

This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organization, or the World Health Organization.
Concise International Chemical Assessment Document 65
First draft prepared by Mr Paul Howe, Centre for Ecology & Hydrology, Monks Wood, United Kingdom; and Mr Peter Watts, Toxicology Advice & Consulting Ltd, Surrey, United Kingdom
Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 2005
The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals.
The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and Agriculture Organization of the United Nations, WHO, the United Nations Industrial Development Organization, the United Nations Institute for Training and Research, and the Organisation for Economic Co-operation and Development (Participating Organizations), following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase coordination in the field of chemical safety. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment.
WHO Library Cataloguing-in-Publication Data
Tin and inorganic tin compounds.
(Concise international chemical assessment document ; 65)
1.Tin - adverse effects 2.Tin compounds - adverse effects
3.Risk assessment 4.Environmental exposure I.International
Programme on Chemical Safety II.Series.
ISBN 92 4 153065 0 (LC/NLM Classification: QV 618)
ISSN 1020-6167
©World Health Organization 2005
All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 2476; fax: +41 22 791 4857; email: bookorders@who.int). Requests for permission to reproduce or translate WHO publications — whether for sale or for noncommercial distribution — should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; email: permissions@who.int).
The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement.
The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters.
All reasonable precautions have been taken by WHO to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either express or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall the World Health Organization be liable for damages arising from its use.
Risk assessment activities of the International Programme on Chemical Safety, including the production of Concise International Chemical Assessment Documents, are supported financially by the Department of Health and Department for Environment, Food & Rural Affairs, UK, Environmental Protection Agency, Food and Drug Administration, and National Institute of Environmental Health Sciences, USA, European Commission, German Federal Ministry of Environment, Nature Conservation and Nuclear Safety, Health Canada, Japanese Ministry of Health, Labour and Welfare, and Swiss Agency for Environment, Forests and Landscape.
Technically and linguistically edited by Marla Sheffer, Ottawa, Canada, and printed by Wissenchaftliche Verlagsgesellschaft mbH, Stuttgart, Germany
|
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION |
|
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS |
|
11.1.2 Criteria for setting tolerable intakes/concentrations |
Concise International Chemical Assessment Documents (CICADs) are the latest in a family of publications from the International Programme on Chemical Safety (IPCS) — a cooperative programme of the World Health Organization (WHO), the International Labour Organization (ILO), and the United Nations Environment Programme (UNEP). CICADs join the Environmental Health Criteria documents (EHCs) as authoritative documents on the risk assessment of chemicals.
International Chemical Safety Cards on the relevant chemical(s) are attached at the end of the CICAD, to provide the reader with concise information on the protection of human health and on emergency action. They are produced in a separate peer-reviewed procedure at IPCS. They may be complemented by information from IPCS Poison Information Monographs (PIM), similarly produced separately from the CICAD process.
CICADs are concise documents that provide summaries of the relevant scientific information concerning the potential effects of chemicals upon human health and/or the environment. They are usually based on selected national or regional evaluation documents or on existing EHCs. Before acceptance for publication as CICADs by IPCS, these documents undergo extensive peer review by internationally selected experts to ensure their completeness, accuracy in the way in which the original data are represented, and the validity of the conclusions drawn.
The primary objective of CICADs is characterization of hazard and dose–response from exposure to a chemical. CICADs are not a summary of all available data on a particular chemical; rather, they include only that information considered critical for characterization of the risk posed by the chemical. The critical studies are, however, presented in sufficient detail to support the conclusions drawn. For additional information, the reader should consult the identified source documents upon which the CICAD has been based.
Risks to human health and the environment will vary considerably depending upon the type and extent of exposure. Responsible authorities are strongly encouraged to characterize risk on the basis of locally measured or predicted exposure scenarios. To assist the reader, examples of exposure estimation and risk characterization are provided in CICADs, whenever possible. These examples cannot be considered as representing all possible exposure situations, but are provided as guidance only. The reader is referred to EHC 170.1
While every effort is made to ensure that CICADs represent the current status of knowledge, new information is being developed constantly. Unless otherwise stated, CICADs are based on a search of the scientific literature to the date shown in the executive summary. In the event that a reader becomes aware of new information that would change the conclusions drawn in a CICAD, the reader is requested to contact IPCS to inform it of the new information.
Procedures
The flow chart shows the procedures followed to produce a CICAD. These procedures are designed to take advantage of the expertise that exists around the world — expertise that is required to produce the high-quality evaluations of toxicological, exposure, and other data that are necessary for assessing risks to human health and/or the environment. The IPCS Risk Assessment Steering Group advises the Coordinator, IPCS, on the selection of chemicals for an IPCS risk assessment based on the following criteria:
Thus, it is typical of a priority chemical that
|
Advice from Risk Assessment Steering Group Criteria of priority:
Thus, it is typical of a priority chemical that
Special emphasis is placed on avoiding duplication of effort by WHO and other international organizations. A prerequisite of the production of a CICAD is the availability of a recent high-quality national/regional risk assessment document = source document. The source document and the CICAD may be produced in parallel. If the source document does not contain an environmental section, this may be produced de novo, provided it is not controversial. If no source document is available, IPCS may produce a de novo risk assessment document if the cost is justified. Depending on the complexity and extent of controversy of the issues involved, the steering group may advise on different levels of peer review:
|
The Steering Group will also advise IPCS on the appropriate form of the document (i.e., a standard CICAD or a de novo CICAD) and which institution bears the responsibility of the document production, as well as on the type and extent of the international peer review.
The first draft is usually based on an existing national, regional, or international review. When no appropriate source document is available, a CICAD may be produced de novo. Authors of the first draft are usually, but not necessarily, from the institution that developed the original review. A standard outline has been developed to encourage consistency in form. The first draft undergoes primary review by IPCS to ensure that it meets the specified criteria for CICADs.
The second stage involves international peer review by scientists known for their particular expertise and by scientists selected from an international roster compiled by IPCS through recommendations from IPCS national Contact Points and from IPCS Participating Institutions. Adequate time is allowed for the selected experts to undertake a thorough review. Authors are required to take reviewers’ comments into account and revise their draft, if necessary. The resulting second draft is submitted to a Final Review Board together with the reviewers’ comments. At any stage in the international review process, a consultative group may be necessary to address specific areas of the science. When a CICAD is prepared de novo, a consultative group is normally convened.
The CICAD Final Review Board has several important functions:
Board members serve in their personal capacity, not as representatives of any organization, government, or industry. They are selected because of their expertise in human and environmental toxicology or because of their experience in the regulation of chemicals. Boards are chosen according to the range of expertise required for a meeting and the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who participate in the preparation of a CICAD are required to declare any real or potential conflict of interest in relation to the subjects under discussion at any stage of the process. Representatives of nongovernmental organizations may be invited to observe the proceedings of the Final Review Board. Observers may participate in Board discussions only at the invitation of the Chairperson, and they may not participate in the final decision-making process.
This CICAD2 on tin and inorganic tin compounds was prepared jointly by Toxicology Advice & Consulting Ltd and the Centre for Ecology & Hydrology. The CICAD was based on three source documents. The first of these source documents was prepared by the Nordic Expert Group for Criteria Documentation of Health Risks from Chemicals and the Dutch Expert Committee on Occupational Standards and considered the literature identified as of March 2002 (Westrum & Thomassen, 2002). The second source document was the monograph prepared by the 55th meeting of the Joint FAO/WHO Expert Committee on Food Additives, published in 2001 (JECFA, 2001). The third source document was the 2003 draft updated Toxicological profile for tin and compounds, produced by the US Agency for Toxic Substances and Disease Registry (ATSDR, 2003). In December 2003, Toxicology Advice & Consulting Ltd and the Centre for Ecology & Hydrology carried out comprehensive literature searches of online databases to identify any very recent references. Information on the nature of the peer review and the availability of the source documents is presented in Appendix 2. Information on the peer review of this CICAD is presented in Appendix 3. This CICAD was approved as an international assessment at a meeting of the Final Review Board, held in Hanoi, Viet Nam, on 28 September – 1 October 2004. Participants at the Final Review Board meeting are listed in Appendix 4. The International Chemical Safety Cards for tin(II) chloride, tin(II) chloride dihydrate, tin(II) fluoride, tin(II) oxide, tin(IV) chloride, and tin(IV) oxide, produced by the International Programme on Chemical Safety (IPCS, 2004a–f), have also been reproduced in this document.
Tin is a grey-white metal. The most important inorganic tin compounds include the tin(II) and tin(IV) chlorides, tin(II) oxide, tin(II) fluoride, and the potassium and sodium stannates. The 2+ and 4+ oxidation states of tin, also known as tin(II) and tin(IV), are both fairly stable.
The annual world production of tin has been growing slowly in recent years and reached about 268 000 tonnes in 2003. About 10–15% of this figure is recovered metal. The major tin-producing countries are China, Indonesia, Peru, Bolivia, Brazil, and Australia, and significant quantities are also produced in Malaysia and Thailand. The main use of tin, accounting for about 34% of annual global production, is for solder alloys for electrical/electronic and general industrial applications. Tin also finds extensive use (about 25–30% of production) as a protective coating for other metals, especially for food containers. Tin(II) chloride is commercially the most important inorganic compound and is used mainly as a reducing agent in organic and inorganic syntheses and in the manufacture of metallized glazing, glass, and pigments. Tin(IV) chloride is used in organic synthesis, in plastics, as an intermediate in organotin compound manufacture, and in the production of tin(IV) oxide films on glass. Tin(II) fluoride is broadly used in preventive dentistry.
Tin may be released to the atmosphere from both natural and anthropogenic sources. Tin is a component of many soils and may be released in dusts from wind storms, roads, and agricultural activities. Other less significant natural sources include forest fires and volcanic emissions. Gases, dusts, and fumes containing tin may be released from smelting and refining processes, industrial uses of tin, waste incineration, and burning of fossil fuels. The vapour pressure of elemental tin is negligible; tin and inorganic tin compounds are non-volatile under environmental conditions. Tin(II) chloride is soluble in water, whereas other tin compounds tend to be only slightly soluble. Tin compounds are likely to partition to soils and sediments. Inorganic tin may undergo oxidation–reduction, ligand exchange, and precipitation reactions in the environment. The biomethylation of inorganic tin has been demonstrated in pure bacterial cultures, sediments, and decaying plant material. Inorganic tin compounds may be bioconcentrated by organisms, but data are limited.
Average tin concentrations in air are generally below 0.1 µg/m3 (ranging up to 0.8 µg/m3), with higher concentrations near some industrial facilities. In general, tin occurs in trace amounts in natural waters. Higher inorganic tin concentrations are associated with industrial discharges and tributyltin use. In a survey of lakes and rivers, nearly 80% of samples were found to contain inorganic tin at concentrations below 1 µg/litre; higher levels of up to 37 µg/litre were reported near pollution sources. Inorganic tin concentrations ranging from 0.001 to 0.01 µg/litre have been reported for coastal waters, with levels of up to 8 µg/litre near pollution sources. Inorganic tin concentrations in sediment ranged up to 8 mg/kg dry weight in coastal areas and up to 15.5 mg/kg in rivers and lakes. Tin concentrations in the Earth’s crust are approximately 2–3 mg/kg. Total tin concentrations in soil can range from <1 to 200 mg/kg, but levels of 1000 mg/kg may occur in areas of high tin deposits. Certain ore deposits may contain up to 50 000 mg/kg as tin.
For the general population, the diet is the main source of exposure to inorganic tin. JECFA recently concluded that mean tin intakes in seven countries ranged from <1 up to 15 mg/day per person, but maximum daily intakes could reach 50–60 mg for certain individuals who routinely consume canned fruits, vegetables, and juices from unlacquered cans. Drinking-water is not a significant source of inorganic tin and might contribute approximately 0.012–0.02 mg/day. Similarly, the low levels of inorganic tin in air mean that the amount of inhaled tin is very low, probably below approximately 0.01–0.02 mg/day.
In humans and laboratory mammals, absorption of inorganic tin from the gastrointestinal tract is low (generally less than 5%), but is influenced by dose, anion (compound solubility), and the presence of other substances. Unabsorbed ingested tin is mostly (95–99%) excreted in the faeces within 48 h. Absorbed tin distributes mainly to the bone, but also to the lungs, liver, and kidneys. Limited evidence suggests that inorganic tin does not readily cross the blood–brain barrier. Absorbed tin is mainly excreted in the urine, with some additional biliary excretion occurring. In mice, the biological half-life of absorbed inorganic tin was approximately 30 days.
Transient eye and nasal irritation occurred in guinea-pigs exposed to tin(IV) chloride by inhalation. Metallic tin is unlikely to have skin irritation potential, whereas tin(II) and tin(IV) chloride are skin irritants. In some studies, the inclusion of tin(II) chloride in the diet for 4–13 weeks produced gastrointestinal tissue changes indicative of local irritation. The early literature contains reports of gastrointestinal effects (nausea, abdominal cramps, vomiting, and diarrhoea) in humans following consumption of fruit or juice from unlacquered tin cans. The effects appear to result from local gastric irritation due to dissolved tin. This aspect is addressed further below. A small number of individuals have given skin reactions indicative of a local allergic response when patch-tested with tin or tin(II) chloride, but, given its widespread use, tin would not appear to be an important skin allergen.
In the early literature, there are a number of cases where occupational exposure to dust and fumes containing insoluble tin(IV) oxide led to a benign pneumoconiosis (stannosis). This condition is characterized by mottled shadows on the lungs, apparently caused by tin(IV) oxide deposits. Stannosis is not associated with fibrosis or loss of lung function.
In laboratory animals, the repeated ingestion of tin(II) chloride had adverse effects on the body status of copper, iron, zinc, and calcium. Tin salt-induced decreases in the calcium content of bone have led to reduced bone strength. Reductions in haemoglobin and effects on red blood cells, leading to anaemia, have been observed. Certain studies involving repeated administration of tin(II) chloride by the oral route have reported tissue effects in the liver, kidneys, testes, pancreas, and brain. In the most comprehensive of the available lifetime oral studies, there were no microscopic changes in a wide range of tissues of rats or mice given tin at up to about 60 mg/kg body weight per day (rats) or 180–270 mg/kg body weight per day (mice) as tin(II) chloride in the diet. In this study, the NOAELs were 30 mg/kg body weight per day in rats and 130 mg/kg body weight per day in mice, with reduced survival seen at the higher doses.
Tin(II) chloride gave no clear evidence of carcinogenic activity when given in the diet to rats and mice for 2 years. More limited bioassays carried out on tin metal, tin(II) chloride, and a small number of other tin compounds also failed to detect carcinogenic activity. In short-term screening assays for genotoxicity potential, tin(II) chloride did not induce mutations in Ames bacterial tests, mutations or gene conversions in yeast, DNA damage in rat liver cells in culture, mutations in mouse lymphoma cells in vitro, or chromosome damage (micronuclei) in vivo in the bone marrow of mice treated by intraperitoneal injection. In bacterial rec assays (in which activity is an indirect indication of DNA damage), tin(II) chloride was active in Escherichia coli but (along with other tin salts) inactive in Bacillus subtilis. In culture, tin(II) chloride induced chromosome damage and SCEs in hamster ovary cells and DNA damage in human lymphocytes, hamster ovary cells, and plasmid DNA. Tin(IV) chloride tested in vitro did not damage DNA in hamster ovary cells but induced chromosome aberrations, micronuclei, and SCEs in human lymphocytes. Tin(II) fluoride caused DNA damage in cultures of human lymphocytes, but did not induce micronuclei formation in the bone marrow following injection into the peritoneum of mice; Ames tests on this compound gave no convincing evidence of activity. Limited evidence is consistent with the suggestion that tin-induced DNA damage might result from the production of reactive oxygen species. The mechanism underlying tin-induced chromosome damage in cultured mammalian cells is unclear, although it is known that certain inorganic compounds can yield positive results in such assays as a result of pH or ionic changes in the test medium.
Only limited data were identified on the potential of inorganic tin compounds to cause reproductive and developmental toxicity. No adverse effects were found in rats when tin (an uncharacterized form, produced by mixing aqueous tin(II) chloride with casein prior to dietary inclusion) was given in the diet for three generations or when tin(II) fluoride, sodium pentachlorostannite, or sodium pentafluorostannite were given in the diet throughout pregnancy. Similarly, repeated gavage treatment of pregnant rats, mice, and hamsters with tin(II) chloride was without adverse effect on the fetuses.
Limited data are available on the ability of ingested tin to adversely affect zinc absorption in humans. In one volunteer study, plasma appearance of zinc 1–4 h following a zinc dose was unaffected by concomitant ingestion of up to 100 mg tin (as tin(II) chloride). Another study reported that a single dose of 36 mg tin (again, as tin(II) chloride), taken with zinc, resulted in a lower zinc retention. Moderate disturbances in zinc excretion rates were reported in a third study in which the normal diet was supplemented with tin at 50 mg/day (as tin(II) chloride in fruit juice). Although a no-effect level for inhibition of zinc absorption has not been clearly established, the lowest dose reported to have this effect (36 mg) is about 2.5 to >36 times higher than the estimated mean population intakes as summarized by JECFA. However, those who routinely consume canned fruits, vegetables, and juices from unlacquered cans could have tin intakes (50–60 mg) that are similar to the acute (36 mg) or repeated (50 mg) dose levels reported in some studies to affect zinc absorption or balance. Whether this would have any clinical effect is likely to be critically dependent upon an adequate dietary supply of zinc.
The tin doses involved in the early reports of gastrointestinal effects following consumption of canned fruit or juice have been estimated (at 30–200 mg), but confidence in the accuracy of these figures is low. Two recent volunteer studies provide a better insight into effective doses and, perhaps more importantly, concentrations. The first study involved ingestion of tomato juice to which tin(II) chloride had been added to give tin concentrations of 161, 264, or 529 mg/kg (tin doses of about 40, 66, and 132 mg, respectively). At 161 mg/kg, one volunteer (of 18) reported mild gastrointestinal symptoms; typical acute symptoms were seen at 264 and 529 mg/kg. Serum levels of tin did not increase 0.5–4 h post-dosing at any dose, supporting the view that acute effects of tin ingestion are dependent upon concentration (resulting in local gastric irritation) rather than due to systemically absorbed tin. A second study involved ingestion of tomato soup containing tin that had migrated from unlacquered cans. The tin concentrations studied were <0.5, 201, and 267 mg/kg, providing acute tin doses of up to about 67 mg. No evidence of any acute effects was seen in this study. The low-effect or no-effect dose of approximately 67 mg tin in these studies is about 4.5 to >67 times higher than the JECFA estimates of mean population daily intakes and is similar to the estimated daily intake (50–60 mg) of individuals who routinely consume fruits, vegetables, and juices contained in unlacquered cans.
Under environmental speciation conditions, inorganic tin compounds have low toxicity in both aquatic and terrestrial organisms, largely due to their low solubility, poor absorption, low accumulation in tissues, and rapid excretion. Most laboratory testing with aquatic organisms has been carried out with the soluble tin(II) chloride. The most sensitive microalgae are the marine diatoms Skeletonema costatum and Thalassiosira guillardii, with 72-h EC50s of tin(II) cations, based on growth inhibition, of around 0.2 mg/litre. Acute LC/EC50s of tin(II) for aquatic invertebrates range from 3.6 to 140 mg/litre, with a 21-day EC50, based on reproductive success in daphnids, of 1.5 mg of tin(II) per litre. Fish toxicity tests clearly show that tin(IV) chloride is less toxic than the more soluble tin(II) chloride. Ninety-six-hour LC50s for fish range from 35 mg of tin(II) per litre to >1000 mg of tin(IV) per litre. Embryo-larval test results (i.e. 7- to 28-day LC50s) for fish and amphibians range from 0.1 to 2.1 mg/litre for tin(II).
Concentrations showing toxicity to organisms are generally several orders of magnitude higher than those found in the environment. The most sensitive test results were 72-h exposures of diatoms and embryo-larval amphibian studies, with toxic effects seen at 0.1–0.2 mg of tin(II) per litre. Even at these concentrations, toxic effects caused by inorganic tin are unlikely, even near sources of local pollution. It should be noted that where concentrations are expressed as total tin, a percentage is likely to be in the form of organotins (e.g. tributyltin), which are more bioavailable and toxic. For more information on the environmental fate and toxicity of tributyltin, please refer to IPCS (1990, 1999).
Tin (CAS No.
Table 1: Chemical identification of tin and inorganic tin compounds reviewed in this CICAD.
|
Chemical name |
Synonyms |
Chemical formula |
Relative molecular mass |
CAS number |
|
Tin |
Sn |
118.7 |
|
|
|
Potassium stannate |
Dipotassium tin trioxide |
K2SnO3 |
244.9 |
|
|
Potassium stannate |
Potassium stannate trihydrate |
K2Sn(OH)6 |
298.9 |
|
|
Sodium stannate |
Disodium tin trioxide |
Na2SnO3 |
212.7 |
|
|
Sodium stannate |
Sodium stannate trihydrate |
Na2Sn(OH)6 |
266.7 |
|
|
Tin(IV) bromide |
Tin tetrabromide; stannic bromide |
SnBr4 |
438.3 |
|
|
Tin(II) chloride |
Tin dichloride; stannous chloride |
SnCl2 |
189.6 |
|
|
Tin(IV) chloride |
Tin tetrachloride; stannic chloride |
SnCl4 |
260.5 |
|
|
Tin(IV) chloride iodide |
Tin dichloride diiodide; stannic dichloride diiodide |
SnCl2I2 |
443.4 |
|
|
Tin(II) difluoroborate |
Stannous fluoroborate |
Sn(BF4)2 |
292.3 |
|
|
Tin(II) fluoride |
Tin difluoride; stannous fluoride |
SnF2 |
156.7 |
|
|
Tin(II) iodide |
Tin diiodide; stannous iodide |
SnI2 |
372.5 |
|
|
Tin(IV) iodide |
Tin tetraiodide; stannic iodide |
SnI4 |
626.3 |
|
|
Tin(II) oxide |
Tin oxide; stannous oxide |
SnO |
134.7 |
|
|
Tin(IV) oxide |
Tin dioxide; stannic oxide |
SnO2 |
150.7 |
|
|
Tin(II) pyrophosphate |
Stannous pyrophosphate |
Sn2P2O7 |
411.3 |
|
|
Tin(II) sulfate |
Stannous sulfate |
SnSO4 |
214.8 |
|
|
Tin(IV) sulfate |
Stannic sulfate |
Sn(SO4)2 |
310.9 |
|
a CAS number given in Westrum & Thomassen (2002).
Table 2: Chemical identification of some furthera inorganic tin compounds featured briefly in this CICAD.
|
Chemical name |
Synonyms |
Chemical formula |
Relative molecular mass |
CAS number |
|
Sodium pentachlorostannite |
Sodium chlorostannite |
NaSn2Cl5 |
437.7 |
|
|
Sodium hexachlorostannate |
Sodium chlorostannate |
Na2SnCl6 |
3544.4 |
Not found |
|
Sodium pentafluorostannite |
Sodium fluorostannite |
NaSn2F5 |
236.7 |
|
|
Stannane |
Tin tetrahydride |
SnH4 |
122.7 |
|
|
Tin(II) orthophosphate |
Tritin bis(orthophosphate); stannous phosphate |
Sn3(PO4)2 |
546.1 |
|
|
Tin(IV) orthophosphate |
Stannic phosphate |
Sn3(PO4)4 |
736.0 |
Not found |
|
Tin(II) sulfide |
Stannous sulfide; tin monosulfide |
SnS |
150.8 |
|
|
Tin(IV) sulfide |
Stannic sulfide; tin disulfide |
SnS2 |
182.8 |
|
|
Tin(II) hydroxide |
Stannous hydroxide; tin dihydroxide |
Sn(OH)2 |
152.7 |
|
|
Tin(IV) hydroxide |
Stannic hydroxide; tin tetrahydroxide |
Sn(OH)4 |
186.7 |
|
|
Tin(II) chloride dihydrate |
Stannous chloride dihydrate |
SnCl2·2H2O |
225.6 |
|
|
Tin(II) citrate |
Stannous citrate; tritin dicitrate |
Sn3((HO)C(COO)-(CH2COO))2 |
734.3 |
|
|
Tin(IV) citrate |
Stannic citrate |
Not found |
Not found |
Not found |
|
Sodium tin citrate |
Not found |
Not found |
Not found |
Not found |
|
Tin(II) oxalate |
Stannous oxalate |
Sn(COO)2 |
206.7 |
|
|
Tin(II) tartrate |
Stannous tartrate |
Sn(OOC(CHOH)2COO) |
266.8 |
|
|
Tin(II) nitrate |
Stannous nitrate |
Sn(NO3)2 |
242.7 |
Not found |
|
Tin(IV) nitrate |
Stannic nitrate |
Sn(NO3)4 |
366.7 |
Not found |
|
Tin(II) oleate |
Stannous oleate |
Sn(C17H34COO) |
401.2 |
|
|
Tin(II) 2-ethylhexanoate |
Stannous bis(2-ethylhexanoate) |
Sn(OOCCH(C2H5)C4H9)2 |
405.1 |
|
|
Tin(II) phytate |
Stannous phytate |
Not found |
Not found |
Not found |
|
a |
These were not the subject of the source documents and consequently were not included in the search updates. However, data on these are included when encountered, as they can provide insights into the effects of other inorganic tin compounds. |
Pure tin exists in two allotropic crystalline modifications: grey tin (alpha form) and white tin (beta form). At low temperatures (at about 18 °C and below), the grey tin changes to white tin. Physical and chemical properties of tin and some inorganic tin compounds are listed in Table 3.
Table 3: Physical and chemical properties of tin and some inorganic tin compounds.a
|
Compound (formula) |
Melting point (°C) |
Boiling point (°C) |
Solubility in water |
|
Sn |
232 |
2602 |
Insoluble |
|
SnBr4 |
31 |
205 |
Slightly soluble |
|
SnCl2 |
247 |
Decomposes at 623–652 |
Soluble |
|
SnCl4 |
−33 |
114 |
Slightly soluble (reacts with) |
|
SnF2 |
213 |
850 |
Slightly soluble |
|
SnI2 |
320 |
714 |
Slightly soluble |
|
SnI4 |
143 |
365 |
Slightly soluble |
|
SnO |
1080 |
No data |
Insoluble |
|
SnO2 |
1630 |
1900 |
Insoluble |
|
Sn2P2O7 |
Decomposes at 400 |
– |
Insoluble |
|
SnS |
880 |
1210 |
Insoluble |
|
SnSO4 |
Decomposes at >378 |
– |
Reacts with |
a From Lide (1998–1999).
The 2+ (stannous) and 4+ (stannic) oxidation states are both reasonably stable and interconverted by moderately active reagents. The Sn2+/Sn4+ potential is −0.15 V, and tin(II) can act as a mild reducing agent. Due to its amphoteric nature, tin reacts with strong acids and strong bases but remains relatively resistant to neutral solutions. A thin protective oxide film forms on tin exposed to oxygen or dry air at ordinary temperatures; heat accelerates this reaction. Tin is readily attacked by hydrogen iodide and hydrogen bromide and less readily by hydrogen chloride. Hot concentrated sulfuric acid reacts with tin to form tin(II) sulfate, whereas the diluted acid reacts only slowly with tin at room temperature. Reaction of tin with dilute nitric acid yields soluble tin nitrates; in concentrated nitric acid, tin is oxidized to insoluble hydrated tin dioxide. Organic acids such as lactic, citric, tartaric, and oxalic acid attack tin slowly in the presence of air and oxidizing substances (Gaver, 1997). Molten tin reacts with phosphorus, forming a phosphide. Stannates are produced by the action of strong potassium hydroxide or sodium hydroxide on tin (Mark, 1983). Tin(IV) chloride reacts with water to generate colloidal tin oxides (Wiberg et al., 2001).
Tin is readily measured in multielement analyses of air, water, and solid waste samples by ICP-AES. For samples that are free of particulate matter, such as drinking-water, direct aspiration AAS, such as EPA Method 7870, may be used. Other samples, such as groundwater, industrial wastes, soils, sediments, sludges, and other solid wastes, require digestion prior to analysis to determine total and acid-leachable metal (US EPA, 1992). EPA Method 3050B, which describes acid digestion of sediments, sludges, and soils, does not list tin as an analyte; however, it states that other elements and matrices may be analysed by this method if performance is demonstrated for that analyte in that matrix at the concentrations of interest (US EPA, 1996).
The standard methods using either flame atomic absorption (Standard Method 3111B) or electrothermal atomic absorption (Standard Method 3113B) may be used for analysis of tin in water, depending on the sensitivity desired (APHA et al., 1998b,c). Although tin is not specifically listed as an analyte for the ICP-MS method (Standard Method 3125), this method may also be used in most cases and has lower detection limits (APHA et al., 1998a).
The method recommended by NIOSH for measuring airborne inorganic tin and its compounds, except oxides, is filter collection followed by acid digestion and AAS or ICP-AES (NIOSH, 1994a). If the aerosol phase is believed to contain tin(IV) oxide, the acid solution is centrifuged and the tin compounds in the supernatant are determined as above. The precipitate is then treated with alkali, rendering tin(IV) oxide to a soluble stannate, and the determination is made as above (Beliles, 1994). Other acid digestion procedures (aqua regia plus hydrogen fluoride) are available for simultaneous measurements of total tin and other elements by, for example, ICP-AES (Butler & Howe, 1999) or ICP-MS (Schramel et al., 1997). Radiochemical neutron activation analysis has been used for the measurement of tin in human biological materials at background levels (Versieck & Vanballenberghe, 1991). A field portable X-ray fluorescence spectrometer has been developed as a rapid, non-destructive, on-site alternative for analysis of membrane filters used in NIOSH Method No. 7300 (NIOSH, 1994a) for metals (Bernick & Campagna, 1995). An ICP-AES method with a limit of quantification of 30 µg/litre (which equated to 0.8 mg/kg product) has been used successfully to measure total tin in various foods (Perring & Basic-Dvorzak, 2002).
Although not specifically listed, tin can be quantified in water using ICP-MS, according to ISO 17294-2 (ISO, 2003a). ISO guidelines also exist to measure tin in canned milk (e.g. ISO, 2003b) and in fruit (ISO, 1998, 2004).
When selecting samplers for aerosol collection, their sampling characteristics should comply with internationally accepted sampling criteria, such as those outlined by the ISO (2000). NIOSH (1994b) also offers internationally accepted sampling criteria.
Savolainen & Valkonen (1986) reported analysis of tin in tissue (brain and blood) samples down to a detection limit of 5 nmol/kg wet weight. Tissue samples were digested in a mixture of nitric, sulfuric, and perchloric acids (3:1:1 by volume), with a gradual increase in temperature to 275 °C. Tin was then converted to stannane using sodium borohydride and sodium hydroxide and, after argon purging, was analysed by AAS.
Analytical methods for total inorganic tin in water, sediment, and biological material are summarized in Table 4.
Table 4: Analytical methods.
|
Sample matrix |
Preparation method |
Analytical method |
Sample detection limit |
Percent recovery |
Reference |
|
Water |
Acidify with nitric acid |
ICP-MS |
0.05–0.1 ng/g |
103 ± 3% |
Brzezinska-Paudyn & Van Loon (1988) |
|
Water |
Generate hydride with sodium borohydride or electrolytically, sweep into silica cell heated to 700 °C |
AAS |
0.02 µg/litre |
No data |
Rains (1982) |
|
0.5 µg/litre |
Thompson & Thomerson (1974) |
||||
|
Water |
Acidify with nitric acid |
AAS (direct aspiration) |
0.8 mg/litre |
No data |
USEPA (1986, 1992, 1996); APHA et al. (1998c) |
|
Water |
Acidify with nitric acid |
AAS (furnace technique) |
5 µg/litre |
No data |
APHA et al. (1998b) |
|
Watera |
Acidify with nitric acid |
ICP-AES |
No data |
No data |
APHA et al. (1998a) |
|
Sediment |
Digest in oxidizing acid |
ICP-MS |
25–50 ng/g |
Brzezinska-Paudyn & Van Loon (1988) |
|
|
Biological material |
Digest in oxidizing acid |
ICP-MS |
25–50 ng/g |
Brzezinska-Paudyn & Van Loon (1988) |
a Tin not listed specifically as an analyte, but can be determined by ICP-AES.
Tin occurs naturally in the Earth’s crust, with an average concentration of approximately 2–3 mg/kg (Budavari, 2001). Tin compounds are found in various environmental media in both inorganic and organic forms. Tin may be released to the environment from natural and anthropogenic sources. Tin is a component of many soils, and inorganic tin compounds may be released in dusts from wind storms, roads, and agricultural activities. Other less significant natural sources include forest fires and volcanic emissions. Releases of tin to environmental media may occur from the production, use, disposal, and recovery of tin and tin compounds. Gases, dusts, and fumes containing tin may be released from smelting and refining processes, industrial uses of tin, waste incineration, and burning of fossil fuels (Lantzy & Mackenzie, 1979; IPCS, 1980; Byrd & Andreae, 1986; Senesi et al., 1999). Tin may be released to soil from landfilling of tin-containing wastes, including used cans (IPCS, 1980). The application of pretreated municipal sludge and urban refuse as soil amendments may also introduce tin to soils. Inorganic tin can be formed as a breakdown product of organotin degradation (Blunden & Chapman, 1982; Maguire et al., 1983; Maguire & Tkacz, 1985; Kawai et al., 1998).
Other point sources that might introduce tin to the soil include application of manure, corrosion of metal objects, and dispersion of metallic ores during transport (Senesi et al., 1999).
Total global emissions to the atmosphere from anthropogenic sources (industrial emissions and burning of fossil fuels) were estimated at 43 000 tonnes (~90% of total emissions) in the 1970s. Emissions from natural sources include continental dusts (~10% of total emissions), forest fires (<2% of total emissions), and volcanoes (<1% of total emissions) (Lantzy & Mackenzie, 1979). Worldwide emissions of tin to the atmosphere from coal and oil combustion, refuse incineration, and copper/nickel production facilities were estimated at 1470–10 810 tonnes in 1983 (Nriagu & Pacyna, 1988). No more recent data were identified.
Tin is mined chiefly as cassiterite (SnO2). The other ores are complex sulfides such as stannite (Cu2FeSnS4), teallite (PbSnS2), canfieldite (Ag8SnS6), and cylinderite (PbSn4FeSb2S14) (Beliles, 1994). Annual world production of tin was quite stable at approximately 210 000–230 000 tonnes for decades (Westrum & Thomassen, 2002) but is growing slowly and reached 268 000 tonnes in 2003 (K. Nimmo & S. Blunden, personal communication, 2004). Of this, about 10–15% is secondary metal recovered mainly from scrap waste and, to a much lesser degree, detinning (Westrum & Thomassen, 2002; K. Nimmo & S. Blunden, personal communication, 2004). More than 22 countries produce tin, but the 6 largest producers in 2001 were China (36%), Indonesia (23%), Peru (17%), Brazil (6%), Bolivia (6%), and Australia (4%) (ATSDR, 2003). Significant quantities are also produced from smelters in Malaysia and Thailand (Westrum & Thomassen, 2002; K. Nimmo & S. Blunden, personal communication, 2004). In Europe and North America (e.g. Belgium, Russian Federation, USA), the tin produced is mainly secondary; the USA (which is not a primary producer) is believed to be the world’s largest producer of secondary tin. In 2002, about 13 000 tonnes of tin from old and new scrap were recycled (ATSDR, 2003; Carlin, 2003a). Tin mining depends on the character of the deposit. About 20% of the primary deposits are embedded in underground granitic rock, and recovery methods are complex; the more important veins or lodes are secondary deposits (about 80%) in the form of an alluvial mud in the stream beds and placers, and the recovery is simpler (Gaver, 1997). The processing of tin ore following recovery involves smelting. The ore is mixed with salt and roasted at about 600 °C, washed in water, and then mixed with anthracite as a reducing agent and smelted at about 1500 °C. After refining, the tin is cast into bars (Robertson, 1960, 1964). Smelted ore may be further refined by heat treatment or electrolytic processes (Gaver, 1997). Certain ore deposits may contain tin at up to 50 000 mg/kg (K. Nimmo & S. Blunden, personal communication, 2004).
Currently, the major use for tin is for solder alloys for electrical/electronic and general industrial applications; this use accounts for about 34% of the tin produced and is growing with the introduction of lead-free soldering technology. A further 25–30% of tin is used as a protective coating for other metals, especially for food containers (K. Nimmo & S. Blunden, personal communication, 2004). Altogether, about 25 000 million cans are produced and filled in Europe annually, about 20% of these having plain internal (unlacquered) tin-coated bodies. Globally, the total for food packaging is approximately 80 000 million cans (JECFA, 1989; Blunden & Wallace, 2003). Tin is also used in transportation applications (ATSDR, 2003; Carlin, 2003b).
An important property of tin is its ability to form alloys with other metals. Tin alloys cover a wide range of compositions and many applications. Common solder, an alloy of 63% tin and lead, is mainly used in the electrical industry; lead-free tin solders containing up to 5% silver or antimony are used at higher temperatures. A large number of tin alloys are widely employed, including those containing lead, antimony, silver, zinc, or indium; babbit (containing mainly copper, antimony, tin, and lead; Wood’s metal (50% bismuth, 25% lead, 12.5% tin, and 12.5% cadmium); brasses and bronzes (essentially tin–copper alloys); pewter (0–95% tin plus 1–8% bismuth and 0.5–3% copper); and dental amalgams (silver–tin–mercury alloys) (Bulten & Meinema, 1991). Tin alloys are important in the production of coatings by electroplating and hot tinning (the most important of these are tin–zinc, tin–nickel, tin–cobalt, and tin–copper) (Gaver, 1997; ATSDR, 2003). Among the newer alloys are niobium–tin and indium–tin alloys used in superconducting cables and magnets (Stewart & Lassiter, 2001) and indium–tin oxide for metallic photonic crystals (Giessen, 2004). Dental amalgam alloys have been used for centuries. Principally, three-compound (ternary) alloys of silver, tin, and copper with smaller amounts of other elements have been widely used in dentistry. Today’s dental alloys are composed of silver (40–70%), tin (12–30%), copper (12–30%), indium (0–4%), palladium (0.5%), and zinc (0.1%) (Berry et al., 1994). Tin coatings can be applied to most metal surfaces by electrodeposition, while in hot-dipping, molten tin wets and adheres readily to clean iron, steel, copper, and copper-base alloys. This tin coating provides protection against oxidation of the base metal/alloy and aids in subsequent fabrication, because it is ductile and solderable (Mark, 1983).
Tin(II) chloride is obtained by dissolving metallic tin in hydrochloric acid or by reducing a solution of tin(IV) chloride with metallic tin. The anhydrous salt is produced by the direct reaction of chlorine and molten tin or by heating tin with hydrogen chloride gas. It is an important industrial reducing agent, used in the preparation of glass and plastic for metallizing, metallized glazing, and electronic components on a plastic base, as a soldering flux, as a mordant in dyeing, and in the manufacture of tin chemicals, colour pigments, and sensitized paper (Graf, 1987; Gaver, 1997; ILO, 1998a; K. Nimmo & S. Blunden, personal communication, 2004). Tin(II) chloride is added to lyophilized kits to prepare 99mTc-labelled tracers (which account for about 80% of radiopharmaceuticals). It is important in nuclear medicine as an essential component in diagnostic agents used to visualize blood, heart, lung, kidney, and bone (Francis et al., 1981; Popescu et al., 1984; Rao et al., 1986). Tin(II) chloride is also used in certain countries as a food additive (as a preservative and colour retention agent) (ATSDR, 2003). Tin(IV) chloride is produced commercially by the direct chlorination of tin at 110–115 °C and is used as a dehydrating agent in organic synthesis, in the production of organotin compounds, in the production of tin(IV) oxide films on glass, as a mordant in the dyeing of silks, in the manufacture of blueprint and other sensitized paper, and as an antistatic agent in synthetic fibre (Graf, 1987; Gaver, 1997; K. Nimmo & S. Blunden, personal communication, 2004).
Tin(II) oxide is prepared from the precipitation of tin(II) chloride with alkali. It is used as a reducing agent, in the preparation of stannous salts, and in the preparation of gold–tin and copper–tin ruby glass (Graf, 1987; Gaver, 1997). Tin(IV) oxide is produced by the combustion of powdered tin or sprayed molten tin in a hot stream of air. It is used in the polishing of glass and enamels, in the manufacture of milk-coloured ruby and alabaster glass and enamels, as a mordant in printing and dyeing of fabrics, and in fingernail polish (Graf, 1987).
Tin(II) fluoride is produced commercially by the reaction of tin(II) oxide and aqueous hydrofluoric acid or by dissolving tin in anhydrous or aqueous hydrofluoric acid and is used primarily as an ingredient of caries-preventing toothpaste (Gaver, 1997). Sn2+ ions have a profound and long-lasting inhibiting effect on the oral microflora in vivo (Attramadal & Svatun, 1984). Topical application of tin(II) fluoride appears to provide dentine with a layer of tin and fluoride, which might provide mechanical and chemical protection and be of clinical significance in restorative dentistry. Sn2+ ions possess antibacterial activity, whereas Sn4+ ions do not (Svatun et al., 1977; Ferretti et al., 1982; Rolla et al., 1983; Ellingsen & Rolla, 1987; Rykke et al., 1991). Tin(II) pyrophosphate is prepared from pyrophosphoric acid and tin(II) chloride and is used as an ingredient in caries-preventing toothpaste (Budavari, 2001).
The vapour pressure of elemental tin is negligible (Cooper & Stranks, 1966), and the high boiling points of elemental tin and many inorganic tin compounds indicate that they are non-volatile under environmental conditions. However, the wind may carry airborne particles for long distances before deposition, depending on the type of emitting source, physical form and properties (e.g. size, density), physical or chemical changes that may occur during transport, adsorption processes, and meteorological conditions (Senesi et al., 1999).
In the environment, tin compounds are generally only sparingly soluble in water and are likely to partition to soils and sediments. In water, inorganic tin may exist as either divalent (Sn2+) or tetravalent (Sn4+) cations under environmental conditions. Cations such as Sn2+ and Sn4+ will generally be adsorbed by soils to some extent, which reduces their mobility. Tin(II) dominates in reduced (oxygen-poor) water and will readily precipitate as tin(II) sulfide or as tin(II) hydroxide in alkaline water. Tin(IV) readily hydrolyses and can precipitate as tin(IV) hydroxide. The solubility product of tin(IV) hydroxide has been measured at approximately 10–56 g/litre at 25 °C. In general, tin(IV) would be expected to be the only stable ionic species in the weathering cycle (Wedepohl et al., 1978). Tin(II) can be hydrolysed into SnOH+, Sn(OH)20, and Sn(OH)3− at low concentrations, whereas the Sn2(OH)22+ and Sn(OH)42+ polynuclear species predominate at higher concentrations (Seby et al., 2001). On release to estuaries, inorganic tin is principally converted to the insoluble hydroxide and is rapidly scavenged by particles, which are the largest sink for the metal. Subsequent release of inorganic tin from benthic sediments is unlikely, except at highly anoxic sites (Byrd & Andreae, 1982; Andreae, 1983). Inorganic tin, as cassiterite, is usually the predominant form in sediments of estuaries associated with metal mining in south-west England (Bryan & Langston, 1992). In seawater, inorganic tin is most commonly present as SnO(OH3)− (Bruland, 1983).
Tin is generally regarded as being relatively immobile in the environment (IPCS, 1980; Gerritse et al., 1982). However, tin may be transported in water if it partitions to suspended sediments (Cooney, 1988), but the significance of this mechanism has not been studied in detail. Analysis of inorganic tin from an enclosed harbour revealed that a large percentage (up to 93%) was present in particulate form (Langston et al., 1987).
From the information available, it appears likely that inorganic tin will partition to soils and sediments and will not volatilize from water (IPCS, 1980; Cooney, 1988). Transfer coefficients for tin in a soil–plant system were reported to be 0.01–0.1 (Kloke et al., 1984).
Marine plants are also important in the cycling of inorganic tin. Both live macroalgae and decaying plant material accumulate inorganic tin compounds and ultimately remove tin from water and release it to the atmosphere by the formation and release of tetramethyltins (Donard et al., 1987).
Inorganic tin may undergo oxidation–reduction, ligand exchange, and precipitation reactions in the environment (HSDB, 2003). The biomethylation of inorganic tin has been demonstrated in pure bacterial cultures, sediments, and decaying plant material, with a variety of products being detected, including mono-, di-, tri-, and tetramethyltins (Hallas et al., 1982; Tugrul et al., 1983; Gilmour et al., 1987; Falke & Weber, 1994). The net methylation rate was found to be independent of the inorganic tin content of the sediments (Tugrul et al., 1983). Methylation of tin in sediments was found to be positively correlated with increasing organic content in sediment and to follow predominately a biotic pathway (Hadjispyrou et al., 1998). Inorganic tin may also be converted to stannane in extremely anaerobic (oxygen-poor) conditions by decaying algal material (Donard & Weber, 1988). Conversely, methyltin compounds can also be demethylated sequentially to inorganic tin by photolysis (Blunden, 1983).
Inorganic tin compounds may be bioconcentrated, but data are limited. It was estimated that the bioconcentration factors of inorganic tin were 100, 1000, and 3000 for marine and freshwater plants, invertebrates, and fish, respectively (Thompson et al., 1972). Marine macroalgae can bioconcentrate the Sn4+ ion by a factor of 1900 (Seidel et al., 1980). Donard et al. (1987) reported inorganic tin concentrations of up to 4.4 mg/kg dry weight in macroalgae. Tin-resistant bacteria contained tin at 3.7–7.7 g/kg dry weight (Maguire et al., 1984).
There is no information available on the potential transfer of inorganic tin compounds from lower trophic levels to higher levels.
Ambient levels of tin in the environment are generally quite low, except in the vicinity of local pollution sources. Analytical results based on inorganic tin have been included where possible. However, many studies have analysed for total tin only; in these cases, the data are provided for information, while bearing in mind that the concentrations may include some organic tin compounds. The proportion of inorganic tin in total tin concentrations will vary depending on sampling time and site.
Tin is detected in air infrequently and at low concentrations, except in the vicinity of industrial sources. Air concentrations of tin in US cities from several studies were as high as 0.8 µg/m3. Average concentrations are generally below 0.1 µg/m3, with higher concentrations near some industrial facilities (IPCS, 1980; US EPA, 1982). Average concentrations have been estimated to be 0.002–0.03 µg/m3 (Biégo et al., 1999), 0.001 µg/m3 in northern hemisphere air (Byrd & Andreae, 1982), and less than 0.3 µg/m3 (JECFA, 1989). Davison et al. (1974) reported that the total tin content of airborne fly ash from coal-burning power plants ranged from 7 mg/kg (particle diameter >1.7 µm) to 19 mg/kg (particle diameter 3.3–4.7 µm).
Atmospheric tin is associated with particulate matter, and peak concentrations were found on smaller respirable particles (1–3 µm) (IPCS, 1980). Samples of airborne inhalable particulate matter were collected in two urban/industrial areas in Illinois, USA (south-east Chicago and East St. Louis) and a rural area in Bondville, also in Illinois, over a 2-year period. Average total tin concentrations in the coarse (2.5–10 µm) and fine (<2.5 µm) particulate fractions were <7 ng/m3 and 12 ng/m3, respectively, for East St. Louis; and <7 ng/m3 for both the fine and coarse fractions in samples from south-east Chicago as well as the rural site in Bondville (Sweet et al., 1993). The average total tin concentration in highway tunnel exhaust aerosol in the Elbtunnel in Hamburg, Germany, between August 1988 and January 1989 was 10.9 ng/m3 (Dannecker et al., 1990). Tin has been identified in air collected at 6 of the 214 current or former US EPA National Priorities List hazardous waste sites where it was detected in some environmental media (HazDat, 2003).
Tin occurs in trace amounts in natural waters; however, it is seldom measured (NAS, 1977; IPCS, 1980). Higher inorganic tin concentrations are associated with industrial discharges and tributyltin use (IPCS, 1980; Maguire & Tkacz, 1985; Maguire et al., 1986). Inorganic tin concentrations of up to 0.003 µg/litre were reported for rainwater in the USA during 1981 (Tugrul et al., 1983). Tin has been identified in groundwater and surface water at 78 and 36 sites, respectively, of the 214 US EPA National Priorities List hazardous waste sites where it was detected in some environmental media (HazDat, 2003). In surface waters, tin was detected in only 3 of 59 samples from 15 US and Canadian rivers at concentrations ranging from 1.3 to 2.1 µg/litre, and it was not detected in 119 samples from 28 US rivers. A mean tin concentration of 0.038 µg/litre was reported for surface water in Maine, USA (NAS, 1977; IPCS, 1980). In a survey of Canadian waters, nearly 80% of samples were found to contain inorganic tin at concentrations below 1 µg/litre; higher levels of up to 37 µg/litre were reported near pollution sources (Maguire et al., 1986). Mean tin(IV) concentrations in Lake Michigan during 1978 ranged from 0.08 to 0.5 µg/litre (Hodge et al., 1979). Similarly, mean inorganic tin concentrations of 0.004 µg/litre were detected in the Lamas River, Turkey, between 1981 and 1983. Industrial pollution was found to increase inorganic tin levels in the river estuary to up to 0.7 µg/litre (Yemenicioglu et al., 1987).
Total tin is present in seawater at about 0.2–3 µg/litre (NAS, 1977; IPCS, 1980). Inorganic tin concentrations ranging from 0.001 to 0.01 µg/litre have been reported for coastal waters, with levels of up to 8 µg/litre near pollution sources (Tugrul et al., 1983; Valkirs et al., 1986). Tin(IV) concentrations ranging from 0.003 µg/litre for open seawater to 0.04 µg/litre in San Diego Bay, California, USA, have been reported (Hodge et al., 1979). Langston et al. (1987) found that concentrations of dissolved inorganic tin displayed extreme variability both temporally and spatially within an enclosed harbour and were largely influenced by localized inputs. Concentrations generally ranged from <0.005 to 0.2 µg/litre; however, levels of up to 48.7 µg/litre were found near local pollution sources.
Tin concentrations in drinking-water, including the United Kingdom supply, have been reported to be below 10 µg/litre (Sherlock & Smart, 1984; JECFA, 2001). Tin concentrations in public water supplies ranged from 1.1 to 2.2 µg/litre in 42 US cities and from 0.8 to 30 µg/litre in 32 of 175 water supplies in Arizona, USA (NAS, 1977; IPCS, 1980). An average concentration of 6 µg/litre has been reported in US municipal drinking-water (Hadjimarkos, 1967).
Tin concentrations in fresh snow from the French Alps collected in 1998 at different altitudes ranged from 0.16 to 0.44 µg/litre (Veysseyre et al., 2001).
Mean total tin concentrations in Antarctic sediment were 2.1 and 5.1 mg/kg dry weight for the <2 mm and <63 µm fractions, respectively (Giordano et al., 1999). Inorganic tin was detected in 100 of 235 sediment samples collected from Canadian waterways. Concentrations ranged up to 8 mg/kg dry weight in coastal areas and up to 15.5 mg/kg in rivers and lakes (Maguire et al., 1986). Sediment concentrations of inorganic tin in Toronto Harbour, Canada, during 1983 were found to be highest (up to 13.8 mg/kg) near areas of tributyltin contamination (Maguire & Tkacz, 1985). Sediment cores collected in January 1996 from Central Park Lake in New York City, New York, USA, contained average tin concentrations ranging from 4.0 mg/kg at a depth of 44–47 cm to 67 mg/kg at a depth of 22–24 cm. The average tin concentration in surface sediments (0- to 2-cm depth) in Central Park Lake was 32 mg/kg. The similarities between the history of municipal solid waste incineration in New York City and the accumulation of trace metals in the Central Park Lake sediments appear to be consistent with incineration being the major source of emissions of several metals to the New York City atmosphere (Chillrud et al., 1999). Total tin concentrations in sediments from the Wah Chang Ditch and the north-east corner of Swan Lake, an area that received runoff from a tin smelter in Texas, USA, during the 1940s and 1950s, were found to be as high as 8000 mg/kg (Park & Presley, 1997). Total tin concentrations up to 1000 mg/kg dry weight have been reported for metal-rich sediments in estuaries associated with metal mining in south-west England (Bryan & Langston, 1992).
Tin concentrations in soil are generally low, except in areas where tin-containing minerals are present (Bulten & Meinema, 1991). Tin concentrations in the Earth’s crust are approximately 2–3 mg/kg (Budavari, 2001). Crockett (1998) reported total tin concentrations in Antarctic soils ranging from 2.5 to 3.1 mg/kg. Total tin concentrations in soil can range from <1 to 200 mg/kg; however, in areas of high tin deposits, such as south-west England, levels of 1000 mg/kg may occur (IPCS, 1980; Schafer & Femfert, 1984). The mean background soil concentration in the USA is 0.89 mg/kg (Eckel & Langley, 1988).
Tin concentrations in topsoil (0–7.6 cm) from the western end of East St. Louis, Illinois, USA, ranged from <13 to 1130 mg/kg. The raised concentrations are thought to be the result of current or recent industrial facilities, including smelters of ferrous and non-ferrous metals, a coal-fired power plant, chemical producing companies, and petroleum refineries (Kaminski & Landsberger, 2000). Tin has been identified in soil at 120 sites and in sediment at 50 sites collected from 214 US EPA National Priorities List hazardous waste sites where it was detected in some environmental media (HazDat, 2003).
Concentrations of total tin in sewage sludges from countries in Europe and North America ranged from 40 to 700 mg/kg dry weight. Manure and poultry wastes contained tin at concentrations of 3.7–7.4 and 2.0–4.1 mg/kg dry weight, respectively (Senesi et al., 1999)
Total tin concentrations in marine macroalgae varied between 0.5 and 101 mg/kg dry weight and clearly demonstrated that most species of aquatic flora bioconcentrate tin from seawater (Eisler, 1989). Local and imported edible seaweeds obtained in British Columbia, Canada, were found to contain total tin concentrations ranging from <0.01 to 0.46 mg/kg dry weight (van Netten et al., 2000).
Total tin concentrations in muscle and liver samples of juvenile Japanese common squid (Todarodes pacificus) collected from three locations in and near Japanese coasts were 0.04–0.05 mg/kg wet weight and 0.08–0.13 mg/kg wet weight, respectively (Ichihashi et al., 2001). Inorganic tin concentrations in fish collected from the Great Lakes in North America (1982–1984) ranged up to 0.9 mg/kg wet weight (Maguire et al., 1986).
Mean total tin concentrations in the feathers of seabirds from the North Pacific and water birds from the coast of Namibia ranged from 0.2 to 15.2 mg/kg dry weight (Burger & Gochfeld, 2000, 2001; Burger et al., 2001).
Total tin concentrations in the kidneys of mink (Mustela vison) collected from the Kootenay River and lower Fraser River in British Columbia, Canada, were 6.25 and 5.5 µg/g dry weight, respectively. Tin concentrations in the livers of mink from the upper and lower Fraser River were 5.5 and 5.2 µg/g dry weight, respectively. Tin concentrations were <4 µg/g dry weight in the livers of otters (Lontra canadensis) collected from the Kootenay, lower and upper Columbia, and upper Fraser rivers and 2.7 µg/g in livers of otters from the lower Fraser River (Harding et al., 1998). Mean total tin concentrations in striped dolphins (Stenella coeruleoalba) from the Mediterranean Sea ranged from 0.4 mg/kg wet weight in lung tissue to 1.3 mg/kg in liver (Cardellicchio et al., 2002). Total tin concentrations in the livers of Antarctic fur seals (Arctocephalus gazella) were less than 0.4 mg/kg dry weight (<0.1 mg/kg wet weight) (Malcolm et al., 1994).
Data presented in ATSDR (2003) indicate that organic tin accounts for only a small fraction of total tin in most foods. On that basis, in this section, tin figures are total tin, but essentially represent inorganic tin.
In most unprocessed foods, inorganic (and total) tin levels are generally less than 1 mg/kg. Higher concentrations can arise as tin(II) in canned foods due to dissolution of the tin coating or tin plate. Tin levels are usually below 25 mg/kg in lacquered food cans, but may exceed 100 mg/kg in unlacquered cans. Tin concentrations in canned foods increase with storage time and temperature. Once opened, the tin content in foods stored in metal cans increases more quickly over time, since tin can rapidly dissolve in the presence of oxygen. Acidic foods are more aggressive to the tin coating in metal cans, and canned acidic foods have higher tin contents. Oxidizing agents (nitrates, iron salts, copper salts, sulfur) accelerate detinning, whereas tin salts, sugars, and gelatin reduce the dissolution rate. Can size and the nature of the base steel might also affect the detinning rate (JECFA, 1989; Blunden & Wallace, 2003).
Tin concentrations of vegetables, fruits and fruit juices, nuts, dairy products, meat, fish, poultry, eggs, beverages, and other foods not packaged in metal cans are generally below 2 mg/kg. Tin concentrations in pastas and breads have been reported to range from <0.003 to 0.03 mg/kg. Mean tin concentrations ranging from <1 to 1000 mg/kg have been found in foods packaged in unlacquered or partially lacquered cans, whereas the average tin concentration in foods in lacquered cans has been reported to be up to 6.9 mg/kg (Biégo et al., 1999; Ysart et al., 1999; JECFA, 2001). Data from the Can Manufacturers Institute indicate that more than 90% of tin-lined cans used for food today are lacquered (CMI, 1988). Only light-coloured fruit and fruit juices are packed in unlacquered cans, since tin helps maintain the colour of the fruit (JECFA, 2001).
Local and imported edible seaweeds obtained in British Columbia were found to contain tin in concentrations ranging from 0.01 to 0.46 mg/kg dry weight (van Netten et al., 2000). A study in Lithuania in 1990–1992 found an average of 0.22 mg/kg in raw milk (Ramonaityte, 2001). In a dietary tin intake study for adults in France, a range of fresh foods contained tin at concentrations of <0.003–0.2 mg/kg (average 0.03 mg/kg) (Biégo et al., 1999). Foods in lacquered cans contained tin at concentrations generally below 10 mg/kg and ranging from 0.5 mg/kg (in cherries) up to 13.4 mg/kg (in mushrooms) (Biégo et al., 1999). Tin concentrations ranged from 24 to 156 mg/kg in food from unlacquered cans, the highest concentration being detected in tomatoes (Biégo et al., 1999). Canned vegetables and fruit products were found to have mean tin concentrations of 44 and 17 mg/kg fresh weight, respectively, in a 1994 total diet study in the United Kingdom (Ysart et al., 1999). A study of metal concentrations in canned milk products in Lithuania in 1990–1992 found mean tin concentrations in evaporated sterilized milk, concentrated sterilized milk, and sweetened condensed milk of 85, 89, and 40 mg/kg, respectively. Tin concentrations in canned milk were shown to increase during storage (Ramonaityte, 2001).
Studies in the United Kingdom showed mean concentrations of tin in the diet of 1–2 mg/kg. The primary sources of tin were said to be canned goods (Evans & Sherlock, 1987). Tin density figures for selected diets in France, New Zealand, and the United Kingdom ranged from 1.2 to 4.4 mg/kg (JECFA, 2001).
Analysis (using energy-dispersive X-ray fluorescence) of dust in eight US homes found tin at <10 mg/kg (the detection limit) in five cases and at 12, 14, and 73 mg/kg in the other three cases. These houses were selected because they were near the homes of men employed as electric cable splicers. Dust in the homes of these workers contained higher tin concentrations (45–242 mg/kg), and levels were higher in the laundry area (geometric mean 117 mg/kg) than in the rest of the house (66 mg/kg), suggesting that the tin source was occupational contamination of the clothing (Rinehart & Yanagisawa, 1993).
The major source of tin for the general population is food. Intake from the diet is highly dependent on the type and amount of canned food consumed (JECFA, 1989). For those consuming no canned food, intake might be approximately 3 mg/day (Sherlock & Smart, 1984). Individuals who routinely consume canned fruit, vegetables, and juices from unlacquered cans could ingest 50–60 mg tin daily, assuming about four servings per day (Johnson & Greger, 1982; Sherlock & Smart, 1984). An adult consuming 1 litre of juice with a tin content of 100 mg/litre (from unlacquered cans) daily would ingest 100 mg of tin per day from this source alone (JECFA, 1989). People with low incomes or living in institutions such as nursing homes, boarding schools, or prisons may routinely select or be served canned food and juices because of economic factors and ease of storage, and those who consume about four daily servings of food from open, unlacquered cans could consume about 200 mg of tin per day (Greger & Baier, 1981; Greger, 1988; JECFA, 2001).
JECFA has summarized data on estimates of mean dietary tin intakes for Australia, France, Japan, the Netherlands, New Zealand, the United Kingdom, and the USA. In Australia, results of a total diet study produced estimated mean tin intakes (for six sex–age groups) ranging from 0.4 to 5.2 mg/day, with the highest 95th percentile being 7.4 mg/day (Marro, 1996). Using the DIAMOND method of modelling nutrition data, the Australia New Zealand Food Authority estimated a mean intake of 1.9–2.4 mg/day for consumers aged 2–70 years, with a 95th percentile value of 10 mg/day (Baines, 2000). Australian surveys produced intake figures of 2.2–2.7 mg/day, with a 95th percentile of 12 mg/day (Baines, 2000). For French adults, it was estimated that the mean dietary intake of tin would be 0.05 mg/day from a fresh food diet, 0.34 mg/day from a diet with usual canned foods in lacquered cans, and 7.4 mg/day from a diet with usual canned foods in unlacquered cans (Biégo et al., 1999). In Japan, the average tin intake of 456 women in 22 cities and villages during the winters of 1990–1995 was estimated to be 0.64 mg/day (based on duplicate food samples and food composition databases), although this was probably an underestimate, because not all food types were included (Shimbo et al., 1996). Dutch total diet studies, based on the consumption by 18-year-old men of 221 food items within 23 food commodity groups, produced average tin intakes of 1.7 mg/day during 1976–1978 and 0.65 mg/day (maximum 1.8 mg/day) in 1984–1986 (van Dokkum et al., 1989). Based on analysis of selected foods in the 1997–1998 New Zealand total diet study, the estimated mean dietary intakes of tin ranged from 2.9 mg/day (for children aged 1–3 years) to 7.5 mg/day for adult female vegetarians. Canned spaghetti, baked beans, apricots, tomatoes, and peaches contributed 77% of the dietary exposure of men aged 19–24 years and of children aged 1–3 years (Vannoort et al., 2000). In the 1974–1975 New Zealand total diet study, it was estimated that young New Zealand men eating 3.5 kg of food per day might ingest a mean of 15 mg of tin per day (Dick et al., 1978). In the United Kingdom, results from total diet studies suggest that tin intake has been falling (mean daily intakes were 4.4, 2.4, and 1.8 mg in 1976, 1994, and 1997, respectively), possibly due to use of an increasing proportion of lacquered cans (Ysart et al., 1999; UK MAFF, 2000; JECFA, 2001). However, a study in 35 female vegetarians in the United Kingdom (using a duplicate-diet sampling method) found a mean intake of 3.8 mg/day (range 0.03–16 mg/day), about twice as high as reported for the general population (UK MAFF, 2000). JECFA did not identify any reliable estimates for the US diet, noting that tin was not measured in the USA’s total diet study, but small studies provided mean estimates of 1 mg/day from a diet without canned food (Schroeder et al., 1964) and 1.5–3.5 mg/day for (undefined) adults (Schroeder et al., 1964; Tipton et al., 1969). JECFA noted that a small, early study focusing on metabolism had claimed that a diet containing "substantial amounts of canned vegetables, fruit juices, and fish could supply as much as 38 mg/day" (Schroeder et al., 1964).
Drinking-water is not considered a significant source of tin. If it is assumed that concentrations of tin in drinking-water are 6–10 µg/litre (see section 6.1.2) and that adults consume 2 litres/day, these figures suggest an intake of 12–20 µg/day from this source (JECFA, 1989, 2001).
Inhaled air represents very low tin exposure. Based on tin levels in air of <0.3 µg/m3 (JECFA, 1989), an adult inhaling 20 m3 of air per day would in general inhale less than 6 µg/day. Air concentrations of tin in US cities ranging from below the detection limit to 0.8 µg/m3 (US EPA, 1982) imply an inhaled dose of up to about 16 µg/day. Based on estimated average tin concentrations of 0.002–0.03 µg/m3 (Biégo et al., 1999), an adult is unlikely to inhale more than 0.6 µg of tin per day. Exposures are presumably higher near emission sources such as waste incineration and non-ferrous metal production (Byrd & Andreae, 1982) (see section 6.1.1).
Most of the operations associated with the extraction of tin ore are wet processes, but tin and tin(IV) oxide dust and fumes may escape during bagging of concentrate, in ore rooms, and during smelting operations (mixing plant and furnace tapping), as well as during the periodic cleaning of bag filters used to remove particulate matter from smelter furnace flue gas (ILO, 1998a). Tin reclamation from tin-plated steel trimmings, rejects from companies manufacturing tin cans, rejected plating coils from the steel industry, tin drosses and sludges, solder drosses and sludges, used bronze and bronze rejects, and metal-type scrap also involve possible exposure to tin dusts and fumes (ILO, 1998b). Gases, dusts, and fumes containing tin may be released from smelting and refining processes, industrial uses of tin, waste incineration, and burning of fossil fuels (IPCS, 1980; Senesi et al., 1999; ATSDR, 2003).
No systematic data on occupational exposure levels in tin production or processing were found by the authors of the Nordic/Dutch source document (Westrum & Thomassen, 2002). The Norwegian occupational exposure database EXPO contains data from all samples analysed at the National Institute of Occupational Health in Oslo since 1984. Most of these samples have been collected due to the desire of different enterprises to control their exposures and are likely to represent "worst-case measurements" (Rajan et al., 1997). Of the 3407 air filter samples (8-h personal monitoring) analysed for tin, 420 contained amounts above the detection limit (0.002 mg/m3). In Table 5, the branches and job functions with tin exposures above 0.05 mg/m3 are listed, together with the mean and range of concentrations.
Table 5: Branches and job functions in EXPO where tin concentrations of >0.05 mg/m3 air were detected.
|
Branch/job functions |
Number of samples |
Mean tin concentration (mg/m3) |
Tin concentration range (mg/m3) |
|
Defence activities/spraying |
3 |
0.20 |
0.01–0.46 |
|
Metal coating/surface coating |
2 |
0.32 |
0.20–0.45 |
|
Electronic production/surface coating |
2 |
1.51 |
0.09–2.93 |
|
Railway repair/termite welding |
6 |
1.07 |
0.01–5.68 |
|
Metal casting/cleaning |
2 |
0.29 |
0.25–0.34 |
Limited information on past occupational exposure to tin was found in the older literature. Analysis of dust samples collected in the vicinity of a worker grinding tin ore in a tin smelting works in Liverpool, United Kingdom, showed that the dust fraction with particle size <5 µm contained more than 33% metallic tin and no detectable silica. The tin ore handlers were said to be "exposed to considerable quantities of dust" (Robertson, 1960). In this smelting works, concentrations of total tin or tin(II) oxide were not measured, but particles <5 µm in diameter were collected using a Hexlet in "places where dust concentration was likely to be especially high." Preliminary figures for concentrations (in mg/m3) of tin as particles <5 µm in diameter in the workroom air were as follows: check sampling shed, 2.22; and dracco room (an area containing filters for furnace gases), 1.50. In addition, air samples were taken near workers: smelting furnace man, 1.55; refining furnace man, 0.82; orehouse skipman, 0.34; plumber, 0.12; electrician, 0.05; and engineer, 0.02. The methods of sampling and analysis were not described (Robertson, 1964). An environmental survey to determine the type of exposure in a Chilean tin foundry showed air concentrations of metal tin between 8.6 and 14.9 mg/m3 (Oyanguren et al., 1958).
In a review, Alessio et al. (1994) reported that tin concentrations in ambient air in three copper alloy industries ranged from 1 to 4 µg/m3. Atmospheric concentrations of tin of 16 and 0.2 µg/m3 were reported for manual metal arc mild steel welding and arc stainless steel high nickel welding, respectively. A tin concentration of 1 µg/m3 was found for silver brazing.
Tin concentrations in the particulate matter in the ambient air at art glass manufacturing plants measured by personal samples from oven charger and batch mixer workers ranged from 0.1 to 3.5 µg/m3 from three plants that use arsenic as a fining agent (an agent that is added to disperse air bubbles in glass). Tin was not detected in the particulate matter in the air at three other plants that used antimony compounds instead of arsenic (Apostoli et al., 1998).
Tin production may also involve exposure to silica, lead, and arsenic in the mining of the sulfide ores of tin, as well as to bismuth and antimony in the roasting and smelting process. Exposure to these toxic metals might also occur during the preparation and use of tin alloys and solders. Tin mining might involve exposure to radon, thorium, and uranium (Fox et al., 1981; Qiao et al., 1989, 1997; Taylor et al., 1989; Hodgson & Jones, 1990; Oresegun & Babalola, 1990; Forman et al., 1992; Beliles, 1994).
Generally, absorption of tin from the gastrointestinal tract is low in humans and laboratory animals, including rats, mice, rabbits, cats, and dogs (JECFA, 2001; Stewart & Lassiter, 2001), but it may be influenced by aqueous solubility, dose, anion, and the presence of other substances. In vitro studies using rat small intestine suggested that absorption of tin occurs by passive diffusion (Kojima et al., 1978).
In a balance study in which eight healthy volunteers were given a control diet providing 0.11 mg of tin per day for 20 days, mean faecal excretion was 55% of the daily dose, suggesting a mean net absorption of 45% at this low dose (although the range was wide, varying from −4 to 71%). When the diet was supplemented (with tin(II) chloride) to provide an additional 50 mg of tin per day for 20 days, mean faecal excretion was 97% of the daily dose, suggesting a net absorption of 3% (range −7 to 9%) at this higher dose (Johnson & Greger, 1982).
Four human volunteers with tin blood levels of <2 ng/ml (<17 nmol/litre) each consumed 60 mg of tin in the form of fruit juice from an unlacquered can, and blood samples were taken after 2, 5, and 24 h. The two females had detectable tin blood levels (3 ng/ml) only in the 5-h samples. The two males had peak blood tin concentrations of 4.7 ng/ml after 2 h and 3.9 ng/ml after 24 h (Byrne & Kosta, 1979).
Anodic stripping voltammetry was used to study tin concentrations in the urine of 89 men and 85 women (aged 20 years or more) in the Japanese prefecture of Aichi over a 3-year period from 1986 to 1988. For 79 subjects, data were available for each year. Expressed as µg/g creatinine, the mean tin concentration was significantly higher in women (5.9 ± 3.0; range 1.9–16.0) than in men (3.7 ± 2.2; range 0.8–13.4; P < 0.001). Distributions of concentrations were lognormal in both sexes. Mean concentrations tended to increase with age in both men (3.3 ± 2.5 at 20–29 years, 4.7 ± 2.2 at >60 years) and women (5.1 ± 3.6 at 20–29 years, 7.3 ± 2.7 at >60 years). In men, mean concentrations increased significantly, in a dose-related manner, with frequency of fish consumption (2.9 ± 1.8, 3.5 ± 2.0, and 4.7 ± 2.9 for consumption on 1–2, 3–4, and 5–7 days per week, respectively). A similar dose-related increase was seen in females (5.3 ± 1.9, 5.8 ± 3.1, and 6.3 ± 3.2 for consumption on 1–2, 3–4, and 5–7 days per week, respectively), although the differences between groups were not statistically significant. Organotin contamination of fish was the suggested explanation for these findings. Urinary tin concentrations showed no relationship with the level of consumption of canned food (Hayashi et al., 1991).
Following single gavage administration of 113Sn(II) or 113Sn(IV) as the citrate or fluoride to rats, absorption was estimated to be 2.85% and 0.64% of the 20 mg/kg body weight dose for the 2+ and 4+ oxidation states, respectively, based on 48-h recovery of radioactivity in the urine and tissues. Absorption was lower when tin was given as the pyrophosphate, presumably due to this anion’s greater tendency to form insoluble tin complexes (Hiles, 1974). These figures are in line with reports that absorption of tin(II) chloride is generally below 5% (Kutzner & Brod, 1971; Furchner & Drake, 1976; Fritsch et al., 1977; Sullivan et al., 1984). In one case, rats reportedly absorbed 7.65% of a single oral dose of tin(IV) chloride (Kojima et al., 1978). The recovery of 99% of administered tin in the faeces and the lack of detectable urinary tin in the 24 h following ingestion of tin (7–20 mg/kg body weight) in orange juice by rats and cats indicate a very low gastrointestinal absorption of tin (Benoy et al., 1971). The presence of certain organic acids can increase tin absorption from the gastrointestinal tract (Kojima et al., 1978).
No increase in blood levels of tin was seen when male Wistar rats were given tin(II) chloride in the drinking-water at up to 250 mg/litre for 1–18 weeks (tin dose probably between 8 and 21 mg/kg body weight per day). At a tin(II) chloride concentration of 500 mg/litre, tin concentrations in blood rose in the first week and remained at 2–7 µg/litre (equivalent to 2–5 times control values) for the rest of the study. The data suggest that mucosal barriers are effective in preventing tin absorption at low doses but are overcome at higher doses (Savolainen & Valkonen, 1986).
Rabbits fed tin at 2 mg/kg body weight per day for 5 days (as tin(II) chloride) had blood tin concentrations of 2.3 µg/litre after 24 h and 0.7 µg/litre after 120 h. Tin was not detected (detection limit not stated in the source document) in the controls (Zareba & Chmielnicka, 1992).
Adequate data on uptake following inhalation or dermal exposure appear to be lacking (Westrum & Thomassen, 2002).
Inorganic tin distributes mainly to bone, but also to the lungs, liver, kidneys, spleen, lymph nodes, tongue, and skin. Certain data indicate that tin may have a higher affinity for the thymus than for other organs. Laboratory animal data suggest that inorganic tin does not readily cross the blood–brain barrier (Hiles, 1974; Furchner & Drake, 1976; Hasset et al., 1984; Savolainen & Valkonen, 1986; JECFA, 2001).
Tin was seldom present in the lung tissue of newborn babies in the USA. In adults, the tin content in human lung tissue was higher in the USA than in Africa (Schroeder et al., 1964). These data suggest that tin levels in the human lung increase with age, and the likely source might be polluted air.
In an analysis of the tin content in tissue samples from adults who had died in accidents, the highest concentrations were found in the bone ash (4.1 mg/kg), followed by the lymph nodes, lungs, liver, and kidneys (1.5, 0.8, 0.4, and 0.2 mg/kg wet weight, respectively), whereas levels in muscle (0.07 mg/kg wet weight) and brain (0.06 mg/kg wet weight) were lower (Hamilton et al., 1973). Autopsy samples from 78 deceased Spaniards contained mean tin concentrations of 0.47, 0.27, 0.25, 0.24, and 0.16 mg/kg in the bone, brain, kidneys, lungs, and liver, respectively (Garcia et al., 2001). Analysis of tissue samples from 20 deceased Spanish subjects (without known occupational tin exposure) found highest and lowest tin levels in bone (mean 6.2 mg/kg) and brain (mean 1 mg/kg), respectively (Llobet et al., 1998). Median tin concentrations (in mg/kg wet weight) in samples from adult US citizens were as follows: adrenal, 5.1; lung, 3.4; liver, 1.8; kidney, 1.5; spleen, 0.8; muscle, <0.4; and brain, 0.3 (Tipton & Cook, 1963). In healthy Japanese males, tin concentrations of 9.8 mg/kg dry weight in hilar lymph nodes and 1.5 mg/kg dry weight in lung tissue were reported (Teraoka, 1981). Analysis (using AAS) of several human organs from 11–13 adult males found mean concentrations (mg/kg dry weight) as follows: liver, 1.05; kidney cortex, 0.83; heart, 0.75; lung, 0.45; rib bone, 0.61; and testis, 2.08 (Chiba et al., 1991). The tin concentration in liver specimens from 11 US citizens ranged from 0.14 to 0.17 mg/kg wet weight (determined by neutron activation analysis), and the tin concentration in Japanese human liver specimens (n = 23) ranged from 0.08 to 1.12 mg/kg wet weight (determined by AAS) (Chiba et al., 1994). An average tin concentration of 12.8 mg/kg wet weight was reported in the thymus of two children (Sherman et al., 1985). Tin was detected at 4.6–15 mg/kg in adipose tissue samples from nine US subjects in a 1982 survey (Stanley, 1986).
In humans with no occupational exposure to tin compounds, blood tin concentrations of 2–9 µg/litre are reported (detection limit 2 µg/litre) (Hamilton et al., 1973; Kazantzis, 1994). Others reported normal tin concentrations of 11.6 ± 4.4 nmol/litre (mean ± SD) in plasma and 21.7 ± 6.7 nmol/litre in red blood cells in 12 humans (8 women, 4 men, mean age 77.8 years) (Corrigan et al., 1992). Background tin concentrations of <1 µg/litre in serum and urine have been reported (Versieck & Vanballenberghe, 1991; Schramel et al., 1997), and a 95th upper percentile of 20 µg/litre in urine was calculated for a group of 496 US residents (Paschal et al., 1998).
Autopsy analysis of the internal organs of 7 Japanese metal industry workers and 12 Japanese males without occupational exposure found elevated concentrations of tin in the lungs, spleen, liver, and kidneys of chromate plating and chromate refining workers. In one chromate refining worker, marked concentrations of tin were found in the hilar lymph nodes (100 mg/kg dry weight) and lungs (100 mg/kg dry weight) (Teraoka, 1981).
Following a single gavage dose of 20 mg/kg body weight of radiolabelled 113Sn(II) or 113Sn(IV) as the fluoride or citrate, the tissue distribution of tin in rats after 48 h as a percentage of the administered tin(II) or tin(IV), respectively, was as follows: skeleton, 1.0% and 0.24%; liver, 0.08% and 0.02%; and kidneys, 0.09% and 0.02%. When oral tin doses of 20 mg/kg body weight were given on 6 days/week for 4 weeks, only the bone contained higher tin concentrations after day 28 than after day 1. The half-time of tin in the femur was estimated to be 34–40 days. The investigator concluded that, of the soft tissues, only liver and kidneys are likely to accumulate significant amounts of tin as a result of the oral ingestion of tin salts. No 113Sn was found in the brain of rats 48 h following administration of 113Sn(II) or 113Sn(IV) as citrate or fluoride as a single oral dose (4 mg), as oral doses of 20 mg/kg body weight on 6 days/week for 4 weeks, or as a single intravenous dose (0.4 mg) (Hiles, 1974).
Studies on the retention of the radionuclide 113Sn administered as tin(II) chloride intraperitoneally in rats showed that most of the tin retained in the body was deposited in the bone, followed by muscle, pelt, liver, and kidneys. In contrast to all other organs, the relative amount of tin in bone increased during the study (Furchner & Drake, 1976).
A study of the pharmacodynamics of several tin compounds in rabbits, using 113Sn(II) chelates also labelled with 99mTc (meta-stable technetium-99), showed that free Sn2+ ions localize mainly in bone. The distribution of 113Sn in bone was similar to that of calcium and other bone-seeking metal ions (Dewanjee & Wahner, 1979).
Tin concentrations of 0.1–0.29 mg/kg wet weight and 0.69 mg/kg dry weight have been reported in the bone tissue of unexposed mice (Chiba et al., 1991).
The concentrations of tin in the tibias of rats fed diets supplemented with tin(II) chloride (100–2000 mg of tin per kg of diet) were more than 5 times greater than the tin concentrations in the kidneys and nearly 20 times greater than the concentrations in the liver. No other organs were analysed. Tin accumulated in the tibia and kidneys in a dose-dependent manner (Johnson & Greger, 1985).
After lifelong administration of about 0.37 mg of tin per kg body weight per day as tin(II) chloride in the drinking-water of rats, mean tin concentrations were increased approximately 2- to 3-fold in the liver, heart, lungs, and spleen, but the differences were not statistically significant. Bone was not examined (Schroeder et al., 1968). In mice, a similar study (reported intake of about 0.37 mg/kg body weight per day) found tin levels of 1.2–4.5 mg/kg wet tissue in kidneys, liver, heart, lungs, spleen, and thyroid, compared with less than 0.5 mg/kg in tissues in the control group (Schroeder & Balassa, 1967).
A 2-year carcinogenesis study of tin(II) chloride administered at 1000 or 2000 mg/kg in the diet to F344 rats and B6C3F1 mice showed a dose-dependent difference in the concentration of tin in examined organs (i.e. bone, liver, and kidneys). For the low and high doses, tin levels in bone were, respectively, 9 and 38 mg/kg in male rats and 23 and 41 mg/kg in female mice. Tin concentrations in the kidneys were, respectively, 17 and 30 mg/kg in male rats and 0.7 and 0.9 mg/kg in female mice. In the liver, tin concentrations were, respectively, 0.2 and 0.4 mg/kg in male rats and 0.4 and 0.5 mg/kg in female mice. In both species, tissue levels tended to be higher in the females. Untreated rats and mice had tin concentrations that were below the detectable limits, which ranged from 0.01 to 0.1 mg/litre of "digested tissue" (NTP, 1982).
Some data indicate that the tin content is higher in the thymus than in other representative organs. In four young adult dogs, the tin concentration in thymus was about twice the concentrations in the spleen or muscle (Sherman & Cardarelli, 1988). Analysis of unexposed adult Lewis rats, adult COBS mice, and adult A/KI mice showed thymus tin concentrations of 20, 5.5, and 4.3 mg/kg, respectively. Tin concentrated in the thymus gland as the gland atrophied with age (Sherman et al., 1986).
The tin content of the brain (7–19 nmol/kg wet weight) of Wistar rats given tin(II) chloride dihydrate at 100 mg/litre in the drinking-water for 18 weeks was not significantly different from that in control rats (5–10 nmol/kg). At 250 mg/litre, brain tin was increased after 15 weeks (19 ± 8 nmol/kg) and 18 weeks (38 ± 8 nmol/kg). At 500 mg/litre, the tin content of the brain rose steadily throughout the 18 weeks to about 80 nmol/kg (Savolainen & Valkonen, 1986).
In pregnant rats fed tin at 20 mg/kg body weight per day as radioactive tin(II) or tin(IV) fluoride, no tin was found in fetal or placental tissues on day 10 of pregnancy. On day 21, fetuses of dams administered tin(II) fluoride apparently contained approximately 0.2% of the cumulative dose (Hiles, 1974). Fetal tin values were slightly elevated (0.8–1.3 mg/kg body weight) in Sprague-Dawley rats on day 20 of gestation when the maternal diets contained tin salts (tin(II) fluoride, sodium pentachlorostannite, or sodium pentafluorostannite) at 125–625 mg/kg in the feed (about 10–50 mg of tin per kg body weight per day). The increases were generally dose-related. Untreated rats had fetuses containing 0.64 mg of tin per kg body weight (Theuer et al., 1971). Others have reported briefly that "considerable" tin concentrations were noted in embryos of rats exposed to tin(II) chloride (Chmielnicka et al., 1981). Data are very limited but suggest the possibility of a low level of transfer across the placenta (ATSDR, 2003).
Few data on biotransformation are available. The difference in the relative affinity of the kidneys and liver for tin(II) and tin(IV) indicates a valence stability of the administered tin (Hiles, 1974). The difference observed between tin(II) and tin(IV) chloride in their effects on the immune response in C57BL/6J mice (see section 8.7) also suggests that these two oxidation states are not readily interconverted in vivo (Dimitrov et al., 1981). Together, these data suggest that tin cations are not rapidly oxidized or reduced during absorption and systemic transportation in mammals.
Ingested tin is largely unabsorbed and excreted mainly in the faeces, with additional slow elimination of the absorbed fraction in the urine (JECFA, 2001).
In a mineral balance study, eight adult human males ate food providing 0.11 mg or 50 mg of tin per day (as tin(II) chloride) for 20-day periods. Their urinary excretion was 29 ± 13 µg/day (mean ± SD) and 122 ± 52 µg/day, respectively, representing 36% and 2.4% of the dose, respectively. Mean faecal excretion accounted for 55% and 97% at the low and high daily dose, respectively (Johnson & Greger, 1982). A review stated that, in humans, 20% of absorbed tin was cleared with a half-time of 4 days, a further 20% with a half-time of 25 days, and the remaining 60% with a longer half-time of 400 days. No further details were given (Magos, 1986). When nine healthy adults were given diets consisting of fresh foods (10 mg of tin per day), cold-stored canned foods (26 mg of tin per day), or warm-stored canned foods (163 mg of tin per day) for 24 days, faecal excretion accounted for the whole dose, and none was detected in the urine (Calloway & McMullen, 1966).
Rats and cats given (by stomach tube) orange juice supplying tin (derived from containers) at 7–20 mg/kg body weight excreted 99% of the dose in the faeces within 48 h (Benoy et al., 1971).
In laboratory animals, the small proportion of tin that is absorbed following ingestion is mainly excreted via the kidneys (Kutzner & Brod, 1971; Hiles, 1974; Furchner & Drake, 1976; IPCS, 1980; Widdowson, 1992).
In the 48 h following a single oral tin dose of 20 mg/kg body weight as 113Sn(II) or 113Sn(IV) fluoride or citrate, female rats excreted 95% of the radiolabel in the faeces and less than 1% in the urine. After a single intravenous dose of 113Sn(II) or 113Sn(IV) at 2 mg/kg body weight, 35% and 40% of the doses, respectively, were excreted in the urine. Twelve per cent of the tin(II) appeared in the faeces, but only 3% of the tin(IV), indicating that the biliary route is more important for tin(II) than for tin(IV) compounds (Hiles, 1974).
Tin was given as 113Sn(II) chloride orally, intraperitoneally, or intravenously to mice, rats, monkeys, and dogs. After parenteral administration, whole-body activities could be described by four-component exponential expressions, similar for all species studied. Following intravenous injection of tin(II) chloride into rats (11 kBq/rat), elimination proceeded with component half-times of 0.4, 4.9, 25, and 90 days (Furchner & Drake, 1976).
For rat liver and kidney, the biological half-life of tin(II) has been estimated to be 10–20 days. For bone, the half-life of tin(II) and tin(IV) is approximately 20–100 days (Hiles, 1974; Brown et al., 1977; Fritsch et al., 1977; Bulten & Meinema, 1991). A biological half-life of approximately 30 days was estimated for inorganic tin in mice, using a whole-body counting method (Brown et al., 1977).
Biological monitoring requires an understanding of the relationships between exposure, external dose, toxicokinetics, internal dose, and effects. Although adequate ultrasensitive analytical techniques (ICP-MS and radiochemical neutron activation) have been developed for the measurement of tin in tissues and urine (see section 3), relationships between tin dose and biological indicators have not yet been established for inorganic tin (Versieck & Vanballenberghe, 1991; Schramel et al., 1997).
The ICRP has developed a model for ingested tin, based on an empirical model developed by Furchner & Drake (1976). The fraction of ingested tin that is absorbed from the human gastrointestinal tract (uptake to blood) is assumed to be 0.02 (i.e. 2%). Absorbed tin is assumed to enter the blood, from where 50% is immediately transferred to excreta (specific routes not specified in the model), 35% is transferred to bone mineral, and 15% is uniformly distributed to all other tissues. Elimination of tin from any tissue or organ is assumed to occur in three phases, with individual half-times of 4, 25, and 400 days, respectively, during which periods some 20%, 20%, and 60% of tissue burdens, respectively, are eliminated (ICRP, 1981, 2001).
The ICRP has also developed a human model for inhaled tin (ICRP, 1994). Sulfides, oxides, hydroxides, halides, and nitrates of tin and tin(IV) orthophosphate are classed as Type M; all other compounds of tin are classed as Type F. For Type F compounds, rapid 100% absorption is assumed to occur within 10 min of material deposition in the bronchi, bronchiole, and alveolar interstitial regions. Fifty per cent of each Type F compound deposited in the extrathoracic region transfers to the gastrointestinal tract. There is rapid absorption of approximately 25% of the tin deposited in the extrathoracic region during nose breathing and 50% absorption during mouth breathing. For Type M compounds, approximately 70% of the tin deposited in the alveolar interstitial regions is eventually transferred to blood, and there is rapid absorption of about 10% of the tin deposited in the bronchi and bronchiole regions and 5% of the tin deposited in the gastrointestinal tract. Approximately 2.5% of the deposit in the gastrointestinal tract is rapidly absorbed during nose breathing, and 5% is rapidly absorbed during mouth breathing (ICRP, 1994).
By the oral route, tin metal itself is described as practically innocuous (JECFA, 2001). In NTP studies, using groups of five animals per sex, the acute oral LD50 for tin(II) chloride was in excess of 1.5 g/kg body weight (the highest dose tested) in rats. In the corresponding study in mice, the LD50 was in excess of 1.2 g/kg body weight, but all mice died at 2.4 g/kg body weight (NTP, 1982). For tin(II) chloride, other studies report LD50 values equivalent to 1.1–1.7 g of tin per kg body weight in rats. Sodium pentafluorostannite showed higher toxicity, with LD50 values (again expressed as tin) of 0.15–0.38 and 0.40 g/kg body weight in rats and mice, respectively. Acute oral administration induced central nervous system effects, including ataxia, general depression, limb weakness, and flaccid paralysis. Both compounds induced pathology in the kidneys, characterized by swelling, discoloration, and tubular necrosis with subsequent regeneration (Conine et al., 1975; JECFA, 2001; Westrum & Thomassen, 2002). Oral LD50 values have also been recorded in mice as 2.7 g/kg body weight for sodium tin citrate (Omori et al., 1973) and 0.25–1.2 g/kg body weight for tin(II) chloride (Pelikan et al., 1968). Necrosis in the liver and spleen were seen in mice treated orally with a single dose of tin(II) chloride (Pelikan et al., 1968). Tin(II) chloride disrupted haem synthesis when given orally to rabbits at 100 mg of tin per kg body weight, but not at 10 mg/kg body weight (Chmielnicka et al., 1992).
Changes ("widespread tiny densities") were seen on X-ray examination of the lungs of rats 4 months after an intratracheal instillation of 50 mg of metallic tin dust (in saline) from a tin smelting works (no further details on composition were given). These changes were said to be similar to those seen in workers exposed occupationally to the same material. Histologically, there was no fibrous response of any kind up to a year in the rats (Robertson, 1960).
Acute toxicity of inorganic tin compounds by intravenous and intraperitoneal injection is considerably higher than acute toxicity by the oral route. For example, an acute LD50 value of 15 mg of tin per kg body weight has been reported in rats treated with tin(II) chloride by the intravenous route (Conine et al., 1975).
There were no effects on survival, growth, food utilization, blood or urine composition, serum biochemistry, organ weights, or the gross and microscopic appearance of a range of tissues and organs when groups of 10 male and 10 female Wistar rats were fed tin(II) chloride, tin(II) orthophosphate, tin(II) sulfate, tin(II) oxalate, or tin(II) tartrate at up to 0.1% in the diet for 4 weeks. At 0.3% and above, these compounds caused growth retardation, decreased food efficiency, mild anaemia, and slight histological changes in the liver. In similar studies, dietary concentrations of up to 1% tin(II) oleate, tin(II) sulfide, or tin(IV) oxide for 4 weeks were tolerated without adverse effect by rats (10 per sex per group). Overall, the NOEL of the tin salts studied was 0.1%, or 22–33 mg of tin per kg body weight per day in an unsupplemented diet containing "a liberal amount of iron and copper." The investigators suggested that the NOEL might be lower in diets that are marginal in iron and copper. Dietary supplements of iron had a marked protective effect against tin-induced anaemia, whereas a decrease in dietary iron exacerbated this effect. The growth depression caused by tin was not alleviated by enriching the diet with iron and copper (de Groot, 1973).
The NTP has carried out studies in which tin(II) chloride was administered to F344/N rats and B6C3F1/N mice (five per sex per species per group) at 1900, 3800, 7500, 15 000, or 30 000 mg/kg in the diet for 2 weeks (up to about 950 and 2400 mg of tin per kg body weight per day in rats and mice, respectively). All animals survived treatment. The rats showed a dose-related decrease in growth and, at the top dose, lost weight and had roughened coats and distended abdomens. In mice, the only effect was reduced growth in the females at 15 000 mg/kg diet and above (NTP, 1982).
No significant toxicity was seen when Wistar rats (10 per sex per group) were given sodium pentafluorostannite at 20 mg/kg body weight per day for 30 days by stomach tube (equivalent to 13.4 mg of tin per kg body weight per day). At 100 mg/kg body weight per day (67 mg of tin per kg body weight per day) and above, there was a dose-related reduction in growth. At the top dose (175 mg/kg body weight per day, equivalent to 117 mg of tin per kg body weight per day), degenerative changes were seen in the proximal tubular epithelium of the kidneys in 15–20% of the animals. At 15 days, a dose-related decrease in haemoglobin was found, although this was statistically significant only in males given 100 mg/kg body weight per day and above. Serum glucose levels were decreased, possibly related to reduced food intake (Conine et al., 1976).
Dose-related decreases in growth and haemoglobin level were noted in weanling Wistar rats given tin at 0, 250, or 500 mg/kg in the diet (as tin(II) chloride) for 4 weeks (about 0, 15, and 30 mg of tin per kg body weight per day, respectively). Crypt depth, villus length, and cell turnover were increased in parts of the intestine (Janssen et al., 1985).
Several studies report effects of tin administration on the bones. The calcium content of the femur (both epiphysis and diaphysis) was reduced in a dose-related manner in male Wistar rats given tin at 1, 3, 10, or 30 mg/kg body weight (as tin(II) chloride) every 12 h for 3 days. The effect was statistically significant at 6 mg/kg body weight per day and above. Serum calcium was decreased at 20 mg/kg body weight per day and above (Yamaguchi et al., 1980a). The distal epiphysis compressive strength decreased significantly in the femoral bone in Wistar rats administered 300 mg of tin (as tin(II) chloride) per litre drinking-water and laboratory chow contaminated with tin at 52.4 mg/kg for 4 weeks. This effect was not seen at 150 mg of tin per litre drinking-water. Feed and water intake were not reported (Ogoshi et al., 1981). The calcium content in the tibia of rats fed 100 mg of tin per kg in the diet as tin(II) chloride (approximately 7 mg of tin per kg body weight per day) for 28 days was decreased (Johnson & Greger, 1985). When gavage doses of 1 mg of tin per kg body weight (as tin(II) chloride) were given to male Wistar rats at 12-h intervals for 28–30 days (i.e. 2 mg of tin per kg body weight per day), there was an increase in the tin content of the femoral diaphysis and epiphysis, decreased calcium content in bone, and decreased acid and alkaline phosphatase activities in the femoral epiphysis (Yamaguchi & Okada, 1980; Yamaguchi et al., 1981a).
Other investigators have studied the effects of inorganic tin compounds on body balance of important minerals. Plasma and tissue (kidney, spleen, and tibia) concentrations of copper, iron, and zinc were unaffected in a group of seven Wistar rats fed diets containing 1 mg of tin per kg (as tin(II) chloride; about 0.07 mg of tin per kg body weight per day) for 28 days, but were slightly reduced at 10 mg of tin per kg in the diet (about 0.7 mg of tin per kg body weight per day). Greater effects were seen at 50 mg of tin per kg in the diet (about 3.5 mg of tin per kg body weight per day) (Pekelharing et al., 1994). Copper metabolism was unaffected in Sprague-Dawley rats given up to 100 mg of tin per kg in the diet as tin(II) chloride (about 7 mg of tin per kg body weight per day) for 27 days; however, at 500 mg of tin per kg in the diet (about 39 mg of tin per kg body weight per day), copper levels were reduced in the plasma, liver, and kidneys, and zinc retention in the tibia, kidneys, liver, and plasma was decreased. Only small changes in iron metabolism were observed (Johnson & Greger, 1984, 1985). Administration of 100 mg of tin per kg in the diet for 4 weeks to weanling rats reduced copper levels in the duodenum, liver, kidneys, and femur and zinc levels in the kidneys and femur (Reicks & Rader, 1990; Rader et al., 1990). Oral administration of tin(II) chloride (2 mg of tin per kg body weight per day) to rabbits for 1 month decreased zinc and copper concentration in bone marrow and increased iron concentrations in liver and kidneys (Zareba & Chmielnicka, 1989). Iron status (tissue iron, haemoglobin, haematocrit, red blood cell count, plasma iron, total iron binding capacity, and transferrin saturation) in rabbits was not influenced by the inclusion of tin in the diet at <100 mg/kg (as tin(II) chloride) for 28 days, but these parameters were decreased at higher (not specified in the source document) dietary tin concentrations. Food intake and body weights were not reported (Beynen et al., 1992).
A study in Wistar rats fed on diets containing 1, 10, 50, 100, or 200 mg of tin per kg (as tin(II) chloride) for 28 days (1 mg/kg was equivalent to about 0.07 mg of tin per kg body weight per day) found that blood haemoglobin concentration and percentage transferrin saturation decreased in a linear manner with increasing dietary tin concentration (Pekelharing et al., 1994).
Gavage adminitration of tin at 1, 3, 10, or 30 mg/kg body weight (as tin(II) chloride) every 12 h for 3 days to male Wistar rats resulted in dose-related decreases in gastric acid secretion, duodenal alkaline phosphatase, and liver phosphorylase. These reductions were statistically significant only at 20 mg/kg body weight per day and above (Yamaguchi et al., 1980a).
Oral doses of 2 mg of tin per kg body weight per day for 5 days (as tin(II) chloride) did not affect haem biosynthesis in a group of five rabbits, based on examination of ALAD in whole blood, liver, kidneys, brain, spleen, and bone marrow, concentrations of free erythrocyte protoporphyrins, activity of ALA synthetase in the liver and bone marrow, urinary ALA, and co-protoporphyrins (Zareba & Chmielnicka, 1992).
In NTP studies, food grade purity (98.5%) tin(II) chloride was given to F344/N rats (groups of 10 per sex) at 0, 500, 1000, 1900, 3800, or 7500 mg/kg in the diet for 13 weeks. An equivalent study in B6C3F1/N mice was carried out using dietary concentrations of 0, 1900, 3800, 7500, 15 000, and 30 000 mg of tin(II) chloride per kg. A wide range of tissues and organs from the control and top-dose animals were examined microscopically. All rodents survived treatment. No effects were seen in either species at up to 1900 mg/kg diet, equivalent to about 170, 400, and 600 mg/kg body weight per day in rats (both sexes), male mice, and female mice, respectively. Both species showed gross distension of the caecum and reddened gastric mucosa at 3800 mg/kg diet (about 330, 900, and 1200 mg/kg body weight per day in rats, male mice, and female mice, respectively) and above. Growth was reduced at the top dose in each species. Microscopically, the tissues were normal (NTP, 1982).
There were no effects on survival, growth, food utilization, blood or urine composition, serum biochemistry, organ weights, or the gross and microscopic appearance of a range of tissues and organs when Wistar rats (10 per sex per group) were given up to 1% tin(II) oxide or 0.1% tin(II) chloride in the diet for 13 weeks. Growth retardation, decreased food efficiency, slight anaemia, and minor liver tissue changes were seen when tin(II) chloride was given at 0.3% in the diet and above. At 1% tin(II) chloride (about 315 mg of tin per kg body weight per day), marked growth retardation and some deaths occurred. This group showed moderate testicular degeneration, severe pancreatic atrophy, spongy white matter in the brain, acute bronchopneumonia, enteritis, distended intestine, and distinct changes in the liver cytoplasm, with mild proliferation in the bile duct epithelium. The NOEL of tin salts examined was 0.1% or 22–33 mg of tin per kg body weight per day in an unsupplemented diet containing "a liberal amount of iron and copper." The investigators suggested that the NOEL might have been lower in diets that are marginal in iron and copper. Dietary supplements of iron had a marked protective effect against tin-induced anaemia, whereas a decrease in dietary iron exacerbated the effect. The growth depression caused by tin was not alleviated by enriching the diet with iron and copper (de Groot et al., 1973).
Slower growth, mild anaemia, increased relative liver and kidney weights, irritation of the gastrointestinal tract, "mild" histological changes in the liver, and pancreatic damage (ranging from necrosis of individual acinar cells to complete destruction of the pancreas) were observed in Wistar rats fed tin(II) chloride for 13 weeks (gradually increased from 163 mg of tin per kg body weight per day in weeks 0–4 to 310 mg of tin per kg body weight per day in weeks 8–13) (der Meulen et al., 1974).
In a study of the effect of tin(II) chloride on biochemical and bone indices in groups of 5–6 Wistar rats, oral doses of 0.6, 2, or 6 mg of tin per kg body weight per day were given (in two daily doses, 12 h apart) for 90 days. The 6 mg/kg body weight per day dose level caused significant decreases in femur weight, lactate dehydrogenase and alkaline phosphatase activities in serum, succinate dehydrogenase activity in the liver, and calcium content and acid phosphatase activity in the femur. The 2 mg/kg body weight per day dose produced significant reductions in succinate dehydrogenase activity in the liver and in the calcium content and acid phosphatase activity in the femur. At 0.6 mg/kg body weight per day, a slight non-significant decrease in calcium content in the femoral epiphysis was observed. The results suggested a LOEL for inorganic tin orally administered of 0.6 mg/kg body weight per day (Yamaguchi & Okada, 1980; Yamaguchi et al., 1980b). In a study in which tin(II) chloride was added to the diet of male Wistar rats at 0, 10, 50, 100, or 250 mg of tin per kg for 90 days (approximately 0, 0.5, 2.5, 5, and 12.5 mg of tin per kg body weight per day), serum calcium and calcium content of the femoral epiphysis were significantly reduced at 2.5 mg/kg body weight per day and above. At 5 mg/kg body weight per day and above, there were additionally decreases in serum inorganic phosphate, femur diaphysis calcium, and femur epiphysis acid phosphatase. No effects were seen at 0.5 mg/kg body weight per day (Yamaguchi et al., 1981b).
Fatty degeneration with lymphocytic infiltration and atrophy of the exocrine tissue of the pancreas was seen when groups of 10 male Sprague-Dawley CD rats were given tin(II) chloride in the diet at 4000 mg/kg (equivalent to 240 mg/kg body weight per day) for 6 months (Fritsch et al., 1978).
In rabbits, oral administration of 10 mg of tin per kg body weight per day (as tin(II) chloride) for 4 months caused transient anaemia during weeks 6–10. A transient high serum iron concentration and a high total iron binding capacity and saturation index were also observed (Chmielnicka et al., 1993).
In the most comprehensive carcinogenicity study available, F344/N rats and B6C3F1/N mice (groups of 50 males and 50 females per species per dose group) were given food grade purity (98.5%) tin(II) chloride at 0, 1000, or 2000 mg/kg in the diet for 104–106 weeks. A wide range of tissues and organs were examined microscopically. Based on food intake and body weight data given in the original report, estimated average intakes (expressed as mg of tin per kg body weight per day) for the control, 1000, and 2000 mg/kg groups are approximately 0, 30, and 60 for male and female rats, 0, 90, and 180 for male mice, and 0, 130, and 270 for female mice, respectively. Westrum & Thomassen (2002) presented estimated tin doses for male rats and female mice at selected weeks, and these are reproduced in Table 6. Treatment had no effect on food consumption or growth, but survival of the male rats and female mice was somewhat lower in the high-dose group. No evidence of carcinogenic activity was seen in the female rats or male mice. In the male rats, there were apparent increases in thyroid C-cell adenomas and C-cell adenomas/carcinomas combined (see Table 7). However, when compared with the laboratory’s historical control rate (32/288; mean 11.1%, maximum 20%) of thyroid C-cell tumours, only the incidence in the low-dose group was significantly (P < 0.01) raised. The incidence of C-cell hyperplasia did not differ between control and treated groups. The male rats also showed an apparent increase in lung adenomas, with a statistically significant positive trend with dose (see Table 6). However, individual comparisons between the high-dose group and the low-dose or control groups were not statistically significant, the 6% incidence in the high-dose group was within the laboratory’s historical control range (6/289; overall mean 2.1%, range 0–6%), and results of statistical tests on incidences of combined lung adenomas and carcinomas were not significant. In the female mice, statistical trend analysis suggested increases in hepatocellular adenoma/ carcinoma combined and in histiocytic malignant lymphoma (see Table 8). However, when the incidences of total lymphomas or lymphomas/leukaemias were considered, statistical significance no longer remained, and the incidence of liver tumours in the high-dose group did not differ significantly from the laboratory’s historical control incidence (24/297; mean 8%, range 4–18%). Overall, the NTP experts judged tin(II) chloride not to be carcinogenic in this study, but cautioned that the thyroid C-cell tumours in male rats might possibly have been associated with the test chemical (NTP, 1982). (In this study, the incidence of retinal degeneration was considerably increased in the high-dose male rats and low-dose female rats [60–74%] compared with the other groups [4–16%]. This was believed to be due to proximity to fluorescent lighting and may reflect poor group distribution of cage locations [NTP, 1982].)
Table 6: Calculated doses in the NTP 105-week study.a
|
Species/sex |
Study week |
Tin concentration (mg/kg body weight per day) |
|
|
Low dose |
High dose |
||
|
Male rats |
5 |
41 |
89 |
|
25 |
30 |
68 |
|
|
62 |
26 |
55 |
|
|
104 |
20 |
35 |
|
|
Female mice |
5 |
182 |
348 |
|
26 |
134 |
272 |
|
|
65 |
92 |
203 |
|
|
104 |
137 |
290 |
|
a From NTP (1982).
Table 7: Key primary tumours in male rats in the NTP 105-week study.a
|
Tumours |
Control group |
Low-dose group |
High-dose group |
|
C-cell adenoma (thyroid) |
2/50 |
9/49b |
5/50 |
|
C-cell adenoma/ carcinoma combined (thyroid) |
2/50 |
13/49b |
8/50b |
|
Lung adenomac |
0/50 |
0/50 |
3/50 |
a From NTP (1982).
b P < 0.05, Fisher’s exact test.
c P < 0.05, Cochran-Armitage trend test.
Table 8: Key primary tumours in female mice in the NTP 105-week study.a
|
Tumours |
Control group |
Low-dose group |
High-dose group |
|
Hepatic adenoma/ carcinoma combinedb |
3/49 |
4/49 |
8/49c |
|
Histiocytic malignant lymphomad |
0/50 |
0/49 |
4/49 |
a From NTP (1982).
b P < 0.05, life table and incidental tumour trend tests; P = 0.067, Cochran-Armitage trend test.
c P = 0.038 life table pairwise test, comparison with control group.
d P < 0.05, Cochran-Armitage trend test.
Survival, growth, serum chemistry, and urinalysis were normal when groups of 30 male and 30 female Cpb:WU rats were given tin(II) chloride at 0, 20, 40, or 80 mg/kg in the diet for 115 weeks. Haemoglobin and haematocrit values were decreased in all tin groups at weeks 4 and 13, but values during the second year of the study were similar to controls. A complete autopsy was performed, and the principal organs and tissues were examined microscopically. Spleen weight was increased (apparently at the top two dose levels), but the organ was microscopically normal. There was no evidence of carcinogenic activity (Sinkeldam et al., 1981).
In a very limited study, groups of 56 male and 56 female Long-Evans rats were given drinking-water containing tin(II) chloride (at 5 mg of tin per litre, equivalent to about 0.37 mg of tin per kg body weight per day) from weaning until natural death. The control rats (possibly 56 males and 76 females) were given drinking-water without added tin. The control diet contained 0.28 mg of tin per kg, which would probably have supplied about 0.014 mg of tin per kg body weight per day. In the tin group, survival was reduced in the females, and (at 18 months) males had lower body weight. Serum biochemistry and urine composition appeared to be unaffected. Tin-treated rats had a higher incidence of fatty degeneration of the liver and of vacuolar changes in the renal tubules. No evidence of carcinogenicity was seen, but the low dose administered means that the sensitivity of the study is limited (Schroeder et al., 1968; Kanisawa & Schroeder, 1969). It is worth noting that no such liver and kidney changes were found in F344 rats given much higher tin(II) chloride doses (up to 60 mg of tin per kg body weight per day) in the NTP study (NTP, 1982).
In another very limited study, 54 male and 54 female Charles River mice were given tin(II) chloride in the drinking-water (at 5 mg of tin per litre, equivalent to about 0.37 mg of tin per kg body weight per day) from weaning until natural death. Controls (34 males, 46 females) received drinking-water without added tin. For both groups, an additional dose of about 0.02 mg of tin per kg body weight per day was supplied by background levels of tin in the diet. Tin treatment had no adverse effect on growth, survival, or the gross and microscopic appearance of an unspecified range of tissues. There was no evidence of carcinogenicity, but the low dose administered means that the sensitivity of the study is limited (Schroeder & Balassa, 1967).
No significant difference was seen in the development of lung tumours when groups of 20 strain A mice (a strain susceptible to lung tumour development) were given multiple intraperitoneal injections of tin(II) chloride over 30 weeks. Total doses given were 240, 600, and 1200 mg/kg body weight, and the ratios of numbers of surviving animals to initial numbers were 18/20, 12/20, and 4/20, respectively (Stoner et al., 1976).
Several other limited carcinogenicity studies are available. No evidence of carcinogenic activity was seen in mice given 1000 or 5000 mg of tin per litre as sodium hexachlorostannate in the drinking-water or 5000 mg of tin per kg as tin(II) oleate in the diet for 1 year (Walters & Roe, 1965). Similarly, there was no clear evidence of carcinogenicity when small groups (approximately 30) of rats were given sodium hexachlorostannate at 2000 mg/kg in the diet or tin(II) 2-ethylhexanoate at 500–1000 mg/kg in the diet for up to 18 months (Roe et al., 1965). In both the above studies, the small group sizes and/or short treatment duration would have limited the studies’ ability to detect weak carcinogens. Following subcutaneous implantation of tin foil, Wistar rats did not develop tumours (Oppenheimer et al., 1956). Intracranial implantation of metallic tin cylinders in 33 male Marsh mice, of which only 10 mice survived beyond 10 months, resulted in local gliosis, but no local tumours developed (Bischoff & Bryson, 1976a). After intrathoracic injection of tin needles (4 mg) into 43 male Marsh mice, the implants were engulfed by giant cells with some adjacent nodular fibroplasia and a new network of capillaries. Survival (up to 19 months) was unaffected, and no increase in intrathoracic tumours was seen (Bischoff & Bryson, 1976b).
In summary, the available laboratory animal studies, mostly limited in nature, have not shown tin metal, tin(II) chloride, or a small number of other tin compounds to be carcinogenic. However, the most comprehensive study suggested that C-cell tumours of the thyroid gland in male rats might have been associated with the administration of tin(II) chloride. In this lifetime NTP study, a comprehensive tissue examination found no non-neoplastic changes in rats and mice given dietary tin at up to 60 or 270 mg/kg body weight per day, respectively (as tin(II) chloride). A more limited, earlier study reported increased incidences of fatty degeneration of the liver and possibly vacuolar changes in the renal tubules of rats (of a different strain) given tin at about 0.4 mg/kg body weight per day (as tin(II) chloride) in the drinking-water for a lifetime.
In NTP studies, anhydrous tin(II) chloride and tin(II) chloride dihydrate were not mutagenic in Ames tests. Tin(II) chloride was tested at up to 0.33 mg/plate in Salmonella typhimurium strains TA98, TA100, TA1535, and TA1537, with and without metabolic activation fractions (S9) derived from the liver of rats or hamsters. A preincubation step was used, and the top dose was limited by either (not disclosed) solubility or toxicity (Mortelmans et al., 1986). Tin(II) chloride dihydrate was not mutagenic at up to 10 mg/plate (there was no preincubation step) in S. typhimurium strains TA98, TA100, TA1535, TA1537, and TA1538, with and without S9 (Prival et al., 1991). Tin(IV) chloride gave no evidence of mutagenic potential in a more limited study using only S. typhimurium strains TA98 and TA100 (Hamasaki et al., 1993). Tin(II) fluoride gave no convincing evidence of mutagenic activity when tested in S. typhimurium strains TA98, TA100, TA1535, TA1537, and TA1538. There was some evidence of a weak dose–response in strain TA100, but only in the presence of a metabolic activation system (S9 liver fraction from Aroclor-pretreated rats) and on one type of modifed (high-citrate) medium (Gocke et al., 1981). Tin(II) chloride did not induce mutations in Escherichia coli strain WP2 (Prival et al., 1991).
Several tin compounds have failed to give evidence of an ability to induce DNA damage in Bacillus subtilis, as assessed by the relative survival of wild-type and DNA repair-deficient strains (rec assays) on exposure to tin. Using B. subtilis strains H17(rec+) and H45(rec–), four tin salts (tin(II) chloride, tin(IV) chloride, tin(II) sulfate, and sodium stannate) gave no evidence of an ability to cause DNA damage, although the investigators noted that the two chlorides were highly toxic to the bacteria, which would have reduced the sensitivity of the test (Kada et al., 1980). Tin(II) chloride, tin(IV) chloride, and sodium stannate were similarly inactive in this assay in the absence of any added metabolic activation fraction (Nishioka, 1975). The literature contains another report of a rec assay test with B. subtilis in which tin(IV) chloride, when tested at up to 10 mg, gave no evidence of an ability to damage DNA (Hamasaki et al., 1992).
Indirect evidence of the ability of tin(II) chloride to damage bacterial DNA comes from studies of relative survival of treated wild-type and DNA repair-deficient strains of E. coli. When E. coli were incubated with tin(II) chloride at 0–75 µg/ml, there was a concentration-related decrease in survival. At all tested concentrations (5 µg/ml and above), survival of the wild strain (AB 1157) was higher than that of the strains that were deficient in DNA repair ability (AB 1886, AB 2463, AB 2494, AB 2480, and IC 204), suggesting that tin(II) chloride caused DNA damage (Silva et al., 1994, 2002). Other studies showed that tin(II) chloride (0–75 µg/ml) caused lysogenic induction of E. coli K-12 and microscopically visible E. coli B filamentation (Bernardo-Filho et al., 1994).
The SOS chromotest, a simple colorimetric assay of the induction of the bacterial gene sfiA in E. coli, indicated effects at 2–3 mmol of tin(II) chloride per litre, but the high degree of bacterial toxicity complicates interpretation of the data (Olivier & Marzin, 1987). Tin(IV) chloride did not produce DNA damage in an SOS chromotest (Hamasaki et al., 1992).
When incubated with tin(II) chloride, plasmid DNA (pUC9.9) showed a decrease in supercoiling, indicating the induction of single-strand DNA breaks (Silva et al., 2002). Plasmid DNA studies using varying doses of tin(II) chloride and oxygen suggested that the mechanism might involve reactive oxygen species (Dantas et al., 1999).
Tin(II) chloride did not induce mutations or gene conversions in strain D7 of the yeast Saccharomyces cerevisiae (Singh, 1983).
Tin(II) chloride gave no evidence of genotoxicity potential in sex-linked recessive lethal mutation assays in Drosophila melanogaster, conducted under the NTP. The protocols involved 3-day feeding of adult fruitflies at 6540 mg/kg in the diet or intraperitoneal injection at a concentration of 12 180 mg/litre and scoring of lethal mutations in three broods (Foureman et al., 1994). Tin(II) chloride was also non-mutagenic in a wing spot test in D. melanogaster. The protocol involved feeding the larvae for 48 h (Tripathy et al., 1990). Tin(II) fluoride was mutagenic in a sex-linked recessive lethal mutation assay in D. melanogaster when fed to the adult flies at 0–25% for 24 h. However, sodium fluoride was more potent than tin(II) fluoride, suggesting a role for the fluoride anion. Other tin salts were not tested (Mitchell & Gerdes, 1973). There was no induction of sex-linked recessive lethal mutations in three successive broods produced by D. melanogaster fed 1.25 mmol of tin(II) fluoride per litre (described as "close to the LD50") in 5% saccharose (Gocke et al., 1981).
Tin(II) chloride at concentrations of 50, 150, 350, or 500 µmol/litre produced dose-related DNA damage, as detected by alkaline sucrose gradient analysis in Chinese hamster ovary cells. Treatment of cells with tin(IV) as tin(IV) chloride produced no such DNA damage. There was no loss in colony formation 6 days after either treatment (McLean et al., 1983a).
Tin(II) as tin(II) chloride (5, 10, 25, or 50 µmol/litre) was readily taken up by human white blood cells and caused a dose-dependent increase in DNA strand breaks that was more extensive than that caused by equimolar amounts of chromium(VI), a known carcinogen and DNA-damaging agent. Tin(IV) as tin(IV) chloride did not cause DNA damage and, in contrast to other studies, was not taken up by cells (McLean et al., 1983b). Tin(II) chloride, phytate, and fluoride all caused DNA damage (strand breaks) in human white blood cells, although this potential was not realized when the tin was chelated with EDTA (Swierenga & McLean, 1983). Others have reported DNA damage in human peripheral blood nuclear cells treated in vitro with 0.4 µmol of tin(II) chloride per litre (Dantas et al., 1999). In a comet assay (an assay that can detect DNA damage at the single cell level) using K562 human leukaemia cells, incubation with tin(II) chloride at 0.06–0.9 mmol/litre resulted in a concentration-dependent increase in DNA damage and a reduction in K562 cell viability. There was evidence that this DNA damage was repairable (Dantas et al., 2002a).
Tin(II) chloride did not induce unscheduled DNA synthesis in rat liver cells, but enhanced the ability of a known genotoxin to do so (Swierenga & McLean, 1983).
An NTP study found no evidence of mutagenic activity when tin(II) chloride (up to about 0.1 mg/ml; slightly toxic) was incubated with mouse lymphoma cells, with and without rat liver S9 (Myhr & Caspary, 1991).
In NTP studies, tin(II) chloride induced chromosome aberrations and SCEs in Chinese hamster ovary cells, both with and without rat liver S9 (Gulati et al., 1989).
According to a report published only as an abstract, tin(IV) chloride at 10 and 20 µg/ml induced concentration-dependent increases in the frequency of chromosome aberrations, micronuclei, and SCEs in human lymphocytes in vitro (Talukder et al., 1989). Incubation of human lymphocytes from 27 male donors with tin(IV) chloride at 2 or 4 µg/ml for 70 h resulted in 2- to 3-fold increases in the incidences of chromosome aberrations and SCEs (Ganguly et al., 1992). When human lymphocytes from 52 donors were incubated with tin(IV) chloride at 4 µg/ml for 48 h, there were significant elevations of chromosome aberrations and micronuclei formation (Ganguly, 1993). Mitotic index and cell cycle kinetics (replicative index) were depressed in all three studies.
A comet assay found evidence of DNA damage in the peripheral blood cells of patients intravenously injected with a radiopharmaceutical containing tin(II) chloride and radiolabelled with 99mTc. DNA damage increased in the first 2 h following treatment but was not detectable at 24 h. The investigators concluded that damage could be ascribed to both tin(II) chloride and 99mTc. The tin(II) chloride "dose" was reported to range from 0.092 to 0.416 "µM" (Dantas et al., 2002b). This "dose" is in fact a concentration expressed in µmol/litre. If the units should have been reported as µmol, then the doses were only 20–80 µg per person.
Tin(II) chloride did not induce micronuclei in the bone marrow when given by intraperitoneal injection to groups of 4–5 male mice at 0, 26.3, 52.5, 105, or 210 mg/kg body weight per day for 3 days (Shelby et al., 1993). Two intraperitoneal doses of 0, 9.8, 19.6, or 39.5 mg of tin(II) fluoride per kg body weight given 24 h apart to NMRI mice (groups of two per sex per dose level) did not induce micronuclei in the bone marrow erythrocytes (Gocke et al., 1981).
To conclude, in short-term screening assays for genotoxicity potential, tin(II) chloride did not induce mutations in Ames bacterial tests, mutations or gene conversions in yeast, DNA damage in rat liver cells in culture, mutations in mouse lymphoma cells in vitro, or chromosome damage (micronuclei) in vivo in mice treated by intraperitoneal injection. In bacterial rec assays (in which activity is an indirect indication of DNA damage), tin(II) chloride was active in E. coli but (along with other tin salts) inactive in B. subtilis. In culture, tin(II) chloride induced chromosome damage and SCEs in hamster ovary cells and DNA damage in human lymphocytes, hamster ovary cells, and plasmid DNA. Tin(IV) chloride tested in vitro did not damage DNA in hamster ovary cells but induced chromosome aberrations, micronuclei, and SCEs in human lymphocytes. Tin(II) fluoride caused DNA damage in cultures of human lymphocytes, but did not induce micronuclei formation when injected intraperitoneally into mice; Ames tests on this compound gave no convincing evidence of activity. There is some evidence that tin-induced DNA damage may arise from a secondary mechanism involving reactive oxygen species. The mechanism underlying the induction of chromosome damage in mammalian cells in culture has not been determined, although it is recognized that such events can occur as a result of changes in ionic strength or pH in the medium.
In a multigeneration study, CPB:WU rats were given tin in the diet at 0, 200, 400, or 800 mg/kg for three generations. To simulate the "form of the tin likely to be found in canned food," tin(II) chloride was allowed to react in aqueous medium with the casein content of the diet. The iron content was increased for the F2 generation onwards. Tin did not affect growth of the parents, fertility, numbers of offspring per litter, or birth weight (Sinkeldam et al., 1979).
In the multigeneration study in CPB:WU rats described above, tin did not affect numbers of offspring per litter or birth weight. Increased mortality of F2 offspring during the first half of lactation was corrected by increasing the iron content of the mothers’ diet. Tin reduced offspring growth and haemoglobin levels during lactation but not thereafter. On pathological examination of rats from the F3b and F3c generations, the F3b pups showed microscopic changes in the liver and spleen at weaning but not at 4 weeks of age (Sinkeldam et al., 1979).
Within this multigeneration study, a teratogenicity study was carried out using 20 F2b females per dose level. On visceral and skeletal examination, there was no increase in the incidence of fetal malformations (Sinkeldam et al., 1979).
When groups of 9–10 female Sprague-Dawley rats were given diets containing tin at 0, 125, 156, 250, 312, 500, or 625 mg/kg (as tin(II) fluoride) throughout pregnancy (to day 20), there were no effects on the numbers of litters, resorptions, or live fetuses per litter. Mean placental and fetal weights were also unaffected (Theuer et al., 1971).
In the same series of studies, the numbers of litters, resorptions, and live fetuses per litter and the mean placental and fetal weights were unaffected by the inclusion of tin (as sodium pentachlorostannite) at 125, 250, or 500 mg/kg in the diet of groups of nine female Sprague-Dawley rats, throughout pregnancy (Theuer et al., 1971).
Other groups of nine female Sprague-Dawley rats were also given 125, 250, or 500 mg of tin per kg in the diet, but as sodium pentafluorostannite, throughout pregnancy. There were no effects on the numbers of litters or live fetuses or on placental and fetal weights. An apparent increase in resorptions was noted in the low- and high-dose groups, which was due to three rats (one low-dose, two high-dose) producing no live fetuses. The observation was not considered toxicologically significant (Theuer et al., 1971).
Gavage administration of tin(II) chloride at 0, 0.5, 2.3, 11, or 50 mg/kg body weight per day to rats and mice (on days 6–15 of pregnancy) or hamsters (on days 6–10 of pregnancy) did not affect nidation, fetal survival, or the incidences of fetal malformations in soft and skeletal tissue (Food and Drug Research Laboratories, 1972).
Transient irritation of the eyes and nose was noted when guinea-pigs were exposed by inhalation to tin(IV) chloride at 3000 mg/m3 air, 10 min daily for "several months" (Pedley, 1927).
Application of aqueous solutions of 2% tin(II) chloride or 0.5% tin(II) fluoride on pieces of gauze to the intact skin of rabbits for 18 h produced no skin irritation. At abraded skin sites, 0.5% tin(II) chloride or 0.1% tin(II) fluoride caused polymorphonuclear leukocyte infiltration, whereas application of 1% tin(II) chloride or 0.25% tin(II) fluoride resulted in pustule development and complete destruction of the (abraded) epidermis (Stone & Willis, 1968).
Following uncovered application for 1 min (and histological examination 6 h later), a threshold concentration for skin irritation in rats of 5% was established for both tin(II) chloride and tin(IV) chloride (in ethanol). For the oral mucosa, equivalent threshold concentrations for irritation were 3% (tin(II) chloride) and 0.05% (tin(IV) chloride) (Larsson et al., 1990).
Diffusely reddened gastric and duodenal mucosa, as well as mucosal hypertrophy and hyperplasia in the entire small bowel, were seen at autopsy in a study in which Wistar rats were fed a diet containing tin(II) chloride for 13 weeks. The tin dose was gradually increased from 163 mg/kg body weight per day during weeks 0–4 up to 310 mg/kg body weight per day in weeks 8–13 (der Meulen et al., 1974). Ridge-like villi, increased migration of epithelial cells along the villus, increased villus length, a decreased number of villi per unit surface, and increased total length and weight of the small intestine were seen after feeding rats diets containing tin(II) chloride at 250 or 500 mg/kg for 4 weeks (Janssen et al., 1985).
Intraperitoneal or intravenous injections of metallic tin powder (200 mg in saline) produced a striking plasmacellular hyperplasia in the draining lymph nodes and spleen of Lewis rats (Levine & Sowinski, 1982; Levine et al., 1983). Depending on the rat strain, the lymph node response to metallic tin varied from a very mild response to insoluble foreign particles to a marked granulomatous hyperplasia (August rats) and intense plasmacellular hyperplasia (Lewis rats and F1 hybrids of Lewis rats) (Levine & Saltzman, 1996). Pretreatment with tin salts (including tin(II) chloride and tin(II) sulfate) in drinking-water prevented the plasmacellular response to subsequently injected metallic tin for up to 2 months after the pretreatment (Levine & Sowinski, 1983). The production of plasma cell hyperplasia by metallic tin and the prevention of such response by tin salts are apparently unique to this metal (Levine & Saltzman, 1991).
To study the potential of inorganic tin compounds to affect the immune system, male C57BL/6J mice were given tin(II) chloride and tin(IV) chloride at approximately 5 and 3.5 mg of tin per kg body weight, respectively, by intraperitoneal injection. Sheep red blood cells were given by the same route 72 h later, and immunotoxicity assays were carried out on days 5, 7, 10, and 13 following sheep red blood cell injection. In plaque-forming assays using spleen cells, on day 5, tin(IV) chloride significantly suppressed both IgM and IgG antibody production; tin(II) chloride also appeared to suppress (to a non-significant extent) IgM production, but to stimulate IgG production. By day 7, antibody production was unaffected by either compound. In an antigen rosette formation test (measuring agglutination of red blood cells around lymphocytes), the response was significantly stimulated by tin(II) chloride and suppressed by tin(IV) chloride; these effects had disappeared by day 13. Cellular immunity was assessed in a leukocyte adherence inhibition test (measuring a reduction in the proportion of spleen cells adhering to glass). Tin(IV) chloride had no obvious effect in this assay, but tin(II) chloride significantly increased the degree of inhibition, indicating immunostimulation (at days 7–13). Finally, neither compound demonstrated delayed-type hypersensitivity in an assay measuring footpad thickness 24 h following injection of sheep red blood cells into one footpad (Dimitrov et al., 1981).
Other data also suggest that tin chlorides might be able to affect the mouse immune response. For example, intraperitoneal injection of tin(II) chloride (about 20 mg of tin per kg body weight per day for 3 days) suppressed both primary and secondary immune response parameters in mice, suggesting that tin suppresses part of the immune response in which IgM antibody production is important and that the IgG production in the primary response is suppressed or delayed (Hayashi et al., 1984). In mice, the intratracheal instillation of tin(II) chloride (0.01 or 0.1 mg, approximately 6 or 60 µg tin, corresponding to 0.24 and 2.4 mg of tin per kg body weight, respectively) in saline increased the mortality induced by subsequent bacterial infection (by aerosolized Group C Streptococcus sp.). A similar action was reported for intratracheal instillation of fly ash, carbon, bentonite, and a number of metal oxides and inhalation of "soluble metals" (Hatch et al., 1985).
Tin compounds can alter various enzyme activities. For example, tin(II) fluoride inhibited hepatic mixed-function oxidase enzyme activity in Charles River CD albino rats when given at 30 mg of tin per kg body weight by single intraperitoneal dose (Shargel & Masnyj, 1981). When fed to rats, diets containing tin(II) chloride (100 mg of tin per kg diet) for 4 weeks caused reduction in hepatocellular antioxidant metalloenzyme activities of superoxide dismutase and glutathione peroxidase. Impairment in hepatocellular antioxidant protection favours the peroxidation of fatty acids (Reicks & Rader, 1990). In mice, intravenous injection of tin(II) chloride resulted in significant inhibition of the P450 cytochrome-dependent hepatic drug metabolizing enzymes such as azo-reductase and aromatic hydroxylase (Burba, 1983). Pretreatment of mice with tin(II) chloride (50 mg/kg body weight per day for 2 days) induced coumarin 7-hydroxylase in liver and kidney (Emde et al., 1996).
Tin(II) tartrate (20 mg of tin per kg body weight, single intraperitoneal injection) caused a decrease in glutathione in partially hepatectomized Sprague-Dawley rats, allowing an increase in lipid peroxidation, which damaged the hepatocytic membranes (Dwivedi et al., 1984). The inhibitory effect of tin on sulfhydryl-containing enzymes, particularly hepatic glutathione reductase and glucose 6-phosphate dehydrogenase, may be caused by the sulfhydryl group forming a metal mercaptide complex with coordinate covalent bonds, leading to decreased catalytic activity. The depression in enzyme levels may also be due to the interaction of tin with the biological ligands not directly involved in the active centre of the enzyme, through the formation of an unacceptable substrate complex for enzyme catalysis (Dwivedi et al., 1983).
Tin(II) compounds can adversely affect the erythrocytes (Chiba & Kikuchi, 1978; Chiba et al., 1980; Dwivedi et al., 1985b; Johnson & Greger, 1985; Zareba & Chmielnicka, 1985; see section 8.7). Tin(II) chloride (~3–30 mg of tin per kg body weight, single subcutaneous dose) and tin(II) tartrate (~9 mg of tin per kg body weight, single intraperitoneal dose) induced haem oxygenase in rat liver and kidney (Kappas & Maines, 1976; Maines & Kappas, 1977a; Kutty & Maines, 1984; Dwivedi et al., 1985b). The Sn2+ ion is more potent as an inducer of haem oxygenase-1 in rat cardiac tissue than is the Sn4+ ion, administered subcutaneously as tin(IV) citrate (single dose, 60 mg of tin per kg body weight) (Neil et al., 1995). Increased renal haem oxidase activity was observed (together with depleted renal cytochrome P450) in rats given tin(II) chloride (63 mg/kg body weight, subcutaneously, twice weekly for 8–15 weeks) (Sacerdoti et al., 1989; Escalante et al., 1991; Laniado-Schwartzman et al., 1992). Haem is essential for cell respiration, energy generation, and oxidative biotransformation. Metal ions directly regulate cellular content of haem and haem proteins by controlling production of ALA synthetase and haem oxygenase. Thus, metal ions may impair the oxidative function of cells, particularly those dependent on cytochrome P450. As a result, the biological impact of chemicals that are detoxified or metabolically transformed by the P450 system is greatly altered (Maines & Kappas, 1977a; Dwivedi et al., 1985b). Chelation of the metal ion into the porphyrin ring is not necessary in order to regulate the enzymes of haem synthesis and oxidation (Maines & Kappas, 1977b). Tin(II) fluoride and other tin(II) halides form complexes with haemoproteins such as hepatic cytochrome P450 and haemoglobin (Dahl & Hodgson, 1977). Substitution of the central iron atom of haem by tin leads to a synthetic haem analogue (tin(IV)-protoporphyrin) that regulates haem oxygenase in a dual mechanism, which involves competitive inhibition of the enzyme for the natural substrate haem and simultaneous enhancement of new enzyme synthesis (Drummond & Kappas, 1982; Sardana & Kappas, 1987).
The activity of ALAD in the erythrocytes of Harlan-Sprague-Dawley rats fed tin at 2000 mg/kg in the diet (as tin(II) chloride) for 21 days was reduced to 55% of the control value (Johnson & Greger, 1985). When tin(II) chloride was given at 2 mg/kg body weight to Wistar rats by subcutaneous, intraperitoneal, or intragastric routes every other day, ALAD activity was clearly decreased after two doses, whereas seven doses resulted in almost complete enzyme inhibition (Zareba & Chmielnicka, 1985). ALAD was inhibited by tin(II) chloride, but not by tin(IV) chloride. The inhibition was rapidly reversed (Chiba & Kikuchi, 1978; Chiba et al., 1980). ALA synthetase and ALAD were inhibited by tin(II) tartrate (Dwivedi et al., 1985b). Tin(II) concentrations of 1.5 µmol/litre increased the activity of isolated and purified ALAD from human red blood cells by approximately 30%. At greater concentrations, tin was an inhibitor of the enzyme, probably due to binding to allosteric sites (Despaux et al., 1977). A protective effect of zinc with respect to ALAD activity in blood and ALA levels in urine was observed after combined administration of tin(II) chloride and zinc sulfate in rabbits (Chmielnicka et al., 1992). One subunit of the ALAD enzyme contains one zinc atom and eight sulfhydryl groups (Tsukamoto et al., 1979). Tin presumably attacks one sulfhydryl group and binds weakly at the zinc-binding site of the enzyme (Chiba & Kikuchi, 1984). Intraperitoneal injection of selenium (as sodium selenite) simultaneously with tin(II) chloride in ICR mice completely prevents tin-induced ALAD inhibition. It has been suggested that selenium protects essential thiol groups in ALAD that are otherwise blocked by tin (Chiba et al., 1985a, 1985b; Chiba & Shinohara, 1992).
Oral administration of tin(II) chloride (2 mg of tin per kg body weight per day) to rats had inhibitory effects on calcium content, acid and alkaline phosphatase activity, and collagen synthesis in femoral bone (Yamaguchi et al., 1980b, 1981a, 1982a, 1982b). When tin was given orally at 60 mg/kg body weight per day for 3 days (as tin(II) chloride), insulin secretion and hepatic phosphorylase activity were inhibited in rats (Yamaguchi et al., 1978a, 1978b), as were active transport of calcium and mucosal alkaline phosphatase activity in the duodenum, and bile calcium content was increased (Yamaguchi & Yamamoto, 1978; Yamaguchi et al., 1979). In rats, tin directly inhibits bone formation independently of calcium homeostasis. Administration of 1.0 mg of tin(II) chloride per kg body weight to weanling male rats at 12-h intervals for 28 days inhibited collagen synthesis prior to suppression of DNA synthesis in the femoral epiphysis (Yamaguichi et al., 1982b).
Slight but statistically significant increases in cerebral and muscle acetylcholinesterase activity were seen in groups of six male Wistar rats given 1.11 or 2.22 mmol of tin(II) chloride per litre in drinking-water (about 21 and 42 mg of tin per kg body weight per day, respectively) for 18 weeks, whereas no effect was seen at 0.44 mmol/litre (about 8 mg of tin per kg body weight per day) (Savolainen & Valkonen, 1986). Studies of frog neuromuscular transmission suggest that activation of the N-type calcium channel is involved in the tin(II) chloride-induced increase in calcium entry into the nerve terminals (Hattori & Maehashi, 1992). Tin(II) chloride itself may facilitate the transmitter release from nerve terminals in mammalian (mouse) as well as in amphibian (frog) species (Hattori & Maehashi, 1993). An intraperitoneal dose of tin(II) chloride (5–30 mg of tin per kg body weight) suppressed gastric secretion in rats. The mechanism of inhibition was assumed to be associated with an inhibition of nerve transmission as well as reduction of gastrin release from G cells (Yamaguchi et al., 1976, 1978c). Injection of tin(II) chloride can stimulate or depress the central nervous system of rats (Silva et al., 2002).
Tin is ubiquitous in animal tissues. There is evidence that tin is essential for growth in rats (Schwarz et al., 1970; Schwarz, 1974a, 1974b; IPCS, 1980; Yokoi et al., 1990; ATSDR, 2003), but no essential function has been shown in other mammals, including humans (Schwarz et al., 1970; Hiles, 1974; IPCS, 1980; Alfrey, 1981; Nielsen, 1984; Dwivedi et al., 1985a; Sherman et al., 1986; Cardarelli, 1990; Tsangaris & Williams, 1992; ATSDR, 2003).
Studies in rats show that ingestion of inorganic tin (as tin(II) chloride) interferes with the body status and handling of copper, iron, and zinc. The mechanism is unknown, but possibly tin impairs absorption of these metals (Johnson & Greger, 1984; Beynen et al., 1992; Pekelharing et al., 1994; Yu & Beynen, 1995).
Limited data suggest a possible neurotoxic effect of tin(II) chloride. Cerebral and muscle acetylcholinesterase activities were slightly increased in rats given tin(II) chloride in the drinking-water for 18 weeks (Savolainen & Valkonen, 1986). The possible mechanism is unknown, but calcium, magnesium, and manganese cations also activate acetylcholinesterase (Tomlinson et al., 1981), suggesting a possible effect on the deacylation phase of enzyme–substrate reactions (Tomlinson et al., 1981; Savolainen & Valkonen, 1986). Studies of frog neuromuscular transmission suggest that activation of the N-type calcium channel is involved in the tin(II) chloride-induced increase in calcium entry into the nerve terminals (Hattori & Maehashi, 1992). Tin(II) chloride itself may facilitate the transmitter release from nerve terminals in mammalian (mouse) as well as in amphibian (frog) species (Hattori & Maehashi, 1993). Suppressed gastric secretion in rats given an intraperitoneal dose of tin(II) chloride (5–30 mg of tin per kg body weight) might be associated with inhibition of nerve transmission as well as reduction of gastrin release from G cells (Yamaguchi et al., 1976, 1978c).
No clearly irritant reactions were seen when 73 nickel-sensitive patients were patch-tested with metallic tin (Menné et al., 1987) or when other subjects were patch-tested with metallic tin or 1% tin(II) chloride in petrolatum (de Fine Olivarius et al., 1993). Irritant reactions were noted in patients patch-tested with 5% or 10% tin(II) chloride in petrolatum (de Fine Olivarius et al., 1993).
Patch tests with metallic tin in 73 nickel-sensitive patients revealed six positive allergic skin reactions (as well as four "doubtful" reactions) (Menné et al., 1987). Patch-testing with 1% tin(II) chloride in petrolatum and with a tin disc suggested that some patients are sensitized to tin (de Fine Olivarius et al., 1993). In 199 patients with suspected allergic reactions to metals, 13 had positive patch tests with 2% tin(II) chloride in petrolatum (Rammelsberg & Pevny, 1986). One out of 50 craftsmen in the ceramics industry had a positive reaction when patch-tested with 2.5% metallic tin dispersed in petrolatum (Gaddoni et al., 1993). A worker who produced metal patterns for body parts on trucks and was exposed to airborne dust from an alloy that contained tin had dermatitis around the eyes, forehead, and wrists. He had a positive patch test to 1% tin(II) chloride in petrolatum (Nielsen & Skov, 1998). Considering its widespread use, it is unlikely that tin is an important contact allergen.
In groups of 10–11 humans, the acute ingestion of 36 mg tin (as tin(II) chloride) together with 0.5, 4, or 6 mg zinc (as 65ZnCl2 solutions) or with 4 mg of 65Zn (in a turkey-based meal) inhibited 65Zn absorption, as measured by whole-body counting of the retention of 65Zn after 7–10 days. According to the authors, the dose required to inhibit zinc absorption under the conditions in this study was well in excess of that supplied by the normal diet (Valberg et al., 1984). However, others were unable to demonstrate any clear inhibition of the plasma appearance of zinc after 1–4 h when human volunteers ingested a single dose of 25, 50, or 100 mg of tin (as tin(II) chloride) with 12.5 mg of zinc (Solomons et al., 1983). Moderate disturbances in zinc and selenium excretion rates were reported in eight adult males when the normal diet (supplying 0.11 mg of tin per day) was supplemented with 50 mg of tin per day (as tin(II) chloride in fruit juice) for 20 days, in a cross-over design study. Faecal and urinary excretion rates of copper, iron, manganese, magnesium, and calcium were unaffected, as were haematocrit and serum ferritin levels (Greger et al., 1982; Johnson & Greger, 1982; Johnson et al., 1982).
There are a number of reports of acute gastrointestinal illness following the intake of fruit or fruit juice from unlacquered tin cans, as well as a smaller number of controlled volunteer trials. These reports have been summarized comprehensively (JECFA, 2001; Blunden & Wallace, 2003). Corrosion of these containers had led to detinning, with tin concentrations reaching 200–2000 mg/kg in the food (Capar & Boyer, 1980; Greger & Baier, 1981). Ingested doses have been estimated as 30–200 mg (Warburton et al., 1962; Barker & Runte, 1972; Nehring, 1972; Svensson, 1975). Symptoms most frequently reported were nausea, abdominal cramps, vomiting, and diarrhoea. The median incubation period was 1 h (range 15 min to 14 h), and the median duration of symptoms was 12 h (Barker & Runte, 1972). Concentrations may be more critical than dose in causing these effects. JECFA has concluded that limited human data indicate that acute manifestations of gastric irritation may arise, in certain individuals, from the consumption of 150 mg of tin per litre in canned beverages or 250 mg of tin per kg in other canned foods. As some canned foods containing up to 700 mg of tin per kg produced no detectable effects, it may be that certain individuals are particularly sensitive or that the chemical form of tin is important (JECFA, 1989, 2001).
In a randomized, double-blind, cross-over study, a group of 18 healthy volunteers (males and females who had fasted for at least 7 h) consumed 250 ml of tomato juice to which tin(II) chloride had been added to give tin concentrations of 161, 264, or 529 mg/kg. The control juice contained <0.5 mg of tin per kg. The only reaction in the 161 mg/kg group considered to be treatment-related was mild gastrointestinal symptoms in one volunteer (of 18). At 264 mg/kg, 3 of 18 subjects reported a total of 7 gastrointestinal symptoms, of which 2 were mild and 5 were moderate. Treatment at 529 mg/kg was discontinued, after 4 of 5 subjects reported a variety of mild and moderate gastrointestinal symptoms. Blood samples taken before dosing and at 0.5–4 h after dosing did not reveal increased serum levels of tin, supporting the view that effects are due to local irritation rather than to systemically absorbed tin (Boogaard et al., 2003).
In a second such study carried out at the same centre, another group of 23 healthy volunteers consumed 250 ml of tomato soup containing tin that had migrated from the unlacquered cans. Tin concentrations were <0.5 (controls), 201, and 267 mg/kg. The incidences of subjects reporting an adverse effect (3/23, 0/23, and 4/23 in the control, low-dose, and high-dose groups, respectively) bore no clear relationship with dose. The seven self-reported events were distributed among the classifications "gastrointestinal," "central and peripheral system," and "psychiatric," and the study provided no evidence of significant toxicity from the acute ingestion of 267 mg of tin per kg in tomato soup (a tin dose of about 67 mg) (Boogaard et al., 2003).
The differences in toxicity seen in these studies might reflect differences in chemical speciation. In the soup study, 52% of the tin content was present in solid matter, whereas only 15% of the tin was found in solid matter in the freshly prepared mixture of juice and tin(II) chloride that was ingested by the volunteers. Low-molecular-weight species (<1000 daltons) in the supernatant accounted for about 59% and 32% of the tin content of the juice and soup, respectively. Within 24 h, the proportion of tin associated with solid matter in the juice/tin(II) chloride mixture increased from 15% to 35%, indicating gradual complexation. It was suggested that the concentration of low-molecular-weight tin species and the nature of the chemical species formed are important factors determining the extent of gastric irritation (Boogaard et al., 2003).
There are several case-reports of a benign pneumoconiosis called stannosis (identified by chest roentgenograms) in workers exposed to tin(IV) oxide dust and fumes for 3 years or more in tin smelting works, scrap metal recovery plants, and hearth tinning. In general, no information on exposure levels was available (Barták et al., 1948; Pendergrass & Pryde, 1948; Cutter et al., 1949; Dundon & Hughes, 1950; Spencer & Wycoff, 1954; Schuler et al., 1958; Cole & Davies, 1964; Sluis-Cremer et al., 1989). A chest roentgenogram revealed a "peculiar widespread mottling of both lung fields by discrete shadows" in a man who had been employed in the tending of a detinning furnace for 18 years. The employment was terminated 8 years before chest examination. At autopsy 10 years later, the tin content of wet lung tissue was 1100 mg/kg (Dundon & Hughes, 1950).
In a health assessment of the employees, including ex-employee pensioners, from a United Kingdom tin smelting works, chest X-ray examinations provided radiological evidence of a benign pneumoconiosis in 121 out of 215 workers. The X-ray changes were either widespread, tiny, dense shadows or softer, larger, more nodular opacities and were found in workers handling raw ore, smelting furnace house workers, and refinery furnace men. Employment time ranged from 3 to 50 years. None of the affected men had any clinical symptoms or signs of pneumoconiosis, and there was no X-ray evidence of fibrosis or significant emphysema. Only men engaged in "dusty" work experienced X-ray changes. No significant X-ray changes were seen in fitters, joiners, or electricians, who were exposed only to the "general dustiness" of the works for up to 50 years, or in ingot casters, who worked with molten tin (Robertson & Whitaker, 1955; Robertson, 1960). Lung function measurements (forced expiratory volume and airway resistance) were normal. Mortality at the tin smelting works was lower than expected (observed 131, expected 166 deaths) in comparison with the male United Kingdom population for the period 1921–1955. The concentrations of tin (in mg/m3) associated with particle size <5 µm (Hexlet sampler) were given in section 6.3. The number of samples and strategy and methods of sampling and analysis were not described, and total airborne tin concentrations were not measured (Robertson, 1964).
Autopsy findings were given for seven tin workers with abnormal radiographs. None had died of pulmonary disease. Aggregates of macrophages containing dust were seen around respiratory bronchioles and less commonly around segmental bronchi, in the alveoli, in the interlobular septa, and in the perivascular lymphatics. The mild focal emphysema observed was assumed to be clinically insignificant and was considerably less severe than that seen in coal workers’ pneumoconiosis. No fibrosis was present. Chemical and X-ray diffraction analysis showed that the lungs contained tin(IV) oxide. X-ray emission microanalysis identified tin in a minute particle of dust in lung phagocytes (Robertson et al., 1961). A survey carried out over a number of years of workers exposed to condensation aerosols formed during the smelting of tin and consisting mainly of tin(IV) oxide found that the total silica concentration in the aerosols did not exceed 3% and that the total dust concentration in air varied between 3 and 70 mg/m3. Workers developed pneumoconiosis after 6–8 years of employment. No cases of pneumoconiosis were observed 10 years after the dust concentration had been reduced to 10 mg/m3. No further details were given (Hlebnikova, 1957).
Symptoms such as wheezing, cough, chest pain, and dyspnoea on exertion, reported in workers handling tin(IV) chloride, were probably due to elevated levels of hydrogen chloride formed by the combination of tin(IV) chloride and water in the presence of heat (Levy et al., 1985).
The lung cancer experience of tin miners in China (principally in Yunnan province) and England has been assessed. In the English studies (covering 1939–1986), an increased mortality from cancer of the lung was seen in a cohort of Cornish tin miners, but the data indicated the main risk factors to be tobacco smoking and radon exposure (Fox et al., 1981; Hodgson & Jones, 1990). In the Chinese studies of workers at the Yunnan Tin Corporation, 1724 lung cancer cases were registered during the period 1954–1986, of whom 90% had a history of working underground. Tin was not considered a contributing factor; the major causes were believed to be radon, arsenic, tobacco, and diet (Qiao et al., 1989, 1997; Taylor et al., 1989; Forman et al., 1992). A nested case–control study of lung cancer in four Chinese tin mines revealed an increased risk of lung cancer; the main risk factors were smoking and arsenic exposure, along with cumulative exposure to dust containing crystalline silica (Chen & Chen, 2002).
Uraemic patients might be especially prone to accumulate trace elements from environmental sources, and elevated tin levels have been found in muscle, serum, liver, and kidney of such patients. As tin affects kidney enzyme activity in animals, it has been suggested that tin might be involved in a degenerative feedback effect in uraemic patients (Rudolph et al., 1973; Nunnelley et al., 1978). In a Belgian case–control study (n = 272 men and women), a significantly increased risk (odds ratio 3.72, 95% confidence interval 1.22–11.3) of chronic renal failure was found for occupational exposure to tin. Exposures were reconstructed from self-reported occupational histories by three industrial hygienists independently (Nuyts et al., 1995).
Plasma and red blood cell tin concentrations were higher in patients with Alzheimer disease (plasma 21.6 nmol/litre and red blood cells 32 nmol/litre) than in those with multi-infarct dementia (12.4 and 19.9 nmol/litre) and controls (11.6 and 21.7 nmol/litre) (Corrigan et al., 1991, 1992). There were negative correlations between tin levels and the red blood cell polyunsaturated fatty acid levels in the Alzheimer disease patients (16 women, 8 men, mean age 77.4 years, SD 8.3 years), and the authors suggested that tin may be involved in lipid peroxidation in that illness (Corrigan et al., 1991). Later studies involving the analysis of tin in hippocampal tissues obtained postmortem from patients with Alzheimer disease and controls found no significant difference in tin concentrations in the tissues (Corrigan et al., 1993).
In summary, occupational exposure to tin(IV) oxide dust or fumes has induced stannosis, with no indication of fibrosis or apparent disability beyond chest X-ray opacities. In one case–control study, an increased risk of chronic renal failure was reported. Excretion rates of zinc and selenium were moderately changed in subjects who ingested about 0.7 mg of tin per kg body weight per day for 20 days.
Most laboratory testing with aquatic organisms has been carried out with the soluble tin(II) chloride, leading to a classification of moderately toxic to aquatic organisms. However, speciation under environmental conditions favours the tin oxide compounds, which have low toxicity in organisms largely due to their low solubility, poor absorption, low accumulation in tissues, and rapid excretion.
The toxicity of inorganic tin to aquatic organisms is summarized in Table 9. The most sensitive microalgae are the marine diatoms Skeletonema costatum and Thalassiosira guillardii, with 72-h EC50s of tin(II), based on growth inhibition, of around 0.2 mg/litre. Acute LC/EC50s of tin(II) for aquatic invertebrates range from 3.6 to 140 mg/litre, with a 21-day EC50, based on reproductive success in daphnids, of 1.5 mg/litre. The fish toxicity tests clearly show that tin(IV) chloride is less toxic than the more soluble tin(II) chloride. Ninety-six-hour LC50s for fish range from 35 mg/litre for tin(II) to >1000 mg/litre for tin(IV). Embryo-larval test results (7- to 28-day LC50s) for fish and amphibians range from 0.1 to 2.1 mg/litre for tin(II).
Table 9: Toxicity of inorganic tin compounds to aquatic species.
|
Organism |
End-point |
Iona |
Tin concentration (mg/litre) |
Reference |
|
Microorganisms |
||||
|
Bacterium (Pseudomonas fluorescens) |
1-h EC50 (viability) |
Sn4+ b |
245 |
Han & Cooney (1995) |
|
Bacterium (Serratia sp.) |
1-h EC50 (viability) |
Sn4+ b |
287 |
Han & Cooney (1995) |
|
Marine bacterium |
EC50 (bioluminescence) |
Sn2+ |
2.3 |
Thomulka & Lange (1996) |
|
Cyanobacterium |
4-h EC50 (primary productivity) |
Sn2+ |
>5 |
Wong et al. (1982) |
|
4-h EC50 (primary productivity) |
Sn4+ b |
>5 |
Wong et al. (1982) |
|
|
Green alga (Ankistrodesmus falcatus) |
8-day EC50 (growth inhibition) c |
Sn2+ |
12 |
Wong et al. (1982) |
|
4-h EC50 (primary productivity) |
Sn2+ |
14 |
Wong et al. (1982) |
|
|
8-day EC50 (growth inhibition) c |
Sn4+ b |
2 |
Wong et al. (1982) |
|
|
4-h EC50 (primary productivity) |
Sn4+ b |
12 |
Wong et al. (1982) |
|
|
Green alga (Scenedesmus quadricauda) |
4-h EC50 (primary productivity) |
Sn2+ |
>50 |
Wong et al. (1982) |
|
4-h EC50 (primary productivity) |
Sn4+ b |
>50 |
Wong et al. (1982) |
|
|
Diatom (Skeletonema costatum) |
72-h EC50 (growth inhibition) c |
Sn2+ |
0.2 |
Walsh et al. (1985) |
|
Diatom (Thalassiosira guillardii) |
72-h EC50 (growth inhibition) c |
Sn2+ |
0.2 |
Walsh et al. (1985) |
|
Ciliate (Tetrahymena pyriformis) |
3-h EC50 (growth inhibition) |
Sn d |
132 |
Sauvant et al. (1995) |
|
6-h EC50 (growth inhibition) |
Sn d |
80 |
Sauvant et al. (1995) |
|
|
9-h EC50 (growth inhibition) |
Sn d |
90 |
Sauvant et al. (1995) |
|
|
Invertebrates |
||||
|
Pulmonate snail (Taphius glabratus) |
24-h NOEC (behaviour) |
Sn2+ |
10 |
Harry & Aldrich (1963) |
|
Tubificid worm (Tubifex tubifex) |
48-h EC50 (immobilization) |
Sn2+ |
140 |
Khangarot (1991) |
|
96-h EC50 (immobilization) |
Sn2+ |
21 |
Khangarot (1991) |
|
|
48-h LC50 |
Sn2+ |
54.9 |
Fargasova (1994) |
|
|
96-h LC50 |
Sn2+ |
30 |
Fargasova (1994) |
|
|
48-h LC50 |
Sn4+ e |
33.1 |
Fargasova (1994) |
|
|
96-h LC50 |
Sn4+ e |
27.5 |
Fargasova (1994) |
|
|
Amphipod (Crangonyx pseudogracilis) |
48-h LC50 |
Sn2+ |
71.8 |
Martin & Holdich (1986) |
|
96-h LC50 |
Sn2+ |
50.1 |
Martin & Holdich (1986) |
|
|
Water flea (Daphnia magna) |
24-h LC50 |
Sn2+ |
37 |
Khangarot et al. (1987) |
|
48-h LC50 |
Sn2+ |
19.5 |
Khangarot et al. (1987) |
|
|
48-h LC50 |
Sn2+ |
55 |
Biesinger & Christensen (1972) |
|
|
21-day LC50 |
Sn2+ |
42 |
Biesinger & Christensen (1972) |
|
|
21-day EC50 (reproductive inhibition) |
Sn2+ |
1.5 |
Biesinger & Christensen (1972) |
|
|
48-h LC50 |
Sn4+ |
32.9 |
Cabejszek & Stasiak (1960) |
|
|
48-h EC50 (immobilization) |
Sn4+ |
21.6 |
Khangarot & Ray (1989) |
|
|
Midge (Chironomus plumosus) |
48-h LC50 |
Sn2+ |
8.3 |
Fargasova (1994) |
|
96-h LC50 |
Sn2+ |
3.6 |
Fargasova (1994) |
|
|
48-h LC50 |
Sn4+ e |
8.3 |
Fargasova (1994) |
|
|
96-h LC50 |
Sn4+ e |
3 |
Fargasova (1994) |
|
|
Fish |
||||
|
Goldfish (Carassius auratus) |
7-day LC50 (embryo-larval test) |
Sn2+ |
2.1 |
Birge (1978) |
|
Carp (Cyprinus carpio) |
96-h EC50 (hatching success) |
Sn2+ |
295 |
Kapur & Yadav (1982) |
|
Mud dab (Limanda limanda) |
96-h LC50 |
Sn2+ |
35 f |
Taylor et al. (1985) |
|
Largemouth bass (Micropterus salmoides) |
8-day LC50 (embryo-larval test) |
Sn2+ |
1.9 |
Birge et al. (1978) |
|
Rainbow trout (Oncorhynchus mykiss) |
28-day LC50 (embryo-larval test) |
Sn2+ |
0.4 |
Birge (1978); Birge et al. (1978) |
|
Killifish (Oryzias latipes) |
48-h LC50 |
Sn4+ b |
480 g |
Tsuji et al. (1986) |
|
Zebra fish (Brachydanio rerio) |
96-h LC50 h |
Sn4+ b |
>1000 |
Hoechst AG (1995) |
|
Amphibians |
||||
|
Marbled salamander (Ambystoma opacum) |
8-day LC50 (embryo-larval test) |
Sn2+ |
0.9 |
Birge et al. (1978) |
|
Eastern narrow-mouthed toad (Gastrophryne carolinensis) |
7-day LC50 (embryo-larval test) |
Sn2+ |
0.1 |
Birge (1978) |
|
a |
Tin(II) chloride unless otherwise stated. |
|
b |
Tin(IV) chloride. |
|
c |
Based on cell yield. |
|
d |
Salt not stated. |
|
e |
Sodium stannate (Na2SnO3). |
|
f |
24- to 96-h LC50s all 35 mg/litre. |
|
g |
24- and 48-h LC50s at 10 °C, 20 °C, and 30 °C all 480 mg/litre; precipitated in the test solution. |
|
h |
OECD Guideline 203 (fish, acute toxicity test). |
The toxicity of tin(IV) chloride to three pure strains of sulfate-reducing marine bacteria isolated from a tributyltin-polluted sediment was determined. Adverse effects on suspended anaerobic cell cultures were reported at concentrations ranging from 130 mg of tin(IV) per litre (500 µmol/litre) to 156 mg/litre (600 µmol/litre) (Lascourreges et al., 2000).
Pawlik-Skowronska et al. (1997) found that tin(II) and tin(IV) salts inhibited the growth of planktonic cyanobacteria (Synechocystis aquatilis). Toxicity increased with increasing tin concentrations, time of exposure, and pH value of the medium (in the pH range 7.0–9.8); tin(II) seemed to be more toxic than tin(IV). At the lowest tin(II) concentration of 1 mg/litre, there was a 36–40% decrease in growth and chlorophyll a content after 96 h at pH 9.8. The presence of humic acids reduced the toxicity of tin. At high pH values, anionic tin species such as SnO3H−, SnO32−, or Sn(OH)62− exist, whereas at neutral or acidic pH values, cationic or neutral tin species like Sn(OH)+, Sn(OH)22+, Sn(OH)2, or SnO are present.
Kick et al. (1971) found adverse effects on the yield of spring wheat (expressed as dry weight) at soil inorganic tin(II) concentrations of 125 mg/kg; however, the addition of sludge completely eliminated the toxic effect due to an increase in soil nutrient content and a decrease in soil acidity. Sinapis alba seeds showed low sensitivity to inorganic tin, with 72-h EC50s, based on root growth inhibition, of 281 mg/litre for tin(II) (as tin(II) chloride) and 417 mg/litre for tin(IV) (as sodium stannate) (Fargasova, 1994).
Inorganic tin (as tin(II) chloride) had no significant effect on day-old chicks fed a diet containing 200 mg of tin per kg for 21 days (Howell & Hill, 1978). A significant increase in myopathy was reported in ducklings during a 4-week exposure to 1000 mg of tin per kg (as tin(II) chloride) in the diet (Van Vleet, 1982).
Tin(II) chloride was found to cause 60% repellency (percentage of mice refusing to eat more than 50% of wheat treated with 2.0% tin(II) chloride) in house mice (Mus musculus) (Schafer & Bowles, 1985).
Occupational inhalation of particles containing water-insoluble tin compounds has been associated with a benign pneumoconiosis (stannosis) observed as X-ray opacities. Where information was given, this effect was restricted to workers in particularly dusty work areas. This condition is not associated with fibrosis and appears not to be associated with any apparent lung dysfunction. The literature on long-term inhalation of inorganic tin consists mainly of case-reports in humans, with poor exposure assessment and old methods of examination. Reports on effects concerning microscopic pathology and cell toxicity in the respiratory system are scarce. The information is insufficient to assess the health risk to the lungs.
Tin metal is not a skin irritant, but tin(II) chloride was irritating to human skin, and tin(II) and tin(IV) chlorides were irritating to the skin and oral mucosa of rats.
Occasional cases of allergic contact dermatitis in humans have been reported. However, the scarcity of reported reactions, despite its widespread use, suggests that tin is not an important contact allergen.
On acute ingestion, tin salts (e.g. tin(II) chloride) can cause gastrointestinal irritation, nausea, vomiting, abdominal cramps, and diarrhoea in humans.
Tin absorbed from the gut may interfere with the status of other important metallic minerals (e.g. zinc). On repeated ingestion, the critical effect of absorbed tin might be the decrease of calcium content in bone. Interference with the status of minerals has been observed at similar oral doses (0.6–0.7 mg of tin per kg body weight per day) in humans and laboratory animals. In laboratory animals, repeated ingestion has led to changes in enzyme levels in blood, liver, kidney, and bone and degenerative changes in liver and kidney. In an old lifetime study, degenerative changes were reported in the liver and kidneys of rats ingesting about 0.4 mg of tin per kg body weight per day from the drinking-water, but such effects were not seen in a later 2-year study, in which rats and mice ingested tin at much higher (at least 100-fold) doses from the diet. In certain studies, exact daily doses are difficult to estimate due to the study designs and lack of information in the reports. The most comprehensive long-term study of reasonable quality involved continuous administration of tin(II) chloride in the diet of rats and mice at 0, 1000, or 2000 mg/kg for 2 years. Food consumption, growth, survival, and the gross and microscopic appearance of a wide range of tissues and organs were evaluated. In rats, the NOAEL was 1000 mg/kg diet, equivalent to 30 mg/kg body weight per day; at the higher dose, survival was decreased in the males. The male mice showed no effects (NOAEL 180 mg/kg body weight per day). Female mice were normal at 1000 mg/kg diet (NOAEL 130 mg/kg body weight per day), but survival was reduced at 270 mg/kg body weight per day (NTP, 1982).
Good-quality carcinogenicity studies are available on rats and mice administered tin(II) chloride in the diet for 2 years. Other, more limited, investigations of carcinogenicity potential have been carried out, notably on tin metal, tin(II) chloride, and tin(II) 2-ethylhexanoate. None of these studies gave any clear evidence of carcinogenicity, although there is some doubt regarding the C-cell tumours in the thyroid gland in male rats in the best available study.
Investigation of the genotoxicity potential of tin compounds in vivo is limited, but has not revealed any evidence of activity. Several in vitro studies also gave negative results, but inorganic tin compounds have induced DNA damage in human white blood cells, hamster ovary cells, and E. coli bacteria, as well as chromosome damage in hamster ovary cells. Some evidence suggests that the DNA damage may be a secondary effect associated with reactive oxygen species. The mechanism resulting in chromosome damage has not been determined, although it is known that certain inorganic salts can give positive results in such assays as the result of changes in the ionic strength or pH of the test medium.
The very limited information on the levels of exposure in the worker populations where cases of stannosis have been diagnosed does not allow the setting of tolerable concentrations for inhaled tin compounds.
Limited data are available on the ability of ingested tin to adversely affect zinc absorption in humans. In one volunteer study, plasma appearance of zinc 1–4 h following a zinc dose of 12.5 mg was unaffected by concomitant ingestion of up to 100 mg tin (as tin(II) chloride) (Solomons et al., 1983). However, others reported that a single dose of 36 mg tin (again, as tin(II) chloride), taken with up to 6 mg zinc (as radiolabelled zinc dichloride), resulted in a lower zinc retention (whole-body counting) 7–10 days later (Valberg et al., 1984). Moderate disturbances in zinc excretion rates were reported when the normal diet (supplying 0.11 mg of tin per day) of eight men was supplemented with 50 mg of tin per day (as tin(II) chloride in fruit juice) (Greger et al., 1982; Johnson & Greger, 1982; Johnson et al., 1982).
Early literature contains a number of reports of gastrointestinal effects (notably nausea, abdominal cramps, vomiting, and diarrhoea) following the intake of fruit or juice from unlacquered tin cans. Although the tin doses involved have been estimated (at 30–200 mg), confidence in the accuracy of these figures is low.
Two recent volunteer studies provided a better insight into effective doses and, perhaps more importantly, concentrations. The first study involved ingestion of 250 ml of tomato juice to which tin(II) chloride had been added to give tin concentrations of 161, 264, or 529 mg/kg. The control juice contained tin at <0.5 mg/kg. One volunteer experienced mild gastrointestinal symptoms at 161 mg/kg (a dose of about 40 mg tin); typical tin-induced acute symptoms were seen at 264 and 529 mg/kg (about 66 and 132 mg tin). Serum levels of tin did not increase at 0.5–4 h post-dosing at any dose, supporting the view that acute effects of tin ingestion are dependent upon concentration (resulting in local gastric irritation) rather than due to systemically absorbed tin (Boogaard et al., 2003). A second study, published by the same investigators, involved ingestion of tomato soup containing tin that had migrated from unlacquered cans; consequently, the tin species involved might be a better match for those that arise in canned food. The volunteers ingested 250 ml of tomato soup containing migrated tin at <0.5, 201, or 267 mg/kg. There was no evidence that consumption at these concentrations (up to about 67 mg tin) produced any acute effects (Boogaard et al., 2003).
For the general population, the major source of tin is the diet. By comparison, drinking-water and inhaled air contribute insignificant amounts.
From data on mean tin intake from food for the populations of seven countries (Australia, France, Japan, Netherlands, New Zealand, the United Kingdom, and the USA), JECFA (2001) concluded that tin intakes ranged from <1 up to 15 mg/person per day.
Certain individuals who routinely consume canned fruits, vegetables, and juices from unlacquered cans could ingest 50–60 mg of tin daily (Johnson & Greger, 1982; Sherlock & Smart, 1984; JECFA, 2001).
Those who consume about four servings of food stored in open unlacquered cans, on a daily basis, might consume in the region of 200 mg of tin per day (Greger & Baier, 1981; JECFA, 2001).
Although a no-effect level for inhibition of zinc absorption has not been clearly established, the lowest dose reported to have this effect (36 mg) is about 2.5 to >36 times higher than the estimated mean population intakes as summarized by JECFA. However, those who routinely consume canned fruits, vegetables, and juices from unlacquered cans could have tin intakes (50–60 mg) that are similar to the acute (36 mg) or repeated (50 mg) dose levels reported in some studies to affect zinc absorption or balance. Whether this would have any clinical effect is likely to be critically dependent upon an adequate dietary supply of zinc.
Of the two recent studies on the acute gastrointestinal effects of tin, one showed a LOAEL of approximately 66 mg of tin (264 mg/kg food). The other, which may be more relevant, found no evidence of effects at a similar dose of 67 mg of tin (267 mg/kg food). This dose is approximately 4.5 to >67 times higher than the values reported in JECFA (2001) as estimates of mean population dietary intakes for seven countries, but similar to the estimated daily intake (50–60 mg) of those individuals who routinely consume canned fruits, vegetables, and juices from unlacquered cans.
Data on possible effects by inhalation exposure are rare. It is unclear whether occupational exposure to tin poses any extra risk to workers with existing renal disease.
Consumers with a high proportion of the diet consisting of foods or beverages from unlacquered cans may have higher than average exposure, especially with regard to canned acid fruits, juice, tomatoes, and tomato products. These may include people on low incomes, the elderly, and institutionalized individuals.
Chronic tin consumption might affect mineral balance in humans. It is unclear to what extent those individuals whose zinc status is marginal may be at extra risk due to consumption of tin in food. Such populations might include those with only low levels of zinc or those who are in a marginal nutritional status regarding zinc (e.g. the elderly, children, and pregnant women).
The possible significance of the increase in thyroid tumours that developed in rats fed tin(II) chloride in the diet for 2 years has not been firmly established.
Tin compounds have been subjected to only limited testing for genotoxicity potential. There is uncertainty over the mechanism by which some tin compounds apparently induce DNA damage and chromosome damage in mammalian cells in culture.
Ambient levels of tin in the environment are generally quite low, except in the vicinity of local pollution sources. Most monitoring studies have analysed only for total tin, and in these cases the proportion of inorganic tin will vary depending on sampling time and site. Therefore, in order to compare environmental concentrations with toxicity, only analytical results based on inorganic tin have been considered in the evaluation.
Tin may be transported in the atmosphere following the release of particulate matter derived from the combustion of fossil fuels and solid wastes. Inorganic tin compounds are non-volatile under environmental conditions. Average tin concentrations in air are generally below 0.1 µg/m3, with higher concentrations near some industrial facilities.
In general, tin occurs in trace amounts in natural waters; higher inorganic tin concentrations are associated with industrial discharges and tributyltin use (tributyltin degrades ultimately to inorganic tin). In a survey of lakes and rivers, nearly 80% of samples were found to contain inorganic tin at concentrations below 1 µg/litre; higher levels of up to 37 µg/litre were reported near local pollution sources. Inorganic tin concentrations ranging from 0.001 to 0.01 µg/litre have been reported for coastal waters, with levels of up to 8 µg/litre near anthropogenic sources. Concentrations of inorganic tin displayed extreme variability both temporally and spatially within enclosed harbours and were largely influenced by localized inputs. Concentrations generally ranged from <0.005 to 0.2 µg/litre; however, levels of up to 48.7 µg/litre were found near local discharges and significant tributyltin usage.
In the environment, tin compounds are generally only sparingly soluble in water and are likely to partition to soils and sediments. Inorganic tin concentrations in sediment ranged up to 8 mg/kg dry weight in coastal areas and up to 15.5 mg/kg in rivers and lakes. Total tin concentrations in soil can range from <1 to 200 mg/kg, but levels of 1000 mg/kg may occur in areas of high tin deposits.
Inorganic tin may undergo oxidation–reduction, ligand exchange, and precipitation reactions in the environment. The biomethylation of inorganic tin has been demonstrated in pure bacterial cultures, sediments, and decaying plant material. Inorganic tin compounds may be bioconcentrated by organisms, but data are limited.
Under environmental speciation conditions, inorganic tin compounds have low toxicity in both aquatic and terrestrial organisms, largely due to their low solubility, poor absorption, often low accumulation in tissues, and rapid excretion. Most laboratory testing with aquatic organisms has been carried out with the soluble tin(II) chloride. Figure 1 summarizes the toxicity of inorganic tin to a range of aquatic organisms (data from Table 8). The most sensitive microalgae are the marine diatoms, with 72-h EC50s of tin(II), based on growth inhibition, of around 0.2 mg/litre. Acute LC/EC50s of tin(II) for aquatic invertebrates range from 3.6 to 140 mg/litre, with a 21-day EC50, based on reproductive success in daphnids, of 1.5 mg/litre. The fish toxicity tests clearly show that tin(IV) chloride is less toxic than the more soluble tin(II) chloride. Ninety-six-hour LC50s for fish range from 35 mg/litre for tin(II) to >1000 mg/litre for tin(IV). In longer-term embryo-larval tests (7- to 28-day LC50s), results for fish and amphibians range from 0.1 to 2.1 mg/litre for tin(II).
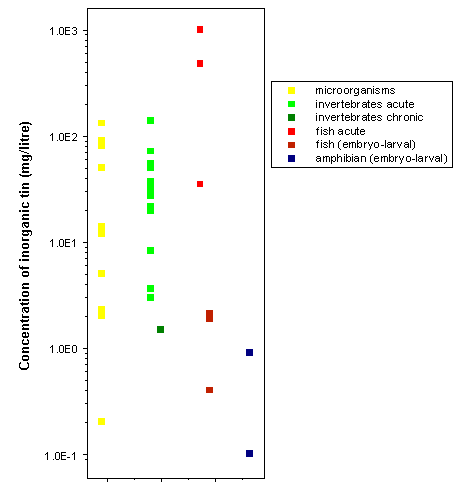
Figure 1: Toxicity of inorganic tin to aquatic organisms.
In the environment, inorganic tin will partition to soil and sediment, and, as a consequence, bioavailability to organisms will tend to be low. Generally, as can be seen from Figure 1, the acute toxicity to aquatic organisms is low to moderate. Concentrations showing toxicity to organisms are generally several orders of magnitude higher than those found in the environment.
The most sensitive test results were 72-h exposures to diatoms and embryo-larval amphibian studies, with toxic effects seen at 0.1 to 0.2 mg/litre for tin(II). Even at these concentrations, toxic effects caused by inorganic tin are unlikely, even near sources of local pollution. It should be noted that where concentrations are expressed as total tin, a percentage is likely to be in the form of organotins, such as tributyltin, which are more bioavailable and toxic. For more information on the environmental fate and toxicity of tributyltin, please refer to IPCS (1990, 1999).
JECFA has reviewed the toxicological literature on inorganic tin on a number of occasions (e.g. JECFA, 1989, 2001, 2005).3
IARC has not evaluated the carcinogenic potential of tin compounds. IARC has evaluated inorganic fluorides, including tin(II) fluoride, and concluded that these are not classifiable as to their carcinogenicity to humans. Evidence for carcinogenicity of inorganic fluorides was considered inadequate for both humans and laboratory animals (IARC, 1987).
Alessio L, Apostoli P, Crippa M (1994) Multiple exposure to solvents and metals. Occupational Hygiene, 1:127–151.
Alfrey A (1981) Aluminium and tin. In: Bronner F, Coburn J, eds. Disorders of mineral metabolism. Vol. 1. New York, NY, Academic Press, pp. 353–368.
Andreae MO (1983) The determination of the chemical species of some of the "hydride elements" (arsenic, antimony, tin and germanium) in seawater: methodology and results. In: Wong CS, Boyle E, Bruland KW, Burton JD, Goldberg ED, eds. Trace metals in sea water. New York, NY, Plenum Press, pp. 1–19.
APHA, AWWA, WEF (1998a) Inductively coupled plasma/mass spectrometry (ICP/MS) method, 3125B. Metals by inductively coupled spectrometry. In: Clesceri LS, Greenberg AE, Eaton AE, eds. Standard methods for the examination of water and wastewater, 20th ed. Washington, DC, American Public Health Association, American Water Works Association, and Water Environment Federation.
APHA, AWWA, WEF (1998b) Metals — electrothermal absorption spectroscopy, 3113B. Electrothermal atomic absorption spectroscopic method. In: Clesceri LS, Greenberg AE, Eaton AE, eds. Standard methods for the examination of water and wastewater, 20th ed. Washington, DC, American Public Health Association, American Water Works Association, and Water Environment Federation.
APHA, AWWA, WEF (1998c) Metals — flame atomic absorption spectroscopy, 3111. Metals by atomic absorption spectroscopy. In: Clesceri LS, Greenberg AE, Eaton AE, eds. Standard methods for the examination of water and wastewater, 20th ed. Washington, DC, American Public Health Association, American Water Works Association, and Water Environment Federation.
Apostoli P, Giusti S, Bartoli D, Perico A, Bavazzano P, Alessio L (1998) Multiple exposure to arsenic, antimony, and other elements in art glass manufacturing. American Journal of Industrial Medicine, 34:65–72.
ATSDR (2003) Toxicological profile for tin and compounds (update). Draft for public comment. Atlanta, GA, US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry.
Attramadal A, Svatun B (1984) In vivo antibacterial effect of tin on the oral microflora. Scandinavian Journal of Dental Research, 92:161–164.
Baines J (2000) Submission to WHO regarding dietary intake of tin. Canberra, Australia New Zealand Food Authority [cited in JECFA, 2001].
Barker W, Runte V (1972) Tomato juice-associated gastroenteritis, Washington and Oregon, 1969. American Journal of Epidemiology, 96:219–226.
Barták F, Tomecka M, Tomisek O (1948) [Stannosis (pneumoconiosis due to tin).] Casopis Lekaru Ceskych, 87:915–920 (in Czech with French summary).
Beliles RP (1994) Tin. In: Clayton GD, Clayton FE, eds. Patty’s industrial hygiene and toxicology, 4th ed. Vol. 2. New York, NY, John Wiley, pp. 2258–2276.
Benoy CJ, Hooper PA, Schneider R (1971) Toxicity of tin in canned fruit juices and solid foods. Food and Cosmetics Toxicology, 9:645–656.
Bernardo-Filho M, Cunha M, Valsa I, Araujo A, Silva F, Fonseca A (1994) Evaluation of potential genotoxicity of stannous chloride: inactivation, filamentation and lysogenic induction of Escherichia coli. Food and Chemical Toxicology, 32:477–479.
Bernick M, Campagna P (1995) Application of field-portable X-ray-fluorescence spectrometers for field-screening air monitoring filters for metals. Journal of Hazardous Materials, 43:91–99.
Berry T, Nicholson J, Troendle K (1994) Almost two centuries with amalgam: where are we today? Journal of the American Dental Association, 125:392–399.
Beynen AC, Pekelharing HLM, Lemmens AG (1992) High intakes of tin lower iron status in rats. Biological Trace Element Research, 35:85–88.
Biégo GH, Joyeux M, Hartmann P (1999) Determination of dietary tin in an adult French citizen. Archives of Environmental Contamination and Toxicology, 36:227–232 [cited in JECFA, 2001].
Biesinger KE, Christensen GM (1972) Effects of various metals on survival, growth, reproduction, and metabolism of Daphnia magna. Journal of the Fisheries Research Board of Canada, 29:1691–1700.
Birge WJ (1978) Aquatic toxicology of trace elements of coal and fly ash. In: Thorp JH, Gibbons JW, eds. Energy and environmental stress in aquatic systems. Augusta, GA, US Department of Energy, pp. 219–240 (DOE Symposium Series 48).
Birge WJ, Hudson JE, Black JA, Westerman AG (1978) Embryo-larval bioassays on inorganic coal elements and in situ biomonitoring of coal-waste effluents. In: Samuel DE, Stauffer JR, Hocutt CH, Mason WT Jr, eds. Surface mining and fish/wildlife needs in the eastern United States. Morgantown, WV, US Fish and Wildlife Service, pp. 97–104 (CONF-781240).
Bischoff F, Bryson G (1976a) Inert foreign-body implants in the mouse CNS. Research Communications in Psychology, Psychiatry and Behavior, 1:187–190.
Bischoff F, Bryson G (1976b) Toxicologic studies of tin needles at the intrathoracic site of mice. Research Communications in Chemical Pathology and Pharmacology, 15:331–340.
Blunden SJ (1983) The ultraviolet degradation of the methyltin chlorides in carbon tetrachloride and water. Journal of Organometallic Chemistry, 248:149–160.
Blunden SJ, Chapman AH (1982) The environmental degradation of organotin compounds — a review. Environmental Technology Letters, 3(6):267–272.
Blunden S, Wallace T (2003) Tin in canned food: a review and understanding of occurrence and effect. Food and Chemical Toxicology, 41:1651–1662.
Boogaard PJ, Boisset M, Blunden S, Davies S, Ong TJ, Taverene J-P (2003) Comparative assessment of gastrointestinal irritant potency in man of tin(II) chloride and tin migrated from packaging. Food and Chemical Toxicology, 41:1663–1670.
Brown RA, Nazario CM, De Tirado RS, Castrillón J, Agard ET (1977) A comparison of the halflife of inorganic and organic tin in the mouse. Environmental Research, 13:56–61.
Bruland KW (1983) Trace elements in sea-water. In: Riley JP, Chester R, eds. Chemical oceanography. Vol. 8. London, Academic Press, pp. 157–220.
Bryan GW, Langston WJ (1992) Bioavailability, accumulation and effects of heavy metals in sediments with special reference to United Kingdom estuaries — a review. Environmental Pollution, 76(2):89–131.
Brzezinska-Paudyn A, Van Loon JC (1988) Determination of tin in environmental samples by graphite furnace atomic absorption and inductively coupled plasma–mass spectrometry. Fresenius’ Journal of Analytical Chemistry, 331:707–712.
Budavari S, ed. (2001) The Merck index. An encyclopedia of chemicals, drugs, and biologicals, 13th ed. Whitehouse Station, NJ, Merck and Co., Inc.
Bulten EJ, Meinema HA (1991) Tin. In: Merian E, ed. Metals and their compounds in the environment: Occurrence, analysis, and biological relevance. Weinheim, VCH, pp. 1243–1259.
Burba J (1983) Inhibition of hepatic azo-reductase and aromatic hydroxylase by radiopharmaceuticals containing tin. Toxicology Letters, 18:269–272.
Burger J, Gochfeld M (2000) Metals in Laysan albatrosses from Midway Atoll. Archives of Environmental Contamination and Toxicology, 38(2):254–259.
Burger J, Gochfeld M (2001) Metal levels in feathers of cormorants, flamingos and gulls from the coast of Namibia in southern Africa. Environmental Monitoring and Assessment, 69(2):195–203.
Burger J, Shukla T, Dixon C, Shukla S, McMahon MJ, Ramos R, Gochfeld M (2001) Metals in feathers of sooty tern, white tern, gray-backed tern, and brown noddy from islands in the North Pacific. Environmental Monitoring and Assessment, 71(1):71–89.
Butler OT, Howe AM (1999) Development of an international standard for the determination of metals and metalloids in workplace air using ICP-AES: evaluation of sample dissolution procedures through an interlaboratory trial. Journal of Environmental Monitoring, 1:23–32.
Byrd JT, Andreae MO (1982) Tin and methyltin species in sea water. Concentration and fluxes. Science, 218:565–569.
Byrd JT, Andreae MO (1986) Concentrations and fluxes of tin in aerosols and rain. Atmospheric Environment, 20(5):931–939.
Byrne AR, Kosta L (1979) On the vanadium and tin contents of diet and human blood. The Science of the Total Environment, 13:87–90.
Cabejszek I, Stasiak M (1960) [Studies on the toxic effects of some metals on the water biocenosis — Daphnia magna employed as index (Part II).] Roczniki Panstwowego Zakladu Higieny Warsaw, 11:533–540 (in Polish).
Calloway DH, McMullen JJ (1966) Fecal excretion of iron and tin by men fed stored canned foods. American Journal of Clinical Nutrition, 18(1):1–6.
Capar SG, Boyer KW (1980) Multi-element analysis of food stored in their open cans. Journal of Food Safety, 2:105–118.
Cardarelli NF (1990) Tin and the thymus gland: a review. Thymus, 15: 223–231.
Cardellicchio N, Decataldo A, Di Leo A, Giandomenico S (2002) Trace elements in organs and tissues of striped dolphins (Stenella coeruleoalba) from the Mediterranean Sea (southern Italy). Chemosphere, 49(1):85–90.
Carlin JF Jr (2003a) Tin. In: US Geological Survey mineral commodity summaries, 2003. Reston, VA, US Department of the Interior, US Geological Survey, pp. 176–177 (http://minerals.er.usgs.gov:80/minerals/pubs/commodity/tin/660303.pdf) [cited in ATSDR, 2003].
Carlin JF Jr (2003b) Tin. In: US Geological Survey minerals yearbook — 2001. Reston, VA, US Department of the Interior, US Geological Survey, pp. 78.2–78.8 plus Tables 1–10 (http://minerals.er.usgs.gov/minerals/pubs/commodity/tin/tinmyb01.pdf) [cited in ATSDR, 2003].
Chen W, Chen J (2002) Nested case–control study of lung cancer in four Chinese tin mines. Occupational and Environmental Medicine, 59:113–118.
Chiba M, Kikuchi M (1978) Effect of tin compounds on activity of 5-aminolevulinate dehydratase in blood. Biochemical and Biophysical Research Communications, 82:1057–1061.
Chiba M, Kikuchi M (1984) The in vitro effects of zinc and manganese on delta-aminolevulinic acid dehydratase activity inhibited by lead or tin. Toxicology and Applied Pharmacology, 73:388–394.
Chiba M, Shinohara A (1992) Inhibition of erythrocyte 5-aminolaevulinate hydrolase activity by tin and its prevention by selenite. British Journal of Industrial Medicine, 49:355–358.
Chiba M, Ogihara K, Kikuchi M (1980) Effect of tin on porphyrin biosynthesis. Archives of Toxicology, 45:189–195.
Chiba M, Fujimoto N, Kikuchi M (1985a) Protective effect of selenium on the inhibition of erythrocyte 5-aminolevulinate dehydratase activity by tin. Toxicology Letters, 24:235–241.
Chiba M, Fujimoto N, Oyamada N, Kikuchi M (1985b) Interactions between selenium and tin, selenium and lead, and their effects on ALAD activity in blood. Biological Trace Element Research, 8:263–282.
Chiba M, Shinohara A, Ujiie C (1991) Tin concentrations in various organs in humans, dogs, and mice. Biomedical Trace Element Research, 2:257–258 [possibly incorrectly cited in Westrum & Thomassen, 2002].
Chiba M, Iyengar V, Greenberg RR, Gills T (1994) Determination of tin in biological materials by atomic absorption spectrophotometry and neutron activation analysis. The Science of the Total Environment, 148:39–44.
Chillrud SN, Bopp RF, Simpson HJ, Ross JM, Shuster EL, Chaky DA, Walsh DC, Choy CC, Tolley LR, Yarme A (1999) Twentieth century atmospheric metal fluxes into Central Park Lake, New York City. Environmental Science & Technology, 33(5):657–662.
Chmielnicka J, Szymanska JA, Sniec J (1981) Distribution of tin in the rat and disturbances in the metabolism of zinc and copper due to repeated exposure to SnCl2. Archives of Toxicology, 47:263–268 [cited in JECFA, 2001].
Chmielnicka J, Zareba G, Grabowska U (1992) Protective effect of zinc on heme biosynthesis disturbances in rabbits after administration per os of tin. Ecotoxicology and Environmental Safety, 24:266–274.
Chmielnicka J, Zareba G, Polkowska-Kulesza E, Najder M, Korycka A (1993) Comparison of tin and lead toxic action on erythropoietic system in blood and bone marrow of rabbits. Biological Trace Element Research, 36:73–87.
CMI (1988) Metal can shipments — 1988. Washington, DC, Can Manufacturers Institute.
Cole CWD, Davies JVSA (1964) Stannosis in hearth tinners. British Journal of Industrial Medicine, 21:235–241.
Conine DL, Yum M, Martz RC, Stookey GK, Muhler JC, Forney RB (1975) Toxicity of sodium pentafluorostannite, a new anticariogenic agent. I. Comparison of the acute toxicity of sodium pentafluorostannite, sodium fluoride, and stannous chloride in mice and/or rats. Toxicology and Applied Pharmacology, 33:21–26.
Conine DL, Yum M, Martz RC, Stookey GK, Forney RB (1976) Toxicity of sodium pentafluorostannite. A new anticariogenic agent. III. 30-day toxicity study in rats. Toxicology and Applied Pharmacology, 35:21–28.
Cooney JJ (1988) Microbial transformations of tin and tin compounds. Journal of Industrial Microbiology, 3(4):195–204.
Cooper R, Stranks DR (1966) Vapor pressure measurements. In: Jonassen H, Weissberger A, eds. Technique of inorganic chemistry. Vol. VI. New York, NY, John Wiley & Sons, pp. 1–82.
Corrigan FM, Van Rhijn AG, Ijomah G, McIntyre F, Skinner ER, Horrobin DF, Ward NI (1991) Tin and fatty acids in dementia. Prostaglandins, Leukotrienes, and Essential Fatty Acids, 43:229–238.
Corrigan FM, Crichton JS, Ward NI, Horrobin DF (1992) Blood tin concentrations in Alzheimer’s disease. Biological Psychiatry, 31:749–750.
Corrigan FM, Reynolds GP, Ward NI (1993) Hippocampal tin, aluminum and zinc in Alzheimer’s disease. Biometals, 6:149–154.
Crockett AB (1998) Background levels of metals in soils, McMurdo Station, Antarctica. Environmental Monitoring and Assessment, 50(3):289–296.
Cutter HC, Faller WW, Stocklen JB, Wilson WL (1949) Benign pneumoconiosis in a tin oxide recovery plant. Journal of Industrial Hygiene and Toxicology, 31:139–141.
Dahl AR, Hodgson E (1977) Complexes of stannous fluoride and other group IVB dihalides with mammalian hemoproteins. Science, 197:1376–1378.
Dannecker W, Schroder B, Stechmann H (1990) Organic and inorganic substances in highway tunnel exhaust air. The Science of the Total Environment, 93:293–300.
Dantas FJS, Moraes MO, Carvalho EF, Bernardo-Filho M, Caldiera-de-Araujo A (1999) Stannous chloride mediated single strand breaks in plasmid DNA through reactive oxygen species formation. Toxicology Letters, 110:129–136.
Dantas FJS, de Mattos JCP, Moraes MO, Viana ME, Lage CAS, Cabral-Neto JB, Leitao AC, Bernardo-Filho M, Bezerra RJAC, Carvalho JJ, Caldeira-de-Araujo A (2002a) Genotoxic effects of stannous chloride (SnCl2) in K562 cell line. Food and Chemical Toxicology, 40:1493–1498.
Dantas FJS, de Mattos JCP, Moraes MO, Boasquevisques E, Rodrigues MP, Lage CAS, Cabral-Neto JB, Leitao AC, Bernardo-Filho M, Bezerra RJAC, Carvalho JJ, Caldeira-de-Araujo A (2002b) DNA damage in peripheral blood nuclear cells assessed by comet assay from individuals submitted to scintigraphic examinations. Cellular and Molecular Biology, 48:789–791.
Davison RL, Natusch DFS, Wallace JR (1974) Trace elements in fly ash: Dependence of concentration on particle size. Environmental Science & Technology, 8:1107–1113.
de Bièvre P, Barnes IL (1985) Table of the isotopic composition of the elements as determined by mass spectrometry. International Journal of Mass Spectrometry and Ion Processes, 65:211–230.
de Fine Olivarius F, Balslev E, Menné T (1993) Skin reactivity to tin chloride and metallic tin. Contact Dermatitis, 29:110–111.
de Groot AP (1973) Subacute toxicity of inorganic tin is influenced by dietary levels of iron and copper. Food and Cosmetics Toxicology, 11:955–962.
de Groot AP, Feron VJ, Til HP (1973) Short-term toxicity studies on some salts and oxides of tin in rats. Food and Cosmetics Toxicology, 11:19–30.
der Meulen HC, Feron VJ, Til HP (1974) Pancreatic atrophy and other pathological changes in rats following the feeding of stannous chloride. Pathologia Europaea, 9:185–192.
Despaux N, Bohuon C, Comoy E, Boudene C (1977) Postulated mode of action of metals on purified human ALA-dehydratase (EC 4-2-1-24). Biomedicine, 27:358–361.
Dewanjee MK, Wahner HW (1979) Pharmacodynamics of stannous chelates administered with 99mTc-labeled chelates. Radiology, 132:711–716.
Dick GL, Hughes JT, Mitchell JW, Davidson F (1978) Survey of trace elements and pesticide residues in the New Zealand diet. I. Trace element content. New Zealand Journal of Science, 21:57–69.
Dimitrov NV, Meyer C, Nahhas F, Miller C, Averill BA (1981) Effect of tin on immune responses of mice. Clinical Immunology and Immunopathology, 20:39–48.
Donard OFX, Weber JH (1988) Volatilization of tin as stannane in anoxic environments. Nature, 332(6162):339–341.
Donard OFX, Short FT, Weber JH (1987) Regulation of tin and methyltin compounds by the green alga Enteromorpha under simulated estuarine conditions. Canadian Journal of Fisheries and Aquatic Sciences, 44(1):140–145.
Drummond GS, Kappas A (1982) Chemoprevention of neonatal jaundice: potency of tin-protoporphyrin in an animal model. Science, 217:1250–1252.
Dundon CC, Hughes JP (1950) Stannic oxide pneumoconiosis. American Journal of Roentgenology and Radium Therapy, 63:797–812.
Dwivedi RS, Kaur G, Srivastava RC, Krishna Murti CR (1983) Effects of tin on sulfhydryl containing enzymes in liver of rats. Chemosphere, 12:333–340.
Dwivedi RS, Kaur G, Srivastava RC, Krishna Murti CR (1984) Lipid peroxidation in tin intoxicated partially hepatectomized rats. Bulletin of Environmental Contamination and Toxicology, 33:200–209.
Dwivedi RS, Kaur G, Srivastava RC, Krishna Murti CR (1985a) Metabolic activity of lysosomes in tin-intoxicated regenerating rat liver. Toxicology Letters, 28:133–138.
Dwivedi RS, Kaur G, Srivastava RC, Krishna Murti CR (1985b) Role of tin on heme and drug biotransformation mechanism of partially hepatectomized rats. Industrial Health, 23:1–7.
Eckel WP, Langley WD (1988) A background-based ranking technique for assessment of elemental enrichment in soils at hazardous waste sites. In: Superfund ’88: Proceedings of the 9th national conference. Washington, DC, The Hazardous Materials Control Research Institute.
Eisler R (1989) Tin hazards to fish, wildlife, and invertebrates: a synoptic review. Laurel, MD, US Fish and Wildlife Service, Patuxent Wildlife Research Center (PB-89-139620/XAB).
Ellingsen JE, Rolla G (1987) Treatment of dentin with stannous fluoride — SEM and electron microprobe study. Scandinavian Journal of Dental Research, 95:281–286.
Emde B, Tegtmeier M, Hahnemann B, Legrum W (1996) Inorganic tin — a new selective inducer of the murine coumarin 7-hydroxylase (CYP2A5). Toxicology, 108:73–78.
Escalante B, Sacerdoti D, Davidian MM, Laniado-Schwartzman M, McGiff JC (1991) Chronic treatment with tin normalizes blood pressure in spontaneously hypertensive rats. Hypertension, 17:776–779.
Evans WH, Sherlock JC (1987) Relationships between elemental intakes within the United Kingdom total diet study and other adult dietary studies. Food Additives and Contaminants, 4:1–8.
Falke AM, Weber JH (1994) Methylation of inorganic tin by decaying Spartina alterniflora in estuarine water and by estuarine water. Applied Organometallic Chemistry, 8(4):351–359.
Fargasova A (1994) A comparative study of the toxicity and inhibitory effects of inorganic tin compounds on various biological subjects. Biologia, 49(3):307–311.
Ferretti GA, Tanzer JM, Tinanoff N (1982) The effect of fluoride and stannous ions on Streptococcus mutans. Viability, growth, acid, glucan production, and adherence. Caries Research, 16:298–307.
Food and Drug Research Laboratories (1972) Teratological evaluation of FDA 71-33 (stannous chloride) in mice, rats and hamsters. Report prepared by Food and Drug Research Laboratories for the US Food and Drug Administration (FDA Contract 71-260; unpublished report submitted to WHO) [cited in JECFA, 1982].
Forman MR, Yao SX, Graubard BI, Qiao YL, McAdams M, Mao BL, Taylor PR (1992) The effect of dietary intake of fruits and vegetables on the odds ratio of lung cancer among Yunnan tin miners. International Journal of Epidemiology, 21:437–441.
Foureman P, Mason JM, Valencia R, Zimmering S (1994) Chemical mutagenesis testing in Drosophila. X. Results of 70 coded chemicals tested for the National Toxicology Program. Environmental and Molecular Mutagenesis, 23:208–227.
Fox AJ, Goldblatt P, Kinlen LJ (1981) A study of the mortality of Cornish tin miners. British Journal of Industrial Medicine, 38:378–380.
Francis MD, Tofe AJ, Hiles RA, Birch CG, Bevan JA, Grabenstetter RJ (1981) Inorganic tin: chemistry, disposition and role in nuclear medicine diagnostic skeletal imaging agents. International Journal of Nuclear Medicine and Biology, 8:145–152.
Fritsch P, De Saint Blanquat G, Derache R (1977) Effects of various dietary components on absorption and tissue distribution of orally administered inorganic tin in rats. Food and Cosmetics Toxicology, 15:147–149.
Fritsch P, De Saint Blanquat G, Derache R (1978) Nutritional and toxicological impacts of administered inorganic tin for six months in rats. Toxicological European Research, 1:253–261.
Furchner JE, Drake GA (1976) Comparative metabolism of radionuclides in mammals — XI. Retention of 113Sn in the mouse, rat, monkey and dog. Health Physics, 31:219–224.
Gaddoni G, Baldassari L, Francesconi E, Motolese A (1993) Contact dermatitis among decorators and enamellers in hand-made ceramic decorations. Contact Dermatitis, 28:127–128.
Ganguly BB (1993) Cell division, chromosomal aberration, and micronuclei formation in human peripheral blood lymphocytes. Effect of stannic chloride on donor’s age. Biological Trace Element Research, 38:55–62.
Ganguly BB, Talukdar G, Sharma A (1992) Cytotoxicity of tin on human peripheral lymphocytes in vitro. Mutation Research, 282:61–67.
Garcia F, Ortega A, Domingo JL, Corbella J (2001) Accumulation of metals in autopsy tissues of subjects living in Tarragona County, Spain. Journal of Environmental Science and Health Part A, 36(9):1767–1786.
Gaver CC Jr (1997) Tin and tin alloys. In: Kirk-Othmer encyclopedia of chemical technology, 4th ed. Vol. 4. New York, NY, Wiley & Sons, pp. 105–122.
Gerritse RG, Vriesema R, Daleberg JW (1982) Effect of sewage sludge on trace element mobility in soils. Journal of Environmental Quality, 11:359–364.
Giessen H (2004) Ultrafast nano-optics: optical properties and applications of metallic photonic crystals. Bonn, University of Bonn, Institute of Applied Physics (http://www.iap.uni-bonn.de/ag_giessen/index.html?research/photoniccrystals.html).
Gilmour CC, Tuttle JH, Means JC (1987) Anaerobic microbial methylation of inorganic tin in estuarine sediment slurries. Microbial Ecology, 14:233–242.
Giordano R, Lombardi G, Ciaralli L, Beccaloni E, Sepe A, Ciprotti M, Costantini S (1999) Major and trace elements in sediments from Terra Nova Bay, Antarctica. The Science of the Total Environment, 227(1):29–40.
Gocke E, King MT, Eckhardt K, Wild D (1981) Mutagenicity of cosmetics ingredients licensed by the European Communities. Mutation Research, 90:91–109.
Graf GG (1987) Tin, tin alloys, and tin compounds. In: Ullman’s encyclopedia of industrial chemistry. Vol. A27. Weinheim, VHC, pp. 79–81.
Greger JL (1988) Tin and aluminium. In: Smith KT, ed. Trace minerals in foods. New York, NY, Marcel Dekker [cited in JECFA, 2001].
Greger JL, Baier MJ (1981) Tin and iron content of canned and bottled foods. Journal of Food Science, 46:1751–1754, 1765.
Greger JL, Smith SA, Johnson MA, Baier MJ (1982) Effects of dietary tin and aluminium on selenium utilization by adult males. Biological Trace Element Research, 4:269–278.
Gulati DK, Witt K, Anderson B, Zeiger E, Shelby MD (1989) Chromosome aberration and sister chromatid exchange tests in Chinese hamster ovary cells in vitro. III. Results with 27 chemicals. Environmental and Molecular Mutagenesis, 13:133–193.
Hadjimarkos DM (1967) Effect of trace elements in drinking water on dental caries. Journal of Pediatrics, 70:967–969 [cited in JECFA, 2001].
Hadjispyrou SA, Anagnostopoulos A, Nicholson K, Nimfopoulos MK, Michailidis KM (1998) Correlation of the methylating capacity of river and marine sediments to their organic sediment index. Environmental Geochemistry and Health, 20(1):19–27.
Hallas LE, Means JC, Cooney JJ (1982) Methylation of tin by estuarine microorganisms. Science, 215:1505–1507.
Hamasaki T, Sato T, Nagase H, Kito H (1992) The genotoxicity of organotin compounds in SOS chromotest and rec-assay. Mutation Research, 280:195–203.
Hamasaki T, Sato T, Nagase H, Kito H (1993) The mutagenicity of organotin compounds as environmental pollutants. Mutation Research, 300(3):265–271.
Hamilton EI, Minski MJ, Cleary JJ (1973) The concentration and distribution of some stable elements in healthy human tissues from the United Kingdom. An environmental study. The Science of the Total Environment, 1(4):341–374.
Han GC, Cooney JJ (1995) Effects of butyltins and inorganic tin on chemotaxis of aquatic bacteria. Journal of Industrial Microbiology, 14(3–4):293–299.
Harding LE, Harris ML, Elliott JE (1998) Heavy and trace metals in wild mink (Mustela vison) and river otter (Lontra canadensis) captured on rivers receiving metals discharges. Bulletin of Environmental Contamination and Toxicology, 61(5):600–607.
Harry HW, Aldrich DV (1963) The distress syndrome in Taphius glabratus (Say) as a reaction to toxic concentrations of inorganic ions. Malacologia, 1(2):283–289.
Hasset JM, Johnson D, Myers JA, Al-Mudamgha A, Melcer ME, Kutscher CL, Sembrat MM (1984) The exposure of rats to inorganic tin: Behavioral and systemic effects of different levels and modes of exposure. Trace Substances and Environmental Health, 8:487–496.
Hatch GE, Boykin E, Graham JA, Lewtas J, Pott F, Loud K, Mumford JL (1985) Inhalable particles and pulmonary host defense: In vivo and in vitro effects of ambient air and combustion particles. Environmental Research, 36:67–80.
Hattori T, Maehashi H (1992) Interaction between stannous chloride and calcium channel blockers in frog neuromuscular transmission. Research Communications in Chemical Pathology and Pharmacology, 75:243–246.
Hattori T, Maehashi H (1993) Facilitation of transmitter release from mouse motor nerve terminals by stannous chloride. Research Communications in Chemical Pathology and Pharmacology, 82:121–124.
Hayashi O, Chiba M, Kikuchi M (1984) The effects of stannous chloride on the humoral immune response of mice. Toxicology Letters, 21:279–285.
Hayashi R, Shima S, Hayakawa K (1991) A study on urinary tin in healthy adults: Relationship between the concentration of urinary tin and life style. Japanese Journal of Hygiene, 46(4):898–904 (in Japanese with limited information given in English; partial translation supplied by Dr Susumu Ishimitsu, National Institute of Health Sciences, Japan).
HazDat (2003) Hazardous Substance Release and Health Effects Database. Atlanta, GA, US Department of Health and Human Services, Agency for Toxic Substances and Disease Registry (http://www.atsdr.cdc.gov/hazdat.html).
Hiles RA (1974) Absorption, distribution and excretion of inorganic tin in rats. Toxicology and Applied Pharmacology, 27:366–379.
Hlebnikova MJ (1957) [Hygienic characteristics of dust in the production of tin.] Gigiena Truda i Professional'nye Zabolevaniia, 2:53–83 (in Russian) [cited in IPCS, 1980].
Hodge VF, Seidel SL, Goldberg ED (1979) Determination of tin(IV) and organotin compounds in natural waters, coastal sediments and macro algae by atomic absorption spectrometry. Analytical Chemistry, 51(8):1256–1259.
Hodgson JT, Jones RD (1990) Mortality of a cohort of tin miners 1941–86. British Journal of Industrial Medicine, 47:665–676.
Hoechst AG (1995) Unveröffentl. Unters. (Report No. 95.0046) [cited in IUCLID (2000) IUCLID dataset: Tin tetrachloride. Ispra, European Commission, European Chemicals Bureau, International Uniform Chemical Information Database].
Howell GO, Hill CH (1978) Biological interaction of selenium with other trace elements in chicks. Environmental Health Perspectives, 25:147–150.
HSDB (2003) Tin. Environmental standards and regulations. Bethesda, MD, National Library of Medicine, Hazardous Substances Data Bank (http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB.htm).
IARC (1987) Fluorides (inorganic, used in drinking-water) (group 3). In: Overall evaluations of carcinogenicity: an updating of IARC Monographs Volumes 1 to 42. Lyon, International Agency for Research on Cancer, pp. 208–210 (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Suppl. 7).
Ichihashi H, Nakamura Y, Kannan K, Tsumura A, Yamasaki S (2001) Multi-elemental concentrations in tissues of Japanese common squid (Todarodes pacificus). Archives of Environmental Contamination and Toxicology, 41(4):483–490.
ICRP (1981) Metabolic data for tin. International Commission on Radiological Protection. Annals of the ICRP, 6(2/3):43–45 (http://www.sciencedirect.com/science/journal/01466453).
ICRP (1994) Human respiratory tract model for radiological protection. International Commission on Radiological Protection. Annals of the ICRP, 24(1–3) (ICRP Publication 66) (http://www.sciencedirect.com/science/journal/01466453).
ICRP (2001) The ICRP database of dose coefficients: Workers and members of the public. International Commission on Radiological Protection. Amsterdam, Elsevier Science Ltd. (CD-ROM).
ILO (1998a) Tin. In: Stellman J, ed. Encyclopedia of occupational health and safety, 4th ed. Vol. 63. Geneva, International Labour Organization, p. 41.
ILO (1998b) Tin reclamation. In: Stellman J, ed. Encyclopedia of occupational health and safety, 4th ed. Vol. 82. Geneva, International Labour Organization, pp. 51–52.
IPCS (1980) Tin and organotin compounds: a preliminary review. Geneva, World Health Organization, International Programme on Chemical Safety (Environmental Health Criteria 15).
IPCS (1990) Tributyltin compounds. Geneva, World Health Organization, International Programme on Chemical Safety (Environmental Health Criteria 116).
IPCS (1999) Tributyltin oxide. Geneva, World Health Organization, International Programme on Chemical Safety (Concise International Chemical Assessment Document 14).
IPCS (2004a) International Chemical Safety Card — Tin (II) chloride dihydrate. Geneva, World Health Organization, International Programme on Chemical Safety (ICSC 0738) (http://www.ilo.org/public/english/protection/safework/cis/products/icsc/dtasht/_icsc07/icsc0738.pdf).
IPCS (2004b) International Chemical Safety Card — Tin (II) fluoride. Geneva, World Health Organization, International Programme on Chemical Safety (ICSC 0860) (http://www.ilo.org/public/english/protection/safework/cis/products/icsc/dtasht/_icsc08/icsc0860.pdf).
IPCS (2004c) International Chemical Safety Card — Tin(IV) chloride (anhydrous). Geneva, World Health Organization, International Programme on Chemical Safety (ICSC 0953) (http://www.ilo.org/public/english/protection/safework/cis/products/icsc/dtasht/_icsc09/icsc0953.pdf).
IPCS (2004d) International Chemical Safety Card — Tin(IV) oxide. Geneva, World Health Organization, International Programme on Chemical Safety (ICSC 0954) (http://www.ilo.org/public/english/protection/safework/cis/products/icsc/dtasht/_icsc09/icsc0954.pdf).
IPCS (2004e) International Chemical Safety Card — Tin (II) chloride (anhydrous). Geneva, World Health Organization, International Programme on Chemical Safety (ICSC 0955) (http://www.ilo.org/public/english/protection/safework/cis/products/icsc/dtasht/_icsc09/icsc0955.pdf).
IPCS (2004f) International Chemical Safety Card — Tin(II) oxide. Geneva, World Health Organization, International Programme on Chemical Safety (ICSC 0956) (http://www.ilo.org/public/english/protection/safework/cis/products/icsc/dtasht/_icsc09/icsc0956.pdf).
ISO (1998) 2447:1998. Fruit and vegetable products — Determination of tin content. Geneva, International Organization for Standardization (http://www.iso.org/iso/en/CatalogueDetailPage.CatalogueDetail?CSNUMBER=7361).
ISO (2000) Workplace air — Determination of metals and metalloids in airborne particulate matter by inductively coupled plasma atomic emission spectrometry — Part 1: Sampling.
Geneva, International Organization for Standardization (ISO 15202-1; http://www.iso.ch/iso/en/ISOOnline.frontpage).
ISO (2003a) 17294-2:2003. Water quality — Application of inductively coupled plasma mass spectrometry (ICP-MS) — Part 2: Determination of 62 elements. Geneva, International Organization for Standardization (http://www.iso.ch/iso/en/ISOOnline.frontpage).
ISO (2003b) 9941:2003. Milk and canned evaporated milk — Determination of tin content — Spectrometric method. Geneva, International Organization for Standardization (http://www.iso.ch/iso/en/ISOOnline.frontpage).
ISO (2004) 17240:2004. Fruit and vegetable products — Determination of tin content — Method using flame atomic absorption spectrometry. Geneva, International Organization for Standardization (http://www.iso.org/iso/en/CatalogueDetailPage.CatalogueDetail?CSNUMBER=38288).
Janssen PJ, Bosland MC, van Hees JP, Spit BJ, Willems MI, Kuper CF (1985) Effects of feeding stannous chloride on different parts of the gastrointestinal tract of the rat. Toxicology and Applied Pharmacology, 78:19–28.
JECFA (1982) Tin and stannous chloride. In: Toxicological evaluation of certain food additives and contaminants. Prepared by the 26th meeting of the Joint FAO/WHO Expert Committee on Food Additives. Geneva, World Health Organization (WHO Food Additive Series 17) (http://www.inchem.org/documents/jecfa/jecmono/v17je32.htm).
JECFA (1989) Toxicological evaluation of certain food additives and contaminants. Prepared by the 33rd meeting of the Joint FAO/WHO Expert Committee on Food Additives. Geneva, World Health Organization (WHO Food Additive Series 24) (http://www.inchem.org/documents/jecfa/jecmono/v024je01.htm).
JECFA (2001) Safety evaluation of certain food additives and contaminants. Prepared by the 55th meeting of the Joint FAO/WHO Expert Committee on Food Additives. Geneva, World Health Organization (WHO Food Additive Series 46) (http://www.inchem.org/documents/jecfa/jecmono/v46je01.htm).
JECFA (2005) Joint FAO/WHO Expert Committee on Food Additives. Sixty-fourth meeting. Rome, 8–17 February 2005. Summary and conclusions. Rome, Food and Agriculture Organization of the United Nations; Geneva, World Health Organization (http://www.who.int/ipcs/food/jecfa/summaries/en/summary_report_64_final.pdf).
Johnson MA, Greger JL (1982) Effects of dietary tin on tin and calcium metabolism of adult males. American Journal of Clinical Nutrition, 35:655–660.
Johnson MA, Greger JL (1984) Absorption, distribution and endogenous excretion of zinc by rats fed various dietary levels of inorganic tin and zinc. Journal of Nutrition, 114:1843–1852.
Johnson MA, Greger JL (1985) Tin, copper, iron and calcium metabolism of rats fed various dietary levels of inorganic tin and zinc. Journal of Nutrition, 115:615–624.
Johnson MA, Baier MJ, Greger JL (1982) Effects of dietary tin on zinc, copper, iron, manganese, and magnesium metabolism of adult males. American Journal of Clinical Nutrition, 35:1332–1338.
Kada T, Hirano K, Shirasu Y (1980) Screening of environmental chemical mutagens by the rec-assay system with Bacillus subtilis. Chemical Mutagenesis, 6:149–173.
Kaminski MD, Landsberger S (2000) Heavy metals in urban soils of East St. Louis, IL. Part I: Total concentration of heavy metals in soils. Journal of the Air & Waste Management Association, 50(9):1667–1679.
Kanisawa M, Schroeder HA (1969) Life term studies on the effect of trace elements on spontaneous tumors in mice and rats. Cancer Research, 29:892–895.
Kappas A, Maines MD (1976) Tin: a potent inducer of heme oxygenase in kidney. Science, 192:60–62.
Kapur K, Yadav NA (1982) The effects of certain heavy metal salts on the development of eggs in common carp, Cyprinus carpio var. communis. Acta Hydrochimica et Hydrobiologia, 10(5):517–522.
Kawai S, Kurokawa Y, Harino H, Fukushima M (1998) Degradation of tributyltin by a bacterial strain isolated from polluted river water. Environmental Pollution, 102:259–263.
Kazantzis G (1994) Thallium and tin. In: Alessio L, Berlin A, Roi R, van der Venne MT, eds. Biological indicators for the assessment of human exposure to industrial chemicals. Luxembourg, European Commission, Office for Official Publications of the European Communities.
Khangarot BS (1991) Toxicity of metals to a freshwater tubificid worm, Tubifex tubifex (Muller). Bulletin of Environmental Contamination and Toxicology, 46:906–912.
Khangarot BS, Ray PK (1989) Investigation of correlation between physicochemical properties of metals and their toxicity to the water flea Daphnia magna Straus. Ecotoxicology and Environmental Safety, 18(2):109–120.
Khangarot BS, Ray PK, Chandra H (1987) Daphnia magna as a model to assess heavy metal toxicity: Comparative assessment with mouse system. Acta Hydrochimica et Hydrobiologia, 15(4):427–432.
Kick H, Nosbers R, Warnusz J (1971) The availability of Cr, Ni, Zn, Cd, Sn and Pb for plants. In: Proceedings of the international symposium on soil fertility evaluation, New Delhi. New Delhi, Indian Society of Soil Science, pp. 1039–1045.
Kloke A, Sauerbeck DR, Vetter H (1984) The contamination of plants and soil with heavy metals and the transport of metals in terrestrial food chain. In: Nriagu JO, ed. Changing metal cycles and human health. Berlin, Springer-Verlag, pp. 113–141.
Kojima S, Saito K, Kiyozumi M (1978) [Studies on poisonous metals. IV. Absorption of stannic chloride from rat alimentary tract and effect of various food components on its absorption (author’s translation).] Yakugaku Zasshi, 98:495–502 (in Japanese).
Kutty RK, Maines MD (1984) Effects of induction of heme oxygenase by cobalt and tin on the in vivo degradation of myoglobin. Biochemical Pharmacology, 33:2924–2926.
Kutzner J, Brod KH (1971) [Resorption and excretion of tin after oral administration of tin-133.] Nuclear Medicine, 10:286–297 (in German).
Langston WJ, Burt GR, Zhou M (1987) Tin and organotin in water, sediments, and benthic organisms of Poole Harbour. Marine Pollution Bulletin, 18(12):634–639.
Laniado-Schwartzman M, Abraham NG, Sacerdoti D, Escalante B, McGiff JC (1992) Effect of acute and chronic treatment of tin on blood pressure in spontaneously hypertensive rats. Tohoku Journal of Experimental Medicine, 166:85–91.
Lantzy RJ, Mackenzie FT (1979) Atmospheric trace metals: global cycles and assessment of man’s impact. Geochimica et Cosmochimica Acta, 43:511–525.
Larsson Å, Kinnby B, Könsberg R, Peszkowski MJ, Warfvinge G (1990) Irritant and sensitizing potential of copper, mercury and tin salts in experimental contact stomatitis of rat oral mucosa. Contact Dermatitis, 23:146–153.
Lascourreges JF, Caumette P, Donard OFX (2000) Toxicity of butyltin, phenyltin and inorganic tin compounds to sulfate-reducing bacteria isolated from anoxic marine sediments. Applied Organometallic Chemistry, 14(2):98–107.
Levine S, Saltzman A (1991) Tin compounds inhibit the plasma cell response to metallic tin. Transfer of inhibition by parabiosis. Biological Trace Element Research, 28:165–172.
Levine S, Saltzman A (1996) Metallic tin-induced lymphadenopathy in rat strains and hybrids. Biological Trace Element Research, 52:303–308.
Levine S, Sowinski R (1982) Plasmacellular lymphadenopathy produced in rats by tin. Experimental and Molecular Pathology, 36:86–98.
Levine S, Sowinski R (1983) Tin salts prevent the plasma cell response to metallic tin in Lewis rats. Toxicology and Applied Pharmacology, 68:110–115.
Levine S, Sowinski R, Koulish S (1983) Plasmacellular and granulomatous splenomegaly produced in rats by tin. Experimental and Molecular Pathology, 39:364–376.
Levy BS, Davis F, Johnson B (1985) Respiratory symptoms among glass bottle makers exposed to stannic chloride solution and other potentially hazardous substances. Journal of Occupational Medicine, 27:277–282.
Lide DR, ed. (1998–1999) CRC handbook of chemistry and physics, 79th ed. New York, NY, CRC Press.
Llobet JM, Granero S, Schuhmacher M, Corbella J, Domingo J (1998) Biological monitoring of environmental pollution and human exposure to metals in Tarragona, Spain. II. Levels in autopsy tissues. Trace Elements and Electrolytes, 15(1):44–49.
Magos L (1986) Tin. In: Friberg L, Nordberg G, Vouk V, eds. Handbook on the toxicology of metals. Vol. 2. Amsterdam, Elsevier, pp. 568–593.
Maguire RJ, Tkacz RJ (1985) Degradation of the tri-n-butyltin species in water and sediment from Toronto Harbor. Journal of Agricultural and Food Chemistry, 33:947–953.
Maguire RJ, Carey JH, Hale EJ (1983) Degradation of the tri-n-butyltin species in water. Journal of Agricultural and Food Chemistry, 31:1060–1065.
Maguire RJ, Wong PTS, Rhamey JS (1984) Accumulation and metabolism of tri-normal-butyltin cation by a green alga, Ankistrodesmus falcatus. Canadian Journal of Fisheries and Aquatic Sciences, 41(3):537–540.
Maguire RJ, Tkacz RJ, Chau YK, Bengert GA, Wong PTS, Chau YK, Kramar O, Bengert GA (1986) Occurrence of organotin compounds in water and sediment in Canada. Chemosphere, 15:253–274.
Maines MD, Kappas A (1977a) Metals as regulators of heme metabolism. Science, 198:1215–1221.
Maines MD, Kappas A (1977b) Regulation of heme pathway enzymes and cellular glutathione content by metals that do not chelate with tetrapyrroles: blockade of metal effects by thiols. Proceedings of the National Academy of Sciences of the United States of America, 74:1875–1878.
Malcolm HM, Boyd IL, Osborn D, French MC, Freestone P (1994) Trace metals in Antarctic fur seal (Arctocephalus gazella) livers from Bird Island, South Georgia. Marine Pollution Bulletin, 28(6):375–380.
Mark H, ed. (1983) Encyclopedia of chemical technology, 3rd ed. Vol. 23. New York, NY, John Wiley & Sons, pp. 18–78.
Marro N (1996) The 1994 Australian Market Basket Survey. Canberra, Australia New Zealand Food Authority [cited in JECFA, 2001].
Martin TR, Holdich DM (1986) The acute lethal toxicity of heavy metals to peracarid crustaceans (with particular reference to fresh-water asellids and gammarids). Water Research, 20(9):1137–1147.
McLean JR, Blakey DH, Douglas GR, Kaplan JG (1983a) The effect of stannous and stannic (tin) chloride on DNA in Chinese hamster ovary cells. Mutation Research, 119:195–201.
McLean JR, Birnboim HC, Pontefact R, Kaplan JG (1983b) The effect of tin chloride on the structure and function of DNA in human white blood cells. Chemico-Biological Interactions, 46:189–200.
Menné T, Andersen KE, Kaaber K, Osmundsen PE, Andersen JR, Yding F, Valeur G (1987) An overlooked contact sensitizer? Contact Dermatitis, 16:9–10.
Mitchell B, Gerdes RA (1973) Mutagenic effects of sodium and stannous fluoride upon Drosophila melanogaster. Fluoride, 6(2):113–117.
Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B, Zeiger E (1986) Salmonella mutagenicity tests: II. Results from the testing of 270 chemicals. Environmental Mutagenesis, 8(Suppl. 7):1–119.
Myhr BC, Caspary WJ (1991) Chemical mutagenesis at the thymidine kinase locus in L5178Y mouse lymphoma cells: results for 31 coded compounds in the National Toxicology Program. Environmental and Molecular Mutagenesis, 18:51–83.
NAS (1977) Tin. In: Drinking water and health. Washington, DC, National Academy of Sciences, National Academy Press, pp. 292–296, 315.
Nehring P (1972) Tin in peach preserves. Die Industrielle Obst- und Gemüseverwertung, 57:489–492.
Neil TK, Abraham NG, Levere RD, Kappas A (1995) Differential heme oxygenase induction by stannous and stannic ions in the heart. Journal of Cell Biochemistry, 57:409–414.
Nielsen FH (1984) Ultratrace elements in nutrition. Annual Review of Nutrition, 4:21–41.
Nielsen NH, Skov L (1998) Occupational allergic contact dermatitis in a patient with a positive patch test to tin. Contact Dermatitis, 39:99–100.
NIOSH (1994a) Method No. 7300: Elements by ICP. In: Schlecht PC, O’Connor PF, eds. NIOSH manual of analytical methods, 4th ed. Cincinnati, OH, US Department of Health and Human Services, National Institute for Occupational Safety and Health (DHHS (NIOSH) Publication 94-113) (http://www.cdc.gov/niosh/nmam/).
NIOSH (1994b) 1st Supplement Publication 96-135, 2nd Supplement Publication 98-119, 3rd Supplement Publication 2003-154. In: Schlecht PC, O’Connor PF, eds. NIOSH manual of analytical methods, 4th ed. Cincinnati, OH, US Department of Health and Human Services, National Institute for Occupational Safety and Health (DHHS (NIOSH) Publication 94-113) (http://www.cdc.gov/niosh/nmam/).
Nishioka H (1975) Mutagenic activities of metal compounds in bacteria. Mutation Research, 31:185–189.
Nriagu JO, Pacyna JM (1988) Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature, 333:134–139.
NTP (1982) Carcinogenesis bioassay of stannous chloride (CAS No.
Nunnelley LL, Smythe WR, Alfrey AC, Ibels LS (1978) Uremic hyperstannum: elevated tissue tin levels associated with uremia. Journal of Laboratory and Clinical Medicine, 91:72–75.
Nuyts GD, Van Vlem E, Thys J, De Leersnjider D, D’Haese PC, Elseviers MM, De Broe ME (1995) New occupational risk factors for chronic renal failure. Lancet, 346:7–11.
Ogoshi K, Kurumatani N, Aoki Y, Moriyama T, Nanzai T (1981) Decrease in compressive strength of the femoral bone in rats administered stannous chloride for a short period. Toxicology and Applied Pharmacology, 58:331–332.
Olivier P, Marzin D (1987) Study of the genotoxic potential of 48 inorganic derivatives with the SOS chromotest. Mutation Research, 189:263–269.
Omori Y, Takanaka A, Tanaka S, Ikeda Y (1973) Experimental studies on toxicity of tin in canned orange juice. Journal of the Food Hygiene Society of Japan, 14:69–74 [cited in JECFA, 2001].
Oppenheimer BS, Oppenheimer ET, Danishefsky I, Stout AP (1956) Carcinogenic effect of metals in rodents. Cancer Research, 16:439–441.
Oresegun MO, Babalola AI (1990) Occupational radiation exposure associated with milling of Th-U-rich Sn ore in Nigeria. Health Physics, 58:213–215.
Oyanguren H, Haddad R, Maass H (1958) Stannosis. Industrial Medicine & Surgery, 27:427–431.
Park J, Presley BJ (1997) Trace metals contamination of sediments and organisms from the Swan Lake area of Galveston Bay. Environmental Pollution, 98(2):209–221.
Paschal DC, Ting BG, Morrow JC, Pirkle JL, Jackson RJ, Sampson EJ, Miller DT, Caldwell KL (1998) Trace metals in urine of United States residents: reference range concentrations. Environmental Research, 76:53–59.
Pawlik-Skowronska B, Kaczorowska R, Skowronski T (1997) The impact of inorganic tin on the planktonic cyanobacterium Synechocystis aquatilis: The effect of pH and humic acid. Environmental Pollution, 97(1–2):65–69.
Pedley FG (1927) Chronic poisoning by tin and its salts. Journal of Industrial Hygiene, 9:43–47.
Pekelharing HLM, Lemmens AG, Beynen AC (1994) Iron, copper and zinc status in rats fed on diets containing various concentrations of tin. British Journal of Nutrition, 71:103–109.
Pelikan Z, Halacka K, Cerny E (1968) Acute toxic effects of stannous chloride on white mice. Science and Medicine, 41:351–356.
Pendergrass EP, Pryde AW (1948) Benign pneumoconiosis due to tin oxide. A case report with experimental investigation of the radiographic density of the tin oxide dust. Journal of Industrial Hygiene and Toxicology, 30:119–123.
Perring L, Basic-Dvorzak M (2002) Determination of total tin in canned food using inductively coupled plasma atomic emission spectroscopy. Analytical and Bioanalytical Chemistry, 374:235–243.
Popescu HI, Lessem J, Erjavec M, Fuger GF (1984) In vivo labelling of RBC with 99mTc for blood pool imaging using different stannous radiopharmaceuticals. European Journal of Nuclear Medicine, 9:295–299.
Prival MJ, Simmon VF, Mortelmans KE (1991) Bacterial mutagenicity testing of 49 food ingredients gives very few positive results. Mutation Research, 260:321–329.
Qiao YL, Taylor PR, Yao SX, Schatzkin A, Mao BL, Lubin J, Rao JY, McAdams M, Xuan XZ, Li JY (1989) Relation of radon exposure and tobacco use to lung cancer among tin miners in Yunnan Province, China. American Journal of Industrial Medicine, 16:511–521.
Qiao YL, Taylor PR, Yao SX, Erozan YS, Luo XC, Barrett MJ, Yan QY, Giffen CA, Huang SQ, Maher MM, Forman MR, Tockman MS (1997) Risk factors and early detection of lung cancer in a cohort of Chinese tin miners. Annals of Epidemiology, 7:533–541.
Rader JI, Hight SC, Capar SG (1990) Copper depletion in Long-Evans rats fed inorganic tin. Journal of Trace Elements in Experimental Medicine, 3:193–202.
Rains TC (1982) Atomic absorption spectrometry. In: Minear RA, Keith LH, eds. Water analysis. Vol. II. Inorganic species. New York, NY, Academic Press, pp. 235–273.
Rajan B, Alesbury R, Carton B, Gerin M, Litske H, Marquardt H, Olsen E, Scheffers T, Stamm R, Woldbaek T (1997) European proposal for core information for the storage and exchange of workplace exposure on chemical agents. Applied Occupational and Environmental Hygiene, 12:31–39.
Rammelsberg P, Pevny I (1986) Metall-Allergien. Epicutantestergebnisse von 1981 bis 1984. Dermatosen, 34:160–162 (with English summary).
Ramonaityte DT (2001) Copper, zinc, tin and lead in canned evaporated milk, produced in Lithuania: the initial content and its change at storage. Food Additives and Contaminants, 18(1):31–37.
Rao SA, Knobel J, Collier BD, Isitman AT (1986) Effect of Sn(II) ion concentration and heparin on technetium-99m red blood cell labeling. Journal of Nuclear Medicine, 27:1202–1206.
Reicks M, Rader JI (1990) Effects of dietary tin and copper on rat hepatocellular antioxidant protection. Proceedings of the Society of Experimental Biology and Medicine, 195:123–128.
Rinehart RD, Yanagisawa Y (1993) Paraoccupational exposures to lead and tin carried by electric cable splicers. American Industrial Hygiene Association Journal, 54:593–599.
Robertson AJ (1960) Pneumoconiosis due to tin oxide. In: King EJ, Fletcher CM, eds. Symposium on industrial pulmonary diseases. Boston, MA, Little Brown, pp. 168–184.
Robertson AJ (1964) The romance of tin. Lancet, 1:1229–1237, 1289–1293.
Robertson AJ, Whitaker PH (1955) Radiological changes in pneumoconiosis due to tin oxide. Journal of the Faculty of Radiologists, 6:224–233.
Robertson AJ, Rivers D, Nagelschmidt G, Duncumb P (1961) Stannosis: Benign pneumoconiosis due to tin oxide. Lancet, 1:1089–1093.
Roe FJC, Boyland E, Millican K (1965) Effects of oral administration of two tin compounds to rats over prolonged periods. Food and Cosmetics Toxicology, 3:277–280.
Rolla G, Amsbaugh SM, Monell-Torrens E, Ellingsen JE, Afseth J, Ciardi JE, Bowen WH (1983) Effect of topical application of stannous fluoride, stannous chloride and stannous tartrate on rat caries. Scandinavian Journal of Dental Research, 91:351–355.
Rudolph H, Alfrey AC, Smythe WR (1973) Muscle and serum trace element profile in uremia. Transactions of the American Society for Artificial Internal Organs, 19:456–465.
Rykke M, Ellingsen JE, Sonju T (1991) Chemical analysis and scanning electron microscopy of acquired pellicle formed in vivo on stannous fluoride treated enamel. Scandinavian Journal of Dental Research, 99:205–211.
Sacerdoti D, Escalante B, Abraham NG, McGiff JC, Levere RD, Schwartzman ML (1989) Treatment with tin prevents the development of hypertension in spontaneously hypertensive rats. Science, 243:388–390.
Sardana MK, Kappas A (1987) Dual control mechanism for heme oxygenase: tin(IV)-protoporphyrin potently inhibits enzyme activity while markedly increasing content of enzyme protein in liver. Proceedings of the National Academy of Sciences of the United States of America, 84:2464–2468.
Sauvant MP, Pepin D, Groliere CA, Bohatier J (1995) Effects of organic and inorganic substances on the cell proliferation of L-929 fibroblasts and Tetrahymena pyriformis GL protozoa used for toxicological bioassays. Bulletin of Environmental Contamination and Toxicology, 55(2):171–178.
Savolainen H, Valkonen S (1986) Dose-dependent brain tin concentration in rats given stannous chloride in drinking water. Toxicology Letters, 30:35–39.
Schafer EW, Bowles WA (1985) Acute oral toxicity and repellency of 933 chemicals to house and deer mice. Archives of Environmental Contamination and Toxicology, 14:111–129.
Schafer SG, Femfert U (1984) Tin — a toxic heavy metal? A review of the literature. Regulatory Toxicology and Pharmacology, 4:57–69.
Schramel P, Wendler I, Angerer J (1997) The determination of metals (antimony, bismuth, lead, cadmium, mercury, palladium, platinum, tellurium, thallium, tin and tungsten) in urine samples by inductively coupled plasma–mass spectrometry. International Archives of Occupational and Environmental Health, 69:219–223.
Schroeder HA, Balassa JJ (1967) Arsenic, germanium, tin and vanadium in mice: effects on growth, survival and tissue levels. Journal of Nutrition, 92:245–252.
Schroeder HA, Balassa JJ, Tipton I (1964) Abnormal trace elements in man: Tin. Journal of Chronic Diseases, 17:483–502.
Schroeder HA, Kanisawa M, Frost DV, Mitchener M (1968) Germanium, tin and arsenic in rats: effects on growth, survival, pathological lesions and life span. Journal of Nutrition, 96:37–45.
Schuler P, Cruz E, Guijon C, Maturana V, Valenzuela A (1958) Stannosis. Benign pneumoconiosis owing to inhalation of tin dust and fume. Industrial Medicine & Surgery, 27:432–435.
Schwarz K (1974a) New essential trace elements (Sn, V, F, Si): Progress report and outlook. In: Hoekstra WG, ed. Trace element metabolism in animals — 2. Proceedings of the second international symposium on trace element metabolism in animals, held in Madison, Wisconsin, on 18–22 June 1973. Baltimore, MD, University Park Press, pp. 355–380.
Schwarz K (1974b) Proceedings: Recent dietary trace element research, exemplified by tin, fluorine, and silicon. Federation Proceedings, 33:1748–1757.
Schwarz K, Milne DB, Vinyard E (1970) Growth effects of tin compounds in rats maintained in a trace element-controlled environment. Biochemical and Biophysical Research Communications, 40:22–29.
Seby F, Potin-Gautier M, Giffaut E, Donard OFX (2001) A critical review of thermodynamic data for inorganic tin species. Geochimica et Cosmochimica Acta, 65(18):3041–3053.
Seidel SL, Hodge VF, Goldberg ED (1980) Tin as an environmental pollutant. Thalassia Jugoslavica, 16:209–223.
Senesi GS, Baldassarre G, Senesi N, Radina B (1999) Trace element inputs into soils by anthropogenic activities and implications for human health. Chemosphere, 39(2):343–377.
Shargel L, Masnyj J (1981) Effect of stannous fluoride and sodium fluoride on hepatic mixed-function oxidase activities in the rat. Toxicology and Applied Pharmacology, 59:452–456.
Shelby MD, Erexson GL, Hook GJ, Tice RR (1993) Evaluation of a three-exposure mouse bone marrow micronucleus protocol: Results with 49 chemicals. Environmental and Molecular Mutagenesis, 21:160–179.
Sherlock JC, Smart GA (1984) Tin in foods and the diet. Food Additives and Contaminants, 1:277–282.
Sherman LR, Cardarelli NF (1988) Tin in the thymus gland of dogs. Thymus, 12:131–134.
Sherman LR, Bilgicer KI, Cardarelli NF (1985) Analyses of tin in mouse and human organs. Journal of Nutrition, Growth and Cancer, 2:107–115.
Sherman LR, Masters J, Peterson R, Levine S (1986) Tin concentration in the thymus glands of rats and mice and its relation to the involution of the gland. Journal of Analytical Toxicology, 10:6–9.
Shimbo S, Hayase A, Murakami M, Hatai I, Higashikawa K, Moon CS, Zhang ZW, Watanabe T, Iguchi H, Ikeda M (1996) Use of a food composition database to estimate daily dietary intake of nutrient or trace elements in Japan, with reference to its limitation. Food Additives and Contaminants, 13:775–786 [cited in JECFA, 2001].
Silva CR, Oliveira MBN, Melo SF, Dantas FJS, de Mattos JCP, Bezerra RJAC, Caldeira-de-Araujo A, Duatti A, Bernardo-Filho M (2002) Biological effects of stannous chloride, a substance that can produce stimulation or depression of the central nervous system. Brain Research Bulletin, 59(3):213–216.
Silva FC, Fonseca AS, Correa AS, Lee CC, De Araujo AC, Valsa JO, Bernardo-Filho M, Favre A (1994) Near-UV light protection effect against lethality induced by stannous chloride in Escherichia coli. Microbios, 79:241–244.
Singh NP (1983) Induction of reverse mutation and mitotic gene conversion by some metal compounds in Saccharomyces cerevisiae. Mutation Research, 117:149–152.
Sinkeldam EJ, Koeter HBWM, Willems ML (1979) Multigeneration study with stannous chloride in rats. Zeist, Central Institute for Nutrition and Food Research (Report No. R6281; unpublished report submitted to WHO) [cited in JECFA, 1982].
Sinkeldam EJ, Dreef-van der Meulen EJ, Willems ML (1981) Chronic (115 week) oral toxicity study with stannous chloride in rats. Zeist, Central Institute for Nutrition and Food Research (Report No. R6372; unpublished report submitted to WHO) [cited in JECFA, 1982].
Sluis-Cremer GK, Thomas RG, Goldstein B, Solomon A (1989) Stannosis. A report of 2 cases. South African Medical Journal, 75:124–126.
Solomons NW, Marchini JS, Duarte-Favaro RM, Vannuchi H, de Oliveira JED (1983) Studies on the bioavailability of zinc in humans: intestinal interaction of tin and zinc. American Journal of Clinical Nutrition, 37:566–571.
Spencer GE, Wycoff WC (1954) Benign tin oxide pneumoconiosis. Archives of Industrial Hygiene and Occupational Medicine, 10:295–297.
Stanley JS (1986) Broad scan analysis of the FY82 national human adipose tissue survey specimens. Vol. I. Executive summary. Report to Office of Toxic Substances, US Environmental Protection Agency, Washington, DC, by Midwest Research Institute, Kansas City, MO (EPA-566/5-86-035).
Stewart JH , Lassiter DV (2001) Germanium, tin, and copper. In: Patty’s toxicology, 5th ed. New York, NY, John Wiley & Sons, pp. 567–610.
Stone OJ, Willis CJ (1968) The effect of stannous fluoride and stannous chloride on inflammation. Toxicology and Applied Pharmacology, 13:332–338.
Stoner GD, Shimkin MB, Troxell MC, Thompson TL, Terry LS (1976) Test for carcinogenicity of metallic compounds by the pulmonary tumor response in strain A mice. Cancer Research, 36:1744–1747.
Sullivan MF, Miller BM, Goebel JC (1984) Gastrointestinal absorption of metals (51Cr, 65Zn, 95mTc, 109Cd, 113Sn, 147Pm, and 238Pu) by rats and swine. Environmental Research, 35:439–453.
Svatun B, Gjermo P, Eriksen HM, Rolla G (1977) A comparison of the plaque-inhibiting effect of stannous fluoride and chlorhexidine. Acta Odontologica Scandinavica, 35:247–250.
Svensson V (1975) Tin poisoning caused by canned peaches. Hygien och miljö, 6:25–27.
Sweet CW, Vermette SJ, Landsberger S (1993) Sources of toxic trace elements in urban air in Illinois. Environmental Science & Technology, 27(12):2502–2510.
Swierenga SHH, McLean JR (1983) Genotoxicity of stannous chloride in mammalian cell tests. In: Brown SS, Savory J, eds. Chemical toxicology and clinical chemistry of metals. London, Academic Press.
Talukder G, Ghosh BB, Sharma A (1989) Comparative clastogenic effects of organic and inorganic tin salts in vitro. Environmental and Molecular Mutagenesis, 14(Suppl. 15):197 (abstract).
Taylor D, Maddock BG, Mance G (1985) The acute toxicity of nine "grey list" metals (arsenic, boron, chromium, copper, lead, nickel, tin, vanadium and zinc) to two marine fish species: dab (Limanda limanda) and grey mullet (Chelon labrosus). Aquatic Toxicology, 7:135–144.
Taylor PR, Qiao YL, Schatzkin A, Yao SX, Lubin J, Mao BL, Rao JY, McAdams M, Xuan XZ, Li JY (1989) Relation of arsenic exposure to lung cancer among tin miners in Yunnan Province, China. British Journal of Industrial Medicine, 46:881–886.
Teraoka H (1981) Distribution of 24 elements in the internal organs of normal males and the metallic workers in Japan. Archives of Environmental Health, 36:155–165.
Theuer RC, Mahoney AW, Sarett HP (1971) Placental transfer of fluoride and tin in rats given various fluoride and tin salts. Journal of Nutrition, 101:525–532.
Thompson KC, Thomerson DR (1974) Atomic absorption studies on the determination of antimony, arsenic, bismuth, germanium, lead, selenium, tellurium and tin by utilizing the generation of covalent hydrides. Analyst, 99:595–601.
Thompson SE, Burton CA, Quinn DJ (1972) Concentration factors of chemical elements in edible aquatic organisms. Livermore, CA, University of California, Lawrence Livermore Laboratory, Biomedical Division.
Thomulka KW, Lange JH (1996) A mixture toxicity study employing combinations of tributyltin chloride, dibutyltin dichloride, and tin chloride using the marine bacterium Vibrio harveyi as the test organism. Ecotoxicology and Environmental Safety, 34(1):76–84.
Tipton IH, Cook MJ (1963) Trace elements in human tissue. Part II. Adult subjects from the United States. Health Physics, 9:103–145.
Tipton IH, Stewart PI, Dickson J (1969) Patterns of elemental excretion in long term balance studies. Health Physics, 16:455–462.
Tomlinson G, Mutus B, McLennan I (1981) Activation and inactivation of acetylcholinesterase by metal ions. Canadian Journal of Biochemistry, 59:728–735 [cited in Savolainen & Valkonen, 1986].
Tripathy NK, Wurgler FE, Frei H (1990) Genetic toxicity of six carcinogens in the Drosophila wing spot test. Mutation Research, 242:169–180.
Tsangaris JM, Williams DR (1992) Tin in pharmacy and nutrition. Applied Organometallic Chemistry, 6:3–18.
Tsuji S, Tonogai Y, Ito Y, Kanoh S (1986) [The influence of rearing temperatures on the toxicity of various environmental pollutants for killifish (Oryzias latipes).] Eisei Kagaku, 32(1):46–53 (in Japanese).
Tsukamoto I, Yoshinaga T, Sano S (1979) The role of zinc with special reference to the essential thiol groups in delta-aminolevulinic acid dehydratase of bovine liver. Biochimica et Biophysica Acta, 570:167–178.
Tugrul S, Balkas TI, Goldberg ED (1983) Methyltins in the marine environment. Marine Pollution Bulletin, 14:297–303.
UK MAFF (2000) MAFF UK — Duplicate diet study of vegetarians — dietary exposures to 12 metals and other elements. London, United Kingdom Ministry of Agriculture, Fisheries and Food, Food Standards Agency (Food Surveillance Information Sheet No. 193; http://www.foodstandards.gov.uk/science/surveillance/maffinfo/2000/maff-2000-193) [cited in JECFA, 2001].
US EPA (1982) Eleventh report of the interagency testing committee to the administrator; receipt of reports and request for comments regarding priority list of chemicals. Federal Register, 47:54626–54636.
US EPA (1986) Tin (atomic absorption, direct aspiration) — method 7870. In: Test methods for evaluating solid waste. Washington, DC, US Environmental Protection Agency, Office of Solid Waste and Emergency Response.
US EPA (1992) Atomic absorption methods — method 7000A revision 1. Washington, DC, US Environmental Protection Agency.
US EPA (1996) Inorganic analytes. In: Test methods for evaluating solid waste, physical/chemical methods. Washington, DC, US Environmental Protection Agency (Report No. SW-846; http://www.epa.gov/SW-846/sw846.htm).
Valberg LS, Flanagan PR, Chamberlain MJ (1984) Effects of iron, tin and copper on zinc absorption in humans. American Journal of Clinical Nutrition, 40:536–541.
Valkirs AO, Seligman PF, Stang PM, Homer V, Lieberman SH, Vata G, Dooley CA (1986) Measurement of butyltin compounds in San Diego Bay. Marine Pollution Bulletin, 17:319–324.
van Dokkum W, de Vos PH, Muys T, Westra JA (1989) Minerals and trace elements in total diets in The Netherlands. British Journal of Nutrition, 61:7–15 [cited in JECFA, 2001].
van Netten C, Cann SAH, Morley DR, van Netten JP (2000) Elemental and radioactive analysis of commercially available seaweed. The Science of the Total Environment, 255(1–3):169–175.
Vannoort R, Cressey P, Silvers K (2000) 1997/98 New Zealand Total Diet Survey. Part 2: Elements. Wellington, New Zealand Ministry of Health [cited in JECFA, 2001].
van Vleet JF (1982) Amounts of twelve elements required to induce selenium–vitamin E deficiency in ducklings. American Journal of Veterinary Research, 43(5):851–857.
Versieck J, Vanballenberghe L (1991) Determination of tin in human blood serum by radiochemical neutron activation analysis. Analytical Chemistry, 63:1143–1146.
Veysseyre A, Moutard K, Ferrari C, Van de Velde K, Barbante C, Cozzi G, Capodaglio G, Boutron C (2001) Heavy metals in fresh snow collected at different altitudes in the Chamonix and Maurienne valleys, French Alps: initial results. Atmospheric Environment, 35(2):415–425.
Walsh GE, McLaughlan LL, Lores EM, Louie MK, Deans CH (1985) Effects of organotins on growth and survival of 2 marine diatoms, Skeletonema costatum and Thalassiosira pseudonana. Chemosphere, 14(3–4):383–392.
Walters M, Roe FJC (1965) A study of the effects of zinc and tin administered orally to mice over a prolonged period. Food and Cosmetics Toxicology, 3:271–276 [cited in JECFA, 1982].
Warburton S, Udler W, Ewert RM, Haynes WS (1962) Outbreak of foodborne illness attributed to tin. Public Health Reports, 77:798–800.
Wedepohl KH, Correns CW, Shaw DM (1978) Behavior during weathering and rock alteration. In: Handbook of geochemistry. Vol II. New York, NY, Springer-Verlag.
Westrum B, Thomassen Y (2002) 130. Tin and inorganic tin compounds. Prepared by the Nordic Expert Group for Criteria Documentation of Health Risks from Chemicals and the Dutch Expert Committee on Occupational Standards. Published on behalf of the Nordic Council of Ministers by the National Institute for Working Life, Stockholm (Arbete och Hälsa 2002:10; http://www.arbetslivsinstitutet.se/publikationer/en/detaljerad.asp?ID=1123).
Wiberg N, Holleman AF, Wiberg E, eds. (2001) Holleman-Wiberg’s inorganic chemistry. New York, NY, Academic Press.
Widdowson EM (1992) Absorption, excretion and storage of trace elements: studies over 50 years. Food Chemistry, 43:203–207.
Wong PTS, Chau YK, Kramar O, Bengert GA (1982) Structure–toxicity relationship of tin compounds on algae. Canadian Journal of Fisheries and Aquatic Sciences, 39(3):483–488.
Yamaguchi M, Okada S (1980) Effect of stannous chloride on rat femur. Toxicology Letters, 6:177–180.
Yamaguchi M, Yamamoto T (1978) Effect of tin on calcium content in the bile of rats. Toxicology and Applied Pharmacology, 45:611–616.
Yamaguchi M, Naganawa M, Yamamoto T (1976) Decreased gastric secretion in rats treated with stannous chloride. Toxicology and Applied Pharmacology, 36:199–200.
Yamaguchi M, Suzuki H, Yamamoto T (1978a) Suppression of insulin secretion by oral administration of stannous chloride. Toxicology Letters, 2:111–113.
Yamaguchi M, Suzuki H, Yamamoto T (1978b) Decrease of phosphorylase activity in the liver of rats orally administered stannous chloride. Toxicology Letters, 2:171–174.
Yamaguchi M, Endo T, Yamamoto T (1978c) Action of tin on the secretory effect of agents which stimulate gastric acid secretion in rats. Toxicology and Applied Pharmacology, 43:417–423.
Yamaguchi M, Kubo Y, Yamamoto T (1979) Inhibitory effect of tin on intestinal calcium absorption in rats. Toxicology and Applied Pharmacology, 47:441–444.
Yamaguchi M, Kitade M, Okada S (1980a) The oral administration of stannous chloride to rats. Toxicology Letters, 5:275–278.
Yamaguchi M, Saito R, Okada S (1980b) Dose–effect of inorganic tin on biochemical indices in rats. Toxicology, 16:267–273.
Yamaguchi M, Sugii K, Okada S (1981a) Changes of mineral composition and its related enzyme activity in the femur of rats orally administered stannous chloride. Journal of Pharmacobio-dynamics, 4:874–878.
Yamaguchi M, Sugii K, Okada S (1981b) Inorganic tin in the diet affects the femur in rats. Toxicology Letters, 9:207–209.
Yamaguchi M, Sugii K, Okada S (1982a) Inhibition of collagen synthesis in the femur of rats orally administered stannous chloride. Journal of Pharmacobiodynamics, 5:388–393.
Yamaguchi M, Sugii K, Okada S (1982b) Tin decreases femoral calcium independently of calcium homeostasis in rats. Toxicology Letters, 10:7–10.
Yemenicioglu S, Saydam C, Salihoglu I (1987) Distribution of tin in the northeastern Mediterranean. Chemosphere, 16:429–443.
Yokoi K, Kimura M, Itokawa Y (1990) Effect of dietary tin deficiency on growth and mineral status in rats. Biological Trace Element Research, 24:223–231.
Ysart G, Miller P, Crews H, Robb P, Baxter M, L’Argy CD, Lofthouse S, Sargent C, Harrison N (1999) Dietary exposure estimates of 30 elements from the Total Diet Study. Food Additives and Contaminants, 16:391–403 [cited in JECFA, 2001].
Yu S, Beynen C (1995) High tin intake reduces copper status in rats through inhibition of copper absorption. British Journal of Nutrition, 73:863–869.
Zareba G, Chmielnicka J (1985) Aminolevulinic acid dehydratase activity in the blood of rats exposed to tin and zinc. Ecotoxicology and Environmental Safety, 9:40–46.
Zareba G, Chmielnicka J (1989) Effects of tin and lead on organ levels of essential minerals in rabbits. Biological Trace Element Research, 20:233–242.
Zareba G, Chmielnicka J (1992) Disturbances in heme biosynthesis in rabbits after administration per os of low doses of tin or lead. Biological Trace Element Research, 34:115–122.
|
AAS |
atomic absorption spectroscopy |
|
AES |
atomic emission spectroscopy |
|
ALA |
delta-aminolaevulinic acid |
|
ALAD |
delta-aminolaevulinic acid dehydratase |
|
ATSDR |
Agency for Toxic Substances and Disease Registry |
|
CANCERLIT |
Cancer Literature Online |
|
CAS |
Chemical Abstracts Service |
|
CCRIS |
Chemical Carcinogenesis Research Information System |
|
CICAD |
Concise International Chemical Assessment Document |
|
DART |
Developmental & Reproductive Toxicology |
|
DNA |
deoxyribonucleic acid |
|
EC50 |
median effective concentration |
|
EDTA |
ethylenediaminetetraacetic acid |
|
EHC |
Environmental Health Criteria monographs |
|
EPA |
Environmental Protection Agency (USA) |
|
ETIC |
Environmental Teratology Information Center |
|
FAO |
Food and Agriculture Organization of the United Nations |
|
GENE-TOX |
Genetic Toxicology |
|
HSDB |
Hazardous Substances Data Bank |
|
IARC |
International Agency for Research on Cancer |
|
ICP |
inductively coupled plasma |
|
ICRP |
International Commission on Radiological Protection |
|
Ig |
immunoglobulin |
|
ILO |
International Labour Organization |
|
IPCS |
International Programme on Chemical Safety |
|
IRIS |
Integrated Risk Information System (USA) |
|
ISO |
International Organization for Standardization |
|
JECFA |
Joint FAO/WHO Expert Committee on Food Additives |
|
JMPR |
Joint FAO/WHO Meeting on Pesticide Residues |
|
LC50 |
median lethal concentration |
|
LD50 |
median lethal dose |
|
LOAEL |
lowest-observed-adverse-effect level |
|
LOEL |
lowest-observed-effect level |
|
MS |
mass spectrometry |
|
NIOSH |
National Institute for Occupational Safety and Health (USA) |
|
NOAEL |
no-observed-adverse-effect level |
|
NOEC |
no-observed-effect concentration |
|
NOEL |
no-observed-effect level |
|
NTP |
National Toxicology Program (USA) |
|
OECD |
Organisation for Economic Co-operation and Development |
|
PIM |
Poison Information Monograph |
|
PMTDI |
provisional maximum tolerable daily intake |
|
PTWI |
provisional tolerable weekly intake |
|
RTECS |
Registry of Toxic Effects of Chemical Substances |
|
S9 |
metabolic activation fraction |
|
SCE |
sister chromatid exchange |
|
SD |
standard deviation |
|
SIDS |
Screening Information Data Set |
|
TSCA |
Toxic Substances Control Act |
|
UNEP |
United Nations Environment Programme |
|
USA |
United States of America |
|
WHO |
World Health Organization |
Three publications served as source documents for this CICAD.
Westrum & Thomassen (2002)
The first source document was prepared by the Nordic Expert Group for Criteria Documentation of Health Risks from Chemicals and the Dutch Expert Committee on Occupational Standards and considered the literature identified as of March 2002. This publication was a result of an agreement between the Nordic Expert Group for Criteria Documentation of Health Risks from Chemicals and the Dutch Expert Committee on Occupational Standards to write joint scientific criteria documents that can be used by the national regulatory authorities in both the Netherlands and the Nordic countries. The report (Arbete och Hälsa 2002:10) was authored by B. Westrum and Y. Thomassen and published on behalf of the Nordic Council of Ministers by the National Institute for Working Life (Arbetslivinstitut), Stockholm, Sweden. Hard copies are available from the institute. Electronic copies can be obtained at http://www.arbetslivsinstitutet.se/.
***
JECFA (2001)
The second source document was the monograph prepared by JECFA, published in 2001 following the 55th meeting held in 2000. The first draft of this monograph was prepared by Dr J.B. Greig, Food Standards Agency, London, United Kingdom, and Dr J.A. Pennington, Division of Nutrition Research Coordination, National Institutes of Health, Bethesda, Maryland, USA. The monograph was published in Safety evaluation of certain food additives and contaminants (WHO Food Additive Series 46). An electronic copy can be obtained at http://www.inchem.org/documents/jecfa/jecmono/v46je01.htm.
The list of experts at the 55th JECFA meeting follows:
Members
Ms J. Baines, Australia New Zealand Food Authority, Australia
Professor J.R. Bend, University of Western Ontario, Canada
Dr J. Chen, Chinese Academy of Preventive Medicine, China
Dr S.M. Dagher, American University of Beirut, Lebanon
Dr C.E. Fisher, Hatfield, Hertfordshire, England
Dr D.G. Hattan, Food and Drug Administration, USA (Rapporteur)
Dr Y. Kawamura, National Institute of Health Sciences, Japan
Dr A.G.A.C. Knaap, National Institute of Public Health and the Environment, The Netherlands
Dr P.M. Kuznesof, Food and Drug Administration, USA (Vice-Chairman)
Dr J.C. Larsen, Ministry of Food, Agriculture and Fisheries, Denmark
Mrs I. Meyland, Ministry of Food, Agriculture and Fisheries, Denmark (Rapporteur)
Dr G. Pascal, National Institute for Agricultural Research, France
Dr A. Pintér, Director, National Institute of Environmental Health, Hungary
Professor R. Walker, University of Surrey, England (Chairman)
Secretariat
Dr P.J. Abbott, Australia New Zealand Food Authority, Australia (WHO Temporary Adviser)
Dr L.M. Barraj, Novigen Sciences Inc., USA (WHO Temporary Adviser)
Dr David C. Bellinger, Boston Children’s Hospital Neuroepidemiology Unit, USA (WHO Temporary Adviser)
Dr M. Bolger, Food and Drug Administration, USA (WHO Temporary Adviser)
Ms M.L. Costarrica, FAO, Italy (FAO Joint Secretary)
Dr M. DiNovi, Food and Drug Administration, USA (WHO Temporary Adviser)
Dr R.L. Ellis, FAO, Italy
Dr R. Goyer, Chapel Hill, NC, USA (WHO Temporary Adviser)
Dr J. Greig, Food Standards Agency, England (WHO Temporary Adviser)
Mr E.F.F. Hecker, Ministry of Agriculture, Nature Management and Fisheries, The Netherlands (WHO Temporary Adviser)
Dr J.L. Herrman, WHO, Switzerland (WHO Joint Secretary)
Dr J.H. Hotchkiss, Cornell University, USA (FAO Consultant)
Dr F. Kayama, Jichi Medical School, Japan (WHO Temporary Adviser)
Dr A. Mattia, Food and Drug Administration, USA (WHO Temporary Adviser)
Dr G. Moy, WHO, Switzerland
Dr I.C. Munro, CanTox Health Sciences International, Canada (WHO Temporary Adviser)
Dr A. Nishikawa, National Institute of Health Sciences, Japan (WHO Temporary Adviser)
Dr J.A. Pennington, National Institutes of Health, USA (FAO Consultant)
Dr M.V. Rao, Dubai Food and Environment Laboratory, United Arab Emirates (FAO Consultant)
Professor A.G. Renwick, University of Southampton, England (WHO Temporary Adviser)
Dr S. Resnik, Department of Industry, Argentina (FAO Consultant)
Dr H. Sakurai, Japan Industrial Safety and Health Association, Japan (WHO Temporary Adviser)
Professor I.G. Sipes, University of Arizona, USA (WHO Temporary Adviser)
Dr G.J.A. Speijers, National Institute of Public Health and Environmental Protection, The Netherlands (WHO Temporary Adviser)
Ms E. Vavasour, Health Canada, Canada (WHO Temporary Adviser)
Dr P.J.P. Verger, National Institute for Agricultural Research, France (FAO Consultant)
Mrs H. Wallin, National Food Administration, Finland (FAO Consultant)
Dr D.B. Whitehouse, Bowdon, Cheshire, England (FAO Consultant)
***
ATSDR (2003)
The third source document was the draft Toxicological profile for tin and compounds (update), prepared by ATSDR through a contract with the Syracruse Research Corporation. Copies of the profile can be obtained from the ATSDR web site (http://www.atsdr.cdc.gov/toxprofiles/) or from:
Division of Toxicology
Agency for Toxic Substances and Disease Registry
Division of Toxicology/Toxicology Information Branch
US Department of Health and Human Services
1600 Clifton Road NE, Mailstop E-29
Atlanta, Georgia 30333
USA
A peer review panel was assembled for tin and compounds. The panel consisted of the following members:
Michael Aschner, PhD, Wake Forest University School of Medicine, Winston-Salem, North Carolina
Olen Brown, PhD, University of Missouri-Columbia, Columbia, Missouri
Bruce Jarnot, PhD, DABT, American Petroleum Institute, Washington, DC
These experts collectively have knowledge of tin and its compounds’ physical and chemical properties, toxicokinetics, key health end-points, mechanisms of action, human and animal exposure, and quantification of risk to humans. All reviewers were selected in conformity with the conditions for peer review specified in Section 104(I)(13) of the Comprehensive Environmental Response, Compensation, and Liability Act, as amended. Scientists from the ATSDR reviewed the peer reviewers’ comments and determined which comments would be included in the profile. A listing of the peer reviewers’ comments not incorporated in the profile, with a brief explanation of the rationale for their exclusion, exists as part of the administrative record for this compound. A list of databases reviewed and a list of unpublished documents cited are also included in the administrative record. The citation of the peer review panel should not be understood to imply its approval of the profile’s final content. The responsibility for the content of this profile lies with the ATSDR.
***
In December 2003, a comprehensive literature search was conducted by Toxicology Advice & Consulting Ltd in order to identify critical data published since publication of the source documents. Databases searched included:
Critical papers on mammalian toxicity were purchased, assessed, and included in the CICAD, where appropriate, by Toxicology Advice & Consulting Ltd.
The draft CICAD on tin and inorganic tin compounds was sent for review to institutions and organizations identified by IPCS after contact with IPCS national Contact Points and Participating Institutions, as well as to identified experts. Comments were received from:
L. Alessio, Institute of Occupational Health, University of Brescia, Brescia, Italy
M. Baril, Institut de recherche Robert Sauvé en santé et en sécurité du travail, Montreal, Canada
R. Benson, US Environmental Protection Agency, Denver, CO, USA
M. Berlin, University of Lund, Lund, Sweden
S. Blunden, Tin Information Ltd, Uxbridge, United Kingdom
R. Chhabra, National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA
I. Desi, Department of Public Health, Budapest, Hungary
J. Donohue, US Environmental Protection Agency, Washington, DC, USA
L. Fishbein, Fairfax, VA, USA
P. Grandjean, University of Southern Denmark, Odense, Denmark
S. Hahn, Fraunhofer Institute for Toxicology and Experimental Medicine, Hanover, Germany
R. Hertel, Bundesinstitut für Risikobewertung, Berlin, Germany
G. Koennecker, Fraunhofer Institute for Toxicology and Experimental Medicine, Hanover, Germany
National Institute for Occupational Safety and Health, Cincinnati, OH, USA
K. Nimmo, Tin Technology Ltd, St Albans, Hertfordshire, United Kingdom
M. Nordberg, Karolinska Institute, Stockholm, Sweden
H. Savolainen, Ministry of Social Affairs and Health, Tampere, Finland
J. Stauber, CSIRO Energy Technology, Menai, New South Wales, Australia
M.H. Sweeney, Hanoi, Viet Nam
S. Tao, US Food & Drug Administration, College Park, MD, USA
J.-P. Taverne, Association of European Producers of Steel for Packaging, Brussels, Belgium (on behalf of the Tin Inter-industry Working Group)
M. Vojtisek, National Institute of Public Health, Prague, Czech Republic
K. Ziegler-Skylakakis, European Commission, Luxembourg
Hanoi, Viet Nam
28 September – 1 October 2004
Members
Mr D.T. Bai, Centre of Environmental Protection & Chemical Safety, Institute of Industrial Chemistry, Hanoi, Viet Nam
Dr R. Chhabra, National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA
Mr P. Copestake, Toxicology Advice & Consulting Ltd, Surrey, United Kingdom
Dr C. De Rosa, Agency for Toxic Substances and Disease Registry, Centres for Disease Control and Prevention, Atlanta, GA, USA
Dr S. Dobson, Centre for Ecology & Hydrology, Monks Wood, Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr G. Dura, National Institute of Environmental Health of József Fodor Public Health Centre, Budapest, Hungary
Ms C.W. Fang, National Institute of Occupational Safety and Health Malaysia, Selangor, Malaysia
Dr L. Fishbein, Fairfax, VA, USA
Dr L. Fruchtengarten, Poison Control Center of Sao Paulo, Sao Paulo, Brazil
Dr C.L. Geraci, Document Development Branch, Centers for Disease Control and Prevention / National Institute for Occupational Safety and Health, Cincinnati, OH, USA
Dr H. Gibb, Sciences International, Alexandria, VA, USA
Dr R.F. Hertel, Federal Institute for Risk Assessment, Berlin, Germany
Mr P. Howe, Centre for Ecology & Hydrology, Monks Wood, Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr S. Ishimitsu, Division of Safety Information on Drug, Food and Chemicals, National Institute of Health Sciences, Tokyo, Japan
Dr J. Kielhorn, Fraunhofer Institute for Toxicology and Experimental Medicine, Hanover, Germany
Dr S. Kunarattanapruke, Food & Drug Administration, Ministry of Public Health, Nonthaburi, Thailand
Dr Y. Liang, Department of Occupational Health, Fudan University School of Public Health, Shanghai, China
Ms B. Meek, Existing Substances Division, Environmental Health Directorate, Health Canada, Ottawa, Ontario, Canada
Mr F.K. Muchiri, Directorate of Occupational Health and Safety Services, Nairobi, Kenya
Dr O. Sabzevari, Food and Drug Control Labs, Ministry of Health & Medical Education, Tehran, Islamic Republic of Iran
Dr J. Stauber, CSIRO Energy Technology, Menai, New South Wales, Australia
Dr M.H. Sweeney, US Embassy, Hanoi, Viet Nam
Mr P. Watts, Toxicology Advice & Consulting Ltd, Surrey, United Kingdom
Ms D. Willcocks, National Industrial Chemicals Notification and Assessment Scheme, Sydney, New South Wales, Australia
Dr K. Ziegler-Skylakakis, European Commission, Luxembourg
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
TIN (II) CHLORIDE DIHYDRATE ICSC:0738
TIN (IV) CHLORIDE (ANHYDROUS) ICSC:0953
TIN (II) CHLORIDE (ANHYDROUS) ICSC:0955
Ce CICAD4 concernant l’étain et les composés inorganiques de l’étain a été préparé conjointement par Toxicology Advice & Consulting Ltd et le Centre for Ecology & Hydrology. Ce CICAD s’appuie sur trois documents bibliographiques. Le premier de ces documents source a été rédigé par le Nordic Expert Group for Criteria Documentation of Health Risks from Chemicals ainsi que le Dutch Expert Committee on Occupational Standards et a tenu compte des publications identifiées jusqu’en mars 2002 (Westrum & Thomassen, 2002). Le deuxième de ces documents source est la monographie rédigée à l’occasion de la cinquante-cinquième réunion du Comité mixte FAO/OMS d’experts des Additifs alimentaires, publié en 2001 (JECFA, 2001). Le troisième document source est le projet remis à jour en 2003 de Toxicological profile for tin and compounds, élaboré par la Agency for Toxic Substances and Disease Registry des Etats-Unis d’Amérique (ATSDR, 2003). En décembre 2003, Toxicology Advice & Consulting Ltd et le Centre for Ecology & Hydrology ont procédé à une recherche bibliographique approfondie des bases de données en ligne pour identifier les dernières références bibliographiques. Des précisions sur l’examen par des pairs et l’existence de documents source figurent à l’Appendice 2. Concernant le présent CICAD, l’information sur l’examen par des pairs est fournie à l’Appendice 3. Ce CICAD a été approuvé en tant qu’évaluation internationale lors d’une réunion du Comité d’évaluation finale qui s’est tenue à Hanoï (Viet Nam) du 28 septembre au 1er octobre 2004. La liste des participants à cette réunion figure à l’Appendice 4. La fiche d’information internationale sur la sécurité chimique (ICSC) établie pour le chlorure d’étain (II), le chlorure d’étain (II) à deux molécules d’eau, le fluorure d’étain (II), l’oxyde d’étain (II), le chlorure d’étain (IV) et l’oxyde d’étain (IV) par le Programme international sur la sécurité chimique (IPCS, 2004a-f) est également reproduite dans ce document.
L’étain est un métal gris-blanc. Les composés inorganiques de l’étain les plus importants sont notamment les chlorures d’étain (II) et (IV), l’oxyde d’étain (II) et le fluorure d’étain (II), ainsi que les stannates de potassium et de sodium. Les oxydes d’étain 2+ et 4+, connus également sous le nom d’étain (II) et étain (IV), sont tous deux relativement stables.
La production annuelle mondiale d’étain est en lente augmentation depuis quelques années et a atteint 268 000 tonnes en 2003. Environ 10-15 % de ce tonnage représentent le métal extrait par récupération. Les pays producteurs d’étain les plus importants sont la Chine, l’Indonésie, le Pérou, la Bolivie, le Brésil et l’Australie; des quantités importantes sont également produites par la Malaisie et la Thaïlande. L’utilisation principale de l’étain, soit environ 34 % de la production mondiale annuelle, est la fabrication d’alliages utilisés pour la soudure avec des applications industrielles électriques/ électroniques et générales. L’étain est également très utilisé (25-30 % de la production) comme revêtement protecteur d’autres métaux, en particulier dans les récipients alimentaires. Le chlorure d’étain (II) est le composé inorganique le plus important commercialement et est surtout utilisé comme agent réducteur en synthèse organique et inorganique ainsi que dans la fabrication des vernis, des verres et des pigments métallisés. Le chlorure d’étain (IV) est utilisé en synthèse organique dans les matières plastiques, comme intermédiaire dans la fabrication des organostanniques et pour la production de films d’oxyde d’étain (IV) sur le verre. Le fluorure d’étain (II) est largement utilisé en dentisterie préventive.
L’étain peut être libéré dans l’atmosphère à partir de sources naturelles ou anthropiques. On le trouve dans de nombreux sols et il peut se retrouver dans les poussières soulevées par le vent, la circulation routière et les activités agricoles. Il existe d’autres sources naturelles moins importantes, notamment les feux de forêt et les émissions volcaniques. Des gaz, des poussières et des fumées contenant de l’étain peuvent être émis pendant les processus de soudage et de raffinage, les usages industriels de l’étain, l’incinération des déchets et la combustion des combustibles fossiles. La tension de vapeur de l’étain élémentaire est négligeable; l’étain et les composés inorganiques de l’étain ne sont pas volatils dans les conditions environnementales. Le chlorure d’étain (II) est soluble dans l’eau, les autres composés stanniques n’étant que peu solubles. Les composés de l’étain sont susceptibles de se répartir dans les sols et les sédiments. L’étain inorganique peut subir une oxydo-réduction, un échange de ligand et des réactions de précipitation dans l’environnement. La biométhylation de l’étain inorganique a été mise en évidence dans des cultures bactériennes pures, dans des sédiments et des végétaux en décomposition. Les composés inorganiques de l’étain pourraient être bioconcentrés par les organismes vivants, mais les données sont limitées.
La concentration moyenne en étain dans l’air est généralement inférieure à 0,1 μg/m3 (jusqu’à 0,8 μg/m3), la concentration maximale s’observant à proximité de certains établissements industriels. En général, l’étain est présent à l’état de traces dans les eaux naturelles. Les concentrations plus élevées en étain inorganique sont liées à la présence de décharges industrielles et à l’utilisation du tributylétain. Au cours d’une campagne de prélèvements dans des lacs et des rivières, près de 80 % des échantillons contenaient des concentrations d’étain inorganique inférieures à 1 μg/litre. Des concentrations atteignant 37 μg/litre ont ιté signalées près de sources de pollution. Dans les eaux côtières, on a signalé des concentrations en étain inorganique de 0,001 à 0,01 μg/litre, avec une concentration allant jusqu’ΰ 8 μg/litre prθs des sources de pollution. La concentration en étain inorganique dans les sédiments allait jusqu’à 8 mg/kg de poids sec dans les zones côtières et jusqu’à 15,5 mg/kg dans les rivières et les lacs. La concentration en étain dans la croûte terrestre est d’environ 2-3 mg/kg. Dans le sol, la concentration totale en étain se situe entre des valeurs <1 et 200 mg/kg; des chiffres de 1000 mg/kg peuvent cependant être observés dans les gisements à haute teneur en étain. Dans certains gisements de minerai, elle peut s’élever jusqu’à 50 000 mg/kg.
Pour la population générale, c’est l’alimentation qui est la principale source d’exposition à l’étain inorganique. Le JECFA a conclu récemment que l’apport moyen en étain dans sept pays était de moins de 1 à 15 mg/jour par personne, mais que l’apport maximum journalier pouvait atteindre 50-60 mg chez certaines personnes qui consomment régulièrement des fruits, des légumes et des jus de fruits en boîte métallique non vernie. L’eau de boisson n’est pas une source importante d’étain inorganique et l’apport pourrait se situer au voisinage de 0,012-0,02 mg/jour. De même, la concentration en étain inorganique dans l’air étant faible, la quantité inhalée est très faible, probablement inférieure à 0,01-0,02 mg/jour environ.
Chez l’homme et les mammifères de laboratoire, l’absorption digestive de l’étain inorganique est faible (en général inférieure à 5 %) mais dépend de la dose, de la nature de l’anion lié à l’étain (solubilité du composé) et de la présence d’autres substances. L’essentiel de l’étain ingéré et non absorbé est essentiellement (95-99 %) excrété dans les fèces dans les 48 heures. La fraction absorbée est répartie entre les os, les poumons, le foie et les reins. D’après un certain nombre d’arguments, l’étain inorganique ne traverse pas facilement la barrière hémato-encéphalique. L’étain absorbé est surtout excrété dans l’urine, et en partie dans la bile. Chez la souris, la demi-vie biologique de l’étain inorganique absorbé est d’environ 30 jours.
Une irritation oculaire et nasale passagère a été observée chez le cobaye exposé au chlorure d’étain (IV) par inhalation. L’étain métal ne provoque probablement pas d’irritation cutanée, tandis que le chlorure d’étain (II) et le chlorure d’étain (IV) sont des irritants cutanés. Dans certaines études, l’inclusion de chlorure d’étain (II) dans l’alimentation pendant 4-13 semaines a provoqué des lésions des tissus digestifs indiquant une irritation locale. Les publications anciennes contiennent des observations faisant état d’effets gastro-intestinaux (nausées, crampes abdominales, vomissements et diarrhée) chez l’homme après consommation de fruits ou de jus de fruit en boîte de métal non vernie. Les effets semblent résulter d’une irritation gastrique locale due à l’étain en solution. Cette question est traitée plus loin. Un petit nombre de personnes ont eu des réactions cutanées indiquant une réponse allergique locale avec un test cutané de provocation à l’étain et au chlorure d’étain (II), mais, bien que très répandu, l’étain ne semble pas être un allergène cutané important.
On trouve décrit dans la littérature ancienne un certain nombre de cas d’exposition professionnelle à des poussières et à des fumées contenant de l’oxyde d’étain (IV) insoluble ayant entraîné une pneumoconiose bénigne (stannose). L’affection est caractérisée par des opacités micronodulaires des poumons, provoquée apparemment par les dépôts d’oxyde d’étain (IV). La stannose n’est associée ni à une fibrose, ni à la diminution de la fonction pulmonaire.
Chez les animaux de laboratoire, l’ingestion répétée de chlorure d’étain (II) a montré des effets indésirables sur le bilan organique du cuivre, du fer, du zinc et du calcium. Une diminution de la teneur des os en calcium induite par les sels d’étain a entraîné une plus faible résistance des os. On a observé une diminution de la concentration de l’hémoglobine et un effet sur les hématies, entraînant une anémie. Certaines études comportant l’administration per os répétée de chlorure d’étain (II) ont signalé un effet sur les tissus du foie, des reins, des testicules, du pancréas et du cerveau. Dans l’étude la plus complète parmi les études connues d’administration per os sur la vie entière, aucune modification microscopique n’a été observée dans une grande variété de tissus chez le rat et la souris ayant reçu des doses d’étain sous forme de chlorure d’étain (II) dans l’alimentation allant jusqu’à près de 60 mg/kg de poids corporel par jour (rat) et 180-270 mg/kg de poids corporel par jour (souris). Dans cette étude, les NOAEL (dose sans effet nocif observé) étaient de 30 mg/kg de poids corporel par jour pour le rat et de 130 mg/kg de poids corporel par jour pour la souris, avec une diminution de la survie aux doses les plus élevées.
Aucune activité cancérogène du chlorure d’étain (II) n’est apparue clairement lorsqu’il est administré dans l’alimentation à des rats et des souris pendant 2 ans. Des essais biologiques plus limités sur l’étain métal, le chlorure d’étain (II) et un petit nombre d’autres composés stanniques n’ont eux non plus pas montré d’activité cancérogène. Dans des essais à court terme sur la génotoxicité, le chlorure d’étain (II) n’a pas induit de mutations dans les tests de Ames sur les bactéries, ni de mutations ou de conversions géniques chez les levures, ni de lésions de l’ADN dans les cultures cellulaires d’hépatocytes de rats, ni de mutations chez les cellules de lymphome murin cultivées in vitro, ni de lésions chromosomiques (micronoyaux) in vivo dans la moelle osseuse de souris traitées par injection intrapéritonéale. Dans les « rec-assays » sur les bactéries (dans lesquelles l’activité est un indicateur indirect des lésions de l’ADN), le chlorure d’étain (II) était actif chez Escherichia coli, mais (comme un certain nombre d’autres sels d’étain) inactif sur Bacillus subtilis. En culture, le chlorure d’étain (II) a provoqué des lésions chromosomiques et des échanges entre chromatides soeurs dans les cellules ovariennes de hamster ainsi que des lésions de l’ADN de lymphocytes humains, de cellules ovariennes de hamster et de plasmides. Le chlorure d’étain (IV) testé in vitro n’a pas provoqué de lésion de l’ADN dans les cellules ovariennes de hamster mais des aberrations chromosomiques et la formation de micronoyaux ainsi que des échanges de chromatides soeurs dans les lymphocytes humains. Le fluorure d’étain (II) a provoqué des lésions de l’ADN dans des cultures de lymphocytes humains, mais n’a pas induit chez la souris la formation de micronoyaux dans la moelle osseuse après injection intrapéritonéale; les tests de Ames réalisés avec ces composés n’ont pas donné de signe convaincant de leur activité. D’après certaines observations, les lésions de l’ADN induites par l’étain pourraient résulter de la formation de radicaux libres. Le mécanisme à l’origine des lésions chromosomiques provoquées par l’étain dans les cellules de mammifères en culture n’est pas clair, même si l’on sait que certains composés inorganiques peuvent donner des résultats positifs dans ces essais par suite d’une modification des ions, ou du pH du milieu.
On n’a pu identifier que des données limitées concernant l’effet toxique potentiel des composés inorganiques de l’étain sur la reproduction et le développement. Aucun effet indésirable n’a été observé chez le rat lorsque l’étain (sous une forme non caractérisée, obtenue en mélangeant du chlorure d’étain (II) en solution aqueuse et de la caséine avant l’introduction dans l’alimentation) a été donné dans l’alimentation pendant trois générations ou lorsque du fluorure d’étain (II), du pentachlorostannite de sodium ou du pentafluorostannite de sodium ont été administrés dans l’alimentation tout au long de la gestation. De même, le traitement répété par gavage de femelles gestantes, rats, souris et hamsters, par le chlorure d’étain (II) n’a pas eu d’effet indésirable sur les foetus.
Les données disponibles sur les troubles chez l’homme de l’absorption du zinc dus à l’étain ingéré sont limitées. Dans une étude chez des volontaires, l’apparition du zinc dans le plasma 1 à 4 heures après l’administration d’une dose de zinc n’a pas été modifiée par l’ingestion concomitante d’étain jusqu’à la dose de 100 mg (sous forme de chlorure d’étain (II)). Une autre étude a signalé qu’une dose unique de 36 mg d’étain (également sous forme de chlorure d’étain (II)), administrée avec du zinc, a entraîné une plus faible rétention du zinc. Une perturbation modérée de l’excrétion du zinc a été notée dans une troisième étude au cours de laquelle l’alimentation normale était supplémentée par une dose de 50 mg d’étain/jour (sous forme de chlorure d’étain (II) dans le jus de fruit). S’il n’a pas été nettement fixé de dose sans effet sur l’inhibition de l’absorption du zinc, la dose minimale signalée comme ayant cet effet (36 mg) est environ 2,5 à >36 fois plus élevée que l’apport moyen estimé pour la population comme l’a indiqué le JECFA. Toutefois, les personnes qui consomment habituellement des fruits, des légumes et des jus de fruits en boîte métallique non laquée peuvent avoir un apport en étain (50-60 mg) comparable aux doses aiguës (36 mg) ou répétées (50 mg) qui dans certaines études modifient l’absorption du zinc ou son bilan. La présence ou non d’un effet clinique pourrait dépendre fortement de l’apport alimentaire en zinc.
Les doses d’étain en cause dans les anciennes observations de troubles gastro-intestinaux après consommation de fruits ou de jus de fruits en boîte ont été estimées (30-200 mg), mais ces chiffres ne peuvent pas être considérés comme parfaitement fiables. Deux études récentes sur des volontaires donnent une meilleure idée des doses efficaces et, ce qui est peut-être plus important, des concentrations. La première étude comportait l’ingestion de jus de tomate dans lequel du chlorure d’étain (II) avait été ajouté pour obtenir une concentration en étain de 161, 264 et 529 mg/kg (doses d’étain de 40, 66 et 132 mg respectivement). A la dose de 161 mg/kg, l’un des volontaires (sur 18) a signalé des symptômes gastro-intestinaux bénins; des symptômes aigus typiques ont été observés aux doses de 264 et 529 mg/kg. La quantité d’étain présent dans le sérum n’a pas augmenté entre 0,5 et 4 heures après l’administration, quelle que soit la dose, confortant l’hypothèse que les effets aigus après ingestion d’étain dépendent de la concentration (entraînant une irritation gastrique locale) plutôt que d’un effet généralisé dû à l’étain absorbé. Une deuxième étude comportait l’ingestion de soupe à la tomate contenant de l’étain provenant d’une boîte de conserve non vernie. Les concentrations étudiées en étain étaient <0,5, 201 et 267 mg/kg, correspondant à des doses aiguës d’étain allant jusqu’à 67 mg. Rien n’indiquait d’effet aigu dans cette étude. La dose faible effet ou la dose sans effet d’environ 67 mg d’étain dans ces études est de 4,5 à >67 fois plus grande que les estimations du JECFA pour l’apport moyen journalier estimé pour la population et est comparable à l’apport journalier estimé (50-60 mg) chez les personnes qui consomment habituellement des fruits, des légumes et des jus de fruits conservés dans des boîtes métalliques non vernies.
Dans les conditions de spéciation qui sont celles de l’environnement, les composés inorganiques de l’étain ont une faible toxicité pour les organismes aquatiques et terrestres, en raison principalement de leur faible solubilité, de leur mauvaise absorption, du faible taux d’accumulation dans les tissus et de leur excrétion rapide. La plupart des analyses de laboratoire avec les organismes aquatiques ont porté sur le chlorure d’étain (II) qui est soluble. Les microalgues les plus sensibles sont les diatomées marines Skeletonema costatum et Thalassiosira guillardii, avec une CE50 (72 heures) pour les cations liés à l’étain (II) sur la croissance d’environ 0,2 mg/litre. La CL/CE50 de l’étain (II) pour les invertébrés aquatiques est de 3,6 à 140 mg/litre avec une CE50 (21 jours) sur la reproduction des daphnies de 1,5 mg d’étain (II) par litre. Les tests de toxicité chez les poissons montrent clairement que le chlorure d’étain (IV) est moins toxique que le chlorure d’étain (II), lequel est plus soluble. La CL50s (96 heures) chez les poissons va de 35 mg d’étain (II) par litre à plus de 1000 mg d’étain (IV) par litre. Les résultats des tests chez les larves et les embryons (CL50 7 à 28 jours) pour les poissons et les amphibiens sont de 0,1 à 2,1 mg/litre pour l’étain (II).
Les concentrations qui se révèlent toxiques pour les organismes sont en général plusieurs fois supérieures à celles qui s’observent dans l’environnement. Les tests les plus sensibles étaient l’exposition des diatomées pendant 72 heures et les études sur les larves et les embryons d’amphibiens, avec l’observation d’un effet toxique à 0,1-0,2 mg d’étain (II) par litre. Même à de telles concentrations, les effets toxiques provoqués par l’étain inorganique sont improbables, y compris à proximité des sources de pollution locales. On remarquera que lorsque les concentrations sont exprimées en étain total, une fraction de celui-ci a des chances de se trouver sous forme d’étain organique (tributylétain par exemple), dont la toxicité et la biodisponibilité sont plus grandes. Pour plus d’informations sur le devenir et la toxicité du tributylétain dans l’environnement, on se reportera aux publications de l’IPCS (1990, 1999).
Este CICAD5 sobre el estaño y sus compuestos inorgánicos fue preparado por Toxicology Advice & Consulting Ltd y el Centro de Ecología e Hidrología. Este CICAD se basó en tres documentos originales. El primero de dichos documentos, preparado por el Grupo Nórdico de Expertos para la documentación de criterios sobre los riesgos de los productos químicos para la salud y el Comité de Expertos Neerlandeses en Normas del Trabajo, comprendía la bibliografía identificada hasta marzo de 2002 (Westrum & Thomassen, 2002). El segundo documento original era la monografía preparada por la 55Ş reunión del Comité Mixto FAO/OMS de Expertos en Aditivos Alimentarios, publicada en 2001 (JECFA, 2001). El tercer documento original era el proyecto de Perfil toxicológico del estaño y sus compuestos, actualizado en 2003, preparado por la Agencia para el Registro de Sustancias Tóxicas y Enfermedades de los Estados Unidos (ATSDR, 2003). En diciembre de 2003, Toxicology Advice & Consulting Ltd y el Centro de Ecología e Hidrología llevaron a cabo una búsqueda bibliográfica amplia de las bases de datos en línea para localizar las referencias más recientes. La información sobre el carácter del examen colegiado y la disponibilidad de los documentos originales se presenta en el apéndice 2. La información sobre el examen colegiado de este CICAD figura en el apéndice 3. Este CICAD se aprobó como evaluación internacional en una reunión de la Junta de Evaluación Final celebrada en Hanoi (Viet Nam) del 28 de septiembre al 1ş de octubre de 2004. La lista de participantes en esta reunión figura en el apéndice 4. También se reproducen en este documento las Fichas internacionales de seguridad química para el cloruro de estaño (II), el cloruro de estaño (II) dihidrato, el fluoruro de estaño (II), el óxido de estaño (II), el cloruro de estaño (IV) y el óxido de estaño (IV), preparadas por el Programa Internacional de Seguridad de las Sustancias Químicas (IPCS, 2004a–f).
El estaño es un metal blanco grisáceo. Los compuestos de estaño inorgánico más importantes son los cloruros de estaño (II) y (IV), el óxido de estaño (II), el fluoruro de estaño (II) y los estannatos de potasio y de sodio. Los estados de oxidación del estaño 2+ y 4+, conocidos también como estaño (II) y estaño (IV), son bastante estables.
La producción mundial anual de estaño ha ido creciendo lentamente en los últimos años, alcanzando un volumen de unas 268 000 toneladas en 2003. Alrededor del 10–15% de esta cifra es metal recuperado. Los principales países productores son China, Indonesia, Perú, Bolivia, Brasil y Australia y también se producen cantidades importantes en Malasia y Tailandia. La principal aplicación del estaño, que absorbe alrededor del 34% de la producción mundial anual, son las aleaciones para soldadura eléctrica/electrónica y las aplicaciones industriales en general. El estaño también se utiliza mucho (alrededor del 25-30% de la producción) como revestimiento protector de otros metales, en particular para recipientes de alimentos. El cloruro de estaño (II) es el compuesto inorgánico más importante desde el punto de vista comercial y se usa sobre todo como agente reductor en síntesis orgánicas e inorgánicas y en la manufactura de vidrio metalizado, vidrio y pigmentos. El cloruro de estaño (IV) se utiliza en procesos de síntesis orgánica, en plásticos, como intermediario en la fabricación de compuestos de estaño orgánico y en la producción de películas de óxido de estaño (IV) sobre vidrio. El fluoruro de estaño (II) se utiliza ampliamente en la odontología preventiva.
El estaño se puede liberar en la atmósfera a partir de fuentes naturales y antropogénicas. Es un componente de muchos suelos y se puede liberar en el polvo de los vendavales, los caminos y las actividades agrícolas. Otras fuentes naturales menos significativas son los incendios forestales y las emisiones volcánicas. Puede haber emisiones de gases, polvo y humo que contienen estaño procedentes de procesos de fusión y refinado, de usos industriales del estaño, de la incineración de desechos y de la quema de combustibles fósiles. La presión de vapor del estaño elemental es insignificante; el estaño y sus compuestos inorgánicos no son volátiles en las condiciones del medio ambiente. El cloruro de estaño (II) es soluble en agua, mientras que otros compuestos suelen ser sólo ligeramente solubles. Los compuestos de estaño probablemente se reparten entre el suelo y los sedimentos. El estaño inorgánico puede sufrir procesos de oxidación-reducción, intercambio de ligandos y reacciones de precipitación en el medio ambiente. Se ha demostrado la biometilación del estaño inorgánico en cultivos bacterianos puros, sedimentos y material vegetal en descomposición. Los compuestos inorgánicos de estaño pueden experimentar procesos de bioconcentración en los microorganismos, pero los datos son limitados.
Las concentraciones medias de estaño en el aire se suelen mantener por debajo de 0,1 µg/m3 (alcanzando valores de hasta 0,8 µg/m3), con concentraciones más altas cerca de algunas instalaciones industriales. En general, el estaño se encuentra en cantidades insignificantes en las aguas naturales. Las concentraciones más elevadas de estaño inorgánico están asociadas con descargas industriales y con el empleo de tributilestaño. En un estudio de lagos y ríos se encontró que casi el 80% de las muestras contenían estaño inorgánico en concentraciones inferiores a 1 µg/l; se notificaron concentraciones más elevadas, de hasta 37 µg/l, cerca de fuentes de contaminación. Se ha informado de concentraciones de estaño inorgánico de 0,001 a 0,01 µg/l en las aguas costeras, con niveles de hasta 8 µg/l cerca de fuentes de contaminación. Las concentraciones de estaño inorgánico en los sedimentos oscilaban entre 8 mg/kg de peso seco en zonas costeras y hasta 15,5 mg/kg en ríos y lagos. La concentración de estaño en la corteza terrestre es aproximadamente de 2–3 mg/kg. La concentración total de estaño en el suelo varía entre <1 y 200 mg/kg, pero se pueden encontrar niveles de 1000 mg/kg en zonas con depósitos elevados de estaño. Algunos depósitos minerales pueden contener hasta 50 000 mg/kg como estaño.
Los alimentos son la fuente principal de exposición al estaño inorgánico para la población general. El JECFA ha llegado recientemente a la conclusión de que la ingesta media de estaño en siete países se situaba entre <1 y 15 mg/día por persona, pero determinadas personas que habitualmente consumían frutas y hortalizas enlatadas o zumos procedentes de latas no barnizadas podían alcanzar valores máximos diarios de 50–60 mg. El agua de bebida no es una fuente importante de estaño inorgánico y podría contribuir con alrededor de 0,012–0,02 mg/día. Igualmente, los bajos niveles de estaño inorgánico en el aire indican que la cantidad inhalada de estaño es muy baja, probablemente inferior a unos 0,01–0,02 mg/día.
La absorción de estaño inorgánico a partir del tracto gastrointestinal es baja en las personas y los mamíferos de laboratorio (en general menos del 5%), pero depende de la dosis, los aniones (solubilidad del compuesto) y la presencia de otras substancias. El estaño ingerido que no se absorbe se excreta fundamentalmente (95–99%) en las heces en un plazo de 48 h. El estaño absorbido se distribuye sobre todo en los huesos, pero también en los pulmones, el hígado y los riñones. Hay pruebas limitadas que parecen indicar que el estaño inorgánico no atraviesa la barrera hematoencefálica. El estaño absorbido se excreta básicamente en la orina, con alguna excreción biliar adicional. En ratones, la semivida biológica del estaño inorgánico absorbido fue de unos 30 días.
En cobayas expuestos a cloruro de estaño (IV) por inhalación se observó irritación ocular y nasal transitoria. Es poco probable que el estaño metálico pueda provocar irritación cutánea, mientras que los cloruros de estaño (II) y (IV) sí tienen esta propiedad. En algunos estudios, la inclusión de cloruro de estaño (II) en la alimentación durante 4–13 semanas produjo cambios en el tejido gastrointestinal indicativos de irritación local. Las primeras referencias bibliográficas contienen informes de efectos gastrointestinales (náuseas, calambres abdominales, vómitos y diarrea) en las personas tras el consumo de fruta o zumo de latas sin barnizar. Los efectos parecen ser el resultado de la irritación gástrica local debida al estaño disuelto. Este aspecto se examina con más detenimiento más adelante. Un pequeño número de personas sufrieron reacciones cutáneas indicativas de una respuesta alérgica local cuando se sometieron a pruebas con parches de estaño o cloruro de estaño (II), pero, dado su empleo generalizado, no parece que el estaño sea un alergeno cutáneo importante.
En las primeras referencias bibliográficas hay algunos casos de exposición ocupacional a polvo y humo que contenían óxido de estaño (IV) insoluble que indujo una pneumoconiosis benigna (estannosis). Este trastorno se caracteriza por sombras moteadas en los pulmones, al parecer debidas a depósitos de óxido de estaño (IV). La estannosis no está asociada con fibrosis o pérdida de función pulmonar.
En animales de laboratorio, la ingestión repetida de cloruro de estaño (II) tuvo efectos adversos en la presencia de cobre, hierro, zinc y calcio en el organismo. La disminución del contenido de calcio de los huesos inducida por las sales de estaño provocó una reducción de su resistencia. Se han observado reducciones de la hemoglobina y efectos en los glóbulos rojos que dan lugar a anemia. En ciertos estudios con administración repetida de cloruro de estaño (II) por vía oral se han notificado efectos en el hígado, los riñones, los testículos, el páncreas y el cerebro. En el más amplio de los estudios disponibles por vía oral a lo largo de toda la vida, no se observaron cambios microscópicos en una gran variedad de tejidos de ratas o ratones a los que se administró estaño en los alimentos en concentraciones de hasta alrededor de 60 mg/kg de peso corporal al día (ratas) o 180–270 mg/kg de peso corporal al día (ratones) en forma de cloruro de estaño (II). En este estudio, las NOAEL fueron de 30 mg/kg de peso corporal al día en ratas y de 130 mg/kg de peso corporal al día en ratones, observándose una supervivencia reducida con las dosis más altas.
El cloruro de estaño (II) no dio pruebas claras de actividad carcinogénica cuando se administró durante dos años a ratas y ratones con los alimentos. En biovaloraciones más limitadas llevadas a cabo con estaño metálico, cloruro de estaño (II) y un pequeño número de otros compuestos de estaño tampoco se logró detectar actividad carcinogénica. En valoraciones breves de detección del potencial genotóxico, el cloruro de estaño (II) no indujo mutaciones en las pruebas bacterianas de Ames, mutaciones o conversiones de genes en levaduras, daños en el ADN de células de hígado de rata cultivadas, mutaciones en células de linfoma de ratón in vitro o daños cromosómicos (micronúcleos) in vivo en la médula ósea de ratones tratados por inyección intraperitoneal. En valoraciones de reparación por recombinación en bacterias (en las cuales la actividad es una indicación indirecta de daño en el ADN), el cloruro de estaño (II) fue activo en Escherichia coli, pero (junto con otras sales de estaño) inactivo en Bacillus subtilis. En cultivos, el cloruro de estaño (II) indujo daños cromosómicos e intercambio de cromátidas hermanas en células de ovario de hámster y daños en el ADN de linfocitos humanos, células de ovario de hámster y ADN de plasmidios. El cloruro de estaño (IV) sometido a prueba in vitro no provocó daños en el ADN de células de ovario de hámster, pero indujo aberraciones cromosómicas, micronúcleos e intercambio de cromátidas hermanas en linfocitos humanos. El fluoruro de estaño (II) provocó daños en el ADN de linfocitos humanos cultivados, pero no indujo formación de micronúcleos en la médula ósea tras la inyección en el peritoneo de ratones; en las pruebas de Ames sobre este compuesto no se demostró de manera convincente que tuviera actividad. Hay pruebas limitadas que respaldan la idea de que el daño en el ADN inducido por el estaño podría ser consecuencia de la producción de especies de oxígeno reactivo. No está claro el mecanismo mediante el cual el estaño induce daños cromosómicos en células de mamífero cultivadas, aunque se sabe que ciertos compuestos inorgánicos pueden dar resultado positivo en dichas valoraciones debido a cambios en el pH o el estado iónico del medio de prueba.
Sólo se encontraron datos limitados sobre el potencial de los compuestos de estaño inorgánico para provocar toxicidad reproductiva y en el desarrollo. No se detectaron efectos adversos en ratas cuando se les administró estaño (una forma no caracterizada obtenida mezclando cloruro de estaño(II) acuoso con caseína antes de su inclusión en la alimentación) en los alimentos durante tres generaciones o cuando se les administró fluoruro de estaño (II), pentacloroestannito de sodio o pentafluoroestannito de sodio con los alimentos durante toda la gestación. Igualmente, el tratamiento repetido de ratas, ratones y hámsteres con cloruro de estaño (II) mediante sonda no dio lugar a efectos adversos en los fetos.
Son limitados los datos disponibles sobre la capacidad del estaño ingerido para afectar negativamente a la absorción del zinc en las personas. En un estudio con voluntarios se observó que la aparición de zinc en el plasma entre una y cuatro horas después de la administración de una dosis de zinc no se vio afectada por la ingestión simultánea de hasta 100 mg de estaño (en forma de cloruro de estaño (II)). En otro estudio se notificó que una dosis única de 36 mg de estaño (también en forma de cloruro de estaño (II)), ingerida con zinc había dado lugar a una menor retención del zinc. En un tercer estudio en el que la alimentación normal se complementó con 50 mg/día de estaño (en forma de cloruro de estaño (II) en zumo de fruta), se detectaron trastornos moderados de los índices de excreción del zinc. Aunque no se ha establecido claramente un nivel sin efectos para la inhibición de la absorción del zinc, la dosis más baja notificada con este efecto (36 mg) es alrededor de 2,5 a >36 veces superior a la ingesta media estimada de la población según el JECFA. Sin embargo, quienes habitualmente consumen frutas y hortalizas enlatadas y zumos de latas no barnizadas podrían tener ingestas de estaño (50–60 mg) similares a los niveles de la dosis aguda (36 mg) o repetida (50 mg) que, según lo notificado en algunos estudios, afectan a la absorción o el equilibrio del zinc. El hecho de que tenga o no algún efecto clínico es probable que dependa básicamente de un suministro adecuado de zinc con los alimentos.
Se han estimado (en 30–200 mg) las dosis de estaño correspondientes a los primeros informes de los efectos gastrointestinales tras el consumo de fruta o zumo enlatados, pero la confianza en la exactitud de estas cifras es escasa. Dos estudios recientes con voluntarios proporcionan un análisis mejor de las dosis efectivas y, lo que tal vez sea más importante, las concentraciones. El primer estudio consistía en la ingestión de zumo de tomate al que se había añadido cloruro de estaño (II) con objeto de obtener concentraciones de estaño de 161, 264 ó 529 mg/kg (dosis de estaño de unos 40, 66 y 132 mg, respectivamente). Con 161 mg/kg, un voluntario (de 18) informó de síntomas gastrointestinales ligeros; se observaron síntomas agudos característicos con 264 y 529 mg/kg. Los niveles de estaño en el suero no aumentaron con ninguna de las dosis en el intervalo de 0,5–4 h después de la administración, lo que respalda la opinión de que los efectos agudos de la ingestión de estaño dependen más de la concentración (que produce irritación gástrica local) que de la absorción sistémica de estaño. Un segundo estudio consistió en la ingestión de sopa de tomate con estaño que se había desplazado desde latas no barnizadas. Las concentraciones de estaño estudiadas fueron <0,5, 201 y 267 mg/kg, que proporcionaron dosis agudas de estaño de hasta unos 67 mg. No hay pruebas de que se hayan observado efectos agudos en este estudio. La dosis con efectos escasos o nulos en estos estudios, de unos 67 mg de estaño, es de alrededor de 4,5 a >67 veces superior a las estimaciones del JECFA para la ingesta diaria media de la población y similar a la ingesta diaria estimada (50–60 mg) de las personas que consumen habitualmente fruta, hortalizas y zumos contenidos en latas sin barnizar.
En condiciones de especiación en el medio ambiente, los compuestos de estaño inorgánico tienen una toxicidad baja para los organismos tanto acuáticos como terrestres, debido fundamentalmente a su baja solubilidad, su escasa absorción, su baja acumulación en los tejidos y su rápida excreción. La mayor parte de las pruebas de laboratorio con organismos acuáticos se han realizado con el cloruro de estaño (II) soluble. Las microalgas más sensibles son las diatomeas marinas Skeletonema costatum y Thalassiosira guillardii, con valores de la CE50 de los cationes de estaño (II) a las 72 h, basados en la inhibición del crecimiento, de unos 0,2 mg/l. Las CL/CE50 agudas del estaño (II) para los invertebrados acuáticos están en la gama de 3,6 a 140 mg/l, con una CE50 a los 21 días, basada en el éxito reproductivo en los dáfnidos, de 1,5 mg de estaño (II) por litro. Las pruebas de toxicidad en los peces demuestran claramente que el cloruro de estaño (IV) es menos tóxico que el cloruro de estaño (II), más soluble. Las CL50 a las 96 horas para los peces varía entre 35 mg de estaño (II) por litro y >1000 mg de estaño (IV) por litro. Los resultados de las pruebas embriolarvarias (es decir, las CL50 a los 7–28 días) en el caso de los peces y anfibios son de 0,1 a 2,1 mg/l para el estaño (II).
Las concentraciones que muestran toxicidad para los organismos son en general varios órdenes de magnitud más elevadas que las que se encuentran en el medio ambiente. Los resultados de las pruebas más sensibles fueron exposiciones de diatomeas de 72 h y estudios embriolarvarios de anfibios, habiéndose observado efectos tóxicos con 0,1–0,2 mg de estaño (II) por litro. Incluso con esas concentraciones, es poco probable que el estaño inorgánico provoque efectos tóxicos, incluso cerca de fuentes de contaminación local. Hay que señalar que cuando las concentraciones se expresan como estaño total, es probable que un porcentaje se encuentre en forma de estaños orgánicos (por ejemplo, tributilestaño), que son formas más biodisponibles y tóxicas. Para más información sobre el destino del tributilestaño en el medio ambiente y su toxicidad, véase el IPCS (1990, 1999).
FOOTNOTES:
See Also:
Toxicological Abbreviations