
Concise International Chemical Assessment Document 19
PHENYLHYDRAZINE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by
Mr R. Cary, Health and Safety Executive, Liverpool, United Kingdom,
Dr S. Dobson, Institute of Terrestrial Ecology, Huntingdon, United
Kingdom, and
Dr I. Brooke, Health and Safety Executive, Liverpool, United Kingdom
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 2000
The International Programme on Chemical Safety (IPCS),
established in 1980, is a joint venture of the United Nations
Environment Programme (UNEP), the International Labour Organisation
(ILO), and the World Health Organization (WHO). The overall objectives
of the IPCS are to establish the scientific basis for assessment of
the risk to human health and the environment from exposure to
chemicals, through international peer review processes, as a
prerequisite for the promotion of chemical safety, and to provide
technical assistance in strengthening national capacities for the
sound management of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization, the United Nations
Institute for Training and Research, and the Organisation for Economic
Co-operation and Development (Participating Organizations), following
recommendations made by the 1992 UN Conference on Environment and
Development to strengthen cooperation and increase coordination in the
field of chemical safety. The purpose of the IOMC is to promote
coordination of the policies and activities pursued by the
Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing-in-Publication Data
Phenylhydrazine.
(Concise international chemical assessment document ; 19)
1.Phenylhydrazines - toxicology 2.No-observed-adverse-effect level
3.Risk assessment 4.Environmental exposure I.International Programme
on Chemical Safety II.Series
ISBN 92 4 153019 7 (NLM classification: QV 180)
ISSN 1020-6167
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 2000
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city, or area
or of its authorities, or concerning the delimitation of its frontiers
or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
TABLE OF CONTENTS
FOREWORD
1. EXECUTIVE SUMMARY
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
3. ANALYTICAL METHODS
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1. Environmental levels
6.2. Human exposure
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposure
8.2. Irritation and sensitization
8.3. Short-term exposure
8.4. Long-term exposure
8.4.1. Subchronic exposure
8.4.2. Chronic exposure and carcinogenicity
8.5. Genotoxicity and related end-points
8.6. Reproductive and developmental toxicity
8.7. Immunological and neurological effects
9. EFFECTS ON HUMANS
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1. Aquatic environment
10.2. Terrestrial environment
11. EFFECTS EVALUATION
11.1. Evaluation of health effects
11.1.1. Hazard identification and dose-response assessment
11.1.2. Criteria for setting guidance values for phenylhydrazine
11.1.3. Sample risk characterization
11.2. Evaluation of environmental effects
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
13.1. Human health hazards
13.2. Advice to physicians
13.3. Health surveillance advice
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
INTERNATIONAL CHEMICAL SAFETY CARD
REFERENCES
APPENDIX 1 - SOURCE DOCUMENTS
APPENDIX 2 - CICAD PEER REVIEW
APPENDIX 3 - CICAD FINAL REVIEW BOARD
RÉSUMÉ D'ORIENTATION
RESUMEN DE ORIENTACION
FOREWORD
Concise International Chemical Assessment Documents (CICADs) are
the latest in a family of publications from the International
Programme on Chemical Safety (IPCS) - a cooperative programme of the
World Health Organization (WHO), the International Labour Organisation
(ILO), and the United Nations Environment Programme (UNEP). CICADs
join the Environmental Health Criteria documents (EHCs) as
authoritative documents on the risk assessment of chemicals.
CICADs are concise documents that provide summaries of the
relevant scientific information concerning the potential effects of
chemicals upon human health and/or the environment. They are based on
selected national or regional evaluation documents or on existing
EHCs. Before acceptance for publication as CICADs by IPCS, these
documents undergo extensive peer review by internationally selected
experts to ensure their completeness, accuracy in the way in which the
original data are represented, and the validity of the conclusions
drawn.
The primary objective of CICADs is characterization of hazard and
dose-response from exposure to a chemical. CICADs are not a summary of
all available data on a particular chemical; rather, they include only
that information considered critical for characterization of the risk
posed by the chemical. The critical studies are, however, presented in
sufficient detail to support the conclusions drawn. For additional
information, the reader should consult the identified source documents
upon which the CICAD has been based.
Risks to human health and the environment will vary considerably
depending upon the type and extent of exposure. Responsible
authorities are strongly encouraged to characterize risk on the basis
of locally measured or predicted exposure scenarios. To assist the
reader, examples of exposure estimation and risk characterization are
provided in CICADs, whenever possible. These examples cannot be
considered as representing all possible exposure situations, but are
provided as guidance only. The reader is referred to EHC 1701 for
advice on the derivation of health-based guidance values.
While every effort is made to ensure that CICADs represent the
current status of knowledge, new information is being developed
constantly. Unless otherwise stated, CICADs are based on a search of
the scientific literature to the date shown in the executive summary.
In the event that a reader becomes aware of new information that would
change the conclusions drawn in a CICAD, the reader is requested to
contact IPCS to inform it of the new information.
1 International Programme on Chemical Safety (1994)
Assessing human health risks of chemicals: deriviation of
guidance values for health-based exposure limits. Geneva, World
Health Organization (Environmental Health Criteria 170).
Procedures
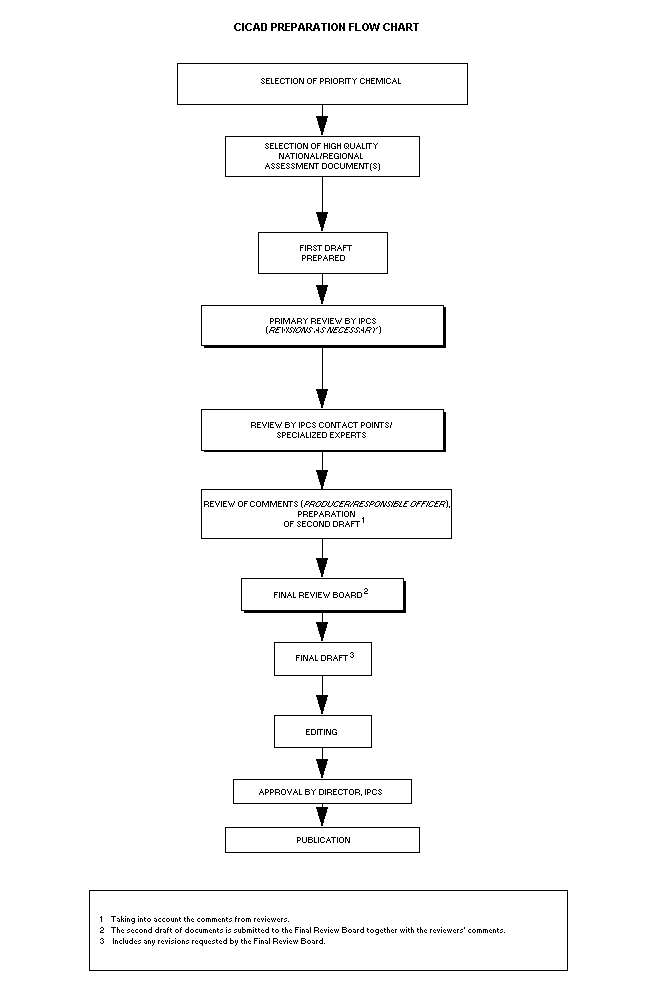
The flow chart shows the procedures followed to produce a CICAD.
These procedures are designed to take advantage of the expertise that
exists around the world - expertise that is required to produce the
high-quality evaluations of toxicological, exposure, and other data
that are necessary for assessing risks to human health and/or the
environment.
The first draft is based on an existing national, regional, or
international review. Authors of the first draft are usually, but not
necessarily, from the institution that developed the original review.
A standard outline has been developed to encourage consistency in
form. The first draft undergoes primary review by IPCS to ensure that
it meets the specified criteria for CICADs.
The second stage involves international peer review by scientists
known for their particular expertise and by scientists selected from
an international roster compiled by IPCS through recommendations from
IPCS national Contact Points and from IPCS Participating Institutions.
Adequate time is allowed for the selected experts to undertake a
thorough review. Authors are required to take reviewers' comments into
account and revise their draft, if necessary. The resulting second
draft is submitted to a Final Review Board together with the
reviewers' comments.
The CICAD Final Review Board has several important functions:
- to ensure that each CICAD has been subjected to an appropriate
and thorough peer review;
- to verify that the peer reviewers' comments have been addressed
appropriately;
- to provide guidance to those responsible for the preparation of
CICADs on how to resolve any remaining issues if, in the opinion
of the Board, the author has not adequately addressed all
comments of the reviewers; and
- to approve CICADs as international assessments.
Board members serve in their personal capacity, not as representatives
of any organization, government, or industry. They are selected
because of their expertise in human and environmental toxicology or
because of their experience in the regulation of chemicals. Boards are
chosen according to the range of expertise required for a meeting and
the need for balanced geographic representation.
 Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
1. EXECUTIVE SUMMARY
This CICAD on phenylhydrazine was based on a review of human
health concerns (primarily occupational) prepared by the United
Kingdom's Health and Safety Executive (Brooke et al., 1997) and a
report prepared for the German Advisory Committee on Existing
Chemicals of Environmental Relevance (BUA, 1995). Hence, this document
focuses on exposures via routes relevant to occupational settings but
also contains environmental information. Data identified as of
December 1993 and December 1994, respectively, were covered. A further
literature search was performed up to January 1998 to identify any
extra information published since these reviews were completed.
Information on the nature of the peer review and availability of the
source documents is presented in Appendix 1. Information on the peer
review of this CICAD is presented in Appendix 2. This CICAD was
approved as an international assessment at a meeting of the Final
Review Board, held in Washington, DC, USA, on 8-11 December 1998.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 0938) for
phenylhydrazine, produced by the International Programme on Chemical
Safety (IPCS, 1993), has also been reproduced in this document.
Phenylhydrazine (CAS No. 100-63-0) exists as yellow to pale brown
crystals or as a yellowish oily liquid. It is sparingly soluble in
water and is miscible with other organic solvents.
Phenylhydrazine is used worldwide mainly as a chemical
intermediate in the pharmaceutical, agrochemical, and chemical
industries.
The number of persons potentially exposed to phenylhydrazine or
its hydrochloride salt is not known, but it is expected to be small.
No personal exposure data were available, although the Estimation and
Assessment of Substance Exposure (EASE) model predicted exposure
(8-h time-weighted average) to be around 2.3 mg/m3 (0.5 ppm). In
practice, the 8-h time-weighted average exposure will be less than
this figure.
The limited data on toxicokinetics indicate that phenylhydrazine
is well absorbed by inhalation, oral, and dermal routes and binds
readily to haemoglobin in red blood cells. Metabolism seems to occur
via ring hydroxylation and conjugation, probably with glucuronic acid.
Excretion is primarily via the urine.
Phenylhydrazine is toxic by single exposure via the oral route
(LD50 80-188 mg/kg body weight) and is expected to be toxic by the
inhalation and dermal routes (data from these routes of exposure are
less clear). Phenylhydrazine has potential for skin and eye
irritation, and there is evidence that it has skin-sensitizing
properties in humans. Exposure to phenylhydrazine may cause damage to
red blood cells, potentially resulting in anaemia and consequential
secondary involvement of other tissues, such as the spleen and liver.
Phenylhydrazine is mutagenic in vitro, and there is some evidence to
indicate that it may express genotoxic activity in vivo. The
substance is clearly carcinogenic in mice following oral dosing,
inducing tumours of the vascular system. The mechanism for tumour
formation is unclear, but a genotoxic component cannot be excluded.
Hence, it is not considered possible to reliably identify a level of
exposure at which there will be no risk of carcinogenic or genotoxic
effects.
There are no adequate data available regarding reproductive or
developmental effects; hence, it is not possible to evaluate the risk
to human health for these end-points.
The level of risk in occupational settings is uncertain; as a
result, there is a continuing requirement to reduce exposure levels as
much as is reasonably practicable with the technology that is
currently available.
The lack of available data to serve as a basis for estimation of
indirect exposure of individuals to phenylhydrazine from the general
environment precludes the characterization of potential cancer risks
for the general population.
No atmospheric effects are expected given the release of
phenylhydrazine predominantly to water, its extremely low
volatilization from water to the atmosphere, and its rapid calculated
atmospheric half-life following reaction with hydroxyl radicals.
Phenylhydrazine is degraded photochemically and autoxidizes in
water. It is readily biodegradable, and this is expected to be the
major route of breakdown in the environment. There is minimal sorption
to particulates.
Phenylhydrazine is toxic to aquatic organisms, with the lowest
reported no-observed-effect concentration (NOEC) in standard acute
fish tests at 0.01 mg/litre; fish are generally more sensitive than
either daphnids or bacteria. A NOEC of 0.49 µg/litre has been reported
for embryo-larval stages of the zebra fish ( Brachydanio rerio).
The risk to aquatic organisms is expected to be low, based on
very conservative assumptions.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
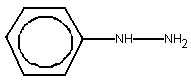
Phenylhydrazine (C6H8N2; molecular weight 108; CAS No.
100-63-0; see structural diagram below) exists as yellow to pale brown
crystals or as a yellowish oily liquid, with a freezing point of
19.6°C, a boiling point of 243.4°C, and a vapour pressure of 133 Pa at
72°C. It is soluble in water (values ranging from 145 to 837 g/litre
at 24°C have been reported) and is miscible with alcohol, ether,
chloroform, benzene, and acetone. The conversion factor for
phenylhydrazine is 1 ppm = 4.5 mg/m3 (at 20°C, 101 kPa). Additional
physical/chemical properties of phenylhydrazine are presented in the
International Chemical Safety Card reproduced in this document.
Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
1. EXECUTIVE SUMMARY
This CICAD on phenylhydrazine was based on a review of human
health concerns (primarily occupational) prepared by the United
Kingdom's Health and Safety Executive (Brooke et al., 1997) and a
report prepared for the German Advisory Committee on Existing
Chemicals of Environmental Relevance (BUA, 1995). Hence, this document
focuses on exposures via routes relevant to occupational settings but
also contains environmental information. Data identified as of
December 1993 and December 1994, respectively, were covered. A further
literature search was performed up to January 1998 to identify any
extra information published since these reviews were completed.
Information on the nature of the peer review and availability of the
source documents is presented in Appendix 1. Information on the peer
review of this CICAD is presented in Appendix 2. This CICAD was
approved as an international assessment at a meeting of the Final
Review Board, held in Washington, DC, USA, on 8-11 December 1998.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 0938) for
phenylhydrazine, produced by the International Programme on Chemical
Safety (IPCS, 1993), has also been reproduced in this document.
Phenylhydrazine (CAS No. 100-63-0) exists as yellow to pale brown
crystals or as a yellowish oily liquid. It is sparingly soluble in
water and is miscible with other organic solvents.
Phenylhydrazine is used worldwide mainly as a chemical
intermediate in the pharmaceutical, agrochemical, and chemical
industries.
The number of persons potentially exposed to phenylhydrazine or
its hydrochloride salt is not known, but it is expected to be small.
No personal exposure data were available, although the Estimation and
Assessment of Substance Exposure (EASE) model predicted exposure
(8-h time-weighted average) to be around 2.3 mg/m3 (0.5 ppm). In
practice, the 8-h time-weighted average exposure will be less than
this figure.
The limited data on toxicokinetics indicate that phenylhydrazine
is well absorbed by inhalation, oral, and dermal routes and binds
readily to haemoglobin in red blood cells. Metabolism seems to occur
via ring hydroxylation and conjugation, probably with glucuronic acid.
Excretion is primarily via the urine.
Phenylhydrazine is toxic by single exposure via the oral route
(LD50 80-188 mg/kg body weight) and is expected to be toxic by the
inhalation and dermal routes (data from these routes of exposure are
less clear). Phenylhydrazine has potential for skin and eye
irritation, and there is evidence that it has skin-sensitizing
properties in humans. Exposure to phenylhydrazine may cause damage to
red blood cells, potentially resulting in anaemia and consequential
secondary involvement of other tissues, such as the spleen and liver.
Phenylhydrazine is mutagenic in vitro, and there is some evidence to
indicate that it may express genotoxic activity in vivo. The
substance is clearly carcinogenic in mice following oral dosing,
inducing tumours of the vascular system. The mechanism for tumour
formation is unclear, but a genotoxic component cannot be excluded.
Hence, it is not considered possible to reliably identify a level of
exposure at which there will be no risk of carcinogenic or genotoxic
effects.
There are no adequate data available regarding reproductive or
developmental effects; hence, it is not possible to evaluate the risk
to human health for these end-points.
The level of risk in occupational settings is uncertain; as a
result, there is a continuing requirement to reduce exposure levels as
much as is reasonably practicable with the technology that is
currently available.
The lack of available data to serve as a basis for estimation of
indirect exposure of individuals to phenylhydrazine from the general
environment precludes the characterization of potential cancer risks
for the general population.
No atmospheric effects are expected given the release of
phenylhydrazine predominantly to water, its extremely low
volatilization from water to the atmosphere, and its rapid calculated
atmospheric half-life following reaction with hydroxyl radicals.
Phenylhydrazine is degraded photochemically and autoxidizes in
water. It is readily biodegradable, and this is expected to be the
major route of breakdown in the environment. There is minimal sorption
to particulates.
Phenylhydrazine is toxic to aquatic organisms, with the lowest
reported no-observed-effect concentration (NOEC) in standard acute
fish tests at 0.01 mg/litre; fish are generally more sensitive than
either daphnids or bacteria. A NOEC of 0.49 µg/litre has been reported
for embryo-larval stages of the zebra fish ( Brachydanio rerio).
The risk to aquatic organisms is expected to be low, based on
very conservative assumptions.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
Phenylhydrazine (C6H8N2; molecular weight 108; CAS No.
100-63-0; see structural diagram below) exists as yellow to pale brown
crystals or as a yellowish oily liquid, with a freezing point of
19.6°C, a boiling point of 243.4°C, and a vapour pressure of 133 Pa at
72°C. It is soluble in water (values ranging from 145 to 837 g/litre
at 24°C have been reported) and is miscible with alcohol, ether,
chloroform, benzene, and acetone. The conversion factor for
phenylhydrazine is 1 ppm = 4.5 mg/m3 (at 20°C, 101 kPa). Additional
physical/chemical properties of phenylhydrazine are presented in the
International Chemical Safety Card reproduced in this document.
 3. ANALYTICAL METHODS
For measurement of phenylhydrazine in water, reduction of Cu(II)
to Cu(I) by phenylhydrazine has been used as the basis for
spectrometric analytical methods measuring coloured complexes (Besada,
1988; Hasan, 1988). The methods are not specific and react to other
reducing substances. A detection limit of 10 µg/litre is given for one
method (Hasan, 1988).
In a method published by NIOSH (1994) for measurement of
phenylhydrazine in workplace air, the air is sampled into a midget
bubbler containing hydrochloric acid. Phosphomolybdic acid is added to
the resulting solution, and the reaction with phenylhydrazine causes
the formation of a bluish-green complex that can be measured at 730 nm
with a spectrophotometer. This method has a detection limit of about 5
mg/m3 (about 1 ppm), based on a 100-litre sample. Potential
interferences are listed as other hydrazine derivatives, aldehydes,
and ketones.
Both the MIRAN 1B and the Bruel and Kjaer 1302 Multigas Monitor
may be used to measure phenylhydrazine in air, with a detection limit
of around 13.5 mg/m3 (3 ppm) (Brooke et al., 1997). Any other
substance having similar infrared absorbances can be expected to
interfere with the measurement.
There are no published biological monitoring methods available
for phenylhydrazine.
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
There are a few reports of the natural occurrence of
phenylhydrazine in plants (BUA, 1995). Phenylhydrazine is produced
commercially by the diazotization of aniline followed by reduction of
the azo compound.
Production figures for 1990-1992 in Germany were about 3000-4000
t/year; use figures for Western Europe in 1988 totalled 6650 t (BUA,
1995). Use patterns for Western Europe in 1988 and for Germany in
1990-1992 are shown in Table 1.
Table 1: Phenylhydrazine use patterns in Western Europe
and Germany.
Phenylhydrazine use (%)
Industry Western Europe, Germany,
1988 1990-1992
Pharmaceuticals 37.6 70.2
Agrochemicals 42.9 7.2
Dyes 15 21.8
Others 4.5 0.8
From production and processing of phenylhydrazine in Germany
during 1990-1992, an estimated 50 kg and <13 t were emitted to the
atmosphere and the hydrosphere, respectively, each year. Less than
50 t of phenylhydrazinium chloride were released to water each year in
the same period (BUA, 1995).
There are now no manufacturers of phenylhydrazine or the
phenylhydrazine hydrochloride salt in the United Kingdom
(Brooke et al., 1997). Two firms are known to import phenylhydrazine
into the United Kingdom, one from its manufacturing site in Germany
and the other from Japan. The total market for phenylhydrazine in the
United Kingdom is thought to be about 20 t/year, whereas the market
for phenylhydrazine hydrochloride is not known. The market for
phenylhydrazine has been static for several years.
Phenylhydrazine is used worldwide mainly as a chemical
intermediate in the pharmaceutical, agrochemical, and chemical
industries. The United Kingdom's pattern of use appears
representative. One company uses phenylhydrazine to produce a chemical
intermediate for use in the photographic industry. Another
manufacturer uses the chemical in the synthesis of organic chemicals.
Phenylhydrazine is also used as a chemical intermediate in the
pharmaceutical and agrochemical industries in the United Kingdom. In
addition, there is some laboratory-scale use of this chemical.
There are no known consumer uses of phenylhydrazine or its
hydrochloride salt in the United Kingdom or Germany. No information is
available regarding the potential for consumer exposure in other
countries.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Most emissions of phenylhydrazine into the environment are into
the hydrosphere. At acidic pH, phenylhydrazine occurs as the salt
(BUA, 1995).
In the atmosphere, phenylhydrazine would exist solely in the
vapour phase (HSDB, 1998). Calculated half-lives of 3.1 h (BUA, 1995)
and 9 h (Meylan & Howard, 1993) have been reported for phenylhydrazine
following reaction with hydroxyl radicals in the atmosphere.
Phenylhydrazine strongly absorbs ultraviolet light in the
environmentally significant range, suggesting that it may photolyse in
sunlight (HSDB, 1998); slow photodecomposition in diffuse daylight in
the absence of oxygen is deduced in BUA (1995). In the presence of
oxygen, phenylhydrazine is subject to autoxidation, the reaction being
accelerated by light and heat; the substance becomes reddish brown on
exposure to air as a result of this autoxidation (Ullmann, 1977).
No hydrolysis is expected to occur (BUA, 1995).
The Henry's law constant for phenylhydrazine has been calculated
at 9.69 × 10-3 Pa.m3/mol (BUA, 1995). This is equivalent to a
dimensionless Henry's law constant (air/water partition coefficient)
of 3.92 × 10-6. These values indicate that phenylhydrazine is
essentially non-volatile from water surfaces.
Reported log octanol/water partition coefficients (log Kow)
range from 1.25 to 1.90 (BUA, 1995); an estimated bioconcentration
factor of 5 was based on the lower value (HSDB, 1998), indicating a
low capacity for bioaccumulation. However, sorption based on chemical
binding is possible, which could lead to some bioaccumulation (BUA,
1995). The sorption coefficient ( Koc) can be calculated to range
between 7.3 (Organisation for Economic Co-operation and Development
[OECD] Technical Guidance Manual) and 11 (Karickhoff et al., 1979),
indicating little sorption to particulates and a capacity for mobility
in soil. However, the regression equations on which these estimates
are based derive from hydrophobic compounds and may not adequately
reflect the likely sorption of the hydrophilic phenylhydrazine.
In a modified OECD ready biodegradability screening test (OECD
301E), phenylhydrazine was "readily biodegradable"; elimination was
77% after 10 days and 97% after 28 days using non-adapted inoculum.
Elimination through abiotic processes in controls was 11% after both
10 and 28 days (BASF, 1993). In the Zahn-Wellens test for inherent
biodegradability (OECD 302B), 20-30% elimination occurred over 3 h
(sorption), with 80% chemical oxygen demand achieved over 15 days
using non-acclimatized industrial activated sludge. Using acclimatized
activated sludge, 85% elimination was seen after 10 days (Hoechst,
1980, 1992). A similar value of 85% elimination in 9-13 days was
reported in the same test by Wellens (1990).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
Phenylhydrazine was not detected (detection limit 0.002 µg/ml
with high-performance liquid chromatography) in 30 samples of surface
water in the 1986 monitoring of the general environment by the Japan
Environment Agency (1987). It was also not detected in 30 samples of
sediment (detection limit 0.2 µg/kg with high-performance liquid
chromatography).
Monitoring of wastewater at the Hoechst production plant in
Germany failed to detect the compound in either inflow or outflow
wastewater (detection limit 500 µg/litre) (BUA, 1995).
6.2 Human exposure
The number of persons potentially exposed to phenylhydrazine or
its hydrochloride salt is not known, but it is expected to be small
(Brooke et al., 1997). Industry in the United Kingdom has not been
able to provide any personal exposure data, although it has been
indicated that exposure to airborne phenylhydrazine between 1993 and
1994 was controlled by process enclosure, the provision of local
exhaust ventilation, and personal protective equipment.
As there are no measured data available, the sections below
describe the use of computer-modelled exposure data from the EASE
model. This is a general-purpose predictive model for workplace
exposure assessments, which is used when measured exposure data are
limited or not available. In its present form, the model is in
widespread use across the European Union for the occupational exposure
assessment of new and existing substances.
Following descriptions of precautions taken during use, the most
appropriate parameters for the use of the EASE model are
non-dispersive use with local exhaust ventilation in place. Exposure
between 25 and 40°C with these assumptions is predicted to be within
the range 2.3-13.5 mg/m3 (0.5-3 ppm) (8-h time-weighted average).
Further, as only small quantities are involved, and as extensive
containment is provided by the combination of a vessel open only at
the bung hole and transfer being achieved by vacuum transfer, exposure
will be at the low end of this range (i.e., 2.3 mg/m3 [0.5 ppm] 8-h
time-weighted average). In practice, the 8-h time-weighted average
exposure will be less than this figure, as the activities involving
exposure to phenylhydrazine will take place for only part of the
shift.
These predicted exposures would be even lower for work carried
out in fume cupboards and would be extensively mitigated at the
operator by use of respiratory protective equipment; air-fed suits
would effectively reduce exposures of these magnitudes to zero.
For direct handling and non-dispersive use with a contact level
assumed to be incidental from the process descriptions, EASE predicts
dermal exposures to range from 0 to 0.1 mg/cm2 per day. If direct
handling is eliminated, dermal exposure is very low. Again, these
exposures would effectively be reduced to zero by adoption of
high-quality personal protective equipment and distancing procedures
described in this assessment.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
Phenylhydrazine reacts readily with the carbonyl group, -C=O,
which is common among biological molecules. It is therefore expected
that direct binding to biological molecules would occur.
There is only limited information available on the toxicokinetics
of phenylhydrazine. Evidence from toxicokinetic and toxicity studies
and from human experience indicates that phenylhydrazine is well
absorbed by the inhalation, oral, and dermal routes in animals and
humans.
Once absorbed, some phenylhydrazine appears to be rapidly taken
up by red blood cells, where destructive intracellular reactions may
occur.
Evidence from a number of studies in vitro and in vivo
suggests that phenylhydrazine interacts with haemoglobin and
cytochrome P-450 in an oxidation reaction, resulting in the generation
of destructive free radicals, which are responsible for subsequent
haemolysis (e.g., Itano et al., 1975; Valenzuela et al., 1977, 1981;
Goldberg et al., 1979; Jain & Hochstein, 1979; Jonen et al., 1982;
Hill, 1985; Marks, 1985; Di Cola et al., 1988, 1989; Maples et al.,
1988).
There is little information available on tissue distribution.
There is only one study available that investigates the
metabolism and excretion of phenylhydrazine, following oral dosing in
rabbits (McIsaac et al., 1958). This study shows that phenylhydrazine
is extensively metabolized following oral administration, although the
complete metabolic pathway has not been characterized. The main
reactions identified in this study were hydroxylation of the aromatic
ring to p-hydroxyphenylhydrazine, followed by conjugation, probably
with glucuronic acid, and production of phenylhydrazones, by reaction
with natural keto acids.
This study also indicated that the major route of excretion is
via the urine. A significant proportion of a single dose was excreted
relatively slowly; 50% of the dose was excreted within 4 days of
dosing. There are insufficient data to determine whether there is any
accumulation of phenylhydrazine in body tissues on repeated exposure.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
Many of the studies reported for phenylhydrazine have been
conducted using phenylhydrazine hydrochloride. This salt is a weak,
complex-forming compound, and either the salt or the free base will
form depending on the physiological medium, regardless of the form in
which the phenylhydrazine is administered (NIOSH, 1978). The
toxicological properties of the salt can therefore be considered to be
at least equivalent to those of free phenylhydrazine. Differences in
toxicity may arise when properties such as pH or solubility contribute
to the expression of toxicity. All dose values quoted throughout this
document refer to free phenylhydrazine.
8.1 Single exposure
In relation to inhalation exposure, there is only one very poorly
reported study, which reports LC50 values for an unstated exposure
period of 2745 mg/m3 (610 ppm) in the rat and 2093 mg/m3 (465 ppm)
in the mouse (Pham, 1979). However, it is expected that the marked
toxicity seen following oral and dermal exposure would also be
expressed following inhalation. Phenylhydrazine is toxic by oral
administration, and oral LD50 values in the range 80-188 mg/kg body
weight have been reported for the rat, mouse, guinea-pig, and rabbit
(Ekshtat, 1965; Pham, 1979). Clinical signs reported were motor
excitation and tonic/clonic spasms. In rabbits, dermal exposure to 380
mg phenylhydrazine/kg body weight for 24 h resulted in 20-30%
mortality, although no deaths occurred in rats at this dose
(Derelanko et al., 1987). The toxic effects are characterized by
destruction of red blood cells, causing a reduction in erythrocyte
count, increased reticulocyte count, methaemoglobin formation, and the
formation of Heinz bodies, and a cyanotic external appearance may
develop. Enlargement and dark coloration of the spleen are also
reported, effects that are considered to be secondary to the
erythrocyte damage.
8.2 Irritation and sensitization
In the single-dose dermal toxicity study in rabbits and rats
reported in the previous section, phenylhydrazine hydrochloride was
applied to the skin as a solid moistened with distilled water, under
either an occlusive or semi-occlusive dressing, for 24 h (Derelanko et
al., 1987). Skin irritation was seen in all rabbits, with some
necrosis at the treated site at 24 h post-application and sloughing of
the skin reported in some animals. Skin irritation, which appeared
within 24 h and persisted for up to 7 days post-exposure, was seen in
a high proportion of rats. Necrosis developed in a small number of
rats.
The quality of skin irritation data from other studies is
limited; overall, however, the results support the conclusion reached
by Derelanko et al. (1987) - that phenylhydrazine should be considered
a skin irritant (Jadassohn, 1930; von Oettingen & Deichmann-Gruebler,
1936; Roudabush et al., 1965; Schuckmann, 1969; Derelanko et al.,
1987). Further details of these studies can be obtained in the source
document (Brooke et al., 1997).
The only available information on the eye irritation potential of
phenylhydrazine in animals comes from a poorly described study in
which application of a 50% solution of phenylhydrazine to the eyes of
rabbits was reported to cause severe suppurative conjunctivitis (Pham,
1979).
There are no good-quality studies available in animals that
investigate the skin sensitization potential of phenylhydrazine. Only
one poorly described study in guinea-pigs is available (Jadassohn,
1930). When a 10% solution of phenylhydrazine in alcohol was painted
on a skin site that had been pretreated 2-3 weeks previously with
undiluted phenylhydrazine, very intense erythema and swelling,
followed by scaling and encrustation, were consistently seen. This
response to 10% phenylhydrazine was more severe than that described in
animals that had not been pretreated.
No information is available in relation to respiratory tract
sensitization.
8.3 Short-term exposure
There are only very limited, poor-quality data available in
relation to short-term repeated-dose toxicity. The effects seen are
similar to those seen following single exposure - in particular,
destruction of circulating red blood cells. Toxicity to the spleen,
liver, and kidney has also been observed in animal studies, possibly
secondary to haemolysis.
Kelly et al. (1969) administered phenylhydrazine hydrochloride to
21 mice by oral gavage once weekly for 8 weeks, at an estimated dose
of 85 mg/kg body weight per week. There were 10 saline-treated
controls. The study report described only tumour-related findings.
There was 30% mortality in treated mice compared with none in
controls.
Haematological changes were reported in four dogs administered 60
mg phenylhydrazine/kg body weight, either as a single dose or as 2, 3,
or 10 equal doses on consecutive days (Giffin & Allen, 1928). There
was a marked reduction in erythrocyte count that was comparable in
magnitude in all dogs at the end of 10 days, but that occurred at a
faster rate after a single high dose compared with repeated lower
doses.
In another dog study, 60 mg phenylhydrazine/kg body weight was
administered daily to three dogs for 5 days (Allen & Giffin, 1928).
One animal was moribund at sacrifice on the fifth day and one animal
died on the fifth day, although it is not specified that death was
treatment related. Full necropsy was performed on only one animal, in
which it was found that the blood was brown and did not coagulate
readily. Several organs, including the liver and kidneys, were darkly
coloured, and several organs contained capillaries engorged with
blood. Blood pigment and partially destroyed erythrocytes were found
in the spleen. Hepatic cell atrophy was noted, and there was an
increase in the iron content of the liver. Similar liver effects were
seen in the two other dogs.
Bolton (1935) briefly reported the effect of repeated oral
administration of 14 mg phenylhydrazine hydrochloride/kg body weight
to one dog on 4 consecutive days. There was a reduction in erythrocyte
count and haemoglobin concentration, whereas white cell count
gradually increased. These parameters had returned towards
pretreatment values 12 days after the last dose. Pathological findings
were reported to be non-conclusive, but no details were given.
Overall, the frequency, duration, and level of exposure often
varied throughout the studies reported above, so that interpretation
of results is very difficult. In all studies, phenylhydrazine as
phenylhydrazine hydrochloride was administered either by stomach tube
or by subcutaneous injection. The authors report that similar findings
were obtained regardless of route. There were no control animals. None
of the treated animals showed clinical signs of toxicity, and there
was no excessive weight loss or gain reported.
There have been a number of studies that investigate the effect
of short-term repeated parenteral administration of phenylhydrazine
(Bodansky, 1923; von Oettingen & Deichmann-Gruebler, 1936; Säterborg,
1974; Ades & Cascarano, 1979; Jain & Hochstein, 1979; Goldstein et
al., 1980; Nishida et al., 1982; Dornfest et al., 1986). These studies
confirm the ability of phenylhydrazine to damage red blood cells but
otherwise do not provide any other information in relation to the
toxicity of phenylhydrazine administered by occupationally relevant
routes of exposure.
8.4 Long-term exposure
8.4.1 Subchronic exposuren
In a very poorly reported study from which limited conclusions
can be drawn, rats, mice, guinea-pigs, and rabbits were exposed to
phenylhydrazine vapour at 0, 0.1, 15.8, 22.5, or 225 mg/m3 (0, 0.03,
3.5, 5, or 50 ppm) (Pham, 1979). Group sizes, duration of exposure,
and exposure regime were not given, although it can be inferred that
some animals were exposed for at least 6 months. Deaths were reported
to occur in animals exposed to 225 mg phenylhydrazine/m3 (species not
specified). Severe weight loss and unspecified haematological changes
and changes in central nervous system function were reported to
precede death, and there was evidence of haemolysis and dystrophic
changes in the liver, spleen, and cerebrum. Animals exposed to 15.8
and 22.5 mg/m3 were reported to have a reduction in erythrocyte count
and haemoglobin concentration, an increase in reticulocytes, and
methaemoglobinaemia; these changes were reversible at 15.8 mg/m3.
Haemolysis and dystrophic changes in the liver and other unspecified
organs were also reported for animals exposed to 22.5 mg/m3. No
further information is available on pathological changes at 0.1
mg/m3. It is not clear if there were lung effects at any of the
exposure concentrations.
It is not possible to draw firm conclusions from a poorly
reported study in three dogs in which the effect of phenylhydrazine
administration on renal and hepatic function and on erythropoiesis was
investigated (Allen & Giffin, 1928). Three dogs were administered
phenylhydrazine in 146 daily doses over a period of 8 months, to give
a total dose of 950 mg/kg body weight; the dosing regimen included a
period of about 60 days of uninterrupted administration of a single
dose level or of two dose levels (6-12 mg/kg body weight per day).
Again, the route of administration was unclear. Kidney function and
hepatic function were unaffected by treatment. Erythrocyte count was
reduced by treatment but recovered after cessation of treatment, at a
rate that was unrelated to the duration of exposure, thus indicating
that the administered dose had no effect on erythropoietic function.
Pathological examination was conducted on two of these dogs at 12 or
13 months. There was evidence of spleen toxicity, liver congestion,
and kidney damage.
In a very briefly reported study, phenylhydrazine was
administered to 25 female Swiss mice by oral gavage, 5 days/week for
40 weeks, at an estimated daily dose of 17-33 mg/kg body weight
(Roe et al., 1967). There were 85 untreated controls. Marked anaemia
necessitated a reduction in the dose during the sixth week of
treatment. No other toxic effects were observed.
8.4.2 Chronic exposure and carcinogenicity
Phenylhydrazine hydrochloride was administered daily by stomach
tube for 42 weeks to 30 BALB/c mice, at an estimated dose level of 25
mg phenylhydrazine/kg body weight (Clayson et al., 1966). Thirty
control animals were included in the study, but a control animal was
killed whenever a treated animal died, to match survival rates. There
was a statistically significant increase in the incidence of animals
with lung tumours in the treated group (53%) compared with controls
(13%). There was also a slight increase in the average number of
tumours per mouse, and the majority of treated mice had multiple
pulmonary tumours. Adenomas accounted for 83% of pulmonary tumours in
the treated group, half of which were judged to be becoming malignant,
and 17% of tumours were carcinomas.
Phenylhydrazine hydrochloride was administered in drinking-water
to 100 Swiss mice for their lifetime, at an estimated daily dose of 22
mg/kg body weight (Toth & Shimizu, 1976). There were 200 control mice.
Complete necropsy was performed on all animals. All organs were
examined macroscopically, and histological analysis was performed on a
wide range of tissues as well as on any organ showing gross pathology.
Phenylhydrazine was reported to decrease survival in comparison with
controls, and many of the treated decedents showed splenomegaly,
although numbers were not given. There was a statistically significant
increased incidence of blood vessel tumours (mainly angiosarcomas and
angiomas) in the liver of treated animals (21%) compared with
controls (0%).
8.5 Genotoxicity and related end-points
Phenylhydrazine and phenylhydrazine hydrochloride have been
investigated in a number of Ames tests, in a variety of strains, and
in the presence and absence of exogenous metabolic activation using up
to 1000 µg phenylhydrazine or phenylhydrazine hydrochloride per plate
(Shimizu et al., 1978; Tosk et al., 1979; De Flora, 1981; Parodi et
al., 1981; Levin et al., 1982; Malca-Mor & Stark, 1982; Rogan et al.,
1982; De Flora et al., 1984a,b; Wilcox et al., 1990; Muller et al.,
1993). The quality of these studies is generally high, and the studies
were apparently conducted according to standard methodology, although
detailed reporting of the results is not always available.
There is some variability in the findings, although positive
results have been obtained in Salmonella typhimurium strains TA97,
TA100, TA102, TA1537, and TA1538 in the absence of exogenous metabolic
activation. In addition, positive results were obtained in the
presence of metabolic activation in TA98 and TA1535. Some
investigators have reported that the mutagenic action is slightly
decreased by the presence of exogenous metabolic activation (Parodi et
al., 1981; Malca-Mor & Stark, 1982; De Flora et al., 1984a,b).
However, one study reports an increase in mutagenic activity in the
presence of metabolic activation (Rogan et al., 1982).
Phenylhydrazine has also given positive results in a number of
other, less well validated bacterial assays (using S. typhimurium
strains such as TA2638, TP138, BA9, and BA13), in the presence and
absence of exogenous metabolic activation (De Flora et al., 1984b;
Ulitzur et al., 1984; Ruiz-Rubio et al., 1985; Levi et al., 1986;
Muller et al., 1993).
Phenylhydrazine has not been tested in an in vitro chromosomal
aberration assay. In a brief abstract of a mammalian cell gene
mutation assay in V79 cells, with and without metabolic activation, a
positive result was reported for phenylhydrazine (Kuszynski et al.,
1981). However, no firm conclusions can be drawn from this report
because of deficiencies in the reporting.
In an unscheduled DNA synthesis assay in rat and mouse primary
hepatocytes, concentrations of 0.0144-144 mg phenylhydrazine
hydrochloride/litre were assessed (Mori et al., 1988). Although
toxicity was measured, no details were given, and quantitative data
were not reported. A positive result was obtained in both cell types,
although the effect was small.
Phenylhydrazine was tested in a micronucleus assay in vitro
using primary mouse bone marrow cells (Suzuki, 1985). Bone marrow
cells from the femur were exposed to 1-50 µg phenylhydrazine/ml for 30
min, in the presence and absence of metabolic activation. A total of
1500 polychromatic erythrocytes (PCEs) per concentration was scored
for the presence of micronuclei. There was no measure of cytotoxicity.
The percentage of micronucleated PCEs was statistically
significantly increased, in the presence of S9 only, at
phenylhydrazine concentrations of 5 µg/ml and greater in a
concentration-related manner.
BALB/c mice were administered a single intraperitoneal injection
of phenylhydrazine, and the incidence of micronucleated PCEs in the
bone marrow was measured at 24 and 48 h (Suzuki, 1985).
Phenylhydrazine was reported to be positive in this test, but no
details of the results or of the test were given. In view of the poor
reporting, no firm conclusions can be drawn from this study.
Groups of 11-12 female BALB/c mice were given a single
intraperitoneal injection of 50 mg phenylhydrazine/kg body weight in
saline (Steinheider et al., 1985). Blood smears of tail vein blood
were prepared at 24-h intervals for 7 or 11 days, and reticulocytes
and micronuclei in normochromatic erythrocytes (NCEs) and PCEs were
counted. It is not stated how many cells were counted per mouse. There
was no reporting of toxicity.
Phenylhydrazine caused a statistically significant increase in
the reticulocyte count on days 2-4 post-injection and in PCEs on day
3. There was a statistically significant increase in the incidence of
micronucleated PCEs at 24 h post-injection (from 1 to 4.7 per 1000)
and in micronucleated NCEs at 48 h post-injection (from 0.7 to 2.3 per
1000).
However, similar increases in micronucleated NCEs were also seen
following bleeding of the animals and splenectomy. The authors suggest
that the increase in micronuclei seen following phenylhydrazine
treatment was due at least partly to stimulation of erythropoiesis
because of the haemolysis induced by phenylhydrazine, thus leading to
more errors of nuclear expulsion; hence, the results do not
necessarily indicate a direct genotoxic action of phenylhydrazine.
Groups of 7-12 mice were given a single intraperitoneal injection
of either 85 or 170 mg phenylhydrazine/kg body weight and killed 1 and
6 h, respectively, after treatment (Parodi et al., 1981). In addition,
six mice were given a series of five daily intraperitoneal injections
of 7.6 mg phenylhydrazine/kg body weight and sacrificed 6 h after the
last injection. Control animals were injected with saline only. DNA
damage was assessed by measurement of the alkaline elution rate of
single-strand DNA from liver and lung tissue extracts.
A statistically significant change in the elution rate of liver
and lung DNA was seen in all groups of treated animals compared with
controls, except in the case of lung tissue DNA from mice given a
single dose of 85 mg phenylhydrazine/kg body weight. Phenylhydrazine
is considered to give a positive result in this assay for DNA damage.
The formation of DNA adducts ( N7-methylguanine and a trace of
O6-methylguanine) in the liver was demonstrated in rats receiving
65 mg phenylhydrazine/kg body weight by oral gavage (Mathison et al.,
1994). Other tissues were not examined.
8.6 Reproductive and developmental toxicity
Each of three dogs (one dog per dose group) received 20, 30, or
40 mg phenylhydrazine/kg body weight in saline by subcutaneous
injection on 2 consecutive days (Witchett, 1975). Two control animals
were not injected. At necropsy, performed on all three animals within
a few days of dosing, a "striking" reduction in spermatogenesis was
reported, with an absence of sperm in the epididymis. The validity of
this result is not clear, given the apparent extreme rapidity of the
effect.
Groups of 8-12 pregnant Wistar rats were given an intraperitoneal
injection of 7.5 mg phenylhydrazine/kg body weight as phenylhydrazine
hydrochloride on days 17, 18, and 19 of gestation; or 15 mg
phenylhydrazine/kg body weight as phenylhydrazine hydrochloride on
days 18 and 19 of gestation (Tamaki et al., 1974). Control animals
were not treated. There was no reporting of maternal toxicity or of
the effect of treatment on gestation or pup viability. Toxicity of the
pups was reported only insofar as there was incidence of jaundice
and/or anaemia among the offspring of treated animals. Twelve male
offspring with severe jaundice and anaemia, selected from treated
dams, and nine males from control dams were assessed at 9-22 weeks of
age for functional and behavioural status.
Although the authors reported that experimental animals showed
statistically significant differences from controls in some tests,
these findings are not considered to be reliable because of the small
numbers of animals used and the exclusion of a control animal from the
analysis. In addition, it is noted that only a brief part of the
gestation period was covered by the treatment regime (days 17-19); no
explanation is available for this choice of dosing regime.
Yamamura et al. (1973) reported in a brief abstract that
intraperitoneal injection of pregnant rats with 15 mg
phenylhydrazine/kg body weight as phenylhydrazine hydrochloride on
days 18 and 19 of gestation produced hyperbilirubinaemia (resulting
from haemolysis) in fetuses and newborns.
8.7 Immunological and neurological effects
No studies are available that specifically investigate the
immunological and neurological end-points, and there is no relevant
information from toxicity studies in animals.
9. EFFECTS ON HUMANS
In humans, no information is available in relation to single
exposure via the inhalation or oral routes, although effects similar
to those seen following dermal exposure would be expected to occur.
Systemic toxicity developed in humans after dermal exposure to liquid
phenylhydrazine, despite immediate attempts to reduce exposure by
removal of contaminated clothing and washing of skin (Schuckmann,
1969). Toxicity was manifest by damage to red blood cells, in one case
resulting in haemolytic jaundice. No such systemic effects were
reported in two cases of skin contamination with solid phenylhydrazine
hydrochloride.
In relation to skin irritation, information is available from
worker exposure data. There was no reporting of irritancy effects in
workers exposed to liquid phenylhydrazine following accidental
exposure, although systemic effects were seen (Schuckmann, 1969). Skin
irritation following contact with phenylhydrazine hydrochloride powder
was reported in two workers following accidental exposure. Local
irritation, superficial erythema, and partly bullous-papular changes
were noted in one case following spillage of powder on arms; multiple
burn marks and small blisters at the site of contact were reported in
the second case in which phenylhydrazine hydrochloride had spilled
into the worker's gloves and shoes. The author also refers to medical
records at the works that describe a number of cases of skin
irritation of differing severity due to phenylhydrazine hydrochloride,
but no details are given.
There are no data available on the eye irritation potential of
phenylhydrazine in humans.
There are a number of case reports of skin hypersensitivity
reactions to phenylhydrazine and its hydrochloride salt in humans.
Solomons (1946) conducted a patch test in one subject with a
phenylhydrazine crystal placed on the forearm under a dressing for an
unstated exposure period. Marked erythema and some oedema developed on
the exposure site after 18 h, with the formation of vesicles after 30
h and crusting after a further 24 h.
Similar hypersensitive skin reactions were reported following
individual exposures to solid or aqueous solutions of phenylhydrazine
or phenylhydrazine salts (Wright & Joyner, 1930; Frost & Hjorth, 1959;
Pevny & Peter, 1983).
There is also evidence that cross-sensitization can occur between
hydrazine compounds, so that subjects already sensitized to hydrazine,
a known skin sensitizer, are also sensitized to hydrazine derivatives,
including phenylhydrazine (Malten, 1962; Van Ketel, 1964; Hovding,
1967; Rothe, 1988).
No data are available on the potential of phenylhydrazine to
cause respiratory tract sensitization.
Earlier this century, phenylhydrazine and phenylhydrazine
hydrochloride were administered orally (usually around 100-200 mg/day)
for the treatment of blood disorders (e.g., Giffin & Allen, 1933). In
some cases, treatment was effective; in others, however, the outcome
was fatal (e.g., Giffin & Conner, 1929). The effects seen (beneficial
or otherwise) may have been related to the disease process and cannot
be attributed entirely to phenylhydrazine.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1 Aquatic environment
Results of acute toxicity tests on aquatic organisms are
summarized in Table 2. All concentrations are nominal.
In the early life stage test summarized in Table 2 (Xiu et al.,
1992), newly fertilized eggs of the zebra fish were exposed to
concentrations of phenylhydrazine through hatch and into the larval
stage (total exposure time was 16 days). A NOEC (survival) was
established for eggs at 5 days post-fertilization at 0.0039 mg/litre,
with a lowest-observed-effect concentration (LOEC) at 0.0078 mg/litre.
At the end of the test (16 days), the NOEC for larvae was 0.000 49
mg/litre, and the LOEC was 0.000 98 mg/litre. The study was conducted
according to a Swedish standard protocol (ss 028193).
In a study on the goldfish ( Carassius auratus), 40% of fish
died when exposed to phenylhydrazine at a nominal concentration of 1
mg/litre for 48 h. Signs of toxicity included erratic swimming,
sinking to the bottom of the test tank, and slowed and erratic
respiration. No gross lesions were found following dissection,
although all viscera showed focal haemorrhaging (Houston et al.,
1988).
Effects on blood cells and the haematopoietic system, comparable
to those found in mammals, were seen in fish injected with
phenylhydrazine. Chinook salmon ( Oncorhynchus tshawytscha) juveniles
were injected with 12.5 mg phenylhydrazine/kg body weight. Red cell
count, haemoglobin, and haematocrit fell to 1-5% of their normal
values within 10 days of treatment; a slight improvement was reported
15 days following injection, and values had returned to normal 95 days
after treatment (Smith et al., 1971).
10.2 Terrestrial environment
Phenylhydrazine at 21.6 mg/litre in a nutrient culture medium had
no effect on the growth of various soil fungi (Zsolnai, 1975).
A phenylhydrazine concentration of 50 mg/litre in a hydroponic
culture solution inhibited germination of Hordeum seeds for at least
6 days, whereas growth was stimulated in Lepidium. At 100 mg/litre,
seedling growth was stimulated in Hordeum but not in Lepidium. At
500 mg/litre, phenylhydrazine inhibited growth in seedlings of both
species, although the seedlings were still apparently "healthy"
(Bokorny, 1933).
Exposure of soil nematodes Caenorhabditis briggsae in culture
to phenylhydrazine at 50 mg/litre of medium resulted in reduced growth
of all four larval stages (the effect was most marked on the last
larval instar) and therefore delayed development to adults. However,
the adults formed were capable of reproduction, although the number of
progeny was reduced. A concentration of 15 mg/litre also delayed
development, although to a lesser degree compared with the higher
concentration (Kampfe et al., 1986a,b). A 6-day EC50 of 12 mg/litre
was reported for production of progeny in culture for the same
nematode (Kreil, 1982).
No dietary or oral toxicity studies have been performed on birds.
However, injection studies have shown haemolytic anaemia and reduced
white cell counts in birds. In contrast to mammals, cell division in
erythropoietic tissue is unaffected by phenylhydrazine (Williams,
1972; Clark et al., 1988; Datta et al., 1989, 1990).
Table 2: Acute toxicity of phenylhydrazine to aquatic organisms.
Organism End-point Concentration Reference
(mg/litre)
Bacteria
Photobacterium phosphoreum 30-min EC50 66.9 Kaiser et al.
(luminescence) (1987)
Facultative anaerobes 24-h toxic 60 Hoechst (1980)
(mixed culture) threshold
Escherichia coli minimum 109.3 Romero & Canada
inhibitory (1991)
concentration
Escherichia coli, minimum >3000 Zemek et al.
Micrococcus luteus, inhibitory (1978)
Bacillus licheniformis concentration
Invertebrates
water flea LC50 2-5 Hoechst (1980)
(Daphnia magna) (immobilization)
Fish
zebra fish 96-h LC50 0.16-0.25 Hoechst (1982)
(Brachydanio rerio) 96-h NOEC 0.1
zebra fish 5-day NOEC (eggs) 0.0039 Xiu et al.
(Brachydanio rerio) 16-day NOEC (larvae) 0.000 49 (1992)
Japanese killifish 48-h LC50 15.7 Tonogai et al.
(Oryzias latipes) (1982)
Table 2 (cont'd)
Organism End-point Concentration Reference
(mg/litre)
common carp 24-h LC100 1.0 Menzie (1979)a
(Cyprinus carpio) 96-h NOEC 0.1
bluegill 48-h LC50 0.1 Menzie (1979)a
(Lepomis 96-h NOEC 0.01
macrochirus)
a Menzie C (1979) Value taken from the DIMDI/ECDIN database. Test performed by the
United States Fish and Wildlife Service, Bureau of Sports, Fisheries and Wildlife,
Department of the Interior, Washington, DC [cited in BUA, 1995].
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Phenylhydrazine is toxic by single exposure via the oral route
(LD50 80-188 mg/kg body weight) and is expected to be toxic by the
inhalation and dermal routes (data from these routes of exposure are
less clear). Phenylhydrazine solution was severely irritating to
rabbit eyes; hence, it is reasonable to predict that it would have
significant eye irritation potential in humans.
Phenylhydrazine also has skin irritation potential, and there is
evidence from human case reports that it has skin sensitizing
properties. Exposure to phenylhydrazine may cause damage to red blood
cells, potentially resulting in anaemia and consequential secondary
involvement of other tissues, such as the spleen and liver. The dose
(exposure)-response characteristics for the induction of damage to the
red blood cells are poorly defined, and a no-effect level has not been
identified. Where phenylhydrazine has been used therapeutically in
humans, via the oral route, for the treatment of blood disorders,
daily doses of the order of 1.5-4 mg/kg body weight per day have
caused a reduction in the numbers of red blood cells; given the health
status of the individuals concerned, however, these data are of
limited use. Phenylhydrazine is mutagenic in vitro, and, although not
conclusive, there is some evidence to indicate that it may express
genotoxic activity in vivo. The substance is clearly carcinogenic in
mice following oral dosing, inducing tumours of the vascular system.
The mechanism for tumour formation is unclear, and, given the
genotoxic profile of phenylhydrazine, a genotoxic component cannot be
excluded. Carcinogenic potential in humans cannot be excluded given
the profile of genotoxicity and animal carcinogenicity, particularly
as other expressions of phenylhydrazine toxicity are common to a
number of species, including humans.
No conclusions can be drawn from the available information on
fertility or development.
11.1.2 Criteria for setting guidance values fon phenylhydrazine
There are no adequate data available regarding reproductive or
developmental effects; hence, it is not possible to evaluate the risk
to human health for these end-points.
The use pattern and physical/chemical characteristics of the
compound suggest that exposure of the general population would be
negligible.
Using United Kingdom workplace conditions as an example (section
6.2), exposure to phenylhydrazine vapour during most occupational
processes would result in a body burden of up to 0.33 mg/kg body
weight per day, assuming a 70-kg worker breathes 10 m3 of air in a
working day and that 100% phenylhydrazine is absorbed. There are no
data available from which to estimate the contribution to body burden
from dermal uptake, although this is expected to be negligible. A
threshold for the induction of red blood cell damage probably exists
but has not been identified, although daily oral doses calculated at
about 1.5 mg/kg body weight per day and above are associated with such
effects. Overall, at these levels of predicted inhalation exposure,
the risk of developing damage to the red blood cells is considered to
be low; if the levels were exceeded (e.g., of the order of a few ppm,
approximately 15-20 mg/m3), however, then this would be cause for
some concern.
On the basis that the carcinogenicity of phenylhydrazine may
involve a genotoxic mechanism, it is not possible to reliably identify
a threshold below which occupational exposure to phenylhydrazine would
not result in some risk to human health.
11.1.3 Sample risk characterization
The scenario chosen as an example is occupational exposure in the
United Kingdom.
The main health concerns associated with exposure to
phenylhydrazine are damage to the red blood cells, deleterious effects
on genetic material, and the development of cancer.
It is recognized that there are a number of different approaches
to assessing the risks to human health for genotoxic and carcinogenic
substances and in the subsequent risk management steps that may be
taken. In addition, although not used in the United Kingdom, there are
models for characterizing potency that may be of some benefit in
priority-setting schemes. In the United Kingdom occupational setting,
a Maximum Exposure Limit or MEL (which is not a health-based standard)
has been proposed at 0.9 mg/m3 (0.2 ppm), 8-h time-weighted average.
The numerical value for the MEL was based on a level of control that
was deemed (by tripartite agreement) to be reasonably practicable
under United Kingdom workplace conditions, and in the United Kingdom
there is a continuing requirement to reduce exposure levels as far as
reasonably practicable with the technology that is currently
available.
Phenylhydrazine also possesses skin and eye irritant properties
and possibly skin sensitizing potential. The information available
indicates that local exposure of these tissues is unlikely; if it did
occur, however, then there would be risk of irritation to the eyes and
the development of irritant and/or allergic dermatitis.
11.2 Evaluation of environmental effects
No atmospheric effects are expected given the release of
phenylhydrazine predominantly to water, its extremely low
volatilization from water to the atmosphere, and its rapid calculated
atmospheric half-life following reaction with hydroxyl radicals.
Few toxicity studies are available for terrestrial organisms, and
little emission to land is expected; on this basis, no quantitative
risk assessment can be attempted for the terrestrial environment.
Phenylhydrazine is degraded photochemically and autoxidizes in
water. It is readily biodegradable, and this is expected to be the
major route of breakdown in the environment. There is minimal sorption
to particulates.
Phenylhydrazine is toxic to aquatic organisms, with the lowest
reported NOEC in acute fish tests at 0.01 mg/litre; fish are generally
more sensitive than either daphnids or bacteria. A NOEC for
embryo-larval stages following 16 days of exposure from fertilization
has been reported at 0.000 49 mg/litre for the zebra fish.
There are no reported measurements of phenylhydrazine in
environmental media. Monitoring studies of both inflow and outflow to
the wastewater treatment plant of the Hoechst Hochst production plant
in Germany showed no detectable phenylhydrazine (detection limit 500
µg/litre) in weekly samples. Maximum emission to wastewater was
estimated at 13 t/year, and this will be used as a worst-case example.
Based on this emission rate, and using mainly default values from
the OECD Technical Guidance Manual, the initial predicted
environmental concentration of phenylhydrazine in river water
(PEClocal (water), in g/litre) would be as follows:
Ceffluent
PEClocal (water) =
(1 + Kp(susp) × Csusp) × D
where:
* Ceffluent is the concentration of phenylhydrazine in the
wastewater treatment plant effluent (g/litre), calculated as
Ceffluent = W × (100 - P)/(100 × Q), where:
W = emission rate (35.6 kg/day)
P = percent removal in the wastewater treatment plant
(based on the "ready biodegradability" of the
compound, 91%)
Q = volume of wastewater in m3/day (default 200
litre/day per capita for a population of 10 000
inhabitants; wastewater volume for the production
plant is unknown)
* Kp(susp) is the suspended matter/water adsorption coefficient,
calculated as Kp(susp) = Koc × foc(susp), where:
Koc = organic carbon/water partition
coefficient (7.3)
foc(susp) = fraction of organic carbon in suspended matter
(default 0.1)
* Csusp is the concentration of suspended matter in the river
water (in kg/litre; default 15 mg/litre)
* D is the dilution factor for river flow (flow rate for the
River Main averages 188 m3/s compared with the estimated flow
rate of the wastewater at 0.02 m3/s; dilution factor
approximately 10 000)
Under these very conservative conditions, PEClocal (water)
= 0.16 µg/litre.
Of the reported acute toxicity test results for organisms in the
environment, those for the majority of fish tested are substantially
lower than those for other organisms tested. The predicted no-effect
concentration (PNEC) will therefore be based on the fish results.
No long-term test results are available. Applying an uncertainty
factor of 1000 to the lowest reported standard acute LC50 value of
0.1 mg/litre for the bluegill ( Lepomis macrochirus) would give a
PNEC of 0.1 µg/litre. This is a factor of 100 lower than the lowest
reported NOEC for the same species. Alternatively, applying an
uncertainty factor of 10 to the NOEC for the early life stage test on
the zebra fish larvae gives a PNEC of 0.049 µg/litre. The more
conservative value will be used in estimating risk.
The low PEC of 0.16 µg/litre would not have been detected in the
monitoring at the site. Assuming that this value is the worst case, a
PEC/PNEC ratio of 3.2 is generated. This indicates that the risk to
aquatic organisms is low, based on very conservative assumptions. The
distribution of reported toxicity test results against the worst-case
PEC is plotted in Figure 1, illustrating the safety margin.
3. ANALYTICAL METHODS
For measurement of phenylhydrazine in water, reduction of Cu(II)
to Cu(I) by phenylhydrazine has been used as the basis for
spectrometric analytical methods measuring coloured complexes (Besada,
1988; Hasan, 1988). The methods are not specific and react to other
reducing substances. A detection limit of 10 µg/litre is given for one
method (Hasan, 1988).
In a method published by NIOSH (1994) for measurement of
phenylhydrazine in workplace air, the air is sampled into a midget
bubbler containing hydrochloric acid. Phosphomolybdic acid is added to
the resulting solution, and the reaction with phenylhydrazine causes
the formation of a bluish-green complex that can be measured at 730 nm
with a spectrophotometer. This method has a detection limit of about 5
mg/m3 (about 1 ppm), based on a 100-litre sample. Potential
interferences are listed as other hydrazine derivatives, aldehydes,
and ketones.
Both the MIRAN 1B and the Bruel and Kjaer 1302 Multigas Monitor
may be used to measure phenylhydrazine in air, with a detection limit
of around 13.5 mg/m3 (3 ppm) (Brooke et al., 1997). Any other
substance having similar infrared absorbances can be expected to
interfere with the measurement.
There are no published biological monitoring methods available
for phenylhydrazine.
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
There are a few reports of the natural occurrence of
phenylhydrazine in plants (BUA, 1995). Phenylhydrazine is produced
commercially by the diazotization of aniline followed by reduction of
the azo compound.
Production figures for 1990-1992 in Germany were about 3000-4000
t/year; use figures for Western Europe in 1988 totalled 6650 t (BUA,
1995). Use patterns for Western Europe in 1988 and for Germany in
1990-1992 are shown in Table 1.
Table 1: Phenylhydrazine use patterns in Western Europe
and Germany.
Phenylhydrazine use (%)
Industry Western Europe, Germany,
1988 1990-1992
Pharmaceuticals 37.6 70.2
Agrochemicals 42.9 7.2
Dyes 15 21.8
Others 4.5 0.8
From production and processing of phenylhydrazine in Germany
during 1990-1992, an estimated 50 kg and <13 t were emitted to the
atmosphere and the hydrosphere, respectively, each year. Less than
50 t of phenylhydrazinium chloride were released to water each year in
the same period (BUA, 1995).
There are now no manufacturers of phenylhydrazine or the
phenylhydrazine hydrochloride salt in the United Kingdom
(Brooke et al., 1997). Two firms are known to import phenylhydrazine
into the United Kingdom, one from its manufacturing site in Germany
and the other from Japan. The total market for phenylhydrazine in the
United Kingdom is thought to be about 20 t/year, whereas the market
for phenylhydrazine hydrochloride is not known. The market for
phenylhydrazine has been static for several years.
Phenylhydrazine is used worldwide mainly as a chemical
intermediate in the pharmaceutical, agrochemical, and chemical
industries. The United Kingdom's pattern of use appears
representative. One company uses phenylhydrazine to produce a chemical
intermediate for use in the photographic industry. Another
manufacturer uses the chemical in the synthesis of organic chemicals.
Phenylhydrazine is also used as a chemical intermediate in the
pharmaceutical and agrochemical industries in the United Kingdom. In
addition, there is some laboratory-scale use of this chemical.
There are no known consumer uses of phenylhydrazine or its
hydrochloride salt in the United Kingdom or Germany. No information is
available regarding the potential for consumer exposure in other
countries.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Most emissions of phenylhydrazine into the environment are into
the hydrosphere. At acidic pH, phenylhydrazine occurs as the salt
(BUA, 1995).
In the atmosphere, phenylhydrazine would exist solely in the
vapour phase (HSDB, 1998). Calculated half-lives of 3.1 h (BUA, 1995)
and 9 h (Meylan & Howard, 1993) have been reported for phenylhydrazine
following reaction with hydroxyl radicals in the atmosphere.
Phenylhydrazine strongly absorbs ultraviolet light in the
environmentally significant range, suggesting that it may photolyse in
sunlight (HSDB, 1998); slow photodecomposition in diffuse daylight in
the absence of oxygen is deduced in BUA (1995). In the presence of
oxygen, phenylhydrazine is subject to autoxidation, the reaction being
accelerated by light and heat; the substance becomes reddish brown on
exposure to air as a result of this autoxidation (Ullmann, 1977).
No hydrolysis is expected to occur (BUA, 1995).
The Henry's law constant for phenylhydrazine has been calculated
at 9.69 × 10-3 Pa.m3/mol (BUA, 1995). This is equivalent to a
dimensionless Henry's law constant (air/water partition coefficient)
of 3.92 × 10-6. These values indicate that phenylhydrazine is
essentially non-volatile from water surfaces.
Reported log octanol/water partition coefficients (log Kow)
range from 1.25 to 1.90 (BUA, 1995); an estimated bioconcentration
factor of 5 was based on the lower value (HSDB, 1998), indicating a
low capacity for bioaccumulation. However, sorption based on chemical
binding is possible, which could lead to some bioaccumulation (BUA,
1995). The sorption coefficient ( Koc) can be calculated to range
between 7.3 (Organisation for Economic Co-operation and Development
[OECD] Technical Guidance Manual) and 11 (Karickhoff et al., 1979),
indicating little sorption to particulates and a capacity for mobility
in soil. However, the regression equations on which these estimates
are based derive from hydrophobic compounds and may not adequately
reflect the likely sorption of the hydrophilic phenylhydrazine.
In a modified OECD ready biodegradability screening test (OECD
301E), phenylhydrazine was "readily biodegradable"; elimination was
77% after 10 days and 97% after 28 days using non-adapted inoculum.
Elimination through abiotic processes in controls was 11% after both
10 and 28 days (BASF, 1993). In the Zahn-Wellens test for inherent
biodegradability (OECD 302B), 20-30% elimination occurred over 3 h
(sorption), with 80% chemical oxygen demand achieved over 15 days
using non-acclimatized industrial activated sludge. Using acclimatized
activated sludge, 85% elimination was seen after 10 days (Hoechst,
1980, 1992). A similar value of 85% elimination in 9-13 days was
reported in the same test by Wellens (1990).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
Phenylhydrazine was not detected (detection limit 0.002 µg/ml
with high-performance liquid chromatography) in 30 samples of surface
water in the 1986 monitoring of the general environment by the Japan
Environment Agency (1987). It was also not detected in 30 samples of
sediment (detection limit 0.2 µg/kg with high-performance liquid
chromatography).
Monitoring of wastewater at the Hoechst production plant in
Germany failed to detect the compound in either inflow or outflow
wastewater (detection limit 500 µg/litre) (BUA, 1995).
6.2 Human exposure
The number of persons potentially exposed to phenylhydrazine or
its hydrochloride salt is not known, but it is expected to be small
(Brooke et al., 1997). Industry in the United Kingdom has not been
able to provide any personal exposure data, although it has been
indicated that exposure to airborne phenylhydrazine between 1993 and
1994 was controlled by process enclosure, the provision of local
exhaust ventilation, and personal protective equipment.
As there are no measured data available, the sections below
describe the use of computer-modelled exposure data from the EASE
model. This is a general-purpose predictive model for workplace
exposure assessments, which is used when measured exposure data are
limited or not available. In its present form, the model is in
widespread use across the European Union for the occupational exposure
assessment of new and existing substances.
Following descriptions of precautions taken during use, the most
appropriate parameters for the use of the EASE model are
non-dispersive use with local exhaust ventilation in place. Exposure
between 25 and 40°C with these assumptions is predicted to be within
the range 2.3-13.5 mg/m3 (0.5-3 ppm) (8-h time-weighted average).
Further, as only small quantities are involved, and as extensive
containment is provided by the combination of a vessel open only at
the bung hole and transfer being achieved by vacuum transfer, exposure
will be at the low end of this range (i.e., 2.3 mg/m3 [0.5 ppm] 8-h
time-weighted average). In practice, the 8-h time-weighted average
exposure will be less than this figure, as the activities involving
exposure to phenylhydrazine will take place for only part of the
shift.
These predicted exposures would be even lower for work carried
out in fume cupboards and would be extensively mitigated at the
operator by use of respiratory protective equipment; air-fed suits
would effectively reduce exposures of these magnitudes to zero.
For direct handling and non-dispersive use with a contact level
assumed to be incidental from the process descriptions, EASE predicts
dermal exposures to range from 0 to 0.1 mg/cm2 per day. If direct
handling is eliminated, dermal exposure is very low. Again, these
exposures would effectively be reduced to zero by adoption of
high-quality personal protective equipment and distancing procedures
described in this assessment.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
Phenylhydrazine reacts readily with the carbonyl group, -C=O,
which is common among biological molecules. It is therefore expected
that direct binding to biological molecules would occur.
There is only limited information available on the toxicokinetics
of phenylhydrazine. Evidence from toxicokinetic and toxicity studies
and from human experience indicates that phenylhydrazine is well
absorbed by the inhalation, oral, and dermal routes in animals and
humans.
Once absorbed, some phenylhydrazine appears to be rapidly taken
up by red blood cells, where destructive intracellular reactions may
occur.
Evidence from a number of studies in vitro and in vivo
suggests that phenylhydrazine interacts with haemoglobin and
cytochrome P-450 in an oxidation reaction, resulting in the generation
of destructive free radicals, which are responsible for subsequent
haemolysis (e.g., Itano et al., 1975; Valenzuela et al., 1977, 1981;
Goldberg et al., 1979; Jain & Hochstein, 1979; Jonen et al., 1982;
Hill, 1985; Marks, 1985; Di Cola et al., 1988, 1989; Maples et al.,
1988).
There is little information available on tissue distribution.
There is only one study available that investigates the
metabolism and excretion of phenylhydrazine, following oral dosing in
rabbits (McIsaac et al., 1958). This study shows that phenylhydrazine
is extensively metabolized following oral administration, although the
complete metabolic pathway has not been characterized. The main
reactions identified in this study were hydroxylation of the aromatic
ring to p-hydroxyphenylhydrazine, followed by conjugation, probably
with glucuronic acid, and production of phenylhydrazones, by reaction
with natural keto acids.
This study also indicated that the major route of excretion is
via the urine. A significant proportion of a single dose was excreted
relatively slowly; 50% of the dose was excreted within 4 days of
dosing. There are insufficient data to determine whether there is any
accumulation of phenylhydrazine in body tissues on repeated exposure.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
Many of the studies reported for phenylhydrazine have been
conducted using phenylhydrazine hydrochloride. This salt is a weak,
complex-forming compound, and either the salt or the free base will
form depending on the physiological medium, regardless of the form in
which the phenylhydrazine is administered (NIOSH, 1978). The
toxicological properties of the salt can therefore be considered to be
at least equivalent to those of free phenylhydrazine. Differences in
toxicity may arise when properties such as pH or solubility contribute
to the expression of toxicity. All dose values quoted throughout this
document refer to free phenylhydrazine.
8.1 Single exposure
In relation to inhalation exposure, there is only one very poorly
reported study, which reports LC50 values for an unstated exposure
period of 2745 mg/m3 (610 ppm) in the rat and 2093 mg/m3 (465 ppm)
in the mouse (Pham, 1979). However, it is expected that the marked
toxicity seen following oral and dermal exposure would also be
expressed following inhalation. Phenylhydrazine is toxic by oral
administration, and oral LD50 values in the range 80-188 mg/kg body
weight have been reported for the rat, mouse, guinea-pig, and rabbit
(Ekshtat, 1965; Pham, 1979). Clinical signs reported were motor
excitation and tonic/clonic spasms. In rabbits, dermal exposure to 380
mg phenylhydrazine/kg body weight for 24 h resulted in 20-30%
mortality, although no deaths occurred in rats at this dose
(Derelanko et al., 1987). The toxic effects are characterized by
destruction of red blood cells, causing a reduction in erythrocyte
count, increased reticulocyte count, methaemoglobin formation, and the
formation of Heinz bodies, and a cyanotic external appearance may
develop. Enlargement and dark coloration of the spleen are also
reported, effects that are considered to be secondary to the
erythrocyte damage.
8.2 Irritation and sensitization
In the single-dose dermal toxicity study in rabbits and rats
reported in the previous section, phenylhydrazine hydrochloride was
applied to the skin as a solid moistened with distilled water, under
either an occlusive or semi-occlusive dressing, for 24 h (Derelanko et
al., 1987). Skin irritation was seen in all rabbits, with some
necrosis at the treated site at 24 h post-application and sloughing of
the skin reported in some animals. Skin irritation, which appeared
within 24 h and persisted for up to 7 days post-exposure, was seen in
a high proportion of rats. Necrosis developed in a small number of
rats.
The quality of skin irritation data from other studies is
limited; overall, however, the results support the conclusion reached
by Derelanko et al. (1987) - that phenylhydrazine should be considered
a skin irritant (Jadassohn, 1930; von Oettingen & Deichmann-Gruebler,
1936; Roudabush et al., 1965; Schuckmann, 1969; Derelanko et al.,
1987). Further details of these studies can be obtained in the source
document (Brooke et al., 1997).
The only available information on the eye irritation potential of
phenylhydrazine in animals comes from a poorly described study in
which application of a 50% solution of phenylhydrazine to the eyes of
rabbits was reported to cause severe suppurative conjunctivitis (Pham,
1979).
There are no good-quality studies available in animals that
investigate the skin sensitization potential of phenylhydrazine. Only
one poorly described study in guinea-pigs is available (Jadassohn,
1930). When a 10% solution of phenylhydrazine in alcohol was painted
on a skin site that had been pretreated 2-3 weeks previously with
undiluted phenylhydrazine, very intense erythema and swelling,
followed by scaling and encrustation, were consistently seen. This
response to 10% phenylhydrazine was more severe than that described in
animals that had not been pretreated.
No information is available in relation to respiratory tract
sensitization.
8.3 Short-term exposure
There are only very limited, poor-quality data available in
relation to short-term repeated-dose toxicity. The effects seen are
similar to those seen following single exposure - in particular,
destruction of circulating red blood cells. Toxicity to the spleen,
liver, and kidney has also been observed in animal studies, possibly
secondary to haemolysis.
Kelly et al. (1969) administered phenylhydrazine hydrochloride to
21 mice by oral gavage once weekly for 8 weeks, at an estimated dose
of 85 mg/kg body weight per week. There were 10 saline-treated
controls. The study report described only tumour-related findings.
There was 30% mortality in treated mice compared with none in
controls.
Haematological changes were reported in four dogs administered 60
mg phenylhydrazine/kg body weight, either as a single dose or as 2, 3,
or 10 equal doses on consecutive days (Giffin & Allen, 1928). There
was a marked reduction in erythrocyte count that was comparable in
magnitude in all dogs at the end of 10 days, but that occurred at a
faster rate after a single high dose compared with repeated lower
doses.
In another dog study, 60 mg phenylhydrazine/kg body weight was
administered daily to three dogs for 5 days (Allen & Giffin, 1928).
One animal was moribund at sacrifice on the fifth day and one animal
died on the fifth day, although it is not specified that death was
treatment related. Full necropsy was performed on only one animal, in
which it was found that the blood was brown and did not coagulate
readily. Several organs, including the liver and kidneys, were darkly
coloured, and several organs contained capillaries engorged with
blood. Blood pigment and partially destroyed erythrocytes were found
in the spleen. Hepatic cell atrophy was noted, and there was an
increase in the iron content of the liver. Similar liver effects were
seen in the two other dogs.
Bolton (1935) briefly reported the effect of repeated oral
administration of 14 mg phenylhydrazine hydrochloride/kg body weight
to one dog on 4 consecutive days. There was a reduction in erythrocyte
count and haemoglobin concentration, whereas white cell count
gradually increased. These parameters had returned towards
pretreatment values 12 days after the last dose. Pathological findings
were reported to be non-conclusive, but no details were given.
Overall, the frequency, duration, and level of exposure often
varied throughout the studies reported above, so that interpretation
of results is very difficult. In all studies, phenylhydrazine as
phenylhydrazine hydrochloride was administered either by stomach tube
or by subcutaneous injection. The authors report that similar findings
were obtained regardless of route. There were no control animals. None
of the treated animals showed clinical signs of toxicity, and there
was no excessive weight loss or gain reported.
There have been a number of studies that investigate the effect
of short-term repeated parenteral administration of phenylhydrazine
(Bodansky, 1923; von Oettingen & Deichmann-Gruebler, 1936; Säterborg,
1974; Ades & Cascarano, 1979; Jain & Hochstein, 1979; Goldstein et
al., 1980; Nishida et al., 1982; Dornfest et al., 1986). These studies
confirm the ability of phenylhydrazine to damage red blood cells but
otherwise do not provide any other information in relation to the
toxicity of phenylhydrazine administered by occupationally relevant
routes of exposure.
8.4 Long-term exposure
8.4.1 Subchronic exposuren
In a very poorly reported study from which limited conclusions
can be drawn, rats, mice, guinea-pigs, and rabbits were exposed to
phenylhydrazine vapour at 0, 0.1, 15.8, 22.5, or 225 mg/m3 (0, 0.03,
3.5, 5, or 50 ppm) (Pham, 1979). Group sizes, duration of exposure,
and exposure regime were not given, although it can be inferred that
some animals were exposed for at least 6 months. Deaths were reported
to occur in animals exposed to 225 mg phenylhydrazine/m3 (species not
specified). Severe weight loss and unspecified haematological changes
and changes in central nervous system function were reported to
precede death, and there was evidence of haemolysis and dystrophic
changes in the liver, spleen, and cerebrum. Animals exposed to 15.8
and 22.5 mg/m3 were reported to have a reduction in erythrocyte count
and haemoglobin concentration, an increase in reticulocytes, and
methaemoglobinaemia; these changes were reversible at 15.8 mg/m3.
Haemolysis and dystrophic changes in the liver and other unspecified
organs were also reported for animals exposed to 22.5 mg/m3. No
further information is available on pathological changes at 0.1
mg/m3. It is not clear if there were lung effects at any of the
exposure concentrations.
It is not possible to draw firm conclusions from a poorly
reported study in three dogs in which the effect of phenylhydrazine
administration on renal and hepatic function and on erythropoiesis was
investigated (Allen & Giffin, 1928). Three dogs were administered
phenylhydrazine in 146 daily doses over a period of 8 months, to give
a total dose of 950 mg/kg body weight; the dosing regimen included a
period of about 60 days of uninterrupted administration of a single
dose level or of two dose levels (6-12 mg/kg body weight per day).
Again, the route of administration was unclear. Kidney function and
hepatic function were unaffected by treatment. Erythrocyte count was
reduced by treatment but recovered after cessation of treatment, at a
rate that was unrelated to the duration of exposure, thus indicating
that the administered dose had no effect on erythropoietic function.
Pathological examination was conducted on two of these dogs at 12 or
13 months. There was evidence of spleen toxicity, liver congestion,
and kidney damage.
In a very briefly reported study, phenylhydrazine was
administered to 25 female Swiss mice by oral gavage, 5 days/week for
40 weeks, at an estimated daily dose of 17-33 mg/kg body weight
(Roe et al., 1967). There were 85 untreated controls. Marked anaemia
necessitated a reduction in the dose during the sixth week of
treatment. No other toxic effects were observed.
8.4.2 Chronic exposure and carcinogenicity
Phenylhydrazine hydrochloride was administered daily by stomach
tube for 42 weeks to 30 BALB/c mice, at an estimated dose level of 25
mg phenylhydrazine/kg body weight (Clayson et al., 1966). Thirty
control animals were included in the study, but a control animal was
killed whenever a treated animal died, to match survival rates. There
was a statistically significant increase in the incidence of animals
with lung tumours in the treated group (53%) compared with controls
(13%). There was also a slight increase in the average number of
tumours per mouse, and the majority of treated mice had multiple
pulmonary tumours. Adenomas accounted for 83% of pulmonary tumours in
the treated group, half of which were judged to be becoming malignant,
and 17% of tumours were carcinomas.
Phenylhydrazine hydrochloride was administered in drinking-water
to 100 Swiss mice for their lifetime, at an estimated daily dose of 22
mg/kg body weight (Toth & Shimizu, 1976). There were 200 control mice.
Complete necropsy was performed on all animals. All organs were
examined macroscopically, and histological analysis was performed on a
wide range of tissues as well as on any organ showing gross pathology.
Phenylhydrazine was reported to decrease survival in comparison with
controls, and many of the treated decedents showed splenomegaly,
although numbers were not given. There was a statistically significant
increased incidence of blood vessel tumours (mainly angiosarcomas and
angiomas) in the liver of treated animals (21%) compared with
controls (0%).
8.5 Genotoxicity and related end-points
Phenylhydrazine and phenylhydrazine hydrochloride have been
investigated in a number of Ames tests, in a variety of strains, and
in the presence and absence of exogenous metabolic activation using up
to 1000 µg phenylhydrazine or phenylhydrazine hydrochloride per plate
(Shimizu et al., 1978; Tosk et al., 1979; De Flora, 1981; Parodi et
al., 1981; Levin et al., 1982; Malca-Mor & Stark, 1982; Rogan et al.,
1982; De Flora et al., 1984a,b; Wilcox et al., 1990; Muller et al.,
1993). The quality of these studies is generally high, and the studies
were apparently conducted according to standard methodology, although
detailed reporting of the results is not always available.
There is some variability in the findings, although positive
results have been obtained in Salmonella typhimurium strains TA97,
TA100, TA102, TA1537, and TA1538 in the absence of exogenous metabolic
activation. In addition, positive results were obtained in the
presence of metabolic activation in TA98 and TA1535. Some
investigators have reported that the mutagenic action is slightly
decreased by the presence of exogenous metabolic activation (Parodi et
al., 1981; Malca-Mor & Stark, 1982; De Flora et al., 1984a,b).
However, one study reports an increase in mutagenic activity in the
presence of metabolic activation (Rogan et al., 1982).
Phenylhydrazine has also given positive results in a number of
other, less well validated bacterial assays (using S. typhimurium
strains such as TA2638, TP138, BA9, and BA13), in the presence and
absence of exogenous metabolic activation (De Flora et al., 1984b;
Ulitzur et al., 1984; Ruiz-Rubio et al., 1985; Levi et al., 1986;
Muller et al., 1993).
Phenylhydrazine has not been tested in an in vitro chromosomal
aberration assay. In a brief abstract of a mammalian cell gene
mutation assay in V79 cells, with and without metabolic activation, a
positive result was reported for phenylhydrazine (Kuszynski et al.,
1981). However, no firm conclusions can be drawn from this report
because of deficiencies in the reporting.
In an unscheduled DNA synthesis assay in rat and mouse primary
hepatocytes, concentrations of 0.0144-144 mg phenylhydrazine
hydrochloride/litre were assessed (Mori et al., 1988). Although
toxicity was measured, no details were given, and quantitative data
were not reported. A positive result was obtained in both cell types,
although the effect was small.
Phenylhydrazine was tested in a micronucleus assay in vitro
using primary mouse bone marrow cells (Suzuki, 1985). Bone marrow
cells from the femur were exposed to 1-50 µg phenylhydrazine/ml for 30
min, in the presence and absence of metabolic activation. A total of
1500 polychromatic erythrocytes (PCEs) per concentration was scored
for the presence of micronuclei. There was no measure of cytotoxicity.
The percentage of micronucleated PCEs was statistically
significantly increased, in the presence of S9 only, at
phenylhydrazine concentrations of 5 µg/ml and greater in a
concentration-related manner.
BALB/c mice were administered a single intraperitoneal injection
of phenylhydrazine, and the incidence of micronucleated PCEs in the
bone marrow was measured at 24 and 48 h (Suzuki, 1985).
Phenylhydrazine was reported to be positive in this test, but no
details of the results or of the test were given. In view of the poor
reporting, no firm conclusions can be drawn from this study.
Groups of 11-12 female BALB/c mice were given a single
intraperitoneal injection of 50 mg phenylhydrazine/kg body weight in
saline (Steinheider et al., 1985). Blood smears of tail vein blood
were prepared at 24-h intervals for 7 or 11 days, and reticulocytes
and micronuclei in normochromatic erythrocytes (NCEs) and PCEs were
counted. It is not stated how many cells were counted per mouse. There
was no reporting of toxicity.
Phenylhydrazine caused a statistically significant increase in
the reticulocyte count on days 2-4 post-injection and in PCEs on day
3. There was a statistically significant increase in the incidence of
micronucleated PCEs at 24 h post-injection (from 1 to 4.7 per 1000)
and in micronucleated NCEs at 48 h post-injection (from 0.7 to 2.3 per
1000).
However, similar increases in micronucleated NCEs were also seen
following bleeding of the animals and splenectomy. The authors suggest
that the increase in micronuclei seen following phenylhydrazine
treatment was due at least partly to stimulation of erythropoiesis
because of the haemolysis induced by phenylhydrazine, thus leading to
more errors of nuclear expulsion; hence, the results do not
necessarily indicate a direct genotoxic action of phenylhydrazine.
Groups of 7-12 mice were given a single intraperitoneal injection
of either 85 or 170 mg phenylhydrazine/kg body weight and killed 1 and
6 h, respectively, after treatment (Parodi et al., 1981). In addition,
six mice were given a series of five daily intraperitoneal injections
of 7.6 mg phenylhydrazine/kg body weight and sacrificed 6 h after the
last injection. Control animals were injected with saline only. DNA
damage was assessed by measurement of the alkaline elution rate of
single-strand DNA from liver and lung tissue extracts.
A statistically significant change in the elution rate of liver
and lung DNA was seen in all groups of treated animals compared with
controls, except in the case of lung tissue DNA from mice given a
single dose of 85 mg phenylhydrazine/kg body weight. Phenylhydrazine
is considered to give a positive result in this assay for DNA damage.
The formation of DNA adducts ( N7-methylguanine and a trace of
O6-methylguanine) in the liver was demonstrated in rats receiving
65 mg phenylhydrazine/kg body weight by oral gavage (Mathison et al.,
1994). Other tissues were not examined.
8.6 Reproductive and developmental toxicity
Each of three dogs (one dog per dose group) received 20, 30, or
40 mg phenylhydrazine/kg body weight in saline by subcutaneous
injection on 2 consecutive days (Witchett, 1975). Two control animals
were not injected. At necropsy, performed on all three animals within
a few days of dosing, a "striking" reduction in spermatogenesis was
reported, with an absence of sperm in the epididymis. The validity of
this result is not clear, given the apparent extreme rapidity of the
effect.
Groups of 8-12 pregnant Wistar rats were given an intraperitoneal
injection of 7.5 mg phenylhydrazine/kg body weight as phenylhydrazine
hydrochloride on days 17, 18, and 19 of gestation; or 15 mg
phenylhydrazine/kg body weight as phenylhydrazine hydrochloride on
days 18 and 19 of gestation (Tamaki et al., 1974). Control animals
were not treated. There was no reporting of maternal toxicity or of
the effect of treatment on gestation or pup viability. Toxicity of the
pups was reported only insofar as there was incidence of jaundice
and/or anaemia among the offspring of treated animals. Twelve male
offspring with severe jaundice and anaemia, selected from treated
dams, and nine males from control dams were assessed at 9-22 weeks of
age for functional and behavioural status.
Although the authors reported that experimental animals showed
statistically significant differences from controls in some tests,
these findings are not considered to be reliable because of the small
numbers of animals used and the exclusion of a control animal from the
analysis. In addition, it is noted that only a brief part of the
gestation period was covered by the treatment regime (days 17-19); no
explanation is available for this choice of dosing regime.
Yamamura et al. (1973) reported in a brief abstract that
intraperitoneal injection of pregnant rats with 15 mg
phenylhydrazine/kg body weight as phenylhydrazine hydrochloride on
days 18 and 19 of gestation produced hyperbilirubinaemia (resulting
from haemolysis) in fetuses and newborns.
8.7 Immunological and neurological effects
No studies are available that specifically investigate the
immunological and neurological end-points, and there is no relevant
information from toxicity studies in animals.
9. EFFECTS ON HUMANS
In humans, no information is available in relation to single
exposure via the inhalation or oral routes, although effects similar
to those seen following dermal exposure would be expected to occur.
Systemic toxicity developed in humans after dermal exposure to liquid
phenylhydrazine, despite immediate attempts to reduce exposure by
removal of contaminated clothing and washing of skin (Schuckmann,
1969). Toxicity was manifest by damage to red blood cells, in one case
resulting in haemolytic jaundice. No such systemic effects were
reported in two cases of skin contamination with solid phenylhydrazine
hydrochloride.
In relation to skin irritation, information is available from
worker exposure data. There was no reporting of irritancy effects in
workers exposed to liquid phenylhydrazine following accidental
exposure, although systemic effects were seen (Schuckmann, 1969). Skin
irritation following contact with phenylhydrazine hydrochloride powder
was reported in two workers following accidental exposure. Local
irritation, superficial erythema, and partly bullous-papular changes
were noted in one case following spillage of powder on arms; multiple
burn marks and small blisters at the site of contact were reported in
the second case in which phenylhydrazine hydrochloride had spilled
into the worker's gloves and shoes. The author also refers to medical
records at the works that describe a number of cases of skin
irritation of differing severity due to phenylhydrazine hydrochloride,
but no details are given.
There are no data available on the eye irritation potential of
phenylhydrazine in humans.
There are a number of case reports of skin hypersensitivity
reactions to phenylhydrazine and its hydrochloride salt in humans.
Solomons (1946) conducted a patch test in one subject with a
phenylhydrazine crystal placed on the forearm under a dressing for an
unstated exposure period. Marked erythema and some oedema developed on
the exposure site after 18 h, with the formation of vesicles after 30
h and crusting after a further 24 h.
Similar hypersensitive skin reactions were reported following
individual exposures to solid or aqueous solutions of phenylhydrazine
or phenylhydrazine salts (Wright & Joyner, 1930; Frost & Hjorth, 1959;
Pevny & Peter, 1983).
There is also evidence that cross-sensitization can occur between
hydrazine compounds, so that subjects already sensitized to hydrazine,
a known skin sensitizer, are also sensitized to hydrazine derivatives,
including phenylhydrazine (Malten, 1962; Van Ketel, 1964; Hovding,
1967; Rothe, 1988).
No data are available on the potential of phenylhydrazine to
cause respiratory tract sensitization.
Earlier this century, phenylhydrazine and phenylhydrazine
hydrochloride were administered orally (usually around 100-200 mg/day)
for the treatment of blood disorders (e.g., Giffin & Allen, 1933). In
some cases, treatment was effective; in others, however, the outcome
was fatal (e.g., Giffin & Conner, 1929). The effects seen (beneficial
or otherwise) may have been related to the disease process and cannot
be attributed entirely to phenylhydrazine.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1 Aquatic environment
Results of acute toxicity tests on aquatic organisms are
summarized in Table 2. All concentrations are nominal.
In the early life stage test summarized in Table 2 (Xiu et al.,
1992), newly fertilized eggs of the zebra fish were exposed to
concentrations of phenylhydrazine through hatch and into the larval
stage (total exposure time was 16 days). A NOEC (survival) was
established for eggs at 5 days post-fertilization at 0.0039 mg/litre,
with a lowest-observed-effect concentration (LOEC) at 0.0078 mg/litre.
At the end of the test (16 days), the NOEC for larvae was 0.000 49
mg/litre, and the LOEC was 0.000 98 mg/litre. The study was conducted
according to a Swedish standard protocol (ss 028193).
In a study on the goldfish ( Carassius auratus), 40% of fish
died when exposed to phenylhydrazine at a nominal concentration of 1
mg/litre for 48 h. Signs of toxicity included erratic swimming,
sinking to the bottom of the test tank, and slowed and erratic
respiration. No gross lesions were found following dissection,
although all viscera showed focal haemorrhaging (Houston et al.,
1988).
Effects on blood cells and the haematopoietic system, comparable
to those found in mammals, were seen in fish injected with
phenylhydrazine. Chinook salmon ( Oncorhynchus tshawytscha) juveniles
were injected with 12.5 mg phenylhydrazine/kg body weight. Red cell
count, haemoglobin, and haematocrit fell to 1-5% of their normal
values within 10 days of treatment; a slight improvement was reported
15 days following injection, and values had returned to normal 95 days
after treatment (Smith et al., 1971).
10.2 Terrestrial environment
Phenylhydrazine at 21.6 mg/litre in a nutrient culture medium had
no effect on the growth of various soil fungi (Zsolnai, 1975).
A phenylhydrazine concentration of 50 mg/litre in a hydroponic
culture solution inhibited germination of Hordeum seeds for at least
6 days, whereas growth was stimulated in Lepidium. At 100 mg/litre,
seedling growth was stimulated in Hordeum but not in Lepidium. At
500 mg/litre, phenylhydrazine inhibited growth in seedlings of both
species, although the seedlings were still apparently "healthy"
(Bokorny, 1933).
Exposure of soil nematodes Caenorhabditis briggsae in culture
to phenylhydrazine at 50 mg/litre of medium resulted in reduced growth
of all four larval stages (the effect was most marked on the last
larval instar) and therefore delayed development to adults. However,
the adults formed were capable of reproduction, although the number of
progeny was reduced. A concentration of 15 mg/litre also delayed
development, although to a lesser degree compared with the higher
concentration (Kampfe et al., 1986a,b). A 6-day EC50 of 12 mg/litre
was reported for production of progeny in culture for the same
nematode (Kreil, 1982).
No dietary or oral toxicity studies have been performed on birds.
However, injection studies have shown haemolytic anaemia and reduced
white cell counts in birds. In contrast to mammals, cell division in
erythropoietic tissue is unaffected by phenylhydrazine (Williams,
1972; Clark et al., 1988; Datta et al., 1989, 1990).
Table 2: Acute toxicity of phenylhydrazine to aquatic organisms.
Organism End-point Concentration Reference
(mg/litre)
Bacteria
Photobacterium phosphoreum 30-min EC50 66.9 Kaiser et al.
(luminescence) (1987)
Facultative anaerobes 24-h toxic 60 Hoechst (1980)
(mixed culture) threshold
Escherichia coli minimum 109.3 Romero & Canada
inhibitory (1991)
concentration
Escherichia coli, minimum >3000 Zemek et al.
Micrococcus luteus, inhibitory (1978)
Bacillus licheniformis concentration
Invertebrates
water flea LC50 2-5 Hoechst (1980)
(Daphnia magna) (immobilization)
Fish
zebra fish 96-h LC50 0.16-0.25 Hoechst (1982)
(Brachydanio rerio) 96-h NOEC 0.1
zebra fish 5-day NOEC (eggs) 0.0039 Xiu et al.
(Brachydanio rerio) 16-day NOEC (larvae) 0.000 49 (1992)
Japanese killifish 48-h LC50 15.7 Tonogai et al.
(Oryzias latipes) (1982)
Table 2 (cont'd)
Organism End-point Concentration Reference
(mg/litre)
common carp 24-h LC100 1.0 Menzie (1979)a
(Cyprinus carpio) 96-h NOEC 0.1
bluegill 48-h LC50 0.1 Menzie (1979)a
(Lepomis 96-h NOEC 0.01
macrochirus)
a Menzie C (1979) Value taken from the DIMDI/ECDIN database. Test performed by the
United States Fish and Wildlife Service, Bureau of Sports, Fisheries and Wildlife,
Department of the Interior, Washington, DC [cited in BUA, 1995].
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Phenylhydrazine is toxic by single exposure via the oral route
(LD50 80-188 mg/kg body weight) and is expected to be toxic by the
inhalation and dermal routes (data from these routes of exposure are
less clear). Phenylhydrazine solution was severely irritating to
rabbit eyes; hence, it is reasonable to predict that it would have
significant eye irritation potential in humans.
Phenylhydrazine also has skin irritation potential, and there is
evidence from human case reports that it has skin sensitizing
properties. Exposure to phenylhydrazine may cause damage to red blood
cells, potentially resulting in anaemia and consequential secondary
involvement of other tissues, such as the spleen and liver. The dose
(exposure)-response characteristics for the induction of damage to the
red blood cells are poorly defined, and a no-effect level has not been
identified. Where phenylhydrazine has been used therapeutically in
humans, via the oral route, for the treatment of blood disorders,
daily doses of the order of 1.5-4 mg/kg body weight per day have
caused a reduction in the numbers of red blood cells; given the health
status of the individuals concerned, however, these data are of
limited use. Phenylhydrazine is mutagenic in vitro, and, although not
conclusive, there is some evidence to indicate that it may express
genotoxic activity in vivo. The substance is clearly carcinogenic in
mice following oral dosing, inducing tumours of the vascular system.
The mechanism for tumour formation is unclear, and, given the
genotoxic profile of phenylhydrazine, a genotoxic component cannot be
excluded. Carcinogenic potential in humans cannot be excluded given
the profile of genotoxicity and animal carcinogenicity, particularly
as other expressions of phenylhydrazine toxicity are common to a
number of species, including humans.
No conclusions can be drawn from the available information on
fertility or development.
11.1.2 Criteria for setting guidance values fon phenylhydrazine
There are no adequate data available regarding reproductive or
developmental effects; hence, it is not possible to evaluate the risk
to human health for these end-points.
The use pattern and physical/chemical characteristics of the
compound suggest that exposure of the general population would be
negligible.
Using United Kingdom workplace conditions as an example (section
6.2), exposure to phenylhydrazine vapour during most occupational
processes would result in a body burden of up to 0.33 mg/kg body
weight per day, assuming a 70-kg worker breathes 10 m3 of air in a
working day and that 100% phenylhydrazine is absorbed. There are no
data available from which to estimate the contribution to body burden
from dermal uptake, although this is expected to be negligible. A
threshold for the induction of red blood cell damage probably exists
but has not been identified, although daily oral doses calculated at
about 1.5 mg/kg body weight per day and above are associated with such
effects. Overall, at these levels of predicted inhalation exposure,
the risk of developing damage to the red blood cells is considered to
be low; if the levels were exceeded (e.g., of the order of a few ppm,
approximately 15-20 mg/m3), however, then this would be cause for
some concern.
On the basis that the carcinogenicity of phenylhydrazine may
involve a genotoxic mechanism, it is not possible to reliably identify
a threshold below which occupational exposure to phenylhydrazine would
not result in some risk to human health.
11.1.3 Sample risk characterization
The scenario chosen as an example is occupational exposure in the
United Kingdom.
The main health concerns associated with exposure to
phenylhydrazine are damage to the red blood cells, deleterious effects
on genetic material, and the development of cancer.
It is recognized that there are a number of different approaches
to assessing the risks to human health for genotoxic and carcinogenic
substances and in the subsequent risk management steps that may be
taken. In addition, although not used in the United Kingdom, there are
models for characterizing potency that may be of some benefit in
priority-setting schemes. In the United Kingdom occupational setting,
a Maximum Exposure Limit or MEL (which is not a health-based standard)
has been proposed at 0.9 mg/m3 (0.2 ppm), 8-h time-weighted average.
The numerical value for the MEL was based on a level of control that
was deemed (by tripartite agreement) to be reasonably practicable
under United Kingdom workplace conditions, and in the United Kingdom
there is a continuing requirement to reduce exposure levels as far as
reasonably practicable with the technology that is currently
available.
Phenylhydrazine also possesses skin and eye irritant properties
and possibly skin sensitizing potential. The information available
indicates that local exposure of these tissues is unlikely; if it did
occur, however, then there would be risk of irritation to the eyes and
the development of irritant and/or allergic dermatitis.
11.2 Evaluation of environmental effects
No atmospheric effects are expected given the release of
phenylhydrazine predominantly to water, its extremely low
volatilization from water to the atmosphere, and its rapid calculated
atmospheric half-life following reaction with hydroxyl radicals.
Few toxicity studies are available for terrestrial organisms, and
little emission to land is expected; on this basis, no quantitative
risk assessment can be attempted for the terrestrial environment.
Phenylhydrazine is degraded photochemically and autoxidizes in
water. It is readily biodegradable, and this is expected to be the
major route of breakdown in the environment. There is minimal sorption
to particulates.
Phenylhydrazine is toxic to aquatic organisms, with the lowest
reported NOEC in acute fish tests at 0.01 mg/litre; fish are generally
more sensitive than either daphnids or bacteria. A NOEC for
embryo-larval stages following 16 days of exposure from fertilization
has been reported at 0.000 49 mg/litre for the zebra fish.
There are no reported measurements of phenylhydrazine in
environmental media. Monitoring studies of both inflow and outflow to
the wastewater treatment plant of the Hoechst Hochst production plant
in Germany showed no detectable phenylhydrazine (detection limit 500
µg/litre) in weekly samples. Maximum emission to wastewater was
estimated at 13 t/year, and this will be used as a worst-case example.
Based on this emission rate, and using mainly default values from
the OECD Technical Guidance Manual, the initial predicted
environmental concentration of phenylhydrazine in river water
(PEClocal (water), in g/litre) would be as follows:
Ceffluent
PEClocal (water) =
(1 + Kp(susp) × Csusp) × D
where:
* Ceffluent is the concentration of phenylhydrazine in the
wastewater treatment plant effluent (g/litre), calculated as
Ceffluent = W × (100 - P)/(100 × Q), where:
W = emission rate (35.6 kg/day)
P = percent removal in the wastewater treatment plant
(based on the "ready biodegradability" of the
compound, 91%)
Q = volume of wastewater in m3/day (default 200
litre/day per capita for a population of 10 000
inhabitants; wastewater volume for the production
plant is unknown)
* Kp(susp) is the suspended matter/water adsorption coefficient,
calculated as Kp(susp) = Koc × foc(susp), where:
Koc = organic carbon/water partition
coefficient (7.3)
foc(susp) = fraction of organic carbon in suspended matter
(default 0.1)
* Csusp is the concentration of suspended matter in the river
water (in kg/litre; default 15 mg/litre)
* D is the dilution factor for river flow (flow rate for the
River Main averages 188 m3/s compared with the estimated flow
rate of the wastewater at 0.02 m3/s; dilution factor
approximately 10 000)
Under these very conservative conditions, PEClocal (water)
= 0.16 µg/litre.
Of the reported acute toxicity test results for organisms in the
environment, those for the majority of fish tested are substantially
lower than those for other organisms tested. The predicted no-effect
concentration (PNEC) will therefore be based on the fish results.
No long-term test results are available. Applying an uncertainty
factor of 1000 to the lowest reported standard acute LC50 value of
0.1 mg/litre for the bluegill ( Lepomis macrochirus) would give a
PNEC of 0.1 µg/litre. This is a factor of 100 lower than the lowest
reported NOEC for the same species. Alternatively, applying an
uncertainty factor of 10 to the NOEC for the early life stage test on
the zebra fish larvae gives a PNEC of 0.049 µg/litre. The more
conservative value will be used in estimating risk.
The low PEC of 0.16 µg/litre would not have been detected in the
monitoring at the site. Assuming that this value is the worst case, a
PEC/PNEC ratio of 3.2 is generated. This indicates that the risk to
aquatic organisms is low, based on very conservative assumptions. The
distribution of reported toxicity test results against the worst-case
PEC is plotted in Figure 1, illustrating the safety margin.
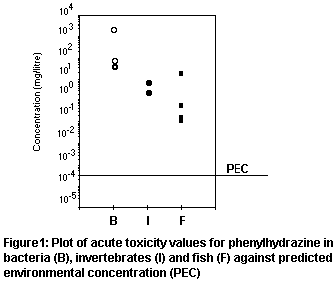 12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Previous evaluations by other international bodies were not
identified. Information on international hazard classification and
labelling is included in the International Chemical Safety Card (ICSC
0938) reproduced in this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventive and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0938) reproduced in this
document.
13.1 Human health hazards
Phenylhydrazine induces damage to red blood cells. Repeated or
prolonged contact with the substance causes skin sensitization, and
there is cause for concern for carcinogenicity.
13.2 Advice to physicians
Phenylhydrazine is a haemolytic agent. There is no specific
antidote, but treatment should be supportive.
13.3 Health surveillance advice
Periodic medical examination of the area of the skin exposed to
phenylhydrazine and annual blood count should be included in the
health surveillance programme.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards
may be obtained from UNEP Chemicals (IRPTC), Geneva.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
PHENYLHYDRAZINE ICSC: 0938
November 1998
CAS # 100-63-0 Hydrazinobenzene
RTECS # MV8925000 Monophenylhydrazine
UN # 2572 C6H8N2/C6H5NHNH2
Molecular mass: 108.1
TYPES OF HAZARD ACUTE HAZARDS/ PREVENTION FIRST AID/
SYMPTOMS FIRE FIGHTING
FIRE Combustible. Gives off NO open flames. Water spray, alcohol-resistant
irritating or toxic foam, powder, carbon dioxide.
fumes (or gases) in
a fire.
EXPLOSION Above 88°C explosive Above 88°C use In case of fire: keep drums, etc.,
vapour/air mixtures a closed system, cool by spraying with water.
may be formed. ventilation.
EXPOSURE STRICT HYGIENE!
Inhalation Cough. Laboured Local exhaust or Fresh air, rest. Refer for
breathing. Sore breathing medical attention.
throat. Cyanosis. protection.
Skin MAY BE ABSORBED! Protective gloves. Remove contaminated clothes.
Dry skin. Redness. Protective clothing. Rinse skin with plenty of water
Pain. or shower. Refer for medical
attention.
Eyes Redness. Pain. Blurred Face shield, or eye First rinse with plenty of
vision protection in water for several minutes
combination with (remove contact lenses if easily
breathing possible), then take to a
protection. doctor.
Ingestion Abdominal pain. Diarrhoea. Do not eat, drink or Rest. Refer for medical
Nausea. Vomiting. Weakness. smoke during work attention.
Vertigo.
SPILLAGE DISPOSAL PACKAGING & LABELLING
If the substance is melted: collect leaking and Airtight. Do not transport with food and feedstuffs.
spilled liquid in sealable containers as far as EU Classification:
possible. Absorb remaining liquid in sand or inert Symbol: T, N
absorbent and remove to safe place. Do NOT wash R: 23/24/25-36-50
away into sewer. If the substance is solid: sweep S: (1/2-)28-45-61
spilled substance into container, carefully collect UN Classification
remainder, then remove to a safe place. Do NOT let UN Hazard Class: 6.1
this chemical enter the environment. (Extra personal UN Pack Group: II
protection: complete protective clothing including
self-contained breathing apparatus).
EMERGENCY RESPONSE STORAGE
Transport Emergency Card: TEC (R)-61/G61b Separated from strong oxidants, food and
NFPA Code: H3; F2; R0; feedstuffs. Cool. Keep in the dark.
IMPORTANT DATA
PHYSICAL STATE; APPEARANCE: ROUTES OF EXPOSURE:
COLOURLESS TO YELLOW OILY LIQUID OR CRYSTALS. The substance can be absorbed into the
TURNS BROWN RED ON EXPOSURE TO AIR AND LIGHT. body by inhalation of its aerosol, through
the skin, by ingestion.
CHEMICAL DANGERS: INHALATION RISK:
The substance decomposes on heating and on A harmful contamination of the air can be reached
burning producing toxic fumes including nitrogen rather quickly on evaporation of this substance at 20°C.
oxides. Reacts with oxidants. Reacts violently
with lead dioxide.
OCCUPATIONAL EXPOSURE LIMITS: EFFECTS OF SHORT-TERM EXPOSURE:
TLV: 0.1 ppm; 0.44 mg/m3 A3 (skin)(ACGIH 1998). The substance irritates the eyes, the skin, the
respiratory tract. The substance may cause effects on
the blood, resulting in hemolysis, kidney impairment,
liver impairment. The effects may be delayed. Medical
observation is indicated.
EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
Repeated or prolonged contact with skin may cause
dermatitis. Repeated or prolonged contact may cause
skin sensitization. The substance may have effects
on the blood, resulting in anaemia.
PHYSICAL PROPERTIES
Boiling point (decomposes): 243.5°C Octanol/water partition coefficient as log Pow: 1.25
Melting point: 19.5°C
Relative density (water = 1): 1.09
Solubility in water: poor
Vapour pressure, Pa at 71.8°C: 133
Relative vapour density
(air = 1): 3.7
Flash point: 88°C c.c.
Auto-ignition temperature: 174°C
ENVIRONMENTAL DATA
The substance is toxic to aquatic organisms.
NOTES
The symptoms of hemolysis do not become manifest until hours have passed.
ADDITIONAL INFORMATION
LEGAL NOTICE Neither the CEC nor the IPCS nor any person acting on behalf of the CEC
or the IPCS is responsible for the use which might be made of this
information.
(c) IPCS, CEC 1999
REFERENCES
Ades I, Cascarano J (1979) Mitochondrial alterations in heart, kidney
and liver of rats subjected to anemic hypoxia. Experimental molecular
pathology, 30:94-109.
Allen E, Giffin H (1928) Experiments with phenylhydrazine. II. Studies
on renal and hepatic function and erythropoiesis. American journal of
medical science, 185:677-682.
BASF (1993) Abschlussbericht. Prufung der biologischen Abbaubarkeit
von Phenylhydrazin im modifizierten OECD-Screeningtest. Ludwigshafen,
BASF AG.
Besada A (1988) Analytical use of copper(II)-neocuproine in the
spectrophotometric determination of hydrazines. Analytical letters,
21:1917-1925.
Bodansky M (1923) The action of hydrazine and some of its derivatives
in producing liver injury as measured by the effect on levulose
tolerance. Journal of biological chemistry, 58:799-811.
Bokorny T (1933) Uber den Einfluss verschiedener Substanzen auf die
Keimung der Pflanzensamen. Wachstumsforderung durch einige. II
Mitteilung. Biochemische Zeitschrift, 50:49-61.
Bolton V (1935) A laboratory study of amidopyrine, barbital,
phenylhydrazine, and benzene in relation to agranulocytic angina.
Journal of laboratory and clinical medicine, 20:1199-1203.
Brooke I, Cain J, Cocker J, Groves J (1997) Phenylhydrazine.
Sudbury, Suffolk, HSE Books (Risk Assessment Document EH72/1; ISBN 0
7176 1355 0).
BUA (1995) Phenylhydrazine. Beratergremium fur Umweltrelevante
Altstoffe (BUA). GDCh Advisory Committee on Existing Chemicals of
Environmental Relevance. Stuttgart, S. Hirzel, Wissenschaftliche
Verlagsgesellschaft (Report No. 120; ISBN 3-7776-0691-X).
Clark M, Gildersleeve R, Thaxton J, Parkhurst C, McRee D (1988)
Hematological effects of ethyl methanesulfate, paraquat and
phenylhydrazine in Japanese quail. Comparative biochemistry and
physiology, 89C:15-30.
Clayson D, Biancifiori C, Milia U, Santilli F (1966) The induction of
pulmonary tumours in BALB/c/Cb/Se mice by derivatives of hydrazine.
In: Lung tumours in animals. Proceedings of the 3rd Perugia
Quadrennial International Conference on Cancer, University of Perugia,
Perugia, pp. 869-880.
Datta K, Soni J, Awadhiya R, Datta I (1989) Erythrophagocytosis in
phenylhydrazine-induced acute anaemia in chickens. Research in
veterinary science, 47:136-137.
Datta K, Soni J, Datta I (1990) Sequential morphological changes in
chicken erythrocytes after in vivo and in vitro exposure to
phenylhydrazine hydrochloride. Research in veterinary science,
48:12-17.
De Flora S (1981) Study of 106 organic and inorganic compounds in the
Salmonella/microsome test. Carcinogenesis, 2:283-298.
De Flora S, Camoirano A, Zanacchi P, Bennicelli C (1984a) Mutagenicity
testing with TA97 and TA102 of 30 DNA-damaging compounds, negative
with other Salmonella strains. Mutation research, 134:159-165.
De Flora S, Zanacchi P, Camoirano A, Bennicelli C, Badolati G (1984b)
Genotoxic activity and potency of 135 compounds in the Ames reversion
test and in a bacterial DNA-repair test. Mutation research,
133:161-198.
Derelanko M, Gad S, Gavigan F, Babich P, Rinehart W (1987) Toxicity of
hydroxylamine sulfate following dermal exposure: variability with
exposure method and species. Fundamental and applied toxicology,
8:583-594.
Di Cola D, Sacchetta P, Battista P (1988) Proteolysis in human
erythrocytes is triggered only by selected oxidative stressing agents.
Italian journal of biochemistry, 37:129-138.
Di Cola D, Battista P, Santarone S, Sacchetta P (1989) Fragmentation
of human hemoglobin by oxidative stress produced by phenylhydrazine.
Free radical research communications, 6:379-386.
Dornfest B, Lapin D, Naughton B, Adu S, Korn L, Gordon A (1986)
Phenylhydrazine-induced leukocytosis in the rat. Journal of leukaemia
biology, 39:37-48.
Ekshtat B (1965) Maximum permissible concentrations of hydrazine
hydrate and phenylhydrazine in water bodies. Hygiene and sanitation
(USSR), 30:191-197.
Frost J, Hjorth N (1959) Contact dermatitis from hydrazine
hydrochloride in soldering flux. Cross sensitization to Apresoline and
isoniazid. Acta Dermato-Venereologica, 39:82-86.
Giffin H, Allen E (1928) Experiments with phenylhydrazine. I. Studies
on the blood. Annals of internal medicine, 1:655-676.
Giffin H, Allen E (1933) The control and complete remission of
polycythemia vera following the prolonged administration of
phenylhydrazine hydrochloride. American journal of medical science,
185:1-13.
Giffin H, Conner H (1929) The untoward effects of treatment by
phenylhydrazine hydrochloride. Journal of the American Medical
Association, 92:1505-1507.
Goldberg B, Stern A, Peisach J, Blumberg W (1979) The detection of
superoxide anion from the reaction of oxyhemoglobin and
phenylhydrazine using EPR spectroscopy. Experientia, 35:488-489.
Goldstein B, Rozen M, Kunis R (1980) Role of red cell membrane lipid
peroxidation in hemolysis due to phenylhydrazine. Biochemical
pharmacology, 29:1355-1359.
Hasan T (1988) Resin bead detection and spectrophotometric
determination of phenylhydrazine using inorganic reagents. Analytical
letters, 21:633-640.
Hill H (1985) Dioxygen-derived radicals in biological systems.
Philosophical transactions of the Royal Society of London, B
311:605-615.
Hoechst (1980) Abwasserbiologische Untersuchung von Phenylhydrazin,
Unveroffentlichte Untersuchung der Abt. RWL vom 15.04.1980 und vom
03.06.1980. Frankfurt/Main, Hoechst AG.
Hoechst (1982) Phenylhydrazin-Reinbase D, Prufung der akuten
Toxizitat am Fisch Brachydanio rerio (Hamilton-Buchanan) uber 96
Stunden. Unverffentlichter Bericht Nr 691/82 der Abt. Pharma
Forschung Toxicologie. Frankfurt/Main, Hoechst AG.
Hoechst (1992) Okologische Daten zu Phenylhydrazin. Bericht uber
Ergebnisse zuruckliegender Versuche anhand der vorliegenden
Laboraufzeichungen und Angaben in der UWS. Frankfurt/Main, Hoechst
AG.
Houston A, Murad A, Gray J (1988) Induction of anemia in goldfish,
Carassius auratus L., by immersion in phenylhydrazine hydrochloride.
Canadian journal of zoology, 66:729-736.
Hovding G (1967) Occupational dermatitis from hydrazine hydrate used
in boiler protection. Acta Dermato-Venereologica, 47:293-297.
HSDB (1998) Hazardous substances data bank. Micromedex Inc. (CD-ROM
version).
IPCS (1993) International Chemical Safety Card - Phenylhydrazine.
Geneva, World Health Organization, International Programme on Chemical
Safety (ICSC 0938).
Itano H, Hirota K, Hosokawa K (1975) Mechanism of induction of
haemolytic anaemia by phenylhydrazine. Nature, 256:665-667.
Jadassohn W (1930) Sensitization of guinea-pig skin to
phenylhydrazine. Klinische Wochenschrift, 9:551.
Jain S, Hochstein P (1979) Generation of superoxide radicals by
hydrazine. Its role in phenylhydrazine-induced hemolytic anemia.
Biochimica et Biophysica Acta, 586:128-136.
Japan Environment Agency (1987) Chemicals in the environment, a
report of nationwide surveys. Environmental Health and Safety
Division, Environmental Health Department, December.
Jonen H, Werringloer J, Prough R, Estabrook R (1982) The reaction of
phenylhydrazine with microsomal cytochrome P-450. Catalysis of heme
modification. Journal of biological chemistry, 257:4404-4411.
Kaiser K, Palabrica V, Robi J (1987) QSAR of acute toxicity of
mono-substituted benzene derivatives to Photobacterium phosphoreum.
In: Kaiser KLE, ed. QSAR environmental toxicology II. Dordrecht, D.
Reidel Publishing Company, pp. 153-168.
Kampfe L, Bode K, Ullrich H-L (1986a) Zur Wirkung einiger Lathyrogene
auf Nematoden. Mededelingen van de Faculteit Landbouwwetenschappen,
Rijksuniversiteit Gent, 51(3b):1267-1277.
Kampfe L, Kreil V, Ullrich, H-L (1986b) Wirkung lathyrogener
Substanzen und des Synergisten Piperonylbutoxid (PBO) auf die
freilebended Nematoden Caenorhabditis briggsae und Rhabditis
oxycerca. Zoologische Jahrbuecher, Abteilung fuer Allgemeine
Zoologie und Physiologie der Tiere, 90:257-271.
Karickhoff S, Brown D, Scott T (1979) Sorption of hydrophobic
pollutants on natural sediments. Water research, 13:241-248.
Kelly M, O'Gara R, Yancey S, Gadekar K, Botkin C, Oliverio V (1969)
Comparative carcinogenicity of N-isopropyl-alpha-(2-methyl
hydrazino)- p-toluamide.HCl (procarbazine hydrochloride), its
degradation products, other hydrazines, and isonicotinic acid
hydrazide. Journal of the National Cancer Institute, 42:337-344.
Kreil V (1982) Prufung xenobiotischer Substazen auf ihre Wirkung
gegenuber Caenorhabditis briggsae unter exenischen
Kultivierungsbedingungen. Thesis, University of Greifswald,
Greifswald [cited in Kampfe et al., 1986b].
Kuszynski C, Langenbach R, Malick L, Tompa A, Toth B (1981) Liver
cell-mediated mutagenesis of V79 cells by hydrazine and related
compounds. Environmental mutagenesis, 3:323-324.
Levi B, Kuhn J, Ulitzur S (1986) Determination of the activity of 16
hydrazine derivatives in the bioluminescence test for genotoxic
agents. Mutation research, 173:233-237.
Levin D, Hollstein M, Christman M, Schwiers E, Ames B (1982) A new
Salmonella tester strain (TA102) with A-T base pairs at the site of
mutation detects oxidative mutagens. Proceedings of the National
Academy of Sciences of the United States of America, 79:7445-7449.
Malca-Mor L, Stark A (1982) Mutagenicity and toxicity of carcinogenic
and other hydrazine derivatives: correlation between toxic potency in
animals and toxic potency in Salmonella typhimurium TA1538. Applied
environmental microbiology, 44:801-808.
Malten K (1962) Industrial contact dermatitis caused by hydrazine
derivatives, with group hypersensitivity to hydralazine (Apresoline)
and isoniazid (INH). Nederlands Tijdschrift voor Geneeskunde,
106:2219-2222.
Maples K, Jordan S, Mason R (1988) In vivo rat hemoglobin thiyl free
radical formation following phenylhydrazine administration. Molecular
pharmacology, 33:344-350.
Marks G (1985) Exposure to toxic agents: the heme biosynthetic pathway
and hemoproteins as indicator. CRC critical reviews in toxicology,
15:151-179.
Mathison B, Murphy S, Shank R (1994) Hydralazine and other hydrazine
derivatives and the formation of DNA adducts. Toxicology and applied
pharmacology, 127:91-98.
McIsaac W, Parke D, Williams R (1958) Studies in detoxication. 77. The
metabolism of phenylhydrazine and some phenylhydrazones. Biochemical
journal, 70:688-697.
Meylan W, Howard P (1993) Computer estimation of the atmospheric
gas-phase reaction rate of organic compounds with hydroxyl radicals
and ozone. Chemosphere, 26:2293-2299.
Mori H, Sugie S, Yoshimi N, Iwata H, Nishikawa A, Matsukubo K, Shimizu
H, Hirono I (1988) Genotoxicity of a variety of hydrazine derivatives
in the hepatocyte primary culture/DNA repair test using rat and mouse
hepatocytes. Japanese journal of cancer research (Gann), 79:204-211.
Muller W, Englehart G, Herbold B, Jackh R, Jung R (1993) Evaluation of
mutagenicity testing with Salmonella typhimurium TA102 in three
different laboratories. Environmental health perspectives,
101(3):33-36.
NIOSH (1978) Criteria for a recommended standard...occupational
exposure to hydrazines. Cincinnati, OH, National Institute for
Occupational Safety and Health (DHEW (NIOSH) Publication No. 78-172).
NIOSH (1994) Method 3518, Phenylhydrazine. In: Manual of analytical
methods, 4th ed. Cincinnati, OH, National Institute for Occupational
Safety and Health.
Nishida N, Nakamura I, Kudo Y, Kagami M (1982) Effects of aromatic
nitro and amino compounds on the osmotic fragility of red cells.
Japanese journal of industrial health, 24:172-180.
Parodi S, De Flora S, Cavanna M, Pino A, Robbiano L, Bennicelli C,
Brambilla G (1981) DNA-damaging activity in vivo and bacterial
mutagenicity of sixteen hydrazine derivatives as related
quantitatively to their carcinogenicity. Cancer research,
41:1469-1482.
Pevny I, Peter G (1983) Allergic contact dermatitis to derivatives of
pyridine and hydrazine. Dermatitis, 31:78-83.
Pham K-C (1979) Toxicity of phenylhydrazine in inhalatory exposure.
Gigiena Truda i Professional'nye Zabolevaniya, 3:45-47.
Roe F, Grant G, Millican D (1967) Carcinogenicity of hydrazine and
1,1-dimethylhydrazine for mouse lung. Nature, 216:375-376.
Rogan E, Walker B, Gingell R, Nagel D, Toth B (1982) Microbial
mutagenicity of selected hydrazines. Mutation research, 102:413-424.
Romero M, Canada A (1991) RCI-1, a GSH-deficient mutant of
Escherichia coli B: response to oxidants and thiol-reacting
compounds. Current microbiology, 23:85-88.
Rothe A (1988) Contact dermatitis from
N-(alpha-chlorobenzylidene)-phenylhydrazine. Contact dermatitis,
18:16-19.
Roudabush R, Terhaar C, Fassett D, Dziuba S (1965) Comparative acute
effects of some chemicals on the skin of rabbits and guinea pigs.
Toxicology and applied pharmacology, 7:559-565.
Ruiz-Rubio M, Alejandre-Duràn E, Pueyo C (1985) Oxidative mutagens
specific for A-T base pairs induce forward mutations to L-arabinose
resistance in Salmonella typhimurium. Mutation research,
147:153-163.
Säterborg N (1974) Bone marrow abnormalities after phenylhydrazine
induced hemolysis in rabbits. Acta Radiologica, Therapy, Physics,
Biology, 13:345-356.
Schuckmann F (1969) Observations on the various forms of poisoning by
phenylhydrazine. Zentralblatt fuer Arbeitsmedizin und Arbeitsschutz,
11:338-341.
Shimizu H, Hayashi K, Takemura N (1978) Relationships between the
mutagenic and carcinogenic effects of hydrazine derivatives. Japanese
journal of hygiene, 33:474-485.
Smith C, McLain L, Zaugg W (1971) Phenylhydrazine-induced anemia in
chinook salmon. Toxicology and applied pharmacology, 20:73-81.
Solomons B (1946) A case of allergy to phenylhydrazine. British
journal of dermatology, 58:236-237.
Steinheider G, Neth R, Marquardt H (1985) Evaluation of nongenotoxic
and genotoxic factors modulating the frequency of micronucleated
erythrocytes in the peripheral blood of mice. Cell biology and
toxicology, 1:197-211.
Suzuki Y (1985) The development of a sensitive micronucleus test (Part
II): an in vitro method using cultured bone marrow cells. Tokyo
Jikeikai medical journal, 100:709-719.
Tamaki Y, Ito M, Semba R, Yamamura H, Kiyono S (1974) Functional
disturbances in adult rats suffered from icterus gravis neonatorum due
to maternal application of phenylhydrazine hydrochloride. Congenital
anomalies, 14:95-103.
Tonogai Y, Ogawa S, Ito Y, Iwaida M (1982) Actual survey of TLm
(median Tolerance Limit) values of environmental pollutants,
especially amines, nitriles, aromatic nitrogen compounds and
artificial dyes. Journal of toxicological sciences, 7:193-203.
Tosk J, Schmeltz I, Hoffmann D (1979) Hydrazines as mutagens in a
histidine-requiring auxotroph of Salmonella typhimurium. Mutation
research, 66:247-252.
Toth B, Shimizu H (1976) Tumorigenic effects of chronic administration
of benzylhydrazine dihydrochloride and phenylhydrazine hydrochloride
in Swiss mice. Zeitschrift fuer Krebsforschung, 87:267-273.
Ulitzur S, Weiser I, Levi B, Barak M (1984) Determination of 100
chemicals by the improved bioluminescence test for mutagenic agents.
In: Analytical and applied bioluminescence and chemiluminescence.
Proceedings of the 3rd International Symposium. New York, NY,
Academic Press, pp. 533-536.
Ullmann (1977) In: Ullmann's Encyklopadie der technischen Chemie. 4.
Neubearbeitete und erweiterte Auflage. Weinheim, VCH
Verlagsgesellschaft mbH.
Valenzuela A, Rios H, Neiman G (1977) Evidence that superoxide
radicals are involved in the hemolytic mechanism of phenylhydrazine.
Experientia, 33:962-963.
Valenzuela A, Guerra R, Fernandez N (1981) Evidence that peroxide
radicals and hydroxide radicals are involved in the hemolytic action
of phenylhydrazine. IRCS medical science, 9:342-343.
van Ketel W (1964) Contact dermatitis from a hydrazine-derivative in a
stain remover. Cross sensitization to Apresoline and isoniazid. Acta
Dermato-Venereologica, 44:49-53.
von Oettingen W, Deichmann-Gruebler W (1936) On the relation between
the chemical constitution and pharmacological action of
phenylhydrazine derivatives. Journal of industrial hygiene and
toxicology, 18:1-16.
Wellens H (1990) Biodegradability of monosubstituted and disubstituted
benzene derivatives. Zeitschrift fuer Wasser und Abwasser Forschung,
23:85-98.
Wilcox P, Naidoo A, Wedd D, Gatehouse D (1990) Comparison of
Salmonella typhimurium TA102 with Escherichia coli WP2 tester
strains. Mutagenesis, 5:285-291.
Williams A (1972) The nature of immature avian erythrocytes in severe
anaemia induced by phenylhydrazine. Journal of cell science,
11:771-776.
Witchett C (1975) Exposure of dog erythrocytes in vivo to
phenylhydrazine and monomethylhydrazine: a freeze-etch study of
erythrocyte damage. Wright-Patterson Air Force Base, OH, Aerospace
Medical Research Laboratory (Report AMRL-TR-74-88).
Wright I, Joyner E (1930) Skin hypersensitivity to phenylhydrazine
hydrochloride. Report of a case. American journal of medical
science, 179:683-687.
Xiu R, Gao S, Xu Y, Ren G (1992) Toxicity of hydrazine and
phenylhydrazine to embryos and larvae of zebrafish. Journal of
environmental science (Beijing), 13(6):67-69.
Yamamura H, Semba R, Keino H, Ohta K, Murakami U (1973) Experimental
studies on the developmenal disorder due to icterus gravis neonatorum:
I. Perinatal hemolytic jaundice and its effect on postnatal
development. Teratology, 8:110.
Zemek J, Bilik V, Kucar S, Linek K, Augustin J (1978) Antibacterial
effect of some phenylhydrazones. Folia Microbiologica (Prague),
23:304-307.
Zsolnai T (1975) Neure Antimycotika. I Phenylhydrazin-Derivate.
Zentralblatt fuer Bakteriologie, Parasitenkunde,
Infektionskrankheiten und Hygiene, Abteilung 1: Originale, Reihe A,
232:119-128.
APPENDIX 1 - SOURCE DOCUMENTS
Brooke I, Cain J, Cocker J, Groves J (1997) Phenylhydrazine.
Sudbury, Suffolk, HSE Books (Risk Assessment Document EH72/1; ISBN
0 7176 1355 0)
The author's draft version is initially reviewed internally by a
group of approximately 10 Health and Safety Executive experts - mainly
toxicologists, but also experts in other relevant disciplines, such as
epidemiology and occupational hygiene. The toxicology section of the
amended draft is then reviewed by toxicologists from the United
Kingdom Department of Health. Subsequently, the entire Criteria
Document is reviewed by a tripartite advisory committee to the United
Kingdom Health and Safety Commission, the Working Group for the
Assessment of Toxic Chemicals (WATCH). This committee is composed of
experts in toxicology and occupational health and hygiene from
industry, trade unions, and academia.
The members of the WATCH committee at the time of the peer review
were Mr S.R. Bailey, Independent Consultant; Professor J. Bridges,
University of Surrey; Dr H. Cross, Trade Unions Congress; Dr A.
Fletcher, Trade Unions Congress; Dr I.G. Guest, Chemical Industries
Association; Dr A. Hay, Trade Unions Congress; Dr J. Leeser, Chemical
Industries Association; Dr L. Levy, Institute of Occupational Hygiene,
Birmingham; Mr A. Moses, Chemical Industries Association; Dr R. Owen,
Trade Unions Congress; Mr J. Sanderson, Independent Consultant; and Dr
M. Sharratt, University of Surrey.
BUA (1995) Phenylhydrazine. Beratergremium fur Umweltrelevante
Altstoffe (BUA). GDCh Advisory Committee on Existing Chemicals of
Environmental Relevance. Stuttgart, S. Hirzel, Wissenschaftliche
Verlagsgesellschaft (Report No. 120; ISBN 3-7776-0691-X)
For the BUA review process, the company that is in charge of
writing the report (usually the largest producer in Germany) prepares
a draft report using literature from an extensive literature search as
well as internal company studies. This draft is subject to a peer
review during several readings of a working group consisting of
representatives from government agencies, the scientific community,
and industry.
APPENDIX 2 - CICAD PEER REVIEW
The draft CICAD on phenylhydrazine was sent for review to
institutions and organizations identified by IPCS after contact with
IPCS national Contact Points and Participating Institutions, as well
as to identified experts. Comments were received from:
Department of Health, London, United Kingdom
Federal Institute for Health Protection of Consumers & Veterinary
Medicine, Berlin, Germany
Institut de Recherches en Santé et Sécurité du Travail du Québec,
Montreal, Canada
Institute of Occupational Medicine, Chinese Academy of Preventive
Medicine, Ministry of Health, Beijing, People's Republic of China
National Institute of Health Sciences, Tokyo, Japan
Senatskommission der Deutschen GSF-Forschungszentrum für Umwelt und
Gesundheit GmbH, Institut für Toxikologie, Oberscheissheim, Germany
United States Department of Health and Human Services (National
Institute for Occupational Safety and Health, Cincinnati; National
Institute of Environmental Health Sciences, Research Triangle Park;
Agency for Toxic Substances and Disease Registry, Atlanta), USA
United States Environmental Protection Agency (National Center for
Environmental Assessment, Washington, DC; Region VIII), USA
APPENDIX 3 - CICAD FINAL REVIEW BOARD
Washington, DC, USA, 8-11 December 1998
Members
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
( Vice-Chairperson)
Mr R. Cary, Toxicology Unit, Health Directorate, Health and Safety
Executive, Bootle, Merseyside, United Kingdom ( Rapporteur)
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr O. Faroon, Agency for Toxic Substances and Disease Registry,
Centers for Disease Control and Prevention, Atlanta, GA, USA
Dr G. Foureman, National Center for Environmental Assessment, US
Environmental Protection Agency, Research Triangle Park, NC, USA
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA ( Chairperson)
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany
Dr A. Nishikawa, Division of Pathology, National Institute of Health
Sciences, Tokyo, Japan
Dr E.V. Ohanian, Office of Water/Office of Science and Technology,
Health and Ecological Criteria Division, US Environmental Protection
Agency, Washington, DC, USA
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan
Professor P. Yao, Institute of Occupational Medicine, Chinese Academy
of Preventive Medicine, Ministry of Health, Beijing, People's Republic
of China
Observers
Dr K. Austin, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr I. Daly (ICCA representative), Regulatory and Technical Associates,
Lebanon, NJ, USA
Ms K.L. Lang (CEFIC, European Chemical Industry Council,
representative), Shell International, London, United Kingdom
Ms K. Roberts (ICCA representative), Chemical Self-funded Technical
Advocacy and Research (CHEMSTAR), Chemical Manufacturers Association,
Arlington, VA, USA
Dr W. Snellings (ICCA representative), Union Carbide Corporation,
Danbury, CN, USA
Dr M. Sweeney, Document Development Branch, National Institute for
Occupational Safety and Health, Cincinnati, OH, USA
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Secretariat
Dr M. Baril, Institut de Recherches en Santé et Sécurité du Travail du
Québec (IRSST), Montreal, Quebec, Canada
Dr H. Galal-Gorchev, Chevy Chase, MD, USA
Ms M. Godden, Health and Safety Executive, Bootle, Merseyside, United
Kingdom
Dr R.G. Liteplo, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Ms L. Regis, Programme for the Promotion of Chemical Safety, World
Health Organization, Geneva, Switzerland
Mr A. Strawson, Health and Safety Executive, London, United Kingdom
Dr P. Toft, Programme for the Promotion of Chemical Safety, World
Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
Ce CICAD relatif à la phénylhydrazine résulte d'une étude des
risques pour la santé humaine (principalement dans un cadre
professionnel) rédigée par le Health and Safety Executive du
Royaume-Uni (Brooke et al., 1997) et d'un rapport préparé par le
Comité consultatif allemand sur les substances chimiques importantes
pour l'environnement (BUA, 1995). Il est donc consacré aux divers
types d'exposition par les voies existant sur les lieux de travail,
mais contient également des données concernant l'environnement
général. Les données prises en compte dans ces deux documents de base
remontent respectivement à décembre 1993 et décembre 1994. Une étude
bibliographique complémentaire arrêtée à janvier 1998 a été effectuée
à la recherche de données supplémentaires qui auraient pu être
publiées postérieurement à ces documents. On trouvera à l'appendice 1
des indications sur le mode d'examen par des pairs ainsi que sur les
sources documentaires utilisées. Les renseignements concernant
l'examen du CICAD par les pairs font l'objet de l'appendice 2. Ce
CICAD a été approuvé en tant qu'évaluation internationale lors de la
réunion du Comité d'évaluation finale qui s'est tenue à Washington du
8 au 11 décembre 1998. La liste des participants à cette réunion
figure à l'appendice 3. La fiche d'information internationale sur la
sécurité chimique (ICSC No 0938) relative à la phénylhydrazine,
établie par le Programme international sur la sécurité chimique (IPCS,
1993), est également reproduite dans ce document.
La phénylhydrazine (CAS No 100-63-0) se présente sous la forme de
cristaux de couleur jaune à brun pâle ou d'un liquide jaunâtre de
consistance huileuse. Il est légèrement soluble dans l'eau et miscible
aux solvants organiques.
La phénylhydrazine est utilisée dans le monde entier comme
intermédiaire dans l'industrie chimique, pharmaceutique et
agrochimique.
On ignore combien de personnes sont susceptibles d'être exposées
à la phénylhydrazine ou à son chlorhydrate, mais elles sont
vraisemblablement un petit nombre. On ne dispose d'aucune donnée sur
l'exposition individuelle, mais le modèle EASE ( Estimation and
Assessment of Substance Exposure) permet de prédire une exposition
d'environ 2,3 mg/m3 (0,5 ppm) en moyenne pondérée par rapport au
temps sur 8 h. En pratique, l'exposition moyenne pondérée par rapport
au temps sur 8 h est inférieure à cette valeur.
Les données limitées dont on dispose au sujet de la
pharmacocinétique de la phénylhydrazine indiquent que ce composé est
bien absorbé après inhalation, ingestion ou par voie percutanée et
qu'il se combine facilement à l'hémoglobine des hématies. Il semble
que sa métabolisation implique une hydroxylation du cycle suivie
probablement de la formation d'un glucuro-conjugué. Il est
principalement éliminé par voie urinaire.
L'ingestion d'une seule dose peut provoquer une intoxication
(DL50 : 80-188 mg/kg de poids corporel) et la phénylhydrazine est sans
doute également toxique si elle est inhalée ou entre en contact avec
la peau (les données concernant ces deux voies d'exposition sont moins
précises). La phénylhydrazine pourrait être irritante pour la peau et
les yeux et on est fondé à penser qu'elle produit une sensibilisation
cutanée chez l'homme. L'exposition à ce composé peut endommager les
hématies, d'où un risque d'anémie et d'atteinte secondaire d'autres
tissus comme le tissu splénique et le tissu hépatique. La
phénylhydrazine est mutagène in vitro et selon certaines données,
elle pourrait également avoir une activité génotoxique in vivo. Elle
est de toute évidence cancérogène pour la souris (administration par
voie orale) et provoque des tumeurs vasculaires. On ne peut, à la
lumière de toutes ces données, indiquer le niveau d'exposition en
dessous duquel il n'y a pas de risque d'effets cancérogènes ou
génotoxiques.
On ne possède pas de données suffisantes concernant les effets
sur la reproduction ou le développement; il n'est donc pas possible de
dire si l'homme court des risques de cette nature.
Le degré de risque professionnel n'est pas connu avec certitude.
Il s'ensuit qu'il est toujours nécessaire de réduire le niveau
d'exposition au minimum raisonnable compte tenu de l'état actuel de la
technique.
Comme on ne dispose pas de données qui puissent servir à estimer
l'exposition individuelle à la phénylhydrazine présente dans
l'environnement général, on ne peut pas préciser quel risque de cancer
elle représente pour la population.
On n'envisage pas d'effets atmosphériques, étant donné que le
composé est essentiellement libéré dans l'eau et qu'une fois dans ce
milieu, il ne s'en évapore que très faiblement. En outre, le calcul
montre que sa demi-vie atmosphérique est très courte par suite de sa
combinaison avec les radicaux hydroxyles.
La phénylhydrazine subit une décomposition photochimique et
s'oxyde dans l'eau. Elle est facilement biodégradable et c'est
probablement ainsi qu'elle se décompose dans l'environnement. Sa
sorption par les matières particulaires est minime.
La phénylhydrazine est toxique pour les organismes aquatiques, la
concentration sans effet observable (NOEC) la plus faible qui ait été
indiquée lors d'épreuves classique de toxicité aiguë sur des poissons
étant de 0,01 mg/litre. Les poissons sont habituellement plus
sensibles que les daphnies ou les bactéries. On a fait état d'une NOEC
de 0,49 µg/litre pour les stades embryo-larvaires d'un poisson, le
danio ( Brachydanio rerio).
Le risque pour les organismes aquatiques devrait être faible,
même dans l'hypothèse la plus prudente.
RESUMEN DE ORIENTACION
El presente CICAD sobre la fenilhidrazina se basa en un examen de
problemas relativos a la salud humana (fundamentalmente ocupacionales)
preparado por el Health and Safety Executive del Reino Unido
(Brooke et al., 1997) y un informe preparado por el Comité Consultivo
Alemán sobre las Sustancias Químicas Importantes para el Medio
Ambiente (BUA, 1995). Por consiguiente, este informe se concentra en
la exposición a través de las vías de interés para los entornos
ocupacionales, pero también contiene información medioambiental.
Incluye los datos identificados a partir de diciembre de 1993 y
diciembre de 1994, respectivamente. Se realizó una nueva búsqueda en
lo publicado hasta enero de 1998 para localizar toda la información
aparecida desde la terminación de estos exámenes. La información
relativa al carácter del examen colegiado y a la disponibilidad de los
documentos originales figura en el apéndice 1. La información sobre el
examen colegiado de este CICAD aparece en el apéndice 2. Este CICAD se
aprobó como evaluación internacional en una reunión de la Junta de
Evaluación Final celebrada en Washington, DC (Estados Unidos de
América), del 8 a 11 de diciembre de 1998. En el apéndice 3 figura la
lista de los participantes en esta reunión. La ficha internacional de
seguridad química (ICSC No 0938) para la fenilhidrazina, preparada
por el Programa Internacional de Seguridad de las Sustancias Químicas
(IPCS, 1993), también se reproduce en el presente documento.
La fenilhidrazina (CAS No 100-63-0) se encuentra en forma de
cristales de un color entre amarillo y marrón claro o como líquido
oleoso amarillento. Es moderadamente soluble en el agua y es miscible
con otros disolventes orgánicos.
La fenilhidrazina se usa en todo el mundo, principalmente como
intermediario químico en las industrias farmacéutica, agroquímica y
química.
No se conoce el número de personas potencialmente expuestas a la
fenilhidrazina o sus hidrocloruros, pero se supone que es bajo. No hay
datos disponibles sobre la exposición personal, aunque el modelo EASE
( Estimation and Assessment of Substance Exposure) predecía (como
promedio ponderado en función del tiempo durante ocho horas) una
exposición de unos 2,3 mg/m3 (0,5 ppm). En la práctica, la exposición
promedio ponderada en función del tiempo durante ocho horas es
inferior a esa cifra.
Los limitados datos sobre la toxicocinética indican que la
fenilhidrazina se absorbe bien por inhalación y por vía oral y cutánea
y se une fácilmente a la hemoglobina en las glóbulos rojos. El
metabolismo parece que se produce por hidroxilación del anillo y
conjugación, probablemente con el ácido glucurónico. La excreción
tiene lugar fundamentalmente por vía urinaria.
La fenilhidrazina es tóxica en una exposición única por vía oral
(DL50 80-188 mg/kg de peso corporal) y es de prever que sea tóxica
por inhalación y por vía cutánea (los datos relativos a esas vías de
exposición son menos claros). Puede provocar irritación cutánea y
ocular y hay pruebas de que tiene propiedades de sensibilización
cutánea en el ser humano. La exposición a la fenilhidrazina puede
provocar daños en los glóbulos rojos, pudiendo producir anemia y en
consecuencia afectar de manera secundaria a otros tejidos, por ejemplo
los del bazo o del hígado. La fenilhidrazina es mutagénica in vitro
y hay algunos indicios de que puede mostrar actividad genotóxica
in vivo. Esta sustancia es claramente carcinogénica en ratones tras
la administración oral, induciendo la formación de tumores en el
sistema vascular. No está claro el mecanismo de formación de tumores,
pero no se puede descartarr un componente genotóxico. Por
consiguiente, no parece que sea posible identificar con seguridad un
nivel de exposición para el cual no haya riesgo de efectos
carcinogénicos o genotóxicos.
No se dispone de datos adecuados relativos a los efectos en la
reproducción o el desarrollo; no es posible, pues, evaluar el riesgo
para la salud humana de esos efectos finales.
El nivel de riesgo en el entorno ocupacional es incierto; en
consecuencia es constante la necesidad de reducir los niveles de
exposición todo lo que sea razonablemente posible con la tecnología
disponible en la actualidad.
La falta de datos disponibles que sirvan como base para la
estimación de la exposición indirecta de las personas a la
fenilhidrazina a partir del medio ambiente impide determinar los
riesgos potenciales de cáncer para la población general.
No son de prever efectos atmosféricos, debido a que la
fenilhidrazina se libera fundamentalmente en el agua, a su escasa
volatilización del agua a la atmósfera y a su rápida semivida en la
atmósfera calculada tras la reacción con los radicales hidroxilo.
La fenilhidrazina se degrada por vía fotoquímica y se autooxida
en el agua. Es fácilmente biodegradable y se supone que es ésta la
principal ruta de descomposición en el medio ambiente. La sorción en
partículas es mínima.
La fenilhidrazina es tóxica para los organismos acuáticos, siendo
de 0,01 mg/litro la concentración sin efectos observados (NOEC) más
baja notificada en pruebas normalizadas de toxicidad aguda en peces;
en general, los peces son más sensibles que los dáfnidos o las
bacterias. Se ha notificado una NOEC de 0,49 µg/litro para las fases
embriolarvarias del pez Brachydanio rerio.
Se supone que el riesgo para los organismos acuáticos es bajo, a
partir de hipótesis muy prudentes.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Previous evaluations by other international bodies were not
identified. Information on international hazard classification and
labelling is included in the International Chemical Safety Card (ICSC
0938) reproduced in this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventive and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0938) reproduced in this
document.
13.1 Human health hazards
Phenylhydrazine induces damage to red blood cells. Repeated or
prolonged contact with the substance causes skin sensitization, and
there is cause for concern for carcinogenicity.
13.2 Advice to physicians
Phenylhydrazine is a haemolytic agent. There is no specific
antidote, but treatment should be supportive.
13.3 Health surveillance advice
Periodic medical examination of the area of the skin exposed to
phenylhydrazine and annual blood count should be included in the
health surveillance programme.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards
may be obtained from UNEP Chemicals (IRPTC), Geneva.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
PHENYLHYDRAZINE ICSC: 0938
November 1998
CAS # 100-63-0 Hydrazinobenzene
RTECS # MV8925000 Monophenylhydrazine
UN # 2572 C6H8N2/C6H5NHNH2
Molecular mass: 108.1
TYPES OF HAZARD ACUTE HAZARDS/ PREVENTION FIRST AID/
SYMPTOMS FIRE FIGHTING
FIRE Combustible. Gives off NO open flames. Water spray, alcohol-resistant
irritating or toxic foam, powder, carbon dioxide.
fumes (or gases) in
a fire.
EXPLOSION Above 88°C explosive Above 88°C use In case of fire: keep drums, etc.,
vapour/air mixtures a closed system, cool by spraying with water.
may be formed. ventilation.
EXPOSURE STRICT HYGIENE!
Inhalation Cough. Laboured Local exhaust or Fresh air, rest. Refer for
breathing. Sore breathing medical attention.
throat. Cyanosis. protection.
Skin MAY BE ABSORBED! Protective gloves. Remove contaminated clothes.
Dry skin. Redness. Protective clothing. Rinse skin with plenty of water
Pain. or shower. Refer for medical
attention.
Eyes Redness. Pain. Blurred Face shield, or eye First rinse with plenty of
vision protection in water for several minutes
combination with (remove contact lenses if easily
breathing possible), then take to a
protection. doctor.
Ingestion Abdominal pain. Diarrhoea. Do not eat, drink or Rest. Refer for medical
Nausea. Vomiting. Weakness. smoke during work attention.
Vertigo.
SPILLAGE DISPOSAL PACKAGING & LABELLING
If the substance is melted: collect leaking and Airtight. Do not transport with food and feedstuffs.
spilled liquid in sealable containers as far as EU Classification:
possible. Absorb remaining liquid in sand or inert Symbol: T, N
absorbent and remove to safe place. Do NOT wash R: 23/24/25-36-50
away into sewer. If the substance is solid: sweep S: (1/2-)28-45-61
spilled substance into container, carefully collect UN Classification
remainder, then remove to a safe place. Do NOT let UN Hazard Class: 6.1
this chemical enter the environment. (Extra personal UN Pack Group: II
protection: complete protective clothing including
self-contained breathing apparatus).
EMERGENCY RESPONSE STORAGE
Transport Emergency Card: TEC (R)-61/G61b Separated from strong oxidants, food and
NFPA Code: H3; F2; R0; feedstuffs. Cool. Keep in the dark.
IMPORTANT DATA
PHYSICAL STATE; APPEARANCE: ROUTES OF EXPOSURE:
COLOURLESS TO YELLOW OILY LIQUID OR CRYSTALS. The substance can be absorbed into the
TURNS BROWN RED ON EXPOSURE TO AIR AND LIGHT. body by inhalation of its aerosol, through
the skin, by ingestion.
CHEMICAL DANGERS: INHALATION RISK:
The substance decomposes on heating and on A harmful contamination of the air can be reached
burning producing toxic fumes including nitrogen rather quickly on evaporation of this substance at 20°C.
oxides. Reacts with oxidants. Reacts violently
with lead dioxide.
OCCUPATIONAL EXPOSURE LIMITS: EFFECTS OF SHORT-TERM EXPOSURE:
TLV: 0.1 ppm; 0.44 mg/m3 A3 (skin)(ACGIH 1998). The substance irritates the eyes, the skin, the
respiratory tract. The substance may cause effects on
the blood, resulting in hemolysis, kidney impairment,
liver impairment. The effects may be delayed. Medical
observation is indicated.
EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
Repeated or prolonged contact with skin may cause
dermatitis. Repeated or prolonged contact may cause
skin sensitization. The substance may have effects
on the blood, resulting in anaemia.
PHYSICAL PROPERTIES
Boiling point (decomposes): 243.5°C Octanol/water partition coefficient as log Pow: 1.25
Melting point: 19.5°C
Relative density (water = 1): 1.09
Solubility in water: poor
Vapour pressure, Pa at 71.8°C: 133
Relative vapour density
(air = 1): 3.7
Flash point: 88°C c.c.
Auto-ignition temperature: 174°C
ENVIRONMENTAL DATA
The substance is toxic to aquatic organisms.
NOTES
The symptoms of hemolysis do not become manifest until hours have passed.
ADDITIONAL INFORMATION
LEGAL NOTICE Neither the CEC nor the IPCS nor any person acting on behalf of the CEC
or the IPCS is responsible for the use which might be made of this
information.
(c) IPCS, CEC 1999
REFERENCES
Ades I, Cascarano J (1979) Mitochondrial alterations in heart, kidney
and liver of rats subjected to anemic hypoxia. Experimental molecular
pathology, 30:94-109.
Allen E, Giffin H (1928) Experiments with phenylhydrazine. II. Studies
on renal and hepatic function and erythropoiesis. American journal of
medical science, 185:677-682.
BASF (1993) Abschlussbericht. Prufung der biologischen Abbaubarkeit
von Phenylhydrazin im modifizierten OECD-Screeningtest. Ludwigshafen,
BASF AG.
Besada A (1988) Analytical use of copper(II)-neocuproine in the
spectrophotometric determination of hydrazines. Analytical letters,
21:1917-1925.
Bodansky M (1923) The action of hydrazine and some of its derivatives
in producing liver injury as measured by the effect on levulose
tolerance. Journal of biological chemistry, 58:799-811.
Bokorny T (1933) Uber den Einfluss verschiedener Substanzen auf die
Keimung der Pflanzensamen. Wachstumsforderung durch einige. II
Mitteilung. Biochemische Zeitschrift, 50:49-61.
Bolton V (1935) A laboratory study of amidopyrine, barbital,
phenylhydrazine, and benzene in relation to agranulocytic angina.
Journal of laboratory and clinical medicine, 20:1199-1203.
Brooke I, Cain J, Cocker J, Groves J (1997) Phenylhydrazine.
Sudbury, Suffolk, HSE Books (Risk Assessment Document EH72/1; ISBN 0
7176 1355 0).
BUA (1995) Phenylhydrazine. Beratergremium fur Umweltrelevante
Altstoffe (BUA). GDCh Advisory Committee on Existing Chemicals of
Environmental Relevance. Stuttgart, S. Hirzel, Wissenschaftliche
Verlagsgesellschaft (Report No. 120; ISBN 3-7776-0691-X).
Clark M, Gildersleeve R, Thaxton J, Parkhurst C, McRee D (1988)
Hematological effects of ethyl methanesulfate, paraquat and
phenylhydrazine in Japanese quail. Comparative biochemistry and
physiology, 89C:15-30.
Clayson D, Biancifiori C, Milia U, Santilli F (1966) The induction of
pulmonary tumours in BALB/c/Cb/Se mice by derivatives of hydrazine.
In: Lung tumours in animals. Proceedings of the 3rd Perugia
Quadrennial International Conference on Cancer, University of Perugia,
Perugia, pp. 869-880.
Datta K, Soni J, Awadhiya R, Datta I (1989) Erythrophagocytosis in
phenylhydrazine-induced acute anaemia in chickens. Research in
veterinary science, 47:136-137.
Datta K, Soni J, Datta I (1990) Sequential morphological changes in
chicken erythrocytes after in vivo and in vitro exposure to
phenylhydrazine hydrochloride. Research in veterinary science,
48:12-17.
De Flora S (1981) Study of 106 organic and inorganic compounds in the
Salmonella/microsome test. Carcinogenesis, 2:283-298.
De Flora S, Camoirano A, Zanacchi P, Bennicelli C (1984a) Mutagenicity
testing with TA97 and TA102 of 30 DNA-damaging compounds, negative
with other Salmonella strains. Mutation research, 134:159-165.
De Flora S, Zanacchi P, Camoirano A, Bennicelli C, Badolati G (1984b)
Genotoxic activity and potency of 135 compounds in the Ames reversion
test and in a bacterial DNA-repair test. Mutation research,
133:161-198.
Derelanko M, Gad S, Gavigan F, Babich P, Rinehart W (1987) Toxicity of
hydroxylamine sulfate following dermal exposure: variability with
exposure method and species. Fundamental and applied toxicology,
8:583-594.
Di Cola D, Sacchetta P, Battista P (1988) Proteolysis in human
erythrocytes is triggered only by selected oxidative stressing agents.
Italian journal of biochemistry, 37:129-138.
Di Cola D, Battista P, Santarone S, Sacchetta P (1989) Fragmentation
of human hemoglobin by oxidative stress produced by phenylhydrazine.
Free radical research communications, 6:379-386.
Dornfest B, Lapin D, Naughton B, Adu S, Korn L, Gordon A (1986)
Phenylhydrazine-induced leukocytosis in the rat. Journal of leukaemia
biology, 39:37-48.
Ekshtat B (1965) Maximum permissible concentrations of hydrazine
hydrate and phenylhydrazine in water bodies. Hygiene and sanitation
(USSR), 30:191-197.
Frost J, Hjorth N (1959) Contact dermatitis from hydrazine
hydrochloride in soldering flux. Cross sensitization to Apresoline and
isoniazid. Acta Dermato-Venereologica, 39:82-86.
Giffin H, Allen E (1928) Experiments with phenylhydrazine. I. Studies
on the blood. Annals of internal medicine, 1:655-676.
Giffin H, Allen E (1933) The control and complete remission of
polycythemia vera following the prolonged administration of
phenylhydrazine hydrochloride. American journal of medical science,
185:1-13.
Giffin H, Conner H (1929) The untoward effects of treatment by
phenylhydrazine hydrochloride. Journal of the American Medical
Association, 92:1505-1507.
Goldberg B, Stern A, Peisach J, Blumberg W (1979) The detection of
superoxide anion from the reaction of oxyhemoglobin and
phenylhydrazine using EPR spectroscopy. Experientia, 35:488-489.
Goldstein B, Rozen M, Kunis R (1980) Role of red cell membrane lipid
peroxidation in hemolysis due to phenylhydrazine. Biochemical
pharmacology, 29:1355-1359.
Hasan T (1988) Resin bead detection and spectrophotometric
determination of phenylhydrazine using inorganic reagents. Analytical
letters, 21:633-640.
Hill H (1985) Dioxygen-derived radicals in biological systems.
Philosophical transactions of the Royal Society of London, B
311:605-615.
Hoechst (1980) Abwasserbiologische Untersuchung von Phenylhydrazin,
Unveroffentlichte Untersuchung der Abt. RWL vom 15.04.1980 und vom
03.06.1980. Frankfurt/Main, Hoechst AG.
Hoechst (1982) Phenylhydrazin-Reinbase D, Prufung der akuten
Toxizitat am Fisch Brachydanio rerio (Hamilton-Buchanan) uber 96
Stunden. Unverffentlichter Bericht Nr 691/82 der Abt. Pharma
Forschung Toxicologie. Frankfurt/Main, Hoechst AG.
Hoechst (1992) Okologische Daten zu Phenylhydrazin. Bericht uber
Ergebnisse zuruckliegender Versuche anhand der vorliegenden
Laboraufzeichungen und Angaben in der UWS. Frankfurt/Main, Hoechst
AG.
Houston A, Murad A, Gray J (1988) Induction of anemia in goldfish,
Carassius auratus L., by immersion in phenylhydrazine hydrochloride.
Canadian journal of zoology, 66:729-736.
Hovding G (1967) Occupational dermatitis from hydrazine hydrate used
in boiler protection. Acta Dermato-Venereologica, 47:293-297.
HSDB (1998) Hazardous substances data bank. Micromedex Inc. (CD-ROM
version).
IPCS (1993) International Chemical Safety Card - Phenylhydrazine.
Geneva, World Health Organization, International Programme on Chemical
Safety (ICSC 0938).
Itano H, Hirota K, Hosokawa K (1975) Mechanism of induction of
haemolytic anaemia by phenylhydrazine. Nature, 256:665-667.
Jadassohn W (1930) Sensitization of guinea-pig skin to
phenylhydrazine. Klinische Wochenschrift, 9:551.
Jain S, Hochstein P (1979) Generation of superoxide radicals by
hydrazine. Its role in phenylhydrazine-induced hemolytic anemia.
Biochimica et Biophysica Acta, 586:128-136.
Japan Environment Agency (1987) Chemicals in the environment, a
report of nationwide surveys. Environmental Health and Safety
Division, Environmental Health Department, December.
Jonen H, Werringloer J, Prough R, Estabrook R (1982) The reaction of
phenylhydrazine with microsomal cytochrome P-450. Catalysis of heme
modification. Journal of biological chemistry, 257:4404-4411.
Kaiser K, Palabrica V, Robi J (1987) QSAR of acute toxicity of
mono-substituted benzene derivatives to Photobacterium phosphoreum.
In: Kaiser KLE, ed. QSAR environmental toxicology II. Dordrecht, D.
Reidel Publishing Company, pp. 153-168.
Kampfe L, Bode K, Ullrich H-L (1986a) Zur Wirkung einiger Lathyrogene
auf Nematoden. Mededelingen van de Faculteit Landbouwwetenschappen,
Rijksuniversiteit Gent, 51(3b):1267-1277.
Kampfe L, Kreil V, Ullrich, H-L (1986b) Wirkung lathyrogener
Substanzen und des Synergisten Piperonylbutoxid (PBO) auf die
freilebended Nematoden Caenorhabditis briggsae und Rhabditis
oxycerca. Zoologische Jahrbuecher, Abteilung fuer Allgemeine
Zoologie und Physiologie der Tiere, 90:257-271.
Karickhoff S, Brown D, Scott T (1979) Sorption of hydrophobic
pollutants on natural sediments. Water research, 13:241-248.
Kelly M, O'Gara R, Yancey S, Gadekar K, Botkin C, Oliverio V (1969)
Comparative carcinogenicity of N-isopropyl-alpha-(2-methyl
hydrazino)- p-toluamide.HCl (procarbazine hydrochloride), its
degradation products, other hydrazines, and isonicotinic acid
hydrazide. Journal of the National Cancer Institute, 42:337-344.
Kreil V (1982) Prufung xenobiotischer Substazen auf ihre Wirkung
gegenuber Caenorhabditis briggsae unter exenischen
Kultivierungsbedingungen. Thesis, University of Greifswald,
Greifswald [cited in Kampfe et al., 1986b].
Kuszynski C, Langenbach R, Malick L, Tompa A, Toth B (1981) Liver
cell-mediated mutagenesis of V79 cells by hydrazine and related
compounds. Environmental mutagenesis, 3:323-324.
Levi B, Kuhn J, Ulitzur S (1986) Determination of the activity of 16
hydrazine derivatives in the bioluminescence test for genotoxic
agents. Mutation research, 173:233-237.
Levin D, Hollstein M, Christman M, Schwiers E, Ames B (1982) A new
Salmonella tester strain (TA102) with A-T base pairs at the site of
mutation detects oxidative mutagens. Proceedings of the National
Academy of Sciences of the United States of America, 79:7445-7449.
Malca-Mor L, Stark A (1982) Mutagenicity and toxicity of carcinogenic
and other hydrazine derivatives: correlation between toxic potency in
animals and toxic potency in Salmonella typhimurium TA1538. Applied
environmental microbiology, 44:801-808.
Malten K (1962) Industrial contact dermatitis caused by hydrazine
derivatives, with group hypersensitivity to hydralazine (Apresoline)
and isoniazid (INH). Nederlands Tijdschrift voor Geneeskunde,
106:2219-2222.
Maples K, Jordan S, Mason R (1988) In vivo rat hemoglobin thiyl free
radical formation following phenylhydrazine administration. Molecular
pharmacology, 33:344-350.
Marks G (1985) Exposure to toxic agents: the heme biosynthetic pathway
and hemoproteins as indicator. CRC critical reviews in toxicology,
15:151-179.
Mathison B, Murphy S, Shank R (1994) Hydralazine and other hydrazine
derivatives and the formation of DNA adducts. Toxicology and applied
pharmacology, 127:91-98.
McIsaac W, Parke D, Williams R (1958) Studies in detoxication. 77. The
metabolism of phenylhydrazine and some phenylhydrazones. Biochemical
journal, 70:688-697.
Meylan W, Howard P (1993) Computer estimation of the atmospheric
gas-phase reaction rate of organic compounds with hydroxyl radicals
and ozone. Chemosphere, 26:2293-2299.
Mori H, Sugie S, Yoshimi N, Iwata H, Nishikawa A, Matsukubo K, Shimizu
H, Hirono I (1988) Genotoxicity of a variety of hydrazine derivatives
in the hepatocyte primary culture/DNA repair test using rat and mouse
hepatocytes. Japanese journal of cancer research (Gann), 79:204-211.
Muller W, Englehart G, Herbold B, Jackh R, Jung R (1993) Evaluation of
mutagenicity testing with Salmonella typhimurium TA102 in three
different laboratories. Environmental health perspectives,
101(3):33-36.
NIOSH (1978) Criteria for a recommended standard...occupational
exposure to hydrazines. Cincinnati, OH, National Institute for
Occupational Safety and Health (DHEW (NIOSH) Publication No. 78-172).
NIOSH (1994) Method 3518, Phenylhydrazine. In: Manual of analytical
methods, 4th ed. Cincinnati, OH, National Institute for Occupational
Safety and Health.
Nishida N, Nakamura I, Kudo Y, Kagami M (1982) Effects of aromatic
nitro and amino compounds on the osmotic fragility of red cells.
Japanese journal of industrial health, 24:172-180.
Parodi S, De Flora S, Cavanna M, Pino A, Robbiano L, Bennicelli C,
Brambilla G (1981) DNA-damaging activity in vivo and bacterial
mutagenicity of sixteen hydrazine derivatives as related
quantitatively to their carcinogenicity. Cancer research,
41:1469-1482.
Pevny I, Peter G (1983) Allergic contact dermatitis to derivatives of
pyridine and hydrazine. Dermatitis, 31:78-83.
Pham K-C (1979) Toxicity of phenylhydrazine in inhalatory exposure.
Gigiena Truda i Professional'nye Zabolevaniya, 3:45-47.
Roe F, Grant G, Millican D (1967) Carcinogenicity of hydrazine and
1,1-dimethylhydrazine for mouse lung. Nature, 216:375-376.
Rogan E, Walker B, Gingell R, Nagel D, Toth B (1982) Microbial
mutagenicity of selected hydrazines. Mutation research, 102:413-424.
Romero M, Canada A (1991) RCI-1, a GSH-deficient mutant of
Escherichia coli B: response to oxidants and thiol-reacting
compounds. Current microbiology, 23:85-88.
Rothe A (1988) Contact dermatitis from
N-(alpha-chlorobenzylidene)-phenylhydrazine. Contact dermatitis,
18:16-19.
Roudabush R, Terhaar C, Fassett D, Dziuba S (1965) Comparative acute
effects of some chemicals on the skin of rabbits and guinea pigs.
Toxicology and applied pharmacology, 7:559-565.
Ruiz-Rubio M, Alejandre-Duràn E, Pueyo C (1985) Oxidative mutagens
specific for A-T base pairs induce forward mutations to L-arabinose
resistance in Salmonella typhimurium. Mutation research,
147:153-163.
Säterborg N (1974) Bone marrow abnormalities after phenylhydrazine
induced hemolysis in rabbits. Acta Radiologica, Therapy, Physics,
Biology, 13:345-356.
Schuckmann F (1969) Observations on the various forms of poisoning by
phenylhydrazine. Zentralblatt fuer Arbeitsmedizin und Arbeitsschutz,
11:338-341.
Shimizu H, Hayashi K, Takemura N (1978) Relationships between the
mutagenic and carcinogenic effects of hydrazine derivatives. Japanese
journal of hygiene, 33:474-485.
Smith C, McLain L, Zaugg W (1971) Phenylhydrazine-induced anemia in
chinook salmon. Toxicology and applied pharmacology, 20:73-81.
Solomons B (1946) A case of allergy to phenylhydrazine. British
journal of dermatology, 58:236-237.
Steinheider G, Neth R, Marquardt H (1985) Evaluation of nongenotoxic
and genotoxic factors modulating the frequency of micronucleated
erythrocytes in the peripheral blood of mice. Cell biology and
toxicology, 1:197-211.
Suzuki Y (1985) The development of a sensitive micronucleus test (Part
II): an in vitro method using cultured bone marrow cells. Tokyo
Jikeikai medical journal, 100:709-719.
Tamaki Y, Ito M, Semba R, Yamamura H, Kiyono S (1974) Functional
disturbances in adult rats suffered from icterus gravis neonatorum due
to maternal application of phenylhydrazine hydrochloride. Congenital
anomalies, 14:95-103.
Tonogai Y, Ogawa S, Ito Y, Iwaida M (1982) Actual survey of TLm
(median Tolerance Limit) values of environmental pollutants,
especially amines, nitriles, aromatic nitrogen compounds and
artificial dyes. Journal of toxicological sciences, 7:193-203.
Tosk J, Schmeltz I, Hoffmann D (1979) Hydrazines as mutagens in a
histidine-requiring auxotroph of Salmonella typhimurium. Mutation
research, 66:247-252.
Toth B, Shimizu H (1976) Tumorigenic effects of chronic administration
of benzylhydrazine dihydrochloride and phenylhydrazine hydrochloride
in Swiss mice. Zeitschrift fuer Krebsforschung, 87:267-273.
Ulitzur S, Weiser I, Levi B, Barak M (1984) Determination of 100
chemicals by the improved bioluminescence test for mutagenic agents.
In: Analytical and applied bioluminescence and chemiluminescence.
Proceedings of the 3rd International Symposium. New York, NY,
Academic Press, pp. 533-536.
Ullmann (1977) In: Ullmann's Encyklopadie der technischen Chemie. 4.
Neubearbeitete und erweiterte Auflage. Weinheim, VCH
Verlagsgesellschaft mbH.
Valenzuela A, Rios H, Neiman G (1977) Evidence that superoxide
radicals are involved in the hemolytic mechanism of phenylhydrazine.
Experientia, 33:962-963.
Valenzuela A, Guerra R, Fernandez N (1981) Evidence that peroxide
radicals and hydroxide radicals are involved in the hemolytic action
of phenylhydrazine. IRCS medical science, 9:342-343.
van Ketel W (1964) Contact dermatitis from a hydrazine-derivative in a
stain remover. Cross sensitization to Apresoline and isoniazid. Acta
Dermato-Venereologica, 44:49-53.
von Oettingen W, Deichmann-Gruebler W (1936) On the relation between
the chemical constitution and pharmacological action of
phenylhydrazine derivatives. Journal of industrial hygiene and
toxicology, 18:1-16.
Wellens H (1990) Biodegradability of monosubstituted and disubstituted
benzene derivatives. Zeitschrift fuer Wasser und Abwasser Forschung,
23:85-98.
Wilcox P, Naidoo A, Wedd D, Gatehouse D (1990) Comparison of
Salmonella typhimurium TA102 with Escherichia coli WP2 tester
strains. Mutagenesis, 5:285-291.
Williams A (1972) The nature of immature avian erythrocytes in severe
anaemia induced by phenylhydrazine. Journal of cell science,
11:771-776.
Witchett C (1975) Exposure of dog erythrocytes in vivo to
phenylhydrazine and monomethylhydrazine: a freeze-etch study of
erythrocyte damage. Wright-Patterson Air Force Base, OH, Aerospace
Medical Research Laboratory (Report AMRL-TR-74-88).
Wright I, Joyner E (1930) Skin hypersensitivity to phenylhydrazine
hydrochloride. Report of a case. American journal of medical
science, 179:683-687.
Xiu R, Gao S, Xu Y, Ren G (1992) Toxicity of hydrazine and
phenylhydrazine to embryos and larvae of zebrafish. Journal of
environmental science (Beijing), 13(6):67-69.
Yamamura H, Semba R, Keino H, Ohta K, Murakami U (1973) Experimental
studies on the developmenal disorder due to icterus gravis neonatorum:
I. Perinatal hemolytic jaundice and its effect on postnatal
development. Teratology, 8:110.
Zemek J, Bilik V, Kucar S, Linek K, Augustin J (1978) Antibacterial
effect of some phenylhydrazones. Folia Microbiologica (Prague),
23:304-307.
Zsolnai T (1975) Neure Antimycotika. I Phenylhydrazin-Derivate.
Zentralblatt fuer Bakteriologie, Parasitenkunde,
Infektionskrankheiten und Hygiene, Abteilung 1: Originale, Reihe A,
232:119-128.
APPENDIX 1 - SOURCE DOCUMENTS
Brooke I, Cain J, Cocker J, Groves J (1997) Phenylhydrazine.
Sudbury, Suffolk, HSE Books (Risk Assessment Document EH72/1; ISBN
0 7176 1355 0)
The author's draft version is initially reviewed internally by a
group of approximately 10 Health and Safety Executive experts - mainly
toxicologists, but also experts in other relevant disciplines, such as
epidemiology and occupational hygiene. The toxicology section of the
amended draft is then reviewed by toxicologists from the United
Kingdom Department of Health. Subsequently, the entire Criteria
Document is reviewed by a tripartite advisory committee to the United
Kingdom Health and Safety Commission, the Working Group for the
Assessment of Toxic Chemicals (WATCH). This committee is composed of
experts in toxicology and occupational health and hygiene from
industry, trade unions, and academia.
The members of the WATCH committee at the time of the peer review
were Mr S.R. Bailey, Independent Consultant; Professor J. Bridges,
University of Surrey; Dr H. Cross, Trade Unions Congress; Dr A.
Fletcher, Trade Unions Congress; Dr I.G. Guest, Chemical Industries
Association; Dr A. Hay, Trade Unions Congress; Dr J. Leeser, Chemical
Industries Association; Dr L. Levy, Institute of Occupational Hygiene,
Birmingham; Mr A. Moses, Chemical Industries Association; Dr R. Owen,
Trade Unions Congress; Mr J. Sanderson, Independent Consultant; and Dr
M. Sharratt, University of Surrey.
BUA (1995) Phenylhydrazine. Beratergremium fur Umweltrelevante
Altstoffe (BUA). GDCh Advisory Committee on Existing Chemicals of
Environmental Relevance. Stuttgart, S. Hirzel, Wissenschaftliche
Verlagsgesellschaft (Report No. 120; ISBN 3-7776-0691-X)
For the BUA review process, the company that is in charge of
writing the report (usually the largest producer in Germany) prepares
a draft report using literature from an extensive literature search as
well as internal company studies. This draft is subject to a peer
review during several readings of a working group consisting of
representatives from government agencies, the scientific community,
and industry.
APPENDIX 2 - CICAD PEER REVIEW
The draft CICAD on phenylhydrazine was sent for review to
institutions and organizations identified by IPCS after contact with
IPCS national Contact Points and Participating Institutions, as well
as to identified experts. Comments were received from:
Department of Health, London, United Kingdom
Federal Institute for Health Protection of Consumers & Veterinary
Medicine, Berlin, Germany
Institut de Recherches en Santé et Sécurité du Travail du Québec,
Montreal, Canada
Institute of Occupational Medicine, Chinese Academy of Preventive
Medicine, Ministry of Health, Beijing, People's Republic of China
National Institute of Health Sciences, Tokyo, Japan
Senatskommission der Deutschen GSF-Forschungszentrum für Umwelt und
Gesundheit GmbH, Institut für Toxikologie, Oberscheissheim, Germany
United States Department of Health and Human Services (National
Institute for Occupational Safety and Health, Cincinnati; National
Institute of Environmental Health Sciences, Research Triangle Park;
Agency for Toxic Substances and Disease Registry, Atlanta), USA
United States Environmental Protection Agency (National Center for
Environmental Assessment, Washington, DC; Region VIII), USA
APPENDIX 3 - CICAD FINAL REVIEW BOARD
Washington, DC, USA, 8-11 December 1998
Members
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
( Vice-Chairperson)
Mr R. Cary, Toxicology Unit, Health Directorate, Health and Safety
Executive, Bootle, Merseyside, United Kingdom ( Rapporteur)
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr O. Faroon, Agency for Toxic Substances and Disease Registry,
Centers for Disease Control and Prevention, Atlanta, GA, USA
Dr G. Foureman, National Center for Environmental Assessment, US
Environmental Protection Agency, Research Triangle Park, NC, USA
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA ( Chairperson)
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany
Dr A. Nishikawa, Division of Pathology, National Institute of Health
Sciences, Tokyo, Japan
Dr E.V. Ohanian, Office of Water/Office of Science and Technology,
Health and Ecological Criteria Division, US Environmental Protection
Agency, Washington, DC, USA
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan
Professor P. Yao, Institute of Occupational Medicine, Chinese Academy
of Preventive Medicine, Ministry of Health, Beijing, People's Republic
of China
Observers
Dr K. Austin, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr I. Daly (ICCA representative), Regulatory and Technical Associates,
Lebanon, NJ, USA
Ms K.L. Lang (CEFIC, European Chemical Industry Council,
representative), Shell International, London, United Kingdom
Ms K. Roberts (ICCA representative), Chemical Self-funded Technical
Advocacy and Research (CHEMSTAR), Chemical Manufacturers Association,
Arlington, VA, USA
Dr W. Snellings (ICCA representative), Union Carbide Corporation,
Danbury, CN, USA
Dr M. Sweeney, Document Development Branch, National Institute for
Occupational Safety and Health, Cincinnati, OH, USA
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Secretariat
Dr M. Baril, Institut de Recherches en Santé et Sécurité du Travail du
Québec (IRSST), Montreal, Quebec, Canada
Dr H. Galal-Gorchev, Chevy Chase, MD, USA
Ms M. Godden, Health and Safety Executive, Bootle, Merseyside, United
Kingdom
Dr R.G. Liteplo, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Ms L. Regis, Programme for the Promotion of Chemical Safety, World
Health Organization, Geneva, Switzerland
Mr A. Strawson, Health and Safety Executive, London, United Kingdom
Dr P. Toft, Programme for the Promotion of Chemical Safety, World
Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
Ce CICAD relatif à la phénylhydrazine résulte d'une étude des
risques pour la santé humaine (principalement dans un cadre
professionnel) rédigée par le Health and Safety Executive du
Royaume-Uni (Brooke et al., 1997) et d'un rapport préparé par le
Comité consultatif allemand sur les substances chimiques importantes
pour l'environnement (BUA, 1995). Il est donc consacré aux divers
types d'exposition par les voies existant sur les lieux de travail,
mais contient également des données concernant l'environnement
général. Les données prises en compte dans ces deux documents de base
remontent respectivement à décembre 1993 et décembre 1994. Une étude
bibliographique complémentaire arrêtée à janvier 1998 a été effectuée
à la recherche de données supplémentaires qui auraient pu être
publiées postérieurement à ces documents. On trouvera à l'appendice 1
des indications sur le mode d'examen par des pairs ainsi que sur les
sources documentaires utilisées. Les renseignements concernant
l'examen du CICAD par les pairs font l'objet de l'appendice 2. Ce
CICAD a été approuvé en tant qu'évaluation internationale lors de la
réunion du Comité d'évaluation finale qui s'est tenue à Washington du
8 au 11 décembre 1998. La liste des participants à cette réunion
figure à l'appendice 3. La fiche d'information internationale sur la
sécurité chimique (ICSC No 0938) relative à la phénylhydrazine,
établie par le Programme international sur la sécurité chimique (IPCS,
1993), est également reproduite dans ce document.
La phénylhydrazine (CAS No 100-63-0) se présente sous la forme de
cristaux de couleur jaune à brun pâle ou d'un liquide jaunâtre de
consistance huileuse. Il est légèrement soluble dans l'eau et miscible
aux solvants organiques.
La phénylhydrazine est utilisée dans le monde entier comme
intermédiaire dans l'industrie chimique, pharmaceutique et
agrochimique.
On ignore combien de personnes sont susceptibles d'être exposées
à la phénylhydrazine ou à son chlorhydrate, mais elles sont
vraisemblablement un petit nombre. On ne dispose d'aucune donnée sur
l'exposition individuelle, mais le modèle EASE ( Estimation and
Assessment of Substance Exposure) permet de prédire une exposition
d'environ 2,3 mg/m3 (0,5 ppm) en moyenne pondérée par rapport au
temps sur 8 h. En pratique, l'exposition moyenne pondérée par rapport
au temps sur 8 h est inférieure à cette valeur.
Les données limitées dont on dispose au sujet de la
pharmacocinétique de la phénylhydrazine indiquent que ce composé est
bien absorbé après inhalation, ingestion ou par voie percutanée et
qu'il se combine facilement à l'hémoglobine des hématies. Il semble
que sa métabolisation implique une hydroxylation du cycle suivie
probablement de la formation d'un glucuro-conjugué. Il est
principalement éliminé par voie urinaire.
L'ingestion d'une seule dose peut provoquer une intoxication
(DL50 : 80-188 mg/kg de poids corporel) et la phénylhydrazine est sans
doute également toxique si elle est inhalée ou entre en contact avec
la peau (les données concernant ces deux voies d'exposition sont moins
précises). La phénylhydrazine pourrait être irritante pour la peau et
les yeux et on est fondé à penser qu'elle produit une sensibilisation
cutanée chez l'homme. L'exposition à ce composé peut endommager les
hématies, d'où un risque d'anémie et d'atteinte secondaire d'autres
tissus comme le tissu splénique et le tissu hépatique. La
phénylhydrazine est mutagène in vitro et selon certaines données,
elle pourrait également avoir une activité génotoxique in vivo. Elle
est de toute évidence cancérogène pour la souris (administration par
voie orale) et provoque des tumeurs vasculaires. On ne peut, à la
lumière de toutes ces données, indiquer le niveau d'exposition en
dessous duquel il n'y a pas de risque d'effets cancérogènes ou
génotoxiques.
On ne possède pas de données suffisantes concernant les effets
sur la reproduction ou le développement; il n'est donc pas possible de
dire si l'homme court des risques de cette nature.
Le degré de risque professionnel n'est pas connu avec certitude.
Il s'ensuit qu'il est toujours nécessaire de réduire le niveau
d'exposition au minimum raisonnable compte tenu de l'état actuel de la
technique.
Comme on ne dispose pas de données qui puissent servir à estimer
l'exposition individuelle à la phénylhydrazine présente dans
l'environnement général, on ne peut pas préciser quel risque de cancer
elle représente pour la population.
On n'envisage pas d'effets atmosphériques, étant donné que le
composé est essentiellement libéré dans l'eau et qu'une fois dans ce
milieu, il ne s'en évapore que très faiblement. En outre, le calcul
montre que sa demi-vie atmosphérique est très courte par suite de sa
combinaison avec les radicaux hydroxyles.
La phénylhydrazine subit une décomposition photochimique et
s'oxyde dans l'eau. Elle est facilement biodégradable et c'est
probablement ainsi qu'elle se décompose dans l'environnement. Sa
sorption par les matières particulaires est minime.
La phénylhydrazine est toxique pour les organismes aquatiques, la
concentration sans effet observable (NOEC) la plus faible qui ait été
indiquée lors d'épreuves classique de toxicité aiguë sur des poissons
étant de 0,01 mg/litre. Les poissons sont habituellement plus
sensibles que les daphnies ou les bactéries. On a fait état d'une NOEC
de 0,49 µg/litre pour les stades embryo-larvaires d'un poisson, le
danio ( Brachydanio rerio).
Le risque pour les organismes aquatiques devrait être faible,
même dans l'hypothèse la plus prudente.
RESUMEN DE ORIENTACION
El presente CICAD sobre la fenilhidrazina se basa en un examen de
problemas relativos a la salud humana (fundamentalmente ocupacionales)
preparado por el Health and Safety Executive del Reino Unido
(Brooke et al., 1997) y un informe preparado por el Comité Consultivo
Alemán sobre las Sustancias Químicas Importantes para el Medio
Ambiente (BUA, 1995). Por consiguiente, este informe se concentra en
la exposición a través de las vías de interés para los entornos
ocupacionales, pero también contiene información medioambiental.
Incluye los datos identificados a partir de diciembre de 1993 y
diciembre de 1994, respectivamente. Se realizó una nueva búsqueda en
lo publicado hasta enero de 1998 para localizar toda la información
aparecida desde la terminación de estos exámenes. La información
relativa al carácter del examen colegiado y a la disponibilidad de los
documentos originales figura en el apéndice 1. La información sobre el
examen colegiado de este CICAD aparece en el apéndice 2. Este CICAD se
aprobó como evaluación internacional en una reunión de la Junta de
Evaluación Final celebrada en Washington, DC (Estados Unidos de
América), del 8 a 11 de diciembre de 1998. En el apéndice 3 figura la
lista de los participantes en esta reunión. La ficha internacional de
seguridad química (ICSC No 0938) para la fenilhidrazina, preparada
por el Programa Internacional de Seguridad de las Sustancias Químicas
(IPCS, 1993), también se reproduce en el presente documento.
La fenilhidrazina (CAS No 100-63-0) se encuentra en forma de
cristales de un color entre amarillo y marrón claro o como líquido
oleoso amarillento. Es moderadamente soluble en el agua y es miscible
con otros disolventes orgánicos.
La fenilhidrazina se usa en todo el mundo, principalmente como
intermediario químico en las industrias farmacéutica, agroquímica y
química.
No se conoce el número de personas potencialmente expuestas a la
fenilhidrazina o sus hidrocloruros, pero se supone que es bajo. No hay
datos disponibles sobre la exposición personal, aunque el modelo EASE
( Estimation and Assessment of Substance Exposure) predecía (como
promedio ponderado en función del tiempo durante ocho horas) una
exposición de unos 2,3 mg/m3 (0,5 ppm). En la práctica, la exposición
promedio ponderada en función del tiempo durante ocho horas es
inferior a esa cifra.
Los limitados datos sobre la toxicocinética indican que la
fenilhidrazina se absorbe bien por inhalación y por vía oral y cutánea
y se une fácilmente a la hemoglobina en las glóbulos rojos. El
metabolismo parece que se produce por hidroxilación del anillo y
conjugación, probablemente con el ácido glucurónico. La excreción
tiene lugar fundamentalmente por vía urinaria.
La fenilhidrazina es tóxica en una exposición única por vía oral
(DL50 80-188 mg/kg de peso corporal) y es de prever que sea tóxica
por inhalación y por vía cutánea (los datos relativos a esas vías de
exposición son menos claros). Puede provocar irritación cutánea y
ocular y hay pruebas de que tiene propiedades de sensibilización
cutánea en el ser humano. La exposición a la fenilhidrazina puede
provocar daños en los glóbulos rojos, pudiendo producir anemia y en
consecuencia afectar de manera secundaria a otros tejidos, por ejemplo
los del bazo o del hígado. La fenilhidrazina es mutagénica in vitro
y hay algunos indicios de que puede mostrar actividad genotóxica
in vivo. Esta sustancia es claramente carcinogénica en ratones tras
la administración oral, induciendo la formación de tumores en el
sistema vascular. No está claro el mecanismo de formación de tumores,
pero no se puede descartarr un componente genotóxico. Por
consiguiente, no parece que sea posible identificar con seguridad un
nivel de exposición para el cual no haya riesgo de efectos
carcinogénicos o genotóxicos.
No se dispone de datos adecuados relativos a los efectos en la
reproducción o el desarrollo; no es posible, pues, evaluar el riesgo
para la salud humana de esos efectos finales.
El nivel de riesgo en el entorno ocupacional es incierto; en
consecuencia es constante la necesidad de reducir los niveles de
exposición todo lo que sea razonablemente posible con la tecnología
disponible en la actualidad.
La falta de datos disponibles que sirvan como base para la
estimación de la exposición indirecta de las personas a la
fenilhidrazina a partir del medio ambiente impide determinar los
riesgos potenciales de cáncer para la población general.
No son de prever efectos atmosféricos, debido a que la
fenilhidrazina se libera fundamentalmente en el agua, a su escasa
volatilización del agua a la atmósfera y a su rápida semivida en la
atmósfera calculada tras la reacción con los radicales hidroxilo.
La fenilhidrazina se degrada por vía fotoquímica y se autooxida
en el agua. Es fácilmente biodegradable y se supone que es ésta la
principal ruta de descomposición en el medio ambiente. La sorción en
partículas es mínima.
La fenilhidrazina es tóxica para los organismos acuáticos, siendo
de 0,01 mg/litro la concentración sin efectos observados (NOEC) más
baja notificada en pruebas normalizadas de toxicidad aguda en peces;
en general, los peces son más sensibles que los dáfnidos o las
bacterias. Se ha notificado una NOEC de 0,49 µg/litro para las fases
embriolarvarias del pez Brachydanio rerio.
Se supone que el riesgo para los organismos acuáticos es bajo, a
partir de hipótesis muy prudentes.
See Also:
Toxicological Abbreviations
Phenylhydrazine (ICSC)
 Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
1. EXECUTIVE SUMMARY
This CICAD on phenylhydrazine was based on a review of human
health concerns (primarily occupational) prepared by the United
Kingdom's Health and Safety Executive (Brooke et al., 1997) and a
report prepared for the German Advisory Committee on Existing
Chemicals of Environmental Relevance (BUA, 1995). Hence, this document
focuses on exposures via routes relevant to occupational settings but
also contains environmental information. Data identified as of
December 1993 and December 1994, respectively, were covered. A further
literature search was performed up to January 1998 to identify any
extra information published since these reviews were completed.
Information on the nature of the peer review and availability of the
source documents is presented in Appendix 1. Information on the peer
review of this CICAD is presented in Appendix 2. This CICAD was
approved as an international assessment at a meeting of the Final
Review Board, held in Washington, DC, USA, on 8-11 December 1998.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 0938) for
phenylhydrazine, produced by the International Programme on Chemical
Safety (IPCS, 1993), has also been reproduced in this document.
Phenylhydrazine (CAS No.
Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
1. EXECUTIVE SUMMARY
This CICAD on phenylhydrazine was based on a review of human
health concerns (primarily occupational) prepared by the United
Kingdom's Health and Safety Executive (Brooke et al., 1997) and a
report prepared for the German Advisory Committee on Existing
Chemicals of Environmental Relevance (BUA, 1995). Hence, this document
focuses on exposures via routes relevant to occupational settings but
also contains environmental information. Data identified as of
December 1993 and December 1994, respectively, were covered. A further
literature search was performed up to January 1998 to identify any
extra information published since these reviews were completed.
Information on the nature of the peer review and availability of the
source documents is presented in Appendix 1. Information on the peer
review of this CICAD is presented in Appendix 2. This CICAD was
approved as an international assessment at a meeting of the Final
Review Board, held in Washington, DC, USA, on 8-11 December 1998.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 0938) for
phenylhydrazine, produced by the International Programme on Chemical
Safety (IPCS, 1993), has also been reproduced in this document.
Phenylhydrazine (CAS No.  3. ANALYTICAL METHODS
For measurement of phenylhydrazine in water, reduction of Cu(II)
to Cu(I) by phenylhydrazine has been used as the basis for
spectrometric analytical methods measuring coloured complexes (Besada,
1988; Hasan, 1988). The methods are not specific and react to other
reducing substances. A detection limit of 10 µg/litre is given for one
method (Hasan, 1988).
In a method published by NIOSH (1994) for measurement of
phenylhydrazine in workplace air, the air is sampled into a midget
bubbler containing hydrochloric acid. Phosphomolybdic acid is added to
the resulting solution, and the reaction with phenylhydrazine causes
the formation of a bluish-green complex that can be measured at 730 nm
with a spectrophotometer. This method has a detection limit of about 5
mg/m3 (about 1 ppm), based on a 100-litre sample. Potential
interferences are listed as other hydrazine derivatives, aldehydes,
and ketones.
Both the MIRAN 1B and the Bruel and Kjaer 1302 Multigas Monitor
may be used to measure phenylhydrazine in air, with a detection limit
of around 13.5 mg/m3 (3 ppm) (Brooke et al., 1997). Any other
substance having similar infrared absorbances can be expected to
interfere with the measurement.
There are no published biological monitoring methods available
for phenylhydrazine.
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
There are a few reports of the natural occurrence of
phenylhydrazine in plants (BUA, 1995). Phenylhydrazine is produced
commercially by the diazotization of aniline followed by reduction of
the azo compound.
Production figures for 1990-1992 in Germany were about 3000-4000
t/year; use figures for Western Europe in 1988 totalled 6650 t (BUA,
1995). Use patterns for Western Europe in 1988 and for Germany in
1990-1992 are shown in Table 1.
Table 1: Phenylhydrazine use patterns in Western Europe
and Germany.
Phenylhydrazine use (%)
Industry Western Europe, Germany,
1988 1990-1992
Pharmaceuticals 37.6 70.2
Agrochemicals 42.9 7.2
Dyes 15 21.8
Others 4.5 0.8
From production and processing of phenylhydrazine in Germany
during 1990-1992, an estimated 50 kg and <13 t were emitted to the
atmosphere and the hydrosphere, respectively, each year. Less than
50 t of phenylhydrazinium chloride were released to water each year in
the same period (BUA, 1995).
There are now no manufacturers of phenylhydrazine or the
phenylhydrazine hydrochloride salt in the United Kingdom
(Brooke et al., 1997). Two firms are known to import phenylhydrazine
into the United Kingdom, one from its manufacturing site in Germany
and the other from Japan. The total market for phenylhydrazine in the
United Kingdom is thought to be about 20 t/year, whereas the market
for phenylhydrazine hydrochloride is not known. The market for
phenylhydrazine has been static for several years.
Phenylhydrazine is used worldwide mainly as a chemical
intermediate in the pharmaceutical, agrochemical, and chemical
industries. The United Kingdom's pattern of use appears
representative. One company uses phenylhydrazine to produce a chemical
intermediate for use in the photographic industry. Another
manufacturer uses the chemical in the synthesis of organic chemicals.
Phenylhydrazine is also used as a chemical intermediate in the
pharmaceutical and agrochemical industries in the United Kingdom. In
addition, there is some laboratory-scale use of this chemical.
There are no known consumer uses of phenylhydrazine or its
hydrochloride salt in the United Kingdom or Germany. No information is
available regarding the potential for consumer exposure in other
countries.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Most emissions of phenylhydrazine into the environment are into
the hydrosphere. At acidic pH, phenylhydrazine occurs as the salt
(BUA, 1995).
In the atmosphere, phenylhydrazine would exist solely in the
vapour phase (HSDB, 1998). Calculated half-lives of 3.1 h (BUA, 1995)
and 9 h (Meylan & Howard, 1993) have been reported for phenylhydrazine
following reaction with hydroxyl radicals in the atmosphere.
Phenylhydrazine strongly absorbs ultraviolet light in the
environmentally significant range, suggesting that it may photolyse in
sunlight (HSDB, 1998); slow photodecomposition in diffuse daylight in
the absence of oxygen is deduced in BUA (1995). In the presence of
oxygen, phenylhydrazine is subject to autoxidation, the reaction being
accelerated by light and heat; the substance becomes reddish brown on
exposure to air as a result of this autoxidation (Ullmann, 1977).
No hydrolysis is expected to occur (BUA, 1995).
The Henry's law constant for phenylhydrazine has been calculated
at 9.69 × 10-3 Pa.m3/mol (BUA, 1995). This is equivalent to a
dimensionless Henry's law constant (air/water partition coefficient)
of 3.92 × 10-6. These values indicate that phenylhydrazine is
essentially non-volatile from water surfaces.
Reported log octanol/water partition coefficients (log Kow)
range from 1.25 to 1.90 (BUA, 1995); an estimated bioconcentration
factor of 5 was based on the lower value (HSDB, 1998), indicating a
low capacity for bioaccumulation. However, sorption based on chemical
binding is possible, which could lead to some bioaccumulation (BUA,
1995). The sorption coefficient ( Koc) can be calculated to range
between 7.3 (Organisation for Economic Co-operation and Development
[OECD] Technical Guidance Manual) and 11 (Karickhoff et al., 1979),
indicating little sorption to particulates and a capacity for mobility
in soil. However, the regression equations on which these estimates
are based derive from hydrophobic compounds and may not adequately
reflect the likely sorption of the hydrophilic phenylhydrazine.
In a modified OECD ready biodegradability screening test (OECD
301E), phenylhydrazine was "readily biodegradable"; elimination was
77% after 10 days and 97% after 28 days using non-adapted inoculum.
Elimination through abiotic processes in controls was 11% after both
10 and 28 days (BASF, 1993). In the Zahn-Wellens test for inherent
biodegradability (OECD 302B), 20-30% elimination occurred over 3 h
(sorption), with 80% chemical oxygen demand achieved over 15 days
using non-acclimatized industrial activated sludge. Using acclimatized
activated sludge, 85% elimination was seen after 10 days (Hoechst,
1980, 1992). A similar value of 85% elimination in 9-13 days was
reported in the same test by Wellens (1990).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
Phenylhydrazine was not detected (detection limit 0.002 µg/ml
with high-performance liquid chromatography) in 30 samples of surface
water in the 1986 monitoring of the general environment by the Japan
Environment Agency (1987). It was also not detected in 30 samples of
sediment (detection limit 0.2 µg/kg with high-performance liquid
chromatography).
Monitoring of wastewater at the Hoechst production plant in
Germany failed to detect the compound in either inflow or outflow
wastewater (detection limit 500 µg/litre) (BUA, 1995).
6.2 Human exposure
The number of persons potentially exposed to phenylhydrazine or
its hydrochloride salt is not known, but it is expected to be small
(Brooke et al., 1997). Industry in the United Kingdom has not been
able to provide any personal exposure data, although it has been
indicated that exposure to airborne phenylhydrazine between 1993 and
1994 was controlled by process enclosure, the provision of local
exhaust ventilation, and personal protective equipment.
As there are no measured data available, the sections below
describe the use of computer-modelled exposure data from the EASE
model. This is a general-purpose predictive model for workplace
exposure assessments, which is used when measured exposure data are
limited or not available. In its present form, the model is in
widespread use across the European Union for the occupational exposure
assessment of new and existing substances.
Following descriptions of precautions taken during use, the most
appropriate parameters for the use of the EASE model are
non-dispersive use with local exhaust ventilation in place. Exposure
between 25 and 40°C with these assumptions is predicted to be within
the range 2.3-13.5 mg/m3 (0.5-3 ppm) (8-h time-weighted average).
Further, as only small quantities are involved, and as extensive
containment is provided by the combination of a vessel open only at
the bung hole and transfer being achieved by vacuum transfer, exposure
will be at the low end of this range (i.e., 2.3 mg/m3 [0.5 ppm] 8-h
time-weighted average). In practice, the 8-h time-weighted average
exposure will be less than this figure, as the activities involving
exposure to phenylhydrazine will take place for only part of the
shift.
These predicted exposures would be even lower for work carried
out in fume cupboards and would be extensively mitigated at the
operator by use of respiratory protective equipment; air-fed suits
would effectively reduce exposures of these magnitudes to zero.
For direct handling and non-dispersive use with a contact level
assumed to be incidental from the process descriptions, EASE predicts
dermal exposures to range from 0 to 0.1 mg/cm2 per day. If direct
handling is eliminated, dermal exposure is very low. Again, these
exposures would effectively be reduced to zero by adoption of
high-quality personal protective equipment and distancing procedures
described in this assessment.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
Phenylhydrazine reacts readily with the carbonyl group, -C=O,
which is common among biological molecules. It is therefore expected
that direct binding to biological molecules would occur.
There is only limited information available on the toxicokinetics
of phenylhydrazine. Evidence from toxicokinetic and toxicity studies
and from human experience indicates that phenylhydrazine is well
absorbed by the inhalation, oral, and dermal routes in animals and
humans.
Once absorbed, some phenylhydrazine appears to be rapidly taken
up by red blood cells, where destructive intracellular reactions may
occur.
Evidence from a number of studies in vitro and in vivo
suggests that phenylhydrazine interacts with haemoglobin and
cytochrome P-450 in an oxidation reaction, resulting in the generation
of destructive free radicals, which are responsible for subsequent
haemolysis (e.g., Itano et al., 1975; Valenzuela et al., 1977, 1981;
Goldberg et al., 1979; Jain & Hochstein, 1979; Jonen et al., 1982;
Hill, 1985; Marks, 1985; Di Cola et al., 1988, 1989; Maples et al.,
1988).
There is little information available on tissue distribution.
There is only one study available that investigates the
metabolism and excretion of phenylhydrazine, following oral dosing in
rabbits (McIsaac et al., 1958). This study shows that phenylhydrazine
is extensively metabolized following oral administration, although the
complete metabolic pathway has not been characterized. The main
reactions identified in this study were hydroxylation of the aromatic
ring to p-hydroxyphenylhydrazine, followed by conjugation, probably
with glucuronic acid, and production of phenylhydrazones, by reaction
with natural keto acids.
This study also indicated that the major route of excretion is
via the urine. A significant proportion of a single dose was excreted
relatively slowly; 50% of the dose was excreted within 4 days of
dosing. There are insufficient data to determine whether there is any
accumulation of phenylhydrazine in body tissues on repeated exposure.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
Many of the studies reported for phenylhydrazine have been
conducted using phenylhydrazine hydrochloride. This salt is a weak,
complex-forming compound, and either the salt or the free base will
form depending on the physiological medium, regardless of the form in
which the phenylhydrazine is administered (NIOSH, 1978). The
toxicological properties of the salt can therefore be considered to be
at least equivalent to those of free phenylhydrazine. Differences in
toxicity may arise when properties such as pH or solubility contribute
to the expression of toxicity. All dose values quoted throughout this
document refer to free phenylhydrazine.
8.1 Single exposure
In relation to inhalation exposure, there is only one very poorly
reported study, which reports LC50 values for an unstated exposure
period of 2745 mg/m3 (610 ppm) in the rat and 2093 mg/m3 (465 ppm)
in the mouse (Pham, 1979). However, it is expected that the marked
toxicity seen following oral and dermal exposure would also be
expressed following inhalation. Phenylhydrazine is toxic by oral
administration, and oral LD50 values in the range 80-188 mg/kg body
weight have been reported for the rat, mouse, guinea-pig, and rabbit
(Ekshtat, 1965; Pham, 1979). Clinical signs reported were motor
excitation and tonic/clonic spasms. In rabbits, dermal exposure to 380
mg phenylhydrazine/kg body weight for 24 h resulted in 20-30%
mortality, although no deaths occurred in rats at this dose
(Derelanko et al., 1987). The toxic effects are characterized by
destruction of red blood cells, causing a reduction in erythrocyte
count, increased reticulocyte count, methaemoglobin formation, and the
formation of Heinz bodies, and a cyanotic external appearance may
develop. Enlargement and dark coloration of the spleen are also
reported, effects that are considered to be secondary to the
erythrocyte damage.
8.2 Irritation and sensitization
In the single-dose dermal toxicity study in rabbits and rats
reported in the previous section, phenylhydrazine hydrochloride was
applied to the skin as a solid moistened with distilled water, under
either an occlusive or semi-occlusive dressing, for 24 h (Derelanko et
al., 1987). Skin irritation was seen in all rabbits, with some
necrosis at the treated site at 24 h post-application and sloughing of
the skin reported in some animals. Skin irritation, which appeared
within 24 h and persisted for up to 7 days post-exposure, was seen in
a high proportion of rats. Necrosis developed in a small number of
rats.
The quality of skin irritation data from other studies is
limited; overall, however, the results support the conclusion reached
by Derelanko et al. (1987) - that phenylhydrazine should be considered
a skin irritant (Jadassohn, 1930; von Oettingen & Deichmann-Gruebler,
1936; Roudabush et al., 1965; Schuckmann, 1969; Derelanko et al.,
1987). Further details of these studies can be obtained in the source
document (Brooke et al., 1997).
The only available information on the eye irritation potential of
phenylhydrazine in animals comes from a poorly described study in
which application of a 50% solution of phenylhydrazine to the eyes of
rabbits was reported to cause severe suppurative conjunctivitis (Pham,
1979).
There are no good-quality studies available in animals that
investigate the skin sensitization potential of phenylhydrazine. Only
one poorly described study in guinea-pigs is available (Jadassohn,
1930). When a 10% solution of phenylhydrazine in alcohol was painted
on a skin site that had been pretreated 2-3 weeks previously with
undiluted phenylhydrazine, very intense erythema and swelling,
followed by scaling and encrustation, were consistently seen. This
response to 10% phenylhydrazine was more severe than that described in
animals that had not been pretreated.
No information is available in relation to respiratory tract
sensitization.
8.3 Short-term exposure
There are only very limited, poor-quality data available in
relation to short-term repeated-dose toxicity. The effects seen are
similar to those seen following single exposure - in particular,
destruction of circulating red blood cells. Toxicity to the spleen,
liver, and kidney has also been observed in animal studies, possibly
secondary to haemolysis.
Kelly et al. (1969) administered phenylhydrazine hydrochloride to
21 mice by oral gavage once weekly for 8 weeks, at an estimated dose
of 85 mg/kg body weight per week. There were 10 saline-treated
controls. The study report described only tumour-related findings.
There was 30% mortality in treated mice compared with none in
controls.
Haematological changes were reported in four dogs administered 60
mg phenylhydrazine/kg body weight, either as a single dose or as 2, 3,
or 10 equal doses on consecutive days (Giffin & Allen, 1928). There
was a marked reduction in erythrocyte count that was comparable in
magnitude in all dogs at the end of 10 days, but that occurred at a
faster rate after a single high dose compared with repeated lower
doses.
In another dog study, 60 mg phenylhydrazine/kg body weight was
administered daily to three dogs for 5 days (Allen & Giffin, 1928).
One animal was moribund at sacrifice on the fifth day and one animal
died on the fifth day, although it is not specified that death was
treatment related. Full necropsy was performed on only one animal, in
which it was found that the blood was brown and did not coagulate
readily. Several organs, including the liver and kidneys, were darkly
coloured, and several organs contained capillaries engorged with
blood. Blood pigment and partially destroyed erythrocytes were found
in the spleen. Hepatic cell atrophy was noted, and there was an
increase in the iron content of the liver. Similar liver effects were
seen in the two other dogs.
Bolton (1935) briefly reported the effect of repeated oral
administration of 14 mg phenylhydrazine hydrochloride/kg body weight
to one dog on 4 consecutive days. There was a reduction in erythrocyte
count and haemoglobin concentration, whereas white cell count
gradually increased. These parameters had returned towards
pretreatment values 12 days after the last dose. Pathological findings
were reported to be non-conclusive, but no details were given.
Overall, the frequency, duration, and level of exposure often
varied throughout the studies reported above, so that interpretation
of results is very difficult. In all studies, phenylhydrazine as
phenylhydrazine hydrochloride was administered either by stomach tube
or by subcutaneous injection. The authors report that similar findings
were obtained regardless of route. There were no control animals. None
of the treated animals showed clinical signs of toxicity, and there
was no excessive weight loss or gain reported.
There have been a number of studies that investigate the effect
of short-term repeated parenteral administration of phenylhydrazine
(Bodansky, 1923; von Oettingen & Deichmann-Gruebler, 1936; Säterborg,
1974; Ades & Cascarano, 1979; Jain & Hochstein, 1979; Goldstein et
al., 1980; Nishida et al., 1982; Dornfest et al., 1986). These studies
confirm the ability of phenylhydrazine to damage red blood cells but
otherwise do not provide any other information in relation to the
toxicity of phenylhydrazine administered by occupationally relevant
routes of exposure.
8.4 Long-term exposure
8.4.1 Subchronic exposuren
In a very poorly reported study from which limited conclusions
can be drawn, rats, mice, guinea-pigs, and rabbits were exposed to
phenylhydrazine vapour at 0, 0.1, 15.8, 22.5, or 225 mg/m3 (0, 0.03,
3.5, 5, or 50 ppm) (Pham, 1979). Group sizes, duration of exposure,
and exposure regime were not given, although it can be inferred that
some animals were exposed for at least 6 months. Deaths were reported
to occur in animals exposed to 225 mg phenylhydrazine/m3 (species not
specified). Severe weight loss and unspecified haematological changes
and changes in central nervous system function were reported to
precede death, and there was evidence of haemolysis and dystrophic
changes in the liver, spleen, and cerebrum. Animals exposed to 15.8
and 22.5 mg/m3 were reported to have a reduction in erythrocyte count
and haemoglobin concentration, an increase in reticulocytes, and
methaemoglobinaemia; these changes were reversible at 15.8 mg/m3.
Haemolysis and dystrophic changes in the liver and other unspecified
organs were also reported for animals exposed to 22.5 mg/m3. No
further information is available on pathological changes at 0.1
mg/m3. It is not clear if there were lung effects at any of the
exposure concentrations.
It is not possible to draw firm conclusions from a poorly
reported study in three dogs in which the effect of phenylhydrazine
administration on renal and hepatic function and on erythropoiesis was
investigated (Allen & Giffin, 1928). Three dogs were administered
phenylhydrazine in 146 daily doses over a period of 8 months, to give
a total dose of 950 mg/kg body weight; the dosing regimen included a
period of about 60 days of uninterrupted administration of a single
dose level or of two dose levels (6-12 mg/kg body weight per day).
Again, the route of administration was unclear. Kidney function and
hepatic function were unaffected by treatment. Erythrocyte count was
reduced by treatment but recovered after cessation of treatment, at a
rate that was unrelated to the duration of exposure, thus indicating
that the administered dose had no effect on erythropoietic function.
Pathological examination was conducted on two of these dogs at 12 or
13 months. There was evidence of spleen toxicity, liver congestion,
and kidney damage.
In a very briefly reported study, phenylhydrazine was
administered to 25 female Swiss mice by oral gavage, 5 days/week for
40 weeks, at an estimated daily dose of 17-33 mg/kg body weight
(Roe et al., 1967). There were 85 untreated controls. Marked anaemia
necessitated a reduction in the dose during the sixth week of
treatment. No other toxic effects were observed.
8.4.2 Chronic exposure and carcinogenicity
Phenylhydrazine hydrochloride was administered daily by stomach
tube for 42 weeks to 30 BALB/c mice, at an estimated dose level of 25
mg phenylhydrazine/kg body weight (Clayson et al., 1966). Thirty
control animals were included in the study, but a control animal was
killed whenever a treated animal died, to match survival rates. There
was a statistically significant increase in the incidence of animals
with lung tumours in the treated group (53%) compared with controls
(13%). There was also a slight increase in the average number of
tumours per mouse, and the majority of treated mice had multiple
pulmonary tumours. Adenomas accounted for 83% of pulmonary tumours in
the treated group, half of which were judged to be becoming malignant,
and 17% of tumours were carcinomas.
Phenylhydrazine hydrochloride was administered in drinking-water
to 100 Swiss mice for their lifetime, at an estimated daily dose of 22
mg/kg body weight (Toth & Shimizu, 1976). There were 200 control mice.
Complete necropsy was performed on all animals. All organs were
examined macroscopically, and histological analysis was performed on a
wide range of tissues as well as on any organ showing gross pathology.
Phenylhydrazine was reported to decrease survival in comparison with
controls, and many of the treated decedents showed splenomegaly,
although numbers were not given. There was a statistically significant
increased incidence of blood vessel tumours (mainly angiosarcomas and
angiomas) in the liver of treated animals (21%) compared with
controls (0%).
8.5 Genotoxicity and related end-points
Phenylhydrazine and phenylhydrazine hydrochloride have been
investigated in a number of Ames tests, in a variety of strains, and
in the presence and absence of exogenous metabolic activation using up
to 1000 µg phenylhydrazine or phenylhydrazine hydrochloride per plate
(Shimizu et al., 1978; Tosk et al., 1979; De Flora, 1981; Parodi et
al., 1981; Levin et al., 1982; Malca-Mor & Stark, 1982; Rogan et al.,
1982; De Flora et al., 1984a,b; Wilcox et al., 1990; Muller et al.,
1993). The quality of these studies is generally high, and the studies
were apparently conducted according to standard methodology, although
detailed reporting of the results is not always available.
There is some variability in the findings, although positive
results have been obtained in Salmonella typhimurium strains TA97,
TA100, TA102, TA1537, and TA1538 in the absence of exogenous metabolic
activation. In addition, positive results were obtained in the
presence of metabolic activation in TA98 and TA1535. Some
investigators have reported that the mutagenic action is slightly
decreased by the presence of exogenous metabolic activation (Parodi et
al., 1981; Malca-Mor & Stark, 1982; De Flora et al., 1984a,b).
However, one study reports an increase in mutagenic activity in the
presence of metabolic activation (Rogan et al., 1982).
Phenylhydrazine has also given positive results in a number of
other, less well validated bacterial assays (using S. typhimurium
strains such as TA2638, TP138, BA9, and BA13), in the presence and
absence of exogenous metabolic activation (De Flora et al., 1984b;
Ulitzur et al., 1984; Ruiz-Rubio et al., 1985; Levi et al., 1986;
Muller et al., 1993).
Phenylhydrazine has not been tested in an in vitro chromosomal
aberration assay. In a brief abstract of a mammalian cell gene
mutation assay in V79 cells, with and without metabolic activation, a
positive result was reported for phenylhydrazine (Kuszynski et al.,
1981). However, no firm conclusions can be drawn from this report
because of deficiencies in the reporting.
In an unscheduled DNA synthesis assay in rat and mouse primary
hepatocytes, concentrations of 0.0144-144 mg phenylhydrazine
hydrochloride/litre were assessed (Mori et al., 1988). Although
toxicity was measured, no details were given, and quantitative data
were not reported. A positive result was obtained in both cell types,
although the effect was small.
Phenylhydrazine was tested in a micronucleus assay in vitro
using primary mouse bone marrow cells (Suzuki, 1985). Bone marrow
cells from the femur were exposed to 1-50 µg phenylhydrazine/ml for 30
min, in the presence and absence of metabolic activation. A total of
1500 polychromatic erythrocytes (PCEs) per concentration was scored
for the presence of micronuclei. There was no measure of cytotoxicity.
The percentage of micronucleated PCEs was statistically
significantly increased, in the presence of S9 only, at
phenylhydrazine concentrations of 5 µg/ml and greater in a
concentration-related manner.
BALB/c mice were administered a single intraperitoneal injection
of phenylhydrazine, and the incidence of micronucleated PCEs in the
bone marrow was measured at 24 and 48 h (Suzuki, 1985).
Phenylhydrazine was reported to be positive in this test, but no
details of the results or of the test were given. In view of the poor
reporting, no firm conclusions can be drawn from this study.
Groups of 11-12 female BALB/c mice were given a single
intraperitoneal injection of 50 mg phenylhydrazine/kg body weight in
saline (Steinheider et al., 1985). Blood smears of tail vein blood
were prepared at 24-h intervals for 7 or 11 days, and reticulocytes
and micronuclei in normochromatic erythrocytes (NCEs) and PCEs were
counted. It is not stated how many cells were counted per mouse. There
was no reporting of toxicity.
Phenylhydrazine caused a statistically significant increase in
the reticulocyte count on days 2-4 post-injection and in PCEs on day
3. There was a statistically significant increase in the incidence of
micronucleated PCEs at 24 h post-injection (from 1 to 4.7 per 1000)
and in micronucleated NCEs at 48 h post-injection (from 0.7 to 2.3 per
1000).
However, similar increases in micronucleated NCEs were also seen
following bleeding of the animals and splenectomy. The authors suggest
that the increase in micronuclei seen following phenylhydrazine
treatment was due at least partly to stimulation of erythropoiesis
because of the haemolysis induced by phenylhydrazine, thus leading to
more errors of nuclear expulsion; hence, the results do not
necessarily indicate a direct genotoxic action of phenylhydrazine.
Groups of 7-12 mice were given a single intraperitoneal injection
of either 85 or 170 mg phenylhydrazine/kg body weight and killed 1 and
6 h, respectively, after treatment (Parodi et al., 1981). In addition,
six mice were given a series of five daily intraperitoneal injections
of 7.6 mg phenylhydrazine/kg body weight and sacrificed 6 h after the
last injection. Control animals were injected with saline only. DNA
damage was assessed by measurement of the alkaline elution rate of
single-strand DNA from liver and lung tissue extracts.
A statistically significant change in the elution rate of liver
and lung DNA was seen in all groups of treated animals compared with
controls, except in the case of lung tissue DNA from mice given a
single dose of 85 mg phenylhydrazine/kg body weight. Phenylhydrazine
is considered to give a positive result in this assay for DNA damage.
The formation of DNA adducts ( N7-methylguanine and a trace of
O6-methylguanine) in the liver was demonstrated in rats receiving
65 mg phenylhydrazine/kg body weight by oral gavage (Mathison et al.,
1994). Other tissues were not examined.
8.6 Reproductive and developmental toxicity
Each of three dogs (one dog per dose group) received 20, 30, or
40 mg phenylhydrazine/kg body weight in saline by subcutaneous
injection on 2 consecutive days (Witchett, 1975). Two control animals
were not injected. At necropsy, performed on all three animals within
a few days of dosing, a "striking" reduction in spermatogenesis was
reported, with an absence of sperm in the epididymis. The validity of
this result is not clear, given the apparent extreme rapidity of the
effect.
Groups of 8-12 pregnant Wistar rats were given an intraperitoneal
injection of 7.5 mg phenylhydrazine/kg body weight as phenylhydrazine
hydrochloride on days 17, 18, and 19 of gestation; or 15 mg
phenylhydrazine/kg body weight as phenylhydrazine hydrochloride on
days 18 and 19 of gestation (Tamaki et al., 1974). Control animals
were not treated. There was no reporting of maternal toxicity or of
the effect of treatment on gestation or pup viability. Toxicity of the
pups was reported only insofar as there was incidence of jaundice
and/or anaemia among the offspring of treated animals. Twelve male
offspring with severe jaundice and anaemia, selected from treated
dams, and nine males from control dams were assessed at 9-22 weeks of
age for functional and behavioural status.
Although the authors reported that experimental animals showed
statistically significant differences from controls in some tests,
these findings are not considered to be reliable because of the small
numbers of animals used and the exclusion of a control animal from the
analysis. In addition, it is noted that only a brief part of the
gestation period was covered by the treatment regime (days 17-19); no
explanation is available for this choice of dosing regime.
Yamamura et al. (1973) reported in a brief abstract that
intraperitoneal injection of pregnant rats with 15 mg
phenylhydrazine/kg body weight as phenylhydrazine hydrochloride on
days 18 and 19 of gestation produced hyperbilirubinaemia (resulting
from haemolysis) in fetuses and newborns.
8.7 Immunological and neurological effects
No studies are available that specifically investigate the
immunological and neurological end-points, and there is no relevant
information from toxicity studies in animals.
9. EFFECTS ON HUMANS
In humans, no information is available in relation to single
exposure via the inhalation or oral routes, although effects similar
to those seen following dermal exposure would be expected to occur.
Systemic toxicity developed in humans after dermal exposure to liquid
phenylhydrazine, despite immediate attempts to reduce exposure by
removal of contaminated clothing and washing of skin (Schuckmann,
1969). Toxicity was manifest by damage to red blood cells, in one case
resulting in haemolytic jaundice. No such systemic effects were
reported in two cases of skin contamination with solid phenylhydrazine
hydrochloride.
In relation to skin irritation, information is available from
worker exposure data. There was no reporting of irritancy effects in
workers exposed to liquid phenylhydrazine following accidental
exposure, although systemic effects were seen (Schuckmann, 1969). Skin
irritation following contact with phenylhydrazine hydrochloride powder
was reported in two workers following accidental exposure. Local
irritation, superficial erythema, and partly bullous-papular changes
were noted in one case following spillage of powder on arms; multiple
burn marks and small blisters at the site of contact were reported in
the second case in which phenylhydrazine hydrochloride had spilled
into the worker's gloves and shoes. The author also refers to medical
records at the works that describe a number of cases of skin
irritation of differing severity due to phenylhydrazine hydrochloride,
but no details are given.
There are no data available on the eye irritation potential of
phenylhydrazine in humans.
There are a number of case reports of skin hypersensitivity
reactions to phenylhydrazine and its hydrochloride salt in humans.
Solomons (1946) conducted a patch test in one subject with a
phenylhydrazine crystal placed on the forearm under a dressing for an
unstated exposure period. Marked erythema and some oedema developed on
the exposure site after 18 h, with the formation of vesicles after 30
h and crusting after a further 24 h.
Similar hypersensitive skin reactions were reported following
individual exposures to solid or aqueous solutions of phenylhydrazine
or phenylhydrazine salts (Wright & Joyner, 1930; Frost & Hjorth, 1959;
Pevny & Peter, 1983).
There is also evidence that cross-sensitization can occur between
hydrazine compounds, so that subjects already sensitized to hydrazine,
a known skin sensitizer, are also sensitized to hydrazine derivatives,
including phenylhydrazine (Malten, 1962; Van Ketel, 1964; Hovding,
1967; Rothe, 1988).
No data are available on the potential of phenylhydrazine to
cause respiratory tract sensitization.
Earlier this century, phenylhydrazine and phenylhydrazine
hydrochloride were administered orally (usually around 100-200 mg/day)
for the treatment of blood disorders (e.g., Giffin & Allen, 1933). In
some cases, treatment was effective; in others, however, the outcome
was fatal (e.g., Giffin & Conner, 1929). The effects seen (beneficial
or otherwise) may have been related to the disease process and cannot
be attributed entirely to phenylhydrazine.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1 Aquatic environment
Results of acute toxicity tests on aquatic organisms are
summarized in Table 2. All concentrations are nominal.
In the early life stage test summarized in Table 2 (Xiu et al.,
1992), newly fertilized eggs of the zebra fish were exposed to
concentrations of phenylhydrazine through hatch and into the larval
stage (total exposure time was 16 days). A NOEC (survival) was
established for eggs at 5 days post-fertilization at 0.0039 mg/litre,
with a lowest-observed-effect concentration (LOEC) at 0.0078 mg/litre.
At the end of the test (16 days), the NOEC for larvae was 0.000 49
mg/litre, and the LOEC was 0.000 98 mg/litre. The study was conducted
according to a Swedish standard protocol (ss 028193).
In a study on the goldfish ( Carassius auratus), 40% of fish
died when exposed to phenylhydrazine at a nominal concentration of 1
mg/litre for 48 h. Signs of toxicity included erratic swimming,
sinking to the bottom of the test tank, and slowed and erratic
respiration. No gross lesions were found following dissection,
although all viscera showed focal haemorrhaging (Houston et al.,
1988).
Effects on blood cells and the haematopoietic system, comparable
to those found in mammals, were seen in fish injected with
phenylhydrazine. Chinook salmon ( Oncorhynchus tshawytscha) juveniles
were injected with 12.5 mg phenylhydrazine/kg body weight. Red cell
count, haemoglobin, and haematocrit fell to 1-5% of their normal
values within 10 days of treatment; a slight improvement was reported
15 days following injection, and values had returned to normal 95 days
after treatment (Smith et al., 1971).
10.2 Terrestrial environment
Phenylhydrazine at 21.6 mg/litre in a nutrient culture medium had
no effect on the growth of various soil fungi (Zsolnai, 1975).
A phenylhydrazine concentration of 50 mg/litre in a hydroponic
culture solution inhibited germination of Hordeum seeds for at least
6 days, whereas growth was stimulated in Lepidium. At 100 mg/litre,
seedling growth was stimulated in Hordeum but not in Lepidium. At
500 mg/litre, phenylhydrazine inhibited growth in seedlings of both
species, although the seedlings were still apparently "healthy"
(Bokorny, 1933).
Exposure of soil nematodes Caenorhabditis briggsae in culture
to phenylhydrazine at 50 mg/litre of medium resulted in reduced growth
of all four larval stages (the effect was most marked on the last
larval instar) and therefore delayed development to adults. However,
the adults formed were capable of reproduction, although the number of
progeny was reduced. A concentration of 15 mg/litre also delayed
development, although to a lesser degree compared with the higher
concentration (Kampfe et al., 1986a,b). A 6-day EC50 of 12 mg/litre
was reported for production of progeny in culture for the same
nematode (Kreil, 1982).
No dietary or oral toxicity studies have been performed on birds.
However, injection studies have shown haemolytic anaemia and reduced
white cell counts in birds. In contrast to mammals, cell division in
erythropoietic tissue is unaffected by phenylhydrazine (Williams,
1972; Clark et al., 1988; Datta et al., 1989, 1990).
Table 2: Acute toxicity of phenylhydrazine to aquatic organisms.
Organism End-point Concentration Reference
(mg/litre)
Bacteria
Photobacterium phosphoreum 30-min EC50 66.9 Kaiser et al.
(luminescence) (1987)
Facultative anaerobes 24-h toxic 60 Hoechst (1980)
(mixed culture) threshold
Escherichia coli minimum 109.3 Romero & Canada
inhibitory (1991)
concentration
Escherichia coli, minimum >3000 Zemek et al.
Micrococcus luteus, inhibitory (1978)
Bacillus licheniformis concentration
Invertebrates
water flea LC50 2-5 Hoechst (1980)
(Daphnia magna) (immobilization)
Fish
zebra fish 96-h LC50 0.16-0.25 Hoechst (1982)
(Brachydanio rerio) 96-h NOEC 0.1
zebra fish 5-day NOEC (eggs) 0.0039 Xiu et al.
(Brachydanio rerio) 16-day NOEC (larvae) 0.000 49 (1992)
Japanese killifish 48-h LC50 15.7 Tonogai et al.
(Oryzias latipes) (1982)
Table 2 (cont'd)
Organism End-point Concentration Reference
(mg/litre)
common carp 24-h LC100 1.0 Menzie (1979)a
(Cyprinus carpio) 96-h NOEC 0.1
bluegill 48-h LC50 0.1 Menzie (1979)a
(Lepomis 96-h NOEC 0.01
macrochirus)
a Menzie C (1979) Value taken from the DIMDI/ECDIN database. Test performed by the
United States Fish and Wildlife Service, Bureau of Sports, Fisheries and Wildlife,
Department of the Interior, Washington, DC [cited in BUA, 1995].
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Phenylhydrazine is toxic by single exposure via the oral route
(LD50 80-188 mg/kg body weight) and is expected to be toxic by the
inhalation and dermal routes (data from these routes of exposure are
less clear). Phenylhydrazine solution was severely irritating to
rabbit eyes; hence, it is reasonable to predict that it would have
significant eye irritation potential in humans.
Phenylhydrazine also has skin irritation potential, and there is
evidence from human case reports that it has skin sensitizing
properties. Exposure to phenylhydrazine may cause damage to red blood
cells, potentially resulting in anaemia and consequential secondary
involvement of other tissues, such as the spleen and liver. The dose
(exposure)-response characteristics for the induction of damage to the
red blood cells are poorly defined, and a no-effect level has not been
identified. Where phenylhydrazine has been used therapeutically in
humans, via the oral route, for the treatment of blood disorders,
daily doses of the order of 1.5-4 mg/kg body weight per day have
caused a reduction in the numbers of red blood cells; given the health
status of the individuals concerned, however, these data are of
limited use. Phenylhydrazine is mutagenic in vitro, and, although not
conclusive, there is some evidence to indicate that it may express
genotoxic activity in vivo. The substance is clearly carcinogenic in
mice following oral dosing, inducing tumours of the vascular system.
The mechanism for tumour formation is unclear, and, given the
genotoxic profile of phenylhydrazine, a genotoxic component cannot be
excluded. Carcinogenic potential in humans cannot be excluded given
the profile of genotoxicity and animal carcinogenicity, particularly
as other expressions of phenylhydrazine toxicity are common to a
number of species, including humans.
No conclusions can be drawn from the available information on
fertility or development.
11.1.2 Criteria for setting guidance values fon phenylhydrazine
There are no adequate data available regarding reproductive or
developmental effects; hence, it is not possible to evaluate the risk
to human health for these end-points.
The use pattern and physical/chemical characteristics of the
compound suggest that exposure of the general population would be
negligible.
Using United Kingdom workplace conditions as an example (section
6.2), exposure to phenylhydrazine vapour during most occupational
processes would result in a body burden of up to 0.33 mg/kg body
weight per day, assuming a 70-kg worker breathes 10 m3 of air in a
working day and that 100% phenylhydrazine is absorbed. There are no
data available from which to estimate the contribution to body burden
from dermal uptake, although this is expected to be negligible. A
threshold for the induction of red blood cell damage probably exists
but has not been identified, although daily oral doses calculated at
about 1.5 mg/kg body weight per day and above are associated with such
effects. Overall, at these levels of predicted inhalation exposure,
the risk of developing damage to the red blood cells is considered to
be low; if the levels were exceeded (e.g., of the order of a few ppm,
approximately 15-20 mg/m3), however, then this would be cause for
some concern.
On the basis that the carcinogenicity of phenylhydrazine may
involve a genotoxic mechanism, it is not possible to reliably identify
a threshold below which occupational exposure to phenylhydrazine would
not result in some risk to human health.
11.1.3 Sample risk characterization
The scenario chosen as an example is occupational exposure in the
United Kingdom.
The main health concerns associated with exposure to
phenylhydrazine are damage to the red blood cells, deleterious effects
on genetic material, and the development of cancer.
It is recognized that there are a number of different approaches
to assessing the risks to human health for genotoxic and carcinogenic
substances and in the subsequent risk management steps that may be
taken. In addition, although not used in the United Kingdom, there are
models for characterizing potency that may be of some benefit in
priority-setting schemes. In the United Kingdom occupational setting,
a Maximum Exposure Limit or MEL (which is not a health-based standard)
has been proposed at 0.9 mg/m3 (0.2 ppm), 8-h time-weighted average.
The numerical value for the MEL was based on a level of control that
was deemed (by tripartite agreement) to be reasonably practicable
under United Kingdom workplace conditions, and in the United Kingdom
there is a continuing requirement to reduce exposure levels as far as
reasonably practicable with the technology that is currently
available.
Phenylhydrazine also possesses skin and eye irritant properties
and possibly skin sensitizing potential. The information available
indicates that local exposure of these tissues is unlikely; if it did
occur, however, then there would be risk of irritation to the eyes and
the development of irritant and/or allergic dermatitis.
11.2 Evaluation of environmental effects
No atmospheric effects are expected given the release of
phenylhydrazine predominantly to water, its extremely low
volatilization from water to the atmosphere, and its rapid calculated
atmospheric half-life following reaction with hydroxyl radicals.
Few toxicity studies are available for terrestrial organisms, and
little emission to land is expected; on this basis, no quantitative
risk assessment can be attempted for the terrestrial environment.
Phenylhydrazine is degraded photochemically and autoxidizes in
water. It is readily biodegradable, and this is expected to be the
major route of breakdown in the environment. There is minimal sorption
to particulates.
Phenylhydrazine is toxic to aquatic organisms, with the lowest
reported NOEC in acute fish tests at 0.01 mg/litre; fish are generally
more sensitive than either daphnids or bacteria. A NOEC for
embryo-larval stages following 16 days of exposure from fertilization
has been reported at 0.000 49 mg/litre for the zebra fish.
There are no reported measurements of phenylhydrazine in
environmental media. Monitoring studies of both inflow and outflow to
the wastewater treatment plant of the Hoechst Hochst production plant
in Germany showed no detectable phenylhydrazine (detection limit 500
µg/litre) in weekly samples. Maximum emission to wastewater was
estimated at 13 t/year, and this will be used as a worst-case example.
Based on this emission rate, and using mainly default values from
the OECD Technical Guidance Manual, the initial predicted
environmental concentration of phenylhydrazine in river water
(PEClocal (water), in g/litre) would be as follows:
Ceffluent
PEClocal (water) =
(1 + Kp(susp) × Csusp) × D
where:
* Ceffluent is the concentration of phenylhydrazine in the
wastewater treatment plant effluent (g/litre), calculated as
Ceffluent = W × (100 - P)/(100 × Q), where:
W = emission rate (35.6 kg/day)
P = percent removal in the wastewater treatment plant
(based on the "ready biodegradability" of the
compound, 91%)
Q = volume of wastewater in m3/day (default 200
litre/day per capita for a population of 10 000
inhabitants; wastewater volume for the production
plant is unknown)
* Kp(susp) is the suspended matter/water adsorption coefficient,
calculated as Kp(susp) = Koc × foc(susp), where:
Koc = organic carbon/water partition
coefficient (7.3)
foc(susp) = fraction of organic carbon in suspended matter
(default 0.1)
* Csusp is the concentration of suspended matter in the river
water (in kg/litre; default 15 mg/litre)
* D is the dilution factor for river flow (flow rate for the
River Main averages 188 m3/s compared with the estimated flow
rate of the wastewater at 0.02 m3/s; dilution factor
approximately 10 000)
Under these very conservative conditions, PEClocal (water)
= 0.16 µg/litre.
Of the reported acute toxicity test results for organisms in the
environment, those for the majority of fish tested are substantially
lower than those for other organisms tested. The predicted no-effect
concentration (PNEC) will therefore be based on the fish results.
No long-term test results are available. Applying an uncertainty
factor of 1000 to the lowest reported standard acute LC50 value of
0.1 mg/litre for the bluegill ( Lepomis macrochirus) would give a
PNEC of 0.1 µg/litre. This is a factor of 100 lower than the lowest
reported NOEC for the same species. Alternatively, applying an
uncertainty factor of 10 to the NOEC for the early life stage test on
the zebra fish larvae gives a PNEC of 0.049 µg/litre. The more
conservative value will be used in estimating risk.
The low PEC of 0.16 µg/litre would not have been detected in the
monitoring at the site. Assuming that this value is the worst case, a
PEC/PNEC ratio of 3.2 is generated. This indicates that the risk to
aquatic organisms is low, based on very conservative assumptions. The
distribution of reported toxicity test results against the worst-case
PEC is plotted in Figure 1, illustrating the safety margin.
3. ANALYTICAL METHODS
For measurement of phenylhydrazine in water, reduction of Cu(II)
to Cu(I) by phenylhydrazine has been used as the basis for
spectrometric analytical methods measuring coloured complexes (Besada,
1988; Hasan, 1988). The methods are not specific and react to other
reducing substances. A detection limit of 10 µg/litre is given for one
method (Hasan, 1988).
In a method published by NIOSH (1994) for measurement of
phenylhydrazine in workplace air, the air is sampled into a midget
bubbler containing hydrochloric acid. Phosphomolybdic acid is added to
the resulting solution, and the reaction with phenylhydrazine causes
the formation of a bluish-green complex that can be measured at 730 nm
with a spectrophotometer. This method has a detection limit of about 5
mg/m3 (about 1 ppm), based on a 100-litre sample. Potential
interferences are listed as other hydrazine derivatives, aldehydes,
and ketones.
Both the MIRAN 1B and the Bruel and Kjaer 1302 Multigas Monitor
may be used to measure phenylhydrazine in air, with a detection limit
of around 13.5 mg/m3 (3 ppm) (Brooke et al., 1997). Any other
substance having similar infrared absorbances can be expected to
interfere with the measurement.
There are no published biological monitoring methods available
for phenylhydrazine.
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
There are a few reports of the natural occurrence of
phenylhydrazine in plants (BUA, 1995). Phenylhydrazine is produced
commercially by the diazotization of aniline followed by reduction of
the azo compound.
Production figures for 1990-1992 in Germany were about 3000-4000
t/year; use figures for Western Europe in 1988 totalled 6650 t (BUA,
1995). Use patterns for Western Europe in 1988 and for Germany in
1990-1992 are shown in Table 1.
Table 1: Phenylhydrazine use patterns in Western Europe
and Germany.
Phenylhydrazine use (%)
Industry Western Europe, Germany,
1988 1990-1992
Pharmaceuticals 37.6 70.2
Agrochemicals 42.9 7.2
Dyes 15 21.8
Others 4.5 0.8
From production and processing of phenylhydrazine in Germany
during 1990-1992, an estimated 50 kg and <13 t were emitted to the
atmosphere and the hydrosphere, respectively, each year. Less than
50 t of phenylhydrazinium chloride were released to water each year in
the same period (BUA, 1995).
There are now no manufacturers of phenylhydrazine or the
phenylhydrazine hydrochloride salt in the United Kingdom
(Brooke et al., 1997). Two firms are known to import phenylhydrazine
into the United Kingdom, one from its manufacturing site in Germany
and the other from Japan. The total market for phenylhydrazine in the
United Kingdom is thought to be about 20 t/year, whereas the market
for phenylhydrazine hydrochloride is not known. The market for
phenylhydrazine has been static for several years.
Phenylhydrazine is used worldwide mainly as a chemical
intermediate in the pharmaceutical, agrochemical, and chemical
industries. The United Kingdom's pattern of use appears
representative. One company uses phenylhydrazine to produce a chemical
intermediate for use in the photographic industry. Another
manufacturer uses the chemical in the synthesis of organic chemicals.
Phenylhydrazine is also used as a chemical intermediate in the
pharmaceutical and agrochemical industries in the United Kingdom. In
addition, there is some laboratory-scale use of this chemical.
There are no known consumer uses of phenylhydrazine or its
hydrochloride salt in the United Kingdom or Germany. No information is
available regarding the potential for consumer exposure in other
countries.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Most emissions of phenylhydrazine into the environment are into
the hydrosphere. At acidic pH, phenylhydrazine occurs as the salt
(BUA, 1995).
In the atmosphere, phenylhydrazine would exist solely in the
vapour phase (HSDB, 1998). Calculated half-lives of 3.1 h (BUA, 1995)
and 9 h (Meylan & Howard, 1993) have been reported for phenylhydrazine
following reaction with hydroxyl radicals in the atmosphere.
Phenylhydrazine strongly absorbs ultraviolet light in the
environmentally significant range, suggesting that it may photolyse in
sunlight (HSDB, 1998); slow photodecomposition in diffuse daylight in
the absence of oxygen is deduced in BUA (1995). In the presence of
oxygen, phenylhydrazine is subject to autoxidation, the reaction being
accelerated by light and heat; the substance becomes reddish brown on
exposure to air as a result of this autoxidation (Ullmann, 1977).
No hydrolysis is expected to occur (BUA, 1995).
The Henry's law constant for phenylhydrazine has been calculated
at 9.69 × 10-3 Pa.m3/mol (BUA, 1995). This is equivalent to a
dimensionless Henry's law constant (air/water partition coefficient)
of 3.92 × 10-6. These values indicate that phenylhydrazine is
essentially non-volatile from water surfaces.
Reported log octanol/water partition coefficients (log Kow)
range from 1.25 to 1.90 (BUA, 1995); an estimated bioconcentration
factor of 5 was based on the lower value (HSDB, 1998), indicating a
low capacity for bioaccumulation. However, sorption based on chemical
binding is possible, which could lead to some bioaccumulation (BUA,
1995). The sorption coefficient ( Koc) can be calculated to range
between 7.3 (Organisation for Economic Co-operation and Development
[OECD] Technical Guidance Manual) and 11 (Karickhoff et al., 1979),
indicating little sorption to particulates and a capacity for mobility
in soil. However, the regression equations on which these estimates
are based derive from hydrophobic compounds and may not adequately
reflect the likely sorption of the hydrophilic phenylhydrazine.
In a modified OECD ready biodegradability screening test (OECD
301E), phenylhydrazine was "readily biodegradable"; elimination was
77% after 10 days and 97% after 28 days using non-adapted inoculum.
Elimination through abiotic processes in controls was 11% after both
10 and 28 days (BASF, 1993). In the Zahn-Wellens test for inherent
biodegradability (OECD 302B), 20-30% elimination occurred over 3 h
(sorption), with 80% chemical oxygen demand achieved over 15 days
using non-acclimatized industrial activated sludge. Using acclimatized
activated sludge, 85% elimination was seen after 10 days (Hoechst,
1980, 1992). A similar value of 85% elimination in 9-13 days was
reported in the same test by Wellens (1990).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
Phenylhydrazine was not detected (detection limit 0.002 µg/ml
with high-performance liquid chromatography) in 30 samples of surface
water in the 1986 monitoring of the general environment by the Japan
Environment Agency (1987). It was also not detected in 30 samples of
sediment (detection limit 0.2 µg/kg with high-performance liquid
chromatography).
Monitoring of wastewater at the Hoechst production plant in
Germany failed to detect the compound in either inflow or outflow
wastewater (detection limit 500 µg/litre) (BUA, 1995).
6.2 Human exposure
The number of persons potentially exposed to phenylhydrazine or
its hydrochloride salt is not known, but it is expected to be small
(Brooke et al., 1997). Industry in the United Kingdom has not been
able to provide any personal exposure data, although it has been
indicated that exposure to airborne phenylhydrazine between 1993 and
1994 was controlled by process enclosure, the provision of local
exhaust ventilation, and personal protective equipment.
As there are no measured data available, the sections below
describe the use of computer-modelled exposure data from the EASE
model. This is a general-purpose predictive model for workplace
exposure assessments, which is used when measured exposure data are
limited or not available. In its present form, the model is in
widespread use across the European Union for the occupational exposure
assessment of new and existing substances.
Following descriptions of precautions taken during use, the most
appropriate parameters for the use of the EASE model are
non-dispersive use with local exhaust ventilation in place. Exposure
between 25 and 40°C with these assumptions is predicted to be within
the range 2.3-13.5 mg/m3 (0.5-3 ppm) (8-h time-weighted average).
Further, as only small quantities are involved, and as extensive
containment is provided by the combination of a vessel open only at
the bung hole and transfer being achieved by vacuum transfer, exposure
will be at the low end of this range (i.e., 2.3 mg/m3 [0.5 ppm] 8-h
time-weighted average). In practice, the 8-h time-weighted average
exposure will be less than this figure, as the activities involving
exposure to phenylhydrazine will take place for only part of the
shift.
These predicted exposures would be even lower for work carried
out in fume cupboards and would be extensively mitigated at the
operator by use of respiratory protective equipment; air-fed suits
would effectively reduce exposures of these magnitudes to zero.
For direct handling and non-dispersive use with a contact level
assumed to be incidental from the process descriptions, EASE predicts
dermal exposures to range from 0 to 0.1 mg/cm2 per day. If direct
handling is eliminated, dermal exposure is very low. Again, these
exposures would effectively be reduced to zero by adoption of
high-quality personal protective equipment and distancing procedures
described in this assessment.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
Phenylhydrazine reacts readily with the carbonyl group, -C=O,
which is common among biological molecules. It is therefore expected
that direct binding to biological molecules would occur.
There is only limited information available on the toxicokinetics
of phenylhydrazine. Evidence from toxicokinetic and toxicity studies
and from human experience indicates that phenylhydrazine is well
absorbed by the inhalation, oral, and dermal routes in animals and
humans.
Once absorbed, some phenylhydrazine appears to be rapidly taken
up by red blood cells, where destructive intracellular reactions may
occur.
Evidence from a number of studies in vitro and in vivo
suggests that phenylhydrazine interacts with haemoglobin and
cytochrome P-450 in an oxidation reaction, resulting in the generation
of destructive free radicals, which are responsible for subsequent
haemolysis (e.g., Itano et al., 1975; Valenzuela et al., 1977, 1981;
Goldberg et al., 1979; Jain & Hochstein, 1979; Jonen et al., 1982;
Hill, 1985; Marks, 1985; Di Cola et al., 1988, 1989; Maples et al.,
1988).
There is little information available on tissue distribution.
There is only one study available that investigates the
metabolism and excretion of phenylhydrazine, following oral dosing in
rabbits (McIsaac et al., 1958). This study shows that phenylhydrazine
is extensively metabolized following oral administration, although the
complete metabolic pathway has not been characterized. The main
reactions identified in this study were hydroxylation of the aromatic
ring to p-hydroxyphenylhydrazine, followed by conjugation, probably
with glucuronic acid, and production of phenylhydrazones, by reaction
with natural keto acids.
This study also indicated that the major route of excretion is
via the urine. A significant proportion of a single dose was excreted
relatively slowly; 50% of the dose was excreted within 4 days of
dosing. There are insufficient data to determine whether there is any
accumulation of phenylhydrazine in body tissues on repeated exposure.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
Many of the studies reported for phenylhydrazine have been
conducted using phenylhydrazine hydrochloride. This salt is a weak,
complex-forming compound, and either the salt or the free base will
form depending on the physiological medium, regardless of the form in
which the phenylhydrazine is administered (NIOSH, 1978). The
toxicological properties of the salt can therefore be considered to be
at least equivalent to those of free phenylhydrazine. Differences in
toxicity may arise when properties such as pH or solubility contribute
to the expression of toxicity. All dose values quoted throughout this
document refer to free phenylhydrazine.
8.1 Single exposure
In relation to inhalation exposure, there is only one very poorly
reported study, which reports LC50 values for an unstated exposure
period of 2745 mg/m3 (610 ppm) in the rat and 2093 mg/m3 (465 ppm)
in the mouse (Pham, 1979). However, it is expected that the marked
toxicity seen following oral and dermal exposure would also be
expressed following inhalation. Phenylhydrazine is toxic by oral
administration, and oral LD50 values in the range 80-188 mg/kg body
weight have been reported for the rat, mouse, guinea-pig, and rabbit
(Ekshtat, 1965; Pham, 1979). Clinical signs reported were motor
excitation and tonic/clonic spasms. In rabbits, dermal exposure to 380
mg phenylhydrazine/kg body weight for 24 h resulted in 20-30%
mortality, although no deaths occurred in rats at this dose
(Derelanko et al., 1987). The toxic effects are characterized by
destruction of red blood cells, causing a reduction in erythrocyte
count, increased reticulocyte count, methaemoglobin formation, and the
formation of Heinz bodies, and a cyanotic external appearance may
develop. Enlargement and dark coloration of the spleen are also
reported, effects that are considered to be secondary to the
erythrocyte damage.
8.2 Irritation and sensitization
In the single-dose dermal toxicity study in rabbits and rats
reported in the previous section, phenylhydrazine hydrochloride was
applied to the skin as a solid moistened with distilled water, under
either an occlusive or semi-occlusive dressing, for 24 h (Derelanko et
al., 1987). Skin irritation was seen in all rabbits, with some
necrosis at the treated site at 24 h post-application and sloughing of
the skin reported in some animals. Skin irritation, which appeared
within 24 h and persisted for up to 7 days post-exposure, was seen in
a high proportion of rats. Necrosis developed in a small number of
rats.
The quality of skin irritation data from other studies is
limited; overall, however, the results support the conclusion reached
by Derelanko et al. (1987) - that phenylhydrazine should be considered
a skin irritant (Jadassohn, 1930; von Oettingen & Deichmann-Gruebler,
1936; Roudabush et al., 1965; Schuckmann, 1969; Derelanko et al.,
1987). Further details of these studies can be obtained in the source
document (Brooke et al., 1997).
The only available information on the eye irritation potential of
phenylhydrazine in animals comes from a poorly described study in
which application of a 50% solution of phenylhydrazine to the eyes of
rabbits was reported to cause severe suppurative conjunctivitis (Pham,
1979).
There are no good-quality studies available in animals that
investigate the skin sensitization potential of phenylhydrazine. Only
one poorly described study in guinea-pigs is available (Jadassohn,
1930). When a 10% solution of phenylhydrazine in alcohol was painted
on a skin site that had been pretreated 2-3 weeks previously with
undiluted phenylhydrazine, very intense erythema and swelling,
followed by scaling and encrustation, were consistently seen. This
response to 10% phenylhydrazine was more severe than that described in
animals that had not been pretreated.
No information is available in relation to respiratory tract
sensitization.
8.3 Short-term exposure
There are only very limited, poor-quality data available in
relation to short-term repeated-dose toxicity. The effects seen are
similar to those seen following single exposure - in particular,
destruction of circulating red blood cells. Toxicity to the spleen,
liver, and kidney has also been observed in animal studies, possibly
secondary to haemolysis.
Kelly et al. (1969) administered phenylhydrazine hydrochloride to
21 mice by oral gavage once weekly for 8 weeks, at an estimated dose
of 85 mg/kg body weight per week. There were 10 saline-treated
controls. The study report described only tumour-related findings.
There was 30% mortality in treated mice compared with none in
controls.
Haematological changes were reported in four dogs administered 60
mg phenylhydrazine/kg body weight, either as a single dose or as 2, 3,
or 10 equal doses on consecutive days (Giffin & Allen, 1928). There
was a marked reduction in erythrocyte count that was comparable in
magnitude in all dogs at the end of 10 days, but that occurred at a
faster rate after a single high dose compared with repeated lower
doses.
In another dog study, 60 mg phenylhydrazine/kg body weight was
administered daily to three dogs for 5 days (Allen & Giffin, 1928).
One animal was moribund at sacrifice on the fifth day and one animal
died on the fifth day, although it is not specified that death was
treatment related. Full necropsy was performed on only one animal, in
which it was found that the blood was brown and did not coagulate
readily. Several organs, including the liver and kidneys, were darkly
coloured, and several organs contained capillaries engorged with
blood. Blood pigment and partially destroyed erythrocytes were found
in the spleen. Hepatic cell atrophy was noted, and there was an
increase in the iron content of the liver. Similar liver effects were
seen in the two other dogs.
Bolton (1935) briefly reported the effect of repeated oral
administration of 14 mg phenylhydrazine hydrochloride/kg body weight
to one dog on 4 consecutive days. There was a reduction in erythrocyte
count and haemoglobin concentration, whereas white cell count
gradually increased. These parameters had returned towards
pretreatment values 12 days after the last dose. Pathological findings
were reported to be non-conclusive, but no details were given.
Overall, the frequency, duration, and level of exposure often
varied throughout the studies reported above, so that interpretation
of results is very difficult. In all studies, phenylhydrazine as
phenylhydrazine hydrochloride was administered either by stomach tube
or by subcutaneous injection. The authors report that similar findings
were obtained regardless of route. There were no control animals. None
of the treated animals showed clinical signs of toxicity, and there
was no excessive weight loss or gain reported.
There have been a number of studies that investigate the effect
of short-term repeated parenteral administration of phenylhydrazine
(Bodansky, 1923; von Oettingen & Deichmann-Gruebler, 1936; Säterborg,
1974; Ades & Cascarano, 1979; Jain & Hochstein, 1979; Goldstein et
al., 1980; Nishida et al., 1982; Dornfest et al., 1986). These studies
confirm the ability of phenylhydrazine to damage red blood cells but
otherwise do not provide any other information in relation to the
toxicity of phenylhydrazine administered by occupationally relevant
routes of exposure.
8.4 Long-term exposure
8.4.1 Subchronic exposuren
In a very poorly reported study from which limited conclusions
can be drawn, rats, mice, guinea-pigs, and rabbits were exposed to
phenylhydrazine vapour at 0, 0.1, 15.8, 22.5, or 225 mg/m3 (0, 0.03,
3.5, 5, or 50 ppm) (Pham, 1979). Group sizes, duration of exposure,
and exposure regime were not given, although it can be inferred that
some animals were exposed for at least 6 months. Deaths were reported
to occur in animals exposed to 225 mg phenylhydrazine/m3 (species not
specified). Severe weight loss and unspecified haematological changes
and changes in central nervous system function were reported to
precede death, and there was evidence of haemolysis and dystrophic
changes in the liver, spleen, and cerebrum. Animals exposed to 15.8
and 22.5 mg/m3 were reported to have a reduction in erythrocyte count
and haemoglobin concentration, an increase in reticulocytes, and
methaemoglobinaemia; these changes were reversible at 15.8 mg/m3.
Haemolysis and dystrophic changes in the liver and other unspecified
organs were also reported for animals exposed to 22.5 mg/m3. No
further information is available on pathological changes at 0.1
mg/m3. It is not clear if there were lung effects at any of the
exposure concentrations.
It is not possible to draw firm conclusions from a poorly
reported study in three dogs in which the effect of phenylhydrazine
administration on renal and hepatic function and on erythropoiesis was
investigated (Allen & Giffin, 1928). Three dogs were administered
phenylhydrazine in 146 daily doses over a period of 8 months, to give
a total dose of 950 mg/kg body weight; the dosing regimen included a
period of about 60 days of uninterrupted administration of a single
dose level or of two dose levels (6-12 mg/kg body weight per day).
Again, the route of administration was unclear. Kidney function and
hepatic function were unaffected by treatment. Erythrocyte count was
reduced by treatment but recovered after cessation of treatment, at a
rate that was unrelated to the duration of exposure, thus indicating
that the administered dose had no effect on erythropoietic function.
Pathological examination was conducted on two of these dogs at 12 or
13 months. There was evidence of spleen toxicity, liver congestion,
and kidney damage.
In a very briefly reported study, phenylhydrazine was
administered to 25 female Swiss mice by oral gavage, 5 days/week for
40 weeks, at an estimated daily dose of 17-33 mg/kg body weight
(Roe et al., 1967). There were 85 untreated controls. Marked anaemia
necessitated a reduction in the dose during the sixth week of
treatment. No other toxic effects were observed.
8.4.2 Chronic exposure and carcinogenicity
Phenylhydrazine hydrochloride was administered daily by stomach
tube for 42 weeks to 30 BALB/c mice, at an estimated dose level of 25
mg phenylhydrazine/kg body weight (Clayson et al., 1966). Thirty
control animals were included in the study, but a control animal was
killed whenever a treated animal died, to match survival rates. There
was a statistically significant increase in the incidence of animals
with lung tumours in the treated group (53%) compared with controls
(13%). There was also a slight increase in the average number of
tumours per mouse, and the majority of treated mice had multiple
pulmonary tumours. Adenomas accounted for 83% of pulmonary tumours in
the treated group, half of which were judged to be becoming malignant,
and 17% of tumours were carcinomas.
Phenylhydrazine hydrochloride was administered in drinking-water
to 100 Swiss mice for their lifetime, at an estimated daily dose of 22
mg/kg body weight (Toth & Shimizu, 1976). There were 200 control mice.
Complete necropsy was performed on all animals. All organs were
examined macroscopically, and histological analysis was performed on a
wide range of tissues as well as on any organ showing gross pathology.
Phenylhydrazine was reported to decrease survival in comparison with
controls, and many of the treated decedents showed splenomegaly,
although numbers were not given. There was a statistically significant
increased incidence of blood vessel tumours (mainly angiosarcomas and
angiomas) in the liver of treated animals (21%) compared with
controls (0%).
8.5 Genotoxicity and related end-points
Phenylhydrazine and phenylhydrazine hydrochloride have been
investigated in a number of Ames tests, in a variety of strains, and
in the presence and absence of exogenous metabolic activation using up
to 1000 µg phenylhydrazine or phenylhydrazine hydrochloride per plate
(Shimizu et al., 1978; Tosk et al., 1979; De Flora, 1981; Parodi et
al., 1981; Levin et al., 1982; Malca-Mor & Stark, 1982; Rogan et al.,
1982; De Flora et al., 1984a,b; Wilcox et al., 1990; Muller et al.,
1993). The quality of these studies is generally high, and the studies
were apparently conducted according to standard methodology, although
detailed reporting of the results is not always available.
There is some variability in the findings, although positive
results have been obtained in Salmonella typhimurium strains TA97,
TA100, TA102, TA1537, and TA1538 in the absence of exogenous metabolic
activation. In addition, positive results were obtained in the
presence of metabolic activation in TA98 and TA1535. Some
investigators have reported that the mutagenic action is slightly
decreased by the presence of exogenous metabolic activation (Parodi et
al., 1981; Malca-Mor & Stark, 1982; De Flora et al., 1984a,b).
However, one study reports an increase in mutagenic activity in the
presence of metabolic activation (Rogan et al., 1982).
Phenylhydrazine has also given positive results in a number of
other, less well validated bacterial assays (using S. typhimurium
strains such as TA2638, TP138, BA9, and BA13), in the presence and
absence of exogenous metabolic activation (De Flora et al., 1984b;
Ulitzur et al., 1984; Ruiz-Rubio et al., 1985; Levi et al., 1986;
Muller et al., 1993).
Phenylhydrazine has not been tested in an in vitro chromosomal
aberration assay. In a brief abstract of a mammalian cell gene
mutation assay in V79 cells, with and without metabolic activation, a
positive result was reported for phenylhydrazine (Kuszynski et al.,
1981). However, no firm conclusions can be drawn from this report
because of deficiencies in the reporting.
In an unscheduled DNA synthesis assay in rat and mouse primary
hepatocytes, concentrations of 0.0144-144 mg phenylhydrazine
hydrochloride/litre were assessed (Mori et al., 1988). Although
toxicity was measured, no details were given, and quantitative data
were not reported. A positive result was obtained in both cell types,
although the effect was small.
Phenylhydrazine was tested in a micronucleus assay in vitro
using primary mouse bone marrow cells (Suzuki, 1985). Bone marrow
cells from the femur were exposed to 1-50 µg phenylhydrazine/ml for 30
min, in the presence and absence of metabolic activation. A total of
1500 polychromatic erythrocytes (PCEs) per concentration was scored
for the presence of micronuclei. There was no measure of cytotoxicity.
The percentage of micronucleated PCEs was statistically
significantly increased, in the presence of S9 only, at
phenylhydrazine concentrations of 5 µg/ml and greater in a
concentration-related manner.
BALB/c mice were administered a single intraperitoneal injection
of phenylhydrazine, and the incidence of micronucleated PCEs in the
bone marrow was measured at 24 and 48 h (Suzuki, 1985).
Phenylhydrazine was reported to be positive in this test, but no
details of the results or of the test were given. In view of the poor
reporting, no firm conclusions can be drawn from this study.
Groups of 11-12 female BALB/c mice were given a single
intraperitoneal injection of 50 mg phenylhydrazine/kg body weight in
saline (Steinheider et al., 1985). Blood smears of tail vein blood
were prepared at 24-h intervals for 7 or 11 days, and reticulocytes
and micronuclei in normochromatic erythrocytes (NCEs) and PCEs were
counted. It is not stated how many cells were counted per mouse. There
was no reporting of toxicity.
Phenylhydrazine caused a statistically significant increase in
the reticulocyte count on days 2-4 post-injection and in PCEs on day
3. There was a statistically significant increase in the incidence of
micronucleated PCEs at 24 h post-injection (from 1 to 4.7 per 1000)
and in micronucleated NCEs at 48 h post-injection (from 0.7 to 2.3 per
1000).
However, similar increases in micronucleated NCEs were also seen
following bleeding of the animals and splenectomy. The authors suggest
that the increase in micronuclei seen following phenylhydrazine
treatment was due at least partly to stimulation of erythropoiesis
because of the haemolysis induced by phenylhydrazine, thus leading to
more errors of nuclear expulsion; hence, the results do not
necessarily indicate a direct genotoxic action of phenylhydrazine.
Groups of 7-12 mice were given a single intraperitoneal injection
of either 85 or 170 mg phenylhydrazine/kg body weight and killed 1 and
6 h, respectively, after treatment (Parodi et al., 1981). In addition,
six mice were given a series of five daily intraperitoneal injections
of 7.6 mg phenylhydrazine/kg body weight and sacrificed 6 h after the
last injection. Control animals were injected with saline only. DNA
damage was assessed by measurement of the alkaline elution rate of
single-strand DNA from liver and lung tissue extracts.
A statistically significant change in the elution rate of liver
and lung DNA was seen in all groups of treated animals compared with
controls, except in the case of lung tissue DNA from mice given a
single dose of 85 mg phenylhydrazine/kg body weight. Phenylhydrazine
is considered to give a positive result in this assay for DNA damage.
The formation of DNA adducts ( N7-methylguanine and a trace of
O6-methylguanine) in the liver was demonstrated in rats receiving
65 mg phenylhydrazine/kg body weight by oral gavage (Mathison et al.,
1994). Other tissues were not examined.
8.6 Reproductive and developmental toxicity
Each of three dogs (one dog per dose group) received 20, 30, or
40 mg phenylhydrazine/kg body weight in saline by subcutaneous
injection on 2 consecutive days (Witchett, 1975). Two control animals
were not injected. At necropsy, performed on all three animals within
a few days of dosing, a "striking" reduction in spermatogenesis was
reported, with an absence of sperm in the epididymis. The validity of
this result is not clear, given the apparent extreme rapidity of the
effect.
Groups of 8-12 pregnant Wistar rats were given an intraperitoneal
injection of 7.5 mg phenylhydrazine/kg body weight as phenylhydrazine
hydrochloride on days 17, 18, and 19 of gestation; or 15 mg
phenylhydrazine/kg body weight as phenylhydrazine hydrochloride on
days 18 and 19 of gestation (Tamaki et al., 1974). Control animals
were not treated. There was no reporting of maternal toxicity or of
the effect of treatment on gestation or pup viability. Toxicity of the
pups was reported only insofar as there was incidence of jaundice
and/or anaemia among the offspring of treated animals. Twelve male
offspring with severe jaundice and anaemia, selected from treated
dams, and nine males from control dams were assessed at 9-22 weeks of
age for functional and behavioural status.
Although the authors reported that experimental animals showed
statistically significant differences from controls in some tests,
these findings are not considered to be reliable because of the small
numbers of animals used and the exclusion of a control animal from the
analysis. In addition, it is noted that only a brief part of the
gestation period was covered by the treatment regime (days 17-19); no
explanation is available for this choice of dosing regime.
Yamamura et al. (1973) reported in a brief abstract that
intraperitoneal injection of pregnant rats with 15 mg
phenylhydrazine/kg body weight as phenylhydrazine hydrochloride on
days 18 and 19 of gestation produced hyperbilirubinaemia (resulting
from haemolysis) in fetuses and newborns.
8.7 Immunological and neurological effects
No studies are available that specifically investigate the
immunological and neurological end-points, and there is no relevant
information from toxicity studies in animals.
9. EFFECTS ON HUMANS
In humans, no information is available in relation to single
exposure via the inhalation or oral routes, although effects similar
to those seen following dermal exposure would be expected to occur.
Systemic toxicity developed in humans after dermal exposure to liquid
phenylhydrazine, despite immediate attempts to reduce exposure by
removal of contaminated clothing and washing of skin (Schuckmann,
1969). Toxicity was manifest by damage to red blood cells, in one case
resulting in haemolytic jaundice. No such systemic effects were
reported in two cases of skin contamination with solid phenylhydrazine
hydrochloride.
In relation to skin irritation, information is available from
worker exposure data. There was no reporting of irritancy effects in
workers exposed to liquid phenylhydrazine following accidental
exposure, although systemic effects were seen (Schuckmann, 1969). Skin
irritation following contact with phenylhydrazine hydrochloride powder
was reported in two workers following accidental exposure. Local
irritation, superficial erythema, and partly bullous-papular changes
were noted in one case following spillage of powder on arms; multiple
burn marks and small blisters at the site of contact were reported in
the second case in which phenylhydrazine hydrochloride had spilled
into the worker's gloves and shoes. The author also refers to medical
records at the works that describe a number of cases of skin
irritation of differing severity due to phenylhydrazine hydrochloride,
but no details are given.
There are no data available on the eye irritation potential of
phenylhydrazine in humans.
There are a number of case reports of skin hypersensitivity
reactions to phenylhydrazine and its hydrochloride salt in humans.
Solomons (1946) conducted a patch test in one subject with a
phenylhydrazine crystal placed on the forearm under a dressing for an
unstated exposure period. Marked erythema and some oedema developed on
the exposure site after 18 h, with the formation of vesicles after 30
h and crusting after a further 24 h.
Similar hypersensitive skin reactions were reported following
individual exposures to solid or aqueous solutions of phenylhydrazine
or phenylhydrazine salts (Wright & Joyner, 1930; Frost & Hjorth, 1959;
Pevny & Peter, 1983).
There is also evidence that cross-sensitization can occur between
hydrazine compounds, so that subjects already sensitized to hydrazine,
a known skin sensitizer, are also sensitized to hydrazine derivatives,
including phenylhydrazine (Malten, 1962; Van Ketel, 1964; Hovding,
1967; Rothe, 1988).
No data are available on the potential of phenylhydrazine to
cause respiratory tract sensitization.
Earlier this century, phenylhydrazine and phenylhydrazine
hydrochloride were administered orally (usually around 100-200 mg/day)
for the treatment of blood disorders (e.g., Giffin & Allen, 1933). In
some cases, treatment was effective; in others, however, the outcome
was fatal (e.g., Giffin & Conner, 1929). The effects seen (beneficial
or otherwise) may have been related to the disease process and cannot
be attributed entirely to phenylhydrazine.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1 Aquatic environment
Results of acute toxicity tests on aquatic organisms are
summarized in Table 2. All concentrations are nominal.
In the early life stage test summarized in Table 2 (Xiu et al.,
1992), newly fertilized eggs of the zebra fish were exposed to
concentrations of phenylhydrazine through hatch and into the larval
stage (total exposure time was 16 days). A NOEC (survival) was
established for eggs at 5 days post-fertilization at 0.0039 mg/litre,
with a lowest-observed-effect concentration (LOEC) at 0.0078 mg/litre.
At the end of the test (16 days), the NOEC for larvae was 0.000 49
mg/litre, and the LOEC was 0.000 98 mg/litre. The study was conducted
according to a Swedish standard protocol (ss 028193).
In a study on the goldfish ( Carassius auratus), 40% of fish
died when exposed to phenylhydrazine at a nominal concentration of 1
mg/litre for 48 h. Signs of toxicity included erratic swimming,
sinking to the bottom of the test tank, and slowed and erratic
respiration. No gross lesions were found following dissection,
although all viscera showed focal haemorrhaging (Houston et al.,
1988).
Effects on blood cells and the haematopoietic system, comparable
to those found in mammals, were seen in fish injected with
phenylhydrazine. Chinook salmon ( Oncorhynchus tshawytscha) juveniles
were injected with 12.5 mg phenylhydrazine/kg body weight. Red cell
count, haemoglobin, and haematocrit fell to 1-5% of their normal
values within 10 days of treatment; a slight improvement was reported
15 days following injection, and values had returned to normal 95 days
after treatment (Smith et al., 1971).
10.2 Terrestrial environment
Phenylhydrazine at 21.6 mg/litre in a nutrient culture medium had
no effect on the growth of various soil fungi (Zsolnai, 1975).
A phenylhydrazine concentration of 50 mg/litre in a hydroponic
culture solution inhibited germination of Hordeum seeds for at least
6 days, whereas growth was stimulated in Lepidium. At 100 mg/litre,
seedling growth was stimulated in Hordeum but not in Lepidium. At
500 mg/litre, phenylhydrazine inhibited growth in seedlings of both
species, although the seedlings were still apparently "healthy"
(Bokorny, 1933).
Exposure of soil nematodes Caenorhabditis briggsae in culture
to phenylhydrazine at 50 mg/litre of medium resulted in reduced growth
of all four larval stages (the effect was most marked on the last
larval instar) and therefore delayed development to adults. However,
the adults formed were capable of reproduction, although the number of
progeny was reduced. A concentration of 15 mg/litre also delayed
development, although to a lesser degree compared with the higher
concentration (Kampfe et al., 1986a,b). A 6-day EC50 of 12 mg/litre
was reported for production of progeny in culture for the same
nematode (Kreil, 1982).
No dietary or oral toxicity studies have been performed on birds.
However, injection studies have shown haemolytic anaemia and reduced
white cell counts in birds. In contrast to mammals, cell division in
erythropoietic tissue is unaffected by phenylhydrazine (Williams,
1972; Clark et al., 1988; Datta et al., 1989, 1990).
Table 2: Acute toxicity of phenylhydrazine to aquatic organisms.
Organism End-point Concentration Reference
(mg/litre)
Bacteria
Photobacterium phosphoreum 30-min EC50 66.9 Kaiser et al.
(luminescence) (1987)
Facultative anaerobes 24-h toxic 60 Hoechst (1980)
(mixed culture) threshold
Escherichia coli minimum 109.3 Romero & Canada
inhibitory (1991)
concentration
Escherichia coli, minimum >3000 Zemek et al.
Micrococcus luteus, inhibitory (1978)
Bacillus licheniformis concentration
Invertebrates
water flea LC50 2-5 Hoechst (1980)
(Daphnia magna) (immobilization)
Fish
zebra fish 96-h LC50 0.16-0.25 Hoechst (1982)
(Brachydanio rerio) 96-h NOEC 0.1
zebra fish 5-day NOEC (eggs) 0.0039 Xiu et al.
(Brachydanio rerio) 16-day NOEC (larvae) 0.000 49 (1992)
Japanese killifish 48-h LC50 15.7 Tonogai et al.
(Oryzias latipes) (1982)
Table 2 (cont'd)
Organism End-point Concentration Reference
(mg/litre)
common carp 24-h LC100 1.0 Menzie (1979)a
(Cyprinus carpio) 96-h NOEC 0.1
bluegill 48-h LC50 0.1 Menzie (1979)a
(Lepomis 96-h NOEC 0.01
macrochirus)
a Menzie C (1979) Value taken from the DIMDI/ECDIN database. Test performed by the
United States Fish and Wildlife Service, Bureau of Sports, Fisheries and Wildlife,
Department of the Interior, Washington, DC [cited in BUA, 1995].
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Phenylhydrazine is toxic by single exposure via the oral route
(LD50 80-188 mg/kg body weight) and is expected to be toxic by the
inhalation and dermal routes (data from these routes of exposure are
less clear). Phenylhydrazine solution was severely irritating to
rabbit eyes; hence, it is reasonable to predict that it would have
significant eye irritation potential in humans.
Phenylhydrazine also has skin irritation potential, and there is
evidence from human case reports that it has skin sensitizing
properties. Exposure to phenylhydrazine may cause damage to red blood
cells, potentially resulting in anaemia and consequential secondary
involvement of other tissues, such as the spleen and liver. The dose
(exposure)-response characteristics for the induction of damage to the
red blood cells are poorly defined, and a no-effect level has not been
identified. Where phenylhydrazine has been used therapeutically in
humans, via the oral route, for the treatment of blood disorders,
daily doses of the order of 1.5-4 mg/kg body weight per day have
caused a reduction in the numbers of red blood cells; given the health
status of the individuals concerned, however, these data are of
limited use. Phenylhydrazine is mutagenic in vitro, and, although not
conclusive, there is some evidence to indicate that it may express
genotoxic activity in vivo. The substance is clearly carcinogenic in
mice following oral dosing, inducing tumours of the vascular system.
The mechanism for tumour formation is unclear, and, given the
genotoxic profile of phenylhydrazine, a genotoxic component cannot be
excluded. Carcinogenic potential in humans cannot be excluded given
the profile of genotoxicity and animal carcinogenicity, particularly
as other expressions of phenylhydrazine toxicity are common to a
number of species, including humans.
No conclusions can be drawn from the available information on
fertility or development.
11.1.2 Criteria for setting guidance values fon phenylhydrazine
There are no adequate data available regarding reproductive or
developmental effects; hence, it is not possible to evaluate the risk
to human health for these end-points.
The use pattern and physical/chemical characteristics of the
compound suggest that exposure of the general population would be
negligible.
Using United Kingdom workplace conditions as an example (section
6.2), exposure to phenylhydrazine vapour during most occupational
processes would result in a body burden of up to 0.33 mg/kg body
weight per day, assuming a 70-kg worker breathes 10 m3 of air in a
working day and that 100% phenylhydrazine is absorbed. There are no
data available from which to estimate the contribution to body burden
from dermal uptake, although this is expected to be negligible. A
threshold for the induction of red blood cell damage probably exists
but has not been identified, although daily oral doses calculated at
about 1.5 mg/kg body weight per day and above are associated with such
effects. Overall, at these levels of predicted inhalation exposure,
the risk of developing damage to the red blood cells is considered to
be low; if the levels were exceeded (e.g., of the order of a few ppm,
approximately 15-20 mg/m3), however, then this would be cause for
some concern.
On the basis that the carcinogenicity of phenylhydrazine may
involve a genotoxic mechanism, it is not possible to reliably identify
a threshold below which occupational exposure to phenylhydrazine would
not result in some risk to human health.
11.1.3 Sample risk characterization
The scenario chosen as an example is occupational exposure in the
United Kingdom.
The main health concerns associated with exposure to
phenylhydrazine are damage to the red blood cells, deleterious effects
on genetic material, and the development of cancer.
It is recognized that there are a number of different approaches
to assessing the risks to human health for genotoxic and carcinogenic
substances and in the subsequent risk management steps that may be
taken. In addition, although not used in the United Kingdom, there are
models for characterizing potency that may be of some benefit in
priority-setting schemes. In the United Kingdom occupational setting,
a Maximum Exposure Limit or MEL (which is not a health-based standard)
has been proposed at 0.9 mg/m3 (0.2 ppm), 8-h time-weighted average.
The numerical value for the MEL was based on a level of control that
was deemed (by tripartite agreement) to be reasonably practicable
under United Kingdom workplace conditions, and in the United Kingdom
there is a continuing requirement to reduce exposure levels as far as
reasonably practicable with the technology that is currently
available.
Phenylhydrazine also possesses skin and eye irritant properties
and possibly skin sensitizing potential. The information available
indicates that local exposure of these tissues is unlikely; if it did
occur, however, then there would be risk of irritation to the eyes and
the development of irritant and/or allergic dermatitis.
11.2 Evaluation of environmental effects
No atmospheric effects are expected given the release of
phenylhydrazine predominantly to water, its extremely low
volatilization from water to the atmosphere, and its rapid calculated
atmospheric half-life following reaction with hydroxyl radicals.
Few toxicity studies are available for terrestrial organisms, and
little emission to land is expected; on this basis, no quantitative
risk assessment can be attempted for the terrestrial environment.
Phenylhydrazine is degraded photochemically and autoxidizes in
water. It is readily biodegradable, and this is expected to be the
major route of breakdown in the environment. There is minimal sorption
to particulates.
Phenylhydrazine is toxic to aquatic organisms, with the lowest
reported NOEC in acute fish tests at 0.01 mg/litre; fish are generally
more sensitive than either daphnids or bacteria. A NOEC for
embryo-larval stages following 16 days of exposure from fertilization
has been reported at 0.000 49 mg/litre for the zebra fish.
There are no reported measurements of phenylhydrazine in
environmental media. Monitoring studies of both inflow and outflow to
the wastewater treatment plant of the Hoechst Hochst production plant
in Germany showed no detectable phenylhydrazine (detection limit 500
µg/litre) in weekly samples. Maximum emission to wastewater was
estimated at 13 t/year, and this will be used as a worst-case example.
Based on this emission rate, and using mainly default values from
the OECD Technical Guidance Manual, the initial predicted
environmental concentration of phenylhydrazine in river water
(PEClocal (water), in g/litre) would be as follows:
Ceffluent
PEClocal (water) =
(1 + Kp(susp) × Csusp) × D
where:
* Ceffluent is the concentration of phenylhydrazine in the
wastewater treatment plant effluent (g/litre), calculated as
Ceffluent = W × (100 - P)/(100 × Q), where:
W = emission rate (35.6 kg/day)
P = percent removal in the wastewater treatment plant
(based on the "ready biodegradability" of the
compound, 91%)
Q = volume of wastewater in m3/day (default 200
litre/day per capita for a population of 10 000
inhabitants; wastewater volume for the production
plant is unknown)
* Kp(susp) is the suspended matter/water adsorption coefficient,
calculated as Kp(susp) = Koc × foc(susp), where:
Koc = organic carbon/water partition
coefficient (7.3)
foc(susp) = fraction of organic carbon in suspended matter
(default 0.1)
* Csusp is the concentration of suspended matter in the river
water (in kg/litre; default 15 mg/litre)
* D is the dilution factor for river flow (flow rate for the
River Main averages 188 m3/s compared with the estimated flow
rate of the wastewater at 0.02 m3/s; dilution factor
approximately 10 000)
Under these very conservative conditions, PEClocal (water)
= 0.16 µg/litre.
Of the reported acute toxicity test results for organisms in the
environment, those for the majority of fish tested are substantially
lower than those for other organisms tested. The predicted no-effect
concentration (PNEC) will therefore be based on the fish results.
No long-term test results are available. Applying an uncertainty
factor of 1000 to the lowest reported standard acute LC50 value of
0.1 mg/litre for the bluegill ( Lepomis macrochirus) would give a
PNEC of 0.1 µg/litre. This is a factor of 100 lower than the lowest
reported NOEC for the same species. Alternatively, applying an
uncertainty factor of 10 to the NOEC for the early life stage test on
the zebra fish larvae gives a PNEC of 0.049 µg/litre. The more
conservative value will be used in estimating risk.
The low PEC of 0.16 µg/litre would not have been detected in the
monitoring at the site. Assuming that this value is the worst case, a
PEC/PNEC ratio of 3.2 is generated. This indicates that the risk to
aquatic organisms is low, based on very conservative assumptions. The
distribution of reported toxicity test results against the worst-case
PEC is plotted in Figure 1, illustrating the safety margin.
 12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Previous evaluations by other international bodies were not
identified. Information on international hazard classification and
labelling is included in the International Chemical Safety Card (ICSC
0938) reproduced in this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventive and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0938) reproduced in this
document.
13.1 Human health hazards
Phenylhydrazine induces damage to red blood cells. Repeated or
prolonged contact with the substance causes skin sensitization, and
there is cause for concern for carcinogenicity.
13.2 Advice to physicians
Phenylhydrazine is a haemolytic agent. There is no specific
antidote, but treatment should be supportive.
13.3 Health surveillance advice
Periodic medical examination of the area of the skin exposed to
phenylhydrazine and annual blood count should be included in the
health surveillance programme.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards
may be obtained from UNEP Chemicals (IRPTC), Geneva.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
PHENYLHYDRAZINE ICSC: 0938
November 1998
CAS #
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Previous evaluations by other international bodies were not
identified. Information on international hazard classification and
labelling is included in the International Chemical Safety Card (ICSC
0938) reproduced in this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventive and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0938) reproduced in this
document.
13.1 Human health hazards
Phenylhydrazine induces damage to red blood cells. Repeated or
prolonged contact with the substance causes skin sensitization, and
there is cause for concern for carcinogenicity.
13.2 Advice to physicians
Phenylhydrazine is a haemolytic agent. There is no specific
antidote, but treatment should be supportive.
13.3 Health surveillance advice
Periodic medical examination of the area of the skin exposed to
phenylhydrazine and annual blood count should be included in the
health surveillance programme.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards
may be obtained from UNEP Chemicals (IRPTC), Geneva.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
PHENYLHYDRAZINE ICSC: 0938
November 1998
CAS # 