
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organization, or the World Health Organization.
Concise International Chemical Assessment Document 53
First draft prepared by Dr C.-H. Selene J. Chou, Agency for Toxic Substances and Disease Registry, Atlanta, Georgia, USA
Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 2003
The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals.
The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and Agriculture Organization of the United Nations, WHO, the United Nations Industrial Development Organization, the United Nations Institute for Training and Research, and the Organisation for Economic Co-operation and Development (Participating Organizations), following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase coordination in the field of chemical safety. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment.
WHO Library Cataloguing-in-Publication Data
Hydrogen sulfide : Human health aspects.
(Concise international chemical assessment document ; 53)
1.Hydrogen sulfide - adverse effects 2.Hydrogen sulfide - toxicity 3.Risk assessment 4.Environmental exposure I.International Programme on Chemical Safety II.Series
ISBN 92 4 153053 7 (LC/NLM Classification: QV 662)
ISSN 1020-6167
©World Health Organization 2003
All rights reserved. Publications of the World Health Organization can be obtained from Marketing and Dissemination, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 2476; fax: +41 22 791 4857; email: ). Requests for permission to reproduce or translate WHO publications — whether for sale or for noncommercial distribution — should be addressed to Publications, at the above address (fax: +41 22 791 4806; email: permissions@who.int).
The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement.
The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters.
The World Health Organization does not warrant that the information contained in this publication is complete and correct and shall not be liable for any damages incurred as a result of its use.
The Federal Ministry for the Environment, Nature Conservation and Nuclear Safety, Germany, provided financial support for the printing of this publication.
Concise International Chemical Assessment Documents (CICADs) are the latest in a family of publications from the International Programme on Chemical Safety (IPCS) — a cooperative programme of the World Health Organization (WHO), the International Labour Organization (ILO), and the United Nations Environment Programme (UNEP). CICADs join the Environmental Health Criteria documents (EHCs) as authoritative documents on the risk assessment of chemicals.
International Chemical Safety Cards on the relevant chemical(s) are attached at the end of the CICAD, to provide the reader with concise information on the protection of human health and on emergency action. They are produced in a separate peer-reviewed procedure at IPCS. They may be complemented by information from IPCS Poison Information Monographs (PIM), similarly produced separately from the CICAD process.
CICADs are concise documents that provide summaries of the relevant scientific information concerning the potential effects of chemicals upon human health and/or the environment. They are based on selected national or regional evaluation documents or on existing EHCs. Before acceptance for publication as CICADs by IPCS, these documents undergo extensive peer review by internationally selected experts to ensure their completeness, accuracy in the way in which the original data are represented, and the validity of the conclusions drawn.
The primary objective of CICADs is characterization of hazard and dose–response from exposure to a chemical. CICADs are not a summary of all available data on a particular chemical; rather, they include only that information considered critical for characterization of the risk posed by the chemical. The critical studies are, however, presented in sufficient detail to support the conclusions drawn. For additional information, the reader should consult the identified source documents upon which the CICAD has been based.
Risks to human health and the environment will vary considerably depending upon the type and extent of exposure. Responsible authorities are strongly encouraged to characterize risk on the basis of locally measured or predicted exposure scenarios. To assist the reader, examples of exposure estimation and risk characterization are provided in CICADs, whenever possible. These examples cannot be considered as representing all possible exposure situations, but are provided as guidance only. The reader is referred to EHC 170.fn1
While every effort is made to ensure that CICADs represent the current status of knowledge, new information is being developed constantly. Unless otherwise stated, CICADs are based on a search of the scientific literature to the date shown in the executive summary. In the event that a reader becomes aware of new information that would change the conclusions drawn in a CICAD, the reader is requested to contact IPCS to inform it of the new information.
Procedures
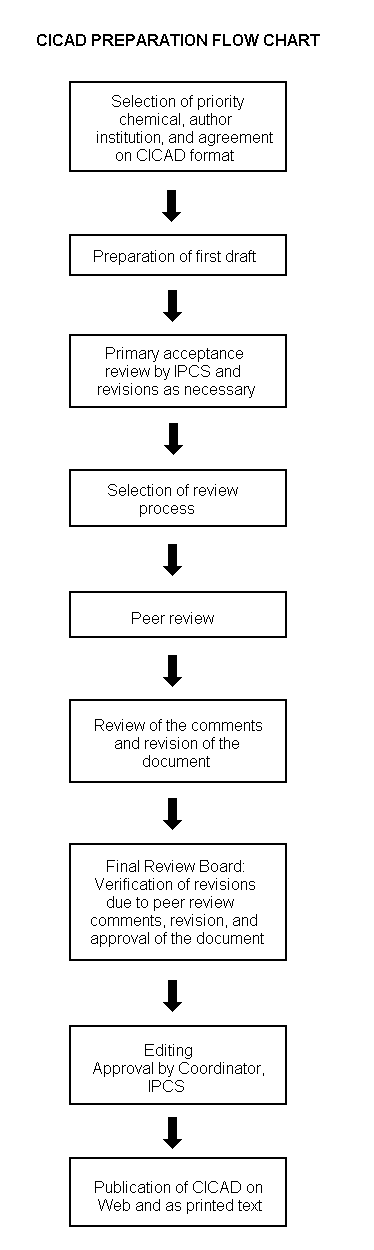
The flow chart on page 2 shows the procedures followed to produce a CICAD. These procedures are designed to take advantage of the expertise that exists around the world — expertise that is required to produce the high-quality evaluations of toxicological, exposure, and other data that are necessary for assessing risks to human health and/or the environment. The IPCS Risk Assessment Steering Group advises the Coordinator, IPCS, on the selection of chemicals for an IPCS risk assessment based on the following criteria:
Thus, it is typical of a priority chemical that
The Steering Group will also advise IPCS on the appropriate form of the document (i.e., EHC or CICAD) and which institution bears the responsibility of the document production, as well as on the type and extent of the international peer review.
The first draft is based on an existing national, regional, or international review. Authors of the first draft are usually, but not necessarily, from the institution that developed the original review. A standard outline has been developed to encourage consistency in form. The first draft undergoes primary review by IPCS to ensure that it meets the specified criteria for CICADs.

|
Advice from Risk Assessment Steering Group Criteria of priority:
Thus, it is typical of a priority chemical that
Special emphasis is placed on avoiding duplication of effort by WHO and other international organizations. A prerequisite of the production of a CICAD is the availability of a recent high-quality national/regional risk assessment document = source document. The source document and the CICAD may be produced in parallel. If the source document does not contain an environmental section, this may be produced de novo, provided it is not controversial. If no source document is available, IPCS may produce a de novo risk assessment document if the cost is justified. Depending on the complexity and extent of controversy of the issues involved, the steering group may advise on different levels of peer review:
|
The second stage involves international peer review by scientists known for their particular expertise and by scientists selected from an international roster compiled by IPCS through recommendations from IPCS national Contact Points and from IPCS Participating Institutions. Adequate time is allowed for the selected experts to undertake a thorough review. Authors are required to take reviewers’ comments into account and revise their draft, if necessary. The resulting second draft is submitted to a Final Review Board together with the reviewers’ comments. At any stage in the international review process, a consultative group may be necessary to address specific areas of the science.
The CICAD Final Review Board has several important functions:
Board members serve in their personal capacity, not as representatives of any organization, government, or industry. They are selected because of their expertise in human and environmental toxicology or because of their experience in the regulation of chemicals. Boards are chosen according to the range of expertise required for a meeting and the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who participate in the preparation of a CICAD are required to declare any real or potential conflict of interest in relation to the subjects under discussion at any stage of the process. Representatives of nongovernmental organizations may be invited to observe the proceedings of the Final Review Board. Observers may participate in Board discussions only at the invitation of the Chairperson, and they may not participate in the final decision-making process.
This CICAD on hydrogen sulfide was prepared by the US Agency for Toxic Substances and Disease Registry. Data identified as of 1998 were considered in the source document (ATSDR, 1999). A comprehensive literature search of several on-line databases was conducted in March 2002 to identify any references published subsequent to those incorporated in the source document. Information on the nature of the peer review and the availability of the source document is presented in Appendix 1. Information on the peer review of this CICAD is presented in Appendix 2. This CICAD was approved as an international assessment at a meeting of the Final Review Board, held in Monks Wood, United Kingdom, on 16–19 September 2002. Participants at the Final Review Board meeting are listed in Appendix 3. The International Chemical Safety Card (ICSC 0165) for hydrogen sulfide, produced by the International Programme on Chemical Safety (IPCS, 2000), has also been reproduced in this document.
Hydrogen sulfide (CAS No.
Hydrogen sulfide may be produced by a variety of commercial methods. The principal source of hydrogen sulfide is recovery as a by-product in the purification of natural and refinery gases. It is also a by-product of kraft pulp and paper manufacturing and carbon disulfide production. It is used as an intermediate in the manufacture of sulfuric acid and inorganic sulfides and as an agricultural disinfectant. Hydrogen sulfide is also produced as a decomposition product of xanthates (used in the mining industry) when they come into contact with water.
Accidental release or improper disposal of materials resulting from these processes may result in hydrogen sulfide emissions. Releases to the environment are primarily in emissions to the ambient air, where the chemical is likely to remain for less than 1 day, but may persist for as long as 42 days in winter. Hydrogen sulfide may evaporate easily from water, depending on temperature and pH. It is unlikely to bioconcentrate and biomagnify in the food-chain.
The concentration of hydrogen sulfide in air in unpolluted areas is very low, between 0.03 and 0.1 µg/m3.
Humans may be exposed to hydrogen sulfide from endogenous production and from exogenous sources. Most endogenous production results from the metabolism of sulfhydryl-containing amino acids (e.g., cysteine) by bacteria present in both the intestinal tract and the mouth. Hydrogen sulfide is also produced in the brain and several smooth muscles (e.g., thoracic aorta, ileum, and portal vein) by enzymes found in these tissues. In the rat, the endogenous level of hydrogen sulfide is 50–160 µmol/litre in the brain and 1 mmol/litre in the ileum.
Human exposure to exogenous hydrogen sulfide is principally via inhalation, and the gas is rapidly absorbed through the lungs. Hydrogen sulfide is metabolized through three pathways: oxidation, methylation, and reactions with metalloproteins or disulfide-containing proteins. Oxidation in the liver is the major detoxification pathway. The major oxidation product is thiosulfate, which is then converted to sulfate and excreted in the urine. The methylation pathway also serves as a detoxification route. The toxicity of hydrogen sulfide is a result of its reaction with metalloenzymes. In the mitochondria, cytochrome oxidase, the final enzyme in the respiratory chain, is inhibited by hydrogen sulfide; this disrupts the electron transport chain and impairs oxidative metabolism. Nervous and cardiac tissues, which have the highest oxygen demand, are especially sensitive to the disruption of oxidative metabolism. In the central nervous system, this effect may result in death from respiratory arrest.
In experimental animals, single inhalation exposures to hydrogen sulfide result in death and respiratory, immunological/lymphoreticular, cardiovascular, and neurological effects. Reported health effects in animals following short-term exposures include ocular, cardiovascular, neurological, metabolic, hepatic, and developmental effects. Medium-duration inhalation studies of hydrogen sulfide in animals have reported respiratory, neurological, and olfactory effects. There are no long-term inhalation studies in animals. The most sensitive target organ for medium-term exposure in animals is the nasal olfactory mucosa. Nasal olfactory lesions were reported in Sprague-Dawley CD rats exposed to hydrogen sulfide at 42 or 110 mg/m3; the no-observed-adverse-effect level (NOAEL) was 14 mg/m3. This NOAEL is used as a basis for the development of a medium-term tolerable concentration.
Most human data are derived from acute poisoning case reports, occupational exposures, and limited community studies. The odour threshold varies depending on the individual; the geometric mean odour threshold is 11 µg/m3. At concentrations greater than 140 mg/m3, olfactory paralysis occurs, making hydrogen sulfide very dangerous, because a few breaths at 700 mg/m3 can be fatal. Short-term inhalation exposure to high concentrations of hydrogen sulfide causes health effects in many systems; reported health effects in humans following exposure to hydrogen sulfide include death and respiratory, ocular, neurological, cardiovascular, metabolic, and reproductive effects. Respiratory, neurological, and ocular effects are the most sensitive end-points in humans following inhalation exposure. The lowest-observed-adverse-effect level (LOAEL) is 2.8 mg/m3 in asthmatic individuals for respiratory and neurological effects. This LOAEL is used as a basis for the development of a short-term tolerable concentration.
Ingestion is of no relevance for humans. There are no human ingestion data.
The genotoxicity of hydrogen sulfide has been inadequately investigated; there is only a single negative Salmonella mutagenicity test. It is not possible to evaluate the carcinogenic potential of hydrogen sulfide, as long-term animal studies are missing and studies on human populations are inadequate.
Tolerable concentrations for hydrogen sulfide in air of 100 µg/m3 and 20 µg/m3, respectively, have been derived based on respiratory effects for short-term (for exposure durations of 1–14 days) and medium-term (for exposure durations of up to 90 days) inhalation exposures.
Environmental exposures to malodorous emissions are usually to a mixture of sulfur-containing gases. The exact concentration of hydrogen sulfide in these types of mixtures cannot be determined. In estimating exposure, there is also uncertainty about the dose and duration of exposure. Based on limited information, rodents appear to be less sensitive to hydrogen sulfide than humans. Since the respiratory tract is the major target organ of hydrogen sulfide toxicity, humans with asthma, the elderly, and young children with compromised respiratory function represent sensitive subpopulations. Due to the serious toxic effects associated with exposures to high concentrations of hydrogen sulfide for very short durations, all exposure should be avoided.
Hydrogen sulfide (H2S; CAS No.
Hydrogen sulfide is a colourless, flammable gas with a characteristic odour of rotten eggs. Hydrogen sulfide’s relative molecular mass is 34.08. Its vapour pressure at 21.9 °C is 1929 Pa. It is soluble in water; the water solubility at 20 °C is 1 g in 242 ml. The taste threshold for hydrogen sulfide in water is between 0.05 and 0.1 mg/litre (WHO, 1993). Hydrogen sulfide is also soluble in alcohol, ether, glycerol, gasoline, kerosene, crude oil, and carbon disulfide. The Henry’s law constant at 20 °C is given as 468 atm/mole fraction in ATSDR (1999). Other physical and chemical properties can be found in the International Chemical Safety Card (ICSC 165), which is reproduced in this document.
The conversion factorsfn2 for hydrogen sulfide in air (20 °C, 101.3 kPa) are as follows:
1 mg/m3 = 0.71 ppm
1 ppm = 1.4 mg/m3
Hydrogen sulfide can be measured in biological samples such as human breath (as expired air), biological tissues, and fluids, including blood and saliva. Commonly used methods include gas chromatography coupled with flame ionization detection (GC/FID), iodometric titration, potentiometry with ion-selective electrodes, spectrophotometry, and high-performance liquid chromatography (HPLC).
The methods most commonly used to measure hydrogen sulfide in environmental samples, such as air, water, sediment, and sludge, include gas chromatography with flame photometric detection (GC/FPD), gas chromatography with electrochemical detection (GC/ECD), iodometric methods, the methylene blue colorimetric or spectrophotometric method, the spot method using paper or tiles impregnated with lead acetate or mercuric chloride, ion chromatography with conductivity, and potentiometric titration with a sulfide ion-selective electrode. Accurate measurements of hydrogen sulfide in water are usually complicated by the presence of other sulfide compounds. A method of determining sulfide concentration in wastewater by first transforming to hydrogen sulfide and then measuring the product using atomic absorption spectroscopy (AAS) has been published (Parvinen & Lajunen, 1994).
Detection limits reported for the analysis of hydrogen sulfide in air (breath) include 10 µg/m3 (GC/FID), 0.2 µg/m3 (spectrophotometry), 1 µg/m3 (iodometric titration), 5–13 µg/m3 (GC/FPD), and 0.7 µg/m3 (spot method using mercuric chloride-impregnated filter paper tape); those for blood include 40 µg/litre (1.2 µmol/litre) (iodometric titration) and 10 µg/litre (0.3 µmol/litre) (GC/ECD). For the analysis of thiosulfate in urine, detection limits of 10 µg/litre (0.3 µmol/litre) (GC/ECD) and 680–1704 µg/litre (20–50 µmol/litre) (HPLC) have been reported (ATSDR, 1999). For occupational measurements of airborne concentrations of hydrogen sulfide, NIOSH (1977) recommended the use of a midget impinger for sampling breathing zone air and the methylene blue/spectrophotometric method; the detection limit was 0.2 µg/m3, and the sampling time was 10 min. The detection limit of OSHA’s (2002) recommended method 141 for workplace air (silver nitrate/differential pulse polarography) is 0.56 mg/m3; the sampling time was 15 min for peak ceiling concentrations and 60 min for time-weighted-average concentrations. The detection limit reported for analysis of hydrogen sulfide in water was 2 × 10–5 µg/litre (0.6 pmol/litre) (GC/FPD) (Radford-Knoery & Cutter, 1993) and 25 µg (AAS) in water and sludge samples (Parvinen & Lajunen, 1994).
Hydrogen sulfide is produced naturally and as a result of human activity. Natural sources account for about 90% of the total hydrogen sulfide in the atmosphere (US EPA, 1993). Hydrogen sulfide is produced naturally through non-specific and anaerobic bacterial reduction of sulfates and sulfur-containing organic compounds (Hill, 1973). It is released primarily as a gas and is found naturally in crude petroleum, natural gas, volcanic gases, and hot springs. Hydrogen sulfide is also found in groundwater (OSU, 2001).
Hydrogen sulfide is emitted from stagnant or polluted waters and manure or coal pits with low oxygen content. Hydrogen sulfide is emitted by some plant species as a by-product of sulfite metabolism (Wilson et al., 1978; Takemoto et al., 1986). Estimates of the terrestrial emission rate of hydrogen sulfide range from 53 to 100 million tonnes of sulfur per year (Hill, 1973). Estimates of the emission rate from oceans range from 27 to 150 million tonnes of sulfur per year (Hill, 1973).
Hydrogen sulfide may be produced by a variety of commercial methods, including reacting dilute sulfuric acid with iron sulfite, heating hydrogen and sulfur into their vapour phase, and heating sulfur with paraffin. The principal source of hydrogen sulfide is recovery as a by-product in the purification of natural and refinery gases (Beauchamp et al., 1984). It is a by-product of kraft pulp and paper manufacturing and of carbon disulfide production. It is used as an intermediate in the manufacture of sulfuric acid and inorganic sulfides (Tyagi et al., 1988; Kauppinen et al., 1997; HSDB, 1998) and as an agricultural disinfectant. Hydrogen sulfide is also produced as a decomposition product of xanthates (used in the mining industry) when they come into contact with water (NICNAS, 1995). Accidental release or improper disposal of materials resulting from these processes may result in hydrogen sulfide emissions. Ambient hydrogen sulfide concentrations in the air near landfills indicate that landfills may be a source as well (HazDat, 1997).
Since hydrogen sulfide exists as a gas at atmospheric pressure, partitioning to the air is likely to occur after environmental releases. However, it is also soluble in oil and water; therefore, it may partition to surface waters, groundwaters, or moist soils and subsequently travel great distances. In addition, sorption of hydrogen sulfide from air onto soils (Cihacek & Bremner, 1993) and plant foliage may occur (De Kok et al., 1983, 1988, 1991).
Hydrogen sulfide may evaporate easily from water, depending on factors such as temperature and pH. In general, low pH and high temperature tend to favour evaporation (HSDB, 1998).
Transport of hydrogen sulfide in water occurs readily in moist soils and aquatic and marine environments because of its solubility. Hydrogen sulfide may also become adsorbed onto clay or organic matter. Several species of soil, aquatic, and marine microorganisms oxidize hydrogen sulfide to elemental sulfur, and its half-life in these environments usually ranges from 1 h to several hours (Jørgensen, 1982). Food-chain bioconcentration and biomagnification are unlikely (HSDB, 1998).
Hydrogen sulfide oxidation by oxygen may readily occur in surface waters. Oxidation by hydrogen peroxide may also occur, primarily in rainwater and marine aerosols, where concentrations of hydrogen peroxide are relatively high (Millero et al., 1989). Hydrogen sulfide in wastewater may be controlled by addition of oxidizing chemicals, which react to form harmless by-products (Tomar & Abdullah, 1994). In warm, damp environments such as manholes and gravity sewers, hydrogen sulfide may be oxidized by autotrophic bacteria to sulfuric acid (Boon, 1992).
Ionization of hydrogen sulfide in water may occur, depending primarily upon pH. The predominant chemical form under typical environmental conditions is hydrogen sulfide, although the sulfhydryl anion (SH–) becomes more abundant with increasing pH (Hill, 1973).
Hydrogen sulfide in the air is oxidized by molecular oxygen and hydroxyl radicals, forming the sulfhydryl radical and ultimately sulfur dioxide or sulfate compounds (Hill, 1973; NSF, 1976). Sulfur dioxide and sulfates are eventually removed from the atmosphere through absorption by plants and soils or through precipitation. The atmospheric residence time of hydrogen sulfide is typically less than 1 day (Hill, 1973), but may be as high as 42 days in winter (Bottenheim & Strausz, 1980).
Soils may adsorb considerable amounts of hydrogen sulfide from the air, retaining most of it in the form of elemental sulfur (Cihacek & Bremner, 1993). A number of microorganisms have been found to degrade hydrogen sulfide to elemental sulfur or sulfate. Among these are a heterotrophic bacterium isolated from dimethyldisulfide-acclimated peat (Cho et al., 1992), heterotrophic fungi (Phae & Shoda, 1991), and the marine isopod Saduria (Mesidotea) entomon (Vismann, 1991).
Concentrations of hydrogen sulfide in ambient air as a result of natural sources have been estimated to be between 0.14 and 0.4 µg/m3 (US EPA, 1993). In an unpolluted area of Colorado, USA, concentrations between 0.03 and 0.1 µg/m3 were measured (Hill, 1973). Near ground level, samples taken around a sulfurous New Zealand lake charged by an active underground geothermal vent had hydrogen sulfide levels of 175–5500 µg/m3 (Siegel et al., 1986).
Air monitored using lead acetate tape at a wastewater treatment plant in Australia had time-averaged hydrogen sulfide levels of 1.4–2.8 mg/m3 near the primary clarifiers and inlet structure and levels below 1.4 mg/m3 at various other locations in the 10-ha plant site (Koe, 1985). However, the method used is generally semiquantitative and subject to many interferences. Thus, the levels of 1.4–2.8 mg/m3 may be inaccurate. Landfills are also a common source of ambient hydrogen sulfide. Hydrogen sulfide levels in air on some US National Priorities List sites ranged from 1.3 to 1130 mg/m3 (HazDat, 1997).
Hydrogen sulfide readily evaporates from surface waters and is not likely to persist in highly oxygenated waters; levels in these environments are expected to be low. Groundwater samples from an area receiving acid mine drainage in Colorado, USA, averaged 0.9 mg hydrogen sulfide/litre, while samples from a power plant site averaged 0.03 mg/litre (Patterson & Runnells, 1992).
In wastewater, concentrations of hydrogen sulfide (as sulfide sulfur) ranging from 3.1 to 5.1 mg/litre were reported (Parvinen & Lajunen, 1994). Total sulfide levels in samples from the Mississippi River, USA, were about 0.92 mg/litre, while levels in pond and well water in St. Paul, Minnesota, USA, were 1.6 and 1.9 mg/litre, respectively (Slooff et al., 1991).
Levels as high as 11.7 mg/litre in soil solution were measured in Louisiana, USA, rice fields (Hollis, 1985). The hydrogen sulfide in these samples was presumably bound to colloidal clay or organic matter, as these levels were higher than typical solubility would predict and were not accompanied by the characteristic hydrogen sulfide odour. Sediment pore water from the Grand Calumet River in an industrialized area of Indiana, USA, contained 0.2–1.5 µg hydrogen sulfide/litre (Hoke et al., 1993). In general, undisturbed anoxic sediment pore water may contain up to 100 µg hydrogen sulfide/litre, while disturbed sediments typically contain pore water concentrations of 1–30 µg/litre (Dillon et al., 1993).
Concentrations of hydrogen sulfide in soil gas from samples taken at some US National Priorities List sites ranged from 110 to 66 000 mg/m3 (HazDat, 1997).
Hydrogen sulfide is commonly found in coal and petroleum deposits and may be mobilized by human manipulation of these resources.
Humans may be exposed to hydrogen sulfide both from exogenous sources and from its endogenous production. Hydrogen sulfide tends to be a problem in communities located near certain types of industrial sites. The general population may be exposed to hydrogen sulfide by accidental release from natural gas wells during drilling operations near residential areas (Layton & Cederwall, 1986; Leahey & Schroeder, 1986). Maximum ground-level downwind hydrogen sulfide concentrations resulting from two sour gas well blowouts were estimated to be 3 and 20 mg/m3. Workers may be occupationally exposed to hazardous levels of hydrogen sulfide from fermenting manure (Morse et al., 1981) or stagnant wells (McDonald & McIntosh, 1951), as well as in poorly ventilated areas of wastewater treatment facilities (NIOSH, 1984, 1985a, 1990), extruded rubber plants (NIOSH, 1985b), and petroleum refineries (NIOSH, 1982a, 1982b). Hydrogen sulfide levels reported were >310 mg/m3 in a stagnant well;
70–300 mg/m3 in open maintenance ports at an oil refinery; and >700 mg/m3 at a wastewater treatment facility where a fatal accident occurred.
Exposures have occurred through the mixing of acid and base drain cleaners and through the use of acid drain cleaner to remove sludge-clogged drains, but these incidents have been rare (Oderda, 1975). Hydrogen sulfide was probably generated from the reaction between sodium sulfite in the sewage and sulfuric acid.
Hydrogen sulfide is produced endogenously in the brain from cysteine by cystathionine β-synthetase (Abe & Kimura, 1996). In the rat, the endogenous brain sulfide concentration was reported to be around 1.6 mg/kg (Warenycia et al., 1989), and the endogenous level of hydrogen sulfide was 50–160 µmol/litre (Hosoki et al., 1997). Hydrogen sulfide is also produced in several smooth muscles (e.g., thoracic aorta, ileum, and portal vein) by enzymes found in these tissues. In the rat, the endogenous level of hydrogen sulfide is 1 mmol/litre in the ileum (Abe & Kimura, 1996; Hosoki et al., 1997). Hydrogen sulfide is produced in the large intestine of mammals by metabolism of sulfhydryl proteins by anaerobic bacteria. The average levels recorded in intestinal gas have been between 1.4 and 5.6 mg/m3 (US EPA, 1978; Beauchamp et al., 1984). Hydrogen sulfide is also produced in the human mouth by microbial putrefaction. In the mouth, air levels between 1.4 and 140 µg/m3 have been found (Rosenberg et al., 1991).
Inhalation is the most common route of exogenous hydrogen sulfide exposure. Hydrogen sulfide is rapidly absorbed through the lungs in humans. It can also be absorbed through the gastrointestinal tract (ATSDR, 1999). At physiological pH, hydrogen sulfide is dissociated to the hydrogen sulfide anion in the circulation, which is probably the absorbed form (WHO, 2000). In animals, absorption of hydrogen sulfide via the lungs also occurs readily and rapidly (Beck et al., 1979; Khan et al., 1990; Kage et al., 1992). The distribution of inhaled hydrogen sulfide is rapid and widespread; storage of hydrogen sulfide in the body is limited by rapid metabolism and excretion (Nagata et al., 1990). Male Wistar rats exposed through an inhaler to hydrogen sulfide at 110 mg/m3 for 20, 40, or 60 min showed essentially the same ratios of distribution of hydrogen sulfide, irrespective of duration. The hydrogen sulfide concentration was highest in the heart, and the level in brain was comparable to the levels in lung, liver, kidney, and spleen. The tissue levels after 20 min of exposure were 10 µg/ml in blood, 25 µg/g in brain, 20 µg/g in lung, 37 µg/g in heart, 20 µg/g in liver, 25 µg/g in spleen, and 30 µg/g in kidney (Kohno et al., 1991). Hydrogen sulfide levels of 0.92 µg/g in blood, 1.06 µg/g in brain, 0.34 µg/g in kidney, and 0.38 µg/g in liver were detected at autopsy in a man who was overcome by hydrogen sulfide after working for 5 min in a tank (Winek et al., 1968). Hydrogen sulfide concentrations in the tank after the accident were 2700–8500 mg/m3.
Hydrogen sulfide is metabolized through three pathways: oxidation, methylation, and reactions with metalloproteins or disulfide-containing proteins (Beauchamp et al., 1984). The major metabolic pathway for detoxification of hydrogen sulfide is oxidation in the liver; the major oxidation product of sulfide is thiosulfate, which is then converted to sulfate and subsequently excreted in urine (Bartholomew et al., 1980). The methylation pathway also serves as a detoxification route (Weisiger & Jacoby, 1980; US EPA, 1987). Reaction with metalloproteins is a major mechanism of toxicity of hydrogen sulfide. Hydrogen also reduces disulfide bridges in proteins. Oxidized glutathione protects against hydrogen sulfide poisoning (see section 8.7). Hydrogen sulfide is excreted primarily as sulfate (free sulfate or thiosulfate) in the urine. It is also excreted unchanged in exhaled air and in faeces and flatus. Thiosulfate in urine is a useful indicator of hydrogen sulfide exposure (Kage et al., 1997). Thiosulfate excretion was measured in volunteers exposed to 11, 25, or 42 mg hydrogen sulfide/m3 for 30–45 min and compared with that of unexposed individuals at a pelt processing plant (Kangas & Savolainen, 1987). The study did not report the summary results of all exposed individuals; however, data from one individual exposed to 25 mg hydrogen sulfide/m3 for 30 min found urinary thiosulfate concentrations of approximately 2, 4, 7, 50, and 5 mmol/mol creatinine at 1, 2, 5, 15, and 17 h post-exposure, respectively. Urinary thiosulfate excreted in controls was 2.9 ± 2.5 (standard deviation [SD]) mmol/mol creatinine (n = 29). In this one individual, therefore, the highest urinary thiosulfate level occurred 15 h after exposure and dropped to control levels by 17 h post-exposure; most absorbed hydrogen sulfide was already oxidized by 15 h post-exposure. The delayed oxidation product thiosulfate buildup is consistent with the metabolic pathway of hydrogen sulfide, which included at least two oxidation steps (Beauchamp et al., 1984).
Evidence for the methylation of hydrogen sulfide comes primarily from in vitro studies of Sprague-Dawley rat intestinal mucosa (Weisiger et al., 1980). Thiol S-methyltransferase catalysed the methylation of hydrogen sulfide to methanethiol (CH3SH). Methanethiol can act as a substrate for another methylation that is also catalysed by thiol S-methyltransferase, yielding dimethylsulfide (CH3SCH3) (Weisiger & Jacoby, 1980; US EPA, 1987). The activity of thiol S-methyltransferase was widely distributed, with the greatest activity being found in the caecal and colonic mucosa, liver, lung, and kidney; enzyme activity was also found in other parts of the intestine and stomach, spleen, heart, and skeletal muscle. No enzyme activity was found in the faeces. Although it has been postulated that methylation is a method of detoxification of hydrogen sulfide, a constituent of human flatus produced in the intestine, the extent to which the toxicity of exogenous hydrogen sulfide is attenuated by methylation is not known.
Single exposure of animals to hydrogen sulfide by inhalation has caused death and respiratory, immunological/lymphoreticular, cardiovascular, and neurological effects. The respiratory tract is the most sensitive target organ in animals following single inhalation exposure. A summary of the effects of single exposures of laboratory mammals to hydrogen sulfide is presented in Table 1.
Table 1: Effects of single exposures of laboratory mammals to hydrogen sulfide.
|
Species / strain |
Exposure |
Duration |
Effects |
Reference |
|
Rat, Wistar |
1100 |
12 min |
10/10 died |
Beck et al., 1979 |
|
Rat, Fischer-344 |
700–1000 |
4 h |
6/6 died |
Khan et al., 1990 |
|
Rat, Sprague-Dawley |
820 |
2 h |
LC50 |
Prior et al., 1988 |
|
Rat, Fischer-344 |
700 |
4 h |
LC50 |
|
|
Rat, Long-Evans |
470 |
6 h |
LC50 |
|
|
Mouse, CD-1 |
1000 |
50 min |
6/6 died |
Smith & Gosselin, 1964 |
|
Rabbit, Japanese White |
700–1400 |
14–30 min |
5/5 died |
Kage et al., 1992 |
|
Rat, Fischer-344 |
280 |
4 h |
Focal areas of perivascular oedema and proteinaceous material in alveoli |
Green et al., 1991 |
|
Rat, Wistar |
140 |
1 h |
Increased blood pressure and respiration rate; biochemical changes in respiratory tissues and fluids |
Higuchi & Fukamachi, 1977 |
|
280 |
2 h |
Decreased response rate in conditioned avoidance task |
||
|
Rat, Fischer-344 |
14 |
4 h |
NOAEL |
Khan et al., 1990 |
|
70 |
4 h |
Reduced lung cytochrome oxidase activity (15%) |
||
|
280 |
4 h |
Inhibition of succinate oxidase |
||
|
Rat, Fischer-344 |
70 |
4 h |
NOAEL |
Khan et al., 1991 |
|
280 |
4 h |
Decreased viable pulmonary alveolar macrophages |
||
|
Rat, Wistar |
110 |
1 h |
Cardiac arrhythmia |
Kohno et al., 1991 |
|
110 |
1 h |
Slight pulmonary oedema |
||
|
Rat, Fischer-344 |
14 |
4 h |
Increased cellularity in nasal lavage fluid |
Lopez et al., 1987 |
|
280 |
4 h |
Increased lactate dehydrogenase activity |
||
|
560 |
4 h |
Increased alkaline phosphatase activity in bronchoalveolar lavage fluid |
||
|
Rat, Fischer-344 |
>280 |
4 h |
Severe inflammation and necrosis of respiratory and olfactory epithelium |
Lopez et al., 1988b |
|
560 |
4 h |
Lethargy |
||
|
Rat, Fischer-344 |
120 |
4 h |
Mild perivascular oedema |
Lopez et al., 1988a |
|
Rat, Fischer-344 |
530 |
4 h |
Pulmonary oedema |
Prior et al., 1990 |
|
Rabbit (mixed-breed) |
100 |
1.5 h |
Unconscious; ventricular repolarization |
Kosmider et al., 1967 |
Inhalation exposure of Sprague-Dawley rats to 2300 mg/m3 killed all five animals within 3 min (Lopez et al., 1989). All male Fischer-344 rats (4–6 used) exposed to 700–1000 mg hydrogen sulfide/m3 for 4 h died (Khan et al., 1990). Male Wistar rats lost consciousness after exposure to 1100 mg hydrogen sulfide/m3 for 7–19.3 min (mean 10.5 min); breathing ceased within 1 min of unconsciousness (Beck et al., 1979). LC50s of 470–820 mg/m3 have been reported in Sprague-Dawley, Fischer-344, and Long-Evans rats exposed to hydrogen sulfide for 2- to 6-h periods (Prior et al., 1988). All six mice exposed to 1000 mg hydrogen sulfide/m3 for 50 min died, while a group of six mice died in 10 min at 2600 mg/m3 (Smith & Gosselin, 1964). All five Japanese White rabbits died within 30 min of exposure to 700–1400 mg/m3 (Kage et al., 1992). Lethargy was reported in Fischer-344 rats exposed to 560 mg hydrogen sulfide/m3 for 4 h (Lopez et al., 1988b). Mixed-breed rabbits exposed to 100 mg/m3 for 1.5 h became unconscious (Kosmider et al., 1967).
Exposure of Wistar rats to 140–280 mg hydrogen sulfide/m3 for 1 h caused an increase in respiration rate and histological and biochemical changes in the respiratory tissues and fluids (Higuchi & Fukamachi, 1977). Cytotoxicity to nasal lavage, bronchoalveolar lavage, and pulmonary alveolar macrophages was evaluated in male Fischer-344 rats exposed to 14, 280, or 560 mg hydrogen sulfide/m3 for 4 h and examined at 1, 20, or 44 h post-exposure (Lopez et al., 1987). Changes in lactate dehydrogenase and alkaline phosphatase and cytomorphology of epithelial cells in nasal and bronchoalveolar lavage fluids were used as cell injury markers. Cellularity of nasal lavage fluid was increased by 139%, 483%, and 817% 1 h post-exposure at 14, 280, and 560 mg/m3, respectively. However, cell counts returned to baseline levels by 20 h post-exposure in rats exposed to 14 and 280 mg/m3. The nasal lavage cell count was the only significant observation following exposure to 14 mg/m3. Altered pulmonary vascular permeability was noted in animals exposed to 560 mg/m3, but this condition resolved by 20 h post-exposure. The observed increased lactate dehydrogenase activity (at exposure levels of 280 and 560 mg/m3) and alkaline phosphatase activity in bronchoalveolar lavage fluid (at an exposure level of 560 mg/m3) were indicative of toxic effects on the pulmonary epithelium. These respiratory effects in Fischer-344 rats were confirmed by Green et al. (1991), who reported significant increases in lavage fluid protein concentrations and lactate dehydrogenase activity in male Fischer-344 rats exposed to 280 or 400 mg hydrogen sulfide/m3 for 4 h. Focal areas of perivascular oedema and proteinaceous material in the alveoli were also seen in the lungs of the exposed animals.
Histological changes have been reported in the nasal cavity of Fischer-344 rats exposed for 4 h (Lopez et al., 1988b). Necrosis and exfoliation of the respiratory and olfactory mucosal cells were observed 1 h post-exposure at concentrations above 280 mg/m3; by 20 h post-exposure, the necrosis ultimately ulcerated the respiratory epithelium, exposing the basement membrane. Histological changes were also reported in the lungs of Fischer-344 rats exposed to 120 or 610 mg hydrogen sulfide/m3 for 4 h (Lopez et al., 1988a). Moderate to massive pulmonary oedema was evident in male Fischer-344 rats exposed to 525 ± 87 or 559 ± 144 mg/m3 for 4 h (Prior et al., 1990), and slight pulmonary congestion was found in male Wistar rats exposed to 110 mg hydrogen sulfide/m3 for 1 h (Kohno et al., 1991).
Cytochrome oxidase activity in lung mitochondria of Fischer-344 rats was significantly decreased at hydrogen sulfide concentrations of 70 mg/m3 (15%), 280 mg/m3 (43%), and 560 mg/m3 (68%) compared with controls after a 4-h exposure (Khan et al., 1990). At 280 and 560 mg/m3, succinate oxidase activities were also significantly inhibited; no effects were observed on succinate-cytochrome c reductase or NADH-cytochrome c reductase activities.
Significant decreases in numbers of viable pulmonary alveolar macrophages were noted in the lung lavage fluid of male rats exposed for 4 h to 280 mg hydrogen sulfide/m3 (Khan et al., 1991). This study also showed complete abolition of zymosan-induced stimulation of respiratory rates of pulmonary alveolar macrophages in animals exposed to 280 or 560 mg/m3. It should be pointed out that these changes were induced by high, nearly lethal concentrations. No changes were noted after exposure to 70 mg/m3.
A 1.5-h inhalation exposure of mixed-breed rabbits to 100 mg hydrogen sulfide/m3 resulted in ventricular repolarization (Kosmider et al., 1967). Histochemical staining of the myocardial cells revealed a reduction in adenosine triphosphate (ATP) phosphohydrolase and NADPH2 oxidoreductase. Cardiac arrhythmia was observed in male rats exposed to 110 mg hydrogen sulfide/m3 for up to 60 min (Kohno et al., 1991). Heart rates in these animals were also 10–27% less than in controls during exposure and up to 1 h post-exposure. A temporary yet marked increase in blood pressure was noted in male Wistar rats exposed to 140–280 mg hydrogen sulfide/m3 for 1 h (Higuchi & Fukamachi, 1977).
Behavioural effects on male Wistar rats exposed to sublethal concentrations of hydrogen sulfide were evaluated. When the rats were exposed to 280 mg/m3 and higher for 2 h, their conditioned avoidance behaviour was suppressed in the Sidman-type conditioning avoidance and the discriminated avoidance tests (Higuchi & Fukamachi, 1977).
Short-term inhalation studies of hydrogen sulfide in animals have reported ocular, neurological, cardiovascular, metabolic, reproductive, and developmental effects. The lowest concentration tested was 28 mg/m3.
Exposure to 28 mg hydrogen sulfide/m3 for 1 h/day for 11 days caused eye irritation, fatigue, drowsiness, dizziness, and itching in guinea-pigs; the cerebral hemisphere and brain stem total lipids and phospholipids were decreased (Haider et al., 1980).
Mixed-breed rabbits exposed half an hour per day for 5 days to 100 mg hydrogen sulfide/m3 experienced cardiac arrhythmia (Kosmider et al., 1967). The electrocardiograms were made on the fifth day of the exposure.
In Sprague-Dawley rat dams exposed to 28, 70, or 110 mg hydrogen sulfide/m3 for 7 h/day from gestation day 1 through postnatal day 21, blood glucose levels were increased about 50% at all exposure concentrations; no effects on blood glucose were noted in the offspring (Hayden et al., 1990a). At 70 mg/m3, serum triglyceride was decreased in the pups by 20% and in the dams by 25% on day 21 postpartum. No changes in serum protein, lactate dehydrogenase, glutamic–oxaloacetic transaminase, or alkaline phosphatase activities were noted. Maternal liver cholesterol levels were increased on day 21 postpartum in the dams exposed to 110 mg/m3 (2.88 ± 0.11 mg/g tissue, P < 0.05), but not 70 mg/m3. There was no significant change in dams’ body weight gain or liver weight over controls (Hayden et al., 1990b). The parturition time was increased approximately 10, 20, and 42% over matched controls at 28, 70, and 110 mg/m3, respectively.
Medium-duration inhalation studies of hydrogen sulfide in animals have reported respiratory, neurological, and olfactory effects. The most sensitive target organ for medium-term exposure in animals is the nasal olfactory mucosa.
B6C3F1 mice (10 males and 12 females per group) were exposed to hydrogen sulfide concentrations at 14, 43, and 110 mg/m3 for 6 h/day, 5 days/week, for 90 days (CIIT, 1983a). The mice in the control group were exposed to clean air only. Minimal to mild inflammation of the nasal mucosa in the anterior segments of the nose was noted in 89% of male and 78% of female mice exposed at 110 mg/m3. No nasal lesions were noted in the 43 mg/m3 and the control groups of mice. Other organs, including brain, kidney, spleen, liver, heart, and ovaries/testes, were also examined. There were no other notable histopathological lesions that could be attributed to exposure to hydrogen sulfide. All work was conducted in conformity with Good Laboratory Practice. The NOAEL for the respiratory effects in this study was 43 mg/m3 (CIIT, 1983a). Eye irritation was not noted. Neurological function evaluation included posture, gait, facial muscle tone, extensor thrust, and crossed-extensor thrust reflexes. Two animals at 110 mg/m3 did not respond to artificial light stimulus, and two others exhibited an irregular gait. At 110 mg/m3, 7–14% depression of mean body weight gain was also observed.
In Fischer-344 rats (15 males and 15 females per group) exposed as described for the B6C3F1 mice above, no significant respiratory effects were observed (CIIT, 1983b).
In Sprague-Dawley rats (15 males and 15 females per group) also exposed as described for the B6C3F1 mice above, decreased body weight gain was noted at 110 mg/m3 (CIIT, 1983c). No significant effects on neurological function (posture and gait, facial tone, papillary reflex, palpebral reflex, extensor thrust reflex, and crossed-extensor thrust reflex) were noted. No treatment-related changes were noted in haematological parameters, in the skeletal muscle, bone marrow, or bone, in the spleen or lymph nodes, in the kidneys, in the pituitary, adrenal, thyroid, or parathyroid glands, or in the skin.
Recently, olfactory toxicity in adult male Sprague-Dawley CD rats following medium-term inhalation exposure to hydrogen sulfide was reported (Brenneman et al., 2000). Rats (n = 12 per group) were exposed to 0, 14, 42, or 110 mg hydrogen sulfide/m3 for 5 h/day, 7 days/week, for 10 weeks in inhalation exposure chambers. A significant increase in nasal lesions in the olfactory mucosa was observed in 11/12 rats exposed to 42 mg/m3 and 12/12 rats exposed to 110 mg/m3. The lesions included olfactory neuron loss and basal cell hyperplasia. They were multifocal and bilaterally symmetrical with a rostrocaudal distribution. The dorsal medial meatus and the dorsal and medial portions of ethmoid recess of the nasal cavity were affected. The olfactory neuron loss was mild to moderate in severity in the 42 mg/m3 group and moderate to severe in the 110 mg/m3 group. The NOAEL for olfactory lesions in this study was 14 mg/m3. Other than the nasal and olfactory system, no other end-points were evaluated. This study was used as the basis for derivation of a medium-term tolerable concentration in section 10.2.
Three crossbred pigs were exposed to 12 mg hydrogen sulfide/m3 continuously for 17 days in inhalation chambers (Curtis et al., 1975). No histopathological changes were noted in representative respiratory tract tissues examined. There was no effect on the rate of weight gain.
There are no studies on effects of long-term exposure to hydrogen sulfide in animals. There are no studies on cancer effects in animals exposed to hydrogen sulfide.
The genotoxicity of hydrogen sulfide has been inadequately investigated. No mutagenicity was observed in the only available study, an Ames test with Salmonella typhimurium TA97, TA98, and TA100 strains with hydrogen sulfide at 17, 57, 175, 582, and 1750 µg/plate, either with or without S9 liver fractions from male Syrian Golden hamsters or Sprague-Dawley rats that had been induced with 500 mg Aroclor 1254/kg body weight (US EPA, 1984). The toxic potential of hydrogen sulfide was not tested in this study.
No treatment-related histopathological changes were found in male or female reproductive organs of Fischer-344 or Sprague-Dawley rats or B6C3F1 mice exposed to 14, 43, or 110 mg hydrogen sulfide/m3 for 6 h/day, 5 days/week, for 90 days (CIIT, 1983a, 1983b, 1983c).
Pregnant Sprague-Dawley rats exposed to 28, 70, or 110 mg hydrogen sulfide/m3 for 7 h/day on gestation day 6 until day 21 postpartum (Hayden et al., 1990b) had a dose-dependent increase in mean parturition time and difficult delivery of 10, 20, and 42% over matched controls handled under the same conditions. Prolonged labour was observed in 6/18 animals from all exposure concentrations, compared with 1/17 controls. No threshold for the effect on parturition time could be determined. However, parturition time was variable (means of 82.5–124 min) among control groups (95.2 ± 7.6 min for the 28 mg/m3 control dams, 124 ± 32 min for the 70 mg/m3 control dams, and 82.5 ± 7.5 min for the 220 mg/m3 control dams) and was not analysed statistically. The mean parturition time of the exposed animals was 105–148.8 min. There was no difference in maternal body weight gain between the exposed groups and controls. No additional data on maternal toxicity were identified.
In a recent reproductive and developmental toxicity study by Dorman et al. (2000), male and female Sprague-Dawley rats (12 per sex per concentration) were exposed to 0, 14, 42, or 110 mg hydrogen sulfide/m3 for 6 h/day, 7 days/week, for 2 weeks prior to mating. Exposure continued during a 2-week mating period and then from gestation days 0 through 19. Exposure of dams and offspring resumed between postnatal days 5 and 18. Adult male rats were exposed for 70 consecutive days. No reproductive toxicity was observed in the exposed female F0 rats as assessed by the number of females with live pups, litter size, average length of gestation, and the number of implants per pregnant female. No reproductive toxicity was observed in exposed male F0 rats as assessed by percent motile sperm, percent normal sperm, daily sperm production, cauda sperm count, or reproductive organ tissue weight. However, there was a higher (but not statistically significant) incidence of testicular tubular degeneration in the 110 mg/m3 group (42%) compared with controls (17%). Findings on developmental neurotoxicity effects in this study are presented in section 8.6.2.
In Sprague-Dawley rat pups exposed to 28, 70, or 110 mg hydrogen sulfide/m3 for 7 h/day in utero and neonatally to day 21 postpartum, significant decreases in time for pinna detachment in exposed pups at 28 mg/m3 and hair growth at 28 and 70 mg/m3 were noted (Hayden et al., 1990b). No other changes in development, including incisor eruption, eyelid opening, and surface righting, were noted through day 21 postpartum. The LOAEL for developmental effects in this study was 28 mg/m3.
No external fetal anomalies were noted in a dose range-finding developmental study in which pregnant Sprague-Dawley rats were exposed to 210 mg hydrogen sulfide/m3 for 6 h/day on gestation days 6–20, despite body weight loss in the dams (Saillenfait et al., 1989). A significant (P < 0.01) but slight decrease (4% of the control value) in fetal body weight was noted.
A morphological examination of cerebellar Purkinje cells from Sprague-Dawley rat pups exposed to 28–70 mg hydrogen sulfide/m3 for 7 h/day from gestation day 5 through postpartum day 21 showed severe alterations in the architecture and growth characteristic of the Purkinje cell dendritic fields compared with controls (Hannah & Roth, 1991). These findings suggest that developing neurons exposed to low levels of hydrogen sulfide are at risk of developing severe deficits. The LOAEL for the developmental effect in this study is 28 mg/m3.
Two studies by Hannah et al. (1989, 1990) examined the effects of prenatal exposure to hydrogen sulfide on amino acid levels in the brain. In the first study, pregnant Sprague-Dawley rats were exposed to 110 mg hydrogen sulfide/m3 for 7 h/day from gestation day 5 to postpartum day 21 (Hannah et al., 1989). Aspartate, glutamate, and gamma aminobutyric acid levels in the cerebrum and cerebellum were reduced by about 20% compared with controls by postpartum day 21. Taurine levels of the offspring were initially 25% higher than those of controls but had returned to control range by postpartum day 21; taurine levels in dams were not measured. In the second study, pregnant rats were exposed to 70 mg hydrogen sulfide/m3 for 7 h/day from gestation day 6 to postpartum day 21. The taurine level in maternal plasma was 30% higher than in controls; taurine levels were not determined in offspring, so relating these levels to high taurine levels found in offspring in the 1989 study is inferential. Alteration of brain amino acids during the critical phase of development could lead to behavioural and structural abnormalities.
Developmental neurochemical effects were also investigated by Skrajny et al. (1992). Pregnant Sprague-Dawley rats were exposed to 28 or 110 mg hydrogen sulfide/m3 for 7 h/day from gestation day 5 to postpartum day 21; separate control groups were used for each exposure level. Exposure to 110 mg/m3 caused significant increases compared with controls in serotonin and norepinephrine levels in the cerebellum and frontal cortex on postpartum days 14 and 21. At 28 mg/m3, norepinephrine levels were below control levels on days 14 and 21; the serotonin levels in the frontal cortex were increased on day 21. In a subsequent study using the same exposure regimen, but following the monoamine levels in various regions of the brain up to postnatal day 60, Roth et al. (1995) found that the monoamine levels observed at postnatal day 21 gradually returned to control values by day 45. Since monoamines influence neural development, alteration of monoamines could lead to alteration of neural growth. It is not known whether the alteration of neural growth caused by alteration of monoamine levels during a critical stage of development is also reversible after exposure stops.
Exposure of Sprague-Dawley rat pups prenatally and perinatally to hydrogen sulfide at 0, 14, 42, or 110 mg/m3, as described above in section 8.6.1 (Dorman et al., 2000), did not affect pup growth, development, or behavioural performance as evaluated on postnatal days 60–62 by motor activity, passive avoidance, functional observation battery, acoustic startle response, and neuropathology. For information on maternal toxicity in this study, please see section 8.6.1.
Inhaled hydrogen sulfide rapidly enters into circulation, where it dissociates in part into hydrosulfide ion. The free hydrogen sulfide remaining in the blood interacts with metalloproteins, disulfide-containing proteins, and thio-S-methyltransferase, forming methyl sulfides (Beauchamp et al., 1984; Guidotti, 1996; Hoffman & Guidotti, 1997). Hydrosulfide ion binds to haem compounds and is metabolized by oxidation to sulfate. The interaction between the hydrosulfide ion and methaemoglobin (forming sulfmethaemoglobin) is a detoxification pathway (Smith & Gosselin, 1979). Methylation is also considered detoxification. The toxicity of hydrogen sulfide has been proposed to be primarily the result of inhibition of cytochrome oxidase, an enzyme critical for cellular mitochondrial respiration (Khan et al., 1990); however, others suggested that toxicity results from complex reactions with many enzymes (Reiffenstein et al., 1992). In the mitochondria, cytochrome oxidase, the final enzyme in the respiratory chain, is inhibited by hydrogen sulfide as a result of the oxygen reduction of one of the enzymatic haems (Chance & Schoener, 1965; Nicholls, 1975; Smith et al., 1977); thus, the electron transport chain is disrupted by preventing oxygen from acting as the final electron acceptor and causing blockage of oxidative metabolism, leading to anaerobic metabolism, decreased ATP production with curtailed cellular energy generation, and the generation of lactic acid. Nervous and cardiac tissues, which have the highest oxygen demand, are especially sensitive to the disruption of oxidative metabolism (Ammann, 1986). In the central nervous system, this effect may result in death from respiratory arrest. Hydrogen sulfide also reduces disulfide bridges in proteins (e.g., succinic dehydrogenase). Oxidized glutathione protects against hydrogen sulfide poisoning, presumably through reaction of the disulfide linkage with hydrosulfide, thereby preventing reaction with other, more critical enzymatic sites (Beauchamp et al., 1984). It has been shown in rats injected with sodium hydrosulfide that the lung, not the brain, is the primary site of action of hydrogen sulfide; an afferent neural signal from the lung via the vagus induces apnoea (Almeida & Guidotti, 1999). In rats injected intraperitoneally with sodium hydrosulfide, substantial changes in neurotransmitter amino acids in the brain stem responsible for neuron control of breathing were noted (Kombian et al., 1988). These changes could lead to central respiratory arrest. Sodium sulfide has also been shown to strongly inhibit neuronal cytochrome oxidase and carbonic anhydrase, causing disruption to respiratory and mitochondrial functions in the mammalian brain in vitro (Nicholson et al., 1998).
Because hydrogen sulfide is a gas, inhalation is the major route of exposure to hydrogen sulfide. Most human data are derived from acute poisoning case reports, occupational exposures, and limited community studies. In confined spaces, human acute poisonings continue to occur. Single inhalation exposures to high concentrations of hydrogen sulfide cause health effects in many systems. Health effects that have been observed in humans following exposure to hydrogen sulfide include death and respiratory, ocular, neurological, cardiovascular, metabolic, and reproductive effects. Respiratory, neurological, and ocular effects are the most sensitive end-points in humans following inhalation exposures. There are no adequate data on carcinogenicity. A summary of human health effects resulting from exposure to hydrogen sulfide is presented in Table 2.
Table 2: Human health effects at various hydrogen sulfide concentrations.
|
Exposure (mg/m3) |
Effect / observation |
Reference |
|
0.011 |
Odour threshold |
Amoore & Hautala, 1983 |
|
2.8 |
Bronchial constriction in asthmatic individuals |
Jappinen et al., 1990 |
|
5.0 |
Increased eye complaints |
Vanhoorne et al., 1995 |
|
7 or 14 |
Increased blood lactate concentration, decreased skeletal muscle citrate synthase activity, decreased oxygen uptake |
Bhambhani & Singh, 1991; Bhambhani et al., 1996b, 1997 |
|
5–29 |
Eye irritation |
IPCS, 1981 |
|
28 |
Fatigue, loss of appetite, headache, irritability, poor memory, dizziness |
Ahlhorg, 1951 |
|
>140 |
Olfactory paralysis |
Hirsch & Zavala, 1999 |
|
>560 |
Respiratory distress |
Spolyar, 1951 |
|
>700 |
Death |
Beauchamp et al., 1984 |
There have been numerous case reports of human deaths after single exposures to high concentrations (>700 mg/m3) of hydrogen sulfide gas (Beauchamp et al., 1984). Most fatal cases associated with hydrogen sulfide exposure have occurred in relatively confined spaces; the victims lost consciousness quickly after inhalation of hydrogen sulfide, sometimes after only one or two breaths (the so-called "slaughterhouse sledgehammer" effect). Many of the case-studies involved assumed accidental poisonings for which the exposure concentrations and/or durations were not known. Death occurring after single exposures to high concentrations of hydrogen sulfide appears to be the result of respiratory failure or arrest, with most cases initially presenting with respiratory insufficiency, non-cardiogenic pulmonary oedema, coma, and cyanosis.
Three men lost consciousness and died after entering a sewer containing high concentrations of hydrogen sulfide; cyanosis and pulmonary oedema were noted at autopsy (Adelson & Sunshine, 1966). After being exposed to hydrogen sulfide in a bathroom connected to a manure pit, a man developed nausea, vomiting, dizziness, and dyspnoea and died a few hours later; haemorrhagic bronchitis and asphyxiation were noted as the cause of death (Parra et al., 1991). Two maintenance workers at a tanning company collapsed and died no more than 45 min after entering a sewer manhole; a hydrogen sulfide concentration of 280 mg/m3 was measured just inside the manhole 6 days after the accident (NIOSH, 1991). A worker at a poultry feather processing plant died after being exposed to hydrogen sulfide gas for an estimated 15–20 min (Breysse, 1961). Testing performed later in the area where the exposure occurred indicated that the hydrogen sulfide concentrations ranged from 2800 to 5600 mg/m3. Pulmonary, intracranial, and cerebral oedema and cyanosis were noted at autopsy.
Of 221 workers’ compensation claims for hydrogen sulfide exposure that occurred over a 5-year period (1969–1973) in Alberta, Canada, primarily among petrochemical workers, 14 deaths were noted (Burnett et al., 1977). Acute effects noted included coma, disequilibrium, and respiratory insufficiency with pulmonary oedema. A descriptive retrospective analysis of 250 workers’ claims for hydrogen sulfide exposure from 1979 to 1983 found seven fatalities that involved the central nervous and respiratory systems; hepatic congestion and cardiac petechiae were also noted (Arnold et al., 1985).
Bates et al. (1997), taking advantage of the fact that the New Zealand city of Rotorua is in a geothermally active area where geothermal energy is used for heating purposes, conducted an ecological epidemiological study in which they compared the mortality for selected diseases between residents in Rotorua and the rest of New Zealand. Monitoring during the 1970s found levels of hydrogen sulfide as high as 1 mg/m3; the median concentration was 30 µg/m3, with 35% of the measurements over 70 µg/m3 and 10% over 400 µg/m3. Mortality data on the respiratory system showed a significantly elevated standardized mortality ratio (SMR = 1.18; P < 0.001). Because the population in the Rotorua area has markedly more Maori than the population in the rest of New Zealand and because disease and mortality rates are relatively higher in the Maori population than in the non-Maori population, further analysis was carried out with an adjustment for ethnicity. When the data were stratified by sex and ethnicity, female Maoris had an SMR of 1.61 (P = 0.001). However, the authors indicated that the prevalence of smoking was not evaluated as a potential confounder and that there may also have been some misclassification of study subjects with regard to ethnicity.
Hydrogen sulfide is an irritant. Ocular effects are believed to have resulted from direct contact of the eye with hydrogen sulfide gas. The effect of hydrogen sulfide on the eye is of considerable importance, because ocular effects occur at concentrations that provide few other observable systemic effects (NIOSH, 1977). A significantly higher prevalence of eye complaints has been reported for workers exposed to hydrogen sulfide above 5 mg/m3 than for unexposed workers (Vanhoorne et al., 1995).
Keratoconjunctivitis, punctate corneal erosion, blepharospasm, lacrimation, and photophobia have developed in individuals exposed briefly to high concentrations of hydrogen sulfide gas (Ahlborg, 1951; Luck & Kaye, 1989). Stinging has also been reported in acute occupational hydrogen sulfide poisoning (Audeau et al., 1985). Eye irritation was reported in workers exposed to hydrogen sulfide at 15–29 mg/m3 for 6–7 h (IPCS, 1981). Exposure at concentrations greater than 70 mg/m3 for 1 h or more can severely damage eye tissues (Riffat et al., 1999). Eighteen percent of 250 Canadian workers who submitted workers’ compensation claims for hydrogen sulfide exposure had developed conjunctivitis, which in some cases persisted for several days (Arnold et al., 1985).
Jaakkola et al. (1990) reported that people exposed to hydrogen sulfide while living in a community around a paper mill reported 12 times more eye irritation than people without exposure. These effects were observed at a mean annual hydrogen sulfide concentration of 6 µg/m3. However, the reported ocular symptoms may have been due to exposure to peak concentrations of hydrogen sulfide (daily peaks as high as 100 µg/m3) or may have been due to co-exposure to methyl mercaptan and methyl sulfides. Due to the co-exposure to other substances, this study cannot serve as a basis for a LOAEL.
Bates et al. (1998) compared hospital-recorded incidence of selected diseases in Rotorua over the decade 1981–1990 with the incidences in the rest of New Zealand over the same period. Statistically significantly elevated standardized incidence ratios (SIR) were found in Rotorua residents compared with the rest of New Zealand for cataract (SIR = 1.26; P < 0.001), conjunctiva disorders (SIR = 2.09; P < 0.001), and orbit disorders (SIR = 1.69; P = 0.005). The median concentration of hydrogen sulfide was 30 µg/m3, with 35% of the measurements above 70 µg/m3 and 10% above 400 µg/m3 (Bates et al., 1997). There were no data on ambient air levels of other geothermal gases such as mercury and radon; thus, there were insufficient exposure data, and there was also concern about systematic biases in the recording of data.
With single accidental exposures to high concentrations of hydrogen sulfide, numerous respiratory effects are observed. Single exposures to >700 mg hydrogen sulfide/m3 are considered to cause rapid respiratory failure (Beauchamp et al., 1984). Respiratory distress was noted in two workers exposed to >56 mg hydrogen sulfide/m3 for <25 min (Spolyar, 1951). Other respiratory effects of single exposures to high concentrations of hydrogen sulfide include non-cardiogenic pulmonary oedema, sore throat, cough, and dyspnoea. Pulmonary function tests were performed on persons with asthma exposed to 2.8 mg hydrogen sulfide/m3 for 30 min (Jappinen et al., 1990). The patients had had bronchial asthma for 1–13 years (mean 3.7 years) and had been taking medication. Patients with severe asthma were not included in the study, because the patients did not take medication for 2 days prior to exposure to hydrogen sulfide. Airway resistance (Raw) and specific airway conductance (SGaw) were assessed by a body plethysmograph, and the ventilatory capacities were measured with a flow volume spirometer. Raw was slightly decreased in two and increased in eight subjects; the difference ranged from –5.95% to +137.78%, averaging an increase of 26.3%. After exposure, there was an increase of 25% in mean Raw. SGaw was decreased in six and increased in four subjects; an average decrease of 8.4% with a range of –57.7% to 28.9% was noted. These changes were not statistically significant as a group. However, 2 of 10 subjects showed changes in excess of 30% in both Raw and SGaw; the authors considered the changes an indication of bronchial obstruction. No notable changes were noted in forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), and forced expiratory flow. Pulmonary function was unaffected following the same exposure protocol in 26 pulp mill workers who had previously had daily occupational hydrogen sulfide exposures of <14 mg/m3. No significant changes were noted in FVC, FEV1, or bronchial responsiveness to histamine challenge. The LOAEL of 2.8 mg/m3 for respiratory effects in asthmatics was used as the basis for the derivation of a short-term tolerable concentration in section 10.2.
Evaluation of the lung function of 47 workers exposed during an accidental release to unknown concentrations of hydrogen sulfide found that 23% of the subjects had reduction in residual volume (RV), while the other parameters of lung function were normal (Buick et al., 2000). The mean (SD) of RV was 1.57 (0.51). An index that fell outside 1.65 SD of the predicted value was considered abnormal. The reduction in RV in the presence of other normal lung function indices was suggested to be a subclinical manifestation of hydrogen sulfide intoxication.
Inhalation of 2.8–14 mg hydrogen sulfide/m3 for 16–30 min does not affect pulmonary function in healthy men and women. Healthy male volunteers were exposed by oral inhalation to hydrogen sulfide at concentrations up to 7 mg/m3 for more than 16 min after graded exercise that was performed to exhaustion (Bhambhani & Singh, 1991). No effects on expired ventilation or maximum power output were noted, but exposure to 7 mg/m3 resulted in a significant increase in maximum oxygen uptake compared with controls. At exposures to 2.8 and 7 mg/m3, the respiratory exchange ratio (RER) decreased significantly. The study authors attributed this to a non-significant trend towards increased oxygen uptake and decreased carbon dioxide output. Another study examined the effects of inhalation of 7 mg hydrogen sulfide/m3 on respiratory physiological parameters and found no changes in partial pressure of oxygen, partial pressure of carbon dioxide, oxygen uptake, percentage of oxygen uptake, uptake of carbon dioxide, or RER in male or female volunteers during 30 min of submaximal exercise (Bhambhani et al., 1994). A third study found that inhalation of 14 mg hydrogen sulfide/m3 for 15 min at elevated metabolic and ventilation rates did not result in significantly altered pulmonary function test results in men and women (Bhambhani et al., 1996a). It should be noted that the study subjects were exposed by oral inhalation, they were unable to smell the hydrogen sulfide, and their eyes were not exposed to the gas.
Hessel et al. (1997) examined the pulmonary health effects of hydrogen sulfide exposure in 175 Canadian oil and gas workers who were employed at the time of the study. Exposure to hydrogen sulfide was assessed by a questionnaire on the occurrence of respiratory symptoms. Lung health was assessed via spirometric testing and by skin prick testing for six common antigens. The workers were divided into three exposure groups: none (n = 110), gas exposure sufficient to produce symptoms (n = 51), and knockdown (n = 14; history of exposure sufficient to cause unconsciousness). Cigarette pack-years and length of employment did not differ significantly between the groups. None of the lung function indicators (FEV1, FVC, or FEV1/FVC) differed signiicantly among the three groups. Significantly increased odds ratios (ORs) for respiratory symptoms were seen only in those having had a knockdown who showed significant excess for shortness of breath while hurrying on the level or walking up a slight hill (OR = 3.55; 95% confidence interval [CI] = 1.02–12.4); wheeze with chest tightness (OR = 5.15; 95% CI = 1.29–20.6); and attacks of wheeze (OR = 5.08; 95% CI = 1.28–20.2).
In a cross-sectional study of sewer and water treatment workers, Richardson (1995) evaluated the association of hydrogen sulfide exposures with reduced lung function using spirometric testing. Job titles were used to categorize sewer workers into high-, medium-, and low-exposure groups. Water treatment workers not occupationally exposed to hydrogen sulfide were chosen as a comparison group. Significant differences between spirometric values (FEV1/FVC) of sewer and water treatment workers were seen across a number of age strata, irrespective of smoking status, although smoking status reduced the impact somewhat. The prevalence OR for obstructive lung disease was 21.0 (95% CI = 2.4–237.8) in non-smoking sewer workers with presumed high hydrogen sulfide exposures compared with non-smoking water treatment workers. The prevalence OR was adjusted for age, height, race, and smoking habits.
A series of studies (Jaakkola et al., 1990; Haahtela et al., 1992; Marttila et al., 1994a, 1994b, 1995; Partti-Pellinen et al., 1996) reported the results of the South Karelia Air Pollution Study, which began in 1986 to evaluate the effects of a low-level mixture of air pollutants from pulp mills in South Karelia, Finland, on human health. The pollutant mixture included particulates, sulfur dioxide, and a number of malodorous compounds, including hydrogen sulfide, methyl mercaptan, and methyl sulfides. In the early studies of this series, levels of hydrogen sulfide, sulfur dioxide, particulates, and methyl mercaptan were individually reported. In the later studies, a complex mixture of "malodorous sulfur components" was monitored as total reduced sulfur (TRS) using a method that first removes any sulfur dioxide, then oxidizes the TRS compounds to sulfur dioxide and reports the results as micrograms per cubic metre. It is not possible, from the information provided, to determine precisely what proportion of the TRS is actually hydrogen sulfide, although the authors indicate that it is about two-thirds (Marttila et al., 1994a). These studies demonstrated that low levels of hydrogen sulfide in combination with other sulfur-containing pollutants, and possibly in combination with particulates, and/or sulfur dioxide can have an adverse effect on respiratory health. However, it is not possible at this time to determine whether it is the low annual average values of 1–2 µg TRS/m3 or the daily average concentrations (56 µg TRS/m3) that are associated with these findings. A recent follow-up study provided further evidence that long-term exposure to low levels of malodorous sulfur compounds increases the risk of acute respiratory infection and symptoms of the respiratory tract (Jaakkola et al., 1999).
Single exposure to high concentrations of hydrogen sulfide can cause nausea, headaches, delirium, disturbed equilibrium, poor memory, neurobehavioural changes, olfactory paralysis, loss of consciousness, tremors, and convulsions. Fatigue, poor memory, dizziness, and irritability have been observed in workers chronically exposed to hydrogen sulfide (Beauchamp et al., 1984). The odour threshold for the rotten eggs odour of hydrogen sulfide varies according to the individual; the geometric mean of available literature data is 11 µg/m3, omitting extreme points and duplicate quotations; the standard error is 2.1 (Amoore & Hautala, 1983). At concentrations greater than 140 mg/m3, olfactory paralysis occurs, causing a loss of odour perception; this makes hydrogen sulfide very dangerous, because a few breaths at 700 mg/m3 can be fatal. Deficits of the olfactory system resulting from single exposures to high concentrations of hydrogen sulfide have been reported in workers 3 years following exposure (Hirsch & Zavala, 1999).
Available information on the neurotoxic effects of single exposures to high concentrations of hydrogen sulfide in humans comes primarily from case reports. In most instances, exposure concentrations were either unknown or estimated. Three men accidentally exposed to hydrogen sulfide in excess of 350 mg/m3 after a few minutes became unconscious (McDonald & McIntosh, 1951). Loss of consciousness has also been reported with single exposures to estimated concentrations of 700–2800 mg hydrogen sulfide/m3 (Spolyar, 1951; Milby, 1962; Krekel, 1964; Deng & Chang, 1987). Other described neurological effects in case reports included disturbed equilibrium, nausea, headache, poor memory, insomnia, irritability, delirium, vertigo, unusual sweating, neuropsychological symptoms, convulsions, and tremors (Krekel, 1964; Arnold et al., 1985).
The neurological effects following single inhalation exposures to high concentrations of hydrogen sulfide may be permanent or persistent. A 5- to 10-year follow-up re-examination of several workers who become unconscious after exposure to unspecified concentrations of hydrogen sulfide revealed permanent neurological symptoms (Tvedt et al., 1991a, 1991b), including vision and memory impairment, rigid movements, reduced motor function, slight tremor, ataxia, psychosis, abnormal learning, retention, and motor function, and slight cerebral atrophy. The probable exposure concentration in one of the patients may have exceeded 280 mg/m3 (as measured 2.5 h after exposure).
In a study of the possible effects of exposure to low concentrations of hydrogen sulfide, 3/10 asthmatic volunteers complained of headache after being exposed in a sealed chamber to 2.8 mg hydrogen sulfide/m3 for 30 min (Jappinen et al., 1990). The data were collected by self-reporting.
Neurological effects resulting from long-term exposure to hydrogen sulfide in the shale industry have also been reported (Ahlborg, 1951). Symptoms observed in workers exposed to daily concentrations of hydrogen sulfide that often exceeded 28 mg/m3 included fatigue, loss of appetite, headache, irritability, poor memory, and dizziness. The frequency of fatigue increased with length of employment and the degree of hydrogen sulfide exposure.
In the South Karelia Air Pollution Study, described in more detail in section 9.2, all of the reports found significant increases in the incidence of headaches or migraines in a polluted community compared with a non-polluted community (Jaakkola et al., 1990; Marttila et al., 1994b, 1995; Partti-Pellinen et al., 1996). The residents of the polluted community showed a significantly increased risk of headache during both the previous 4-week period (OR = 1.83; 95% CI = 1.06–3.15) and the preceding 12 months (OR = 1.70; 95% CI = 1.01–2.64), compared with the residents of the reference community, even after adjusting for differences in age, gender, smoking, history of allergic diseases, education, and marital status between the two communities.
Residents in Rotorua, a New Zealand city that uses geothermal energy for industrial and domestic heating purposes, had significant increases in the incidence of diseases of the nervous system and sense organs compared with the rest of New Zealand residents (SIR = 1.11; P < 0.001) (Bates et al., 1998). When the data were stratified by gender and ethnicity, the increased risks remained significant for all but non-Maori men. When incidence rates were examined for minor disease groupings within nervous system diseases, significantly increased risks were seen for other disorders of the central nervous system (SIR = 1.22; P < 0.001) and disorders of the peripheral nervous system (SIR = 1.35; P < 0.001). At the level of individual diseases, statistically significant SIRs were found for infant cerebral palsy, migraine, other conditions of the brain, mononeuritis of the upper limbs and lower limbs, and mononeuritis multiplex. As described above in section 9.1, the median concentration of hydrogen sulfide was 30 µg/m3, with 35% of the measurements >70 µg/m3 and 10% >400 µg/m3 (Bates et al., 1997). There were no data on ambient air levels of other geothermal gases such as mercury and radon; thus, there were insufficient exposure data. The authors also had concern about systematic biases in the recording of data.
Chest pain and bradycardia have been reported after single exposures to high levels of hydrogen sulfide via inhalation (Arnold et al., 1985). Cardiac arrhythmias, cardiac irregularities, and increase in blood pressure have been reported in workers after brief exposures (Krekel, 1964; Thoman, 1969; Audeau et al., 1985). However, there was no information on hydrogen sulfide concentration. No adverse cardiovascular effects were found when healthy volunteers were exposed to 7 or 14 mg hydrogen sulfide/m3 by oral inhalation during 30 min of submaximal exercise (Bhambhani et al., 1994, 1997).
Exposure of healthy volunteers to 7 or 14 mg hydrogen sulfide/m3 via oral breathing for two 30-min sessions when exercising at 50% maximum aerobic power resulted in increases in blood lactate concentrations, a decrease in oxygen uptake, and a decrease in skeletal muscle citrate synthase activity, indicative of an inhibition of the aerobic capacity of the exercising muscle and a tendency to shift the metabolic profile of skeletal muscle from aerobic towards anaerobic metabolism (Bhambhani & Singh, 1991; Bhambhani et al., 1996b, 1997). The study subjects were primarily undergraduate and graduate students: 13–16 males, average age 25 years; and 12–13 females, average age 22 years. The men appeared to be more sensitive to this effect, showing a small response at 7 mg/m3, whereas women did not show an effect until the 14 mg/m3 level. The decrease in oxygen uptake was seen in 73% of the men and 70% of the women in the study; the magnitude of the decrease ranged from 5% to 18%.
Evidence suggests that occupational exposure to hydrogen sulfide may be associated with an increase in the rate of spontaneous abortion. Hemminki & Niemi (1982) examined the spontaneous abortion rate in relationship to maternal and paternal occupation and residential environmental pollution in an industrial community in Finland. Women who were employed in rayon textile and paper products jobs had an increased rate of spontaneous abortions (P < 0.10), as did women whose husbands worked in rayon textile or chemical processing jobs. Pollutants examined were sulfur dioxide, hydrogen sulfide, and carbon disulfide. More spontaneous abortions in areas with annual levels of hydrogen sulfide above 4 µg/m3 were recorded; however, the difference was not large enough to be significant. In a retrospective study of spontaneous abortions in a large population of women working in the petrochemical industry in China, Xu et al. (1998) reported a significantly increased risk of spontaneous abortion with frequent exposure to petrochemicals (OR = 2.7; 95% CI = 1.8–3.9) after adjustment for potential confounders, including age, educational level, plant, shift of work, standing and kneeling hours at work, noise level, dust concentration, passive smoking, and diet. When the risk associated with exposure to specific chemicals was examined, exposure to hydrogen sulfide was found to have an OR of 2.3 (95% CI = 1.2–4.4). Significantly increased risk of spontaneous abortion was also found to be associated with exposure to benzene and gasoline. There was no information on the exposure concentrations during the first trimester of the pregnancy.
No increase in cancer incidence was noted in a residential cohort study of individuals living downwind from natural gas refineries in Alberta, Canada, from 1970 to 1984 (Schechter et al., 1989). In a retrospective epidemiological study using the cancer registry from 1981 to 1990, Bates et al. (1998) evaluated the risk of cancer to known target organ systems of hydrogen sulfide toxicity in residents of Rotorua, a New Zealand city that uses geothermal energy for industrial and domestic heating purposes. The exposures to hydrogen sulfide and mercury from geothermal sources could have a health impact. A significantly increased risk of nasal cancers (SIR = 3.17; P = 0.01) was found among Rotorua residents compared with the rest of the population of New Zealand. However, this is a rare cancer, and this finding was based on only four cases. Because the population of Rotorua has a higher percentage of Maoris than the rest of New Zealand, these researchers also examined their data stratified by ethnicity and sex and found a significantly increased risk of cancers of the trachea, bronchus, and lung (SIR = 1.48; P = 0.02) among female Maoris in Rotorua compared with female Maoris in the rest of New Zealand. Differences in smoking history between the two populations were not sufficient to explain the observed differences in risk. The authors concluded that there are inadequate data on exposure to permit conclusions on possible causal relationships between hydrogen sulfide and cancer incidence. In total, it is not possible to evaluate the carcinogenic potential of hydrogen sulfide on the basis of the human studies.
Humans may be exposed to hydrogen sulfide from endogenous production and from exogenous sources. Most endogenous production results from the metabolism of sulfhydryl-containing amino acids (e.g., cysteine) by bacteria present in both the intestinal tract and the mouth (Tonzetich & Carpenter, 1971; Beauchamp et al., 1984). Hydrogen sulfide is also produced in the brain and several smooth muscles (e.g., thoracic aorta, ileum, and portal vein) by enzymes found in these tissues (Abe & Kimura, 1996; Hosoki et al., 1997). In the rat, the endogenous level of hydrogen sulfide is 50–160 µmol/litre in the brain and 1 mmol/litre in the ileum (Abe & Kimura, 1996).
The inhalation route is the major route of exogenous intake of hydrogen sulfide. The oral route is of no practical relevance. The principal adverse health effects noted in humans exposed for short periods to high concentrations of hydrogen sulfide by inhalation include respiratory and neurological effects; death may result as a consequence of respiratory failure. Hydrogen sulfide is also an ocular and respiratory tract irritant. There is also some evidence that exposure to hydrogen sulfide may be associated with an increased rate of spontaneous abortion. The LOAEL for single exposures is 2.8 mg/m3 for respiratory effects in asthmatic individuals exposed for 30 min in an exposure chamber (Jappinen et al., 1990).
Effects observed in animals are similar to those that have been observed in humans. Death has occurred in animals after inhalation of high concentrations of hydrogen sulfide. Single, short-term and medium-term inhalation exposures to hydrogen sulfide have also resulted in respiratory, olfactory, cardiovascular, neurological, hepatic, and developmental neurochemical effects and abnormal growth in developing cerebellar Purkinje cells in animals. There are no data on medium-term exposure of humans to hydrogen sulfide. Animal data showed that respiratory effects are the most sensitive end-point. For the B6C3 mice exposed 6 h/day, 5 days/week, for 90 days, the LOAEL was 110 mg/m3 for nasal mucosa inflammation; the NOAEL was 43 mg/m3 (CIIT, 1983a). Recently, a LOAEL of 42 mg/m3 was reported for nasal olfactory lesions in male Sprague-Dawley CD rats exposed 6 h/day, 7 days/week, for 10 weeks; the NOAEL was 14 mg/m3 (Brenneman et al., 2000).
There are no long-term animal studies. Health effects in human populations exposed for long periods to low levels of hydrogen sulfide cannot serve as a basis for setting tolerable concentrations (see section 10.2) because of either co-exposure to several substances or insufficient exposure characterization.
There are no human data on oral exposure to hydrogen sulfide. The only reported oral animal study (Wetterau et al., 1964) is of questionable validity.
Insufficient data exist with which to evaluate the carcinogenic or genotoxic potential of hydrogen sulfide.
Inhalation is the major route of exposure to hydrogen sulfide in the environment. Hydrogen sulfide is disruptive to the mitochondrial electron transport system and is thus expected to affect all systems; the most sensitive systems are the respiratory and central nervous systems. Hydrogen sulfide is also an ocular and respiratory tract irritant. For single exposures to high concentrations, the concentration drives toxicity, and duration is much less significant. Hydrogen sulfide is not mutagenic. No studies are available that demonstrate clear evidence of cancer in humans after exposure to hydrogen sulfide. Therefore, tolerable concentrations for hydrogen sulfide should be based on non-cancer effects from available studies with the most sensitive end-point. Human data are preferred. In the absence of adequate human data, experimental animal data can be used. The tolerable concentrations calculated herein are appropriate for the setting of ambient air limits.
A short-term tolerable concentration can be derived based on the LOAEL of 2.8 mg/m3 for bronchial obstruction in asthmatic individuals (Jappinen et al., 1990). The short-term tolerable concentration is for exposure durations from 1 to 14 days. The effect on respiratory function in 10 asthmatic individuals (7 women and 3 men) exposed to 2.8 mg hydrogen sulfide/m3 for 30 min in an exposure chamber was evaluated. The patients had had bronchial asthma for 1–13 years (mean 3.7 years) and had been taking medication. Patients with severe asthma were not included in the study because the patients did not take medication for 2 days prior to exposure to hydrogen sulfide. Raw and SGaw were assessed by a body plethysmograph, and the ventilatory capacities were measured with a flow volume spirometer. No statistically significant changes in FVC, FEV1, or forced expiratory flow were seen in these individuals. Average Raw was increased by 26.5%, and SGaw was decreased by 8.4%. These changes were not statistically significant. However, changes greater than 30% in both Raw and SGaw were seen in two subjects, indicating bronchial obstruction. Three out of the 10 subjects also reported headaches after exposure.
The asthmatic individuals represent a sensitive population, and the observed effects are of relevance to humans. Thus, the study is suitable for derivation of a tolerable concentration. An uncertainty factor (UF) of 30 is applied to the LOAEL of 2.8 mg/m3 (10 for using a LOAEL, and 3 for human variability), resulting in a short-term tolerable concentration of approximately 100 µg/m3 (LOAEL/UF = 2.8 mg/m3 / 30 ≈ 100 µg/m3). An uncertainty factor of 3 was used for human variability to protect all subpopulations, including the most sensitive population (e.g., persons with severe asthma). The LOAEL was not time-adjusted to 24-h exposure because for single exposures to high concentrations, the hydrogen sulfide toxicity is concentration- rather than duration-dependent (Guidotti, 1996).
A medium-term tolerable concentration can be derived based on the NOAEL of 14 mg/m3 for nasal lesions in the olfactory mucosa in the Brenneman et al. (2000) study in which male Sprague-Dawley CD rats were exposed 6 h/day, 7 days/week, for 10 weeks. The medium-term tolerable concentration is for exposure durations up to 90 days. Rats (n = 12 per group) were exposed to 0, 14, 42, or 110 mg hydrogen sulfide/m3. A significant increase in nasal lesions in the olfactory mucosa was observed in rats exposed to 42 and 110 mg/m3. The lesions included olfactory neuron loss and basal cell hyperplasia. They were multifocal, bilaterally symmetrical, and distributed rostrocaudally. The dorsal medial meatus and the dorsal and medial portions of ethmoid recess of the nasal cavity were affected. The NOAEL is adjusted for intermittent exposure:
NOAELADJ = 14 mg/m3 × 6 h/24 h = 3.5 mg/m3
The human equivalent NOAEL (NOAELHEC) is then calculated for a "gas:respiratory" effect in the extrathoracic region (US EPA, 1994), as described below.
The regional gas dose ratio for the extrathoracic region [RGDR(ET)] is given as:
RGDR(ET) = (VE/SA(ET))a ÷ (VE/SA(ET))h
where VE = the minute volume and SA(ET) = the surface area of the extrathoracic region for the rat (a) and human (h).
VE(a) = 0.275 m3/day
VE(h) = 20 m3/day
SA(ET)a = 15 cm2
SA(ET)h = 200 cm2
RGDR(ET) = (0.275/15) ÷ (20/200) = 0.18
NOAELHEC = NOAELADJ × RGDR(ET) = 3.5 mg/m3 × 0.18 = 0.63 mg/m3
An uncertainty factor of 30 is applied: 3 for extrapolation from animals to humans and 10 for human variability. An uncertainty factor of 3 is used for interspecies extrapolation because dosimetry adjustment was applied when calculating the NOAELHEC. An uncertainty factor of 10 was used for human variability to protect the sensitive population. The medium-term tolerable concentration is therefore 20 µg/m3:
NOAELHEC ÷ UF = 0.63 mg/m3 ÷ 30 = 0.02 mg/m3 = 20 µg/m3
Ambient air concentrations of hydrogen sulfide in the USA are in the range of 0.14–0.4 µg/m3 (US EPA, 1993). These levels are well below both the short-term and medium-term tolerable concentrations of 100 and 20 µg/m3. The general population may be exposed to hydrogen sulfide by accidental release from natural gas wells during drilling operations near residential areas (Layton & Cederwall, 1986; Leahey & Schroeder, 1986). Maximum ground-level downwind hydrogen sulfide concentrations resulting from two sour gas well blowouts were estimated to be 2.8 and 20 mg/m3. Workers may be occupationally exposed to hazardous levels of hydrogen sulfide from fermenting manure (Morse et al., 1981) or stagnant wells (McDonald & McIntosh, 1951), as well as in poorly ventilated areas of wastewater treatment facilities (NIOSH, 1984, 1985a, 1990), extruded rubber plants (NIOSH, 1985b), and petroleum refineries (NIOSH, 1982a, 1982b). Hydrogen sulfide levels reported were >310 mg/m3 in a stagnant well; 70–280 mg/m3 in open maintenance ports at an oil refinery; and >700 mg/m3 at a wastewater treatment facility. The US ceiling recommended exposure limit (REL) for hydrogen sulfide is currently 14 mg/m3 for 10 min (NIOSH, 1997).
Environmental exposures to malodorous emissions are usually to a mixture of sulfur-containing gases. The exact concentration of hydrogen sulfide in these types of mixtures cannot be determined. In estimating exposure, there is also uncertainty about the dose and duration of exposure. Based on limited information, rodents appear to be less sensitive to hydrogen sulfide than humans. Since the respiratory tract is the major target organ of hydrogen sulfide toxicity, humans with asthma, the elderly, and young children with compromised respiratory function represent the sensitive subpopulations.
The LOAEL of 2.8 mg/m3 for bronchial constriction in 2 of 10 asthmatics in the Jappinen et al. (1990) study was selected as the basis to derive the short-term tolerable concentration. Three of the 10 subjects also complained of headaches after exposure. The study subjects had had asthma for 1–13 years and had been taking medication. Patients with severe asthma were not included in the study because the study subjects had to stop taking medication for 2 days prior to exposure. The subjects were exposed in an exposure chamber for 30 min. The asthmatics represent a sensitive population, and the route of exposure is relevant. This study is limited by the fact that there was a small number of study subjects, the study has not been repeated, and there were no obvious clinical symptoms. However, it should be noted that it would not be ethical to expose severe asthmatics to higher concentrations of hydrogen sulfide. Bhambhani & Singh (1991) and Bhambhani et al. (1996b, 1997) reported metabolic effects in young healthy volunteers exposed to 7 mg hydrogen sulfide/m3 for 30 min. However, the exposure route employed by Bhambhani & Singh (1991) and Bhambhani et al. (1996b, 1997) was by oral inhalation. The study subjects could not smell the hydrogen sulfide, and their eyes were not exposed. This LOAEL was therefore not used as the basis to derive the short-term tolerable concentration. Short-term tolerable concentrations derived from either study would not differ markedly.
Due to the serious toxic effects associated with exposures to high concentrations of hydrogen sulfide for very short durations, all exposure should be avoided. No toxicity data exist on medium- or long-term exposures of humans to low levels of hydrogen sulfide. This kind of data is of priority for evaluating health risks of exposure to hydrogen sulfide for populations living in the vicinity of hazardous waste sites and other potential sources of hydrogen sulfide, such as hot springs and wastewater treatment plants.
The WHO air quality guideline for hydrogen sulfide is 150 µg/m3 for an average concentration over 24 h. The health end-point was eye irritation. To avoid odour annoyance, a 30-min average ambient air concentration not exceeding 7 µg/m3 is recommended (WHO, 2000).
In the WHO guidelines for drinking-water (WHO, 1993), it is concluded that it is unlikely that anyone could consume a harmful dose of hydrogen sulfide in drinking-water; thus, no health-based guideline was proposed. However, it was stated that hydrogen sulfide should not be detected in drinking-water by taste or odour, and the taste and odour threshold for hydrogen sulfide in water was considered to be between 0.05 and 0.1 mg/litre.
Abe K, Kimura H (1996) The possible role of hydrogen sulfide as an endogenous neuromodulator. Journal of Neuroscience, 16:1066–1071.
Adelson L, Sunshine I (1966) Fatal hydrogen sulfide intoxication: report of three cases occurring in a sewer. Archives of Pathology, 81:375–380.
Ahlborg G (1951) Hydrogen sulfide poisoning in shale oil industry. Archives of Industrial Hygiene and Occupational Medicine, 3:247–266.
Almeida AF, Guidotti TL (1999) Differential sensitivity of lung and brain to sulfide exposure: a peripheral mechanism for apnea. Toxicological Sciences, 50:287–293.
Ammann HM (1986) A new look at physiologic respiratory response to hydrogen sulfide poisoning. Journal of Hazardous Materials, 13:369–374.
Amoore JE, Hautala E (1983) Odor as an aid to chemical safety: odor thresholds compared with threshold limit values and volatilities for 214 industrial chemicals in air and water dilution. Journal of Applied Toxicology, 3:272–290.
Arnold IM, Dufresne RM, Alleyne BC, Stuart PJ (1985) Health implication of occupational exposures to hydrogen sulfide. Journal of Occupational Medicine, 27(5):373–376.
ATSDR (1999) Toxicological profile for hydrogen sulfide. Atlanta, GA, US Department of Health and Human Services, Agency for Toxic Substances and Disease Registry.
Audeau FM, Gnanaharan C, Davey K (1985) Hydrogen sulphide poisoning: associated with pelt processing. New Zealand Medical Journal, 98:145–147.
Bartholomew TC, Powell GM, Dodgson KS, Curtis CG (1980) Oxidation of sodium sulphide by rat liver, lungs and kidney. Biochemical Pharmacology, 29:2431–2437.
Bates MN, Garrett N, Graham B, Read D (1997) Air pollution and mortality in the Rotorua geothermal area. Australia and New Zealand Journal of Public Health, 21:581–586.
Bates MN, Garrett N, Graham B, Read D (1998) Cancer incidence, morbidity and geothermal air pollution in Rotorua, New Zealand. International Journal of Epidemiology, 27:10–14.
Beauchamp RO Jr, Bus JS, Popp JA, Boreiko CJ, Andjelkovich DA (1984) A critical review of the literature on hydrogen sulfide toxicity. Critical Reviews in Toxicology, 13:25–97.
Beck JF, Cormier F, Donini JC (1979) The combined toxicity of ethanol and hydrogen sulfide. Toxicology Letters, 3:311–313.
Bhambhani Y, Singh M (1991) Physiological effects of hydrogen sulfide inhalation during exercise in healthy men. Journal of Applied Physiology, 71:1872–1877.
Bhambhani Y, Burnham R, Snydmiller G, MacLean I, Martin T (1994) Comparative physiological responses of exercising men and women to 5 ppm hydrogen sulfide exposure. American Industrial Hygiene Association Journal, 55:1030–1035.
Bhambhani Y, Burnham R, Snydmiller G, MacLean I, Lovlin R (1996a) Effects of 10-ppm hydrogen sulfide inhalation on pulmonary function in healthy men and women. Journal of Occupational and Environmental Medicine, 38:1012–1017.
Bhambhani Y, Burnham R, Snydmiller G, MacLean I, Martin T (1996b) Effects of 5 ppm hydrogen sulfide inhalation on biochemical properties of skeletal muscle in exercising men and women. American Industrial Hygiene Association Journal, 57:464–468.
Bhambhani Y, Burnham R, Snydmiller G, MacLean I (1997) Effects of 10-ppm hydrogen sulfide inhalation in exercising men and women. Journal of Occupational and Environmental Medicine, 39:122–129.
Boon AG (1992) Septicity in sewers: causes, consequences and containment. Journal of Water Engineering and Management, 6:79–90.
Bottenheim JW, Strausz OP (1980) Gas-phase chemistry of clean air at 55°N latitude. Environmental Science and Technology, 14:709–718.
Brenneman KA, James A, Gross EA, Dorman DC (2000) Olfactory neuron loss in adult male CD rats following subchronic inhalation exposure to hydrogen sulfide. Toxicologic Pathology, 28:326–333.
Breysse PA (1961) Hydrogen sulfide fatality in a poultry feather fertilizer plant. American Industrial Hygiene Association Journal, 22:220–222.
Buick JB, Lowry RC, Magee TR (2000) Is a reduction in residual volume a sub-clinical manifestation of hydrogen sulfide intoxication. American Journal of Industrial Medicine, 37:296–299.
Burnett WW, King EG, Grace M, Hall WF (1977) Hydrogen sulfide poisoning: Review of 5 years’ experience. Canadian Medical Association Journal, 117:1277–1280.
Chance B, Schoener B (1965) High and low energy states of cytochromes. I. In mitochondria. Journal of Biological Chemistry, 241:4567–4573.
Cho KS, Hirai M, Shoda M (1992) Degradation of hydrogen sulfide by Xanthomonas sp. strain DY44 isolated from peat. Applied Environmental Microbiology, 58:1183–1189.
Cihacek LJ, Bremner JM (1993) Characterization of the sulfur retained by soils exposed to hydrogen sulfide. Communications in Soil Science and Plant Analysis, 24:85–92.
CIIT (1983a) 90 day vapor inhalation toxicity study of hydrogen sulfide in B6C3F1 mice. Report to the Chemical Industry Institute of Toxicology, Research Triangle Park, NC, by ToxiGenics, Inc. (CIIT Docket No. 42063).
CIIT (1983b) 90 day vapor inhalation toxicity study of hydrogen sulfide in Fischer 344 rats. Report to the Chemical Industry Institute of Toxicology, Research Triangle Park, NC, by ToxiGenics, Inc. (CIIT Docket No. 22063).
CIIT (1983c) 90 day vapor inhalation toxicity study of hydrogen sulfide in Sprague-Dawley rats. Report to the Chemical Industry Institute of Toxicology, Research Triangle Park, NC, by ToxiGenics, Inc. (CIIT Docket No. 32063).
Curtis SE, Anderson CR, Simon J, Jensen AH, Day DL, Kelley KW (1975) Effects of aerial ammonia, hydrogen sulfide and swine-house dust on rate of gain and respiratory-tract structure in swine. Journal of Animal Science, 41:735–739.
De Kok LJ, Thompson CR, Mudd JB, Kats G (1983) Effect of hydrogen sulfide fumigation on water-soluble sulfhydryl compounds in shoots of crop plants. Zeitschrift für Pflanzenphysiologie und Bodenkunde, 111:85–89.
De Kok LJ, Maas FM, Stulen I, Juiper PJC (1988) Sulfur containing air pollutants and their effects on plant metabolism. Commission of European Communities, 11244:620–625.
De Kok LJ, Rennenberg H, Kuiper PJC (1991) The internal resistance in spinach leaves to atmospheric hydrogen sulfide deposition is determined by metabolic processes. Plant Physiology and Biochemistry, 29:463–470.
Deng JF, Chang SC (1987) Hydrogen sulfide poisonings in hotspring reservoir cleaning: two case reports. American Journal of Industrial Medicine, 11:447–451.
Dillon TM, Moore DW, Gibson AB (1993) Development of a chronic sublethal bioassay for evaluating contaminated sediment with the marine polychaete worm Nereis (Neanthes) arenaceodentata. Environmental Toxicology and Chemistry, 12:589–605.
Dorman DC, Brenneman KA, Struve MF, Miller KL, James RA, Marshall MW, Foster PM (2000) Fertility and developmental neurotoxicity effects of inhaled hydrogen sulfide in Sprague-Dawley rats. Neurotoxicology and Teratology, 22:71–84.
Green FHY, Schurch S, De Sanctis GT, Wallace JA, Cheng S, Prior M (1991) Effects of hydrogen sulfide exposure on surface properties of lung surfactant. Journal of Applied Physiology, 70:1943–1949.
Guidotti TL (1996) Hydrogen sulphide. Occupational Medicine, 46:367–371.
Haahtela T, Marttila O, Vilkka V, Jappinen P, Jaakkola JJ (1992) The South Karelia Air Pollution Study: acute health effects of malodorous sulfur air pollutants released by a pulp mill. American Journal of Public Health, 82:603–605.
Haider SS, Hasan M, Islam F (1980) Effect of air pollutant hydrogen sulfide on the levels of total lipids, phospholipids & cholesterol in different regions of the guineapig brain. Indian Journal of Experimental Biology, 18:418–420.
Hannah RS, Roth SH (1991) Chronic exposure to low concentrations of hydrogen sulfide produces abnormal growth in developing cerebellar Purkinje cells. Neuroscience Letters, 122:225–228.
Hannah RS, Hayden LJ, Roth SH (1989) Hydrogen sulfide exposure alters the amino acid content in developing rat CNS. Neuroscience Letters, 99:323–327.
Hannah RS, Bennington R, Roth SH (1990) A relationship between hydrogen sulfide exposure and taurine levels in maternal rats. Proceedings of the Western Pharmacology Society, 33:177–179.
Hayden LJ, Goeden H, Roth SH (1990a) Exposure to low levels of hydrogen sulfide elevates circulating glucose in maternal rats. Journal of Toxicology and Environmental Health, 31:45–52.
Hayden LJ, Goeden H, Roth SH (1990b) Growth and development in the rat during subchronic exposure to low levels of hydrogen sulfide. Toxicology and Industrial Health, 6:389–401.
HazDat (1997) Hazardous substance release and health effects database. Atlanta, GA, US Department of Health and Human Services, Agency for Toxic Substances and Disease Registry.
Hemminki K, Niemi ML (1982) Community study of spontaneous abortions: relation to occupation and air pollution by sulfur dioxide, hydrogen sulfide, and carbon disulfide. International Archives of Occupational and Environmental Health, 51:55–63.
Hessel PA, Herbert FA, Melenka LS, Yoshida K, Nakaza M (1997) Lung health in relation to hydrogen sulfide exposure in oil and gas workers in Alberta, Canada. American Journal of Industrial Medicine, 31:554–557.
Higuchi Y, Fukamachi M (1977) [Behavioral studies on toxicity of hydrogen sulfide by means of conditioned avoidance responses in rats.] Folia Pharmacologica Japonica, 73:307–319 (in Japanese).
Hill FB (1973) Atmospheric sulfur and its links to the biota. Brookhaven Symposia in Biology, 30:159–181.
Hirsch AR, Zavala G (1999) Long term effects on the olfactory system of exposure to hydrogen sulphide. Occupational and Environmental Medicine, 56:284–287.
Hoffman HE, Guidotti TL (1997) Natural gas. In: Greenberg MI, ed. Occupational, industrial, and environmental toxicology. Mosby, Philadelphia, PA, pp. 359–366.
Hoke RA, Giesy JP, Zabik M, Unger M (1993) Toxicity of sediments and sediment pore waters from the Grand Calumet River–Indiana Harbor, Indiana area of concern. Ecotoxicology and Environmental Safety, 26:86–112.
Hollis JP Jr (1985) Hydrogen sulfide in Louisiana rice fields. Acta Phytopathologica Academiae Scientiarum Hungaricae, 20:321–326.
Hosoki R, Matsuki N, Kimura H (1997) The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochemical and Biophysical Research Communications, 237:527–531.
HSDB (1998) Hazardous substances data bank. Bethesda, MD, National Library of Medicine, National Toxicology Program, 25 February.
IPCS (1981) Hydrogen sulfide. Geneva, World Health Organization, International Programme on Chemical Safety (Environmental Health Criteria 19).
IPCS (2000) International Chemical Safety Card — Hydrogen sulfide. Geneva, World Health Organization, International Programme on Chemical Safety (ICSC 0165).
Jaakkola JJ, Vilkka V, Marttila O, Jappinen P, Haahtela T (1990) The South Karelia Air Pollution Study: the effects of malodorous sulfur compounds from pulp mills on respiratory and other symptoms. American Review of Respiratory Diseases, 142:1344–1350.
Jaakkola JJ, Partti-Pellinen K, Marttila O (1999) The South Karelia Air Pollution Study: changes in respiratory health in relation to emission reduction of malodorous sulfur compounds from pulp mills. Archives of Environmental Health, 54:254–263.
Jappinen P, Vilkka V, Marttila O, Haahtela T (1990) Exposure to hydrogen sulphide and respiratory function. British Journal of Industrial Medicine, 47:824–828.
Jørgensen BB (1982) Ecology of the bacteria of the sulphur cycle with special reference to anoxic–oxic interface environments. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences, 298:543–561.
Kage S, Nagata T, Kimura K, Kudo K, Imamura T (1992) Usefulness of thiosulfate as an indicator of hydrogen sulfide poisoning in forensic toxicological examination: a study with animal experiments. Japanese Journal of Forensic Toxicology, 10(3):223–227.
Kage S, Takekawa K, Kurosaki K, Imamura T, Kudo K (1997) The usefulness of thiosulfate as an indicator of hydrogen sulfide poisoning: three cases. International Journal of Legal Medicine, 110:220–222.
Kangas J, Savolainen H (1987) Urinary thiosulphate as an indicator of exposure to hydrogen sulphide vapour. Clinica Chimica Acta, 164:710.
Kauppinen T, Teschke K, Savela A, Kogevinas M, Boffetta P (1997) International data base of exposure measurements in the pulp, paper and paper product industries. International Archives of Occupational and Environmental Health, 70:119–127.
Khan AA, Schuler MM, Prior MG, Yong S, Coppock RW, Florence LZ, Lillie LE (1990) Effects of hydrogen sulfide exposure on lung mitochondrial respiratory chain enzymes in rats. Toxicology and Applied Pharmacology, 103:482–490.
Khan AA, Yong S, Prior MG, Lillie LE (1991) Cytotoxic effects of hydrogen sulfide on pulmonary alveolar macrophages in rats. Journal of Toxicology and Environmental Health, 33:57–64.
Koe LCC (1985) Ambient hydrogen sulfide levels at a wastewater treatment plant. Environmental Monitoring and Assessment, 5:101–108.
Kohno M, Tanaka E, Nakamura T, Shimojo N, Misawa S (1991) [Influence of short-term inhalation of hydrogen sulfide in rats.] Eisei Kagaku (Japanese Journal of Toxicology and Environmental Health), 37:103–106 (in Japanese).
Kombian SB, Warenycia MW, Mele FG, Reiffenstein RJ (1988) Effects of acute intoxication with hydrogen sulphide on central amino acid transmitter systems. Neurotoxicology, 9:587–596.
Kosmider S, Rogala E, Pacholek A (1967) Electrocardiographic and histochemical studies of the heart muscle in acute experimental hydrogen sulfide poisoning. Archivum Immunologiae et Therapiae Experimentalis,15:731–740.
Krekel K (1964) [Electrocardiographic (ECG) changes in two workers after hydrogen sulfide poisoning.] Zentralblatt für Arbeitsmedizin und Arbeitsschutz, 14:159–163 (in German).
Layton DW, Cederwall RT (1986) Assessing and managing the risks of accidental releases of hazardous gas: a case study of natural gas wells contaminated with hydrogen sulfide. Environment International, 12:519–532.
Leahey DM, Schroeder MB (1986) Predictions of maximum ground-level hydrogen sulfide concentrations resulting from two sour gas well blowouts. Journal of the Air Pollution Control Association, 36:1147–1149.
Lopez A, Prior M, Yong S, Albassam M, Lillie LE (1987) Biochemical and cytological alterations in the respiratory tract of rats exposed for 4 hours to hydrogen sulfide. Fundamental and Applied Toxicology, 9:753–762.
Lopez A, Prior M, Lillie LE, Gulayets C, Atwal OS (1988a) Histologic and ultrastructural alterations in lungs of rats exposed to sub-lethal concentrations of hydrogen sulfide. Veterinary Pathology, 25:376–384.
Lopez A, Prior M, Yong S, Lillie LE, Lefebvre M (1988b) Nasal lesions in rats exposed to hydrogen sulfide for four hours. American Journal of Veterinary Research, 49:1107–1111.
Lopez A, Prior M, Reiffenstein R, Goodwin LR (1989) Peracute toxic effects of inhaled hydrogen sulfide and injected sodium hydrosulfide on the lungs of rats. Fundamental and Applied Toxicology, 12:367–373.
Luck J, Kaye SB (1989) An unrecognized form of hydrogen sulphide keratoconjunctivitis. British Journal of Industrial Medicine, 46:748–749.
Marttila O, Haahtela T, Silakoski I, Vaittinen S, Suominen O (1994a) The South Karelia Air Pollution Study: relationship of outdoor and indoor concentrations of malodorous sulfur compounds released by pulp mills. Journal of the Air and Waste Management Association, 44:1093–1096.
Marttila O, Jaakkola JK, Vilkka V, Jäppinen P, Haahtela T (1994b) The South Karelia Air Pollution Study: the effects of malodorous sulfur compounds from pulp mills on respiratory and other symptoms in children. Environmental Research, 66:152–159.
Marttila O, Jaakkola JK, Partti-Pellinen K, Vilkka V, Haahtela T (1995) South Karelia Air Pollution Study: daily symptom intensity in relation to exposure levels of malodorous sulfur compounds from pulp mills. Environmental Research, 71:122–127.
McDonald JM, McIntosh AP (1951) Fatalities from hydrogen sulfide in wells. Archives of Industrial Hygiene and Occupational Medicine, 3:445–447.
Milby TH (1962) Hydrogen sulfide intoxication: review of the literature and report of an unusual accident resulting in two cases of nonfatal poisoning. Journal of Occupational Medicine, 4:431–437.
Millero FJ, LeFerriere A, Fernandez M, Hubinger S, Hershey JP (1989) Oxidation of hydrogen sulfide with H2O2 in natural waters. Environmental Science and Technology, 23(2):209–213.
Morse DL, Woodbury MA, Rentmeester K, Farmer D (1981) Death caused by fermenting manure. Journal of the American Medical Association, 245:63–64.
Nagata T, Kage S, Kimura K, Kudo K, Noda M (1990) Sulfide concentrations in postmortem mammalian tissues. Journal of Forensic Sciences, 35:706–712.
Nicholls P (1975) The effect of sulphide on cytochrome aa3. Isoteric and allosteric shifts of the reduced a-peak. Biochimica et Biophysica Acta, 396:24–35.
Nicholson RA, Roth SH, Jian Zheng AZ (1998) Inhibition of respiratory and bioenergetic mechanisms by hydrogen sulfide in mammalian brain. Journal of Toxicology and Environmental Health, 54:491–507.
NICNAS (1995) Full public report: Sodium ethyl xanthate. Canberra, Australian Government Printing Services (National Industrial Chemicals Notification Assessment Scheme Priority Existing Chemical No. 5).
NIOSH (1977) Criteria for a recommended standard: occupational exposure to hydrogen sulfide. Cincinnati, OH, US Department of Health and Human Services, National Institute for Occupational Safety and Health (NIOSH 77158; NTIS Publication No. PB274196).
NIOSH (1982a) Control technology assessment for coal gasification and liquefaction processes, Rockwell International, Santa Susana, California. Cincinnati, OH, US Department of Health and Human Services, National Institute for Occupational Safety and Health (NTIS Publication No. PB84-182724).
NIOSH (1982b) Health hazard evaluation report HETA 81-327-1161, Caribbean Gulf Refining Corporation, Bayamon, Puerto Rico. Cincinnati, OH, US Department of Health and Human Services, National Institute for Occupational Safety and Health (NTIS Publication No. PB84-150333).
NIOSH (1984) Health hazard evaluation report HETA 83-440-1537, Papillion Creek Wastewater Treatment Plant, Omaha, Nebraska. Cincinnati, OH, US Department of Health and Human Services, National Institute for Occupational Safety and Health (NTIS Publication No. PB85-208270).
NIOSH (1985a) Fatal accident circumstances and epidemiology (FACE) report: Two sanitation employees die in confined space in Kentucky, August 24, 1985. Morgantown, WV, US Department of Health and Human Services, National Institute for Occupational Safety and Health (Report No. FACE-85-44; NTIS Publication No. PB91-197848).
NIOSH (1985b) Health hazard evaluation report HETA 80-13/81-147-1644, Schlegel Tennessee, Inc., Maryville, Tennessee. Cincinnati, OH, US Department of Health and Human Services, National Institute for Occupational Safety and Health (NTIS Publication No. PB86-221355).
NIOSH (1990) Hazard evaluation and technical assistance report HETA 89-379 and 90-282-L2074, Stone Container Corporation, Missoula, Montana. Cincinnati, OH, US Department of Health and Human Services, National Institute for Occupational Safety and Health (NTIS Publication No. PB91212761).
NIOSH (1991) Fatal accident circumstances and epidemiology (FACE) report: two maintenance workers die after inhaling hydrogen sulfide in manhole, January 31, 1989. Morgantown, WV, US Department of Health and Human Services, National Institute for Occupational Safety and Health (NTIS Publication No. PB91212761).
NIOSH (1997) NIOSH pocket guide to chemical hazards. Cincinnati, OH, US Department of Health and Human Services, National Institute for Occupational Safety and Health (DHHS (NIOSH) Publication No. 94116).
NSF (1976) Behavior of hydrogen sulfide in the atmosphere and its effects on vegetation. Report to the National Science Foundation, Research Applied to National Needs, Washington, DC, by Thompson RC, University of California, Statewide Air Pollution Research Center, Riverside, CA (Report No. NSF/RA760398; NTIS Publication No. PB-262733).
Oderda GM (1975) Fatality produced by accidental inhalation of drain cleaner fumes. Clinical Toxicology, 8:547–551.
OSHA (2002) Hydrogen sulfide in workplace atmospheres. Method number ID-141. US Department of Labor, Occupational Safety & Health Administration, at website http://www.osha.gov/dts/sltc/methods/inorganic/id141/id141.html.
OSU (2001) Ohio State University fact sheet: Wyandot County ground-water resources. Columbus, OH, Ohio State University, at website (Fact Sheet No. AEX-490.88-97).
Parra O, Monso E, Gallego M, Morera J (1991) Inhalation of hydrogen sulphide: a case of subacute manifestations and long term sequelae. British Journal of Industrial Medicine, 48:286–287.
Partti-Pellinen K, Marttila O, Vilkka V, Jaakkola JK, Jäppinen P, Haahtela T (1996) The South Karelia Air Pollution Study: effects of low-level exposure to malodorous sulfur compounds on symptoms. Archives of Environmental Health, 51:315–320.
Parvinen P, Lajunen LHJ (1994) Determination of sulfide as hydrogen sulfide in water and sludge samples by gas phase molecular AS. Atomic Spectroscopy, 15:83–86.
Patterson CG, Runnells DD (1992) Dissolved gases in groundwater as indicators of redox conditions. In: Kharaka YK, Maest AS, eds. Water–rock interaction, proceedings of the 7th international symposium, Vol. 1. Rotterdam, A.A. Balkema, pp. 517–520.
Phae CG, Shoda M (1991) A new fungus which degrades hydrogen sulfide, methanethiol, dimethyl sulfide and dimethyl disulfide. Biotechnology Letters, 13:375–380.
Prior MP, Sharma AK, Yong S, Lopez A (1988) Concentration–time interactions in hydrogen sulphide toxicity in rats. Canadian Journal of Veterinary Research, 52:375–379.
Prior M, Green F, Lopez A, Balu A, De Sanctis GT, Fick G (1990) Capsaicin pretreatment modifies hydrogen sulphide-induced pulmonary injury in rats. Toxicologic Pathology, 18:279–288.
Radford-Knoery J, Cutter GA (1993) Determination of carbonyl sulfide and hydrogen sulfide species in natural waters using specialized collection procedures and gas chromatography with flame photometric detection. Analytical Chemistry, 65:976–982.
Reiffenstein RJ, Hulbert WC, Roth SH (1992) Toxicology of hydrogen sulfide. Annual Review of Pharmacology and Toxicology, 32:109–134.
Richardson DB (1995) Respiratory effects of chronic hydrogen sulfide exposure. American Journal of Industrial Medicine, 28:99–108.
Riffat R, Weeks JL, Brady P (1999) Safety alert. Research indicates that even long-term, low-level exposure to hydrogen sulfide emissions can cause significant health effects. Industrial Wastewater, May/June, pp. 31–34.
Rosenberg M, Septon I, Eli I, Bar-Ness R, Gelernter I, Brenner S, Gabbay J (1991) Halitosis measurement by an industrial sulphide monitor. Journal of Periodontology, 62:487–489.
Roth SH, Skrajny B, Reiffenstein RJ (1995) Alteration of the morphology and neurochemistry of the developing mammalian nervous system by hydrogen sulphide. Clinical and Experimental Pharmacology and Physiology, 22:379–380.
Saillenfait AM, Bonnet P, de Ceaurriz J (1989) Effects of inhalation exposure to carbon disulfide and its combination with hydrogen sulfide on embryonal and fetal development in rats. Toxicology Letters, 48:57–66.
Schechter MT, Spitzer WO, Hutcheon ME, Dales RE, Eastridge LM, Steinmetz M, Tousignant P, Hobbs C (1989) Cancer downwind from sour gas refineries: the perception and the reality of an epidemic. Environmental Health Perspectives, 79:283–290.
Siegel SM, Penny P, Siegel BZ, Penny D (1986) Atmospheric hydrogen sulfide levels at the Sulfur Bay Wildlife area, Lake Rotorua, New Zealand. Water, Air, and Soil Pollution, 28:385–391.
Skrajny B, Hannah RS, Roth SH (1992) Low concentrations of hydrogen sulphide alter monoamine levels in the developing rat central nervous system. Canadian Journal of Physiology and Pharmacology, 70:1515–1518.
Slooff W, Bont PFH, Janus JA, Ros JPM (1991) Exploratory report, hydrogen sulphide. Bilthoven, National Institute of Public Health and Environmental Protection (Report No. RIVM710401011; PB92209105).
Smith L, Kruszyna H, Smith RP (1977) The effect of methemoglobin on the inhibition of cytochrome c oxidase by cyanide, sulfide or azide. Biochemical Pharmacology, 26:2247–2250.
Smith RP, Gosselin RE (1964) The influence of methemoglobinemia on the lethality of some toxic anions. II. Sulfide. Toxicology and Applied Pharmacology, 6:584–592.
Smith RP, Gosselin RE (1979) Hydrogen sulfide poisoning. Journal of Occupational Medicine, 21:93–97.
Spolyar LW (1951) Three men overcome by hydrogen sulfide in starch plant. Industrial Health Monthly, 11:116–117.
Takemoto BK, Noble RD, Harrington HM (1986) Differential sensitivity of duckweeds (Lemnaceae) to sulfite: II. Thiol production and hydrogen sulphide emission as factors influencing sulphite phytotoxicity under low and high irradiance. New Phytologist, 103:541–548.
Thoman M (1969) Sewer gas: hydrogen sulfide intoxication. Clinical Toxicology, 2:383–386.
Tomar M, Abdullah THA (1994) Evaluation of chemicals to control the generation of malodorous hydrogen sulfide in waste water. Water Research, 28:2545–2552.
Tonzetich J, Carpenter PAW (1971) Production of volatile sulphur compounds from cysteine, cystine and methionine by human dental plaque. Archives of Oral Biology, 16:599–607.
Tvedt B, Edland A, Skyberg K, Forberg O (1991a) Delayed neuropsychiatric sequelae after acute hydrogen sulfide poisoning: affection of motor function, memory, vision and hearing. Acta Neurologica Scandinavica, 84:348–351.
Tvedt B, Skyberg K, Aaserud O, Hobbesland A, Mathiesen T (1991b) Brain damage caused by hydrogen sulfide: a follow-up study of six patients. American Journal of Industrial Medicine, 20:91–101.
Tyagi RD, Tran FT, Polprasert C (1988) Bioconversion of lignosulphonate into lignin and hydrogen sulfide by mutualistic bacterial system. Journal of Microbial Biotechnology, 3:90–98.
US EPA (1978) Hydrogen sulfide. Report to the Health Effects Research Laboratory, US Environmental Protection Agency, Research Triangle Park, NC, by the Committee on Medical and Biologic Effects of Environmental Pollutants, National Research Council, Washington, DC (EPA-600/1-78018; NTIS Publication No. PB278576).
US EPA (1984) Validation of chemical and biological techniques for evaluation of vapors in ambient air/mutagenicity testing of twelve (12) vapor-phase compounds. Report to the Health Effects Research Laboratory, US Environmental Protection Agency, Research Triangle Park, NC, by Hughes TJ, Sparacino C, Frazier S, Research Triangle Institute, Research Triangle Park, NC (EPA-600/1-84-005; NTIS Publication No. PB84-164219).
US EPA (1987) A new look at physiologic respiratory response to hydrogen sulfide poisoning. Research Triangle Park, NC, US Environmental Protection Agency, Office of Research and Development.
US EPA (1993) Report to Congress on hydrogen sulfide air emissions associated with the extraction of oil and natural gas. Research Triangle Park, NC, US Environmental Protection Agency, Office of Air Quality Planning and Standards (EPA/453/R93045; NTIS Publication No. PB941312240).
US EPA (1994) Methods for derivation of inhalation reference concentrations and application of inhalation dosimetry. Washington, DC, US Environmental Protection Agency, Office of Research and Development (EPA/600/8-90/066F).
Vanhoorne M, De Rouck A, De Bacquer D (1995) Epidemiological study of eye irritation by hydrogen sulfide and/or carbon disulphide exposure in viscose rayon workers. Annals of Occupational Hygiene, 3:307–315.
Vismann B (1991) Physiology of sulfide detoxification in the isopod Saduria (Mesidotea) entomon. Marine Ecology Progress Series, 76:283–293.
Warenycia MW, Goodwin LR, Benishin CG, Reiffenstein RJ, Francom DM, Taylor JD, Dieken FP (1989) Acute hydrogen sulfide poisoning: demonstration of selective uptake of sulfide by the brainstem by measurement of brain sulfide levels. Biochemical Pharmacology, 38:973–981.
Weisiger RA, Jakoby WB (1980) Thiol-S-methyltransferase from rat liver. Archives of Biochemistry and Biophysics, 196:631–637.
Weisiger RA, Pinkus LM, Jakoby WB (1980) Thiol S-methyltransferase: suggested role in detoxication of intestinal hydrogen sulfide. Biochemical Pharmacology, 29:2885–2887.
Wetterau H, Oekert W, Knape UG (1964) [Tests for the application of dried green fodder with higher hydrogen sulfide content (experiments with poultry and fattened pigs).] Fetterung, 5:383–393 (in German).
WHO (1993) Guidelines for drinking-water quality, 2nd ed. Vol. 2. Health criteria and other supporting information. Geneva, World Health Organization, p. 48.
WHO (2000) Air quality guidelines for Europe, 2nd ed. Copenhagen, World Health Organization Regional Publications, European Series.
Wilson LG, Bressan RA, Filner P (1978) Light-dependent emission of hydrogen sulfide from plants. Plant Physiology, 61:184–189.
Winek CL, Collom WD, Wecht CH (1968) Death from hydrogen sulfide fumes. The Lancet, 1:1096.
Xu X, Cho SI, Sammel M, You L, Cui S, Huang Y, Ma G, Padungtod C, Pothier L, Niu T, Christiani D, Smith T, Ryan L, Wang L (1998) Association of petrochemical exposure with spontaneous abortion. Occupational and Environmental Medicine, 55:31–36.
Agency for Toxic Substances and Disease Registry (ATSDR, 1999)
Copies of the ATSDR toxicological profile for hydrogen sulfide (ATSDR, 1999) may be obtained from:
Agency for Toxic Substances and Disease Registry
Division of Toxicology/Toxicology Information Branch
1600 Clifton Road, NE, MS E-29
Atlanta, Georgia 30333
USA
The profile has undergone the following ATSDR internal reviews: Health Effects Review, Minimal Risk Level Review, and Data Needs Review. In addition, a peer review panel, which included Dr Alan Hall (University of Colorado), Mr Edwin Kinkead (private consultant, USA), and Dr James Way (Texas A & M University), was assembled.
The draft CICAD on hydrogen sulfide was sent for review to institutions and organizations identified by IPCS after contact with IPCS national Contact Points and Participating Institutions, as well as to identified experts. Comments were received from:
M. Baril, International Programme on Chemical Safety/Institut de Recherche en Santé et en Sécurité du Travail du Québec, Montreal, Quebec, Canada
S. Batt, National Industrial Chemicals Notification and Assessment Scheme, Sydney, Australia
R. Benson, Drinking Water Program, US Environmental Protection Agency, Denver, CO, USA
R. Cary, Health and Safety Executive, Bootle, Merseyside, United Kingdom
N. Cherry, University of Alberta, Edmonton, Alberta, Canada
R. Chhabra, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC, USA
S. Clark, Hydrogen Sulfide Panel, The American Chemistry Council, Arlington, VA, USA
J. Curless, National Institute of Occupational Safety and Health, Cincinnati, OH, USA
H. Gibb, National Center for Environmental Assessment, US Environmental Protection Agency, Washington, DC, USA
R. Hertel, Federal Institute for Health Protection of Consumers and Veterinary Medicine, Berlin, Germany
J. Kielhorn, Fraunhofer Institute of Toxicology and Aerosol Research, Hanover, Germany
H. Nagy, National Institute of Occupational Safety and Health, Cincinnati, OH, USA
P.I. Rabbani, Division of Risk Assessment, US Food and Drug Administration, Washington, DC, USA
D. Willcocks, National Industrial Chemicals Notification and Assessment Scheme, Sydney, Australia
K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umvelt und Gesundheit, Neuherberg, Oberschleissheim, Germany
Monks Wood, United Kingdom
16–19 September 2002
Members
Dr R. Benson, US Environmental Protection Agency, Region VIII, Denver, CO, USA
Mr R. Cary, Health and Safety Executive, Bootle, Merseyside, United Kingdom
Dr R. Chhabra, National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA
Dr S. Chou, Agency for Toxic Substances and Disease Registry (ATSDR), Atlanta, GA, USA
Dr S. Czerczak, Nofer Institute of Occupational Medicine, Lodz, Poland
Dr S. Dobson, Centre for Ecology and Hydrology, Monks Wood, Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr G. Dura, National Institute of Environmental Health, Jozsef Fodor Public Health Centre, Budapest, Hungary
Dr L. Fishbein, Fairfax, VA, USA
Dr H. Gibb, National Center for Environmental Assessment, US Environmental Protection Agency, Washington, DC, USA
Dr Y. Hayashi, Division of Chem-Bio Informatics, National Institute of Health Sciences, Ministry of Health, Labour and Welfare, Tokyo, Japan
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers and Veterinary Medicine, Berlin, Germany
Dr A. Hirose, Division of Risk Assessment, National Institute of Health Sciences, Tokyo, Japan
Mr P. Howe, Centre for Ecology and Hydrology, Monks Wood, Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Prof. J. Jeyaratnam, Colombo, Sri Lanka
Dr J. Kielhorn, Fraunhofer Institute of Toxicology and Aerosol Research, Hanover, Germany
Prof. Y.-X. Liang, School of Public Health, Fudan University, Shanghai Medical College, Shanghai, People’s Republic of China
Dr R. Liteplo, Existing Substances Division, Environmental Contaminants Bureau, Health Canada, Ottawa, Ontario, Canada
Ms M.E. Meek, Existing Substances Division, Safe Environments Programme, Health Canada, Ottawa, Ontario, Canada
Mr F.K. Muchiri, Directorate of Occupational Health and Safety Services, Nairobi, Kenya
Dr O. Sabzevari, Department of Toxicology & Pharmacology, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute of Health Sciences, Tokyo, Japan
Dr F.P. Simeonova, Sofia, Bulgaria
Dr J. Stauber, CSIRO Energy Technology, Centre for Advanced Analytical Chemistry, Bangor, Australia
Dr M.H. Sweeney, Document Development Branch, Education and Information Division, National Institute for Occupational Safety and Health, Cincinnati, OH, USA
Dr K. Ziegler-Skylakakis, European Commission, DG Employment & Social Affairs, Luxembourg
Resource Persons
Dr C. Cowles, Health and Safety Executive, Industrial Chemicals Unit HD, Bootle, Merseyside, United Kingdom
Dr C. Elliott-Minty, Health and Safety Executive, Industrial Chemicals Unit HD, Bootle, Merseyside, United Kingdom
Dr K. Fuller, Health and Safety Executive, Industrial Chemicals Unit HD, Bootle, Merseyside, United Kingdom
Observers
Mr A.G. Berends, Solvay S.A., Brussels, Belgium; European Chemical Industry Council / European Centre for Ecotoxicology and Toxicology of Chemicals (CEFIC/ECETOC)
Mr W. Gulledge, American Chemistry Council, Arlington, VA, USA
Mr C. Newsome, Dow Chemical Company Limited, West Drayton, Middlesex, United Kingdom; European Chemical Industry Council / European Centre for Ecotoxicology and Toxicology of Chemicals (CEFIC/ECETOC)
Mr M.A. Pemberton, Wilmslow, United Kingdom; European Chemical Industry Council / European Centre for Ecotoxicology and Toxicology of Chemicals (CEFIC/ECETOC)
Mr W. Stott, Dow Chemical Company, Midland, MI, USA; European Chemical Industry Council / European Centre for Ecotoxicology and Toxicology of Chemicals (CEFIC/ECETOC)
Mr J.M. Waechter, Jr, The Dow Chemical Company, Midland, MI, USA; European Chemical Industry Council / European Centre for Ecotoxicology and Toxicology of Chemicals (CEFIC/ECETOC)
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Mr T. Ehara, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Mr H. Malcolm, Centre for Ecology and Hydrology, Monks Wood, Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Ms C. Vickers, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
|
ATP |
adenosine triphosphate |
|
CI |
confidence interval |
|
CICAD |
Concise International Chemical Assessment Document |
|
FEV1 |
forced expiratory volume in 1 s |
|
FVC |
forced vital capacity |
|
ICSC |
International Chemical Safety Card |
|
IPCS |
International Programme on Chemical Safety |
|
LC50 |
median lethal concentration |
|
LOAEL |
lowest-observed-adverse-effect level |
|
NADH |
nicotinamide adenine dinucleotide, reduced form |
|
NADPH2 |
nicotinamide adenine dinucleotide phosphate, reduced form |
|
NOAEL |
no-observed-adverse-effect level |
|
NOAELADJ |
NOAEL adjusted for intermittent exposure |
|
NOAELHEC |
human equivalent NOAEL |
|
OR |
odds ratio |
|
Raw |
airway resistance |
|
REL |
recommended exposure limit |
|
RER |
respiratory exchange ratio |
|
RGDR(ET) |
regional gas dose ratio for the extrathoracic region |
|
RV |
residual volume |
|
SA(ET)a |
surface area of the extrathoracic region for animal |
|
SA(ET)h |
surface area of the extrathoracic region for human |
|
SD |
standard deviation |
|
SGaw |
specific airway conductance |
|
SIR |
standardized incidence ratio |
|
SMR |
standardized mortality ratio |
|
TRS |
total reduced sulfur |
|
UF |
uncertainty factor |
|
VE(a) |
minute volume for animal |
|
VE(h) |
minute volume for human |
|
WHO |
World Health Organization |
FEV1 – Forced expiratory volume at 1 s. The volume of air that can be forcibly exhaled during the first second of expiration following a maximal inspiration.
FVC – Forced vital capacity. The maximal volume of air that can be exhaled as forcibly and rapidly as possible after a maximal inspiration.
LC50 – Median lethal concentration. A calculated concentration of a chemical in air to which exposure for a specific length of time is expected to cause death in 50% of a defined experimental animal population.
LOAEL – Lowest-observed-adverse-effect level. The lowest exposure level of chemical in a study that produces statistically or biologically significant increases in frequency or severity of adverse effects between the exposed population and its appropriate control.
Medium-term exposure – Exposure to a chemical for a duration up to 50% of the life span of animals (e.g., 90-day studies).
NOAEL – No-observed-adverse-effect level. The dose of chemical at which there were no statistically or biologically significant increases in frequency or severity of adverse effects seen between the exposed population and its appropriate control. Effects may be produced at this dose, but they are not considered to be adverse.
REL – A National Institute for Occupational Safety and Health time-weighted-average concentration for up to a 10-h workday during a 40-h workweek.
RGDR – Regional gas dose ratio. The ratio of the deposited gas dose in a respiratory tract region for laboratory animals to that of humans.
Short-term exposure – Exposure to a chemical for a duration up to 28 days.
Single exposure – Exposure to a chemical for up to 24 h.
UF – Uncertainty factor. A factor used in deriving health guidance values from experimental data. UFs are intended to account for the variation in sensitivity among the members of the human population, the uncertainty in extrapolating animal data to the case of humans, the uncertainty in extrapolating from data obtained in a study that is of less than lifetime exposure, and the uncertainty in using LOAEL data rather than NOAEL data.
Ce CICAD sur le sulfure d’hydrogène a été préparé par l’Agency for Toxic Substances and Disease Registry des Etats-Unis. Le document original prend en compte les données répertoriées jusqu’en 1998 (ATDSR, 1999). En mars 2002, il a été procédé à une recherche bibliographique approfondie portant sur plusieurs bases de données en ligne afin de repérer toute référence sur le sujet publiée après celles qui ont été utilisées pour la rédaction du document original. Des informations sur la nature de l’examen par des pairs et sur la disponibilité du document original sont données à l’appendice 1. L’appendice 2 donne des renseignements sur l’examen par des pairs du présent CICAD. Ce CICAD a été approuvé en tant qu’évaluation internationale lors de la réunion du Comité d’évaluation finale qui s’est tenue à Monks Wood (Royaume-Uni), du 16 au 19 septembre 2002. La liste des participants à cette réunion figure à l’appendice 3. La fiche internationale sur la sécurité chimique du sulfure d’hydrogène (ICSC 0165) établie par le Programme international sur la sécurité chimique (IPCS, 2000), est également reproduite dans le présent document.
Le sulfure d’hydrogène ou hydrogène sulfuré (No CAS
Il existe diverses méthodes de préparation industrielle du sulfure d’hydrogène. La principale source de ce gaz réside dans sa récupération comme sous-produit de la purification du gaz naturel et des gaz de raffinerie. C’est également un sous-produit de l’industrie de la pâte à papier selon le procédé Kraft et de la production de sulfure de carbone. On l’utilise comme intermédiaire dans la production de l’acide sulfurique et des sulfures minéraux ainsi que comme désinfectant en agriculture. La décomposition par l’eau des xanthates (utilisés dans l’industrie minière) produit également du sulfure d’hydrogène.
La libération accidentelle ou l’élimination dans de mauvaises conditions des produits résultant de ces divers processus peuvent également entraîner des émissions de sulfure d’hydrogène. La libération d’hydrogène sulfuré dans l’environnement résulte principalement d’émissions dans l’air ambiant où ce composé ne devrait probablement pas rester plus d’une journée, encore qu’il puisse y persister jusqu’à 42 jours pendant l’hiver. Il peut facilement s’évaporer de l’eau, en fonction de la température et du pH. Il est peu probable qu’il subisse une bioconcentration et une biomagnification le long de la chaîne alimentaire.
La concentration de l’hydrogène sulfuré dans l’air des zones non polluées est très faible, se situant entre 0,03 et 0,1 μg/m3.
L’Homme peut être exposé à du sulfure d’hydrogène d’origine endogène ou exogène. La production endogène résulte majoritairement du métabolisme des acides aminés porteurs de groupements sulfhydryle, comme la cystéine, par les bactéries présentes dans l’intestin et dans la cavité buccale. De l’hydrogène sulfuré prend également naissance dans le cerveau et dans plusieurs muscles lisses (par ex. l’aorte thoracique, l’iléon et la veine porte) sous l’action des enzymes présentes dans ces tissus. Chez le rat, la concentration de sulfure d’hydrogène endogène est de 50-160 μmol/litre dans l’encéphale et de 1 mmol/litre dans l’iléon.
L’inhalation constitue la principale voie d’exposition humaine, le gaz étant ensuite rapidement résorbé au niveau du poumon. Le sulfure d’hydrogène comporte trois voies métaboliques : oxydation, méthylation et réactions avec des métalloprotéines ou des protéines à pont disulfure. La voie de détoxication prédominante consiste en une oxydation au niveau du foie, avec pour métabolite principal le thiosulfate, qui est ensuite converti en sulfate puis excrété dans l’urine. La méthylation constitue également une voie de détoxication. La toxicité du sulfure d’hydrogène résulte de sa réaction avec les métalloenzymes. Dans les mitochondries, la cytochrome-oxydase, qui constitue l’enzyme ultime de la chaîne respiratoire, est inhibée par le sulfure d’hydrogène; cette action rompt la chaîne de transport des électrons et inhibe le métabolisme oxydatif. Les tissus nerveux et cardiaques, dont les besoins en oxygène sont les plus importants, sont particulièrement sensibles à cette perturbation du métabolisme oxydatif. Au niveau du système nerveux central, il peut en résulter un arrêt respiratoire fatal.
L’expérimentation animale montre qu’une seule et unique exposition à du sulfure d’hydrogène provoque des effets respiratoires, immunologiques/lymphoréticulaires, cardiovasculaires et neurologiques dont l’issue peut être fatale. Les effets observés chez l’animal après exposition de brève durée à ce gaz sont de nature oculaire, cardiovasculaire, neurologique, métabolique ou hépatique et peuvent également se manifester au niveau du développement. Selon des études sur l’animal comportant une exposition de durée moyenne par inhalation, les effets sont de nature respiratoire, neurologique ou olfactive. On ne dispose pas d’études sur l’animal comportant une exposition respiratoire de longue durée. En cas d’exposition de durée moyenne, l’expérimentation animale montre que l’organe le plus sensible est la muqueuse olfactive nasale. On a ainsi observé des lésions de cette muqueuse chez des rats Sprague-Dawley CD exposés à du sulfure d’hydrogène à des concentrations de 42 ou 110 mg/m3; la dose sans effet nocif observable (NOAEL) se situait à 14 mg/m3. Cette valeur de la NOAEL est utilisée pour établir la concentration tolérable à moyen terme.
La plupart des données relatives aux effets sur la santé humaine proviennent de rapports médicaux concernant des cas d’intoxication aiguë et d’exposition professionnelle ou encore d’études communautaires de portée limitée. Le seuil olfactif varie selon les individus; le seuil moyen géométrique est de 11 μg/m3; à partir de 140 mg/m3, il y a paralysie du nerf olfactif, ce qui rend le sulfure d’hydrogène très dangereux car à la concentration de 700 mg/m3, quelques bouffées peuvent être mortelles. Chez l’Homme, une exposition respiratoire de brève durée à une forte concentration de sulfure d’hydrogène provoque des effets au niveau de nombreux systèmes ou appareils; on observe notamment des effets respiratoires, oculaires, neurologiques, cardiovasculaires, métaboliques ou génésiques, dont l’issue peut être fatale. En cas d’exposition par inhalation, les points d’aboutissement les plus sensibles de l’action toxique du sulfure d’hydrogène sont de nature respiratoire, neurologique et oculaire. La dose la plus faible produisant un effet nocif observable (LOAEL) est de 2,8 mg/m3 chez les asthmatiques en ce qui concerne les effets respiratoires et neurologiques. Cette valeur de la LOAEL est utilisée pour établir la concentration tolérable à court terme.
La question de l’ingestion n’est pas à considérer chez l’Homme et il n’existe d’ailleurs pas de données à ce sujet.
La génotoxicité du sulfure d’hydrogène n’a pas été suffisamment étudiée; on n’a connaissance que d’un seul test de mutagénicité sur Salmonella, ayant d’ailleurs donné un résultat négatif. Il sera impossible d’évaluer le pouvoir cancérogène du sulfure d’hydrogène tant que l’on manquera d’études de longue durée sur l’animal et que les études sur les populations humaines resteront limitées.
La concentration tolérable de sulfure d’hydrogène dans l’air, établie sur la base des effets respiratoires de ce gaz, est respectivement égale à 100 μg/m3 pour une exposition par inhalation de brève durée (de 1 à 14 jours) et à 20 μg/m3 pour une exposition de longue durée (jusqu’à 90 jours).
Les émissions malodorantes auxquelles on peut être exposé dans l’environnement sont en fait des mélanges de gaz soufrés. Il n’est pas possible de déterminer quelle est la concentration exacte de sulfure d’hydrogène dans ces mélanges. En ce qui concerne l’estimation de l’exposition, il y a également incertitude sur la dose et la durée. D’après les données limitées dont on dispose, il semblerait que les rongeurs soient moins sensibles au sulfure d’hydrogène que l’être humain. Comme le principal organe cible de l’action toxique de ce gaz est l’appareil respiratoire, ce sont les asthmatiques, les personnes âgées et les enfants en bas âge souffrant de troubles respiratoires qui constituent les sous-populations sensibles. En raison des graves effets toxiques qu’entraîne l’exposition à des concentrations élevées de ce gaz pendant de très brèves durées, toute exposition doit être évitée.
Este CICAD sobre el ácido sulfhídrico fue preparado por la Agencia para el Registro de Sustancias Tóxicas y Enfermedades de los Estados Unidos. En el documento original se incluyeron los datos identificados hasta 1998 (ATSDR, 1999). Se realizó una búsqueda bibliográfica amplia en diversas bases de datos en línea en marzo de 2002 para localizar cualquier referencia publicada después de las incorporadas al documento original. La información relativa al carácter del examen colegiado y a la disponibilidad del documento original se presenta en el apéndice 1. La información sobre el examen colegiado de este CICAD aparece en el apéndice 2. Este CICAD se aprobó como evaluación internacional en una reunión de la Junta de Evaluación Final, celebrada en Monks Wood (Reino Unido) del 16 al 19 de septiembre de 2002. La lista de participantes en esta reunión figura en el apéndice 3. También se reproduce en este documento la Ficha internacional de seguridad química para el ácido sulfhídrico (ICSC 0165), preparada por el Programa Internacional de Seguridad de las Sustancias Químicas (IPCS, 2000).
El ácido sulfhídrico (CAS Nº
Se puede producir ácido sulfhídrico mediante diversos métodos comerciales. Su fuente principal es la recuperación como subproducto en la purificación de gases naturales y de refinería. Es también un subproducto de la fabricación de pasta y papel kraft y de la producción de sulfuro de carbono. Se utiliza como intermediario en la fabricación de ácido sulfúrico y de sulfuros inorgánicos y como desinfectante agrícola. También se obtiene ácido sulfhídrico como producto de la descomposición de los xantatos (utilizados en la industria de la minería) cuando se ponen en contacto con el agua.
Se pueden producir emisiones de ácido sulfhídrico por liberación accidental o eliminación inadecuada de materiales derivados de estos procesos. Las emisiones al medio ambiente se realizan sobre todo al aire ambiente, donde es probable que el producto químico permanezca durante menos de un día, pero puede persistir hasta 42 días en invierno. El ácido sulfhídrico se evapora fácilmente del agua, en función de la temperatura y el pH. No es probable su bioconcentración o bioamplificación en la cadena trófica.
La concentración de ácido sulfhídrico en el aire de zonas no contaminadas es muy baja, entre 0,03 y 0,1 µg/m3.
Las personas pueden verse expuestas al ácido sulfhídrico de producción endógena y a partir de fuentes exógenas. La mayor parte de la producción endógena se debe al metabolismo de los aminoácidos con radicales sulfhidrilo (por ejemplo, la cisteína) por parte de bacterias presentes tanto en el tracto intestinal como en la boca. También se produce ácido sulfhídrico en el cerebro y en diversos músculos de fibra lisa (por ejemplo, la aorta torácica, el íleon y la vena porta) por la acción de enzimas que se encuentran en estos tejidos. En la rata, el nivel endógeno de ácido sulfhídrico es de 50-160 µmol/l en el cerebro y de 1 mmol/l en el íleon.
La exposición humana al ácido sulfhídrico exógeno se produce principalmente por inhalación, y el gas se absorbe con rapidez a través de los pulmones. El ácido sulfhídrico se metaboliza mediante tres vías: oxidación, metilación y reacciones con metaloproteínas o proteínas que contienen disulfuro. La oxidación en el hígado es la vía principal de desintoxicación. El principal producto de la oxidación es el tiosulfato, que luego pasa a sulfato y se excreta en la orina. La vía de la metilación también sirve como sistema de desintoxicación. La toxicidad del ácido sulfhídrico se debe a su reacción con las enzimas metálicas. En las mitocondrias, inhibe la citocromo oxidasa, enzima final de la cadena respiratoria. Los tejidos nervioso y cardíaco, cuya demanda de oxígeno es máxima, son especialmente sensibles a la perturbación del metabolismo oxidativo. En el sistema nervioso central, este efecto puede provocar la muerte por paro respiratorio.
En animales de experimentación, la exposición aislada por inhalación al ácido sulfhídrico provoca efectos respiratorios, inmunológicos/linforreticulares, cardiovasculares y neurológicos y la muerte. Se han notificado efectos en la salud de los animales tras exposiciones breves, en particular efectos oculares, cardiovasculares, neurológicos, metabólicos, hepáticos y del desarrollo. En estudios de inhalación de ácido sulfhídrico de duración media en animales se notificaron efectos respiratorios, neurológicos y olfativos. No hay estudios de inhalación de duración prolongada en animales. El órgano destinatario más sensible para la exposición de duración media en animales es la mucosa olfatoria nasal. Se notificaron lesiones de la mucosa olfatoria nasal en ratas Sprague-Dawley CD expuestas a concentraciones de ácido sulfhídrico de 42 ó 110 mg/m3; la concentración sin efectos adversos observados (NOAEL) fue de 14 mg/m3. Esta NOAEL se utiliza como base para la obtención de una concentración tolerable a plazo medio.
La mayoría de los datos humanos proceden de las notificaciones de casos de intoxicación aguda, exposiciones profesionales y estudios comunitarios limitados. El umbral de olor varía en función de las personas; la media geométrica del umbral para el olor es de 11 µg/m3. las concentraciones superiores a 140 mg/m3 provocan parálisis olfatoria, convirtiendo al ácido sulfhídrico en una sustancia muy peligrosa, porque un pequeño número de inhalaciones a 700 mg/m3 puede ser fatal. La exposición de duración breve por inhalación a concentraciones elevadas de ácido sulfhídrico provoca efectos patológicos en muchos sistemas; entre los efectos notificados en la salud de las personas tras la exposición al ácido sulfhídrico figuran efectos respiratorios, oculares, neurológicos, cardiovasculares, metabólicos y de la reproducción, así como la muerte. Los efectos finales más sensibles en las personas tras la exposición por inhalación son los trastornos respiratorios, neurológicos y oculares. En personas asmáticas, la concentración más baja con efectos adversos observados (LOAEL) es de 2,8 mg/m3 para los efectos respiratorios y neurológicos. Esta LOAEL se utiliza como base para la obtención de una concentración tolerable en períodos breves.
La ingestión no es importante para las personas. No hay datos relativos a la ingestión humana.
No se ha investigado suficientemente la genotoxicidad del ácido sulfhídrico; hay sólo una prueba única de mutagenicidad de Salmonella. No es posible evaluar el potencial carcinogénico del ácido sulfhídrico, puesto que no se dispone de estudios prolongados en animales y los estudios en poblaciones humanas son deficientes.
Basándose en los efectos respiratorios para exposiciones por inhalación de duración breve (de 1 a 14 días) y media (de hasta 90 días) se han obtenido concentraciones tolerables para el ácido sulfhídrico en el aire de 100 µg/m3 y 20 µg/m3, respectivamente.
La exposición en el medio ambiente a emisiones malolientes suele deberse a una mezcla de gases que contiene azufre. No se puede determinar la concentración exacta de ácido sulfhídrico en estos tipos de mezclas. Al estimar la exposición, hay también incertidumbre acerca de las dosis y la duración de la exposición. Basándose en una información limitada, los roedores parecen ser menos sensibles al ácido sulfhídrico que las personas. Dado que el sistema respiratorio es el órgano destinatario principal de la toxicidad del ácido sulfhídrico, las subpoblaciones más sensibles son las personas asmáticas, los ancianos y los niños pequeños con problemas respiratorios. Se debería evitar toda exposición, debido a los serios efectos tóxicos asociados con la exposición a concentraciones elevadas de ácido sulfhídrico de duración muy breve.
ENDNOTES
|
International Programme on Chemical Safety (1994) Assessing human health risks of chemicals: derivation of guidance values for health-based exposure limits. Geneva, World Health Organization (Environmental Health Criteria 170) (also available at http://www.who.int/pcs/). |
|
|
In keeping with WHO policy, which is to provide measurements in SI units, all concentrations of gaseous chemicals in air will be given in SI units in the CICAD series. Where the original study or source document has provided concentrations in SI units, these will be cited here. Where the original study or source document has provided concentrations in volumetric units, conversions will be done using the conversion factors given here, assuming a temperature of 20 °C and a pressure of 101.3 kPa. Conversions are to no more than two significant digits. |
See Also:
Toxicological Abbreviations