
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
CONCISE INTERNATIONAL CHEMICAL ASSESSMENT DOCUMENT NO. 8
TRIGLYCIDYL ISOCYANURATE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
INTER-ORGANIZATION PROGRAMME FOR THE SOUND MANAGEMENT OF CHEMICALS
A cooperative agreement among UNEP, ILO, FAO, WHO, UNIDO, UNITAR and
OECD
First draft prepared by Ms D. Willcocks, Ms L. Onyon, Ms C. Jenkins,
and Dr B. Diver, Chemical Assessment Division, National Occupational
Health and Safety Commission, Australia
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization Geneva, 1998
The International Programme on Chemical Safety (IPCS),
established in 1980, is a joint venture of the United Nations
Environment Programme (UNEP), the International Labour Organisation
(ILO), and the World Health Organization (WHO). The overall
objectives of the IPCS are to establish the scientific basis for
assessment of the risk to human health and the environment from
exposure to chemicals, through international peer review processes, as
a prerequisite for the promotion of chemical safety, and to provide
technical assistance in strengthening national capacities for the
sound management of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization, the United Nations
Institute for Training and Research, and the Organisation for Economic
Co-operation and Development (Participating Organizations), following
recommendations made by the 1992 UN Conference on Environment and
Development to strengthen cooperation and increase coordination in the
field of chemical safety. The purpose of the IOMC is to promote
coordination of the policies and activities pursued by the
Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing in Publication Data
Triglycidyl isocyanurate.
(Concise international chemical assessment document ; 8)
First draft prepared by D. Willcocks, L. Onyon, C. Jenkins and
B. Diver
1.Triazines - adverse effects 2.Triazines -
toxicity 3.Environmental exposure I.Willcocks, D. II.Series
ISBN 92 4 153008 1 (NLM Classification: QD 401)
ISSN 1020-6167
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1998
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city, or area
or of its authorities, or concerning the delimitation of its frontiers
or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
The Federal Ministry for the Environment, Nature Conservation and
Nuclear Safety, Germany, provided financial support for the printing
of this publication.
TABLE OF CONTENTS
FOREWORD
1. EXECUTIVE SUMMARY
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
3. ANALYTICAL METHODS
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1. Environmental levels
6.2. Human exposure
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposure
8.2. Irritation and sensitization
8.3. Short-term exposure
8.4. Long-term exposure
8.5. Genotoxicity and related end-points
8.6. Reproductive and developmental toxicity
9. EFFECTS ON HUMANS
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
11. EFFECTS EVALUATION
11.1. Evaluation of health effects
11.1.1. Hazard identification and dose-response assessment
11.1.2. Criteria for setting guidance values for triglycidyl isocyanurate
11.1.3. Sample risk characterization
11.2. Evaluation of environmental effects
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
13.1. Human health hazards
13.2. Advice to physicians
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
INTERNATIONAL CHEMICAL SAFETY CARD
REFERENCES
APPENDIX 1 - SOURCE DOCUMENT
APPENDIX 2 - CICAD PEER REVIEW
APPENDIX 3 - CICAD FINAL REVIEW BOARD
RÉSUMÉ D'ORIENTATION
RESUMEN DE ORIENTACION
FOREWORD
Concise International Chemical Assessment Documents (CICADs) are
the latest in a family of publications from the International
Programme on Chemical Safety (IPCS) - a cooperative programme of the
World Health Organization (WHO), the International Labour Organisation
(ILO), and the United Nations Environment Programme (UNEP). CICADs
join the Environmental Health Criteria documents (EHCs) as
authoritative documents on the risk assessment of chemicals.
CICADs are concise documents that provide summaries of the
relevant scientific information concerning the potential effects of
chemicals upon human health and/or the environment. They are based on
selected national or regional evaluation documents or on existing
EHCs. Before acceptance for publication as CICADs by IPCS, these
documents undergo extensive peer review by internationally selected
experts to ensure their completeness, accuracy in the way in which the
original data are represented, and the validity of the conclusions
drawn.
The primary objective of CICADs is characterization of hazard and
dose-response from exposure to a chemical. CICADs are not a summary
of all available data on a particular chemical; rather, they include
only that information considered critical for characterization of the
risk posed by the chemical. The critical studies are, however,
presented in sufficient detail to support the conclusions drawn. For
additional information, the reader should consult the identified
source documents upon which the CICAD has been based.
Risks to human health and the environment will vary considerably
depending upon the type and extent of exposure. Responsible
authorities are strongly encouraged to characterize risk on the basis
of locally measured or predicted exposure scenarios. To assist the
reader, examples of exposure estimation and risk characterization are
provided in CICADs, whenever possible. These examples cannot be
considered as representing all possible exposure situations, but are
provided as guidance only. The reader is referred to EHC 1701 for
advice on the derivation of health-based guidance values.
While every effort is made to ensure that CICADs represent the
current status of knowledge, new information is being developed
constantly. Unless otherwise stated, CICADs are based on a search of
the scientific literature to the date shown in the executive summary.
In the event that a reader becomes aware of new information that would
change the conclusions drawn in a CICAD, the reader is requested to
contact the IPCS to inform it of the new information.
1 International Programme on Chemical Safety (1994) Assessing
human health risks of chemicals: derivation of guidance values for
health-based exposure limits. Geneva, World Health Organization
(Environmental Health Criteria 170).
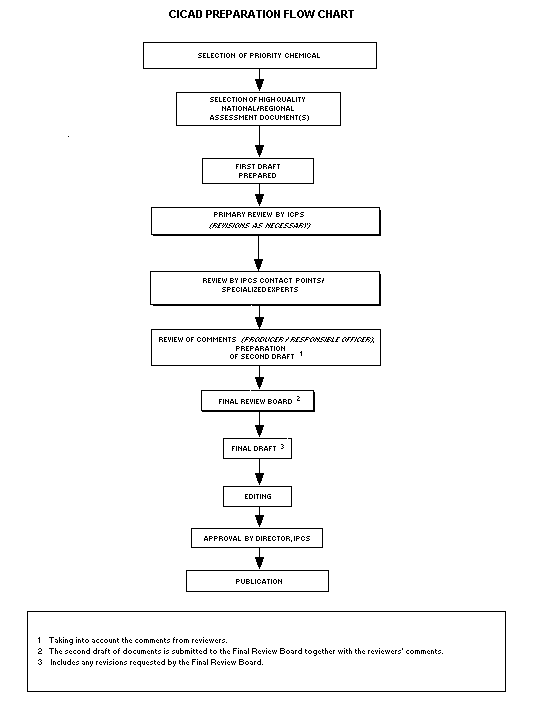
Procedures
The flow chart shows the procedures followed to produce a CICAD.
These procedures are designed to take advantage of the expertise that
exists around the world - expertise that is required to produce the
high-quality evaluations of toxicological, exposure, and other data
that are necessary for assessing risks to human health and/or the
environment.
The first draft is based on an existing national, regional, or
international review. Authors of the first draft are usually, but not
necessarily, from the institution that developed the original review.
A standard outline has been developed to encourage consistency in
form. The first draft undergoes primary review by IPCS to ensure that
it meets the specified criteria for CICADs.
The second stage involves international peer review by scientists
known for their particular expertise and by scientists selected from
an international roster compiled by IPCS through recommendations from
IPCS national Contact Points and from IPCS Participating Institutions.
Adequate time is allowed for the selected experts to undertake a
thorough review. Authors are required to take reviewers' comments
into account and revise their draft, if necessary. The resulting
second draft is submitted to a Final Review Board together with the
reviewers' comments.
The CICAD Final Review Board has several important functions:
- to ensure that each CICAD has been subjected to an appropriate
and thorough peer review;
- to verify that the peer reviewers' comments have been addressed
appropriately;
- to provide guidance to those responsible for the preparation of
CICADs on how to resolve any remaining issues if, in the opinion
of the Board, the author has not adequately addressed all
comments of the reviewers; and
- to approve CICADs as international assessments.
Board members serve in their personal capacity, not as representatives
of any organization, government, or industry. They are selected
because of their expertise in human and environmental toxicology or
because of their experience in the regulation of chemicals. Boards
are chosen according to the range of expertise required for a meeting
and the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
 1. EXECUTIVE SUMMARY
This CICAD was based principally on the assessment of triglycidyl
isocyanurate completed under the Australian National Industrial
Chemicals Notification and Assessment Scheme (NICNAS) and published in
April 1994 (NICNAS, 1994). Information that has become available
since completion of the NICNAS report, identified up to November 1997,
has also been assessed and included in this CICAD. Some additional
information from the United Kingdom Health and Safety Executive's
toxicity review of triglycidyl isocyanurate (HSE, 1992) has also been
included. Information on the nature of peer review and availability
of the NICNAS report is presented in Appendix 1. Information on the
peer review of this CICAD is presented in Appendix 2. This CICAD was
approved as an international assessment at a meeting of the Final
Review Board, held in Berlin, Germany, on 26-28 November 1997.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 1274) for triglycidyl
isocyanurate, produced by the International Programme on Chemical
Safety (IPCS, 1997), has also been reproduced in this document.
Triglycidyl isocyanurate (CAS no. 2451-62-9), a synthetic white
powder or granule with no discernible odour at room temperature, is
used mainly as a three-dimensional cross-linking or curing agent in
polyester powder coatings (paints). These powder coatings usually
contain between 4 and 10% triglycidyl isocyanurate. Triglycidyl 2
isocyanurate is also used in solder "mask" inks in the printed circuit
board industry. The two-part inks contain approximately 60%
triglycidyl isocyanurate in the hardener component. Much of the
triglycidyl isocyanurate in powder coatings and solder inks is
immobilized through cross-linking in an insoluble matrix.
Exposure of the general population to triglycidyl isocyanurate is
expected to be minimal; however, there is potential for occupational
exposure during the manufacture of triglycidyl isocyanurate and the
manufacture and use of products containing the chemical.
Little information is available on the effects of triglycidyl
isocyanurate on humans. Several cases of allergic contact dermatitis
and one case of respiratory sensitization caused by occupational
exposure to triglycidyl isocyanurate have been reported.
In laboratory animals, triglycidyl isocyanurate is acutely toxic
by ingestion and inhalation and can cause serious eye damage. It is a
skin sensitizer but not a skin irritant. The data for
repeated-exposure toxicity are limited. In short-term (5-7 days)
repeated-exposure studies in rats and mice, effects on the kidneys,
liver, lungs, gastrointestinal tract, and spermatogonial cells were
observed. In a 13-week toxicity/fertility study in male rats, a
dose-related reduction in the number of spermatozoa was observed.
In vitro and in vivo genotoxicity studies indicate that
triglycidyl isocyanurate is a direct-acting mutagen capable of
affecting the reproductive organs. In view of its potential
genotoxicity, all appropriate measures should be taken to minimize
human exposure to triglycidyl isocyanurate.
Owing to its low persistence and probable low ecotoxicity,
triglycidyl isocyanurate is unlikely to pose a significant hazard to
the environment, except in the case of an accident or inappropriate
disposal.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
Triglycidyl isocyanurate (CAS no. 2451-62-9; C12H15N3O6;
1,3,5-triglycidyl isocyanurate, tris(2,3-epoxypropyl) isocyanurate) is
a synthetic chemical, manufactured and supplied as the technical
grades TEPIC and Araldite PT 810. Technical-grade triglycidyl
isocyanurate is a mixture of two diastereomers, alpha and beta. Some
physical and chemical properties of triglycidyl isocyanurate
(technical grades) are presented in Table 1. Additional properties
are presented in the International Chemical Safety Card reproduced in
this document.
Triglycidyl isocyanurate is a white powder or granule with no
discernible odour at room temperature. The solubility of Araldite PT
810 in various solvents at 25°C is as follows: epichlorohydrin, <22%;
methanol, 7.3%; toluene, 3%; isopropanol, 1%. Triglycidyl
isocyanurate reacts rapidly with primary and secondary amines,
carboxylic acids and anhydrides, thiols, phenols, and alcohols. It
can be polymerized by catalysts and may undergo violent
autopolymerization. Combustion products include carbon dioxide,
carbon monoxide, and oxides of nitrogen.
Table 1: Physical and chemical properties of triglycidyl isocyanurate
(technical grades).
Araldite PT
Property TEPICa 810b
Degree of purity (%
triglycidyl isocyanurate) 90 (approx.) >97
Melting point (°C) 90-125 95
Vapour pressure
(kPa at 20°C) n.a.c 7.2 × 10-9
Solubility in water
(g/litre at 25°C)d 9 8.7
Partition coefficient
(log Kow) -0.8 n.a.
a Information provided by Nissan Chemical Industries (Nissan,
no date).
b Information provided by Ciba-Geigy (1991).
c n.a. = not available.
d The alpha and beta isomers have different water solubilities:
10.1 and 0.53 g/litre, respectively (Atassi et al., 1980).
3. ANALYTICAL METHODS
Methods of detection and analysis include infrared spectroscopy,
mass spectroscopy, epoxy equivalent weight, gas chromatography, and
high-performance liquid chromatography. Methodology for the sampling
and analysis of triglycidyl isocyanurate in air has been developed by
its manufacturers and involves the collection of the dust on a glass
fibre filter, followed by high-performance liquid chromatography with
ultraviolet detection (detection limit 0.8 µg/m3) (NICNAS, 1994).
Methodology for the analysis of triglycidyl isocyanurate in other
environmental media is not available.
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Triglycidyl isocyanurate does not occur naturally. It is
produced industrially by reacting cyanuric acid with excess
epichlorohydrin. The worldwide production of triglycidyl isocyanurate
is approximately 7000-8000 tonnes per year. Australia imports
100-1000 tonnes per year as technical-grade triglycidyl isocyanurate
for the manufacture of polyester powder coatings or as an ingredient
in powder coatings. The United Kingdom imports approximately 400
tonnes of triglycidyl isocyanurate per year for use in powder
coatings. In the United Kingdom, approximately 30 tonnes of solder
"mask" inks containing triglycidyl isocyanurate are manufactured per
year by four or five companies (NICNAS, 1994).
The main use of triglycidyl isocyanurate is as a
three-dimensional cross-linking or curing agent in polyester powder
coatings (paints). In the manufacture of powder coatings, triglycidyl
isocyanurate granules are mixed with other ingredients; the mix is
then melted and extruded as a brittle sheet, chipped, and packaged.
Generally, the particle size of 90-95% of the powder coating is
>10 µm.
Powder coatings usually contain 4-10% triglycidyl isocyanurate
and are sprayed onto metal objects by an electrostatic process. The
coated metal objects are then treated in an oven to a temperature of
about 200°C. This heating causes the powder coatings to melt, flow,
and chemically cross-link. The coatings are durable and resist
ultraviolet damage; as a result, they are typically used in outdoor
applications. Triglycidyl isocyanurate is also used in solder "mask"
inks in the printed circuit board industry. The two-part inks contain
approximately 60% triglycidyl isocyanurate in the hardener component.
The inks are applied by curtain coating, electrostatic spraying, or
screen printing. The coated circuit board is finally passed through
an oven at 150°C to complete the curing.
Release of the chemical to air is expected to be minimal. During
the manufacture of triglycidyl isocyanurate and the formulation of
products containing triglycidyl isocyanurate, dust extractors and
other pollution control devices remove particulate waste for disposal.
Triglycidyl isocyanurate contained in such waste is effectively
immobilized after consignment to landfill, particularly if waste
powder is heat-cured beforehand. Release to the environment during
normal use of powder coatings in spray painting workplaces is expected
to be low, as electrostatic application is an efficient application
method. Triglycidyl isocyanurate may be released into water by
facilities that manufacture, process, or use this chemical.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Much of the triglycidyl isocyanurate in powder coatings and
solder inks is immobilized through cross-linking in an insoluble
matrix. As triglycidyl isocyanurate is an epoxide, any residues not
captured in this way are expected to be rapidly degraded through
microbial action or abiotic hydrolysis.
Triglycidyl isocyanurate did not satisfy criteria for ready
biodegradability in the modified Sturm test. When exposed for 28 days
to bacteria from a sewage treatment plant, 9% and 48% of theoretical
amounts of carbon dioxide were evolved from solutions of 10 and 20 mg
triglycidyl isocyanurate/litre, respectively (Ciba-Geigy, 1988d).
Although these results indicate incomplete mineralization, they are
likely to reflect complete primary degradation, with slow opening of
the triazine ring restricting the rate of mineralization, as has been
noted for triazine herbicides (Scheunert, 1992).
In a modified Zahn-Wellers test measuring carbon dioxide
evolution rather than loss of dissolved organic carbon, triglycidyl
isocyanurate was inherently biodegradable at 11.3 mg/litre, but not at
21.1 mg/litre (44% and 1%, respectively, after 28 days) (Ciba-Geigy,
1993b). As the solubility of triglycidyl isocyanurate in this test
was said to be poor, necessitating the use of an emulsifier to achieve
the stated test concentration, the results of this study should be
treated with caution. Triglycidyl isocyanurate is not expected to
accumulate in soil or sediment because of high mobility and limited
persistence. High mobility may be predicted by analogy with the
triazine herbicide hexazinone, a chemical known to leach into
groundwater. The oxirane substituents are not expected to retard the
mobility significantly, as methyloxirane has a low soil organic matter
adsorption coefficient, generally between 3 and 30 (Howard, 1989).
Persistence in the aquatic environment is expected to be limited,
predicted by analogy with methyloxirane, which has a half-life in
fresh surface waters of 6.6 days at pH 5 and 11.6 days at pH 7-9
(Howard, 1989). Hydrolysis proceeds more rapidly in the marine
environment because of more rapid ring opening by chloride ions. The
reactivity of triglycidyl isocyanurate precludes any possibility of
bioaccumulation.
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
Information on levels of triglycidyl isocyanurate in the general
environment was not available.
6.2 Human exposure
Exposure of the general population to triglycidyl isocyanurate is
expected to be minimal. There is potential for occupational exposure,
most likely via inhalation, during the manufacture of triglycidyl
isocyanurate and the manufacture and use of products containing
triglycidyl isocyanurate. In powder coatings, triglycidyl
isocyanurate is partially cross-linked to the polyester resin before
application, and it is only the unbound triglycidyl isocyanurate that
is bioavailable. The amount of unbound triglycidyl isocyanurate
varies between different powder coatings. The triglycidyl
isocyanurate in powder coatings after application to metal particles
is fully cross-linked and is bound in a solid matrix, from which it
would not be bioavailable.
No monitoring data are available for the manufacture of
triglycidyl isocyanurate; however, monitoring data are available for
exposure to triglycidyl isocyanurate during the manufacture of powder
coatings containing the chemical. In an Australian plant, triglycidyl
isocyanurate levels in air ranged from 0.02 to 1.34 mg/m3
(time-weighted average) during July/August 1991 (NICNAS, 1994). Two
months later, levels of triglycidyl isocyanurate in air were <0.03
mg/m3 (time-weighted average) after changes, primarily improved work
practices, were implemented in the plant. In a plant in Japan,
triglycidyl isocyanurate levels up to 0.035 mg/m3 (time-weighted
average) were measured in 1991 (NICNAS, 1994). In a survey of five
plants by the United Kingdom Health and Safety Executive in 1994,
triglycidyl isocyanurate levels ranged from 0.01 to 0.44 mg/m3
(time-weighted average; mean 0.1 mg/m3), with corresponding total
inhalable particulate levels of 1.1-64 mg/m3 (mean 9.4 mg/m3) (HSE,
1994).
There are limited air monitoring data available for the
application of powder coatings containing triglycidyl isocyanurate.
In a survey of eight spray painting workplaces in Australia in 1991,
triglycidyl isocyanurate levels up to 6.5 mg/m3 (time-weighted
average) were measured (NICNAS, 1994). In a survey of 16 similar
workplaces by the Health and Safety Executive in the United Kingdom in
1994, triglycidyl isocyanurate levels ranged from 0.001 to 1.5 mg/m3
(time-weighted average; mean 0.24 mg/m3), with corresponding total
inhalable particulate levels between 0.2 and 131 mg/m3 (mean 13
mg/m3) (HSE, 1994). Air monitoring data for the printed circuit
board industry were not available.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS
AND HUMANS
The only available human data are from clinical trials with
alpha-triglycidyl isocyanurate (intravenous administration), which
indicate that alpha-triglycidyl isocyanurate has a mean half-life in
the blood of approximately 1 min and a mean total body clearance of
5.7 litres/min (Ames et al., 1984; Neidhart, 1984; Rubin et al.,
1987). Less than 1% of the administered dose was recovered unchanged
in urine within 24 h (Ames et al., 1984).
In an oral (gavage) study in mice, at least 17% of the
administered dose was absorbed within 24 h, with blood analysis
indicating that the absorption of triglycidyl isocyanurate
administered in aqueous solution was twice that of triglycidyl
isocyanurate in sesame oil. Triglycidyl isocyanurate was distributed
to the liver, stomach, and testes (the only tissues studied). Blood
plasma analysis indicated that triglycidyl isocyanurate was
metabolized by hydrolysis to the diol diepoxide, the bis-diol epoxide,
and the fully hydrolysed tris-diol, with no free triglycidyl
isocyanurate detected 8 h after treatment (Ciba-Geigy, 1990c).
In oral (gavage) and intravenous studies with
[14C]alpha-triglycidyl isocyanurate in rabbits, the radioactivity
recovered in urine within 24 h was approximately 30% and 60-70%,
respectively. In the intravenous study, the half-life of triglycidyl
isocyanurate in the blood was <5 min (Ames et al., 1984).
In in vitro studies, rapid hydrolysis of triglycidyl
isocyanurate involving the enzyme epoxide hydrolase was observed in
mouse liver preparations (Ciba-Geigy, 1990c). Hydrolysis was also
observed in rat liver preparations but not in rat lung preparations
(Ames et al., 1984). Microsomal epoxide hydrolase activity with
triglycidyl isocyanurate as substrate measured in two human livers
obtained from kidney donors was found to be greater than the activity
in rat liver (Ciba-Geigy, 1993a).
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
Triglycidyl isocyanurate is toxic to laboratory animals following
acute oral administration and inhalation exposure. The oral LD50 for
triglycidyl isocyanurate ranges from 188 to 715 mg/kg body weight in
rats (Ciba-Geigy, 1975a, 1982a, 1990a; Safepharm, 1988a). Clinical
signs of toxicity observed prior to death included sedation, dyspnoea,
and emaciation. Pathological findings included oedematous and
haemorrhagic lungs, haemorrhagic thymus, intestines, and testes,
involuted testes, and enlarged kidney.
In rat inhalation (nose-only) studies, an LC50 of 650 mg/m3 was
obtained with triglycidyl isocyanurate in dust form (Ciba-Geigy,
1979a), and an LC50 of >300 mg/m3 was found with triglycidyl
isocyanurate in liquid aerosol form (Ciba-Geigy, 1979b). Slight
inflammation of the nasal mucosa in the upper dose groups was
observed. There were no substance-related gross organ changes in
sacrificed animals, and partial haemorrhage only was observed in the
lungs of animals who died during the study. An LC50 of 2000 mg/m3
was obtained for the mouse (whole-body exposure to dust, particle size
range 3.2-3.9 µm) (Bushy Run, 1991). Clinical signs included
hypoactivity and ocular and respiratory irritation. Pathological
observations included perinasal/periocular/perioral encrustation and
lung discoloration.
The dermal LD50 for rats was >2000 mg/kg body weight
(Ciba-Geigy, 1975b, 1990b; Safepharm, 1988b). There were no deaths or
exposure-related adverse clinical signs; at necropsy, no gross organ
changes were observed.
8.2 Irritation and sensitization
In several rabbit studies, triglycidyl isocyanurate caused slight
skin irritation, with both very slight erythema and very slight oedema
observed in the intact skin of some animals up to 72 h
post-application (Ciba-Geigy, 1979c,d, 1982b; Safepharm, 1988c,d).
Triglycidyl isocyanurate caused serious eye damage in rabbits,
including severe corneal opacity and chemosis (Ciba-Geigy, 1979e,
1982c).
Triglycidyl isocyanurate (commercial grade) was positive for skin
sensitization in guinea-pigs in two modified Magnusson and Kligman
studies (Ciba-Geigy, 1988b; Safepharm, 1988e). In both studies,
groups of 10 male and 10 female guinea-pigs were initially exposed to
triglycidyl isocyanurate and challenged 2 weeks after induction.
Positive responses were observed in 25% of the test animals in one
study (Ciba-Geigy, 1988b) and in 60% of the test animals in the other
study (Safepharm, 1988e). No skin reactions were noted in the control
group at induction or when challenged with triglycidyl isocyanurate.
8.3 Short-term exposure
In a 7-day oral rat study, gross pathology was recorded for the
lungs, kidney, liver, stomach, and intestines. Renal tubular damage
and haemorrhagic and degenerative changes involving the gastric and
duodenal mucosa were observed at the high triglycidyl isocyanurate
dose (216 mg/kg body weight per day for males and 172 mg/kg body
weight per day for females). Less marked changes to the renal tubules
were noted at the low dose (54 mg/kg body weight per day for males and
43 mg/kg body weight per day for females) (Shell, 1971).
In a study in which male CD-1 mice were exposed (nose-only) to
triglycidyl isocyanurate at 0, 10, 40, or 140 mg/m3, 6 h/day for 5
days, mortality, body weight loss, and lung damage occurred at the
highest concentrations (40 and 140 mg/m3). No exposure-related
effects were observed at 10 mg/m3. Clinical signs of toxicity
observed in the intermediate- and high-concentration groups included
lethargy, ptosis, decreased respiratory rate, and noisy or gasping
respiration. Gross pathology and organ weights were recorded for the
lungs, liver, kidney, and testes. Pathological findings included dark
or reddened lungs, pale liver, pale kidneys, and congestion of the
small intestine (Safepharm, 1991).
8.4 Long-term exposure
Information from general subchronic or chronic toxicity studies
was not available.1
8.5 Genotoxicity and related end-points
The genotoxicity of triglycidyl isocyanurate has been
investigated in a wide range of in vitro and in vivo assays
(Tables 2 and 3). Triglycidyl isocyanurate was mutagenic in
Salmonella typhimurium and mouse lymphoma cells and clastogenic in
Chinese hamster ovary cells in vitro. It induced chromosomal
aberrations in bone marrow cells in hamsters and in germ cells in mice
following oral administration. Available data also indicate that
triglycidyl isocyanurate has the potential to alkylate DNA.
In two reverse mutation assays, commercial-grade triglycidyl
isocyanurate induced mutations in the presence and absence of
metabolic activation in S. typhimurium strains TA1535, TA1538, TA98,
and TA100, with the effect more pronounced in the latter two strains
(Ciba-Geigy, 1982d; Hazleton, 1987). Triglycidyl isocyanurate was not
mutagenic in TA1537. In one of these studies, triglycidyl
isocyanurate did not induce back mutation in Escherichia coli
1 The final results of a chronic toxicity/carcinogenicity bioassay
in rats conducted by the Centre Internationale de Toxicologie (CIT) in
France were not available at the time this CICAD was prepared.
WP2uvrA, with or without metabolic activation (Ciba-Geigy, 1982d).
All studies were well conducted, using appropriate negative and
positive controls, with the results indicating that triglycidyl
isocyanurate is a direct-acting mutagen.
In a mouse lymphoma cell assay, triglycidyl isocyanurate induced
forward mutations in the presence of metabolic activation at 6.0 µg/ml
and in the absence of metabolic activation at 2.8 µg/ml (Ciba-Geigy,
1983a). Triglycidyl isocyanurate with and without metabolic
activation induced sister chromatid exchanges and chromosomal
aberrations in Chinese hamster ovary cells (Loveday et al., 1990;
Sofuni et al., 1990). Triglycidyl isocyanurate also tested positive
for the induction of chromosomal aberrations without metabolic
activation in Chinese hamster lung cells but was negative with
metabolic activation (Sofuni et al., 1990). In an unscheduled DNA
synthesis assay in rat hepatocytes, a clear dose-response relationship
was noted for triglycidyl isocyanurate over the range 5-20 µg/ml
(Ciba-Geigy, 1988c). However, in a similar study conducted with human
fibroblasts, triglycidyl isocyanurate did not induce unscheduled DNA
synthesis at concentrations up to 400 µg/ml (Ciba-Geigy, 1988a). In
two cell transformation studies in mouse embryo fibroblasts,
triglycidyl isocyanurate did not induce any significant increase in
either transformed colony number or size in the concentration range
8.8-5000 ng/ml (Ciba-Geigy, 1983b, 1986a).
Triglycidyl isocyanurate did not induce chromosomal aberrations
in human lymphocytes at concentrations up to 2500 ng/ml. Only one
aberration was reported at each of the two higher concentrations of
5000 and 10 000 ng/ml. Significant numbers of aberrations were
observed with the positive controls. As only a very late sampling
time was used, the results are considered questionable (Ciba-Geigy,
1985).
In a gavage study, male and female Chinese hamsters were
administered 0, 140, 280, or 560 mg triglycidyl isocyanurate/kg body
weight per day for 2 days. Triglycidyl isocyanurate induced small but
significant increases in nuclear anomalies in bone marrow cells at the
two highest doses, indicative of clastogenicity (Ciba-Geigy, 1983c).
Two gavage studies were conducted to determine the ability of
triglycidyl isocyanurate to induce sister chromatid exchanges in bone
marrow cells in male and female Chinese hamsters. In one study, no
increase in the number of sister chromatid exchanges was observed in
animals administered a single dose of 0, 35, 70, or 140 mg triglycidyl
isocyanurate/kg body weight (Ciba-Geigy, 1984). In the study in which
triglycidyl isocyanurate was administered at a single dose of 0, 140,
280, or 560 mg/kg body weight, a dose-related increase in sister
chromatid exchange in bone marrow cells (with statistically
significant increases) was observed at all exposures (Ciba-Geigy,
1983d). The results of the mouse spot test were negative when
triglycidyl isocyanurate was administered at doses of 13.5, 27, or 54
mg/kg body weight in a single intraperitoneal injection to pregnant
mice on the 10th day after conception (Ciba-Geigy, 1986d).
Table 2: Genotoxicity of triglycidyl isocyanurate in vitro.
Resultsa
With Without
Species (test system) End-point Test concentration activation activation Reference
Procaryotic systems
Salmonella typhimurium Gene mutation 1-10 000 µg/plate + + Hazleton, 1987
(TA1535, TA1538, TA98, TA100) 5-5000 µg/plate + + Ciba-Geigy, 1982d
Escherichia coli WP2uvrA Gene mutation 5-5000 µg/plate - - Ciba-Geigy, 1982d
Animal systems
Mouse lymphoma Gene mutation 0.375-6.0 µg/ml + NT Ciba-Geigy, 1983a
0.175-2.8 µg/ml NT +
Rat hepatocytes Unscheduled 0.20-20 µg/ml NT + Ciba-Geigy, 1988c
DNA synthesis
Chinese hamster ovary cells Sister chromatid 1.98-19.8 µg/ml + NT Loveday et al.,
exchanges 0.066-0.66 µg/ml NT + 1990
Chinese hamster ovary cells Chromosomal 10-100 µg/ml + NT Loveday et al.,
aberrations 3-30 µg/ml NT + 1990
Chinese hamster ovary cells Chromosomal 10-100 µg/ml + NT Sofuni et al., 1990
aberrations 3-50 µg/ml NT +
Chinese hamster lung cells Chromosomal 1.25-5 µg/ml - + Sofuni et al., 1990
aberrations
Mouse embryo fibroblasts Cell 8.75-140 ng/ml NT - Ciba-Geigy, 1983b
transformation 0.3125-5 µg/ml - NT Ciba-Geigy, 1986a
Table 2: (continued)
Resultsa
With Without
Species (test system) End-point Test concentration activation activation Reference
Human cells
Human lymphocytes Chromosomal 62.5-10 000 ng/ml NT Equivocal Ciba-Geigy, 1985
aberrations
Human fibroblasts Unscheduled 2.7-400 µg/ml NT - Ciba-Geigy, 1988a
DNA synthesis
a NT = not tested; - = negative result; + = positive result.
Table 3: Genotoxicity of triglycidyl isocyanurate in vivo.
Species (test Route of
system) End-point exposure Dose Resultsa Reference
Chinese hamster Nuclear Oral 0, 140, 280, 560 mg/kg body weight + Ciba-Geigy, 1983c
bone marrow anomalies
Chinese hamster Sister Oral 0, 35, 70, 140 mg/kg body weight - Ciba-Geigy, 1984
bone marrow chromatid Oral 0, 140, 280, 560 mg/kg body weight + Ciba-Geigy, 1983d
exchange
Mouse Chromosomal Oral 0, 43, 128 mg/kg body weight + Ciba-Geigy, 1986b
spermatogonial aberrations Oral 0, 30, 125, 350 mg/kg body weight + Hazleton, 1989a
cells Oral 0, 29, 58, 115 mg/kg body weight + Hazleton, 1991
Oral 115 mg/kg body weight + Safepharm, 1992
Inhalation 0, 2.5, 10, 50 mg/m3 Equivocal Bushy Run, 1992a
Inhalation 0, 7.8 mg/m3 - Safepharm, 1992
Mouse Chromosomal Oral 0, 32, 96 mg/kg body weight - Ciba-Geigy, 1986e
spermatocytes aberrations
Mouse spot test Gene mutation Intraperitoneal 13.5, 27, 54 mg/kg body weight - Ciba-Geigy, 1986d
Mouse Dominant Oral 0, 160, 480 mg/kg body weight Equivocalb Ciba-Geigy, 1986c
lethal Oral 0, 138, 275, 550 mg/kg body weight -b Hazleton, 1989b
mutations Inhalation 0.25, 10, 50 mg/m3 - Bushy Run, 1992b
Mouse stomach, DNA binding Oral 5, 17, 200 mg/kg body weight + Ciba-Geigy, 1990c
liver, and
testis
Rat liver DNA binding Oral, 20 mg/kg body weight + Ciba-Geigy, 1993a
intraperitoneal
a NT = not tested; - = negative result; + = positive result.
b Mating period included only first 3 weeks post-treatment.
A number of oral studies have been conducted to investigate the
potential of triglycidyl isocyanurate to induce chromosomal
aberrations in mouse germ cells. In male ICR mice administered 0, 30,
125, or 350 mg triglycidyl isocyanurate/kg body weight for 5 days,
chromosomal aberrations were induced in spermatogonial cells at the
two highest doses (Hazleton, 1989a). In another study in which male
B6D2F1 mice were administered 0, 29, 58, or 115 mg triglycidyl
isocyanurate/kg body weight for 5 days, chromosomal aberrations in
spermatogonial cells were significantly increased at all doses
(Hazleton, 1991). When male TifMAGf mice were administered 0, 43, or
128 mg triglycidyl isocyanurate/kg body weight for 5 days, a
dose-related increase in chromosomal aberrations was observed in
spermatogonial cells; however, a statistical analysis was not
conducted (Ciba-Geigy, 1986b). Chromosomal aberrations were not
induced in the spermatocytes of male mice administered (by gavage)
0, 32, or 96 mg triglycidyl isocyanurate/kg body weight for 4 days
(Ciba-Geigy, 1986e). In a single-dose oral study, chromosomal
aberrations were induced in mouse spermatogonia at 115 mg triglycidyl
isocyanurate/kg body weight (Safepharm, 1992).
In a (whole-body) inhalation study, mice were exposed to 0, 2.5,
10, or 50 mg triglycidyl isocyanurate/m3 (particle size range 2.5-3.5
µm) for 6 h/day for 5 days. Effects on mouse spermatogonial cells
were measured by the induction of chromosomal aberrations. The results
of this study were inconclusive (Bushy Run, 1992a). The number of
chromosomal aberrations in the control group was high, there was a
very low number of scorable cells at the highest concentrations (10
and 50 mg/m3), and cytotoxicity was not clearly established, as the
cytotoxic ratio was not measured. At 2.5 mg/m3, the number of
chromosomal aberrations was only slightly higher than in the controls,
which was unusually high. In an (nose-only) inhalation study, male
CD-1 mice were exposed to 0 or 7.8 mg triglycidyl isocyanurate/m3
(mean particle size 1.6 µm) for 6 h/day for 5 days. Chromosomal
aberrations were not induced in spermatogonial cells at the single
concentration tested (Safepharm, 1992). In both these studies, body
weight gain was unaffected, no deaths occurred, and no adverse
clinical signs were observed.
In a dominant lethal test, male TifMAGf(SPF) mice were
administered (by gavage) 0, 160, or 480 mg triglycidyl isocyanurate/kg
body weight. In females mated to the high-dose males, there was a
significant increase in the number of embryonic deaths when mating
occurred during the 1st week after exposure, but not when mating took
place 2-3 weeks after the males were exposed (Ciba-Geigy, 1986c). No
increase in embryonic deaths was noted in females mated to the
low-dose males 1-3 weeks after exposure. The results of this study are
considered equivocal. In a study in which male ICR mice were
administered (by gavage) 0, 138, 275, or 550 mg triglycidyl
isocyanurate/kg body weight, no significant increase in the number of
embryonic deaths was observed in the mated females (Hazleton, 1989b).
These studies are of limited value, as the mating periods covered only
the first 3 weeks after the males had been exposed. In another study,
male CD-1 mice were exposed (by inhalation) to 0.25, 10, or 50 mg
triglycidyl isocyanurate/m3. A reduction in male fertility (i.e.
number of sperm-positive and pregnant females) was observed in
high-dose males in the first 3 weeks and 6th week post-exposure, as
well as in mid-dose males in the 3rd week post-exposure only. No
dominant lethal effects were observed; however, the results are
suggestive of an effect on mature sperm, maturing spermatids, and Type
B spermatogonia. This is the only dominant lethal study in which the
mating period covered all stages of the spermatogenic cycle (Bushy
Run, 1992b).
The molecular structure of triglycidyl isocyanurate indicates a
potential for alkylating DNA. A dose-dependent increase in
triglycidyl isocyanurate-DNA adduct formation was observed in a study
in which male TifMAGf(SPF) mice were orally administered 5, 17, or 200
mg triglycidyl isocyanurate/kg body weight. DNA alkylation was
measured as the covalent binding index. For the highest dose, the
ratio of covalent binding indices for the stomach, liver, and testes
at 3 h after exposure was approximately 30 : 7 : 1. The highest
covalent binding index, for the stomach, was 8.9, compared with
covalent binding indices of 20 000 for the potent liver carcinogen
aflatoxin B1 and 200 for the moderate carcinogen 2-acetylaminofluorene
(Ciba-Geigy, 1990c). In a second DNA alkylation study, male
TifRAIf(SPF) rats were pretreated with trans-stilbene oxide at 0,
100, or 400 mg/kg body weight to induce epoxide hydrolase activity,
followed by triglycidyl isocyanurate administered orally or
intraperitoneally (20 mg/kg body weight). The dose-dependent increase
in liver microsomal epoxide hydrolase activity was associated with a
dose-dependent decrease in DNA binding by triglycidyl isocyanurate,
calculated as the covalent binding index. However, the relatively low
covalent binding indices suggested that only a small proportion of
triglycidyl isocyanurate binds to DNA. As mentioned in section 7,
microsomal epoxide hydrolase activity with triglycidyl isocyanurate as
substrate measured in two human livers was greater than the activity
in non-induced rat liver (Ciba-Geigy, 1993a).
8.6 Reproductive and developmental toxicity
In a 13-week toxicity/fertility study, groups of 10 male rats
were given diets containing 0, 10, 30, or 100 ppm (mg/kg) triglycidyl
isocyanurate (CIT, 1995). This study followed a preliminary 19-day
range-finding investigation in which signs of toxicity (large
mesenteric lymph nodes, small prostate and seminal vesicles) were
observed in animals administered diets containing 160 or 640 ppm
(mg/kg) triglycidyl isocyanurate. In the full study after 64 days of
treatment, each male was placed with two females until mating
occurred. The females were then allocated to two subgroups (caesarean
or normal delivery) on day 19 of pregnancy. Females from the
caesarean group were killed on day 20 of pregnancy and the ovaries and
uterus examined to determine number of corpora lutea, live and dead
fetuses, resorptions, and implantation sites. The other group was
allowed to deliver normally; litter size was noted, pups were examined
for presence of clinical signs, and their development was recorded.
Between 22 and 25 days postpartum, the females in the normal delivery
group were sacrificed, the main thoracic and abdominal organs were
examined, and the number of implantation sites was noted. In males at
autopsy, all organ weights and macroscopic and microscopic changes
were recorded in the control and highest-dose groups, with selected
organs examined in the other test groups.
No exposure-related clinical effects or deaths were observed.
Body weight gain was slightly lower over the first 6 weeks in animals
from the 100 ppm (mg/kg) test group. A dose-related reduction in the
number of spermatozoa was noted; compared with the unexposed controls,
there was a 5%, 13%, and 23% reduction in spermatozoa in males
administered diets containing 10, 30, and 100 ppm (mg/kg) triglycidyl
isocyanurate, respectively. Spermatozoa viability was similar in the
triglycidyl isocyanurate-exposed and control groups. No
exposure-related infertility was noted in males, and no effects on
embryonic and pup development were observed in the offspring of
females mated with triglycidyl isocyanurate-exposed males (CIT, 1995).
However, it should be noted that the highest concentration used in
this study (100 ppm; mg/kg) was not a maximum tolerated dose.
There were no other data available on the developmental toxicity
of triglycidyl isocyanurate.
9. EFFECTS ON HUMANS
Allergic contact dermatitis has been reported in several
case-studies of exposure to triglycidyl isocyanurate and powder
coatings containing triglycidyl isocyanurate. Exposure occurred
during the manufacture of triglycidyl isocyanurate (Nishioka et al.,
1988) and powdered paint coatings (Foulds & Koh, 1992; Munro &
Lawrence, 1992), during the application of products containing
triglycidyl isocyanurate, including powder coatings (Matthias, 1988;
McFadden & Rycroft, 1993) and hardener for epoxy acrylate ink (Jolanki
et al., 1994), and while cleaning equipment contaminated with
triglycidyl isocyanurate (Dooms-Goossens et al., 1989). Symptoms
included dermatitis, itchy rashes, and swelling on the face, hands,
arms, neck, and thighs. All subjects tested positive to patch testing
with triglycidyl isocyanurate.
A case-study reported asthma-like symptoms in a spray painter who
had been using powder coatings containing triglycidyl isocyanurate for
4 years (Piirila et al., 1997). Symptoms included elevated blood
eosinophils and serum immunoglobulin E (IgE), moderate bronchial
hyper-reactivity, and a reduction in forced expiratory volume in 1
second (FEV1) of 23% and 19% when challenged with 4% triglycidyl
isocyanurate and 4% triglycidyl isocyanurate mixed with lactose,
respectively. The worker also had eczema on his hands, face, and
body; the skin prick test was negative.
Human trials were performed during clinical development of
alpha-triglycidyl isocyanurate as an antitumour agent. In these
studies, alpha-triglycidyl isocyanurate was administered intravenously
to cancer patients at doses up to 900 mg/kg body weight using a
variety of dosing regimes. Toxic signs included myelosuppression,
nausea and vomiting, and, rarely, alopecia and leucopenia at high
doses (>600 mg/kg body weight). Owing to its severe local toxicity
(thrombophlebitis) at the site of injection, the use of
alpha-triglycidyl isocyanurate as an antitumour agent was not pursued
(HSE, 1992).
There were no epidemiological studies on triglycidyl isocyanurate
available.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
Only limited ecotoxicological data for triglycidyl isocyanurate
are available. No deaths were observed at the only concentration
tested in a 96-h static test with zebra fish (Brachydanio rerio);
the no-observed-effect concentration (NOEC) was >77 mg/litre
(Ciba-Geigy, 1988e). The 24-h EC50 in a static immobilization test
with Daphnia magna was >100 mg/litre, with a NOEC of 58 mg/litre
(Ciba-Geigy, 1988f). These results indicate that triglycidyl
isocyanurate is, at most, slightly toxic to aquatic fauna under
conditions of acute exposure. Chronic effects would not be expected
because of limited aquatic persistence.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
The only reported health effects in humans are contact dermatitis
and one case of respiratory sensitization.
Acute animal toxicity studies reveal that triglycidyl
isocyanurate is toxic by oral and inhalation routes of exposure but
has low acute dermal toxicity. Triglycidyl isocyanurate produces
serious eye irritation. It is a skin sensitizer but not a skin
irritant. Short-term repeated-dose studies revealed renal, lung,
gastric/duodenal, and sperm cell damage. In a subchronic
toxicity/fertility study conducted in rats, a dose-dependent reduction
in the number of spermatozoa was the only effect observed at
concentrations of up to 100 ppm (mg/kg) triglycidyl isocyanurate in
the diet.
Triglycidyl isocyanurate has structural alerts suggesting
direct-acting mutagenicity. DNA binding of triglycidyl isocyanurate
has been measured in mouse liver, testes, and stomach following oral
administration. The chemical has produced positive results in a range
of in vitro genotoxicity studies (gene mutation in bacterial and
mammalian cells, unscheduled DNA synthesis, sister chromatid
exchanges, and chromosomal aberration assays). Genotoxic effects have
also been observed in vivo in somatic (bone marrow) cells and germ
cells in the testes. Triglycidyl isocyanurate therefore causes
heritable mutations, and the demonstrated ability for triglycidyl
isocyanurate to cause genetic damage also raises concern over
potential carcinogenic effects.
Genotoxicity studies have revealed that the inhalation of
triglycidyl isocyanurate produces cytotoxicity and chromosomal
aberrations in mouse spermatogonia, and therefore there may be a risk
of reproductive effects. A 13-week dietary study in rats has
indicated no effects on male fertility, but no firm conclusions can be
drawn in view of the low doses tested. Overall, the reproductive
toxicity of triglycidyl isocyanurate has not been adequately
investigated. As triglycidyl isocyanurate is a direct-acting
in vivo mutagen, identification of a no-observed-effect level for
the non-critical end-points of systemic toxicity may not be
appropriate.
11.1.2 Criteria for setting guidance values for triglycidyl
isocyanurate
Exposure of the general public to triglycidyl isocyanurate is
likely to be minimal. The main risk to human health is through
occupational exposure via inhalation. Data from human and animal
studies upon which to base a guidance value for occupational exposure
to triglycidyl isocyanurate are very limited. Based on the
information available from animal studies, the critical effect for
chronic exposure is the potential genotoxicity of triglycidyl
isocyanurate. Triglycidyl isocyanurate is a direct-acting in vivo
mutagen, and it is not possible to identify a level of exposure below
which there would be no risk to human health.
11.1.3 Sample risk characterization
Because triglycidyl isocyanurate is genotoxic, a
no-observed-effect level cannot be determined, and a quantitative risk
characterization is not considered appropriate. Public exposure to
the chemical is likely to be very low. Owing to its genotoxic and
sensitization effects, workplace exposures should be maintained at the
lowest practicable level.
11.2 Evaluation of environmental effects
Triglycidyl isocyanurate is not expected to produce adverse
effects, as environmental exposure is low. Much of the triglycidyl
isocyanurate contained in waste will be effectively immobile, as it is
bound in the coating matrix of triglycidyl isocyanurate-containing
materials. Amounts released into the environment will have limited
persistence because of rapid biodegradation.
Some triglycidyl isocyanurate and triglycidyl
isocyanurate-containing powder coatings may also be released to the
atmosphere and the sewage system. The limited ecotoxicological data
indicate that the acute toxicity of triglycidyl isocyanurate in
aquatic fauna is low (NOEC >58 mg/litre). Chronic effects are not
expected because of limited aquatic persistence.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Previous evaluations of triglycidyl isocyanurate by international
bodies were not identified. Information on international hazard
classification and labelling is included in the International Chemical
Safety Card reproduced in this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventative and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 1274) reproduced in this
document.
13.1 Human health hazards
Triglycidyl isocyanurate is a skin sensitizer and has the
potential to cause heritable mutations in humans. It may also cause
serious eye damage.
13.2 Advice to physicians
In case of intoxication, the treatment is supportive.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards
can be found in the International Register of Potentially Toxic
Chemicals (IRPTC), available from UNEP Chemicals (IRPTC), Geneva.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
TRIGLYCIDYL ISOCYANURATE ICSC: 1274
08.10.1997
CAS # 2451-62-9
RTECS # XZ1994900
1,3,5-Triglycidyl isocyanurate
s-Triazine-2,4,6(1H,3H,5H)-trione
Tris(epoxypropyl)isocyanurate
C12H15N3O6
Molecular mass: 297.3
TYPES OF HAZARD/ ACUTE HAZARDS/ PREVENTION FIRST AID/FIRE FIGHTING
EXPOSURE SYMPTOMS
FIRE Combustible. Gives off irritating or NO open flames. Foam, powder, carbon dioxide.
toxic fumes (or gases) in a fire.
EXPLOSION Finely dispersed particles form Prevent deposition of dust; closed
explosive mixtures in air. system, dust explosion-proof
electrical equipment and lighting.
EXPOSURE PREVENT DISPERSION OF DUST!
AVOID ALL CONTACT!
Inhalation Local exhaust or breathing protection Fresh air, rest.
Skin Protective gloves. Protective Remove contaminated clothes. Rinse skin
clothing. with plenty of water or shower.
Eyes Redness. Pain. Safety goggles, or eye protection in First rinse with plenty of water for
combination with breathing several minutes (remove contact lenses if
protection. easily possible), then take to a doctor.
Ingestion Do not eat, drink, or smoke during Rinse mouth. Induce vomiting (ONLY IN)
work. CONSCIOUS PERSONS!). Refer for medical
attention.
(continued)
SPILLAGE DISPOSAL PACKAGING & LABELLING
Sweep spilled substance into sealable containers. Carefully collect Symbol
remainder, then remove to safe place. Do NOT let this chemical enter R: 23/25-41-43-46
the environment (extra personal protection: P2 filter respirator for S:
harmful particles). UN Hazard Class:
UN Subsidiary Risks:
UN Pack Group:
EMERGENCY RESPONSE STORAGE
Well closed.
IMPORTANT DATA
PHYSICAL STATE: APPEARANCE ROUTES OF EXPOSURE:
WHITE POWDER OR GRANULE The substance can be absorbed into the body by inhalation of its
aerosol and by ingestion.
PHYSICAL DANGERS: INHALATION RISK:
Dust explosion possible if in powder or granular form, No indication can be given about the rate in which a harmful
mixed with air. concentration in the air is reached on evaporation of this
substance at 20°C.
CHEMICAL DANGERS: EFFECTS OF SHORT-TERM EXPOSURE:
The substance may polymerize due to heating to more than 120°C The substance irritates severely the eyes. The substance may
for more than 12 hours, or under the influence of catalysts. cause effects on the central nervous system, kidneys, liver,
The substance decomposes on burning producing toxic fumes lungs and gastrointestinal tract, resulting in tissue lesions.
including nitrogen oxides. Molten triglycidyl isocyanurate
reacts rapidly with primary and secondary amines, carboxylic
acids and anhydrides, thiols, phenols and alcohols.
OCCUPATIONAL EXPOSURE LIMITS: EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
TLV not established. Repeated or prolonged contact may cause skin sensitization. May
cause heritable genetic damage in humans.
(continued)
PHYSICAL PROPERTIES
Melting point: 95°C
Density: 1.5 g/cm3
Solubility in water,
g/100 ml at 25°C: 0.9 (technical grade)
Flash point: >170°C (technical grade)
Auto-ignition temperature: >200°C (technical grade)
Octanol/water partition
coefficient as log Pow: -0.8 (technical grade)
ENVIRONMENTAL DATA
NOTES
Technical grade of this substance is a mixture of alpha and beta isomers.
REFERENCES
Ames M, Kovach JS, Rubin J (1984) Pharmacological characterization of
Teroxirone, a triepoxide antitumour agent in rats, rabbits and humans.
Cancer research, 44:4151-4156.
Atassi G, Spreafico F, Dumont P, Nayer P, Klatersky J (1980)
Antitumoral effect in mice of a new triepoxide derivative:
1,3,5-triglycidyl- S-triazinetrione (NSC 296934). European journal
of cancer, 16(12):1561-1567.
Bushy Run (1991) PL90-810: Acute dust inhalation toxicity test in
mice. Export, PA, Bushy Run Research Center.
Bushy Run (1992a) PL90-810: Chromosomal aberrations assay in mouse
spermatogonial cells, Project No. 54-520. Export, PA, Bushy Run
Research Center.
Bushy Run (1992b) Dominant lethal assay of inhaled PL 90-810 dust in
CD-1 mice (No. 54-515). Export, PA, Bushy Run Research Center.
Ciba-Geigy (1975a) Acute oral toxicity in the rat of TK 10622 (No.
SISS 4735). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1975b) Acute dermal toxicity of TK 10622 in the rat.
Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1979a) Acute aerosol inhalation toxicity in the rat of
TK 10622/3 (No. 790339). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1979b) Acute aerosol inhalation toxicity in the rat of
TK 10622 (No. 790336). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1979c) Skin irritation in the rabbit after single
application of TK 10622/3 (No. 790340). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1979d) Skin irritation in the rabbit after single
application of TK 10622 (No. 790337). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1979e) Eye irritation in the rabbit after single
application of TK 10622/3 (No. 790341). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1982a) Acute oral toxicity in the rat (No. 820694) with
TK 10622. Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1982b) Acute skin irritation in the rabbit with TK 10622
(No. 820697). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1982c) Acute eye irritation study in the rabbit with TK
10622 (No. 820696). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1982d) Salmonella and Escherichia/ liver microsome test
with TK 10622. Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1983a) L5178Y/TK+/œ mouse lymphoma mutagenicity test
with TK 10622 (No. 830797). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1983b) BALB/3T3 cell transformation assay with TK 10622
(No. 820934). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1983c) Nucleus anomaly test in somatic interphase nuclei
of Chinese hamster with TK 10622 (No. 820931). Basel, Ciba-Geigy
Ltd.
Ciba-Geigy (1983d) Sister chromatid exchange studies on somatic
cells of the Chinese hamster (No. 820932). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1984) Sister chromatid exchange studies on somatic cells
of Chinese hamster (No. 830989). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1985) Chromosome studies on human lymphocytes in vitro
with TK 10622 (No. 850071). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1986a) Transformation/liver microsome test with TK 10622
(No. 850072). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1986b) Chromosome studies on male germinal epithelium of
mouse spermatogonia (No. 850067). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1986c) Dominant lethal test, mouse, three weeks (No.
850069). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1986d) Mammalian spot test, mouse, 8 weeks (No. 850070)
with TK 10622. Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1986e) Chromosome studies on male epithelium mouse
spermatocytes (No. 850068) with TK 10622. Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1988a) Autoradiographic DNA repair test on human
fibroblasts with TK 10622 (No. 874267). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1988b) Skin sensitization test in the guinea pig,
modified maximization test (No. 884210). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1988c) Autoradiographic DNA repair test on rat
hepatocytes with TK 10622 (No. 874266). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1988d) Report on the test for ready biodegradability of
TK 10622 in the modified Sturm test (Report No. 884053). Basel,
Ciba-Geigy Ltd.
Ciba-Geigy (1988e) Report on the test for acute toxicity of TK 10622
to zebra fish (Brachydanio rerio). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1988f) Report on the test for acute toxicity of TK 10622
to Daphnia magna. Basel, Ciba-Geigy Ltd, March.
Ciba-Geigy (1990a) Acute oral toxicity in the rat (No. 894516) of TK
10622 (Araldite PT 810). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1990b) Acute dermal toxicity in the rat (No. 894517)
with TK 10622 (Araldite PT 810). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1990c) Potential for DNA binding of Araldite PT 810 (No.
PS32-01903). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1991) Analytical method, Araldite PT 810 pure in powder
coatings. Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1993a) Hazard assessment with Araldite PT 810:
Inactivation by subcellular liver fractions and binding to liver
DNA, Project No. 924004. Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1993b) Report on the test for inherent biodegradability
in a combined Zahn-Wellers/carbon dioxide evolution test of Araldite
PT 810. Basel, Ciba-Geigy Ltd.
CIT (1995) 13-week toxicity study and fertility study by oral route
(dietary admixture) in male rats with PT 810(R) (TGIC). Miserey,
Centre Internationale de Toxicologie.
Dooms-Goossens A, Bedert R, Vandaele M, Degreef H (1989) Airborne
contact dermatitis due to triglycidyl isocyanurate. Contact
dermatitis, 21(3):202-203.
Foulds IS, Koh D (1992) Allergic contact dermatitis from resin
hardeners during the manufacture of thermosetting coating paints.
Contact dermatitis, 26:87-90.
Hazleton (1987) Mutagenicity test on Araldite PT 810 in the Ames
Salmonella/microsome reverse mutation assay (No. E-9782-0-401).
Veenendaal, Hazleton Biotechnologies.
Hazleton (1989a) Mutagenicity test on PL88-810 in the mouse
spermatogonial cell cytogenetic assay (No. 10386-0-474). Kensington,
MD, Hazleton Laboratories America Inc.
Hazleton (1989b) Mutagenicity test on PL88-810 in the dominant
lethal assay (No. 10386-0-471). Kensington, MD, Hazleton
Laboratories America Inc.
Hazleton (1991) Study to evaluate the chromosome damaging potential
of TK 10622 (PT810 [TGIC, 97%]) by its effects on the spermatogonial
cells of treated mice. Heslington, York, Hazleton Microtest.
Howard P (1989) Handbook of environmental fate and exposure data for
organic chemicals. Vol.1. Large production and priority pollutants.
Chelsea, MI, Lewis Publishers, p. 485.
HSE (1992) Toxicity review 27 - Part 1: Triglycidyl isocyanurate.
United Kingdom, Health and Safety Executive, HMSO Publication (ISBN 0
11 886343 6).
HSE (1994) Triglycidyl isocyanurate, a review of use and exposure.
United Kingdom, Health and Safety Executive, Hygiene Technology and
Chemical Agents Unit.
IPCS (1997) International Chemical Safety Card - Triglycidyl
isocyanurate. Geneva, World Health Organization, International
Programme on Chemical Safety (No. 1274).
Jolanki R, Kanerva L, Estlander T, Tarvainen K (1994) Concomitant
sensitization to triglycidyl isocyanurate, diaminodiphenylmethane and
2-hydroxyethyl methacrylate from silk-screen printing coatings in the
manufacture of circuit boards. Contact dermatitis, 30:12-15.
Loveday KS, Anderson BE, Resnick MA, Zeiger E (1990) Chromosome
aberration and sister chromatid exchange tests in Chinese hamster
ovary cells in vitro. V: Results with 46 chemicals. Environmental
and molecular mutagenesis, 16:272-303.
Matthias CG (1988) Allergic contact dermatitis from triglycidyl
isocyanurate in polyester paint pigments. Contact dermatitis,
19(1):67-68.
McFadden JP, Rycroft RJG (1993) Occupational contact dermatitis from
triglycidyl isocyanurate in a powder paint sprayer. Contact
dermatitis, 28:251.
Munro CS, Lawrence CM (1992) Occupational contact dermatitis from
triglycidyl isocyanurate in a powder paint factory. Contact
dermatitis, 26:59.
Neidhart JA (1984) Phase I trial of Teroxirone. Cancer treatment
reports, 68:1115-1119.
NICNAS (1994) Priority Existing Chemical No.1 - Triglycidyl
isocyanurate, full public report, National Industrial Chemicals
Notification and Assessment Scheme. Canberra, Australian Government
Publishing Service, April.
Nishioka K, Ogasawara M, Asagami C (1988) Occupational contact allergy
to triglycidyl isocyanurate (TGIC, TEPIC). Contact dermatitis,
19(5):379-380.
Nissan (no date) TEPIC, information on basic properties. Tokyo,
Nissan Chemical Industries Ltd.
Piirila P, Estlander T, Keskinen H, Jolanki R, Laakkonen A, Pfaffli P,
Tupasela O, Tuppurainen M, Nordman H (1997) Occupational asthma caused
by triglycidyl isocyanurate (TGIC). Clinical and experimental
allergy, 27:510-514.
Rubin J, Kovach JS, Ames MM, Moertel CG, Creagan ET, O'Connell MJ
(1987) Phase I study of two schedules of Teroxirone. Cancer
treatment reports, 71:489-492.
Safepharm (1988a) TEPIC-G: Acute oral toxicity test in the rat
(No. 14/10). Derby, Safepharm Laboratories Ltd.
Safepharm (1988b) TEPIC-G: Acute dermal toxicity (limit test) in
the rat (No. 14/11). Derby, Safepharm Laboratories Ltd.
Safepharm (1988c) TEPIC-G: Acute dermal irritation test in the
rabbit (No. 14/12). Derby, Safepharm Laboratories Ltd.
Safepharm (1988d) TEPIC-G: Acute dermal irritation test in the
rabbit (24-hour exposure) (No. 14/13). Derby, Safepharm Laboratories
Ltd.
Safepharm (1988e) TEPIC-G: Magnusson and Kligman maximisation study
in the guinea pig (No. 14/14). Derby, Safepharm Laboratories Ltd.
Safepharm (1991) TEPIC SP: Five-day repeat exposure inhalation
toxicity study in the male mouse (No. 14/68). Derby, Safepharm
Laboratories Ltd.
Safepharm (1992) TGIC technical and TGIC ten per cent powder:
Chromosome analysis in mouse spermatogonial cells, comparative
inhalation study (No. 14/75) [draft]. Derby, Safepharm Laboratories
Ltd.
Scheunert I (1992) Transformation and degradation of pesticides in
soil. In: Ebing W, ed. Terrestrial behavior of pesticides. Berlin,
Springer-Verlag, p. 64.
Shell (1971) Toxicity studies on the diglycidyl esters of
tetrahydrophthalic and hexahydrophthalic acids and of
triglycidylisocyanurate: Repeated dosing experiments with rats
(TLGR.0019.71). London, Shell Research Ltd.
Sofuni T, Matsuoka A, Sawada M, Ishidate M Jr, Zeiger E, Shelby MD
(1990) A comparison of chromosome aberration induction by 25 compounds
tested by two Chinese hamster cell (CHL and CHO) systems in culture.
Mutation research, 241:175-213.
APPENDIX 1 - SOURCE DOCUMENT
National Industrial Chemicals Notification and Assessment Scheme -
Triglycidyl Isocyanurate, Priority Existing Chemical No. 1, Full
Public Report (1994)
Copies of the NICNAS (1994) report on triglycidyl isocyanurate
(prepared by D. Willcocks, L. Onyon, C. Jenkins, and B. Diver) may be
obtained from:
Australian Government Publishing Service
Mail Order Service
GPO Box 84
Canberra 2601, Australia
NICNAS reports are prepared to meet the requirements of the
Industrial Chemicals Notification and Assessment Act, 1989, as
amended. In the preparation of the assessment report, both internal
and external peer review are undertaken. Under the NICNAS
legislation, applicants for the assessment of a chemical (i.e.
importers and manufacturers of the chemical) may apply for variations
to the draft report. The following companies participated in the
review of the assessment at this stage: Ciba-Geigy Australia Ltd,
Dulux Powder Coatings, Evode Powder Coatings Pty Ltd, Itochu Australia
Ltd, Jotun Powder Coatings Pty Ltd, Sumitomo Australia Ltd, Taubmans
Pty Ltd, and Western Coatings Ltd.
In the assessment of triglycidyl isocyanurate, Ciba-Geigy
Australia Ltd made several requests to vary the assessment report -
notably in the areas of acute toxicity and mutagenicity - which the
Director of NICNAS refused. Consequently, Ciba-Geigy lodged an
application for review of the Director's decision with the independent
Administrative Appeals Tribunal (members included Justice D.F.
O'Connor and Professor G. Johnston, Sydney University). The
Administrative Appeals Tribunal looked at all the material relevant to
the assessment decisions in question and upheld the Director's
decisions.1 During the Administrative Appeals Tribunal review
process, the following expert witnesses were called upon: Dr D.J.
Birkett, Flinders University School of Medicine, Australia; Dr M.E.
McManus, University of Queensland, Australia; Dr H.J. Weideli,
Ciba-Geigy Ltd, Basel, Switzerland; Dr C. Winder, University of New
South Wales, Australia; and Dr E. Zeiger, National Institute of
Environmental Health Sciences, Research Triangle Park, NC, USA.
1 Administrative Appeals Tribunal, No. P93/339, Re Ciba-Geigy
Australia Ltd (Applicant) and Worksafe Australia (Respondent), March
1994.
APPENDIX 2 - CICAD PEER REVIEW
The draft CICAD on triglycidyl isocyanurate was sent for review
to institutions and organizations identified by IPCS after contact
with IPCS national Contact Points and Participating Institutions, as
well as to identified experts. Comments were received from:
Ciba Speciality Chemicals Inc., Basel, Switzerland
Department of Health, London, United Kingdom
Department of Public Health Promotion, Prague, Czech Republic
Environment Canada, Ottawa, Canada
Health and Safety Executive, Bootle, United Kingdom
Health Canada, Ottawa, Canada
National Chemicals Inspectorate (KEMI), Solna, Sweden
National Institute of Occupational Health, Budapest, Hungary
National Institute of Public Health, Oslo, Norway
United States Department of Health and Human Services (National
Institute for Occupational Safety and Health, Cincinnati, USA;
National Institute of Environmental Health Sciences, Research
Triangle Park, USA)
United States Environmental Protection Agency (National Center
for Environmental Assessment, Office of Research and Development,
Research Triangle Park, USA)
APPENDIX 3 - CICAD FINAL REVIEW BOARD
Berlin, Germany, 26-28 November 1997
Members
Dr H. Ahlers, Education and Information Division, National Institute
for Occupational Safety and Health, Cincinnati, OH, USA
Mr R. Cary, Health Directorate, Health and Safety Executive, Bootle,
United Kingdom
Dr S. Dobson, Institute of Terrestrial Ecology, Huntingdon, United
Kingdom
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany (Chairperson)
Mr J.R. Hickman, Health Protection Branch, Health Canada, Ottawa,
Ontario, Canada
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany
Ms M.E. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada (Rapporteur)
Dr K. Paksy, Department of Reproductive Toxicology, National Institute
of Occupational Health, Budapest, Hungary
Mr V. Quarg, Ministry for the Environment, Nature Conservation &
Nuclear Safety, Bonn, Germany
Mr D. Renshaw, Department of Health, London, United Kingdom
Dr J. Sekizawa, Division of Chemo-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan
Prof S. Soliman, Department of Pesticide Chemistry, Alexandria
University, Alexandria, Egypt (Vice-Chairperson)
Dr M. Wallen, National Chemicals Inspectorate (KEMI), Solna, Sweden
Ms D. Willcocks, Chemical Assessment Division, Worksafe Australia,
Camperdown, Australia
Dr M. Williams-Johnson, Division of Toxicology, Agency for Toxic
Substances and Disease Registry, Atlanta, GA, USA
Dr K. Ziegler-Skylakakis, Senatskommission der Deutschen
Forschungsgemeinschaft zuer Pruefung gesundheitsschaedlicher
Arbeitsstoffe, GSF-Institut fuer Toxikologie, Neuherberg,
Oberschleissheim, Germany
Observers
Mrs B. Dinham,1 The Pesticide Trust, London, United Kingdom
Dr R. Ebert, KSU Ps-Toxicology, Huels AG, Marl, Germany (representing
ECETOC, the European Centre for Ecotoxicology and Toxicology of
Chemicals)
Mr R. Green,1 International Federation of Chemical, Energy, Mine and
General Workers' Unions, Brussels, Belgium
Dr B. Hansen,1 European Chemicals Bureau, European Commission, Ispra,
Italy
Dr J. Heuer, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Mr T. Jacob,1 DuPont, Washington, DC, USA
Ms L. Onyon, Environment Directorate, Organisation for Economic
Co-operation and Development, Paris, France
Dr H.J. Weideli, Ciba Speciality Chemicals Inc., Basel, Switzerland
(representing CEFIC, the European Chemical Industry Council)
Secretariat
Dr M. Baril, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr R.G. Liteplo, Health Canada, Ottawa, Ontario, Canada
Ms L. Regis, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Mr A. Strawson, Health and Safety Executive, London, United Kingdom
Dr P. Toft, Associate Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
1 Invited but unable to attend.
RÉSUMÉ D'ORIENTATION
Ce CICAD est fondé principalement sur l'évaluation de
l'isocyanurate de triglycidyle réalisée dans le cadre du NICNAS
(Australian National Industrial Chemicals Notification and Assessment
Scheme) et publiée en avril 1994 (NICNAS, 1994). De nouvelles
informations parues entre l'achèvement du rapport NICNAS et
novembre 1997 ont également été évaluées et incorporées dans ce CICAD,
de même que des informations complémentaires extraites de l'étude du
United Kingdom Health and Safety Executive (HSE, 1992) portant sur la
toxicité de cette substance. Des informations relatives à l'examen du
rapport NICNAS par les pairs et à sa disponibilité figurent à
l'appendice 1. Les renseignements concernant l'examen du CICAD par
les pairs font l'objet de l'appendice 2. Ce CICAD a été approuvé en
tant qu'évaluation internationale lors d'une une réunion du Comité
d'évaluation finale qui s'est tenue à Berlin (Allemagne) du 26 au
28 novembre 1997. La liste des participants à cette réunion figure à
l'appendice 3. La fiche d'information sur la sécurité chimique de
l'isocyanurate de triglycidyle (ICSC 1274), établie par le Programme
international sur la Sécurité chimique (IPCS, 1997), est également
reproduite dans le présent document.
L'isocyanurate de triglycidyle (CAS N° 2451-62-9) est une
substance chimique de synthèse qui se présente sous la forme d'une
poudre blanche ou d'un granulé pratiquement inodore à la température
ambiante. Il est employé principalement comme agent de polymérisation
ou de réticulation tridimensionnelle dans les revêtements de polyester
en poudre (peinture). Ces revêtements en poudre contiennent
généralement de 4 à 10 % d'isocyanurate de triglycidyle. Il est
également utilisé dans les encres servant à la fabrication de masques
dans l'industrie des circuits imprimés. L'encre est constituée de
deux parties dont l'élément durcisseur contient approximativement 60 %
d'isocyanurate de triglycidyle. La plus grande partie de
l'isocyanurate de triglycidyle présent dans ces revêtements en poudre
et ces encres est immobilisée par réticulation dans une matrice
insoluble.
En principe, l'exposition de la population générale à
l'isocyanurate de triglycidyle devrait être minime. Par contre, il
existe un risque d'exposition professionnelle lors de la fabrication
et de l'utilisation de cette substance et des produits qui en
contiennent.
On dispose de peu d'informations sur les effets de l'isocyanurate
de triglycidyle chez l'homme. On a signalé plusieurs cas de dermatite
allergique de contact et un cas de sensibilisation respiratoire à la
suite d'une exposition professionnelle.
L'isocyanurate de triglycidyle est très toxique par ingestion et
inhalation chez les animaux de laboratoire et il peut provoquer de
graves lésions oculaires. Sur la peau, il agit comme sensibilisant,
mais non comme irritant. Les données sur sa toxicité à la suite d'une
exposition répétée sont limitées. Chez des rats et des souris soumis
à des expositions répétées pendant une courte période (5-7 jours), on
a constaté des effets sur les reins, le foie, les poumons, le tube
digestif et les spermatogonies. Une étude de 13 semaines menée sur
des rats mâles pour évaluer la toxicité de l'isocyanurate de
triglycidyle et ses effets sur la fécondité a révélé une réduction de
nombre de spermatozoïdes liée à la dose.
Des études de génotoxicité in vitro et in vivo ont montré que
l'isocyanurate de triglycidyle est un mutagène à action directe
capable d'agir sur les organes de la reproduction. Compte tenu de sa
génotoxicité potentielle, toutes les mesures appropriées doivent être
prises pour réduire au minimum l'exposition humaine à cette substance.
Compte tenu de sa faible persistance et d'une écotoxicité
probablement faible, l'isocyanurate de triglycidyle ne devrait pas
présenter de risque significatif pour l'environnement, sauf en cas de
rejet accidentel ou d'élimination dans des conditions inadaptées.
RESUMEN DE ORIENTACION
Este CICAD se basa principalmente en la evaluación del
isocianurato de triglicidilo realizada en el marco del Plan Nacional
Australiano de Notificación y Evaluación de Sustancias Químicas
Industriales (NICNAS) y publicada en abril de 1994 (NICNAS, 1994).
También se ha evaluado e incorporado a este CICAD la información
aparecida desde la terminación del informe del NICNAS hasta noviembre
de 1997. Se ha incluido asimismo alguna información adicional
procedente del examen de la toxicidad del isocianurato de triglicidilo
de la Dirección de Salud y Seguridad del Reino Unido (HSE, 1992). La
información relativa al examen colegiado del informe del NICNAS y a su
disponibilidad figura en el Apéndice 1. La información sobre el
examen colegiado de este CICAD se presenta en el Apéndice 2. Este
CICAD se aprobó como evaluación internacional en una reunión de la
Junta de Evaluación Final celebrada en Berlín (Alemania) los días
26-28 de noviembre de 1997. La lista de participantes en la Junta de
Evaluación Final figura en el Apéndice 3. La Ficha internacional de
seguridad química (ICSC 1274) para el isocianurato de triglicidilo,
preparada por el Programa Internacional de Seguridad de las Sustancias
Químicas (IPCS, 1997), también se reproduce en este documento.
El isocianurato de triglicidilo (CAS Nº 2451-62-9), sustancia
química sintética en forma de polvo blanco o granulada prácticamente
inodora a temperatura ambiente, se utiliza sobre todo como agente de
polimerización o de reticulación tridimensional en los revestimientos
de poliéster en polvo (pintura). Estos revestimientos en polvo suelen
contener entre un 4% y un 10% de isocianurato de triglicidilo.
También se utiliza éste en las tintas que sirven para la fabricación
de "máscaras" de soldadura en la industria de los circuitos impresos.
Las tintas constan de dos partes, cuyo elemento endurecedor contiene
alrededor de un 60% de isocianurato de triglicidilo. La mayor parte
de esta sustancia presente en los revestimientos en polvo y las tintas
mencionados se inmoviliza mediante la reticulación en una matriz
insoluble.
La exposición de la población general al isocianurato de
triglicidilo se supone que es mínima; sin embargo, hay riesgo de
exposición profesional durante la fabricación y la utilización de esta
sustancia y de los productos que la contienen.
Se dispone de escasa información sobre los efectos del
isocianurato de triglicidilo en el ser humano. Se han notificado
varios casos de dermatitis alérgica por contacto y un caso de
sensibilización respiratoria debidos a exposición profesional.
El isocianurato de triglicidilo es muy tóxico en animales de
laboratorio por ingestión e inhalación y puede provocar lesiones
oculares graves. Es sensibilizador cutáneo, pero no irritante de la
piel. Los datos sobre toxicidad por exposición repetida son
limitados. En estudios de exposición repetida de corta duración (5-7
días) en ratas y ratones, se observaron efectos en los riñones, el
hígado, los pulmones, el aparato digestivo y los espermatogonios. En
un estudio de toxicidad/fecundidad de 13 semanas en ratas machos se
observó una reducción del número de espermatozoides relacionada con la
dosis.
Los estudios de genotoxicidad in vitro e in vivo indican que
el isocianurato de triglicidilo es un mutágeno de acción directa capaz
de afectar los órganos de la reproducción. A la vista de su
genotoxicidad potencial, deben tomarse todas las medidas apropiadas
para reducir al mínimo la exposición humana a esta sustancia.
Debido a su escasa persistencia y su ecotoxicidad probablemente
baja, no es probable que el isocianurato de triglicidilo represente un
riesgo significativo para el medio ambiente, excepto en el caso de un
accidente o de una eliminación inapropiada.
1. EXECUTIVE SUMMARY
This CICAD was based principally on the assessment of triglycidyl
isocyanurate completed under the Australian National Industrial
Chemicals Notification and Assessment Scheme (NICNAS) and published in
April 1994 (NICNAS, 1994). Information that has become available
since completion of the NICNAS report, identified up to November 1997,
has also been assessed and included in this CICAD. Some additional
information from the United Kingdom Health and Safety Executive's
toxicity review of triglycidyl isocyanurate (HSE, 1992) has also been
included. Information on the nature of peer review and availability
of the NICNAS report is presented in Appendix 1. Information on the
peer review of this CICAD is presented in Appendix 2. This CICAD was
approved as an international assessment at a meeting of the Final
Review Board, held in Berlin, Germany, on 26-28 November 1997.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 1274) for triglycidyl
isocyanurate, produced by the International Programme on Chemical
Safety (IPCS, 1997), has also been reproduced in this document.
Triglycidyl isocyanurate (CAS no. 2451-62-9), a synthetic white
powder or granule with no discernible odour at room temperature, is
used mainly as a three-dimensional cross-linking or curing agent in
polyester powder coatings (paints). These powder coatings usually
contain between 4 and 10% triglycidyl isocyanurate. Triglycidyl 2
isocyanurate is also used in solder "mask" inks in the printed circuit
board industry. The two-part inks contain approximately 60%
triglycidyl isocyanurate in the hardener component. Much of the
triglycidyl isocyanurate in powder coatings and solder inks is
immobilized through cross-linking in an insoluble matrix.
Exposure of the general population to triglycidyl isocyanurate is
expected to be minimal; however, there is potential for occupational
exposure during the manufacture of triglycidyl isocyanurate and the
manufacture and use of products containing the chemical.
Little information is available on the effects of triglycidyl
isocyanurate on humans. Several cases of allergic contact dermatitis
and one case of respiratory sensitization caused by occupational
exposure to triglycidyl isocyanurate have been reported.
In laboratory animals, triglycidyl isocyanurate is acutely toxic
by ingestion and inhalation and can cause serious eye damage. It is a
skin sensitizer but not a skin irritant. The data for
repeated-exposure toxicity are limited. In short-term (5-7 days)
repeated-exposure studies in rats and mice, effects on the kidneys,
liver, lungs, gastrointestinal tract, and spermatogonial cells were
observed. In a 13-week toxicity/fertility study in male rats, a
dose-related reduction in the number of spermatozoa was observed.
In vitro and in vivo genotoxicity studies indicate that
triglycidyl isocyanurate is a direct-acting mutagen capable of
affecting the reproductive organs. In view of its potential
genotoxicity, all appropriate measures should be taken to minimize
human exposure to triglycidyl isocyanurate.
Owing to its low persistence and probable low ecotoxicity,
triglycidyl isocyanurate is unlikely to pose a significant hazard to
the environment, except in the case of an accident or inappropriate
disposal.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
Triglycidyl isocyanurate (CAS no. 2451-62-9; C12H15N3O6;
1,3,5-triglycidyl isocyanurate, tris(2,3-epoxypropyl) isocyanurate) is
a synthetic chemical, manufactured and supplied as the technical
grades TEPIC and Araldite PT 810. Technical-grade triglycidyl
isocyanurate is a mixture of two diastereomers, alpha and beta. Some
physical and chemical properties of triglycidyl isocyanurate
(technical grades) are presented in Table 1. Additional properties
are presented in the International Chemical Safety Card reproduced in
this document.
Triglycidyl isocyanurate is a white powder or granule with no
discernible odour at room temperature. The solubility of Araldite PT
810 in various solvents at 25°C is as follows: epichlorohydrin, <22%;
methanol, 7.3%; toluene, 3%; isopropanol, 1%. Triglycidyl
isocyanurate reacts rapidly with primary and secondary amines,
carboxylic acids and anhydrides, thiols, phenols, and alcohols. It
can be polymerized by catalysts and may undergo violent
autopolymerization. Combustion products include carbon dioxide,
carbon monoxide, and oxides of nitrogen.
Table 1: Physical and chemical properties of triglycidyl isocyanurate
(technical grades).
Araldite PT
Property TEPICa 810b
Degree of purity (%
triglycidyl isocyanurate) 90 (approx.) >97
Melting point (°C) 90-125 95
Vapour pressure
(kPa at 20°C) n.a.c 7.2 × 10-9
Solubility in water
(g/litre at 25°C)d 9 8.7
Partition coefficient
(log Kow) -0.8 n.a.
a Information provided by Nissan Chemical Industries (Nissan,
no date).
b Information provided by Ciba-Geigy (1991).
c n.a. = not available.
d The alpha and beta isomers have different water solubilities:
10.1 and 0.53 g/litre, respectively (Atassi et al., 1980).
3. ANALYTICAL METHODS
Methods of detection and analysis include infrared spectroscopy,
mass spectroscopy, epoxy equivalent weight, gas chromatography, and
high-performance liquid chromatography. Methodology for the sampling
and analysis of triglycidyl isocyanurate in air has been developed by
its manufacturers and involves the collection of the dust on a glass
fibre filter, followed by high-performance liquid chromatography with
ultraviolet detection (detection limit 0.8 µg/m3) (NICNAS, 1994).
Methodology for the analysis of triglycidyl isocyanurate in other
environmental media is not available.
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Triglycidyl isocyanurate does not occur naturally. It is
produced industrially by reacting cyanuric acid with excess
epichlorohydrin. The worldwide production of triglycidyl isocyanurate
is approximately 7000-8000 tonnes per year. Australia imports
100-1000 tonnes per year as technical-grade triglycidyl isocyanurate
for the manufacture of polyester powder coatings or as an ingredient
in powder coatings. The United Kingdom imports approximately 400
tonnes of triglycidyl isocyanurate per year for use in powder
coatings. In the United Kingdom, approximately 30 tonnes of solder
"mask" inks containing triglycidyl isocyanurate are manufactured per
year by four or five companies (NICNAS, 1994).
The main use of triglycidyl isocyanurate is as a
three-dimensional cross-linking or curing agent in polyester powder
coatings (paints). In the manufacture of powder coatings, triglycidyl
isocyanurate granules are mixed with other ingredients; the mix is
then melted and extruded as a brittle sheet, chipped, and packaged.
Generally, the particle size of 90-95% of the powder coating is
>10 µm.
Powder coatings usually contain 4-10% triglycidyl isocyanurate
and are sprayed onto metal objects by an electrostatic process. The
coated metal objects are then treated in an oven to a temperature of
about 200°C. This heating causes the powder coatings to melt, flow,
and chemically cross-link. The coatings are durable and resist
ultraviolet damage; as a result, they are typically used in outdoor
applications. Triglycidyl isocyanurate is also used in solder "mask"
inks in the printed circuit board industry. The two-part inks contain
approximately 60% triglycidyl isocyanurate in the hardener component.
The inks are applied by curtain coating, electrostatic spraying, or
screen printing. The coated circuit board is finally passed through
an oven at 150°C to complete the curing.
Release of the chemical to air is expected to be minimal. During
the manufacture of triglycidyl isocyanurate and the formulation of
products containing triglycidyl isocyanurate, dust extractors and
other pollution control devices remove particulate waste for disposal.
Triglycidyl isocyanurate contained in such waste is effectively
immobilized after consignment to landfill, particularly if waste
powder is heat-cured beforehand. Release to the environment during
normal use of powder coatings in spray painting workplaces is expected
to be low, as electrostatic application is an efficient application
method. Triglycidyl isocyanurate may be released into water by
facilities that manufacture, process, or use this chemical.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Much of the triglycidyl isocyanurate in powder coatings and
solder inks is immobilized through cross-linking in an insoluble
matrix. As triglycidyl isocyanurate is an epoxide, any residues not
captured in this way are expected to be rapidly degraded through
microbial action or abiotic hydrolysis.
Triglycidyl isocyanurate did not satisfy criteria for ready
biodegradability in the modified Sturm test. When exposed for 28 days
to bacteria from a sewage treatment plant, 9% and 48% of theoretical
amounts of carbon dioxide were evolved from solutions of 10 and 20 mg
triglycidyl isocyanurate/litre, respectively (Ciba-Geigy, 1988d).
Although these results indicate incomplete mineralization, they are
likely to reflect complete primary degradation, with slow opening of
the triazine ring restricting the rate of mineralization, as has been
noted for triazine herbicides (Scheunert, 1992).
In a modified Zahn-Wellers test measuring carbon dioxide
evolution rather than loss of dissolved organic carbon, triglycidyl
isocyanurate was inherently biodegradable at 11.3 mg/litre, but not at
21.1 mg/litre (44% and 1%, respectively, after 28 days) (Ciba-Geigy,
1993b). As the solubility of triglycidyl isocyanurate in this test
was said to be poor, necessitating the use of an emulsifier to achieve
the stated test concentration, the results of this study should be
treated with caution. Triglycidyl isocyanurate is not expected to
accumulate in soil or sediment because of high mobility and limited
persistence. High mobility may be predicted by analogy with the
triazine herbicide hexazinone, a chemical known to leach into
groundwater. The oxirane substituents are not expected to retard the
mobility significantly, as methyloxirane has a low soil organic matter
adsorption coefficient, generally between 3 and 30 (Howard, 1989).
Persistence in the aquatic environment is expected to be limited,
predicted by analogy with methyloxirane, which has a half-life in
fresh surface waters of 6.6 days at pH 5 and 11.6 days at pH 7-9
(Howard, 1989). Hydrolysis proceeds more rapidly in the marine
environment because of more rapid ring opening by chloride ions. The
reactivity of triglycidyl isocyanurate precludes any possibility of
bioaccumulation.
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
Information on levels of triglycidyl isocyanurate in the general
environment was not available.
6.2 Human exposure
Exposure of the general population to triglycidyl isocyanurate is
expected to be minimal. There is potential for occupational exposure,
most likely via inhalation, during the manufacture of triglycidyl
isocyanurate and the manufacture and use of products containing
triglycidyl isocyanurate. In powder coatings, triglycidyl
isocyanurate is partially cross-linked to the polyester resin before
application, and it is only the unbound triglycidyl isocyanurate that
is bioavailable. The amount of unbound triglycidyl isocyanurate
varies between different powder coatings. The triglycidyl
isocyanurate in powder coatings after application to metal particles
is fully cross-linked and is bound in a solid matrix, from which it
would not be bioavailable.
No monitoring data are available for the manufacture of
triglycidyl isocyanurate; however, monitoring data are available for
exposure to triglycidyl isocyanurate during the manufacture of powder
coatings containing the chemical. In an Australian plant, triglycidyl
isocyanurate levels in air ranged from 0.02 to 1.34 mg/m3
(time-weighted average) during July/August 1991 (NICNAS, 1994). Two
months later, levels of triglycidyl isocyanurate in air were <0.03
mg/m3 (time-weighted average) after changes, primarily improved work
practices, were implemented in the plant. In a plant in Japan,
triglycidyl isocyanurate levels up to 0.035 mg/m3 (time-weighted
average) were measured in 1991 (NICNAS, 1994). In a survey of five
plants by the United Kingdom Health and Safety Executive in 1994,
triglycidyl isocyanurate levels ranged from 0.01 to 0.44 mg/m3
(time-weighted average; mean 0.1 mg/m3), with corresponding total
inhalable particulate levels of 1.1-64 mg/m3 (mean 9.4 mg/m3) (HSE,
1994).
There are limited air monitoring data available for the
application of powder coatings containing triglycidyl isocyanurate.
In a survey of eight spray painting workplaces in Australia in 1991,
triglycidyl isocyanurate levels up to 6.5 mg/m3 (time-weighted
average) were measured (NICNAS, 1994). In a survey of 16 similar
workplaces by the Health and Safety Executive in the United Kingdom in
1994, triglycidyl isocyanurate levels ranged from 0.001 to 1.5 mg/m3
(time-weighted average; mean 0.24 mg/m3), with corresponding total
inhalable particulate levels between 0.2 and 131 mg/m3 (mean 13
mg/m3) (HSE, 1994). Air monitoring data for the printed circuit
board industry were not available.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS
AND HUMANS
The only available human data are from clinical trials with
alpha-triglycidyl isocyanurate (intravenous administration), which
indicate that alpha-triglycidyl isocyanurate has a mean half-life in
the blood of approximately 1 min and a mean total body clearance of
5.7 litres/min (Ames et al., 1984; Neidhart, 1984; Rubin et al.,
1987). Less than 1% of the administered dose was recovered unchanged
in urine within 24 h (Ames et al., 1984).
In an oral (gavage) study in mice, at least 17% of the
administered dose was absorbed within 24 h, with blood analysis
indicating that the absorption of triglycidyl isocyanurate
administered in aqueous solution was twice that of triglycidyl
isocyanurate in sesame oil. Triglycidyl isocyanurate was distributed
to the liver, stomach, and testes (the only tissues studied). Blood
plasma analysis indicated that triglycidyl isocyanurate was
metabolized by hydrolysis to the diol diepoxide, the bis-diol epoxide,
and the fully hydrolysed tris-diol, with no free triglycidyl
isocyanurate detected 8 h after treatment (Ciba-Geigy, 1990c).
In oral (gavage) and intravenous studies with
[14C]alpha-triglycidyl isocyanurate in rabbits, the radioactivity
recovered in urine within 24 h was approximately 30% and 60-70%,
respectively. In the intravenous study, the half-life of triglycidyl
isocyanurate in the blood was <5 min (Ames et al., 1984).
In in vitro studies, rapid hydrolysis of triglycidyl
isocyanurate involving the enzyme epoxide hydrolase was observed in
mouse liver preparations (Ciba-Geigy, 1990c). Hydrolysis was also
observed in rat liver preparations but not in rat lung preparations
(Ames et al., 1984). Microsomal epoxide hydrolase activity with
triglycidyl isocyanurate as substrate measured in two human livers
obtained from kidney donors was found to be greater than the activity
in rat liver (Ciba-Geigy, 1993a).
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
Triglycidyl isocyanurate is toxic to laboratory animals following
acute oral administration and inhalation exposure. The oral LD50 for
triglycidyl isocyanurate ranges from 188 to 715 mg/kg body weight in
rats (Ciba-Geigy, 1975a, 1982a, 1990a; Safepharm, 1988a). Clinical
signs of toxicity observed prior to death included sedation, dyspnoea,
and emaciation. Pathological findings included oedematous and
haemorrhagic lungs, haemorrhagic thymus, intestines, and testes,
involuted testes, and enlarged kidney.
In rat inhalation (nose-only) studies, an LC50 of 650 mg/m3 was
obtained with triglycidyl isocyanurate in dust form (Ciba-Geigy,
1979a), and an LC50 of >300 mg/m3 was found with triglycidyl
isocyanurate in liquid aerosol form (Ciba-Geigy, 1979b). Slight
inflammation of the nasal mucosa in the upper dose groups was
observed. There were no substance-related gross organ changes in
sacrificed animals, and partial haemorrhage only was observed in the
lungs of animals who died during the study. An LC50 of 2000 mg/m3
was obtained for the mouse (whole-body exposure to dust, particle size
range 3.2-3.9 µm) (Bushy Run, 1991). Clinical signs included
hypoactivity and ocular and respiratory irritation. Pathological
observations included perinasal/periocular/perioral encrustation and
lung discoloration.
The dermal LD50 for rats was >2000 mg/kg body weight
(Ciba-Geigy, 1975b, 1990b; Safepharm, 1988b). There were no deaths or
exposure-related adverse clinical signs; at necropsy, no gross organ
changes were observed.
8.2 Irritation and sensitization
In several rabbit studies, triglycidyl isocyanurate caused slight
skin irritation, with both very slight erythema and very slight oedema
observed in the intact skin of some animals up to 72 h
post-application (Ciba-Geigy, 1979c,d, 1982b; Safepharm, 1988c,d).
Triglycidyl isocyanurate caused serious eye damage in rabbits,
including severe corneal opacity and chemosis (Ciba-Geigy, 1979e,
1982c).
Triglycidyl isocyanurate (commercial grade) was positive for skin
sensitization in guinea-pigs in two modified Magnusson and Kligman
studies (Ciba-Geigy, 1988b; Safepharm, 1988e). In both studies,
groups of 10 male and 10 female guinea-pigs were initially exposed to
triglycidyl isocyanurate and challenged 2 weeks after induction.
Positive responses were observed in 25% of the test animals in one
study (Ciba-Geigy, 1988b) and in 60% of the test animals in the other
study (Safepharm, 1988e). No skin reactions were noted in the control
group at induction or when challenged with triglycidyl isocyanurate.
8.3 Short-term exposure
In a 7-day oral rat study, gross pathology was recorded for the
lungs, kidney, liver, stomach, and intestines. Renal tubular damage
and haemorrhagic and degenerative changes involving the gastric and
duodenal mucosa were observed at the high triglycidyl isocyanurate
dose (216 mg/kg body weight per day for males and 172 mg/kg body
weight per day for females). Less marked changes to the renal tubules
were noted at the low dose (54 mg/kg body weight per day for males and
43 mg/kg body weight per day for females) (Shell, 1971).
In a study in which male CD-1 mice were exposed (nose-only) to
triglycidyl isocyanurate at 0, 10, 40, or 140 mg/m3, 6 h/day for 5
days, mortality, body weight loss, and lung damage occurred at the
highest concentrations (40 and 140 mg/m3). No exposure-related
effects were observed at 10 mg/m3. Clinical signs of toxicity
observed in the intermediate- and high-concentration groups included
lethargy, ptosis, decreased respiratory rate, and noisy or gasping
respiration. Gross pathology and organ weights were recorded for the
lungs, liver, kidney, and testes. Pathological findings included dark
or reddened lungs, pale liver, pale kidneys, and congestion of the
small intestine (Safepharm, 1991).
8.4 Long-term exposure
Information from general subchronic or chronic toxicity studies
was not available.1
8.5 Genotoxicity and related end-points
The genotoxicity of triglycidyl isocyanurate has been
investigated in a wide range of in vitro and in vivo assays
(Tables 2 and 3). Triglycidyl isocyanurate was mutagenic in
Salmonella typhimurium and mouse lymphoma cells and clastogenic in
Chinese hamster ovary cells in vitro. It induced chromosomal
aberrations in bone marrow cells in hamsters and in germ cells in mice
following oral administration. Available data also indicate that
triglycidyl isocyanurate has the potential to alkylate DNA.
In two reverse mutation assays, commercial-grade triglycidyl
isocyanurate induced mutations in the presence and absence of
metabolic activation in S. typhimurium strains TA1535, TA1538, TA98,
and TA100, with the effect more pronounced in the latter two strains
(Ciba-Geigy, 1982d; Hazleton, 1987). Triglycidyl isocyanurate was not
mutagenic in TA1537. In one of these studies, triglycidyl
isocyanurate did not induce back mutation in Escherichia coli
1 The final results of a chronic toxicity/carcinogenicity bioassay
in rats conducted by the Centre Internationale de Toxicologie (CIT) in
France were not available at the time this CICAD was prepared.
WP2uvrA, with or without metabolic activation (Ciba-Geigy, 1982d).
All studies were well conducted, using appropriate negative and
positive controls, with the results indicating that triglycidyl
isocyanurate is a direct-acting mutagen.
In a mouse lymphoma cell assay, triglycidyl isocyanurate induced
forward mutations in the presence of metabolic activation at 6.0 µg/ml
and in the absence of metabolic activation at 2.8 µg/ml (Ciba-Geigy,
1983a). Triglycidyl isocyanurate with and without metabolic
activation induced sister chromatid exchanges and chromosomal
aberrations in Chinese hamster ovary cells (Loveday et al., 1990;
Sofuni et al., 1990). Triglycidyl isocyanurate also tested positive
for the induction of chromosomal aberrations without metabolic
activation in Chinese hamster lung cells but was negative with
metabolic activation (Sofuni et al., 1990). In an unscheduled DNA
synthesis assay in rat hepatocytes, a clear dose-response relationship
was noted for triglycidyl isocyanurate over the range 5-20 µg/ml
(Ciba-Geigy, 1988c). However, in a similar study conducted with human
fibroblasts, triglycidyl isocyanurate did not induce unscheduled DNA
synthesis at concentrations up to 400 µg/ml (Ciba-Geigy, 1988a). In
two cell transformation studies in mouse embryo fibroblasts,
triglycidyl isocyanurate did not induce any significant increase in
either transformed colony number or size in the concentration range
8.8-5000 ng/ml (Ciba-Geigy, 1983b, 1986a).
Triglycidyl isocyanurate did not induce chromosomal aberrations
in human lymphocytes at concentrations up to 2500 ng/ml. Only one
aberration was reported at each of the two higher concentrations of
5000 and 10 000 ng/ml. Significant numbers of aberrations were
observed with the positive controls. As only a very late sampling
time was used, the results are considered questionable (Ciba-Geigy,
1985).
In a gavage study, male and female Chinese hamsters were
administered 0, 140, 280, or 560 mg triglycidyl isocyanurate/kg body
weight per day for 2 days. Triglycidyl isocyanurate induced small but
significant increases in nuclear anomalies in bone marrow cells at the
two highest doses, indicative of clastogenicity (Ciba-Geigy, 1983c).
Two gavage studies were conducted to determine the ability of
triglycidyl isocyanurate to induce sister chromatid exchanges in bone
marrow cells in male and female Chinese hamsters. In one study, no
increase in the number of sister chromatid exchanges was observed in
animals administered a single dose of 0, 35, 70, or 140 mg triglycidyl
isocyanurate/kg body weight (Ciba-Geigy, 1984). In the study in which
triglycidyl isocyanurate was administered at a single dose of 0, 140,
280, or 560 mg/kg body weight, a dose-related increase in sister
chromatid exchange in bone marrow cells (with statistically
significant increases) was observed at all exposures (Ciba-Geigy,
1983d). The results of the mouse spot test were negative when
triglycidyl isocyanurate was administered at doses of 13.5, 27, or 54
mg/kg body weight in a single intraperitoneal injection to pregnant
mice on the 10th day after conception (Ciba-Geigy, 1986d).
Table 2: Genotoxicity of triglycidyl isocyanurate in vitro.
Resultsa
With Without
Species (test system) End-point Test concentration activation activation Reference
Procaryotic systems
Salmonella typhimurium Gene mutation 1-10 000 µg/plate + + Hazleton, 1987
(TA1535, TA1538, TA98, TA100) 5-5000 µg/plate + + Ciba-Geigy, 1982d
Escherichia coli WP2uvrA Gene mutation 5-5000 µg/plate - - Ciba-Geigy, 1982d
Animal systems
Mouse lymphoma Gene mutation 0.375-6.0 µg/ml + NT Ciba-Geigy, 1983a
0.175-2.8 µg/ml NT +
Rat hepatocytes Unscheduled 0.20-20 µg/ml NT + Ciba-Geigy, 1988c
DNA synthesis
Chinese hamster ovary cells Sister chromatid 1.98-19.8 µg/ml + NT Loveday et al.,
exchanges 0.066-0.66 µg/ml NT + 1990
Chinese hamster ovary cells Chromosomal 10-100 µg/ml + NT Loveday et al.,
aberrations 3-30 µg/ml NT + 1990
Chinese hamster ovary cells Chromosomal 10-100 µg/ml + NT Sofuni et al., 1990
aberrations 3-50 µg/ml NT +
Chinese hamster lung cells Chromosomal 1.25-5 µg/ml - + Sofuni et al., 1990
aberrations
Mouse embryo fibroblasts Cell 8.75-140 ng/ml NT - Ciba-Geigy, 1983b
transformation 0.3125-5 µg/ml - NT Ciba-Geigy, 1986a
Table 2: (continued)
Resultsa
With Without
Species (test system) End-point Test concentration activation activation Reference
Human cells
Human lymphocytes Chromosomal 62.5-10 000 ng/ml NT Equivocal Ciba-Geigy, 1985
aberrations
Human fibroblasts Unscheduled 2.7-400 µg/ml NT - Ciba-Geigy, 1988a
DNA synthesis
a NT = not tested; - = negative result; + = positive result.
Table 3: Genotoxicity of triglycidyl isocyanurate in vivo.
Species (test Route of
system) End-point exposure Dose Resultsa Reference
Chinese hamster Nuclear Oral 0, 140, 280, 560 mg/kg body weight + Ciba-Geigy, 1983c
bone marrow anomalies
Chinese hamster Sister Oral 0, 35, 70, 140 mg/kg body weight - Ciba-Geigy, 1984
bone marrow chromatid Oral 0, 140, 280, 560 mg/kg body weight + Ciba-Geigy, 1983d
exchange
Mouse Chromosomal Oral 0, 43, 128 mg/kg body weight + Ciba-Geigy, 1986b
spermatogonial aberrations Oral 0, 30, 125, 350 mg/kg body weight + Hazleton, 1989a
cells Oral 0, 29, 58, 115 mg/kg body weight + Hazleton, 1991
Oral 115 mg/kg body weight + Safepharm, 1992
Inhalation 0, 2.5, 10, 50 mg/m3 Equivocal Bushy Run, 1992a
Inhalation 0, 7.8 mg/m3 - Safepharm, 1992
Mouse Chromosomal Oral 0, 32, 96 mg/kg body weight - Ciba-Geigy, 1986e
spermatocytes aberrations
Mouse spot test Gene mutation Intraperitoneal 13.5, 27, 54 mg/kg body weight - Ciba-Geigy, 1986d
Mouse Dominant Oral 0, 160, 480 mg/kg body weight Equivocalb Ciba-Geigy, 1986c
lethal Oral 0, 138, 275, 550 mg/kg body weight -b Hazleton, 1989b
mutations Inhalation 0.25, 10, 50 mg/m3 - Bushy Run, 1992b
Mouse stomach, DNA binding Oral 5, 17, 200 mg/kg body weight + Ciba-Geigy, 1990c
liver, and
testis
Rat liver DNA binding Oral, 20 mg/kg body weight + Ciba-Geigy, 1993a
intraperitoneal
a NT = not tested; - = negative result; + = positive result.
b Mating period included only first 3 weeks post-treatment.
A number of oral studies have been conducted to investigate the
potential of triglycidyl isocyanurate to induce chromosomal
aberrations in mouse germ cells. In male ICR mice administered 0, 30,
125, or 350 mg triglycidyl isocyanurate/kg body weight for 5 days,
chromosomal aberrations were induced in spermatogonial cells at the
two highest doses (Hazleton, 1989a). In another study in which male
B6D2F1 mice were administered 0, 29, 58, or 115 mg triglycidyl
isocyanurate/kg body weight for 5 days, chromosomal aberrations in
spermatogonial cells were significantly increased at all doses
(Hazleton, 1991). When male TifMAGf mice were administered 0, 43, or
128 mg triglycidyl isocyanurate/kg body weight for 5 days, a
dose-related increase in chromosomal aberrations was observed in
spermatogonial cells; however, a statistical analysis was not
conducted (Ciba-Geigy, 1986b). Chromosomal aberrations were not
induced in the spermatocytes of male mice administered (by gavage)
0, 32, or 96 mg triglycidyl isocyanurate/kg body weight for 4 days
(Ciba-Geigy, 1986e). In a single-dose oral study, chromosomal
aberrations were induced in mouse spermatogonia at 115 mg triglycidyl
isocyanurate/kg body weight (Safepharm, 1992).
In a (whole-body) inhalation study, mice were exposed to 0, 2.5,
10, or 50 mg triglycidyl isocyanurate/m3 (particle size range 2.5-3.5
µm) for 6 h/day for 5 days. Effects on mouse spermatogonial cells
were measured by the induction of chromosomal aberrations. The results
of this study were inconclusive (Bushy Run, 1992a). The number of
chromosomal aberrations in the control group was high, there was a
very low number of scorable cells at the highest concentrations (10
and 50 mg/m3), and cytotoxicity was not clearly established, as the
cytotoxic ratio was not measured. At 2.5 mg/m3, the number of
chromosomal aberrations was only slightly higher than in the controls,
which was unusually high. In an (nose-only) inhalation study, male
CD-1 mice were exposed to 0 or 7.8 mg triglycidyl isocyanurate/m3
(mean particle size 1.6 µm) for 6 h/day for 5 days. Chromosomal
aberrations were not induced in spermatogonial cells at the single
concentration tested (Safepharm, 1992). In both these studies, body
weight gain was unaffected, no deaths occurred, and no adverse
clinical signs were observed.
In a dominant lethal test, male TifMAGf(SPF) mice were
administered (by gavage) 0, 160, or 480 mg triglycidyl isocyanurate/kg
body weight. In females mated to the high-dose males, there was a
significant increase in the number of embryonic deaths when mating
occurred during the 1st week after exposure, but not when mating took
place 2-3 weeks after the males were exposed (Ciba-Geigy, 1986c). No
increase in embryonic deaths was noted in females mated to the
low-dose males 1-3 weeks after exposure. The results of this study are
considered equivocal. In a study in which male ICR mice were
administered (by gavage) 0, 138, 275, or 550 mg triglycidyl
isocyanurate/kg body weight, no significant increase in the number of
embryonic deaths was observed in the mated females (Hazleton, 1989b).
These studies are of limited value, as the mating periods covered only
the first 3 weeks after the males had been exposed. In another study,
male CD-1 mice were exposed (by inhalation) to 0.25, 10, or 50 mg
triglycidyl isocyanurate/m3. A reduction in male fertility (i.e.
number of sperm-positive and pregnant females) was observed in
high-dose males in the first 3 weeks and 6th week post-exposure, as
well as in mid-dose males in the 3rd week post-exposure only. No
dominant lethal effects were observed; however, the results are
suggestive of an effect on mature sperm, maturing spermatids, and Type
B spermatogonia. This is the only dominant lethal study in which the
mating period covered all stages of the spermatogenic cycle (Bushy
Run, 1992b).
The molecular structure of triglycidyl isocyanurate indicates a
potential for alkylating DNA. A dose-dependent increase in
triglycidyl isocyanurate-DNA adduct formation was observed in a study
in which male TifMAGf(SPF) mice were orally administered 5, 17, or 200
mg triglycidyl isocyanurate/kg body weight. DNA alkylation was
measured as the covalent binding index. For the highest dose, the
ratio of covalent binding indices for the stomach, liver, and testes
at 3 h after exposure was approximately 30 : 7 : 1. The highest
covalent binding index, for the stomach, was 8.9, compared with
covalent binding indices of 20 000 for the potent liver carcinogen
aflatoxin B1 and 200 for the moderate carcinogen 2-acetylaminofluorene
(Ciba-Geigy, 1990c). In a second DNA alkylation study, male
TifRAIf(SPF) rats were pretreated with trans-stilbene oxide at 0,
100, or 400 mg/kg body weight to induce epoxide hydrolase activity,
followed by triglycidyl isocyanurate administered orally or
intraperitoneally (20 mg/kg body weight). The dose-dependent increase
in liver microsomal epoxide hydrolase activity was associated with a
dose-dependent decrease in DNA binding by triglycidyl isocyanurate,
calculated as the covalent binding index. However, the relatively low
covalent binding indices suggested that only a small proportion of
triglycidyl isocyanurate binds to DNA. As mentioned in section 7,
microsomal epoxide hydrolase activity with triglycidyl isocyanurate as
substrate measured in two human livers was greater than the activity
in non-induced rat liver (Ciba-Geigy, 1993a).
8.6 Reproductive and developmental toxicity
In a 13-week toxicity/fertility study, groups of 10 male rats
were given diets containing 0, 10, 30, or 100 ppm (mg/kg) triglycidyl
isocyanurate (CIT, 1995). This study followed a preliminary 19-day
range-finding investigation in which signs of toxicity (large
mesenteric lymph nodes, small prostate and seminal vesicles) were
observed in animals administered diets containing 160 or 640 ppm
(mg/kg) triglycidyl isocyanurate. In the full study after 64 days of
treatment, each male was placed with two females until mating
occurred. The females were then allocated to two subgroups (caesarean
or normal delivery) on day 19 of pregnancy. Females from the
caesarean group were killed on day 20 of pregnancy and the ovaries and
uterus examined to determine number of corpora lutea, live and dead
fetuses, resorptions, and implantation sites. The other group was
allowed to deliver normally; litter size was noted, pups were examined
for presence of clinical signs, and their development was recorded.
Between 22 and 25 days postpartum, the females in the normal delivery
group were sacrificed, the main thoracic and abdominal organs were
examined, and the number of implantation sites was noted. In males at
autopsy, all organ weights and macroscopic and microscopic changes
were recorded in the control and highest-dose groups, with selected
organs examined in the other test groups.
No exposure-related clinical effects or deaths were observed.
Body weight gain was slightly lower over the first 6 weeks in animals
from the 100 ppm (mg/kg) test group. A dose-related reduction in the
number of spermatozoa was noted; compared with the unexposed controls,
there was a 5%, 13%, and 23% reduction in spermatozoa in males
administered diets containing 10, 30, and 100 ppm (mg/kg) triglycidyl
isocyanurate, respectively. Spermatozoa viability was similar in the
triglycidyl isocyanurate-exposed and control groups. No
exposure-related infertility was noted in males, and no effects on
embryonic and pup development were observed in the offspring of
females mated with triglycidyl isocyanurate-exposed males (CIT, 1995).
However, it should be noted that the highest concentration used in
this study (100 ppm; mg/kg) was not a maximum tolerated dose.
There were no other data available on the developmental toxicity
of triglycidyl isocyanurate.
9. EFFECTS ON HUMANS
Allergic contact dermatitis has been reported in several
case-studies of exposure to triglycidyl isocyanurate and powder
coatings containing triglycidyl isocyanurate. Exposure occurred
during the manufacture of triglycidyl isocyanurate (Nishioka et al.,
1988) and powdered paint coatings (Foulds & Koh, 1992; Munro &
Lawrence, 1992), during the application of products containing
triglycidyl isocyanurate, including powder coatings (Matthias, 1988;
McFadden & Rycroft, 1993) and hardener for epoxy acrylate ink (Jolanki
et al., 1994), and while cleaning equipment contaminated with
triglycidyl isocyanurate (Dooms-Goossens et al., 1989). Symptoms
included dermatitis, itchy rashes, and swelling on the face, hands,
arms, neck, and thighs. All subjects tested positive to patch testing
with triglycidyl isocyanurate.
A case-study reported asthma-like symptoms in a spray painter who
had been using powder coatings containing triglycidyl isocyanurate for
4 years (Piirila et al., 1997). Symptoms included elevated blood
eosinophils and serum immunoglobulin E (IgE), moderate bronchial
hyper-reactivity, and a reduction in forced expiratory volume in 1
second (FEV1) of 23% and 19% when challenged with 4% triglycidyl
isocyanurate and 4% triglycidyl isocyanurate mixed with lactose,
respectively. The worker also had eczema on his hands, face, and
body; the skin prick test was negative.
Human trials were performed during clinical development of
alpha-triglycidyl isocyanurate as an antitumour agent. In these
studies, alpha-triglycidyl isocyanurate was administered intravenously
to cancer patients at doses up to 900 mg/kg body weight using a
variety of dosing regimes. Toxic signs included myelosuppression,
nausea and vomiting, and, rarely, alopecia and leucopenia at high
doses (>600 mg/kg body weight). Owing to its severe local toxicity
(thrombophlebitis) at the site of injection, the use of
alpha-triglycidyl isocyanurate as an antitumour agent was not pursued
(HSE, 1992).
There were no epidemiological studies on triglycidyl isocyanurate
available.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
Only limited ecotoxicological data for triglycidyl isocyanurate
are available. No deaths were observed at the only concentration
tested in a 96-h static test with zebra fish (Brachydanio rerio);
the no-observed-effect concentration (NOEC) was >77 mg/litre
(Ciba-Geigy, 1988e). The 24-h EC50 in a static immobilization test
with Daphnia magna was >100 mg/litre, with a NOEC of 58 mg/litre
(Ciba-Geigy, 1988f). These results indicate that triglycidyl
isocyanurate is, at most, slightly toxic to aquatic fauna under
conditions of acute exposure. Chronic effects would not be expected
because of limited aquatic persistence.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
The only reported health effects in humans are contact dermatitis
and one case of respiratory sensitization.
Acute animal toxicity studies reveal that triglycidyl
isocyanurate is toxic by oral and inhalation routes of exposure but
has low acute dermal toxicity. Triglycidyl isocyanurate produces
serious eye irritation. It is a skin sensitizer but not a skin
irritant. Short-term repeated-dose studies revealed renal, lung,
gastric/duodenal, and sperm cell damage. In a subchronic
toxicity/fertility study conducted in rats, a dose-dependent reduction
in the number of spermatozoa was the only effect observed at
concentrations of up to 100 ppm (mg/kg) triglycidyl isocyanurate in
the diet.
Triglycidyl isocyanurate has structural alerts suggesting
direct-acting mutagenicity. DNA binding of triglycidyl isocyanurate
has been measured in mouse liver, testes, and stomach following oral
administration. The chemical has produced positive results in a range
of in vitro genotoxicity studies (gene mutation in bacterial and
mammalian cells, unscheduled DNA synthesis, sister chromatid
exchanges, and chromosomal aberration assays). Genotoxic effects have
also been observed in vivo in somatic (bone marrow) cells and germ
cells in the testes. Triglycidyl isocyanurate therefore causes
heritable mutations, and the demonstrated ability for triglycidyl
isocyanurate to cause genetic damage also raises concern over
potential carcinogenic effects.
Genotoxicity studies have revealed that the inhalation of
triglycidyl isocyanurate produces cytotoxicity and chromosomal
aberrations in mouse spermatogonia, and therefore there may be a risk
of reproductive effects. A 13-week dietary study in rats has
indicated no effects on male fertility, but no firm conclusions can be
drawn in view of the low doses tested. Overall, the reproductive
toxicity of triglycidyl isocyanurate has not been adequately
investigated. As triglycidyl isocyanurate is a direct-acting
in vivo mutagen, identification of a no-observed-effect level for
the non-critical end-points of systemic toxicity may not be
appropriate.
11.1.2 Criteria for setting guidance values for triglycidyl
isocyanurate
Exposure of the general public to triglycidyl isocyanurate is
likely to be minimal. The main risk to human health is through
occupational exposure via inhalation. Data from human and animal
studies upon which to base a guidance value for occupational exposure
to triglycidyl isocyanurate are very limited. Based on the
information available from animal studies, the critical effect for
chronic exposure is the potential genotoxicity of triglycidyl
isocyanurate. Triglycidyl isocyanurate is a direct-acting in vivo
mutagen, and it is not possible to identify a level of exposure below
which there would be no risk to human health.
11.1.3 Sample risk characterization
Because triglycidyl isocyanurate is genotoxic, a
no-observed-effect level cannot be determined, and a quantitative risk
characterization is not considered appropriate. Public exposure to
the chemical is likely to be very low. Owing to its genotoxic and
sensitization effects, workplace exposures should be maintained at the
lowest practicable level.
11.2 Evaluation of environmental effects
Triglycidyl isocyanurate is not expected to produce adverse
effects, as environmental exposure is low. Much of the triglycidyl
isocyanurate contained in waste will be effectively immobile, as it is
bound in the coating matrix of triglycidyl isocyanurate-containing
materials. Amounts released into the environment will have limited
persistence because of rapid biodegradation.
Some triglycidyl isocyanurate and triglycidyl
isocyanurate-containing powder coatings may also be released to the
atmosphere and the sewage system. The limited ecotoxicological data
indicate that the acute toxicity of triglycidyl isocyanurate in
aquatic fauna is low (NOEC >58 mg/litre). Chronic effects are not
expected because of limited aquatic persistence.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Previous evaluations of triglycidyl isocyanurate by international
bodies were not identified. Information on international hazard
classification and labelling is included in the International Chemical
Safety Card reproduced in this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventative and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 1274) reproduced in this
document.
13.1 Human health hazards
Triglycidyl isocyanurate is a skin sensitizer and has the
potential to cause heritable mutations in humans. It may also cause
serious eye damage.
13.2 Advice to physicians
In case of intoxication, the treatment is supportive.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards
can be found in the International Register of Potentially Toxic
Chemicals (IRPTC), available from UNEP Chemicals (IRPTC), Geneva.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
TRIGLYCIDYL ISOCYANURATE ICSC: 1274
08.10.1997
CAS # 2451-62-9
RTECS # XZ1994900
1,3,5-Triglycidyl isocyanurate
s-Triazine-2,4,6(1H,3H,5H)-trione
Tris(epoxypropyl)isocyanurate
C12H15N3O6
Molecular mass: 297.3
TYPES OF HAZARD/ ACUTE HAZARDS/ PREVENTION FIRST AID/FIRE FIGHTING
EXPOSURE SYMPTOMS
FIRE Combustible. Gives off irritating or NO open flames. Foam, powder, carbon dioxide.
toxic fumes (or gases) in a fire.
EXPLOSION Finely dispersed particles form Prevent deposition of dust; closed
explosive mixtures in air. system, dust explosion-proof
electrical equipment and lighting.
EXPOSURE PREVENT DISPERSION OF DUST!
AVOID ALL CONTACT!
Inhalation Local exhaust or breathing protection Fresh air, rest.
Skin Protective gloves. Protective Remove contaminated clothes. Rinse skin
clothing. with plenty of water or shower.
Eyes Redness. Pain. Safety goggles, or eye protection in First rinse with plenty of water for
combination with breathing several minutes (remove contact lenses if
protection. easily possible), then take to a doctor.
Ingestion Do not eat, drink, or smoke during Rinse mouth. Induce vomiting (ONLY IN)
work. CONSCIOUS PERSONS!). Refer for medical
attention.
(continued)
SPILLAGE DISPOSAL PACKAGING & LABELLING
Sweep spilled substance into sealable containers. Carefully collect Symbol
remainder, then remove to safe place. Do NOT let this chemical enter R: 23/25-41-43-46
the environment (extra personal protection: P2 filter respirator for S:
harmful particles). UN Hazard Class:
UN Subsidiary Risks:
UN Pack Group:
EMERGENCY RESPONSE STORAGE
Well closed.
IMPORTANT DATA
PHYSICAL STATE: APPEARANCE ROUTES OF EXPOSURE:
WHITE POWDER OR GRANULE The substance can be absorbed into the body by inhalation of its
aerosol and by ingestion.
PHYSICAL DANGERS: INHALATION RISK:
Dust explosion possible if in powder or granular form, No indication can be given about the rate in which a harmful
mixed with air. concentration in the air is reached on evaporation of this
substance at 20°C.
CHEMICAL DANGERS: EFFECTS OF SHORT-TERM EXPOSURE:
The substance may polymerize due to heating to more than 120°C The substance irritates severely the eyes. The substance may
for more than 12 hours, or under the influence of catalysts. cause effects on the central nervous system, kidneys, liver,
The substance decomposes on burning producing toxic fumes lungs and gastrointestinal tract, resulting in tissue lesions.
including nitrogen oxides. Molten triglycidyl isocyanurate
reacts rapidly with primary and secondary amines, carboxylic
acids and anhydrides, thiols, phenols and alcohols.
OCCUPATIONAL EXPOSURE LIMITS: EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
TLV not established. Repeated or prolonged contact may cause skin sensitization. May
cause heritable genetic damage in humans.
(continued)
PHYSICAL PROPERTIES
Melting point: 95°C
Density: 1.5 g/cm3
Solubility in water,
g/100 ml at 25°C: 0.9 (technical grade)
Flash point: >170°C (technical grade)
Auto-ignition temperature: >200°C (technical grade)
Octanol/water partition
coefficient as log Pow: -0.8 (technical grade)
ENVIRONMENTAL DATA
NOTES
Technical grade of this substance is a mixture of alpha and beta isomers.
REFERENCES
Ames M, Kovach JS, Rubin J (1984) Pharmacological characterization of
Teroxirone, a triepoxide antitumour agent in rats, rabbits and humans.
Cancer research, 44:4151-4156.
Atassi G, Spreafico F, Dumont P, Nayer P, Klatersky J (1980)
Antitumoral effect in mice of a new triepoxide derivative:
1,3,5-triglycidyl- S-triazinetrione (NSC 296934). European journal
of cancer, 16(12):1561-1567.
Bushy Run (1991) PL90-810: Acute dust inhalation toxicity test in
mice. Export, PA, Bushy Run Research Center.
Bushy Run (1992a) PL90-810: Chromosomal aberrations assay in mouse
spermatogonial cells, Project No. 54-520. Export, PA, Bushy Run
Research Center.
Bushy Run (1992b) Dominant lethal assay of inhaled PL 90-810 dust in
CD-1 mice (No. 54-515). Export, PA, Bushy Run Research Center.
Ciba-Geigy (1975a) Acute oral toxicity in the rat of TK 10622 (No.
SISS 4735). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1975b) Acute dermal toxicity of TK 10622 in the rat.
Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1979a) Acute aerosol inhalation toxicity in the rat of
TK 10622/3 (No. 790339). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1979b) Acute aerosol inhalation toxicity in the rat of
TK 10622 (No. 790336). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1979c) Skin irritation in the rabbit after single
application of TK 10622/3 (No. 790340). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1979d) Skin irritation in the rabbit after single
application of TK 10622 (No. 790337). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1979e) Eye irritation in the rabbit after single
application of TK 10622/3 (No. 790341). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1982a) Acute oral toxicity in the rat (No. 820694) with
TK 10622. Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1982b) Acute skin irritation in the rabbit with TK 10622
(No. 820697). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1982c) Acute eye irritation study in the rabbit with TK
10622 (No. 820696). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1982d) Salmonella and Escherichia/ liver microsome test
with TK 10622. Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1983a) L5178Y/TK+/œ mouse lymphoma mutagenicity test
with TK 10622 (No. 830797). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1983b) BALB/3T3 cell transformation assay with TK 10622
(No. 820934). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1983c) Nucleus anomaly test in somatic interphase nuclei
of Chinese hamster with TK 10622 (No. 820931). Basel, Ciba-Geigy
Ltd.
Ciba-Geigy (1983d) Sister chromatid exchange studies on somatic
cells of the Chinese hamster (No. 820932). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1984) Sister chromatid exchange studies on somatic cells
of Chinese hamster (No. 830989). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1985) Chromosome studies on human lymphocytes in vitro
with TK 10622 (No. 850071). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1986a) Transformation/liver microsome test with TK 10622
(No. 850072). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1986b) Chromosome studies on male germinal epithelium of
mouse spermatogonia (No. 850067). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1986c) Dominant lethal test, mouse, three weeks (No.
850069). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1986d) Mammalian spot test, mouse, 8 weeks (No. 850070)
with TK 10622. Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1986e) Chromosome studies on male epithelium mouse
spermatocytes (No. 850068) with TK 10622. Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1988a) Autoradiographic DNA repair test on human
fibroblasts with TK 10622 (No. 874267). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1988b) Skin sensitization test in the guinea pig,
modified maximization test (No. 884210). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1988c) Autoradiographic DNA repair test on rat
hepatocytes with TK 10622 (No. 874266). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1988d) Report on the test for ready biodegradability of
TK 10622 in the modified Sturm test (Report No. 884053). Basel,
Ciba-Geigy Ltd.
Ciba-Geigy (1988e) Report on the test for acute toxicity of TK 10622
to zebra fish (Brachydanio rerio). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1988f) Report on the test for acute toxicity of TK 10622
to Daphnia magna. Basel, Ciba-Geigy Ltd, March.
Ciba-Geigy (1990a) Acute oral toxicity in the rat (No. 894516) of TK
10622 (Araldite PT 810). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1990b) Acute dermal toxicity in the rat (No. 894517)
with TK 10622 (Araldite PT 810). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1990c) Potential for DNA binding of Araldite PT 810 (No.
PS32-01903). Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1991) Analytical method, Araldite PT 810 pure in powder
coatings. Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1993a) Hazard assessment with Araldite PT 810:
Inactivation by subcellular liver fractions and binding to liver
DNA, Project No. 924004. Basel, Ciba-Geigy Ltd.
Ciba-Geigy (1993b) Report on the test for inherent biodegradability
in a combined Zahn-Wellers/carbon dioxide evolution test of Araldite
PT 810. Basel, Ciba-Geigy Ltd.
CIT (1995) 13-week toxicity study and fertility study by oral route
(dietary admixture) in male rats with PT 810(R) (TGIC). Miserey,
Centre Internationale de Toxicologie.
Dooms-Goossens A, Bedert R, Vandaele M, Degreef H (1989) Airborne
contact dermatitis due to triglycidyl isocyanurate. Contact
dermatitis, 21(3):202-203.
Foulds IS, Koh D (1992) Allergic contact dermatitis from resin
hardeners during the manufacture of thermosetting coating paints.
Contact dermatitis, 26:87-90.
Hazleton (1987) Mutagenicity test on Araldite PT 810 in the Ames
Salmonella/microsome reverse mutation assay (No. E-9782-0-401).
Veenendaal, Hazleton Biotechnologies.
Hazleton (1989a) Mutagenicity test on PL88-810 in the mouse
spermatogonial cell cytogenetic assay (No. 10386-0-474). Kensington,
MD, Hazleton Laboratories America Inc.
Hazleton (1989b) Mutagenicity test on PL88-810 in the dominant
lethal assay (No. 10386-0-471). Kensington, MD, Hazleton
Laboratories America Inc.
Hazleton (1991) Study to evaluate the chromosome damaging potential
of TK 10622 (PT810 [TGIC, 97%]) by its effects on the spermatogonial
cells of treated mice. Heslington, York, Hazleton Microtest.
Howard P (1989) Handbook of environmental fate and exposure data for
organic chemicals. Vol.1. Large production and priority pollutants.
Chelsea, MI, Lewis Publishers, p. 485.
HSE (1992) Toxicity review 27 - Part 1: Triglycidyl isocyanurate.
United Kingdom, Health and Safety Executive, HMSO Publication (ISBN 0
11 886343 6).
HSE (1994) Triglycidyl isocyanurate, a review of use and exposure.
United Kingdom, Health and Safety Executive, Hygiene Technology and
Chemical Agents Unit.
IPCS (1997) International Chemical Safety Card - Triglycidyl
isocyanurate. Geneva, World Health Organization, International
Programme on Chemical Safety (No. 1274).
Jolanki R, Kanerva L, Estlander T, Tarvainen K (1994) Concomitant
sensitization to triglycidyl isocyanurate, diaminodiphenylmethane and
2-hydroxyethyl methacrylate from silk-screen printing coatings in the
manufacture of circuit boards. Contact dermatitis, 30:12-15.
Loveday KS, Anderson BE, Resnick MA, Zeiger E (1990) Chromosome
aberration and sister chromatid exchange tests in Chinese hamster
ovary cells in vitro. V: Results with 46 chemicals. Environmental
and molecular mutagenesis, 16:272-303.
Matthias CG (1988) Allergic contact dermatitis from triglycidyl
isocyanurate in polyester paint pigments. Contact dermatitis,
19(1):67-68.
McFadden JP, Rycroft RJG (1993) Occupational contact dermatitis from
triglycidyl isocyanurate in a powder paint sprayer. Contact
dermatitis, 28:251.
Munro CS, Lawrence CM (1992) Occupational contact dermatitis from
triglycidyl isocyanurate in a powder paint factory. Contact
dermatitis, 26:59.
Neidhart JA (1984) Phase I trial of Teroxirone. Cancer treatment
reports, 68:1115-1119.
NICNAS (1994) Priority Existing Chemical No.1 - Triglycidyl
isocyanurate, full public report, National Industrial Chemicals
Notification and Assessment Scheme. Canberra, Australian Government
Publishing Service, April.
Nishioka K, Ogasawara M, Asagami C (1988) Occupational contact allergy
to triglycidyl isocyanurate (TGIC, TEPIC). Contact dermatitis,
19(5):379-380.
Nissan (no date) TEPIC, information on basic properties. Tokyo,
Nissan Chemical Industries Ltd.
Piirila P, Estlander T, Keskinen H, Jolanki R, Laakkonen A, Pfaffli P,
Tupasela O, Tuppurainen M, Nordman H (1997) Occupational asthma caused
by triglycidyl isocyanurate (TGIC). Clinical and experimental
allergy, 27:510-514.
Rubin J, Kovach JS, Ames MM, Moertel CG, Creagan ET, O'Connell MJ
(1987) Phase I study of two schedules of Teroxirone. Cancer
treatment reports, 71:489-492.
Safepharm (1988a) TEPIC-G: Acute oral toxicity test in the rat
(No. 14/10). Derby, Safepharm Laboratories Ltd.
Safepharm (1988b) TEPIC-G: Acute dermal toxicity (limit test) in
the rat (No. 14/11). Derby, Safepharm Laboratories Ltd.
Safepharm (1988c) TEPIC-G: Acute dermal irritation test in the
rabbit (No. 14/12). Derby, Safepharm Laboratories Ltd.
Safepharm (1988d) TEPIC-G: Acute dermal irritation test in the
rabbit (24-hour exposure) (No. 14/13). Derby, Safepharm Laboratories
Ltd.
Safepharm (1988e) TEPIC-G: Magnusson and Kligman maximisation study
in the guinea pig (No. 14/14). Derby, Safepharm Laboratories Ltd.
Safepharm (1991) TEPIC SP: Five-day repeat exposure inhalation
toxicity study in the male mouse (No. 14/68). Derby, Safepharm
Laboratories Ltd.
Safepharm (1992) TGIC technical and TGIC ten per cent powder:
Chromosome analysis in mouse spermatogonial cells, comparative
inhalation study (No. 14/75) [draft]. Derby, Safepharm Laboratories
Ltd.
Scheunert I (1992) Transformation and degradation of pesticides in
soil. In: Ebing W, ed. Terrestrial behavior of pesticides. Berlin,
Springer-Verlag, p. 64.
Shell (1971) Toxicity studies on the diglycidyl esters of
tetrahydrophthalic and hexahydrophthalic acids and of
triglycidylisocyanurate: Repeated dosing experiments with rats
(TLGR.0019.71). London, Shell Research Ltd.
Sofuni T, Matsuoka A, Sawada M, Ishidate M Jr, Zeiger E, Shelby MD
(1990) A comparison of chromosome aberration induction by 25 compounds
tested by two Chinese hamster cell (CHL and CHO) systems in culture.
Mutation research, 241:175-213.
APPENDIX 1 - SOURCE DOCUMENT
National Industrial Chemicals Notification and Assessment Scheme -
Triglycidyl Isocyanurate, Priority Existing Chemical No. 1, Full
Public Report (1994)
Copies of the NICNAS (1994) report on triglycidyl isocyanurate
(prepared by D. Willcocks, L. Onyon, C. Jenkins, and B. Diver) may be
obtained from:
Australian Government Publishing Service
Mail Order Service
GPO Box 84
Canberra 2601, Australia
NICNAS reports are prepared to meet the requirements of the
Industrial Chemicals Notification and Assessment Act, 1989, as
amended. In the preparation of the assessment report, both internal
and external peer review are undertaken. Under the NICNAS
legislation, applicants for the assessment of a chemical (i.e.
importers and manufacturers of the chemical) may apply for variations
to the draft report. The following companies participated in the
review of the assessment at this stage: Ciba-Geigy Australia Ltd,
Dulux Powder Coatings, Evode Powder Coatings Pty Ltd, Itochu Australia
Ltd, Jotun Powder Coatings Pty Ltd, Sumitomo Australia Ltd, Taubmans
Pty Ltd, and Western Coatings Ltd.
In the assessment of triglycidyl isocyanurate, Ciba-Geigy
Australia Ltd made several requests to vary the assessment report -
notably in the areas of acute toxicity and mutagenicity - which the
Director of NICNAS refused. Consequently, Ciba-Geigy lodged an
application for review of the Director's decision with the independent
Administrative Appeals Tribunal (members included Justice D.F.
O'Connor and Professor G. Johnston, Sydney University). The
Administrative Appeals Tribunal looked at all the material relevant to
the assessment decisions in question and upheld the Director's
decisions.1 During the Administrative Appeals Tribunal review
process, the following expert witnesses were called upon: Dr D.J.
Birkett, Flinders University School of Medicine, Australia; Dr M.E.
McManus, University of Queensland, Australia; Dr H.J. Weideli,
Ciba-Geigy Ltd, Basel, Switzerland; Dr C. Winder, University of New
South Wales, Australia; and Dr E. Zeiger, National Institute of
Environmental Health Sciences, Research Triangle Park, NC, USA.
1 Administrative Appeals Tribunal, No. P93/339, Re Ciba-Geigy
Australia Ltd (Applicant) and Worksafe Australia (Respondent), March
1994.
APPENDIX 2 - CICAD PEER REVIEW
The draft CICAD on triglycidyl isocyanurate was sent for review
to institutions and organizations identified by IPCS after contact
with IPCS national Contact Points and Participating Institutions, as
well as to identified experts. Comments were received from:
Ciba Speciality Chemicals Inc., Basel, Switzerland
Department of Health, London, United Kingdom
Department of Public Health Promotion, Prague, Czech Republic
Environment Canada, Ottawa, Canada
Health and Safety Executive, Bootle, United Kingdom
Health Canada, Ottawa, Canada
National Chemicals Inspectorate (KEMI), Solna, Sweden
National Institute of Occupational Health, Budapest, Hungary
National Institute of Public Health, Oslo, Norway
United States Department of Health and Human Services (National
Institute for Occupational Safety and Health, Cincinnati, USA;
National Institute of Environmental Health Sciences, Research
Triangle Park, USA)
United States Environmental Protection Agency (National Center
for Environmental Assessment, Office of Research and Development,
Research Triangle Park, USA)
APPENDIX 3 - CICAD FINAL REVIEW BOARD
Berlin, Germany, 26-28 November 1997
Members
Dr H. Ahlers, Education and Information Division, National Institute
for Occupational Safety and Health, Cincinnati, OH, USA
Mr R. Cary, Health Directorate, Health and Safety Executive, Bootle,
United Kingdom
Dr S. Dobson, Institute of Terrestrial Ecology, Huntingdon, United
Kingdom
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany (Chairperson)
Mr J.R. Hickman, Health Protection Branch, Health Canada, Ottawa,
Ontario, Canada
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany
Ms M.E. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada (Rapporteur)
Dr K. Paksy, Department of Reproductive Toxicology, National Institute
of Occupational Health, Budapest, Hungary
Mr V. Quarg, Ministry for the Environment, Nature Conservation &
Nuclear Safety, Bonn, Germany
Mr D. Renshaw, Department of Health, London, United Kingdom
Dr J. Sekizawa, Division of Chemo-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan
Prof S. Soliman, Department of Pesticide Chemistry, Alexandria
University, Alexandria, Egypt (Vice-Chairperson)
Dr M. Wallen, National Chemicals Inspectorate (KEMI), Solna, Sweden
Ms D. Willcocks, Chemical Assessment Division, Worksafe Australia,
Camperdown, Australia
Dr M. Williams-Johnson, Division of Toxicology, Agency for Toxic
Substances and Disease Registry, Atlanta, GA, USA
Dr K. Ziegler-Skylakakis, Senatskommission der Deutschen
Forschungsgemeinschaft zuer Pruefung gesundheitsschaedlicher
Arbeitsstoffe, GSF-Institut fuer Toxikologie, Neuherberg,
Oberschleissheim, Germany
Observers
Mrs B. Dinham,1 The Pesticide Trust, London, United Kingdom
Dr R. Ebert, KSU Ps-Toxicology, Huels AG, Marl, Germany (representing
ECETOC, the European Centre for Ecotoxicology and Toxicology of
Chemicals)
Mr R. Green,1 International Federation of Chemical, Energy, Mine and
General Workers' Unions, Brussels, Belgium
Dr B. Hansen,1 European Chemicals Bureau, European Commission, Ispra,
Italy
Dr J. Heuer, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Mr T. Jacob,1 DuPont, Washington, DC, USA
Ms L. Onyon, Environment Directorate, Organisation for Economic
Co-operation and Development, Paris, France
Dr H.J. Weideli, Ciba Speciality Chemicals Inc., Basel, Switzerland
(representing CEFIC, the European Chemical Industry Council)
Secretariat
Dr M. Baril, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr R.G. Liteplo, Health Canada, Ottawa, Ontario, Canada
Ms L. Regis, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Mr A. Strawson, Health and Safety Executive, London, United Kingdom
Dr P. Toft, Associate Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
1 Invited but unable to attend.
RÉSUMÉ D'ORIENTATION
Ce CICAD est fondé principalement sur l'évaluation de
l'isocyanurate de triglycidyle réalisée dans le cadre du NICNAS
(Australian National Industrial Chemicals Notification and Assessment
Scheme) et publiée en avril 1994 (NICNAS, 1994). De nouvelles
informations parues entre l'achèvement du rapport NICNAS et
novembre 1997 ont également été évaluées et incorporées dans ce CICAD,
de même que des informations complémentaires extraites de l'étude du
United Kingdom Health and Safety Executive (HSE, 1992) portant sur la
toxicité de cette substance. Des informations relatives à l'examen du
rapport NICNAS par les pairs et à sa disponibilité figurent à
l'appendice 1. Les renseignements concernant l'examen du CICAD par
les pairs font l'objet de l'appendice 2. Ce CICAD a été approuvé en
tant qu'évaluation internationale lors d'une une réunion du Comité
d'évaluation finale qui s'est tenue à Berlin (Allemagne) du 26 au
28 novembre 1997. La liste des participants à cette réunion figure à
l'appendice 3. La fiche d'information sur la sécurité chimique de
l'isocyanurate de triglycidyle (ICSC 1274), établie par le Programme
international sur la Sécurité chimique (IPCS, 1997), est également
reproduite dans le présent document.
L'isocyanurate de triglycidyle (CAS N° 2451-62-9) est une
substance chimique de synthèse qui se présente sous la forme d'une
poudre blanche ou d'un granulé pratiquement inodore à la température
ambiante. Il est employé principalement comme agent de polymérisation
ou de réticulation tridimensionnelle dans les revêtements de polyester
en poudre (peinture). Ces revêtements en poudre contiennent
généralement de 4 à 10 % d'isocyanurate de triglycidyle. Il est
également utilisé dans les encres servant à la fabrication de masques
dans l'industrie des circuits imprimés. L'encre est constituée de
deux parties dont l'élément durcisseur contient approximativement 60 %
d'isocyanurate de triglycidyle. La plus grande partie de
l'isocyanurate de triglycidyle présent dans ces revêtements en poudre
et ces encres est immobilisée par réticulation dans une matrice
insoluble.
En principe, l'exposition de la population générale à
l'isocyanurate de triglycidyle devrait être minime. Par contre, il
existe un risque d'exposition professionnelle lors de la fabrication
et de l'utilisation de cette substance et des produits qui en
contiennent.
On dispose de peu d'informations sur les effets de l'isocyanurate
de triglycidyle chez l'homme. On a signalé plusieurs cas de dermatite
allergique de contact et un cas de sensibilisation respiratoire à la
suite d'une exposition professionnelle.
L'isocyanurate de triglycidyle est très toxique par ingestion et
inhalation chez les animaux de laboratoire et il peut provoquer de
graves lésions oculaires. Sur la peau, il agit comme sensibilisant,
mais non comme irritant. Les données sur sa toxicité à la suite d'une
exposition répétée sont limitées. Chez des rats et des souris soumis
à des expositions répétées pendant une courte période (5-7 jours), on
a constaté des effets sur les reins, le foie, les poumons, le tube
digestif et les spermatogonies. Une étude de 13 semaines menée sur
des rats mâles pour évaluer la toxicité de l'isocyanurate de
triglycidyle et ses effets sur la fécondité a révélé une réduction de
nombre de spermatozoïdes liée à la dose.
Des études de génotoxicité in vitro et in vivo ont montré que
l'isocyanurate de triglycidyle est un mutagène à action directe
capable d'agir sur les organes de la reproduction. Compte tenu de sa
génotoxicité potentielle, toutes les mesures appropriées doivent être
prises pour réduire au minimum l'exposition humaine à cette substance.
Compte tenu de sa faible persistance et d'une écotoxicité
probablement faible, l'isocyanurate de triglycidyle ne devrait pas
présenter de risque significatif pour l'environnement, sauf en cas de
rejet accidentel ou d'élimination dans des conditions inadaptées.
RESUMEN DE ORIENTACION
Este CICAD se basa principalmente en la evaluación del
isocianurato de triglicidilo realizada en el marco del Plan Nacional
Australiano de Notificación y Evaluación de Sustancias Químicas
Industriales (NICNAS) y publicada en abril de 1994 (NICNAS, 1994).
También se ha evaluado e incorporado a este CICAD la información
aparecida desde la terminación del informe del NICNAS hasta noviembre
de 1997. Se ha incluido asimismo alguna información adicional
procedente del examen de la toxicidad del isocianurato de triglicidilo
de la Dirección de Salud y Seguridad del Reino Unido (HSE, 1992). La
información relativa al examen colegiado del informe del NICNAS y a su
disponibilidad figura en el Apéndice 1. La información sobre el
examen colegiado de este CICAD se presenta en el Apéndice 2. Este
CICAD se aprobó como evaluación internacional en una reunión de la
Junta de Evaluación Final celebrada en Berlín (Alemania) los días
26-28 de noviembre de 1997. La lista de participantes en la Junta de
Evaluación Final figura en el Apéndice 3. La Ficha internacional de
seguridad química (ICSC 1274) para el isocianurato de triglicidilo,
preparada por el Programa Internacional de Seguridad de las Sustancias
Químicas (IPCS, 1997), también se reproduce en este documento.
El isocianurato de triglicidilo (CAS Nº 2451-62-9), sustancia
química sintética en forma de polvo blanco o granulada prácticamente
inodora a temperatura ambiente, se utiliza sobre todo como agente de
polimerización o de reticulación tridimensional en los revestimientos
de poliéster en polvo (pintura). Estos revestimientos en polvo suelen
contener entre un 4% y un 10% de isocianurato de triglicidilo.
También se utiliza éste en las tintas que sirven para la fabricación
de "máscaras" de soldadura en la industria de los circuitos impresos.
Las tintas constan de dos partes, cuyo elemento endurecedor contiene
alrededor de un 60% de isocianurato de triglicidilo. La mayor parte
de esta sustancia presente en los revestimientos en polvo y las tintas
mencionados se inmoviliza mediante la reticulación en una matriz
insoluble.
La exposición de la población general al isocianurato de
triglicidilo se supone que es mínima; sin embargo, hay riesgo de
exposición profesional durante la fabricación y la utilización de esta
sustancia y de los productos que la contienen.
Se dispone de escasa información sobre los efectos del
isocianurato de triglicidilo en el ser humano. Se han notificado
varios casos de dermatitis alérgica por contacto y un caso de
sensibilización respiratoria debidos a exposición profesional.
El isocianurato de triglicidilo es muy tóxico en animales de
laboratorio por ingestión e inhalación y puede provocar lesiones
oculares graves. Es sensibilizador cutáneo, pero no irritante de la
piel. Los datos sobre toxicidad por exposición repetida son
limitados. En estudios de exposición repetida de corta duración (5-7
días) en ratas y ratones, se observaron efectos en los riñones, el
hígado, los pulmones, el aparato digestivo y los espermatogonios. En
un estudio de toxicidad/fecundidad de 13 semanas en ratas machos se
observó una reducción del número de espermatozoides relacionada con la
dosis.
Los estudios de genotoxicidad in vitro e in vivo indican que
el isocianurato de triglicidilo es un mutágeno de acción directa capaz
de afectar los órganos de la reproducción. A la vista de su
genotoxicidad potencial, deben tomarse todas las medidas apropiadas
para reducir al mínimo la exposición humana a esta sustancia.
Debido a su escasa persistencia y su ecotoxicidad probablemente
baja, no es probable que el isocianurato de triglicidilo represente un
riesgo significativo para el medio ambiente, excepto en el caso de un
accidente o de una eliminación inapropiada.
See Also:
Toxicological Abbreviations
Triglycidyl isocyanurate (ICSC)
 1. EXECUTIVE SUMMARY
This CICAD was based principally on the assessment of triglycidyl
isocyanurate completed under the Australian National Industrial
Chemicals Notification and Assessment Scheme (NICNAS) and published in
April 1994 (NICNAS, 1994). Information that has become available
since completion of the NICNAS report, identified up to November 1997,
has also been assessed and included in this CICAD. Some additional
information from the United Kingdom Health and Safety Executive's
toxicity review of triglycidyl isocyanurate (HSE, 1992) has also been
included. Information on the nature of peer review and availability
of the NICNAS report is presented in Appendix 1. Information on the
peer review of this CICAD is presented in Appendix 2. This CICAD was
approved as an international assessment at a meeting of the Final
Review Board, held in Berlin, Germany, on 26-28 November 1997.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 1274) for triglycidyl
isocyanurate, produced by the International Programme on Chemical
Safety (IPCS, 1997), has also been reproduced in this document.
Triglycidyl isocyanurate (CAS no.
1. EXECUTIVE SUMMARY
This CICAD was based principally on the assessment of triglycidyl
isocyanurate completed under the Australian National Industrial
Chemicals Notification and Assessment Scheme (NICNAS) and published in
April 1994 (NICNAS, 1994). Information that has become available
since completion of the NICNAS report, identified up to November 1997,
has also been assessed and included in this CICAD. Some additional
information from the United Kingdom Health and Safety Executive's
toxicity review of triglycidyl isocyanurate (HSE, 1992) has also been
included. Information on the nature of peer review and availability
of the NICNAS report is presented in Appendix 1. Information on the
peer review of this CICAD is presented in Appendix 2. This CICAD was
approved as an international assessment at a meeting of the Final
Review Board, held in Berlin, Germany, on 26-28 November 1997.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 1274) for triglycidyl
isocyanurate, produced by the International Programme on Chemical
Safety (IPCS, 1997), has also been reproduced in this document.
Triglycidyl isocyanurate (CAS no. 