
Concise International Chemical Assessment Document 20
MONONITROPHENOLS
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by Dr A. Boehncke, Dr G. Koennecker, Dr I.
Mangelsdorf, and Dr A. Wibbertmann, Fraunhofer Institute for
Toxicology and Aerosol Research, Hanover, Germany
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 2000
The International Programme on Chemical Safety (IPCS),
established in 1980, is a joint venture of the United Nations
Environment Programme (UNEP), the International Labour Organisation
(ILO), and the World Health Organization (WHO). The overall objectives
of the IPCS are to establish the scientific basis for assessment of
the risk to human health and the environment from exposure to
chemicals, through international peer review processes, as a
prerequisite for the promotion of chemical safety, and to provide
technical assistance in strengthening national capacities for the
sound management of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization, the United Nations
Institute for Training and Research, and the Organisation for Economic
Co-operation and Development (Participating Organizations), following
recommendations made by the 1992 UN Conference on Environment and
Development to strengthen cooperation and increase coordination in the
field of chemical safety. The purpose of the IOMC is to promote
coordination of the policies and activities pursued by the
Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing-in-Publication Data
Mononitrophenols.
(Concise international chemical assessment document ; 20)
1.Nitrophenols - toxicity 2.Risk assessment 3.Environmental
exposure I.International Programme on Chemical Safety II.Series
ISBN 92 4 153020 0 (NLM classification: QD 341.P5)
ISSN 1020-6167
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 2000
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city, or area
or of its authorities, or concerning the delimitation of its frontiers
or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
TABLE OF CONTENTS
FOREWORD
1. EXECUTIVE SUMMARY
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
3. ANALYTICAL METHODS
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1. Environmental levels
6.2. Human exposure
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
7.1. 2-Nitrophenol
7.2. 4-Nitrophenol
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposure
8.2. Irritation and sensitization
8.3. Short-term exposure
8.3.1. Oral exposure
8.3.2. Inhalation exposure
8.3.2.1 2-Nitrophenol
8.3.2.2 4-Nitrophenol
8.3.3. Dermal exposure
8.4. Long-term exposure
8.4.1. Subchronic exposure
8.4.2. Chronic exposure and carcinogenicity
8.5. Genotoxicity and related end-points
8.6. Reproductive and developmental toxicity
8.6.1. Reproductive toxicity
8.6.2. Developmental toxicity
8.6.2.1 2-Nitrophenol
8.6.2.2 4-Nitrophenol
8.7. Immunological and neurological effects
8.8. Methaemoglobin formation
9. EFFECTS ON HUMANS
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1. Aquatic environment
10.2. Terrestrial environment
11. EFFECTS EVALUATION
11.1. Evaluation of health effects
11.1.1. Hazard identification and dose-response assessment
11.1.2. Criteria for setting guidance values for 2- and 4-nitrophenol
11.1.3. Sample risk characterization
11.2. Evaluation of environmental effects
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
INTERNATIONAL CHEMICAL SAFETY CARD
REFERENCES
APPENDIX 1 - 3-NITROPHENOL
APPENDIX 2 - SOURCE DOCUMENTS
APPENDIX 3 - CICAD PEER REVIEW
APPENDIX 4 - CICAD FINAL REVIEW BOARD
RÉSUMÉ D'ORIENTATION
RESUMEN DE ORIENTACION
FOREWORD
Concise International Chemical Assessment Documents (CICADs) are
the latest in a family of publications from the International
Programme on Chemical Safety (IPCS) -- a cooperative programme of the
World Health Organization (WHO), the International Labour Organisation
(ILO), and the United Nations Environment Programme (UNEP). CICADs
join the Environmental Health Criteria documents (EHCs) as
authoritative documents on the risk assessment of chemicals.
CICADs are concise documents that provide summaries of the
relevant scientific information concerning the potential effects of
chemicals upon human health and/or the environment. They are based on
selected national or regional evaluation documents or on existing
EHCs. Before acceptance for publication as CICADs by IPCS, these
documents undergo extensive peer review by internationally selected
experts to ensure their completeness, accuracy in the way in which the
original data are represented, and the validity of the conclusions
drawn.
The primary objective of CICADs is characterization of hazard and
dose-response from exposure to a chemical. CICADs are not a summary of
all available data on a particular chemical; rather, they include only
that information considered critical for characterization of the risk
posed by the chemical. The critical studies are, however, presented in
sufficient detail to support the conclusions drawn. For additional
information, the reader should consult the identified source documents
upon which the CICAD has been based.
Risks to human health and the environment will vary considerably
depending upon the type and extent of exposure. Responsible
authorities are strongly encouraged to characterize risk on the basis
of locally measured or predicted exposure scenarios. To assist the
reader, examples of exposure estimation and risk characterization are
provided in CICADs, whenever possible. These examples cannot be
considered as representing all possible exposure situations, but are
provided as guidance only. The reader is referred to EHC 1701 for
advice on the derivation of health-based guidance values.
While every effort is made to ensure that CICADs represent the
current status of knowledge, new information is being developed
constantly. Unless otherwise stated, CICADs are based on a search of
the scientific literature to the date shown in the executive summary.
In the event that a reader becomes aware of new information that would
change the conclusions drawn in a CICAD, the reader is requested to
contact IPCS to inform it of the new information.
1 International Programme on Chemical Safety (1994)
Assessing human health risks of chemicals: deriviation of
guidance values for health-based exposure limits. Geneva, World
Health Organization (Environmental Health Criteria 170).
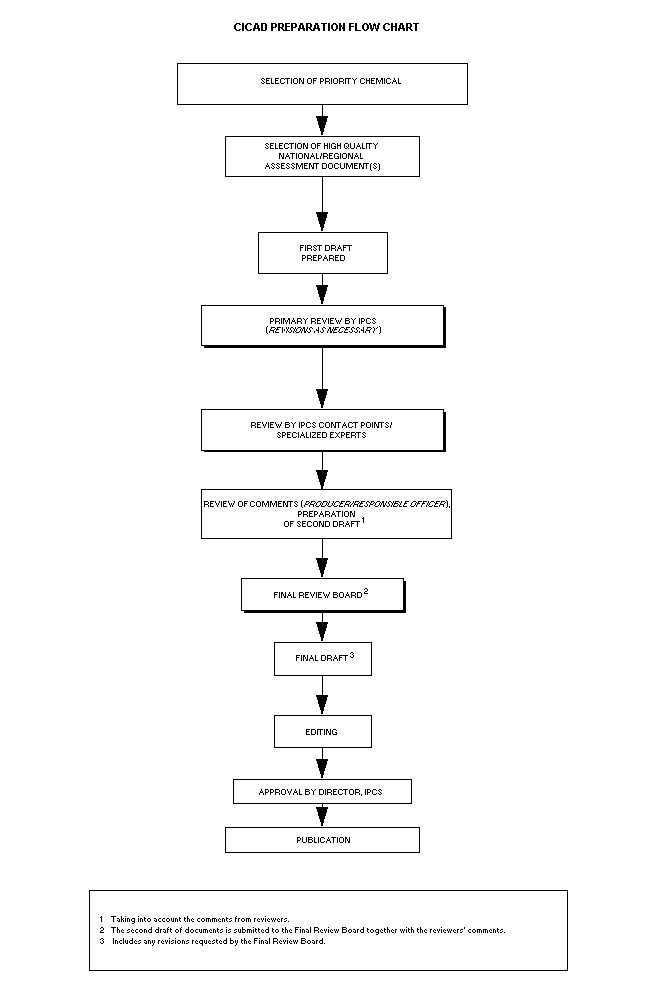
Procedures
The flow chart shows the procedures followed to produce a CICAD.
These procedures are designed to take advantage of the expertise that
exists around the world -- expertise that is required to produce the
high-quality evaluations of toxicological, exposure, and other data
that are necessary for assessing risks to human health and/or the
environment.
The first draft is based on an existing national, regional, or
international review. Authors of the first draft are usually, but not
necessarily, from the institution that developed the original review.
A standard outline has been developed to encourage consistency in
form. The first draft undergoes primary review by IPCS to ensure that
it meets the specified criteria for CICADs.
The second stage involves international peer review by scientists
known for their particular expertise and by scientists selected from
an international roster compiled by IPCS through recommendations from
IPCS national Contact Points and from IPCS Participating Institutions.
Adequate time is allowed for the selected experts to undertake a
thorough review. Authors are required to take reviewers' comments into
account and revise their draft, if necessary. The resulting second
draft is submitted to a Final Review Board together with the
reviewers' comments.
The CICAD Final Review Board has several important functions:
- to ensure that each CICAD has been subjected to an appropriate
and thorough peer review;
- to verify that the peer reviewers' comments have been addressed
appropriately;
- to provide guidance to those responsible for the preparation of
CICADs on how to resolve any remaining issues if, in the opinion
of the Board, the author has not adequately addressed all
comments of the reviewers; and
- to approve CICADs as international assessments.
Board members serve in their personal capacity, not as representatives
of any organization, government, or industry. They are selected
because of their expertise in human and environmental toxicology or
because of their experience in the regulation of chemicals. Boards are
chosen according to the range of expertise required for a meeting and
the need for balanced geographic representation.
 Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
1. EXECUTIVE SUMMARY
This CICAD on the isomers 2-, 3-, and 4-nitrophenol was prepared
by the Fraunhofer Institute for Toxicology and Aerosol Research,
Hanover, Germany. It was based on reviews compiled by the German
Advisory Committee on Existing Chemicals of Environmental Relevance
(BUA, 1992) and the US Agency for Toxic Substances and Disease
Registry (ATSDR, 1992) to assess the potential effects of 2- and
4-nitrophenol on the environment and on human health. Data identified
up to 1992 were considered in these reviews. A comprehensive
literature search of several databases was conducted in 1998 to
identify any relevant references on 2- and 4-nitrophenol published
subsequent to those in the source documents and to identify all
references containing relevant data on the isomer 3-nitrophenol.
Information found on 3-nitrophenol was very scarce, precluding a
meaningful assessment. As a result, data on this isomer are
summarized in Appendix 1. Information on the nature of the peer review
and the availability of the source documents is presented in Appendix
2. Information on the peer review of this CICAD is presented in
Appendix 3. This CICAD was approved as an international assessment at
a meeting of the Final Review Board, held in Washington, DC, USA, on
8-11 December 1998. Participants at the Final Review Board meeting are
listed in Appendix 4. The International Chemical Safety Card (ICSC
1342) for mononitrophenols, produced by the International Programme on
Chemical Safety (IPCS, 1998), has also been reproduced in this
document.
The nitrophenol isomers are water-soluble solids that are
moderately acidic in water as a result of dissociation. 2-Nitrophenol
and 4-nitrophenol are used as intermediates in the synthesis of a
number of organophosphorus pesticides and some medical products.
Releases into the environment are primarily emissions into air, water,
and soil from diffuse sources, such as vehicle traffic and hydrolytic
and photolytic degradation of the respective pesticides. Further
releases into the hydrosphere and the geosphere are caused by the dry
and wet deposition of airborne nitrophenols from the atmosphere. The
photo-oxidative formation of 2- and 4-nitrophenol in the atmosphere is
still under discussion.
From the available data, only a slow rate of volatilization of
2-nitrophenol and no significant volatilization of 4-nitrophenol from
water to air are to be expected. 2-Nitrophenol is enriched in the
liquid phase of clouds, whereas more 4-nitrophenol than expected from
physicochemical data can be found in the gas phase owing to extensive
binding to particles. In view of the water solubilities and the
expected occurrence in the vapour phase, wet deposition of
nitrophenols from air to surface waters and soil is to be expected.
The major transformation pathway for 2-nitrophenol emitted to the
troposphere should be rapid nitration to 2,4-dinitrophenol, whereas
the major portion of airborne 4-nitrophenol is expected to be particle
bound and therefore only to a minor extent available for photochemical
reactions. Most of the 4-nitrophenol should be washed out from air by
wet and dry deposition. Nitrophenols are not considered to contribute
directly to the depletion of the stratospheric ozone layer or to
global warming. Measured half-lives for the photochemical
decomposition of 4-nitrophenol in water ranged from 2.8 to 13.7 days.
Numerous studies on the biodegradation of 2- and 4-nitrophenol
indicate the isomers to be inherently biodegradable in water under
aerobic conditions. Mineralization of nitrophenols under anaerobic
conditions requires extended adaptation of microbial communities.
Measured coefficients of soil sorption ( Koc) in the range of
44-530 indicate a low to moderate potential for soil sorption.
Nitrophenols released to soil should be biodecomposed under aerobic
conditions. Infiltration into groundwater is expected only under
conditions unfavourable to biodegradation. For 2- and 4-nitrophenol,
measured bioconcentration factors ranging from 11 to 76 indicate a low
potential for bioaccumulation.
There is only limited information concerning the toxicological
profiles of 2- and 4-nitrophenol. In experimental animals given
4-nitrophenol orally, intravenously, or intraperitoneally, most of the
applied dose was excreted via the urine within 24-48 h as glucuronide
and sulfate conjugates, while only very small amounts were excreted
via faeces or as unchanged 4-nitrophenol. The percentages of
glucuronide and sulfate conjugates were shown to be species and dose
dependent. After oral dosing in rabbits, 4-nitrophenol undergoes
reduction to p-aminophenol as well as glucuronidation and sulfation.
The available data from in vivo and in vitro studies give an
indication for dermal uptake of 4-nitrophenol. The data for
2-nitrophenol are very limited. However, based on the available data,
a comparable metabolic transformation is assumed. Bioaccumulation of
2- and 4-nitrophenol in organisms is not to be expected owing to their
rapid metabolism and excretion.
In acute studies, 4-nitrophenol is harmful after oral uptake and
was found to be more toxic than 2-nitrophenol. A dose-dependent
increase in the formation of methaemoglobin was seen in cats after
oral exposure to 2-nitrophenol and in rats after exposure by
inhalation to 4-nitrophenol. After repeated exposure to 4-nitrophenol,
the formation of methaemoglobin was shown to be the most critical
end-point for exposure by inhalation and is assumed to be relevant for
oral exposure too. Other noted effects included decreases in body
weight gain, differences in organ weights, focal fatty degeneration of
the liver, and haematological changes. For these effects, it was not
possible to identify a clear dose-response or reliable
no-observed-(adverse-)effect levels (NO(A)ELs).
2-Nitrophenol is slightly irritating to the skin but
non-irritating to the eye. The substance proved to have no sensitizing
effects in a Buehler test. Based on valid studies with experimental
animals, irritating effects on skin and eye are assumed for
4-nitrophenol. In a guinea-pig maximization test, 4-nitrophenol was
considered as slightly sensitizing. In humans, a possible
sensitization after contact with 4-nitrophenol cannot be excluded,
especially as skin sensitization has been found in patch tests on
factory workers who may have been exposed to 4-nitrophenol.
Neither of the two isomers of nitrophenol has been fully tested
for genotoxicity. Insufficient data are available on 2-nitrophenol to
allow any conclusions to be made about its possible mutagenicity. More
mutagenicity studies are available for 4-nitrophenol, although some
were inadequately reported. There is evidence to suggest that
4-nitrophenol can cause chromosomal aberrations in vitro. In the
absence of any in vivo mutagenicity studies in mammals, it is not
possible to conclude whether or not the mutagenic potential of
4-nitrophenol is expressed in vivo.
In mice, the dermal application of 4-nitrophenol for 78 weeks
gave no indication of carcinogenic effects. In another study with
mice, which has several limitations, no skin tumours were noted after
dermal application of 2- or 4-nitrophenol over 12 weeks.
Carcinogenicity studies using the oral or inhalation routes were not
available for either of the isomers.
For 4-nitrophenol, the available data gave no evidence of
specific or statistically significant reproductive or developmental
toxicity effects after dermal or oral application to rats and mice. In
an oral study with rats, 2-nitrophenol induced developmental effects
in the offspring only at doses that also produced maternal toxicity.
However, in these studies, the fetuses were not examined for internal
malformations.
The database for 2-nitrophenol is extremely limited, and the
database for 4-nitrophenol is insufficient for deriving reliable
NO(A)EL values. Therefore, at present, no tolerable daily intakes
(TDIs) or tolerable concentrations (TCs) can be derived for either
2- or 4-nitrophenol.
From valid test results available on the toxicity of 2- and
4-nitrophenol to various aquatic organisms, nitrophenols can be
classified as substances exhibiting moderate to high toxicity in the
aquatic compartment. The lowest effect concentrations found in chronic
studies with freshwater organisms ( Scenedesmus subspicatus, 96-h
EC50: 0.39 mg 2-nitrophenol/litre; Entosiphon sulcatum, 72-h
minimum inhibitory concentration, or MIC: 0.83 mg 4-nitrophenol/litre)
were 40-50 times higher than maximum levels determined in a densely
populated and highly industrialized Asian river basin (0.0072 mg
2-nitrophenol/litre and 0.019 mg 4-nitrophenol/litre). Therefore,
despite biotic and photochemical decomposition, nitrophenols emitted
to water could pose some risk to sensitive aquatic organisms,
particularly under surface water conditions not favouring both
elimination pathways. Because of their use patterns and release
scenarios, it is likely that nitrophenols pose only a minor risk to
aquatic organisms.
The available data indicate only a moderate toxicity potential of
nitrophenols in the terrestrial environment. From calculations of the
toxicity exposure ratio (TER) of nitrophenols from the degradation of
pesticides, only a minor risk for organisms in this compartment is to
be expected, even under a worst-case scenario.
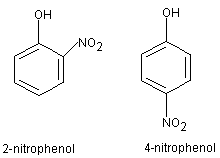
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
2-Nitrophenol (CAS No. 88-75-5; 2-hydroxy-1-nitrobenzene,
o-nitrophenol) and 4-nitrophenol (CAS No. 100-02-7;
4-hydroxy-1-nitrobenzene, p-nitrophenol) share the empirical formula
C6H5NO3. Their structural formulas are shown below.
Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
1. EXECUTIVE SUMMARY
This CICAD on the isomers 2-, 3-, and 4-nitrophenol was prepared
by the Fraunhofer Institute for Toxicology and Aerosol Research,
Hanover, Germany. It was based on reviews compiled by the German
Advisory Committee on Existing Chemicals of Environmental Relevance
(BUA, 1992) and the US Agency for Toxic Substances and Disease
Registry (ATSDR, 1992) to assess the potential effects of 2- and
4-nitrophenol on the environment and on human health. Data identified
up to 1992 were considered in these reviews. A comprehensive
literature search of several databases was conducted in 1998 to
identify any relevant references on 2- and 4-nitrophenol published
subsequent to those in the source documents and to identify all
references containing relevant data on the isomer 3-nitrophenol.
Information found on 3-nitrophenol was very scarce, precluding a
meaningful assessment. As a result, data on this isomer are
summarized in Appendix 1. Information on the nature of the peer review
and the availability of the source documents is presented in Appendix
2. Information on the peer review of this CICAD is presented in
Appendix 3. This CICAD was approved as an international assessment at
a meeting of the Final Review Board, held in Washington, DC, USA, on
8-11 December 1998. Participants at the Final Review Board meeting are
listed in Appendix 4. The International Chemical Safety Card (ICSC
1342) for mononitrophenols, produced by the International Programme on
Chemical Safety (IPCS, 1998), has also been reproduced in this
document.
The nitrophenol isomers are water-soluble solids that are
moderately acidic in water as a result of dissociation. 2-Nitrophenol
and 4-nitrophenol are used as intermediates in the synthesis of a
number of organophosphorus pesticides and some medical products.
Releases into the environment are primarily emissions into air, water,
and soil from diffuse sources, such as vehicle traffic and hydrolytic
and photolytic degradation of the respective pesticides. Further
releases into the hydrosphere and the geosphere are caused by the dry
and wet deposition of airborne nitrophenols from the atmosphere. The
photo-oxidative formation of 2- and 4-nitrophenol in the atmosphere is
still under discussion.
From the available data, only a slow rate of volatilization of
2-nitrophenol and no significant volatilization of 4-nitrophenol from
water to air are to be expected. 2-Nitrophenol is enriched in the
liquid phase of clouds, whereas more 4-nitrophenol than expected from
physicochemical data can be found in the gas phase owing to extensive
binding to particles. In view of the water solubilities and the
expected occurrence in the vapour phase, wet deposition of
nitrophenols from air to surface waters and soil is to be expected.
The major transformation pathway for 2-nitrophenol emitted to the
troposphere should be rapid nitration to 2,4-dinitrophenol, whereas
the major portion of airborne 4-nitrophenol is expected to be particle
bound and therefore only to a minor extent available for photochemical
reactions. Most of the 4-nitrophenol should be washed out from air by
wet and dry deposition. Nitrophenols are not considered to contribute
directly to the depletion of the stratospheric ozone layer or to
global warming. Measured half-lives for the photochemical
decomposition of 4-nitrophenol in water ranged from 2.8 to 13.7 days.
Numerous studies on the biodegradation of 2- and 4-nitrophenol
indicate the isomers to be inherently biodegradable in water under
aerobic conditions. Mineralization of nitrophenols under anaerobic
conditions requires extended adaptation of microbial communities.
Measured coefficients of soil sorption ( Koc) in the range of
44-530 indicate a low to moderate potential for soil sorption.
Nitrophenols released to soil should be biodecomposed under aerobic
conditions. Infiltration into groundwater is expected only under
conditions unfavourable to biodegradation. For 2- and 4-nitrophenol,
measured bioconcentration factors ranging from 11 to 76 indicate a low
potential for bioaccumulation.
There is only limited information concerning the toxicological
profiles of 2- and 4-nitrophenol. In experimental animals given
4-nitrophenol orally, intravenously, or intraperitoneally, most of the
applied dose was excreted via the urine within 24-48 h as glucuronide
and sulfate conjugates, while only very small amounts were excreted
via faeces or as unchanged 4-nitrophenol. The percentages of
glucuronide and sulfate conjugates were shown to be species and dose
dependent. After oral dosing in rabbits, 4-nitrophenol undergoes
reduction to p-aminophenol as well as glucuronidation and sulfation.
The available data from in vivo and in vitro studies give an
indication for dermal uptake of 4-nitrophenol. The data for
2-nitrophenol are very limited. However, based on the available data,
a comparable metabolic transformation is assumed. Bioaccumulation of
2- and 4-nitrophenol in organisms is not to be expected owing to their
rapid metabolism and excretion.
In acute studies, 4-nitrophenol is harmful after oral uptake and
was found to be more toxic than 2-nitrophenol. A dose-dependent
increase in the formation of methaemoglobin was seen in cats after
oral exposure to 2-nitrophenol and in rats after exposure by
inhalation to 4-nitrophenol. After repeated exposure to 4-nitrophenol,
the formation of methaemoglobin was shown to be the most critical
end-point for exposure by inhalation and is assumed to be relevant for
oral exposure too. Other noted effects included decreases in body
weight gain, differences in organ weights, focal fatty degeneration of
the liver, and haematological changes. For these effects, it was not
possible to identify a clear dose-response or reliable
no-observed-(adverse-)effect levels (NO(A)ELs).
2-Nitrophenol is slightly irritating to the skin but
non-irritating to the eye. The substance proved to have no sensitizing
effects in a Buehler test. Based on valid studies with experimental
animals, irritating effects on skin and eye are assumed for
4-nitrophenol. In a guinea-pig maximization test, 4-nitrophenol was
considered as slightly sensitizing. In humans, a possible
sensitization after contact with 4-nitrophenol cannot be excluded,
especially as skin sensitization has been found in patch tests on
factory workers who may have been exposed to 4-nitrophenol.
Neither of the two isomers of nitrophenol has been fully tested
for genotoxicity. Insufficient data are available on 2-nitrophenol to
allow any conclusions to be made about its possible mutagenicity. More
mutagenicity studies are available for 4-nitrophenol, although some
were inadequately reported. There is evidence to suggest that
4-nitrophenol can cause chromosomal aberrations in vitro. In the
absence of any in vivo mutagenicity studies in mammals, it is not
possible to conclude whether or not the mutagenic potential of
4-nitrophenol is expressed in vivo.
In mice, the dermal application of 4-nitrophenol for 78 weeks
gave no indication of carcinogenic effects. In another study with
mice, which has several limitations, no skin tumours were noted after
dermal application of 2- or 4-nitrophenol over 12 weeks.
Carcinogenicity studies using the oral or inhalation routes were not
available for either of the isomers.
For 4-nitrophenol, the available data gave no evidence of
specific or statistically significant reproductive or developmental
toxicity effects after dermal or oral application to rats and mice. In
an oral study with rats, 2-nitrophenol induced developmental effects
in the offspring only at doses that also produced maternal toxicity.
However, in these studies, the fetuses were not examined for internal
malformations.
The database for 2-nitrophenol is extremely limited, and the
database for 4-nitrophenol is insufficient for deriving reliable
NO(A)EL values. Therefore, at present, no tolerable daily intakes
(TDIs) or tolerable concentrations (TCs) can be derived for either
2- or 4-nitrophenol.
From valid test results available on the toxicity of 2- and
4-nitrophenol to various aquatic organisms, nitrophenols can be
classified as substances exhibiting moderate to high toxicity in the
aquatic compartment. The lowest effect concentrations found in chronic
studies with freshwater organisms ( Scenedesmus subspicatus, 96-h
EC50: 0.39 mg 2-nitrophenol/litre; Entosiphon sulcatum, 72-h
minimum inhibitory concentration, or MIC: 0.83 mg 4-nitrophenol/litre)
were 40-50 times higher than maximum levels determined in a densely
populated and highly industrialized Asian river basin (0.0072 mg
2-nitrophenol/litre and 0.019 mg 4-nitrophenol/litre). Therefore,
despite biotic and photochemical decomposition, nitrophenols emitted
to water could pose some risk to sensitive aquatic organisms,
particularly under surface water conditions not favouring both
elimination pathways. Because of their use patterns and release
scenarios, it is likely that nitrophenols pose only a minor risk to
aquatic organisms.
The available data indicate only a moderate toxicity potential of
nitrophenols in the terrestrial environment. From calculations of the
toxicity exposure ratio (TER) of nitrophenols from the degradation of
pesticides, only a minor risk for organisms in this compartment is to
be expected, even under a worst-case scenario.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
2-Nitrophenol (CAS No. 88-75-5; 2-hydroxy-1-nitrobenzene,
o-nitrophenol) and 4-nitrophenol (CAS No. 100-02-7;
4-hydroxy-1-nitrobenzene, p-nitrophenol) share the empirical formula
C6H5NO3. Their structural formulas are shown below.
 Technical-grade 2- and 4-nitrophenol from the German producer
have a typical purity of >99%. Named impurities are the corresponding
isomer for each product (0.3%) and traces of 3-nitrochlorobenzene
(<0.05%). Polychlorinated dibenzo- p-dioxin/dibenzofuran (PCDD/PCDF)
and tetrachlorodibenzo- p-dioxin/dibenzofuran (TCDD/TCDF) isomers
were not detected at detection limits between 0.1 and 0.4 µg/kg
product (BUA, 1992).
The pure nitrophenol isomers form pale yellow to yellow crystals
at room temperature. The substances are characterized by the
physicochemical properties given in Table 1 (Sax & Lewis, 1987).
Additional physicochemical properties for mononitrophenols are
presented in the International Chemical Safety Card (ICSC 1342)
reproduced in this document.
Table 1: Physicochemical properties of 2- and 4-nitrophenol.
Parameter 2-Nitrophenol 4-Nitrophenol
Molecular mass (g/mol) 139.11 139.11
Melting point (°C) 44-45 (1)(2)(3) 113-114 (1)(2)(3)
Boiling point (°C) 214-217 (1) 279 (decomposition)(3)
Vapour pressure (kPa) 6.8 × 10-3
(19.8 °C) (4) 3.2 × 10-6
(20 °C) (5)
Water solubility 1.26 12.4
(g/litre) (20 °C) (4) (20 °C) (6)
n-Octanol/water
partition
coefficient (log Kow) 1.77-1.89 (7) 1.85-2.04 (7)
Dissociation constant 7.23 7.08
(pKa) (21.5 °C) (8) (21.5 °C) (8)
Ultraviolet spectrum deltamax (water): deltamax (methanol):
230; 276 nm; no absorption
log epsilonmax: 3.57; maxima <290 nm (9)
3.80 (9)
Conversion factors 1 mg/m3 = 0.173 ppmv
1 ppmv = 5.78 mg/m3
References: (1) Budavari et al. (1996); (2) Booth (1991);
(3) Verschueren (1983); (4) Koerdel et al. (1981);
(5) Sewekow (1983); (6) Andrae et al. (1981);
(7) BUA (1992); (8) Schwarzenbach et al. (1988); (9) Weast (1979)
3. ANALYTICAL METHODS
The nitrophenol isomers are usually determined by gas
chromatography combined with mass spectrometric detection, flame
ionization detection, electron capture detection, or
nitrogen-sensitive detection, which are generally applied after
derivatization (BUA, 1992; Nick & Schoeler, 1992; Geissler & Schoeler,
1994; Harrison et al., 1994; Luettke & Levsen, 1994; Mussmann et al.,
1994). For liquid samples (water, urine, blood), high-performance
liquid chromatography in combination with concentration-gradient
elution (acetonitrile/methanol or ammonium acetate, acetic acid with
potassium chloride/methanol) and ultraviolet or electrochemical
detection, which can be carried out without derivatization, is also
used (BUA, 1992; Nasseredine-Sebaei et al., 1993; Ruana et al., 1993;
Paterson et al., 1996; Pocurull et al., 1996; Thompson et al., 1996).
The separation of the different isomers is carried out either by steam
distillation (BUA, 1992) or by the formation and subsequent extraction
of different ion pairs (León-González et al., 1992).
The following enrichment techniques are used (BUA, 1992; see also
review by Puig & Barcelo, 1996):
* solid-phase adsorption with thermal or liquid extraction for air
and water samples (Luettke & Levsen, 1994; Mussmann et al., 1994)
* liquid/liquid extraction after derivatization for water samples
(initial purification by acid/base fractionation of highly
polluted samples, increased recovery rates with continuous
extraction methods) (León-González et al., 1992; Nick & Schoeler,
1992; Geissler & Schoeler, 1994; Harrison et al., 1994)
* liquid extraction with acid/base fractionation or solid-phase
enrichment and subsequent desorption following aqueous extraction
for soil samples (Vozñáková et al., 1996)
* acid hydrolysis of the glucuronide with subsequent
derivatization for blood and urine samples or denaturation
(Nasseredine-Sebaei et al., 1993; Thompson et al., 1996).
The detection limits are <10 ng/m3 for air, 0.03-10 µg/litre
for water, and 200-1600 µg/kg for soil. A detection limit for the
determination of the nitrophenol isomers in biological materials was
given only for rat liver perfusate (0.5-1 mg/litre; Thompson et al.,
1996).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
There are no known natural sources of the nitrophenol isomers.
Within the European Union, 2- and 4-nitrophenol are produced
mainly by three companies. Six other large manufacturers are known in
the USA and Japan (as of 1989). In 1983, the production volume for
Western Europe was estimated at about 6400 t 2-nitrophenol and about
20 500 t 4-nitrophenol. In 1988-89, the German production volumes
originating from one manufacturer were approximately 500 t
2-nitrophenol and about 2000 t 4-nitrophenol, with about 20 t of each
being exported. Both 2- and 4-nitrophenol are intermediates in the
synthesis of azo dyes and a number of pesticides, mainly insecticides
(2-nitrophenol: carbofuran, phosalon; 4-nitrophenol: parathion,
parathion-methyl, fluorodifen) and, to a lesser extent, herbicides
(4-nitrophenol: nitrofen, bifenox). The corresponding aminophenols
that are gained by reduction are used as a photographic developer
(2-aminophenol) and as an intermediate in the synthesis of the
tuberculostatic 4-aminosalicylic acid and the analgesic
4-acetaminophenol (paracetamol) (4-aminophenol) (see also Booth,
1991). In the 1980s, the production volumes for 2- and 4-nitrophenol
showed a decreasing tendency in Germany as a result of changes in and
termination of the production of some organophosphorus pesticides.
The releases of 2- and 4-nitrophenol during production and
processing at the only German manufacturer appear to be of minor
importance. In 1988-89, about 2.5 kg 2-nitrophenol and 10 kg
4-nitrophenol were emitted to air, and <93 kg 2-nitrophenol and
<64 kg 4-nitrophenol were emitted to surface water.
For 1996, the following releases of 2- and 4-nitrophenol to the
environment were reported by manufacturers in the USA (TRI, 1998):
* 2-nitrophenol: from three manufacturers (one production site
each) with production volumes between 450 and 45 000 kg/year,
total releases of 15 kg to air and 23 kg into water were
reported.
* 4-nitrophenol: from three manufacturers (six production sites)
with production volumes of 45-450 kg/year up to 45 000-450 000
kg/year, a total release of 420 kg to air was reported. Data on
releases into water were not given.
2-Nitrophenol and 4-nitrophenol have been detected in the exhaust
gases of light-duty gasoline and diesel vehicles. Depending on the
motor load, the exhaust concentrations of the isomers were <50 µg/m3
exhaust gas (idle) and about 1000 µg 4-nitrophenol/m3 and 2000 µg
2-nitrophenol/m3 (driving at constant velocity) (Nojima et al., 1983;
Tremp et al., 1993). A regulated three-way catalytic converter reduced
the nitrophenol emissions to about 8% at high motor load and to about
2% at normal motor load (Tremp et al., 1993). A rough estimation
combining the above-mentioned exhaust gas concentrations with
estimations of the total exhaust gas volumes from vehicle traffic for
Germany resulted in an airborne nitrophenol load of at least several
tonnes per year from this source (BUA, 1992). Data concerning
nitrophenol releases from other combustion processes (heating, burning
of refuse) were not identified.
From laboratory experiments, there is some evidence that 2- and
4-nitrophenol are generated in the atmosphere during the photochemical
degradation of aromatic compounds such as benzene and toluene in the
presence of nitric oxide or hydroxyl radicals and nitrous dioxide.
These results were at least partly obtained in model experiments with
unrealistically high nitric oxide concentrations, and there are
competing reactions without nitrophenol formation for which the rate
constant is not known (BUA, 1992). However, smog chamber experiments
confirmed the formation of nitrophenol isomers during irradiation
(Leone & Seinfeld, 1985; Leone et al., 1985). Recent cloud water model
experiments showed that 2- and 4-nitrophenol are also formed from the
reaction of phenol with nitrogen pentoxide or monochloronitrogen
dioxide, especially under alkaline conditions (Scheer et al., 1996).
Estimations of the contribution of photochemically formed nitrophenols
to total emissions into the atmosphere are not possible with the
available data.
Significant releases of 4-nitrophenol into the hydrosphere may
occur from the hydrolytic degradation of the insecticides parathion
and parathion-methyl and -- although to a lesser extent -- from the
photolytic degradation of the herbicides nitrofen and bifenox.
Quantification of releases is not possible with the available data.
Furthermore, a considerable portion of airborne nitrophenols,
especially 4-nitrophenol, can be released to the hydrosphere and the
geosphere by wet and dry deposition (see section 5) (Herterich &
Herrmann, 1990; Luettke et al., 1997). Numerous studies concerning the
concentrations of 2- and 4-nitrophenol in wet deposition samples are
available (see section 6.1). From precipitation data (average 746 mm
rain per year for land masses, according to Baumgartner & Liebscher,
1990) and the measured concentrations of 2- and 4-nitrophenol in
rainwater, the release of nitrophenols via rain can be estimated to be
at least in the order of several thousand tonnes per year on a global
basis.
The application of the herbicides nitrofen and bifenox, which are
photolytically degraded to 4-nitrophenol in aqueous solutions, may
especially lead to emissions into the geosphere and the biosphere.
Further, nitrophenol-contaminated rain, snow, and other wet and dry
deposition may contribute to nitrophenol levels in soils. Data
concerning the release of nitrophenols into the biosphere are not
available.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Environmental releases of nitrophenols are mostly to ambient air,
surface waters, and -- to a smaller extent -- soil. Using a
non-steady-state equilibrium model, the following distribution of
4-nitrophenol in different environmental compartments was predicted:
air, 0.0006%; water, 94.6%; sediment, 4.44%; soil, 0.95%; biota,
0.000 09% (Yoshida et al., 1983). The distribution patterns of 2- and
4-nitrophenol sprayed on a natural soil in a standardized terrestrial
ecosystem were determined via radiotracer technique (14C). Of the
applied radioactivity (2-nitrophenol/4-nitrophenol), 49.45%/20.01% was
recovered in air, 27.38%/40.21% in soil (including animals),
12.73%/7.57% in plants, and 0.05%/0.02% in leachate (Figge et al.,
1985). Distribution of 4-nitrophenol in a terrestrial microcosm
chamber with artificial soil largely corresponded to this result (Gile
& Gillett, 1981). Owing to the expected decomposition within the
incubation periods of 30 and 28 days, respectively, it can be assumed
that most of the recovered radioactivity referred to breakdown
products of the applied nitrophenols.
In volatility experiments conducted according to Organisation for
Economic Co-operation and Development (OECD) guidelines, half-lives of
2-nitrophenol in water ranged from 14.5 to 27.3 days, indicating a
slow rate of volatilization (Koerdel et al., 1981; Rippen et al.,
1984; Scheunert, 1984; Schoene & Steinhanses, 1984). Measurements
concerning the partitioning between the gas and liquid phases of
clouds during different rain events showed that 2-nitrophenol is
enriched in the liquid phase to a larger extent than would be
predicted from its water solubility and vapour pressure. On the other
hand, 4-nitrophenol is extensively adsorbed to particles. Therefore,
elevated levels of this isomer are detected in the gaseous phase of
clouds (Luettke et al., 1997). From the available data, a significant
volatilization of 4-nitrophenol from water to air is not expected.
Since nitrophenols dissociate in aqueous solution, volatilization may
further decrease with increasing pH in surface waters. This leads to
the conclusion that dry and wet deposition of nitrophenols from air to
surface waters and soil are to be expected. The occurrence of this
partition mechanism is supported by the detection of 2- and
4-nitrophenol in rainwater and wet deposition samples (see section
6.1).
From experimental results on direct photodegradation (Koerdel et
al., 1981) and the atmospheric photo-oxidation by hydroxyl radicals
(Zetzsch et al., 1984), both pathways were found to be of minor
importance for the removal of 2-nitrophenol emitted to the
troposphere. Thus, the major degradation pathway for airborne
2-nitrophenol should be rapid nitration to 2,4-dinitrophenol
(Herterich & Herrmann, 1990; Luettke et al., 1997). The major portion
of airborne 4-nitrophenol is expected to be particle bound and
therefore only to a minor extent available for photochemical
reactions. Thus, most of the 4-nitrophenol can be washed out from air
by wet and dry deposition. Measured half-lives for the photochemical
decomposition of 4-nitrophenol in water exposed to sunlight ranged
from 2.8 to 13.7 days (Hustert et al., 1981; Mansour, 1996), being
longer with increasing pH (Hustert et al., 1981). Traces of
4-aminophenol were found as a photoproduct in river water (Mansour,
1996). In experiments conducted according to OECD guidelines, Andrae
et al. (1981) and Koerdel et al. (1981) found no hydrolysis of 2- or
4-nitrophenol under environmental conditions.
Numerous studies on the biodegradation of 2- and 4-nitrophenol
have been conducted. Standardized tests on ready or inherent
biodegradability provide data of large variability, indicating 2- and
4-nitrophenol to be inherently biodegradable under aerobic conditions
(depending on origin and density of inoculum and the applied test
method) (see Table 2). Results from different tests point to a
possible bacteriotoxic effect of 4-nitrophenol at concentrations above
300 mg/litre (Gerike & Fischer, 1979; Nyholm et al., 1984; Kayser et
al., 1994).
Non-standardized experiments with different inocula (e.g.,
natural water, soil, sediment) showed that microbial decomposition of
nitrophenols can occur in different environmental compartments after
adaptation of the microflora (Rubin et al., 1982; Subba-Rao et al.,
1982; Van Veld & Spain, 1983; Spain et al., 1984; Ou, 1985; Hoover et
al., 1986; Aelion et al., 1987; Wiggins et al., 1987). Time for
acclimation and degree of removal depended mostly on substance
concentration, microbial population, climate, and additional
substrates.
Biotic degradation of nitrophenols under anaerobic conditions
requires extended acclimatization of microbial communities. In tests
with sewage sludge and sludge from the primary anaerobic stage of a
municipal sewage treatment plant, respectively, initial 2- and
4-nitrophenol concentrations in the range of 96.5-579 mg/litre were
not degraded at all within 7-60 days (Wagner & Braeutigam, 1981;
Battersby & Wilson, 1989). Boyd et al. (1983) found complete anaerobic
removal of 50 mg/litre for all nitrophenol isomers within 1 week, but
complete mineralization was demonstrated only if the incubation period
was extended to 10 weeks. Anaerobic degradation even of high initial
nitrophenol concentrations was found by Tseng & Lin (1994), who
observed >90% removal of 2- and 4-nitrophenol (350-650 mg/litre) in a
biological fluidized bed reactor with three different kinds of
wastewater. From the available results, a slow degradation of
nitrophenols under anaerobic conditions by adapted microorganisms can
be expected.
Table 2: Biotic degradation of nitrophenols under aerobic conditions.
Test Substance Concentration Additional Test duration Removal Reference
(mg/litre) carbon source (days) (%)
Tests on ready biodegradability
AFNOR test 2-NP 40 OC no 14 16 Gerike & Fischer (1979)
Sturm test 2-NP 10 no 28 32 Gerike & Fischer (1979)
MITI I 2-NP 100 no 14 0 Urano & Kato (1986)
50 no 14 7 Gerike & Fischer (1979)
Closed bottle 4-NP 2 no 28 55 Rott et al. (1982)
test
Modified OECD 4-NP 20 DOC no 28 1 Rott et al. (1982)
screening test
Shake flask test 4-NP 20 OC no 21 50 Means & Anderson (1981)
AFNOR test 4-NP 40 OC no 14 97 Gerike & Fischer (1979)
Sturm test 4-NP 10 no 28 90 Gerike & Fischer (1979)
MITI I 4-NP 50 no 14 1 Gerike & Fischer (1979)
100 no 14 0 Urano & Kato (1986)
100 no 14 4.3 CITI (1992)
Tests on inherent biodegradability
Zahn-Wellens 2-NP 400 no 14 80 Gerike & Fischer (1979)
test
SCAS test 2-NP 20 TOC yes 24 107 Broecker et al. (1984)
13.3 TOC yes 24 110
Table 2 (continued)
Test Substance Concentration Additional Test duration Removal Reference
(mg/litre) carbon source (days) (%)
Bunch & Chambers 2-NP 5-10 yes 28 100 Tabak et al. (1981)
Coupled units 2-NP 12 OC yes 7 61 Gerike & Fischer (1979)
test
Batch test, 2-NP 200 COD no 5 97 Pitter (1976)
aerated
Zahn-Wellens 4-NP 300 no 14 8 Andrae et al. (1981)
test 100 DOC no 28 100 Pagga et al. (1982)
Activated sludge 4-NP 50 no 19 100 Means & Anderson
test 100 no 19 90 (1981)
SCAS test 4-NP 20 TOC yes 33 >90 Marquart et al. (1984)
27 >97 Scheubel (1984)
25/39 100 Ballhorn et al. (1984)
12-15 100 Koerdel et al. (1984)
Coupled units 4-NP 12 OC yes 7 100 Gerike & Fischer
test (1979)
Batch test, 4-NP 200 COD no 5 95 Pitter (1976)
aerated
Abbreviations used: 2-NP = 2-nitrophenol; 4-NP = 4-nitrophenol; OC = organic carbon; DOC = dissolved organic carbon;
TOC = total organic carbon; COD = chemical oxygen demand.
Soil sorption coefficients ( Koc) were found to increase with
increasing organic carbon content. Measured Koc values ranged from
44 to 230 (2-nitrophenol) and from 56 to 530 (4-nitrophenol) (Boyd,
1982; Broecker et al., 1984; Koerdel et al., 1984; Lokke, 1984;
Marquart et al., 1984). Nitrophenols emitted to soil are expected to
be biodecomposed under aerobic conditions. Infiltration into
groundwater is expected only under conditions unfavourable for
biodegradation (e.g., anaerobic conditions). From the available
experimental results, nitrophenols have to be classified as substances
with a low to moderate potential for soil sorption.
A low potential for bioaccumulation is to be expected from the
available valid test results for 2- and 4-nitrophenol.
Bioconcentration factors ranging from 14.6 to 24.4 were determined for
2-nitrophenol in a semistatic test system with zebra fish
( Brachydanio rerio) (Koerdel et al., 1984); in a flow-through
experiment, bioconcentration factors ranged from 30 to 76 for common
carp ( Cyprinus carpio), including possible conjugates (Broecker et
al., 1984). In static tests, accumulation factors for 4-nitrophenol of
11 for the green alga Chlorella fusca after 1 day (Geyer et al.,
1981) and 57 for the freshwater golden orfe ( Leuciscus idus
melanotus) after 3 days of exposure were determined (Freitag et al.,
1982). Zebra fish exposed in tap and river water nearly completely
eliminated the accumulated 14C-4-nitrophenol within 48 h (Ensenbach &
Nagel, 1991). Star fish ( Pisaster ochraceus) and sea urchin
( Strongylocentrotus purpuratus) eliminated 89% and 36%,
respectively, of injected 14C-4-nitrophenol (3.48 and 3.70 mg/kg body
weight, respectively) within 8 h (Landrum & Crosby, 1981).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
From the concentrations in rainwater, the total atmospheric
nitrophenol pollution in Switzerland is estimated at about 1 µg/m3
(Leuenberger et al., 1988). Recent measurements in the air of remote
areas in Europe (German Alps, Fichtelgebirge, Germany; Mount Brocken,
Germany; Great Dun Fell summit, United Kingdom) gave 2-nitrophenol
concentrations between 0.8 and 25 ng/m3 and 4-nitrophenol levels
between 1.2 and 360 ng/m3 (Herterich & Herrmann, 1990; Luettke et
al., 1997). The higher atmospheric 4-nitrophenol levels are apparently
due to the higher photochemical stability of this isomer (see section
5). 2-Nitrophenol was found in 22 out of 27 samples of air (range
1-140 ng/m3; detection limit 1 ng/m3) in Japan in 1994, and
4-nitrophenol was detected in 27 out of 27 air samples (range 1-71
ng/m3; detection limit 1 ng/m3) (Japan Environment Agency, 1995).
In street dust samples from a Japanese city, up to 3.9 mg
2-nitrophenol/kg and up to 42 mg 4-nitrophenol/kg were detected
(Nojima et al., 1983).
Numerous studies deal with the distribution, deposition, and
degradation behaviour of airborne 2- and 4-nitrophenol in clouds and
rainwater. 2-Nitrophenol levels in rainwater and snow between 0.03 and
5.7 µg/litre and 4-nitrophenol concentrations from <0.5 to
19 µg/litre are given in reports mainly from Germany and the USA (BUA,
1992). The recent measurements in rainwater, cloud water, and "fog"
(water vapour; not further characterized) from rural and urban areas
in Europe confirm these concentration ranges (Herterich & Herrmann,
1990; Levsen et al., 1990; Richartz et al., 1990; Capel et al., 1991;
Geissler & Schoeler, 1993; Levsen et al., 1993; Luettke et al., 1997).
The 2-nitrophenol levels are mostly below or slightly above the
detection limit (i.e., <0.1 µg/litre), whereas mean 4-nitrophenol
concentrations of about 5 µg/litre rainwater and cloud water and 20
µg/litre fog water were detected. The nitrophenol concentrations in
fog are significantly higher than those in rainwater or cloud water
owing to the higher droplet surface and longer residence times of the
droplets in air compared with rain. The lower concentrations of
2-nitrophenol in the deposition samples compared with 4-nitrophenol
are presumably due to the lower photochemical stability of this
compound (see section 5).
In the 1970s and early 1980s, the 2- and 4-nitrophenol
concentrations in the German and Dutch parts of the river Rhine and
some of its tributaries were between 0.1 and 1 µg/litre (BUA, 1992).
2-Nitrophenol and 4-nitrophenol were not detected in 177 samples of
Japanese surface waters (detection limits 0.04-10 µg/litre) or in 177
sediment samples (detection limits between 0.002 and 0.8 µg/kg) in
1978, 1979, and 1994 (Japan Environment Agency, 1979, 1980, 1995).
Whereas 4-nitrophenol was not detected in 129 fish samples (detection
limits 0.005-0.2 µg/kg) in Japan in 1979 and 1994, 2-nitrophenol was
detected in 1 out of 129 saltwater fish samples (detection limits
0.005-0.3 µg/kg) in 1994 (Japan Environment Agency, 1980, 1995).
2-Nitrophenol concentrations between <0.15 µg/litre (detection limit)
and 7.2 µg/litre and 4-nitrophenol levels between <0.1 and 18.8
µg/litre were reported for the densely populated and highly
industrialized Malaysian Klang river basin in 1990 and 1991 (Tan &
Chong, 1993).
6.2 Human exposure
Workers may be exposed to 2- and 4-nitrophenol via inhalation and
skin contact during production and processing (mainly in the
manufacturing of pesticides). However, data on nitrophenol
concentrations at the workplace were not identified.
Based on the measured concentrations given in section 6.1, an
exposure of the general population to nitrophenols via the environment
-- predominantly through ambient air and drinking-water -- cannot be
excluded.
4-Nitrophenol accumulates in fog, whereas 2-nitrophenol is
rapidly photochemically transformed (see sections 5 and 6.1). The mean
measured level of 4-nitrophenol in fog water is about 20 µg/litre.
In Dutch drinking-water samples, maximum concentrations of 1 µg
2-nitrophenol/litre and <0.1 µg 4-nitrophenol/litre were reported in
1988 (BUA, 1992). Further data are not available.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND
HUMANS
Studies providing quantitative information on the absorption,
metabolism, or elimination of 2- or 4-nitrophenol in humans were not
identified.
7.1 2-Nitrophenol
There is only very limited information available for
2-nitrophenol. In rabbits given a single dose of 200-330 mg/kg body
weight via gavage, most of the applied dose (>>80%) was excreted via
the urine within 24 h. About 71% was conjugated with glucuronic acid
and about 11% with sulfate, whereas about 3% was reduced to
aminophenols (Robinson et al., 1951).
Skin permeation for 2-nitrophenol was shown in several in vitro
experiments (Huq et al., 1986; Jetzer et al., 1986; Ohkura et al.,
1990).
Although the information is limited, bioaccumulation of
2-nitrophenol in organisms is not to be expected owing to its rapid
metabolism and excretion.
7.2 4-Nitrophenol
After oral, dermal, intravenous, or intraperitoneal application
of 4-nitrophenol to several test species (rats, mice, dogs, or
rabbits), most of the applied dose (up to 95%) was excreted as
glucuronide and sulfate conjugates of 4-nitrophenol via the urine
within 24-48 h. Only small amounts were excreted via faeces (about 1%)
or as unchanged 4-nitrophenol (about 2-7%). The percentages of
glucuronide and sulfate conjugates were shown to be species, sex, and
dose dependent. Although sulfate conjugation dominates at lower
4-nitrophenol concentrations, the percentage of glucuronide conjugates
increases at higher dosages (Robinson et al., 1951; Gessner & Hamada,
1970; Machida et al., 1982; Rush et al., 1983; Snodgrass, 1983;
Tremaine et al., 1984; Meerman et al., 1987). As shown in rabbits
after oral dosing, 4-nitrophenol undergoes reduction to 4-aminophenol
as well as glucuronidation and sulfation. Up to 14% of the
administered dose was detected as amino compounds in the urine
(Robinson et al., 1951). After intraperitoneal administration in mice,
4-nitrophenyl glucoside was identified as a minor metabolite of
4-nitrophenol (about 1-2% of the administered dose) (Gessner & Hamada,
1970).
For 4-nitrophenol, the pretreatment of laboratory animals with
ethanol (induction of cytochrome P-450) resulted in a marked increase
in hepatic microsomal hydroxylation. The 4-nitrocatechol then formed
competed with 4-nitrophenol for the glucuronidation and sulfation
pathways (Reinke & Moyer, 1985; Koop, 1986; McCoy & Koop, 1988; Koop &
Laethem, 1992).
Specific investigations on dermal resorption under non-occlusive
conditions showed dermal uptake of about 35% and 11% of the applied
dose of 14C-4-nitrophenol within 7 days in rabbits and dogs,
respectively. Skin permeation for 4-nitrophenol was also shown in
several in vitro experiments (Huq et al., 1986; Jetzer et al., 1986;
Ohkura et al., 1990).
Owing to its rapid metabolism and excretion, bioaccumulation of
4-nitrophenol in organisms is not to be expected.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
For 2-nitrophenol, the oral LD50 is in the range of
2830-5376 mg/kg body weight in rats (BASF AG, 1970; Vasilenko et al.,
1976; Vernot et al., 1977; Koerdel et al., 1981) and 1300-2080 mg/kg
body weight in mice (Vasilenko et al., 1976; Vernot et al., 1977).
Clinical signs following oral exposure were unspecific and included
dyspnoea, staggering, trembling, somnolence, apathy, and cramps. The
macroscopic examination performed in some studies revealed congestion
in liver and kidneys and ulcers of the stomach in high-dose rats. The
inhalation exposure of rats to an atmosphere saturated with the test
substance at 20°C for 8 h (no further information available) resulted
in no mortality and no signs of toxicity (BASF AG, 1970). In a limit
test, the dermal LD50 for the rat was >5000 mg/kg body weight
(Koerdel et al., 1981). In cats (two animals per dose group), the oral
application of 2-nitrophenol (50, 100, or 250 mg/kg body weight; no
controls) resulted in a dose-dependent increase in methaemoglobin (6,
44, and 57%, respectively).1 One animal dosed with 250 mg/kg body
weight died. No formation of methaemoglobin was detected after dermal
application of a 50% solution of 2-nitrophenol in water to rabbits
(dose not specified, exposure time 1 min to 20 h on the back or 20 h
on the ear) (BASF AG, 1970).
The oral LD50 of 4-nitrophenol is in the range of 220-620 mg/kg
body weight in rats (BASF AG, 1969; Vasilenko et al., 1976; Hoechst
AG, 1977a; Vernot et al., 1977; Andrae et al., 1981) and 380-470 mg/kg
body weight in mice (Vasilenko et al., 1976; Vernot et al., 1977).
Clinical signs following oral exposure of rats were unspecific and
included tachypnoea and cramps, and the macroscopic examination
performed in some studies revealed a greyish discoloration with dark
red patches of the lungs. No mortality was observed in rats after
single exposure (head only) to 4700 mg/m3 (application as dust
[sodium salt]; particle size not given) for 4 h. In four of six rats,
a corneal opacity was observed at the end of exposure, which persisted
through the 14-day observation period. In two extra rats exposed to
1510 mg/m3, the methaemoglobin concentrations were not altered
compared with controls. A determination of methaemoglobin
concentrations after exposure to 4700 mg/m3 was not performed (Smith
et al., 1988). In another inhalation study with rats (exposure to an
atmosphere saturated with the test substance at 20°C for 8 h; no
further information available), no mortality and no signs of toxicity
1 Methaemoglobin formation is discussed in greater detail in
section 8.8.
were seen (BASF AG, 1969). The dermal LD50 for rats and guinea-pigs
is >1000 mg/kg body weight (Hoechst AG, 1977b; Eastman Kodak Co.,
1980; Andrae et al., 1981). In contrast to 2-nitrophenol, no formation
of methaemoglobin was noted in cats (two animals per dose group) after
oral dosing with 100, 200, or 500 mg 4-nitrophenol/kg body weight. The
mortality rate was 0/2, 1/2, and 2/2, respectively (BASF AG, 1969).
8.2 Irritation and sensitization
From studies comparable to OECD Guidelines 404 and 405, it can be
concluded that 2-nitrophenol is slightly irritating to the skin but
not to the eye (scores not given). In a Buehler test with guinea-pigs
comparable to OECD Guideline 406, the substance showed no
skin-sensitizing effects (Koerdel et al., 1981).
In a study performed according to US Food and Drug Administration
(FDA) guidelines, non-dissolved 4-nitrophenol was slightly irritating
to the skin (score 2 of 8) (Hoechst AG, 1977c); in another study
comparable to OECD Guideline 404, however, the non-dissolved substance
showed no skin-irritating effects (score 0 of 4) (Andrae et al.,
1981). 4-Nitrophenol applied as a 10% solution to the eyes was
slightly irritating in a test conducted according to FDA guidelines
(scores not given; Hoechst AG, 1977c). Results with the non-dissolved
substance were either strongly irritating in a test conducted
according to FDA guidelines (scores not given; Hoechst AG, 1977c) or
slightly irritating in a test comparable to OECD Guideline 405 (score
1-2 of 4; Andrae et al., 1981).
In a guinea-pig maximization test comparable to OECD Guideline
406, skin sensitization was shown in 5 of 20 animals (Andrae et al.,
1981).
Data on respiratory tract sensitization for 2- and 4-nitrophenol
were not identified in the literature.
8.3 Short-term exposure
8.3.1 Oral exposure
The effect of 2-nitrophenol in rats was studied in a 28-day study
to evaluate OECD Guideline 407 (five animals per sex per dose group;
daily oral doses of 0, 22, 67, or 200 mg/kg body weight via gavage).
Food intake decreased in high-dose males and in mid- and high-dose
females, and final body weight decreased non-significantly in all
dosed animals. The absolute liver and kidney weights were decreased in
mid-dose animals, and the relative testes weight increased in low- and
mid-dose males and decreased in high-dose males. In all dosed animals,
the relative and absolute weights of the adrenal glands increased. The
haematological examination, clinical chemistry, and histopathological
examination of the major organs and tissues did not give any
indication of a substance-related toxic effect in comparison with
controls (Koerdel et al., 1981). Owing to insufficient documentation
and the fact that there were minor effects (weight of adrenal glands)
shown by all exposed animals, a reliable NO(A)EL cannot be deduced.
In a 28-day study that was also conducted to evaluate OECD
Guideline 407, Sprague-Dawley rats (10 per sex per dose group)
received daily oral doses of 0, 70, 210, or 630 mg 4-nitrophenol/kg
body weight via gavage. After dosing, locomotor inhibition, which
lasted for about 2 h, was seen in mid- and high-dose animals. In
mid-dose animals, 1/10 males died; in high-dose males and females, the
mortality rate was 4/10 and 6/10, respectively (specific signs of
intoxication were not given). In the lowest dose group, the
macroscopic examination revealed seven cases of pale liver, and the
histopathological examination showed 14 cases of finely dispersed
fatty degeneration. A focal fatty degeneration of the liver was also
observed in 13/20 rats of the mid-dose group, but not in high-dose
animals. However, it must be noted that finely dispersed fatty
degeneration was also seen in 6/20 control animals. In 4/10 high-dose
males but not females, a hydropic liver cell swelling was noted, and
all high-dose rats that died before the end of the study showed
vascular congestion of the liver. A slight increase in the leukocyte
count was seen at 210 and 630 mg/kg body weight in males and females;
the increase was significant in high-dose females. In high-dose males,
the alanine aminotransferase (ALAT) activity was significantly
increased. Other substance-related effects in high-dose animals
included increased nephrosis (two males and five females), testicular
atrophy and inhibition of spermatogenesis (one and two males,
respectively), and follicular atresia in the ovaries (four females)
(Andrae et al., 1981). Because of unclear effects in the liver, a
NO(A)EL cannot be deduced.
8.3.2 Inhalation exposure
8.3.2.1 2-Nitrophenol
In Sprague-Dawley rats (15 per sex per group), no mortality was
observed after exposure to 0, 5, 30, or 60 mg 2-nitrophenol vapour/m3
("whole body" exposure; to generate the vapour, melted 2-nitrophenol
was used) for 6 h/day, 5 days/week, over a period of 4 weeks. Except
for squamous metaplasia of the epithelium lining the maxilloturbinates
and nasoturbinates in all high-dose animals, the clinical and
histopathological examinations gave no consistent exposure-related
effects. The methaemoglobin values determined after the 11th exposure
were significantly increased only in low-dose animals (males: 1.0,
2.3, 1.8, and 1.6%; females: 2.0, 4.1, 2.1, and 1.1%), but were within
control values at the end of the study (Hazleton Lab., 1984).
8.3.2.2 4-Nitrophenol
No mortality was observed in male albino Crl:CDR rats (10 per
group) after exposure to 0, 340, or 2470 mg 4-nitrophenol dust/m3
(application as sodium salt; "head only" exposure; mass median
aerodynamic diameter [MMAD] 4.6-7.5 µm) for 6 h/day, 5 days/week, over
a period of 2 weeks. Both exposure concentrations resulted in signs of
irritation (not further specified). After exposure to 340 and 2470
mg/m3, darker urine, proteinuria, elevated aspartate aminotransferase
(ASAT) values, and a dose-dependent increase in methaemoglobin values
were observed. These effects were still evident after a 14-day
recovery period; however, the methaemoglobin value was then still
elevated in only 2/5 high-dose animals. The methaemoglobin values were
0.2, 0.87, and 1.53% after 10 exposures and 0.2, 0.13, and 0.7% after
14 days' recovery. The erythrocyte, haemoglobin, and haematocrit
values decreased during exposure but were elevated after the 14-day
recovery period. In treated rats, the urine volume decreased in a
dose-dependent manner during exposure and during the 14-day recovery
period. In high-dose animals, the absolute spleen weight was
significantly lower than that of controls after 10 exposures, and the
absolute/relative spleen and lung weights were significantly lower in
comparison with controls at the end of the recovery period. According
to the authors, the biological significance of the changes in organ
weights is unknown owing to the absence of corroborating pathological
effects (Smith et al., 1988).
In a second trial (exposure to 0, 30, or 130 mg/m3; MMAD 4.0-4.8
µm), both exposure concentrations again resulted in signs of
irritation (not further specified). Methaemoglobinaemia, an effect
that was reversible within a 14-day recovery period, was seen only at
130 mg/m3. The methaemoglobin values were 0.5, 0.3, and 1.5% after 10
exposures and 0.4, 0.5, and 0.2% after 14 days' recovery. The gross
and histopathological examination revealed no adverse effects in any
dose group. From these results, the authors of the study decided upon
a NO(A)EL of 30 mg/m3 (Smith et al., 1988).
Groups of Sprague-Dawley rats (15 per sex) were exposed to 0, 1,
5, or 30 mg 4-nitrophenol dust/m3 ("whole body" exposure; MMAD
5.2-6.7 µm) for 6 h/day, 5 days/week, over a period of 4 weeks. The
exposure resulted in no deaths, and no exposure-related effects were
noted in terms of haematology or clinical chemistry values, gross
examination, histopathology, and body or organ weights. In high-dose
animals, unilateral and bilateral diffuse anterior capsular cataracts
were observed. The methaemoglobin values determined after 2 weeks of
exposure showed great variability and appeared to be unusually high
(>3 %) in some unexposed controls. However, the group total
methaemoglobin value was increased at a concentration of 5 mg/m3,
which was significant in males and not significant in females (males:
0.8, 0.5, 2.2, and 1.1%; females: 1.3, 1.1, 2.0, and 1.0%) (Hazleton
Lab., 1983). Therefore, a NO(A)EL of 5 mg/m3 can be derived for local
effects (cataracts), whereas the NO(A)EL for systemic effects
(formation of methaemoglobin) may be lower.
8.3.3 Dermal exposure
Data concerning short-term dermal exposure were not identified in
the literature.
8.4 Long-term exposure
In the literature, subchronic and chronic studies are available
only for 4-nitrophenol.
8.4.1 Subchronic exposuren
In a 13-week gavage study with Sprague-Dawley rats (20 per sex
per dose group) given 0, 25, 70, or 140 mg 4-nitrophenol/kg body
weight in water 5 days/week, premature deaths were seen in animals
dosed with 70 and 140 mg/kg body weight (1 male/1 female at 70 mg/kg
body weight and 15 males/6 females at 140 mg/kg body weight); these
were usually preceded by clinical signs, including pale appearance,
languid behaviour, prostration, wheezing, and dyspnoea, shortly after
dosing. The histopathological examination of these animals revealed
minimal to moderately severe congestion in the lung, liver, kidney,
adrenal cortex, and pituitary; in surviving animals, no
treatment-related changes compared with controls were reported. A
statement concerning altered methaemoglobin values cannot be given
owing to a non-reliable analytical method (about 13% in controls at
week 7) (Hazleton Lab., 1989). Therefore, only a provisional NO(A)EL
(changes in liver, kidneys, and lungs) of 25 mg/kg body weight can be
derived from this study. The NO(A)EL based on the formation of
methaemoglobin may be lower.
The dermal application of 4-nitrophenol to Swiss-Webster mice (10
per sex and dose group; given 0, 22, 44, 88, 175, or 350 mg/kg body
weight in acetone, 3 times per week over 13 weeks) resulted in
dose-dependent mortality as well as skin irritation/inflammation and
necrosis at >175 mg/kg body weight.1
1 Gulf South Research Institute, not dated;
no further information available; results cited from
NTP (1993).
8.4.2 Chronic exposure and carcinogenicityn
In a long-term study with Swiss-Webster mice (50 per sex per dose
group), 4-nitrophenol in acetone was applied to the interscapular skin
at doses of 0, 40, 80, or 160 mg/kg body weight, 3 days/week for 78
weeks. At termination of the study, the survival rates were 29/60,
17/60, 26/60, and 24/60 for males and 35/60, 26/60, 33/60, and 27/60
for females. The increased mortality after 60 weeks was due to a
generalized amyloidosis (the severity of the amyloidosis was similar
among dosed and control animals) and secondary kidney failure. The
final mean body weights of the dosed animals were similar to those of
the controls. NTP (1993) stated that there were no substance-related
neoplastic or non-neoplastic effects associated with the dermal
administration of 4-nitrophenol and that there was no evidence of a
carcinogenic activity of the substance in male or female mice.
In another study, which had several procedural deficiencies (only
the skin was examined; only 12 weeks of exposure), no skin tumours
were observed in 31 female Sutter mice after dermal application of a
20% solution (25 µl of solution applied twice weekly) of 2- or
4-nitrophenol in dioxane (Boutwell & Bosch, 1959).
8.5 Genotoxicity and related end-points
The available in vitro and in vivo genotoxicity studies on
2- and 4-nitrophenol are summarized in Table 3.
2-Nitrophenol showed no mutagenicity in several limited bacterial
assays. From the available data, it is not possible to draw any
conclusions regarding its mutagenicity.
For 4-nitrophenol, positive results were obtained in in vitro
tests for chromosomal aberrations in mammalian cells. However, apart
from one well-documented study published by NTP (1993), the other
available assays were inadequately reported. 4-Nitrophenol was shown
to be mutagenic in some but not all of the bacterial assays, whereas
other studies (i.e., host-mediated bacterial assay, mouse lymphoma
assay, unscheduled DNA synthesis assay [apparently in vitro], sister
chromatid exchange assay, sex-linked recessive lethal [SLRL] assay in
Drosophila) gave negative results. In the absence of any in vivo
mutagenicity studies in mammals, it is not possible to conclude
whether or not the mutagenic potential of 4-nitrophenol is expressed
in vivo.
Table 3: Genotoxicity of 2- and 4-nitrophenol in vitro and in vivo.
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
2-Nitrophenol
(in vitro studies)
delta phage DNA Induction of 35 mg - 0 Yamada et al.
DNA breakage (1987)
Bacillus subtilis Recombination 0.01-0.5 - 0 Shimizu & Yano
H17, M45 assay mg/plate (1986)
Salmonella Reverse 0.003-2.5 - - Koerdel et al. (1981);
typhimurium mutations mg/plate Haworth et al. (1983);
TA1535, TA1537 Shimizu & Yano (1986)
Salmonella Reverse 0.01-2.5 - - Koerdel et al. (1981);
typhimurium mutations mg/plate Shimizu & Yano (1986)
TA1538
Salmonella Reverse 0.0007-5 - - Suzuki et al. Chiu et al. (1978);
typhimurium mutations mg/plate (1983) also Koerdel et al. (1981);
TA98, TA100 tested both Haworth et al. (1983);
strains in Suzuki et al. (1983);
the presence Shimizu & Yano (1986);
of norharman, Kawai et al. (1987);
which also Dellarco & Prival (1989);
gave negative Massey et al. (1994)
results
Table 3 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
2-Nitrophenol (in vivo studies)
Drosophila SLRL assay via feed (400 and 500 - Foureman et al. (1994)
melanogaster ppm) or injection
(2500 and 5000 ppm)
4-Nitrophenol (in vitro studies)
delta phage DNA Induction of 35 mg - 0 Yamada et al. (1987)
DNA breakage
Bacillus subtilis Recombination 0.01-5 mg/plate + 0 positive Shimizu
H17, M45 assay at 0.5 & Yano (1986)
mg/plate
Escherichia coli Gene mutation 0.001-2.5 - - Hoechst AG (1980)
WP2uvrA mg/plate
Escherichia coli Gene mutation 0.125-2 mg/plate - 0 Rashid & Mumma (1986)
K-12 (Pol
A1+/Pol1-), WP2
(WP2, WP2uvrA,
WP67, CM611,
CM571)
Escherichia coli DNA cell 7 or 70 mg + + positive at Kubinski et al.
Q13 binding assay 70 mg (1981)
Table 3 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
Saccharomyces Mitotic gene 2.9 mg/ml (+) 0 Fahrig (1974)
cerevisiae conversion
ade 2, trp 5
Salmonella DNA damage up to 0.75 - - Nakamura et al.
typhimurium (umu test) mg/ml (1987)
TA1535/pSK 1002
Salmonella Reverse 0.001-2.5 + - positive Hoechst
typhimurium mutation mg/plate at >0.1 AG (1980)
TA1538 mg/plate
Salmonella Reverse 0.01-5 - - Andrae et al. (1981);
typhimurium mutation mg/plate Shimizu & Yano (1986)
TA1538
Salmonella Reverse 0.125-2 - 0 Rashid & Mumma (1986)
typhimurium mutation mg/plate
TA1538, TA1978
Salmonella Reverse 0.0007- - - Suzuki et McCann et al. (1975);
typhimurium mutation 5 mg/plate al. (1983) Hoechst AG (1980);
TA98, TA100 also tested Andrae et al. (1981);
both strains Haworth et al. (1983);
in the Suzuki et al. (1983);
presence of Shimizu & Yano (1986);
norharman, Kawai et al. (1987);
which also Dellarco & Prival (1989);
gave negative Massey et al. (1994)
results
Table 3 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
Salmonella Reverse 0.001-5 - - McCann et al. (1975);
typhimurium mutation mg/plate Hoechst AG (1980);
TA1535, TA1537 Andrae et al. (1981);
Haworth et al. (1983);
Shimizu & Yano (1986)
Rat hepatocytes DNA damage 42-417 mg (+) 0 Weakly Storer
(alkaline positive at et al. (1996)
elution) >97 mg
Rat hepatocytes DNA repair 4.2-417 mg - 0 Andrae et al. (1981)
Chinese hamster Chromosomal without S9 mix: - + NTP (1993)
ovary (CHO) aberration 0.1-0.5 mg/ml
cells with S9 mix:
1.25-2 mg/ml
Chinese hamster Sister chromatid without S9 mix: - - NTP (1993)
ovary (CHO) cells exchange 0.00017-0.025 mg/ml
with S9 mix:
0.05-1.5 mg/ml
Mouse lymphoma Forward mutation without S9 mix: - - Oberly et al. (1984)
assay 0.7-1.5 mg/ml
L5178Y with S9 mix:
TK+/- cells 0.0001-0.03
mg/ml
Table 3 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
Mouse lymphoma Forward mutation 0.06-0.78 mg/ml 0 - Amacher & Turner (1982)
assay L5178Y
TK+/- cells
Rat hepatocytes Unscheduled DNA 0.00007-0.14 mg/ml - 0 Probst et al. (1981)
synthesis
Human lymphocytes Chromosomal not given + No data about Huang et al. (1996)
aberration metabolic
activation;
validity
cannot be
judged
(documentation
and study
design
insufficient
for
assessment)
lowest
positive
concentration:
1.4 mg/ml
Table 3 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
Human Chromosomal 0.001-0.3 mg/ml + No data about Huang et al. (1995)
lymphocytes aberration metabolic
activation;
validity cannot
be judged
(documentation
and study
design
insufficient
for assessment)
Human fibroblasts DNA repair 0.14-139 mg + No data about Poirier et al. (1975)
(WI-38) metabolic
activation;
validity
cannot be
judged
(documentation
insufficient
for assessment)
positive at
>13.9 mg
Table 3 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
4-Nitrophenol (in vivo studies)
NMRI mice Host-mediated single subcutaneous - application of Buselmaier et al. (1972)
assay (tester injection of test substance
strains 75 mg/kg immediately
Salmonella body weight after the
typhimurium G 46 bacteria had
and Serratia been injected
marcescens a into the
21 Leu-) abdominal
cavities;
test duration
3 h
Drosophila SLRL assay via feed (1000, - Zimmering et al. (1985);
melanogaster 2500, 6000, or Foureman et al. (1994)
7500 ppm) or
injection (1000
or 1500 ppm)
a -, negative; +, positive; (+), weakly positive; 0, not tested.
8.6 Reproductive and developmental toxicity
8.6.1 Reproductive toxicity
In a valid two-generation study with groups of 24 female and 12
male Sprague-Dawley rats carried out by Angerhofer (1985),
4-nitrophenol dissolved in ethanol was applied dermally at doses of 0,
50, 100, or 250 mg/kg body weight per day, 5 days/week. The F0
generation was exposed over a period of 140 days before mating. Dosing
of the F0 females continued throughout breeding, gestation, and
lactation. Groups of 26 females and 13 males of the F1 generation
were then exposed for 168 days in the same manner as had been the F0
rats; the females were again exposed throughout breeding, gestation,
and lactation. Apart from dose-related signs of skin irritation
(erythema, scaling, scabbing, and cracking) in dosed animals, the
gross and histopathological examinations provided no indication of
significant adverse effects. The calculated indices concerning
fertility, gestation, viability, and lactation were not different from
those of controls. The testis to body weight ratios in the F0
generation were not affected, and histological lesions were not
observed in the testes. In a 28-day study in rats (see section 8.3.1),
testicular atrophy and inhibition of spermatogenesis were observed in
some animals after oral dosing at a level of 630 mg/kg body weight,
but not at 210 mg/kg body weight.
8.6.2 Developmental toxicity
8.6.2.1 2-Nitrophenol
In a range-finding study with Charles River COBS(c) CD(c) rats
(five dams per group; application of 0, 50, 125, 250, 500, or
1000 mg/kg body weight via gavage from day 6 to day 15 of gestation;
uterine examination on day 20), dose levels of 500 and 1000 mg/kg body
weight caused signs of maternal toxicity (transient but dose-related
decrease in weight gain early during treatment). One high-dose animal
died, but no cause of death could be determined. Other clinical
findings included darkly coloured urine at >250 mg/kg body weight
and yellow staining of haircoat (at the nose, mouth, anogenital area)
at >125 mg/kg body weight; the necropsy findings gave no
biologically meaningful differences in surviving dams. At the highest
dose level of 1000 mg/kg body weight, a slight but statistically
significant (also compared with historical controls) increase in group
mean post-implantation losses (13.8% versus 8.2% in controls) and mean
early resorptions (2.3 versus 1.2 in controls) was seen. No effects
were observed on the number of viable fetuses, implantations, or
corpora lutea (International Research and Developmental Corporation,
1983).
8.6.2.2 4-Nitrophenol
In both studies cited below, a complete examination of the pups
for possible teratogenic effects was not performed. In addition, owing
to limitations of these studies (i.e., use of only one dose group or
exposure to a mixture), reliable NO(A)EL values cannot be derived.
In a study performed by Booth et al. (1983), groups of 50 female
CD-1 mice received daily oral doses of 400 mg 4-nitrophenol/kg body
weight via gavage from day 7 to day 14 of gestation. The survival rate
in pregnant mice ( n = 36) was 81% versus 100% in controls, and dosed
animals showed less maternal weight gain. No changes were observed in
the reproductive index (ratio between survivors delivered and pregnant
survivors). The average number of live pups per litter was slightly
decreased, but 4-nitrophenol produced no gross abnormalities.
Kavlock (1990) studied the developmental toxicity of
4-nitrophenol in Sprague-Dawley rats. The substance (dissolved in a
mixture of water, Tween 20, propylene glycol, and ethanol [4:4:1:1])
was applied via gavage to groups of 12-13 animals at doses of 0, 100,
333, 667, or 1000 mg/kg body weight on day 11 of gestation. Endpoints
concerning maternal toxicity included signs of toxicity, mortality,
body weight gain, and the number of implantation scars in the uteri at
weaning. In the offspring, viability, body weight on postnatal days
1-6, overt malformations, and perinatal loss were recorded. In dams,
the mortality was increased at a dose level of >667 mg/kg body
weight; at a dose level of >333 mg/kg body weight, the litter size
on postnatal days 1 and 6 was non-significantly decreased.
8.7 Immunological and neurological effects
There are no studies available dealing specifically with
immunological or neurological effects. There is an indication from an
in vitro study that 4-nitrophenol may act as a suppressor of
cell-mediated immune response (Pruett & Chambers, 1988). However, the
biological significance is uncertain.
8.8 Methaemoglobin formation
Methaemoglobin formation by 2-nitrophenol and 4-nitrophenol has
been tested in several studies using different species, routes, and
durations of applications. An overview is given in Table 4.
2-Nitrophenol clearly leads to the formation of methaemoglobin in
a dose-dependent manner in cats (BASF AG, 1970), the most sensitive
species. The lowest dose tested, 50 mg/kg body weight, produced
increased methaemoglobin levels. In inhalation experiments in rats,
elevated methaemoglobin levels were observed at an exposure level of 5
mg/m3; methaemoglobin levels were less elevated at exposure levels of
30 and 60 mg/m3 (Hazelton Lab., 1984).
4-Nitrophenol, in contrast, did not lead to methaemoglobin
formation in cats at concentrations up to 500 mg/kg body weight (BASF
AG, 1969). In rats, at high concentrations in inhalation experiments,
the methaemoglobin-forming capacity seemed to be very low (1.5% at
2470 mg/m3). In conclusion, 4-nitrophenol may induce methaemoglobin
formation, but the effect seems to be rather weak, without clear
dose-response.
9. EFFECTS ON HUMANS
Naniwa (1979) performed patch tests with 4-nitrophenol,
4-aminophenol, 2-amino-4-chlorophenol,
3'-chlorodiphenylamine-2-carboxylic acid, and 4-dichloronitrobenzene
(0.1, 0.5, or 1% in petrolatum) on 31 employees probably exposed to
these chemicals in a chemical factory and on 5 control persons. In
four employees, a positive reaction to 4-nitrophenol was observed,
although none of these persons reacted positively to all three tested
concentrations. All four employees also reacted positively to
2-amino-4-chlorophenol, which was shown to be a strong sensitizer.
Therefore, 2-amino-4-chlorophenol may act as the primary allergen, and
the effects observed with 4-nitrophenol may be due to
cross-sensitization.
In 27 patients primarily sensitized to
1-chloro-2,4-dinitrobenzene, no cross-sensitization due to
4-nitrophenol (1-2% in petrolatum) was observed. In addition, 15
patients with a chloramphenicol allergy failed to react to
4-nitrophenol (Eriksen, 1978).
Table 4: Methaemoglobin formation by 2-nitrophenol and 4-nitrophenol.
Species Route Frequency/duration Dose Results Reference
(strain/number/dose/sex) (% metHb)
2-Nitrophenol
cat oral 1 × 50 mg/kg body weight 6 BASF AG (1970)
2 100 44
sex not given 250 57
rabbit dermal 1 × 50% solution no increase BASF AG (1970)
number and sex in water
not given
rat inhalation 6 h/day m f Hazleton Lab.
Sprague-Dawley 5 days/week 0 mg/m3 1.0 2.0 (1984)
15 m/15 f 4 weeks 5 2.3 4.1
30 1.8 2.1
60 1.6 1.1
11th day of
treatment
4-Nitrophenol
cat oral 1 × 100 mg/kg body no increase BASF AG (1969)
2 200 weight
sex not given 500
rat oral 5 days/week 0 mg/kg body weight analytical Hazleton Lab.
Sprague-Dawley 13 weeks 25 method not (1989)
20 m/20 f 70 reliable (13%
140 in controls)
Table 4: Methaemoglobin formation by 2-nitrophenol and 4-nitrophenol.
Species Route Frequency/duration Dose Results Reference
(strain/number/dose/sex) (% metHb)
rat inhalation 6 h/day 0 mg/m3 0.2 0.2 Smith et al.
Crl:CDR 5 days/week 340 0.87 0.13 (1988)
10 m 2 weeks 2470 1.53 0.7
end of treatment
and after 14
days of recovery
rat inhalation 6 h/day 0 mg/m3 0.5 0.4 Smith et al.
Crl:CDR 5 days/week 30 0.3 0.5 (1988)
10 m 2 weeks 130 1.5 0.2
end of treatment
and after 14
days of recovery
rat inhalation 6 h/day m f Hazleton Lab.
Sprague-Dawley 5 days/week 0 mg/m3 0.8 1.3 (1983)
15 m/15 f 4 weeks 1 0.5 1.1
5 2.2 2.0
30 1.1 1.0
after 2 weeks of
exposure, values
unusually high
in some control
animals
Abbreviations used: m = male; f = female; metHb = methaemoglobin.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1 Aquatic environment
Experimental test results for the most sensitive species are
summarized in Table 5. Additional data on the toxicity of 2- and
4-nitrophenol to aquatic organisms are cited in BUA (1992). Among all
tested organisms, the protozoan Entosiphon sulcatum and the green
alga Scenedesmus subspicatus proved to be most sensitive in chronic
cell multiplication inhibition tests with freshwater species. Daphnia
magna exhibited a 21-day lowest-observed-effect concentration (LOEC)
of 1.0 mg 2-nitrophenol/litre in the Daphnia reproduction test
(Koerdel et al., 1984). The entoproct Barentsia matsushimana was the
most sensitive marine invertebrate species tested, exhibiting a 49-day
EC50 value of 0.21 mg 4-nitrophenol/litre and a minimal effective
concentration (ECm) of 0.03 mg/litre (end-point: growth of
germinated spores) (Scholz, 1986). Freshwater fish showed less
sensitivity. The lowest 96-h LC50 value of 3.8 mg 4-nitrophenol/litre
was determined for rainbow trout ( Oncorhynchus mykiss) (Howe et
al., 1994). The measured no-observed-effect concentration (NOEC) for
behavioural changes in a 28-day flow-through test with zebra fish was
2 mg 2-nitrophenol/litre (Broecker et al., 1984). After prolonged
exposure of zebra fish to 4-nitrophenol, minor morphological
alterations of the liver, even at a concentration of 0.1 mg/litre,
were observed. At 1 and 5 mg/litre, about 25% of the animals showed
symptoms of degenerative transformation of the liver tissue (Braunbeck
et al., 1989).
10.2 Terrestrial environment
The toxicity of 2- and 4-nitrophenol on higher plants according
to OECD Guideline 208 was tested in independent studies. After
incubation of seeds with different test substance concentrations,
14-day EC50 values for reduced fresh weight of grown shoots were in
the range of 52-420 mg 2-nitrophenol/kg soil (Broecker et al., 1984;
Koerdel et al., 1984) and 35-260 mg 4-nitrophenol/kg soil (Ballhorn et
al., 1984; Marquart et al., 1984). The 14-day EC10 value for
2-nitrophenol was 10 mg/kg soil for both species. Overall, turnip
( Brassica rapa) proved to be more sensitive than oat
( Avena sativa).
In tests conducted according to OECD Guideline 207, the adverse
effects of 2- and 4-nitrophenol on earthworms were examined in several
independent studies. In the contact test, in which the animals are
exposed on filter paper soaked with the test substance, Neuhauser
et al. (1985) established a 48-h LC50 value of 5.9 µg/cm2 for the
toxicity of 2-nitrophenol on Eisenia fetida. For 4-nitrophenol, the
48-h LC50 values were in the range of 0.7-2.7 µg/cm2, with
Table 5: Aquatic toxicity of nitrophenols.
Most sensitive species
(test method/end-point) Substance Effective Reference
concentration
(mg/litre)
Bacteria
Pseudomonas putida 2-NP 16-h MICa: 0.9 Bringmann & Kuehn (1977)
(cell multiplication 4-NP 16-h MIC: 4.0
inhibition test)
Protozoa
Entosiphon sulcatumn 2-NP 72-h MIC: 0.40 Bringmann (1978);
(cell multiplication 4-NP 72-h MIC: 0.83 Bringmann et al. (1980)
inhibition test)
Algae
Scenedesmus subspicatus 2-NP 96-h EC50: 0.39 Broecker et al. (1984);
Chlorella vulgaris 2-NP 6-h EC50: 1.53 Kramer et al. (1986)
(cell multiplication 4-NP 6-h EC50: 6.97
inhibition test)
Invertebrates
Moina macrocopa (acute) 2-NP 3-h LC50: 1.9 Yoshioka et al. (1985)
(immobilization) 4-NP 3-h LC50: 1.3
Daphnia magna (long-term) 2-NP 21-day LOEC: 1.0 Koerdel et al. (1984)
(immobilization/reproduction) 4-NP 21-day NOEC: 1.3
Barentsia matsushimana (marine) 4-NP 49-day EC50: 0.21 Kuehn et al. (1988)
(growth of germinated spores) 4-NP 49-day ECmb: 0.03 Scholz (1986)
Table 5 (cont'd)
Most sensitive species
(test method/end-point) Substance Effective Reference
concentration
(mg/litre)
Fish
Cyprinus carpio (static) 2-NP 96-h LC50: 36.6 Lang et al. (1996)
Oncorhynchus mykiss (static) 4-NP 96-h LC50: 3.8 Howe et al. (1994)
Oncorhynchus mykiss (flow-through) 4-NP 96-h LC50: 7.93
Abbreviations used: 2-NP = 2-nitrophenol; 4-NP = 4-nitrophenol.
a MIC = minimum inhibitory concentration.
b ECm = minimal effective concentration.
Eisenia fetida and Eudrilus eugeniae being the most sensitive
species tested (Roberts & Dorough, 1984; Neuhauser et al., 1985,
1986). When exposed in an artificial soil mixture, 28-day LC50 values
for 2-nitrophenol were in the range of 250-500 mg/kg soil ( Eisenia
fetida) (Broecker et al., 1984; Koerdel et al., 1984), and 14-day
LC50 values for 4-nitrophenol were in the range of 38-67 mg/kg soil,
again with Eisenia fetida and Eudrilus eugeniae as the most
sensitive species tested (Ballhorn et al., 1984; Marquart et al.,
1984; Neuhauser et al., 1985, 1986).
The environmental relevance, particularly of the earthworm
contact test, seems questionable. Critical results from this test, as
sole effect data on terrestrial organisms, should not justify a
classification of tested substances as highly toxic to earthworms or
other soil organisms. The available data on microorganisms and plants
indicate only a moderate toxicity potential in the terrestrial
environment.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessmentn
In general, there is only limited information concerning the
toxicological profiles of 2- and 4-nitrophenol.
In experimental animals given 4-nitrophenol orally,
intravenously, or intraperitoneally, most of the applied dose was
excreted via the urine within 24-48 h as glucuronide and sulfate
conjugates, while only very small amounts were excreted via faeces or
as unchanged 4-nitrophenol. In rabbits, after oral dosing,
4-nitrophenol undergoes reduction to 4-aminophenol as well as
glucuronidation and sulfation. In vivo and in vitro studies gave
an indication for dermal uptake. For 2-nitrophenol, the information is
very limited. However, a comparable metabolic transformation is
assumed based on the available data. Owing to their rapid metabolism
and excretion, bioaccumulation of 2- and 4-nitrophenol in organisms is
not to be expected.
With oral LD50 values of 220-620 mg/kg body weight in rats and
380-470 mg/kg body weight in mice, 4-nitrophenol is harmful after oral
uptake and was found to be more toxic than 2-nitrophenol. A
dose-dependent increase in the formation of methaemoglobin was seen in
cats after oral exposure to 2-nitrophenol -- but not after exposure to
4-nitrophenol -- and in rats after exposure by inhalation to
4-nitrophenol.
Most of the studies concerning skin- or eye-irritating effects in
experimental animals are limited as a result of insufficient
documentation. However, from the available data, it can be concluded
that 2-nitrophenol is slightly irritating to the skin but
non-irritating to the eye, and the substance proved to have no
sensitizing effects. For 4-nitrophenol, irritating effects on skin and
eye are assumed based on the studies performed according to OECD/FDA
guidelines; in addition, signs of irritation were reported after
exposure by inhalation as well as subchronic dermal exposure. In a
guinea-pig maximization test, 4-nitrophenol was considered to be
sensitizing. Positive patch tests were recorded in humans exposed to
4-nitrophenol. Although this may have been due to cross-sensitization,
sensitization to 4-nitrophenol in humans cannot be excluded.
Only a few limited studies concerning repeated oral exposure to
2- and 4-nitrophenol in experimental animals were identified. With
2-nitrophenol, decreases in body weight gain accompanied by decreased
food consumption and differences in organ weights without clear dose
dependency were found. However, the haematological examination,
clinical chemistry, and histopathological examination of the major
organs and tissues gave no indication for a substance-related toxic
effect compared with controls. In rats dosed with 4-nitrophenol, a
focal fatty degeneration of the liver as well as congestion in several
organs were the major histopathological findings. Other reported
effects included haematological changes, nephrosis, testicular
atrophy, and follicular atresia in the ovaries. The exposure by
inhalation to 2-nitrophenol vapour caused squamous metaplasia of the
epithelium of the upper respiratory tract; with 4-nitrophenol dust
(applied as sodium salt), haematological changes, increased
methaemoglobin values, and differences in organ weights were noted.
For the effects given in these studies, it was not possible to
identify a clear dose-response or reliable NO(A)EL values.
Insufficient data are available on 2-nitrophenol to allow any
conclusions to be made about its possible mutagenicity. For
4-nitrophenol, more mutagenicity studies are available, and the
substance was shown to be mutagenic in some but not all of the
bacterial assays. In addition, positive results were obtained in
in vitro tests for chromosomal aberrations in mammalian cells;
however, apart from one well-documented study, the available assays
were inadequately reported. In the absence of any in vivo
mutagenicity studies in mammals, it is not possible to conclude
whether or not the mutagenic potential of 4-nitrophenol is expressed
in vivo.
4-Nitrophenol was not carcinogenic in male or female mice after
dermal application over 78 weeks. In a limited study with female mice,
no skin tumours were seen after dermal application of 2- or
4-nitrophenol over 12 weeks. No carcinogenicity studies using the oral
or inhalation routes were available for either of the isomers.
No reproductive effects were observed in rats exposed to
4-nitrophenol in a two-generation study. For developmental toxicity,
the available studies were inadequately performed (i.e., only one dose
was applied, or animals were dosed only on one day with a mixture). In
an oral study with rats, 2-nitrophenol induced developmental effects
in the offspring only at doses that also produced maternal toxicity.
However, the fetuses were not examined for internal malformations.
Data on humans relevant for the assessment of potential adverse
effects are limited to some patch tests performed with 4-nitrophenol.
11.1.2 Criteria for setting guidance values for 2- and 4-nitrophenol
As given in section 8, the database for 2-nitrophenol is
inadequate for calculating a tolerable daily intake (TDI) or a
tolerable concentration (TC).
For 4-nitrophenol, the formation of methaemoglobin was shown to
be the most critical end-point after exposure by inhalation and is
assumed to be relevant for oral exposure too. However, owing to the
inaccuracy of the analytical method used in the 13-week study with
oral application, a reliable NO(A)EL cannot be derived. Therefore, at
present, no TDI for 4-nitrophenol can be developed owing to inadequacy
of the database.
Longer-term toxicity studies concerning inhalation exposure were
not identified in the literature, and the NO(A)EL values derived for
4-nitrophenol from short-term studies gave considerable differences
(2-week exposure: NO(A)EL of about 30 mg/m3; 4-week exposure: NO(A)EL
of about 5 mg/m3). The NO(A)EL of 5 mg/m3 was derived for local
effects (cataracts), whereas the NO(A)EL for systemic effects
(formation of methaemoglobin) may be lower. Therefore, a reliable TC
for exposure by inhalation cannot be calculated, as the formation of
methaemoglobin is the critical end-point.
11.1.3 Sample risk characterization
As given in section 6.2, workers may be exposed to 2- and
4-nitrophenol via inhalation and skin contact during production and
processing (mainly in the manufacturing of pesticides). However, data
on nitrophenol concentrations at the workplace were not identified.
For the general population, an exposure to nitrophenols via the
environment cannot be excluded (see also section 6.2). Assuming an
ambient atmospheric concentration of about 1 µg/m3, an inhalation
uptake of 100%, a daily respiratory volume of 22 m3 for adults, a
mean body weight of 64 kg for males and females, and that 4 of 24 h
are spent outdoors (IPCS, 1994), the uptake by inhalation of
nitrophenols is calculated to be 0.06 µg/kg body weight per day. In
addition, 4-nitrophenol accumulates in fog. From the mean measured
level of 20 µg/litre, the uptake of the substance by inhalation (using
the same assumptions as above) can be calculated to be about 8 ng
during a 1-h exposure period (i.e., 0.12 ng/kg body weight), assuming
a maximum water content of fog of 0.1 g/m3 (Pruppacher & Klett,
1978). The uptake via drinking-water for 2- and 4-nitrophenol can be
calculated to be about 0.02 µg/kg body weight per day, assuming a
maximum concentration of 1 µg/litre drinking-water, a daily
drinking-water consumption of 1.4 litres, and a mean body weight of
64 kg for males and females.
From these data, it can be concluded that exposure of the general
population to the nitrophenol isomers is mainly through ambient air
and drinking-water.
11.2 Evaluation of environmental effects
Releases of 2- and 4-nitrophenol into the environment are
primarily emissions into air, water, and soil from diffuse sources,
such as vehicle traffic and hydrolytic and photolytic degradation of
the respective pesticides.
2-Nitrophenol emitted to the troposphere will stay predominantly
in the gaseous phase and should be rapidly removed by nitration. The
major portion of airborne 4-nitrophenol is expected to be particle
bound and can be washed out to surface waters and soil by wet and dry
deposition. Because of their removal from air and their insignificant
volatility, nitrophenols are not considered to contribute directly to
the depletion of the stratospheric ozone layer or to global warming.
Measured bioconcentration factors indicate a low potential for
bioaccumulation.
Nitrophenols exhibit moderate to high toxicity to aquatic
organisms, with lowest effect concentrations reported from chronic
studies on algae, Daphnia, and aquatic invertebrates. The lowest
effect concentrations found in chronic studies with freshwater
organisms ( Scenedesmus subspicatus, 96-h EC50: 0.39 mg
2-nitrophenol/litre; Entosiphon sulcatum, 72-h MIC: 0.83 mg
4-nitrophenol/litre) were 40-50 times higher than maximum levels
determined in a densely populated and highly industrialized Asian
river basin (0.0072 mg 2-nitrophenol/litre and 0.019 mg
4-nitrophenol/litre). From these data, the safety margin between the
LOEC and maximum surface water concentrations is insufficient to
exclude any risk for sensitive aquatic organisms, particularly under
surface water conditions not favouring both elimination pathways.
Taking into account the missing chronic effect data for fish, an
uncertainty/assessment factor of 100 has to be applied to derive a
predicted no-effect concentration (PNEC) according to standard
procedures for environmental risk assessment. From acute tests (see
section 10.1), however, fish obviously seem to be the least sensitive
aquatic species tested. Thus, an assessment factor of 10 might be
appropriate. Furthermore, the use pattern and the release scenario
outlined in section 4 lead to the conclusion that nitrophenols emitted
to surface waters will pose only a minor risk to aquatic organisms.
There were no data available on the occurrence of nitrophenols in
the terrestrial compartment. Therefore, an assessment of possible
effects on organisms for this compartment could be conducted only with
regard to the degradation of pesticides. For the insecticides
mentioned in section 4 (parathion, parathion-methyl, carbofuran,
phosalon, fluorodifen) and the herbicides bifenox and nitrofen,
predicted soil concentrations were calculated from the maximum
application rates (taken from Domsch, 1992) according to the EPPO
(1993) guidelines for environmental risk assessment of plant
protection products. Based on the relative molecular mass of the
pesticides, the maximum concentration of nitrophenols in the top 5 cm
of soil was calculated (worst case; one application). For pesticides
that are applied at times when the soil is plant covered to a high
degree, it is assumed that only half of the amount applied reaches the
soil. Thus, the predicted environmental concentration (PEC) for the
insecticides was reduced by 50%. Dividing the PECsoil by the lowest
LC50 value for a terrestrial species gives the toxicity exposure
ratio (TER). The lowest LC50 value for earthworms (38 mg/kg body
weight; see section 10.2) has to be corrected by a factor of 2 because
of the higher organic matter content in artificial soil compared with
natural agricultural soil. The following TERs were derived:
Insecticides Herbicides
Parathion: 244 Bifenox: 131
Parathion-methyl: 557 Nitrofen: 18
Carbofuran: 47
Phosalon: 36
Fluorodifen: 69
According to the EPPO (1993) guidelines, the trigger value for
concern is <10. Therefore, these pesticides are expected to pose only
a minor risk to earthworms, even under a worst-case scenario.
Furthermore, the herbicide nitrofen and the insecticides phosalon and
fluorodifen are no longer manufactured or marketed for crop protection
use.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Previous evaluations of mononitrophenols by international bodies
were not identified.
Information on international hazard classification and labelling
for mononitrophenols is included in the International Chemical Safety
Card reproduced in this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventative and protective
measures and first aid recommendations, are presented on the enclosed
International Chemical Safety Card (ICSC 1342) reproduced in this
document.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards is
available from the International Register of Potentially Toxic
Chemicals (IRPTC) legal file.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
INTERNATIONAL CHEMICAL SAFETY CARD
MONONITROPHENOLS ICSC: 1342
November 1998
CAS # 25154-55-6 Nitrophenols (mixed Isomers)
RTECS # Nitrophenols
UN # 1883 C6H5O3N
Molecular mass: 139.1
TYPES OF HAZARD ACUTE HAZARDS/ PREVENTION FIRST AID/ FIRE
/EXPOSURE SYMPTOMS FIGHTING
FIRE Combustible. Gives off NO open flames. Powder, water spray, foam,
irritating or toxic fumes carbon dioxide.
(or gases) in a fire.
EXPLOSION Finely dispersed particles Prevent deposition In case of fire: keep drums,
form explosive mixtures of dust; closed etc., cool by spraying
in air. system, dust explosion-proof with water.
electrical equipment and
lighting.
EXPOSURE PREVENT DISPERSION OF
DUST! STRICT HYGIENE!
Inhalation Blue lips or finger nails. Local exhaust or breathing Fresh air, rest.
Blue skin. Confusion. protection. Refer for medical attention.
Convulsions. Cough.
Dizziness. Headache.
Nausea. Sore throat.
Unconsciousness.
Skin MAY BE ABSORBED! Protective gloves. Protective Remove contaminated clothes.
clothing. Rinse and then wash skin with
water and soap. Refer for medical
attention.
Eyes Redness. Pain. Safety goggles, or eye First rinse with plenty of water
protection in combination for several minutes (remove contact
with breathing protection. lenses if easily
possible), then take to a doctor.
Ingestion Abdominal pain. Sore Do not eat, drink, or smoke Rinse mouth. Rest. Refer
throat. Vomiting. during work. for medical attention.
(see Inhalation).
SPILLAGE DISPOSAL PACKAGING & LABELLING
Sweep spilled substance into sealable Do not transport with food and feedstuffs.
containers. Carefully collect EU Classification
remainder, then remove to safe place. UN Classification
Do NOT let this chemical UN Hazard Class: 6.1
enter the environment. UN Pack Group: III
(Extra personal protection: P2 filter
respirator for harmful particles).
EMERGENCY RESPONSE STORAGE
Separated from combustible and reducing
substances, food and feedstuffs. Dry.
Well closed.
IMPORTANT DATA
PHYSICAL STATE; APPEARANCE: ROUTES OF EXPOSURE:
YELLOW CRYSTALS The substance can be absorbed into the
body by inhalation of its aerosol through
the skin and by ingestion.
PHYSICAL DANGERS:
Dust explosion possible if in powder INHALATION RISK:
or granular form, mixed with air. Evaporation at 20°C is negligible;
a harmful concentration of airborne
particles can, however, be reached quickly.
CHEMICAL DANGERS: EFFECTS OF SHORT-TERM EXPOSURE:
May explode on heating. On combustion, The substance irritates the eyes and the
forms nitrogen oxides. The skin and the respiratory tract. The
substance decomposes on heating substance may cause effects on the blood,
producing toxic fumes including resulting in formation of
nitrogen oxides. Reacts with strong oxidants. methaemoglobin. The effects may be delayed.
Medical observation is indicated.
OCCUPATIONAL EXPOSURE LIMITS: EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
TLV not established. Repeated or prolonged contact may cause skin sensitization.
PHYSICAL PROPERTIES
Boiling point: 194-279
Melting point: 44-116°C
Density: 1.5 g/cm3
Solubility in water, g/100 ml: 0.13-1.2
Vapour pressure, Pa at 20°C: 0.0032 - 7
Relative vapour density (air = 1): 4.81
Flash point: 169°C
ENVIRONMENTAL DATA
The substance is toxic to aquatic organisms. Avoid release to the environment in circumstances different to normal use.
NOTES
Depending on the degree of exposure, periodic medical examination is indicated. Specific treatment is necessary in
case of poisoning with this substance; the appropriate means with instructions must be available.
ADDITIONAL INFORMATION
LEGAL NOTICE Neither the CEC nor the IPCS nor any person acting on behalf of the CEC or the IPCS is responsible
for the use which might be made of this information
(c) IPCS, CEC 1999
REFERENCES
Aelion CM, Swindoll CM, Pfaender FK (1987) Adaptation to and
biodegradation of xenobiotic compounds by microbial communities from a
pristine aquifer. Applied environmental microbiology, 53:2212-2217.
Amacher DE, Turner GN (1982) Mutagenic evaluation of carcinogens and
non-carcinogens in the L5178Y/TK assay utilizing postmitochondrial
fractions (S9) from normal rat liver. Mutation research, 97:49-65.
Andrae U, Bieniek D, Freitag D, Goeggelmann W, Huber W, Klein W,
Kotzias D, Lahaniatis E, Mansour M, Parlar H, Politzki G, Rohleder H,
Rott B, Scheunert I, Spieser H, Viswanathan R (1981) Feasibility of
test guidelines and evidence of the base-set testing according to the
chemicals legislation. Muenchen, Gesellschaft für Strahlen- und
Umweltfoschung mbH (in German).
Angerhofer RA (1985) Final phase: Effect of dermal applications of
paranitrophenol on the reproductive functions of rats. Aberdeen
Proving Ground, MD, US Army Environmental Hygiene Agency (Study No.
75-51-0047-85).
ATSDR (1992) Toxicological profile for nitrophenols: 2- and
4-nitrophenol. Atlanta, GA, US Department of Health and Human
Services, Public Health Service, Agency for Toxic Substances and
Disease Registry (Report No. TP-91/23).
Ballhorn D, Freitag D, Geyer H, Quast I, Rott B, Scheunert I, Spieser
H, Viswanathan R (1984) Assessment of the feasibility and evidence of
the test methods of levels I and II of the chemicals act. Berlin,
Umweltbundesamt (in German).
BASF AG (1969) Results of the toxicological testing of
Rongal-Stabilisator A. Ludwigshafen (unpublished report) (in German).
BASF AG (1970) Results of the toxicological testing of
o -nitrophenol. Ludwigshafen (unpublished report) (in German).
Battersby NS, Wilson V (1989) Survey of the anaerobic biodegradation
potential of organic chemicals in digesting sludge. Applied
environmental microbiology, 55:433-439.
Baumgartner A, Liebscher H-J (1990) Allgemeine Hydrologie.
Quantitative Hydrologie. Berlin, Gebrueder Borntraeger Verlag, pp.
93-96.
Booth G (1991) Nitro compounds, aromatic. In: Ullmann's encyclopedia
of industrial chemistry. Vol. A17. Weinheim, VCH VerlagsGmbH, pp.
411-455.
Booth GM, Bradshaw WS, Carter MW (1983) Screening of priority
chemicals for potential reproductive hazard. Orem, UT, MESA Corp.
(Report No. PB83-213017).
Boutwell RK, Bosch DK (1959) The tumor-promoting action of phenol and
related compounds for mouse skin. Cancer research, 19:413-424.
Boyd SA (1982) Adsorption of substituted phenols by soil. Soil
science, 134:337-343.
Boyd SA, Shelton DR, Berry D, Tiedje JM (1983) Anaerobic
biodegradation of phenolic compounds in digested sludge. Applied
environmental microbiology, 46:50-54.
Braunbeck T, Storch V, Nagel R (1989) Sex-specific reaction of liver
ultrastructure in zebra fish ( Brachydanio rerio) after prolonged
sublethal exposure to 4-nitrophenol. Aquatic toxicology, 14:185-202.
Bringmann G (1978) Determination of the toxicity of water pollutants
on protozoa. I. Bacteriovorous flagellates. Zeitschrift fuer Wasser
und Abwasser Forschung, 11:210-215 (in German).
Bringmann G, Kuehn R (1977) Results of the damaging effects of water
pollutants on Daphnia magna. Zeitschrift fuer Wasser und Abwasser
Forschung, 10:161-165 (in German).
Bringmann G, Kuehn R, Winter A (1980) Determination of the biological
effect of water pollutants in protozoa. III. Saprozoic flagellates.
Zeitschrift fuer Wasser und Abwasser Forschung, 13:170-173
(in German).
Broecker B, Fischer R, Gerber HG, Goerlitz G, Markert M, Wellens H
(1984) Assessment of the feasibility and evidence of the test method
of level 1 and 2 of the chemical act. Berlin, Umweltbundesamt
(in German).
BUA (1992) BUA-Stoffbericht 2- und 4-Nitrophenol. Beratergremium
fuer Umweltrelevante Altstoffe. Weinheim, VCH VerlagsGmbH (Report No.
75; February 1992).
Budavari S, O'Neil MJ, Smith A, Heckelmann PE, Kinneary JE, eds.
(1996) The Merck Index. An encyclopedia of chemicals, drugs and
biologicals, 12th ed. Whitehouse Station, NJ, Merck & Co. Inc.
Buselmaier W, Roehrborn G, Propping P (1972)
Mutagenitaets-Untersuchungen mit Pestiziden im Host-mediated assay und
mit dem Dominanten Letaltest an der Maus. Biologisches Zentralblatt,
91:311-325.
Capel PD, Leuenberger C, Giger W (1991) Hydrophobic organic chemicals
in urban fog. Atmospheric environment, 25A:1335-1346.
Chiu CW, Lee LH, Wang CY, Bryan GT (1978) Mutagenicity of some
commercially available nitro compounds for Salmonella typhimurium.
Mutation research, 58:11-22.
CITI (1992) Biodegradation and bioaccumulation. Data of existing
chemicals based on the CSCL Japan. Tokyo, Japan Chemical Industry
Ecology-Toxicology & Information Center, Chemicals Inspection &
Testing Institute.
Dellarco VL, Prival MJ (1989) Mutagenicity of nitro compounds in
Salmonella typhimurium in the presence of flavin mononucleotide in a
preincubation assay. Environmental molecular mutagenesis,
13:116-127.
Domsch KH (1992) Pesticides in soil. Weinheim, VCH-Verlag (in
German).
Eastman Kodak Co. (1980) Toxicology Laboratory Report No. 125877V; US
Environmental Protection Agency Report No. 86-890000202. Rochester, NY
[cited in Cantilli R (1991) Drinking water health advisory for
p -nitrophenol. Washington, DC, US Environmental Protection Agency
(Report No. PB92-135490)].
Ensenbach U, Nagel R (1991) Toxicokinetics of xenobiotics in zebrafish
- comparison between tap and river water. Comparative biochemistry
and physiology, 100C:49-53.
EPPO (1993) Decision-making scheme for the environmental risk
assessment of plant protection products. Paris, European and
Mediterranean Plant Protection Organization.
Eriksen K (1978) Cross allergy between paranitro compounds with
special reference to DNCB and chloramphenicol. Contact dermatitis,
4:29-32.
Fahrig R (1974) Comparative mutagenicity studies with pesticides.
IARC scientific publications, 10:161-181.
Figge K, Klahn J, Koch J (1985) Chemicals in ecosystems. Inventory,
evaluation and application of distribution models. Society for Water,
Soil and Air Hygiene, 61:1-234 (in German).
Foureman P, Mason JM, Valencia R, Zimmering S (1994) Chemical
mutagenesis testing in Drosophila. IX. Results of 50 coded compounds
tested for the National Toxicology Program. Environmental molecular
mutagenesis, 23:51-63.
Freitag D, Geyer H, Kraus A, Viswanathan R, Kotzias D, Attar A, Klein
W, Korte F (1982) Ecotoxicological profile analysis. VII. Screening
chemicals for their environmental behavior by comparative evaluation.
Ecotoxicology and environmental safety, 6:60-81.
Geissler A, Schoeler HF (1993) Atmospheric deposition of pesticides
and nitrophenols in the Rhine/Sieg-area (Germany). Vom Wasser,
80:357-370.
Geissler A, Schoeler HF (1994) Gas chromatographic determination of
phenol, methylphenols, chlorophenols, nitrophenols and nitroquinones
in water at 0.1 µg l-1. Water research, 28:2047-2053.
Gerike P, Fischer WK (1979) A correlation study of biodegradability
determination with various chemicals in various tests. Ecotoxicology
and environmental safety, 3:159-173.
Gessner T, Hamada N (1970) Identification of p-nitrophenol glucoside
as a urinary metabolite. Journal of pharmaceutical sciences,
59:1528-1529.
Geyer H, Viswanathan R, Freitag D, Korte F (1981) Relationship between
water solubility of organic chemicals and their bioaccumulation by the
alga Chlorella. Chemosphere, 14:1307-1313.
Gile JD, Gillett JW (1981) Transport and fate of organophosphate
insecticides in a laboratory model ecosystem. Journal of agricultural
and food chemistry, 29:616-621.
Hansch C, Leo A (1979) Substituent constants for correlation analysis
in chemistry and biology. New York, NY, John Wiley & Sons.
Harrison I, Leader RU, Higgo JJW, Tjell JC (1994) Determination of
organic pollutants in small samples of groundwaters by liquid-liquid
extraction and capillary gas chromatography. Journal of
chromatography, 688:181-188.
Haworth S, Lawlor T, Mortelmans K, Speck W, Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals.
Environmental mutagenesis, 5 (Suppl. 1):3-142.
Hazleton Lab. (1983) Subacute dust inhalation toxicity study in rats.
p -Nitrophenol. Final report. Sponsored by Monsanto Co., St. Louis,
MO (HLA Study No. 82-242).
Hazleton Lab. (1984) Subacute inhalation toxicity study in rats.
o -Nitrophenol. Final report. Sponsored by Monsanto Co., St. Louis,
MO (HLA Study No. 82-254).
Hazleton Lab. (1989) Subchronic toxicity study in rats with
paranitrophenol. Sponsored by Monsanto Co., St. Louis, MO (HLA Study
No. 241-221).
Herterich R, Herrmann R (1990) Comparing the distribution of nitrated
phenols in the atmosphere of two German hill sites. Environmental
technology letters, 11:961-972.
Hoechst AG (1977a) Acute oral toxicity of p -nitrophenol in female
SPF-Wistar rats. Frankfurt/Main, Hoechst AG (unpublished report) (in
German).
Hoechst AG (1977b) Acute dermal toxicity of p -nitrophenol in female
SPF-Wistar rats. Frankfurt/Main, Hoechst AG (unpublished report)
(in German).
Hoechst AG (1977c) Skin and eye irritating effects of p -nitrophenol
in rabbits. Frankfurt/Main, Hoechst AG (unpublished report) (in
German).
Hoechst AG (1980) A mutagenicity screening of 408 / 80 A in bacteria
(Ames test). Frankfurt/Main, Hoechst AG (unpublished report).
Hoover DG, Borgonovi GE, Jones SH, Alexander M (1986) Anomalies in
mineralization of low concentrations of organic compounds in lake
water and sewage. Applied environmental microbiology, 51:226-232.
Howe GE, Marking LL, Bills TD, Rach JJ, Mayer FLJ (1994) Effects of
water temperature and pH on toxicity of terbufos, trichlorfon,
4-nitrophenol and 2,4-dinitrophenol to the amphipod Gammarus
pseudolimnaeus and rainbow trout ( Oncorhynchus mykiss).
Environmental toxicology and chemistry, 13:51-66.
HSDB (1998) Hazardous substances data bank. Bethesda, MD, National
Library of Medicine.
Huang Q, Wang L, Han S (1995) The genotoxicity of substituted
nitrobenzenes and the quantitative structure-activity relationship
studies. Chemosphere, 30:915-923.
Huang Q-G, Kong L-R, Liu Y-B, Wang L-S (1996) Relationships between
molecular structure and chromosomal aberrations in in vitro human
lymphocytes induced by substituted nitrobenzenes. Bulletin of
environmental contamination and toxicology, 57:349-353.
Huq AS, Ho NFH, Husari N, Flynn GL, Jetzer WE, Condie LJ Jr (1986)
Permeation of water contaminative phenols through hairless mouse skin.
Archives of environmental contamination and toxicology, 15:557-566.
Hustert K, Mansour M, Parlar H, Korte F (1981) The EPA test - A method
for the determination of the photochemical degradation of organic
compounds in aquatic systems. Chemosphere, 10(9):995-998 (in
German).
International Research and Developmental Corporation (1983)
Range-finding teratology study in rats. Sponsored by Monsanto Co.,
St. Louis, MO (IR-83-100).
IPCS (1994) Assessing human health risks of chemicals: derivation of
guidance values for health-based exposure limits. Geneva, World
Health Organization, International Programme on Chemical Safety
(Environmental Health Criteria 170).
IPCS (1998) International Chemical Safety Card - Mononitrophenols.
Geneva, World Health Organization, International Programme on
Chemical Safety (ICSC 1342).
Japan Environment Agency (1979) Chemicals in the environment.
Monitoring of the general environment in Japan 1978. Tokyo.
Japan Environment Agency (1980) Chemicals in the environment.
Monitoring of the general environment in Japan 1979. Tokyo.
Japan Environment Agency (1995) Chemicals in the environment.
Monitoring of the general environment in Japan 1994. Tokyo.
Jetzer WE, Huq AS, Ho NFH, Flynn GL, Duraiswamy N, Condie L Jr (1986)
Permeation of mouse skin and silicone rubber membranes by phenols:
relationship to in vitro partitioning. Journal of pharmaceutical
sciences, 75:1098-1103.
Kavlock RJ (1990) Structure-activity relationships in the
developmental toxicity of substituted phenols: In vivo effects.
Teratology, 41:43-59.
Kawai A, Goto S, Matsumoto Y, Matsushita H (1987) Mutagenicity of
aliphatic and aromatic nitro compounds. Japanese journal of
industrial health, 29:34-54.
Kayser G, Koch M, Ruck W (1994) Simultaneous quantitative measurement
of biodegradation and toxicity of environmental chemicals.
Vom Wasser, 82:219-232.
Koerdel W, Schoene K, Bruckert J, Pfeiffer U, Schreiber G, Rittmann D,
Hochrainer D, Otto F, Spielberg T, Fingerhut R, Kuhnen-Clausen D,
Koenig J (1981) Assessment of the feasibility of test guidelines as
well as the evidence of the base set of the law on chemicals.
Hanover, Fraunhofer Institute for Toxicology and Aerosol Research (in
German).
Koerdel W, Kuhnen-Clausen D, Fabig W, Otto F (1984) Evaluation of
test guidelines for environmental chemicals. Schmallenberg,
Fraunhofer Institute for Environmental Chemistry and Ecotoxicology (in
German).
Koop DR (1986) Hydroxylation of p-nitrophenol by rabbit
ethanol-inducible cytochrome P-450 isozyme 3a. Molecular
pharmacology, 29:399-404.
Koop DR, Laethem CL (1992) Inhibition of rabbit microsomal cytochrome
P-450 2E1-dependent p-nitrophenol hydroxylation by substituted
benzene derivatives. Drug metabolism and disposition, 20:775-777.
Kramer CR, Truemper l, Berger L (1986) Quantitative structure-activity
relations for the autotrophic growth inhibition of synchrone
Chlorella vulgaris suspensions by monosubstituted nitrobenzenes.
Biochemie und Physiologie der Pflanzen, 181:411-420 (in German).
Kubinski H, Gutzke GE, Kubinski ZO (1981) DNA-cell-binding (DCB) assay
for suspected carcinogens and mutagens. Mutation research,
89:95-136.
Kuehn R, Pattard M, Pernak K-D, Winter A (1988) Damaging effects of
environmental chemicals in the Daphnia reproductive toxicity test as
a basis for the evaluation of environmental hazards in aquatic
systems. Berlin, Institute for Water, Soil and Air Hygiene (in
German).
Landrum PF, Crosby DG (1981) Comparison of the disposition of several
nitrogen-containing compounds in the sea-urchin and other marine
invertebrates. Xenobiotica, 11:351-361.
Lang P-Z, Ma X-F, Lu G-H, Wang YI, Bian Y (1996) QSAR for the acute
toxicity of nitroaromatics to the carp ( Cyprinus carpio).
Chemosphere, 32:1547-1552.
Leone JA, Seinfeld JH (1985) Comparative analysis of chemical reaction
mechanism for photochemical smog. Atmospheric environment,
19:437-464.
Leone JA, Flagan RC, Grosjean D, Seinfeld JH (1985) An outdoor smog
chamber and modeling study of toluene-NOx photooxidation.
International journal of chemical kinetics, 17:177-216.
León-González ME, Perez-Arribas LV, Santos-Delgado MJ, Polo-Diez LM
(1992) Simultaneous flow-injection determination of o- and
p-nitrophenol using a photodiode-array detector. Analytica Chimica
Acta, 258:269-273.
Leuenberger C, Czuczwa J, Tremp J, Giger W (1988) Nitrated phenols in
rain: atmospheric occurrence of phytotoxic pollutants. Chemosphere,
17(3):511-516.
Levsen K, Behnert S, Priess B, Svoboda M, Winkeler H-D, Zietlow J
(1990) Organic compounds in precipitation. Chemosphere,
21:1037-1061.
Levsen K, Behnert S, Mussmann P, Raabe M, Priess B (1993) Organic
compounds in cloud and rain water. International journal of
environmental analytical chemistry, 52:87-97.
Lokke H (1984) Sorption of selected organic pollutants in Danish
soils. Ecotoxicology and environmental safety, 8:395-409.
Luettke J, Levsen K (1994) Partitioning of phenol and nitrophenols in
gas and liquid phase of clouds. In: Borrell PM, Borrell P, Cvitas T,
Seiler W, eds. Transport and transformation of pollutants in the
troposphere. Proceedings of EUROTRAC Symposium '94
Garmisch-Patenkirchen, 11-15 April 1994. Garmisch-Patenkirchen, SPB
Academic Publishing bv / EUROTRAC International Scientific Secretariat
(ISS); Fraunhofer Institute for Atmospheric Environmental Research,
pp.1075-1078.
Luettke J, Scheer V, Levsen K, Wuensch G, Cape JN, Hargreaves KJ,
Storeton-West RL, Acker K, Wieprecht W, Jones B (1997) Occurrence and
formation of nitrated phenols in and out of cloud. Atmospheric
environment, 31:2637-2648.
Machida M, Morita Y, Hayashi M, Awazu S (1982) Pharmacokinetic
evidence for the occurrence of extrahepatic conjugative metabolism of
p-nitrophenol in rats. Biochemical pharmacology, 31:787-791.
Mansour M (1996) Abiotic degradation of pesticides and other organic
chemicals in aquatic systems. Pesticide outlook, 7:9-10.
Marquart HW, Sewekow B, Hamburger B, Harzdorf C, Hellbusch HD (1984)
Assessment of the feasibility and evidence of the test methods of
levels I and II of the chemicals act. Part II. Leverkusen, Bayer-AG,
pp. 1-128 (in German).
Massey IJ, Aitken MD, Ball LM, Heck PE (1994) Mutagenicity screening
of reaction products from the enzyme-catalyzed oxidation of phenolic
pollutants. Environmental toxicology and chemistry, 13:1743-1752.
McCann J, Choi E, Yamasaki E, Ames BN (1975) Detection of carcinogens
as mutagens in the Salmonella/microsome test: assay of 300
chemicals. Proceedings of the National Academy of Sciences of the
United States of America, 72:5135-5139.
McCoy GD, Koop DR (1988) Biochemical and immunochemical evidence for
the induction of an ethanol-inducible cytochrome P-450 isoenzyme in
male Syrian golden hamsters. Biochemical pharmacology, 37:1563-1568.
Means JL, Anderson SJ (1981) Comparison of five different methods for
measuring biodegradability in aqueous environment. Water, air, and
soil pollution, 16:301-315.
Meerman JH, Nijland C, Mulder GJ (1987) Sex differences in sulfation
and glucuronidation of phenol, 4-nitrophenol and
N-hydroxy-2-acetylaminofluorene in the rat in vivo. Biochemical
pharmacology, 36:2605-2608.
Mussmann P, Levsen K, Radeck W (1994) Gas-chromatographic
determination of phenols in aqueous samples after solid phase
extraction. Fresenius journal of analytical chemistry, 348:654-659.
Nakamura S, Oda Y, Shimada T, Oki I, Sugimoto K (1987) SOS-inducing
activity of chemical carcinogens and mutagens in Salmonella
typhimurium TA1535/pSK1002: examination with 151 chemicals. Mutation
research, 192:239-246.
Naniwa S (1979) Industrial contact dermatitis due to nitro and amino
derivatives. 1st report: mass-examination of a factory. Journal of
dermatology, 6:59-63.
Nasseredine-Sebaei SM, Crider AM, Carroll RT, Hinko CN (1993)
Determination of m-nitrophenol and nipecotic acid in mouse tissues
by high-performance liquid chromatography after administration of the
anticonvulsant m-nitrophenyl-3-piperidinecarboxylate hydrochloride.
Journal of pharmaceutical sciences, 82:39-43.
Neuhauser EF, Loehr RC, Malecki MR, Milligan DL, Durkin PR (1985) The
toxicity of selected organic chemicals to the earthworm Eisenia
fetida. Journal of environmental quality, 14:383-388.
Neuhauser EF, Durkin PR, Malecki MR, Anatra M (1986) Comparative
toxicity of ten organic chemicals to four earthworm species.
Comparative biochemistry and physiology, C 83:197-200.
Nick K, Schoeler HF (1992) Gas-chromatographic determination of
nitrophenols after derivatization with diazomethane. Fresenius
journal of analytical chemistry, 343:304-307.
Nojima K, Kawaguchi A, Ohya T, Kanno S, Hirobe M (1983) Studies on
photochemical reaction of air pollutants. X. Identification of
nitrophenols in suspended particulates. Chemical and pharmaceutical
bulletin, 31:1047-1051.
NTP (1993) Toxicology and carcinogenesis studies of p -nitrophenol
(CAS No. 100-02-7) in Swiss Webster mice (dermal studies). Research
Triangle Park, NC, US Department of Health and Human Services,
National Toxicology Program (NTP Report No. TR-417).
Nyholm N, Lindgaard-Joergensen P, Hansen N (1984) Biodegradation of
4-nitrophenol in standardized aquatic degradation tests.
Ecotoxicology and environmental safety, 8:451-470.
Oberly TJ, Bewsey BJ, Probst GS (1984) An evaluation of the L5178Y
TK+/- mouse lymphoma forward mutation assay using 42 chemicals.
Mutation research, 125:291-306.
Ohkura K, Iwamoto K, Terada H (1990) Transcellular permeation of
nitrophenols through newborn rat skin epidermal cells in monolayer
culture. Chemical and pharmaceutical bulletin, 38:2788-2791.
Ou L-T (1985) Methyl parathion degradation and metabolism in soil:
Influence of high soil-water contents. Soil biology and
biochemistry, 17:241-243.
Pagga U, Haltrich WG, Guenthner W (1982) Investigations of the effect
of 4-nitrophenol on activated sludge. Vom Wasser, 59:51-65 (in
German).
Paterson B, Cowie CE, Jackson PE (1996) Determination of phenols in
environmental waters using liquid chromatography with electrochemical
detection. Journal of chromatography, A 731:95-102.
Pitter P (1976) Determination of biological degradability of organic
substances. Water research, 10:231-235.
Pocurull E, Marce RM, Borrull F (1996) Determination of phenolic
compounds in natural waters by liquid chromatography with ultraviolet
and electrochemical detection after on-line trace enrichment. Journal
of chromatography, 738:1-9.
Poirier MC, De Cicco BT, Lieberman MW (1975) Nonspecific inhibition of
DNA repair synthesis by tumor promoters in human diploid fibroblasts
damaged with N-acetoxy-2-acetylaminofluorene. Cancer research,
35:1392-1397.
Probst GS, McMahon RE, Hill LE, Thompson CZ, Epp JK, Neal SB (1981)
Chemically-induced unscheduled DNA synthesis in primary rat hepatocyte
cultures: a comparison with bacterial mutagenicity using 218
compounds. Environmental mutagenesis, 3:11-32.
Pruett SB, Chambers JE (1988) Effects of paraoxon, p-nitrophenol,
phenyl saligenin cyclic phosphate, and phenol on the rat interleukin 2
system. Toxicology letters, 40:11-20.
Pruppacher HR, Klett JD (1978) Microphysics of clouds and
precipitation. Dordrecht/Boston/London, D. Reidel Publishing Co.
Puig D, Barcelo D (1996) Determination of phenolic compounds in water
and waste water. Trends in analytical chemistry, 15:362-375.
Rashid KA, Mumma RO (1986) Screening pesticides for their ability to
damage bacterial DNA. Journal of environmental science and health,
21:319-334.
Reinke LA, Moyer MJ (1985) p-Nitrophenol hydroxylation. A microsomal
oxidation which is highly inducible by ethanol. Drug metabolism and
disposition, 13:548-552.
Richartz H, Reischl A, Trautner F, Hutzinger O (1990) Nitrated phenols
in fog. Atmospheric environment, 24:3067-3072.
Rippen G, Flothmann D, Witt W (1984) Improvement of the OECD test
guideline A 80/9 and comparative evaluation of other relevant methods
for the measurement of volatility. Frankfurt a. M., Batelle-Institut
e.V. (Report No. 106 02 024/06) (in German).
Roberts BL, Dorough HW (1984) Relative toxicities of chemicals to the
earthworm Eisenia fetida. Environmental toxicology and chemistry,
3:67-78.
Robinson D, Smith JN, Williams RT (1951) Studies in detoxication. 39.
Nitro compounds. (a) The metabolism of o-, m-, and
p-nitrophenols in the rabbit. (b) The glucuronides of the
mononitrophenols and observations on the anomalous optical rotations
of triacetyl -- o-nitrophenylglucuronide and its methyl ester.
Biochemical journal, 50:221-227.
Rott B, Viswanathan R, Freitag D, Korte F (1982) Comparative
investigation on the feasibility of different tests for the evaluation
of the degradation of environmental chemicals. Chemosphere,
11:531-538 (in German).
Ruana J, Urbe I, Borrull F (1993) Determination of phenols at the ng/l
level in drinking and river waters by liquid chromatography with UV
and electrochemical detection. Journal of chromatography, A
655:217-226.
Rubin HE, Subba-Rao RV, Alexander M (1982) Rates of mineralization of
trace concentrations of aromatic compounds in lake water and sewage
samples. Applied environmental microbiology, 43:1133-1138.
Rush GF, Newton JF, Hook JB (1983) Sex differences in the excretion of
glucuronide conjugates: The role of intrarenal glucuronidation.
Journal of pharmacology and experimental therapeutics, 227:658-662.
Sax NI, Lewis RJ (1987) Hawley's condensed chemical dictionary, 11th
ed. New York, NY, Van Nostrand Reinhold Co.
Scheer V, Luettke J, George C, Levsen K, Frenzel A, Behnke W, Zetzsch
C (1996) Atmospheric nitration of phenol in clouds by N2O5 and
ClNO2. In: Borrell PM, Borrell P, Kelly K, Cvitas T, Seiler W, eds.
Transport and transformation of pollutants in the troposphere.
Proceedings of EUROTRAC Symposium -96. Garmisch-Patenkirchen, 25-29
March 1996. Southhampton, Computational Mechanics Publications.
Scheubel JB (1984) Assessment of the feasibility and evidence of the
test method of level 1 and 2 of the chemical act. Marl, Chemische
Werke Huels AG (Report No. 106 04 011/5 CWH) (in German).
Scheunert I (1984) Examination and optimization of the
"GSF-Cold-Finger-Method" and comparative calculations of the
volatility. Gesellschaft fuer Strahlen- und Umweltforschung (Report
No. 10602024/08) (in German).
Schoene K, Steinhanses J (1984) Comparative measurements of the
volatility in open systems. Schmallenberg, Fraunhofer Institute for
Toxicology and Aerosol Research (Report No. 10602024/7 Part II) (in
German).
Scholz N (1986) Development of test guidelines on marine species for
ecotoxicological studies according to the chemicals act
- Bryozota/Camptozoa. Berlin, Umweltbundesamt (Report No.
10603042/02) (in German).
Schwarzenbach RP, Stierli R, Folsom BR, Zeyer J (1988) Compound
properties relevant for assessing the environmental partitioning of
nitrophenols. Environmental science and technology, 22:83-92.
Sewekow B (1983) Feasibility of test guidelines and evidence of the
base-set testing according to the chemicals legislation. Muenchen,
Gesellschaft fuer Strahlen- und Umweltforschung (in German).
Shimizu M, Yano E (1986) Mutagenicity of mono-nitrobenzene derivatives
in the Ames test and rec assay. Mutation research, 170:11-22.
Smith LW, Hall GT, Kennedy GL (1988) Acute and repeated dose
inhalation toxicity of para-nitrophenol sodium salt in rats. Drug
chemistry and toxicology, 11:319-327.
Snodgrass HL Jr (1983) Phase I, dermal penetration and distribution
of 14C-labeled paranitrophenol (PNP). Aberdeen Proving Ground, MD,
US Army Environmental Hygiene Agency (Study No. 75-51-0047-84).
Spain JC, van Veld PA, Monti CA, Pritchard PH, Cripe CR (1984)
Comparison of p-nitrophenol biodegradation in field and laboratory
test systems. Applied environmental microbiology, 48:944-950.
Storer RD, McKelvey TW, Kraynak AR, Elia MC, Barnum JE, Harmon LS,
Nichols WW, DeLuca JG (1996) Revalidation of the in vitro alkaline
elution/rat hepatocyte assay for DNA damage: improved criteria for
assessment of cytotoxicity and genotoxicity and results for 81
compounds. Mutation research, 368:59-101.
Subba-Rao RV, Rubin HE, Alexander M (1982) Kinetics and extent of
mineralization of organic chemicals at trace levels in freshwater and
sewage. Applied environmental microbiology, 43:1139-1150.
Suzuki J, Koyama T, Suzuki S (1983) Mutagenicities of
mono-nitrobenzene derivatives in the presence of norharman. Mutation
research, 120:105-110.
Tabak HH, Quave SA, Mashni CI, Barth EF (1981) Biodegradability
studies with organic priority pollutant compounds. Journal of the
Water Pollution Control Federation, 52:1503-1518.
Tan GH, Chong CL (1993) Trace monitoring of water-borne phenolics in
the Klang River basin. Environmental monitoring and assessment,
24:267-277.
Thompson MJ, Ballinger LN, Cross SE, Roberts MS (1996)
High-performance liquid chromatographic determination of phenol,
4-nitrophenol, beta-naphthol and a number of their glucuronide and
sulfate conjugates in organ perfusate. Journal of chromatography, B
677:117-122.
Tremaine LM, Diamond GL, Quebbemann AJ (1984) In vivo quantification
of renal glucuronide and sulfate conjugation of 1-naphthol and
p-nitrophenol in the rat. Biochemical pharmacology, 33:419-427.
Tremp J, Mattrel P, Fingler S, Giger W (1993) Phenols and nitrophenols
as tropospheric pollutants: Emissions from automobile exhausts and
phase transfer in the atmosphere. Water, air, and soil pollution,
68:113-123.
TRI (1998) Toxics release inventory. Washington, DC, US
Environmental Protection Agency, Office of Toxic Substances
(17 December 1998).
Tseng S, Lin M (1994) Treatment of organic wastewater by anaerobic
biological fluidized bed reactor. Water science and technology,
29:157-166.
Urano K, Kato Z (1986) Evaluation of biodegradation ranks of priority
organic compounds. Journal of hazardous materials, 13:147-159.
Van Veld PA, Spain JC (1983) Degradation of selected xenobiotic
compounds in three types of aquatic test systems. Chemosphere,
12:1291-1305.
Vasilenko NM, Volodchenko VA, Baturina TS, Kolodub FA (1976)
Toxicological peculiarities of mononitrophenols with regard for their
isomeric form. Farmakologiya i Toksikologiya, 39:718-721.
Vernot EH, MacEwen JD, Haun CC, Kinkead ER (1977) Acute toxicity and
skin corrosion data for some organic and inorganic compounds and
aqueous solutions. Toxicology and applied pharmacology, 42:417-423.
Verschueren K, ed. (1983) Handbook of environmental data on organic
chemicals, 2nd ed. New York, NY, Van Nostrand Reinhold Co.
Vozñáková Z, Podehradská J, Kohlicková M (1996) Determination of
nitrophenols in soil. Chemosphere, 33:285-291.
Wagner R, Braeutigam H-J (1981) Development and testing of a method
for studying the degradation of organic compounds under anaerobic
conditions (report no. 03 7221). In: Biehl HM, Fuehr F, Seibert K,
eds. Methods for the ecotoxicological evaluation of chemicals, Part
1, Aquatic systems. Juelich, Forschungszentrum, pp. 20-41 (in
German).
Weast RC (1979) CRC handbook of chemistry and physics, 69th ed. Boca
Raton, FL, CRC Press, Inc.
Wiggins BA, Jones SH, Alexander M (1987) Explanations for the
acclimation period preceding the mineralization of organic chemicals
in aquatic environments. Applied environmental microbiology,
43:791-796.
Yamada K, Murakami H, Yasumura K, Shirahata S, Shinohara K, Omura H
(1987) Production of DNA-breaking substance after treatment of
monophenols with sodium nitrite and then with dimethyl sulfoxide.
Agricultural and biological chemistry, 51:247-248.
Yoshida K, Shigeoka T, Yamauchi F (1983) Non-steady-state equilibrium
model for the preliminary prediction of the fate of chemicals in the
environment. Ecotoxicology and environmental safety, 7:179-190.
Yoshioka Y, Ose Y, Sato T (1985) Testing for the toxicity of chemicals
with Tetrahymena pyriformis. The science of the total environment,
43:149-157.
Zetzsch C, Rinke M, Scharpring H, Schueler P, Urbanik E, Wahner A,
Wiedelmann A, Witte F (1984) Upper limits of the persistence of
chemicals in the atmosphere from their reactivity against OH
radicals. University of Bochum, Bochum, pp. 1-20 (BMFT Report No. PTU
037253).
Zimmering S, Mason JM, Valencia R, Woodruff RC (1985) Chemical
mutagenesis testing in Drosophila. II. Results of 20 coded compounds
tested for the National Toxicology Program. Environmental
mutagenesis, 7:87-100.
APPENDIX 1 - 3-NITROPHENOL
Identity and physical/chemical properties
3-Nitrophenol (CAS No. 554-84-7; 3-hydroxy-1-nitrobenzene,
m-nitrophenol) has the empirical formula C6H5NO3. Its structural
formula is shown below:
Technical-grade 2- and 4-nitrophenol from the German producer
have a typical purity of >99%. Named impurities are the corresponding
isomer for each product (0.3%) and traces of 3-nitrochlorobenzene
(<0.05%). Polychlorinated dibenzo- p-dioxin/dibenzofuran (PCDD/PCDF)
and tetrachlorodibenzo- p-dioxin/dibenzofuran (TCDD/TCDF) isomers
were not detected at detection limits between 0.1 and 0.4 µg/kg
product (BUA, 1992).
The pure nitrophenol isomers form pale yellow to yellow crystals
at room temperature. The substances are characterized by the
physicochemical properties given in Table 1 (Sax & Lewis, 1987).
Additional physicochemical properties for mononitrophenols are
presented in the International Chemical Safety Card (ICSC 1342)
reproduced in this document.
Table 1: Physicochemical properties of 2- and 4-nitrophenol.
Parameter 2-Nitrophenol 4-Nitrophenol
Molecular mass (g/mol) 139.11 139.11
Melting point (°C) 44-45 (1)(2)(3) 113-114 (1)(2)(3)
Boiling point (°C) 214-217 (1) 279 (decomposition)(3)
Vapour pressure (kPa) 6.8 × 10-3
(19.8 °C) (4) 3.2 × 10-6
(20 °C) (5)
Water solubility 1.26 12.4
(g/litre) (20 °C) (4) (20 °C) (6)
n-Octanol/water
partition
coefficient (log Kow) 1.77-1.89 (7) 1.85-2.04 (7)
Dissociation constant 7.23 7.08
(pKa) (21.5 °C) (8) (21.5 °C) (8)
Ultraviolet spectrum deltamax (water): deltamax (methanol):
230; 276 nm; no absorption
log epsilonmax: 3.57; maxima <290 nm (9)
3.80 (9)
Conversion factors 1 mg/m3 = 0.173 ppmv
1 ppmv = 5.78 mg/m3
References: (1) Budavari et al. (1996); (2) Booth (1991);
(3) Verschueren (1983); (4) Koerdel et al. (1981);
(5) Sewekow (1983); (6) Andrae et al. (1981);
(7) BUA (1992); (8) Schwarzenbach et al. (1988); (9) Weast (1979)
3. ANALYTICAL METHODS
The nitrophenol isomers are usually determined by gas
chromatography combined with mass spectrometric detection, flame
ionization detection, electron capture detection, or
nitrogen-sensitive detection, which are generally applied after
derivatization (BUA, 1992; Nick & Schoeler, 1992; Geissler & Schoeler,
1994; Harrison et al., 1994; Luettke & Levsen, 1994; Mussmann et al.,
1994). For liquid samples (water, urine, blood), high-performance
liquid chromatography in combination with concentration-gradient
elution (acetonitrile/methanol or ammonium acetate, acetic acid with
potassium chloride/methanol) and ultraviolet or electrochemical
detection, which can be carried out without derivatization, is also
used (BUA, 1992; Nasseredine-Sebaei et al., 1993; Ruana et al., 1993;
Paterson et al., 1996; Pocurull et al., 1996; Thompson et al., 1996).
The separation of the different isomers is carried out either by steam
distillation (BUA, 1992) or by the formation and subsequent extraction
of different ion pairs (León-González et al., 1992).
The following enrichment techniques are used (BUA, 1992; see also
review by Puig & Barcelo, 1996):
* solid-phase adsorption with thermal or liquid extraction for air
and water samples (Luettke & Levsen, 1994; Mussmann et al., 1994)
* liquid/liquid extraction after derivatization for water samples
(initial purification by acid/base fractionation of highly
polluted samples, increased recovery rates with continuous
extraction methods) (León-González et al., 1992; Nick & Schoeler,
1992; Geissler & Schoeler, 1994; Harrison et al., 1994)
* liquid extraction with acid/base fractionation or solid-phase
enrichment and subsequent desorption following aqueous extraction
for soil samples (Vozñáková et al., 1996)
* acid hydrolysis of the glucuronide with subsequent
derivatization for blood and urine samples or denaturation
(Nasseredine-Sebaei et al., 1993; Thompson et al., 1996).
The detection limits are <10 ng/m3 for air, 0.03-10 µg/litre
for water, and 200-1600 µg/kg for soil. A detection limit for the
determination of the nitrophenol isomers in biological materials was
given only for rat liver perfusate (0.5-1 mg/litre; Thompson et al.,
1996).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
There are no known natural sources of the nitrophenol isomers.
Within the European Union, 2- and 4-nitrophenol are produced
mainly by three companies. Six other large manufacturers are known in
the USA and Japan (as of 1989). In 1983, the production volume for
Western Europe was estimated at about 6400 t 2-nitrophenol and about
20 500 t 4-nitrophenol. In 1988-89, the German production volumes
originating from one manufacturer were approximately 500 t
2-nitrophenol and about 2000 t 4-nitrophenol, with about 20 t of each
being exported. Both 2- and 4-nitrophenol are intermediates in the
synthesis of azo dyes and a number of pesticides, mainly insecticides
(2-nitrophenol: carbofuran, phosalon; 4-nitrophenol: parathion,
parathion-methyl, fluorodifen) and, to a lesser extent, herbicides
(4-nitrophenol: nitrofen, bifenox). The corresponding aminophenols
that are gained by reduction are used as a photographic developer
(2-aminophenol) and as an intermediate in the synthesis of the
tuberculostatic 4-aminosalicylic acid and the analgesic
4-acetaminophenol (paracetamol) (4-aminophenol) (see also Booth,
1991). In the 1980s, the production volumes for 2- and 4-nitrophenol
showed a decreasing tendency in Germany as a result of changes in and
termination of the production of some organophosphorus pesticides.
The releases of 2- and 4-nitrophenol during production and
processing at the only German manufacturer appear to be of minor
importance. In 1988-89, about 2.5 kg 2-nitrophenol and 10 kg
4-nitrophenol were emitted to air, and <93 kg 2-nitrophenol and
<64 kg 4-nitrophenol were emitted to surface water.
For 1996, the following releases of 2- and 4-nitrophenol to the
environment were reported by manufacturers in the USA (TRI, 1998):
* 2-nitrophenol: from three manufacturers (one production site
each) with production volumes between 450 and 45 000 kg/year,
total releases of 15 kg to air and 23 kg into water were
reported.
* 4-nitrophenol: from three manufacturers (six production sites)
with production volumes of 45-450 kg/year up to 45 000-450 000
kg/year, a total release of 420 kg to air was reported. Data on
releases into water were not given.
2-Nitrophenol and 4-nitrophenol have been detected in the exhaust
gases of light-duty gasoline and diesel vehicles. Depending on the
motor load, the exhaust concentrations of the isomers were <50 µg/m3
exhaust gas (idle) and about 1000 µg 4-nitrophenol/m3 and 2000 µg
2-nitrophenol/m3 (driving at constant velocity) (Nojima et al., 1983;
Tremp et al., 1993). A regulated three-way catalytic converter reduced
the nitrophenol emissions to about 8% at high motor load and to about
2% at normal motor load (Tremp et al., 1993). A rough estimation
combining the above-mentioned exhaust gas concentrations with
estimations of the total exhaust gas volumes from vehicle traffic for
Germany resulted in an airborne nitrophenol load of at least several
tonnes per year from this source (BUA, 1992). Data concerning
nitrophenol releases from other combustion processes (heating, burning
of refuse) were not identified.
From laboratory experiments, there is some evidence that 2- and
4-nitrophenol are generated in the atmosphere during the photochemical
degradation of aromatic compounds such as benzene and toluene in the
presence of nitric oxide or hydroxyl radicals and nitrous dioxide.
These results were at least partly obtained in model experiments with
unrealistically high nitric oxide concentrations, and there are
competing reactions without nitrophenol formation for which the rate
constant is not known (BUA, 1992). However, smog chamber experiments
confirmed the formation of nitrophenol isomers during irradiation
(Leone & Seinfeld, 1985; Leone et al., 1985). Recent cloud water model
experiments showed that 2- and 4-nitrophenol are also formed from the
reaction of phenol with nitrogen pentoxide or monochloronitrogen
dioxide, especially under alkaline conditions (Scheer et al., 1996).
Estimations of the contribution of photochemically formed nitrophenols
to total emissions into the atmosphere are not possible with the
available data.
Significant releases of 4-nitrophenol into the hydrosphere may
occur from the hydrolytic degradation of the insecticides parathion
and parathion-methyl and -- although to a lesser extent -- from the
photolytic degradation of the herbicides nitrofen and bifenox.
Quantification of releases is not possible with the available data.
Furthermore, a considerable portion of airborne nitrophenols,
especially 4-nitrophenol, can be released to the hydrosphere and the
geosphere by wet and dry deposition (see section 5) (Herterich &
Herrmann, 1990; Luettke et al., 1997). Numerous studies concerning the
concentrations of 2- and 4-nitrophenol in wet deposition samples are
available (see section 6.1). From precipitation data (average 746 mm
rain per year for land masses, according to Baumgartner & Liebscher,
1990) and the measured concentrations of 2- and 4-nitrophenol in
rainwater, the release of nitrophenols via rain can be estimated to be
at least in the order of several thousand tonnes per year on a global
basis.
The application of the herbicides nitrofen and bifenox, which are
photolytically degraded to 4-nitrophenol in aqueous solutions, may
especially lead to emissions into the geosphere and the biosphere.
Further, nitrophenol-contaminated rain, snow, and other wet and dry
deposition may contribute to nitrophenol levels in soils. Data
concerning the release of nitrophenols into the biosphere are not
available.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Environmental releases of nitrophenols are mostly to ambient air,
surface waters, and -- to a smaller extent -- soil. Using a
non-steady-state equilibrium model, the following distribution of
4-nitrophenol in different environmental compartments was predicted:
air, 0.0006%; water, 94.6%; sediment, 4.44%; soil, 0.95%; biota,
0.000 09% (Yoshida et al., 1983). The distribution patterns of 2- and
4-nitrophenol sprayed on a natural soil in a standardized terrestrial
ecosystem were determined via radiotracer technique (14C). Of the
applied radioactivity (2-nitrophenol/4-nitrophenol), 49.45%/20.01% was
recovered in air, 27.38%/40.21% in soil (including animals),
12.73%/7.57% in plants, and 0.05%/0.02% in leachate (Figge et al.,
1985). Distribution of 4-nitrophenol in a terrestrial microcosm
chamber with artificial soil largely corresponded to this result (Gile
& Gillett, 1981). Owing to the expected decomposition within the
incubation periods of 30 and 28 days, respectively, it can be assumed
that most of the recovered radioactivity referred to breakdown
products of the applied nitrophenols.
In volatility experiments conducted according to Organisation for
Economic Co-operation and Development (OECD) guidelines, half-lives of
2-nitrophenol in water ranged from 14.5 to 27.3 days, indicating a
slow rate of volatilization (Koerdel et al., 1981; Rippen et al.,
1984; Scheunert, 1984; Schoene & Steinhanses, 1984). Measurements
concerning the partitioning between the gas and liquid phases of
clouds during different rain events showed that 2-nitrophenol is
enriched in the liquid phase to a larger extent than would be
predicted from its water solubility and vapour pressure. On the other
hand, 4-nitrophenol is extensively adsorbed to particles. Therefore,
elevated levels of this isomer are detected in the gaseous phase of
clouds (Luettke et al., 1997). From the available data, a significant
volatilization of 4-nitrophenol from water to air is not expected.
Since nitrophenols dissociate in aqueous solution, volatilization may
further decrease with increasing pH in surface waters. This leads to
the conclusion that dry and wet deposition of nitrophenols from air to
surface waters and soil are to be expected. The occurrence of this
partition mechanism is supported by the detection of 2- and
4-nitrophenol in rainwater and wet deposition samples (see section
6.1).
From experimental results on direct photodegradation (Koerdel et
al., 1981) and the atmospheric photo-oxidation by hydroxyl radicals
(Zetzsch et al., 1984), both pathways were found to be of minor
importance for the removal of 2-nitrophenol emitted to the
troposphere. Thus, the major degradation pathway for airborne
2-nitrophenol should be rapid nitration to 2,4-dinitrophenol
(Herterich & Herrmann, 1990; Luettke et al., 1997). The major portion
of airborne 4-nitrophenol is expected to be particle bound and
therefore only to a minor extent available for photochemical
reactions. Thus, most of the 4-nitrophenol can be washed out from air
by wet and dry deposition. Measured half-lives for the photochemical
decomposition of 4-nitrophenol in water exposed to sunlight ranged
from 2.8 to 13.7 days (Hustert et al., 1981; Mansour, 1996), being
longer with increasing pH (Hustert et al., 1981). Traces of
4-aminophenol were found as a photoproduct in river water (Mansour,
1996). In experiments conducted according to OECD guidelines, Andrae
et al. (1981) and Koerdel et al. (1981) found no hydrolysis of 2- or
4-nitrophenol under environmental conditions.
Numerous studies on the biodegradation of 2- and 4-nitrophenol
have been conducted. Standardized tests on ready or inherent
biodegradability provide data of large variability, indicating 2- and
4-nitrophenol to be inherently biodegradable under aerobic conditions
(depending on origin and density of inoculum and the applied test
method) (see Table 2). Results from different tests point to a
possible bacteriotoxic effect of 4-nitrophenol at concentrations above
300 mg/litre (Gerike & Fischer, 1979; Nyholm et al., 1984; Kayser et
al., 1994).
Non-standardized experiments with different inocula (e.g.,
natural water, soil, sediment) showed that microbial decomposition of
nitrophenols can occur in different environmental compartments after
adaptation of the microflora (Rubin et al., 1982; Subba-Rao et al.,
1982; Van Veld & Spain, 1983; Spain et al., 1984; Ou, 1985; Hoover et
al., 1986; Aelion et al., 1987; Wiggins et al., 1987). Time for
acclimation and degree of removal depended mostly on substance
concentration, microbial population, climate, and additional
substrates.
Biotic degradation of nitrophenols under anaerobic conditions
requires extended acclimatization of microbial communities. In tests
with sewage sludge and sludge from the primary anaerobic stage of a
municipal sewage treatment plant, respectively, initial 2- and
4-nitrophenol concentrations in the range of 96.5-579 mg/litre were
not degraded at all within 7-60 days (Wagner & Braeutigam, 1981;
Battersby & Wilson, 1989). Boyd et al. (1983) found complete anaerobic
removal of 50 mg/litre for all nitrophenol isomers within 1 week, but
complete mineralization was demonstrated only if the incubation period
was extended to 10 weeks. Anaerobic degradation even of high initial
nitrophenol concentrations was found by Tseng & Lin (1994), who
observed >90% removal of 2- and 4-nitrophenol (350-650 mg/litre) in a
biological fluidized bed reactor with three different kinds of
wastewater. From the available results, a slow degradation of
nitrophenols under anaerobic conditions by adapted microorganisms can
be expected.
Table 2: Biotic degradation of nitrophenols under aerobic conditions.
Test Substance Concentration Additional Test duration Removal Reference
(mg/litre) carbon source (days) (%)
Tests on ready biodegradability
AFNOR test 2-NP 40 OC no 14 16 Gerike & Fischer (1979)
Sturm test 2-NP 10 no 28 32 Gerike & Fischer (1979)
MITI I 2-NP 100 no 14 0 Urano & Kato (1986)
50 no 14 7 Gerike & Fischer (1979)
Closed bottle 4-NP 2 no 28 55 Rott et al. (1982)
test
Modified OECD 4-NP 20 DOC no 28 1 Rott et al. (1982)
screening test
Shake flask test 4-NP 20 OC no 21 50 Means & Anderson (1981)
AFNOR test 4-NP 40 OC no 14 97 Gerike & Fischer (1979)
Sturm test 4-NP 10 no 28 90 Gerike & Fischer (1979)
MITI I 4-NP 50 no 14 1 Gerike & Fischer (1979)
100 no 14 0 Urano & Kato (1986)
100 no 14 4.3 CITI (1992)
Tests on inherent biodegradability
Zahn-Wellens 2-NP 400 no 14 80 Gerike & Fischer (1979)
test
SCAS test 2-NP 20 TOC yes 24 107 Broecker et al. (1984)
13.3 TOC yes 24 110
Table 2 (continued)
Test Substance Concentration Additional Test duration Removal Reference
(mg/litre) carbon source (days) (%)
Bunch & Chambers 2-NP 5-10 yes 28 100 Tabak et al. (1981)
Coupled units 2-NP 12 OC yes 7 61 Gerike & Fischer (1979)
test
Batch test, 2-NP 200 COD no 5 97 Pitter (1976)
aerated
Zahn-Wellens 4-NP 300 no 14 8 Andrae et al. (1981)
test 100 DOC no 28 100 Pagga et al. (1982)
Activated sludge 4-NP 50 no 19 100 Means & Anderson
test 100 no 19 90 (1981)
SCAS test 4-NP 20 TOC yes 33 >90 Marquart et al. (1984)
27 >97 Scheubel (1984)
25/39 100 Ballhorn et al. (1984)
12-15 100 Koerdel et al. (1984)
Coupled units 4-NP 12 OC yes 7 100 Gerike & Fischer
test (1979)
Batch test, 4-NP 200 COD no 5 95 Pitter (1976)
aerated
Abbreviations used: 2-NP = 2-nitrophenol; 4-NP = 4-nitrophenol; OC = organic carbon; DOC = dissolved organic carbon;
TOC = total organic carbon; COD = chemical oxygen demand.
Soil sorption coefficients ( Koc) were found to increase with
increasing organic carbon content. Measured Koc values ranged from
44 to 230 (2-nitrophenol) and from 56 to 530 (4-nitrophenol) (Boyd,
1982; Broecker et al., 1984; Koerdel et al., 1984; Lokke, 1984;
Marquart et al., 1984). Nitrophenols emitted to soil are expected to
be biodecomposed under aerobic conditions. Infiltration into
groundwater is expected only under conditions unfavourable for
biodegradation (e.g., anaerobic conditions). From the available
experimental results, nitrophenols have to be classified as substances
with a low to moderate potential for soil sorption.
A low potential for bioaccumulation is to be expected from the
available valid test results for 2- and 4-nitrophenol.
Bioconcentration factors ranging from 14.6 to 24.4 were determined for
2-nitrophenol in a semistatic test system with zebra fish
( Brachydanio rerio) (Koerdel et al., 1984); in a flow-through
experiment, bioconcentration factors ranged from 30 to 76 for common
carp ( Cyprinus carpio), including possible conjugates (Broecker et
al., 1984). In static tests, accumulation factors for 4-nitrophenol of
11 for the green alga Chlorella fusca after 1 day (Geyer et al.,
1981) and 57 for the freshwater golden orfe ( Leuciscus idus
melanotus) after 3 days of exposure were determined (Freitag et al.,
1982). Zebra fish exposed in tap and river water nearly completely
eliminated the accumulated 14C-4-nitrophenol within 48 h (Ensenbach &
Nagel, 1991). Star fish ( Pisaster ochraceus) and sea urchin
( Strongylocentrotus purpuratus) eliminated 89% and 36%,
respectively, of injected 14C-4-nitrophenol (3.48 and 3.70 mg/kg body
weight, respectively) within 8 h (Landrum & Crosby, 1981).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
From the concentrations in rainwater, the total atmospheric
nitrophenol pollution in Switzerland is estimated at about 1 µg/m3
(Leuenberger et al., 1988). Recent measurements in the air of remote
areas in Europe (German Alps, Fichtelgebirge, Germany; Mount Brocken,
Germany; Great Dun Fell summit, United Kingdom) gave 2-nitrophenol
concentrations between 0.8 and 25 ng/m3 and 4-nitrophenol levels
between 1.2 and 360 ng/m3 (Herterich & Herrmann, 1990; Luettke et
al., 1997). The higher atmospheric 4-nitrophenol levels are apparently
due to the higher photochemical stability of this isomer (see section
5). 2-Nitrophenol was found in 22 out of 27 samples of air (range
1-140 ng/m3; detection limit 1 ng/m3) in Japan in 1994, and
4-nitrophenol was detected in 27 out of 27 air samples (range 1-71
ng/m3; detection limit 1 ng/m3) (Japan Environment Agency, 1995).
In street dust samples from a Japanese city, up to 3.9 mg
2-nitrophenol/kg and up to 42 mg 4-nitrophenol/kg were detected
(Nojima et al., 1983).
Numerous studies deal with the distribution, deposition, and
degradation behaviour of airborne 2- and 4-nitrophenol in clouds and
rainwater. 2-Nitrophenol levels in rainwater and snow between 0.03 and
5.7 µg/litre and 4-nitrophenol concentrations from <0.5 to
19 µg/litre are given in reports mainly from Germany and the USA (BUA,
1992). The recent measurements in rainwater, cloud water, and "fog"
(water vapour; not further characterized) from rural and urban areas
in Europe confirm these concentration ranges (Herterich & Herrmann,
1990; Levsen et al., 1990; Richartz et al., 1990; Capel et al., 1991;
Geissler & Schoeler, 1993; Levsen et al., 1993; Luettke et al., 1997).
The 2-nitrophenol levels are mostly below or slightly above the
detection limit (i.e., <0.1 µg/litre), whereas mean 4-nitrophenol
concentrations of about 5 µg/litre rainwater and cloud water and 20
µg/litre fog water were detected. The nitrophenol concentrations in
fog are significantly higher than those in rainwater or cloud water
owing to the higher droplet surface and longer residence times of the
droplets in air compared with rain. The lower concentrations of
2-nitrophenol in the deposition samples compared with 4-nitrophenol
are presumably due to the lower photochemical stability of this
compound (see section 5).
In the 1970s and early 1980s, the 2- and 4-nitrophenol
concentrations in the German and Dutch parts of the river Rhine and
some of its tributaries were between 0.1 and 1 µg/litre (BUA, 1992).
2-Nitrophenol and 4-nitrophenol were not detected in 177 samples of
Japanese surface waters (detection limits 0.04-10 µg/litre) or in 177
sediment samples (detection limits between 0.002 and 0.8 µg/kg) in
1978, 1979, and 1994 (Japan Environment Agency, 1979, 1980, 1995).
Whereas 4-nitrophenol was not detected in 129 fish samples (detection
limits 0.005-0.2 µg/kg) in Japan in 1979 and 1994, 2-nitrophenol was
detected in 1 out of 129 saltwater fish samples (detection limits
0.005-0.3 µg/kg) in 1994 (Japan Environment Agency, 1980, 1995).
2-Nitrophenol concentrations between <0.15 µg/litre (detection limit)
and 7.2 µg/litre and 4-nitrophenol levels between <0.1 and 18.8
µg/litre were reported for the densely populated and highly
industrialized Malaysian Klang river basin in 1990 and 1991 (Tan &
Chong, 1993).
6.2 Human exposure
Workers may be exposed to 2- and 4-nitrophenol via inhalation and
skin contact during production and processing (mainly in the
manufacturing of pesticides). However, data on nitrophenol
concentrations at the workplace were not identified.
Based on the measured concentrations given in section 6.1, an
exposure of the general population to nitrophenols via the environment
-- predominantly through ambient air and drinking-water -- cannot be
excluded.
4-Nitrophenol accumulates in fog, whereas 2-nitrophenol is
rapidly photochemically transformed (see sections 5 and 6.1). The mean
measured level of 4-nitrophenol in fog water is about 20 µg/litre.
In Dutch drinking-water samples, maximum concentrations of 1 µg
2-nitrophenol/litre and <0.1 µg 4-nitrophenol/litre were reported in
1988 (BUA, 1992). Further data are not available.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND
HUMANS
Studies providing quantitative information on the absorption,
metabolism, or elimination of 2- or 4-nitrophenol in humans were not
identified.
7.1 2-Nitrophenol
There is only very limited information available for
2-nitrophenol. In rabbits given a single dose of 200-330 mg/kg body
weight via gavage, most of the applied dose (>>80%) was excreted via
the urine within 24 h. About 71% was conjugated with glucuronic acid
and about 11% with sulfate, whereas about 3% was reduced to
aminophenols (Robinson et al., 1951).
Skin permeation for 2-nitrophenol was shown in several in vitro
experiments (Huq et al., 1986; Jetzer et al., 1986; Ohkura et al.,
1990).
Although the information is limited, bioaccumulation of
2-nitrophenol in organisms is not to be expected owing to its rapid
metabolism and excretion.
7.2 4-Nitrophenol
After oral, dermal, intravenous, or intraperitoneal application
of 4-nitrophenol to several test species (rats, mice, dogs, or
rabbits), most of the applied dose (up to 95%) was excreted as
glucuronide and sulfate conjugates of 4-nitrophenol via the urine
within 24-48 h. Only small amounts were excreted via faeces (about 1%)
or as unchanged 4-nitrophenol (about 2-7%). The percentages of
glucuronide and sulfate conjugates were shown to be species, sex, and
dose dependent. Although sulfate conjugation dominates at lower
4-nitrophenol concentrations, the percentage of glucuronide conjugates
increases at higher dosages (Robinson et al., 1951; Gessner & Hamada,
1970; Machida et al., 1982; Rush et al., 1983; Snodgrass, 1983;
Tremaine et al., 1984; Meerman et al., 1987). As shown in rabbits
after oral dosing, 4-nitrophenol undergoes reduction to 4-aminophenol
as well as glucuronidation and sulfation. Up to 14% of the
administered dose was detected as amino compounds in the urine
(Robinson et al., 1951). After intraperitoneal administration in mice,
4-nitrophenyl glucoside was identified as a minor metabolite of
4-nitrophenol (about 1-2% of the administered dose) (Gessner & Hamada,
1970).
For 4-nitrophenol, the pretreatment of laboratory animals with
ethanol (induction of cytochrome P-450) resulted in a marked increase
in hepatic microsomal hydroxylation. The 4-nitrocatechol then formed
competed with 4-nitrophenol for the glucuronidation and sulfation
pathways (Reinke & Moyer, 1985; Koop, 1986; McCoy & Koop, 1988; Koop &
Laethem, 1992).
Specific investigations on dermal resorption under non-occlusive
conditions showed dermal uptake of about 35% and 11% of the applied
dose of 14C-4-nitrophenol within 7 days in rabbits and dogs,
respectively. Skin permeation for 4-nitrophenol was also shown in
several in vitro experiments (Huq et al., 1986; Jetzer et al., 1986;
Ohkura et al., 1990).
Owing to its rapid metabolism and excretion, bioaccumulation of
4-nitrophenol in organisms is not to be expected.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
For 2-nitrophenol, the oral LD50 is in the range of
2830-5376 mg/kg body weight in rats (BASF AG, 1970; Vasilenko et al.,
1976; Vernot et al., 1977; Koerdel et al., 1981) and 1300-2080 mg/kg
body weight in mice (Vasilenko et al., 1976; Vernot et al., 1977).
Clinical signs following oral exposure were unspecific and included
dyspnoea, staggering, trembling, somnolence, apathy, and cramps. The
macroscopic examination performed in some studies revealed congestion
in liver and kidneys and ulcers of the stomach in high-dose rats. The
inhalation exposure of rats to an atmosphere saturated with the test
substance at 20°C for 8 h (no further information available) resulted
in no mortality and no signs of toxicity (BASF AG, 1970). In a limit
test, the dermal LD50 for the rat was >5000 mg/kg body weight
(Koerdel et al., 1981). In cats (two animals per dose group), the oral
application of 2-nitrophenol (50, 100, or 250 mg/kg body weight; no
controls) resulted in a dose-dependent increase in methaemoglobin (6,
44, and 57%, respectively).1 One animal dosed with 250 mg/kg body
weight died. No formation of methaemoglobin was detected after dermal
application of a 50% solution of 2-nitrophenol in water to rabbits
(dose not specified, exposure time 1 min to 20 h on the back or 20 h
on the ear) (BASF AG, 1970).
The oral LD50 of 4-nitrophenol is in the range of 220-620 mg/kg
body weight in rats (BASF AG, 1969; Vasilenko et al., 1976; Hoechst
AG, 1977a; Vernot et al., 1977; Andrae et al., 1981) and 380-470 mg/kg
body weight in mice (Vasilenko et al., 1976; Vernot et al., 1977).
Clinical signs following oral exposure of rats were unspecific and
included tachypnoea and cramps, and the macroscopic examination
performed in some studies revealed a greyish discoloration with dark
red patches of the lungs. No mortality was observed in rats after
single exposure (head only) to 4700 mg/m3 (application as dust
[sodium salt]; particle size not given) for 4 h. In four of six rats,
a corneal opacity was observed at the end of exposure, which persisted
through the 14-day observation period. In two extra rats exposed to
1510 mg/m3, the methaemoglobin concentrations were not altered
compared with controls. A determination of methaemoglobin
concentrations after exposure to 4700 mg/m3 was not performed (Smith
et al., 1988). In another inhalation study with rats (exposure to an
atmosphere saturated with the test substance at 20°C for 8 h; no
further information available), no mortality and no signs of toxicity
1 Methaemoglobin formation is discussed in greater detail in
section 8.8.
were seen (BASF AG, 1969). The dermal LD50 for rats and guinea-pigs
is >1000 mg/kg body weight (Hoechst AG, 1977b; Eastman Kodak Co.,
1980; Andrae et al., 1981). In contrast to 2-nitrophenol, no formation
of methaemoglobin was noted in cats (two animals per dose group) after
oral dosing with 100, 200, or 500 mg 4-nitrophenol/kg body weight. The
mortality rate was 0/2, 1/2, and 2/2, respectively (BASF AG, 1969).
8.2 Irritation and sensitization
From studies comparable to OECD Guidelines 404 and 405, it can be
concluded that 2-nitrophenol is slightly irritating to the skin but
not to the eye (scores not given). In a Buehler test with guinea-pigs
comparable to OECD Guideline 406, the substance showed no
skin-sensitizing effects (Koerdel et al., 1981).
In a study performed according to US Food and Drug Administration
(FDA) guidelines, non-dissolved 4-nitrophenol was slightly irritating
to the skin (score 2 of 8) (Hoechst AG, 1977c); in another study
comparable to OECD Guideline 404, however, the non-dissolved substance
showed no skin-irritating effects (score 0 of 4) (Andrae et al.,
1981). 4-Nitrophenol applied as a 10% solution to the eyes was
slightly irritating in a test conducted according to FDA guidelines
(scores not given; Hoechst AG, 1977c). Results with the non-dissolved
substance were either strongly irritating in a test conducted
according to FDA guidelines (scores not given; Hoechst AG, 1977c) or
slightly irritating in a test comparable to OECD Guideline 405 (score
1-2 of 4; Andrae et al., 1981).
In a guinea-pig maximization test comparable to OECD Guideline
406, skin sensitization was shown in 5 of 20 animals (Andrae et al.,
1981).
Data on respiratory tract sensitization for 2- and 4-nitrophenol
were not identified in the literature.
8.3 Short-term exposure
8.3.1 Oral exposure
The effect of 2-nitrophenol in rats was studied in a 28-day study
to evaluate OECD Guideline 407 (five animals per sex per dose group;
daily oral doses of 0, 22, 67, or 200 mg/kg body weight via gavage).
Food intake decreased in high-dose males and in mid- and high-dose
females, and final body weight decreased non-significantly in all
dosed animals. The absolute liver and kidney weights were decreased in
mid-dose animals, and the relative testes weight increased in low- and
mid-dose males and decreased in high-dose males. In all dosed animals,
the relative and absolute weights of the adrenal glands increased. The
haematological examination, clinical chemistry, and histopathological
examination of the major organs and tissues did not give any
indication of a substance-related toxic effect in comparison with
controls (Koerdel et al., 1981). Owing to insufficient documentation
and the fact that there were minor effects (weight of adrenal glands)
shown by all exposed animals, a reliable NO(A)EL cannot be deduced.
In a 28-day study that was also conducted to evaluate OECD
Guideline 407, Sprague-Dawley rats (10 per sex per dose group)
received daily oral doses of 0, 70, 210, or 630 mg 4-nitrophenol/kg
body weight via gavage. After dosing, locomotor inhibition, which
lasted for about 2 h, was seen in mid- and high-dose animals. In
mid-dose animals, 1/10 males died; in high-dose males and females, the
mortality rate was 4/10 and 6/10, respectively (specific signs of
intoxication were not given). In the lowest dose group, the
macroscopic examination revealed seven cases of pale liver, and the
histopathological examination showed 14 cases of finely dispersed
fatty degeneration. A focal fatty degeneration of the liver was also
observed in 13/20 rats of the mid-dose group, but not in high-dose
animals. However, it must be noted that finely dispersed fatty
degeneration was also seen in 6/20 control animals. In 4/10 high-dose
males but not females, a hydropic liver cell swelling was noted, and
all high-dose rats that died before the end of the study showed
vascular congestion of the liver. A slight increase in the leukocyte
count was seen at 210 and 630 mg/kg body weight in males and females;
the increase was significant in high-dose females. In high-dose males,
the alanine aminotransferase (ALAT) activity was significantly
increased. Other substance-related effects in high-dose animals
included increased nephrosis (two males and five females), testicular
atrophy and inhibition of spermatogenesis (one and two males,
respectively), and follicular atresia in the ovaries (four females)
(Andrae et al., 1981). Because of unclear effects in the liver, a
NO(A)EL cannot be deduced.
8.3.2 Inhalation exposure
8.3.2.1 2-Nitrophenol
In Sprague-Dawley rats (15 per sex per group), no mortality was
observed after exposure to 0, 5, 30, or 60 mg 2-nitrophenol vapour/m3
("whole body" exposure; to generate the vapour, melted 2-nitrophenol
was used) for 6 h/day, 5 days/week, over a period of 4 weeks. Except
for squamous metaplasia of the epithelium lining the maxilloturbinates
and nasoturbinates in all high-dose animals, the clinical and
histopathological examinations gave no consistent exposure-related
effects. The methaemoglobin values determined after the 11th exposure
were significantly increased only in low-dose animals (males: 1.0,
2.3, 1.8, and 1.6%; females: 2.0, 4.1, 2.1, and 1.1%), but were within
control values at the end of the study (Hazleton Lab., 1984).
8.3.2.2 4-Nitrophenol
No mortality was observed in male albino Crl:CDR rats (10 per
group) after exposure to 0, 340, or 2470 mg 4-nitrophenol dust/m3
(application as sodium salt; "head only" exposure; mass median
aerodynamic diameter [MMAD] 4.6-7.5 µm) for 6 h/day, 5 days/week, over
a period of 2 weeks. Both exposure concentrations resulted in signs of
irritation (not further specified). After exposure to 340 and 2470
mg/m3, darker urine, proteinuria, elevated aspartate aminotransferase
(ASAT) values, and a dose-dependent increase in methaemoglobin values
were observed. These effects were still evident after a 14-day
recovery period; however, the methaemoglobin value was then still
elevated in only 2/5 high-dose animals. The methaemoglobin values were
0.2, 0.87, and 1.53% after 10 exposures and 0.2, 0.13, and 0.7% after
14 days' recovery. The erythrocyte, haemoglobin, and haematocrit
values decreased during exposure but were elevated after the 14-day
recovery period. In treated rats, the urine volume decreased in a
dose-dependent manner during exposure and during the 14-day recovery
period. In high-dose animals, the absolute spleen weight was
significantly lower than that of controls after 10 exposures, and the
absolute/relative spleen and lung weights were significantly lower in
comparison with controls at the end of the recovery period. According
to the authors, the biological significance of the changes in organ
weights is unknown owing to the absence of corroborating pathological
effects (Smith et al., 1988).
In a second trial (exposure to 0, 30, or 130 mg/m3; MMAD 4.0-4.8
µm), both exposure concentrations again resulted in signs of
irritation (not further specified). Methaemoglobinaemia, an effect
that was reversible within a 14-day recovery period, was seen only at
130 mg/m3. The methaemoglobin values were 0.5, 0.3, and 1.5% after 10
exposures and 0.4, 0.5, and 0.2% after 14 days' recovery. The gross
and histopathological examination revealed no adverse effects in any
dose group. From these results, the authors of the study decided upon
a NO(A)EL of 30 mg/m3 (Smith et al., 1988).
Groups of Sprague-Dawley rats (15 per sex) were exposed to 0, 1,
5, or 30 mg 4-nitrophenol dust/m3 ("whole body" exposure; MMAD
5.2-6.7 µm) for 6 h/day, 5 days/week, over a period of 4 weeks. The
exposure resulted in no deaths, and no exposure-related effects were
noted in terms of haematology or clinical chemistry values, gross
examination, histopathology, and body or organ weights. In high-dose
animals, unilateral and bilateral diffuse anterior capsular cataracts
were observed. The methaemoglobin values determined after 2 weeks of
exposure showed great variability and appeared to be unusually high
(>3 %) in some unexposed controls. However, the group total
methaemoglobin value was increased at a concentration of 5 mg/m3,
which was significant in males and not significant in females (males:
0.8, 0.5, 2.2, and 1.1%; females: 1.3, 1.1, 2.0, and 1.0%) (Hazleton
Lab., 1983). Therefore, a NO(A)EL of 5 mg/m3 can be derived for local
effects (cataracts), whereas the NO(A)EL for systemic effects
(formation of methaemoglobin) may be lower.
8.3.3 Dermal exposure
Data concerning short-term dermal exposure were not identified in
the literature.
8.4 Long-term exposure
In the literature, subchronic and chronic studies are available
only for 4-nitrophenol.
8.4.1 Subchronic exposuren
In a 13-week gavage study with Sprague-Dawley rats (20 per sex
per dose group) given 0, 25, 70, or 140 mg 4-nitrophenol/kg body
weight in water 5 days/week, premature deaths were seen in animals
dosed with 70 and 140 mg/kg body weight (1 male/1 female at 70 mg/kg
body weight and 15 males/6 females at 140 mg/kg body weight); these
were usually preceded by clinical signs, including pale appearance,
languid behaviour, prostration, wheezing, and dyspnoea, shortly after
dosing. The histopathological examination of these animals revealed
minimal to moderately severe congestion in the lung, liver, kidney,
adrenal cortex, and pituitary; in surviving animals, no
treatment-related changes compared with controls were reported. A
statement concerning altered methaemoglobin values cannot be given
owing to a non-reliable analytical method (about 13% in controls at
week 7) (Hazleton Lab., 1989). Therefore, only a provisional NO(A)EL
(changes in liver, kidneys, and lungs) of 25 mg/kg body weight can be
derived from this study. The NO(A)EL based on the formation of
methaemoglobin may be lower.
The dermal application of 4-nitrophenol to Swiss-Webster mice (10
per sex and dose group; given 0, 22, 44, 88, 175, or 350 mg/kg body
weight in acetone, 3 times per week over 13 weeks) resulted in
dose-dependent mortality as well as skin irritation/inflammation and
necrosis at >175 mg/kg body weight.1
1 Gulf South Research Institute, not dated;
no further information available; results cited from
NTP (1993).
8.4.2 Chronic exposure and carcinogenicityn
In a long-term study with Swiss-Webster mice (50 per sex per dose
group), 4-nitrophenol in acetone was applied to the interscapular skin
at doses of 0, 40, 80, or 160 mg/kg body weight, 3 days/week for 78
weeks. At termination of the study, the survival rates were 29/60,
17/60, 26/60, and 24/60 for males and 35/60, 26/60, 33/60, and 27/60
for females. The increased mortality after 60 weeks was due to a
generalized amyloidosis (the severity of the amyloidosis was similar
among dosed and control animals) and secondary kidney failure. The
final mean body weights of the dosed animals were similar to those of
the controls. NTP (1993) stated that there were no substance-related
neoplastic or non-neoplastic effects associated with the dermal
administration of 4-nitrophenol and that there was no evidence of a
carcinogenic activity of the substance in male or female mice.
In another study, which had several procedural deficiencies (only
the skin was examined; only 12 weeks of exposure), no skin tumours
were observed in 31 female Sutter mice after dermal application of a
20% solution (25 µl of solution applied twice weekly) of 2- or
4-nitrophenol in dioxane (Boutwell & Bosch, 1959).
8.5 Genotoxicity and related end-points
The available in vitro and in vivo genotoxicity studies on
2- and 4-nitrophenol are summarized in Table 3.
2-Nitrophenol showed no mutagenicity in several limited bacterial
assays. From the available data, it is not possible to draw any
conclusions regarding its mutagenicity.
For 4-nitrophenol, positive results were obtained in in vitro
tests for chromosomal aberrations in mammalian cells. However, apart
from one well-documented study published by NTP (1993), the other
available assays were inadequately reported. 4-Nitrophenol was shown
to be mutagenic in some but not all of the bacterial assays, whereas
other studies (i.e., host-mediated bacterial assay, mouse lymphoma
assay, unscheduled DNA synthesis assay [apparently in vitro], sister
chromatid exchange assay, sex-linked recessive lethal [SLRL] assay in
Drosophila) gave negative results. In the absence of any in vivo
mutagenicity studies in mammals, it is not possible to conclude
whether or not the mutagenic potential of 4-nitrophenol is expressed
in vivo.
Table 3: Genotoxicity of 2- and 4-nitrophenol in vitro and in vivo.
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
2-Nitrophenol
(in vitro studies)
delta phage DNA Induction of 35 mg - 0 Yamada et al.
DNA breakage (1987)
Bacillus subtilis Recombination 0.01-0.5 - 0 Shimizu & Yano
H17, M45 assay mg/plate (1986)
Salmonella Reverse 0.003-2.5 - - Koerdel et al. (1981);
typhimurium mutations mg/plate Haworth et al. (1983);
TA1535, TA1537 Shimizu & Yano (1986)
Salmonella Reverse 0.01-2.5 - - Koerdel et al. (1981);
typhimurium mutations mg/plate Shimizu & Yano (1986)
TA1538
Salmonella Reverse 0.0007-5 - - Suzuki et al. Chiu et al. (1978);
typhimurium mutations mg/plate (1983) also Koerdel et al. (1981);
TA98, TA100 tested both Haworth et al. (1983);
strains in Suzuki et al. (1983);
the presence Shimizu & Yano (1986);
of norharman, Kawai et al. (1987);
which also Dellarco & Prival (1989);
gave negative Massey et al. (1994)
results
Table 3 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
2-Nitrophenol (in vivo studies)
Drosophila SLRL assay via feed (400 and 500 - Foureman et al. (1994)
melanogaster ppm) or injection
(2500 and 5000 ppm)
4-Nitrophenol (in vitro studies)
delta phage DNA Induction of 35 mg - 0 Yamada et al. (1987)
DNA breakage
Bacillus subtilis Recombination 0.01-5 mg/plate + 0 positive Shimizu
H17, M45 assay at 0.5 & Yano (1986)
mg/plate
Escherichia coli Gene mutation 0.001-2.5 - - Hoechst AG (1980)
WP2uvrA mg/plate
Escherichia coli Gene mutation 0.125-2 mg/plate - 0 Rashid & Mumma (1986)
K-12 (Pol
A1+/Pol1-), WP2
(WP2, WP2uvrA,
WP67, CM611,
CM571)
Escherichia coli DNA cell 7 or 70 mg + + positive at Kubinski et al.
Q13 binding assay 70 mg (1981)
Table 3 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
Saccharomyces Mitotic gene 2.9 mg/ml (+) 0 Fahrig (1974)
cerevisiae conversion
ade 2, trp 5
Salmonella DNA damage up to 0.75 - - Nakamura et al.
typhimurium (umu test) mg/ml (1987)
TA1535/pSK 1002
Salmonella Reverse 0.001-2.5 + - positive Hoechst
typhimurium mutation mg/plate at >0.1 AG (1980)
TA1538 mg/plate
Salmonella Reverse 0.01-5 - - Andrae et al. (1981);
typhimurium mutation mg/plate Shimizu & Yano (1986)
TA1538
Salmonella Reverse 0.125-2 - 0 Rashid & Mumma (1986)
typhimurium mutation mg/plate
TA1538, TA1978
Salmonella Reverse 0.0007- - - Suzuki et McCann et al. (1975);
typhimurium mutation 5 mg/plate al. (1983) Hoechst AG (1980);
TA98, TA100 also tested Andrae et al. (1981);
both strains Haworth et al. (1983);
in the Suzuki et al. (1983);
presence of Shimizu & Yano (1986);
norharman, Kawai et al. (1987);
which also Dellarco & Prival (1989);
gave negative Massey et al. (1994)
results
Table 3 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
Salmonella Reverse 0.001-5 - - McCann et al. (1975);
typhimurium mutation mg/plate Hoechst AG (1980);
TA1535, TA1537 Andrae et al. (1981);
Haworth et al. (1983);
Shimizu & Yano (1986)
Rat hepatocytes DNA damage 42-417 mg (+) 0 Weakly Storer
(alkaline positive at et al. (1996)
elution) >97 mg
Rat hepatocytes DNA repair 4.2-417 mg - 0 Andrae et al. (1981)
Chinese hamster Chromosomal without S9 mix: - + NTP (1993)
ovary (CHO) aberration 0.1-0.5 mg/ml
cells with S9 mix:
1.25-2 mg/ml
Chinese hamster Sister chromatid without S9 mix: - - NTP (1993)
ovary (CHO) cells exchange 0.00017-0.025 mg/ml
with S9 mix:
0.05-1.5 mg/ml
Mouse lymphoma Forward mutation without S9 mix: - - Oberly et al. (1984)
assay 0.7-1.5 mg/ml
L5178Y with S9 mix:
TK+/- cells 0.0001-0.03
mg/ml
Table 3 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
Mouse lymphoma Forward mutation 0.06-0.78 mg/ml 0 - Amacher & Turner (1982)
assay L5178Y
TK+/- cells
Rat hepatocytes Unscheduled DNA 0.00007-0.14 mg/ml - 0 Probst et al. (1981)
synthesis
Human lymphocytes Chromosomal not given + No data about Huang et al. (1996)
aberration metabolic
activation;
validity
cannot be
judged
(documentation
and study
design
insufficient
for
assessment)
lowest
positive
concentration:
1.4 mg/ml
Table 3 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
Human Chromosomal 0.001-0.3 mg/ml + No data about Huang et al. (1995)
lymphocytes aberration metabolic
activation;
validity cannot
be judged
(documentation
and study
design
insufficient
for assessment)
Human fibroblasts DNA repair 0.14-139 mg + No data about Poirier et al. (1975)
(WI-38) metabolic
activation;
validity
cannot be
judged
(documentation
insufficient
for assessment)
positive at
>13.9 mg
Table 3 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
4-Nitrophenol (in vivo studies)
NMRI mice Host-mediated single subcutaneous - application of Buselmaier et al. (1972)
assay (tester injection of test substance
strains 75 mg/kg immediately
Salmonella body weight after the
typhimurium G 46 bacteria had
and Serratia been injected
marcescens a into the
21 Leu-) abdominal
cavities;
test duration
3 h
Drosophila SLRL assay via feed (1000, - Zimmering et al. (1985);
melanogaster 2500, 6000, or Foureman et al. (1994)
7500 ppm) or
injection (1000
or 1500 ppm)
a -, negative; +, positive; (+), weakly positive; 0, not tested.
8.6 Reproductive and developmental toxicity
8.6.1 Reproductive toxicity
In a valid two-generation study with groups of 24 female and 12
male Sprague-Dawley rats carried out by Angerhofer (1985),
4-nitrophenol dissolved in ethanol was applied dermally at doses of 0,
50, 100, or 250 mg/kg body weight per day, 5 days/week. The F0
generation was exposed over a period of 140 days before mating. Dosing
of the F0 females continued throughout breeding, gestation, and
lactation. Groups of 26 females and 13 males of the F1 generation
were then exposed for 168 days in the same manner as had been the F0
rats; the females were again exposed throughout breeding, gestation,
and lactation. Apart from dose-related signs of skin irritation
(erythema, scaling, scabbing, and cracking) in dosed animals, the
gross and histopathological examinations provided no indication of
significant adverse effects. The calculated indices concerning
fertility, gestation, viability, and lactation were not different from
those of controls. The testis to body weight ratios in the F0
generation were not affected, and histological lesions were not
observed in the testes. In a 28-day study in rats (see section 8.3.1),
testicular atrophy and inhibition of spermatogenesis were observed in
some animals after oral dosing at a level of 630 mg/kg body weight,
but not at 210 mg/kg body weight.
8.6.2 Developmental toxicity
8.6.2.1 2-Nitrophenol
In a range-finding study with Charles River COBS(c) CD(c) rats
(five dams per group; application of 0, 50, 125, 250, 500, or
1000 mg/kg body weight via gavage from day 6 to day 15 of gestation;
uterine examination on day 20), dose levels of 500 and 1000 mg/kg body
weight caused signs of maternal toxicity (transient but dose-related
decrease in weight gain early during treatment). One high-dose animal
died, but no cause of death could be determined. Other clinical
findings included darkly coloured urine at >250 mg/kg body weight
and yellow staining of haircoat (at the nose, mouth, anogenital area)
at >125 mg/kg body weight; the necropsy findings gave no
biologically meaningful differences in surviving dams. At the highest
dose level of 1000 mg/kg body weight, a slight but statistically
significant (also compared with historical controls) increase in group
mean post-implantation losses (13.8% versus 8.2% in controls) and mean
early resorptions (2.3 versus 1.2 in controls) was seen. No effects
were observed on the number of viable fetuses, implantations, or
corpora lutea (International Research and Developmental Corporation,
1983).
8.6.2.2 4-Nitrophenol
In both studies cited below, a complete examination of the pups
for possible teratogenic effects was not performed. In addition, owing
to limitations of these studies (i.e., use of only one dose group or
exposure to a mixture), reliable NO(A)EL values cannot be derived.
In a study performed by Booth et al. (1983), groups of 50 female
CD-1 mice received daily oral doses of 400 mg 4-nitrophenol/kg body
weight via gavage from day 7 to day 14 of gestation. The survival rate
in pregnant mice ( n = 36) was 81% versus 100% in controls, and dosed
animals showed less maternal weight gain. No changes were observed in
the reproductive index (ratio between survivors delivered and pregnant
survivors). The average number of live pups per litter was slightly
decreased, but 4-nitrophenol produced no gross abnormalities.
Kavlock (1990) studied the developmental toxicity of
4-nitrophenol in Sprague-Dawley rats. The substance (dissolved in a
mixture of water, Tween 20, propylene glycol, and ethanol [4:4:1:1])
was applied via gavage to groups of 12-13 animals at doses of 0, 100,
333, 667, or 1000 mg/kg body weight on day 11 of gestation. Endpoints
concerning maternal toxicity included signs of toxicity, mortality,
body weight gain, and the number of implantation scars in the uteri at
weaning. In the offspring, viability, body weight on postnatal days
1-6, overt malformations, and perinatal loss were recorded. In dams,
the mortality was increased at a dose level of >667 mg/kg body
weight; at a dose level of >333 mg/kg body weight, the litter size
on postnatal days 1 and 6 was non-significantly decreased.
8.7 Immunological and neurological effects
There are no studies available dealing specifically with
immunological or neurological effects. There is an indication from an
in vitro study that 4-nitrophenol may act as a suppressor of
cell-mediated immune response (Pruett & Chambers, 1988). However, the
biological significance is uncertain.
8.8 Methaemoglobin formation
Methaemoglobin formation by 2-nitrophenol and 4-nitrophenol has
been tested in several studies using different species, routes, and
durations of applications. An overview is given in Table 4.
2-Nitrophenol clearly leads to the formation of methaemoglobin in
a dose-dependent manner in cats (BASF AG, 1970), the most sensitive
species. The lowest dose tested, 50 mg/kg body weight, produced
increased methaemoglobin levels. In inhalation experiments in rats,
elevated methaemoglobin levels were observed at an exposure level of 5
mg/m3; methaemoglobin levels were less elevated at exposure levels of
30 and 60 mg/m3 (Hazelton Lab., 1984).
4-Nitrophenol, in contrast, did not lead to methaemoglobin
formation in cats at concentrations up to 500 mg/kg body weight (BASF
AG, 1969). In rats, at high concentrations in inhalation experiments,
the methaemoglobin-forming capacity seemed to be very low (1.5% at
2470 mg/m3). In conclusion, 4-nitrophenol may induce methaemoglobin
formation, but the effect seems to be rather weak, without clear
dose-response.
9. EFFECTS ON HUMANS
Naniwa (1979) performed patch tests with 4-nitrophenol,
4-aminophenol, 2-amino-4-chlorophenol,
3'-chlorodiphenylamine-2-carboxylic acid, and 4-dichloronitrobenzene
(0.1, 0.5, or 1% in petrolatum) on 31 employees probably exposed to
these chemicals in a chemical factory and on 5 control persons. In
four employees, a positive reaction to 4-nitrophenol was observed,
although none of these persons reacted positively to all three tested
concentrations. All four employees also reacted positively to
2-amino-4-chlorophenol, which was shown to be a strong sensitizer.
Therefore, 2-amino-4-chlorophenol may act as the primary allergen, and
the effects observed with 4-nitrophenol may be due to
cross-sensitization.
In 27 patients primarily sensitized to
1-chloro-2,4-dinitrobenzene, no cross-sensitization due to
4-nitrophenol (1-2% in petrolatum) was observed. In addition, 15
patients with a chloramphenicol allergy failed to react to
4-nitrophenol (Eriksen, 1978).
Table 4: Methaemoglobin formation by 2-nitrophenol and 4-nitrophenol.
Species Route Frequency/duration Dose Results Reference
(strain/number/dose/sex) (% metHb)
2-Nitrophenol
cat oral 1 × 50 mg/kg body weight 6 BASF AG (1970)
2 100 44
sex not given 250 57
rabbit dermal 1 × 50% solution no increase BASF AG (1970)
number and sex in water
not given
rat inhalation 6 h/day m f Hazleton Lab.
Sprague-Dawley 5 days/week 0 mg/m3 1.0 2.0 (1984)
15 m/15 f 4 weeks 5 2.3 4.1
30 1.8 2.1
60 1.6 1.1
11th day of
treatment
4-Nitrophenol
cat oral 1 × 100 mg/kg body no increase BASF AG (1969)
2 200 weight
sex not given 500
rat oral 5 days/week 0 mg/kg body weight analytical Hazleton Lab.
Sprague-Dawley 13 weeks 25 method not (1989)
20 m/20 f 70 reliable (13%
140 in controls)
Table 4: Methaemoglobin formation by 2-nitrophenol and 4-nitrophenol.
Species Route Frequency/duration Dose Results Reference
(strain/number/dose/sex) (% metHb)
rat inhalation 6 h/day 0 mg/m3 0.2 0.2 Smith et al.
Crl:CDR 5 days/week 340 0.87 0.13 (1988)
10 m 2 weeks 2470 1.53 0.7
end of treatment
and after 14
days of recovery
rat inhalation 6 h/day 0 mg/m3 0.5 0.4 Smith et al.
Crl:CDR 5 days/week 30 0.3 0.5 (1988)
10 m 2 weeks 130 1.5 0.2
end of treatment
and after 14
days of recovery
rat inhalation 6 h/day m f Hazleton Lab.
Sprague-Dawley 5 days/week 0 mg/m3 0.8 1.3 (1983)
15 m/15 f 4 weeks 1 0.5 1.1
5 2.2 2.0
30 1.1 1.0
after 2 weeks of
exposure, values
unusually high
in some control
animals
Abbreviations used: m = male; f = female; metHb = methaemoglobin.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1 Aquatic environment
Experimental test results for the most sensitive species are
summarized in Table 5. Additional data on the toxicity of 2- and
4-nitrophenol to aquatic organisms are cited in BUA (1992). Among all
tested organisms, the protozoan Entosiphon sulcatum and the green
alga Scenedesmus subspicatus proved to be most sensitive in chronic
cell multiplication inhibition tests with freshwater species. Daphnia
magna exhibited a 21-day lowest-observed-effect concentration (LOEC)
of 1.0 mg 2-nitrophenol/litre in the Daphnia reproduction test
(Koerdel et al., 1984). The entoproct Barentsia matsushimana was the
most sensitive marine invertebrate species tested, exhibiting a 49-day
EC50 value of 0.21 mg 4-nitrophenol/litre and a minimal effective
concentration (ECm) of 0.03 mg/litre (end-point: growth of
germinated spores) (Scholz, 1986). Freshwater fish showed less
sensitivity. The lowest 96-h LC50 value of 3.8 mg 4-nitrophenol/litre
was determined for rainbow trout ( Oncorhynchus mykiss) (Howe et
al., 1994). The measured no-observed-effect concentration (NOEC) for
behavioural changes in a 28-day flow-through test with zebra fish was
2 mg 2-nitrophenol/litre (Broecker et al., 1984). After prolonged
exposure of zebra fish to 4-nitrophenol, minor morphological
alterations of the liver, even at a concentration of 0.1 mg/litre,
were observed. At 1 and 5 mg/litre, about 25% of the animals showed
symptoms of degenerative transformation of the liver tissue (Braunbeck
et al., 1989).
10.2 Terrestrial environment
The toxicity of 2- and 4-nitrophenol on higher plants according
to OECD Guideline 208 was tested in independent studies. After
incubation of seeds with different test substance concentrations,
14-day EC50 values for reduced fresh weight of grown shoots were in
the range of 52-420 mg 2-nitrophenol/kg soil (Broecker et al., 1984;
Koerdel et al., 1984) and 35-260 mg 4-nitrophenol/kg soil (Ballhorn et
al., 1984; Marquart et al., 1984). The 14-day EC10 value for
2-nitrophenol was 10 mg/kg soil for both species. Overall, turnip
( Brassica rapa) proved to be more sensitive than oat
( Avena sativa).
In tests conducted according to OECD Guideline 207, the adverse
effects of 2- and 4-nitrophenol on earthworms were examined in several
independent studies. In the contact test, in which the animals are
exposed on filter paper soaked with the test substance, Neuhauser
et al. (1985) established a 48-h LC50 value of 5.9 µg/cm2 for the
toxicity of 2-nitrophenol on Eisenia fetida. For 4-nitrophenol, the
48-h LC50 values were in the range of 0.7-2.7 µg/cm2, with
Table 5: Aquatic toxicity of nitrophenols.
Most sensitive species
(test method/end-point) Substance Effective Reference
concentration
(mg/litre)
Bacteria
Pseudomonas putida 2-NP 16-h MICa: 0.9 Bringmann & Kuehn (1977)
(cell multiplication 4-NP 16-h MIC: 4.0
inhibition test)
Protozoa
Entosiphon sulcatumn 2-NP 72-h MIC: 0.40 Bringmann (1978);
(cell multiplication 4-NP 72-h MIC: 0.83 Bringmann et al. (1980)
inhibition test)
Algae
Scenedesmus subspicatus 2-NP 96-h EC50: 0.39 Broecker et al. (1984);
Chlorella vulgaris 2-NP 6-h EC50: 1.53 Kramer et al. (1986)
(cell multiplication 4-NP 6-h EC50: 6.97
inhibition test)
Invertebrates
Moina macrocopa (acute) 2-NP 3-h LC50: 1.9 Yoshioka et al. (1985)
(immobilization) 4-NP 3-h LC50: 1.3
Daphnia magna (long-term) 2-NP 21-day LOEC: 1.0 Koerdel et al. (1984)
(immobilization/reproduction) 4-NP 21-day NOEC: 1.3
Barentsia matsushimana (marine) 4-NP 49-day EC50: 0.21 Kuehn et al. (1988)
(growth of germinated spores) 4-NP 49-day ECmb: 0.03 Scholz (1986)
Table 5 (cont'd)
Most sensitive species
(test method/end-point) Substance Effective Reference
concentration
(mg/litre)
Fish
Cyprinus carpio (static) 2-NP 96-h LC50: 36.6 Lang et al. (1996)
Oncorhynchus mykiss (static) 4-NP 96-h LC50: 3.8 Howe et al. (1994)
Oncorhynchus mykiss (flow-through) 4-NP 96-h LC50: 7.93
Abbreviations used: 2-NP = 2-nitrophenol; 4-NP = 4-nitrophenol.
a MIC = minimum inhibitory concentration.
b ECm = minimal effective concentration.
Eisenia fetida and Eudrilus eugeniae being the most sensitive
species tested (Roberts & Dorough, 1984; Neuhauser et al., 1985,
1986). When exposed in an artificial soil mixture, 28-day LC50 values
for 2-nitrophenol were in the range of 250-500 mg/kg soil ( Eisenia
fetida) (Broecker et al., 1984; Koerdel et al., 1984), and 14-day
LC50 values for 4-nitrophenol were in the range of 38-67 mg/kg soil,
again with Eisenia fetida and Eudrilus eugeniae as the most
sensitive species tested (Ballhorn et al., 1984; Marquart et al.,
1984; Neuhauser et al., 1985, 1986).
The environmental relevance, particularly of the earthworm
contact test, seems questionable. Critical results from this test, as
sole effect data on terrestrial organisms, should not justify a
classification of tested substances as highly toxic to earthworms or
other soil organisms. The available data on microorganisms and plants
indicate only a moderate toxicity potential in the terrestrial
environment.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessmentn
In general, there is only limited information concerning the
toxicological profiles of 2- and 4-nitrophenol.
In experimental animals given 4-nitrophenol orally,
intravenously, or intraperitoneally, most of the applied dose was
excreted via the urine within 24-48 h as glucuronide and sulfate
conjugates, while only very small amounts were excreted via faeces or
as unchanged 4-nitrophenol. In rabbits, after oral dosing,
4-nitrophenol undergoes reduction to 4-aminophenol as well as
glucuronidation and sulfation. In vivo and in vitro studies gave
an indication for dermal uptake. For 2-nitrophenol, the information is
very limited. However, a comparable metabolic transformation is
assumed based on the available data. Owing to their rapid metabolism
and excretion, bioaccumulation of 2- and 4-nitrophenol in organisms is
not to be expected.
With oral LD50 values of 220-620 mg/kg body weight in rats and
380-470 mg/kg body weight in mice, 4-nitrophenol is harmful after oral
uptake and was found to be more toxic than 2-nitrophenol. A
dose-dependent increase in the formation of methaemoglobin was seen in
cats after oral exposure to 2-nitrophenol -- but not after exposure to
4-nitrophenol -- and in rats after exposure by inhalation to
4-nitrophenol.
Most of the studies concerning skin- or eye-irritating effects in
experimental animals are limited as a result of insufficient
documentation. However, from the available data, it can be concluded
that 2-nitrophenol is slightly irritating to the skin but
non-irritating to the eye, and the substance proved to have no
sensitizing effects. For 4-nitrophenol, irritating effects on skin and
eye are assumed based on the studies performed according to OECD/FDA
guidelines; in addition, signs of irritation were reported after
exposure by inhalation as well as subchronic dermal exposure. In a
guinea-pig maximization test, 4-nitrophenol was considered to be
sensitizing. Positive patch tests were recorded in humans exposed to
4-nitrophenol. Although this may have been due to cross-sensitization,
sensitization to 4-nitrophenol in humans cannot be excluded.
Only a few limited studies concerning repeated oral exposure to
2- and 4-nitrophenol in experimental animals were identified. With
2-nitrophenol, decreases in body weight gain accompanied by decreased
food consumption and differences in organ weights without clear dose
dependency were found. However, the haematological examination,
clinical chemistry, and histopathological examination of the major
organs and tissues gave no indication for a substance-related toxic
effect compared with controls. In rats dosed with 4-nitrophenol, a
focal fatty degeneration of the liver as well as congestion in several
organs were the major histopathological findings. Other reported
effects included haematological changes, nephrosis, testicular
atrophy, and follicular atresia in the ovaries. The exposure by
inhalation to 2-nitrophenol vapour caused squamous metaplasia of the
epithelium of the upper respiratory tract; with 4-nitrophenol dust
(applied as sodium salt), haematological changes, increased
methaemoglobin values, and differences in organ weights were noted.
For the effects given in these studies, it was not possible to
identify a clear dose-response or reliable NO(A)EL values.
Insufficient data are available on 2-nitrophenol to allow any
conclusions to be made about its possible mutagenicity. For
4-nitrophenol, more mutagenicity studies are available, and the
substance was shown to be mutagenic in some but not all of the
bacterial assays. In addition, positive results were obtained in
in vitro tests for chromosomal aberrations in mammalian cells;
however, apart from one well-documented study, the available assays
were inadequately reported. In the absence of any in vivo
mutagenicity studies in mammals, it is not possible to conclude
whether or not the mutagenic potential of 4-nitrophenol is expressed
in vivo.
4-Nitrophenol was not carcinogenic in male or female mice after
dermal application over 78 weeks. In a limited study with female mice,
no skin tumours were seen after dermal application of 2- or
4-nitrophenol over 12 weeks. No carcinogenicity studies using the oral
or inhalation routes were available for either of the isomers.
No reproductive effects were observed in rats exposed to
4-nitrophenol in a two-generation study. For developmental toxicity,
the available studies were inadequately performed (i.e., only one dose
was applied, or animals were dosed only on one day with a mixture). In
an oral study with rats, 2-nitrophenol induced developmental effects
in the offspring only at doses that also produced maternal toxicity.
However, the fetuses were not examined for internal malformations.
Data on humans relevant for the assessment of potential adverse
effects are limited to some patch tests performed with 4-nitrophenol.
11.1.2 Criteria for setting guidance values for 2- and 4-nitrophenol
As given in section 8, the database for 2-nitrophenol is
inadequate for calculating a tolerable daily intake (TDI) or a
tolerable concentration (TC).
For 4-nitrophenol, the formation of methaemoglobin was shown to
be the most critical end-point after exposure by inhalation and is
assumed to be relevant for oral exposure too. However, owing to the
inaccuracy of the analytical method used in the 13-week study with
oral application, a reliable NO(A)EL cannot be derived. Therefore, at
present, no TDI for 4-nitrophenol can be developed owing to inadequacy
of the database.
Longer-term toxicity studies concerning inhalation exposure were
not identified in the literature, and the NO(A)EL values derived for
4-nitrophenol from short-term studies gave considerable differences
(2-week exposure: NO(A)EL of about 30 mg/m3; 4-week exposure: NO(A)EL
of about 5 mg/m3). The NO(A)EL of 5 mg/m3 was derived for local
effects (cataracts), whereas the NO(A)EL for systemic effects
(formation of methaemoglobin) may be lower. Therefore, a reliable TC
for exposure by inhalation cannot be calculated, as the formation of
methaemoglobin is the critical end-point.
11.1.3 Sample risk characterization
As given in section 6.2, workers may be exposed to 2- and
4-nitrophenol via inhalation and skin contact during production and
processing (mainly in the manufacturing of pesticides). However, data
on nitrophenol concentrations at the workplace were not identified.
For the general population, an exposure to nitrophenols via the
environment cannot be excluded (see also section 6.2). Assuming an
ambient atmospheric concentration of about 1 µg/m3, an inhalation
uptake of 100%, a daily respiratory volume of 22 m3 for adults, a
mean body weight of 64 kg for males and females, and that 4 of 24 h
are spent outdoors (IPCS, 1994), the uptake by inhalation of
nitrophenols is calculated to be 0.06 µg/kg body weight per day. In
addition, 4-nitrophenol accumulates in fog. From the mean measured
level of 20 µg/litre, the uptake of the substance by inhalation (using
the same assumptions as above) can be calculated to be about 8 ng
during a 1-h exposure period (i.e., 0.12 ng/kg body weight), assuming
a maximum water content of fog of 0.1 g/m3 (Pruppacher & Klett,
1978). The uptake via drinking-water for 2- and 4-nitrophenol can be
calculated to be about 0.02 µg/kg body weight per day, assuming a
maximum concentration of 1 µg/litre drinking-water, a daily
drinking-water consumption of 1.4 litres, and a mean body weight of
64 kg for males and females.
From these data, it can be concluded that exposure of the general
population to the nitrophenol isomers is mainly through ambient air
and drinking-water.
11.2 Evaluation of environmental effects
Releases of 2- and 4-nitrophenol into the environment are
primarily emissions into air, water, and soil from diffuse sources,
such as vehicle traffic and hydrolytic and photolytic degradation of
the respective pesticides.
2-Nitrophenol emitted to the troposphere will stay predominantly
in the gaseous phase and should be rapidly removed by nitration. The
major portion of airborne 4-nitrophenol is expected to be particle
bound and can be washed out to surface waters and soil by wet and dry
deposition. Because of their removal from air and their insignificant
volatility, nitrophenols are not considered to contribute directly to
the depletion of the stratospheric ozone layer or to global warming.
Measured bioconcentration factors indicate a low potential for
bioaccumulation.
Nitrophenols exhibit moderate to high toxicity to aquatic
organisms, with lowest effect concentrations reported from chronic
studies on algae, Daphnia, and aquatic invertebrates. The lowest
effect concentrations found in chronic studies with freshwater
organisms ( Scenedesmus subspicatus, 96-h EC50: 0.39 mg
2-nitrophenol/litre; Entosiphon sulcatum, 72-h MIC: 0.83 mg
4-nitrophenol/litre) were 40-50 times higher than maximum levels
determined in a densely populated and highly industrialized Asian
river basin (0.0072 mg 2-nitrophenol/litre and 0.019 mg
4-nitrophenol/litre). From these data, the safety margin between the
LOEC and maximum surface water concentrations is insufficient to
exclude any risk for sensitive aquatic organisms, particularly under
surface water conditions not favouring both elimination pathways.
Taking into account the missing chronic effect data for fish, an
uncertainty/assessment factor of 100 has to be applied to derive a
predicted no-effect concentration (PNEC) according to standard
procedures for environmental risk assessment. From acute tests (see
section 10.1), however, fish obviously seem to be the least sensitive
aquatic species tested. Thus, an assessment factor of 10 might be
appropriate. Furthermore, the use pattern and the release scenario
outlined in section 4 lead to the conclusion that nitrophenols emitted
to surface waters will pose only a minor risk to aquatic organisms.
There were no data available on the occurrence of nitrophenols in
the terrestrial compartment. Therefore, an assessment of possible
effects on organisms for this compartment could be conducted only with
regard to the degradation of pesticides. For the insecticides
mentioned in section 4 (parathion, parathion-methyl, carbofuran,
phosalon, fluorodifen) and the herbicides bifenox and nitrofen,
predicted soil concentrations were calculated from the maximum
application rates (taken from Domsch, 1992) according to the EPPO
(1993) guidelines for environmental risk assessment of plant
protection products. Based on the relative molecular mass of the
pesticides, the maximum concentration of nitrophenols in the top 5 cm
of soil was calculated (worst case; one application). For pesticides
that are applied at times when the soil is plant covered to a high
degree, it is assumed that only half of the amount applied reaches the
soil. Thus, the predicted environmental concentration (PEC) for the
insecticides was reduced by 50%. Dividing the PECsoil by the lowest
LC50 value for a terrestrial species gives the toxicity exposure
ratio (TER). The lowest LC50 value for earthworms (38 mg/kg body
weight; see section 10.2) has to be corrected by a factor of 2 because
of the higher organic matter content in artificial soil compared with
natural agricultural soil. The following TERs were derived:
Insecticides Herbicides
Parathion: 244 Bifenox: 131
Parathion-methyl: 557 Nitrofen: 18
Carbofuran: 47
Phosalon: 36
Fluorodifen: 69
According to the EPPO (1993) guidelines, the trigger value for
concern is <10. Therefore, these pesticides are expected to pose only
a minor risk to earthworms, even under a worst-case scenario.
Furthermore, the herbicide nitrofen and the insecticides phosalon and
fluorodifen are no longer manufactured or marketed for crop protection
use.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Previous evaluations of mononitrophenols by international bodies
were not identified.
Information on international hazard classification and labelling
for mononitrophenols is included in the International Chemical Safety
Card reproduced in this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventative and protective
measures and first aid recommendations, are presented on the enclosed
International Chemical Safety Card (ICSC 1342) reproduced in this
document.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards is
available from the International Register of Potentially Toxic
Chemicals (IRPTC) legal file.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
INTERNATIONAL CHEMICAL SAFETY CARD
MONONITROPHENOLS ICSC: 1342
November 1998
CAS # 25154-55-6 Nitrophenols (mixed Isomers)
RTECS # Nitrophenols
UN # 1883 C6H5O3N
Molecular mass: 139.1
TYPES OF HAZARD ACUTE HAZARDS/ PREVENTION FIRST AID/ FIRE
/EXPOSURE SYMPTOMS FIGHTING
FIRE Combustible. Gives off NO open flames. Powder, water spray, foam,
irritating or toxic fumes carbon dioxide.
(or gases) in a fire.
EXPLOSION Finely dispersed particles Prevent deposition In case of fire: keep drums,
form explosive mixtures of dust; closed etc., cool by spraying
in air. system, dust explosion-proof with water.
electrical equipment and
lighting.
EXPOSURE PREVENT DISPERSION OF
DUST! STRICT HYGIENE!
Inhalation Blue lips or finger nails. Local exhaust or breathing Fresh air, rest.
Blue skin. Confusion. protection. Refer for medical attention.
Convulsions. Cough.
Dizziness. Headache.
Nausea. Sore throat.
Unconsciousness.
Skin MAY BE ABSORBED! Protective gloves. Protective Remove contaminated clothes.
clothing. Rinse and then wash skin with
water and soap. Refer for medical
attention.
Eyes Redness. Pain. Safety goggles, or eye First rinse with plenty of water
protection in combination for several minutes (remove contact
with breathing protection. lenses if easily
possible), then take to a doctor.
Ingestion Abdominal pain. Sore Do not eat, drink, or smoke Rinse mouth. Rest. Refer
throat. Vomiting. during work. for medical attention.
(see Inhalation).
SPILLAGE DISPOSAL PACKAGING & LABELLING
Sweep spilled substance into sealable Do not transport with food and feedstuffs.
containers. Carefully collect EU Classification
remainder, then remove to safe place. UN Classification
Do NOT let this chemical UN Hazard Class: 6.1
enter the environment. UN Pack Group: III
(Extra personal protection: P2 filter
respirator for harmful particles).
EMERGENCY RESPONSE STORAGE
Separated from combustible and reducing
substances, food and feedstuffs. Dry.
Well closed.
IMPORTANT DATA
PHYSICAL STATE; APPEARANCE: ROUTES OF EXPOSURE:
YELLOW CRYSTALS The substance can be absorbed into the
body by inhalation of its aerosol through
the skin and by ingestion.
PHYSICAL DANGERS:
Dust explosion possible if in powder INHALATION RISK:
or granular form, mixed with air. Evaporation at 20°C is negligible;
a harmful concentration of airborne
particles can, however, be reached quickly.
CHEMICAL DANGERS: EFFECTS OF SHORT-TERM EXPOSURE:
May explode on heating. On combustion, The substance irritates the eyes and the
forms nitrogen oxides. The skin and the respiratory tract. The
substance decomposes on heating substance may cause effects on the blood,
producing toxic fumes including resulting in formation of
nitrogen oxides. Reacts with strong oxidants. methaemoglobin. The effects may be delayed.
Medical observation is indicated.
OCCUPATIONAL EXPOSURE LIMITS: EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
TLV not established. Repeated or prolonged contact may cause skin sensitization.
PHYSICAL PROPERTIES
Boiling point: 194-279
Melting point: 44-116°C
Density: 1.5 g/cm3
Solubility in water, g/100 ml: 0.13-1.2
Vapour pressure, Pa at 20°C: 0.0032 - 7
Relative vapour density (air = 1): 4.81
Flash point: 169°C
ENVIRONMENTAL DATA
The substance is toxic to aquatic organisms. Avoid release to the environment in circumstances different to normal use.
NOTES
Depending on the degree of exposure, periodic medical examination is indicated. Specific treatment is necessary in
case of poisoning with this substance; the appropriate means with instructions must be available.
ADDITIONAL INFORMATION
LEGAL NOTICE Neither the CEC nor the IPCS nor any person acting on behalf of the CEC or the IPCS is responsible
for the use which might be made of this information
(c) IPCS, CEC 1999
REFERENCES
Aelion CM, Swindoll CM, Pfaender FK (1987) Adaptation to and
biodegradation of xenobiotic compounds by microbial communities from a
pristine aquifer. Applied environmental microbiology, 53:2212-2217.
Amacher DE, Turner GN (1982) Mutagenic evaluation of carcinogens and
non-carcinogens in the L5178Y/TK assay utilizing postmitochondrial
fractions (S9) from normal rat liver. Mutation research, 97:49-65.
Andrae U, Bieniek D, Freitag D, Goeggelmann W, Huber W, Klein W,
Kotzias D, Lahaniatis E, Mansour M, Parlar H, Politzki G, Rohleder H,
Rott B, Scheunert I, Spieser H, Viswanathan R (1981) Feasibility of
test guidelines and evidence of the base-set testing according to the
chemicals legislation. Muenchen, Gesellschaft für Strahlen- und
Umweltfoschung mbH (in German).
Angerhofer RA (1985) Final phase: Effect of dermal applications of
paranitrophenol on the reproductive functions of rats. Aberdeen
Proving Ground, MD, US Army Environmental Hygiene Agency (Study No.
75-51-0047-85).
ATSDR (1992) Toxicological profile for nitrophenols: 2- and
4-nitrophenol. Atlanta, GA, US Department of Health and Human
Services, Public Health Service, Agency for Toxic Substances and
Disease Registry (Report No. TP-91/23).
Ballhorn D, Freitag D, Geyer H, Quast I, Rott B, Scheunert I, Spieser
H, Viswanathan R (1984) Assessment of the feasibility and evidence of
the test methods of levels I and II of the chemicals act. Berlin,
Umweltbundesamt (in German).
BASF AG (1969) Results of the toxicological testing of
Rongal-Stabilisator A. Ludwigshafen (unpublished report) (in German).
BASF AG (1970) Results of the toxicological testing of
o -nitrophenol. Ludwigshafen (unpublished report) (in German).
Battersby NS, Wilson V (1989) Survey of the anaerobic biodegradation
potential of organic chemicals in digesting sludge. Applied
environmental microbiology, 55:433-439.
Baumgartner A, Liebscher H-J (1990) Allgemeine Hydrologie.
Quantitative Hydrologie. Berlin, Gebrueder Borntraeger Verlag, pp.
93-96.
Booth G (1991) Nitro compounds, aromatic. In: Ullmann's encyclopedia
of industrial chemistry. Vol. A17. Weinheim, VCH VerlagsGmbH, pp.
411-455.
Booth GM, Bradshaw WS, Carter MW (1983) Screening of priority
chemicals for potential reproductive hazard. Orem, UT, MESA Corp.
(Report No. PB83-213017).
Boutwell RK, Bosch DK (1959) The tumor-promoting action of phenol and
related compounds for mouse skin. Cancer research, 19:413-424.
Boyd SA (1982) Adsorption of substituted phenols by soil. Soil
science, 134:337-343.
Boyd SA, Shelton DR, Berry D, Tiedje JM (1983) Anaerobic
biodegradation of phenolic compounds in digested sludge. Applied
environmental microbiology, 46:50-54.
Braunbeck T, Storch V, Nagel R (1989) Sex-specific reaction of liver
ultrastructure in zebra fish ( Brachydanio rerio) after prolonged
sublethal exposure to 4-nitrophenol. Aquatic toxicology, 14:185-202.
Bringmann G (1978) Determination of the toxicity of water pollutants
on protozoa. I. Bacteriovorous flagellates. Zeitschrift fuer Wasser
und Abwasser Forschung, 11:210-215 (in German).
Bringmann G, Kuehn R (1977) Results of the damaging effects of water
pollutants on Daphnia magna. Zeitschrift fuer Wasser und Abwasser
Forschung, 10:161-165 (in German).
Bringmann G, Kuehn R, Winter A (1980) Determination of the biological
effect of water pollutants in protozoa. III. Saprozoic flagellates.
Zeitschrift fuer Wasser und Abwasser Forschung, 13:170-173
(in German).
Broecker B, Fischer R, Gerber HG, Goerlitz G, Markert M, Wellens H
(1984) Assessment of the feasibility and evidence of the test method
of level 1 and 2 of the chemical act. Berlin, Umweltbundesamt
(in German).
BUA (1992) BUA-Stoffbericht 2- und 4-Nitrophenol. Beratergremium
fuer Umweltrelevante Altstoffe. Weinheim, VCH VerlagsGmbH (Report No.
75; February 1992).
Budavari S, O'Neil MJ, Smith A, Heckelmann PE, Kinneary JE, eds.
(1996) The Merck Index. An encyclopedia of chemicals, drugs and
biologicals, 12th ed. Whitehouse Station, NJ, Merck & Co. Inc.
Buselmaier W, Roehrborn G, Propping P (1972)
Mutagenitaets-Untersuchungen mit Pestiziden im Host-mediated assay und
mit dem Dominanten Letaltest an der Maus. Biologisches Zentralblatt,
91:311-325.
Capel PD, Leuenberger C, Giger W (1991) Hydrophobic organic chemicals
in urban fog. Atmospheric environment, 25A:1335-1346.
Chiu CW, Lee LH, Wang CY, Bryan GT (1978) Mutagenicity of some
commercially available nitro compounds for Salmonella typhimurium.
Mutation research, 58:11-22.
CITI (1992) Biodegradation and bioaccumulation. Data of existing
chemicals based on the CSCL Japan. Tokyo, Japan Chemical Industry
Ecology-Toxicology & Information Center, Chemicals Inspection &
Testing Institute.
Dellarco VL, Prival MJ (1989) Mutagenicity of nitro compounds in
Salmonella typhimurium in the presence of flavin mononucleotide in a
preincubation assay. Environmental molecular mutagenesis,
13:116-127.
Domsch KH (1992) Pesticides in soil. Weinheim, VCH-Verlag (in
German).
Eastman Kodak Co. (1980) Toxicology Laboratory Report No. 125877V; US
Environmental Protection Agency Report No. 86-890000202. Rochester, NY
[cited in Cantilli R (1991) Drinking water health advisory for
p -nitrophenol. Washington, DC, US Environmental Protection Agency
(Report No. PB92-135490)].
Ensenbach U, Nagel R (1991) Toxicokinetics of xenobiotics in zebrafish
- comparison between tap and river water. Comparative biochemistry
and physiology, 100C:49-53.
EPPO (1993) Decision-making scheme for the environmental risk
assessment of plant protection products. Paris, European and
Mediterranean Plant Protection Organization.
Eriksen K (1978) Cross allergy between paranitro compounds with
special reference to DNCB and chloramphenicol. Contact dermatitis,
4:29-32.
Fahrig R (1974) Comparative mutagenicity studies with pesticides.
IARC scientific publications, 10:161-181.
Figge K, Klahn J, Koch J (1985) Chemicals in ecosystems. Inventory,
evaluation and application of distribution models. Society for Water,
Soil and Air Hygiene, 61:1-234 (in German).
Foureman P, Mason JM, Valencia R, Zimmering S (1994) Chemical
mutagenesis testing in Drosophila. IX. Results of 50 coded compounds
tested for the National Toxicology Program. Environmental molecular
mutagenesis, 23:51-63.
Freitag D, Geyer H, Kraus A, Viswanathan R, Kotzias D, Attar A, Klein
W, Korte F (1982) Ecotoxicological profile analysis. VII. Screening
chemicals for their environmental behavior by comparative evaluation.
Ecotoxicology and environmental safety, 6:60-81.
Geissler A, Schoeler HF (1993) Atmospheric deposition of pesticides
and nitrophenols in the Rhine/Sieg-area (Germany). Vom Wasser,
80:357-370.
Geissler A, Schoeler HF (1994) Gas chromatographic determination of
phenol, methylphenols, chlorophenols, nitrophenols and nitroquinones
in water at 0.1 µg l-1. Water research, 28:2047-2053.
Gerike P, Fischer WK (1979) A correlation study of biodegradability
determination with various chemicals in various tests. Ecotoxicology
and environmental safety, 3:159-173.
Gessner T, Hamada N (1970) Identification of p-nitrophenol glucoside
as a urinary metabolite. Journal of pharmaceutical sciences,
59:1528-1529.
Geyer H, Viswanathan R, Freitag D, Korte F (1981) Relationship between
water solubility of organic chemicals and their bioaccumulation by the
alga Chlorella. Chemosphere, 14:1307-1313.
Gile JD, Gillett JW (1981) Transport and fate of organophosphate
insecticides in a laboratory model ecosystem. Journal of agricultural
and food chemistry, 29:616-621.
Hansch C, Leo A (1979) Substituent constants for correlation analysis
in chemistry and biology. New York, NY, John Wiley & Sons.
Harrison I, Leader RU, Higgo JJW, Tjell JC (1994) Determination of
organic pollutants in small samples of groundwaters by liquid-liquid
extraction and capillary gas chromatography. Journal of
chromatography, 688:181-188.
Haworth S, Lawlor T, Mortelmans K, Speck W, Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals.
Environmental mutagenesis, 5 (Suppl. 1):3-142.
Hazleton Lab. (1983) Subacute dust inhalation toxicity study in rats.
p -Nitrophenol. Final report. Sponsored by Monsanto Co., St. Louis,
MO (HLA Study No. 82-242).
Hazleton Lab. (1984) Subacute inhalation toxicity study in rats.
o -Nitrophenol. Final report. Sponsored by Monsanto Co., St. Louis,
MO (HLA Study No. 82-254).
Hazleton Lab. (1989) Subchronic toxicity study in rats with
paranitrophenol. Sponsored by Monsanto Co., St. Louis, MO (HLA Study
No. 241-221).
Herterich R, Herrmann R (1990) Comparing the distribution of nitrated
phenols in the atmosphere of two German hill sites. Environmental
technology letters, 11:961-972.
Hoechst AG (1977a) Acute oral toxicity of p -nitrophenol in female
SPF-Wistar rats. Frankfurt/Main, Hoechst AG (unpublished report) (in
German).
Hoechst AG (1977b) Acute dermal toxicity of p -nitrophenol in female
SPF-Wistar rats. Frankfurt/Main, Hoechst AG (unpublished report)
(in German).
Hoechst AG (1977c) Skin and eye irritating effects of p -nitrophenol
in rabbits. Frankfurt/Main, Hoechst AG (unpublished report) (in
German).
Hoechst AG (1980) A mutagenicity screening of 408 / 80 A in bacteria
(Ames test). Frankfurt/Main, Hoechst AG (unpublished report).
Hoover DG, Borgonovi GE, Jones SH, Alexander M (1986) Anomalies in
mineralization of low concentrations of organic compounds in lake
water and sewage. Applied environmental microbiology, 51:226-232.
Howe GE, Marking LL, Bills TD, Rach JJ, Mayer FLJ (1994) Effects of
water temperature and pH on toxicity of terbufos, trichlorfon,
4-nitrophenol and 2,4-dinitrophenol to the amphipod Gammarus
pseudolimnaeus and rainbow trout ( Oncorhynchus mykiss).
Environmental toxicology and chemistry, 13:51-66.
HSDB (1998) Hazardous substances data bank. Bethesda, MD, National
Library of Medicine.
Huang Q, Wang L, Han S (1995) The genotoxicity of substituted
nitrobenzenes and the quantitative structure-activity relationship
studies. Chemosphere, 30:915-923.
Huang Q-G, Kong L-R, Liu Y-B, Wang L-S (1996) Relationships between
molecular structure and chromosomal aberrations in in vitro human
lymphocytes induced by substituted nitrobenzenes. Bulletin of
environmental contamination and toxicology, 57:349-353.
Huq AS, Ho NFH, Husari N, Flynn GL, Jetzer WE, Condie LJ Jr (1986)
Permeation of water contaminative phenols through hairless mouse skin.
Archives of environmental contamination and toxicology, 15:557-566.
Hustert K, Mansour M, Parlar H, Korte F (1981) The EPA test - A method
for the determination of the photochemical degradation of organic
compounds in aquatic systems. Chemosphere, 10(9):995-998 (in
German).
International Research and Developmental Corporation (1983)
Range-finding teratology study in rats. Sponsored by Monsanto Co.,
St. Louis, MO (IR-83-100).
IPCS (1994) Assessing human health risks of chemicals: derivation of
guidance values for health-based exposure limits. Geneva, World
Health Organization, International Programme on Chemical Safety
(Environmental Health Criteria 170).
IPCS (1998) International Chemical Safety Card - Mononitrophenols.
Geneva, World Health Organization, International Programme on
Chemical Safety (ICSC 1342).
Japan Environment Agency (1979) Chemicals in the environment.
Monitoring of the general environment in Japan 1978. Tokyo.
Japan Environment Agency (1980) Chemicals in the environment.
Monitoring of the general environment in Japan 1979. Tokyo.
Japan Environment Agency (1995) Chemicals in the environment.
Monitoring of the general environment in Japan 1994. Tokyo.
Jetzer WE, Huq AS, Ho NFH, Flynn GL, Duraiswamy N, Condie L Jr (1986)
Permeation of mouse skin and silicone rubber membranes by phenols:
relationship to in vitro partitioning. Journal of pharmaceutical
sciences, 75:1098-1103.
Kavlock RJ (1990) Structure-activity relationships in the
developmental toxicity of substituted phenols: In vivo effects.
Teratology, 41:43-59.
Kawai A, Goto S, Matsumoto Y, Matsushita H (1987) Mutagenicity of
aliphatic and aromatic nitro compounds. Japanese journal of
industrial health, 29:34-54.
Kayser G, Koch M, Ruck W (1994) Simultaneous quantitative measurement
of biodegradation and toxicity of environmental chemicals.
Vom Wasser, 82:219-232.
Koerdel W, Schoene K, Bruckert J, Pfeiffer U, Schreiber G, Rittmann D,
Hochrainer D, Otto F, Spielberg T, Fingerhut R, Kuhnen-Clausen D,
Koenig J (1981) Assessment of the feasibility of test guidelines as
well as the evidence of the base set of the law on chemicals.
Hanover, Fraunhofer Institute for Toxicology and Aerosol Research (in
German).
Koerdel W, Kuhnen-Clausen D, Fabig W, Otto F (1984) Evaluation of
test guidelines for environmental chemicals. Schmallenberg,
Fraunhofer Institute for Environmental Chemistry and Ecotoxicology (in
German).
Koop DR (1986) Hydroxylation of p-nitrophenol by rabbit
ethanol-inducible cytochrome P-450 isozyme 3a. Molecular
pharmacology, 29:399-404.
Koop DR, Laethem CL (1992) Inhibition of rabbit microsomal cytochrome
P-450 2E1-dependent p-nitrophenol hydroxylation by substituted
benzene derivatives. Drug metabolism and disposition, 20:775-777.
Kramer CR, Truemper l, Berger L (1986) Quantitative structure-activity
relations for the autotrophic growth inhibition of synchrone
Chlorella vulgaris suspensions by monosubstituted nitrobenzenes.
Biochemie und Physiologie der Pflanzen, 181:411-420 (in German).
Kubinski H, Gutzke GE, Kubinski ZO (1981) DNA-cell-binding (DCB) assay
for suspected carcinogens and mutagens. Mutation research,
89:95-136.
Kuehn R, Pattard M, Pernak K-D, Winter A (1988) Damaging effects of
environmental chemicals in the Daphnia reproductive toxicity test as
a basis for the evaluation of environmental hazards in aquatic
systems. Berlin, Institute for Water, Soil and Air Hygiene (in
German).
Landrum PF, Crosby DG (1981) Comparison of the disposition of several
nitrogen-containing compounds in the sea-urchin and other marine
invertebrates. Xenobiotica, 11:351-361.
Lang P-Z, Ma X-F, Lu G-H, Wang YI, Bian Y (1996) QSAR for the acute
toxicity of nitroaromatics to the carp ( Cyprinus carpio).
Chemosphere, 32:1547-1552.
Leone JA, Seinfeld JH (1985) Comparative analysis of chemical reaction
mechanism for photochemical smog. Atmospheric environment,
19:437-464.
Leone JA, Flagan RC, Grosjean D, Seinfeld JH (1985) An outdoor smog
chamber and modeling study of toluene-NOx photooxidation.
International journal of chemical kinetics, 17:177-216.
León-González ME, Perez-Arribas LV, Santos-Delgado MJ, Polo-Diez LM
(1992) Simultaneous flow-injection determination of o- and
p-nitrophenol using a photodiode-array detector. Analytica Chimica
Acta, 258:269-273.
Leuenberger C, Czuczwa J, Tremp J, Giger W (1988) Nitrated phenols in
rain: atmospheric occurrence of phytotoxic pollutants. Chemosphere,
17(3):511-516.
Levsen K, Behnert S, Priess B, Svoboda M, Winkeler H-D, Zietlow J
(1990) Organic compounds in precipitation. Chemosphere,
21:1037-1061.
Levsen K, Behnert S, Mussmann P, Raabe M, Priess B (1993) Organic
compounds in cloud and rain water. International journal of
environmental analytical chemistry, 52:87-97.
Lokke H (1984) Sorption of selected organic pollutants in Danish
soils. Ecotoxicology and environmental safety, 8:395-409.
Luettke J, Levsen K (1994) Partitioning of phenol and nitrophenols in
gas and liquid phase of clouds. In: Borrell PM, Borrell P, Cvitas T,
Seiler W, eds. Transport and transformation of pollutants in the
troposphere. Proceedings of EUROTRAC Symposium '94
Garmisch-Patenkirchen, 11-15 April 1994. Garmisch-Patenkirchen, SPB
Academic Publishing bv / EUROTRAC International Scientific Secretariat
(ISS); Fraunhofer Institute for Atmospheric Environmental Research,
pp.1075-1078.
Luettke J, Scheer V, Levsen K, Wuensch G, Cape JN, Hargreaves KJ,
Storeton-West RL, Acker K, Wieprecht W, Jones B (1997) Occurrence and
formation of nitrated phenols in and out of cloud. Atmospheric
environment, 31:2637-2648.
Machida M, Morita Y, Hayashi M, Awazu S (1982) Pharmacokinetic
evidence for the occurrence of extrahepatic conjugative metabolism of
p-nitrophenol in rats. Biochemical pharmacology, 31:787-791.
Mansour M (1996) Abiotic degradation of pesticides and other organic
chemicals in aquatic systems. Pesticide outlook, 7:9-10.
Marquart HW, Sewekow B, Hamburger B, Harzdorf C, Hellbusch HD (1984)
Assessment of the feasibility and evidence of the test methods of
levels I and II of the chemicals act. Part II. Leverkusen, Bayer-AG,
pp. 1-128 (in German).
Massey IJ, Aitken MD, Ball LM, Heck PE (1994) Mutagenicity screening
of reaction products from the enzyme-catalyzed oxidation of phenolic
pollutants. Environmental toxicology and chemistry, 13:1743-1752.
McCann J, Choi E, Yamasaki E, Ames BN (1975) Detection of carcinogens
as mutagens in the Salmonella/microsome test: assay of 300
chemicals. Proceedings of the National Academy of Sciences of the
United States of America, 72:5135-5139.
McCoy GD, Koop DR (1988) Biochemical and immunochemical evidence for
the induction of an ethanol-inducible cytochrome P-450 isoenzyme in
male Syrian golden hamsters. Biochemical pharmacology, 37:1563-1568.
Means JL, Anderson SJ (1981) Comparison of five different methods for
measuring biodegradability in aqueous environment. Water, air, and
soil pollution, 16:301-315.
Meerman JH, Nijland C, Mulder GJ (1987) Sex differences in sulfation
and glucuronidation of phenol, 4-nitrophenol and
N-hydroxy-2-acetylaminofluorene in the rat in vivo. Biochemical
pharmacology, 36:2605-2608.
Mussmann P, Levsen K, Radeck W (1994) Gas-chromatographic
determination of phenols in aqueous samples after solid phase
extraction. Fresenius journal of analytical chemistry, 348:654-659.
Nakamura S, Oda Y, Shimada T, Oki I, Sugimoto K (1987) SOS-inducing
activity of chemical carcinogens and mutagens in Salmonella
typhimurium TA1535/pSK1002: examination with 151 chemicals. Mutation
research, 192:239-246.
Naniwa S (1979) Industrial contact dermatitis due to nitro and amino
derivatives. 1st report: mass-examination of a factory. Journal of
dermatology, 6:59-63.
Nasseredine-Sebaei SM, Crider AM, Carroll RT, Hinko CN (1993)
Determination of m-nitrophenol and nipecotic acid in mouse tissues
by high-performance liquid chromatography after administration of the
anticonvulsant m-nitrophenyl-3-piperidinecarboxylate hydrochloride.
Journal of pharmaceutical sciences, 82:39-43.
Neuhauser EF, Loehr RC, Malecki MR, Milligan DL, Durkin PR (1985) The
toxicity of selected organic chemicals to the earthworm Eisenia
fetida. Journal of environmental quality, 14:383-388.
Neuhauser EF, Durkin PR, Malecki MR, Anatra M (1986) Comparative
toxicity of ten organic chemicals to four earthworm species.
Comparative biochemistry and physiology, C 83:197-200.
Nick K, Schoeler HF (1992) Gas-chromatographic determination of
nitrophenols after derivatization with diazomethane. Fresenius
journal of analytical chemistry, 343:304-307.
Nojima K, Kawaguchi A, Ohya T, Kanno S, Hirobe M (1983) Studies on
photochemical reaction of air pollutants. X. Identification of
nitrophenols in suspended particulates. Chemical and pharmaceutical
bulletin, 31:1047-1051.
NTP (1993) Toxicology and carcinogenesis studies of p -nitrophenol
(CAS No. 100-02-7) in Swiss Webster mice (dermal studies). Research
Triangle Park, NC, US Department of Health and Human Services,
National Toxicology Program (NTP Report No. TR-417).
Nyholm N, Lindgaard-Joergensen P, Hansen N (1984) Biodegradation of
4-nitrophenol in standardized aquatic degradation tests.
Ecotoxicology and environmental safety, 8:451-470.
Oberly TJ, Bewsey BJ, Probst GS (1984) An evaluation of the L5178Y
TK+/- mouse lymphoma forward mutation assay using 42 chemicals.
Mutation research, 125:291-306.
Ohkura K, Iwamoto K, Terada H (1990) Transcellular permeation of
nitrophenols through newborn rat skin epidermal cells in monolayer
culture. Chemical and pharmaceutical bulletin, 38:2788-2791.
Ou L-T (1985) Methyl parathion degradation and metabolism in soil:
Influence of high soil-water contents. Soil biology and
biochemistry, 17:241-243.
Pagga U, Haltrich WG, Guenthner W (1982) Investigations of the effect
of 4-nitrophenol on activated sludge. Vom Wasser, 59:51-65 (in
German).
Paterson B, Cowie CE, Jackson PE (1996) Determination of phenols in
environmental waters using liquid chromatography with electrochemical
detection. Journal of chromatography, A 731:95-102.
Pitter P (1976) Determination of biological degradability of organic
substances. Water research, 10:231-235.
Pocurull E, Marce RM, Borrull F (1996) Determination of phenolic
compounds in natural waters by liquid chromatography with ultraviolet
and electrochemical detection after on-line trace enrichment. Journal
of chromatography, 738:1-9.
Poirier MC, De Cicco BT, Lieberman MW (1975) Nonspecific inhibition of
DNA repair synthesis by tumor promoters in human diploid fibroblasts
damaged with N-acetoxy-2-acetylaminofluorene. Cancer research,
35:1392-1397.
Probst GS, McMahon RE, Hill LE, Thompson CZ, Epp JK, Neal SB (1981)
Chemically-induced unscheduled DNA synthesis in primary rat hepatocyte
cultures: a comparison with bacterial mutagenicity using 218
compounds. Environmental mutagenesis, 3:11-32.
Pruett SB, Chambers JE (1988) Effects of paraoxon, p-nitrophenol,
phenyl saligenin cyclic phosphate, and phenol on the rat interleukin 2
system. Toxicology letters, 40:11-20.
Pruppacher HR, Klett JD (1978) Microphysics of clouds and
precipitation. Dordrecht/Boston/London, D. Reidel Publishing Co.
Puig D, Barcelo D (1996) Determination of phenolic compounds in water
and waste water. Trends in analytical chemistry, 15:362-375.
Rashid KA, Mumma RO (1986) Screening pesticides for their ability to
damage bacterial DNA. Journal of environmental science and health,
21:319-334.
Reinke LA, Moyer MJ (1985) p-Nitrophenol hydroxylation. A microsomal
oxidation which is highly inducible by ethanol. Drug metabolism and
disposition, 13:548-552.
Richartz H, Reischl A, Trautner F, Hutzinger O (1990) Nitrated phenols
in fog. Atmospheric environment, 24:3067-3072.
Rippen G, Flothmann D, Witt W (1984) Improvement of the OECD test
guideline A 80/9 and comparative evaluation of other relevant methods
for the measurement of volatility. Frankfurt a. M., Batelle-Institut
e.V. (Report No. 106 02 024/06) (in German).
Roberts BL, Dorough HW (1984) Relative toxicities of chemicals to the
earthworm Eisenia fetida. Environmental toxicology and chemistry,
3:67-78.
Robinson D, Smith JN, Williams RT (1951) Studies in detoxication. 39.
Nitro compounds. (a) The metabolism of o-, m-, and
p-nitrophenols in the rabbit. (b) The glucuronides of the
mononitrophenols and observations on the anomalous optical rotations
of triacetyl -- o-nitrophenylglucuronide and its methyl ester.
Biochemical journal, 50:221-227.
Rott B, Viswanathan R, Freitag D, Korte F (1982) Comparative
investigation on the feasibility of different tests for the evaluation
of the degradation of environmental chemicals. Chemosphere,
11:531-538 (in German).
Ruana J, Urbe I, Borrull F (1993) Determination of phenols at the ng/l
level in drinking and river waters by liquid chromatography with UV
and electrochemical detection. Journal of chromatography, A
655:217-226.
Rubin HE, Subba-Rao RV, Alexander M (1982) Rates of mineralization of
trace concentrations of aromatic compounds in lake water and sewage
samples. Applied environmental microbiology, 43:1133-1138.
Rush GF, Newton JF, Hook JB (1983) Sex differences in the excretion of
glucuronide conjugates: The role of intrarenal glucuronidation.
Journal of pharmacology and experimental therapeutics, 227:658-662.
Sax NI, Lewis RJ (1987) Hawley's condensed chemical dictionary, 11th
ed. New York, NY, Van Nostrand Reinhold Co.
Scheer V, Luettke J, George C, Levsen K, Frenzel A, Behnke W, Zetzsch
C (1996) Atmospheric nitration of phenol in clouds by N2O5 and
ClNO2. In: Borrell PM, Borrell P, Kelly K, Cvitas T, Seiler W, eds.
Transport and transformation of pollutants in the troposphere.
Proceedings of EUROTRAC Symposium -96. Garmisch-Patenkirchen, 25-29
March 1996. Southhampton, Computational Mechanics Publications.
Scheubel JB (1984) Assessment of the feasibility and evidence of the
test method of level 1 and 2 of the chemical act. Marl, Chemische
Werke Huels AG (Report No. 106 04 011/5 CWH) (in German).
Scheunert I (1984) Examination and optimization of the
"GSF-Cold-Finger-Method" and comparative calculations of the
volatility. Gesellschaft fuer Strahlen- und Umweltforschung (Report
No. 10602024/08) (in German).
Schoene K, Steinhanses J (1984) Comparative measurements of the
volatility in open systems. Schmallenberg, Fraunhofer Institute for
Toxicology and Aerosol Research (Report No. 10602024/7 Part II) (in
German).
Scholz N (1986) Development of test guidelines on marine species for
ecotoxicological studies according to the chemicals act
- Bryozota/Camptozoa. Berlin, Umweltbundesamt (Report No.
10603042/02) (in German).
Schwarzenbach RP, Stierli R, Folsom BR, Zeyer J (1988) Compound
properties relevant for assessing the environmental partitioning of
nitrophenols. Environmental science and technology, 22:83-92.
Sewekow B (1983) Feasibility of test guidelines and evidence of the
base-set testing according to the chemicals legislation. Muenchen,
Gesellschaft fuer Strahlen- und Umweltforschung (in German).
Shimizu M, Yano E (1986) Mutagenicity of mono-nitrobenzene derivatives
in the Ames test and rec assay. Mutation research, 170:11-22.
Smith LW, Hall GT, Kennedy GL (1988) Acute and repeated dose
inhalation toxicity of para-nitrophenol sodium salt in rats. Drug
chemistry and toxicology, 11:319-327.
Snodgrass HL Jr (1983) Phase I, dermal penetration and distribution
of 14C-labeled paranitrophenol (PNP). Aberdeen Proving Ground, MD,
US Army Environmental Hygiene Agency (Study No. 75-51-0047-84).
Spain JC, van Veld PA, Monti CA, Pritchard PH, Cripe CR (1984)
Comparison of p-nitrophenol biodegradation in field and laboratory
test systems. Applied environmental microbiology, 48:944-950.
Storer RD, McKelvey TW, Kraynak AR, Elia MC, Barnum JE, Harmon LS,
Nichols WW, DeLuca JG (1996) Revalidation of the in vitro alkaline
elution/rat hepatocyte assay for DNA damage: improved criteria for
assessment of cytotoxicity and genotoxicity and results for 81
compounds. Mutation research, 368:59-101.
Subba-Rao RV, Rubin HE, Alexander M (1982) Kinetics and extent of
mineralization of organic chemicals at trace levels in freshwater and
sewage. Applied environmental microbiology, 43:1139-1150.
Suzuki J, Koyama T, Suzuki S (1983) Mutagenicities of
mono-nitrobenzene derivatives in the presence of norharman. Mutation
research, 120:105-110.
Tabak HH, Quave SA, Mashni CI, Barth EF (1981) Biodegradability
studies with organic priority pollutant compounds. Journal of the
Water Pollution Control Federation, 52:1503-1518.
Tan GH, Chong CL (1993) Trace monitoring of water-borne phenolics in
the Klang River basin. Environmental monitoring and assessment,
24:267-277.
Thompson MJ, Ballinger LN, Cross SE, Roberts MS (1996)
High-performance liquid chromatographic determination of phenol,
4-nitrophenol, beta-naphthol and a number of their glucuronide and
sulfate conjugates in organ perfusate. Journal of chromatography, B
677:117-122.
Tremaine LM, Diamond GL, Quebbemann AJ (1984) In vivo quantification
of renal glucuronide and sulfate conjugation of 1-naphthol and
p-nitrophenol in the rat. Biochemical pharmacology, 33:419-427.
Tremp J, Mattrel P, Fingler S, Giger W (1993) Phenols and nitrophenols
as tropospheric pollutants: Emissions from automobile exhausts and
phase transfer in the atmosphere. Water, air, and soil pollution,
68:113-123.
TRI (1998) Toxics release inventory. Washington, DC, US
Environmental Protection Agency, Office of Toxic Substances
(17 December 1998).
Tseng S, Lin M (1994) Treatment of organic wastewater by anaerobic
biological fluidized bed reactor. Water science and technology,
29:157-166.
Urano K, Kato Z (1986) Evaluation of biodegradation ranks of priority
organic compounds. Journal of hazardous materials, 13:147-159.
Van Veld PA, Spain JC (1983) Degradation of selected xenobiotic
compounds in three types of aquatic test systems. Chemosphere,
12:1291-1305.
Vasilenko NM, Volodchenko VA, Baturina TS, Kolodub FA (1976)
Toxicological peculiarities of mononitrophenols with regard for their
isomeric form. Farmakologiya i Toksikologiya, 39:718-721.
Vernot EH, MacEwen JD, Haun CC, Kinkead ER (1977) Acute toxicity and
skin corrosion data for some organic and inorganic compounds and
aqueous solutions. Toxicology and applied pharmacology, 42:417-423.
Verschueren K, ed. (1983) Handbook of environmental data on organic
chemicals, 2nd ed. New York, NY, Van Nostrand Reinhold Co.
Vozñáková Z, Podehradská J, Kohlicková M (1996) Determination of
nitrophenols in soil. Chemosphere, 33:285-291.
Wagner R, Braeutigam H-J (1981) Development and testing of a method
for studying the degradation of organic compounds under anaerobic
conditions (report no. 03 7221). In: Biehl HM, Fuehr F, Seibert K,
eds. Methods for the ecotoxicological evaluation of chemicals, Part
1, Aquatic systems. Juelich, Forschungszentrum, pp. 20-41 (in
German).
Weast RC (1979) CRC handbook of chemistry and physics, 69th ed. Boca
Raton, FL, CRC Press, Inc.
Wiggins BA, Jones SH, Alexander M (1987) Explanations for the
acclimation period preceding the mineralization of organic chemicals
in aquatic environments. Applied environmental microbiology,
43:791-796.
Yamada K, Murakami H, Yasumura K, Shirahata S, Shinohara K, Omura H
(1987) Production of DNA-breaking substance after treatment of
monophenols with sodium nitrite and then with dimethyl sulfoxide.
Agricultural and biological chemistry, 51:247-248.
Yoshida K, Shigeoka T, Yamauchi F (1983) Non-steady-state equilibrium
model for the preliminary prediction of the fate of chemicals in the
environment. Ecotoxicology and environmental safety, 7:179-190.
Yoshioka Y, Ose Y, Sato T (1985) Testing for the toxicity of chemicals
with Tetrahymena pyriformis. The science of the total environment,
43:149-157.
Zetzsch C, Rinke M, Scharpring H, Schueler P, Urbanik E, Wahner A,
Wiedelmann A, Witte F (1984) Upper limits of the persistence of
chemicals in the atmosphere from their reactivity against OH
radicals. University of Bochum, Bochum, pp. 1-20 (BMFT Report No. PTU
037253).
Zimmering S, Mason JM, Valencia R, Woodruff RC (1985) Chemical
mutagenesis testing in Drosophila. II. Results of 20 coded compounds
tested for the National Toxicology Program. Environmental
mutagenesis, 7:87-100.
APPENDIX 1 - 3-NITROPHENOL
Identity and physical/chemical properties
3-Nitrophenol (CAS No. 554-84-7; 3-hydroxy-1-nitrobenzene,
m-nitrophenol) has the empirical formula C6H5NO3. Its structural
formula is shown below:
 Physicochemical properties of 3-nitrophenol are given in
Table A-1.
Table A-1: Physicochemical properties of 3-nitrophenol.
Parameter Value
Molecular mass (g/mol) 139.11
Melting point (°C) 96-97 (1)(2)
Boiling point (°C) 194 (1)
Vapour pressure (kPa; 20 °C) 0.10 (3)
Water solubility (g/litre; 25 °C) 13.5 (1)
n-Octanol/water partition coefficient (log Kow) 2.00 (4)
Dissociation constant (pKa) (18 °C) 8.34 (2)
Conversion factors 1 mg/m3 = 0.173 ppmv
1 ppmv = 5.78 mg/m3
References: (1) Verschueren (1983); (2) Budavari et al. (1996);
(3) HSDB (1998); (4) Hansch & Leo (1979)
Environmental transport, distribution, and transformation
Data on the abiotic degradation of 3-nitrophenol were not
available.
Three studies on biotic degradation, summarized in Table A-2,
indicate the isomer to be inherently biodegradable in water under
aerobic conditions.
In tests on biotic degradation under anaerobic conditions using
sewage sludge and sludge from the primary anaerobic stage of a
municipal sewage treatment plant, respectively, initial 3-nitrophenol
concentrations in the range of 96.5-579 mg/litre were not degraded at
all within 7-60 days (Wagner & Braeutigam, 1981; Battersby & Wilson,
1989). Boyd et al. (1983), however, found complete anaerobic removal
of 50 mg/litre within 1 week of incubation. In this test,
mineralization was demonstrated only if the incubation period was
extended to 10 weeks. Anaerobic degradation, even of high initial
nitrophenol concentrations, was found by Tseng & Lin (1994), who
observed 90% removal of 3-nitrophenol (350-650 mg/litre) in a
biological fluidized bed reactor with three different kinds of
wastewater. From the available results, a slow mineralization of
3-nitrophenol under anaerobic conditions by adapted microorganisms can
be expected.
A soil sorption coefficient ( Koc) of 52.83, determined by Boyd
(1982), and the n-octanol/water partition coefficient (log Kow)
of 2.0, reported by Hansch & Leo (1979), indicate a low to moderate
potential for soil sorption as well as for bioaccumulation.
Environmental levels
3-Nitrophenol was not detected in 27 samples of air (detection
limit 8 ng/m3) in Japan in 1994 (Japan Environment Agency, 1995). It
was not detected in 177 samples of Japanese surface waters (detection
limits 0.04-10 µg/litre) or in 177 sediment samples (detection limits
0.002-0.8 µg/kg) in 1978, 1979, and 1994 (Japan Environment Agency,
1979, 1980, 1995). 3-Nitrophenol was not detected in 129 fish samples
(detection limits 0.005-0.2 µg/kg) in Japan in 1979 and 1994 (Japan
Environment Agency, 1980, 1995).
Comparative kinetics and metabolism in laboratory animals and humans
Studies providing quantitative information on the absorption,
metabolism, or elimination of 3-nitrophenol in humans were not
identified. In addition, there is only very limited information
available for experimental animals. In rabbits given a single dose of
150-200 mg/kg body weight via gavage, most of the applied dose
(>>80%) was excreted via the urine within 24 h. About 68-86% was
conjugated with glucuronic acid and sulfonic acid, whereas about 7-13%
was reduced to aminophenols (Robinson et al., 1951). Skin permeation
was shown in several in vitro experiments (Huq et al., 1986; Jetzer
et al., 1986; Ohkura et al., 1990). Although the information is
limited, bioaccumulation of 3-nitrophenol in organisms is not to be
expected owing to the isomer's rapid metabolism and excretion.
Effects on laboratory mammals and in vitro test systems
The oral LD50 of 3-nitrophenol is quoted to be >930 mg/kg
body weight for rats (Vasilenko et al., 1976; Vernot et al., 1977) and
>1070 mg/kg body weight for mice (Vasilenko et al., 1976; Vernot et
al., 1977).
The available in vitro and in vivo genotoxicity studies on
3-nitrophenol are summarized in Table A-3. 3-Nitrophenol was shown to
be mutagenic in a rec-assay and gave inconsistent results in
Salmonella/microsome assays. One study showed it to be non-mutagenic
in the Salmonella typhimurium TA98 and TA100 strains, whereas
another study showed mutagenicity in both of these strains in both the
presence and absence of metabolic activation. In view of the
conflicting results from Salmonella/microsome assays and the absence
of any data on clastogenicity, no conclusions can be made regarding
the mutagenicity of 3-nitrophenol.
For 3-nitrophenol, there are no studies available concerning
irritating or sensitizing effects, repeated exposure, reproductive and
developmental toxicity, or effects on humans.
Effects on aquatic species
In tests performed on the toxicity of 3-nitrophenol to various
aquatic organisms (see Table A-4), 3-nitrophenol exhibited a moderate
to high toxicity.
Table A-2: Biotic degradation of 3-nitrophenol under aerobic conditions.
Test Concentration Additional Test duration Removal Reference
(mg/litre) carbon source (days) (%)
Tests on ready biodegradability
MITI I 100 no 14 0 Gerike & Fischer (1979);
Urano & Kato (1986)
Tests on inherent biodegradability
Batch test, aerated 200 CODa no 5 95 Pitter (1976)
Respirometric test 300 yes 10 44 Kayser et al. (1994)
a COD = chemical oxygen demand.
Table A-3: Genotoxicity of 3-nitrophenol in vitro and in vivo.
Resultsa
Species End-point Concentration Without With Remarks References
(test system) range metabolic metabolic
activation activation
In vitro studies
Bacillus subtilis Recombination 0.01-5 mg/plate + 0 positive at Shimizu & Yano
H17, M45 assay >0.5 (1986)
mg/plate
Salmonella Reverse 0.01-5 mg/plate - - Shimizu & Yano
typhimurium mutations (1986)
TA1535, TA1537,
TA1538
Salmonella Reverse 0.1-5 mg/plate + + Study in Kawai et al.
typhimurium mutations Japanese (data (1987)
TA98, TA100 taken from
tables)
Salmonella Reverse 0.01-5 mg/plate - - Suzuki et al. Suzuki et al.
typhimurium TA98, mutations (1983) also (1983);
TA100 tested both Shimizu & Yano
strains in the (1986)
presence of
norharman,
which also gave
negative results
Table A-3 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks References
(test system) range metabolic metabolic
activation activation
In vivo studies
Drosophila SLRL assay via feed Foureman et al.
melanogaster (5000 ppm) (1994)
or injection
(1200 ppm)
a -, negative; +, positive; 0, not tested.
Table A-4: Aquatic toxicity of 3-nitrophenol.
Species
(test method/end-point) Effective concentration Reference
(mg/litre)
Bacteria
Pseudomonas putida 16-h MICa: 7.0 Bringmann & Kuehn (1977)
(cell multiplication inhibition test)
Protozoa
Entosiphon sulcatum 72-h MIC: 0.97 Bringmann (1978);
(cell multiplication inhibition test) Bringmann et al. (1980)
Algae
Scenedesmus subspicatus
Chlorella vulgaris 6-h EC50: 6.21 Kramer et al. (1986)
(cell multiplication inhibition test)
Invertebrates
Moina macrocopa (acute) 3-h LC50: 1.7 Yoshioka et al. (1985)
(immobilization)
Fish
Cyprinus carpio (static) 96-h LC50: 17.5 Lang et al. (1996)
a MIC = minimum inhibitory concentration.
APPENDIX 2 - SOURCE DOCUMENTS
BUA (1992): BUA-Stoffbericht 2- und 4-Nitrophenol.
Beratergremium fuer Umweltrelevante Altstoffe. Weinheim, VCH
VerlagsGmbH (Report No. 75; February 1992)
For the BUA review process, the company that is in charge of
writing the report (usually the largest producer in Germany) prepares
a draft report using literature from an extensive literature search as
well as internal company studies. This draft is subject to a peer
review during several readings of a working group consisting of
representatives from government agencies, the scientific community,
and industry.
The English translation of BUA Report No. 75 ( BUA Report 2- and
4-Nitrophenol. GDCh-Advisory Committee on Existing Chemicals of
Environmental Relevance. Stuttgart, Hirzel Verlag [February 1992]) was
released in 1993.
ATSDR (1992): Toxicological profile for nitrophenols:
2- and 4-nitrophenol. Atlanta, GA, US Department of Health and Human
Services, Public Health Service, Agency for Toxic Substances and
Disease Registry (Report No. TP-91/23)
Copies of the ATSDR Toxicological profile for nitrophenols:
2- and 4-nitrophenol (ATSDR, 1992) may be obtained from the:
Agency for Toxic Substances and Disease Registry
Division of Toxicology
1600 Clifton Road, E-29
Atlanta, Georgia 30333
USA
Initial drafts of the Toxicology profile for nitrophenols:
2- and 4-nitrophenol were reviewed by scientists from the Agency for
Toxic Substances and Disease Registry, the US Centers for Disease
Control, the US National Toxicology Program, and other federal
agencies. The document was also reviewed by an expert panel of
nongovernmental reviewers, consisting of the following members:
Dr Martin Alexander, Cornell University
Dr Gary Booth, Brigham Young University
Dr Samuel Cohen, University of Nebraska Medical Center
Dr Loren Koller, Oregon State University
Dr Frederick Oehme, Kansas State University
APPENDIX 3 - CICAD PEER REVIEW
The draft CICAD on mononitrophenols was sent for review to
institutions and organizations identified by IPCS after contact with
IPCS national Contact Points and Participating Institutions, as well
as to identified experts. Comments were received from:
Federal Institute for Health Protection of Consumers & Veterinary
Medicine, Berlin, Germany
Gesellschaft Deutscher Chemiker, Frankfurt, Germany
Institute of Occupational Medicine, Chinese Academy of Preventive
Medicine, Ministry of Health, Beijing, People's Republic of China
Institute of Terrestrial Ecology, Huntingdon, United Kingdom
Joint Food Safety and Standards Group, Department of Health,
London, United Kingdom
National Institute of Health Sciences, Tokyo, Japan
National Institute of Public Health, Prague, Czech Republic
United States Department of Health and Human Services (National
Institute of Environmental Health Sciences, Research Triangle
Park), USA
United States Environmental Protection Agency (National Center
for Environmental Assessment, Washington, DC; Region VIII), USA
World Health Organization/International Programme on Chemical
Safety, Montreal, Canada
APPENDIX 4 - CICAD FINAL REVIEW BOARD
Washington, DC, USA, 8-11 December 1998
Members
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
( Vice-Chairperson)
Mr R. Cary, Toxicology Unit, Health Directorate, Health and Safety
Executive, Bootle, Merseyside, United Kingdom ( Rapporteur)
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr O. Faroon, Agency for Toxic Substances and Disease Registry,
Centers for Disease Control and Prevention, Atlanta, GA, USA
Dr G. Foureman, National Center for Environmental Assessment, US
Environmental Protection Agency, Research Triangle Park, NC, USA
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA ( Chairperson)
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany
Dr A. Nishikawa, Division of Pathology, National Institute of Health
Sciences, Tokyo, Japan
Dr E.V. Ohanian, Office of Water/Office of Science and Technology,
Health and Ecological Criteria Division, US Environmental Protection
Agency, Washington, DC, USA
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan
Professor P. Yao, Institute of Occupational Medicine, Chinese Academy
of Preventive Medicine, Ministry of Health, Beijing, People's Republic
of China
Observers
Dr K. Austin, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr I. Daly (ICCA representative), Regulatory and Technical Associates,
Lebanon, NJ, USA
Ms K.L. Lang (CEFIC, European Chemical Industry Council,
representative), Shell International, London, United Kingdom
Ms K. Roberts (ICCA representative), Chemical Self-funded Technical
Advocacy and Research (CHEMSTAR), Chemical Manufacturers Association,
Arlington, VA, USA
Dr W. Snellings (ICCA representative), Union Carbide Corporation,
Danbury, CN, USA
Dr M. Sweeney, Document Development Branch, National Institute for
Occupational Safety and Health, Cincinnati, OH, USA
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Secretariat
Dr M. Baril, Institut de Recherches en Santé et Sécurité du Travail du
Québec (IRSST), Montreal, Quebec, Canada
Dr H. Galal-Gorchev, Chevy Chase, MD, USA
Ms M. Godden, Health and Safety Executive, Bootle, Merseyside, United
Kingdom
Dr R.G. Liteplo, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Ms L. Regis, Programme for the Promotion of Chemical Safety, World
Health Organization, Geneva, Switzerland
Mr A. Strawson, Health and Safety Executive, London, United Kingdom
Dr P. Toft, Programme for the Promotion of Chemical Safety, World
Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
Ce CICAD relatif aux isomères en position 2-, 3- et 4- du
nitrophénol a été préparé par l'Institut Fraunhofer de recherche en
toxicologie et sur les aérosols de Hanovre (Allemagne). Il est basé
sur des mises au point rédigées par le Comité consultatif allemand sur
les produits chimiques qui posent un problème écologique (BUA, 1992)
et par l'US Agency for Toxic Substances and Disease Registry (ATSDR,
1992) afin d'évaluer les effets potentiels du 2- et du 4-nitrophénol
sur l'environnement et la santé humaine. Les données prises en compte
dans ces mises au point vont jusqu'en 1992. Une recherche
bibliographique exhaustive a été effectuée en 1998 sur plusieurs bases
de données afin de relever les références intéressantes sur le 2- et
le 4-nitrophénol publiées après celles qui figurent dans les documents
de base et d'obtenir toutes celles qui contiennent des données utiles
sur le 3-nitrophénol. On a trouvé très peu de données sur cet isomère,
ce qui rend impossible une véritable évaluation. Les données
concernant cet isomère sont donc récapitulées à l'appendice 1. On
trouvera à l'appendice 2 des indications sur le mode d'examen par des
pairs ainsi que sur les sources documentaires utilisées. Les
renseignements concernant l'examen du CICAD par des pairs font l'objet
de l'appendice 3. Ce CICAD a été approuvé en tant qu'évaluation
internationale lors de la réunion du Comité d'évaluation finale qui
s'est tenue à Washington du 8 au 11 décembre 1998. La liste des
participants à cette réunion figure à l'appendice 4. La fiche
d'information internationale sur la sécurité chimique (ICSC No 1342)
relative au mélange d'isomères du nitrophénol, établie par le
Programme international sur la sécurité chimique (IPCS, 1998) est
également reproduite dans ce document.
Les isomères du nitrophénol sont des solides solubles dans l'eau
qui sont légèrement acides dans ce solvant par suite de leur
dissociation. Les isomères 2- et 4- sont utilisés comme intermédiaires
dans la synthèse d'un certain nombre d'insecticides organophosphorés
et de composés à usage médical. Lorsqu'ils passent dans
l'environnement, c'est principalement par suite d'émissions dans
l'eau, l'air et le sol provenant de sources diffuses comme la
circulation automobile ou la décomposition par photolyse ou hydrolyse
de certains insecticides. Le dépôt par voie sèche ou humide de
nitrophénols présents dans l'atmosphère constitue un apport
supplémentaire dans l'hydrosphère et la géosphère. La formation
photo-oxydative du 2- et du 4-nitrophénol dans l'atmosphère est encore
débattue.
Les données disponibles montrent que le 2-nitrophénol ne devrait
se volatiliser que lentement dans l'atmosphère et qu'il ne passe sans
doute qu'en quantité négligeable de l'eau à l'air selon ce processus.
On constate un enrichissement en 2-nitrophénol de la phase aqueuse des
nuages; en revanche, dans la phase gazeuse, la proportion de
4-nitrophénol est plus importante qu'on pourrait le penser en fonction
des données physico-chimiques, par suite d'une importante fixation du
composé sur les particules. Compte tenu de leur solubilité dans l'eau
et de leur concentration dans la phase gazeuse, on peut s'attendre à
ce que les nitrophénols présents dans l'atmosphère se déposent par
voie humide sur la surface du sol et de l'eau. La principale voie de
transformation du 2-nitrophénol présent dans l'atmosphère est
vraisemblablement sa nitration rapide en 2,4-dinitrophénol, le
4-nitrophénol étant quant à lui en majeure partie fixé aux particules
aéroportées et donc disponible en petites quantités seulement pour des
réactions photochimiques. La majeure partie du 4-nitrophénol devrait
d'ailleurs disparaître de l'atmosphère en se déposant soit par voie
sèche, soit par voie humide. Il ne semble pas que les nitrophénols
contribuent directement à la dégradation de la couche d'ozone
stratosphérique ni au réchauffement général de la planète. Soumis à
une photodécomposition en milieu aqueux, le 4-nitrophénol a une
demi-vie qui peut aller de 2,8 à 13,7 jours selon les mesures. Les
nombreuses études consacrées à la biodégradation du 2- et du
4-nitrophénol montrent que ces deux isomères sont intrinsèquement
biodégradables dans l'eau en aérobiose. La minéralisation des
nitrophénols en anaérobiose exige à l'évidence une importante
adaptation des populations microbiennes.
La valeur du coefficient de sorption par les particules du sol
( Koc), qui se situe entre 44 et 530, indique que le potentiel de
sorption est faible à modéré. Les nitrophénols qui passent dans le sol
vont vraisemblablement subir une biodégradation aérobie. Il ne devrait
y avoir infiltration dans les eaux souterraines que lorsque les
conditions ne sont pas favorables à une biodégradation. Pour le 2- et
le 4-nitrophénol, la mesure du facteur de bioconcentration donne des
valeurs allant de 11 à 76, ce qui indique un faible potentiel de
bioconcentration.
On connaît plutôt mal le profil toxicologique du 2- et du
4-nitrophénol. Lorsqu'il est administré à des animaux de laboratoire
par voie orale, intraveineuse ou intrapéritonéale, le 4-nitrophénol
est en majeure partie excrété dans les urines en l'espace de 24 à 48 h
sous forme de glucuronide ou de sulfo-conjugué, une faible partie
seulement passant dans les matières fécale ou restant inchangée. On a
montré que la proportion de glucuronide et de sulfo-conjugués variait
selon les espèces. Après administration par voie orale à des lapins,
le 4-nitrophénol est réduit en p-aminophénol et subit aussi une
transformation en glucuronide et sulfo-conjugués. Les données tirées
des études in vivo et in vitro donnent une indication sur la
résorption du 4-nitrophénol par la voie transcutanée. En revanche, les
données concernant le 2-nitrophénol sont très limitées. Quoi qu'il en
soit, on peut considérer, en se basant sur les données disponibles,
que les deux isomères ont un métabolisme comparable. Le 2- et le
4-nitrophénol ne devraient pas s'accumuler dans l'organisme en raison
de leur métabolisation et de leur excrétion rapides.
Les études de toxicité aiguë montrent que le 4-nitrophénol a un
effet nocif après ingestion et qu'il est plus toxique que le
2-nitrophénol. Chez des chats, on a constaté une augmentation du taux
de méthémoglobine liée à la dose après ingestion de 2-nitrophénol; la
même constatation a été faite chez des rats après inhalation de
4-nitrophénol. Une exposition répétée à du 4-nitrophénol a montré que
la formation de méthémoglobine est l'effet le plus déterminant d'une
exposition par la voie respiratoire et cela vaut sans doute aussi pour
la voie orale. Parmi les autres effets observés, on peut citer un
moindre gain de poids, une modification du poids des organes, une
dégénérescence graisseuse du foie et des anomalies hématologiques. Il
n'a pas été possible de dégager une véritable relation dose-réponse ni
de déterminer de manière fiable la dose sans effet (nocif) observable
(NO(A)EL) correspondant à ces effets.
Le 2-nitrophénol est légèrement irritant pour la peau mais il
n'irrite pas la muqueuse oculaire. Le test de Buehler montre que le
composé n'a pas non plus d'effet sensibilisateur. En s'appuyant sur
des études valables effectuées sur l'animal, on peut conclure que le
4-nitrophénol a par contre une légère action irritante sur la peau et
les yeux. Un test de maximalisation sur le cobaye a montré que le
4-nitrophénol avait également une légère action sensibilisatrice. Chez
l'homme, on ne peut exclure une légère sensibilisation après un
contact avec le composé, d'autant plus que la pose d'un timbre cutané
chez des ouvriers pouvant avoir été en contact avec du 4-nitrophénol a
permis de constater une telle sensibilisation.
Aucun des deux isomères n'a fait l'objet d'épreuves de
génotoxicité suffisamment complètes. S'agissant du 2-nitrophénol, les
données sont insuffisantes pour que l'on puisse tirer la moindre
conclusion concernant une mutagénicité éventuelle. Dans le cas du
4-nitrophénol, les études de mutagénicité sont plus nombreuses, mais
le compte rendu en est parfois insuffisant. On est fondé à penser que
ce composé est susceptible de provoquer des aberrations chromosomiques
in vitro. Faute d'études de mutagénicité in vivo sur des
mammifères, il n'est pas possible de savoir si le pouvoir mutagène de
cet isomère peut s'exprimer in vivo.
Chez la souris l'application cutanée de 4-nitrophénol pendant une
durée de 78 semaines n'a pas donné d'indices d'effets cancérogènes.
Dans une autre étude sur la souris, qui présentait toutefois un
certain nombre d'insuffisances, on n'a pas non plus observé de tumeurs
cutanées après application cutanée de ces deux isomères pendant 12
semaines. Aucune étude de cancérogénicité utilisant la voie orale ou
respiratoire n'était disponible.
Les données relatives au 4-nitrophénol ne révèlent aucun effet
indésirable sur la reproduction ou le développement qui soit
statistiquement significatif après exposition de rats et de souris par
voie orale ou cutanée. Après administration par voie orale de
2-nitrophénol à des rats, on a constaté dans la progéniture des
animaux des effets indésirables sur le développement, mais seulement
aux doses toxiques pour les mères. On n'a toutefois pas recherché la
présence de malformations internes.
La base de données relative au 2-nitrophénol est extrêmement
limitée et celle qui concerne le 4-nitrophénol est insuffisante pour
qu'on puisse en tirer une valeur fiable de la NO(A)EL. Il est donc
impossible de fixer pour l'instant une valeur pour la dose journalière
tolérable (DJT) ou pour la concentration tolérable (CT) de ces deux
isomères.
D'après les résultats des études toxicologiques valables
effectuées sur divers organismes aquatiques, on peut considérer que
ces deux nitrophénols sont modérément à fortement toxiques pour la vie
aquatique. La concentration sans effet la plus faible qui ait été
obtenue lors d'études de longue durée sur des organismes d'eau douce
( Scenedesmus subspicatus, EC50 à 96 h : 0,39 mg de
2-nitrophénol/litre; Entosiphon sulcatum, concentration minimale
inhibitrice à 72 h ou CMI : 0,83 mg de 4-nitrophénol/l) était 40 à 50
fois plus forte que la valeur maximale obtenue dans un bassin fluvial
d'Asie situé dans une zone très industrialisée et densément peuplée
(respectivement 0,0072 mg/l et 0,019 mg/l). Par conséquent, malgré la
biodégradation et la décomposition photochimique, les nitrophénols
déversés dans l'eau peuvent présenter un certain risque pour les
organismes aquatiques sensibles, notamment dans des eaux
superficielles où les conditions ne sont pas favorables à ces deux
modes d'élimination. Cependant, compte tenu de leurs usages et de
leurs possibilités de libération dans l'environnement, ces deux
nitrophénols ne présentent qu'un risque mineur pour les organismes
aquatiques.
Les données disponibles indiquent que la toxicité potentielle de
ces nitrophénols est modérée dans l'environnement terrestre. Le calcul
du rapport d'exposition toxique (TER) des nitrophénols provenant de la
décomposition de certains pesticides montre que, dans ce milieu, le
risque reste faible pour la faune et la flore, même dans la pire des
hypothèses.
RESUMEN DE ORIENTACION
Preparó el presente CICAD sobre los isómeros 2-, 3- y
4-nitrofenol el Instituto Fraunhofer de Toxicología y de Investigación
sobre los Aerosoles de Hannover, Alemania. Se basa en los exámenes
compilados por el Comité Consultivo Alemán sobre las Sustancias
Químicas Importantes para el Medio Ambiente (BUA, 1992) y la Agencia
para el Registro de Sustancias Tóxicas y Enfermedades de los Estados
Unidos (ATSDR, 1992) para evaluar los efectos potenciales del 2- y el
4-nitrofenol en el medio ambiente y en el ser humano. En estos
exámenes se incluyeron los datos identificados hasta 1992. En 1998 se
realizó una búsqueda bibliográfica amplia de varias bases de datos
para identificar todas las referencias importantes relativas al 2- y
el 4-nitrofenol publicadas con posterioridad a las que figuran en los
documentos originales y para conocer todas las referencias con datos
pertinentes sobre el isómero 3-nitrofenol. La información obtenida
sobre el 3-nitrofenol fue muy escasa, lo que impide una evaluación
válida. En consecuencia, los datos sobre este isómero se resumen en el
apéndice 1. La información relativa al carácter del examen colegiado y
a la disponibilidad de los documentos originales figura en el apéndice
2. La información sobre el examen colegiado de este CICAD se presenta
en el apéndice 3. Este CICAD se aprobó como evaluación internacional
en una reunión de la Junta de Evaluación Final celebrada en
Washington, DC, Estados Unidos, los días 8-11 de diciembre de 1998. La
lista de participantes en esta reunión figura en el apéndice 4. La
Ficha internacional de seguridad química (ICSC 1342) para las mezclas
de isómeros de nitrofenoles, preparada por el Programa Internacional
de Seguridad de las Sustancias Químicas (IPCS, 1998), también se
reproduce en el presente documento.
Los isómeros del nitrofenol son sólidos hidrosolubles con una
acidez moderada en agua debido a la disociación. El 2-nitrofenol y el
4-nitrofenol se utilizan como intermediarios en la síntesis de
diversos plaguicidas órganofosforados y algunos productos médicos. La
liberación en el medio ambiente se produce fundamentalmente por
emisiones en el aire, el agua y el suelo a partir de fuentes difusas,
como el tráfico de vehículos y la degradación hidrolítica y fotolítica
de los respectivos plaguicidas. También se produce liberación en la
hidrosfera y la geosfera a partir de la atmósfera debido a la
deposición seca y húmeda de nitrofenoles suspendidos en el aire. La
formación fotooxidativa de 2- y 4-nitrofenol en la atmósfera es
todavía objeto de estudio.
Con los datos disponibles sólo cabe esperar una volatilización
lenta desde el agua hacia el aire para el 2-nitrofenol y no
significativa para el 4-nitrofenol. El 2-nitrofenol se enriquece en la
fase líquida de las nubes, mientras que es posible encontrar más
4-nitrofenol del previsto a partir de los datos fisicoquímicos en la
fase gaseosa de las nubes, debido a una amplia unión a partículas.
Habida cuenta de la solubilidad en agua y la presencia prevista en la
fase de vapor, cabe esperar una deposición húmeda de nitrofenoles del
aire en las aguas superficiales y en el suelo. La vía principal de
transformación del 2-nitrofenol emitido a la troposfera debe ser la
nitración rápida a 2,4-dinitrofenol, mientras que se supone que la
mayor parte del 4-nitrofenol suspendido en el aire se encuentra unido
a partículas y, por consiguiente, disponible solamente en menor
cantidad para reacciones fotoquímicas. La mayor parte del 4-nitrofenol
del aire debe precipitar por deposición húmeda y seca. No se considera
que los nitrofenoles contribuyan directamente al agotamiento de la
capa de ozono estratosférico o al calentamiento mundial. La semivida
medida para la descomposición fotoquímica del 4-nitrofenol en agua
osciló entre 2,8 y 13,7 días. Numerosos estudios sobre la
biodegradación del 2- y el 4-nitrofenol indican que los isómeros son
inherentemente biodegradables en agua en condiciones aerobias. La
mineralización de los nitrofenoles en condiciones anaerobias requiere
evidentemente una amplia adaptación de las comunidades microbianas.
Los coeficientes de sorción en el suelo ( Koc) medidos del
orden de 44-530 indican un potencial de bajo a moderado para la
sorción en el suelo. Los nitrofenoles liberados al suelo se deben
biodescomponer en condiciones aerobias. Cabe prever filtración hacia
el agua freática sólo en condiciones desfavorables para la
biodegradación. Para el 2- y el 4-nitrofenol, los factores de
bioconcentración medidos de 11 a 76 ponen de manifiesto un potencial
bajo de bioacumulación.
Se dispone sólo de información limitada relativa a los perfiles
toxicológicos del 2- y el 4-nitrofenol. Los animales experimentales a
los que se administró 4-nitrofenol por vía oral, intravenosa o
intraperitoneal excretaron la mayor parte de la dosis aplicada por vía
urinaria en un plazo de 24-48 horas en forma de conjugados de
glucurónidos y sulfatos, mientras que por las heces se excretaron
solamente cantidades muy pequeñas, o bien como 4-nitrofenol
inalterado. Se observó que los porcentajes de conjugados de
glucurónidos y sulfatos eran dependientes de la especie y de la dosis.
Tras la administración oral a conejos, el 4-nitrofenol sufre una
reducción a p-aminofenol, así como una glucuronización y
sulfatación. Los datos disponibles de estudios in vivo e in vitro
dan una idea de la absorción cutánea del 4-nitrofenol. Los datos para
el 2-nitrofenol son muy limitados. Sin embargo, teniendo cuenta los
datos disponibles, se supone que se produce una transformación
metabólica comparable. No cabe prever bioacumulación de 2- y
4-nitrofenol en los organismos debido a la rapidez de su metabolismo y
excreción.
En estudios de toxicidad aguda, el 4-nitrofenol es perjudicial
tras la absorción oral, y se observó que era más tóxico que el
2-nitrofenol. Se detectó un aumento en la formación de metahemoglobina
dependiente de la dosis en gatos tras la exposición oral al
2-nitrofenol y en ratas tras la exposición por inhalación al
4-nitrofenol. Después de una exposición repetida al 4-nitrofenol, se
observó la formación de metahemoglobina como el efecto final más
importante de la exposición por inhalación, y se considera que esto es
aplicable también a la exposición oral. Otros efectos observados
fueron la reducción del aumento del peso corporal, diferencias en el
peso de los órganos, degeneración adiposa focal del hígado y cambios
hematológicos. Para esos efectos no fue posible determinar una
relación clara dosis-respuesta o concentraciones sin efectos
(adversos) observados (NO(A)EL) fidedignas.
El 2-nitrofenol es ligeramente irritante para la piel, pero no
para los ojos. En una prueba de Buehler no se observó efecto
sensibilizador. Tomando como base los estudios válidos con animales
experimentales, se supone que el 4-nitrofenol tiene efectos irritantes
en la piel y los ojos. En una prueba de maximización en cobayas, se
observó que el 4-nitrofenol era ligeramente sensibilizante. En el ser
humano no se puede excluir una posible sensibilización tras el
contacto con 4-nitrofenol, especialmente teniendo en cuenta que se ha
observado sensibilización cutánea en pruebas de parche en trabajadores
de fábricas que podían haber estado expuestos al 4-nitrofenol.
No se ha sometido a pruebas completas de genotoxicidad ninguno de
los dos isómeros del nitrofenol. Los datos disponibles sobre el
2-nitrofenol son insuficientes para poder sacar conclusiones acerca de
su posible mutagenicidad. Hay más estudios de mutagenicidad para el
4-nitrofenol, aunque algunos se notificaron de manera inadecuada. Hay
pruebas que parecen indicar que el 4-nitrofenol puede producir
aberraciones cromosómicas in vitro. En ausencia de estudios de
mutagenicidad in vivo en mamíferos, no es posible llegar a la
conclusión de si se expresa o no in vivo el potencial mutagénico del
4-nitrofenol.
Tras la aplicación cutánea de 4-nitrofenol a ratones durante 78
semanas no se observaron efectos carcinogénicos. En otro estudio con
ratones, que tiene varias limitaciones, no se detectaron tumores
cutáneos tras la aplicación en la piel de 2- ó 4-nitrofenol durante 12
semanas. Para ninguno de los isómeros había estudios de
carcinogenicidad utilizando la vía oral o la inhalación.
Los datos disponibles para el 4-nitrofenol no pusieron de
manifiesto efectos específicos o estadísticamente significativos de
toxicidad reproductiva o del desarrollo tras la administración cutánea
u oral a ratas y ratones. En un estudio de administración por vía oral
a ratas, el 2-nitrofenol indujo efectos en el desarrollo de la camada
sólo con dosis que también producían toxicidad materna. Sin embargo,
en estos estudios no se examinaron los fetos para investigar
malformaciones internas.
La base de datos para el 2-nitrofenol es enormemente limitada y
la relativa al 4-nitrofenol es insuficiente para deducir valores
fidedignos de la (NO(A)EL). Por consiguiente, es imposible determinar
en este momento la ingesta diaria tolerable o las concentraciones
tolerables para el 2- ó el 4-nitrofenol.
De los resultados disponibles de pruebas válidas sobre la
toxicidad del 2-y el 4-nitrofenol para diversos organismos acuáticos,
los nitrofenoles se pueden clasificar como sustancias con una
toxicidad entre moderada y alta en el compartimento acuático. Las
concentraciones más bajas con efectos obtenidas en estudios crónicos
con organismos de agua dulce ( Scenedesmus subspicatus, CE50: 0,39
mg de 2-nitrofenol/litro a las 96 h; Entosiphon sulcatum,
concentración inhibitoria mínima a las 72 horas: 83 mg de
4-nitrofenol/litro) fueron 40-50 veces superiores a los niveles
máximos determinados en una cuenca fluvial asiática densamente poblada
y muy industrializada (0,0072 mg de 2-nitrofenol/litro y 0,019 mg de
4-nitrofenol/litro). Por consiguiente, a pesar de la descomposición
biótica y fotoquímica, los nitrofenoles emitidos al agua pueden
representar algún peligro para los organismos acuáticos sensibles,
particularmente en las condiciones de las aguas superficiales que no
favorecen ambas vías de eliminación. Habida cuenta de sus pautas de
uso y sus características de liberación, probablemente los
nitrofenoles plantean sólo un pequeño riesgo para los organismos
acuáticos.
Los datos disponibles indican solamente una toxicidad potencial
moderada de los nitrofenoles en el medio ambiente terrestre. A partir
de los cálculos de la razón exposición-toxicidad de los nitrofenoles a
partir de la degradación de plaguicidas, sólo cabe esperar un pequeño
riesgo para los organismos en este compartimento, incluso en el peor
de los casos.
Physicochemical properties of 3-nitrophenol are given in
Table A-1.
Table A-1: Physicochemical properties of 3-nitrophenol.
Parameter Value
Molecular mass (g/mol) 139.11
Melting point (°C) 96-97 (1)(2)
Boiling point (°C) 194 (1)
Vapour pressure (kPa; 20 °C) 0.10 (3)
Water solubility (g/litre; 25 °C) 13.5 (1)
n-Octanol/water partition coefficient (log Kow) 2.00 (4)
Dissociation constant (pKa) (18 °C) 8.34 (2)
Conversion factors 1 mg/m3 = 0.173 ppmv
1 ppmv = 5.78 mg/m3
References: (1) Verschueren (1983); (2) Budavari et al. (1996);
(3) HSDB (1998); (4) Hansch & Leo (1979)
Environmental transport, distribution, and transformation
Data on the abiotic degradation of 3-nitrophenol were not
available.
Three studies on biotic degradation, summarized in Table A-2,
indicate the isomer to be inherently biodegradable in water under
aerobic conditions.
In tests on biotic degradation under anaerobic conditions using
sewage sludge and sludge from the primary anaerobic stage of a
municipal sewage treatment plant, respectively, initial 3-nitrophenol
concentrations in the range of 96.5-579 mg/litre were not degraded at
all within 7-60 days (Wagner & Braeutigam, 1981; Battersby & Wilson,
1989). Boyd et al. (1983), however, found complete anaerobic removal
of 50 mg/litre within 1 week of incubation. In this test,
mineralization was demonstrated only if the incubation period was
extended to 10 weeks. Anaerobic degradation, even of high initial
nitrophenol concentrations, was found by Tseng & Lin (1994), who
observed 90% removal of 3-nitrophenol (350-650 mg/litre) in a
biological fluidized bed reactor with three different kinds of
wastewater. From the available results, a slow mineralization of
3-nitrophenol under anaerobic conditions by adapted microorganisms can
be expected.
A soil sorption coefficient ( Koc) of 52.83, determined by Boyd
(1982), and the n-octanol/water partition coefficient (log Kow)
of 2.0, reported by Hansch & Leo (1979), indicate a low to moderate
potential for soil sorption as well as for bioaccumulation.
Environmental levels
3-Nitrophenol was not detected in 27 samples of air (detection
limit 8 ng/m3) in Japan in 1994 (Japan Environment Agency, 1995). It
was not detected in 177 samples of Japanese surface waters (detection
limits 0.04-10 µg/litre) or in 177 sediment samples (detection limits
0.002-0.8 µg/kg) in 1978, 1979, and 1994 (Japan Environment Agency,
1979, 1980, 1995). 3-Nitrophenol was not detected in 129 fish samples
(detection limits 0.005-0.2 µg/kg) in Japan in 1979 and 1994 (Japan
Environment Agency, 1980, 1995).
Comparative kinetics and metabolism in laboratory animals and humans
Studies providing quantitative information on the absorption,
metabolism, or elimination of 3-nitrophenol in humans were not
identified. In addition, there is only very limited information
available for experimental animals. In rabbits given a single dose of
150-200 mg/kg body weight via gavage, most of the applied dose
(>>80%) was excreted via the urine within 24 h. About 68-86% was
conjugated with glucuronic acid and sulfonic acid, whereas about 7-13%
was reduced to aminophenols (Robinson et al., 1951). Skin permeation
was shown in several in vitro experiments (Huq et al., 1986; Jetzer
et al., 1986; Ohkura et al., 1990). Although the information is
limited, bioaccumulation of 3-nitrophenol in organisms is not to be
expected owing to the isomer's rapid metabolism and excretion.
Effects on laboratory mammals and in vitro test systems
The oral LD50 of 3-nitrophenol is quoted to be >930 mg/kg
body weight for rats (Vasilenko et al., 1976; Vernot et al., 1977) and
>1070 mg/kg body weight for mice (Vasilenko et al., 1976; Vernot et
al., 1977).
The available in vitro and in vivo genotoxicity studies on
3-nitrophenol are summarized in Table A-3. 3-Nitrophenol was shown to
be mutagenic in a rec-assay and gave inconsistent results in
Salmonella/microsome assays. One study showed it to be non-mutagenic
in the Salmonella typhimurium TA98 and TA100 strains, whereas
another study showed mutagenicity in both of these strains in both the
presence and absence of metabolic activation. In view of the
conflicting results from Salmonella/microsome assays and the absence
of any data on clastogenicity, no conclusions can be made regarding
the mutagenicity of 3-nitrophenol.
For 3-nitrophenol, there are no studies available concerning
irritating or sensitizing effects, repeated exposure, reproductive and
developmental toxicity, or effects on humans.
Effects on aquatic species
In tests performed on the toxicity of 3-nitrophenol to various
aquatic organisms (see Table A-4), 3-nitrophenol exhibited a moderate
to high toxicity.
Table A-2: Biotic degradation of 3-nitrophenol under aerobic conditions.
Test Concentration Additional Test duration Removal Reference
(mg/litre) carbon source (days) (%)
Tests on ready biodegradability
MITI I 100 no 14 0 Gerike & Fischer (1979);
Urano & Kato (1986)
Tests on inherent biodegradability
Batch test, aerated 200 CODa no 5 95 Pitter (1976)
Respirometric test 300 yes 10 44 Kayser et al. (1994)
a COD = chemical oxygen demand.
Table A-3: Genotoxicity of 3-nitrophenol in vitro and in vivo.
Resultsa
Species End-point Concentration Without With Remarks References
(test system) range metabolic metabolic
activation activation
In vitro studies
Bacillus subtilis Recombination 0.01-5 mg/plate + 0 positive at Shimizu & Yano
H17, M45 assay >0.5 (1986)
mg/plate
Salmonella Reverse 0.01-5 mg/plate - - Shimizu & Yano
typhimurium mutations (1986)
TA1535, TA1537,
TA1538
Salmonella Reverse 0.1-5 mg/plate + + Study in Kawai et al.
typhimurium mutations Japanese (data (1987)
TA98, TA100 taken from
tables)
Salmonella Reverse 0.01-5 mg/plate - - Suzuki et al. Suzuki et al.
typhimurium TA98, mutations (1983) also (1983);
TA100 tested both Shimizu & Yano
strains in the (1986)
presence of
norharman,
which also gave
negative results
Table A-3 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks References
(test system) range metabolic metabolic
activation activation
In vivo studies
Drosophila SLRL assay via feed Foureman et al.
melanogaster (5000 ppm) (1994)
or injection
(1200 ppm)
a -, negative; +, positive; 0, not tested.
Table A-4: Aquatic toxicity of 3-nitrophenol.
Species
(test method/end-point) Effective concentration Reference
(mg/litre)
Bacteria
Pseudomonas putida 16-h MICa: 7.0 Bringmann & Kuehn (1977)
(cell multiplication inhibition test)
Protozoa
Entosiphon sulcatum 72-h MIC: 0.97 Bringmann (1978);
(cell multiplication inhibition test) Bringmann et al. (1980)
Algae
Scenedesmus subspicatus
Chlorella vulgaris 6-h EC50: 6.21 Kramer et al. (1986)
(cell multiplication inhibition test)
Invertebrates
Moina macrocopa (acute) 3-h LC50: 1.7 Yoshioka et al. (1985)
(immobilization)
Fish
Cyprinus carpio (static) 96-h LC50: 17.5 Lang et al. (1996)
a MIC = minimum inhibitory concentration.
APPENDIX 2 - SOURCE DOCUMENTS
BUA (1992): BUA-Stoffbericht 2- und 4-Nitrophenol.
Beratergremium fuer Umweltrelevante Altstoffe. Weinheim, VCH
VerlagsGmbH (Report No. 75; February 1992)
For the BUA review process, the company that is in charge of
writing the report (usually the largest producer in Germany) prepares
a draft report using literature from an extensive literature search as
well as internal company studies. This draft is subject to a peer
review during several readings of a working group consisting of
representatives from government agencies, the scientific community,
and industry.
The English translation of BUA Report No. 75 ( BUA Report 2- and
4-Nitrophenol. GDCh-Advisory Committee on Existing Chemicals of
Environmental Relevance. Stuttgart, Hirzel Verlag [February 1992]) was
released in 1993.
ATSDR (1992): Toxicological profile for nitrophenols:
2- and 4-nitrophenol. Atlanta, GA, US Department of Health and Human
Services, Public Health Service, Agency for Toxic Substances and
Disease Registry (Report No. TP-91/23)
Copies of the ATSDR Toxicological profile for nitrophenols:
2- and 4-nitrophenol (ATSDR, 1992) may be obtained from the:
Agency for Toxic Substances and Disease Registry
Division of Toxicology
1600 Clifton Road, E-29
Atlanta, Georgia 30333
USA
Initial drafts of the Toxicology profile for nitrophenols:
2- and 4-nitrophenol were reviewed by scientists from the Agency for
Toxic Substances and Disease Registry, the US Centers for Disease
Control, the US National Toxicology Program, and other federal
agencies. The document was also reviewed by an expert panel of
nongovernmental reviewers, consisting of the following members:
Dr Martin Alexander, Cornell University
Dr Gary Booth, Brigham Young University
Dr Samuel Cohen, University of Nebraska Medical Center
Dr Loren Koller, Oregon State University
Dr Frederick Oehme, Kansas State University
APPENDIX 3 - CICAD PEER REVIEW
The draft CICAD on mononitrophenols was sent for review to
institutions and organizations identified by IPCS after contact with
IPCS national Contact Points and Participating Institutions, as well
as to identified experts. Comments were received from:
Federal Institute for Health Protection of Consumers & Veterinary
Medicine, Berlin, Germany
Gesellschaft Deutscher Chemiker, Frankfurt, Germany
Institute of Occupational Medicine, Chinese Academy of Preventive
Medicine, Ministry of Health, Beijing, People's Republic of China
Institute of Terrestrial Ecology, Huntingdon, United Kingdom
Joint Food Safety and Standards Group, Department of Health,
London, United Kingdom
National Institute of Health Sciences, Tokyo, Japan
National Institute of Public Health, Prague, Czech Republic
United States Department of Health and Human Services (National
Institute of Environmental Health Sciences, Research Triangle
Park), USA
United States Environmental Protection Agency (National Center
for Environmental Assessment, Washington, DC; Region VIII), USA
World Health Organization/International Programme on Chemical
Safety, Montreal, Canada
APPENDIX 4 - CICAD FINAL REVIEW BOARD
Washington, DC, USA, 8-11 December 1998
Members
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
( Vice-Chairperson)
Mr R. Cary, Toxicology Unit, Health Directorate, Health and Safety
Executive, Bootle, Merseyside, United Kingdom ( Rapporteur)
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr O. Faroon, Agency for Toxic Substances and Disease Registry,
Centers for Disease Control and Prevention, Atlanta, GA, USA
Dr G. Foureman, National Center for Environmental Assessment, US
Environmental Protection Agency, Research Triangle Park, NC, USA
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA ( Chairperson)
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany
Dr A. Nishikawa, Division of Pathology, National Institute of Health
Sciences, Tokyo, Japan
Dr E.V. Ohanian, Office of Water/Office of Science and Technology,
Health and Ecological Criteria Division, US Environmental Protection
Agency, Washington, DC, USA
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan
Professor P. Yao, Institute of Occupational Medicine, Chinese Academy
of Preventive Medicine, Ministry of Health, Beijing, People's Republic
of China
Observers
Dr K. Austin, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr I. Daly (ICCA representative), Regulatory and Technical Associates,
Lebanon, NJ, USA
Ms K.L. Lang (CEFIC, European Chemical Industry Council,
representative), Shell International, London, United Kingdom
Ms K. Roberts (ICCA representative), Chemical Self-funded Technical
Advocacy and Research (CHEMSTAR), Chemical Manufacturers Association,
Arlington, VA, USA
Dr W. Snellings (ICCA representative), Union Carbide Corporation,
Danbury, CN, USA
Dr M. Sweeney, Document Development Branch, National Institute for
Occupational Safety and Health, Cincinnati, OH, USA
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Secretariat
Dr M. Baril, Institut de Recherches en Santé et Sécurité du Travail du
Québec (IRSST), Montreal, Quebec, Canada
Dr H. Galal-Gorchev, Chevy Chase, MD, USA
Ms M. Godden, Health and Safety Executive, Bootle, Merseyside, United
Kingdom
Dr R.G. Liteplo, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Ms L. Regis, Programme for the Promotion of Chemical Safety, World
Health Organization, Geneva, Switzerland
Mr A. Strawson, Health and Safety Executive, London, United Kingdom
Dr P. Toft, Programme for the Promotion of Chemical Safety, World
Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
Ce CICAD relatif aux isomères en position 2-, 3- et 4- du
nitrophénol a été préparé par l'Institut Fraunhofer de recherche en
toxicologie et sur les aérosols de Hanovre (Allemagne). Il est basé
sur des mises au point rédigées par le Comité consultatif allemand sur
les produits chimiques qui posent un problème écologique (BUA, 1992)
et par l'US Agency for Toxic Substances and Disease Registry (ATSDR,
1992) afin d'évaluer les effets potentiels du 2- et du 4-nitrophénol
sur l'environnement et la santé humaine. Les données prises en compte
dans ces mises au point vont jusqu'en 1992. Une recherche
bibliographique exhaustive a été effectuée en 1998 sur plusieurs bases
de données afin de relever les références intéressantes sur le 2- et
le 4-nitrophénol publiées après celles qui figurent dans les documents
de base et d'obtenir toutes celles qui contiennent des données utiles
sur le 3-nitrophénol. On a trouvé très peu de données sur cet isomère,
ce qui rend impossible une véritable évaluation. Les données
concernant cet isomère sont donc récapitulées à l'appendice 1. On
trouvera à l'appendice 2 des indications sur le mode d'examen par des
pairs ainsi que sur les sources documentaires utilisées. Les
renseignements concernant l'examen du CICAD par des pairs font l'objet
de l'appendice 3. Ce CICAD a été approuvé en tant qu'évaluation
internationale lors de la réunion du Comité d'évaluation finale qui
s'est tenue à Washington du 8 au 11 décembre 1998. La liste des
participants à cette réunion figure à l'appendice 4. La fiche
d'information internationale sur la sécurité chimique (ICSC No 1342)
relative au mélange d'isomères du nitrophénol, établie par le
Programme international sur la sécurité chimique (IPCS, 1998) est
également reproduite dans ce document.
Les isomères du nitrophénol sont des solides solubles dans l'eau
qui sont légèrement acides dans ce solvant par suite de leur
dissociation. Les isomères 2- et 4- sont utilisés comme intermédiaires
dans la synthèse d'un certain nombre d'insecticides organophosphorés
et de composés à usage médical. Lorsqu'ils passent dans
l'environnement, c'est principalement par suite d'émissions dans
l'eau, l'air et le sol provenant de sources diffuses comme la
circulation automobile ou la décomposition par photolyse ou hydrolyse
de certains insecticides. Le dépôt par voie sèche ou humide de
nitrophénols présents dans l'atmosphère constitue un apport
supplémentaire dans l'hydrosphère et la géosphère. La formation
photo-oxydative du 2- et du 4-nitrophénol dans l'atmosphère est encore
débattue.
Les données disponibles montrent que le 2-nitrophénol ne devrait
se volatiliser que lentement dans l'atmosphère et qu'il ne passe sans
doute qu'en quantité négligeable de l'eau à l'air selon ce processus.
On constate un enrichissement en 2-nitrophénol de la phase aqueuse des
nuages; en revanche, dans la phase gazeuse, la proportion de
4-nitrophénol est plus importante qu'on pourrait le penser en fonction
des données physico-chimiques, par suite d'une importante fixation du
composé sur les particules. Compte tenu de leur solubilité dans l'eau
et de leur concentration dans la phase gazeuse, on peut s'attendre à
ce que les nitrophénols présents dans l'atmosphère se déposent par
voie humide sur la surface du sol et de l'eau. La principale voie de
transformation du 2-nitrophénol présent dans l'atmosphère est
vraisemblablement sa nitration rapide en 2,4-dinitrophénol, le
4-nitrophénol étant quant à lui en majeure partie fixé aux particules
aéroportées et donc disponible en petites quantités seulement pour des
réactions photochimiques. La majeure partie du 4-nitrophénol devrait
d'ailleurs disparaître de l'atmosphère en se déposant soit par voie
sèche, soit par voie humide. Il ne semble pas que les nitrophénols
contribuent directement à la dégradation de la couche d'ozone
stratosphérique ni au réchauffement général de la planète. Soumis à
une photodécomposition en milieu aqueux, le 4-nitrophénol a une
demi-vie qui peut aller de 2,8 à 13,7 jours selon les mesures. Les
nombreuses études consacrées à la biodégradation du 2- et du
4-nitrophénol montrent que ces deux isomères sont intrinsèquement
biodégradables dans l'eau en aérobiose. La minéralisation des
nitrophénols en anaérobiose exige à l'évidence une importante
adaptation des populations microbiennes.
La valeur du coefficient de sorption par les particules du sol
( Koc), qui se situe entre 44 et 530, indique que le potentiel de
sorption est faible à modéré. Les nitrophénols qui passent dans le sol
vont vraisemblablement subir une biodégradation aérobie. Il ne devrait
y avoir infiltration dans les eaux souterraines que lorsque les
conditions ne sont pas favorables à une biodégradation. Pour le 2- et
le 4-nitrophénol, la mesure du facteur de bioconcentration donne des
valeurs allant de 11 à 76, ce qui indique un faible potentiel de
bioconcentration.
On connaît plutôt mal le profil toxicologique du 2- et du
4-nitrophénol. Lorsqu'il est administré à des animaux de laboratoire
par voie orale, intraveineuse ou intrapéritonéale, le 4-nitrophénol
est en majeure partie excrété dans les urines en l'espace de 24 à 48 h
sous forme de glucuronide ou de sulfo-conjugué, une faible partie
seulement passant dans les matières fécale ou restant inchangée. On a
montré que la proportion de glucuronide et de sulfo-conjugués variait
selon les espèces. Après administration par voie orale à des lapins,
le 4-nitrophénol est réduit en p-aminophénol et subit aussi une
transformation en glucuronide et sulfo-conjugués. Les données tirées
des études in vivo et in vitro donnent une indication sur la
résorption du 4-nitrophénol par la voie transcutanée. En revanche, les
données concernant le 2-nitrophénol sont très limitées. Quoi qu'il en
soit, on peut considérer, en se basant sur les données disponibles,
que les deux isomères ont un métabolisme comparable. Le 2- et le
4-nitrophénol ne devraient pas s'accumuler dans l'organisme en raison
de leur métabolisation et de leur excrétion rapides.
Les études de toxicité aiguë montrent que le 4-nitrophénol a un
effet nocif après ingestion et qu'il est plus toxique que le
2-nitrophénol. Chez des chats, on a constaté une augmentation du taux
de méthémoglobine liée à la dose après ingestion de 2-nitrophénol; la
même constatation a été faite chez des rats après inhalation de
4-nitrophénol. Une exposition répétée à du 4-nitrophénol a montré que
la formation de méthémoglobine est l'effet le plus déterminant d'une
exposition par la voie respiratoire et cela vaut sans doute aussi pour
la voie orale. Parmi les autres effets observés, on peut citer un
moindre gain de poids, une modification du poids des organes, une
dégénérescence graisseuse du foie et des anomalies hématologiques. Il
n'a pas été possible de dégager une véritable relation dose-réponse ni
de déterminer de manière fiable la dose sans effet (nocif) observable
(NO(A)EL) correspondant à ces effets.
Le 2-nitrophénol est légèrement irritant pour la peau mais il
n'irrite pas la muqueuse oculaire. Le test de Buehler montre que le
composé n'a pas non plus d'effet sensibilisateur. En s'appuyant sur
des études valables effectuées sur l'animal, on peut conclure que le
4-nitrophénol a par contre une légère action irritante sur la peau et
les yeux. Un test de maximalisation sur le cobaye a montré que le
4-nitrophénol avait également une légère action sensibilisatrice. Chez
l'homme, on ne peut exclure une légère sensibilisation après un
contact avec le composé, d'autant plus que la pose d'un timbre cutané
chez des ouvriers pouvant avoir été en contact avec du 4-nitrophénol a
permis de constater une telle sensibilisation.
Aucun des deux isomères n'a fait l'objet d'épreuves de
génotoxicité suffisamment complètes. S'agissant du 2-nitrophénol, les
données sont insuffisantes pour que l'on puisse tirer la moindre
conclusion concernant une mutagénicité éventuelle. Dans le cas du
4-nitrophénol, les études de mutagénicité sont plus nombreuses, mais
le compte rendu en est parfois insuffisant. On est fondé à penser que
ce composé est susceptible de provoquer des aberrations chromosomiques
in vitro. Faute d'études de mutagénicité in vivo sur des
mammifères, il n'est pas possible de savoir si le pouvoir mutagène de
cet isomère peut s'exprimer in vivo.
Chez la souris l'application cutanée de 4-nitrophénol pendant une
durée de 78 semaines n'a pas donné d'indices d'effets cancérogènes.
Dans une autre étude sur la souris, qui présentait toutefois un
certain nombre d'insuffisances, on n'a pas non plus observé de tumeurs
cutanées après application cutanée de ces deux isomères pendant 12
semaines. Aucune étude de cancérogénicité utilisant la voie orale ou
respiratoire n'était disponible.
Les données relatives au 4-nitrophénol ne révèlent aucun effet
indésirable sur la reproduction ou le développement qui soit
statistiquement significatif après exposition de rats et de souris par
voie orale ou cutanée. Après administration par voie orale de
2-nitrophénol à des rats, on a constaté dans la progéniture des
animaux des effets indésirables sur le développement, mais seulement
aux doses toxiques pour les mères. On n'a toutefois pas recherché la
présence de malformations internes.
La base de données relative au 2-nitrophénol est extrêmement
limitée et celle qui concerne le 4-nitrophénol est insuffisante pour
qu'on puisse en tirer une valeur fiable de la NO(A)EL. Il est donc
impossible de fixer pour l'instant une valeur pour la dose journalière
tolérable (DJT) ou pour la concentration tolérable (CT) de ces deux
isomères.
D'après les résultats des études toxicologiques valables
effectuées sur divers organismes aquatiques, on peut considérer que
ces deux nitrophénols sont modérément à fortement toxiques pour la vie
aquatique. La concentration sans effet la plus faible qui ait été
obtenue lors d'études de longue durée sur des organismes d'eau douce
( Scenedesmus subspicatus, EC50 à 96 h : 0,39 mg de
2-nitrophénol/litre; Entosiphon sulcatum, concentration minimale
inhibitrice à 72 h ou CMI : 0,83 mg de 4-nitrophénol/l) était 40 à 50
fois plus forte que la valeur maximale obtenue dans un bassin fluvial
d'Asie situé dans une zone très industrialisée et densément peuplée
(respectivement 0,0072 mg/l et 0,019 mg/l). Par conséquent, malgré la
biodégradation et la décomposition photochimique, les nitrophénols
déversés dans l'eau peuvent présenter un certain risque pour les
organismes aquatiques sensibles, notamment dans des eaux
superficielles où les conditions ne sont pas favorables à ces deux
modes d'élimination. Cependant, compte tenu de leurs usages et de
leurs possibilités de libération dans l'environnement, ces deux
nitrophénols ne présentent qu'un risque mineur pour les organismes
aquatiques.
Les données disponibles indiquent que la toxicité potentielle de
ces nitrophénols est modérée dans l'environnement terrestre. Le calcul
du rapport d'exposition toxique (TER) des nitrophénols provenant de la
décomposition de certains pesticides montre que, dans ce milieu, le
risque reste faible pour la faune et la flore, même dans la pire des
hypothèses.
RESUMEN DE ORIENTACION
Preparó el presente CICAD sobre los isómeros 2-, 3- y
4-nitrofenol el Instituto Fraunhofer de Toxicología y de Investigación
sobre los Aerosoles de Hannover, Alemania. Se basa en los exámenes
compilados por el Comité Consultivo Alemán sobre las Sustancias
Químicas Importantes para el Medio Ambiente (BUA, 1992) y la Agencia
para el Registro de Sustancias Tóxicas y Enfermedades de los Estados
Unidos (ATSDR, 1992) para evaluar los efectos potenciales del 2- y el
4-nitrofenol en el medio ambiente y en el ser humano. En estos
exámenes se incluyeron los datos identificados hasta 1992. En 1998 se
realizó una búsqueda bibliográfica amplia de varias bases de datos
para identificar todas las referencias importantes relativas al 2- y
el 4-nitrofenol publicadas con posterioridad a las que figuran en los
documentos originales y para conocer todas las referencias con datos
pertinentes sobre el isómero 3-nitrofenol. La información obtenida
sobre el 3-nitrofenol fue muy escasa, lo que impide una evaluación
válida. En consecuencia, los datos sobre este isómero se resumen en el
apéndice 1. La información relativa al carácter del examen colegiado y
a la disponibilidad de los documentos originales figura en el apéndice
2. La información sobre el examen colegiado de este CICAD se presenta
en el apéndice 3. Este CICAD se aprobó como evaluación internacional
en una reunión de la Junta de Evaluación Final celebrada en
Washington, DC, Estados Unidos, los días 8-11 de diciembre de 1998. La
lista de participantes en esta reunión figura en el apéndice 4. La
Ficha internacional de seguridad química (ICSC 1342) para las mezclas
de isómeros de nitrofenoles, preparada por el Programa Internacional
de Seguridad de las Sustancias Químicas (IPCS, 1998), también se
reproduce en el presente documento.
Los isómeros del nitrofenol son sólidos hidrosolubles con una
acidez moderada en agua debido a la disociación. El 2-nitrofenol y el
4-nitrofenol se utilizan como intermediarios en la síntesis de
diversos plaguicidas órganofosforados y algunos productos médicos. La
liberación en el medio ambiente se produce fundamentalmente por
emisiones en el aire, el agua y el suelo a partir de fuentes difusas,
como el tráfico de vehículos y la degradación hidrolítica y fotolítica
de los respectivos plaguicidas. También se produce liberación en la
hidrosfera y la geosfera a partir de la atmósfera debido a la
deposición seca y húmeda de nitrofenoles suspendidos en el aire. La
formación fotooxidativa de 2- y 4-nitrofenol en la atmósfera es
todavía objeto de estudio.
Con los datos disponibles sólo cabe esperar una volatilización
lenta desde el agua hacia el aire para el 2-nitrofenol y no
significativa para el 4-nitrofenol. El 2-nitrofenol se enriquece en la
fase líquida de las nubes, mientras que es posible encontrar más
4-nitrofenol del previsto a partir de los datos fisicoquímicos en la
fase gaseosa de las nubes, debido a una amplia unión a partículas.
Habida cuenta de la solubilidad en agua y la presencia prevista en la
fase de vapor, cabe esperar una deposición húmeda de nitrofenoles del
aire en las aguas superficiales y en el suelo. La vía principal de
transformación del 2-nitrofenol emitido a la troposfera debe ser la
nitración rápida a 2,4-dinitrofenol, mientras que se supone que la
mayor parte del 4-nitrofenol suspendido en el aire se encuentra unido
a partículas y, por consiguiente, disponible solamente en menor
cantidad para reacciones fotoquímicas. La mayor parte del 4-nitrofenol
del aire debe precipitar por deposición húmeda y seca. No se considera
que los nitrofenoles contribuyan directamente al agotamiento de la
capa de ozono estratosférico o al calentamiento mundial. La semivida
medida para la descomposición fotoquímica del 4-nitrofenol en agua
osciló entre 2,8 y 13,7 días. Numerosos estudios sobre la
biodegradación del 2- y el 4-nitrofenol indican que los isómeros son
inherentemente biodegradables en agua en condiciones aerobias. La
mineralización de los nitrofenoles en condiciones anaerobias requiere
evidentemente una amplia adaptación de las comunidades microbianas.
Los coeficientes de sorción en el suelo ( Koc) medidos del
orden de 44-530 indican un potencial de bajo a moderado para la
sorción en el suelo. Los nitrofenoles liberados al suelo se deben
biodescomponer en condiciones aerobias. Cabe prever filtración hacia
el agua freática sólo en condiciones desfavorables para la
biodegradación. Para el 2- y el 4-nitrofenol, los factores de
bioconcentración medidos de 11 a 76 ponen de manifiesto un potencial
bajo de bioacumulación.
Se dispone sólo de información limitada relativa a los perfiles
toxicológicos del 2- y el 4-nitrofenol. Los animales experimentales a
los que se administró 4-nitrofenol por vía oral, intravenosa o
intraperitoneal excretaron la mayor parte de la dosis aplicada por vía
urinaria en un plazo de 24-48 horas en forma de conjugados de
glucurónidos y sulfatos, mientras que por las heces se excretaron
solamente cantidades muy pequeñas, o bien como 4-nitrofenol
inalterado. Se observó que los porcentajes de conjugados de
glucurónidos y sulfatos eran dependientes de la especie y de la dosis.
Tras la administración oral a conejos, el 4-nitrofenol sufre una
reducción a p-aminofenol, así como una glucuronización y
sulfatación. Los datos disponibles de estudios in vivo e in vitro
dan una idea de la absorción cutánea del 4-nitrofenol. Los datos para
el 2-nitrofenol son muy limitados. Sin embargo, teniendo cuenta los
datos disponibles, se supone que se produce una transformación
metabólica comparable. No cabe prever bioacumulación de 2- y
4-nitrofenol en los organismos debido a la rapidez de su metabolismo y
excreción.
En estudios de toxicidad aguda, el 4-nitrofenol es perjudicial
tras la absorción oral, y se observó que era más tóxico que el
2-nitrofenol. Se detectó un aumento en la formación de metahemoglobina
dependiente de la dosis en gatos tras la exposición oral al
2-nitrofenol y en ratas tras la exposición por inhalación al
4-nitrofenol. Después de una exposición repetida al 4-nitrofenol, se
observó la formación de metahemoglobina como el efecto final más
importante de la exposición por inhalación, y se considera que esto es
aplicable también a la exposición oral. Otros efectos observados
fueron la reducción del aumento del peso corporal, diferencias en el
peso de los órganos, degeneración adiposa focal del hígado y cambios
hematológicos. Para esos efectos no fue posible determinar una
relación clara dosis-respuesta o concentraciones sin efectos
(adversos) observados (NO(A)EL) fidedignas.
El 2-nitrofenol es ligeramente irritante para la piel, pero no
para los ojos. En una prueba de Buehler no se observó efecto
sensibilizador. Tomando como base los estudios válidos con animales
experimentales, se supone que el 4-nitrofenol tiene efectos irritantes
en la piel y los ojos. En una prueba de maximización en cobayas, se
observó que el 4-nitrofenol era ligeramente sensibilizante. En el ser
humano no se puede excluir una posible sensibilización tras el
contacto con 4-nitrofenol, especialmente teniendo en cuenta que se ha
observado sensibilización cutánea en pruebas de parche en trabajadores
de fábricas que podían haber estado expuestos al 4-nitrofenol.
No se ha sometido a pruebas completas de genotoxicidad ninguno de
los dos isómeros del nitrofenol. Los datos disponibles sobre el
2-nitrofenol son insuficientes para poder sacar conclusiones acerca de
su posible mutagenicidad. Hay más estudios de mutagenicidad para el
4-nitrofenol, aunque algunos se notificaron de manera inadecuada. Hay
pruebas que parecen indicar que el 4-nitrofenol puede producir
aberraciones cromosómicas in vitro. En ausencia de estudios de
mutagenicidad in vivo en mamíferos, no es posible llegar a la
conclusión de si se expresa o no in vivo el potencial mutagénico del
4-nitrofenol.
Tras la aplicación cutánea de 4-nitrofenol a ratones durante 78
semanas no se observaron efectos carcinogénicos. En otro estudio con
ratones, que tiene varias limitaciones, no se detectaron tumores
cutáneos tras la aplicación en la piel de 2- ó 4-nitrofenol durante 12
semanas. Para ninguno de los isómeros había estudios de
carcinogenicidad utilizando la vía oral o la inhalación.
Los datos disponibles para el 4-nitrofenol no pusieron de
manifiesto efectos específicos o estadísticamente significativos de
toxicidad reproductiva o del desarrollo tras la administración cutánea
u oral a ratas y ratones. En un estudio de administración por vía oral
a ratas, el 2-nitrofenol indujo efectos en el desarrollo de la camada
sólo con dosis que también producían toxicidad materna. Sin embargo,
en estos estudios no se examinaron los fetos para investigar
malformaciones internas.
La base de datos para el 2-nitrofenol es enormemente limitada y
la relativa al 4-nitrofenol es insuficiente para deducir valores
fidedignos de la (NO(A)EL). Por consiguiente, es imposible determinar
en este momento la ingesta diaria tolerable o las concentraciones
tolerables para el 2- ó el 4-nitrofenol.
De los resultados disponibles de pruebas válidas sobre la
toxicidad del 2-y el 4-nitrofenol para diversos organismos acuáticos,
los nitrofenoles se pueden clasificar como sustancias con una
toxicidad entre moderada y alta en el compartimento acuático. Las
concentraciones más bajas con efectos obtenidas en estudios crónicos
con organismos de agua dulce ( Scenedesmus subspicatus, CE50: 0,39
mg de 2-nitrofenol/litro a las 96 h; Entosiphon sulcatum,
concentración inhibitoria mínima a las 72 horas: 83 mg de
4-nitrofenol/litro) fueron 40-50 veces superiores a los niveles
máximos determinados en una cuenca fluvial asiática densamente poblada
y muy industrializada (0,0072 mg de 2-nitrofenol/litro y 0,019 mg de
4-nitrofenol/litro). Por consiguiente, a pesar de la descomposición
biótica y fotoquímica, los nitrofenoles emitidos al agua pueden
representar algún peligro para los organismos acuáticos sensibles,
particularmente en las condiciones de las aguas superficiales que no
favorecen ambas vías de eliminación. Habida cuenta de sus pautas de
uso y sus características de liberación, probablemente los
nitrofenoles plantean sólo un pequeño riesgo para los organismos
acuáticos.
Los datos disponibles indican solamente una toxicidad potencial
moderada de los nitrofenoles en el medio ambiente terrestre. A partir
de los cálculos de la razón exposición-toxicidad de los nitrofenoles a
partir de la degradación de plaguicidas, sólo cabe esperar un pequeño
riesgo para los organismos en este compartimento, incluso en el peor
de los casos.
 Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
1. EXECUTIVE SUMMARY
This CICAD on the isomers 2-, 3-, and 4-nitrophenol was prepared
by the Fraunhofer Institute for Toxicology and Aerosol Research,
Hanover, Germany. It was based on reviews compiled by the German
Advisory Committee on Existing Chemicals of Environmental Relevance
(BUA, 1992) and the US Agency for Toxic Substances and Disease
Registry (ATSDR, 1992) to assess the potential effects of 2- and
4-nitrophenol on the environment and on human health. Data identified
up to 1992 were considered in these reviews. A comprehensive
literature search of several databases was conducted in 1998 to
identify any relevant references on 2- and 4-nitrophenol published
subsequent to those in the source documents and to identify all
references containing relevant data on the isomer 3-nitrophenol.
Information found on 3-nitrophenol was very scarce, precluding a
meaningful assessment. As a result, data on this isomer are
summarized in Appendix 1. Information on the nature of the peer review
and the availability of the source documents is presented in Appendix
2. Information on the peer review of this CICAD is presented in
Appendix 3. This CICAD was approved as an international assessment at
a meeting of the Final Review Board, held in Washington, DC, USA, on
8-11 December 1998. Participants at the Final Review Board meeting are
listed in Appendix 4. The International Chemical Safety Card (ICSC
1342) for mononitrophenols, produced by the International Programme on
Chemical Safety (IPCS, 1998), has also been reproduced in this
document.
The nitrophenol isomers are water-soluble solids that are
moderately acidic in water as a result of dissociation. 2-Nitrophenol
and 4-nitrophenol are used as intermediates in the synthesis of a
number of organophosphorus pesticides and some medical products.
Releases into the environment are primarily emissions into air, water,
and soil from diffuse sources, such as vehicle traffic and hydrolytic
and photolytic degradation of the respective pesticides. Further
releases into the hydrosphere and the geosphere are caused by the dry
and wet deposition of airborne nitrophenols from the atmosphere. The
photo-oxidative formation of 2- and 4-nitrophenol in the atmosphere is
still under discussion.
From the available data, only a slow rate of volatilization of
2-nitrophenol and no significant volatilization of 4-nitrophenol from
water to air are to be expected. 2-Nitrophenol is enriched in the
liquid phase of clouds, whereas more 4-nitrophenol than expected from
physicochemical data can be found in the gas phase owing to extensive
binding to particles. In view of the water solubilities and the
expected occurrence in the vapour phase, wet deposition of
nitrophenols from air to surface waters and soil is to be expected.
The major transformation pathway for 2-nitrophenol emitted to the
troposphere should be rapid nitration to 2,4-dinitrophenol, whereas
the major portion of airborne 4-nitrophenol is expected to be particle
bound and therefore only to a minor extent available for photochemical
reactions. Most of the 4-nitrophenol should be washed out from air by
wet and dry deposition. Nitrophenols are not considered to contribute
directly to the depletion of the stratospheric ozone layer or to
global warming. Measured half-lives for the photochemical
decomposition of 4-nitrophenol in water ranged from 2.8 to 13.7 days.
Numerous studies on the biodegradation of 2- and 4-nitrophenol
indicate the isomers to be inherently biodegradable in water under
aerobic conditions. Mineralization of nitrophenols under anaerobic
conditions requires extended adaptation of microbial communities.
Measured coefficients of soil sorption ( Koc) in the range of
44-530 indicate a low to moderate potential for soil sorption.
Nitrophenols released to soil should be biodecomposed under aerobic
conditions. Infiltration into groundwater is expected only under
conditions unfavourable to biodegradation. For 2- and 4-nitrophenol,
measured bioconcentration factors ranging from 11 to 76 indicate a low
potential for bioaccumulation.
There is only limited information concerning the toxicological
profiles of 2- and 4-nitrophenol. In experimental animals given
4-nitrophenol orally, intravenously, or intraperitoneally, most of the
applied dose was excreted via the urine within 24-48 h as glucuronide
and sulfate conjugates, while only very small amounts were excreted
via faeces or as unchanged 4-nitrophenol. The percentages of
glucuronide and sulfate conjugates were shown to be species and dose
dependent. After oral dosing in rabbits, 4-nitrophenol undergoes
reduction to p-aminophenol as well as glucuronidation and sulfation.
The available data from in vivo and in vitro studies give an
indication for dermal uptake of 4-nitrophenol. The data for
2-nitrophenol are very limited. However, based on the available data,
a comparable metabolic transformation is assumed. Bioaccumulation of
2- and 4-nitrophenol in organisms is not to be expected owing to their
rapid metabolism and excretion.
In acute studies, 4-nitrophenol is harmful after oral uptake and
was found to be more toxic than 2-nitrophenol. A dose-dependent
increase in the formation of methaemoglobin was seen in cats after
oral exposure to 2-nitrophenol and in rats after exposure by
inhalation to 4-nitrophenol. After repeated exposure to 4-nitrophenol,
the formation of methaemoglobin was shown to be the most critical
end-point for exposure by inhalation and is assumed to be relevant for
oral exposure too. Other noted effects included decreases in body
weight gain, differences in organ weights, focal fatty degeneration of
the liver, and haematological changes. For these effects, it was not
possible to identify a clear dose-response or reliable
no-observed-(adverse-)effect levels (NO(A)ELs).
2-Nitrophenol is slightly irritating to the skin but
non-irritating to the eye. The substance proved to have no sensitizing
effects in a Buehler test. Based on valid studies with experimental
animals, irritating effects on skin and eye are assumed for
4-nitrophenol. In a guinea-pig maximization test, 4-nitrophenol was
considered as slightly sensitizing. In humans, a possible
sensitization after contact with 4-nitrophenol cannot be excluded,
especially as skin sensitization has been found in patch tests on
factory workers who may have been exposed to 4-nitrophenol.
Neither of the two isomers of nitrophenol has been fully tested
for genotoxicity. Insufficient data are available on 2-nitrophenol to
allow any conclusions to be made about its possible mutagenicity. More
mutagenicity studies are available for 4-nitrophenol, although some
were inadequately reported. There is evidence to suggest that
4-nitrophenol can cause chromosomal aberrations in vitro. In the
absence of any in vivo mutagenicity studies in mammals, it is not
possible to conclude whether or not the mutagenic potential of
4-nitrophenol is expressed in vivo.
In mice, the dermal application of 4-nitrophenol for 78 weeks
gave no indication of carcinogenic effects. In another study with
mice, which has several limitations, no skin tumours were noted after
dermal application of 2- or 4-nitrophenol over 12 weeks.
Carcinogenicity studies using the oral or inhalation routes were not
available for either of the isomers.
For 4-nitrophenol, the available data gave no evidence of
specific or statistically significant reproductive or developmental
toxicity effects after dermal or oral application to rats and mice. In
an oral study with rats, 2-nitrophenol induced developmental effects
in the offspring only at doses that also produced maternal toxicity.
However, in these studies, the fetuses were not examined for internal
malformations.
The database for 2-nitrophenol is extremely limited, and the
database for 4-nitrophenol is insufficient for deriving reliable
NO(A)EL values. Therefore, at present, no tolerable daily intakes
(TDIs) or tolerable concentrations (TCs) can be derived for either
2- or 4-nitrophenol.
From valid test results available on the toxicity of 2- and
4-nitrophenol to various aquatic organisms, nitrophenols can be
classified as substances exhibiting moderate to high toxicity in the
aquatic compartment. The lowest effect concentrations found in chronic
studies with freshwater organisms ( Scenedesmus subspicatus, 96-h
EC50: 0.39 mg 2-nitrophenol/litre; Entosiphon sulcatum, 72-h
minimum inhibitory concentration, or MIC: 0.83 mg 4-nitrophenol/litre)
were 40-50 times higher than maximum levels determined in a densely
populated and highly industrialized Asian river basin (0.0072 mg
2-nitrophenol/litre and 0.019 mg 4-nitrophenol/litre). Therefore,
despite biotic and photochemical decomposition, nitrophenols emitted
to water could pose some risk to sensitive aquatic organisms,
particularly under surface water conditions not favouring both
elimination pathways. Because of their use patterns and release
scenarios, it is likely that nitrophenols pose only a minor risk to
aquatic organisms.
The available data indicate only a moderate toxicity potential of
nitrophenols in the terrestrial environment. From calculations of the
toxicity exposure ratio (TER) of nitrophenols from the degradation of
pesticides, only a minor risk for organisms in this compartment is to
be expected, even under a worst-case scenario.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
2-Nitrophenol (CAS No.
Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
1. EXECUTIVE SUMMARY
This CICAD on the isomers 2-, 3-, and 4-nitrophenol was prepared
by the Fraunhofer Institute for Toxicology and Aerosol Research,
Hanover, Germany. It was based on reviews compiled by the German
Advisory Committee on Existing Chemicals of Environmental Relevance
(BUA, 1992) and the US Agency for Toxic Substances and Disease
Registry (ATSDR, 1992) to assess the potential effects of 2- and
4-nitrophenol on the environment and on human health. Data identified
up to 1992 were considered in these reviews. A comprehensive
literature search of several databases was conducted in 1998 to
identify any relevant references on 2- and 4-nitrophenol published
subsequent to those in the source documents and to identify all
references containing relevant data on the isomer 3-nitrophenol.
Information found on 3-nitrophenol was very scarce, precluding a
meaningful assessment. As a result, data on this isomer are
summarized in Appendix 1. Information on the nature of the peer review
and the availability of the source documents is presented in Appendix
2. Information on the peer review of this CICAD is presented in
Appendix 3. This CICAD was approved as an international assessment at
a meeting of the Final Review Board, held in Washington, DC, USA, on
8-11 December 1998. Participants at the Final Review Board meeting are
listed in Appendix 4. The International Chemical Safety Card (ICSC
1342) for mononitrophenols, produced by the International Programme on
Chemical Safety (IPCS, 1998), has also been reproduced in this
document.
The nitrophenol isomers are water-soluble solids that are
moderately acidic in water as a result of dissociation. 2-Nitrophenol
and 4-nitrophenol are used as intermediates in the synthesis of a
number of organophosphorus pesticides and some medical products.
Releases into the environment are primarily emissions into air, water,
and soil from diffuse sources, such as vehicle traffic and hydrolytic
and photolytic degradation of the respective pesticides. Further
releases into the hydrosphere and the geosphere are caused by the dry
and wet deposition of airborne nitrophenols from the atmosphere. The
photo-oxidative formation of 2- and 4-nitrophenol in the atmosphere is
still under discussion.
From the available data, only a slow rate of volatilization of
2-nitrophenol and no significant volatilization of 4-nitrophenol from
water to air are to be expected. 2-Nitrophenol is enriched in the
liquid phase of clouds, whereas more 4-nitrophenol than expected from
physicochemical data can be found in the gas phase owing to extensive
binding to particles. In view of the water solubilities and the
expected occurrence in the vapour phase, wet deposition of
nitrophenols from air to surface waters and soil is to be expected.
The major transformation pathway for 2-nitrophenol emitted to the
troposphere should be rapid nitration to 2,4-dinitrophenol, whereas
the major portion of airborne 4-nitrophenol is expected to be particle
bound and therefore only to a minor extent available for photochemical
reactions. Most of the 4-nitrophenol should be washed out from air by
wet and dry deposition. Nitrophenols are not considered to contribute
directly to the depletion of the stratospheric ozone layer or to
global warming. Measured half-lives for the photochemical
decomposition of 4-nitrophenol in water ranged from 2.8 to 13.7 days.
Numerous studies on the biodegradation of 2- and 4-nitrophenol
indicate the isomers to be inherently biodegradable in water under
aerobic conditions. Mineralization of nitrophenols under anaerobic
conditions requires extended adaptation of microbial communities.
Measured coefficients of soil sorption ( Koc) in the range of
44-530 indicate a low to moderate potential for soil sorption.
Nitrophenols released to soil should be biodecomposed under aerobic
conditions. Infiltration into groundwater is expected only under
conditions unfavourable to biodegradation. For 2- and 4-nitrophenol,
measured bioconcentration factors ranging from 11 to 76 indicate a low
potential for bioaccumulation.
There is only limited information concerning the toxicological
profiles of 2- and 4-nitrophenol. In experimental animals given
4-nitrophenol orally, intravenously, or intraperitoneally, most of the
applied dose was excreted via the urine within 24-48 h as glucuronide
and sulfate conjugates, while only very small amounts were excreted
via faeces or as unchanged 4-nitrophenol. The percentages of
glucuronide and sulfate conjugates were shown to be species and dose
dependent. After oral dosing in rabbits, 4-nitrophenol undergoes
reduction to p-aminophenol as well as glucuronidation and sulfation.
The available data from in vivo and in vitro studies give an
indication for dermal uptake of 4-nitrophenol. The data for
2-nitrophenol are very limited. However, based on the available data,
a comparable metabolic transformation is assumed. Bioaccumulation of
2- and 4-nitrophenol in organisms is not to be expected owing to their
rapid metabolism and excretion.
In acute studies, 4-nitrophenol is harmful after oral uptake and
was found to be more toxic than 2-nitrophenol. A dose-dependent
increase in the formation of methaemoglobin was seen in cats after
oral exposure to 2-nitrophenol and in rats after exposure by
inhalation to 4-nitrophenol. After repeated exposure to 4-nitrophenol,
the formation of methaemoglobin was shown to be the most critical
end-point for exposure by inhalation and is assumed to be relevant for
oral exposure too. Other noted effects included decreases in body
weight gain, differences in organ weights, focal fatty degeneration of
the liver, and haematological changes. For these effects, it was not
possible to identify a clear dose-response or reliable
no-observed-(adverse-)effect levels (NO(A)ELs).
2-Nitrophenol is slightly irritating to the skin but
non-irritating to the eye. The substance proved to have no sensitizing
effects in a Buehler test. Based on valid studies with experimental
animals, irritating effects on skin and eye are assumed for
4-nitrophenol. In a guinea-pig maximization test, 4-nitrophenol was
considered as slightly sensitizing. In humans, a possible
sensitization after contact with 4-nitrophenol cannot be excluded,
especially as skin sensitization has been found in patch tests on
factory workers who may have been exposed to 4-nitrophenol.
Neither of the two isomers of nitrophenol has been fully tested
for genotoxicity. Insufficient data are available on 2-nitrophenol to
allow any conclusions to be made about its possible mutagenicity. More
mutagenicity studies are available for 4-nitrophenol, although some
were inadequately reported. There is evidence to suggest that
4-nitrophenol can cause chromosomal aberrations in vitro. In the
absence of any in vivo mutagenicity studies in mammals, it is not
possible to conclude whether or not the mutagenic potential of
4-nitrophenol is expressed in vivo.
In mice, the dermal application of 4-nitrophenol for 78 weeks
gave no indication of carcinogenic effects. In another study with
mice, which has several limitations, no skin tumours were noted after
dermal application of 2- or 4-nitrophenol over 12 weeks.
Carcinogenicity studies using the oral or inhalation routes were not
available for either of the isomers.
For 4-nitrophenol, the available data gave no evidence of
specific or statistically significant reproductive or developmental
toxicity effects after dermal or oral application to rats and mice. In
an oral study with rats, 2-nitrophenol induced developmental effects
in the offspring only at doses that also produced maternal toxicity.
However, in these studies, the fetuses were not examined for internal
malformations.
The database for 2-nitrophenol is extremely limited, and the
database for 4-nitrophenol is insufficient for deriving reliable
NO(A)EL values. Therefore, at present, no tolerable daily intakes
(TDIs) or tolerable concentrations (TCs) can be derived for either
2- or 4-nitrophenol.
From valid test results available on the toxicity of 2- and
4-nitrophenol to various aquatic organisms, nitrophenols can be
classified as substances exhibiting moderate to high toxicity in the
aquatic compartment. The lowest effect concentrations found in chronic
studies with freshwater organisms ( Scenedesmus subspicatus, 96-h
EC50: 0.39 mg 2-nitrophenol/litre; Entosiphon sulcatum, 72-h
minimum inhibitory concentration, or MIC: 0.83 mg 4-nitrophenol/litre)
were 40-50 times higher than maximum levels determined in a densely
populated and highly industrialized Asian river basin (0.0072 mg
2-nitrophenol/litre and 0.019 mg 4-nitrophenol/litre). Therefore,
despite biotic and photochemical decomposition, nitrophenols emitted
to water could pose some risk to sensitive aquatic organisms,
particularly under surface water conditions not favouring both
elimination pathways. Because of their use patterns and release
scenarios, it is likely that nitrophenols pose only a minor risk to
aquatic organisms.
The available data indicate only a moderate toxicity potential of
nitrophenols in the terrestrial environment. From calculations of the
toxicity exposure ratio (TER) of nitrophenols from the degradation of
pesticides, only a minor risk for organisms in this compartment is to
be expected, even under a worst-case scenario.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
2-Nitrophenol (CAS No.  Technical-grade 2- and 4-nitrophenol from the German producer
have a typical purity of >99%. Named impurities are the corresponding
isomer for each product (0.3%) and traces of 3-nitrochlorobenzene
(<0.05%). Polychlorinated dibenzo- p-dioxin/dibenzofuran (PCDD/PCDF)
and tetrachlorodibenzo- p-dioxin/dibenzofuran (TCDD/TCDF) isomers
were not detected at detection limits between 0.1 and 0.4 µg/kg
product (BUA, 1992).
The pure nitrophenol isomers form pale yellow to yellow crystals
at room temperature. The substances are characterized by the
physicochemical properties given in Table 1 (Sax & Lewis, 1987).
Additional physicochemical properties for mononitrophenols are
presented in the International Chemical Safety Card (ICSC 1342)
reproduced in this document.
Table 1: Physicochemical properties of 2- and 4-nitrophenol.
Parameter 2-Nitrophenol 4-Nitrophenol
Molecular mass (g/mol) 139.11 139.11
Melting point (°C) 44-45 (1)(2)(3) 113-114 (1)(2)(3)
Boiling point (°C) 214-217 (1) 279 (decomposition)(3)
Vapour pressure (kPa) 6.8 × 10-3
(19.8 °C) (4) 3.2 × 10-6
(20 °C) (5)
Water solubility 1.26 12.4
(g/litre) (20 °C) (4) (20 °C) (6)
n-Octanol/water
partition
coefficient (log Kow) 1.77-1.89 (7) 1.85-2.04 (7)
Dissociation constant 7.23 7.08
(pKa) (21.5 °C) (8) (21.5 °C) (8)
Ultraviolet spectrum deltamax (water): deltamax (methanol):
230; 276 nm; no absorption
log epsilonmax: 3.57; maxima <290 nm (9)
3.80 (9)
Conversion factors 1 mg/m3 = 0.173 ppmv
1 ppmv = 5.78 mg/m3
References: (1) Budavari et al. (1996); (2) Booth (1991);
(3) Verschueren (1983); (4) Koerdel et al. (1981);
(5) Sewekow (1983); (6) Andrae et al. (1981);
(7) BUA (1992); (8) Schwarzenbach et al. (1988); (9) Weast (1979)
3. ANALYTICAL METHODS
The nitrophenol isomers are usually determined by gas
chromatography combined with mass spectrometric detection, flame
ionization detection, electron capture detection, or
nitrogen-sensitive detection, which are generally applied after
derivatization (BUA, 1992; Nick & Schoeler, 1992; Geissler & Schoeler,
1994; Harrison et al., 1994; Luettke & Levsen, 1994; Mussmann et al.,
1994). For liquid samples (water, urine, blood), high-performance
liquid chromatography in combination with concentration-gradient
elution (acetonitrile/methanol or ammonium acetate, acetic acid with
potassium chloride/methanol) and ultraviolet or electrochemical
detection, which can be carried out without derivatization, is also
used (BUA, 1992; Nasseredine-Sebaei et al., 1993; Ruana et al., 1993;
Paterson et al., 1996; Pocurull et al., 1996; Thompson et al., 1996).
The separation of the different isomers is carried out either by steam
distillation (BUA, 1992) or by the formation and subsequent extraction
of different ion pairs (León-González et al., 1992).
The following enrichment techniques are used (BUA, 1992; see also
review by Puig & Barcelo, 1996):
* solid-phase adsorption with thermal or liquid extraction for air
and water samples (Luettke & Levsen, 1994; Mussmann et al., 1994)
* liquid/liquid extraction after derivatization for water samples
(initial purification by acid/base fractionation of highly
polluted samples, increased recovery rates with continuous
extraction methods) (León-González et al., 1992; Nick & Schoeler,
1992; Geissler & Schoeler, 1994; Harrison et al., 1994)
* liquid extraction with acid/base fractionation or solid-phase
enrichment and subsequent desorption following aqueous extraction
for soil samples (Vozñáková et al., 1996)
* acid hydrolysis of the glucuronide with subsequent
derivatization for blood and urine samples or denaturation
(Nasseredine-Sebaei et al., 1993; Thompson et al., 1996).
The detection limits are <10 ng/m3 for air, 0.03-10 µg/litre
for water, and 200-1600 µg/kg for soil. A detection limit for the
determination of the nitrophenol isomers in biological materials was
given only for rat liver perfusate (0.5-1 mg/litre; Thompson et al.,
1996).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
There are no known natural sources of the nitrophenol isomers.
Within the European Union, 2- and 4-nitrophenol are produced
mainly by three companies. Six other large manufacturers are known in
the USA and Japan (as of 1989). In 1983, the production volume for
Western Europe was estimated at about 6400 t 2-nitrophenol and about
20 500 t 4-nitrophenol. In 1988-89, the German production volumes
originating from one manufacturer were approximately 500 t
2-nitrophenol and about 2000 t 4-nitrophenol, with about 20 t of each
being exported. Both 2- and 4-nitrophenol are intermediates in the
synthesis of azo dyes and a number of pesticides, mainly insecticides
(2-nitrophenol: carbofuran, phosalon; 4-nitrophenol: parathion,
parathion-methyl, fluorodifen) and, to a lesser extent, herbicides
(4-nitrophenol: nitrofen, bifenox). The corresponding aminophenols
that are gained by reduction are used as a photographic developer
(2-aminophenol) and as an intermediate in the synthesis of the
tuberculostatic 4-aminosalicylic acid and the analgesic
4-acetaminophenol (paracetamol) (4-aminophenol) (see also Booth,
1991). In the 1980s, the production volumes for 2- and 4-nitrophenol
showed a decreasing tendency in Germany as a result of changes in and
termination of the production of some organophosphorus pesticides.
The releases of 2- and 4-nitrophenol during production and
processing at the only German manufacturer appear to be of minor
importance. In 1988-89, about 2.5 kg 2-nitrophenol and 10 kg
4-nitrophenol were emitted to air, and <93 kg 2-nitrophenol and
<64 kg 4-nitrophenol were emitted to surface water.
For 1996, the following releases of 2- and 4-nitrophenol to the
environment were reported by manufacturers in the USA (TRI, 1998):
* 2-nitrophenol: from three manufacturers (one production site
each) with production volumes between 450 and 45 000 kg/year,
total releases of 15 kg to air and 23 kg into water were
reported.
* 4-nitrophenol: from three manufacturers (six production sites)
with production volumes of 45-450 kg/year up to 45 000-450 000
kg/year, a total release of 420 kg to air was reported. Data on
releases into water were not given.
2-Nitrophenol and 4-nitrophenol have been detected in the exhaust
gases of light-duty gasoline and diesel vehicles. Depending on the
motor load, the exhaust concentrations of the isomers were <50 µg/m3
exhaust gas (idle) and about 1000 µg 4-nitrophenol/m3 and 2000 µg
2-nitrophenol/m3 (driving at constant velocity) (Nojima et al., 1983;
Tremp et al., 1993). A regulated three-way catalytic converter reduced
the nitrophenol emissions to about 8% at high motor load and to about
2% at normal motor load (Tremp et al., 1993). A rough estimation
combining the above-mentioned exhaust gas concentrations with
estimations of the total exhaust gas volumes from vehicle traffic for
Germany resulted in an airborne nitrophenol load of at least several
tonnes per year from this source (BUA, 1992). Data concerning
nitrophenol releases from other combustion processes (heating, burning
of refuse) were not identified.
From laboratory experiments, there is some evidence that 2- and
4-nitrophenol are generated in the atmosphere during the photochemical
degradation of aromatic compounds such as benzene and toluene in the
presence of nitric oxide or hydroxyl radicals and nitrous dioxide.
These results were at least partly obtained in model experiments with
unrealistically high nitric oxide concentrations, and there are
competing reactions without nitrophenol formation for which the rate
constant is not known (BUA, 1992). However, smog chamber experiments
confirmed the formation of nitrophenol isomers during irradiation
(Leone & Seinfeld, 1985; Leone et al., 1985). Recent cloud water model
experiments showed that 2- and 4-nitrophenol are also formed from the
reaction of phenol with nitrogen pentoxide or monochloronitrogen
dioxide, especially under alkaline conditions (Scheer et al., 1996).
Estimations of the contribution of photochemically formed nitrophenols
to total emissions into the atmosphere are not possible with the
available data.
Significant releases of 4-nitrophenol into the hydrosphere may
occur from the hydrolytic degradation of the insecticides parathion
and parathion-methyl and -- although to a lesser extent -- from the
photolytic degradation of the herbicides nitrofen and bifenox.
Quantification of releases is not possible with the available data.
Furthermore, a considerable portion of airborne nitrophenols,
especially 4-nitrophenol, can be released to the hydrosphere and the
geosphere by wet and dry deposition (see section 5) (Herterich &
Herrmann, 1990; Luettke et al., 1997). Numerous studies concerning the
concentrations of 2- and 4-nitrophenol in wet deposition samples are
available (see section 6.1). From precipitation data (average 746 mm
rain per year for land masses, according to Baumgartner & Liebscher,
1990) and the measured concentrations of 2- and 4-nitrophenol in
rainwater, the release of nitrophenols via rain can be estimated to be
at least in the order of several thousand tonnes per year on a global
basis.
The application of the herbicides nitrofen and bifenox, which are
photolytically degraded to 4-nitrophenol in aqueous solutions, may
especially lead to emissions into the geosphere and the biosphere.
Further, nitrophenol-contaminated rain, snow, and other wet and dry
deposition may contribute to nitrophenol levels in soils. Data
concerning the release of nitrophenols into the biosphere are not
available.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Environmental releases of nitrophenols are mostly to ambient air,
surface waters, and -- to a smaller extent -- soil. Using a
non-steady-state equilibrium model, the following distribution of
4-nitrophenol in different environmental compartments was predicted:
air, 0.0006%; water, 94.6%; sediment, 4.44%; soil, 0.95%; biota,
0.000 09% (Yoshida et al., 1983). The distribution patterns of 2- and
4-nitrophenol sprayed on a natural soil in a standardized terrestrial
ecosystem were determined via radiotracer technique (14C). Of the
applied radioactivity (2-nitrophenol/4-nitrophenol), 49.45%/20.01% was
recovered in air, 27.38%/40.21% in soil (including animals),
12.73%/7.57% in plants, and 0.05%/0.02% in leachate (Figge et al.,
1985). Distribution of 4-nitrophenol in a terrestrial microcosm
chamber with artificial soil largely corresponded to this result (Gile
& Gillett, 1981). Owing to the expected decomposition within the
incubation periods of 30 and 28 days, respectively, it can be assumed
that most of the recovered radioactivity referred to breakdown
products of the applied nitrophenols.
In volatility experiments conducted according to Organisation for
Economic Co-operation and Development (OECD) guidelines, half-lives of
2-nitrophenol in water ranged from 14.5 to 27.3 days, indicating a
slow rate of volatilization (Koerdel et al., 1981; Rippen et al.,
1984; Scheunert, 1984; Schoene & Steinhanses, 1984). Measurements
concerning the partitioning between the gas and liquid phases of
clouds during different rain events showed that 2-nitrophenol is
enriched in the liquid phase to a larger extent than would be
predicted from its water solubility and vapour pressure. On the other
hand, 4-nitrophenol is extensively adsorbed to particles. Therefore,
elevated levels of this isomer are detected in the gaseous phase of
clouds (Luettke et al., 1997). From the available data, a significant
volatilization of 4-nitrophenol from water to air is not expected.
Since nitrophenols dissociate in aqueous solution, volatilization may
further decrease with increasing pH in surface waters. This leads to
the conclusion that dry and wet deposition of nitrophenols from air to
surface waters and soil are to be expected. The occurrence of this
partition mechanism is supported by the detection of 2- and
4-nitrophenol in rainwater and wet deposition samples (see section
6.1).
From experimental results on direct photodegradation (Koerdel et
al., 1981) and the atmospheric photo-oxidation by hydroxyl radicals
(Zetzsch et al., 1984), both pathways were found to be of minor
importance for the removal of 2-nitrophenol emitted to the
troposphere. Thus, the major degradation pathway for airborne
2-nitrophenol should be rapid nitration to 2,4-dinitrophenol
(Herterich & Herrmann, 1990; Luettke et al., 1997). The major portion
of airborne 4-nitrophenol is expected to be particle bound and
therefore only to a minor extent available for photochemical
reactions. Thus, most of the 4-nitrophenol can be washed out from air
by wet and dry deposition. Measured half-lives for the photochemical
decomposition of 4-nitrophenol in water exposed to sunlight ranged
from 2.8 to 13.7 days (Hustert et al., 1981; Mansour, 1996), being
longer with increasing pH (Hustert et al., 1981). Traces of
4-aminophenol were found as a photoproduct in river water (Mansour,
1996). In experiments conducted according to OECD guidelines, Andrae
et al. (1981) and Koerdel et al. (1981) found no hydrolysis of 2- or
4-nitrophenol under environmental conditions.
Numerous studies on the biodegradation of 2- and 4-nitrophenol
have been conducted. Standardized tests on ready or inherent
biodegradability provide data of large variability, indicating 2- and
4-nitrophenol to be inherently biodegradable under aerobic conditions
(depending on origin and density of inoculum and the applied test
method) (see Table 2). Results from different tests point to a
possible bacteriotoxic effect of 4-nitrophenol at concentrations above
300 mg/litre (Gerike & Fischer, 1979; Nyholm et al., 1984; Kayser et
al., 1994).
Non-standardized experiments with different inocula (e.g.,
natural water, soil, sediment) showed that microbial decomposition of
nitrophenols can occur in different environmental compartments after
adaptation of the microflora (Rubin et al., 1982; Subba-Rao et al.,
1982; Van Veld & Spain, 1983; Spain et al., 1984; Ou, 1985; Hoover et
al., 1986; Aelion et al., 1987; Wiggins et al., 1987). Time for
acclimation and degree of removal depended mostly on substance
concentration, microbial population, climate, and additional
substrates.
Biotic degradation of nitrophenols under anaerobic conditions
requires extended acclimatization of microbial communities. In tests
with sewage sludge and sludge from the primary anaerobic stage of a
municipal sewage treatment plant, respectively, initial 2- and
4-nitrophenol concentrations in the range of 96.5-579 mg/litre were
not degraded at all within 7-60 days (Wagner & Braeutigam, 1981;
Battersby & Wilson, 1989). Boyd et al. (1983) found complete anaerobic
removal of 50 mg/litre for all nitrophenol isomers within 1 week, but
complete mineralization was demonstrated only if the incubation period
was extended to 10 weeks. Anaerobic degradation even of high initial
nitrophenol concentrations was found by Tseng & Lin (1994), who
observed >90% removal of 2- and 4-nitrophenol (350-650 mg/litre) in a
biological fluidized bed reactor with three different kinds of
wastewater. From the available results, a slow degradation of
nitrophenols under anaerobic conditions by adapted microorganisms can
be expected.
Table 2: Biotic degradation of nitrophenols under aerobic conditions.
Test Substance Concentration Additional Test duration Removal Reference
(mg/litre) carbon source (days) (%)
Tests on ready biodegradability
AFNOR test 2-NP 40 OC no 14 16 Gerike & Fischer (1979)
Sturm test 2-NP 10 no 28 32 Gerike & Fischer (1979)
MITI I 2-NP 100 no 14 0 Urano & Kato (1986)
50 no 14 7 Gerike & Fischer (1979)
Closed bottle 4-NP 2 no 28 55 Rott et al. (1982)
test
Modified OECD 4-NP 20 DOC no 28 1 Rott et al. (1982)
screening test
Shake flask test 4-NP 20 OC no 21 50 Means & Anderson (1981)
AFNOR test 4-NP 40 OC no 14 97 Gerike & Fischer (1979)
Sturm test 4-NP 10 no 28 90 Gerike & Fischer (1979)
MITI I 4-NP 50 no 14 1 Gerike & Fischer (1979)
100 no 14 0 Urano & Kato (1986)
100 no 14 4.3 CITI (1992)
Tests on inherent biodegradability
Zahn-Wellens 2-NP 400 no 14 80 Gerike & Fischer (1979)
test
SCAS test 2-NP 20 TOC yes 24 107 Broecker et al. (1984)
13.3 TOC yes 24 110
Table 2 (continued)
Test Substance Concentration Additional Test duration Removal Reference
(mg/litre) carbon source (days) (%)
Bunch & Chambers 2-NP 5-10 yes 28 100 Tabak et al. (1981)
Coupled units 2-NP 12 OC yes 7 61 Gerike & Fischer (1979)
test
Batch test, 2-NP 200 COD no 5 97 Pitter (1976)
aerated
Zahn-Wellens 4-NP 300 no 14 8 Andrae et al. (1981)
test 100 DOC no 28 100 Pagga et al. (1982)
Activated sludge 4-NP 50 no 19 100 Means & Anderson
test 100 no 19 90 (1981)
SCAS test 4-NP 20 TOC yes 33 >90 Marquart et al. (1984)
27 >97 Scheubel (1984)
25/39 100 Ballhorn et al. (1984)
12-15 100 Koerdel et al. (1984)
Coupled units 4-NP 12 OC yes 7 100 Gerike & Fischer
test (1979)
Batch test, 4-NP 200 COD no 5 95 Pitter (1976)
aerated
Abbreviations used: 2-NP = 2-nitrophenol; 4-NP = 4-nitrophenol; OC = organic carbon; DOC = dissolved organic carbon;
TOC = total organic carbon; COD = chemical oxygen demand.
Soil sorption coefficients ( Koc) were found to increase with
increasing organic carbon content. Measured Koc values ranged from
44 to 230 (2-nitrophenol) and from 56 to 530 (4-nitrophenol) (Boyd,
1982; Broecker et al., 1984; Koerdel et al., 1984; Lokke, 1984;
Marquart et al., 1984). Nitrophenols emitted to soil are expected to
be biodecomposed under aerobic conditions. Infiltration into
groundwater is expected only under conditions unfavourable for
biodegradation (e.g., anaerobic conditions). From the available
experimental results, nitrophenols have to be classified as substances
with a low to moderate potential for soil sorption.
A low potential for bioaccumulation is to be expected from the
available valid test results for 2- and 4-nitrophenol.
Bioconcentration factors ranging from 14.6 to 24.4 were determined for
2-nitrophenol in a semistatic test system with zebra fish
( Brachydanio rerio) (Koerdel et al., 1984); in a flow-through
experiment, bioconcentration factors ranged from 30 to 76 for common
carp ( Cyprinus carpio), including possible conjugates (Broecker et
al., 1984). In static tests, accumulation factors for 4-nitrophenol of
11 for the green alga Chlorella fusca after 1 day (Geyer et al.,
1981) and 57 for the freshwater golden orfe ( Leuciscus idus
melanotus) after 3 days of exposure were determined (Freitag et al.,
1982). Zebra fish exposed in tap and river water nearly completely
eliminated the accumulated 14C-4-nitrophenol within 48 h (Ensenbach &
Nagel, 1991). Star fish ( Pisaster ochraceus) and sea urchin
( Strongylocentrotus purpuratus) eliminated 89% and 36%,
respectively, of injected 14C-4-nitrophenol (3.48 and 3.70 mg/kg body
weight, respectively) within 8 h (Landrum & Crosby, 1981).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
From the concentrations in rainwater, the total atmospheric
nitrophenol pollution in Switzerland is estimated at about 1 µg/m3
(Leuenberger et al., 1988). Recent measurements in the air of remote
areas in Europe (German Alps, Fichtelgebirge, Germany; Mount Brocken,
Germany; Great Dun Fell summit, United Kingdom) gave 2-nitrophenol
concentrations between 0.8 and 25 ng/m3 and 4-nitrophenol levels
between 1.2 and 360 ng/m3 (Herterich & Herrmann, 1990; Luettke et
al., 1997). The higher atmospheric 4-nitrophenol levels are apparently
due to the higher photochemical stability of this isomer (see section
5). 2-Nitrophenol was found in 22 out of 27 samples of air (range
1-140 ng/m3; detection limit 1 ng/m3) in Japan in 1994, and
4-nitrophenol was detected in 27 out of 27 air samples (range 1-71
ng/m3; detection limit 1 ng/m3) (Japan Environment Agency, 1995).
In street dust samples from a Japanese city, up to 3.9 mg
2-nitrophenol/kg and up to 42 mg 4-nitrophenol/kg were detected
(Nojima et al., 1983).
Numerous studies deal with the distribution, deposition, and
degradation behaviour of airborne 2- and 4-nitrophenol in clouds and
rainwater. 2-Nitrophenol levels in rainwater and snow between 0.03 and
5.7 µg/litre and 4-nitrophenol concentrations from <0.5 to
19 µg/litre are given in reports mainly from Germany and the USA (BUA,
1992). The recent measurements in rainwater, cloud water, and "fog"
(water vapour; not further characterized) from rural and urban areas
in Europe confirm these concentration ranges (Herterich & Herrmann,
1990; Levsen et al., 1990; Richartz et al., 1990; Capel et al., 1991;
Geissler & Schoeler, 1993; Levsen et al., 1993; Luettke et al., 1997).
The 2-nitrophenol levels are mostly below or slightly above the
detection limit (i.e., <0.1 µg/litre), whereas mean 4-nitrophenol
concentrations of about 5 µg/litre rainwater and cloud water and 20
µg/litre fog water were detected. The nitrophenol concentrations in
fog are significantly higher than those in rainwater or cloud water
owing to the higher droplet surface and longer residence times of the
droplets in air compared with rain. The lower concentrations of
2-nitrophenol in the deposition samples compared with 4-nitrophenol
are presumably due to the lower photochemical stability of this
compound (see section 5).
In the 1970s and early 1980s, the 2- and 4-nitrophenol
concentrations in the German and Dutch parts of the river Rhine and
some of its tributaries were between 0.1 and 1 µg/litre (BUA, 1992).
2-Nitrophenol and 4-nitrophenol were not detected in 177 samples of
Japanese surface waters (detection limits 0.04-10 µg/litre) or in 177
sediment samples (detection limits between 0.002 and 0.8 µg/kg) in
1978, 1979, and 1994 (Japan Environment Agency, 1979, 1980, 1995).
Whereas 4-nitrophenol was not detected in 129 fish samples (detection
limits 0.005-0.2 µg/kg) in Japan in 1979 and 1994, 2-nitrophenol was
detected in 1 out of 129 saltwater fish samples (detection limits
0.005-0.3 µg/kg) in 1994 (Japan Environment Agency, 1980, 1995).
2-Nitrophenol concentrations between <0.15 µg/litre (detection limit)
and 7.2 µg/litre and 4-nitrophenol levels between <0.1 and 18.8
µg/litre were reported for the densely populated and highly
industrialized Malaysian Klang river basin in 1990 and 1991 (Tan &
Chong, 1993).
6.2 Human exposure
Workers may be exposed to 2- and 4-nitrophenol via inhalation and
skin contact during production and processing (mainly in the
manufacturing of pesticides). However, data on nitrophenol
concentrations at the workplace were not identified.
Based on the measured concentrations given in section 6.1, an
exposure of the general population to nitrophenols via the environment
-- predominantly through ambient air and drinking-water -- cannot be
excluded.
4-Nitrophenol accumulates in fog, whereas 2-nitrophenol is
rapidly photochemically transformed (see sections 5 and 6.1). The mean
measured level of 4-nitrophenol in fog water is about 20 µg/litre.
In Dutch drinking-water samples, maximum concentrations of 1 µg
2-nitrophenol/litre and <0.1 µg 4-nitrophenol/litre were reported in
1988 (BUA, 1992). Further data are not available.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND
HUMANS
Studies providing quantitative information on the absorption,
metabolism, or elimination of 2- or 4-nitrophenol in humans were not
identified.
7.1 2-Nitrophenol
There is only very limited information available for
2-nitrophenol. In rabbits given a single dose of 200-330 mg/kg body
weight via gavage, most of the applied dose (>>80%) was excreted via
the urine within 24 h. About 71% was conjugated with glucuronic acid
and about 11% with sulfate, whereas about 3% was reduced to
aminophenols (Robinson et al., 1951).
Skin permeation for 2-nitrophenol was shown in several in vitro
experiments (Huq et al., 1986; Jetzer et al., 1986; Ohkura et al.,
1990).
Although the information is limited, bioaccumulation of
2-nitrophenol in organisms is not to be expected owing to its rapid
metabolism and excretion.
7.2 4-Nitrophenol
After oral, dermal, intravenous, or intraperitoneal application
of 4-nitrophenol to several test species (rats, mice, dogs, or
rabbits), most of the applied dose (up to 95%) was excreted as
glucuronide and sulfate conjugates of 4-nitrophenol via the urine
within 24-48 h. Only small amounts were excreted via faeces (about 1%)
or as unchanged 4-nitrophenol (about 2-7%). The percentages of
glucuronide and sulfate conjugates were shown to be species, sex, and
dose dependent. Although sulfate conjugation dominates at lower
4-nitrophenol concentrations, the percentage of glucuronide conjugates
increases at higher dosages (Robinson et al., 1951; Gessner & Hamada,
1970; Machida et al., 1982; Rush et al., 1983; Snodgrass, 1983;
Tremaine et al., 1984; Meerman et al., 1987). As shown in rabbits
after oral dosing, 4-nitrophenol undergoes reduction to 4-aminophenol
as well as glucuronidation and sulfation. Up to 14% of the
administered dose was detected as amino compounds in the urine
(Robinson et al., 1951). After intraperitoneal administration in mice,
4-nitrophenyl glucoside was identified as a minor metabolite of
4-nitrophenol (about 1-2% of the administered dose) (Gessner & Hamada,
1970).
For 4-nitrophenol, the pretreatment of laboratory animals with
ethanol (induction of cytochrome P-450) resulted in a marked increase
in hepatic microsomal hydroxylation. The 4-nitrocatechol then formed
competed with 4-nitrophenol for the glucuronidation and sulfation
pathways (Reinke & Moyer, 1985; Koop, 1986; McCoy & Koop, 1988; Koop &
Laethem, 1992).
Specific investigations on dermal resorption under non-occlusive
conditions showed dermal uptake of about 35% and 11% of the applied
dose of 14C-4-nitrophenol within 7 days in rabbits and dogs,
respectively. Skin permeation for 4-nitrophenol was also shown in
several in vitro experiments (Huq et al., 1986; Jetzer et al., 1986;
Ohkura et al., 1990).
Owing to its rapid metabolism and excretion, bioaccumulation of
4-nitrophenol in organisms is not to be expected.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
For 2-nitrophenol, the oral LD50 is in the range of
2830-5376 mg/kg body weight in rats (BASF AG, 1970; Vasilenko et al.,
1976; Vernot et al., 1977; Koerdel et al., 1981) and 1300-2080 mg/kg
body weight in mice (Vasilenko et al., 1976; Vernot et al., 1977).
Clinical signs following oral exposure were unspecific and included
dyspnoea, staggering, trembling, somnolence, apathy, and cramps. The
macroscopic examination performed in some studies revealed congestion
in liver and kidneys and ulcers of the stomach in high-dose rats. The
inhalation exposure of rats to an atmosphere saturated with the test
substance at 20°C for 8 h (no further information available) resulted
in no mortality and no signs of toxicity (BASF AG, 1970). In a limit
test, the dermal LD50 for the rat was >5000 mg/kg body weight
(Koerdel et al., 1981). In cats (two animals per dose group), the oral
application of 2-nitrophenol (50, 100, or 250 mg/kg body weight; no
controls) resulted in a dose-dependent increase in methaemoglobin (6,
44, and 57%, respectively).1 One animal dosed with 250 mg/kg body
weight died. No formation of methaemoglobin was detected after dermal
application of a 50% solution of 2-nitrophenol in water to rabbits
(dose not specified, exposure time 1 min to 20 h on the back or 20 h
on the ear) (BASF AG, 1970).
The oral LD50 of 4-nitrophenol is in the range of 220-620 mg/kg
body weight in rats (BASF AG, 1969; Vasilenko et al., 1976; Hoechst
AG, 1977a; Vernot et al., 1977; Andrae et al., 1981) and 380-470 mg/kg
body weight in mice (Vasilenko et al., 1976; Vernot et al., 1977).
Clinical signs following oral exposure of rats were unspecific and
included tachypnoea and cramps, and the macroscopic examination
performed in some studies revealed a greyish discoloration with dark
red patches of the lungs. No mortality was observed in rats after
single exposure (head only) to 4700 mg/m3 (application as dust
[sodium salt]; particle size not given) for 4 h. In four of six rats,
a corneal opacity was observed at the end of exposure, which persisted
through the 14-day observation period. In two extra rats exposed to
1510 mg/m3, the methaemoglobin concentrations were not altered
compared with controls. A determination of methaemoglobin
concentrations after exposure to 4700 mg/m3 was not performed (Smith
et al., 1988). In another inhalation study with rats (exposure to an
atmosphere saturated with the test substance at 20°C for 8 h; no
further information available), no mortality and no signs of toxicity
1 Methaemoglobin formation is discussed in greater detail in
section 8.8.
were seen (BASF AG, 1969). The dermal LD50 for rats and guinea-pigs
is >1000 mg/kg body weight (Hoechst AG, 1977b; Eastman Kodak Co.,
1980; Andrae et al., 1981). In contrast to 2-nitrophenol, no formation
of methaemoglobin was noted in cats (two animals per dose group) after
oral dosing with 100, 200, or 500 mg 4-nitrophenol/kg body weight. The
mortality rate was 0/2, 1/2, and 2/2, respectively (BASF AG, 1969).
8.2 Irritation and sensitization
From studies comparable to OECD Guidelines 404 and 405, it can be
concluded that 2-nitrophenol is slightly irritating to the skin but
not to the eye (scores not given). In a Buehler test with guinea-pigs
comparable to OECD Guideline 406, the substance showed no
skin-sensitizing effects (Koerdel et al., 1981).
In a study performed according to US Food and Drug Administration
(FDA) guidelines, non-dissolved 4-nitrophenol was slightly irritating
to the skin (score 2 of 8) (Hoechst AG, 1977c); in another study
comparable to OECD Guideline 404, however, the non-dissolved substance
showed no skin-irritating effects (score 0 of 4) (Andrae et al.,
1981). 4-Nitrophenol applied as a 10% solution to the eyes was
slightly irritating in a test conducted according to FDA guidelines
(scores not given; Hoechst AG, 1977c). Results with the non-dissolved
substance were either strongly irritating in a test conducted
according to FDA guidelines (scores not given; Hoechst AG, 1977c) or
slightly irritating in a test comparable to OECD Guideline 405 (score
1-2 of 4; Andrae et al., 1981).
In a guinea-pig maximization test comparable to OECD Guideline
406, skin sensitization was shown in 5 of 20 animals (Andrae et al.,
1981).
Data on respiratory tract sensitization for 2- and 4-nitrophenol
were not identified in the literature.
8.3 Short-term exposure
8.3.1 Oral exposure
The effect of 2-nitrophenol in rats was studied in a 28-day study
to evaluate OECD Guideline 407 (five animals per sex per dose group;
daily oral doses of 0, 22, 67, or 200 mg/kg body weight via gavage).
Food intake decreased in high-dose males and in mid- and high-dose
females, and final body weight decreased non-significantly in all
dosed animals. The absolute liver and kidney weights were decreased in
mid-dose animals, and the relative testes weight increased in low- and
mid-dose males and decreased in high-dose males. In all dosed animals,
the relative and absolute weights of the adrenal glands increased. The
haematological examination, clinical chemistry, and histopathological
examination of the major organs and tissues did not give any
indication of a substance-related toxic effect in comparison with
controls (Koerdel et al., 1981). Owing to insufficient documentation
and the fact that there were minor effects (weight of adrenal glands)
shown by all exposed animals, a reliable NO(A)EL cannot be deduced.
In a 28-day study that was also conducted to evaluate OECD
Guideline 407, Sprague-Dawley rats (10 per sex per dose group)
received daily oral doses of 0, 70, 210, or 630 mg 4-nitrophenol/kg
body weight via gavage. After dosing, locomotor inhibition, which
lasted for about 2 h, was seen in mid- and high-dose animals. In
mid-dose animals, 1/10 males died; in high-dose males and females, the
mortality rate was 4/10 and 6/10, respectively (specific signs of
intoxication were not given). In the lowest dose group, the
macroscopic examination revealed seven cases of pale liver, and the
histopathological examination showed 14 cases of finely dispersed
fatty degeneration. A focal fatty degeneration of the liver was also
observed in 13/20 rats of the mid-dose group, but not in high-dose
animals. However, it must be noted that finely dispersed fatty
degeneration was also seen in 6/20 control animals. In 4/10 high-dose
males but not females, a hydropic liver cell swelling was noted, and
all high-dose rats that died before the end of the study showed
vascular congestion of the liver. A slight increase in the leukocyte
count was seen at 210 and 630 mg/kg body weight in males and females;
the increase was significant in high-dose females. In high-dose males,
the alanine aminotransferase (ALAT) activity was significantly
increased. Other substance-related effects in high-dose animals
included increased nephrosis (two males and five females), testicular
atrophy and inhibition of spermatogenesis (one and two males,
respectively), and follicular atresia in the ovaries (four females)
(Andrae et al., 1981). Because of unclear effects in the liver, a
NO(A)EL cannot be deduced.
8.3.2 Inhalation exposure
8.3.2.1 2-Nitrophenol
In Sprague-Dawley rats (15 per sex per group), no mortality was
observed after exposure to 0, 5, 30, or 60 mg 2-nitrophenol vapour/m3
("whole body" exposure; to generate the vapour, melted 2-nitrophenol
was used) for 6 h/day, 5 days/week, over a period of 4 weeks. Except
for squamous metaplasia of the epithelium lining the maxilloturbinates
and nasoturbinates in all high-dose animals, the clinical and
histopathological examinations gave no consistent exposure-related
effects. The methaemoglobin values determined after the 11th exposure
were significantly increased only in low-dose animals (males: 1.0,
2.3, 1.8, and 1.6%; females: 2.0, 4.1, 2.1, and 1.1%), but were within
control values at the end of the study (Hazleton Lab., 1984).
8.3.2.2 4-Nitrophenol
No mortality was observed in male albino Crl:CDR rats (10 per
group) after exposure to 0, 340, or 2470 mg 4-nitrophenol dust/m3
(application as sodium salt; "head only" exposure; mass median
aerodynamic diameter [MMAD] 4.6-7.5 µm) for 6 h/day, 5 days/week, over
a period of 2 weeks. Both exposure concentrations resulted in signs of
irritation (not further specified). After exposure to 340 and 2470
mg/m3, darker urine, proteinuria, elevated aspartate aminotransferase
(ASAT) values, and a dose-dependent increase in methaemoglobin values
were observed. These effects were still evident after a 14-day
recovery period; however, the methaemoglobin value was then still
elevated in only 2/5 high-dose animals. The methaemoglobin values were
0.2, 0.87, and 1.53% after 10 exposures and 0.2, 0.13, and 0.7% after
14 days' recovery. The erythrocyte, haemoglobin, and haematocrit
values decreased during exposure but were elevated after the 14-day
recovery period. In treated rats, the urine volume decreased in a
dose-dependent manner during exposure and during the 14-day recovery
period. In high-dose animals, the absolute spleen weight was
significantly lower than that of controls after 10 exposures, and the
absolute/relative spleen and lung weights were significantly lower in
comparison with controls at the end of the recovery period. According
to the authors, the biological significance of the changes in organ
weights is unknown owing to the absence of corroborating pathological
effects (Smith et al., 1988).
In a second trial (exposure to 0, 30, or 130 mg/m3; MMAD 4.0-4.8
µm), both exposure concentrations again resulted in signs of
irritation (not further specified). Methaemoglobinaemia, an effect
that was reversible within a 14-day recovery period, was seen only at
130 mg/m3. The methaemoglobin values were 0.5, 0.3, and 1.5% after 10
exposures and 0.4, 0.5, and 0.2% after 14 days' recovery. The gross
and histopathological examination revealed no adverse effects in any
dose group. From these results, the authors of the study decided upon
a NO(A)EL of 30 mg/m3 (Smith et al., 1988).
Groups of Sprague-Dawley rats (15 per sex) were exposed to 0, 1,
5, or 30 mg 4-nitrophenol dust/m3 ("whole body" exposure; MMAD
5.2-6.7 µm) for 6 h/day, 5 days/week, over a period of 4 weeks. The
exposure resulted in no deaths, and no exposure-related effects were
noted in terms of haematology or clinical chemistry values, gross
examination, histopathology, and body or organ weights. In high-dose
animals, unilateral and bilateral diffuse anterior capsular cataracts
were observed. The methaemoglobin values determined after 2 weeks of
exposure showed great variability and appeared to be unusually high
(>3 %) in some unexposed controls. However, the group total
methaemoglobin value was increased at a concentration of 5 mg/m3,
which was significant in males and not significant in females (males:
0.8, 0.5, 2.2, and 1.1%; females: 1.3, 1.1, 2.0, and 1.0%) (Hazleton
Lab., 1983). Therefore, a NO(A)EL of 5 mg/m3 can be derived for local
effects (cataracts), whereas the NO(A)EL for systemic effects
(formation of methaemoglobin) may be lower.
8.3.3 Dermal exposure
Data concerning short-term dermal exposure were not identified in
the literature.
8.4 Long-term exposure
In the literature, subchronic and chronic studies are available
only for 4-nitrophenol.
8.4.1 Subchronic exposuren
In a 13-week gavage study with Sprague-Dawley rats (20 per sex
per dose group) given 0, 25, 70, or 140 mg 4-nitrophenol/kg body
weight in water 5 days/week, premature deaths were seen in animals
dosed with 70 and 140 mg/kg body weight (1 male/1 female at 70 mg/kg
body weight and 15 males/6 females at 140 mg/kg body weight); these
were usually preceded by clinical signs, including pale appearance,
languid behaviour, prostration, wheezing, and dyspnoea, shortly after
dosing. The histopathological examination of these animals revealed
minimal to moderately severe congestion in the lung, liver, kidney,
adrenal cortex, and pituitary; in surviving animals, no
treatment-related changes compared with controls were reported. A
statement concerning altered methaemoglobin values cannot be given
owing to a non-reliable analytical method (about 13% in controls at
week 7) (Hazleton Lab., 1989). Therefore, only a provisional NO(A)EL
(changes in liver, kidneys, and lungs) of 25 mg/kg body weight can be
derived from this study. The NO(A)EL based on the formation of
methaemoglobin may be lower.
The dermal application of 4-nitrophenol to Swiss-Webster mice (10
per sex and dose group; given 0, 22, 44, 88, 175, or 350 mg/kg body
weight in acetone, 3 times per week over 13 weeks) resulted in
dose-dependent mortality as well as skin irritation/inflammation and
necrosis at >175 mg/kg body weight.1
1 Gulf South Research Institute, not dated;
no further information available; results cited from
NTP (1993).
8.4.2 Chronic exposure and carcinogenicityn
In a long-term study with Swiss-Webster mice (50 per sex per dose
group), 4-nitrophenol in acetone was applied to the interscapular skin
at doses of 0, 40, 80, or 160 mg/kg body weight, 3 days/week for 78
weeks. At termination of the study, the survival rates were 29/60,
17/60, 26/60, and 24/60 for males and 35/60, 26/60, 33/60, and 27/60
for females. The increased mortality after 60 weeks was due to a
generalized amyloidosis (the severity of the amyloidosis was similar
among dosed and control animals) and secondary kidney failure. The
final mean body weights of the dosed animals were similar to those of
the controls. NTP (1993) stated that there were no substance-related
neoplastic or non-neoplastic effects associated with the dermal
administration of 4-nitrophenol and that there was no evidence of a
carcinogenic activity of the substance in male or female mice.
In another study, which had several procedural deficiencies (only
the skin was examined; only 12 weeks of exposure), no skin tumours
were observed in 31 female Sutter mice after dermal application of a
20% solution (25 µl of solution applied twice weekly) of 2- or
4-nitrophenol in dioxane (Boutwell & Bosch, 1959).
8.5 Genotoxicity and related end-points
The available in vitro and in vivo genotoxicity studies on
2- and 4-nitrophenol are summarized in Table 3.
2-Nitrophenol showed no mutagenicity in several limited bacterial
assays. From the available data, it is not possible to draw any
conclusions regarding its mutagenicity.
For 4-nitrophenol, positive results were obtained in in vitro
tests for chromosomal aberrations in mammalian cells. However, apart
from one well-documented study published by NTP (1993), the other
available assays were inadequately reported. 4-Nitrophenol was shown
to be mutagenic in some but not all of the bacterial assays, whereas
other studies (i.e., host-mediated bacterial assay, mouse lymphoma
assay, unscheduled DNA synthesis assay [apparently in vitro], sister
chromatid exchange assay, sex-linked recessive lethal [SLRL] assay in
Drosophila) gave negative results. In the absence of any in vivo
mutagenicity studies in mammals, it is not possible to conclude
whether or not the mutagenic potential of 4-nitrophenol is expressed
in vivo.
Table 3: Genotoxicity of 2- and 4-nitrophenol in vitro and in vivo.
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
2-Nitrophenol
(in vitro studies)
delta phage DNA Induction of 35 mg - 0 Yamada et al.
DNA breakage (1987)
Bacillus subtilis Recombination 0.01-0.5 - 0 Shimizu & Yano
H17, M45 assay mg/plate (1986)
Salmonella Reverse 0.003-2.5 - - Koerdel et al. (1981);
typhimurium mutations mg/plate Haworth et al. (1983);
TA1535, TA1537 Shimizu & Yano (1986)
Salmonella Reverse 0.01-2.5 - - Koerdel et al. (1981);
typhimurium mutations mg/plate Shimizu & Yano (1986)
TA1538
Salmonella Reverse 0.0007-5 - - Suzuki et al. Chiu et al. (1978);
typhimurium mutations mg/plate (1983) also Koerdel et al. (1981);
TA98, TA100 tested both Haworth et al. (1983);
strains in Suzuki et al. (1983);
the presence Shimizu & Yano (1986);
of norharman, Kawai et al. (1987);
which also Dellarco & Prival (1989);
gave negative Massey et al. (1994)
results
Table 3 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
2-Nitrophenol (in vivo studies)
Drosophila SLRL assay via feed (400 and 500 - Foureman et al. (1994)
melanogaster ppm) or injection
(2500 and 5000 ppm)
4-Nitrophenol (in vitro studies)
delta phage DNA Induction of 35 mg - 0 Yamada et al. (1987)
DNA breakage
Bacillus subtilis Recombination 0.01-5 mg/plate + 0 positive Shimizu
H17, M45 assay at 0.5 & Yano (1986)
mg/plate
Escherichia coli Gene mutation 0.001-2.5 - - Hoechst AG (1980)
WP2uvrA mg/plate
Escherichia coli Gene mutation 0.125-2 mg/plate - 0 Rashid & Mumma (1986)
K-12 (Pol
A1+/Pol1-), WP2
(WP2, WP2uvrA,
WP67, CM611,
CM571)
Escherichia coli DNA cell 7 or 70 mg + + positive at Kubinski et al.
Q13 binding assay 70 mg (1981)
Table 3 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
Saccharomyces Mitotic gene 2.9 mg/ml (+) 0 Fahrig (1974)
cerevisiae conversion
ade 2, trp 5
Salmonella DNA damage up to 0.75 - - Nakamura et al.
typhimurium (umu test) mg/ml (1987)
TA1535/pSK 1002
Salmonella Reverse 0.001-2.5 + - positive Hoechst
typhimurium mutation mg/plate at >0.1 AG (1980)
TA1538 mg/plate
Salmonella Reverse 0.01-5 - - Andrae et al. (1981);
typhimurium mutation mg/plate Shimizu & Yano (1986)
TA1538
Salmonella Reverse 0.125-2 - 0 Rashid & Mumma (1986)
typhimurium mutation mg/plate
TA1538, TA1978
Salmonella Reverse 0.0007- - - Suzuki et McCann et al. (1975);
typhimurium mutation 5 mg/plate al. (1983) Hoechst AG (1980);
TA98, TA100 also tested Andrae et al. (1981);
both strains Haworth et al. (1983);
in the Suzuki et al. (1983);
presence of Shimizu & Yano (1986);
norharman, Kawai et al. (1987);
which also Dellarco & Prival (1989);
gave negative Massey et al. (1994)
results
Table 3 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
Salmonella Reverse 0.001-5 - - McCann et al. (1975);
typhimurium mutation mg/plate Hoechst AG (1980);
TA1535, TA1537 Andrae et al. (1981);
Haworth et al. (1983);
Shimizu & Yano (1986)
Rat hepatocytes DNA damage 42-417 mg (+) 0 Weakly Storer
(alkaline positive at et al. (1996)
elution) >97 mg
Rat hepatocytes DNA repair 4.2-417 mg - 0 Andrae et al. (1981)
Chinese hamster Chromosomal without S9 mix: - + NTP (1993)
ovary (CHO) aberration 0.1-0.5 mg/ml
cells with S9 mix:
1.25-2 mg/ml
Chinese hamster Sister chromatid without S9 mix: - - NTP (1993)
ovary (CHO) cells exchange 0.00017-0.025 mg/ml
with S9 mix:
0.05-1.5 mg/ml
Mouse lymphoma Forward mutation without S9 mix: - - Oberly et al. (1984)
assay 0.7-1.5 mg/ml
L5178Y with S9 mix:
TK+/- cells 0.0001-0.03
mg/ml
Table 3 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
Mouse lymphoma Forward mutation 0.06-0.78 mg/ml 0 - Amacher & Turner (1982)
assay L5178Y
TK+/- cells
Rat hepatocytes Unscheduled DNA 0.00007-0.14 mg/ml - 0 Probst et al. (1981)
synthesis
Human lymphocytes Chromosomal not given + No data about Huang et al. (1996)
aberration metabolic
activation;
validity
cannot be
judged
(documentation
and study
design
insufficient
for
assessment)
lowest
positive
concentration:
1.4 mg/ml
Table 3 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
Human Chromosomal 0.001-0.3 mg/ml + No data about Huang et al. (1995)
lymphocytes aberration metabolic
activation;
validity cannot
be judged
(documentation
and study
design
insufficient
for assessment)
Human fibroblasts DNA repair 0.14-139 mg + No data about Poirier et al. (1975)
(WI-38) metabolic
activation;
validity
cannot be
judged
(documentation
insufficient
for assessment)
positive at
>13.9 mg
Table 3 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
4-Nitrophenol (in vivo studies)
NMRI mice Host-mediated single subcutaneous - application of Buselmaier et al. (1972)
assay (tester injection of test substance
strains 75 mg/kg immediately
Salmonella body weight after the
typhimurium G 46 bacteria had
and Serratia been injected
marcescens a into the
21 Leu-) abdominal
cavities;
test duration
3 h
Drosophila SLRL assay via feed (1000, - Zimmering et al. (1985);
melanogaster 2500, 6000, or Foureman et al. (1994)
7500 ppm) or
injection (1000
or 1500 ppm)
a -, negative; +, positive; (+), weakly positive; 0, not tested.
8.6 Reproductive and developmental toxicity
8.6.1 Reproductive toxicity
In a valid two-generation study with groups of 24 female and 12
male Sprague-Dawley rats carried out by Angerhofer (1985),
4-nitrophenol dissolved in ethanol was applied dermally at doses of 0,
50, 100, or 250 mg/kg body weight per day, 5 days/week. The F0
generation was exposed over a period of 140 days before mating. Dosing
of the F0 females continued throughout breeding, gestation, and
lactation. Groups of 26 females and 13 males of the F1 generation
were then exposed for 168 days in the same manner as had been the F0
rats; the females were again exposed throughout breeding, gestation,
and lactation. Apart from dose-related signs of skin irritation
(erythema, scaling, scabbing, and cracking) in dosed animals, the
gross and histopathological examinations provided no indication of
significant adverse effects. The calculated indices concerning
fertility, gestation, viability, and lactation were not different from
those of controls. The testis to body weight ratios in the F0
generation were not affected, and histological lesions were not
observed in the testes. In a 28-day study in rats (see section 8.3.1),
testicular atrophy and inhibition of spermatogenesis were observed in
some animals after oral dosing at a level of 630 mg/kg body weight,
but not at 210 mg/kg body weight.
8.6.2 Developmental toxicity
8.6.2.1 2-Nitrophenol
In a range-finding study with Charles River COBS(c) CD(c) rats
(five dams per group; application of 0, 50, 125, 250, 500, or
1000 mg/kg body weight via gavage from day 6 to day 15 of gestation;
uterine examination on day 20), dose levels of 500 and 1000 mg/kg body
weight caused signs of maternal toxicity (transient but dose-related
decrease in weight gain early during treatment). One high-dose animal
died, but no cause of death could be determined. Other clinical
findings included darkly coloured urine at >250 mg/kg body weight
and yellow staining of haircoat (at the nose, mouth, anogenital area)
at >125 mg/kg body weight; the necropsy findings gave no
biologically meaningful differences in surviving dams. At the highest
dose level of 1000 mg/kg body weight, a slight but statistically
significant (also compared with historical controls) increase in group
mean post-implantation losses (13.8% versus 8.2% in controls) and mean
early resorptions (2.3 versus 1.2 in controls) was seen. No effects
were observed on the number of viable fetuses, implantations, or
corpora lutea (International Research and Developmental Corporation,
1983).
8.6.2.2 4-Nitrophenol
In both studies cited below, a complete examination of the pups
for possible teratogenic effects was not performed. In addition, owing
to limitations of these studies (i.e., use of only one dose group or
exposure to a mixture), reliable NO(A)EL values cannot be derived.
In a study performed by Booth et al. (1983), groups of 50 female
CD-1 mice received daily oral doses of 400 mg 4-nitrophenol/kg body
weight via gavage from day 7 to day 14 of gestation. The survival rate
in pregnant mice ( n = 36) was 81% versus 100% in controls, and dosed
animals showed less maternal weight gain. No changes were observed in
the reproductive index (ratio between survivors delivered and pregnant
survivors). The average number of live pups per litter was slightly
decreased, but 4-nitrophenol produced no gross abnormalities.
Kavlock (1990) studied the developmental toxicity of
4-nitrophenol in Sprague-Dawley rats. The substance (dissolved in a
mixture of water, Tween 20, propylene glycol, and ethanol [4:4:1:1])
was applied via gavage to groups of 12-13 animals at doses of 0, 100,
333, 667, or 1000 mg/kg body weight on day 11 of gestation. Endpoints
concerning maternal toxicity included signs of toxicity, mortality,
body weight gain, and the number of implantation scars in the uteri at
weaning. In the offspring, viability, body weight on postnatal days
1-6, overt malformations, and perinatal loss were recorded. In dams,
the mortality was increased at a dose level of >667 mg/kg body
weight; at a dose level of >333 mg/kg body weight, the litter size
on postnatal days 1 and 6 was non-significantly decreased.
8.7 Immunological and neurological effects
There are no studies available dealing specifically with
immunological or neurological effects. There is an indication from an
in vitro study that 4-nitrophenol may act as a suppressor of
cell-mediated immune response (Pruett & Chambers, 1988). However, the
biological significance is uncertain.
8.8 Methaemoglobin formation
Methaemoglobin formation by 2-nitrophenol and 4-nitrophenol has
been tested in several studies using different species, routes, and
durations of applications. An overview is given in Table 4.
2-Nitrophenol clearly leads to the formation of methaemoglobin in
a dose-dependent manner in cats (BASF AG, 1970), the most sensitive
species. The lowest dose tested, 50 mg/kg body weight, produced
increased methaemoglobin levels. In inhalation experiments in rats,
elevated methaemoglobin levels were observed at an exposure level of 5
mg/m3; methaemoglobin levels were less elevated at exposure levels of
30 and 60 mg/m3 (Hazelton Lab., 1984).
4-Nitrophenol, in contrast, did not lead to methaemoglobin
formation in cats at concentrations up to 500 mg/kg body weight (BASF
AG, 1969). In rats, at high concentrations in inhalation experiments,
the methaemoglobin-forming capacity seemed to be very low (1.5% at
2470 mg/m3). In conclusion, 4-nitrophenol may induce methaemoglobin
formation, but the effect seems to be rather weak, without clear
dose-response.
9. EFFECTS ON HUMANS
Naniwa (1979) performed patch tests with 4-nitrophenol,
4-aminophenol, 2-amino-4-chlorophenol,
3'-chlorodiphenylamine-2-carboxylic acid, and 4-dichloronitrobenzene
(0.1, 0.5, or 1% in petrolatum) on 31 employees probably exposed to
these chemicals in a chemical factory and on 5 control persons. In
four employees, a positive reaction to 4-nitrophenol was observed,
although none of these persons reacted positively to all three tested
concentrations. All four employees also reacted positively to
2-amino-4-chlorophenol, which was shown to be a strong sensitizer.
Therefore, 2-amino-4-chlorophenol may act as the primary allergen, and
the effects observed with 4-nitrophenol may be due to
cross-sensitization.
In 27 patients primarily sensitized to
1-chloro-2,4-dinitrobenzene, no cross-sensitization due to
4-nitrophenol (1-2% in petrolatum) was observed. In addition, 15
patients with a chloramphenicol allergy failed to react to
4-nitrophenol (Eriksen, 1978).
Table 4: Methaemoglobin formation by 2-nitrophenol and 4-nitrophenol.
Species Route Frequency/duration Dose Results Reference
(strain/number/dose/sex) (% metHb)
2-Nitrophenol
cat oral 1 × 50 mg/kg body weight 6 BASF AG (1970)
2 100 44
sex not given 250 57
rabbit dermal 1 × 50% solution no increase BASF AG (1970)
number and sex in water
not given
rat inhalation 6 h/day m f Hazleton Lab.
Sprague-Dawley 5 days/week 0 mg/m3 1.0 2.0 (1984)
15 m/15 f 4 weeks 5 2.3 4.1
30 1.8 2.1
60 1.6 1.1
11th day of
treatment
4-Nitrophenol
cat oral 1 × 100 mg/kg body no increase BASF AG (1969)
2 200 weight
sex not given 500
rat oral 5 days/week 0 mg/kg body weight analytical Hazleton Lab.
Sprague-Dawley 13 weeks 25 method not (1989)
20 m/20 f 70 reliable (13%
140 in controls)
Table 4: Methaemoglobin formation by 2-nitrophenol and 4-nitrophenol.
Species Route Frequency/duration Dose Results Reference
(strain/number/dose/sex) (% metHb)
rat inhalation 6 h/day 0 mg/m3 0.2 0.2 Smith et al.
Crl:CDR 5 days/week 340 0.87 0.13 (1988)
10 m 2 weeks 2470 1.53 0.7
end of treatment
and after 14
days of recovery
rat inhalation 6 h/day 0 mg/m3 0.5 0.4 Smith et al.
Crl:CDR 5 days/week 30 0.3 0.5 (1988)
10 m 2 weeks 130 1.5 0.2
end of treatment
and after 14
days of recovery
rat inhalation 6 h/day m f Hazleton Lab.
Sprague-Dawley 5 days/week 0 mg/m3 0.8 1.3 (1983)
15 m/15 f 4 weeks 1 0.5 1.1
5 2.2 2.0
30 1.1 1.0
after 2 weeks of
exposure, values
unusually high
in some control
animals
Abbreviations used: m = male; f = female; metHb = methaemoglobin.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1 Aquatic environment
Experimental test results for the most sensitive species are
summarized in Table 5. Additional data on the toxicity of 2- and
4-nitrophenol to aquatic organisms are cited in BUA (1992). Among all
tested organisms, the protozoan Entosiphon sulcatum and the green
alga Scenedesmus subspicatus proved to be most sensitive in chronic
cell multiplication inhibition tests with freshwater species. Daphnia
magna exhibited a 21-day lowest-observed-effect concentration (LOEC)
of 1.0 mg 2-nitrophenol/litre in the Daphnia reproduction test
(Koerdel et al., 1984). The entoproct Barentsia matsushimana was the
most sensitive marine invertebrate species tested, exhibiting a 49-day
EC50 value of 0.21 mg 4-nitrophenol/litre and a minimal effective
concentration (ECm) of 0.03 mg/litre (end-point: growth of
germinated spores) (Scholz, 1986). Freshwater fish showed less
sensitivity. The lowest 96-h LC50 value of 3.8 mg 4-nitrophenol/litre
was determined for rainbow trout ( Oncorhynchus mykiss) (Howe et
al., 1994). The measured no-observed-effect concentration (NOEC) for
behavioural changes in a 28-day flow-through test with zebra fish was
2 mg 2-nitrophenol/litre (Broecker et al., 1984). After prolonged
exposure of zebra fish to 4-nitrophenol, minor morphological
alterations of the liver, even at a concentration of 0.1 mg/litre,
were observed. At 1 and 5 mg/litre, about 25% of the animals showed
symptoms of degenerative transformation of the liver tissue (Braunbeck
et al., 1989).
10.2 Terrestrial environment
The toxicity of 2- and 4-nitrophenol on higher plants according
to OECD Guideline 208 was tested in independent studies. After
incubation of seeds with different test substance concentrations,
14-day EC50 values for reduced fresh weight of grown shoots were in
the range of 52-420 mg 2-nitrophenol/kg soil (Broecker et al., 1984;
Koerdel et al., 1984) and 35-260 mg 4-nitrophenol/kg soil (Ballhorn et
al., 1984; Marquart et al., 1984). The 14-day EC10 value for
2-nitrophenol was 10 mg/kg soil for both species. Overall, turnip
( Brassica rapa) proved to be more sensitive than oat
( Avena sativa).
In tests conducted according to OECD Guideline 207, the adverse
effects of 2- and 4-nitrophenol on earthworms were examined in several
independent studies. In the contact test, in which the animals are
exposed on filter paper soaked with the test substance, Neuhauser
et al. (1985) established a 48-h LC50 value of 5.9 µg/cm2 for the
toxicity of 2-nitrophenol on Eisenia fetida. For 4-nitrophenol, the
48-h LC50 values were in the range of 0.7-2.7 µg/cm2, with
Table 5: Aquatic toxicity of nitrophenols.
Most sensitive species
(test method/end-point) Substance Effective Reference
concentration
(mg/litre)
Bacteria
Pseudomonas putida 2-NP 16-h MICa: 0.9 Bringmann & Kuehn (1977)
(cell multiplication 4-NP 16-h MIC: 4.0
inhibition test)
Protozoa
Entosiphon sulcatumn 2-NP 72-h MIC: 0.40 Bringmann (1978);
(cell multiplication 4-NP 72-h MIC: 0.83 Bringmann et al. (1980)
inhibition test)
Algae
Scenedesmus subspicatus 2-NP 96-h EC50: 0.39 Broecker et al. (1984);
Chlorella vulgaris 2-NP 6-h EC50: 1.53 Kramer et al. (1986)
(cell multiplication 4-NP 6-h EC50: 6.97
inhibition test)
Invertebrates
Moina macrocopa (acute) 2-NP 3-h LC50: 1.9 Yoshioka et al. (1985)
(immobilization) 4-NP 3-h LC50: 1.3
Daphnia magna (long-term) 2-NP 21-day LOEC: 1.0 Koerdel et al. (1984)
(immobilization/reproduction) 4-NP 21-day NOEC: 1.3
Barentsia matsushimana (marine) 4-NP 49-day EC50: 0.21 Kuehn et al. (1988)
(growth of germinated spores) 4-NP 49-day ECmb: 0.03 Scholz (1986)
Table 5 (cont'd)
Most sensitive species
(test method/end-point) Substance Effective Reference
concentration
(mg/litre)
Fish
Cyprinus carpio (static) 2-NP 96-h LC50: 36.6 Lang et al. (1996)
Oncorhynchus mykiss (static) 4-NP 96-h LC50: 3.8 Howe et al. (1994)
Oncorhynchus mykiss (flow-through) 4-NP 96-h LC50: 7.93
Abbreviations used: 2-NP = 2-nitrophenol; 4-NP = 4-nitrophenol.
a MIC = minimum inhibitory concentration.
b ECm = minimal effective concentration.
Eisenia fetida and Eudrilus eugeniae being the most sensitive
species tested (Roberts & Dorough, 1984; Neuhauser et al., 1985,
1986). When exposed in an artificial soil mixture, 28-day LC50 values
for 2-nitrophenol were in the range of 250-500 mg/kg soil ( Eisenia
fetida) (Broecker et al., 1984; Koerdel et al., 1984), and 14-day
LC50 values for 4-nitrophenol were in the range of 38-67 mg/kg soil,
again with Eisenia fetida and Eudrilus eugeniae as the most
sensitive species tested (Ballhorn et al., 1984; Marquart et al.,
1984; Neuhauser et al., 1985, 1986).
The environmental relevance, particularly of the earthworm
contact test, seems questionable. Critical results from this test, as
sole effect data on terrestrial organisms, should not justify a
classification of tested substances as highly toxic to earthworms or
other soil organisms. The available data on microorganisms and plants
indicate only a moderate toxicity potential in the terrestrial
environment.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessmentn
In general, there is only limited information concerning the
toxicological profiles of 2- and 4-nitrophenol.
In experimental animals given 4-nitrophenol orally,
intravenously, or intraperitoneally, most of the applied dose was
excreted via the urine within 24-48 h as glucuronide and sulfate
conjugates, while only very small amounts were excreted via faeces or
as unchanged 4-nitrophenol. In rabbits, after oral dosing,
4-nitrophenol undergoes reduction to 4-aminophenol as well as
glucuronidation and sulfation. In vivo and in vitro studies gave
an indication for dermal uptake. For 2-nitrophenol, the information is
very limited. However, a comparable metabolic transformation is
assumed based on the available data. Owing to their rapid metabolism
and excretion, bioaccumulation of 2- and 4-nitrophenol in organisms is
not to be expected.
With oral LD50 values of 220-620 mg/kg body weight in rats and
380-470 mg/kg body weight in mice, 4-nitrophenol is harmful after oral
uptake and was found to be more toxic than 2-nitrophenol. A
dose-dependent increase in the formation of methaemoglobin was seen in
cats after oral exposure to 2-nitrophenol -- but not after exposure to
4-nitrophenol -- and in rats after exposure by inhalation to
4-nitrophenol.
Most of the studies concerning skin- or eye-irritating effects in
experimental animals are limited as a result of insufficient
documentation. However, from the available data, it can be concluded
that 2-nitrophenol is slightly irritating to the skin but
non-irritating to the eye, and the substance proved to have no
sensitizing effects. For 4-nitrophenol, irritating effects on skin and
eye are assumed based on the studies performed according to OECD/FDA
guidelines; in addition, signs of irritation were reported after
exposure by inhalation as well as subchronic dermal exposure. In a
guinea-pig maximization test, 4-nitrophenol was considered to be
sensitizing. Positive patch tests were recorded in humans exposed to
4-nitrophenol. Although this may have been due to cross-sensitization,
sensitization to 4-nitrophenol in humans cannot be excluded.
Only a few limited studies concerning repeated oral exposure to
2- and 4-nitrophenol in experimental animals were identified. With
2-nitrophenol, decreases in body weight gain accompanied by decreased
food consumption and differences in organ weights without clear dose
dependency were found. However, the haematological examination,
clinical chemistry, and histopathological examination of the major
organs and tissues gave no indication for a substance-related toxic
effect compared with controls. In rats dosed with 4-nitrophenol, a
focal fatty degeneration of the liver as well as congestion in several
organs were the major histopathological findings. Other reported
effects included haematological changes, nephrosis, testicular
atrophy, and follicular atresia in the ovaries. The exposure by
inhalation to 2-nitrophenol vapour caused squamous metaplasia of the
epithelium of the upper respiratory tract; with 4-nitrophenol dust
(applied as sodium salt), haematological changes, increased
methaemoglobin values, and differences in organ weights were noted.
For the effects given in these studies, it was not possible to
identify a clear dose-response or reliable NO(A)EL values.
Insufficient data are available on 2-nitrophenol to allow any
conclusions to be made about its possible mutagenicity. For
4-nitrophenol, more mutagenicity studies are available, and the
substance was shown to be mutagenic in some but not all of the
bacterial assays. In addition, positive results were obtained in
in vitro tests for chromosomal aberrations in mammalian cells;
however, apart from one well-documented study, the available assays
were inadequately reported. In the absence of any in vivo
mutagenicity studies in mammals, it is not possible to conclude
whether or not the mutagenic potential of 4-nitrophenol is expressed
in vivo.
4-Nitrophenol was not carcinogenic in male or female mice after
dermal application over 78 weeks. In a limited study with female mice,
no skin tumours were seen after dermal application of 2- or
4-nitrophenol over 12 weeks. No carcinogenicity studies using the oral
or inhalation routes were available for either of the isomers.
No reproductive effects were observed in rats exposed to
4-nitrophenol in a two-generation study. For developmental toxicity,
the available studies were inadequately performed (i.e., only one dose
was applied, or animals were dosed only on one day with a mixture). In
an oral study with rats, 2-nitrophenol induced developmental effects
in the offspring only at doses that also produced maternal toxicity.
However, the fetuses were not examined for internal malformations.
Data on humans relevant for the assessment of potential adverse
effects are limited to some patch tests performed with 4-nitrophenol.
11.1.2 Criteria for setting guidance values for 2- and 4-nitrophenol
As given in section 8, the database for 2-nitrophenol is
inadequate for calculating a tolerable daily intake (TDI) or a
tolerable concentration (TC).
For 4-nitrophenol, the formation of methaemoglobin was shown to
be the most critical end-point after exposure by inhalation and is
assumed to be relevant for oral exposure too. However, owing to the
inaccuracy of the analytical method used in the 13-week study with
oral application, a reliable NO(A)EL cannot be derived. Therefore, at
present, no TDI for 4-nitrophenol can be developed owing to inadequacy
of the database.
Longer-term toxicity studies concerning inhalation exposure were
not identified in the literature, and the NO(A)EL values derived for
4-nitrophenol from short-term studies gave considerable differences
(2-week exposure: NO(A)EL of about 30 mg/m3; 4-week exposure: NO(A)EL
of about 5 mg/m3). The NO(A)EL of 5 mg/m3 was derived for local
effects (cataracts), whereas the NO(A)EL for systemic effects
(formation of methaemoglobin) may be lower. Therefore, a reliable TC
for exposure by inhalation cannot be calculated, as the formation of
methaemoglobin is the critical end-point.
11.1.3 Sample risk characterization
As given in section 6.2, workers may be exposed to 2- and
4-nitrophenol via inhalation and skin contact during production and
processing (mainly in the manufacturing of pesticides). However, data
on nitrophenol concentrations at the workplace were not identified.
For the general population, an exposure to nitrophenols via the
environment cannot be excluded (see also section 6.2). Assuming an
ambient atmospheric concentration of about 1 µg/m3, an inhalation
uptake of 100%, a daily respiratory volume of 22 m3 for adults, a
mean body weight of 64 kg for males and females, and that 4 of 24 h
are spent outdoors (IPCS, 1994), the uptake by inhalation of
nitrophenols is calculated to be 0.06 µg/kg body weight per day. In
addition, 4-nitrophenol accumulates in fog. From the mean measured
level of 20 µg/litre, the uptake of the substance by inhalation (using
the same assumptions as above) can be calculated to be about 8 ng
during a 1-h exposure period (i.e., 0.12 ng/kg body weight), assuming
a maximum water content of fog of 0.1 g/m3 (Pruppacher & Klett,
1978). The uptake via drinking-water for 2- and 4-nitrophenol can be
calculated to be about 0.02 µg/kg body weight per day, assuming a
maximum concentration of 1 µg/litre drinking-water, a daily
drinking-water consumption of 1.4 litres, and a mean body weight of
64 kg for males and females.
From these data, it can be concluded that exposure of the general
population to the nitrophenol isomers is mainly through ambient air
and drinking-water.
11.2 Evaluation of environmental effects
Releases of 2- and 4-nitrophenol into the environment are
primarily emissions into air, water, and soil from diffuse sources,
such as vehicle traffic and hydrolytic and photolytic degradation of
the respective pesticides.
2-Nitrophenol emitted to the troposphere will stay predominantly
in the gaseous phase and should be rapidly removed by nitration. The
major portion of airborne 4-nitrophenol is expected to be particle
bound and can be washed out to surface waters and soil by wet and dry
deposition. Because of their removal from air and their insignificant
volatility, nitrophenols are not considered to contribute directly to
the depletion of the stratospheric ozone layer or to global warming.
Measured bioconcentration factors indicate a low potential for
bioaccumulation.
Nitrophenols exhibit moderate to high toxicity to aquatic
organisms, with lowest effect concentrations reported from chronic
studies on algae, Daphnia, and aquatic invertebrates. The lowest
effect concentrations found in chronic studies with freshwater
organisms ( Scenedesmus subspicatus, 96-h EC50: 0.39 mg
2-nitrophenol/litre; Entosiphon sulcatum, 72-h MIC: 0.83 mg
4-nitrophenol/litre) were 40-50 times higher than maximum levels
determined in a densely populated and highly industrialized Asian
river basin (0.0072 mg 2-nitrophenol/litre and 0.019 mg
4-nitrophenol/litre). From these data, the safety margin between the
LOEC and maximum surface water concentrations is insufficient to
exclude any risk for sensitive aquatic organisms, particularly under
surface water conditions not favouring both elimination pathways.
Taking into account the missing chronic effect data for fish, an
uncertainty/assessment factor of 100 has to be applied to derive a
predicted no-effect concentration (PNEC) according to standard
procedures for environmental risk assessment. From acute tests (see
section 10.1), however, fish obviously seem to be the least sensitive
aquatic species tested. Thus, an assessment factor of 10 might be
appropriate. Furthermore, the use pattern and the release scenario
outlined in section 4 lead to the conclusion that nitrophenols emitted
to surface waters will pose only a minor risk to aquatic organisms.
There were no data available on the occurrence of nitrophenols in
the terrestrial compartment. Therefore, an assessment of possible
effects on organisms for this compartment could be conducted only with
regard to the degradation of pesticides. For the insecticides
mentioned in section 4 (parathion, parathion-methyl, carbofuran,
phosalon, fluorodifen) and the herbicides bifenox and nitrofen,
predicted soil concentrations were calculated from the maximum
application rates (taken from Domsch, 1992) according to the EPPO
(1993) guidelines for environmental risk assessment of plant
protection products. Based on the relative molecular mass of the
pesticides, the maximum concentration of nitrophenols in the top 5 cm
of soil was calculated (worst case; one application). For pesticides
that are applied at times when the soil is plant covered to a high
degree, it is assumed that only half of the amount applied reaches the
soil. Thus, the predicted environmental concentration (PEC) for the
insecticides was reduced by 50%. Dividing the PECsoil by the lowest
LC50 value for a terrestrial species gives the toxicity exposure
ratio (TER). The lowest LC50 value for earthworms (38 mg/kg body
weight; see section 10.2) has to be corrected by a factor of 2 because
of the higher organic matter content in artificial soil compared with
natural agricultural soil. The following TERs were derived:
Insecticides Herbicides
Parathion: 244 Bifenox: 131
Parathion-methyl: 557 Nitrofen: 18
Carbofuran: 47
Phosalon: 36
Fluorodifen: 69
According to the EPPO (1993) guidelines, the trigger value for
concern is <10. Therefore, these pesticides are expected to pose only
a minor risk to earthworms, even under a worst-case scenario.
Furthermore, the herbicide nitrofen and the insecticides phosalon and
fluorodifen are no longer manufactured or marketed for crop protection
use.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Previous evaluations of mononitrophenols by international bodies
were not identified.
Information on international hazard classification and labelling
for mononitrophenols is included in the International Chemical Safety
Card reproduced in this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventative and protective
measures and first aid recommendations, are presented on the enclosed
International Chemical Safety Card (ICSC 1342) reproduced in this
document.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards is
available from the International Register of Potentially Toxic
Chemicals (IRPTC) legal file.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
INTERNATIONAL CHEMICAL SAFETY CARD
MONONITROPHENOLS ICSC: 1342
November 1998
CAS #
Technical-grade 2- and 4-nitrophenol from the German producer
have a typical purity of >99%. Named impurities are the corresponding
isomer for each product (0.3%) and traces of 3-nitrochlorobenzene
(<0.05%). Polychlorinated dibenzo- p-dioxin/dibenzofuran (PCDD/PCDF)
and tetrachlorodibenzo- p-dioxin/dibenzofuran (TCDD/TCDF) isomers
were not detected at detection limits between 0.1 and 0.4 µg/kg
product (BUA, 1992).
The pure nitrophenol isomers form pale yellow to yellow crystals
at room temperature. The substances are characterized by the
physicochemical properties given in Table 1 (Sax & Lewis, 1987).
Additional physicochemical properties for mononitrophenols are
presented in the International Chemical Safety Card (ICSC 1342)
reproduced in this document.
Table 1: Physicochemical properties of 2- and 4-nitrophenol.
Parameter 2-Nitrophenol 4-Nitrophenol
Molecular mass (g/mol) 139.11 139.11
Melting point (°C) 44-45 (1)(2)(3) 113-114 (1)(2)(3)
Boiling point (°C) 214-217 (1) 279 (decomposition)(3)
Vapour pressure (kPa) 6.8 × 10-3
(19.8 °C) (4) 3.2 × 10-6
(20 °C) (5)
Water solubility 1.26 12.4
(g/litre) (20 °C) (4) (20 °C) (6)
n-Octanol/water
partition
coefficient (log Kow) 1.77-1.89 (7) 1.85-2.04 (7)
Dissociation constant 7.23 7.08
(pKa) (21.5 °C) (8) (21.5 °C) (8)
Ultraviolet spectrum deltamax (water): deltamax (methanol):
230; 276 nm; no absorption
log epsilonmax: 3.57; maxima <290 nm (9)
3.80 (9)
Conversion factors 1 mg/m3 = 0.173 ppmv
1 ppmv = 5.78 mg/m3
References: (1) Budavari et al. (1996); (2) Booth (1991);
(3) Verschueren (1983); (4) Koerdel et al. (1981);
(5) Sewekow (1983); (6) Andrae et al. (1981);
(7) BUA (1992); (8) Schwarzenbach et al. (1988); (9) Weast (1979)
3. ANALYTICAL METHODS
The nitrophenol isomers are usually determined by gas
chromatography combined with mass spectrometric detection, flame
ionization detection, electron capture detection, or
nitrogen-sensitive detection, which are generally applied after
derivatization (BUA, 1992; Nick & Schoeler, 1992; Geissler & Schoeler,
1994; Harrison et al., 1994; Luettke & Levsen, 1994; Mussmann et al.,
1994). For liquid samples (water, urine, blood), high-performance
liquid chromatography in combination with concentration-gradient
elution (acetonitrile/methanol or ammonium acetate, acetic acid with
potassium chloride/methanol) and ultraviolet or electrochemical
detection, which can be carried out without derivatization, is also
used (BUA, 1992; Nasseredine-Sebaei et al., 1993; Ruana et al., 1993;
Paterson et al., 1996; Pocurull et al., 1996; Thompson et al., 1996).
The separation of the different isomers is carried out either by steam
distillation (BUA, 1992) or by the formation and subsequent extraction
of different ion pairs (León-González et al., 1992).
The following enrichment techniques are used (BUA, 1992; see also
review by Puig & Barcelo, 1996):
* solid-phase adsorption with thermal or liquid extraction for air
and water samples (Luettke & Levsen, 1994; Mussmann et al., 1994)
* liquid/liquid extraction after derivatization for water samples
(initial purification by acid/base fractionation of highly
polluted samples, increased recovery rates with continuous
extraction methods) (León-González et al., 1992; Nick & Schoeler,
1992; Geissler & Schoeler, 1994; Harrison et al., 1994)
* liquid extraction with acid/base fractionation or solid-phase
enrichment and subsequent desorption following aqueous extraction
for soil samples (Vozñáková et al., 1996)
* acid hydrolysis of the glucuronide with subsequent
derivatization for blood and urine samples or denaturation
(Nasseredine-Sebaei et al., 1993; Thompson et al., 1996).
The detection limits are <10 ng/m3 for air, 0.03-10 µg/litre
for water, and 200-1600 µg/kg for soil. A detection limit for the
determination of the nitrophenol isomers in biological materials was
given only for rat liver perfusate (0.5-1 mg/litre; Thompson et al.,
1996).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
There are no known natural sources of the nitrophenol isomers.
Within the European Union, 2- and 4-nitrophenol are produced
mainly by three companies. Six other large manufacturers are known in
the USA and Japan (as of 1989). In 1983, the production volume for
Western Europe was estimated at about 6400 t 2-nitrophenol and about
20 500 t 4-nitrophenol. In 1988-89, the German production volumes
originating from one manufacturer were approximately 500 t
2-nitrophenol and about 2000 t 4-nitrophenol, with about 20 t of each
being exported. Both 2- and 4-nitrophenol are intermediates in the
synthesis of azo dyes and a number of pesticides, mainly insecticides
(2-nitrophenol: carbofuran, phosalon; 4-nitrophenol: parathion,
parathion-methyl, fluorodifen) and, to a lesser extent, herbicides
(4-nitrophenol: nitrofen, bifenox). The corresponding aminophenols
that are gained by reduction are used as a photographic developer
(2-aminophenol) and as an intermediate in the synthesis of the
tuberculostatic 4-aminosalicylic acid and the analgesic
4-acetaminophenol (paracetamol) (4-aminophenol) (see also Booth,
1991). In the 1980s, the production volumes for 2- and 4-nitrophenol
showed a decreasing tendency in Germany as a result of changes in and
termination of the production of some organophosphorus pesticides.
The releases of 2- and 4-nitrophenol during production and
processing at the only German manufacturer appear to be of minor
importance. In 1988-89, about 2.5 kg 2-nitrophenol and 10 kg
4-nitrophenol were emitted to air, and <93 kg 2-nitrophenol and
<64 kg 4-nitrophenol were emitted to surface water.
For 1996, the following releases of 2- and 4-nitrophenol to the
environment were reported by manufacturers in the USA (TRI, 1998):
* 2-nitrophenol: from three manufacturers (one production site
each) with production volumes between 450 and 45 000 kg/year,
total releases of 15 kg to air and 23 kg into water were
reported.
* 4-nitrophenol: from three manufacturers (six production sites)
with production volumes of 45-450 kg/year up to 45 000-450 000
kg/year, a total release of 420 kg to air was reported. Data on
releases into water were not given.
2-Nitrophenol and 4-nitrophenol have been detected in the exhaust
gases of light-duty gasoline and diesel vehicles. Depending on the
motor load, the exhaust concentrations of the isomers were <50 µg/m3
exhaust gas (idle) and about 1000 µg 4-nitrophenol/m3 and 2000 µg
2-nitrophenol/m3 (driving at constant velocity) (Nojima et al., 1983;
Tremp et al., 1993). A regulated three-way catalytic converter reduced
the nitrophenol emissions to about 8% at high motor load and to about
2% at normal motor load (Tremp et al., 1993). A rough estimation
combining the above-mentioned exhaust gas concentrations with
estimations of the total exhaust gas volumes from vehicle traffic for
Germany resulted in an airborne nitrophenol load of at least several
tonnes per year from this source (BUA, 1992). Data concerning
nitrophenol releases from other combustion processes (heating, burning
of refuse) were not identified.
From laboratory experiments, there is some evidence that 2- and
4-nitrophenol are generated in the atmosphere during the photochemical
degradation of aromatic compounds such as benzene and toluene in the
presence of nitric oxide or hydroxyl radicals and nitrous dioxide.
These results were at least partly obtained in model experiments with
unrealistically high nitric oxide concentrations, and there are
competing reactions without nitrophenol formation for which the rate
constant is not known (BUA, 1992). However, smog chamber experiments
confirmed the formation of nitrophenol isomers during irradiation
(Leone & Seinfeld, 1985; Leone et al., 1985). Recent cloud water model
experiments showed that 2- and 4-nitrophenol are also formed from the
reaction of phenol with nitrogen pentoxide or monochloronitrogen
dioxide, especially under alkaline conditions (Scheer et al., 1996).
Estimations of the contribution of photochemically formed nitrophenols
to total emissions into the atmosphere are not possible with the
available data.
Significant releases of 4-nitrophenol into the hydrosphere may
occur from the hydrolytic degradation of the insecticides parathion
and parathion-methyl and -- although to a lesser extent -- from the
photolytic degradation of the herbicides nitrofen and bifenox.
Quantification of releases is not possible with the available data.
Furthermore, a considerable portion of airborne nitrophenols,
especially 4-nitrophenol, can be released to the hydrosphere and the
geosphere by wet and dry deposition (see section 5) (Herterich &
Herrmann, 1990; Luettke et al., 1997). Numerous studies concerning the
concentrations of 2- and 4-nitrophenol in wet deposition samples are
available (see section 6.1). From precipitation data (average 746 mm
rain per year for land masses, according to Baumgartner & Liebscher,
1990) and the measured concentrations of 2- and 4-nitrophenol in
rainwater, the release of nitrophenols via rain can be estimated to be
at least in the order of several thousand tonnes per year on a global
basis.
The application of the herbicides nitrofen and bifenox, which are
photolytically degraded to 4-nitrophenol in aqueous solutions, may
especially lead to emissions into the geosphere and the biosphere.
Further, nitrophenol-contaminated rain, snow, and other wet and dry
deposition may contribute to nitrophenol levels in soils. Data
concerning the release of nitrophenols into the biosphere are not
available.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Environmental releases of nitrophenols are mostly to ambient air,
surface waters, and -- to a smaller extent -- soil. Using a
non-steady-state equilibrium model, the following distribution of
4-nitrophenol in different environmental compartments was predicted:
air, 0.0006%; water, 94.6%; sediment, 4.44%; soil, 0.95%; biota,
0.000 09% (Yoshida et al., 1983). The distribution patterns of 2- and
4-nitrophenol sprayed on a natural soil in a standardized terrestrial
ecosystem were determined via radiotracer technique (14C). Of the
applied radioactivity (2-nitrophenol/4-nitrophenol), 49.45%/20.01% was
recovered in air, 27.38%/40.21% in soil (including animals),
12.73%/7.57% in plants, and 0.05%/0.02% in leachate (Figge et al.,
1985). Distribution of 4-nitrophenol in a terrestrial microcosm
chamber with artificial soil largely corresponded to this result (Gile
& Gillett, 1981). Owing to the expected decomposition within the
incubation periods of 30 and 28 days, respectively, it can be assumed
that most of the recovered radioactivity referred to breakdown
products of the applied nitrophenols.
In volatility experiments conducted according to Organisation for
Economic Co-operation and Development (OECD) guidelines, half-lives of
2-nitrophenol in water ranged from 14.5 to 27.3 days, indicating a
slow rate of volatilization (Koerdel et al., 1981; Rippen et al.,
1984; Scheunert, 1984; Schoene & Steinhanses, 1984). Measurements
concerning the partitioning between the gas and liquid phases of
clouds during different rain events showed that 2-nitrophenol is
enriched in the liquid phase to a larger extent than would be
predicted from its water solubility and vapour pressure. On the other
hand, 4-nitrophenol is extensively adsorbed to particles. Therefore,
elevated levels of this isomer are detected in the gaseous phase of
clouds (Luettke et al., 1997). From the available data, a significant
volatilization of 4-nitrophenol from water to air is not expected.
Since nitrophenols dissociate in aqueous solution, volatilization may
further decrease with increasing pH in surface waters. This leads to
the conclusion that dry and wet deposition of nitrophenols from air to
surface waters and soil are to be expected. The occurrence of this
partition mechanism is supported by the detection of 2- and
4-nitrophenol in rainwater and wet deposition samples (see section
6.1).
From experimental results on direct photodegradation (Koerdel et
al., 1981) and the atmospheric photo-oxidation by hydroxyl radicals
(Zetzsch et al., 1984), both pathways were found to be of minor
importance for the removal of 2-nitrophenol emitted to the
troposphere. Thus, the major degradation pathway for airborne
2-nitrophenol should be rapid nitration to 2,4-dinitrophenol
(Herterich & Herrmann, 1990; Luettke et al., 1997). The major portion
of airborne 4-nitrophenol is expected to be particle bound and
therefore only to a minor extent available for photochemical
reactions. Thus, most of the 4-nitrophenol can be washed out from air
by wet and dry deposition. Measured half-lives for the photochemical
decomposition of 4-nitrophenol in water exposed to sunlight ranged
from 2.8 to 13.7 days (Hustert et al., 1981; Mansour, 1996), being
longer with increasing pH (Hustert et al., 1981). Traces of
4-aminophenol were found as a photoproduct in river water (Mansour,
1996). In experiments conducted according to OECD guidelines, Andrae
et al. (1981) and Koerdel et al. (1981) found no hydrolysis of 2- or
4-nitrophenol under environmental conditions.
Numerous studies on the biodegradation of 2- and 4-nitrophenol
have been conducted. Standardized tests on ready or inherent
biodegradability provide data of large variability, indicating 2- and
4-nitrophenol to be inherently biodegradable under aerobic conditions
(depending on origin and density of inoculum and the applied test
method) (see Table 2). Results from different tests point to a
possible bacteriotoxic effect of 4-nitrophenol at concentrations above
300 mg/litre (Gerike & Fischer, 1979; Nyholm et al., 1984; Kayser et
al., 1994).
Non-standardized experiments with different inocula (e.g.,
natural water, soil, sediment) showed that microbial decomposition of
nitrophenols can occur in different environmental compartments after
adaptation of the microflora (Rubin et al., 1982; Subba-Rao et al.,
1982; Van Veld & Spain, 1983; Spain et al., 1984; Ou, 1985; Hoover et
al., 1986; Aelion et al., 1987; Wiggins et al., 1987). Time for
acclimation and degree of removal depended mostly on substance
concentration, microbial population, climate, and additional
substrates.
Biotic degradation of nitrophenols under anaerobic conditions
requires extended acclimatization of microbial communities. In tests
with sewage sludge and sludge from the primary anaerobic stage of a
municipal sewage treatment plant, respectively, initial 2- and
4-nitrophenol concentrations in the range of 96.5-579 mg/litre were
not degraded at all within 7-60 days (Wagner & Braeutigam, 1981;
Battersby & Wilson, 1989). Boyd et al. (1983) found complete anaerobic
removal of 50 mg/litre for all nitrophenol isomers within 1 week, but
complete mineralization was demonstrated only if the incubation period
was extended to 10 weeks. Anaerobic degradation even of high initial
nitrophenol concentrations was found by Tseng & Lin (1994), who
observed >90% removal of 2- and 4-nitrophenol (350-650 mg/litre) in a
biological fluidized bed reactor with three different kinds of
wastewater. From the available results, a slow degradation of
nitrophenols under anaerobic conditions by adapted microorganisms can
be expected.
Table 2: Biotic degradation of nitrophenols under aerobic conditions.
Test Substance Concentration Additional Test duration Removal Reference
(mg/litre) carbon source (days) (%)
Tests on ready biodegradability
AFNOR test 2-NP 40 OC no 14 16 Gerike & Fischer (1979)
Sturm test 2-NP 10 no 28 32 Gerike & Fischer (1979)
MITI I 2-NP 100 no 14 0 Urano & Kato (1986)
50 no 14 7 Gerike & Fischer (1979)
Closed bottle 4-NP 2 no 28 55 Rott et al. (1982)
test
Modified OECD 4-NP 20 DOC no 28 1 Rott et al. (1982)
screening test
Shake flask test 4-NP 20 OC no 21 50 Means & Anderson (1981)
AFNOR test 4-NP 40 OC no 14 97 Gerike & Fischer (1979)
Sturm test 4-NP 10 no 28 90 Gerike & Fischer (1979)
MITI I 4-NP 50 no 14 1 Gerike & Fischer (1979)
100 no 14 0 Urano & Kato (1986)
100 no 14 4.3 CITI (1992)
Tests on inherent biodegradability
Zahn-Wellens 2-NP 400 no 14 80 Gerike & Fischer (1979)
test
SCAS test 2-NP 20 TOC yes 24 107 Broecker et al. (1984)
13.3 TOC yes 24 110
Table 2 (continued)
Test Substance Concentration Additional Test duration Removal Reference
(mg/litre) carbon source (days) (%)
Bunch & Chambers 2-NP 5-10 yes 28 100 Tabak et al. (1981)
Coupled units 2-NP 12 OC yes 7 61 Gerike & Fischer (1979)
test
Batch test, 2-NP 200 COD no 5 97 Pitter (1976)
aerated
Zahn-Wellens 4-NP 300 no 14 8 Andrae et al. (1981)
test 100 DOC no 28 100 Pagga et al. (1982)
Activated sludge 4-NP 50 no 19 100 Means & Anderson
test 100 no 19 90 (1981)
SCAS test 4-NP 20 TOC yes 33 >90 Marquart et al. (1984)
27 >97 Scheubel (1984)
25/39 100 Ballhorn et al. (1984)
12-15 100 Koerdel et al. (1984)
Coupled units 4-NP 12 OC yes 7 100 Gerike & Fischer
test (1979)
Batch test, 4-NP 200 COD no 5 95 Pitter (1976)
aerated
Abbreviations used: 2-NP = 2-nitrophenol; 4-NP = 4-nitrophenol; OC = organic carbon; DOC = dissolved organic carbon;
TOC = total organic carbon; COD = chemical oxygen demand.
Soil sorption coefficients ( Koc) were found to increase with
increasing organic carbon content. Measured Koc values ranged from
44 to 230 (2-nitrophenol) and from 56 to 530 (4-nitrophenol) (Boyd,
1982; Broecker et al., 1984; Koerdel et al., 1984; Lokke, 1984;
Marquart et al., 1984). Nitrophenols emitted to soil are expected to
be biodecomposed under aerobic conditions. Infiltration into
groundwater is expected only under conditions unfavourable for
biodegradation (e.g., anaerobic conditions). From the available
experimental results, nitrophenols have to be classified as substances
with a low to moderate potential for soil sorption.
A low potential for bioaccumulation is to be expected from the
available valid test results for 2- and 4-nitrophenol.
Bioconcentration factors ranging from 14.6 to 24.4 were determined for
2-nitrophenol in a semistatic test system with zebra fish
( Brachydanio rerio) (Koerdel et al., 1984); in a flow-through
experiment, bioconcentration factors ranged from 30 to 76 for common
carp ( Cyprinus carpio), including possible conjugates (Broecker et
al., 1984). In static tests, accumulation factors for 4-nitrophenol of
11 for the green alga Chlorella fusca after 1 day (Geyer et al.,
1981) and 57 for the freshwater golden orfe ( Leuciscus idus
melanotus) after 3 days of exposure were determined (Freitag et al.,
1982). Zebra fish exposed in tap and river water nearly completely
eliminated the accumulated 14C-4-nitrophenol within 48 h (Ensenbach &
Nagel, 1991). Star fish ( Pisaster ochraceus) and sea urchin
( Strongylocentrotus purpuratus) eliminated 89% and 36%,
respectively, of injected 14C-4-nitrophenol (3.48 and 3.70 mg/kg body
weight, respectively) within 8 h (Landrum & Crosby, 1981).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
From the concentrations in rainwater, the total atmospheric
nitrophenol pollution in Switzerland is estimated at about 1 µg/m3
(Leuenberger et al., 1988). Recent measurements in the air of remote
areas in Europe (German Alps, Fichtelgebirge, Germany; Mount Brocken,
Germany; Great Dun Fell summit, United Kingdom) gave 2-nitrophenol
concentrations between 0.8 and 25 ng/m3 and 4-nitrophenol levels
between 1.2 and 360 ng/m3 (Herterich & Herrmann, 1990; Luettke et
al., 1997). The higher atmospheric 4-nitrophenol levels are apparently
due to the higher photochemical stability of this isomer (see section
5). 2-Nitrophenol was found in 22 out of 27 samples of air (range
1-140 ng/m3; detection limit 1 ng/m3) in Japan in 1994, and
4-nitrophenol was detected in 27 out of 27 air samples (range 1-71
ng/m3; detection limit 1 ng/m3) (Japan Environment Agency, 1995).
In street dust samples from a Japanese city, up to 3.9 mg
2-nitrophenol/kg and up to 42 mg 4-nitrophenol/kg were detected
(Nojima et al., 1983).
Numerous studies deal with the distribution, deposition, and
degradation behaviour of airborne 2- and 4-nitrophenol in clouds and
rainwater. 2-Nitrophenol levels in rainwater and snow between 0.03 and
5.7 µg/litre and 4-nitrophenol concentrations from <0.5 to
19 µg/litre are given in reports mainly from Germany and the USA (BUA,
1992). The recent measurements in rainwater, cloud water, and "fog"
(water vapour; not further characterized) from rural and urban areas
in Europe confirm these concentration ranges (Herterich & Herrmann,
1990; Levsen et al., 1990; Richartz et al., 1990; Capel et al., 1991;
Geissler & Schoeler, 1993; Levsen et al., 1993; Luettke et al., 1997).
The 2-nitrophenol levels are mostly below or slightly above the
detection limit (i.e., <0.1 µg/litre), whereas mean 4-nitrophenol
concentrations of about 5 µg/litre rainwater and cloud water and 20
µg/litre fog water were detected. The nitrophenol concentrations in
fog are significantly higher than those in rainwater or cloud water
owing to the higher droplet surface and longer residence times of the
droplets in air compared with rain. The lower concentrations of
2-nitrophenol in the deposition samples compared with 4-nitrophenol
are presumably due to the lower photochemical stability of this
compound (see section 5).
In the 1970s and early 1980s, the 2- and 4-nitrophenol
concentrations in the German and Dutch parts of the river Rhine and
some of its tributaries were between 0.1 and 1 µg/litre (BUA, 1992).
2-Nitrophenol and 4-nitrophenol were not detected in 177 samples of
Japanese surface waters (detection limits 0.04-10 µg/litre) or in 177
sediment samples (detection limits between 0.002 and 0.8 µg/kg) in
1978, 1979, and 1994 (Japan Environment Agency, 1979, 1980, 1995).
Whereas 4-nitrophenol was not detected in 129 fish samples (detection
limits 0.005-0.2 µg/kg) in Japan in 1979 and 1994, 2-nitrophenol was
detected in 1 out of 129 saltwater fish samples (detection limits
0.005-0.3 µg/kg) in 1994 (Japan Environment Agency, 1980, 1995).
2-Nitrophenol concentrations between <0.15 µg/litre (detection limit)
and 7.2 µg/litre and 4-nitrophenol levels between <0.1 and 18.8
µg/litre were reported for the densely populated and highly
industrialized Malaysian Klang river basin in 1990 and 1991 (Tan &
Chong, 1993).
6.2 Human exposure
Workers may be exposed to 2- and 4-nitrophenol via inhalation and
skin contact during production and processing (mainly in the
manufacturing of pesticides). However, data on nitrophenol
concentrations at the workplace were not identified.
Based on the measured concentrations given in section 6.1, an
exposure of the general population to nitrophenols via the environment
-- predominantly through ambient air and drinking-water -- cannot be
excluded.
4-Nitrophenol accumulates in fog, whereas 2-nitrophenol is
rapidly photochemically transformed (see sections 5 and 6.1). The mean
measured level of 4-nitrophenol in fog water is about 20 µg/litre.
In Dutch drinking-water samples, maximum concentrations of 1 µg
2-nitrophenol/litre and <0.1 µg 4-nitrophenol/litre were reported in
1988 (BUA, 1992). Further data are not available.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND
HUMANS
Studies providing quantitative information on the absorption,
metabolism, or elimination of 2- or 4-nitrophenol in humans were not
identified.
7.1 2-Nitrophenol
There is only very limited information available for
2-nitrophenol. In rabbits given a single dose of 200-330 mg/kg body
weight via gavage, most of the applied dose (>>80%) was excreted via
the urine within 24 h. About 71% was conjugated with glucuronic acid
and about 11% with sulfate, whereas about 3% was reduced to
aminophenols (Robinson et al., 1951).
Skin permeation for 2-nitrophenol was shown in several in vitro
experiments (Huq et al., 1986; Jetzer et al., 1986; Ohkura et al.,
1990).
Although the information is limited, bioaccumulation of
2-nitrophenol in organisms is not to be expected owing to its rapid
metabolism and excretion.
7.2 4-Nitrophenol
After oral, dermal, intravenous, or intraperitoneal application
of 4-nitrophenol to several test species (rats, mice, dogs, or
rabbits), most of the applied dose (up to 95%) was excreted as
glucuronide and sulfate conjugates of 4-nitrophenol via the urine
within 24-48 h. Only small amounts were excreted via faeces (about 1%)
or as unchanged 4-nitrophenol (about 2-7%). The percentages of
glucuronide and sulfate conjugates were shown to be species, sex, and
dose dependent. Although sulfate conjugation dominates at lower
4-nitrophenol concentrations, the percentage of glucuronide conjugates
increases at higher dosages (Robinson et al., 1951; Gessner & Hamada,
1970; Machida et al., 1982; Rush et al., 1983; Snodgrass, 1983;
Tremaine et al., 1984; Meerman et al., 1987). As shown in rabbits
after oral dosing, 4-nitrophenol undergoes reduction to 4-aminophenol
as well as glucuronidation and sulfation. Up to 14% of the
administered dose was detected as amino compounds in the urine
(Robinson et al., 1951). After intraperitoneal administration in mice,
4-nitrophenyl glucoside was identified as a minor metabolite of
4-nitrophenol (about 1-2% of the administered dose) (Gessner & Hamada,
1970).
For 4-nitrophenol, the pretreatment of laboratory animals with
ethanol (induction of cytochrome P-450) resulted in a marked increase
in hepatic microsomal hydroxylation. The 4-nitrocatechol then formed
competed with 4-nitrophenol for the glucuronidation and sulfation
pathways (Reinke & Moyer, 1985; Koop, 1986; McCoy & Koop, 1988; Koop &
Laethem, 1992).
Specific investigations on dermal resorption under non-occlusive
conditions showed dermal uptake of about 35% and 11% of the applied
dose of 14C-4-nitrophenol within 7 days in rabbits and dogs,
respectively. Skin permeation for 4-nitrophenol was also shown in
several in vitro experiments (Huq et al., 1986; Jetzer et al., 1986;
Ohkura et al., 1990).
Owing to its rapid metabolism and excretion, bioaccumulation of
4-nitrophenol in organisms is not to be expected.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
For 2-nitrophenol, the oral LD50 is in the range of
2830-5376 mg/kg body weight in rats (BASF AG, 1970; Vasilenko et al.,
1976; Vernot et al., 1977; Koerdel et al., 1981) and 1300-2080 mg/kg
body weight in mice (Vasilenko et al., 1976; Vernot et al., 1977).
Clinical signs following oral exposure were unspecific and included
dyspnoea, staggering, trembling, somnolence, apathy, and cramps. The
macroscopic examination performed in some studies revealed congestion
in liver and kidneys and ulcers of the stomach in high-dose rats. The
inhalation exposure of rats to an atmosphere saturated with the test
substance at 20°C for 8 h (no further information available) resulted
in no mortality and no signs of toxicity (BASF AG, 1970). In a limit
test, the dermal LD50 for the rat was >5000 mg/kg body weight
(Koerdel et al., 1981). In cats (two animals per dose group), the oral
application of 2-nitrophenol (50, 100, or 250 mg/kg body weight; no
controls) resulted in a dose-dependent increase in methaemoglobin (6,
44, and 57%, respectively).1 One animal dosed with 250 mg/kg body
weight died. No formation of methaemoglobin was detected after dermal
application of a 50% solution of 2-nitrophenol in water to rabbits
(dose not specified, exposure time 1 min to 20 h on the back or 20 h
on the ear) (BASF AG, 1970).
The oral LD50 of 4-nitrophenol is in the range of 220-620 mg/kg
body weight in rats (BASF AG, 1969; Vasilenko et al., 1976; Hoechst
AG, 1977a; Vernot et al., 1977; Andrae et al., 1981) and 380-470 mg/kg
body weight in mice (Vasilenko et al., 1976; Vernot et al., 1977).
Clinical signs following oral exposure of rats were unspecific and
included tachypnoea and cramps, and the macroscopic examination
performed in some studies revealed a greyish discoloration with dark
red patches of the lungs. No mortality was observed in rats after
single exposure (head only) to 4700 mg/m3 (application as dust
[sodium salt]; particle size not given) for 4 h. In four of six rats,
a corneal opacity was observed at the end of exposure, which persisted
through the 14-day observation period. In two extra rats exposed to
1510 mg/m3, the methaemoglobin concentrations were not altered
compared with controls. A determination of methaemoglobin
concentrations after exposure to 4700 mg/m3 was not performed (Smith
et al., 1988). In another inhalation study with rats (exposure to an
atmosphere saturated with the test substance at 20°C for 8 h; no
further information available), no mortality and no signs of toxicity
1 Methaemoglobin formation is discussed in greater detail in
section 8.8.
were seen (BASF AG, 1969). The dermal LD50 for rats and guinea-pigs
is >1000 mg/kg body weight (Hoechst AG, 1977b; Eastman Kodak Co.,
1980; Andrae et al., 1981). In contrast to 2-nitrophenol, no formation
of methaemoglobin was noted in cats (two animals per dose group) after
oral dosing with 100, 200, or 500 mg 4-nitrophenol/kg body weight. The
mortality rate was 0/2, 1/2, and 2/2, respectively (BASF AG, 1969).
8.2 Irritation and sensitization
From studies comparable to OECD Guidelines 404 and 405, it can be
concluded that 2-nitrophenol is slightly irritating to the skin but
not to the eye (scores not given). In a Buehler test with guinea-pigs
comparable to OECD Guideline 406, the substance showed no
skin-sensitizing effects (Koerdel et al., 1981).
In a study performed according to US Food and Drug Administration
(FDA) guidelines, non-dissolved 4-nitrophenol was slightly irritating
to the skin (score 2 of 8) (Hoechst AG, 1977c); in another study
comparable to OECD Guideline 404, however, the non-dissolved substance
showed no skin-irritating effects (score 0 of 4) (Andrae et al.,
1981). 4-Nitrophenol applied as a 10% solution to the eyes was
slightly irritating in a test conducted according to FDA guidelines
(scores not given; Hoechst AG, 1977c). Results with the non-dissolved
substance were either strongly irritating in a test conducted
according to FDA guidelines (scores not given; Hoechst AG, 1977c) or
slightly irritating in a test comparable to OECD Guideline 405 (score
1-2 of 4; Andrae et al., 1981).
In a guinea-pig maximization test comparable to OECD Guideline
406, skin sensitization was shown in 5 of 20 animals (Andrae et al.,
1981).
Data on respiratory tract sensitization for 2- and 4-nitrophenol
were not identified in the literature.
8.3 Short-term exposure
8.3.1 Oral exposure
The effect of 2-nitrophenol in rats was studied in a 28-day study
to evaluate OECD Guideline 407 (five animals per sex per dose group;
daily oral doses of 0, 22, 67, or 200 mg/kg body weight via gavage).
Food intake decreased in high-dose males and in mid- and high-dose
females, and final body weight decreased non-significantly in all
dosed animals. The absolute liver and kidney weights were decreased in
mid-dose animals, and the relative testes weight increased in low- and
mid-dose males and decreased in high-dose males. In all dosed animals,
the relative and absolute weights of the adrenal glands increased. The
haematological examination, clinical chemistry, and histopathological
examination of the major organs and tissues did not give any
indication of a substance-related toxic effect in comparison with
controls (Koerdel et al., 1981). Owing to insufficient documentation
and the fact that there were minor effects (weight of adrenal glands)
shown by all exposed animals, a reliable NO(A)EL cannot be deduced.
In a 28-day study that was also conducted to evaluate OECD
Guideline 407, Sprague-Dawley rats (10 per sex per dose group)
received daily oral doses of 0, 70, 210, or 630 mg 4-nitrophenol/kg
body weight via gavage. After dosing, locomotor inhibition, which
lasted for about 2 h, was seen in mid- and high-dose animals. In
mid-dose animals, 1/10 males died; in high-dose males and females, the
mortality rate was 4/10 and 6/10, respectively (specific signs of
intoxication were not given). In the lowest dose group, the
macroscopic examination revealed seven cases of pale liver, and the
histopathological examination showed 14 cases of finely dispersed
fatty degeneration. A focal fatty degeneration of the liver was also
observed in 13/20 rats of the mid-dose group, but not in high-dose
animals. However, it must be noted that finely dispersed fatty
degeneration was also seen in 6/20 control animals. In 4/10 high-dose
males but not females, a hydropic liver cell swelling was noted, and
all high-dose rats that died before the end of the study showed
vascular congestion of the liver. A slight increase in the leukocyte
count was seen at 210 and 630 mg/kg body weight in males and females;
the increase was significant in high-dose females. In high-dose males,
the alanine aminotransferase (ALAT) activity was significantly
increased. Other substance-related effects in high-dose animals
included increased nephrosis (two males and five females), testicular
atrophy and inhibition of spermatogenesis (one and two males,
respectively), and follicular atresia in the ovaries (four females)
(Andrae et al., 1981). Because of unclear effects in the liver, a
NO(A)EL cannot be deduced.
8.3.2 Inhalation exposure
8.3.2.1 2-Nitrophenol
In Sprague-Dawley rats (15 per sex per group), no mortality was
observed after exposure to 0, 5, 30, or 60 mg 2-nitrophenol vapour/m3
("whole body" exposure; to generate the vapour, melted 2-nitrophenol
was used) for 6 h/day, 5 days/week, over a period of 4 weeks. Except
for squamous metaplasia of the epithelium lining the maxilloturbinates
and nasoturbinates in all high-dose animals, the clinical and
histopathological examinations gave no consistent exposure-related
effects. The methaemoglobin values determined after the 11th exposure
were significantly increased only in low-dose animals (males: 1.0,
2.3, 1.8, and 1.6%; females: 2.0, 4.1, 2.1, and 1.1%), but were within
control values at the end of the study (Hazleton Lab., 1984).
8.3.2.2 4-Nitrophenol
No mortality was observed in male albino Crl:CDR rats (10 per
group) after exposure to 0, 340, or 2470 mg 4-nitrophenol dust/m3
(application as sodium salt; "head only" exposure; mass median
aerodynamic diameter [MMAD] 4.6-7.5 µm) for 6 h/day, 5 days/week, over
a period of 2 weeks. Both exposure concentrations resulted in signs of
irritation (not further specified). After exposure to 340 and 2470
mg/m3, darker urine, proteinuria, elevated aspartate aminotransferase
(ASAT) values, and a dose-dependent increase in methaemoglobin values
were observed. These effects were still evident after a 14-day
recovery period; however, the methaemoglobin value was then still
elevated in only 2/5 high-dose animals. The methaemoglobin values were
0.2, 0.87, and 1.53% after 10 exposures and 0.2, 0.13, and 0.7% after
14 days' recovery. The erythrocyte, haemoglobin, and haematocrit
values decreased during exposure but were elevated after the 14-day
recovery period. In treated rats, the urine volume decreased in a
dose-dependent manner during exposure and during the 14-day recovery
period. In high-dose animals, the absolute spleen weight was
significantly lower than that of controls after 10 exposures, and the
absolute/relative spleen and lung weights were significantly lower in
comparison with controls at the end of the recovery period. According
to the authors, the biological significance of the changes in organ
weights is unknown owing to the absence of corroborating pathological
effects (Smith et al., 1988).
In a second trial (exposure to 0, 30, or 130 mg/m3; MMAD 4.0-4.8
µm), both exposure concentrations again resulted in signs of
irritation (not further specified). Methaemoglobinaemia, an effect
that was reversible within a 14-day recovery period, was seen only at
130 mg/m3. The methaemoglobin values were 0.5, 0.3, and 1.5% after 10
exposures and 0.4, 0.5, and 0.2% after 14 days' recovery. The gross
and histopathological examination revealed no adverse effects in any
dose group. From these results, the authors of the study decided upon
a NO(A)EL of 30 mg/m3 (Smith et al., 1988).
Groups of Sprague-Dawley rats (15 per sex) were exposed to 0, 1,
5, or 30 mg 4-nitrophenol dust/m3 ("whole body" exposure; MMAD
5.2-6.7 µm) for 6 h/day, 5 days/week, over a period of 4 weeks. The
exposure resulted in no deaths, and no exposure-related effects were
noted in terms of haematology or clinical chemistry values, gross
examination, histopathology, and body or organ weights. In high-dose
animals, unilateral and bilateral diffuse anterior capsular cataracts
were observed. The methaemoglobin values determined after 2 weeks of
exposure showed great variability and appeared to be unusually high
(>3 %) in some unexposed controls. However, the group total
methaemoglobin value was increased at a concentration of 5 mg/m3,
which was significant in males and not significant in females (males:
0.8, 0.5, 2.2, and 1.1%; females: 1.3, 1.1, 2.0, and 1.0%) (Hazleton
Lab., 1983). Therefore, a NO(A)EL of 5 mg/m3 can be derived for local
effects (cataracts), whereas the NO(A)EL for systemic effects
(formation of methaemoglobin) may be lower.
8.3.3 Dermal exposure
Data concerning short-term dermal exposure were not identified in
the literature.
8.4 Long-term exposure
In the literature, subchronic and chronic studies are available
only for 4-nitrophenol.
8.4.1 Subchronic exposuren
In a 13-week gavage study with Sprague-Dawley rats (20 per sex
per dose group) given 0, 25, 70, or 140 mg 4-nitrophenol/kg body
weight in water 5 days/week, premature deaths were seen in animals
dosed with 70 and 140 mg/kg body weight (1 male/1 female at 70 mg/kg
body weight and 15 males/6 females at 140 mg/kg body weight); these
were usually preceded by clinical signs, including pale appearance,
languid behaviour, prostration, wheezing, and dyspnoea, shortly after
dosing. The histopathological examination of these animals revealed
minimal to moderately severe congestion in the lung, liver, kidney,
adrenal cortex, and pituitary; in surviving animals, no
treatment-related changes compared with controls were reported. A
statement concerning altered methaemoglobin values cannot be given
owing to a non-reliable analytical method (about 13% in controls at
week 7) (Hazleton Lab., 1989). Therefore, only a provisional NO(A)EL
(changes in liver, kidneys, and lungs) of 25 mg/kg body weight can be
derived from this study. The NO(A)EL based on the formation of
methaemoglobin may be lower.
The dermal application of 4-nitrophenol to Swiss-Webster mice (10
per sex and dose group; given 0, 22, 44, 88, 175, or 350 mg/kg body
weight in acetone, 3 times per week over 13 weeks) resulted in
dose-dependent mortality as well as skin irritation/inflammation and
necrosis at >175 mg/kg body weight.1
1 Gulf South Research Institute, not dated;
no further information available; results cited from
NTP (1993).
8.4.2 Chronic exposure and carcinogenicityn
In a long-term study with Swiss-Webster mice (50 per sex per dose
group), 4-nitrophenol in acetone was applied to the interscapular skin
at doses of 0, 40, 80, or 160 mg/kg body weight, 3 days/week for 78
weeks. At termination of the study, the survival rates were 29/60,
17/60, 26/60, and 24/60 for males and 35/60, 26/60, 33/60, and 27/60
for females. The increased mortality after 60 weeks was due to a
generalized amyloidosis (the severity of the amyloidosis was similar
among dosed and control animals) and secondary kidney failure. The
final mean body weights of the dosed animals were similar to those of
the controls. NTP (1993) stated that there were no substance-related
neoplastic or non-neoplastic effects associated with the dermal
administration of 4-nitrophenol and that there was no evidence of a
carcinogenic activity of the substance in male or female mice.
In another study, which had several procedural deficiencies (only
the skin was examined; only 12 weeks of exposure), no skin tumours
were observed in 31 female Sutter mice after dermal application of a
20% solution (25 µl of solution applied twice weekly) of 2- or
4-nitrophenol in dioxane (Boutwell & Bosch, 1959).
8.5 Genotoxicity and related end-points
The available in vitro and in vivo genotoxicity studies on
2- and 4-nitrophenol are summarized in Table 3.
2-Nitrophenol showed no mutagenicity in several limited bacterial
assays. From the available data, it is not possible to draw any
conclusions regarding its mutagenicity.
For 4-nitrophenol, positive results were obtained in in vitro
tests for chromosomal aberrations in mammalian cells. However, apart
from one well-documented study published by NTP (1993), the other
available assays were inadequately reported. 4-Nitrophenol was shown
to be mutagenic in some but not all of the bacterial assays, whereas
other studies (i.e., host-mediated bacterial assay, mouse lymphoma
assay, unscheduled DNA synthesis assay [apparently in vitro], sister
chromatid exchange assay, sex-linked recessive lethal [SLRL] assay in
Drosophila) gave negative results. In the absence of any in vivo
mutagenicity studies in mammals, it is not possible to conclude
whether or not the mutagenic potential of 4-nitrophenol is expressed
in vivo.
Table 3: Genotoxicity of 2- and 4-nitrophenol in vitro and in vivo.
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
2-Nitrophenol
(in vitro studies)
delta phage DNA Induction of 35 mg - 0 Yamada et al.
DNA breakage (1987)
Bacillus subtilis Recombination 0.01-0.5 - 0 Shimizu & Yano
H17, M45 assay mg/plate (1986)
Salmonella Reverse 0.003-2.5 - - Koerdel et al. (1981);
typhimurium mutations mg/plate Haworth et al. (1983);
TA1535, TA1537 Shimizu & Yano (1986)
Salmonella Reverse 0.01-2.5 - - Koerdel et al. (1981);
typhimurium mutations mg/plate Shimizu & Yano (1986)
TA1538
Salmonella Reverse 0.0007-5 - - Suzuki et al. Chiu et al. (1978);
typhimurium mutations mg/plate (1983) also Koerdel et al. (1981);
TA98, TA100 tested both Haworth et al. (1983);
strains in Suzuki et al. (1983);
the presence Shimizu & Yano (1986);
of norharman, Kawai et al. (1987);
which also Dellarco & Prival (1989);
gave negative Massey et al. (1994)
results
Table 3 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
2-Nitrophenol (in vivo studies)
Drosophila SLRL assay via feed (400 and 500 - Foureman et al. (1994)
melanogaster ppm) or injection
(2500 and 5000 ppm)
4-Nitrophenol (in vitro studies)
delta phage DNA Induction of 35 mg - 0 Yamada et al. (1987)
DNA breakage
Bacillus subtilis Recombination 0.01-5 mg/plate + 0 positive Shimizu
H17, M45 assay at 0.5 & Yano (1986)
mg/plate
Escherichia coli Gene mutation 0.001-2.5 - - Hoechst AG (1980)
WP2uvrA mg/plate
Escherichia coli Gene mutation 0.125-2 mg/plate - 0 Rashid & Mumma (1986)
K-12 (Pol
A1+/Pol1-), WP2
(WP2, WP2uvrA,
WP67, CM611,
CM571)
Escherichia coli DNA cell 7 or 70 mg + + positive at Kubinski et al.
Q13 binding assay 70 mg (1981)
Table 3 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
Saccharomyces Mitotic gene 2.9 mg/ml (+) 0 Fahrig (1974)
cerevisiae conversion
ade 2, trp 5
Salmonella DNA damage up to 0.75 - - Nakamura et al.
typhimurium (umu test) mg/ml (1987)
TA1535/pSK 1002
Salmonella Reverse 0.001-2.5 + - positive Hoechst
typhimurium mutation mg/plate at >0.1 AG (1980)
TA1538 mg/plate
Salmonella Reverse 0.01-5 - - Andrae et al. (1981);
typhimurium mutation mg/plate Shimizu & Yano (1986)
TA1538
Salmonella Reverse 0.125-2 - 0 Rashid & Mumma (1986)
typhimurium mutation mg/plate
TA1538, TA1978
Salmonella Reverse 0.0007- - - Suzuki et McCann et al. (1975);
typhimurium mutation 5 mg/plate al. (1983) Hoechst AG (1980);
TA98, TA100 also tested Andrae et al. (1981);
both strains Haworth et al. (1983);
in the Suzuki et al. (1983);
presence of Shimizu & Yano (1986);
norharman, Kawai et al. (1987);
which also Dellarco & Prival (1989);
gave negative Massey et al. (1994)
results
Table 3 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
Salmonella Reverse 0.001-5 - - McCann et al. (1975);
typhimurium mutation mg/plate Hoechst AG (1980);
TA1535, TA1537 Andrae et al. (1981);
Haworth et al. (1983);
Shimizu & Yano (1986)
Rat hepatocytes DNA damage 42-417 mg (+) 0 Weakly Storer
(alkaline positive at et al. (1996)
elution) >97 mg
Rat hepatocytes DNA repair 4.2-417 mg - 0 Andrae et al. (1981)
Chinese hamster Chromosomal without S9 mix: - + NTP (1993)
ovary (CHO) aberration 0.1-0.5 mg/ml
cells with S9 mix:
1.25-2 mg/ml
Chinese hamster Sister chromatid without S9 mix: - - NTP (1993)
ovary (CHO) cells exchange 0.00017-0.025 mg/ml
with S9 mix:
0.05-1.5 mg/ml
Mouse lymphoma Forward mutation without S9 mix: - - Oberly et al. (1984)
assay 0.7-1.5 mg/ml
L5178Y with S9 mix:
TK+/- cells 0.0001-0.03
mg/ml
Table 3 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
Mouse lymphoma Forward mutation 0.06-0.78 mg/ml 0 - Amacher & Turner (1982)
assay L5178Y
TK+/- cells
Rat hepatocytes Unscheduled DNA 0.00007-0.14 mg/ml - 0 Probst et al. (1981)
synthesis
Human lymphocytes Chromosomal not given + No data about Huang et al. (1996)
aberration metabolic
activation;
validity
cannot be
judged
(documentation
and study
design
insufficient
for
assessment)
lowest
positive
concentration:
1.4 mg/ml
Table 3 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
Human Chromosomal 0.001-0.3 mg/ml + No data about Huang et al. (1995)
lymphocytes aberration metabolic
activation;
validity cannot
be judged
(documentation
and study
design
insufficient
for assessment)
Human fibroblasts DNA repair 0.14-139 mg + No data about Poirier et al. (1975)
(WI-38) metabolic
activation;
validity
cannot be
judged
(documentation
insufficient
for assessment)
positive at
>13.9 mg
Table 3 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
4-Nitrophenol (in vivo studies)
NMRI mice Host-mediated single subcutaneous - application of Buselmaier et al. (1972)
assay (tester injection of test substance
strains 75 mg/kg immediately
Salmonella body weight after the
typhimurium G 46 bacteria had
and Serratia been injected
marcescens a into the
21 Leu-) abdominal
cavities;
test duration
3 h
Drosophila SLRL assay via feed (1000, - Zimmering et al. (1985);
melanogaster 2500, 6000, or Foureman et al. (1994)
7500 ppm) or
injection (1000
or 1500 ppm)
a -, negative; +, positive; (+), weakly positive; 0, not tested.
8.6 Reproductive and developmental toxicity
8.6.1 Reproductive toxicity
In a valid two-generation study with groups of 24 female and 12
male Sprague-Dawley rats carried out by Angerhofer (1985),
4-nitrophenol dissolved in ethanol was applied dermally at doses of 0,
50, 100, or 250 mg/kg body weight per day, 5 days/week. The F0
generation was exposed over a period of 140 days before mating. Dosing
of the F0 females continued throughout breeding, gestation, and
lactation. Groups of 26 females and 13 males of the F1 generation
were then exposed for 168 days in the same manner as had been the F0
rats; the females were again exposed throughout breeding, gestation,
and lactation. Apart from dose-related signs of skin irritation
(erythema, scaling, scabbing, and cracking) in dosed animals, the
gross and histopathological examinations provided no indication of
significant adverse effects. The calculated indices concerning
fertility, gestation, viability, and lactation were not different from
those of controls. The testis to body weight ratios in the F0
generation were not affected, and histological lesions were not
observed in the testes. In a 28-day study in rats (see section 8.3.1),
testicular atrophy and inhibition of spermatogenesis were observed in
some animals after oral dosing at a level of 630 mg/kg body weight,
but not at 210 mg/kg body weight.
8.6.2 Developmental toxicity
8.6.2.1 2-Nitrophenol
In a range-finding study with Charles River COBS(c) CD(c) rats
(five dams per group; application of 0, 50, 125, 250, 500, or
1000 mg/kg body weight via gavage from day 6 to day 15 of gestation;
uterine examination on day 20), dose levels of 500 and 1000 mg/kg body
weight caused signs of maternal toxicity (transient but dose-related
decrease in weight gain early during treatment). One high-dose animal
died, but no cause of death could be determined. Other clinical
findings included darkly coloured urine at >250 mg/kg body weight
and yellow staining of haircoat (at the nose, mouth, anogenital area)
at >125 mg/kg body weight; the necropsy findings gave no
biologically meaningful differences in surviving dams. At the highest
dose level of 1000 mg/kg body weight, a slight but statistically
significant (also compared with historical controls) increase in group
mean post-implantation losses (13.8% versus 8.2% in controls) and mean
early resorptions (2.3 versus 1.2 in controls) was seen. No effects
were observed on the number of viable fetuses, implantations, or
corpora lutea (International Research and Developmental Corporation,
1983).
8.6.2.2 4-Nitrophenol
In both studies cited below, a complete examination of the pups
for possible teratogenic effects was not performed. In addition, owing
to limitations of these studies (i.e., use of only one dose group or
exposure to a mixture), reliable NO(A)EL values cannot be derived.
In a study performed by Booth et al. (1983), groups of 50 female
CD-1 mice received daily oral doses of 400 mg 4-nitrophenol/kg body
weight via gavage from day 7 to day 14 of gestation. The survival rate
in pregnant mice ( n = 36) was 81% versus 100% in controls, and dosed
animals showed less maternal weight gain. No changes were observed in
the reproductive index (ratio between survivors delivered and pregnant
survivors). The average number of live pups per litter was slightly
decreased, but 4-nitrophenol produced no gross abnormalities.
Kavlock (1990) studied the developmental toxicity of
4-nitrophenol in Sprague-Dawley rats. The substance (dissolved in a
mixture of water, Tween 20, propylene glycol, and ethanol [4:4:1:1])
was applied via gavage to groups of 12-13 animals at doses of 0, 100,
333, 667, or 1000 mg/kg body weight on day 11 of gestation. Endpoints
concerning maternal toxicity included signs of toxicity, mortality,
body weight gain, and the number of implantation scars in the uteri at
weaning. In the offspring, viability, body weight on postnatal days
1-6, overt malformations, and perinatal loss were recorded. In dams,
the mortality was increased at a dose level of >667 mg/kg body
weight; at a dose level of >333 mg/kg body weight, the litter size
on postnatal days 1 and 6 was non-significantly decreased.
8.7 Immunological and neurological effects
There are no studies available dealing specifically with
immunological or neurological effects. There is an indication from an
in vitro study that 4-nitrophenol may act as a suppressor of
cell-mediated immune response (Pruett & Chambers, 1988). However, the
biological significance is uncertain.
8.8 Methaemoglobin formation
Methaemoglobin formation by 2-nitrophenol and 4-nitrophenol has
been tested in several studies using different species, routes, and
durations of applications. An overview is given in Table 4.
2-Nitrophenol clearly leads to the formation of methaemoglobin in
a dose-dependent manner in cats (BASF AG, 1970), the most sensitive
species. The lowest dose tested, 50 mg/kg body weight, produced
increased methaemoglobin levels. In inhalation experiments in rats,
elevated methaemoglobin levels were observed at an exposure level of 5
mg/m3; methaemoglobin levels were less elevated at exposure levels of
30 and 60 mg/m3 (Hazelton Lab., 1984).
4-Nitrophenol, in contrast, did not lead to methaemoglobin
formation in cats at concentrations up to 500 mg/kg body weight (BASF
AG, 1969). In rats, at high concentrations in inhalation experiments,
the methaemoglobin-forming capacity seemed to be very low (1.5% at
2470 mg/m3). In conclusion, 4-nitrophenol may induce methaemoglobin
formation, but the effect seems to be rather weak, without clear
dose-response.
9. EFFECTS ON HUMANS
Naniwa (1979) performed patch tests with 4-nitrophenol,
4-aminophenol, 2-amino-4-chlorophenol,
3'-chlorodiphenylamine-2-carboxylic acid, and 4-dichloronitrobenzene
(0.1, 0.5, or 1% in petrolatum) on 31 employees probably exposed to
these chemicals in a chemical factory and on 5 control persons. In
four employees, a positive reaction to 4-nitrophenol was observed,
although none of these persons reacted positively to all three tested
concentrations. All four employees also reacted positively to
2-amino-4-chlorophenol, which was shown to be a strong sensitizer.
Therefore, 2-amino-4-chlorophenol may act as the primary allergen, and
the effects observed with 4-nitrophenol may be due to
cross-sensitization.
In 27 patients primarily sensitized to
1-chloro-2,4-dinitrobenzene, no cross-sensitization due to
4-nitrophenol (1-2% in petrolatum) was observed. In addition, 15
patients with a chloramphenicol allergy failed to react to
4-nitrophenol (Eriksen, 1978).
Table 4: Methaemoglobin formation by 2-nitrophenol and 4-nitrophenol.
Species Route Frequency/duration Dose Results Reference
(strain/number/dose/sex) (% metHb)
2-Nitrophenol
cat oral 1 × 50 mg/kg body weight 6 BASF AG (1970)
2 100 44
sex not given 250 57
rabbit dermal 1 × 50% solution no increase BASF AG (1970)
number and sex in water
not given
rat inhalation 6 h/day m f Hazleton Lab.
Sprague-Dawley 5 days/week 0 mg/m3 1.0 2.0 (1984)
15 m/15 f 4 weeks 5 2.3 4.1
30 1.8 2.1
60 1.6 1.1
11th day of
treatment
4-Nitrophenol
cat oral 1 × 100 mg/kg body no increase BASF AG (1969)
2 200 weight
sex not given 500
rat oral 5 days/week 0 mg/kg body weight analytical Hazleton Lab.
Sprague-Dawley 13 weeks 25 method not (1989)
20 m/20 f 70 reliable (13%
140 in controls)
Table 4: Methaemoglobin formation by 2-nitrophenol and 4-nitrophenol.
Species Route Frequency/duration Dose Results Reference
(strain/number/dose/sex) (% metHb)
rat inhalation 6 h/day 0 mg/m3 0.2 0.2 Smith et al.
Crl:CDR 5 days/week 340 0.87 0.13 (1988)
10 m 2 weeks 2470 1.53 0.7
end of treatment
and after 14
days of recovery
rat inhalation 6 h/day 0 mg/m3 0.5 0.4 Smith et al.
Crl:CDR 5 days/week 30 0.3 0.5 (1988)
10 m 2 weeks 130 1.5 0.2
end of treatment
and after 14
days of recovery
rat inhalation 6 h/day m f Hazleton Lab.
Sprague-Dawley 5 days/week 0 mg/m3 0.8 1.3 (1983)
15 m/15 f 4 weeks 1 0.5 1.1
5 2.2 2.0
30 1.1 1.0
after 2 weeks of
exposure, values
unusually high
in some control
animals
Abbreviations used: m = male; f = female; metHb = methaemoglobin.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1 Aquatic environment
Experimental test results for the most sensitive species are
summarized in Table 5. Additional data on the toxicity of 2- and
4-nitrophenol to aquatic organisms are cited in BUA (1992). Among all
tested organisms, the protozoan Entosiphon sulcatum and the green
alga Scenedesmus subspicatus proved to be most sensitive in chronic
cell multiplication inhibition tests with freshwater species. Daphnia
magna exhibited a 21-day lowest-observed-effect concentration (LOEC)
of 1.0 mg 2-nitrophenol/litre in the Daphnia reproduction test
(Koerdel et al., 1984). The entoproct Barentsia matsushimana was the
most sensitive marine invertebrate species tested, exhibiting a 49-day
EC50 value of 0.21 mg 4-nitrophenol/litre and a minimal effective
concentration (ECm) of 0.03 mg/litre (end-point: growth of
germinated spores) (Scholz, 1986). Freshwater fish showed less
sensitivity. The lowest 96-h LC50 value of 3.8 mg 4-nitrophenol/litre
was determined for rainbow trout ( Oncorhynchus mykiss) (Howe et
al., 1994). The measured no-observed-effect concentration (NOEC) for
behavioural changes in a 28-day flow-through test with zebra fish was
2 mg 2-nitrophenol/litre (Broecker et al., 1984). After prolonged
exposure of zebra fish to 4-nitrophenol, minor morphological
alterations of the liver, even at a concentration of 0.1 mg/litre,
were observed. At 1 and 5 mg/litre, about 25% of the animals showed
symptoms of degenerative transformation of the liver tissue (Braunbeck
et al., 1989).
10.2 Terrestrial environment
The toxicity of 2- and 4-nitrophenol on higher plants according
to OECD Guideline 208 was tested in independent studies. After
incubation of seeds with different test substance concentrations,
14-day EC50 values for reduced fresh weight of grown shoots were in
the range of 52-420 mg 2-nitrophenol/kg soil (Broecker et al., 1984;
Koerdel et al., 1984) and 35-260 mg 4-nitrophenol/kg soil (Ballhorn et
al., 1984; Marquart et al., 1984). The 14-day EC10 value for
2-nitrophenol was 10 mg/kg soil for both species. Overall, turnip
( Brassica rapa) proved to be more sensitive than oat
( Avena sativa).
In tests conducted according to OECD Guideline 207, the adverse
effects of 2- and 4-nitrophenol on earthworms were examined in several
independent studies. In the contact test, in which the animals are
exposed on filter paper soaked with the test substance, Neuhauser
et al. (1985) established a 48-h LC50 value of 5.9 µg/cm2 for the
toxicity of 2-nitrophenol on Eisenia fetida. For 4-nitrophenol, the
48-h LC50 values were in the range of 0.7-2.7 µg/cm2, with
Table 5: Aquatic toxicity of nitrophenols.
Most sensitive species
(test method/end-point) Substance Effective Reference
concentration
(mg/litre)
Bacteria
Pseudomonas putida 2-NP 16-h MICa: 0.9 Bringmann & Kuehn (1977)
(cell multiplication 4-NP 16-h MIC: 4.0
inhibition test)
Protozoa
Entosiphon sulcatumn 2-NP 72-h MIC: 0.40 Bringmann (1978);
(cell multiplication 4-NP 72-h MIC: 0.83 Bringmann et al. (1980)
inhibition test)
Algae
Scenedesmus subspicatus 2-NP 96-h EC50: 0.39 Broecker et al. (1984);
Chlorella vulgaris 2-NP 6-h EC50: 1.53 Kramer et al. (1986)
(cell multiplication 4-NP 6-h EC50: 6.97
inhibition test)
Invertebrates
Moina macrocopa (acute) 2-NP 3-h LC50: 1.9 Yoshioka et al. (1985)
(immobilization) 4-NP 3-h LC50: 1.3
Daphnia magna (long-term) 2-NP 21-day LOEC: 1.0 Koerdel et al. (1984)
(immobilization/reproduction) 4-NP 21-day NOEC: 1.3
Barentsia matsushimana (marine) 4-NP 49-day EC50: 0.21 Kuehn et al. (1988)
(growth of germinated spores) 4-NP 49-day ECmb: 0.03 Scholz (1986)
Table 5 (cont'd)
Most sensitive species
(test method/end-point) Substance Effective Reference
concentration
(mg/litre)
Fish
Cyprinus carpio (static) 2-NP 96-h LC50: 36.6 Lang et al. (1996)
Oncorhynchus mykiss (static) 4-NP 96-h LC50: 3.8 Howe et al. (1994)
Oncorhynchus mykiss (flow-through) 4-NP 96-h LC50: 7.93
Abbreviations used: 2-NP = 2-nitrophenol; 4-NP = 4-nitrophenol.
a MIC = minimum inhibitory concentration.
b ECm = minimal effective concentration.
Eisenia fetida and Eudrilus eugeniae being the most sensitive
species tested (Roberts & Dorough, 1984; Neuhauser et al., 1985,
1986). When exposed in an artificial soil mixture, 28-day LC50 values
for 2-nitrophenol were in the range of 250-500 mg/kg soil ( Eisenia
fetida) (Broecker et al., 1984; Koerdel et al., 1984), and 14-day
LC50 values for 4-nitrophenol were in the range of 38-67 mg/kg soil,
again with Eisenia fetida and Eudrilus eugeniae as the most
sensitive species tested (Ballhorn et al., 1984; Marquart et al.,
1984; Neuhauser et al., 1985, 1986).
The environmental relevance, particularly of the earthworm
contact test, seems questionable. Critical results from this test, as
sole effect data on terrestrial organisms, should not justify a
classification of tested substances as highly toxic to earthworms or
other soil organisms. The available data on microorganisms and plants
indicate only a moderate toxicity potential in the terrestrial
environment.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessmentn
In general, there is only limited information concerning the
toxicological profiles of 2- and 4-nitrophenol.
In experimental animals given 4-nitrophenol orally,
intravenously, or intraperitoneally, most of the applied dose was
excreted via the urine within 24-48 h as glucuronide and sulfate
conjugates, while only very small amounts were excreted via faeces or
as unchanged 4-nitrophenol. In rabbits, after oral dosing,
4-nitrophenol undergoes reduction to 4-aminophenol as well as
glucuronidation and sulfation. In vivo and in vitro studies gave
an indication for dermal uptake. For 2-nitrophenol, the information is
very limited. However, a comparable metabolic transformation is
assumed based on the available data. Owing to their rapid metabolism
and excretion, bioaccumulation of 2- and 4-nitrophenol in organisms is
not to be expected.
With oral LD50 values of 220-620 mg/kg body weight in rats and
380-470 mg/kg body weight in mice, 4-nitrophenol is harmful after oral
uptake and was found to be more toxic than 2-nitrophenol. A
dose-dependent increase in the formation of methaemoglobin was seen in
cats after oral exposure to 2-nitrophenol -- but not after exposure to
4-nitrophenol -- and in rats after exposure by inhalation to
4-nitrophenol.
Most of the studies concerning skin- or eye-irritating effects in
experimental animals are limited as a result of insufficient
documentation. However, from the available data, it can be concluded
that 2-nitrophenol is slightly irritating to the skin but
non-irritating to the eye, and the substance proved to have no
sensitizing effects. For 4-nitrophenol, irritating effects on skin and
eye are assumed based on the studies performed according to OECD/FDA
guidelines; in addition, signs of irritation were reported after
exposure by inhalation as well as subchronic dermal exposure. In a
guinea-pig maximization test, 4-nitrophenol was considered to be
sensitizing. Positive patch tests were recorded in humans exposed to
4-nitrophenol. Although this may have been due to cross-sensitization,
sensitization to 4-nitrophenol in humans cannot be excluded.
Only a few limited studies concerning repeated oral exposure to
2- and 4-nitrophenol in experimental animals were identified. With
2-nitrophenol, decreases in body weight gain accompanied by decreased
food consumption and differences in organ weights without clear dose
dependency were found. However, the haematological examination,
clinical chemistry, and histopathological examination of the major
organs and tissues gave no indication for a substance-related toxic
effect compared with controls. In rats dosed with 4-nitrophenol, a
focal fatty degeneration of the liver as well as congestion in several
organs were the major histopathological findings. Other reported
effects included haematological changes, nephrosis, testicular
atrophy, and follicular atresia in the ovaries. The exposure by
inhalation to 2-nitrophenol vapour caused squamous metaplasia of the
epithelium of the upper respiratory tract; with 4-nitrophenol dust
(applied as sodium salt), haematological changes, increased
methaemoglobin values, and differences in organ weights were noted.
For the effects given in these studies, it was not possible to
identify a clear dose-response or reliable NO(A)EL values.
Insufficient data are available on 2-nitrophenol to allow any
conclusions to be made about its possible mutagenicity. For
4-nitrophenol, more mutagenicity studies are available, and the
substance was shown to be mutagenic in some but not all of the
bacterial assays. In addition, positive results were obtained in
in vitro tests for chromosomal aberrations in mammalian cells;
however, apart from one well-documented study, the available assays
were inadequately reported. In the absence of any in vivo
mutagenicity studies in mammals, it is not possible to conclude
whether or not the mutagenic potential of 4-nitrophenol is expressed
in vivo.
4-Nitrophenol was not carcinogenic in male or female mice after
dermal application over 78 weeks. In a limited study with female mice,
no skin tumours were seen after dermal application of 2- or
4-nitrophenol over 12 weeks. No carcinogenicity studies using the oral
or inhalation routes were available for either of the isomers.
No reproductive effects were observed in rats exposed to
4-nitrophenol in a two-generation study. For developmental toxicity,
the available studies were inadequately performed (i.e., only one dose
was applied, or animals were dosed only on one day with a mixture). In
an oral study with rats, 2-nitrophenol induced developmental effects
in the offspring only at doses that also produced maternal toxicity.
However, the fetuses were not examined for internal malformations.
Data on humans relevant for the assessment of potential adverse
effects are limited to some patch tests performed with 4-nitrophenol.
11.1.2 Criteria for setting guidance values for 2- and 4-nitrophenol
As given in section 8, the database for 2-nitrophenol is
inadequate for calculating a tolerable daily intake (TDI) or a
tolerable concentration (TC).
For 4-nitrophenol, the formation of methaemoglobin was shown to
be the most critical end-point after exposure by inhalation and is
assumed to be relevant for oral exposure too. However, owing to the
inaccuracy of the analytical method used in the 13-week study with
oral application, a reliable NO(A)EL cannot be derived. Therefore, at
present, no TDI for 4-nitrophenol can be developed owing to inadequacy
of the database.
Longer-term toxicity studies concerning inhalation exposure were
not identified in the literature, and the NO(A)EL values derived for
4-nitrophenol from short-term studies gave considerable differences
(2-week exposure: NO(A)EL of about 30 mg/m3; 4-week exposure: NO(A)EL
of about 5 mg/m3). The NO(A)EL of 5 mg/m3 was derived for local
effects (cataracts), whereas the NO(A)EL for systemic effects
(formation of methaemoglobin) may be lower. Therefore, a reliable TC
for exposure by inhalation cannot be calculated, as the formation of
methaemoglobin is the critical end-point.
11.1.3 Sample risk characterization
As given in section 6.2, workers may be exposed to 2- and
4-nitrophenol via inhalation and skin contact during production and
processing (mainly in the manufacturing of pesticides). However, data
on nitrophenol concentrations at the workplace were not identified.
For the general population, an exposure to nitrophenols via the
environment cannot be excluded (see also section 6.2). Assuming an
ambient atmospheric concentration of about 1 µg/m3, an inhalation
uptake of 100%, a daily respiratory volume of 22 m3 for adults, a
mean body weight of 64 kg for males and females, and that 4 of 24 h
are spent outdoors (IPCS, 1994), the uptake by inhalation of
nitrophenols is calculated to be 0.06 µg/kg body weight per day. In
addition, 4-nitrophenol accumulates in fog. From the mean measured
level of 20 µg/litre, the uptake of the substance by inhalation (using
the same assumptions as above) can be calculated to be about 8 ng
during a 1-h exposure period (i.e., 0.12 ng/kg body weight), assuming
a maximum water content of fog of 0.1 g/m3 (Pruppacher & Klett,
1978). The uptake via drinking-water for 2- and 4-nitrophenol can be
calculated to be about 0.02 µg/kg body weight per day, assuming a
maximum concentration of 1 µg/litre drinking-water, a daily
drinking-water consumption of 1.4 litres, and a mean body weight of
64 kg for males and females.
From these data, it can be concluded that exposure of the general
population to the nitrophenol isomers is mainly through ambient air
and drinking-water.
11.2 Evaluation of environmental effects
Releases of 2- and 4-nitrophenol into the environment are
primarily emissions into air, water, and soil from diffuse sources,
such as vehicle traffic and hydrolytic and photolytic degradation of
the respective pesticides.
2-Nitrophenol emitted to the troposphere will stay predominantly
in the gaseous phase and should be rapidly removed by nitration. The
major portion of airborne 4-nitrophenol is expected to be particle
bound and can be washed out to surface waters and soil by wet and dry
deposition. Because of their removal from air and their insignificant
volatility, nitrophenols are not considered to contribute directly to
the depletion of the stratospheric ozone layer or to global warming.
Measured bioconcentration factors indicate a low potential for
bioaccumulation.
Nitrophenols exhibit moderate to high toxicity to aquatic
organisms, with lowest effect concentrations reported from chronic
studies on algae, Daphnia, and aquatic invertebrates. The lowest
effect concentrations found in chronic studies with freshwater
organisms ( Scenedesmus subspicatus, 96-h EC50: 0.39 mg
2-nitrophenol/litre; Entosiphon sulcatum, 72-h MIC: 0.83 mg
4-nitrophenol/litre) were 40-50 times higher than maximum levels
determined in a densely populated and highly industrialized Asian
river basin (0.0072 mg 2-nitrophenol/litre and 0.019 mg
4-nitrophenol/litre). From these data, the safety margin between the
LOEC and maximum surface water concentrations is insufficient to
exclude any risk for sensitive aquatic organisms, particularly under
surface water conditions not favouring both elimination pathways.
Taking into account the missing chronic effect data for fish, an
uncertainty/assessment factor of 100 has to be applied to derive a
predicted no-effect concentration (PNEC) according to standard
procedures for environmental risk assessment. From acute tests (see
section 10.1), however, fish obviously seem to be the least sensitive
aquatic species tested. Thus, an assessment factor of 10 might be
appropriate. Furthermore, the use pattern and the release scenario
outlined in section 4 lead to the conclusion that nitrophenols emitted
to surface waters will pose only a minor risk to aquatic organisms.
There were no data available on the occurrence of nitrophenols in
the terrestrial compartment. Therefore, an assessment of possible
effects on organisms for this compartment could be conducted only with
regard to the degradation of pesticides. For the insecticides
mentioned in section 4 (parathion, parathion-methyl, carbofuran,
phosalon, fluorodifen) and the herbicides bifenox and nitrofen,
predicted soil concentrations were calculated from the maximum
application rates (taken from Domsch, 1992) according to the EPPO
(1993) guidelines for environmental risk assessment of plant
protection products. Based on the relative molecular mass of the
pesticides, the maximum concentration of nitrophenols in the top 5 cm
of soil was calculated (worst case; one application). For pesticides
that are applied at times when the soil is plant covered to a high
degree, it is assumed that only half of the amount applied reaches the
soil. Thus, the predicted environmental concentration (PEC) for the
insecticides was reduced by 50%. Dividing the PECsoil by the lowest
LC50 value for a terrestrial species gives the toxicity exposure
ratio (TER). The lowest LC50 value for earthworms (38 mg/kg body
weight; see section 10.2) has to be corrected by a factor of 2 because
of the higher organic matter content in artificial soil compared with
natural agricultural soil. The following TERs were derived:
Insecticides Herbicides
Parathion: 244 Bifenox: 131
Parathion-methyl: 557 Nitrofen: 18
Carbofuran: 47
Phosalon: 36
Fluorodifen: 69
According to the EPPO (1993) guidelines, the trigger value for
concern is <10. Therefore, these pesticides are expected to pose only
a minor risk to earthworms, even under a worst-case scenario.
Furthermore, the herbicide nitrofen and the insecticides phosalon and
fluorodifen are no longer manufactured or marketed for crop protection
use.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Previous evaluations of mononitrophenols by international bodies
were not identified.
Information on international hazard classification and labelling
for mononitrophenols is included in the International Chemical Safety
Card reproduced in this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventative and protective
measures and first aid recommendations, are presented on the enclosed
International Chemical Safety Card (ICSC 1342) reproduced in this
document.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards is
available from the International Register of Potentially Toxic
Chemicals (IRPTC) legal file.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
INTERNATIONAL CHEMICAL SAFETY CARD
MONONITROPHENOLS ICSC: 1342
November 1998
CAS #  Physicochemical properties of 3-nitrophenol are given in
Table A-1.
Table A-1: Physicochemical properties of 3-nitrophenol.
Parameter Value
Molecular mass (g/mol) 139.11
Melting point (°C) 96-97 (1)(2)
Boiling point (°C) 194 (1)
Vapour pressure (kPa; 20 °C) 0.10 (3)
Water solubility (g/litre; 25 °C) 13.5 (1)
n-Octanol/water partition coefficient (log Kow) 2.00 (4)
Dissociation constant (pKa) (18 °C) 8.34 (2)
Conversion factors 1 mg/m3 = 0.173 ppmv
1 ppmv = 5.78 mg/m3
References: (1) Verschueren (1983); (2) Budavari et al. (1996);
(3) HSDB (1998); (4) Hansch & Leo (1979)
Environmental transport, distribution, and transformation
Data on the abiotic degradation of 3-nitrophenol were not
available.
Three studies on biotic degradation, summarized in Table A-2,
indicate the isomer to be inherently biodegradable in water under
aerobic conditions.
In tests on biotic degradation under anaerobic conditions using
sewage sludge and sludge from the primary anaerobic stage of a
municipal sewage treatment plant, respectively, initial 3-nitrophenol
concentrations in the range of 96.5-579 mg/litre were not degraded at
all within 7-60 days (Wagner & Braeutigam, 1981; Battersby & Wilson,
1989). Boyd et al. (1983), however, found complete anaerobic removal
of 50 mg/litre within 1 week of incubation. In this test,
mineralization was demonstrated only if the incubation period was
extended to 10 weeks. Anaerobic degradation, even of high initial
nitrophenol concentrations, was found by Tseng & Lin (1994), who
observed 90% removal of 3-nitrophenol (350-650 mg/litre) in a
biological fluidized bed reactor with three different kinds of
wastewater. From the available results, a slow mineralization of
3-nitrophenol under anaerobic conditions by adapted microorganisms can
be expected.
A soil sorption coefficient ( Koc) of 52.83, determined by Boyd
(1982), and the n-octanol/water partition coefficient (log Kow)
of 2.0, reported by Hansch & Leo (1979), indicate a low to moderate
potential for soil sorption as well as for bioaccumulation.
Environmental levels
3-Nitrophenol was not detected in 27 samples of air (detection
limit 8 ng/m3) in Japan in 1994 (Japan Environment Agency, 1995). It
was not detected in 177 samples of Japanese surface waters (detection
limits 0.04-10 µg/litre) or in 177 sediment samples (detection limits
0.002-0.8 µg/kg) in 1978, 1979, and 1994 (Japan Environment Agency,
1979, 1980, 1995). 3-Nitrophenol was not detected in 129 fish samples
(detection limits 0.005-0.2 µg/kg) in Japan in 1979 and 1994 (Japan
Environment Agency, 1980, 1995).
Comparative kinetics and metabolism in laboratory animals and humans
Studies providing quantitative information on the absorption,
metabolism, or elimination of 3-nitrophenol in humans were not
identified. In addition, there is only very limited information
available for experimental animals. In rabbits given a single dose of
150-200 mg/kg body weight via gavage, most of the applied dose
(>>80%) was excreted via the urine within 24 h. About 68-86% was
conjugated with glucuronic acid and sulfonic acid, whereas about 7-13%
was reduced to aminophenols (Robinson et al., 1951). Skin permeation
was shown in several in vitro experiments (Huq et al., 1986; Jetzer
et al., 1986; Ohkura et al., 1990). Although the information is
limited, bioaccumulation of 3-nitrophenol in organisms is not to be
expected owing to the isomer's rapid metabolism and excretion.
Effects on laboratory mammals and in vitro test systems
The oral LD50 of 3-nitrophenol is quoted to be >930 mg/kg
body weight for rats (Vasilenko et al., 1976; Vernot et al., 1977) and
>1070 mg/kg body weight for mice (Vasilenko et al., 1976; Vernot et
al., 1977).
The available in vitro and in vivo genotoxicity studies on
3-nitrophenol are summarized in Table A-3. 3-Nitrophenol was shown to
be mutagenic in a rec-assay and gave inconsistent results in
Salmonella/microsome assays. One study showed it to be non-mutagenic
in the Salmonella typhimurium TA98 and TA100 strains, whereas
another study showed mutagenicity in both of these strains in both the
presence and absence of metabolic activation. In view of the
conflicting results from Salmonella/microsome assays and the absence
of any data on clastogenicity, no conclusions can be made regarding
the mutagenicity of 3-nitrophenol.
For 3-nitrophenol, there are no studies available concerning
irritating or sensitizing effects, repeated exposure, reproductive and
developmental toxicity, or effects on humans.
Effects on aquatic species
In tests performed on the toxicity of 3-nitrophenol to various
aquatic organisms (see Table A-4), 3-nitrophenol exhibited a moderate
to high toxicity.
Table A-2: Biotic degradation of 3-nitrophenol under aerobic conditions.
Test Concentration Additional Test duration Removal Reference
(mg/litre) carbon source (days) (%)
Tests on ready biodegradability
MITI I 100 no 14 0 Gerike & Fischer (1979);
Urano & Kato (1986)
Tests on inherent biodegradability
Batch test, aerated 200 CODa no 5 95 Pitter (1976)
Respirometric test 300 yes 10 44 Kayser et al. (1994)
a COD = chemical oxygen demand.
Table A-3: Genotoxicity of 3-nitrophenol in vitro and in vivo.
Resultsa
Species End-point Concentration Without With Remarks References
(test system) range metabolic metabolic
activation activation
In vitro studies
Bacillus subtilis Recombination 0.01-5 mg/plate + 0 positive at Shimizu & Yano
H17, M45 assay >0.5 (1986)
mg/plate
Salmonella Reverse 0.01-5 mg/plate - - Shimizu & Yano
typhimurium mutations (1986)
TA1535, TA1537,
TA1538
Salmonella Reverse 0.1-5 mg/plate + + Study in Kawai et al.
typhimurium mutations Japanese (data (1987)
TA98, TA100 taken from
tables)
Salmonella Reverse 0.01-5 mg/plate - - Suzuki et al. Suzuki et al.
typhimurium TA98, mutations (1983) also (1983);
TA100 tested both Shimizu & Yano
strains in the (1986)
presence of
norharman,
which also gave
negative results
Table A-3 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks References
(test system) range metabolic metabolic
activation activation
In vivo studies
Drosophila SLRL assay via feed Foureman et al.
melanogaster (5000 ppm) (1994)
or injection
(1200 ppm)
a -, negative; +, positive; 0, not tested.
Table A-4: Aquatic toxicity of 3-nitrophenol.
Species
(test method/end-point) Effective concentration Reference
(mg/litre)
Bacteria
Pseudomonas putida 16-h MICa: 7.0 Bringmann & Kuehn (1977)
(cell multiplication inhibition test)
Protozoa
Entosiphon sulcatum 72-h MIC: 0.97 Bringmann (1978);
(cell multiplication inhibition test) Bringmann et al. (1980)
Algae
Scenedesmus subspicatus
Chlorella vulgaris 6-h EC50: 6.21 Kramer et al. (1986)
(cell multiplication inhibition test)
Invertebrates
Moina macrocopa (acute) 3-h LC50: 1.7 Yoshioka et al. (1985)
(immobilization)
Fish
Cyprinus carpio (static) 96-h LC50: 17.5 Lang et al. (1996)
a MIC = minimum inhibitory concentration.
APPENDIX 2 - SOURCE DOCUMENTS
BUA (1992): BUA-Stoffbericht 2- und 4-Nitrophenol.
Beratergremium fuer Umweltrelevante Altstoffe. Weinheim, VCH
VerlagsGmbH (Report No. 75; February 1992)
For the BUA review process, the company that is in charge of
writing the report (usually the largest producer in Germany) prepares
a draft report using literature from an extensive literature search as
well as internal company studies. This draft is subject to a peer
review during several readings of a working group consisting of
representatives from government agencies, the scientific community,
and industry.
The English translation of BUA Report No. 75 ( BUA Report 2- and
4-Nitrophenol. GDCh-Advisory Committee on Existing Chemicals of
Environmental Relevance. Stuttgart, Hirzel Verlag [February 1992]) was
released in 1993.
ATSDR (1992): Toxicological profile for nitrophenols:
2- and 4-nitrophenol. Atlanta, GA, US Department of Health and Human
Services, Public Health Service, Agency for Toxic Substances and
Disease Registry (Report No. TP-91/23)
Copies of the ATSDR Toxicological profile for nitrophenols:
2- and 4-nitrophenol (ATSDR, 1992) may be obtained from the:
Agency for Toxic Substances and Disease Registry
Division of Toxicology
1600 Clifton Road, E-29
Atlanta, Georgia 30333
USA
Initial drafts of the Toxicology profile for nitrophenols:
2- and 4-nitrophenol were reviewed by scientists from the Agency for
Toxic Substances and Disease Registry, the US Centers for Disease
Control, the US National Toxicology Program, and other federal
agencies. The document was also reviewed by an expert panel of
nongovernmental reviewers, consisting of the following members:
Dr Martin Alexander, Cornell University
Dr Gary Booth, Brigham Young University
Dr Samuel Cohen, University of Nebraska Medical Center
Dr Loren Koller, Oregon State University
Dr Frederick Oehme, Kansas State University
APPENDIX 3 - CICAD PEER REVIEW
The draft CICAD on mononitrophenols was sent for review to
institutions and organizations identified by IPCS after contact with
IPCS national Contact Points and Participating Institutions, as well
as to identified experts. Comments were received from:
Federal Institute for Health Protection of Consumers & Veterinary
Medicine, Berlin, Germany
Gesellschaft Deutscher Chemiker, Frankfurt, Germany
Institute of Occupational Medicine, Chinese Academy of Preventive
Medicine, Ministry of Health, Beijing, People's Republic of China
Institute of Terrestrial Ecology, Huntingdon, United Kingdom
Joint Food Safety and Standards Group, Department of Health,
London, United Kingdom
National Institute of Health Sciences, Tokyo, Japan
National Institute of Public Health, Prague, Czech Republic
United States Department of Health and Human Services (National
Institute of Environmental Health Sciences, Research Triangle
Park), USA
United States Environmental Protection Agency (National Center
for Environmental Assessment, Washington, DC; Region VIII), USA
World Health Organization/International Programme on Chemical
Safety, Montreal, Canada
APPENDIX 4 - CICAD FINAL REVIEW BOARD
Washington, DC, USA, 8-11 December 1998
Members
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
( Vice-Chairperson)
Mr R. Cary, Toxicology Unit, Health Directorate, Health and Safety
Executive, Bootle, Merseyside, United Kingdom ( Rapporteur)
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr O. Faroon, Agency for Toxic Substances and Disease Registry,
Centers for Disease Control and Prevention, Atlanta, GA, USA
Dr G. Foureman, National Center for Environmental Assessment, US
Environmental Protection Agency, Research Triangle Park, NC, USA
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA ( Chairperson)
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany
Dr A. Nishikawa, Division of Pathology, National Institute of Health
Sciences, Tokyo, Japan
Dr E.V. Ohanian, Office of Water/Office of Science and Technology,
Health and Ecological Criteria Division, US Environmental Protection
Agency, Washington, DC, USA
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan
Professor P. Yao, Institute of Occupational Medicine, Chinese Academy
of Preventive Medicine, Ministry of Health, Beijing, People's Republic
of China
Observers
Dr K. Austin, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr I. Daly (ICCA representative), Regulatory and Technical Associates,
Lebanon, NJ, USA
Ms K.L. Lang (CEFIC, European Chemical Industry Council,
representative), Shell International, London, United Kingdom
Ms K. Roberts (ICCA representative), Chemical Self-funded Technical
Advocacy and Research (CHEMSTAR), Chemical Manufacturers Association,
Arlington, VA, USA
Dr W. Snellings (ICCA representative), Union Carbide Corporation,
Danbury, CN, USA
Dr M. Sweeney, Document Development Branch, National Institute for
Occupational Safety and Health, Cincinnati, OH, USA
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Secretariat
Dr M. Baril, Institut de Recherches en Santé et Sécurité du Travail du
Québec (IRSST), Montreal, Quebec, Canada
Dr H. Galal-Gorchev, Chevy Chase, MD, USA
Ms M. Godden, Health and Safety Executive, Bootle, Merseyside, United
Kingdom
Dr R.G. Liteplo, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Ms L. Regis, Programme for the Promotion of Chemical Safety, World
Health Organization, Geneva, Switzerland
Mr A. Strawson, Health and Safety Executive, London, United Kingdom
Dr P. Toft, Programme for the Promotion of Chemical Safety, World
Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
Ce CICAD relatif aux isomères en position 2-, 3- et 4- du
nitrophénol a été préparé par l'Institut Fraunhofer de recherche en
toxicologie et sur les aérosols de Hanovre (Allemagne). Il est basé
sur des mises au point rédigées par le Comité consultatif allemand sur
les produits chimiques qui posent un problème écologique (BUA, 1992)
et par l'US Agency for Toxic Substances and Disease Registry (ATSDR,
1992) afin d'évaluer les effets potentiels du 2- et du 4-nitrophénol
sur l'environnement et la santé humaine. Les données prises en compte
dans ces mises au point vont jusqu'en 1992. Une recherche
bibliographique exhaustive a été effectuée en 1998 sur plusieurs bases
de données afin de relever les références intéressantes sur le 2- et
le 4-nitrophénol publiées après celles qui figurent dans les documents
de base et d'obtenir toutes celles qui contiennent des données utiles
sur le 3-nitrophénol. On a trouvé très peu de données sur cet isomère,
ce qui rend impossible une véritable évaluation. Les données
concernant cet isomère sont donc récapitulées à l'appendice 1. On
trouvera à l'appendice 2 des indications sur le mode d'examen par des
pairs ainsi que sur les sources documentaires utilisées. Les
renseignements concernant l'examen du CICAD par des pairs font l'objet
de l'appendice 3. Ce CICAD a été approuvé en tant qu'évaluation
internationale lors de la réunion du Comité d'évaluation finale qui
s'est tenue à Washington du 8 au 11 décembre 1998. La liste des
participants à cette réunion figure à l'appendice 4. La fiche
d'information internationale sur la sécurité chimique (ICSC No 1342)
relative au mélange d'isomères du nitrophénol, établie par le
Programme international sur la sécurité chimique (IPCS, 1998) est
également reproduite dans ce document.
Les isomères du nitrophénol sont des solides solubles dans l'eau
qui sont légèrement acides dans ce solvant par suite de leur
dissociation. Les isomères 2- et 4- sont utilisés comme intermédiaires
dans la synthèse d'un certain nombre d'insecticides organophosphorés
et de composés à usage médical. Lorsqu'ils passent dans
l'environnement, c'est principalement par suite d'émissions dans
l'eau, l'air et le sol provenant de sources diffuses comme la
circulation automobile ou la décomposition par photolyse ou hydrolyse
de certains insecticides. Le dépôt par voie sèche ou humide de
nitrophénols présents dans l'atmosphère constitue un apport
supplémentaire dans l'hydrosphère et la géosphère. La formation
photo-oxydative du 2- et du 4-nitrophénol dans l'atmosphère est encore
débattue.
Les données disponibles montrent que le 2-nitrophénol ne devrait
se volatiliser que lentement dans l'atmosphère et qu'il ne passe sans
doute qu'en quantité négligeable de l'eau à l'air selon ce processus.
On constate un enrichissement en 2-nitrophénol de la phase aqueuse des
nuages; en revanche, dans la phase gazeuse, la proportion de
4-nitrophénol est plus importante qu'on pourrait le penser en fonction
des données physico-chimiques, par suite d'une importante fixation du
composé sur les particules. Compte tenu de leur solubilité dans l'eau
et de leur concentration dans la phase gazeuse, on peut s'attendre à
ce que les nitrophénols présents dans l'atmosphère se déposent par
voie humide sur la surface du sol et de l'eau. La principale voie de
transformation du 2-nitrophénol présent dans l'atmosphère est
vraisemblablement sa nitration rapide en 2,4-dinitrophénol, le
4-nitrophénol étant quant à lui en majeure partie fixé aux particules
aéroportées et donc disponible en petites quantités seulement pour des
réactions photochimiques. La majeure partie du 4-nitrophénol devrait
d'ailleurs disparaître de l'atmosphère en se déposant soit par voie
sèche, soit par voie humide. Il ne semble pas que les nitrophénols
contribuent directement à la dégradation de la couche d'ozone
stratosphérique ni au réchauffement général de la planète. Soumis à
une photodécomposition en milieu aqueux, le 4-nitrophénol a une
demi-vie qui peut aller de 2,8 à 13,7 jours selon les mesures. Les
nombreuses études consacrées à la biodégradation du 2- et du
4-nitrophénol montrent que ces deux isomères sont intrinsèquement
biodégradables dans l'eau en aérobiose. La minéralisation des
nitrophénols en anaérobiose exige à l'évidence une importante
adaptation des populations microbiennes.
La valeur du coefficient de sorption par les particules du sol
( Koc), qui se situe entre 44 et 530, indique que le potentiel de
sorption est faible à modéré. Les nitrophénols qui passent dans le sol
vont vraisemblablement subir une biodégradation aérobie. Il ne devrait
y avoir infiltration dans les eaux souterraines que lorsque les
conditions ne sont pas favorables à une biodégradation. Pour le 2- et
le 4-nitrophénol, la mesure du facteur de bioconcentration donne des
valeurs allant de 11 à 76, ce qui indique un faible potentiel de
bioconcentration.
On connaît plutôt mal le profil toxicologique du 2- et du
4-nitrophénol. Lorsqu'il est administré à des animaux de laboratoire
par voie orale, intraveineuse ou intrapéritonéale, le 4-nitrophénol
est en majeure partie excrété dans les urines en l'espace de 24 à 48 h
sous forme de glucuronide ou de sulfo-conjugué, une faible partie
seulement passant dans les matières fécale ou restant inchangée. On a
montré que la proportion de glucuronide et de sulfo-conjugués variait
selon les espèces. Après administration par voie orale à des lapins,
le 4-nitrophénol est réduit en p-aminophénol et subit aussi une
transformation en glucuronide et sulfo-conjugués. Les données tirées
des études in vivo et in vitro donnent une indication sur la
résorption du 4-nitrophénol par la voie transcutanée. En revanche, les
données concernant le 2-nitrophénol sont très limitées. Quoi qu'il en
soit, on peut considérer, en se basant sur les données disponibles,
que les deux isomères ont un métabolisme comparable. Le 2- et le
4-nitrophénol ne devraient pas s'accumuler dans l'organisme en raison
de leur métabolisation et de leur excrétion rapides.
Les études de toxicité aiguë montrent que le 4-nitrophénol a un
effet nocif après ingestion et qu'il est plus toxique que le
2-nitrophénol. Chez des chats, on a constaté une augmentation du taux
de méthémoglobine liée à la dose après ingestion de 2-nitrophénol; la
même constatation a été faite chez des rats après inhalation de
4-nitrophénol. Une exposition répétée à du 4-nitrophénol a montré que
la formation de méthémoglobine est l'effet le plus déterminant d'une
exposition par la voie respiratoire et cela vaut sans doute aussi pour
la voie orale. Parmi les autres effets observés, on peut citer un
moindre gain de poids, une modification du poids des organes, une
dégénérescence graisseuse du foie et des anomalies hématologiques. Il
n'a pas été possible de dégager une véritable relation dose-réponse ni
de déterminer de manière fiable la dose sans effet (nocif) observable
(NO(A)EL) correspondant à ces effets.
Le 2-nitrophénol est légèrement irritant pour la peau mais il
n'irrite pas la muqueuse oculaire. Le test de Buehler montre que le
composé n'a pas non plus d'effet sensibilisateur. En s'appuyant sur
des études valables effectuées sur l'animal, on peut conclure que le
4-nitrophénol a par contre une légère action irritante sur la peau et
les yeux. Un test de maximalisation sur le cobaye a montré que le
4-nitrophénol avait également une légère action sensibilisatrice. Chez
l'homme, on ne peut exclure une légère sensibilisation après un
contact avec le composé, d'autant plus que la pose d'un timbre cutané
chez des ouvriers pouvant avoir été en contact avec du 4-nitrophénol a
permis de constater une telle sensibilisation.
Aucun des deux isomères n'a fait l'objet d'épreuves de
génotoxicité suffisamment complètes. S'agissant du 2-nitrophénol, les
données sont insuffisantes pour que l'on puisse tirer la moindre
conclusion concernant une mutagénicité éventuelle. Dans le cas du
4-nitrophénol, les études de mutagénicité sont plus nombreuses, mais
le compte rendu en est parfois insuffisant. On est fondé à penser que
ce composé est susceptible de provoquer des aberrations chromosomiques
in vitro. Faute d'études de mutagénicité in vivo sur des
mammifères, il n'est pas possible de savoir si le pouvoir mutagène de
cet isomère peut s'exprimer in vivo.
Chez la souris l'application cutanée de 4-nitrophénol pendant une
durée de 78 semaines n'a pas donné d'indices d'effets cancérogènes.
Dans une autre étude sur la souris, qui présentait toutefois un
certain nombre d'insuffisances, on n'a pas non plus observé de tumeurs
cutanées après application cutanée de ces deux isomères pendant 12
semaines. Aucune étude de cancérogénicité utilisant la voie orale ou
respiratoire n'était disponible.
Les données relatives au 4-nitrophénol ne révèlent aucun effet
indésirable sur la reproduction ou le développement qui soit
statistiquement significatif après exposition de rats et de souris par
voie orale ou cutanée. Après administration par voie orale de
2-nitrophénol à des rats, on a constaté dans la progéniture des
animaux des effets indésirables sur le développement, mais seulement
aux doses toxiques pour les mères. On n'a toutefois pas recherché la
présence de malformations internes.
La base de données relative au 2-nitrophénol est extrêmement
limitée et celle qui concerne le 4-nitrophénol est insuffisante pour
qu'on puisse en tirer une valeur fiable de la NO(A)EL. Il est donc
impossible de fixer pour l'instant une valeur pour la dose journalière
tolérable (DJT) ou pour la concentration tolérable (CT) de ces deux
isomères.
D'après les résultats des études toxicologiques valables
effectuées sur divers organismes aquatiques, on peut considérer que
ces deux nitrophénols sont modérément à fortement toxiques pour la vie
aquatique. La concentration sans effet la plus faible qui ait été
obtenue lors d'études de longue durée sur des organismes d'eau douce
( Scenedesmus subspicatus, EC50 à 96 h : 0,39 mg de
2-nitrophénol/litre; Entosiphon sulcatum, concentration minimale
inhibitrice à 72 h ou CMI : 0,83 mg de 4-nitrophénol/l) était 40 à 50
fois plus forte que la valeur maximale obtenue dans un bassin fluvial
d'Asie situé dans une zone très industrialisée et densément peuplée
(respectivement 0,0072 mg/l et 0,019 mg/l). Par conséquent, malgré la
biodégradation et la décomposition photochimique, les nitrophénols
déversés dans l'eau peuvent présenter un certain risque pour les
organismes aquatiques sensibles, notamment dans des eaux
superficielles où les conditions ne sont pas favorables à ces deux
modes d'élimination. Cependant, compte tenu de leurs usages et de
leurs possibilités de libération dans l'environnement, ces deux
nitrophénols ne présentent qu'un risque mineur pour les organismes
aquatiques.
Les données disponibles indiquent que la toxicité potentielle de
ces nitrophénols est modérée dans l'environnement terrestre. Le calcul
du rapport d'exposition toxique (TER) des nitrophénols provenant de la
décomposition de certains pesticides montre que, dans ce milieu, le
risque reste faible pour la faune et la flore, même dans la pire des
hypothèses.
RESUMEN DE ORIENTACION
Preparó el presente CICAD sobre los isómeros 2-, 3- y
4-nitrofenol el Instituto Fraunhofer de Toxicología y de Investigación
sobre los Aerosoles de Hannover, Alemania. Se basa en los exámenes
compilados por el Comité Consultivo Alemán sobre las Sustancias
Químicas Importantes para el Medio Ambiente (BUA, 1992) y la Agencia
para el Registro de Sustancias Tóxicas y Enfermedades de los Estados
Unidos (ATSDR, 1992) para evaluar los efectos potenciales del 2- y el
4-nitrofenol en el medio ambiente y en el ser humano. En estos
exámenes se incluyeron los datos identificados hasta 1992. En 1998 se
realizó una búsqueda bibliográfica amplia de varias bases de datos
para identificar todas las referencias importantes relativas al 2- y
el 4-nitrofenol publicadas con posterioridad a las que figuran en los
documentos originales y para conocer todas las referencias con datos
pertinentes sobre el isómero 3-nitrofenol. La información obtenida
sobre el 3-nitrofenol fue muy escasa, lo que impide una evaluación
válida. En consecuencia, los datos sobre este isómero se resumen en el
apéndice 1. La información relativa al carácter del examen colegiado y
a la disponibilidad de los documentos originales figura en el apéndice
2. La información sobre el examen colegiado de este CICAD se presenta
en el apéndice 3. Este CICAD se aprobó como evaluación internacional
en una reunión de la Junta de Evaluación Final celebrada en
Washington, DC, Estados Unidos, los días 8-11 de diciembre de 1998. La
lista de participantes en esta reunión figura en el apéndice 4. La
Ficha internacional de seguridad química (ICSC 1342) para las mezclas
de isómeros de nitrofenoles, preparada por el Programa Internacional
de Seguridad de las Sustancias Químicas (IPCS, 1998), también se
reproduce en el presente documento.
Los isómeros del nitrofenol son sólidos hidrosolubles con una
acidez moderada en agua debido a la disociación. El 2-nitrofenol y el
4-nitrofenol se utilizan como intermediarios en la síntesis de
diversos plaguicidas órganofosforados y algunos productos médicos. La
liberación en el medio ambiente se produce fundamentalmente por
emisiones en el aire, el agua y el suelo a partir de fuentes difusas,
como el tráfico de vehículos y la degradación hidrolítica y fotolítica
de los respectivos plaguicidas. También se produce liberación en la
hidrosfera y la geosfera a partir de la atmósfera debido a la
deposición seca y húmeda de nitrofenoles suspendidos en el aire. La
formación fotooxidativa de 2- y 4-nitrofenol en la atmósfera es
todavía objeto de estudio.
Con los datos disponibles sólo cabe esperar una volatilización
lenta desde el agua hacia el aire para el 2-nitrofenol y no
significativa para el 4-nitrofenol. El 2-nitrofenol se enriquece en la
fase líquida de las nubes, mientras que es posible encontrar más
4-nitrofenol del previsto a partir de los datos fisicoquímicos en la
fase gaseosa de las nubes, debido a una amplia unión a partículas.
Habida cuenta de la solubilidad en agua y la presencia prevista en la
fase de vapor, cabe esperar una deposición húmeda de nitrofenoles del
aire en las aguas superficiales y en el suelo. La vía principal de
transformación del 2-nitrofenol emitido a la troposfera debe ser la
nitración rápida a 2,4-dinitrofenol, mientras que se supone que la
mayor parte del 4-nitrofenol suspendido en el aire se encuentra unido
a partículas y, por consiguiente, disponible solamente en menor
cantidad para reacciones fotoquímicas. La mayor parte del 4-nitrofenol
del aire debe precipitar por deposición húmeda y seca. No se considera
que los nitrofenoles contribuyan directamente al agotamiento de la
capa de ozono estratosférico o al calentamiento mundial. La semivida
medida para la descomposición fotoquímica del 4-nitrofenol en agua
osciló entre 2,8 y 13,7 días. Numerosos estudios sobre la
biodegradación del 2- y el 4-nitrofenol indican que los isómeros son
inherentemente biodegradables en agua en condiciones aerobias. La
mineralización de los nitrofenoles en condiciones anaerobias requiere
evidentemente una amplia adaptación de las comunidades microbianas.
Los coeficientes de sorción en el suelo ( Koc) medidos del
orden de 44-530 indican un potencial de bajo a moderado para la
sorción en el suelo. Los nitrofenoles liberados al suelo se deben
biodescomponer en condiciones aerobias. Cabe prever filtración hacia
el agua freática sólo en condiciones desfavorables para la
biodegradación. Para el 2- y el 4-nitrofenol, los factores de
bioconcentración medidos de 11 a 76 ponen de manifiesto un potencial
bajo de bioacumulación.
Se dispone sólo de información limitada relativa a los perfiles
toxicológicos del 2- y el 4-nitrofenol. Los animales experimentales a
los que se administró 4-nitrofenol por vía oral, intravenosa o
intraperitoneal excretaron la mayor parte de la dosis aplicada por vía
urinaria en un plazo de 24-48 horas en forma de conjugados de
glucurónidos y sulfatos, mientras que por las heces se excretaron
solamente cantidades muy pequeñas, o bien como 4-nitrofenol
inalterado. Se observó que los porcentajes de conjugados de
glucurónidos y sulfatos eran dependientes de la especie y de la dosis.
Tras la administración oral a conejos, el 4-nitrofenol sufre una
reducción a p-aminofenol, así como una glucuronización y
sulfatación. Los datos disponibles de estudios in vivo e in vitro
dan una idea de la absorción cutánea del 4-nitrofenol. Los datos para
el 2-nitrofenol son muy limitados. Sin embargo, teniendo cuenta los
datos disponibles, se supone que se produce una transformación
metabólica comparable. No cabe prever bioacumulación de 2- y
4-nitrofenol en los organismos debido a la rapidez de su metabolismo y
excreción.
En estudios de toxicidad aguda, el 4-nitrofenol es perjudicial
tras la absorción oral, y se observó que era más tóxico que el
2-nitrofenol. Se detectó un aumento en la formación de metahemoglobina
dependiente de la dosis en gatos tras la exposición oral al
2-nitrofenol y en ratas tras la exposición por inhalación al
4-nitrofenol. Después de una exposición repetida al 4-nitrofenol, se
observó la formación de metahemoglobina como el efecto final más
importante de la exposición por inhalación, y se considera que esto es
aplicable también a la exposición oral. Otros efectos observados
fueron la reducción del aumento del peso corporal, diferencias en el
peso de los órganos, degeneración adiposa focal del hígado y cambios
hematológicos. Para esos efectos no fue posible determinar una
relación clara dosis-respuesta o concentraciones sin efectos
(adversos) observados (NO(A)EL) fidedignas.
El 2-nitrofenol es ligeramente irritante para la piel, pero no
para los ojos. En una prueba de Buehler no se observó efecto
sensibilizador. Tomando como base los estudios válidos con animales
experimentales, se supone que el 4-nitrofenol tiene efectos irritantes
en la piel y los ojos. En una prueba de maximización en cobayas, se
observó que el 4-nitrofenol era ligeramente sensibilizante. En el ser
humano no se puede excluir una posible sensibilización tras el
contacto con 4-nitrofenol, especialmente teniendo en cuenta que se ha
observado sensibilización cutánea en pruebas de parche en trabajadores
de fábricas que podían haber estado expuestos al 4-nitrofenol.
No se ha sometido a pruebas completas de genotoxicidad ninguno de
los dos isómeros del nitrofenol. Los datos disponibles sobre el
2-nitrofenol son insuficientes para poder sacar conclusiones acerca de
su posible mutagenicidad. Hay más estudios de mutagenicidad para el
4-nitrofenol, aunque algunos se notificaron de manera inadecuada. Hay
pruebas que parecen indicar que el 4-nitrofenol puede producir
aberraciones cromosómicas in vitro. En ausencia de estudios de
mutagenicidad in vivo en mamíferos, no es posible llegar a la
conclusión de si se expresa o no in vivo el potencial mutagénico del
4-nitrofenol.
Tras la aplicación cutánea de 4-nitrofenol a ratones durante 78
semanas no se observaron efectos carcinogénicos. En otro estudio con
ratones, que tiene varias limitaciones, no se detectaron tumores
cutáneos tras la aplicación en la piel de 2- ó 4-nitrofenol durante 12
semanas. Para ninguno de los isómeros había estudios de
carcinogenicidad utilizando la vía oral o la inhalación.
Los datos disponibles para el 4-nitrofenol no pusieron de
manifiesto efectos específicos o estadísticamente significativos de
toxicidad reproductiva o del desarrollo tras la administración cutánea
u oral a ratas y ratones. En un estudio de administración por vía oral
a ratas, el 2-nitrofenol indujo efectos en el desarrollo de la camada
sólo con dosis que también producían toxicidad materna. Sin embargo,
en estos estudios no se examinaron los fetos para investigar
malformaciones internas.
La base de datos para el 2-nitrofenol es enormemente limitada y
la relativa al 4-nitrofenol es insuficiente para deducir valores
fidedignos de la (NO(A)EL). Por consiguiente, es imposible determinar
en este momento la ingesta diaria tolerable o las concentraciones
tolerables para el 2- ó el 4-nitrofenol.
De los resultados disponibles de pruebas válidas sobre la
toxicidad del 2-y el 4-nitrofenol para diversos organismos acuáticos,
los nitrofenoles se pueden clasificar como sustancias con una
toxicidad entre moderada y alta en el compartimento acuático. Las
concentraciones más bajas con efectos obtenidas en estudios crónicos
con organismos de agua dulce ( Scenedesmus subspicatus, CE50: 0,39
mg de 2-nitrofenol/litro a las 96 h; Entosiphon sulcatum,
concentración inhibitoria mínima a las 72 horas: 83 mg de
4-nitrofenol/litro) fueron 40-50 veces superiores a los niveles
máximos determinados en una cuenca fluvial asiática densamente poblada
y muy industrializada (0,0072 mg de 2-nitrofenol/litro y 0,019 mg de
4-nitrofenol/litro). Por consiguiente, a pesar de la descomposición
biótica y fotoquímica, los nitrofenoles emitidos al agua pueden
representar algún peligro para los organismos acuáticos sensibles,
particularmente en las condiciones de las aguas superficiales que no
favorecen ambas vías de eliminación. Habida cuenta de sus pautas de
uso y sus características de liberación, probablemente los
nitrofenoles plantean sólo un pequeño riesgo para los organismos
acuáticos.
Los datos disponibles indican solamente una toxicidad potencial
moderada de los nitrofenoles en el medio ambiente terrestre. A partir
de los cálculos de la razón exposición-toxicidad de los nitrofenoles a
partir de la degradación de plaguicidas, sólo cabe esperar un pequeño
riesgo para los organismos en este compartimento, incluso en el peor
de los casos.
Physicochemical properties of 3-nitrophenol are given in
Table A-1.
Table A-1: Physicochemical properties of 3-nitrophenol.
Parameter Value
Molecular mass (g/mol) 139.11
Melting point (°C) 96-97 (1)(2)
Boiling point (°C) 194 (1)
Vapour pressure (kPa; 20 °C) 0.10 (3)
Water solubility (g/litre; 25 °C) 13.5 (1)
n-Octanol/water partition coefficient (log Kow) 2.00 (4)
Dissociation constant (pKa) (18 °C) 8.34 (2)
Conversion factors 1 mg/m3 = 0.173 ppmv
1 ppmv = 5.78 mg/m3
References: (1) Verschueren (1983); (2) Budavari et al. (1996);
(3) HSDB (1998); (4) Hansch & Leo (1979)
Environmental transport, distribution, and transformation
Data on the abiotic degradation of 3-nitrophenol were not
available.
Three studies on biotic degradation, summarized in Table A-2,
indicate the isomer to be inherently biodegradable in water under
aerobic conditions.
In tests on biotic degradation under anaerobic conditions using
sewage sludge and sludge from the primary anaerobic stage of a
municipal sewage treatment plant, respectively, initial 3-nitrophenol
concentrations in the range of 96.5-579 mg/litre were not degraded at
all within 7-60 days (Wagner & Braeutigam, 1981; Battersby & Wilson,
1989). Boyd et al. (1983), however, found complete anaerobic removal
of 50 mg/litre within 1 week of incubation. In this test,
mineralization was demonstrated only if the incubation period was
extended to 10 weeks. Anaerobic degradation, even of high initial
nitrophenol concentrations, was found by Tseng & Lin (1994), who
observed 90% removal of 3-nitrophenol (350-650 mg/litre) in a
biological fluidized bed reactor with three different kinds of
wastewater. From the available results, a slow mineralization of
3-nitrophenol under anaerobic conditions by adapted microorganisms can
be expected.
A soil sorption coefficient ( Koc) of 52.83, determined by Boyd
(1982), and the n-octanol/water partition coefficient (log Kow)
of 2.0, reported by Hansch & Leo (1979), indicate a low to moderate
potential for soil sorption as well as for bioaccumulation.
Environmental levels
3-Nitrophenol was not detected in 27 samples of air (detection
limit 8 ng/m3) in Japan in 1994 (Japan Environment Agency, 1995). It
was not detected in 177 samples of Japanese surface waters (detection
limits 0.04-10 µg/litre) or in 177 sediment samples (detection limits
0.002-0.8 µg/kg) in 1978, 1979, and 1994 (Japan Environment Agency,
1979, 1980, 1995). 3-Nitrophenol was not detected in 129 fish samples
(detection limits 0.005-0.2 µg/kg) in Japan in 1979 and 1994 (Japan
Environment Agency, 1980, 1995).
Comparative kinetics and metabolism in laboratory animals and humans
Studies providing quantitative information on the absorption,
metabolism, or elimination of 3-nitrophenol in humans were not
identified. In addition, there is only very limited information
available for experimental animals. In rabbits given a single dose of
150-200 mg/kg body weight via gavage, most of the applied dose
(>>80%) was excreted via the urine within 24 h. About 68-86% was
conjugated with glucuronic acid and sulfonic acid, whereas about 7-13%
was reduced to aminophenols (Robinson et al., 1951). Skin permeation
was shown in several in vitro experiments (Huq et al., 1986; Jetzer
et al., 1986; Ohkura et al., 1990). Although the information is
limited, bioaccumulation of 3-nitrophenol in organisms is not to be
expected owing to the isomer's rapid metabolism and excretion.
Effects on laboratory mammals and in vitro test systems
The oral LD50 of 3-nitrophenol is quoted to be >930 mg/kg
body weight for rats (Vasilenko et al., 1976; Vernot et al., 1977) and
>1070 mg/kg body weight for mice (Vasilenko et al., 1976; Vernot et
al., 1977).
The available in vitro and in vivo genotoxicity studies on
3-nitrophenol are summarized in Table A-3. 3-Nitrophenol was shown to
be mutagenic in a rec-assay and gave inconsistent results in
Salmonella/microsome assays. One study showed it to be non-mutagenic
in the Salmonella typhimurium TA98 and TA100 strains, whereas
another study showed mutagenicity in both of these strains in both the
presence and absence of metabolic activation. In view of the
conflicting results from Salmonella/microsome assays and the absence
of any data on clastogenicity, no conclusions can be made regarding
the mutagenicity of 3-nitrophenol.
For 3-nitrophenol, there are no studies available concerning
irritating or sensitizing effects, repeated exposure, reproductive and
developmental toxicity, or effects on humans.
Effects on aquatic species
In tests performed on the toxicity of 3-nitrophenol to various
aquatic organisms (see Table A-4), 3-nitrophenol exhibited a moderate
to high toxicity.
Table A-2: Biotic degradation of 3-nitrophenol under aerobic conditions.
Test Concentration Additional Test duration Removal Reference
(mg/litre) carbon source (days) (%)
Tests on ready biodegradability
MITI I 100 no 14 0 Gerike & Fischer (1979);
Urano & Kato (1986)
Tests on inherent biodegradability
Batch test, aerated 200 CODa no 5 95 Pitter (1976)
Respirometric test 300 yes 10 44 Kayser et al. (1994)
a COD = chemical oxygen demand.
Table A-3: Genotoxicity of 3-nitrophenol in vitro and in vivo.
Resultsa
Species End-point Concentration Without With Remarks References
(test system) range metabolic metabolic
activation activation
In vitro studies
Bacillus subtilis Recombination 0.01-5 mg/plate + 0 positive at Shimizu & Yano
H17, M45 assay >0.5 (1986)
mg/plate
Salmonella Reverse 0.01-5 mg/plate - - Shimizu & Yano
typhimurium mutations (1986)
TA1535, TA1537,
TA1538
Salmonella Reverse 0.1-5 mg/plate + + Study in Kawai et al.
typhimurium mutations Japanese (data (1987)
TA98, TA100 taken from
tables)
Salmonella Reverse 0.01-5 mg/plate - - Suzuki et al. Suzuki et al.
typhimurium TA98, mutations (1983) also (1983);
TA100 tested both Shimizu & Yano
strains in the (1986)
presence of
norharman,
which also gave
negative results
Table A-3 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks References
(test system) range metabolic metabolic
activation activation
In vivo studies
Drosophila SLRL assay via feed Foureman et al.
melanogaster (5000 ppm) (1994)
or injection
(1200 ppm)
a -, negative; +, positive; 0, not tested.
Table A-4: Aquatic toxicity of 3-nitrophenol.
Species
(test method/end-point) Effective concentration Reference
(mg/litre)
Bacteria
Pseudomonas putida 16-h MICa: 7.0 Bringmann & Kuehn (1977)
(cell multiplication inhibition test)
Protozoa
Entosiphon sulcatum 72-h MIC: 0.97 Bringmann (1978);
(cell multiplication inhibition test) Bringmann et al. (1980)
Algae
Scenedesmus subspicatus
Chlorella vulgaris 6-h EC50: 6.21 Kramer et al. (1986)
(cell multiplication inhibition test)
Invertebrates
Moina macrocopa (acute) 3-h LC50: 1.7 Yoshioka et al. (1985)
(immobilization)
Fish
Cyprinus carpio (static) 96-h LC50: 17.5 Lang et al. (1996)
a MIC = minimum inhibitory concentration.
APPENDIX 2 - SOURCE DOCUMENTS
BUA (1992): BUA-Stoffbericht 2- und 4-Nitrophenol.
Beratergremium fuer Umweltrelevante Altstoffe. Weinheim, VCH
VerlagsGmbH (Report No. 75; February 1992)
For the BUA review process, the company that is in charge of
writing the report (usually the largest producer in Germany) prepares
a draft report using literature from an extensive literature search as
well as internal company studies. This draft is subject to a peer
review during several readings of a working group consisting of
representatives from government agencies, the scientific community,
and industry.
The English translation of BUA Report No. 75 ( BUA Report 2- and
4-Nitrophenol. GDCh-Advisory Committee on Existing Chemicals of
Environmental Relevance. Stuttgart, Hirzel Verlag [February 1992]) was
released in 1993.
ATSDR (1992): Toxicological profile for nitrophenols:
2- and 4-nitrophenol. Atlanta, GA, US Department of Health and Human
Services, Public Health Service, Agency for Toxic Substances and
Disease Registry (Report No. TP-91/23)
Copies of the ATSDR Toxicological profile for nitrophenols:
2- and 4-nitrophenol (ATSDR, 1992) may be obtained from the:
Agency for Toxic Substances and Disease Registry
Division of Toxicology
1600 Clifton Road, E-29
Atlanta, Georgia 30333
USA
Initial drafts of the Toxicology profile for nitrophenols:
2- and 4-nitrophenol were reviewed by scientists from the Agency for
Toxic Substances and Disease Registry, the US Centers for Disease
Control, the US National Toxicology Program, and other federal
agencies. The document was also reviewed by an expert panel of
nongovernmental reviewers, consisting of the following members:
Dr Martin Alexander, Cornell University
Dr Gary Booth, Brigham Young University
Dr Samuel Cohen, University of Nebraska Medical Center
Dr Loren Koller, Oregon State University
Dr Frederick Oehme, Kansas State University
APPENDIX 3 - CICAD PEER REVIEW
The draft CICAD on mononitrophenols was sent for review to
institutions and organizations identified by IPCS after contact with
IPCS national Contact Points and Participating Institutions, as well
as to identified experts. Comments were received from:
Federal Institute for Health Protection of Consumers & Veterinary
Medicine, Berlin, Germany
Gesellschaft Deutscher Chemiker, Frankfurt, Germany
Institute of Occupational Medicine, Chinese Academy of Preventive
Medicine, Ministry of Health, Beijing, People's Republic of China
Institute of Terrestrial Ecology, Huntingdon, United Kingdom
Joint Food Safety and Standards Group, Department of Health,
London, United Kingdom
National Institute of Health Sciences, Tokyo, Japan
National Institute of Public Health, Prague, Czech Republic
United States Department of Health and Human Services (National
Institute of Environmental Health Sciences, Research Triangle
Park), USA
United States Environmental Protection Agency (National Center
for Environmental Assessment, Washington, DC; Region VIII), USA
World Health Organization/International Programme on Chemical
Safety, Montreal, Canada
APPENDIX 4 - CICAD FINAL REVIEW BOARD
Washington, DC, USA, 8-11 December 1998
Members
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
( Vice-Chairperson)
Mr R. Cary, Toxicology Unit, Health Directorate, Health and Safety
Executive, Bootle, Merseyside, United Kingdom ( Rapporteur)
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr O. Faroon, Agency for Toxic Substances and Disease Registry,
Centers for Disease Control and Prevention, Atlanta, GA, USA
Dr G. Foureman, National Center for Environmental Assessment, US
Environmental Protection Agency, Research Triangle Park, NC, USA
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA ( Chairperson)
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany
Dr A. Nishikawa, Division of Pathology, National Institute of Health
Sciences, Tokyo, Japan
Dr E.V. Ohanian, Office of Water/Office of Science and Technology,
Health and Ecological Criteria Division, US Environmental Protection
Agency, Washington, DC, USA
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan
Professor P. Yao, Institute of Occupational Medicine, Chinese Academy
of Preventive Medicine, Ministry of Health, Beijing, People's Republic
of China
Observers
Dr K. Austin, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr I. Daly (ICCA representative), Regulatory and Technical Associates,
Lebanon, NJ, USA
Ms K.L. Lang (CEFIC, European Chemical Industry Council,
representative), Shell International, London, United Kingdom
Ms K. Roberts (ICCA representative), Chemical Self-funded Technical
Advocacy and Research (CHEMSTAR), Chemical Manufacturers Association,
Arlington, VA, USA
Dr W. Snellings (ICCA representative), Union Carbide Corporation,
Danbury, CN, USA
Dr M. Sweeney, Document Development Branch, National Institute for
Occupational Safety and Health, Cincinnati, OH, USA
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Secretariat
Dr M. Baril, Institut de Recherches en Santé et Sécurité du Travail du
Québec (IRSST), Montreal, Quebec, Canada
Dr H. Galal-Gorchev, Chevy Chase, MD, USA
Ms M. Godden, Health and Safety Executive, Bootle, Merseyside, United
Kingdom
Dr R.G. Liteplo, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Ms L. Regis, Programme for the Promotion of Chemical Safety, World
Health Organization, Geneva, Switzerland
Mr A. Strawson, Health and Safety Executive, London, United Kingdom
Dr P. Toft, Programme for the Promotion of Chemical Safety, World
Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
Ce CICAD relatif aux isomères en position 2-, 3- et 4- du
nitrophénol a été préparé par l'Institut Fraunhofer de recherche en
toxicologie et sur les aérosols de Hanovre (Allemagne). Il est basé
sur des mises au point rédigées par le Comité consultatif allemand sur
les produits chimiques qui posent un problème écologique (BUA, 1992)
et par l'US Agency for Toxic Substances and Disease Registry (ATSDR,
1992) afin d'évaluer les effets potentiels du 2- et du 4-nitrophénol
sur l'environnement et la santé humaine. Les données prises en compte
dans ces mises au point vont jusqu'en 1992. Une recherche
bibliographique exhaustive a été effectuée en 1998 sur plusieurs bases
de données afin de relever les références intéressantes sur le 2- et
le 4-nitrophénol publiées après celles qui figurent dans les documents
de base et d'obtenir toutes celles qui contiennent des données utiles
sur le 3-nitrophénol. On a trouvé très peu de données sur cet isomère,
ce qui rend impossible une véritable évaluation. Les données
concernant cet isomère sont donc récapitulées à l'appendice 1. On
trouvera à l'appendice 2 des indications sur le mode d'examen par des
pairs ainsi que sur les sources documentaires utilisées. Les
renseignements concernant l'examen du CICAD par des pairs font l'objet
de l'appendice 3. Ce CICAD a été approuvé en tant qu'évaluation
internationale lors de la réunion du Comité d'évaluation finale qui
s'est tenue à Washington du 8 au 11 décembre 1998. La liste des
participants à cette réunion figure à l'appendice 4. La fiche
d'information internationale sur la sécurité chimique (ICSC No 1342)
relative au mélange d'isomères du nitrophénol, établie par le
Programme international sur la sécurité chimique (IPCS, 1998) est
également reproduite dans ce document.
Les isomères du nitrophénol sont des solides solubles dans l'eau
qui sont légèrement acides dans ce solvant par suite de leur
dissociation. Les isomères 2- et 4- sont utilisés comme intermédiaires
dans la synthèse d'un certain nombre d'insecticides organophosphorés
et de composés à usage médical. Lorsqu'ils passent dans
l'environnement, c'est principalement par suite d'émissions dans
l'eau, l'air et le sol provenant de sources diffuses comme la
circulation automobile ou la décomposition par photolyse ou hydrolyse
de certains insecticides. Le dépôt par voie sèche ou humide de
nitrophénols présents dans l'atmosphère constitue un apport
supplémentaire dans l'hydrosphère et la géosphère. La formation
photo-oxydative du 2- et du 4-nitrophénol dans l'atmosphère est encore
débattue.
Les données disponibles montrent que le 2-nitrophénol ne devrait
se volatiliser que lentement dans l'atmosphère et qu'il ne passe sans
doute qu'en quantité négligeable de l'eau à l'air selon ce processus.
On constate un enrichissement en 2-nitrophénol de la phase aqueuse des
nuages; en revanche, dans la phase gazeuse, la proportion de
4-nitrophénol est plus importante qu'on pourrait le penser en fonction
des données physico-chimiques, par suite d'une importante fixation du
composé sur les particules. Compte tenu de leur solubilité dans l'eau
et de leur concentration dans la phase gazeuse, on peut s'attendre à
ce que les nitrophénols présents dans l'atmosphère se déposent par
voie humide sur la surface du sol et de l'eau. La principale voie de
transformation du 2-nitrophénol présent dans l'atmosphère est
vraisemblablement sa nitration rapide en 2,4-dinitrophénol, le
4-nitrophénol étant quant à lui en majeure partie fixé aux particules
aéroportées et donc disponible en petites quantités seulement pour des
réactions photochimiques. La majeure partie du 4-nitrophénol devrait
d'ailleurs disparaître de l'atmosphère en se déposant soit par voie
sèche, soit par voie humide. Il ne semble pas que les nitrophénols
contribuent directement à la dégradation de la couche d'ozone
stratosphérique ni au réchauffement général de la planète. Soumis à
une photodécomposition en milieu aqueux, le 4-nitrophénol a une
demi-vie qui peut aller de 2,8 à 13,7 jours selon les mesures. Les
nombreuses études consacrées à la biodégradation du 2- et du
4-nitrophénol montrent que ces deux isomères sont intrinsèquement
biodégradables dans l'eau en aérobiose. La minéralisation des
nitrophénols en anaérobiose exige à l'évidence une importante
adaptation des populations microbiennes.
La valeur du coefficient de sorption par les particules du sol
( Koc), qui se situe entre 44 et 530, indique que le potentiel de
sorption est faible à modéré. Les nitrophénols qui passent dans le sol
vont vraisemblablement subir une biodégradation aérobie. Il ne devrait
y avoir infiltration dans les eaux souterraines que lorsque les
conditions ne sont pas favorables à une biodégradation. Pour le 2- et
le 4-nitrophénol, la mesure du facteur de bioconcentration donne des
valeurs allant de 11 à 76, ce qui indique un faible potentiel de
bioconcentration.
On connaît plutôt mal le profil toxicologique du 2- et du
4-nitrophénol. Lorsqu'il est administré à des animaux de laboratoire
par voie orale, intraveineuse ou intrapéritonéale, le 4-nitrophénol
est en majeure partie excrété dans les urines en l'espace de 24 à 48 h
sous forme de glucuronide ou de sulfo-conjugué, une faible partie
seulement passant dans les matières fécale ou restant inchangée. On a
montré que la proportion de glucuronide et de sulfo-conjugués variait
selon les espèces. Après administration par voie orale à des lapins,
le 4-nitrophénol est réduit en p-aminophénol et subit aussi une
transformation en glucuronide et sulfo-conjugués. Les données tirées
des études in vivo et in vitro donnent une indication sur la
résorption du 4-nitrophénol par la voie transcutanée. En revanche, les
données concernant le 2-nitrophénol sont très limitées. Quoi qu'il en
soit, on peut considérer, en se basant sur les données disponibles,
que les deux isomères ont un métabolisme comparable. Le 2- et le
4-nitrophénol ne devraient pas s'accumuler dans l'organisme en raison
de leur métabolisation et de leur excrétion rapides.
Les études de toxicité aiguë montrent que le 4-nitrophénol a un
effet nocif après ingestion et qu'il est plus toxique que le
2-nitrophénol. Chez des chats, on a constaté une augmentation du taux
de méthémoglobine liée à la dose après ingestion de 2-nitrophénol; la
même constatation a été faite chez des rats après inhalation de
4-nitrophénol. Une exposition répétée à du 4-nitrophénol a montré que
la formation de méthémoglobine est l'effet le plus déterminant d'une
exposition par la voie respiratoire et cela vaut sans doute aussi pour
la voie orale. Parmi les autres effets observés, on peut citer un
moindre gain de poids, une modification du poids des organes, une
dégénérescence graisseuse du foie et des anomalies hématologiques. Il
n'a pas été possible de dégager une véritable relation dose-réponse ni
de déterminer de manière fiable la dose sans effet (nocif) observable
(NO(A)EL) correspondant à ces effets.
Le 2-nitrophénol est légèrement irritant pour la peau mais il
n'irrite pas la muqueuse oculaire. Le test de Buehler montre que le
composé n'a pas non plus d'effet sensibilisateur. En s'appuyant sur
des études valables effectuées sur l'animal, on peut conclure que le
4-nitrophénol a par contre une légère action irritante sur la peau et
les yeux. Un test de maximalisation sur le cobaye a montré que le
4-nitrophénol avait également une légère action sensibilisatrice. Chez
l'homme, on ne peut exclure une légère sensibilisation après un
contact avec le composé, d'autant plus que la pose d'un timbre cutané
chez des ouvriers pouvant avoir été en contact avec du 4-nitrophénol a
permis de constater une telle sensibilisation.
Aucun des deux isomères n'a fait l'objet d'épreuves de
génotoxicité suffisamment complètes. S'agissant du 2-nitrophénol, les
données sont insuffisantes pour que l'on puisse tirer la moindre
conclusion concernant une mutagénicité éventuelle. Dans le cas du
4-nitrophénol, les études de mutagénicité sont plus nombreuses, mais
le compte rendu en est parfois insuffisant. On est fondé à penser que
ce composé est susceptible de provoquer des aberrations chromosomiques
in vitro. Faute d'études de mutagénicité in vivo sur des
mammifères, il n'est pas possible de savoir si le pouvoir mutagène de
cet isomère peut s'exprimer in vivo.
Chez la souris l'application cutanée de 4-nitrophénol pendant une
durée de 78 semaines n'a pas donné d'indices d'effets cancérogènes.
Dans une autre étude sur la souris, qui présentait toutefois un
certain nombre d'insuffisances, on n'a pas non plus observé de tumeurs
cutanées après application cutanée de ces deux isomères pendant 12
semaines. Aucune étude de cancérogénicité utilisant la voie orale ou
respiratoire n'était disponible.
Les données relatives au 4-nitrophénol ne révèlent aucun effet
indésirable sur la reproduction ou le développement qui soit
statistiquement significatif après exposition de rats et de souris par
voie orale ou cutanée. Après administration par voie orale de
2-nitrophénol à des rats, on a constaté dans la progéniture des
animaux des effets indésirables sur le développement, mais seulement
aux doses toxiques pour les mères. On n'a toutefois pas recherché la
présence de malformations internes.
La base de données relative au 2-nitrophénol est extrêmement
limitée et celle qui concerne le 4-nitrophénol est insuffisante pour
qu'on puisse en tirer une valeur fiable de la NO(A)EL. Il est donc
impossible de fixer pour l'instant une valeur pour la dose journalière
tolérable (DJT) ou pour la concentration tolérable (CT) de ces deux
isomères.
D'après les résultats des études toxicologiques valables
effectuées sur divers organismes aquatiques, on peut considérer que
ces deux nitrophénols sont modérément à fortement toxiques pour la vie
aquatique. La concentration sans effet la plus faible qui ait été
obtenue lors d'études de longue durée sur des organismes d'eau douce
( Scenedesmus subspicatus, EC50 à 96 h : 0,39 mg de
2-nitrophénol/litre; Entosiphon sulcatum, concentration minimale
inhibitrice à 72 h ou CMI : 0,83 mg de 4-nitrophénol/l) était 40 à 50
fois plus forte que la valeur maximale obtenue dans un bassin fluvial
d'Asie situé dans une zone très industrialisée et densément peuplée
(respectivement 0,0072 mg/l et 0,019 mg/l). Par conséquent, malgré la
biodégradation et la décomposition photochimique, les nitrophénols
déversés dans l'eau peuvent présenter un certain risque pour les
organismes aquatiques sensibles, notamment dans des eaux
superficielles où les conditions ne sont pas favorables à ces deux
modes d'élimination. Cependant, compte tenu de leurs usages et de
leurs possibilités de libération dans l'environnement, ces deux
nitrophénols ne présentent qu'un risque mineur pour les organismes
aquatiques.
Les données disponibles indiquent que la toxicité potentielle de
ces nitrophénols est modérée dans l'environnement terrestre. Le calcul
du rapport d'exposition toxique (TER) des nitrophénols provenant de la
décomposition de certains pesticides montre que, dans ce milieu, le
risque reste faible pour la faune et la flore, même dans la pire des
hypothèses.
RESUMEN DE ORIENTACION
Preparó el presente CICAD sobre los isómeros 2-, 3- y
4-nitrofenol el Instituto Fraunhofer de Toxicología y de Investigación
sobre los Aerosoles de Hannover, Alemania. Se basa en los exámenes
compilados por el Comité Consultivo Alemán sobre las Sustancias
Químicas Importantes para el Medio Ambiente (BUA, 1992) y la Agencia
para el Registro de Sustancias Tóxicas y Enfermedades de los Estados
Unidos (ATSDR, 1992) para evaluar los efectos potenciales del 2- y el
4-nitrofenol en el medio ambiente y en el ser humano. En estos
exámenes se incluyeron los datos identificados hasta 1992. En 1998 se
realizó una búsqueda bibliográfica amplia de varias bases de datos
para identificar todas las referencias importantes relativas al 2- y
el 4-nitrofenol publicadas con posterioridad a las que figuran en los
documentos originales y para conocer todas las referencias con datos
pertinentes sobre el isómero 3-nitrofenol. La información obtenida
sobre el 3-nitrofenol fue muy escasa, lo que impide una evaluación
válida. En consecuencia, los datos sobre este isómero se resumen en el
apéndice 1. La información relativa al carácter del examen colegiado y
a la disponibilidad de los documentos originales figura en el apéndice
2. La información sobre el examen colegiado de este CICAD se presenta
en el apéndice 3. Este CICAD se aprobó como evaluación internacional
en una reunión de la Junta de Evaluación Final celebrada en
Washington, DC, Estados Unidos, los días 8-11 de diciembre de 1998. La
lista de participantes en esta reunión figura en el apéndice 4. La
Ficha internacional de seguridad química (ICSC 1342) para las mezclas
de isómeros de nitrofenoles, preparada por el Programa Internacional
de Seguridad de las Sustancias Químicas (IPCS, 1998), también se
reproduce en el presente documento.
Los isómeros del nitrofenol son sólidos hidrosolubles con una
acidez moderada en agua debido a la disociación. El 2-nitrofenol y el
4-nitrofenol se utilizan como intermediarios en la síntesis de
diversos plaguicidas órganofosforados y algunos productos médicos. La
liberación en el medio ambiente se produce fundamentalmente por
emisiones en el aire, el agua y el suelo a partir de fuentes difusas,
como el tráfico de vehículos y la degradación hidrolítica y fotolítica
de los respectivos plaguicidas. También se produce liberación en la
hidrosfera y la geosfera a partir de la atmósfera debido a la
deposición seca y húmeda de nitrofenoles suspendidos en el aire. La
formación fotooxidativa de 2- y 4-nitrofenol en la atmósfera es
todavía objeto de estudio.
Con los datos disponibles sólo cabe esperar una volatilización
lenta desde el agua hacia el aire para el 2-nitrofenol y no
significativa para el 4-nitrofenol. El 2-nitrofenol se enriquece en la
fase líquida de las nubes, mientras que es posible encontrar más
4-nitrofenol del previsto a partir de los datos fisicoquímicos en la
fase gaseosa de las nubes, debido a una amplia unión a partículas.
Habida cuenta de la solubilidad en agua y la presencia prevista en la
fase de vapor, cabe esperar una deposición húmeda de nitrofenoles del
aire en las aguas superficiales y en el suelo. La vía principal de
transformación del 2-nitrofenol emitido a la troposfera debe ser la
nitración rápida a 2,4-dinitrofenol, mientras que se supone que la
mayor parte del 4-nitrofenol suspendido en el aire se encuentra unido
a partículas y, por consiguiente, disponible solamente en menor
cantidad para reacciones fotoquímicas. La mayor parte del 4-nitrofenol
del aire debe precipitar por deposición húmeda y seca. No se considera
que los nitrofenoles contribuyan directamente al agotamiento de la
capa de ozono estratosférico o al calentamiento mundial. La semivida
medida para la descomposición fotoquímica del 4-nitrofenol en agua
osciló entre 2,8 y 13,7 días. Numerosos estudios sobre la
biodegradación del 2- y el 4-nitrofenol indican que los isómeros son
inherentemente biodegradables en agua en condiciones aerobias. La
mineralización de los nitrofenoles en condiciones anaerobias requiere
evidentemente una amplia adaptación de las comunidades microbianas.
Los coeficientes de sorción en el suelo ( Koc) medidos del
orden de 44-530 indican un potencial de bajo a moderado para la
sorción en el suelo. Los nitrofenoles liberados al suelo se deben
biodescomponer en condiciones aerobias. Cabe prever filtración hacia
el agua freática sólo en condiciones desfavorables para la
biodegradación. Para el 2- y el 4-nitrofenol, los factores de
bioconcentración medidos de 11 a 76 ponen de manifiesto un potencial
bajo de bioacumulación.
Se dispone sólo de información limitada relativa a los perfiles
toxicológicos del 2- y el 4-nitrofenol. Los animales experimentales a
los que se administró 4-nitrofenol por vía oral, intravenosa o
intraperitoneal excretaron la mayor parte de la dosis aplicada por vía
urinaria en un plazo de 24-48 horas en forma de conjugados de
glucurónidos y sulfatos, mientras que por las heces se excretaron
solamente cantidades muy pequeñas, o bien como 4-nitrofenol
inalterado. Se observó que los porcentajes de conjugados de
glucurónidos y sulfatos eran dependientes de la especie y de la dosis.
Tras la administración oral a conejos, el 4-nitrofenol sufre una
reducción a p-aminofenol, así como una glucuronización y
sulfatación. Los datos disponibles de estudios in vivo e in vitro
dan una idea de la absorción cutánea del 4-nitrofenol. Los datos para
el 2-nitrofenol son muy limitados. Sin embargo, teniendo cuenta los
datos disponibles, se supone que se produce una transformación
metabólica comparable. No cabe prever bioacumulación de 2- y
4-nitrofenol en los organismos debido a la rapidez de su metabolismo y
excreción.
En estudios de toxicidad aguda, el 4-nitrofenol es perjudicial
tras la absorción oral, y se observó que era más tóxico que el
2-nitrofenol. Se detectó un aumento en la formación de metahemoglobina
dependiente de la dosis en gatos tras la exposición oral al
2-nitrofenol y en ratas tras la exposición por inhalación al
4-nitrofenol. Después de una exposición repetida al 4-nitrofenol, se
observó la formación de metahemoglobina como el efecto final más
importante de la exposición por inhalación, y se considera que esto es
aplicable también a la exposición oral. Otros efectos observados
fueron la reducción del aumento del peso corporal, diferencias en el
peso de los órganos, degeneración adiposa focal del hígado y cambios
hematológicos. Para esos efectos no fue posible determinar una
relación clara dosis-respuesta o concentraciones sin efectos
(adversos) observados (NO(A)EL) fidedignas.
El 2-nitrofenol es ligeramente irritante para la piel, pero no
para los ojos. En una prueba de Buehler no se observó efecto
sensibilizador. Tomando como base los estudios válidos con animales
experimentales, se supone que el 4-nitrofenol tiene efectos irritantes
en la piel y los ojos. En una prueba de maximización en cobayas, se
observó que el 4-nitrofenol era ligeramente sensibilizante. En el ser
humano no se puede excluir una posible sensibilización tras el
contacto con 4-nitrofenol, especialmente teniendo en cuenta que se ha
observado sensibilización cutánea en pruebas de parche en trabajadores
de fábricas que podían haber estado expuestos al 4-nitrofenol.
No se ha sometido a pruebas completas de genotoxicidad ninguno de
los dos isómeros del nitrofenol. Los datos disponibles sobre el
2-nitrofenol son insuficientes para poder sacar conclusiones acerca de
su posible mutagenicidad. Hay más estudios de mutagenicidad para el
4-nitrofenol, aunque algunos se notificaron de manera inadecuada. Hay
pruebas que parecen indicar que el 4-nitrofenol puede producir
aberraciones cromosómicas in vitro. En ausencia de estudios de
mutagenicidad in vivo en mamíferos, no es posible llegar a la
conclusión de si se expresa o no in vivo el potencial mutagénico del
4-nitrofenol.
Tras la aplicación cutánea de 4-nitrofenol a ratones durante 78
semanas no se observaron efectos carcinogénicos. En otro estudio con
ratones, que tiene varias limitaciones, no se detectaron tumores
cutáneos tras la aplicación en la piel de 2- ó 4-nitrofenol durante 12
semanas. Para ninguno de los isómeros había estudios de
carcinogenicidad utilizando la vía oral o la inhalación.
Los datos disponibles para el 4-nitrofenol no pusieron de
manifiesto efectos específicos o estadísticamente significativos de
toxicidad reproductiva o del desarrollo tras la administración cutánea
u oral a ratas y ratones. En un estudio de administración por vía oral
a ratas, el 2-nitrofenol indujo efectos en el desarrollo de la camada
sólo con dosis que también producían toxicidad materna. Sin embargo,
en estos estudios no se examinaron los fetos para investigar
malformaciones internas.
La base de datos para el 2-nitrofenol es enormemente limitada y
la relativa al 4-nitrofenol es insuficiente para deducir valores
fidedignos de la (NO(A)EL). Por consiguiente, es imposible determinar
en este momento la ingesta diaria tolerable o las concentraciones
tolerables para el 2- ó el 4-nitrofenol.
De los resultados disponibles de pruebas válidas sobre la
toxicidad del 2-y el 4-nitrofenol para diversos organismos acuáticos,
los nitrofenoles se pueden clasificar como sustancias con una
toxicidad entre moderada y alta en el compartimento acuático. Las
concentraciones más bajas con efectos obtenidas en estudios crónicos
con organismos de agua dulce ( Scenedesmus subspicatus, CE50: 0,39
mg de 2-nitrofenol/litro a las 96 h; Entosiphon sulcatum,
concentración inhibitoria mínima a las 72 horas: 83 mg de
4-nitrofenol/litro) fueron 40-50 veces superiores a los niveles
máximos determinados en una cuenca fluvial asiática densamente poblada
y muy industrializada (0,0072 mg de 2-nitrofenol/litro y 0,019 mg de
4-nitrofenol/litro). Por consiguiente, a pesar de la descomposición
biótica y fotoquímica, los nitrofenoles emitidos al agua pueden
representar algún peligro para los organismos acuáticos sensibles,
particularmente en las condiciones de las aguas superficiales que no
favorecen ambas vías de eliminación. Habida cuenta de sus pautas de
uso y sus características de liberación, probablemente los
nitrofenoles plantean sólo un pequeño riesgo para los organismos
acuáticos.
Los datos disponibles indican solamente una toxicidad potencial
moderada de los nitrofenoles en el medio ambiente terrestre. A partir
de los cálculos de la razón exposición-toxicidad de los nitrofenoles a
partir de la degradación de plaguicidas, sólo cabe esperar un pequeño
riesgo para los organismos en este compartimento, incluso en el peor
de los casos.
