Louis, MO, to Office of Toxic
Substances, US Environmental Protection Agency (Document
Identification No. 87-7800282; Microfiche No. OTS200290).
Litton Bionetics Inc. (1985) Evaluation of 1D in the in vitro
transformation of BALB/3T3 cells assay. Final report. Submitted by
Chemical Manufacturers Association, Washington, DC, to US
Environmental Protection Agency (Document Identification No.
40+8526206; Microfiche No. OTS0509537).
Mackay D, Shiu WY, Ma KC (1995) Illustrated handbook of
physical-chemical properties and environmental fate for organic
chemicals -- Vol. IV. Oxygen, nitrogen, and sulfur containing
compounds. Boca Raton, FL, CRC Press, Inc., Lewis Publishers,
pp.-702-705 (ISBN 1-56670-035-3).
MAFF (1987) Survey of plasticiser levels in food contact materials
and in foods. The 21st report of the Ministry of Agriculture,
Fisheries and Food Steering Group on Food Surveillance / The Working
Party on Chemical Contaminants from Food Contact Materials / Sub Group
on Plasticisers. London, HMSO, 105 pp. (Food Surveillance Paper No.
21).
MAFF (1996a) Phthalates in food. Food Safety Directorate, Ministry
of Agriculture, Fisheries and Food, 9 pp. (Food Surveillance
Information Sheet No. 82).
MAFF (1996b) Phthalates in infant formulae. Food Safety Directorate,
Ministry of Agriculture, Fisheries and Food, 9 pp. (Food Surveillance
Information Sheet No. 83).
Mallette FS, von Haam E (1952) Studies on the toxicity and skin
effects of compounds used in the rubber and plastics industries. II.
Plasticizers. Archives of industrial hygiene and occupational
medicine, 6(3):231-236.
Martin F, inventor (1996) Top nail coat composition containing
cellulose esters. Almell Limited, 5 pp. (US Patent 5,512,273;
04-30-96). In: Chemical Abstracts (online) Accession No.
CA12426352355Y.
Meek MD, Clemons J, Wu ZF, Zacharewski TR (1996) Assessment of the
alleged estrogen receptor-mediated activity of phthalate esters. In:
17th annual meeting of the Society of Environmental Toxicology and
Chemistry, abstract book, p. 85 (Abstract 443).
Mikuriya H, Ikemoto I, Tanaka A (1988) Urinary metabolites
contributing to testicular damage induced by butylbenzyl phthalate.
Jikeikai medical journal, 35:403-409.
Milligan SR, Balasubramanian AV, Kalita JC (1998) Relative potency of
xenobiotic estrogens in an acute in vivo mammalian assay.
Environmental health perspectives, 106(1):23-26.
Monsanto Company (1978) Acute toxicity of Santicizer 160 (butyl
benzyl phthalate) to Daphnia magna in the presence of fulvic acid.
St. Louis, MO (Report No. ES-SS-78-11).
Monsanto Company (1980a) Report of a short-term (90-day) study in
rats with Santicizer 160 with cover memo. Study done under contract
by British Industrial Biological Research Association. Submitted by
Monsanto, St. Louis, MO, to Office of Toxic Substances, US
Environmental Protection Agency (Document Identification No.
878213602; Microfiche No. 206416).
Monsanto Company (1980b) 14-day algal bottle assay. Bioassay data
summary. St. Louis, MO.
Monsanto Company (1981a) Acute toxicity studies on S-160 using two
midge species as the test organisms. St. Louis, MO (SR-83-X-059).
Monsanto Company (1981b) Acute toxicity of Santicizer 160 to the
midge Paratanytarsus parthenogenetica. St. Louis, MO (Report No.
ES-81-SS-8).
Monsanto Company (1981c) Acute toxicity of GLP-1 (9AB981018) to
Chironomus tentans. Static acute bioassay. St. Louis, MO (Report
No. 27145).
Monsanto Company (1982a) Thirteen-week inhalation toxicity of
Santicizer 160 plasticizer vapor-aerosol to Sprague-Dawley rats with
cover memo. Submitted by Monsanto, St. Louis, MO, to Office of Toxic
Substances, US Environmental Protection Agency (Document
Identification No. 878213601; Microfiche No. OTS 206416).
Monsanto Company (1982b) Acute toxicity of Santicizer 160 to midge
(Paratanytarsus parthenogenetica). St. Louis, MO (Report No.
ES-82-SS-79).
Monsanto Company (1982c) Chronic toxicity of Santicizer 160 to midge
Daphnia magna : 21-day chronic renewal study. St. Louis, MO (Report
No. ES-82-SS-103).
Monsanto Company (1986a) 96-h flow-through toxicity study of
butylbenzyl phthalate to Hydra littoralis. St. Louis, MO, 13 pp.
(Final Flow-through Acute Toxicity Report No. 34168).
Monsanto Company (1986b) Acute toxicity of butylbenzyl phthalate to
polychaetes (Nereis/Neanthes virens ) under flow-through
conditions. St. Louis, MO, 28 pp. (Bionomics Report No.
BW-86-7-2094).
Monsanto Company (1986c) Acute toxicity of 14C-butylbenzyl
phthalate to eastern oysters (Crassostrea virginica ). St. Louis,
MO, 28 pp. (Report No. BW-86-7-2083).
Monsanto Company (1986d) Acute toxicity of butylbenzyl phthalate to
grass shrimp (Paleomonetes vulgaris ) under flow-through
conditions. St. Louis, MO, 28 pp. (Bionomics Report No.
BW-86-7-2087).
Monsanto Company (1986e) 96-h flow-through toxicity study of
butylbenzyl phthalate to the freshwater crayfish, Procambarus sp.
St. Louis, MO, 13 pp. (Final Flow-through Acute Toxicity Report No.
34166).
Monsanto Company (1986f) 96-h flow-through toxicity study of
butylbenzyl phthalate to the mayfly, Hexagenia sp. St. Louis, MO,
13 pp. (Final Flow-through Acute Toxicity Report No. 34167).
Monsanto Company (1986g) Early life stage toxicity of
14C-butylbenzyl phthalate to rainbow trout (Salmo gairdneri ) in a
flow-through system. St. Louis, MO, 31 pp. (Early Life Stage
Toxicity Final Report No. 33996).
Monsanto Company (1988) Acute toxicity of butylbenzyl phthalate to
mysid shrimp (Mysidopsis bahia ) under flow-through conditions. St.
Louis, MO, 31 pp. (Report No. 87-10-2525).
Monsanto Europe SA (1995a) Study to evaluate the effect of butyl
benzyl phthalate on uterine growth in immature female rats after
oral administration. Study conducted for Monsanto Europe SA by
Central Toxicology Laboratory, Cheshire, 15 pp. (Report No.
CTL/R/1280).
Monsanto Europe SA (1995b) Study to evaluate the effect of
monobenzyl phthalate on uterine growth in immature female rats after
oral administration. Study conducted for Monsanto Europe SA by
Central Toxicology Laboratory, Cheshire, 16 pp. (Report No.
CTL/R/1281).
Monsanto Europe SA (1996a) Study to evaluate the effect of butyl
benzyl phthalate on uterine growth in immature female rats after
subcutaneous administration. Study conducted for Monsanto Europe SA
by Central Toxicology Laboratory, Cheshire, 15 pp. (Report No.
CTL/R/1278).
Monsanto Europe SA (1996b) Study to evaluate the effect of monobutyl
phthalate on uterine growth in immature female rats after oral
administration. Study conducted for Monsanto Europe SA by Central
Toxicology Laboratory, Cheshire, 15 pp. (Report No. CTL/R/1279).
Müller J, Kördel W (1993) Occurrence and fate of phthalates in soil
and plants. The science of the total environment, Suppl. (Part
1):431-437.
Munro JR, Foster MG, Pawson T, Stelzig A, Tseng T, King L (1985) St.
Clair River point source survey, 1979-1980. Toronto, Ontario,
Ontario Ministry of the Environment/Environment Canada.194 pp.
Myhr BC, Caspary WJ (1991) Chemical mutagenesis at the thymidine
kinase locus in L1578Y mouse lymphoma cells: results for 31 coded
compounds in the National Toxicology Program. Environmental and
molecular mutagenesis, 18(1):51-83.
Myhr BC, Bowers LR, Caspary WJ (1986) Results from the testing of
coded chemicals in the L5178Y TK+/- mouse lymphoma mutagenesis
assay [abstract]. Environmental mutagenesis, 8 (Suppl. 6):58.
Nabholz JV (1987) The acute and chronic toxicity of dialkyl
phthalate esters to daphnids. Interagency memorandum to "whom it may
concern." Washington, DC, US Environmental Protection Agency, Health
and Environmental Review Division, Environmental Effects Branch,
Office of Toxic Substances (TS-796).
Nielsen J, Akesson B, Skerfving S (1985) Phthalate ester exposure
-- air levels and health of workers processing polyvinylchloride.
American Industrial Hygiene Association journal, 46(11):643-647.
NIWR (1996) Occurrence of phthalates and organotins in sediments and
water in Norway. Oslo, Norwegian Institute for Water Research
(Report No. 0-96006).
NPRI (1996) National Pollutant Release Inventory. Ottawa, Ontario,
Environment Canada.
NTP (1982) Carcinogenesis bioassay of butyl benzyl phthalate (CAS
No. 85-68-7) in F344/N rats and B6C3F1 mice (feed study). Research
Triangle Park, NC, National Institutes of Health, National Toxicology
Program (NTP Technical Report No. 213; National Technical Information
Service Publication No. PB83-118398).
NTP (1989) Developmental toxicity evaluation of butyl benzyl
phthalate (CAS No. 85-68-7) administered in feed to CD rats on
gestational days 6 to 15. Research Triangle Park, NC, National
Institutes of Health, National Toxicology Program (Report No.
NTP-89-246; National Technical Information Service Publication No.
PB90-115346).
NTP (1990) Final report on the developmental toxicity of butyl
benzyl phthalate (CAS No. 85-68-7) in CD-1-Swiss mice. Research
Triangle Park, NC, National Institutes of Health, National Toxicology
Program (NTP Report No. 90-114; National Technical Information Service
Publication No. PB91-129999).
NTP (1997a) Toxicology and carcinogenesis studies of butyl benzyl
phthalate (CAS No. 85-68-7) in F344/N rats (feed studies). Research
Triangle Park, NC, National Institutes of Health, National Toxicology
Program (NTP Technical Report No. 458; NIH Publication No. 97-3374).
NTP (1997b) Effect of dietary restriction on toxicology and
carcinogenesis studies in F344/N rats and B6C3F1 mice. Research
Triangle Park, NC, National Institutes of Health, National Toxicology
Program (NTP Technical Report No. 460; NIH Publication No. 97-3376).
OMOE (1988) Thirty-seven municipal pollution control plants. Pilot
monitoring study. Vol. 1. Interim report. Ontario Ministry of the
Environment, Municipal/Industrial Strategy for Abatement (MISA).
OMOE (1990) Second report on the monitoring data for the petroleum
refining sector. July 1990. Ontario Ministry of the Environment,
Municipal/Industrial Strategy for Abatement (MISA).
OMOE (1991) Organic chemical manufacturing sector twelve month
report. Data from October 1/89 to September 30/90. Ontario
Ministry of the Environment, Municipal/Industrial Strategy for
Abatement (MISA).
Oppelt ET (1987) Incineration of hazard waste. A critical review.
Journal of the Air Pollution Control Association, 37:558-586.
Ozretich RJ, Randall RC, Boese BL, Schroeder WP, Smith JR (1983) Acute
toxicity of butylbenzyl phthalate to shiner perch ( Cymatogaster
aggregata). Archives of environmental contamination and
toxicology, 12:655-660.
Page BD, Lacroix GM (1995) The occurrence of phthalate ester and
di-2-ethylhexyl adipate plasticizers in Canadian packaging and food
sampled in 1985-1989: a survey. Food additives and contaminants,
12(1):129-151.
Painter SE, Jones WJ (1990) Anaerobic bioconversion of phthalic acid
esters by natural inocula. Environmental technology, 11:1015-1026.
Pankow JF, Ligocki MP, Rosen ME, Isabelle LM, Hart KM (1988)
Adsorption/thermal desorption with small cartridges for the
determination of trace aqueous semivolatile organic compounds.
Analytical chemistry, 60:40-47.
Pickering QH (1983) Chronic toxicity to fathead minnow Pimephales
promelas of wastewater from a conventional wastewater treatment
system receiving organic priority pollutants. Environmental
pollution (Series A), 31:105-117.
Piersma AH, Verhoef A, Dortant PM (1995) Evaluation of the OECD 421
reproductive toxicity screening test protocol using butyl benzyl
phthalate. Toxicology, 99(3):191-197.
Plumb RH (1991) The occurrence of Appendix IX organic constituents in
disposal site ground water. Ground water monitoring review,
11:157-164.
Price CJ, Field EA, Marr MC, Myers CB, Morrissey RE, Schwetz BA (1990)
Developmental toxicity of butyl benzyl phthalate (BBP) in mice and
rats. Teratology, 41(5):586 (Abstract P51).
Randall RC, Ozretich RJ, Boese BL (1983) Acute toxicity of butyl
benzyl phthalate to the saltwater fish English sole, Parophrys
vetulus. Environmental science and technology, 17:670-672.
Rhodes JE, Adams WJ, Biddinger GR, Robillard KA, Gorsuch JW (1995)
Chronic toxicity of 14 phthalate esters to Daphnia magna and rainbow
trout ( Oncorhynchus mykiss). Environmental toxicology and
chemistry, 14:1967-1976.
Ritsema R, Cofino WP, Frintrop PCM, Brinkman UAT (1989) Trace-level
analysis of phthalate esters in surface water and suspended
particulate matter by means of capillary gas chromatography with
electron-capture and mass-selective detection. Chemosphere,
18:2161-2175.
Robinson EC (1991) Lack of neuropathological changes in rats after
exposure to butyl benzyl phthalate. Journal of toxicology and
environmental health, 32(3):345-347.
Rubin RJ, Kozumbo W, Kroll R (1979) Ames mutagenic assay of a series
of phthalic acid esters: positive response of the dimethyl and diethyl
esters in TA100 [abstract]. Toxicology and applied pharmacology, 48
(1 Part 2):A133.
Saeger VW, Tucker ES (1976) Biodegradation of phthalic acid esters in
river water and activated sludge. Applied environmental
microbiology, 31:29-34.
Sharpe RM, Fisher JS, Millar MM, Jobling S, Sumpter JP (1995)
Gestational and lactational exposure of rats to xenoestrogens results
in reduced testicular size and sperm production. Environmental
health perspectives, 103(12):1136-1143.
Shelton DR, Tiedje JM (1984) General method for determining anaerobic
biodegradation potential. Applied environmental microbiology,
47:850-857.
SIGMA (1985) Study of the characterization of wastes and discharges
from selected organic chemical plants. Draft report prepared by
SIGMA Resource Consultants Limited for Environmental Protection
Service, Environment Canada, March 1985 (SRCL 3479).
Skinner JP (1992) Final report on the safety assessment of butyl
benzyl phthalate. Journal of the American College of Toxicology,
11(1):1-23.
Soto AM, Sonnenschein C, Chung KL, Fernandez MF, Olea N, Serrano FO
(1995) The E-SCREEN assay as a tool to identify estrogens: an update
on estrogenic environmental pollutants. Environmental health
perspectives, 103 (Suppl. 7):113-122.
Springborn Bionomics (1984) Chronic toxicity of fourteen phthalate
esters to Daphnia magna. Toxicity test report submitted to Chemical
Manufacturers Association, Washington, DC (Report No. BW-84-5-1567).
Springborn Bionomics (1986a) Chronic toxicity of butylbenzyl
phthalate to mysid shrimp (Mysidopsis bahia ). Toxicity test report
submitted to Monsanto Company, St. Louis, MO (Report No.
BW-86-7-2074).
Springborn Bionomics (1986b) Acute toxicity of butylbenzyl phthalate
to pink shrimp (Penaeus duorarum ) under flow-through conditions.
Toxicity test report submitted to Monsanto Company, St. Louis, MO
(Report No. BW-86-7-2093).
Staples CA, Werner AF, Hoogheem TJ (1985) Assessment of priority
pollutant concentrations in the United States using STORET database.
Environmental toxicology and chemistry, 4:131-142.
Statsek NK (1974) Hygienic investigations of certain esters of
phthalic acid and of polyvinylchloride materials plastificated
thereby. Gigiena i Sanitariya, 39(6):25-28 [cited in US NLM (1994)
Hazardous substances data bank. Bethesda, MD, National Library of
Medicine, National Toxicology Information Program.].
Stein VB, Amin TA, Narang RS (1987) Simplified method for determining
polychlorinated biphenyls, phthalates, and hexachlorocyclohexanes in
air. Journal of the Association of Official Analytical Chemists,
70(4):721-723.
Suggatt RH, Foote K (1981) Comprehensive review of acute aquatic
toxicity data on phthalate esters. Final report. Syracuse, NY,
Syracuse Research Corporation (Contract SRC TR81-537) [cited in
Staples CA, Adams WJ, Parkerton TF, Gorsuch JW, Biddinger GR, Reinert
KH (1997) Aquatic toxicity of eighteen phthalate esters.
Environmental toxicology and chemistry, 16(5):875-891].
Sun K, Krause GF, Mayer FL, Ellersieck MR, Basu AP (1995) Predicting
chronic lethality of chemicals to fishes from acute toxicity test
data: theory of accelerated life testing. Environmental toxicology
and chemistry, 14:1745-1752.
Swain LG, Walton DG (1990) Report on the 1989 Boundary Bay
Monitoring Program. British Columbia Department of the Environment.
Tetra Tech Inc. (1986) Development of sediment quality values for
Puget Sound. Vol. 1. Bellevue, WA, 128 pp.
TNO Biotechnology and Chemistry Institute (1993) Dietary
one-generation reproduction study with butyl benzyl phthalate in
rats. Contract for Monsanto Company. Submitted by Monsanto to Office
of Toxic Substances, US Environmental Protection Agency (Document
Identification No. 86-930000189; Microfiche No. OTS0538169).
TNO Nutrition and Food Research Institute (1997) Protocol for an
oral developmental reproduction study with butyl benzyl phthalate in
Wistar rats, 21 pp. (Project No. 470839; Study No. 1899).
TRI93 (1995) Toxic chemicals release inventory. Bethesda, MD,
National Library of Medicine, National Toxicology Information Program.
Tucker MW, Mosher RG, Adams WJ (1985) Acute toxicity of S-160 (butyl
benzyl phthalate) to the freshwater green alga, Selenastrum
capricornutum. St. Louis, MO, Monsanto Company (Report No.
ESC-EAG-85-38).
US EPA (1995) The use of the benchmark dose approach in health risk
assessment. Office of Research and Development, US Environmental
Protection Agency (EPA/630/R-94/007).
US EPA (1996) EPA Superfund Record of Decision: Petoskey Municipal
Well Field Superfund Site, Petoskey, MI, 6/14/1995. US Environmental
Protection Agency (EPA/ROD/R05-95/274; PB95-964102).
Valencia R, Mason JM, Woodruff RC, Zimmering S (1985) Chemical
mutagenesis testing in Drosophila. III. Results of 48 coded
compounds tested for the National Toxicology Program. Environmental
mutagenesis, 7(3):325-348.
Veith GD, Macek KJ, Petrocelli SR, Carroll J (1980) An evaluation of
using partition coefficients and solubility to estimate
bioconcentration factors for organic chemicals in fish. In: Eaton JG,
Parrish PR, Hendricks AC, eds. Aquatic toxicology. Philadelphia, PA,
American Society for Testing and Materials, pp. 116-129 (ASTM STP
707).
Verschueren K (1983) Handbook of environmental data on organic
chemicals, 2nd ed. New York, NY, Van Nostrand Reinhold Co.
Vitali M, Guidotti M, Macilenti G, Cremisini C (1997) Phthalate esters
in freshwaters as markers of contamination sources -- a site study in
Italy. Environment international, 23(3):337-347.
Volskay VT, Grady CPL (1988) Toxicity of selected RCRA compounds to
activated sludge microorganisms. Journal of the Water Pollution
Control Federation, 60:1850-1856.
Volskay VT, Grady CPL, Tabak HH (1990) Effect of selected RCRA
compounds on activated sludge activity. Research journal of the
Water Pollution Control Federation, 62:654-664.
Webber MD, Wang C (1995) Industrial organic compounds in selected
Canadian soils. Canadian journal of soil science, 75(4):513-524.
Weschler CJ (1984) Indoor-outdoor relationships for nonpolar organic
constituents of aerosol particles. Environmental science and
technology, 18:648-652.
Wild SR, Jones KC (1992) Organic chemicals entering agricultural soils
in sewage sludges: screening for their potential to transfer to crop
plants and livestock. The science of the total environment,
119:85-119.
Ziegenfuss PS, Renaudette WJ, Adams WJ (1986) Methodology for
assessing the acute toxicity of chemicals sorbed to sediments: testing
the equilibrium partitioning theory. In: Poston TM, Purdy R, eds.
Aquatic toxicology and environmental fate. Vol. 9. Philadelphia, PA,
American Society for Testing and Materials, pp. 479-493 (ASTM STP
921).
Zeiger E, Haworth S, Speck W, Mortelmans K (1982) Phthalate ester
testing in the National Toxicology Program's Environmental Mutagenesis
Test Development Program. Environmental health perspectives,
45:99-101.
Zeiger E, Haworth S, Mortelmans K, Speck W (1985) Mutagenicity testing
of di(2-ethylhexyl)phthalate and related chemicals in Salmonella.
Environmental mutagenesis, 7(2):213-232.
Zurmühl T, Durner W, Herrmann R (1991) Transport of phthalate-esters
in undisturbed and unsaturated soil columns. Journal of contaminant
hydrology, 8:111-133.
APPENDIX 1 -- SOURCE DOCUMENTS
Government of Canada (in press)
Copies of the Canadian Environmental Protection Act Priority
Substances List assessment report (Government of Canada, in press)
and unpublished supporting documentation for BBP may be obtained from:
Commercial Chemicals Branch
Environment Canada
14th Floor, Place Vincent Massey
351 St. Joseph Blvd.
Hull, Quebec
Canada K1A 0H3
or
Environmental Health Centre
Health Canada
Address Locator: 0801A
Tunney's Pasture
Ottawa, Ontario
Canada K1A 0L2
Initial drafts of the supporting documentation and Assessment
Report for BBP were prepared by staff of Health Canada and Environment
Canada.
The environmental sections were reviewed externally by Dr G.
Coyle (Monsanto Company), Dr T. Parkerton (Exxon Biomedical Sciences
Inc.), Mr A. Sardella (Monsanto Canada), and Dr D. Spry (Ontario
Ministry of Environment and Energy).
Sections of the supporting documentation pertaining to human
health were reviewed externally by Dr R. Nair (Solutia Inc.) to
address adequacy of coverage. Accuracy of reporting, adequacy of
coverage, and defensibility of conclusions with respect to hazard
identification and dose-response analyses were considered in written
review by staff of the Information Department of BIBRA International
and at a panel meeting of the following members convened by Toxicology
Excellence for Risk Assessment on 27 April 1998 in Cincinnati, Ohio,
USA:
Dr M. Abdel-Raman, University of Medicine and Dentistry of New
Jersey
Dr J. Christopher, California Environmental Protection Agency
Dr G. Datson, Procter & Gamble Co.
Dr J. Donohue, US Environmental Protection Agency
Dr M. Dourson, Toxicology Excellence for Risk Assessment
Ms D. Proctor, ChemRisk
Ms R. Rudel, Silent Spring Institute (submitted written comments;
not available to attend panel meeting)
Dr A. Stern, New Jersey Department of Environmental Protection
APPENDIX 2 -- CICAD PEER REVIEW
The draft CICAD on BBP was sent for review to institutions and
organizations identified by IPCS after contact with IPCS national
Contact Points and Participating Institutions, as well as to
identified experts. Comments were received from:
Chemical Industry Institute of Toxicology (CIIT), Research
Triangle Park, USA
Department of Health, London, United Kingdom
Fraunhofer Institute of Toxicology and Aerosol Research, Hanover,
Germany
Health and Safety Executive, Bootle, United Kingdom
Health Canada, Ottawa, Canada
József Fodor National Center of Public Health, Budapest, Hungary
Karolinska Institute, Stockholm, Sweden
National Chemicals Inspectorate (KEMI), Solna, Sweden
National Food Administration, Uppsala, Sweden
National Institute for Working Life, Solna, Sweden
National Institute of Public Health, Oslo, Norway
National Institute of Public Health and Environmental Protection,
Bilthoven, The Netherlands
Nofer Institute of Occupational Medicine, Lodz, Poland
Norwegian University of Science and Technology, Trondheim, Norway
Mr Frank Sullivan, Consultant Toxicologist, Brighton, United
Kingdom
United States Department of Health and Human Services (Agency for
Toxic Substances and Disease Registry, Atlanta, USA; National
Institute of Environmental Health Sciences, Research Triangle
Park, USA)
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
Tokyo, Japan, 30 June - 2 July 1998
Members
Dr R. Benson, Drinking Water Program, United States Environmental
Protection Agency, Denver, CO, USA
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
Mr R. Cary, Health Directorate, Health and Safety Executive,
Merseyside, United Kingdom
Dr C. DeRosa, Agency for Toxic Substances and Disease Registry, Center
for Disease Control and Prevention, Atlanta, GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Cambridgeshire, United
Kingdom
Dr H. Gibb, National Center for Environmental Assessment, United
States Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany
Ms M.E. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada ( Chairperson)
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan ( Vice-Chairperson)
Professor S.A. Soliman, Department of Pesticide Chemistry, Alexandria
University, Alexandria, Egypt
Ms D. Willcocks, Chemical Assessment Division, Worksafe Australia,
Camperdown, Australia ( Rapporteur)
Professor P. Yao, Chinese Academy of Preventive Medicine, Institute of
Occupational Medicine, Beijing, People's Republic of China
Observers
Professor F.M.C. Carpanini,1 Secretary-General, ECETOC (European
Centre for Ecotoxicology and Toxicology of Chemicals), Brussels,
Belgium
1 Invited but unable to attend.
Dr M. Ema, Division of Biological Evaluation, National Institute of
Health Sciences, Osakai, Japan
Mr R. Green,1 International Federation of Chemical, Energy, Mine and
General Workers' Unions, Brussels, Belgium
Dr B. Hansen,1 European Chemicals Bureau, European Commission, Ispra,
Italy
Mr T. Jacob,1 Dupont, Washington, DC, USA
Dr H. Koeter, Organisation for Economic Co-operation and Development,
Paris, France
Mr H. Kondo, Chemical Safety Policy Office, Ministry of International
Trade and Industry, Tokyo, Japan
Ms J. Matsui, Chemical Safety Policy Office, Ministry of International
Trade and Industry, Tokyo, Japan
Mr R. Montaigne,1 European Chemical Industry Council (CEFIC),
Brussels, Belgium
Dr A. Nishikawa, Division of Pathology, National Institute of Health
Sciences, Tokyo, Japan
Dr H. Nishimura, Environmental Health Science Laboratory, National
Institute of Health Sciences, Osaka, Japan
Ms C. Ohtake, Chem-Bio Informatics, National Institute of Health
Sciences, Tokyo, Japan
Dr T. Suzuki, Division of Food, National Institute of Health Sciences,
Tokyo, Japan
Dr K. Takeda, Mitsubishikagaku Institute of Toxicological and
Environmental Sciences, Yokohama, Japan
Dr K. Tasaka, Department of Chemistry, International Christian
University, Tokyo, Japan
Dr H. Yamada, Environment Conservation Division, National Research
Institute of Fisheries Science, Kanagawa, Japan
Dr M. Yamamoto, Chem-Bio Informatics, National Institute of Health
Sciences, Tokyo, Japan
Dr M. Yasuno, School of Environmental Science, The University of Shiga
Prefecture, Hikone, Japan
1 Invited but unable to attend.
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Secretariat
Ms L. Regis, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Mr A. Strawson, Health and Safety Executive, London, United Kingdom
Dr P. Toft, Associate Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
Ce CICAD relatif au phtalate de butyle et de benzyle a été
préparé conjointement par la Direction de l'Hygične du Milieu (Santé
Canada) et par la Direction de l'Evaluation des Produits chimiques
commerciaux (Environnement Canada) d'aprčs la documentation préparée
parallčlement dans le cadre du Programme d'évaluation des substances
d'intéręt prioritaire mené en vertu de la Loi canadienne sur la
protection de l'environnement (LCPE). L'objectif de l'évaluation des
substances d'intéręt prioritaire en vertu de la LCPE est d'évaluer les
effets potentiels sur la santé humaine de l'exposition indirecte aux
substances présentes dans l'environnement, ainsi que les effets de ces
substances sur l'environnement. Les analyses ont porté sur les données
connues ŕ fin avril 1998. On trouvera ŕ l'appendice 1 des informations
sur les modalités de l'examen par des pairs et sur les sources
documentaires. Les renseignements concernant l'examen du CICAD par des
pairs font l'objet de l'appendice 2. Ce CICAD a été approuvé en tant
qu'évaluation internationale lors d'une réunion du Comité d'évaluation
finale qui s'est tenue ŕ Tokyo (Japon) du 30 juin au 2 juillet 1998.
La liste des participants ŕ cette réunion figure ŕ l'appendice 3. La
fiche d'information internationale sur la sécurité chimique (ICSC
No 0834) pour le phtalate de butyle et de benzyle, établie par le
Programme international sur la Sécurité chimique (IPCS, 1993) est
également reproduite dans ce document.
Le phtalate de butyle et de benzyle (No CAS 85-68-7), ou PBB,
est un liquide huileux limpide utilisé comme plastifiant
principalement dans le poly(chlorure de vinyle) (PVC) pour les
revętements de sol, les mousses de vinyle et les sous-couches de tapis
et, dans une moindre mesure, dans les plastiques cellulosiques et le
polyuréthane. La plupart des rejets se font dans l'air. Une fois dans
l'environnement, le PBB se répartit entre l'atmosphčre, le sol, les
eaux de surface, les sédiments et les biotes, et a été détecté dans
chacun de ces compartiments.
Le PBB est éliminé de l'atmosphčre par photo-oxydation et par la
pluie, avec une demi-vie de quelques heures ŕ quelques jours. En
aérobiose, il ne persiste pas dans l'eau, les sédiments ni les sols,
sa demi-vie étant de quelques jours. En anaérobiose, il est plus
persistant, avec une demi-vie de quelques mois. Les vertébrés et les
invertébrés le métabolisent facilement. Les facteurs de
bioconcentration signalés sont inférieurs ŕ 1000 si l'on tient compte
des résidus totaux, et sont bien inférieurs ŕ 100 d'aprčs les résidus
intacts.
Les données disponibles sur l'homme sont insuffisantes pour
servir de base ŕ l'évaluation des effets d'une exposition ŕ long terme
au PBB sur les populations humaines.
La toxicité aiguë du PBB est relativement faible, la DL50 orale
chez le rat étant supérieure ŕ 2 g/kg de poids corporel. Aprčs
exposition aiguë, les cibles principales sont le sang et le systčme
nerveux central.
Les données disponibles sont insuffisantes pour évaluer les
effets irritants et sensibilisants du PBB chez l'animal.
La toxicité du PBB aprčs administration de doses répétées a été
largement étudiée lors d'études récentes, principalement chez le rat,
espčce pour laquelle une relation dose-réponse a été bien
caractérisée. Les effets réguličrement observés consistaient en une
diminution de la prise de poids (souvent accompagnée d'une diminution
de la consommation alimentaire) et en une augmentation du rapport du
poids des organes (notamment du foie et des reins) au poids total. On
a également observé des effets histopathologiques sur le pancréas et
les reins et des effets hématologiques. Aux fortes doses, une
dégénérescence des testicules et parfois des effets histopathologiques
sur le foie ont été rapportés. Des investigations spécialisées ont
montré une prolifération des peroxysomes du foie, bien que le PBB ait
fait preuve dans cette étude d'une activité plus faible que les autres
phtalates, par exemple le phtalate de bis(2-éthylhexyle).
La toxicité chronique et la cancérogénicité du PBB ont été
étudiées lors d'essais biologiques de l'US National Toxicology Program
(NTP) chez le rat (avec des protocoles alimentaires standard et
restrictifs) et chez la souris. Il a été conclu qu'il y avait
"certaines preuves" de cancérogénicité chez le rat mâle, d'aprčs une
incidence accrue des tumeurs du pancréas, et des preuves non
concluantes chez les rats femelles d'aprčs une augmentation marginale
des tumeurs du pancréas et de la vessie. L'administration d'une
alimentation restreinte empęchait l'expression complčte des tumeurs du
pancréas et retardait l'apparition des tumeurs de la vessie. Aucune
preuve de cancérogénicité n'a été trouvée chez la souris.
Les résultats des études de génotoxicité portant sur le PBB sont
clairement négatifs. Cependant, les données disponibles sont
insuffisantes pour permettre de conclure formellement ŕ l'absence de
clastogénicité du PBB, bien que dans certaines études il ait induit au
maximum une activité clastogčne faible, d'une intensité compatible
avec des effets secondaires sur l'ADN.
En résumé, le PBB a induit une augmentation des tumeurs du
pancréas chez les animaux d'un sexe d'une espčce, effet dont
l'expression totale était empęchée par une restriction alimentaire, et
une augmentation marginale des tumeurs de la vessie chez les animaux
de l'autre sexe, effet retardé par la restriction alimentaire. En ce
qui concerne la génotoxicité, les résultats ont été négatifs et, bien
qu'on ne puisse exclure un faible potentiel clastogčne, les données
disponibles permettent de penser que le composé n'exerce pas
d'interaction directe avec l'ADN. D'aprčs ces résultats, le PBB peut
ętre considéré au maximum comme éventuellement cancérogčne pour
l'homme, susceptible d'induire des tumeurs par un mécanisme non
génotoxique (mais inconnu).
Dans diverses études, y compris celles portant sur les effets du
PBB sur les testicules et les hormones endocrines chez le rat mâle,
des études avec un protocole modifié d'accouplement réalisées par le
NTP, et une étude sur une seule génération, on n'a en général observé
d'effets indésirables sur les testicules et par conséquent sur la
fécondité qu'aux doses supérieures ŕ celles qui induisent des effets
sur les autres organes (comme le foie et les reins), bien qu'une
diminution du nombre de spermatozoďdes ait été observée ŕ des doses
analogues ŕ celles qui induisent des effets sur le rein et le foie.
Ces résultats sont compatibles avec ceux des études de toxicité
portant sur des doses répétées.
Une diminution du poids des testicules et de la production
quotidienne de spermatozoďdes chez la descendance a été observée ŕ une
dose relativement faible chez des rats exposés in utero et pendant
l'allaitement lors d'une étude dans laquelle la relation dose-réponse
n'était pas examinée. Cependant, de tels effets n'ont pas été observés
lors d'une étude récente de conception similaire mais non identique
réalisée sur une autre souche de rats, chez lesquels seule une
augmentation du poids relatif et absolu du foie a été observée
90 jours aprčs la naissance. Des investigations supplémentaires sur
les effets potentiels du composé sur le systčme reproducteur d'animaux
mâles et femelles exposés in utero et pendant l'allaitement dans le
cadre d'études portant également sur la relation dose-réponse sont
souhaitables, et sont en cours.
Bien que le PBB ait des effets estrogéniques dans des lignées de
cellules humaines de cancer du sein in vitro, les résultats en
cellules de levure sont peu concluants. Ni le PBB ni ses principaux
métabolites ne sont utérotrophiques in vivo chez le rat ou la
souris. Si les données disponibles ne permettent pas de conclure que
le PBB a des propriétés estrogéniques, d'autres effets potentiels ŕ
médiation endocrinienne, par exemple un effet anti-androgčne associé
au phtalate de dibutyle, ne sont pas exclus.
On s'intéresse actuellement ŕ la mise au point de cadres plus
sensibles d'essai et d'évaluation des substances perturbant
l'équilibre endocrinien; des composés comme les phtalates sont
susceptibles d'ętre parmi les premiers candidats ŕ soumettre ŕ des
essais supplémentaires.
Lors de plusieurs études bien conduites chez des rats et des
souris, le PBB a induit des effets notables sur le développement, mais
seulement ŕ des doses qui entraînent une toxicité significative chez
la mčre.
Bien que la neurotoxicité potentielle du PBB n'ait pas été
largement explorée, il n'a pas été observé d'effets histopathologiques
sur le systčme nerveux central et périphérique aprčs exposition ŕ
court terme ŕ des doses relativement élevées dans l'alimentation. Les
données disponibles sont insuffisantes pour permettre d'évaluer la
toxicité immunologique potentielle du PBB.
Une dose journaličre tolérable estimative (DJT) de 1300 µg/kg de
poids corporel par jour a été calculée pour le PBB, d'aprčs la limite
inférieure de confiance ŕ 95% pour la dose associée ŕ une augmentation
de 5% de l'incidence des lésions pancréatiques chez le rat mâle lors
d'un essai biologique subchronique par voie orale, divisée par un
facteur d'incertitude de 100 (10 pour la variation interspécifique et
10 pour la variation intraspécifique). D'aprčs les concentrations
rencontrées dans les divers milieux de l'environnement, il apparaît
(d'aprčs des estimations) que la totalité de l'apport estimé est
imputable ŕ l'alimentation; cet apport est évalué, pour la population
générale, ŕ 2-6 µg/kg de poids corporel par jour. Ces estimations sont
200 ŕ 650 fois plus faibles que la DJT. Les données sont insuffisantes
pour estimer l'exposition dans le milieu de travail ou par des
produits de consommation.
Divers tests de toxicité réalisés sur des organismes aquatiques
ont montré que des effets indésirables se produisent lors
d'expositions ŕ des concentrations supérieures ou égales ŕ
100 µg/litre. Comme les concentrations de PBB dans les eaux de surface
sont en général inférieures ŕ 1 µg/litre, il est probable que ce
composé comportera peu de risques pour les organismes aquatiques.
On ne dispose d'aucune information sur les effets du PBB sur les
organismes benthiques, les invertébrés du sol, les plantes terrestres
ou les oiseaux, qui permettrait d'estimer le risque pour ces
organismes.
RESUMEN DE ORIENTACION
Este CICAD sobre el butil-bencil-ftalato, preparado conjuntamente
por la Dirección de Higiene del Medio del Ministerio de Sanidad del
Canadá y la División de Evaluación de Productos Químicos Comerciales
del Ministerio de Medio Ambiente del Canadá, se basa en la
documentación preparada al mismo tiempo como parte del Programa de
Sustancias Prioritarias en el marco de la Ley Canadiense de
Protección del Medio Ambiente (CEPA). Las evaluaciones de sustancias
prioritarias prevista en la CEPA tienen por objeto valorar los efectos
potenciales para la salud humana de la exposición indirecta en el
medio ambiente general, así como los efectos ecológicos. En estos
exámenes se incluyen los datos identificados hasta el final de abril
de 1998. La información relativa al carácter del examen colegiado del
documento original y su disponibilidad figura en el apéndice 1. La
información sobre el examen colegiado de este CICAD aparece en el
apéndice 2. Este CICAD se aprobó como evaluación internacional en una
reunión de la Junta de Evaluación Final, celebrada en Tokio (Japón)
del 30 de junio al 2 de julio de 1998. La lista de participantes en
esta reunión figura en el apéndice 3. La ficha internacional de
seguridad química (ICSC 0834) para el butil-bencil-ftalato, preparada
por el Programa Internacional de Seguridad de las Sustancias Químicas
(IPCS, 1993), también se reproduce en este documento.
El butil-bencil-ftalato (CAS No 85-68-7) o BBP es un líquido
oleoso transparente que se utiliza como plastificante sobre todo en el
cloruro de polivinilo (PVC) para la fabricación de baldosas de vinilo,
espumas de vinilo y entramado para alfombras y en menor medida también
en los plásticos de celulosa y el poliuretano. La mayor parte del que
se libera en el medio ambiente va al aire. Una vez en el medio
ambiente, el BBP se distribuye entre la atmósfera, el suelo, el agua
superficial, los sedimentos y la biota, y se ha detectado en cada uno
de estos compartimentos.
El BBP se elimina de la atmósfera por fotooxidación y por el agua
de lluvia, con una semivida que oscila entre algunas horas y varios
días. No es persistente en el agua, los sedimentos o el suelo en
condiciones aerobias, con una semivida de varios días. En condiciones
anaerobias, el BBP es más persistente, siendo su semivida de varios
meses. Los vertebrados e invertebrados lo metabolizan fácilmente. Se
han notificado factores de bioconcentración inferiores a 1000, tomando
como base los residuos totales, y muy por debajo de 100 a partir de
los residuos de BBP intactos.
Los datos disponibles en el ser humano son insuficientes para
poder evaluar los efectos de la exposición prolongada al BBP en
poblaciones humanas.
La toxicidad aguda del BBP es relativamente baja, con valores de
la DL50 por vía oral superiores a 2 g/kg de peso corporal en ratas.
Los órganos afectados tras la exposición aguda son el sistema
hematológico y el sistema nervioso central.
Los datos disponibles son insuficientes para evaluar los efectos
irritantes y sensibilizantes del BBP en especies de animales.
En estudios recientes se ha investigado a fondo la toxicidad de
dosis repetidas de BBP, particularmente en la rata, en la cual está
bien caracterizada la relación dosis-respuesta. Los efectos observados
han sido siempre una disminución del aumento del peso corporal (con
frecuencia acompańada de una reducción del consumo de alimentos) y un
incremento de la razón peso de los órganos/peso corporal,
especialmente para el rińón y el hígado. Se han observado asimismo
efectos en el páncreas y el rińón, así como hematológicos. Con dosis
más elevadas, se han notificado efectos degenerativos en los
testículos y, ocasionalmente, efectos histopatológicos en el hígado.
En investigaciones especializadas se ha observado proliferación de
peroxisomas en el hígado, aunque la potencia a este respecto fue
inferior a la de otros ftalatos, como el bis(2-etilhexil)ftalato
(DEHP).
Se han investigado la toxicidad crónica y la carcinogenicidad del
BBP en biovaloraciones realizadas por el Programa Nacional de
Toxicología de los Estados Unidos de América (con inclusión de
protocolos normales y de alimentación limitada) en ratas y en ratones.
Se llegó a la conclusión de que había "algunos indicios" de
carcinogenicidad en ratas macho, basados en una mayor incidencia de
tumores pancreáticos, e indicios equívocos en ratas hembra, basadas en
un aumento marginal de la incidencia de tumores pancreáticos y de
vejiga. La limitación de la alimentación impidió la expresión completa
de los tumores pancreáticos y retrasó la aparición de los tumores de
vejiga. No hubo indicios de carcinogenicidad en ratones.
El valor demostrativo de los indicios de genotoxicidad del BBP es
claramente negativo. Sin embargo, los datos disponibles son
insuficientes para llegar a la conclusión inequívoca de que el BBP no
es clastogénico, aunque en determinados estudios ha inducido, como
máximo, una actividad débil de magnitud compatible con los efectos
secundarios en el ADN.
Por consiguiente, el BBP ha inducido un aumento de los tumores
pancreáticos fundamentalmente en un sexo de una especie, cuya
expresión completa se evitó mediante un protocolo de alimentación
limitada, y un aumento marginal de los tumores de vejiga en el otro
sexo, que se retrasó con la limitación de la alimentación. El valor
demostrativo de los indicios de genotoxicidad es negativo y, aunque no
se puede descartar el potencial clastogénico, los datos disponibles
son compatibles con el hecho de que el compuesto no tiene una
interacción directa con el ADN. De acuerdo con esto, el BBP puede
considerarse, como máximo, posiblemente carcinogénico para el ser
humano, probablemente induciendo tumores a través de un mecanismo no
genotóxico (aunque desconocido).
En una serie de estudios, en particular los diseńados para
investigar los efectos reproductivos del BBP en los testículos y las
hormonas endocrinas de las ratas macho, un protocolo modificado de
acoplamiento realizado por el NTP y un estudio de una generación, en
general se han observado efectos adversos en los testículos y, por
consiguiente, en la fecundidad sólo con dosis superiores a las que
inducen efectos en otros órganos (como el rińón y el hígado), si bien
se ha puesto de manifiesto una reducción en el recuento de
espermatozoides con dosis semejantes a las que inducen efectos en el
rińón y el hígado. Esto está en consonancia con los resultados de los
estudios de toxicidad con dosis repetidas.
En un estudio en el que no se investigó la relación
dosis-respuesta se observó una reducción del peso de los testículos y
de la producción diaria de espermatozoides en crías de ratas expuestas
a una concentración relativamente baja en el útero y durante la
lactancia. Sin embargo, estos efectos no se observaron en un estudio
reciente de diseńo parecido, pero no idéntico, realizado con otra
estirpe de ratas en la cual sólo se observó un aumento del peso
absoluto y relativo del hígado a los 90 días del nacimiento. Son
convenientes, y se han emprendido ya, nuevas investigaciones de los
efectos potenciales en el sistema reproductor de animales machos y
hembras expuestos en el útero y durante la lactancia en estudios
encaminados a examinar la relación dosis-respuesta.
Si bien el BBP ha sido estrogénico en líneas de células de cáncer
de mama humano in vitro, los resultados en células de levadura han
sido contradictorios. Ni el BBP ni sus principales metabolitos han
sido uterotróficos in vivo en ratas o ratones. Aunque los datos
disponibles no permiten llegar a la conclusión de que el BBP es
estrogénico, no se pueden descartar otros posibles efectos debidos a
factores endocrinos, como la actividad antiandrogénica asociada al
dibutil-ftalato (DBP).
En la actualidad se concede una importancia considerable a la
creación de sistemas más sensibles de prueba y evaluación de
sustancias perturbadoras del sistema endocrino; compuestos como los
ftalatos probablemente serán de los primeros que se someterán a
pruebas adicionales.
En varios estudios bien realizados en ratas y ratones, el BBP
indujo efectos pronunciados en el desarrollo, pero sólo a
concentraciones que producen una toxicidad materna significativa.
Aunque no se ha investigado bien la posible neurotoxicidad del
BBP, no se han observado efectos histopatológicos en los sistemas
nerviosos central y periférico tras una exposición breve a
concentraciones relativamente altas en los alimentos. Los datos
disponibles son insuficientes para evaluar la posible inmunotoxicidad
del BBP.
Se ha estimado para el BBP una ingesta diaria tolerable (IDT)
muestral de 1300 µg/kg de peso corporal al día. Se basa en un límite
de confianza inferior del 95% para la dosis de referencia asociada con
un aumento del 5% de la incidencia de lesiones pancreáticas en ratas
macho en una biovaloración subcrónica por vía oral, dividido por un
factor de incertidumbre de 100 (10 por la variación interespecífica y
10 por la intraespecífica). Teniendo en cuenta las concentraciones en
diversos medios parece (a partir de estimaciones muestrales) que los
alimentos son la fuente de toda la ingesta estimada, que se considera
que oscila, para la población general, entre 2 y 6 µg/kg de peso
corporal al día. Estas estimaciones son 200-650 veces inferiores a la
IDT. Los datos fueron insuficientes para estimar la exposición en el
entorno ocupacional o a partir de productos de consumo.
En una serie de pruebas de toxicidad con organismos acuáticos se
ha puesto de manifiesto que se producen efectos adversos a
concentraciones de exposición iguales o superiores a 100 µg/l. Habida
cuenta de que la concentración en el agua superficial suele ser
inferior a 1 µg/l, es probable que el BBP represente un riesgo bajo
para los organismos acuáticos.
No se dispone de información acerca de los efectos del BBP en los
organismos de los sedimentos, los invertebrados del suelo, las plantas
terrestres o las aves en la que pueda basarse una estimación del
riesgo para estos organismos.
1. EXECUTIVE SUMMARY
This CICAD on butyl benzyl phthalate was prepared jointly by the
Environmental Health Directorate of Health Canada and the Commercial
Chemicals Evaluation Division of Environment Canada based on
documentation prepared concurrently as part of the Priority Substances
Program under the Canadian Environmental Protection Act (CEPA). The
objective of assessments on Priority Substances under CEPA is
to assess potential effects of indirect exposure in the general
environment on human health as well as environmental effects. Data
identified as of the end of April 1998 were considered in these
reviews. Information on the nature of the peer review and availability
of the source document is presented in Appendix 1. Information on the
peer review of this CICAD is presented in Appendix 2. This CICAD was
approved as an international assessment at a meeting of the Final
Review Board, held in Tokyo, Japan, on 30 June - 2 July 1998.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 0834) for butyl benzyl
phthalate, produced by the International Programme on Chemical Safety
(IPCS, 1993), has also been reproduced in this document.
Butyl benzyl phthalate (CAS No. 85-68-7), or BBP, is a clear,
oily liquid that is used as a plasticizer mainly in polyvinyl chloride
(PVC) for vinyl floor tile, vinyl foams, and carpet backing and to a
minor extent also in cellulose plastics and polyurethane. Most
environmental release is to the air. Once in the environment, BBP
partitions to the atmosphere, soil, surface water, sediments, and
biota and has been detected in each of these compartments.
BBP is removed from the atmosphere by photooxidation and by
rainwater, with a half-life of a few hours to a few days. BBP is not
persistent in water, sediments, or soil under aerobic conditions, with
a half-life of a few days. Under anaerobic conditions, BBP is more
persistent, with a half-life of a few months. BBP is readily
metabolized by vertebrates and invertebrates. Reported
bioconcentration factors (BCFs) are less than 1000 based on total
residues and well under 100 based on intact BBP residues.
Available data in humans are inadequate to serve as a basis for
assessment of the effects of long-term exposure to BBP in human
populations.
The acute toxicity of BBP is relatively low, with oral LD50
values in rats being greater than 2 g/kg body weight. Target organs
following acute exposure include the haematological and central
nervous systems.
Available data are inadequate to assess the irritant and
sensitizing effects of BBP in animal species.
The repeated-dose toxicity of BBP has been well investigated in
recent studies, primarily in the rat, in which dose-response was well
characterized. Effects observed consistently have been decreases in
body weight gain (often accompanied by decreases in food consumption)
and increases in organ to body weight ratios, particularly for the
kidney and liver. Histopathological effects on the pancreas and kidney
and haematological effects have also been observed. At higher doses,
degenerative effects on the testes and, occasionally,
histopathological effects on the liver have been reported. In
specialized investigations, peroxisomal proliferation in the liver has
been observed, although potency in this regard was less than that for
other phthalates, such as bis(2-ethylhexyl) phthalate (DEHP).
The chronic toxicity and carcinogenicity of BBP have been
investigated in US National Toxicology Program (NTP) bioassays in rats
(including standard and feed-restricted protocols) and mice. It was
concluded that there was "some evidence" of carcinogenicity in male
rats, based on an increased incidence of pancreatic tumours, and
equivocal evidence in female rats, based on marginal increases in
pancreatic and bladder tumours. Dietary restriction prevented full
expression of the pancreatic tumours and delayed appearance of the
bladder tumours. There was no evidence of carcinogenicity in mice.
The weight of evidence of the genotoxicity of BBP is clearly
negative. However, available data are inadequate to conclude
unequivocally that BBP is not clastogenic, although in identified
studies it has induced, at most, weak activity of a magnitude
consistent with secondary effects on DNA.
Therefore, BBP has induced an increase in pancreatic tumours
primarily in one sex of one species, the full expression of which was
prevented in a dietary restriction protocol, and a marginal increase
in bladder tumours in the other sex, which was delayed upon dietary
restriction. The weight of evidence of genotoxicity is negative, and,
although weak clastogenic potential cannot be ruled out, available
data are consistent with the compound not interacting directly with
DNA. On this basis, BBP can be considered, at most, possibly
carcinogenic to humans, likely inducing tumours through a
non-genotoxic (although unknown) mechanism.
In a range of studies, including those designed to investigate
the reproductive effects of BBP on the testes and endocrine hormones
of male rats, a modified mating protocol conducted by the NTP, and a
one-generation study, adverse effects on the testes and, consequently,
fertility have generally been observed only at doses higher than those
that induce effects on other organs (such as the kidney and liver),
although decreases in sperm counts have been observed at doses similar
to those that induce effects in the kidney and liver. This is
consistent with the results of repeated-dose toxicity studies.
Reductions in testes weight and daily sperm production in
offspring were reported at a relatively low level in rats exposed in
utero and during lactation in a study in which dose-response was not
investigated. However, such effects were not observed in a recent
study of similar, but not identical, design in another strain of rats
in which only increases in absolute and relative liver weights were
observed at postnatal day 90. Additional investigation of potential
effects on the reproductive systems of male and female animals exposed
in utero and during lactation in studies designed to address
dose-response is desirable and is under way.
Although BBP has been estrogenic in human breast cell cancer
lines in vitro, results in yeast cells have been mixed. Neither BBP
nor its principal metabolites have been uterotrophic in vivo in rats
or mice. Although available data do not support the conclusion that
BBP is estrogenic, other potential endocrine-mediated effects such as
anti-androgenic activity associated with dibutyl phthalate (DBP) are
not precluded.
There is considerable emphasis currently on development of more
sensitive frameworks for testing and assessment of
endocrine-disrupting substances; compounds such as phthalates are
likely early candidates for additional testing.
In several well-conducted studies in rats and mice, BBP has
induced marked developmental effects, but only at dose levels that
induce significant maternal toxicity.
Although the potential neurotoxicity of BBP has not been well
investigated, histopathological effects on the central and peripheral
nervous systems have not been observed following short-term exposure
to relatively high dietary concentrations. Available data are
inadequate to assess the potential immunotoxicity of BBP.
A sample tolerable daily intake (TDI) of 1300 µg/kg body weight
per day has been derived for BBP. It is based upon the lower 95%
confidence limit for the benchmark dose associated with a 5% increase
in the incidence of pancreatic lesions in male rats in an oral
subchronic bioassay divided by an uncertainty factor of 100 (10 for
interspecies variation and 10 for intraspecies variation). Based upon
concentrations in various environmental media, it appears (from sample
estimates) that food contributes all of the estimated intake, which is
considered, for the general population, to range from 2 to 6 µg/kg
body weight per day. These estimates are 200-650 times less than the
TDI. Data were inadequate to estimate exposure in the occupational
environment or from consumer products.
A range of toxicity tests with aquatic organisms has indicated
that adverse effects occur at exposure concentrations equal to or
greater than 100 µg/litre. As concentrations in surface waters are
generally less than 1 µg/litre, it is likely that BBP poses low risk
to aquatic organisms.
No information about the effects of BBP on sediment-dwelling
organisms, soil invertebrates, terrestrial plants, or birds has been
identified on which to base an estimate of risk to these organisms.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
The physical and chemical properties of BBP have been summarized
by Skinner (1992). BBP (CAS No. 85-68-7) is an aromatic ester that
conforms to the formula C19H20O4. Synonyms include
1,2-benzenedicarboxylic acid, butyl phenylmethyl ester, and benzyl
n-butyl phthalate. BBP is a clear, oily liquid at room temperature
with a molecular weight of 312.4 g/mol. Reported log octanol/water
partition coefficients (log Kow) range from 3.6 to 5.8; 4.91 is a
measured value, whereas the 4.77 provided in the International
Chemical Safety Card is an estimated value. Additional physical/
chemical properties are presented in the International Chemical Safety
Card reproduced in this document.
3. ANALYTICAL METHODS
Analytical methods have been reviewed by Skinner (1992). BBP may
be analysed by gas chromatography/mass spectrometry and by
high-performance liquid chromatography. It is determined in water by
an enrichment procedure using sequential reverse osmosis, followed by
extraction and analysis by gas chromatography/mass spectrometry, or
after adsorption onto Tenax material and thermal desorption to a fused
silica capillary gas chromatography column under whole-column
cryotrapping conditions (Pankow et al., 1988). In air, BBP has been
determined by liquid chromatographic separation using a florisil
adsorbent and 10% 2-propanol in hexane as the eluent followed by gas
chromatographic analysis with detection by 63Ni electron capture
(Stein et al., 1987). BBP can be determined in mixtures of
semivolatile organic pollutants using gas chromatography/Fourier
transform infrared spectroscopy with wall-coated open tubular
capillary columns. Combining this technique with gas
chromatography/mass spectrometry allows for better and faster
identification of the components of complex mixtures of environmental
pollutants.
In reports of analyses of environmental media for BBP, detection
limits were 1 µg/litre for samples of drinking-water (G. Halina,
personal communication, 1994; method not specified) and 0.2 mg/kg dry
weight for soil (gas chromatography/mass spectrometry; Webber & Wang,
1995). Detection limits for analyses (by gas chromatography/flame
ionization detection) of table-ready foods were 0.5 µg/g (butter), 0.2
µg/g (vegetables and fruits), and 0.1 µg/g (meat and fish) (Page &
Lacroix, 1995). The detection limits varied with the fat content of
the food and food matrix interferences.
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
BBP is manufactured by the reaction of the monobutyl ester of
phthalic acid with benzyl chloride (Skinner, 1992). In the USA,
Monsanto Company is the sole manufacturer of BBP (Anon., 1996). BBP is
used mainly as a plasticizer in PVC for vinyl floor tile, vinyl foams,
and carpet backing. Other polymers plasticized with BBP include
cellulose plastics, polyvinyl acetate, polysulfides, and polyurethane.
Consumption of BBP in Europe is approximately 18 000-45 000 tonnes per
year (Harris et al., 1997).
BBP is released from facilities that manufacture the substance or
blend it with PVC (Howard, 1990). Releases may also occur through
diffusion of BBP from PVC products.
Total on-site environmental releases of BBP reported to the
Canadian National Pollutant Release Inventory by 11 facilities using
BBP amounted to 3.7 tonnes in 1994, all to the atmosphere. Total
transfers of BBP for off-site disposal were much higher, amounting to
33.3 tonnes in 1994, with 25.1 tonnes going to incinerators and the
remainder, 8.2 tonnes, to landfill. A reported total of 3.7 tonnes of
BBP was sent for recovery in 1994, 2.3 tonnes for energy recovery and
1.4 tonnes for recovery, reuse, or recycling (NPRI, 1996).
In the USA, it was estimated that manufacturing facilities
released approximately 176 tonnes to the environment in 1993, with
about 99% released to the atmosphere (TRI93, 1995).
BBP may be released to air through automobile emissions and from
combustion of refuse (Graedel et al., 1986). It has also been detected
in stack emissions from hazardous waste combustion facilities and from
coal-fired power plants in the USA (Oppelt, 1987). Reasonable
worst-case emissions of BBP from incinerators, boilers, and industrial
furnaces burning such wastes were predicted to be 3 µg/m3 waste gas
(Dempsey & Oppelt, 1993). In a study of four US coal-fired utility
boiler plants, the emission rates for BBP in flue gases ranged from
210 to 3400 mg/h (Haile et al., 1984). BBP was identified, but not
quantified, in extracts of municipal incinerator fly ash from the
Netherlands, but it was not detected in extracts from Japan or Ontario
(Eiceman et al., 1979).
In leachate from municipal landfills in the USA, BBP was
detected, but not quantified (Brown & Donnelly, 1988). BBP has also
been detected (detection limits not reported) in groundwater at
disposal sites in the USA (Plumb, 1991). BBP was also detected in 2 of
44 groundwater samples at a Superfund site in Michigan, USA, at
estimated concentrations of 0.6 and 1.0 µg/litre (US EPA, 1996).
In Canada, BBP has been detected in storm sewer effluents at
concentrations up to 50 µg/litre (Hargesheimer & Lewis, 1987) and in
effluents from municipal sewage treatment plants and industrial plants
at concentrations up to 25 µg/litre (Munro et al., 1985; SIGMA, 1985;
OMOE, 1988, 1990, 1991).1 BBP has also been detected in sludges from
Canadian sewage treatment plants at concentrations up to 914 498 ng/g
dry weight (OMOE, 1988).
BBP can be emitted from products containing the substance. For
example, BBP has been detected in emissions from carpets (Bayer &
Papanicolopoulos, 1990), PVC floorings (Bremer et al., 1993), and
vinyl wall coverings (Etkin, 1995), although quantitative data were
not identified. It is also a component of some consumer products, such
as nail polish (Martin, 1996). The possibility that toys made of
plastic might contain BBP is currently being investigated, although
quantitative data are not yet available.
1 Additional data from ENVIRODAT, Surveys and Information Systems
Branch, Environment Canada, 1993.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Fugacity modelling was based upon the assumption of continuous
emissions of 1000 kg/h to air, water, or soil (DMER & AEL, 1996). Most
environmental releases of BBP are to the atmosphere. The Level III
calculations of the EQC fugacity model predict that when BBP is
emitted into air, approximately 72% is found in soil, 22% in air, 4%
in water, and 2% in sediment. When BBP is emitted into water, 65% is
found in water, while about 35% partitions to sediment and a very
small fraction to soil. When BBP is released to soil, more than 99% is
found in the soil. Values for input parameters were as follows:
molecular weight, 312.4 g/mol; vapour pressure, 0.001 15 Pa; water
solubility, 2.69 mg/litre; melting point, -35°C; and log Kow, 4.9.
Average degrading reaction half-lives were assumed to be 55 h in air,
170 h in water, 550 h in soil, and 1700 h in sediment (Mackay et al.,
1995). The calculated organic carbon/water partition coefficient (log
Koc) is 4.51 (based on the correlation Koc = 0.41 Kow), and
the Henry's law constant is 0.133 Pa.m3/mol at 25°C.
Photooxidation is the most important process for the breakdown of
BBP in the atmosphere (Atkinson, 1987). Howard et al. (1991) estimated
a half-life in air of 6-60 h for BBP based on photooxidation rates.
BBP is also readily removed from air by rain (Ligocki et al., 1985a).
BBP is readily biodegraded in aerobic surface water, with a
half-life of about 1-7 days (Saeger & Tucker, 1976; Gledhill et al.,
1980; Howard et al., 1991; Adams & Saeger, 1993). Biodegradation is
considerably slower in cold water, as BBP was almost completely
biodegraded after 7 days in Rhine River water at 20°C but was not
biodegraded in the same water after 10 days at 4°C (Ritsema et al.,
1989). BBP is expected to adsorb to suspended matter, sediments, and
biota.
Biodegradation is the most important degradation pathway in
sediments (Gledhill et al., 1980; Adams & Saeger, 1993). In a river
water/sediment microcosm, the degradation pathway appeared to be
BBP -> monobutyl/monobenzyl phthalate -> phthalic acid ->
4,5-dihydroxyphthalic acid -> oxalic acid -> formic acid -> carbon
dioxide (Adams et al., 1986, 1989; Adams & Saeger, 1993). The
half-life for complete mineralization of BBP in this study was 13 days
(Adams & Saeger, 1993). BBP can also be biodegraded in sediment under
anaerobic conditions (Shelton & Tiedje, 1984; Painter & Jones, 1990;
Ejlertsson et al., 1996), with an estimated half-life of about 1 day
to 6 months (Howard et al., 1991).
Biodegradation of BBP occurs readily in aerobic soils, with a
half-life of about 1-7 days at room temperature (Howard et al., 1991).
It is also biodegraded in anaerobic soils. For the removal of BBP in a
silt loam, Kincannon & Lin (1985) determined a half-life of 59.2 days.
BBP sorbs to soil, so soil leaching should not be significant (Zurmhhl
et al., 1991).
With reported log Kow values ranging from 3.6 to 5.8, BBP
would appear to have a high potential for bioaccumulation. However,
reported BCFs in oysters, microorganisms, and several species of fish
are less than 1000, because BBP is readily metabolized, with a
depuration half-life of less than 2 days (Barrows et al., 1980; Veith
et al., 1980). The highest reported BCF was 776 for bluegill
( Lepomis macrochirus) (Veith et al., 1980).
Based on physical/chemical properties of BBP, Wild & Jones (1992)
predicted that retention of the substance by root surfaces of plants
would be high, but that subsequent uptake by plants would be low. This
prediction was confirmed by Müller and Kördel (1993), who demonstrated
that plants grown on phthalate-enriched soil did not take up BBP from
the soil through the roots. However, plants exposed to
phthalate-treated dust did take up BBP through leaf cuticles
(quantitative data not available).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
In air samples from Greater Vancouver, British Columbia, Canada,
BBP was detected at concentrations ranging from 0.38 to 1.78 ng/m3
(W. Belzer, personal communication, 1997). Concentrations of BBP in
air up to 9.6 ng/m3 (in the aerosol phase at Portland, Oregon, USA)
have been reported (Ligocki et al., 1985a,b). BBP has been identified
in ambient air in Barcelona, Spain; concentrations of 1.0 and 8.0
ng/m3 in winter and 0.25 and 2.0 ng/m3 in summer, associated with
coarse (>7.2 µm) and fine (<0.5 µm) aerosol fractions, respectively,
have been reported (Aceves & Grimalt, 1993).
In Canadian surface waters, BBP was detected at concentrations up
to 1 µg/litre.1 Gledhill et al. (1980) reported a concentration of
2.4 µg/litre in the Mississippi River south of St. Louis, Missouri,
USA. In central Italy, BBP was detected at concentrations up to
6.6 µg/litre in Lake Scandarello (Vitali et al., 1997). In water
samples collected from the Rhine River and its tributaries, levels
ranged up to 5.2 µg/litre (ECPI, 1996). In samples of inflow and
outflow from sewage treatment plants in Sweden and Norway,
concentrations of BBP up to 2.4 µg/litre and 0.58 µg/litre,
respectively, were reported (ECPI, 1996; NIWR, 1996).
BBP has been reported in marine sediments from British Columbia,
Canada, at concentrations up to 370 ng/g dry weight (Axys Analytical
Services Limited, 1992; D. Goyette, personal communication, 1993).
Outside Canada, the highest reported concentration of BBP in sediments
was 3800 ng/g dry weight, in sediments from the Lower Passaic River,
Newark, New Jersey, USA, adjacent to combined sewer overflow outfalls
(Iannuzzi et al., 1997).
In limited surveys of soils from agricultural and typical urban
residential and parkland locations in Canada, concentrations of BBP
were less than 0.3 µg/g (Golder Associates, 1987; Webber & Wang,
1995).
At a lime disposal area of a refinery in Regina, BBP
concentrations in soil of 0.15 and 0.55 µg/g were reported.2 In soil
in the neighbourhood of three phthalate-emitting plants in Germany
from 1986 to 1989, the highest concentration in an individual sample
was 100 µg/kg; the highest mean value for a single site was 30 µg/kg
(Müller & Kördel, 1993).
1 Data from ENVIRODAT, Surveys and Information Systems Branch,
Environment Canada, 1993.
2 Letter from D. Fast, Saskatchewan Department of Environment and
Public Safety, to Senes Consultants Limited, Richmond Hill, Ontario,
1989.
In Canadian biota, BBP has been detected at concentrations up to
1470 ng/g wet weight (in butter sole, Isopsetta [ Pleuronectes]
isolepis, from Boundary Bay, British Columbia; Swain & Walton,
1990). BBP was detected in US biota at 3% of 182 STORET stations, with
a median concentration of <2500 ng/g (Staples et al., 1985).
6.2 Human exposure
In 125 homes in California, USA, two 12-h indoor air samples were
collected during daytime and overnight periods. In indoor air, median
daytime and nighttime concentrations were 34 and 35 ng/m3,
respectively. In a subset of 65 homes, outdoor air samples were also
collected. In outdoor air, the median (for both daytime and nighttime
sampling) was below the method quantifiable limit of 5.1 ng/m3; the
90th percentiles were 5.3 and 6.7 ng/m3 for daytime and nighttime
sampling, respectively (California Environmental Protection Agency,
1992). In an early study, BBP concentrations of 1 and 20 ng/m3 were
reported in office air at two locations in the USA, although the
compound was not detected (detection limit not reported) in ambient
air (Weschler, 1984).
In surveys of drinking-water primarily from surface water
supplies conducted at over 300 sites in two provinces in Canada
between 1985 and 1994, BBP was detected in only one sample in 1991
(2.8 µg/litre; limits of detection 1-3 µg/litre) (D. Spink, personal
communication, 1986; G. Halina, personal communication, 1994; A.
Riopel, unpublished data, 1994, 1996).
Of approximately 100 foodstuffs (generally single composite
samples from four supermarkets) purchased in Ontario, Canada, in 1985
and 1988 in a total diet study, BBP was detected only in yoghurt
(0.6 µg/g), cheddar cheese (1.6 µg/g), butter (0.64 µg/g), and
crackers (0.48 µg/g) (detection limits ranged from 0.005 to 0.5 µg/g;
Page & Lacroix, 1995).
In foods purchased at retail stores in the United Kingdom and
stored in their packaging until their "best before" date, BBP was not
detected in chocolate or sugar confectioneries, although it was
detected in baked savouries (1.5 mg/kg), meat pies (4.8 mg/kg), and
sandwiches (14 mg/kg) (MAFF, 1987). In stored samples of composite
fatty foods in a total diet study in the United Kingdom, BBP was
detected in carcass meat (0.09 mg/kg), poultry (0.03 mg/kg), eggs
(0.09 mg/kg), and milk (0.002 mg/kg) (MAFF, 1996a). Concentrations in
59 individual samples of 15 different brands of infant formula from
retail outlets in five towns across the United Kingdom ranged from
<0.004 to 0.25 mg/kg (MAFF, 1996b).
An example of indirect exposure in the general environment is
presented here. Exposure of the general population to BBP in
environmental media may be estimated based upon concentrations
determined in various media and reference values for body weight and
consumption patterns. Owing to the availability of relevant data,
exposure has been estimated based primarily upon data from Canada.
However, countries are encouraged to estimate total exposure on the
basis of national data, possibly in a manner similar to that outlined
here. Indeed, estimates based on the data on concentrations in
foodstuffs determined in the United Kingdom presented above would be
higher than those provided as examples here.
Although concentrations of BBP in air (both ambient and indoor),
drinking-water, and soil have been reported, they are so low that
intakes from these routes are essentially negligible. Estimates of
exposure for the general population are based almost entirely upon the
estimates for intake from food. The estimates presented here are based
upon identified concentrations for foodstuffs in Canada, as well as
assumed concentrations of zero or method detection limits for foods in
which BBP was not identified (minimum and maximum estimates,
respectively). Adults are assumed to breathe 15.8 m3 of air per day
(Allan, 1995), weigh 70 kg, drink 1.4 litres of water per day, ingest
20 mg soil per day, and consume, on a daily basis, 13.61 g butter,
3.81 g processed cheddar cheese, 1.54 g yoghurt, 22.73 g fresh pork,
and 3.45 g crackers (Health Canada, 1994). Estimated intake for adults
is 2 µg/kg body weight per day; intake values for infants and children
are up to threefold higher. Data are inadequate to estimate intake in
breast-fed infants.
Identified data on concentrations of BBP in the occupational
environment are inadequate as a basis for estimation of exposure.
Similarly, data are inadequate for estimation of exposure from
consumer products, although it should be noted that inclusion of
information on levels in indoor air in the estimates of exposure for
the general population presented here should account at least
partially for exposure from consumer products.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND
HUMANS
No data were identified concerning the absorption, metabolism, or
elimination of BBP in humans.
Based upon a limited number of studies (Erickson, 1965; Kluwe,
1984; Eigenberg et al., 1986; Mikuriya et al., 1988; Elsisi et al.,
1989) conducted principally in rats following oral administration, BBP
is readily hydrolysed in the gastrointestinal tract and the liver to
the corresponding monobutyl or benzyl ester. These phthalate
monoesters are then rapidly eliminated (90% in 24 h) in the excreta in
ratios of approximately 80% in urine and 20% in faeces, although
results of one study indicate that the fraction eliminated in faeces
increases at higher doses (of approximately 2 g/kg body weight)
(Eigenberg et al., 1986). The monobutyl ester is generally present in
highest amounts; for example, the ratio of monobutyl to monobenzyl
phthalate in rats in one study was 5:3 (Mikuriya et al., 1988).
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
The acute toxicity of BBP is relatively low. Reported oral LD50
values for rats range from 2 to 20 g/kg body weight (NTP, 1982;
Hammond et al., 1987); a dermal LD50 of 6.7 g/kg in both rats and
mice has been reported (Statsek, 1974). Clinical signs at or near
lethal doses following oral exposure of rats included weight loss,
apathy, and leukocytosis. Histological examination revealed toxic
splenitis and degenerative lesions of the central nervous system with
congestive encephalopathy, myelin degeneration, and glial
proliferation.
8.2 Irritation and sensitization
No tests have been conducted according to validated international
protocols.
Exposure to BBP did not cause immediate or delayed
hypersensitivity in mice in a series of studies following
administration of an initiating dose either intraperitoneally or by
application to the abdomen or footpad and a challenging dose to the
dorsal side of the ear up to 15 days later. Similarly, there was no
immediate or delayed hypersensitivity in guinea-pigs initiated on the
footpad and receiving a challenge dose on shaved abdominal skin. BBP
did not form a detectable amount of hapten-protein complex when
assayed with bovine serum albumin, although results were equivocal
following intradermal initiation and challenge (24 h later) of mice
with serum from BBP-exposed (intraperitoneally) mice (Little & Little,
1983).
Data reported in accounts of early studies on the irritancy of
BBP, the results of which were inconsistent, are inadequate for
evaluation (Dueva & Aldyreva, 1969; Hammond et al., 1987). Calley et
al. (1966) injected BBP intradermally into the backs of rabbits,
followed by trypan blue into the ear vein. Moderate irritation was
indicated by extravasated trypan blue at the site of administration.
8.3 Short-term exposure
In short-term investigations by the oral route (excluding those
addressing specifically reproductive effects or peroxisomal
proliferation, which are addressed elsewhere) for which the range of
end-points examined was often limited, consistent effects on body
weight gain in rats were observed at doses of approximately 1000 mg/kg
body weight per day and above, sometimes accompanied by a decrease in
food consumption (NTP, 1982; Hammond et al., 1987). Although some
effects on body weight gain were observed at lower doses, the pattern
was inconsistent. In one study, minimal testicular changes were
observed in one of six male rats at 480 mg/kg body weight per day
(Bibra, 1978; Hammond et al., 19871). In general, though, testicular
effects were observed only at much higher doses (i.e., atrophy at 1600
mg/kg body weight per day; Hammond et al., 1987). In a 6-week
investigation, there were no adverse histopathological effects on the
nervous system of rats exposed to 3000 mg/kg body weight per day,
although reversible clinical signs were observed (Robinson, 1991).
In an inhalation study in rats, there were no effects upon
haematology, urinalysis, blood chemistry, or histopathology following
exposure for 4 weeks to 144 mg/m3 (Hammond et al., 1987). At 526
mg/m3, effects included reduced body weight gain and reduced serum
glucose. In a similar study, exposure to 2100 mg/m3 resulted in death
of some animals; effects after 4 weeks included reduced body weight
gain and atrophy of the spleen and testes (Hammond et al., 1987).
8.4 Long-term exposure
Detailed descriptions of the protocol and effect levels are
presented here for critical studies only. Experimental details and
effect levels for all key subchronic and chronic studies via ingestion
are provided in Table 1.
8.4.1 Subchronic exposure
The protocol of an early study in Charles River rats included
examination of body weight, clinical signs, organ weight, and limited
histopathology of liver, spleen, kidney, adrenal, stomach, and small
and large intestine (Hazleton Laboratories, 1958). The only effect
observed was a decrease in body weight gain in males at the highest
dose (1253 mg/kg body weight per day).
In a range-finding NTP subchronic (13-week) dietary bioassay in
F344 rats, the only adverse effects observed upon examination of body
weight and clinical signs and histopathological examination of control
and high-dose animals were depressed weight gain and testicular
degeneration (nature of degeneration not specified) at the highest
dose (1250 mg/kg body weight per day) (NTP, 1982).
Sprague-Dawley rats were administered BBP in the diet for 3
months at dose levels of 0, 188, 375, 750, 1125, or 1500 mg/kg body
weight per day in males and females (Hammond et al., 1987). End-points
examined included body weight gain, haematology, urinalysis, and
histopathology (control and high-dose groups only). No
compound-related lesions were observed at necropsy or upon
histopathological examination. In females, the increase in liver to
body weight ratio was significant at 750 mg/kg body weight per day and
higher; in males, the increase was significant at 1125 mg/kg body
1 Full study reports for several of the investigations described
therein were available to the authors.
weight per day and higher. No change occurred in kidney to body weight
ratio in females, but there was a significant increase in males at 750
mg/kg body weight per day and higher.
A subchronic dietary study was also conducted in Wistar rats
(Monsanto Company, 1980a; Hammond et al., 1987) at dose levels of 0,
151, 381, or 960 mg/kg body weight per day in males and 0, 171, 422,
or 1069 mg/kg body weight per day in females for 3 months. Intake of
BBP, based on body weight and food consumption, was calculated at
4-day intervals throughout the study. Observations included slight
anaemia in males at the highest dose and decreased urinary pH in males
at the mid and high doses. At the highest dose, no reduction in food
consumption was apparent, suggesting that the reduced body weight gain
in those groups may have been compound related. Liver to body weight
ratio was significantly increased at all dose levels in females and at
the highest dose in males. A significant increase in kidney to body
weight ratio occurred in a dose-related manner in both sexes at the
mid and high doses. The caecum to body weight ratio was unaffected in
males but increased at all dose levels in females in a dose-related
manner. Gross pathological lesions were limited to increased incidence
of red spots on the liver of mid- and high-dose males.
Histopathological lesions of the pancreas were observed in males at
the mid and high doses and included islet enlargement with cell
vacuolization and peri-islet congestion. The liver of high-dose males
had small areas of cellular necrosis. No histopathological lesions
were described for females. The lowest-observed-adverse-effect level
(LOAEL) is 381 mg/kg body weight per day, based upon histopathological
effects in the pancreas in males. The lowest-observed-effect level
(LOEL) in females is 171 mg/kg body weight per day, based upon
increases in organ to body weight ratio at all doses for the liver and
caecum (the no-observed-effect level, or NOEL, in males is 151 mg/kg
body weight per day).
In a 6-month dietary study in male F344 rats (NTP, 1997a),
effects on haematological parameters were reported at 550 mg/kg body
weight per day. Only transitory changes in haematological parameters
were reported at 180 mg/kg body weight per day.
In a 3-month dietary study in dogs (Hammond et al., 1987),
decreases in body weight gain were associated with decreases in food
consumption at the highest dose (1852 and 1973 mg/kg body weight per
day for males and females, respectively).
In a 90-day study in mice, there were decreases in body weight
gain at 208 mg/kg body weight per day and greater in males, although
no histopathological effects were observed and food consumption was
not reported (NTP, 1982). End-points included clinical observations,
body weight, and histopathology (control and high dose).
One subchronic inhalation bioassay was identified, in which
groups of 25 male or 25 female Sprague-Dawley rats were exposed to
concentrations of 0, 51, 218, or 789 mg/m3 for 6 h/day, 5 days/week,
for a total of 59 exposures. End-points examined were limited to organ
weight changes and histopathological examination of control and
high-dose groups (Monsanto Company, 1982a; Hammond et al., 1987). A
LOEL of 218 mg/m3 was reported for male rats, based upon increases in
kidney weight, measured at interim sacrifice only, although no
dose-related histopathological changes were observed in any group. The
NOEL was 51 mg/m3.
8.4.2 Chronic exposure and carcinogenicity
A carcinogenicity bioassay was conducted by the NTP (1982) in
F344 rats. Fifty rats per sex per group were administered BBP via the
diet, at levels of 0, 6000, or 12 000 ppm (0, 300, and 600 mg/kg body
weight per day,1 respectively). Females were exposed for 103 weeks.
Because of poor survival, all males were sacrificed at weeks 29-30;
this part of the study was later repeated (NTP, 1997a).
Only females were examined histopathologically. The incidence of
mononuclear cell leukaemias was increased in the high-dose group
( P = 0.011); the trend was significant ( P = 0.006). (Incidences
for the control, low-dose, and high-dose groups were 7/49, 7/49, and
18/50, respectively.) The incidence in the high-dose group and the
overall trend remained significant ( P = 0.008 and P = 0.019,
respectively) when compared with historical control data. The NTP
concluded that BBP was "probably carcinogenic for female F344/N rats,
causing an increased incidence of mononuclear cell leukemias" (NTP,
1982).
However, these results were not repeated in the 2-year dietary
study in F344/N rats recently completed by the NTP (1997a). The
average daily doses (reported by the authors) were 0, 120, 240, or 500
mg/kg body weight per day for males and 0, 300, 600, or 1200 mg/kg
body weight per day for females. The protocol included periodic
haematological evaluation and hormonal assays and a 15-month interim
sacrifice.
There were no differences in survival between exposed groups and
their controls. A mild decrease in triiodothyronine concentration in
the high-dose females at 6 and 15 months and at termination was
considered to be related to a non-thyroidal disorder. Changes in
haematological parameters were sporadic and minor. In this bioassay,
there was no increase in the incidence of mononuclear cell leukaemia
in female rats, as was reported in the earlier bioassay (NTP, 1982),
although the level of exposure (600 mg/kg body weight per day) at
which the incidence was observed in the early bioassay was common to
both studies.
1 Conversion factor; 1 ppm in food = 0.05 mg/kg body weight per day
(Health Canada, 1994).
Table 1: Effect levels in subchronic, chronic, reproductive, and developmental studies by the ingestion route.
Study protocol Effect levela Critical end-point/comments Reference
Subchronic exposure
Charles River rats LOAEL (males) = 1253 mg/kg Limited range of end-points, Hazleton
(10/sex/group) body weight per day including body weight, food Laboratories, 1958
90 days NOEL (females) = 1270 mg/kg consumption, organ weight, and
median intake body weight per day histopathological examination of
in diet: (highest doses administered) only seven organs/tissues,
males: 0, 447, or excluding the testes.
1253 mg/kg body weight
females: 0, 462, or
1270 mg/kg body weight
F344/N rats LOAEL (males) = 1250 mg/kg body Histopathological degeneration of NTP, 1982
(10/sex/group) weight per day the testes and depressed weight
13 weeks NOEL = 625 mg/kg body weight gain. End-points examined were
approximate intake in per day restricted to clinical
diet: 0, 80, 155, 315, observations, body weight gain,
625, or 1250 mg/kg body and histopathological observation
weight per day of control and high-dose animals.
Sprague-Dawley rats LOEL (females) = 750 mg/kg Significant increases in liver to Hammond et al., 1987
(10/sex/group) body weight per day body weight ratio (females) and
3 months NOEL = 375 mg/kg body kidney to body weight ratio
approximate intake in weight per day (males). No histopathological
diet: 0, 188, 375, 750, changes.
1125, or 1500 mg/kg
body weight per day
Wistar rats LOAEL (males) (pancreas) = 381 Histopathological changes in Monsanto Company,
(27-45/sex/group) mg/kg body weight per day the pancreas in males from 1980a;
(15-27 exposed for LOEL (females) = 171 mg/kg body the two highest dose groups. Hammond et al.,
entire period) weight per day (lowest dose Increases in organ to body 1987
3 months administered) weight ratio at all doses
Table 1 (continued)
Study protocol Effect levela Critical end-point/comments Reference
approximate intake for the liver (females) and
in diet: caecum (females). No
males: 0, 151, 381, histopathological effects in either
or 960 mg/kg body of these organs at higher doses in
weight per day the same sex.
females: 0, 171, 422,
or 1069 mg/kg body
weight per day
Male F344 rats LOEL = 550 mg/kg body weight Effects on haematological parameters NTP, 1997a
(15/group) 26 weeks per day (significant increase in mean cell
approximate intake NOAEL = 180 mg/kg body weight haemoglobin and mean cell
in diet for four per day haemoglobin concentration) and
lowest doses: 0, 30, increased relative liver weight.
60, 180, or 550 mg/kg Transitory changes only in
body weight per day haematological parameters at the
lower dose (NOAEL).
Beagle dogs (3 males NOAEL (males) = 1852 mg/kg Decreases in body weight gain at Hammond et al., 1987
and 3 females) body weight per day the highest doses associated with
3 months NOAEL (females) = 1973 mg/kg decreases in food consumption.
approximate intake in body weight per day (Body weight increased but remained
diet or by capsule (highest doses administered) depressed in relation to controls
(high-dose group): during 2 months of administration
males: 0, 400, 1000, by capsule.)
or 1852 mg/kg body
weight per day
females: 0, 700, 1270,
or 1973 mg/kg body
weight per day
Table 1 (continued)
Study protocol Effect levela Critical end-point/comments Reference
B6C3F1 mice LOEL (males) = 208 mg/kg Decreases in body weight (of NTP, 1982
(10/sex/group) body weight per day unspecified statistical
13 weeks LOEL (females) = 1625 mg/kg significance), but no
approximate intake body weight per day histopathological effects;
in diet: 0, 208, 403, food consumption was not
819, 1625, or 3250 reported. End-points examined
mg/kg body weight restricted to body weight gain,
per day clinical observations, and
histopathology in control
and high-dose groups.
Chronic exposure
F344/N rats LOEL (females) = 300 mg/kg Increased nephropathy (the latter NTP, 1997a
(60/sex/group) body weight per day observed at all dose levels
2 years LOEL (males) = 120 mg/kg in females). Relative kidney weight
approximate intake in body weight per day increased at all doses in males at
diet: interim sacrifice (not determined
males: 0, 120, 240, at terminal sacrifice). At high
or 500 mg/kg body dose, increase in severity of renal
weight per day tubular pigmentation in both sexes.
females: 0, 300, 600,
or 1200 mg/kg body
weight per day
B6C3F1 mice LOEL = 780 mg/kg Decrease in body weight gain NTP, 1982
(50/sex/group) body weight per day (unspecified statistical
103 weeks significance). End-points
approximate intake examined restricted to clinical
in diet: 0, 780, or signs, body weight, and
1560 mg/kg body weight histopathology.
per day
Table 1 (continued)
Study protocol Effect levela Critical end-point/comments Reference
Reproductive/developmental studies
RIVM-bred WU rats LOAEL = 1000 mg/kg body weight At top dose, reduction in body weight Piersma et al.,
(10/sex/group) per day gain, fluctuation in food consumption, 1995
14 days prior to and NOAEL = 500 mg/kg body weight reduction in weight of testis and
throughout mating per day epididymis, and increase in testicular
(OECD 421 - combined degeneration (males). At top dose,
reproductive/developmental decrease in body weight gain and
toxicity screening protocol) effects on food consumption; adverse
gavage in corn oil: 0, 250, effects on reproductive indices
500, or 1000 mg/kg body (females). The only effect observed at
weight per day 500 mg/kg body weight per day was a
transient decrease (day 1) in
pup weight.
Male F344 rats LOAEL = 312.5 mg/kg body weight Dose-related increases in relative Kluwe et al., 1984;
(10/group) per day weights of kidney and liver at all Agarwal et al., 1985
14 days doses; increase in absolute kidney
approximate intake in diet: weight at two lowest doses, and
0, 312.5, 625, 1250, or decrease in absolute kidney weight
2500 mg/kg body weight at two highest doses. Proximal
per day tubular regeneration and
histopathological changes in the
thymus were also observed at all
dose levels; however, latter was
minimal and not considered dose
related. Histopathological effects
on the liver, testes, epididymis,
seminal vesicles, and prostate were
observed only at higher concentrations.
Table 1 (continued)
Study protocol Effect levela Critical end-point/comments Reference
Male F344/N rats NOEL = 20 mg/kg body weight Significant dose-related decrease in NTP, 1997a
(15/group) per day epididymal spermatozoal concentration
10 weeks prior to mating LOAEL = 200 mg/kg body weight at the two highest dose levels.
(modified mating protocol) per day Histopathological evidence of
approximate intake in diet: (it should be noted that hypospermia and a decrease in fertility
0, 20, 200, or 2200 mg/kg dose spacing was poor in index were observed at the highest dose
body weight per day the study) only (2200 mg/kg body weight per day).
Wistar rats (12 males, Reproductive effects: No effects on reproductive performance
24 females per group) NOAEL (males) = 418 mg/kg body and development of offspring.
one-generation reproductive weight per day TNO Biotechnology and
study approximate intake NOAEL (females) = 446 mg/kg body Chemistry Institute,
in diet: weight per day 1993
males: 0, 108, 206, or (highest doses administered)
418 mg/kg body weight per day
females: 0, 106, 217, or Parental effects: At top dose, significant increase in
446 mg/kg body weight NOEL (males) = 418 mg/kg body relative weight of livers and
per day weight per day decrease in food consumption
NOAEL (females) = 217 mg/kg and body weight (females).
body weight per day
LOEL (females) = 446 mg/kg
body weight per day
Examination of effects on Reduction in testes weight Dose-response was not investigated. Sharpe et al., 1995
the testes of offspring of and daily sperm production;
female Wistar rats (number effects were not replicated
unspecified) administered in the study of similar design
0 or 1000 µg BBP/litre in in another strain of rats
drinking-water (estimated by Ashby et al. (1997a)
to be approximately 126-366
µg/kg body weight per day)
for 2 weeks prior to mating,
throughout mating and
gestation, and until 22
days after giving birth
Table 1 (continued)
Study protocol Effect levela Critical end-point/comments Reference
Examination of effects on Reversible increase in absolute and Dose-response was not investigated. Ashby et al., 1997a
reproductive systems of relative liver weight at postnatal
male and female offspring day 90 in male offspring
of female Alpk:APfSD rats
(n = 19) exposed during
gestation and lactation
to 0 or 1000 µg BBP/litre
in drinking-water (estimated
to be 183 µg/kg body weight
per day)
Sprague-Dawley rats NOAEL (maternal and offspring) An increase in the percentage of NTP, 1989; Price et
(30 females/group) = 420 mg/kg body weight per day fetuses with variations per litter. al., 1990
gestational days 6-15 LOAEL (significant maternal and Maternal toxicity was evident at the
approximate intake in diet: minimal developmental effects) mid and high dose levels (decreased
0, 420, 1100, or 1640 mg/kg = 1100 mg/kg body weight per day maternal weight gain, increased
body weight per day relative liver weight, increased food
and water consumption).
Swiss albino mice (30 NOAEL (maternal and Increased percentage of late fetal NTP, 1990; Price et
females/group) gestational developmental) = 182 mg/kg body deaths per litter and non-live al., 1990
days 6-15 weight per day implants per litter, decreased number
approximate intake in diet: LOAEL (maternal and developmental) of live fetuses per litter, increased
0, 182, 910, or 2330 mg/kg = 910 mg/kg body weight per day percentage of litters with malformed
body weight per day fetuses, and an increased percentage
of malformed fetuses per litter.
Decreased maternal weight gain at two
highest doses; increased relative
kidney and liver weight in mothers
at top dose.
Table 1 (continued)
Study protocol Effect levela Critical end-point/comments Reference
Wistar rats (15-19 LOAEL (maternal) = 654 mg/kg Significant dose-related reduction Ema et al., 1990
females/group) body weight per day in maternal body weight gain and
gestational days 0-20 NOEL (embryo/fetal toxicity) reduced food consumption at three
approximate intake in = 654 mg/kg body weight per day highest doses (significant only at
diet: 180, 375, 654, Significant reduction in the two highest doses when adjusted
or 974 mg/kg body number of live fetuses per for gravid uterus). Additional
weight per day litter at 375 and 654 mg/kg studies conducted by these
body weight per day investigators in which effects
(administration on days 0-20 in offspring were examined
in study of unconventional design) following administration of
(maternal LOEL) doses greater than 500 mg/kg
NOEL (reproduction) = 185 mg/kg body weight per day either in
body weight per day the diet or by gavage during
various periods of gestation are
not additionally informative with
respect to effect levels.
Peroxisomal proliferation
F344 rats (5/sex/group) LOEL (males) = 639 mg/kg Increase in relative liver BIBRA, 1985
21 days body weight per day and kidney weights in males
approximate intake LOEL (females) = 679 mg/kg and females; increase in
in diet: body weight per day cyanide-insensitive
males: 0, 639, 1277, or (lowest doses administered) palmitoyl-CoA oxidation;
2450 mg/kg body weight increase in lauric acid
per day 11- and 12-hydroxylase activity
females: 0, 679, 1346, in males.
or 2628 mg/kg body weight
per day
Table 1 (continued)
Study protocol Effect levela Critical end-point/comments Reference
female F344/N rats (5 or 10) LOEL = 300 mg/kg body weight Increase in peroxisomal NTP, 1997a
1 or 12 months in the diet per day proliferation (carnitine
approximate intake in diet: (lowest dose administered) acetyl transferase activity).
300, 600, or 1200 mg/kg body
weight per day
a NOEL = no-observed-effect level; NOAEL = no-observed-adverse-effect level; LOEL = lowest-observed-effect level;
LOAEL = lowest-observed-adverse-effect level.
At the 15-month interim sacrifice, the absolute weight of the
right kidney in the females at 600 mg/kg body weight per day and the
relative weight in all exposed males were significantly greater than
in controls. The severity of renal tubule pigmentation in high-dose
males and females was greater than in controls, both at 15 months and
at 2 years. The incidence of mineralization in kidney was
significantly less than in controls in low- and high-dose females at 2
years; severity decreased in all groups of exposed females. The
incidence of nephropathy was significantly increased in all groups of
exposed females (34/50, 47/50, 43/50, and 45/50 in control, 300, 600,
and 1200 mg/kg body weight per day groups, respectively) (see Table 3
in section 11.1.2). The incidence of transitional cell hyperplasia
(0/50, 3/50, 7/50, and 4/50 in control, 300, 600, and 1200 mg/kg body
weight per day groups, respectively) was significantly increased at
600 mg/kg body weight per day.
At final necropsy, the incidences of pancreatic acinar cell
adenoma (3/50, 2/49, 3/50, and 10/50 in control, 120, 240, and 500
mg/kg body weight per day groups, respectively) and pancreatic acinar
cell adenoma or carcinoma (combined) (3/50, 2/49, 3/50, and 11/50 in
control, 120, 240, and 500 mg/kg body weight per day groups,
respectively) in the high-dose males were significantly greater than
in the controls and exceeded those in the ranges of historical
controls from NTP 2-year feeding studies. One carcinoma was observed
in a high-dose male; this neoplasm had never been observed in the
historical controls. The incidence of focal hyperplasia of the
pancreatic acinar cell in the high-dose males was also significantly
greater than in the controls (4/50, 0/49, 9/50, and 12/50 in control,
120, 240, and 500 mg/kg body weight per day groups, respectively). Two
pancreatic acinar cell adenomas were observed in the high-dose
females.
The incidences of transitional cell papilloma of the urinary
bladder in female rats at 2 years were 1/50, 0/50, 0/50, and 2/50 in
control, 300, 600, and 1200 mg/kg body weight per day groups,
respectively.
The authors concluded that there was "some evidence of
carcinogenic activity" in male rats, based upon the increased
incidences of pancreatic acinar cell adenoma and of acinar cell
adenoma or carcinoma (combined). There was "equivocal evidence of
carcinogenic activity" in female rats, based upon the marginally
increased incidences of pancreatic acinar cell adenoma and of
transitional cell papilloma of the urinary bladder.
The NTP (1997b) has released a technical report of a study that
compared outcomes when chemicals were evaluated under typical NTP
bioassay conditions as well as under protocols employing dietary
restriction. The experiments were designed to evaluate the effect of
dietary restriction on the sensitivity of bioassays towards
chemical-induced chronic toxicity and carcinogenicity and to evaluate
the effect of weight-matched control groups on the sensitivity of the
bioassays. BBP was included in the protocol; the results were
summarized as follows:
"Butyl benzyl phthalate caused an increased incidence of pancreatic
acinar cell neoplasms in ad libitum-fed male rats relative to ad
libitum-fed and weight-matched controls. This change did not occur
in rats in the restricted feed protocol after 2 years ... Butyl benzyl
phthalate also caused an increased incidence of urinary bladder
neoplasms in female rats in the 32-month restricted feed protocol. The
incidences of urinary bladder neoplasms were not significantly
increased in female rats in any of the 2-year protocols, suggesting
that the length of the study, and not body weight, was the primary
factor in the detection of this carcinogenic response."
Fifty B6C3F1 mice per sex per group were exposed to 0, 6000, or
12 000 ppm BBP (0, 780, or 1560 mg/kg body weight per day1) via the
diet for 103 weeks (NTP, 1982). Approximately 35 tissues were examined
histopathologically. The only compound-related sign of exposure was a
dose-related decrease (statistical significance not specified) in body
weight in both sexes. Survival was not affected, and there was no
increased incidence of any neoplasm that was compound related. As
well, non-neoplastic changes were all within the normal limits of
incidence for B6C3F1 mice. The NTP concluded that, under the
conditions of the bioassay, BBP "was not carcinogenic for B6C3F1 mice
of either sex."
8.5 Genotoxicity and related end-points
In the (few) published reports of Ames assays with BBP, results
have been negative (Litton Bionetics Inc., 1976; Rubin et al., 1979;
Kozumbo et al., 1982; Zeiger et al., 1982, 1985). Negative results
have also been reported for mouse lymphoma assays (Litton Bionetics
Inc., 1977; Hazleton Biotechnologies Company, 1986), although
equivocal findings have also been published (Myhr et al., 1986; Myhr &
Caspary, 1991). In an assay for in vitro transformation of
Balb/c-3T3 cells (Litton Bionetics Inc., 1985), results were negative.
In an assay for chromosomal aberrations and sister chromatid exchanges
in Chinese hamster ovary cells (Galloway et al., 1987), there was
slight evidence for a trend in one sister chromatid exchange test
without activation, but no convincing evidence for positive results
for sister chromatid exchanges or aberrations.
1 Conversion factor: 1 ppm in food = 0.13 mg/kg body weight per day
(Health Canada, 1994).
The results from the mouse lymphoma (Myhr et al., 1986; Myhr &
Caspary, 1991) and chromosomal aberration (Galloway et al., 1987)
assays are equivocal. For the mouse lymphoma assay, the NTP concluded
that "Increases in mutant colonies were observed in the absence of S9
in cultures treated with concentrations that produced precipitation,
but such responses were not considered valid by experimental quality
control parameters." However, it is difficult to dismiss the observed
dose-response in several studies as spurious, although the repeat
tests were negative, particularly in view of inconsistencies of
results of the latter. In repeat studies ( n = 5) in the absence of
S9, there was limited evidence of activity in only one case; however,
although BBP was positive at 80 nl/ml in the second trial, it was
toxic at concentrations above 30 nl/ml in the third. The
inconsistently observed increase in small colony mutants and percent
damaged Chinese hamster ovary cells may be indicative of weak
clastogenic activity, which warrants proper confirmation in
well-conducted assays.
A negative response was reported for an assay for the induction
of sex-linked recessive lethals in Drosophila melanogaster (Valencia
et al., 1985). Recently, the NTP (1997a) published summary results of
mouse bone marrow tests for sister chromatid exchanges and induction
of chromosomal aberrations; responses were weak, and the sister
chromatid exchange test was not repeated. Both of these responses,
although statistically significant, were small and indicative of only
weak clastogenic activity. Ashby et al. (1997a) reported negative
results in a micronucleus assay in rats.
8.6 Reproductive and developmental toxicity
Detailed descriptions of the protocol and effect levels are
presented here for critical studies only. Experimental details and
effect levels for all key reproductive and developmental studies via
ingestion are provided in Table 1.
With respect to reproductive effects, in repeated-dose toxicity
studies by the oral route, decreases in the weight of the testes and
histopathological effects in the testes have been observed, although
only at doses greater than those that induce other effects, such as
variations in organ to body weight ratios for the kidney and liver or
histopathological effects in the pancreas or kidney. With the
exception of a short-term gavage study in which minimal
histopathological effects in the testes of rats were observed at 480
mg/kg body weight per day in one of six animals (control data not
presented, and no statistical analysis) (Hammond et al., 1987),
testicular atrophy or degeneration has been observed only in rats only
at doses exceeding 1250 mg/kg body weight per day (NTP, 1982, 1997a;
Hammond et al., 1987).
In a combined reproductive/developmental screening protocol, at
1000 mg/kg body weight per day there was a decrease in body weight
gain, fluctuation in food consumption, reduction in the weight of
testis and epididymis, and increase in testicular degeneration in
males. In females at this dose, there was a decrease in body weight
gain, effects on food consumption, and adverse effects on reproductive
indices. With the exception of a transient decrease in pup weight,
there were no effects on the parental generation or offspring at
500 mg/kg body weight per day (Piersma et al., 1995).
Reproductive effects of BBP in male Fischer 344 rats have been
investigated by the NTP (Kluwe et al., 1984; Agarwal et al., 1985).
Groups of 10 males were administered 0, 0.625, 1.25, 2.5, or 5.0% (0,
312.5, 625, 1250, or 2500 mg/kg body weight per day1) in the diet
for 14 days. The protocol included measurement of endocrine hormones
and histopathological examination of brain, liver, kidney, spleen,
thyroid, thymus, pituitary, testes, epididymis, prostate, seminal
vesicles, and mesenteric lymph nodes. Bone marrow was also examined.
No deaths occurred during the study. Body weight was reduced in
the two highest dose groups. Food consumption was consistently reduced
in the highest dose group throughout the experiment. Absolute weights
of testis, epididymis, prostate, and seminal vesicles were
significantly reduced at the two highest dose levels in a dose-related
manner and were accompanied by "generalized histological atrophy."
Statistical analyses were presented for histopathological changes in
testis (aspermatogenesis/seminiferous tubular atrophy), seminal
vesicles (atrophy), and prostate (atrophy); significant changes were
consistently observed at the two highest doses. The authors noted a
"clear relationship" between dose and severity of morphological
changes in testis, seminal vesicles, and prostate; the changes
occurred only at the two highest dose levels. Similarly, effects on
the epididymis were observed in only the two highest dose groups.
Absolute weight of liver was increased at the two lowest doses
and decreased at the highest dose. The relative weight was increased
at all levels of exposure, in a dose-related manner. Histopathological
changes (mild multifocal chronic hepatitis) were described only for
the highest dose. Absolute weight of kidney was also increased at the
two lowest doses and decreased at the two highest. The relative weight
was increased at all levels of exposure, in a dose-related manner.
Proximal tubular regeneration was observed at all dose levels. Thymic
weight was reduced at the two highest doses in a dose-related manner.
Although histopathological changes were described for all dose groups,
atrophy was observed only in the highest dose group. There were no
effects upon absolute or relative pituitary weight, nor were
morphological changes observed in thyroid, pituitary, spleen, or lymph
nodes. Statistical analyses were not presented for histopathological
observation of these organs.
1 Conversion factor: 1 ppm in food = 0.05 mg/kg body weight per day
(Health Canada, 1994).
Plasma testosterone was decreased at the highest dose.
Follicle-stimulating hormone was increased at the two highest doses in
a dose-related manner. Luteinizing hormone was increased at the lowest
dose and at the two highest doses; there was a limited number of
samples at the high dose. No effects were observed upon such
haematological parameters as red blood cell count, packed cell volume,
haemoglobin, mean corpuscular volume, or white blood cell count. There
was no significant effect upon blood clotting ability, as measured by
prothrombin time. Bone marrow cell count was reduced at the two
highest doses.
At the lowest dose (312.5 mg/kg body weight per day), there was a
significant increase in both the absolute and relative weights of both
liver and kidney. There was proximal tubular regeneration at all
levels of exposure. Focal thymic medullary haemorrhage (minimal
severity) was observed in a small number of animals in all BBP-exposed
groups, but the incidences were not dose related. Based upon these
observations, the LOAEL is 312.5 mg/kg body weight per day for effects
on the liver and kidney.
Male F344/N rats (15 per group) were administered BBP via the
diet for 10 weeks, then each mated to 2 unexposed females (NTP,
1997a). Dietary concentrations were relatively widely spaced at 0,
300, 2800, or 25 000 ppm, which were reported by the authors to be
equivalent to 0, 20, 200, or 2200 mg/kg body weight per day. The final
body weight and body weight gain of the high-dose group were
significantly lower than in the controls. Minimal changes in
haematological parameters were observed in the high-dose group. Both
the absolute and relative weights of prostate and testis were
significantly decreased in the high-dose group (2200 mg/kg body weight
per day). (Other lower organ weights in this group were attributed to
the lower mean body weight.) Other effects observed at the high dose
included degeneration of the seminiferous tubular germinal epithelium
and significantly reduced weight of right cauda, right epididymis, and
right testis. Epididymal spermatozoal concentrations were
significantly reduced in a dose-related manner at the two highest
doses. However, histopathological evidence of hypospermia and a
decrease in fertility index were observed only at the highest dose.
Ten of 30 females mated to high-dose males were found to be sperm
positive, but none was pregnant at necropsy. Fertility indices were
significantly lower at the high dose. At the lower two doses, there
were no exposure-related effects observed on maternal body weight,
maternal clinical observations, or litter data. A NOEL of 20 mg/kg
body weight per day can be designated, based upon a significant and
dose-related decrease in epididymal spermatozoal concentration at the
two highest doses and associated effects on fertility at the highest
dose (LOAEL = 200 mg/kg body weight per day).
Concentrations of BBP of 0.2, 0.4, and 0.8% (108, 206, and 418
mg/kg body weight per day for males; 106, 217, and 446 mg/kg body
weight per day for females) were administered in the diet to males for
10 weeks and to females for 2 weeks premating. Two litters were
produced, and no adverse effects were observed on fertility,
pregnancy, or offspring development (TNO Biotechnology and Chemistry
Institute, 1993).
Sharpe et al. (1995) administered a single dose level of BBP via
drinking-water to pregnant Wistar rats, to determine the effects of
gestational and lactational exposure upon male offspring. Dams were
exposed for 2 weeks prior to mating and throughout gestation until
weaning. This procedure was then repeated on the same dams, and
observations were also carried out on the second litters. Based upon
measurement of drinking-water consumption in six animals, intake of
BBP was estimated to range from 126 to 366 µg/kg body weight per day,
from postnatal days 1-2 to postnatal days 20-21, respectively. There
was a significant reduction in daily sperm production in the
BBP-exposed animals examined at 90-95 days. Sperm production in the
positive control group, which received 100 µg diethylstilbestrol
(DES)/litre in drinking-water, was also reduced ( P < 0.01); the
negative control group (which received 1000 µg octylphenol
polyethoxylate/litre) was not evaluated. The authors questioned the
relevance of the effects to humans on the basis that this would
require detailed dose-response data and measurement of the actual
levels of the administered chemical in the male rats. Moreover, these
results vary from those reported by Sharpe et al. (1995) in an
investigation of effects on male offspring.
Ashby et al. (1997a) exposed Alpk:APfSD rats during gestation
and lactation to 1000 µg BBP/litre in drinking-water or 50 µg
DES/litre in drinking-water (positive control). Negative controls
received 100 µl ethanol/litre in drinking-water. Glass drinking-water
bottles were used in the experiment, as the authors had determined
that 60% of BBP was adsorbed onto plastic drinking-water bottles
within 24 h (Ashby et al., 1997b). The authors reported that the
overall exposures were 183 µg BBP/kg body weight per day and 8.6 µg
DES/kg body weight per day. There were no effects upon weight of right
testis after decapsulation, total sperm count (right testis), sperm
count per gram of right testis, or total sperm count in right cauda on
either postnatal day 90 or postnatal day 137. The authors noted the
contrast between these results and those reported by Sharpe et al.
(1995).1
1 It is noted that TNO Nutrition and Food Research Institute (1997)
is conducting an experiment in which the protocol was designed "to
investigate the reproducibility of, and expand on, the findings of
Sharpe et al. ... related to the development of the reproductive
system in Wistar rats exposed in utero and during lactation to
butyl benzyl phthalate in drinking water." Data from this study have
not yet been published.
It should be noted that there were significant differences
between these studies with respect to exposure of the dams. In the
Sharpe et al. (1995) study, the dams were exposed for 2 weeks prior to
mating, throughout gestation and weaning, and subsequently for another
2 weeks prior to mating, during gestation, and during lactation. In
the study by Ashby et al. (1997a), dams were exposed only during
gestation and lactation.
Although BBP has been estrogenic in human breast cancer cells
in vitro (Jobling et al., 1995; Soto et al., 1995; Meek et al.,
1996), results in yeast have been both positive (Coldham et al., 1997;
Harris et al., 1997) and negative, the latter for both BBP and its
principal metabolites (Gaido et al., 1997); it should be noted,
however, that administered concentrations were unclear in two of the
studies (Coldham et al., 1997; Harris et al., 1997). However, neither
BBP nor its metabolites monobutyl benzyl phthalate and monobenzyl
phthalate have been uterotrophic in vivo in rats (Monsanto Europe
SA, 1995a, 1996a) or in rats and mice (Monsanto Europe SA, 1995b,
1996b), respectively. There was no estrogenic effect in an acute in
vivo assay (Milligan et al., 1998) in mice (stimulation of increased
uterine vascular permeability).
The developmental toxicity of BBP following dietary
administration has been well investigated by the NTP in studies in
both rats and mice (NTP, 1989, 1990; Price et al., 1990) and in a
series of investigations in rats by Ema et al. (1990, 1991a,b,c, 1993,
1994, 1995) following both dietary and gavage administration. In
general, developmental effects of BBP have been observed only at dose
levels that induced significant maternal toxicity; in pair feeding
studies, however, malformations observed at high doses were not fully
attributable to maternal toxicity (Ema et al., 1992). In a
well-conducted NTP (1989) study in rats, at 1100 mg/kg body weight per
day there were significant effects in the mothers but minimal effects
in the offspring. At the highest dose (1640 mg/kg body weight per
day), there was an increased incidence of rudimentary extra lumbar
ribs. Results of studies by Ema and colleagues in which BBP was
administered in the diet to rats for various periods, including the
full 21 days of gestation, were similar. Although the
no-observed-adverse-effect levels (NOAELs) for both maternal and
developmental toxicity were less in the NTP (1990) study in mice (182
mg/kg body weight per day), this was primarily a function of wide dose
spacing, with maternal (decreased weight gain) and developmental
effects being observed at 910 mg/kg body weight per day. At both 910
and 2330 mg/kg body weight per day, there was a significant increase
in the percentage of fetuses malformed per litter. Ema et al. (1998)
observed a significant decrease in uterine decidual growth at 750
mg/kg body weight and higher following administration on days 0-8 to
pseudopregnant rats. Functional effects have not been investigated in
available studies.
Metabolites of BBP have induced effects on the testes similar to
those of BBP, with the monobutyl ester being more potent in this
regard (Mikuriya et al., 1988). Similarly, profiles of effects (e.g.,
fusion of sternebrae, cleft palate) in the offspring observed at
maternally toxic doses of the metabolites of BBP are similar to those
induced by BBP itself, with effects being observed at lower doses of
monobenzyl than of monobutyl phthalate (see, for example, Ema et al.,
1996a,b,c).
In a recent multigeneration study by continuous breeding in rats
exposed to DBP for which the sole metabolite is monobutyl phthalate,
testicular effects observed in the F1 generation were attributed to
impairment of normal androgen signalling in the fetus, although
available data were considered insufficient to conclude that the
monoester was responsible (Foster, 1997). Multigeneration studies for
BBP have not been identified.
8.7 Peroxisomal proliferation
BIBRA (1985) investigated peroxisomal proliferation of BBP and
reported an increase in relative liver and relative kidney weights, an
increase in cyanide-insensitive palmitoyl-CoA oxidation, and an
increase in lauric acid 11- and 12-hydroxylase activity in male F344
rats at 639 mg/kg body weight per day. In female rats, an increase in
relative liver and relative kidney weights was reported at 679 mg/kg
body weight per day. These were the lowest levels of exposure. The NTP
(1997a) reported an increase in peroxisomal proliferation in female
F344/N rats after exposure to 300 mg/kg body weight per day for either
1 or 12 months. In a comparative study, whereas DEHP induced a "very
marked" increase in peroxisomal proliferation, that for BBP was
considered "moderate" (Barber et al., 1987).
8.8 Immunological and neurological effects
Data additional to those presented in sections 8.2 and 8.3
relevant to assessment of the potential immunotoxicity and
neurotoxicity of BBP were not identified.
9. EFFECTS ON HUMANS
Although in an early study (Mallette & von Haam, 1952) BBP was
reported to have a moderately irritating effect upon 15-30 volunteers,
Hammond et al. (1987) observed neither primary irritation nor
sensitization reactions in a patch test with 200 volunteers. Other
identified data in humans relevant to the assessment of the potential
adverse effects of BBP are restricted to limited studies of
respiratory/neurological effects or cancer in populations of workers
generally exposed to mixtures of plasticizers, of which BBP was a
minor component (Nielsen et al., 1985; Hagmar et al., 1990).
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1 Aquatic environment
10.1.1 Pelagic organisms
Data on acute toxicity are available for approximately two dozen
species, including microorganisms, algae, invertebrates, and fish
(Table 2). The lowest reported acute toxicity value was a 96-h LC50
of 510 µg/litre for the shiner perch ( Cymatogaster aggregata) in a
flow-through study using measured concentrations (Ozretich et al.,
1983). Values of the LC50 for most other fish species exceeded 1000
µg/litre. The most sensitive invertebrate species in acute toxicity
tests was the mysid shrimp ( Mysidopsis bahia), with a 96-h LC50 of
900 µg/litre in a static bioassay using nominal concentrations
(Gledhill et al., 1980). Reported LC50 values for other invertebrates
exceeded 1000 µg/litre.
Data on chronic toxicity are available for about a dozen species,
including algae, invertebrates, and fish. The lowest reported chronic
toxicity value was a 96-h EC50 of 110 µg/litre, reported for the
green alga Selenastrum, based on chlorophyll a measurements and
cell number reductions using nominal concentrations (Suggatt & Foote,
1981). The most sensitive invertebrate species in chronic toxicity
tests was the mysid shrimp ( Mysidopsis bahia), with a 28-day
lowest-observed-effect concentration (LOEC) of 170 µg/litre, based on
reproduction and growth in a flow-through study using measured
concentrations (Springborn Bionomics, 1986a). The most sensitive fish
species in chronic toxicity tests was the fathead minnow ( Pimephales
promelas), with a 30-day LOEC of 360 µg/litre based on hatching of
eggs and survival and growth of larvae using measured concentrations
(LeBlanc, 1984).
Table 2: Toxicity to aquatic organisms.
Organism Effect Reference
Acute toxicity
mixed microbial cultures 8% inhibition of oxygen consumption Volskay & Grady, 1988;
at solubility limit (2900 µg/litre) Volskay et al., 1990
with Organisation for Economic
Co-operation and Development (OECD)
Method 209 and Respiration Inhibition
Kinetic Analysis (RIKA)a Screening Test
selected pure bacterial cultures little or no inhibition of growth in Painter & Jones, 1990
cultures amended with 625-625 000
µg/litre
unacclimated wastewater treatment 8.4% chemical oxygen demand (COD) removal Adams
plant sludge inhibition after 3 h at 3000 µg/litre, & Bianchini-Akbeg,
no effect on nitrification of ammonia 1989
Photobacterium phosphoreum 5-min Apparent Effects Thresholdb (AET), Tetra Tech Inc., 1986;
Puget Sound (reduced sediment Barrick et al., 1988
luminescence), 63 ng/g dry weight
sediment
Photobacterium phosphoreum 5-min, Puget Sound (reduced sediment
luminescence), 4900 ng/g organic carbon Barrick et al., 1988
variety of algae, invertebrates, and fish acute toxicity = 500-5000 µg/litre TOXNETc
Hydra littoralis 96-h EC50 = 1100 µg/litre (mortality Monsanto Company, 1986a
and presence of "tulip" stage)
polychaetes (Nereis/Neanthes virens) 96-h LC50 > 3000 µg/litre Monsanto Company, 1986b
Table 2 (continued)
Organism Effect Reference
oyster (Crassostrea gigas) AET, Puget Sound (increased Tetra Tech Inc., 1986;
abnormalities), >470 ng/g dry weight Barrick et al., 1988
sediment
oyster (Crassostrea gigas) AET, Puget Sound (increased Barrick et al., 1988
abnormalities), >9200 ng/g organic carbon
oyster (Crassostrea virginica) 96-h EC50 (shell deposition) Monsanto Company, 1986c
= 1300 µg/litre
amphipod (Rhepoxynius abronius) AET, Puget Sound (increased mortality), Tetra Tech Inc., 1986
>470 ng/g dry weight sediment
amphipod (Rhepoxynius abronius) AET, Puget Sound (increased mortality), Barrick et al., 1988
900 ng/g dry weight sediment
amphipod (Rhepoxynius abronius) AET, Puget Sound (increased mortality), Barrick et al., 1988
42 000 ng/g organic carbon
daphnids 48-h LC50 = 1000-3700 µg/litre Nabholz, 1987
Daphnia magna 24-h LC50 > 460 000 µg/litre LeBlanc, 1980
Daphnia magna 24-h EC50 = 3800 µg/litre Adams & Heidolph, 1985
Daphnia magna 48-h EC50 > 960 µg/litre Adams et al., 1995
Daphnia magna 48-h EC50 > 1400 µg/litre CMA, 1984
Daphnia magna 48-h LC50 = 1800 µg/litre Zeigenfuss et al., 1986
Daphnia magna 48-h EC50 = 1800 µg/litre Adams & Heidolph, 1985
Daphnia magna 48-h EC50 = 3700 µg/litre Gledhill et al., 1980
Table 2 (continued)
Organism Effect Reference
Daphnia magna 48-h LC50 = 92 000 µg/litre LeBlanc, 1980
Daphnia magna 48-h LC50 = 1600 µg/litre (no food) to Barera & Adams, 1983
>10 000 µg/litre (2000 µg/litre algae
or 30 000 µg/litre
trout chow/alfalfa yeast as food)
Daphnia magna 48-h LC50 = 1000 µg/litre (no organic Barera & Adams, 1983
solvent) to 2200 µg/litre (triethylene
glycol solvent)
Daphnia magna 2-, 7-, 14-, and 21-day EC50 > 760 Adams & Heidolph, 1985
µg/litre (flow-through); 21-day EC50
= 680 µg/litre (static renewal)
Daphnia magna 48-h LC50 = 3700 µg/litre (no fulvic acid) Monsanto Company, 1978
48-h LC50 = 2430 µg/litre (250 mg natural
fulvic acid/litre)
48-h LC50 = 1910 µg/litre (250 mg
purchased fulvic acid/litre)
Mysid shrimp (Mysidopsis bahia) 48-h LC50 = 1700 µg/litre CMA, 1984
Mysid shrimp (Mysidopsis bahia) 96-h LC50 = 900 µg/litre Gledhill et al., 1980
Mysid shrimp (Mysidopsis bahia) 96-h LC50 > 740 µg/litre Monsanto Company, 1988
(estimate = 1100 µg/litre)
Mysid shrimp (Mysidopsis bahia) 96-h LC50 = 9630 µg/litre Suggatt & Foote, 1981
Grass shrimp (Paleomonetes vulgaris) 96-h LC50 > 2700 µg/litre Monsanto Company, 1986d
Pink shrimp (Penaeus duorarum) 96-h LC50 > 3400 µg/litre Springborn Bionomics, 1986b
Table 2 (continued)
Organism Effect Reference
Crayfish (Procambarus sp.) 96-h LC50 > 2400 µg/litre Monsanto Company, 1986e
Chironomus tentans 48-h LC50 = 1600 µg/litre Zeigenfuss et al., 1986
Chironomus tentans 48-h LC50 = 1640 µg/litre Monsanto Company, 1982b
Chironomus tentans 48-h LC50 = 3600 µg/litre Monsanto Company, 1981a
Paratanytarsus dissimilis 48-h LC50 > 3600 µg/litre Monsanto Company, 1981a
Midge (Paratanytarsus parthenogenetica) 48-h LC50 = 7200 µg/litre Monsanto Company, 1981b;
CMA, 1984
Midge (Paratanytarsus parthenogenetica) 48-h LC50 = 13 400 µg/litre Monsanto Company, 1981c
Midge (Paratanytarsus parthenogenetica) 96-h LC50 > 3600 µg/litre Adams et al., 1995
Mayfly (Hexagenia sp.) 96-h LC50 = 1100 µg/litre Monsanto Company, 1986f
Fathead minnow (Pimephales promelas) 96-h LC50 = 2100 µg/litre (hardness Gledhill et al., 1980
40 000 µg calcium carbonate/litre)
Fathead minnow (Pimephales promelas) 96-h LC50 = 5300 µg/litre (hardness Gledhill et al., 1980
160 000 µg calcium carbonate/litre)
Fathead minnow (Pimephales promelas) 96-h LC50 > 780 µg/litre (static test) Adams et al., 1995
Fathead minnow (Pimephales promelas) 96-h LC50 = 1500 µg/litre CMA, 1984; Adams et al., 1995
(flow-through test)
Fathead minnow (Pimephales promelas) 96-h LC50 > 1600 µg/litre (static test) CMA, 1984
Fathead minnow (Pimephales promelas) 96-h LC50 = 2320 µg/litre Gledhill et al., 1980
Fathead minnow (Pimephales promelas) 14-day LC50 = 2250 µg/litre Gledhill et al., 1980
Table 2 (continued)
Organism Effect Reference
Bluegill (Lepomis macrochirus) 24-h LC50 = 62 000 µg/litre Buccafusco et al., 1981
Bluegill (Lepomis macrochirus) 96-h LC50 = 43 000 µg/litre Buccafusco et al., 1981
Bluegill (Lepomis macrochirus) 96-h LC50 = 1700 µg/litre Gledhill et al., 1980;
CMA, 1984
Rainbow trout (Oncorhynchus mykiss) 96-h LC50 = 3300 µg/litre Gledhill et al., 1980
Rainbow trout (Oncorhynchus mykiss) 96-h LC50 = 820 µg/litre (flow-through test) CMA, 1984; Adams et al., 1995
Sheepshead minnow (Cyprinodon variegatus) 48-h LC50 = 3300 µg/litre AQUIREd
Sheepshead minnow (Cyprinodon variegatus) 96-h LC50 > 680 µg/litre (static test) CMA, 1984; Adams et al., 1995
Sheepshead minnow (Cyprinodon variegatus) 96-h LC50 = 3000 µg/litre Gledhill et al., 1980
Sheepshead minnow (Cyprinodon variegatus) 96-h LC50 = 440 000 µg/litre Heitmuller et al., 1981
Shiner perch (Cymatogaster aggregata) 96-h LC50 = 510 µg/litre Ozretich et al., 1983
English sole (Parophrys vetulus) 96-h LC50 = 660 µg/litre (static Randall et al., 1983
replenish), 550 µg/litre (flow-through)
Chronic toxicity
Algae 96-h EC50 = 200 µg/litre CMA, 1984
Anacystis 96-h EC50 = 1000 µg/litre (cell count) AQUIREd
Microcystis 96-h EC50 = 1 000 000 µg/litre (cell count) Gledhill et al., 1980
Dunaliella 96-h EC50 = 1000 µg/litre (cell count) Gledhill et al., 1980
Navicula 96-h EC50 = 600 µg/litre (cell count) Gledhill et al., 1980
Table 2 (continued)
Organism Effect Reference
Skeletonema 96-h EC50 = 600 µg/litre (cell count) Gledhill et al., 1980
Skeletonema EC50 = 190 µg/litre (cell count) Suggatt & Foote, 1981
Skeletonema EC50 = 170 µg/litre (chlorophyll a) Suggatt & Foote, 1981
Selenastrum 96-h EC50= 400 µg/litre (cell count) Gledhill et al., 1980
Selenastrum 96-h EC50= 110 µg/litre (chlorophyll a) Suggatt & Foote, 1981
Selenastrum 96-h EC50 = 130 µg/litre (cell count) Suggatt & Foote, 1981
Selenastrum 96-h EC50 = 600 µg/litre (cell count) AQUIREd
Selenastrum capricornutum 96-h EC50 = 210 µg/litre (cell count) Adams et al., 1995
Selenastrum capricornutum 96-h EC50 = 520 µg/litre (red blood cells, Tucker et al., 1985
reduced dry weight)
Selenastrum capricornutum 5-day EC50 = 720 µg/litre (cell count) Monsanto Company, 1980b
Selenastrum capricornutum 14-day EC50 = 520 µg/litre (cell count) Monsanto Company, 1980b
Daphnids 21-day NOEC = 440-630 µg/litre Nabholz, 1987
Daphnia and fathead minnow (Pimephales promelas) chronic toxicity = 100-800 µg/litre TOXNETc
Daphnia magna 21-day LOEC = 350 µg/litre; NOEC = 220
µg/litre (reproduction) (chronic renewal) Monsanto Company, 1982c
Daphnia magna 21-day LOEC = 760 µg/litre; NOEC = 260 Adams & Heidolph, 1985
µg/litre (reproduction) (flow-through)
Table 2 (continued)
Organism Effect Reference
Daphnia magna 21-day LOEC = 700 µg/litre; NOEC = 350 Adams & Heidolph, 1985
µg/litre (growth, survival, and
reproduction) (static)
Daphnia magna 14-day (Springborn = 21-day) LOEC = 1400 CMA, 1984; Springborn Bionomics,
µg/litre; NOEC = 280 µg/litre (survival 1984; Rhodes et al., 1995
and reproduction)
Daphnia magna 42-day LOEC = 760 µg/litre; NOEC = Gledhill et al., 1980
260 µg/litre (decreased reproduction,
both generations; decreased survival in
second generation)
Mysid shrimp (Mysidopsis bahia) 28-day LOEC = 170 µg/litre; NOEC = Springborn Bionomics, 1986a
75 µg/litre (reproduction and growth)
Fathead minnow (Pimephales promelas) 30-day LOEC = 360 µg/litre; NOEC = LeBlanc, 1984
140 µg/litre (decreased growth,
embryo-larvae study)
Fathead minnow (Pimephales promelas) maximum acceptable toxicant concentration Sun et al., 1995
(MATC) > 360 µg/litre; chronic LC01 =
547 µg/litre (estimated)
Fathead minnow (Pimephales promelas) 30-day mean chronic value = 220 µg/litre Pickering, 1983
Bluegill (Lepomis macrochirus) NOEC = 380 µg/litre Verschueren, 1983
Table 2 (continued)
Organism Effect Reference
Rainbow trout (Oncorhynchus mykiss) 109-day NOEC > 200 µg/litre (hatchability, Monsanto Company, 1986g;
growth, survival) Rhodes et al., 1995
English sole (Parophrys vetulus) sublethal effects at all exposures, TOXNETc
lowest = 100 µg/litre
a According to Volskay et al. (1990).
b The concentration above which statistically significant adverse effects are always expected relative
to appropriate reference conditions.
c Toxicology Data Network, National Library of Medicine, US Department of Health and Human Services,
Bethesda, MD.
d Aquatic Information Retrieval Database, US Environmental Protection Agency.
10.1.2 Benthic organisms
There were no acute or chronic toxicity studies identified for
BBP in sediments.
Tetra Tech Inc. (1986) calculated a sediment quality value1 of
55 000 ng BBP/g dry weight for sediment containing 1% organic carbon
using the equilibrium partitioning approach. The assumption behind
this approach is that non-polar organic compounds partition to the
organic carbon fraction of the sediments to varying degrees depending
upon their organic carbon/water partition coefficients (Di Toro et
al., 1991).
10.2 Terrestrial environment
No studies on the effects of BBP on wild mammals were identified.
Information about the effects of BBP on laboratory mammals is
presented in section 8.
No studies on the effects of BBP on plants were identified.
1 Sediment quality values represent concentrations of chemicals in
sediments that are expected to be associated with adverse biological
effects based either on field evidence or on theoretical predictions
(Tetra Tech Inc., 1986).
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Following oral administration to rats, BBP is readily hydrolysed
in the gastrointestinal tract and the liver to phthalate monoesters
(monobutyl and monobenzyl phthalate), which are rapidly eliminated,
predominantly in urine.
Available data in humans are inadequate to serve as a basis for
assessment of the effects of long-term exposure to BBP in human
populations. The remainder of this section, therefore, addresses
effects in experimental animals.
The acute toxicity of BBP is relatively low, with oral LD50
values in rats being greater than 2 g/kg body weight. Target organs
following acute exposure include the haematological and central
nervous systems.
Available data are inadequate to assess the irritant or
sensitizing effects of BBP in animal species.
The repeated-dose toxicity of BBP has been well investigated in
recent studies, primarily in the rat, in which dose-response was well
characterized. Effects observed consistently have been decreases in
body weight gain (often accompanied by decreases in food consumption)
and increases in organ to body weight ratios, particularly for the
kidney and liver. In addition, histopathological effects on the
pancreas and kidney and haematological effects have also been
observed. At higher doses, degenerative effects on the testes and,
occasionally, histopathological effects on the liver have been
reported. In specialized investigations, peroxisomal proliferation in
the liver has been observed, although potency in this regard was less
than that for other phthalates, such as DEHP.
The chronic toxicity and carcinogenicity of BBP have been
investigated in NTP bioassays in rats (including standard and
feed-restricted protocols) and mice. An increase in mononuclear cell
leukaemias observed in female F344 rats was not confirmed in a repeat
study. It was concluded that there was "some evidence" of
carcinogenicity in male rats, based on an increased incidence of
pancreatic tumours, and equivocal evidence in female rats, based on
marginal increases in pancreatic and bladder tumours. Dietary
restriction prevented full expression of the pancreatic tumours and
delayed appearance of the bladder tumours. There was no evidence of
carcinogenicity in mice.
The weight of evidence of the genotoxicity of BBP is clearly
negative. However, available data are inadequate to conclude
unequivocally that BBP is not clastogenic, although in identified
studies it has induced, at most, weak activity of a magnitude
consistent with secondary effects on DNA.
Therefore, BBP has induced an increase in pancreatic tumours
primarily in one sex of one species, the full expression of which was
prevented in a dietary restriction protocol, and a marginal increase
in bladder tumours in the other sex, which was delayed upon dietary
restriction. Available data are consistent with the compound not
interacting directly with DNA. On this basis, BBP can be considered,
at most, possibly carcinogenic to humans, likely inducing tumours
through a non-genotoxic (although unknown) mechanism.
In a range of studies, including those designed to investigate
the reproductive effects of BBP on the testes and endocrine hormones
of male rats, a modified mating protocol conducted by the NTP, and a
one-generation study, adverse effects on the testes and, consequently,
fertility have generally been observed only at doses higher than those
that induce effects on other organs (such as the kidney and liver),
although decreases in sperm counts have been observed at doses similar
to those that induce effects in the kidney and liver. This is
consistent with the results of repeated-dose toxicity studies.
Reductions in testes weight and daily sperm production in
offspring were reported at a relatively low level in rats exposed in
utero and during lactation in a study in which dose-response was not
investigated. However, such effects were not observed in a recent
study in another strain of rats in which only increases in absolute
and relative liver weights were observed at postnatal day 90.
Additional investigation of potential effects on the reproductive
systems of male and female animals exposed in utero and during
lactation in studies designed to address dose-response is desirable
and under way.
Although BBP has been estrogenic in human breast cell cancer
lines in vitro, results in yeast cells have been mixed. Neither BBP
nor its principal metabolites have been uterotrophic in vivo in rats
or mice. Although available data do not support the conclusion that
BBP is estrogenic, other potential endocrine-mediated effects such as
anti-androgenic activity associated with DBP are not precluded.
There is considerable emphasis currently on development of more
sensitive frameworks for testing and assessment of
endocrine-disrupting substances; compounds such as phthalates are
likely early candidates for additional testing.
In several well-conducted studies in rats and mice, BBP has
induced marked developmental effects, but only at dose levels that
induce significant maternal toxicity.
Although the potential neurotoxicity of BBP has not been well
investigated, histopathological effects on the central and peripheral
nervous systems have not been observed following short-term exposure
to relatively high dietary concentrations. Available data are
inadequate to assess the potential immunotoxicity of BBP.
Effect levels in available studies for the oral route are
summarized in Table 1. Based on consideration of the complete database
on repeated-dose toxicity by the oral route (including subchronic,
chronic, and reproductive/developmental studies), effects that occur
at lowest concentrations in rats are increases in organ to body weight
ratios, primarily for the liver and kidney, and histopathological
effects on the pancreas and kidney at dose levels in the range of 120
to just greater than 300 mg/kg body weight per day. Specifically,
these include increases in ratios of liver to body weight and
pancreatic lesions in the Wistar rat observed in a 90-day study
(Hammond et al., 1987), increases in (absolute and relative) kidney
weight and relative liver weight and proximal tubular regeneration in
a 2-week reproductive study (Agarwal et al., 1985), and increased
relative kidney weight at interim (15 month; not determined at
termination) sacrifice in male F344 rats in the 2-year NTP bioassay
(NTP, 1997a). Although nephropathy was also increased at all doses
(300 mg/kg body weight per day and higher) in the kidney of female
F344 rats in the 2-year NTP bioassay (NTP, 1997a), incidence was high
in all groups, with no evidence of dose-response (incidence or
severity) and no increase in severity between the interim and final
sacrifices.
Increases in hepatic peroxisomal proliferation in F344 rats also
occur at doses similar to those at which the effects mentioned above
have been observed following exposure for 1 or 12 months (NTP, 1997a).
Decreases in body weight in mice (of unspecified statistical
significance) have also been observed in this dose range in a 90-day
study, although food consumption was not reported (NTP, 1982).
Decreases in epididymal spermatozoal concentrations have also been
reported at these levels, although without accompanying
histopathological effects on the testes or adverse impact on fertility
(NTP, 1997a).
11.1.2 Criteria for setting guidance values for BBP
The following guidance is provided as a possible basis for
derivation of limits of exposure and judgement of the quality of
environmental media by relevant authorities.
Benchmark doses have been developed for histopathological lesions
in the pancreas of male Wistar rats in the 90-day study (Hammond et
al., 1987) and renal lesions in male F344 rats in the 2-week
reproductive study conducted by the NTP (Agarwal et al., 1985).
Primarily for purposes of comparison, a benchmark dose for renal
lesions in female F344/N rats in the 2-year carcinogenesis bioassays
conducted by the NTP is also presented (NTP, 1997a). Information on
the incidence of these lesions, resulting benchmark doses calculated
using the THRESH program (Howe, 1995), and associated parameter
estimates and statistics of fit are presented in Table 3. Each
benchmark dose is based upon a 5% effect level; 95% lower confidence
limits are also presented.
Histopathological effects have not been associated with increases
in organ to body weight ratios in the same sex except at much higher
doses, nor have decreases in epididymal spermatozoal concentrations at
lowest doses been accompanied by histopathological effects and adverse
impact on fertility. Owing principally to these considerations and
less to the inadequacy of current statistical techniques to adequately
model continuous data for these end-points and for peroxisomal
proliferation, benchmark doses for these end-points have not been
developed; for completeness, however, relevant effect levels are
included in Table 3 for comparison.
The fit of the model was best for pancreatic lesions in male
Wistar rats in the subchronic study by Hammond et al. (1987)
( P = 0.98), adequate for proximal tubular regeneration in the kidney
of male rats in the 2-week reproductive protocol (Agarwal et al.,
1985) ( P = 0.39), and inadequate for nephropathy in female rats in
the 2-year bioassay (NTP, 1997a) ( P = 0.03). Inadequate fit for the
latter is attributable to high incidence in all dose groups and little
evidence of dose-response. On this basis and consideration of the fact
that pancreatic lesions and tumours were also observed in the NTP
2-year bioassay in males of another strain of rats, the pancreatic
lesions in the Hammond et al. (1987) study have been selected as the
point of departure for development of a tolerable intake.
For comparison, a benchmark dose calculated using the THRESH
program (Howe, 1995) and associated parameter estimates and statistics
of fit for pancreatic focal hyperplasia (acinus) in male F344 rats in
the 2-year NTP bioassay are presented in Table 4. Although the
benchmark dose and associated lower 95% confidence limit are slightly
less than those calculated on the basis of the Hammond et al. (1987)
study in Wistar rats, it should be noted that the distinction between
hyperplasia and adenomas in the carcinogenesis bioassay was not
readily apparent. A tumorigenic dose (TD05) calculated on the basis
of multistage modelling (Global 82) of the incidence of pancreatic
adenomas or carcinomas (acinus) in male rats in the NTP bioassay is
also presented in Table 4 and, as would be expected, is greater than
the benchmark dose for hyperplasia. Data presented in the published
account were insufficient to develop a benchmark dose on the basis of
hyperplasia and adenomas combined.
Table 3: Benchmark doses for non-neoplastic effects.
Study Data for calculating Parameter estimates
benchmark dosea
(reference) Effect levels Dose Response Benchmark dose Goodness of fit
Subchronic dietary LOAEL = 381 mg/kg body Males: Lesions in 5% dose: 167 mg/kg Chi-square goodness
study weight per day (based pancreas: body weight per day of fit: 9.3 × 10-4
Wistar rats, upon histopathological
27-45/group lesions in males in control 0/27 (0%) 95% lower confidence Degrees of freedom:
3-month duration pancreas at two highest 151 mg/kg body 0/14 (0%) limit: 132 mg/kg body 1
doses) (males) weight per day 8/15 (53%) weight per day P-value: 0.98
381 mg/kg body 13/14 (93%)
weight per day
960 mg/kg body
weight per day
(Monsanto Company, LOEL = 171 mg/kg body
1980a; Hammond et al., weight per day (based
1987) on increases in organ
to body weight ratios
at all doses for the
kidney, liver, and
caecum) (females)
Reproductive study LOAEL = 312.5 mg/kg Males: Kidney,
F344 male rats, body weight per day proximal
10/group (based upon significant control tubular 5% dose: 228 mg/kg Chi-square goodness
14-day dietary increase in the relative regeneration: body weight per day of fit: 3.01
administration weight of liver and both 312.5 mg/kg body 0/10
the absolute and weight per day 2/10
(Kluwe et al., 1984; relative weights of 625 mg/kg body 2/10 95% lower confidence Degrees of freedom: 3
Agarwal et al., 1985) kidney and proximal weight per day 4/10 limit:
tubular regeneration 1250 mg/kg body 3/10 117 mg/kg body weight P-value: 0.39
at all levels of weight per day per day
exposure) 2500 mg/kg body
weight per day
Table 3 (continued)
Study Data for calculating Parameter estimates
benchmark dosea
(reference) Effect levels Dose Response Benchmark dose Goodness of fit
Carcinogenicity LOEL = 120 mg/kg body Females: 2-year sacrifice; 5% dose: 50 mg/kg body Chi-square goodness
bioassay weight per day (based kidney weight per day of fit: 7.09
F344/N rats, on increased relative nephropathy:
60/sex/group kidney weight in males
Dietary at interim sacrifice)
administration (not determined at
for 2 years terminal sacrifice)
(NTP, 1997a) Increase in renal control 34/50 95% lower confidence Degrees of freedom: 2
nephropathy in females 300 mg/kg body 47/50 (P < 0.01) limit: 28 mg/kg body
at all doses (300 mg/kg weight per day 43/50 (P < 0.05) weight per day P-value: 2.9 × 10-2
body weight and above); 600 mg/kg body 45/50 (P < 0.01)
however, unacceptable weight per day
goodness of fit for 1200 mg/kg body
benchmark dose weight per day
F344/N female LOEL = 300 mg/kg body
rats, 5/group weight per day (based
Dietary administration on increase in
for 1 or 12 months peroxisomal
proliferation)
(NTP, 1997a)
Table 3 (continued)
Study Data for calculating Parameter estimates
benchmark dosea
(reference) Effect levels Dose Response Benchmark dose Goodness of fit
F344 males, 15/group LOAEL = 200 mg/kg body
Modified mating weight per day (decrease
protocol in epididymal
spermatozoal
concentration without
histopathological
evidence of hypospermia
(NTP, 1997a) or decrease in fertility)
(It should be noted that
the dose levels in this
protocol increase by a
factor of 10.)
a Benchmark doses were calculated with the THRESH program (Howe, 1995). The approach to the use of benchmark doses in risk
assessment is described by US EPA (1995).
The TDI is developed, therefore, as follows:
TDI = 132 mg/kg body weight per day
100
= 1.3 mg/kg body weight per day
where:
132 mg/kg body weight per day is the lower 95% confidence limit for
the benchmark dose (167 mg/kg body weight per day) associated with a
5% increase in the incidence of pancreatic lesions in male Wistar rats
in the subchronic study of Hammond et al. (1987). It is noted that
increased excretion in faeces at higher doses observed in one study
might impact on the dose-response curve and resulting benchmark dose,
although it was not possible to address this quantitatively.
100 is the uncertainty factor (×10 for intraspecies variation; ×10 for
interspecies variation). An additional factor for extrapolation from
subchronic to chronic has not been incorporated as, on the basis of a
fairly robust database, there is no indication that effect levels are
lower in chronic studies than in investigations of shorter duration;
moreover, the compound is rapidly eliminated. Also, the incidence of
pancreatic lesions in the Wistar rat in the subchronic study on which
the benchmark dose is based is higher than that observed in the F344/N
rat in the 2-year carcinogenesis bioassay. Available data were
considered insufficient to replace default values for toxicokinetic
and toxicodynamic components of interspecies and intraspecies
variation with data-derived values.
This TDI is similar to values that could be developed based on
the LOELs for the continuous end-points such as peroxisomal
proliferation and increases in organ to body weight ratios included in
Table 1.
Data on the toxicity of BBP following repeated exposure by
inhalation are limited, with information relevant to characterization
of exposure-response being confined to results of two short-term and
one subchronic study in rats, with the range of end-points examined in
the latter investigation being more limited (Hammond et al., 1987). In
the short-term study with lowest concentrations, effects on body
weight gain and serum glucose were observed at 526 mg/m3; there were
no effects on haematology, blood chemistry, urinalysis, organ weights,
or histopathology at 144 mg/m3. Increases in organ weights were
observed at 218 mg/m3 in the subchronic study, although there were no
histopathological effects at the highest dose (789 mg/m3); the NOEL
was 51 mg/m3. Although the database for inhalation is somewhat
limited, it is of interest to note that the NOELs for effects by this
route are similar to those for ingestion. For example, the NOEL in the
investigation in which the range of end-points examined was more
extensive (144 mg/m3) is equivalent to a dose approximately threefold
less than the point of departure (i.e., the 95% lower confidence
limit) for the TDI presented above.1
11.1.3 Sample risk characterization
Based upon a sample estimate (section 6.2), intake of BBP for the
general population ranges from 2 µg/kg body weight per day in adults
to 6 µg/kg body weight per day in children. Food is by far the
greatest source, contributing essentially all of the intake.
The maximum and minimum estimates of total daily intake are 200
and 650 times less, respectively, than the TDI derived above for the
general population.
Identified data were inadequate to provide sample estimates of
exposure to BBP in the occupational environment or from consumer
products and hence sample risk characterizations for these scenarios.
It should be noted, though, that the inclusion of concentrations in
indoor air in the estimates of exposure for the general population
should account, at least to some extent, for exposure from consumer
products.
11.2 Evaluation of environmental effects
BBP may be released to the environment from a number of
industrial and municipal sources. Most releases are reported to be to
the atmosphere, but BBP is also released to the aquatic environment
from industrial and municipal liquid effluents.
Once in the environment, BBP partitions to soil, surface water,
sediments, and biota, and the substance has been detected in each of
these compartments. Likely sinks are soil and sediment.
BBP is removed from the atmosphere by photooxidation and by
rainwater, with a half-life of a few hours to a few days. BBP is not
persistent in water, sediments, or soil under aerobic conditions, with
a half-life of a few days. Under anaerobic conditions, BBP is more
persistent, with a half-life of a few months. BBP is readily
metabolized by vertebrates and invertebrates. Reported BCFs are less
than 1000, based on total residues, and well under 100, based on
intact BBP residues.
1 Based on the following conversion: 1 mg/m3 in air = 0.31 mg/kg
body weight per day ingested in rats (Health Canada, 1994).
Table 4: Benchmark dose for pancreatic hyperplasia and tumorigenic dose (NTP 2-year bioassay).
Study Data for calculating benchmark dose Parameter estimates
(reference) Effect levels Dose Response Benchmark dose Goodness of fit
Carcinogenicity Pancreas, acinus, Males: Incidence: 5% dose: 130 mg/kg P-value for lack of
bioassay focal hyperplasia body weight per day fit: 0.916
F344/N rats control 4/50
Dietary 120 mg/kg body 7/49 95% lower confidence
administration weight per day 9/50 limit: 73 mg/kg body
for 2 years 240 mg/kg body weight 12/50 weight per day
per day (P < 0.05)
(NTP, 1997a) 500 mg/kg body weight
per day
Carcinogenicity Pancreas, acinus, Males: Incidence: TD05: 320 mg/kg body P-value for lack of
bioassay adenoma, or carcinoma weight per day fit: 0.854
F344/N rats control 3/50
Dietary 120 mg/kg body weight 2/49 95% lower confidence
administration per day 3/50 limit:
for 2 years 240 mg/kg body weight 11/50 (P = 0.014) 160 mg/kg body weight
per day per day
(NTP, 1997a) 500 mg/kg body weight
per day
P = 0.003
for trend
In acute toxicity tests on approximately two dozen species and
chronic tests on about a dozen species, adverse effects occur at
exposure concentrations equal to or greater than 100 µg/litre.
Although higher concentrations have sometimes been reported,
concentrations in surface waters are generally less than 1 µg/litre.
Therefore, it is likely that BBP poses low risk to aquatic organisms.
No information about the effects of BBP on sediment-dwelling
organisms, soil invertebrates, terrestrial plants, or birds has been
identified on which to base an estimate of risk to these organisms.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
IARC (1987) has classified BBP in Group 3: "the agent is not
classifiable as to its carcinogenicity to humans." There were no
adequate data for humans, and evidence in animals was inadequate.
Information on international hazard classification and labelling
is included in the International Chemical Safety Card reproduced in
this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventive and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0834) reproduced in this
document.
13.1 Human health hazards
BBP has the potential to adversely affect reproductive function,
although effects on kidney, liver, and pancreas are noted at generally
lower doses.
13.2 Advice to physicians
In case of poisoning, treatment is supportive.
13.3 Spillage
In the event of spillage, measures should be undertaken to
prevent BBP from reaching drains or watercourses because of its
toxicity to aquatic organisms.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards
may be obtained from UNEP Chemicals (IRPTC), Geneva. The reader should
be aware that regulatory decisions about chemicals taken in a certain
country can be fully understood only in the framework of the
legislation of that country. The regulations and guidelines of all
countries are subject to change and should always be verified with
appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
BUTYL BENZYL PHTHALATE ICSC: 0834
March 1998
CAS # 85-68-7 Benzyl Butyl phthalate
RTECS # TH9990000 1,2-Benzenedicarboxylic acid, butyl phenymethyl ester
BBP
1,2-C6H4(COOCH2C6H5)(COOC4H9)/C19H20O4
Molecular Mass: 312.4
TYPES OF HAZARD/ ACUTE HAZARDS/ PREVENTION FIRST AID/
EXPOSURE SYMPTOMS FIRE FIGHTING
FIRE Combustible. Gives off NO open flames. Water spray, powder,
irritating or toxic fumes carbon dioxide.
(or gases) in a fire.
EXPLOSION
EXPOSURE
Inhalation Cough. Sore throat. Ventilation, local exhaust, Fresh air, rest.
or breathing protection.
Skin Redness. Protective gloves. Remove contaminated
clothes. Rinse and then
wash skin with water and
soap.
Eyes Redness. Safety spectacles. First rinse with plenty
of water for several
minutes (remove contact
lenses if easily possible),
then take to a doctor.
Ingestion Do not eat, drink, or smoke Rinse mouth. Rest.
during work.
SPILLAGE DISPOSAL PACKAGING & LABELLING
Collect leaking and spilled liquid in sealable metal Marine pollutant.
containers as far as possible. Absorb remaining liquid EU Classification
in sand or inert absorbent and remove to safe place. Symbol:
Do NOT let this chemical enter the environment. (Extra UN Classification
personal protection: A/P2 filter respirator for organic
vapour and harmful dust).
EMERGENCY RESPONSE STORAGE
NFPA Code: H1; F1; R0; Separated from strong oxidants.
IMPORTANT DATA
PHYSICAL STATE; APPEARANCE: ROUTES OF EXPOSURE:
COLOURLESS OILY LIQUID The substance can be absorbed into the body
by inhalation of its vapour.
CHEMICAL DANGERS: INHALATION RISK:
The substance decomposes on heating producing toxic No indication can be given about the rate in
fumes (phthalic anhydride). Reacts with oxidants. which a harmful concentration in the air is
reached on evaporation of this substance
at 20°C.
OCCUPATIONAL EXPOSURE LIMITS: EFFECTS OF SHORT-TERM EXPOSURE:
TLV not established. The substance irritates the eyes, the skin
and the respiratory tract.
EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
The substance may have effects on the liver and
kidneys, resulting in impaired functions.
PHYSICAL PROPERTIES
Boiling point: 370°C
Melting point: -35°C
Relative density (water = 1): 1.1
Solubility in water: none
Vapour pressure, Pa at 20°C: <0.1
Relative vapour density (air = 1): 10.8
Flash point: 199°C
Octanol/water partition
coefficient as log Pow: 4.77
ENVIRONMENTAL DATA
The substance is very toxic to aquatic organisms. In the food chain important to humans,
bioaccumulation takes place, specifically in fish.
NOTES
Saniticizer 160, Sicol 160, Unimoll BB and Palatinol BB are trade names. Also consult
ICSC #0271 Di(2-ethylhexyl)phthalate.
ADDITIONAL INFORMATION
LEGAL NOTICE: Neither the CEC nor the IPCS nor any person acting on behalf of the CEC
or the IPCS is responsible for the use which might be made of this
information.
REFERENCES
Aceves M, Grimalt JO (1993) Large and small particle size screening of
organic compounds in urban air. Atmospheric environment,
27B(2):251-263.
Adams WJ, Bianchini-Akbeg M (1989) Determination of inhibition of
nitrification and COD removal in an activated sludge waste treatment
plant by butylbenzyl phthalate, CAS #85687. St. Louis, MO, Monsanto
Company (Report No. ESC 98-49).
Adams WJ, Heidolph BB (1985) Short-cut chronic toxicity estimates
using Daphnia magna. In: Cardwell RD, Purdy R, Bahner RC, eds.
Aquatic toxicology and hazard assessment: seventh symposium.
Philadelphia, PA, American Society for Testing and Materials, pp.
87-103 (ASTM STP 854).
Adams WJ, Saeger VW (1993) Utility of laboratory microcosms for
predicting the environmental fate of chemicals: a comparison of two
microcosm designs with butyl benzyl phthalate. In: Gorsuch JW, Dwyer
FJ, Ingersoll CG, La Point TW, eds. Environmental toxicology and
risk assessment. Vol. 2. Philadelphia, PA, American Society for
Testing and Materials, pp. 103-119 (ASTM STP 1216).
Adams WJ, Renaudette WJ, Doi JD, Stepro MG, Tucker MW (1986)
Experimental freshwater microcosm biodegradability study of butyl
benzyl phthalate. St. Louis, MO, Monsanto Company, 104 pp. (Report
No. ESC-EAG-86-01).
Adams WJ, Renaudette WJ, Doi JD, Stepro MG, Tucker MW, Kimerle RA,
Franklin BB, Nabholz JV (1989) Experimental freshwater microcosm
biodegradability study of butyl benzyl phthalate. In: Suter GW, II,
Lewis MA, eds. Aquatic toxicology and environmental fate. Vol. 11.
Philadelphia, PA, American Society for Testing and Materials, pp.
19-40 (ASTM STP 1007).
Adams WJ, Biddinger GR, Robillard KA, Gorsuch JW (1995) A summary of
the acute toxicity of 14 phthalate esters to representative aquatic
organisms. Environmental toxicology and chemistry, 14:1569-1574.
Agarwal DK, Maronpot RR, Lamb JC, Kluwe WM (1985) Adverse effects of
butyl benzyl phthalate on the reproductive and hematopoietic systems
of male rats. Toxicology, 35(3):189-206.
Allan M (1995) Probabilistic assessment of 24-hour breathing rates.
Unpublished report prepared by Cornerstone Engineering and Consulting
Inc. for Environmental Health Directorate, Health Canada, October
1995.
Anon. (1996) Reporting relief denied by Massachusetts due to possible
health threat from plasticizer. Chemical regulation reporter,
20(22):766.
Ashby J, Tinwell H, Lefevre PA, Odum J, Paton D, Millward SW,
Tittensor S, Brooks AN (1997a) Normal sexual development of rats
exposed to butyl benzyl phthalate from conception to weaning.
Regulatory toxicology and pharmacology, 26:102-118.
Ashby J, Odum J, Tinwell H, Lefevre PA (1997b) Assessing the risks of
adverse endocrine-mediated effects: where to from here? Regulatory
toxicology and pharmacology, 26:80-93.
Atkinson R (1987) Structure-activity relationship for the estimation
of rate constants for the gas-phase reactions of OH radicals with
organic compounds. International journal of chemical kinetics,
19:799-828.
Axys Analytical Services Limited (1992) Phthalate esters analysis
report. Report prepared for Environment Canada, Conservation and
Protection (Axys File: AS1171).
Barber ED, Astill BD, Moran EJ, Schneider BF, Gray TJB, Lake BG, Evans
JG (1987) Peroxisome induction studies on seven phthalate esters.
Toxicology and industrial health, 3(2):7-24.
Barera Y, Adams WJ (1983) Resolving some practical questions about
Daphnia acute toxicity tests. In: Bishop WE, Cardwell RD, Heidolph
BB, eds. Aquatic toxicology and hazard assessment: sixth symposium.
Philadelphia, PA, American Society for Testing and Materials, pp.
509-518 (ASTM STP 802).
Barrick R, Becker S, Brown L, Beller H, Pastorok R (1988) Sediment
quality values refinement: Vol. 1. 1988 update and evaluation of
Puget Sound AET. Final report. Bellevue, WA, PTI Environmental
Services (NTIS PB89-200398).
Barrows ME, Petrocelli SR, Macek KJ (1980) Bioconcentration and
elimination of selected water pollutants by bluegill sunfish
( Lepomis macrochirus). In: Haque R, ed. Dynamics, exposure, and
hazard assessment of toxic chemicals. Ann Arbor, MI, Ann Arbor
Science Publishers Inc., pp. 379-392.
Bayer CW, Papanicolopoulos CD (1990) Exposure assessments to volatile
organic compound emissions from textile products. In: Indoor air
'90. Proceedings of the 5th international conference on indoor air
quality and climate. Vol. 3. Toronto, Ontario, 29 July - 3 August
1990, pp. 725-730.
BIBRA (1978) Studies on the metabolism and biological effects of
n -butyl benzyl phthalate in the rat. Surrey, British Industrial
Biological Research Association, 44 pp. (BIBRA Report No. 232/78).
BIBRA (1985) A 21-day feeding study of butyl benzyl phthalate to
rats: Effects on the liver and liver lipids. Report of the British
Industrial Biological Research Association to the Chemical
Manufacturers Association, Washington, DC (Project No. 3.0495.1;
Report No. 0495/1/84; CMA Reference PE 28.0-BT-BIB; US Environmental
Protection Agency Document No. 40+8626201; Office of Toxic Substances
Fiche No. OTS050943).
Bremer J, Witte E, Schneider D (1993) Measurement and characterisation
of emissions from PVC materials for indoor use. In: Indoor air '93.
Proceedings of the 6th international conference on indoor air
quality and climate. Vol. 2. Chemicals in indoor air, material
emissions. Helsinki, 4-8 July 1993, pp. 419-424.
Brown KW, Donnelly KC (1988) An estimation of the risk associated with
the organic constituents of hazardous and municipal waste landfill
leachates. Hazardous waste & hazardous materials, 5:1-30.
Buccafusco RJ, Ells SJ, LeBlanc GA (1981) Acute toxicity of priority
pollutants to bluegill ( Lepomis macrochirus). Bulletin of
environmental contamination and toxicology, 26:446-452.
California Environmental Protection Agency (1992) PTEAM: Monitoring
of phthalates and PAHs in indoor and outdoor air samples in
Riverside, California. Final report, Vol. II. Prepared under
contract by Research Triangle Institute, Research Triangle Park, NC,
for Air Resources Board, Research Division (Contract No. A933-144).
Calley D, Autian J, Guess WL (1966) Toxicology of a series of
phthalate esters. Journal of pharmacy science, 55(2):158-162.
CMA (1984) Generation of environmental fate and effects data base on
14 phthalate esters. Summary report -- Environmental studies
-- Phase I. December 15, 1984. Washington, DC, Chemical
Manufacturers Association, Phthalate Esters Program Panel.
Coldham NG, Dave M, Sivapathasundaram S, McDonnell DP, Connor C, Sauer
MJ (1997) Evaluation of a recombinant yeast cell estrogen screening
assay. Environmental health perspectives, 105(7):734-742.
Dempsey CR, Oppelt ET (1993) Incineration of hazardous waste: a
critical review update. Air & Waste, 43:25-73.
Di Toro DM, Zarba CS, Hansen DJ, Berry WJ, Swartz RC, Cowan CE, Pavlou
SP, Allen HE, Thomas NA, Paquin PR (1991) Technical basis for
establishing sediment quality criteria for nonionic organic chemicals
using equilibrium partitioning. Environmental toxicology and
chemistry, 10:1541-1583.
DMER, AEL (1996) Pathways analysis of butyl benzyl phthalate for the
second Priority Substances List using fugacity modelling. Report
prepared for Chemicals Evaluation Division, Commercial Chemicals
Evaluation Branch, Environment Canada, by Don Mackay Environmental
Research, Peterborough, Ontario, and Angus Environmental Limited, Don
Mills, Ontario. March 1996.
Dueva LA, Aldyreva MV (1969) Experimental assessment of sensitizing
and irritating action of phthalate plasticizers. Gigiena Truda i
Professional'nye Zabolevaniya, 13(10):7-19 (in Russian with English
abstract).
ECPI (1996) Phthalates in the aquatic environment. Brussels,
European Council for Plasticisers and Intermediates.
Eiceman GA, Clement RE, Karasek FW (1979) Analysis of fly ash from
municipal incinerators for trace organic compounds. Analytical
chemistry, 51:2343-2350.
Eigenberg DA, Bozigian HP, Carter DE, Sipes IG (1986) Distribution,
excretion, and metabolism of butylbenzyl phthalate in the rat.
Journal of toxicology and environmental health, 17(4):445-456.
Ejlertsson J, Johansson E, Karlsson A, Meyerson U, Svensson BH (1996)
Anaerobic degradation of xenobiotics by organisms from municipal solid
waste under landfilling conditions. Antonie van Leeuwenhoek,
69:67-74.
Elsisi AE, Carter DE, Sipes IG (1989) Dermal absorption of phthalate
diesters in rats. Fundamental and applied toxicology, 12(1):70-77.
Ema M, Murai T, Itami T, Kawasaki H (1990) Evaluation of the
teratogenic potential of the plasticizer butyl benzyl phthalate in
rats. Journal of applied toxicology, 10(5):339-343.
Ema M, Itami T, Kawasaki H (1991a) Embryolethality of butyl benzyl
phthalate in rats [abstract]. The FASEB journal, 5(5):A1237.
Ema M, Itami T, Kawasaki H (1991b) Evaluation of the embryolethality
of butyl benzyl phthalate by conventional and pair-feeding studies in
rats. Journal of applied toxicology, 11(1):39-42.
Ema M, Itami T, Kawasaki H (1991c) Teratogenicity of butyl benzyl
phthalate in rats [abstract]. Teratology, 44(6):16B.
Ema M, Itami T, Kawasaki H (1992) Effect of period of exposure on the
developmental toxicity of butyl benzyl phthalate in rats. Journal of
applied toxicology, 12(1):57-61.
Ema M, Itami T, Kawasaki H (1993) Teratogenic phase specificity of
butyl benzyl phthalate in rats. Toxicology, 79(1):11-19.
Ema M, Kurosaka R, Amano H, Ogawa Y (1994) Embryolethality of butyl
benzyl phthalate during early pregnancy in rats. Reproductive
toxicology, 8(3):231-236.
Ema M, Kurosaka R, Amano H, Ogawa Y (1995) Comparative developmental
toxicity of n-butyl benzyl phthalate and di- n-butyl phthalate in
rats. Archives of environmental contamination and toxicology,
28(2):223-228.
Ema M, Harazono A, Miyawaki E, Ogawa Y (1996a) Developmental toxicity
of mono- n-benzyl phthalate, one of the major metabolites of the
plasticizer n-butyl benzyl phthalate, in rats. Toxicology letters,
86(1):19-25.
Ema M, Harazono A, Miyawaki E, Ogawa Y (1996b) Characterization of
developmental toxicity of mono- n-benzyl phthalate in rats.
Reproductive toxicology, 10(5):365-372.
Ema M, Kurosaka R, Harazono A, Amano H, Ogawa Y (1996c) Phase
specificity of developmental toxicity after oral administration of
mono- n-butyl phthalate in rats. Archives of environmental
contamination and toxicology, 31(2):170-176.
Ema M, Miyawaki E, Kawashima K (1998) Reproductive effects of butyl
benzyl phthalate in pregnant and pseudopregnant rats. Reproductive
toxicology, 12(2):127-132.
Erickson NG (1965) The metabolism of diphenyl phthalate and
butylbenzyl phthalate in the beagle dog. Dissertation abstracts,
26(5):3014-3015.
Etkin DS (1995) Chemical contaminants and their health effects. Indoor
air quality in schools. In: Indoor air quality update: A guide to
the practical control of indoor air problems. Arlington, MA, Cutter
Information Corporation, pp. 27-45.
Foster PMD (1997) Assessing the effects of chemicals on male
reproduction: lessons learned from di- n-butyl phthalate. CIIT
activities, 17(9):1-9.
Gaido KW, Leonard LS, Lovell S, Gould JC, Babai D, Portier CJ,
McDonnell DP (1997) Evaluation of chemicals with endocrine modulating
activity in a yeast-based steroid hormone receptor gene transcription
assay. Toxicology and applied pharmacology, 143(1):205-212.
Galloway SM, Armstrong MJ, Reuben C, Colman S, Brown B, Cannon C,
Bloom AD, Nakamura F, Ahmed M, Duk S, Rimpo J, Margolin BH, Resnick
MA, Anderson B, Zeiger E (1987) Chromosome aberrations and sister
chromatid exchanges in Chinese hamster ovary cells: evaluations of 108
chemicals. Environmental and molecular mutagenesis, 10 (Suppl.
10):1-175.
Gledhill WE, Kaley RG, Adams WJ, Hicks O, Michael PR, Saeger VW (1980)
An environmental safety assessment of butyl benzyl phthalate.
Environmental science and technology, 14:301-305.
Golder Associates (1987) Testing of specific organic compounds in
soils in urban areas. Port Credit and Oakville/Burlington, Ontario.
Draft working paper to Shell Canada Limited and Texaco Canada Limited,
Mississauga, Ontario.
Government of Canada (in press) Canadian Environmental Protection Act
Priority Substances List assessment report -- Butyl benzyl
phthalate. Priority Substances Program, Ottawa, Canada.
Graedel TE, Hawkins DT, Claxton LD (1986) Atmospheric chemical
compounds: Sources, occurrence, and bioassay. New York, NY, Academic
Press, Inc., Harcourt Brace Jovanovich Publishers.
Hagmar L, Akesson B, Nielsen J, Andersson C, Linden K, Attewell R,
Moller T (1990) Mortality and cancer morbidity in workers exposed to
low levels of vinyl chloride monomer at a polyvinyl choride processing
plant. American journal of industrial medicine, 17(5):553-565.
Haile CL, Stanley JS, Magin AM, Northcutt RV, Redford DP (1984)
Emissions of organic pollutants from coal-fired utility boiler plants.
In: Identification and analysis of organic pollutants in air.
Conference proceedings. Boston, MA, Lawrence H. Butterworth, pp.
443-458.
Hammond BG, Levinskas GJ, Robinson EC, Johannsen FR (1987) A review of
the subchronic toxicity of butyl benzyl phthalate. Toxicology and
industrial health, 3(2):79-98.
Hargesheimer EE, Lewis CM (1987) Comparative source water quality
monitoring at two surface reservoirs. In: Proceedings of the
American Water Works Association annual conference. Kansas City, MO,
14-18 June 1987, pp. 153-175.
Harris CA, Henttu P, Parker MG, Sumpter JP (1997) The estrogenic
activity of phthalate esters in vitro. Environmental health
perspectives, 105(8):802-811.
Hazleton Biotechnologies Company (1986) Mutagenicity of 1D in a
mouse lymphoma mutation assay. Final report. Submitted by Chemical
Manufacturers Association, Washington, DC, to Office of Toxic
Substances, US Environmental Protection Agency (Document
Identification No. 40-8626225; Microfiche No. OTS0510527).
Hazleton Laboratories (1958) Final report. Subacute feeding
-- Albino rats. Sponsored by Monsanto Chemical Company. Office of
Toxic Substances, US Environmental Protection Agency (Document No.
878213590; Microfiche No. 206416).
Health Canada (1994) Human health risk assessment for Priority
Substances. Canada Communications Group, Ottawa, Ontario.
Heitmuller PT, Hollister TA, Parrish PR (1981) Acute toxicity of 54
industrial chemicals to sheepshead minnows ( Cyprinodon variegatus).
Bulletin of environmental contamination and toxicology, 27:596-604.
Howard PH (1990) Handbook of environmental fate and exposure data
for organic chemicals. Vol. 1. Large production and priority
pollutants. Chelsea, MI, Lewis Publishers Inc.
Howard PH, Boethling RS, Jarvis WF, Meylan WM, Michalenko EM (1991)
Handbook of environmental degradation rates. Chelsea, MI, Lewis
Publishers Inc.
Howe RB (1995) THRESH: A computer program to compute a reference
dose from quantal animal toxicity data using the benchmark dose
method. Ruston, LA, ICF Kaiser Engineers, Inc.
Iannuzzi TJ, Huntley SL, Schmidt CW, Finley BL, McNutt RP, Burton SJ
(1997) Combined sewer overflows (CSOs) as sources of sediment
contamination in the lower Passaic River, New Jersey. I. Priority
pollutants and inorganic chemicals. Chemosphere, 34:213-231.
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs volumes 1 to 42. Lyon, International Agency for
Research on Cancer (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Supplement No. 7).
IPCS (1993) International Chemical Safety Card -- Butyl benzyl
phthalate. Geneva, World Health Organization, International
Programme on Chemical Safety (ICSC 0834).
Jobling S, Reynolds T, White R, Parker MG, Sumpter JP (1995) A variety
of environmentally persistent chemicals, including some phthalate
plasticizers, are weakly estrogenic. Environmental health
perspectives, 103:582-588.
Kincannon DF, Lin YS (1985) Microbial degradation of hazardous wastes
by land treatment. In: Proceedings of the 40th industrial waste
conference, Purdue University, West Lafayette, Indiana. Boston, MA,
Butterworths, pp. 607-619.
Kluwe W (1984) Comprehensive evaluation of the biological effects of
phthalate esters. Presented at the International Conference on
Phthalic Acid Esters, University of Surrey, Guildford, England [cited
in Woodward K (1988) Phthalate esters: Toxicity and metabolism. Vol.
1. Boca Raton, FL, CRC Press].
Kluwe WM, Maronpot RR, Lamb JC, Agarwal DK (1984) Adverse effects of
butyl benzyl phthalate on bone marrow and the male reproductive system
[abstract]. The Toxicologist, 4:136.
Kozumbo WJ, Kroll R, Rubin RJ (1982) Assessment of the mutagenicity of
phthalate esters. Environmental health perspectives, 45:103-109.
LeBlanc GA (1980) Acute toxicity of priority pollutants to water flea
( Daphnia magna). Bulletin of environmental contamination and
toxicology, 24:684-691.
LeBlanc GA (1984) Comparative structure-toxicity relationships between
acute and chronic effects to aquatic organisms. In: Kaiser KLE, ed.
QSAR in environmental toxicology. Proceedings of the Workshop on
Quantitative Structure-Activity Relationships (QSAR) in Environmental
Toxicology, held at McMaster University, Hamilton, Ontario, 16-18
August 1983. Boston, MA, D. Reidel Publishing Company, pp. 235-260.
Ligocki MP, Leuenberger C, Pankow JF (1985a) Trace organic compounds
in rain -- II. Gas scavenging of neutral organic compounds.
Atmospheric environment, 19:1609-1617.
Ligocki MP, Leuenberger C, Pankow JF (1985b) Trace organic compounds
in rain -- III. Particle scavenging of neutral organic compounds.
Atmospheric environment, 19:1619-1626.
Little KD, Little JR (1983) Investigation of Santicizer 160 (benzyl
butyl phthalate) as a potential allergen. St. Louis, MO, Washington
University School of Medicine. Report to Monsanto Company (Project No.
JH-81-302).
Litton Bionetics Inc. (1976) Mutagenicity evaluation of BIO-76-17
Santicizer 160. NB 259784. Final report. Submitted by Monsanto
Company, St. Louis, MO, to Office of Toxic Substances, US
Environmental Protection Agency (Document Identification No.
87-7800282; Microfiche No. OTS200290).
Litton Bionetics Inc. (1977) Mutagenicity evaluation of BIO-76-243
CP731 (Santicizer 160) in the mouse lymphoma assay. Final report.
Submitted by Monsanto Company, St. Louis, MO, to Office of Toxic
Substances, US Environmental Protection Agency (Document
Identification No. 87-7800282; Microfiche No. OTS200290).
Litton Bionetics Inc. (1985) Evaluation of 1D in the in vitro
transformation of BALB/3T3 cells assay. Final report. Submitted by
Chemical Manufacturers Association, Washington, DC, to US
Environmental Protection Agency (Document Identification No.
40+8526206; Microfiche No. OTS0509537).
Mackay D, Shiu WY, Ma KC (1995) Illustrated handbook of
physical-chemical properties and environmental fate for organic
chemicals -- Vol. IV. Oxygen, nitrogen, and sulfur containing
compounds. Boca Raton, FL, CRC Press, Inc., Lewis Publishers,
pp.-702-705 (ISBN 1-56670-035-3).
MAFF (1987) Survey of plasticiser levels in food contact materials
and in foods. The 21st report of the Ministry of Agriculture,
Fisheries and Food Steering Group on Food Surveillance / The Working
Party on Chemical Contaminants from Food Contact Materials / Sub Group
on Plasticisers. London, HMSO, 105 pp. (Food Surveillance Paper No.
21).
MAFF (1996a) Phthalates in food. Food Safety Directorate, Ministry
of Agriculture, Fisheries and Food, 9 pp. (Food Surveillance
Information Sheet No. 82).
MAFF (1996b) Phthalates in infant formulae. Food Safety Directorate,
Ministry of Agriculture, Fisheries and Food, 9 pp. (Food Surveillance
Information Sheet No. 83).
Mallette FS, von Haam E (1952) Studies on the toxicity and skin
effects of compounds used in the rubber and plastics industries. II.
Plasticizers. Archives of industrial hygiene and occupational
medicine, 6(3):231-236.
Martin F, inventor (1996) Top nail coat composition containing
cellulose esters. Almell Limited, 5 pp. (US Patent 5,512,273;
04-30-96). In: Chemical Abstracts (online) Accession No.
CA12426352355Y.
Meek MD, Clemons J, Wu ZF, Zacharewski TR (1996) Assessment of the
alleged estrogen receptor-mediated activity of phthalate esters. In:
17th annual meeting of the Society of Environmental Toxicology and
Chemistry, abstract book, p. 85 (Abstract 443).
Mikuriya H, Ikemoto I, Tanaka A (1988) Urinary metabolites
contributing to testicular damage induced by butylbenzyl phthalate.
Jikeikai medical journal, 35:403-409.
Milligan SR, Balasubramanian AV, Kalita JC (1998) Relative potency of
xenobiotic estrogens in an acute in vivo mammalian assay.
Environmental health perspectives, 106(1):23-26.
Monsanto Company (1978) Acute toxicity of Santicizer 160 (butyl
benzyl phthalate) to Daphnia magna in the presence of fulvic acid.
St. Louis, MO (Report No. ES-SS-78-11).
Monsanto Company (1980a) Report of a short-term (90-day) study in
rats with Santicizer 160 with cover memo. Study done under contract
by British Industrial Biological Research Association. Submitted by
Monsanto, St. Louis, MO, to Office of Toxic Substances, US
Environmental Protection Agency (Document Identification No.
878213602; Microfiche No. 206416).
Monsanto Company (1980b) 14-day algal bottle assay. Bioassay data
summary. St. Louis, MO.
Monsanto Company (1981a) Acute toxicity studies on S-160 using two
midge species as the test organisms. St. Louis, MO (SR-83-X-059).
Monsanto Company (1981b) Acute toxicity of Santicizer 160 to the
midge Paratanytarsus parthenogenetica. St. Louis, MO (Report No.
ES-81-SS-8).
Monsanto Company (1981c) Acute toxicity of GLP-1 (9AB981018) to
Chironomus tentans. Static acute bioassay. St. Louis, MO (Report
No. 27145).
Monsanto Company (1982a) Thirteen-week inhalation toxicity of
Santicizer 160 plasticizer vapor-aerosol to Sprague-Dawley rats with
cover memo. Submitted by Monsanto, St. Louis, MO, to Office of Toxic
Substances, US Environmental Protection Agency (Document
Identification No. 878213601; Microfiche No. OTS 206416).
Monsanto Company (1982b) Acute toxicity of Santicizer 160 to midge
(Paratanytarsus parthenogenetica). St. Louis, MO (Report No.
ES-82-SS-79).
Monsanto Company (1982c) Chronic toxicity of Santicizer 160 to midge
Daphnia magna : 21-day chronic renewal study. St. Louis, MO (Report
No. ES-82-SS-103).
Monsanto Company (1986a) 96-h flow-through toxicity study of
butylbenzyl phthalate to Hydra littoralis. St. Louis, MO, 13 pp.
(Final Flow-through Acute Toxicity Report No. 34168).
Monsanto Company (1986b) Acute toxicity of butylbenzyl phthalate to
polychaetes (Nereis/Neanthes virens ) under flow-through
conditions. St. Louis, MO, 28 pp. (Bionomics Report No.
BW-86-7-2094).
Monsanto Company (1986c) Acute toxicity of 14C-butylbenzyl
phthalate to eastern oysters (Crassostrea virginica ). St. Louis,
MO, 28 pp. (Report No. BW-86-7-2083).
Monsanto Company (1986d) Acute toxicity of butylbenzyl phthalate to
grass shrimp (Paleomonetes vulgaris ) under flow-through
conditions. St. Louis, MO, 28 pp. (Bionomics Report No.
BW-86-7-2087).
Monsanto Company (1986e) 96-h flow-through toxicity study of
butylbenzyl phthalate to the freshwater crayfish, Procambarus sp.
St. Louis, MO, 13 pp. (Final Flow-through Acute Toxicity Report No.
34166).
Monsanto Company (1986f) 96-h flow-through toxicity study of
butylbenzyl phthalate to the mayfly, Hexagenia sp. St. Louis, MO,
13 pp. (Final Flow-through Acute Toxicity Report No. 34167).
Monsanto Company (1986g) Early life stage toxicity of
14C-butylbenzyl phthalate to rainbow trout (Salmo gairdneri ) in a
flow-through system. St. Louis, MO, 31 pp. (Early Life Stage
Toxicity Final Report No. 33996).
Monsanto Company (1988) Acute toxicity of butylbenzyl phthalate to
mysid shrimp (Mysidopsis bahia ) under flow-through conditions. St.
Louis, MO, 31 pp. (Report No. 87-10-2525).
Monsanto Europe SA (1995a) Study to evaluate the effect of butyl
benzyl phthalate on uterine growth in immature female rats after
oral administration. Study conducted for Monsanto Europe SA by
Central Toxicology Laboratory, Cheshire, 15 pp. (Report No.
CTL/R/1280).
Monsanto Europe SA (1995b) Study to evaluate the effect of
monobenzyl phthalate on uterine growth in immature female rats after
oral administration. Study conducted for Monsanto Europe SA by
Central Toxicology Laboratory, Cheshire, 16 pp. (Report No.
CTL/R/1281).
Monsanto Europe SA (1996a) Study to evaluate the effect of butyl
benzyl phthalate on uterine growth in immature female rats after
subcutaneous administration. Study conducted for Monsanto Europe SA
by Central Toxicology Laboratory, Cheshire, 15 pp. (Report No.
CTL/R/1278).
Monsanto Europe SA (1996b) Study to evaluate the effect of monobutyl
phthalate on uterine growth in immature female rats after oral
administration. Study conducted for Monsanto Europe SA by Central
Toxicology Laboratory, Cheshire, 15 pp. (Report No. CTL/R/1279).
Müller J, Kördel W (1993) Occurrence and fate of phthalates in soil
and plants. The science of the total environment, Suppl. (Part
1):431-437.
Munro JR, Foster MG, Pawson T, Stelzig A, Tseng T, King L (1985) St.
Clair River point source survey, 1979-1980. Toronto, Ontario,
Ontario Ministry of the Environment/Environment Canada.194 pp.
Myhr BC, Caspary WJ (1991) Chemical mutagenesis at the thymidine
kinase locus in L1578Y mouse lymphoma cells: results for 31 coded
compounds in the National Toxicology Program. Environmental and
molecular mutagenesis, 18(1):51-83.
Myhr BC, Bowers LR, Caspary WJ (1986) Results from the testing of
coded chemicals in the L5178Y TK+/- mouse lymphoma mutagenesis
assay [abstract]. Environmental mutagenesis, 8 (Suppl. 6):58.
Nabholz JV (1987) The acute and chronic toxicity of dialkyl
phthalate esters to daphnids. Interagency memorandum to "whom it may
concern." Washington, DC, US Environmental Protection Agency, Health
and Environmental Review Division, Environmental Effects Branch,
Office of Toxic Substances (TS-796).
Nielsen J, Akesson B, Skerfving S (1985) Phthalate ester exposure
-- air levels and health of workers processing polyvinylchloride.
American Industrial Hygiene Association journal, 46(11):643-647.
NIWR (1996) Occurrence of phthalates and organotins in sediments and
water in Norway. Oslo, Norwegian Institute for Water Research
(Report No. 0-96006).
NPRI (1996) National Pollutant Release Inventory. Ottawa, Ontario,
Environment Canada.
NTP (1982) Carcinogenesis bioassay of butyl benzyl phthalate (CAS
No. 85-68-7) in F344/N rats and B6C3F1 mice (feed study). Research
Triangle Park, NC, National Institutes of Health, National Toxicology
Program (NTP Technical Report No. 213; National Technical Information
Service Publication No. PB83-118398).
NTP (1989) Developmental toxicity evaluation of butyl benzyl
phthalate (CAS No. 85-68-7) administered in feed to CD rats on
gestational days 6 to 15. Research Triangle Park, NC, National
Institutes of Health, National Toxicology Program (Report No.
NTP-89-246; National Technical Information Service Publication No.
PB90-115346).
NTP (1990) Final report on the developmental toxicity of butyl
benzyl phthalate (CAS No. 85-68-7) in CD-1-Swiss mice. Research
Triangle Park, NC, National Institutes of Health, National Toxicology
Program (NTP Report No. 90-114; National Technical Information Service
Publication No. PB91-129999).
NTP (1997a) Toxicology and carcinogenesis studies of butyl benzyl
phthalate (CAS No. 85-68-7) in F344/N rats (feed studies). Research
Triangle Park, NC, National Institutes of Health, National Toxicology
Program (NTP Technical Report No. 458; NIH Publication No. 97-3374).
NTP (1997b) Effect of dietary restriction on toxicology and
carcinogenesis studies in F344/N rats and B6C3F1 mice. Research
Triangle Park, NC, National Institutes of Health, National Toxicology
Program (NTP Technical Report No. 460; NIH Publication No. 97-3376).
OMOE (1988) Thirty-seven municipal pollution control plants. Pilot
monitoring study. Vol. 1. Interim report. Ontario Ministry of the
Environment, Municipal/Industrial Strategy for Abatement (MISA).
OMOE (1990) Second report on the monitoring data for the petroleum
refining sector. July 1990. Ontario Ministry of the Environment,
Municipal/Industrial Strategy for Abatement (MISA).
OMOE (1991) Organic chemical manufacturing sector twelve month
report. Data from October 1/89 to September 30/90. Ontario
Ministry of the Environment, Municipal/Industrial Strategy for
Abatement (MISA).
Oppelt ET (1987) Incineration of hazard waste. A critical review.
Journal of the Air Pollution Control Association, 37:558-586.
Ozretich RJ, Randall RC, Boese BL, Schroeder WP, Smith JR (1983) Acute
toxicity of butylbenzyl phthalate to shiner perch ( Cymatogaster
aggregata). Archives of environmental contamination and
toxicology, 12:655-660.
Page BD, Lacroix GM (1995) The occurrence of phthalate ester and
di-2-ethylhexyl adipate plasticizers in Canadian packaging and food
sampled in 1985-1989: a survey. Food additives and contaminants,
12(1):129-151.
Painter SE, Jones WJ (1990) Anaerobic bioconversion of phthalic acid
esters by natural inocula. Environmental technology, 11:1015-1026.
Pankow JF, Ligocki MP, Rosen ME, Isabelle LM, Hart KM (1988)
Adsorption/thermal desorption with small cartridges for the
determination of trace aqueous semivolatile organic compounds.
Analytical chemistry, 60:40-47.
Pickering QH (1983) Chronic toxicity to fathead minnow Pimephales
promelas of wastewater from a conventional wastewater treatment
system receiving organic priority pollutants. Environmental
pollution (Series A), 31:105-117.
Piersma AH, Verhoef A, Dortant PM (1995) Evaluation of the OECD 421
reproductive toxicity screening test protocol using butyl benzyl
phthalate. Toxicology, 99(3):191-197.
Plumb RH (1991) The occurrence of Appendix IX organic constituents in
disposal site ground water. Ground water monitoring review,
11:157-164.
Price CJ, Field EA, Marr MC, Myers CB, Morrissey RE, Schwetz BA (1990)
Developmental toxicity of butyl benzyl phthalate (BBP) in mice and
rats. Teratology, 41(5):586 (Abstract P51).
Randall RC, Ozretich RJ, Boese BL (1983) Acute toxicity of butyl
benzyl phthalate to the saltwater fish English sole, Parophrys
vetulus. Environmental science and technology, 17:670-672.
Rhodes JE, Adams WJ, Biddinger GR, Robillard KA, Gorsuch JW (1995)
Chronic toxicity of 14 phthalate esters to Daphnia magna and rainbow
trout ( Oncorhynchus mykiss). Environmental toxicology and
chemistry, 14:1967-1976.
Ritsema R, Cofino WP, Frintrop PCM, Brinkman UAT (1989) Trace-level
analysis of phthalate esters in surface water and suspended
particulate matter by means of capillary gas chromatography with
electron-capture and mass-selective detection. Chemosphere,
18:2161-2175.
Robinson EC (1991) Lack of neuropathological changes in rats after
exposure to butyl benzyl phthalate. Journal of toxicology and
environmental health, 32(3):345-347.
Rubin RJ, Kozumbo W, Kroll R (1979) Ames mutagenic assay of a series
of phthalic acid esters: positive response of the dimethyl and diethyl
esters in TA100 [abstract]. Toxicology and applied pharmacology, 48
(1 Part 2):A133.
Saeger VW, Tucker ES (1976) Biodegradation of phthalic acid esters in
river water and activated sludge. Applied environmental
microbiology, 31:29-34.
Sharpe RM, Fisher JS, Millar MM, Jobling S, Sumpter JP (1995)
Gestational and lactational exposure of rats to xenoestrogens results
in reduced testicular size and sperm production. Environmental
health perspectives, 103(12):1136-1143.
Shelton DR, Tiedje JM (1984) General method for determining anaerobic
biodegradation potential. Applied environmental microbiology,
47:850-857.
SIGMA (1985) Study of the characterization of wastes and discharges
from selected organic chemical plants. Draft report prepared by
SIGMA Resource Consultants Limited for Environmental Protection
Service, Environment Canada, March 1985 (SRCL 3479).
Skinner JP (1992) Final report on the safety assessment of butyl
benzyl phthalate. Journal of the American College of Toxicology,
11(1):1-23.
Soto AM, Sonnenschein C, Chung KL, Fernandez MF, Olea N, Serrano FO
(1995) The E-SCREEN assay as a tool to identify estrogens: an update
on estrogenic environmental pollutants. Environmental health
perspectives, 103 (Suppl. 7):113-122.
Springborn Bionomics (1984) Chronic toxicity of fourteen phthalate
esters to Daphnia magna. Toxicity test report submitted to Chemical
Manufacturers Association, Washington, DC (Report No. BW-84-5-1567).
Springborn Bionomics (1986a) Chronic toxicity of butylbenzyl
phthalate to mysid shrimp (Mysidopsis bahia ). Toxicity test report
submitted to Monsanto Company, St. Louis, MO (Report No.
BW-86-7-2074).
Springborn Bionomics (1986b) Acute toxicity of butylbenzyl phthalate
to pink shrimp (Penaeus duorarum ) under flow-through conditions.
Toxicity test report submitted to Monsanto Company, St. Louis, MO
(Report No. BW-86-7-2093).
Staples CA, Werner AF, Hoogheem TJ (1985) Assessment of priority
pollutant concentrations in the United States using STORET database.
Environmental toxicology and chemistry, 4:131-142.
Statsek NK (1974) Hygienic investigations of certain esters of
phthalic acid and of polyvinylchloride materials plastificated
thereby. Gigiena i Sanitariya, 39(6):25-28 [cited in US NLM (1994)
Hazardous substances data bank. Bethesda, MD, National Library of
Medicine, National Toxicology Information Program.].
Stein VB, Amin TA, Narang RS (1987) Simplified method for determining
polychlorinated biphenyls, phthalates, and hexachlorocyclohexanes in
air. Journal of the Association of Official Analytical Chemists,
70(4):721-723.
Suggatt RH, Foote K (1981) Comprehensive review of acute aquatic
toxicity data on phthalate esters. Final report. Syracuse, NY,
Syracuse Research Corporation (Contract SRC TR81-537) [cited in
Staples CA, Adams WJ, Parkerton TF, Gorsuch JW, Biddinger GR, Reinert
KH (1997) Aquatic toxicity of eighteen phthalate esters.
Environmental toxicology and chemistry, 16(5):875-891].
Sun K, Krause GF, Mayer FL, Ellersieck MR, Basu AP (1995) Predicting
chronic lethality of chemicals to fishes from acute toxicity test
data: theory of accelerated life testing. Environmental toxicology
and chemistry, 14:1745-1752.
Swain LG, Walton DG (1990) Report on the 1989 Boundary Bay
Monitoring Program. British Columbia Department of the Environment.
Tetra Tech Inc. (1986) Development of sediment quality values for
Puget Sound. Vol. 1. Bellevue, WA, 128 pp.
TNO Biotechnology and Chemistry Institute (1993) Dietary
one-generation reproduction study with butyl benzyl phthalate in
rats. Contract for Monsanto Company. Submitted by Monsanto to Office
of Toxic Substances, US Environmental Protection Agency (Document
Identification No. 86-930000189; Microfiche No. OTS0538169).
TNO Nutrition and Food Research Institute (1997) Protocol for an
oral developmental reproduction study with butyl benzyl phthalate in
Wistar rats, 21 pp. (Project No. 470839; Study No. 1899).
TRI93 (1995) Toxic chemicals release inventory. Bethesda, MD,
National Library of Medicine, National Toxicology Information Program.
Tucker MW, Mosher RG, Adams WJ (1985) Acute toxicity of S-160 (butyl
benzyl phthalate) to the freshwater green alga, Selenastrum
capricornutum. St. Louis, MO, Monsanto Company (Report No.
ESC-EAG-85-38).
US EPA (1995) The use of the benchmark dose approach in health risk
assessment. Office of Research and Development, US Environmental
Protection Agency (EPA/630/R-94/007).
US EPA (1996) EPA Superfund Record of Decision: Petoskey Municipal
Well Field Superfund Site, Petoskey, MI, 6/14/1995. US Environmental
Protection Agency (EPA/ROD/R05-95/274; PB95-964102).
Valencia R, Mason JM, Woodruff RC, Zimmering S (1985) Chemical
mutagenesis testing in Drosophila. III. Results of 48 coded
compounds tested for the National Toxicology Program. Environmental
mutagenesis, 7(3):325-348.
Veith GD, Macek KJ, Petrocelli SR, Carroll J (1980) An evaluation of
using partition coefficients and solubility to estimate
bioconcentration factors for organic chemicals in fish. In: Eaton JG,
Parrish PR, Hendricks AC, eds. Aquatic toxicology. Philadelphia, PA,
American Society for Testing and Materials, pp. 116-129 (ASTM STP
707).
Verschueren K (1983) Handbook of environmental data on organic
chemicals, 2nd ed. New York, NY, Van Nostrand Reinhold Co.
Vitali M, Guidotti M, Macilenti G, Cremisini C (1997) Phthalate esters
in freshwaters as markers of contamination sources -- a site study in
Italy. Environment international, 23(3):337-347.
Volskay VT, Grady CPL (1988) Toxicity of selected RCRA compounds to
activated sludge microorganisms. Journal of the Water Pollution
Control Federation, 60:1850-1856.
Volskay VT, Grady CPL, Tabak HH (1990) Effect of selected RCRA
compounds on activated sludge activity. Research journal of the
Water Pollution Control Federation, 62:654-664.
Webber MD, Wang C (1995) Industrial organic compounds in selected
Canadian soils. Canadian journal of soil science, 75(4):513-524.
Weschler CJ (1984) Indoor-outdoor relationships for nonpolar organic
constituents of aerosol particles. Environmental science and
technology, 18:648-652.
Wild SR, Jones KC (1992) Organic chemicals entering agricultural soils
in sewage sludges: screening for their potential to transfer to crop
plants and livestock. The science of the total environment,
119:85-119.
Ziegenfuss PS, Renaudette WJ, Adams WJ (1986) Methodology for
assessing the acute toxicity of chemicals sorbed to sediments: testing
the equilibrium partitioning theory. In: Poston TM, Purdy R, eds.
Aquatic toxicology and environmental fate. Vol. 9. Philadelphia, PA,
American Society for Testing and Materials, pp. 479-493 (ASTM STP
921).
Zeiger E, Haworth S, Speck W, Mortelmans K (1982) Phthalate ester
testing in the National Toxicology Program's Environmental Mutagenesis
Test Development Program. Environmental health perspectives,
45:99-101.
Zeiger E, Haworth S, Mortelmans K, Speck W (1985) Mutagenicity testing
of di(2-ethylhexyl)phthalate and related chemicals in Salmonella.
Environmental mutagenesis, 7(2):213-232.
Zurmühl T, Durner W, Herrmann R (1991) Transport of phthalate-esters
in undisturbed and unsaturated soil columns. Journal of contaminant
hydrology, 8:111-133.
APPENDIX 1 -- SOURCE DOCUMENTS
Government of Canada (in press)
Copies of the Canadian Environmental Protection Act Priority
Substances List assessment report (Government of Canada, in press)
and unpublished supporting documentation for BBP may be obtained from:
Commercial Chemicals Branch
Environment Canada
14th Floor, Place Vincent Massey
351 St. Joseph Blvd.
Hull, Quebec
Canada K1A 0H3
or
Environmental Health Centre
Health Canada
Address Locator: 0801A
Tunney's Pasture
Ottawa, Ontario
Canada K1A 0L2
Initial drafts of the supporting documentation and Assessment
Report for BBP were prepared by staff of Health Canada and Environment
Canada.
The environmental sections were reviewed externally by Dr G.
Coyle (Monsanto Company), Dr T. Parkerton (Exxon Biomedical Sciences
Inc.), Mr A. Sardella (Monsanto Canada), and Dr D. Spry (Ontario
Ministry of Environment and Energy).
Sections of the supporting documentation pertaining to human
health were reviewed externally by Dr R. Nair (Solutia Inc.) to
address adequacy of coverage. Accuracy of reporting, adequacy of
coverage, and defensibility of conclusions with respect to hazard
identification and dose-response analyses were considered in written
review by staff of the Information Department of BIBRA International
and at a panel meeting of the following members convened by Toxicology
Excellence for Risk Assessment on 27 April 1998 in Cincinnati, Ohio,
USA:
Dr M. Abdel-Raman, University of Medicine and Dentistry of New
Jersey
Dr J. Christopher, California Environmental Protection Agency
Dr G. Datson, Procter & Gamble Co.
Dr J. Donohue, US Environmental Protection Agency
Dr M. Dourson, Toxicology Excellence for Risk Assessment
Ms D. Proctor, ChemRisk
Ms R. Rudel, Silent Spring Institute (submitted written comments;
not available to attend panel meeting)
Dr A. Stern, New Jersey Department of Environmental Protection
APPENDIX 2 -- CICAD PEER REVIEW
The draft CICAD on BBP was sent for review to institutions and
organizations identified by IPCS after contact with IPCS national
Contact Points and Participating Institutions, as well as to
identified experts. Comments were received from:
Chemical Industry Institute of Toxicology (CIIT), Research
Triangle Park, USA
Department of Health, London, United Kingdom
Fraunhofer Institute of Toxicology and Aerosol Research, Hanover,
Germany
Health and Safety Executive, Bootle, United Kingdom
Health Canada, Ottawa, Canada
József Fodor National Center of Public Health, Budapest, Hungary
Karolinska Institute, Stockholm, Sweden
National Chemicals Inspectorate (KEMI), Solna, Sweden
National Food Administration, Uppsala, Sweden
National Institute for Working Life, Solna, Sweden
National Institute of Public Health, Oslo, Norway
National Institute of Public Health and Environmental Protection,
Bilthoven, The Netherlands
Nofer Institute of Occupational Medicine, Lodz, Poland
Norwegian University of Science and Technology, Trondheim, Norway
Mr Frank Sullivan, Consultant Toxicologist, Brighton, United
Kingdom
United States Department of Health and Human Services (Agency for
Toxic Substances and Disease Registry, Atlanta, USA; National
Institute of Environmental Health Sciences, Research Triangle
Park, USA)
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
Tokyo, Japan, 30 June - 2 July 1998
Members
Dr R. Benson, Drinking Water Program, United States Environmental
Protection Agency, Denver, CO, USA
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
Mr R. Cary, Health Directorate, Health and Safety Executive,
Merseyside, United Kingdom
Dr C. DeRosa, Agency for Toxic Substances and Disease Registry, Center
for Disease Control and Prevention, Atlanta, GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Cambridgeshire, United
Kingdom
Dr H. Gibb, National Center for Environmental Assessment, United
States Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany
Ms M.E. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada ( Chairperson)
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan ( Vice-Chairperson)
Professor S.A. Soliman, Department of Pesticide Chemistry, Alexandria
University, Alexandria, Egypt
Ms D. Willcocks, Chemical Assessment Division, Worksafe Australia,
Camperdown, Australia ( Rapporteur)
Professor P. Yao, Chinese Academy of Preventive Medicine, Institute of
Occupational Medicine, Beijing, People's Republic of China
Observers
Professor F.M.C. Carpanini,1 Secretary-General, ECETOC (European
Centre for Ecotoxicology and Toxicology of Chemicals), Brussels,
Belgium
1 Invited but unable to attend.
Dr M. Ema, Division of Biological Evaluation, National Institute of
Health Sciences, Osakai, Japan
Mr R. Green,1 International Federation of Chemical, Energy, Mine and
General Workers' Unions, Brussels, Belgium
Dr B. Hansen,1 European Chemicals Bureau, European Commission, Ispra,
Italy
Mr T. Jacob,1 Dupont, Washington, DC, USA
Dr H. Koeter, Organisation for Economic Co-operation and Development,
Paris, France
Mr H. Kondo, Chemical Safety Policy Office, Ministry of International
Trade and Industry, Tokyo, Japan
Ms J. Matsui, Chemical Safety Policy Office, Ministry of International
Trade and Industry, Tokyo, Japan
Mr R. Montaigne,1 European Chemical Industry Council (CEFIC),
Brussels, Belgium
Dr A. Nishikawa, Division of Pathology, National Institute of Health
Sciences, Tokyo, Japan
Dr H. Nishimura, Environmental Health Science Laboratory, National
Institute of Health Sciences, Osaka, Japan
Ms C. Ohtake, Chem-Bio Informatics, National Institute of Health
Sciences, Tokyo, Japan
Dr T. Suzuki, Division of Food, National Institute of Health Sciences,
Tokyo, Japan
Dr K. Takeda, Mitsubishikagaku Institute of Toxicological and
Environmental Sciences, Yokohama, Japan
Dr K. Tasaka, Department of Chemistry, International Christian
University, Tokyo, Japan
Dr H. Yamada, Environment Conservation Division, National Research
Institute of Fisheries Science, Kanagawa, Japan
Dr M. Yamamoto, Chem-Bio Informatics, National Institute of Health
Sciences, Tokyo, Japan
Dr M. Yasuno, School of Environmental Science, The University of Shiga
Prefecture, Hikone, Japan
1 Invited but unable to attend.
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Secretariat
Ms L. Regis, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Mr A. Strawson, Health and Safety Executive, London, United Kingdom
Dr P. Toft, Associate Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
Ce CICAD relatif au phtalate de butyle et de benzyle a été
préparé conjointement par la Direction de l'Hygične du Milieu (Santé
Canada) et par la Direction de l'Evaluation des Produits chimiques
commerciaux (Environnement Canada) d'aprčs la documentation préparée
parallčlement dans le cadre du Programme d'évaluation des substances
d'intéręt prioritaire mené en vertu de la Loi canadienne sur la
protection de l'environnement (LCPE). L'objectif de l'évaluation des
substances d'intéręt prioritaire en vertu de la LCPE est d'évaluer les
effets potentiels sur la santé humaine de l'exposition indirecte aux
substances présentes dans l'environnement, ainsi que les effets de ces
substances sur l'environnement. Les analyses ont porté sur les données
connues ŕ fin avril 1998. On trouvera ŕ l'appendice 1 des informations
sur les modalités de l'examen par des pairs et sur les sources
documentaires. Les renseignements concernant l'examen du CICAD par des
pairs font l'objet de l'appendice 2. Ce CICAD a été approuvé en tant
qu'évaluation internationale lors d'une réunion du Comité d'évaluation
finale qui s'est tenue ŕ Tokyo (Japon) du 30 juin au 2 juillet 1998.
La liste des participants ŕ cette réunion figure ŕ l'appendice 3. La
fiche d'information internationale sur la sécurité chimique (ICSC
No 0834) pour le phtalate de butyle et de benzyle, établie par le
Programme international sur la Sécurité chimique (IPCS, 1993) est
également reproduite dans ce document.
Le phtalate de butyle et de benzyle (No CAS 85-68-7), ou PBB,
est un liquide huileux limpide utilisé comme plastifiant
principalement dans le poly(chlorure de vinyle) (PVC) pour les
revętements de sol, les mousses de vinyle et les sous-couches de tapis
et, dans une moindre mesure, dans les plastiques cellulosiques et le
polyuréthane. La plupart des rejets se font dans l'air. Une fois dans
l'environnement, le PBB se répartit entre l'atmosphčre, le sol, les
eaux de surface, les sédiments et les biotes, et a été détecté dans
chacun de ces compartiments.
Le PBB est éliminé de l'atmosphčre par photo-oxydation et par la
pluie, avec une demi-vie de quelques heures ŕ quelques jours. En
aérobiose, il ne persiste pas dans l'eau, les sédiments ni les sols,
sa demi-vie étant de quelques jours. En anaérobiose, il est plus
persistant, avec une demi-vie de quelques mois. Les vertébrés et les
invertébrés le métabolisent facilement. Les facteurs de
bioconcentration signalés sont inférieurs ŕ 1000 si l'on tient compte
des résidus totaux, et sont bien inférieurs ŕ 100 d'aprčs les résidus
intacts.
Les données disponibles sur l'homme sont insuffisantes pour
servir de base ŕ l'évaluation des effets d'une exposition ŕ long terme
au PBB sur les populations humaines.
La toxicité aiguë du PBB est relativement faible, la DL50 orale
chez le rat étant supérieure ŕ 2 g/kg de poids corporel. Aprčs
exposition aiguë, les cibles principales sont le sang et le systčme
nerveux central.
Les données disponibles sont insuffisantes pour évaluer les
effets irritants et sensibilisants du PBB chez l'animal.
La toxicité du PBB aprčs administration de doses répétées a été
largement étudiée lors d'études récentes, principalement chez le rat,
espčce pour laquelle une relation dose-réponse a été bien
caractérisée. Les effets réguličrement observés consistaient en une
diminution de la prise de poids (souvent accompagnée d'une diminution
de la consommation alimentaire) et en une augmentation du rapport du
poids des organes (notamment du foie et des reins) au poids total. On
a également observé des effets histopathologiques sur le pancréas et
les reins et des effets hématologiques. Aux fortes doses, une
dégénérescence des testicules et parfois des effets histopathologiques
sur le foie ont été rapportés. Des investigations spécialisées ont
montré une prolifération des peroxysomes du foie, bien que le PBB ait
fait preuve dans cette étude d'une activité plus faible que les autres
phtalates, par exemple le phtalate de bis(2-éthylhexyle).
La toxicité chronique et la cancérogénicité du PBB ont été
étudiées lors d'essais biologiques de l'US National Toxicology Program
(NTP) chez le rat (avec des protocoles alimentaires standard et
restrictifs) et chez la souris. Il a été conclu qu'il y avait
"certaines preuves" de cancérogénicité chez le rat mâle, d'aprčs une
incidence accrue des tumeurs du pancréas, et des preuves non
concluantes chez les rats femelles d'aprčs une augmentation marginale
des tumeurs du pancréas et de la vessie. L'administration d'une
alimentation restreinte empęchait l'expression complčte des tumeurs du
pancréas et retardait l'apparition des tumeurs de la vessie. Aucune
preuve de cancérogénicité n'a été trouvée chez la souris.
Les résultats des études de génotoxicité portant sur le PBB sont
clairement négatifs. Cependant, les données disponibles sont
insuffisantes pour permettre de conclure formellement ŕ l'absence de
clastogénicité du PBB, bien que dans certaines études il ait induit au
maximum une activité clastogčne faible, d'une intensité compatible
avec des effets secondaires sur l'ADN.
En résumé, le PBB a induit une augmentation des tumeurs du
pancréas chez les animaux d'un sexe d'une espčce, effet dont
l'expression totale était empęchée par une restriction alimentaire, et
une augmentation marginale des tumeurs de la vessie chez les animaux
de l'autre sexe, effet retardé par la restriction alimentaire. En ce
qui concerne la génotoxicité, les résultats ont été négatifs et, bien
qu'on ne puisse exclure un faible potentiel clastogčne, les données
disponibles permettent de penser que le composé n'exerce pas
d'interaction directe avec l'ADN. D'aprčs ces résultats, le PBB peut
ętre considéré au maximum comme éventuellement cancérogčne pour
l'homme, susceptible d'induire des tumeurs par un mécanisme non
génotoxique (mais inconnu).
Dans diverses études, y compris celles portant sur les effets du
PBB sur les testicules et les hormones endocrines chez le rat mâle,
des études avec un protocole modifié d'accouplement réalisées par le
NTP, et une étude sur une seule génération, on n'a en général observé
d'effets indésirables sur les testicules et par conséquent sur la
fécondité qu'aux doses supérieures ŕ celles qui induisent des effets
sur les autres organes (comme le foie et les reins), bien qu'une
diminution du nombre de spermatozoďdes ait été observée ŕ des doses
analogues ŕ celles qui induisent des effets sur le rein et le foie.
Ces résultats sont compatibles avec ceux des études de toxicité
portant sur des doses répétées.
Une diminution du poids des testicules et de la production
quotidienne de spermatozoďdes chez la descendance a été observée ŕ une
dose relativement faible chez des rats exposés in utero et pendant
l'allaitement lors d'une étude dans laquelle la relation dose-réponse
n'était pas examinée. Cependant, de tels effets n'ont pas été observés
lors d'une étude récente de conception similaire mais non identique
réalisée sur une autre souche de rats, chez lesquels seule une
augmentation du poids relatif et absolu du foie a été observée
90 jours aprčs la naissance. Des investigations supplémentaires sur
les effets potentiels du composé sur le systčme reproducteur d'animaux
mâles et femelles exposés in utero et pendant l'allaitement dans le
cadre d'études portant également sur la relation dose-réponse sont
souhaitables, et sont en cours.
Bien que le PBB ait des effets estrogéniques dans des lignées de
cellules humaines de cancer du sein in vitro, les résultats en
cellules de levure sont peu concluants. Ni le PBB ni ses principaux
métabolites ne sont utérotrophiques in vivo chez le rat ou la
souris. Si les données disponibles ne permettent pas de conclure que
le PBB a des propriétés estrogéniques, d'autres effets potentiels ŕ
médiation endocrinienne, par exemple un effet anti-androgčne associé
au phtalate de dibutyle, ne sont pas exclus.
On s'intéresse actuellement ŕ la mise au point de cadres plus
sensibles d'essai et d'évaluation des substances perturbant
l'équilibre endocrinien; des composés comme les phtalates sont
susceptibles d'ętre parmi les premiers candidats ŕ soumettre ŕ des
essais supplémentaires.
Lors de plusieurs études bien conduites chez des rats et des
souris, le PBB a induit des effets notables sur le développement, mais
seulement ŕ des doses qui entraînent une toxicité significative chez
la mčre.
Bien que la neurotoxicité potentielle du PBB n'ait pas été
largement explorée, il n'a pas été observé d'effets histopathologiques
sur le systčme nerveux central et périphérique aprčs exposition ŕ
court terme ŕ des doses relativement élevées dans l'alimentation. Les
données disponibles sont insuffisantes pour permettre d'évaluer la
toxicité immunologique potentielle du PBB.
Une dose journaličre tolérable estimative (DJT) de 1300 µg/kg de
poids corporel par jour a été calculée pour le PBB, d'aprčs la limite
inférieure de confiance ŕ 95% pour la dose associée ŕ une augmentation
de 5% de l'incidence des lésions pancréatiques chez le rat mâle lors
d'un essai biologique subchronique par voie orale, divisée par un
facteur d'incertitude de 100 (10 pour la variation interspécifique et
10 pour la variation intraspécifique). D'aprčs les concentrations
rencontrées dans les divers milieux de l'environnement, il apparaît
(d'aprčs des estimations) que la totalité de l'apport estimé est
imputable ŕ l'alimentation; cet apport est évalué, pour la population
générale, ŕ 2-6 µg/kg de poids corporel par jour. Ces estimations sont
200 ŕ 650 fois plus faibles que la DJT. Les données sont insuffisantes
pour estimer l'exposition dans le milieu de travail ou par des
produits de consommation.
Divers tests de toxicité réalisés sur des organismes aquatiques
ont montré que des effets indésirables se produisent lors
d'expositions ŕ des concentrations supérieures ou égales ŕ
100 µg/litre. Comme les concentrations de PBB dans les eaux de surface
sont en général inférieures ŕ 1 µg/litre, il est probable que ce
composé comportera peu de risques pour les organismes aquatiques.
On ne dispose d'aucune information sur les effets du PBB sur les
organismes benthiques, les invertébrés du sol, les plantes terrestres
ou les oiseaux, qui permettrait d'estimer le risque pour ces
organismes.
RESUMEN DE ORIENTACION
Este CICAD sobre el butil-bencil-ftalato, preparado conjuntamente
por la Dirección de Higiene del Medio del Ministerio de Sanidad del
Canadá y la División de Evaluación de Productos Químicos Comerciales
del Ministerio de Medio Ambiente del Canadá, se basa en la
documentación preparada al mismo tiempo como parte del Programa de
Sustancias Prioritarias en el marco de la Ley Canadiense de
Protección del Medio Ambiente (CEPA). Las evaluaciones de sustancias
prioritarias prevista en la CEPA tienen por objeto valorar los efectos
potenciales para la salud humana de la exposición indirecta en el
medio ambiente general, así como los efectos ecológicos. En estos
exámenes se incluyen los datos identificados hasta el final de abril
de 1998. La información relativa al carácter del examen colegiado del
documento original y su disponibilidad figura en el apéndice 1. La
información sobre el examen colegiado de este CICAD aparece en el
apéndice 2. Este CICAD se aprobó como evaluación internacional en una
reunión de la Junta de Evaluación Final, celebrada en Tokio (Japón)
del 30 de junio al 2 de julio de 1998. La lista de participantes en
esta reunión figura en el apéndice 3. La ficha internacional de
seguridad química (ICSC 0834) para el butil-bencil-ftalato, preparada
por el Programa Internacional de Seguridad de las Sustancias Químicas
(IPCS, 1993), también se reproduce en este documento.
El butil-bencil-ftalato (CAS No 85-68-7) o BBP es un líquido
oleoso transparente que se utiliza como plastificante sobre todo en el
cloruro de polivinilo (PVC) para la fabricación de baldosas de vinilo,
espumas de vinilo y entramado para alfombras y en menor medida también
en los plásticos de celulosa y el poliuretano. La mayor parte del que
se libera en el medio ambiente va al aire. Una vez en el medio
ambiente, el BBP se distribuye entre la atmósfera, el suelo, el agua
superficial, los sedimentos y la biota, y se ha detectado en cada uno
de estos compartimentos.
El BBP se elimina de la atmósfera por fotooxidación y por el agua
de lluvia, con una semivida que oscila entre algunas horas y varios
días. No es persistente en el agua, los sedimentos o el suelo en
condiciones aerobias, con una semivida de varios días. En condiciones
anaerobias, el BBP es más persistente, siendo su semivida de varios
meses. Los vertebrados e invertebrados lo metabolizan fácilmente. Se
han notificado factores de bioconcentración inferiores a 1000, tomando
como base los residuos totales, y muy por debajo de 100 a partir de
los residuos de BBP intactos.
Los datos disponibles en el ser humano son insuficientes para
poder evaluar los efectos de la exposición prolongada al BBP en
poblaciones humanas.
La toxicidad aguda del BBP es relativamente baja, con valores de
la DL50 por vía oral superiores a 2 g/kg de peso corporal en ratas.
Los órganos afectados tras la exposición aguda son el sistema
hematológico y el sistema nervioso central.
Los datos disponibles son insuficientes para evaluar los efectos
irritantes y sensibilizantes del BBP en especies de animales.
En estudios recientes se ha investigado a fondo la toxicidad de
dosis repetidas de BBP, particularmente en la rata, en la cual está
bien caracterizada la relación dosis-respuesta. Los efectos observados
han sido siempre una disminución del aumento del peso corporal (con
frecuencia acompańada de una reducción del consumo de alimentos) y un
incremento de la razón peso de los órganos/peso corporal,
especialmente para el rińón y el hígado. Se han observado asimismo
efectos en el páncreas y el rińón, así como hematológicos. Con dosis
más elevadas, se han notificado efectos degenerativos en los
testículos y, ocasionalmente, efectos histopatológicos en el hígado.
En investigaciones especializadas se ha observado proliferación de
peroxisomas en el hígado, aunque la potencia a este respecto fue
inferior a la de otros ftalatos, como el bis(2-etilhexil)ftalato
(DEHP).
Se han investigado la toxicidad crónica y la carcinogenicidad del
BBP en biovaloraciones realizadas por el Programa Nacional de
Toxicología de los Estados Unidos de América (con inclusión de
protocolos normales y de alimentación limitada) en ratas y en ratones.
Se llegó a la conclusión de que había "algunos indicios" de
carcinogenicidad en ratas macho, basados en una mayor incidencia de
tumores pancreáticos, e indicios equívocos en ratas hembra, basadas en
un aumento marginal de la incidencia de tumores pancreáticos y de
vejiga. La limitación de la alimentación impidió la expresión completa
de los tumores pancreáticos y retrasó la aparición de los tumores de
vejiga. No hubo indicios de carcinogenicidad en ratones.
El valor demostrativo de los indicios de genotoxicidad del BBP es
claramente negativo. Sin embargo, los datos disponibles son
insuficientes para llegar a la conclusión inequívoca de que el BBP no
es clastogénico, aunque en determinados estudios ha inducido, como
máximo, una actividad débil de magnitud compatible con los efectos
secundarios en el ADN.
Por consiguiente, el BBP ha inducido un aumento de los tumores
pancreáticos fundamentalmente en un sexo de una especie, cuya
expresión completa se evitó mediante un protocolo de alimentación
limitada, y un aumento marginal de los tumores de vejiga en el otro
sexo, que se retrasó con la limitación de la alimentación. El valor
demostrativo de los indicios de genotoxicidad es negativo y, aunque no
se puede descartar el potencial clastogénico, los datos disponibles
son compatibles con el hecho de que el compuesto no tiene una
interacción directa con el ADN. De acuerdo con esto, el BBP puede
considerarse, como máximo, posiblemente carcinogénico para el ser
humano, probablemente induciendo tumores a través de un mecanismo no
genotóxico (aunque desconocido).
En una serie de estudios, en particular los diseńados para
investigar los efectos reproductivos del BBP en los testículos y las
hormonas endocrinas de las ratas macho, un protocolo modificado de
acoplamiento realizado por el NTP y un estudio de una generación, en
general se han observado efectos adversos en los testículos y, por
consiguiente, en la fecundidad sólo con dosis superiores a las que
inducen efectos en otros órganos (como el rińón y el hígado), si bien
se ha puesto de manifiesto una reducción en el recuento de
espermatozoides con dosis semejantes a las que inducen efectos en el
rińón y el hígado. Esto está en consonancia con los resultados de los
estudios de toxicidad con dosis repetidas.
En un estudio en el que no se investigó la relación
dosis-respuesta se observó una reducción del peso de los testículos y
de la producción diaria de espermatozoides en crías de ratas expuestas
a una concentración relativamente baja en el útero y durante la
lactancia. Sin embargo, estos efectos no se observaron en un estudio
reciente de diseńo parecido, pero no idéntico, realizado con otra
estirpe de ratas en la cual sólo se observó un aumento del peso
absoluto y relativo del hígado a los 90 días del nacimiento. Son
convenientes, y se han emprendido ya, nuevas investigaciones de los
efectos potenciales en el sistema reproductor de animales machos y
hembras expuestos en el útero y durante la lactancia en estudios
encaminados a examinar la relación dosis-respuesta.
Si bien el BBP ha sido estrogénico en líneas de células de cáncer
de mama humano in vitro, los resultados en células de levadura han
sido contradictorios. Ni el BBP ni sus principales metabolitos han
sido uterotróficos in vivo en ratas o ratones. Aunque los datos
disponibles no permiten llegar a la conclusión de que el BBP es
estrogénico, no se pueden descartar otros posibles efectos debidos a
factores endocrinos, como la actividad antiandrogénica asociada al
dibutil-ftalato (DBP).
En la actualidad se concede una importancia considerable a la
creación de sistemas más sensibles de prueba y evaluación de
sustancias perturbadoras del sistema endocrino; compuestos como los
ftalatos probablemente serán de los primeros que se someterán a
pruebas adicionales.
En varios estudios bien realizados en ratas y ratones, el BBP
indujo efectos pronunciados en el desarrollo, pero sólo a
concentraciones que producen una toxicidad materna significativa.
Aunque no se ha investigado bien la posible neurotoxicidad del
BBP, no se han observado efectos histopatológicos en los sistemas
nerviosos central y periférico tras una exposición breve a
concentraciones relativamente altas en los alimentos. Los datos
disponibles son insuficientes para evaluar la posible inmunotoxicidad
del BBP.
Se ha estimado para el BBP una ingesta diaria tolerable (IDT)
muestral de 1300 µg/kg de peso corporal al día. Se basa en un límite
de confianza inferior del 95% para la dosis de referencia asociada con
un aumento del 5% de la incidencia de lesiones pancreáticas en ratas
macho en una biovaloración subcrónica por vía oral, dividido por un
factor de incertidumbre de 100 (10 por la variación interespecífica y
10 por la intraespecífica). Teniendo en cuenta las concentraciones en
diversos medios parece (a partir de estimaciones muestrales) que los
alimentos son la fuente de toda la ingesta estimada, que se considera
que oscila, para la población general, entre 2 y 6 µg/kg de peso
corporal al día. Estas estimaciones son 200-650 veces inferiores a la
IDT. Los datos fueron insuficientes para estimar la exposición en el
entorno ocupacional o a partir de productos de consumo.
En una serie de pruebas de toxicidad con organismos acuáticos se
ha puesto de manifiesto que se producen efectos adversos a
concentraciones de exposición iguales o superiores a 100 µg/l. Habida
cuenta de que la concentración en el agua superficial suele ser
inferior a 1 µg/l, es probable que el BBP represente un riesgo bajo
para los organismos acuáticos.
No se dispone de información acerca de los efectos del BBP en los
organismos de los sedimentos, los invertebrados del suelo, las plantas
terrestres o las aves en la que pueda basarse una estimación del
riesgo para estos organismos.
 1. EXECUTIVE SUMMARY
This CICAD on butyl benzyl phthalate was prepared jointly by the
Environmental Health Directorate of Health Canada and the Commercial
Chemicals Evaluation Division of Environment Canada based on
documentation prepared concurrently as part of the Priority Substances
Program under the Canadian Environmental Protection Act (CEPA). The
objective of assessments on Priority Substances under CEPA is
to assess potential effects of indirect exposure in the general
environment on human health as well as environmental effects. Data
identified as of the end of April 1998 were considered in these
reviews. Information on the nature of the peer review and availability
of the source document is presented in Appendix 1. Information on the
peer review of this CICAD is presented in Appendix 2. This CICAD was
approved as an international assessment at a meeting of the Final
Review Board, held in Tokyo, Japan, on 30 June - 2 July 1998.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 0834) for butyl benzyl
phthalate, produced by the International Programme on Chemical Safety
(IPCS, 1993), has also been reproduced in this document.
Butyl benzyl phthalate (CAS No.
1. EXECUTIVE SUMMARY
This CICAD on butyl benzyl phthalate was prepared jointly by the
Environmental Health Directorate of Health Canada and the Commercial
Chemicals Evaluation Division of Environment Canada based on
documentation prepared concurrently as part of the Priority Substances
Program under the Canadian Environmental Protection Act (CEPA). The
objective of assessments on Priority Substances under CEPA is
to assess potential effects of indirect exposure in the general
environment on human health as well as environmental effects. Data
identified as of the end of April 1998 were considered in these
reviews. Information on the nature of the peer review and availability
of the source document is presented in Appendix 1. Information on the
peer review of this CICAD is presented in Appendix 2. This CICAD was
approved as an international assessment at a meeting of the Final
Review Board, held in Tokyo, Japan, on 30 June - 2 July 1998.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 0834) for butyl benzyl
phthalate, produced by the International Programme on Chemical Safety
(IPCS, 1993), has also been reproduced in this document.
Butyl benzyl phthalate (CAS No. 