
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
CONCISE INTERNATIONAL CHEMICAL ASSESSMENT DOCUMENT NO. 24
CRYSTALLINE SILICA, QUARTZ
INTER-ORGANIZATION PROGRAMME FOR THE SOUND MANAGEMENT OF CHEMICALS
A cooperative agreement among UNEP, ILO, FAO, WHO, UNIDO, UNITAR and
OECD
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organization, or the World Health Organization.
First draft prepared by Ms F. Rice, National Institute of Occupational
Safety and Health, Cincinnati, OH, USA
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organization, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 2000
The International Programme on Chemical Safety (IPCS),
established in 1980, is a joint venture of the United Nations
Environment Programme (UNEP), the International Labour Organization
(ILO), and the World Health Organization (WHO). The overall objectives
of the IPCS are to establish the scientific basis for assessment of
the risk to human health and the environment from exposure to
chemicals, through international peer review processes, as a
prerequisite for the promotion of chemical safety, and to provide
technical assistance in strengthening national capacities for the
sound management of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization, the United Nations
Institute for Training and Research, and the Organisation for Economic
Co-operation and Development (Participating Organizations), following
recommendations made by the 1992 UN Conference on Environment and
Development to strengthen cooperation and increase coordination in the
field of chemical safety. The purpose of the IOMC is to promote
coordination of the policies and activities pursued by the
Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing-in-Publication Data
Crystalline silica, quartz.
(Concise international chemical assessment document ; 24)
1.Quartz - toxicity 2.Quartz - adverse effects
3.Risk assessment 4.Occupational exposure
5.Environmental exposure 6.Epidemiologic studies
I.Programme on Chemical Safety II.Series
ISBN 92 4 153023 5 (NLM Classification: QV 633)
ISSN 1020-6167
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 2000
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city, or area
or of its authorities, or concerning the delimitation of its frontiers
or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
The Federal Ministry for the Environment, Nature Conservation and
Nuclear Safety, Germany, provided financial support for the printing
of this publication.
TABLE OF CONTENTS
FOREWORD
1. EXECUTIVE SUMMARY
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
3. ANALYTICAL METHODS
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposure
8.2. Short-term exposure
8.3. Long-term exposure and carcinogenicity
8.3.1. Interaction with other compounds
8.4. Genotoxicity and related end-points
8.5. Reproductive and developmental toxicity
8.6. Immunological and neurological effects
9. EFFECTS ON HUMANS
9.1. Case reports
9.2. Epidemiological studies
9.2.1. Silicosis
9.2.2. Pulmonary tuberculosis and other infections
9.2.3. Lung cancer
9.2.4. Autoimmune-related disease
9.2.5. Renal disease
9.2.6. Chronic obstructive pulmonary disease
9.2.7. Other adverse health effects
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
11. EFFECTS EVALUATION
11.1. Evaluation of health effects
11.1.1. Hazard identification and dose-response assessment
11.1.2. Criteria for setting tolerable intakes or guidance values for quartz
11.1.3. Sample risk characterization
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
APPENDIX 1 -- SOURCE DOCUMENTS
APPENDIX 2 -- CICAD PEER REVIEW
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
APPENDIX 4 -- INTERNATIONAL CHEMICAL SAFETY CARD
RÉSUMÉ D'ORIENTATION
RESUMEN DE ORIENTACI²N
FOREWORD
Concise International Chemical Assessment Documents (CICADs) are
the latest in a family of publications from the International
Programme on Chemical Safety (IPCS) -- a cooperative programme of the
World Health Organization (WHO), the International Labour Organization
(ILO), and the United Nations Environment Programme (UNEP). CICADs
join the Environmental Health Criteria documents (EHCs) as
authoritative documents on the risk assessment of chemicals.
CICADs are concise documents that provide summaries of the
relevant scientific information concerning the potential effects of
chemicals upon human health and/or the environment. They are based on
selected national or regional evaluation documents or on existing
EHCs. Before acceptance for publication as CICADs by IPCS, these
documents undergo extensive peer review by internationally selected
experts to ensure their completeness, accuracy in the way in which the
original data are represented, and the validity of the conclusions
drawn.
The primary objective of CICADs is characterization of hazard and
dose-response from exposure to a chemical. CICADs are not a summary of
all available data on a particular chemical; rather, they include only
that information considered critical for characterization of the risk
posed by the chemical. The critical studies are, however, presented in
sufficient detail to support the conclusions drawn. For additional
information, the reader should consult the identified source documents
upon which the CICAD has been based.
Risks to human health and the environment will vary considerably
depending upon the type and extent of exposure. Responsible
authorities are strongly encouraged to characterize risk on the basis
of locally measured or predicted exposure scenarios. To assist the
reader, examples of exposure estimation and risk characterization are
provided in CICADs, whenever possible. These examples cannot be
considered as representing all possible exposure situations, but are
provided as guidance only. The reader is referred to EHC 1701 for
advice on the derivation of health-based tolerable intakes and
guidance values.
1 International Programme on Chemical Safety (1994) Assessing human
health risks of chemicals: derivation of guidance values for
health-based exposure limits. Geneva, World Health Organization
(Environmental Health Criteria 170).
While every effort is made to ensure that CICADs represent the
current status of knowledge, new information is being developed
constantly. Unless otherwise stated, CICADs are based on a search of
the scientific literature to the date shown in the executive summary.
In the event that a reader becomes aware of new information that would
change the conclusions drawn in a CICAD, the reader is requested to
contact IPCS to inform it of the new information.
Procedures
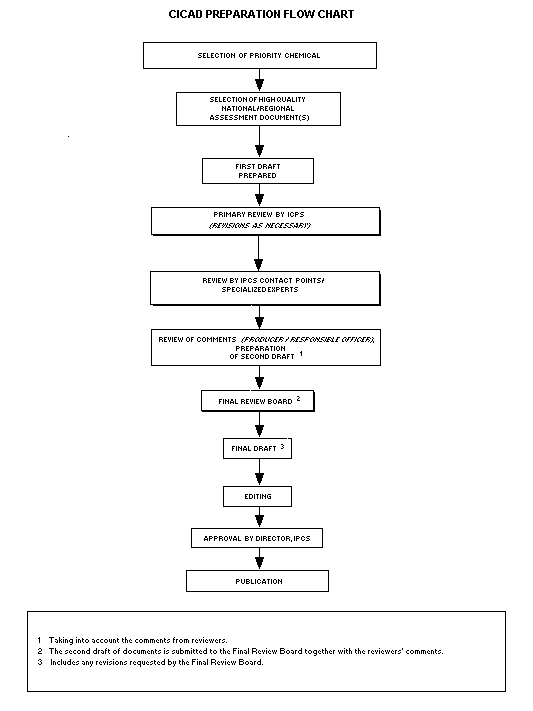
The flow chart shows the procedures followed to produce a CICAD.
These procedures are designed to take advantage of the expertise that
exists around the world -- expertise that is required to produce the
high-quality evaluations of toxicological, exposure, and other data
that are necessary for assessing risks to human health and/or the
environment.
The first draft is based on an existing national, regional, or
international review. Authors of the first draft are usually, but not
necessarily, from the institution that developed the original review.
A standard outline has been developed to encourage consistency in
form. The first draft undergoes primary review by IPCS to ensure that
it meets the specified criteria for CICADs.
The second stage involves international peer review by scientists
known for their particular expertise and by scientists selected from
an international roster compiled by IPCS through recommendations from
IPCS national Contact Points and from IPCS Participating Institutions.
Adequate time is allowed for the selected experts to undertake a
thorough review. Authors are required to take reviewers' comments into
account and revise their draft, if necessary. The resulting second
draft is submitted to a Final Review Board together with the
reviewers' comments.
The CICAD Final Review Board has several important functions:
- to ensure that each CICAD has been subjected to an appropriate
and thorough peer review;
- to verify that the peer reviewers' comments have been addressed
appropriately;
- to provide guidance to those responsible for the preparation of
CICADs on how to resolve any remaining issues if, in the opinion
of the Board, the author has not adequately addressed all
comments of the reviewers; and
- to approve CICADs as international assessments.
Board members serve in their personal capacity, not as representatives
of any organization, government, or industry. They are selected
because of their expertise in human and environmental toxicology or
because of their experience in the regulation of chemicals. Boards are
chosen according to the range of expertise required for a meeting and
the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
 1. EXECUTIVE SUMMARY
This CICAD on crystalline silica, quartz was based on the
following three extensive peer-reviewed documents on the health
effects of crystalline silica, including quartz: (1) a review of
published human studies and reports on the adverse health effects of
quartz exposure (NIOSH, forthcoming), (2) a review of the
carcinogenicity studies conducted by the International Agency for
Research on Cancer (IARC, 1997), and (3) a review of the non-cancer
health effects of ambient quartz (US EPA, 1996). The source documents
had different emphases on different end-points, and the CICAD was
developed to assess all the adverse health effects identified in these
documents. It is to be noted that despite the different emphases, the
final conclusions of all source documents were very similar. A
comprehensive literature search of several on-line databases was
conducted. Data identified as of March 1999 are included in this
review.
This CICAD considers the most common form of crystalline silica
(i.e., quartz). It does not consider experimental studies of the
effects of other forms of crystalline silica (e.g., cristobalite,
tridymite, stishovite, or coesite), coal dust, diatomaceous earth, or
amorphous silica, because their in vitro toxicities differ from that
of quartz. Differences in induction of fibrogenicity of quartz,
cristobalite, and tridymite were demonstrated in vivo in an early
rat study. However, there are virtually no experimental studies that
systematically evaluated exactly the same material to which humans are
exposed. The IARC Working Group considered the possibility that there
may be differences in the carcinogenic potential among polymorphs of
crystalline silica. However, some of the epidemiological studies
evaluated lung cancer among workers in "mixed environments" where
quartz may be heated and varying degrees of conversion to cristobalite
or tridymite can occur (e.g., ceramics, pottery, and refractory brick
industries), and exposures specifically to quartz or cristobalite were
not delineated. Although there were some indications that cancer risks
varied by industry and process in a manner suggestive of
polymorph-specific risks, the Working Group could reach only a single
conclusion for quartz and cristobalite. The CICAD reflects the
discussion and conclusion of that source document; therefore, when
considering the carcinogenicity of quartz in the occupational setting,
it does not distinguish between epidemiological studies of quartz and
those of cristobalite.
The peer review process for this CICAD was targeted to include
review by an international group of experts selected for their
knowledge about the current controversies and issues surrounding
quartz. Information on the nature of the peer review and the
availability of the source documents is presented in Appendix 1.
Information on the peer review of this CICAD is presented in
Appendix 2. This CICAD was approved as an international assessment at
a meeting of the Final Review Board, held in Sydney, Australia, on
21-24 November 1999. Participants at the Final Review Board meeting
are listed in Appendix 3. The International Chemical Safety Card
(ICSC 0808) for crystalline silica, quartz has been reproduced in
Appendix 4 (IPCS, 1993).
Quartz (CAS No. 14808-60-7) is a frequently occurring solid
component of most natural mineral dusts. Human exposures to quartz
occur most often during occupational activities that involve movement
of earth, disturbance of silica-containing products (e.g., masonry,
concrete), or use or manufacture of silica-containing products.
Environmental exposure to ambient quartz dust can occur during
natural, industrial, and agricultural activities. Respirable quartz
dust particles can be inhaled and deposited in the lung; however, no
conclusions have been made about the clearance kinetics of quartz
particles in humans.
Quartz dust induces cellular inflammation in vivo. Short-term
experimental studies of rats have found that intratracheal
instillation of quartz particles leads to the formation of discrete
silicotic nodules in rats, mice, and hamsters. Inhalation of
aerosolized quartz particles impairs alveolar macrophage clearance
functions and leads to progressive lesions and pneumonitis. Oxidative
stress (i.e., increased formation of hydroxyl radicals, reactive
oxygen species, or reactive nitrogen species) has been observed in
rats after intratracheal instillation or inhalation of quartz. Many
experimental in vitro studies have found that the surface
characteristics of the crystalline silica particle influence its
fibrogenic activity and a number of features related to its
cytotoxicity. Although many potential contributory mechanisms have
been described in the literature, the mechanisms responsible for
cellular damage by quartz particles are complex and not completely
understood.
Long-term inhalation studies of rats and mice have shown that
quartz particles produce cellular proliferation, nodule formation,
suppressed immune functions, and alveolar proteinosis. Experimental
studies of rats reported the occurrence of adenocarcinomas and
squamous cell carcinomas after the inhalation or intratracheal
instillation of quartz. Pulmonary tumours were not observed in
experiments with hamsters or mice. Adequate dose-response data (e.g.,
no-adverse-effect or lowest-adverse-effect levels) for rats or other
rodents are not available because few multiple-dose carcinogenicity
studies have been performed.
Quartz did not test positively in standard bacterial mutagenesis
assays. Results of genotoxicity studies of quartz conflict, and a
direct genotoxic effect for quartz has not been confirmed or ruled
out.
In experimental studies of particles, results may vary depending
on the test material, particle size of the material, concentration
administered, and species tested. The experiments with quartz
particles involved specimens from various sources, using various
doses, particle sizes, and species, which could have affected the
observations.
Data on the reproductive and developmental effects of quartz in
laboratory animals are not available.
The adverse effects of quartz in aquatic organisms and
terrestrial mammals have not been studied.
There are many epidemiological studies of occupational cohorts
exposed to respirable quartz dust. Silicosis, lung cancer, and
pulmonary tuberculosis are associated with occupational exposure to
quartz dust. IARC classified inhaled crystalline silica (quartz or
cristobalite) from occupational sources as a Group 1 carcinogen based
on sufficient evidence of carcinogenicity in humans and experimental
animals; "in making the overall evaluation, the Working Group noted
that carcinogenicity in humans was not detected in all industrial
circumstances studied. Carcinogenicity may be dependent on inherent
characteristics of the crystalline silica or on external factors
affecting its biological activity or distribution of its polymorphs"
(IARC, 1997).
Statistically significant increases in deaths or cases of
bronchitis, emphysema, chronic obstructive pulmonary disease,
autoimmune-related diseases (i.e., scleroderma, rheumatoid arthritis,
systemic lupus erythematosus), and renal diseases have been reported.
Silicosis is the critical effect for hazard identification and
exposure-response assessment. There are sufficient epidemiological
data to allow the risk of silicosis to be quantitatively estimated,
but not to permit accurate estimations of risks for other health
effects mentioned above. (A pooled risk assessment of epidemiological
studies of silica and lung cancer is in progress at IARC.)
The risk estimates for silicosis prevalence for a working
lifetime of exposure to respirable quartz dust concentrations of about
0.05 or 0.10 mg/m3 in the occupational environment vary widely (i.e.,
2-90%). Regarding exposure to ambient quartz in the general
environment, a benchmark dose analysis predicted that the silicosis
risk for a continuous 70-year lifetime exposure to 0.008 mg/m3
(estimated high crystalline silica concentration in US metropolitan
areas) is less than 3% for healthy individuals not compromised by
other respiratory diseases or conditions and for ambient environment
(US EPA, 1996). The silicosis risk for persons with respiratory
diseases exposed to ambient quartz in the general environment was not
evaluated.
Uncertainties exist in the evaluation of epidemiological studies
and the risk assessment of health effects related to quartz dust
exposure. The difficulties, many of which are inherent to the study of
respiratory diseases in occupational populations, include limitations
in the amount and quality of historical exposure data, deficiencies in
data on potentially confounding factors, such as cigarette smoking,
and difficulties in the interpretation of chest radiographs as
evidence of exposure. In addition, occupational exposures to quartz
dust are complex because workers are frequently exposed to dust
mixtures that contain quartz and other mineral varieties. Properties
of the dust (e.g., particle size, surface properties, crystalline
form) may differ according to geological source and can also change
during industrial processing. Such variations can affect the
biological activity of the inhaled dust. The IARC Working Group
evaluated the carcinogenicity of crystalline silica (including quartz)
and focused on epidemiological studies that were the least likely to
have been affected by confounding and selection biases and that
evaluated exposure-response relationships (IARC, 1997).
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
"Silica," or silicon dioxide (SiO2), occurs in either a
crystalline or non-crystalline (amorphous) form. Crystalline silica
may be found in more than one form (polymorphism), depending on the
orientation and position of the tetrahedra (i.e., the
three-dimensional basic unit of all forms of crystalline silica). The
natural crystalline forms of silica are alpha-quartz, ß-quartz,
alpha-, ß1-, and ß2-tridymite, alpha- and ß-cristobalite, coesite,
stishovite, and moganite (IARC, 1997). This document discusses the
most common form of naturally occurring crystalline silica -- quartz
(CAS No. 14808-60-7). Cristobalite (CAS No. 14464-46-1) and tridymite
(CAS No. 15468-32-3) exist in nature, but they can also be created
during industrial processes, such as the calcination of diatomaceous
earth, ceramics manufacturing, foundry processes, silicon carbide
manufacturing, and any other process in which quartz is heated to high
temperatures (NIOSH, 1974; Altieri et al., 1984; Virta, 1993;
Weill et al., 1994; IARC, 1997).
Quartz is a colourless, odourless, non-combustible solid and a
component of many mineral dusts (NIOSH, 1997). It is insoluble in
water (NIOSH, 1997). When quartz is cut, ground, or milled, the
crystal is fractured, and Si and Si-O radicals may be generated on the
cleavage surfaces (Castranova et al., 1996). Trace metal impurities,
such as iron and aluminium, can modify the surface reactivity of
quartz (Fubini et al., 1995; Fubini, 1997, 1998; IARC, 1997; Donaldson
& Borm, 1998).
Most of the experimental studies described in section 8 used
Min-U-Sil or DQ 12 quartz. Min-U-Sil is a trade name, and the number
that follows (e.g., Min-U-Sil 5) describes the particle size of the
sample (e.g., Min-U-Sil 5 is <5 µm in diameter). The purity is 99%
quartz (IARC, 1997). However, the geological sources of crystals have
varied; consequently, the associated impurities may have varied. The
particle size distributions of Min-U-Sil and several other reference
standards for the quantification of quartz in coal mine dust have been
investigated (Huggins et al., 1985), but a comprehensive report has
not been published on the analytical characteristics of a standard
sample of Min-U-Sil and the reproducibility of its aliquots
(Saffiotti et al., 1993). DQ 12 is a quartz sand that contains 87%
crystalline silica; the remaining proportion is amorphous silica, with
small contaminations of kaolinite. All DQ 12 samples originate from
the same source, but its particle size and composition have not been
reported recently (IARC, 1997). Furthermore, many experimental and
epidemiological studies do not state the source and properties of the
quartz that is used as a test material or collected in the workplace
(Mossman & Churg, 1998).
Additional physical and chemical properties of quartz are
presented in the International Chemical Safety Card (ICSC 0808)
reproduced in this document (Appendix 4).
3. ANALYTICAL METHODS
Mineral dust particles, such as quartz particles, are typically
described by diameter size (e.g., geometric mean diameter) and
aerodynamic diameter. Both characteristics are important in
determining whether the particle is respirable (IARC, 1997). Analysis
for airborne quartz is usually by X-ray diffraction or infrared
spectrophotometry in combination with filter collection methods
(IARC, 1997). Dust levels can be based on counts from an impinger or
on mass collected on a filter (IARC, 1997). Currently, the latter
method is more commonly used. Most countries (e.g., the USA, the
United Kingdom, Germany, Japan, and Australia) require that the sample
be restricted to the respirable fraction (IARC, 1997). The estimated
detection limit for quartz in respirable dust samples is 0.005 mg
using US National Institute for Occupational Safety and Health (NIOSH)
method 7500 (i.e., X-ray powder diffraction) (NIOSH, 1994a). The
estimated detection limit for quartz in respirable dust samples with
NIOSH method 7602 (infrared absorption spectrophotometry) is also
0.005 mg (NIOSH, 1994b).
4. SOURCES OF HUMAN AND ENVIRONMENTAL
EXPOSURE
Quartz is abundant in most rocks, sands, and soils (IARC, 1997).
The extensive natural occurrence of quartz and the wide uses of the
materials that contain quartz are directly related to potential
occupational exposures to quartz for workers in many industries and
occupations. Virtually any process that involves movement of earth
(e.g., mining, farming, construction), disturbance of
silica-containing products such as masonry and concrete, or use of
sand and other silica-containing products (e.g., foundry processes)
may potentially expose a worker to quartz (IARC, 1997).
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND
TRANSFORMATION
Environmental exposures to quartz can occur when ambient quartz
is emitted into the air as a component of particulate emissions
produced by natural, industrial, and farming activities
(US EPA, 1996). These activities include construction and demolition,
quarrying and mining, dust from travel on paved and unpaved roads,
electrical power generation, agricultural tilling, forest fires,
volcanic eruptions, and wind erosion (US EPA, 1996; IARC, 1997).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Ambient quartz is emitted to the environment as a component of
particulate emissions. The available data on concentrations of quartz
in the non-occupational environment (i.e., ambient air), including
data collected by the US Environmental Protection Agency (US EPA), are
limited (US EPA, 1996). The US EPA's Inhalable Particulate Network
provides a data set of quartz concentrations that were collected from
high-volume or dichotomous samples of ambient aerosols in 25 US cities
in 1980 (Davis et al., 1984). Average quartz concentrations were
highest (and most variable) in air masses in continental interior
sites. Ambient quartz concentrations collected from high-volume filter
samples of total suspended particulates in 10 US cities ranged from
0 µg/m3 (Portland, Oregon) to 15.8 µg/m3 (Akron, Ohio)
(Davis et al., 1984). Both of those findings were based on one sample
in each city (US EPA, 1996).
Non-occupational inhalation of quartz may occur while using a
variety of commercial products, such as cleansers, cosmetics,
art clays and glazes, pet litter, talcum powder, caulk, putty, paint,
and mortar (US Department of the Interior, 1992). Data representing
quantitative exposure levels of respirable quartz during
non-occupational uses of commercial products are not available.
Although quartz particles may be present in water, quantitative
data on concentrations of quartz in potable or other forms of
drinking-water are not available (IARC, 1997).
Occupational quartz dust exposure is probably one of the most
documented workplace exposures. Nearly every mineral deposit contains
some quartz (Greskevitch et al., 1992); thus, most quartz exposures
are to mixed dust with a variable quartz content that must be measured
by dust collection and analysis (Wagner, 1995). Compliance officers
for the US Occupational Safety and Health Administration measured
respirable quartz in 255 industries that were targeted for inspection,
excluding mining and agriculture. In 48% of the industries, average
overall exposure exceeded the permissible exposure level (10 mg
respirable dust/m3 divided by % silica + 2) (Freeman & Grossman,
1995).
Respirable quartz levels exceeding 0.1 mg/m3 have been reported
in many industries worldwide and are most frequently found in metal,
non-metal, and coal mines and mills; in granite quarrying and
processing, crushed stone and related industries; in foundries; in the
ceramics industry; and in construction and sandblasting operations
(IARC, 1997). IARC summarized data from the main industries for which
quantitative quartz exposure levels were available in the published
literature or where major occupational health studies were conducted
(IARC, 1997). The IARC review is condensed here and presented by
industry. Many processes in these industries include potential
exposures to other substances with known adverse health effects,
including carcinogenicity. Information about the health hazards for a
particular industry, the variability of the proportion of quartz found
in total dust samples from different industries, and the estimated
proportion of workers exposed to defined concentrations is available
elsewhere (e.g., IARC, 1984, 1987, 1997; Burgess, 1995; Linch et al.,
1998).
The mean respirable quartz level in mining operations (i.e.,
underground and surface mining, milling operations) inspected in the
USA from 1988 to 1992 was usually less than 0.10 mg/m3, but a
significant percentage of samples exceeded the permissible exposure
limit (see above) (Watts & Parker, 1995; IARC, 1997). Estimated
arithmetic mean respirable crystalline silica levels (form of
"crystalline silica" was not specified) for 1950-1959 and 1981-1987 in
20 Chinese mines (10 tungsten, 6 iron-copper, and 4 tin) decreased
about 10-fold between those periods. The estimated arithmetic mean
level of respirable silica (mg/m3) for the older period and the more
recent period, respectively, were as follows: underground mining,
4.89, 0.39; surface mining, 1.75, 0.27; ore dressing, 3.45, 0.42;
tungsten mines, 4.99, 0.46; iron and copper mines, 0.75, 0.20; and tin
mines, 3.49, 0.45 (Dosemeci et al., 1995; IARC, 1997). Respirable
quartz concentrations in underground dust from South African gold
mines ranged from 0.05 to 0.58 mg/m3 in surveys taken during
1965-1967 (Beadle & Bradley, 1970). In a copper mine in Finland, the
mean concentration of respirable quartz in the general mine air was
about 0.16 mg/m3 until 1965, 0.12 mg/m3 in 1966-1975, and 0.08
mg/m3 after 1981 (Ahlman et al., 1991).
Exposure to respirable quartz dust can occur in granite quarrying
and processing, including crushed stone and related industries.
Geometric mean air concentrations and air concentrations of quartz
from personal breathing-zone samples collected during various jobs in
the granite quarrying and processing industries and crushed stone and
related industries in Finland, the USA, and the United Kingdom ranged
from 0.03 to 1.5 mg/m3 and from not detectable to 135 mg/m3,
respectively (Donaldson et al., 1982; Eisen et al., 1984; Koskela et
al., 1987; Davies et al., 1994; Kullman et al., 1995; IARC, 1997). In
US granite quarries and sheds, control measures implemented in the
late 1930s and the 1940s resulted in 10- to 100-fold reductions of
formerly high dust levels (Davis et al., 1983; IARC, 1997).
In India, personal respirable dust levels of 0.06-1.12 mg/m3
(average 0.61 mg/m3 with a free silica content of 15%; form of silica
not specified) were generated during the manufacture of slate pencils
from natural rock. Average personal dust concentrations measured in
previous surveys in 1977 and 1982 were 10- to 100-fold higher (Fulekar
& Alam Kham, 1995; IARC, 1997).
Foundry occupations can involve exposure to quartz-containing
sands and parting powders (e.g., silica flour). The quartz content of
sands ranges from 5% to nearly 100% (IARC, 1997). Foundry occupations
with particularly high potential exposures to quartz (e.g., sand or
silica flour) are those jobs that involve sand preparation and
reclamation, knocking-out or shaking-out, cleaning of castings (i.e.,
fettling, grinding, sandblasting), and furnace and ladle refractory
relining and repair (IARC, 1997). Mean personal respirable quartz
levels in iron, steel, aluminium, brass, and other types of foundries
ranged from 0.19 to 5.26 mg/m3 in Finland (Siltanen et al., 1976;
IARC, 1997) and from 0.13 to 0.63 mg/m3 in Sweden (Gerhardsson, 1976;
IARC, 1997); in Canadian iron and steel foundries, the mean personal
respirable quartz concentration was 0.086 mg/m3 (Oudyk, 1995; IARC,
1997).
IARC presented respirable quartz dust levels for jobs in
ceramics, brick, cement, or glass industries in China (Dosemeci et
al., 1995), Italy (Cavariani et al., 1995), the Netherlands (Buringh
et al., 1990), South Africa (Myers et al., 1989; Rees et al., 1992),
the United Kingdom (Bloor et al., 1971; Fox et al., 1975; Higgins et
al., 1985), and the USA (Anderson et al., 1980; Salisbury & Melius,
1982; Cooper et al., 1993) and noted that jobs involving mixing,
moulding, glaze spraying, and finishing were associated with higher
exposure levels, often in the range of 0.1-0.3 mg/m3 (IARC, 1997). In
ceramic and pottery manufacturing facilities, exposures are mainly to
quartz, but potential exposures to cristobalite may occur where high
temperatures are used (e.g., ovens) (IARC, 1997). In refractory brick
and diatomaceous earth processing facilities, the raw materials
(amorphous or crystalline silica) are processed at temperatures near
1000°C, with varying degrees of conversion to cristobalite (IARC,
1997).
In the construction industry, drilling, sandblasting, sawing,
grinding, cleaning, and many other actions that are applied to
concrete, mortar surfaces, brick, rock, and other silica-containing
substances and products can result in the generation of a fine
airborne dust (Lofgren, 1993; Linch & Cocalis, 1994; NIOSH, 1996; IARC
1997). Concrete finishers and masons in the USA (Lofgren, 1993),
caisson workers in Hong Kong (Ng et al., 1987a), and construction site
cleaners in Finland (Riala, 1988) have had respirable quartz exposure
levels of at least 0.10 mg/m3, and some exposures were many times
higher.
Sandblasters in US steel fabrication yards were exposed to a mean
exposure of 4.8 mg/m3 of respirable free silica (type of silica not
specified). Samples were collected from the workers' breathing zones,
inside and outside protective hoods. Other yard workers had mean
respirable free silica exposures ranging from 0.06 to 0.7 mg/m3
(Samimi et al., 1974).
In the USA, average personal respirable quartz exposures ranged
from 0.02 to 0.07 mg/m3 during rice farming activities (Lawson et
al., 1995), and median airborne quartz levels during fruit harvesting
ranged from 0.007 to 0.11 mg/m3 (Popendorf et al., 1982; Stopford &
Stopford, 1995).
Exposures to respirable quartz have been noted in "miscellaneous"
operations (IARC, 1997). Studies of US waste incinerator workers
(Bresnitz et al., 1992), US wildland firefighters (Kelly, 1992;
Materna et al., 1992), and workers in Canadian silicon carbide
manufacturing plants (Dufresne et al., 1987) reported respirable
quartz levels that were generally below 0.1 mg/m3. Gemstone workers
in Hong Kong (Ng et al., 1987b), US workers involved with refuse
burning, transfer, and landfill activities (Mozzon et al., 1987), and
US maintenance-of-way railroad workers (i.e., broom operators and
ballast regulators) (Tucker et al., 1995) had exposures to respirable
quartz above 0.10 mg/m3.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS
AND HUMANS
Quartz enters the body as a particle. Usually the particle is
inhaled, and it may be deposited in the lung. Solid particulates such
as quartz are often described by size range. For example, "coarse"
particles are usually described as particles with a diameter more than
2 µm; "fine" particles are those with diameters in the range of
0.1-2.0 µm; and "ultrafine" particles are described as particles with
a diameter less than 0.1 µm. While particle size is often described as
geometric mean diameter in inhalation studies, the aerodynamic
characteristics of the particle are important, too. In humans,
inhalation of "respirable" particles involves exposure to the
particles in a mineral dust that are able to penetrate into the
alveolar spaces of the lungs. It is generally considered that
respirable particles have an aerodynamic diameter of <3-4 µm, while
most particles larger than 5 µm may be deposited in the
tracheobronchial airways and thus not reach the alveolar region (IARC,
1997). Particles deposited in the respiratory bronchioles and proximal
alveoli are cleared more slowly and are more likely to injure the
lung.
There are few data on quartz dust burdens in human lungs, and no
conclusions have been drawn about the clearance kinetics of quartz
particles in humans (IARC, 1997). It has been observed that the
deposition and clearance of quartz and other inhaled particles in
other animals vary with species (IARC, 1997; Oberdörster, 1997).
Short-term inhalation exposures (i.e., <10 days) in rats have shown
that respirable quartz particles can be deposited in the lung and
translocated into epithelial cells and to the interstitium and may
eventually accumulate in the lymph nodes (IARC, 1997). Experimental
particle inhalation studies of laboratory animals, particularly
Fischer 344 rats, have demonstrated a phenomenon known as "particle
overload," which may occur when the pulmonary defences are overwhelmed
by very high exposures (Donaldson & Borm, 1998) and which may reduce
the accuracy of linear exposure-response extrapolations to low levels
(US EPA, 1996). The implications of particle overload for non-rodent
species, such as humans, are not known, but in rats it is
characterized by the suppression of particle transport by alveolar
macrophages and the development of concurrent events such as increased
interstitial dust uptake and prolonged inflammatory response (Morrow,
1988, 1992).
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
The biological response to quartz particles depends on a variety
of factors. It is currently thought that the surface of the quartz
particle is of prime importance in determining its biological effects
because the surface makes contact with biological molecules and cell
surfaces (Fubini et al., 1995; Fubini, 1997; Donaldson & Borm, 1998).
Many experimental in vitro studies have investigated the surface
characteristics of crystalline silica particles, including quartz, and
their influence on fibrogenic activity and have found that a number of
features may be related to cytotoxicity (Fubini et al., 1995;
Bolsaitis & Wallace, 1996; Castranova et al., 1996; Fubini, 1997,
1998; Donaldson & Borm, 1998; Erdogdu & Hasirci, 1998). Some factors
are inherent to the quartz particle itself (e.g., particle size,
micromorphology, external surface defects, origin of the sample,
thermal treatments, and grinding, ball milling, or etching of the
particles), while other factors are external (e.g., contact,
association, contamination, or coating by substances other than
quartz) (Iler, 1979; Fubini, 1998). It has been suggested that
intimate contact between quartz and carbon or metals could modify the
nature of the surface sites (Fubini, 1998) and thus affect the
biological response to quartz. Freshly fractured surfaces are more
reactive than aged ones (IARC, 1997). Further research is needed to
associate the surface characteristics with occupational exposure
situations and health effects (Donaldson & Borm, 1998; NIOSH,
forthcoming), such as work processes that produce freshly fractured
silica surfaces (Vallyathan et al., 1995; Bolsaitis & Wallace, 1996)
or where quartz may be contaminated with trace elements such as iron
(Castranova et al., 1997). There is also a need for experimental
studies to fully describe the sources and properties of quartz in
products used in experimental studies (Mossman & Churg, 1998).
Experimental studies of the effects of other forms of crystalline
silica, such as cristobalite, tridymite, stishovite, and coesite, as
well as coal dust, diatomaceous earth, and amorphous silica, are not
discussed because their in vitro toxicities differ from that of
quartz (Parkes, 1982; Wiessner et al., 1988; Driscoll, 1995; Fubini et
al., 1995; Donaldson & Borm, 1998; Hart & Hesterberg, 1998).
Differences in induction of fibrogenicity of quartz, cristobalite, and
tridymite were demonstrated in vivo in an early rat study (King et
al., 1953).
8.1 Single exposure
No useful data are available on lethal doses of quartz in
experimental animals.
8.2 Short-term exposure
Evidence of cellular proliferation and 3-fold (or higher)
increases in the water, protein, and phospholipid content of male rat
lungs were observed within 28 days after a single 50-mg intratracheal
injection of quartz (i.e., Min-U-Sil with particle diameter less than
5 µm) (Dethloff et al., 1986a,b; Hook & Viviano, 1996). Discrete
silicotic granulomas were observed in rats (both sexes) 21-30 days
after administration of a single intratracheal instillation of 12 mg
of quartz (Min-U-Sil; particle size <5 µm in diameter)
(Saffiotti et al., 1996). When the same research team administered a
single intratracheal instillation of 10 mg of quartz (Min-U-Sil;
particle size <5 µm in diameter) to male mice, the histopathological
changes were not as pronounced on day 30 as in the experimental rats,
but silicotic nodules and some fibrosis were present
(Saffiotti et al., 1996). However, hamsters at the same facility had
silicotic granulomas, but not fibrosis or epithelial reactions,
30 days after a single intratracheal instillation of 20 mg of quartz
(Min-U-Sil; particle size <5 µm in diameter) (Saffiotti et al.,
1996).
A short-term inhalation bioassay of the pulmonary toxicity of
aerosolized quartz particles (Berkeley Min-U-Sil(R) particles with
mass median aerodynamic diameter of 3.7 µm; particle size range not
reported) in rats found that brief exposure produced a persistent
pulmonary inflammatory response and impairment of alveolar macrophage
clearance functions (Warheit & Hartsky, 1997). Progressive lesions
were observed within 1 month after a 3-day (6 h per day) aerosolized
quartz exposure of 100 mg/m3. Two months after exposure, the lesions
had progressed and developed into a multifocal, granulomatous-type
pneumonitis. Rats with a 3-day exposure (6 h per day) to 100 mg
carbonyl iron particles/m3 (negative controls) had no cellular,
cytotoxic, or membrane permeability changes at any time after exposure
(Warheit & Hartsky, 1997).
Silica-induced apoptosis (i.e., programmed cell death) was
observed in three in vivo experiments with 60 (Leigh et al., 1998a)
or 20 (Leigh et al., 1997; Wang et al., 1997a) male Wistar rats that
were divided into groups of equal size and intratracheally instilled
with 0.5 ml saline as a control or doses of Min-U-Sil 5 quartz
suspended in 0.5 ml saline and ranging from 2.5 to 22.5 mg per group.
Apoptotic cells were observed among lavaged cells (both alveolar and
granulomatous) at various time periods, ranging from 1 to 56 days
after instillation. The proportion of apoptotic cells generally
appeared to increase with increasing quartz dose (Leigh et al., 1997),
and it was proposed that apoptosis and subsequent engulfment of
apoptotic cells by macrophages may be involved in the silica-induced
inflammatory response, both acutely and chronically (Leigh et al.,
1997; Wang et al., 1997a).
8.3 Long-term exposure and carcinogenicity
Several end-points have been selected to measure the fibrogenic
potential of quartz in animals: pulmonary toxicity, lung weight,
development of fibrous tissue, collagen content, cytotoxicity, and
biochemical changes in the lungs (US EPA, 1996; Gift & Faust, 1997).
Table 1 presents critical non-cancer effects found in subchronic and
chronic quartz inhalation studies of rats and mice (US EPA, 1996; Gift
& Faust, 1997). All studies showed either fibrosis, increased
collagen, and increased elastin content of lungs or impaired
phagocytic ability of alveolar macrophages. (Table 1 also presents
estimates of human equivalent concentrations [HECs] for environmental
exposures, which are discussed in section 11.1.1.)
Tests of the carcinogenicity of quartz by different routes of
exposure have been conducted. Different quartz specimens (i.e.,
Min-U-Sil 5, Novaculite, DQ 12, hydrogen fluoride-etched Min-U-Sil 5,
Min-U-Sil with polyvinylpyridine- N-oxide, DQ 12 with
polyvinylpyridine- N-oxide, and Sikron F-300 quartz) with particle
sizes in the respirable range were tested in five experiments with
rats by inhalation (Holland et al., 1983, 1986; Dagle et al., 1986;
Muhle et al., 1989, 1991, 1995; Reuzel et al., 1991; Spiethoff et al.,
1992) and in four experiments with rats by intratracheal instillation
(Holland et al., 1983; Groth et al., 1986; Saffiotti, 1990, 1992;
Pott et al., 1994; Saffiotti et al., 1996). The results of these
experiments and others are summarized in Tables 2-4. Significant
increases in the incidence of adenocarcinomas and squamous cell
carcinomas of the lung were found in eight of the nine experiments.
Marked, dense pulmonary fibrosis was part of the response
(IARC, 1997). (The IARC Working Group noted that the experiment by
Reuzel et al. [1991], in which only one respiratory tract tumour was
observed, had a short duration and lacked information on survival;
in addition, only a small proportion of the quartz particles was
respirable by rats.) (Note: Level of statistical significance of
tumour incidence in treated animals compared with control animals was
reported only by Groth et al. [1986]. P-value was less than 0.001
for their Min-U-Sil 5 and Novaculite experiments.)
Although pulmonary granulomatous inflammation and slight to
moderate fibrosis of the alveolar septa were observed in three
experiments on hamsters that used repeated intratracheal instillation
of quartz dusts, no pulmonary tumours were observed (Holland et al.,
1983; Renne et al., 1985; Niemeier et al., 1986).
In experiments with mice, no statistically significant increase
was seen in the incidence of lung tumours in a strain A mouse (i.e.,
male A/J mice from Jackson Laboratories, Bar Harbor, ME, USA) lung
adenoma assay with one sample of quartz (McNeill et al., 1990) or with
a sample of quartz in a limited inhalation study of BALB/cBYJ female
mice (Wilson et al., 1986). Fibrosis was not observed; however, the
lungs of quartz-treated mice did have silicotic granulomas, and
lymphoid cuffing was observed around airways (IARC, 1997).
Thoracic and abdominal malignant lymphomas, primarily of the
histiocytic type, were found in several studies in rats using single
intrapleural or intraperitoneal injection of suspensions of several
types of quartz (Wagner & Berry, 1969; J.C. Wagner, 1970; Wagner &
Wagner, 1972; M.M.F. Wagner, 1976; Wagner et al., 1980;
Jaurand et al., 1987; IARC, 1997).
It is important to note the species differences observed in the
tumour response to quartz particles. In rats, quartz is clearly
carcinogenic, but there is less or no malignant tumour response in
mice and hamsters (Donaldson & Borm, 1998). Particle-induced lung
tumours have been noted in rats, but not to the same degree in mice or
hamsters (IARC, 1997). Currently, there is a limited understanding of
the mechanisms of quartz toxicity, including mechanisms of the rat
lung response (IARC, 1997). Several mechanisms for the carcinogenicity
of quartz in rats have been proposed, included the hypothetical
inflammation-based mechanism (Figure 1; IARC, 1997). The rat model is
the best model currently available for studying the effects of quartz,
because it demonstrates the carcinogenic response observed in some
human studies (Donaldson & Borm, 1998).
8.3.1 Interaction with other compounds
Other tests of carcinogenicity have been conducted using mixtures
of quartz with known carcinogens. When aerosol concentrations of
quartz (Dörentrup DQ 12) were administered by inhalation to rats for
29 days and followed by a single intravenous injection of Thorotrast
(an alpha-radiation-emitting material) at the end of the inhalation
period, there was a pronounced interactive effect of Thorotrast with
quartz (DQ 12) that included the occurrence of tumours in the lung
(i.e., bronchioloalveolar adenomas, bronchioloalveolar carcinomas, and
squamous cell carcinomas), liver, and spleen (Spiethoff et al., 1992;
IARC, 1997). In experiments with hamsters, benzo[ a]pyrene with
quartz and ferric oxide with quartz were administered by intratracheal
instillation. No pulmonary tumours were observed in hamsters given
mixtures of quartz and ferric oxide (1 : 1) (Niemeier et al., 1986).
However, the number of respiratory tract tumours in hamsters given
Min-U-Sil quartz and benzo[ a]pyrene or Sil-Co-Sil and
benzo[ a]pyrene was significantly higher (P < 0.01) than the
number found in hamsters that received saline and benzo[ a]pyrene
(Niemeier et al., 1986).
Table 1: Human equivalent concentrations (HECs) for environmental exposures and non-cancer and
non-tumour effects for LOAELsa reported in subchronic (<3 months) and chronic quartz inhalation
studies in experimental animals.b
Species, strain, Exposure, dose, LOAEL(mg/m3) LOAELHECc
number, sex duration (mg/m3) Critical effect Reference
Rat 1 mg/m3 of DQ 12 1 0.18 Subpleural and Muhle et al., 1989
Fischer 344 for 6 h/day, 5 peribronchial
50/sex days/week, for fibrosis, focal
24 months lipoproteinosis
cholesterol clefts,
enlarged lymph
nodes, and
granulomatous
lesions in the
walls of large bronchi;
doubling of lung
collagen content.
(Quantitative data were
not reported for these
effects.)
Mouse 2 mg/m3 of 2 0.36 Suppressed response to Scheuchenzuber
BALB/c Min-U-Sil aerosol of Escherichia et al., 1985
Female for 8 h/day, coli (i.e., formation
5 days/week, of plaque-forming cells
for 150, 300, in spleen) at 150, 300,
or 570 days and 570 days; reduced
ability of alveolar
macrophages to
phagocytize
Staphylococcus aureus
at 570 days; reduced
T-lymphocyte-mediated
cytolysis of allogeneic
tumour cells at 185 days.
Table 1 (cont'd)
Species, strain, Exposure, dose, LOAEL(mg/m3) LOAELHECc
number, sex duration (mg/m3) Critical effect Reference
Mouse 4932.4 + 235.4 5 0.90 Suppressed response to Burns et al., 1980
BALB/c µg/m3 of Min-U-Sil aerosol of Escherichia
Female 5 for 6 h/day, coli (i.e., formation of
5 days/week, for plaque-forming cells in
3, 9, 15, 27, spleen) at 15, 27, 33,
33, or 39 weeks and 39 weeks; increased
spleen/body ratios at
15, 21, and 27 weeks;
induced pulmonary fibrosis
(fibrotic nodules of
collagen, fibroblasts,
lymphocytes,
silica-filled
macrophages) at 39 weeks.
Rat 0, 2, 10, or 20 2 0.36 Increased collagen and Drew & Kutzman,
Fischer 344 mg/m3 of Min-U-Sil elastin content of lungs; 1984b
Both sexes 5 for 6 h/day, caused birefringent
5 days/week, for crystals in foamy
6 months cytoplasm of macrophages
that had accumulated in
end airway luminal
spaces; induced Type II
cell hyperplasia in
alveolar compartment and
intralymphatic
microgranulomas around
bronchioles in some
animals. Quartz-dependent
increases in collagen and
elastin were 110%, 111%,
and 116% for collagen
Table 1 (cont'd)
Species, strain, Exposure, dose, LOAEL(mg/m3) LOAELHECc
number, sex duration (mg/m3) Critical effect Reference
(as hydroxyproline) and
102%, 109%, and 109% for
elastin, respectively, for
each exposure group
relative to controls
(US EPA, 1996).
Rat 0, 2, 10, or 2 0.36 Increased weight and Drew & Kutzman,
Fischer 344 20 mg/m3 collagen, elastin, 1984a
Both sexes of Min-U-Sil 5 deoxyribonucleic acid,
for 6 h/day, and protein content of
5 days/week, lungs (particularly at
for 6 months, higher exposures of 10
plus 6-month and 20 mg/m3),
incubation period indicating continued
tissue proliferation and
fibrogenesis during
incubation; increased
number of silica
particles and
inflammation at end
airways, focal fibrosis
and intralymphatic
granulomata, and overall
severity and frequency of
lesions. Alveolar
proteinosis observed in
the 20 mg/m3 group.
Quartz increases in
collagen and elastin
were 116%, 128%, and
Table 1 (cont'd)
Species, strain, Exposure, dose, LOAEL(mg/m3) LOAELHECc
number, sex duration (mg/m3) Critical effect Reference
136% for collagen (as
hydroxyproline) and 107%,
119%, and 130% for
elastin, respectively,
for each exposure group
relative to controls
(US EPA, 1996).
a LOAEL = lowest-observed-adverse-effect level. No-observed-adverse-effect levels (NOAELs) were not reported.
b Adapted from Gift & Faust (1997).
c HEC calculated using methods described in US EPA (1994) and summarized in section 11.1.1.
Table 2: Summary of data on lung tumours induced in rats by quartz.a
Incidence of lung tumoursb
Treatment sample Exposure conditions Rat strain Sex Treated Controls Reference
Quartz Intratracheal instillation Sprague-Dawley not 6/36c 0/58 Holland
(Min-U-Sil 5) (suspended in 0.2 ml saline) reported et al., 1983
of 7 mg weekly for 10 weeks
Inhalation (nose only), Fischer 344 F 20/60d 0/54 Holland
12 + 5 mg/m3 for up to 2 years et al., 1986
Inhalation of 51.6 mg/m3 for Fischer 344 F 10/53e 0/47 Dagle et al.,
various durations; sacrificed M 1/47f 0/42 1986
at 24 months
Intratracheal instillation Fischer 344 M 30/67g 1/75h Groth et al.,
(volume of suspension not 1986
reported) of 20 mg in left
lung, sacrificed at 12, 18,
or 22 months or found dead
Novaculite Intratracheal instillation Fischer 344 M 21/72i 1/75h Groth et al.,
(i.e., of 20 mg (volume of 1986
microcrystalline suspension not reported)
quartz) in left lung, sacrificed at
12, 18, or 22 months or
found dead
Quartz (DQ 12) Inhalation of 1 mg/m3 Fischer 344 F 12/50j 3/100k (male Muhle et al.,
for 24 months M 6/50l and female) 1989
Inhalation (nose only) of Wistar F 62/82m 0/85 Spiethoff
6 mg/m3 for 29 days, followed et al., 1992
by lifetime observation
Table 2 (cont'd)
Incidence of lung tumoursb
Treatment sample Exposure conditions Rat strain Sex Treated Controls Reference
Inhalation (nose only) of Wistar F 69/82n 0/85 Spiethoff
30 mg/m3 for 29 days, et al., 1992
followed by lifetime
observation
Quartzo (Sikron Inhalation of 58.5 + 0.7 mg/m3, Wistar F 1/70p 0/70 Reuzel
F300 from Quartz 6 h/day, 5 days/week, M 0/70 0/70 et al., 1991
Werke, Frechen, for 13 weeks
Germany)
a Adapted from Saffiotti et al. (1996); IARC (1997).
b Number of lung tumours per number of rats observed.
c One adenoma and five carcinomas.
d Six adenomas, 11 adenocarcinomas, and three epidermoid carcinomas.
e All epidermoid carcinomas.
f One epidermoid carcinoma.
g All adenocarcinomas.
h One adenocarcinoma.
i Twenty adenocarcinomas and one epidermoid carcinoma.
j Two keratinizing cystic squamous cell tumours, two adenomas, and eight adenocarcinomas.
k Two adenomas and one adenocarcinoma.
l Two keratinizing cystic squamous cell tumours, two adenocarcinomas, one adenosquamous carcinoma,
and one epidermoid carcinoma.
m Eight adenomas, 17 bronchioloalveolar carcinomas, and 37 squamous cell carcinomas.
n Three adenomas, 26 bronchioloalveolar carcinomas, and 30 squamous carcinomas.
o IARC Working Group noted that only a small proportion of particles were respirable in rats.
p One squamous cell carcinoma, 1 year after the end of the exposure period.
Table 3: Lung tumours induced in Fischer 344 rats by a single intratracheal instillation of quartz.a
Treatment Treatment Sex Observation Incidence of Total number of Histological
sample doseb time lung tumoursc lung tumoursd types
Untreated None M Died after 0/32 0
17 months
None F Died after 1/20 (5%) 1 1 adenoma
17 months
Quartz 12 mg M Sacrificed 3/18 (17%) 37 6 adenomas,
(Min-U-Sil 5) 11 months 25 adenocarcinomas,
1 undifferentiated
Sacrificed 6/19 (32%) carcinoma, 2 mixed
17 months carcinomas, and 3
epidermoid
Died after 12/14 (86%) carcinomas
17 months
12 mg F Sacrificed 8/19 (42%) 59 2 adenomas,
11 months 46 adenocarcinomas,
3 undifferentiated
Sacrificed 10/17 (59%) carcinomas, 5 mixed
17 months carcinomas, and
3 epidermoid
Died after 8/9 (89%) carcinomas
17 months
20 mg F Died after 6/8 (75%) 13 1 adenoma, 10
17 months adenocarcinomas, 1 mixed
carcinoma, and 1 epidermoid
carcinoma
Table 3 (cont'd)
Treatment Treatment Sex Observation Incidence of Total number of Histological
sample doseb time lung tumoursc lung tumoursd types
Quartz 12 mg M Sacrificed 2/18 (11%) 20 5 adenomas,
(hydrogen 11 months 14 adenocarcinomas,
fluoride-etched and 1 mixed carcinoma
Min-U-Sil 5) Sacrificed 7/19 (37%)
17 months
Died after 7/9 (78%)
17 months
12 mg F Sacrificed 7/18 (39%) 45 1 adenoma,
11 months 36 adenocarcinomas,
3 mixed carcinomas,
Sacrificed 13/16 (81%) and 5 epidermoid
17 months carcinomas
Died after 8/8 (100%)
17 months
a From Saffiotti et al. (1993, 1996).
b As mg quartz suspended in 0.3 ml saline.
c Number of rats with lung tumours per number of rats observed.
d At all observation times.
Table 4: Incidence of lung tumours in female Wistar rats after intratracheal instillation of quartz.a
Number and percentage of rats with primary epithelial lung tumoursb
Material Surface Number of Number of Adenoma Adenocarcinoma Benign Squamous Total Other
area instillations rats CKSCTd cell (%) tumourse
(m2/g) (× mg)c examined carcinoma
Quartz
(DQ 12) 9.4 15 × 3 37 0 1z 11 1 + 1y 38 1
Quartz
(DQ 12) + PVNOf 9.4 15 × 3 38 0 1 + 3z 8 + 1x 4 + 1x + 3y + 1z 58 2
Quartz
(DQ 12) 9.4 1 × 45 40 0 1 7 1 23 2
Quartz
(Min-U-Sil) 15 × 3 39 1 4 + 4z 6 1 + 2y + 2z + 1y,z 54 3
Quartz
(Min-U-Sil)
+ PVNO 15 × 3 35 1 2 + 1x 8 5 + 1x + 1y + 1z 57 3
Quartz
Sykron (F600) 3.7 15 × 3 40 0 3 5 3 + 1z 30 1
0.9% sodium
chloride - 15 39 0 0 0 0 0 5
a From Pott et al. (1994); IARC (1997).
b If an animal was found to bear more than one primary epithelial lung tumour type, this was indicated as follows: x adenoma;
y adenocarcinoma; z benign CKSCT.
c Dusts were suspended in 0.9% sodium chloride solution with ultrasonication for 1-5 min.
d CKSCT, cystic keratinizing squamous cell tumour.
e Other types of tumours in the lung: fibrosarcoma, lymphosarcoma, mesothelioma or lung metastases from tumours at other sites
f PVNO, polyvinylpyridine-N-oxide; PVNO was administered subcutaneously in seven injections of 2 ml each of 2% PVNO in saline.
No PVNO control group was included.
8.4 Genotoxicity and related end-points
Although silica (form not specified) has not tested positive in
standard bacterial mutagenesis assays (IARC, 1987, 1997; Rabovsky,
1997), chromosomal changes, including DNA damage, have been observed
in experimental systems, both in vitro and in vivo. (Study results
are presented in Table 5.) Although the results of some studies
(Daniel et al., 1993, 1995; Saffiotti et al., 1993; Shi et al., 1994)
demonstrated that quartz caused damage (i.e., strand breakage) to
isolated DNA in acellular systems, the IARC Working Group (IARC, 1997)
stated that the relevance of these assays to assess quartz-related
genetic effects in vivo was "questionable." Uncertainties existed
because the non-physiological experimental conditions did not apply to
intracellular silica exposure and because very high doses of silica
were used in the DNA breakage assays (IARC, 1997). However, a recent
study not included in the IARC review found that by using the alkaline
single cell gel/comet assay, crystalline silica (Min-U-Sil 5) induced
DNA damage (i.e., DNA migration) in cultured Chinese hamster lung
fibroblasts (V79 cells) and human embryonic lung fibroblasts (Hel 299
cells) at concentrations ranging from 17.2 to 103.4 µg/cm2 (Zhong et
al., 1997). Since the time of the IARC review, Liu et al. (1996, 1998)
applied experimental conditions (i.e., Chinese hamster lung
fibroblasts challenged with dusts pretreated with a phospholipid
surfactant) to simulate the condition of particles immediately after
deposition on the pulmonary alveolar surface. Results of the
experiments showed that untreated Min-U-Sil 5 and Min-U-Sil 10 induced
micronucleus formation in a dose-dependent manner, but surfactant
pretreatment suppressed that activity (Liu et al., 1996). A subsequent
experiment found that surfactant pretreatment suppressed
quartz-induced DNA damage in lavaged rat pulmonary macrophages, but
DNA-damaging activity was restored with time as the phospholipid
surfactant was removed by intercellular digestion (Liu et al., 1998).
In vitro cellular transformation systems model the in vivo
process of carcinogenesis (Gu & Ong, 1996; Gao et al., 1997). The
ability of quartz to induce dose-dependent morphological
transformation of cells in vitro has been demonstrated in
experiments with Syrian hamster embryo cells (Hesterberg & Barrett,
1984) and mouse embryo BALB/c-3T3 cells (Saffiotti & Ahmed, 1995). Gu
& Ong (1996) also reported a significant increase in the frequency of
transformed foci of mouse embryo BALB/c-3T3 cells after treatment with
Min-U-Sil 5 quartz. These studies indicate that quartz can
morphologically transform mammalian cells. However, further studies
are needed to determine whether the transforming activity of quartz is
related to its carcinogenic potential.
1. EXECUTIVE SUMMARY
This CICAD on crystalline silica, quartz was based on the
following three extensive peer-reviewed documents on the health
effects of crystalline silica, including quartz: (1) a review of
published human studies and reports on the adverse health effects of
quartz exposure (NIOSH, forthcoming), (2) a review of the
carcinogenicity studies conducted by the International Agency for
Research on Cancer (IARC, 1997), and (3) a review of the non-cancer
health effects of ambient quartz (US EPA, 1996). The source documents
had different emphases on different end-points, and the CICAD was
developed to assess all the adverse health effects identified in these
documents. It is to be noted that despite the different emphases, the
final conclusions of all source documents were very similar. A
comprehensive literature search of several on-line databases was
conducted. Data identified as of March 1999 are included in this
review.
This CICAD considers the most common form of crystalline silica
(i.e., quartz). It does not consider experimental studies of the
effects of other forms of crystalline silica (e.g., cristobalite,
tridymite, stishovite, or coesite), coal dust, diatomaceous earth, or
amorphous silica, because their in vitro toxicities differ from that
of quartz. Differences in induction of fibrogenicity of quartz,
cristobalite, and tridymite were demonstrated in vivo in an early
rat study. However, there are virtually no experimental studies that
systematically evaluated exactly the same material to which humans are
exposed. The IARC Working Group considered the possibility that there
may be differences in the carcinogenic potential among polymorphs of
crystalline silica. However, some of the epidemiological studies
evaluated lung cancer among workers in "mixed environments" where
quartz may be heated and varying degrees of conversion to cristobalite
or tridymite can occur (e.g., ceramics, pottery, and refractory brick
industries), and exposures specifically to quartz or cristobalite were
not delineated. Although there were some indications that cancer risks
varied by industry and process in a manner suggestive of
polymorph-specific risks, the Working Group could reach only a single
conclusion for quartz and cristobalite. The CICAD reflects the
discussion and conclusion of that source document; therefore, when
considering the carcinogenicity of quartz in the occupational setting,
it does not distinguish between epidemiological studies of quartz and
those of cristobalite.
The peer review process for this CICAD was targeted to include
review by an international group of experts selected for their
knowledge about the current controversies and issues surrounding
quartz. Information on the nature of the peer review and the
availability of the source documents is presented in Appendix 1.
Information on the peer review of this CICAD is presented in
Appendix 2. This CICAD was approved as an international assessment at
a meeting of the Final Review Board, held in Sydney, Australia, on
21-24 November 1999. Participants at the Final Review Board meeting
are listed in Appendix 3. The International Chemical Safety Card
(ICSC 0808) for crystalline silica, quartz has been reproduced in
Appendix 4 (IPCS, 1993).
Quartz (CAS No. 14808-60-7) is a frequently occurring solid
component of most natural mineral dusts. Human exposures to quartz
occur most often during occupational activities that involve movement
of earth, disturbance of silica-containing products (e.g., masonry,
concrete), or use or manufacture of silica-containing products.
Environmental exposure to ambient quartz dust can occur during
natural, industrial, and agricultural activities. Respirable quartz
dust particles can be inhaled and deposited in the lung; however, no
conclusions have been made about the clearance kinetics of quartz
particles in humans.
Quartz dust induces cellular inflammation in vivo. Short-term
experimental studies of rats have found that intratracheal
instillation of quartz particles leads to the formation of discrete
silicotic nodules in rats, mice, and hamsters. Inhalation of
aerosolized quartz particles impairs alveolar macrophage clearance
functions and leads to progressive lesions and pneumonitis. Oxidative
stress (i.e., increased formation of hydroxyl radicals, reactive
oxygen species, or reactive nitrogen species) has been observed in
rats after intratracheal instillation or inhalation of quartz. Many
experimental in vitro studies have found that the surface
characteristics of the crystalline silica particle influence its
fibrogenic activity and a number of features related to its
cytotoxicity. Although many potential contributory mechanisms have
been described in the literature, the mechanisms responsible for
cellular damage by quartz particles are complex and not completely
understood.
Long-term inhalation studies of rats and mice have shown that
quartz particles produce cellular proliferation, nodule formation,
suppressed immune functions, and alveolar proteinosis. Experimental
studies of rats reported the occurrence of adenocarcinomas and
squamous cell carcinomas after the inhalation or intratracheal
instillation of quartz. Pulmonary tumours were not observed in
experiments with hamsters or mice. Adequate dose-response data (e.g.,
no-adverse-effect or lowest-adverse-effect levels) for rats or other
rodents are not available because few multiple-dose carcinogenicity
studies have been performed.
Quartz did not test positively in standard bacterial mutagenesis
assays. Results of genotoxicity studies of quartz conflict, and a
direct genotoxic effect for quartz has not been confirmed or ruled
out.
In experimental studies of particles, results may vary depending
on the test material, particle size of the material, concentration
administered, and species tested. The experiments with quartz
particles involved specimens from various sources, using various
doses, particle sizes, and species, which could have affected the
observations.
Data on the reproductive and developmental effects of quartz in
laboratory animals are not available.
The adverse effects of quartz in aquatic organisms and
terrestrial mammals have not been studied.
There are many epidemiological studies of occupational cohorts
exposed to respirable quartz dust. Silicosis, lung cancer, and
pulmonary tuberculosis are associated with occupational exposure to
quartz dust. IARC classified inhaled crystalline silica (quartz or
cristobalite) from occupational sources as a Group 1 carcinogen based
on sufficient evidence of carcinogenicity in humans and experimental
animals; "in making the overall evaluation, the Working Group noted
that carcinogenicity in humans was not detected in all industrial
circumstances studied. Carcinogenicity may be dependent on inherent
characteristics of the crystalline silica or on external factors
affecting its biological activity or distribution of its polymorphs"
(IARC, 1997).
Statistically significant increases in deaths or cases of
bronchitis, emphysema, chronic obstructive pulmonary disease,
autoimmune-related diseases (i.e., scleroderma, rheumatoid arthritis,
systemic lupus erythematosus), and renal diseases have been reported.
Silicosis is the critical effect for hazard identification and
exposure-response assessment. There are sufficient epidemiological
data to allow the risk of silicosis to be quantitatively estimated,
but not to permit accurate estimations of risks for other health
effects mentioned above. (A pooled risk assessment of epidemiological
studies of silica and lung cancer is in progress at IARC.)
The risk estimates for silicosis prevalence for a working
lifetime of exposure to respirable quartz dust concentrations of about
0.05 or 0.10 mg/m3 in the occupational environment vary widely (i.e.,
2-90%). Regarding exposure to ambient quartz in the general
environment, a benchmark dose analysis predicted that the silicosis
risk for a continuous 70-year lifetime exposure to 0.008 mg/m3
(estimated high crystalline silica concentration in US metropolitan
areas) is less than 3% for healthy individuals not compromised by
other respiratory diseases or conditions and for ambient environment
(US EPA, 1996). The silicosis risk for persons with respiratory
diseases exposed to ambient quartz in the general environment was not
evaluated.
Uncertainties exist in the evaluation of epidemiological studies
and the risk assessment of health effects related to quartz dust
exposure. The difficulties, many of which are inherent to the study of
respiratory diseases in occupational populations, include limitations
in the amount and quality of historical exposure data, deficiencies in
data on potentially confounding factors, such as cigarette smoking,
and difficulties in the interpretation of chest radiographs as
evidence of exposure. In addition, occupational exposures to quartz
dust are complex because workers are frequently exposed to dust
mixtures that contain quartz and other mineral varieties. Properties
of the dust (e.g., particle size, surface properties, crystalline
form) may differ according to geological source and can also change
during industrial processing. Such variations can affect the
biological activity of the inhaled dust. The IARC Working Group
evaluated the carcinogenicity of crystalline silica (including quartz)
and focused on epidemiological studies that were the least likely to
have been affected by confounding and selection biases and that
evaluated exposure-response relationships (IARC, 1997).
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
"Silica," or silicon dioxide (SiO2), occurs in either a
crystalline or non-crystalline (amorphous) form. Crystalline silica
may be found in more than one form (polymorphism), depending on the
orientation and position of the tetrahedra (i.e., the
three-dimensional basic unit of all forms of crystalline silica). The
natural crystalline forms of silica are alpha-quartz, ß-quartz,
alpha-, ß1-, and ß2-tridymite, alpha- and ß-cristobalite, coesite,
stishovite, and moganite (IARC, 1997). This document discusses the
most common form of naturally occurring crystalline silica -- quartz
(CAS No. 14808-60-7). Cristobalite (CAS No. 14464-46-1) and tridymite
(CAS No. 15468-32-3) exist in nature, but they can also be created
during industrial processes, such as the calcination of diatomaceous
earth, ceramics manufacturing, foundry processes, silicon carbide
manufacturing, and any other process in which quartz is heated to high
temperatures (NIOSH, 1974; Altieri et al., 1984; Virta, 1993;
Weill et al., 1994; IARC, 1997).
Quartz is a colourless, odourless, non-combustible solid and a
component of many mineral dusts (NIOSH, 1997). It is insoluble in
water (NIOSH, 1997). When quartz is cut, ground, or milled, the
crystal is fractured, and Si and Si-O radicals may be generated on the
cleavage surfaces (Castranova et al., 1996). Trace metal impurities,
such as iron and aluminium, can modify the surface reactivity of
quartz (Fubini et al., 1995; Fubini, 1997, 1998; IARC, 1997; Donaldson
& Borm, 1998).
Most of the experimental studies described in section 8 used
Min-U-Sil or DQ 12 quartz. Min-U-Sil is a trade name, and the number
that follows (e.g., Min-U-Sil 5) describes the particle size of the
sample (e.g., Min-U-Sil 5 is <5 µm in diameter). The purity is 99%
quartz (IARC, 1997). However, the geological sources of crystals have
varied; consequently, the associated impurities may have varied. The
particle size distributions of Min-U-Sil and several other reference
standards for the quantification of quartz in coal mine dust have been
investigated (Huggins et al., 1985), but a comprehensive report has
not been published on the analytical characteristics of a standard
sample of Min-U-Sil and the reproducibility of its aliquots
(Saffiotti et al., 1993). DQ 12 is a quartz sand that contains 87%
crystalline silica; the remaining proportion is amorphous silica, with
small contaminations of kaolinite. All DQ 12 samples originate from
the same source, but its particle size and composition have not been
reported recently (IARC, 1997). Furthermore, many experimental and
epidemiological studies do not state the source and properties of the
quartz that is used as a test material or collected in the workplace
(Mossman & Churg, 1998).
Additional physical and chemical properties of quartz are
presented in the International Chemical Safety Card (ICSC 0808)
reproduced in this document (Appendix 4).
3. ANALYTICAL METHODS
Mineral dust particles, such as quartz particles, are typically
described by diameter size (e.g., geometric mean diameter) and
aerodynamic diameter. Both characteristics are important in
determining whether the particle is respirable (IARC, 1997). Analysis
for airborne quartz is usually by X-ray diffraction or infrared
spectrophotometry in combination with filter collection methods
(IARC, 1997). Dust levels can be based on counts from an impinger or
on mass collected on a filter (IARC, 1997). Currently, the latter
method is more commonly used. Most countries (e.g., the USA, the
United Kingdom, Germany, Japan, and Australia) require that the sample
be restricted to the respirable fraction (IARC, 1997). The estimated
detection limit for quartz in respirable dust samples is 0.005 mg
using US National Institute for Occupational Safety and Health (NIOSH)
method 7500 (i.e., X-ray powder diffraction) (NIOSH, 1994a). The
estimated detection limit for quartz in respirable dust samples with
NIOSH method 7602 (infrared absorption spectrophotometry) is also
0.005 mg (NIOSH, 1994b).
4. SOURCES OF HUMAN AND ENVIRONMENTAL
EXPOSURE
Quartz is abundant in most rocks, sands, and soils (IARC, 1997).
The extensive natural occurrence of quartz and the wide uses of the
materials that contain quartz are directly related to potential
occupational exposures to quartz for workers in many industries and
occupations. Virtually any process that involves movement of earth
(e.g., mining, farming, construction), disturbance of
silica-containing products such as masonry and concrete, or use of
sand and other silica-containing products (e.g., foundry processes)
may potentially expose a worker to quartz (IARC, 1997).
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND
TRANSFORMATION
Environmental exposures to quartz can occur when ambient quartz
is emitted into the air as a component of particulate emissions
produced by natural, industrial, and farming activities
(US EPA, 1996). These activities include construction and demolition,
quarrying and mining, dust from travel on paved and unpaved roads,
electrical power generation, agricultural tilling, forest fires,
volcanic eruptions, and wind erosion (US EPA, 1996; IARC, 1997).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Ambient quartz is emitted to the environment as a component of
particulate emissions. The available data on concentrations of quartz
in the non-occupational environment (i.e., ambient air), including
data collected by the US Environmental Protection Agency (US EPA), are
limited (US EPA, 1996). The US EPA's Inhalable Particulate Network
provides a data set of quartz concentrations that were collected from
high-volume or dichotomous samples of ambient aerosols in 25 US cities
in 1980 (Davis et al., 1984). Average quartz concentrations were
highest (and most variable) in air masses in continental interior
sites. Ambient quartz concentrations collected from high-volume filter
samples of total suspended particulates in 10 US cities ranged from
0 µg/m3 (Portland, Oregon) to 15.8 µg/m3 (Akron, Ohio)
(Davis et al., 1984). Both of those findings were based on one sample
in each city (US EPA, 1996).
Non-occupational inhalation of quartz may occur while using a
variety of commercial products, such as cleansers, cosmetics,
art clays and glazes, pet litter, talcum powder, caulk, putty, paint,
and mortar (US Department of the Interior, 1992). Data representing
quantitative exposure levels of respirable quartz during
non-occupational uses of commercial products are not available.
Although quartz particles may be present in water, quantitative
data on concentrations of quartz in potable or other forms of
drinking-water are not available (IARC, 1997).
Occupational quartz dust exposure is probably one of the most
documented workplace exposures. Nearly every mineral deposit contains
some quartz (Greskevitch et al., 1992); thus, most quartz exposures
are to mixed dust with a variable quartz content that must be measured
by dust collection and analysis (Wagner, 1995). Compliance officers
for the US Occupational Safety and Health Administration measured
respirable quartz in 255 industries that were targeted for inspection,
excluding mining and agriculture. In 48% of the industries, average
overall exposure exceeded the permissible exposure level (10 mg
respirable dust/m3 divided by % silica + 2) (Freeman & Grossman,
1995).
Respirable quartz levels exceeding 0.1 mg/m3 have been reported
in many industries worldwide and are most frequently found in metal,
non-metal, and coal mines and mills; in granite quarrying and
processing, crushed stone and related industries; in foundries; in the
ceramics industry; and in construction and sandblasting operations
(IARC, 1997). IARC summarized data from the main industries for which
quantitative quartz exposure levels were available in the published
literature or where major occupational health studies were conducted
(IARC, 1997). The IARC review is condensed here and presented by
industry. Many processes in these industries include potential
exposures to other substances with known adverse health effects,
including carcinogenicity. Information about the health hazards for a
particular industry, the variability of the proportion of quartz found
in total dust samples from different industries, and the estimated
proportion of workers exposed to defined concentrations is available
elsewhere (e.g., IARC, 1984, 1987, 1997; Burgess, 1995; Linch et al.,
1998).
The mean respirable quartz level in mining operations (i.e.,
underground and surface mining, milling operations) inspected in the
USA from 1988 to 1992 was usually less than 0.10 mg/m3, but a
significant percentage of samples exceeded the permissible exposure
limit (see above) (Watts & Parker, 1995; IARC, 1997). Estimated
arithmetic mean respirable crystalline silica levels (form of
"crystalline silica" was not specified) for 1950-1959 and 1981-1987 in
20 Chinese mines (10 tungsten, 6 iron-copper, and 4 tin) decreased
about 10-fold between those periods. The estimated arithmetic mean
level of respirable silica (mg/m3) for the older period and the more
recent period, respectively, were as follows: underground mining,
4.89, 0.39; surface mining, 1.75, 0.27; ore dressing, 3.45, 0.42;
tungsten mines, 4.99, 0.46; iron and copper mines, 0.75, 0.20; and tin
mines, 3.49, 0.45 (Dosemeci et al., 1995; IARC, 1997). Respirable
quartz concentrations in underground dust from South African gold
mines ranged from 0.05 to 0.58 mg/m3 in surveys taken during
1965-1967 (Beadle & Bradley, 1970). In a copper mine in Finland, the
mean concentration of respirable quartz in the general mine air was
about 0.16 mg/m3 until 1965, 0.12 mg/m3 in 1966-1975, and 0.08
mg/m3 after 1981 (Ahlman et al., 1991).
Exposure to respirable quartz dust can occur in granite quarrying
and processing, including crushed stone and related industries.
Geometric mean air concentrations and air concentrations of quartz
from personal breathing-zone samples collected during various jobs in
the granite quarrying and processing industries and crushed stone and
related industries in Finland, the USA, and the United Kingdom ranged
from 0.03 to 1.5 mg/m3 and from not detectable to 135 mg/m3,
respectively (Donaldson et al., 1982; Eisen et al., 1984; Koskela et
al., 1987; Davies et al., 1994; Kullman et al., 1995; IARC, 1997). In
US granite quarries and sheds, control measures implemented in the
late 1930s and the 1940s resulted in 10- to 100-fold reductions of
formerly high dust levels (Davis et al., 1983; IARC, 1997).
In India, personal respirable dust levels of 0.06-1.12 mg/m3
(average 0.61 mg/m3 with a free silica content of 15%; form of silica
not specified) were generated during the manufacture of slate pencils
from natural rock. Average personal dust concentrations measured in
previous surveys in 1977 and 1982 were 10- to 100-fold higher (Fulekar
& Alam Kham, 1995; IARC, 1997).
Foundry occupations can involve exposure to quartz-containing
sands and parting powders (e.g., silica flour). The quartz content of
sands ranges from 5% to nearly 100% (IARC, 1997). Foundry occupations
with particularly high potential exposures to quartz (e.g., sand or
silica flour) are those jobs that involve sand preparation and
reclamation, knocking-out or shaking-out, cleaning of castings (i.e.,
fettling, grinding, sandblasting), and furnace and ladle refractory
relining and repair (IARC, 1997). Mean personal respirable quartz
levels in iron, steel, aluminium, brass, and other types of foundries
ranged from 0.19 to 5.26 mg/m3 in Finland (Siltanen et al., 1976;
IARC, 1997) and from 0.13 to 0.63 mg/m3 in Sweden (Gerhardsson, 1976;
IARC, 1997); in Canadian iron and steel foundries, the mean personal
respirable quartz concentration was 0.086 mg/m3 (Oudyk, 1995; IARC,
1997).
IARC presented respirable quartz dust levels for jobs in
ceramics, brick, cement, or glass industries in China (Dosemeci et
al., 1995), Italy (Cavariani et al., 1995), the Netherlands (Buringh
et al., 1990), South Africa (Myers et al., 1989; Rees et al., 1992),
the United Kingdom (Bloor et al., 1971; Fox et al., 1975; Higgins et
al., 1985), and the USA (Anderson et al., 1980; Salisbury & Melius,
1982; Cooper et al., 1993) and noted that jobs involving mixing,
moulding, glaze spraying, and finishing were associated with higher
exposure levels, often in the range of 0.1-0.3 mg/m3 (IARC, 1997). In
ceramic and pottery manufacturing facilities, exposures are mainly to
quartz, but potential exposures to cristobalite may occur where high
temperatures are used (e.g., ovens) (IARC, 1997). In refractory brick
and diatomaceous earth processing facilities, the raw materials
(amorphous or crystalline silica) are processed at temperatures near
1000°C, with varying degrees of conversion to cristobalite (IARC,
1997).
In the construction industry, drilling, sandblasting, sawing,
grinding, cleaning, and many other actions that are applied to
concrete, mortar surfaces, brick, rock, and other silica-containing
substances and products can result in the generation of a fine
airborne dust (Lofgren, 1993; Linch & Cocalis, 1994; NIOSH, 1996; IARC
1997). Concrete finishers and masons in the USA (Lofgren, 1993),
caisson workers in Hong Kong (Ng et al., 1987a), and construction site
cleaners in Finland (Riala, 1988) have had respirable quartz exposure
levels of at least 0.10 mg/m3, and some exposures were many times
higher.
Sandblasters in US steel fabrication yards were exposed to a mean
exposure of 4.8 mg/m3 of respirable free silica (type of silica not
specified). Samples were collected from the workers' breathing zones,
inside and outside protective hoods. Other yard workers had mean
respirable free silica exposures ranging from 0.06 to 0.7 mg/m3
(Samimi et al., 1974).
In the USA, average personal respirable quartz exposures ranged
from 0.02 to 0.07 mg/m3 during rice farming activities (Lawson et
al., 1995), and median airborne quartz levels during fruit harvesting
ranged from 0.007 to 0.11 mg/m3 (Popendorf et al., 1982; Stopford &
Stopford, 1995).
Exposures to respirable quartz have been noted in "miscellaneous"
operations (IARC, 1997). Studies of US waste incinerator workers
(Bresnitz et al., 1992), US wildland firefighters (Kelly, 1992;
Materna et al., 1992), and workers in Canadian silicon carbide
manufacturing plants (Dufresne et al., 1987) reported respirable
quartz levels that were generally below 0.1 mg/m3. Gemstone workers
in Hong Kong (Ng et al., 1987b), US workers involved with refuse
burning, transfer, and landfill activities (Mozzon et al., 1987), and
US maintenance-of-way railroad workers (i.e., broom operators and
ballast regulators) (Tucker et al., 1995) had exposures to respirable
quartz above 0.10 mg/m3.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS
AND HUMANS
Quartz enters the body as a particle. Usually the particle is
inhaled, and it may be deposited in the lung. Solid particulates such
as quartz are often described by size range. For example, "coarse"
particles are usually described as particles with a diameter more than
2 µm; "fine" particles are those with diameters in the range of
0.1-2.0 µm; and "ultrafine" particles are described as particles with
a diameter less than 0.1 µm. While particle size is often described as
geometric mean diameter in inhalation studies, the aerodynamic
characteristics of the particle are important, too. In humans,
inhalation of "respirable" particles involves exposure to the
particles in a mineral dust that are able to penetrate into the
alveolar spaces of the lungs. It is generally considered that
respirable particles have an aerodynamic diameter of <3-4 µm, while
most particles larger than 5 µm may be deposited in the
tracheobronchial airways and thus not reach the alveolar region (IARC,
1997). Particles deposited in the respiratory bronchioles and proximal
alveoli are cleared more slowly and are more likely to injure the
lung.
There are few data on quartz dust burdens in human lungs, and no
conclusions have been drawn about the clearance kinetics of quartz
particles in humans (IARC, 1997). It has been observed that the
deposition and clearance of quartz and other inhaled particles in
other animals vary with species (IARC, 1997; Oberdörster, 1997).
Short-term inhalation exposures (i.e., <10 days) in rats have shown
that respirable quartz particles can be deposited in the lung and
translocated into epithelial cells and to the interstitium and may
eventually accumulate in the lymph nodes (IARC, 1997). Experimental
particle inhalation studies of laboratory animals, particularly
Fischer 344 rats, have demonstrated a phenomenon known as "particle
overload," which may occur when the pulmonary defences are overwhelmed
by very high exposures (Donaldson & Borm, 1998) and which may reduce
the accuracy of linear exposure-response extrapolations to low levels
(US EPA, 1996). The implications of particle overload for non-rodent
species, such as humans, are not known, but in rats it is
characterized by the suppression of particle transport by alveolar
macrophages and the development of concurrent events such as increased
interstitial dust uptake and prolonged inflammatory response (Morrow,
1988, 1992).
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
The biological response to quartz particles depends on a variety
of factors. It is currently thought that the surface of the quartz
particle is of prime importance in determining its biological effects
because the surface makes contact with biological molecules and cell
surfaces (Fubini et al., 1995; Fubini, 1997; Donaldson & Borm, 1998).
Many experimental in vitro studies have investigated the surface
characteristics of crystalline silica particles, including quartz, and
their influence on fibrogenic activity and have found that a number of
features may be related to cytotoxicity (Fubini et al., 1995;
Bolsaitis & Wallace, 1996; Castranova et al., 1996; Fubini, 1997,
1998; Donaldson & Borm, 1998; Erdogdu & Hasirci, 1998). Some factors
are inherent to the quartz particle itself (e.g., particle size,
micromorphology, external surface defects, origin of the sample,
thermal treatments, and grinding, ball milling, or etching of the
particles), while other factors are external (e.g., contact,
association, contamination, or coating by substances other than
quartz) (Iler, 1979; Fubini, 1998). It has been suggested that
intimate contact between quartz and carbon or metals could modify the
nature of the surface sites (Fubini, 1998) and thus affect the
biological response to quartz. Freshly fractured surfaces are more
reactive than aged ones (IARC, 1997). Further research is needed to
associate the surface characteristics with occupational exposure
situations and health effects (Donaldson & Borm, 1998; NIOSH,
forthcoming), such as work processes that produce freshly fractured
silica surfaces (Vallyathan et al., 1995; Bolsaitis & Wallace, 1996)
or where quartz may be contaminated with trace elements such as iron
(Castranova et al., 1997). There is also a need for experimental
studies to fully describe the sources and properties of quartz in
products used in experimental studies (Mossman & Churg, 1998).
Experimental studies of the effects of other forms of crystalline
silica, such as cristobalite, tridymite, stishovite, and coesite, as
well as coal dust, diatomaceous earth, and amorphous silica, are not
discussed because their in vitro toxicities differ from that of
quartz (Parkes, 1982; Wiessner et al., 1988; Driscoll, 1995; Fubini et
al., 1995; Donaldson & Borm, 1998; Hart & Hesterberg, 1998).
Differences in induction of fibrogenicity of quartz, cristobalite, and
tridymite were demonstrated in vivo in an early rat study (King et
al., 1953).
8.1 Single exposure
No useful data are available on lethal doses of quartz in
experimental animals.
8.2 Short-term exposure
Evidence of cellular proliferation and 3-fold (or higher)
increases in the water, protein, and phospholipid content of male rat
lungs were observed within 28 days after a single 50-mg intratracheal
injection of quartz (i.e., Min-U-Sil with particle diameter less than
5 µm) (Dethloff et al., 1986a,b; Hook & Viviano, 1996). Discrete
silicotic granulomas were observed in rats (both sexes) 21-30 days
after administration of a single intratracheal instillation of 12 mg
of quartz (Min-U-Sil; particle size <5 µm in diameter)
(Saffiotti et al., 1996). When the same research team administered a
single intratracheal instillation of 10 mg of quartz (Min-U-Sil;
particle size <5 µm in diameter) to male mice, the histopathological
changes were not as pronounced on day 30 as in the experimental rats,
but silicotic nodules and some fibrosis were present
(Saffiotti et al., 1996). However, hamsters at the same facility had
silicotic granulomas, but not fibrosis or epithelial reactions,
30 days after a single intratracheal instillation of 20 mg of quartz
(Min-U-Sil; particle size <5 µm in diameter) (Saffiotti et al.,
1996).
A short-term inhalation bioassay of the pulmonary toxicity of
aerosolized quartz particles (Berkeley Min-U-Sil(R) particles with
mass median aerodynamic diameter of 3.7 µm; particle size range not
reported) in rats found that brief exposure produced a persistent
pulmonary inflammatory response and impairment of alveolar macrophage
clearance functions (Warheit & Hartsky, 1997). Progressive lesions
were observed within 1 month after a 3-day (6 h per day) aerosolized
quartz exposure of 100 mg/m3. Two months after exposure, the lesions
had progressed and developed into a multifocal, granulomatous-type
pneumonitis. Rats with a 3-day exposure (6 h per day) to 100 mg
carbonyl iron particles/m3 (negative controls) had no cellular,
cytotoxic, or membrane permeability changes at any time after exposure
(Warheit & Hartsky, 1997).
Silica-induced apoptosis (i.e., programmed cell death) was
observed in three in vivo experiments with 60 (Leigh et al., 1998a)
or 20 (Leigh et al., 1997; Wang et al., 1997a) male Wistar rats that
were divided into groups of equal size and intratracheally instilled
with 0.5 ml saline as a control or doses of Min-U-Sil 5 quartz
suspended in 0.5 ml saline and ranging from 2.5 to 22.5 mg per group.
Apoptotic cells were observed among lavaged cells (both alveolar and
granulomatous) at various time periods, ranging from 1 to 56 days
after instillation. The proportion of apoptotic cells generally
appeared to increase with increasing quartz dose (Leigh et al., 1997),
and it was proposed that apoptosis and subsequent engulfment of
apoptotic cells by macrophages may be involved in the silica-induced
inflammatory response, both acutely and chronically (Leigh et al.,
1997; Wang et al., 1997a).
8.3 Long-term exposure and carcinogenicity
Several end-points have been selected to measure the fibrogenic
potential of quartz in animals: pulmonary toxicity, lung weight,
development of fibrous tissue, collagen content, cytotoxicity, and
biochemical changes in the lungs (US EPA, 1996; Gift & Faust, 1997).
Table 1 presents critical non-cancer effects found in subchronic and
chronic quartz inhalation studies of rats and mice (US EPA, 1996; Gift
& Faust, 1997). All studies showed either fibrosis, increased
collagen, and increased elastin content of lungs or impaired
phagocytic ability of alveolar macrophages. (Table 1 also presents
estimates of human equivalent concentrations [HECs] for environmental
exposures, which are discussed in section 11.1.1.)
Tests of the carcinogenicity of quartz by different routes of
exposure have been conducted. Different quartz specimens (i.e.,
Min-U-Sil 5, Novaculite, DQ 12, hydrogen fluoride-etched Min-U-Sil 5,
Min-U-Sil with polyvinylpyridine- N-oxide, DQ 12 with
polyvinylpyridine- N-oxide, and Sikron F-300 quartz) with particle
sizes in the respirable range were tested in five experiments with
rats by inhalation (Holland et al., 1983, 1986; Dagle et al., 1986;
Muhle et al., 1989, 1991, 1995; Reuzel et al., 1991; Spiethoff et al.,
1992) and in four experiments with rats by intratracheal instillation
(Holland et al., 1983; Groth et al., 1986; Saffiotti, 1990, 1992;
Pott et al., 1994; Saffiotti et al., 1996). The results of these
experiments and others are summarized in Tables 2-4. Significant
increases in the incidence of adenocarcinomas and squamous cell
carcinomas of the lung were found in eight of the nine experiments.
Marked, dense pulmonary fibrosis was part of the response
(IARC, 1997). (The IARC Working Group noted that the experiment by
Reuzel et al. [1991], in which only one respiratory tract tumour was
observed, had a short duration and lacked information on survival;
in addition, only a small proportion of the quartz particles was
respirable by rats.) (Note: Level of statistical significance of
tumour incidence in treated animals compared with control animals was
reported only by Groth et al. [1986]. P-value was less than 0.001
for their Min-U-Sil 5 and Novaculite experiments.)
Although pulmonary granulomatous inflammation and slight to
moderate fibrosis of the alveolar septa were observed in three
experiments on hamsters that used repeated intratracheal instillation
of quartz dusts, no pulmonary tumours were observed (Holland et al.,
1983; Renne et al., 1985; Niemeier et al., 1986).
In experiments with mice, no statistically significant increase
was seen in the incidence of lung tumours in a strain A mouse (i.e.,
male A/J mice from Jackson Laboratories, Bar Harbor, ME, USA) lung
adenoma assay with one sample of quartz (McNeill et al., 1990) or with
a sample of quartz in a limited inhalation study of BALB/cBYJ female
mice (Wilson et al., 1986). Fibrosis was not observed; however, the
lungs of quartz-treated mice did have silicotic granulomas, and
lymphoid cuffing was observed around airways (IARC, 1997).
Thoracic and abdominal malignant lymphomas, primarily of the
histiocytic type, were found in several studies in rats using single
intrapleural or intraperitoneal injection of suspensions of several
types of quartz (Wagner & Berry, 1969; J.C. Wagner, 1970; Wagner &
Wagner, 1972; M.M.F. Wagner, 1976; Wagner et al., 1980;
Jaurand et al., 1987; IARC, 1997).
It is important to note the species differences observed in the
tumour response to quartz particles. In rats, quartz is clearly
carcinogenic, but there is less or no malignant tumour response in
mice and hamsters (Donaldson & Borm, 1998). Particle-induced lung
tumours have been noted in rats, but not to the same degree in mice or
hamsters (IARC, 1997). Currently, there is a limited understanding of
the mechanisms of quartz toxicity, including mechanisms of the rat
lung response (IARC, 1997). Several mechanisms for the carcinogenicity
of quartz in rats have been proposed, included the hypothetical
inflammation-based mechanism (Figure 1; IARC, 1997). The rat model is
the best model currently available for studying the effects of quartz,
because it demonstrates the carcinogenic response observed in some
human studies (Donaldson & Borm, 1998).
8.3.1 Interaction with other compounds
Other tests of carcinogenicity have been conducted using mixtures
of quartz with known carcinogens. When aerosol concentrations of
quartz (Dörentrup DQ 12) were administered by inhalation to rats for
29 days and followed by a single intravenous injection of Thorotrast
(an alpha-radiation-emitting material) at the end of the inhalation
period, there was a pronounced interactive effect of Thorotrast with
quartz (DQ 12) that included the occurrence of tumours in the lung
(i.e., bronchioloalveolar adenomas, bronchioloalveolar carcinomas, and
squamous cell carcinomas), liver, and spleen (Spiethoff et al., 1992;
IARC, 1997). In experiments with hamsters, benzo[ a]pyrene with
quartz and ferric oxide with quartz were administered by intratracheal
instillation. No pulmonary tumours were observed in hamsters given
mixtures of quartz and ferric oxide (1 : 1) (Niemeier et al., 1986).
However, the number of respiratory tract tumours in hamsters given
Min-U-Sil quartz and benzo[ a]pyrene or Sil-Co-Sil and
benzo[ a]pyrene was significantly higher (P < 0.01) than the
number found in hamsters that received saline and benzo[ a]pyrene
(Niemeier et al., 1986).
Table 1: Human equivalent concentrations (HECs) for environmental exposures and non-cancer and
non-tumour effects for LOAELsa reported in subchronic (<3 months) and chronic quartz inhalation
studies in experimental animals.b
Species, strain, Exposure, dose, LOAEL(mg/m3) LOAELHECc
number, sex duration (mg/m3) Critical effect Reference
Rat 1 mg/m3 of DQ 12 1 0.18 Subpleural and Muhle et al., 1989
Fischer 344 for 6 h/day, 5 peribronchial
50/sex days/week, for fibrosis, focal
24 months lipoproteinosis
cholesterol clefts,
enlarged lymph
nodes, and
granulomatous
lesions in the
walls of large bronchi;
doubling of lung
collagen content.
(Quantitative data were
not reported for these
effects.)
Mouse 2 mg/m3 of 2 0.36 Suppressed response to Scheuchenzuber
BALB/c Min-U-Sil aerosol of Escherichia et al., 1985
Female for 8 h/day, coli (i.e., formation
5 days/week, of plaque-forming cells
for 150, 300, in spleen) at 150, 300,
or 570 days and 570 days; reduced
ability of alveolar
macrophages to
phagocytize
Staphylococcus aureus
at 570 days; reduced
T-lymphocyte-mediated
cytolysis of allogeneic
tumour cells at 185 days.
Table 1 (cont'd)
Species, strain, Exposure, dose, LOAEL(mg/m3) LOAELHECc
number, sex duration (mg/m3) Critical effect Reference
Mouse 4932.4 + 235.4 5 0.90 Suppressed response to Burns et al., 1980
BALB/c µg/m3 of Min-U-Sil aerosol of Escherichia
Female 5 for 6 h/day, coli (i.e., formation of
5 days/week, for plaque-forming cells in
3, 9, 15, 27, spleen) at 15, 27, 33,
33, or 39 weeks and 39 weeks; increased
spleen/body ratios at
15, 21, and 27 weeks;
induced pulmonary fibrosis
(fibrotic nodules of
collagen, fibroblasts,
lymphocytes,
silica-filled
macrophages) at 39 weeks.
Rat 0, 2, 10, or 20 2 0.36 Increased collagen and Drew & Kutzman,
Fischer 344 mg/m3 of Min-U-Sil elastin content of lungs; 1984b
Both sexes 5 for 6 h/day, caused birefringent
5 days/week, for crystals in foamy
6 months cytoplasm of macrophages
that had accumulated in
end airway luminal
spaces; induced Type II
cell hyperplasia in
alveolar compartment and
intralymphatic
microgranulomas around
bronchioles in some
animals. Quartz-dependent
increases in collagen and
elastin were 110%, 111%,
and 116% for collagen
Table 1 (cont'd)
Species, strain, Exposure, dose, LOAEL(mg/m3) LOAELHECc
number, sex duration (mg/m3) Critical effect Reference
(as hydroxyproline) and
102%, 109%, and 109% for
elastin, respectively, for
each exposure group
relative to controls
(US EPA, 1996).
Rat 0, 2, 10, or 2 0.36 Increased weight and Drew & Kutzman,
Fischer 344 20 mg/m3 collagen, elastin, 1984a
Both sexes of Min-U-Sil 5 deoxyribonucleic acid,
for 6 h/day, and protein content of
5 days/week, lungs (particularly at
for 6 months, higher exposures of 10
plus 6-month and 20 mg/m3),
incubation period indicating continued
tissue proliferation and
fibrogenesis during
incubation; increased
number of silica
particles and
inflammation at end
airways, focal fibrosis
and intralymphatic
granulomata, and overall
severity and frequency of
lesions. Alveolar
proteinosis observed in
the 20 mg/m3 group.
Quartz increases in
collagen and elastin
were 116%, 128%, and
Table 1 (cont'd)
Species, strain, Exposure, dose, LOAEL(mg/m3) LOAELHECc
number, sex duration (mg/m3) Critical effect Reference
136% for collagen (as
hydroxyproline) and 107%,
119%, and 130% for
elastin, respectively,
for each exposure group
relative to controls
(US EPA, 1996).
a LOAEL = lowest-observed-adverse-effect level. No-observed-adverse-effect levels (NOAELs) were not reported.
b Adapted from Gift & Faust (1997).
c HEC calculated using methods described in US EPA (1994) and summarized in section 11.1.1.
Table 2: Summary of data on lung tumours induced in rats by quartz.a
Incidence of lung tumoursb
Treatment sample Exposure conditions Rat strain Sex Treated Controls Reference
Quartz Intratracheal instillation Sprague-Dawley not 6/36c 0/58 Holland
(Min-U-Sil 5) (suspended in 0.2 ml saline) reported et al., 1983
of 7 mg weekly for 10 weeks
Inhalation (nose only), Fischer 344 F 20/60d 0/54 Holland
12 + 5 mg/m3 for up to 2 years et al., 1986
Inhalation of 51.6 mg/m3 for Fischer 344 F 10/53e 0/47 Dagle et al.,
various durations; sacrificed M 1/47f 0/42 1986
at 24 months
Intratracheal instillation Fischer 344 M 30/67g 1/75h Groth et al.,
(volume of suspension not 1986
reported) of 20 mg in left
lung, sacrificed at 12, 18,
or 22 months or found dead
Novaculite Intratracheal instillation Fischer 344 M 21/72i 1/75h Groth et al.,
(i.e., of 20 mg (volume of 1986
microcrystalline suspension not reported)
quartz) in left lung, sacrificed at
12, 18, or 22 months or
found dead
Quartz (DQ 12) Inhalation of 1 mg/m3 Fischer 344 F 12/50j 3/100k (male Muhle et al.,
for 24 months M 6/50l and female) 1989
Inhalation (nose only) of Wistar F 62/82m 0/85 Spiethoff
6 mg/m3 for 29 days, followed et al., 1992
by lifetime observation
Table 2 (cont'd)
Incidence of lung tumoursb
Treatment sample Exposure conditions Rat strain Sex Treated Controls Reference
Inhalation (nose only) of Wistar F 69/82n 0/85 Spiethoff
30 mg/m3 for 29 days, et al., 1992
followed by lifetime
observation
Quartzo (Sikron Inhalation of 58.5 + 0.7 mg/m3, Wistar F 1/70p 0/70 Reuzel
F300 from Quartz 6 h/day, 5 days/week, M 0/70 0/70 et al., 1991
Werke, Frechen, for 13 weeks
Germany)
a Adapted from Saffiotti et al. (1996); IARC (1997).
b Number of lung tumours per number of rats observed.
c One adenoma and five carcinomas.
d Six adenomas, 11 adenocarcinomas, and three epidermoid carcinomas.
e All epidermoid carcinomas.
f One epidermoid carcinoma.
g All adenocarcinomas.
h One adenocarcinoma.
i Twenty adenocarcinomas and one epidermoid carcinoma.
j Two keratinizing cystic squamous cell tumours, two adenomas, and eight adenocarcinomas.
k Two adenomas and one adenocarcinoma.
l Two keratinizing cystic squamous cell tumours, two adenocarcinomas, one adenosquamous carcinoma,
and one epidermoid carcinoma.
m Eight adenomas, 17 bronchioloalveolar carcinomas, and 37 squamous cell carcinomas.
n Three adenomas, 26 bronchioloalveolar carcinomas, and 30 squamous carcinomas.
o IARC Working Group noted that only a small proportion of particles were respirable in rats.
p One squamous cell carcinoma, 1 year after the end of the exposure period.
Table 3: Lung tumours induced in Fischer 344 rats by a single intratracheal instillation of quartz.a
Treatment Treatment Sex Observation Incidence of Total number of Histological
sample doseb time lung tumoursc lung tumoursd types
Untreated None M Died after 0/32 0
17 months
None F Died after 1/20 (5%) 1 1 adenoma
17 months
Quartz 12 mg M Sacrificed 3/18 (17%) 37 6 adenomas,
(Min-U-Sil 5) 11 months 25 adenocarcinomas,
1 undifferentiated
Sacrificed 6/19 (32%) carcinoma, 2 mixed
17 months carcinomas, and 3
epidermoid
Died after 12/14 (86%) carcinomas
17 months
12 mg F Sacrificed 8/19 (42%) 59 2 adenomas,
11 months 46 adenocarcinomas,
3 undifferentiated
Sacrificed 10/17 (59%) carcinomas, 5 mixed
17 months carcinomas, and
3 epidermoid
Died after 8/9 (89%) carcinomas
17 months
20 mg F Died after 6/8 (75%) 13 1 adenoma, 10
17 months adenocarcinomas, 1 mixed
carcinoma, and 1 epidermoid
carcinoma
Table 3 (cont'd)
Treatment Treatment Sex Observation Incidence of Total number of Histological
sample doseb time lung tumoursc lung tumoursd types
Quartz 12 mg M Sacrificed 2/18 (11%) 20 5 adenomas,
(hydrogen 11 months 14 adenocarcinomas,
fluoride-etched and 1 mixed carcinoma
Min-U-Sil 5) Sacrificed 7/19 (37%)
17 months
Died after 7/9 (78%)
17 months
12 mg F Sacrificed 7/18 (39%) 45 1 adenoma,
11 months 36 adenocarcinomas,
3 mixed carcinomas,
Sacrificed 13/16 (81%) and 5 epidermoid
17 months carcinomas
Died after 8/8 (100%)
17 months
a From Saffiotti et al. (1993, 1996).
b As mg quartz suspended in 0.3 ml saline.
c Number of rats with lung tumours per number of rats observed.
d At all observation times.
Table 4: Incidence of lung tumours in female Wistar rats after intratracheal instillation of quartz.a
Number and percentage of rats with primary epithelial lung tumoursb
Material Surface Number of Number of Adenoma Adenocarcinoma Benign Squamous Total Other
area instillations rats CKSCTd cell (%) tumourse
(m2/g) (× mg)c examined carcinoma
Quartz
(DQ 12) 9.4 15 × 3 37 0 1z 11 1 + 1y 38 1
Quartz
(DQ 12) + PVNOf 9.4 15 × 3 38 0 1 + 3z 8 + 1x 4 + 1x + 3y + 1z 58 2
Quartz
(DQ 12) 9.4 1 × 45 40 0 1 7 1 23 2
Quartz
(Min-U-Sil) 15 × 3 39 1 4 + 4z 6 1 + 2y + 2z + 1y,z 54 3
Quartz
(Min-U-Sil)
+ PVNO 15 × 3 35 1 2 + 1x 8 5 + 1x + 1y + 1z 57 3
Quartz
Sykron (F600) 3.7 15 × 3 40 0 3 5 3 + 1z 30 1
0.9% sodium
chloride - 15 39 0 0 0 0 0 5
a From Pott et al. (1994); IARC (1997).
b If an animal was found to bear more than one primary epithelial lung tumour type, this was indicated as follows: x adenoma;
y adenocarcinoma; z benign CKSCT.
c Dusts were suspended in 0.9% sodium chloride solution with ultrasonication for 1-5 min.
d CKSCT, cystic keratinizing squamous cell tumour.
e Other types of tumours in the lung: fibrosarcoma, lymphosarcoma, mesothelioma or lung metastases from tumours at other sites
f PVNO, polyvinylpyridine-N-oxide; PVNO was administered subcutaneously in seven injections of 2 ml each of 2% PVNO in saline.
No PVNO control group was included.
8.4 Genotoxicity and related end-points
Although silica (form not specified) has not tested positive in
standard bacterial mutagenesis assays (IARC, 1987, 1997; Rabovsky,
1997), chromosomal changes, including DNA damage, have been observed
in experimental systems, both in vitro and in vivo. (Study results
are presented in Table 5.) Although the results of some studies
(Daniel et al., 1993, 1995; Saffiotti et al., 1993; Shi et al., 1994)
demonstrated that quartz caused damage (i.e., strand breakage) to
isolated DNA in acellular systems, the IARC Working Group (IARC, 1997)
stated that the relevance of these assays to assess quartz-related
genetic effects in vivo was "questionable." Uncertainties existed
because the non-physiological experimental conditions did not apply to
intracellular silica exposure and because very high doses of silica
were used in the DNA breakage assays (IARC, 1997). However, a recent
study not included in the IARC review found that by using the alkaline
single cell gel/comet assay, crystalline silica (Min-U-Sil 5) induced
DNA damage (i.e., DNA migration) in cultured Chinese hamster lung
fibroblasts (V79 cells) and human embryonic lung fibroblasts (Hel 299
cells) at concentrations ranging from 17.2 to 103.4 µg/cm2 (Zhong et
al., 1997). Since the time of the IARC review, Liu et al. (1996, 1998)
applied experimental conditions (i.e., Chinese hamster lung
fibroblasts challenged with dusts pretreated with a phospholipid
surfactant) to simulate the condition of particles immediately after
deposition on the pulmonary alveolar surface. Results of the
experiments showed that untreated Min-U-Sil 5 and Min-U-Sil 10 induced
micronucleus formation in a dose-dependent manner, but surfactant
pretreatment suppressed that activity (Liu et al., 1996). A subsequent
experiment found that surfactant pretreatment suppressed
quartz-induced DNA damage in lavaged rat pulmonary macrophages, but
DNA-damaging activity was restored with time as the phospholipid
surfactant was removed by intercellular digestion (Liu et al., 1998).
In vitro cellular transformation systems model the in vivo
process of carcinogenesis (Gu & Ong, 1996; Gao et al., 1997). The
ability of quartz to induce dose-dependent morphological
transformation of cells in vitro has been demonstrated in
experiments with Syrian hamster embryo cells (Hesterberg & Barrett,
1984) and mouse embryo BALB/c-3T3 cells (Saffiotti & Ahmed, 1995). Gu
& Ong (1996) also reported a significant increase in the frequency of
transformed foci of mouse embryo BALB/c-3T3 cells after treatment with
Min-U-Sil 5 quartz. These studies indicate that quartz can
morphologically transform mammalian cells. However, further studies
are needed to determine whether the transforming activity of quartz is
related to its carcinogenic potential.
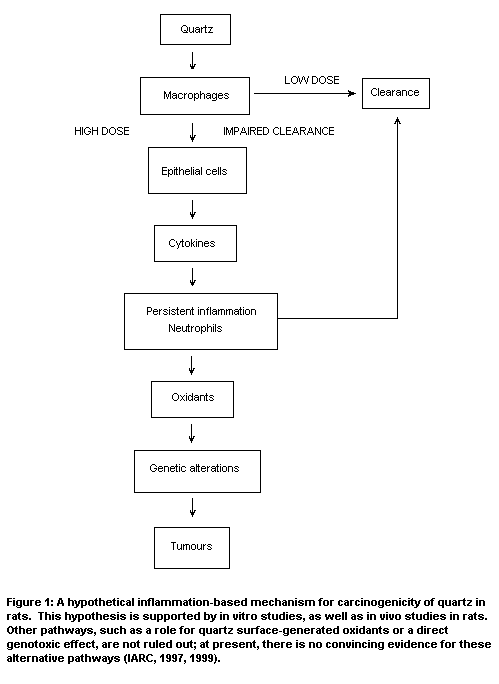 Table 5: Genetic and related effects of silica.a
Test system Resultb Dose Reference
(LED/HID)c
DNA strand breaks, gamma-HindIII-digested DNA + 30 000d Daniel et al., 1993
DNA strand breaks, herring sperm genomic DNA + 10 000d Daniel et al., 1993
DNA strand breaks, gamma-HindIII-digested DNA + 9 500d Daniel et al., 1995
DNA strand breaks, PM2 supercoiled DNA + 9 500d Daniel et al., 1995
GIA, Gene mutation, hprt locus, rat RLE-6TN
alveolar epithelial cells in vitro - NG Driscoll et al., 1997
SIC, Sister chromatid exchange,
Chinese hamster V79-4 cells in vitro - 15e Price-Jones et al., 1980
SHL, Sister chromatid exchange, human lymphocytes in vitro - 100d Pairon et al., 1990
SIH, Sister chromatid exchange, human lymphocytes
and monocytes in vitro - 100d Pairon et al., 1990
MIA, Micronucleus test, Syrian hamster embryo cells in vitro - 18.75f Oshimura et al., 1984
MIA, Micronucleus test, Syrian hamster embryo cells in vitro + 70e Hesterberg et al., 1986
MIA, Micronucleus test, Chinese hamster lung fibroblasts
(V79) in vitro + 200g Nagalakshmi et al., 1995
CIC, Chromosomal aberrations, Chinese hamster lung
fibroblasts (V79) in vitro - 1 600g Nagalakshmi et al., 1995
CIS, Chromosomal aberrations, Syrian hamster embryo
cells in vitro - 18.75f Oshimura et al., 1984
Table 5 (cont'd)
Test system Resultb Dose Reference
(LED/HID)c
AIA, Aneuploidy, Chinese hamster lung cells (V79-4) in vitro - 15e Price-Jones et al., 1980
AIA, Aneuploidy, Syrian hamster embryo cells in vitro - 18.75f Oshimura et al., 1984
AIA, Tetraploidy, Syrian hamster embryo cells in vitro - 70e Hesterberg et al., 1986
TBM, Cell transformation, BALB/3T3/31-1-1 mouse cells in vitro + 30d,h,i Saffiotti & Ahmed, 1995
TBM, Cell transformation, BALB/3T3/31-1-1 mouse cells in vitro + 60j,k Saffiotti & Ahmed, 1995
TCS, Cell transformation, Syrian hamster embryo cells in vitro + 18e Hesterberg & Barrett, 1984
TCS, Cell transformation, Syrian hamster embryo cells in vitro + 70f Hesterberg & Barrett, 1984
TCL, Cell transformation, fetal rat lung epithelial cells
in vitro (+) NGd Williams et al., 1996
MIH, Micronucleus test, human embryonic lung (Hel 299)
cells in vitro + 800g Nagalakshmi et al., 1995
CIH, Chromosomal aberrations, human embryonic lung
(Hel 299) cells in vitro - 1 600g Nagalakshmi et al., 1995
DVA, 8-hydroxy-2'-deoxyguanosine DNA extract from
lung tissue, male Wistar rats + 50 × 1 itd Yamano et al., 1995
DVA, 8-hydroxy-2'-deoxyguanosine DNA extract from
peripheral blood leukocytes, male Wistar rats - 50 × 1 itd Yamano et al., 1995
GVA, Gene mutation, hprt locus, rat alveolar
epithelial cells in vivo + 100 × 1 it Driscoll et al., 1995
Table 5 (cont'd)
Test system Resultb Dose Reference
(LED/HID)c
GVA, Gene mutation, hprt locus, rat alveolar
epithelial cells in vivo + 5 × 2 it Driscoll et al., 1997
MVM, Micronucleus test, albino mice in vivo - 500 ip Vanchugova et al., 1985
SLH, Sister chromatid exchange, human lymphocytes in vivo + NG Sobti & Bhardwaj, 1991
CLH, Chromosomal aberrations, human lymphocytes in vivo + NG Sobti & Bhardwaj, 1991
BID, Calf thymus DNA binding in vitro + 200l Mao et al., 1994
ICR, Metabolic cooperation using 8-azaguanine-resistant
cells, Chinese hamster lung cells (V79-4) in vitro - 50 Chamberlain, 1983
a Source: IARC (1997). See Appendix 1 of IARC (1997), Test system code words, for definitions of
the abbreviations used in column 1.
b Results without exogenous metabolic system: +, positive; (+), weakly positive; -, negative.
c LED, lowest effective dose; HID, highest ineffective dose; in vitro tests, µg/ml; in vivo tests,
mg/kg body weight per day; NG, not given; it, intratracheal; ip, intraperitoneal.
d Min-U-Sil 5.
e Min-U-Sil unspecified.
f alpha-Quartz.
g Min-U-Sil 5 and Min-U-Sil 10.
h Min-U-Sil 5, hydrofluoric acid-etched.
i A Chinese standard quartz sample.
j DQ 12, a standard German quartz sample.
k F600 quartz.
l Min-U-Sil 5 or Chinese standard quartz.
Some studies have demonstrated the ability of quartz to induce
micronuclei in mammalian cells in culture (i.e., Oshimura et al.,
1984; Hesterberg et al., 1986; Nagalakshmi et al., 1995) and were
reviewed by IARC (Table 5). However, other in vitro studies did not
observe chromosomal aberration (Oshimura et al., 1984;
Nagalakshmi et al., 1995), hprt (hypoxanthine-guanine phosphoribosyl
transferase) gene mutation (Driscoll et al., 1997), or aneuploid or
tetraploid cells (Price-Jones et al., 1980; Oshimura et al., 1984;
Hesterberg et al., 1986).
Pairon et al. (1990) tested quartz (i.e., Min-U-Sil 5) particles
for their ability to induce a significant number of sister chromatid
exchanges in cultures of human lymphocytes plus monocytes or of human
purified lymphocytes. The results were not "clear cut" for any of the
three doses tested (i.e., 0.5, 5.0, and 50 µg/cm2) (Pairon et al.,
1990) (Table 5).
An in vivo treatment of rats with quartz induced mutation in
rat alveolar epithelial cells (Table 5) (Driscoll et al., 1995, 1997).
Nehls et al. (1997) reported results of tests for DNA modifications by
quartz that were not reviewed by IARC (1997). Quartz (2.5 mg of DQ 12
suspended in 0.5 ml of physiological saline) or corundum (2.5 mg), a
non-carcinogenic particle, was intratracheally instilled into the
lungs of Wistar rats (10 rats per exposure and per time period).
Control animals were exposed to saline solution or not treated. Rats
were sacrificed 7, 21, and 90 days after treatment, then lung tissue
sections were analysed with immunocytological assay to determine the
level of 8-hydroxydeoxyguanosine in DNA extracts. Reactive oxygen
species can induce 8-hydroxydeoxyguanosine and other mutagenic DNA
oxidation products, which may be converted to mutations in
proliferating cells (Nehls et al., 1997). Exposure to quartz induced
levels of 8-hydroxydeoxyguanosine in the DNA of alveolar lung cells
that were significantly higher (P-value not reported) at all time
points than levels found in cells of untreated rats or rats treated
with corundum or saline. The number of total cells in bronchoalveolar
lavage fluid was 3-4 times higher in the quartz-treated groups at all
time points than in corundum-exposed rats and control rats exposed to
saline.
Other in vivo studies not reviewed by IARC (1997) found that
quartz induced micronuclei in pulmonary alveolar macrophages of male
Wistar rats in a time-dependent (Leigh et al., 1998b) and
dose-dependent manner (Wang et al., 1997b).
In summary, results of genotoxicity studies of quartz conflict,
and a direct genotoxic effect for quartz has not been confirmed or
ruled out.
8.5 Reproductive and developmental toxicity
There are no data available on the reproductive or developmental
effects of quartz in laboratory animals (IARC, 1997).
8.6 Immunological and neurological effects
Data on the neurological effects of quartz have not been
identified. In vitro studies have shown that quartz can stimulate
release of cytokines and growth factors from macrophages and
epithelial cells, and there is evidence that these events may occur
in vivo and contribute to disease (IARC, 1997). The immunological
response to quartz in experimental animals is a complex subject with
uncertain implications for humans, and detailed reviews are available
elsewhere (i.e., Davis, 1991, 1996; Haslam, 1994; Heppleston, 1994;
Weill et al., 1994; Driscoll, 1996; Gu & Ong, 1996; Hook & Viviano,
1996; Iyer & Holian, 1996; Kane, 1996; Sweeney & Brain, 1996;
Weissman et al., 1996; Mossman & Churg, 1998).
9. EFFECTS ON HUMANS
9.1 Case reports
There are many published case reports of adverse health effects
from occupational exposure to quartz. These health effects include
silicosis (acute and chronic) and lung cancer. Case reports of
silicosis and lung cancer are not mentioned further, because these
diseases have been researched in depth in epidemiological studies
(section 9.2).
There are numerous published case reports of several autoimmune
disorders in workers or patients who had been occupationally exposed
to crystalline silica, including quartz dust (NIOSH, forthcoming). The
most frequently noted autoimmune diseases in those reports were
scleroderma, systemic lupus erythematosus (i.e., lupus), rheumatoid
arthritis, autoimmune haemolytic anaemia (Muramatsu et al., 1989), and
dermatomyositis or dermatopolymyositis (Robbins, 1974; Koeger et al.,
1991). Case reports have also described health effects that may be
related to the immunological abnormalities observed in patients with
silicosis, such as chronic renal disease (Saita & Zavaglia, 1951;
Giles et al., 1978; Hauglustaine et al., 1980; Bolton et al., 1981;
Banks et al., 1983; Slavin et al., 1985; Bonnin et al., 1987;
Osorio et al., 1987; Arnalich et al., 1989; Sherson & Jorgensen, 1989;
Dracon et al., 1990; Pouthier et al., 1991; Rispal et al., 1991;
Neyer et al., 1994; Wilke et al., 1996), ataxic sensory neuropathy
(Tokumaru et al., 1990), chronic thyroiditis (Masuda, 1981),
hyperthyroidism (i.e., Graves' disease) (Koeger et al., 1996),
monoclonal gammopathy (Fukata et al., 1983, 1987; Aoki et al., 1988),
and polyarteritis nodosa (Arnalich et al., 1989).
9.2 Epidemiological studies
9.2.1 Silicosis
Most, if not all, of the several hundred epidemiological studies
of exposure to quartz dust are studies of occupational cohorts. The
majority of studies investigated the occurrence of silicosis morbidity
or mortality. These studies have conclusively linked occupational
quartz dust exposure with silicosis. Silicosis (i.e., nodular
pulmonary fibrosis) is a fibrotic lung disease, sometimes
asymptomatic, that is caused by the inhalation and deposition of
respirable crystalline silica particles (i.e., particles <10 µm in
diameter) (Ziskind et al., 1976; IARC, 1987).
A worker may develop one of three types of silicosis, depending
on the airborne concentration of respirable crystalline silica: (1)
chronic silicosis, which usually occurs after 10 or more years of
exposure at relatively low concentrations; (2) accelerated silicosis,
which develops 5-10 years after the first exposure; or (3) acute
silicosis, which develops after exposure to high concentrations of
respirable crystalline silica and results in symptoms within a few
weeks to 4 or 5 years after the initial exposure (Ziskind et al.,
1976; Peters, 1986; NIOSH, 1992a,b, 1996). Acute silicosis is a risk
for workers with a history of high exposures from performing
occupational processes that produce small particles of airborne dust
with a high silica content, such as during sandblasting, rock
drilling, or quartz milling, or any other process with high exposures
to small particles of airborne dust with a high quartz content
(Davis, 1996).
A recent study of 67 paraffin-embedded lung tissue samples from
silicotic patients found a significant linear relationship
(P <0.001) between lung quartz concentration and silicosis severity in
gold miners; although several types of mineral particles were found in
the lungs, quartz was the only significant indicator of silicosis
severity. The silicosis cases included 39 patients without lung cancer
and 28 patients with lung cancer. All of the cases were gold miners in
Canada (Dufresne et al., 1998a,b).
The epidemiological studies of silicosis usually define the
profusion of small opacities present in the disease according to a
standard system used by trained readers and developed by the
International Labour Organization for classification of chest
radiographs of pneumoconioses (ILO, 1980). Each reader assesses the
profusion according to a 12-point scale of severity. Categories 0/-
and 0/0 are the first and second points on the scale and represent a
normal chest radiograph. The third point, category 0/1, represents the
borderline between normality and abnormality, and category 1/0, the
fourth point, represents definite, but slight, abnormality
(Love et al., 1994). The shape (rounded or irregular) and size of the opacities
can also be described by the readers.
The critical studies of chronic silicosis, a progressive disease,
are those occupational epidemiological studies where (1) quantitative
quartz exposure data were available and used for risk analysis,
(2) exposure-response relationships were investigated, or (3) the
exposure-response relationships were documented with sufficient detail
for a health effects benchmark, including (4) application of data to
mathematical models that predicted silicosis prevalence at increasing
concentrations of cumulative quartz exposure. (The predicted
prevalences reported in the studies are discussed in section 11.1.3.)
Studies that selected workers from a broad spectrum of occupations and
included many workers that were exposed to different combinations of
various minerals, such as studies of "dusty trades" workers
(i.e., Rice et al., 1986), were excluded from consideration for risk
assessment of quartz and silicosis. Epidemiological studies that
provided evidence of an exposure-response relationship for silica and
silicosis based on other kinds of exposure data (e.g., there is a
positive relationship between development of chronic silicosis and
duration of exposure) have been reviewed elsewhere (WHO, 1986;
Goldsmith, 1994; Hughes, 1995; Rice & Stayner, 1995; Seaton, 1995;
Steenland & Brown, 1995a; Davis, 1996; US EPA, 1996).
The two critical cross-sectional studies (i.e., Kreiss & Zhen,
1996; Rosenman et al., 1996 -- see Table 6) found that the prevalence
of radiographic silicosis (ILO category >1/0 or >1/1) was
dose-related. That is, the prevalence of radiographic silicosis
increased with average silica dust exposure, cumulative quartz
exposure, duration of employment, or all of these measures. The actual
prevalences varied greatly among the studies, and conclusions
concerning quartz dust concentrations that may or may not induce
silicosis cannot be drawn from simple "eyeball" analysis of the
prevalences in the following two worker populations:
* Kreiss & Zhen (1996) conducted a community-based random sample
survey of 134 male residents at least 40 years old, living in a
hardrock (i.e., molybdenum, lead, zinc, and gold) mining town in
Colorado, USA. Of the 134 residents, 100 were silica-exposed
hardrock miners (including 32 silicosis cases) and 34 were
community "controls" without occupational dust exposure. Nearly
all (97%) of the dust-exposed subjects were 20 years since first
exposure. The estimated crystalline silica content (polymorph not
reported) of the total dust was 12.3%. Exposure was assessed with
information from occupational histories, gravimetric dust
exposure data from 1974-1982, and a cumulative silica exposure
index. Pre-1974 exposure estimates were based on job-specific
gravimetric data collected after 1974. Exposures were also
estimated for mines where no exposure data were available (17.1%
of person-years of follow-up). Thirty-two per cent of the 100
dust-exposed subjects had silicosis (defined as radiological
profusion of small opacities of ILO category >1/0). Prevalence
of silicosis was related to average silica dust exposure. Among
the 94 dust-exposed subjects with data on cumulative and average
dust exposures, those subjects with average silica exposure
<0.05 mg/m3 had 10% prevalence of silicosis; subjects with
>0.05-0.10 mg/m3 had a prevalence of 22.5%; subjects with
greater than 0.10 mg/m3 average silica exposure had a silicosis
prevalence of 48.6% (P = 0.01). (See Table 6 for predicted
prevalences when silicosis was defined as radiological profusion
of small opacities of ILO category >1/1.) It is not known
whether the small sample of 134 residents was representative of
all miners or if the exposure estimates for mines where no
exposure data were available (17.1% of person-years of follow-up)
were representative.
* Rosenman et al. (1996) conducted a cross-sectional study in 1991
of 549 current, 497 retired, and 26 current salaried workers that
were former production workers in a US grey iron foundry that
produced automotive engine blocks (total workers = 1072).
Twenty-eight cases (2.9%) of silicosis, defined as rounded
opacities >ILO category 1/0, were identified by at least two
of three "B" readers of a total of 952 chest radiographs. More
than half (18/28) of the cases were found in retired workers.
Silicosis prevalence was positively related to mean silica (i.e.,
quartz) exposure (P < 0.0001). Of the workers with mean quartz
exposure less than 0.05 mg/m3, 0.8% had silicosis, while 6.3%
of foundry workers with mean quartz exposure greater than 0.45
mg/m3 had silicosis. Silicosis prevalence also increased with
years of employment at the foundry, cumulative silica exposure,
work area within the foundry, and cigarette smoking (i.e., smoker
vs. non-smoker). Exposure estimates were derived from conversions
of "early silica exposure data" collected by impingers. Quartz
content of total dust was not reported. Weighted total dust
exposure from impinger data was converted to an estimate of
silica exposure in mass units (mg/m3) by multiplying it by the
average percentage of quartz in bulk samples.
Results of cohort studies of gold miners in South Africa, Canada,
and the USA (see Table 7) also demonstrated an exposure-response
relationship for radiographic silicosis (US EPA, 1996):
* A cohort study was conducted of 2235 white South African
underground gold miners, 45-54 years old at the time of medical
examination in 1968-1971, who started working after 1938, worked
>10 years, and were followed until 1991 (Hnizdo &
Sluis-Cremer, 1993). More than 300 (n = 313) of the 2235 miners
were followed to the time when radiological signs developed, 658
miners were followed up to death, and 1264 miners were followed
to the year of the most recent radiograph. Radiographs were read
blindly by two independent readers. Silicosis was defined as the
presence of rounded opacities of ILO category >1/1.
Radiographs were read blindly by two readers initially, then one
reader was chosen because his readings more closely matched the
autopsy data. Mean respirable dust concentrations, after heat and
acid treatment, in milligrams per cubic metre per shift were
calculated for nine gold mining occupations. The concentrations
were based on a study of shift-long dust exposure that measured
the surface area of the respirable mine dust and the number of
respirable particles (i.e., incombustible and acid-insoluble dust
particles) per cubic metre in a random sample of 20 South African
gold mines (Beadle, 1965, 1971). After heat and acid treatment,
the respirable dust in South African gold mines was found to
contain about 30% quartz (Beadle & Bradley, 1970). Cumulative
dust exposure for the miners was calculated in milligrams per
cubic metre-year by using data for mean mass respirable dust
concentrations for the nine occupational categories, the average
number of hours underground, and the number of dusty 8-h shifts.
Of the 2235 miners studied by Hnizdo & Sluis-Cremer (1993),
313 developed radiologically diagnosed silicosis (rounded
opacities with profusion of ILO category >1/1) during the
follow-up period (i.e., 1968-1971 to 1991). The onset of
silicosis occurred after an average (i.e., mean) of 27 years of
Table 6: Predicted prevalence of silicosis (ILO category >1/1)
following exposure to respirable quartz dust based on modelling of cumulative exposure
at mean concentrations of 0.05 or 0.10 mg/m3 over a 45-year working lifetime.
Cross-sectional study Mean concentration of Predicted prevalence of Cohort's mean time Cohort's maximum time
and cohort respirable quartz dust silicosis, ILO category since first quartz since first quartz
(mg/m3) >1/1 (cases per exposure (years) exposure (years)
100 workers)
Kreiss & Zhen, 1996 0.05 approx. 30a silicotic miners: silicotic miners:
41.6 66
100 US hardrock 0.10 approx. 90a non-silicotic non-silicotic
miners and 34 miners: 33.5 miners: 68
community controls
Rosenman et al., 0.05 2b,c 28 >30
1996
1072 US grey 0.10 3b,c
iron foundry workers
a Based on cumulative silica exposure model with 10 years of post-employment follow-up.
b ILO category >1/0.
c Based on a 40-year working lifetime and controlling for pack-years of cigarette smoking, race,
and silica exposure other than in the foundry under study.
Table 7: Predicted number of silicosis cases (ILO category >1/1) following exposure to respirable
quartz dust based on modelling of cumulative exposure at mean concentrations of 0.05 or 0.10 mg/m3
over a 45-year working lifetime.
Cohort study Mean concentration of Silicosis cases, Mean time since first Maximum time since
and population respirable quartz ILO category >1/1, quartz exposure (years) first quartz exposure
dust (mg/m3) per 100 workers (years)
Hnizdo & 0.05 13a silicotic miners: 36 silicotic miners: 50
Sluis-Cremer, 1993
2235 South African 0.10 approx. 70
gold miners
Muir et al., 0.05 0.09-0.62a,b 18 silicotic miners: 38
1989a,b;
Muir, 1991
2109 Canadian
gold and
uranium miners
Steenland & 0.05 10c 37 73d
Brown, 1995a
3330 US gold 0.09 47c
miners
a Estimate was reported in Rice & Stayner (1995).
b No post-employment follow-up and no retired miners included. The range includes five estimates
(one for each reader).
c The predicted number of silicosis cases does not account for effects of age or calendar time
(K. Steenland, personal communication, 1997).
d K. Steenland, personal communication, 1998.
net service, at a mean age of 56 years. For more than half of the
miners (n = 178; 57%), the onset occurred an average of
7.4 years (standard deviation 5.5; range 0.1-25 years) after
their employment at the mines, at 59 years of age (range
44-74 years). For the other miners (n = 135; 43%), the onset
of silicosis occurred while they were still mining, at 51 years
of age (range 39-61 years). These results show that the majority
of the cases occurred in miners who were no longer employed at
the mine and who were at least 50 years old
(Hnizdo & Sluis-Cremer, 1993).
* Muir and colleagues conducted a study of 2109 current Ontario
miners from 21 gold and uranium mines who started working and
worked more than 5 years between 1940 and 1959 and were followed
to 1982 or to the end of their dust exposure, whichever came
first (Muir et al., 1989a,b; Muir, 1991). Any uranium miner with
more than 2 weeks of exposure was also included (Muir et al.,
1989a). The quartz content of respirable gold mine dust was 6.0%,
and that of uranium mine dust was 8.4%. Retired and former miners
were not included in the study. Sources of data for this study
were full-sized annual chest radiographs taken for all miners
after 1927 and periodic (pre-1959) and semi-annual mine dust
measurements obtained with a konimeter (which is an instantaneous
dust sampler that measures the number of particles per unit
volume of air; Verma et al., 1989). Konimeter dust measurements
taken from 1940 to 1952 were expressed in particles per cubic
centimetre of air (ppcc). Verma et al. (1989) initiated an
extensive, side-by-side comparison of the konimetric and
gravimetric (i.e., milligrams of silica per cubic metre) sampling
to derive a konimetric/gravimetric silica conversion curve. A
total of 2360 filter (i.e., nylon cyclone-filter assembly in a
constant-flow pump) samples and 90 000 konimeter samples were
taken in a 2-year period in two gold and uranium mines, in
existing conditions as well as in an experimental simulation of
the high-dust conditions of the past caused by dry drilling
(Verma et al., 1989). The results of the conversion relationship
were non-linear and may have reflected the limitations of the
konimeter in measuring high dust (i.e., high count)
concentrations and the limitations of the gravimetric sampler in
measuring low dust concentrations. There were different
relationships for the gold and uranium mines, possibly because of
the different fractional silica concentrations in the host rock.
The conversion of the historical konimeter counts to gravimetric
respirable silica equivalents was used to derive a cumulative
respirable silica dose for each miner based on the miner's
respirable silica dose for each year, mine, and task in his work
history (Verma et al., 1989).
Thirty-two of the 2109 hardrock miners studied by Muir and
colleagues were considered by at least one of five readers to
have silicosis (small, rounded opacities with profusion of ILO
category >1/1). However, the results differed among the five
readers and "complicated the analysis" (Muir et al., 1989b). One
of the five readers identified only seven cases of silicosis
(Muir et al., 1989b). The results were presented by individual
reader and by consensus. A consensus of all of the five readers
with respect to identification of silicosis was reached on only
six cases (Muir et al., 1989b). Average respirable quartz dust
exposure for the cases was not reported.
* A cohort study of 3330 white male underground gold miners from
South Dakota employed for at least 1 year between 1940 and 1965
and followed through 1990 found 170 cases of silicosis (128 cases
were identified on death certificates, 29 cases were found during
X-ray surveys of workers conducted in 1960 and 1976, and 13 cases
were identified on both X-ray and death certificate). Cases were
defined as (1) an underlying or contributing cause of death of
silicosis, silico-tuberculosis, respiratory tuberculosis, or
pneumoconiosis, and/or (2) ILO category >1/1 silicosis
identified in the 1976 radiographic survey or "small opacities"
or "large opacities" identified in the 1960 radiographic survey
(Steenland & Brown, 1995a). The miners were exposed to a median
quartz level of 0.05 mg/m3 (0.15 mg/m3 for workers hired prior
to 1930). The average length of follow-up was 37 years, and the
average length of employment underground was 9 years. Quartz
exposure was estimated by converting dust particle counts to
gravimetric measurements (i.e., mg/m3), based on an estimate of
13% quartz content of total dust. A job-exposure matrix was
created to estimate dust exposures for each job over time, then
average dust exposures for the job categories were calculated
using existing measurements for each year from 1937 to 1975. The
estimated daily dust exposures (constant over each year) were
weighted to account for daily time spent underground. Summation
of the estimated daily dust levels over time provided an estimate
of cumulative quartz exposure (Steenland & Brown, 1995a). The
risk of silicosis was less than 1% for miners with a cumulative
exposure less than 0.5 mg/m3-years. The risk increased to 68-84%
for the highest cumulative exposure category (i.e.,
4 mg/m3-years) (Steenland & Brown, 1995a). Silicosis risk
estimates could have been affected by (1) combining silicosis
deaths with silicosis cases detected by cross-sectional
radiographic surveys, (2) differences in quartz content of dust
in early years, and (3) lack of dust measurements before 1937.
A cohort study of a subcohort of the South Dakota gold
miners described above analysed cases of silicosis that were
reported as the underlying cause of death on the death
certificates. Forty cases of silicosis, as well as 49 cases of
tuberculosis, were ascertained among the 1321 miners employed for
at least 21 years and followed through 1973. There was a linear
trend in risk of about 2.4% for each 0.1 mg/m3 of silica
exposure. However, this study does not meet the criteria for a
critical study because risk by cumulative quartz exposure was not
calculated (McDonald & Oakes, 1984).
In the five critical studies described above, the number of cases
identified depended upon the definition of silicosis (radiographic
category and whether irregular opacities were included), the quality
of the evaluation of the chest radiographs (e.g., number and training
of readers), the duration of dust exposure, and the duration of
follow-up after the end of exposure. Interstudy variation exists for
each of these factors. In addition, exposure assessments in these
studies were accompanied by uncertainties, such as the use of
conversion equations (i.e., converting particle count data to mass
concentrations; application of equations from one industry to a
different industry) and estimation of quartz content of the dust. It
is not uncommon for epidemiological studies to lack characterization
of the source and properties of the mineral dusts collected in the
workplace (Mossman & Churg, 1998). Nevertheless, the critical studies
found an exposure-response relationship for respirable quartz dust
that, when modelled, predicts the occurrence of silicosis cases in
various industries at exposures close to regulatory levels.
9.2.2 Pulmonary tuberculosis and other infections
The association between tuberculosis and silicosis has been
firmly established by the results of epidemiological studies conducted
during this century (Balmes, 1990). In recent studies of silicotics,
the association was well supported by the results of a survey of
tuberculosis deaths among silicotics in the USA for the period
1979-1991 (Althouse et al., 1995), a mortality study of 590 California
silicosis claimants (Goldsmith et al., 1995a), and a retrospective
study of silicotic miners from the Freegold mines in South Africa
(Kleinschmidt & Churchyard, 1997).
In studies of workers without silicosis, there is some limited
evidence that long exposures or high cumulative exposures to quartz
dust may increase the risk of developing tuberculosis. Two
epidemiological studies reported 3-fold higher incidences of pulmonary
tuberculosis cases in 5424 non-silicotic silica-exposed Danish foundry
workers employed 25 or more years (Sherson & Lander, 1990) and among
335 non-silicotic black South African gold miners with a median
underground employment of 26 years (Cowie, 1994). Westerholm et al.
(1986) found 13 cases of tuberculosis among 428 silicotic Swedish iron
and steel workers and one case of tuberculosis in a comparison group
of 476 Swedish iron and steel workers with normal chest radiographs
(level of statistical significance not reported). Both groups had been
exposed to silica for at least 5 years.
A study of tuberculosis incidence in 2255 white South African
gold miners included 1296 miners who had an autopsy. The
smoking-adjusted relative risk for pulmonary tuberculosis in miners
without silicotic nodules on autopsy examination (n = 577) increased
slightly with quartiles of cumulative dust exposure (relative risk
[RR] = 1.38; 95% confidence interval [CI] = 0.33-5.62) for the highest
quartile of cumulative exposure). For miners without radiologically
diagnosed silicosis (n = 1934), the smoking-adjusted relative risk
increased to 4.01 (95% CI = 2.04-7.88) in the highest quartile of
cumulative dust exposure (Hnizdo & Murray, 1998, 1999). Radiological
silicosis was defined as ILO category >1/1; detailed ILO grading
was not performed (Hnizdo & Murray, 1998, 1999). Tuberculosis was
diagnosed on average 7.6 years after the end of dust exposure and
6.8 years after the onset of radiological silicosis -- a result that
supports the need for medical surveillance of workers after the end of
exposure to silica dust (Hnizdo & Murray, 1998). (Miners who developed
tuberculosis before completing 10 years of underground employment were
excluded because they were not allowed to continue working underground
after diagnosis) (Hnizdo & Murray, 1998). It is not clear whether
"dust" exposure refers to quartz exposure or exposure to gold mine
dust.
Chen et al. (1997) conducted a case-control study (8740 cases;
83 338 controls) with US National Occupational Mortality Surveillance
data for the years 1983-1992 that controlled for confounding from age,
gender, race, socioeconomic status, potential exposure to active
tuberculosis, and the presence of silicosis and other pneumoconioses.
The potential for exposure to silica was based on potential exposure
data from the National Occupational Exposure Survey (Seta et al.,
1988) and the National Occupational Health Survey of Mining
(Greskevitch et al., 1996) and was categorized as "high,"
"intermediate," or "low or no" potential exposure. The study found
that decedents with potential high exposure to silica and with no
documentation of silicosis on the death certificate had a 30% greater
odds of mortality from respiratory tuberculosis than decedents with no
potential exposure to silica after adjustment by logistic regression
for the possible confounders mentioned above (odds ratio [OR] = 1.3;
95% CI = 1.14-1.48). The results also suggested the presence of an
exposure-response relationship of silica exposure, in the absence of
silicosis, with death from respiratory tuberculosis (Chen et al.,
1997).
In summary, the relationship between quartz exposure and
tuberculosis risk in the absence of radiographic silicosis in
silica-exposed workers has not been well defined or quantitatively
described by existing epidemiological studies.
A recent case-control study of tuberculosis and pulmonary disease
caused by non-tuberculous mycobacteria (NTM) in South African gold
miners found that radiographic silicosis, focal radiological scarring,
and human immunodeficiency virus (HIV) infection were significant risk
factors for NTM disease and for tuberculosis (Corbett et al., 1999).
Past medical history of tuberculosis treatment (OR = 15.1;
95% CI = 7.64-29.93) and current employment in a "dusty job" at the
mines (OR = 2.5; 95% CI = 1.46-4.44) were significant risk factors for
NTM. The study included 206 NTM patients and 381 tuberculosis patients
of known HIV status admitted to a South African hospital and
180 controls that were HIV-tested surgical or trauma patients admitted
to that hospital during the same time period. Odds ratios for NTM
and tuberculosis increased with increasing years of employment
(range of ORs: 1.0-9.4 for NTM, and 1.0-4.1 for tuberculosis).
9.2.3 Lung cancer
Lung cancer is associated with occupational exposures to inhaled
quartz (IARC, 1997).
Following a comprehensive review of the large body of published
epidemiological studies, IARC (1997) found that the following
epidemiological studies provide the least confounded investigations of
an association between occupational crystalline silica exposure and
lung cancer risk:
* US gold miners (Steenland & Brown, 1995b)
* Danish stone industry workers (Guenel et al., 1989)
* US granite shed and quarry workers (Costello & Graham, 1988)
* US crushed stone industry workers (Costello et al., 1995)
* US diatomaceous earth industry workers (Checkoway et al., 1993,
1996)
* Chinese refractory brick workers (Dong et al., 1995)
* Italian refractory brick workers (Puntoni et al., 1988;
Merlo et al., 1991)
* United Kingdom pottery workers (Cherry et al., 1995, 1997;
McDonald et al., 1995, 1997; Burgess et al., 1997)
* Chinese pottery workers (McLaughlin et al., 1992)
* cohorts of registered silicotics from North Carolina
(Amandus et al., 1991, 1992) and Finland (Kurppa et al., 1986;
Partanen et al., 1994)1
Although a few of those studies did not find a statistically
significant association between occupational crystalline silica
exposure and lung cancer risk, most of the studies did. Some
non-uniformity of results is not unusual when a large number of
epidemiological studies are reviewed and a variety of populations and
1 This list includes studies of diatomaceous earth industry workers
and workers in the ceramics, refractory brick, and pottery
manufacturing industries where the primary silica exposure may have
been to cristobalite rather than quartz. In most studies, exposure
data for quartz as well as cristobalite or tridymite were not
available to support that assumption.
work environments are studied (IARC, 1997). In addition, IARC noted
that the carcinogenicity of quartz (or cristobalite) "may be dependent
on inherent characteristics of the crystalline silica or on external
factors affecting its biological activity or distribution of its
polymorphs" (IARC, 1997). (A detailed critique of the studies listed
above is available in Soutar et al., 1997.)
Some of the least-confounded studies reported that lung cancer
risk tended to increase with:
* cumulative exposure to respirable silica (i.e., Checkoway et al.,
1993, 1996)
* duration of exposure (i.e., Costello & Graham, 1988;
Merlo et al., 1991; Partenen et al., 1994; Costello et al., 1995;
Dong et al., 1995)
* peak intensity of exposure (Burgess et al., 1997; Cherry et al.,
1997; McDonald et al., 1997)
* the presence of radiographically defined silicosis
(Amandus et al., 1992; Dong et al., 1995)
* length of follow-up time from date of silicosis diagnosis
(Partenen et al., 1994)
The observed associations noted above, including the
exposure-response associations, are unlikely to be explained by
confounding or other biases; therefore, the epidemiological studies,
overall, support increased lung cancer risks from occupational
exposure to inhaled respirable crystalline silica (i.e., quartz and
cristobalite) (IARC, 1997).
Three studies published since the IARC review investigated
exposure-response associations for lung cancer and occupational
exposure to quartz.
Cherry et al. (1998) published a final report of the preliminary
results of a nested case-control study of 52 lung cancer deaths in a
cohort of 5115 pottery workers (i.e., Burgess et al., 1997; Cherry et
al., 1997; McDonald et al., 1997). After adjustment for smoking and
inclusion of a 20-year-, 10-year-, or 0-year lag period, mean silica
concentration (i.e., estimated daily 8-h time-weighted airborne
concentrations in µg/m3; polymorph not specified) was associated with
lung cancer (OR = 1.60, 95% CI = 1.11-2.31; OR = 1.66,
95% CI = 1.14-2.41; OR = 1.67, 95% CI = 1.13-2.47, for 20-year,
10-year, and 0-year lag periods, respectively; P < 0.008 for each
lag period). However, duration of exposure and cumulative silica dust
exposure were not significantly associated with lung cancer mortality,
regardless of lag time (Cherry et al., 1998). The presence of small
parenchymal radiographic opacities (category >1/0; shape not
reported) was not related to lung cancer mortality, before
(P = 0.78) or after (P = 0.68) adjustment for smoking.
The authors concluded that the results imply "that crystalline silica
may well be a human carcinogen" (Cherry et al., 1998). The study did
not differentiate quartz exposures from cristobalite exposures.
De Klerk & Musk (1998) conducted a cohort study of 2297 surface
and underground gold miners in western Australia who participated in
surveys of respiratory symptoms, smoking habits, and lung function in
1961, 1974, and 1975. Eighty-nine per cent of the cohort was traced to
the end of 1993 for trachea, bronchus, and lung cancer mortality and
incidence of compensated silicosis (i.e., compensation awarded by the
Pneumoconiosis Medical Board). A nested case-control analysis of the
138 lung cancer deaths found that lung cancer mortality was related to
log total cumulative silica dust exposure after adjustment for smoking
(cigarette, pipe, or cigar) and for the presence of bronchitis at
survey (relative rate = 1.31; 95% CI = 1.01-1.70). However, the effect
of total cumulative silica dust exposure on lung cancer mortality was
not significant after adjustment for smoking, bronchitis, and
compensation for silicosis (relative rate = 1.20; 95% CI = 0.92-1.56).
Lung cancer mortality was not significantly related (P > 0.15) to
other silica exposure variables (i.e., duration of underground or
surface employment, intensity of underground or surface exposure)
after adjustment for smoking and bronchitis. Cigarette smoking
(relative rate = 32.5; 95% CI = 4.4-241.2 for >25 cigarettes smoked
per day), incidence of a compensation award for silicosis after lung
cancer diagnosis (relative rate = 1.59; 95% CI = 1.10-2.28), and
presence of bronchitis at survey (relative rate = 1.60;
95% CI = 1.09-2.33) were significantly related to lung cancer
mortality (de Klerk & Musk, 1998). The results of this study do not
support a relationship between lung cancer and silica exposure in the
absence of silicosis (i.e., a compensation award for silicosis after
lung cancer diagnosis).
Hnizdo et al. (1997) conducted a nested case-control study of
lung cancer deaths in a cohort of 2260 white South African underground
gold miners. (A lung cancer mortality cohort study was conducted
earlier [Hnizdo & Sluis-Cremer, 1991].) The mineral content of the
rock in the gold mines was mostly quartz (70-90%), silicates (10-30%),
pyrite (1-4%), and heavy minerals with grains of gold and
uranium-bearing minerals (2-4%). Seventy-eight lung cancer deaths
(69 of the 78 miners had a necropsy) that occurred during 1970-1986
were matched by year of birth with 386 control subjects from the same
cohort (Hnizdo et al., 1997). Lung cancer mortality risk and a
relationship with cigarette smoking (i.e., pack-years), cumulative
"dust" exposure (mg/m3-years), years of underground mining, incidence
of radiographic silicosis (i.e., ILO category >1/1 diagnosed up to
3 years before death of a matched case), and uranium production or
uranium grade of the ore in the gold mine were analysed with
conditional logistic regression models. Radon daughter measurements in
the gold mines were not available.
Lung cancer mortality was associated with cigarette smoking,
cumulative dust exposure (lagged 20 years from death), duration of
underground mining (lagged 20 years from death), and silicosis. The
best-fitting model predicted relative risks of 1.0, 3.5
(95% CI = 0.7-16.8), 5.7 (95% CI = 1.3-25.8), and 13.2
(95% CI = 3.1-56.2) for <6.5, 6.5-20, 21-30, and >30 pack-years of
smoking, respectively, and 2.45 (95% CI = 1.2-5.2) for silicosis. The
authors stated that variables representing uranium mining were not
significantly related to lung cancer mortality (modelling results for
these variables were not presented) (Hnizdo et al., 1997). The authors
proposed three explanations for their results: (1) miners with high
dust exposure who develop silicosis have increased lung cancer risk,
(2) high silica dust exposure concentrations may be important in the
pathogenesis of lung cancer, and silicosis is coincidental, and (3)
high levels of silica dust exposure may be a surrogate measure of
exposure to radon daughters (Hnizdo et al., 1997).
Meta-analyses of the epidemiological studies of silica exposure
and lung cancer reported a moderate summary risk of 1.3 for
silica-exposed workers (Steenland & Stayner, 1997) and higher summary
relative risks of 2.2-2.3 for studies of silicotic workers (Smith et
al., 1995; Steenland & Stayner, 1997). Tsuda et al. (1997) pooled lung
cancer risk estimates from 32 mortality studies of pneumoconiosis or
silicosis (excluding asbestosis) published from 1980 to 1994. The
estimated rate ratios of 2.74 (95% CI = 2.60-2.90) for all studies,
2.77 (95% CI = 2.61-2.94) for cohort studies only (25 of 32 studies),
and 2.84 (95% CI = 2.25-3.59) for case-control studies (5 of 32
studies) were similar to the estimates reported by Steenland & Stayner
(1997) and Smith et al. (1995).
Reasons for the higher risks in silicotics are not known;
similarly, the question of whether fibrosis is a precursor to the
development of lung cancer in humans has not been resolved. Various
hypothetical mechanistic pathways have been proposed to explain the
occurrence of lung tumours in rats and lung cancer in humans; however,
there is no convincing evidence for any specific proposed pathway
(IARC, 1997).
Selection bias is a common criticism of epidemiological studies
of lung cancer in compensated silicotics, because workers who sought
compensation for their disease may differ from the group of all
silicotics in symptoms, radiographic changes, social and psychological
factors, and industry (McDonald, 1995; Weill & McDonald, 1996).
However, Goldsmith (1998) reviewed this question and concluded that
lung cancer risk estimates were not higher in compensated silicotics
when results of studies of compensated silicotics were compared with
results of studies of silicotics ascertained from other clinical
sources (i.e., hospital or registry data).
9.2.4 Autoimmune-related disease
In humans, immune activation by occupational exposure to
respirable quartz may be linked to scleroderma, rheumatoid arthritis,
polyarthritis, mixed connective tissue disease, systemic lupus
erythematosus, Sjögren's syndrome, polymyositis, and fibrositis
(Haustein et al., 1990; Ziegler & Haustein, 1992; Otsuki et al.,
1998). The cellular mechanism that leads from quartz dust exposure to
autoimmune diseases is not known (Otsuki et al., 1998; NIOSH,
forthcoming). One theory is that when respirable silica particles are
encapsulated by macrophages, fibrogenic proteins and growth factors
are generated, and ultimately the immune system is activated (Haustein
et al., 1992; Ziegler & Haustein, 1992; Haustein & Anderegg, 1998).
Several epidemiological studies have reported statistically
significant numbers of excess deaths or cases of autoimmune-related
diseases such as scleroderma (Sluis-Cremer et al., 1985; Steenland &
Brown, 1995b), rheumatoid arthritis (Sluis-Cremer et al., 1986;
Klockars et al., 1987), and systemic lupus erythematosus (Steenland &
Brown, 1995b) in silica-exposed workers.
9.2.5 Renal disease
Recent epidemiological studies have found statistically
significant associations between occupational exposure to crystalline
silica dust and renal diseases and subclinical renal changes
(Steenland et al., 1992; Ng et al., 1993; Boujemaa et al., 1994; Hotz
et al., 1995; Nuyts et al., 1995; Steenland & Brown, 1995b; Steenland
& Goldsmith, 1995; Calvert et al., 1997).
9.2.6 Chronic obstructive pulmonary disease
Occupational exposure to respirable crystalline silica dust is
associated with chronic obstructive pulmonary disease, including
bronchitis and emphysema. Although these health effects are also
associated with tobacco smoking, some epidemiological studies suggest
that they may be present to a significant extent in non-smokers with
occupational exposure to quartz (Wiles & Faure, 1977; Becklake et al.,
1987; Holman et al., 1987; Kreiss et al., 1989; Cowie & Mabena, 1991).
9.2.7 Other adverse health effects
Cor pulmonale (i.e., enlargement of the right ventricle of the
heart because of structural or functional abnormalities of the lungs)
may occur as a complication of silicosis (Green & Vallyathan, 1996)
and other pneumoconioses (Kusiak et al., 1993). It is usually preceded
by pulmonary arterial hypertension. An epidemiological case-control
study of 732 white South African autopsied gold miners reported a
statistically significant association (P < 0.05) of cor pulmonale
with "extensive" silicosis and "slight" silicosis (Murray et al.,
1993).
Other adverse health effects or complications of silicosis have
been studied or identified in epidemiological studies of workers that
may have been exposed to quartz dust, but evidence of an association
with quartz exposure is inconclusive. These adverse effects include
dental abrasion (Petersen & Henmar, 1988), nasopharyngeal or
pharyngeal cancer (Carta et al., 1991; Chen et al., 1992), salivary
gland cancer (Zheng et al., 1996), liver cancer (Chen et al., 1992;
Hua et al., 1992), bone cancer (Steenland & Beaumont, 1986; Forastiere
et al., 1989), pancreatic cancer (Kauppinen et al., 1995), skin cancer
(Partanen et al., 1994), oesophageal cancer (Belli et al., 1989;
Xu et al., 1996; Pan et al., 1999), stomach cancer
(Parent et al., 1998), cancers of the digestive system
(Decoufle & Wood, 1979), intestinal or peritoneal cancer
(Amandus et al., 1991; Costello et al., 1995; Goldsmith et al.,
1995a), lymphopoietic or haematopoietic cancers (Silverstein et al.,
1986; Steenland & Brown, 1995b), and bladder cancer (Bravo et al.,
1987).
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
Terrestrial mammals (i.e., horses, camels) and birds exposed to
quartz in the natural environment, especially in desert or coastal
areas, show pathological lesions sometimes described as "silicosis"
that are similar to those seen in humans with silicosis
(Schwartz et al., 1981; Evans et al., 1988; Hansen et al., 1989;
Berry et al., 1991; Xu et al., 1993; Green & Vallyathan, 1996). Rats,
hamsters, guinea-pigs, monkeys, and mice exposed to quartz under
experimental conditions develop lung conditions and nodules similar to
those found in humans (reviewed by Green & Vallyathan, 1996).
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
The extensive body of in vitro and in vivo research
evaluating the effects of quartz on mammalian cells is summarized here
(IARC, 1997; NIOSH, forthcoming). Quartz deposited in the lungs causes
epithelial and macrophage injury and activation, and it translocates
to the interstitium and the regional lymph nodes. Recruitment of
inflammatory cells occurs in a dose-dependent manner. Oxidative stress
(i.e., increased formation of reactive oxygen species, including
hydroxyl radicals, or reactive nitrogen species) has been observed in
rats after intratracheal instillation (Blackford et al., 1994;
Schapira et al., 1995) or inhalation (Vallyathan et al., 1995) of
quartz. Several mechanisms have been proposed to explain the cause of
the cellular damage by quartz particles (Lapp & Castranova, 1993):
(1) direct cytotoxicity of quartz, (2) stimulation of the alveolar
macrophages by quartz, which results in the release of cytotoxic
enzymes or oxidants, (3) stimulation of the alveolar macrophages to
release inflammatory factors (e.g., interleukin-8, leukotriene B4,
platelet-activating factor, tumour necrosis factor, platelet-derived
growth factor) that recruit polymorphonuclear leukocytes, which may
release cytotoxins, (4) stimulation of the alveolar macrophages to
release factors that initiate fibroblast production and collagen
synthesis (e.g., interleukin-1, tumour necrosis factor,
platelet-derived growth factor, fibronectin, alveolar
macrophage-derived growth factor), and, more recently, (5) induction
by quartz of apoptosis and subsequent engulfment by macrophages to
regulate the evolution of inflammation and fibrosis (Leigh et al.,
1997).
Silicosis is indisputably causally related to occupational quartz
exposure, and the dose-response assessments of the adverse health
effects of quartz are based on epidemiological studies of occupational
cohorts with silicosis. To date, there are no known adverse health
effects associated with non-occupational exposure to quartz dust.
Silicosis is the critical effect for hazard identification and
dose-response assessment, for two reasons. First, although IARC
classified inhaled quartz from occupational sources as a Group 1
carcinogen, there are very few published risk assessments
(toxicological or epidemiological) with a quantitative dose-response
assessment of lung cancer risk at various levels of quartz exposure. A
pooled exposure-response assessment of a number of epidemiological
studies with quantitative data for quartz exposures and lung cancer is
currently being conducted by IARC.
Secondly, epidemiological studies of quartz-exposed workers
reported statistically significant numbers of excess deaths or cases
of renal disease or subclinical renal changes (Steenland et al., 1992;
Ng et al., 1993; Boujemaa et al., 1994; Hotz et al., 1995;
Nuyts et al., 1995; Steenland & Brown, 1995b; Calvert et al., 1997),
mycobacterial infections (tuberculous and non-tuberculous), or fungal
infections (Ziskind et al., 1976; Parkes, 1982; Parker, 1994; Althouse
et al., 1995; Goldsmith et al., 1995a; NIOSH, 1996; American Thoracic
Society, 1997; Kleinschmidt & Churchyard, 1997; Hnizdo & Murray, 1998;
Corbett et al., 1999), immunological disorders and autoimmune diseases
(i.e., scleroderma) (Sluis-Cremer et al., 1985; Cowie, 1987; Steenland
& Brown, 1995b), rheumatoid arthritis (Sluis-Cremer et al., 1986;
Klockars et al., 1987), and systemic lupus erythematosus (Steenland &
Brown, 1995b), but sufficient epidemiological or toxicological data do
not currently exist for quantitative assessment of the
exposure-response relationship for these health effects.
The US EPA calculated lowest-observed-adverse-effect level
(LOAEL) human equivalent concentrations (HECs) for non-cancer effects
reported in subchronic (<3 months) and chronic inhalation studies
that administered less than 20 mg quartz/m3 to experimental rats and
mice (Table 1). The results varied widely -- from 0.18 mg/m3 (based
on rat study) to 0.90 mg/m3 (based on mouse study). Some of this
variation may have been due to differences in dose, species, and the
quartz specimens. The method for determining the HEC was based on a
series of empirical equations described elsewhere (US EPA, 1994) and
summarized here. It represents an example of the calculation of an HEC
and may not necessarily be a method used worldwide. The equations
estimate fractional deposition of relatively insoluble particles and
adjust for dosimetric differences between species by incorporating
normalizing factors such as body weight or surface area (US EPA,
1994). In summary:
NOAEL[HEC] (mg/m3) = NOAEL[ADJ] (mg/m3) × RDDRr
where:
* NOAEL[HEC] = the no-observed-adverse-effect level (NOAEL) human
equivalent concentration, dosimetrically adjusted
* NOAEL[ADJ] = the NOAEL adjusted for duration: E (mg/m3) × D
(h/24 h) × W (days/7 days), where E = experimental exposure level
* RDDRr = the regional deposited dose ratio of particles for the
respiratory tract region (r)
* RDDR = (RDDTH/Normalizing Factor)A/(RDDTH/Normalizing
Factor)H, the ratio of regional deposited dose (RDDr) in the
thoracic (TH) region in the animal (A) species to that of humans
(H) (US EPA, 1994)
* r = region of dose deposition, in this case thoracic
* RDDr = 10-6 × Ci × VE × Fr, where:
Ci = concentration (mg/m3)
VE = minute volume (ml/min)
Fr = fractional deposition in region r
The literature contains a few additional risk assessments of lung
cancer and quartz, and all were based on results of inhalation studies
of rats (Collins & Marty, 1995, 1997; Goldsmith et al., 1995b).
However, human data introduce less uncertainty than extrapolation from
animals to humans (Goldsmith et al., 1995b; Goldsmith &
Hertz-Picciotto, 1997). For quartz, the uncertainty can be attributed
in part to the lack of (1) experimental studies that systematically
evaluated exactly the same material to which humans are exposed (IARC,
1997), (2) understanding of the mechanism for induction of rat lung
tumours (IARC, 1997), (3) understanding of the species differences
observed in the fibrogenic response (Gift & Faust, 1997), and
(4) experimental studies that administered doses in concentrations
similar to known occupational exposures (US EPA, 1996).
Epidemiological studies that used cumulative exposure estimates
represent the best currently available source of information for
characterizing the dose-response relationship for silicosis or lung
cancer in occupational, as well as non-occupational, cohorts.
Exposure-response models based on cumulative exposure data can
provide predictions of silicosis risk for a particular silica dust
exposure over a period of time. Table 6 presents results of logistic
regression models of data from the cross-sectional epidemiological
studies described in section 9.2. The models predicted the prevalence
of radiographic silicosis (ILO category >1/1 or >1/0) from
cumulative exposure to respirable quartz. All models predicted the
occurrence of at least one case of radiographic silicosis per 100
workers at cumulative respirable quartz dust exposures of 0.05 or 0.10
mg/m3 over a 45-year working lifetime. Logistic regression assumes
that the change in the level of quartz exposure affects risk (or
predicted prevalence) in a multiplicative manner (as compared with
linear models, which predict deviations from additivity)
(Kleinbaum et al., 1982).
Table 7 displays the results of three studies that followed
cohorts of miners for silicosis over time or retrospectively. In these
studies, the miners with silicosis had their first quartz exposure
18-37 years (mean) prior to radiographic diagnosis. The models applied
to the data collected in these studies predicted that the risk of
chronic silicosis increases exponentially with increasing cumulative
dose of silica dust (US EPA, 1996). Data from the study of South
African gold miners (Hnizdo & Sluis-Cremer, 1993) and Canadian gold
and uranium miners were analysed with models that assumed some risk of
silicosis at any exposure. The study of US gold miners (Steenland &
Brown, 1995a) estimated silicosis rates (cases per person-time at
risk) for seven cumulative exposure categories, stratified by 5-year
age and calendar time intervals. Poisson regression was used to adjust
the crude rates for age and calendar time. Although age and calendar
time were highly correlated with exposure, it is not likely that they
confounded the exposure-response analysis, because silicosis has no
background rate for non-exposed populations that changes with age or
calendar time (US EPA, 1996).
In summary, the risk estimates for silicosis prevalence for a
working lifetime of exposure to respirable quartz dust concentrations
of about 0.05 or 0.10 mg/m3 in the occupational environment vary
widely (i.e., 2-90%). Epidemiological studies varied in the definition
of silicosis cases, radiographic interpretation methods, cohort
follow-up periods, and statistical methods. The variability in the
risk estimates cannot be solely attributed to differences in follow-up
periods; however, it must be recognized that chronic silicosis is a
progressive disease. Therefore, the development of silicosis after a
long latency period and after workers leave employment must be
accounted for in epidemiological studies. Results of a study of
autopsied South African gold miners found that the diagnostic
sensitivity of radiological examination is particularly poor (Hnizdo
et al., 1993). For example, when the radiological findings for
profusion of rounded opacities (ILO category >1/1) were compared
with pathological findings for silicosis in 326 miners with an average
of 2.7 years between the radiological and pathological examination,
silicosis was not diagnosed radiographically for at least 61% of the
miners with slight to marked silicosis at autopsy. The probability of
a false-negative reading increased with years of mining and the
average concentration of respirable dust (Hnizdo et al., 1993).
Experimental studies of rats also reported lack of agreement between
histopathological indicators of silica dust exposure and radiographic
readings (Drew & Kutzman, 1984a,b).
Improved exposure assessment methods and data analyses that
account for variations and deficiencies in exposure data would improve
the risk estimates for silica-exposed workers (Agius et al., 1992;
Checkoway, 1995). Although epidemiological studies that used
cumulative exposure estimates represent the best available source of
information for characterizing the dose-response relationship in
occupational cohorts, peak exposures may predict silicosis risk better
than cumulative exposures (Checkoway & Rice, 1992), but data are
rarely available.
11.1.2 Criteria for setting tolerable intakes or guidance values
for quartz
Results of genotoxicity tests of quartz, as well as other
in vitro and in vivo evidence, suggest that persistent
inflammation and epithelial proliferation are related to the tumour
response in rats. Other pathways for tumour induction, such as a
direct genotoxic effect, have not been ruled out. Because a pathway
has not been determined, and it is unclear from results of
epidemiological studies whether silicosis is a precursor to
lung cancer, it cannot be assumed that there is a threshold (i.e.,
tolerable concentration, or TC) at which exposure to quartz would not
result in silicosis and/or lung cancer. In addition, available data
indicate that occupational quartz exposure is associated with the
development of tuberculosis, especially in workers with silicosis;
however, the association has not been quantified for that outcome or
for other quartz-related diseases. Therefore, occupational exposure to
respirable quartz dust should be reduced to the extent possible.
11.1.3 Sample risk characterization
It is recognized that there are many different methods for
assessing the risk to human health posed by environmental and
occupational substances. The example presented here applies to healthy
individuals not compromised by respiratory ailments and who breathe
the ambient air in the USA. It is based on the results of the three
cohort studies of miners presented in Table 7 (section 11.1.1). Using
a high estimate of 10% crystalline silica content of particulate
matter with a mass median aerodynamic diameter not greater than 10 µm
(PM10) from US metropolitan areas, the highest cumulative crystalline
silica exposure expected from continuous human lifetime exposure at or
below the annual US national ambient air quality standard (NAAQS) for
particulate matter of 50 µg/m3 is 1 mg/m3-year (US EPA, 1996).
The US EPA applied a mathematical model (i.e., benchmark dose
[BMD] analysis) to determine a concentration and lower confidence
bound associated with a predefined effect level (i.e., 1%, 5%, and 10%
risk of silicosis) (US EPA, 1996). After consideration of
cross-sectional, longitudinal, retrospective cohort, and case-control
epidemiological studies, the BMD was conducted on data from the
previously described study of radiographic silicosis in a cohort of
2235 South African gold miners (Hnizdo & Sluis-Cremer, 1993). This
study was selected for several reasons: (1) continuous and
longitudinal investigation of silicosis, (2) miners had multiple X-ray
examinations, and (3) autopsy data were available for more accurate
interpretation of the radiographic results, which lack sensitivity (US
EPA, 1996).1
1 The cross-sectional studies described in Table 6 and sections
9.2.1 and 11.1.1 were not available at the time of the US EPA
assessment. These studies modelled the cumulative quartz exposures
of hardrock miners (Kreiss & Zhen, 1996) and grey iron foundry
workers (Rosenman et al., 1996) and predicted a 30% prevalence of
ILO category > 1/1 silicosis over a 45-year working lifetime with
10 years of post-employment follow-up (Kreiss & Zhen, 1996) or a
2% prevalence of ILO category > 1/0 silicosis over a 40-year
working lifetime and controlling for pack-years of cigarette
smoking, race, and silica exposure other than in the foundry under
study (Rosenman et al., 1996). Both predictions are for a mean
quartz dust concentration of 0.05 mg/m3.
Using methods described by US EPA (1996), the BMD analysis
predicted that the silicosis risk for a continuous 70-year lifetime
exposure to 0.008 mg/m3 (estimated high crystalline silica
concentration in US metropolitan areas) is less than 3% for healthy
individuals not compromised by other respiratory diseases or
conditions and for ambient environment (US EPA, 1996). (Risks were not
calculated for other groups, such as people with respiratory
illnesses.) This risk estimate for exposure to ambient quartz may be
conservative, because quartz particles in the occupational environment
may be finer or "freshly fractured," occupational exposures may
involve high "peak" exposures, and, thus, the potential for disease
development may be greater.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
IARC has classified inhaled crystalline silica (quartz or
cristobalite) from occupational sources as a Group 1 carcinogen based
on sufficient evident of carcinogenicity in humans and experimental
animals. In addition, "in making the overall evaluation, the Working
Group noted that carcinogenicity in humans was not detected in all
industrial circumstances studied. Carcinogenicity may be dependent on
inherent characteristics of the crystalline silica or on external
factors affecting its biological activity or distribution of its
polymorphs" (IARC, 1997).
In 1991, the ILO published a document describing methods for
prevention and control of occupational lung diseases, including
silicosis (ILO, 1991), and in 1993, the Office of Occupational Health
of the World Health Organization (WHO) called for increased medical
surveillance of workers exposed to mineral dusts to prevent
pneumoconioses such as silicosis and asbestosis (WHO, 1993). WHO also
recently published information about risk factors for pulmonary
tuberculosis, including occupational exposure to respirable
crystalline silica (WHO, 1996).
In 1986, a WHO study group recommended a limit of 40 µg/m3
(time-weighted average of an 8-h shift) for occupational exposure to
respirable crystalline silica dust (WHO, 1986).
REFERENCES
Agius RM, Love RG, Davies LST, Hutchison PA, Cherrie JW, Robertson A,
Cowie HA, Hurley JF, Seaton A, Soutar CA (1992) Epidemiological
studies of respiratory health and dust exposure in hard rock quarry
workers and ex-workers. Edinburgh, Institute of Occupational
Medicine (HSE Contract No. 1/LMD/126/146/88; Report No. TM/92/10).
Ahlman K, Koskela R-S, Kuikka P, Koponen M, Annanmäki M (1991)
Mortality among sulfide ore miners. American journal of industrial
medicine, 19:603-617.
Althouse RB, Bang KM, Castellan RM (1995) Tuberculosis comortality
with silicosis -- United States, 1979-1991. Applied occupational and
environmental hygiene, 10(12):1037-1041.
Altieri A, Sperduto B, Verdel U, Porceli D (1984) Identification of
cristobalite and quartz in the production of silicon carbide. Rivista
degli Infortuni e delle Malattie Professionali, 71(1-2):131-135.
Amandus HE, Shy C, Wing S, Blair A, Heineman EF (1991) Silicosis and
lung cancer in North Carolina dusty trades workers. American journal
of industrial medicine, 20:57-70.
Amandus HE, Castellan RM, Shy C, Heineman EF, Blair A (1992)
Reevaluation of silicosis and lung cancer in North Carolina dusty
trades workers. American journal of industrial medicine, 22:147-153.
American Thoracic Society (1997) Adverse effects of crystalline silica
exposure. American journal of respiratory and critical care medicine,
155:761-765.
Anderson LJ, Donaldson HM, Jones JH, Stringer WT, Wallingford KM
(1980) North Carolina Brick Industry Industrial Hygiene and
Respiratory Disease Morbidity Survey, 1974-1975. Cincinnati, OH,
National Institute for Occupational Safety and Health (National
Technical Information Service Report No. PB83-181735).
Aoki A, Sirai A, Sakamoto H, Igarashi T, Matsunaga K, Ishigatsubo Y,
Tani K, Okubo T (1988) A case of silicosis associated with
polymyositis and benign monoclonal gammopathy. Ryumachi,
28(5):373-378.
Arnalich F, Lahoz C, Picazo ML, Monereo A, Arribas JR, Martinez Ara J,
Vazquez JJ (1989) Polyarteritis nodosa and necrotizing
glomerulonephritis associated with long-standing silicosis. Nephron,
51(4):544-547.
Balmes J (1990) Silica exposure and tuberculosis: an old problem with
some new twists [editorial]. Journal of occupational medicine,
32(2):114-115.
Banks DE, Milutinovic J, Desnick RJ, Grabowski GA, Lapp NL, Boehlecke
BA (1983) Silicon nephropathy mimicking Fabry's disease.
American journal of nephrology, 3(5):279-284.
Beadle DG (1965) An epidemiological study of the relationship between
the amount of dust breathed and the incidence of silicosis in South
African gold miners. In: Davies CN, ed. Inhaled particles and
vapours II. Oxford, Pergamon Press, pp. 479-492.
Beadle DG (1971) The relationship between the amount of dust breathed
and the development of radiological signs of silicosis: an
epidemiological study in South African gold miners. In: Walton WH, ed.
Inhaled particles III: Proceedings of an international symposium
organized by the British Occupational Hygiene Society, Vol. II.
Surrey, Unwin Brothers Limited, pp. 953-964.
Beadle DG, Bradley AA (1970) The composition of airborne dust in South
African gold mines. In: Shapiro HA, ed. Pneumoconiosis: Proceedings
of the international conference, Johannesburg, 1969. Oxford, Oxford
University Press, pp. 462-466.
Becklake MR, Irwig L, Kielkowski D, Webster I, De Beer M, Landau S
(1987) The predictors of emphysema in South African gold miners.
American review of respiratory disease, 135:1234-1241.
Belli S, Comba P, Germani D, Grignoli M, Lagorio S, Paganoni R,
Ronchin M (1989) [Mortality study among lead-zinc Italian (Val
Seriana) miners.] Medicina del Lavoro, 80(6):467-478 (in Italian).
Berry CR, O'Brien TR, Madigan JE, Hager DA (1991) Thoracic
radiographic features of silicosis in 19 horses. Journal of
veterinary internal medicine, 5(4):248-256.
Blackford JA, Antonini JM, Castranova V, Dey RD (1994) Intratracheal
instillation of silica up-regulates inducible nitric oxide synthase
gene expression and increases nitric oxide production in alveolar
macrophages and neutrophils. American journal of respiratory cell and
molecular biology, 11(4):426-431.
Bloor WA, Eardley RE, Dinsdale A (1971) Environmental conditions in
sanitary whiteware casting shops. Annals of occupational hygiene,
14(4):321-327.
Bolsaitis PP, Wallace WE (1996) The structure of silica surfaces in
relation to cytotoxicity. In: Castranova V, Vallyathan V, Wallace WE,
eds. Silica and silica-induced lung diseases. Boca Raton, FL,
CRC Press, pp. 79-89.
Bolton WK, Suratt PM, Sturgill BC (1981) Rapidly progressive silicon
nephropathy. American journal of medicine, 71:823-828.
Bonnin A, Mousson C, Justrabo E, Tanter Y, Chalopin JM, Rifle G (1987)
Silicosis associated with crescentic IgA mesangial nephropathy
[letter]. Nephron, 47(3):229-230.
Boujemaa W, Lauwerys R, Bernard A (1994) Early indicators of renal
dysfunction in silicotic workers. Scandinavian journal of work,
environment and health, 20(3):180-183.
Bravo MP, Del Rey Calero J, Conde M (1987) Silice y cancer de vejiga
en varones. Archivos Espanoles de Urologia, 40(9):635-637.
Bresnitz EA, Roseman J, Becker D, Gracely E (1992) Morbidity among
municipal waste incinerator workers . American journal of industrial
medicine, 22:363-378.
Burgess GL, Turner S, McDonald JC, Cherry NM (1997) Cohort mortality
study of Staffordshire pottery workers: (I) Radiographic validation of
an exposure matrix for respirable crystalline silica. Annals of
occupational hygiene, 41 (Suppl. 1):403-407.
Burgess WA (1995) Recognition of health hazards in industry: a review
of materials and processes, 2nd ed. New York, NY, John Wiley & Sons,
pp. 106-139, 411-422, 423-434, 475-482.
Buringh E, van de Belt R, van der Wal JF (1990) Dust control measures
in Dutch brickworks. Annals of occupational hygiene, 34:483-497.
Burns CA, Zarkower A, Ferguson FG (1980) Murine immunological and
histological changes in response to chronic silica exposure.
Environmental research, 21(2):298-307.
Calvert GM, Steenland K, Palu S (1997) End-stage renal disease among
silica-exposed gold miners. Journal of the American Medical
Association, 277(15):1219-1223.
Carta P, Cocco PL, Casula D (1991) Mortality from lung cancer among
Sardinian patients with silicosis. British journal of industrial
medicine, 48(2):122-129.
Castranova V, Dalal NS, Vallyathan V (1996) Role of surface free
radicals in the pathogenicity of silica. In: Castranova V, Vallyathan
V, Wallace WE, eds. Silica and silica-induced lung diseases. Boca
Raton, FL, CRC Press, pp. 91-105.
Castranova V, Vallyathan V, Ramsey DM, McLaurin JL, Pack D, Leonard S,
Barger MW, Ma JYC, Dalal NS, Teass A (1997) Augmentation of pulmonary
reactions to quartz inhalation by trace amounts of iron-containing
particles. Environmental health perspectives, 105 (Suppl.
5):1319-1324.
Cavariani F, Di Pietro A, Miceli M, Forastiere F, Biggeri A, Scavalli
P, Petti A, Borgia P (1995) Incidence of silicosis among ceramic
workers in central Italy. Scandinavian journal of work, environment
and health, 21 (Suppl. 2):58-62.
Chamberlain M (1983) Effect of mineral dusts on metabolic cooperation
between Chinese hamster V79 cells in vitro. Environmental health
perspectives, 51:5-9.
Checkoway H (1995) Methodological considerations relevant to
epidemiology studies of silica and lung cancer. Applied occupational
and environmental hygiene, 10(12):1049-1055.
Checkoway H, Rice CH (1992) Time-weighted averages, peaks, and other
indices of exposure in occupational epidemiology. American journal of
industrial medicine, 21:25-33.
Checkoway H, Heyer NJ, Demers PA, Breslow NE (1993) Mortality among
workers in the diatomaceous earth industry. British journal of
industrial medicine, 50:586-597.
Checkoway H, Heyer NJ, Demers PA, Gibbs GW (1996) Reanalysis of
mortality from lung cancer among diatomaceous earth industry workers,
with consideration of potential confounding by asbestos exposure.
Occupational and environmental medicine, 53:645-647.
Chen GX, Burnett CA, Cameron LL, Alterman T, Lalich NR, Tanaka S,
Althouse RB (1997) Tuberculosis mortality and silica exposure: a
case-control study based on a national mortality database for the
years 1983-1992. International journal of occupational and
environmental health, 3:163-170.
Chen J, McLaughlin JK, Zhang JY, Stone BJ, Luo J, Chen RA, Dosemeci M,
Rexing SH, Wu Z, Hearl FJ, McCawley MA, Blot WJ (1992) Mortality among
dust-exposed Chinese mine and pottery workers. Journal of
occupational medicine, 34(3):311-316.
Cherry N, Burgess G, McNamee R, Turner S, McDonald C (1995) Initial
findings from a cohort mortality study of British pottery workers.
Applied occupational and environmental hygiene, 10(12):1042-1045.
Cherry NM, Burgess GL, Turner S, McDonald JC (1997) Cohort study of
Staffordshire pottery workers: (II) Nested case referent analysis of
lung cancer. Annals of occupational hygiene, 41 (Suppl. 1):408-411.
Cherry NM, Burgess GL, Turner S, McDonald JC (1998) Crystalline silica
and risk of lung cancer in the potteries. Occupational and
environmental medicine, 55:779-785.
Collins JF, Marty MA (1995) Cancer risk assessment for crystalline
silica to implement California's Hot Spots Act. Scandinavian
journal of work, environment and health, 21 (Suppl. 2):99-103.
Collins JF, Marty MA (1997) Cancer risk assessment for crystalline
silica. Journal of exposure analysis and environmental epidemiology,
7(3):359-365.
Cooper TC, Gressel MG, Froelich PA, Caplan PE, Mickelsen RL, Valiante
D, Bost P (1993) Successful reduction of silica exposures at a
sanitary ware pottery. American Industrial Hygiene Association
Journal, 54(10):600-606.
Corbett EL, Churchyard GJ, Clayton T, Herselman P, Williams B, Hayes
R, Mulder D, De Cock KM (1999) Risk factors for pulmonary
mycobacterial disease in South African gold miners. American journal
of respiratory and critical care medicine, 159:94-99.
Costello J, Graham WGB (1988) Vermont granite workers' mortality
study. American journal of industrial medicine, 13:483-497.
Costello J, Castellan RM, Swecker GS, Kullman GJ (1995) Mortality of a
cohort of U.S. workers employed in the crushed stone industry,
1940-1980. American journal of industrial medicine, 27:625-640.
Cowie RL (1987) Silica-dust-exposed mine workers with scleroderma
(systemic sclerosis). Chest, 92(2): 260-262.
Cowie RL (1994) The epidemiology of tuberculosis in gold miners with
silicosis. American journal of respiratory and critical care
medicine, 150:1460-1462.
Cowie RL, Mabena SK (1991) Silicosis, chronic airflow limitation, and
chronic bronchitis in South African gold miners. American review of
respiratory disease, 143:80-84.
Dagle GE, Wehner AP, Clark ML, Buschbom RL (1986) Chronic inhalation
exposure of rats to quartz. In: Goldsmith DF, Winn DM, Shy CM, eds.
Silica, silicosis, and cancer: Controversy in occupational medicine.
New York, NY, Praeger Publishers, pp. 255-266 (Cancer Research
Monographs, Vol. 2).
Daniel LN, Mao Y, Saffiotti U (1993) Oxidative DNA damage by
crystalline silica. Free radical biology and medicine, 14:463-472.
Daniel LN, Mao Y, Wang T-CL, Markey CJ, Markey SP, Shi X, Saffiotti U
(1995) DNA strand breakage, thymine glycol production, and hydroxyl
radical generation induced by different samples of crystalline silica
in vitro. Environmental research, 71:60-73.
Davies LST, Robertson A, Agius RM, Cowie HA, Cherrie JW, Hutchison P
(1994) The use of compliance monitoring for assessing quarry workers'
exposures to respirable dust and quartz. Annals of occupational
hygiene, 38 (Suppl. 1):559-570.
Davis BL, Johnson LR, Stevens RK, Courtney WJ, Safriet DW (1984) The
quartz content and elemental composition of aerosols from selected
sites of the EPA inhalable particulate network. Atmospheric
environment, 18(4):771-782.
Davis GS (1991) Immunologic aspects of pneumoconioses in asbestosis
and silicosis. In: Lynch JP, DeRemee RA, eds. Immunologically
mediated pulmonary diseases. Philadelphia, PA, J.B. Lippincott
Company, pp. 111-155.
Davis GS (1996) Silica. In: Harber P, Schenker MB, Balmes JR, eds.
Occupational and environmental respiratory disease, 1st ed. St.
Louis, MO, Mosby, pp. 373-399.
Davis LK, Wegman DH, Monson RR, Froines J (1983) Mortality experience
of Vermont granite workers. American journal of industrial medicine,
4:705-723.
Decoufle P, Wood DJ (1979) Mortality patterns among workers in a gray
iron foundry. American journal of epidemiology, 109(6):667-675.
de Klerk NH, Musk AW (1998) Silica, compensated silicosis, and lung
cancer in Western Australian goldminers. Occupational and
environmental medicine, 55:243-248.
Dethloff LA, Gilmore LB, Gladen BC, George G, Chhabra RS, Hook GER
(1986a) Effects of silica on the composition of the pulmonary
extracellular lining. Toxicology and applied pharmacology,
84(1):66-83.
Dethloff LA, Gilmore LB, Brody AR, Hook GER (1986b) Induction of
intracellular and extracellular phospholipids in the lungs of rats
exposed to silica. Biochemical journal, 233(1):111-118.
Donaldson HM, Wallingford K, Jones JH (1982) Environmental surveys in
the Barre, Vermont and Elberton, Georgia granite industries.
Cincinnati, OH, National Institute for Occupational Safety and Health
(National Technical Information Service Publication No. PB83-179911).
Donaldson K, Borm PJA (1998) The quartz hazard: a variable entity.
Annals of occupational hygiene, 42(5):287-294.
Dong D, Xu G, Sun Y, Hu P (1995) Lung cancer among workers exposed to
silica dust in Chinese refractory plants. Scandinavian journal of
work, environment and health, 21 (Suppl. 2):69-72.
Dosemeci M, McLaughlin JK, Chen J-Q, Hearl F, Chen R-G, McCawley M, Wu
Z, Peng K-L, Chen A-L, Rexing SH, Blot WJ (1995) Historical total and
respirable silica dust exposure levels in mines and pottery factories
in China. Scandinavian journal of work, environment and health, 21
(Suppl. 2):39-43.
Dracon M, Noel C, Wallaert P, Dequiedt P, Lelievre G, Tacquet A (1990)
Rapidly progressive glomerulonephritis in silicotic coal miners.
Nephrologie, 11:61-65.
Drew RT, Kutzman RS (1984a) Final report on a study of Fischer 344
rats exposed to silica dust for six months at concentrations of 0, 2,
10 or 20 mg/m3, then maintained for six months prior to assessment.
Upton, NY, Brookhaven National Laboratory (Report No. BNL 35735;
submitted to the National Toxicology Program under Interagency
Agreement No. 222-Y01-ES-9-0043, November 1984).
Drew RT, Kutzman RS (1984b) Final report on a study of Fischer 344
rats exposed to silica dust for six months at concentrations of 0, 2,
10 or 20 mg/m3. Upton, NY, Brookhaven National Laboratory (Report
No. BNL 34617; submitted to the National Toxicology Program under
Interagency Agreement No. 222-Y01-ES-9-0043, February 1984).
Driscoll KE (1995) The toxicology of crystalline silica studied
in vitro. Applied occupational and environmental hygiene,
10(12):1118-1125.
Driscoll KE (1996) The role of interleukin-1 and tumor necrosis factor
alpha in the lung's response to silica. In: Castranova V, Vallyathan
V, Wallace WE, eds. Silica and silica-induced lung diseases. Boca
Raton, FL, CRC Press, pp. 163-184.
Driscoll KE, Deyo LC, Howard BW, Poynter J, Carter JM (1995)
Characterizing mutagenesis in the hprt gene of rat alveolar
epithelial cells. Experimental lung research, 21:941-956.
Driscoll KE, Deyo LC, Carter JM, Howard BW, Hassenbein DG, Bertram TA
(1997) Effects of particle exposure and particle-elicited inflammatory
cells on mutation in rat alveolar epithelial cells. Carcinogenesis,
18(2):423-430.
Dufresne A, Lesage J, Perrault G (1987) Evaluation of occupational
exposure to mixed dusts and polycyclic aromatic hydrocarbons in
silicon carbide plants. American Industrial Hygiene Association
Journal, 48(2):160-166.
Dufresne A, Loosereewanich P, Bégin R, Dion C, Ecobichon D, Muir DCF,
Ritchie AC, Perrault G (1998a) Tentative explanatory variable of lung
dust concentration in gold miners exposed to crystalline silica.
Journal of exposure analysis and environmental epidemiology,
8(3):375-398.
Dufresne A, Bégin R, Dion C, Jagirdar J, Rom WN, Loosereewanich P,
Muir DCF, Ritchie AC, Perrault G (1998b) Angular and fibrous particles
in lung in relation to silica-induced diseases. International
archives of occupational and environmental health, 71:263-269.
Eisen EA, Smith TJ, Wegman DH, Louis TA, Froines J (1984) Estimation
of long term dust exposures in the Vermont granite sheds.
American Industrial Hygiene Association Journal, 45(2):89-94.
Erdogdu G, Hasirci V (1998) An overview of the role of mineral
solubility in silicosis and asbestosis. Environmental research,
78:38-42.
Evans MG, Slocombe RF, Schwartz LD (1988) Pulmonary silicosis in
captive ring-necked pheasants: definitive diagnosis by electron probe
X-ray microanalysis. Veterinary pathology, 25(3):239-241.
Forastiere F, Lagorio S, Michelozzi P, Perucci CA, Axelson O (1989)
Mortality pattern of silicotic subjects in the Latium region, Italy.
British journal of industrial medicine, 46(12):877-880.
Fox AJ, Greenberg M, Ritchie GL (1975) A survey of respiratory
disease in the pottery industry. London, Her Majesty's Stationery
Office.
Freeman CS, Grossman EA (1995) Silica exposures in the United States
between 1980 and 1992. Scandinavian journal of work, environment and
health, 21 (Suppl. 2):47-49.
Fubini B (1997) Surface reactivity in the pathogenic response to
particulates. Environmental health perspectives, 105 (Suppl.
5):1013-1020.
Fubini B (1998) Surface chemistry and quartz hazard. Annals of
occupational hygiene, 42(8):521-530.
Fubini B, Bolis V, Cavenago A, Volante M (1995) Physicochemical
properties of crystalline silica dusts and their possible implication
in various biological responses. Scandinavian journal of work,
environment and health, 21 (Suppl. 2):9-14.
Fukata S, Matsubayashi S, Nagato H, Sakai K, Ohishi S, Yasuda M, Tamai
H, Nakagawa T (1983) Monoclonal gammopathies associated with
silicosis. Rinsho Ketsueki, 24(1):9-17.
Fukata S, Tamai H, Nagai K, Matsubayashi S, Nagato H, Tashiro T,
Yasuda M, Kumagai LF (1987) A patient with hereditary spherocytosis
and silicosis who developed an IgA(lambda) monoclonal gammopathy.
Japanese journal of medicine, 26(1):81-83.
Fulekar MH, Alam Khan MM (1995) Occupational exposure to dust in slate
pencil manufacture. Annals of occupational hygiene, 39(1):107-114.
Gao H, Brick J, Ong S, Miller M, Whong W-Z, Ong T (1997) Selective
hyperexpression of c-jun oncoprotein by glass fiber- and
silica-transformed BALB/c-3T3 cells. Cancer letters, 112:65-69.
Gerhardsson G (1976) Dust prevention in Swedish foundries.
Staub-Reinhaltung der Luft, 36:433-439.
Gift JS, Faust RA (1997) Noncancer inhalation toxicology of
crystalline silica: exposure-response assessment. Journal of exposure
analysis and environmental epidemiology, 7(3): 345-358.
Giles RD, Sturgill BC, Suratt PM, Bolton WK (1978) Massive proteinuria
and acute renal failure in a patient with acute silicoproteinosis.
American journal of medicine, 64:336-342.
Goldsmith DF (1994) Health effects of silica dust exposure. In: Heaney
PJ, Prewitt CT, Gibbs GV, eds. Silica: physical behavior,
geochemistry, and materials applications. Washington, DC,
Mineralogical Society of America. Reviews in mineralogy, 29:545-606.
Goldsmith DF (1998) Uses of workers' compensation data in epidemiology
research. Occupational medicine state of the art reviews,
13(2):389-415.
Goldsmith DF, Hertz-Picciotto I (1997) Criteria for conducting
quantitative risk assessments for silica. Journal of exposure
analysis and environmental epidemiology, 7(3):367-375.
Goldsmith DF, Beaumont JJ, Morrin LA, Schenker MB (1995a) Respiratory
cancer and other chronic disease mortality among silicotics in
California. American journal of industrial medicine, 28(4):459-467.
Goldsmith DF, Ruble RP, Klein CA (1995b) Comparative cancer potency
for silica from extrapolations of human and animal findings.
Scandinavian journal of work, environment and health, 21 (Suppl.
2):104-107.
Green FHY, Vallyathan V (1996) Pathologic responses to inhaled silica.
In: Castranova V, Vallyathan V, Wallace WE, eds. Silica and
silica-induced lung diseases. Boca Raton, FL, CRC Press, pp. 39-59.
Greskevitch MF, Turk AR, Dieffenbach AL, Roman JM, Groce DW, Hearl FJ
(1992) Quartz analyses of the bulk dust samples collected by the
National Occupational Health Survey of Mining. Applied occupational
and environmental hygiene, 7(8):527-531.
Greskevitch MF, Bajpayee SS, Hale JM, Groce DW, Hearl FJ, eds. (1996)
NIOSH technical report: results from the National Occupational Health
Survey of Mining (NOHSM). Cincinnati, OH, US Department of Health
and Human Services, Public Health Service, Centers for Disease Control
and Prevention, National Institute for Occupational Safety and Health,
Division of Respiratory Disease Studies (DHHS (NIOSH) Publication No.
96-136).
Groth DH, Stettler LE, Platek SF, Lal JB, Burg JR (1986) Lung tumors
in rats treated with quartz by intratracheal instillation. In:
Goldsmith DF, Winn DM, Shy CM, eds. Silica, silicosis, and cancer:
Controversy in occupational medicine. New York, NY, Praeger
Publishers, pp. 243-253 (Cancer Research Monographs, Vol. 2).
Gu Z-W, Ong TO (1996) Potential mechanisms of silica-induced cancer.
In: Castranova V, Vallyathan V, Wallace WE, eds. Silica and
silica-induced lung diseases. Boca Raton, FL, CRC Press, pp.
397-406.
Guenel P, Hojberg G, Lynge E (1989) Cancer incidence among Danish
stone workers. Scandinavian journal of work, environment and health,
15:265-270.
Hansen HJ, Jama FM, Nilsson C, Norrgren L, Abdurahman OS (1989)
Silicate pneumoconiosis in camels (Camelus dromedarius L.).
Zentralblatt für Veterinarmedizin (A), 36(10):789-796.
Hart GA, Hesterberg TW (1998) In vitro toxicity of respirable-size
particles of diatomaceous earth and crystalline silica compared with
asbestos and titanium dioxide. Journal of occupational and
environmental medicine, 40(1):29-42.
Haslam PL (1994) Basic immunology and immunopathology. In: Parkes WR,
ed. Occupational lung disorders, 3rd ed. London,
Butterworth-Heinemann, pp. 50-99.
Hauglustaine D, Van Damme B, Daenens P, Michielsen P (1980) Silicon
nephropathy: a possible occupational hazard. Nephron, 26:219-224.
Haustein UF, Anderegg U (1998) Silica induced scleroderma -- clinical
and experimental aspects. Journal of rheumatology, 25(10):1917-1926.
Haustein UF, Ziegler V, Herrmann K, Mehlhorn J, Schmidt C (1990)
Silica-induced scleroderma. Journal of the American Academy of
Dermatology, 22(3):444-448.
Haustein UF, Ziegler V, Herrmann K (1992) Chemically induced
scleroderma. Der Hautarzt, 43:464-474.
Heppleston AG (1994) Pathogenesis of mineral pneumoconioses. In:
Parkes WR, ed. Occupational lung disorders, 3rd ed. London,
Butterworth-Heinemann, pp. 100-134.
Hesterberg TW, Barrett JC (1984) Dependence of asbestos- and mineral
dust-induced transformation of mammalian cells in culture on fiber
dimension. Cancer research, 44:2170-2180.
Hesterberg TW, Oshimura M, Brody AR, Barrett JC (1986) Asbestos and
silica induce morphological transformation of mammalian cells in
culture: a possible mechanism. In: Goldsmith DF, Winn DM, Shy CM, eds.
Silica, silicosis, and cancer. Controversy in occupational medicine.
New York, NY, Praeger Publishers, pp. 177-190 (Cancer Research
Monographs, Vol. 2).
Higgins RI, Deere MR, Cinkotai FF (1985) Fettlers' exposure to pottery
dust in a factory making sanitary whiteware. Annals of occupational
hygiene, 29(3):365-375.
Hnizdo E, Murray J (1998) Risk of pulmonary tuberculosis relative to
silicosis and exposure to silica dust in South African gold miners.
Occupational and environmental medicine, 55:496-502.
Hnizdo E, Murray J (1999) Correction: Risk of pulmonary tuberculosis
relative to silicosis and exposure to silica dust in South African
gold miners. Occupational and environmental medicine, 56:215-216.
Hnizdo E, Sluis-Cremer GK (1991) Silica exposure, silicosis, and lung
cancer: a mortality study of South African gold miners. British
journal of industrial medicine, 48:53-60.
Hnizdo E, Sluis-Cremer GK (1993) Risk of silicosis in a cohort of
white South African gold miners. American journal of industrial
medicine, 24:447-457.
Hnizdo E, Murray J, Sluis-Cremer GK, Thomas RG (1993) Correlation
between radiological and pathological diagnosis of silicosis: an
autopsy population based study. American journal of industrial
medicine, 24:427-445.
Hnizdo E, Murray J, Klempman S (1997) Lung cancer in relation to
exposure to silica dust, silicosis and uranium production in South
African gold miners. Thorax, 52:271-275.
Holland LM, Gonzales M, Wilson JS, Tillery MI (1983) Pulmonary effects
of shale dusts in experimental animals. In: Wagner WL, Rom WN,
Merchant JA, eds. Health issues related to metal and nonmetallic
mining. Boston, MA, Butterworth, pp. 485-496.
Holland LM, Wilson JS, Tillery MI, Smith DM (1986) Lung cancer in rats
exposed to fibrogenic dusts. In: Goldsmith DF, Winn DM, Shy CM, eds.
Silica, silicosis, and cancer: Controversy in occupational medicine.
New York, NY, Praeger Publishers, pp. 267-279 (Cancer Research
Monographs, Vol. 2).
Holman CDJ, Psaila-Savona P, Roberts M, McNulty JC (1987) Determinants
of chronic bronchitis and lung dysfunction in Western Australian gold
miners. British journal of industrial medicine, 44:810-818.
Hook GER, Viviano CJ (1996) Acute silicosis and the activation of
alveolar type II cells. In: Castranova V, Vallyathan V, Wallace WE,
eds. Silica and silica-induced lung diseases. Boca Raton, FL, CRC
Press, pp. 229-242.
Hotz P, Gonzalez-Lorenzo J, Siles E, Trujillano G, Lauwerys R, Bernard
A (1995) Subclinical signs of kidney dysfunction following short
exposure to silica in the absence of silicosis. Nephron, 70:438-442.
Hua F, Xipeng J, Shunzhang Y, Xueqi G, Kaiguo W, JianY, Shenghua Q
(1992) Quantitative risk assessment for lung cancer from exposure to
metal ore dust. Biomedical and environmental sciences, 5:221-228.
Huggins CW, Johnson SN, Segreti JM, Snyder JG (1985) Bureau of Mines
Report of Investigations 8975: Determination of alpha quartz particle
distribution in respirable coal mine dust samples and reference
standards. Avondale, MD, US Department of the Interior, Bureau of
Mines, 7 pp. (National Technical Information Service Publication No.
PB86-136660).
Hughes JM (1995) Radiographic evidence of silicosis in relation to
silica exposure. Applied occupational and environmental hygiene,
10(12):1064-1069.
IARC (1984) Polynuclear aromatic compounds. Part 3. Industrial
exposures in aluminium production, coal gasification, coke production
and iron and steel founding. Lyon, International Agency for Research
on Cancer, pp. 133-190 (IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Vol. 34).
IARC (1987) Silica and some silicates. Lyon, France, International
Agency for Research on Cancer, pp. 33-143 (IARC Monographs on the
Evaluation of the Carcinogenic Risk of Chemicals to Humans, Vol. 42).
IARC (1997) Silica, some silicates, coal dust and para -aramid
fibrils. Lyon, International Agency for Research on Cancer, pp.
1-242 (IARC Monographs on the Evaluation of Carcinogenic Risks to
Humans, Vol. 68).
Iler RK (1979) The chemistry of silica: solubility, polymerization,
colloid and surface properties, and biochemistry. New York, NY,
Wiley-Interscience Publishers.
ILO (1980) Guidelines for the use of ILO international classification
of radiographs of pneumoconioses, rev. ed. Geneva, International
Labour Organisation, 48 pp. (Occupational Safety and Health Series No.
22).
ILO (1991) Occupational lung diseases: prevention and control.
Geneva, International Labour Organisation, 85 pp. (Occupational
Safety and Health Series No. 67).
IPCS (1993) International Chemical Safety Card -- Quartz. Geneva,
World Health Organization, International Programme on Chemical Safety
(ICSC 0808).
Iyer R, Holian A (1996) Immunological aspects of silicosis. In:
Castranova V, Vallyathan V, Wallace WE, eds. Silica and
silica-induced lung diseases. Boca Raton, FL, CRC Press, pp.
253-267.
Jaurand MC, Fleury J, Monchaux G, Nebut M, Bignon J (1987) Pleural
carcinogenic potency of mineral fibers (asbestos, attapulgite) and
their cytotoxicity on cultured cells. Journal of the National Cancer
Institute, 79(4):797-804.
Kane AB (1996) Questions and controversies about the pathogenesis of
silicosis. In: Castranova V, Vallyathan V, Wallace WE, eds. Silica
and silica-induced lung diseases. Boca Raton, FL, CRC Press, pp.
121-136.
Kauppinen T, Partanen T, Degerth R, Ojajarvi A (1995) Pancreatic
cancer and occupational exposures. Epidemiology, 6(5):498-502.
Kelly J (1992) U.S. Department of the Interior, National Park
Service, New River Gorge National River, West Virginia. Cincinnati,
OH, National Institute for Occupational Safety and Health (HHE Report
No. HETA 92-045-2260),
King EJ, Mohanty GP, Harrison CV, Nagelschmidt G (1953) The action of
different forms of pure silica on the lungs of rats. British journal
of industrial medicine, 10:9-17.
Kleinbaum DG, Kupper LL, Morgenstern H (1982) Epidemiologic research:
principles and quantitative methods. New York, NY, Van Nostrand
Reinhold, p. 428.
Kleinschmidt I, Churchyard G (1997) Variation in incidences of
tuberculosis in subgroups of South African gold miners. Occupational
and environmental medicine, 54:636-641.
Klockars M, Koskela RS, Järvinen E, Kolari PJ, Rossi A (1987) Silica
exposure and rheumatoid arthritis: a follow up study of granite
workers 1940-81. British medical journal, 294:997-1000.
Koeger AC, Alcaix D, Rozenberg S, Bourgeois P (1991) Occupational
exposure to silica and dermatopolymyositis: three cases. Annales de
medecine interne (Paris), 142(6):409-413.
Koeger AC, Alcaix D, Rozenberg S, Bourgeois P (1996) Graves' disease
after silica dust exposure. Journal of rheumatology, 68(11):202.
Koskela R-S, Klockars M, Järvinen E, Kolari PJ, Rossi A (1987)
Mortality and disability among granite workers. Scandinavian journal
of work, environment and health, 13:18-25.
Kreiss K, Zhen B (1996) Risk of silicosis in a Colorado mining
community. American journal of industrial medicine, 30:529-539.
Kreiss K, Greenberg LM, Kogut SJH, Lezotte DC, Irvin CG, Cherniack RM
(1989) Hard-rock mining exposures affect smokers and nonsmokers
differently: results of a community prevalence study. American review
of respiratory disease, 139:1487-1493.
Kullman GJ, Greife AL, Costello J, Hearl FJ (1995) Occupational
exposures to fibers and quartz at 19 crushed stone mining and milling
operations. American journal of industrial medicine, 27:641-660.
Kurppa K, Gudbergsson H, Hannunkari I, Koskinen H, Hernberg S, Koskela
R-S, Ahlman K (1986) Lung cancer among silicotics in Finland. In:
Goldsmith DF, Winn DM, Shy CM, eds. Silica, silicosis, and cancer:
Controversy in occupational medicine. New York, NY, Praeger
Publishers, pp. 311-319 (Cancer Research Monographs, Vol. 2).
Kusiak R, Liss GM, Gailitis MM (1993) Cor pulmonale and pneumoconiotic
lung disease: an investigation using hospital discharge data.
American journal of industrial medicine, 24:161-173.
Lapp NL, Castranova V (1993) How silicosis and coal workers'
pneumoconiosis develops -- a cellular assessment. Occupational
medicine state of the art reviews, 8(1):35-56.
Lawson RJ, Schenker MB, McCurdy SA, Jenkins B, Lischak LA, John W,
Scales D (1995) Exposure to amorphous silica fibers and other
particulate matter during rice farming operations. Applied
occupational and environmental hygiene, 10(8):677-684.
Leigh J, Wang H, Bonin A, Peters M, Ruan X (1997) Silica-induced
apoptosis in alveolar and granulomatous cells in vivo.
Environmental health perspectives, 105 (Suppl. 5):1241-1245.
Leigh J, Wang H, Bonin A, Peters M (1998a) Persistence of
silica-induced inflammation is related to delayed occurrence of
apoptosis in alveolar leukocytes. In: Chiyotani K, Hosoda Y, Aizawa Y,
eds. Advances in the prevention of occupational respiratory diseases.
Amsterdam, Elsevier Science, pp. 890-895 (Excerpta Medica Supplement
53).
Leigh J, Wang H, Bonin A, Peters M (1998b) In vivo genotoxicity of
silica evidenced by progressive development of micronuclei in alveolar
macrophages. In: Chiyotani K, Hosoda Y, Aizawa Y, eds. Advances in
the prevention of occupational respiratory diseases. Amsterdam,
Elsevier Science, pp. 520-525 (Excerpta Medica Supplement 53).
Linch KD, Cocalis JC (1994) An emerging issue: Silicosis prevention in
construction. Applied occupational and environmental hygiene,
9(8):539-542.
Linch KD, Miller WE, Althouse RB, Groce DW, Hale JM (1998)
Surveillance of respirable crystalline silica dust using OSHA
compliance data (1979-1995). American journal of industrial medicine,
34(6):547-558.
Liu X, Keane MJ, Zhong B-Z, Ong T, Wallace WE (1996) Micronucleus
formation in V79 cells treated with respirable silica dispersed in
medium and in simulated pulmonary surfactant. Mutation research,
361:89-94.
Liu X, Keane MJ, Harrison JC, Cilento EV, Ong T, Wallace WE (1998)
Phospholipid surfactant adsorption by respirable quartz and in vitro
expression of cytotoxicity and DNA damage. Toxicology letters, 96,
97:77-84.
Lofgren DJ (1993) Case studies: silica exposure for concrete workers
and masons. Applied occupational and environmental hygiene,
8(10):832-836.
Love RG, Waclawski ER, Maclaren WM, Porteous RH, Groat SK, Wetherill
GZ, Hutchison PA, Kidd MW, Soutar CA (1994) Cross-sectional study of
risks of respiratory disease in relation to exposures of airborne
quartz in the heavy clay industry. Edinburgh, Institute of
Occupational Medicine (IOM Report No. TM/94/07).
Mao Y, Daniel LN, Whittaker N, Saffiotti U (1994) DNA binding to
crystalline silica characterized by Fourier-transform infrared
spectroscopy. Environmental health perspectives, 102
(Suppl. 10):165-171.
Masuda K (1981) Chronic thyroiditis observed in patients with
silicosis as an adjuvant disease of man. Shikoku Acta Medica,
37(1):119-129.
Materna BL, Jones JR, Sutton PM, Rothman N, Harrison RJ (1992)
Occupational exposures in California wildland fire fighting. American
Industrial Hygiene Association journal, 53(1):69-76.
McDonald C (1995) Silica, silicosis, and lung cancer: an
epidemiological update. Applied occupational and environmental
hygiene, 10(12):1056-1063.
McDonald JC, Oakes D (1984) Exposure-response in miners exposed to
silica. In: Sixth International Pneumoconiosis Conference: 1983,
Bochum, Germany. Vol 1. Geneva, International Labour Organisation,
pp. 114-123.
McDonald JC, Cherry N, McNamee R, Burgess G, Turner S (1995)
Preliminary analysis of proportional mortality in a cohort of British
pottery workers exposed to crystalline silica. Scandinavian journal
of work, environment and health, 21 (Suppl. 2):63-65.
McDonald JC, Burgess GL, Turner S, Cherry NM (1997) Cohort study of
Staffordshire pottery workers: (III) Lung cancer, radiographic
changes, silica exposure and smoking habit. Annals of occupational
hygiene, 41 (Suppl. 1):412-414.
McLaughlin JK, Jing-Qiong C, Dosemeci M, Rong-An C, Rexing SH, Zhien
W, Hearl FJ, McCawley MA, Blot WJ (1992) A nested case-control study
of lung cancer among silica exposed workers in China. British journal
of industrial medicine, 49:167-171.
McNeill DA, Chrisp CE, Fisher GL (1990) Pulmonary adenomas in A/J mice
treated with silica. Drug and chemical toxicology, 13(1):87-92.
Merlo F, Costantini M, Reggiardo G, Ceppi M, Puntoni R (1991) Lung
cancer risk among refractory brick workers exposed to crystalline
silica: a retrospective cohort study. Epidemiology, 2(4):299-305.
Morrow PE (1988) Issues: Possible mechanisms to explain dust
overloading of the lungs. Fundamental and applied toxicology,
10:369-384.
Morrow PE (1992) Contemporary issues in toxicology -- dust overloading
of the lungs: update and appraisal. Toxicology and applied
pharmacology, 113:1-12.
Mossman BT, Churg A (1998) State of the art: Mechanisms in the
pathogenesis of asbestosis and silicosis. American journal of
respiratory and critical care medicine, 157:1666-1680.
Mozzon D, Brown DA, Smith JW (1987) Occupational exposure to airborne
dust, respirable quartz and metals arising from refuse handling,
burning and landfilling. American Industrial Hygiene Association
journal, 48(2):111-116.
Muhle H, Takenaka S, Mohr U, Dasenbrock C, Mermelstein R (1989) Lung
tumor induction upon long term low level inhalation of crystalline
silica. American journal of industrial medicine, 15(3):343-346.
Muhle H, Bellmann B, Creutzenberg O, Dasenbrock C, Ernst H, Kilpper R,
Mackenzie JC, Morrow P, Mohr U, Takenaka S, Mermelstein R (1991)
Pulmonary response to toner upon chronic inhalation exposure in rats.
Fundamental and applied toxicology, 17:280-299.
Muhle H, Kittel B, Ernst H, Mohr U, Mermelstein R (1995) Neoplastic
lung lesions in rat after chronic exposure to crystalline silica.
Scandinavian journal of work, environment and health, 21
(Suppl. 2):27-29.
Muir DCF (1991) Correction in cumulative risk in silicosis exposure
assessment. American journal of industrial medicine, 19:555.
Muir DCF, Shannon HS, Julian JA, Verma DK, Sebestyen A, Bernholz CD
(1989a) Silica exposure and silicosis among Ontario hardrock miners:
I. Methodology. American journal of industrial medicine, 16:5-11.
Muir DCF, Shannon HS, Julian JA, Verma DK, Sebestyen A, Bernholz CD
(1989b) Silica exposure and silicosis among Ontario hardrock miners:
III. Analysis and risk estimates. American journal of industrial
medicine, 16:29-43.
Muramatsu K, Yamamoto T, Hasegawa A et al. (1989) [A case of
autoimmune hemolytic anemia associated with silicosis.] Japanese
journal of chest diseases, 48(1):45-50 (in Japanese).
Murray J, Reid G, Kielkowski D, de Beer M (1993) Cor pulmonale and
silicosis: a necropsy based case-control study. British journal of
industrial medicine, 50:544-548.
Myers JE, Lewis P, Hofmeyr W (1989) Respiratory health of brickworkers
in Cape Town, South Africa: background, aims and dust exposure
determinations. Scandinavian journal of work, environment and health,
15(3):180-187.
Nagalakshmi R, Nath J, Ong T, Whong W-Z (1995) Silica-induced
micronuclei and chromosomal aberrations in Chinese hamster lung (V79)
and human lung (Hel 299) cells. Mutation research, 335:27-33.
Nehls P, Seiler F, Rehn B, Greferath R, Bruch J (1997) Formation and
persistence of 8-oxoguanine in rat lung cells as an important
determinant for tumor formation following particle exposure.
Environmental health perspectives, 105 (Suppl. 5):1291-1296.
Neyer U, Woss E, Neuweiler J (1994) Case report: Wegener's
granulomatosis associated with silicosis. Nephrology dialysis
transplantation, 9(5):559-561.
Ng TP, Yeung KH, O'Kelly FJ (1987a) Silica hazard of caisson
construction in Hong Kong. Journal of the Society of Occupational
Medicine, 37:62-65.
Ng TP, Tsin TW, O'Kelly FJ, Chan SL (1987b) A survey of the
respiratory health of silica-exposed gemstone workers in Hong Kong.
American review of respiratory disease, 135:1249-1254.
Ng TP, Lee HS, Phoon WH (1993) Further evidence of human silica
nephrotoxicity in occupationally exposed workers. British journal of
industrial medicine, 50:907-912.
Niemeier RW, Mulligan LT, Rowland J (1986) Cocarcinogenicity of
foundry silica sand in hamsters. In: Goldsmith DF, Winn DM, Shy CM,
eds. Silica, silicosis, and cancer: Controversy in occupational
medicine. New York, NY, Praeger Publishers, pp. 215-227 (Cancer
Research Monographs, Vol. 2).
NIOSH (1974) Criteria for a recommended standard: occupational
exposure to crystalline silica. Cincinnati, OH, US Department of
Health, Education, and Welfare, Health Services and Mental Health
Administration, National Institute for Occupational Safety and Health
(DHEW (NIOSH) Publication No. 75-120).
NIOSH (1992a) NIOSH Alert: Request for assistance in preventing
silicosis and deaths from sandblasting. Cincinnati, OH, US
Department of Health and Human Services, Public Health Service,
Centers for Disease Control, National Institute for Occupational
Safety and Health (DHHS (NIOSH) Publication No. 92-102).
NIOSH (1992b) NIOSH Alert: Request for assistance in preventing
silicosis and deaths in rock drillers. Cincinnati, OH, US Department
of Health and Human Services, Public Health Service, Centers for
Disease Control, National Institute for Occupational Safety and Health
(DHHS (NIOSH) Publication No. 92-107).
NIOSH (1994a) Silica, crystalline, respirable by XRD: Method 7500,
Issue 2 (dated 8/15/94). In: Cassinelli ME, O'Connor PF, eds. NIOSH
manual of analytical methods, 4th ed. Cincinnati, OH, US Department
of Health and Human Services, Public Health Service, Centers for
Disease Control and Prevention, National Institute for Occupational
Safety and Health (DHHS (NIOSH) Publication No. 94-113).
NIOSH (1994b) Silica, crystalline, respirable by IR: Method 7602,
Issue 2 (dated 8/15/94). In: Cassinelli ME, O'Connor PF, eds. NIOSH
manual of analytical methods, 4th ed. Cincinnati, OH, US Department
of Health and Human Services, Public Health Service, Centers for
Disease Control and Prevention, National Institute for Occupational
Safety and Health (DHHS (NIOSH) Publication No. 94-113).
NIOSH (1996) NIOSH Alert: Request for assistance in preventing
silicosis and deaths in construction workers. Cincinnati, OH, US
Department of Health and Human Services, Public Health Service,
Centers for Disease Control and Prevention, National Institute for
Occupational Safety and Health (DHHS (NIOSH) Publication No. 96-112).
NIOSH (1997) Pocket guide to chemical hazards. Cincinnati, OH, US
Department of Health and Human Services, Public Health Service,
Centers for Disease Control and Prevention, National Institute for
Occupational Safety and Health (DHHS (NIOSH) Publication No. 97-140).
NIOSH (forthcoming) NIOSH special hazard review: Health effects of
occupational exposure to respirable crystalline silica. Cincinnati,
OH, US Department of Health and Human Services, Public Health Service,
Centers for Disease Control and Prevention, National Institute for
Occupational Safety and Health (DHHS (NIOSH) Publication).
Nuyts GD, Van Vlem E, De Vos A, Daelemans RA, Rorive G, Elseviers MM,
Schurgers M, Segaert M, D'Haese PC, De Broe ME (1995) Wegener
granulomatosis is associated to exposure to silicon compounds: a
case-control study. Nephrology dialysis transplantation,
10:1162-1165.
Oberdörster G (1997) Pulmonary carcinogenicity of inhaled particles
and the maximum tolerated dose. Environmental health perspectives,
195 (Suppl. 5):1347-1355.
Oshimura M, Hesterberg TW, Tsutsui T, Barrett JC (1984) Correlation of
asbestos-induced cytogenetic effects with cell transformation of
Syrian hamster embryo cells in culture. Cancer research,
44:5017-5022.
Osorio AM, Thun MJ, Novak RF, Van Cura J, Avner ED (1987) Silica and
glomerulonephritis: case report and review of the literature.
American journal of kidney diseases, 9(3):224-230.
Otsuki T, Sakaguchi H, Tomokuni A, Aikoh T, Matsuki T, Kawakami Y,
Kusaka M, Ueki H, Kita S, Ueki A (1998) Soluble Fas mRNA is dominantly
expressed in cases with silicosis. Immunology, 94:258-262.
Oudyk JD (1995) Review of an extensive ferrous foundry silica sampling
program. Applied occupational and environmental hygiene, 10:331-340.
Pairon JC, Jaurand MC, Kheuang L, Janson X, Brochard P, Bignon J
(1990) Sister chromatid exhanges in human lymphocytes treated with
silica. British journal of industrial medicine, 47:110-115.
Pan G, Takahashi K, Feng Y, Liu L, Liu T, Zhang S, Liu N, Okubo T,
Goldsmith DF (1999) Nested case-control study of esophageal cancer in
relation to occupational exposure to silica and other dusts. American
journal of industrial medicine, 35:272-280.
Parent ME, Siemiatycki J, Fritschi L (1998) Occupational exposures and
gastric cancer. Epidemiology, 9(1):48-55.
Parker JE (1994) Silicosis. In: Rakel RE, ed. Conn's current therapy.
Philadelphia, PA, W.B. Saunders, pp. 207-210.
Parkes WR, ed. (1982) Occupational lung disorders, 2nd ed. London,
Butterworths, pp. 134-174.
Partanen T, Pukkala E, Vainio H, Kurppa K, Koskinen H (1994) Increased
incidence of lung and skin cancer in Finnish silicotic patients.
Journal of occupational medicine, 36(6):616-622.
Peters JM (1986) Silicosis. In: Merchant JA, Boehlecke BA, Taylor G,
Pickett-Harner M, eds. Occupational respiratory diseases.
Cincinnati, OH, US Department of Health and Human Services, Public
Health Service, Centers for Disease Control, National Institute for
Occupational Safety and Health, pp. 219-237 (DHHS (NIOSH) Publication
No. 86-102).
Petersen PE, Henmar P (1988) Oral conditions among workers in the
Danish granite industry. Scandinavian journal of work, environment
and health, 14:328-331.
Popendorf WJ, Pryor A, Wenk HR (1982) Mineral dust in manual harvest
operations. Annals of the American Conference of Governmental
Industrial Hygienists, 2:101-115.
Pott F, Dungworth DL, Heinrich U, Muhle H, Kamino K, Germann P-G,
Roller M, Rippe RM, Mohr U (1994) Lung tumours in rats after
intratracheal instillation of dusts. Annals of occupational hygiene,
38 (Suppl. 1):357-363.
Pouthier D, Duhoux P, Van Damme B (1991) Pulmonary silicosis and
glomerular nephropathy: Apropos of 1 case. Nephrologie, 12(1):8-11.
Price-Jones MJ, Gubbings G,Chamberlain M (1980) The genetic effects of
crocidolite asbestos; comparison of chromosome abnormalities and
sister-chromatid exchanges. Mutation research, 79:331-336.
Puntoni R, Goldsmith DG, Valerio F, Vercelli M, Bonassi S, Di Giorgio
F, Ceppi M, Stagnaro E, Filiberti R, Santi L, Merlo F (1988) A cohort
study of workers employed in a refractory brick plant. Tumori,
74:27-33.
Rabovsky J (1997) Laboratory studies on silica induced toxicity and
relationship to carcinogenicity. Journal of exposure analysis and
environmental epidemiology, 7(3):267-278.
Rees D, Cronje R, du Toit RSJ (1992) Dust exposure and pneumoconiosis
in a South African pottery. 1. Study objectives and dust exposure.
British journal of industrial medicine, 49:459-464.
Renne RA, Eldridge SR, Lewis TR, Stevens DL (1985) Fibrogenic
potential of intratracheally instilled quartz, ferric oxide, fibrous
glass, and hydrated alumina in hamsters. Toxicologic pathology,
13(4):306-314.
Reuzel PGJ, Bruijntjes JP, Feron VJ, Woutersen RA (1991) Subchronic
inhalation toxicity of amorphous silicas and quartz dust in rats.
Food and chemical toxicology, 29(5):341-354.
Riala R (1988) Dust and quartz exposure of Finnish construction
cleaners. Annals of occupational hygiene, 32(2):215-220.
Rice CH, Harris RL, Checkoway H, Symons MJ (1986) Dose-response
relationships for silicosis from a case-control study of North
Carolina dusty trades workers. In: Goldsmith DF, Winn DM, Shy CM, eds.
Silica, silicosis, and cancer: Controversy in occupational medicine.
New York, NY, Praeger Publishers, pp. 77-86 (Cancer Research
Monographs, Vol. 2).
Rice FL, Stayner LT (1995) Assessment of silicosis risk for
occupational exposure to crystalline silica. Scandinavian journal of
work, environment and health, 21 (Suppl. 2):87-90.
Rispal P, Wen L, De Precigout V, Aparicio M (1991) Nephropathy with
silica in a dental technician. La presse medicale, 20(4):176.
Robbins SL (1974) Pathologic basis of disease. Philadelphia, PA,
W.B. Saunders Co., pp. 239-240.
Rosenman KD, Reilly MJ, Rice C, Hertzberg V, Tseng C-Y, Anderson HA
(1996) Silicosis among foundry workers: implication for the need to
revise the OSHA standard. American journal of epidemiology,
144(9):890-900.
Saffiotti U (1990) Lung cancer induction by silica in rats, but not in
mice and hamsters: species differences in epithelial and granulomatous
reactions. In: Seemayer NH, Hadnagy W, eds. Environmental hygiene II.
New York, NY, Springer-Verlag, pp. 235-238.
Saffiotti U (1992) Lung cancer induction by crystalline silica. In:
D'Amato RD, Slaga TJ, Farland WH, Henry C, eds. Relevance of animal
studies to the evaluation of human cancer risk. New York, NY,
Wiley-Liss, pp. 51-69.
Saffiotti U, Ahmed N (1995) Neoplastic transformation by quartz in the
BALB/3T3/A31-1-1 cell line and the effects of associated minerals.
Teratogenesis, carcinogenesis, and mutagenesis, 15(6):339-356.
Saffiotti U, Daniel LN, Mao Y, Williams AO, Kaighn ME, Ahmed N,
Knapton AD (1993) Biological studies on the carcinogenic mechanisms of
quartz. In: Guthrie GD, Mossman BT, eds. Health effects of mineral
dusts. Washington, DC, Mineralogical Society of America. Reviews in
mineralogy, 28:523-544.
Saffiotti U, Williams O, Lambert N, Daniel N, Kaighn ME, Mao Y, Shi X
(1996) Carcinogenesis by crystalline silica: animal, cellular, and
molecular studies. In: Castranova V, Vallyathan V, Wallace WE, eds.
Silica and silica-induced lung diseases. Boca Raton, FL, CRC Press,
pp. 345-381.
Saita G, Zavaglia O (1951) Kidney function in silicotics.
Medicina del Lavoro, 42(2):41-48.
Salisbury S, Melius J (1982) Harbison-Walker Refractories, Fairfield,
Alabama, Bessemer, Alabama. Cincinnati, OH, National Institute for
Occupational Safety and Health (HHE Report No. HETA-80-086-1191).
Samimi B, Weill H, Ziskind M (1974) Respirable silica dust exposure of
sandblasters and associated workers in steel fabrication yards.
Archives of environmental health, 29:61-66.
Schapira RM, Ghio AJ, Effros RM, Morrisey J, Almagro UA, Dawson CA,
Hacker AD (1995) Hydroxyl radical production and lung injury in the
rat following silica or titanium dioxide instillation in vivo.
American journal of respiratory cell and molecular biology,
12(2):220-226.
Scheuchenzuber WJ, Eskew ML, Zarkower A (1985) Effects of prolonged
inhalation of silica and olivine dusts on immune functions in the
mouse. Environmental research, 38(2):389-399.
Schwartz LW, Knight HD, Whittig LD, Malloy RL, Abraham JL,Tyler NK
(1981) Silicate pneumoconiosis and pulmonary fibrosis in horses from
the Monterey-Carmel peninsula. Chest, 80 (Suppl. 1):82-85.
Seaton A (1995) Silicosis. In: Morgan WKC, Seaton A, eds.
Occupational lung diseases, 3rd ed. Philadelphia, PA, W.B. Saunders
Co., pp. 222-267.
Seta JA, Sundin DA, Pedersen DH, eds. (1988) National occupational
exposure survey field guidelines. Vol. 1. Cincinnati, OH, US
Department of Health and Human Services, Public Health Service,
Centers for Disease Control, National Institute for Occupational
Safety and Health, Division of Surveillance, Hazard Evaluations, and
Field Studies (DHHS (NIOSH) Publication No. 88-106).
Sherson D, Jorgensen F (1989) Rapidly progressive crescenteric
glomerulonephritis in a sandblaster with silicosis. British journal
of industrial medicine, 46:675-676.
Sherson D, Lander F (1990) Morbidity of pulmonary tuberculosis among
silicotic and nonsilicotic foundry workers in Denmark. Journal of
occupational medicine, 32(2):110-113.
Shi X, Mao Y, Daniel LN, Saffiotti U, Dalal NS, Vallyathan V (1994)
Silica radical-induced DNA damage and lipid peroxidation.
Environmental health perspectives, 102 (Suppl. 10):149-154.
Siltanen E, Koponen M, Kokko A, Engström B, Reponen J (1976) Dust
exposure in Finnish foundries. Scandinavian journal of work,
environment and health, 2 (Suppl. 1):19-31.
Silverstein M, Maizlish N, Park R, Silverstein B, Brodsky L, Mirer F
(1986) Mortality among ferrous foundry workers. American journal of
industrial medicine, 10(1):27-43.
Slavin RE, Swedo JL, Brandes D, Gonzalez-Vitale JC, Osornio-Vargas A
(1985) Extrapulmonary silicosis: a clinical, morphologic, and
ultrastructural study. Human pathology, 16(4):393-412.
Sluis-Cremer GK, Hessel PA, Hnizdo E, Churchill AR, Zeiss EA (1985)
Silica, silicosis, and progressive systemic sclerosis. British
journal of industrial medicine, 42:838-843.
Sluis-Cremer GK, Hessel PA, Hnizdo E, Churchill AR (1986) Relationship
between silicosis and rheumatoid arthritis. Thorax, 41:596-601.
Smith AH, Lopipero PA, Barroga VR (1995) Meta-analysis of studies of
lung cancer among silicotics. Epidemiology, 6(6):617-624.
Sobti RC, Bhardwaj DK (1991) Cytogenetic damage and occupational
exposure: I. Exposure to stone dust. Environmental research,
56:25-30.
Soutar CA, Robertson A, Miller BG, Searl A (1997) Epidemiological
evidence on the carcinogenicity of silica: factors in scientific
judgement. Edinburgh, Institute of Occupational Medicine, 34 pp.
(IOM Report TM/97/09).
Spiethoff A, Wesch H, Wegener K, Klimisch HJ (1992) The effects of
Thorotrast and quartz on the induction of lung tumors in rats.
Health physics, 63(1):101-110.
Steenland K, Beaumont J (1986) A proportionate mortality study of
granite cutters. American journal of industrial medicine, 9:189-201.
Steenland K, Brown D (1995a) Silicosis among gold miners:
exposure-response analyses and risk assessment. American journal of
public health, 85(10):1372-1377.
Steenland K, Brown D (1995b) Mortality study of gold miners exposed to
silica and nonasbestiform amphibole minerals: an update with 14 more
years of follow-up. American journal of industrial medicine,
27:217-229.
Steenland K, Goldsmith DF (1995) Silica exposure and autoimmune
diseases. American journal of industrial medicine, 28:603-608.
Steenland K, Stayner L (1997) Silica, asbestos, man-made mineral
fibers, and cancer. Cancer causes and control, 8:491-503.
Steenland K, Nowlin S, Ryan B, Adams S (1992) Use of multiple-cause
mortality data in epidemiologic analyses: US rate and proportion files
developed by the National Institute for Occupational Safety and Health
and the National Cancer Institute. American journal of epidemiology,
136(7):855-862.
Stopford CM, Stopford W (1995) Respirable quartz content of farm
soils. Applied occupational and environmental hygiene,
10(3):196-199.
Sweeney TD, Brain JD (1996) Lavagable biomarkers of exposure to
fibrogenic dusts. In: Castranova V, Vallyathan V, Wallace WE, eds.
Silica and silica-induced lung diseases. Boca Raton, FL, CRC Press,
pp. 197-207.
Tokumaru Y, Hirayama K, Kita K, Kawamura M, Katayama K (1990) Two
cases of ataxic sensory neuropathy associated with silicosis.
Clinical neurology, 30:933-938.
Tsuda T, Babazono A, Yamamoto E, Mino Y, Matsuoka H (1997) A
meta-analysis on the relationship between pneumoconiosis and lung
cancer. Journal of occupational health, 39:285-294.
Tucker DM, Reger RB, Morgan WKC (1995) Effects of silica exposure
among railroad workers. Applied occupational and environmental
hygiene, 10(12):1080-1085.
US Department of the Interior (1992) Special publication: Crystalline
silica primer. Washington, DC, US Department of the Interior, Bureau
of Mines.
US EPA (1994) Methods for derivation of inhalation reference
concentrations and application of inhalation dosimetry, Section 4.3.5
Dosimetric adjustments for particle exposures. Research Triangle
Park, NC, US Environmental Protection Agency, Office of Health and
Environmental Assessment, Office of Research and Development
(Publication No. EPA/600/8-90/066F).
US EPA (1996) Ambient levels and noncancer health effects of inhaled
crystalline and amorphous silica: health issue assessment.
Washington, DC, US Environmental Protection Agency, Office of
Research and Development (Publication No. EPA/600/R-95/115; National
Technical Information Service Publication No. PB97-188122).
Vallyathan V, Castranova V, Pack D, Leonard S, Shumaker J, Hubbs AF,
Shoemaker DA, Ramsey DM, Pretty JR, McLaurin JL, Khan A, Teass A
(1995) Freshly fractured quartz inhalation leads to enhanced lung
injury and inflammation: Potential role of free radicals. American
journal of respiratory and critical care medicine, 152(3):1003-1009.
Vanchugova NN, Frash VN, Kogan FM (1985) The use of a micronucleus
test as a short-term method in detecting potential blastomogenicity of
asbestos-containing and other mineral fibers. Gigiena Truda i
Professional'nye Zabolevaniya, 6:45-48.
Verma DK, Sebestyen A, Julian JA, Muir DCF, Schmidt H, Bernholz CD,
Shannon HS (1989) Silica exposure and silicosis among Ontario hardrock
miners: II. Exposure estimates. American journal of industrial
medicine, 16:13-28.
Virta RL (1993) Crystalline silica: what it is -- and isn't.
Minerals today, October: 12-16.
Wagner GR (1995) The inexcusable persistence of silicosis [editorial].
American journal of public health, 85(10):1346-1347.
Wagner JC (1970) The pathogenesis of tumors following the intrapleural
injection of asbestos and silica. In: Nettesheim P, Hanna MG Jr,
Deatherage JW Jr, eds. Morphology of experimental respiratory
carcinogenesis. Oak Ridge, TN, US Atomic Energy Commission, pp.
347-358.
Wagner JC, Berry G (1969) Mesotheliomas in rats following inoculation
with asbestos. British journal of cancer, 23:567-581.
Wagner MMF (1976) Pathogenesis of malignant histiocytic lymphoma
induced by silica in a colony of specific-pathogen-free Wistar rats.
Journal of the National Cancer Institute, 57:509-518.
Wagner MMF, Wagner JC (1972) Lymphomas in the Wistar rat after
intrapleural inoculation of silica. Journal of the National Cancer
Institute, 49:81-91.
Wagner MMF, Wagner JC, Davies R, Griffiths DM (1980) Silica-induced
malignant histiocytic lymphoma: incidence linked with strain of rat
and type of silica. British journal of cancer, 41:908-917.
Wang H, Leigh J, Bonin A, Peters M (1997a) Silica-induced
morphological change similar to apoptosis in bronchoalveolar lavage
cells and granulomatous cells. Annals of occupational hygiene, 41
(Suppl. 1):459-464.
Wang H, Leigh J, Bonin A, Peters M (1997b) Silica induced micronuclei
in pulmonary alveolar macrophages in vivo. Annals of occupational
hygiene, 41 (Suppl. 1):434-439.
Warheit DB, Hartsky MA (1997) Initiating the risk assessment process
for inhaled particulate materials: development of short term
inhalation bioassays. Journal of exposure analysis and environmental
epidemiology, 7(3): 313-325.
Watts WF, Parker DR (1995) Quartz exposure trends in metal and
nonmetal mining. Applied occupational and environmental hygiene,
10(12):1009-1018.
Weill H, McDonald JC (1996) Exposure to crystalline silica and risk of
lung cancer: the epidemiological evidence. Thorax, 51:97-102.
Weill H, Jones RN, Parkes WR (1994) Silicosis and related diseases.
In: Parkes WR, ed. Occupational lung disorders, 3rd ed. London,
Butterworth-Heinemann, pp. 285-339.
Weissman DN, Ma JKH, Rojanasakul Y, Hubbs AF (1996) Immune dysfunction
in silicosis: a hypothesis. Applied occupational and environmental
hygiene, 11(7):962-965.
Westerholm P, Ahlmark A, Maasing R, Segelberg I (1986) Silicosis and
risk of lung cancer or lung tuberculosis: a cohort study.
Environmental research, 41:339-350.
WHO (1986) Recommended health-based limits in occupational exposure
to selected mineral dusts (silica, coal): report of a WHO study
group. Geneva, World Health Organization, 80 pp. (WHO Technical
Report Series 734).
WHO (1993) "WHO calls for medical surveillance of workers exposed to
mineral dusts." Geneva, World Health Organization (Press Release
WHO/73, 23 September 1993).
WHO (1996) Groups at risk: WHO report on the tuberculosis epidemic
1996. Geneva, World Health Organization.
Wiessner JH, Henderson JD Jr, Sohnle PG, Mandel NS, Mandel GS (1988)
The effect of crystal structure on mouse lung inflammation and
fibrosis. American review of respiratory disease, 138:445-450.
Wiles FJ, Faure MH (1977) Chronic obstructive lung disease in gold
miners. In: Walton WH, ed. Inhaled particles IV. Part 2. Oxford,
Pergamon Press, pp. 727-735.
Wilke RA, Salisbury S, Abdel-Rahman E, Brazy PC (1996) Lupus-like
autoimmune disease associated with silicosis. Nephrology dialysis and
transplantation, 11:1835-1838.
Williams AO, Knapton AD, Ifon ET, Saffiotti U (1996) Transforming
growth factor beta expression and transformation of rat lung
epithelial cells by crystalline silica (quartz). International
journal of cancer, 65:639-649.
Wilson T, Scheuchenzuber WJ, Eskew ML, Zarkower A (1986) Comparative
pathological aspects of chronic olivine and silica inhalation in mice.
Environmental research, 39(2):331-344.
Xu XZ, Cia XG, Men XS, Yang PY, Yang JF, Jing SL, He JH, Si WY (1993)
A study of siliceous pneumoconiosis in a desert area of Sunan county,
Gansu province, China. Biomedical and environmental sciences,
6:217-222.
Xu Z, Pan G-W, Liu L-M, Brown LM, Gaun D-X, Xiu Q, Sheng J-H, Stone
BJ, Dosemeci M, Fraumeni JF Jr, Blot WJ (1996) Cancer risks among iron
and steel workers in Anshan, China, Part I: proportional mortality
ratio analysis. American journal of industrial medicine, 30:1-6.
Yamano Y, Kagawa J, Hanaoka T, Takahashi T, Kasai H, Tsugane S,
Watanabe S (1995) Oxidative DNA damage induced by silica in vivo.
Environmental research, 69(2):102-107.
Zheng W, Shu XO, Ji BT, Gao YT (1996) Diet and other risk factors for
cancer of the salivary glands: a population-based case-control study.
International journal of cancer, 67:194-198.
Zhong B, Whong W, Ong T (1997) Detection of mineral-dust-induced DNA
damage in two mammalian cell lines using the alkaline single cell
gel/comet assay. Mutation research, 393:181-187.
Ziegler V, Haustein UF (1992) Systemic scleroderma -- a silica induced
occupational disease? Epidemiological, clinical, immunological,
mineralogical, animal and cell culture investigations.
Dermatologische Monatschrift, 178:34-43.
Ziskind M, Jones RN, Weill H (1976) Silicosis. American review of
respiratory disease, 113:643-665.
APPENDIX 1 -- SOURCE DOCUMENTS
IARC (1997)
Copies of Silica, some silicates, coal dust and
para -aramid fibrils (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Vol. 68) may be obtained from:
International Agency for Research on Cancer
150 cours Albert Thomas
69372 Lyon Cedex 08
France
The members of the Working Group on the Evaluation of
Carcinogenic Risks to Humans of silica (including quartz), some
silicates, coal dust and para-aramid fibrils that met in Lyon on
15-22 February 1996 were:
M.D. Attfield, National Institute for Occupational Safety and
Health, USA
P.J. Borm, University of Limburg, The Netherlands
H. Checkoway, University of Washington, USA
K. Donaldson, Napier University, United Kingdom
M. Dosemeci, National Cancer Institute, USA
V.J. Feron, TNO Nutrition and Food Research Institute, The
Netherlands
B.J. Fubini, University of Torino, Italy
M. Gérin, Université de Montréal, Canada
E. Hnizdo, National Centre for Occupational Health, South Africa
A.B. Kane, Brown University, USA
J.C. McDonald, Imperial College, United Kingdom
H. Muhle, Fraunhofer Institute for Toxicology and Aerosol
Research, Germany
S. Olin, International Life Sciences Institute, USA
J.-C. Pairon, INSERM, France
T. Partanen, Institute of Occupational Health, Finland
C. Shy, University of North Carolina, USA
E. Tatrai, National Institute of Occupational Health, Hungary
D.B. Warheit, DuPont Haskell Laboratory for Toxicology and
Industrial Medicine, USA
V. Yermilov, N.N. Petrov Research Institute of Oncology, Russia
US EPA (1996)
Copies of the US Environmental Protection Agency report entitled
Ambient levels and noncancer health effects of inhaled crystalline
and amorphous silica: health issue assessment (NTIS Publication
PB97-188122) may be obtained from:
National Technical Information Service
Springfield, VA 22161
USA
The document was reviewed in accordance with US EPA policy and
approved for publication. The principal authors of the document were:
Dr R.A. Faust, Oak Ridge National Laboratory, USA
Dr J.S. Gift, National Center for Environmental Assessment, USA
Dr D.F. Goldsmith, Western Consortium for Public Health, USA
Dr R. Ruble, Western Consortium for Public Health, USA
The reviewers and contributors from the US EPA were:
Dr D.L. Costa, National Health and Environmental Effects Research
Laboratory (MD-82), USA
Dr J.S. Dollarhide, National Center for Environmental Assessment
Dr J.A. Graham, National Center for Environmental Assessment
Dr A. Koppikar, National Center for Environmental Assessment
Dr W.E. Pepelko, National Center for Environmental Assessment
Dr C. Shoaf, National Center for Environmental Assessment
Mr E.G. Smith, Office of Air Quality Planning and Standards
Dr W. Victery, Region IX
The external peer reviewers of the document were:
Dr D. Gardner, Private Consultant, Raleigh, NC, USA
Ms S. Goodwin, University of Maryland, Baltimore, MD, USA
Dr M. Jacobsen, National Institute for Occupational Safety and
Health, USA
Dr R. Kutzman, MITRE Corporation, McLean, VA, USA
Dr J. Rabovsky, California Environmental Protection Agency, USA
Ms F. Rice, National Institute for Occupational Safety and
Health, USA
Dr J.M. Samet, The Johns Hopkins University, USA
Dr C. Shy, University of North Carolina, USA
Dr J. Vincent, University of Minnesota, USA
Dr J. Watson, Desert Research Institute, USA
Dr H. Weill, Tulane University, USA
NIOSH (forthcoming)
When published, copies of the NIOSH special hazard review:
Health effects of occupational exposure to respirable crystalline
silica will be available from:
National Institute for Occupational Safety and Health
Publications Dissemination
4676 Columbia Parkway
Cincinnati, OH 45226-1998
USA
The draft document has undergone the following NIOSH internal
reviews in accordance with NIOSH policy: intradivisional review and
interdivisional review. The external peer reviewers of the document
were:
W. Beckett, University of Rochester School of Medicine
H. Checkoway, University of Washington
G.S. Davis, University of Vermont College of Medicine
J. Gift, US EPA
D. Goldsmith, George Washington University
E. Hnizdo, National Centre for Occupational Health, South Africa
J. Hughes, Tulane School of Public Health and Tropical Medicine
C. Jones, Mine Safety and Health Administration, US Department of
Labor
W. Kojola, American Federation of Labor and Congress of
Industrial Organizations
A.G. Macneski, Environmental Safety and Health, Bechtel National,
Inc.
M. Schaper, Mine Safety and Health Administration, US Department
of Labor
L. Schuman, Occupational Safety and Health Administration, US
Department of Labor
J. Sharpe, National Stone Association
D.M. Tucker, Norfolk Southern Corporation
J.A. Ulizio, US Silica Company
J.L. Weeks, George Washington University Medical Center
APPENDIX 2 -- CICAD PEER REVIEW
The draft CICAD on crystalline silica, quartz was sent for review
to institutions and organizations identified by IPCS after contact
with IPCS national Contact Points and Participating Institutions, as
well as to identified experts. Comments were received from:
M. Baril, Institut de Recherche en Santé et Sécurité du Travail
du Québec, Montreal, Quebec, Canada
R. Benson, Drinking Water Program, US Environmental Protection
Agency, Denver, CO, USA
T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
P. Borm, Medical Institute for Environmental Hygiene, Department
of Fibre and Particle Toxicology, Dusseldorf University,
Düsseldorf, Germany
R. Brown, Toxservices, Uppingham, United Kingdom
R.S. Chhabra, National Institute of Environmental Health
Sciences/National Institute of Health, Research Triangle Park,
NC, USA
S. Dobson, Institute of Terrestrial Ecology, Cambridgeshire,
United Kingdom
K. Donaldson, Department of Biological Sciences, Napier
University, Edinburgh, Scotland
J. Heuer, Federal Institute for Health Protection of Consumers
and Veterinary Medicine, Berlin, Germany
J. Hurych, National Institute of Public Health, Prague, Czech
Republic
J. Leigh, National Occupational Health and Safety Commission,
Sydney, Australia
M. Moore, The Morgan Crucible Company, Windsor, United Kingdom
F. Morrall, British Ceramic Confederation, Stoke-on-Trent, United
Kingdom
D. Muir, Occupational Health Program, McMaster University,
Hamilton, Ontario, Canada
M. Wyart-Remy, Industrial Minerals Association - Europe,
Brussels, Belgium
P. Yao, Institute of Occupational Medicine, Chinese Academy of
Preventive Medicine, Beijing, People's Republic of China
J. Yoshida, Chemical Safety Management, Office of Environmental
Chemical Safety, Environmental Health Bureau, Ministry of Health
and Welfare, Tokyo, Japan
K. Ziegler-Skylakakis, Beratergremium für Umweltrelevante
Altstoffe, Muenchen, Germany
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
Sydney, Australia, 21-24 November 1999
Members
Dr R. Benson, Drinking Water Program, US Environmental Protection
Agency, Region VIII, Denver, CO, USA
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
Dr R.M. Bruce, National Center for Environmental Assessment, US
Environmental Protection Agency, Cincinnati, OH, USA
Mr R. Cary, Health and Safety Executive, Merseyside, United Kingdom
Dr R.S. Chhabra, National Institute of Environmental Health Sciences,
National Institutes of Health, Research Triangle Park, NC, USA
Dr S. Chou, Agency for Toxic Substances and Disease Registry, Atlanta,
GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood,
Cambridgeshire, United Kingdom
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers
and Veterinary Medicine, Berlin, Germany
Dr J. Kielhorn, Fraunhofer Institute for Toxicology and Aerosol
Research, Hannover, Germany
Dr S. Kristensen, National Occupational Health and Safety Commission
(Worksafe), Sydney, NSW, Australia
Mr C. Lee-Steere, Environment Australia, Canberra, ACT, Australia
Ms M. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada
Ms F. Rice, National Institute for Occupational Safety and Health,
Cincinnati, OH, USA
Dr J. Sekizawa, National Institute of Health Sciences, Tokyo, Japan
Dr D. Willcocks, National Industrial Chemicals Notification and
Assessment Scheme (NICNAS), Sydney, NSW, Australia (Chairperson)
Professor P. Yao, Institute of Occupational Medicine, Chinese Academy
of Preventive Medicine, Beijing, People's Republic of China
Observers
Mr P. Howe, Institute of Terrestrial Ecology, Huntingdon,
Cambridgeshire, United Kingdom
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit, GmbH, Oberschleissheim, Germany
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Ms M. Godden, Health and Safety Executive, Bootle, Merseyside, United
Kingdom
Dr M. Younes, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
APPENDIX 4 -- INTERNATIONAL CHEMICAL SAFETY CARD
QUARTZ ICSC: 0808
October 1997
CAS# 14808-60-7 Crystalline silica, quartz
RTECS# VV7330000 Crystalline silicon dioxide, quartz
Silicic anhydride
SIO2
Molecular mass: 60.1
TYPES OF HAZARD ACUTE HAZARDS/ PREVENTION FIRST AID / FIRE
/ EXPOSURE SYMPTOMS FIGHTING
FIRE Not combustible. In case of fire in the
surroundings, all
extinguishing agents allowed.
EXPLOSION
EXPOSURE PREVENT DISPERSION
OF DUST!
Inhalation Cough. Local exhaust
or breathing
protection.
Skin
Eyes Safety goggles,
or eye protection
in combination with
breathing protection.
Ingestion
SPILLAGE DISPOSAL PACKAGING & LABELLING
Sweep spilled substance into containers; EU Classification
if appropriate, moisten first to prevent UN Classification
dusting. Wash away remainder with plenty
of water. (Extra personal protection:
P3 filter respirator for toxic particles).
EMERGENCY RESPONSE STORAGE
IMPORTANT DATA
PHYSICAL STATE; APPEARANCE: ROUTES OF EXPOSURE:
COLOURLESS WHITE OR VARIABLE BLACK, PURPLE, The substance can be absorbed
GREEN CRYSTALS into the body by inhalation.
CHEMICAL DANGERS: INHALATION RISK:
Reacts with strong oxidants causing fire Evaporation at 20°C is negligible;
and explosion hazard. a harmful concentration of airborne
particles can, however, be reached
quickly when dispersed.
OCCUPATIONAL EXPOSURE LIMITS: EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
TLV: 0.1 mg/m3 (respirable dust) The substance may have effects on the lungs,
(ACGIH 1997). resulting in fibrosis (silicosis). This substance
MAK: 0.15 mg/m3; (1996) is carcinogenic to humans.
PHYSICAL PROPERTIES
Boiling Point: 2230°C
Melting Point: 1610°C
Relative density (water = 1): 2.6
Solubility in water: none
ENVIRONMENTAL DATA
NOTES
Depending on the degree of exposure, periodic medical examination is indicated. CSQZ, DQ 12,
MIN-U-Sil, Sil-Co-Sil, Snowit, Sykron F300, Sykron F600 are trade names.
ADDITIONAL INFORMATION
LEGAL NOTICE Neither the CEC nor the IPCS nor any person acting on
behalf of the CEC or the IPCS is responsible for the use
which might be made of this information.
RÉSUMÉ D'ORIENTATION
Le présent CICAD, relatif à la silice cristallisée ou quartz, est
basé sur trois documents résultant d'un examen approfondi par des
pairs des effets sanitaires de cette substance, à savoir : 1) une mise
au point portant sur les études et rapports consacrés aux effets
nocifs d'une exposition de l'organisme humain au quartz (NIOSH, à
paraître); 2) une revue critique des études de cancérogénicité
effectuées par le Centre international de recherche sur le cancer
(IARC/CIRC, 1997) et 3) un examen des études consacrées aux effets
sanitaires du quartz présent dans le milieu ambiant, à l'exclusion des
cancers (US EPA, 1996). Ces différentes sources documentaires
n'accordant pas la même attention aux divers points d'aboutissement
toxicologiques, on s'est efforcé, dans ce CICAD, de prendre en compte
l'ensemble des effets indésirables mentionnés dans les documents de
base. Il est a noter à cet égard, que même si les trois documents ne
traitent pas tous les effets de manière aussi détaillée, leurs
conclusions finales n'en sont pas moins très voisines. On a utilisé
plusieurs bases de données en ligne pour effectuer une recherche
bibliographique très complète. Le dernier dépouillement remonte à mars
1999.
Le présent CICAD porte sur la forme la plus commune de silice
cristallisée, c'est-à-dire le quartz. Il ne prend pas en compte les
travaux résultant des études expérimentales consacrées aux effets des
différentes autres formes de silice cristallisée comme la
cristobalite, la tridymite, la stishovite ou la coésite, ni à d'autres
variétés comme la terre d'infusoires ou la silice amorphe ni même à la
poussière de charbon car leur toxicité in vitro est différente ce
celle du quartz. Une ancienne étude effectuée sur des rats vivants a
montré que l'induction de la fibrogénicité était différentes selon les
différentes formes : quartz, cristobalite ou tridymite. Il n'existe
toutefois pratiquement aucune étude expérimentale qui ait procédé à
une évaluation systématique du risque que représentent des matériaux
identiques à ceux auxquels l'Homme est exposé. Selon le groupe d'étude
du CIRC, il est possible que les différentes formes cristallines de
silice n'aient pas toutes le même pouvoir cancérogène. Il est vrai que
dans beaucoup d'études, l'évaluation a porté sur les
« environnements mixtes » dans lesquels le quartz a pu être chauffé -
ce qui est susceptible d'avoir produit une conversion en cristobalite
ou en tridymite dans des proportions diverses (par ex. dans
l'industrie de la céramique, de la poterie et de la brique
réfractaire) - et on n'a pas fait de distinction entre l'exposition au
quartz et l'exposition à la cristobalite, par exemple. On peut penser,
au vu de certaines données, que le risque de cancer varie selon le
type d'industrie et de procédé, et ce, selon des modalités qui
laissent supposer que ce risque est spécifique de chacune des formes
cristallines, mais le Groupe n'a pu parvenir à une conclusion que dans
le cas précis du quartz et de la cristobalite. Ce CICAD reprend
l'exposé et les conclusions du document de ce Groupe, aussi
l'évaluation qu'il donne de la cancérogénicité du quartz sur le lieu
de travail ne fait-elle pas de distinction entre les études
épidémiologiques portant sur le quartz et celles qui sont consacrées à
la cristobalite.
Il a été convenu que l'examen par des pairs de la littérature
consacrée au quartz en vue de la rédaction du présent CICAD
comporterait un volet particulier, consistant en une étude critique
par un groupe international de spécialistes choisis en fonction de
leur connaissance des controverses actuelles au sujet du quartz. On
trouvera à l'appendice 1 des indications sur la nature de l'examen par
des pairs et sur les sources documentaires existantes. Des indications
sur l'examen par des pairs du présent CICAD figurent à l'appendice 2.
Ce CICAD a été approuvé en tant qu'évaluation internationale lors de
la réunion du Comité d'évaluation finale qui s'est tenue à Sydney
(Australie) du 21 au 24 novembre 1999. La liste des participants à
cette réunion figure à l'appendice 3. La fiche d'information
internationale sur la sécurité chimique (ICSC No 0808) relative à la
silice cristallisée est également reproduite dans l'appendice 4 (IPCS,
1993).
Le quartz (No CAS 14808-60-7) est un constituant solide souvent
présent dans la plupart des poussières minérales naturelles.
L'exposition humaine au quartz est la plupart du temps liée à des
activités professionnelles qui impliquent le déplacement de masses de
terre, la manipulation de matériaux qui contiennent de la silice
(pierre à bâtir ou béton par ex.); elle peut aussi se produire lors de
l'utilisation ou de la fabrication de produits à base de silice. Il
peut y avoir exposition à la poussière de quartz présente dans
l'environnement lors d'activités naturelles, industrielles ou
agricoles. Les particules de quartz respirables peuvent être inhalées
et se déposer dans les poumons; toutefois on n'a aucune certitude au
sujet de la cinétique d'élimination des particules de quartz chez
l'Homme.
Le quartz provoque une inflammation cellulaire in vivo. Des
études expérimentales à court terme sur des rats, des souris et des
hamsters ont montré que l'instillation intratrachéenne de particules
de quartz conduisait à la formation de nodules silicotiques discrets.
L'inhalation d'aérosols de particules de quartz gêne la fonction de
nettoyage alvéolaire des macrophages et conduit à des lésions
évolutives et à une pneumopathie inflammatoire. Un stress oxydatif
(c'est-à-dire la formation accrue de radicaux hydroxyles et d'espèces
oxygénées ou azotées réactives) a été observé chez des rats après
instillation intratrachéenne ou inhalation de particules de quartz. De
nombreuses études expérimentales in vitro ont permis de constater
que les propriétés de surface des particules de silice cristallisées
influent sur leur activité fibrogène et sur un certain nombre de
caractéristiques de leur activité cytotoxique. De nombreux mécanismes
possibles sont décrits dans la littérature, mais en fait les lésions
cellulaires provoquées par le quartz sont le résultat de mécanismes
complexes dont la nature n'est pas encore totalement élucidée.
Les études d'inhalation à long terme effectuées sur des rats et
des souris montrent que les particules de quartz entraînent une
prolifération cellulaire, la formation de nodules, la dépression des
fonctions immunitaires et une protéinose alvéolaire pulmonaire. Des
études expérimentales sur des rats ont permis de mettre en évidence la
formation d'adénocarcinomes et de carcinomes spinocellulaires
consécutive à l'inhalation ou à l'instillation intratrachéenne de
particules de quartz. Les études sur le hamster ou la souris n'ont pas
permis de mettre en évidence ce genre de tumeurs pulmonaires. On ne
dispose pas de données dose-réponse (par ex. dose sans effet nocif ou
dose minimale produisant un effet nocif) satisfaisantes pour le rat ou
d'autres rongeurs car peu d'études de cancérogénicité comportant une
variété de doses ont été effectuées.
Les tests de mutagénicité classique sur bactérie ne donnent pas
de résultat positif. Les résultats des études de génotoxicité sont
contradictoires et il n'a pas été possible de confirmer ou d'infirmer
l'existence d'un effet génotoxique direct.
Les résultats d'études sur les particules peuvent donner des
résultats variables selon le matériau testé, la granulométrie, la
concentration administrée aux animaux et l'espèce utilisée. Les essais
effectués avec des particules de quartz ont porté sur diverses espèces
avec échantillons d'origine, de concentration et de granulométrie
variée, autant de facteurs qui ont pu avoir une influence sur les
observations.
On ne dispose pas de données concernant les effets que le quartz
pourrait avoir sur la reproduction ou le développement chez des
animaux d'expérience.
Les effets indésirables du quartz sur les organismes aquatiques
et les mammifères terrestres n'ont pas été étudiés non plus.
Il existe beaucoup d'études épidémiologiques portant sur des
cohortes de divers professionnels exposés à des poussières respirables
de quartz. L'exposition professionnelle à la poussière de quartz est
associée à la silicose, au cancer du poumon et à la tuberculose
pulmonaire. Le CIRC a classé la silice cristallisée (quartz ou
cristobalite) inhalée sur le lieu de travail dans le groupe 1 des
produits dont la cancérogénicité pour l'Homme et l'animal repose sur
des preuves suffisantes. Dans son évaluation générale de cette
substance, le Groupe de travail a noté qu'aucun signe de
cancérogénicité n'avait été relevé au cours de toutes ses enquêtes
dans l'industrie. Il est possible que la cancérogénicité de la silice
cristallisée dépende de certaines propriétés intrinsèques de cette
substance ou encore de facteurs extérieurs susceptibles d'influer sur
son activité biologique ou sur la proportion relative des différentes
formes (IARC/CIRC, 1997).
On a fait état d'une augmentation statistiquement significative
des décès ou des cas de bronchite, d'emphysème, de
broncho-pneumopathie chronique obstructive, de maladies à composante
auto-immune (comme la sclérodermie, l'arthrite rhumatoïde ou le lupus
érythémateux disséminé) ou encore de néphropathie.
En ce qui concerne l'identification du risque et la relation
exposition-réponse, c'est la silicose qui constitue l'effet
déterminant. On possède suffisamment de données épidémiologiques pour
permettre une évaluation quantitative du risque de silicose, mais pas
pour donner une estimation précise du risque des autres effets
sanitaires indiqués plus haut (une évaluation groupée utilisant des
études épidémiologiques consacrées à la silice et au cancer du poumon
est en cours au CIRC).
Il y a de grandes variations (par ex. 2 à 90 %) dans les
estimations du risque relatives à la prévalence de la silicose par
suite d'une exposition tout au long de la vie professionnelle à des
particules respirables de quartz de concentration comprise entre 0,05
et 0,10 mg/m3 sur le lieu de travail. En ce qui concerne l'exposition
aux particules présentes dans l'air ambiant de l'environnement
général, une analyse de référence conclut que le risque de silicose
pour une exposition continue pendant 70 ans à 0,008 mg/m3 (c'est à
dire la valeur estimative élevée de la concentration de silice
cristallisée en milieu urbain aux États-Unis), est inférieur à 3 %
pour les individus en bonne santé ne souffrant pas de pathologie
respiratoire (US EPA, 1996). Le risque de silicose pour les personnes
exposées dans les même conditions mais présentant une pathologie
respiratoire n'a pas été évalué.
Il existe un certain nombre d'incertitudes concernant
l'évaluation des études épidémiologiques et l'estimation du risque
d'effets toxiques résultant de l'exposition à des poussières de
quartz. Les difficultés, qui pour une grande part sont liées à l'étude
même des affections respiratoires dans les diverses professions,
tiennent au nombre et à la qualité des données longitudinales
d'exposition, à l'insuffisance des informations sur les facteurs de
confusion possibles - comme le tabagisme à la cigarette, par ex. - et
à l'interprétation des radiographies thoraciques en tant que preuves
de l'exposition. En outre, l'exposition professionnelle à la poussière
de quartz est complexe car les travailleurs sont fréquemment exposés à
des mélanges qui contiennent du quartz à côté d'autres types de
substances minérales. Les propriétés de ces poussières (par ex. la
granulométrie, les caractéristiques de surface, la forme cristalline)
peuvent varier selon leur origine géologique et se même se modifier
lors des divers traitements industriels. Ces variations peuvent
influer sur l'activité biologique des poussières inhalées. Le Groupe
de travail du CIRC a évalué la cancérogénicité de la silice
cristallisée (quartz, notamment) et a concentré son attention sur les
études épidémiologiques les moins susceptibles d'être affectées par
des facteurs de confusion et des biais de sélection et il en a tiré
des relations dose-réponse (IARC/CIRC, 1997).
RESUMEN DE ORIENTACI²N
Este CICAD sobre la sílice cristalina, el cuarzo, se basa en los
tres amplios documentos siguientes objeto de un examen colegiado sobre
los efectos en la salud de la sílice cristalina, incluido el cuarzo:
1) un examen de los estudios e informes publicados sobre los efectos
adversos en la salud humana de la exposición al cuarzo (NIOSH, de
próxima aparición), 2) un examen de los estudios de carcinogenicidad
realizados por el Centro Internacional de Investigaciones sobre el
Cáncer (IARC/CIIC, 1997) y 3) un examen de los efectos en la salud
distintos del cáncer del cuarzo presente en el medio ambiente (US EPA,
1996). En los documentos originales se prestó una atención diferente a
los distintos efectos finales, y el CICAD se elaboró a fin de evaluar
todos los efectos adversos para la salud identificados en esos
documentos. Hay que señalar que, a pesar de esas diferencias, las
conclusiones finales de todos los documentos originales fueron muy
semejantes. Se realizó una búsqueda bibliográfica amplia de varias
bases de datos en línea. El presente examen contiene los datos
obtenidos hasta marzo de 1999.
En este CICAD se examina la forma más común de sílice cristalina
(es decir, el cuarzo). No se tienen en cuenta los estudios
experimentales de los efectos de otras formas de sílice cristalina
(por ejemplo, la cristobalita, la tridimita, la estishovita o la
coesita), el polvo de carbón, la tierra de diatomeas o la sílice
amorfa, porque su toxicidad in vitro es diferente de la del cuarzo .
En un estudio preliminar en ratas in vivo se pusieron de manifiesto
diferencias en la capacidad de inducción de fibrogenicidad del cuarzo,
la cristobalita y la tridimita. Sin embargo, apenas existen estudios
experimentales en los que se evalúe de manera sistemática exactamente
el mismo material al que está expuesto el ser humano. El Grupo de
Trabajo del CIIC examinó la posibilidad de que hubiera diferencias en
el potencial carcinogénico entre los distintos tipos polimórficos de
la sílice cristalina. Sin embargo, en algunos de los estudios
epidemiológicos se evaluó la presencia de cáncer de pulmón entre los
trabajadores de "entornos mixtos", donde el cuarzo se puede calentar y
se pueden producir diversos grados de conversión a cristobalita o
tridimita (por ejemplo, en las industrias de cerámica, alfarería y
ladrillos refractarios) y no se describieron específicamente las
exposiciones al cuarzo o la cristobalita. Aunque hubo algunos indicios
de que los riesgos de cáncer variaban en función de la industria y el
proceso, de manera que parecía indicar riesgos específicos de los
tipos polimórficos, el Grupo de Trabajo pudo llegar solamente a una
conclusión única para el cuarzo y la cristobalita. El CICAD se hace
eco del debate y la conclusión de ese documento original; por
consiguiente, al examinar la carcinogenicidad del cuarzo en el entorno
ocupacional no se distingue entre los estudios epidemiológicos del
cuarzo y los de la cristobalita.
El proceso de examen colegiado para el presente CICAD tenía por
objeto incluir el examen de un grupo internacional de expertos
seleccionados por sus conocimientos acerca de las controversias y las
cuestiones actuales en relación con el cuarzo. La información relativa
al carácter del examen colegiado y a la disponibilidad de los
documentos originales figura en el apéndice 1. La información relativa
al examen colegiado de este CICAD se presenta en el apéndice 2. Este
CICAD se aprobó como evaluación internacional en una reunión de la
Junta de Evaluación Final celebrada en Sydney, Australia, los días
21-24 de noviembre de 1999. En el apéndice 3 figura la lista de
participantes en esta reunión. La Ficha internacional de seguridad
química (ICSC 0808) para la sílice cristalina, cuarzo (IPCS, 1993),
también se reproduce en el apéndice 4.
El cuarzo (CAS No 14808-60-7) es un componente sólido
presente con frecuencia en la gran mayoría de los tipos de polvo
mineral natural. La exposición humana al cuarzo se produce sobre todo
durante las actividades laborales que requieren el desplazamiento de
tierra, la alteración de productos con sílice (por ejemplo, obras de
albañilería, hormigón) o el uso o fabricación de productos con sílice.
Se puede producir exposición al polvo de cuarzo presente en el medio
ambiente durante la realización de actividades físicas, industriales y
agrícolas. Se pueden inhalar partículas de polvo de cuarzo
respirables, que se depositan en el pulmón; sin embargo, no se ha
llegado a ninguna conclusión acerca de su cinética de eliminación en
el ser humano.
El polvo de cuarzo induce inflamación celular in vivo. En
estudios experimentales de corta duración en ratas se ha observado que
la instilación intratraqueal de partículas de cuarzo da lugar a la
formación de nódulos silicóticos dispersos en ratas, ratones y
hámsteres. La inhalación de partículas de cuarzo pulverizadas
dificulta las funciones de limpieza de los macrófagos alveolares y
provoca lesiones progresivas y neumonitis. Se ha observado una tensión
oxidante (es decir, una mayor formación de radicales hidroxilo,
especies de oxígeno reactivo o especies de nitrógeno reactivo) en
ratas tras la instilación intratraqueal o la inhalación de cuarzo.
Numerosos estudios experimentales in vitro han puesto de manifiesto
que las características de la superficie de las partículas de sílice
cristalina influyen en su actividad fibrogénica y en varias
propiedades relacionadas con su citotoxicidad. Aunque en la
bibliografía se han descrito muchos mecanismos que posiblemente
contribuyan a esto, la manera en la cual las partículas de cuarzo
provocan el daño celular es compleja y no se conoce del todo.
En estudios de inhalación prolongados con ratas y ratones se ha
comprobado que las partículas de cuarzo inducen proliferación celular,
formación de nódulos, supresión de las funciones inmunitarias y
proteinosis alveolar. En estudios experimentales con ratas se notificó
la aparición de adenocarcinomas y carcinomas de las células escamosas
tras la inhalación o la instilación intratraqueal de cuarzo.
En experimentos con hámsteres o ratones no se observaron tumores en
los pulmones. No se dispone de datos adecuados sobre la relación
dosis-respuesta (por ejemplo, la concentración sin efectos adversos o
la concentración mas baja con efectos adversos) para ratas u otros
roedores, debido a que se han realizado escasos estudios de
carcinogenicidad con dosis múltiples.
El cuarzo no dio resultado positivo en las valoraciones normales
de mutagénesis en bacterias. Los resultados de los estudios de
genotoxicidad del cuarzo son contradictorios y no se ha confirmado ni
descartado un efecto genotóxico directo.
En estudios experimentales de partículas, los resultados pueden
variar en función del material de prueba, el tamaño de las partículas,
la concentración administrada y la especie objeto de examen. En los
experimentos con partículas de cuarzo se utilizaron ejemplares de
varios orígenes, con diversidad de dosis, tamaños de partículas y
especies, que podrían haber afectado a las observaciones.
No se dispone de datos sobre los efectos reproductivos y en el
desarrollo del cuarzo en animales de laboratorio.
No se han estudiado los efectos adversos del cuarzo en los
organismos acuáticos y los mamíferos terrestres.
Hay numerosos estudios epidemiológicos de cohortes ocupacionales
expuestas a polvo de cuarzo respirable. La silicosis, el cáncer de
pulmón y la tuberculosis pulmonar son enfermedades asociadas con la
exposición ocupacional al polvo de cuarzo. El CIIC clasificó la sílice
cristalina (cuarzo o cristobalita) de procedencia ocupacional como
carcinógeno del grupo 1, basándose en pruebas suficientes de
carcinogenicidad en el ser humano y en los animales experimentales;
"al hacer la evaluación general, el Grupo de Trabajo observó que no se
había detectado carcinogenicidad en el ser humano en todas las
circunstancias industriales estudiadas. La carcinogenicidad puede
depender de características inherentes a la sílice cristalina o de
factores externos que afectan a su actividad biológica o a la
distribución de sus tipos polimórficos" (IARC/CIIC, 1997).
Se han notificado aumentos estadísticamente significativos de
muertes o casos de bronquitis, enfisema, enfermedad pulmonar
obstructiva crónica, enfermedades relacionadas con la autoinmunidad
(es decir, escleroderma, artritis reumatoide, lupus eritematoso
sistémico) y enfermedades renales.
La silicosis es el efecto decisivo para la determinación del
peligro y la evaluación de la relación exposición-respuesta. Hay datos
epidemiológicos suficientes para poder efectuar una estimación
cuantitativa del riesgo de silicosis, pero no estimaciones precisas de
los riesgos de otros efectos para la salud mencionados anteriormente.
(En el CIIC se está realizando una evaluación agrupada del riesgo de
estudios epidemiológicos de la sílice y el cáncer de pulmón.)
Las estimaciones del riesgo relativas a la prevalencia de la
silicosis para la exposición durante toda la vida laboral a
concentraciones de polvo de cuarzo respirable de 0,05 ó 0,10 mg/m3 en
el entorno de trabajo varían ampliamente (es decir, 2%-90%). Con
respecto a la exposición al cuarzo presente en el medio ambiente
general, a partir del análisis de una dosis de referencia se
pronosticó que el riesgo de silicosis para una exposición continua
durante una vida de 70 años a 0,008 mg/m3 (concentración de sílice
cristalina estimada alta en las zonas metropolitanas de los Estados
Unidos) era inferior al 3% para las personas sanas sin otras
enfermedades o trastornos del aparato respiratorio y para el medio
ambiente (US EPA, 1996). No se evaluó el riesgo de silicosis para las
personas con enfermedades respiratorias expuestas al cuarzo en el
medio ambiente general.
Hay incertidumbres en la evaluación de los estudios
epidemiológicos y en la evaluación del riesgo de los efectos para la
salud relacionados con la exposición al polvo de cuarzo. Las
dificultades, muchas de las cuales son inherentes al estudio de las
enfermedades respiratorias en poblaciones ocupacionales, se deben a
limitaciones en la cantidad y la calidad de los datos históricos de
exposición, la falta de datos sobre posibles factores de confusión,
como por ejemplo el humo de los cigarrillos, y dificultades en la
interpretación de las radiografías del tórax como prueba de
exposición. Además, las exposiciones ocupacionales al polvo de cuarzo
son complejas, porque los trabajadores están expuestos con frecuencia
a mezclas de polvo que contienen cuarzo y otras variedades minerales.
Las propiedades del polvo (por ejemplo, el tamaño de las partículas,
las propiedades de la superficie, la forma cristalina) pueden variar
en función del origen geológico y también pueden cambiar durante la
elaboración industrial. Dichas variaciones pueden afectar a la
actividad biológica del polvo inhalado. El Grupo de Trabajo del CIIC
evaluó la carcinogenicidad de la sílice cristalina (incluido el
cuarzo) y se concentró en los estudios epidemiológicos con menor
probabilidad de verse afectados por sesgos de confusión y selección en
los que se analizaban las relaciones exposición-respuesta (IARC/CIIC,
1997).
Table 5: Genetic and related effects of silica.a
Test system Resultb Dose Reference
(LED/HID)c
DNA strand breaks, gamma-HindIII-digested DNA + 30 000d Daniel et al., 1993
DNA strand breaks, herring sperm genomic DNA + 10 000d Daniel et al., 1993
DNA strand breaks, gamma-HindIII-digested DNA + 9 500d Daniel et al., 1995
DNA strand breaks, PM2 supercoiled DNA + 9 500d Daniel et al., 1995
GIA, Gene mutation, hprt locus, rat RLE-6TN
alveolar epithelial cells in vitro - NG Driscoll et al., 1997
SIC, Sister chromatid exchange,
Chinese hamster V79-4 cells in vitro - 15e Price-Jones et al., 1980
SHL, Sister chromatid exchange, human lymphocytes in vitro - 100d Pairon et al., 1990
SIH, Sister chromatid exchange, human lymphocytes
and monocytes in vitro - 100d Pairon et al., 1990
MIA, Micronucleus test, Syrian hamster embryo cells in vitro - 18.75f Oshimura et al., 1984
MIA, Micronucleus test, Syrian hamster embryo cells in vitro + 70e Hesterberg et al., 1986
MIA, Micronucleus test, Chinese hamster lung fibroblasts
(V79) in vitro + 200g Nagalakshmi et al., 1995
CIC, Chromosomal aberrations, Chinese hamster lung
fibroblasts (V79) in vitro - 1 600g Nagalakshmi et al., 1995
CIS, Chromosomal aberrations, Syrian hamster embryo
cells in vitro - 18.75f Oshimura et al., 1984
Table 5 (cont'd)
Test system Resultb Dose Reference
(LED/HID)c
AIA, Aneuploidy, Chinese hamster lung cells (V79-4) in vitro - 15e Price-Jones et al., 1980
AIA, Aneuploidy, Syrian hamster embryo cells in vitro - 18.75f Oshimura et al., 1984
AIA, Tetraploidy, Syrian hamster embryo cells in vitro - 70e Hesterberg et al., 1986
TBM, Cell transformation, BALB/3T3/31-1-1 mouse cells in vitro + 30d,h,i Saffiotti & Ahmed, 1995
TBM, Cell transformation, BALB/3T3/31-1-1 mouse cells in vitro + 60j,k Saffiotti & Ahmed, 1995
TCS, Cell transformation, Syrian hamster embryo cells in vitro + 18e Hesterberg & Barrett, 1984
TCS, Cell transformation, Syrian hamster embryo cells in vitro + 70f Hesterberg & Barrett, 1984
TCL, Cell transformation, fetal rat lung epithelial cells
in vitro (+) NGd Williams et al., 1996
MIH, Micronucleus test, human embryonic lung (Hel 299)
cells in vitro + 800g Nagalakshmi et al., 1995
CIH, Chromosomal aberrations, human embryonic lung
(Hel 299) cells in vitro - 1 600g Nagalakshmi et al., 1995
DVA, 8-hydroxy-2'-deoxyguanosine DNA extract from
lung tissue, male Wistar rats + 50 × 1 itd Yamano et al., 1995
DVA, 8-hydroxy-2'-deoxyguanosine DNA extract from
peripheral blood leukocytes, male Wistar rats - 50 × 1 itd Yamano et al., 1995
GVA, Gene mutation, hprt locus, rat alveolar
epithelial cells in vivo + 100 × 1 it Driscoll et al., 1995
Table 5 (cont'd)
Test system Resultb Dose Reference
(LED/HID)c
GVA, Gene mutation, hprt locus, rat alveolar
epithelial cells in vivo + 5 × 2 it Driscoll et al., 1997
MVM, Micronucleus test, albino mice in vivo - 500 ip Vanchugova et al., 1985
SLH, Sister chromatid exchange, human lymphocytes in vivo + NG Sobti & Bhardwaj, 1991
CLH, Chromosomal aberrations, human lymphocytes in vivo + NG Sobti & Bhardwaj, 1991
BID, Calf thymus DNA binding in vitro + 200l Mao et al., 1994
ICR, Metabolic cooperation using 8-azaguanine-resistant
cells, Chinese hamster lung cells (V79-4) in vitro - 50 Chamberlain, 1983
a Source: IARC (1997). See Appendix 1 of IARC (1997), Test system code words, for definitions of
the abbreviations used in column 1.
b Results without exogenous metabolic system: +, positive; (+), weakly positive; -, negative.
c LED, lowest effective dose; HID, highest ineffective dose; in vitro tests, µg/ml; in vivo tests,
mg/kg body weight per day; NG, not given; it, intratracheal; ip, intraperitoneal.
d Min-U-Sil 5.
e Min-U-Sil unspecified.
f alpha-Quartz.
g Min-U-Sil 5 and Min-U-Sil 10.
h Min-U-Sil 5, hydrofluoric acid-etched.
i A Chinese standard quartz sample.
j DQ 12, a standard German quartz sample.
k F600 quartz.
l Min-U-Sil 5 or Chinese standard quartz.
Some studies have demonstrated the ability of quartz to induce
micronuclei in mammalian cells in culture (i.e., Oshimura et al.,
1984; Hesterberg et al., 1986; Nagalakshmi et al., 1995) and were
reviewed by IARC (Table 5). However, other in vitro studies did not
observe chromosomal aberration (Oshimura et al., 1984;
Nagalakshmi et al., 1995), hprt (hypoxanthine-guanine phosphoribosyl
transferase) gene mutation (Driscoll et al., 1997), or aneuploid or
tetraploid cells (Price-Jones et al., 1980; Oshimura et al., 1984;
Hesterberg et al., 1986).
Pairon et al. (1990) tested quartz (i.e., Min-U-Sil 5) particles
for their ability to induce a significant number of sister chromatid
exchanges in cultures of human lymphocytes plus monocytes or of human
purified lymphocytes. The results were not "clear cut" for any of the
three doses tested (i.e., 0.5, 5.0, and 50 µg/cm2) (Pairon et al.,
1990) (Table 5).
An in vivo treatment of rats with quartz induced mutation in
rat alveolar epithelial cells (Table 5) (Driscoll et al., 1995, 1997).
Nehls et al. (1997) reported results of tests for DNA modifications by
quartz that were not reviewed by IARC (1997). Quartz (2.5 mg of DQ 12
suspended in 0.5 ml of physiological saline) or corundum (2.5 mg), a
non-carcinogenic particle, was intratracheally instilled into the
lungs of Wistar rats (10 rats per exposure and per time period).
Control animals were exposed to saline solution or not treated. Rats
were sacrificed 7, 21, and 90 days after treatment, then lung tissue
sections were analysed with immunocytological assay to determine the
level of 8-hydroxydeoxyguanosine in DNA extracts. Reactive oxygen
species can induce 8-hydroxydeoxyguanosine and other mutagenic DNA
oxidation products, which may be converted to mutations in
proliferating cells (Nehls et al., 1997). Exposure to quartz induced
levels of 8-hydroxydeoxyguanosine in the DNA of alveolar lung cells
that were significantly higher (P-value not reported) at all time
points than levels found in cells of untreated rats or rats treated
with corundum or saline. The number of total cells in bronchoalveolar
lavage fluid was 3-4 times higher in the quartz-treated groups at all
time points than in corundum-exposed rats and control rats exposed to
saline.
Other in vivo studies not reviewed by IARC (1997) found that
quartz induced micronuclei in pulmonary alveolar macrophages of male
Wistar rats in a time-dependent (Leigh et al., 1998b) and
dose-dependent manner (Wang et al., 1997b).
In summary, results of genotoxicity studies of quartz conflict,
and a direct genotoxic effect for quartz has not been confirmed or
ruled out.
8.5 Reproductive and developmental toxicity
There are no data available on the reproductive or developmental
effects of quartz in laboratory animals (IARC, 1997).
8.6 Immunological and neurological effects
Data on the neurological effects of quartz have not been
identified. In vitro studies have shown that quartz can stimulate
release of cytokines and growth factors from macrophages and
epithelial cells, and there is evidence that these events may occur
in vivo and contribute to disease (IARC, 1997). The immunological
response to quartz in experimental animals is a complex subject with
uncertain implications for humans, and detailed reviews are available
elsewhere (i.e., Davis, 1991, 1996; Haslam, 1994; Heppleston, 1994;
Weill et al., 1994; Driscoll, 1996; Gu & Ong, 1996; Hook & Viviano,
1996; Iyer & Holian, 1996; Kane, 1996; Sweeney & Brain, 1996;
Weissman et al., 1996; Mossman & Churg, 1998).
9. EFFECTS ON HUMANS
9.1 Case reports
There are many published case reports of adverse health effects
from occupational exposure to quartz. These health effects include
silicosis (acute and chronic) and lung cancer. Case reports of
silicosis and lung cancer are not mentioned further, because these
diseases have been researched in depth in epidemiological studies
(section 9.2).
There are numerous published case reports of several autoimmune
disorders in workers or patients who had been occupationally exposed
to crystalline silica, including quartz dust (NIOSH, forthcoming). The
most frequently noted autoimmune diseases in those reports were
scleroderma, systemic lupus erythematosus (i.e., lupus), rheumatoid
arthritis, autoimmune haemolytic anaemia (Muramatsu et al., 1989), and
dermatomyositis or dermatopolymyositis (Robbins, 1974; Koeger et al.,
1991). Case reports have also described health effects that may be
related to the immunological abnormalities observed in patients with
silicosis, such as chronic renal disease (Saita & Zavaglia, 1951;
Giles et al., 1978; Hauglustaine et al., 1980; Bolton et al., 1981;
Banks et al., 1983; Slavin et al., 1985; Bonnin et al., 1987;
Osorio et al., 1987; Arnalich et al., 1989; Sherson & Jorgensen, 1989;
Dracon et al., 1990; Pouthier et al., 1991; Rispal et al., 1991;
Neyer et al., 1994; Wilke et al., 1996), ataxic sensory neuropathy
(Tokumaru et al., 1990), chronic thyroiditis (Masuda, 1981),
hyperthyroidism (i.e., Graves' disease) (Koeger et al., 1996),
monoclonal gammopathy (Fukata et al., 1983, 1987; Aoki et al., 1988),
and polyarteritis nodosa (Arnalich et al., 1989).
9.2 Epidemiological studies
9.2.1 Silicosis
Most, if not all, of the several hundred epidemiological studies
of exposure to quartz dust are studies of occupational cohorts. The
majority of studies investigated the occurrence of silicosis morbidity
or mortality. These studies have conclusively linked occupational
quartz dust exposure with silicosis. Silicosis (i.e., nodular
pulmonary fibrosis) is a fibrotic lung disease, sometimes
asymptomatic, that is caused by the inhalation and deposition of
respirable crystalline silica particles (i.e., particles <10 µm in
diameter) (Ziskind et al., 1976; IARC, 1987).
A worker may develop one of three types of silicosis, depending
on the airborne concentration of respirable crystalline silica: (1)
chronic silicosis, which usually occurs after 10 or more years of
exposure at relatively low concentrations; (2) accelerated silicosis,
which develops 5-10 years after the first exposure; or (3) acute
silicosis, which develops after exposure to high concentrations of
respirable crystalline silica and results in symptoms within a few
weeks to 4 or 5 years after the initial exposure (Ziskind et al.,
1976; Peters, 1986; NIOSH, 1992a,b, 1996). Acute silicosis is a risk
for workers with a history of high exposures from performing
occupational processes that produce small particles of airborne dust
with a high silica content, such as during sandblasting, rock
drilling, or quartz milling, or any other process with high exposures
to small particles of airborne dust with a high quartz content
(Davis, 1996).
A recent study of 67 paraffin-embedded lung tissue samples from
silicotic patients found a significant linear relationship
(P <0.001) between lung quartz concentration and silicosis severity in
gold miners; although several types of mineral particles were found in
the lungs, quartz was the only significant indicator of silicosis
severity. The silicosis cases included 39 patients without lung cancer
and 28 patients with lung cancer. All of the cases were gold miners in
Canada (Dufresne et al., 1998a,b).
The epidemiological studies of silicosis usually define the
profusion of small opacities present in the disease according to a
standard system used by trained readers and developed by the
International Labour Organization for classification of chest
radiographs of pneumoconioses (ILO, 1980). Each reader assesses the
profusion according to a 12-point scale of severity. Categories 0/-
and 0/0 are the first and second points on the scale and represent a
normal chest radiograph. The third point, category 0/1, represents the
borderline between normality and abnormality, and category 1/0, the
fourth point, represents definite, but slight, abnormality
(Love et al., 1994). The shape (rounded or irregular) and size of the opacities
can also be described by the readers.
The critical studies of chronic silicosis, a progressive disease,
are those occupational epidemiological studies where (1) quantitative
quartz exposure data were available and used for risk analysis,
(2) exposure-response relationships were investigated, or (3) the
exposure-response relationships were documented with sufficient detail
for a health effects benchmark, including (4) application of data to
mathematical models that predicted silicosis prevalence at increasing
concentrations of cumulative quartz exposure. (The predicted
prevalences reported in the studies are discussed in section 11.1.3.)
Studies that selected workers from a broad spectrum of occupations and
included many workers that were exposed to different combinations of
various minerals, such as studies of "dusty trades" workers
(i.e., Rice et al., 1986), were excluded from consideration for risk
assessment of quartz and silicosis. Epidemiological studies that
provided evidence of an exposure-response relationship for silica and
silicosis based on other kinds of exposure data (e.g., there is a
positive relationship between development of chronic silicosis and
duration of exposure) have been reviewed elsewhere (WHO, 1986;
Goldsmith, 1994; Hughes, 1995; Rice & Stayner, 1995; Seaton, 1995;
Steenland & Brown, 1995a; Davis, 1996; US EPA, 1996).
The two critical cross-sectional studies (i.e., Kreiss & Zhen,
1996; Rosenman et al., 1996 -- see Table 6) found that the prevalence
of radiographic silicosis (ILO category >1/0 or >1/1) was
dose-related. That is, the prevalence of radiographic silicosis
increased with average silica dust exposure, cumulative quartz
exposure, duration of employment, or all of these measures. The actual
prevalences varied greatly among the studies, and conclusions
concerning quartz dust concentrations that may or may not induce
silicosis cannot be drawn from simple "eyeball" analysis of the
prevalences in the following two worker populations:
* Kreiss & Zhen (1996) conducted a community-based random sample
survey of 134 male residents at least 40 years old, living in a
hardrock (i.e., molybdenum, lead, zinc, and gold) mining town in
Colorado, USA. Of the 134 residents, 100 were silica-exposed
hardrock miners (including 32 silicosis cases) and 34 were
community "controls" without occupational dust exposure. Nearly
all (97%) of the dust-exposed subjects were 20 years since first
exposure. The estimated crystalline silica content (polymorph not
reported) of the total dust was 12.3%. Exposure was assessed with
information from occupational histories, gravimetric dust
exposure data from 1974-1982, and a cumulative silica exposure
index. Pre-1974 exposure estimates were based on job-specific
gravimetric data collected after 1974. Exposures were also
estimated for mines where no exposure data were available (17.1%
of person-years of follow-up). Thirty-two per cent of the 100
dust-exposed subjects had silicosis (defined as radiological
profusion of small opacities of ILO category >1/0). Prevalence
of silicosis was related to average silica dust exposure. Among
the 94 dust-exposed subjects with data on cumulative and average
dust exposures, those subjects with average silica exposure
<0.05 mg/m3 had 10% prevalence of silicosis; subjects with
>0.05-0.10 mg/m3 had a prevalence of 22.5%; subjects with
greater than 0.10 mg/m3 average silica exposure had a silicosis
prevalence of 48.6% (P = 0.01). (See Table 6 for predicted
prevalences when silicosis was defined as radiological profusion
of small opacities of ILO category >1/1.) It is not known
whether the small sample of 134 residents was representative of
all miners or if the exposure estimates for mines where no
exposure data were available (17.1% of person-years of follow-up)
were representative.
* Rosenman et al. (1996) conducted a cross-sectional study in 1991
of 549 current, 497 retired, and 26 current salaried workers that
were former production workers in a US grey iron foundry that
produced automotive engine blocks (total workers = 1072).
Twenty-eight cases (2.9%) of silicosis, defined as rounded
opacities >ILO category 1/0, were identified by at least two
of three "B" readers of a total of 952 chest radiographs. More
than half (18/28) of the cases were found in retired workers.
Silicosis prevalence was positively related to mean silica (i.e.,
quartz) exposure (P < 0.0001). Of the workers with mean quartz
exposure less than 0.05 mg/m3, 0.8% had silicosis, while 6.3%
of foundry workers with mean quartz exposure greater than 0.45
mg/m3 had silicosis. Silicosis prevalence also increased with
years of employment at the foundry, cumulative silica exposure,
work area within the foundry, and cigarette smoking (i.e., smoker
vs. non-smoker). Exposure estimates were derived from conversions
of "early silica exposure data" collected by impingers. Quartz
content of total dust was not reported. Weighted total dust
exposure from impinger data was converted to an estimate of
silica exposure in mass units (mg/m3) by multiplying it by the
average percentage of quartz in bulk samples.
Results of cohort studies of gold miners in South Africa, Canada,
and the USA (see Table 7) also demonstrated an exposure-response
relationship for radiographic silicosis (US EPA, 1996):
* A cohort study was conducted of 2235 white South African
underground gold miners, 45-54 years old at the time of medical
examination in 1968-1971, who started working after 1938, worked
>10 years, and were followed until 1991 (Hnizdo &
Sluis-Cremer, 1993). More than 300 (n = 313) of the 2235 miners
were followed to the time when radiological signs developed, 658
miners were followed up to death, and 1264 miners were followed
to the year of the most recent radiograph. Radiographs were read
blindly by two independent readers. Silicosis was defined as the
presence of rounded opacities of ILO category >1/1.
Radiographs were read blindly by two readers initially, then one
reader was chosen because his readings more closely matched the
autopsy data. Mean respirable dust concentrations, after heat and
acid treatment, in milligrams per cubic metre per shift were
calculated for nine gold mining occupations. The concentrations
were based on a study of shift-long dust exposure that measured
the surface area of the respirable mine dust and the number of
respirable particles (i.e., incombustible and acid-insoluble dust
particles) per cubic metre in a random sample of 20 South African
gold mines (Beadle, 1965, 1971). After heat and acid treatment,
the respirable dust in South African gold mines was found to
contain about 30% quartz (Beadle & Bradley, 1970). Cumulative
dust exposure for the miners was calculated in milligrams per
cubic metre-year by using data for mean mass respirable dust
concentrations for the nine occupational categories, the average
number of hours underground, and the number of dusty 8-h shifts.
Of the 2235 miners studied by Hnizdo & Sluis-Cremer (1993),
313 developed radiologically diagnosed silicosis (rounded
opacities with profusion of ILO category >1/1) during the
follow-up period (i.e., 1968-1971 to 1991). The onset of
silicosis occurred after an average (i.e., mean) of 27 years of
Table 6: Predicted prevalence of silicosis (ILO category >1/1)
following exposure to respirable quartz dust based on modelling of cumulative exposure
at mean concentrations of 0.05 or 0.10 mg/m3 over a 45-year working lifetime.
Cross-sectional study Mean concentration of Predicted prevalence of Cohort's mean time Cohort's maximum time
and cohort respirable quartz dust silicosis, ILO category since first quartz since first quartz
(mg/m3) >1/1 (cases per exposure (years) exposure (years)
100 workers)
Kreiss & Zhen, 1996 0.05 approx. 30a silicotic miners: silicotic miners:
41.6 66
100 US hardrock 0.10 approx. 90a non-silicotic non-silicotic
miners and 34 miners: 33.5 miners: 68
community controls
Rosenman et al., 0.05 2b,c 28 >30
1996
1072 US grey 0.10 3b,c
iron foundry workers
a Based on cumulative silica exposure model with 10 years of post-employment follow-up.
b ILO category >1/0.
c Based on a 40-year working lifetime and controlling for pack-years of cigarette smoking, race,
and silica exposure other than in the foundry under study.
Table 7: Predicted number of silicosis cases (ILO category >1/1) following exposure to respirable
quartz dust based on modelling of cumulative exposure at mean concentrations of 0.05 or 0.10 mg/m3
over a 45-year working lifetime.
Cohort study Mean concentration of Silicosis cases, Mean time since first Maximum time since
and population respirable quartz ILO category >1/1, quartz exposure (years) first quartz exposure
dust (mg/m3) per 100 workers (years)
Hnizdo & 0.05 13a silicotic miners: 36 silicotic miners: 50
Sluis-Cremer, 1993
2235 South African 0.10 approx. 70
gold miners
Muir et al., 0.05 0.09-0.62a,b 18 silicotic miners: 38
1989a,b;
Muir, 1991
2109 Canadian
gold and
uranium miners
Steenland & 0.05 10c 37 73d
Brown, 1995a
3330 US gold 0.09 47c
miners
a Estimate was reported in Rice & Stayner (1995).
b No post-employment follow-up and no retired miners included. The range includes five estimates
(one for each reader).
c The predicted number of silicosis cases does not account for effects of age or calendar time
(K. Steenland, personal communication, 1997).
d K. Steenland, personal communication, 1998.
net service, at a mean age of 56 years. For more than half of the
miners (n = 178; 57%), the onset occurred an average of
7.4 years (standard deviation 5.5; range 0.1-25 years) after
their employment at the mines, at 59 years of age (range
44-74 years). For the other miners (n = 135; 43%), the onset
of silicosis occurred while they were still mining, at 51 years
of age (range 39-61 years). These results show that the majority
of the cases occurred in miners who were no longer employed at
the mine and who were at least 50 years old
(Hnizdo & Sluis-Cremer, 1993).
* Muir and colleagues conducted a study of 2109 current Ontario
miners from 21 gold and uranium mines who started working and
worked more than 5 years between 1940 and 1959 and were followed
to 1982 or to the end of their dust exposure, whichever came
first (Muir et al., 1989a,b; Muir, 1991). Any uranium miner with
more than 2 weeks of exposure was also included (Muir et al.,
1989a). The quartz content of respirable gold mine dust was 6.0%,
and that of uranium mine dust was 8.4%. Retired and former miners
were not included in the study. Sources of data for this study
were full-sized annual chest radiographs taken for all miners
after 1927 and periodic (pre-1959) and semi-annual mine dust
measurements obtained with a konimeter (which is an instantaneous
dust sampler that measures the number of particles per unit
volume of air; Verma et al., 1989). Konimeter dust measurements
taken from 1940 to 1952 were expressed in particles per cubic
centimetre of air (ppcc). Verma et al. (1989) initiated an
extensive, side-by-side comparison of the konimetric and
gravimetric (i.e., milligrams of silica per cubic metre) sampling
to derive a konimetric/gravimetric silica conversion curve. A
total of 2360 filter (i.e., nylon cyclone-filter assembly in a
constant-flow pump) samples and 90 000 konimeter samples were
taken in a 2-year period in two gold and uranium mines, in
existing conditions as well as in an experimental simulation of
the high-dust conditions of the past caused by dry drilling
(Verma et al., 1989). The results of the conversion relationship
were non-linear and may have reflected the limitations of the
konimeter in measuring high dust (i.e., high count)
concentrations and the limitations of the gravimetric sampler in
measuring low dust concentrations. There were different
relationships for the gold and uranium mines, possibly because of
the different fractional silica concentrations in the host rock.
The conversion of the historical konimeter counts to gravimetric
respirable silica equivalents was used to derive a cumulative
respirable silica dose for each miner based on the miner's
respirable silica dose for each year, mine, and task in his work
history (Verma et al., 1989).
Thirty-two of the 2109 hardrock miners studied by Muir and
colleagues were considered by at least one of five readers to
have silicosis (small, rounded opacities with profusion of ILO
category >1/1). However, the results differed among the five
readers and "complicated the analysis" (Muir et al., 1989b). One
of the five readers identified only seven cases of silicosis
(Muir et al., 1989b). The results were presented by individual
reader and by consensus. A consensus of all of the five readers
with respect to identification of silicosis was reached on only
six cases (Muir et al., 1989b). Average respirable quartz dust
exposure for the cases was not reported.
* A cohort study of 3330 white male underground gold miners from
South Dakota employed for at least 1 year between 1940 and 1965
and followed through 1990 found 170 cases of silicosis (128 cases
were identified on death certificates, 29 cases were found during
X-ray surveys of workers conducted in 1960 and 1976, and 13 cases
were identified on both X-ray and death certificate). Cases were
defined as (1) an underlying or contributing cause of death of
silicosis, silico-tuberculosis, respiratory tuberculosis, or
pneumoconiosis, and/or (2) ILO category >1/1 silicosis
identified in the 1976 radiographic survey or "small opacities"
or "large opacities" identified in the 1960 radiographic survey
(Steenland & Brown, 1995a). The miners were exposed to a median
quartz level of 0.05 mg/m3 (0.15 mg/m3 for workers hired prior
to 1930). The average length of follow-up was 37 years, and the
average length of employment underground was 9 years. Quartz
exposure was estimated by converting dust particle counts to
gravimetric measurements (i.e., mg/m3), based on an estimate of
13% quartz content of total dust. A job-exposure matrix was
created to estimate dust exposures for each job over time, then
average dust exposures for the job categories were calculated
using existing measurements for each year from 1937 to 1975. The
estimated daily dust exposures (constant over each year) were
weighted to account for daily time spent underground. Summation
of the estimated daily dust levels over time provided an estimate
of cumulative quartz exposure (Steenland & Brown, 1995a). The
risk of silicosis was less than 1% for miners with a cumulative
exposure less than 0.5 mg/m3-years. The risk increased to 68-84%
for the highest cumulative exposure category (i.e.,
4 mg/m3-years) (Steenland & Brown, 1995a). Silicosis risk
estimates could have been affected by (1) combining silicosis
deaths with silicosis cases detected by cross-sectional
radiographic surveys, (2) differences in quartz content of dust
in early years, and (3) lack of dust measurements before 1937.
A cohort study of a subcohort of the South Dakota gold
miners described above analysed cases of silicosis that were
reported as the underlying cause of death on the death
certificates. Forty cases of silicosis, as well as 49 cases of
tuberculosis, were ascertained among the 1321 miners employed for
at least 21 years and followed through 1973. There was a linear
trend in risk of about 2.4% for each 0.1 mg/m3 of silica
exposure. However, this study does not meet the criteria for a
critical study because risk by cumulative quartz exposure was not
calculated (McDonald & Oakes, 1984).
In the five critical studies described above, the number of cases
identified depended upon the definition of silicosis (radiographic
category and whether irregular opacities were included), the quality
of the evaluation of the chest radiographs (e.g., number and training
of readers), the duration of dust exposure, and the duration of
follow-up after the end of exposure. Interstudy variation exists for
each of these factors. In addition, exposure assessments in these
studies were accompanied by uncertainties, such as the use of
conversion equations (i.e., converting particle count data to mass
concentrations; application of equations from one industry to a
different industry) and estimation of quartz content of the dust. It
is not uncommon for epidemiological studies to lack characterization
of the source and properties of the mineral dusts collected in the
workplace (Mossman & Churg, 1998). Nevertheless, the critical studies
found an exposure-response relationship for respirable quartz dust
that, when modelled, predicts the occurrence of silicosis cases in
various industries at exposures close to regulatory levels.
9.2.2 Pulmonary tuberculosis and other infections
The association between tuberculosis and silicosis has been
firmly established by the results of epidemiological studies conducted
during this century (Balmes, 1990). In recent studies of silicotics,
the association was well supported by the results of a survey of
tuberculosis deaths among silicotics in the USA for the period
1979-1991 (Althouse et al., 1995), a mortality study of 590 California
silicosis claimants (Goldsmith et al., 1995a), and a retrospective
study of silicotic miners from the Freegold mines in South Africa
(Kleinschmidt & Churchyard, 1997).
In studies of workers without silicosis, there is some limited
evidence that long exposures or high cumulative exposures to quartz
dust may increase the risk of developing tuberculosis. Two
epidemiological studies reported 3-fold higher incidences of pulmonary
tuberculosis cases in 5424 non-silicotic silica-exposed Danish foundry
workers employed 25 or more years (Sherson & Lander, 1990) and among
335 non-silicotic black South African gold miners with a median
underground employment of 26 years (Cowie, 1994). Westerholm et al.
(1986) found 13 cases of tuberculosis among 428 silicotic Swedish iron
and steel workers and one case of tuberculosis in a comparison group
of 476 Swedish iron and steel workers with normal chest radiographs
(level of statistical significance not reported). Both groups had been
exposed to silica for at least 5 years.
A study of tuberculosis incidence in 2255 white South African
gold miners included 1296 miners who had an autopsy. The
smoking-adjusted relative risk for pulmonary tuberculosis in miners
without silicotic nodules on autopsy examination (n = 577) increased
slightly with quartiles of cumulative dust exposure (relative risk
[RR] = 1.38; 95% confidence interval [CI] = 0.33-5.62) for the highest
quartile of cumulative exposure). For miners without radiologically
diagnosed silicosis (n = 1934), the smoking-adjusted relative risk
increased to 4.01 (95% CI = 2.04-7.88) in the highest quartile of
cumulative dust exposure (Hnizdo & Murray, 1998, 1999). Radiological
silicosis was defined as ILO category >1/1; detailed ILO grading
was not performed (Hnizdo & Murray, 1998, 1999). Tuberculosis was
diagnosed on average 7.6 years after the end of dust exposure and
6.8 years after the onset of radiological silicosis -- a result that
supports the need for medical surveillance of workers after the end of
exposure to silica dust (Hnizdo & Murray, 1998). (Miners who developed
tuberculosis before completing 10 years of underground employment were
excluded because they were not allowed to continue working underground
after diagnosis) (Hnizdo & Murray, 1998). It is not clear whether
"dust" exposure refers to quartz exposure or exposure to gold mine
dust.
Chen et al. (1997) conducted a case-control study (8740 cases;
83 338 controls) with US National Occupational Mortality Surveillance
data for the years 1983-1992 that controlled for confounding from age,
gender, race, socioeconomic status, potential exposure to active
tuberculosis, and the presence of silicosis and other pneumoconioses.
The potential for exposure to silica was based on potential exposure
data from the National Occupational Exposure Survey (Seta et al.,
1988) and the National Occupational Health Survey of Mining
(Greskevitch et al., 1996) and was categorized as "high,"
"intermediate," or "low or no" potential exposure. The study found
that decedents with potential high exposure to silica and with no
documentation of silicosis on the death certificate had a 30% greater
odds of mortality from respiratory tuberculosis than decedents with no
potential exposure to silica after adjustment by logistic regression
for the possible confounders mentioned above (odds ratio [OR] = 1.3;
95% CI = 1.14-1.48). The results also suggested the presence of an
exposure-response relationship of silica exposure, in the absence of
silicosis, with death from respiratory tuberculosis (Chen et al.,
1997).
In summary, the relationship between quartz exposure and
tuberculosis risk in the absence of radiographic silicosis in
silica-exposed workers has not been well defined or quantitatively
described by existing epidemiological studies.
A recent case-control study of tuberculosis and pulmonary disease
caused by non-tuberculous mycobacteria (NTM) in South African gold
miners found that radiographic silicosis, focal radiological scarring,
and human immunodeficiency virus (HIV) infection were significant risk
factors for NTM disease and for tuberculosis (Corbett et al., 1999).
Past medical history of tuberculosis treatment (OR = 15.1;
95% CI = 7.64-29.93) and current employment in a "dusty job" at the
mines (OR = 2.5; 95% CI = 1.46-4.44) were significant risk factors for
NTM. The study included 206 NTM patients and 381 tuberculosis patients
of known HIV status admitted to a South African hospital and
180 controls that were HIV-tested surgical or trauma patients admitted
to that hospital during the same time period. Odds ratios for NTM
and tuberculosis increased with increasing years of employment
(range of ORs: 1.0-9.4 for NTM, and 1.0-4.1 for tuberculosis).
9.2.3 Lung cancer
Lung cancer is associated with occupational exposures to inhaled
quartz (IARC, 1997).
Following a comprehensive review of the large body of published
epidemiological studies, IARC (1997) found that the following
epidemiological studies provide the least confounded investigations of
an association between occupational crystalline silica exposure and
lung cancer risk:
* US gold miners (Steenland & Brown, 1995b)
* Danish stone industry workers (Guenel et al., 1989)
* US granite shed and quarry workers (Costello & Graham, 1988)
* US crushed stone industry workers (Costello et al., 1995)
* US diatomaceous earth industry workers (Checkoway et al., 1993,
1996)
* Chinese refractory brick workers (Dong et al., 1995)
* Italian refractory brick workers (Puntoni et al., 1988;
Merlo et al., 1991)
* United Kingdom pottery workers (Cherry et al., 1995, 1997;
McDonald et al., 1995, 1997; Burgess et al., 1997)
* Chinese pottery workers (McLaughlin et al., 1992)
* cohorts of registered silicotics from North Carolina
(Amandus et al., 1991, 1992) and Finland (Kurppa et al., 1986;
Partanen et al., 1994)1
Although a few of those studies did not find a statistically
significant association between occupational crystalline silica
exposure and lung cancer risk, most of the studies did. Some
non-uniformity of results is not unusual when a large number of
epidemiological studies are reviewed and a variety of populations and
1 This list includes studies of diatomaceous earth industry workers
and workers in the ceramics, refractory brick, and pottery
manufacturing industries where the primary silica exposure may have
been to cristobalite rather than quartz. In most studies, exposure
data for quartz as well as cristobalite or tridymite were not
available to support that assumption.
work environments are studied (IARC, 1997). In addition, IARC noted
that the carcinogenicity of quartz (or cristobalite) "may be dependent
on inherent characteristics of the crystalline silica or on external
factors affecting its biological activity or distribution of its
polymorphs" (IARC, 1997). (A detailed critique of the studies listed
above is available in Soutar et al., 1997.)
Some of the least-confounded studies reported that lung cancer
risk tended to increase with:
* cumulative exposure to respirable silica (i.e., Checkoway et al.,
1993, 1996)
* duration of exposure (i.e., Costello & Graham, 1988;
Merlo et al., 1991; Partenen et al., 1994; Costello et al., 1995;
Dong et al., 1995)
* peak intensity of exposure (Burgess et al., 1997; Cherry et al.,
1997; McDonald et al., 1997)
* the presence of radiographically defined silicosis
(Amandus et al., 1992; Dong et al., 1995)
* length of follow-up time from date of silicosis diagnosis
(Partenen et al., 1994)
The observed associations noted above, including the
exposure-response associations, are unlikely to be explained by
confounding or other biases; therefore, the epidemiological studies,
overall, support increased lung cancer risks from occupational
exposure to inhaled respirable crystalline silica (i.e., quartz and
cristobalite) (IARC, 1997).
Three studies published since the IARC review investigated
exposure-response associations for lung cancer and occupational
exposure to quartz.
Cherry et al. (1998) published a final report of the preliminary
results of a nested case-control study of 52 lung cancer deaths in a
cohort of 5115 pottery workers (i.e., Burgess et al., 1997; Cherry et
al., 1997; McDonald et al., 1997). After adjustment for smoking and
inclusion of a 20-year-, 10-year-, or 0-year lag period, mean silica
concentration (i.e., estimated daily 8-h time-weighted airborne
concentrations in µg/m3; polymorph not specified) was associated with
lung cancer (OR = 1.60, 95% CI = 1.11-2.31; OR = 1.66,
95% CI = 1.14-2.41; OR = 1.67, 95% CI = 1.13-2.47, for 20-year,
10-year, and 0-year lag periods, respectively; P < 0.008 for each
lag period). However, duration of exposure and cumulative silica dust
exposure were not significantly associated with lung cancer mortality,
regardless of lag time (Cherry et al., 1998). The presence of small
parenchymal radiographic opacities (category >1/0; shape not
reported) was not related to lung cancer mortality, before
(P = 0.78) or after (P = 0.68) adjustment for smoking.
The authors concluded that the results imply "that crystalline silica
may well be a human carcinogen" (Cherry et al., 1998). The study did
not differentiate quartz exposures from cristobalite exposures.
De Klerk & Musk (1998) conducted a cohort study of 2297 surface
and underground gold miners in western Australia who participated in
surveys of respiratory symptoms, smoking habits, and lung function in
1961, 1974, and 1975. Eighty-nine per cent of the cohort was traced to
the end of 1993 for trachea, bronchus, and lung cancer mortality and
incidence of compensated silicosis (i.e., compensation awarded by the
Pneumoconiosis Medical Board). A nested case-control analysis of the
138 lung cancer deaths found that lung cancer mortality was related to
log total cumulative silica dust exposure after adjustment for smoking
(cigarette, pipe, or cigar) and for the presence of bronchitis at
survey (relative rate = 1.31; 95% CI = 1.01-1.70). However, the effect
of total cumulative silica dust exposure on lung cancer mortality was
not significant after adjustment for smoking, bronchitis, and
compensation for silicosis (relative rate = 1.20; 95% CI = 0.92-1.56).
Lung cancer mortality was not significantly related (P > 0.15) to
other silica exposure variables (i.e., duration of underground or
surface employment, intensity of underground or surface exposure)
after adjustment for smoking and bronchitis. Cigarette smoking
(relative rate = 32.5; 95% CI = 4.4-241.2 for >25 cigarettes smoked
per day), incidence of a compensation award for silicosis after lung
cancer diagnosis (relative rate = 1.59; 95% CI = 1.10-2.28), and
presence of bronchitis at survey (relative rate = 1.60;
95% CI = 1.09-2.33) were significantly related to lung cancer
mortality (de Klerk & Musk, 1998). The results of this study do not
support a relationship between lung cancer and silica exposure in the
absence of silicosis (i.e., a compensation award for silicosis after
lung cancer diagnosis).
Hnizdo et al. (1997) conducted a nested case-control study of
lung cancer deaths in a cohort of 2260 white South African underground
gold miners. (A lung cancer mortality cohort study was conducted
earlier [Hnizdo & Sluis-Cremer, 1991].) The mineral content of the
rock in the gold mines was mostly quartz (70-90%), silicates (10-30%),
pyrite (1-4%), and heavy minerals with grains of gold and
uranium-bearing minerals (2-4%). Seventy-eight lung cancer deaths
(69 of the 78 miners had a necropsy) that occurred during 1970-1986
were matched by year of birth with 386 control subjects from the same
cohort (Hnizdo et al., 1997). Lung cancer mortality risk and a
relationship with cigarette smoking (i.e., pack-years), cumulative
"dust" exposure (mg/m3-years), years of underground mining, incidence
of radiographic silicosis (i.e., ILO category >1/1 diagnosed up to
3 years before death of a matched case), and uranium production or
uranium grade of the ore in the gold mine were analysed with
conditional logistic regression models. Radon daughter measurements in
the gold mines were not available.
Lung cancer mortality was associated with cigarette smoking,
cumulative dust exposure (lagged 20 years from death), duration of
underground mining (lagged 20 years from death), and silicosis. The
best-fitting model predicted relative risks of 1.0, 3.5
(95% CI = 0.7-16.8), 5.7 (95% CI = 1.3-25.8), and 13.2
(95% CI = 3.1-56.2) for <6.5, 6.5-20, 21-30, and >30 pack-years of
smoking, respectively, and 2.45 (95% CI = 1.2-5.2) for silicosis. The
authors stated that variables representing uranium mining were not
significantly related to lung cancer mortality (modelling results for
these variables were not presented) (Hnizdo et al., 1997). The authors
proposed three explanations for their results: (1) miners with high
dust exposure who develop silicosis have increased lung cancer risk,
(2) high silica dust exposure concentrations may be important in the
pathogenesis of lung cancer, and silicosis is coincidental, and (3)
high levels of silica dust exposure may be a surrogate measure of
exposure to radon daughters (Hnizdo et al., 1997).
Meta-analyses of the epidemiological studies of silica exposure
and lung cancer reported a moderate summary risk of 1.3 for
silica-exposed workers (Steenland & Stayner, 1997) and higher summary
relative risks of 2.2-2.3 for studies of silicotic workers (Smith et
al., 1995; Steenland & Stayner, 1997). Tsuda et al. (1997) pooled lung
cancer risk estimates from 32 mortality studies of pneumoconiosis or
silicosis (excluding asbestosis) published from 1980 to 1994. The
estimated rate ratios of 2.74 (95% CI = 2.60-2.90) for all studies,
2.77 (95% CI = 2.61-2.94) for cohort studies only (25 of 32 studies),
and 2.84 (95% CI = 2.25-3.59) for case-control studies (5 of 32
studies) were similar to the estimates reported by Steenland & Stayner
(1997) and Smith et al. (1995).
Reasons for the higher risks in silicotics are not known;
similarly, the question of whether fibrosis is a precursor to the
development of lung cancer in humans has not been resolved. Various
hypothetical mechanistic pathways have been proposed to explain the
occurrence of lung tumours in rats and lung cancer in humans; however,
there is no convincing evidence for any specific proposed pathway
(IARC, 1997).
Selection bias is a common criticism of epidemiological studies
of lung cancer in compensated silicotics, because workers who sought
compensation for their disease may differ from the group of all
silicotics in symptoms, radiographic changes, social and psychological
factors, and industry (McDonald, 1995; Weill & McDonald, 1996).
However, Goldsmith (1998) reviewed this question and concluded that
lung cancer risk estimates were not higher in compensated silicotics
when results of studies of compensated silicotics were compared with
results of studies of silicotics ascertained from other clinical
sources (i.e., hospital or registry data).
9.2.4 Autoimmune-related disease
In humans, immune activation by occupational exposure to
respirable quartz may be linked to scleroderma, rheumatoid arthritis,
polyarthritis, mixed connective tissue disease, systemic lupus
erythematosus, Sjögren's syndrome, polymyositis, and fibrositis
(Haustein et al., 1990; Ziegler & Haustein, 1992; Otsuki et al.,
1998). The cellular mechanism that leads from quartz dust exposure to
autoimmune diseases is not known (Otsuki et al., 1998; NIOSH,
forthcoming). One theory is that when respirable silica particles are
encapsulated by macrophages, fibrogenic proteins and growth factors
are generated, and ultimately the immune system is activated (Haustein
et al., 1992; Ziegler & Haustein, 1992; Haustein & Anderegg, 1998).
Several epidemiological studies have reported statistically
significant numbers of excess deaths or cases of autoimmune-related
diseases such as scleroderma (Sluis-Cremer et al., 1985; Steenland &
Brown, 1995b), rheumatoid arthritis (Sluis-Cremer et al., 1986;
Klockars et al., 1987), and systemic lupus erythematosus (Steenland &
Brown, 1995b) in silica-exposed workers.
9.2.5 Renal disease
Recent epidemiological studies have found statistically
significant associations between occupational exposure to crystalline
silica dust and renal diseases and subclinical renal changes
(Steenland et al., 1992; Ng et al., 1993; Boujemaa et al., 1994; Hotz
et al., 1995; Nuyts et al., 1995; Steenland & Brown, 1995b; Steenland
& Goldsmith, 1995; Calvert et al., 1997).
9.2.6 Chronic obstructive pulmonary disease
Occupational exposure to respirable crystalline silica dust is
associated with chronic obstructive pulmonary disease, including
bronchitis and emphysema. Although these health effects are also
associated with tobacco smoking, some epidemiological studies suggest
that they may be present to a significant extent in non-smokers with
occupational exposure to quartz (Wiles & Faure, 1977; Becklake et al.,
1987; Holman et al., 1987; Kreiss et al., 1989; Cowie & Mabena, 1991).
9.2.7 Other adverse health effects
Cor pulmonale (i.e., enlargement of the right ventricle of the
heart because of structural or functional abnormalities of the lungs)
may occur as a complication of silicosis (Green & Vallyathan, 1996)
and other pneumoconioses (Kusiak et al., 1993). It is usually preceded
by pulmonary arterial hypertension. An epidemiological case-control
study of 732 white South African autopsied gold miners reported a
statistically significant association (P < 0.05) of cor pulmonale
with "extensive" silicosis and "slight" silicosis (Murray et al.,
1993).
Other adverse health effects or complications of silicosis have
been studied or identified in epidemiological studies of workers that
may have been exposed to quartz dust, but evidence of an association
with quartz exposure is inconclusive. These adverse effects include
dental abrasion (Petersen & Henmar, 1988), nasopharyngeal or
pharyngeal cancer (Carta et al., 1991; Chen et al., 1992), salivary
gland cancer (Zheng et al., 1996), liver cancer (Chen et al., 1992;
Hua et al., 1992), bone cancer (Steenland & Beaumont, 1986; Forastiere
et al., 1989), pancreatic cancer (Kauppinen et al., 1995), skin cancer
(Partanen et al., 1994), oesophageal cancer (Belli et al., 1989;
Xu et al., 1996; Pan et al., 1999), stomach cancer
(Parent et al., 1998), cancers of the digestive system
(Decoufle & Wood, 1979), intestinal or peritoneal cancer
(Amandus et al., 1991; Costello et al., 1995; Goldsmith et al.,
1995a), lymphopoietic or haematopoietic cancers (Silverstein et al.,
1986; Steenland & Brown, 1995b), and bladder cancer (Bravo et al.,
1987).
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
Terrestrial mammals (i.e., horses, camels) and birds exposed to
quartz in the natural environment, especially in desert or coastal
areas, show pathological lesions sometimes described as "silicosis"
that are similar to those seen in humans with silicosis
(Schwartz et al., 1981; Evans et al., 1988; Hansen et al., 1989;
Berry et al., 1991; Xu et al., 1993; Green & Vallyathan, 1996). Rats,
hamsters, guinea-pigs, monkeys, and mice exposed to quartz under
experimental conditions develop lung conditions and nodules similar to
those found in humans (reviewed by Green & Vallyathan, 1996).
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
The extensive body of in vitro and in vivo research
evaluating the effects of quartz on mammalian cells is summarized here
(IARC, 1997; NIOSH, forthcoming). Quartz deposited in the lungs causes
epithelial and macrophage injury and activation, and it translocates
to the interstitium and the regional lymph nodes. Recruitment of
inflammatory cells occurs in a dose-dependent manner. Oxidative stress
(i.e., increased formation of reactive oxygen species, including
hydroxyl radicals, or reactive nitrogen species) has been observed in
rats after intratracheal instillation (Blackford et al., 1994;
Schapira et al., 1995) or inhalation (Vallyathan et al., 1995) of
quartz. Several mechanisms have been proposed to explain the cause of
the cellular damage by quartz particles (Lapp & Castranova, 1993):
(1) direct cytotoxicity of quartz, (2) stimulation of the alveolar
macrophages by quartz, which results in the release of cytotoxic
enzymes or oxidants, (3) stimulation of the alveolar macrophages to
release inflammatory factors (e.g., interleukin-8, leukotriene B4,
platelet-activating factor, tumour necrosis factor, platelet-derived
growth factor) that recruit polymorphonuclear leukocytes, which may
release cytotoxins, (4) stimulation of the alveolar macrophages to
release factors that initiate fibroblast production and collagen
synthesis (e.g., interleukin-1, tumour necrosis factor,
platelet-derived growth factor, fibronectin, alveolar
macrophage-derived growth factor), and, more recently, (5) induction
by quartz of apoptosis and subsequent engulfment by macrophages to
regulate the evolution of inflammation and fibrosis (Leigh et al.,
1997).
Silicosis is indisputably causally related to occupational quartz
exposure, and the dose-response assessments of the adverse health
effects of quartz are based on epidemiological studies of occupational
cohorts with silicosis. To date, there are no known adverse health
effects associated with non-occupational exposure to quartz dust.
Silicosis is the critical effect for hazard identification and
dose-response assessment, for two reasons. First, although IARC
classified inhaled quartz from occupational sources as a Group 1
carcinogen, there are very few published risk assessments
(toxicological or epidemiological) with a quantitative dose-response
assessment of lung cancer risk at various levels of quartz exposure. A
pooled exposure-response assessment of a number of epidemiological
studies with quantitative data for quartz exposures and lung cancer is
currently being conducted by IARC.
Secondly, epidemiological studies of quartz-exposed workers
reported statistically significant numbers of excess deaths or cases
of renal disease or subclinical renal changes (Steenland et al., 1992;
Ng et al., 1993; Boujemaa et al., 1994; Hotz et al., 1995;
Nuyts et al., 1995; Steenland & Brown, 1995b; Calvert et al., 1997),
mycobacterial infections (tuberculous and non-tuberculous), or fungal
infections (Ziskind et al., 1976; Parkes, 1982; Parker, 1994; Althouse
et al., 1995; Goldsmith et al., 1995a; NIOSH, 1996; American Thoracic
Society, 1997; Kleinschmidt & Churchyard, 1997; Hnizdo & Murray, 1998;
Corbett et al., 1999), immunological disorders and autoimmune diseases
(i.e., scleroderma) (Sluis-Cremer et al., 1985; Cowie, 1987; Steenland
& Brown, 1995b), rheumatoid arthritis (Sluis-Cremer et al., 1986;
Klockars et al., 1987), and systemic lupus erythematosus (Steenland &
Brown, 1995b), but sufficient epidemiological or toxicological data do
not currently exist for quantitative assessment of the
exposure-response relationship for these health effects.
The US EPA calculated lowest-observed-adverse-effect level
(LOAEL) human equivalent concentrations (HECs) for non-cancer effects
reported in subchronic (<3 months) and chronic inhalation studies
that administered less than 20 mg quartz/m3 to experimental rats and
mice (Table 1). The results varied widely -- from 0.18 mg/m3 (based
on rat study) to 0.90 mg/m3 (based on mouse study). Some of this
variation may have been due to differences in dose, species, and the
quartz specimens. The method for determining the HEC was based on a
series of empirical equations described elsewhere (US EPA, 1994) and
summarized here. It represents an example of the calculation of an HEC
and may not necessarily be a method used worldwide. The equations
estimate fractional deposition of relatively insoluble particles and
adjust for dosimetric differences between species by incorporating
normalizing factors such as body weight or surface area (US EPA,
1994). In summary:
NOAEL[HEC] (mg/m3) = NOAEL[ADJ] (mg/m3) × RDDRr
where:
* NOAEL[HEC] = the no-observed-adverse-effect level (NOAEL) human
equivalent concentration, dosimetrically adjusted
* NOAEL[ADJ] = the NOAEL adjusted for duration: E (mg/m3) × D
(h/24 h) × W (days/7 days), where E = experimental exposure level
* RDDRr = the regional deposited dose ratio of particles for the
respiratory tract region (r)
* RDDR = (RDDTH/Normalizing Factor)A/(RDDTH/Normalizing
Factor)H, the ratio of regional deposited dose (RDDr) in the
thoracic (TH) region in the animal (A) species to that of humans
(H) (US EPA, 1994)
* r = region of dose deposition, in this case thoracic
* RDDr = 10-6 × Ci × VE × Fr, where:
Ci = concentration (mg/m3)
VE = minute volume (ml/min)
Fr = fractional deposition in region r
The literature contains a few additional risk assessments of lung
cancer and quartz, and all were based on results of inhalation studies
of rats (Collins & Marty, 1995, 1997; Goldsmith et al., 1995b).
However, human data introduce less uncertainty than extrapolation from
animals to humans (Goldsmith et al., 1995b; Goldsmith &
Hertz-Picciotto, 1997). For quartz, the uncertainty can be attributed
in part to the lack of (1) experimental studies that systematically
evaluated exactly the same material to which humans are exposed (IARC,
1997), (2) understanding of the mechanism for induction of rat lung
tumours (IARC, 1997), (3) understanding of the species differences
observed in the fibrogenic response (Gift & Faust, 1997), and
(4) experimental studies that administered doses in concentrations
similar to known occupational exposures (US EPA, 1996).
Epidemiological studies that used cumulative exposure estimates
represent the best currently available source of information for
characterizing the dose-response relationship for silicosis or lung
cancer in occupational, as well as non-occupational, cohorts.
Exposure-response models based on cumulative exposure data can
provide predictions of silicosis risk for a particular silica dust
exposure over a period of time. Table 6 presents results of logistic
regression models of data from the cross-sectional epidemiological
studies described in section 9.2. The models predicted the prevalence
of radiographic silicosis (ILO category >1/1 or >1/0) from
cumulative exposure to respirable quartz. All models predicted the
occurrence of at least one case of radiographic silicosis per 100
workers at cumulative respirable quartz dust exposures of 0.05 or 0.10
mg/m3 over a 45-year working lifetime. Logistic regression assumes
that the change in the level of quartz exposure affects risk (or
predicted prevalence) in a multiplicative manner (as compared with
linear models, which predict deviations from additivity)
(Kleinbaum et al., 1982).
Table 7 displays the results of three studies that followed
cohorts of miners for silicosis over time or retrospectively. In these
studies, the miners with silicosis had their first quartz exposure
18-37 years (mean) prior to radiographic diagnosis. The models applied
to the data collected in these studies predicted that the risk of
chronic silicosis increases exponentially with increasing cumulative
dose of silica dust (US EPA, 1996). Data from the study of South
African gold miners (Hnizdo & Sluis-Cremer, 1993) and Canadian gold
and uranium miners were analysed with models that assumed some risk of
silicosis at any exposure. The study of US gold miners (Steenland &
Brown, 1995a) estimated silicosis rates (cases per person-time at
risk) for seven cumulative exposure categories, stratified by 5-year
age and calendar time intervals. Poisson regression was used to adjust
the crude rates for age and calendar time. Although age and calendar
time were highly correlated with exposure, it is not likely that they
confounded the exposure-response analysis, because silicosis has no
background rate for non-exposed populations that changes with age or
calendar time (US EPA, 1996).
In summary, the risk estimates for silicosis prevalence for a
working lifetime of exposure to respirable quartz dust concentrations
of about 0.05 or 0.10 mg/m3 in the occupational environment vary
widely (i.e., 2-90%). Epidemiological studies varied in the definition
of silicosis cases, radiographic interpretation methods, cohort
follow-up periods, and statistical methods. The variability in the
risk estimates cannot be solely attributed to differences in follow-up
periods; however, it must be recognized that chronic silicosis is a
progressive disease. Therefore, the development of silicosis after a
long latency period and after workers leave employment must be
accounted for in epidemiological studies. Results of a study of
autopsied South African gold miners found that the diagnostic
sensitivity of radiological examination is particularly poor (Hnizdo
et al., 1993). For example, when the radiological findings for
profusion of rounded opacities (ILO category >1/1) were compared
with pathological findings for silicosis in 326 miners with an average
of 2.7 years between the radiological and pathological examination,
silicosis was not diagnosed radiographically for at least 61% of the
miners with slight to marked silicosis at autopsy. The probability of
a false-negative reading increased with years of mining and the
average concentration of respirable dust (Hnizdo et al., 1993).
Experimental studies of rats also reported lack of agreement between
histopathological indicators of silica dust exposure and radiographic
readings (Drew & Kutzman, 1984a,b).
Improved exposure assessment methods and data analyses that
account for variations and deficiencies in exposure data would improve
the risk estimates for silica-exposed workers (Agius et al., 1992;
Checkoway, 1995). Although epidemiological studies that used
cumulative exposure estimates represent the best available source of
information for characterizing the dose-response relationship in
occupational cohorts, peak exposures may predict silicosis risk better
than cumulative exposures (Checkoway & Rice, 1992), but data are
rarely available.
11.1.2 Criteria for setting tolerable intakes or guidance values
for quartz
Results of genotoxicity tests of quartz, as well as other
in vitro and in vivo evidence, suggest that persistent
inflammation and epithelial proliferation are related to the tumour
response in rats. Other pathways for tumour induction, such as a
direct genotoxic effect, have not been ruled out. Because a pathway
has not been determined, and it is unclear from results of
epidemiological studies whether silicosis is a precursor to
lung cancer, it cannot be assumed that there is a threshold (i.e.,
tolerable concentration, or TC) at which exposure to quartz would not
result in silicosis and/or lung cancer. In addition, available data
indicate that occupational quartz exposure is associated with the
development of tuberculosis, especially in workers with silicosis;
however, the association has not been quantified for that outcome or
for other quartz-related diseases. Therefore, occupational exposure to
respirable quartz dust should be reduced to the extent possible.
11.1.3 Sample risk characterization
It is recognized that there are many different methods for
assessing the risk to human health posed by environmental and
occupational substances. The example presented here applies to healthy
individuals not compromised by respiratory ailments and who breathe
the ambient air in the USA. It is based on the results of the three
cohort studies of miners presented in Table 7 (section 11.1.1). Using
a high estimate of 10% crystalline silica content of particulate
matter with a mass median aerodynamic diameter not greater than 10 µm
(PM10) from US metropolitan areas, the highest cumulative crystalline
silica exposure expected from continuous human lifetime exposure at or
below the annual US national ambient air quality standard (NAAQS) for
particulate matter of 50 µg/m3 is 1 mg/m3-year (US EPA, 1996).
The US EPA applied a mathematical model (i.e., benchmark dose
[BMD] analysis) to determine a concentration and lower confidence
bound associated with a predefined effect level (i.e., 1%, 5%, and 10%
risk of silicosis) (US EPA, 1996). After consideration of
cross-sectional, longitudinal, retrospective cohort, and case-control
epidemiological studies, the BMD was conducted on data from the
previously described study of radiographic silicosis in a cohort of
2235 South African gold miners (Hnizdo & Sluis-Cremer, 1993). This
study was selected for several reasons: (1) continuous and
longitudinal investigation of silicosis, (2) miners had multiple X-ray
examinations, and (3) autopsy data were available for more accurate
interpretation of the radiographic results, which lack sensitivity (US
EPA, 1996).1
1 The cross-sectional studies described in Table 6 and sections
9.2.1 and 11.1.1 were not available at the time of the US EPA
assessment. These studies modelled the cumulative quartz exposures
of hardrock miners (Kreiss & Zhen, 1996) and grey iron foundry
workers (Rosenman et al., 1996) and predicted a 30% prevalence of
ILO category > 1/1 silicosis over a 45-year working lifetime with
10 years of post-employment follow-up (Kreiss & Zhen, 1996) or a
2% prevalence of ILO category > 1/0 silicosis over a 40-year
working lifetime and controlling for pack-years of cigarette
smoking, race, and silica exposure other than in the foundry under
study (Rosenman et al., 1996). Both predictions are for a mean
quartz dust concentration of 0.05 mg/m3.
Using methods described by US EPA (1996), the BMD analysis
predicted that the silicosis risk for a continuous 70-year lifetime
exposure to 0.008 mg/m3 (estimated high crystalline silica
concentration in US metropolitan areas) is less than 3% for healthy
individuals not compromised by other respiratory diseases or
conditions and for ambient environment (US EPA, 1996). (Risks were not
calculated for other groups, such as people with respiratory
illnesses.) This risk estimate for exposure to ambient quartz may be
conservative, because quartz particles in the occupational environment
may be finer or "freshly fractured," occupational exposures may
involve high "peak" exposures, and, thus, the potential for disease
development may be greater.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
IARC has classified inhaled crystalline silica (quartz or
cristobalite) from occupational sources as a Group 1 carcinogen based
on sufficient evident of carcinogenicity in humans and experimental
animals. In addition, "in making the overall evaluation, the Working
Group noted that carcinogenicity in humans was not detected in all
industrial circumstances studied. Carcinogenicity may be dependent on
inherent characteristics of the crystalline silica or on external
factors affecting its biological activity or distribution of its
polymorphs" (IARC, 1997).
In 1991, the ILO published a document describing methods for
prevention and control of occupational lung diseases, including
silicosis (ILO, 1991), and in 1993, the Office of Occupational Health
of the World Health Organization (WHO) called for increased medical
surveillance of workers exposed to mineral dusts to prevent
pneumoconioses such as silicosis and asbestosis (WHO, 1993). WHO also
recently published information about risk factors for pulmonary
tuberculosis, including occupational exposure to respirable
crystalline silica (WHO, 1996).
In 1986, a WHO study group recommended a limit of 40 µg/m3
(time-weighted average of an 8-h shift) for occupational exposure to
respirable crystalline silica dust (WHO, 1986).
REFERENCES
Agius RM, Love RG, Davies LST, Hutchison PA, Cherrie JW, Robertson A,
Cowie HA, Hurley JF, Seaton A, Soutar CA (1992) Epidemiological
studies of respiratory health and dust exposure in hard rock quarry
workers and ex-workers. Edinburgh, Institute of Occupational
Medicine (HSE Contract No. 1/LMD/126/146/88; Report No. TM/92/10).
Ahlman K, Koskela R-S, Kuikka P, Koponen M, Annanmäki M (1991)
Mortality among sulfide ore miners. American journal of industrial
medicine, 19:603-617.
Althouse RB, Bang KM, Castellan RM (1995) Tuberculosis comortality
with silicosis -- United States, 1979-1991. Applied occupational and
environmental hygiene, 10(12):1037-1041.
Altieri A, Sperduto B, Verdel U, Porceli D (1984) Identification of
cristobalite and quartz in the production of silicon carbide. Rivista
degli Infortuni e delle Malattie Professionali, 71(1-2):131-135.
Amandus HE, Shy C, Wing S, Blair A, Heineman EF (1991) Silicosis and
lung cancer in North Carolina dusty trades workers. American journal
of industrial medicine, 20:57-70.
Amandus HE, Castellan RM, Shy C, Heineman EF, Blair A (1992)
Reevaluation of silicosis and lung cancer in North Carolina dusty
trades workers. American journal of industrial medicine, 22:147-153.
American Thoracic Society (1997) Adverse effects of crystalline silica
exposure. American journal of respiratory and critical care medicine,
155:761-765.
Anderson LJ, Donaldson HM, Jones JH, Stringer WT, Wallingford KM
(1980) North Carolina Brick Industry Industrial Hygiene and
Respiratory Disease Morbidity Survey, 1974-1975. Cincinnati, OH,
National Institute for Occupational Safety and Health (National
Technical Information Service Report No. PB83-181735).
Aoki A, Sirai A, Sakamoto H, Igarashi T, Matsunaga K, Ishigatsubo Y,
Tani K, Okubo T (1988) A case of silicosis associated with
polymyositis and benign monoclonal gammopathy. Ryumachi,
28(5):373-378.
Arnalich F, Lahoz C, Picazo ML, Monereo A, Arribas JR, Martinez Ara J,
Vazquez JJ (1989) Polyarteritis nodosa and necrotizing
glomerulonephritis associated with long-standing silicosis. Nephron,
51(4):544-547.
Balmes J (1990) Silica exposure and tuberculosis: an old problem with
some new twists [editorial]. Journal of occupational medicine,
32(2):114-115.
Banks DE, Milutinovic J, Desnick RJ, Grabowski GA, Lapp NL, Boehlecke
BA (1983) Silicon nephropathy mimicking Fabry's disease.
American journal of nephrology, 3(5):279-284.
Beadle DG (1965) An epidemiological study of the relationship between
the amount of dust breathed and the incidence of silicosis in South
African gold miners. In: Davies CN, ed. Inhaled particles and
vapours II. Oxford, Pergamon Press, pp. 479-492.
Beadle DG (1971) The relationship between the amount of dust breathed
and the development of radiological signs of silicosis: an
epidemiological study in South African gold miners. In: Walton WH, ed.
Inhaled particles III: Proceedings of an international symposium
organized by the British Occupational Hygiene Society, Vol. II.
Surrey, Unwin Brothers Limited, pp. 953-964.
Beadle DG, Bradley AA (1970) The composition of airborne dust in South
African gold mines. In: Shapiro HA, ed. Pneumoconiosis: Proceedings
of the international conference, Johannesburg, 1969. Oxford, Oxford
University Press, pp. 462-466.
Becklake MR, Irwig L, Kielkowski D, Webster I, De Beer M, Landau S
(1987) The predictors of emphysema in South African gold miners.
American review of respiratory disease, 135:1234-1241.
Belli S, Comba P, Germani D, Grignoli M, Lagorio S, Paganoni R,
Ronchin M (1989) [Mortality study among lead-zinc Italian (Val
Seriana) miners.] Medicina del Lavoro, 80(6):467-478 (in Italian).
Berry CR, O'Brien TR, Madigan JE, Hager DA (1991) Thoracic
radiographic features of silicosis in 19 horses. Journal of
veterinary internal medicine, 5(4):248-256.
Blackford JA, Antonini JM, Castranova V, Dey RD (1994) Intratracheal
instillation of silica up-regulates inducible nitric oxide synthase
gene expression and increases nitric oxide production in alveolar
macrophages and neutrophils. American journal of respiratory cell and
molecular biology, 11(4):426-431.
Bloor WA, Eardley RE, Dinsdale A (1971) Environmental conditions in
sanitary whiteware casting shops. Annals of occupational hygiene,
14(4):321-327.
Bolsaitis PP, Wallace WE (1996) The structure of silica surfaces in
relation to cytotoxicity. In: Castranova V, Vallyathan V, Wallace WE,
eds. Silica and silica-induced lung diseases. Boca Raton, FL,
CRC Press, pp. 79-89.
Bolton WK, Suratt PM, Sturgill BC (1981) Rapidly progressive silicon
nephropathy. American journal of medicine, 71:823-828.
Bonnin A, Mousson C, Justrabo E, Tanter Y, Chalopin JM, Rifle G (1987)
Silicosis associated with crescentic IgA mesangial nephropathy
[letter]. Nephron, 47(3):229-230.
Boujemaa W, Lauwerys R, Bernard A (1994) Early indicators of renal
dysfunction in silicotic workers. Scandinavian journal of work,
environment and health, 20(3):180-183.
Bravo MP, Del Rey Calero J, Conde M (1987) Silice y cancer de vejiga
en varones. Archivos Espanoles de Urologia, 40(9):635-637.
Bresnitz EA, Roseman J, Becker D, Gracely E (1992) Morbidity among
municipal waste incinerator workers . American journal of industrial
medicine, 22:363-378.
Burgess GL, Turner S, McDonald JC, Cherry NM (1997) Cohort mortality
study of Staffordshire pottery workers: (I) Radiographic validation of
an exposure matrix for respirable crystalline silica. Annals of
occupational hygiene, 41 (Suppl. 1):403-407.
Burgess WA (1995) Recognition of health hazards in industry: a review
of materials and processes, 2nd ed. New York, NY, John Wiley & Sons,
pp. 106-139, 411-422, 423-434, 475-482.
Buringh E, van de Belt R, van der Wal JF (1990) Dust control measures
in Dutch brickworks. Annals of occupational hygiene, 34:483-497.
Burns CA, Zarkower A, Ferguson FG (1980) Murine immunological and
histological changes in response to chronic silica exposure.
Environmental research, 21(2):298-307.
Calvert GM, Steenland K, Palu S (1997) End-stage renal disease among
silica-exposed gold miners. Journal of the American Medical
Association, 277(15):1219-1223.
Carta P, Cocco PL, Casula D (1991) Mortality from lung cancer among
Sardinian patients with silicosis. British journal of industrial
medicine, 48(2):122-129.
Castranova V, Dalal NS, Vallyathan V (1996) Role of surface free
radicals in the pathogenicity of silica. In: Castranova V, Vallyathan
V, Wallace WE, eds. Silica and silica-induced lung diseases. Boca
Raton, FL, CRC Press, pp. 91-105.
Castranova V, Vallyathan V, Ramsey DM, McLaurin JL, Pack D, Leonard S,
Barger MW, Ma JYC, Dalal NS, Teass A (1997) Augmentation of pulmonary
reactions to quartz inhalation by trace amounts of iron-containing
particles. Environmental health perspectives, 105 (Suppl.
5):1319-1324.
Cavariani F, Di Pietro A, Miceli M, Forastiere F, Biggeri A, Scavalli
P, Petti A, Borgia P (1995) Incidence of silicosis among ceramic
workers in central Italy. Scandinavian journal of work, environment
and health, 21 (Suppl. 2):58-62.
Chamberlain M (1983) Effect of mineral dusts on metabolic cooperation
between Chinese hamster V79 cells in vitro. Environmental health
perspectives, 51:5-9.
Checkoway H (1995) Methodological considerations relevant to
epidemiology studies of silica and lung cancer. Applied occupational
and environmental hygiene, 10(12):1049-1055.
Checkoway H, Rice CH (1992) Time-weighted averages, peaks, and other
indices of exposure in occupational epidemiology. American journal of
industrial medicine, 21:25-33.
Checkoway H, Heyer NJ, Demers PA, Breslow NE (1993) Mortality among
workers in the diatomaceous earth industry. British journal of
industrial medicine, 50:586-597.
Checkoway H, Heyer NJ, Demers PA, Gibbs GW (1996) Reanalysis of
mortality from lung cancer among diatomaceous earth industry workers,
with consideration of potential confounding by asbestos exposure.
Occupational and environmental medicine, 53:645-647.
Chen GX, Burnett CA, Cameron LL, Alterman T, Lalich NR, Tanaka S,
Althouse RB (1997) Tuberculosis mortality and silica exposure: a
case-control study based on a national mortality database for the
years 1983-1992. International journal of occupational and
environmental health, 3:163-170.
Chen J, McLaughlin JK, Zhang JY, Stone BJ, Luo J, Chen RA, Dosemeci M,
Rexing SH, Wu Z, Hearl FJ, McCawley MA, Blot WJ (1992) Mortality among
dust-exposed Chinese mine and pottery workers. Journal of
occupational medicine, 34(3):311-316.
Cherry N, Burgess G, McNamee R, Turner S, McDonald C (1995) Initial
findings from a cohort mortality study of British pottery workers.
Applied occupational and environmental hygiene, 10(12):1042-1045.
Cherry NM, Burgess GL, Turner S, McDonald JC (1997) Cohort study of
Staffordshire pottery workers: (II) Nested case referent analysis of
lung cancer. Annals of occupational hygiene, 41 (Suppl. 1):408-411.
Cherry NM, Burgess GL, Turner S, McDonald JC (1998) Crystalline silica
and risk of lung cancer in the potteries. Occupational and
environmental medicine, 55:779-785.
Collins JF, Marty MA (1995) Cancer risk assessment for crystalline
silica to implement California's Hot Spots Act. Scandinavian
journal of work, environment and health, 21 (Suppl. 2):99-103.
Collins JF, Marty MA (1997) Cancer risk assessment for crystalline
silica. Journal of exposure analysis and environmental epidemiology,
7(3):359-365.
Cooper TC, Gressel MG, Froelich PA, Caplan PE, Mickelsen RL, Valiante
D, Bost P (1993) Successful reduction of silica exposures at a
sanitary ware pottery. American Industrial Hygiene Association
Journal, 54(10):600-606.
Corbett EL, Churchyard GJ, Clayton T, Herselman P, Williams B, Hayes
R, Mulder D, De Cock KM (1999) Risk factors for pulmonary
mycobacterial disease in South African gold miners. American journal
of respiratory and critical care medicine, 159:94-99.
Costello J, Graham WGB (1988) Vermont granite workers' mortality
study. American journal of industrial medicine, 13:483-497.
Costello J, Castellan RM, Swecker GS, Kullman GJ (1995) Mortality of a
cohort of U.S. workers employed in the crushed stone industry,
1940-1980. American journal of industrial medicine, 27:625-640.
Cowie RL (1987) Silica-dust-exposed mine workers with scleroderma
(systemic sclerosis). Chest, 92(2): 260-262.
Cowie RL (1994) The epidemiology of tuberculosis in gold miners with
silicosis. American journal of respiratory and critical care
medicine, 150:1460-1462.
Cowie RL, Mabena SK (1991) Silicosis, chronic airflow limitation, and
chronic bronchitis in South African gold miners. American review of
respiratory disease, 143:80-84.
Dagle GE, Wehner AP, Clark ML, Buschbom RL (1986) Chronic inhalation
exposure of rats to quartz. In: Goldsmith DF, Winn DM, Shy CM, eds.
Silica, silicosis, and cancer: Controversy in occupational medicine.
New York, NY, Praeger Publishers, pp. 255-266 (Cancer Research
Monographs, Vol. 2).
Daniel LN, Mao Y, Saffiotti U (1993) Oxidative DNA damage by
crystalline silica. Free radical biology and medicine, 14:463-472.
Daniel LN, Mao Y, Wang T-CL, Markey CJ, Markey SP, Shi X, Saffiotti U
(1995) DNA strand breakage, thymine glycol production, and hydroxyl
radical generation induced by different samples of crystalline silica
in vitro. Environmental research, 71:60-73.
Davies LST, Robertson A, Agius RM, Cowie HA, Cherrie JW, Hutchison P
(1994) The use of compliance monitoring for assessing quarry workers'
exposures to respirable dust and quartz. Annals of occupational
hygiene, 38 (Suppl. 1):559-570.
Davis BL, Johnson LR, Stevens RK, Courtney WJ, Safriet DW (1984) The
quartz content and elemental composition of aerosols from selected
sites of the EPA inhalable particulate network. Atmospheric
environment, 18(4):771-782.
Davis GS (1991) Immunologic aspects of pneumoconioses in asbestosis
and silicosis. In: Lynch JP, DeRemee RA, eds. Immunologically
mediated pulmonary diseases. Philadelphia, PA, J.B. Lippincott
Company, pp. 111-155.
Davis GS (1996) Silica. In: Harber P, Schenker MB, Balmes JR, eds.
Occupational and environmental respiratory disease, 1st ed. St.
Louis, MO, Mosby, pp. 373-399.
Davis LK, Wegman DH, Monson RR, Froines J (1983) Mortality experience
of Vermont granite workers. American journal of industrial medicine,
4:705-723.
Decoufle P, Wood DJ (1979) Mortality patterns among workers in a gray
iron foundry. American journal of epidemiology, 109(6):667-675.
de Klerk NH, Musk AW (1998) Silica, compensated silicosis, and lung
cancer in Western Australian goldminers. Occupational and
environmental medicine, 55:243-248.
Dethloff LA, Gilmore LB, Gladen BC, George G, Chhabra RS, Hook GER
(1986a) Effects of silica on the composition of the pulmonary
extracellular lining. Toxicology and applied pharmacology,
84(1):66-83.
Dethloff LA, Gilmore LB, Brody AR, Hook GER (1986b) Induction of
intracellular and extracellular phospholipids in the lungs of rats
exposed to silica. Biochemical journal, 233(1):111-118.
Donaldson HM, Wallingford K, Jones JH (1982) Environmental surveys in
the Barre, Vermont and Elberton, Georgia granite industries.
Cincinnati, OH, National Institute for Occupational Safety and Health
(National Technical Information Service Publication No. PB83-179911).
Donaldson K, Borm PJA (1998) The quartz hazard: a variable entity.
Annals of occupational hygiene, 42(5):287-294.
Dong D, Xu G, Sun Y, Hu P (1995) Lung cancer among workers exposed to
silica dust in Chinese refractory plants. Scandinavian journal of
work, environment and health, 21 (Suppl. 2):69-72.
Dosemeci M, McLaughlin JK, Chen J-Q, Hearl F, Chen R-G, McCawley M, Wu
Z, Peng K-L, Chen A-L, Rexing SH, Blot WJ (1995) Historical total and
respirable silica dust exposure levels in mines and pottery factories
in China. Scandinavian journal of work, environment and health, 21
(Suppl. 2):39-43.
Dracon M, Noel C, Wallaert P, Dequiedt P, Lelievre G, Tacquet A (1990)
Rapidly progressive glomerulonephritis in silicotic coal miners.
Nephrologie, 11:61-65.
Drew RT, Kutzman RS (1984a) Final report on a study of Fischer 344
rats exposed to silica dust for six months at concentrations of 0, 2,
10 or 20 mg/m3, then maintained for six months prior to assessment.
Upton, NY, Brookhaven National Laboratory (Report No. BNL 35735;
submitted to the National Toxicology Program under Interagency
Agreement No. 222-Y01-ES-9-0043, November 1984).
Drew RT, Kutzman RS (1984b) Final report on a study of Fischer 344
rats exposed to silica dust for six months at concentrations of 0, 2,
10 or 20 mg/m3. Upton, NY, Brookhaven National Laboratory (Report
No. BNL 34617; submitted to the National Toxicology Program under
Interagency Agreement No. 222-Y01-ES-9-0043, February 1984).
Driscoll KE (1995) The toxicology of crystalline silica studied
in vitro. Applied occupational and environmental hygiene,
10(12):1118-1125.
Driscoll KE (1996) The role of interleukin-1 and tumor necrosis factor
alpha in the lung's response to silica. In: Castranova V, Vallyathan
V, Wallace WE, eds. Silica and silica-induced lung diseases. Boca
Raton, FL, CRC Press, pp. 163-184.
Driscoll KE, Deyo LC, Howard BW, Poynter J, Carter JM (1995)
Characterizing mutagenesis in the hprt gene of rat alveolar
epithelial cells. Experimental lung research, 21:941-956.
Driscoll KE, Deyo LC, Carter JM, Howard BW, Hassenbein DG, Bertram TA
(1997) Effects of particle exposure and particle-elicited inflammatory
cells on mutation in rat alveolar epithelial cells. Carcinogenesis,
18(2):423-430.
Dufresne A, Lesage J, Perrault G (1987) Evaluation of occupational
exposure to mixed dusts and polycyclic aromatic hydrocarbons in
silicon carbide plants. American Industrial Hygiene Association
Journal, 48(2):160-166.
Dufresne A, Loosereewanich P, Bégin R, Dion C, Ecobichon D, Muir DCF,
Ritchie AC, Perrault G (1998a) Tentative explanatory variable of lung
dust concentration in gold miners exposed to crystalline silica.
Journal of exposure analysis and environmental epidemiology,
8(3):375-398.
Dufresne A, Bégin R, Dion C, Jagirdar J, Rom WN, Loosereewanich P,
Muir DCF, Ritchie AC, Perrault G (1998b) Angular and fibrous particles
in lung in relation to silica-induced diseases. International
archives of occupational and environmental health, 71:263-269.
Eisen EA, Smith TJ, Wegman DH, Louis TA, Froines J (1984) Estimation
of long term dust exposures in the Vermont granite sheds.
American Industrial Hygiene Association Journal, 45(2):89-94.
Erdogdu G, Hasirci V (1998) An overview of the role of mineral
solubility in silicosis and asbestosis. Environmental research,
78:38-42.
Evans MG, Slocombe RF, Schwartz LD (1988) Pulmonary silicosis in
captive ring-necked pheasants: definitive diagnosis by electron probe
X-ray microanalysis. Veterinary pathology, 25(3):239-241.
Forastiere F, Lagorio S, Michelozzi P, Perucci CA, Axelson O (1989)
Mortality pattern of silicotic subjects in the Latium region, Italy.
British journal of industrial medicine, 46(12):877-880.
Fox AJ, Greenberg M, Ritchie GL (1975) A survey of respiratory
disease in the pottery industry. London, Her Majesty's Stationery
Office.
Freeman CS, Grossman EA (1995) Silica exposures in the United States
between 1980 and 1992. Scandinavian journal of work, environment and
health, 21 (Suppl. 2):47-49.
Fubini B (1997) Surface reactivity in the pathogenic response to
particulates. Environmental health perspectives, 105 (Suppl.
5):1013-1020.
Fubini B (1998) Surface chemistry and quartz hazard. Annals of
occupational hygiene, 42(8):521-530.
Fubini B, Bolis V, Cavenago A, Volante M (1995) Physicochemical
properties of crystalline silica dusts and their possible implication
in various biological responses. Scandinavian journal of work,
environment and health, 21 (Suppl. 2):9-14.
Fukata S, Matsubayashi S, Nagato H, Sakai K, Ohishi S, Yasuda M, Tamai
H, Nakagawa T (1983) Monoclonal gammopathies associated with
silicosis. Rinsho Ketsueki, 24(1):9-17.
Fukata S, Tamai H, Nagai K, Matsubayashi S, Nagato H, Tashiro T,
Yasuda M, Kumagai LF (1987) A patient with hereditary spherocytosis
and silicosis who developed an IgA(lambda) monoclonal gammopathy.
Japanese journal of medicine, 26(1):81-83.
Fulekar MH, Alam Khan MM (1995) Occupational exposure to dust in slate
pencil manufacture. Annals of occupational hygiene, 39(1):107-114.
Gao H, Brick J, Ong S, Miller M, Whong W-Z, Ong T (1997) Selective
hyperexpression of c-jun oncoprotein by glass fiber- and
silica-transformed BALB/c-3T3 cells. Cancer letters, 112:65-69.
Gerhardsson G (1976) Dust prevention in Swedish foundries.
Staub-Reinhaltung der Luft, 36:433-439.
Gift JS, Faust RA (1997) Noncancer inhalation toxicology of
crystalline silica: exposure-response assessment. Journal of exposure
analysis and environmental epidemiology, 7(3): 345-358.
Giles RD, Sturgill BC, Suratt PM, Bolton WK (1978) Massive proteinuria
and acute renal failure in a patient with acute silicoproteinosis.
American journal of medicine, 64:336-342.
Goldsmith DF (1994) Health effects of silica dust exposure. In: Heaney
PJ, Prewitt CT, Gibbs GV, eds. Silica: physical behavior,
geochemistry, and materials applications. Washington, DC,
Mineralogical Society of America. Reviews in mineralogy, 29:545-606.
Goldsmith DF (1998) Uses of workers' compensation data in epidemiology
research. Occupational medicine state of the art reviews,
13(2):389-415.
Goldsmith DF, Hertz-Picciotto I (1997) Criteria for conducting
quantitative risk assessments for silica. Journal of exposure
analysis and environmental epidemiology, 7(3):367-375.
Goldsmith DF, Beaumont JJ, Morrin LA, Schenker MB (1995a) Respiratory
cancer and other chronic disease mortality among silicotics in
California. American journal of industrial medicine, 28(4):459-467.
Goldsmith DF, Ruble RP, Klein CA (1995b) Comparative cancer potency
for silica from extrapolations of human and animal findings.
Scandinavian journal of work, environment and health, 21 (Suppl.
2):104-107.
Green FHY, Vallyathan V (1996) Pathologic responses to inhaled silica.
In: Castranova V, Vallyathan V, Wallace WE, eds. Silica and
silica-induced lung diseases. Boca Raton, FL, CRC Press, pp. 39-59.
Greskevitch MF, Turk AR, Dieffenbach AL, Roman JM, Groce DW, Hearl FJ
(1992) Quartz analyses of the bulk dust samples collected by the
National Occupational Health Survey of Mining. Applied occupational
and environmental hygiene, 7(8):527-531.
Greskevitch MF, Bajpayee SS, Hale JM, Groce DW, Hearl FJ, eds. (1996)
NIOSH technical report: results from the National Occupational Health
Survey of Mining (NOHSM). Cincinnati, OH, US Department of Health
and Human Services, Public Health Service, Centers for Disease Control
and Prevention, National Institute for Occupational Safety and Health,
Division of Respiratory Disease Studies (DHHS (NIOSH) Publication No.
96-136).
Groth DH, Stettler LE, Platek SF, Lal JB, Burg JR (1986) Lung tumors
in rats treated with quartz by intratracheal instillation. In:
Goldsmith DF, Winn DM, Shy CM, eds. Silica, silicosis, and cancer:
Controversy in occupational medicine. New York, NY, Praeger
Publishers, pp. 243-253 (Cancer Research Monographs, Vol. 2).
Gu Z-W, Ong TO (1996) Potential mechanisms of silica-induced cancer.
In: Castranova V, Vallyathan V, Wallace WE, eds. Silica and
silica-induced lung diseases. Boca Raton, FL, CRC Press, pp.
397-406.
Guenel P, Hojberg G, Lynge E (1989) Cancer incidence among Danish
stone workers. Scandinavian journal of work, environment and health,
15:265-270.
Hansen HJ, Jama FM, Nilsson C, Norrgren L, Abdurahman OS (1989)
Silicate pneumoconiosis in camels (Camelus dromedarius L.).
Zentralblatt für Veterinarmedizin (A), 36(10):789-796.
Hart GA, Hesterberg TW (1998) In vitro toxicity of respirable-size
particles of diatomaceous earth and crystalline silica compared with
asbestos and titanium dioxide. Journal of occupational and
environmental medicine, 40(1):29-42.
Haslam PL (1994) Basic immunology and immunopathology. In: Parkes WR,
ed. Occupational lung disorders, 3rd ed. London,
Butterworth-Heinemann, pp. 50-99.
Hauglustaine D, Van Damme B, Daenens P, Michielsen P (1980) Silicon
nephropathy: a possible occupational hazard. Nephron, 26:219-224.
Haustein UF, Anderegg U (1998) Silica induced scleroderma -- clinical
and experimental aspects. Journal of rheumatology, 25(10):1917-1926.
Haustein UF, Ziegler V, Herrmann K, Mehlhorn J, Schmidt C (1990)
Silica-induced scleroderma. Journal of the American Academy of
Dermatology, 22(3):444-448.
Haustein UF, Ziegler V, Herrmann K (1992) Chemically induced
scleroderma. Der Hautarzt, 43:464-474.
Heppleston AG (1994) Pathogenesis of mineral pneumoconioses. In:
Parkes WR, ed. Occupational lung disorders, 3rd ed. London,
Butterworth-Heinemann, pp. 100-134.
Hesterberg TW, Barrett JC (1984) Dependence of asbestos- and mineral
dust-induced transformation of mammalian cells in culture on fiber
dimension. Cancer research, 44:2170-2180.
Hesterberg TW, Oshimura M, Brody AR, Barrett JC (1986) Asbestos and
silica induce morphological transformation of mammalian cells in
culture: a possible mechanism. In: Goldsmith DF, Winn DM, Shy CM, eds.
Silica, silicosis, and cancer. Controversy in occupational medicine.
New York, NY, Praeger Publishers, pp. 177-190 (Cancer Research
Monographs, Vol. 2).
Higgins RI, Deere MR, Cinkotai FF (1985) Fettlers' exposure to pottery
dust in a factory making sanitary whiteware. Annals of occupational
hygiene, 29(3):365-375.
Hnizdo E, Murray J (1998) Risk of pulmonary tuberculosis relative to
silicosis and exposure to silica dust in South African gold miners.
Occupational and environmental medicine, 55:496-502.
Hnizdo E, Murray J (1999) Correction: Risk of pulmonary tuberculosis
relative to silicosis and exposure to silica dust in South African
gold miners. Occupational and environmental medicine, 56:215-216.
Hnizdo E, Sluis-Cremer GK (1991) Silica exposure, silicosis, and lung
cancer: a mortality study of South African gold miners. British
journal of industrial medicine, 48:53-60.
Hnizdo E, Sluis-Cremer GK (1993) Risk of silicosis in a cohort of
white South African gold miners. American journal of industrial
medicine, 24:447-457.
Hnizdo E, Murray J, Sluis-Cremer GK, Thomas RG (1993) Correlation
between radiological and pathological diagnosis of silicosis: an
autopsy population based study. American journal of industrial
medicine, 24:427-445.
Hnizdo E, Murray J, Klempman S (1997) Lung cancer in relation to
exposure to silica dust, silicosis and uranium production in South
African gold miners. Thorax, 52:271-275.
Holland LM, Gonzales M, Wilson JS, Tillery MI (1983) Pulmonary effects
of shale dusts in experimental animals. In: Wagner WL, Rom WN,
Merchant JA, eds. Health issues related to metal and nonmetallic
mining. Boston, MA, Butterworth, pp. 485-496.
Holland LM, Wilson JS, Tillery MI, Smith DM (1986) Lung cancer in rats
exposed to fibrogenic dusts. In: Goldsmith DF, Winn DM, Shy CM, eds.
Silica, silicosis, and cancer: Controversy in occupational medicine.
New York, NY, Praeger Publishers, pp. 267-279 (Cancer Research
Monographs, Vol. 2).
Holman CDJ, Psaila-Savona P, Roberts M, McNulty JC (1987) Determinants
of chronic bronchitis and lung dysfunction in Western Australian gold
miners. British journal of industrial medicine, 44:810-818.
Hook GER, Viviano CJ (1996) Acute silicosis and the activation of
alveolar type II cells. In: Castranova V, Vallyathan V, Wallace WE,
eds. Silica and silica-induced lung diseases. Boca Raton, FL, CRC
Press, pp. 229-242.
Hotz P, Gonzalez-Lorenzo J, Siles E, Trujillano G, Lauwerys R, Bernard
A (1995) Subclinical signs of kidney dysfunction following short
exposure to silica in the absence of silicosis. Nephron, 70:438-442.
Hua F, Xipeng J, Shunzhang Y, Xueqi G, Kaiguo W, JianY, Shenghua Q
(1992) Quantitative risk assessment for lung cancer from exposure to
metal ore dust. Biomedical and environmental sciences, 5:221-228.
Huggins CW, Johnson SN, Segreti JM, Snyder JG (1985) Bureau of Mines
Report of Investigations 8975: Determination of alpha quartz particle
distribution in respirable coal mine dust samples and reference
standards. Avondale, MD, US Department of the Interior, Bureau of
Mines, 7 pp. (National Technical Information Service Publication No.
PB86-136660).
Hughes JM (1995) Radiographic evidence of silicosis in relation to
silica exposure. Applied occupational and environmental hygiene,
10(12):1064-1069.
IARC (1984) Polynuclear aromatic compounds. Part 3. Industrial
exposures in aluminium production, coal gasification, coke production
and iron and steel founding. Lyon, International Agency for Research
on Cancer, pp. 133-190 (IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Vol. 34).
IARC (1987) Silica and some silicates. Lyon, France, International
Agency for Research on Cancer, pp. 33-143 (IARC Monographs on the
Evaluation of the Carcinogenic Risk of Chemicals to Humans, Vol. 42).
IARC (1997) Silica, some silicates, coal dust and para -aramid
fibrils. Lyon, International Agency for Research on Cancer, pp.
1-242 (IARC Monographs on the Evaluation of Carcinogenic Risks to
Humans, Vol. 68).
Iler RK (1979) The chemistry of silica: solubility, polymerization,
colloid and surface properties, and biochemistry. New York, NY,
Wiley-Interscience Publishers.
ILO (1980) Guidelines for the use of ILO international classification
of radiographs of pneumoconioses, rev. ed. Geneva, International
Labour Organisation, 48 pp. (Occupational Safety and Health Series No.
22).
ILO (1991) Occupational lung diseases: prevention and control.
Geneva, International Labour Organisation, 85 pp. (Occupational
Safety and Health Series No. 67).
IPCS (1993) International Chemical Safety Card -- Quartz. Geneva,
World Health Organization, International Programme on Chemical Safety
(ICSC 0808).
Iyer R, Holian A (1996) Immunological aspects of silicosis. In:
Castranova V, Vallyathan V, Wallace WE, eds. Silica and
silica-induced lung diseases. Boca Raton, FL, CRC Press, pp.
253-267.
Jaurand MC, Fleury J, Monchaux G, Nebut M, Bignon J (1987) Pleural
carcinogenic potency of mineral fibers (asbestos, attapulgite) and
their cytotoxicity on cultured cells. Journal of the National Cancer
Institute, 79(4):797-804.
Kane AB (1996) Questions and controversies about the pathogenesis of
silicosis. In: Castranova V, Vallyathan V, Wallace WE, eds. Silica
and silica-induced lung diseases. Boca Raton, FL, CRC Press, pp.
121-136.
Kauppinen T, Partanen T, Degerth R, Ojajarvi A (1995) Pancreatic
cancer and occupational exposures. Epidemiology, 6(5):498-502.
Kelly J (1992) U.S. Department of the Interior, National Park
Service, New River Gorge National River, West Virginia. Cincinnati,
OH, National Institute for Occupational Safety and Health (HHE Report
No. HETA 92-045-2260),
King EJ, Mohanty GP, Harrison CV, Nagelschmidt G (1953) The action of
different forms of pure silica on the lungs of rats. British journal
of industrial medicine, 10:9-17.
Kleinbaum DG, Kupper LL, Morgenstern H (1982) Epidemiologic research:
principles and quantitative methods. New York, NY, Van Nostrand
Reinhold, p. 428.
Kleinschmidt I, Churchyard G (1997) Variation in incidences of
tuberculosis in subgroups of South African gold miners. Occupational
and environmental medicine, 54:636-641.
Klockars M, Koskela RS, Järvinen E, Kolari PJ, Rossi A (1987) Silica
exposure and rheumatoid arthritis: a follow up study of granite
workers 1940-81. British medical journal, 294:997-1000.
Koeger AC, Alcaix D, Rozenberg S, Bourgeois P (1991) Occupational
exposure to silica and dermatopolymyositis: three cases. Annales de
medecine interne (Paris), 142(6):409-413.
Koeger AC, Alcaix D, Rozenberg S, Bourgeois P (1996) Graves' disease
after silica dust exposure. Journal of rheumatology, 68(11):202.
Koskela R-S, Klockars M, Järvinen E, Kolari PJ, Rossi A (1987)
Mortality and disability among granite workers. Scandinavian journal
of work, environment and health, 13:18-25.
Kreiss K, Zhen B (1996) Risk of silicosis in a Colorado mining
community. American journal of industrial medicine, 30:529-539.
Kreiss K, Greenberg LM, Kogut SJH, Lezotte DC, Irvin CG, Cherniack RM
(1989) Hard-rock mining exposures affect smokers and nonsmokers
differently: results of a community prevalence study. American review
of respiratory disease, 139:1487-1493.
Kullman GJ, Greife AL, Costello J, Hearl FJ (1995) Occupational
exposures to fibers and quartz at 19 crushed stone mining and milling
operations. American journal of industrial medicine, 27:641-660.
Kurppa K, Gudbergsson H, Hannunkari I, Koskinen H, Hernberg S, Koskela
R-S, Ahlman K (1986) Lung cancer among silicotics in Finland. In:
Goldsmith DF, Winn DM, Shy CM, eds. Silica, silicosis, and cancer:
Controversy in occupational medicine. New York, NY, Praeger
Publishers, pp. 311-319 (Cancer Research Monographs, Vol. 2).
Kusiak R, Liss GM, Gailitis MM (1993) Cor pulmonale and pneumoconiotic
lung disease: an investigation using hospital discharge data.
American journal of industrial medicine, 24:161-173.
Lapp NL, Castranova V (1993) How silicosis and coal workers'
pneumoconiosis develops -- a cellular assessment. Occupational
medicine state of the art reviews, 8(1):35-56.
Lawson RJ, Schenker MB, McCurdy SA, Jenkins B, Lischak LA, John W,
Scales D (1995) Exposure to amorphous silica fibers and other
particulate matter during rice farming operations. Applied
occupational and environmental hygiene, 10(8):677-684.
Leigh J, Wang H, Bonin A, Peters M, Ruan X (1997) Silica-induced
apoptosis in alveolar and granulomatous cells in vivo.
Environmental health perspectives, 105 (Suppl. 5):1241-1245.
Leigh J, Wang H, Bonin A, Peters M (1998a) Persistence of
silica-induced inflammation is related to delayed occurrence of
apoptosis in alveolar leukocytes. In: Chiyotani K, Hosoda Y, Aizawa Y,
eds. Advances in the prevention of occupational respiratory diseases.
Amsterdam, Elsevier Science, pp. 890-895 (Excerpta Medica Supplement
53).
Leigh J, Wang H, Bonin A, Peters M (1998b) In vivo genotoxicity of
silica evidenced by progressive development of micronuclei in alveolar
macrophages. In: Chiyotani K, Hosoda Y, Aizawa Y, eds. Advances in
the prevention of occupational respiratory diseases. Amsterdam,
Elsevier Science, pp. 520-525 (Excerpta Medica Supplement 53).
Linch KD, Cocalis JC (1994) An emerging issue: Silicosis prevention in
construction. Applied occupational and environmental hygiene,
9(8):539-542.
Linch KD, Miller WE, Althouse RB, Groce DW, Hale JM (1998)
Surveillance of respirable crystalline silica dust using OSHA
compliance data (1979-1995). American journal of industrial medicine,
34(6):547-558.
Liu X, Keane MJ, Zhong B-Z, Ong T, Wallace WE (1996) Micronucleus
formation in V79 cells treated with respirable silica dispersed in
medium and in simulated pulmonary surfactant. Mutation research,
361:89-94.
Liu X, Keane MJ, Harrison JC, Cilento EV, Ong T, Wallace WE (1998)
Phospholipid surfactant adsorption by respirable quartz and in vitro
expression of cytotoxicity and DNA damage. Toxicology letters, 96,
97:77-84.
Lofgren DJ (1993) Case studies: silica exposure for concrete workers
and masons. Applied occupational and environmental hygiene,
8(10):832-836.
Love RG, Waclawski ER, Maclaren WM, Porteous RH, Groat SK, Wetherill
GZ, Hutchison PA, Kidd MW, Soutar CA (1994) Cross-sectional study of
risks of respiratory disease in relation to exposures of airborne
quartz in the heavy clay industry. Edinburgh, Institute of
Occupational Medicine (IOM Report No. TM/94/07).
Mao Y, Daniel LN, Whittaker N, Saffiotti U (1994) DNA binding to
crystalline silica characterized by Fourier-transform infrared
spectroscopy. Environmental health perspectives, 102
(Suppl. 10):165-171.
Masuda K (1981) Chronic thyroiditis observed in patients with
silicosis as an adjuvant disease of man. Shikoku Acta Medica,
37(1):119-129.
Materna BL, Jones JR, Sutton PM, Rothman N, Harrison RJ (1992)
Occupational exposures in California wildland fire fighting. American
Industrial Hygiene Association journal, 53(1):69-76.
McDonald C (1995) Silica, silicosis, and lung cancer: an
epidemiological update. Applied occupational and environmental
hygiene, 10(12):1056-1063.
McDonald JC, Oakes D (1984) Exposure-response in miners exposed to
silica. In: Sixth International Pneumoconiosis Conference: 1983,
Bochum, Germany. Vol 1. Geneva, International Labour Organisation,
pp. 114-123.
McDonald JC, Cherry N, McNamee R, Burgess G, Turner S (1995)
Preliminary analysis of proportional mortality in a cohort of British
pottery workers exposed to crystalline silica. Scandinavian journal
of work, environment and health, 21 (Suppl. 2):63-65.
McDonald JC, Burgess GL, Turner S, Cherry NM (1997) Cohort study of
Staffordshire pottery workers: (III) Lung cancer, radiographic
changes, silica exposure and smoking habit. Annals of occupational
hygiene, 41 (Suppl. 1):412-414.
McLaughlin JK, Jing-Qiong C, Dosemeci M, Rong-An C, Rexing SH, Zhien
W, Hearl FJ, McCawley MA, Blot WJ (1992) A nested case-control study
of lung cancer among silica exposed workers in China. British journal
of industrial medicine, 49:167-171.
McNeill DA, Chrisp CE, Fisher GL (1990) Pulmonary adenomas in A/J mice
treated with silica. Drug and chemical toxicology, 13(1):87-92.
Merlo F, Costantini M, Reggiardo G, Ceppi M, Puntoni R (1991) Lung
cancer risk among refractory brick workers exposed to crystalline
silica: a retrospective cohort study. Epidemiology, 2(4):299-305.
Morrow PE (1988) Issues: Possible mechanisms to explain dust
overloading of the lungs. Fundamental and applied toxicology,
10:369-384.
Morrow PE (1992) Contemporary issues in toxicology -- dust overloading
of the lungs: update and appraisal. Toxicology and applied
pharmacology, 113:1-12.
Mossman BT, Churg A (1998) State of the art: Mechanisms in the
pathogenesis of asbestosis and silicosis. American journal of
respiratory and critical care medicine, 157:1666-1680.
Mozzon D, Brown DA, Smith JW (1987) Occupational exposure to airborne
dust, respirable quartz and metals arising from refuse handling,
burning and landfilling. American Industrial Hygiene Association
journal, 48(2):111-116.
Muhle H, Takenaka S, Mohr U, Dasenbrock C, Mermelstein R (1989) Lung
tumor induction upon long term low level inhalation of crystalline
silica. American journal of industrial medicine, 15(3):343-346.
Muhle H, Bellmann B, Creutzenberg O, Dasenbrock C, Ernst H, Kilpper R,
Mackenzie JC, Morrow P, Mohr U, Takenaka S, Mermelstein R (1991)
Pulmonary response to toner upon chronic inhalation exposure in rats.
Fundamental and applied toxicology, 17:280-299.
Muhle H, Kittel B, Ernst H, Mohr U, Mermelstein R (1995) Neoplastic
lung lesions in rat after chronic exposure to crystalline silica.
Scandinavian journal of work, environment and health, 21
(Suppl. 2):27-29.
Muir DCF (1991) Correction in cumulative risk in silicosis exposure
assessment. American journal of industrial medicine, 19:555.
Muir DCF, Shannon HS, Julian JA, Verma DK, Sebestyen A, Bernholz CD
(1989a) Silica exposure and silicosis among Ontario hardrock miners:
I. Methodology. American journal of industrial medicine, 16:5-11.
Muir DCF, Shannon HS, Julian JA, Verma DK, Sebestyen A, Bernholz CD
(1989b) Silica exposure and silicosis among Ontario hardrock miners:
III. Analysis and risk estimates. American journal of industrial
medicine, 16:29-43.
Muramatsu K, Yamamoto T, Hasegawa A et al. (1989) [A case of
autoimmune hemolytic anemia associated with silicosis.] Japanese
journal of chest diseases, 48(1):45-50 (in Japanese).
Murray J, Reid G, Kielkowski D, de Beer M (1993) Cor pulmonale and
silicosis: a necropsy based case-control study. British journal of
industrial medicine, 50:544-548.
Myers JE, Lewis P, Hofmeyr W (1989) Respiratory health of brickworkers
in Cape Town, South Africa: background, aims and dust exposure
determinations. Scandinavian journal of work, environment and health,
15(3):180-187.
Nagalakshmi R, Nath J, Ong T, Whong W-Z (1995) Silica-induced
micronuclei and chromosomal aberrations in Chinese hamster lung (V79)
and human lung (Hel 299) cells. Mutation research, 335:27-33.
Nehls P, Seiler F, Rehn B, Greferath R, Bruch J (1997) Formation and
persistence of 8-oxoguanine in rat lung cells as an important
determinant for tumor formation following particle exposure.
Environmental health perspectives, 105 (Suppl. 5):1291-1296.
Neyer U, Woss E, Neuweiler J (1994) Case report: Wegener's
granulomatosis associated with silicosis. Nephrology dialysis
transplantation, 9(5):559-561.
Ng TP, Yeung KH, O'Kelly FJ (1987a) Silica hazard of caisson
construction in Hong Kong. Journal of the Society of Occupational
Medicine, 37:62-65.
Ng TP, Tsin TW, O'Kelly FJ, Chan SL (1987b) A survey of the
respiratory health of silica-exposed gemstone workers in Hong Kong.
American review of respiratory disease, 135:1249-1254.
Ng TP, Lee HS, Phoon WH (1993) Further evidence of human silica
nephrotoxicity in occupationally exposed workers. British journal of
industrial medicine, 50:907-912.
Niemeier RW, Mulligan LT, Rowland J (1986) Cocarcinogenicity of
foundry silica sand in hamsters. In: Goldsmith DF, Winn DM, Shy CM,
eds. Silica, silicosis, and cancer: Controversy in occupational
medicine. New York, NY, Praeger Publishers, pp. 215-227 (Cancer
Research Monographs, Vol. 2).
NIOSH (1974) Criteria for a recommended standard: occupational
exposure to crystalline silica. Cincinnati, OH, US Department of
Health, Education, and Welfare, Health Services and Mental Health
Administration, National Institute for Occupational Safety and Health
(DHEW (NIOSH) Publication No. 75-120).
NIOSH (1992a) NIOSH Alert: Request for assistance in preventing
silicosis and deaths from sandblasting. Cincinnati, OH, US
Department of Health and Human Services, Public Health Service,
Centers for Disease Control, National Institute for Occupational
Safety and Health (DHHS (NIOSH) Publication No. 92-102).
NIOSH (1992b) NIOSH Alert: Request for assistance in preventing
silicosis and deaths in rock drillers. Cincinnati, OH, US Department
of Health and Human Services, Public Health Service, Centers for
Disease Control, National Institute for Occupational Safety and Health
(DHHS (NIOSH) Publication No. 92-107).
NIOSH (1994a) Silica, crystalline, respirable by XRD: Method 7500,
Issue 2 (dated 8/15/94). In: Cassinelli ME, O'Connor PF, eds. NIOSH
manual of analytical methods, 4th ed. Cincinnati, OH, US Department
of Health and Human Services, Public Health Service, Centers for
Disease Control and Prevention, National Institute for Occupational
Safety and Health (DHHS (NIOSH) Publication No. 94-113).
NIOSH (1994b) Silica, crystalline, respirable by IR: Method 7602,
Issue 2 (dated 8/15/94). In: Cassinelli ME, O'Connor PF, eds. NIOSH
manual of analytical methods, 4th ed. Cincinnati, OH, US Department
of Health and Human Services, Public Health Service, Centers for
Disease Control and Prevention, National Institute for Occupational
Safety and Health (DHHS (NIOSH) Publication No. 94-113).
NIOSH (1996) NIOSH Alert: Request for assistance in preventing
silicosis and deaths in construction workers. Cincinnati, OH, US
Department of Health and Human Services, Public Health Service,
Centers for Disease Control and Prevention, National Institute for
Occupational Safety and Health (DHHS (NIOSH) Publication No. 96-112).
NIOSH (1997) Pocket guide to chemical hazards. Cincinnati, OH, US
Department of Health and Human Services, Public Health Service,
Centers for Disease Control and Prevention, National Institute for
Occupational Safety and Health (DHHS (NIOSH) Publication No. 97-140).
NIOSH (forthcoming) NIOSH special hazard review: Health effects of
occupational exposure to respirable crystalline silica. Cincinnati,
OH, US Department of Health and Human Services, Public Health Service,
Centers for Disease Control and Prevention, National Institute for
Occupational Safety and Health (DHHS (NIOSH) Publication).
Nuyts GD, Van Vlem E, De Vos A, Daelemans RA, Rorive G, Elseviers MM,
Schurgers M, Segaert M, D'Haese PC, De Broe ME (1995) Wegener
granulomatosis is associated to exposure to silicon compounds: a
case-control study. Nephrology dialysis transplantation,
10:1162-1165.
Oberdörster G (1997) Pulmonary carcinogenicity of inhaled particles
and the maximum tolerated dose. Environmental health perspectives,
195 (Suppl. 5):1347-1355.
Oshimura M, Hesterberg TW, Tsutsui T, Barrett JC (1984) Correlation of
asbestos-induced cytogenetic effects with cell transformation of
Syrian hamster embryo cells in culture. Cancer research,
44:5017-5022.
Osorio AM, Thun MJ, Novak RF, Van Cura J, Avner ED (1987) Silica and
glomerulonephritis: case report and review of the literature.
American journal of kidney diseases, 9(3):224-230.
Otsuki T, Sakaguchi H, Tomokuni A, Aikoh T, Matsuki T, Kawakami Y,
Kusaka M, Ueki H, Kita S, Ueki A (1998) Soluble Fas mRNA is dominantly
expressed in cases with silicosis. Immunology, 94:258-262.
Oudyk JD (1995) Review of an extensive ferrous foundry silica sampling
program. Applied occupational and environmental hygiene, 10:331-340.
Pairon JC, Jaurand MC, Kheuang L, Janson X, Brochard P, Bignon J
(1990) Sister chromatid exhanges in human lymphocytes treated with
silica. British journal of industrial medicine, 47:110-115.
Pan G, Takahashi K, Feng Y, Liu L, Liu T, Zhang S, Liu N, Okubo T,
Goldsmith DF (1999) Nested case-control study of esophageal cancer in
relation to occupational exposure to silica and other dusts. American
journal of industrial medicine, 35:272-280.
Parent ME, Siemiatycki J, Fritschi L (1998) Occupational exposures and
gastric cancer. Epidemiology, 9(1):48-55.
Parker JE (1994) Silicosis. In: Rakel RE, ed. Conn's current therapy.
Philadelphia, PA, W.B. Saunders, pp. 207-210.
Parkes WR, ed. (1982) Occupational lung disorders, 2nd ed. London,
Butterworths, pp. 134-174.
Partanen T, Pukkala E, Vainio H, Kurppa K, Koskinen H (1994) Increased
incidence of lung and skin cancer in Finnish silicotic patients.
Journal of occupational medicine, 36(6):616-622.
Peters JM (1986) Silicosis. In: Merchant JA, Boehlecke BA, Taylor G,
Pickett-Harner M, eds. Occupational respiratory diseases.
Cincinnati, OH, US Department of Health and Human Services, Public
Health Service, Centers for Disease Control, National Institute for
Occupational Safety and Health, pp. 219-237 (DHHS (NIOSH) Publication
No. 86-102).
Petersen PE, Henmar P (1988) Oral conditions among workers in the
Danish granite industry. Scandinavian journal of work, environment
and health, 14:328-331.
Popendorf WJ, Pryor A, Wenk HR (1982) Mineral dust in manual harvest
operations. Annals of the American Conference of Governmental
Industrial Hygienists, 2:101-115.
Pott F, Dungworth DL, Heinrich U, Muhle H, Kamino K, Germann P-G,
Roller M, Rippe RM, Mohr U (1994) Lung tumours in rats after
intratracheal instillation of dusts. Annals of occupational hygiene,
38 (Suppl. 1):357-363.
Pouthier D, Duhoux P, Van Damme B (1991) Pulmonary silicosis and
glomerular nephropathy: Apropos of 1 case. Nephrologie, 12(1):8-11.
Price-Jones MJ, Gubbings G,Chamberlain M (1980) The genetic effects of
crocidolite asbestos; comparison of chromosome abnormalities and
sister-chromatid exchanges. Mutation research, 79:331-336.
Puntoni R, Goldsmith DG, Valerio F, Vercelli M, Bonassi S, Di Giorgio
F, Ceppi M, Stagnaro E, Filiberti R, Santi L, Merlo F (1988) A cohort
study of workers employed in a refractory brick plant. Tumori,
74:27-33.
Rabovsky J (1997) Laboratory studies on silica induced toxicity and
relationship to carcinogenicity. Journal of exposure analysis and
environmental epidemiology, 7(3):267-278.
Rees D, Cronje R, du Toit RSJ (1992) Dust exposure and pneumoconiosis
in a South African pottery. 1. Study objectives and dust exposure.
British journal of industrial medicine, 49:459-464.
Renne RA, Eldridge SR, Lewis TR, Stevens DL (1985) Fibrogenic
potential of intratracheally instilled quartz, ferric oxide, fibrous
glass, and hydrated alumina in hamsters. Toxicologic pathology,
13(4):306-314.
Reuzel PGJ, Bruijntjes JP, Feron VJ, Woutersen RA (1991) Subchronic
inhalation toxicity of amorphous silicas and quartz dust in rats.
Food and chemical toxicology, 29(5):341-354.
Riala R (1988) Dust and quartz exposure of Finnish construction
cleaners. Annals of occupational hygiene, 32(2):215-220.
Rice CH, Harris RL, Checkoway H, Symons MJ (1986) Dose-response
relationships for silicosis from a case-control study of North
Carolina dusty trades workers. In: Goldsmith DF, Winn DM, Shy CM, eds.
Silica, silicosis, and cancer: Controversy in occupational medicine.
New York, NY, Praeger Publishers, pp. 77-86 (Cancer Research
Monographs, Vol. 2).
Rice FL, Stayner LT (1995) Assessment of silicosis risk for
occupational exposure to crystalline silica. Scandinavian journal of
work, environment and health, 21 (Suppl. 2):87-90.
Rispal P, Wen L, De Precigout V, Aparicio M (1991) Nephropathy with
silica in a dental technician. La presse medicale, 20(4):176.
Robbins SL (1974) Pathologic basis of disease. Philadelphia, PA,
W.B. Saunders Co., pp. 239-240.
Rosenman KD, Reilly MJ, Rice C, Hertzberg V, Tseng C-Y, Anderson HA
(1996) Silicosis among foundry workers: implication for the need to
revise the OSHA standard. American journal of epidemiology,
144(9):890-900.
Saffiotti U (1990) Lung cancer induction by silica in rats, but not in
mice and hamsters: species differences in epithelial and granulomatous
reactions. In: Seemayer NH, Hadnagy W, eds. Environmental hygiene II.
New York, NY, Springer-Verlag, pp. 235-238.
Saffiotti U (1992) Lung cancer induction by crystalline silica. In:
D'Amato RD, Slaga TJ, Farland WH, Henry C, eds. Relevance of animal
studies to the evaluation of human cancer risk. New York, NY,
Wiley-Liss, pp. 51-69.
Saffiotti U, Ahmed N (1995) Neoplastic transformation by quartz in the
BALB/3T3/A31-1-1 cell line and the effects of associated minerals.
Teratogenesis, carcinogenesis, and mutagenesis, 15(6):339-356.
Saffiotti U, Daniel LN, Mao Y, Williams AO, Kaighn ME, Ahmed N,
Knapton AD (1993) Biological studies on the carcinogenic mechanisms of
quartz. In: Guthrie GD, Mossman BT, eds. Health effects of mineral
dusts. Washington, DC, Mineralogical Society of America. Reviews in
mineralogy, 28:523-544.
Saffiotti U, Williams O, Lambert N, Daniel N, Kaighn ME, Mao Y, Shi X
(1996) Carcinogenesis by crystalline silica: animal, cellular, and
molecular studies. In: Castranova V, Vallyathan V, Wallace WE, eds.
Silica and silica-induced lung diseases. Boca Raton, FL, CRC Press,
pp. 345-381.
Saita G, Zavaglia O (1951) Kidney function in silicotics.
Medicina del Lavoro, 42(2):41-48.
Salisbury S, Melius J (1982) Harbison-Walker Refractories, Fairfield,
Alabama, Bessemer, Alabama. Cincinnati, OH, National Institute for
Occupational Safety and Health (HHE Report No. HETA-80-086-1191).
Samimi B, Weill H, Ziskind M (1974) Respirable silica dust exposure of
sandblasters and associated workers in steel fabrication yards.
Archives of environmental health, 29:61-66.
Schapira RM, Ghio AJ, Effros RM, Morrisey J, Almagro UA, Dawson CA,
Hacker AD (1995) Hydroxyl radical production and lung injury in the
rat following silica or titanium dioxide instillation in vivo.
American journal of respiratory cell and molecular biology,
12(2):220-226.
Scheuchenzuber WJ, Eskew ML, Zarkower A (1985) Effects of prolonged
inhalation of silica and olivine dusts on immune functions in the
mouse. Environmental research, 38(2):389-399.
Schwartz LW, Knight HD, Whittig LD, Malloy RL, Abraham JL,Tyler NK
(1981) Silicate pneumoconiosis and pulmonary fibrosis in horses from
the Monterey-Carmel peninsula. Chest, 80 (Suppl. 1):82-85.
Seaton A (1995) Silicosis. In: Morgan WKC, Seaton A, eds.
Occupational lung diseases, 3rd ed. Philadelphia, PA, W.B. Saunders
Co., pp. 222-267.
Seta JA, Sundin DA, Pedersen DH, eds. (1988) National occupational
exposure survey field guidelines. Vol. 1. Cincinnati, OH, US
Department of Health and Human Services, Public Health Service,
Centers for Disease Control, National Institute for Occupational
Safety and Health, Division of Surveillance, Hazard Evaluations, and
Field Studies (DHHS (NIOSH) Publication No. 88-106).
Sherson D, Jorgensen F (1989) Rapidly progressive crescenteric
glomerulonephritis in a sandblaster with silicosis. British journal
of industrial medicine, 46:675-676.
Sherson D, Lander F (1990) Morbidity of pulmonary tuberculosis among
silicotic and nonsilicotic foundry workers in Denmark. Journal of
occupational medicine, 32(2):110-113.
Shi X, Mao Y, Daniel LN, Saffiotti U, Dalal NS, Vallyathan V (1994)
Silica radical-induced DNA damage and lipid peroxidation.
Environmental health perspectives, 102 (Suppl. 10):149-154.
Siltanen E, Koponen M, Kokko A, Engström B, Reponen J (1976) Dust
exposure in Finnish foundries. Scandinavian journal of work,
environment and health, 2 (Suppl. 1):19-31.
Silverstein M, Maizlish N, Park R, Silverstein B, Brodsky L, Mirer F
(1986) Mortality among ferrous foundry workers. American journal of
industrial medicine, 10(1):27-43.
Slavin RE, Swedo JL, Brandes D, Gonzalez-Vitale JC, Osornio-Vargas A
(1985) Extrapulmonary silicosis: a clinical, morphologic, and
ultrastructural study. Human pathology, 16(4):393-412.
Sluis-Cremer GK, Hessel PA, Hnizdo E, Churchill AR, Zeiss EA (1985)
Silica, silicosis, and progressive systemic sclerosis. British
journal of industrial medicine, 42:838-843.
Sluis-Cremer GK, Hessel PA, Hnizdo E, Churchill AR (1986) Relationship
between silicosis and rheumatoid arthritis. Thorax, 41:596-601.
Smith AH, Lopipero PA, Barroga VR (1995) Meta-analysis of studies of
lung cancer among silicotics. Epidemiology, 6(6):617-624.
Sobti RC, Bhardwaj DK (1991) Cytogenetic damage and occupational
exposure: I. Exposure to stone dust. Environmental research,
56:25-30.
Soutar CA, Robertson A, Miller BG, Searl A (1997) Epidemiological
evidence on the carcinogenicity of silica: factors in scientific
judgement. Edinburgh, Institute of Occupational Medicine, 34 pp.
(IOM Report TM/97/09).
Spiethoff A, Wesch H, Wegener K, Klimisch HJ (1992) The effects of
Thorotrast and quartz on the induction of lung tumors in rats.
Health physics, 63(1):101-110.
Steenland K, Beaumont J (1986) A proportionate mortality study of
granite cutters. American journal of industrial medicine, 9:189-201.
Steenland K, Brown D (1995a) Silicosis among gold miners:
exposure-response analyses and risk assessment. American journal of
public health, 85(10):1372-1377.
Steenland K, Brown D (1995b) Mortality study of gold miners exposed to
silica and nonasbestiform amphibole minerals: an update with 14 more
years of follow-up. American journal of industrial medicine,
27:217-229.
Steenland K, Goldsmith DF (1995) Silica exposure and autoimmune
diseases. American journal of industrial medicine, 28:603-608.
Steenland K, Stayner L (1997) Silica, asbestos, man-made mineral
fibers, and cancer. Cancer causes and control, 8:491-503.
Steenland K, Nowlin S, Ryan B, Adams S (1992) Use of multiple-cause
mortality data in epidemiologic analyses: US rate and proportion files
developed by the National Institute for Occupational Safety and Health
and the National Cancer Institute. American journal of epidemiology,
136(7):855-862.
Stopford CM, Stopford W (1995) Respirable quartz content of farm
soils. Applied occupational and environmental hygiene,
10(3):196-199.
Sweeney TD, Brain JD (1996) Lavagable biomarkers of exposure to
fibrogenic dusts. In: Castranova V, Vallyathan V, Wallace WE, eds.
Silica and silica-induced lung diseases. Boca Raton, FL, CRC Press,
pp. 197-207.
Tokumaru Y, Hirayama K, Kita K, Kawamura M, Katayama K (1990) Two
cases of ataxic sensory neuropathy associated with silicosis.
Clinical neurology, 30:933-938.
Tsuda T, Babazono A, Yamamoto E, Mino Y, Matsuoka H (1997) A
meta-analysis on the relationship between pneumoconiosis and lung
cancer. Journal of occupational health, 39:285-294.
Tucker DM, Reger RB, Morgan WKC (1995) Effects of silica exposure
among railroad workers. Applied occupational and environmental
hygiene, 10(12):1080-1085.
US Department of the Interior (1992) Special publication: Crystalline
silica primer. Washington, DC, US Department of the Interior, Bureau
of Mines.
US EPA (1994) Methods for derivation of inhalation reference
concentrations and application of inhalation dosimetry, Section 4.3.5
Dosimetric adjustments for particle exposures. Research Triangle
Park, NC, US Environmental Protection Agency, Office of Health and
Environmental Assessment, Office of Research and Development
(Publication No. EPA/600/8-90/066F).
US EPA (1996) Ambient levels and noncancer health effects of inhaled
crystalline and amorphous silica: health issue assessment.
Washington, DC, US Environmental Protection Agency, Office of
Research and Development (Publication No. EPA/600/R-95/115; National
Technical Information Service Publication No. PB97-188122).
Vallyathan V, Castranova V, Pack D, Leonard S, Shumaker J, Hubbs AF,
Shoemaker DA, Ramsey DM, Pretty JR, McLaurin JL, Khan A, Teass A
(1995) Freshly fractured quartz inhalation leads to enhanced lung
injury and inflammation: Potential role of free radicals. American
journal of respiratory and critical care medicine, 152(3):1003-1009.
Vanchugova NN, Frash VN, Kogan FM (1985) The use of a micronucleus
test as a short-term method in detecting potential blastomogenicity of
asbestos-containing and other mineral fibers. Gigiena Truda i
Professional'nye Zabolevaniya, 6:45-48.
Verma DK, Sebestyen A, Julian JA, Muir DCF, Schmidt H, Bernholz CD,
Shannon HS (1989) Silica exposure and silicosis among Ontario hardrock
miners: II. Exposure estimates. American journal of industrial
medicine, 16:13-28.
Virta RL (1993) Crystalline silica: what it is -- and isn't.
Minerals today, October: 12-16.
Wagner GR (1995) The inexcusable persistence of silicosis [editorial].
American journal of public health, 85(10):1346-1347.
Wagner JC (1970) The pathogenesis of tumors following the intrapleural
injection of asbestos and silica. In: Nettesheim P, Hanna MG Jr,
Deatherage JW Jr, eds. Morphology of experimental respiratory
carcinogenesis. Oak Ridge, TN, US Atomic Energy Commission, pp.
347-358.
Wagner JC, Berry G (1969) Mesotheliomas in rats following inoculation
with asbestos. British journal of cancer, 23:567-581.
Wagner MMF (1976) Pathogenesis of malignant histiocytic lymphoma
induced by silica in a colony of specific-pathogen-free Wistar rats.
Journal of the National Cancer Institute, 57:509-518.
Wagner MMF, Wagner JC (1972) Lymphomas in the Wistar rat after
intrapleural inoculation of silica. Journal of the National Cancer
Institute, 49:81-91.
Wagner MMF, Wagner JC, Davies R, Griffiths DM (1980) Silica-induced
malignant histiocytic lymphoma: incidence linked with strain of rat
and type of silica. British journal of cancer, 41:908-917.
Wang H, Leigh J, Bonin A, Peters M (1997a) Silica-induced
morphological change similar to apoptosis in bronchoalveolar lavage
cells and granulomatous cells. Annals of occupational hygiene, 41
(Suppl. 1):459-464.
Wang H, Leigh J, Bonin A, Peters M (1997b) Silica induced micronuclei
in pulmonary alveolar macrophages in vivo. Annals of occupational
hygiene, 41 (Suppl. 1):434-439.
Warheit DB, Hartsky MA (1997) Initiating the risk assessment process
for inhaled particulate materials: development of short term
inhalation bioassays. Journal of exposure analysis and environmental
epidemiology, 7(3): 313-325.
Watts WF, Parker DR (1995) Quartz exposure trends in metal and
nonmetal mining. Applied occupational and environmental hygiene,
10(12):1009-1018.
Weill H, McDonald JC (1996) Exposure to crystalline silica and risk of
lung cancer: the epidemiological evidence. Thorax, 51:97-102.
Weill H, Jones RN, Parkes WR (1994) Silicosis and related diseases.
In: Parkes WR, ed. Occupational lung disorders, 3rd ed. London,
Butterworth-Heinemann, pp. 285-339.
Weissman DN, Ma JKH, Rojanasakul Y, Hubbs AF (1996) Immune dysfunction
in silicosis: a hypothesis. Applied occupational and environmental
hygiene, 11(7):962-965.
Westerholm P, Ahlmark A, Maasing R, Segelberg I (1986) Silicosis and
risk of lung cancer or lung tuberculosis: a cohort study.
Environmental research, 41:339-350.
WHO (1986) Recommended health-based limits in occupational exposure
to selected mineral dusts (silica, coal): report of a WHO study
group. Geneva, World Health Organization, 80 pp. (WHO Technical
Report Series 734).
WHO (1993) "WHO calls for medical surveillance of workers exposed to
mineral dusts." Geneva, World Health Organization (Press Release
WHO/73, 23 September 1993).
WHO (1996) Groups at risk: WHO report on the tuberculosis epidemic
1996. Geneva, World Health Organization.
Wiessner JH, Henderson JD Jr, Sohnle PG, Mandel NS, Mandel GS (1988)
The effect of crystal structure on mouse lung inflammation and
fibrosis. American review of respiratory disease, 138:445-450.
Wiles FJ, Faure MH (1977) Chronic obstructive lung disease in gold
miners. In: Walton WH, ed. Inhaled particles IV. Part 2. Oxford,
Pergamon Press, pp. 727-735.
Wilke RA, Salisbury S, Abdel-Rahman E, Brazy PC (1996) Lupus-like
autoimmune disease associated with silicosis. Nephrology dialysis and
transplantation, 11:1835-1838.
Williams AO, Knapton AD, Ifon ET, Saffiotti U (1996) Transforming
growth factor beta expression and transformation of rat lung
epithelial cells by crystalline silica (quartz). International
journal of cancer, 65:639-649.
Wilson T, Scheuchenzuber WJ, Eskew ML, Zarkower A (1986) Comparative
pathological aspects of chronic olivine and silica inhalation in mice.
Environmental research, 39(2):331-344.
Xu XZ, Cia XG, Men XS, Yang PY, Yang JF, Jing SL, He JH, Si WY (1993)
A study of siliceous pneumoconiosis in a desert area of Sunan county,
Gansu province, China. Biomedical and environmental sciences,
6:217-222.
Xu Z, Pan G-W, Liu L-M, Brown LM, Gaun D-X, Xiu Q, Sheng J-H, Stone
BJ, Dosemeci M, Fraumeni JF Jr, Blot WJ (1996) Cancer risks among iron
and steel workers in Anshan, China, Part I: proportional mortality
ratio analysis. American journal of industrial medicine, 30:1-6.
Yamano Y, Kagawa J, Hanaoka T, Takahashi T, Kasai H, Tsugane S,
Watanabe S (1995) Oxidative DNA damage induced by silica in vivo.
Environmental research, 69(2):102-107.
Zheng W, Shu XO, Ji BT, Gao YT (1996) Diet and other risk factors for
cancer of the salivary glands: a population-based case-control study.
International journal of cancer, 67:194-198.
Zhong B, Whong W, Ong T (1997) Detection of mineral-dust-induced DNA
damage in two mammalian cell lines using the alkaline single cell
gel/comet assay. Mutation research, 393:181-187.
Ziegler V, Haustein UF (1992) Systemic scleroderma -- a silica induced
occupational disease? Epidemiological, clinical, immunological,
mineralogical, animal and cell culture investigations.
Dermatologische Monatschrift, 178:34-43.
Ziskind M, Jones RN, Weill H (1976) Silicosis. American review of
respiratory disease, 113:643-665.
APPENDIX 1 -- SOURCE DOCUMENTS
IARC (1997)
Copies of Silica, some silicates, coal dust and
para -aramid fibrils (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Vol. 68) may be obtained from:
International Agency for Research on Cancer
150 cours Albert Thomas
69372 Lyon Cedex 08
France
The members of the Working Group on the Evaluation of
Carcinogenic Risks to Humans of silica (including quartz), some
silicates, coal dust and para-aramid fibrils that met in Lyon on
15-22 February 1996 were:
M.D. Attfield, National Institute for Occupational Safety and
Health, USA
P.J. Borm, University of Limburg, The Netherlands
H. Checkoway, University of Washington, USA
K. Donaldson, Napier University, United Kingdom
M. Dosemeci, National Cancer Institute, USA
V.J. Feron, TNO Nutrition and Food Research Institute, The
Netherlands
B.J. Fubini, University of Torino, Italy
M. Gérin, Université de Montréal, Canada
E. Hnizdo, National Centre for Occupational Health, South Africa
A.B. Kane, Brown University, USA
J.C. McDonald, Imperial College, United Kingdom
H. Muhle, Fraunhofer Institute for Toxicology and Aerosol
Research, Germany
S. Olin, International Life Sciences Institute, USA
J.-C. Pairon, INSERM, France
T. Partanen, Institute of Occupational Health, Finland
C. Shy, University of North Carolina, USA
E. Tatrai, National Institute of Occupational Health, Hungary
D.B. Warheit, DuPont Haskell Laboratory for Toxicology and
Industrial Medicine, USA
V. Yermilov, N.N. Petrov Research Institute of Oncology, Russia
US EPA (1996)
Copies of the US Environmental Protection Agency report entitled
Ambient levels and noncancer health effects of inhaled crystalline
and amorphous silica: health issue assessment (NTIS Publication
PB97-188122) may be obtained from:
National Technical Information Service
Springfield, VA 22161
USA
The document was reviewed in accordance with US EPA policy and
approved for publication. The principal authors of the document were:
Dr R.A. Faust, Oak Ridge National Laboratory, USA
Dr J.S. Gift, National Center for Environmental Assessment, USA
Dr D.F. Goldsmith, Western Consortium for Public Health, USA
Dr R. Ruble, Western Consortium for Public Health, USA
The reviewers and contributors from the US EPA were:
Dr D.L. Costa, National Health and Environmental Effects Research
Laboratory (MD-82), USA
Dr J.S. Dollarhide, National Center for Environmental Assessment
Dr J.A. Graham, National Center for Environmental Assessment
Dr A. Koppikar, National Center for Environmental Assessment
Dr W.E. Pepelko, National Center for Environmental Assessment
Dr C. Shoaf, National Center for Environmental Assessment
Mr E.G. Smith, Office of Air Quality Planning and Standards
Dr W. Victery, Region IX
The external peer reviewers of the document were:
Dr D. Gardner, Private Consultant, Raleigh, NC, USA
Ms S. Goodwin, University of Maryland, Baltimore, MD, USA
Dr M. Jacobsen, National Institute for Occupational Safety and
Health, USA
Dr R. Kutzman, MITRE Corporation, McLean, VA, USA
Dr J. Rabovsky, California Environmental Protection Agency, USA
Ms F. Rice, National Institute for Occupational Safety and
Health, USA
Dr J.M. Samet, The Johns Hopkins University, USA
Dr C. Shy, University of North Carolina, USA
Dr J. Vincent, University of Minnesota, USA
Dr J. Watson, Desert Research Institute, USA
Dr H. Weill, Tulane University, USA
NIOSH (forthcoming)
When published, copies of the NIOSH special hazard review:
Health effects of occupational exposure to respirable crystalline
silica will be available from:
National Institute for Occupational Safety and Health
Publications Dissemination
4676 Columbia Parkway
Cincinnati, OH 45226-1998
USA
The draft document has undergone the following NIOSH internal
reviews in accordance with NIOSH policy: intradivisional review and
interdivisional review. The external peer reviewers of the document
were:
W. Beckett, University of Rochester School of Medicine
H. Checkoway, University of Washington
G.S. Davis, University of Vermont College of Medicine
J. Gift, US EPA
D. Goldsmith, George Washington University
E. Hnizdo, National Centre for Occupational Health, South Africa
J. Hughes, Tulane School of Public Health and Tropical Medicine
C. Jones, Mine Safety and Health Administration, US Department of
Labor
W. Kojola, American Federation of Labor and Congress of
Industrial Organizations
A.G. Macneski, Environmental Safety and Health, Bechtel National,
Inc.
M. Schaper, Mine Safety and Health Administration, US Department
of Labor
L. Schuman, Occupational Safety and Health Administration, US
Department of Labor
J. Sharpe, National Stone Association
D.M. Tucker, Norfolk Southern Corporation
J.A. Ulizio, US Silica Company
J.L. Weeks, George Washington University Medical Center
APPENDIX 2 -- CICAD PEER REVIEW
The draft CICAD on crystalline silica, quartz was sent for review
to institutions and organizations identified by IPCS after contact
with IPCS national Contact Points and Participating Institutions, as
well as to identified experts. Comments were received from:
M. Baril, Institut de Recherche en Santé et Sécurité du Travail
du Québec, Montreal, Quebec, Canada
R. Benson, Drinking Water Program, US Environmental Protection
Agency, Denver, CO, USA
T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
P. Borm, Medical Institute for Environmental Hygiene, Department
of Fibre and Particle Toxicology, Dusseldorf University,
Düsseldorf, Germany
R. Brown, Toxservices, Uppingham, United Kingdom
R.S. Chhabra, National Institute of Environmental Health
Sciences/National Institute of Health, Research Triangle Park,
NC, USA
S. Dobson, Institute of Terrestrial Ecology, Cambridgeshire,
United Kingdom
K. Donaldson, Department of Biological Sciences, Napier
University, Edinburgh, Scotland
J. Heuer, Federal Institute for Health Protection of Consumers
and Veterinary Medicine, Berlin, Germany
J. Hurych, National Institute of Public Health, Prague, Czech
Republic
J. Leigh, National Occupational Health and Safety Commission,
Sydney, Australia
M. Moore, The Morgan Crucible Company, Windsor, United Kingdom
F. Morrall, British Ceramic Confederation, Stoke-on-Trent, United
Kingdom
D. Muir, Occupational Health Program, McMaster University,
Hamilton, Ontario, Canada
M. Wyart-Remy, Industrial Minerals Association - Europe,
Brussels, Belgium
P. Yao, Institute of Occupational Medicine, Chinese Academy of
Preventive Medicine, Beijing, People's Republic of China
J. Yoshida, Chemical Safety Management, Office of Environmental
Chemical Safety, Environmental Health Bureau, Ministry of Health
and Welfare, Tokyo, Japan
K. Ziegler-Skylakakis, Beratergremium für Umweltrelevante
Altstoffe, Muenchen, Germany
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
Sydney, Australia, 21-24 November 1999
Members
Dr R. Benson, Drinking Water Program, US Environmental Protection
Agency, Region VIII, Denver, CO, USA
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
Dr R.M. Bruce, National Center for Environmental Assessment, US
Environmental Protection Agency, Cincinnati, OH, USA
Mr R. Cary, Health and Safety Executive, Merseyside, United Kingdom
Dr R.S. Chhabra, National Institute of Environmental Health Sciences,
National Institutes of Health, Research Triangle Park, NC, USA
Dr S. Chou, Agency for Toxic Substances and Disease Registry, Atlanta,
GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood,
Cambridgeshire, United Kingdom
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers
and Veterinary Medicine, Berlin, Germany
Dr J. Kielhorn, Fraunhofer Institute for Toxicology and Aerosol
Research, Hannover, Germany
Dr S. Kristensen, National Occupational Health and Safety Commission
(Worksafe), Sydney, NSW, Australia
Mr C. Lee-Steere, Environment Australia, Canberra, ACT, Australia
Ms M. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada
Ms F. Rice, National Institute for Occupational Safety and Health,
Cincinnati, OH, USA
Dr J. Sekizawa, National Institute of Health Sciences, Tokyo, Japan
Dr D. Willcocks, National Industrial Chemicals Notification and
Assessment Scheme (NICNAS), Sydney, NSW, Australia (Chairperson)
Professor P. Yao, Institute of Occupational Medicine, Chinese Academy
of Preventive Medicine, Beijing, People's Republic of China
Observers
Mr P. Howe, Institute of Terrestrial Ecology, Huntingdon,
Cambridgeshire, United Kingdom
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit, GmbH, Oberschleissheim, Germany
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Ms M. Godden, Health and Safety Executive, Bootle, Merseyside, United
Kingdom
Dr M. Younes, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
APPENDIX 4 -- INTERNATIONAL CHEMICAL SAFETY CARD
QUARTZ ICSC: 0808
October 1997
CAS# 14808-60-7 Crystalline silica, quartz
RTECS# VV7330000 Crystalline silicon dioxide, quartz
Silicic anhydride
SIO2
Molecular mass: 60.1
TYPES OF HAZARD ACUTE HAZARDS/ PREVENTION FIRST AID / FIRE
/ EXPOSURE SYMPTOMS FIGHTING
FIRE Not combustible. In case of fire in the
surroundings, all
extinguishing agents allowed.
EXPLOSION
EXPOSURE PREVENT DISPERSION
OF DUST!
Inhalation Cough. Local exhaust
or breathing
protection.
Skin
Eyes Safety goggles,
or eye protection
in combination with
breathing protection.
Ingestion
SPILLAGE DISPOSAL PACKAGING & LABELLING
Sweep spilled substance into containers; EU Classification
if appropriate, moisten first to prevent UN Classification
dusting. Wash away remainder with plenty
of water. (Extra personal protection:
P3 filter respirator for toxic particles).
EMERGENCY RESPONSE STORAGE
IMPORTANT DATA
PHYSICAL STATE; APPEARANCE: ROUTES OF EXPOSURE:
COLOURLESS WHITE OR VARIABLE BLACK, PURPLE, The substance can be absorbed
GREEN CRYSTALS into the body by inhalation.
CHEMICAL DANGERS: INHALATION RISK:
Reacts with strong oxidants causing fire Evaporation at 20°C is negligible;
and explosion hazard. a harmful concentration of airborne
particles can, however, be reached
quickly when dispersed.
OCCUPATIONAL EXPOSURE LIMITS: EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
TLV: 0.1 mg/m3 (respirable dust) The substance may have effects on the lungs,
(ACGIH 1997). resulting in fibrosis (silicosis). This substance
MAK: 0.15 mg/m3; (1996) is carcinogenic to humans.
PHYSICAL PROPERTIES
Boiling Point: 2230°C
Melting Point: 1610°C
Relative density (water = 1): 2.6
Solubility in water: none
ENVIRONMENTAL DATA
NOTES
Depending on the degree of exposure, periodic medical examination is indicated. CSQZ, DQ 12,
MIN-U-Sil, Sil-Co-Sil, Snowit, Sykron F300, Sykron F600 are trade names.
ADDITIONAL INFORMATION
LEGAL NOTICE Neither the CEC nor the IPCS nor any person acting on
behalf of the CEC or the IPCS is responsible for the use
which might be made of this information.
RÉSUMÉ D'ORIENTATION
Le présent CICAD, relatif à la silice cristallisée ou quartz, est
basé sur trois documents résultant d'un examen approfondi par des
pairs des effets sanitaires de cette substance, à savoir : 1) une mise
au point portant sur les études et rapports consacrés aux effets
nocifs d'une exposition de l'organisme humain au quartz (NIOSH, à
paraître); 2) une revue critique des études de cancérogénicité
effectuées par le Centre international de recherche sur le cancer
(IARC/CIRC, 1997) et 3) un examen des études consacrées aux effets
sanitaires du quartz présent dans le milieu ambiant, à l'exclusion des
cancers (US EPA, 1996). Ces différentes sources documentaires
n'accordant pas la même attention aux divers points d'aboutissement
toxicologiques, on s'est efforcé, dans ce CICAD, de prendre en compte
l'ensemble des effets indésirables mentionnés dans les documents de
base. Il est a noter à cet égard, que même si les trois documents ne
traitent pas tous les effets de manière aussi détaillée, leurs
conclusions finales n'en sont pas moins très voisines. On a utilisé
plusieurs bases de données en ligne pour effectuer une recherche
bibliographique très complète. Le dernier dépouillement remonte à mars
1999.
Le présent CICAD porte sur la forme la plus commune de silice
cristallisée, c'est-à-dire le quartz. Il ne prend pas en compte les
travaux résultant des études expérimentales consacrées aux effets des
différentes autres formes de silice cristallisée comme la
cristobalite, la tridymite, la stishovite ou la coésite, ni à d'autres
variétés comme la terre d'infusoires ou la silice amorphe ni même à la
poussière de charbon car leur toxicité in vitro est différente ce
celle du quartz. Une ancienne étude effectuée sur des rats vivants a
montré que l'induction de la fibrogénicité était différentes selon les
différentes formes : quartz, cristobalite ou tridymite. Il n'existe
toutefois pratiquement aucune étude expérimentale qui ait procédé à
une évaluation systématique du risque que représentent des matériaux
identiques à ceux auxquels l'Homme est exposé. Selon le groupe d'étude
du CIRC, il est possible que les différentes formes cristallines de
silice n'aient pas toutes le même pouvoir cancérogène. Il est vrai que
dans beaucoup d'études, l'évaluation a porté sur les
« environnements mixtes » dans lesquels le quartz a pu être chauffé -
ce qui est susceptible d'avoir produit une conversion en cristobalite
ou en tridymite dans des proportions diverses (par ex. dans
l'industrie de la céramique, de la poterie et de la brique
réfractaire) - et on n'a pas fait de distinction entre l'exposition au
quartz et l'exposition à la cristobalite, par exemple. On peut penser,
au vu de certaines données, que le risque de cancer varie selon le
type d'industrie et de procédé, et ce, selon des modalités qui
laissent supposer que ce risque est spécifique de chacune des formes
cristallines, mais le Groupe n'a pu parvenir à une conclusion que dans
le cas précis du quartz et de la cristobalite. Ce CICAD reprend
l'exposé et les conclusions du document de ce Groupe, aussi
l'évaluation qu'il donne de la cancérogénicité du quartz sur le lieu
de travail ne fait-elle pas de distinction entre les études
épidémiologiques portant sur le quartz et celles qui sont consacrées à
la cristobalite.
Il a été convenu que l'examen par des pairs de la littérature
consacrée au quartz en vue de la rédaction du présent CICAD
comporterait un volet particulier, consistant en une étude critique
par un groupe international de spécialistes choisis en fonction de
leur connaissance des controverses actuelles au sujet du quartz. On
trouvera à l'appendice 1 des indications sur la nature de l'examen par
des pairs et sur les sources documentaires existantes. Des indications
sur l'examen par des pairs du présent CICAD figurent à l'appendice 2.
Ce CICAD a été approuvé en tant qu'évaluation internationale lors de
la réunion du Comité d'évaluation finale qui s'est tenue à Sydney
(Australie) du 21 au 24 novembre 1999. La liste des participants à
cette réunion figure à l'appendice 3. La fiche d'information
internationale sur la sécurité chimique (ICSC No 0808) relative à la
silice cristallisée est également reproduite dans l'appendice 4 (IPCS,
1993).
Le quartz (No CAS 14808-60-7) est un constituant solide souvent
présent dans la plupart des poussières minérales naturelles.
L'exposition humaine au quartz est la plupart du temps liée à des
activités professionnelles qui impliquent le déplacement de masses de
terre, la manipulation de matériaux qui contiennent de la silice
(pierre à bâtir ou béton par ex.); elle peut aussi se produire lors de
l'utilisation ou de la fabrication de produits à base de silice. Il
peut y avoir exposition à la poussière de quartz présente dans
l'environnement lors d'activités naturelles, industrielles ou
agricoles. Les particules de quartz respirables peuvent être inhalées
et se déposer dans les poumons; toutefois on n'a aucune certitude au
sujet de la cinétique d'élimination des particules de quartz chez
l'Homme.
Le quartz provoque une inflammation cellulaire in vivo. Des
études expérimentales à court terme sur des rats, des souris et des
hamsters ont montré que l'instillation intratrachéenne de particules
de quartz conduisait à la formation de nodules silicotiques discrets.
L'inhalation d'aérosols de particules de quartz gêne la fonction de
nettoyage alvéolaire des macrophages et conduit à des lésions
évolutives et à une pneumopathie inflammatoire. Un stress oxydatif
(c'est-à-dire la formation accrue de radicaux hydroxyles et d'espèces
oxygénées ou azotées réactives) a été observé chez des rats après
instillation intratrachéenne ou inhalation de particules de quartz. De
nombreuses études expérimentales in vitro ont permis de constater
que les propriétés de surface des particules de silice cristallisées
influent sur leur activité fibrogène et sur un certain nombre de
caractéristiques de leur activité cytotoxique. De nombreux mécanismes
possibles sont décrits dans la littérature, mais en fait les lésions
cellulaires provoquées par le quartz sont le résultat de mécanismes
complexes dont la nature n'est pas encore totalement élucidée.
Les études d'inhalation à long terme effectuées sur des rats et
des souris montrent que les particules de quartz entraînent une
prolifération cellulaire, la formation de nodules, la dépression des
fonctions immunitaires et une protéinose alvéolaire pulmonaire. Des
études expérimentales sur des rats ont permis de mettre en évidence la
formation d'adénocarcinomes et de carcinomes spinocellulaires
consécutive à l'inhalation ou à l'instillation intratrachéenne de
particules de quartz. Les études sur le hamster ou la souris n'ont pas
permis de mettre en évidence ce genre de tumeurs pulmonaires. On ne
dispose pas de données dose-réponse (par ex. dose sans effet nocif ou
dose minimale produisant un effet nocif) satisfaisantes pour le rat ou
d'autres rongeurs car peu d'études de cancérogénicité comportant une
variété de doses ont été effectuées.
Les tests de mutagénicité classique sur bactérie ne donnent pas
de résultat positif. Les résultats des études de génotoxicité sont
contradictoires et il n'a pas été possible de confirmer ou d'infirmer
l'existence d'un effet génotoxique direct.
Les résultats d'études sur les particules peuvent donner des
résultats variables selon le matériau testé, la granulométrie, la
concentration administrée aux animaux et l'espèce utilisée. Les essais
effectués avec des particules de quartz ont porté sur diverses espèces
avec échantillons d'origine, de concentration et de granulométrie
variée, autant de facteurs qui ont pu avoir une influence sur les
observations.
On ne dispose pas de données concernant les effets que le quartz
pourrait avoir sur la reproduction ou le développement chez des
animaux d'expérience.
Les effets indésirables du quartz sur les organismes aquatiques
et les mammifères terrestres n'ont pas été étudiés non plus.
Il existe beaucoup d'études épidémiologiques portant sur des
cohortes de divers professionnels exposés à des poussières respirables
de quartz. L'exposition professionnelle à la poussière de quartz est
associée à la silicose, au cancer du poumon et à la tuberculose
pulmonaire. Le CIRC a classé la silice cristallisée (quartz ou
cristobalite) inhalée sur le lieu de travail dans le groupe 1 des
produits dont la cancérogénicité pour l'Homme et l'animal repose sur
des preuves suffisantes. Dans son évaluation générale de cette
substance, le Groupe de travail a noté qu'aucun signe de
cancérogénicité n'avait été relevé au cours de toutes ses enquêtes
dans l'industrie. Il est possible que la cancérogénicité de la silice
cristallisée dépende de certaines propriétés intrinsèques de cette
substance ou encore de facteurs extérieurs susceptibles d'influer sur
son activité biologique ou sur la proportion relative des différentes
formes (IARC/CIRC, 1997).
On a fait état d'une augmentation statistiquement significative
des décès ou des cas de bronchite, d'emphysème, de
broncho-pneumopathie chronique obstructive, de maladies à composante
auto-immune (comme la sclérodermie, l'arthrite rhumatoïde ou le lupus
érythémateux disséminé) ou encore de néphropathie.
En ce qui concerne l'identification du risque et la relation
exposition-réponse, c'est la silicose qui constitue l'effet
déterminant. On possède suffisamment de données épidémiologiques pour
permettre une évaluation quantitative du risque de silicose, mais pas
pour donner une estimation précise du risque des autres effets
sanitaires indiqués plus haut (une évaluation groupée utilisant des
études épidémiologiques consacrées à la silice et au cancer du poumon
est en cours au CIRC).
Il y a de grandes variations (par ex. 2 à 90 %) dans les
estimations du risque relatives à la prévalence de la silicose par
suite d'une exposition tout au long de la vie professionnelle à des
particules respirables de quartz de concentration comprise entre 0,05
et 0,10 mg/m3 sur le lieu de travail. En ce qui concerne l'exposition
aux particules présentes dans l'air ambiant de l'environnement
général, une analyse de référence conclut que le risque de silicose
pour une exposition continue pendant 70 ans à 0,008 mg/m3 (c'est à
dire la valeur estimative élevée de la concentration de silice
cristallisée en milieu urbain aux États-Unis), est inférieur à 3 %
pour les individus en bonne santé ne souffrant pas de pathologie
respiratoire (US EPA, 1996). Le risque de silicose pour les personnes
exposées dans les même conditions mais présentant une pathologie
respiratoire n'a pas été évalué.
Il existe un certain nombre d'incertitudes concernant
l'évaluation des études épidémiologiques et l'estimation du risque
d'effets toxiques résultant de l'exposition à des poussières de
quartz. Les difficultés, qui pour une grande part sont liées à l'étude
même des affections respiratoires dans les diverses professions,
tiennent au nombre et à la qualité des données longitudinales
d'exposition, à l'insuffisance des informations sur les facteurs de
confusion possibles - comme le tabagisme à la cigarette, par ex. - et
à l'interprétation des radiographies thoraciques en tant que preuves
de l'exposition. En outre, l'exposition professionnelle à la poussière
de quartz est complexe car les travailleurs sont fréquemment exposés à
des mélanges qui contiennent du quartz à côté d'autres types de
substances minérales. Les propriétés de ces poussières (par ex. la
granulométrie, les caractéristiques de surface, la forme cristalline)
peuvent varier selon leur origine géologique et se même se modifier
lors des divers traitements industriels. Ces variations peuvent
influer sur l'activité biologique des poussières inhalées. Le Groupe
de travail du CIRC a évalué la cancérogénicité de la silice
cristallisée (quartz, notamment) et a concentré son attention sur les
études épidémiologiques les moins susceptibles d'être affectées par
des facteurs de confusion et des biais de sélection et il en a tiré
des relations dose-réponse (IARC/CIRC, 1997).
RESUMEN DE ORIENTACI²N
Este CICAD sobre la sílice cristalina, el cuarzo, se basa en los
tres amplios documentos siguientes objeto de un examen colegiado sobre
los efectos en la salud de la sílice cristalina, incluido el cuarzo:
1) un examen de los estudios e informes publicados sobre los efectos
adversos en la salud humana de la exposición al cuarzo (NIOSH, de
próxima aparición), 2) un examen de los estudios de carcinogenicidad
realizados por el Centro Internacional de Investigaciones sobre el
Cáncer (IARC/CIIC, 1997) y 3) un examen de los efectos en la salud
distintos del cáncer del cuarzo presente en el medio ambiente (US EPA,
1996). En los documentos originales se prestó una atención diferente a
los distintos efectos finales, y el CICAD se elaboró a fin de evaluar
todos los efectos adversos para la salud identificados en esos
documentos. Hay que señalar que, a pesar de esas diferencias, las
conclusiones finales de todos los documentos originales fueron muy
semejantes. Se realizó una búsqueda bibliográfica amplia de varias
bases de datos en línea. El presente examen contiene los datos
obtenidos hasta marzo de 1999.
En este CICAD se examina la forma más común de sílice cristalina
(es decir, el cuarzo). No se tienen en cuenta los estudios
experimentales de los efectos de otras formas de sílice cristalina
(por ejemplo, la cristobalita, la tridimita, la estishovita o la
coesita), el polvo de carbón, la tierra de diatomeas o la sílice
amorfa, porque su toxicidad in vitro es diferente de la del cuarzo .
En un estudio preliminar en ratas in vivo se pusieron de manifiesto
diferencias en la capacidad de inducción de fibrogenicidad del cuarzo,
la cristobalita y la tridimita. Sin embargo, apenas existen estudios
experimentales en los que se evalúe de manera sistemática exactamente
el mismo material al que está expuesto el ser humano. El Grupo de
Trabajo del CIIC examinó la posibilidad de que hubiera diferencias en
el potencial carcinogénico entre los distintos tipos polimórficos de
la sílice cristalina. Sin embargo, en algunos de los estudios
epidemiológicos se evaluó la presencia de cáncer de pulmón entre los
trabajadores de "entornos mixtos", donde el cuarzo se puede calentar y
se pueden producir diversos grados de conversión a cristobalita o
tridimita (por ejemplo, en las industrias de cerámica, alfarería y
ladrillos refractarios) y no se describieron específicamente las
exposiciones al cuarzo o la cristobalita. Aunque hubo algunos indicios
de que los riesgos de cáncer variaban en función de la industria y el
proceso, de manera que parecía indicar riesgos específicos de los
tipos polimórficos, el Grupo de Trabajo pudo llegar solamente a una
conclusión única para el cuarzo y la cristobalita. El CICAD se hace
eco del debate y la conclusión de ese documento original; por
consiguiente, al examinar la carcinogenicidad del cuarzo en el entorno
ocupacional no se distingue entre los estudios epidemiológicos del
cuarzo y los de la cristobalita.
El proceso de examen colegiado para el presente CICAD tenía por
objeto incluir el examen de un grupo internacional de expertos
seleccionados por sus conocimientos acerca de las controversias y las
cuestiones actuales en relación con el cuarzo. La información relativa
al carácter del examen colegiado y a la disponibilidad de los
documentos originales figura en el apéndice 1. La información relativa
al examen colegiado de este CICAD se presenta en el apéndice 2. Este
CICAD se aprobó como evaluación internacional en una reunión de la
Junta de Evaluación Final celebrada en Sydney, Australia, los días
21-24 de noviembre de 1999. En el apéndice 3 figura la lista de
participantes en esta reunión. La Ficha internacional de seguridad
química (ICSC 0808) para la sílice cristalina, cuarzo (IPCS, 1993),
también se reproduce en el apéndice 4.
El cuarzo (CAS No 14808-60-7) es un componente sólido
presente con frecuencia en la gran mayoría de los tipos de polvo
mineral natural. La exposición humana al cuarzo se produce sobre todo
durante las actividades laborales que requieren el desplazamiento de
tierra, la alteración de productos con sílice (por ejemplo, obras de
albañilería, hormigón) o el uso o fabricación de productos con sílice.
Se puede producir exposición al polvo de cuarzo presente en el medio
ambiente durante la realización de actividades físicas, industriales y
agrícolas. Se pueden inhalar partículas de polvo de cuarzo
respirables, que se depositan en el pulmón; sin embargo, no se ha
llegado a ninguna conclusión acerca de su cinética de eliminación en
el ser humano.
El polvo de cuarzo induce inflamación celular in vivo. En
estudios experimentales de corta duración en ratas se ha observado que
la instilación intratraqueal de partículas de cuarzo da lugar a la
formación de nódulos silicóticos dispersos en ratas, ratones y
hámsteres. La inhalación de partículas de cuarzo pulverizadas
dificulta las funciones de limpieza de los macrófagos alveolares y
provoca lesiones progresivas y neumonitis. Se ha observado una tensión
oxidante (es decir, una mayor formación de radicales hidroxilo,
especies de oxígeno reactivo o especies de nitrógeno reactivo) en
ratas tras la instilación intratraqueal o la inhalación de cuarzo.
Numerosos estudios experimentales in vitro han puesto de manifiesto
que las características de la superficie de las partículas de sílice
cristalina influyen en su actividad fibrogénica y en varias
propiedades relacionadas con su citotoxicidad. Aunque en la
bibliografía se han descrito muchos mecanismos que posiblemente
contribuyan a esto, la manera en la cual las partículas de cuarzo
provocan el daño celular es compleja y no se conoce del todo.
En estudios de inhalación prolongados con ratas y ratones se ha
comprobado que las partículas de cuarzo inducen proliferación celular,
formación de nódulos, supresión de las funciones inmunitarias y
proteinosis alveolar. En estudios experimentales con ratas se notificó
la aparición de adenocarcinomas y carcinomas de las células escamosas
tras la inhalación o la instilación intratraqueal de cuarzo.
En experimentos con hámsteres o ratones no se observaron tumores en
los pulmones. No se dispone de datos adecuados sobre la relación
dosis-respuesta (por ejemplo, la concentración sin efectos adversos o
la concentración mas baja con efectos adversos) para ratas u otros
roedores, debido a que se han realizado escasos estudios de
carcinogenicidad con dosis múltiples.
El cuarzo no dio resultado positivo en las valoraciones normales
de mutagénesis en bacterias. Los resultados de los estudios de
genotoxicidad del cuarzo son contradictorios y no se ha confirmado ni
descartado un efecto genotóxico directo.
En estudios experimentales de partículas, los resultados pueden
variar en función del material de prueba, el tamaño de las partículas,
la concentración administrada y la especie objeto de examen. En los
experimentos con partículas de cuarzo se utilizaron ejemplares de
varios orígenes, con diversidad de dosis, tamaños de partículas y
especies, que podrían haber afectado a las observaciones.
No se dispone de datos sobre los efectos reproductivos y en el
desarrollo del cuarzo en animales de laboratorio.
No se han estudiado los efectos adversos del cuarzo en los
organismos acuáticos y los mamíferos terrestres.
Hay numerosos estudios epidemiológicos de cohortes ocupacionales
expuestas a polvo de cuarzo respirable. La silicosis, el cáncer de
pulmón y la tuberculosis pulmonar son enfermedades asociadas con la
exposición ocupacional al polvo de cuarzo. El CIIC clasificó la sílice
cristalina (cuarzo o cristobalita) de procedencia ocupacional como
carcinógeno del grupo 1, basándose en pruebas suficientes de
carcinogenicidad en el ser humano y en los animales experimentales;
"al hacer la evaluación general, el Grupo de Trabajo observó que no se
había detectado carcinogenicidad en el ser humano en todas las
circunstancias industriales estudiadas. La carcinogenicidad puede
depender de características inherentes a la sílice cristalina o de
factores externos que afectan a su actividad biológica o a la
distribución de sus tipos polimórficos" (IARC/CIIC, 1997).
Se han notificado aumentos estadísticamente significativos de
muertes o casos de bronquitis, enfisema, enfermedad pulmonar
obstructiva crónica, enfermedades relacionadas con la autoinmunidad
(es decir, escleroderma, artritis reumatoide, lupus eritematoso
sistémico) y enfermedades renales.
La silicosis es el efecto decisivo para la determinación del
peligro y la evaluación de la relación exposición-respuesta. Hay datos
epidemiológicos suficientes para poder efectuar una estimación
cuantitativa del riesgo de silicosis, pero no estimaciones precisas de
los riesgos de otros efectos para la salud mencionados anteriormente.
(En el CIIC se está realizando una evaluación agrupada del riesgo de
estudios epidemiológicos de la sílice y el cáncer de pulmón.)
Las estimaciones del riesgo relativas a la prevalencia de la
silicosis para la exposición durante toda la vida laboral a
concentraciones de polvo de cuarzo respirable de 0,05 ó 0,10 mg/m3 en
el entorno de trabajo varían ampliamente (es decir, 2%-90%). Con
respecto a la exposición al cuarzo presente en el medio ambiente
general, a partir del análisis de una dosis de referencia se
pronosticó que el riesgo de silicosis para una exposición continua
durante una vida de 70 años a 0,008 mg/m3 (concentración de sílice
cristalina estimada alta en las zonas metropolitanas de los Estados
Unidos) era inferior al 3% para las personas sanas sin otras
enfermedades o trastornos del aparato respiratorio y para el medio
ambiente (US EPA, 1996). No se evaluó el riesgo de silicosis para las
personas con enfermedades respiratorias expuestas al cuarzo en el
medio ambiente general.
Hay incertidumbres en la evaluación de los estudios
epidemiológicos y en la evaluación del riesgo de los efectos para la
salud relacionados con la exposición al polvo de cuarzo. Las
dificultades, muchas de las cuales son inherentes al estudio de las
enfermedades respiratorias en poblaciones ocupacionales, se deben a
limitaciones en la cantidad y la calidad de los datos históricos de
exposición, la falta de datos sobre posibles factores de confusión,
como por ejemplo el humo de los cigarrillos, y dificultades en la
interpretación de las radiografías del tórax como prueba de
exposición. Además, las exposiciones ocupacionales al polvo de cuarzo
son complejas, porque los trabajadores están expuestos con frecuencia
a mezclas de polvo que contienen cuarzo y otras variedades minerales.
Las propiedades del polvo (por ejemplo, el tamaño de las partículas,
las propiedades de la superficie, la forma cristalina) pueden variar
en función del origen geológico y también pueden cambiar durante la
elaboración industrial. Dichas variaciones pueden afectar a la
actividad biológica del polvo inhalado. El Grupo de Trabajo del CIIC
evaluó la carcinogenicidad de la sílice cristalina (incluido el
cuarzo) y se concentró en los estudios epidemiológicos con menor
probabilidad de verse afectados por sesgos de confusión y selección en
los que se analizaban las relaciones exposición-respuesta (IARC/CIIC,
1997).
See Also:
Toxicological Abbreviations
 1. EXECUTIVE SUMMARY
This CICAD on crystalline silica, quartz was based on the
following three extensive peer-reviewed documents on the health
effects of crystalline silica, including quartz: (1) a review of
published human studies and reports on the adverse health effects of
quartz exposure (NIOSH, forthcoming), (2) a review of the
carcinogenicity studies conducted by the International Agency for
Research on Cancer (IARC, 1997), and (3) a review of the non-cancer
health effects of ambient quartz (US EPA, 1996). The source documents
had different emphases on different end-points, and the CICAD was
developed to assess all the adverse health effects identified in these
documents. It is to be noted that despite the different emphases, the
final conclusions of all source documents were very similar. A
comprehensive literature search of several on-line databases was
conducted. Data identified as of March 1999 are included in this
review.
This CICAD considers the most common form of crystalline silica
(i.e., quartz). It does not consider experimental studies of the
effects of other forms of crystalline silica (e.g., cristobalite,
tridymite, stishovite, or coesite), coal dust, diatomaceous earth, or
amorphous silica, because their in vitro toxicities differ from that
of quartz. Differences in induction of fibrogenicity of quartz,
cristobalite, and tridymite were demonstrated in vivo in an early
rat study. However, there are virtually no experimental studies that
systematically evaluated exactly the same material to which humans are
exposed. The IARC Working Group considered the possibility that there
may be differences in the carcinogenic potential among polymorphs of
crystalline silica. However, some of the epidemiological studies
evaluated lung cancer among workers in "mixed environments" where
quartz may be heated and varying degrees of conversion to cristobalite
or tridymite can occur (e.g., ceramics, pottery, and refractory brick
industries), and exposures specifically to quartz or cristobalite were
not delineated. Although there were some indications that cancer risks
varied by industry and process in a manner suggestive of
polymorph-specific risks, the Working Group could reach only a single
conclusion for quartz and cristobalite. The CICAD reflects the
discussion and conclusion of that source document; therefore, when
considering the carcinogenicity of quartz in the occupational setting,
it does not distinguish between epidemiological studies of quartz and
those of cristobalite.
The peer review process for this CICAD was targeted to include
review by an international group of experts selected for their
knowledge about the current controversies and issues surrounding
quartz. Information on the nature of the peer review and the
availability of the source documents is presented in Appendix 1.
Information on the peer review of this CICAD is presented in
Appendix 2. This CICAD was approved as an international assessment at
a meeting of the Final Review Board, held in Sydney, Australia, on
21-24 November 1999. Participants at the Final Review Board meeting
are listed in Appendix 3. The International Chemical Safety Card
(ICSC 0808) for crystalline silica, quartz has been reproduced in
Appendix 4 (IPCS, 1993).
Quartz (CAS No.
1. EXECUTIVE SUMMARY
This CICAD on crystalline silica, quartz was based on the
following three extensive peer-reviewed documents on the health
effects of crystalline silica, including quartz: (1) a review of
published human studies and reports on the adverse health effects of
quartz exposure (NIOSH, forthcoming), (2) a review of the
carcinogenicity studies conducted by the International Agency for
Research on Cancer (IARC, 1997), and (3) a review of the non-cancer
health effects of ambient quartz (US EPA, 1996). The source documents
had different emphases on different end-points, and the CICAD was
developed to assess all the adverse health effects identified in these
documents. It is to be noted that despite the different emphases, the
final conclusions of all source documents were very similar. A
comprehensive literature search of several on-line databases was
conducted. Data identified as of March 1999 are included in this
review.
This CICAD considers the most common form of crystalline silica
(i.e., quartz). It does not consider experimental studies of the
effects of other forms of crystalline silica (e.g., cristobalite,
tridymite, stishovite, or coesite), coal dust, diatomaceous earth, or
amorphous silica, because their in vitro toxicities differ from that
of quartz. Differences in induction of fibrogenicity of quartz,
cristobalite, and tridymite were demonstrated in vivo in an early
rat study. However, there are virtually no experimental studies that
systematically evaluated exactly the same material to which humans are
exposed. The IARC Working Group considered the possibility that there
may be differences in the carcinogenic potential among polymorphs of
crystalline silica. However, some of the epidemiological studies
evaluated lung cancer among workers in "mixed environments" where
quartz may be heated and varying degrees of conversion to cristobalite
or tridymite can occur (e.g., ceramics, pottery, and refractory brick
industries), and exposures specifically to quartz or cristobalite were
not delineated. Although there were some indications that cancer risks
varied by industry and process in a manner suggestive of
polymorph-specific risks, the Working Group could reach only a single
conclusion for quartz and cristobalite. The CICAD reflects the
discussion and conclusion of that source document; therefore, when
considering the carcinogenicity of quartz in the occupational setting,
it does not distinguish between epidemiological studies of quartz and
those of cristobalite.
The peer review process for this CICAD was targeted to include
review by an international group of experts selected for their
knowledge about the current controversies and issues surrounding
quartz. Information on the nature of the peer review and the
availability of the source documents is presented in Appendix 1.
Information on the peer review of this CICAD is presented in
Appendix 2. This CICAD was approved as an international assessment at
a meeting of the Final Review Board, held in Sydney, Australia, on
21-24 November 1999. Participants at the Final Review Board meeting
are listed in Appendix 3. The International Chemical Safety Card
(ICSC 0808) for crystalline silica, quartz has been reproduced in
Appendix 4 (IPCS, 1993).
Quartz (CAS No.  Table 5: Genetic and related effects of silica.a
Test system Resultb Dose Reference
(LED/HID)c
DNA strand breaks, gamma-HindIII-digested DNA + 30 000d Daniel et al., 1993
DNA strand breaks, herring sperm genomic DNA + 10 000d Daniel et al., 1993
DNA strand breaks, gamma-HindIII-digested DNA + 9 500d Daniel et al., 1995
DNA strand breaks, PM2 supercoiled DNA + 9 500d Daniel et al., 1995
GIA, Gene mutation, hprt locus, rat RLE-6TN
alveolar epithelial cells in vitro - NG Driscoll et al., 1997
SIC, Sister chromatid exchange,
Chinese hamster V79-4 cells in vitro - 15e Price-Jones et al., 1980
SHL, Sister chromatid exchange, human lymphocytes in vitro - 100d Pairon et al., 1990
SIH, Sister chromatid exchange, human lymphocytes
and monocytes in vitro - 100d Pairon et al., 1990
MIA, Micronucleus test, Syrian hamster embryo cells in vitro - 18.75f Oshimura et al., 1984
MIA, Micronucleus test, Syrian hamster embryo cells in vitro + 70e Hesterberg et al., 1986
MIA, Micronucleus test, Chinese hamster lung fibroblasts
(V79) in vitro + 200g Nagalakshmi et al., 1995
CIC, Chromosomal aberrations, Chinese hamster lung
fibroblasts (V79) in vitro - 1 600g Nagalakshmi et al., 1995
CIS, Chromosomal aberrations, Syrian hamster embryo
cells in vitro - 18.75f Oshimura et al., 1984
Table 5 (cont'd)
Test system Resultb Dose Reference
(LED/HID)c
AIA, Aneuploidy, Chinese hamster lung cells (V79-4) in vitro - 15e Price-Jones et al., 1980
AIA, Aneuploidy, Syrian hamster embryo cells in vitro - 18.75f Oshimura et al., 1984
AIA, Tetraploidy, Syrian hamster embryo cells in vitro - 70e Hesterberg et al., 1986
TBM, Cell transformation, BALB/3T3/31-1-1 mouse cells in vitro + 30d,h,i Saffiotti & Ahmed, 1995
TBM, Cell transformation, BALB/3T3/31-1-1 mouse cells in vitro + 60j,k Saffiotti & Ahmed, 1995
TCS, Cell transformation, Syrian hamster embryo cells in vitro + 18e Hesterberg & Barrett, 1984
TCS, Cell transformation, Syrian hamster embryo cells in vitro + 70f Hesterberg & Barrett, 1984
TCL, Cell transformation, fetal rat lung epithelial cells
in vitro (+) NGd Williams et al., 1996
MIH, Micronucleus test, human embryonic lung (Hel 299)
cells in vitro + 800g Nagalakshmi et al., 1995
CIH, Chromosomal aberrations, human embryonic lung
(Hel 299) cells in vitro - 1 600g Nagalakshmi et al., 1995
DVA, 8-hydroxy-2'-deoxyguanosine DNA extract from
lung tissue, male Wistar rats + 50 × 1 itd Yamano et al., 1995
DVA, 8-hydroxy-2'-deoxyguanosine DNA extract from
peripheral blood leukocytes, male Wistar rats - 50 × 1 itd Yamano et al., 1995
GVA, Gene mutation, hprt locus, rat alveolar
epithelial cells in vivo + 100 × 1 it Driscoll et al., 1995
Table 5 (cont'd)
Test system Resultb Dose Reference
(LED/HID)c
GVA, Gene mutation, hprt locus, rat alveolar
epithelial cells in vivo + 5 × 2 it Driscoll et al., 1997
MVM, Micronucleus test, albino mice in vivo - 500 ip Vanchugova et al., 1985
SLH, Sister chromatid exchange, human lymphocytes in vivo + NG Sobti & Bhardwaj, 1991
CLH, Chromosomal aberrations, human lymphocytes in vivo + NG Sobti & Bhardwaj, 1991
BID, Calf thymus DNA binding in vitro + 200l Mao et al., 1994
ICR, Metabolic cooperation using 8-azaguanine-resistant
cells, Chinese hamster lung cells (V79-4) in vitro - 50 Chamberlain, 1983
a Source: IARC (1997). See Appendix 1 of IARC (1997), Test system code words, for definitions of
the abbreviations used in column 1.
b Results without exogenous metabolic system: +, positive; (+), weakly positive; -, negative.
c LED, lowest effective dose; HID, highest ineffective dose; in vitro tests, µg/ml; in vivo tests,
mg/kg body weight per day; NG, not given; it, intratracheal; ip, intraperitoneal.
d Min-U-Sil 5.
e Min-U-Sil unspecified.
f alpha-Quartz.
g Min-U-Sil 5 and Min-U-Sil 10.
h Min-U-Sil 5, hydrofluoric acid-etched.
i A Chinese standard quartz sample.
j DQ 12, a standard German quartz sample.
k F600 quartz.
l Min-U-Sil 5 or Chinese standard quartz.
Some studies have demonstrated the ability of quartz to induce
micronuclei in mammalian cells in culture (i.e., Oshimura et al.,
1984; Hesterberg et al., 1986; Nagalakshmi et al., 1995) and were
reviewed by IARC (Table 5). However, other in vitro studies did not
observe chromosomal aberration (Oshimura et al., 1984;
Nagalakshmi et al., 1995), hprt (hypoxanthine-guanine phosphoribosyl
transferase) gene mutation (Driscoll et al., 1997), or aneuploid or
tetraploid cells (Price-Jones et al., 1980; Oshimura et al., 1984;
Hesterberg et al., 1986).
Pairon et al. (1990) tested quartz (i.e., Min-U-Sil 5) particles
for their ability to induce a significant number of sister chromatid
exchanges in cultures of human lymphocytes plus monocytes or of human
purified lymphocytes. The results were not "clear cut" for any of the
three doses tested (i.e., 0.5, 5.0, and 50 µg/cm2) (Pairon et al.,
1990) (Table 5).
An in vivo treatment of rats with quartz induced mutation in
rat alveolar epithelial cells (Table 5) (Driscoll et al., 1995, 1997).
Nehls et al. (1997) reported results of tests for DNA modifications by
quartz that were not reviewed by IARC (1997). Quartz (2.5 mg of DQ 12
suspended in 0.5 ml of physiological saline) or corundum (2.5 mg), a
non-carcinogenic particle, was intratracheally instilled into the
lungs of Wistar rats (10 rats per exposure and per time period).
Control animals were exposed to saline solution or not treated. Rats
were sacrificed 7, 21, and 90 days after treatment, then lung tissue
sections were analysed with immunocytological assay to determine the
level of 8-hydroxydeoxyguanosine in DNA extracts. Reactive oxygen
species can induce 8-hydroxydeoxyguanosine and other mutagenic DNA
oxidation products, which may be converted to mutations in
proliferating cells (Nehls et al., 1997). Exposure to quartz induced
levels of 8-hydroxydeoxyguanosine in the DNA of alveolar lung cells
that were significantly higher (P-value not reported) at all time
points than levels found in cells of untreated rats or rats treated
with corundum or saline. The number of total cells in bronchoalveolar
lavage fluid was 3-4 times higher in the quartz-treated groups at all
time points than in corundum-exposed rats and control rats exposed to
saline.
Other in vivo studies not reviewed by IARC (1997) found that
quartz induced micronuclei in pulmonary alveolar macrophages of male
Wistar rats in a time-dependent (Leigh et al., 1998b) and
dose-dependent manner (Wang et al., 1997b).
In summary, results of genotoxicity studies of quartz conflict,
and a direct genotoxic effect for quartz has not been confirmed or
ruled out.
8.5 Reproductive and developmental toxicity
There are no data available on the reproductive or developmental
effects of quartz in laboratory animals (IARC, 1997).
8.6 Immunological and neurological effects
Data on the neurological effects of quartz have not been
identified. In vitro studies have shown that quartz can stimulate
release of cytokines and growth factors from macrophages and
epithelial cells, and there is evidence that these events may occur
in vivo and contribute to disease (IARC, 1997). The immunological
response to quartz in experimental animals is a complex subject with
uncertain implications for humans, and detailed reviews are available
elsewhere (i.e., Davis, 1991, 1996; Haslam, 1994; Heppleston, 1994;
Weill et al., 1994; Driscoll, 1996; Gu & Ong, 1996; Hook & Viviano,
1996; Iyer & Holian, 1996; Kane, 1996; Sweeney & Brain, 1996;
Weissman et al., 1996; Mossman & Churg, 1998).
9. EFFECTS ON HUMANS
9.1 Case reports
There are many published case reports of adverse health effects
from occupational exposure to quartz. These health effects include
silicosis (acute and chronic) and lung cancer. Case reports of
silicosis and lung cancer are not mentioned further, because these
diseases have been researched in depth in epidemiological studies
(section 9.2).
There are numerous published case reports of several autoimmune
disorders in workers or patients who had been occupationally exposed
to crystalline silica, including quartz dust (NIOSH, forthcoming). The
most frequently noted autoimmune diseases in those reports were
scleroderma, systemic lupus erythematosus (i.e., lupus), rheumatoid
arthritis, autoimmune haemolytic anaemia (Muramatsu et al., 1989), and
dermatomyositis or dermatopolymyositis (Robbins, 1974; Koeger et al.,
1991). Case reports have also described health effects that may be
related to the immunological abnormalities observed in patients with
silicosis, such as chronic renal disease (Saita & Zavaglia, 1951;
Giles et al., 1978; Hauglustaine et al., 1980; Bolton et al., 1981;
Banks et al., 1983; Slavin et al., 1985; Bonnin et al., 1987;
Osorio et al., 1987; Arnalich et al., 1989; Sherson & Jorgensen, 1989;
Dracon et al., 1990; Pouthier et al., 1991; Rispal et al., 1991;
Neyer et al., 1994; Wilke et al., 1996), ataxic sensory neuropathy
(Tokumaru et al., 1990), chronic thyroiditis (Masuda, 1981),
hyperthyroidism (i.e., Graves' disease) (Koeger et al., 1996),
monoclonal gammopathy (Fukata et al., 1983, 1987; Aoki et al., 1988),
and polyarteritis nodosa (Arnalich et al., 1989).
9.2 Epidemiological studies
9.2.1 Silicosis
Most, if not all, of the several hundred epidemiological studies
of exposure to quartz dust are studies of occupational cohorts. The
majority of studies investigated the occurrence of silicosis morbidity
or mortality. These studies have conclusively linked occupational
quartz dust exposure with silicosis. Silicosis (i.e., nodular
pulmonary fibrosis) is a fibrotic lung disease, sometimes
asymptomatic, that is caused by the inhalation and deposition of
respirable crystalline silica particles (i.e., particles <10 µm in
diameter) (Ziskind et al., 1976; IARC, 1987).
A worker may develop one of three types of silicosis, depending
on the airborne concentration of respirable crystalline silica: (1)
chronic silicosis, which usually occurs after 10 or more years of
exposure at relatively low concentrations; (2) accelerated silicosis,
which develops 5-10 years after the first exposure; or (3) acute
silicosis, which develops after exposure to high concentrations of
respirable crystalline silica and results in symptoms within a few
weeks to 4 or 5 years after the initial exposure (Ziskind et al.,
1976; Peters, 1986; NIOSH, 1992a,b, 1996). Acute silicosis is a risk
for workers with a history of high exposures from performing
occupational processes that produce small particles of airborne dust
with a high silica content, such as during sandblasting, rock
drilling, or quartz milling, or any other process with high exposures
to small particles of airborne dust with a high quartz content
(Davis, 1996).
A recent study of 67 paraffin-embedded lung tissue samples from
silicotic patients found a significant linear relationship
(P <0.001) between lung quartz concentration and silicosis severity in
gold miners; although several types of mineral particles were found in
the lungs, quartz was the only significant indicator of silicosis
severity. The silicosis cases included 39 patients without lung cancer
and 28 patients with lung cancer. All of the cases were gold miners in
Canada (Dufresne et al., 1998a,b).
The epidemiological studies of silicosis usually define the
profusion of small opacities present in the disease according to a
standard system used by trained readers and developed by the
International Labour Organization for classification of chest
radiographs of pneumoconioses (ILO, 1980). Each reader assesses the
profusion according to a 12-point scale of severity. Categories 0/-
and 0/0 are the first and second points on the scale and represent a
normal chest radiograph. The third point, category 0/1, represents the
borderline between normality and abnormality, and category 1/0, the
fourth point, represents definite, but slight, abnormality
(Love et al., 1994). The shape (rounded or irregular) and size of the opacities
can also be described by the readers.
The critical studies of chronic silicosis, a progressive disease,
are those occupational epidemiological studies where (1) quantitative
quartz exposure data were available and used for risk analysis,
(2) exposure-response relationships were investigated, or (3) the
exposure-response relationships were documented with sufficient detail
for a health effects benchmark, including (4) application of data to
mathematical models that predicted silicosis prevalence at increasing
concentrations of cumulative quartz exposure. (The predicted
prevalences reported in the studies are discussed in section 11.1.3.)
Studies that selected workers from a broad spectrum of occupations and
included many workers that were exposed to different combinations of
various minerals, such as studies of "dusty trades" workers
(i.e., Rice et al., 1986), were excluded from consideration for risk
assessment of quartz and silicosis. Epidemiological studies that
provided evidence of an exposure-response relationship for silica and
silicosis based on other kinds of exposure data (e.g., there is a
positive relationship between development of chronic silicosis and
duration of exposure) have been reviewed elsewhere (WHO, 1986;
Goldsmith, 1994; Hughes, 1995; Rice & Stayner, 1995; Seaton, 1995;
Steenland & Brown, 1995a; Davis, 1996; US EPA, 1996).
The two critical cross-sectional studies (i.e., Kreiss & Zhen,
1996; Rosenman et al., 1996 -- see Table 6) found that the prevalence
of radiographic silicosis (ILO category >1/0 or >1/1) was
dose-related. That is, the prevalence of radiographic silicosis
increased with average silica dust exposure, cumulative quartz
exposure, duration of employment, or all of these measures. The actual
prevalences varied greatly among the studies, and conclusions
concerning quartz dust concentrations that may or may not induce
silicosis cannot be drawn from simple "eyeball" analysis of the
prevalences in the following two worker populations:
* Kreiss & Zhen (1996) conducted a community-based random sample
survey of 134 male residents at least 40 years old, living in a
hardrock (i.e., molybdenum, lead, zinc, and gold) mining town in
Colorado, USA. Of the 134 residents, 100 were silica-exposed
hardrock miners (including 32 silicosis cases) and 34 were
community "controls" without occupational dust exposure. Nearly
all (97%) of the dust-exposed subjects were 20 years since first
exposure. The estimated crystalline silica content (polymorph not
reported) of the total dust was 12.3%. Exposure was assessed with
information from occupational histories, gravimetric dust
exposure data from 1974-1982, and a cumulative silica exposure
index. Pre-1974 exposure estimates were based on job-specific
gravimetric data collected after 1974. Exposures were also
estimated for mines where no exposure data were available (17.1%
of person-years of follow-up). Thirty-two per cent of the 100
dust-exposed subjects had silicosis (defined as radiological
profusion of small opacities of ILO category >1/0). Prevalence
of silicosis was related to average silica dust exposure. Among
the 94 dust-exposed subjects with data on cumulative and average
dust exposures, those subjects with average silica exposure
<0.05 mg/m3 had 10% prevalence of silicosis; subjects with
>0.05-0.10 mg/m3 had a prevalence of 22.5%; subjects with
greater than 0.10 mg/m3 average silica exposure had a silicosis
prevalence of 48.6% (P = 0.01). (See Table 6 for predicted
prevalences when silicosis was defined as radiological profusion
of small opacities of ILO category >1/1.) It is not known
whether the small sample of 134 residents was representative of
all miners or if the exposure estimates for mines where no
exposure data were available (17.1% of person-years of follow-up)
were representative.
* Rosenman et al. (1996) conducted a cross-sectional study in 1991
of 549 current, 497 retired, and 26 current salaried workers that
were former production workers in a US grey iron foundry that
produced automotive engine blocks (total workers = 1072).
Twenty-eight cases (2.9%) of silicosis, defined as rounded
opacities >ILO category 1/0, were identified by at least two
of three "B" readers of a total of 952 chest radiographs. More
than half (18/28) of the cases were found in retired workers.
Silicosis prevalence was positively related to mean silica (i.e.,
quartz) exposure (P < 0.0001). Of the workers with mean quartz
exposure less than 0.05 mg/m3, 0.8% had silicosis, while 6.3%
of foundry workers with mean quartz exposure greater than 0.45
mg/m3 had silicosis. Silicosis prevalence also increased with
years of employment at the foundry, cumulative silica exposure,
work area within the foundry, and cigarette smoking (i.e., smoker
vs. non-smoker). Exposure estimates were derived from conversions
of "early silica exposure data" collected by impingers. Quartz
content of total dust was not reported. Weighted total dust
exposure from impinger data was converted to an estimate of
silica exposure in mass units (mg/m3) by multiplying it by the
average percentage of quartz in bulk samples.
Results of cohort studies of gold miners in South Africa, Canada,
and the USA (see Table 7) also demonstrated an exposure-response
relationship for radiographic silicosis (US EPA, 1996):
* A cohort study was conducted of 2235 white South African
underground gold miners, 45-54 years old at the time of medical
examination in 1968-1971, who started working after 1938, worked
>10 years, and were followed until 1991 (Hnizdo &
Sluis-Cremer, 1993). More than 300 (n = 313) of the 2235 miners
were followed to the time when radiological signs developed, 658
miners were followed up to death, and 1264 miners were followed
to the year of the most recent radiograph. Radiographs were read
blindly by two independent readers. Silicosis was defined as the
presence of rounded opacities of ILO category >1/1.
Radiographs were read blindly by two readers initially, then one
reader was chosen because his readings more closely matched the
autopsy data. Mean respirable dust concentrations, after heat and
acid treatment, in milligrams per cubic metre per shift were
calculated for nine gold mining occupations. The concentrations
were based on a study of shift-long dust exposure that measured
the surface area of the respirable mine dust and the number of
respirable particles (i.e., incombustible and acid-insoluble dust
particles) per cubic metre in a random sample of 20 South African
gold mines (Beadle, 1965, 1971). After heat and acid treatment,
the respirable dust in South African gold mines was found to
contain about 30% quartz (Beadle & Bradley, 1970). Cumulative
dust exposure for the miners was calculated in milligrams per
cubic metre-year by using data for mean mass respirable dust
concentrations for the nine occupational categories, the average
number of hours underground, and the number of dusty 8-h shifts.
Of the 2235 miners studied by Hnizdo & Sluis-Cremer (1993),
313 developed radiologically diagnosed silicosis (rounded
opacities with profusion of ILO category >1/1) during the
follow-up period (i.e., 1968-1971 to 1991). The onset of
silicosis occurred after an average (i.e., mean) of 27 years of
Table 6: Predicted prevalence of silicosis (ILO category >1/1)
following exposure to respirable quartz dust based on modelling of cumulative exposure
at mean concentrations of 0.05 or 0.10 mg/m3 over a 45-year working lifetime.
Cross-sectional study Mean concentration of Predicted prevalence of Cohort's mean time Cohort's maximum time
and cohort respirable quartz dust silicosis, ILO category since first quartz since first quartz
(mg/m3) >1/1 (cases per exposure (years) exposure (years)
100 workers)
Kreiss & Zhen, 1996 0.05 approx. 30a silicotic miners: silicotic miners:
41.6 66
100 US hardrock 0.10 approx. 90a non-silicotic non-silicotic
miners and 34 miners: 33.5 miners: 68
community controls
Rosenman et al., 0.05 2b,c 28 >30
1996
1072 US grey 0.10 3b,c
iron foundry workers
a Based on cumulative silica exposure model with 10 years of post-employment follow-up.
b ILO category >1/0.
c Based on a 40-year working lifetime and controlling for pack-years of cigarette smoking, race,
and silica exposure other than in the foundry under study.
Table 7: Predicted number of silicosis cases (ILO category >1/1) following exposure to respirable
quartz dust based on modelling of cumulative exposure at mean concentrations of 0.05 or 0.10 mg/m3
over a 45-year working lifetime.
Cohort study Mean concentration of Silicosis cases, Mean time since first Maximum time since
and population respirable quartz ILO category >1/1, quartz exposure (years) first quartz exposure
dust (mg/m3) per 100 workers (years)
Hnizdo & 0.05 13a silicotic miners: 36 silicotic miners: 50
Sluis-Cremer, 1993
2235 South African 0.10 approx. 70
gold miners
Muir et al., 0.05 0.09-0.62a,b 18 silicotic miners: 38
1989a,b;
Muir, 1991
2109 Canadian
gold and
uranium miners
Steenland & 0.05 10c 37 73d
Brown, 1995a
3330 US gold 0.09 47c
miners
a Estimate was reported in Rice & Stayner (1995).
b No post-employment follow-up and no retired miners included. The range includes five estimates
(one for each reader).
c The predicted number of silicosis cases does not account for effects of age or calendar time
(K. Steenland, personal communication, 1997).
d K. Steenland, personal communication, 1998.
net service, at a mean age of 56 years. For more than half of the
miners (n = 178; 57%), the onset occurred an average of
7.4 years (standard deviation 5.5; range 0.1-25 years) after
their employment at the mines, at 59 years of age (range
44-74 years). For the other miners (n = 135; 43%), the onset
of silicosis occurred while they were still mining, at 51 years
of age (range 39-61 years). These results show that the majority
of the cases occurred in miners who were no longer employed at
the mine and who were at least 50 years old
(Hnizdo & Sluis-Cremer, 1993).
* Muir and colleagues conducted a study of 2109 current Ontario
miners from 21 gold and uranium mines who started working and
worked more than 5 years between 1940 and 1959 and were followed
to 1982 or to the end of their dust exposure, whichever came
first (Muir et al., 1989a,b; Muir, 1991). Any uranium miner with
more than 2 weeks of exposure was also included (Muir et al.,
1989a). The quartz content of respirable gold mine dust was 6.0%,
and that of uranium mine dust was 8.4%. Retired and former miners
were not included in the study. Sources of data for this study
were full-sized annual chest radiographs taken for all miners
after 1927 and periodic (pre-1959) and semi-annual mine dust
measurements obtained with a konimeter (which is an instantaneous
dust sampler that measures the number of particles per unit
volume of air; Verma et al., 1989). Konimeter dust measurements
taken from 1940 to 1952 were expressed in particles per cubic
centimetre of air (ppcc). Verma et al. (1989) initiated an
extensive, side-by-side comparison of the konimetric and
gravimetric (i.e., milligrams of silica per cubic metre) sampling
to derive a konimetric/gravimetric silica conversion curve. A
total of 2360 filter (i.e., nylon cyclone-filter assembly in a
constant-flow pump) samples and 90 000 konimeter samples were
taken in a 2-year period in two gold and uranium mines, in
existing conditions as well as in an experimental simulation of
the high-dust conditions of the past caused by dry drilling
(Verma et al., 1989). The results of the conversion relationship
were non-linear and may have reflected the limitations of the
konimeter in measuring high dust (i.e., high count)
concentrations and the limitations of the gravimetric sampler in
measuring low dust concentrations. There were different
relationships for the gold and uranium mines, possibly because of
the different fractional silica concentrations in the host rock.
The conversion of the historical konimeter counts to gravimetric
respirable silica equivalents was used to derive a cumulative
respirable silica dose for each miner based on the miner's
respirable silica dose for each year, mine, and task in his work
history (Verma et al., 1989).
Thirty-two of the 2109 hardrock miners studied by Muir and
colleagues were considered by at least one of five readers to
have silicosis (small, rounded opacities with profusion of ILO
category >1/1). However, the results differed among the five
readers and "complicated the analysis" (Muir et al., 1989b). One
of the five readers identified only seven cases of silicosis
(Muir et al., 1989b). The results were presented by individual
reader and by consensus. A consensus of all of the five readers
with respect to identification of silicosis was reached on only
six cases (Muir et al., 1989b). Average respirable quartz dust
exposure for the cases was not reported.
* A cohort study of 3330 white male underground gold miners from
South Dakota employed for at least 1 year between 1940 and 1965
and followed through 1990 found 170 cases of silicosis (128 cases
were identified on death certificates, 29 cases were found during
X-ray surveys of workers conducted in 1960 and 1976, and 13 cases
were identified on both X-ray and death certificate). Cases were
defined as (1) an underlying or contributing cause of death of
silicosis, silico-tuberculosis, respiratory tuberculosis, or
pneumoconiosis, and/or (2) ILO category >1/1 silicosis
identified in the 1976 radiographic survey or "small opacities"
or "large opacities" identified in the 1960 radiographic survey
(Steenland & Brown, 1995a). The miners were exposed to a median
quartz level of 0.05 mg/m3 (0.15 mg/m3 for workers hired prior
to 1930). The average length of follow-up was 37 years, and the
average length of employment underground was 9 years. Quartz
exposure was estimated by converting dust particle counts to
gravimetric measurements (i.e., mg/m3), based on an estimate of
13% quartz content of total dust. A job-exposure matrix was
created to estimate dust exposures for each job over time, then
average dust exposures for the job categories were calculated
using existing measurements for each year from 1937 to 1975. The
estimated daily dust exposures (constant over each year) were
weighted to account for daily time spent underground. Summation
of the estimated daily dust levels over time provided an estimate
of cumulative quartz exposure (Steenland & Brown, 1995a). The
risk of silicosis was less than 1% for miners with a cumulative
exposure less than 0.5 mg/m3-years. The risk increased to 68-84%
for the highest cumulative exposure category (i.e.,
4 mg/m3-years) (Steenland & Brown, 1995a). Silicosis risk
estimates could have been affected by (1) combining silicosis
deaths with silicosis cases detected by cross-sectional
radiographic surveys, (2) differences in quartz content of dust
in early years, and (3) lack of dust measurements before 1937.
A cohort study of a subcohort of the South Dakota gold
miners described above analysed cases of silicosis that were
reported as the underlying cause of death on the death
certificates. Forty cases of silicosis, as well as 49 cases of
tuberculosis, were ascertained among the 1321 miners employed for
at least 21 years and followed through 1973. There was a linear
trend in risk of about 2.4% for each 0.1 mg/m3 of silica
exposure. However, this study does not meet the criteria for a
critical study because risk by cumulative quartz exposure was not
calculated (McDonald & Oakes, 1984).
In the five critical studies described above, the number of cases
identified depended upon the definition of silicosis (radiographic
category and whether irregular opacities were included), the quality
of the evaluation of the chest radiographs (e.g., number and training
of readers), the duration of dust exposure, and the duration of
follow-up after the end of exposure. Interstudy variation exists for
each of these factors. In addition, exposure assessments in these
studies were accompanied by uncertainties, such as the use of
conversion equations (i.e., converting particle count data to mass
concentrations; application of equations from one industry to a
different industry) and estimation of quartz content of the dust. It
is not uncommon for epidemiological studies to lack characterization
of the source and properties of the mineral dusts collected in the
workplace (Mossman & Churg, 1998). Nevertheless, the critical studies
found an exposure-response relationship for respirable quartz dust
that, when modelled, predicts the occurrence of silicosis cases in
various industries at exposures close to regulatory levels.
9.2.2 Pulmonary tuberculosis and other infections
The association between tuberculosis and silicosis has been
firmly established by the results of epidemiological studies conducted
during this century (Balmes, 1990). In recent studies of silicotics,
the association was well supported by the results of a survey of
tuberculosis deaths among silicotics in the USA for the period
1979-1991 (Althouse et al., 1995), a mortality study of 590 California
silicosis claimants (Goldsmith et al., 1995a), and a retrospective
study of silicotic miners from the Freegold mines in South Africa
(Kleinschmidt & Churchyard, 1997).
In studies of workers without silicosis, there is some limited
evidence that long exposures or high cumulative exposures to quartz
dust may increase the risk of developing tuberculosis. Two
epidemiological studies reported 3-fold higher incidences of pulmonary
tuberculosis cases in 5424 non-silicotic silica-exposed Danish foundry
workers employed 25 or more years (Sherson & Lander, 1990) and among
335 non-silicotic black South African gold miners with a median
underground employment of 26 years (Cowie, 1994). Westerholm et al.
(1986) found 13 cases of tuberculosis among 428 silicotic Swedish iron
and steel workers and one case of tuberculosis in a comparison group
of 476 Swedish iron and steel workers with normal chest radiographs
(level of statistical significance not reported). Both groups had been
exposed to silica for at least 5 years.
A study of tuberculosis incidence in 2255 white South African
gold miners included 1296 miners who had an autopsy. The
smoking-adjusted relative risk for pulmonary tuberculosis in miners
without silicotic nodules on autopsy examination (n = 577) increased
slightly with quartiles of cumulative dust exposure (relative risk
[RR] = 1.38; 95% confidence interval [CI] = 0.33-5.62) for the highest
quartile of cumulative exposure). For miners without radiologically
diagnosed silicosis (n = 1934), the smoking-adjusted relative risk
increased to 4.01 (95% CI = 2.04-7.88) in the highest quartile of
cumulative dust exposure (Hnizdo & Murray, 1998, 1999). Radiological
silicosis was defined as ILO category >1/1; detailed ILO grading
was not performed (Hnizdo & Murray, 1998, 1999). Tuberculosis was
diagnosed on average 7.6 years after the end of dust exposure and
6.8 years after the onset of radiological silicosis -- a result that
supports the need for medical surveillance of workers after the end of
exposure to silica dust (Hnizdo & Murray, 1998). (Miners who developed
tuberculosis before completing 10 years of underground employment were
excluded because they were not allowed to continue working underground
after diagnosis) (Hnizdo & Murray, 1998). It is not clear whether
"dust" exposure refers to quartz exposure or exposure to gold mine
dust.
Chen et al. (1997) conducted a case-control study (8740 cases;
83 338 controls) with US National Occupational Mortality Surveillance
data for the years 1983-1992 that controlled for confounding from age,
gender, race, socioeconomic status, potential exposure to active
tuberculosis, and the presence of silicosis and other pneumoconioses.
The potential for exposure to silica was based on potential exposure
data from the National Occupational Exposure Survey (Seta et al.,
1988) and the National Occupational Health Survey of Mining
(Greskevitch et al., 1996) and was categorized as "high,"
"intermediate," or "low or no" potential exposure. The study found
that decedents with potential high exposure to silica and with no
documentation of silicosis on the death certificate had a 30% greater
odds of mortality from respiratory tuberculosis than decedents with no
potential exposure to silica after adjustment by logistic regression
for the possible confounders mentioned above (odds ratio [OR] = 1.3;
95% CI = 1.14-1.48). The results also suggested the presence of an
exposure-response relationship of silica exposure, in the absence of
silicosis, with death from respiratory tuberculosis (Chen et al.,
1997).
In summary, the relationship between quartz exposure and
tuberculosis risk in the absence of radiographic silicosis in
silica-exposed workers has not been well defined or quantitatively
described by existing epidemiological studies.
A recent case-control study of tuberculosis and pulmonary disease
caused by non-tuberculous mycobacteria (NTM) in South African gold
miners found that radiographic silicosis, focal radiological scarring,
and human immunodeficiency virus (HIV) infection were significant risk
factors for NTM disease and for tuberculosis (Corbett et al., 1999).
Past medical history of tuberculosis treatment (OR = 15.1;
95% CI = 7.64-29.93) and current employment in a "dusty job" at the
mines (OR = 2.5; 95% CI = 1.46-4.44) were significant risk factors for
NTM. The study included 206 NTM patients and 381 tuberculosis patients
of known HIV status admitted to a South African hospital and
180 controls that were HIV-tested surgical or trauma patients admitted
to that hospital during the same time period. Odds ratios for NTM
and tuberculosis increased with increasing years of employment
(range of ORs: 1.0-9.4 for NTM, and 1.0-4.1 for tuberculosis).
9.2.3 Lung cancer
Lung cancer is associated with occupational exposures to inhaled
quartz (IARC, 1997).
Following a comprehensive review of the large body of published
epidemiological studies, IARC (1997) found that the following
epidemiological studies provide the least confounded investigations of
an association between occupational crystalline silica exposure and
lung cancer risk:
* US gold miners (Steenland & Brown, 1995b)
* Danish stone industry workers (Guenel et al., 1989)
* US granite shed and quarry workers (Costello & Graham, 1988)
* US crushed stone industry workers (Costello et al., 1995)
* US diatomaceous earth industry workers (Checkoway et al., 1993,
1996)
* Chinese refractory brick workers (Dong et al., 1995)
* Italian refractory brick workers (Puntoni et al., 1988;
Merlo et al., 1991)
* United Kingdom pottery workers (Cherry et al., 1995, 1997;
McDonald et al., 1995, 1997; Burgess et al., 1997)
* Chinese pottery workers (McLaughlin et al., 1992)
* cohorts of registered silicotics from North Carolina
(Amandus et al., 1991, 1992) and Finland (Kurppa et al., 1986;
Partanen et al., 1994)1
Although a few of those studies did not find a statistically
significant association between occupational crystalline silica
exposure and lung cancer risk, most of the studies did. Some
non-uniformity of results is not unusual when a large number of
epidemiological studies are reviewed and a variety of populations and
1 This list includes studies of diatomaceous earth industry workers
and workers in the ceramics, refractory brick, and pottery
manufacturing industries where the primary silica exposure may have
been to cristobalite rather than quartz. In most studies, exposure
data for quartz as well as cristobalite or tridymite were not
available to support that assumption.
work environments are studied (IARC, 1997). In addition, IARC noted
that the carcinogenicity of quartz (or cristobalite) "may be dependent
on inherent characteristics of the crystalline silica or on external
factors affecting its biological activity or distribution of its
polymorphs" (IARC, 1997). (A detailed critique of the studies listed
above is available in Soutar et al., 1997.)
Some of the least-confounded studies reported that lung cancer
risk tended to increase with:
* cumulative exposure to respirable silica (i.e., Checkoway et al.,
1993, 1996)
* duration of exposure (i.e., Costello & Graham, 1988;
Merlo et al., 1991; Partenen et al., 1994; Costello et al., 1995;
Dong et al., 1995)
* peak intensity of exposure (Burgess et al., 1997; Cherry et al.,
1997; McDonald et al., 1997)
* the presence of radiographically defined silicosis
(Amandus et al., 1992; Dong et al., 1995)
* length of follow-up time from date of silicosis diagnosis
(Partenen et al., 1994)
The observed associations noted above, including the
exposure-response associations, are unlikely to be explained by
confounding or other biases; therefore, the epidemiological studies,
overall, support increased lung cancer risks from occupational
exposure to inhaled respirable crystalline silica (i.e., quartz and
cristobalite) (IARC, 1997).
Three studies published since the IARC review investigated
exposure-response associations for lung cancer and occupational
exposure to quartz.
Cherry et al. (1998) published a final report of the preliminary
results of a nested case-control study of 52 lung cancer deaths in a
cohort of 5115 pottery workers (i.e., Burgess et al., 1997; Cherry et
al., 1997; McDonald et al., 1997). After adjustment for smoking and
inclusion of a 20-year-, 10-year-, or 0-year lag period, mean silica
concentration (i.e., estimated daily 8-h time-weighted airborne
concentrations in µg/m3; polymorph not specified) was associated with
lung cancer (OR = 1.60, 95% CI = 1.11-2.31; OR = 1.66,
95% CI = 1.14-2.41; OR = 1.67, 95% CI = 1.13-2.47, for 20-year,
10-year, and 0-year lag periods, respectively; P < 0.008 for each
lag period). However, duration of exposure and cumulative silica dust
exposure were not significantly associated with lung cancer mortality,
regardless of lag time (Cherry et al., 1998). The presence of small
parenchymal radiographic opacities (category >1/0; shape not
reported) was not related to lung cancer mortality, before
(P = 0.78) or after (P = 0.68) adjustment for smoking.
The authors concluded that the results imply "that crystalline silica
may well be a human carcinogen" (Cherry et al., 1998). The study did
not differentiate quartz exposures from cristobalite exposures.
De Klerk & Musk (1998) conducted a cohort study of 2297 surface
and underground gold miners in western Australia who participated in
surveys of respiratory symptoms, smoking habits, and lung function in
1961, 1974, and 1975. Eighty-nine per cent of the cohort was traced to
the end of 1993 for trachea, bronchus, and lung cancer mortality and
incidence of compensated silicosis (i.e., compensation awarded by the
Pneumoconiosis Medical Board). A nested case-control analysis of the
138 lung cancer deaths found that lung cancer mortality was related to
log total cumulative silica dust exposure after adjustment for smoking
(cigarette, pipe, or cigar) and for the presence of bronchitis at
survey (relative rate = 1.31; 95% CI = 1.01-1.70). However, the effect
of total cumulative silica dust exposure on lung cancer mortality was
not significant after adjustment for smoking, bronchitis, and
compensation for silicosis (relative rate = 1.20; 95% CI = 0.92-1.56).
Lung cancer mortality was not significantly related (P > 0.15) to
other silica exposure variables (i.e., duration of underground or
surface employment, intensity of underground or surface exposure)
after adjustment for smoking and bronchitis. Cigarette smoking
(relative rate = 32.5; 95% CI = 4.4-241.2 for >25 cigarettes smoked
per day), incidence of a compensation award for silicosis after lung
cancer diagnosis (relative rate = 1.59; 95% CI = 1.10-2.28), and
presence of bronchitis at survey (relative rate = 1.60;
95% CI = 1.09-2.33) were significantly related to lung cancer
mortality (de Klerk & Musk, 1998). The results of this study do not
support a relationship between lung cancer and silica exposure in the
absence of silicosis (i.e., a compensation award for silicosis after
lung cancer diagnosis).
Hnizdo et al. (1997) conducted a nested case-control study of
lung cancer deaths in a cohort of 2260 white South African underground
gold miners. (A lung cancer mortality cohort study was conducted
earlier [Hnizdo & Sluis-Cremer, 1991].) The mineral content of the
rock in the gold mines was mostly quartz (70-90%), silicates (10-30%),
pyrite (1-4%), and heavy minerals with grains of gold and
uranium-bearing minerals (2-4%). Seventy-eight lung cancer deaths
(69 of the 78 miners had a necropsy) that occurred during 1970-1986
were matched by year of birth with 386 control subjects from the same
cohort (Hnizdo et al., 1997). Lung cancer mortality risk and a
relationship with cigarette smoking (i.e., pack-years), cumulative
"dust" exposure (mg/m3-years), years of underground mining, incidence
of radiographic silicosis (i.e., ILO category >1/1 diagnosed up to
3 years before death of a matched case), and uranium production or
uranium grade of the ore in the gold mine were analysed with
conditional logistic regression models. Radon daughter measurements in
the gold mines were not available.
Lung cancer mortality was associated with cigarette smoking,
cumulative dust exposure (lagged 20 years from death), duration of
underground mining (lagged 20 years from death), and silicosis. The
best-fitting model predicted relative risks of 1.0, 3.5
(95% CI = 0.7-16.8), 5.7 (95% CI = 1.3-25.8), and 13.2
(95% CI = 3.1-56.2) for <6.5, 6.5-20, 21-30, and >30 pack-years of
smoking, respectively, and 2.45 (95% CI = 1.2-5.2) for silicosis. The
authors stated that variables representing uranium mining were not
significantly related to lung cancer mortality (modelling results for
these variables were not presented) (Hnizdo et al., 1997). The authors
proposed three explanations for their results: (1) miners with high
dust exposure who develop silicosis have increased lung cancer risk,
(2) high silica dust exposure concentrations may be important in the
pathogenesis of lung cancer, and silicosis is coincidental, and (3)
high levels of silica dust exposure may be a surrogate measure of
exposure to radon daughters (Hnizdo et al., 1997).
Meta-analyses of the epidemiological studies of silica exposure
and lung cancer reported a moderate summary risk of 1.3 for
silica-exposed workers (Steenland & Stayner, 1997) and higher summary
relative risks of 2.2-2.3 for studies of silicotic workers (Smith et
al., 1995; Steenland & Stayner, 1997). Tsuda et al. (1997) pooled lung
cancer risk estimates from 32 mortality studies of pneumoconiosis or
silicosis (excluding asbestosis) published from 1980 to 1994. The
estimated rate ratios of 2.74 (95% CI = 2.60-2.90) for all studies,
2.77 (95% CI = 2.61-2.94) for cohort studies only (25 of 32 studies),
and 2.84 (95% CI = 2.25-3.59) for case-control studies (5 of 32
studies) were similar to the estimates reported by Steenland & Stayner
(1997) and Smith et al. (1995).
Reasons for the higher risks in silicotics are not known;
similarly, the question of whether fibrosis is a precursor to the
development of lung cancer in humans has not been resolved. Various
hypothetical mechanistic pathways have been proposed to explain the
occurrence of lung tumours in rats and lung cancer in humans; however,
there is no convincing evidence for any specific proposed pathway
(IARC, 1997).
Selection bias is a common criticism of epidemiological studies
of lung cancer in compensated silicotics, because workers who sought
compensation for their disease may differ from the group of all
silicotics in symptoms, radiographic changes, social and psychological
factors, and industry (McDonald, 1995; Weill & McDonald, 1996).
However, Goldsmith (1998) reviewed this question and concluded that
lung cancer risk estimates were not higher in compensated silicotics
when results of studies of compensated silicotics were compared with
results of studies of silicotics ascertained from other clinical
sources (i.e., hospital or registry data).
9.2.4 Autoimmune-related disease
In humans, immune activation by occupational exposure to
respirable quartz may be linked to scleroderma, rheumatoid arthritis,
polyarthritis, mixed connective tissue disease, systemic lupus
erythematosus, Sjögren's syndrome, polymyositis, and fibrositis
(Haustein et al., 1990; Ziegler & Haustein, 1992; Otsuki et al.,
1998). The cellular mechanism that leads from quartz dust exposure to
autoimmune diseases is not known (Otsuki et al., 1998; NIOSH,
forthcoming). One theory is that when respirable silica particles are
encapsulated by macrophages, fibrogenic proteins and growth factors
are generated, and ultimately the immune system is activated (Haustein
et al., 1992; Ziegler & Haustein, 1992; Haustein & Anderegg, 1998).
Several epidemiological studies have reported statistically
significant numbers of excess deaths or cases of autoimmune-related
diseases such as scleroderma (Sluis-Cremer et al., 1985; Steenland &
Brown, 1995b), rheumatoid arthritis (Sluis-Cremer et al., 1986;
Klockars et al., 1987), and systemic lupus erythematosus (Steenland &
Brown, 1995b) in silica-exposed workers.
9.2.5 Renal disease
Recent epidemiological studies have found statistically
significant associations between occupational exposure to crystalline
silica dust and renal diseases and subclinical renal changes
(Steenland et al., 1992; Ng et al., 1993; Boujemaa et al., 1994; Hotz
et al., 1995; Nuyts et al., 1995; Steenland & Brown, 1995b; Steenland
& Goldsmith, 1995; Calvert et al., 1997).
9.2.6 Chronic obstructive pulmonary disease
Occupational exposure to respirable crystalline silica dust is
associated with chronic obstructive pulmonary disease, including
bronchitis and emphysema. Although these health effects are also
associated with tobacco smoking, some epidemiological studies suggest
that they may be present to a significant extent in non-smokers with
occupational exposure to quartz (Wiles & Faure, 1977; Becklake et al.,
1987; Holman et al., 1987; Kreiss et al., 1989; Cowie & Mabena, 1991).
9.2.7 Other adverse health effects
Cor pulmonale (i.e., enlargement of the right ventricle of the
heart because of structural or functional abnormalities of the lungs)
may occur as a complication of silicosis (Green & Vallyathan, 1996)
and other pneumoconioses (Kusiak et al., 1993). It is usually preceded
by pulmonary arterial hypertension. An epidemiological case-control
study of 732 white South African autopsied gold miners reported a
statistically significant association (P < 0.05) of cor pulmonale
with "extensive" silicosis and "slight" silicosis (Murray et al.,
1993).
Other adverse health effects or complications of silicosis have
been studied or identified in epidemiological studies of workers that
may have been exposed to quartz dust, but evidence of an association
with quartz exposure is inconclusive. These adverse effects include
dental abrasion (Petersen & Henmar, 1988), nasopharyngeal or
pharyngeal cancer (Carta et al., 1991; Chen et al., 1992), salivary
gland cancer (Zheng et al., 1996), liver cancer (Chen et al., 1992;
Hua et al., 1992), bone cancer (Steenland & Beaumont, 1986; Forastiere
et al., 1989), pancreatic cancer (Kauppinen et al., 1995), skin cancer
(Partanen et al., 1994), oesophageal cancer (Belli et al., 1989;
Xu et al., 1996; Pan et al., 1999), stomach cancer
(Parent et al., 1998), cancers of the digestive system
(Decoufle & Wood, 1979), intestinal or peritoneal cancer
(Amandus et al., 1991; Costello et al., 1995; Goldsmith et al.,
1995a), lymphopoietic or haematopoietic cancers (Silverstein et al.,
1986; Steenland & Brown, 1995b), and bladder cancer (Bravo et al.,
1987).
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
Terrestrial mammals (i.e., horses, camels) and birds exposed to
quartz in the natural environment, especially in desert or coastal
areas, show pathological lesions sometimes described as "silicosis"
that are similar to those seen in humans with silicosis
(Schwartz et al., 1981; Evans et al., 1988; Hansen et al., 1989;
Berry et al., 1991; Xu et al., 1993; Green & Vallyathan, 1996). Rats,
hamsters, guinea-pigs, monkeys, and mice exposed to quartz under
experimental conditions develop lung conditions and nodules similar to
those found in humans (reviewed by Green & Vallyathan, 1996).
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
The extensive body of in vitro and in vivo research
evaluating the effects of quartz on mammalian cells is summarized here
(IARC, 1997; NIOSH, forthcoming). Quartz deposited in the lungs causes
epithelial and macrophage injury and activation, and it translocates
to the interstitium and the regional lymph nodes. Recruitment of
inflammatory cells occurs in a dose-dependent manner. Oxidative stress
(i.e., increased formation of reactive oxygen species, including
hydroxyl radicals, or reactive nitrogen species) has been observed in
rats after intratracheal instillation (Blackford et al., 1994;
Schapira et al., 1995) or inhalation (Vallyathan et al., 1995) of
quartz. Several mechanisms have been proposed to explain the cause of
the cellular damage by quartz particles (Lapp & Castranova, 1993):
(1) direct cytotoxicity of quartz, (2) stimulation of the alveolar
macrophages by quartz, which results in the release of cytotoxic
enzymes or oxidants, (3) stimulation of the alveolar macrophages to
release inflammatory factors (e.g., interleukin-8, leukotriene B4,
platelet-activating factor, tumour necrosis factor, platelet-derived
growth factor) that recruit polymorphonuclear leukocytes, which may
release cytotoxins, (4) stimulation of the alveolar macrophages to
release factors that initiate fibroblast production and collagen
synthesis (e.g., interleukin-1, tumour necrosis factor,
platelet-derived growth factor, fibronectin, alveolar
macrophage-derived growth factor), and, more recently, (5) induction
by quartz of apoptosis and subsequent engulfment by macrophages to
regulate the evolution of inflammation and fibrosis (Leigh et al.,
1997).
Silicosis is indisputably causally related to occupational quartz
exposure, and the dose-response assessments of the adverse health
effects of quartz are based on epidemiological studies of occupational
cohorts with silicosis. To date, there are no known adverse health
effects associated with non-occupational exposure to quartz dust.
Silicosis is the critical effect for hazard identification and
dose-response assessment, for two reasons. First, although IARC
classified inhaled quartz from occupational sources as a Group 1
carcinogen, there are very few published risk assessments
(toxicological or epidemiological) with a quantitative dose-response
assessment of lung cancer risk at various levels of quartz exposure. A
pooled exposure-response assessment of a number of epidemiological
studies with quantitative data for quartz exposures and lung cancer is
currently being conducted by IARC.
Secondly, epidemiological studies of quartz-exposed workers
reported statistically significant numbers of excess deaths or cases
of renal disease or subclinical renal changes (Steenland et al., 1992;
Ng et al., 1993; Boujemaa et al., 1994; Hotz et al., 1995;
Nuyts et al., 1995; Steenland & Brown, 1995b; Calvert et al., 1997),
mycobacterial infections (tuberculous and non-tuberculous), or fungal
infections (Ziskind et al., 1976; Parkes, 1982; Parker, 1994; Althouse
et al., 1995; Goldsmith et al., 1995a; NIOSH, 1996; American Thoracic
Society, 1997; Kleinschmidt & Churchyard, 1997; Hnizdo & Murray, 1998;
Corbett et al., 1999), immunological disorders and autoimmune diseases
(i.e., scleroderma) (Sluis-Cremer et al., 1985; Cowie, 1987; Steenland
& Brown, 1995b), rheumatoid arthritis (Sluis-Cremer et al., 1986;
Klockars et al., 1987), and systemic lupus erythematosus (Steenland &
Brown, 1995b), but sufficient epidemiological or toxicological data do
not currently exist for quantitative assessment of the
exposure-response relationship for these health effects.
The US EPA calculated lowest-observed-adverse-effect level
(LOAEL) human equivalent concentrations (HECs) for non-cancer effects
reported in subchronic (<3 months) and chronic inhalation studies
that administered less than 20 mg quartz/m3 to experimental rats and
mice (Table 1). The results varied widely -- from 0.18 mg/m3 (based
on rat study) to 0.90 mg/m3 (based on mouse study). Some of this
variation may have been due to differences in dose, species, and the
quartz specimens. The method for determining the HEC was based on a
series of empirical equations described elsewhere (US EPA, 1994) and
summarized here. It represents an example of the calculation of an HEC
and may not necessarily be a method used worldwide. The equations
estimate fractional deposition of relatively insoluble particles and
adjust for dosimetric differences between species by incorporating
normalizing factors such as body weight or surface area (US EPA,
1994). In summary:
NOAEL[HEC] (mg/m3) = NOAEL[ADJ] (mg/m3) × RDDRr
where:
* NOAEL[HEC] = the no-observed-adverse-effect level (NOAEL) human
equivalent concentration, dosimetrically adjusted
* NOAEL[ADJ] = the NOAEL adjusted for duration: E (mg/m3) × D
(h/24 h) × W (days/7 days), where E = experimental exposure level
* RDDRr = the regional deposited dose ratio of particles for the
respiratory tract region (r)
* RDDR = (RDDTH/Normalizing Factor)A/(RDDTH/Normalizing
Factor)H, the ratio of regional deposited dose (RDDr) in the
thoracic (TH) region in the animal (A) species to that of humans
(H) (US EPA, 1994)
* r = region of dose deposition, in this case thoracic
* RDDr = 10-6 × Ci × VE × Fr, where:
Ci = concentration (mg/m3)
VE = minute volume (ml/min)
Fr = fractional deposition in region r
The literature contains a few additional risk assessments of lung
cancer and quartz, and all were based on results of inhalation studies
of rats (Collins & Marty, 1995, 1997; Goldsmith et al., 1995b).
However, human data introduce less uncertainty than extrapolation from
animals to humans (Goldsmith et al., 1995b; Goldsmith &
Hertz-Picciotto, 1997). For quartz, the uncertainty can be attributed
in part to the lack of (1) experimental studies that systematically
evaluated exactly the same material to which humans are exposed (IARC,
1997), (2) understanding of the mechanism for induction of rat lung
tumours (IARC, 1997), (3) understanding of the species differences
observed in the fibrogenic response (Gift & Faust, 1997), and
(4) experimental studies that administered doses in concentrations
similar to known occupational exposures (US EPA, 1996).
Epidemiological studies that used cumulative exposure estimates
represent the best currently available source of information for
characterizing the dose-response relationship for silicosis or lung
cancer in occupational, as well as non-occupational, cohorts.
Exposure-response models based on cumulative exposure data can
provide predictions of silicosis risk for a particular silica dust
exposure over a period of time. Table 6 presents results of logistic
regression models of data from the cross-sectional epidemiological
studies described in section 9.2. The models predicted the prevalence
of radiographic silicosis (ILO category >1/1 or >1/0) from
cumulative exposure to respirable quartz. All models predicted the
occurrence of at least one case of radiographic silicosis per 100
workers at cumulative respirable quartz dust exposures of 0.05 or 0.10
mg/m3 over a 45-year working lifetime. Logistic regression assumes
that the change in the level of quartz exposure affects risk (or
predicted prevalence) in a multiplicative manner (as compared with
linear models, which predict deviations from additivity)
(Kleinbaum et al., 1982).
Table 7 displays the results of three studies that followed
cohorts of miners for silicosis over time or retrospectively. In these
studies, the miners with silicosis had their first quartz exposure
18-37 years (mean) prior to radiographic diagnosis. The models applied
to the data collected in these studies predicted that the risk of
chronic silicosis increases exponentially with increasing cumulative
dose of silica dust (US EPA, 1996). Data from the study of South
African gold miners (Hnizdo & Sluis-Cremer, 1993) and Canadian gold
and uranium miners were analysed with models that assumed some risk of
silicosis at any exposure. The study of US gold miners (Steenland &
Brown, 1995a) estimated silicosis rates (cases per person-time at
risk) for seven cumulative exposure categories, stratified by 5-year
age and calendar time intervals. Poisson regression was used to adjust
the crude rates for age and calendar time. Although age and calendar
time were highly correlated with exposure, it is not likely that they
confounded the exposure-response analysis, because silicosis has no
background rate for non-exposed populations that changes with age or
calendar time (US EPA, 1996).
In summary, the risk estimates for silicosis prevalence for a
working lifetime of exposure to respirable quartz dust concentrations
of about 0.05 or 0.10 mg/m3 in the occupational environment vary
widely (i.e., 2-90%). Epidemiological studies varied in the definition
of silicosis cases, radiographic interpretation methods, cohort
follow-up periods, and statistical methods. The variability in the
risk estimates cannot be solely attributed to differences in follow-up
periods; however, it must be recognized that chronic silicosis is a
progressive disease. Therefore, the development of silicosis after a
long latency period and after workers leave employment must be
accounted for in epidemiological studies. Results of a study of
autopsied South African gold miners found that the diagnostic
sensitivity of radiological examination is particularly poor (Hnizdo
et al., 1993). For example, when the radiological findings for
profusion of rounded opacities (ILO category >1/1) were compared
with pathological findings for silicosis in 326 miners with an average
of 2.7 years between the radiological and pathological examination,
silicosis was not diagnosed radiographically for at least 61% of the
miners with slight to marked silicosis at autopsy. The probability of
a false-negative reading increased with years of mining and the
average concentration of respirable dust (Hnizdo et al., 1993).
Experimental studies of rats also reported lack of agreement between
histopathological indicators of silica dust exposure and radiographic
readings (Drew & Kutzman, 1984a,b).
Improved exposure assessment methods and data analyses that
account for variations and deficiencies in exposure data would improve
the risk estimates for silica-exposed workers (Agius et al., 1992;
Checkoway, 1995). Although epidemiological studies that used
cumulative exposure estimates represent the best available source of
information for characterizing the dose-response relationship in
occupational cohorts, peak exposures may predict silicosis risk better
than cumulative exposures (Checkoway & Rice, 1992), but data are
rarely available.
11.1.2 Criteria for setting tolerable intakes or guidance values
for quartz
Results of genotoxicity tests of quartz, as well as other
in vitro and in vivo evidence, suggest that persistent
inflammation and epithelial proliferation are related to the tumour
response in rats. Other pathways for tumour induction, such as a
direct genotoxic effect, have not been ruled out. Because a pathway
has not been determined, and it is unclear from results of
epidemiological studies whether silicosis is a precursor to
lung cancer, it cannot be assumed that there is a threshold (i.e.,
tolerable concentration, or TC) at which exposure to quartz would not
result in silicosis and/or lung cancer. In addition, available data
indicate that occupational quartz exposure is associated with the
development of tuberculosis, especially in workers with silicosis;
however, the association has not been quantified for that outcome or
for other quartz-related diseases. Therefore, occupational exposure to
respirable quartz dust should be reduced to the extent possible.
11.1.3 Sample risk characterization
It is recognized that there are many different methods for
assessing the risk to human health posed by environmental and
occupational substances. The example presented here applies to healthy
individuals not compromised by respiratory ailments and who breathe
the ambient air in the USA. It is based on the results of the three
cohort studies of miners presented in Table 7 (section 11.1.1). Using
a high estimate of 10% crystalline silica content of particulate
matter with a mass median aerodynamic diameter not greater than 10 µm
(PM10) from US metropolitan areas, the highest cumulative crystalline
silica exposure expected from continuous human lifetime exposure at or
below the annual US national ambient air quality standard (NAAQS) for
particulate matter of 50 µg/m3 is 1 mg/m3-year (US EPA, 1996).
The US EPA applied a mathematical model (i.e., benchmark dose
[BMD] analysis) to determine a concentration and lower confidence
bound associated with a predefined effect level (i.e., 1%, 5%, and 10%
risk of silicosis) (US EPA, 1996). After consideration of
cross-sectional, longitudinal, retrospective cohort, and case-control
epidemiological studies, the BMD was conducted on data from the
previously described study of radiographic silicosis in a cohort of
2235 South African gold miners (Hnizdo & Sluis-Cremer, 1993). This
study was selected for several reasons: (1) continuous and
longitudinal investigation of silicosis, (2) miners had multiple X-ray
examinations, and (3) autopsy data were available for more accurate
interpretation of the radiographic results, which lack sensitivity (US
EPA, 1996).1
1 The cross-sectional studies described in Table 6 and sections
9.2.1 and 11.1.1 were not available at the time of the US EPA
assessment. These studies modelled the cumulative quartz exposures
of hardrock miners (Kreiss & Zhen, 1996) and grey iron foundry
workers (Rosenman et al., 1996) and predicted a 30% prevalence of
ILO category > 1/1 silicosis over a 45-year working lifetime with
10 years of post-employment follow-up (Kreiss & Zhen, 1996) or a
2% prevalence of ILO category > 1/0 silicosis over a 40-year
working lifetime and controlling for pack-years of cigarette
smoking, race, and silica exposure other than in the foundry under
study (Rosenman et al., 1996). Both predictions are for a mean
quartz dust concentration of 0.05 mg/m3.
Using methods described by US EPA (1996), the BMD analysis
predicted that the silicosis risk for a continuous 70-year lifetime
exposure to 0.008 mg/m3 (estimated high crystalline silica
concentration in US metropolitan areas) is less than 3% for healthy
individuals not compromised by other respiratory diseases or
conditions and for ambient environment (US EPA, 1996). (Risks were not
calculated for other groups, such as people with respiratory
illnesses.) This risk estimate for exposure to ambient quartz may be
conservative, because quartz particles in the occupational environment
may be finer or "freshly fractured," occupational exposures may
involve high "peak" exposures, and, thus, the potential for disease
development may be greater.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
IARC has classified inhaled crystalline silica (quartz or
cristobalite) from occupational sources as a Group 1 carcinogen based
on sufficient evident of carcinogenicity in humans and experimental
animals. In addition, "in making the overall evaluation, the Working
Group noted that carcinogenicity in humans was not detected in all
industrial circumstances studied. Carcinogenicity may be dependent on
inherent characteristics of the crystalline silica or on external
factors affecting its biological activity or distribution of its
polymorphs" (IARC, 1997).
In 1991, the ILO published a document describing methods for
prevention and control of occupational lung diseases, including
silicosis (ILO, 1991), and in 1993, the Office of Occupational Health
of the World Health Organization (WHO) called for increased medical
surveillance of workers exposed to mineral dusts to prevent
pneumoconioses such as silicosis and asbestosis (WHO, 1993). WHO also
recently published information about risk factors for pulmonary
tuberculosis, including occupational exposure to respirable
crystalline silica (WHO, 1996).
In 1986, a WHO study group recommended a limit of 40 µg/m3
(time-weighted average of an 8-h shift) for occupational exposure to
respirable crystalline silica dust (WHO, 1986).
REFERENCES
Agius RM, Love RG, Davies LST, Hutchison PA, Cherrie JW, Robertson A,
Cowie HA, Hurley JF, Seaton A, Soutar CA (1992) Epidemiological
studies of respiratory health and dust exposure in hard rock quarry
workers and ex-workers. Edinburgh, Institute of Occupational
Medicine (HSE Contract No. 1/LMD/126/146/88; Report No. TM/92/10).
Ahlman K, Koskela R-S, Kuikka P, Koponen M, Annanmäki M (1991)
Mortality among sulfide ore miners. American journal of industrial
medicine, 19:603-617.
Althouse RB, Bang KM, Castellan RM (1995) Tuberculosis comortality
with silicosis -- United States, 1979-1991. Applied occupational and
environmental hygiene, 10(12):1037-1041.
Altieri A, Sperduto B, Verdel U, Porceli D (1984) Identification of
cristobalite and quartz in the production of silicon carbide. Rivista
degli Infortuni e delle Malattie Professionali, 71(1-2):131-135.
Amandus HE, Shy C, Wing S, Blair A, Heineman EF (1991) Silicosis and
lung cancer in North Carolina dusty trades workers. American journal
of industrial medicine, 20:57-70.
Amandus HE, Castellan RM, Shy C, Heineman EF, Blair A (1992)
Reevaluation of silicosis and lung cancer in North Carolina dusty
trades workers. American journal of industrial medicine, 22:147-153.
American Thoracic Society (1997) Adverse effects of crystalline silica
exposure. American journal of respiratory and critical care medicine,
155:761-765.
Anderson LJ, Donaldson HM, Jones JH, Stringer WT, Wallingford KM
(1980) North Carolina Brick Industry Industrial Hygiene and
Respiratory Disease Morbidity Survey, 1974-1975. Cincinnati, OH,
National Institute for Occupational Safety and Health (National
Technical Information Service Report No. PB83-181735).
Aoki A, Sirai A, Sakamoto H, Igarashi T, Matsunaga K, Ishigatsubo Y,
Tani K, Okubo T (1988) A case of silicosis associated with
polymyositis and benign monoclonal gammopathy. Ryumachi,
28(5):373-378.
Arnalich F, Lahoz C, Picazo ML, Monereo A, Arribas JR, Martinez Ara J,
Vazquez JJ (1989) Polyarteritis nodosa and necrotizing
glomerulonephritis associated with long-standing silicosis. Nephron,
51(4):544-547.
Balmes J (1990) Silica exposure and tuberculosis: an old problem with
some new twists [editorial]. Journal of occupational medicine,
32(2):114-115.
Banks DE, Milutinovic J, Desnick RJ, Grabowski GA, Lapp NL, Boehlecke
BA (1983) Silicon nephropathy mimicking Fabry's disease.
American journal of nephrology, 3(5):279-284.
Beadle DG (1965) An epidemiological study of the relationship between
the amount of dust breathed and the incidence of silicosis in South
African gold miners. In: Davies CN, ed. Inhaled particles and
vapours II. Oxford, Pergamon Press, pp. 479-492.
Beadle DG (1971) The relationship between the amount of dust breathed
and the development of radiological signs of silicosis: an
epidemiological study in South African gold miners. In: Walton WH, ed.
Inhaled particles III: Proceedings of an international symposium
organized by the British Occupational Hygiene Society, Vol. II.
Surrey, Unwin Brothers Limited, pp. 953-964.
Beadle DG, Bradley AA (1970) The composition of airborne dust in South
African gold mines. In: Shapiro HA, ed. Pneumoconiosis: Proceedings
of the international conference, Johannesburg, 1969. Oxford, Oxford
University Press, pp. 462-466.
Becklake MR, Irwig L, Kielkowski D, Webster I, De Beer M, Landau S
(1987) The predictors of emphysema in South African gold miners.
American review of respiratory disease, 135:1234-1241.
Belli S, Comba P, Germani D, Grignoli M, Lagorio S, Paganoni R,
Ronchin M (1989) [Mortality study among lead-zinc Italian (Val
Seriana) miners.] Medicina del Lavoro, 80(6):467-478 (in Italian).
Berry CR, O'Brien TR, Madigan JE, Hager DA (1991) Thoracic
radiographic features of silicosis in 19 horses. Journal of
veterinary internal medicine, 5(4):248-256.
Blackford JA, Antonini JM, Castranova V, Dey RD (1994) Intratracheal
instillation of silica up-regulates inducible nitric oxide synthase
gene expression and increases nitric oxide production in alveolar
macrophages and neutrophils. American journal of respiratory cell and
molecular biology, 11(4):426-431.
Bloor WA, Eardley RE, Dinsdale A (1971) Environmental conditions in
sanitary whiteware casting shops. Annals of occupational hygiene,
14(4):321-327.
Bolsaitis PP, Wallace WE (1996) The structure of silica surfaces in
relation to cytotoxicity. In: Castranova V, Vallyathan V, Wallace WE,
eds. Silica and silica-induced lung diseases. Boca Raton, FL,
CRC Press, pp. 79-89.
Bolton WK, Suratt PM, Sturgill BC (1981) Rapidly progressive silicon
nephropathy. American journal of medicine, 71:823-828.
Bonnin A, Mousson C, Justrabo E, Tanter Y, Chalopin JM, Rifle G (1987)
Silicosis associated with crescentic IgA mesangial nephropathy
[letter]. Nephron, 47(3):229-230.
Boujemaa W, Lauwerys R, Bernard A (1994) Early indicators of renal
dysfunction in silicotic workers. Scandinavian journal of work,
environment and health, 20(3):180-183.
Bravo MP, Del Rey Calero J, Conde M (1987) Silice y cancer de vejiga
en varones. Archivos Espanoles de Urologia, 40(9):635-637.
Bresnitz EA, Roseman J, Becker D, Gracely E (1992) Morbidity among
municipal waste incinerator workers . American journal of industrial
medicine, 22:363-378.
Burgess GL, Turner S, McDonald JC, Cherry NM (1997) Cohort mortality
study of Staffordshire pottery workers: (I) Radiographic validation of
an exposure matrix for respirable crystalline silica. Annals of
occupational hygiene, 41 (Suppl. 1):403-407.
Burgess WA (1995) Recognition of health hazards in industry: a review
of materials and processes, 2nd ed. New York, NY, John Wiley & Sons,
pp. 106-139, 411-422, 423-434, 475-482.
Buringh E, van de Belt R, van der Wal JF (1990) Dust control measures
in Dutch brickworks. Annals of occupational hygiene, 34:483-497.
Burns CA, Zarkower A, Ferguson FG (1980) Murine immunological and
histological changes in response to chronic silica exposure.
Environmental research, 21(2):298-307.
Calvert GM, Steenland K, Palu S (1997) End-stage renal disease among
silica-exposed gold miners. Journal of the American Medical
Association, 277(15):1219-1223.
Carta P, Cocco PL, Casula D (1991) Mortality from lung cancer among
Sardinian patients with silicosis. British journal of industrial
medicine, 48(2):122-129.
Castranova V, Dalal NS, Vallyathan V (1996) Role of surface free
radicals in the pathogenicity of silica. In: Castranova V, Vallyathan
V, Wallace WE, eds. Silica and silica-induced lung diseases. Boca
Raton, FL, CRC Press, pp. 91-105.
Castranova V, Vallyathan V, Ramsey DM, McLaurin JL, Pack D, Leonard S,
Barger MW, Ma JYC, Dalal NS, Teass A (1997) Augmentation of pulmonary
reactions to quartz inhalation by trace amounts of iron-containing
particles. Environmental health perspectives, 105 (Suppl.
5):1319-1324.
Cavariani F, Di Pietro A, Miceli M, Forastiere F, Biggeri A, Scavalli
P, Petti A, Borgia P (1995) Incidence of silicosis among ceramic
workers in central Italy. Scandinavian journal of work, environment
and health, 21 (Suppl. 2):58-62.
Chamberlain M (1983) Effect of mineral dusts on metabolic cooperation
between Chinese hamster V79 cells in vitro. Environmental health
perspectives, 51:5-9.
Checkoway H (1995) Methodological considerations relevant to
epidemiology studies of silica and lung cancer. Applied occupational
and environmental hygiene, 10(12):1049-1055.
Checkoway H, Rice CH (1992) Time-weighted averages, peaks, and other
indices of exposure in occupational epidemiology. American journal of
industrial medicine, 21:25-33.
Checkoway H, Heyer NJ, Demers PA, Breslow NE (1993) Mortality among
workers in the diatomaceous earth industry. British journal of
industrial medicine, 50:586-597.
Checkoway H, Heyer NJ, Demers PA, Gibbs GW (1996) Reanalysis of
mortality from lung cancer among diatomaceous earth industry workers,
with consideration of potential confounding by asbestos exposure.
Occupational and environmental medicine, 53:645-647.
Chen GX, Burnett CA, Cameron LL, Alterman T, Lalich NR, Tanaka S,
Althouse RB (1997) Tuberculosis mortality and silica exposure: a
case-control study based on a national mortality database for the
years 1983-1992. International journal of occupational and
environmental health, 3:163-170.
Chen J, McLaughlin JK, Zhang JY, Stone BJ, Luo J, Chen RA, Dosemeci M,
Rexing SH, Wu Z, Hearl FJ, McCawley MA, Blot WJ (1992) Mortality among
dust-exposed Chinese mine and pottery workers. Journal of
occupational medicine, 34(3):311-316.
Cherry N, Burgess G, McNamee R, Turner S, McDonald C (1995) Initial
findings from a cohort mortality study of British pottery workers.
Applied occupational and environmental hygiene, 10(12):1042-1045.
Cherry NM, Burgess GL, Turner S, McDonald JC (1997) Cohort study of
Staffordshire pottery workers: (II) Nested case referent analysis of
lung cancer. Annals of occupational hygiene, 41 (Suppl. 1):408-411.
Cherry NM, Burgess GL, Turner S, McDonald JC (1998) Crystalline silica
and risk of lung cancer in the potteries. Occupational and
environmental medicine, 55:779-785.
Collins JF, Marty MA (1995) Cancer risk assessment for crystalline
silica to implement California's Hot Spots Act. Scandinavian
journal of work, environment and health, 21 (Suppl. 2):99-103.
Collins JF, Marty MA (1997) Cancer risk assessment for crystalline
silica. Journal of exposure analysis and environmental epidemiology,
7(3):359-365.
Cooper TC, Gressel MG, Froelich PA, Caplan PE, Mickelsen RL, Valiante
D, Bost P (1993) Successful reduction of silica exposures at a
sanitary ware pottery. American Industrial Hygiene Association
Journal, 54(10):600-606.
Corbett EL, Churchyard GJ, Clayton T, Herselman P, Williams B, Hayes
R, Mulder D, De Cock KM (1999) Risk factors for pulmonary
mycobacterial disease in South African gold miners. American journal
of respiratory and critical care medicine, 159:94-99.
Costello J, Graham WGB (1988) Vermont granite workers' mortality
study. American journal of industrial medicine, 13:483-497.
Costello J, Castellan RM, Swecker GS, Kullman GJ (1995) Mortality of a
cohort of U.S. workers employed in the crushed stone industry,
1940-1980. American journal of industrial medicine, 27:625-640.
Cowie RL (1987) Silica-dust-exposed mine workers with scleroderma
(systemic sclerosis). Chest, 92(2): 260-262.
Cowie RL (1994) The epidemiology of tuberculosis in gold miners with
silicosis. American journal of respiratory and critical care
medicine, 150:1460-1462.
Cowie RL, Mabena SK (1991) Silicosis, chronic airflow limitation, and
chronic bronchitis in South African gold miners. American review of
respiratory disease, 143:80-84.
Dagle GE, Wehner AP, Clark ML, Buschbom RL (1986) Chronic inhalation
exposure of rats to quartz. In: Goldsmith DF, Winn DM, Shy CM, eds.
Silica, silicosis, and cancer: Controversy in occupational medicine.
New York, NY, Praeger Publishers, pp. 255-266 (Cancer Research
Monographs, Vol. 2).
Daniel LN, Mao Y, Saffiotti U (1993) Oxidative DNA damage by
crystalline silica. Free radical biology and medicine, 14:463-472.
Daniel LN, Mao Y, Wang T-CL, Markey CJ, Markey SP, Shi X, Saffiotti U
(1995) DNA strand breakage, thymine glycol production, and hydroxyl
radical generation induced by different samples of crystalline silica
in vitro. Environmental research, 71:60-73.
Davies LST, Robertson A, Agius RM, Cowie HA, Cherrie JW, Hutchison P
(1994) The use of compliance monitoring for assessing quarry workers'
exposures to respirable dust and quartz. Annals of occupational
hygiene, 38 (Suppl. 1):559-570.
Davis BL, Johnson LR, Stevens RK, Courtney WJ, Safriet DW (1984) The
quartz content and elemental composition of aerosols from selected
sites of the EPA inhalable particulate network. Atmospheric
environment, 18(4):771-782.
Davis GS (1991) Immunologic aspects of pneumoconioses in asbestosis
and silicosis. In: Lynch JP, DeRemee RA, eds. Immunologically
mediated pulmonary diseases. Philadelphia, PA, J.B. Lippincott
Company, pp. 111-155.
Davis GS (1996) Silica. In: Harber P, Schenker MB, Balmes JR, eds.
Occupational and environmental respiratory disease, 1st ed. St.
Louis, MO, Mosby, pp. 373-399.
Davis LK, Wegman DH, Monson RR, Froines J (1983) Mortality experience
of Vermont granite workers. American journal of industrial medicine,
4:705-723.
Decoufle P, Wood DJ (1979) Mortality patterns among workers in a gray
iron foundry. American journal of epidemiology, 109(6):667-675.
de Klerk NH, Musk AW (1998) Silica, compensated silicosis, and lung
cancer in Western Australian goldminers. Occupational and
environmental medicine, 55:243-248.
Dethloff LA, Gilmore LB, Gladen BC, George G, Chhabra RS, Hook GER
(1986a) Effects of silica on the composition of the pulmonary
extracellular lining. Toxicology and applied pharmacology,
84(1):66-83.
Dethloff LA, Gilmore LB, Brody AR, Hook GER (1986b) Induction of
intracellular and extracellular phospholipids in the lungs of rats
exposed to silica. Biochemical journal, 233(1):111-118.
Donaldson HM, Wallingford K, Jones JH (1982) Environmental surveys in
the Barre, Vermont and Elberton, Georgia granite industries.
Cincinnati, OH, National Institute for Occupational Safety and Health
(National Technical Information Service Publication No. PB83-179911).
Donaldson K, Borm PJA (1998) The quartz hazard: a variable entity.
Annals of occupational hygiene, 42(5):287-294.
Dong D, Xu G, Sun Y, Hu P (1995) Lung cancer among workers exposed to
silica dust in Chinese refractory plants. Scandinavian journal of
work, environment and health, 21 (Suppl. 2):69-72.
Dosemeci M, McLaughlin JK, Chen J-Q, Hearl F, Chen R-G, McCawley M, Wu
Z, Peng K-L, Chen A-L, Rexing SH, Blot WJ (1995) Historical total and
respirable silica dust exposure levels in mines and pottery factories
in China. Scandinavian journal of work, environment and health, 21
(Suppl. 2):39-43.
Dracon M, Noel C, Wallaert P, Dequiedt P, Lelievre G, Tacquet A (1990)
Rapidly progressive glomerulonephritis in silicotic coal miners.
Nephrologie, 11:61-65.
Drew RT, Kutzman RS (1984a) Final report on a study of Fischer 344
rats exposed to silica dust for six months at concentrations of 0, 2,
10 or 20 mg/m3, then maintained for six months prior to assessment.
Upton, NY, Brookhaven National Laboratory (Report No. BNL 35735;
submitted to the National Toxicology Program under Interagency
Agreement No. 222-Y01-ES-9-0043, November 1984).
Drew RT, Kutzman RS (1984b) Final report on a study of Fischer 344
rats exposed to silica dust for six months at concentrations of 0, 2,
10 or 20 mg/m3. Upton, NY, Brookhaven National Laboratory (Report
No. BNL 34617; submitted to the National Toxicology Program under
Interagency Agreement No. 222-Y01-ES-9-0043, February 1984).
Driscoll KE (1995) The toxicology of crystalline silica studied
in vitro. Applied occupational and environmental hygiene,
10(12):1118-1125.
Driscoll KE (1996) The role of interleukin-1 and tumor necrosis factor
alpha in the lung's response to silica. In: Castranova V, Vallyathan
V, Wallace WE, eds. Silica and silica-induced lung diseases. Boca
Raton, FL, CRC Press, pp. 163-184.
Driscoll KE, Deyo LC, Howard BW, Poynter J, Carter JM (1995)
Characterizing mutagenesis in the hprt gene of rat alveolar
epithelial cells. Experimental lung research, 21:941-956.
Driscoll KE, Deyo LC, Carter JM, Howard BW, Hassenbein DG, Bertram TA
(1997) Effects of particle exposure and particle-elicited inflammatory
cells on mutation in rat alveolar epithelial cells. Carcinogenesis,
18(2):423-430.
Dufresne A, Lesage J, Perrault G (1987) Evaluation of occupational
exposure to mixed dusts and polycyclic aromatic hydrocarbons in
silicon carbide plants. American Industrial Hygiene Association
Journal, 48(2):160-166.
Dufresne A, Loosereewanich P, Bégin R, Dion C, Ecobichon D, Muir DCF,
Ritchie AC, Perrault G (1998a) Tentative explanatory variable of lung
dust concentration in gold miners exposed to crystalline silica.
Journal of exposure analysis and environmental epidemiology,
8(3):375-398.
Dufresne A, Bégin R, Dion C, Jagirdar J, Rom WN, Loosereewanich P,
Muir DCF, Ritchie AC, Perrault G (1998b) Angular and fibrous particles
in lung in relation to silica-induced diseases. International
archives of occupational and environmental health, 71:263-269.
Eisen EA, Smith TJ, Wegman DH, Louis TA, Froines J (1984) Estimation
of long term dust exposures in the Vermont granite sheds.
American Industrial Hygiene Association Journal, 45(2):89-94.
Erdogdu G, Hasirci V (1998) An overview of the role of mineral
solubility in silicosis and asbestosis. Environmental research,
78:38-42.
Evans MG, Slocombe RF, Schwartz LD (1988) Pulmonary silicosis in
captive ring-necked pheasants: definitive diagnosis by electron probe
X-ray microanalysis. Veterinary pathology, 25(3):239-241.
Forastiere F, Lagorio S, Michelozzi P, Perucci CA, Axelson O (1989)
Mortality pattern of silicotic subjects in the Latium region, Italy.
British journal of industrial medicine, 46(12):877-880.
Fox AJ, Greenberg M, Ritchie GL (1975) A survey of respiratory
disease in the pottery industry. London, Her Majesty's Stationery
Office.
Freeman CS, Grossman EA (1995) Silica exposures in the United States
between 1980 and 1992. Scandinavian journal of work, environment and
health, 21 (Suppl. 2):47-49.
Fubini B (1997) Surface reactivity in the pathogenic response to
particulates. Environmental health perspectives, 105 (Suppl.
5):1013-1020.
Fubini B (1998) Surface chemistry and quartz hazard. Annals of
occupational hygiene, 42(8):521-530.
Fubini B, Bolis V, Cavenago A, Volante M (1995) Physicochemical
properties of crystalline silica dusts and their possible implication
in various biological responses. Scandinavian journal of work,
environment and health, 21 (Suppl. 2):9-14.
Fukata S, Matsubayashi S, Nagato H, Sakai K, Ohishi S, Yasuda M, Tamai
H, Nakagawa T (1983) Monoclonal gammopathies associated with
silicosis. Rinsho Ketsueki, 24(1):9-17.
Fukata S, Tamai H, Nagai K, Matsubayashi S, Nagato H, Tashiro T,
Yasuda M, Kumagai LF (1987) A patient with hereditary spherocytosis
and silicosis who developed an IgA(lambda) monoclonal gammopathy.
Japanese journal of medicine, 26(1):81-83.
Fulekar MH, Alam Khan MM (1995) Occupational exposure to dust in slate
pencil manufacture. Annals of occupational hygiene, 39(1):107-114.
Gao H, Brick J, Ong S, Miller M, Whong W-Z, Ong T (1997) Selective
hyperexpression of c-jun oncoprotein by glass fiber- and
silica-transformed BALB/c-3T3 cells. Cancer letters, 112:65-69.
Gerhardsson G (1976) Dust prevention in Swedish foundries.
Staub-Reinhaltung der Luft, 36:433-439.
Gift JS, Faust RA (1997) Noncancer inhalation toxicology of
crystalline silica: exposure-response assessment. Journal of exposure
analysis and environmental epidemiology, 7(3): 345-358.
Giles RD, Sturgill BC, Suratt PM, Bolton WK (1978) Massive proteinuria
and acute renal failure in a patient with acute silicoproteinosis.
American journal of medicine, 64:336-342.
Goldsmith DF (1994) Health effects of silica dust exposure. In: Heaney
PJ, Prewitt CT, Gibbs GV, eds. Silica: physical behavior,
geochemistry, and materials applications. Washington, DC,
Mineralogical Society of America. Reviews in mineralogy, 29:545-606.
Goldsmith DF (1998) Uses of workers' compensation data in epidemiology
research. Occupational medicine state of the art reviews,
13(2):389-415.
Goldsmith DF, Hertz-Picciotto I (1997) Criteria for conducting
quantitative risk assessments for silica. Journal of exposure
analysis and environmental epidemiology, 7(3):367-375.
Goldsmith DF, Beaumont JJ, Morrin LA, Schenker MB (1995a) Respiratory
cancer and other chronic disease mortality among silicotics in
California. American journal of industrial medicine, 28(4):459-467.
Goldsmith DF, Ruble RP, Klein CA (1995b) Comparative cancer potency
for silica from extrapolations of human and animal findings.
Scandinavian journal of work, environment and health, 21 (Suppl.
2):104-107.
Green FHY, Vallyathan V (1996) Pathologic responses to inhaled silica.
In: Castranova V, Vallyathan V, Wallace WE, eds. Silica and
silica-induced lung diseases. Boca Raton, FL, CRC Press, pp. 39-59.
Greskevitch MF, Turk AR, Dieffenbach AL, Roman JM, Groce DW, Hearl FJ
(1992) Quartz analyses of the bulk dust samples collected by the
National Occupational Health Survey of Mining. Applied occupational
and environmental hygiene, 7(8):527-531.
Greskevitch MF, Bajpayee SS, Hale JM, Groce DW, Hearl FJ, eds. (1996)
NIOSH technical report: results from the National Occupational Health
Survey of Mining (NOHSM). Cincinnati, OH, US Department of Health
and Human Services, Public Health Service, Centers for Disease Control
and Prevention, National Institute for Occupational Safety and Health,
Division of Respiratory Disease Studies (DHHS (NIOSH) Publication No.
96-136).
Groth DH, Stettler LE, Platek SF, Lal JB, Burg JR (1986) Lung tumors
in rats treated with quartz by intratracheal instillation. In:
Goldsmith DF, Winn DM, Shy CM, eds. Silica, silicosis, and cancer:
Controversy in occupational medicine. New York, NY, Praeger
Publishers, pp. 243-253 (Cancer Research Monographs, Vol. 2).
Gu Z-W, Ong TO (1996) Potential mechanisms of silica-induced cancer.
In: Castranova V, Vallyathan V, Wallace WE, eds. Silica and
silica-induced lung diseases. Boca Raton, FL, CRC Press, pp.
397-406.
Guenel P, Hojberg G, Lynge E (1989) Cancer incidence among Danish
stone workers. Scandinavian journal of work, environment and health,
15:265-270.
Hansen HJ, Jama FM, Nilsson C, Norrgren L, Abdurahman OS (1989)
Silicate pneumoconiosis in camels (Camelus dromedarius L.).
Zentralblatt für Veterinarmedizin (A), 36(10):789-796.
Hart GA, Hesterberg TW (1998) In vitro toxicity of respirable-size
particles of diatomaceous earth and crystalline silica compared with
asbestos and titanium dioxide. Journal of occupational and
environmental medicine, 40(1):29-42.
Haslam PL (1994) Basic immunology and immunopathology. In: Parkes WR,
ed. Occupational lung disorders, 3rd ed. London,
Butterworth-Heinemann, pp. 50-99.
Hauglustaine D, Van Damme B, Daenens P, Michielsen P (1980) Silicon
nephropathy: a possible occupational hazard. Nephron, 26:219-224.
Haustein UF, Anderegg U (1998) Silica induced scleroderma -- clinical
and experimental aspects. Journal of rheumatology, 25(10):1917-1926.
Haustein UF, Ziegler V, Herrmann K, Mehlhorn J, Schmidt C (1990)
Silica-induced scleroderma. Journal of the American Academy of
Dermatology, 22(3):444-448.
Haustein UF, Ziegler V, Herrmann K (1992) Chemically induced
scleroderma. Der Hautarzt, 43:464-474.
Heppleston AG (1994) Pathogenesis of mineral pneumoconioses. In:
Parkes WR, ed. Occupational lung disorders, 3rd ed. London,
Butterworth-Heinemann, pp. 100-134.
Hesterberg TW, Barrett JC (1984) Dependence of asbestos- and mineral
dust-induced transformation of mammalian cells in culture on fiber
dimension. Cancer research, 44:2170-2180.
Hesterberg TW, Oshimura M, Brody AR, Barrett JC (1986) Asbestos and
silica induce morphological transformation of mammalian cells in
culture: a possible mechanism. In: Goldsmith DF, Winn DM, Shy CM, eds.
Silica, silicosis, and cancer. Controversy in occupational medicine.
New York, NY, Praeger Publishers, pp. 177-190 (Cancer Research
Monographs, Vol. 2).
Higgins RI, Deere MR, Cinkotai FF (1985) Fettlers' exposure to pottery
dust in a factory making sanitary whiteware. Annals of occupational
hygiene, 29(3):365-375.
Hnizdo E, Murray J (1998) Risk of pulmonary tuberculosis relative to
silicosis and exposure to silica dust in South African gold miners.
Occupational and environmental medicine, 55:496-502.
Hnizdo E, Murray J (1999) Correction: Risk of pulmonary tuberculosis
relative to silicosis and exposure to silica dust in South African
gold miners. Occupational and environmental medicine, 56:215-216.
Hnizdo E, Sluis-Cremer GK (1991) Silica exposure, silicosis, and lung
cancer: a mortality study of South African gold miners. British
journal of industrial medicine, 48:53-60.
Hnizdo E, Sluis-Cremer GK (1993) Risk of silicosis in a cohort of
white South African gold miners. American journal of industrial
medicine, 24:447-457.
Hnizdo E, Murray J, Sluis-Cremer GK, Thomas RG (1993) Correlation
between radiological and pathological diagnosis of silicosis: an
autopsy population based study. American journal of industrial
medicine, 24:427-445.
Hnizdo E, Murray J, Klempman S (1997) Lung cancer in relation to
exposure to silica dust, silicosis and uranium production in South
African gold miners. Thorax, 52:271-275.
Holland LM, Gonzales M, Wilson JS, Tillery MI (1983) Pulmonary effects
of shale dusts in experimental animals. In: Wagner WL, Rom WN,
Merchant JA, eds. Health issues related to metal and nonmetallic
mining. Boston, MA, Butterworth, pp. 485-496.
Holland LM, Wilson JS, Tillery MI, Smith DM (1986) Lung cancer in rats
exposed to fibrogenic dusts. In: Goldsmith DF, Winn DM, Shy CM, eds.
Silica, silicosis, and cancer: Controversy in occupational medicine.
New York, NY, Praeger Publishers, pp. 267-279 (Cancer Research
Monographs, Vol. 2).
Holman CDJ, Psaila-Savona P, Roberts M, McNulty JC (1987) Determinants
of chronic bronchitis and lung dysfunction in Western Australian gold
miners. British journal of industrial medicine, 44:810-818.
Hook GER, Viviano CJ (1996) Acute silicosis and the activation of
alveolar type II cells. In: Castranova V, Vallyathan V, Wallace WE,
eds. Silica and silica-induced lung diseases. Boca Raton, FL, CRC
Press, pp. 229-242.
Hotz P, Gonzalez-Lorenzo J, Siles E, Trujillano G, Lauwerys R, Bernard
A (1995) Subclinical signs of kidney dysfunction following short
exposure to silica in the absence of silicosis. Nephron, 70:438-442.
Hua F, Xipeng J, Shunzhang Y, Xueqi G, Kaiguo W, JianY, Shenghua Q
(1992) Quantitative risk assessment for lung cancer from exposure to
metal ore dust. Biomedical and environmental sciences, 5:221-228.
Huggins CW, Johnson SN, Segreti JM, Snyder JG (1985) Bureau of Mines
Report of Investigations 8975: Determination of alpha quartz particle
distribution in respirable coal mine dust samples and reference
standards. Avondale, MD, US Department of the Interior, Bureau of
Mines, 7 pp. (National Technical Information Service Publication No.
PB86-136660).
Hughes JM (1995) Radiographic evidence of silicosis in relation to
silica exposure. Applied occupational and environmental hygiene,
10(12):1064-1069.
IARC (1984) Polynuclear aromatic compounds. Part 3. Industrial
exposures in aluminium production, coal gasification, coke production
and iron and steel founding. Lyon, International Agency for Research
on Cancer, pp. 133-190 (IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Vol. 34).
IARC (1987) Silica and some silicates. Lyon, France, International
Agency for Research on Cancer, pp. 33-143 (IARC Monographs on the
Evaluation of the Carcinogenic Risk of Chemicals to Humans, Vol. 42).
IARC (1997) Silica, some silicates, coal dust and para -aramid
fibrils. Lyon, International Agency for Research on Cancer, pp.
1-242 (IARC Monographs on the Evaluation of Carcinogenic Risks to
Humans, Vol. 68).
Iler RK (1979) The chemistry of silica: solubility, polymerization,
colloid and surface properties, and biochemistry. New York, NY,
Wiley-Interscience Publishers.
ILO (1980) Guidelines for the use of ILO international classification
of radiographs of pneumoconioses, rev. ed. Geneva, International
Labour Organisation, 48 pp. (Occupational Safety and Health Series No.
22).
ILO (1991) Occupational lung diseases: prevention and control.
Geneva, International Labour Organisation, 85 pp. (Occupational
Safety and Health Series No. 67).
IPCS (1993) International Chemical Safety Card -- Quartz. Geneva,
World Health Organization, International Programme on Chemical Safety
(ICSC 0808).
Iyer R, Holian A (1996) Immunological aspects of silicosis. In:
Castranova V, Vallyathan V, Wallace WE, eds. Silica and
silica-induced lung diseases. Boca Raton, FL, CRC Press, pp.
253-267.
Jaurand MC, Fleury J, Monchaux G, Nebut M, Bignon J (1987) Pleural
carcinogenic potency of mineral fibers (asbestos, attapulgite) and
their cytotoxicity on cultured cells. Journal of the National Cancer
Institute, 79(4):797-804.
Kane AB (1996) Questions and controversies about the pathogenesis of
silicosis. In: Castranova V, Vallyathan V, Wallace WE, eds. Silica
and silica-induced lung diseases. Boca Raton, FL, CRC Press, pp.
121-136.
Kauppinen T, Partanen T, Degerth R, Ojajarvi A (1995) Pancreatic
cancer and occupational exposures. Epidemiology, 6(5):498-502.
Kelly J (1992) U.S. Department of the Interior, National Park
Service, New River Gorge National River, West Virginia. Cincinnati,
OH, National Institute for Occupational Safety and Health (HHE Report
No. HETA 92-045-2260),
King EJ, Mohanty GP, Harrison CV, Nagelschmidt G (1953) The action of
different forms of pure silica on the lungs of rats. British journal
of industrial medicine, 10:9-17.
Kleinbaum DG, Kupper LL, Morgenstern H (1982) Epidemiologic research:
principles and quantitative methods. New York, NY, Van Nostrand
Reinhold, p. 428.
Kleinschmidt I, Churchyard G (1997) Variation in incidences of
tuberculosis in subgroups of South African gold miners. Occupational
and environmental medicine, 54:636-641.
Klockars M, Koskela RS, Järvinen E, Kolari PJ, Rossi A (1987) Silica
exposure and rheumatoid arthritis: a follow up study of granite
workers 1940-81. British medical journal, 294:997-1000.
Koeger AC, Alcaix D, Rozenberg S, Bourgeois P (1991) Occupational
exposure to silica and dermatopolymyositis: three cases. Annales de
medecine interne (Paris), 142(6):409-413.
Koeger AC, Alcaix D, Rozenberg S, Bourgeois P (1996) Graves' disease
after silica dust exposure. Journal of rheumatology, 68(11):202.
Koskela R-S, Klockars M, Järvinen E, Kolari PJ, Rossi A (1987)
Mortality and disability among granite workers. Scandinavian journal
of work, environment and health, 13:18-25.
Kreiss K, Zhen B (1996) Risk of silicosis in a Colorado mining
community. American journal of industrial medicine, 30:529-539.
Kreiss K, Greenberg LM, Kogut SJH, Lezotte DC, Irvin CG, Cherniack RM
(1989) Hard-rock mining exposures affect smokers and nonsmokers
differently: results of a community prevalence study. American review
of respiratory disease, 139:1487-1493.
Kullman GJ, Greife AL, Costello J, Hearl FJ (1995) Occupational
exposures to fibers and quartz at 19 crushed stone mining and milling
operations. American journal of industrial medicine, 27:641-660.
Kurppa K, Gudbergsson H, Hannunkari I, Koskinen H, Hernberg S, Koskela
R-S, Ahlman K (1986) Lung cancer among silicotics in Finland. In:
Goldsmith DF, Winn DM, Shy CM, eds. Silica, silicosis, and cancer:
Controversy in occupational medicine. New York, NY, Praeger
Publishers, pp. 311-319 (Cancer Research Monographs, Vol. 2).
Kusiak R, Liss GM, Gailitis MM (1993) Cor pulmonale and pneumoconiotic
lung disease: an investigation using hospital discharge data.
American journal of industrial medicine, 24:161-173.
Lapp NL, Castranova V (1993) How silicosis and coal workers'
pneumoconiosis develops -- a cellular assessment. Occupational
medicine state of the art reviews, 8(1):35-56.
Lawson RJ, Schenker MB, McCurdy SA, Jenkins B, Lischak LA, John W,
Scales D (1995) Exposure to amorphous silica fibers and other
particulate matter during rice farming operations. Applied
occupational and environmental hygiene, 10(8):677-684.
Leigh J, Wang H, Bonin A, Peters M, Ruan X (1997) Silica-induced
apoptosis in alveolar and granulomatous cells in vivo.
Environmental health perspectives, 105 (Suppl. 5):1241-1245.
Leigh J, Wang H, Bonin A, Peters M (1998a) Persistence of
silica-induced inflammation is related to delayed occurrence of
apoptosis in alveolar leukocytes. In: Chiyotani K, Hosoda Y, Aizawa Y,
eds. Advances in the prevention of occupational respiratory diseases.
Amsterdam, Elsevier Science, pp. 890-895 (Excerpta Medica Supplement
53).
Leigh J, Wang H, Bonin A, Peters M (1998b) In vivo genotoxicity of
silica evidenced by progressive development of micronuclei in alveolar
macrophages. In: Chiyotani K, Hosoda Y, Aizawa Y, eds. Advances in
the prevention of occupational respiratory diseases. Amsterdam,
Elsevier Science, pp. 520-525 (Excerpta Medica Supplement 53).
Linch KD, Cocalis JC (1994) An emerging issue: Silicosis prevention in
construction. Applied occupational and environmental hygiene,
9(8):539-542.
Linch KD, Miller WE, Althouse RB, Groce DW, Hale JM (1998)
Surveillance of respirable crystalline silica dust using OSHA
compliance data (1979-1995). American journal of industrial medicine,
34(6):547-558.
Liu X, Keane MJ, Zhong B-Z, Ong T, Wallace WE (1996) Micronucleus
formation in V79 cells treated with respirable silica dispersed in
medium and in simulated pulmonary surfactant. Mutation research,
361:89-94.
Liu X, Keane MJ, Harrison JC, Cilento EV, Ong T, Wallace WE (1998)
Phospholipid surfactant adsorption by respirable quartz and in vitro
expression of cytotoxicity and DNA damage. Toxicology letters, 96,
97:77-84.
Lofgren DJ (1993) Case studies: silica exposure for concrete workers
and masons. Applied occupational and environmental hygiene,
8(10):832-836.
Love RG, Waclawski ER, Maclaren WM, Porteous RH, Groat SK, Wetherill
GZ, Hutchison PA, Kidd MW, Soutar CA (1994) Cross-sectional study of
risks of respiratory disease in relation to exposures of airborne
quartz in the heavy clay industry. Edinburgh, Institute of
Occupational Medicine (IOM Report No. TM/94/07).
Mao Y, Daniel LN, Whittaker N, Saffiotti U (1994) DNA binding to
crystalline silica characterized by Fourier-transform infrared
spectroscopy. Environmental health perspectives, 102
(Suppl. 10):165-171.
Masuda K (1981) Chronic thyroiditis observed in patients with
silicosis as an adjuvant disease of man. Shikoku Acta Medica,
37(1):119-129.
Materna BL, Jones JR, Sutton PM, Rothman N, Harrison RJ (1992)
Occupational exposures in California wildland fire fighting. American
Industrial Hygiene Association journal, 53(1):69-76.
McDonald C (1995) Silica, silicosis, and lung cancer: an
epidemiological update. Applied occupational and environmental
hygiene, 10(12):1056-1063.
McDonald JC, Oakes D (1984) Exposure-response in miners exposed to
silica. In: Sixth International Pneumoconiosis Conference: 1983,
Bochum, Germany. Vol 1. Geneva, International Labour Organisation,
pp. 114-123.
McDonald JC, Cherry N, McNamee R, Burgess G, Turner S (1995)
Preliminary analysis of proportional mortality in a cohort of British
pottery workers exposed to crystalline silica. Scandinavian journal
of work, environment and health, 21 (Suppl. 2):63-65.
McDonald JC, Burgess GL, Turner S, Cherry NM (1997) Cohort study of
Staffordshire pottery workers: (III) Lung cancer, radiographic
changes, silica exposure and smoking habit. Annals of occupational
hygiene, 41 (Suppl. 1):412-414.
McLaughlin JK, Jing-Qiong C, Dosemeci M, Rong-An C, Rexing SH, Zhien
W, Hearl FJ, McCawley MA, Blot WJ (1992) A nested case-control study
of lung cancer among silica exposed workers in China. British journal
of industrial medicine, 49:167-171.
McNeill DA, Chrisp CE, Fisher GL (1990) Pulmonary adenomas in A/J mice
treated with silica. Drug and chemical toxicology, 13(1):87-92.
Merlo F, Costantini M, Reggiardo G, Ceppi M, Puntoni R (1991) Lung
cancer risk among refractory brick workers exposed to crystalline
silica: a retrospective cohort study. Epidemiology, 2(4):299-305.
Morrow PE (1988) Issues: Possible mechanisms to explain dust
overloading of the lungs. Fundamental and applied toxicology,
10:369-384.
Morrow PE (1992) Contemporary issues in toxicology -- dust overloading
of the lungs: update and appraisal. Toxicology and applied
pharmacology, 113:1-12.
Mossman BT, Churg A (1998) State of the art: Mechanisms in the
pathogenesis of asbestosis and silicosis. American journal of
respiratory and critical care medicine, 157:1666-1680.
Mozzon D, Brown DA, Smith JW (1987) Occupational exposure to airborne
dust, respirable quartz and metals arising from refuse handling,
burning and landfilling. American Industrial Hygiene Association
journal, 48(2):111-116.
Muhle H, Takenaka S, Mohr U, Dasenbrock C, Mermelstein R (1989) Lung
tumor induction upon long term low level inhalation of crystalline
silica. American journal of industrial medicine, 15(3):343-346.
Muhle H, Bellmann B, Creutzenberg O, Dasenbrock C, Ernst H, Kilpper R,
Mackenzie JC, Morrow P, Mohr U, Takenaka S, Mermelstein R (1991)
Pulmonary response to toner upon chronic inhalation exposure in rats.
Fundamental and applied toxicology, 17:280-299.
Muhle H, Kittel B, Ernst H, Mohr U, Mermelstein R (1995) Neoplastic
lung lesions in rat after chronic exposure to crystalline silica.
Scandinavian journal of work, environment and health, 21
(Suppl. 2):27-29.
Muir DCF (1991) Correction in cumulative risk in silicosis exposure
assessment. American journal of industrial medicine, 19:555.
Muir DCF, Shannon HS, Julian JA, Verma DK, Sebestyen A, Bernholz CD
(1989a) Silica exposure and silicosis among Ontario hardrock miners:
I. Methodology. American journal of industrial medicine, 16:5-11.
Muir DCF, Shannon HS, Julian JA, Verma DK, Sebestyen A, Bernholz CD
(1989b) Silica exposure and silicosis among Ontario hardrock miners:
III. Analysis and risk estimates. American journal of industrial
medicine, 16:29-43.
Muramatsu K, Yamamoto T, Hasegawa A et al. (1989) [A case of
autoimmune hemolytic anemia associated with silicosis.] Japanese
journal of chest diseases, 48(1):45-50 (in Japanese).
Murray J, Reid G, Kielkowski D, de Beer M (1993) Cor pulmonale and
silicosis: a necropsy based case-control study. British journal of
industrial medicine, 50:544-548.
Myers JE, Lewis P, Hofmeyr W (1989) Respiratory health of brickworkers
in Cape Town, South Africa: background, aims and dust exposure
determinations. Scandinavian journal of work, environment and health,
15(3):180-187.
Nagalakshmi R, Nath J, Ong T, Whong W-Z (1995) Silica-induced
micronuclei and chromosomal aberrations in Chinese hamster lung (V79)
and human lung (Hel 299) cells. Mutation research, 335:27-33.
Nehls P, Seiler F, Rehn B, Greferath R, Bruch J (1997) Formation and
persistence of 8-oxoguanine in rat lung cells as an important
determinant for tumor formation following particle exposure.
Environmental health perspectives, 105 (Suppl. 5):1291-1296.
Neyer U, Woss E, Neuweiler J (1994) Case report: Wegener's
granulomatosis associated with silicosis. Nephrology dialysis
transplantation, 9(5):559-561.
Ng TP, Yeung KH, O'Kelly FJ (1987a) Silica hazard of caisson
construction in Hong Kong. Journal of the Society of Occupational
Medicine, 37:62-65.
Ng TP, Tsin TW, O'Kelly FJ, Chan SL (1987b) A survey of the
respiratory health of silica-exposed gemstone workers in Hong Kong.
American review of respiratory disease, 135:1249-1254.
Ng TP, Lee HS, Phoon WH (1993) Further evidence of human silica
nephrotoxicity in occupationally exposed workers. British journal of
industrial medicine, 50:907-912.
Niemeier RW, Mulligan LT, Rowland J (1986) Cocarcinogenicity of
foundry silica sand in hamsters. In: Goldsmith DF, Winn DM, Shy CM,
eds. Silica, silicosis, and cancer: Controversy in occupational
medicine. New York, NY, Praeger Publishers, pp. 215-227 (Cancer
Research Monographs, Vol. 2).
NIOSH (1974) Criteria for a recommended standard: occupational
exposure to crystalline silica. Cincinnati, OH, US Department of
Health, Education, and Welfare, Health Services and Mental Health
Administration, National Institute for Occupational Safety and Health
(DHEW (NIOSH) Publication No. 75-120).
NIOSH (1992a) NIOSH Alert: Request for assistance in preventing
silicosis and deaths from sandblasting. Cincinnati, OH, US
Department of Health and Human Services, Public Health Service,
Centers for Disease Control, National Institute for Occupational
Safety and Health (DHHS (NIOSH) Publication No. 92-102).
NIOSH (1992b) NIOSH Alert: Request for assistance in preventing
silicosis and deaths in rock drillers. Cincinnati, OH, US Department
of Health and Human Services, Public Health Service, Centers for
Disease Control, National Institute for Occupational Safety and Health
(DHHS (NIOSH) Publication No. 92-107).
NIOSH (1994a) Silica, crystalline, respirable by XRD: Method 7500,
Issue 2 (dated 8/15/94). In: Cassinelli ME, O'Connor PF, eds. NIOSH
manual of analytical methods, 4th ed. Cincinnati, OH, US Department
of Health and Human Services, Public Health Service, Centers for
Disease Control and Prevention, National Institute for Occupational
Safety and Health (DHHS (NIOSH) Publication No. 94-113).
NIOSH (1994b) Silica, crystalline, respirable by IR: Method 7602,
Issue 2 (dated 8/15/94). In: Cassinelli ME, O'Connor PF, eds. NIOSH
manual of analytical methods, 4th ed. Cincinnati, OH, US Department
of Health and Human Services, Public Health Service, Centers for
Disease Control and Prevention, National Institute for Occupational
Safety and Health (DHHS (NIOSH) Publication No. 94-113).
NIOSH (1996) NIOSH Alert: Request for assistance in preventing
silicosis and deaths in construction workers. Cincinnati, OH, US
Department of Health and Human Services, Public Health Service,
Centers for Disease Control and Prevention, National Institute for
Occupational Safety and Health (DHHS (NIOSH) Publication No. 96-112).
NIOSH (1997) Pocket guide to chemical hazards. Cincinnati, OH, US
Department of Health and Human Services, Public Health Service,
Centers for Disease Control and Prevention, National Institute for
Occupational Safety and Health (DHHS (NIOSH) Publication No. 97-140).
NIOSH (forthcoming) NIOSH special hazard review: Health effects of
occupational exposure to respirable crystalline silica. Cincinnati,
OH, US Department of Health and Human Services, Public Health Service,
Centers for Disease Control and Prevention, National Institute for
Occupational Safety and Health (DHHS (NIOSH) Publication).
Nuyts GD, Van Vlem E, De Vos A, Daelemans RA, Rorive G, Elseviers MM,
Schurgers M, Segaert M, D'Haese PC, De Broe ME (1995) Wegener
granulomatosis is associated to exposure to silicon compounds: a
case-control study. Nephrology dialysis transplantation,
10:1162-1165.
Oberdörster G (1997) Pulmonary carcinogenicity of inhaled particles
and the maximum tolerated dose. Environmental health perspectives,
195 (Suppl. 5):1347-1355.
Oshimura M, Hesterberg TW, Tsutsui T, Barrett JC (1984) Correlation of
asbestos-induced cytogenetic effects with cell transformation of
Syrian hamster embryo cells in culture. Cancer research,
44:5017-5022.
Osorio AM, Thun MJ, Novak RF, Van Cura J, Avner ED (1987) Silica and
glomerulonephritis: case report and review of the literature.
American journal of kidney diseases, 9(3):224-230.
Otsuki T, Sakaguchi H, Tomokuni A, Aikoh T, Matsuki T, Kawakami Y,
Kusaka M, Ueki H, Kita S, Ueki A (1998) Soluble Fas mRNA is dominantly
expressed in cases with silicosis. Immunology, 94:258-262.
Oudyk JD (1995) Review of an extensive ferrous foundry silica sampling
program. Applied occupational and environmental hygiene, 10:331-340.
Pairon JC, Jaurand MC, Kheuang L, Janson X, Brochard P, Bignon J
(1990) Sister chromatid exhanges in human lymphocytes treated with
silica. British journal of industrial medicine, 47:110-115.
Pan G, Takahashi K, Feng Y, Liu L, Liu T, Zhang S, Liu N, Okubo T,
Goldsmith DF (1999) Nested case-control study of esophageal cancer in
relation to occupational exposure to silica and other dusts. American
journal of industrial medicine, 35:272-280.
Parent ME, Siemiatycki J, Fritschi L (1998) Occupational exposures and
gastric cancer. Epidemiology, 9(1):48-55.
Parker JE (1994) Silicosis. In: Rakel RE, ed. Conn's current therapy.
Philadelphia, PA, W.B. Saunders, pp. 207-210.
Parkes WR, ed. (1982) Occupational lung disorders, 2nd ed. London,
Butterworths, pp. 134-174.
Partanen T, Pukkala E, Vainio H, Kurppa K, Koskinen H (1994) Increased
incidence of lung and skin cancer in Finnish silicotic patients.
Journal of occupational medicine, 36(6):616-622.
Peters JM (1986) Silicosis. In: Merchant JA, Boehlecke BA, Taylor G,
Pickett-Harner M, eds. Occupational respiratory diseases.
Cincinnati, OH, US Department of Health and Human Services, Public
Health Service, Centers for Disease Control, National Institute for
Occupational Safety and Health, pp. 219-237 (DHHS (NIOSH) Publication
No. 86-102).
Petersen PE, Henmar P (1988) Oral conditions among workers in the
Danish granite industry. Scandinavian journal of work, environment
and health, 14:328-331.
Popendorf WJ, Pryor A, Wenk HR (1982) Mineral dust in manual harvest
operations. Annals of the American Conference of Governmental
Industrial Hygienists, 2:101-115.
Pott F, Dungworth DL, Heinrich U, Muhle H, Kamino K, Germann P-G,
Roller M, Rippe RM, Mohr U (1994) Lung tumours in rats after
intratracheal instillation of dusts. Annals of occupational hygiene,
38 (Suppl. 1):357-363.
Pouthier D, Duhoux P, Van Damme B (1991) Pulmonary silicosis and
glomerular nephropathy: Apropos of 1 case. Nephrologie, 12(1):8-11.
Price-Jones MJ, Gubbings G,Chamberlain M (1980) The genetic effects of
crocidolite asbestos; comparison of chromosome abnormalities and
sister-chromatid exchanges. Mutation research, 79:331-336.
Puntoni R, Goldsmith DG, Valerio F, Vercelli M, Bonassi S, Di Giorgio
F, Ceppi M, Stagnaro E, Filiberti R, Santi L, Merlo F (1988) A cohort
study of workers employed in a refractory brick plant. Tumori,
74:27-33.
Rabovsky J (1997) Laboratory studies on silica induced toxicity and
relationship to carcinogenicity. Journal of exposure analysis and
environmental epidemiology, 7(3):267-278.
Rees D, Cronje R, du Toit RSJ (1992) Dust exposure and pneumoconiosis
in a South African pottery. 1. Study objectives and dust exposure.
British journal of industrial medicine, 49:459-464.
Renne RA, Eldridge SR, Lewis TR, Stevens DL (1985) Fibrogenic
potential of intratracheally instilled quartz, ferric oxide, fibrous
glass, and hydrated alumina in hamsters. Toxicologic pathology,
13(4):306-314.
Reuzel PGJ, Bruijntjes JP, Feron VJ, Woutersen RA (1991) Subchronic
inhalation toxicity of amorphous silicas and quartz dust in rats.
Food and chemical toxicology, 29(5):341-354.
Riala R (1988) Dust and quartz exposure of Finnish construction
cleaners. Annals of occupational hygiene, 32(2):215-220.
Rice CH, Harris RL, Checkoway H, Symons MJ (1986) Dose-response
relationships for silicosis from a case-control study of North
Carolina dusty trades workers. In: Goldsmith DF, Winn DM, Shy CM, eds.
Silica, silicosis, and cancer: Controversy in occupational medicine.
New York, NY, Praeger Publishers, pp. 77-86 (Cancer Research
Monographs, Vol. 2).
Rice FL, Stayner LT (1995) Assessment of silicosis risk for
occupational exposure to crystalline silica. Scandinavian journal of
work, environment and health, 21 (Suppl. 2):87-90.
Rispal P, Wen L, De Precigout V, Aparicio M (1991) Nephropathy with
silica in a dental technician. La presse medicale, 20(4):176.
Robbins SL (1974) Pathologic basis of disease. Philadelphia, PA,
W.B. Saunders Co., pp. 239-240.
Rosenman KD, Reilly MJ, Rice C, Hertzberg V, Tseng C-Y, Anderson HA
(1996) Silicosis among foundry workers: implication for the need to
revise the OSHA standard. American journal of epidemiology,
144(9):890-900.
Saffiotti U (1990) Lung cancer induction by silica in rats, but not in
mice and hamsters: species differences in epithelial and granulomatous
reactions. In: Seemayer NH, Hadnagy W, eds. Environmental hygiene II.
New York, NY, Springer-Verlag, pp. 235-238.
Saffiotti U (1992) Lung cancer induction by crystalline silica. In:
D'Amato RD, Slaga TJ, Farland WH, Henry C, eds. Relevance of animal
studies to the evaluation of human cancer risk. New York, NY,
Wiley-Liss, pp. 51-69.
Saffiotti U, Ahmed N (1995) Neoplastic transformation by quartz in the
BALB/3T3/A31-1-1 cell line and the effects of associated minerals.
Teratogenesis, carcinogenesis, and mutagenesis, 15(6):339-356.
Saffiotti U, Daniel LN, Mao Y, Williams AO, Kaighn ME, Ahmed N,
Knapton AD (1993) Biological studies on the carcinogenic mechanisms of
quartz. In: Guthrie GD, Mossman BT, eds. Health effects of mineral
dusts. Washington, DC, Mineralogical Society of America. Reviews in
mineralogy, 28:523-544.
Saffiotti U, Williams O, Lambert N, Daniel N, Kaighn ME, Mao Y, Shi X
(1996) Carcinogenesis by crystalline silica: animal, cellular, and
molecular studies. In: Castranova V, Vallyathan V, Wallace WE, eds.
Silica and silica-induced lung diseases. Boca Raton, FL, CRC Press,
pp. 345-381.
Saita G, Zavaglia O (1951) Kidney function in silicotics.
Medicina del Lavoro, 42(2):41-48.
Salisbury S, Melius J (1982) Harbison-Walker Refractories, Fairfield,
Alabama, Bessemer, Alabama. Cincinnati, OH, National Institute for
Occupational Safety and Health (HHE Report No. HETA-80-086-1191).
Samimi B, Weill H, Ziskind M (1974) Respirable silica dust exposure of
sandblasters and associated workers in steel fabrication yards.
Archives of environmental health, 29:61-66.
Schapira RM, Ghio AJ, Effros RM, Morrisey J, Almagro UA, Dawson CA,
Hacker AD (1995) Hydroxyl radical production and lung injury in the
rat following silica or titanium dioxide instillation in vivo.
American journal of respiratory cell and molecular biology,
12(2):220-226.
Scheuchenzuber WJ, Eskew ML, Zarkower A (1985) Effects of prolonged
inhalation of silica and olivine dusts on immune functions in the
mouse. Environmental research, 38(2):389-399.
Schwartz LW, Knight HD, Whittig LD, Malloy RL, Abraham JL,Tyler NK
(1981) Silicate pneumoconiosis and pulmonary fibrosis in horses from
the Monterey-Carmel peninsula. Chest, 80 (Suppl. 1):82-85.
Seaton A (1995) Silicosis. In: Morgan WKC, Seaton A, eds.
Occupational lung diseases, 3rd ed. Philadelphia, PA, W.B. Saunders
Co., pp. 222-267.
Seta JA, Sundin DA, Pedersen DH, eds. (1988) National occupational
exposure survey field guidelines. Vol. 1. Cincinnati, OH, US
Department of Health and Human Services, Public Health Service,
Centers for Disease Control, National Institute for Occupational
Safety and Health, Division of Surveillance, Hazard Evaluations, and
Field Studies (DHHS (NIOSH) Publication No. 88-106).
Sherson D, Jorgensen F (1989) Rapidly progressive crescenteric
glomerulonephritis in a sandblaster with silicosis. British journal
of industrial medicine, 46:675-676.
Sherson D, Lander F (1990) Morbidity of pulmonary tuberculosis among
silicotic and nonsilicotic foundry workers in Denmark. Journal of
occupational medicine, 32(2):110-113.
Shi X, Mao Y, Daniel LN, Saffiotti U, Dalal NS, Vallyathan V (1994)
Silica radical-induced DNA damage and lipid peroxidation.
Environmental health perspectives, 102 (Suppl. 10):149-154.
Siltanen E, Koponen M, Kokko A, Engström B, Reponen J (1976) Dust
exposure in Finnish foundries. Scandinavian journal of work,
environment and health, 2 (Suppl. 1):19-31.
Silverstein M, Maizlish N, Park R, Silverstein B, Brodsky L, Mirer F
(1986) Mortality among ferrous foundry workers. American journal of
industrial medicine, 10(1):27-43.
Slavin RE, Swedo JL, Brandes D, Gonzalez-Vitale JC, Osornio-Vargas A
(1985) Extrapulmonary silicosis: a clinical, morphologic, and
ultrastructural study. Human pathology, 16(4):393-412.
Sluis-Cremer GK, Hessel PA, Hnizdo E, Churchill AR, Zeiss EA (1985)
Silica, silicosis, and progressive systemic sclerosis. British
journal of industrial medicine, 42:838-843.
Sluis-Cremer GK, Hessel PA, Hnizdo E, Churchill AR (1986) Relationship
between silicosis and rheumatoid arthritis. Thorax, 41:596-601.
Smith AH, Lopipero PA, Barroga VR (1995) Meta-analysis of studies of
lung cancer among silicotics. Epidemiology, 6(6):617-624.
Sobti RC, Bhardwaj DK (1991) Cytogenetic damage and occupational
exposure: I. Exposure to stone dust. Environmental research,
56:25-30.
Soutar CA, Robertson A, Miller BG, Searl A (1997) Epidemiological
evidence on the carcinogenicity of silica: factors in scientific
judgement. Edinburgh, Institute of Occupational Medicine, 34 pp.
(IOM Report TM/97/09).
Spiethoff A, Wesch H, Wegener K, Klimisch HJ (1992) The effects of
Thorotrast and quartz on the induction of lung tumors in rats.
Health physics, 63(1):101-110.
Steenland K, Beaumont J (1986) A proportionate mortality study of
granite cutters. American journal of industrial medicine, 9:189-201.
Steenland K, Brown D (1995a) Silicosis among gold miners:
exposure-response analyses and risk assessment. American journal of
public health, 85(10):1372-1377.
Steenland K, Brown D (1995b) Mortality study of gold miners exposed to
silica and nonasbestiform amphibole minerals: an update with 14 more
years of follow-up. American journal of industrial medicine,
27:217-229.
Steenland K, Goldsmith DF (1995) Silica exposure and autoimmune
diseases. American journal of industrial medicine, 28:603-608.
Steenland K, Stayner L (1997) Silica, asbestos, man-made mineral
fibers, and cancer. Cancer causes and control, 8:491-503.
Steenland K, Nowlin S, Ryan B, Adams S (1992) Use of multiple-cause
mortality data in epidemiologic analyses: US rate and proportion files
developed by the National Institute for Occupational Safety and Health
and the National Cancer Institute. American journal of epidemiology,
136(7):855-862.
Stopford CM, Stopford W (1995) Respirable quartz content of farm
soils. Applied occupational and environmental hygiene,
10(3):196-199.
Sweeney TD, Brain JD (1996) Lavagable biomarkers of exposure to
fibrogenic dusts. In: Castranova V, Vallyathan V, Wallace WE, eds.
Silica and silica-induced lung diseases. Boca Raton, FL, CRC Press,
pp. 197-207.
Tokumaru Y, Hirayama K, Kita K, Kawamura M, Katayama K (1990) Two
cases of ataxic sensory neuropathy associated with silicosis.
Clinical neurology, 30:933-938.
Tsuda T, Babazono A, Yamamoto E, Mino Y, Matsuoka H (1997) A
meta-analysis on the relationship between pneumoconiosis and lung
cancer. Journal of occupational health, 39:285-294.
Tucker DM, Reger RB, Morgan WKC (1995) Effects of silica exposure
among railroad workers. Applied occupational and environmental
hygiene, 10(12):1080-1085.
US Department of the Interior (1992) Special publication: Crystalline
silica primer. Washington, DC, US Department of the Interior, Bureau
of Mines.
US EPA (1994) Methods for derivation of inhalation reference
concentrations and application of inhalation dosimetry, Section 4.3.5
Dosimetric adjustments for particle exposures. Research Triangle
Park, NC, US Environmental Protection Agency, Office of Health and
Environmental Assessment, Office of Research and Development
(Publication No. EPA/600/8-90/066F).
US EPA (1996) Ambient levels and noncancer health effects of inhaled
crystalline and amorphous silica: health issue assessment.
Washington, DC, US Environmental Protection Agency, Office of
Research and Development (Publication No. EPA/600/R-95/115; National
Technical Information Service Publication No. PB97-188122).
Vallyathan V, Castranova V, Pack D, Leonard S, Shumaker J, Hubbs AF,
Shoemaker DA, Ramsey DM, Pretty JR, McLaurin JL, Khan A, Teass A
(1995) Freshly fractured quartz inhalation leads to enhanced lung
injury and inflammation: Potential role of free radicals. American
journal of respiratory and critical care medicine, 152(3):1003-1009.
Vanchugova NN, Frash VN, Kogan FM (1985) The use of a micronucleus
test as a short-term method in detecting potential blastomogenicity of
asbestos-containing and other mineral fibers. Gigiena Truda i
Professional'nye Zabolevaniya, 6:45-48.
Verma DK, Sebestyen A, Julian JA, Muir DCF, Schmidt H, Bernholz CD,
Shannon HS (1989) Silica exposure and silicosis among Ontario hardrock
miners: II. Exposure estimates. American journal of industrial
medicine, 16:13-28.
Virta RL (1993) Crystalline silica: what it is -- and isn't.
Minerals today, October: 12-16.
Wagner GR (1995) The inexcusable persistence of silicosis [editorial].
American journal of public health, 85(10):1346-1347.
Wagner JC (1970) The pathogenesis of tumors following the intrapleural
injection of asbestos and silica. In: Nettesheim P, Hanna MG Jr,
Deatherage JW Jr, eds. Morphology of experimental respiratory
carcinogenesis. Oak Ridge, TN, US Atomic Energy Commission, pp.
347-358.
Wagner JC, Berry G (1969) Mesotheliomas in rats following inoculation
with asbestos. British journal of cancer, 23:567-581.
Wagner MMF (1976) Pathogenesis of malignant histiocytic lymphoma
induced by silica in a colony of specific-pathogen-free Wistar rats.
Journal of the National Cancer Institute, 57:509-518.
Wagner MMF, Wagner JC (1972) Lymphomas in the Wistar rat after
intrapleural inoculation of silica. Journal of the National Cancer
Institute, 49:81-91.
Wagner MMF, Wagner JC, Davies R, Griffiths DM (1980) Silica-induced
malignant histiocytic lymphoma: incidence linked with strain of rat
and type of silica. British journal of cancer, 41:908-917.
Wang H, Leigh J, Bonin A, Peters M (1997a) Silica-induced
morphological change similar to apoptosis in bronchoalveolar lavage
cells and granulomatous cells. Annals of occupational hygiene, 41
(Suppl. 1):459-464.
Wang H, Leigh J, Bonin A, Peters M (1997b) Silica induced micronuclei
in pulmonary alveolar macrophages in vivo. Annals of occupational
hygiene, 41 (Suppl. 1):434-439.
Warheit DB, Hartsky MA (1997) Initiating the risk assessment process
for inhaled particulate materials: development of short term
inhalation bioassays. Journal of exposure analysis and environmental
epidemiology, 7(3): 313-325.
Watts WF, Parker DR (1995) Quartz exposure trends in metal and
nonmetal mining. Applied occupational and environmental hygiene,
10(12):1009-1018.
Weill H, McDonald JC (1996) Exposure to crystalline silica and risk of
lung cancer: the epidemiological evidence. Thorax, 51:97-102.
Weill H, Jones RN, Parkes WR (1994) Silicosis and related diseases.
In: Parkes WR, ed. Occupational lung disorders, 3rd ed. London,
Butterworth-Heinemann, pp. 285-339.
Weissman DN, Ma JKH, Rojanasakul Y, Hubbs AF (1996) Immune dysfunction
in silicosis: a hypothesis. Applied occupational and environmental
hygiene, 11(7):962-965.
Westerholm P, Ahlmark A, Maasing R, Segelberg I (1986) Silicosis and
risk of lung cancer or lung tuberculosis: a cohort study.
Environmental research, 41:339-350.
WHO (1986) Recommended health-based limits in occupational exposure
to selected mineral dusts (silica, coal): report of a WHO study
group. Geneva, World Health Organization, 80 pp. (WHO Technical
Report Series 734).
WHO (1993) "WHO calls for medical surveillance of workers exposed to
mineral dusts." Geneva, World Health Organization (Press Release
WHO/73, 23 September 1993).
WHO (1996) Groups at risk: WHO report on the tuberculosis epidemic
1996. Geneva, World Health Organization.
Wiessner JH, Henderson JD Jr, Sohnle PG, Mandel NS, Mandel GS (1988)
The effect of crystal structure on mouse lung inflammation and
fibrosis. American review of respiratory disease, 138:445-450.
Wiles FJ, Faure MH (1977) Chronic obstructive lung disease in gold
miners. In: Walton WH, ed. Inhaled particles IV. Part 2. Oxford,
Pergamon Press, pp. 727-735.
Wilke RA, Salisbury S, Abdel-Rahman E, Brazy PC (1996) Lupus-like
autoimmune disease associated with silicosis. Nephrology dialysis and
transplantation, 11:1835-1838.
Williams AO, Knapton AD, Ifon ET, Saffiotti U (1996) Transforming
growth factor beta expression and transformation of rat lung
epithelial cells by crystalline silica (quartz). International
journal of cancer, 65:639-649.
Wilson T, Scheuchenzuber WJ, Eskew ML, Zarkower A (1986) Comparative
pathological aspects of chronic olivine and silica inhalation in mice.
Environmental research, 39(2):331-344.
Xu XZ, Cia XG, Men XS, Yang PY, Yang JF, Jing SL, He JH, Si WY (1993)
A study of siliceous pneumoconiosis in a desert area of Sunan county,
Gansu province, China. Biomedical and environmental sciences,
6:217-222.
Xu Z, Pan G-W, Liu L-M, Brown LM, Gaun D-X, Xiu Q, Sheng J-H, Stone
BJ, Dosemeci M, Fraumeni JF Jr, Blot WJ (1996) Cancer risks among iron
and steel workers in Anshan, China, Part I: proportional mortality
ratio analysis. American journal of industrial medicine, 30:1-6.
Yamano Y, Kagawa J, Hanaoka T, Takahashi T, Kasai H, Tsugane S,
Watanabe S (1995) Oxidative DNA damage induced by silica in vivo.
Environmental research, 69(2):102-107.
Zheng W, Shu XO, Ji BT, Gao YT (1996) Diet and other risk factors for
cancer of the salivary glands: a population-based case-control study.
International journal of cancer, 67:194-198.
Zhong B, Whong W, Ong T (1997) Detection of mineral-dust-induced DNA
damage in two mammalian cell lines using the alkaline single cell
gel/comet assay. Mutation research, 393:181-187.
Ziegler V, Haustein UF (1992) Systemic scleroderma -- a silica induced
occupational disease? Epidemiological, clinical, immunological,
mineralogical, animal and cell culture investigations.
Dermatologische Monatschrift, 178:34-43.
Ziskind M, Jones RN, Weill H (1976) Silicosis. American review of
respiratory disease, 113:643-665.
APPENDIX 1 -- SOURCE DOCUMENTS
IARC (1997)
Copies of Silica, some silicates, coal dust and
para -aramid fibrils (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Vol. 68) may be obtained from:
International Agency for Research on Cancer
150 cours Albert Thomas
69372 Lyon Cedex 08
France
The members of the Working Group on the Evaluation of
Carcinogenic Risks to Humans of silica (including quartz), some
silicates, coal dust and para-aramid fibrils that met in Lyon on
15-22 February 1996 were:
M.D. Attfield, National Institute for Occupational Safety and
Health, USA
P.J. Borm, University of Limburg, The Netherlands
H. Checkoway, University of Washington, USA
K. Donaldson, Napier University, United Kingdom
M. Dosemeci, National Cancer Institute, USA
V.J. Feron, TNO Nutrition and Food Research Institute, The
Netherlands
B.J. Fubini, University of Torino, Italy
M. Gérin, Université de Montréal, Canada
E. Hnizdo, National Centre for Occupational Health, South Africa
A.B. Kane, Brown University, USA
J.C. McDonald, Imperial College, United Kingdom
H. Muhle, Fraunhofer Institute for Toxicology and Aerosol
Research, Germany
S. Olin, International Life Sciences Institute, USA
J.-C. Pairon, INSERM, France
T. Partanen, Institute of Occupational Health, Finland
C. Shy, University of North Carolina, USA
E. Tatrai, National Institute of Occupational Health, Hungary
D.B. Warheit, DuPont Haskell Laboratory for Toxicology and
Industrial Medicine, USA
V. Yermilov, N.N. Petrov Research Institute of Oncology, Russia
US EPA (1996)
Copies of the US Environmental Protection Agency report entitled
Ambient levels and noncancer health effects of inhaled crystalline
and amorphous silica: health issue assessment (NTIS Publication
PB97-188122) may be obtained from:
National Technical Information Service
Springfield, VA 22161
USA
The document was reviewed in accordance with US EPA policy and
approved for publication. The principal authors of the document were:
Dr R.A. Faust, Oak Ridge National Laboratory, USA
Dr J.S. Gift, National Center for Environmental Assessment, USA
Dr D.F. Goldsmith, Western Consortium for Public Health, USA
Dr R. Ruble, Western Consortium for Public Health, USA
The reviewers and contributors from the US EPA were:
Dr D.L. Costa, National Health and Environmental Effects Research
Laboratory (MD-82), USA
Dr J.S. Dollarhide, National Center for Environmental Assessment
Dr J.A. Graham, National Center for Environmental Assessment
Dr A. Koppikar, National Center for Environmental Assessment
Dr W.E. Pepelko, National Center for Environmental Assessment
Dr C. Shoaf, National Center for Environmental Assessment
Mr E.G. Smith, Office of Air Quality Planning and Standards
Dr W. Victery, Region IX
The external peer reviewers of the document were:
Dr D. Gardner, Private Consultant, Raleigh, NC, USA
Ms S. Goodwin, University of Maryland, Baltimore, MD, USA
Dr M. Jacobsen, National Institute for Occupational Safety and
Health, USA
Dr R. Kutzman, MITRE Corporation, McLean, VA, USA
Dr J. Rabovsky, California Environmental Protection Agency, USA
Ms F. Rice, National Institute for Occupational Safety and
Health, USA
Dr J.M. Samet, The Johns Hopkins University, USA
Dr C. Shy, University of North Carolina, USA
Dr J. Vincent, University of Minnesota, USA
Dr J. Watson, Desert Research Institute, USA
Dr H. Weill, Tulane University, USA
NIOSH (forthcoming)
When published, copies of the NIOSH special hazard review:
Health effects of occupational exposure to respirable crystalline
silica will be available from:
National Institute for Occupational Safety and Health
Publications Dissemination
4676 Columbia Parkway
Cincinnati, OH 45226-1998
USA
The draft document has undergone the following NIOSH internal
reviews in accordance with NIOSH policy: intradivisional review and
interdivisional review. The external peer reviewers of the document
were:
W. Beckett, University of Rochester School of Medicine
H. Checkoway, University of Washington
G.S. Davis, University of Vermont College of Medicine
J. Gift, US EPA
D. Goldsmith, George Washington University
E. Hnizdo, National Centre for Occupational Health, South Africa
J. Hughes, Tulane School of Public Health and Tropical Medicine
C. Jones, Mine Safety and Health Administration, US Department of
Labor
W. Kojola, American Federation of Labor and Congress of
Industrial Organizations
A.G. Macneski, Environmental Safety and Health, Bechtel National,
Inc.
M. Schaper, Mine Safety and Health Administration, US Department
of Labor
L. Schuman, Occupational Safety and Health Administration, US
Department of Labor
J. Sharpe, National Stone Association
D.M. Tucker, Norfolk Southern Corporation
J.A. Ulizio, US Silica Company
J.L. Weeks, George Washington University Medical Center
APPENDIX 2 -- CICAD PEER REVIEW
The draft CICAD on crystalline silica, quartz was sent for review
to institutions and organizations identified by IPCS after contact
with IPCS national Contact Points and Participating Institutions, as
well as to identified experts. Comments were received from:
M. Baril, Institut de Recherche en Santé et Sécurité du Travail
du Québec, Montreal, Quebec, Canada
R. Benson, Drinking Water Program, US Environmental Protection
Agency, Denver, CO, USA
T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
P. Borm, Medical Institute for Environmental Hygiene, Department
of Fibre and Particle Toxicology, Dusseldorf University,
Düsseldorf, Germany
R. Brown, Toxservices, Uppingham, United Kingdom
R.S. Chhabra, National Institute of Environmental Health
Sciences/National Institute of Health, Research Triangle Park,
NC, USA
S. Dobson, Institute of Terrestrial Ecology, Cambridgeshire,
United Kingdom
K. Donaldson, Department of Biological Sciences, Napier
University, Edinburgh, Scotland
J. Heuer, Federal Institute for Health Protection of Consumers
and Veterinary Medicine, Berlin, Germany
J. Hurych, National Institute of Public Health, Prague, Czech
Republic
J. Leigh, National Occupational Health and Safety Commission,
Sydney, Australia
M. Moore, The Morgan Crucible Company, Windsor, United Kingdom
F. Morrall, British Ceramic Confederation, Stoke-on-Trent, United
Kingdom
D. Muir, Occupational Health Program, McMaster University,
Hamilton, Ontario, Canada
M. Wyart-Remy, Industrial Minerals Association - Europe,
Brussels, Belgium
P. Yao, Institute of Occupational Medicine, Chinese Academy of
Preventive Medicine, Beijing, People's Republic of China
J. Yoshida, Chemical Safety Management, Office of Environmental
Chemical Safety, Environmental Health Bureau, Ministry of Health
and Welfare, Tokyo, Japan
K. Ziegler-Skylakakis, Beratergremium für Umweltrelevante
Altstoffe, Muenchen, Germany
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
Sydney, Australia, 21-24 November 1999
Members
Dr R. Benson, Drinking Water Program, US Environmental Protection
Agency, Region VIII, Denver, CO, USA
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
Dr R.M. Bruce, National Center for Environmental Assessment, US
Environmental Protection Agency, Cincinnati, OH, USA
Mr R. Cary, Health and Safety Executive, Merseyside, United Kingdom
Dr R.S. Chhabra, National Institute of Environmental Health Sciences,
National Institutes of Health, Research Triangle Park, NC, USA
Dr S. Chou, Agency for Toxic Substances and Disease Registry, Atlanta,
GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood,
Cambridgeshire, United Kingdom
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers
and Veterinary Medicine, Berlin, Germany
Dr J. Kielhorn, Fraunhofer Institute for Toxicology and Aerosol
Research, Hannover, Germany
Dr S. Kristensen, National Occupational Health and Safety Commission
(Worksafe), Sydney, NSW, Australia
Mr C. Lee-Steere, Environment Australia, Canberra, ACT, Australia
Ms M. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada
Ms F. Rice, National Institute for Occupational Safety and Health,
Cincinnati, OH, USA
Dr J. Sekizawa, National Institute of Health Sciences, Tokyo, Japan
Dr D. Willcocks, National Industrial Chemicals Notification and
Assessment Scheme (NICNAS), Sydney, NSW, Australia (Chairperson)
Professor P. Yao, Institute of Occupational Medicine, Chinese Academy
of Preventive Medicine, Beijing, People's Republic of China
Observers
Mr P. Howe, Institute of Terrestrial Ecology, Huntingdon,
Cambridgeshire, United Kingdom
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit, GmbH, Oberschleissheim, Germany
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Ms M. Godden, Health and Safety Executive, Bootle, Merseyside, United
Kingdom
Dr M. Younes, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
APPENDIX 4 -- INTERNATIONAL CHEMICAL SAFETY CARD
QUARTZ ICSC: 0808
October 1997
CAS#
Table 5: Genetic and related effects of silica.a
Test system Resultb Dose Reference
(LED/HID)c
DNA strand breaks, gamma-HindIII-digested DNA + 30 000d Daniel et al., 1993
DNA strand breaks, herring sperm genomic DNA + 10 000d Daniel et al., 1993
DNA strand breaks, gamma-HindIII-digested DNA + 9 500d Daniel et al., 1995
DNA strand breaks, PM2 supercoiled DNA + 9 500d Daniel et al., 1995
GIA, Gene mutation, hprt locus, rat RLE-6TN
alveolar epithelial cells in vitro - NG Driscoll et al., 1997
SIC, Sister chromatid exchange,
Chinese hamster V79-4 cells in vitro - 15e Price-Jones et al., 1980
SHL, Sister chromatid exchange, human lymphocytes in vitro - 100d Pairon et al., 1990
SIH, Sister chromatid exchange, human lymphocytes
and monocytes in vitro - 100d Pairon et al., 1990
MIA, Micronucleus test, Syrian hamster embryo cells in vitro - 18.75f Oshimura et al., 1984
MIA, Micronucleus test, Syrian hamster embryo cells in vitro + 70e Hesterberg et al., 1986
MIA, Micronucleus test, Chinese hamster lung fibroblasts
(V79) in vitro + 200g Nagalakshmi et al., 1995
CIC, Chromosomal aberrations, Chinese hamster lung
fibroblasts (V79) in vitro - 1 600g Nagalakshmi et al., 1995
CIS, Chromosomal aberrations, Syrian hamster embryo
cells in vitro - 18.75f Oshimura et al., 1984
Table 5 (cont'd)
Test system Resultb Dose Reference
(LED/HID)c
AIA, Aneuploidy, Chinese hamster lung cells (V79-4) in vitro - 15e Price-Jones et al., 1980
AIA, Aneuploidy, Syrian hamster embryo cells in vitro - 18.75f Oshimura et al., 1984
AIA, Tetraploidy, Syrian hamster embryo cells in vitro - 70e Hesterberg et al., 1986
TBM, Cell transformation, BALB/3T3/31-1-1 mouse cells in vitro + 30d,h,i Saffiotti & Ahmed, 1995
TBM, Cell transformation, BALB/3T3/31-1-1 mouse cells in vitro + 60j,k Saffiotti & Ahmed, 1995
TCS, Cell transformation, Syrian hamster embryo cells in vitro + 18e Hesterberg & Barrett, 1984
TCS, Cell transformation, Syrian hamster embryo cells in vitro + 70f Hesterberg & Barrett, 1984
TCL, Cell transformation, fetal rat lung epithelial cells
in vitro (+) NGd Williams et al., 1996
MIH, Micronucleus test, human embryonic lung (Hel 299)
cells in vitro + 800g Nagalakshmi et al., 1995
CIH, Chromosomal aberrations, human embryonic lung
(Hel 299) cells in vitro - 1 600g Nagalakshmi et al., 1995
DVA, 8-hydroxy-2'-deoxyguanosine DNA extract from
lung tissue, male Wistar rats + 50 × 1 itd Yamano et al., 1995
DVA, 8-hydroxy-2'-deoxyguanosine DNA extract from
peripheral blood leukocytes, male Wistar rats - 50 × 1 itd Yamano et al., 1995
GVA, Gene mutation, hprt locus, rat alveolar
epithelial cells in vivo + 100 × 1 it Driscoll et al., 1995
Table 5 (cont'd)
Test system Resultb Dose Reference
(LED/HID)c
GVA, Gene mutation, hprt locus, rat alveolar
epithelial cells in vivo + 5 × 2 it Driscoll et al., 1997
MVM, Micronucleus test, albino mice in vivo - 500 ip Vanchugova et al., 1985
SLH, Sister chromatid exchange, human lymphocytes in vivo + NG Sobti & Bhardwaj, 1991
CLH, Chromosomal aberrations, human lymphocytes in vivo + NG Sobti & Bhardwaj, 1991
BID, Calf thymus DNA binding in vitro + 200l Mao et al., 1994
ICR, Metabolic cooperation using 8-azaguanine-resistant
cells, Chinese hamster lung cells (V79-4) in vitro - 50 Chamberlain, 1983
a Source: IARC (1997). See Appendix 1 of IARC (1997), Test system code words, for definitions of
the abbreviations used in column 1.
b Results without exogenous metabolic system: +, positive; (+), weakly positive; -, negative.
c LED, lowest effective dose; HID, highest ineffective dose; in vitro tests, µg/ml; in vivo tests,
mg/kg body weight per day; NG, not given; it, intratracheal; ip, intraperitoneal.
d Min-U-Sil 5.
e Min-U-Sil unspecified.
f alpha-Quartz.
g Min-U-Sil 5 and Min-U-Sil 10.
h Min-U-Sil 5, hydrofluoric acid-etched.
i A Chinese standard quartz sample.
j DQ 12, a standard German quartz sample.
k F600 quartz.
l Min-U-Sil 5 or Chinese standard quartz.
Some studies have demonstrated the ability of quartz to induce
micronuclei in mammalian cells in culture (i.e., Oshimura et al.,
1984; Hesterberg et al., 1986; Nagalakshmi et al., 1995) and were
reviewed by IARC (Table 5). However, other in vitro studies did not
observe chromosomal aberration (Oshimura et al., 1984;
Nagalakshmi et al., 1995), hprt (hypoxanthine-guanine phosphoribosyl
transferase) gene mutation (Driscoll et al., 1997), or aneuploid or
tetraploid cells (Price-Jones et al., 1980; Oshimura et al., 1984;
Hesterberg et al., 1986).
Pairon et al. (1990) tested quartz (i.e., Min-U-Sil 5) particles
for their ability to induce a significant number of sister chromatid
exchanges in cultures of human lymphocytes plus monocytes or of human
purified lymphocytes. The results were not "clear cut" for any of the
three doses tested (i.e., 0.5, 5.0, and 50 µg/cm2) (Pairon et al.,
1990) (Table 5).
An in vivo treatment of rats with quartz induced mutation in
rat alveolar epithelial cells (Table 5) (Driscoll et al., 1995, 1997).
Nehls et al. (1997) reported results of tests for DNA modifications by
quartz that were not reviewed by IARC (1997). Quartz (2.5 mg of DQ 12
suspended in 0.5 ml of physiological saline) or corundum (2.5 mg), a
non-carcinogenic particle, was intratracheally instilled into the
lungs of Wistar rats (10 rats per exposure and per time period).
Control animals were exposed to saline solution or not treated. Rats
were sacrificed 7, 21, and 90 days after treatment, then lung tissue
sections were analysed with immunocytological assay to determine the
level of 8-hydroxydeoxyguanosine in DNA extracts. Reactive oxygen
species can induce 8-hydroxydeoxyguanosine and other mutagenic DNA
oxidation products, which may be converted to mutations in
proliferating cells (Nehls et al., 1997). Exposure to quartz induced
levels of 8-hydroxydeoxyguanosine in the DNA of alveolar lung cells
that were significantly higher (P-value not reported) at all time
points than levels found in cells of untreated rats or rats treated
with corundum or saline. The number of total cells in bronchoalveolar
lavage fluid was 3-4 times higher in the quartz-treated groups at all
time points than in corundum-exposed rats and control rats exposed to
saline.
Other in vivo studies not reviewed by IARC (1997) found that
quartz induced micronuclei in pulmonary alveolar macrophages of male
Wistar rats in a time-dependent (Leigh et al., 1998b) and
dose-dependent manner (Wang et al., 1997b).
In summary, results of genotoxicity studies of quartz conflict,
and a direct genotoxic effect for quartz has not been confirmed or
ruled out.
8.5 Reproductive and developmental toxicity
There are no data available on the reproductive or developmental
effects of quartz in laboratory animals (IARC, 1997).
8.6 Immunological and neurological effects
Data on the neurological effects of quartz have not been
identified. In vitro studies have shown that quartz can stimulate
release of cytokines and growth factors from macrophages and
epithelial cells, and there is evidence that these events may occur
in vivo and contribute to disease (IARC, 1997). The immunological
response to quartz in experimental animals is a complex subject with
uncertain implications for humans, and detailed reviews are available
elsewhere (i.e., Davis, 1991, 1996; Haslam, 1994; Heppleston, 1994;
Weill et al., 1994; Driscoll, 1996; Gu & Ong, 1996; Hook & Viviano,
1996; Iyer & Holian, 1996; Kane, 1996; Sweeney & Brain, 1996;
Weissman et al., 1996; Mossman & Churg, 1998).
9. EFFECTS ON HUMANS
9.1 Case reports
There are many published case reports of adverse health effects
from occupational exposure to quartz. These health effects include
silicosis (acute and chronic) and lung cancer. Case reports of
silicosis and lung cancer are not mentioned further, because these
diseases have been researched in depth in epidemiological studies
(section 9.2).
There are numerous published case reports of several autoimmune
disorders in workers or patients who had been occupationally exposed
to crystalline silica, including quartz dust (NIOSH, forthcoming). The
most frequently noted autoimmune diseases in those reports were
scleroderma, systemic lupus erythematosus (i.e., lupus), rheumatoid
arthritis, autoimmune haemolytic anaemia (Muramatsu et al., 1989), and
dermatomyositis or dermatopolymyositis (Robbins, 1974; Koeger et al.,
1991). Case reports have also described health effects that may be
related to the immunological abnormalities observed in patients with
silicosis, such as chronic renal disease (Saita & Zavaglia, 1951;
Giles et al., 1978; Hauglustaine et al., 1980; Bolton et al., 1981;
Banks et al., 1983; Slavin et al., 1985; Bonnin et al., 1987;
Osorio et al., 1987; Arnalich et al., 1989; Sherson & Jorgensen, 1989;
Dracon et al., 1990; Pouthier et al., 1991; Rispal et al., 1991;
Neyer et al., 1994; Wilke et al., 1996), ataxic sensory neuropathy
(Tokumaru et al., 1990), chronic thyroiditis (Masuda, 1981),
hyperthyroidism (i.e., Graves' disease) (Koeger et al., 1996),
monoclonal gammopathy (Fukata et al., 1983, 1987; Aoki et al., 1988),
and polyarteritis nodosa (Arnalich et al., 1989).
9.2 Epidemiological studies
9.2.1 Silicosis
Most, if not all, of the several hundred epidemiological studies
of exposure to quartz dust are studies of occupational cohorts. The
majority of studies investigated the occurrence of silicosis morbidity
or mortality. These studies have conclusively linked occupational
quartz dust exposure with silicosis. Silicosis (i.e., nodular
pulmonary fibrosis) is a fibrotic lung disease, sometimes
asymptomatic, that is caused by the inhalation and deposition of
respirable crystalline silica particles (i.e., particles <10 µm in
diameter) (Ziskind et al., 1976; IARC, 1987).
A worker may develop one of three types of silicosis, depending
on the airborne concentration of respirable crystalline silica: (1)
chronic silicosis, which usually occurs after 10 or more years of
exposure at relatively low concentrations; (2) accelerated silicosis,
which develops 5-10 years after the first exposure; or (3) acute
silicosis, which develops after exposure to high concentrations of
respirable crystalline silica and results in symptoms within a few
weeks to 4 or 5 years after the initial exposure (Ziskind et al.,
1976; Peters, 1986; NIOSH, 1992a,b, 1996). Acute silicosis is a risk
for workers with a history of high exposures from performing
occupational processes that produce small particles of airborne dust
with a high silica content, such as during sandblasting, rock
drilling, or quartz milling, or any other process with high exposures
to small particles of airborne dust with a high quartz content
(Davis, 1996).
A recent study of 67 paraffin-embedded lung tissue samples from
silicotic patients found a significant linear relationship
(P <0.001) between lung quartz concentration and silicosis severity in
gold miners; although several types of mineral particles were found in
the lungs, quartz was the only significant indicator of silicosis
severity. The silicosis cases included 39 patients without lung cancer
and 28 patients with lung cancer. All of the cases were gold miners in
Canada (Dufresne et al., 1998a,b).
The epidemiological studies of silicosis usually define the
profusion of small opacities present in the disease according to a
standard system used by trained readers and developed by the
International Labour Organization for classification of chest
radiographs of pneumoconioses (ILO, 1980). Each reader assesses the
profusion according to a 12-point scale of severity. Categories 0/-
and 0/0 are the first and second points on the scale and represent a
normal chest radiograph. The third point, category 0/1, represents the
borderline between normality and abnormality, and category 1/0, the
fourth point, represents definite, but slight, abnormality
(Love et al., 1994). The shape (rounded or irregular) and size of the opacities
can also be described by the readers.
The critical studies of chronic silicosis, a progressive disease,
are those occupational epidemiological studies where (1) quantitative
quartz exposure data were available and used for risk analysis,
(2) exposure-response relationships were investigated, or (3) the
exposure-response relationships were documented with sufficient detail
for a health effects benchmark, including (4) application of data to
mathematical models that predicted silicosis prevalence at increasing
concentrations of cumulative quartz exposure. (The predicted
prevalences reported in the studies are discussed in section 11.1.3.)
Studies that selected workers from a broad spectrum of occupations and
included many workers that were exposed to different combinations of
various minerals, such as studies of "dusty trades" workers
(i.e., Rice et al., 1986), were excluded from consideration for risk
assessment of quartz and silicosis. Epidemiological studies that
provided evidence of an exposure-response relationship for silica and
silicosis based on other kinds of exposure data (e.g., there is a
positive relationship between development of chronic silicosis and
duration of exposure) have been reviewed elsewhere (WHO, 1986;
Goldsmith, 1994; Hughes, 1995; Rice & Stayner, 1995; Seaton, 1995;
Steenland & Brown, 1995a; Davis, 1996; US EPA, 1996).
The two critical cross-sectional studies (i.e., Kreiss & Zhen,
1996; Rosenman et al., 1996 -- see Table 6) found that the prevalence
of radiographic silicosis (ILO category >1/0 or >1/1) was
dose-related. That is, the prevalence of radiographic silicosis
increased with average silica dust exposure, cumulative quartz
exposure, duration of employment, or all of these measures. The actual
prevalences varied greatly among the studies, and conclusions
concerning quartz dust concentrations that may or may not induce
silicosis cannot be drawn from simple "eyeball" analysis of the
prevalences in the following two worker populations:
* Kreiss & Zhen (1996) conducted a community-based random sample
survey of 134 male residents at least 40 years old, living in a
hardrock (i.e., molybdenum, lead, zinc, and gold) mining town in
Colorado, USA. Of the 134 residents, 100 were silica-exposed
hardrock miners (including 32 silicosis cases) and 34 were
community "controls" without occupational dust exposure. Nearly
all (97%) of the dust-exposed subjects were 20 years since first
exposure. The estimated crystalline silica content (polymorph not
reported) of the total dust was 12.3%. Exposure was assessed with
information from occupational histories, gravimetric dust
exposure data from 1974-1982, and a cumulative silica exposure
index. Pre-1974 exposure estimates were based on job-specific
gravimetric data collected after 1974. Exposures were also
estimated for mines where no exposure data were available (17.1%
of person-years of follow-up). Thirty-two per cent of the 100
dust-exposed subjects had silicosis (defined as radiological
profusion of small opacities of ILO category >1/0). Prevalence
of silicosis was related to average silica dust exposure. Among
the 94 dust-exposed subjects with data on cumulative and average
dust exposures, those subjects with average silica exposure
<0.05 mg/m3 had 10% prevalence of silicosis; subjects with
>0.05-0.10 mg/m3 had a prevalence of 22.5%; subjects with
greater than 0.10 mg/m3 average silica exposure had a silicosis
prevalence of 48.6% (P = 0.01). (See Table 6 for predicted
prevalences when silicosis was defined as radiological profusion
of small opacities of ILO category >1/1.) It is not known
whether the small sample of 134 residents was representative of
all miners or if the exposure estimates for mines where no
exposure data were available (17.1% of person-years of follow-up)
were representative.
* Rosenman et al. (1996) conducted a cross-sectional study in 1991
of 549 current, 497 retired, and 26 current salaried workers that
were former production workers in a US grey iron foundry that
produced automotive engine blocks (total workers = 1072).
Twenty-eight cases (2.9%) of silicosis, defined as rounded
opacities >ILO category 1/0, were identified by at least two
of three "B" readers of a total of 952 chest radiographs. More
than half (18/28) of the cases were found in retired workers.
Silicosis prevalence was positively related to mean silica (i.e.,
quartz) exposure (P < 0.0001). Of the workers with mean quartz
exposure less than 0.05 mg/m3, 0.8% had silicosis, while 6.3%
of foundry workers with mean quartz exposure greater than 0.45
mg/m3 had silicosis. Silicosis prevalence also increased with
years of employment at the foundry, cumulative silica exposure,
work area within the foundry, and cigarette smoking (i.e., smoker
vs. non-smoker). Exposure estimates were derived from conversions
of "early silica exposure data" collected by impingers. Quartz
content of total dust was not reported. Weighted total dust
exposure from impinger data was converted to an estimate of
silica exposure in mass units (mg/m3) by multiplying it by the
average percentage of quartz in bulk samples.
Results of cohort studies of gold miners in South Africa, Canada,
and the USA (see Table 7) also demonstrated an exposure-response
relationship for radiographic silicosis (US EPA, 1996):
* A cohort study was conducted of 2235 white South African
underground gold miners, 45-54 years old at the time of medical
examination in 1968-1971, who started working after 1938, worked
>10 years, and were followed until 1991 (Hnizdo &
Sluis-Cremer, 1993). More than 300 (n = 313) of the 2235 miners
were followed to the time when radiological signs developed, 658
miners were followed up to death, and 1264 miners were followed
to the year of the most recent radiograph. Radiographs were read
blindly by two independent readers. Silicosis was defined as the
presence of rounded opacities of ILO category >1/1.
Radiographs were read blindly by two readers initially, then one
reader was chosen because his readings more closely matched the
autopsy data. Mean respirable dust concentrations, after heat and
acid treatment, in milligrams per cubic metre per shift were
calculated for nine gold mining occupations. The concentrations
were based on a study of shift-long dust exposure that measured
the surface area of the respirable mine dust and the number of
respirable particles (i.e., incombustible and acid-insoluble dust
particles) per cubic metre in a random sample of 20 South African
gold mines (Beadle, 1965, 1971). After heat and acid treatment,
the respirable dust in South African gold mines was found to
contain about 30% quartz (Beadle & Bradley, 1970). Cumulative
dust exposure for the miners was calculated in milligrams per
cubic metre-year by using data for mean mass respirable dust
concentrations for the nine occupational categories, the average
number of hours underground, and the number of dusty 8-h shifts.
Of the 2235 miners studied by Hnizdo & Sluis-Cremer (1993),
313 developed radiologically diagnosed silicosis (rounded
opacities with profusion of ILO category >1/1) during the
follow-up period (i.e., 1968-1971 to 1991). The onset of
silicosis occurred after an average (i.e., mean) of 27 years of
Table 6: Predicted prevalence of silicosis (ILO category >1/1)
following exposure to respirable quartz dust based on modelling of cumulative exposure
at mean concentrations of 0.05 or 0.10 mg/m3 over a 45-year working lifetime.
Cross-sectional study Mean concentration of Predicted prevalence of Cohort's mean time Cohort's maximum time
and cohort respirable quartz dust silicosis, ILO category since first quartz since first quartz
(mg/m3) >1/1 (cases per exposure (years) exposure (years)
100 workers)
Kreiss & Zhen, 1996 0.05 approx. 30a silicotic miners: silicotic miners:
41.6 66
100 US hardrock 0.10 approx. 90a non-silicotic non-silicotic
miners and 34 miners: 33.5 miners: 68
community controls
Rosenman et al., 0.05 2b,c 28 >30
1996
1072 US grey 0.10 3b,c
iron foundry workers
a Based on cumulative silica exposure model with 10 years of post-employment follow-up.
b ILO category >1/0.
c Based on a 40-year working lifetime and controlling for pack-years of cigarette smoking, race,
and silica exposure other than in the foundry under study.
Table 7: Predicted number of silicosis cases (ILO category >1/1) following exposure to respirable
quartz dust based on modelling of cumulative exposure at mean concentrations of 0.05 or 0.10 mg/m3
over a 45-year working lifetime.
Cohort study Mean concentration of Silicosis cases, Mean time since first Maximum time since
and population respirable quartz ILO category >1/1, quartz exposure (years) first quartz exposure
dust (mg/m3) per 100 workers (years)
Hnizdo & 0.05 13a silicotic miners: 36 silicotic miners: 50
Sluis-Cremer, 1993
2235 South African 0.10 approx. 70
gold miners
Muir et al., 0.05 0.09-0.62a,b 18 silicotic miners: 38
1989a,b;
Muir, 1991
2109 Canadian
gold and
uranium miners
Steenland & 0.05 10c 37 73d
Brown, 1995a
3330 US gold 0.09 47c
miners
a Estimate was reported in Rice & Stayner (1995).
b No post-employment follow-up and no retired miners included. The range includes five estimates
(one for each reader).
c The predicted number of silicosis cases does not account for effects of age or calendar time
(K. Steenland, personal communication, 1997).
d K. Steenland, personal communication, 1998.
net service, at a mean age of 56 years. For more than half of the
miners (n = 178; 57%), the onset occurred an average of
7.4 years (standard deviation 5.5; range 0.1-25 years) after
their employment at the mines, at 59 years of age (range
44-74 years). For the other miners (n = 135; 43%), the onset
of silicosis occurred while they were still mining, at 51 years
of age (range 39-61 years). These results show that the majority
of the cases occurred in miners who were no longer employed at
the mine and who were at least 50 years old
(Hnizdo & Sluis-Cremer, 1993).
* Muir and colleagues conducted a study of 2109 current Ontario
miners from 21 gold and uranium mines who started working and
worked more than 5 years between 1940 and 1959 and were followed
to 1982 or to the end of their dust exposure, whichever came
first (Muir et al., 1989a,b; Muir, 1991). Any uranium miner with
more than 2 weeks of exposure was also included (Muir et al.,
1989a). The quartz content of respirable gold mine dust was 6.0%,
and that of uranium mine dust was 8.4%. Retired and former miners
were not included in the study. Sources of data for this study
were full-sized annual chest radiographs taken for all miners
after 1927 and periodic (pre-1959) and semi-annual mine dust
measurements obtained with a konimeter (which is an instantaneous
dust sampler that measures the number of particles per unit
volume of air; Verma et al., 1989). Konimeter dust measurements
taken from 1940 to 1952 were expressed in particles per cubic
centimetre of air (ppcc). Verma et al. (1989) initiated an
extensive, side-by-side comparison of the konimetric and
gravimetric (i.e., milligrams of silica per cubic metre) sampling
to derive a konimetric/gravimetric silica conversion curve. A
total of 2360 filter (i.e., nylon cyclone-filter assembly in a
constant-flow pump) samples and 90 000 konimeter samples were
taken in a 2-year period in two gold and uranium mines, in
existing conditions as well as in an experimental simulation of
the high-dust conditions of the past caused by dry drilling
(Verma et al., 1989). The results of the conversion relationship
were non-linear and may have reflected the limitations of the
konimeter in measuring high dust (i.e., high count)
concentrations and the limitations of the gravimetric sampler in
measuring low dust concentrations. There were different
relationships for the gold and uranium mines, possibly because of
the different fractional silica concentrations in the host rock.
The conversion of the historical konimeter counts to gravimetric
respirable silica equivalents was used to derive a cumulative
respirable silica dose for each miner based on the miner's
respirable silica dose for each year, mine, and task in his work
history (Verma et al., 1989).
Thirty-two of the 2109 hardrock miners studied by Muir and
colleagues were considered by at least one of five readers to
have silicosis (small, rounded opacities with profusion of ILO
category >1/1). However, the results differed among the five
readers and "complicated the analysis" (Muir et al., 1989b). One
of the five readers identified only seven cases of silicosis
(Muir et al., 1989b). The results were presented by individual
reader and by consensus. A consensus of all of the five readers
with respect to identification of silicosis was reached on only
six cases (Muir et al., 1989b). Average respirable quartz dust
exposure for the cases was not reported.
* A cohort study of 3330 white male underground gold miners from
South Dakota employed for at least 1 year between 1940 and 1965
and followed through 1990 found 170 cases of silicosis (128 cases
were identified on death certificates, 29 cases were found during
X-ray surveys of workers conducted in 1960 and 1976, and 13 cases
were identified on both X-ray and death certificate). Cases were
defined as (1) an underlying or contributing cause of death of
silicosis, silico-tuberculosis, respiratory tuberculosis, or
pneumoconiosis, and/or (2) ILO category >1/1 silicosis
identified in the 1976 radiographic survey or "small opacities"
or "large opacities" identified in the 1960 radiographic survey
(Steenland & Brown, 1995a). The miners were exposed to a median
quartz level of 0.05 mg/m3 (0.15 mg/m3 for workers hired prior
to 1930). The average length of follow-up was 37 years, and the
average length of employment underground was 9 years. Quartz
exposure was estimated by converting dust particle counts to
gravimetric measurements (i.e., mg/m3), based on an estimate of
13% quartz content of total dust. A job-exposure matrix was
created to estimate dust exposures for each job over time, then
average dust exposures for the job categories were calculated
using existing measurements for each year from 1937 to 1975. The
estimated daily dust exposures (constant over each year) were
weighted to account for daily time spent underground. Summation
of the estimated daily dust levels over time provided an estimate
of cumulative quartz exposure (Steenland & Brown, 1995a). The
risk of silicosis was less than 1% for miners with a cumulative
exposure less than 0.5 mg/m3-years. The risk increased to 68-84%
for the highest cumulative exposure category (i.e.,
4 mg/m3-years) (Steenland & Brown, 1995a). Silicosis risk
estimates could have been affected by (1) combining silicosis
deaths with silicosis cases detected by cross-sectional
radiographic surveys, (2) differences in quartz content of dust
in early years, and (3) lack of dust measurements before 1937.
A cohort study of a subcohort of the South Dakota gold
miners described above analysed cases of silicosis that were
reported as the underlying cause of death on the death
certificates. Forty cases of silicosis, as well as 49 cases of
tuberculosis, were ascertained among the 1321 miners employed for
at least 21 years and followed through 1973. There was a linear
trend in risk of about 2.4% for each 0.1 mg/m3 of silica
exposure. However, this study does not meet the criteria for a
critical study because risk by cumulative quartz exposure was not
calculated (McDonald & Oakes, 1984).
In the five critical studies described above, the number of cases
identified depended upon the definition of silicosis (radiographic
category and whether irregular opacities were included), the quality
of the evaluation of the chest radiographs (e.g., number and training
of readers), the duration of dust exposure, and the duration of
follow-up after the end of exposure. Interstudy variation exists for
each of these factors. In addition, exposure assessments in these
studies were accompanied by uncertainties, such as the use of
conversion equations (i.e., converting particle count data to mass
concentrations; application of equations from one industry to a
different industry) and estimation of quartz content of the dust. It
is not uncommon for epidemiological studies to lack characterization
of the source and properties of the mineral dusts collected in the
workplace (Mossman & Churg, 1998). Nevertheless, the critical studies
found an exposure-response relationship for respirable quartz dust
that, when modelled, predicts the occurrence of silicosis cases in
various industries at exposures close to regulatory levels.
9.2.2 Pulmonary tuberculosis and other infections
The association between tuberculosis and silicosis has been
firmly established by the results of epidemiological studies conducted
during this century (Balmes, 1990). In recent studies of silicotics,
the association was well supported by the results of a survey of
tuberculosis deaths among silicotics in the USA for the period
1979-1991 (Althouse et al., 1995), a mortality study of 590 California
silicosis claimants (Goldsmith et al., 1995a), and a retrospective
study of silicotic miners from the Freegold mines in South Africa
(Kleinschmidt & Churchyard, 1997).
In studies of workers without silicosis, there is some limited
evidence that long exposures or high cumulative exposures to quartz
dust may increase the risk of developing tuberculosis. Two
epidemiological studies reported 3-fold higher incidences of pulmonary
tuberculosis cases in 5424 non-silicotic silica-exposed Danish foundry
workers employed 25 or more years (Sherson & Lander, 1990) and among
335 non-silicotic black South African gold miners with a median
underground employment of 26 years (Cowie, 1994). Westerholm et al.
(1986) found 13 cases of tuberculosis among 428 silicotic Swedish iron
and steel workers and one case of tuberculosis in a comparison group
of 476 Swedish iron and steel workers with normal chest radiographs
(level of statistical significance not reported). Both groups had been
exposed to silica for at least 5 years.
A study of tuberculosis incidence in 2255 white South African
gold miners included 1296 miners who had an autopsy. The
smoking-adjusted relative risk for pulmonary tuberculosis in miners
without silicotic nodules on autopsy examination (n = 577) increased
slightly with quartiles of cumulative dust exposure (relative risk
[RR] = 1.38; 95% confidence interval [CI] = 0.33-5.62) for the highest
quartile of cumulative exposure). For miners without radiologically
diagnosed silicosis (n = 1934), the smoking-adjusted relative risk
increased to 4.01 (95% CI = 2.04-7.88) in the highest quartile of
cumulative dust exposure (Hnizdo & Murray, 1998, 1999). Radiological
silicosis was defined as ILO category >1/1; detailed ILO grading
was not performed (Hnizdo & Murray, 1998, 1999). Tuberculosis was
diagnosed on average 7.6 years after the end of dust exposure and
6.8 years after the onset of radiological silicosis -- a result that
supports the need for medical surveillance of workers after the end of
exposure to silica dust (Hnizdo & Murray, 1998). (Miners who developed
tuberculosis before completing 10 years of underground employment were
excluded because they were not allowed to continue working underground
after diagnosis) (Hnizdo & Murray, 1998). It is not clear whether
"dust" exposure refers to quartz exposure or exposure to gold mine
dust.
Chen et al. (1997) conducted a case-control study (8740 cases;
83 338 controls) with US National Occupational Mortality Surveillance
data for the years 1983-1992 that controlled for confounding from age,
gender, race, socioeconomic status, potential exposure to active
tuberculosis, and the presence of silicosis and other pneumoconioses.
The potential for exposure to silica was based on potential exposure
data from the National Occupational Exposure Survey (Seta et al.,
1988) and the National Occupational Health Survey of Mining
(Greskevitch et al., 1996) and was categorized as "high,"
"intermediate," or "low or no" potential exposure. The study found
that decedents with potential high exposure to silica and with no
documentation of silicosis on the death certificate had a 30% greater
odds of mortality from respiratory tuberculosis than decedents with no
potential exposure to silica after adjustment by logistic regression
for the possible confounders mentioned above (odds ratio [OR] = 1.3;
95% CI = 1.14-1.48). The results also suggested the presence of an
exposure-response relationship of silica exposure, in the absence of
silicosis, with death from respiratory tuberculosis (Chen et al.,
1997).
In summary, the relationship between quartz exposure and
tuberculosis risk in the absence of radiographic silicosis in
silica-exposed workers has not been well defined or quantitatively
described by existing epidemiological studies.
A recent case-control study of tuberculosis and pulmonary disease
caused by non-tuberculous mycobacteria (NTM) in South African gold
miners found that radiographic silicosis, focal radiological scarring,
and human immunodeficiency virus (HIV) infection were significant risk
factors for NTM disease and for tuberculosis (Corbett et al., 1999).
Past medical history of tuberculosis treatment (OR = 15.1;
95% CI = 7.64-29.93) and current employment in a "dusty job" at the
mines (OR = 2.5; 95% CI = 1.46-4.44) were significant risk factors for
NTM. The study included 206 NTM patients and 381 tuberculosis patients
of known HIV status admitted to a South African hospital and
180 controls that were HIV-tested surgical or trauma patients admitted
to that hospital during the same time period. Odds ratios for NTM
and tuberculosis increased with increasing years of employment
(range of ORs: 1.0-9.4 for NTM, and 1.0-4.1 for tuberculosis).
9.2.3 Lung cancer
Lung cancer is associated with occupational exposures to inhaled
quartz (IARC, 1997).
Following a comprehensive review of the large body of published
epidemiological studies, IARC (1997) found that the following
epidemiological studies provide the least confounded investigations of
an association between occupational crystalline silica exposure and
lung cancer risk:
* US gold miners (Steenland & Brown, 1995b)
* Danish stone industry workers (Guenel et al., 1989)
* US granite shed and quarry workers (Costello & Graham, 1988)
* US crushed stone industry workers (Costello et al., 1995)
* US diatomaceous earth industry workers (Checkoway et al., 1993,
1996)
* Chinese refractory brick workers (Dong et al., 1995)
* Italian refractory brick workers (Puntoni et al., 1988;
Merlo et al., 1991)
* United Kingdom pottery workers (Cherry et al., 1995, 1997;
McDonald et al., 1995, 1997; Burgess et al., 1997)
* Chinese pottery workers (McLaughlin et al., 1992)
* cohorts of registered silicotics from North Carolina
(Amandus et al., 1991, 1992) and Finland (Kurppa et al., 1986;
Partanen et al., 1994)1
Although a few of those studies did not find a statistically
significant association between occupational crystalline silica
exposure and lung cancer risk, most of the studies did. Some
non-uniformity of results is not unusual when a large number of
epidemiological studies are reviewed and a variety of populations and
1 This list includes studies of diatomaceous earth industry workers
and workers in the ceramics, refractory brick, and pottery
manufacturing industries where the primary silica exposure may have
been to cristobalite rather than quartz. In most studies, exposure
data for quartz as well as cristobalite or tridymite were not
available to support that assumption.
work environments are studied (IARC, 1997). In addition, IARC noted
that the carcinogenicity of quartz (or cristobalite) "may be dependent
on inherent characteristics of the crystalline silica or on external
factors affecting its biological activity or distribution of its
polymorphs" (IARC, 1997). (A detailed critique of the studies listed
above is available in Soutar et al., 1997.)
Some of the least-confounded studies reported that lung cancer
risk tended to increase with:
* cumulative exposure to respirable silica (i.e., Checkoway et al.,
1993, 1996)
* duration of exposure (i.e., Costello & Graham, 1988;
Merlo et al., 1991; Partenen et al., 1994; Costello et al., 1995;
Dong et al., 1995)
* peak intensity of exposure (Burgess et al., 1997; Cherry et al.,
1997; McDonald et al., 1997)
* the presence of radiographically defined silicosis
(Amandus et al., 1992; Dong et al., 1995)
* length of follow-up time from date of silicosis diagnosis
(Partenen et al., 1994)
The observed associations noted above, including the
exposure-response associations, are unlikely to be explained by
confounding or other biases; therefore, the epidemiological studies,
overall, support increased lung cancer risks from occupational
exposure to inhaled respirable crystalline silica (i.e., quartz and
cristobalite) (IARC, 1997).
Three studies published since the IARC review investigated
exposure-response associations for lung cancer and occupational
exposure to quartz.
Cherry et al. (1998) published a final report of the preliminary
results of a nested case-control study of 52 lung cancer deaths in a
cohort of 5115 pottery workers (i.e., Burgess et al., 1997; Cherry et
al., 1997; McDonald et al., 1997). After adjustment for smoking and
inclusion of a 20-year-, 10-year-, or 0-year lag period, mean silica
concentration (i.e., estimated daily 8-h time-weighted airborne
concentrations in µg/m3; polymorph not specified) was associated with
lung cancer (OR = 1.60, 95% CI = 1.11-2.31; OR = 1.66,
95% CI = 1.14-2.41; OR = 1.67, 95% CI = 1.13-2.47, for 20-year,
10-year, and 0-year lag periods, respectively; P < 0.008 for each
lag period). However, duration of exposure and cumulative silica dust
exposure were not significantly associated with lung cancer mortality,
regardless of lag time (Cherry et al., 1998). The presence of small
parenchymal radiographic opacities (category >1/0; shape not
reported) was not related to lung cancer mortality, before
(P = 0.78) or after (P = 0.68) adjustment for smoking.
The authors concluded that the results imply "that crystalline silica
may well be a human carcinogen" (Cherry et al., 1998). The study did
not differentiate quartz exposures from cristobalite exposures.
De Klerk & Musk (1998) conducted a cohort study of 2297 surface
and underground gold miners in western Australia who participated in
surveys of respiratory symptoms, smoking habits, and lung function in
1961, 1974, and 1975. Eighty-nine per cent of the cohort was traced to
the end of 1993 for trachea, bronchus, and lung cancer mortality and
incidence of compensated silicosis (i.e., compensation awarded by the
Pneumoconiosis Medical Board). A nested case-control analysis of the
138 lung cancer deaths found that lung cancer mortality was related to
log total cumulative silica dust exposure after adjustment for smoking
(cigarette, pipe, or cigar) and for the presence of bronchitis at
survey (relative rate = 1.31; 95% CI = 1.01-1.70). However, the effect
of total cumulative silica dust exposure on lung cancer mortality was
not significant after adjustment for smoking, bronchitis, and
compensation for silicosis (relative rate = 1.20; 95% CI = 0.92-1.56).
Lung cancer mortality was not significantly related (P > 0.15) to
other silica exposure variables (i.e., duration of underground or
surface employment, intensity of underground or surface exposure)
after adjustment for smoking and bronchitis. Cigarette smoking
(relative rate = 32.5; 95% CI = 4.4-241.2 for >25 cigarettes smoked
per day), incidence of a compensation award for silicosis after lung
cancer diagnosis (relative rate = 1.59; 95% CI = 1.10-2.28), and
presence of bronchitis at survey (relative rate = 1.60;
95% CI = 1.09-2.33) were significantly related to lung cancer
mortality (de Klerk & Musk, 1998). The results of this study do not
support a relationship between lung cancer and silica exposure in the
absence of silicosis (i.e., a compensation award for silicosis after
lung cancer diagnosis).
Hnizdo et al. (1997) conducted a nested case-control study of
lung cancer deaths in a cohort of 2260 white South African underground
gold miners. (A lung cancer mortality cohort study was conducted
earlier [Hnizdo & Sluis-Cremer, 1991].) The mineral content of the
rock in the gold mines was mostly quartz (70-90%), silicates (10-30%),
pyrite (1-4%), and heavy minerals with grains of gold and
uranium-bearing minerals (2-4%). Seventy-eight lung cancer deaths
(69 of the 78 miners had a necropsy) that occurred during 1970-1986
were matched by year of birth with 386 control subjects from the same
cohort (Hnizdo et al., 1997). Lung cancer mortality risk and a
relationship with cigarette smoking (i.e., pack-years), cumulative
"dust" exposure (mg/m3-years), years of underground mining, incidence
of radiographic silicosis (i.e., ILO category >1/1 diagnosed up to
3 years before death of a matched case), and uranium production or
uranium grade of the ore in the gold mine were analysed with
conditional logistic regression models. Radon daughter measurements in
the gold mines were not available.
Lung cancer mortality was associated with cigarette smoking,
cumulative dust exposure (lagged 20 years from death), duration of
underground mining (lagged 20 years from death), and silicosis. The
best-fitting model predicted relative risks of 1.0, 3.5
(95% CI = 0.7-16.8), 5.7 (95% CI = 1.3-25.8), and 13.2
(95% CI = 3.1-56.2) for <6.5, 6.5-20, 21-30, and >30 pack-years of
smoking, respectively, and 2.45 (95% CI = 1.2-5.2) for silicosis. The
authors stated that variables representing uranium mining were not
significantly related to lung cancer mortality (modelling results for
these variables were not presented) (Hnizdo et al., 1997). The authors
proposed three explanations for their results: (1) miners with high
dust exposure who develop silicosis have increased lung cancer risk,
(2) high silica dust exposure concentrations may be important in the
pathogenesis of lung cancer, and silicosis is coincidental, and (3)
high levels of silica dust exposure may be a surrogate measure of
exposure to radon daughters (Hnizdo et al., 1997).
Meta-analyses of the epidemiological studies of silica exposure
and lung cancer reported a moderate summary risk of 1.3 for
silica-exposed workers (Steenland & Stayner, 1997) and higher summary
relative risks of 2.2-2.3 for studies of silicotic workers (Smith et
al., 1995; Steenland & Stayner, 1997). Tsuda et al. (1997) pooled lung
cancer risk estimates from 32 mortality studies of pneumoconiosis or
silicosis (excluding asbestosis) published from 1980 to 1994. The
estimated rate ratios of 2.74 (95% CI = 2.60-2.90) for all studies,
2.77 (95% CI = 2.61-2.94) for cohort studies only (25 of 32 studies),
and 2.84 (95% CI = 2.25-3.59) for case-control studies (5 of 32
studies) were similar to the estimates reported by Steenland & Stayner
(1997) and Smith et al. (1995).
Reasons for the higher risks in silicotics are not known;
similarly, the question of whether fibrosis is a precursor to the
development of lung cancer in humans has not been resolved. Various
hypothetical mechanistic pathways have been proposed to explain the
occurrence of lung tumours in rats and lung cancer in humans; however,
there is no convincing evidence for any specific proposed pathway
(IARC, 1997).
Selection bias is a common criticism of epidemiological studies
of lung cancer in compensated silicotics, because workers who sought
compensation for their disease may differ from the group of all
silicotics in symptoms, radiographic changes, social and psychological
factors, and industry (McDonald, 1995; Weill & McDonald, 1996).
However, Goldsmith (1998) reviewed this question and concluded that
lung cancer risk estimates were not higher in compensated silicotics
when results of studies of compensated silicotics were compared with
results of studies of silicotics ascertained from other clinical
sources (i.e., hospital or registry data).
9.2.4 Autoimmune-related disease
In humans, immune activation by occupational exposure to
respirable quartz may be linked to scleroderma, rheumatoid arthritis,
polyarthritis, mixed connective tissue disease, systemic lupus
erythematosus, Sjögren's syndrome, polymyositis, and fibrositis
(Haustein et al., 1990; Ziegler & Haustein, 1992; Otsuki et al.,
1998). The cellular mechanism that leads from quartz dust exposure to
autoimmune diseases is not known (Otsuki et al., 1998; NIOSH,
forthcoming). One theory is that when respirable silica particles are
encapsulated by macrophages, fibrogenic proteins and growth factors
are generated, and ultimately the immune system is activated (Haustein
et al., 1992; Ziegler & Haustein, 1992; Haustein & Anderegg, 1998).
Several epidemiological studies have reported statistically
significant numbers of excess deaths or cases of autoimmune-related
diseases such as scleroderma (Sluis-Cremer et al., 1985; Steenland &
Brown, 1995b), rheumatoid arthritis (Sluis-Cremer et al., 1986;
Klockars et al., 1987), and systemic lupus erythematosus (Steenland &
Brown, 1995b) in silica-exposed workers.
9.2.5 Renal disease
Recent epidemiological studies have found statistically
significant associations between occupational exposure to crystalline
silica dust and renal diseases and subclinical renal changes
(Steenland et al., 1992; Ng et al., 1993; Boujemaa et al., 1994; Hotz
et al., 1995; Nuyts et al., 1995; Steenland & Brown, 1995b; Steenland
& Goldsmith, 1995; Calvert et al., 1997).
9.2.6 Chronic obstructive pulmonary disease
Occupational exposure to respirable crystalline silica dust is
associated with chronic obstructive pulmonary disease, including
bronchitis and emphysema. Although these health effects are also
associated with tobacco smoking, some epidemiological studies suggest
that they may be present to a significant extent in non-smokers with
occupational exposure to quartz (Wiles & Faure, 1977; Becklake et al.,
1987; Holman et al., 1987; Kreiss et al., 1989; Cowie & Mabena, 1991).
9.2.7 Other adverse health effects
Cor pulmonale (i.e., enlargement of the right ventricle of the
heart because of structural or functional abnormalities of the lungs)
may occur as a complication of silicosis (Green & Vallyathan, 1996)
and other pneumoconioses (Kusiak et al., 1993). It is usually preceded
by pulmonary arterial hypertension. An epidemiological case-control
study of 732 white South African autopsied gold miners reported a
statistically significant association (P < 0.05) of cor pulmonale
with "extensive" silicosis and "slight" silicosis (Murray et al.,
1993).
Other adverse health effects or complications of silicosis have
been studied or identified in epidemiological studies of workers that
may have been exposed to quartz dust, but evidence of an association
with quartz exposure is inconclusive. These adverse effects include
dental abrasion (Petersen & Henmar, 1988), nasopharyngeal or
pharyngeal cancer (Carta et al., 1991; Chen et al., 1992), salivary
gland cancer (Zheng et al., 1996), liver cancer (Chen et al., 1992;
Hua et al., 1992), bone cancer (Steenland & Beaumont, 1986; Forastiere
et al., 1989), pancreatic cancer (Kauppinen et al., 1995), skin cancer
(Partanen et al., 1994), oesophageal cancer (Belli et al., 1989;
Xu et al., 1996; Pan et al., 1999), stomach cancer
(Parent et al., 1998), cancers of the digestive system
(Decoufle & Wood, 1979), intestinal or peritoneal cancer
(Amandus et al., 1991; Costello et al., 1995; Goldsmith et al.,
1995a), lymphopoietic or haematopoietic cancers (Silverstein et al.,
1986; Steenland & Brown, 1995b), and bladder cancer (Bravo et al.,
1987).
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
Terrestrial mammals (i.e., horses, camels) and birds exposed to
quartz in the natural environment, especially in desert or coastal
areas, show pathological lesions sometimes described as "silicosis"
that are similar to those seen in humans with silicosis
(Schwartz et al., 1981; Evans et al., 1988; Hansen et al., 1989;
Berry et al., 1991; Xu et al., 1993; Green & Vallyathan, 1996). Rats,
hamsters, guinea-pigs, monkeys, and mice exposed to quartz under
experimental conditions develop lung conditions and nodules similar to
those found in humans (reviewed by Green & Vallyathan, 1996).
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
The extensive body of in vitro and in vivo research
evaluating the effects of quartz on mammalian cells is summarized here
(IARC, 1997; NIOSH, forthcoming). Quartz deposited in the lungs causes
epithelial and macrophage injury and activation, and it translocates
to the interstitium and the regional lymph nodes. Recruitment of
inflammatory cells occurs in a dose-dependent manner. Oxidative stress
(i.e., increased formation of reactive oxygen species, including
hydroxyl radicals, or reactive nitrogen species) has been observed in
rats after intratracheal instillation (Blackford et al., 1994;
Schapira et al., 1995) or inhalation (Vallyathan et al., 1995) of
quartz. Several mechanisms have been proposed to explain the cause of
the cellular damage by quartz particles (Lapp & Castranova, 1993):
(1) direct cytotoxicity of quartz, (2) stimulation of the alveolar
macrophages by quartz, which results in the release of cytotoxic
enzymes or oxidants, (3) stimulation of the alveolar macrophages to
release inflammatory factors (e.g., interleukin-8, leukotriene B4,
platelet-activating factor, tumour necrosis factor, platelet-derived
growth factor) that recruit polymorphonuclear leukocytes, which may
release cytotoxins, (4) stimulation of the alveolar macrophages to
release factors that initiate fibroblast production and collagen
synthesis (e.g., interleukin-1, tumour necrosis factor,
platelet-derived growth factor, fibronectin, alveolar
macrophage-derived growth factor), and, more recently, (5) induction
by quartz of apoptosis and subsequent engulfment by macrophages to
regulate the evolution of inflammation and fibrosis (Leigh et al.,
1997).
Silicosis is indisputably causally related to occupational quartz
exposure, and the dose-response assessments of the adverse health
effects of quartz are based on epidemiological studies of occupational
cohorts with silicosis. To date, there are no known adverse health
effects associated with non-occupational exposure to quartz dust.
Silicosis is the critical effect for hazard identification and
dose-response assessment, for two reasons. First, although IARC
classified inhaled quartz from occupational sources as a Group 1
carcinogen, there are very few published risk assessments
(toxicological or epidemiological) with a quantitative dose-response
assessment of lung cancer risk at various levels of quartz exposure. A
pooled exposure-response assessment of a number of epidemiological
studies with quantitative data for quartz exposures and lung cancer is
currently being conducted by IARC.
Secondly, epidemiological studies of quartz-exposed workers
reported statistically significant numbers of excess deaths or cases
of renal disease or subclinical renal changes (Steenland et al., 1992;
Ng et al., 1993; Boujemaa et al., 1994; Hotz et al., 1995;
Nuyts et al., 1995; Steenland & Brown, 1995b; Calvert et al., 1997),
mycobacterial infections (tuberculous and non-tuberculous), or fungal
infections (Ziskind et al., 1976; Parkes, 1982; Parker, 1994; Althouse
et al., 1995; Goldsmith et al., 1995a; NIOSH, 1996; American Thoracic
Society, 1997; Kleinschmidt & Churchyard, 1997; Hnizdo & Murray, 1998;
Corbett et al., 1999), immunological disorders and autoimmune diseases
(i.e., scleroderma) (Sluis-Cremer et al., 1985; Cowie, 1987; Steenland
& Brown, 1995b), rheumatoid arthritis (Sluis-Cremer et al., 1986;
Klockars et al., 1987), and systemic lupus erythematosus (Steenland &
Brown, 1995b), but sufficient epidemiological or toxicological data do
not currently exist for quantitative assessment of the
exposure-response relationship for these health effects.
The US EPA calculated lowest-observed-adverse-effect level
(LOAEL) human equivalent concentrations (HECs) for non-cancer effects
reported in subchronic (<3 months) and chronic inhalation studies
that administered less than 20 mg quartz/m3 to experimental rats and
mice (Table 1). The results varied widely -- from 0.18 mg/m3 (based
on rat study) to 0.90 mg/m3 (based on mouse study). Some of this
variation may have been due to differences in dose, species, and the
quartz specimens. The method for determining the HEC was based on a
series of empirical equations described elsewhere (US EPA, 1994) and
summarized here. It represents an example of the calculation of an HEC
and may not necessarily be a method used worldwide. The equations
estimate fractional deposition of relatively insoluble particles and
adjust for dosimetric differences between species by incorporating
normalizing factors such as body weight or surface area (US EPA,
1994). In summary:
NOAEL[HEC] (mg/m3) = NOAEL[ADJ] (mg/m3) × RDDRr
where:
* NOAEL[HEC] = the no-observed-adverse-effect level (NOAEL) human
equivalent concentration, dosimetrically adjusted
* NOAEL[ADJ] = the NOAEL adjusted for duration: E (mg/m3) × D
(h/24 h) × W (days/7 days), where E = experimental exposure level
* RDDRr = the regional deposited dose ratio of particles for the
respiratory tract region (r)
* RDDR = (RDDTH/Normalizing Factor)A/(RDDTH/Normalizing
Factor)H, the ratio of regional deposited dose (RDDr) in the
thoracic (TH) region in the animal (A) species to that of humans
(H) (US EPA, 1994)
* r = region of dose deposition, in this case thoracic
* RDDr = 10-6 × Ci × VE × Fr, where:
Ci = concentration (mg/m3)
VE = minute volume (ml/min)
Fr = fractional deposition in region r
The literature contains a few additional risk assessments of lung
cancer and quartz, and all were based on results of inhalation studies
of rats (Collins & Marty, 1995, 1997; Goldsmith et al., 1995b).
However, human data introduce less uncertainty than extrapolation from
animals to humans (Goldsmith et al., 1995b; Goldsmith &
Hertz-Picciotto, 1997). For quartz, the uncertainty can be attributed
in part to the lack of (1) experimental studies that systematically
evaluated exactly the same material to which humans are exposed (IARC,
1997), (2) understanding of the mechanism for induction of rat lung
tumours (IARC, 1997), (3) understanding of the species differences
observed in the fibrogenic response (Gift & Faust, 1997), and
(4) experimental studies that administered doses in concentrations
similar to known occupational exposures (US EPA, 1996).
Epidemiological studies that used cumulative exposure estimates
represent the best currently available source of information for
characterizing the dose-response relationship for silicosis or lung
cancer in occupational, as well as non-occupational, cohorts.
Exposure-response models based on cumulative exposure data can
provide predictions of silicosis risk for a particular silica dust
exposure over a period of time. Table 6 presents results of logistic
regression models of data from the cross-sectional epidemiological
studies described in section 9.2. The models predicted the prevalence
of radiographic silicosis (ILO category >1/1 or >1/0) from
cumulative exposure to respirable quartz. All models predicted the
occurrence of at least one case of radiographic silicosis per 100
workers at cumulative respirable quartz dust exposures of 0.05 or 0.10
mg/m3 over a 45-year working lifetime. Logistic regression assumes
that the change in the level of quartz exposure affects risk (or
predicted prevalence) in a multiplicative manner (as compared with
linear models, which predict deviations from additivity)
(Kleinbaum et al., 1982).
Table 7 displays the results of three studies that followed
cohorts of miners for silicosis over time or retrospectively. In these
studies, the miners with silicosis had their first quartz exposure
18-37 years (mean) prior to radiographic diagnosis. The models applied
to the data collected in these studies predicted that the risk of
chronic silicosis increases exponentially with increasing cumulative
dose of silica dust (US EPA, 1996). Data from the study of South
African gold miners (Hnizdo & Sluis-Cremer, 1993) and Canadian gold
and uranium miners were analysed with models that assumed some risk of
silicosis at any exposure. The study of US gold miners (Steenland &
Brown, 1995a) estimated silicosis rates (cases per person-time at
risk) for seven cumulative exposure categories, stratified by 5-year
age and calendar time intervals. Poisson regression was used to adjust
the crude rates for age and calendar time. Although age and calendar
time were highly correlated with exposure, it is not likely that they
confounded the exposure-response analysis, because silicosis has no
background rate for non-exposed populations that changes with age or
calendar time (US EPA, 1996).
In summary, the risk estimates for silicosis prevalence for a
working lifetime of exposure to respirable quartz dust concentrations
of about 0.05 or 0.10 mg/m3 in the occupational environment vary
widely (i.e., 2-90%). Epidemiological studies varied in the definition
of silicosis cases, radiographic interpretation methods, cohort
follow-up periods, and statistical methods. The variability in the
risk estimates cannot be solely attributed to differences in follow-up
periods; however, it must be recognized that chronic silicosis is a
progressive disease. Therefore, the development of silicosis after a
long latency period and after workers leave employment must be
accounted for in epidemiological studies. Results of a study of
autopsied South African gold miners found that the diagnostic
sensitivity of radiological examination is particularly poor (Hnizdo
et al., 1993). For example, when the radiological findings for
profusion of rounded opacities (ILO category >1/1) were compared
with pathological findings for silicosis in 326 miners with an average
of 2.7 years between the radiological and pathological examination,
silicosis was not diagnosed radiographically for at least 61% of the
miners with slight to marked silicosis at autopsy. The probability of
a false-negative reading increased with years of mining and the
average concentration of respirable dust (Hnizdo et al., 1993).
Experimental studies of rats also reported lack of agreement between
histopathological indicators of silica dust exposure and radiographic
readings (Drew & Kutzman, 1984a,b).
Improved exposure assessment methods and data analyses that
account for variations and deficiencies in exposure data would improve
the risk estimates for silica-exposed workers (Agius et al., 1992;
Checkoway, 1995). Although epidemiological studies that used
cumulative exposure estimates represent the best available source of
information for characterizing the dose-response relationship in
occupational cohorts, peak exposures may predict silicosis risk better
than cumulative exposures (Checkoway & Rice, 1992), but data are
rarely available.
11.1.2 Criteria for setting tolerable intakes or guidance values
for quartz
Results of genotoxicity tests of quartz, as well as other
in vitro and in vivo evidence, suggest that persistent
inflammation and epithelial proliferation are related to the tumour
response in rats. Other pathways for tumour induction, such as a
direct genotoxic effect, have not been ruled out. Because a pathway
has not been determined, and it is unclear from results of
epidemiological studies whether silicosis is a precursor to
lung cancer, it cannot be assumed that there is a threshold (i.e.,
tolerable concentration, or TC) at which exposure to quartz would not
result in silicosis and/or lung cancer. In addition, available data
indicate that occupational quartz exposure is associated with the
development of tuberculosis, especially in workers with silicosis;
however, the association has not been quantified for that outcome or
for other quartz-related diseases. Therefore, occupational exposure to
respirable quartz dust should be reduced to the extent possible.
11.1.3 Sample risk characterization
It is recognized that there are many different methods for
assessing the risk to human health posed by environmental and
occupational substances. The example presented here applies to healthy
individuals not compromised by respiratory ailments and who breathe
the ambient air in the USA. It is based on the results of the three
cohort studies of miners presented in Table 7 (section 11.1.1). Using
a high estimate of 10% crystalline silica content of particulate
matter with a mass median aerodynamic diameter not greater than 10 µm
(PM10) from US metropolitan areas, the highest cumulative crystalline
silica exposure expected from continuous human lifetime exposure at or
below the annual US national ambient air quality standard (NAAQS) for
particulate matter of 50 µg/m3 is 1 mg/m3-year (US EPA, 1996).
The US EPA applied a mathematical model (i.e., benchmark dose
[BMD] analysis) to determine a concentration and lower confidence
bound associated with a predefined effect level (i.e., 1%, 5%, and 10%
risk of silicosis) (US EPA, 1996). After consideration of
cross-sectional, longitudinal, retrospective cohort, and case-control
epidemiological studies, the BMD was conducted on data from the
previously described study of radiographic silicosis in a cohort of
2235 South African gold miners (Hnizdo & Sluis-Cremer, 1993). This
study was selected for several reasons: (1) continuous and
longitudinal investigation of silicosis, (2) miners had multiple X-ray
examinations, and (3) autopsy data were available for more accurate
interpretation of the radiographic results, which lack sensitivity (US
EPA, 1996).1
1 The cross-sectional studies described in Table 6 and sections
9.2.1 and 11.1.1 were not available at the time of the US EPA
assessment. These studies modelled the cumulative quartz exposures
of hardrock miners (Kreiss & Zhen, 1996) and grey iron foundry
workers (Rosenman et al., 1996) and predicted a 30% prevalence of
ILO category > 1/1 silicosis over a 45-year working lifetime with
10 years of post-employment follow-up (Kreiss & Zhen, 1996) or a
2% prevalence of ILO category > 1/0 silicosis over a 40-year
working lifetime and controlling for pack-years of cigarette
smoking, race, and silica exposure other than in the foundry under
study (Rosenman et al., 1996). Both predictions are for a mean
quartz dust concentration of 0.05 mg/m3.
Using methods described by US EPA (1996), the BMD analysis
predicted that the silicosis risk for a continuous 70-year lifetime
exposure to 0.008 mg/m3 (estimated high crystalline silica
concentration in US metropolitan areas) is less than 3% for healthy
individuals not compromised by other respiratory diseases or
conditions and for ambient environment (US EPA, 1996). (Risks were not
calculated for other groups, such as people with respiratory
illnesses.) This risk estimate for exposure to ambient quartz may be
conservative, because quartz particles in the occupational environment
may be finer or "freshly fractured," occupational exposures may
involve high "peak" exposures, and, thus, the potential for disease
development may be greater.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
IARC has classified inhaled crystalline silica (quartz or
cristobalite) from occupational sources as a Group 1 carcinogen based
on sufficient evident of carcinogenicity in humans and experimental
animals. In addition, "in making the overall evaluation, the Working
Group noted that carcinogenicity in humans was not detected in all
industrial circumstances studied. Carcinogenicity may be dependent on
inherent characteristics of the crystalline silica or on external
factors affecting its biological activity or distribution of its
polymorphs" (IARC, 1997).
In 1991, the ILO published a document describing methods for
prevention and control of occupational lung diseases, including
silicosis (ILO, 1991), and in 1993, the Office of Occupational Health
of the World Health Organization (WHO) called for increased medical
surveillance of workers exposed to mineral dusts to prevent
pneumoconioses such as silicosis and asbestosis (WHO, 1993). WHO also
recently published information about risk factors for pulmonary
tuberculosis, including occupational exposure to respirable
crystalline silica (WHO, 1996).
In 1986, a WHO study group recommended a limit of 40 µg/m3
(time-weighted average of an 8-h shift) for occupational exposure to
respirable crystalline silica dust (WHO, 1986).
REFERENCES
Agius RM, Love RG, Davies LST, Hutchison PA, Cherrie JW, Robertson A,
Cowie HA, Hurley JF, Seaton A, Soutar CA (1992) Epidemiological
studies of respiratory health and dust exposure in hard rock quarry
workers and ex-workers. Edinburgh, Institute of Occupational
Medicine (HSE Contract No. 1/LMD/126/146/88; Report No. TM/92/10).
Ahlman K, Koskela R-S, Kuikka P, Koponen M, Annanmäki M (1991)
Mortality among sulfide ore miners. American journal of industrial
medicine, 19:603-617.
Althouse RB, Bang KM, Castellan RM (1995) Tuberculosis comortality
with silicosis -- United States, 1979-1991. Applied occupational and
environmental hygiene, 10(12):1037-1041.
Altieri A, Sperduto B, Verdel U, Porceli D (1984) Identification of
cristobalite and quartz in the production of silicon carbide. Rivista
degli Infortuni e delle Malattie Professionali, 71(1-2):131-135.
Amandus HE, Shy C, Wing S, Blair A, Heineman EF (1991) Silicosis and
lung cancer in North Carolina dusty trades workers. American journal
of industrial medicine, 20:57-70.
Amandus HE, Castellan RM, Shy C, Heineman EF, Blair A (1992)
Reevaluation of silicosis and lung cancer in North Carolina dusty
trades workers. American journal of industrial medicine, 22:147-153.
American Thoracic Society (1997) Adverse effects of crystalline silica
exposure. American journal of respiratory and critical care medicine,
155:761-765.
Anderson LJ, Donaldson HM, Jones JH, Stringer WT, Wallingford KM
(1980) North Carolina Brick Industry Industrial Hygiene and
Respiratory Disease Morbidity Survey, 1974-1975. Cincinnati, OH,
National Institute for Occupational Safety and Health (National
Technical Information Service Report No. PB83-181735).
Aoki A, Sirai A, Sakamoto H, Igarashi T, Matsunaga K, Ishigatsubo Y,
Tani K, Okubo T (1988) A case of silicosis associated with
polymyositis and benign monoclonal gammopathy. Ryumachi,
28(5):373-378.
Arnalich F, Lahoz C, Picazo ML, Monereo A, Arribas JR, Martinez Ara J,
Vazquez JJ (1989) Polyarteritis nodosa and necrotizing
glomerulonephritis associated with long-standing silicosis. Nephron,
51(4):544-547.
Balmes J (1990) Silica exposure and tuberculosis: an old problem with
some new twists [editorial]. Journal of occupational medicine,
32(2):114-115.
Banks DE, Milutinovic J, Desnick RJ, Grabowski GA, Lapp NL, Boehlecke
BA (1983) Silicon nephropathy mimicking Fabry's disease.
American journal of nephrology, 3(5):279-284.
Beadle DG (1965) An epidemiological study of the relationship between
the amount of dust breathed and the incidence of silicosis in South
African gold miners. In: Davies CN, ed. Inhaled particles and
vapours II. Oxford, Pergamon Press, pp. 479-492.
Beadle DG (1971) The relationship between the amount of dust breathed
and the development of radiological signs of silicosis: an
epidemiological study in South African gold miners. In: Walton WH, ed.
Inhaled particles III: Proceedings of an international symposium
organized by the British Occupational Hygiene Society, Vol. II.
Surrey, Unwin Brothers Limited, pp. 953-964.
Beadle DG, Bradley AA (1970) The composition of airborne dust in South
African gold mines. In: Shapiro HA, ed. Pneumoconiosis: Proceedings
of the international conference, Johannesburg, 1969. Oxford, Oxford
University Press, pp. 462-466.
Becklake MR, Irwig L, Kielkowski D, Webster I, De Beer M, Landau S
(1987) The predictors of emphysema in South African gold miners.
American review of respiratory disease, 135:1234-1241.
Belli S, Comba P, Germani D, Grignoli M, Lagorio S, Paganoni R,
Ronchin M (1989) [Mortality study among lead-zinc Italian (Val
Seriana) miners.] Medicina del Lavoro, 80(6):467-478 (in Italian).
Berry CR, O'Brien TR, Madigan JE, Hager DA (1991) Thoracic
radiographic features of silicosis in 19 horses. Journal of
veterinary internal medicine, 5(4):248-256.
Blackford JA, Antonini JM, Castranova V, Dey RD (1994) Intratracheal
instillation of silica up-regulates inducible nitric oxide synthase
gene expression and increases nitric oxide production in alveolar
macrophages and neutrophils. American journal of respiratory cell and
molecular biology, 11(4):426-431.
Bloor WA, Eardley RE, Dinsdale A (1971) Environmental conditions in
sanitary whiteware casting shops. Annals of occupational hygiene,
14(4):321-327.
Bolsaitis PP, Wallace WE (1996) The structure of silica surfaces in
relation to cytotoxicity. In: Castranova V, Vallyathan V, Wallace WE,
eds. Silica and silica-induced lung diseases. Boca Raton, FL,
CRC Press, pp. 79-89.
Bolton WK, Suratt PM, Sturgill BC (1981) Rapidly progressive silicon
nephropathy. American journal of medicine, 71:823-828.
Bonnin A, Mousson C, Justrabo E, Tanter Y, Chalopin JM, Rifle G (1987)
Silicosis associated with crescentic IgA mesangial nephropathy
[letter]. Nephron, 47(3):229-230.
Boujemaa W, Lauwerys R, Bernard A (1994) Early indicators of renal
dysfunction in silicotic workers. Scandinavian journal of work,
environment and health, 20(3):180-183.
Bravo MP, Del Rey Calero J, Conde M (1987) Silice y cancer de vejiga
en varones. Archivos Espanoles de Urologia, 40(9):635-637.
Bresnitz EA, Roseman J, Becker D, Gracely E (1992) Morbidity among
municipal waste incinerator workers . American journal of industrial
medicine, 22:363-378.
Burgess GL, Turner S, McDonald JC, Cherry NM (1997) Cohort mortality
study of Staffordshire pottery workers: (I) Radiographic validation of
an exposure matrix for respirable crystalline silica. Annals of
occupational hygiene, 41 (Suppl. 1):403-407.
Burgess WA (1995) Recognition of health hazards in industry: a review
of materials and processes, 2nd ed. New York, NY, John Wiley & Sons,
pp. 106-139, 411-422, 423-434, 475-482.
Buringh E, van de Belt R, van der Wal JF (1990) Dust control measures
in Dutch brickworks. Annals of occupational hygiene, 34:483-497.
Burns CA, Zarkower A, Ferguson FG (1980) Murine immunological and
histological changes in response to chronic silica exposure.
Environmental research, 21(2):298-307.
Calvert GM, Steenland K, Palu S (1997) End-stage renal disease among
silica-exposed gold miners. Journal of the American Medical
Association, 277(15):1219-1223.
Carta P, Cocco PL, Casula D (1991) Mortality from lung cancer among
Sardinian patients with silicosis. British journal of industrial
medicine, 48(2):122-129.
Castranova V, Dalal NS, Vallyathan V (1996) Role of surface free
radicals in the pathogenicity of silica. In: Castranova V, Vallyathan
V, Wallace WE, eds. Silica and silica-induced lung diseases. Boca
Raton, FL, CRC Press, pp. 91-105.
Castranova V, Vallyathan V, Ramsey DM, McLaurin JL, Pack D, Leonard S,
Barger MW, Ma JYC, Dalal NS, Teass A (1997) Augmentation of pulmonary
reactions to quartz inhalation by trace amounts of iron-containing
particles. Environmental health perspectives, 105 (Suppl.
5):1319-1324.
Cavariani F, Di Pietro A, Miceli M, Forastiere F, Biggeri A, Scavalli
P, Petti A, Borgia P (1995) Incidence of silicosis among ceramic
workers in central Italy. Scandinavian journal of work, environment
and health, 21 (Suppl. 2):58-62.
Chamberlain M (1983) Effect of mineral dusts on metabolic cooperation
between Chinese hamster V79 cells in vitro. Environmental health
perspectives, 51:5-9.
Checkoway H (1995) Methodological considerations relevant to
epidemiology studies of silica and lung cancer. Applied occupational
and environmental hygiene, 10(12):1049-1055.
Checkoway H, Rice CH (1992) Time-weighted averages, peaks, and other
indices of exposure in occupational epidemiology. American journal of
industrial medicine, 21:25-33.
Checkoway H, Heyer NJ, Demers PA, Breslow NE (1993) Mortality among
workers in the diatomaceous earth industry. British journal of
industrial medicine, 50:586-597.
Checkoway H, Heyer NJ, Demers PA, Gibbs GW (1996) Reanalysis of
mortality from lung cancer among diatomaceous earth industry workers,
with consideration of potential confounding by asbestos exposure.
Occupational and environmental medicine, 53:645-647.
Chen GX, Burnett CA, Cameron LL, Alterman T, Lalich NR, Tanaka S,
Althouse RB (1997) Tuberculosis mortality and silica exposure: a
case-control study based on a national mortality database for the
years 1983-1992. International journal of occupational and
environmental health, 3:163-170.
Chen J, McLaughlin JK, Zhang JY, Stone BJ, Luo J, Chen RA, Dosemeci M,
Rexing SH, Wu Z, Hearl FJ, McCawley MA, Blot WJ (1992) Mortality among
dust-exposed Chinese mine and pottery workers. Journal of
occupational medicine, 34(3):311-316.
Cherry N, Burgess G, McNamee R, Turner S, McDonald C (1995) Initial
findings from a cohort mortality study of British pottery workers.
Applied occupational and environmental hygiene, 10(12):1042-1045.
Cherry NM, Burgess GL, Turner S, McDonald JC (1997) Cohort study of
Staffordshire pottery workers: (II) Nested case referent analysis of
lung cancer. Annals of occupational hygiene, 41 (Suppl. 1):408-411.
Cherry NM, Burgess GL, Turner S, McDonald JC (1998) Crystalline silica
and risk of lung cancer in the potteries. Occupational and
environmental medicine, 55:779-785.
Collins JF, Marty MA (1995) Cancer risk assessment for crystalline
silica to implement California's Hot Spots Act. Scandinavian
journal of work, environment and health, 21 (Suppl. 2):99-103.
Collins JF, Marty MA (1997) Cancer risk assessment for crystalline
silica. Journal of exposure analysis and environmental epidemiology,
7(3):359-365.
Cooper TC, Gressel MG, Froelich PA, Caplan PE, Mickelsen RL, Valiante
D, Bost P (1993) Successful reduction of silica exposures at a
sanitary ware pottery. American Industrial Hygiene Association
Journal, 54(10):600-606.
Corbett EL, Churchyard GJ, Clayton T, Herselman P, Williams B, Hayes
R, Mulder D, De Cock KM (1999) Risk factors for pulmonary
mycobacterial disease in South African gold miners. American journal
of respiratory and critical care medicine, 159:94-99.
Costello J, Graham WGB (1988) Vermont granite workers' mortality
study. American journal of industrial medicine, 13:483-497.
Costello J, Castellan RM, Swecker GS, Kullman GJ (1995) Mortality of a
cohort of U.S. workers employed in the crushed stone industry,
1940-1980. American journal of industrial medicine, 27:625-640.
Cowie RL (1987) Silica-dust-exposed mine workers with scleroderma
(systemic sclerosis). Chest, 92(2): 260-262.
Cowie RL (1994) The epidemiology of tuberculosis in gold miners with
silicosis. American journal of respiratory and critical care
medicine, 150:1460-1462.
Cowie RL, Mabena SK (1991) Silicosis, chronic airflow limitation, and
chronic bronchitis in South African gold miners. American review of
respiratory disease, 143:80-84.
Dagle GE, Wehner AP, Clark ML, Buschbom RL (1986) Chronic inhalation
exposure of rats to quartz. In: Goldsmith DF, Winn DM, Shy CM, eds.
Silica, silicosis, and cancer: Controversy in occupational medicine.
New York, NY, Praeger Publishers, pp. 255-266 (Cancer Research
Monographs, Vol. 2).
Daniel LN, Mao Y, Saffiotti U (1993) Oxidative DNA damage by
crystalline silica. Free radical biology and medicine, 14:463-472.
Daniel LN, Mao Y, Wang T-CL, Markey CJ, Markey SP, Shi X, Saffiotti U
(1995) DNA strand breakage, thymine glycol production, and hydroxyl
radical generation induced by different samples of crystalline silica
in vitro. Environmental research, 71:60-73.
Davies LST, Robertson A, Agius RM, Cowie HA, Cherrie JW, Hutchison P
(1994) The use of compliance monitoring for assessing quarry workers'
exposures to respirable dust and quartz. Annals of occupational
hygiene, 38 (Suppl. 1):559-570.
Davis BL, Johnson LR, Stevens RK, Courtney WJ, Safriet DW (1984) The
quartz content and elemental composition of aerosols from selected
sites of the EPA inhalable particulate network. Atmospheric
environment, 18(4):771-782.
Davis GS (1991) Immunologic aspects of pneumoconioses in asbestosis
and silicosis. In: Lynch JP, DeRemee RA, eds. Immunologically
mediated pulmonary diseases. Philadelphia, PA, J.B. Lippincott
Company, pp. 111-155.
Davis GS (1996) Silica. In: Harber P, Schenker MB, Balmes JR, eds.
Occupational and environmental respiratory disease, 1st ed. St.
Louis, MO, Mosby, pp. 373-399.
Davis LK, Wegman DH, Monson RR, Froines J (1983) Mortality experience
of Vermont granite workers. American journal of industrial medicine,
4:705-723.
Decoufle P, Wood DJ (1979) Mortality patterns among workers in a gray
iron foundry. American journal of epidemiology, 109(6):667-675.
de Klerk NH, Musk AW (1998) Silica, compensated silicosis, and lung
cancer in Western Australian goldminers. Occupational and
environmental medicine, 55:243-248.
Dethloff LA, Gilmore LB, Gladen BC, George G, Chhabra RS, Hook GER
(1986a) Effects of silica on the composition of the pulmonary
extracellular lining. Toxicology and applied pharmacology,
84(1):66-83.
Dethloff LA, Gilmore LB, Brody AR, Hook GER (1986b) Induction of
intracellular and extracellular phospholipids in the lungs of rats
exposed to silica. Biochemical journal, 233(1):111-118.
Donaldson HM, Wallingford K, Jones JH (1982) Environmental surveys in
the Barre, Vermont and Elberton, Georgia granite industries.
Cincinnati, OH, National Institute for Occupational Safety and Health
(National Technical Information Service Publication No. PB83-179911).
Donaldson K, Borm PJA (1998) The quartz hazard: a variable entity.
Annals of occupational hygiene, 42(5):287-294.
Dong D, Xu G, Sun Y, Hu P (1995) Lung cancer among workers exposed to
silica dust in Chinese refractory plants. Scandinavian journal of
work, environment and health, 21 (Suppl. 2):69-72.
Dosemeci M, McLaughlin JK, Chen J-Q, Hearl F, Chen R-G, McCawley M, Wu
Z, Peng K-L, Chen A-L, Rexing SH, Blot WJ (1995) Historical total and
respirable silica dust exposure levels in mines and pottery factories
in China. Scandinavian journal of work, environment and health, 21
(Suppl. 2):39-43.
Dracon M, Noel C, Wallaert P, Dequiedt P, Lelievre G, Tacquet A (1990)
Rapidly progressive glomerulonephritis in silicotic coal miners.
Nephrologie, 11:61-65.
Drew RT, Kutzman RS (1984a) Final report on a study of Fischer 344
rats exposed to silica dust for six months at concentrations of 0, 2,
10 or 20 mg/m3, then maintained for six months prior to assessment.
Upton, NY, Brookhaven National Laboratory (Report No. BNL 35735;
submitted to the National Toxicology Program under Interagency
Agreement No. 222-Y01-ES-9-0043, November 1984).
Drew RT, Kutzman RS (1984b) Final report on a study of Fischer 344
rats exposed to silica dust for six months at concentrations of 0, 2,
10 or 20 mg/m3. Upton, NY, Brookhaven National Laboratory (Report
No. BNL 34617; submitted to the National Toxicology Program under
Interagency Agreement No. 222-Y01-ES-9-0043, February 1984).
Driscoll KE (1995) The toxicology of crystalline silica studied
in vitro. Applied occupational and environmental hygiene,
10(12):1118-1125.
Driscoll KE (1996) The role of interleukin-1 and tumor necrosis factor
alpha in the lung's response to silica. In: Castranova V, Vallyathan
V, Wallace WE, eds. Silica and silica-induced lung diseases. Boca
Raton, FL, CRC Press, pp. 163-184.
Driscoll KE, Deyo LC, Howard BW, Poynter J, Carter JM (1995)
Characterizing mutagenesis in the hprt gene of rat alveolar
epithelial cells. Experimental lung research, 21:941-956.
Driscoll KE, Deyo LC, Carter JM, Howard BW, Hassenbein DG, Bertram TA
(1997) Effects of particle exposure and particle-elicited inflammatory
cells on mutation in rat alveolar epithelial cells. Carcinogenesis,
18(2):423-430.
Dufresne A, Lesage J, Perrault G (1987) Evaluation of occupational
exposure to mixed dusts and polycyclic aromatic hydrocarbons in
silicon carbide plants. American Industrial Hygiene Association
Journal, 48(2):160-166.
Dufresne A, Loosereewanich P, Bégin R, Dion C, Ecobichon D, Muir DCF,
Ritchie AC, Perrault G (1998a) Tentative explanatory variable of lung
dust concentration in gold miners exposed to crystalline silica.
Journal of exposure analysis and environmental epidemiology,
8(3):375-398.
Dufresne A, Bégin R, Dion C, Jagirdar J, Rom WN, Loosereewanich P,
Muir DCF, Ritchie AC, Perrault G (1998b) Angular and fibrous particles
in lung in relation to silica-induced diseases. International
archives of occupational and environmental health, 71:263-269.
Eisen EA, Smith TJ, Wegman DH, Louis TA, Froines J (1984) Estimation
of long term dust exposures in the Vermont granite sheds.
American Industrial Hygiene Association Journal, 45(2):89-94.
Erdogdu G, Hasirci V (1998) An overview of the role of mineral
solubility in silicosis and asbestosis. Environmental research,
78:38-42.
Evans MG, Slocombe RF, Schwartz LD (1988) Pulmonary silicosis in
captive ring-necked pheasants: definitive diagnosis by electron probe
X-ray microanalysis. Veterinary pathology, 25(3):239-241.
Forastiere F, Lagorio S, Michelozzi P, Perucci CA, Axelson O (1989)
Mortality pattern of silicotic subjects in the Latium region, Italy.
British journal of industrial medicine, 46(12):877-880.
Fox AJ, Greenberg M, Ritchie GL (1975) A survey of respiratory
disease in the pottery industry. London, Her Majesty's Stationery
Office.
Freeman CS, Grossman EA (1995) Silica exposures in the United States
between 1980 and 1992. Scandinavian journal of work, environment and
health, 21 (Suppl. 2):47-49.
Fubini B (1997) Surface reactivity in the pathogenic response to
particulates. Environmental health perspectives, 105 (Suppl.
5):1013-1020.
Fubini B (1998) Surface chemistry and quartz hazard. Annals of
occupational hygiene, 42(8):521-530.
Fubini B, Bolis V, Cavenago A, Volante M (1995) Physicochemical
properties of crystalline silica dusts and their possible implication
in various biological responses. Scandinavian journal of work,
environment and health, 21 (Suppl. 2):9-14.
Fukata S, Matsubayashi S, Nagato H, Sakai K, Ohishi S, Yasuda M, Tamai
H, Nakagawa T (1983) Monoclonal gammopathies associated with
silicosis. Rinsho Ketsueki, 24(1):9-17.
Fukata S, Tamai H, Nagai K, Matsubayashi S, Nagato H, Tashiro T,
Yasuda M, Kumagai LF (1987) A patient with hereditary spherocytosis
and silicosis who developed an IgA(lambda) monoclonal gammopathy.
Japanese journal of medicine, 26(1):81-83.
Fulekar MH, Alam Khan MM (1995) Occupational exposure to dust in slate
pencil manufacture. Annals of occupational hygiene, 39(1):107-114.
Gao H, Brick J, Ong S, Miller M, Whong W-Z, Ong T (1997) Selective
hyperexpression of c-jun oncoprotein by glass fiber- and
silica-transformed BALB/c-3T3 cells. Cancer letters, 112:65-69.
Gerhardsson G (1976) Dust prevention in Swedish foundries.
Staub-Reinhaltung der Luft, 36:433-439.
Gift JS, Faust RA (1997) Noncancer inhalation toxicology of
crystalline silica: exposure-response assessment. Journal of exposure
analysis and environmental epidemiology, 7(3): 345-358.
Giles RD, Sturgill BC, Suratt PM, Bolton WK (1978) Massive proteinuria
and acute renal failure in a patient with acute silicoproteinosis.
American journal of medicine, 64:336-342.
Goldsmith DF (1994) Health effects of silica dust exposure. In: Heaney
PJ, Prewitt CT, Gibbs GV, eds. Silica: physical behavior,
geochemistry, and materials applications. Washington, DC,
Mineralogical Society of America. Reviews in mineralogy, 29:545-606.
Goldsmith DF (1998) Uses of workers' compensation data in epidemiology
research. Occupational medicine state of the art reviews,
13(2):389-415.
Goldsmith DF, Hertz-Picciotto I (1997) Criteria for conducting
quantitative risk assessments for silica. Journal of exposure
analysis and environmental epidemiology, 7(3):367-375.
Goldsmith DF, Beaumont JJ, Morrin LA, Schenker MB (1995a) Respiratory
cancer and other chronic disease mortality among silicotics in
California. American journal of industrial medicine, 28(4):459-467.
Goldsmith DF, Ruble RP, Klein CA (1995b) Comparative cancer potency
for silica from extrapolations of human and animal findings.
Scandinavian journal of work, environment and health, 21 (Suppl.
2):104-107.
Green FHY, Vallyathan V (1996) Pathologic responses to inhaled silica.
In: Castranova V, Vallyathan V, Wallace WE, eds. Silica and
silica-induced lung diseases. Boca Raton, FL, CRC Press, pp. 39-59.
Greskevitch MF, Turk AR, Dieffenbach AL, Roman JM, Groce DW, Hearl FJ
(1992) Quartz analyses of the bulk dust samples collected by the
National Occupational Health Survey of Mining. Applied occupational
and environmental hygiene, 7(8):527-531.
Greskevitch MF, Bajpayee SS, Hale JM, Groce DW, Hearl FJ, eds. (1996)
NIOSH technical report: results from the National Occupational Health
Survey of Mining (NOHSM). Cincinnati, OH, US Department of Health
and Human Services, Public Health Service, Centers for Disease Control
and Prevention, National Institute for Occupational Safety and Health,
Division of Respiratory Disease Studies (DHHS (NIOSH) Publication No.
96-136).
Groth DH, Stettler LE, Platek SF, Lal JB, Burg JR (1986) Lung tumors
in rats treated with quartz by intratracheal instillation. In:
Goldsmith DF, Winn DM, Shy CM, eds. Silica, silicosis, and cancer:
Controversy in occupational medicine. New York, NY, Praeger
Publishers, pp. 243-253 (Cancer Research Monographs, Vol. 2).
Gu Z-W, Ong TO (1996) Potential mechanisms of silica-induced cancer.
In: Castranova V, Vallyathan V, Wallace WE, eds. Silica and
silica-induced lung diseases. Boca Raton, FL, CRC Press, pp.
397-406.
Guenel P, Hojberg G, Lynge E (1989) Cancer incidence among Danish
stone workers. Scandinavian journal of work, environment and health,
15:265-270.
Hansen HJ, Jama FM, Nilsson C, Norrgren L, Abdurahman OS (1989)
Silicate pneumoconiosis in camels (Camelus dromedarius L.).
Zentralblatt für Veterinarmedizin (A), 36(10):789-796.
Hart GA, Hesterberg TW (1998) In vitro toxicity of respirable-size
particles of diatomaceous earth and crystalline silica compared with
asbestos and titanium dioxide. Journal of occupational and
environmental medicine, 40(1):29-42.
Haslam PL (1994) Basic immunology and immunopathology. In: Parkes WR,
ed. Occupational lung disorders, 3rd ed. London,
Butterworth-Heinemann, pp. 50-99.
Hauglustaine D, Van Damme B, Daenens P, Michielsen P (1980) Silicon
nephropathy: a possible occupational hazard. Nephron, 26:219-224.
Haustein UF, Anderegg U (1998) Silica induced scleroderma -- clinical
and experimental aspects. Journal of rheumatology, 25(10):1917-1926.
Haustein UF, Ziegler V, Herrmann K, Mehlhorn J, Schmidt C (1990)
Silica-induced scleroderma. Journal of the American Academy of
Dermatology, 22(3):444-448.
Haustein UF, Ziegler V, Herrmann K (1992) Chemically induced
scleroderma. Der Hautarzt, 43:464-474.
Heppleston AG (1994) Pathogenesis of mineral pneumoconioses. In:
Parkes WR, ed. Occupational lung disorders, 3rd ed. London,
Butterworth-Heinemann, pp. 100-134.
Hesterberg TW, Barrett JC (1984) Dependence of asbestos- and mineral
dust-induced transformation of mammalian cells in culture on fiber
dimension. Cancer research, 44:2170-2180.
Hesterberg TW, Oshimura M, Brody AR, Barrett JC (1986) Asbestos and
silica induce morphological transformation of mammalian cells in
culture: a possible mechanism. In: Goldsmith DF, Winn DM, Shy CM, eds.
Silica, silicosis, and cancer. Controversy in occupational medicine.
New York, NY, Praeger Publishers, pp. 177-190 (Cancer Research
Monographs, Vol. 2).
Higgins RI, Deere MR, Cinkotai FF (1985) Fettlers' exposure to pottery
dust in a factory making sanitary whiteware. Annals of occupational
hygiene, 29(3):365-375.
Hnizdo E, Murray J (1998) Risk of pulmonary tuberculosis relative to
silicosis and exposure to silica dust in South African gold miners.
Occupational and environmental medicine, 55:496-502.
Hnizdo E, Murray J (1999) Correction: Risk of pulmonary tuberculosis
relative to silicosis and exposure to silica dust in South African
gold miners. Occupational and environmental medicine, 56:215-216.
Hnizdo E, Sluis-Cremer GK (1991) Silica exposure, silicosis, and lung
cancer: a mortality study of South African gold miners. British
journal of industrial medicine, 48:53-60.
Hnizdo E, Sluis-Cremer GK (1993) Risk of silicosis in a cohort of
white South African gold miners. American journal of industrial
medicine, 24:447-457.
Hnizdo E, Murray J, Sluis-Cremer GK, Thomas RG (1993) Correlation
between radiological and pathological diagnosis of silicosis: an
autopsy population based study. American journal of industrial
medicine, 24:427-445.
Hnizdo E, Murray J, Klempman S (1997) Lung cancer in relation to
exposure to silica dust, silicosis and uranium production in South
African gold miners. Thorax, 52:271-275.
Holland LM, Gonzales M, Wilson JS, Tillery MI (1983) Pulmonary effects
of shale dusts in experimental animals. In: Wagner WL, Rom WN,
Merchant JA, eds. Health issues related to metal and nonmetallic
mining. Boston, MA, Butterworth, pp. 485-496.
Holland LM, Wilson JS, Tillery MI, Smith DM (1986) Lung cancer in rats
exposed to fibrogenic dusts. In: Goldsmith DF, Winn DM, Shy CM, eds.
Silica, silicosis, and cancer: Controversy in occupational medicine.
New York, NY, Praeger Publishers, pp. 267-279 (Cancer Research
Monographs, Vol. 2).
Holman CDJ, Psaila-Savona P, Roberts M, McNulty JC (1987) Determinants
of chronic bronchitis and lung dysfunction in Western Australian gold
miners. British journal of industrial medicine, 44:810-818.
Hook GER, Viviano CJ (1996) Acute silicosis and the activation of
alveolar type II cells. In: Castranova V, Vallyathan V, Wallace WE,
eds. Silica and silica-induced lung diseases. Boca Raton, FL, CRC
Press, pp. 229-242.
Hotz P, Gonzalez-Lorenzo J, Siles E, Trujillano G, Lauwerys R, Bernard
A (1995) Subclinical signs of kidney dysfunction following short
exposure to silica in the absence of silicosis. Nephron, 70:438-442.
Hua F, Xipeng J, Shunzhang Y, Xueqi G, Kaiguo W, JianY, Shenghua Q
(1992) Quantitative risk assessment for lung cancer from exposure to
metal ore dust. Biomedical and environmental sciences, 5:221-228.
Huggins CW, Johnson SN, Segreti JM, Snyder JG (1985) Bureau of Mines
Report of Investigations 8975: Determination of alpha quartz particle
distribution in respirable coal mine dust samples and reference
standards. Avondale, MD, US Department of the Interior, Bureau of
Mines, 7 pp. (National Technical Information Service Publication No.
PB86-136660).
Hughes JM (1995) Radiographic evidence of silicosis in relation to
silica exposure. Applied occupational and environmental hygiene,
10(12):1064-1069.
IARC (1984) Polynuclear aromatic compounds. Part 3. Industrial
exposures in aluminium production, coal gasification, coke production
and iron and steel founding. Lyon, International Agency for Research
on Cancer, pp. 133-190 (IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Vol. 34).
IARC (1987) Silica and some silicates. Lyon, France, International
Agency for Research on Cancer, pp. 33-143 (IARC Monographs on the
Evaluation of the Carcinogenic Risk of Chemicals to Humans, Vol. 42).
IARC (1997) Silica, some silicates, coal dust and para -aramid
fibrils. Lyon, International Agency for Research on Cancer, pp.
1-242 (IARC Monographs on the Evaluation of Carcinogenic Risks to
Humans, Vol. 68).
Iler RK (1979) The chemistry of silica: solubility, polymerization,
colloid and surface properties, and biochemistry. New York, NY,
Wiley-Interscience Publishers.
ILO (1980) Guidelines for the use of ILO international classification
of radiographs of pneumoconioses, rev. ed. Geneva, International
Labour Organisation, 48 pp. (Occupational Safety and Health Series No.
22).
ILO (1991) Occupational lung diseases: prevention and control.
Geneva, International Labour Organisation, 85 pp. (Occupational
Safety and Health Series No. 67).
IPCS (1993) International Chemical Safety Card -- Quartz. Geneva,
World Health Organization, International Programme on Chemical Safety
(ICSC 0808).
Iyer R, Holian A (1996) Immunological aspects of silicosis. In:
Castranova V, Vallyathan V, Wallace WE, eds. Silica and
silica-induced lung diseases. Boca Raton, FL, CRC Press, pp.
253-267.
Jaurand MC, Fleury J, Monchaux G, Nebut M, Bignon J (1987) Pleural
carcinogenic potency of mineral fibers (asbestos, attapulgite) and
their cytotoxicity on cultured cells. Journal of the National Cancer
Institute, 79(4):797-804.
Kane AB (1996) Questions and controversies about the pathogenesis of
silicosis. In: Castranova V, Vallyathan V, Wallace WE, eds. Silica
and silica-induced lung diseases. Boca Raton, FL, CRC Press, pp.
121-136.
Kauppinen T, Partanen T, Degerth R, Ojajarvi A (1995) Pancreatic
cancer and occupational exposures. Epidemiology, 6(5):498-502.
Kelly J (1992) U.S. Department of the Interior, National Park
Service, New River Gorge National River, West Virginia. Cincinnati,
OH, National Institute for Occupational Safety and Health (HHE Report
No. HETA 92-045-2260),
King EJ, Mohanty GP, Harrison CV, Nagelschmidt G (1953) The action of
different forms of pure silica on the lungs of rats. British journal
of industrial medicine, 10:9-17.
Kleinbaum DG, Kupper LL, Morgenstern H (1982) Epidemiologic research:
principles and quantitative methods. New York, NY, Van Nostrand
Reinhold, p. 428.
Kleinschmidt I, Churchyard G (1997) Variation in incidences of
tuberculosis in subgroups of South African gold miners. Occupational
and environmental medicine, 54:636-641.
Klockars M, Koskela RS, Järvinen E, Kolari PJ, Rossi A (1987) Silica
exposure and rheumatoid arthritis: a follow up study of granite
workers 1940-81. British medical journal, 294:997-1000.
Koeger AC, Alcaix D, Rozenberg S, Bourgeois P (1991) Occupational
exposure to silica and dermatopolymyositis: three cases. Annales de
medecine interne (Paris), 142(6):409-413.
Koeger AC, Alcaix D, Rozenberg S, Bourgeois P (1996) Graves' disease
after silica dust exposure. Journal of rheumatology, 68(11):202.
Koskela R-S, Klockars M, Järvinen E, Kolari PJ, Rossi A (1987)
Mortality and disability among granite workers. Scandinavian journal
of work, environment and health, 13:18-25.
Kreiss K, Zhen B (1996) Risk of silicosis in a Colorado mining
community. American journal of industrial medicine, 30:529-539.
Kreiss K, Greenberg LM, Kogut SJH, Lezotte DC, Irvin CG, Cherniack RM
(1989) Hard-rock mining exposures affect smokers and nonsmokers
differently: results of a community prevalence study. American review
of respiratory disease, 139:1487-1493.
Kullman GJ, Greife AL, Costello J, Hearl FJ (1995) Occupational
exposures to fibers and quartz at 19 crushed stone mining and milling
operations. American journal of industrial medicine, 27:641-660.
Kurppa K, Gudbergsson H, Hannunkari I, Koskinen H, Hernberg S, Koskela
R-S, Ahlman K (1986) Lung cancer among silicotics in Finland. In:
Goldsmith DF, Winn DM, Shy CM, eds. Silica, silicosis, and cancer:
Controversy in occupational medicine. New York, NY, Praeger
Publishers, pp. 311-319 (Cancer Research Monographs, Vol. 2).
Kusiak R, Liss GM, Gailitis MM (1993) Cor pulmonale and pneumoconiotic
lung disease: an investigation using hospital discharge data.
American journal of industrial medicine, 24:161-173.
Lapp NL, Castranova V (1993) How silicosis and coal workers'
pneumoconiosis develops -- a cellular assessment. Occupational
medicine state of the art reviews, 8(1):35-56.
Lawson RJ, Schenker MB, McCurdy SA, Jenkins B, Lischak LA, John W,
Scales D (1995) Exposure to amorphous silica fibers and other
particulate matter during rice farming operations. Applied
occupational and environmental hygiene, 10(8):677-684.
Leigh J, Wang H, Bonin A, Peters M, Ruan X (1997) Silica-induced
apoptosis in alveolar and granulomatous cells in vivo.
Environmental health perspectives, 105 (Suppl. 5):1241-1245.
Leigh J, Wang H, Bonin A, Peters M (1998a) Persistence of
silica-induced inflammation is related to delayed occurrence of
apoptosis in alveolar leukocytes. In: Chiyotani K, Hosoda Y, Aizawa Y,
eds. Advances in the prevention of occupational respiratory diseases.
Amsterdam, Elsevier Science, pp. 890-895 (Excerpta Medica Supplement
53).
Leigh J, Wang H, Bonin A, Peters M (1998b) In vivo genotoxicity of
silica evidenced by progressive development of micronuclei in alveolar
macrophages. In: Chiyotani K, Hosoda Y, Aizawa Y, eds. Advances in
the prevention of occupational respiratory diseases. Amsterdam,
Elsevier Science, pp. 520-525 (Excerpta Medica Supplement 53).
Linch KD, Cocalis JC (1994) An emerging issue: Silicosis prevention in
construction. Applied occupational and environmental hygiene,
9(8):539-542.
Linch KD, Miller WE, Althouse RB, Groce DW, Hale JM (1998)
Surveillance of respirable crystalline silica dust using OSHA
compliance data (1979-1995). American journal of industrial medicine,
34(6):547-558.
Liu X, Keane MJ, Zhong B-Z, Ong T, Wallace WE (1996) Micronucleus
formation in V79 cells treated with respirable silica dispersed in
medium and in simulated pulmonary surfactant. Mutation research,
361:89-94.
Liu X, Keane MJ, Harrison JC, Cilento EV, Ong T, Wallace WE (1998)
Phospholipid surfactant adsorption by respirable quartz and in vitro
expression of cytotoxicity and DNA damage. Toxicology letters, 96,
97:77-84.
Lofgren DJ (1993) Case studies: silica exposure for concrete workers
and masons. Applied occupational and environmental hygiene,
8(10):832-836.
Love RG, Waclawski ER, Maclaren WM, Porteous RH, Groat SK, Wetherill
GZ, Hutchison PA, Kidd MW, Soutar CA (1994) Cross-sectional study of
risks of respiratory disease in relation to exposures of airborne
quartz in the heavy clay industry. Edinburgh, Institute of
Occupational Medicine (IOM Report No. TM/94/07).
Mao Y, Daniel LN, Whittaker N, Saffiotti U (1994) DNA binding to
crystalline silica characterized by Fourier-transform infrared
spectroscopy. Environmental health perspectives, 102
(Suppl. 10):165-171.
Masuda K (1981) Chronic thyroiditis observed in patients with
silicosis as an adjuvant disease of man. Shikoku Acta Medica,
37(1):119-129.
Materna BL, Jones JR, Sutton PM, Rothman N, Harrison RJ (1992)
Occupational exposures in California wildland fire fighting. American
Industrial Hygiene Association journal, 53(1):69-76.
McDonald C (1995) Silica, silicosis, and lung cancer: an
epidemiological update. Applied occupational and environmental
hygiene, 10(12):1056-1063.
McDonald JC, Oakes D (1984) Exposure-response in miners exposed to
silica. In: Sixth International Pneumoconiosis Conference: 1983,
Bochum, Germany. Vol 1. Geneva, International Labour Organisation,
pp. 114-123.
McDonald JC, Cherry N, McNamee R, Burgess G, Turner S (1995)
Preliminary analysis of proportional mortality in a cohort of British
pottery workers exposed to crystalline silica. Scandinavian journal
of work, environment and health, 21 (Suppl. 2):63-65.
McDonald JC, Burgess GL, Turner S, Cherry NM (1997) Cohort study of
Staffordshire pottery workers: (III) Lung cancer, radiographic
changes, silica exposure and smoking habit. Annals of occupational
hygiene, 41 (Suppl. 1):412-414.
McLaughlin JK, Jing-Qiong C, Dosemeci M, Rong-An C, Rexing SH, Zhien
W, Hearl FJ, McCawley MA, Blot WJ (1992) A nested case-control study
of lung cancer among silica exposed workers in China. British journal
of industrial medicine, 49:167-171.
McNeill DA, Chrisp CE, Fisher GL (1990) Pulmonary adenomas in A/J mice
treated with silica. Drug and chemical toxicology, 13(1):87-92.
Merlo F, Costantini M, Reggiardo G, Ceppi M, Puntoni R (1991) Lung
cancer risk among refractory brick workers exposed to crystalline
silica: a retrospective cohort study. Epidemiology, 2(4):299-305.
Morrow PE (1988) Issues: Possible mechanisms to explain dust
overloading of the lungs. Fundamental and applied toxicology,
10:369-384.
Morrow PE (1992) Contemporary issues in toxicology -- dust overloading
of the lungs: update and appraisal. Toxicology and applied
pharmacology, 113:1-12.
Mossman BT, Churg A (1998) State of the art: Mechanisms in the
pathogenesis of asbestosis and silicosis. American journal of
respiratory and critical care medicine, 157:1666-1680.
Mozzon D, Brown DA, Smith JW (1987) Occupational exposure to airborne
dust, respirable quartz and metals arising from refuse handling,
burning and landfilling. American Industrial Hygiene Association
journal, 48(2):111-116.
Muhle H, Takenaka S, Mohr U, Dasenbrock C, Mermelstein R (1989) Lung
tumor induction upon long term low level inhalation of crystalline
silica. American journal of industrial medicine, 15(3):343-346.
Muhle H, Bellmann B, Creutzenberg O, Dasenbrock C, Ernst H, Kilpper R,
Mackenzie JC, Morrow P, Mohr U, Takenaka S, Mermelstein R (1991)
Pulmonary response to toner upon chronic inhalation exposure in rats.
Fundamental and applied toxicology, 17:280-299.
Muhle H, Kittel B, Ernst H, Mohr U, Mermelstein R (1995) Neoplastic
lung lesions in rat after chronic exposure to crystalline silica.
Scandinavian journal of work, environment and health, 21
(Suppl. 2):27-29.
Muir DCF (1991) Correction in cumulative risk in silicosis exposure
assessment. American journal of industrial medicine, 19:555.
Muir DCF, Shannon HS, Julian JA, Verma DK, Sebestyen A, Bernholz CD
(1989a) Silica exposure and silicosis among Ontario hardrock miners:
I. Methodology. American journal of industrial medicine, 16:5-11.
Muir DCF, Shannon HS, Julian JA, Verma DK, Sebestyen A, Bernholz CD
(1989b) Silica exposure and silicosis among Ontario hardrock miners:
III. Analysis and risk estimates. American journal of industrial
medicine, 16:29-43.
Muramatsu K, Yamamoto T, Hasegawa A et al. (1989) [A case of
autoimmune hemolytic anemia associated with silicosis.] Japanese
journal of chest diseases, 48(1):45-50 (in Japanese).
Murray J, Reid G, Kielkowski D, de Beer M (1993) Cor pulmonale and
silicosis: a necropsy based case-control study. British journal of
industrial medicine, 50:544-548.
Myers JE, Lewis P, Hofmeyr W (1989) Respiratory health of brickworkers
in Cape Town, South Africa: background, aims and dust exposure
determinations. Scandinavian journal of work, environment and health,
15(3):180-187.
Nagalakshmi R, Nath J, Ong T, Whong W-Z (1995) Silica-induced
micronuclei and chromosomal aberrations in Chinese hamster lung (V79)
and human lung (Hel 299) cells. Mutation research, 335:27-33.
Nehls P, Seiler F, Rehn B, Greferath R, Bruch J (1997) Formation and
persistence of 8-oxoguanine in rat lung cells as an important
determinant for tumor formation following particle exposure.
Environmental health perspectives, 105 (Suppl. 5):1291-1296.
Neyer U, Woss E, Neuweiler J (1994) Case report: Wegener's
granulomatosis associated with silicosis. Nephrology dialysis
transplantation, 9(5):559-561.
Ng TP, Yeung KH, O'Kelly FJ (1987a) Silica hazard of caisson
construction in Hong Kong. Journal of the Society of Occupational
Medicine, 37:62-65.
Ng TP, Tsin TW, O'Kelly FJ, Chan SL (1987b) A survey of the
respiratory health of silica-exposed gemstone workers in Hong Kong.
American review of respiratory disease, 135:1249-1254.
Ng TP, Lee HS, Phoon WH (1993) Further evidence of human silica
nephrotoxicity in occupationally exposed workers. British journal of
industrial medicine, 50:907-912.
Niemeier RW, Mulligan LT, Rowland J (1986) Cocarcinogenicity of
foundry silica sand in hamsters. In: Goldsmith DF, Winn DM, Shy CM,
eds. Silica, silicosis, and cancer: Controversy in occupational
medicine. New York, NY, Praeger Publishers, pp. 215-227 (Cancer
Research Monographs, Vol. 2).
NIOSH (1974) Criteria for a recommended standard: occupational
exposure to crystalline silica. Cincinnati, OH, US Department of
Health, Education, and Welfare, Health Services and Mental Health
Administration, National Institute for Occupational Safety and Health
(DHEW (NIOSH) Publication No. 75-120).
NIOSH (1992a) NIOSH Alert: Request for assistance in preventing
silicosis and deaths from sandblasting. Cincinnati, OH, US
Department of Health and Human Services, Public Health Service,
Centers for Disease Control, National Institute for Occupational
Safety and Health (DHHS (NIOSH) Publication No. 92-102).
NIOSH (1992b) NIOSH Alert: Request for assistance in preventing
silicosis and deaths in rock drillers. Cincinnati, OH, US Department
of Health and Human Services, Public Health Service, Centers for
Disease Control, National Institute for Occupational Safety and Health
(DHHS (NIOSH) Publication No. 92-107).
NIOSH (1994a) Silica, crystalline, respirable by XRD: Method 7500,
Issue 2 (dated 8/15/94). In: Cassinelli ME, O'Connor PF, eds. NIOSH
manual of analytical methods, 4th ed. Cincinnati, OH, US Department
of Health and Human Services, Public Health Service, Centers for
Disease Control and Prevention, National Institute for Occupational
Safety and Health (DHHS (NIOSH) Publication No. 94-113).
NIOSH (1994b) Silica, crystalline, respirable by IR: Method 7602,
Issue 2 (dated 8/15/94). In: Cassinelli ME, O'Connor PF, eds. NIOSH
manual of analytical methods, 4th ed. Cincinnati, OH, US Department
of Health and Human Services, Public Health Service, Centers for
Disease Control and Prevention, National Institute for Occupational
Safety and Health (DHHS (NIOSH) Publication No. 94-113).
NIOSH (1996) NIOSH Alert: Request for assistance in preventing
silicosis and deaths in construction workers. Cincinnati, OH, US
Department of Health and Human Services, Public Health Service,
Centers for Disease Control and Prevention, National Institute for
Occupational Safety and Health (DHHS (NIOSH) Publication No. 96-112).
NIOSH (1997) Pocket guide to chemical hazards. Cincinnati, OH, US
Department of Health and Human Services, Public Health Service,
Centers for Disease Control and Prevention, National Institute for
Occupational Safety and Health (DHHS (NIOSH) Publication No. 97-140).
NIOSH (forthcoming) NIOSH special hazard review: Health effects of
occupational exposure to respirable crystalline silica. Cincinnati,
OH, US Department of Health and Human Services, Public Health Service,
Centers for Disease Control and Prevention, National Institute for
Occupational Safety and Health (DHHS (NIOSH) Publication).
Nuyts GD, Van Vlem E, De Vos A, Daelemans RA, Rorive G, Elseviers MM,
Schurgers M, Segaert M, D'Haese PC, De Broe ME (1995) Wegener
granulomatosis is associated to exposure to silicon compounds: a
case-control study. Nephrology dialysis transplantation,
10:1162-1165.
Oberdörster G (1997) Pulmonary carcinogenicity of inhaled particles
and the maximum tolerated dose. Environmental health perspectives,
195 (Suppl. 5):1347-1355.
Oshimura M, Hesterberg TW, Tsutsui T, Barrett JC (1984) Correlation of
asbestos-induced cytogenetic effects with cell transformation of
Syrian hamster embryo cells in culture. Cancer research,
44:5017-5022.
Osorio AM, Thun MJ, Novak RF, Van Cura J, Avner ED (1987) Silica and
glomerulonephritis: case report and review of the literature.
American journal of kidney diseases, 9(3):224-230.
Otsuki T, Sakaguchi H, Tomokuni A, Aikoh T, Matsuki T, Kawakami Y,
Kusaka M, Ueki H, Kita S, Ueki A (1998) Soluble Fas mRNA is dominantly
expressed in cases with silicosis. Immunology, 94:258-262.
Oudyk JD (1995) Review of an extensive ferrous foundry silica sampling
program. Applied occupational and environmental hygiene, 10:331-340.
Pairon JC, Jaurand MC, Kheuang L, Janson X, Brochard P, Bignon J
(1990) Sister chromatid exhanges in human lymphocytes treated with
silica. British journal of industrial medicine, 47:110-115.
Pan G, Takahashi K, Feng Y, Liu L, Liu T, Zhang S, Liu N, Okubo T,
Goldsmith DF (1999) Nested case-control study of esophageal cancer in
relation to occupational exposure to silica and other dusts. American
journal of industrial medicine, 35:272-280.
Parent ME, Siemiatycki J, Fritschi L (1998) Occupational exposures and
gastric cancer. Epidemiology, 9(1):48-55.
Parker JE (1994) Silicosis. In: Rakel RE, ed. Conn's current therapy.
Philadelphia, PA, W.B. Saunders, pp. 207-210.
Parkes WR, ed. (1982) Occupational lung disorders, 2nd ed. London,
Butterworths, pp. 134-174.
Partanen T, Pukkala E, Vainio H, Kurppa K, Koskinen H (1994) Increased
incidence of lung and skin cancer in Finnish silicotic patients.
Journal of occupational medicine, 36(6):616-622.
Peters JM (1986) Silicosis. In: Merchant JA, Boehlecke BA, Taylor G,
Pickett-Harner M, eds. Occupational respiratory diseases.
Cincinnati, OH, US Department of Health and Human Services, Public
Health Service, Centers for Disease Control, National Institute for
Occupational Safety and Health, pp. 219-237 (DHHS (NIOSH) Publication
No. 86-102).
Petersen PE, Henmar P (1988) Oral conditions among workers in the
Danish granite industry. Scandinavian journal of work, environment
and health, 14:328-331.
Popendorf WJ, Pryor A, Wenk HR (1982) Mineral dust in manual harvest
operations. Annals of the American Conference of Governmental
Industrial Hygienists, 2:101-115.
Pott F, Dungworth DL, Heinrich U, Muhle H, Kamino K, Germann P-G,
Roller M, Rippe RM, Mohr U (1994) Lung tumours in rats after
intratracheal instillation of dusts. Annals of occupational hygiene,
38 (Suppl. 1):357-363.
Pouthier D, Duhoux P, Van Damme B (1991) Pulmonary silicosis and
glomerular nephropathy: Apropos of 1 case. Nephrologie, 12(1):8-11.
Price-Jones MJ, Gubbings G,Chamberlain M (1980) The genetic effects of
crocidolite asbestos; comparison of chromosome abnormalities and
sister-chromatid exchanges. Mutation research, 79:331-336.
Puntoni R, Goldsmith DG, Valerio F, Vercelli M, Bonassi S, Di Giorgio
F, Ceppi M, Stagnaro E, Filiberti R, Santi L, Merlo F (1988) A cohort
study of workers employed in a refractory brick plant. Tumori,
74:27-33.
Rabovsky J (1997) Laboratory studies on silica induced toxicity and
relationship to carcinogenicity. Journal of exposure analysis and
environmental epidemiology, 7(3):267-278.
Rees D, Cronje R, du Toit RSJ (1992) Dust exposure and pneumoconiosis
in a South African pottery. 1. Study objectives and dust exposure.
British journal of industrial medicine, 49:459-464.
Renne RA, Eldridge SR, Lewis TR, Stevens DL (1985) Fibrogenic
potential of intratracheally instilled quartz, ferric oxide, fibrous
glass, and hydrated alumina in hamsters. Toxicologic pathology,
13(4):306-314.
Reuzel PGJ, Bruijntjes JP, Feron VJ, Woutersen RA (1991) Subchronic
inhalation toxicity of amorphous silicas and quartz dust in rats.
Food and chemical toxicology, 29(5):341-354.
Riala R (1988) Dust and quartz exposure of Finnish construction
cleaners. Annals of occupational hygiene, 32(2):215-220.
Rice CH, Harris RL, Checkoway H, Symons MJ (1986) Dose-response
relationships for silicosis from a case-control study of North
Carolina dusty trades workers. In: Goldsmith DF, Winn DM, Shy CM, eds.
Silica, silicosis, and cancer: Controversy in occupational medicine.
New York, NY, Praeger Publishers, pp. 77-86 (Cancer Research
Monographs, Vol. 2).
Rice FL, Stayner LT (1995) Assessment of silicosis risk for
occupational exposure to crystalline silica. Scandinavian journal of
work, environment and health, 21 (Suppl. 2):87-90.
Rispal P, Wen L, De Precigout V, Aparicio M (1991) Nephropathy with
silica in a dental technician. La presse medicale, 20(4):176.
Robbins SL (1974) Pathologic basis of disease. Philadelphia, PA,
W.B. Saunders Co., pp. 239-240.
Rosenman KD, Reilly MJ, Rice C, Hertzberg V, Tseng C-Y, Anderson HA
(1996) Silicosis among foundry workers: implication for the need to
revise the OSHA standard. American journal of epidemiology,
144(9):890-900.
Saffiotti U (1990) Lung cancer induction by silica in rats, but not in
mice and hamsters: species differences in epithelial and granulomatous
reactions. In: Seemayer NH, Hadnagy W, eds. Environmental hygiene II.
New York, NY, Springer-Verlag, pp. 235-238.
Saffiotti U (1992) Lung cancer induction by crystalline silica. In:
D'Amato RD, Slaga TJ, Farland WH, Henry C, eds. Relevance of animal
studies to the evaluation of human cancer risk. New York, NY,
Wiley-Liss, pp. 51-69.
Saffiotti U, Ahmed N (1995) Neoplastic transformation by quartz in the
BALB/3T3/A31-1-1 cell line and the effects of associated minerals.
Teratogenesis, carcinogenesis, and mutagenesis, 15(6):339-356.
Saffiotti U, Daniel LN, Mao Y, Williams AO, Kaighn ME, Ahmed N,
Knapton AD (1993) Biological studies on the carcinogenic mechanisms of
quartz. In: Guthrie GD, Mossman BT, eds. Health effects of mineral
dusts. Washington, DC, Mineralogical Society of America. Reviews in
mineralogy, 28:523-544.
Saffiotti U, Williams O, Lambert N, Daniel N, Kaighn ME, Mao Y, Shi X
(1996) Carcinogenesis by crystalline silica: animal, cellular, and
molecular studies. In: Castranova V, Vallyathan V, Wallace WE, eds.
Silica and silica-induced lung diseases. Boca Raton, FL, CRC Press,
pp. 345-381.
Saita G, Zavaglia O (1951) Kidney function in silicotics.
Medicina del Lavoro, 42(2):41-48.
Salisbury S, Melius J (1982) Harbison-Walker Refractories, Fairfield,
Alabama, Bessemer, Alabama. Cincinnati, OH, National Institute for
Occupational Safety and Health (HHE Report No. HETA-80-086-1191).
Samimi B, Weill H, Ziskind M (1974) Respirable silica dust exposure of
sandblasters and associated workers in steel fabrication yards.
Archives of environmental health, 29:61-66.
Schapira RM, Ghio AJ, Effros RM, Morrisey J, Almagro UA, Dawson CA,
Hacker AD (1995) Hydroxyl radical production and lung injury in the
rat following silica or titanium dioxide instillation in vivo.
American journal of respiratory cell and molecular biology,
12(2):220-226.
Scheuchenzuber WJ, Eskew ML, Zarkower A (1985) Effects of prolonged
inhalation of silica and olivine dusts on immune functions in the
mouse. Environmental research, 38(2):389-399.
Schwartz LW, Knight HD, Whittig LD, Malloy RL, Abraham JL,Tyler NK
(1981) Silicate pneumoconiosis and pulmonary fibrosis in horses from
the Monterey-Carmel peninsula. Chest, 80 (Suppl. 1):82-85.
Seaton A (1995) Silicosis. In: Morgan WKC, Seaton A, eds.
Occupational lung diseases, 3rd ed. Philadelphia, PA, W.B. Saunders
Co., pp. 222-267.
Seta JA, Sundin DA, Pedersen DH, eds. (1988) National occupational
exposure survey field guidelines. Vol. 1. Cincinnati, OH, US
Department of Health and Human Services, Public Health Service,
Centers for Disease Control, National Institute for Occupational
Safety and Health, Division of Surveillance, Hazard Evaluations, and
Field Studies (DHHS (NIOSH) Publication No. 88-106).
Sherson D, Jorgensen F (1989) Rapidly progressive crescenteric
glomerulonephritis in a sandblaster with silicosis. British journal
of industrial medicine, 46:675-676.
Sherson D, Lander F (1990) Morbidity of pulmonary tuberculosis among
silicotic and nonsilicotic foundry workers in Denmark. Journal of
occupational medicine, 32(2):110-113.
Shi X, Mao Y, Daniel LN, Saffiotti U, Dalal NS, Vallyathan V (1994)
Silica radical-induced DNA damage and lipid peroxidation.
Environmental health perspectives, 102 (Suppl. 10):149-154.
Siltanen E, Koponen M, Kokko A, Engström B, Reponen J (1976) Dust
exposure in Finnish foundries. Scandinavian journal of work,
environment and health, 2 (Suppl. 1):19-31.
Silverstein M, Maizlish N, Park R, Silverstein B, Brodsky L, Mirer F
(1986) Mortality among ferrous foundry workers. American journal of
industrial medicine, 10(1):27-43.
Slavin RE, Swedo JL, Brandes D, Gonzalez-Vitale JC, Osornio-Vargas A
(1985) Extrapulmonary silicosis: a clinical, morphologic, and
ultrastructural study. Human pathology, 16(4):393-412.
Sluis-Cremer GK, Hessel PA, Hnizdo E, Churchill AR, Zeiss EA (1985)
Silica, silicosis, and progressive systemic sclerosis. British
journal of industrial medicine, 42:838-843.
Sluis-Cremer GK, Hessel PA, Hnizdo E, Churchill AR (1986) Relationship
between silicosis and rheumatoid arthritis. Thorax, 41:596-601.
Smith AH, Lopipero PA, Barroga VR (1995) Meta-analysis of studies of
lung cancer among silicotics. Epidemiology, 6(6):617-624.
Sobti RC, Bhardwaj DK (1991) Cytogenetic damage and occupational
exposure: I. Exposure to stone dust. Environmental research,
56:25-30.
Soutar CA, Robertson A, Miller BG, Searl A (1997) Epidemiological
evidence on the carcinogenicity of silica: factors in scientific
judgement. Edinburgh, Institute of Occupational Medicine, 34 pp.
(IOM Report TM/97/09).
Spiethoff A, Wesch H, Wegener K, Klimisch HJ (1992) The effects of
Thorotrast and quartz on the induction of lung tumors in rats.
Health physics, 63(1):101-110.
Steenland K, Beaumont J (1986) A proportionate mortality study of
granite cutters. American journal of industrial medicine, 9:189-201.
Steenland K, Brown D (1995a) Silicosis among gold miners:
exposure-response analyses and risk assessment. American journal of
public health, 85(10):1372-1377.
Steenland K, Brown D (1995b) Mortality study of gold miners exposed to
silica and nonasbestiform amphibole minerals: an update with 14 more
years of follow-up. American journal of industrial medicine,
27:217-229.
Steenland K, Goldsmith DF (1995) Silica exposure and autoimmune
diseases. American journal of industrial medicine, 28:603-608.
Steenland K, Stayner L (1997) Silica, asbestos, man-made mineral
fibers, and cancer. Cancer causes and control, 8:491-503.
Steenland K, Nowlin S, Ryan B, Adams S (1992) Use of multiple-cause
mortality data in epidemiologic analyses: US rate and proportion files
developed by the National Institute for Occupational Safety and Health
and the National Cancer Institute. American journal of epidemiology,
136(7):855-862.
Stopford CM, Stopford W (1995) Respirable quartz content of farm
soils. Applied occupational and environmental hygiene,
10(3):196-199.
Sweeney TD, Brain JD (1996) Lavagable biomarkers of exposure to
fibrogenic dusts. In: Castranova V, Vallyathan V, Wallace WE, eds.
Silica and silica-induced lung diseases. Boca Raton, FL, CRC Press,
pp. 197-207.
Tokumaru Y, Hirayama K, Kita K, Kawamura M, Katayama K (1990) Two
cases of ataxic sensory neuropathy associated with silicosis.
Clinical neurology, 30:933-938.
Tsuda T, Babazono A, Yamamoto E, Mino Y, Matsuoka H (1997) A
meta-analysis on the relationship between pneumoconiosis and lung
cancer. Journal of occupational health, 39:285-294.
Tucker DM, Reger RB, Morgan WKC (1995) Effects of silica exposure
among railroad workers. Applied occupational and environmental
hygiene, 10(12):1080-1085.
US Department of the Interior (1992) Special publication: Crystalline
silica primer. Washington, DC, US Department of the Interior, Bureau
of Mines.
US EPA (1994) Methods for derivation of inhalation reference
concentrations and application of inhalation dosimetry, Section 4.3.5
Dosimetric adjustments for particle exposures. Research Triangle
Park, NC, US Environmental Protection Agency, Office of Health and
Environmental Assessment, Office of Research and Development
(Publication No. EPA/600/8-90/066F).
US EPA (1996) Ambient levels and noncancer health effects of inhaled
crystalline and amorphous silica: health issue assessment.
Washington, DC, US Environmental Protection Agency, Office of
Research and Development (Publication No. EPA/600/R-95/115; National
Technical Information Service Publication No. PB97-188122).
Vallyathan V, Castranova V, Pack D, Leonard S, Shumaker J, Hubbs AF,
Shoemaker DA, Ramsey DM, Pretty JR, McLaurin JL, Khan A, Teass A
(1995) Freshly fractured quartz inhalation leads to enhanced lung
injury and inflammation: Potential role of free radicals. American
journal of respiratory and critical care medicine, 152(3):1003-1009.
Vanchugova NN, Frash VN, Kogan FM (1985) The use of a micronucleus
test as a short-term method in detecting potential blastomogenicity of
asbestos-containing and other mineral fibers. Gigiena Truda i
Professional'nye Zabolevaniya, 6:45-48.
Verma DK, Sebestyen A, Julian JA, Muir DCF, Schmidt H, Bernholz CD,
Shannon HS (1989) Silica exposure and silicosis among Ontario hardrock
miners: II. Exposure estimates. American journal of industrial
medicine, 16:13-28.
Virta RL (1993) Crystalline silica: what it is -- and isn't.
Minerals today, October: 12-16.
Wagner GR (1995) The inexcusable persistence of silicosis [editorial].
American journal of public health, 85(10):1346-1347.
Wagner JC (1970) The pathogenesis of tumors following the intrapleural
injection of asbestos and silica. In: Nettesheim P, Hanna MG Jr,
Deatherage JW Jr, eds. Morphology of experimental respiratory
carcinogenesis. Oak Ridge, TN, US Atomic Energy Commission, pp.
347-358.
Wagner JC, Berry G (1969) Mesotheliomas in rats following inoculation
with asbestos. British journal of cancer, 23:567-581.
Wagner MMF (1976) Pathogenesis of malignant histiocytic lymphoma
induced by silica in a colony of specific-pathogen-free Wistar rats.
Journal of the National Cancer Institute, 57:509-518.
Wagner MMF, Wagner JC (1972) Lymphomas in the Wistar rat after
intrapleural inoculation of silica. Journal of the National Cancer
Institute, 49:81-91.
Wagner MMF, Wagner JC, Davies R, Griffiths DM (1980) Silica-induced
malignant histiocytic lymphoma: incidence linked with strain of rat
and type of silica. British journal of cancer, 41:908-917.
Wang H, Leigh J, Bonin A, Peters M (1997a) Silica-induced
morphological change similar to apoptosis in bronchoalveolar lavage
cells and granulomatous cells. Annals of occupational hygiene, 41
(Suppl. 1):459-464.
Wang H, Leigh J, Bonin A, Peters M (1997b) Silica induced micronuclei
in pulmonary alveolar macrophages in vivo. Annals of occupational
hygiene, 41 (Suppl. 1):434-439.
Warheit DB, Hartsky MA (1997) Initiating the risk assessment process
for inhaled particulate materials: development of short term
inhalation bioassays. Journal of exposure analysis and environmental
epidemiology, 7(3): 313-325.
Watts WF, Parker DR (1995) Quartz exposure trends in metal and
nonmetal mining. Applied occupational and environmental hygiene,
10(12):1009-1018.
Weill H, McDonald JC (1996) Exposure to crystalline silica and risk of
lung cancer: the epidemiological evidence. Thorax, 51:97-102.
Weill H, Jones RN, Parkes WR (1994) Silicosis and related diseases.
In: Parkes WR, ed. Occupational lung disorders, 3rd ed. London,
Butterworth-Heinemann, pp. 285-339.
Weissman DN, Ma JKH, Rojanasakul Y, Hubbs AF (1996) Immune dysfunction
in silicosis: a hypothesis. Applied occupational and environmental
hygiene, 11(7):962-965.
Westerholm P, Ahlmark A, Maasing R, Segelberg I (1986) Silicosis and
risk of lung cancer or lung tuberculosis: a cohort study.
Environmental research, 41:339-350.
WHO (1986) Recommended health-based limits in occupational exposure
to selected mineral dusts (silica, coal): report of a WHO study
group. Geneva, World Health Organization, 80 pp. (WHO Technical
Report Series 734).
WHO (1993) "WHO calls for medical surveillance of workers exposed to
mineral dusts." Geneva, World Health Organization (Press Release
WHO/73, 23 September 1993).
WHO (1996) Groups at risk: WHO report on the tuberculosis epidemic
1996. Geneva, World Health Organization.
Wiessner JH, Henderson JD Jr, Sohnle PG, Mandel NS, Mandel GS (1988)
The effect of crystal structure on mouse lung inflammation and
fibrosis. American review of respiratory disease, 138:445-450.
Wiles FJ, Faure MH (1977) Chronic obstructive lung disease in gold
miners. In: Walton WH, ed. Inhaled particles IV. Part 2. Oxford,
Pergamon Press, pp. 727-735.
Wilke RA, Salisbury S, Abdel-Rahman E, Brazy PC (1996) Lupus-like
autoimmune disease associated with silicosis. Nephrology dialysis and
transplantation, 11:1835-1838.
Williams AO, Knapton AD, Ifon ET, Saffiotti U (1996) Transforming
growth factor beta expression and transformation of rat lung
epithelial cells by crystalline silica (quartz). International
journal of cancer, 65:639-649.
Wilson T, Scheuchenzuber WJ, Eskew ML, Zarkower A (1986) Comparative
pathological aspects of chronic olivine and silica inhalation in mice.
Environmental research, 39(2):331-344.
Xu XZ, Cia XG, Men XS, Yang PY, Yang JF, Jing SL, He JH, Si WY (1993)
A study of siliceous pneumoconiosis in a desert area of Sunan county,
Gansu province, China. Biomedical and environmental sciences,
6:217-222.
Xu Z, Pan G-W, Liu L-M, Brown LM, Gaun D-X, Xiu Q, Sheng J-H, Stone
BJ, Dosemeci M, Fraumeni JF Jr, Blot WJ (1996) Cancer risks among iron
and steel workers in Anshan, China, Part I: proportional mortality
ratio analysis. American journal of industrial medicine, 30:1-6.
Yamano Y, Kagawa J, Hanaoka T, Takahashi T, Kasai H, Tsugane S,
Watanabe S (1995) Oxidative DNA damage induced by silica in vivo.
Environmental research, 69(2):102-107.
Zheng W, Shu XO, Ji BT, Gao YT (1996) Diet and other risk factors for
cancer of the salivary glands: a population-based case-control study.
International journal of cancer, 67:194-198.
Zhong B, Whong W, Ong T (1997) Detection of mineral-dust-induced DNA
damage in two mammalian cell lines using the alkaline single cell
gel/comet assay. Mutation research, 393:181-187.
Ziegler V, Haustein UF (1992) Systemic scleroderma -- a silica induced
occupational disease? Epidemiological, clinical, immunological,
mineralogical, animal and cell culture investigations.
Dermatologische Monatschrift, 178:34-43.
Ziskind M, Jones RN, Weill H (1976) Silicosis. American review of
respiratory disease, 113:643-665.
APPENDIX 1 -- SOURCE DOCUMENTS
IARC (1997)
Copies of Silica, some silicates, coal dust and
para -aramid fibrils (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Vol. 68) may be obtained from:
International Agency for Research on Cancer
150 cours Albert Thomas
69372 Lyon Cedex 08
France
The members of the Working Group on the Evaluation of
Carcinogenic Risks to Humans of silica (including quartz), some
silicates, coal dust and para-aramid fibrils that met in Lyon on
15-22 February 1996 were:
M.D. Attfield, National Institute for Occupational Safety and
Health, USA
P.J. Borm, University of Limburg, The Netherlands
H. Checkoway, University of Washington, USA
K. Donaldson, Napier University, United Kingdom
M. Dosemeci, National Cancer Institute, USA
V.J. Feron, TNO Nutrition and Food Research Institute, The
Netherlands
B.J. Fubini, University of Torino, Italy
M. Gérin, Université de Montréal, Canada
E. Hnizdo, National Centre for Occupational Health, South Africa
A.B. Kane, Brown University, USA
J.C. McDonald, Imperial College, United Kingdom
H. Muhle, Fraunhofer Institute for Toxicology and Aerosol
Research, Germany
S. Olin, International Life Sciences Institute, USA
J.-C. Pairon, INSERM, France
T. Partanen, Institute of Occupational Health, Finland
C. Shy, University of North Carolina, USA
E. Tatrai, National Institute of Occupational Health, Hungary
D.B. Warheit, DuPont Haskell Laboratory for Toxicology and
Industrial Medicine, USA
V. Yermilov, N.N. Petrov Research Institute of Oncology, Russia
US EPA (1996)
Copies of the US Environmental Protection Agency report entitled
Ambient levels and noncancer health effects of inhaled crystalline
and amorphous silica: health issue assessment (NTIS Publication
PB97-188122) may be obtained from:
National Technical Information Service
Springfield, VA 22161
USA
The document was reviewed in accordance with US EPA policy and
approved for publication. The principal authors of the document were:
Dr R.A. Faust, Oak Ridge National Laboratory, USA
Dr J.S. Gift, National Center for Environmental Assessment, USA
Dr D.F. Goldsmith, Western Consortium for Public Health, USA
Dr R. Ruble, Western Consortium for Public Health, USA
The reviewers and contributors from the US EPA were:
Dr D.L. Costa, National Health and Environmental Effects Research
Laboratory (MD-82), USA
Dr J.S. Dollarhide, National Center for Environmental Assessment
Dr J.A. Graham, National Center for Environmental Assessment
Dr A. Koppikar, National Center for Environmental Assessment
Dr W.E. Pepelko, National Center for Environmental Assessment
Dr C. Shoaf, National Center for Environmental Assessment
Mr E.G. Smith, Office of Air Quality Planning and Standards
Dr W. Victery, Region IX
The external peer reviewers of the document were:
Dr D. Gardner, Private Consultant, Raleigh, NC, USA
Ms S. Goodwin, University of Maryland, Baltimore, MD, USA
Dr M. Jacobsen, National Institute for Occupational Safety and
Health, USA
Dr R. Kutzman, MITRE Corporation, McLean, VA, USA
Dr J. Rabovsky, California Environmental Protection Agency, USA
Ms F. Rice, National Institute for Occupational Safety and
Health, USA
Dr J.M. Samet, The Johns Hopkins University, USA
Dr C. Shy, University of North Carolina, USA
Dr J. Vincent, University of Minnesota, USA
Dr J. Watson, Desert Research Institute, USA
Dr H. Weill, Tulane University, USA
NIOSH (forthcoming)
When published, copies of the NIOSH special hazard review:
Health effects of occupational exposure to respirable crystalline
silica will be available from:
National Institute for Occupational Safety and Health
Publications Dissemination
4676 Columbia Parkway
Cincinnati, OH 45226-1998
USA
The draft document has undergone the following NIOSH internal
reviews in accordance with NIOSH policy: intradivisional review and
interdivisional review. The external peer reviewers of the document
were:
W. Beckett, University of Rochester School of Medicine
H. Checkoway, University of Washington
G.S. Davis, University of Vermont College of Medicine
J. Gift, US EPA
D. Goldsmith, George Washington University
E. Hnizdo, National Centre for Occupational Health, South Africa
J. Hughes, Tulane School of Public Health and Tropical Medicine
C. Jones, Mine Safety and Health Administration, US Department of
Labor
W. Kojola, American Federation of Labor and Congress of
Industrial Organizations
A.G. Macneski, Environmental Safety and Health, Bechtel National,
Inc.
M. Schaper, Mine Safety and Health Administration, US Department
of Labor
L. Schuman, Occupational Safety and Health Administration, US
Department of Labor
J. Sharpe, National Stone Association
D.M. Tucker, Norfolk Southern Corporation
J.A. Ulizio, US Silica Company
J.L. Weeks, George Washington University Medical Center
APPENDIX 2 -- CICAD PEER REVIEW
The draft CICAD on crystalline silica, quartz was sent for review
to institutions and organizations identified by IPCS after contact
with IPCS national Contact Points and Participating Institutions, as
well as to identified experts. Comments were received from:
M. Baril, Institut de Recherche en Santé et Sécurité du Travail
du Québec, Montreal, Quebec, Canada
R. Benson, Drinking Water Program, US Environmental Protection
Agency, Denver, CO, USA
T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
P. Borm, Medical Institute for Environmental Hygiene, Department
of Fibre and Particle Toxicology, Dusseldorf University,
Düsseldorf, Germany
R. Brown, Toxservices, Uppingham, United Kingdom
R.S. Chhabra, National Institute of Environmental Health
Sciences/National Institute of Health, Research Triangle Park,
NC, USA
S. Dobson, Institute of Terrestrial Ecology, Cambridgeshire,
United Kingdom
K. Donaldson, Department of Biological Sciences, Napier
University, Edinburgh, Scotland
J. Heuer, Federal Institute for Health Protection of Consumers
and Veterinary Medicine, Berlin, Germany
J. Hurych, National Institute of Public Health, Prague, Czech
Republic
J. Leigh, National Occupational Health and Safety Commission,
Sydney, Australia
M. Moore, The Morgan Crucible Company, Windsor, United Kingdom
F. Morrall, British Ceramic Confederation, Stoke-on-Trent, United
Kingdom
D. Muir, Occupational Health Program, McMaster University,
Hamilton, Ontario, Canada
M. Wyart-Remy, Industrial Minerals Association - Europe,
Brussels, Belgium
P. Yao, Institute of Occupational Medicine, Chinese Academy of
Preventive Medicine, Beijing, People's Republic of China
J. Yoshida, Chemical Safety Management, Office of Environmental
Chemical Safety, Environmental Health Bureau, Ministry of Health
and Welfare, Tokyo, Japan
K. Ziegler-Skylakakis, Beratergremium für Umweltrelevante
Altstoffe, Muenchen, Germany
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
Sydney, Australia, 21-24 November 1999
Members
Dr R. Benson, Drinking Water Program, US Environmental Protection
Agency, Region VIII, Denver, CO, USA
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
Dr R.M. Bruce, National Center for Environmental Assessment, US
Environmental Protection Agency, Cincinnati, OH, USA
Mr R. Cary, Health and Safety Executive, Merseyside, United Kingdom
Dr R.S. Chhabra, National Institute of Environmental Health Sciences,
National Institutes of Health, Research Triangle Park, NC, USA
Dr S. Chou, Agency for Toxic Substances and Disease Registry, Atlanta,
GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood,
Cambridgeshire, United Kingdom
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers
and Veterinary Medicine, Berlin, Germany
Dr J. Kielhorn, Fraunhofer Institute for Toxicology and Aerosol
Research, Hannover, Germany
Dr S. Kristensen, National Occupational Health and Safety Commission
(Worksafe), Sydney, NSW, Australia
Mr C. Lee-Steere, Environment Australia, Canberra, ACT, Australia
Ms M. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada
Ms F. Rice, National Institute for Occupational Safety and Health,
Cincinnati, OH, USA
Dr J. Sekizawa, National Institute of Health Sciences, Tokyo, Japan
Dr D. Willcocks, National Industrial Chemicals Notification and
Assessment Scheme (NICNAS), Sydney, NSW, Australia (Chairperson)
Professor P. Yao, Institute of Occupational Medicine, Chinese Academy
of Preventive Medicine, Beijing, People's Republic of China
Observers
Mr P. Howe, Institute of Terrestrial Ecology, Huntingdon,
Cambridgeshire, United Kingdom
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit, GmbH, Oberschleissheim, Germany
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Ms M. Godden, Health and Safety Executive, Bootle, Merseyside, United
Kingdom
Dr M. Younes, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
APPENDIX 4 -- INTERNATIONAL CHEMICAL SAFETY CARD
QUARTZ ICSC: 0808
October 1997
CAS# 