
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 103
2-PROPANOL
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Organization
Geneva, 1990
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
2-Propanol.
(Environmental health criteria ; 103)
1.Alcohol,propyl
I.Series
ISBN 92 4 157103 9 (NLM Classification: QD 305.A4)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1990
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR 2-PROPANOL
1. SUMMARY
1.1. Identity, physical and chemical properties, analytical
methods
1.2. Sources of human and environmental exposure
1.3. Environmental transport, distribution, and transformation
1.4. Environmental levels and human exposure
1.5. Kinetics and metabolism
1.6. Effects on organisms in the environment
1.7. Effects on experimental animals and in vitro test
systems
1.8. Health effects in human beings
1.9. Summary of evaluation
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Man-made sources
3.2.1. Production levels and processes
3.2.1.1 Production levels
3.2.1.2 Production processes
3.2.2. Uses
3.2.3. Waste disposal
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and distribution between media
4.2. Abiotic degradation
4.3. Biotransformation
4.3.1. Biodegradation
4.3.2. Bioaccumulation
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.2. General population exposure
5.2.1. Exposure via food
5.2.2. Exposure via other consumer products
5.3. Occupational exposure
6. KINETICS AND METABOLISM
6.1. Absorption
6.1.1. Animals
6.1.2. Human beings
6.2. Distribution
6.2.1. Animals
6.2.2. Human beings
6.3. Metabolism
6.3.1. Animals
6.3.2. Human beings
6.4. Elimination and excretion
6.4.1. Animals
6.4.2. Human beings
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Aquatic organisms
7.2. Terrestrial organisms
7.2.1. Microorganisms
7.2.2. Insects
7.2.3. Plants
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposures
8.1.1. Mortality
8.1.2. Signs of intoxication
8.1.3. Skin, eye, and respiratory tract irritation
8.2. Continuous or repeated exposures
8.3. Neurotoxicity and behavioural effects
8.4. Biochemical effects
8.4.1. Effects on lipids in liver and blood
8.4.2. Effects on microsomal enzymes
8.4.3. Other biochemical findings
8.5. Immunological effects
8.6. Reproduction, embryotoxicity, and teratogenicity
8.7. Mutagenicity
8.8. Carcinogenicity
8.9. Factors modifying toxicity
9. EFFECTS ON MAN
9.1. General population exposure
9.1.1. Poisoning incidents
9.1.2. Controlled exposures
9.1.3. Skin irritation; sensitization
9.2. Occupational exposure
9.2.1. Epidemiology studies
9.2.2. Interacting agents
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE
ENVIRONMENT
10.1. Evaluation of human health risks
10.1.1. Exposure
10.1.2. Health effects
10.2. Evaluation of effects on the environment
11. RECOMMENDATIONS
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
RESUME
RESUMEN
WHO TASK GROUP MEETING ON ENVIRONMENTAL HEALTH CRITERIA FOR
2-PROPANOL
Members
Dr R. Drew, Department of Clinical Pharmacology, Flinders
University of South Australia, Bedford Park, South Australia,
Australia
Dr B. Gilbert, Company for Development of Technology Transfer
(CODETEC), City University, Campinas, Brazil (Rapporteur)
Dr B. Hardin, Document Development Branch, Division of Standards
Development and Technology Transfer, National Institute for
Occupational Safety and Health, Cincinnati, Ohio, USA (Chairman)
Dr S.K. Kashyap, National Institute of Occupational Health,
Ahmedabad, India
Professor M. Noweir, Occupational Health Research Centre, High
Institute of Public Health, University of Alexandria,
Alexandria, Egypt
Dr L. Rosenstein, Office of Toxic Substances, US Environmental
Protection Agency, Washington, DC, USA
Professor I.V. Sanotsky, Chief, Department of Toxicology, Institute
of Industrial Hygiene and Occupational Diseases, Moscow, USSR
(Vice-Chairman)
Dr J. Sokal, Division of Industrial Toxicology, Institute of
Occupational Medicine, Lodz, Poland
Dr H.J. Wiegand, Toxicology Department, Huls AG, Marl, Federal
Republic of Germany
Dr K. Woodward, Department of Health, Medical Toxicology and
Environmental Health Division, London, United Kingdom
Observers
Dr K. Miller (Representing International Commission on Occupational
Health (ICOH)), British Industrial Biological Research
Association, Carshalton, Surrey, United Kingdom
Secretariat
Professor F. Valic , Consultant, IPCS, World Health Organization,
Geneva, Switzerland, also Vice-Rector, University of Zagreb,
Zagreb, Yugoslavia (Secretary)
Dr T. Vermeire, National Institute of Public Health and
Environmental Hygiene, Bilthoven, Holland
Host Organization
Dr S.D. Gangolli, British Industrial Biological Research
Association, Carshalton, Surrey, United Kingdom
Dr D. Anderson, British Industrial Biological Research Association,
Carshalton, Surrey, United Kingdom
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the
criteria documents as accurately as possible without unduly
delaying their publication. In the interest of all users of the
environmental health criteria documents, readers are kindly
requested to communicate any errors that may have occurred to the
Manager of the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland, in order that they may be
included in corrigenda, which will appear in subsequent volumes.
* * *
A detailed data profile and a legal file can be obtained from
the International Register of Potentially Toxic Chemicals, Palais
des Nations, 1211 Geneva 10, Switzerland (Telephone no. 7988400/
7985850).
ENVIRONMENTAL HEALTH CRITERIA FOR 2-PROPANOL
A WHO Task Group on Environmental Health Criteria for
2-Propanol met at the British Industrial Biological Research
Association (BIBRA), Carshalton, Surrey, United Kingdom, from 10 to
14 April 1989. Dr S.D. Gangolli, who opened the meeting, welcomed
the participants on behalf of the Department of Health, and
Dr D. Anderson on behalf of BIBRA, the host institution.
Dr F. Valic greeted the participants on behalf of the heads of the
three IPCS cooperating organizations (UNEP/ILO/WHO). The Task
Group reviewed and revised the draft criteria document and made an
evaluation of the human health risks and effects on the environment
of exposure to 1-propanol.
The drafts of this document were prepared by Dr T. VERMEIRE,
National Institute of Public Health and Environmental Hygiene,
Bilthoven, Netherlands. Dr F. VALIC was responsible for the
overall scientific content of the document and Mrs M.O. HEAD of
Oxford, England, for the editing.
The efforts of all who helped in the preparation and
finalization of the document are gratefully acknowledged.
* * *
Partial financial support for the publication of this criteria
document was kindly provided by the United States Department of
Health and Human Services, through a contract from the National
Institute of Environmental Health Sciences, Research Triangle Park,
North Carolina, USA - a WHO Collaborating Centre for Environmental
Health Effects. The United Kingdom Department of Health and
Social Security generously supported the cost of printing.
1. SUMMARY
1.1 Identity, Physical and Chemical Properties, Analytical Methods
2-Propanol is a colourless highly flammable liquid with an
odour resembling that of a mixture of ethanol and acetone. The
compound is completely miscible with water, ethanol, acetone,
chloroform, and benzene. Analytical methods are available for the
detection of 2-propanol in various media (air, water, blood, serum,
and urine) with detection limits for air, water, and blood of
2 x 10-5 mg/m3, 0.04 mg/litre, and 1 mg/litre, respectively. Gas
chromatographic methods (primarily using flame ionization
detection) as well as paper electrophoresis and photoionization ion
mobility spectrometry methods are available for the determination
of 2-propanol in the various media.
1.2 Sources of Human and Environmental Exposure
World production of 2-propanol in 1975 was estimated to be more
than 1100 kilotonnes and the global production capacity in 1984 was
estimated to be more than 2000 kilotonnes. 2-Propanol is commonly
manufactured from propene. The previously used strong acid and
weak acid processes, which involved potentially hazardous
intermediates and by-products, have now largely been replaced by
the catalytic hydration process. The catalytic reduction of
acetone is an alternative process.
2-Propanol has been identified as a metabolic product of a
variety of microorganisms.
The compound has widespread solvent applications, and is used
as a component of household and personal products including aerosol
sprays, topically applied pharmaceutical products, and cosmetics.
2-Propanol is also used in the production of acetone and other
chemicals, as a de-icing agent, as a preservative, in windscreen
wiper concentrates, and as a flavour volatile in foodstuffs.
2-Propanol may enter the atmosphere, water, or soil following
waste disposal and has been identified in the air and leachates
from hazardous waste sites and landfills. It is emitted in waste
gases and waste water from industrial sources, and may be removed
from the latter by biological oxidation or reverse osmosis.
Disperse airborne emissions will occur during the use of 2-propanol
in consumer products.
1.3 Environmental Transport, Distribution, and Transformation
The main pathway of entry of 2-propanol into the environment is
through its emission into the atmosphere during production,
processing, storage, transport, use, and disposal. Emissions into
soil and water also occur. The emissions to each environmental
compartment are difficult to estimate. However, in 1976, the total
release of this compound into the atmosphere was estimated to
exceed 50% of the 2-propanol produced.
2-Propanol is rapidly removed from the atmosphere by reaction
with hydroxyl radicals and by rain-out. The latter is responsible
for the transport of 2-propanol from the atmosphere to soil or
water. Once in the soil, it is likely to be very mobile and it
increases soil permeability to some aromatic hydrocarbons.
2-Propanol is readily biodegradable, both aerobically and
anaerobically.
It is not expected to bioaccumulate because it is biodegradable
and is completely miscible in water, with a log n-octanol/water
partition coefficient of 0.14 and a bioconcentration factor of 0.5.
1.4 Environmental Levels and Human Exposure
Exposure of the general population occurs through accidental or
intentional ingestion, through the ingestion of food containing
2-propanol as a natural or added flavour volatile or as a solvent
residue, and through inhalation during use. Concentrations have
been found of between 0.2 and 325 mg/litre in non-alcoholic
beverages and 50 - 3000 mg/kg in foods following the use of
2-propanol as a solvent in their production. Exposure of the
general population through inhalation of ambient air is low,
because of its rapid removal and degradation. Various sites have
been monitored and time-weighted average concentrations of up to
35 mg/m3 have been measured for urban sites.
Workers are exposed to 2-propanol during the production of the
compound itself, and of acetone and other derivatives, and also
during its use as a solvent. In the USA, it was estimated by the
National Occupational Exposure Survey (1980 - 83) that over 1.8
million workers were potentially exposed. Concentrations of up to
1350 mg/m3 have been measured at work-places, with time-weighted
averages of up to about 500 mg/m3.
1.5 Kinetics and Metabolism
2-Propanol is rapidly absorbed and distributed throughout
the body after inhalation or ingestion. At high doses,
gastrointestinal absorption is delayed. Blood levels of 2-propanol
(detectable when ethanol is ingested simultaneously) or of its
metabolite, acetone, are related to the exposure levels. Human
volunteers who ingested a dose of 3.75 mg/kg (with 1200 mg
ethanol/kg) in orange juice exhibited a peak level of 0.8 ± 0.3 mg
free 2-propanol/litre in the blood, and 2.3 ± 1.4 mg/litre after
incubation with aryl sulfatase, suggesting sulfation. Workers
exposed to vapour (8 - 647 mg/m3) showed concentrations of 3 - 270
mg/m3 in the alveolar air but, in this case, acetone, not
2-propanol, was found in the blood and urine. In treated
laboratory animals, 2-propanol was detected not only in the blood
but also in the spinal fluid, liver, kidneys, and brain. It passes
the blood-brain barrier twice as effectively as ethanol.
2-Propanol is excreted partly unchanged and partly as acetone,
mainly via the lungs, but also via saliva and gastric juice.
Reabsorption may follow excretion via the last 2 routes. The
metabolism to acetone via liver alcohol dehydrogenase (ADH) is
rather slow since the relative affinity of ADH for 2-propanol is
lower than it is for ethanol. In vitro, human ADH with 2-propanol
showed 9 - 10% of the activity of the enzyme with ethanol as
substrate. In vitro, rat liver microsomal oxidases are also
capable of oxidizing 2-propanol. In human beings, acetone is
excreted unchanged, primarily via the lungs, and minimally by the
kidneys. Acetone levels in alveolar air, blood, and urine increase
with the extent and the duration of exposure to 2-propanol. The
elimination of 2-propanol and acetone from the body is first order,
and half-lives in human beings are 2.5 - 6.4 h and 22 h,
respectively.
1.6 Effects on Organisms in the Environment
The toxicity of 2-propanol for aquatic organisms, insects, and
plants is low. The inhibitory threshold for cell multiplication of
a sensitive protozoan species ranged from 104 to 4930 mg/litre
under various experimental conditions. Progressing higher through
the phylogenetic chain, various species of crustacea, including
Daphnia magna, showed EC50s at levels ranging from 2285 to 9714
mg/litre. LC50s (96-h) for freshwater fish ranged from 4200 to
11 130 mg/litre. Data obtained for fruit fly species showed LC50s
ranging between 10 200 and 13 340 mg/litre of nutrient media.
The LC50 for third instar mosquito larvae (Aedes aegypti) was
25 - 120 mg/litre in a 4-h static test.
The effects on plants of exposure to 2-propanol at
concentrations between 2100 mg/litre and more than 36 000 mg/litre
ranged from no effect to complete inhibition of germination.
1.7 Effects on Experimental Animals and In Vitro Test Systems
The acute toxicity of 2-propanol for mammals, based on
mortality, is low, whether exposure is via the oral, dermal, or
respiratory route. The LD50 values for several animal species
after oral administration varied between 4475 and 7990 mg/kg body
weight; the inhalation 8-h LC50s for rats ranged from 46 000 to
55 000 mg/m3 air. At these lethal levels, rats showed severe
irritation of the mucous membranes and severe depression of the
central nervous system. Death was caused by respiratory or cardiac
arrest. Histopathological lesions included congestion and oedema
of the lungs, and cell degeneration in the liver.
Single oral doses of 3000 or 6000 mg 2-propanol/kg body weight
resulted in a reversible accumulation of triglycerides in the liver
of rats. Microsomal enzyme induction was observed in rats at an
oral dose level of 390 mg/kg.
Undiluted 2-propanol appeared non-irritant when applied to the
clipped or abraded skin of rabbits for 4 h. However, 2-propanol
caused irritation when 0.1 ml of undiluted compound was applied to
the rabbit eye. High vapour concentrations of 2-propanol caused
irritation of the respiratory tract in mice, and the respiratory
rate was decreased by 50% at concentrations of 12 300 - 43 525
mg/m3 of air.
Repeated exposure studies on the effects of 2-propanol in
animals are rather limited. After inhalation of 500 mg
2-propanol/m3 for 5 days/week and 4 h/day over 4 months, irritation
of the respiratory tract, haematological changes, and
histopathological alterations in the liver and spleen were seen in
rats. In another study group, 5 rats of each sex received
drinking-water containing 2-propanol for 27 weeks. Comparison of
animals receiving approximately 600 or 2300 mg/kg per day (males)
and 1000 or 3900 mg/kg per day (females) with untreated controls
showed growth retardation only in both exposed female groups. No
further adverse effects were found.
The available evidence suggests that the effects of 2-propanol
on the central nervous system (CNS) are similar to those of
ethanol. The oral ED50 for narcosis in rabbits is 2280 mg/kg, the
intraperitoneal ED50 for loss of righting reflex in mice is 165
mg/kg, and the intraperitoneal threshold for induction of ataxia in
rats is 1106 mg/kg. These values are approximately two times lower
than those for ethanol. Inhalation of 2-propanol at 739 mg/m3 for
6 h/day, 5 days/week for 15 weeks did not result in any adverse
effects in an open field test.
2-Propanol was evaluated in a 2-generation study on rats by the
administration of 1290, 1380, or 1470 mg/kg per day in the
drinking-water to both generations. The only adverse effect noted
was a transient reduction in growth rate in the F0 generation. In
contrast, other research workers observed an increase in
malformations in a teratology study after pregnant rats were dosed
orally with 252 or 1008 mg 2-propanol/kg per day (maternal
toxicity was not discussed). Both of these doses, administered in
the drinking-water for 45 days, were also reported to increase the
estrous cycle to 5 days (versus 4 days in controls). Increased
total embryonic mortality was seen when female rats received
drinking-water doses of 1800 mg/kg per day for 6 months prior to
breeding; various effects on intrauterine and postnatal survival
were reported at a dose as low as 0.18 mg/kg per day, but no
consistent pattern was apparent. Pregnant rats were exposed to
airborne 2-propanol at concentrations of 9001, 18 327, or 23 210
mg/m3 (3659, 7450, or 9435 ppm). The two higher concentrations
were toxic to the maternal animals, but 9001 mg/m3 was not.
Developmental toxicity was seen at all three concentrations.
2-Propanol gave negative results in a test at 0.18 mg per plate
for point mutations in S. typhimurium and a test for sister
chromatid exchange in Chinese hamster lung fibroblasts. It induced
mitotic abnormalities in rat bone marrow cells and in onion root
tip cells in vitro. No other mutagenicity data were available.
2-Propanol was tested in several limited carcinogenicity
studies in the mouse using the dermal (3 times weekly for 1 year),
inhalation (7700 mg/m3 for 3 - 7 h/day, 5 days/week, over 5 - 8
months) and subcutaneous (20 mg undiluted, weekly for 20 - 40
weeks) routes of exposure. The occurrence of tumours was
investigated in the three studies in the skin, lung, and at the
injection site, respectively. There was no evidence of any
carcinogenic effects. There are no adequate epidemiology data with
which to assess the carcinogenicity of 2-propanol for human beings.
The available data suggest that di-2-propyl sulfate, an
intermediate in the strong and weak acid processes for the
production of 2-propanol, may be causally associated with the
induction of paranasal sinus cancer in human beings.
1.8 Health Effects in Human Beings
Several cases of intoxication have been reported after oral
ingestion and also in febrile children who were sponged with
2-propanol preparations. In cases of poisoning, the major signs
are those of alcoholic intoxication including nausea, vomiting,
abdominal pain, gastritis, hypotension, and hypothermia.
2-Propanol depresses the central nervous system about twice as much
as ethanol, causing unconsciousness, ending in deep coma; death may
follow due to respiratory depression. Other compound-related
effects are hyperglycaemia, elevated protein levels in
cerebrospinal fluid, and atelectasis. Although skin absorption has
been deemed insignificant, a case report on a child intoxicated
after being sponged with 2-propanol suggested that dermal
absorption should not be underestimated, particularly in children.
No adverse effects were observed in healthy volunteers who drank
syrup containing 2.6 or 6.4 mg 2-propanol/kg, daily, for 6 weeks.
A group of male volunteers, when exposed to 2-propanol vapours at
concentrations of 490, 980, or 1970 mg/m3 air for 3 - 5 min, judged
irritation to be "mild" at 980 mg/m3 and to be "satisfactory" for
their own 8-h occupational exposure.
Skin irritation in the form of erythema, 2nd and 3rd degree
burns, and blisters was reported in premature infants following
prolonged contact with 2-propanol. Occasionally, cases of allergic
contact dermatitis have also been reported.
Few epidemiological studies were available on mortality from
cancer or from other causes. In a group of 71 workers employed for
over 5 years in a plant manufacturing 2-propanol by the strong acid
process, 7 cancer cases were reported including 4 cases of
paranasal sinus cancer. In a cohort study on 779 workers at a
similar plant, the age- and sex-adjusted incidences of sinus and
laryngeal cancer were 21 times higher than expected. The minimum
latency period was 10 years. In another retrospective cohort study
at another plant using the strong acid process, there were more
than 4000 person-years at risk. The results showed that mortality
rates due to all causes and due to neoplasms were not significantly
higher than expected. A retrospective cohort study was undertaken
in a plant manufacturing 2-propanol by the weak acid process. More
than 11 000 person-years were at risk. The mortality rate due to
all causes was lower than expected. No excess mortality due to all
cancers was observed. However, the incidence of buccal and
pharyngeal cancer was 4 times higher than expected. The cohort
studies collectively suggest a cancer hazard related to the strong
acid manufacturing process but, in two small case-control studies,
no evidence of an association between exposure to 2-propanol and
the incidence of gliomas or lymphatic leukaemia was reported.
There are reports suggesting that combined exposure to carbon
tetrachloride and 2-propanol in workers results in potentiation of
the toxicity of the former.
1.9 Summary of Evaluation
Exposure of human beings to 2-propanol may occur through
inhalation during manufacture, processing, and both occupational
and household use. Exposure to a potentially lethal level in the
general population may result from accidental or intentional
ingestion and children may be exposed when sponged with 2-propanol
preparations (rubbing alcohol).
2-Propanol is rapidly absorbed and distributed throughout the
body, partly as acetone. Exposure-effect data on human beings
under conditions of acute overexposure are scarce and show great
variation. The major effects are gastritis, depression of the
central nervous system with hypothermia and respiratory depression,
and hypotension. The acute mortality data on experimental animals
indicate that the toxicity of 2-propanol is low, the oral LD50
values in various species ranging between 4475 and 7990 mg/kg, and
the inhalation LC50 values for rats being around 50 000 mg/m3. In
rabbits 2-propanol did not irritate the skin, but the application
of 0.1 ml undiluted 2-propanol irritated the eyes.
In man, the most likely acute effects of exposure to high
levels of 2-propanol through ingestion or inhalation are alcoholic
intoxication and narcosis.
No adequate animal studies are available from which an
evaluation can be made of the human health risks associated with
repeated exposure to 2-propanol. However, the results of two
short-term studies on rats, including inhalation exposure (500
mg/m3 for 4 h/day, 5 days per week, for 4 months) and oral exposure
(600 - 3900 mg/kg in the drinking-water), suggest that exposure to
2-propanol at some of the very high occupational exposure levels
reported should be avoided.
Inhalation exposure of pregnant rats to 2-propanol provided a
lowest-observed-effect level (LOEL) of 18 327 mg/m3 (7450 ppm) and
a no-observed-effect level (NOEL) of 9001 mg/m3 (3659 ppm) for
maternal toxicity. In the same study, 9001 mg/m3 (3659 ppm) was a
LOEL for developmental toxicity, with no demonstration of a NOEL.
These concentrations are higher than those likely to be encountered
under conditions of human exposure.
2-Propanol was negative in genotoxicity tests but induced
mitotic aberrations in the bone marrow of rats. Although these
findings suggest that the substance does not have any genotoxic
potential, no adequate assessment of mutagenicity can be made on
the basis of the limited data.
The available data are inadequate to assess the carcinogenicity
of 2-propanol in experimental animals. There are no data to assess
the carcinogenicity of 2-propanol in human beings.
It is unlikely that 2-propanol will pose a serious health risk
for the general population under exposure conditions likely to be
normally encountered.
2-Propanol disappears rapidly (half-time <2.5 days) from the
atmosphere and removal of 2-propanol from water and soil occurs
rapidly by aerobic and anaerobic biodegradation, especially after
adaptation of initially seeded microorganisms. In view of the
physical properties of 2-propanol, its potential for
bioaccumulation is low. It does not present a risk to naturally
occurring organisms at concentrations that usually occur in the
environment.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Chemical formula: C3H8O
Chemical structure: H H H
| | |
H - C - C - C - H
| | |
H OH H
Common name: isopropyl alcohol
Common synonyms: dimethylcarbinol, isopropanol, 2-propanol
(IUPAC and CAS name), propanol-2, propan-2-ol,
sec-propyl-alcohol
Common trade names: Alcojel, Alcosolve 2, Avantin(e), Chromar,
Combi-Schutz, E 501, Hartosol, Imsol A, IPS-1,
Isohol, Lutosol, Perspirit, Petrohol, PRO,
Propol, Spectrar, Takineocol, UN 1219
Abbreviation: IPA
CAS registry number: 67-63-0
Specifications: three commercial grades with different water
contents are available in the USA: 91% and 95%
by volume, and anhydrous 2-propanol (IARC,
1977); the anhydrous grade typically contains
99.5% or more of 2-propanol, and water (0.5%
by weight) and aldehydes and ketones (0.1% by
weight as acetone) as the main impurities [47].
Conversion factors: 1 mg 2-propanol/m3 air = 0.41 ppm at 25 °C and
101.3 kPa (760 mmHg); 1 ppm = 2.46 mg/m3 air.
2.2 Physical and Chemical Properties
2-Propanol is a highly flammable liquid at room temperature and
standard atmospheric pressure. Its odour resembles that of a
mixture of ethanol and acetone, and its taste is slightly bitter.
The compound is completely miscible with water, ethanol, acetone,
chloroform, and benzene. 2-Propanol and water form a constant
boiling mixture that contains 88% by weight (91% by volume) of
2-propanol and boils at 80 - 81 °C. 2-Propanol undergoes all
chemical reactions typical of secondary alcohols. It reacts
violently with strong oxidizing agents. In a fire, it may
decompose to form toxic gases, such as carbon monoxide. Physical
and chemical data on 2-propanol are given in Table 1.
Table 1. Some physical and chemical properties of 2-propanol
-------------------------------------------------------------------
Physical state liquid
Colour colourless
Relative molecular mass 60.09
Odour perception threshold 7.990 mg/m3a
Odour recognition threshold 18.4-120 mg/m3a
Boiling point (°C) 82
Water solubility infinite
log n-octanol/water partition coefficient 0.14b
Specific density (20 °C) 0.785
Relative vapour density 2.07
Vapour pressure (20 °C) 4.4 kPa (33 mmHg)
Flash point (°C) 12 (closed cup)
17 (open cup)c
Flammability limits 2-12% by volume
-------------------------------------------------------------------
a From: May [185], Oelert & Florian [196], and Hellman & Small
[109].
b Experimentally derived by Veith et al. [262].
c From: Kirk & Othmer [137].
2.3 Analytical Methods
A summary of methods for the determination of 2-propanol in
air, water, and biological media is presented in Table 2.
Kring et al. [144] evaluated the US NIOSH charcoal tube
sampling method after having identified several shortcomings in the
NIOSH validation procedure, including dry air dilution and small
sample sizes, because of short sampling periods (15 min). The
overall accuracy for the determination of 2-propanol was 30.4% at
concentrations of 128 and 428 mg/m3, 80% relative humidity, and
sampling periods of 6 h.
The sensitivity of the gas chromatographic determination of
alcohols with electron capture or photoionization detection can be
greatly improved by prior derivatization with pentafluorophenyl-
dimethylsilyl chloride [145].
Ramsey & Flanagan [211] reported a method for the detection and
identification of 2-propanol and other volatile organic compounds
in the headspace of blood, plasma, or serum, using gas chromatography
with flame-ionization and electron-capture detection. The method is
applicable to samples obtained from victims of poisoning, for which a
high sensitivity is not required. After preincubation of the samples
with a proteolytic enzyme, the method can be used for the analysis
of tissues.
Table 2. Sampling, preparation, and determination of 2-propanol
-------------------------------------------------------------------------------------------------------------------------------------------
Medium Sampling method Analytical method Detection limit Sample size Comments Reference
-------------------------------------------------------------------------------------------------------------------------------------------
Air sampling on charcoal, gas chromatography with flame 0.01 mg/sample 0.0002-0.003 m3 suitable for personal and [259]
desorption by carbon ionization detection, packing area monitoring validated
disulfide containing 1% by FFAP on Chromosorb W over the range of
butanol 165-3300 mg/m3
Air sampling on charcoal, gas chromatography with flame 0.25 mg/m3 0.024 m3 suitable for area [152]
desorption by a 1:1 mixture ionization detection, packing monitoring, applicable to
of carbon disulfide and by Oronite NIW on Carbopack B mixtures of both polar and
water non-polar solvents
Air sampling on porous polymer, gas chromatography with mass 0.0012 mg/m3 0.002 m3 suitable for area [123]
based on 2,6-diphenyl- p- spectrometric detection and monitoring, designed for
phenylene oxide, desorption OV-101, SE-30, or SP-1000 the analysis of ambient
by heating capillary columns and indoor air
Air cryogenic sampling on two-dimensional gas 2 x 10-5 mg/m3 0.002-0.003 m3 suitable for area [126]
chromosorb WAW coated with chromatography with monitoring, identification
trifluoropropylmethyl- photoionization and flame of unknown compounds by
silicone, desorption by ionization detection, column mass spectometry; designed
heating 1: 1,2,3-tris(2-cyanoethoxy)- for the analysis of a wide
propane on Chromosorb WAW, range of low-molecular mass
column 2: OV-101 packed compounds; oxygenates in
capillary ambient air
Air direct injection photoionization-ion mobility 0.025 mg/m3 working range, 25-2500 [154]
spectrometry mg/m3
Water direct injection gas chromatography with flame 1 mg/litre 0.001 ml applicable to a mixture [139]
ionization detection, packing of a wide variety of
by porous polymer Tenax GC compounds
Water direct injection gas chromatography with steam 0.04 mg/litre 0.002 ml applicable to a mixture of [256]
as a carrier and flame aliphatic compounds
ionization detection, packing
by Chromosorb PAW modified
with phosphoric acid
-------------------------------------------------------------------------------------------------------------------------------------------
Table 2. (contd.)
-------------------------------------------------------------------------------------------------------------------------------------------
Medium Sampling method Analytical method Detection limit Sample size Comments Reference
-------------------------------------------------------------------------------------------------------------------------------------------
Water extraction by micro steam gas chromatography with flame 0.2 mg/litre 50 ml applicable to the analysis [260]
distillation with ethyl ionization detection, packing of soft drinks
ether by Carbowax 20M; confirmation
by mass spectrometry
Water derivatization by 2-fluoro- paper electrophoresis with 39 mg/litre 0.2 ml applicable to the analysis [20]
1-methyl-pyridinium p- detection by Dragendorff's of mixtures of primary and
toluene, sulfonate in reagent secondary alcohols, such
presence of tridodecylamine as in alcoholic beverages
Blood sampled blood placed in gas chromatography with flame 1 mg/litre 0.0005 ml headspace method [150]
aluminium capsule, capsule ionization, detection packing
heated in GC injector and with Carbowax 20M on
and pierced Chromosorb WAW-DMCS
Serum direct injection of gas chromatography with flame 60 mg/litre 0.2 ml applicable to a mixture of [236]
deproteinized sample with ionization detection, bonded aliphatic alcohols and
n-propanol added as methyl-silicone-coated acetone
internal standard column capillary
Blood, headspace sampling, 1% gas chromatography with flame not reported 0.2 ml applicable to a mixture of [180]
urine dioxane in water added to ionization detection, packing aliphatic alcohols,
samples as internal with Carbowax 20M on acetone, and acetaldehyde
standard Carbopack B
-------------------------------------------------------------------------------------------------------------------------------------------
Gas chromatographic methods, using flame-ionization detection,
are available for the determination of 2-propanol in milk and milk
products [201], in fruits [45], in oilseed meals and flours [84],
in solid fish protein [3, 237], in drugs [115], and in drug raw
materials [183]. The determination of C1 - C4 alcohols in gasoline
can be done by direct injection into a gas chromatograph with an
ion-trap detector [231]. Another direct method for the
determination of C1 - C3 alcohols and water in gasoline is size-
exclusion liquid chromatography and detection by a differential
refractometry [292]. One gas chromatographic method, using thermal
conductivity detection, is described for the determination of
2-propanol in aerosol products [142]. Methods for the
identification of 2-propanol as flavour volatile are also described
[115] (Table 6; section 5.2).
On the basis of the correlation found between alveolar and
blood acetone levels in rats and human beings and 2-propanol
exposure levels (section 6.3), it can be concluded that these
acetone levels can be used for biological monitoring. Acetone
concentrations in the saliva of human beings have also been shown
to be well correlated with 2-propanol exposure levels [250].
Although 2-propanol levels in the breath and saliva are equally
well correlated with environmental 2-propanol concentrations, the
half-life of 2-propanol is much shorter than that of acetone
(section 6.4).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural Occurrence
2-Propanol has been identified as a metabolic product of a
variety of microorganisms and as a flavour volatile in foodstuffs,
primarily plant products (section 5).
3.2 Man-Made Sources
3.2.1 Production levels and processes
3.2.1.1 Production levels
Estimated production capacities for 2-propanol in 1984 in the
USA and western Europe were 1129 and nearly 1000 kilotonnes,
respectively [72, 223], although current production may be lower.
The major producers in western Europe are the Federal Republic of
Germany, France, the Netherlands, and the United Kingdom, with
estimated production capacities of 29, 13, 29, and 24% of the total
western European capacity in 1981, respectively [137]. In the USA,
real production gradually declined from 878 kilotonnes in 1976 to
550 kilotonnes in 1983 [223, 275]. Japan was reported to produce
58 kilotonnes in 1975 [120] and 96 kilotonnes in 1982 [199]. On
the basis of data from Japan, western Europe, and the USA [120,
223], world production in 1975 can be estimated to have exceeded
1100 kilotonnes.
3.2.1.2 Production processes
2-Propanol can be produced from propene by 2 different
processes, i.e., indirect hydration and direct catalytic hydration.
The former process is believed to have been replaced by the direct
hydration process in Japan, the USA, and in western Europe.
2-Propanol is also produced by the catalytic hydrogenation of
acetone [120, 223]. Initially, indirect hydration involved the
feeding of 88 - 93% sulfuric acid and propene gas into a reactor to
produce a mixture of isopropyl and diisopropyl sulfates, which were
hydrolysed with water to 2-propanol. Principal by-products were
diisopropyl ether and isopropyl oils consisting mainly of
polypropylenes of high relative molecular mass [257]. Acetone and
other by-products of low molecular mass, as well as sulfur dioxide
were formed and these gave rise to further condensation products.
This so-called strong-acid process has been causally related with
an excess risk of cancer of the paranasal sinuses [120] (section
9.2.1). It has gradually been replaced by the weak-acid process,
in which propene gas is absorbed in, and reacted with, 60% sulfuric
acid and the resulting sulfates hydrolysed in a single-step
process. 2-Propanol is stripped and refined from the condensate,
which also contains diisopropyl ether, acetone, and polymer oils of
low relative molecular mass [257]. The current major process,
catalytic hydration of propene with water, has three variants:
gas-phase hydration using a fixed-bed supported phosphoric acid
catalyst, a mixed-phase reaction using a cation-exchange resin
catalyst, and a liquid phase reaction in the presence of a
dissolved tungsten catalyst [137]. Catalytic hydration largely
avoids the corrosion and effluent problems associated with the
sulfuric acid processes.
3.2.2 Uses
2-Propanol is mainly used as a solvent, and in pharmaceutical,
household, and personal products [14, 120, 137, 223]. It is a low-
cost solvent with many consumer and industrial applications (Table
3) and it has been estimated that, in 1975, between 35 and 45% of
the total consumption of 2-propanol in Japan, western Europe, and
the USA, was used in this way [120]. Apart from its solvent
properties, 2-propanol also possesses cooling, antipyretic,
rubefacient, cleansing, and antiseptic properties [202].
Table 3. Solvent application of 2-propanola
------------------------------------------------------------------------------------------
Function Application
------------------------------------------------------------------------------------------
1. Process solvent - extraction and purification of natural products, such as vegetable
and animal oils and fats, gums resins, waxes, colours, flavourings,
alkaloids, vitamins, kelp and alginates
- carrier in the manufacture of food products
- purification, crystallization and precipitation of organic
chemicals
2. Coating and dye - in synthetic polymers, such as phenolic varnishes and
solvent nitrocellulose lacquers
- in cements, primers, paints, and inks
3. Cleaning and - in the manufacture of electronic parts, for metals and photographic
drying agent films and papers, in glass cleaners, liquid soaps and detergents,
and in aerosols (see 5)
4. Solvent in - in pharmaceutical products: embrocations, massage solutions, such
topically as rubbing alcohol (70% 2-propanol aerosols (see 5))
applied - in cosmetics: hair tonics, perfumes, skin lotions, hair dye rinses
preparations and permanent wave lotions, skin cleaners and deodorants, nail
polishes, shampoos
5. Aerosol solvent - cleaners, waxes, polishes, paints, de-icers, shoe and sock sprays,
insect repellants, hair sprays, deodorants, air-fresheners
- medical and veterinary products: antiseptics, foot fungicides,
first aid and medical vapour sprays, skin soothers, veterinary pink
eye, wound, and dehorning sprays, house and garden type
insecticides
------------------------------------------------------------------------------------------
a From: Anon. [14], CEC [47], Kirk & Othmer [137], Zakhari [291].
2-Propanol is used in the production of acetone and its
derivatives and in the manufacture of other chemicals, such as
isopropyl acetate, isopropylamine, diisopropyl ether, isopropyl
xanthate, fatty acid esters of 2-propanol, herbicidal esters, and
aluminium isopropoxide [47, 137].
Other uses include the application of 2-propanol as: a
denaturant in industrial solvents, a coolant in beer manufacture, a
coupling agent, a dehydrating agent, a polymerization modifier in
the production of polyvinyl fluoride, a foam inhibitor, a de-icing
agent, a preservative, a heat-exchange medium, and in windscreen
wiper concentrates [47, 137, 291]. It is also used as a flavouring
agent in, for example, tea and beer [47, 242].
3.2.3 Waste disposal
2-Propanol may enter the atmosphere, water, and soil following
waste disposal (section 4.1). At hazardous waste sites and
landfills, 2-propanol was identified in the air and in leachate
(section 5.1). Emissions of 2-propanol via waste gases and waste
water occur in industry and diffuse airborne emissions will occur
during the use of the compound in consumer products (section 4.1).
Air emissions can be controlled by incineration, gas stripping,
or biological oxidation in biofiltration systems [72]. 2-Propanol
can be removed from waste water by biodegradation (section 4.3.1).
Activated carbon adsorption is not feasible as adsorption on this
compound is poor [97]. Removal of the compound from waste water by
reverse osmosis (hyperfiltration) can be successful, depending on
the type of membrane used. Cellulose acetate membranes yielded
40 - 60% separation of 2-propanol, while cross-linked
polyethyleneimine and aromatic polyamine membranes yielded 80 - 90%
separation [76, 80].
Ozonization of 2-propanol appears to be too slow a process to
be of any significance for water treatment [114].
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and Distribution Between Media
In view of the physical properties and the use pattern of
2-propanol, it can be concluded that the main pathway of entry of
this compound into the environment is through its emission into the
atmosphere during production, handling, storage, transport, and
use, and following waste disposal. Second in importance will be
its emission to water and soil. In the USA, it was estimated that
1.5% of the production in 1976 was lost to the environment [74].
Emission registration data from the Netherlands over the years
1974 - 79 indicated that industrial airborne emissions amounted to
3.3% of the 1975 production volume, and emissions into water to
0.2%. From more recent data, a total industrial release into the
environment of approximately 0.6% of the 1985 production capacity
was estimated [72]. However, this figure is hardly significant,
because of the wide use of 2-propanol in a considerable range of
consumer products; disposal of wastes will also account for large
emissions. For example, in the Netherlands, an emission factor for
the domestic use of 2-propanol in aerosol sprays was estimated to
be 430 mg per inhabitant per day [212]. This source alone would
result in an annual emission into the air of 2.1% of the 1975
production volume [72]. In 1976 in the USA, 50% of the 2-propanol
produced was estimated to be released into the atmosphere [74].
Intercompartmental transfer of 2-propanol can occur between
water, soil or waste and air, and between soil or waste and water.
Volatilization of the compound will be considerable in view of its
rather high vapour pressure. Jones & McGugan [125] measured the
evaporation rate of 2-propanol, undiluted or as a 1:1 (v/v) mixture
with water, from a shallow pool or from pulverized domestic waste,
under controlled conditions. The rate of evaporation of undiluted
2-propanol from a pool was 1.1 kg/m2 per h at a wind speed of
0.5 m/second, an ambient air temperature of 12 °C, and a pool
temperature of 13 °C. The evaporation rate of diluted 2-propanol
was 1.5 kg/m2 per hour at a wind speed of 4.5 m/second, a pool
temperature of 20 °C, and an ambient air temperature of 22 °C.
Addition of domestic waste to both the diluted and the undiluted
2-propanol initially increased the evaporation rate, but strongly
attenuated the release of vapour within 2 h.
Transport of 2-propanol from the atmosphere to soil or water
will occur via rain-out, as it is highly soluble in water. Data on
the behaviour of 2-propanol in soil are scarce. With respect to
adsorption, there is one study showing that the compound is poorly
adsorbed on activated carbon [97]. Since 2-propanol is completely
miscible with water, it can be expected to be very mobile in the
soil [72] and it has been shown to increase the permeability of
soil to aromatic hydrocarbons [81].
4.2 Abiotic Degradation
Once in the atmosphere, 2-propanol will be degraded mainly by
hydroxyl radicals. It is not expected to react at appreciable
rates with other reactive species, such as ozone, and hydroperoxy-,
alkyl-, and alkoxy-radicals. Since the compound does not absorb
ultraviolet radiation within the solar spectrum, photolysis is not
expected [46]. Experimentally determined rate constants for the
reaction between 2-propanol and hydroxyl radicals are 0.71 x 10-11
ml/molecule per second at 32 °C [164], and 0.54 x 10-11 ml/molecule
per second at 23 °C [200]. On the basis of these rate constants,
atmospheric residence times of 1.4 and 2.3 days, respectively, can
be calculated [61]. These short lifetimes will prevent migration
of the chemical into the stratosphere.
The initial reaction product of 2-propanol with a hydroxy
radical is an alpha-hydroxy-2-propyl radical. By analogy with the
irradiation of 2-butanol in an NOx--air atmosphere, these radicals
are expected to react with oxygen almost exclusively with hydrogen
abstraction from the hydroxyl-group to produce acetone, or with
loss of methyl to give acetaldehyde. Follow-up reactions will
produce small quantities of peroxyacetyl nitrate, formaldehyde,
methyl nitrate, and formic acid [46].
Hydrolysis or light-induced degradation of 2-propanol in water
cannot be expected. No data are available on abiotic degradation in
soil.
4.3 Biotransformation
4.3.1 Biodegradation
The results of the determination of the biological oxygen
demand (BOD) by dilution methods at 20 °C are summarized in Table
4. Unless otherwise stated, the results are expressed as a
percentage of the theoretical oxygen demand (ThOD), which is 2.40 g
oxygen/g 2-propanol. The chemical oxygen demand (COD) was reported
to be 96 and 93% of the ThOD by Price et al. [208] and Bridie et
al. [33], respectively.
Adaptation of the seed material to the chemical enhances the
rate of biodegradation considerably. Gerhold & Malaney [95] added
2-propanol to undiluted activated sludge and found an oxygen uptake
of 10% of the ThOD in 24 h. Mack [175] measured total degradation
of 2-propanol within 96 h and total degradation of the initial
product acetone within 120 - 144 h following incubation in a
standard medium that had been inoculated by effluent from a water
purification plant. In another study, 2-propanol (50 mg/litre) was
added as the sole source of carbon to a mineral medium in a
continuous flow reactor, seeded by a culture isolated from
activated sludge using methanol, phenol, acetone, and 2-propanol as
substrates. Growth was extremely poor. However, when acetone (100
mg/litre) was added as well, almost 100% degradation of the 2
chemicals was achieved within a minimum of 2.9 h. The authors
concluded that, in this case, the oxidation of 2-propanol was
dependent on the simultaneous oxidation of acetone. When 2-propanol
was added to the same medium, seeded by a culture isolated using
2-propanol as the sole substrate, almost 100% degradation was
achieved within a minimum of 4.3 h [281].
Table 4. BOD and COD of 2-propanol
--------------------------------------------------------------------
Dilution Source or seed Adaptation BODxa Value Reference
water (+/-) (% of
ThOD)
--------------------------------------------------------------------
Fresh municipal waste water - BOD5 7 [289]
- BOD20 70
domestic waste water - BOD5 28 [208]
- BOD20 78
domestic waste water - BOD5 66 [268]
domestic waste water - BOD5 74 [269]
activated sludge + BOD5 99b [203]
effluent from a + BOD5 72 [33]
biological waste - BOD5 49
treatment plant
Salt domestic waste water - BOD5 13 [208]
- BOD20 72
--------------------------------------------------------------------
a BODx = biological oxygen demand after x days of incubation.
b Expressed as percentage of the COD.
In a water treatment facility of a plant manufacturing organic
chemicals, a typical removal efficiency for 2-propanol was 76%,
using an aerated, non-flocculent, biological stabilization process
[25]. After conversion to an activated sludge facility, the
removal efficiency increased to 96% [147].
There are two reports on anaerobic biodegradation. Typical
2-propanol removal efficiencies for an anaerobic lagoon treatment
facility, with a retention time of 15 days, were 50% after loading
with dilute waste, and 69 and 74% after loading with concentrated
wastes [118]. In closed bottle studies, 2-propanol was completely
degraded anaerobically by an acetate-enriched culture, derived from
a seed of domestic sludge. The culture started to use cross-fed
2-propanol, after 4 days, at a rate of 200 mg/litre per day. In a
mixed reactor with a 20-day retention time, seeded by the same
culture, 56% removal was achieved in the 20 days following 70 days
of acclimation to a final 2-propanol concentration of 10 000
mg/litre [55].
4.3.2 Bioaccumulation
2-Propanol is completely miscible with water. Its log
n-octanol/water partition coefficient is 0.14 [263]. A
bioconcentration factor of 0.5 can be calculated using the formula
of Veith & Kosian [262]. In addition, the compound is
biodegradable. In view of these data, no bioaccumulation is
expected.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental Levels
The rapid removal of 2-propanol from air and water is reflected
in the few reports indicating its presence in these compartments
(sections 4.2 and 4.3). No data are available on the occurrence of
the compound in soil.
2-Propanol was detected, at a level of 95 mg/m3 air, at the
outlet of the main chimney of a paint-manufacturing plant in France
in 1980. The compound was not detected at a distance of 10 - 30 m
from this chimney [51]. In 1970, Gorlova [98] reported that
average atmospheric levels of 2-propanol at distances of 500 and
5000 m from a plant producing 2-propanol by indirect hydration,
were 1.7 and 0.2 mg/m3 air, with maxima of 3 and 0.5 mg/m3 air,
respectively. In 260 1-h samples of air from 4 sites in Stockholm,
Sweden, in 1983, 2-propanol concentrations ranging between 0.61 and
108 mg/m3 were measured with averages of between 1.52 and 35.2
mg/m3. In 56 air samples from a monitoring site near dense traffic
12 km outside central Stockholm, concentrations of between 0.12 and
2.93 mg/m3 were measured, the average being 0.74 mg/m3. The
2-propanol levels were not correlated with typical vehicle exhaust
compound levels. However, a correlation was found between
2-propanol levels and the use of anti-freezing agents for
windscreen washers at a similar site [126].
2-Propanol was detected in the air beneath the surface of 2 out
of 6 landfill sites sampled in the United Kingdom. At these 2
sites, used for the disposal of domestic waste, the 2-propanol
concentrations were 17 mg/m3 and more than 46 mg/m3, respectively
[288]. Analysis of a total of 82 air samples at 5 hazardous waste
sites in New Jersey, USA, revealed the presence of 2-propanol at 4
sites [151]. Leaching from landfills may result in ground water
pollution. In 1982-83, 2-propanol concentrations of up to 8.8
mg/litre water were measured in 6 out of 7 samples of leachate,
obtained from test wells in 1 of 5 landfills sampled in the United
Kingdom [225].
2-Propanol has been identified as a metabolic product in
microorganisms, as shown in Table 5.
Table 5. 2-Propanol production by microorganisms
--------------------------------------------------------------------------------------
Type Species Reference
--------------------------------------------------------------------------------------
Aerobic fish spoilage bacteria Pseudomonas spp., Moraxella-like, [7]
Flavobacterium, Micrococcus, Coryneforms,
Vibrio
Aerobic beef spoilage bacteria Pseudomonas spp. [63]
Aerobic potato tuber soft rot Erwinia carotovora [273]
bacteria
--------------------------------------------------------------------------------------
Table 5. (contd.)
--------------------------------------------------------------------------------------
Type Species Reference
--------------------------------------------------------------------------------------
Anaerobic bacteria Clostridium beijerinckii [92]
Clostridium aurantibutyricum
Anaerobic methylotrophic, [181]
propane fed bacteria
Fungi, mushroom Leucocoprinus elaedis [93]
Yeast Kluyveromyces lactis [107]
--------------------------------------------------------------------------------------
5.2 General Population Exposure
5.2.1 Exposure via food
When 2-propanol is used as an extraction or carrier solvent for
food constituents, the compound may be found in the final product.
It was detected in 8 out of 17 samples of lemonade, prepared with
natural essence extracted with 2-propanol. Concentrations of
between 0.2 and 82 mg/litre were measured in 7 samples and 325
mg/litre in one sample [260]. The compound was also identified in
fish protein concentrate, extracted by 2-propanol [237]. The
Scientific Committee for Food of the Commission of the European
Communities has published industry-derived residue levels in
foodstuffs following the use of 2-propanol as an extraction and/or
carrier solvent. Typical levels are: 250 mg/kg of dry fish
protein concentrate, 50 mg/kg of meat product as consumed, 750
mg/kg of meat after use of 2-propanol as a smoke flavour carrier
and before drying, 1200 mg/kg of jam, and 3000 mg/kg of jelly [47].
Studies showing the presence of 2-propanol as a flavour
volatile in a variety of foodstuffs are summarized in Table 6.
Table 6. 2-Propanol as a flavour volatile in foodstuffs
-------------------------------------------------------------------
Foodstuff Reference
Common name Scientific name
-------------------------------------------------------------------
Reunion geranium oil Pelargonium roseum Bourbon [249]
Rooibos tea Asphalathus linearis [104]
Winged bean (raw/roasted) Psophocarpus tetragonalobus [70]
Soybean (raw/roasted) Glycine max
Virginia peanut (raw) [166]
Peanut (raw/roasted) Arachis hypogaea [156]
Filbert (roasted) Corylus avellana [136]
-------------------------------------------------------------------
Table 6. (contd.)
-------------------------------------------------------------------
Foodstuff Reference
Common name Scientific name
-------------------------------------------------------------------
Babaco fruit Carica pentagona [229]
Apple Malus [242]
Tomato Lycopersicum
Endive Cichorium endivia [99]
Lime essence Citrus arantifolia [189]
Grapefruit essence Citrus paradisi [58]
Grapefruit aroma oil
Mushroom (fresh/edible) Leucocoprinus elaedis [93]
Kefir culture [201]
Yoghurt culture
Swiss Gruyere cheese [31]
Feta cheese [116]
-------------------------------------------------------------------
Microbial metabolism may be responsible for the presence of
2-propanol in certain foodstuffs, such as cheese, as suggested by
Bosset & Liardon [31]. Approximately 25% of the 2-propanol found
in beer is added as a flavouring agent during its manufacture [242].
5.2.2 Exposure via other consumer products
The general population is potentially exposed to a wide variety
of consumer products containing 2-propanol (section 3.2.2). In a
1980 survey in Japan, commercial heterogeneous solvent products
were collected throughout the country. Of 102 products, 12 out of
59 samples of paint, 6 out of 18 samples of ink, 1 out of 12
samples of adhesives, and 3 out of 13 samples of other products
contained 2-propanol [146]. Not surprisingly, 2-propanol was one
of over 250 compounds found in the indoor air of homes in 2 urban
areas of the USA, where the levels were all less than 0.25 mg/m3
[123].
Intentional or accidental poisoning by 2-propanol in consumer
products has been reported frequently. Several cases will be
discussed in section 9.1. The subject of non-beverage alcohol use
has been reviewed recently by Egbert et al. [77], who reported that
10 - 15% of a specified group of alcoholics in the USA (admitted to
a Veteran Administration detoxication unit) were found to have
consumed non-beverage alcohols. Addiction to a disinfectant
containing 2-propanol was reported in one case [133]. In Canada,
the incidence of cases of exposure to 2-propanol in rubbing alcohol
reported to the Poison Control Centres increased from 254 in 1973
to 338 in 1976 [151]. Rubbing alcohol is also the single most
frequent source of 2-propanol intoxication in small children. This
can occur from accidental ingestion, or via inhalation following
sponging for fever reduction [163].
5.3 Occupational Exposure
Workers are potentially exposed to 2-propanol during production
of the compound itself or of acetone and other derivatives, or
during its use in solvent type applications. In the USA, NIOSH
estimated, on the basis of the 1980 - 83 National Occupational
Exposure Survey, that over 1.8 million workers, of whom over 1.1
million were females, were potentially exposed to this compound
[259]. Inhalation exposure of workers in various industries where
2-propanol or 2-propanol-containing products are used, is
summarized in Table 7. In most cases, these workers were also
exposed to other chemicals. No data are available on the exposure
of workers in 2-propanol- or acetone-producing industries, except
for the report of Guseinov [103] pertaining to a plant in the USSR
producing 2-propanol by indirect hydration.
Table 7. Occupational inhalation exposure to 2-propanol
------------------------------------------------------------------------------------------
Job description Country Sampling Concentration (mg/m3) Reference
(number of workers)
------------------------------------------------------------------------------------------
Car painting (40) Finland personal 7.1 (average) [148]
209 (maximum)
Printing (12) Italy area 8-647 [41]
15-493 (time-weighted average)
Ink production (41) Italy area 6.3-32.8 [42]
Paint manufacture (3) Sweden personal 6-258 (time-weighted average) [168]
129 (time-weighted average-average)
Work in hospital United area 8.8 (average) [106]
operating theatre Kingdom 30 (maximum)
Tractor painting (28) USA personal NDa-697 [102]
area 33-332
Higher aromatic booth USA personal 4.7 (time-weighted average-average) [279]
spray painting (14) 32 (time-weighted average-maximum)
54 (maximum)
Lower aromatic booth USA personal 10.6 (time-weighted average-average) [279]
spray painting (16) 125 (time-weighted average-maximum)
605 (maximum)
Solvent wiping (11) USA personal 2.5 (time-weighted average-average) [279]
13 (maximum)
------------------------------------------------------------------------------------------
Table 7. (contd.)
------------------------------------------------------------------------------------------
Job description Country Sampling Concentration (mg/m3) Reference
(number of workers)
------------------------------------------------------------------------------------------
Paint mixing (3) USA personal 4.2 (time-weighted average-average) [276]
10 (maximum)
Spraying paint, USA personal <2.5 (time-weighted average-average) [177]
lacquer
Printing (26) USA personal 396 (time-weighted average-average) [177]
Printing (2) USA personal 33-67 [140]
Printing (7) USA personal 85-293 [101]
area 236
Printing (4) USA personal 0.5-3.7 [152]
area 2.2-16.5
Printing (8) USA personal NDa-519 [282]
area 15-307
Printed circuit USA personal 5.8-23 [280]
boards manufacture
(5)
Furniture stripping USA personal 42-160 [179]
(7)
Degreasing metal (14) USA personal 2.2-10.6 [271]
Manufacture of rubber USA personal NDa-34 [278]
weather strips (67) area 6.5-140
Chemicals packaging USA area 150-1350 [83]
2-Propanol production USSR area 92 (average)b [103]
area 165 (average)c
Chloramphenicol USSR area 10-36 (average)d [173]
production NDa-120
Sulfite additive USSR area 7.1-14.6 (average) [17]
production
------------------------------------------------------------------------------------------
a ND = not detected.
b Wintertime.
c Summertime.
d Average of positive samples.
6. KINETICS AND METABOLISM
6.1 Absorption
6.1.1 Animals
Exposure of dogs, rabbits, and rats via various routes resulted
in detectable levels of 2-propanol in the blood within 0.5 h of the
start of the exposure [1, 121, 150, 151, 158, 181, 195].
Maximum blood concentrations of 2-propanol of up to 2950
µg/litre were attained in dogs within 0.5 - 2 h following single
oral doses of the compound in water of up to 2940 mg/kg body
weight. The blood concentration was directly related to the dose
level [158]. Following single oral dosing of rats with 2-propanol
in water at 2000 mg/kg body weight [121] or 6000 mg/kg body weight
[195], peak blood concentrations were 1080 µg/litre after 1 h and
4800 - 6000 µg/litre after 8 h, respectively. Apparently,
gastrointestinal absorption is delayed at high doses.
Blood levels of 2-propanol were determined in groups of 3 adult
(200 - 300 g) female Sprague-Dawley rats following 1, 10, or 19
consecutive 7-h daily exposures to measured concentrations of 7636,
18 327, or 23 210 mg/m3 (3104, 7450, or 9435 ppm). Immature
(approximately 90 g) females of the same strain were also evaluated
following a single 7-h exposure to 23 210 mg/m3 (9435 ppm). In the
immature females, the blood level of 2-propanol averaged 9600 µg/
litre. The blood levels in adult rats following a single exposure
were not detectable at 7636 mg/m3 (3104 ppm), 6800 µg/litre at
18 327 mg/m3 (7450 ppm), and 7900 µg/litre at 23 210 mg/m3 (9435
ppm). Following 10 and 19 consecutive daily exposures, blood
levels in adult rats were consistently not detected at 7636 mg/m3
(3104 ppm); 5800 and 5700 µg/litre at 18 327 mg/m3 (7450 ppm), and
7000 and 6400 µg/litre at 23 210 (9435 ppm) [193], respectively.
The Task Group noted that these blood 2-propanol levels appeared to
be exceptionally high.
When rats inhaled 2-propanol at concentrations of between 1230
and 19 680 mg/m3 for 4 or 8 h, maximum blood concentrations
attained at the end of the exposure period at the highest exposure
level were 235 and 760 µg/litre, respectively [151].
Wax et al. [274] injected equal volumes of 2-propanol in saline
into each of several ligated loops of the intestines and into the
stomach loop of anaesthetized dogs. The concentration of the
solutions varied, the total dose always being 980 mg/kg body
weight. Absorption from the intestines was 67 - 91% of the dose
within 30 min and 99% within 2 h. Absorption from the stomach loop
was 41% within 30 min.
Rats exposed intraperitoneally to 1000 mg 2-propanol/kg body
weight in saline showed peak blood concentrations of 1020 - 1300
µg/litre within 1 h [1, 195].
Groups of 3 rabbits received 2-propanol in water orally at 2 or
4 mg/kg body weight or were exposed to 2-propanol in towels, one
applied to the chest and others on the floor of the inhalation
chamber, with or without a plastic layer to prevent skin contact.
The highest blood levels of 2-propanol were produced by the oral
exposures, followed by the combined dermal and inhalation exposure
[181].
6.1.2 Human beings
Ten human volunteers drank orange juice containing doses of
3.75 mg 2-propanol/kg and 1200 mg ethanol/kg body weight over a
period of 2 h. At the end of this period, the average peak blood
concentration of 2-propanol was 0.83 ± 0.34 (mean ± standard
deviation) mg/litre. When the blood was analysed after incubation
with aryl sulfatase (EC 3.1.6.1), an average peak concentration of
2.27 ± 1.43 mg/litre was measured 1 h after exposure [27]. These
data provide limited evidence for the sulfation of 2-propanol.
Brugnone et al. [41] analysed the alveolar air, blood, and
urine of 12 printing workers, exposed to 2-propanol at
concentrations of between 8 and 647 mg/m3 air. The alveolar
2-propanol concentration was highly correlated with the exposure
level at any time of exposure, the ratio of the two concentrations
being 0.418. The alveolar uptake (0.03 - 6.6 mg/min) showed a
linear increase with exposure levels.
6.2 Distribution
6.2.1 Animals
2-Propanol, a compound with infinite water solubility, is
rapidly distributed throughout the body [1].
Wax et al. [274] recovered 2-propanol from the blood, spinal
fluid, liver, kidneys, and brain of dogs, 30 min after exposure via
injection into ligated loops of the gastrointestinal tract. Three
hours after a single oral exposure of rats, the compound was found
in the blood, liver, kidneys, and brain [121]. No other tissues
were analysed in either study.
The permeability of the blood-brain barrier for 2-propanol
was investigated in monkeys and rabbits. Anaesthetized monkeys
received a single injection of 0.2 ml of an 11C-labelled alcohol in
the carotid artery, followed by an injection of 15O-labelled water.
At a cerebral blood flow of 50 ml/100 g per min, about 99% of
2-propanol, 97% of ethanol, and 93% of labelled water exchanged
freely with the brain during a single capillary transit. On the
basis of these data, the blood-brain barrier permeabilities for
these three compounds were estimated to be 5, 2.5, and 1.8 x 10-4
cm/second, respectively [209].
6.2.2 Human beings
Following ingestion of an unknown amount of rubbing alcohol,
2-propanol as well as its metabolite acetone were found in the
spinal fluid of 2 persons at levels similar to those in the serum
[5, 191].
6.3 Metabolism
6.3.1 Animals
The metabolism and elimination of 2-propanol in mammals is
summarized in Fig. 1. It has been well established that 2-propanol
is metabolized to acetone in the rat, dog, and rabbit [1, 121, 150,
151, 195, 224, 232]. Oral (0.2, 1.0 ml) and inhalation exposure
(1230 - 19 680 mg/m3, for 4 h) (500 - 8000 ppm) of rats produced
dose-related increases in blood levels of 2-propanol and its
metabolite acetone. Following inhalation, the acetone/2-propanol
ratio in blood decreased with increasing 2-propanol concentrations
indicating saturation of the oxidative metabolic pathway above
concentrations of approximately 9840 mg/m3 (4000 ppm) [150, 151].
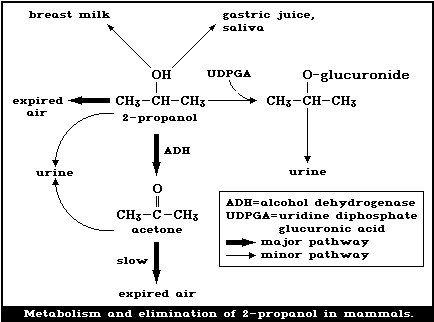 Since adequate balance studies have not been conducted, the
extent of this metabolism to acetone is not known. Siebert et al.
[232] injected 750 or 1300 mg/kg body weight intravenously in
rabbits and estimated that 64 - 84% of the dose was oxidized to
acetone, confirming the earlier findings of Pohl [204].
Evidence of another metabolic pathway was found in rabbits
given an oral dose of 2-propanol of 3000 mg/kg body weight, 10.2%
of the dose was found in the urine as beta-isopropyl-glucuronide
[129].
Sufficient evidence is available to show that 2-propanol is
oxidized to acetone mainly by the non-specific cytosolic enzyme
alcohol dehydrogenase (ADH) (EC 1.1.1.1). When either pyrazole or
4-methylpyrazole (inhibitors of ADH) was administered to rats prior
to exposure to 2-propanol, both the elimination of 2-propanol and
the production of acetone were retarded [121, 193]. The
elimination of 2-propanol by rats was retarded when it was given in
combination with ethanol; both compounds are substrates for ADH [2,
121]. Enzyme kinetic data show that 2-propanol is a poorer
substrate of ADH than ethanol. In vitro, the Michaelis-Menten
constant (Km) for horse liver ADH was 0.0126 mol/litre, using
2-propanol as a substrate, and 0.00018 mol/litre, using ethanol as
a substrate [64]. For rat liver ADH in vivo, Km was 0.034
mol/litre, using 2-propanol as a substrate, and 0.00192 mol/litre,
using ethanol as a substrate. The maximum initial velocity (Vmax)
of rat liver ADH and the substrate 2-propanol in vivo was 1.7 times
less than that with the substrate ethanol. It was further shown
that the ratio of the relative rates of oxidation of 2-propanol and
deuterated 2-propanol-d7 were about the same in vitro and in vivo.
From this, it can be concluded that ADH activity is the rate-
limiting factor in 2-propanol metabolism [54].
It has been shown that rat liver microsomal oxidases are also
capable of oxidizing 2-propanol, but that the compound is not an
effective substrate for the peroxidative activity of catalase
(EC 1.11.1.6) [48, 193].
6.3.2 Human beings
Acetone has also been found in the blood of human beings after
exposure to 2-propanol [e.g., 11, 41, 65, 131]. Furthermore,
acetone was detected in the spinal fluid, at levels similar to
those in serum, in 2 persons after ingestion of 2-propanol [5, 191].
In the investigations of Brugnone et al. [41] (section 6.1.2),
elevated acetone levels were measured in the blood, alveolar air,
and urine of 12 printing workers. 2-Propanol was not detected in
the blood or urine. The concentrations of acetone ranged between
0.76 and 15.6 mg/litre in the blood and between 3 and 93 mg/m3 in
the alveolar air. The acetone levels in alveolar air and blood
increased with the increasing exposure period and were linearly
related to the alveolar 2-propanol levels. Urinary acetone levels
ranged from 0.85 to 53.7 mg/litre in overnight pooled urine samples
and were highly correlated with the alveolar 2-propanol uptake and
with blood acetone levels. Pulmonary and renal clearance of
acetone were 41 - 97 and 0.1 - 3 ml/min, respectively, for 8 of the
workers, showing that acetone is mainly excreted via the lungs.
Pulmonary acetone excretion varied from 10.7 to 39.8% of the
uptake and was inversely related to the exposure level [41]. It
is known that the metabolic capacity of the human liver for acetone
is limited [161].
2-Propanol and acetone have also been found in the returns of
gastric lavage [5] and in saliva [250]. Reabsorption may follow
excretion via these routes.
When 10 volunteers drank orange juice containing doses that
gave 3.75 mg 2-propanol and 1200 mg ethanol/kg body weight over
2 h, 2-propanol was detected in the blood, partly as sulfate
(section 6.1.2), and in the urine, partly as glucuronide. The
total urinary excretion of 2-propanol was 1.9% of the dose [27, 28].
Human data support the importance of the ADH pathway for the
oxidation of 2-propanol as observed in experimental animals. In
the studies of Bonte et al. [27, 28] described above, 2-propanol
was only detected in the blood when ethanol was ingested
simultaneously, indicating a retarded elimination. Among 74
persons intoxicated by 2-propanol, significantly lower acetone
values were found in the blood of those who had also been exposed
to ethanol [11, 131]. In in vitro studies, the activity of human
ADH with 2-propanol was 9 - 10% of the activity of the enzyme with
ethanol as substrate [121].
Endogenous formation of 2-propanol has been revealed at
autopsies of individuals not previously exposed to this compound.
This observation and the results of additional studies on rats show
that 2-propanol can result from the reduction of acetone by liver
ADH, especially when high levels of acetone and high NADH/NAD+
ratios occur. Such conditions are found in diabetes mellitus,
starvation, high fat feeding, chronic alcoholism, and dehydration
[68, 161, 248].
6.4 Elimination and Excretion
6.4.1 Animals
In a review article, Rietbrock & Abshagen [218] concluded that
urinary excretion of both 2-propanol and its metabolite acetone is
limited and in each case does not exceed 4% of the dose in the rat,
rabbit, and dog. The major route of excretion is via the lungs.
2-Propanol is excreted via the gastric juice and saliva in the dog
[158] and through breast milk in the rat, as shown by tissue levels
in the newborn [159].
The disappearance of 2-propanol from the blood of experimental
animals was found to be a first-order process at doses <1500 mg/kg
[1, 232]. In rats, the half-life of 2-propanol was 1.5 h at an
intraperitoneal dose of 500 mg/kg body weight and increased to
2.5 h at a dose of 1500 mg/kg body weight. This can be explained
by a limited metabolic capacity as suggested in section 6.3.1
[218]. Simultaneous administration of ethanol to rats increased
the half-life of 2-propanol in the blood approximately 5-fold
following acute exposure [2]. A half-life of 4 h was determined in
dogs administered 1000 mg 2-propanol/kg body weight intravenously [1].
The biotransformation of 2-propanol and the elimination of the
acetone produced are both slow processes. Peak acetone levels in
the blood of various species, following single exposures via
different routes, were only reached several hours after exposure,
though acetone was already detectable shortly after the start of
exposure [1, 121, 150, 151, 193, 232]. The elimination of acetone
in the dog and the rat was found to be a first-order process with
half-lives of 11 and 5 h, respectively [1].
After prolonged administration of 2-propanol to dogs [159] and
rats [224], the elimination rate of 2-propanol was increased.
Simultaneous exposure of rats to ethanol in the drinking-water (5%)
and atmospheric 2-propanol at 738 mg/m3 (300 ppm) for 5 - 21 weeks
significantly increased the rates of elimination of 2-propanol and
acetone [224].
6.4.2 Human beings
The elimination of 2-propanol in human beings also appears to
follow first-order kinetics. In two alcoholics who had ingested
rubbing alcohol, 2-propanol was eliminated from the blood with
half-lives of 2.5 and 3 h, respectively. Acetone levels declined
slowly over the next 30 h; no ethanol was detected [65]. A half-
life of 2-propanol of 6.4 h was determined in a non-alcoholic, who
also had ingested rubbing alcohol. The acetone level in the blood
reached a maximum 30 h after admission to hospital. The half-life
of acetone was 22 h. Ethanol was also found in the blood. The
differences in the half-lives in these three cases may reflect
metabolic adaptation in the alcoholics, or genetic variations in
ADH [191].
When 4 volunteers ingested an artificial liquor containing 40%
2-propanol, acetone was detectable in exhaled air from 15 min after
exposure and in the urine from 1 h after exposure [132].
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Aquatic Organisms
A summary of acute aquatic toxicity data is presented in Table 8.
The concentration of 2-propanol was reported to have been measured
in only 3 of the studies [34, 111, 263] and in no case were
potential metabolites considered. In view of the volatility of the
compound, it can be expected that the toxic effects observed in the
other open systems studied occurred at lower concentrations than
the nominal ones.
Several short- and long-term studies have also been conducted.
Seiler et al. [227] determined the breakpoint of bioinhibition for
a total of 20 strains of bacterial groups prevalent in a waste-
water treatment plant in the chemical industry, i.e., Zoogloea,
Alcaligenes, and Pseudomonas. After one week of static exposure to
2-propanol in an open system at 30 °C, 100% growth inhibition
occurred at concentrations of 80 000 - 100 000 mg/litre of medium.
No analysis for the compound was reported. The cell multiplication
of blue algae (Microcystis aeruginosa) and green algae (Scenedesmus
quadricauda) was just inhibited after 8 days of static exposure to
1000 and 1800 mg/litre water, respectively, in a closed system at
27 °C and a pH of 7 [35, 37]. When water fleas (Daphnia magna)
were exposed to 2-propanol for 16 days in a semi-static test at
19 °C and a water hardness of 100 mg CaCO3/litre, the highest
concentration that did not result in a significant reduction in
growth was 141 mg/litre water. The water was analysed for the
compound just before and after each renewal of test solution [111].
A 7-day LC50 of 7060 mg 2-propanol/litre water was determined for
2 - 3-month-old guppies (Poecilia reticulata) in a semi-static
test. No analysis for 2-propanol was reported for this open system
[141].
7.2 Terrestrial Organisms
7.2.1 Microorganisms
The sensitivity of 4 soil fungi, i.e., Chrysosporium
crassitunicatum, Nannizzia fulva(+), Nannizzia fulva(-), and
Trichophyton equinum, to saturated 2-propanol vapour was
determined at 28 °C and a pH of 7. Mycelial growth of
Chrysosporium was stimulated after 14 days of exposure, while that
of the other strains was inhibited. Sporulation was poor in
Chrysosporium and in Trichophyton, and fair or good in the other
strains [233].
Table 8. Acute aquatic toxicity of 2-propanol
---------------------------------------------------------------------------------------------------------------------------------------
Organism Description Temperature pH Dissolved Hardness Stat/flow Exposure Parameter Nominal Reference
(°C) oxygen (mg CaCO3/ open/closeda period concentration
(mg/litre) litre) (mg/litre)
---------------------------------------------------------------------------------------------------------------------------------------
FRESHWATER
Bacteria Pseudomonas 25 7 stat, closed 16 h TTb 1 050 [37]
putida
Micro- activated 21 7.4-8 stat, closed 3 h 50% inhibition 1 000 [138]
organisms sludge of respiration
rate
Protozoa Entosiphon 25 6.9 stat, closed 72 h TTb 4 930 [36]
sulcatum
Protozoa Chilomonas 20 6.9 stat, closed 48 h TTb 104 [40]
paramecium
Protozoa Uronema 25 6.9 stat, closed 20 h TTb 3 425 [38]
parduczi
Crustacea water flea 20 8 2 250 stat, open 24 h EC50c 9 714 [39]
(Daphnia EC0 5 102
magna) EC100 10 000
Crustacea water flea 22 100 stat 44 h EC50c,d 2 285 [110]
(Daphnia
magna)
Amphibia frog tadpole 20 stat threshold 22 530 [190]
(Rana pipiens) narcotic
concentration
Fish fathead minnow 18-22 4 stat, open 96 h LC50 11 130 [184]
(Pimephales 24 h LC50 11 160
promelas)
Fish fathead minnow 25 7.5 5 45 flow, open 96 h LC50 9 640-10 400f [262]
(Pimephales
promelas)
---------------------------------------------------------------------------------------------------------------------------------------
Table 8. (contd.)
---------------------------------------------------------------------------------------------------------------------------------------
Organism Description Temperature pH Dissolved Hardness Stat/flow Exposure Parameter Nominal Reference
(°C) oxygen (mg CaCO3/ open/closeda period concentration
(mg/litre) litre) (mg/litre)
---------------------------------------------------------------------------------------------------------------------------------------
Fish goldfish 20 6-8 4 280 stat, open 24 h LC50 5 000f [34]
(Carassius
auratus)
Fish golden orfe 20 7-8 5 200-300 stat 48 h LC50 8 970 [127]
(Leuciscus
idus melanotus)
Fish harlequin fish 20 8.1 20 flow, open 96 h LC50 4 200 [251]
(Rasbora 96 h LC10 1 500
heteromorpha
duncker)
Fish Creek chub 15-21 8.3 98 stat, open 24 h LC0 900 [96]
(Semotitus
atromaculatus)
SEA WATER
Bacteria Photobacterium 15 7 stat, closed 2 min TTh 15 000 [43]
phosphoreum 15 stat, closed 5 min 50% light 35 000 [44, 62]
reduction 42 000
Worm Tubifex 20 stat 2 min EC100c 51 080 [56]
tubifex
Crustacea Brine shrimp 24 stat, open 24 h LC50 10 000 [208]
(Artemia
salina)
Crustacea Brown shrimp semi-stat, 96 h LC50 1 150 [26]
(Crangon open
crangon)
---------------------------------------------------------------------------------------------------------------------------------------
a Static or flow-through test; open or closed system. e Concentration at which touching the tadpoles failed to cause
b TT = toxic threshold for inhibition of cell multiplication. motion; exposure period and conditions; and the method of
c Effect is complete immobilization. calculation of the threshold not specified.
d Calculated value based on a quantitative structure-activity f Analysis for 2-propanol reported.
relationship between the n-octanol/water partition coefficient of g Test compound was Imsol A (90% 2-propanol, remainder unknown)
a group of 19 organic chemicals and their anaesthetic potency. h TT = toxic threshold for light reduction.
7.2.2 Insects
The 4-h LC50 for third instar mosquito larvae (Aedes aegypti)
was 25 120 mg/litre water in a static test at 22 - 24 °C [143].
The 48-h LC50 values for the fruit fly strains Drosophila
melanogaster and Drosophila simulans were between 10 200 and 13 340
mg/litre of nutrient medium in static tests [66]. Exposure of
Drosophila melanogaster eggs and larvae to 7850 mg 2-propanol/litre
of nutrient medium caused an 87% decrease in the activity of the
non-specific enzyme alcohol dehydrogenase (EC 1.1.1.1) in the 14-
day-old larvae. At the same time, viability was decreased by 74%.
The development of Drosophila simulans was completely inhibited at
the same concentration. Gas chromatographic analysis revealed
that, after 4 days of exposure, acetone appeared as the 2-propanol
concentration decreased. The appearance of acetone was associated
with an observed decrease in enzyme activity [265]. Anderson &
McDonald [13] also found a decrease in the specific activity of
alcohol dehydrogenase in Drosophila melanogaster after 1 - 4 days
of exposure to 2-propanol. On the other hand, they found that the
stability and concentration of the enzyme increased. The authors
explained the findings as an adaptation of the fruit fly to its
environment.
7.2.3 Plants
The effects of 2-propanol on the rate of seed germination were
investigated on several occasions. Total inhibition of the
germination of barley grains was reached after incubation for 4
days at 18 °C on filter papers, absorbing a solution containing
39 420 mg 2-propanol/litre water [56]. The germination of white
amaranth (Amaranthus albus) seeds was not affected after 5 h of
incubation at 25 °C on filter papers moistened with a solution
containing 36 050 mg 2-propanol/litre of water [49]. Reynolds
[216] measured 50% inhibition of germination in lettuce (Lactuca
sativa) seeds after incubation for 3 days at 30 °C on agar
containing 2100 mg 2-propanol/litre. At 6000 mg/litre, no
germination at all was observed. However, above 18 030 mg/litre,
germination was again observed and reached a maximum of 62% at
26 440 mg/litre. Inhibition of the growth of hypocotyls and
rootlets after 6 days of incubation gradually increased with
concentration. ED50 values were above 36 000 mg/litre.
In a 28-day study on cell suspensions of root sections of
soybean, (Glycine max), at 26 °C and a pH of 5.6, onset of growth
was delayed for 1 and 2 weeks at 2-propanol concentrations of
10 000 and 20 000 mg/litre of nutrient medium, respectively [67].
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single Exposures
8.1.1 Mortality
Available acute mortality data are summarized in Table 9.
Except where otherwise indicated, the vehicle was water or saline
solution. LD50 values for several animal species after oral
administration vary between 4475 and 7990 mg/kg body weight. Using
14-day-old rats of both sexes, Kimura et al. [134] established an
oral LD50 of 4396 mg/kg body weight for undiluted 2-propanol; this
was not significantly different from that obtained for young male
adults (4710 mg/kg body weight) or older male adults (5338 mg/kg
body weight).
8.1.2 Signs of intoxication
Sprague-Dawley rats of both sexes, inhaling 2-propanol for 8 h
at concentrations between 19 680 and 64 206 mg/m3, showed severe
irritation of the mucous membranes and depression of the central
nervous system indicated by subsequent ataxia, prostration, and
narcosis. These effects were concentration- and time-dependent.
Rats surviving the narcosis recovered. Transient paralysis of the
hind limbs was observed at levels between 49 200 and 54 120 mg/m3.
The rats died at and above 44 280 mg/m3, usually within 2 days,
with females dying earlier than males. All rats were autopsied,
including survivors, 15 days after exposure. At non-lethal levels,
congestion of the liver, lung, and spleen were observed, especially
in males. At lethal levels, vacuolation of the liver, acute
pneumonitis, and spleen oedema were found. These effects were most
pronounced in survivors. At 51 660 mg/m3, severe cytoplasmic
degeneration of the liver and oedema of the lung and brain occurred
in all rats [151]. Deep narcosis occurred in rats exposed through
inhalation to 40 mg/litre for 2 h. Significant and lasting
hypothermia was induced in Sprague-Dawley rats exposed for 4 h to
vapour concentrations of 19 680 mg/m3 or more [151].
Rats and mice of unknown strains, exposed orally or through
inhalation to lethal levels of 2-propanol, showed unspecified signs
of irritation and died through respiratory arrest while under
narcosis, usually within 24 h following exposure. Necropsy
revealed oedema, haemorrhage, inflammation, and dystrophy in the
interstitial tissues of parenchymal organs. In the lung,
infiltration, oedema, and thinning of the alveolar walls were
observed [103].
Table 9. Lethality of 2-propanol
---------------------------------------------------------------------------------------------------
Species/strain Sex Route of exposure Observation LD50 LC50 Reference
period (mg/kg body (mg/m3)
weight)
---------------------------------------------------------------------------------------------------
Rat not reported oral 3 days 5 280 - [157]
Sherman rat not reported oral 14 days 5 480 - [239]
Sprague-Dawley rat male oral 7 days 4 710 - [134]
Rat not reported oral 14 days 5 500 - [103]
Mouse not reported oral 14 days 4 475 - [103]
Rabbit not reported oral 3 days 5 030 - [157]
Rabbit male oral 1 day 7 990 [190]
Dog not reported oral 3 days 4 830 - [157]
Wistar rat male intravenous 5 days 1 088 - [247]
H mouse male intravenous 5 days 1 580 - [247]
H mouse female intravenous not reported 1 860 - [56]
Chinchilla rabbit male, female intravenous 5 days 1 184 - [247]
Wistar rat male intraperitoneal 5 days 2 830 - [247]
H mouse male intraperitoneal 5 days 4 868 - [247]
Syrian hamster male intraperitoneal 5 days 3 467 - [247]
Rabbit not reported dermal not reported 12 870 - [239]
Rat not reported inhalation (4 h) 14 days - 72 600 [103]
Sprague-Dawley rat male inhalation (8 h) 15 days - 46 740 [150]
Sprague-Dawley rat female inhalation (8 h) 15 days - 55 350 [150]
Mouse not reported inhalation (2 h) 14 days - 53 000 [103]
---------------------------------------------------------------------------------------------------
The effects of 2-propanol on the mucociliary system of the
trachea [197] and the middle ear [198] were investigated in two
separate studies. Groups of 20 or 24 Hartley guinea-pigs were
exposed to 2-propanol vapour at measured concentrations of 0, 969,
or 13 382 mg/m3 for 24 consecutive hours. Four animals from each
of the 3 groups were killed by decapitation at 12 h, 24 h, and 3,
7, and 14 days after the exposure period. A concentration-related
deterioration of ciliary activity and mucosal degeneration were
observed in both the trachea and in the middle ear. At the lower
exposure level, the effects completely reversed within 2 weeks of
exposure, but, at the higher exposure level, they did not.
2-Propanol can enter the trachea and deeper lung structures by
aspiration through the oral and nasal cavities. Anaesthetized rats
were made to inspire 110 or 140 mg of the compound in water, or 160
mg of the undiluted compound. Survivors were sacrificed 24 h later
for lung examination. At the lower doses, 1 out of 10 rats in each
group died. At the highest dose, 6 out of 10 rats died of cardiac
or respiratory arrest. All controls survived. It was not reported
whether the latter were sham-exposed or not. The average absolute
lung weight in the high-dose group was increased by 75% [94].
8.1.3 Skin, eye and respiratory tract irritation
In primary irritation patch tests on groups of 6 rabbits and 6
Hartley guinea-pigs, 0.5 ml of undiluted 2-propanol was applied to
areas of clipped or abraded skin. No irritation was observed for
up to 48 h after a 4-h exposure [194]. Erythema and changes in
vascular permeability did not occur when the clipped skin of
guinea-pigs was exposed for 2 min to discs of filter paper soaked
in undiluted 2-propanol [240].
Marzulli & Ruggles [182] reported on an interlaboratory
evaluation of the ocular irritancy of 2-propanol, according to
Draize scores, in groups of 6 rabbits, 1 - 7 days after application
of 0.1 ml of a 70% solution on the cornea. One day after exposure,
mean Draize scores were approximately 0.6 for corneal opacity, 0.3
for iritis, 1.5 for conjunctival redness, and 1.3 for chemosis.
The maximum total Draize score was 10.1. In each laboratory,
67 - 97% of the rabbits met the criteria for eye irritation, which
decreased over time. In another Draize test, groups of 6 - 9 New
Zealand rabbits received doses of 70% 2-propanol at levels of 0.01,
0.03, or 0.10 ml per eye. Maximum total Draize scores were 21, 36,
and 37, respectively, the maximum on the Draize scale being 110.
Using irritation categories based on the number of days required
for the effects to disappear, the compound was substantially
irritating (clearing within 14 days) at the higher doses and
moderately irritating (clearing within 7 days) at the lowest dose
[100].
The eye irritancy of 2-propanol was evaluated using Stauffland
albino rabbits, each of which received 0.1 ml of undiluted propanol
in one eye. Eye irritation was scored according to Draize up to 21
days after application. The exact scores were not reported, but
2-propanol was found to be corrosive according to the US EPA
criteria used in 1981, implying corneal involvement and irritation
or eye damage that persisted for more than 21 days after treatment
[188].
2-Propanol was tested on several occasions in in vitro
cytotoxicity assays and the results showed good agreement with
those in vivo [29, 30, 213].
The potency of 2-propanol as a sensory irritant was evaluated,
using a 50% reflex decrease in the respiratory rate of mice (RD50)
as an index. The mice were exposed by their heads only. An
exposure-related effect was found with RD50 values of 12 300 mg/m3
for Swiss OF1 mice and 43 525 mg/m3 for Swiss Webster mice [61, 130].
8.2 Continuous or Repeated Exposures
Groups of rats were exposed to 2-propanol vapour at
concentrations of 0, 100, or 500 mg/m3 air for 5 days/week and
4 h/day over 4 months [103]. Strain, sex, and group size were not
given, but were presumably in accordance with CMEA standards. No
deaths were reported. At the end of 4 months of exposure to 500
mg/m3 air, growth was reduced significantly by 10% and the
respiratory rate was increased by 22%. The white blood cell count
was decreased at both exposure levels in an exposure-related
manner. At 500 mg/m3, this was attributed to a decreased absolute
and relative number of lymphocytes. Decreases in hippuric acid
excretion and in total serum protein, and an increase in blood
acetylcholine were found at both exposure levels. The decrease in
total serum protein was exposure-related and could partly be
accounted for by the observed decrease in alpha1- and alpha2-
globulins and in albumin at 500 mg/m3. Blood glucose levels were
decreased at 500 mg/m3. Macroscopic and microscopic changes,
observed at 500 mg/m3, included irritant effects on the respiratory
system, such as thinning of the alveolar walls, perivascular
infiltration, pneumonia, and bronchitis. Dystrophic changes and
perivascular cell reactions were seen in the liver. Follicular
hyperplasia was observed in the spleen [103].
Another report described the continuous exposure of groups of
15 rats of unknown strain and sex to 2-propanol vapour at
concentrations of 0, 0.6, 2.5, or 20 mg/m3 air for 86 days. The
data were not statistically analysed. No deaths were reported. At
the highest exposure level, changes in the latency period of
unconditional reaction and an increase in the number of fluorescent
leukocytes were found. Effects reported at this exposure level
included an increase in sulfobromophthalein retention and decreased
blood levels of nucleic acids. Microscopy only revealed adverse
effects at 20 mg/m3 with liver dystrophy, degenerative changes in
the cerebral cortex, and spleen hyperplasia [19].
These studies [19, 103] lack a number of essential details
concerning the protocol, the effects observed, the incidence of
these effects, and the statistical analysis.
A "coefficient of accumulation" (the ratio between cumulative
LD50 and single dose LD50) reported for repeated oral exposure to
2-propanol was 4.0 in rats and 4.9 in mice [10].
Groups of 5 white rats of each sex and of unknown strain
received drinking-water containing 2-propanol for 27 weeks.
Average daily intakes were 0, 600, or 2300 mg/kg body weight for
males and 0, 1000, or 3900 mg/kg body weight for females. Only
males died: 2 at the lower dose and 3 at the higher dose. It was
reported that extensive postmortem changes prevented an accurate
diagnosis of the cause of death. At the end of the exposure
period, all exposed females showed growth retardation. Males
showed a slight decrease in growth initially but overtook the
controls in body weight towards the end of the exposure period. No
adverse effects were found on food intake, behaviour, and
histopathology [157].
8.3 Neurotoxicity and Behavioural Effects
2-Propanol passes the blood-brain barrier twice as effectively
as ethanol (section 6.2.1). The oral ED50 for narcosis in rabbits
exposed to 2-propanol was 2280 mg/kg body weight, i.e., 2.5 times
lower than that for ethanol [190]. The threshold of an acute
effect on the CNS was observed in rats after a 4-h exposure at 1450
mg/m3 using the method of flexor reflexes, the "summation threshold
method" [10]. The intraperitoneal ED50 for loss of righting reflex
in Swiss Webster mice, which was 164 mg/kg body weight, was 1.8
times lower than that for ethanol [169]. The threshold for the
induction of ataxia in Sprague-Dawley rats following intraperitoneal
exposure was 1106 mg/kg body weight [171]. In 2 tilted plane
tests, the performance of rats decreased by an average of 30 - 40%
after oral or intraperitoneal exposure to 2000 and 1800 mg/kg body
weight, respectively [178, 270]. On a molar basis, 2-propanol was
2.7 times as intoxicating as ethanol [270]. When C57BL/6J or
DBA/2J mice were exposed intraperitoneally to a single dose of 2-
propanol at 3 dose levels, the former strain showed increased
activity in the open field test at 392 mg/kg body weight, no effect
on activity at 785 mg/kg body weight, and narcosis at 1570 mg/kg
body weight. DBA/2J mice showed increased activity at the middle
dose level and narcosis at the high dose level [243]. Wistar rats,
inhaling 2-propanol at a concentration of 739 mg/m3 for 6 h/day, 5
days/week, for up to 15 weeks, did not show any adverse effects in
the open field test [224].
The depressive action of 2-propanol on the central nervous
system was related by several investigators to interactions with
neuronal membranes in vitro and in vivo. Lyon et al. [169]
observed a high correlation between the narcotic potencies of
aliphatic alcohols in mice and their ability to disorder brain
synaptosomal plasma membrane in vitro, as measured by electron
paramagnetic resonance, which was, in turn, related to membrane
solubility. In Sprague-Dawley rats, no change in synaptosomal
membrane fluidity was measured via in vitro registration of the
fluorescence polarization of 1,6-diphenyl-1,3,5-hexatriene, 20 h
after a single oral dose of 3000 mg/kg body weight. When
2-propanol was added subsequently, a change in membrane fluidity
was observed. This effect was related to membrane solubility.
The activity of synaptosomal Na+/K+-transporting
adenosinetriphosphatase (EC 3.6.1.37) was increased both in vitro
and in vivo [24].
Functional loss due to disruption of membrane integrity by
2-propanol was also observed in vitro by the blocking of the action
potentials of sciatic nerves in the toad, Bufo marinus [214], and
by the inhibition of membrane-bound guanylate cyclase (EC 4.6.1.2)
in intact murine neuroblastoma N1E-115 cells [241]. It was also
indicated by the interference of 2-propanol with the transport of
Ca2+ ions across biological membranes, as shown, in vitro, by the
inhibition of Ca2+ ion-induced contractions of guinea-pig ileum
(Yashuda et al., 1976), and, in vivo, in rats by a decrease in
regional brain Ca2+ ion levels, 30 min after one intraperitoneal
dose of 2000 mg/kg body weight [221].
Several neurochemical parameters were measured in the brain or
spinal cord axon of Wistar rats, inhaling 2-propanol at a
concentration of 739 mg/m3 air for 6 h/day, 5 days/week, over 5,
10, 16, or 21 weeks. In cerebellar homogenates, the activities of
superoxide dismutase (EC 1.15.1.1) and azoreductase (EC 1.6.6.7)
were decreased at weeks 16 and 21, but no effects were found on the
activity of dihydrolipoamide dehydrogenase (EC 1.8.1.4). In
cerebellar glial cells, the activity of acid proteinase (EC
3.4.21.1) was stimulated at weeks 5 and 10, while the glutathione
concentration was unaffected at all times. In the spinal cord
axon, the phosphate/cholesterol ratio of lipid was reduced by 25%
[224].
8.4 Biochemical Effects
8.4.1 Effects on lipids in liver and blood
Oral administration of single doses of 3000 or 6000 mg
2-propanol/kg body weight to Wistar rats caused a reversible
accumulation of triglycerides in the liver [21, 22, 23, 90]. At
the 6000 mg/kg body weight dose, a slight fatty infiltration was
observed histologically [90]. Observations that could explain this
effect were increased hepatic uptake of palmitate [23], increased
incorporation of palmitate into hepatic triglycerides, decreased
hepatic palmitate oxidation, and, at a later stage of intoxication,
interference with the synthesis and excretion of very dense
lipoproteins [21, 22, 23].
Decreased palmitate oxidation was related to the observed
increase in the hepatic b-hydroxybutyrate/acetoacetate ratio,
implying a decrease in the intramitochondrial NAD+/NADH ratio [21,
23, 195]. Excess extramitochondrial reducing equivalents,
indicated by an increased lactate/pyruvate ratio, was also observed
in vivo [21] and in vitro [85]. However, all these changes in the
redox state of the liver were very modest and therefore were not
thought to be fully responsible for the fatty livers induced.
More significant seemed to be the observation that the
incorporation of palmitate and glycerol into serum triglycerides
and the incorporation of palmitate into serum and hepatic
phospholipids were inhibited. This indicates an impaired secretion
of lipo-proteins, partly explained by the observed disturbance of
the biosynthesis of phospholipids, and partly by the observed
inhibition of hepatic protein synthesis [21, 22, 23]. Inhibition
of hepatic protein synthesis, as shown by the synthesis of the
marker enzymes ornithine decarboxylase (EC 4.1.1.17) and tyrosine
amino-transferase (EC 2.6.1.5), was observed in hepatectomized rats
following oral exposure to 2300 mg 2-propanol/kg body weight [205].
Acetone administration to rats up to a blood level comparable
to that in 2-propanol-dosed rats only slightly increased the level
of liver triglycerides; therefore acetone does not seem to play a
major role in the induction of fatty liver by 2-propanol [22].
8.4.2 Effects on microsomal enzymes
Oral exposure of Sprague-Dawley rats to 390 mg 2-propanol/kg
body weight, once or on 4 subsequent days, caused a slight but
significant increase in the hepatic cytochrome P-450 content and a
2 - 3-fold increase in the activities of the microsomal liver
enzymes (EC 1.14.14.1) aniline hydroxylase and 7-ethoxycoumarin
O-deethylase, 18 h after exposure [255]. Direct activation of these
enzymes does not offer a satisfactory explanation, as it was shown
in vitro that 2-propanol is an inhibitor of several microsomal
enzymes via an interaction with cytochrome P-450, which causes a
change in the reverse Type I spectrum [8, 57, 222, 246, 254, 285].
It was therefore concluded that, as acetone also does not appear to
be involved in aniline hydroxylase induction in vivo, 2-propanol
induces one or more forms of cytochrome P-450 [255]. In young
Sprague-Dawley rats, receiving 2-propanol once at a level of 1960
or 3140 mg/kg body weight and sacrificed 22 - 24 h later, the
hepatic cytochrome P-450 content increased, as well as the
activities of several nitrosamine N-demethylases, benzphetamine
demethylase, ethylmorphine demethylase, p-nitroanisole demethylase,
and 7-ethoxycoumarin- O-deethylase. A slight increase was observed
in the activity of NADPH cytochrome c reductase (EC 1.6.2.4).
Acetone appeared to play a role in this induction. From several
lines of observations, it was concluded that the enhanced activity
of nitrosodimethylamine N-demethylase was due to the induction of
specific forms of P-450 isozymes and P-450-dependent enzymes [255].
In several earlier studies on rats, at comparable or higher
oral doses, induction of microsomal liver enzymes was observed,
including a slight induction of NADPH cytochrome c reductase, but
no increase in hepatic cytochrome P-450 content [206, 207, 234].
Ueng et al. [255] explained the latter observation by suggesting
that the species of cytochrome P-450 induced must be normally
present in low concentrations, so that a several-fold increase in
catalytic activity could be induced without markedly increasing the
total content of the haemoprotein.
Sprague-Dawley rats were also exposed by inhalation to
2-propanol at concentrations of 490, 4920, or 19 680 mg/m3 air for
6 days/week, 6 h/day over 2 weeks. In the liver and kidneys, the
contents of cytochrome P-450 and cytochrome b5 were increased as
well as the activity of NADPH cytochrome c reductase at 4920 and
19 680 mg/m3. These effects were completely reversible in the
liver of rats exposed at 19 680 mg/m3 for 2 weeks and allowed to
recover for 4 weeks. However, they persisted in the kidneys [290].
8.4.3 Other biochemical findings
Differential effects have been observed on the glutathione
status of the liver following exposure of rats to 2-propanol. In
Sprague-Dawley rats inhaling 2-propanol at a concentration of 4920
or 19 680 mg/m3 for 6 days/week, 6 h/day, over 2 weeks, the reduced
glutathione concentration in the liver and kidneys increased
slightly while, at 19 680 mg/m3, the activity of glutathione
S-transferase (EC 2.5.1.18) increased by 13% in the liver only
[290]. The glutathione level in the liver of Wistar rats that had
received a single oral dose of 3110 mg 2-propanol/kg body weight
was reduced 6 h following exposure, while there was a 10% increase
in lipid peroxidation, as indicated by the formation of diene
conjugates [264]. A homogenate of normal or regenerating rat liver
did not show any increase in malondialdehyde production after
incubation with 2-propanol [6].
The activity of hepatic and kidney alanine aminotransferase (EC
2.6.1.2) in rats was unchanged 18 h after exposure to 1 or 4 daily
doses of 392 mg 2-propanol/kg body weight [255] or 4 h after
exposure to a single dose of 2300 mg/kg body weight [205]. Liver
damage was not observed in guinea-pigs histopathologically or by
any increase in serum ornithine carbamoyltransferase (EC 2.1.3.3),
24 h after receiving intraperitoneal doses of up to 1000 mg
2-propanol/kg body weight [73].
In Charles River rats, 2-propanol (785 mg/kg body weight ip)
induced hepatic metallothionein and caused low blood zinc levels
[244]. These changes were not prevented by adrenalectomy.
8.5 Immunological Effects
2-Propanol and acetone both enhanced the incorporation of
labelled thymidine into concanavalin A-stimulated murine spleen
cells in vitro [210]. 2-Propanol inhibited the killing of YAC-1
tumour cells by natural killer effector cells from mouse or rat
spleens [219]. Inhibition of the synthesis and/or the secretion
and/or function of at least one monocyte-derived substance that
inhibited cell proliferation was suggested as an explanation of
these findings [210].
8.6 Reproduction, Embryotoxicity, and Teratogenicity
Groups of 13 - 15 pregnant Sprague-Dawley rats were exposed to
2-propanol for 7 h/day on gestation days 1 - 19 at measured
concentrations of 9001, 18 327, or 23 210 mg/m3 (3659, 7450, or
9435 ppm). At the highest concentration, rats were completely
narcotized at the end of the first exposures, but by the end of the
19 days the effect was slight. Initial exposures to 18 327 mg/m3
(7450 ppm) caused an unsteady gait, but this was not noticeable by
the end of 19 days of exposure. Food consumption and maternal body
weight gain were significantly reduced by exposure to 18 327 and
23 210 mg/m3 (7450 and 9435 ppm). In the group exposed to 23 210
mg/m3 (9435 ppm): 6 out of 15 mated rats were not pregnant at
term, which was attributed to an exposure-induced failure of
implantation; 4 out of 9 pregnancies were totally resorbed; and
resorptions per litter were significantly increased. Fetal body
weights were significantly reduced, in a concentration-related
pattern, at all 3 concentrations. The incidence of cervical ribs
was significantly increased in the 23 210 mg/m3 (9435 ppm) group
[193].
A group of 6 female and 3 male rats (38 - 40 days old), of
unspecified strain, received drinking-water containing 2-propanol.
They were mated after approximately 2 months of exposure. This
schedule was repeated through 2 further generations. Average daily
intakes for the 3 generations were 1470, 1380, and 1290 mg/kg body
weight, respectively. The growth of the first generation was
retarded initially, but returned to normal by the 13th week.
Otherwise, no effects were found on growth and reproductive
functions [159].
When female hybrid rats received oral doses of 252 or 1008 mg
2-propanol/kg body weight per day for 45 days, the length of the
estrus cycle was increased by 23 - 24% [15]. Five female hybrid
rats also received daily doses of 1800 mg/kg body weight for 3
months before mating. Together with 6 controls they were
sacrificed on day 21 of pregnancy. The total embryonic mortality
rate was increased 2-fold, which was statistically significant [15].
In a 6-month drinking-water study, 2-propanol was administered
to hybrid rats of both sexes, at daily doses of 0.018, 0.18, 1.8,
or 18 mg/kg body weight. Mating of males with 5 - 7 females per
group occurred after the exposure period. When both sexes were
exposed to 18 mg/kg body weight, the litter size was increased.
The neonatal mortality rate was increased at 0.18 and 1.8 mg/kg,
when females only were exposed, at 18 mg/kg body weight, when males
only were exposed, and at the 2 highest exposure levels, when both
sexes were exposed. Neonates from the last group showed a dose-
related decrease in the rate of reaction to electric stimuli
(unconditioned defence reaction), but no other effects on their
development [15].
Groups of 10 - 13 female hybrid rats received 252 or 1008 mg
2-propanol/kg body weight from day 1 to day 20 of pregnancy. They
were sacrificed on day 21 of pregnancy together with 11 controls.
Litter size was reduced at both exposure levels. General embryonic
toxicity was 18 and 31%, respectively (10% in controls). At 1008
mg/kg body weight, total embryonic mortality rate was trebled and
10 out of 70 fetuses showed developmental anomalies compared with
none out of 90 controls. The anomalies found were brain damage in
3 fetuses, kidney damage in 5 fetuses, and gastrointestinal damage
in 2 fetuses. The lesions were not specified further. Data on
maternal toxicity were not reported [15].
8.7 Mutagenicity
A reverse mutation spot test with the Salmonella typhimurium
strains TA 98, TA 100, TA 1525, and TA 1537 was negative at 0.18 mg
per plate, with and without metabolic activation by S9 rat liver
[82].
Rat bone marrow cells were examined after 4 months of exposure
of rats to 2-propanol vapour at concentrations of 0, 1.03, or 10.2
mg/m3 air for 4 h/day. Statistically significant increases were
counted in the percentage of mitotic aberrations [18]. However,
the authors did not report the number of rats exposed, their sex,
or strain.
Root tip cells of onion (Allium cepa) exposed to 2-propanol
showed a 2.8-fold increase in the mitotic aberration rate [230].
2-Propanol did not increase sister chromatid exchange
frequencies in vitro in V79 Chinese hamster lung fibroblasts, with
or without S9 mix, when tested at concentrations of 3.3, 10, 33.3,
and 100 mmol/litre [267].
A dose-related increase in the inhibition of metabolic
cooperation in hamster V79 cells, a phenomenon believed to reflect
carcinogenic promotion ability and not to be indicative of
genotoxic potential, was observed by Chen et al. [53]. This may be
due to the membrane effects of 2-propanol.
8.8 Carcinogenicity
Several limited carcinogenicity studies on the mouse have been
conducted with 2-propanol, using the inhalation, dermal, and
subcutaneous routes of exposure.
Groups of 3 month-old, male C3H, ABC, and C57/BL mice were
exposed to 2-propanol vapour at a concentration of 7700 mg/m3 air
for 3 - 7 h/day, 5 days/week, over 5 - 8 months. The sizes of the
experimental and control groups were not reported. The occurrence
of lung tumours was examined microscopically in mice that had
survived until killed at the age of 8 months (36 exposed and 69
control C3H mice, 49 exposed and 120 control ABC mice), or 12
months (47 exposed and 52 control C57/BL mice) and showed
macroscopic lesions. The mice were not examined for the occurrence
of sinus tumours as occurred in workers engaged in the manufacture
of 2-propanol (section 9.2.1). No excess of lung tumours was
observed [276].
2-Propanol was painted on the clipped backs of 30 Rockland
mice, 3 times/week for 1 year. Sex, dose, and observation period
were not specified. Skin papillomas developed in 3 control mice,
one of which also had a skin carcinoma, while there was no response
in treated mice (Weil, C.S., written communication to US NIOSH [257]).
Groups of 3-month-old male C3H, ABC, and C57/BL mice received
20 mg undiluted 2-propanol subcutaneously once a week, for
20 - 40 weeks. The sizes of the treated and untreated control
groups were not reported. Surviving mice that showed macroscopic
lung lesions were examined microscopically for lung tumours. No
excess of lung tumours was observed. Lung tumour incidence in the
control group was high [276].
Doses of 20 mg undiluted 2-propanol were injected
subcutaneously in 40 C3H mice, once a week, over a period of 20
weeks. Controls were untreated. The occurrence of lung tumours
was examined in mice that survived until killed 5 months after the
first injection (22 exposed and 33 control mice). No excess of
lung tumours was observed (Weil, C.S., written communication to US
NIOSH [257]).
In these studies, only lung tumours and, in the case of dermal
exposure, skin tumours were investigated. Moreover, the exposure
periods, where given, were too short to allow for tumour induction.
Experimental details including size of experimental and control
groups, sex ratio, and observation periods were not quoted for some
of the studies. Because of these shortcomings, the studies are
inadequate for the assessment of carcinogenic potential.
8.9 Factors Modifying Toxicity
As a result of the induction of specific isozymes of cytochrome
P-450 (section 8.4.2) by 2-propanol and/or acetone, the
hepatotoxicity of carbon tetrachloride, 1,1,2-trichloroethane,
chloroform (CHCl3), trichloroethylene, and dimethylnitrosamine in
rodents is enhanced [60, 165, 234, 252, 253, 254]. The metabolic
activation of n-hexane by liver and kidney microsomes is also
increased by 2-propanol pretreatment [290].
9. EFFECTS ON MAN
9.1 General Population Exposure
9.1.1 Poisoning incidents
The subject of non-beverage alcohol use has already been
addressed in section 5.2.2. The clinical literature has been
reviewed [149, 176, 245, 163]. A brief summary of cases of
intoxication and major symptoms is presented in Table 10.
Table 10. Acute intoxications by 2-propanola
------------------------------------------------------------
No. of No. of Observationsb Reference
cases alcoholics 1 2 3 4 5 6 7
------------------------------------------------------------
Ingestion
57 46 +57 [11]
5 2 +5 +5 +4 +2 +4 +2 [4]
1c 0 - + + + + + + [186]
3 3 - +3 - +2 +2 +1 +2 [170]
1 0 - + + + + + - [191]
2 0 - +2 +2 +2 +1 [149]
1 1 - + + - + - + [245]
17 14 - +2 +3 +10 - - [131]
1 - + + + - [88]
1 1 - + + + [135]
1c 0 - + + - + - - [266]
1 1 - + + - - + [261]
1 0 - + + [75]
1 0 - + - - - [220]
2 1 - - + +2 - +2 +1 [5]
1 1 - - - + - + - [128]
1 1 - - - + + [108]
Sponging
1c - - + + + + + [228]
1c - - + + + - - + [160]
1c - - + + + [91]
1c - - + + - - + [172]
1c - - + [181]
1c - - + + + + - + [16]
--------------------------------------------------------------
a +N = observed in N cases; - = not observed; no sign = not
reported.
b 1 = death; 2 = coma; 3 = hypotension, meaning < 100 mm Hg systolic
pressure and/or < 60 mmHg diastolic pressure; 4 = tachycardia,
meaning > 100 beats per min; 5 = hypothermia, meaning < 36.5 °C;
6 = gastritis, characterized by nausea, vomiting, abdominal pain;
and/or gastric haemorrhage; 7 = elevated blood glucose, meaning
> 1500 mg/litre.
c Child below 2.5 years of age.
Intoxications have been reported after oral ingestion (Table
10), rectal administration [59], and inhalation by children who
have been sponged for several hours with 2-propanol preparations
for fever reduction (Table 10). Skin absorption in 2-propanol
sponging has been deemed insignificant [e.g., 149, 176]. However,
a case report on a child with an almost lethal blood level of
2-propanol after sponge bathing for fever reduction, and
questionable ingestion, suggested to the authors that the
importance of dermal absorption, compared with inhalation, should
not be underestimated [181].
2-Propanol depresses the central nervous system. At comparable
doses, 2-propanol is thought to be approximately twice as active as
ethanol in this respect with a longer duration of the coma due to
slower metabolism and to the contribution of acetone to the
depression of the central nervous system [149, 163, 176, 245]. As
with ethanol, excessive intoxication with 2-propanol causes
unconsciousness, usually, but not always ending in a deep coma [5,
59, 108, 128]; death may follow due to respiratory depression [4].
In most cases, decreased or absent reflexes are reported. Pupil
size is variable, but most often miotic [16, 88, 149, 220, 228,
266]. Early gastrointestinal problems and hypothermia frequently
occur. Cardiovascular effects include severe hypotension, shock
[4, 266], and even cardiac arrest [176, 266]. Tachycardia is found
as a secondary effect. Other possible compound-related effects are
hyperglycaemia, elevated protein levels in cerebrospinal fluid [5,
170, 266], and atelectasis [191, 220]. Lung congestion was
observed in fatal cases [4, 11].
In cases of 2-propanol intoxication, hypotension can be a grave
prognostic sign [4], which some authors suggest requires immediate
action by gastric lavage, haemodialysis [5, 22, 88, 135, 176], or
peritoneal dialysis [75, 186, 245]. Following treatment, recovery
is usually complete, unless the hypotension is severe and persistent.
In such cases, renal injury and death may occur [4, 128].
Acetone can be detected in the blood, urine, and breath.
Because other ketone bodies are not found in significant quantities,
early acidosis does not usually occur, serum bicarbonate, anion
gap, and blood pH remaining normal. Sometimes, a mild metabolic
acidosis and anion gap may develop as a result of lactic acid
accumulation. Acetonaemia and/or acetonuria without metabolic
acidosis and with normal or slightly elevated blood glucose levels
differentiate 2-propanol poisoning from diabetic ketoacidosis and
poisoning by other alcohols [16, 163, 176]. In addition, a
significant osmolality gap may be observed [16, 261].
None of the biochemical findings could be related to blood
levels of 2-propanol or acetone. 2-Propanol levels, measured at
different times after exposure, varied between undetectable
concentrations and 5600 mg/litre [176]. Acetone levels varied
between undetectable concentrations and 18 780 mg/litre [75].
Alexander et al. [11] suggested that combined levels of 2-propanol
and acetone may allow greater accuracy in predicting the clinical
course of the condition of a patient.
Adults have been reported to survive ingestion of 700 ml of
2-propanol [88] and to die after ingestion of 400 ml of 2-propanol
[4]. The lowest dose reported to be life-threatening was in an
18-month-old child who ingested approximately 170 ml of 2-propanol
[186].
9.1.2 Controlled exposures
No adverse effects were observed in groups of 8 healthy male
volunteers (24 - 57 years of age) who drank a daily dose of 2.6 or
6.4 mg 2-propanol/kg body weight in diluted syrup for 6 weeks.
Investigations included haematology, blood chemistry, urinalysis,
and ophthalmoscopy [283].
Another group of 10 healthy male volunteers (age not stated)
was exposed to 2-propanol vapour at nominal concentrations of 490,
980, or 1970 mg/m3 air for 3 - 5 min. After each exposure, the
participants were asked to classify the effects of the vapour on
the eyes, nose, and throat. The volunteers judged irritation to be
"mild" at 980 mg/m3 and "not severe" at 1970 mg/m3. They also
judged 490 mg/m3 to be "satisfactory" for their own 8-h
occupational exposure [192]. The validity of these results is
doubtful, for various reasons including the subjective criteria
used, the lack of control exposures, and the unreliability of the
exposure levels.
9.1.3 Skin irritation; sensitization
No skin irritation was observed in 6 human volunteers when 0.5
ml of undiluted 2-propanol was applied to a 4 cm square area on the
back and evaluated after 4 h, 24 h and 48 h [194]. Closed patch
tests with 10-min applications of 0.1 - 0.3 ml undiluted 2-propanol
on the dry skin of 10 healthy volunteers and 12 persons receiving
disulfiram therapy for the treatment of chronic alcoholism did not
result in any reaction. After immersion of the skin in tepid water
for 10 min, a transient erythema rapidly appeared on the site of
application in 19 subjects [105].
Skin irritation was also reported in a total of 6 premature
infants with a gestational age of less than 27 weeks. They were
exposed via swabs used for conduction in ECG recording or by the
application of 2-propanol on the umbilical area with subsequent
soaking of the diaper. Erythema and second or third degree burns
or blisters were observed in areas of prolonged contact with
2-propanol. Hypoperfusion of the skin was suggested to be a
contributing factor [226, 277].
In 1968, Wasilewski [272] reported a case of alleged allergic
contact dermatitis following skin disinfection with a 70%
2-propanol swab. The patient was treated for allergic rhinitis.
Patch tests were positive for 2-propanol. However, controls were
not tested and there was no information on the purity of the
alcohol. This report was followed by several others concerning a
total of 8 persons who showed dermatitis after contact with medi-
swabs containing 2-propanol. Patch testing revealed that another
volatile agent, presumably propylene oxide, caused the effect [174,
124, 217]. Only one of these 8 persons was also hypersensitive to
2-propanol of unknown purity [124].
Itching eczematous lesions developed in a laboratory worker, in
a company manufacturing hair cosmetics, in patch tests with
chemically pure 2-propanol solutions (2.5 - 99.7% by volume). This
person also reacted to 1-propanol, 1-butanol, 2-butanol, and
formaldehyde, but not to ethanol and methanol. Controls were not
tested [167]. Two out of 4 confirmed cases of hypersensitivity to
primary alcohols were also found to be hypersensitive to secondary
alcohols. Both cases had a history of contact allergies.
Recurrent exposures to 2-propanol and/or other alcohols occurred
through consumption of alcoholic beverages, pre-injection
disinfection, via a hair lotion in one case and occupationally in
the other case. Patch tests were positive for pure undiluted
2-propanol and 2-butanol. Twenty controls did not show any
reactions to secondary alcohols [86, 87]. The mechanism of alcohol
hypersensitivity is not clear. Fregert et al. [87] pointed out
that it is difficult to understand how alcohols can act as haptens.
9.2 Occupational Exposure
9.2.1 Epidemiology studies
A retrospective cohort study was reported among 182 workers at
a plant, in the USA, manufacturing 2-propanol by the strong acid
process over the period 1928 - 50. In a subgroup of 71 men, who
had been employed for more than 5 years, 7 cases of cancer were
observed: 4 cancers of the paranasal sinuses, 1 lung carcinoma, 1
laryngeal carcinoma, and 1 laryngeal papilloma. The minimum
latency period was 6 years [276]. According to USA vital
statistics for 1948, 0.0014 paranasal sinus cancers would have been
expected for the total cohort [286].
Two cases of paranasal sinus cancer and 2 cases of laryngeal
cancer were reported in 1966 in a cohort of 779 workers in a
similar plant in the USA that had been in operation since 1927.
The minimum latency period was 10 years. The age- and sex-adjusted
incidence of sinus and laryngeal cancer in this group was 21 times
higher than expected [119].
Another retrospective cohort study was undertaken among 262 men
who had worked for at least one year in a 2-propanol-manufacturing
plant in the United Kingdom using the strong acid procedure over
the period 1949 - 76. There were more than 4000 person-years at
risk. No person was lost to follow-up, which was over an average
of 15.5 years. The mortality rates due to all causes and due to
neoplasms were not significantly higher than expected according to
national vital statistics. One person died from nasal cancer
against 0.02 expected. Other significant findings were 2 "kidney
and adrenal malignancies" and 2 cancers of "the brain and the
central nervous system" [9].
A fourth retrospective cohort study was conducted over the
years 1966 - 78 among 433 workers in a 2-propanol manufacturing
plant, in the USA, who were exposed for at least 3 months during
the period 1941 - 65. The strong acid process used in 1941 had
been gradually changed to the weak acid process by 1965. More than
11 000 person-years were at risk. The mortality rate due to all
causes was lower than expected on the basis of state vital
statistics. No excess mortality due to all cancers was observed,
but the incidence of buccal and pharyngeal cancer was 4 times
higher than expected (2 versus 0.5). There was a slight excess of
lung cancer (7 versus 5.94) [79].
These cohort studies collectively suggest a cancer hazard
related to the strong acid manufacturing process. The excess in
respiratory cancers was initially attributed to isopropyl oils
[119, 276]. However, the experimental basis for this assumption is
weak (section 8.8) and more recently diisopropyl sulfate, an
intermediate produced in the strong acid process, has been proposed
as a more likely causative agent. The concentration of this
chemical is high in the strong acid process and low in the weak
acid process [79, 286].
Two small case-control studies were conducted in the USA: one
to consider the risk of lymphocytic leukaemia associated with 24
solvents among rubber industry workers [52], and the other to
consider the risk of brain gliomas associated with exposure
conditions during work at a chemical plant [155]. Although there
was no evidence of an association between exposure to 2-propanol
and the incidence of gliomas or lymphocytic leukaemia, the small
number of subjects and multiple exposures mean that no conclusions
can be drawn from these studies.
9.2.2 Interacting agents
Fourteen out of 43 workers in a 2-propanol-packaging plant
became ill when carbon tetrachloride was used for cleaning
equipment. The incidence and the severity of the effects increased
where exposure to carbon tetrachloride was the highest, and where,
on another occasion, the mean concentration of 2-propanol in air
was also highest, i.e., 1010 mg/m3. In 4 cases, renal failure or
hepatitis developed [83]. Another similar incident was reported in
a colour printing factory, 17 workers had abnormal liver function
and 3 developed acute hepatitis following combined exposure to
carbon tetrachloride and 2-propanol [71]. These reports suggest
potentiation of the toxicity of carbon tetrachloride by 2-propanol,
which was also found in experimental animals (section 8.9).
Some shaving lotions containing 2-propanol and other ingredients
(e.g., menthol, camphor, methyl salicylate, naphthalene) have been
reported to produce central stimulation with motor restlessness,
extreme apprehension, hallucinations, and general disorientation
[89, 238].
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of Human Health Risks
10.1.1 Exposure
Exposure of human beings to 2-propanol may occur through
inhalation during manufacture, processing, and both occupational
and household use. Average concentrations of up to 35 mg/m3 in the
ambient urban air and up to 500 mg/m3 in the occupational
environment have been measured. Concentrations between 0.2 and 325
mg/litre have been found in non-alcoholic beverages, and between 50
and 3000 mg/kg in foods (section 5).
Exposure of the general public to a potentially lethal level
may result from accidental or intentional ingestion and children
may be exposed when sponged with 2-propanol preparations (rubbing
alcohol) (sections 6 and 9).
10.1.2 Health effects
2-Propanol is rapidly absorbed after inhalation or ingestion
and distributed throughout the body as such, sulfated, or as its
metabolite, acetone (section 6). The possibility of dermal
absorption should not be neglected.
The acute toxicity of 2-propanol for animals is low (based on
lethality estimates) whether exposure occurs via the dermal, oral,
or respiratory route.
In man, the most likely acute effects of exposure to high
levels of 2-propanol by ingestion or inhalation are alcoholic
intoxication and narcosis. The results of animal studies indicate
that 2-propanol is approximately twice as intoxicating as ethanol
with an oral ED50 for rabbits of 2280 mg/kg and a threshold for
ataxia in rats of 1106 mg/kg intraperitoneally (section 8.3). Oral
LD50 values in various species vary between 4475 and 7990 mg/kg and
inhalation LC50 values between 50 000 and 70 000 mg/m3 (section
8.1.1).
Because ethanol retards the elimination of 2-propanol and is
also a CNS depressant, interaction between ethanol and propanol may
be expected to increase the CNS effects of either agent. The
majority of acute intoxication cases involved ingestion by known
alcoholics. Febrile children have experienced life-threatening
intoxications when treated by skin sponging with 2-propanol. In
these cases, skin absorption may also be an important route of
exposure in addition to inhalation (section 9.1.1).
Exposure-effect data on human beings in an acute overexposure
situation are scarce and show great variation. The major effects
are gastritis, depression of the central nervous system with
hypothermia and respiratory depression, and hypotension (section
9.1.1).
In rabbits, 2-propanol did not irritate the skin but did
irritate the eyes when 0.1 ml undiluted compound was applied
(section 8.1.3). Care is required in the use of 2-propanol as a
disinfectant on premature babies as it may cause severe skin
irritation following prolonged contact.
No adequate animal studies are available to make an evaluation
of the human health risks associated with repeated exposure to
2-propanol. However, 2 studies in rats with inhalation (500 mg/m3,
4 h/day, 5 days per week, for 4 months) or oral (600 - 3900 mg/kg
in drinking-water) exposure suggest that exposure to 2-propanol at
some of the very high occupational exposures reported should be
avoided (section 8.2).
2-Propanol administered in the drinking-water has been tested
for reproductive effects in a number of studies with conflicting
results. In one study, impaired neonatal survival was reported
after 6 months exposure of female rats to 0.18 mg/kg per day. In a
multi-generation study there were no adverse effects at drinking-
water doses as high as 1470 mg/kg per day. In a teratology study,
drinking-water doses of 252 and 1008 mg/kg per day produced
developmental toxicity, but this was not related to maternal
toxicity. Inhalation exposure of pregnant rats to 2-propanol
provided a LOEL of 18 327 mg/m3 (7450 ppm) and a NOEL of 9001 mg/m3
(3659 ppm) for maternal toxicity. In the same study, 9001 mg/m3
(3659 ppm) was a LOEL for developmental toxicity, with no
demonstration of a NOEL (section 8.6). These concentrations are
higher than those likely to be encountered under conditions of
human exposure.
2-Propanol was negative in a bacterial spot test and in a test
for sister chromatid exchange in mammalian cells in vitro. It
induced mitotic aberrations in the bone marrow of rats. Although
these findings suggest that the substance has no genotoxic
potential, no adequate assessment of mutagenicity can be made on
the basis of the limited data available. An in vitro test said to
predict promotional activity was negative (section 8.7).
The data available are inadequate to assess the carcinogenicity
of 2-propanol in experimental animals (section 8.8). There are no
data to assess the carcinogenicity of 2-propanol itself in human
beings. There are adequate data to indicate that the strong acid
process for the production of 2-propanol is causally associated
with the induction of paranasal sinus cancer in human beings,
probably due to exposure to the intermediate, di-2-propyl sulfate,
an alkylating agent and not to 2-propanol itself (section 9.2.1).
2-Propanol is shown to potentiate the hepatic toxicity of
halocarbons, such as carbon tetrachloride. Therefore simultaneous
exposure to 2-propanol and halocarbons should be avoided (section
9.2.2).
The Task Group considers it unlikely that 2-propanol will pose
a serious health risk to the general population under exposure
conditions likely to be encountered.
10.2 Evaluation of Effects on the Environment
By reacting with hydroxyl radicals and through rain-out,
2-propanol will disappear rapidly from the atmosphere, with a
residence time of less than 2.5 days (section 4.2). Hydrolysis and
photolysis are not important in the removal of 2-propanol from
water and soil but removal occurs quite rapidly by aerobic and
anaerobic biodegradation, especially after adaptation of initially
seeded microorganisms (section 4.3.1). Adsorption of 2-propanol on
soil particles is poor but it should be mobile in soil and it has
been shown to increase the permeability of soil to some aromatic
hydrocarbons. In view of the physical properties of 2-propanol,
its potential for bioaccumulation is low (section 4.3.2).
Toxicity in aquatic organisms was observed at levels ranging
from 104 mg/litre for one protozoan to over 50 000 mg/litre for
Tubifex worms. Insects and plant seeds were only affected at
concentrations above 2000 mg/litre (section 7).
On the basis of these data, it can be concluded that, except in
cases of accident and inappropriate disposal, 2-propanol does not
present a risk to naturally occurring organisms at concentrations
that usually occur in the environment.
11. RECOMMENDATIONS
1. 2-Propanol has not shown mutagenic potential in the small number
of assays performed. A full array of modern genotoxicity tests
should be completed.
2. Several studies of the carcinogenic activity of 2-propanol have
been published but all are seriously flawed and cannot be used
to evaluate the potential carcinogenicity of 2-propanol. The
desirability of a carcinogenesis bioassay of 2-propanol should
be considered based on the outcome of genotoxicity tests.
3. Inhalation exposure to overtly toxic concentrations of
2-propanol produced reproductive and developmental toxicity.
Additionally, the data available from drinking-water studies
are conflicting. In view of the potential for environmental
and drinking-water contamination, reproductive and
developmental toxicity should be conducted using oral dosing.
4. Epidemiological studies including precise exposure data would
assist in an assessment of the occupational hazards from
2-propanol.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
An evaluation of the carcinogenicity of 2-Propanol by the
International Agency for Research on Cancera was reported as
follows:
"A. Evidence for carcinogenicity to humans (sufficient for the
manufacture of isopropyl alcohol by the strong-acid process;
inadequate for isopropyl alcohol and isopropyl oils).
An increased incidence of cancer of the paranasal sinuses was
observed in workers at factories where isopropyl alcohol was
manufactured by the strong-acid process. The risk for
laryngeal cancer may also have been elevated in these workers.
It is unclear whether the cancer risk is due to the presence of
diisopropyl sulfate, which is an intermediate in the process,
to isopropyl oils, which are formed as by-products, or to other
factors, such as sulfuric acid. Epidemiological data
concerning the manufacture of isopropyl alcohol by the weak-
acid process are insufficient for an evaluation of
carcinogenicity."
"B. Evidence for carcinogenicity to animals (inadequate for
isopropyl alcohol and isopropyl oils).
Isopropyl oils, formed during the manufacture of isopropyl
alcohol by both the strong-acid and weak-acid processes, were
tested inadequately in mice by inhalation, skin application and
subcutaneous administration. Isopropyl oils formed during the
strong-acid process were also tested inadequately in dogs by
inhalation and instillation into the sinuses.
The available data on isopropyl alcohol were inadequate for
evaluation."
"C. Other relevant data
No data were available to the Working Group."
-------------------------------------------------------------------
a International Agency for Research on Cancer, Overall Evaluations
of Carcinogenicity: An updating of IARC Monographs volumes 1 to
42. Lyon, France, 1987 (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Supplement 7)
REFERENCES
1. ABSHAGEN, U. & RIETBROCK, N. (1969) [Elimination of
2-propanol in dogs and rats.] Naunyn-Schmiedebergs Arch.
Pharmakol. exp. Pathol., 264: 110-118 (in German).
2. ABSHAGEN, U. & RIETBROCK, N. (1970) [The mechanism of the
2-propanol oxidation.] Naunyn-Schmiedebergs Arch. Pharmakol.
exp. Pathol., 265: 411-424 (in German).
3. ACKMAN, R.G., HINLEY, H.J., & POWER, H.E. (1967)
Determination of isopropyl alcohol in fish protein
concentrate by solvent extraction and gas-liquid
chromatography. J. Fish. Res. Board Can., 24: 1521-1529.
4. ADELSON, L. (1962) Fatal intoxication with isopropyl
alcohol (rubbing alcohol). Am J. clin. Pathol., 38: 144-151.
5. AGARWAL, S.K. (1979) Non-acidotic acetonemia: a syndrome
due to isopropyl alcohol intoxication. J. Med. Soc. New
Jersey, 76: 914-916.
6. AGOSTINI, C. (1982) Effects of various inhibitors on lipid
peroxidation by homogenates of normal, regenerating and
hepatomous rat liver, by liver slices and by hepatoma cells.
Med. Biol. Environ., 10: 3-15.
7. AHAMED, A. & MATCHES, J.R. (1983) Alcohol production by
fish spoilage bacteria. J. food Prot., 46: 1055-1059.
8. AKHREM, A.A., POPOVA, E.M., & METELITSA, D.I. (1978)
[Interaction of aliphatic alcohols with cytochrome P-450
from rat liver microsomes.] Biokhimiya (Mosk), 43: 1485-1491
(in Russian).
9. ALDERSON, M.R. & RATTAN, N.S. (1980) Mortality of workers
on an isopropyl alcohol plant and two MEK dewaxing plants.
Br. J. ind. Med., 37: 85-89.
10. ALEKPEROV, I.I. & GUSEINOV, V.G. (1980) Toxicological
characteristics of isopropanol as an industrial poison, All
Union Foundation Conference on Toxicology, Moscow, 25-27
November 1980, p. 33.
11. ALEXANDER, C.B., MCBAY, A.J., & HUDSON, R.P. (1982)
Isopropanol and isopropanol deaths: ten years' experience.
J. forensic Sci., 27: 541-548.
12. ANDERS, M.W. & HARRIS, R.N. (1981) Effect of 2-propanol
treatment on carbon tetrachloride metabolism and toxicity.
Adv. exp. Med. Biol., 136A: 591-602.
13. ANDERSON, S.M. & MCDONALD, J.F. (1981) Effect of
environmental alcohol on in vivo properties of Drosophila
alcohol dehydrogenase. Biochem. Genet., 19: 421-430.
14. ANON. (1987) Chemical market profile: isopropanol. Chem.
Market. Rep., 31 August: 46.
15. ANTONOVA, V.I. & SALMINA, Z.A. (1978) [The maximum
permissible concentration of isopropyl alcohol in water
bodies with due regard for its action on the gonads and the
progeny.] Gig. i Sanit., 43: 8-11 (in Russian).
16. ARDITI, M. & KILLMER, M.S. (1987) Coma following use of
rubbing alcohol for fever control. Am. J. DC, 141: 237-238.
17. ARISTOV, V.N. (1982) [Combined effect of toluene, isopropanol,
and sulfur dioxide in conditions of petrochemical production.]
Gig. Tr. Prof. Zabol., ISS9: 5-9 (in Russian).
18. ARISTOV, V.N., REDKIN, Y.V., BRUSKIN, Z.Z., & OGLEZNEV, G.A.
(1981) [Experimental data on the mutagenous effects of
toluene, isopropanol, and sulfur dioxide.] Gig. Tr. Prof.
Zabol., 25: 33-36 (in Russian).
19. BAIKOV, B.K., GORLOVA, O.E., GUSEV, M.I., NOVIKOV, Y.V.,
YUDINA, T.V., & SERGEEV, A.N. (1974) [Hygienic
standardization of the daily average maximal permissible
concentrations of propyl and isopropyl alcohols in the
atmosphere.] Gig. i Sanit., 4: 6-13 (in Russian).
20. BALD, E. & MAZURKIEWICZ, B. (1980) Analytical utility of
2-halopyridinium salts. Part III. Paper electrophoretic
characterization of alcohols as 2-alkoxy-1-methylpyridinium
p-toluenesulfonates. Chromatographia, 13: 295-297.
21. BEAUGE, F., CLEMENT, M., RENAUD, G., NORDMANN, R., &
NORDMANN, J. (1975) Action de l'isopropanol sur le
métabolisme lipidique chez le rat: études complémentaires
sur les méchanismes impliqués dans l'accumulation hépatique
des triacylglycérides. Arch. int. Physiol. Biochim., 83:
573-591.
22. BEAUGE, F., CLEMENT, M., NORDMANN, J., & NORDMANN, R.
(1977) Etudes comparatives des actions de l'acétone et de
l'isopropanol sur le métabolisme lipidique chez le rat.
Arch. int. Physiol. Biochim., 85: 931-940.
23. BEAUGE, F., CLEMENT, M., NORDMANN, J., & NORDMANN, R.
(1979) Comparative effects of ethanol, n-propanol and
isopropanol on lipid disposal by rat liver. Chem.-biol.
Interact., 26: 155-166.
24. BEAUGE, F., FLEURET, C., BARIN, F., & NORDMANN, R. (1984)
Brain membrane disordering after acute in vivo administration
of ethanol, isopropanol or t-butanol in rats. Biochem.
Pharmacol., 33: 3591-3595.
25. BESS, F.D. & CONWAY, R.A. (1966) Aerated stabilization of
synthetic organic chemical wastes. J. Water Pollut. Control
Fed., 38: 939-956.
26. BLACKMAN, R.A.A. (1974) Toxicity of oil-sinking agents.
Mar. Pollut. Bull., 5: 116-118.
27. BONTE, W., RUDELL, E., SPRUNG, R., FRAUENRATH, C., BLANKE,
E., KUPILAS, G., WOCHNIK, J. & ZAH, G. (1981a)
[Experimental investigations concerning the analytical
detection of small doses of higher aliphatic alcohols in
blood in man.] Blutalkohol, 18: 399-411 (in German).
28. BONTE, W., SPRUNG, R., RUDELL, E., FRAUENRATH, C., BLANKE,
E., KUPILAS, G., WOCHNIK, J., & ZAH, G. (1981b)
[Experimental investigations concerning the analytical
detection of small doses of higher aliphatic alcohols in
human urine.] Blutalkohol, 18: 412-426 (in German).
29. BORENFREUND, E. & BORRERO, O. (1984) In vitro cytotoxicity
assays. Potential alternatives to the Draize ocular allergy
test. Cell Biol. Toxicol., 1: 55-65.
30. BORENFREUND, E. & SHOPSIS, C. (1985) Toxicity monitored
with a correlated set of cell-culture assays. Xenobiotica,
15: 705-711.
31. BOSSET, J.O. & LIARDON, R. (1984) The aroma composition of
Swiss Gruyere cheese. II. The neutral volatile components.
Lebensm.-Wiss. Technol., 17: 359-362.
32. BOUGHTON, L.L. (1944) The relative toxicity of ethyl and
isopropyl alcohols as determined by long term rat feeding
and external application. Am. pharm. Assoc., 33: 111-113.
33. BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979a) BOD and
COD of some petrochemicals. Water Res., 13: 627-630.
34. BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979b) The acute
toxicity of some petrochemicals to goldfish. Water Res., 13:
623-626.
35. BRINGMANN, G. (1975) [Determination of the harmful
biological action of water-endangering substances through
inhibition of cell multiplication in the blue alga
Microcystis.] Ges.-Ing., 96: 238-241 (in German).
36. BRINGMANN, G. (1978) [Determination of the harmful
biological action of water-endangering substances on
protozoa. I. Bacteria fed flagellates.] Z. Wasser-Abwasser
Forsch., 11: 210-215 (in German).
37. BRINGMANN, G. & KUHN, R. (1977) [Limiting values of the
harmful action of water-endangering substances on bacteria
(Pseudomonas putida) and green algae (Scenedesmus
quadricauda) in the cell multiplication inhibition test.] Z.
Wasser-Abwasser Forsch., 10: 87-98 (in German).
38. BRINGMANN, G. & KUHN, R. (1980) [Determination of the
harmful biological action of water-endangering substances on
protozoa. II. Bacteria fed ciliates.] Z. Wasser-Abwasser
Forsch., 13: 26-31 (in German).
39. BRINGMANN, G. & KUHN, R. (1982) [Findings regarding the
harmful action of water-endangering substances on Daphnia
magna in a further developed standardised test.] Z. Wasser-
Abwasser Forsch., 15: 1-16 (in German).
40. BRINGMANN, G., KUHN, R. & WINTER, A. (1980) [Determination
of the harmful biological action of water-endangering
substances on protozoa. III. Saprozoic flagellates.] Z.
Wasser-Abwasser Forsch., 13: 170-173 (in German).
41. BRUGNONE, F., PERBELLINI, L., APOSTOLI, P., BELLOMI, M., &
CARETTA, D. (1983) Isopropanol exposure: environmental and
biological monitoring in a printing works. Br. J. ind. Med.,
40: 160-168.
42. BUFFONI, F., SANTONI, G., ALBANESE, V., & DOLARA, P. (1983)
Urinary mercapturic acid in chemical workers and in control
subjects. J. appl. Toxicol., 3: 63-65.
43. BULICH, A.A. (1979) Use of luminescent bacteria for
determining toxicity in aquatic environments. In: Marking,
L.L. & Kimerle, R.A., ed. Aquatic toxicology, American
Society for Testing and Materials, pp. 98-106 (ASTM STP 667).
44. BULICH, A.A., GREENE, M.W., & ISENBERG, D.L. (1981)
Reliability of the bacterial luminescence assay for
determination of the toxicity of pure compounds and complex
effluents. In: Branson, D.R. & Dickson, K.L., ed. Aquatic
toxicology and hazard assessment. Fourth Conference,
American Society for Testing and Materials, pp. 338-347
(ASTM STP 737).
45. CALHOUN, M.J. & DELLAMONICA, E.S. (1974) Determination of
2-propanol residue in some fruits dewaxed with alcohol
vapors. J. Assoc. Off. Anal. Chem., 57: 1342-1345.
46. CARTER, W.P.L., DARNALL, K.R., GRAHAM, R.A., WINER, A.M., &
PITTS, J.N. (1979) Reactions of C2 and C4 alpha-hydroxy
radicals with oxygen. J. phys. Chem., 83: 2305-2311.
47. CEC (1982) Propan-2-ol: chemico-physical data, toxicity
data, environmental occurrence, and permissible levels. In:
Report of the Scientific Committee for Food on Extraction
Solvents, Brussels, Belgium, Commission of the European
Communities, Directorate General for Internal Market and
Industrial Affairs, pp. 46-72.
48. CEDERBAUM, A.I., QURESHI, A., & MESSENGER, P. (1981)
Oxidation of isopropanol by rat liver microsomes. Possible
role of hydroxyl radicals. Biochem. Pharmacol., 30: 825-831.
49. CHADOEUF-HANNEL, R. & TAYLORSON, R.B. (1985) Anaesthetic
stimulation of Amaranthus albus seed germination:
interaction with phytochrome. Physiol. Plant, 65: 451-454.
50. CHARBONNEAU, M., IJIMA, M., COTE, M.G., & PLAA, G.L. (1985)
Temporal analysis of rat liver injury following potentiation
of carbon tetrachloride hepatotoxicity with ketonic or
ketogenic compounds. Toxicology, 35: 95-112.
51. CHARRETON, M. (1981) Estimation des rejets de vapeurs de
solvants au voisinage d'une usine de peintures. Double
Liaison - Chimie des Peintures, 28: 233-241.
52. CHECKOWAY, H., WILCOSKY, T., WOLF, P., & TYROLER, H. (1984)
An evaluation of associations of leukemia and rubber
industry solvent exposures. Am. J. ind. Med., 5: 239-249.
53. CHEN, T.-H., KAVANAGH, T.J., CHANG, C.C., & TROSKO, J.E.
(1984) Inhibition of metabolic cooperation in Chinese
hamster V79 cells by various organic solvents and simple
compounds. Cell Biol. Toxicol., 1: 155-171.
54. CHEN, W.-S. & PLAPP, B.V. (1980) Kinetics and control of
alcohol oxidation in rats. Adv. exp. Med. Biol., 132:
543-549.
55. CHOU, W.L., SPEECE, R.E., & SIDDIQI, R.H. (1978)
Acclimation and degradation of petrochemical wastewater
components by methane fermentation. Biotechnol. Bioeng.
Symp., 8: 391-414.
56. CHVAPIL, M., ZAHRADNIK, R., & CMUCHALOVA, B. (1962)
Influence of alcohols and potassium salts of xanthogenic
acids on various biological objects. Arch. int. Pharmacodyn.
Ther., 135: 330-343.
57. COHEN, G.M. & MANNERING, G.J. (1973) Involvement of a
hydrophobic site in the inhibition of the microsomal
p-hydroxylation of aniline by alcohols. Mol. Pharmacol., 9:
383-397.
58. COLEMAN, R.L., LUND, E.D., & SHAW, P.E. (1972) Analysis of
grapefruit essence and aroma oils. J. agric. food Chem., 20:
100-103.
59. CORBETT, J. & MEIER, G. (1968) Suicide attempted by rectal
administration of drug. J. Am. Med. Assoc., 206: 2320-2321.
60. CORNISH, H.H. & ADEFUIN, J. (1967) Potentiation of carbon
tetrachloride toxicity by aliphatic alcohols. Arch. environ.
Health, 14: 447-449.
61. CUPITT, L.T. (1980) Fate of toxic and hazardous materials
in the air environment, Research Triangle Park, North
Carolina, US Environmental Protection Agency, Environmental
Sciences Laboratory, Office of Research and Development (EPA
600/3-80-084, PB 80-221948).
62. CURTIS, C., LIMA, A., LOZANO, S.J., & VEITH, G.D. (1982)
Evaluation of a bacterial bioluminiscence bioassay as a
method for predicting acute toxicity of organic chemicals to
fish. In: Pearson, J.G., Foster, R.B., & Bishop, W.E., ed.
Aquatic Toxicology and Hazard Assessment. Fifth Conference,
American Society for Testing and Materials, pp. 170-178
(ASTM STP 766).
63. DAINTY, R.H., EDWARDS, R.A., & HIBBARD, C.M. (1984)
Volatile compounds associated with the aerobic growth of
some Pseudomonas species on beef. J. appl. Bacteriol., 57:
75-81.
64. DALZIEL, K. & DICKINSON, F.M. (1966) The kinetics and
mechanism of liver alcohol dehydrogenase with primary and
secondary alcohols as substrates. Biochem. J., 100: 34-46.
65. DANIEL, D.R., MCANALLEY, B.H., & GARRIOTT, J.C. (1981)
Isopropyl alcohol metabolism after acute intoxication in
humans. J. anal. Toxicol., 5: 110-112.
66. DAVID, J. & BOCQUET, C. (1976) Compared toxicities of
different alcohols for two Drosophila sibling species: D.
melanogaster and D. simulans. Comp. Biochem. Physiol., 54C:
71-74.
67. DAVIS, D.G., WERGIN, W.P., & DUSBABEK, K.E. (1978) Effects
of organic solvents on growth and ultrastructure of plant
cell suspensions. Pestic. Biochem. Physiol., 8: 84-97.
68. DAVIS, P.L., DAL CORTIVO, L.A., & MATURO, J. (1984)
Endogenous isopropanol: forensic and biochemical
implications. J. anal. Toxicol., 8: 209-212.
69. DE CEAURRIZ, J.C., MICILLINO, J.C., BONNET, P., & GUENIER,
J.P. (1981) Sensory irritation caused by various industrial
airborne chemicals. Toxicol. Lett., 9: 137-143.
70. DEL ROSARIO, R., DE LUMEN, B.O., HABU, T., FLATH, R.A., MON,
T.R., & TERANISHI, R. (1984) Comparison of headspace
volatiles from winged beans and soybeans. J. agric. food
Chem., 32: 1011-1015.
71. DENG, J.-F., WANG, J.-D., SHIH, T.-S., & LAN, F.-L. (1987)
Outbreak of carbon tetrachloride poisoning in a color
printing factory related to the use of isopropyl alcohol and
an air conditioning system in Taiwan. Am. J. ind. Med., 12:
11-19.
72. DGEP (1987) Review of literature data on 2-propanol,
Leidschendam, Netherlands, Directorate-General of
Environmental Protection, Ministry of Housing, Physical
Planning and Environment.
73. DIVINCENZO, G.D. & KRASAVAGE, W.J. (1974) Serum ornithine
carbamyl transferase as a liver response test for exposure
to organic solvents. Am. Ind. Hyg. Assoc. J., 35: 21-29.
74. DORIGAN, J., FULLER, B., & DUFFY, R. (1976) Scoring of
organic air pollutants. Chemistry, production and toxicity
of selected synthetic organic chemicals, The MITRE
Corporation (MITRE Technical Report MTR-7248, Rev. 1,
Appendix III).
75. DUA, S.L. (1974) Peritoneal dialysis for isopropyl alcohol
poisoning. J. Am. Med. Assoc., 230: 35.
76. DUVEL, W.A. & HELFGOTT, T. (1975) Removal of wastewater
organics by reverse osmosis. J. Water Pollut. Control Fed.,
47: 57-65.
77. EGBERT, A.M., REED, J.S., POWELL, B.J., LISKOW, B.I., &
LIESE, B.S. (1985) Alcoholics who drink mouthwash: the
spectrum of nonbeverage alcohol use. J. Stud. Alcohol, 46:
473-481.
78. EGOROV, Y.L. (1966) Vision of workers engaged in the
production of synthetic fatty acids and issues relative to
setting hygienic standards for the alcohol content in the
air. Gig. Tr. Prof. Zabol., 7: 33-38.
79. ENTERLINE, P.E. (1982) Importance of sequential exposure in
the production of epichlorohydrin and isopropanol. Ann. N.Y.
Acad. Sci., 381: 344-349.
80. FANG, H.H.P & CHIAN, E.S.K. (1976) Reverse osmosis
separation of polar organic compounds in aqueous solution.
Environ. Sci. Technol., 10: 364-369.
81. FERNANDEZ, F. & QUIGLEY, R.M. (1985) Hydraulic conductivity
of natural clays permeated with simple liquid hydrocarbons.
Can. Geotech. J., 22: 205-214.
82. FLORIN, I., RUTBERG, L., CURVALL, M., & ENZELL, C.R. (1980)
Screening of tobacco smoke constituents for mutagenicity
using the Ames test. Toxicology, 18: 219-232.
83. FOLLAND, D.S., SCHAFFNER, W., GINN, E., CROFFORD, O.B., &
MCMURRAY, D.R. (1976) Carbon tetrachloride toxicity
potentiated by isopropyl alcohol. Investigation of an
industrial outbreak. J. Am. Med. Assoc., 236: 1853-1856.
84. FORE, S.P., RAYNER, E.T., & DUPUY, H.P. (1971)
Determination of residual solvent in oilseed meals and
flours. III. Isopropanol. J. Am. Oil Chem. Soc., 48:
140-142.
85. FORSANDER, O.A. (1967) Influence of some aliphatic
alcohols on the metabolism of rat liver slices. Biochem. J.,
105: 93-97.
86. FREGERT, S., GROTH, O., HJORTH, N., MAGNUSSON, B., RORSMAN,
H., & OVRUM, P. (1969) Alcohol dermatitis. Acta
dermatovenereol., 49: 493-497.
87. FREGERT, S., GROTH, O., GRUVBERGER, B., MAGNUSSON, B.,
MOBACKEN, H., & RORSMAN, H. (1971) Hypersensitivity to
secondary alcohols. Acta dermatovenereol., 51: 271-272.
88. FREIREICH, A.W., CINQUE, T.J., XANTHAKY, G., & LANDAU, D.
(1967) Hemodialysis for isopropanol poisoning. New Engl. J.
Med., 277: 699-700.
89. GADSDEN, R.H., MELETTE, R.R., & MILLER, W.C. (1958) Scrap
iron intoxication. J. Am. Med. Assoc., 168: 1220-1224.
90. GAILLARD, D. & DERACHE, R. (1966) Action de quelques
alcools aliphatiques sur la mobilisation de différentes
fractions lipidiques chez la rate. Food cosmet. Toxicol., 4:
515-520.
91. GARRISON, R.F. (1953) Acute poisoning from use of isopropyl
alcohol in tepid sponging. J. Am. Med. Assoc., 152: 317-318.
92. GEORGE, H.A., JOHNSON, J.L., MOORE, W.E.C., HOLDEMAN, L.V.,
& CHEN, J.S. (1983) Acetone, isopropanol, and butanol
production by Clostridium beijerinckii (syn. Clostridium
butylicum) and Clostridium aurantibutyricum. Appl. environ.
Microbiol., 45: 1160-1163.
93. GEORGE, V., SHARMA, S.D., TRIPATHI, A.K., & ABRAHAM, S.P.
(1985) Flavour components of some edible fungi from
Kashmir. I. Pafai J., 7: 27-30.
94. GERARDE, H.W., AHLSTROM, D.B., & LINDEN, N.J. (1966) The
aspiration hazard and toxicity of a homologous series of
alcohols. Arch. environ. Health, 13: 457-461.
95. GERHOLD, R.M. & MALANEY, G.W. (1966) Structural
determinants in the oxidation of aliphatic compounds by
activated sludge. J. Water Pollut. Control Fed., 38:
562-579.
96. GILLETTE, L.A., MILLER, D.L., & REDMAN, H.E. (1952)
Appraisal of a chemical waste problem by fish toxicity
tests. Sewage ind. Waste, 24: 1397-1401.
97. GIUSTI, D.M., CONWAY, R.A., & LAWSON, C.T. (1974) Activated
carbon adsorption of petrochemicals. J. Water Pollut.
Control Fed., 46: 947-965.
98. GORLOVA, O.E. (1970) [Hygienic assessment of isopropyl
alcohol as an atmospheric pollutant.] Gig. i Sanit., 35:
9-14 (in Russian).
99. GOTZ-SCHMIDT, E.-M. & SCHREIER, P. (1986) Neutral volatiles
from blended endive ( Cichorium endivia L.). J. agric. food
Chem., 34: 212-215.
100. GRIFFITH, J.F., NIXON, G.A., BRUCE, R.D., REER, P.J., &
BANNAN, E.A. (1980) Dose-response studies with chemical
irritants in the albino rabbit eye as a basis for selecting
optimum testing conditions for predicting hazard to the human
eye. Toxicol. appl. Pharmacol., 55: 501-513.
101. GUNTER, B. (1982) Health hazard evaluation: Jeppesen
Sanderson Inc., Cincinnati, Ohio, US National Institute for
Occupational Safety and Health (HETA 81-261-1085, PB 83-
201749).
102. GUNTER, B.J., LIGO, R.L., & RUHE, R.L. (1976) Health hazard
evaluation determination, Steiger Tractor Inc., Cincinnati,
Ohio, US National Institute for Occupational Safety and
Health (NIOSH-TR-HHE-75-30-266, PB 273711).
103. GUSEINOV, V.G. (1985) [Toxicological hygienic characteristics
of isopropyl alcohol.] Gig. Tr. Prof. Zabol., (7): 60-62
(in Russian).
104. HABU, T., FLATH, R.A., MON, T.R., & MORTON, J.F. (1985)
Volatile components of Rooibos tea (Asphalathus linearis). J.
agric. food Chem., 33: 249-254.
105. HADDOCK, N.F. & WILKIN J.K. (1982) Cutaneous reactions to
lower aliphatic alcohols before and during disulfiram
therapy. Arch. Dermatol., 118: 157-159.
106. HALLIDAY, M.M. & CARTER, K.B. (1978) A chemical adsorption
system for the sampling of gaseous organic pollutants in
operating theatre atmospheres. Br. J. Anaesth., 50:
1013-1018.
107. HANSSEN, H.-P., SPRECHER, E., & KLINGENBERG, A. (1984)
Accumulation of volatile flavour compounds in liquid cultures
of Kluyveromyces lactis strains. Z. Naturforsch., 39c:
1030-1033.
108. HAWLEY, P.C. & FALKO, J.M. (1982) "Pseudo" renal failure
after isopropyl alcohol intoxication. South. med. J., 75:
630-631.
109. HELLMAN, T.M. & SMALL, F.H. (1974) Characterization of the
odor properties of 101 petrochemicals using sensory methods.
J. Air Pollut. Control Assoc., 24: 979-982.
110. HERMENS, J. CANTON, H., JANSSEN, P., & DE JONG, R. (1984)
Quantitative structure-activity relationships and toxicity
studies of mixtures of chemicals with anaesthetic potency:
acute lethal and sublethal toxicity to Daphnia magna. Aquat.
Toxicol., 5: 143-154.
111. HERMENS, J., BROEKHUYZEN, E., CANTON, H., & WEGMAN, R.
(1985) Quantitative structure-activity relationships and
mixture toxicity studies of mixtures of alcohols and
chlorohydrocarbons: effects on growth of Daphnia magna.
Aquat. Toxicol., 6: 209-217.
112. HO, Y.H., SCHWARZE, I., & SOEHRING, K. (1970) [The influence
of low aliphatic alcohols on the chloral hydrate metabolism
in rat liver sections.] Arzneim.-Forsch., 20: 1507-1509 (in
German).
113. HODGE, H.C. & STERNER, J.H. (1943) Am. Ind. Hyg. Assoc. J.,
10: 93.
114. HOIGNE, J. & BADER, H. (1983) Rate constants of reactions of
ozone with organic and inorganic compounds in water. I. Non-
dissociating organic compounds. Water Res., 17: 173-183.
115. HORWITZ, W., ed. (1975) Official methods of analysis of the
Association of Official Analytical Chemists, 12th ed.,
Washington DC, Association of Official Analytical Chemists,
pp. 328, 337-338, 343, 656-658.
116. HORWOOD, J.F., LLOYD, G.T., & STARK, W. (1981) Some flavour
components of feta cheese. Aust. J. dairy Technol., 36:
34-37.
117. HOU, C.T., PATEL, R.N., LASKIN, A.I., MARCZAK, I., & BARNABE,
N. (1981) Microbial oxidation of gaseous hydrocarbons:
production of alcohols and methyl ketones from their
corresponding n-alkanes by methylotrophic bacteria. Can. J.
Microbiol., 27: 107-115.
118. HOVIOUS, J.C., CONWAY, R.A., & GANZE, C.W. (1973) Anaerobic
lagoon pretreatment of petrochemical wastes. J. Water Pollut.
Control Fed., 45: 71-84.
119. HUEPER, W.C. (1966) Occupational and environmental cancers
of the respiratory system. Recent results. Cancer Res., 3:
105-107.
120. IARC (1977) Some fumigants, the herbicides 2,4-D and 2,4,5-
T, chlorinated dibenzodioxins and miscellaneous industrial
chemicals, Lyons, International Agency for Research on
Cancer, pp. 223-243 (Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Man, 15).
121. IDOTA, S. (1985) [Studies on isopropanol metabolism and
poisoning.] J. Nihon Univ. Med. Assoc., 44: 39-47 (in
Japanese).
122. IRPTC (1987) Data profile on 2-propanol, Geneva,
Switzerland, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
123. JARKE, F.H., DRAVNIEKS, A., & GORDON, S.M. (1981) Organic
contaminants in indoor air and their relation to outdoor
contaminants. ASHRAE Trans., 87: 153-166.
124. JENSEN, O. (1981) Contact allergy to propylene oxide and
isopropyl alcohol in a skin disinfectant swab. Contact
Dermat., 7: 148-150.
125. JONES, C.J. & MCGUGAN, P.J. (1977/1978) An investigation of
the evaporation of some volatile solvents from domestic
waste. J. hazard. Mater., 2: 235-251.
126. JONSSON, A., PERSSON, K.A. & GRIGORIADIS, V. (1985)
Measurements of some low molecular-weight oxygenated,
aromatic, and chlorinated hydrocarbons in ambient air and in
vehicle emissions. Environ. Int., 11: 383-392.
127. JUHNKE, I. & LUDEMANN, D. (1978) [Results of examination of
200 chemical compounds for acute toxicity towards fish by
means of the golden orfe test.] Z. Wasser-Abwasser Forsch.,
11: 161-164 (in German).
128. JUNCOS, L. & TAGUCHI, J.T. (1968) Isopropyl alcohol
intoxication. Report of a case associated with myopathy,
renal failure, and hemolytic anemia. J. Am. Med. Assoc., 204:
186-188.
129. KAMIL, I.A., SMITH, J.N., & WILLIAMS, R.T. (1953) The
metabolism of aliphatic alcohols. The glucuronic acid
conjugation of acyclic aliphatic alcohols. Biochem. J., 53:
129-136.
130. KANE, L.E., DOMBROSKE, R., & ALARIE, Y. (1980) Evaluation of
sensory irritation from some common industrial solvents. Am.
Ind. Hyg. Assoc. J., 41: 451-455.
131. KELNER, M. & BAILEY, D.N. (1983) Isopropanol ingestion:
interpretation of blood concentrations and clinical findings.
J. Toxicol.-clin. Toxicol., 20: 497-507.
132. KEMAL, H. (1927) [Contribution to investigations into the
fate of isopropyl alcohol in the human body.] Biochem. Z.,
187: 461-466 (in German).
133. KHAN, J.S., WILSON, M.C., & TAYLOR, T.V. (1979) A case of
dettol addiction. Br. med. J., 1: 791-792.
134. KIMURA, E.T., EBERT, D.M., & DODGE, P.W. (1971) Acute
toxicity and limits of solvent residue for sixteen organic
solvents. Toxicol. appl. Pharmacol., 19: 699-703.
135. KING, L.H., BRADLEY, K.P., & SHIRES, D.L. (1970)
Hemodialysis for isopropyl alcohol poisoning. J. Am. Med.
Assoc., 211: 1855.
136. KINLIN, T.E., MURALIDHARA, R., PITTET, A.O., SANDERSON, A., &
WALRADT, J.P. (1972) Volatile components of roasted
filberts. J. agric. food Chem., 20: 1021-1028.
137. KIRK, R.E. & OTHMER, D.F., ed. (1978-1984) Encyclopedia of
chemical technology, 3rd ed., New York, Wiley Interscience.
138. KLECKA, G.M. & LANDI, L.P. (1985) Evaluation of the OECD
activated sludge, respiration inhibition test. Chemosphere,
14: 1239-1251.
139. KNUTH, M.L. & HOGLUND, M.D. (1984) Quantitative analysis of
68 polar compounds from ten chemical classes by direct
aqueous injection gas chromatography. J. Chromatogr., 285:
153-160.
140. KOMINSKY, J., LOVE, J., & ANDERSON, K. (1982) Health hazard
Evaluation, Tweddle Litho Company,Cincinnati, Ohio, US
National Institute for Occupational Safety and Health (HETA
81-117-1087, PB 83-202390).
141. KONEMANN, H. (1981) Quantitative structure-activity
relationships in fish toxicity studies. Toxicology, 19:
209-221.
142. KONIG, H. & HERMES, M. (1981) [Separation, identification
and estimation of propellant gases and solvents in aerosol
products by gas chromatography.] Chromatographia, 14: 351-354
(in German).
143. KRAMER, V.C., SCHNELL, D.J., & NICKERSON, K.W. (1983)
Relative toxicity of organic solvents to Aedes aegyptilarvae.
J. Invertebr. Pathol., 42: 285-287.
144. KRING, E.V., ANSUL, G.R., HENRY, T.J., MORELLO, J.A., DIXON,
S.W., VASTA, J.F., & HEMINGWAY, R.E. (1984) Evaluation of
the standard NIOSH type charcoal tube sampling method for
organic vapors in air. Am. Ind. Hyg. Assoc. J., 45: 250-259.
145. KRULL, I.S., SWARTZ, M., & DRISCOLL, J.N. (1984)
Derivatizations for improved detection of alcohols by gas
chromatography and photoionization detection. Anal. Lett.,
17(A20): 2369-2384.
146. KUMAI, M., KOIZUMI, A., SAITO, K., SAKURAI, H., INOUE, T.,
TAKEUCHI, Y., HARA, I., OGATA, M., MATSUSHITA, T., & IKEDA,
M. (1983) A nationwide survey on organic solvent components
in various solvent products. Part 2. Heterogeneous products
such as paints, inks, and adhesives. Ind. Health, 21:
185-197.
147. KUMKE, G.W., HALL, J.F., & OEBEN, R.W. (1968) Conversion to
activated sludge at Union Carbide's institute plant. J. Water
Pollut. Control Fed., 40: 1408-1422.
148. KURPPA, K. & HUSMAN, K. (1982) Car painters' exposure to a
mixture of organic solvents. Serum activities of liver
enzymes. Scand. J. Work environ. Health, 8: 137-140.
149. LACOUTURE, P.G., WASON, S., ABRAMS, A., & LOVEJOY, F.H.
(1983) Acute isopropyl alcohol intoxication. Diagnosis and
management. Am. J. Med., 75: 680-686.
150. LAHAM, S., POTVIN, M., & SCHRADER, K. (1979) Microméthode de
dosage simultané de l'alcool isopropylique et de son
metabolite l'acétone. Chemosphere, 2: 79-87.
151. LAHAM, S., POTVIN, M., SCHRADER, K., & MARINO, I. (1980)
Studies on inhalation toxicity of 2-propanol. Drug Chem.
Toxicol., 3: 343-360.
152. LANGVARDT, P.W. & MELCHER, R. (1979) Simultaneous
determination of polar and non-polar solvents in air using a
two-phase desorption from charcoal. Am. Ind. Hyg. Assoc. J.,
40: 1006-1012.
153. LAREGINA, J., BOZZELLI, J.W., HARKOV, R., & GIANTI, S.
(1986) Volatile organic compounds at hazardous waste sites
and a sanitary landfill in New Jersey. An up-to-date review
of the present situation. Environ. Progr., 5: 18-27.
154. LEASURE, C.S., FLEISCHER, M.E., ANDERSON, G.K., & EICEMAN,
G.A. (1986) Photoionization in air with ion mobility
spectrometry using a hydrogen discharge lamp. Anal. Chem.,
58: 2142-2147.
155. LEFFINGWELL, S.S., WAXWEILER, R., ALEXANDER, V., LUDWIG, H.R.
& HALPERIN, W. (1983) Case-control study of gliomas of the
brain among workers employed by a Texas City, Texas chemical
plant. Neuroepidemiology, 2: 179-195.
156. LEGENDRE, M.G. & DUPUY, H.P. (1981) Flavor volatiles as
measured by rapid instrumental techniques, American Chemical
Society, pp. 41-49 (ACS Symposium Series No. 147).
157. LEHMAN, A.J. & CHASE, H.F. (1944) The acute and chronic
toxicity of isopropyl alcohol. J. Lab. clin. Med., 29:
561-567.
158. LEHMAN, A.J., SCHWERMA, H., & RICKARDS, E. (1944) Isopropyl
alcohol: rate of disappearance from the blood stream of dogs
after intravenous and oral administration. J. Pharmacol. exp.
Ther., 82: 196-201.
159. LEHMAN, A.J., SCHWERMA, H., & RICKARDS, E. (1945) Acquired
tolerance in dogs, rate of disappearance from the blood
stream in various species, and effects on successive
generation of rats. J. Pharmacol. exp. Ther., 85: 61-69.
160. LEWIN, G.A., OPPENHEIMER, P.R., & WINGERT, W.A. (1977) Coma
from alcohol sponging. J.A.C.E.P., 6: 165-167.
161. LEWIS, G.D., LAUFMAN, A.K., MCANALLY, B.H., & GARRIOTT, J.C.
(1984) Metabolism of acetone to isopropyl alcohol in rats
and humans. J. forensic Sci., 29: 541-549.
162. LINDSTROM, T.D. & ANDERS, M.W. (1978) Effect of agents known
to alter carbon tetrachloride hepatotoxicity and cytochrome
P-450 levels on carbon tetrachloride-stimulated lipid
peroxidation and ethane expiration in the intact rat.
Biochem. Pharmacol., 27: 563-567.
163. LITOVITZ, T. (1986) The alcohols: ethanol, methanol, iso-
propanol, ethylene glycol. Pediatr. Clin. N. Am., 33:
311-323.
164. LLOYD, A.C., DARNALL, K.R. WINER, A.M., & PITTS, J.N. (1976)
Relative rate constants for the reactions of OH radicals with
isopropyl alcohol, diethyl and di- n-propyl ether at 305 ±
20 °K. Chem. Phys. Lett., 42: 205-209.
165. LORR, N.A., MILLER, K.W., CHUNG, H.R., & YANG, C.S. (1984)
Potentiation of the hepatotoxicity of N-nitrosodimethylamine
by fasting, diabetes, acetone, and isopropanol. Toxicol.
appl. Pharmacol., 73: 423-431.
166. LOVEGREN, N.V., VINNETT, C.H., & ANGELO, A.J.S. (1982) Gas
chromatographic profile of good quality raw peanuts. Peanut
Sci., 9: 93-96,
167. LUDWIG, E. & HAUSEN, B.M. (1977) Sensitivity to isopropyl
alcohol. Contact Dermat., 3: 240-244.
168. LUNDBERG, I. & HAKANSSON, M. (1985) Normal serum activities
of liver enzymes in Swedish paint industry workers with heavy
exposure to organic solvents. Br. J. ind. Med., 42: 596-600.
169. LYON, R.C., MCCOMB, J.A., SCHREURS, J., & GOLDSTEIN, D.B.
(1981) A relationship between alcohol intoxication and the
disordering of brain membranes by a series of short-chain
alcohols. J. Pharmacol. exp. Ther., 218: 669-675.
170. MCCORD, W.M., SWITZER, P.K., & BRILL, H.H. (1948) Isopropyl
alcohol intoxication. South med. J., 41: 639-642.
171. MCCREERY, M.J. & HUNT, W.A. (1978) Physico-chemical
correlates of alcohol intoxication. Neuropharmacology, 17:
451-461.
172. MCFADDEN, S.W. & HADDOW, J.E. (1969) Coma produced by
topical application of isopropanol. Pediatrics, 43: 622-623.
173. MACHYULITE, N.I. (1978) [Hygienic characterization of
conditions of work in production of levomycetin.] Gig. Tr.
Prof. Zabol., 12: 8-12 (in Russian).
174. MCINNES, A. (1973) Skin reaction to isopropyl alcohol. Br.
med. J., 1: 357.
175. MACK, K. (1973) The problem of waste water purification in
the chemical pharmaceutical industry. Prog. Water Technol.,
3: 239-249.
176. MACK, R.B. (1985) Pervasive procrustianism-isopropyl alcohol
intoxication. N.C. med. J., 46: 101-102.
177. MAIZLISH, N.A., LANGOLF, G.D., WHITEHEAD, L.W., FINE, L.J.,
ALBERS, J.W., GOLDBERG, J. & SMITH, P. (1985) Behavioural
evaluation of workers exposed to mixtures of organic
solvents. Br. J. ind. Med., 42: 579-590.
178. MALILA, A. (1978) Intoxicating effects of three aliphatic
alcohols and barbital on two rat strains genetically selected
for their ethanol intake. Pharmacol. Biochem. Behav., 8:
197-201.
179. MARKEL, H. (1982) Health hazard evaluation, Federal
Correctional Institution, Cincinnati, Ohio, US National
Institute for Occupational Safety and Health (HETA 80-119-
1066, PB 83-199398).
180. MARTIN, M. & HAERDI, W. (1982) Determination de composes
volatils toxiques dans le sang et dans l'urine par
chromatographie en phase gazeuse, methode de l'espace de tete
(head-space). Trav. Chim. aliment. Hyg., 73: 212-217.
181. MARTINEZ, T.T., JAEGER, R.W., DECASTRO, F.J., THOMPSON, M.W.,
& HAMILTON, M.F. (1986) A comparison of the absorption and
metabolism of isopropyl alcohol by oral, dermal, and
inhalation routes. Vet. human Toxicol., 28: 233-236.
182. MARZULLI, F.N. & RUGGLES, D.I. (1973) Rabbit eye irritation
test: collaborative study. J. Assoc. Off. Anal. Chem., 56:
905-914.
183. MATSUI, F., LOVERING, E.G., WATSON, J.R., BLACK, D.B., &
SEARS, R.W. (1984) Gas chromatographic method for solvent
residues in drug raw materials. J. pharm. Sci., 73:
1664-1666.
184. MATTSON, V.R., ARTHUR, J.W., & WALBRIDGE, C.T. (1976) Acute
toxicity of selected organic compounds to fathead minnows,
Duluth, Minnesota, US Environmental Protection Agency,
Environmental Research Laboratory (EPA 600/3-76-097; PB
262897).
185. MAY, J. (1966) [Odour thresholds of solvents for the
judgement of solvent odour in air.] Staub Reinhalt. Luft, 26:
385-389 (in German).
186. MECIKALSKI, M.B. & DEPNER, T.A. (1982) Peritoneal dialysis
for isopropanol poisoning. West. J. Med., 137: 322-324.
187. MENDELSON, J., WEXLER, D., LEIDERMAN, P.H., & SOLOMON, P.
(1957) A study of addiction to nonethyl alcohols and other
poisonous compounds. Quart. J. Stud. Alcohol, 18: 561-580.
188. MORGAN, R.L., SORENSON, S.S., & CASTLES, T.R. (1987)
Prediction of ocular irritation by corneal pachymetry. Food
chem. Toxicol., 25: 609-613.
189. MOSHONAS, M.G. & SHAW, P.E. (1972) Analysis of flavor
constituents from lemon and lime essence. J. agric. food
Chem., 20: 1029-1030.
190. MUNCH, J.C. (1972) Aliphatic alcohols and alkyl esters:
narcotic and lethal potencies to tadpoles and to rabbits.
Ind. Med., 41: 31-33.
191. NATOWICZ, M., DONAHUE, J., GORMAN, L., KANE, M., MCKISSICK,
J. & SHAW, L. (1985) Pharmacokinetic analysis of a case of
isopropanol intoxication. Clin. Med., 31: 326-328.
192. NELSON, K.W., EGE, J.F., ROSS, M., WOODMAN, L.E., &
SILVERMAN, L. (1943) Sensory response to certain industrial
solvent vapors. J. ind. Hyg. Toxicol., 25: 282-285.
193. NELSON, B.K., BRIGHTWELL, W.S., MACKENZIE-TAYLOR, D.R., KHAN,
A., BURG, J.R., WEIGEL, W.W., & GOAD, P.T. (1988)
Teratogenicity of n-propanol and isopropanol administered at
high inhalation concentrations to rats. Food Chem. Toxicol.,
26: 247-254.
194. NIXON, G.A., TYSON, C.A., & WERTZ, W.C. (1975) Interspecies
comparisons of skin irritancy. Toxicol. appl. Pharmacol., 31:
481-490.
195. NORDMANN, R., RIBIERE, C., ROUACH, H., BEAUGE, F.,
GIUDICELLI, Y., & NORDMANN, J. (1973) Metabolic pathways
involved in the oxidation of isopropanol into acetone by the
intact rat. Life Sci., 13: 919-932.
196. OELERT, H.H. & FLORIAN, T. (1972) [Recording and valuation
of the inconvenience caused by odours from diesel exhaust.]
Staub Reinhalt. Luft, 32: 400-407 (in German).
197. OHASHI, Y., NAKAI, Y., IKEOKA, H., KOSHIMO, H., ESAKI, Y.,
HORIGUCHI, S., TERAMOTO, K., & NAKASEKO, H. (1987a)
Recovery process of tracheal mucosa of guinea-pigs exposed to
isopropyl alcohol. Arch. Toxicol., 61: 12-20.
198. OHASHI, Y., NAKAI, Y., IKEOKA, H., KOSHIMO, H., ESAKI, Y.,
HORIGUCHI, S., TERAMOTO, K., & NAKASEKO, H. (1987b) Acute
effects of isopropyl alcohol exposure on the middle ear
mucosa. J. appl. Toxicol., 7: 205-211.
199. OLSON, B.A. (1982) Effects of organic solvents on
behavioural performance of workers in the paint industry.
Neurobehav. Toxicol. Teratol., 4: 703-708.
200. OVEREND, R. & PARASKEVOPOULOS, G. (1978) Rates of OH radical
reactions. IV. Reactions with methanol, ethanol, 1-propanol,
and 2-propanol at 296 ° K. J. phys. Chem., 82: 1329-1333.
201. PALO, V. & ILKOVA, H. (1970) Direct gas chromatographic
estimation of lower alcohols, acetaldehyde, acetone and
diacetyl in milk products. J. Chromatogr., 53: 363-367.
202. PARKER, W.A. (1982-1983) Alcohol-containing pharmaceuticals.
Am. J. drug alcohol Abuse, 9: 195-209.
203. PITTER, P. (1976) Determination of biological degradability
of organic substances. Water Res., 10: 231-235.
204. POHL, J. (1922) [Investigations into the fate of methyl and
isopropyl alcohol.] Biochem. Z., 127: 66-71 (in German).
205. POSO, H. & POSO, A.R. (1980) Inhibition by aliphatic
alcohols of the stimulated activity of ornithine
decarboxylase and tyrosine amino-transferase occurring in
regenerating rat liver. Biochem. Pharmacol., 29: 2799-2803.
206. POWIS, G. (1975) Effect of a single oral dose of methanol,
ethanol and propan-2-ol on the hepatic microsomal metabolism
of foreign compounds in the rat. Biochem. J., 148: 269-277.
207. POWIS, G. & GRANT, L. (1976) The effect of inhibitors of
alcohol metabolism upon the changes in the hepatic microsomal
metabolism of foreign compounds produced by the acute
administration of some alcohols to the rat. Biochem.
Pharmacol., 25: 2197-2201.
208. PRICE, K.S., WAGGY, G.T., & CONWAY, R.A. (1974) Brine shrimp
bioassay and seawater BOD of petrochemicals. J. Water Pollut.
Control Fed., 46: 63-77.
209. RAICHLE, M.E., EICHLING, J.O., STRAATMANN, M.G., WELCH, M.J.,
LARSON, K.B., & TER-POGOSSIAN, M.M. (1976) Blood-brain
barrier permeability of 11C-labeled alcohols and 15U-labeled
water. Am. J. Physiol., 230: 543-552.
210. RAILE, A., HAMMAN, K.P., SCHEINER, O., SCHULTZ, T., ERDEI,
A., & DIERICH, M.P. (1982) Differential effect of low
molecular weight alcohols on the Con A stimulation of mouse
spleen cells. Immunol. Lett., 4: 305-309.
211. RAMSEY, J.D. & FLANAGAN, R.J. (1982) Detection and
identification of volatile organic compounds in blood by
headspace gas chromatograpy as an aid to the diagnosis of
solvent abuse. J. Chromatogr., 240: 423-444.
212. REINDERS, M.E. (1980) Handbook of emission factors. Part I.
Non-industrial sources, The Hague, Netherlands, Ministry of
Health and Environmental Protection.
213. REINHARDT, C.A., PELLI, D.A., & ZBINDEN, G. (1985)
Interpretation of cell toxicity data for the estimation of
potential irritation. Food chem. Toxicol., 23: 247-252.
214. REQUENA, J., VELAZ, M.E., GUERRERO, J.R., & MEDINA, J.D.
(1985) Isomers of long-chain alkane derivatives and nervous
impulse blockage. J. Membr. Biol., 84: 229-238.
215. REYNOLDS, E.S., MOSLEN, M.T., & TREINEN, R.J. (1982)
Isopropanol enhancement of carbon tetrachloride metabolism in
vivo. Life Sci., 31: 661-669.
216. REYNOLDS, T. (1977) An anomalous effect of isopropanol on
lettuce germination. Plant Sci. Lett., 15: 25-28.
217. RICHARDSON, D.R., CARAVATI, C.M., PEYTON, E., & WEARY, P.E.
(1969) Allergic contact dermatitis to "alcohol" swabs.
Cutis, 5: 1115-1118.
218. RIETBROCK, N. & ABSHAGEN, U. (1971) [Pharmacokinetics and
metabolism of aliphatic alcohols.] Arzneim.-Forsch.,
21: 1309-1319 (in German).
219. RISTOW, S.S., STARKEY, J.R., & HASS, G.M. (1982) Inhibition
of natural killer cell activity in vitro by alcohols.
Biochem. Biophys. Res. Commun., 105: 1315-1321.
220. ROSANSKY, S.J. (1982) Isopropyl alcohol poisoning treated
with haemodialysis: kinetics of isopropyl alcohol and acetone
removal. J. Toxicol.-clin. Toxicol., 19: 265-271.
221. ROSS, D.H. (1976) Selective action of alcohols on cerebral
calcium levels. Ann. N.Y. Acad. Sci., 273: 280-294.
222. SABLJIC, A. & PROTIC-SABLIC, M. (1983) Quantitative
structure-activity study on the mechanism of inhibition of
microsomal p-hydroxylation of aniline by alcohols. Mol.
Pharmacol., 23: 213-218.
223. SANTODONATO, J. (1985) Monograph on human exposure to
chemicals in the workplace: isopropyl alcohol, Syracuse, New
York, Center for Chemical Hazard Assessment, Syracuse
Research Corporation (SRC-TR-84-1043, PB 86-143401).
224. SAVOLAINEN, H., PEKARI, K., & HELOJOKI, H. (1979)
Neurochemical and behavioural effects of extended exposure to
isopropanol vapour with simultaneous ethanol intake. Chem.-
biol. Interact., 28: 237-248.
225. SAWHNEY, B.L. & KOZLOSKI, R.P. (1984) Organic pollutants in
leachates from landfill sites. J. environ. Qual., 13:
349-352.
226. SCHICK, J.B. & MILSTEIN, J.M. (1981) Burn hazard of
isopropyl alcohol in the neonate. Pediatrics, 68: 587-588.
227. SEILER, H., BLAIM, H., & BUSSE, M. (1984) [Antibacterial
effects on predominant taxa in the activated sludge system of
a chemical combine.] Z. Wasser-Abwasser Forsch., 17: 127-133
(in German).
228. SENZ, E.H. & GOLDFARB, D.L. (1958) Coma in a child following
use of isopropyl alcohol in sponging. J. Pediatr., 53:
322-323.
229. SHAW, G.J., ALLEN, J.M., & VISSER, F.R. (1985) Volatile
flavor components of Babaco fruit ( Carica pentagona,
Heilborn). J. agric. food Chem., 33: 795-797.
230. SHEHAB, A.S. (1980) Comparative cytological studies of the
effect of some aliphatic alcohols and the fatty alcohols from
Euphorbia granulata and Pulicaria crispa on mitosis of Allium
cepa. Cytologia, 45: 507-513.
231. SHOFSTAHL, J.H. & HARDY, J.K. (1986) Determination of C1-C4
alcohols in gasoline using multiple ion detection. Anal.
Chem., 58: 2412-2414.
232. SIEBERT, H., SIEBERT, G., & BOHN, G. (1972) [Animal
experimental investigations into the metabolism of
propan-2-ol.] D tsch. Apoth.-ZTG., 112: 1040-1041 (in German).
233. SINGH, K.V. & AGRAWAL, S.C. (1981) Growth responses of
keratinophilic fungi to some volatile substances. Mykosen,
24: 630-634.
234. SIPES, I.G., STRIPP, B., KRISHNA, G., MALING, H., & GILLETTE,
J.R. (1973) Enhanced hepatic microsomal activity by
pretreatment of rats with acetone or isopropanol. Proc. Soc.
Exp. Biol. Med., 142: 237-240.
235. SMEA (1982) [Problems in industrial toxicology.] (in
Russian).
236. SMITH, N.B. (1984) Determination of volatile alcohols and
acetone in serum by non-polar capillary gas chromatography
after direct sample injection. Clin. Chem., 30: 1672-1674.
237. SMITH, P. & BROWN, N.L. (1969) Determination of isopropyl
alcohol in solid fish protein concentrate by gas-liquid
chromatography. J. agric. food Chem., 17: 34-37.
238. SMITH, R.P. (1959) Poisoning with Old Spice shaving lotion.
A case report. Bull. Suppl. Mat. clin. Toxicol. comm. Prod.,
2(11): 12.
239. SMYTH, H.F. & CARPENTER, C.P. (1948) Further experience with
the range finding test in the industrial toxicology
laboratory. J. ind. Hyg. Toxicol., 30: 63-70.
240. STEELE, R.H. & WILHELM, D.L. (1966) The inflammatory
reaction in chemical injury. I. Increased vascular
permeability and erythema induced by various chemicals. Br.
J. exp. Pathol., 47: 612-623.
241. STENSTROM, S., ENLOE, L., PFENNING, M., & RICHELSON, E.
(1986) Acute effects of ethanol and other short-chain
alcohols on the guanylate cyclase system of murine
neuroblastoma cells (clone N1E-115). J. Pharmacol. exp.
Ther., 236: 458-463.
242. STOFBERG, J. & GRUNDSCHOBER, F. (1984) Consumption ratio and
food predominance of flavoring materials-second cumulative
series. Perfumer & Flavorist, 9: 53-83.
243. STRANGE, A.W., SCHNEIDER, C.W., & GOLDBORT, R. (1976)
Selection of C3 alcohols by high and low ethanol selecting
mouse strains and the effects on open field activity.
Pharmacol. Biochem. Behav., 4: 527-530.
244. SWERDEL, M.R. & COUSINS, R.J. (1984) Changes in rat liver
metallothionein and metallothionein mRNA induced by
isopropanol. Proc. Soc. Exp. Biol. Med., 175: 522-529.
245. TAYLOR, C.D., COWART, C.O., & RYAN, N.T. (1985) Isopropanol
intoxication: managing the coma. Hosp. Pract., 20: 173-175.
246. TESTA, B. (1981) Structural and electronic factors
influencing the inhibition of aniline hydroxylation by
alcohols and their binding to cytochrome P-450. Chem.-biol.
Interact., 34: 287-300.
247. TICHY, M., TRCKA, V. ROTH, Z., & KRIVUCOVA, M. (1985) QSAR
analysis and data extrapolation among mammals in a series of
aliphatic alcohols. Environ. Health Perspect., 61: 321-328.
248. TIESS, D. & HAMMER, U. (1985) [On endogenous acetone
(propane-2-on) and isopropanol (propane-2-ol) levels in the
human body after ketoacidic states.] Z. gesamte Hyg., 31:
527-529 (in German).
249. TIMMER, R., TER HEIDE, R., DE VALOIS, P.J., & WOBBEN, H.J.
(1971) Qualitative analysis of the most volatile neutral
components of Reunion geranium oil ( Pelargonium roseum
Bourbon). J. agric. food Chem., 19: 1066-1068.
250. TOMITA, M. & NISHIMURA, M. (1982) Using saliva to estimate
human exposure to organic solvents. Bull. Tokyo dent. Coll.,
23: 175-188.
251. TOOBY, T.E., HURSEY, P.A., & ALABASTER, J.S. (1975) The
acute toxicity of 102 pesticides and miscellaneous substances
to fish. Chem. Ind., 12: 523-526.
252. TRAIGER, G.J. & PLAA, G.L. (1972) Relationship of alcohol
metabolism to the potentiation of CCl4 hepatotoxicity induced
by aliphatic alcohols. J. Pharmacol. exp. Ther., 183:
481-488.
253. TRAIGER, G.J. & PLAA, G.L. (1974) Chlorinated hydrocarbon
toxicity. Potentiation by isopropyl alcohol and acetone.
Arch. environ. Health, 28: 276-278.
254. TU, Y.Y., PENG, R., CHANG, Z.-F., & YANG, C.S. (1983)
Induction of a high affinity nitrosamine demethylase in rat
liver microsomes by acetone and isopropanol. Chem.-biol.
Interact., 44: 247-260.
255. UENG, T.-H., MOORE, L., ELVES, R.G., & ALVARES, A.P. (1983)
Isopropanol enhancement of cytochrome P-450-dependent
monooxygenase activities and its effects on carbon
tetrachloride intoxication. Toxicol. appl. Pharmacol., 71:
204-214.
256. URANO, K., OGURA, K., & WADA, H. (1981) Direct analytical
method for aliphatic compounds in water by steam carrier gas
chromatography. Water Res., 15: 225-231.
257. US NIOSH (1976) Criteria for a recommended standard:
occupational exposure to isopropyl alcohol, Cincinnati, Ohio,
US National Institute of Occupational Safety and Health, US
Department of Health, Education, and Welfare, Public Health
Services, Center for Disease Control (DHEW Publication No.
(NIOSH)76-142).
258. US NIOSH (1977) Manual of analytical methods, 2nd ed.,
Cincinnati, Ohio, US National Institute for Occupational
Safety and Health, US Department of Health, Education, and
Welfare, Vol. 2, pp. S185.
259. US NIOSH (1984) Method 1400. In: Eller, P.M., ed. NIOSH
Manual of analytical methods, 3rd ed., Cincinnati, Ohio,
National Institute for Occupational Safety and Health, Vol.
1, pp. 1400-1-1400-5.
260. VAN RILLAER, W.G. & BEERNAERT, H. (1983) Determination of
residual isopropanol and propylene glycol in soft drinks by
glass capillary gas chromatography. Z. Lebensm. Unters.
Forsch., 177: 196-199.
261. VASILIADES, J., POLLOCK, J., & ROBINSON, C.A. (1978)
Pitfalls of the alcohol dehydrogenase procedure for the
emergency assay of alcohol: a case study of isopropanol
overdose. Clin. Chem., 24: 383-385.
262. VEITH, G.D. & KOSIAN, P. (1983) Estimating bioconcentration
potential from octanol/water partition coefficients. In:
Mackay et al., ed. Physical behaviour of PCBs in the Great
Lakes, Ann Arbor, Michigan, Ann Arbor Science, pp. 269-282.
263. VEITH, G.D., CALL, D.J., & BROOKE, L.T. (1983) Structure-
toxicity relationships for the fathead minnow, Pimephales
promelas: narcotic industrial chemicals. Can. J. Fish. aquat.
Sci., 40: 743-748.
264. VIDELA, L.A., FERNANDEZ, V., & DE MARINIS, A. (1982) Liver
peroxidative pressure and glutathione status following
acetaldehyde and aliphatic alcohols pretreatment in the rat.
Biochem. Biophys. Res. Commun., 104: 965-970.
265. VILAGELIU ARQUES, L. & GONZALEZ DUARTE, R. (1980) Effect of
ethanol and isopropanol on the activity of alcohol
dehydrogenase, viability and life-span in Drosophila
melanogaster and Drosophila funebris. Experientia, 36:
828-830.
266. VISUDHIPAN, P. & KAUFMAN, H. (1971) Increased cerebrospinal
fluid protein following isopropyl alcohol intoxication. N.Y.
State J. Med., 71: 887-880.
267. VON DER HUDE, W., SCHEUTWINKEL, M., GRAMLICH, U., FISSLER,
B., & BUSLER, A. (1987) Genotoxicity of three carbon
compounds evaluated in the SCE test in vitro. Environ.
Mutagen., 9: 401-410.
268. WAGNER, R. (1974) [Investigations into the degradation
behaviour of organic compounds using the respirometric
dilution method. I. Monohydric alcohols.] Vom Wasser, 42:
271-305 (in German).
269. WAGNER, R. (1976) [Investigations into the degradation
behaviour of organic compounds using the respirometric
dilution method. II. The degradation kinetics of the test
compounds.] Vom Wasser, 47: 241-265 (in German).
270. WALLGREN, H. (1960) Relative intoxicating effects on rats of
ethyl, propyl and butyl alcohols. Acta pharmacol. toxicol.,
16: 217-222.
271. WALLINGFORD, K.M. (1983) Health hazard evaluation, Xomox
Corporation, Cincinnati, Ohio, US National Institute for
Occupational Safety and Health (HETA 83-170-1346, PB 85-
163434).
272. WASILEWSKI, C. (1968) Allergic contact dermatitis from
isopropyl alcohol. Arch. Dermatol., 98: 502-504.
273. WATERER, D.R. & PRITCHARD, M.K. (1984) Monitoring of
volatiles: a technique for detection of soft rot (Erwinia
carotovora) in potato tubers. Can. J. Plant. Pathol., 6:
165-171.
274. WAX, J., ELLIS, F.W., & LEHMAN, A.J. (1949) Absorption and
distribution of isopropyl alcohol. J. Pathol. exp. Ther., 97:
229-237.
275. WEBBER, D. (1984) Basic chemical output returns to growth.
Top 50 chemical products. Chem. eng. News, May 7: 8-10.
276. WEIL, C.S., SMYTH, H.F., & NALE, T.W. (1952) Quest for a
suspected industrial carcinogen. Arch. ind. Hyg. occup. Med.,
5: 535-547.
277. WEINTRAUB, Z. & IANCU, T.C. (1982) Isopropyl alcohol burns.
Pediatrics, 69: 506.
278. WHITE, G.A., EMMETT, E.A., KOMINSKY, J.R., & SINGAL, M.
(1983) Health hazard evaluation, Inland Division, GMC,
Cincinnati, Ohio, US National Instituue for Occupational
Safety and Health (HETA 77-011-1338, PB 85-101319).
279. WHITEHEAD, L.W., BALL, G.L., FINE, L.J., & LANGOLF, G.D.
(1984) Solvent vapor exposures in booth spray painting and
spray glueing, and associated operations. Am. ind. Hyg.
Assoc. J., 45: 767-772.
280. WILKINSON, C. & IGLEWICZ, R. (1982) Health hazard
evaluation, Syntrex Corporation, Cincinnati, Ohio, US
National Institute for Occupational Safety and Health (HETA
81-370-1050, PB 83-198424).
281. WILKINSON, T. & HAMER, G. (1979) The microbial oxidation of
mixtures of methanol, phenol, acetone, and isopropanol with
reference to effluent purification. J. chem. Technol.
Biotechnol., 29: 56-67.
282. WILLIAMS, T.M., HICKEY, J.L.S., & SHY,C.M. (1982) Health
hazard evaluation, Dittler Brothers, Inc., Cincinnati, Ohio,
US National Institute for Occupational Safety and Health
(HETA 81-173-1051, PB 83-198473).
283. WILLS, J.H., JAMESON, E.M., & COULSTON, F. (1969) Effects on
man of daily ingestion of small doses of isopropyl alcohol.
Toxicol. appl. Pharmacol., 15: 560-565.
284. WINEK, C.L. & JANSSEN, J.K. (1982) Blood versus bone marrow
isopropanol concentrations in rabbits. Forensic Sci. Int.,
20: 11-20.
285. WOLFF, T. (1978) In vitro inhibition of monooxygenase
dependent reactions by organic solvents. Int. Congr. Ser.
Excerpta Med., 440: 196-199.
286. WRIGHT, U. (1979) The hidden carcinogen in the manufacture
of isopropyl alcohol. Dev. Toxicol. environ. Sci., 4: 93-98.
287. YASHUDA, Y., CABRAL, A.M., & ANTONIO, A. (1976) Inhibitory
action of aliphatic alcohols on smooth muscle contraction.
Pharmacology, 14: 473-478.
288. YOUNG, P.J. & PARKER, A. (1983) The identification and
possible environmental impact of trace gases and vapours in
landfill gas. Waste Management Res., 1: 213-226.
289. YOUNG, R.H.F., RYCKMAN, D.W., & BUZZELL, J.C. (1968) An
improved tool for measuring biodegradability. J. Water
Pollut. Control Fed., 40: R354-R368.
290. ZAHLSEN, K., AARSTAD, K., & NILSEN, O.G. (1985) Inhalation
of isopropanol: induction of activating and deactivating
enzymes in rat kidney and liver, increased microsomal
metabolism of n-hexane. Toxicology, 34: 57-66.
291. ZAKHARI, S. (1977) Isopropanol and ketones in the
environment, Oxford, England, CRC Press.
292. ZINBO, M. (1984) Determination of one-carbon to three-carbon
alcohols and water in gasoline/alcohol blends by liquid
chromatography. Anal. Chem., 56: 244-247.
RESUME
1. Identité, propriétés physiques et chimiques, méthodes d'analyse
Le propanol-2 est un liquide incolore, très inflammable dont
l'odeur rappelle celle d'un mélange d'éthanol et d'acétone. Il est
entièrement miscible à l'eau, à l'éthanol, à l'acétone, au
chloroforme et au benzène. Il existe des méthodes d'analyse pour
la recherche du propanol-2 dans divers milieux (air, eau, sang,
sérum et urine), avec des limites de détection dans l'air, l'eau et
le sang respectivement égales à 2 x 10-5 mg/m3, 0,04 mg/litre et 1
mg/litre. La chromatographie en phase gazeuse (essentiellement
avec détection par ionisation de flamme) ainsi que l'électrophorèse
sur papier et la spectrométrie de mobilité ionique par
photoionisation permettent de doser le propanol-2 dans divers
mileux.
2. Sources d'exposition humaine et environnementale
On estime qu'en 1975, la production mondiale de propanol-2
dépassait 1100 kilotonnes, la capacité mondiale de production étant
supérieure à 2000 kilotonnes en 1984. Le propanol-2 est couramment
produit à partir du propène. Les procédés antérieurs de
fabrication qui reposaient sur l'utilisation d'acides forts ou
d'acides faibles, et donnaient naissance à des produits
intermédiaires et à des sous-produits potentiellement dangereux,
sont désormais largement supplantés par le procédé d'hydratation
catalytique. On peut également procéder par réduction catalytique
de l'acétone.
Le propanol-2 est un produit du métabolisme de divers micro-
organismes.
Il a de nombreuses applications comme solvant et il entre dans
la composition de différents produits ménagers et produits de soins
personnels, sous forme d'aérosols et d'excipients pour produits
pharmaceutiques à usage externe et pour cosmétiques. Le propanol-2
est également utilisé pour la production de l'acétone et autres
produits chimiques, comme agent de dégivrage, comme conservateur et
il entre dans la composition des concentrés pour le nettoyage des
pare-brise et de certains aromatisants alimentaires.
Le propanol-2 peut pénétrer dans l'atmosphère, dans l'eau et
dans le sol lors du rejet de déchets et on en a trouvé dans l'air
et dans les eaux de lessivage de décharges mal protégées. Présent
dans les gaz et eaux résiduaires industriels, on peut l'en éliminer
par oxydation biologique ou osmose inverse. Il peut être dissipé
dans l'atmosphère lors de l'utilisation de produits de consommation
qui en contiennent.
3. Transport et distribution dans l'environnement
C'est principalement lors d'opérations telles que la
production, la transformation, le stockage, le transport,
l'utilisation et le rejet de déchets que le propanol-2 pénètre dans
l'environnement atmosphérique. Il peut être également déchargé
dans le sol et l'eau. Il est difficile d'évaluer la part qui
revient à chaque compartiment du milieu. Toutefois on estimait en
1976 que plus de 50 % du propanol-2 produit finissait par être
libéré dans l'atmosphère.
Le propanol-2 est rapidement éliminé de l'atmosphère par
réaction sur les radicaux hydroxyles et entraînement par les
précipitations. Ce sont ces dernières qui sont responsables du
transport de ce composé de l'atmosphère dans le sol ou l'eau. Une
fois dans le sol, il doit y être très mobile et augmente la
perméabilité du sol à certains hydrocarbures aromatiques. Le
propanol-2 est facilement biodégradable par voie aérobie ou
anaérobie.
Comme il est biodégradable et complètement miscible à l'eau,
avec un coefficient de partition octanol/eau logarithmique de 0,14
et un facteur de bioconcentration de 0,5, il est peu probable qu'il
donne lieu à une bioaccumulation.
4. Niveaux dans l'environnement et exposition humaine
L'exposition de la population en général peut se produire par
ingestion accidentelle ou volontaire, par absorption de nourriture
contenant du propanol-2 d'origine naturelle, ou sous forme
d'aromatisant volatil ou de résidus de solvant, ou encore par
inhalation lors de l'utilisation de produits qui en contiennent.
On en a trouvé aux concentrations de 0,2 à 325 mg/litre dans des
boissons non alcoolisées et aux concentrations de 50 à 3000 mg/kg
dans des denrées alimentaires pour la production desquelles on
l'avait utilisé comme solvant. L'exposition de la population en
général par inhalation de l'air ambiant est faible, du fait de
l'élimination et de la dégradation rapides de ce produit. En
procédant à des contrôles en divers lieux et, en particulier, dans
des sites urbains, on a obtenu des concentrations moyennes
pondérées par rapport au temps allant jusqu'à 35 mg/m3.
Les travailleurs peuvent être exposés au propanol-2 au cours de
la production du composé lui-même, lors de la fabrication de
l'acétone ou d'autres dérivés et également lorsqu'on utilise ce
produit comme solvant. Aux Etats-Unis, une enquête (National
Occupational Exposure Survey) effectuée en 1980-83 a montré que
plus de 1,8 million de travailleurs pouvaient être exposés. On a
mesuré sur les lieux de travail des concentrations atteignant 1350
mg/m3 avec des moyennes pondérées par rapport au temps allant
jusqu'á 500 mg/m3.
5. Cinétique et métabolisme
Le propanol-2 est rapidement absorbé et se répartit dans tout
l'organisme par inhalation et ingestion. A forte dose,
l'absorption dans les voies digestives est retardée. Les taux
sanguins de propanol-2 (décelables lorsqu'il y a ingestion
simultanée d'éthanol) ou de son métabolite, l'acétone, sont
corrélés avec l'intensité de l'exposition. Des volontaires qui
avaient ingéré une dose de 3,75 mg/kg de propanol-2 (avec 1200 mg
d'éthanol/kg) dans du jus d'orange, présentaient une concentration
sanguine maximale de propanol-2 libre de 0,8 ± 0,3 mg/litre, et de
2,3 ± 1,4 mg/litre après incubation en présence d'arylsulfatase, ce
qui témoigne d'une sulfatation. Chez les ouvriers exposés à des
vapeurs de propanol-2 (8 - 647 mg/m3), on a observé des
concentrations de 3 - 270 mg/m3 dans l'air alvéolaire, mais dans ce
cas, c'est de l'acétone et non du propanol-2 que l'on a trouvé dans
le sang et les urines. Chez des animaux de laboratoire exposés au
propanol-2, on a retrouvé celui-ci non seulement dans le sang mais
également dans le liquide céphalo-rachidien, dans le foie, les
reins et le cerveau. Le propanol-2 traverse la barrière
hémoméningée deux fois plus facilement que l'éthanol. Le propanol-
2 est excrété en partie tel quel et en partie sous forme d'acétone,
essentiellement au niveau des poumons mais également dans la salive
et le suc gastrique. Il peut y avoir réabsorption après excrétion
par ces deux dernières voies. La métabolisation en acétone en
présence d'alcool-déshydrogénase (ADH) hépatique est assez lente,
du fait que l'ADH a une moindre affinité pour le propanol-2 que
pour l'éthanol. In vitro, l'activité de l'ADH humaine vis-à-vis du
propanol-2 correspond à 9 - 10 % de l'activité de cette enzyme
lorsque le substrat est de l'éthanol. Les oxydases microsomiques
du foie de rat sont également capables d'oxyder le propanol-2
in vitro. Chez l'homme, l'acétone est excrétée telle quelle,
essentiellement au niveau des poumons et en quantité minime au
niveau des reins. Plus l'exposition au propanol-2 se prolonge,
plus la concentration d'acétone dans l'air alvéolaire, dans le sang
et dans les urines est élevée. Le propanol-2 et l'acétone sont
éliminés de l'organisme selon une cinétique du premier ordre et
leur demi-vie chez l'homme est de 2,5 - 6,4 heures et 22 heures,
respectivement.
6. Effets sur les êtres vivants dans leur milieu naturel
Le propanol-2 est peu toxique pour la faune et la flore
aquatiques, les insectes et les plantes. Son seuil d'inhibition de
la multiplication cellulaire, mesuré chez une espèce sensible de
protozoaire, varie de 104 à 4930 mg/litre selon les conditions
expérimentales. Si l'on s'élève dans l'arbre phylogénétique, on
constate que diverses espèces de crustacés, notamment Daphnia
magna, présentent des CE50 allant de 2285 à 9714 mg/litre. Pour
des poissons d'eau douce, on a obtenu des CL50 à 96 heures allant
de 4200 à 11 130 mg/litre. Chez la drosophile, les CL50 vont de
10 200 à 13 340 mg/litre de milieu nutritif. Pour le troisième
stade larvaire du moustique Aedes aegypti, on a obtenu, lors d'une
épreuve statique de 4 heures, des valeurs de la CL50 allant de 25 à
120 mg/litre.
L'exposition de végétaux à du propanol-2 à des concentrations
comprises entre 2100 mg/litre et plus de 36 000 mg/litre, a
provoqué toute une gamme d'effets allant de l'absence totale
d'anomalies à une inhibition complète de la germination.
7. Effets sur les animaux d'expérience et les systèmes d'épreuves
in vitro
A en juger d'après la mortalité qu'il provoque, le propanol-2
présente une faible toxicité aiguë pour les mammifères, que
l'exposition ait lieu par voie orale, percutanée ou respiratoire.
Chez plusieurs espèces animales on a obtenu, après administration
par voie orale, des valeurs de la DL50 allant de 4475 à 7990 mg par
kg de poids corporel; la CL50 pour une inhalation de 8 heures
allait de 46 000 à 56 000 mg/m3 d'air chez le rat. A ces doses
mortelles, les rats présentaient une forte irritation des muqueuses
et une grave dépression du système nerveux central. La mort est
survenue par arrêt cardiaque ou respiratoire. Entre autres lésions
histopathologiques, on notait une congestion et un oedème du poumon
ainsi qu' une dégénérescence des hépatocytes.
Administré en dose unique par voie orale à des rats à raison de
3000 ou 6000 mg par kg de poids corporel, le propanol-2 a provoqué
une accumulation réversible de triglycérides dans le foie. On a
également observé chez ces rats une induction des enzymes
microsomiques à la dose de 390 mg/kg.
Non dilué, le propanol-2 n'est pas irritant en applications de
4 heures sur la peau, intacte ou abrasée, de lapins tondus.
Toutefois, en instillations oculaires de 0,1 ml, le propanol-2 non
dilué a provoqué une irritation chez le lapin. De fortes
concentrations de vapeurs de propanol-2 ont provoqué une irritation
respiratoire chez la souris et l'on a noté une réduction de 50 % du
rythme respiratoire à des concentrations allant de 12 300 à 43 525
mg/m3 d'air.
Les études conscrées aux effets sur l'animal d'exposition
répétées au propanol-2 sont plutôt limitées. Après inhalation par
des rats de 500 mg/m3 de propanol-2, cinq jours par semaine et
quatre heures par jour pendant quatre mois, on a noté une
irritation des voies respiratoires, des anomalies haématologiques
et des altérations histopathologiques au niveau du foie et de la
rate. Un autre groupe expérimental composé de cinq rats de chaque
sexe a reçu pendant 27 semaines du propanol-2 dans son eau de
boisson. En comparant les animaux qui recevaient environ 600 ou
2300 mg/kg par jour (mâles) et 1000 ou 3900 mg/kg par jour
(femelles) de propanol-2 à des témoins non traités, on a constaté
un retard de croissance, mais uniquement chez les deux groupes de
femelles traitées. Aucun autre effet indésirable n'a été constaté.
Les données disponibles donnent à penser que le propanol-2
produit sur le système nerveux central (SNC) des effets analogues à
ceux de l'éthanol. La DE50 d'anésthésie par voie orale est de 2280
mg/kg chez le lapin, la DE50 par voie intrapéritonéale
correspondant à la perte du réflexe de redressement chez la souris
est égale à 165 mg/kg et le seuil d'induction de l'ataxie par voie
intrapéritonéale est de 1106 mg/kg chez le rat. Ces valeurs sont
sont environ deux fois plus faibles que pour l'éthanol. Lors d'une
expérience menée à l'air libre, on a constaté que l'inhalation de
propanol-2 à la dose de 739 mg/m3, dix heures par jour, et cinq
jours par semaine pendant 15 semaines ne produisait aucun effet
indésirable.
Le propanol-2 a été soumis à une étude portant sur deux
générations de rats qui ont reçu dans leur eau de boisson des doses
quotidiennes de 1290, 1380 ou 1479 mg de ce produit par kg de poids
corporel. Les seuls effets indésirables qui ont été notés
consistaient dans une réduction passagère du taux de croissance
dans la génération F0. En revanche, d'autres chercheurs ont
constaté une augmentation des malformations lors d'une étude
tératogènicité, au cours de laquelle on avait administré à des
rates gravides, des doses orales quotidiennes de 252 ou 1008 mg de
propanol-2 par kg de poids corporel (toxicité maternelle non
étudiée). On a également indiqué que ces deux doses, administrées
pendant 45 jours dans l'eau de boisson, faisaient passer le cycle
oestral à cinq jours (contre quatre chez les témoins). Chez des
rates ayant reçu pendant six mois dans leur eau de boisson, des
doses quotidiennes de propanol-2 de 1800 mg/kg avant de mettre bas,
on a constaté un accroissement de la mortalité embryonnaire totale;
divers effets ont également été signalés concernant la survie
intra-utérine et postnatale à des doses aussi basses que 0,8 mg/kg,
sans qu'on puisse toutefois dégager une tendance précise. Des
rates gravides ont été exposées à de l'air contenant du propanol-2
aux concentrations respectives de 9001, 18 327 et 23 210 mg/m3
(3659, 7450 ou 9435 ppm). Les deux concentrations les plus fortes
se sont révélées toxiques pour les mères, la concentration de 9001
mg/m3 ne produisant aucun effet. A toutes les concentrations on a
constaté un effet nocif sur le développement.
Une épreuve de recherche des mutations ponctuelles utilisant
S. typhimurium a donné des résultats négatifs avec du propanol-2 à
la dose de 0,18 mg par boîte; la recherche d'échanges entre
chromatides soeurs sur fibroblastes de hamster chinois a également
été négative. Le propanol-2 a provoqué des anomalies de la mitose
dans des cellules médullaires de rat ainsi que des cellules de
l'extrêmité radiculaire d'oignons in vitro. On ne dispose d'aucune
autre donnée sur la mutagénicité de ce produit.
Un certain nombre d'études restreintes ont été consacrées au
pouvoir cancérogène du propanol-2, au cours desquelles des souris
ont été exposées à cette substance par voie percutanée (3 fois par
semaine pendant un an), par voie respiratoire (7700 mg/m3, 3 à 7
heures par jour, cinq jours par semaine pendant 5 à 8 mois) et par
voie sous-cutanée (20 mg de propanol non dilué par semaine pendant
20 à 40 semaines). Au cours de ces trois études, on a recherché la
présence de tumeurs sur la peau, dans les poumons et au point
d'injection. Aucun signe d'effets cancérogènes n'a été observé.
On ne dispose pas de données épidémiologiques suffisantes pour
évaluer la cancérogénicité du propanol-2 chez l'homme. A la
lumière des données disponibles, on peut penser que le sulfate de
dipropyl-2, un produit intermédiaire de la fabrication du
propanol-2 par le procédé aux acides forts et faibles, pourrait
être à l'origine de cancers du sinus maxillaire chez l'homme.
8. Effets sur la santé de l'homme
On a signalé plusieurs cas d'intoxication consécutifs à
l'ingestion de propanol-2 ou à l'utilisation de lotions à base de
ce produit pour rafraîchir des enfants fébriles. Les principaux
signes d'intoxication rappellent ceux de l'intoxication alcoolique:
nausées, vomissements, douleurs abdominales, gastrite, hypotension
et hypothermie. La dépression du SNC par le propanol-2 est deux
fois plus intense qu'avec l'éthanol et entraîne l'inconscience puis
un coma profond; la mort peut survenir par dépression respiratoire.
Parmi les autres effets on peut noter l'hyperglycémie, un taux
élevé de protéines dans le liquide céphalorachidien et une
atélectasie. Il semblerait que l'absorption percutanée soit
négligeable mais on connaît le cas d'un enfant qui a été intoxiqué
après avoir été lotionné avec du propanol-2, ce qui donne à penser
qu'il ne faut pas négliger l'absorption percutanée, notamment chez
les enfants. Aucun effet indésirable n'a été observé chez des
volontaires en bonne santé qui avaient bu tous les jours pendant
six semaines un sirop contenant du propanol-2 à des doses
corrrespondant respectivement à 2,6 et 6,4 mg de propanol-2 par kg
de poids corporel. Des volontaires du sexe masculin, exposés 3 à 5
minutes à des vapeurs de propanol-2 correspondant à des
concentrations de 490, 980 ou 1970 mg/m3 d'air, ont estimé qu'ils
ressentaient une irritation légère à 980 mg/m3 et que la situation
était "satisfaisante" pendant les 8 heures correspondant à leur
propre exposition professionnelle.
Des enfants prématurés ayant subi un contact prolongé avec du
propanol-2 ont présenté une irritation cutanée prenant la forme
d'érythèmes, voire de brûlures du 2ème et du 3ème degré et de
phlyctènes. On a également signalé ça et là des cas de dermatites
allergiques de contact.
Il n'existe de peu d'études épidémiologiques consacrées à la
mortalité par cancers ou autres maladies provoquées par le
propanol-2. Parmi un groupe de 61 travailleurs, employés pendant
plus de cinq ans dans un atelier de fabrication de propanol-2 par
le procédé à l'acide fort, on a observé sept cas de cancer dont
quatre de cancer des sinus maxillaires. Une étude portant sur une
cohorte de 779 travailleurs d'un atelier analogue a révélé que
l'incidence des cancers du sinus et du larynx, corrigée de
l'influence de l'âge et du sexe, était 21 fois plus forte que
prévue. La période minimale de latence était de dix ans. Dans une
autre étude rétrospective de cohorte portant sur le personnel d'une
autre usine où on utilisait le procédé à l'acide fort, on a
constitué une cohorte représentant plus de 4000 années-hommes
exposés au risque. Les résultats de cette étude ont montré que les
taux de mortalité pour toutes causes et les taux de mortalité par
cancer n'étaient pas sensiblement plus élevés que les taux
prévisibles. Une autre étude rétrospective a été menée dans une
usine qui fabriquait du propanol-2 par le procédé à l'acide faible.
Cette fois, on comptait plus de 11 000 années-hommes exposés au
risque. Dans ce cas, le taux de mortalité pour toutes causes était
inférieur aux prévisions et l'on ne constatait pas de surmortalité
attribuable aux cancers en général. Toutefois, l'incidence des
cancers de la bouche et du pharynx était 4 fois supérieure à la
normale. Dans l'ensemble, ces études de cohorte donnent à penser
qu'il existe un risque de cancer imputable au procédé à l'acide
fort; toutefois lors de deux petites études castémoins, on n'a noté
aucune association entre l'exposition au propanol-2 et l'incidence
des gliomes ou de la leucémie lymphatique.
Certaines études font état d'une potentialisation de la
toxicité du tétrachlorure de carbone chez des ouvriers
simultanément exposés au propanol-2.
9. Résumé de l'évaluation
L'homme peut être exposé au propanol-2 par inhalation lors de
la fabrication, de la transformation ou de l'utilisation de cette
substance dans le cadre professionnel ou domestique. En ce qui
concerne la population en général, l'exposition à des doses
potentiellement mortelles peut se produire par suite d'ingestion
accidentelle ou volontaire de cette substance et les enfants
peuvent être exposés par application de lotions à base de propanol-
2.
Le propanol-2 est vite absorbé et se répartit rapidement dans
l'ensemble de l'organisme, en partie sous forme d'acétone. Les
données relatives aux effets aigus consécutifs à l'exposition
d'êtres humains à des doses excessives sont rares et contrastées.
Les principaux effets consistent en gastrite, dépression du système
nerveux central, hypothermie, dépression respiratoire et
hypotension. Les données de mortalité aiguë obtenues sur des
animaux de laboratoire indiquent que le propanol-2 est peu toxique,
les valeurs de la DL50 par voie orale chez diverses espèces vont de
4475 à 7990 mg/kg, et les valeurs de la CL50 par inhalation se
situent aux alentours de 50 000 mg/m3 chez le rat. Chez le lapin,
le propanol-2 ne provoque pas d'irritation cutanée, toutefois
l'instillation de 0,1 ml de cette substance non diluée dans les
yeux a provoqué une irritation.
Chez l'homme, les effets aigus les plus probables d'une
exposition de fortes concentrations de propanol-2 par ingestion ou
inhalation, consistent en une intoxication de type alcoolique
aboutissant à la narcose.
Les études sur l'animal sont insuffisantes pour qu'on puisse
évaluer les risques encourus par l'homme à la suite d'expositions
répétées au propanol-2. Toutefois les résultats de deux études à
court terme chez le rat, au cours desquelles on a fait a) inhaler
500 mg/m3 de cette substance, 4 heures par jour, 5 heures par
semaine pendant 4 mois et b) ingérer le même produit à raison de
600 à 3900 mg/kg dans l'eau de boisson, donnent à penser qu'il
serait préférable d'éviter de s'exposer aux fortes concentrations
en propanol-2 signalées dans le cadre de certaines activités
professionnelles.
En faisant inhaler du propanol-2 à des rattes gravides on a
constaté que le seuil d'apparition d'un effet se situait à 18 327
mg/m3 (7450 ppm), la dose sans effet observable était de 9001 mg/m3
(3659 ppm), la toxicité maternelle étant prise comme critère. Au
cours de la même étude, le seuil d'apparition d'effets s'est situé
à 9001 mg/m3 (3659 ppm) pour les anomalies du développement et
aucune dose sans effet observable n'a pu être mise en évidence.
Ces concentrations sont plus élevées que celles auxquelles l'homme
est susceptible d'être exposé.
Les épreuves de génotoxicité ont donné des résultats négatifs
dans le cas du propanol-2, cependant on a observé des anomalies de
la mitose dans des cellules médullaires de rats. Ces résultats
indiquent que le propanol-2 n'est pas du tout génétoxique mais les
données sont trop limitées pour qu'on puisse se prononcer
véritablement sur le pouvoir mutagène de cette substance.
Les données existantes sont insuffisantes pour permettre une
évaluation de la cancérogénicité du propanol-2 chez l'animal
d'expérience. On ne dispose pas de données permettant d'évaluer
cette cancérogénicité chez l'homme.
Le propanol-2 ne fait probablement pas courir de risque
important à la population dans son ensemble dans les conditions
d'exposition qui sont susceptibles de se produire.
Le propanol-2 disparaît rapidement (demi-vie, 5 jours) de
l'atmosphère et s'élimine à bref délai de l'eau et du sol par
biodégradation aérobie ou anaérobie, en particulier une fois que
les microorganismes préalablement ensemencés se sont adaptés.
Compte tenu de ses propriétés physiques, le propanol-2 n'a qu'une
faible tendance à la bioaccumulation. Il ne présente pas de risque
pour la faune et la flore aux concentrations auxquelles il est
habituellement présent dans l'environnement.
RESUMEN
1. Identidad, propiedades físicas y químicas, métodos analíticos
El 2-propanol es un líquido incoloro, sumamente inflamable, con
un olor que recuerda al de la mezcla de etanol y acetona. El
compuesto es completamente miscible con agua, etanol, acetona,
cloroformo y benceno. Se dispone de métodos analíticos para
detectar el 2-propanol en diversos medios (aire, agua, sangre,
suero y orina) con límites de detección de 2 x 10-5 mg/m3, 0,04
mg/litro y 1 mg/litro en el aire, el agua y la sangre,
respectivamente. Existen métodos de cromatografía de gases (que se
sirven principalmente de la detección de ionización de llama) así
como métodos de electroforesis en papel y de espectrometría de
movilidad iónica inducida por fotoionización para determinar el
2-propanol en los distintos medios.
2. Fuentes de exposición humana y ambiental
La producción mundial estimada de 2-propanol en 1975 fue
superior a 1100 kilotoneladas y la capacidad de producción mundial
en 1984 se cifró en más de 2000 kilotoneladas. El 2-propanol se
fabrica comúnmente a partir del propeno. Los antiguos procesos
basados en ácidos fuertes y débiles, en los que se generaban
productos intermedios y desechos potencialmente peligrosos, se han
sustituido actualmente en gran medida por el proceso de hidratación
catalítica. La reducción catalítica de la acetona es otro proceso
posible.
El 2-propanol se ha identificado como producto metabólico de
diversos microorganismos.
El compuesto tiene amplias aplicaciones como disolvente y se
utiliza como componente de productos domésticos y personales, entre
ellos vaporizadores de aerosoles, productos farmacéuticos de
aplicación tópica y cosméticos. El 2-propanol se utiliza también
para producir acetona y otras sustancias químicas, como agente
descongelante, como conservante, en concentrados para
limpiaparabrisas y como aromatizante volátil en alimentos.
El 2-propanol puede ingresar en la atmósfera, el agua o el
suelo por la evacuación de desechos y se ha aislado en el aire y en
el líquido que rezuma de basureros y terraplenados. Se encuentra
en los gases y las aguas residuales que emiten algunas industrias,
y puede extraerse de esas aguas por oxidación biológica o por
ósmosis inversa. Durante el uso de 2-propanol en productos de
consumo se producen emisiones dispersas a la atmósfera.
3. Transporte, distribución y transformación en el medio ambiente
La vía principal de entrada del 2-propanol en el medio ambiente
es su emisión a la atmósfera durante la producción, el tratamiento,
el almacenamiento, el transporte, el uso y la evacuación. También
se producen emisiones al suelo y al agua. Es difícil calcular el
volumen que ingresa en cada compartimiento ambiental. No obstante,
se calculó que en 1976 la liberación total de este compuesto en la
atmósfera fue superior al 50% del 2-propanol producido.
El 2-propanol desaparece rápidamente de la atmósfera por
reacción con radicales hidroxilo y arrastrado por la lluvia. A
este último proceso se debe el transporte del 2-propanol desde la
atmósfera hasta el suelo o el agua. Una vez que está en el suelo,
se cree que es muy móvil y que aumenta la permeabilidad del suelo a
algunos hidrocarburos aromáticos. El 2-propanol es fácilmente
biodegradable, en condiciones tanto aerobias como anaerobias.
La bioacumulación del compuesto no es probable, dados su
carácter biodegradable y su miscibilidad total con el agua; su
coeficiente de reparto log n-octanol/agua es de 0,14 y su factor de
bioconcentración de 0,5.
4. Niveles ambientales y exposición humana
La exposición de la población general se produce por ingestión
accidental o intencionada, por la ingestión de alimentos que lo
contengan como aromatizante volátil natural o añadido o como
residuo de disolvente, y por inhalación durante su uso. Se han
encontrado concentraciones de 0,2 a 325 mg por litro en bebidas no
alcohólicas y de 50 a 3000 mg por kg en alimentos tras el uso de
2-propanol como disolvente en su producción. La exposición de la
población general por inhalación de aire ambiental es baja a causa
de su rápida desaparición y degradación. Se han estudiado diversas
localizaciones y se han medido concentraciones medias ponderadas en
función del tiempo de hasta 35 mg/m3 en emplazamientos urbanos.
Los trabajadores se ven expuestos al 2-propanol durante la
producción del propio compuesto y de acetona y otros derivados, así
como durante su uso como disolvente. En la encuesta nacional de
exposición ocupacional (1980 - 83) realizada en los Estados Unidos,
se estimó que más de 1,8 millones de trabajadores estaban
potencialmente expuestos. En ciertos lugares de trabajo se han
medido concentraciones de hasta 1350 mg/m3, con promedios
ponderados en función del tiempo de hasta 500 mg/m3.
5. Cinética y metabolismo
El 2-propanol se absorbe y distribuye rápidamente por todo el
organismo tras su inhalación o ingestión. A dosis elevadas se
retrasa la absorción gastrointestinal. Las concentraciones
sanguíneas de 2-propanol (detectables cuando se ingiere etanol
simultáneamente) o de su metabolito, la acetona, guardan relación
con los niveles de exposición. En voluntarios que ingirieron una
dosis de 3,75 mg/kg (con 1200 mg de etanol/kg) en zumo de naranja,
se observó un nivel máximo de 0,8 ± 0,3 mg de 2-propanol libre por
litro en la sangre, y de 2,3 ± 1,4 mg por litro tras la incubación
con arilsulfatasa, lo que es un signo de sulfatación. Los
trabajadores expuestos a vapores (8 - 647 mg/m3) mostraron
concentraciones de 3 - 270 mg/m3 en el aire alveolar, pero en este
caso se encontró acetona y no 2-propanol en la sangre y la orina.
En animales de laboratorio tratados, el 2-propanol se detectó no
sólo en la sangre sino también en el líquido espinal, el hígado,
los riñones y el cerebro. Atraviesa la barrera hematocerebral dos
veces mejor que el etanol. El 2-propanol se excreta en parte como
tal y en parte como acetona, principalmente por vía pulmonar, pero
también en la saliva y el jugo gástrico. La reabsorción puede
producirse después de la excreción por las últimas dos vías. La
transformación en acetona por medio de la deshidrogenasa alcohólica
del hígado es más bien lenta, porque la afinidad relativa de la
deshidrogenasa por el 2-propanol es más baja que por el etanol.
In vitro, la actividad enzimática de la deshidrogenasa humana con
2-propanol fue del 9 - 10% de la actividad que exhibe cuando el
sustrato es el etanol. In vitro las oxidasas microsómicas de
hígado de rata también son capaces de oxidar el 2-propanol. En el
hombre, la acetona se excreta sin cambios, principalmente por los
pulmones y en cantidad mínima por los riñones. La concentración de
acetona en el aire alveolar, la sangre y la orina aumenta con la
intensidad y la duración de la exposición al 2-propanol. La
eliminación de 2-propanol y de acetona del organismo es de primer
orden, y los periodos de semieliminación en el hombre son de
2,5 - 6,4 horas y 22 horas, respectivamente.
6. Efectos en los organismos en el medio ambiente
La toxicidad del 2-propanol para organismos acuáticos, insectos
y plantas es baja. El umbral inhibitorio para la multiplicación
celular de una especie de protozoo sensible varió de 104 a 4930 mg
por litro en diversas condiciones experimentales. Avanzando en la
cadena filogenética, varias especies de crustáceos, incluida
Daphnia magna, mostraron CE50 a concentraciones que iban desde 2285
hasta 9714 mg por litro. Las CL50 (96 h) para peces de agua dulce
variaron desde 4200 hasta 11 130 mg por litro. Los datos obtenidos
para especies de moscas de la fruta mostraron CL50 comprendidas
entre 10 200 y 13 340 mg por litro de medio nutritivo. La CL50
para larvas de mosquito ( Aedes aegypti) en la tercera etapa de
desarrollo fue de 25 - 120 mg/litro en un ensayo estático de 4
horas.
Los efectos que tiene en las plantas la exposición a 2-propanol
en concentraciones entre 2100 mg/litro y más de 36 000 mg/litro
variaron entre la ausencia de efecto y la inhibición total de la
germinación.
7. Efectos en animales de experimentación y en sistemas de ensayo
in vitro
La toxicidad aguda del 2-propanol para los mamíferos, a juzgar
por la mortalidad, es baja, sea cual sea la vía de exposición oral,
cutánea o respiratoria. Los valores de la DL50 para varias
especies animales tras la administración oral variaron entre 4475 y
7990 mg por kg de peso corporal; La CL50 de inhalación durante 8
horas en la rata varió de 46 000 a 55 000 mg por m3 de aire. A
estos niveles letales, las ratas mostraron grave irritación de las
mucosas y depresión profunda del sistema nervioso central. La
muerte fue provocada por paro respiratorio o cardiaco. Entre las
lesiones histopatológicas figuraron la congestión y el edema
pulmonar, así como la degeneración celular en el hígado.
Con dosis orales únicas de 3000 ó 6000 mg de 2-propanol por kg
de peso corporal se produjo una acumulación reversible de
triglicéridos en el hígado de la rata. En la rata se observó
inducción de enzimas microsómicas a dosis orales de 390 mg/kg.
El 2-propanol sin diluir no produjo irritaciones cuando se
aplicó a la piel cortada o raspada del conejo durante 4 horas. En
cambio, se observó irritación cuando se aplicó 0,1 ml de compuesto
sin diluir en el ojo del conejo. Con concentraciones de vapor
elevadas de 2-propanol se produjo irritación del tracto
respiratorio en el ratón, y el ritmo respiratorio disminuyó en un
50% a concentraciones de 12 300 - 43 525 mg/m3 de aire.
Se han hecho escasos estudios de exposición repetida sobre los
efectos del 2-propanol en animales. Tras la inhalación de 500 mg
de 2-propanol/m3 durante 5 días a la semana y 4 horas al día
durante más de 4 meses, se observaron irritación del tracto
respiratorio, cambios hematológicos y alteraciones histopatológicas
en el hígado y el bazo de la rata. En otro grupo de estudio, se
administró a 5 ratas de cada sexo agua de bebida que contenía
2-propanol durante 27 semanas. La comparación de animales que
recibían aproximadamente 600 ó 2300 mg por kg al día (machos) y
1000 ó 3900 mg por kg al día (hembras) con grupos de control no
tratados reveló un retraso del crecimiento sólo en ambos grupos de
hembras expuestas. No se observaron otros efectos adversos.
Los datos disponibles indican que los efectos del 2-propanol en
el sistema nervioso central son semejantes a los del etanol. La
DE50 por vía oral para la narcosis en conejos es de 2280 mg/kg; la
DE50 intraperitoneal correspondiente a la pérdida del reflejo de
enderezamiento en el ratón es de 165 mg/kg, y el umbral
intraperitoneal de inducción de ataxia en la rata es de 1106 mg/kg.
Estos valores son aproximadamente dos veces más bajos que los
correspondientes al etanol. La inhalación de 2-propanol a una
concentración de 739 mg/m3 durante 6 horas al día y 5 días a la
semana durante 15 semanas no originó ningún resultado adverso en un
ensayo en campo abierto.
El 2-propanol se evaluó en un estudio de 2 generaciones de
ratas mediante la administración de 1290, 1380 ó 1470 mg por kg al
día en el agua de bebida a ambas generaciones. El único efecto
adverso observado fue una reducción transitoria del ritmo de
crecimiento en la generación Fo. En cambio, otros investigadores
observaron un aumento de las malformaciones en un estudio de la
teratogénesis después de administrar por vía oral a ratas gestantes
252 ó 1008 mg de 2-propanol por kg al día (no se formularon
observaciones sobre la toxicidad materna). Ambas dosis,
administradas en el agua de bebida durante 45 días, también
aumentaron la duración del ciclo estrual hasta 5 días (frente a 4
días en los sujetos de control). Se observó una mortalidad
embrionaria total mayor cuando se administraban a la rata hembra
dosis de 1800 mg/kg en el agua de bebida al día durante 6 meses
antes de criar; se notificaron diversos efectos en la supervivencia
intrauterina y puerperal a dosis tan bajas como 0,18 mg/kg al día,
pero no se observó ninguna pauta coherente. Se expusieron ratas
gestantes a 2-propanol atmosférico a concentraciones de 9001,
18 327 ó 23 210 mg por m3 (3659, 7450 ó 9435 ppm). Las dos
concentraciones más elevadas fueron tóxicas para las madres, pero
no así la de 9001 mg/m3. Se observó toxicidad en el desarrollo con
las tres concentraciones.
El 2-propanol dio resultados negativos en una prueba con 0,18
mg por placa para detectar mutuaciones puntuales en S.
typhimurium y en una prueba de intercambio de cromátidas hermanas
en fibroblastos pulmonares de hámster chino. Indujo anomalías
mitóticas en células de médula ósea de rata y en células de ápice
radicular de cebolla in vitro. No se dispone de otros datos sobre
mutagenicidad.
El 2-propanol se ensayó en varios estudios limitados de
carcinogenicidad en el ratón utilizando las vías de exposición
cutánea (3 veces a la semana durante un año), inhalación (7700
mg/m3 durante 3 - 7 h/día, 5 días/semana, durante 5 - 8 meses) y
subcutánea (20 mg sin diluir, semanalmente durante 20 - 40
semanas). La aparición de tumores se investigó en los tres
estudios en la piel, el pulmón y el lugar de inyección,
respectivamente. No se observaron efectos carcinogénicos. No se
dispone de datos epidemiológicos adecuados con los que evaluar la
carcinogenicidad del 2-propanol para el ser humano. Los datos
disponibles indican que el di-2-propilsulfato, un producto
intermedio en los procesos de ácidos fuertes y débiles para
producir 2-propanol, puede estar asociado causalmente con la
inducción de cáncer del seno paranasal en el ser humano.
8. Efectos en la salud humana
Se han notificado varios casos de intoxicación tras la
ingestión oral y también en niños con fiebre a los que se refrescó
con esponjas impregnadas con preparaciones con 2-propanol. En
casos de envenenamiento, los principales signos son los de la
intoxicación alcohólica, en particular náuseas, vómitos, dolores
abdominales, gastritis, hipotensión e hipotermia. El 2-propanol
deprime el sistema nervioso central unas dos veces más que el
etanol, provocando una inconsciencia que termina en coma profundo;
puede sobrevenir la muerte por depresión respiratoria. Otros
efectos relacionados con el compuesto son la hiperglucemia,
elevados niveles de proteínas en el líquido cefalorraquídeo y
atelectasia. Aunque se considera que la absorción por la piel es
insignificante, en un informe sobre un caso de un niño intoxicado
tras refrescársele con una esponja impregnada con 2-propanol, se
indicaba que no conviene subestimar el riesgo de absorción dérmica,
especialmente en los niños. No se observaron efectos adversos en
voluntarios sanos que bebieron diariamente durante 6 semanas un
jarabe que contenía 2,6 ó 6,4 mg de 2-propanol/kg. Un grupo de
varones voluntarios, cuando se expusieron a vapores de 2-propanol
en concentraciones de 490, 980 ó 1970 mg por m3 de aire durante
3 - 5 minutos juzgaron que la irritación era "leve" a 980 mg/m3 y
"satisactoria" para su propia exposición ocupacional de 8 horas.
Las irritaciones de la piel en forma de eritema, quemaduras de
segundo y tercer grado y ampollas se notificaron en niños
prematuros tras un contacto prolongado con 2-propanol. En
ocasiones también se han notificado casos de dermatitis alérgica
por contacto.
Se dispone de pocos estudios epidemiológicos sobre mortalidad
por cáncer o por otras causas. En un grupo de 71 trabajadores
empleados durante más de 5 años en una fábrica de 2-propanol por el
proceso del ácido fuerte, se notificaron 7 casos de cáncer, entre
ellos 4 de cáncer del seno paranasal. En un estudio de cohortes
realizado sobre 779 trabajadores en una fábrica similar, las
incidencias reajustadas en función de la edad y del sexo de cáncer
del seno y de la laringe fueron 21 veces mayores de lo esperado.
El periodo mínimo de latencia fue de 10 años. En otro estudio
retrospectivo de cohortes realizado en otra fábrica que utilizaba
el proceso del ácido fuerte, había más de 4000 personas-años
expuestas. Los resultados mostraron que las tasas de mortalidad
por todas las causas y por neoplasmas no eran significativamente
mayores de lo previsto. Se llevó a cabo un estudio retrospectivo
de cohortes en una planta que fabricaba 2-propanol por el proceso
del ácido débil. Había más de 11 000 personas-años expuestas. La
tasa de mortalidad debida a todas las causas fue más baja de lo
esperado. No se observó mortalidad excesiva por todos los
cánceres. Sin embargo, la incidencia del cáncer de la boca y de la
faringe fue 4 veces más elevada de lo previsto. Los estudios de
cohortes indican en conjunto un riesgo de cáncer relacionado con el
proceso de fabricación con ácido fuerte, pero, en dos pequeños
estudios controlados de casos, no se observó correlación alguna
entre la exposición a 2-propanol y la incidencia de gliomas o de
leucemia linfática.
Algunos informes parecen indicar que la exposición combinada a
tetracloruro de carbono y 2-propanol en los trabajadores potencia
la toxicidad del primero.
9. Resumen de la evaluación
La exposición del hombre al 2-propanol puede producirse por
inhalación durante la fabricación, el tratamiento y el uso tanto
ocupacional como doméstico. La exposición a un nivel
potencialmente letal en la población general puede producirse por
ingestión accidental o intencionada y los niños pueden estar
expuestos cuando se les refresca con esponjas impregnadas con
preparaciones a base de 2-propanol (alcohol para friegas).
El 2-propanol se absorbe y distribuye rápidamente por todo el
organismo, en parte en forma de acetona. Los datos sobre
exposición-efecto en el hombre en condiciones de sobreexposición
aguda son escasos y muestran grandes variaciones. Los principales
efectos son la gastritis, la depresión del sistema nervioso central
con hipotermia y depresión respiratoria, y la hipotensión. Los
datos de mortalidad aguda en animales de experimentación indican
que la toxicidad del 2-propanol es baja, siendo los valores de DL50
orales en diversas especies entre 4475 y 7990 mg/kg, y los valores
de CL50 de inhalación en ratas alrededor de 50 000 mg/m3. En el
conejo, el 2-propanol no produjo irritaciones cutáneas, pero la
aplicación de 0,1 ml de 2-propanol sin diluir produjo irritación en
los ojos.
En el hombre, los efectos agudos más probables de la exposición
a concentraciones elevadas de 2-propanol por ingestión o inhalación
son la intoxicación alcohólica y la narcosis.
No se han hecho suficientes estudios en animales como para
evaluar los riesgos que entraña para la salud humana la exposición
repetida al 2-propanol. No obstante, los resultados de dos
estudios a corto plazo en la rata, incluida la exposición por
inhalación (500 mg/m3 durante 4 horas al día y 5 días a la semana
durante 4 meses) y la exposición oral (600 - 3900 mg/kg en el agua
de bebida) indican que debe evitarse la exposición al 2-propanol en
algunos de los muy elevados niveles de exposición ocupacional que
se han notificado.
La exposición por inhalación de ratas gestantes a 2-propanol
dio un nivel mínimo de observación de efectos de 18 327 mg/m3 (7450
ppm) y un nivel sin efectos observados de 9001 mg/m3 (3659 ppm)
respecto a la toxicidad materna. En el mismo estudio, 9001 mg/m3
(3659 ppm) fue el nivel más bajo de observación de efectos en lo
que respecta a la toxicidad de desarrollo; no se indicó nivel sin
efectos observados. Estas concentraciones son más elevadas que las
que normalmente se registran en condiciones de exposición humana.
El 2-propanol dio resultado negativo en las pruebas de
genotoxicidad, pero indujo aberraciones mitóticas en la médula ósea
de la rata. Aunque estos resultados indican que la sustancia no
tiene potencial genotóxico, no puede hacerse una evaluación
correcta de la mutagenicidad basándose en datos tan limitados.
Los datos disponibles no bastan para evaluar la carcinogenicidad
del 2-propanol en animales de experimentación. No se dispone de
datos para evaluar la carcinogenicidad del 2-propanol en el ser
humano.
Es poco probable que el 2-propanol plantee un riesgo grave para
la salud de la población general en las condiciones de exposición
que se producen normalmente.
El 2-propanol desaparece rápidamente (periodo de semieliminación
2,5 días) de la atmósfera y su desaparición del agua y del suelo se
produce rápidamente por biodegradación aerobia y anaerobia,
especialmente tras la adaptación de microorganismos inicialmente
sembrados. En vista de las propiedades físicas del 2-propanol, su
potencial de bioacumulación es bajo. No representa un riesgo para
los organismos naturales en las concentraciones en que suele
encontrarse en el medio ambiente.
Since adequate balance studies have not been conducted, the
extent of this metabolism to acetone is not known. Siebert et al.
[232] injected 750 or 1300 mg/kg body weight intravenously in
rabbits and estimated that 64 - 84% of the dose was oxidized to
acetone, confirming the earlier findings of Pohl [204].
Evidence of another metabolic pathway was found in rabbits
given an oral dose of 2-propanol of 3000 mg/kg body weight, 10.2%
of the dose was found in the urine as beta-isopropyl-glucuronide
[129].
Sufficient evidence is available to show that 2-propanol is
oxidized to acetone mainly by the non-specific cytosolic enzyme
alcohol dehydrogenase (ADH) (EC 1.1.1.1). When either pyrazole or
4-methylpyrazole (inhibitors of ADH) was administered to rats prior
to exposure to 2-propanol, both the elimination of 2-propanol and
the production of acetone were retarded [121, 193]. The
elimination of 2-propanol by rats was retarded when it was given in
combination with ethanol; both compounds are substrates for ADH [2,
121]. Enzyme kinetic data show that 2-propanol is a poorer
substrate of ADH than ethanol. In vitro, the Michaelis-Menten
constant (Km) for horse liver ADH was 0.0126 mol/litre, using
2-propanol as a substrate, and 0.00018 mol/litre, using ethanol as
a substrate [64]. For rat liver ADH in vivo, Km was 0.034
mol/litre, using 2-propanol as a substrate, and 0.00192 mol/litre,
using ethanol as a substrate. The maximum initial velocity (Vmax)
of rat liver ADH and the substrate 2-propanol in vivo was 1.7 times
less than that with the substrate ethanol. It was further shown
that the ratio of the relative rates of oxidation of 2-propanol and
deuterated 2-propanol-d7 were about the same in vitro and in vivo.
From this, it can be concluded that ADH activity is the rate-
limiting factor in 2-propanol metabolism [54].
It has been shown that rat liver microsomal oxidases are also
capable of oxidizing 2-propanol, but that the compound is not an
effective substrate for the peroxidative activity of catalase
(EC 1.11.1.6) [48, 193].
6.3.2 Human beings
Acetone has also been found in the blood of human beings after
exposure to 2-propanol [e.g., 11, 41, 65, 131]. Furthermore,
acetone was detected in the spinal fluid, at levels similar to
those in serum, in 2 persons after ingestion of 2-propanol [5, 191].
In the investigations of Brugnone et al. [41] (section 6.1.2),
elevated acetone levels were measured in the blood, alveolar air,
and urine of 12 printing workers. 2-Propanol was not detected in
the blood or urine. The concentrations of acetone ranged between
0.76 and 15.6 mg/litre in the blood and between 3 and 93 mg/m3 in
the alveolar air. The acetone levels in alveolar air and blood
increased with the increasing exposure period and were linearly
related to the alveolar 2-propanol levels. Urinary acetone levels
ranged from 0.85 to 53.7 mg/litre in overnight pooled urine samples
and were highly correlated with the alveolar 2-propanol uptake and
with blood acetone levels. Pulmonary and renal clearance of
acetone were 41 - 97 and 0.1 - 3 ml/min, respectively, for 8 of the
workers, showing that acetone is mainly excreted via the lungs.
Pulmonary acetone excretion varied from 10.7 to 39.8% of the
uptake and was inversely related to the exposure level [41]. It
is known that the metabolic capacity of the human liver for acetone
is limited [161].
2-Propanol and acetone have also been found in the returns of
gastric lavage [5] and in saliva [250]. Reabsorption may follow
excretion via these routes.
When 10 volunteers drank orange juice containing doses that
gave 3.75 mg 2-propanol and 1200 mg ethanol/kg body weight over
2 h, 2-propanol was detected in the blood, partly as sulfate
(section 6.1.2), and in the urine, partly as glucuronide. The
total urinary excretion of 2-propanol was 1.9% of the dose [27, 28].
Human data support the importance of the ADH pathway for the
oxidation of 2-propanol as observed in experimental animals. In
the studies of Bonte et al. [27, 28] described above, 2-propanol
was only detected in the blood when ethanol was ingested
simultaneously, indicating a retarded elimination. Among 74
persons intoxicated by 2-propanol, significantly lower acetone
values were found in the blood of those who had also been exposed
to ethanol [11, 131]. In in vitro studies, the activity of human
ADH with 2-propanol was 9 - 10% of the activity of the enzyme with
ethanol as substrate [121].
Endogenous formation of 2-propanol has been revealed at
autopsies of individuals not previously exposed to this compound.
This observation and the results of additional studies on rats show
that 2-propanol can result from the reduction of acetone by liver
ADH, especially when high levels of acetone and high NADH/NAD+
ratios occur. Such conditions are found in diabetes mellitus,
starvation, high fat feeding, chronic alcoholism, and dehydration
[68, 161, 248].
6.4 Elimination and Excretion
6.4.1 Animals
In a review article, Rietbrock & Abshagen [218] concluded that
urinary excretion of both 2-propanol and its metabolite acetone is
limited and in each case does not exceed 4% of the dose in the rat,
rabbit, and dog. The major route of excretion is via the lungs.
2-Propanol is excreted via the gastric juice and saliva in the dog
[158] and through breast milk in the rat, as shown by tissue levels
in the newborn [159].
The disappearance of 2-propanol from the blood of experimental
animals was found to be a first-order process at doses <1500 mg/kg
[1, 232]. In rats, the half-life of 2-propanol was 1.5 h at an
intraperitoneal dose of 500 mg/kg body weight and increased to
2.5 h at a dose of 1500 mg/kg body weight. This can be explained
by a limited metabolic capacity as suggested in section 6.3.1
[218]. Simultaneous administration of ethanol to rats increased
the half-life of 2-propanol in the blood approximately 5-fold
following acute exposure [2]. A half-life of 4 h was determined in
dogs administered 1000 mg 2-propanol/kg body weight intravenously [1].
The biotransformation of 2-propanol and the elimination of the
acetone produced are both slow processes. Peak acetone levels in
the blood of various species, following single exposures via
different routes, were only reached several hours after exposure,
though acetone was already detectable shortly after the start of
exposure [1, 121, 150, 151, 193, 232]. The elimination of acetone
in the dog and the rat was found to be a first-order process with
half-lives of 11 and 5 h, respectively [1].
After prolonged administration of 2-propanol to dogs [159] and
rats [224], the elimination rate of 2-propanol was increased.
Simultaneous exposure of rats to ethanol in the drinking-water (5%)
and atmospheric 2-propanol at 738 mg/m3 (300 ppm) for 5 - 21 weeks
significantly increased the rates of elimination of 2-propanol and
acetone [224].
6.4.2 Human beings
The elimination of 2-propanol in human beings also appears to
follow first-order kinetics. In two alcoholics who had ingested
rubbing alcohol, 2-propanol was eliminated from the blood with
half-lives of 2.5 and 3 h, respectively. Acetone levels declined
slowly over the next 30 h; no ethanol was detected [65]. A half-
life of 2-propanol of 6.4 h was determined in a non-alcoholic, who
also had ingested rubbing alcohol. The acetone level in the blood
reached a maximum 30 h after admission to hospital. The half-life
of acetone was 22 h. Ethanol was also found in the blood. The
differences in the half-lives in these three cases may reflect
metabolic adaptation in the alcoholics, or genetic variations in
ADH [191].
When 4 volunteers ingested an artificial liquor containing 40%
2-propanol, acetone was detectable in exhaled air from 15 min after
exposure and in the urine from 1 h after exposure [132].
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Aquatic Organisms
A summary of acute aquatic toxicity data is presented in Table 8.
The concentration of 2-propanol was reported to have been measured
in only 3 of the studies [34, 111, 263] and in no case were
potential metabolites considered. In view of the volatility of the
compound, it can be expected that the toxic effects observed in the
other open systems studied occurred at lower concentrations than
the nominal ones.
Several short- and long-term studies have also been conducted.
Seiler et al. [227] determined the breakpoint of bioinhibition for
a total of 20 strains of bacterial groups prevalent in a waste-
water treatment plant in the chemical industry, i.e., Zoogloea,
Alcaligenes, and Pseudomonas. After one week of static exposure to
2-propanol in an open system at 30 °C, 100% growth inhibition
occurred at concentrations of 80 000 - 100 000 mg/litre of medium.
No analysis for the compound was reported. The cell multiplication
of blue algae (Microcystis aeruginosa) and green algae (Scenedesmus
quadricauda) was just inhibited after 8 days of static exposure to
1000 and 1800 mg/litre water, respectively, in a closed system at
27 °C and a pH of 7 [35, 37]. When water fleas (Daphnia magna)
were exposed to 2-propanol for 16 days in a semi-static test at
19 °C and a water hardness of 100 mg CaCO3/litre, the highest
concentration that did not result in a significant reduction in
growth was 141 mg/litre water. The water was analysed for the
compound just before and after each renewal of test solution [111].
A 7-day LC50 of 7060 mg 2-propanol/litre water was determined for
2 - 3-month-old guppies (Poecilia reticulata) in a semi-static
test. No analysis for 2-propanol was reported for this open system
[141].
7.2 Terrestrial Organisms
7.2.1 Microorganisms
The sensitivity of 4 soil fungi, i.e., Chrysosporium
crassitunicatum, Nannizzia fulva(+), Nannizzia fulva(-), and
Trichophyton equinum, to saturated 2-propanol vapour was
determined at 28 °C and a pH of 7. Mycelial growth of
Chrysosporium was stimulated after 14 days of exposure, while that
of the other strains was inhibited. Sporulation was poor in
Chrysosporium and in Trichophyton, and fair or good in the other
strains [233].
Table 8. Acute aquatic toxicity of 2-propanol
---------------------------------------------------------------------------------------------------------------------------------------
Organism Description Temperature pH Dissolved Hardness Stat/flow Exposure Parameter Nominal Reference
(°C) oxygen (mg CaCO3/ open/closeda period concentration
(mg/litre) litre) (mg/litre)
---------------------------------------------------------------------------------------------------------------------------------------
FRESHWATER
Bacteria Pseudomonas 25 7 stat, closed 16 h TTb 1 050 [37]
putida
Micro- activated 21 7.4-8 stat, closed 3 h 50% inhibition 1 000 [138]
organisms sludge of respiration
rate
Protozoa Entosiphon 25 6.9 stat, closed 72 h TTb 4 930 [36]
sulcatum
Protozoa Chilomonas 20 6.9 stat, closed 48 h TTb 104 [40]
paramecium
Protozoa Uronema 25 6.9 stat, closed 20 h TTb 3 425 [38]
parduczi
Crustacea water flea 20 8 2 250 stat, open 24 h EC50c 9 714 [39]
(Daphnia EC0 5 102
magna) EC100 10 000
Crustacea water flea 22 100 stat 44 h EC50c,d 2 285 [110]
(Daphnia
magna)
Amphibia frog tadpole 20 stat threshold 22 530 [190]
(Rana pipiens) narcotic
concentration
Fish fathead minnow 18-22 4 stat, open 96 h LC50 11 130 [184]
(Pimephales 24 h LC50 11 160
promelas)
Fish fathead minnow 25 7.5 5 45 flow, open 96 h LC50 9 640-10 400f [262]
(Pimephales
promelas)
---------------------------------------------------------------------------------------------------------------------------------------
Table 8. (contd.)
---------------------------------------------------------------------------------------------------------------------------------------
Organism Description Temperature pH Dissolved Hardness Stat/flow Exposure Parameter Nominal Reference
(°C) oxygen (mg CaCO3/ open/closeda period concentration
(mg/litre) litre) (mg/litre)
---------------------------------------------------------------------------------------------------------------------------------------
Fish goldfish 20 6-8 4 280 stat, open 24 h LC50 5 000f [34]
(Carassius
auratus)
Fish golden orfe 20 7-8 5 200-300 stat 48 h LC50 8 970 [127]
(Leuciscus
idus melanotus)
Fish harlequin fish 20 8.1 20 flow, open 96 h LC50 4 200 [251]
(Rasbora 96 h LC10 1 500
heteromorpha
duncker)
Fish Creek chub 15-21 8.3 98 stat, open 24 h LC0 900 [96]
(Semotitus
atromaculatus)
SEA WATER
Bacteria Photobacterium 15 7 stat, closed 2 min TTh 15 000 [43]
phosphoreum 15 stat, closed 5 min 50% light 35 000 [44, 62]
reduction 42 000
Worm Tubifex 20 stat 2 min EC100c 51 080 [56]
tubifex
Crustacea Brine shrimp 24 stat, open 24 h LC50 10 000 [208]
(Artemia
salina)
Crustacea Brown shrimp semi-stat, 96 h LC50 1 150 [26]
(Crangon open
crangon)
---------------------------------------------------------------------------------------------------------------------------------------
a Static or flow-through test; open or closed system. e Concentration at which touching the tadpoles failed to cause
b TT = toxic threshold for inhibition of cell multiplication. motion; exposure period and conditions; and the method of
c Effect is complete immobilization. calculation of the threshold not specified.
d Calculated value based on a quantitative structure-activity f Analysis for 2-propanol reported.
relationship between the n-octanol/water partition coefficient of g Test compound was Imsol A (90% 2-propanol, remainder unknown)
a group of 19 organic chemicals and their anaesthetic potency. h TT = toxic threshold for light reduction.
7.2.2 Insects
The 4-h LC50 for third instar mosquito larvae (Aedes aegypti)
was 25 120 mg/litre water in a static test at 22 - 24 °C [143].
The 48-h LC50 values for the fruit fly strains Drosophila
melanogaster and Drosophila simulans were between 10 200 and 13 340
mg/litre of nutrient medium in static tests [66]. Exposure of
Drosophila melanogaster eggs and larvae to 7850 mg 2-propanol/litre
of nutrient medium caused an 87% decrease in the activity of the
non-specific enzyme alcohol dehydrogenase (EC 1.1.1.1) in the 14-
day-old larvae. At the same time, viability was decreased by 74%.
The development of Drosophila simulans was completely inhibited at
the same concentration. Gas chromatographic analysis revealed
that, after 4 days of exposure, acetone appeared as the 2-propanol
concentration decreased. The appearance of acetone was associated
with an observed decrease in enzyme activity [265]. Anderson &
McDonald [13] also found a decrease in the specific activity of
alcohol dehydrogenase in Drosophila melanogaster after 1 - 4 days
of exposure to 2-propanol. On the other hand, they found that the
stability and concentration of the enzyme increased. The authors
explained the findings as an adaptation of the fruit fly to its
environment.
7.2.3 Plants
The effects of 2-propanol on the rate of seed germination were
investigated on several occasions. Total inhibition of the
germination of barley grains was reached after incubation for 4
days at 18 °C on filter papers, absorbing a solution containing
39 420 mg 2-propanol/litre water [56]. The germination of white
amaranth (Amaranthus albus) seeds was not affected after 5 h of
incubation at 25 °C on filter papers moistened with a solution
containing 36 050 mg 2-propanol/litre of water [49]. Reynolds
[216] measured 50% inhibition of germination in lettuce (Lactuca
sativa) seeds after incubation for 3 days at 30 °C on agar
containing 2100 mg 2-propanol/litre. At 6000 mg/litre, no
germination at all was observed. However, above 18 030 mg/litre,
germination was again observed and reached a maximum of 62% at
26 440 mg/litre. Inhibition of the growth of hypocotyls and
rootlets after 6 days of incubation gradually increased with
concentration. ED50 values were above 36 000 mg/litre.
In a 28-day study on cell suspensions of root sections of
soybean, (Glycine max), at 26 °C and a pH of 5.6, onset of growth
was delayed for 1 and 2 weeks at 2-propanol concentrations of
10 000 and 20 000 mg/litre of nutrient medium, respectively [67].
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single Exposures
8.1.1 Mortality
Available acute mortality data are summarized in Table 9.
Except where otherwise indicated, the vehicle was water or saline
solution. LD50 values for several animal species after oral
administration vary between 4475 and 7990 mg/kg body weight. Using
14-day-old rats of both sexes, Kimura et al. [134] established an
oral LD50 of 4396 mg/kg body weight for undiluted 2-propanol; this
was not significantly different from that obtained for young male
adults (4710 mg/kg body weight) or older male adults (5338 mg/kg
body weight).
8.1.2 Signs of intoxication
Sprague-Dawley rats of both sexes, inhaling 2-propanol for 8 h
at concentrations between 19 680 and 64 206 mg/m3, showed severe
irritation of the mucous membranes and depression of the central
nervous system indicated by subsequent ataxia, prostration, and
narcosis. These effects were concentration- and time-dependent.
Rats surviving the narcosis recovered. Transient paralysis of the
hind limbs was observed at levels between 49 200 and 54 120 mg/m3.
The rats died at and above 44 280 mg/m3, usually within 2 days,
with females dying earlier than males. All rats were autopsied,
including survivors, 15 days after exposure. At non-lethal levels,
congestion of the liver, lung, and spleen were observed, especially
in males. At lethal levels, vacuolation of the liver, acute
pneumonitis, and spleen oedema were found. These effects were most
pronounced in survivors. At 51 660 mg/m3, severe cytoplasmic
degeneration of the liver and oedema of the lung and brain occurred
in all rats [151]. Deep narcosis occurred in rats exposed through
inhalation to 40 mg/litre for 2 h. Significant and lasting
hypothermia was induced in Sprague-Dawley rats exposed for 4 h to
vapour concentrations of 19 680 mg/m3 or more [151].
Rats and mice of unknown strains, exposed orally or through
inhalation to lethal levels of 2-propanol, showed unspecified signs
of irritation and died through respiratory arrest while under
narcosis, usually within 24 h following exposure. Necropsy
revealed oedema, haemorrhage, inflammation, and dystrophy in the
interstitial tissues of parenchymal organs. In the lung,
infiltration, oedema, and thinning of the alveolar walls were
observed [103].
Table 9. Lethality of 2-propanol
---------------------------------------------------------------------------------------------------
Species/strain Sex Route of exposure Observation LD50 LC50 Reference
period (mg/kg body (mg/m3)
weight)
---------------------------------------------------------------------------------------------------
Rat not reported oral 3 days 5 280 - [157]
Sherman rat not reported oral 14 days 5 480 - [239]
Sprague-Dawley rat male oral 7 days 4 710 - [134]
Rat not reported oral 14 days 5 500 - [103]
Mouse not reported oral 14 days 4 475 - [103]
Rabbit not reported oral 3 days 5 030 - [157]
Rabbit male oral 1 day 7 990 [190]
Dog not reported oral 3 days 4 830 - [157]
Wistar rat male intravenous 5 days 1 088 - [247]
H mouse male intravenous 5 days 1 580 - [247]
H mouse female intravenous not reported 1 860 - [56]
Chinchilla rabbit male, female intravenous 5 days 1 184 - [247]
Wistar rat male intraperitoneal 5 days 2 830 - [247]
H mouse male intraperitoneal 5 days 4 868 - [247]
Syrian hamster male intraperitoneal 5 days 3 467 - [247]
Rabbit not reported dermal not reported 12 870 - [239]
Rat not reported inhalation (4 h) 14 days - 72 600 [103]
Sprague-Dawley rat male inhalation (8 h) 15 days - 46 740 [150]
Sprague-Dawley rat female inhalation (8 h) 15 days - 55 350 [150]
Mouse not reported inhalation (2 h) 14 days - 53 000 [103]
---------------------------------------------------------------------------------------------------
The effects of 2-propanol on the mucociliary system of the
trachea [197] and the middle ear [198] were investigated in two
separate studies. Groups of 20 or 24 Hartley guinea-pigs were
exposed to 2-propanol vapour at measured concentrations of 0, 969,
or 13 382 mg/m3 for 24 consecutive hours. Four animals from each
of the 3 groups were killed by decapitation at 12 h, 24 h, and 3,
7, and 14 days after the exposure period. A concentration-related
deterioration of ciliary activity and mucosal degeneration were
observed in both the trachea and in the middle ear. At the lower
exposure level, the effects completely reversed within 2 weeks of
exposure, but, at the higher exposure level, they did not.
2-Propanol can enter the trachea and deeper lung structures by
aspiration through the oral and nasal cavities. Anaesthetized rats
were made to inspire 110 or 140 mg of the compound in water, or 160
mg of the undiluted compound. Survivors were sacrificed 24 h later
for lung examination. At the lower doses, 1 out of 10 rats in each
group died. At the highest dose, 6 out of 10 rats died of cardiac
or respiratory arrest. All controls survived. It was not reported
whether the latter were sham-exposed or not. The average absolute
lung weight in the high-dose group was increased by 75% [94].
8.1.3 Skin, eye and respiratory tract irritation
In primary irritation patch tests on groups of 6 rabbits and 6
Hartley guinea-pigs, 0.5 ml of undiluted 2-propanol was applied to
areas of clipped or abraded skin. No irritation was observed for
up to 48 h after a 4-h exposure [194]. Erythema and changes in
vascular permeability did not occur when the clipped skin of
guinea-pigs was exposed for 2 min to discs of filter paper soaked
in undiluted 2-propanol [240].
Marzulli & Ruggles [182] reported on an interlaboratory
evaluation of the ocular irritancy of 2-propanol, according to
Draize scores, in groups of 6 rabbits, 1 - 7 days after application
of 0.1 ml of a 70% solution on the cornea. One day after exposure,
mean Draize scores were approximately 0.6 for corneal opacity, 0.3
for iritis, 1.5 for conjunctival redness, and 1.3 for chemosis.
The maximum total Draize score was 10.1. In each laboratory,
67 - 97% of the rabbits met the criteria for eye irritation, which
decreased over time. In another Draize test, groups of 6 - 9 New
Zealand rabbits received doses of 70% 2-propanol at levels of 0.01,
0.03, or 0.10 ml per eye. Maximum total Draize scores were 21, 36,
and 37, respectively, the maximum on the Draize scale being 110.
Using irritation categories based on the number of days required
for the effects to disappear, the compound was substantially
irritating (clearing within 14 days) at the higher doses and
moderately irritating (clearing within 7 days) at the lowest dose
[100].
The eye irritancy of 2-propanol was evaluated using Stauffland
albino rabbits, each of which received 0.1 ml of undiluted propanol
in one eye. Eye irritation was scored according to Draize up to 21
days after application. The exact scores were not reported, but
2-propanol was found to be corrosive according to the US EPA
criteria used in 1981, implying corneal involvement and irritation
or eye damage that persisted for more than 21 days after treatment
[188].
2-Propanol was tested on several occasions in in vitro
cytotoxicity assays and the results showed good agreement with
those in vivo [29, 30, 213].
The potency of 2-propanol as a sensory irritant was evaluated,
using a 50% reflex decrease in the respiratory rate of mice (RD50)
as an index. The mice were exposed by their heads only. An
exposure-related effect was found with RD50 values of 12 300 mg/m3
for Swiss OF1 mice and 43 525 mg/m3 for Swiss Webster mice [61, 130].
8.2 Continuous or Repeated Exposures
Groups of rats were exposed to 2-propanol vapour at
concentrations of 0, 100, or 500 mg/m3 air for 5 days/week and
4 h/day over 4 months [103]. Strain, sex, and group size were not
given, but were presumably in accordance with CMEA standards. No
deaths were reported. At the end of 4 months of exposure to 500
mg/m3 air, growth was reduced significantly by 10% and the
respiratory rate was increased by 22%. The white blood cell count
was decreased at both exposure levels in an exposure-related
manner. At 500 mg/m3, this was attributed to a decreased absolute
and relative number of lymphocytes. Decreases in hippuric acid
excretion and in total serum protein, and an increase in blood
acetylcholine were found at both exposure levels. The decrease in
total serum protein was exposure-related and could partly be
accounted for by the observed decrease in alpha1- and alpha2-
globulins and in albumin at 500 mg/m3. Blood glucose levels were
decreased at 500 mg/m3. Macroscopic and microscopic changes,
observed at 500 mg/m3, included irritant effects on the respiratory
system, such as thinning of the alveolar walls, perivascular
infiltration, pneumonia, and bronchitis. Dystrophic changes and
perivascular cell reactions were seen in the liver. Follicular
hyperplasia was observed in the spleen [103].
Another report described the continuous exposure of groups of
15 rats of unknown strain and sex to 2-propanol vapour at
concentrations of 0, 0.6, 2.5, or 20 mg/m3 air for 86 days. The
data were not statistically analysed. No deaths were reported. At
the highest exposure level, changes in the latency period of
unconditional reaction and an increase in the number of fluorescent
leukocytes were found. Effects reported at this exposure level
included an increase in sulfobromophthalein retention and decreased
blood levels of nucleic acids. Microscopy only revealed adverse
effects at 20 mg/m3 with liver dystrophy, degenerative changes in
the cerebral cortex, and spleen hyperplasia [19].
These studies [19, 103] lack a number of essential details
concerning the protocol, the effects observed, the incidence of
these effects, and the statistical analysis.
A "coefficient of accumulation" (the ratio between cumulative
LD50 and single dose LD50) reported for repeated oral exposure to
2-propanol was 4.0 in rats and 4.9 in mice [10].
Groups of 5 white rats of each sex and of unknown strain
received drinking-water containing 2-propanol for 27 weeks.
Average daily intakes were 0, 600, or 2300 mg/kg body weight for
males and 0, 1000, or 3900 mg/kg body weight for females. Only
males died: 2 at the lower dose and 3 at the higher dose. It was
reported that extensive postmortem changes prevented an accurate
diagnosis of the cause of death. At the end of the exposure
period, all exposed females showed growth retardation. Males
showed a slight decrease in growth initially but overtook the
controls in body weight towards the end of the exposure period. No
adverse effects were found on food intake, behaviour, and
histopathology [157].
8.3 Neurotoxicity and Behavioural Effects
2-Propanol passes the blood-brain barrier twice as effectively
as ethanol (section 6.2.1). The oral ED50 for narcosis in rabbits
exposed to 2-propanol was 2280 mg/kg body weight, i.e., 2.5 times
lower than that for ethanol [190]. The threshold of an acute
effect on the CNS was observed in rats after a 4-h exposure at 1450
mg/m3 using the method of flexor reflexes, the "summation threshold
method" [10]. The intraperitoneal ED50 for loss of righting reflex
in Swiss Webster mice, which was 164 mg/kg body weight, was 1.8
times lower than that for ethanol [169]. The threshold for the
induction of ataxia in Sprague-Dawley rats following intraperitoneal
exposure was 1106 mg/kg body weight [171]. In 2 tilted plane
tests, the performance of rats decreased by an average of 30 - 40%
after oral or intraperitoneal exposure to 2000 and 1800 mg/kg body
weight, respectively [178, 270]. On a molar basis, 2-propanol was
2.7 times as intoxicating as ethanol [270]. When C57BL/6J or
DBA/2J mice were exposed intraperitoneally to a single dose of 2-
propanol at 3 dose levels, the former strain showed increased
activity in the open field test at 392 mg/kg body weight, no effect
on activity at 785 mg/kg body weight, and narcosis at 1570 mg/kg
body weight. DBA/2J mice showed increased activity at the middle
dose level and narcosis at the high dose level [243]. Wistar rats,
inhaling 2-propanol at a concentration of 739 mg/m3 for 6 h/day, 5
days/week, for up to 15 weeks, did not show any adverse effects in
the open field test [224].
The depressive action of 2-propanol on the central nervous
system was related by several investigators to interactions with
neuronal membranes in vitro and in vivo. Lyon et al. [169]
observed a high correlation between the narcotic potencies of
aliphatic alcohols in mice and their ability to disorder brain
synaptosomal plasma membrane in vitro, as measured by electron
paramagnetic resonance, which was, in turn, related to membrane
solubility. In Sprague-Dawley rats, no change in synaptosomal
membrane fluidity was measured via in vitro registration of the
fluorescence polarization of 1,6-diphenyl-1,3,5-hexatriene, 20 h
after a single oral dose of 3000 mg/kg body weight. When
2-propanol was added subsequently, a change in membrane fluidity
was observed. This effect was related to membrane solubility.
The activity of synaptosomal Na+/K+-transporting
adenosinetriphosphatase (EC 3.6.1.37) was increased both in vitro
and in vivo [24].
Functional loss due to disruption of membrane integrity by
2-propanol was also observed in vitro by the blocking of the action
potentials of sciatic nerves in the toad, Bufo marinus [214], and
by the inhibition of membrane-bound guanylate cyclase (EC 4.6.1.2)
in intact murine neuroblastoma N1E-115 cells [241]. It was also
indicated by the interference of 2-propanol with the transport of
Ca2+ ions across biological membranes, as shown, in vitro, by the
inhibition of Ca2+ ion-induced contractions of guinea-pig ileum
(Yashuda et al., 1976), and, in vivo, in rats by a decrease in
regional brain Ca2+ ion levels, 30 min after one intraperitoneal
dose of 2000 mg/kg body weight [221].
Several neurochemical parameters were measured in the brain or
spinal cord axon of Wistar rats, inhaling 2-propanol at a
concentration of 739 mg/m3 air for 6 h/day, 5 days/week, over 5,
10, 16, or 21 weeks. In cerebellar homogenates, the activities of
superoxide dismutase (EC 1.15.1.1) and azoreductase (EC 1.6.6.7)
were decreased at weeks 16 and 21, but no effects were found on the
activity of dihydrolipoamide dehydrogenase (EC 1.8.1.4). In
cerebellar glial cells, the activity of acid proteinase (EC
3.4.21.1) was stimulated at weeks 5 and 10, while the glutathione
concentration was unaffected at all times. In the spinal cord
axon, the phosphate/cholesterol ratio of lipid was reduced by 25%
[224].
8.4 Biochemical Effects
8.4.1 Effects on lipids in liver and blood
Oral administration of single doses of 3000 or 6000 mg
2-propanol/kg body weight to Wistar rats caused a reversible
accumulation of triglycerides in the liver [21, 22, 23, 90]. At
the 6000 mg/kg body weight dose, a slight fatty infiltration was
observed histologically [90]. Observations that could explain this
effect were increased hepatic uptake of palmitate [23], increased
incorporation of palmitate into hepatic triglycerides, decreased
hepatic palmitate oxidation, and, at a later stage of intoxication,
interference with the synthesis and excretion of very dense
lipoproteins [21, 22, 23].
Decreased palmitate oxidation was related to the observed
increase in the hepatic b-hydroxybutyrate/acetoacetate ratio,
implying a decrease in the intramitochondrial NAD+/NADH ratio [21,
23, 195]. Excess extramitochondrial reducing equivalents,
indicated by an increased lactate/pyruvate ratio, was also observed
in vivo [21] and in vitro [85]. However, all these changes in the
redox state of the liver were very modest and therefore were not
thought to be fully responsible for the fatty livers induced.
More significant seemed to be the observation that the
incorporation of palmitate and glycerol into serum triglycerides
and the incorporation of palmitate into serum and hepatic
phospholipids were inhibited. This indicates an impaired secretion
of lipo-proteins, partly explained by the observed disturbance of
the biosynthesis of phospholipids, and partly by the observed
inhibition of hepatic protein synthesis [21, 22, 23]. Inhibition
of hepatic protein synthesis, as shown by the synthesis of the
marker enzymes ornithine decarboxylase (EC 4.1.1.17) and tyrosine
amino-transferase (EC 2.6.1.5), was observed in hepatectomized rats
following oral exposure to 2300 mg 2-propanol/kg body weight [205].
Acetone administration to rats up to a blood level comparable
to that in 2-propanol-dosed rats only slightly increased the level
of liver triglycerides; therefore acetone does not seem to play a
major role in the induction of fatty liver by 2-propanol [22].
8.4.2 Effects on microsomal enzymes
Oral exposure of Sprague-Dawley rats to 390 mg 2-propanol/kg
body weight, once or on 4 subsequent days, caused a slight but
significant increase in the hepatic cytochrome P-450 content and a
2 - 3-fold increase in the activities of the microsomal liver
enzymes (EC 1.14.14.1) aniline hydroxylase and 7-ethoxycoumarin
O-deethylase, 18 h after exposure [255]. Direct activation of these
enzymes does not offer a satisfactory explanation, as it was shown
in vitro that 2-propanol is an inhibitor of several microsomal
enzymes via an interaction with cytochrome P-450, which causes a
change in the reverse Type I spectrum [8, 57, 222, 246, 254, 285].
It was therefore concluded that, as acetone also does not appear to
be involved in aniline hydroxylase induction in vivo, 2-propanol
induces one or more forms of cytochrome P-450 [255]. In young
Sprague-Dawley rats, receiving 2-propanol once at a level of 1960
or 3140 mg/kg body weight and sacrificed 22 - 24 h later, the
hepatic cytochrome P-450 content increased, as well as the
activities of several nitrosamine N-demethylases, benzphetamine
demethylase, ethylmorphine demethylase, p-nitroanisole demethylase,
and 7-ethoxycoumarin- O-deethylase. A slight increase was observed
in the activity of NADPH cytochrome c reductase (EC 1.6.2.4).
Acetone appeared to play a role in this induction. From several
lines of observations, it was concluded that the enhanced activity
of nitrosodimethylamine N-demethylase was due to the induction of
specific forms of P-450 isozymes and P-450-dependent enzymes [255].
In several earlier studies on rats, at comparable or higher
oral doses, induction of microsomal liver enzymes was observed,
including a slight induction of NADPH cytochrome c reductase, but
no increase in hepatic cytochrome P-450 content [206, 207, 234].
Ueng et al. [255] explained the latter observation by suggesting
that the species of cytochrome P-450 induced must be normally
present in low concentrations, so that a several-fold increase in
catalytic activity could be induced without markedly increasing the
total content of the haemoprotein.
Sprague-Dawley rats were also exposed by inhalation to
2-propanol at concentrations of 490, 4920, or 19 680 mg/m3 air for
6 days/week, 6 h/day over 2 weeks. In the liver and kidneys, the
contents of cytochrome P-450 and cytochrome b5 were increased as
well as the activity of NADPH cytochrome c reductase at 4920 and
19 680 mg/m3. These effects were completely reversible in the
liver of rats exposed at 19 680 mg/m3 for 2 weeks and allowed to
recover for 4 weeks. However, they persisted in the kidneys [290].
8.4.3 Other biochemical findings
Differential effects have been observed on the glutathione
status of the liver following exposure of rats to 2-propanol. In
Sprague-Dawley rats inhaling 2-propanol at a concentration of 4920
or 19 680 mg/m3 for 6 days/week, 6 h/day, over 2 weeks, the reduced
glutathione concentration in the liver and kidneys increased
slightly while, at 19 680 mg/m3, the activity of glutathione
S-transferase (EC 2.5.1.18) increased by 13% in the liver only
[290]. The glutathione level in the liver of Wistar rats that had
received a single oral dose of 3110 mg 2-propanol/kg body weight
was reduced 6 h following exposure, while there was a 10% increase
in lipid peroxidation, as indicated by the formation of diene
conjugates [264]. A homogenate of normal or regenerating rat liver
did not show any increase in malondialdehyde production after
incubation with 2-propanol [6].
The activity of hepatic and kidney alanine aminotransferase (EC
2.6.1.2) in rats was unchanged 18 h after exposure to 1 or 4 daily
doses of 392 mg 2-propanol/kg body weight [255] or 4 h after
exposure to a single dose of 2300 mg/kg body weight [205]. Liver
damage was not observed in guinea-pigs histopathologically or by
any increase in serum ornithine carbamoyltransferase (EC 2.1.3.3),
24 h after receiving intraperitoneal doses of up to 1000 mg
2-propanol/kg body weight [73].
In Charles River rats, 2-propanol (785 mg/kg body weight ip)
induced hepatic metallothionein and caused low blood zinc levels
[244]. These changes were not prevented by adrenalectomy.
8.5 Immunological Effects
2-Propanol and acetone both enhanced the incorporation of
labelled thymidine into concanavalin A-stimulated murine spleen
cells in vitro [210]. 2-Propanol inhibited the killing of YAC-1
tumour cells by natural killer effector cells from mouse or rat
spleens [219]. Inhibition of the synthesis and/or the secretion
and/or function of at least one monocyte-derived substance that
inhibited cell proliferation was suggested as an explanation of
these findings [210].
8.6 Reproduction, Embryotoxicity, and Teratogenicity
Groups of 13 - 15 pregnant Sprague-Dawley rats were exposed to
2-propanol for 7 h/day on gestation days 1 - 19 at measured
concentrations of 9001, 18 327, or 23 210 mg/m3 (3659, 7450, or
9435 ppm). At the highest concentration, rats were completely
narcotized at the end of the first exposures, but by the end of the
19 days the effect was slight. Initial exposures to 18 327 mg/m3
(7450 ppm) caused an unsteady gait, but this was not noticeable by
the end of 19 days of exposure. Food consumption and maternal body
weight gain were significantly reduced by exposure to 18 327 and
23 210 mg/m3 (7450 and 9435 ppm). In the group exposed to 23 210
mg/m3 (9435 ppm): 6 out of 15 mated rats were not pregnant at
term, which was attributed to an exposure-induced failure of
implantation; 4 out of 9 pregnancies were totally resorbed; and
resorptions per litter were significantly increased. Fetal body
weights were significantly reduced, in a concentration-related
pattern, at all 3 concentrations. The incidence of cervical ribs
was significantly increased in the 23 210 mg/m3 (9435 ppm) group
[193].
A group of 6 female and 3 male rats (38 - 40 days old), of
unspecified strain, received drinking-water containing 2-propanol.
They were mated after approximately 2 months of exposure. This
schedule was repeated through 2 further generations. Average daily
intakes for the 3 generations were 1470, 1380, and 1290 mg/kg body
weight, respectively. The growth of the first generation was
retarded initially, but returned to normal by the 13th week.
Otherwise, no effects were found on growth and reproductive
functions [159].
When female hybrid rats received oral doses of 252 or 1008 mg
2-propanol/kg body weight per day for 45 days, the length of the
estrus cycle was increased by 23 - 24% [15]. Five female hybrid
rats also received daily doses of 1800 mg/kg body weight for 3
months before mating. Together with 6 controls they were
sacrificed on day 21 of pregnancy. The total embryonic mortality
rate was increased 2-fold, which was statistically significant [15].
In a 6-month drinking-water study, 2-propanol was administered
to hybrid rats of both sexes, at daily doses of 0.018, 0.18, 1.8,
or 18 mg/kg body weight. Mating of males with 5 - 7 females per
group occurred after the exposure period. When both sexes were
exposed to 18 mg/kg body weight, the litter size was increased.
The neonatal mortality rate was increased at 0.18 and 1.8 mg/kg,
when females only were exposed, at 18 mg/kg body weight, when males
only were exposed, and at the 2 highest exposure levels, when both
sexes were exposed. Neonates from the last group showed a dose-
related decrease in the rate of reaction to electric stimuli
(unconditioned defence reaction), but no other effects on their
development [15].
Groups of 10 - 13 female hybrid rats received 252 or 1008 mg
2-propanol/kg body weight from day 1 to day 20 of pregnancy. They
were sacrificed on day 21 of pregnancy together with 11 controls.
Litter size was reduced at both exposure levels. General embryonic
toxicity was 18 and 31%, respectively (10% in controls). At 1008
mg/kg body weight, total embryonic mortality rate was trebled and
10 out of 70 fetuses showed developmental anomalies compared with
none out of 90 controls. The anomalies found were brain damage in
3 fetuses, kidney damage in 5 fetuses, and gastrointestinal damage
in 2 fetuses. The lesions were not specified further. Data on
maternal toxicity were not reported [15].
8.7 Mutagenicity
A reverse mutation spot test with the Salmonella typhimurium
strains TA 98, TA 100, TA 1525, and TA 1537 was negative at 0.18 mg
per plate, with and without metabolic activation by S9 rat liver
[82].
Rat bone marrow cells were examined after 4 months of exposure
of rats to 2-propanol vapour at concentrations of 0, 1.03, or 10.2
mg/m3 air for 4 h/day. Statistically significant increases were
counted in the percentage of mitotic aberrations [18]. However,
the authors did not report the number of rats exposed, their sex,
or strain.
Root tip cells of onion (Allium cepa) exposed to 2-propanol
showed a 2.8-fold increase in the mitotic aberration rate [230].
2-Propanol did not increase sister chromatid exchange
frequencies in vitro in V79 Chinese hamster lung fibroblasts, with
or without S9 mix, when tested at concentrations of 3.3, 10, 33.3,
and 100 mmol/litre [267].
A dose-related increase in the inhibition of metabolic
cooperation in hamster V79 cells, a phenomenon believed to reflect
carcinogenic promotion ability and not to be indicative of
genotoxic potential, was observed by Chen et al. [53]. This may be
due to the membrane effects of 2-propanol.
8.8 Carcinogenicity
Several limited carcinogenicity studies on the mouse have been
conducted with 2-propanol, using the inhalation, dermal, and
subcutaneous routes of exposure.
Groups of 3 month-old, male C3H, ABC, and C57/BL mice were
exposed to 2-propanol vapour at a concentration of 7700 mg/m3 air
for 3 - 7 h/day, 5 days/week, over 5 - 8 months. The sizes of the
experimental and control groups were not reported. The occurrence
of lung tumours was examined microscopically in mice that had
survived until killed at the age of 8 months (36 exposed and 69
control C3H mice, 49 exposed and 120 control ABC mice), or 12
months (47 exposed and 52 control C57/BL mice) and showed
macroscopic lesions. The mice were not examined for the occurrence
of sinus tumours as occurred in workers engaged in the manufacture
of 2-propanol (section 9.2.1). No excess of lung tumours was
observed [276].
2-Propanol was painted on the clipped backs of 30 Rockland
mice, 3 times/week for 1 year. Sex, dose, and observation period
were not specified. Skin papillomas developed in 3 control mice,
one of which also had a skin carcinoma, while there was no response
in treated mice (Weil, C.S., written communication to US NIOSH [257]).
Groups of 3-month-old male C3H, ABC, and C57/BL mice received
20 mg undiluted 2-propanol subcutaneously once a week, for
20 - 40 weeks. The sizes of the treated and untreated control
groups were not reported. Surviving mice that showed macroscopic
lung lesions were examined microscopically for lung tumours. No
excess of lung tumours was observed. Lung tumour incidence in the
control group was high [276].
Doses of 20 mg undiluted 2-propanol were injected
subcutaneously in 40 C3H mice, once a week, over a period of 20
weeks. Controls were untreated. The occurrence of lung tumours
was examined in mice that survived until killed 5 months after the
first injection (22 exposed and 33 control mice). No excess of
lung tumours was observed (Weil, C.S., written communication to US
NIOSH [257]).
In these studies, only lung tumours and, in the case of dermal
exposure, skin tumours were investigated. Moreover, the exposure
periods, where given, were too short to allow for tumour induction.
Experimental details including size of experimental and control
groups, sex ratio, and observation periods were not quoted for some
of the studies. Because of these shortcomings, the studies are
inadequate for the assessment of carcinogenic potential.
8.9 Factors Modifying Toxicity
As a result of the induction of specific isozymes of cytochrome
P-450 (section 8.4.2) by 2-propanol and/or acetone, the
hepatotoxicity of carbon tetrachloride, 1,1,2-trichloroethane,
chloroform (CHCl3), trichloroethylene, and dimethylnitrosamine in
rodents is enhanced [60, 165, 234, 252, 253, 254]. The metabolic
activation of n-hexane by liver and kidney microsomes is also
increased by 2-propanol pretreatment [290].
9. EFFECTS ON MAN
9.1 General Population Exposure
9.1.1 Poisoning incidents
The subject of non-beverage alcohol use has already been
addressed in section 5.2.2. The clinical literature has been
reviewed [149, 176, 245, 163]. A brief summary of cases of
intoxication and major symptoms is presented in Table 10.
Table 10. Acute intoxications by 2-propanola
------------------------------------------------------------
No. of No. of Observationsb Reference
cases alcoholics 1 2 3 4 5 6 7
------------------------------------------------------------
Ingestion
57 46 +57 [11]
5 2 +5 +5 +4 +2 +4 +2 [4]
1c 0 - + + + + + + [186]
3 3 - +3 - +2 +2 +1 +2 [170]
1 0 - + + + + + - [191]
2 0 - +2 +2 +2 +1 [149]
1 1 - + + - + - + [245]
17 14 - +2 +3 +10 - - [131]
1 - + + + - [88]
1 1 - + + + [135]
1c 0 - + + - + - - [266]
1 1 - + + - - + [261]
1 0 - + + [75]
1 0 - + - - - [220]
2 1 - - + +2 - +2 +1 [5]
1 1 - - - + - + - [128]
1 1 - - - + + [108]
Sponging
1c - - + + + + + [228]
1c - - + + + - - + [160]
1c - - + + + [91]
1c - - + + - - + [172]
1c - - + [181]
1c - - + + + + - + [16]
--------------------------------------------------------------
a +N = observed in N cases; - = not observed; no sign = not
reported.
b 1 = death; 2 = coma; 3 = hypotension, meaning < 100 mm Hg systolic
pressure and/or < 60 mmHg diastolic pressure; 4 = tachycardia,
meaning > 100 beats per min; 5 = hypothermia, meaning < 36.5 °C;
6 = gastritis, characterized by nausea, vomiting, abdominal pain;
and/or gastric haemorrhage; 7 = elevated blood glucose, meaning
> 1500 mg/litre.
c Child below 2.5 years of age.
Intoxications have been reported after oral ingestion (Table
10), rectal administration [59], and inhalation by children who
have been sponged for several hours with 2-propanol preparations
for fever reduction (Table 10). Skin absorption in 2-propanol
sponging has been deemed insignificant [e.g., 149, 176]. However,
a case report on a child with an almost lethal blood level of
2-propanol after sponge bathing for fever reduction, and
questionable ingestion, suggested to the authors that the
importance of dermal absorption, compared with inhalation, should
not be underestimated [181].
2-Propanol depresses the central nervous system. At comparable
doses, 2-propanol is thought to be approximately twice as active as
ethanol in this respect with a longer duration of the coma due to
slower metabolism and to the contribution of acetone to the
depression of the central nervous system [149, 163, 176, 245]. As
with ethanol, excessive intoxication with 2-propanol causes
unconsciousness, usually, but not always ending in a deep coma [5,
59, 108, 128]; death may follow due to respiratory depression [4].
In most cases, decreased or absent reflexes are reported. Pupil
size is variable, but most often miotic [16, 88, 149, 220, 228,
266]. Early gastrointestinal problems and hypothermia frequently
occur. Cardiovascular effects include severe hypotension, shock
[4, 266], and even cardiac arrest [176, 266]. Tachycardia is found
as a secondary effect. Other possible compound-related effects are
hyperglycaemia, elevated protein levels in cerebrospinal fluid [5,
170, 266], and atelectasis [191, 220]. Lung congestion was
observed in fatal cases [4, 11].
In cases of 2-propanol intoxication, hypotension can be a grave
prognostic sign [4], which some authors suggest requires immediate
action by gastric lavage, haemodialysis [5, 22, 88, 135, 176], or
peritoneal dialysis [75, 186, 245]. Following treatment, recovery
is usually complete, unless the hypotension is severe and persistent.
In such cases, renal injury and death may occur [4, 128].
Acetone can be detected in the blood, urine, and breath.
Because other ketone bodies are not found in significant quantities,
early acidosis does not usually occur, serum bicarbonate, anion
gap, and blood pH remaining normal. Sometimes, a mild metabolic
acidosis and anion gap may develop as a result of lactic acid
accumulation. Acetonaemia and/or acetonuria without metabolic
acidosis and with normal or slightly elevated blood glucose levels
differentiate 2-propanol poisoning from diabetic ketoacidosis and
poisoning by other alcohols [16, 163, 176]. In addition, a
significant osmolality gap may be observed [16, 261].
None of the biochemical findings could be related to blood
levels of 2-propanol or acetone. 2-Propanol levels, measured at
different times after exposure, varied between undetectable
concentrations and 5600 mg/litre [176]. Acetone levels varied
between undetectable concentrations and 18 780 mg/litre [75].
Alexander et al. [11] suggested that combined levels of 2-propanol
and acetone may allow greater accuracy in predicting the clinical
course of the condition of a patient.
Adults have been reported to survive ingestion of 700 ml of
2-propanol [88] and to die after ingestion of 400 ml of 2-propanol
[4]. The lowest dose reported to be life-threatening was in an
18-month-old child who ingested approximately 170 ml of 2-propanol
[186].
9.1.2 Controlled exposures
No adverse effects were observed in groups of 8 healthy male
volunteers (24 - 57 years of age) who drank a daily dose of 2.6 or
6.4 mg 2-propanol/kg body weight in diluted syrup for 6 weeks.
Investigations included haematology, blood chemistry, urinalysis,
and ophthalmoscopy [283].
Another group of 10 healthy male volunteers (age not stated)
was exposed to 2-propanol vapour at nominal concentrations of 490,
980, or 1970 mg/m3 air for 3 - 5 min. After each exposure, the
participants were asked to classify the effects of the vapour on
the eyes, nose, and throat. The volunteers judged irritation to be
"mild" at 980 mg/m3 and "not severe" at 1970 mg/m3. They also
judged 490 mg/m3 to be "satisfactory" for their own 8-h
occupational exposure [192]. The validity of these results is
doubtful, for various reasons including the subjective criteria
used, the lack of control exposures, and the unreliability of the
exposure levels.
9.1.3 Skin irritation; sensitization
No skin irritation was observed in 6 human volunteers when 0.5
ml of undiluted 2-propanol was applied to a 4 cm square area on the
back and evaluated after 4 h, 24 h and 48 h [194]. Closed patch
tests with 10-min applications of 0.1 - 0.3 ml undiluted 2-propanol
on the dry skin of 10 healthy volunteers and 12 persons receiving
disulfiram therapy for the treatment of chronic alcoholism did not
result in any reaction. After immersion of the skin in tepid water
for 10 min, a transient erythema rapidly appeared on the site of
application in 19 subjects [105].
Skin irritation was also reported in a total of 6 premature
infants with a gestational age of less than 27 weeks. They were
exposed via swabs used for conduction in ECG recording or by the
application of 2-propanol on the umbilical area with subsequent
soaking of the diaper. Erythema and second or third degree burns
or blisters were observed in areas of prolonged contact with
2-propanol. Hypoperfusion of the skin was suggested to be a
contributing factor [226, 277].
In 1968, Wasilewski [272] reported a case of alleged allergic
contact dermatitis following skin disinfection with a 70%
2-propanol swab. The patient was treated for allergic rhinitis.
Patch tests were positive for 2-propanol. However, controls were
not tested and there was no information on the purity of the
alcohol. This report was followed by several others concerning a
total of 8 persons who showed dermatitis after contact with medi-
swabs containing 2-propanol. Patch testing revealed that another
volatile agent, presumably propylene oxide, caused the effect [174,
124, 217]. Only one of these 8 persons was also hypersensitive to
2-propanol of unknown purity [124].
Itching eczematous lesions developed in a laboratory worker, in
a company manufacturing hair cosmetics, in patch tests with
chemically pure 2-propanol solutions (2.5 - 99.7% by volume). This
person also reacted to 1-propanol, 1-butanol, 2-butanol, and
formaldehyde, but not to ethanol and methanol. Controls were not
tested [167]. Two out of 4 confirmed cases of hypersensitivity to
primary alcohols were also found to be hypersensitive to secondary
alcohols. Both cases had a history of contact allergies.
Recurrent exposures to 2-propanol and/or other alcohols occurred
through consumption of alcoholic beverages, pre-injection
disinfection, via a hair lotion in one case and occupationally in
the other case. Patch tests were positive for pure undiluted
2-propanol and 2-butanol. Twenty controls did not show any
reactions to secondary alcohols [86, 87]. The mechanism of alcohol
hypersensitivity is not clear. Fregert et al. [87] pointed out
that it is difficult to understand how alcohols can act as haptens.
9.2 Occupational Exposure
9.2.1 Epidemiology studies
A retrospective cohort study was reported among 182 workers at
a plant, in the USA, manufacturing 2-propanol by the strong acid
process over the period 1928 - 50. In a subgroup of 71 men, who
had been employed for more than 5 years, 7 cases of cancer were
observed: 4 cancers of the paranasal sinuses, 1 lung carcinoma, 1
laryngeal carcinoma, and 1 laryngeal papilloma. The minimum
latency period was 6 years [276]. According to USA vital
statistics for 1948, 0.0014 paranasal sinus cancers would have been
expected for the total cohort [286].
Two cases of paranasal sinus cancer and 2 cases of laryngeal
cancer were reported in 1966 in a cohort of 779 workers in a
similar plant in the USA that had been in operation since 1927.
The minimum latency period was 10 years. The age- and sex-adjusted
incidence of sinus and laryngeal cancer in this group was 21 times
higher than expected [119].
Another retrospective cohort study was undertaken among 262 men
who had worked for at least one year in a 2-propanol-manufacturing
plant in the United Kingdom using the strong acid procedure over
the period 1949 - 76. There were more than 4000 person-years at
risk. No person was lost to follow-up, which was over an average
of 15.5 years. The mortality rates due to all causes and due to
neoplasms were not significantly higher than expected according to
national vital statistics. One person died from nasal cancer
against 0.02 expected. Other significant findings were 2 "kidney
and adrenal malignancies" and 2 cancers of "the brain and the
central nervous system" [9].
A fourth retrospective cohort study was conducted over the
years 1966 - 78 among 433 workers in a 2-propanol manufacturing
plant, in the USA, who were exposed for at least 3 months during
the period 1941 - 65. The strong acid process used in 1941 had
been gradually changed to the weak acid process by 1965. More than
11 000 person-years were at risk. The mortality rate due to all
causes was lower than expected on the basis of state vital
statistics. No excess mortality due to all cancers was observed,
but the incidence of buccal and pharyngeal cancer was 4 times
higher than expected (2 versus 0.5). There was a slight excess of
lung cancer (7 versus 5.94) [79].
These cohort studies collectively suggest a cancer hazard
related to the strong acid manufacturing process. The excess in
respiratory cancers was initially attributed to isopropyl oils
[119, 276]. However, the experimental basis for this assumption is
weak (section 8.8) and more recently diisopropyl sulfate, an
intermediate produced in the strong acid process, has been proposed
as a more likely causative agent. The concentration of this
chemical is high in the strong acid process and low in the weak
acid process [79, 286].
Two small case-control studies were conducted in the USA: one
to consider the risk of lymphocytic leukaemia associated with 24
solvents among rubber industry workers [52], and the other to
consider the risk of brain gliomas associated with exposure
conditions during work at a chemical plant [155]. Although there
was no evidence of an association between exposure to 2-propanol
and the incidence of gliomas or lymphocytic leukaemia, the small
number of subjects and multiple exposures mean that no conclusions
can be drawn from these studies.
9.2.2 Interacting agents
Fourteen out of 43 workers in a 2-propanol-packaging plant
became ill when carbon tetrachloride was used for cleaning
equipment. The incidence and the severity of the effects increased
where exposure to carbon tetrachloride was the highest, and where,
on another occasion, the mean concentration of 2-propanol in air
was also highest, i.e., 1010 mg/m3. In 4 cases, renal failure or
hepatitis developed [83]. Another similar incident was reported in
a colour printing factory, 17 workers had abnormal liver function
and 3 developed acute hepatitis following combined exposure to
carbon tetrachloride and 2-propanol [71]. These reports suggest
potentiation of the toxicity of carbon tetrachloride by 2-propanol,
which was also found in experimental animals (section 8.9).
Some shaving lotions containing 2-propanol and other ingredients
(e.g., menthol, camphor, methyl salicylate, naphthalene) have been
reported to produce central stimulation with motor restlessness,
extreme apprehension, hallucinations, and general disorientation
[89, 238].
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of Human Health Risks
10.1.1 Exposure
Exposure of human beings to 2-propanol may occur through
inhalation during manufacture, processing, and both occupational
and household use. Average concentrations of up to 35 mg/m3 in the
ambient urban air and up to 500 mg/m3 in the occupational
environment have been measured. Concentrations between 0.2 and 325
mg/litre have been found in non-alcoholic beverages, and between 50
and 3000 mg/kg in foods (section 5).
Exposure of the general public to a potentially lethal level
may result from accidental or intentional ingestion and children
may be exposed when sponged with 2-propanol preparations (rubbing
alcohol) (sections 6 and 9).
10.1.2 Health effects
2-Propanol is rapidly absorbed after inhalation or ingestion
and distributed throughout the body as such, sulfated, or as its
metabolite, acetone (section 6). The possibility of dermal
absorption should not be neglected.
The acute toxicity of 2-propanol for animals is low (based on
lethality estimates) whether exposure occurs via the dermal, oral,
or respiratory route.
In man, the most likely acute effects of exposure to high
levels of 2-propanol by ingestion or inhalation are alcoholic
intoxication and narcosis. The results of animal studies indicate
that 2-propanol is approximately twice as intoxicating as ethanol
with an oral ED50 for rabbits of 2280 mg/kg and a threshold for
ataxia in rats of 1106 mg/kg intraperitoneally (section 8.3). Oral
LD50 values in various species vary between 4475 and 7990 mg/kg and
inhalation LC50 values between 50 000 and 70 000 mg/m3 (section
8.1.1).
Because ethanol retards the elimination of 2-propanol and is
also a CNS depressant, interaction between ethanol and propanol may
be expected to increase the CNS effects of either agent. The
majority of acute intoxication cases involved ingestion by known
alcoholics. Febrile children have experienced life-threatening
intoxications when treated by skin sponging with 2-propanol. In
these cases, skin absorption may also be an important route of
exposure in addition to inhalation (section 9.1.1).
Exposure-effect data on human beings in an acute overexposure
situation are scarce and show great variation. The major effects
are gastritis, depression of the central nervous system with
hypothermia and respiratory depression, and hypotension (section
9.1.1).
In rabbits, 2-propanol did not irritate the skin but did
irritate the eyes when 0.1 ml undiluted compound was applied
(section 8.1.3). Care is required in the use of 2-propanol as a
disinfectant on premature babies as it may cause severe skin
irritation following prolonged contact.
No adequate animal studies are available to make an evaluation
of the human health risks associated with repeated exposure to
2-propanol. However, 2 studies in rats with inhalation (500 mg/m3,
4 h/day, 5 days per week, for 4 months) or oral (600 - 3900 mg/kg
in drinking-water) exposure suggest that exposure to 2-propanol at
some of the very high occupational exposures reported should be
avoided (section 8.2).
2-Propanol administered in the drinking-water has been tested
for reproductive effects in a number of studies with conflicting
results. In one study, impaired neonatal survival was reported
after 6 months exposure of female rats to 0.18 mg/kg per day. In a
multi-generation study there were no adverse effects at drinking-
water doses as high as 1470 mg/kg per day. In a teratology study,
drinking-water doses of 252 and 1008 mg/kg per day produced
developmental toxicity, but this was not related to maternal
toxicity. Inhalation exposure of pregnant rats to 2-propanol
provided a LOEL of 18 327 mg/m3 (7450 ppm) and a NOEL of 9001 mg/m3
(3659 ppm) for maternal toxicity. In the same study, 9001 mg/m3
(3659 ppm) was a LOEL for developmental toxicity, with no
demonstration of a NOEL (section 8.6). These concentrations are
higher than those likely to be encountered under conditions of
human exposure.
2-Propanol was negative in a bacterial spot test and in a test
for sister chromatid exchange in mammalian cells in vitro. It
induced mitotic aberrations in the bone marrow of rats. Although
these findings suggest that the substance has no genotoxic
potential, no adequate assessment of mutagenicity can be made on
the basis of the limited data available. An in vitro test said to
predict promotional activity was negative (section 8.7).
The data available are inadequate to assess the carcinogenicity
of 2-propanol in experimental animals (section 8.8). There are no
data to assess the carcinogenicity of 2-propanol itself in human
beings. There are adequate data to indicate that the strong acid
process for the production of 2-propanol is causally associated
with the induction of paranasal sinus cancer in human beings,
probably due to exposure to the intermediate, di-2-propyl sulfate,
an alkylating agent and not to 2-propanol itself (section 9.2.1).
2-Propanol is shown to potentiate the hepatic toxicity of
halocarbons, such as carbon tetrachloride. Therefore simultaneous
exposure to 2-propanol and halocarbons should be avoided (section
9.2.2).
The Task Group considers it unlikely that 2-propanol will pose
a serious health risk to the general population under exposure
conditions likely to be encountered.
10.2 Evaluation of Effects on the Environment
By reacting with hydroxyl radicals and through rain-out,
2-propanol will disappear rapidly from the atmosphere, with a
residence time of less than 2.5 days (section 4.2). Hydrolysis and
photolysis are not important in the removal of 2-propanol from
water and soil but removal occurs quite rapidly by aerobic and
anaerobic biodegradation, especially after adaptation of initially
seeded microorganisms (section 4.3.1). Adsorption of 2-propanol on
soil particles is poor but it should be mobile in soil and it has
been shown to increase the permeability of soil to some aromatic
hydrocarbons. In view of the physical properties of 2-propanol,
its potential for bioaccumulation is low (section 4.3.2).
Toxicity in aquatic organisms was observed at levels ranging
from 104 mg/litre for one protozoan to over 50 000 mg/litre for
Tubifex worms. Insects and plant seeds were only affected at
concentrations above 2000 mg/litre (section 7).
On the basis of these data, it can be concluded that, except in
cases of accident and inappropriate disposal, 2-propanol does not
present a risk to naturally occurring organisms at concentrations
that usually occur in the environment.
11. RECOMMENDATIONS
1. 2-Propanol has not shown mutagenic potential in the small number
of assays performed. A full array of modern genotoxicity tests
should be completed.
2. Several studies of the carcinogenic activity of 2-propanol have
been published but all are seriously flawed and cannot be used
to evaluate the potential carcinogenicity of 2-propanol. The
desirability of a carcinogenesis bioassay of 2-propanol should
be considered based on the outcome of genotoxicity tests.
3. Inhalation exposure to overtly toxic concentrations of
2-propanol produced reproductive and developmental toxicity.
Additionally, the data available from drinking-water studies
are conflicting. In view of the potential for environmental
and drinking-water contamination, reproductive and
developmental toxicity should be conducted using oral dosing.
4. Epidemiological studies including precise exposure data would
assist in an assessment of the occupational hazards from
2-propanol.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
An evaluation of the carcinogenicity of 2-Propanol by the
International Agency for Research on Cancera was reported as
follows:
"A. Evidence for carcinogenicity to humans (sufficient for the
manufacture of isopropyl alcohol by the strong-acid process;
inadequate for isopropyl alcohol and isopropyl oils).
An increased incidence of cancer of the paranasal sinuses was
observed in workers at factories where isopropyl alcohol was
manufactured by the strong-acid process. The risk for
laryngeal cancer may also have been elevated in these workers.
It is unclear whether the cancer risk is due to the presence of
diisopropyl sulfate, which is an intermediate in the process,
to isopropyl oils, which are formed as by-products, or to other
factors, such as sulfuric acid. Epidemiological data
concerning the manufacture of isopropyl alcohol by the weak-
acid process are insufficient for an evaluation of
carcinogenicity."
"B. Evidence for carcinogenicity to animals (inadequate for
isopropyl alcohol and isopropyl oils).
Isopropyl oils, formed during the manufacture of isopropyl
alcohol by both the strong-acid and weak-acid processes, were
tested inadequately in mice by inhalation, skin application and
subcutaneous administration. Isopropyl oils formed during the
strong-acid process were also tested inadequately in dogs by
inhalation and instillation into the sinuses.
The available data on isopropyl alcohol were inadequate for
evaluation."
"C. Other relevant data
No data were available to the Working Group."
-------------------------------------------------------------------
a International Agency for Research on Cancer, Overall Evaluations
of Carcinogenicity: An updating of IARC Monographs volumes 1 to
42. Lyon, France, 1987 (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Supplement 7)
REFERENCES
1. ABSHAGEN, U. & RIETBROCK, N. (1969) [Elimination of
2-propanol in dogs and rats.] Naunyn-Schmiedebergs Arch.
Pharmakol. exp. Pathol., 264: 110-118 (in German).
2. ABSHAGEN, U. & RIETBROCK, N. (1970) [The mechanism of the
2-propanol oxidation.] Naunyn-Schmiedebergs Arch. Pharmakol.
exp. Pathol., 265: 411-424 (in German).
3. ACKMAN, R.G., HINLEY, H.J., & POWER, H.E. (1967)
Determination of isopropyl alcohol in fish protein
concentrate by solvent extraction and gas-liquid
chromatography. J. Fish. Res. Board Can., 24: 1521-1529.
4. ADELSON, L. (1962) Fatal intoxication with isopropyl
alcohol (rubbing alcohol). Am J. clin. Pathol., 38: 144-151.
5. AGARWAL, S.K. (1979) Non-acidotic acetonemia: a syndrome
due to isopropyl alcohol intoxication. J. Med. Soc. New
Jersey, 76: 914-916.
6. AGOSTINI, C. (1982) Effects of various inhibitors on lipid
peroxidation by homogenates of normal, regenerating and
hepatomous rat liver, by liver slices and by hepatoma cells.
Med. Biol. Environ., 10: 3-15.
7. AHAMED, A. & MATCHES, J.R. (1983) Alcohol production by
fish spoilage bacteria. J. food Prot., 46: 1055-1059.
8. AKHREM, A.A., POPOVA, E.M., & METELITSA, D.I. (1978)
[Interaction of aliphatic alcohols with cytochrome P-450
from rat liver microsomes.] Biokhimiya (Mosk), 43: 1485-1491
(in Russian).
9. ALDERSON, M.R. & RATTAN, N.S. (1980) Mortality of workers
on an isopropyl alcohol plant and two MEK dewaxing plants.
Br. J. ind. Med., 37: 85-89.
10. ALEKPEROV, I.I. & GUSEINOV, V.G. (1980) Toxicological
characteristics of isopropanol as an industrial poison, All
Union Foundation Conference on Toxicology, Moscow, 25-27
November 1980, p. 33.
11. ALEXANDER, C.B., MCBAY, A.J., & HUDSON, R.P. (1982)
Isopropanol and isopropanol deaths: ten years' experience.
J. forensic Sci., 27: 541-548.
12. ANDERS, M.W. & HARRIS, R.N. (1981) Effect of 2-propanol
treatment on carbon tetrachloride metabolism and toxicity.
Adv. exp. Med. Biol., 136A: 591-602.
13. ANDERSON, S.M. & MCDONALD, J.F. (1981) Effect of
environmental alcohol on in vivo properties of Drosophila
alcohol dehydrogenase. Biochem. Genet., 19: 421-430.
14. ANON. (1987) Chemical market profile: isopropanol. Chem.
Market. Rep., 31 August: 46.
15. ANTONOVA, V.I. & SALMINA, Z.A. (1978) [The maximum
permissible concentration of isopropyl alcohol in water
bodies with due regard for its action on the gonads and the
progeny.] Gig. i Sanit., 43: 8-11 (in Russian).
16. ARDITI, M. & KILLMER, M.S. (1987) Coma following use of
rubbing alcohol for fever control. Am. J. DC, 141: 237-238.
17. ARISTOV, V.N. (1982) [Combined effect of toluene, isopropanol,
and sulfur dioxide in conditions of petrochemical production.]
Gig. Tr. Prof. Zabol., ISS9: 5-9 (in Russian).
18. ARISTOV, V.N., REDKIN, Y.V., BRUSKIN, Z.Z., & OGLEZNEV, G.A.
(1981) [Experimental data on the mutagenous effects of
toluene, isopropanol, and sulfur dioxide.] Gig. Tr. Prof.
Zabol., 25: 33-36 (in Russian).
19. BAIKOV, B.K., GORLOVA, O.E., GUSEV, M.I., NOVIKOV, Y.V.,
YUDINA, T.V., & SERGEEV, A.N. (1974) [Hygienic
standardization of the daily average maximal permissible
concentrations of propyl and isopropyl alcohols in the
atmosphere.] Gig. i Sanit., 4: 6-13 (in Russian).
20. BALD, E. & MAZURKIEWICZ, B. (1980) Analytical utility of
2-halopyridinium salts. Part III. Paper electrophoretic
characterization of alcohols as 2-alkoxy-1-methylpyridinium
p-toluenesulfonates. Chromatographia, 13: 295-297.
21. BEAUGE, F., CLEMENT, M., RENAUD, G., NORDMANN, R., &
NORDMANN, J. (1975) Action de l'isopropanol sur le
métabolisme lipidique chez le rat: études complémentaires
sur les méchanismes impliqués dans l'accumulation hépatique
des triacylglycérides. Arch. int. Physiol. Biochim., 83:
573-591.
22. BEAUGE, F., CLEMENT, M., NORDMANN, J., & NORDMANN, R.
(1977) Etudes comparatives des actions de l'acétone et de
l'isopropanol sur le métabolisme lipidique chez le rat.
Arch. int. Physiol. Biochim., 85: 931-940.
23. BEAUGE, F., CLEMENT, M., NORDMANN, J., & NORDMANN, R.
(1979) Comparative effects of ethanol, n-propanol and
isopropanol on lipid disposal by rat liver. Chem.-biol.
Interact., 26: 155-166.
24. BEAUGE, F., FLEURET, C., BARIN, F., & NORDMANN, R. (1984)
Brain membrane disordering after acute in vivo administration
of ethanol, isopropanol or t-butanol in rats. Biochem.
Pharmacol., 33: 3591-3595.
25. BESS, F.D. & CONWAY, R.A. (1966) Aerated stabilization of
synthetic organic chemical wastes. J. Water Pollut. Control
Fed., 38: 939-956.
26. BLACKMAN, R.A.A. (1974) Toxicity of oil-sinking agents.
Mar. Pollut. Bull., 5: 116-118.
27. BONTE, W., RUDELL, E., SPRUNG, R., FRAUENRATH, C., BLANKE,
E., KUPILAS, G., WOCHNIK, J. & ZAH, G. (1981a)
[Experimental investigations concerning the analytical
detection of small doses of higher aliphatic alcohols in
blood in man.] Blutalkohol, 18: 399-411 (in German).
28. BONTE, W., SPRUNG, R., RUDELL, E., FRAUENRATH, C., BLANKE,
E., KUPILAS, G., WOCHNIK, J., & ZAH, G. (1981b)
[Experimental investigations concerning the analytical
detection of small doses of higher aliphatic alcohols in
human urine.] Blutalkohol, 18: 412-426 (in German).
29. BORENFREUND, E. & BORRERO, O. (1984) In vitro cytotoxicity
assays. Potential alternatives to the Draize ocular allergy
test. Cell Biol. Toxicol., 1: 55-65.
30. BORENFREUND, E. & SHOPSIS, C. (1985) Toxicity monitored
with a correlated set of cell-culture assays. Xenobiotica,
15: 705-711.
31. BOSSET, J.O. & LIARDON, R. (1984) The aroma composition of
Swiss Gruyere cheese. II. The neutral volatile components.
Lebensm.-Wiss. Technol., 17: 359-362.
32. BOUGHTON, L.L. (1944) The relative toxicity of ethyl and
isopropyl alcohols as determined by long term rat feeding
and external application. Am. pharm. Assoc., 33: 111-113.
33. BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979a) BOD and
COD of some petrochemicals. Water Res., 13: 627-630.
34. BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979b) The acute
toxicity of some petrochemicals to goldfish. Water Res., 13:
623-626.
35. BRINGMANN, G. (1975) [Determination of the harmful
biological action of water-endangering substances through
inhibition of cell multiplication in the blue alga
Microcystis.] Ges.-Ing., 96: 238-241 (in German).
36. BRINGMANN, G. (1978) [Determination of the harmful
biological action of water-endangering substances on
protozoa. I. Bacteria fed flagellates.] Z. Wasser-Abwasser
Forsch., 11: 210-215 (in German).
37. BRINGMANN, G. & KUHN, R. (1977) [Limiting values of the
harmful action of water-endangering substances on bacteria
(Pseudomonas putida) and green algae (Scenedesmus
quadricauda) in the cell multiplication inhibition test.] Z.
Wasser-Abwasser Forsch., 10: 87-98 (in German).
38. BRINGMANN, G. & KUHN, R. (1980) [Determination of the
harmful biological action of water-endangering substances on
protozoa. II. Bacteria fed ciliates.] Z. Wasser-Abwasser
Forsch., 13: 26-31 (in German).
39. BRINGMANN, G. & KUHN, R. (1982) [Findings regarding the
harmful action of water-endangering substances on Daphnia
magna in a further developed standardised test.] Z. Wasser-
Abwasser Forsch., 15: 1-16 (in German).
40. BRINGMANN, G., KUHN, R. & WINTER, A. (1980) [Determination
of the harmful biological action of water-endangering
substances on protozoa. III. Saprozoic flagellates.] Z.
Wasser-Abwasser Forsch., 13: 170-173 (in German).
41. BRUGNONE, F., PERBELLINI, L., APOSTOLI, P., BELLOMI, M., &
CARETTA, D. (1983) Isopropanol exposure: environmental and
biological monitoring in a printing works. Br. J. ind. Med.,
40: 160-168.
42. BUFFONI, F., SANTONI, G., ALBANESE, V., & DOLARA, P. (1983)
Urinary mercapturic acid in chemical workers and in control
subjects. J. appl. Toxicol., 3: 63-65.
43. BULICH, A.A. (1979) Use of luminescent bacteria for
determining toxicity in aquatic environments. In: Marking,
L.L. & Kimerle, R.A., ed. Aquatic toxicology, American
Society for Testing and Materials, pp. 98-106 (ASTM STP 667).
44. BULICH, A.A., GREENE, M.W., & ISENBERG, D.L. (1981)
Reliability of the bacterial luminescence assay for
determination of the toxicity of pure compounds and complex
effluents. In: Branson, D.R. & Dickson, K.L., ed. Aquatic
toxicology and hazard assessment. Fourth Conference,
American Society for Testing and Materials, pp. 338-347
(ASTM STP 737).
45. CALHOUN, M.J. & DELLAMONICA, E.S. (1974) Determination of
2-propanol residue in some fruits dewaxed with alcohol
vapors. J. Assoc. Off. Anal. Chem., 57: 1342-1345.
46. CARTER, W.P.L., DARNALL, K.R., GRAHAM, R.A., WINER, A.M., &
PITTS, J.N. (1979) Reactions of C2 and C4 alpha-hydroxy
radicals with oxygen. J. phys. Chem., 83: 2305-2311.
47. CEC (1982) Propan-2-ol: chemico-physical data, toxicity
data, environmental occurrence, and permissible levels. In:
Report of the Scientific Committee for Food on Extraction
Solvents, Brussels, Belgium, Commission of the European
Communities, Directorate General for Internal Market and
Industrial Affairs, pp. 46-72.
48. CEDERBAUM, A.I., QURESHI, A., & MESSENGER, P. (1981)
Oxidation of isopropanol by rat liver microsomes. Possible
role of hydroxyl radicals. Biochem. Pharmacol., 30: 825-831.
49. CHADOEUF-HANNEL, R. & TAYLORSON, R.B. (1985) Anaesthetic
stimulation of Amaranthus albus seed germination:
interaction with phytochrome. Physiol. Plant, 65: 451-454.
50. CHARBONNEAU, M., IJIMA, M., COTE, M.G., & PLAA, G.L. (1985)
Temporal analysis of rat liver injury following potentiation
of carbon tetrachloride hepatotoxicity with ketonic or
ketogenic compounds. Toxicology, 35: 95-112.
51. CHARRETON, M. (1981) Estimation des rejets de vapeurs de
solvants au voisinage d'une usine de peintures. Double
Liaison - Chimie des Peintures, 28: 233-241.
52. CHECKOWAY, H., WILCOSKY, T., WOLF, P., & TYROLER, H. (1984)
An evaluation of associations of leukemia and rubber
industry solvent exposures. Am. J. ind. Med., 5: 239-249.
53. CHEN, T.-H., KAVANAGH, T.J., CHANG, C.C., & TROSKO, J.E.
(1984) Inhibition of metabolic cooperation in Chinese
hamster V79 cells by various organic solvents and simple
compounds. Cell Biol. Toxicol., 1: 155-171.
54. CHEN, W.-S. & PLAPP, B.V. (1980) Kinetics and control of
alcohol oxidation in rats. Adv. exp. Med. Biol., 132:
543-549.
55. CHOU, W.L., SPEECE, R.E., & SIDDIQI, R.H. (1978)
Acclimation and degradation of petrochemical wastewater
components by methane fermentation. Biotechnol. Bioeng.
Symp., 8: 391-414.
56. CHVAPIL, M., ZAHRADNIK, R., & CMUCHALOVA, B. (1962)
Influence of alcohols and potassium salts of xanthogenic
acids on various biological objects. Arch. int. Pharmacodyn.
Ther., 135: 330-343.
57. COHEN, G.M. & MANNERING, G.J. (1973) Involvement of a
hydrophobic site in the inhibition of the microsomal
p-hydroxylation of aniline by alcohols. Mol. Pharmacol., 9:
383-397.
58. COLEMAN, R.L., LUND, E.D., & SHAW, P.E. (1972) Analysis of
grapefruit essence and aroma oils. J. agric. food Chem., 20:
100-103.
59. CORBETT, J. & MEIER, G. (1968) Suicide attempted by rectal
administration of drug. J. Am. Med. Assoc., 206: 2320-2321.
60. CORNISH, H.H. & ADEFUIN, J. (1967) Potentiation of carbon
tetrachloride toxicity by aliphatic alcohols. Arch. environ.
Health, 14: 447-449.
61. CUPITT, L.T. (1980) Fate of toxic and hazardous materials
in the air environment, Research Triangle Park, North
Carolina, US Environmental Protection Agency, Environmental
Sciences Laboratory, Office of Research and Development (EPA
600/3-80-084, PB 80-221948).
62. CURTIS, C., LIMA, A., LOZANO, S.J., & VEITH, G.D. (1982)
Evaluation of a bacterial bioluminiscence bioassay as a
method for predicting acute toxicity of organic chemicals to
fish. In: Pearson, J.G., Foster, R.B., & Bishop, W.E., ed.
Aquatic Toxicology and Hazard Assessment. Fifth Conference,
American Society for Testing and Materials, pp. 170-178
(ASTM STP 766).
63. DAINTY, R.H., EDWARDS, R.A., & HIBBARD, C.M. (1984)
Volatile compounds associated with the aerobic growth of
some Pseudomonas species on beef. J. appl. Bacteriol., 57:
75-81.
64. DALZIEL, K. & DICKINSON, F.M. (1966) The kinetics and
mechanism of liver alcohol dehydrogenase with primary and
secondary alcohols as substrates. Biochem. J., 100: 34-46.
65. DANIEL, D.R., MCANALLEY, B.H., & GARRIOTT, J.C. (1981)
Isopropyl alcohol metabolism after acute intoxication in
humans. J. anal. Toxicol., 5: 110-112.
66. DAVID, J. & BOCQUET, C. (1976) Compared toxicities of
different alcohols for two Drosophila sibling species: D.
melanogaster and D. simulans. Comp. Biochem. Physiol., 54C:
71-74.
67. DAVIS, D.G., WERGIN, W.P., & DUSBABEK, K.E. (1978) Effects
of organic solvents on growth and ultrastructure of plant
cell suspensions. Pestic. Biochem. Physiol., 8: 84-97.
68. DAVIS, P.L., DAL CORTIVO, L.A., & MATURO, J. (1984)
Endogenous isopropanol: forensic and biochemical
implications. J. anal. Toxicol., 8: 209-212.
69. DE CEAURRIZ, J.C., MICILLINO, J.C., BONNET, P., & GUENIER,
J.P. (1981) Sensory irritation caused by various industrial
airborne chemicals. Toxicol. Lett., 9: 137-143.
70. DEL ROSARIO, R., DE LUMEN, B.O., HABU, T., FLATH, R.A., MON,
T.R., & TERANISHI, R. (1984) Comparison of headspace
volatiles from winged beans and soybeans. J. agric. food
Chem., 32: 1011-1015.
71. DENG, J.-F., WANG, J.-D., SHIH, T.-S., & LAN, F.-L. (1987)
Outbreak of carbon tetrachloride poisoning in a color
printing factory related to the use of isopropyl alcohol and
an air conditioning system in Taiwan. Am. J. ind. Med., 12:
11-19.
72. DGEP (1987) Review of literature data on 2-propanol,
Leidschendam, Netherlands, Directorate-General of
Environmental Protection, Ministry of Housing, Physical
Planning and Environment.
73. DIVINCENZO, G.D. & KRASAVAGE, W.J. (1974) Serum ornithine
carbamyl transferase as a liver response test for exposure
to organic solvents. Am. Ind. Hyg. Assoc. J., 35: 21-29.
74. DORIGAN, J., FULLER, B., & DUFFY, R. (1976) Scoring of
organic air pollutants. Chemistry, production and toxicity
of selected synthetic organic chemicals, The MITRE
Corporation (MITRE Technical Report MTR-7248, Rev. 1,
Appendix III).
75. DUA, S.L. (1974) Peritoneal dialysis for isopropyl alcohol
poisoning. J. Am. Med. Assoc., 230: 35.
76. DUVEL, W.A. & HELFGOTT, T. (1975) Removal of wastewater
organics by reverse osmosis. J. Water Pollut. Control Fed.,
47: 57-65.
77. EGBERT, A.M., REED, J.S., POWELL, B.J., LISKOW, B.I., &
LIESE, B.S. (1985) Alcoholics who drink mouthwash: the
spectrum of nonbeverage alcohol use. J. Stud. Alcohol, 46:
473-481.
78. EGOROV, Y.L. (1966) Vision of workers engaged in the
production of synthetic fatty acids and issues relative to
setting hygienic standards for the alcohol content in the
air. Gig. Tr. Prof. Zabol., 7: 33-38.
79. ENTERLINE, P.E. (1982) Importance of sequential exposure in
the production of epichlorohydrin and isopropanol. Ann. N.Y.
Acad. Sci., 381: 344-349.
80. FANG, H.H.P & CHIAN, E.S.K. (1976) Reverse osmosis
separation of polar organic compounds in aqueous solution.
Environ. Sci. Technol., 10: 364-369.
81. FERNANDEZ, F. & QUIGLEY, R.M. (1985) Hydraulic conductivity
of natural clays permeated with simple liquid hydrocarbons.
Can. Geotech. J., 22: 205-214.
82. FLORIN, I., RUTBERG, L., CURVALL, M., & ENZELL, C.R. (1980)
Screening of tobacco smoke constituents for mutagenicity
using the Ames test. Toxicology, 18: 219-232.
83. FOLLAND, D.S., SCHAFFNER, W., GINN, E., CROFFORD, O.B., &
MCMURRAY, D.R. (1976) Carbon tetrachloride toxicity
potentiated by isopropyl alcohol. Investigation of an
industrial outbreak. J. Am. Med. Assoc., 236: 1853-1856.
84. FORE, S.P., RAYNER, E.T., & DUPUY, H.P. (1971)
Determination of residual solvent in oilseed meals and
flours. III. Isopropanol. J. Am. Oil Chem. Soc., 48:
140-142.
85. FORSANDER, O.A. (1967) Influence of some aliphatic
alcohols on the metabolism of rat liver slices. Biochem. J.,
105: 93-97.
86. FREGERT, S., GROTH, O., HJORTH, N., MAGNUSSON, B., RORSMAN,
H., & OVRUM, P. (1969) Alcohol dermatitis. Acta
dermatovenereol., 49: 493-497.
87. FREGERT, S., GROTH, O., GRUVBERGER, B., MAGNUSSON, B.,
MOBACKEN, H., & RORSMAN, H. (1971) Hypersensitivity to
secondary alcohols. Acta dermatovenereol., 51: 271-272.
88. FREIREICH, A.W., CINQUE, T.J., XANTHAKY, G., & LANDAU, D.
(1967) Hemodialysis for isopropanol poisoning. New Engl. J.
Med., 277: 699-700.
89. GADSDEN, R.H., MELETTE, R.R., & MILLER, W.C. (1958) Scrap
iron intoxication. J. Am. Med. Assoc., 168: 1220-1224.
90. GAILLARD, D. & DERACHE, R. (1966) Action de quelques
alcools aliphatiques sur la mobilisation de différentes
fractions lipidiques chez la rate. Food cosmet. Toxicol., 4:
515-520.
91. GARRISON, R.F. (1953) Acute poisoning from use of isopropyl
alcohol in tepid sponging. J. Am. Med. Assoc., 152: 317-318.
92. GEORGE, H.A., JOHNSON, J.L., MOORE, W.E.C., HOLDEMAN, L.V.,
& CHEN, J.S. (1983) Acetone, isopropanol, and butanol
production by Clostridium beijerinckii (syn. Clostridium
butylicum) and Clostridium aurantibutyricum. Appl. environ.
Microbiol., 45: 1160-1163.
93. GEORGE, V., SHARMA, S.D., TRIPATHI, A.K., & ABRAHAM, S.P.
(1985) Flavour components of some edible fungi from
Kashmir. I. Pafai J., 7: 27-30.
94. GERARDE, H.W., AHLSTROM, D.B., & LINDEN, N.J. (1966) The
aspiration hazard and toxicity of a homologous series of
alcohols. Arch. environ. Health, 13: 457-461.
95. GERHOLD, R.M. & MALANEY, G.W. (1966) Structural
determinants in the oxidation of aliphatic compounds by
activated sludge. J. Water Pollut. Control Fed., 38:
562-579.
96. GILLETTE, L.A., MILLER, D.L., & REDMAN, H.E. (1952)
Appraisal of a chemical waste problem by fish toxicity
tests. Sewage ind. Waste, 24: 1397-1401.
97. GIUSTI, D.M., CONWAY, R.A., & LAWSON, C.T. (1974) Activated
carbon adsorption of petrochemicals. J. Water Pollut.
Control Fed., 46: 947-965.
98. GORLOVA, O.E. (1970) [Hygienic assessment of isopropyl
alcohol as an atmospheric pollutant.] Gig. i Sanit., 35:
9-14 (in Russian).
99. GOTZ-SCHMIDT, E.-M. & SCHREIER, P. (1986) Neutral volatiles
from blended endive ( Cichorium endivia L.). J. agric. food
Chem., 34: 212-215.
100. GRIFFITH, J.F., NIXON, G.A., BRUCE, R.D., REER, P.J., &
BANNAN, E.A. (1980) Dose-response studies with chemical
irritants in the albino rabbit eye as a basis for selecting
optimum testing conditions for predicting hazard to the human
eye. Toxicol. appl. Pharmacol., 55: 501-513.
101. GUNTER, B. (1982) Health hazard evaluation: Jeppesen
Sanderson Inc., Cincinnati, Ohio, US National Institute for
Occupational Safety and Health (HETA 81-261-1085, PB 83-
201749).
102. GUNTER, B.J., LIGO, R.L., & RUHE, R.L. (1976) Health hazard
evaluation determination, Steiger Tractor Inc., Cincinnati,
Ohio, US National Institute for Occupational Safety and
Health (NIOSH-TR-HHE-75-30-266, PB 273711).
103. GUSEINOV, V.G. (1985) [Toxicological hygienic characteristics
of isopropyl alcohol.] Gig. Tr. Prof. Zabol., (7): 60-62
(in Russian).
104. HABU, T., FLATH, R.A., MON, T.R., & MORTON, J.F. (1985)
Volatile components of Rooibos tea (Asphalathus linearis). J.
agric. food Chem., 33: 249-254.
105. HADDOCK, N.F. & WILKIN J.K. (1982) Cutaneous reactions to
lower aliphatic alcohols before and during disulfiram
therapy. Arch. Dermatol., 118: 157-159.
106. HALLIDAY, M.M. & CARTER, K.B. (1978) A chemical adsorption
system for the sampling of gaseous organic pollutants in
operating theatre atmospheres. Br. J. Anaesth., 50:
1013-1018.
107. HANSSEN, H.-P., SPRECHER, E., & KLINGENBERG, A. (1984)
Accumulation of volatile flavour compounds in liquid cultures
of Kluyveromyces lactis strains. Z. Naturforsch., 39c:
1030-1033.
108. HAWLEY, P.C. & FALKO, J.M. (1982) "Pseudo" renal failure
after isopropyl alcohol intoxication. South. med. J., 75:
630-631.
109. HELLMAN, T.M. & SMALL, F.H. (1974) Characterization of the
odor properties of 101 petrochemicals using sensory methods.
J. Air Pollut. Control Assoc., 24: 979-982.
110. HERMENS, J. CANTON, H., JANSSEN, P., & DE JONG, R. (1984)
Quantitative structure-activity relationships and toxicity
studies of mixtures of chemicals with anaesthetic potency:
acute lethal and sublethal toxicity to Daphnia magna. Aquat.
Toxicol., 5: 143-154.
111. HERMENS, J., BROEKHUYZEN, E., CANTON, H., & WEGMAN, R.
(1985) Quantitative structure-activity relationships and
mixture toxicity studies of mixtures of alcohols and
chlorohydrocarbons: effects on growth of Daphnia magna.
Aquat. Toxicol., 6: 209-217.
112. HO, Y.H., SCHWARZE, I., & SOEHRING, K. (1970) [The influence
of low aliphatic alcohols on the chloral hydrate metabolism
in rat liver sections.] Arzneim.-Forsch., 20: 1507-1509 (in
German).
113. HODGE, H.C. & STERNER, J.H. (1943) Am. Ind. Hyg. Assoc. J.,
10: 93.
114. HOIGNE, J. & BADER, H. (1983) Rate constants of reactions of
ozone with organic and inorganic compounds in water. I. Non-
dissociating organic compounds. Water Res., 17: 173-183.
115. HORWITZ, W., ed. (1975) Official methods of analysis of the
Association of Official Analytical Chemists, 12th ed.,
Washington DC, Association of Official Analytical Chemists,
pp. 328, 337-338, 343, 656-658.
116. HORWOOD, J.F., LLOYD, G.T., & STARK, W. (1981) Some flavour
components of feta cheese. Aust. J. dairy Technol., 36:
34-37.
117. HOU, C.T., PATEL, R.N., LASKIN, A.I., MARCZAK, I., & BARNABE,
N. (1981) Microbial oxidation of gaseous hydrocarbons:
production of alcohols and methyl ketones from their
corresponding n-alkanes by methylotrophic bacteria. Can. J.
Microbiol., 27: 107-115.
118. HOVIOUS, J.C., CONWAY, R.A., & GANZE, C.W. (1973) Anaerobic
lagoon pretreatment of petrochemical wastes. J. Water Pollut.
Control Fed., 45: 71-84.
119. HUEPER, W.C. (1966) Occupational and environmental cancers
of the respiratory system. Recent results. Cancer Res., 3:
105-107.
120. IARC (1977) Some fumigants, the herbicides 2,4-D and 2,4,5-
T, chlorinated dibenzodioxins and miscellaneous industrial
chemicals, Lyons, International Agency for Research on
Cancer, pp. 223-243 (Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Man, 15).
121. IDOTA, S. (1985) [Studies on isopropanol metabolism and
poisoning.] J. Nihon Univ. Med. Assoc., 44: 39-47 (in
Japanese).
122. IRPTC (1987) Data profile on 2-propanol, Geneva,
Switzerland, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
123. JARKE, F.H., DRAVNIEKS, A., & GORDON, S.M. (1981) Organic
contaminants in indoor air and their relation to outdoor
contaminants. ASHRAE Trans., 87: 153-166.
124. JENSEN, O. (1981) Contact allergy to propylene oxide and
isopropyl alcohol in a skin disinfectant swab. Contact
Dermat., 7: 148-150.
125. JONES, C.J. & MCGUGAN, P.J. (1977/1978) An investigation of
the evaporation of some volatile solvents from domestic
waste. J. hazard. Mater., 2: 235-251.
126. JONSSON, A., PERSSON, K.A. & GRIGORIADIS, V. (1985)
Measurements of some low molecular-weight oxygenated,
aromatic, and chlorinated hydrocarbons in ambient air and in
vehicle emissions. Environ. Int., 11: 383-392.
127. JUHNKE, I. & LUDEMANN, D. (1978) [Results of examination of
200 chemical compounds for acute toxicity towards fish by
means of the golden orfe test.] Z. Wasser-Abwasser Forsch.,
11: 161-164 (in German).
128. JUNCOS, L. & TAGUCHI, J.T. (1968) Isopropyl alcohol
intoxication. Report of a case associated with myopathy,
renal failure, and hemolytic anemia. J. Am. Med. Assoc., 204:
186-188.
129. KAMIL, I.A., SMITH, J.N., & WILLIAMS, R.T. (1953) The
metabolism of aliphatic alcohols. The glucuronic acid
conjugation of acyclic aliphatic alcohols. Biochem. J., 53:
129-136.
130. KANE, L.E., DOMBROSKE, R., & ALARIE, Y. (1980) Evaluation of
sensory irritation from some common industrial solvents. Am.
Ind. Hyg. Assoc. J., 41: 451-455.
131. KELNER, M. & BAILEY, D.N. (1983) Isopropanol ingestion:
interpretation of blood concentrations and clinical findings.
J. Toxicol.-clin. Toxicol., 20: 497-507.
132. KEMAL, H. (1927) [Contribution to investigations into the
fate of isopropyl alcohol in the human body.] Biochem. Z.,
187: 461-466 (in German).
133. KHAN, J.S., WILSON, M.C., & TAYLOR, T.V. (1979) A case of
dettol addiction. Br. med. J., 1: 791-792.
134. KIMURA, E.T., EBERT, D.M., & DODGE, P.W. (1971) Acute
toxicity and limits of solvent residue for sixteen organic
solvents. Toxicol. appl. Pharmacol., 19: 699-703.
135. KING, L.H., BRADLEY, K.P., & SHIRES, D.L. (1970)
Hemodialysis for isopropyl alcohol poisoning. J. Am. Med.
Assoc., 211: 1855.
136. KINLIN, T.E., MURALIDHARA, R., PITTET, A.O., SANDERSON, A., &
WALRADT, J.P. (1972) Volatile components of roasted
filberts. J. agric. food Chem., 20: 1021-1028.
137. KIRK, R.E. & OTHMER, D.F., ed. (1978-1984) Encyclopedia of
chemical technology, 3rd ed., New York, Wiley Interscience.
138. KLECKA, G.M. & LANDI, L.P. (1985) Evaluation of the OECD
activated sludge, respiration inhibition test. Chemosphere,
14: 1239-1251.
139. KNUTH, M.L. & HOGLUND, M.D. (1984) Quantitative analysis of
68 polar compounds from ten chemical classes by direct
aqueous injection gas chromatography. J. Chromatogr., 285:
153-160.
140. KOMINSKY, J., LOVE, J., & ANDERSON, K. (1982) Health hazard
Evaluation, Tweddle Litho Company,Cincinnati, Ohio, US
National Institute for Occupational Safety and Health (HETA
81-117-1087, PB 83-202390).
141. KONEMANN, H. (1981) Quantitative structure-activity
relationships in fish toxicity studies. Toxicology, 19:
209-221.
142. KONIG, H. & HERMES, M. (1981) [Separation, identification
and estimation of propellant gases and solvents in aerosol
products by gas chromatography.] Chromatographia, 14: 351-354
(in German).
143. KRAMER, V.C., SCHNELL, D.J., & NICKERSON, K.W. (1983)
Relative toxicity of organic solvents to Aedes aegyptilarvae.
J. Invertebr. Pathol., 42: 285-287.
144. KRING, E.V., ANSUL, G.R., HENRY, T.J., MORELLO, J.A., DIXON,
S.W., VASTA, J.F., & HEMINGWAY, R.E. (1984) Evaluation of
the standard NIOSH type charcoal tube sampling method for
organic vapors in air. Am. Ind. Hyg. Assoc. J., 45: 250-259.
145. KRULL, I.S., SWARTZ, M., & DRISCOLL, J.N. (1984)
Derivatizations for improved detection of alcohols by gas
chromatography and photoionization detection. Anal. Lett.,
17(A20): 2369-2384.
146. KUMAI, M., KOIZUMI, A., SAITO, K., SAKURAI, H., INOUE, T.,
TAKEUCHI, Y., HARA, I., OGATA, M., MATSUSHITA, T., & IKEDA,
M. (1983) A nationwide survey on organic solvent components
in various solvent products. Part 2. Heterogeneous products
such as paints, inks, and adhesives. Ind. Health, 21:
185-197.
147. KUMKE, G.W., HALL, J.F., & OEBEN, R.W. (1968) Conversion to
activated sludge at Union Carbide's institute plant. J. Water
Pollut. Control Fed., 40: 1408-1422.
148. KURPPA, K. & HUSMAN, K. (1982) Car painters' exposure to a
mixture of organic solvents. Serum activities of liver
enzymes. Scand. J. Work environ. Health, 8: 137-140.
149. LACOUTURE, P.G., WASON, S., ABRAMS, A., & LOVEJOY, F.H.
(1983) Acute isopropyl alcohol intoxication. Diagnosis and
management. Am. J. Med., 75: 680-686.
150. LAHAM, S., POTVIN, M., & SCHRADER, K. (1979) Microméthode de
dosage simultané de l'alcool isopropylique et de son
metabolite l'acétone. Chemosphere, 2: 79-87.
151. LAHAM, S., POTVIN, M., SCHRADER, K., & MARINO, I. (1980)
Studies on inhalation toxicity of 2-propanol. Drug Chem.
Toxicol., 3: 343-360.
152. LANGVARDT, P.W. & MELCHER, R. (1979) Simultaneous
determination of polar and non-polar solvents in air using a
two-phase desorption from charcoal. Am. Ind. Hyg. Assoc. J.,
40: 1006-1012.
153. LAREGINA, J., BOZZELLI, J.W., HARKOV, R., & GIANTI, S.
(1986) Volatile organic compounds at hazardous waste sites
and a sanitary landfill in New Jersey. An up-to-date review
of the present situation. Environ. Progr., 5: 18-27.
154. LEASURE, C.S., FLEISCHER, M.E., ANDERSON, G.K., & EICEMAN,
G.A. (1986) Photoionization in air with ion mobility
spectrometry using a hydrogen discharge lamp. Anal. Chem.,
58: 2142-2147.
155. LEFFINGWELL, S.S., WAXWEILER, R., ALEXANDER, V., LUDWIG, H.R.
& HALPERIN, W. (1983) Case-control study of gliomas of the
brain among workers employed by a Texas City, Texas chemical
plant. Neuroepidemiology, 2: 179-195.
156. LEGENDRE, M.G. & DUPUY, H.P. (1981) Flavor volatiles as
measured by rapid instrumental techniques, American Chemical
Society, pp. 41-49 (ACS Symposium Series No. 147).
157. LEHMAN, A.J. & CHASE, H.F. (1944) The acute and chronic
toxicity of isopropyl alcohol. J. Lab. clin. Med., 29:
561-567.
158. LEHMAN, A.J., SCHWERMA, H., & RICKARDS, E. (1944) Isopropyl
alcohol: rate of disappearance from the blood stream of dogs
after intravenous and oral administration. J. Pharmacol. exp.
Ther., 82: 196-201.
159. LEHMAN, A.J., SCHWERMA, H., & RICKARDS, E. (1945) Acquired
tolerance in dogs, rate of disappearance from the blood
stream in various species, and effects on successive
generation of rats. J. Pharmacol. exp. Ther., 85: 61-69.
160. LEWIN, G.A., OPPENHEIMER, P.R., & WINGERT, W.A. (1977) Coma
from alcohol sponging. J.A.C.E.P., 6: 165-167.
161. LEWIS, G.D., LAUFMAN, A.K., MCANALLY, B.H., & GARRIOTT, J.C.
(1984) Metabolism of acetone to isopropyl alcohol in rats
and humans. J. forensic Sci., 29: 541-549.
162. LINDSTROM, T.D. & ANDERS, M.W. (1978) Effect of agents known
to alter carbon tetrachloride hepatotoxicity and cytochrome
P-450 levels on carbon tetrachloride-stimulated lipid
peroxidation and ethane expiration in the intact rat.
Biochem. Pharmacol., 27: 563-567.
163. LITOVITZ, T. (1986) The alcohols: ethanol, methanol, iso-
propanol, ethylene glycol. Pediatr. Clin. N. Am., 33:
311-323.
164. LLOYD, A.C., DARNALL, K.R. WINER, A.M., & PITTS, J.N. (1976)
Relative rate constants for the reactions of OH radicals with
isopropyl alcohol, diethyl and di- n-propyl ether at 305 ±
20 °K. Chem. Phys. Lett., 42: 205-209.
165. LORR, N.A., MILLER, K.W., CHUNG, H.R., & YANG, C.S. (1984)
Potentiation of the hepatotoxicity of N-nitrosodimethylamine
by fasting, diabetes, acetone, and isopropanol. Toxicol.
appl. Pharmacol., 73: 423-431.
166. LOVEGREN, N.V., VINNETT, C.H., & ANGELO, A.J.S. (1982) Gas
chromatographic profile of good quality raw peanuts. Peanut
Sci., 9: 93-96,
167. LUDWIG, E. & HAUSEN, B.M. (1977) Sensitivity to isopropyl
alcohol. Contact Dermat., 3: 240-244.
168. LUNDBERG, I. & HAKANSSON, M. (1985) Normal serum activities
of liver enzymes in Swedish paint industry workers with heavy
exposure to organic solvents. Br. J. ind. Med., 42: 596-600.
169. LYON, R.C., MCCOMB, J.A., SCHREURS, J., & GOLDSTEIN, D.B.
(1981) A relationship between alcohol intoxication and the
disordering of brain membranes by a series of short-chain
alcohols. J. Pharmacol. exp. Ther., 218: 669-675.
170. MCCORD, W.M., SWITZER, P.K., & BRILL, H.H. (1948) Isopropyl
alcohol intoxication. South med. J., 41: 639-642.
171. MCCREERY, M.J. & HUNT, W.A. (1978) Physico-chemical
correlates of alcohol intoxication. Neuropharmacology, 17:
451-461.
172. MCFADDEN, S.W. & HADDOW, J.E. (1969) Coma produced by
topical application of isopropanol. Pediatrics, 43: 622-623.
173. MACHYULITE, N.I. (1978) [Hygienic characterization of
conditions of work in production of levomycetin.] Gig. Tr.
Prof. Zabol., 12: 8-12 (in Russian).
174. MCINNES, A. (1973) Skin reaction to isopropyl alcohol. Br.
med. J., 1: 357.
175. MACK, K. (1973) The problem of waste water purification in
the chemical pharmaceutical industry. Prog. Water Technol.,
3: 239-249.
176. MACK, R.B. (1985) Pervasive procrustianism-isopropyl alcohol
intoxication. N.C. med. J., 46: 101-102.
177. MAIZLISH, N.A., LANGOLF, G.D., WHITEHEAD, L.W., FINE, L.J.,
ALBERS, J.W., GOLDBERG, J. & SMITH, P. (1985) Behavioural
evaluation of workers exposed to mixtures of organic
solvents. Br. J. ind. Med., 42: 579-590.
178. MALILA, A. (1978) Intoxicating effects of three aliphatic
alcohols and barbital on two rat strains genetically selected
for their ethanol intake. Pharmacol. Biochem. Behav., 8:
197-201.
179. MARKEL, H. (1982) Health hazard evaluation, Federal
Correctional Institution, Cincinnati, Ohio, US National
Institute for Occupational Safety and Health (HETA 80-119-
1066, PB 83-199398).
180. MARTIN, M. & HAERDI, W. (1982) Determination de composes
volatils toxiques dans le sang et dans l'urine par
chromatographie en phase gazeuse, methode de l'espace de tete
(head-space). Trav. Chim. aliment. Hyg., 73: 212-217.
181. MARTINEZ, T.T., JAEGER, R.W., DECASTRO, F.J., THOMPSON, M.W.,
& HAMILTON, M.F. (1986) A comparison of the absorption and
metabolism of isopropyl alcohol by oral, dermal, and
inhalation routes. Vet. human Toxicol., 28: 233-236.
182. MARZULLI, F.N. & RUGGLES, D.I. (1973) Rabbit eye irritation
test: collaborative study. J. Assoc. Off. Anal. Chem., 56:
905-914.
183. MATSUI, F., LOVERING, E.G., WATSON, J.R., BLACK, D.B., &
SEARS, R.W. (1984) Gas chromatographic method for solvent
residues in drug raw materials. J. pharm. Sci., 73:
1664-1666.
184. MATTSON, V.R., ARTHUR, J.W., & WALBRIDGE, C.T. (1976) Acute
toxicity of selected organic compounds to fathead minnows,
Duluth, Minnesota, US Environmental Protection Agency,
Environmental Research Laboratory (EPA 600/3-76-097; PB
262897).
185. MAY, J. (1966) [Odour thresholds of solvents for the
judgement of solvent odour in air.] Staub Reinhalt. Luft, 26:
385-389 (in German).
186. MECIKALSKI, M.B. & DEPNER, T.A. (1982) Peritoneal dialysis
for isopropanol poisoning. West. J. Med., 137: 322-324.
187. MENDELSON, J., WEXLER, D., LEIDERMAN, P.H., & SOLOMON, P.
(1957) A study of addiction to nonethyl alcohols and other
poisonous compounds. Quart. J. Stud. Alcohol, 18: 561-580.
188. MORGAN, R.L., SORENSON, S.S., & CASTLES, T.R. (1987)
Prediction of ocular irritation by corneal pachymetry. Food
chem. Toxicol., 25: 609-613.
189. MOSHONAS, M.G. & SHAW, P.E. (1972) Analysis of flavor
constituents from lemon and lime essence. J. agric. food
Chem., 20: 1029-1030.
190. MUNCH, J.C. (1972) Aliphatic alcohols and alkyl esters:
narcotic and lethal potencies to tadpoles and to rabbits.
Ind. Med., 41: 31-33.
191. NATOWICZ, M., DONAHUE, J., GORMAN, L., KANE, M., MCKISSICK,
J. & SHAW, L. (1985) Pharmacokinetic analysis of a case of
isopropanol intoxication. Clin. Med., 31: 326-328.
192. NELSON, K.W., EGE, J.F., ROSS, M., WOODMAN, L.E., &
SILVERMAN, L. (1943) Sensory response to certain industrial
solvent vapors. J. ind. Hyg. Toxicol., 25: 282-285.
193. NELSON, B.K., BRIGHTWELL, W.S., MACKENZIE-TAYLOR, D.R., KHAN,
A., BURG, J.R., WEIGEL, W.W., & GOAD, P.T. (1988)
Teratogenicity of n-propanol and isopropanol administered at
high inhalation concentrations to rats. Food Chem. Toxicol.,
26: 247-254.
194. NIXON, G.A., TYSON, C.A., & WERTZ, W.C. (1975) Interspecies
comparisons of skin irritancy. Toxicol. appl. Pharmacol., 31:
481-490.
195. NORDMANN, R., RIBIERE, C., ROUACH, H., BEAUGE, F.,
GIUDICELLI, Y., & NORDMANN, J. (1973) Metabolic pathways
involved in the oxidation of isopropanol into acetone by the
intact rat. Life Sci., 13: 919-932.
196. OELERT, H.H. & FLORIAN, T. (1972) [Recording and valuation
of the inconvenience caused by odours from diesel exhaust.]
Staub Reinhalt. Luft, 32: 400-407 (in German).
197. OHASHI, Y., NAKAI, Y., IKEOKA, H., KOSHIMO, H., ESAKI, Y.,
HORIGUCHI, S., TERAMOTO, K., & NAKASEKO, H. (1987a)
Recovery process of tracheal mucosa of guinea-pigs exposed to
isopropyl alcohol. Arch. Toxicol., 61: 12-20.
198. OHASHI, Y., NAKAI, Y., IKEOKA, H., KOSHIMO, H., ESAKI, Y.,
HORIGUCHI, S., TERAMOTO, K., & NAKASEKO, H. (1987b) Acute
effects of isopropyl alcohol exposure on the middle ear
mucosa. J. appl. Toxicol., 7: 205-211.
199. OLSON, B.A. (1982) Effects of organic solvents on
behavioural performance of workers in the paint industry.
Neurobehav. Toxicol. Teratol., 4: 703-708.
200. OVEREND, R. & PARASKEVOPOULOS, G. (1978) Rates of OH radical
reactions. IV. Reactions with methanol, ethanol, 1-propanol,
and 2-propanol at 296 ° K. J. phys. Chem., 82: 1329-1333.
201. PALO, V. & ILKOVA, H. (1970) Direct gas chromatographic
estimation of lower alcohols, acetaldehyde, acetone and
diacetyl in milk products. J. Chromatogr., 53: 363-367.
202. PARKER, W.A. (1982-1983) Alcohol-containing pharmaceuticals.
Am. J. drug alcohol Abuse, 9: 195-209.
203. PITTER, P. (1976) Determination of biological degradability
of organic substances. Water Res., 10: 231-235.
204. POHL, J. (1922) [Investigations into the fate of methyl and
isopropyl alcohol.] Biochem. Z., 127: 66-71 (in German).
205. POSO, H. & POSO, A.R. (1980) Inhibition by aliphatic
alcohols of the stimulated activity of ornithine
decarboxylase and tyrosine amino-transferase occurring in
regenerating rat liver. Biochem. Pharmacol., 29: 2799-2803.
206. POWIS, G. (1975) Effect of a single oral dose of methanol,
ethanol and propan-2-ol on the hepatic microsomal metabolism
of foreign compounds in the rat. Biochem. J., 148: 269-277.
207. POWIS, G. & GRANT, L. (1976) The effect of inhibitors of
alcohol metabolism upon the changes in the hepatic microsomal
metabolism of foreign compounds produced by the acute
administration of some alcohols to the rat. Biochem.
Pharmacol., 25: 2197-2201.
208. PRICE, K.S., WAGGY, G.T., & CONWAY, R.A. (1974) Brine shrimp
bioassay and seawater BOD of petrochemicals. J. Water Pollut.
Control Fed., 46: 63-77.
209. RAICHLE, M.E., EICHLING, J.O., STRAATMANN, M.G., WELCH, M.J.,
LARSON, K.B., & TER-POGOSSIAN, M.M. (1976) Blood-brain
barrier permeability of 11C-labeled alcohols and 15U-labeled
water. Am. J. Physiol., 230: 543-552.
210. RAILE, A., HAMMAN, K.P., SCHEINER, O., SCHULTZ, T., ERDEI,
A., & DIERICH, M.P. (1982) Differential effect of low
molecular weight alcohols on the Con A stimulation of mouse
spleen cells. Immunol. Lett., 4: 305-309.
211. RAMSEY, J.D. & FLANAGAN, R.J. (1982) Detection and
identification of volatile organic compounds in blood by
headspace gas chromatograpy as an aid to the diagnosis of
solvent abuse. J. Chromatogr., 240: 423-444.
212. REINDERS, M.E. (1980) Handbook of emission factors. Part I.
Non-industrial sources, The Hague, Netherlands, Ministry of
Health and Environmental Protection.
213. REINHARDT, C.A., PELLI, D.A., & ZBINDEN, G. (1985)
Interpretation of cell toxicity data for the estimation of
potential irritation. Food chem. Toxicol., 23: 247-252.
214. REQUENA, J., VELAZ, M.E., GUERRERO, J.R., & MEDINA, J.D.
(1985) Isomers of long-chain alkane derivatives and nervous
impulse blockage. J. Membr. Biol., 84: 229-238.
215. REYNOLDS, E.S., MOSLEN, M.T., & TREINEN, R.J. (1982)
Isopropanol enhancement of carbon tetrachloride metabolism in
vivo. Life Sci., 31: 661-669.
216. REYNOLDS, T. (1977) An anomalous effect of isopropanol on
lettuce germination. Plant Sci. Lett., 15: 25-28.
217. RICHARDSON, D.R., CARAVATI, C.M., PEYTON, E., & WEARY, P.E.
(1969) Allergic contact dermatitis to "alcohol" swabs.
Cutis, 5: 1115-1118.
218. RIETBROCK, N. & ABSHAGEN, U. (1971) [Pharmacokinetics and
metabolism of aliphatic alcohols.] Arzneim.-Forsch.,
21: 1309-1319 (in German).
219. RISTOW, S.S., STARKEY, J.R., & HASS, G.M. (1982) Inhibition
of natural killer cell activity in vitro by alcohols.
Biochem. Biophys. Res. Commun., 105: 1315-1321.
220. ROSANSKY, S.J. (1982) Isopropyl alcohol poisoning treated
with haemodialysis: kinetics of isopropyl alcohol and acetone
removal. J. Toxicol.-clin. Toxicol., 19: 265-271.
221. ROSS, D.H. (1976) Selective action of alcohols on cerebral
calcium levels. Ann. N.Y. Acad. Sci., 273: 280-294.
222. SABLJIC, A. & PROTIC-SABLIC, M. (1983) Quantitative
structure-activity study on the mechanism of inhibition of
microsomal p-hydroxylation of aniline by alcohols. Mol.
Pharmacol., 23: 213-218.
223. SANTODONATO, J. (1985) Monograph on human exposure to
chemicals in the workplace: isopropyl alcohol, Syracuse, New
York, Center for Chemical Hazard Assessment, Syracuse
Research Corporation (SRC-TR-84-1043, PB 86-143401).
224. SAVOLAINEN, H., PEKARI, K., & HELOJOKI, H. (1979)
Neurochemical and behavioural effects of extended exposure to
isopropanol vapour with simultaneous ethanol intake. Chem.-
biol. Interact., 28: 237-248.
225. SAWHNEY, B.L. & KOZLOSKI, R.P. (1984) Organic pollutants in
leachates from landfill sites. J. environ. Qual., 13:
349-352.
226. SCHICK, J.B. & MILSTEIN, J.M. (1981) Burn hazard of
isopropyl alcohol in the neonate. Pediatrics, 68: 587-588.
227. SEILER, H., BLAIM, H., & BUSSE, M. (1984) [Antibacterial
effects on predominant taxa in the activated sludge system of
a chemical combine.] Z. Wasser-Abwasser Forsch., 17: 127-133
(in German).
228. SENZ, E.H. & GOLDFARB, D.L. (1958) Coma in a child following
use of isopropyl alcohol in sponging. J. Pediatr., 53:
322-323.
229. SHAW, G.J., ALLEN, J.M., & VISSER, F.R. (1985) Volatile
flavor components of Babaco fruit ( Carica pentagona,
Heilborn). J. agric. food Chem., 33: 795-797.
230. SHEHAB, A.S. (1980) Comparative cytological studies of the
effect of some aliphatic alcohols and the fatty alcohols from
Euphorbia granulata and Pulicaria crispa on mitosis of Allium
cepa. Cytologia, 45: 507-513.
231. SHOFSTAHL, J.H. & HARDY, J.K. (1986) Determination of C1-C4
alcohols in gasoline using multiple ion detection. Anal.
Chem., 58: 2412-2414.
232. SIEBERT, H., SIEBERT, G., & BOHN, G. (1972) [Animal
experimental investigations into the metabolism of
propan-2-ol.] D tsch. Apoth.-ZTG., 112: 1040-1041 (in German).
233. SINGH, K.V. & AGRAWAL, S.C. (1981) Growth responses of
keratinophilic fungi to some volatile substances. Mykosen,
24: 630-634.
234. SIPES, I.G., STRIPP, B., KRISHNA, G., MALING, H., & GILLETTE,
J.R. (1973) Enhanced hepatic microsomal activity by
pretreatment of rats with acetone or isopropanol. Proc. Soc.
Exp. Biol. Med., 142: 237-240.
235. SMEA (1982) [Problems in industrial toxicology.] (in
Russian).
236. SMITH, N.B. (1984) Determination of volatile alcohols and
acetone in serum by non-polar capillary gas chromatography
after direct sample injection. Clin. Chem., 30: 1672-1674.
237. SMITH, P. & BROWN, N.L. (1969) Determination of isopropyl
alcohol in solid fish protein concentrate by gas-liquid
chromatography. J. agric. food Chem., 17: 34-37.
238. SMITH, R.P. (1959) Poisoning with Old Spice shaving lotion.
A case report. Bull. Suppl. Mat. clin. Toxicol. comm. Prod.,
2(11): 12.
239. SMYTH, H.F. & CARPENTER, C.P. (1948) Further experience with
the range finding test in the industrial toxicology
laboratory. J. ind. Hyg. Toxicol., 30: 63-70.
240. STEELE, R.H. & WILHELM, D.L. (1966) The inflammatory
reaction in chemical injury. I. Increased vascular
permeability and erythema induced by various chemicals. Br.
J. exp. Pathol., 47: 612-623.
241. STENSTROM, S., ENLOE, L., PFENNING, M., & RICHELSON, E.
(1986) Acute effects of ethanol and other short-chain
alcohols on the guanylate cyclase system of murine
neuroblastoma cells (clone N1E-115). J. Pharmacol. exp.
Ther., 236: 458-463.
242. STOFBERG, J. & GRUNDSCHOBER, F. (1984) Consumption ratio and
food predominance of flavoring materials-second cumulative
series. Perfumer & Flavorist, 9: 53-83.
243. STRANGE, A.W., SCHNEIDER, C.W., & GOLDBORT, R. (1976)
Selection of C3 alcohols by high and low ethanol selecting
mouse strains and the effects on open field activity.
Pharmacol. Biochem. Behav., 4: 527-530.
244. SWERDEL, M.R. & COUSINS, R.J. (1984) Changes in rat liver
metallothionein and metallothionein mRNA induced by
isopropanol. Proc. Soc. Exp. Biol. Med., 175: 522-529.
245. TAYLOR, C.D., COWART, C.O., & RYAN, N.T. (1985) Isopropanol
intoxication: managing the coma. Hosp. Pract., 20: 173-175.
246. TESTA, B. (1981) Structural and electronic factors
influencing the inhibition of aniline hydroxylation by
alcohols and their binding to cytochrome P-450. Chem.-biol.
Interact., 34: 287-300.
247. TICHY, M., TRCKA, V. ROTH, Z., & KRIVUCOVA, M. (1985) QSAR
analysis and data extrapolation among mammals in a series of
aliphatic alcohols. Environ. Health Perspect., 61: 321-328.
248. TIESS, D. & HAMMER, U. (1985) [On endogenous acetone
(propane-2-on) and isopropanol (propane-2-ol) levels in the
human body after ketoacidic states.] Z. gesamte Hyg., 31:
527-529 (in German).
249. TIMMER, R., TER HEIDE, R., DE VALOIS, P.J., & WOBBEN, H.J.
(1971) Qualitative analysis of the most volatile neutral
components of Reunion geranium oil ( Pelargonium roseum
Bourbon). J. agric. food Chem., 19: 1066-1068.
250. TOMITA, M. & NISHIMURA, M. (1982) Using saliva to estimate
human exposure to organic solvents. Bull. Tokyo dent. Coll.,
23: 175-188.
251. TOOBY, T.E., HURSEY, P.A., & ALABASTER, J.S. (1975) The
acute toxicity of 102 pesticides and miscellaneous substances
to fish. Chem. Ind., 12: 523-526.
252. TRAIGER, G.J. & PLAA, G.L. (1972) Relationship of alcohol
metabolism to the potentiation of CCl4 hepatotoxicity induced
by aliphatic alcohols. J. Pharmacol. exp. Ther., 183:
481-488.
253. TRAIGER, G.J. & PLAA, G.L. (1974) Chlorinated hydrocarbon
toxicity. Potentiation by isopropyl alcohol and acetone.
Arch. environ. Health, 28: 276-278.
254. TU, Y.Y., PENG, R., CHANG, Z.-F., & YANG, C.S. (1983)
Induction of a high affinity nitrosamine demethylase in rat
liver microsomes by acetone and isopropanol. Chem.-biol.
Interact., 44: 247-260.
255. UENG, T.-H., MOORE, L., ELVES, R.G., & ALVARES, A.P. (1983)
Isopropanol enhancement of cytochrome P-450-dependent
monooxygenase activities and its effects on carbon
tetrachloride intoxication. Toxicol. appl. Pharmacol., 71:
204-214.
256. URANO, K., OGURA, K., & WADA, H. (1981) Direct analytical
method for aliphatic compounds in water by steam carrier gas
chromatography. Water Res., 15: 225-231.
257. US NIOSH (1976) Criteria for a recommended standard:
occupational exposure to isopropyl alcohol, Cincinnati, Ohio,
US National Institute of Occupational Safety and Health, US
Department of Health, Education, and Welfare, Public Health
Services, Center for Disease Control (DHEW Publication No.
(NIOSH)76-142).
258. US NIOSH (1977) Manual of analytical methods, 2nd ed.,
Cincinnati, Ohio, US National Institute for Occupational
Safety and Health, US Department of Health, Education, and
Welfare, Vol. 2, pp. S185.
259. US NIOSH (1984) Method 1400. In: Eller, P.M., ed. NIOSH
Manual of analytical methods, 3rd ed., Cincinnati, Ohio,
National Institute for Occupational Safety and Health, Vol.
1, pp. 1400-1-1400-5.
260. VAN RILLAER, W.G. & BEERNAERT, H. (1983) Determination of
residual isopropanol and propylene glycol in soft drinks by
glass capillary gas chromatography. Z. Lebensm. Unters.
Forsch., 177: 196-199.
261. VASILIADES, J., POLLOCK, J., & ROBINSON, C.A. (1978)
Pitfalls of the alcohol dehydrogenase procedure for the
emergency assay of alcohol: a case study of isopropanol
overdose. Clin. Chem., 24: 383-385.
262. VEITH, G.D. & KOSIAN, P. (1983) Estimating bioconcentration
potential from octanol/water partition coefficients. In:
Mackay et al., ed. Physical behaviour of PCBs in the Great
Lakes, Ann Arbor, Michigan, Ann Arbor Science, pp. 269-282.
263. VEITH, G.D., CALL, D.J., & BROOKE, L.T. (1983) Structure-
toxicity relationships for the fathead minnow, Pimephales
promelas: narcotic industrial chemicals. Can. J. Fish. aquat.
Sci., 40: 743-748.
264. VIDELA, L.A., FERNANDEZ, V., & DE MARINIS, A. (1982) Liver
peroxidative pressure and glutathione status following
acetaldehyde and aliphatic alcohols pretreatment in the rat.
Biochem. Biophys. Res. Commun., 104: 965-970.
265. VILAGELIU ARQUES, L. & GONZALEZ DUARTE, R. (1980) Effect of
ethanol and isopropanol on the activity of alcohol
dehydrogenase, viability and life-span in Drosophila
melanogaster and Drosophila funebris. Experientia, 36:
828-830.
266. VISUDHIPAN, P. & KAUFMAN, H. (1971) Increased cerebrospinal
fluid protein following isopropyl alcohol intoxication. N.Y.
State J. Med., 71: 887-880.
267. VON DER HUDE, W., SCHEUTWINKEL, M., GRAMLICH, U., FISSLER,
B., & BUSLER, A. (1987) Genotoxicity of three carbon
compounds evaluated in the SCE test in vitro. Environ.
Mutagen., 9: 401-410.
268. WAGNER, R. (1974) [Investigations into the degradation
behaviour of organic compounds using the respirometric
dilution method. I. Monohydric alcohols.] Vom Wasser, 42:
271-305 (in German).
269. WAGNER, R. (1976) [Investigations into the degradation
behaviour of organic compounds using the respirometric
dilution method. II. The degradation kinetics of the test
compounds.] Vom Wasser, 47: 241-265 (in German).
270. WALLGREN, H. (1960) Relative intoxicating effects on rats of
ethyl, propyl and butyl alcohols. Acta pharmacol. toxicol.,
16: 217-222.
271. WALLINGFORD, K.M. (1983) Health hazard evaluation, Xomox
Corporation, Cincinnati, Ohio, US National Institute for
Occupational Safety and Health (HETA 83-170-1346, PB 85-
163434).
272. WASILEWSKI, C. (1968) Allergic contact dermatitis from
isopropyl alcohol. Arch. Dermatol., 98: 502-504.
273. WATERER, D.R. & PRITCHARD, M.K. (1984) Monitoring of
volatiles: a technique for detection of soft rot (Erwinia
carotovora) in potato tubers. Can. J. Plant. Pathol., 6:
165-171.
274. WAX, J., ELLIS, F.W., & LEHMAN, A.J. (1949) Absorption and
distribution of isopropyl alcohol. J. Pathol. exp. Ther., 97:
229-237.
275. WEBBER, D. (1984) Basic chemical output returns to growth.
Top 50 chemical products. Chem. eng. News, May 7: 8-10.
276. WEIL, C.S., SMYTH, H.F., & NALE, T.W. (1952) Quest for a
suspected industrial carcinogen. Arch. ind. Hyg. occup. Med.,
5: 535-547.
277. WEINTRAUB, Z. & IANCU, T.C. (1982) Isopropyl alcohol burns.
Pediatrics, 69: 506.
278. WHITE, G.A., EMMETT, E.A., KOMINSKY, J.R., & SINGAL, M.
(1983) Health hazard evaluation, Inland Division, GMC,
Cincinnati, Ohio, US National Instituue for Occupational
Safety and Health (HETA 77-011-1338, PB 85-101319).
279. WHITEHEAD, L.W., BALL, G.L., FINE, L.J., & LANGOLF, G.D.
(1984) Solvent vapor exposures in booth spray painting and
spray glueing, and associated operations. Am. ind. Hyg.
Assoc. J., 45: 767-772.
280. WILKINSON, C. & IGLEWICZ, R. (1982) Health hazard
evaluation, Syntrex Corporation, Cincinnati, Ohio, US
National Institute for Occupational Safety and Health (HETA
81-370-1050, PB 83-198424).
281. WILKINSON, T. & HAMER, G. (1979) The microbial oxidation of
mixtures of methanol, phenol, acetone, and isopropanol with
reference to effluent purification. J. chem. Technol.
Biotechnol., 29: 56-67.
282. WILLIAMS, T.M., HICKEY, J.L.S., & SHY,C.M. (1982) Health
hazard evaluation, Dittler Brothers, Inc., Cincinnati, Ohio,
US National Institute for Occupational Safety and Health
(HETA 81-173-1051, PB 83-198473).
283. WILLS, J.H., JAMESON, E.M., & COULSTON, F. (1969) Effects on
man of daily ingestion of small doses of isopropyl alcohol.
Toxicol. appl. Pharmacol., 15: 560-565.
284. WINEK, C.L. & JANSSEN, J.K. (1982) Blood versus bone marrow
isopropanol concentrations in rabbits. Forensic Sci. Int.,
20: 11-20.
285. WOLFF, T. (1978) In vitro inhibition of monooxygenase
dependent reactions by organic solvents. Int. Congr. Ser.
Excerpta Med., 440: 196-199.
286. WRIGHT, U. (1979) The hidden carcinogen in the manufacture
of isopropyl alcohol. Dev. Toxicol. environ. Sci., 4: 93-98.
287. YASHUDA, Y., CABRAL, A.M., & ANTONIO, A. (1976) Inhibitory
action of aliphatic alcohols on smooth muscle contraction.
Pharmacology, 14: 473-478.
288. YOUNG, P.J. & PARKER, A. (1983) The identification and
possible environmental impact of trace gases and vapours in
landfill gas. Waste Management Res., 1: 213-226.
289. YOUNG, R.H.F., RYCKMAN, D.W., & BUZZELL, J.C. (1968) An
improved tool for measuring biodegradability. J. Water
Pollut. Control Fed., 40: R354-R368.
290. ZAHLSEN, K., AARSTAD, K., & NILSEN, O.G. (1985) Inhalation
of isopropanol: induction of activating and deactivating
enzymes in rat kidney and liver, increased microsomal
metabolism of n-hexane. Toxicology, 34: 57-66.
291. ZAKHARI, S. (1977) Isopropanol and ketones in the
environment, Oxford, England, CRC Press.
292. ZINBO, M. (1984) Determination of one-carbon to three-carbon
alcohols and water in gasoline/alcohol blends by liquid
chromatography. Anal. Chem., 56: 244-247.
RESUME
1. Identité, propriétés physiques et chimiques, méthodes d'analyse
Le propanol-2 est un liquide incolore, très inflammable dont
l'odeur rappelle celle d'un mélange d'éthanol et d'acétone. Il est
entièrement miscible à l'eau, à l'éthanol, à l'acétone, au
chloroforme et au benzène. Il existe des méthodes d'analyse pour
la recherche du propanol-2 dans divers milieux (air, eau, sang,
sérum et urine), avec des limites de détection dans l'air, l'eau et
le sang respectivement égales à 2 x 10-5 mg/m3, 0,04 mg/litre et 1
mg/litre. La chromatographie en phase gazeuse (essentiellement
avec détection par ionisation de flamme) ainsi que l'électrophorèse
sur papier et la spectrométrie de mobilité ionique par
photoionisation permettent de doser le propanol-2 dans divers
mileux.
2. Sources d'exposition humaine et environnementale
On estime qu'en 1975, la production mondiale de propanol-2
dépassait 1100 kilotonnes, la capacité mondiale de production étant
supérieure à 2000 kilotonnes en 1984. Le propanol-2 est couramment
produit à partir du propène. Les procédés antérieurs de
fabrication qui reposaient sur l'utilisation d'acides forts ou
d'acides faibles, et donnaient naissance à des produits
intermédiaires et à des sous-produits potentiellement dangereux,
sont désormais largement supplantés par le procédé d'hydratation
catalytique. On peut également procéder par réduction catalytique
de l'acétone.
Le propanol-2 est un produit du métabolisme de divers micro-
organismes.
Il a de nombreuses applications comme solvant et il entre dans
la composition de différents produits ménagers et produits de soins
personnels, sous forme d'aérosols et d'excipients pour produits
pharmaceutiques à usage externe et pour cosmétiques. Le propanol-2
est également utilisé pour la production de l'acétone et autres
produits chimiques, comme agent de dégivrage, comme conservateur et
il entre dans la composition des concentrés pour le nettoyage des
pare-brise et de certains aromatisants alimentaires.
Le propanol-2 peut pénétrer dans l'atmosphère, dans l'eau et
dans le sol lors du rejet de déchets et on en a trouvé dans l'air
et dans les eaux de lessivage de décharges mal protégées. Présent
dans les gaz et eaux résiduaires industriels, on peut l'en éliminer
par oxydation biologique ou osmose inverse. Il peut être dissipé
dans l'atmosphère lors de l'utilisation de produits de consommation
qui en contiennent.
3. Transport et distribution dans l'environnement
C'est principalement lors d'opérations telles que la
production, la transformation, le stockage, le transport,
l'utilisation et le rejet de déchets que le propanol-2 pénètre dans
l'environnement atmosphérique. Il peut être également déchargé
dans le sol et l'eau. Il est difficile d'évaluer la part qui
revient à chaque compartiment du milieu. Toutefois on estimait en
1976 que plus de 50 % du propanol-2 produit finissait par être
libéré dans l'atmosphère.
Le propanol-2 est rapidement éliminé de l'atmosphère par
réaction sur les radicaux hydroxyles et entraînement par les
précipitations. Ce sont ces dernières qui sont responsables du
transport de ce composé de l'atmosphère dans le sol ou l'eau. Une
fois dans le sol, il doit y être très mobile et augmente la
perméabilité du sol à certains hydrocarbures aromatiques. Le
propanol-2 est facilement biodégradable par voie aérobie ou
anaérobie.
Comme il est biodégradable et complètement miscible à l'eau,
avec un coefficient de partition octanol/eau logarithmique de 0,14
et un facteur de bioconcentration de 0,5, il est peu probable qu'il
donne lieu à une bioaccumulation.
4. Niveaux dans l'environnement et exposition humaine
L'exposition de la population en général peut se produire par
ingestion accidentelle ou volontaire, par absorption de nourriture
contenant du propanol-2 d'origine naturelle, ou sous forme
d'aromatisant volatil ou de résidus de solvant, ou encore par
inhalation lors de l'utilisation de produits qui en contiennent.
On en a trouvé aux concentrations de 0,2 à 325 mg/litre dans des
boissons non alcoolisées et aux concentrations de 50 à 3000 mg/kg
dans des denrées alimentaires pour la production desquelles on
l'avait utilisé comme solvant. L'exposition de la population en
général par inhalation de l'air ambiant est faible, du fait de
l'élimination et de la dégradation rapides de ce produit. En
procédant à des contrôles en divers lieux et, en particulier, dans
des sites urbains, on a obtenu des concentrations moyennes
pondérées par rapport au temps allant jusqu'à 35 mg/m3.
Les travailleurs peuvent être exposés au propanol-2 au cours de
la production du composé lui-même, lors de la fabrication de
l'acétone ou d'autres dérivés et également lorsqu'on utilise ce
produit comme solvant. Aux Etats-Unis, une enquête (National
Occupational Exposure Survey) effectuée en 1980-83 a montré que
plus de 1,8 million de travailleurs pouvaient être exposés. On a
mesuré sur les lieux de travail des concentrations atteignant 1350
mg/m3 avec des moyennes pondérées par rapport au temps allant
jusqu'á 500 mg/m3.
5. Cinétique et métabolisme
Le propanol-2 est rapidement absorbé et se répartit dans tout
l'organisme par inhalation et ingestion. A forte dose,
l'absorption dans les voies digestives est retardée. Les taux
sanguins de propanol-2 (décelables lorsqu'il y a ingestion
simultanée d'éthanol) ou de son métabolite, l'acétone, sont
corrélés avec l'intensité de l'exposition. Des volontaires qui
avaient ingéré une dose de 3,75 mg/kg de propanol-2 (avec 1200 mg
d'éthanol/kg) dans du jus d'orange, présentaient une concentration
sanguine maximale de propanol-2 libre de 0,8 ± 0,3 mg/litre, et de
2,3 ± 1,4 mg/litre après incubation en présence d'arylsulfatase, ce
qui témoigne d'une sulfatation. Chez les ouvriers exposés à des
vapeurs de propanol-2 (8 - 647 mg/m3), on a observé des
concentrations de 3 - 270 mg/m3 dans l'air alvéolaire, mais dans ce
cas, c'est de l'acétone et non du propanol-2 que l'on a trouvé dans
le sang et les urines. Chez des animaux de laboratoire exposés au
propanol-2, on a retrouvé celui-ci non seulement dans le sang mais
également dans le liquide céphalo-rachidien, dans le foie, les
reins et le cerveau. Le propanol-2 traverse la barrière
hémoméningée deux fois plus facilement que l'éthanol. Le propanol-
2 est excrété en partie tel quel et en partie sous forme d'acétone,
essentiellement au niveau des poumons mais également dans la salive
et le suc gastrique. Il peut y avoir réabsorption après excrétion
par ces deux dernières voies. La métabolisation en acétone en
présence d'alcool-déshydrogénase (ADH) hépatique est assez lente,
du fait que l'ADH a une moindre affinité pour le propanol-2 que
pour l'éthanol. In vitro, l'activité de l'ADH humaine vis-à-vis du
propanol-2 correspond à 9 - 10 % de l'activité de cette enzyme
lorsque le substrat est de l'éthanol. Les oxydases microsomiques
du foie de rat sont également capables d'oxyder le propanol-2
in vitro. Chez l'homme, l'acétone est excrétée telle quelle,
essentiellement au niveau des poumons et en quantité minime au
niveau des reins. Plus l'exposition au propanol-2 se prolonge,
plus la concentration d'acétone dans l'air alvéolaire, dans le sang
et dans les urines est élevée. Le propanol-2 et l'acétone sont
éliminés de l'organisme selon une cinétique du premier ordre et
leur demi-vie chez l'homme est de 2,5 - 6,4 heures et 22 heures,
respectivement.
6. Effets sur les êtres vivants dans leur milieu naturel
Le propanol-2 est peu toxique pour la faune et la flore
aquatiques, les insectes et les plantes. Son seuil d'inhibition de
la multiplication cellulaire, mesuré chez une espèce sensible de
protozoaire, varie de 104 à 4930 mg/litre selon les conditions
expérimentales. Si l'on s'élève dans l'arbre phylogénétique, on
constate que diverses espèces de crustacés, notamment Daphnia
magna, présentent des CE50 allant de 2285 à 9714 mg/litre. Pour
des poissons d'eau douce, on a obtenu des CL50 à 96 heures allant
de 4200 à 11 130 mg/litre. Chez la drosophile, les CL50 vont de
10 200 à 13 340 mg/litre de milieu nutritif. Pour le troisième
stade larvaire du moustique Aedes aegypti, on a obtenu, lors d'une
épreuve statique de 4 heures, des valeurs de la CL50 allant de 25 à
120 mg/litre.
L'exposition de végétaux à du propanol-2 à des concentrations
comprises entre 2100 mg/litre et plus de 36 000 mg/litre, a
provoqué toute une gamme d'effets allant de l'absence totale
d'anomalies à une inhibition complète de la germination.
7. Effets sur les animaux d'expérience et les systèmes d'épreuves
in vitro
A en juger d'après la mortalité qu'il provoque, le propanol-2
présente une faible toxicité aiguë pour les mammifères, que
l'exposition ait lieu par voie orale, percutanée ou respiratoire.
Chez plusieurs espèces animales on a obtenu, après administration
par voie orale, des valeurs de la DL50 allant de 4475 à 7990 mg par
kg de poids corporel; la CL50 pour une inhalation de 8 heures
allait de 46 000 à 56 000 mg/m3 d'air chez le rat. A ces doses
mortelles, les rats présentaient une forte irritation des muqueuses
et une grave dépression du système nerveux central. La mort est
survenue par arrêt cardiaque ou respiratoire. Entre autres lésions
histopathologiques, on notait une congestion et un oedème du poumon
ainsi qu' une dégénérescence des hépatocytes.
Administré en dose unique par voie orale à des rats à raison de
3000 ou 6000 mg par kg de poids corporel, le propanol-2 a provoqué
une accumulation réversible de triglycérides dans le foie. On a
également observé chez ces rats une induction des enzymes
microsomiques à la dose de 390 mg/kg.
Non dilué, le propanol-2 n'est pas irritant en applications de
4 heures sur la peau, intacte ou abrasée, de lapins tondus.
Toutefois, en instillations oculaires de 0,1 ml, le propanol-2 non
dilué a provoqué une irritation chez le lapin. De fortes
concentrations de vapeurs de propanol-2 ont provoqué une irritation
respiratoire chez la souris et l'on a noté une réduction de 50 % du
rythme respiratoire à des concentrations allant de 12 300 à 43 525
mg/m3 d'air.
Les études conscrées aux effets sur l'animal d'exposition
répétées au propanol-2 sont plutôt limitées. Après inhalation par
des rats de 500 mg/m3 de propanol-2, cinq jours par semaine et
quatre heures par jour pendant quatre mois, on a noté une
irritation des voies respiratoires, des anomalies haématologiques
et des altérations histopathologiques au niveau du foie et de la
rate. Un autre groupe expérimental composé de cinq rats de chaque
sexe a reçu pendant 27 semaines du propanol-2 dans son eau de
boisson. En comparant les animaux qui recevaient environ 600 ou
2300 mg/kg par jour (mâles) et 1000 ou 3900 mg/kg par jour
(femelles) de propanol-2 à des témoins non traités, on a constaté
un retard de croissance, mais uniquement chez les deux groupes de
femelles traitées. Aucun autre effet indésirable n'a été constaté.
Les données disponibles donnent à penser que le propanol-2
produit sur le système nerveux central (SNC) des effets analogues à
ceux de l'éthanol. La DE50 d'anésthésie par voie orale est de 2280
mg/kg chez le lapin, la DE50 par voie intrapéritonéale
correspondant à la perte du réflexe de redressement chez la souris
est égale à 165 mg/kg et le seuil d'induction de l'ataxie par voie
intrapéritonéale est de 1106 mg/kg chez le rat. Ces valeurs sont
sont environ deux fois plus faibles que pour l'éthanol. Lors d'une
expérience menée à l'air libre, on a constaté que l'inhalation de
propanol-2 à la dose de 739 mg/m3, dix heures par jour, et cinq
jours par semaine pendant 15 semaines ne produisait aucun effet
indésirable.
Le propanol-2 a été soumis à une étude portant sur deux
générations de rats qui ont reçu dans leur eau de boisson des doses
quotidiennes de 1290, 1380 ou 1479 mg de ce produit par kg de poids
corporel. Les seuls effets indésirables qui ont été notés
consistaient dans une réduction passagère du taux de croissance
dans la génération F0. En revanche, d'autres chercheurs ont
constaté une augmentation des malformations lors d'une étude
tératogènicité, au cours de laquelle on avait administré à des
rates gravides, des doses orales quotidiennes de 252 ou 1008 mg de
propanol-2 par kg de poids corporel (toxicité maternelle non
étudiée). On a également indiqué que ces deux doses, administrées
pendant 45 jours dans l'eau de boisson, faisaient passer le cycle
oestral à cinq jours (contre quatre chez les témoins). Chez des
rates ayant reçu pendant six mois dans leur eau de boisson, des
doses quotidiennes de propanol-2 de 1800 mg/kg avant de mettre bas,
on a constaté un accroissement de la mortalité embryonnaire totale;
divers effets ont également été signalés concernant la survie
intra-utérine et postnatale à des doses aussi basses que 0,8 mg/kg,
sans qu'on puisse toutefois dégager une tendance précise. Des
rates gravides ont été exposées à de l'air contenant du propanol-2
aux concentrations respectives de 9001, 18 327 et 23 210 mg/m3
(3659, 7450 ou 9435 ppm). Les deux concentrations les plus fortes
se sont révélées toxiques pour les mères, la concentration de 9001
mg/m3 ne produisant aucun effet. A toutes les concentrations on a
constaté un effet nocif sur le développement.
Une épreuve de recherche des mutations ponctuelles utilisant
S. typhimurium a donné des résultats négatifs avec du propanol-2 à
la dose de 0,18 mg par boîte; la recherche d'échanges entre
chromatides soeurs sur fibroblastes de hamster chinois a également
été négative. Le propanol-2 a provoqué des anomalies de la mitose
dans des cellules médullaires de rat ainsi que des cellules de
l'extrêmité radiculaire d'oignons in vitro. On ne dispose d'aucune
autre donnée sur la mutagénicité de ce produit.
Un certain nombre d'études restreintes ont été consacrées au
pouvoir cancérogène du propanol-2, au cours desquelles des souris
ont été exposées à cette substance par voie percutanée (3 fois par
semaine pendant un an), par voie respiratoire (7700 mg/m3, 3 à 7
heures par jour, cinq jours par semaine pendant 5 à 8 mois) et par
voie sous-cutanée (20 mg de propanol non dilué par semaine pendant
20 à 40 semaines). Au cours de ces trois études, on a recherché la
présence de tumeurs sur la peau, dans les poumons et au point
d'injection. Aucun signe d'effets cancérogènes n'a été observé.
On ne dispose pas de données épidémiologiques suffisantes pour
évaluer la cancérogénicité du propanol-2 chez l'homme. A la
lumière des données disponibles, on peut penser que le sulfate de
dipropyl-2, un produit intermédiaire de la fabrication du
propanol-2 par le procédé aux acides forts et faibles, pourrait
être à l'origine de cancers du sinus maxillaire chez l'homme.
8. Effets sur la santé de l'homme
On a signalé plusieurs cas d'intoxication consécutifs à
l'ingestion de propanol-2 ou à l'utilisation de lotions à base de
ce produit pour rafraîchir des enfants fébriles. Les principaux
signes d'intoxication rappellent ceux de l'intoxication alcoolique:
nausées, vomissements, douleurs abdominales, gastrite, hypotension
et hypothermie. La dépression du SNC par le propanol-2 est deux
fois plus intense qu'avec l'éthanol et entraîne l'inconscience puis
un coma profond; la mort peut survenir par dépression respiratoire.
Parmi les autres effets on peut noter l'hyperglycémie, un taux
élevé de protéines dans le liquide céphalorachidien et une
atélectasie. Il semblerait que l'absorption percutanée soit
négligeable mais on connaît le cas d'un enfant qui a été intoxiqué
après avoir été lotionné avec du propanol-2, ce qui donne à penser
qu'il ne faut pas négliger l'absorption percutanée, notamment chez
les enfants. Aucun effet indésirable n'a été observé chez des
volontaires en bonne santé qui avaient bu tous les jours pendant
six semaines un sirop contenant du propanol-2 à des doses
corrrespondant respectivement à 2,6 et 6,4 mg de propanol-2 par kg
de poids corporel. Des volontaires du sexe masculin, exposés 3 à 5
minutes à des vapeurs de propanol-2 correspondant à des
concentrations de 490, 980 ou 1970 mg/m3 d'air, ont estimé qu'ils
ressentaient une irritation légère à 980 mg/m3 et que la situation
était "satisfaisante" pendant les 8 heures correspondant à leur
propre exposition professionnelle.
Des enfants prématurés ayant subi un contact prolongé avec du
propanol-2 ont présenté une irritation cutanée prenant la forme
d'érythèmes, voire de brûlures du 2ème et du 3ème degré et de
phlyctènes. On a également signalé ça et là des cas de dermatites
allergiques de contact.
Il n'existe de peu d'études épidémiologiques consacrées à la
mortalité par cancers ou autres maladies provoquées par le
propanol-2. Parmi un groupe de 61 travailleurs, employés pendant
plus de cinq ans dans un atelier de fabrication de propanol-2 par
le procédé à l'acide fort, on a observé sept cas de cancer dont
quatre de cancer des sinus maxillaires. Une étude portant sur une
cohorte de 779 travailleurs d'un atelier analogue a révélé que
l'incidence des cancers du sinus et du larynx, corrigée de
l'influence de l'âge et du sexe, était 21 fois plus forte que
prévue. La période minimale de latence était de dix ans. Dans une
autre étude rétrospective de cohorte portant sur le personnel d'une
autre usine où on utilisait le procédé à l'acide fort, on a
constitué une cohorte représentant plus de 4000 années-hommes
exposés au risque. Les résultats de cette étude ont montré que les
taux de mortalité pour toutes causes et les taux de mortalité par
cancer n'étaient pas sensiblement plus élevés que les taux
prévisibles. Une autre étude rétrospective a été menée dans une
usine qui fabriquait du propanol-2 par le procédé à l'acide faible.
Cette fois, on comptait plus de 11 000 années-hommes exposés au
risque. Dans ce cas, le taux de mortalité pour toutes causes était
inférieur aux prévisions et l'on ne constatait pas de surmortalité
attribuable aux cancers en général. Toutefois, l'incidence des
cancers de la bouche et du pharynx était 4 fois supérieure à la
normale. Dans l'ensemble, ces études de cohorte donnent à penser
qu'il existe un risque de cancer imputable au procédé à l'acide
fort; toutefois lors de deux petites études castémoins, on n'a noté
aucune association entre l'exposition au propanol-2 et l'incidence
des gliomes ou de la leucémie lymphatique.
Certaines études font état d'une potentialisation de la
toxicité du tétrachlorure de carbone chez des ouvriers
simultanément exposés au propanol-2.
9. Résumé de l'évaluation
L'homme peut être exposé au propanol-2 par inhalation lors de
la fabrication, de la transformation ou de l'utilisation de cette
substance dans le cadre professionnel ou domestique. En ce qui
concerne la population en général, l'exposition à des doses
potentiellement mortelles peut se produire par suite d'ingestion
accidentelle ou volontaire de cette substance et les enfants
peuvent être exposés par application de lotions à base de propanol-
2.
Le propanol-2 est vite absorbé et se répartit rapidement dans
l'ensemble de l'organisme, en partie sous forme d'acétone. Les
données relatives aux effets aigus consécutifs à l'exposition
d'êtres humains à des doses excessives sont rares et contrastées.
Les principaux effets consistent en gastrite, dépression du système
nerveux central, hypothermie, dépression respiratoire et
hypotension. Les données de mortalité aiguë obtenues sur des
animaux de laboratoire indiquent que le propanol-2 est peu toxique,
les valeurs de la DL50 par voie orale chez diverses espèces vont de
4475 à 7990 mg/kg, et les valeurs de la CL50 par inhalation se
situent aux alentours de 50 000 mg/m3 chez le rat. Chez le lapin,
le propanol-2 ne provoque pas d'irritation cutanée, toutefois
l'instillation de 0,1 ml de cette substance non diluée dans les
yeux a provoqué une irritation.
Chez l'homme, les effets aigus les plus probables d'une
exposition de fortes concentrations de propanol-2 par ingestion ou
inhalation, consistent en une intoxication de type alcoolique
aboutissant à la narcose.
Les études sur l'animal sont insuffisantes pour qu'on puisse
évaluer les risques encourus par l'homme à la suite d'expositions
répétées au propanol-2. Toutefois les résultats de deux études à
court terme chez le rat, au cours desquelles on a fait a) inhaler
500 mg/m3 de cette substance, 4 heures par jour, 5 heures par
semaine pendant 4 mois et b) ingérer le même produit à raison de
600 à 3900 mg/kg dans l'eau de boisson, donnent à penser qu'il
serait préférable d'éviter de s'exposer aux fortes concentrations
en propanol-2 signalées dans le cadre de certaines activités
professionnelles.
En faisant inhaler du propanol-2 à des rattes gravides on a
constaté que le seuil d'apparition d'un effet se situait à 18 327
mg/m3 (7450 ppm), la dose sans effet observable était de 9001 mg/m3
(3659 ppm), la toxicité maternelle étant prise comme critère. Au
cours de la même étude, le seuil d'apparition d'effets s'est situé
à 9001 mg/m3 (3659 ppm) pour les anomalies du développement et
aucune dose sans effet observable n'a pu être mise en évidence.
Ces concentrations sont plus élevées que celles auxquelles l'homme
est susceptible d'être exposé.
Les épreuves de génotoxicité ont donné des résultats négatifs
dans le cas du propanol-2, cependant on a observé des anomalies de
la mitose dans des cellules médullaires de rats. Ces résultats
indiquent que le propanol-2 n'est pas du tout génétoxique mais les
données sont trop limitées pour qu'on puisse se prononcer
véritablement sur le pouvoir mutagène de cette substance.
Les données existantes sont insuffisantes pour permettre une
évaluation de la cancérogénicité du propanol-2 chez l'animal
d'expérience. On ne dispose pas de données permettant d'évaluer
cette cancérogénicité chez l'homme.
Le propanol-2 ne fait probablement pas courir de risque
important à la population dans son ensemble dans les conditions
d'exposition qui sont susceptibles de se produire.
Le propanol-2 disparaît rapidement (demi-vie, 5 jours) de
l'atmosphère et s'élimine à bref délai de l'eau et du sol par
biodégradation aérobie ou anaérobie, en particulier une fois que
les microorganismes préalablement ensemencés se sont adaptés.
Compte tenu de ses propriétés physiques, le propanol-2 n'a qu'une
faible tendance à la bioaccumulation. Il ne présente pas de risque
pour la faune et la flore aux concentrations auxquelles il est
habituellement présent dans l'environnement.
RESUMEN
1. Identidad, propiedades físicas y químicas, métodos analíticos
El 2-propanol es un líquido incoloro, sumamente inflamable, con
un olor que recuerda al de la mezcla de etanol y acetona. El
compuesto es completamente miscible con agua, etanol, acetona,
cloroformo y benceno. Se dispone de métodos analíticos para
detectar el 2-propanol en diversos medios (aire, agua, sangre,
suero y orina) con límites de detección de 2 x 10-5 mg/m3, 0,04
mg/litro y 1 mg/litro en el aire, el agua y la sangre,
respectivamente. Existen métodos de cromatografía de gases (que se
sirven principalmente de la detección de ionización de llama) así
como métodos de electroforesis en papel y de espectrometría de
movilidad iónica inducida por fotoionización para determinar el
2-propanol en los distintos medios.
2. Fuentes de exposición humana y ambiental
La producción mundial estimada de 2-propanol en 1975 fue
superior a 1100 kilotoneladas y la capacidad de producción mundial
en 1984 se cifró en más de 2000 kilotoneladas. El 2-propanol se
fabrica comúnmente a partir del propeno. Los antiguos procesos
basados en ácidos fuertes y débiles, en los que se generaban
productos intermedios y desechos potencialmente peligrosos, se han
sustituido actualmente en gran medida por el proceso de hidratación
catalítica. La reducción catalítica de la acetona es otro proceso
posible.
El 2-propanol se ha identificado como producto metabólico de
diversos microorganismos.
El compuesto tiene amplias aplicaciones como disolvente y se
utiliza como componente de productos domésticos y personales, entre
ellos vaporizadores de aerosoles, productos farmacéuticos de
aplicación tópica y cosméticos. El 2-propanol se utiliza también
para producir acetona y otras sustancias químicas, como agente
descongelante, como conservante, en concentrados para
limpiaparabrisas y como aromatizante volátil en alimentos.
El 2-propanol puede ingresar en la atmósfera, el agua o el
suelo por la evacuación de desechos y se ha aislado en el aire y en
el líquido que rezuma de basureros y terraplenados. Se encuentra
en los gases y las aguas residuales que emiten algunas industrias,
y puede extraerse de esas aguas por oxidación biológica o por
ósmosis inversa. Durante el uso de 2-propanol en productos de
consumo se producen emisiones dispersas a la atmósfera.
3. Transporte, distribución y transformación en el medio ambiente
La vía principal de entrada del 2-propanol en el medio ambiente
es su emisión a la atmósfera durante la producción, el tratamiento,
el almacenamiento, el transporte, el uso y la evacuación. También
se producen emisiones al suelo y al agua. Es difícil calcular el
volumen que ingresa en cada compartimiento ambiental. No obstante,
se calculó que en 1976 la liberación total de este compuesto en la
atmósfera fue superior al 50% del 2-propanol producido.
El 2-propanol desaparece rápidamente de la atmósfera por
reacción con radicales hidroxilo y arrastrado por la lluvia. A
este último proceso se debe el transporte del 2-propanol desde la
atmósfera hasta el suelo o el agua. Una vez que está en el suelo,
se cree que es muy móvil y que aumenta la permeabilidad del suelo a
algunos hidrocarburos aromáticos. El 2-propanol es fácilmente
biodegradable, en condiciones tanto aerobias como anaerobias.
La bioacumulación del compuesto no es probable, dados su
carácter biodegradable y su miscibilidad total con el agua; su
coeficiente de reparto log n-octanol/agua es de 0,14 y su factor de
bioconcentración de 0,5.
4. Niveles ambientales y exposición humana
La exposición de la población general se produce por ingestión
accidental o intencionada, por la ingestión de alimentos que lo
contengan como aromatizante volátil natural o añadido o como
residuo de disolvente, y por inhalación durante su uso. Se han
encontrado concentraciones de 0,2 a 325 mg por litro en bebidas no
alcohólicas y de 50 a 3000 mg por kg en alimentos tras el uso de
2-propanol como disolvente en su producción. La exposición de la
población general por inhalación de aire ambiental es baja a causa
de su rápida desaparición y degradación. Se han estudiado diversas
localizaciones y se han medido concentraciones medias ponderadas en
función del tiempo de hasta 35 mg/m3 en emplazamientos urbanos.
Los trabajadores se ven expuestos al 2-propanol durante la
producción del propio compuesto y de acetona y otros derivados, así
como durante su uso como disolvente. En la encuesta nacional de
exposición ocupacional (1980 - 83) realizada en los Estados Unidos,
se estimó que más de 1,8 millones de trabajadores estaban
potencialmente expuestos. En ciertos lugares de trabajo se han
medido concentraciones de hasta 1350 mg/m3, con promedios
ponderados en función del tiempo de hasta 500 mg/m3.
5. Cinética y metabolismo
El 2-propanol se absorbe y distribuye rápidamente por todo el
organismo tras su inhalación o ingestión. A dosis elevadas se
retrasa la absorción gastrointestinal. Las concentraciones
sanguíneas de 2-propanol (detectables cuando se ingiere etanol
simultáneamente) o de su metabolito, la acetona, guardan relación
con los niveles de exposición. En voluntarios que ingirieron una
dosis de 3,75 mg/kg (con 1200 mg de etanol/kg) en zumo de naranja,
se observó un nivel máximo de 0,8 ± 0,3 mg de 2-propanol libre por
litro en la sangre, y de 2,3 ± 1,4 mg por litro tras la incubación
con arilsulfatasa, lo que es un signo de sulfatación. Los
trabajadores expuestos a vapores (8 - 647 mg/m3) mostraron
concentraciones de 3 - 270 mg/m3 en el aire alveolar, pero en este
caso se encontró acetona y no 2-propanol en la sangre y la orina.
En animales de laboratorio tratados, el 2-propanol se detectó no
sólo en la sangre sino también en el líquido espinal, el hígado,
los riñones y el cerebro. Atraviesa la barrera hematocerebral dos
veces mejor que el etanol. El 2-propanol se excreta en parte como
tal y en parte como acetona, principalmente por vía pulmonar, pero
también en la saliva y el jugo gástrico. La reabsorción puede
producirse después de la excreción por las últimas dos vías. La
transformación en acetona por medio de la deshidrogenasa alcohólica
del hígado es más bien lenta, porque la afinidad relativa de la
deshidrogenasa por el 2-propanol es más baja que por el etanol.
In vitro, la actividad enzimática de la deshidrogenasa humana con
2-propanol fue del 9 - 10% de la actividad que exhibe cuando el
sustrato es el etanol. In vitro las oxidasas microsómicas de
hígado de rata también son capaces de oxidar el 2-propanol. En el
hombre, la acetona se excreta sin cambios, principalmente por los
pulmones y en cantidad mínima por los riñones. La concentración de
acetona en el aire alveolar, la sangre y la orina aumenta con la
intensidad y la duración de la exposición al 2-propanol. La
eliminación de 2-propanol y de acetona del organismo es de primer
orden, y los periodos de semieliminación en el hombre son de
2,5 - 6,4 horas y 22 horas, respectivamente.
6. Efectos en los organismos en el medio ambiente
La toxicidad del 2-propanol para organismos acuáticos, insectos
y plantas es baja. El umbral inhibitorio para la multiplicación
celular de una especie de protozoo sensible varió de 104 a 4930 mg
por litro en diversas condiciones experimentales. Avanzando en la
cadena filogenética, varias especies de crustáceos, incluida
Daphnia magna, mostraron CE50 a concentraciones que iban desde 2285
hasta 9714 mg por litro. Las CL50 (96 h) para peces de agua dulce
variaron desde 4200 hasta 11 130 mg por litro. Los datos obtenidos
para especies de moscas de la fruta mostraron CL50 comprendidas
entre 10 200 y 13 340 mg por litro de medio nutritivo. La CL50
para larvas de mosquito ( Aedes aegypti) en la tercera etapa de
desarrollo fue de 25 - 120 mg/litro en un ensayo estático de 4
horas.
Los efectos que tiene en las plantas la exposición a 2-propanol
en concentraciones entre 2100 mg/litro y más de 36 000 mg/litro
variaron entre la ausencia de efecto y la inhibición total de la
germinación.
7. Efectos en animales de experimentación y en sistemas de ensayo
in vitro
La toxicidad aguda del 2-propanol para los mamíferos, a juzgar
por la mortalidad, es baja, sea cual sea la vía de exposición oral,
cutánea o respiratoria. Los valores de la DL50 para varias
especies animales tras la administración oral variaron entre 4475 y
7990 mg por kg de peso corporal; La CL50 de inhalación durante 8
horas en la rata varió de 46 000 a 55 000 mg por m3 de aire. A
estos niveles letales, las ratas mostraron grave irritación de las
mucosas y depresión profunda del sistema nervioso central. La
muerte fue provocada por paro respiratorio o cardiaco. Entre las
lesiones histopatológicas figuraron la congestión y el edema
pulmonar, así como la degeneración celular en el hígado.
Con dosis orales únicas de 3000 ó 6000 mg de 2-propanol por kg
de peso corporal se produjo una acumulación reversible de
triglicéridos en el hígado de la rata. En la rata se observó
inducción de enzimas microsómicas a dosis orales de 390 mg/kg.
El 2-propanol sin diluir no produjo irritaciones cuando se
aplicó a la piel cortada o raspada del conejo durante 4 horas. En
cambio, se observó irritación cuando se aplicó 0,1 ml de compuesto
sin diluir en el ojo del conejo. Con concentraciones de vapor
elevadas de 2-propanol se produjo irritación del tracto
respiratorio en el ratón, y el ritmo respiratorio disminuyó en un
50% a concentraciones de 12 300 - 43 525 mg/m3 de aire.
Se han hecho escasos estudios de exposición repetida sobre los
efectos del 2-propanol en animales. Tras la inhalación de 500 mg
de 2-propanol/m3 durante 5 días a la semana y 4 horas al día
durante más de 4 meses, se observaron irritación del tracto
respiratorio, cambios hematológicos y alteraciones histopatológicas
en el hígado y el bazo de la rata. En otro grupo de estudio, se
administró a 5 ratas de cada sexo agua de bebida que contenía
2-propanol durante 27 semanas. La comparación de animales que
recibían aproximadamente 600 ó 2300 mg por kg al día (machos) y
1000 ó 3900 mg por kg al día (hembras) con grupos de control no
tratados reveló un retraso del crecimiento sólo en ambos grupos de
hembras expuestas. No se observaron otros efectos adversos.
Los datos disponibles indican que los efectos del 2-propanol en
el sistema nervioso central son semejantes a los del etanol. La
DE50 por vía oral para la narcosis en conejos es de 2280 mg/kg; la
DE50 intraperitoneal correspondiente a la pérdida del reflejo de
enderezamiento en el ratón es de 165 mg/kg, y el umbral
intraperitoneal de inducción de ataxia en la rata es de 1106 mg/kg.
Estos valores son aproximadamente dos veces más bajos que los
correspondientes al etanol. La inhalación de 2-propanol a una
concentración de 739 mg/m3 durante 6 horas al día y 5 días a la
semana durante 15 semanas no originó ningún resultado adverso en un
ensayo en campo abierto.
El 2-propanol se evaluó en un estudio de 2 generaciones de
ratas mediante la administración de 1290, 1380 ó 1470 mg por kg al
día en el agua de bebida a ambas generaciones. El único efecto
adverso observado fue una reducción transitoria del ritmo de
crecimiento en la generación Fo. En cambio, otros investigadores
observaron un aumento de las malformaciones en un estudio de la
teratogénesis después de administrar por vía oral a ratas gestantes
252 ó 1008 mg de 2-propanol por kg al día (no se formularon
observaciones sobre la toxicidad materna). Ambas dosis,
administradas en el agua de bebida durante 45 días, también
aumentaron la duración del ciclo estrual hasta 5 días (frente a 4
días en los sujetos de control). Se observó una mortalidad
embrionaria total mayor cuando se administraban a la rata hembra
dosis de 1800 mg/kg en el agua de bebida al día durante 6 meses
antes de criar; se notificaron diversos efectos en la supervivencia
intrauterina y puerperal a dosis tan bajas como 0,18 mg/kg al día,
pero no se observó ninguna pauta coherente. Se expusieron ratas
gestantes a 2-propanol atmosférico a concentraciones de 9001,
18 327 ó 23 210 mg por m3 (3659, 7450 ó 9435 ppm). Las dos
concentraciones más elevadas fueron tóxicas para las madres, pero
no así la de 9001 mg/m3. Se observó toxicidad en el desarrollo con
las tres concentraciones.
El 2-propanol dio resultados negativos en una prueba con 0,18
mg por placa para detectar mutuaciones puntuales en S.
typhimurium y en una prueba de intercambio de cromátidas hermanas
en fibroblastos pulmonares de hámster chino. Indujo anomalías
mitóticas en células de médula ósea de rata y en células de ápice
radicular de cebolla in vitro. No se dispone de otros datos sobre
mutagenicidad.
El 2-propanol se ensayó en varios estudios limitados de
carcinogenicidad en el ratón utilizando las vías de exposición
cutánea (3 veces a la semana durante un año), inhalación (7700
mg/m3 durante 3 - 7 h/día, 5 días/semana, durante 5 - 8 meses) y
subcutánea (20 mg sin diluir, semanalmente durante 20 - 40
semanas). La aparición de tumores se investigó en los tres
estudios en la piel, el pulmón y el lugar de inyección,
respectivamente. No se observaron efectos carcinogénicos. No se
dispone de datos epidemiológicos adecuados con los que evaluar la
carcinogenicidad del 2-propanol para el ser humano. Los datos
disponibles indican que el di-2-propilsulfato, un producto
intermedio en los procesos de ácidos fuertes y débiles para
producir 2-propanol, puede estar asociado causalmente con la
inducción de cáncer del seno paranasal en el ser humano.
8. Efectos en la salud humana
Se han notificado varios casos de intoxicación tras la
ingestión oral y también en niños con fiebre a los que se refrescó
con esponjas impregnadas con preparaciones con 2-propanol. En
casos de envenenamiento, los principales signos son los de la
intoxicación alcohólica, en particular náuseas, vómitos, dolores
abdominales, gastritis, hipotensión e hipotermia. El 2-propanol
deprime el sistema nervioso central unas dos veces más que el
etanol, provocando una inconsciencia que termina en coma profundo;
puede sobrevenir la muerte por depresión respiratoria. Otros
efectos relacionados con el compuesto son la hiperglucemia,
elevados niveles de proteínas en el líquido cefalorraquídeo y
atelectasia. Aunque se considera que la absorción por la piel es
insignificante, en un informe sobre un caso de un niño intoxicado
tras refrescársele con una esponja impregnada con 2-propanol, se
indicaba que no conviene subestimar el riesgo de absorción dérmica,
especialmente en los niños. No se observaron efectos adversos en
voluntarios sanos que bebieron diariamente durante 6 semanas un
jarabe que contenía 2,6 ó 6,4 mg de 2-propanol/kg. Un grupo de
varones voluntarios, cuando se expusieron a vapores de 2-propanol
en concentraciones de 490, 980 ó 1970 mg por m3 de aire durante
3 - 5 minutos juzgaron que la irritación era "leve" a 980 mg/m3 y
"satisactoria" para su propia exposición ocupacional de 8 horas.
Las irritaciones de la piel en forma de eritema, quemaduras de
segundo y tercer grado y ampollas se notificaron en niños
prematuros tras un contacto prolongado con 2-propanol. En
ocasiones también se han notificado casos de dermatitis alérgica
por contacto.
Se dispone de pocos estudios epidemiológicos sobre mortalidad
por cáncer o por otras causas. En un grupo de 71 trabajadores
empleados durante más de 5 años en una fábrica de 2-propanol por el
proceso del ácido fuerte, se notificaron 7 casos de cáncer, entre
ellos 4 de cáncer del seno paranasal. En un estudio de cohortes
realizado sobre 779 trabajadores en una fábrica similar, las
incidencias reajustadas en función de la edad y del sexo de cáncer
del seno y de la laringe fueron 21 veces mayores de lo esperado.
El periodo mínimo de latencia fue de 10 años. En otro estudio
retrospectivo de cohortes realizado en otra fábrica que utilizaba
el proceso del ácido fuerte, había más de 4000 personas-años
expuestas. Los resultados mostraron que las tasas de mortalidad
por todas las causas y por neoplasmas no eran significativamente
mayores de lo previsto. Se llevó a cabo un estudio retrospectivo
de cohortes en una planta que fabricaba 2-propanol por el proceso
del ácido débil. Había más de 11 000 personas-años expuestas. La
tasa de mortalidad debida a todas las causas fue más baja de lo
esperado. No se observó mortalidad excesiva por todos los
cánceres. Sin embargo, la incidencia del cáncer de la boca y de la
faringe fue 4 veces más elevada de lo previsto. Los estudios de
cohortes indican en conjunto un riesgo de cáncer relacionado con el
proceso de fabricación con ácido fuerte, pero, en dos pequeños
estudios controlados de casos, no se observó correlación alguna
entre la exposición a 2-propanol y la incidencia de gliomas o de
leucemia linfática.
Algunos informes parecen indicar que la exposición combinada a
tetracloruro de carbono y 2-propanol en los trabajadores potencia
la toxicidad del primero.
9. Resumen de la evaluación
La exposición del hombre al 2-propanol puede producirse por
inhalación durante la fabricación, el tratamiento y el uso tanto
ocupacional como doméstico. La exposición a un nivel
potencialmente letal en la población general puede producirse por
ingestión accidental o intencionada y los niños pueden estar
expuestos cuando se les refresca con esponjas impregnadas con
preparaciones a base de 2-propanol (alcohol para friegas).
El 2-propanol se absorbe y distribuye rápidamente por todo el
organismo, en parte en forma de acetona. Los datos sobre
exposición-efecto en el hombre en condiciones de sobreexposición
aguda son escasos y muestran grandes variaciones. Los principales
efectos son la gastritis, la depresión del sistema nervioso central
con hipotermia y depresión respiratoria, y la hipotensión. Los
datos de mortalidad aguda en animales de experimentación indican
que la toxicidad del 2-propanol es baja, siendo los valores de DL50
orales en diversas especies entre 4475 y 7990 mg/kg, y los valores
de CL50 de inhalación en ratas alrededor de 50 000 mg/m3. En el
conejo, el 2-propanol no produjo irritaciones cutáneas, pero la
aplicación de 0,1 ml de 2-propanol sin diluir produjo irritación en
los ojos.
En el hombre, los efectos agudos más probables de la exposición
a concentraciones elevadas de 2-propanol por ingestión o inhalación
son la intoxicación alcohólica y la narcosis.
No se han hecho suficientes estudios en animales como para
evaluar los riesgos que entraña para la salud humana la exposición
repetida al 2-propanol. No obstante, los resultados de dos
estudios a corto plazo en la rata, incluida la exposición por
inhalación (500 mg/m3 durante 4 horas al día y 5 días a la semana
durante 4 meses) y la exposición oral (600 - 3900 mg/kg en el agua
de bebida) indican que debe evitarse la exposición al 2-propanol en
algunos de los muy elevados niveles de exposición ocupacional que
se han notificado.
La exposición por inhalación de ratas gestantes a 2-propanol
dio un nivel mínimo de observación de efectos de 18 327 mg/m3 (7450
ppm) y un nivel sin efectos observados de 9001 mg/m3 (3659 ppm)
respecto a la toxicidad materna. En el mismo estudio, 9001 mg/m3
(3659 ppm) fue el nivel más bajo de observación de efectos en lo
que respecta a la toxicidad de desarrollo; no se indicó nivel sin
efectos observados. Estas concentraciones son más elevadas que las
que normalmente se registran en condiciones de exposición humana.
El 2-propanol dio resultado negativo en las pruebas de
genotoxicidad, pero indujo aberraciones mitóticas en la médula ósea
de la rata. Aunque estos resultados indican que la sustancia no
tiene potencial genotóxico, no puede hacerse una evaluación
correcta de la mutagenicidad basándose en datos tan limitados.
Los datos disponibles no bastan para evaluar la carcinogenicidad
del 2-propanol en animales de experimentación. No se dispone de
datos para evaluar la carcinogenicidad del 2-propanol en el ser
humano.
Es poco probable que el 2-propanol plantee un riesgo grave para
la salud de la población general en las condiciones de exposición
que se producen normalmente.
El 2-propanol desaparece rápidamente (periodo de semieliminación
2,5 días) de la atmósfera y su desaparición del agua y del suelo se
produce rápidamente por biodegradación aerobia y anaerobia,
especialmente tras la adaptación de microorganismos inicialmente
sembrados. En vista de las propiedades físicas del 2-propanol, su
potencial de bioacumulación es bajo. No representa un riesgo para
los organismos naturales en las concentraciones en que suele
encontrarse en el medio ambiente.
 Since adequate balance studies have not been conducted, the
extent of this metabolism to acetone is not known. Siebert et al.
[232] injected 750 or 1300 mg/kg body weight intravenously in
rabbits and estimated that 64 - 84% of the dose was oxidized to
acetone, confirming the earlier findings of Pohl [204].
Evidence of another metabolic pathway was found in rabbits
given an oral dose of 2-propanol of 3000 mg/kg body weight, 10.2%
of the dose was found in the urine as beta-isopropyl-glucuronide
[129].
Sufficient evidence is available to show that 2-propanol is
oxidized to acetone mainly by the non-specific cytosolic enzyme
alcohol dehydrogenase (ADH) (EC 1.1.1.1). When either pyrazole or
4-methylpyrazole (inhibitors of ADH) was administered to rats prior
to exposure to 2-propanol, both the elimination of 2-propanol and
the production of acetone were retarded [121, 193]. The
elimination of 2-propanol by rats was retarded when it was given in
combination with ethanol; both compounds are substrates for ADH [2,
121]. Enzyme kinetic data show that 2-propanol is a poorer
substrate of ADH than ethanol. In vitro, the Michaelis-Menten
constant (Km) for horse liver ADH was 0.0126 mol/litre, using
2-propanol as a substrate, and 0.00018 mol/litre, using ethanol as
a substrate [64]. For rat liver ADH in vivo, Km was 0.034
mol/litre, using 2-propanol as a substrate, and 0.00192 mol/litre,
using ethanol as a substrate. The maximum initial velocity (Vmax)
of rat liver ADH and the substrate 2-propanol in vivo was 1.7 times
less than that with the substrate ethanol. It was further shown
that the ratio of the relative rates of oxidation of 2-propanol and
deuterated 2-propanol-d7 were about the same in vitro and in vivo.
From this, it can be concluded that ADH activity is the rate-
limiting factor in 2-propanol metabolism [54].
It has been shown that rat liver microsomal oxidases are also
capable of oxidizing 2-propanol, but that the compound is not an
effective substrate for the peroxidative activity of catalase
(EC 1.11.1.6) [48, 193].
6.3.2 Human beings
Acetone has also been found in the blood of human beings after
exposure to 2-propanol [e.g., 11, 41, 65, 131]. Furthermore,
acetone was detected in the spinal fluid, at levels similar to
those in serum, in 2 persons after ingestion of 2-propanol [5, 191].
In the investigations of Brugnone et al. [41] (section 6.1.2),
elevated acetone levels were measured in the blood, alveolar air,
and urine of 12 printing workers. 2-Propanol was not detected in
the blood or urine. The concentrations of acetone ranged between
0.76 and 15.6 mg/litre in the blood and between 3 and 93 mg/m3 in
the alveolar air. The acetone levels in alveolar air and blood
increased with the increasing exposure period and were linearly
related to the alveolar 2-propanol levels. Urinary acetone levels
ranged from 0.85 to 53.7 mg/litre in overnight pooled urine samples
and were highly correlated with the alveolar 2-propanol uptake and
with blood acetone levels. Pulmonary and renal clearance of
acetone were 41 - 97 and 0.1 - 3 ml/min, respectively, for 8 of the
workers, showing that acetone is mainly excreted via the lungs.
Pulmonary acetone excretion varied from 10.7 to 39.8% of the
uptake and was inversely related to the exposure level [41]. It
is known that the metabolic capacity of the human liver for acetone
is limited [161].
2-Propanol and acetone have also been found in the returns of
gastric lavage [5] and in saliva [250]. Reabsorption may follow
excretion via these routes.
When 10 volunteers drank orange juice containing doses that
gave 3.75 mg 2-propanol and 1200 mg ethanol/kg body weight over
2 h, 2-propanol was detected in the blood, partly as sulfate
(section 6.1.2), and in the urine, partly as glucuronide. The
total urinary excretion of 2-propanol was 1.9% of the dose [27, 28].
Human data support the importance of the ADH pathway for the
oxidation of 2-propanol as observed in experimental animals. In
the studies of Bonte et al. [27, 28] described above, 2-propanol
was only detected in the blood when ethanol was ingested
simultaneously, indicating a retarded elimination. Among 74
persons intoxicated by 2-propanol, significantly lower acetone
values were found in the blood of those who had also been exposed
to ethanol [11, 131]. In in vitro studies, the activity of human
ADH with 2-propanol was 9 - 10% of the activity of the enzyme with
ethanol as substrate [121].
Endogenous formation of 2-propanol has been revealed at
autopsies of individuals not previously exposed to this compound.
This observation and the results of additional studies on rats show
that 2-propanol can result from the reduction of acetone by liver
ADH, especially when high levels of acetone and high NADH/NAD+
ratios occur. Such conditions are found in diabetes mellitus,
starvation, high fat feeding, chronic alcoholism, and dehydration
[68, 161, 248].
6.4 Elimination and Excretion
6.4.1 Animals
In a review article, Rietbrock & Abshagen [218] concluded that
urinary excretion of both 2-propanol and its metabolite acetone is
limited and in each case does not exceed 4% of the dose in the rat,
rabbit, and dog. The major route of excretion is via the lungs.
2-Propanol is excreted via the gastric juice and saliva in the dog
[158] and through breast milk in the rat, as shown by tissue levels
in the newborn [159].
The disappearance of 2-propanol from the blood of experimental
animals was found to be a first-order process at doses <1500 mg/kg
[1, 232]. In rats, the half-life of 2-propanol was 1.5 h at an
intraperitoneal dose of 500 mg/kg body weight and increased to
2.5 h at a dose of 1500 mg/kg body weight. This can be explained
by a limited metabolic capacity as suggested in section 6.3.1
[218]. Simultaneous administration of ethanol to rats increased
the half-life of 2-propanol in the blood approximately 5-fold
following acute exposure [2]. A half-life of 4 h was determined in
dogs administered 1000 mg 2-propanol/kg body weight intravenously [1].
The biotransformation of 2-propanol and the elimination of the
acetone produced are both slow processes. Peak acetone levels in
the blood of various species, following single exposures via
different routes, were only reached several hours after exposure,
though acetone was already detectable shortly after the start of
exposure [1, 121, 150, 151, 193, 232]. The elimination of acetone
in the dog and the rat was found to be a first-order process with
half-lives of 11 and 5 h, respectively [1].
After prolonged administration of 2-propanol to dogs [159] and
rats [224], the elimination rate of 2-propanol was increased.
Simultaneous exposure of rats to ethanol in the drinking-water (5%)
and atmospheric 2-propanol at 738 mg/m3 (300 ppm) for 5 - 21 weeks
significantly increased the rates of elimination of 2-propanol and
acetone [224].
6.4.2 Human beings
The elimination of 2-propanol in human beings also appears to
follow first-order kinetics. In two alcoholics who had ingested
rubbing alcohol, 2-propanol was eliminated from the blood with
half-lives of 2.5 and 3 h, respectively. Acetone levels declined
slowly over the next 30 h; no ethanol was detected [65]. A half-
life of 2-propanol of 6.4 h was determined in a non-alcoholic, who
also had ingested rubbing alcohol. The acetone level in the blood
reached a maximum 30 h after admission to hospital. The half-life
of acetone was 22 h. Ethanol was also found in the blood. The
differences in the half-lives in these three cases may reflect
metabolic adaptation in the alcoholics, or genetic variations in
ADH [191].
When 4 volunteers ingested an artificial liquor containing 40%
2-propanol, acetone was detectable in exhaled air from 15 min after
exposure and in the urine from 1 h after exposure [132].
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Aquatic Organisms
A summary of acute aquatic toxicity data is presented in Table 8.
The concentration of 2-propanol was reported to have been measured
in only 3 of the studies [34, 111, 263] and in no case were
potential metabolites considered. In view of the volatility of the
compound, it can be expected that the toxic effects observed in the
other open systems studied occurred at lower concentrations than
the nominal ones.
Several short- and long-term studies have also been conducted.
Seiler et al. [227] determined the breakpoint of bioinhibition for
a total of 20 strains of bacterial groups prevalent in a waste-
water treatment plant in the chemical industry, i.e., Zoogloea,
Alcaligenes, and Pseudomonas. After one week of static exposure to
2-propanol in an open system at 30 °C, 100% growth inhibition
occurred at concentrations of 80 000 - 100 000 mg/litre of medium.
No analysis for the compound was reported. The cell multiplication
of blue algae (Microcystis aeruginosa) and green algae (Scenedesmus
quadricauda) was just inhibited after 8 days of static exposure to
1000 and 1800 mg/litre water, respectively, in a closed system at
27 °C and a pH of 7 [35, 37]. When water fleas (Daphnia magna)
were exposed to 2-propanol for 16 days in a semi-static test at
19 °C and a water hardness of 100 mg CaCO3/litre, the highest
concentration that did not result in a significant reduction in
growth was 141 mg/litre water. The water was analysed for the
compound just before and after each renewal of test solution [111].
A 7-day LC50 of 7060 mg 2-propanol/litre water was determined for
2 - 3-month-old guppies (Poecilia reticulata) in a semi-static
test. No analysis for 2-propanol was reported for this open system
[141].
7.2 Terrestrial Organisms
7.2.1 Microorganisms
The sensitivity of 4 soil fungi, i.e., Chrysosporium
crassitunicatum, Nannizzia fulva(+), Nannizzia fulva(-), and
Trichophyton equinum, to saturated 2-propanol vapour was
determined at 28 °C and a pH of 7. Mycelial growth of
Chrysosporium was stimulated after 14 days of exposure, while that
of the other strains was inhibited. Sporulation was poor in
Chrysosporium and in Trichophyton, and fair or good in the other
strains [233].
Table 8. Acute aquatic toxicity of 2-propanol
---------------------------------------------------------------------------------------------------------------------------------------
Organism Description Temperature pH Dissolved Hardness Stat/flow Exposure Parameter Nominal Reference
(°C) oxygen (mg CaCO3/ open/closeda period concentration
(mg/litre) litre) (mg/litre)
---------------------------------------------------------------------------------------------------------------------------------------
FRESHWATER
Bacteria Pseudomonas 25 7 stat, closed 16 h TTb 1 050 [37]
putida
Micro- activated 21 7.4-8 stat, closed 3 h 50% inhibition 1 000 [138]
organisms sludge of respiration
rate
Protozoa Entosiphon 25 6.9 stat, closed 72 h TTb 4 930 [36]
sulcatum
Protozoa Chilomonas 20 6.9 stat, closed 48 h TTb 104 [40]
paramecium
Protozoa Uronema 25 6.9 stat, closed 20 h TTb 3 425 [38]
parduczi
Crustacea water flea 20 8 2 250 stat, open 24 h EC50c 9 714 [39]
(Daphnia EC0 5 102
magna) EC100 10 000
Crustacea water flea 22 100 stat 44 h EC50c,d 2 285 [110]
(Daphnia
magna)
Amphibia frog tadpole 20 stat threshold 22 530 [190]
(Rana pipiens) narcotic
concentration
Fish fathead minnow 18-22 4 stat, open 96 h LC50 11 130 [184]
(Pimephales 24 h LC50 11 160
promelas)
Fish fathead minnow 25 7.5 5 45 flow, open 96 h LC50 9 640-10 400f [262]
(Pimephales
promelas)
---------------------------------------------------------------------------------------------------------------------------------------
Table 8. (contd.)
---------------------------------------------------------------------------------------------------------------------------------------
Organism Description Temperature pH Dissolved Hardness Stat/flow Exposure Parameter Nominal Reference
(°C) oxygen (mg CaCO3/ open/closeda period concentration
(mg/litre) litre) (mg/litre)
---------------------------------------------------------------------------------------------------------------------------------------
Fish goldfish 20 6-8 4 280 stat, open 24 h LC50 5 000f [34]
(Carassius
auratus)
Fish golden orfe 20 7-8 5 200-300 stat 48 h LC50 8 970 [127]
(Leuciscus
idus melanotus)
Fish harlequin fish 20 8.1 20 flow, open 96 h LC50 4 200 [251]
(Rasbora 96 h LC10 1 500
heteromorpha
duncker)
Fish Creek chub 15-21 8.3 98 stat, open 24 h LC0 900 [96]
(Semotitus
atromaculatus)
SEA WATER
Bacteria Photobacterium 15 7 stat, closed 2 min TTh 15 000 [43]
phosphoreum 15 stat, closed 5 min 50% light 35 000 [44, 62]
reduction 42 000
Worm Tubifex 20 stat 2 min EC100c 51 080 [56]
tubifex
Crustacea Brine shrimp 24 stat, open 24 h LC50 10 000 [208]
(Artemia
salina)
Crustacea Brown shrimp semi-stat, 96 h LC50 1 150 [26]
(Crangon open
crangon)
---------------------------------------------------------------------------------------------------------------------------------------
a Static or flow-through test; open or closed system. e Concentration at which touching the tadpoles failed to cause
b TT = toxic threshold for inhibition of cell multiplication. motion; exposure period and conditions; and the method of
c Effect is complete immobilization. calculation of the threshold not specified.
d Calculated value based on a quantitative structure-activity f Analysis for 2-propanol reported.
relationship between the n-octanol/water partition coefficient of g Test compound was Imsol A (90% 2-propanol, remainder unknown)
a group of 19 organic chemicals and their anaesthetic potency. h TT = toxic threshold for light reduction.
7.2.2 Insects
The 4-h LC50 for third instar mosquito larvae (Aedes aegypti)
was 25 120 mg/litre water in a static test at 22 - 24 °C [143].
The 48-h LC50 values for the fruit fly strains Drosophila
melanogaster and Drosophila simulans were between 10 200 and 13 340
mg/litre of nutrient medium in static tests [66]. Exposure of
Drosophila melanogaster eggs and larvae to 7850 mg 2-propanol/litre
of nutrient medium caused an 87% decrease in the activity of the
non-specific enzyme alcohol dehydrogenase (EC 1.1.1.1) in the 14-
day-old larvae. At the same time, viability was decreased by 74%.
The development of Drosophila simulans was completely inhibited at
the same concentration. Gas chromatographic analysis revealed
that, after 4 days of exposure, acetone appeared as the 2-propanol
concentration decreased. The appearance of acetone was associated
with an observed decrease in enzyme activity [265]. Anderson &
McDonald [13] also found a decrease in the specific activity of
alcohol dehydrogenase in Drosophila melanogaster after 1 - 4 days
of exposure to 2-propanol. On the other hand, they found that the
stability and concentration of the enzyme increased. The authors
explained the findings as an adaptation of the fruit fly to its
environment.
7.2.3 Plants
The effects of 2-propanol on the rate of seed germination were
investigated on several occasions. Total inhibition of the
germination of barley grains was reached after incubation for 4
days at 18 °C on filter papers, absorbing a solution containing
39 420 mg 2-propanol/litre water [56]. The germination of white
amaranth (Amaranthus albus) seeds was not affected after 5 h of
incubation at 25 °C on filter papers moistened with a solution
containing 36 050 mg 2-propanol/litre of water [49]. Reynolds
[216] measured 50% inhibition of germination in lettuce (Lactuca
sativa) seeds after incubation for 3 days at 30 °C on agar
containing 2100 mg 2-propanol/litre. At 6000 mg/litre, no
germination at all was observed. However, above 18 030 mg/litre,
germination was again observed and reached a maximum of 62% at
26 440 mg/litre. Inhibition of the growth of hypocotyls and
rootlets after 6 days of incubation gradually increased with
concentration. ED50 values were above 36 000 mg/litre.
In a 28-day study on cell suspensions of root sections of
soybean, (Glycine max), at 26 °C and a pH of 5.6, onset of growth
was delayed for 1 and 2 weeks at 2-propanol concentrations of
10 000 and 20 000 mg/litre of nutrient medium, respectively [67].
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single Exposures
8.1.1 Mortality
Available acute mortality data are summarized in Table 9.
Except where otherwise indicated, the vehicle was water or saline
solution. LD50 values for several animal species after oral
administration vary between 4475 and 7990 mg/kg body weight. Using
14-day-old rats of both sexes, Kimura et al. [134] established an
oral LD50 of 4396 mg/kg body weight for undiluted 2-propanol; this
was not significantly different from that obtained for young male
adults (4710 mg/kg body weight) or older male adults (5338 mg/kg
body weight).
8.1.2 Signs of intoxication
Sprague-Dawley rats of both sexes, inhaling 2-propanol for 8 h
at concentrations between 19 680 and 64 206 mg/m3, showed severe
irritation of the mucous membranes and depression of the central
nervous system indicated by subsequent ataxia, prostration, and
narcosis. These effects were concentration- and time-dependent.
Rats surviving the narcosis recovered. Transient paralysis of the
hind limbs was observed at levels between 49 200 and 54 120 mg/m3.
The rats died at and above 44 280 mg/m3, usually within 2 days,
with females dying earlier than males. All rats were autopsied,
including survivors, 15 days after exposure. At non-lethal levels,
congestion of the liver, lung, and spleen were observed, especially
in males. At lethal levels, vacuolation of the liver, acute
pneumonitis, and spleen oedema were found. These effects were most
pronounced in survivors. At 51 660 mg/m3, severe cytoplasmic
degeneration of the liver and oedema of the lung and brain occurred
in all rats [151]. Deep narcosis occurred in rats exposed through
inhalation to 40 mg/litre for 2 h. Significant and lasting
hypothermia was induced in Sprague-Dawley rats exposed for 4 h to
vapour concentrations of 19 680 mg/m3 or more [151].
Rats and mice of unknown strains, exposed orally or through
inhalation to lethal levels of 2-propanol, showed unspecified signs
of irritation and died through respiratory arrest while under
narcosis, usually within 24 h following exposure. Necropsy
revealed oedema, haemorrhage, inflammation, and dystrophy in the
interstitial tissues of parenchymal organs. In the lung,
infiltration, oedema, and thinning of the alveolar walls were
observed [103].
Table 9. Lethality of 2-propanol
---------------------------------------------------------------------------------------------------
Species/strain Sex Route of exposure Observation LD50 LC50 Reference
period (mg/kg body (mg/m3)
weight)
---------------------------------------------------------------------------------------------------
Rat not reported oral 3 days 5 280 - [157]
Sherman rat not reported oral 14 days 5 480 - [239]
Sprague-Dawley rat male oral 7 days 4 710 - [134]
Rat not reported oral 14 days 5 500 - [103]
Mouse not reported oral 14 days 4 475 - [103]
Rabbit not reported oral 3 days 5 030 - [157]
Rabbit male oral 1 day 7 990 [190]
Dog not reported oral 3 days 4 830 - [157]
Wistar rat male intravenous 5 days 1 088 - [247]
H mouse male intravenous 5 days 1 580 - [247]
H mouse female intravenous not reported 1 860 - [56]
Chinchilla rabbit male, female intravenous 5 days 1 184 - [247]
Wistar rat male intraperitoneal 5 days 2 830 - [247]
H mouse male intraperitoneal 5 days 4 868 - [247]
Syrian hamster male intraperitoneal 5 days 3 467 - [247]
Rabbit not reported dermal not reported 12 870 - [239]
Rat not reported inhalation (4 h) 14 days - 72 600 [103]
Sprague-Dawley rat male inhalation (8 h) 15 days - 46 740 [150]
Sprague-Dawley rat female inhalation (8 h) 15 days - 55 350 [150]
Mouse not reported inhalation (2 h) 14 days - 53 000 [103]
---------------------------------------------------------------------------------------------------
The effects of 2-propanol on the mucociliary system of the
trachea [197] and the middle ear [198] were investigated in two
separate studies. Groups of 20 or 24 Hartley guinea-pigs were
exposed to 2-propanol vapour at measured concentrations of 0, 969,
or 13 382 mg/m3 for 24 consecutive hours. Four animals from each
of the 3 groups were killed by decapitation at 12 h, 24 h, and 3,
7, and 14 days after the exposure period. A concentration-related
deterioration of ciliary activity and mucosal degeneration were
observed in both the trachea and in the middle ear. At the lower
exposure level, the effects completely reversed within 2 weeks of
exposure, but, at the higher exposure level, they did not.
2-Propanol can enter the trachea and deeper lung structures by
aspiration through the oral and nasal cavities. Anaesthetized rats
were made to inspire 110 or 140 mg of the compound in water, or 160
mg of the undiluted compound. Survivors were sacrificed 24 h later
for lung examination. At the lower doses, 1 out of 10 rats in each
group died. At the highest dose, 6 out of 10 rats died of cardiac
or respiratory arrest. All controls survived. It was not reported
whether the latter were sham-exposed or not. The average absolute
lung weight in the high-dose group was increased by 75% [94].
8.1.3 Skin, eye and respiratory tract irritation
In primary irritation patch tests on groups of 6 rabbits and 6
Hartley guinea-pigs, 0.5 ml of undiluted 2-propanol was applied to
areas of clipped or abraded skin. No irritation was observed for
up to 48 h after a 4-h exposure [194]. Erythema and changes in
vascular permeability did not occur when the clipped skin of
guinea-pigs was exposed for 2 min to discs of filter paper soaked
in undiluted 2-propanol [240].
Marzulli & Ruggles [182] reported on an interlaboratory
evaluation of the ocular irritancy of 2-propanol, according to
Draize scores, in groups of 6 rabbits, 1 - 7 days after application
of 0.1 ml of a 70% solution on the cornea. One day after exposure,
mean Draize scores were approximately 0.6 for corneal opacity, 0.3
for iritis, 1.5 for conjunctival redness, and 1.3 for chemosis.
The maximum total Draize score was 10.1. In each laboratory,
67 - 97% of the rabbits met the criteria for eye irritation, which
decreased over time. In another Draize test, groups of 6 - 9 New
Zealand rabbits received doses of 70% 2-propanol at levels of 0.01,
0.03, or 0.10 ml per eye. Maximum total Draize scores were 21, 36,
and 37, respectively, the maximum on the Draize scale being 110.
Using irritation categories based on the number of days required
for the effects to disappear, the compound was substantially
irritating (clearing within 14 days) at the higher doses and
moderately irritating (clearing within 7 days) at the lowest dose
[100].
The eye irritancy of 2-propanol was evaluated using Stauffland
albino rabbits, each of which received 0.1 ml of undiluted propanol
in one eye. Eye irritation was scored according to Draize up to 21
days after application. The exact scores were not reported, but
2-propanol was found to be corrosive according to the US EPA
criteria used in 1981, implying corneal involvement and irritation
or eye damage that persisted for more than 21 days after treatment
[188].
2-Propanol was tested on several occasions in in vitro
cytotoxicity assays and the results showed good agreement with
those in vivo [29, 30, 213].
The potency of 2-propanol as a sensory irritant was evaluated,
using a 50% reflex decrease in the respiratory rate of mice (RD50)
as an index. The mice were exposed by their heads only. An
exposure-related effect was found with RD50 values of 12 300 mg/m3
for Swiss OF1 mice and 43 525 mg/m3 for Swiss Webster mice [61, 130].
8.2 Continuous or Repeated Exposures
Groups of rats were exposed to 2-propanol vapour at
concentrations of 0, 100, or 500 mg/m3 air for 5 days/week and
4 h/day over 4 months [103]. Strain, sex, and group size were not
given, but were presumably in accordance with CMEA standards. No
deaths were reported. At the end of 4 months of exposure to 500
mg/m3 air, growth was reduced significantly by 10% and the
respiratory rate was increased by 22%. The white blood cell count
was decreased at both exposure levels in an exposure-related
manner. At 500 mg/m3, this was attributed to a decreased absolute
and relative number of lymphocytes. Decreases in hippuric acid
excretion and in total serum protein, and an increase in blood
acetylcholine were found at both exposure levels. The decrease in
total serum protein was exposure-related and could partly be
accounted for by the observed decrease in alpha1- and alpha2-
globulins and in albumin at 500 mg/m3. Blood glucose levels were
decreased at 500 mg/m3. Macroscopic and microscopic changes,
observed at 500 mg/m3, included irritant effects on the respiratory
system, such as thinning of the alveolar walls, perivascular
infiltration, pneumonia, and bronchitis. Dystrophic changes and
perivascular cell reactions were seen in the liver. Follicular
hyperplasia was observed in the spleen [103].
Another report described the continuous exposure of groups of
15 rats of unknown strain and sex to 2-propanol vapour at
concentrations of 0, 0.6, 2.5, or 20 mg/m3 air for 86 days. The
data were not statistically analysed. No deaths were reported. At
the highest exposure level, changes in the latency period of
unconditional reaction and an increase in the number of fluorescent
leukocytes were found. Effects reported at this exposure level
included an increase in sulfobromophthalein retention and decreased
blood levels of nucleic acids. Microscopy only revealed adverse
effects at 20 mg/m3 with liver dystrophy, degenerative changes in
the cerebral cortex, and spleen hyperplasia [19].
These studies [19, 103] lack a number of essential details
concerning the protocol, the effects observed, the incidence of
these effects, and the statistical analysis.
A "coefficient of accumulation" (the ratio between cumulative
LD50 and single dose LD50) reported for repeated oral exposure to
2-propanol was 4.0 in rats and 4.9 in mice [10].
Groups of 5 white rats of each sex and of unknown strain
received drinking-water containing 2-propanol for 27 weeks.
Average daily intakes were 0, 600, or 2300 mg/kg body weight for
males and 0, 1000, or 3900 mg/kg body weight for females. Only
males died: 2 at the lower dose and 3 at the higher dose. It was
reported that extensive postmortem changes prevented an accurate
diagnosis of the cause of death. At the end of the exposure
period, all exposed females showed growth retardation. Males
showed a slight decrease in growth initially but overtook the
controls in body weight towards the end of the exposure period. No
adverse effects were found on food intake, behaviour, and
histopathology [157].
8.3 Neurotoxicity and Behavioural Effects
2-Propanol passes the blood-brain barrier twice as effectively
as ethanol (section 6.2.1). The oral ED50 for narcosis in rabbits
exposed to 2-propanol was 2280 mg/kg body weight, i.e., 2.5 times
lower than that for ethanol [190]. The threshold of an acute
effect on the CNS was observed in rats after a 4-h exposure at 1450
mg/m3 using the method of flexor reflexes, the "summation threshold
method" [10]. The intraperitoneal ED50 for loss of righting reflex
in Swiss Webster mice, which was 164 mg/kg body weight, was 1.8
times lower than that for ethanol [169]. The threshold for the
induction of ataxia in Sprague-Dawley rats following intraperitoneal
exposure was 1106 mg/kg body weight [171]. In 2 tilted plane
tests, the performance of rats decreased by an average of 30 - 40%
after oral or intraperitoneal exposure to 2000 and 1800 mg/kg body
weight, respectively [178, 270]. On a molar basis, 2-propanol was
2.7 times as intoxicating as ethanol [270]. When C57BL/6J or
DBA/2J mice were exposed intraperitoneally to a single dose of 2-
propanol at 3 dose levels, the former strain showed increased
activity in the open field test at 392 mg/kg body weight, no effect
on activity at 785 mg/kg body weight, and narcosis at 1570 mg/kg
body weight. DBA/2J mice showed increased activity at the middle
dose level and narcosis at the high dose level [243]. Wistar rats,
inhaling 2-propanol at a concentration of 739 mg/m3 for 6 h/day, 5
days/week, for up to 15 weeks, did not show any adverse effects in
the open field test [224].
The depressive action of 2-propanol on the central nervous
system was related by several investigators to interactions with
neuronal membranes in vitro and in vivo. Lyon et al. [169]
observed a high correlation between the narcotic potencies of
aliphatic alcohols in mice and their ability to disorder brain
synaptosomal plasma membrane in vitro, as measured by electron
paramagnetic resonance, which was, in turn, related to membrane
solubility. In Sprague-Dawley rats, no change in synaptosomal
membrane fluidity was measured via in vitro registration of the
fluorescence polarization of 1,6-diphenyl-1,3,5-hexatriene, 20 h
after a single oral dose of 3000 mg/kg body weight. When
2-propanol was added subsequently, a change in membrane fluidity
was observed. This effect was related to membrane solubility.
The activity of synaptosomal Na+/K+-transporting
adenosinetriphosphatase (EC 3.6.1.37) was increased both in vitro
and in vivo [24].
Functional loss due to disruption of membrane integrity by
2-propanol was also observed in vitro by the blocking of the action
potentials of sciatic nerves in the toad, Bufo marinus [214], and
by the inhibition of membrane-bound guanylate cyclase (EC 4.6.1.2)
in intact murine neuroblastoma N1E-115 cells [241]. It was also
indicated by the interference of 2-propanol with the transport of
Ca2+ ions across biological membranes, as shown, in vitro, by the
inhibition of Ca2+ ion-induced contractions of guinea-pig ileum
(Yashuda et al., 1976), and, in vivo, in rats by a decrease in
regional brain Ca2+ ion levels, 30 min after one intraperitoneal
dose of 2000 mg/kg body weight [221].
Several neurochemical parameters were measured in the brain or
spinal cord axon of Wistar rats, inhaling 2-propanol at a
concentration of 739 mg/m3 air for 6 h/day, 5 days/week, over 5,
10, 16, or 21 weeks. In cerebellar homogenates, the activities of
superoxide dismutase (EC 1.15.1.1) and azoreductase (EC 1.6.6.7)
were decreased at weeks 16 and 21, but no effects were found on the
activity of dihydrolipoamide dehydrogenase (EC 1.8.1.4). In
cerebellar glial cells, the activity of acid proteinase (EC
3.4.21.1) was stimulated at weeks 5 and 10, while the glutathione
concentration was unaffected at all times. In the spinal cord
axon, the phosphate/cholesterol ratio of lipid was reduced by 25%
[224].
8.4 Biochemical Effects
8.4.1 Effects on lipids in liver and blood
Oral administration of single doses of 3000 or 6000 mg
2-propanol/kg body weight to Wistar rats caused a reversible
accumulation of triglycerides in the liver [21, 22, 23, 90]. At
the 6000 mg/kg body weight dose, a slight fatty infiltration was
observed histologically [90]. Observations that could explain this
effect were increased hepatic uptake of palmitate [23], increased
incorporation of palmitate into hepatic triglycerides, decreased
hepatic palmitate oxidation, and, at a later stage of intoxication,
interference with the synthesis and excretion of very dense
lipoproteins [21, 22, 23].
Decreased palmitate oxidation was related to the observed
increase in the hepatic b-hydroxybutyrate/acetoacetate ratio,
implying a decrease in the intramitochondrial NAD+/NADH ratio [21,
23, 195]. Excess extramitochondrial reducing equivalents,
indicated by an increased lactate/pyruvate ratio, was also observed
in vivo [21] and in vitro [85]. However, all these changes in the
redox state of the liver were very modest and therefore were not
thought to be fully responsible for the fatty livers induced.
More significant seemed to be the observation that the
incorporation of palmitate and glycerol into serum triglycerides
and the incorporation of palmitate into serum and hepatic
phospholipids were inhibited. This indicates an impaired secretion
of lipo-proteins, partly explained by the observed disturbance of
the biosynthesis of phospholipids, and partly by the observed
inhibition of hepatic protein synthesis [21, 22, 23]. Inhibition
of hepatic protein synthesis, as shown by the synthesis of the
marker enzymes ornithine decarboxylase (EC 4.1.1.17) and tyrosine
amino-transferase (EC 2.6.1.5), was observed in hepatectomized rats
following oral exposure to 2300 mg 2-propanol/kg body weight [205].
Acetone administration to rats up to a blood level comparable
to that in 2-propanol-dosed rats only slightly increased the level
of liver triglycerides; therefore acetone does not seem to play a
major role in the induction of fatty liver by 2-propanol [22].
8.4.2 Effects on microsomal enzymes
Oral exposure of Sprague-Dawley rats to 390 mg 2-propanol/kg
body weight, once or on 4 subsequent days, caused a slight but
significant increase in the hepatic cytochrome P-450 content and a
2 - 3-fold increase in the activities of the microsomal liver
enzymes (EC 1.14.14.1) aniline hydroxylase and 7-ethoxycoumarin
O-deethylase, 18 h after exposure [255]. Direct activation of these
enzymes does not offer a satisfactory explanation, as it was shown
in vitro that 2-propanol is an inhibitor of several microsomal
enzymes via an interaction with cytochrome P-450, which causes a
change in the reverse Type I spectrum [8, 57, 222, 246, 254, 285].
It was therefore concluded that, as acetone also does not appear to
be involved in aniline hydroxylase induction in vivo, 2-propanol
induces one or more forms of cytochrome P-450 [255]. In young
Sprague-Dawley rats, receiving 2-propanol once at a level of 1960
or 3140 mg/kg body weight and sacrificed 22 - 24 h later, the
hepatic cytochrome P-450 content increased, as well as the
activities of several nitrosamine N-demethylases, benzphetamine
demethylase, ethylmorphine demethylase, p-nitroanisole demethylase,
and 7-ethoxycoumarin- O-deethylase. A slight increase was observed
in the activity of NADPH cytochrome c reductase (EC 1.6.2.4).
Acetone appeared to play a role in this induction. From several
lines of observations, it was concluded that the enhanced activity
of nitrosodimethylamine N-demethylase was due to the induction of
specific forms of P-450 isozymes and P-450-dependent enzymes [255].
In several earlier studies on rats, at comparable or higher
oral doses, induction of microsomal liver enzymes was observed,
including a slight induction of NADPH cytochrome c reductase, but
no increase in hepatic cytochrome P-450 content [206, 207, 234].
Ueng et al. [255] explained the latter observation by suggesting
that the species of cytochrome P-450 induced must be normally
present in low concentrations, so that a several-fold increase in
catalytic activity could be induced without markedly increasing the
total content of the haemoprotein.
Sprague-Dawley rats were also exposed by inhalation to
2-propanol at concentrations of 490, 4920, or 19 680 mg/m3 air for
6 days/week, 6 h/day over 2 weeks. In the liver and kidneys, the
contents of cytochrome P-450 and cytochrome b5 were increased as
well as the activity of NADPH cytochrome c reductase at 4920 and
19 680 mg/m3. These effects were completely reversible in the
liver of rats exposed at 19 680 mg/m3 for 2 weeks and allowed to
recover for 4 weeks. However, they persisted in the kidneys [290].
8.4.3 Other biochemical findings
Differential effects have been observed on the glutathione
status of the liver following exposure of rats to 2-propanol. In
Sprague-Dawley rats inhaling 2-propanol at a concentration of 4920
or 19 680 mg/m3 for 6 days/week, 6 h/day, over 2 weeks, the reduced
glutathione concentration in the liver and kidneys increased
slightly while, at 19 680 mg/m3, the activity of glutathione
S-transferase (EC 2.5.1.18) increased by 13% in the liver only
[290]. The glutathione level in the liver of Wistar rats that had
received a single oral dose of 3110 mg 2-propanol/kg body weight
was reduced 6 h following exposure, while there was a 10% increase
in lipid peroxidation, as indicated by the formation of diene
conjugates [264]. A homogenate of normal or regenerating rat liver
did not show any increase in malondialdehyde production after
incubation with 2-propanol [6].
The activity of hepatic and kidney alanine aminotransferase (EC
2.6.1.2) in rats was unchanged 18 h after exposure to 1 or 4 daily
doses of 392 mg 2-propanol/kg body weight [255] or 4 h after
exposure to a single dose of 2300 mg/kg body weight [205]. Liver
damage was not observed in guinea-pigs histopathologically or by
any increase in serum ornithine carbamoyltransferase (EC 2.1.3.3),
24 h after receiving intraperitoneal doses of up to 1000 mg
2-propanol/kg body weight [73].
In Charles River rats, 2-propanol (785 mg/kg body weight ip)
induced hepatic metallothionein and caused low blood zinc levels
[244]. These changes were not prevented by adrenalectomy.
8.5 Immunological Effects
2-Propanol and acetone both enhanced the incorporation of
labelled thymidine into concanavalin A-stimulated murine spleen
cells in vitro [210]. 2-Propanol inhibited the killing of YAC-1
tumour cells by natural killer effector cells from mouse or rat
spleens [219]. Inhibition of the synthesis and/or the secretion
and/or function of at least one monocyte-derived substance that
inhibited cell proliferation was suggested as an explanation of
these findings [210].
8.6 Reproduction, Embryotoxicity, and Teratogenicity
Groups of 13 - 15 pregnant Sprague-Dawley rats were exposed to
2-propanol for 7 h/day on gestation days 1 - 19 at measured
concentrations of 9001, 18 327, or 23 210 mg/m3 (3659, 7450, or
9435 ppm). At the highest concentration, rats were completely
narcotized at the end of the first exposures, but by the end of the
19 days the effect was slight. Initial exposures to 18 327 mg/m3
(7450 ppm) caused an unsteady gait, but this was not noticeable by
the end of 19 days of exposure. Food consumption and maternal body
weight gain were significantly reduced by exposure to 18 327 and
23 210 mg/m3 (7450 and 9435 ppm). In the group exposed to 23 210
mg/m3 (9435 ppm): 6 out of 15 mated rats were not pregnant at
term, which was attributed to an exposure-induced failure of
implantation; 4 out of 9 pregnancies were totally resorbed; and
resorptions per litter were significantly increased. Fetal body
weights were significantly reduced, in a concentration-related
pattern, at all 3 concentrations. The incidence of cervical ribs
was significantly increased in the 23 210 mg/m3 (9435 ppm) group
[193].
A group of 6 female and 3 male rats (38 - 40 days old), of
unspecified strain, received drinking-water containing 2-propanol.
They were mated after approximately 2 months of exposure. This
schedule was repeated through 2 further generations. Average daily
intakes for the 3 generations were 1470, 1380, and 1290 mg/kg body
weight, respectively. The growth of the first generation was
retarded initially, but returned to normal by the 13th week.
Otherwise, no effects were found on growth and reproductive
functions [159].
When female hybrid rats received oral doses of 252 or 1008 mg
2-propanol/kg body weight per day for 45 days, the length of the
estrus cycle was increased by 23 - 24% [15]. Five female hybrid
rats also received daily doses of 1800 mg/kg body weight for 3
months before mating. Together with 6 controls they were
sacrificed on day 21 of pregnancy. The total embryonic mortality
rate was increased 2-fold, which was statistically significant [15].
In a 6-month drinking-water study, 2-propanol was administered
to hybrid rats of both sexes, at daily doses of 0.018, 0.18, 1.8,
or 18 mg/kg body weight. Mating of males with 5 - 7 females per
group occurred after the exposure period. When both sexes were
exposed to 18 mg/kg body weight, the litter size was increased.
The neonatal mortality rate was increased at 0.18 and 1.8 mg/kg,
when females only were exposed, at 18 mg/kg body weight, when males
only were exposed, and at the 2 highest exposure levels, when both
sexes were exposed. Neonates from the last group showed a dose-
related decrease in the rate of reaction to electric stimuli
(unconditioned defence reaction), but no other effects on their
development [15].
Groups of 10 - 13 female hybrid rats received 252 or 1008 mg
2-propanol/kg body weight from day 1 to day 20 of pregnancy. They
were sacrificed on day 21 of pregnancy together with 11 controls.
Litter size was reduced at both exposure levels. General embryonic
toxicity was 18 and 31%, respectively (10% in controls). At 1008
mg/kg body weight, total embryonic mortality rate was trebled and
10 out of 70 fetuses showed developmental anomalies compared with
none out of 90 controls. The anomalies found were brain damage in
3 fetuses, kidney damage in 5 fetuses, and gastrointestinal damage
in 2 fetuses. The lesions were not specified further. Data on
maternal toxicity were not reported [15].
8.7 Mutagenicity
A reverse mutation spot test with the Salmonella typhimurium
strains TA 98, TA 100, TA 1525, and TA 1537 was negative at 0.18 mg
per plate, with and without metabolic activation by S9 rat liver
[82].
Rat bone marrow cells were examined after 4 months of exposure
of rats to 2-propanol vapour at concentrations of 0, 1.03, or 10.2
mg/m3 air for 4 h/day. Statistically significant increases were
counted in the percentage of mitotic aberrations [18]. However,
the authors did not report the number of rats exposed, their sex,
or strain.
Root tip cells of onion (Allium cepa) exposed to 2-propanol
showed a 2.8-fold increase in the mitotic aberration rate [230].
2-Propanol did not increase sister chromatid exchange
frequencies in vitro in V79 Chinese hamster lung fibroblasts, with
or without S9 mix, when tested at concentrations of 3.3, 10, 33.3,
and 100 mmol/litre [267].
A dose-related increase in the inhibition of metabolic
cooperation in hamster V79 cells, a phenomenon believed to reflect
carcinogenic promotion ability and not to be indicative of
genotoxic potential, was observed by Chen et al. [53]. This may be
due to the membrane effects of 2-propanol.
8.8 Carcinogenicity
Several limited carcinogenicity studies on the mouse have been
conducted with 2-propanol, using the inhalation, dermal, and
subcutaneous routes of exposure.
Groups of 3 month-old, male C3H, ABC, and C57/BL mice were
exposed to 2-propanol vapour at a concentration of 7700 mg/m3 air
for 3 - 7 h/day, 5 days/week, over 5 - 8 months. The sizes of the
experimental and control groups were not reported. The occurrence
of lung tumours was examined microscopically in mice that had
survived until killed at the age of 8 months (36 exposed and 69
control C3H mice, 49 exposed and 120 control ABC mice), or 12
months (47 exposed and 52 control C57/BL mice) and showed
macroscopic lesions. The mice were not examined for the occurrence
of sinus tumours as occurred in workers engaged in the manufacture
of 2-propanol (section 9.2.1). No excess of lung tumours was
observed [276].
2-Propanol was painted on the clipped backs of 30 Rockland
mice, 3 times/week for 1 year. Sex, dose, and observation period
were not specified. Skin papillomas developed in 3 control mice,
one of which also had a skin carcinoma, while there was no response
in treated mice (Weil, C.S., written communication to US NIOSH [257]).
Groups of 3-month-old male C3H, ABC, and C57/BL mice received
20 mg undiluted 2-propanol subcutaneously once a week, for
20 - 40 weeks. The sizes of the treated and untreated control
groups were not reported. Surviving mice that showed macroscopic
lung lesions were examined microscopically for lung tumours. No
excess of lung tumours was observed. Lung tumour incidence in the
control group was high [276].
Doses of 20 mg undiluted 2-propanol were injected
subcutaneously in 40 C3H mice, once a week, over a period of 20
weeks. Controls were untreated. The occurrence of lung tumours
was examined in mice that survived until killed 5 months after the
first injection (22 exposed and 33 control mice). No excess of
lung tumours was observed (Weil, C.S., written communication to US
NIOSH [257]).
In these studies, only lung tumours and, in the case of dermal
exposure, skin tumours were investigated. Moreover, the exposure
periods, where given, were too short to allow for tumour induction.
Experimental details including size of experimental and control
groups, sex ratio, and observation periods were not quoted for some
of the studies. Because of these shortcomings, the studies are
inadequate for the assessment of carcinogenic potential.
8.9 Factors Modifying Toxicity
As a result of the induction of specific isozymes of cytochrome
P-450 (section 8.4.2) by 2-propanol and/or acetone, the
hepatotoxicity of carbon tetrachloride, 1,1,2-trichloroethane,
chloroform (CHCl3), trichloroethylene, and dimethylnitrosamine in
rodents is enhanced [60, 165, 234, 252, 253, 254]. The metabolic
activation of n-hexane by liver and kidney microsomes is also
increased by 2-propanol pretreatment [290].
9. EFFECTS ON MAN
9.1 General Population Exposure
9.1.1 Poisoning incidents
The subject of non-beverage alcohol use has already been
addressed in section 5.2.2. The clinical literature has been
reviewed [149, 176, 245, 163]. A brief summary of cases of
intoxication and major symptoms is presented in Table 10.
Table 10. Acute intoxications by 2-propanola
------------------------------------------------------------
No. of No. of Observationsb Reference
cases alcoholics 1 2 3 4 5 6 7
------------------------------------------------------------
Ingestion
57 46 +57 [11]
5 2 +5 +5 +4 +2 +4 +2 [4]
1c 0 - + + + + + + [186]
3 3 - +3 - +2 +2 +1 +2 [170]
1 0 - + + + + + - [191]
2 0 - +2 +2 +2 +1 [149]
1 1 - + + - + - + [245]
17 14 - +2 +3 +10 - - [131]
1 - + + + - [88]
1 1 - + + + [135]
1c 0 - + + - + - - [266]
1 1 - + + - - + [261]
1 0 - + + [75]
1 0 - + - - - [220]
2 1 - - + +2 - +2 +1 [5]
1 1 - - - + - + - [128]
1 1 - - - + + [108]
Sponging
1c - - + + + + + [228]
1c - - + + + - - + [160]
1c - - + + + [91]
1c - - + + - - + [172]
1c - - + [181]
1c - - + + + + - + [16]
--------------------------------------------------------------
a +N = observed in N cases; - = not observed; no sign = not
reported.
b 1 = death; 2 = coma; 3 = hypotension, meaning < 100 mm Hg systolic
pressure and/or < 60 mmHg diastolic pressure; 4 = tachycardia,
meaning > 100 beats per min; 5 = hypothermia, meaning < 36.5 °C;
6 = gastritis, characterized by nausea, vomiting, abdominal pain;
and/or gastric haemorrhage; 7 = elevated blood glucose, meaning
> 1500 mg/litre.
c Child below 2.5 years of age.
Intoxications have been reported after oral ingestion (Table
10), rectal administration [59], and inhalation by children who
have been sponged for several hours with 2-propanol preparations
for fever reduction (Table 10). Skin absorption in 2-propanol
sponging has been deemed insignificant [e.g., 149, 176]. However,
a case report on a child with an almost lethal blood level of
2-propanol after sponge bathing for fever reduction, and
questionable ingestion, suggested to the authors that the
importance of dermal absorption, compared with inhalation, should
not be underestimated [181].
2-Propanol depresses the central nervous system. At comparable
doses, 2-propanol is thought to be approximately twice as active as
ethanol in this respect with a longer duration of the coma due to
slower metabolism and to the contribution of acetone to the
depression of the central nervous system [149, 163, 176, 245]. As
with ethanol, excessive intoxication with 2-propanol causes
unconsciousness, usually, but not always ending in a deep coma [5,
59, 108, 128]; death may follow due to respiratory depression [4].
In most cases, decreased or absent reflexes are reported. Pupil
size is variable, but most often miotic [16, 88, 149, 220, 228,
266]. Early gastrointestinal problems and hypothermia frequently
occur. Cardiovascular effects include severe hypotension, shock
[4, 266], and even cardiac arrest [176, 266]. Tachycardia is found
as a secondary effect. Other possible compound-related effects are
hyperglycaemia, elevated protein levels in cerebrospinal fluid [5,
170, 266], and atelectasis [191, 220]. Lung congestion was
observed in fatal cases [4, 11].
In cases of 2-propanol intoxication, hypotension can be a grave
prognostic sign [4], which some authors suggest requires immediate
action by gastric lavage, haemodialysis [5, 22, 88, 135, 176], or
peritoneal dialysis [75, 186, 245]. Following treatment, recovery
is usually complete, unless the hypotension is severe and persistent.
In such cases, renal injury and death may occur [4, 128].
Acetone can be detected in the blood, urine, and breath.
Because other ketone bodies are not found in significant quantities,
early acidosis does not usually occur, serum bicarbonate, anion
gap, and blood pH remaining normal. Sometimes, a mild metabolic
acidosis and anion gap may develop as a result of lactic acid
accumulation. Acetonaemia and/or acetonuria without metabolic
acidosis and with normal or slightly elevated blood glucose levels
differentiate 2-propanol poisoning from diabetic ketoacidosis and
poisoning by other alcohols [16, 163, 176]. In addition, a
significant osmolality gap may be observed [16, 261].
None of the biochemical findings could be related to blood
levels of 2-propanol or acetone. 2-Propanol levels, measured at
different times after exposure, varied between undetectable
concentrations and 5600 mg/litre [176]. Acetone levels varied
between undetectable concentrations and 18 780 mg/litre [75].
Alexander et al. [11] suggested that combined levels of 2-propanol
and acetone may allow greater accuracy in predicting the clinical
course of the condition of a patient.
Adults have been reported to survive ingestion of 700 ml of
2-propanol [88] and to die after ingestion of 400 ml of 2-propanol
[4]. The lowest dose reported to be life-threatening was in an
18-month-old child who ingested approximately 170 ml of 2-propanol
[186].
9.1.2 Controlled exposures
No adverse effects were observed in groups of 8 healthy male
volunteers (24 - 57 years of age) who drank a daily dose of 2.6 or
6.4 mg 2-propanol/kg body weight in diluted syrup for 6 weeks.
Investigations included haematology, blood chemistry, urinalysis,
and ophthalmoscopy [283].
Another group of 10 healthy male volunteers (age not stated)
was exposed to 2-propanol vapour at nominal concentrations of 490,
980, or 1970 mg/m3 air for 3 - 5 min. After each exposure, the
participants were asked to classify the effects of the vapour on
the eyes, nose, and throat. The volunteers judged irritation to be
"mild" at 980 mg/m3 and "not severe" at 1970 mg/m3. They also
judged 490 mg/m3 to be "satisfactory" for their own 8-h
occupational exposure [192]. The validity of these results is
doubtful, for various reasons including the subjective criteria
used, the lack of control exposures, and the unreliability of the
exposure levels.
9.1.3 Skin irritation; sensitization
No skin irritation was observed in 6 human volunteers when 0.5
ml of undiluted 2-propanol was applied to a 4 cm square area on the
back and evaluated after 4 h, 24 h and 48 h [194]. Closed patch
tests with 10-min applications of 0.1 - 0.3 ml undiluted 2-propanol
on the dry skin of 10 healthy volunteers and 12 persons receiving
disulfiram therapy for the treatment of chronic alcoholism did not
result in any reaction. After immersion of the skin in tepid water
for 10 min, a transient erythema rapidly appeared on the site of
application in 19 subjects [105].
Skin irritation was also reported in a total of 6 premature
infants with a gestational age of less than 27 weeks. They were
exposed via swabs used for conduction in ECG recording or by the
application of 2-propanol on the umbilical area with subsequent
soaking of the diaper. Erythema and second or third degree burns
or blisters were observed in areas of prolonged contact with
2-propanol. Hypoperfusion of the skin was suggested to be a
contributing factor [226, 277].
In 1968, Wasilewski [272] reported a case of alleged allergic
contact dermatitis following skin disinfection with a 70%
2-propanol swab. The patient was treated for allergic rhinitis.
Patch tests were positive for 2-propanol. However, controls were
not tested and there was no information on the purity of the
alcohol. This report was followed by several others concerning a
total of 8 persons who showed dermatitis after contact with medi-
swabs containing 2-propanol. Patch testing revealed that another
volatile agent, presumably propylene oxide, caused the effect [174,
124, 217]. Only one of these 8 persons was also hypersensitive to
2-propanol of unknown purity [124].
Itching eczematous lesions developed in a laboratory worker, in
a company manufacturing hair cosmetics, in patch tests with
chemically pure 2-propanol solutions (2.5 - 99.7% by volume). This
person also reacted to 1-propanol, 1-butanol, 2-butanol, and
formaldehyde, but not to ethanol and methanol. Controls were not
tested [167]. Two out of 4 confirmed cases of hypersensitivity to
primary alcohols were also found to be hypersensitive to secondary
alcohols. Both cases had a history of contact allergies.
Recurrent exposures to 2-propanol and/or other alcohols occurred
through consumption of alcoholic beverages, pre-injection
disinfection, via a hair lotion in one case and occupationally in
the other case. Patch tests were positive for pure undiluted
2-propanol and 2-butanol. Twenty controls did not show any
reactions to secondary alcohols [86, 87]. The mechanism of alcohol
hypersensitivity is not clear. Fregert et al. [87] pointed out
that it is difficult to understand how alcohols can act as haptens.
9.2 Occupational Exposure
9.2.1 Epidemiology studies
A retrospective cohort study was reported among 182 workers at
a plant, in the USA, manufacturing 2-propanol by the strong acid
process over the period 1928 - 50. In a subgroup of 71 men, who
had been employed for more than 5 years, 7 cases of cancer were
observed: 4 cancers of the paranasal sinuses, 1 lung carcinoma, 1
laryngeal carcinoma, and 1 laryngeal papilloma. The minimum
latency period was 6 years [276]. According to USA vital
statistics for 1948, 0.0014 paranasal sinus cancers would have been
expected for the total cohort [286].
Two cases of paranasal sinus cancer and 2 cases of laryngeal
cancer were reported in 1966 in a cohort of 779 workers in a
similar plant in the USA that had been in operation since 1927.
The minimum latency period was 10 years. The age- and sex-adjusted
incidence of sinus and laryngeal cancer in this group was 21 times
higher than expected [119].
Another retrospective cohort study was undertaken among 262 men
who had worked for at least one year in a 2-propanol-manufacturing
plant in the United Kingdom using the strong acid procedure over
the period 1949 - 76. There were more than 4000 person-years at
risk. No person was lost to follow-up, which was over an average
of 15.5 years. The mortality rates due to all causes and due to
neoplasms were not significantly higher than expected according to
national vital statistics. One person died from nasal cancer
against 0.02 expected. Other significant findings were 2 "kidney
and adrenal malignancies" and 2 cancers of "the brain and the
central nervous system" [9].
A fourth retrospective cohort study was conducted over the
years 1966 - 78 among 433 workers in a 2-propanol manufacturing
plant, in the USA, who were exposed for at least 3 months during
the period 1941 - 65. The strong acid process used in 1941 had
been gradually changed to the weak acid process by 1965. More than
11 000 person-years were at risk. The mortality rate due to all
causes was lower than expected on the basis of state vital
statistics. No excess mortality due to all cancers was observed,
but the incidence of buccal and pharyngeal cancer was 4 times
higher than expected (2 versus 0.5). There was a slight excess of
lung cancer (7 versus 5.94) [79].
These cohort studies collectively suggest a cancer hazard
related to the strong acid manufacturing process. The excess in
respiratory cancers was initially attributed to isopropyl oils
[119, 276]. However, the experimental basis for this assumption is
weak (section 8.8) and more recently diisopropyl sulfate, an
intermediate produced in the strong acid process, has been proposed
as a more likely causative agent. The concentration of this
chemical is high in the strong acid process and low in the weak
acid process [79, 286].
Two small case-control studies were conducted in the USA: one
to consider the risk of lymphocytic leukaemia associated with 24
solvents among rubber industry workers [52], and the other to
consider the risk of brain gliomas associated with exposure
conditions during work at a chemical plant [155]. Although there
was no evidence of an association between exposure to 2-propanol
and the incidence of gliomas or lymphocytic leukaemia, the small
number of subjects and multiple exposures mean that no conclusions
can be drawn from these studies.
9.2.2 Interacting agents
Fourteen out of 43 workers in a 2-propanol-packaging plant
became ill when carbon tetrachloride was used for cleaning
equipment. The incidence and the severity of the effects increased
where exposure to carbon tetrachloride was the highest, and where,
on another occasion, the mean concentration of 2-propanol in air
was also highest, i.e., 1010 mg/m3. In 4 cases, renal failure or
hepatitis developed [83]. Another similar incident was reported in
a colour printing factory, 17 workers had abnormal liver function
and 3 developed acute hepatitis following combined exposure to
carbon tetrachloride and 2-propanol [71]. These reports suggest
potentiation of the toxicity of carbon tetrachloride by 2-propanol,
which was also found in experimental animals (section 8.9).
Some shaving lotions containing 2-propanol and other ingredients
(e.g., menthol, camphor, methyl salicylate, naphthalene) have been
reported to produce central stimulation with motor restlessness,
extreme apprehension, hallucinations, and general disorientation
[89, 238].
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of Human Health Risks
10.1.1 Exposure
Exposure of human beings to 2-propanol may occur through
inhalation during manufacture, processing, and both occupational
and household use. Average concentrations of up to 35 mg/m3 in the
ambient urban air and up to 500 mg/m3 in the occupational
environment have been measured. Concentrations between 0.2 and 325
mg/litre have been found in non-alcoholic beverages, and between 50
and 3000 mg/kg in foods (section 5).
Exposure of the general public to a potentially lethal level
may result from accidental or intentional ingestion and children
may be exposed when sponged with 2-propanol preparations (rubbing
alcohol) (sections 6 and 9).
10.1.2 Health effects
2-Propanol is rapidly absorbed after inhalation or ingestion
and distributed throughout the body as such, sulfated, or as its
metabolite, acetone (section 6). The possibility of dermal
absorption should not be neglected.
The acute toxicity of 2-propanol for animals is low (based on
lethality estimates) whether exposure occurs via the dermal, oral,
or respiratory route.
In man, the most likely acute effects of exposure to high
levels of 2-propanol by ingestion or inhalation are alcoholic
intoxication and narcosis. The results of animal studies indicate
that 2-propanol is approximately twice as intoxicating as ethanol
with an oral ED50 for rabbits of 2280 mg/kg and a threshold for
ataxia in rats of 1106 mg/kg intraperitoneally (section 8.3). Oral
LD50 values in various species vary between 4475 and 7990 mg/kg and
inhalation LC50 values between 50 000 and 70 000 mg/m3 (section
8.1.1).
Because ethanol retards the elimination of 2-propanol and is
also a CNS depressant, interaction between ethanol and propanol may
be expected to increase the CNS effects of either agent. The
majority of acute intoxication cases involved ingestion by known
alcoholics. Febrile children have experienced life-threatening
intoxications when treated by skin sponging with 2-propanol. In
these cases, skin absorption may also be an important route of
exposure in addition to inhalation (section 9.1.1).
Exposure-effect data on human beings in an acute overexposure
situation are scarce and show great variation. The major effects
are gastritis, depression of the central nervous system with
hypothermia and respiratory depression, and hypotension (section
9.1.1).
In rabbits, 2-propanol did not irritate the skin but did
irritate the eyes when 0.1 ml undiluted compound was applied
(section 8.1.3). Care is required in the use of 2-propanol as a
disinfectant on premature babies as it may cause severe skin
irritation following prolonged contact.
No adequate animal studies are available to make an evaluation
of the human health risks associated with repeated exposure to
2-propanol. However, 2 studies in rats with inhalation (500 mg/m3,
4 h/day, 5 days per week, for 4 months) or oral (600 - 3900 mg/kg
in drinking-water) exposure suggest that exposure to 2-propanol at
some of the very high occupational exposures reported should be
avoided (section 8.2).
2-Propanol administered in the drinking-water has been tested
for reproductive effects in a number of studies with conflicting
results. In one study, impaired neonatal survival was reported
after 6 months exposure of female rats to 0.18 mg/kg per day. In a
multi-generation study there were no adverse effects at drinking-
water doses as high as 1470 mg/kg per day. In a teratology study,
drinking-water doses of 252 and 1008 mg/kg per day produced
developmental toxicity, but this was not related to maternal
toxicity. Inhalation exposure of pregnant rats to 2-propanol
provided a LOEL of 18 327 mg/m3 (7450 ppm) and a NOEL of 9001 mg/m3
(3659 ppm) for maternal toxicity. In the same study, 9001 mg/m3
(3659 ppm) was a LOEL for developmental toxicity, with no
demonstration of a NOEL (section 8.6). These concentrations are
higher than those likely to be encountered under conditions of
human exposure.
2-Propanol was negative in a bacterial spot test and in a test
for sister chromatid exchange in mammalian cells in vitro. It
induced mitotic aberrations in the bone marrow of rats. Although
these findings suggest that the substance has no genotoxic
potential, no adequate assessment of mutagenicity can be made on
the basis of the limited data available. An in vitro test said to
predict promotional activity was negative (section 8.7).
The data available are inadequate to assess the carcinogenicity
of 2-propanol in experimental animals (section 8.8). There are no
data to assess the carcinogenicity of 2-propanol itself in human
beings. There are adequate data to indicate that the strong acid
process for the production of 2-propanol is causally associated
with the induction of paranasal sinus cancer in human beings,
probably due to exposure to the intermediate, di-2-propyl sulfate,
an alkylating agent and not to 2-propanol itself (section 9.2.1).
2-Propanol is shown to potentiate the hepatic toxicity of
halocarbons, such as carbon tetrachloride. Therefore simultaneous
exposure to 2-propanol and halocarbons should be avoided (section
9.2.2).
The Task Group considers it unlikely that 2-propanol will pose
a serious health risk to the general population under exposure
conditions likely to be encountered.
10.2 Evaluation of Effects on the Environment
By reacting with hydroxyl radicals and through rain-out,
2-propanol will disappear rapidly from the atmosphere, with a
residence time of less than 2.5 days (section 4.2). Hydrolysis and
photolysis are not important in the removal of 2-propanol from
water and soil but removal occurs quite rapidly by aerobic and
anaerobic biodegradation, especially after adaptation of initially
seeded microorganisms (section 4.3.1). Adsorption of 2-propanol on
soil particles is poor but it should be mobile in soil and it has
been shown to increase the permeability of soil to some aromatic
hydrocarbons. In view of the physical properties of 2-propanol,
its potential for bioaccumulation is low (section 4.3.2).
Toxicity in aquatic organisms was observed at levels ranging
from 104 mg/litre for one protozoan to over 50 000 mg/litre for
Tubifex worms. Insects and plant seeds were only affected at
concentrations above 2000 mg/litre (section 7).
On the basis of these data, it can be concluded that, except in
cases of accident and inappropriate disposal, 2-propanol does not
present a risk to naturally occurring organisms at concentrations
that usually occur in the environment.
11. RECOMMENDATIONS
1. 2-Propanol has not shown mutagenic potential in the small number
of assays performed. A full array of modern genotoxicity tests
should be completed.
2. Several studies of the carcinogenic activity of 2-propanol have
been published but all are seriously flawed and cannot be used
to evaluate the potential carcinogenicity of 2-propanol. The
desirability of a carcinogenesis bioassay of 2-propanol should
be considered based on the outcome of genotoxicity tests.
3. Inhalation exposure to overtly toxic concentrations of
2-propanol produced reproductive and developmental toxicity.
Additionally, the data available from drinking-water studies
are conflicting. In view of the potential for environmental
and drinking-water contamination, reproductive and
developmental toxicity should be conducted using oral dosing.
4. Epidemiological studies including precise exposure data would
assist in an assessment of the occupational hazards from
2-propanol.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
An evaluation of the carcinogenicity of 2-Propanol by the
International Agency for Research on Cancera was reported as
follows:
"A. Evidence for carcinogenicity to humans (sufficient for the
manufacture of isopropyl alcohol by the strong-acid process;
inadequate for isopropyl alcohol and isopropyl oils).
An increased incidence of cancer of the paranasal sinuses was
observed in workers at factories where isopropyl alcohol was
manufactured by the strong-acid process. The risk for
laryngeal cancer may also have been elevated in these workers.
It is unclear whether the cancer risk is due to the presence of
diisopropyl sulfate, which is an intermediate in the process,
to isopropyl oils, which are formed as by-products, or to other
factors, such as sulfuric acid. Epidemiological data
concerning the manufacture of isopropyl alcohol by the weak-
acid process are insufficient for an evaluation of
carcinogenicity."
"B. Evidence for carcinogenicity to animals (inadequate for
isopropyl alcohol and isopropyl oils).
Isopropyl oils, formed during the manufacture of isopropyl
alcohol by both the strong-acid and weak-acid processes, were
tested inadequately in mice by inhalation, skin application and
subcutaneous administration. Isopropyl oils formed during the
strong-acid process were also tested inadequately in dogs by
inhalation and instillation into the sinuses.
The available data on isopropyl alcohol were inadequate for
evaluation."
"C. Other relevant data
No data were available to the Working Group."
-------------------------------------------------------------------
a International Agency for Research on Cancer, Overall Evaluations
of Carcinogenicity: An updating of IARC Monographs volumes 1 to
42. Lyon, France, 1987 (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Supplement 7)
REFERENCES
1. ABSHAGEN, U. & RIETBROCK, N. (1969) [Elimination of
2-propanol in dogs and rats.] Naunyn-Schmiedebergs Arch.
Pharmakol. exp. Pathol., 264: 110-118 (in German).
2. ABSHAGEN, U. & RIETBROCK, N. (1970) [The mechanism of the
2-propanol oxidation.] Naunyn-Schmiedebergs Arch. Pharmakol.
exp. Pathol., 265: 411-424 (in German).
3. ACKMAN, R.G., HINLEY, H.J., & POWER, H.E. (1967)
Determination of isopropyl alcohol in fish protein
concentrate by solvent extraction and gas-liquid
chromatography. J. Fish. Res. Board Can., 24: 1521-1529.
4. ADELSON, L. (1962) Fatal intoxication with isopropyl
alcohol (rubbing alcohol). Am J. clin. Pathol., 38: 144-151.
5. AGARWAL, S.K. (1979) Non-acidotic acetonemia: a syndrome
due to isopropyl alcohol intoxication. J. Med. Soc. New
Jersey, 76: 914-916.
6. AGOSTINI, C. (1982) Effects of various inhibitors on lipid
peroxidation by homogenates of normal, regenerating and
hepatomous rat liver, by liver slices and by hepatoma cells.
Med. Biol. Environ., 10: 3-15.
7. AHAMED, A. & MATCHES, J.R. (1983) Alcohol production by
fish spoilage bacteria. J. food Prot., 46: 1055-1059.
8. AKHREM, A.A., POPOVA, E.M., & METELITSA, D.I. (1978)
[Interaction of aliphatic alcohols with cytochrome P-450
from rat liver microsomes.] Biokhimiya (Mosk), 43: 1485-1491
(in Russian).
9. ALDERSON, M.R. & RATTAN, N.S. (1980) Mortality of workers
on an isopropyl alcohol plant and two MEK dewaxing plants.
Br. J. ind. Med., 37: 85-89.
10. ALEKPEROV, I.I. & GUSEINOV, V.G. (1980) Toxicological
characteristics of isopropanol as an industrial poison, All
Union Foundation Conference on Toxicology, Moscow, 25-27
November 1980, p. 33.
11. ALEXANDER, C.B., MCBAY, A.J., & HUDSON, R.P. (1982)
Isopropanol and isopropanol deaths: ten years' experience.
J. forensic Sci., 27: 541-548.
12. ANDERS, M.W. & HARRIS, R.N. (1981) Effect of 2-propanol
treatment on carbon tetrachloride metabolism and toxicity.
Adv. exp. Med. Biol., 136A: 591-602.
13. ANDERSON, S.M. & MCDONALD, J.F. (1981) Effect of
environmental alcohol on in vivo properties of Drosophila
alcohol dehydrogenase. Biochem. Genet., 19: 421-430.
14. ANON. (1987) Chemical market profile: isopropanol. Chem.
Market. Rep., 31 August: 46.
15. ANTONOVA, V.I. & SALMINA, Z.A. (1978) [The maximum
permissible concentration of isopropyl alcohol in water
bodies with due regard for its action on the gonads and the
progeny.] Gig. i Sanit., 43: 8-11 (in Russian).
16. ARDITI, M. & KILLMER, M.S. (1987) Coma following use of
rubbing alcohol for fever control. Am. J. DC, 141: 237-238.
17. ARISTOV, V.N. (1982) [Combined effect of toluene, isopropanol,
and sulfur dioxide in conditions of petrochemical production.]
Gig. Tr. Prof. Zabol., ISS9: 5-9 (in Russian).
18. ARISTOV, V.N., REDKIN, Y.V., BRUSKIN, Z.Z., & OGLEZNEV, G.A.
(1981) [Experimental data on the mutagenous effects of
toluene, isopropanol, and sulfur dioxide.] Gig. Tr. Prof.
Zabol., 25: 33-36 (in Russian).
19. BAIKOV, B.K., GORLOVA, O.E., GUSEV, M.I., NOVIKOV, Y.V.,
YUDINA, T.V., & SERGEEV, A.N. (1974) [Hygienic
standardization of the daily average maximal permissible
concentrations of propyl and isopropyl alcohols in the
atmosphere.] Gig. i Sanit., 4: 6-13 (in Russian).
20. BALD, E. & MAZURKIEWICZ, B. (1980) Analytical utility of
2-halopyridinium salts. Part III. Paper electrophoretic
characterization of alcohols as 2-alkoxy-1-methylpyridinium
p-toluenesulfonates. Chromatographia, 13: 295-297.
21. BEAUGE, F., CLEMENT, M., RENAUD, G., NORDMANN, R., &
NORDMANN, J. (1975) Action de l'isopropanol sur le
métabolisme lipidique chez le rat: études complémentaires
sur les méchanismes impliqués dans l'accumulation hépatique
des triacylglycérides. Arch. int. Physiol. Biochim., 83:
573-591.
22. BEAUGE, F., CLEMENT, M., NORDMANN, J., & NORDMANN, R.
(1977) Etudes comparatives des actions de l'acétone et de
l'isopropanol sur le métabolisme lipidique chez le rat.
Arch. int. Physiol. Biochim., 85: 931-940.
23. BEAUGE, F., CLEMENT, M., NORDMANN, J., & NORDMANN, R.
(1979) Comparative effects of ethanol, n-propanol and
isopropanol on lipid disposal by rat liver. Chem.-biol.
Interact., 26: 155-166.
24. BEAUGE, F., FLEURET, C., BARIN, F., & NORDMANN, R. (1984)
Brain membrane disordering after acute in vivo administration
of ethanol, isopropanol or t-butanol in rats. Biochem.
Pharmacol., 33: 3591-3595.
25. BESS, F.D. & CONWAY, R.A. (1966) Aerated stabilization of
synthetic organic chemical wastes. J. Water Pollut. Control
Fed., 38: 939-956.
26. BLACKMAN, R.A.A. (1974) Toxicity of oil-sinking agents.
Mar. Pollut. Bull., 5: 116-118.
27. BONTE, W., RUDELL, E., SPRUNG, R., FRAUENRATH, C., BLANKE,
E., KUPILAS, G., WOCHNIK, J. & ZAH, G. (1981a)
[Experimental investigations concerning the analytical
detection of small doses of higher aliphatic alcohols in
blood in man.] Blutalkohol, 18: 399-411 (in German).
28. BONTE, W., SPRUNG, R., RUDELL, E., FRAUENRATH, C., BLANKE,
E., KUPILAS, G., WOCHNIK, J., & ZAH, G. (1981b)
[Experimental investigations concerning the analytical
detection of small doses of higher aliphatic alcohols in
human urine.] Blutalkohol, 18: 412-426 (in German).
29. BORENFREUND, E. & BORRERO, O. (1984) In vitro cytotoxicity
assays. Potential alternatives to the Draize ocular allergy
test. Cell Biol. Toxicol., 1: 55-65.
30. BORENFREUND, E. & SHOPSIS, C. (1985) Toxicity monitored
with a correlated set of cell-culture assays. Xenobiotica,
15: 705-711.
31. BOSSET, J.O. & LIARDON, R. (1984) The aroma composition of
Swiss Gruyere cheese. II. The neutral volatile components.
Lebensm.-Wiss. Technol., 17: 359-362.
32. BOUGHTON, L.L. (1944) The relative toxicity of ethyl and
isopropyl alcohols as determined by long term rat feeding
and external application. Am. pharm. Assoc., 33: 111-113.
33. BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979a) BOD and
COD of some petrochemicals. Water Res., 13: 627-630.
34. BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979b) The acute
toxicity of some petrochemicals to goldfish. Water Res., 13:
623-626.
35. BRINGMANN, G. (1975) [Determination of the harmful
biological action of water-endangering substances through
inhibition of cell multiplication in the blue alga
Microcystis.] Ges.-Ing., 96: 238-241 (in German).
36. BRINGMANN, G. (1978) [Determination of the harmful
biological action of water-endangering substances on
protozoa. I. Bacteria fed flagellates.] Z. Wasser-Abwasser
Forsch., 11: 210-215 (in German).
37. BRINGMANN, G. & KUHN, R. (1977) [Limiting values of the
harmful action of water-endangering substances on bacteria
(Pseudomonas putida) and green algae (Scenedesmus
quadricauda) in the cell multiplication inhibition test.] Z.
Wasser-Abwasser Forsch., 10: 87-98 (in German).
38. BRINGMANN, G. & KUHN, R. (1980) [Determination of the
harmful biological action of water-endangering substances on
protozoa. II. Bacteria fed ciliates.] Z. Wasser-Abwasser
Forsch., 13: 26-31 (in German).
39. BRINGMANN, G. & KUHN, R. (1982) [Findings regarding the
harmful action of water-endangering substances on Daphnia
magna in a further developed standardised test.] Z. Wasser-
Abwasser Forsch., 15: 1-16 (in German).
40. BRINGMANN, G., KUHN, R. & WINTER, A. (1980) [Determination
of the harmful biological action of water-endangering
substances on protozoa. III. Saprozoic flagellates.] Z.
Wasser-Abwasser Forsch., 13: 170-173 (in German).
41. BRUGNONE, F., PERBELLINI, L., APOSTOLI, P., BELLOMI, M., &
CARETTA, D. (1983) Isopropanol exposure: environmental and
biological monitoring in a printing works. Br. J. ind. Med.,
40: 160-168.
42. BUFFONI, F., SANTONI, G., ALBANESE, V., & DOLARA, P. (1983)
Urinary mercapturic acid in chemical workers and in control
subjects. J. appl. Toxicol., 3: 63-65.
43. BULICH, A.A. (1979) Use of luminescent bacteria for
determining toxicity in aquatic environments. In: Marking,
L.L. & Kimerle, R.A., ed. Aquatic toxicology, American
Society for Testing and Materials, pp. 98-106 (ASTM STP 667).
44. BULICH, A.A., GREENE, M.W., & ISENBERG, D.L. (1981)
Reliability of the bacterial luminescence assay for
determination of the toxicity of pure compounds and complex
effluents. In: Branson, D.R. & Dickson, K.L., ed. Aquatic
toxicology and hazard assessment. Fourth Conference,
American Society for Testing and Materials, pp. 338-347
(ASTM STP 737).
45. CALHOUN, M.J. & DELLAMONICA, E.S. (1974) Determination of
2-propanol residue in some fruits dewaxed with alcohol
vapors. J. Assoc. Off. Anal. Chem., 57: 1342-1345.
46. CARTER, W.P.L., DARNALL, K.R., GRAHAM, R.A., WINER, A.M., &
PITTS, J.N. (1979) Reactions of C2 and C4 alpha-hydroxy
radicals with oxygen. J. phys. Chem., 83: 2305-2311.
47. CEC (1982) Propan-2-ol: chemico-physical data, toxicity
data, environmental occurrence, and permissible levels. In:
Report of the Scientific Committee for Food on Extraction
Solvents, Brussels, Belgium, Commission of the European
Communities, Directorate General for Internal Market and
Industrial Affairs, pp. 46-72.
48. CEDERBAUM, A.I., QURESHI, A., & MESSENGER, P. (1981)
Oxidation of isopropanol by rat liver microsomes. Possible
role of hydroxyl radicals. Biochem. Pharmacol., 30: 825-831.
49. CHADOEUF-HANNEL, R. & TAYLORSON, R.B. (1985) Anaesthetic
stimulation of Amaranthus albus seed germination:
interaction with phytochrome. Physiol. Plant, 65: 451-454.
50. CHARBONNEAU, M., IJIMA, M., COTE, M.G., & PLAA, G.L. (1985)
Temporal analysis of rat liver injury following potentiation
of carbon tetrachloride hepatotoxicity with ketonic or
ketogenic compounds. Toxicology, 35: 95-112.
51. CHARRETON, M. (1981) Estimation des rejets de vapeurs de
solvants au voisinage d'une usine de peintures. Double
Liaison - Chimie des Peintures, 28: 233-241.
52. CHECKOWAY, H., WILCOSKY, T., WOLF, P., & TYROLER, H. (1984)
An evaluation of associations of leukemia and rubber
industry solvent exposures. Am. J. ind. Med., 5: 239-249.
53. CHEN, T.-H., KAVANAGH, T.J., CHANG, C.C., & TROSKO, J.E.
(1984) Inhibition of metabolic cooperation in Chinese
hamster V79 cells by various organic solvents and simple
compounds. Cell Biol. Toxicol., 1: 155-171.
54. CHEN, W.-S. & PLAPP, B.V. (1980) Kinetics and control of
alcohol oxidation in rats. Adv. exp. Med. Biol., 132:
543-549.
55. CHOU, W.L., SPEECE, R.E., & SIDDIQI, R.H. (1978)
Acclimation and degradation of petrochemical wastewater
components by methane fermentation. Biotechnol. Bioeng.
Symp., 8: 391-414.
56. CHVAPIL, M., ZAHRADNIK, R., & CMUCHALOVA, B. (1962)
Influence of alcohols and potassium salts of xanthogenic
acids on various biological objects. Arch. int. Pharmacodyn.
Ther., 135: 330-343.
57. COHEN, G.M. & MANNERING, G.J. (1973) Involvement of a
hydrophobic site in the inhibition of the microsomal
p-hydroxylation of aniline by alcohols. Mol. Pharmacol., 9:
383-397.
58. COLEMAN, R.L., LUND, E.D., & SHAW, P.E. (1972) Analysis of
grapefruit essence and aroma oils. J. agric. food Chem., 20:
100-103.
59. CORBETT, J. & MEIER, G. (1968) Suicide attempted by rectal
administration of drug. J. Am. Med. Assoc., 206: 2320-2321.
60. CORNISH, H.H. & ADEFUIN, J. (1967) Potentiation of carbon
tetrachloride toxicity by aliphatic alcohols. Arch. environ.
Health, 14: 447-449.
61. CUPITT, L.T. (1980) Fate of toxic and hazardous materials
in the air environment, Research Triangle Park, North
Carolina, US Environmental Protection Agency, Environmental
Sciences Laboratory, Office of Research and Development (EPA
600/3-80-084, PB 80-221948).
62. CURTIS, C., LIMA, A., LOZANO, S.J., & VEITH, G.D. (1982)
Evaluation of a bacterial bioluminiscence bioassay as a
method for predicting acute toxicity of organic chemicals to
fish. In: Pearson, J.G., Foster, R.B., & Bishop, W.E., ed.
Aquatic Toxicology and Hazard Assessment. Fifth Conference,
American Society for Testing and Materials, pp. 170-178
(ASTM STP 766).
63. DAINTY, R.H., EDWARDS, R.A., & HIBBARD, C.M. (1984)
Volatile compounds associated with the aerobic growth of
some Pseudomonas species on beef. J. appl. Bacteriol., 57:
75-81.
64. DALZIEL, K. & DICKINSON, F.M. (1966) The kinetics and
mechanism of liver alcohol dehydrogenase with primary and
secondary alcohols as substrates. Biochem. J., 100: 34-46.
65. DANIEL, D.R., MCANALLEY, B.H., & GARRIOTT, J.C. (1981)
Isopropyl alcohol metabolism after acute intoxication in
humans. J. anal. Toxicol., 5: 110-112.
66. DAVID, J. & BOCQUET, C. (1976) Compared toxicities of
different alcohols for two Drosophila sibling species: D.
melanogaster and D. simulans. Comp. Biochem. Physiol., 54C:
71-74.
67. DAVIS, D.G., WERGIN, W.P., & DUSBABEK, K.E. (1978) Effects
of organic solvents on growth and ultrastructure of plant
cell suspensions. Pestic. Biochem. Physiol., 8: 84-97.
68. DAVIS, P.L., DAL CORTIVO, L.A., & MATURO, J. (1984)
Endogenous isopropanol: forensic and biochemical
implications. J. anal. Toxicol., 8: 209-212.
69. DE CEAURRIZ, J.C., MICILLINO, J.C., BONNET, P., & GUENIER,
J.P. (1981) Sensory irritation caused by various industrial
airborne chemicals. Toxicol. Lett., 9: 137-143.
70. DEL ROSARIO, R., DE LUMEN, B.O., HABU, T., FLATH, R.A., MON,
T.R., & TERANISHI, R. (1984) Comparison of headspace
volatiles from winged beans and soybeans. J. agric. food
Chem., 32: 1011-1015.
71. DENG, J.-F., WANG, J.-D., SHIH, T.-S., & LAN, F.-L. (1987)
Outbreak of carbon tetrachloride poisoning in a color
printing factory related to the use of isopropyl alcohol and
an air conditioning system in Taiwan. Am. J. ind. Med., 12:
11-19.
72. DGEP (1987) Review of literature data on 2-propanol,
Leidschendam, Netherlands, Directorate-General of
Environmental Protection, Ministry of Housing, Physical
Planning and Environment.
73. DIVINCENZO, G.D. & KRASAVAGE, W.J. (1974) Serum ornithine
carbamyl transferase as a liver response test for exposure
to organic solvents. Am. Ind. Hyg. Assoc. J., 35: 21-29.
74. DORIGAN, J., FULLER, B., & DUFFY, R. (1976) Scoring of
organic air pollutants. Chemistry, production and toxicity
of selected synthetic organic chemicals, The MITRE
Corporation (MITRE Technical Report MTR-7248, Rev. 1,
Appendix III).
75. DUA, S.L. (1974) Peritoneal dialysis for isopropyl alcohol
poisoning. J. Am. Med. Assoc., 230: 35.
76. DUVEL, W.A. & HELFGOTT, T. (1975) Removal of wastewater
organics by reverse osmosis. J. Water Pollut. Control Fed.,
47: 57-65.
77. EGBERT, A.M., REED, J.S., POWELL, B.J., LISKOW, B.I., &
LIESE, B.S. (1985) Alcoholics who drink mouthwash: the
spectrum of nonbeverage alcohol use. J. Stud. Alcohol, 46:
473-481.
78. EGOROV, Y.L. (1966) Vision of workers engaged in the
production of synthetic fatty acids and issues relative to
setting hygienic standards for the alcohol content in the
air. Gig. Tr. Prof. Zabol., 7: 33-38.
79. ENTERLINE, P.E. (1982) Importance of sequential exposure in
the production of epichlorohydrin and isopropanol. Ann. N.Y.
Acad. Sci., 381: 344-349.
80. FANG, H.H.P & CHIAN, E.S.K. (1976) Reverse osmosis
separation of polar organic compounds in aqueous solution.
Environ. Sci. Technol., 10: 364-369.
81. FERNANDEZ, F. & QUIGLEY, R.M. (1985) Hydraulic conductivity
of natural clays permeated with simple liquid hydrocarbons.
Can. Geotech. J., 22: 205-214.
82. FLORIN, I., RUTBERG, L., CURVALL, M., & ENZELL, C.R. (1980)
Screening of tobacco smoke constituents for mutagenicity
using the Ames test. Toxicology, 18: 219-232.
83. FOLLAND, D.S., SCHAFFNER, W., GINN, E., CROFFORD, O.B., &
MCMURRAY, D.R. (1976) Carbon tetrachloride toxicity
potentiated by isopropyl alcohol. Investigation of an
industrial outbreak. J. Am. Med. Assoc., 236: 1853-1856.
84. FORE, S.P., RAYNER, E.T., & DUPUY, H.P. (1971)
Determination of residual solvent in oilseed meals and
flours. III. Isopropanol. J. Am. Oil Chem. Soc., 48:
140-142.
85. FORSANDER, O.A. (1967) Influence of some aliphatic
alcohols on the metabolism of rat liver slices. Biochem. J.,
105: 93-97.
86. FREGERT, S., GROTH, O., HJORTH, N., MAGNUSSON, B., RORSMAN,
H., & OVRUM, P. (1969) Alcohol dermatitis. Acta
dermatovenereol., 49: 493-497.
87. FREGERT, S., GROTH, O., GRUVBERGER, B., MAGNUSSON, B.,
MOBACKEN, H., & RORSMAN, H. (1971) Hypersensitivity to
secondary alcohols. Acta dermatovenereol., 51: 271-272.
88. FREIREICH, A.W., CINQUE, T.J., XANTHAKY, G., & LANDAU, D.
(1967) Hemodialysis for isopropanol poisoning. New Engl. J.
Med., 277: 699-700.
89. GADSDEN, R.H., MELETTE, R.R., & MILLER, W.C. (1958) Scrap
iron intoxication. J. Am. Med. Assoc., 168: 1220-1224.
90. GAILLARD, D. & DERACHE, R. (1966) Action de quelques
alcools aliphatiques sur la mobilisation de différentes
fractions lipidiques chez la rate. Food cosmet. Toxicol., 4:
515-520.
91. GARRISON, R.F. (1953) Acute poisoning from use of isopropyl
alcohol in tepid sponging. J. Am. Med. Assoc., 152: 317-318.
92. GEORGE, H.A., JOHNSON, J.L., MOORE, W.E.C., HOLDEMAN, L.V.,
& CHEN, J.S. (1983) Acetone, isopropanol, and butanol
production by Clostridium beijerinckii (syn. Clostridium
butylicum) and Clostridium aurantibutyricum. Appl. environ.
Microbiol., 45: 1160-1163.
93. GEORGE, V., SHARMA, S.D., TRIPATHI, A.K., & ABRAHAM, S.P.
(1985) Flavour components of some edible fungi from
Kashmir. I. Pafai J., 7: 27-30.
94. GERARDE, H.W., AHLSTROM, D.B., & LINDEN, N.J. (1966) The
aspiration hazard and toxicity of a homologous series of
alcohols. Arch. environ. Health, 13: 457-461.
95. GERHOLD, R.M. & MALANEY, G.W. (1966) Structural
determinants in the oxidation of aliphatic compounds by
activated sludge. J. Water Pollut. Control Fed., 38:
562-579.
96. GILLETTE, L.A., MILLER, D.L., & REDMAN, H.E. (1952)
Appraisal of a chemical waste problem by fish toxicity
tests. Sewage ind. Waste, 24: 1397-1401.
97. GIUSTI, D.M., CONWAY, R.A., & LAWSON, C.T. (1974) Activated
carbon adsorption of petrochemicals. J. Water Pollut.
Control Fed., 46: 947-965.
98. GORLOVA, O.E. (1970) [Hygienic assessment of isopropyl
alcohol as an atmospheric pollutant.] Gig. i Sanit., 35:
9-14 (in Russian).
99. GOTZ-SCHMIDT, E.-M. & SCHREIER, P. (1986) Neutral volatiles
from blended endive ( Cichorium endivia L.). J. agric. food
Chem., 34: 212-215.
100. GRIFFITH, J.F., NIXON, G.A., BRUCE, R.D., REER, P.J., &
BANNAN, E.A. (1980) Dose-response studies with chemical
irritants in the albino rabbit eye as a basis for selecting
optimum testing conditions for predicting hazard to the human
eye. Toxicol. appl. Pharmacol., 55: 501-513.
101. GUNTER, B. (1982) Health hazard evaluation: Jeppesen
Sanderson Inc., Cincinnati, Ohio, US National Institute for
Occupational Safety and Health (HETA 81-261-1085, PB 83-
201749).
102. GUNTER, B.J., LIGO, R.L., & RUHE, R.L. (1976) Health hazard
evaluation determination, Steiger Tractor Inc., Cincinnati,
Ohio, US National Institute for Occupational Safety and
Health (NIOSH-TR-HHE-75-30-266, PB 273711).
103. GUSEINOV, V.G. (1985) [Toxicological hygienic characteristics
of isopropyl alcohol.] Gig. Tr. Prof. Zabol., (7): 60-62
(in Russian).
104. HABU, T., FLATH, R.A., MON, T.R., & MORTON, J.F. (1985)
Volatile components of Rooibos tea (Asphalathus linearis). J.
agric. food Chem., 33: 249-254.
105. HADDOCK, N.F. & WILKIN J.K. (1982) Cutaneous reactions to
lower aliphatic alcohols before and during disulfiram
therapy. Arch. Dermatol., 118: 157-159.
106. HALLIDAY, M.M. & CARTER, K.B. (1978) A chemical adsorption
system for the sampling of gaseous organic pollutants in
operating theatre atmospheres. Br. J. Anaesth., 50:
1013-1018.
107. HANSSEN, H.-P., SPRECHER, E., & KLINGENBERG, A. (1984)
Accumulation of volatile flavour compounds in liquid cultures
of Kluyveromyces lactis strains. Z. Naturforsch., 39c:
1030-1033.
108. HAWLEY, P.C. & FALKO, J.M. (1982) "Pseudo" renal failure
after isopropyl alcohol intoxication. South. med. J., 75:
630-631.
109. HELLMAN, T.M. & SMALL, F.H. (1974) Characterization of the
odor properties of 101 petrochemicals using sensory methods.
J. Air Pollut. Control Assoc., 24: 979-982.
110. HERMENS, J. CANTON, H., JANSSEN, P., & DE JONG, R. (1984)
Quantitative structure-activity relationships and toxicity
studies of mixtures of chemicals with anaesthetic potency:
acute lethal and sublethal toxicity to Daphnia magna. Aquat.
Toxicol., 5: 143-154.
111. HERMENS, J., BROEKHUYZEN, E., CANTON, H., & WEGMAN, R.
(1985) Quantitative structure-activity relationships and
mixture toxicity studies of mixtures of alcohols and
chlorohydrocarbons: effects on growth of Daphnia magna.
Aquat. Toxicol., 6: 209-217.
112. HO, Y.H., SCHWARZE, I., & SOEHRING, K. (1970) [The influence
of low aliphatic alcohols on the chloral hydrate metabolism
in rat liver sections.] Arzneim.-Forsch., 20: 1507-1509 (in
German).
113. HODGE, H.C. & STERNER, J.H. (1943) Am. Ind. Hyg. Assoc. J.,
10: 93.
114. HOIGNE, J. & BADER, H. (1983) Rate constants of reactions of
ozone with organic and inorganic compounds in water. I. Non-
dissociating organic compounds. Water Res., 17: 173-183.
115. HORWITZ, W., ed. (1975) Official methods of analysis of the
Association of Official Analytical Chemists, 12th ed.,
Washington DC, Association of Official Analytical Chemists,
pp. 328, 337-338, 343, 656-658.
116. HORWOOD, J.F., LLOYD, G.T., & STARK, W. (1981) Some flavour
components of feta cheese. Aust. J. dairy Technol., 36:
34-37.
117. HOU, C.T., PATEL, R.N., LASKIN, A.I., MARCZAK, I., & BARNABE,
N. (1981) Microbial oxidation of gaseous hydrocarbons:
production of alcohols and methyl ketones from their
corresponding n-alkanes by methylotrophic bacteria. Can. J.
Microbiol., 27: 107-115.
118. HOVIOUS, J.C., CONWAY, R.A., & GANZE, C.W. (1973) Anaerobic
lagoon pretreatment of petrochemical wastes. J. Water Pollut.
Control Fed., 45: 71-84.
119. HUEPER, W.C. (1966) Occupational and environmental cancers
of the respiratory system. Recent results. Cancer Res., 3:
105-107.
120. IARC (1977) Some fumigants, the herbicides 2,4-D and 2,4,5-
T, chlorinated dibenzodioxins and miscellaneous industrial
chemicals, Lyons, International Agency for Research on
Cancer, pp. 223-243 (Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Man, 15).
121. IDOTA, S. (1985) [Studies on isopropanol metabolism and
poisoning.] J. Nihon Univ. Med. Assoc., 44: 39-47 (in
Japanese).
122. IRPTC (1987) Data profile on 2-propanol, Geneva,
Switzerland, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
123. JARKE, F.H., DRAVNIEKS, A., & GORDON, S.M. (1981) Organic
contaminants in indoor air and their relation to outdoor
contaminants. ASHRAE Trans., 87: 153-166.
124. JENSEN, O. (1981) Contact allergy to propylene oxide and
isopropyl alcohol in a skin disinfectant swab. Contact
Dermat., 7: 148-150.
125. JONES, C.J. & MCGUGAN, P.J. (1977/1978) An investigation of
the evaporation of some volatile solvents from domestic
waste. J. hazard. Mater., 2: 235-251.
126. JONSSON, A., PERSSON, K.A. & GRIGORIADIS, V. (1985)
Measurements of some low molecular-weight oxygenated,
aromatic, and chlorinated hydrocarbons in ambient air and in
vehicle emissions. Environ. Int., 11: 383-392.
127. JUHNKE, I. & LUDEMANN, D. (1978) [Results of examination of
200 chemical compounds for acute toxicity towards fish by
means of the golden orfe test.] Z. Wasser-Abwasser Forsch.,
11: 161-164 (in German).
128. JUNCOS, L. & TAGUCHI, J.T. (1968) Isopropyl alcohol
intoxication. Report of a case associated with myopathy,
renal failure, and hemolytic anemia. J. Am. Med. Assoc., 204:
186-188.
129. KAMIL, I.A., SMITH, J.N., & WILLIAMS, R.T. (1953) The
metabolism of aliphatic alcohols. The glucuronic acid
conjugation of acyclic aliphatic alcohols. Biochem. J., 53:
129-136.
130. KANE, L.E., DOMBROSKE, R., & ALARIE, Y. (1980) Evaluation of
sensory irritation from some common industrial solvents. Am.
Ind. Hyg. Assoc. J., 41: 451-455.
131. KELNER, M. & BAILEY, D.N. (1983) Isopropanol ingestion:
interpretation of blood concentrations and clinical findings.
J. Toxicol.-clin. Toxicol., 20: 497-507.
132. KEMAL, H. (1927) [Contribution to investigations into the
fate of isopropyl alcohol in the human body.] Biochem. Z.,
187: 461-466 (in German).
133. KHAN, J.S., WILSON, M.C., & TAYLOR, T.V. (1979) A case of
dettol addiction. Br. med. J., 1: 791-792.
134. KIMURA, E.T., EBERT, D.M., & DODGE, P.W. (1971) Acute
toxicity and limits of solvent residue for sixteen organic
solvents. Toxicol. appl. Pharmacol., 19: 699-703.
135. KING, L.H., BRADLEY, K.P., & SHIRES, D.L. (1970)
Hemodialysis for isopropyl alcohol poisoning. J. Am. Med.
Assoc., 211: 1855.
136. KINLIN, T.E., MURALIDHARA, R., PITTET, A.O., SANDERSON, A., &
WALRADT, J.P. (1972) Volatile components of roasted
filberts. J. agric. food Chem., 20: 1021-1028.
137. KIRK, R.E. & OTHMER, D.F., ed. (1978-1984) Encyclopedia of
chemical technology, 3rd ed., New York, Wiley Interscience.
138. KLECKA, G.M. & LANDI, L.P. (1985) Evaluation of the OECD
activated sludge, respiration inhibition test. Chemosphere,
14: 1239-1251.
139. KNUTH, M.L. & HOGLUND, M.D. (1984) Quantitative analysis of
68 polar compounds from ten chemical classes by direct
aqueous injection gas chromatography. J. Chromatogr., 285:
153-160.
140. KOMINSKY, J., LOVE, J., & ANDERSON, K. (1982) Health hazard
Evaluation, Tweddle Litho Company,Cincinnati, Ohio, US
National Institute for Occupational Safety and Health (HETA
81-117-1087, PB 83-202390).
141. KONEMANN, H. (1981) Quantitative structure-activity
relationships in fish toxicity studies. Toxicology, 19:
209-221.
142. KONIG, H. & HERMES, M. (1981) [Separation, identification
and estimation of propellant gases and solvents in aerosol
products by gas chromatography.] Chromatographia, 14: 351-354
(in German).
143. KRAMER, V.C., SCHNELL, D.J., & NICKERSON, K.W. (1983)
Relative toxicity of organic solvents to Aedes aegyptilarvae.
J. Invertebr. Pathol., 42: 285-287.
144. KRING, E.V., ANSUL, G.R., HENRY, T.J., MORELLO, J.A., DIXON,
S.W., VASTA, J.F., & HEMINGWAY, R.E. (1984) Evaluation of
the standard NIOSH type charcoal tube sampling method for
organic vapors in air. Am. Ind. Hyg. Assoc. J., 45: 250-259.
145. KRULL, I.S., SWARTZ, M., & DRISCOLL, J.N. (1984)
Derivatizations for improved detection of alcohols by gas
chromatography and photoionization detection. Anal. Lett.,
17(A20): 2369-2384.
146. KUMAI, M., KOIZUMI, A., SAITO, K., SAKURAI, H., INOUE, T.,
TAKEUCHI, Y., HARA, I., OGATA, M., MATSUSHITA, T., & IKEDA,
M. (1983) A nationwide survey on organic solvent components
in various solvent products. Part 2. Heterogeneous products
such as paints, inks, and adhesives. Ind. Health, 21:
185-197.
147. KUMKE, G.W., HALL, J.F., & OEBEN, R.W. (1968) Conversion to
activated sludge at Union Carbide's institute plant. J. Water
Pollut. Control Fed., 40: 1408-1422.
148. KURPPA, K. & HUSMAN, K. (1982) Car painters' exposure to a
mixture of organic solvents. Serum activities of liver
enzymes. Scand. J. Work environ. Health, 8: 137-140.
149. LACOUTURE, P.G., WASON, S., ABRAMS, A., & LOVEJOY, F.H.
(1983) Acute isopropyl alcohol intoxication. Diagnosis and
management. Am. J. Med., 75: 680-686.
150. LAHAM, S., POTVIN, M., & SCHRADER, K. (1979) Microméthode de
dosage simultané de l'alcool isopropylique et de son
metabolite l'acétone. Chemosphere, 2: 79-87.
151. LAHAM, S., POTVIN, M., SCHRADER, K., & MARINO, I. (1980)
Studies on inhalation toxicity of 2-propanol. Drug Chem.
Toxicol., 3: 343-360.
152. LANGVARDT, P.W. & MELCHER, R. (1979) Simultaneous
determination of polar and non-polar solvents in air using a
two-phase desorption from charcoal. Am. Ind. Hyg. Assoc. J.,
40: 1006-1012.
153. LAREGINA, J., BOZZELLI, J.W., HARKOV, R., & GIANTI, S.
(1986) Volatile organic compounds at hazardous waste sites
and a sanitary landfill in New Jersey. An up-to-date review
of the present situation. Environ. Progr., 5: 18-27.
154. LEASURE, C.S., FLEISCHER, M.E., ANDERSON, G.K., & EICEMAN,
G.A. (1986) Photoionization in air with ion mobility
spectrometry using a hydrogen discharge lamp. Anal. Chem.,
58: 2142-2147.
155. LEFFINGWELL, S.S., WAXWEILER, R., ALEXANDER, V., LUDWIG, H.R.
& HALPERIN, W. (1983) Case-control study of gliomas of the
brain among workers employed by a Texas City, Texas chemical
plant. Neuroepidemiology, 2: 179-195.
156. LEGENDRE, M.G. & DUPUY, H.P. (1981) Flavor volatiles as
measured by rapid instrumental techniques, American Chemical
Society, pp. 41-49 (ACS Symposium Series No. 147).
157. LEHMAN, A.J. & CHASE, H.F. (1944) The acute and chronic
toxicity of isopropyl alcohol. J. Lab. clin. Med., 29:
561-567.
158. LEHMAN, A.J., SCHWERMA, H., & RICKARDS, E. (1944) Isopropyl
alcohol: rate of disappearance from the blood stream of dogs
after intravenous and oral administration. J. Pharmacol. exp.
Ther., 82: 196-201.
159. LEHMAN, A.J., SCHWERMA, H., & RICKARDS, E. (1945) Acquired
tolerance in dogs, rate of disappearance from the blood
stream in various species, and effects on successive
generation of rats. J. Pharmacol. exp. Ther., 85: 61-69.
160. LEWIN, G.A., OPPENHEIMER, P.R., & WINGERT, W.A. (1977) Coma
from alcohol sponging. J.A.C.E.P., 6: 165-167.
161. LEWIS, G.D., LAUFMAN, A.K., MCANALLY, B.H., & GARRIOTT, J.C.
(1984) Metabolism of acetone to isopropyl alcohol in rats
and humans. J. forensic Sci., 29: 541-549.
162. LINDSTROM, T.D. & ANDERS, M.W. (1978) Effect of agents known
to alter carbon tetrachloride hepatotoxicity and cytochrome
P-450 levels on carbon tetrachloride-stimulated lipid
peroxidation and ethane expiration in the intact rat.
Biochem. Pharmacol., 27: 563-567.
163. LITOVITZ, T. (1986) The alcohols: ethanol, methanol, iso-
propanol, ethylene glycol. Pediatr. Clin. N. Am., 33:
311-323.
164. LLOYD, A.C., DARNALL, K.R. WINER, A.M., & PITTS, J.N. (1976)
Relative rate constants for the reactions of OH radicals with
isopropyl alcohol, diethyl and di- n-propyl ether at 305 ±
20 °K. Chem. Phys. Lett., 42: 205-209.
165. LORR, N.A., MILLER, K.W., CHUNG, H.R., & YANG, C.S. (1984)
Potentiation of the hepatotoxicity of N-nitrosodimethylamine
by fasting, diabetes, acetone, and isopropanol. Toxicol.
appl. Pharmacol., 73: 423-431.
166. LOVEGREN, N.V., VINNETT, C.H., & ANGELO, A.J.S. (1982) Gas
chromatographic profile of good quality raw peanuts. Peanut
Sci., 9: 93-96,
167. LUDWIG, E. & HAUSEN, B.M. (1977) Sensitivity to isopropyl
alcohol. Contact Dermat., 3: 240-244.
168. LUNDBERG, I. & HAKANSSON, M. (1985) Normal serum activities
of liver enzymes in Swedish paint industry workers with heavy
exposure to organic solvents. Br. J. ind. Med., 42: 596-600.
169. LYON, R.C., MCCOMB, J.A., SCHREURS, J., & GOLDSTEIN, D.B.
(1981) A relationship between alcohol intoxication and the
disordering of brain membranes by a series of short-chain
alcohols. J. Pharmacol. exp. Ther., 218: 669-675.
170. MCCORD, W.M., SWITZER, P.K., & BRILL, H.H. (1948) Isopropyl
alcohol intoxication. South med. J., 41: 639-642.
171. MCCREERY, M.J. & HUNT, W.A. (1978) Physico-chemical
correlates of alcohol intoxication. Neuropharmacology, 17:
451-461.
172. MCFADDEN, S.W. & HADDOW, J.E. (1969) Coma produced by
topical application of isopropanol. Pediatrics, 43: 622-623.
173. MACHYULITE, N.I. (1978) [Hygienic characterization of
conditions of work in production of levomycetin.] Gig. Tr.
Prof. Zabol., 12: 8-12 (in Russian).
174. MCINNES, A. (1973) Skin reaction to isopropyl alcohol. Br.
med. J., 1: 357.
175. MACK, K. (1973) The problem of waste water purification in
the chemical pharmaceutical industry. Prog. Water Technol.,
3: 239-249.
176. MACK, R.B. (1985) Pervasive procrustianism-isopropyl alcohol
intoxication. N.C. med. J., 46: 101-102.
177. MAIZLISH, N.A., LANGOLF, G.D., WHITEHEAD, L.W., FINE, L.J.,
ALBERS, J.W., GOLDBERG, J. & SMITH, P. (1985) Behavioural
evaluation of workers exposed to mixtures of organic
solvents. Br. J. ind. Med., 42: 579-590.
178. MALILA, A. (1978) Intoxicating effects of three aliphatic
alcohols and barbital on two rat strains genetically selected
for their ethanol intake. Pharmacol. Biochem. Behav., 8:
197-201.
179. MARKEL, H. (1982) Health hazard evaluation, Federal
Correctional Institution, Cincinnati, Ohio, US National
Institute for Occupational Safety and Health (HETA 80-119-
1066, PB 83-199398).
180. MARTIN, M. & HAERDI, W. (1982) Determination de composes
volatils toxiques dans le sang et dans l'urine par
chromatographie en phase gazeuse, methode de l'espace de tete
(head-space). Trav. Chim. aliment. Hyg., 73: 212-217.
181. MARTINEZ, T.T., JAEGER, R.W., DECASTRO, F.J., THOMPSON, M.W.,
& HAMILTON, M.F. (1986) A comparison of the absorption and
metabolism of isopropyl alcohol by oral, dermal, and
inhalation routes. Vet. human Toxicol., 28: 233-236.
182. MARZULLI, F.N. & RUGGLES, D.I. (1973) Rabbit eye irritation
test: collaborative study. J. Assoc. Off. Anal. Chem., 56:
905-914.
183. MATSUI, F., LOVERING, E.G., WATSON, J.R., BLACK, D.B., &
SEARS, R.W. (1984) Gas chromatographic method for solvent
residues in drug raw materials. J. pharm. Sci., 73:
1664-1666.
184. MATTSON, V.R., ARTHUR, J.W., & WALBRIDGE, C.T. (1976) Acute
toxicity of selected organic compounds to fathead minnows,
Duluth, Minnesota, US Environmental Protection Agency,
Environmental Research Laboratory (EPA 600/3-76-097; PB
262897).
185. MAY, J. (1966) [Odour thresholds of solvents for the
judgement of solvent odour in air.] Staub Reinhalt. Luft, 26:
385-389 (in German).
186. MECIKALSKI, M.B. & DEPNER, T.A. (1982) Peritoneal dialysis
for isopropanol poisoning. West. J. Med., 137: 322-324.
187. MENDELSON, J., WEXLER, D., LEIDERMAN, P.H., & SOLOMON, P.
(1957) A study of addiction to nonethyl alcohols and other
poisonous compounds. Quart. J. Stud. Alcohol, 18: 561-580.
188. MORGAN, R.L., SORENSON, S.S., & CASTLES, T.R. (1987)
Prediction of ocular irritation by corneal pachymetry. Food
chem. Toxicol., 25: 609-613.
189. MOSHONAS, M.G. & SHAW, P.E. (1972) Analysis of flavor
constituents from lemon and lime essence. J. agric. food
Chem., 20: 1029-1030.
190. MUNCH, J.C. (1972) Aliphatic alcohols and alkyl esters:
narcotic and lethal potencies to tadpoles and to rabbits.
Ind. Med., 41: 31-33.
191. NATOWICZ, M., DONAHUE, J., GORMAN, L., KANE, M., MCKISSICK,
J. & SHAW, L. (1985) Pharmacokinetic analysis of a case of
isopropanol intoxication. Clin. Med., 31: 326-328.
192. NELSON, K.W., EGE, J.F., ROSS, M., WOODMAN, L.E., &
SILVERMAN, L. (1943) Sensory response to certain industrial
solvent vapors. J. ind. Hyg. Toxicol., 25: 282-285.
193. NELSON, B.K., BRIGHTWELL, W.S., MACKENZIE-TAYLOR, D.R., KHAN,
A., BURG, J.R., WEIGEL, W.W., & GOAD, P.T. (1988)
Teratogenicity of n-propanol and isopropanol administered at
high inhalation concentrations to rats. Food Chem. Toxicol.,
26: 247-254.
194. NIXON, G.A., TYSON, C.A., & WERTZ, W.C. (1975) Interspecies
comparisons of skin irritancy. Toxicol. appl. Pharmacol., 31:
481-490.
195. NORDMANN, R., RIBIERE, C., ROUACH, H., BEAUGE, F.,
GIUDICELLI, Y., & NORDMANN, J. (1973) Metabolic pathways
involved in the oxidation of isopropanol into acetone by the
intact rat. Life Sci., 13: 919-932.
196. OELERT, H.H. & FLORIAN, T. (1972) [Recording and valuation
of the inconvenience caused by odours from diesel exhaust.]
Staub Reinhalt. Luft, 32: 400-407 (in German).
197. OHASHI, Y., NAKAI, Y., IKEOKA, H., KOSHIMO, H., ESAKI, Y.,
HORIGUCHI, S., TERAMOTO, K., & NAKASEKO, H. (1987a)
Recovery process of tracheal mucosa of guinea-pigs exposed to
isopropyl alcohol. Arch. Toxicol., 61: 12-20.
198. OHASHI, Y., NAKAI, Y., IKEOKA, H., KOSHIMO, H., ESAKI, Y.,
HORIGUCHI, S., TERAMOTO, K., & NAKASEKO, H. (1987b) Acute
effects of isopropyl alcohol exposure on the middle ear
mucosa. J. appl. Toxicol., 7: 205-211.
199. OLSON, B.A. (1982) Effects of organic solvents on
behavioural performance of workers in the paint industry.
Neurobehav. Toxicol. Teratol., 4: 703-708.
200. OVEREND, R. & PARASKEVOPOULOS, G. (1978) Rates of OH radical
reactions. IV. Reactions with methanol, ethanol, 1-propanol,
and 2-propanol at 296 ° K. J. phys. Chem., 82: 1329-1333.
201. PALO, V. & ILKOVA, H. (1970) Direct gas chromatographic
estimation of lower alcohols, acetaldehyde, acetone and
diacetyl in milk products. J. Chromatogr., 53: 363-367.
202. PARKER, W.A. (1982-1983) Alcohol-containing pharmaceuticals.
Am. J. drug alcohol Abuse, 9: 195-209.
203. PITTER, P. (1976) Determination of biological degradability
of organic substances. Water Res., 10: 231-235.
204. POHL, J. (1922) [Investigations into the fate of methyl and
isopropyl alcohol.] Biochem. Z., 127: 66-71 (in German).
205. POSO, H. & POSO, A.R. (1980) Inhibition by aliphatic
alcohols of the stimulated activity of ornithine
decarboxylase and tyrosine amino-transferase occurring in
regenerating rat liver. Biochem. Pharmacol., 29: 2799-2803.
206. POWIS, G. (1975) Effect of a single oral dose of methanol,
ethanol and propan-2-ol on the hepatic microsomal metabolism
of foreign compounds in the rat. Biochem. J., 148: 269-277.
207. POWIS, G. & GRANT, L. (1976) The effect of inhibitors of
alcohol metabolism upon the changes in the hepatic microsomal
metabolism of foreign compounds produced by the acute
administration of some alcohols to the rat. Biochem.
Pharmacol., 25: 2197-2201.
208. PRICE, K.S., WAGGY, G.T., & CONWAY, R.A. (1974) Brine shrimp
bioassay and seawater BOD of petrochemicals. J. Water Pollut.
Control Fed., 46: 63-77.
209. RAICHLE, M.E., EICHLING, J.O., STRAATMANN, M.G., WELCH, M.J.,
LARSON, K.B., & TER-POGOSSIAN, M.M. (1976) Blood-brain
barrier permeability of 11C-labeled alcohols and 15U-labeled
water. Am. J. Physiol., 230: 543-552.
210. RAILE, A., HAMMAN, K.P., SCHEINER, O., SCHULTZ, T., ERDEI,
A., & DIERICH, M.P. (1982) Differential effect of low
molecular weight alcohols on the Con A stimulation of mouse
spleen cells. Immunol. Lett., 4: 305-309.
211. RAMSEY, J.D. & FLANAGAN, R.J. (1982) Detection and
identification of volatile organic compounds in blood by
headspace gas chromatograpy as an aid to the diagnosis of
solvent abuse. J. Chromatogr., 240: 423-444.
212. REINDERS, M.E. (1980) Handbook of emission factors. Part I.
Non-industrial sources, The Hague, Netherlands, Ministry of
Health and Environmental Protection.
213. REINHARDT, C.A., PELLI, D.A., & ZBINDEN, G. (1985)
Interpretation of cell toxicity data for the estimation of
potential irritation. Food chem. Toxicol., 23: 247-252.
214. REQUENA, J., VELAZ, M.E., GUERRERO, J.R., & MEDINA, J.D.
(1985) Isomers of long-chain alkane derivatives and nervous
impulse blockage. J. Membr. Biol., 84: 229-238.
215. REYNOLDS, E.S., MOSLEN, M.T., & TREINEN, R.J. (1982)
Isopropanol enhancement of carbon tetrachloride metabolism in
vivo. Life Sci., 31: 661-669.
216. REYNOLDS, T. (1977) An anomalous effect of isopropanol on
lettuce germination. Plant Sci. Lett., 15: 25-28.
217. RICHARDSON, D.R., CARAVATI, C.M., PEYTON, E., & WEARY, P.E.
(1969) Allergic contact dermatitis to "alcohol" swabs.
Cutis, 5: 1115-1118.
218. RIETBROCK, N. & ABSHAGEN, U. (1971) [Pharmacokinetics and
metabolism of aliphatic alcohols.] Arzneim.-Forsch.,
21: 1309-1319 (in German).
219. RISTOW, S.S., STARKEY, J.R., & HASS, G.M. (1982) Inhibition
of natural killer cell activity in vitro by alcohols.
Biochem. Biophys. Res. Commun., 105: 1315-1321.
220. ROSANSKY, S.J. (1982) Isopropyl alcohol poisoning treated
with haemodialysis: kinetics of isopropyl alcohol and acetone
removal. J. Toxicol.-clin. Toxicol., 19: 265-271.
221. ROSS, D.H. (1976) Selective action of alcohols on cerebral
calcium levels. Ann. N.Y. Acad. Sci., 273: 280-294.
222. SABLJIC, A. & PROTIC-SABLIC, M. (1983) Quantitative
structure-activity study on the mechanism of inhibition of
microsomal p-hydroxylation of aniline by alcohols. Mol.
Pharmacol., 23: 213-218.
223. SANTODONATO, J. (1985) Monograph on human exposure to
chemicals in the workplace: isopropyl alcohol, Syracuse, New
York, Center for Chemical Hazard Assessment, Syracuse
Research Corporation (SRC-TR-84-1043, PB 86-143401).
224. SAVOLAINEN, H., PEKARI, K., & HELOJOKI, H. (1979)
Neurochemical and behavioural effects of extended exposure to
isopropanol vapour with simultaneous ethanol intake. Chem.-
biol. Interact., 28: 237-248.
225. SAWHNEY, B.L. & KOZLOSKI, R.P. (1984) Organic pollutants in
leachates from landfill sites. J. environ. Qual., 13:
349-352.
226. SCHICK, J.B. & MILSTEIN, J.M. (1981) Burn hazard of
isopropyl alcohol in the neonate. Pediatrics, 68: 587-588.
227. SEILER, H., BLAIM, H., & BUSSE, M. (1984) [Antibacterial
effects on predominant taxa in the activated sludge system of
a chemical combine.] Z. Wasser-Abwasser Forsch., 17: 127-133
(in German).
228. SENZ, E.H. & GOLDFARB, D.L. (1958) Coma in a child following
use of isopropyl alcohol in sponging. J. Pediatr., 53:
322-323.
229. SHAW, G.J., ALLEN, J.M., & VISSER, F.R. (1985) Volatile
flavor components of Babaco fruit ( Carica pentagona,
Heilborn). J. agric. food Chem., 33: 795-797.
230. SHEHAB, A.S. (1980) Comparative cytological studies of the
effect of some aliphatic alcohols and the fatty alcohols from
Euphorbia granulata and Pulicaria crispa on mitosis of Allium
cepa. Cytologia, 45: 507-513.
231. SHOFSTAHL, J.H. & HARDY, J.K. (1986) Determination of C1-C4
alcohols in gasoline using multiple ion detection. Anal.
Chem., 58: 2412-2414.
232. SIEBERT, H., SIEBERT, G., & BOHN, G. (1972) [Animal
experimental investigations into the metabolism of
propan-2-ol.] D tsch. Apoth.-ZTG., 112: 1040-1041 (in German).
233. SINGH, K.V. & AGRAWAL, S.C. (1981) Growth responses of
keratinophilic fungi to some volatile substances. Mykosen,
24: 630-634.
234. SIPES, I.G., STRIPP, B., KRISHNA, G., MALING, H., & GILLETTE,
J.R. (1973) Enhanced hepatic microsomal activity by
pretreatment of rats with acetone or isopropanol. Proc. Soc.
Exp. Biol. Med., 142: 237-240.
235. SMEA (1982) [Problems in industrial toxicology.] (in
Russian).
236. SMITH, N.B. (1984) Determination of volatile alcohols and
acetone in serum by non-polar capillary gas chromatography
after direct sample injection. Clin. Chem., 30: 1672-1674.
237. SMITH, P. & BROWN, N.L. (1969) Determination of isopropyl
alcohol in solid fish protein concentrate by gas-liquid
chromatography. J. agric. food Chem., 17: 34-37.
238. SMITH, R.P. (1959) Poisoning with Old Spice shaving lotion.
A case report. Bull. Suppl. Mat. clin. Toxicol. comm. Prod.,
2(11): 12.
239. SMYTH, H.F. & CARPENTER, C.P. (1948) Further experience with
the range finding test in the industrial toxicology
laboratory. J. ind. Hyg. Toxicol., 30: 63-70.
240. STEELE, R.H. & WILHELM, D.L. (1966) The inflammatory
reaction in chemical injury. I. Increased vascular
permeability and erythema induced by various chemicals. Br.
J. exp. Pathol., 47: 612-623.
241. STENSTROM, S., ENLOE, L., PFENNING, M., & RICHELSON, E.
(1986) Acute effects of ethanol and other short-chain
alcohols on the guanylate cyclase system of murine
neuroblastoma cells (clone N1E-115). J. Pharmacol. exp.
Ther., 236: 458-463.
242. STOFBERG, J. & GRUNDSCHOBER, F. (1984) Consumption ratio and
food predominance of flavoring materials-second cumulative
series. Perfumer & Flavorist, 9: 53-83.
243. STRANGE, A.W., SCHNEIDER, C.W., & GOLDBORT, R. (1976)
Selection of C3 alcohols by high and low ethanol selecting
mouse strains and the effects on open field activity.
Pharmacol. Biochem. Behav., 4: 527-530.
244. SWERDEL, M.R. & COUSINS, R.J. (1984) Changes in rat liver
metallothionein and metallothionein mRNA induced by
isopropanol. Proc. Soc. Exp. Biol. Med., 175: 522-529.
245. TAYLOR, C.D., COWART, C.O., & RYAN, N.T. (1985) Isopropanol
intoxication: managing the coma. Hosp. Pract., 20: 173-175.
246. TESTA, B. (1981) Structural and electronic factors
influencing the inhibition of aniline hydroxylation by
alcohols and their binding to cytochrome P-450. Chem.-biol.
Interact., 34: 287-300.
247. TICHY, M., TRCKA, V. ROTH, Z., & KRIVUCOVA, M. (1985) QSAR
analysis and data extrapolation among mammals in a series of
aliphatic alcohols. Environ. Health Perspect., 61: 321-328.
248. TIESS, D. & HAMMER, U. (1985) [On endogenous acetone
(propane-2-on) and isopropanol (propane-2-ol) levels in the
human body after ketoacidic states.] Z. gesamte Hyg., 31:
527-529 (in German).
249. TIMMER, R., TER HEIDE, R., DE VALOIS, P.J., & WOBBEN, H.J.
(1971) Qualitative analysis of the most volatile neutral
components of Reunion geranium oil ( Pelargonium roseum
Bourbon). J. agric. food Chem., 19: 1066-1068.
250. TOMITA, M. & NISHIMURA, M. (1982) Using saliva to estimate
human exposure to organic solvents. Bull. Tokyo dent. Coll.,
23: 175-188.
251. TOOBY, T.E., HURSEY, P.A., & ALABASTER, J.S. (1975) The
acute toxicity of 102 pesticides and miscellaneous substances
to fish. Chem. Ind., 12: 523-526.
252. TRAIGER, G.J. & PLAA, G.L. (1972) Relationship of alcohol
metabolism to the potentiation of CCl4 hepatotoxicity induced
by aliphatic alcohols. J. Pharmacol. exp. Ther., 183:
481-488.
253. TRAIGER, G.J. & PLAA, G.L. (1974) Chlorinated hydrocarbon
toxicity. Potentiation by isopropyl alcohol and acetone.
Arch. environ. Health, 28: 276-278.
254. TU, Y.Y., PENG, R., CHANG, Z.-F., & YANG, C.S. (1983)
Induction of a high affinity nitrosamine demethylase in rat
liver microsomes by acetone and isopropanol. Chem.-biol.
Interact., 44: 247-260.
255. UENG, T.-H., MOORE, L., ELVES, R.G., & ALVARES, A.P. (1983)
Isopropanol enhancement of cytochrome P-450-dependent
monooxygenase activities and its effects on carbon
tetrachloride intoxication. Toxicol. appl. Pharmacol., 71:
204-214.
256. URANO, K., OGURA, K., & WADA, H. (1981) Direct analytical
method for aliphatic compounds in water by steam carrier gas
chromatography. Water Res., 15: 225-231.
257. US NIOSH (1976) Criteria for a recommended standard:
occupational exposure to isopropyl alcohol, Cincinnati, Ohio,
US National Institute of Occupational Safety and Health, US
Department of Health, Education, and Welfare, Public Health
Services, Center for Disease Control (DHEW Publication No.
(NIOSH)76-142).
258. US NIOSH (1977) Manual of analytical methods, 2nd ed.,
Cincinnati, Ohio, US National Institute for Occupational
Safety and Health, US Department of Health, Education, and
Welfare, Vol. 2, pp. S185.
259. US NIOSH (1984) Method 1400. In: Eller, P.M., ed. NIOSH
Manual of analytical methods, 3rd ed., Cincinnati, Ohio,
National Institute for Occupational Safety and Health, Vol.
1, pp. 1400-1-1400-5.
260. VAN RILLAER, W.G. & BEERNAERT, H. (1983) Determination of
residual isopropanol and propylene glycol in soft drinks by
glass capillary gas chromatography. Z. Lebensm. Unters.
Forsch., 177: 196-199.
261. VASILIADES, J., POLLOCK, J., & ROBINSON, C.A. (1978)
Pitfalls of the alcohol dehydrogenase procedure for the
emergency assay of alcohol: a case study of isopropanol
overdose. Clin. Chem., 24: 383-385.
262. VEITH, G.D. & KOSIAN, P. (1983) Estimating bioconcentration
potential from octanol/water partition coefficients. In:
Mackay et al., ed. Physical behaviour of PCBs in the Great
Lakes, Ann Arbor, Michigan, Ann Arbor Science, pp. 269-282.
263. VEITH, G.D., CALL, D.J., & BROOKE, L.T. (1983) Structure-
toxicity relationships for the fathead minnow, Pimephales
promelas: narcotic industrial chemicals. Can. J. Fish. aquat.
Sci., 40: 743-748.
264. VIDELA, L.A., FERNANDEZ, V., & DE MARINIS, A. (1982) Liver
peroxidative pressure and glutathione status following
acetaldehyde and aliphatic alcohols pretreatment in the rat.
Biochem. Biophys. Res. Commun., 104: 965-970.
265. VILAGELIU ARQUES, L. & GONZALEZ DUARTE, R. (1980) Effect of
ethanol and isopropanol on the activity of alcohol
dehydrogenase, viability and life-span in Drosophila
melanogaster and Drosophila funebris. Experientia, 36:
828-830.
266. VISUDHIPAN, P. & KAUFMAN, H. (1971) Increased cerebrospinal
fluid protein following isopropyl alcohol intoxication. N.Y.
State J. Med., 71: 887-880.
267. VON DER HUDE, W., SCHEUTWINKEL, M., GRAMLICH, U., FISSLER,
B., & BUSLER, A. (1987) Genotoxicity of three carbon
compounds evaluated in the SCE test in vitro. Environ.
Mutagen., 9: 401-410.
268. WAGNER, R. (1974) [Investigations into the degradation
behaviour of organic compounds using the respirometric
dilution method. I. Monohydric alcohols.] Vom Wasser, 42:
271-305 (in German).
269. WAGNER, R. (1976) [Investigations into the degradation
behaviour of organic compounds using the respirometric
dilution method. II. The degradation kinetics of the test
compounds.] Vom Wasser, 47: 241-265 (in German).
270. WALLGREN, H. (1960) Relative intoxicating effects on rats of
ethyl, propyl and butyl alcohols. Acta pharmacol. toxicol.,
16: 217-222.
271. WALLINGFORD, K.M. (1983) Health hazard evaluation, Xomox
Corporation, Cincinnati, Ohio, US National Institute for
Occupational Safety and Health (HETA 83-170-1346, PB 85-
163434).
272. WASILEWSKI, C. (1968) Allergic contact dermatitis from
isopropyl alcohol. Arch. Dermatol., 98: 502-504.
273. WATERER, D.R. & PRITCHARD, M.K. (1984) Monitoring of
volatiles: a technique for detection of soft rot (Erwinia
carotovora) in potato tubers. Can. J. Plant. Pathol., 6:
165-171.
274. WAX, J., ELLIS, F.W., & LEHMAN, A.J. (1949) Absorption and
distribution of isopropyl alcohol. J. Pathol. exp. Ther., 97:
229-237.
275. WEBBER, D. (1984) Basic chemical output returns to growth.
Top 50 chemical products. Chem. eng. News, May 7: 8-10.
276. WEIL, C.S., SMYTH, H.F., & NALE, T.W. (1952) Quest for a
suspected industrial carcinogen. Arch. ind. Hyg. occup. Med.,
5: 535-547.
277. WEINTRAUB, Z. & IANCU, T.C. (1982) Isopropyl alcohol burns.
Pediatrics, 69: 506.
278. WHITE, G.A., EMMETT, E.A., KOMINSKY, J.R., & SINGAL, M.
(1983) Health hazard evaluation, Inland Division, GMC,
Cincinnati, Ohio, US National Instituue for Occupational
Safety and Health (HETA 77-011-1338, PB 85-101319).
279. WHITEHEAD, L.W., BALL, G.L., FINE, L.J., & LANGOLF, G.D.
(1984) Solvent vapor exposures in booth spray painting and
spray glueing, and associated operations. Am. ind. Hyg.
Assoc. J., 45: 767-772.
280. WILKINSON, C. & IGLEWICZ, R. (1982) Health hazard
evaluation, Syntrex Corporation, Cincinnati, Ohio, US
National Institute for Occupational Safety and Health (HETA
81-370-1050, PB 83-198424).
281. WILKINSON, T. & HAMER, G. (1979) The microbial oxidation of
mixtures of methanol, phenol, acetone, and isopropanol with
reference to effluent purification. J. chem. Technol.
Biotechnol., 29: 56-67.
282. WILLIAMS, T.M., HICKEY, J.L.S., & SHY,C.M. (1982) Health
hazard evaluation, Dittler Brothers, Inc., Cincinnati, Ohio,
US National Institute for Occupational Safety and Health
(HETA 81-173-1051, PB 83-198473).
283. WILLS, J.H., JAMESON, E.M., & COULSTON, F. (1969) Effects on
man of daily ingestion of small doses of isopropyl alcohol.
Toxicol. appl. Pharmacol., 15: 560-565.
284. WINEK, C.L. & JANSSEN, J.K. (1982) Blood versus bone marrow
isopropanol concentrations in rabbits. Forensic Sci. Int.,
20: 11-20.
285. WOLFF, T. (1978) In vitro inhibition of monooxygenase
dependent reactions by organic solvents. Int. Congr. Ser.
Excerpta Med., 440: 196-199.
286. WRIGHT, U. (1979) The hidden carcinogen in the manufacture
of isopropyl alcohol. Dev. Toxicol. environ. Sci., 4: 93-98.
287. YASHUDA, Y., CABRAL, A.M., & ANTONIO, A. (1976) Inhibitory
action of aliphatic alcohols on smooth muscle contraction.
Pharmacology, 14: 473-478.
288. YOUNG, P.J. & PARKER, A. (1983) The identification and
possible environmental impact of trace gases and vapours in
landfill gas. Waste Management Res., 1: 213-226.
289. YOUNG, R.H.F., RYCKMAN, D.W., & BUZZELL, J.C. (1968) An
improved tool for measuring biodegradability. J. Water
Pollut. Control Fed., 40: R354-R368.
290. ZAHLSEN, K., AARSTAD, K., & NILSEN, O.G. (1985) Inhalation
of isopropanol: induction of activating and deactivating
enzymes in rat kidney and liver, increased microsomal
metabolism of n-hexane. Toxicology, 34: 57-66.
291. ZAKHARI, S. (1977) Isopropanol and ketones in the
environment, Oxford, England, CRC Press.
292. ZINBO, M. (1984) Determination of one-carbon to three-carbon
alcohols and water in gasoline/alcohol blends by liquid
chromatography. Anal. Chem., 56: 244-247.
RESUME
1. Identité, propriétés physiques et chimiques, méthodes d'analyse
Le propanol-2 est un liquide incolore, très inflammable dont
l'odeur rappelle celle d'un mélange d'éthanol et d'acétone. Il est
entièrement miscible à l'eau, à l'éthanol, à l'acétone, au
chloroforme et au benzène. Il existe des méthodes d'analyse pour
la recherche du propanol-2 dans divers milieux (air, eau, sang,
sérum et urine), avec des limites de détection dans l'air, l'eau et
le sang respectivement égales à 2 x 10-5 mg/m3, 0,04 mg/litre et 1
mg/litre. La chromatographie en phase gazeuse (essentiellement
avec détection par ionisation de flamme) ainsi que l'électrophorèse
sur papier et la spectrométrie de mobilité ionique par
photoionisation permettent de doser le propanol-2 dans divers
mileux.
2. Sources d'exposition humaine et environnementale
On estime qu'en 1975, la production mondiale de propanol-2
dépassait 1100 kilotonnes, la capacité mondiale de production étant
supérieure à 2000 kilotonnes en 1984. Le propanol-2 est couramment
produit à partir du propène. Les procédés antérieurs de
fabrication qui reposaient sur l'utilisation d'acides forts ou
d'acides faibles, et donnaient naissance à des produits
intermédiaires et à des sous-produits potentiellement dangereux,
sont désormais largement supplantés par le procédé d'hydratation
catalytique. On peut également procéder par réduction catalytique
de l'acétone.
Le propanol-2 est un produit du métabolisme de divers micro-
organismes.
Il a de nombreuses applications comme solvant et il entre dans
la composition de différents produits ménagers et produits de soins
personnels, sous forme d'aérosols et d'excipients pour produits
pharmaceutiques à usage externe et pour cosmétiques. Le propanol-2
est également utilisé pour la production de l'acétone et autres
produits chimiques, comme agent de dégivrage, comme conservateur et
il entre dans la composition des concentrés pour le nettoyage des
pare-brise et de certains aromatisants alimentaires.
Le propanol-2 peut pénétrer dans l'atmosphère, dans l'eau et
dans le sol lors du rejet de déchets et on en a trouvé dans l'air
et dans les eaux de lessivage de décharges mal protégées. Présent
dans les gaz et eaux résiduaires industriels, on peut l'en éliminer
par oxydation biologique ou osmose inverse. Il peut être dissipé
dans l'atmosphère lors de l'utilisation de produits de consommation
qui en contiennent.
3. Transport et distribution dans l'environnement
C'est principalement lors d'opérations telles que la
production, la transformation, le stockage, le transport,
l'utilisation et le rejet de déchets que le propanol-2 pénètre dans
l'environnement atmosphérique. Il peut être également déchargé
dans le sol et l'eau. Il est difficile d'évaluer la part qui
revient à chaque compartiment du milieu. Toutefois on estimait en
1976 que plus de 50 % du propanol-2 produit finissait par être
libéré dans l'atmosphère.
Le propanol-2 est rapidement éliminé de l'atmosphère par
réaction sur les radicaux hydroxyles et entraînement par les
précipitations. Ce sont ces dernières qui sont responsables du
transport de ce composé de l'atmosphère dans le sol ou l'eau. Une
fois dans le sol, il doit y être très mobile et augmente la
perméabilité du sol à certains hydrocarbures aromatiques. Le
propanol-2 est facilement biodégradable par voie aérobie ou
anaérobie.
Comme il est biodégradable et complètement miscible à l'eau,
avec un coefficient de partition octanol/eau logarithmique de 0,14
et un facteur de bioconcentration de 0,5, il est peu probable qu'il
donne lieu à une bioaccumulation.
4. Niveaux dans l'environnement et exposition humaine
L'exposition de la population en général peut se produire par
ingestion accidentelle ou volontaire, par absorption de nourriture
contenant du propanol-2 d'origine naturelle, ou sous forme
d'aromatisant volatil ou de résidus de solvant, ou encore par
inhalation lors de l'utilisation de produits qui en contiennent.
On en a trouvé aux concentrations de 0,2 à 325 mg/litre dans des
boissons non alcoolisées et aux concentrations de 50 à 3000 mg/kg
dans des denrées alimentaires pour la production desquelles on
l'avait utilisé comme solvant. L'exposition de la population en
général par inhalation de l'air ambiant est faible, du fait de
l'élimination et de la dégradation rapides de ce produit. En
procédant à des contrôles en divers lieux et, en particulier, dans
des sites urbains, on a obtenu des concentrations moyennes
pondérées par rapport au temps allant jusqu'à 35 mg/m3.
Les travailleurs peuvent être exposés au propanol-2 au cours de
la production du composé lui-même, lors de la fabrication de
l'acétone ou d'autres dérivés et également lorsqu'on utilise ce
produit comme solvant. Aux Etats-Unis, une enquête (National
Occupational Exposure Survey) effectuée en 1980-83 a montré que
plus de 1,8 million de travailleurs pouvaient être exposés. On a
mesuré sur les lieux de travail des concentrations atteignant 1350
mg/m3 avec des moyennes pondérées par rapport au temps allant
jusqu'á 500 mg/m3.
5. Cinétique et métabolisme
Le propanol-2 est rapidement absorbé et se répartit dans tout
l'organisme par inhalation et ingestion. A forte dose,
l'absorption dans les voies digestives est retardée. Les taux
sanguins de propanol-2 (décelables lorsqu'il y a ingestion
simultanée d'éthanol) ou de son métabolite, l'acétone, sont
corrélés avec l'intensité de l'exposition. Des volontaires qui
avaient ingéré une dose de 3,75 mg/kg de propanol-2 (avec 1200 mg
d'éthanol/kg) dans du jus d'orange, présentaient une concentration
sanguine maximale de propanol-2 libre de 0,8 ± 0,3 mg/litre, et de
2,3 ± 1,4 mg/litre après incubation en présence d'arylsulfatase, ce
qui témoigne d'une sulfatation. Chez les ouvriers exposés à des
vapeurs de propanol-2 (8 - 647 mg/m3), on a observé des
concentrations de 3 - 270 mg/m3 dans l'air alvéolaire, mais dans ce
cas, c'est de l'acétone et non du propanol-2 que l'on a trouvé dans
le sang et les urines. Chez des animaux de laboratoire exposés au
propanol-2, on a retrouvé celui-ci non seulement dans le sang mais
également dans le liquide céphalo-rachidien, dans le foie, les
reins et le cerveau. Le propanol-2 traverse la barrière
hémoméningée deux fois plus facilement que l'éthanol. Le propanol-
2 est excrété en partie tel quel et en partie sous forme d'acétone,
essentiellement au niveau des poumons mais également dans la salive
et le suc gastrique. Il peut y avoir réabsorption après excrétion
par ces deux dernières voies. La métabolisation en acétone en
présence d'alcool-déshydrogénase (ADH) hépatique est assez lente,
du fait que l'ADH a une moindre affinité pour le propanol-2 que
pour l'éthanol. In vitro, l'activité de l'ADH humaine vis-à-vis du
propanol-2 correspond à 9 - 10 % de l'activité de cette enzyme
lorsque le substrat est de l'éthanol. Les oxydases microsomiques
du foie de rat sont également capables d'oxyder le propanol-2
in vitro. Chez l'homme, l'acétone est excrétée telle quelle,
essentiellement au niveau des poumons et en quantité minime au
niveau des reins. Plus l'exposition au propanol-2 se prolonge,
plus la concentration d'acétone dans l'air alvéolaire, dans le sang
et dans les urines est élevée. Le propanol-2 et l'acétone sont
éliminés de l'organisme selon une cinétique du premier ordre et
leur demi-vie chez l'homme est de 2,5 - 6,4 heures et 22 heures,
respectivement.
6. Effets sur les êtres vivants dans leur milieu naturel
Le propanol-2 est peu toxique pour la faune et la flore
aquatiques, les insectes et les plantes. Son seuil d'inhibition de
la multiplication cellulaire, mesuré chez une espèce sensible de
protozoaire, varie de 104 à 4930 mg/litre selon les conditions
expérimentales. Si l'on s'élève dans l'arbre phylogénétique, on
constate que diverses espèces de crustacés, notamment Daphnia
magna, présentent des CE50 allant de 2285 à 9714 mg/litre. Pour
des poissons d'eau douce, on a obtenu des CL50 à 96 heures allant
de 4200 à 11 130 mg/litre. Chez la drosophile, les CL50 vont de
10 200 à 13 340 mg/litre de milieu nutritif. Pour le troisième
stade larvaire du moustique Aedes aegypti, on a obtenu, lors d'une
épreuve statique de 4 heures, des valeurs de la CL50 allant de 25 à
120 mg/litre.
L'exposition de végétaux à du propanol-2 à des concentrations
comprises entre 2100 mg/litre et plus de 36 000 mg/litre, a
provoqué toute une gamme d'effets allant de l'absence totale
d'anomalies à une inhibition complète de la germination.
7. Effets sur les animaux d'expérience et les systèmes d'épreuves
in vitro
A en juger d'après la mortalité qu'il provoque, le propanol-2
présente une faible toxicité aiguë pour les mammifères, que
l'exposition ait lieu par voie orale, percutanée ou respiratoire.
Chez plusieurs espèces animales on a obtenu, après administration
par voie orale, des valeurs de la DL50 allant de 4475 à 7990 mg par
kg de poids corporel; la CL50 pour une inhalation de 8 heures
allait de 46 000 à 56 000 mg/m3 d'air chez le rat. A ces doses
mortelles, les rats présentaient une forte irritation des muqueuses
et une grave dépression du système nerveux central. La mort est
survenue par arrêt cardiaque ou respiratoire. Entre autres lésions
histopathologiques, on notait une congestion et un oedème du poumon
ainsi qu' une dégénérescence des hépatocytes.
Administré en dose unique par voie orale à des rats à raison de
3000 ou 6000 mg par kg de poids corporel, le propanol-2 a provoqué
une accumulation réversible de triglycérides dans le foie. On a
également observé chez ces rats une induction des enzymes
microsomiques à la dose de 390 mg/kg.
Non dilué, le propanol-2 n'est pas irritant en applications de
4 heures sur la peau, intacte ou abrasée, de lapins tondus.
Toutefois, en instillations oculaires de 0,1 ml, le propanol-2 non
dilué a provoqué une irritation chez le lapin. De fortes
concentrations de vapeurs de propanol-2 ont provoqué une irritation
respiratoire chez la souris et l'on a noté une réduction de 50 % du
rythme respiratoire à des concentrations allant de 12 300 à 43 525
mg/m3 d'air.
Les études conscrées aux effets sur l'animal d'exposition
répétées au propanol-2 sont plutôt limitées. Après inhalation par
des rats de 500 mg/m3 de propanol-2, cinq jours par semaine et
quatre heures par jour pendant quatre mois, on a noté une
irritation des voies respiratoires, des anomalies haématologiques
et des altérations histopathologiques au niveau du foie et de la
rate. Un autre groupe expérimental composé de cinq rats de chaque
sexe a reçu pendant 27 semaines du propanol-2 dans son eau de
boisson. En comparant les animaux qui recevaient environ 600 ou
2300 mg/kg par jour (mâles) et 1000 ou 3900 mg/kg par jour
(femelles) de propanol-2 à des témoins non traités, on a constaté
un retard de croissance, mais uniquement chez les deux groupes de
femelles traitées. Aucun autre effet indésirable n'a été constaté.
Les données disponibles donnent à penser que le propanol-2
produit sur le système nerveux central (SNC) des effets analogues à
ceux de l'éthanol. La DE50 d'anésthésie par voie orale est de 2280
mg/kg chez le lapin, la DE50 par voie intrapéritonéale
correspondant à la perte du réflexe de redressement chez la souris
est égale à 165 mg/kg et le seuil d'induction de l'ataxie par voie
intrapéritonéale est de 1106 mg/kg chez le rat. Ces valeurs sont
sont environ deux fois plus faibles que pour l'éthanol. Lors d'une
expérience menée à l'air libre, on a constaté que l'inhalation de
propanol-2 à la dose de 739 mg/m3, dix heures par jour, et cinq
jours par semaine pendant 15 semaines ne produisait aucun effet
indésirable.
Le propanol-2 a été soumis à une étude portant sur deux
générations de rats qui ont reçu dans leur eau de boisson des doses
quotidiennes de 1290, 1380 ou 1479 mg de ce produit par kg de poids
corporel. Les seuls effets indésirables qui ont été notés
consistaient dans une réduction passagère du taux de croissance
dans la génération F0. En revanche, d'autres chercheurs ont
constaté une augmentation des malformations lors d'une étude
tératogènicité, au cours de laquelle on avait administré à des
rates gravides, des doses orales quotidiennes de 252 ou 1008 mg de
propanol-2 par kg de poids corporel (toxicité maternelle non
étudiée). On a également indiqué que ces deux doses, administrées
pendant 45 jours dans l'eau de boisson, faisaient passer le cycle
oestral à cinq jours (contre quatre chez les témoins). Chez des
rates ayant reçu pendant six mois dans leur eau de boisson, des
doses quotidiennes de propanol-2 de 1800 mg/kg avant de mettre bas,
on a constaté un accroissement de la mortalité embryonnaire totale;
divers effets ont également été signalés concernant la survie
intra-utérine et postnatale à des doses aussi basses que 0,8 mg/kg,
sans qu'on puisse toutefois dégager une tendance précise. Des
rates gravides ont été exposées à de l'air contenant du propanol-2
aux concentrations respectives de 9001, 18 327 et 23 210 mg/m3
(3659, 7450 ou 9435 ppm). Les deux concentrations les plus fortes
se sont révélées toxiques pour les mères, la concentration de 9001
mg/m3 ne produisant aucun effet. A toutes les concentrations on a
constaté un effet nocif sur le développement.
Une épreuve de recherche des mutations ponctuelles utilisant
S. typhimurium a donné des résultats négatifs avec du propanol-2 à
la dose de 0,18 mg par boîte; la recherche d'échanges entre
chromatides soeurs sur fibroblastes de hamster chinois a également
été négative. Le propanol-2 a provoqué des anomalies de la mitose
dans des cellules médullaires de rat ainsi que des cellules de
l'extrêmité radiculaire d'oignons in vitro. On ne dispose d'aucune
autre donnée sur la mutagénicité de ce produit.
Un certain nombre d'études restreintes ont été consacrées au
pouvoir cancérogène du propanol-2, au cours desquelles des souris
ont été exposées à cette substance par voie percutanée (3 fois par
semaine pendant un an), par voie respiratoire (7700 mg/m3, 3 à 7
heures par jour, cinq jours par semaine pendant 5 à 8 mois) et par
voie sous-cutanée (20 mg de propanol non dilué par semaine pendant
20 à 40 semaines). Au cours de ces trois études, on a recherché la
présence de tumeurs sur la peau, dans les poumons et au point
d'injection. Aucun signe d'effets cancérogènes n'a été observé.
On ne dispose pas de données épidémiologiques suffisantes pour
évaluer la cancérogénicité du propanol-2 chez l'homme. A la
lumière des données disponibles, on peut penser que le sulfate de
dipropyl-2, un produit intermédiaire de la fabrication du
propanol-2 par le procédé aux acides forts et faibles, pourrait
être à l'origine de cancers du sinus maxillaire chez l'homme.
8. Effets sur la santé de l'homme
On a signalé plusieurs cas d'intoxication consécutifs à
l'ingestion de propanol-2 ou à l'utilisation de lotions à base de
ce produit pour rafraîchir des enfants fébriles. Les principaux
signes d'intoxication rappellent ceux de l'intoxication alcoolique:
nausées, vomissements, douleurs abdominales, gastrite, hypotension
et hypothermie. La dépression du SNC par le propanol-2 est deux
fois plus intense qu'avec l'éthanol et entraîne l'inconscience puis
un coma profond; la mort peut survenir par dépression respiratoire.
Parmi les autres effets on peut noter l'hyperglycémie, un taux
élevé de protéines dans le liquide céphalorachidien et une
atélectasie. Il semblerait que l'absorption percutanée soit
négligeable mais on connaît le cas d'un enfant qui a été intoxiqué
après avoir été lotionné avec du propanol-2, ce qui donne à penser
qu'il ne faut pas négliger l'absorption percutanée, notamment chez
les enfants. Aucun effet indésirable n'a été observé chez des
volontaires en bonne santé qui avaient bu tous les jours pendant
six semaines un sirop contenant du propanol-2 à des doses
corrrespondant respectivement à 2,6 et 6,4 mg de propanol-2 par kg
de poids corporel. Des volontaires du sexe masculin, exposés 3 à 5
minutes à des vapeurs de propanol-2 correspondant à des
concentrations de 490, 980 ou 1970 mg/m3 d'air, ont estimé qu'ils
ressentaient une irritation légère à 980 mg/m3 et que la situation
était "satisfaisante" pendant les 8 heures correspondant à leur
propre exposition professionnelle.
Des enfants prématurés ayant subi un contact prolongé avec du
propanol-2 ont présenté une irritation cutanée prenant la forme
d'érythèmes, voire de brûlures du 2ème et du 3ème degré et de
phlyctènes. On a également signalé ça et là des cas de dermatites
allergiques de contact.
Il n'existe de peu d'études épidémiologiques consacrées à la
mortalité par cancers ou autres maladies provoquées par le
propanol-2. Parmi un groupe de 61 travailleurs, employés pendant
plus de cinq ans dans un atelier de fabrication de propanol-2 par
le procédé à l'acide fort, on a observé sept cas de cancer dont
quatre de cancer des sinus maxillaires. Une étude portant sur une
cohorte de 779 travailleurs d'un atelier analogue a révélé que
l'incidence des cancers du sinus et du larynx, corrigée de
l'influence de l'âge et du sexe, était 21 fois plus forte que
prévue. La période minimale de latence était de dix ans. Dans une
autre étude rétrospective de cohorte portant sur le personnel d'une
autre usine où on utilisait le procédé à l'acide fort, on a
constitué une cohorte représentant plus de 4000 années-hommes
exposés au risque. Les résultats de cette étude ont montré que les
taux de mortalité pour toutes causes et les taux de mortalité par
cancer n'étaient pas sensiblement plus élevés que les taux
prévisibles. Une autre étude rétrospective a été menée dans une
usine qui fabriquait du propanol-2 par le procédé à l'acide faible.
Cette fois, on comptait plus de 11 000 années-hommes exposés au
risque. Dans ce cas, le taux de mortalité pour toutes causes était
inférieur aux prévisions et l'on ne constatait pas de surmortalité
attribuable aux cancers en général. Toutefois, l'incidence des
cancers de la bouche et du pharynx était 4 fois supérieure à la
normale. Dans l'ensemble, ces études de cohorte donnent à penser
qu'il existe un risque de cancer imputable au procédé à l'acide
fort; toutefois lors de deux petites études castémoins, on n'a noté
aucune association entre l'exposition au propanol-2 et l'incidence
des gliomes ou de la leucémie lymphatique.
Certaines études font état d'une potentialisation de la
toxicité du tétrachlorure de carbone chez des ouvriers
simultanément exposés au propanol-2.
9. Résumé de l'évaluation
L'homme peut être exposé au propanol-2 par inhalation lors de
la fabrication, de la transformation ou de l'utilisation de cette
substance dans le cadre professionnel ou domestique. En ce qui
concerne la population en général, l'exposition à des doses
potentiellement mortelles peut se produire par suite d'ingestion
accidentelle ou volontaire de cette substance et les enfants
peuvent être exposés par application de lotions à base de propanol-
2.
Le propanol-2 est vite absorbé et se répartit rapidement dans
l'ensemble de l'organisme, en partie sous forme d'acétone. Les
données relatives aux effets aigus consécutifs à l'exposition
d'êtres humains à des doses excessives sont rares et contrastées.
Les principaux effets consistent en gastrite, dépression du système
nerveux central, hypothermie, dépression respiratoire et
hypotension. Les données de mortalité aiguë obtenues sur des
animaux de laboratoire indiquent que le propanol-2 est peu toxique,
les valeurs de la DL50 par voie orale chez diverses espèces vont de
4475 à 7990 mg/kg, et les valeurs de la CL50 par inhalation se
situent aux alentours de 50 000 mg/m3 chez le rat. Chez le lapin,
le propanol-2 ne provoque pas d'irritation cutanée, toutefois
l'instillation de 0,1 ml de cette substance non diluée dans les
yeux a provoqué une irritation.
Chez l'homme, les effets aigus les plus probables d'une
exposition de fortes concentrations de propanol-2 par ingestion ou
inhalation, consistent en une intoxication de type alcoolique
aboutissant à la narcose.
Les études sur l'animal sont insuffisantes pour qu'on puisse
évaluer les risques encourus par l'homme à la suite d'expositions
répétées au propanol-2. Toutefois les résultats de deux études à
court terme chez le rat, au cours desquelles on a fait a) inhaler
500 mg/m3 de cette substance, 4 heures par jour, 5 heures par
semaine pendant 4 mois et b) ingérer le même produit à raison de
600 à 3900 mg/kg dans l'eau de boisson, donnent à penser qu'il
serait préférable d'éviter de s'exposer aux fortes concentrations
en propanol-2 signalées dans le cadre de certaines activités
professionnelles.
En faisant inhaler du propanol-2 à des rattes gravides on a
constaté que le seuil d'apparition d'un effet se situait à 18 327
mg/m3 (7450 ppm), la dose sans effet observable était de 9001 mg/m3
(3659 ppm), la toxicité maternelle étant prise comme critère. Au
cours de la même étude, le seuil d'apparition d'effets s'est situé
à 9001 mg/m3 (3659 ppm) pour les anomalies du développement et
aucune dose sans effet observable n'a pu être mise en évidence.
Ces concentrations sont plus élevées que celles auxquelles l'homme
est susceptible d'être exposé.
Les épreuves de génotoxicité ont donné des résultats négatifs
dans le cas du propanol-2, cependant on a observé des anomalies de
la mitose dans des cellules médullaires de rats. Ces résultats
indiquent que le propanol-2 n'est pas du tout génétoxique mais les
données sont trop limitées pour qu'on puisse se prononcer
véritablement sur le pouvoir mutagène de cette substance.
Les données existantes sont insuffisantes pour permettre une
évaluation de la cancérogénicité du propanol-2 chez l'animal
d'expérience. On ne dispose pas de données permettant d'évaluer
cette cancérogénicité chez l'homme.
Le propanol-2 ne fait probablement pas courir de risque
important à la population dans son ensemble dans les conditions
d'exposition qui sont susceptibles de se produire.
Le propanol-2 disparaît rapidement (demi-vie, 5 jours) de
l'atmosphère et s'élimine à bref délai de l'eau et du sol par
biodégradation aérobie ou anaérobie, en particulier une fois que
les microorganismes préalablement ensemencés se sont adaptés.
Compte tenu de ses propriétés physiques, le propanol-2 n'a qu'une
faible tendance à la bioaccumulation. Il ne présente pas de risque
pour la faune et la flore aux concentrations auxquelles il est
habituellement présent dans l'environnement.
RESUMEN
1. Identidad, propiedades físicas y químicas, métodos analíticos
El 2-propanol es un líquido incoloro, sumamente inflamable, con
un olor que recuerda al de la mezcla de etanol y acetona. El
compuesto es completamente miscible con agua, etanol, acetona,
cloroformo y benceno. Se dispone de métodos analíticos para
detectar el 2-propanol en diversos medios (aire, agua, sangre,
suero y orina) con límites de detección de 2 x 10-5 mg/m3, 0,04
mg/litro y 1 mg/litro en el aire, el agua y la sangre,
respectivamente. Existen métodos de cromatografía de gases (que se
sirven principalmente de la detección de ionización de llama) así
como métodos de electroforesis en papel y de espectrometría de
movilidad iónica inducida por fotoionización para determinar el
2-propanol en los distintos medios.
2. Fuentes de exposición humana y ambiental
La producción mundial estimada de 2-propanol en 1975 fue
superior a 1100 kilotoneladas y la capacidad de producción mundial
en 1984 se cifró en más de 2000 kilotoneladas. El 2-propanol se
fabrica comúnmente a partir del propeno. Los antiguos procesos
basados en ácidos fuertes y débiles, en los que se generaban
productos intermedios y desechos potencialmente peligrosos, se han
sustituido actualmente en gran medida por el proceso de hidratación
catalítica. La reducción catalítica de la acetona es otro proceso
posible.
El 2-propanol se ha identificado como producto metabólico de
diversos microorganismos.
El compuesto tiene amplias aplicaciones como disolvente y se
utiliza como componente de productos domésticos y personales, entre
ellos vaporizadores de aerosoles, productos farmacéuticos de
aplicación tópica y cosméticos. El 2-propanol se utiliza también
para producir acetona y otras sustancias químicas, como agente
descongelante, como conservante, en concentrados para
limpiaparabrisas y como aromatizante volátil en alimentos.
El 2-propanol puede ingresar en la atmósfera, el agua o el
suelo por la evacuación de desechos y se ha aislado en el aire y en
el líquido que rezuma de basureros y terraplenados. Se encuentra
en los gases y las aguas residuales que emiten algunas industrias,
y puede extraerse de esas aguas por oxidación biológica o por
ósmosis inversa. Durante el uso de 2-propanol en productos de
consumo se producen emisiones dispersas a la atmósfera.
3. Transporte, distribución y transformación en el medio ambiente
La vía principal de entrada del 2-propanol en el medio ambiente
es su emisión a la atmósfera durante la producción, el tratamiento,
el almacenamiento, el transporte, el uso y la evacuación. También
se producen emisiones al suelo y al agua. Es difícil calcular el
volumen que ingresa en cada compartimiento ambiental. No obstante,
se calculó que en 1976 la liberación total de este compuesto en la
atmósfera fue superior al 50% del 2-propanol producido.
El 2-propanol desaparece rápidamente de la atmósfera por
reacción con radicales hidroxilo y arrastrado por la lluvia. A
este último proceso se debe el transporte del 2-propanol desde la
atmósfera hasta el suelo o el agua. Una vez que está en el suelo,
se cree que es muy móvil y que aumenta la permeabilidad del suelo a
algunos hidrocarburos aromáticos. El 2-propanol es fácilmente
biodegradable, en condiciones tanto aerobias como anaerobias.
La bioacumulación del compuesto no es probable, dados su
carácter biodegradable y su miscibilidad total con el agua; su
coeficiente de reparto log n-octanol/agua es de 0,14 y su factor de
bioconcentración de 0,5.
4. Niveles ambientales y exposición humana
La exposición de la población general se produce por ingestión
accidental o intencionada, por la ingestión de alimentos que lo
contengan como aromatizante volátil natural o añadido o como
residuo de disolvente, y por inhalación durante su uso. Se han
encontrado concentraciones de 0,2 a 325 mg por litro en bebidas no
alcohólicas y de 50 a 3000 mg por kg en alimentos tras el uso de
2-propanol como disolvente en su producción. La exposición de la
población general por inhalación de aire ambiental es baja a causa
de su rápida desaparición y degradación. Se han estudiado diversas
localizaciones y se han medido concentraciones medias ponderadas en
función del tiempo de hasta 35 mg/m3 en emplazamientos urbanos.
Los trabajadores se ven expuestos al 2-propanol durante la
producción del propio compuesto y de acetona y otros derivados, así
como durante su uso como disolvente. En la encuesta nacional de
exposición ocupacional (1980 - 83) realizada en los Estados Unidos,
se estimó que más de 1,8 millones de trabajadores estaban
potencialmente expuestos. En ciertos lugares de trabajo se han
medido concentraciones de hasta 1350 mg/m3, con promedios
ponderados en función del tiempo de hasta 500 mg/m3.
5. Cinética y metabolismo
El 2-propanol se absorbe y distribuye rápidamente por todo el
organismo tras su inhalación o ingestión. A dosis elevadas se
retrasa la absorción gastrointestinal. Las concentraciones
sanguíneas de 2-propanol (detectables cuando se ingiere etanol
simultáneamente) o de su metabolito, la acetona, guardan relación
con los niveles de exposición. En voluntarios que ingirieron una
dosis de 3,75 mg/kg (con 1200 mg de etanol/kg) en zumo de naranja,
se observó un nivel máximo de 0,8 ± 0,3 mg de 2-propanol libre por
litro en la sangre, y de 2,3 ± 1,4 mg por litro tras la incubación
con arilsulfatasa, lo que es un signo de sulfatación. Los
trabajadores expuestos a vapores (8 - 647 mg/m3) mostraron
concentraciones de 3 - 270 mg/m3 en el aire alveolar, pero en este
caso se encontró acetona y no 2-propanol en la sangre y la orina.
En animales de laboratorio tratados, el 2-propanol se detectó no
sólo en la sangre sino también en el líquido espinal, el hígado,
los riñones y el cerebro. Atraviesa la barrera hematocerebral dos
veces mejor que el etanol. El 2-propanol se excreta en parte como
tal y en parte como acetona, principalmente por vía pulmonar, pero
también en la saliva y el jugo gástrico. La reabsorción puede
producirse después de la excreción por las últimas dos vías. La
transformación en acetona por medio de la deshidrogenasa alcohólica
del hígado es más bien lenta, porque la afinidad relativa de la
deshidrogenasa por el 2-propanol es más baja que por el etanol.
In vitro, la actividad enzimática de la deshidrogenasa humana con
2-propanol fue del 9 - 10% de la actividad que exhibe cuando el
sustrato es el etanol. In vitro las oxidasas microsómicas de
hígado de rata también son capaces de oxidar el 2-propanol. En el
hombre, la acetona se excreta sin cambios, principalmente por los
pulmones y en cantidad mínima por los riñones. La concentración de
acetona en el aire alveolar, la sangre y la orina aumenta con la
intensidad y la duración de la exposición al 2-propanol. La
eliminación de 2-propanol y de acetona del organismo es de primer
orden, y los periodos de semieliminación en el hombre son de
2,5 - 6,4 horas y 22 horas, respectivamente.
6. Efectos en los organismos en el medio ambiente
La toxicidad del 2-propanol para organismos acuáticos, insectos
y plantas es baja. El umbral inhibitorio para la multiplicación
celular de una especie de protozoo sensible varió de 104 a 4930 mg
por litro en diversas condiciones experimentales. Avanzando en la
cadena filogenética, varias especies de crustáceos, incluida
Daphnia magna, mostraron CE50 a concentraciones que iban desde 2285
hasta 9714 mg por litro. Las CL50 (96 h) para peces de agua dulce
variaron desde 4200 hasta 11 130 mg por litro. Los datos obtenidos
para especies de moscas de la fruta mostraron CL50 comprendidas
entre 10 200 y 13 340 mg por litro de medio nutritivo. La CL50
para larvas de mosquito ( Aedes aegypti) en la tercera etapa de
desarrollo fue de 25 - 120 mg/litro en un ensayo estático de 4
horas.
Los efectos que tiene en las plantas la exposición a 2-propanol
en concentraciones entre 2100 mg/litro y más de 36 000 mg/litro
variaron entre la ausencia de efecto y la inhibición total de la
germinación.
7. Efectos en animales de experimentación y en sistemas de ensayo
in vitro
La toxicidad aguda del 2-propanol para los mamíferos, a juzgar
por la mortalidad, es baja, sea cual sea la vía de exposición oral,
cutánea o respiratoria. Los valores de la DL50 para varias
especies animales tras la administración oral variaron entre 4475 y
7990 mg por kg de peso corporal; La CL50 de inhalación durante 8
horas en la rata varió de 46 000 a 55 000 mg por m3 de aire. A
estos niveles letales, las ratas mostraron grave irritación de las
mucosas y depresión profunda del sistema nervioso central. La
muerte fue provocada por paro respiratorio o cardiaco. Entre las
lesiones histopatológicas figuraron la congestión y el edema
pulmonar, así como la degeneración celular en el hígado.
Con dosis orales únicas de 3000 ó 6000 mg de 2-propanol por kg
de peso corporal se produjo una acumulación reversible de
triglicéridos en el hígado de la rata. En la rata se observó
inducción de enzimas microsómicas a dosis orales de 390 mg/kg.
El 2-propanol sin diluir no produjo irritaciones cuando se
aplicó a la piel cortada o raspada del conejo durante 4 horas. En
cambio, se observó irritación cuando se aplicó 0,1 ml de compuesto
sin diluir en el ojo del conejo. Con concentraciones de vapor
elevadas de 2-propanol se produjo irritación del tracto
respiratorio en el ratón, y el ritmo respiratorio disminuyó en un
50% a concentraciones de 12 300 - 43 525 mg/m3 de aire.
Se han hecho escasos estudios de exposición repetida sobre los
efectos del 2-propanol en animales. Tras la inhalación de 500 mg
de 2-propanol/m3 durante 5 días a la semana y 4 horas al día
durante más de 4 meses, se observaron irritación del tracto
respiratorio, cambios hematológicos y alteraciones histopatológicas
en el hígado y el bazo de la rata. En otro grupo de estudio, se
administró a 5 ratas de cada sexo agua de bebida que contenía
2-propanol durante 27 semanas. La comparación de animales que
recibían aproximadamente 600 ó 2300 mg por kg al día (machos) y
1000 ó 3900 mg por kg al día (hembras) con grupos de control no
tratados reveló un retraso del crecimiento sólo en ambos grupos de
hembras expuestas. No se observaron otros efectos adversos.
Los datos disponibles indican que los efectos del 2-propanol en
el sistema nervioso central son semejantes a los del etanol. La
DE50 por vía oral para la narcosis en conejos es de 2280 mg/kg; la
DE50 intraperitoneal correspondiente a la pérdida del reflejo de
enderezamiento en el ratón es de 165 mg/kg, y el umbral
intraperitoneal de inducción de ataxia en la rata es de 1106 mg/kg.
Estos valores son aproximadamente dos veces más bajos que los
correspondientes al etanol. La inhalación de 2-propanol a una
concentración de 739 mg/m3 durante 6 horas al día y 5 días a la
semana durante 15 semanas no originó ningún resultado adverso en un
ensayo en campo abierto.
El 2-propanol se evaluó en un estudio de 2 generaciones de
ratas mediante la administración de 1290, 1380 ó 1470 mg por kg al
día en el agua de bebida a ambas generaciones. El único efecto
adverso observado fue una reducción transitoria del ritmo de
crecimiento en la generación Fo. En cambio, otros investigadores
observaron un aumento de las malformaciones en un estudio de la
teratogénesis después de administrar por vía oral a ratas gestantes
252 ó 1008 mg de 2-propanol por kg al día (no se formularon
observaciones sobre la toxicidad materna). Ambas dosis,
administradas en el agua de bebida durante 45 días, también
aumentaron la duración del ciclo estrual hasta 5 días (frente a 4
días en los sujetos de control). Se observó una mortalidad
embrionaria total mayor cuando se administraban a la rata hembra
dosis de 1800 mg/kg en el agua de bebida al día durante 6 meses
antes de criar; se notificaron diversos efectos en la supervivencia
intrauterina y puerperal a dosis tan bajas como 0,18 mg/kg al día,
pero no se observó ninguna pauta coherente. Se expusieron ratas
gestantes a 2-propanol atmosférico a concentraciones de 9001,
18 327 ó 23 210 mg por m3 (3659, 7450 ó 9435 ppm). Las dos
concentraciones más elevadas fueron tóxicas para las madres, pero
no así la de 9001 mg/m3. Se observó toxicidad en el desarrollo con
las tres concentraciones.
El 2-propanol dio resultados negativos en una prueba con 0,18
mg por placa para detectar mutuaciones puntuales en S.
typhimurium y en una prueba de intercambio de cromátidas hermanas
en fibroblastos pulmonares de hámster chino. Indujo anomalías
mitóticas en células de médula ósea de rata y en células de ápice
radicular de cebolla in vitro. No se dispone de otros datos sobre
mutagenicidad.
El 2-propanol se ensayó en varios estudios limitados de
carcinogenicidad en el ratón utilizando las vías de exposición
cutánea (3 veces a la semana durante un año), inhalación (7700
mg/m3 durante 3 - 7 h/día, 5 días/semana, durante 5 - 8 meses) y
subcutánea (20 mg sin diluir, semanalmente durante 20 - 40
semanas). La aparición de tumores se investigó en los tres
estudios en la piel, el pulmón y el lugar de inyección,
respectivamente. No se observaron efectos carcinogénicos. No se
dispone de datos epidemiológicos adecuados con los que evaluar la
carcinogenicidad del 2-propanol para el ser humano. Los datos
disponibles indican que el di-2-propilsulfato, un producto
intermedio en los procesos de ácidos fuertes y débiles para
producir 2-propanol, puede estar asociado causalmente con la
inducción de cáncer del seno paranasal en el ser humano.
8. Efectos en la salud humana
Se han notificado varios casos de intoxicación tras la
ingestión oral y también en niños con fiebre a los que se refrescó
con esponjas impregnadas con preparaciones con 2-propanol. En
casos de envenenamiento, los principales signos son los de la
intoxicación alcohólica, en particular náuseas, vómitos, dolores
abdominales, gastritis, hipotensión e hipotermia. El 2-propanol
deprime el sistema nervioso central unas dos veces más que el
etanol, provocando una inconsciencia que termina en coma profundo;
puede sobrevenir la muerte por depresión respiratoria. Otros
efectos relacionados con el compuesto son la hiperglucemia,
elevados niveles de proteínas en el líquido cefalorraquídeo y
atelectasia. Aunque se considera que la absorción por la piel es
insignificante, en un informe sobre un caso de un niño intoxicado
tras refrescársele con una esponja impregnada con 2-propanol, se
indicaba que no conviene subestimar el riesgo de absorción dérmica,
especialmente en los niños. No se observaron efectos adversos en
voluntarios sanos que bebieron diariamente durante 6 semanas un
jarabe que contenía 2,6 ó 6,4 mg de 2-propanol/kg. Un grupo de
varones voluntarios, cuando se expusieron a vapores de 2-propanol
en concentraciones de 490, 980 ó 1970 mg por m3 de aire durante
3 - 5 minutos juzgaron que la irritación era "leve" a 980 mg/m3 y
"satisactoria" para su propia exposición ocupacional de 8 horas.
Las irritaciones de la piel en forma de eritema, quemaduras de
segundo y tercer grado y ampollas se notificaron en niños
prematuros tras un contacto prolongado con 2-propanol. En
ocasiones también se han notificado casos de dermatitis alérgica
por contacto.
Se dispone de pocos estudios epidemiológicos sobre mortalidad
por cáncer o por otras causas. En un grupo de 71 trabajadores
empleados durante más de 5 años en una fábrica de 2-propanol por el
proceso del ácido fuerte, se notificaron 7 casos de cáncer, entre
ellos 4 de cáncer del seno paranasal. En un estudio de cohortes
realizado sobre 779 trabajadores en una fábrica similar, las
incidencias reajustadas en función de la edad y del sexo de cáncer
del seno y de la laringe fueron 21 veces mayores de lo esperado.
El periodo mínimo de latencia fue de 10 años. En otro estudio
retrospectivo de cohortes realizado en otra fábrica que utilizaba
el proceso del ácido fuerte, había más de 4000 personas-años
expuestas. Los resultados mostraron que las tasas de mortalidad
por todas las causas y por neoplasmas no eran significativamente
mayores de lo previsto. Se llevó a cabo un estudio retrospectivo
de cohortes en una planta que fabricaba 2-propanol por el proceso
del ácido débil. Había más de 11 000 personas-años expuestas. La
tasa de mortalidad debida a todas las causas fue más baja de lo
esperado. No se observó mortalidad excesiva por todos los
cánceres. Sin embargo, la incidencia del cáncer de la boca y de la
faringe fue 4 veces más elevada de lo previsto. Los estudios de
cohortes indican en conjunto un riesgo de cáncer relacionado con el
proceso de fabricación con ácido fuerte, pero, en dos pequeños
estudios controlados de casos, no se observó correlación alguna
entre la exposición a 2-propanol y la incidencia de gliomas o de
leucemia linfática.
Algunos informes parecen indicar que la exposición combinada a
tetracloruro de carbono y 2-propanol en los trabajadores potencia
la toxicidad del primero.
9. Resumen de la evaluación
La exposición del hombre al 2-propanol puede producirse por
inhalación durante la fabricación, el tratamiento y el uso tanto
ocupacional como doméstico. La exposición a un nivel
potencialmente letal en la población general puede producirse por
ingestión accidental o intencionada y los niños pueden estar
expuestos cuando se les refresca con esponjas impregnadas con
preparaciones a base de 2-propanol (alcohol para friegas).
El 2-propanol se absorbe y distribuye rápidamente por todo el
organismo, en parte en forma de acetona. Los datos sobre
exposición-efecto en el hombre en condiciones de sobreexposición
aguda son escasos y muestran grandes variaciones. Los principales
efectos son la gastritis, la depresión del sistema nervioso central
con hipotermia y depresión respiratoria, y la hipotensión. Los
datos de mortalidad aguda en animales de experimentación indican
que la toxicidad del 2-propanol es baja, siendo los valores de DL50
orales en diversas especies entre 4475 y 7990 mg/kg, y los valores
de CL50 de inhalación en ratas alrededor de 50 000 mg/m3. En el
conejo, el 2-propanol no produjo irritaciones cutáneas, pero la
aplicación de 0,1 ml de 2-propanol sin diluir produjo irritación en
los ojos.
En el hombre, los efectos agudos más probables de la exposición
a concentraciones elevadas de 2-propanol por ingestión o inhalación
son la intoxicación alcohólica y la narcosis.
No se han hecho suficientes estudios en animales como para
evaluar los riesgos que entraña para la salud humana la exposición
repetida al 2-propanol. No obstante, los resultados de dos
estudios a corto plazo en la rata, incluida la exposición por
inhalación (500 mg/m3 durante 4 horas al día y 5 días a la semana
durante 4 meses) y la exposición oral (600 - 3900 mg/kg en el agua
de bebida) indican que debe evitarse la exposición al 2-propanol en
algunos de los muy elevados niveles de exposición ocupacional que
se han notificado.
La exposición por inhalación de ratas gestantes a 2-propanol
dio un nivel mínimo de observación de efectos de 18 327 mg/m3 (7450
ppm) y un nivel sin efectos observados de 9001 mg/m3 (3659 ppm)
respecto a la toxicidad materna. En el mismo estudio, 9001 mg/m3
(3659 ppm) fue el nivel más bajo de observación de efectos en lo
que respecta a la toxicidad de desarrollo; no se indicó nivel sin
efectos observados. Estas concentraciones son más elevadas que las
que normalmente se registran en condiciones de exposición humana.
El 2-propanol dio resultado negativo en las pruebas de
genotoxicidad, pero indujo aberraciones mitóticas en la médula ósea
de la rata. Aunque estos resultados indican que la sustancia no
tiene potencial genotóxico, no puede hacerse una evaluación
correcta de la mutagenicidad basándose en datos tan limitados.
Los datos disponibles no bastan para evaluar la carcinogenicidad
del 2-propanol en animales de experimentación. No se dispone de
datos para evaluar la carcinogenicidad del 2-propanol en el ser
humano.
Es poco probable que el 2-propanol plantee un riesgo grave para
la salud de la población general en las condiciones de exposición
que se producen normalmente.
El 2-propanol desaparece rápidamente (periodo de semieliminación
2,5 días) de la atmósfera y su desaparición del agua y del suelo se
produce rápidamente por biodegradación aerobia y anaerobia,
especialmente tras la adaptación de microorganismos inicialmente
sembrados. En vista de las propiedades físicas del 2-propanol, su
potencial de bioacumulación es bajo. No representa un riesgo para
los organismos naturales en las concentraciones en que suele
encontrarse en el medio ambiente.
Since adequate balance studies have not been conducted, the
extent of this metabolism to acetone is not known. Siebert et al.
[232] injected 750 or 1300 mg/kg body weight intravenously in
rabbits and estimated that 64 - 84% of the dose was oxidized to
acetone, confirming the earlier findings of Pohl [204].
Evidence of another metabolic pathway was found in rabbits
given an oral dose of 2-propanol of 3000 mg/kg body weight, 10.2%
of the dose was found in the urine as beta-isopropyl-glucuronide
[129].
Sufficient evidence is available to show that 2-propanol is
oxidized to acetone mainly by the non-specific cytosolic enzyme
alcohol dehydrogenase (ADH) (EC 1.1.1.1). When either pyrazole or
4-methylpyrazole (inhibitors of ADH) was administered to rats prior
to exposure to 2-propanol, both the elimination of 2-propanol and
the production of acetone were retarded [121, 193]. The
elimination of 2-propanol by rats was retarded when it was given in
combination with ethanol; both compounds are substrates for ADH [2,
121]. Enzyme kinetic data show that 2-propanol is a poorer
substrate of ADH than ethanol. In vitro, the Michaelis-Menten
constant (Km) for horse liver ADH was 0.0126 mol/litre, using
2-propanol as a substrate, and 0.00018 mol/litre, using ethanol as
a substrate [64]. For rat liver ADH in vivo, Km was 0.034
mol/litre, using 2-propanol as a substrate, and 0.00192 mol/litre,
using ethanol as a substrate. The maximum initial velocity (Vmax)
of rat liver ADH and the substrate 2-propanol in vivo was 1.7 times
less than that with the substrate ethanol. It was further shown
that the ratio of the relative rates of oxidation of 2-propanol and
deuterated 2-propanol-d7 were about the same in vitro and in vivo.
From this, it can be concluded that ADH activity is the rate-
limiting factor in 2-propanol metabolism [54].
It has been shown that rat liver microsomal oxidases are also
capable of oxidizing 2-propanol, but that the compound is not an
effective substrate for the peroxidative activity of catalase
(EC 1.11.1.6) [48, 193].
6.3.2 Human beings
Acetone has also been found in the blood of human beings after
exposure to 2-propanol [e.g., 11, 41, 65, 131]. Furthermore,
acetone was detected in the spinal fluid, at levels similar to
those in serum, in 2 persons after ingestion of 2-propanol [5, 191].
In the investigations of Brugnone et al. [41] (section 6.1.2),
elevated acetone levels were measured in the blood, alveolar air,
and urine of 12 printing workers. 2-Propanol was not detected in
the blood or urine. The concentrations of acetone ranged between
0.76 and 15.6 mg/litre in the blood and between 3 and 93 mg/m3 in
the alveolar air. The acetone levels in alveolar air and blood
increased with the increasing exposure period and were linearly
related to the alveolar 2-propanol levels. Urinary acetone levels
ranged from 0.85 to 53.7 mg/litre in overnight pooled urine samples
and were highly correlated with the alveolar 2-propanol uptake and
with blood acetone levels. Pulmonary and renal clearance of
acetone were 41 - 97 and 0.1 - 3 ml/min, respectively, for 8 of the
workers, showing that acetone is mainly excreted via the lungs.
Pulmonary acetone excretion varied from 10.7 to 39.8% of the
uptake and was inversely related to the exposure level [41]. It
is known that the metabolic capacity of the human liver for acetone
is limited [161].
2-Propanol and acetone have also been found in the returns of
gastric lavage [5] and in saliva [250]. Reabsorption may follow
excretion via these routes.
When 10 volunteers drank orange juice containing doses that
gave 3.75 mg 2-propanol and 1200 mg ethanol/kg body weight over
2 h, 2-propanol was detected in the blood, partly as sulfate
(section 6.1.2), and in the urine, partly as glucuronide. The
total urinary excretion of 2-propanol was 1.9% of the dose [27, 28].
Human data support the importance of the ADH pathway for the
oxidation of 2-propanol as observed in experimental animals. In
the studies of Bonte et al. [27, 28] described above, 2-propanol
was only detected in the blood when ethanol was ingested
simultaneously, indicating a retarded elimination. Among 74
persons intoxicated by 2-propanol, significantly lower acetone
values were found in the blood of those who had also been exposed
to ethanol [11, 131]. In in vitro studies, the activity of human
ADH with 2-propanol was 9 - 10% of the activity of the enzyme with
ethanol as substrate [121].
Endogenous formation of 2-propanol has been revealed at
autopsies of individuals not previously exposed to this compound.
This observation and the results of additional studies on rats show
that 2-propanol can result from the reduction of acetone by liver
ADH, especially when high levels of acetone and high NADH/NAD+
ratios occur. Such conditions are found in diabetes mellitus,
starvation, high fat feeding, chronic alcoholism, and dehydration
[68, 161, 248].
6.4 Elimination and Excretion
6.4.1 Animals
In a review article, Rietbrock & Abshagen [218] concluded that
urinary excretion of both 2-propanol and its metabolite acetone is
limited and in each case does not exceed 4% of the dose in the rat,
rabbit, and dog. The major route of excretion is via the lungs.
2-Propanol is excreted via the gastric juice and saliva in the dog
[158] and through breast milk in the rat, as shown by tissue levels
in the newborn [159].
The disappearance of 2-propanol from the blood of experimental
animals was found to be a first-order process at doses <1500 mg/kg
[1, 232]. In rats, the half-life of 2-propanol was 1.5 h at an
intraperitoneal dose of 500 mg/kg body weight and increased to
2.5 h at a dose of 1500 mg/kg body weight. This can be explained
by a limited metabolic capacity as suggested in section 6.3.1
[218]. Simultaneous administration of ethanol to rats increased
the half-life of 2-propanol in the blood approximately 5-fold
following acute exposure [2]. A half-life of 4 h was determined in
dogs administered 1000 mg 2-propanol/kg body weight intravenously [1].
The biotransformation of 2-propanol and the elimination of the
acetone produced are both slow processes. Peak acetone levels in
the blood of various species, following single exposures via
different routes, were only reached several hours after exposure,
though acetone was already detectable shortly after the start of
exposure [1, 121, 150, 151, 193, 232]. The elimination of acetone
in the dog and the rat was found to be a first-order process with
half-lives of 11 and 5 h, respectively [1].
After prolonged administration of 2-propanol to dogs [159] and
rats [224], the elimination rate of 2-propanol was increased.
Simultaneous exposure of rats to ethanol in the drinking-water (5%)
and atmospheric 2-propanol at 738 mg/m3 (300 ppm) for 5 - 21 weeks
significantly increased the rates of elimination of 2-propanol and
acetone [224].
6.4.2 Human beings
The elimination of 2-propanol in human beings also appears to
follow first-order kinetics. In two alcoholics who had ingested
rubbing alcohol, 2-propanol was eliminated from the blood with
half-lives of 2.5 and 3 h, respectively. Acetone levels declined
slowly over the next 30 h; no ethanol was detected [65]. A half-
life of 2-propanol of 6.4 h was determined in a non-alcoholic, who
also had ingested rubbing alcohol. The acetone level in the blood
reached a maximum 30 h after admission to hospital. The half-life
of acetone was 22 h. Ethanol was also found in the blood. The
differences in the half-lives in these three cases may reflect
metabolic adaptation in the alcoholics, or genetic variations in
ADH [191].
When 4 volunteers ingested an artificial liquor containing 40%
2-propanol, acetone was detectable in exhaled air from 15 min after
exposure and in the urine from 1 h after exposure [132].
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Aquatic Organisms
A summary of acute aquatic toxicity data is presented in Table 8.
The concentration of 2-propanol was reported to have been measured
in only 3 of the studies [34, 111, 263] and in no case were
potential metabolites considered. In view of the volatility of the
compound, it can be expected that the toxic effects observed in the
other open systems studied occurred at lower concentrations than
the nominal ones.
Several short- and long-term studies have also been conducted.
Seiler et al. [227] determined the breakpoint of bioinhibition for
a total of 20 strains of bacterial groups prevalent in a waste-
water treatment plant in the chemical industry, i.e., Zoogloea,
Alcaligenes, and Pseudomonas. After one week of static exposure to
2-propanol in an open system at 30 °C, 100% growth inhibition
occurred at concentrations of 80 000 - 100 000 mg/litre of medium.
No analysis for the compound was reported. The cell multiplication
of blue algae (Microcystis aeruginosa) and green algae (Scenedesmus
quadricauda) was just inhibited after 8 days of static exposure to
1000 and 1800 mg/litre water, respectively, in a closed system at
27 °C and a pH of 7 [35, 37]. When water fleas (Daphnia magna)
were exposed to 2-propanol for 16 days in a semi-static test at
19 °C and a water hardness of 100 mg CaCO3/litre, the highest
concentration that did not result in a significant reduction in
growth was 141 mg/litre water. The water was analysed for the
compound just before and after each renewal of test solution [111].
A 7-day LC50 of 7060 mg 2-propanol/litre water was determined for
2 - 3-month-old guppies (Poecilia reticulata) in a semi-static
test. No analysis for 2-propanol was reported for this open system
[141].
7.2 Terrestrial Organisms
7.2.1 Microorganisms
The sensitivity of 4 soil fungi, i.e., Chrysosporium
crassitunicatum, Nannizzia fulva(+), Nannizzia fulva(-), and
Trichophyton equinum, to saturated 2-propanol vapour was
determined at 28 °C and a pH of 7. Mycelial growth of
Chrysosporium was stimulated after 14 days of exposure, while that
of the other strains was inhibited. Sporulation was poor in
Chrysosporium and in Trichophyton, and fair or good in the other
strains [233].
Table 8. Acute aquatic toxicity of 2-propanol
---------------------------------------------------------------------------------------------------------------------------------------
Organism Description Temperature pH Dissolved Hardness Stat/flow Exposure Parameter Nominal Reference
(°C) oxygen (mg CaCO3/ open/closeda period concentration
(mg/litre) litre) (mg/litre)
---------------------------------------------------------------------------------------------------------------------------------------
FRESHWATER
Bacteria Pseudomonas 25 7 stat, closed 16 h TTb 1 050 [37]
putida
Micro- activated 21 7.4-8 stat, closed 3 h 50% inhibition 1 000 [138]
organisms sludge of respiration
rate
Protozoa Entosiphon 25 6.9 stat, closed 72 h TTb 4 930 [36]
sulcatum
Protozoa Chilomonas 20 6.9 stat, closed 48 h TTb 104 [40]
paramecium
Protozoa Uronema 25 6.9 stat, closed 20 h TTb 3 425 [38]
parduczi
Crustacea water flea 20 8 2 250 stat, open 24 h EC50c 9 714 [39]
(Daphnia EC0 5 102
magna) EC100 10 000
Crustacea water flea 22 100 stat 44 h EC50c,d 2 285 [110]
(Daphnia
magna)
Amphibia frog tadpole 20 stat threshold 22 530 [190]
(Rana pipiens) narcotic
concentration
Fish fathead minnow 18-22 4 stat, open 96 h LC50 11 130 [184]
(Pimephales 24 h LC50 11 160
promelas)
Fish fathead minnow 25 7.5 5 45 flow, open 96 h LC50 9 640-10 400f [262]
(Pimephales
promelas)
---------------------------------------------------------------------------------------------------------------------------------------
Table 8. (contd.)
---------------------------------------------------------------------------------------------------------------------------------------
Organism Description Temperature pH Dissolved Hardness Stat/flow Exposure Parameter Nominal Reference
(°C) oxygen (mg CaCO3/ open/closeda period concentration
(mg/litre) litre) (mg/litre)
---------------------------------------------------------------------------------------------------------------------------------------
Fish goldfish 20 6-8 4 280 stat, open 24 h LC50 5 000f [34]
(Carassius
auratus)
Fish golden orfe 20 7-8 5 200-300 stat 48 h LC50 8 970 [127]
(Leuciscus
idus melanotus)
Fish harlequin fish 20 8.1 20 flow, open 96 h LC50 4 200 [251]
(Rasbora 96 h LC10 1 500
heteromorpha
duncker)
Fish Creek chub 15-21 8.3 98 stat, open 24 h LC0 900 [96]
(Semotitus
atromaculatus)
SEA WATER
Bacteria Photobacterium 15 7 stat, closed 2 min TTh 15 000 [43]
phosphoreum 15 stat, closed 5 min 50% light 35 000 [44, 62]
reduction 42 000
Worm Tubifex 20 stat 2 min EC100c 51 080 [56]
tubifex
Crustacea Brine shrimp 24 stat, open 24 h LC50 10 000 [208]
(Artemia
salina)
Crustacea Brown shrimp semi-stat, 96 h LC50 1 150 [26]
(Crangon open
crangon)
---------------------------------------------------------------------------------------------------------------------------------------
a Static or flow-through test; open or closed system. e Concentration at which touching the tadpoles failed to cause
b TT = toxic threshold for inhibition of cell multiplication. motion; exposure period and conditions; and the method of
c Effect is complete immobilization. calculation of the threshold not specified.
d Calculated value based on a quantitative structure-activity f Analysis for 2-propanol reported.
relationship between the n-octanol/water partition coefficient of g Test compound was Imsol A (90% 2-propanol, remainder unknown)
a group of 19 organic chemicals and their anaesthetic potency. h TT = toxic threshold for light reduction.
7.2.2 Insects
The 4-h LC50 for third instar mosquito larvae (Aedes aegypti)
was 25 120 mg/litre water in a static test at 22 - 24 °C [143].
The 48-h LC50 values for the fruit fly strains Drosophila
melanogaster and Drosophila simulans were between 10 200 and 13 340
mg/litre of nutrient medium in static tests [66]. Exposure of
Drosophila melanogaster eggs and larvae to 7850 mg 2-propanol/litre
of nutrient medium caused an 87% decrease in the activity of the
non-specific enzyme alcohol dehydrogenase (EC 1.1.1.1) in the 14-
day-old larvae. At the same time, viability was decreased by 74%.
The development of Drosophila simulans was completely inhibited at
the same concentration. Gas chromatographic analysis revealed
that, after 4 days of exposure, acetone appeared as the 2-propanol
concentration decreased. The appearance of acetone was associated
with an observed decrease in enzyme activity [265]. Anderson &
McDonald [13] also found a decrease in the specific activity of
alcohol dehydrogenase in Drosophila melanogaster after 1 - 4 days
of exposure to 2-propanol. On the other hand, they found that the
stability and concentration of the enzyme increased. The authors
explained the findings as an adaptation of the fruit fly to its
environment.
7.2.3 Plants
The effects of 2-propanol on the rate of seed germination were
investigated on several occasions. Total inhibition of the
germination of barley grains was reached after incubation for 4
days at 18 °C on filter papers, absorbing a solution containing
39 420 mg 2-propanol/litre water [56]. The germination of white
amaranth (Amaranthus albus) seeds was not affected after 5 h of
incubation at 25 °C on filter papers moistened with a solution
containing 36 050 mg 2-propanol/litre of water [49]. Reynolds
[216] measured 50% inhibition of germination in lettuce (Lactuca
sativa) seeds after incubation for 3 days at 30 °C on agar
containing 2100 mg 2-propanol/litre. At 6000 mg/litre, no
germination at all was observed. However, above 18 030 mg/litre,
germination was again observed and reached a maximum of 62% at
26 440 mg/litre. Inhibition of the growth of hypocotyls and
rootlets after 6 days of incubation gradually increased with
concentration. ED50 values were above 36 000 mg/litre.
In a 28-day study on cell suspensions of root sections of
soybean, (Glycine max), at 26 °C and a pH of 5.6, onset of growth
was delayed for 1 and 2 weeks at 2-propanol concentrations of
10 000 and 20 000 mg/litre of nutrient medium, respectively [67].
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single Exposures
8.1.1 Mortality
Available acute mortality data are summarized in Table 9.
Except where otherwise indicated, the vehicle was water or saline
solution. LD50 values for several animal species after oral
administration vary between 4475 and 7990 mg/kg body weight. Using
14-day-old rats of both sexes, Kimura et al. [134] established an
oral LD50 of 4396 mg/kg body weight for undiluted 2-propanol; this
was not significantly different from that obtained for young male
adults (4710 mg/kg body weight) or older male adults (5338 mg/kg
body weight).
8.1.2 Signs of intoxication
Sprague-Dawley rats of both sexes, inhaling 2-propanol for 8 h
at concentrations between 19 680 and 64 206 mg/m3, showed severe
irritation of the mucous membranes and depression of the central
nervous system indicated by subsequent ataxia, prostration, and
narcosis. These effects were concentration- and time-dependent.
Rats surviving the narcosis recovered. Transient paralysis of the
hind limbs was observed at levels between 49 200 and 54 120 mg/m3.
The rats died at and above 44 280 mg/m3, usually within 2 days,
with females dying earlier than males. All rats were autopsied,
including survivors, 15 days after exposure. At non-lethal levels,
congestion of the liver, lung, and spleen were observed, especially
in males. At lethal levels, vacuolation of the liver, acute
pneumonitis, and spleen oedema were found. These effects were most
pronounced in survivors. At 51 660 mg/m3, severe cytoplasmic
degeneration of the liver and oedema of the lung and brain occurred
in all rats [151]. Deep narcosis occurred in rats exposed through
inhalation to 40 mg/litre for 2 h. Significant and lasting
hypothermia was induced in Sprague-Dawley rats exposed for 4 h to
vapour concentrations of 19 680 mg/m3 or more [151].
Rats and mice of unknown strains, exposed orally or through
inhalation to lethal levels of 2-propanol, showed unspecified signs
of irritation and died through respiratory arrest while under
narcosis, usually within 24 h following exposure. Necropsy
revealed oedema, haemorrhage, inflammation, and dystrophy in the
interstitial tissues of parenchymal organs. In the lung,
infiltration, oedema, and thinning of the alveolar walls were
observed [103].
Table 9. Lethality of 2-propanol
---------------------------------------------------------------------------------------------------
Species/strain Sex Route of exposure Observation LD50 LC50 Reference
period (mg/kg body (mg/m3)
weight)
---------------------------------------------------------------------------------------------------
Rat not reported oral 3 days 5 280 - [157]
Sherman rat not reported oral 14 days 5 480 - [239]
Sprague-Dawley rat male oral 7 days 4 710 - [134]
Rat not reported oral 14 days 5 500 - [103]
Mouse not reported oral 14 days 4 475 - [103]
Rabbit not reported oral 3 days 5 030 - [157]
Rabbit male oral 1 day 7 990 [190]
Dog not reported oral 3 days 4 830 - [157]
Wistar rat male intravenous 5 days 1 088 - [247]
H mouse male intravenous 5 days 1 580 - [247]
H mouse female intravenous not reported 1 860 - [56]
Chinchilla rabbit male, female intravenous 5 days 1 184 - [247]
Wistar rat male intraperitoneal 5 days 2 830 - [247]
H mouse male intraperitoneal 5 days 4 868 - [247]
Syrian hamster male intraperitoneal 5 days 3 467 - [247]
Rabbit not reported dermal not reported 12 870 - [239]
Rat not reported inhalation (4 h) 14 days - 72 600 [103]
Sprague-Dawley rat male inhalation (8 h) 15 days - 46 740 [150]
Sprague-Dawley rat female inhalation (8 h) 15 days - 55 350 [150]
Mouse not reported inhalation (2 h) 14 days - 53 000 [103]
---------------------------------------------------------------------------------------------------
The effects of 2-propanol on the mucociliary system of the
trachea [197] and the middle ear [198] were investigated in two
separate studies. Groups of 20 or 24 Hartley guinea-pigs were
exposed to 2-propanol vapour at measured concentrations of 0, 969,
or 13 382 mg/m3 for 24 consecutive hours. Four animals from each
of the 3 groups were killed by decapitation at 12 h, 24 h, and 3,
7, and 14 days after the exposure period. A concentration-related
deterioration of ciliary activity and mucosal degeneration were
observed in both the trachea and in the middle ear. At the lower
exposure level, the effects completely reversed within 2 weeks of
exposure, but, at the higher exposure level, they did not.
2-Propanol can enter the trachea and deeper lung structures by
aspiration through the oral and nasal cavities. Anaesthetized rats
were made to inspire 110 or 140 mg of the compound in water, or 160
mg of the undiluted compound. Survivors were sacrificed 24 h later
for lung examination. At the lower doses, 1 out of 10 rats in each
group died. At the highest dose, 6 out of 10 rats died of cardiac
or respiratory arrest. All controls survived. It was not reported
whether the latter were sham-exposed or not. The average absolute
lung weight in the high-dose group was increased by 75% [94].
8.1.3 Skin, eye and respiratory tract irritation
In primary irritation patch tests on groups of 6 rabbits and 6
Hartley guinea-pigs, 0.5 ml of undiluted 2-propanol was applied to
areas of clipped or abraded skin. No irritation was observed for
up to 48 h after a 4-h exposure [194]. Erythema and changes in
vascular permeability did not occur when the clipped skin of
guinea-pigs was exposed for 2 min to discs of filter paper soaked
in undiluted 2-propanol [240].
Marzulli & Ruggles [182] reported on an interlaboratory
evaluation of the ocular irritancy of 2-propanol, according to
Draize scores, in groups of 6 rabbits, 1 - 7 days after application
of 0.1 ml of a 70% solution on the cornea. One day after exposure,
mean Draize scores were approximately 0.6 for corneal opacity, 0.3
for iritis, 1.5 for conjunctival redness, and 1.3 for chemosis.
The maximum total Draize score was 10.1. In each laboratory,
67 - 97% of the rabbits met the criteria for eye irritation, which
decreased over time. In another Draize test, groups of 6 - 9 New
Zealand rabbits received doses of 70% 2-propanol at levels of 0.01,
0.03, or 0.10 ml per eye. Maximum total Draize scores were 21, 36,
and 37, respectively, the maximum on the Draize scale being 110.
Using irritation categories based on the number of days required
for the effects to disappear, the compound was substantially
irritating (clearing within 14 days) at the higher doses and
moderately irritating (clearing within 7 days) at the lowest dose
[100].
The eye irritancy of 2-propanol was evaluated using Stauffland
albino rabbits, each of which received 0.1 ml of undiluted propanol
in one eye. Eye irritation was scored according to Draize up to 21
days after application. The exact scores were not reported, but
2-propanol was found to be corrosive according to the US EPA
criteria used in 1981, implying corneal involvement and irritation
or eye damage that persisted for more than 21 days after treatment
[188].
2-Propanol was tested on several occasions in in vitro
cytotoxicity assays and the results showed good agreement with
those in vivo [29, 30, 213].
The potency of 2-propanol as a sensory irritant was evaluated,
using a 50% reflex decrease in the respiratory rate of mice (RD50)
as an index. The mice were exposed by their heads only. An
exposure-related effect was found with RD50 values of 12 300 mg/m3
for Swiss OF1 mice and 43 525 mg/m3 for Swiss Webster mice [61, 130].
8.2 Continuous or Repeated Exposures
Groups of rats were exposed to 2-propanol vapour at
concentrations of 0, 100, or 500 mg/m3 air for 5 days/week and
4 h/day over 4 months [103]. Strain, sex, and group size were not
given, but were presumably in accordance with CMEA standards. No
deaths were reported. At the end of 4 months of exposure to 500
mg/m3 air, growth was reduced significantly by 10% and the
respiratory rate was increased by 22%. The white blood cell count
was decreased at both exposure levels in an exposure-related
manner. At 500 mg/m3, this was attributed to a decreased absolute
and relative number of lymphocytes. Decreases in hippuric acid
excretion and in total serum protein, and an increase in blood
acetylcholine were found at both exposure levels. The decrease in
total serum protein was exposure-related and could partly be
accounted for by the observed decrease in alpha1- and alpha2-
globulins and in albumin at 500 mg/m3. Blood glucose levels were
decreased at 500 mg/m3. Macroscopic and microscopic changes,
observed at 500 mg/m3, included irritant effects on the respiratory
system, such as thinning of the alveolar walls, perivascular
infiltration, pneumonia, and bronchitis. Dystrophic changes and
perivascular cell reactions were seen in the liver. Follicular
hyperplasia was observed in the spleen [103].
Another report described the continuous exposure of groups of
15 rats of unknown strain and sex to 2-propanol vapour at
concentrations of 0, 0.6, 2.5, or 20 mg/m3 air for 86 days. The
data were not statistically analysed. No deaths were reported. At
the highest exposure level, changes in the latency period of
unconditional reaction and an increase in the number of fluorescent
leukocytes were found. Effects reported at this exposure level
included an increase in sulfobromophthalein retention and decreased
blood levels of nucleic acids. Microscopy only revealed adverse
effects at 20 mg/m3 with liver dystrophy, degenerative changes in
the cerebral cortex, and spleen hyperplasia [19].
These studies [19, 103] lack a number of essential details
concerning the protocol, the effects observed, the incidence of
these effects, and the statistical analysis.
A "coefficient of accumulation" (the ratio between cumulative
LD50 and single dose LD50) reported for repeated oral exposure to
2-propanol was 4.0 in rats and 4.9 in mice [10].
Groups of 5 white rats of each sex and of unknown strain
received drinking-water containing 2-propanol for 27 weeks.
Average daily intakes were 0, 600, or 2300 mg/kg body weight for
males and 0, 1000, or 3900 mg/kg body weight for females. Only
males died: 2 at the lower dose and 3 at the higher dose. It was
reported that extensive postmortem changes prevented an accurate
diagnosis of the cause of death. At the end of the exposure
period, all exposed females showed growth retardation. Males
showed a slight decrease in growth initially but overtook the
controls in body weight towards the end of the exposure period. No
adverse effects were found on food intake, behaviour, and
histopathology [157].
8.3 Neurotoxicity and Behavioural Effects
2-Propanol passes the blood-brain barrier twice as effectively
as ethanol (section 6.2.1). The oral ED50 for narcosis in rabbits
exposed to 2-propanol was 2280 mg/kg body weight, i.e., 2.5 times
lower than that for ethanol [190]. The threshold of an acute
effect on the CNS was observed in rats after a 4-h exposure at 1450
mg/m3 using the method of flexor reflexes, the "summation threshold
method" [10]. The intraperitoneal ED50 for loss of righting reflex
in Swiss Webster mice, which was 164 mg/kg body weight, was 1.8
times lower than that for ethanol [169]. The threshold for the
induction of ataxia in Sprague-Dawley rats following intraperitoneal
exposure was 1106 mg/kg body weight [171]. In 2 tilted plane
tests, the performance of rats decreased by an average of 30 - 40%
after oral or intraperitoneal exposure to 2000 and 1800 mg/kg body
weight, respectively [178, 270]. On a molar basis, 2-propanol was
2.7 times as intoxicating as ethanol [270]. When C57BL/6J or
DBA/2J mice were exposed intraperitoneally to a single dose of 2-
propanol at 3 dose levels, the former strain showed increased
activity in the open field test at 392 mg/kg body weight, no effect
on activity at 785 mg/kg body weight, and narcosis at 1570 mg/kg
body weight. DBA/2J mice showed increased activity at the middle
dose level and narcosis at the high dose level [243]. Wistar rats,
inhaling 2-propanol at a concentration of 739 mg/m3 for 6 h/day, 5
days/week, for up to 15 weeks, did not show any adverse effects in
the open field test [224].
The depressive action of 2-propanol on the central nervous
system was related by several investigators to interactions with
neuronal membranes in vitro and in vivo. Lyon et al. [169]
observed a high correlation between the narcotic potencies of
aliphatic alcohols in mice and their ability to disorder brain
synaptosomal plasma membrane in vitro, as measured by electron
paramagnetic resonance, which was, in turn, related to membrane
solubility. In Sprague-Dawley rats, no change in synaptosomal
membrane fluidity was measured via in vitro registration of the
fluorescence polarization of 1,6-diphenyl-1,3,5-hexatriene, 20 h
after a single oral dose of 3000 mg/kg body weight. When
2-propanol was added subsequently, a change in membrane fluidity
was observed. This effect was related to membrane solubility.
The activity of synaptosomal Na+/K+-transporting
adenosinetriphosphatase (EC 3.6.1.37) was increased both in vitro
and in vivo [24].
Functional loss due to disruption of membrane integrity by
2-propanol was also observed in vitro by the blocking of the action
potentials of sciatic nerves in the toad, Bufo marinus [214], and
by the inhibition of membrane-bound guanylate cyclase (EC 4.6.1.2)
in intact murine neuroblastoma N1E-115 cells [241]. It was also
indicated by the interference of 2-propanol with the transport of
Ca2+ ions across biological membranes, as shown, in vitro, by the
inhibition of Ca2+ ion-induced contractions of guinea-pig ileum
(Yashuda et al., 1976), and, in vivo, in rats by a decrease in
regional brain Ca2+ ion levels, 30 min after one intraperitoneal
dose of 2000 mg/kg body weight [221].
Several neurochemical parameters were measured in the brain or
spinal cord axon of Wistar rats, inhaling 2-propanol at a
concentration of 739 mg/m3 air for 6 h/day, 5 days/week, over 5,
10, 16, or 21 weeks. In cerebellar homogenates, the activities of
superoxide dismutase (EC 1.15.1.1) and azoreductase (EC 1.6.6.7)
were decreased at weeks 16 and 21, but no effects were found on the
activity of dihydrolipoamide dehydrogenase (EC 1.8.1.4). In
cerebellar glial cells, the activity of acid proteinase (EC
3.4.21.1) was stimulated at weeks 5 and 10, while the glutathione
concentration was unaffected at all times. In the spinal cord
axon, the phosphate/cholesterol ratio of lipid was reduced by 25%
[224].
8.4 Biochemical Effects
8.4.1 Effects on lipids in liver and blood
Oral administration of single doses of 3000 or 6000 mg
2-propanol/kg body weight to Wistar rats caused a reversible
accumulation of triglycerides in the liver [21, 22, 23, 90]. At
the 6000 mg/kg body weight dose, a slight fatty infiltration was
observed histologically [90]. Observations that could explain this
effect were increased hepatic uptake of palmitate [23], increased
incorporation of palmitate into hepatic triglycerides, decreased
hepatic palmitate oxidation, and, at a later stage of intoxication,
interference with the synthesis and excretion of very dense
lipoproteins [21, 22, 23].
Decreased palmitate oxidation was related to the observed
increase in the hepatic b-hydroxybutyrate/acetoacetate ratio,
implying a decrease in the intramitochondrial NAD+/NADH ratio [21,
23, 195]. Excess extramitochondrial reducing equivalents,
indicated by an increased lactate/pyruvate ratio, was also observed
in vivo [21] and in vitro [85]. However, all these changes in the
redox state of the liver were very modest and therefore were not
thought to be fully responsible for the fatty livers induced.
More significant seemed to be the observation that the
incorporation of palmitate and glycerol into serum triglycerides
and the incorporation of palmitate into serum and hepatic
phospholipids were inhibited. This indicates an impaired secretion
of lipo-proteins, partly explained by the observed disturbance of
the biosynthesis of phospholipids, and partly by the observed
inhibition of hepatic protein synthesis [21, 22, 23]. Inhibition
of hepatic protein synthesis, as shown by the synthesis of the
marker enzymes ornithine decarboxylase (EC 4.1.1.17) and tyrosine
amino-transferase (EC 2.6.1.5), was observed in hepatectomized rats
following oral exposure to 2300 mg 2-propanol/kg body weight [205].
Acetone administration to rats up to a blood level comparable
to that in 2-propanol-dosed rats only slightly increased the level
of liver triglycerides; therefore acetone does not seem to play a
major role in the induction of fatty liver by 2-propanol [22].
8.4.2 Effects on microsomal enzymes
Oral exposure of Sprague-Dawley rats to 390 mg 2-propanol/kg
body weight, once or on 4 subsequent days, caused a slight but
significant increase in the hepatic cytochrome P-450 content and a
2 - 3-fold increase in the activities of the microsomal liver
enzymes (EC 1.14.14.1) aniline hydroxylase and 7-ethoxycoumarin
O-deethylase, 18 h after exposure [255]. Direct activation of these
enzymes does not offer a satisfactory explanation, as it was shown
in vitro that 2-propanol is an inhibitor of several microsomal
enzymes via an interaction with cytochrome P-450, which causes a
change in the reverse Type I spectrum [8, 57, 222, 246, 254, 285].
It was therefore concluded that, as acetone also does not appear to
be involved in aniline hydroxylase induction in vivo, 2-propanol
induces one or more forms of cytochrome P-450 [255]. In young
Sprague-Dawley rats, receiving 2-propanol once at a level of 1960
or 3140 mg/kg body weight and sacrificed 22 - 24 h later, the
hepatic cytochrome P-450 content increased, as well as the
activities of several nitrosamine N-demethylases, benzphetamine
demethylase, ethylmorphine demethylase, p-nitroanisole demethylase,
and 7-ethoxycoumarin- O-deethylase. A slight increase was observed
in the activity of NADPH cytochrome c reductase (EC 1.6.2.4).
Acetone appeared to play a role in this induction. From several
lines of observations, it was concluded that the enhanced activity
of nitrosodimethylamine N-demethylase was due to the induction of
specific forms of P-450 isozymes and P-450-dependent enzymes [255].
In several earlier studies on rats, at comparable or higher
oral doses, induction of microsomal liver enzymes was observed,
including a slight induction of NADPH cytochrome c reductase, but
no increase in hepatic cytochrome P-450 content [206, 207, 234].
Ueng et al. [255] explained the latter observation by suggesting
that the species of cytochrome P-450 induced must be normally
present in low concentrations, so that a several-fold increase in
catalytic activity could be induced without markedly increasing the
total content of the haemoprotein.
Sprague-Dawley rats were also exposed by inhalation to
2-propanol at concentrations of 490, 4920, or 19 680 mg/m3 air for
6 days/week, 6 h/day over 2 weeks. In the liver and kidneys, the
contents of cytochrome P-450 and cytochrome b5 were increased as
well as the activity of NADPH cytochrome c reductase at 4920 and
19 680 mg/m3. These effects were completely reversible in the
liver of rats exposed at 19 680 mg/m3 for 2 weeks and allowed to
recover for 4 weeks. However, they persisted in the kidneys [290].
8.4.3 Other biochemical findings
Differential effects have been observed on the glutathione
status of the liver following exposure of rats to 2-propanol. In
Sprague-Dawley rats inhaling 2-propanol at a concentration of 4920
or 19 680 mg/m3 for 6 days/week, 6 h/day, over 2 weeks, the reduced
glutathione concentration in the liver and kidneys increased
slightly while, at 19 680 mg/m3, the activity of glutathione
S-transferase (EC 2.5.1.18) increased by 13% in the liver only
[290]. The glutathione level in the liver of Wistar rats that had
received a single oral dose of 3110 mg 2-propanol/kg body weight
was reduced 6 h following exposure, while there was a 10% increase
in lipid peroxidation, as indicated by the formation of diene
conjugates [264]. A homogenate of normal or regenerating rat liver
did not show any increase in malondialdehyde production after
incubation with 2-propanol [6].
The activity of hepatic and kidney alanine aminotransferase (EC
2.6.1.2) in rats was unchanged 18 h after exposure to 1 or 4 daily
doses of 392 mg 2-propanol/kg body weight [255] or 4 h after
exposure to a single dose of 2300 mg/kg body weight [205]. Liver
damage was not observed in guinea-pigs histopathologically or by
any increase in serum ornithine carbamoyltransferase (EC 2.1.3.3),
24 h after receiving intraperitoneal doses of up to 1000 mg
2-propanol/kg body weight [73].
In Charles River rats, 2-propanol (785 mg/kg body weight ip)
induced hepatic metallothionein and caused low blood zinc levels
[244]. These changes were not prevented by adrenalectomy.
8.5 Immunological Effects
2-Propanol and acetone both enhanced the incorporation of
labelled thymidine into concanavalin A-stimulated murine spleen
cells in vitro [210]. 2-Propanol inhibited the killing of YAC-1
tumour cells by natural killer effector cells from mouse or rat
spleens [219]. Inhibition of the synthesis and/or the secretion
and/or function of at least one monocyte-derived substance that
inhibited cell proliferation was suggested as an explanation of
these findings [210].
8.6 Reproduction, Embryotoxicity, and Teratogenicity
Groups of 13 - 15 pregnant Sprague-Dawley rats were exposed to
2-propanol for 7 h/day on gestation days 1 - 19 at measured
concentrations of 9001, 18 327, or 23 210 mg/m3 (3659, 7450, or
9435 ppm). At the highest concentration, rats were completely
narcotized at the end of the first exposures, but by the end of the
19 days the effect was slight. Initial exposures to 18 327 mg/m3
(7450 ppm) caused an unsteady gait, but this was not noticeable by
the end of 19 days of exposure. Food consumption and maternal body
weight gain were significantly reduced by exposure to 18 327 and
23 210 mg/m3 (7450 and 9435 ppm). In the group exposed to 23 210
mg/m3 (9435 ppm): 6 out of 15 mated rats were not pregnant at
term, which was attributed to an exposure-induced failure of
implantation; 4 out of 9 pregnancies were totally resorbed; and
resorptions per litter were significantly increased. Fetal body
weights were significantly reduced, in a concentration-related
pattern, at all 3 concentrations. The incidence of cervical ribs
was significantly increased in the 23 210 mg/m3 (9435 ppm) group
[193].
A group of 6 female and 3 male rats (38 - 40 days old), of
unspecified strain, received drinking-water containing 2-propanol.
They were mated after approximately 2 months of exposure. This
schedule was repeated through 2 further generations. Average daily
intakes for the 3 generations were 1470, 1380, and 1290 mg/kg body
weight, respectively. The growth of the first generation was
retarded initially, but returned to normal by the 13th week.
Otherwise, no effects were found on growth and reproductive
functions [159].
When female hybrid rats received oral doses of 252 or 1008 mg
2-propanol/kg body weight per day for 45 days, the length of the
estrus cycle was increased by 23 - 24% [15]. Five female hybrid
rats also received daily doses of 1800 mg/kg body weight for 3
months before mating. Together with 6 controls they were
sacrificed on day 21 of pregnancy. The total embryonic mortality
rate was increased 2-fold, which was statistically significant [15].
In a 6-month drinking-water study, 2-propanol was administered
to hybrid rats of both sexes, at daily doses of 0.018, 0.18, 1.8,
or 18 mg/kg body weight. Mating of males with 5 - 7 females per
group occurred after the exposure period. When both sexes were
exposed to 18 mg/kg body weight, the litter size was increased.
The neonatal mortality rate was increased at 0.18 and 1.8 mg/kg,
when females only were exposed, at 18 mg/kg body weight, when males
only were exposed, and at the 2 highest exposure levels, when both
sexes were exposed. Neonates from the last group showed a dose-
related decrease in the rate of reaction to electric stimuli
(unconditioned defence reaction), but no other effects on their
development [15].
Groups of 10 - 13 female hybrid rats received 252 or 1008 mg
2-propanol/kg body weight from day 1 to day 20 of pregnancy. They
were sacrificed on day 21 of pregnancy together with 11 controls.
Litter size was reduced at both exposure levels. General embryonic
toxicity was 18 and 31%, respectively (10% in controls). At 1008
mg/kg body weight, total embryonic mortality rate was trebled and
10 out of 70 fetuses showed developmental anomalies compared with
none out of 90 controls. The anomalies found were brain damage in
3 fetuses, kidney damage in 5 fetuses, and gastrointestinal damage
in 2 fetuses. The lesions were not specified further. Data on
maternal toxicity were not reported [15].
8.7 Mutagenicity
A reverse mutation spot test with the Salmonella typhimurium
strains TA 98, TA 100, TA 1525, and TA 1537 was negative at 0.18 mg
per plate, with and without metabolic activation by S9 rat liver
[82].
Rat bone marrow cells were examined after 4 months of exposure
of rats to 2-propanol vapour at concentrations of 0, 1.03, or 10.2
mg/m3 air for 4 h/day. Statistically significant increases were
counted in the percentage of mitotic aberrations [18]. However,
the authors did not report the number of rats exposed, their sex,
or strain.
Root tip cells of onion (Allium cepa) exposed to 2-propanol
showed a 2.8-fold increase in the mitotic aberration rate [230].
2-Propanol did not increase sister chromatid exchange
frequencies in vitro in V79 Chinese hamster lung fibroblasts, with
or without S9 mix, when tested at concentrations of 3.3, 10, 33.3,
and 100 mmol/litre [267].
A dose-related increase in the inhibition of metabolic
cooperation in hamster V79 cells, a phenomenon believed to reflect
carcinogenic promotion ability and not to be indicative of
genotoxic potential, was observed by Chen et al. [53]. This may be
due to the membrane effects of 2-propanol.
8.8 Carcinogenicity
Several limited carcinogenicity studies on the mouse have been
conducted with 2-propanol, using the inhalation, dermal, and
subcutaneous routes of exposure.
Groups of 3 month-old, male C3H, ABC, and C57/BL mice were
exposed to 2-propanol vapour at a concentration of 7700 mg/m3 air
for 3 - 7 h/day, 5 days/week, over 5 - 8 months. The sizes of the
experimental and control groups were not reported. The occurrence
of lung tumours was examined microscopically in mice that had
survived until killed at the age of 8 months (36 exposed and 69
control C3H mice, 49 exposed and 120 control ABC mice), or 12
months (47 exposed and 52 control C57/BL mice) and showed
macroscopic lesions. The mice were not examined for the occurrence
of sinus tumours as occurred in workers engaged in the manufacture
of 2-propanol (section 9.2.1). No excess of lung tumours was
observed [276].
2-Propanol was painted on the clipped backs of 30 Rockland
mice, 3 times/week for 1 year. Sex, dose, and observation period
were not specified. Skin papillomas developed in 3 control mice,
one of which also had a skin carcinoma, while there was no response
in treated mice (Weil, C.S., written communication to US NIOSH [257]).
Groups of 3-month-old male C3H, ABC, and C57/BL mice received
20 mg undiluted 2-propanol subcutaneously once a week, for
20 - 40 weeks. The sizes of the treated and untreated control
groups were not reported. Surviving mice that showed macroscopic
lung lesions were examined microscopically for lung tumours. No
excess of lung tumours was observed. Lung tumour incidence in the
control group was high [276].
Doses of 20 mg undiluted 2-propanol were injected
subcutaneously in 40 C3H mice, once a week, over a period of 20
weeks. Controls were untreated. The occurrence of lung tumours
was examined in mice that survived until killed 5 months after the
first injection (22 exposed and 33 control mice). No excess of
lung tumours was observed (Weil, C.S., written communication to US
NIOSH [257]).
In these studies, only lung tumours and, in the case of dermal
exposure, skin tumours were investigated. Moreover, the exposure
periods, where given, were too short to allow for tumour induction.
Experimental details including size of experimental and control
groups, sex ratio, and observation periods were not quoted for some
of the studies. Because of these shortcomings, the studies are
inadequate for the assessment of carcinogenic potential.
8.9 Factors Modifying Toxicity
As a result of the induction of specific isozymes of cytochrome
P-450 (section 8.4.2) by 2-propanol and/or acetone, the
hepatotoxicity of carbon tetrachloride, 1,1,2-trichloroethane,
chloroform (CHCl3), trichloroethylene, and dimethylnitrosamine in
rodents is enhanced [60, 165, 234, 252, 253, 254]. The metabolic
activation of n-hexane by liver and kidney microsomes is also
increased by 2-propanol pretreatment [290].
9. EFFECTS ON MAN
9.1 General Population Exposure
9.1.1 Poisoning incidents
The subject of non-beverage alcohol use has already been
addressed in section 5.2.2. The clinical literature has been
reviewed [149, 176, 245, 163]. A brief summary of cases of
intoxication and major symptoms is presented in Table 10.
Table 10. Acute intoxications by 2-propanola
------------------------------------------------------------
No. of No. of Observationsb Reference
cases alcoholics 1 2 3 4 5 6 7
------------------------------------------------------------
Ingestion
57 46 +57 [11]
5 2 +5 +5 +4 +2 +4 +2 [4]
1c 0 - + + + + + + [186]
3 3 - +3 - +2 +2 +1 +2 [170]
1 0 - + + + + + - [191]
2 0 - +2 +2 +2 +1 [149]
1 1 - + + - + - + [245]
17 14 - +2 +3 +10 - - [131]
1 - + + + - [88]
1 1 - + + + [135]
1c 0 - + + - + - - [266]
1 1 - + + - - + [261]
1 0 - + + [75]
1 0 - + - - - [220]
2 1 - - + +2 - +2 +1 [5]
1 1 - - - + - + - [128]
1 1 - - - + + [108]
Sponging
1c - - + + + + + [228]
1c - - + + + - - + [160]
1c - - + + + [91]
1c - - + + - - + [172]
1c - - + [181]
1c - - + + + + - + [16]
--------------------------------------------------------------
a +N = observed in N cases; - = not observed; no sign = not
reported.
b 1 = death; 2 = coma; 3 = hypotension, meaning < 100 mm Hg systolic
pressure and/or < 60 mmHg diastolic pressure; 4 = tachycardia,
meaning > 100 beats per min; 5 = hypothermia, meaning < 36.5 °C;
6 = gastritis, characterized by nausea, vomiting, abdominal pain;
and/or gastric haemorrhage; 7 = elevated blood glucose, meaning
> 1500 mg/litre.
c Child below 2.5 years of age.
Intoxications have been reported after oral ingestion (Table
10), rectal administration [59], and inhalation by children who
have been sponged for several hours with 2-propanol preparations
for fever reduction (Table 10). Skin absorption in 2-propanol
sponging has been deemed insignificant [e.g., 149, 176]. However,
a case report on a child with an almost lethal blood level of
2-propanol after sponge bathing for fever reduction, and
questionable ingestion, suggested to the authors that the
importance of dermal absorption, compared with inhalation, should
not be underestimated [181].
2-Propanol depresses the central nervous system. At comparable
doses, 2-propanol is thought to be approximately twice as active as
ethanol in this respect with a longer duration of the coma due to
slower metabolism and to the contribution of acetone to the
depression of the central nervous system [149, 163, 176, 245]. As
with ethanol, excessive intoxication with 2-propanol causes
unconsciousness, usually, but not always ending in a deep coma [5,
59, 108, 128]; death may follow due to respiratory depression [4].
In most cases, decreased or absent reflexes are reported. Pupil
size is variable, but most often miotic [16, 88, 149, 220, 228,
266]. Early gastrointestinal problems and hypothermia frequently
occur. Cardiovascular effects include severe hypotension, shock
[4, 266], and even cardiac arrest [176, 266]. Tachycardia is found
as a secondary effect. Other possible compound-related effects are
hyperglycaemia, elevated protein levels in cerebrospinal fluid [5,
170, 266], and atelectasis [191, 220]. Lung congestion was
observed in fatal cases [4, 11].
In cases of 2-propanol intoxication, hypotension can be a grave
prognostic sign [4], which some authors suggest requires immediate
action by gastric lavage, haemodialysis [5, 22, 88, 135, 176], or
peritoneal dialysis [75, 186, 245]. Following treatment, recovery
is usually complete, unless the hypotension is severe and persistent.
In such cases, renal injury and death may occur [4, 128].
Acetone can be detected in the blood, urine, and breath.
Because other ketone bodies are not found in significant quantities,
early acidosis does not usually occur, serum bicarbonate, anion
gap, and blood pH remaining normal. Sometimes, a mild metabolic
acidosis and anion gap may develop as a result of lactic acid
accumulation. Acetonaemia and/or acetonuria without metabolic
acidosis and with normal or slightly elevated blood glucose levels
differentiate 2-propanol poisoning from diabetic ketoacidosis and
poisoning by other alcohols [16, 163, 176]. In addition, a
significant osmolality gap may be observed [16, 261].
None of the biochemical findings could be related to blood
levels of 2-propanol or acetone. 2-Propanol levels, measured at
different times after exposure, varied between undetectable
concentrations and 5600 mg/litre [176]. Acetone levels varied
between undetectable concentrations and 18 780 mg/litre [75].
Alexander et al. [11] suggested that combined levels of 2-propanol
and acetone may allow greater accuracy in predicting the clinical
course of the condition of a patient.
Adults have been reported to survive ingestion of 700 ml of
2-propanol [88] and to die after ingestion of 400 ml of 2-propanol
[4]. The lowest dose reported to be life-threatening was in an
18-month-old child who ingested approximately 170 ml of 2-propanol
[186].
9.1.2 Controlled exposures
No adverse effects were observed in groups of 8 healthy male
volunteers (24 - 57 years of age) who drank a daily dose of 2.6 or
6.4 mg 2-propanol/kg body weight in diluted syrup for 6 weeks.
Investigations included haematology, blood chemistry, urinalysis,
and ophthalmoscopy [283].
Another group of 10 healthy male volunteers (age not stated)
was exposed to 2-propanol vapour at nominal concentrations of 490,
980, or 1970 mg/m3 air for 3 - 5 min. After each exposure, the
participants were asked to classify the effects of the vapour on
the eyes, nose, and throat. The volunteers judged irritation to be
"mild" at 980 mg/m3 and "not severe" at 1970 mg/m3. They also
judged 490 mg/m3 to be "satisfactory" for their own 8-h
occupational exposure [192]. The validity of these results is
doubtful, for various reasons including the subjective criteria
used, the lack of control exposures, and the unreliability of the
exposure levels.
9.1.3 Skin irritation; sensitization
No skin irritation was observed in 6 human volunteers when 0.5
ml of undiluted 2-propanol was applied to a 4 cm square area on the
back and evaluated after 4 h, 24 h and 48 h [194]. Closed patch
tests with 10-min applications of 0.1 - 0.3 ml undiluted 2-propanol
on the dry skin of 10 healthy volunteers and 12 persons receiving
disulfiram therapy for the treatment of chronic alcoholism did not
result in any reaction. After immersion of the skin in tepid water
for 10 min, a transient erythema rapidly appeared on the site of
application in 19 subjects [105].
Skin irritation was also reported in a total of 6 premature
infants with a gestational age of less than 27 weeks. They were
exposed via swabs used for conduction in ECG recording or by the
application of 2-propanol on the umbilical area with subsequent
soaking of the diaper. Erythema and second or third degree burns
or blisters were observed in areas of prolonged contact with
2-propanol. Hypoperfusion of the skin was suggested to be a
contributing factor [226, 277].
In 1968, Wasilewski [272] reported a case of alleged allergic
contact dermatitis following skin disinfection with a 70%
2-propanol swab. The patient was treated for allergic rhinitis.
Patch tests were positive for 2-propanol. However, controls were
not tested and there was no information on the purity of the
alcohol. This report was followed by several others concerning a
total of 8 persons who showed dermatitis after contact with medi-
swabs containing 2-propanol. Patch testing revealed that another
volatile agent, presumably propylene oxide, caused the effect [174,
124, 217]. Only one of these 8 persons was also hypersensitive to
2-propanol of unknown purity [124].
Itching eczematous lesions developed in a laboratory worker, in
a company manufacturing hair cosmetics, in patch tests with
chemically pure 2-propanol solutions (2.5 - 99.7% by volume). This
person also reacted to 1-propanol, 1-butanol, 2-butanol, and
formaldehyde, but not to ethanol and methanol. Controls were not
tested [167]. Two out of 4 confirmed cases of hypersensitivity to
primary alcohols were also found to be hypersensitive to secondary
alcohols. Both cases had a history of contact allergies.
Recurrent exposures to 2-propanol and/or other alcohols occurred
through consumption of alcoholic beverages, pre-injection
disinfection, via a hair lotion in one case and occupationally in
the other case. Patch tests were positive for pure undiluted
2-propanol and 2-butanol. Twenty controls did not show any
reactions to secondary alcohols [86, 87]. The mechanism of alcohol
hypersensitivity is not clear. Fregert et al. [87] pointed out
that it is difficult to understand how alcohols can act as haptens.
9.2 Occupational Exposure
9.2.1 Epidemiology studies
A retrospective cohort study was reported among 182 workers at
a plant, in the USA, manufacturing 2-propanol by the strong acid
process over the period 1928 - 50. In a subgroup of 71 men, who
had been employed for more than 5 years, 7 cases of cancer were
observed: 4 cancers of the paranasal sinuses, 1 lung carcinoma, 1
laryngeal carcinoma, and 1 laryngeal papilloma. The minimum
latency period was 6 years [276]. According to USA vital
statistics for 1948, 0.0014 paranasal sinus cancers would have been
expected for the total cohort [286].
Two cases of paranasal sinus cancer and 2 cases of laryngeal
cancer were reported in 1966 in a cohort of 779 workers in a
similar plant in the USA that had been in operation since 1927.
The minimum latency period was 10 years. The age- and sex-adjusted
incidence of sinus and laryngeal cancer in this group was 21 times
higher than expected [119].
Another retrospective cohort study was undertaken among 262 men
who had worked for at least one year in a 2-propanol-manufacturing
plant in the United Kingdom using the strong acid procedure over
the period 1949 - 76. There were more than 4000 person-years at
risk. No person was lost to follow-up, which was over an average
of 15.5 years. The mortality rates due to all causes and due to
neoplasms were not significantly higher than expected according to
national vital statistics. One person died from nasal cancer
against 0.02 expected. Other significant findings were 2 "kidney
and adrenal malignancies" and 2 cancers of "the brain and the
central nervous system" [9].
A fourth retrospective cohort study was conducted over the
years 1966 - 78 among 433 workers in a 2-propanol manufacturing
plant, in the USA, who were exposed for at least 3 months during
the period 1941 - 65. The strong acid process used in 1941 had
been gradually changed to the weak acid process by 1965. More than
11 000 person-years were at risk. The mortality rate due to all
causes was lower than expected on the basis of state vital
statistics. No excess mortality due to all cancers was observed,
but the incidence of buccal and pharyngeal cancer was 4 times
higher than expected (2 versus 0.5). There was a slight excess of
lung cancer (7 versus 5.94) [79].
These cohort studies collectively suggest a cancer hazard
related to the strong acid manufacturing process. The excess in
respiratory cancers was initially attributed to isopropyl oils
[119, 276]. However, the experimental basis for this assumption is
weak (section 8.8) and more recently diisopropyl sulfate, an
intermediate produced in the strong acid process, has been proposed
as a more likely causative agent. The concentration of this
chemical is high in the strong acid process and low in the weak
acid process [79, 286].
Two small case-control studies were conducted in the USA: one
to consider the risk of lymphocytic leukaemia associated with 24
solvents among rubber industry workers [52], and the other to
consider the risk of brain gliomas associated with exposure
conditions during work at a chemical plant [155]. Although there
was no evidence of an association between exposure to 2-propanol
and the incidence of gliomas or lymphocytic leukaemia, the small
number of subjects and multiple exposures mean that no conclusions
can be drawn from these studies.
9.2.2 Interacting agents
Fourteen out of 43 workers in a 2-propanol-packaging plant
became ill when carbon tetrachloride was used for cleaning
equipment. The incidence and the severity of the effects increased
where exposure to carbon tetrachloride was the highest, and where,
on another occasion, the mean concentration of 2-propanol in air
was also highest, i.e., 1010 mg/m3. In 4 cases, renal failure or
hepatitis developed [83]. Another similar incident was reported in
a colour printing factory, 17 workers had abnormal liver function
and 3 developed acute hepatitis following combined exposure to
carbon tetrachloride and 2-propanol [71]. These reports suggest
potentiation of the toxicity of carbon tetrachloride by 2-propanol,
which was also found in experimental animals (section 8.9).
Some shaving lotions containing 2-propanol and other ingredients
(e.g., menthol, camphor, methyl salicylate, naphthalene) have been
reported to produce central stimulation with motor restlessness,
extreme apprehension, hallucinations, and general disorientation
[89, 238].
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of Human Health Risks
10.1.1 Exposure
Exposure of human beings to 2-propanol may occur through
inhalation during manufacture, processing, and both occupational
and household use. Average concentrations of up to 35 mg/m3 in the
ambient urban air and up to 500 mg/m3 in the occupational
environment have been measured. Concentrations between 0.2 and 325
mg/litre have been found in non-alcoholic beverages, and between 50
and 3000 mg/kg in foods (section 5).
Exposure of the general public to a potentially lethal level
may result from accidental or intentional ingestion and children
may be exposed when sponged with 2-propanol preparations (rubbing
alcohol) (sections 6 and 9).
10.1.2 Health effects
2-Propanol is rapidly absorbed after inhalation or ingestion
and distributed throughout the body as such, sulfated, or as its
metabolite, acetone (section 6). The possibility of dermal
absorption should not be neglected.
The acute toxicity of 2-propanol for animals is low (based on
lethality estimates) whether exposure occurs via the dermal, oral,
or respiratory route.
In man, the most likely acute effects of exposure to high
levels of 2-propanol by ingestion or inhalation are alcoholic
intoxication and narcosis. The results of animal studies indicate
that 2-propanol is approximately twice as intoxicating as ethanol
with an oral ED50 for rabbits of 2280 mg/kg and a threshold for
ataxia in rats of 1106 mg/kg intraperitoneally (section 8.3). Oral
LD50 values in various species vary between 4475 and 7990 mg/kg and
inhalation LC50 values between 50 000 and 70 000 mg/m3 (section
8.1.1).
Because ethanol retards the elimination of 2-propanol and is
also a CNS depressant, interaction between ethanol and propanol may
be expected to increase the CNS effects of either agent. The
majority of acute intoxication cases involved ingestion by known
alcoholics. Febrile children have experienced life-threatening
intoxications when treated by skin sponging with 2-propanol. In
these cases, skin absorption may also be an important route of
exposure in addition to inhalation (section 9.1.1).
Exposure-effect data on human beings in an acute overexposure
situation are scarce and show great variation. The major effects
are gastritis, depression of the central nervous system with
hypothermia and respiratory depression, and hypotension (section
9.1.1).
In rabbits, 2-propanol did not irritate the skin but did
irritate the eyes when 0.1 ml undiluted compound was applied
(section 8.1.3). Care is required in the use of 2-propanol as a
disinfectant on premature babies as it may cause severe skin
irritation following prolonged contact.
No adequate animal studies are available to make an evaluation
of the human health risks associated with repeated exposure to
2-propanol. However, 2 studies in rats with inhalation (500 mg/m3,
4 h/day, 5 days per week, for 4 months) or oral (600 - 3900 mg/kg
in drinking-water) exposure suggest that exposure to 2-propanol at
some of the very high occupational exposures reported should be
avoided (section 8.2).
2-Propanol administered in the drinking-water has been tested
for reproductive effects in a number of studies with conflicting
results. In one study, impaired neonatal survival was reported
after 6 months exposure of female rats to 0.18 mg/kg per day. In a
multi-generation study there were no adverse effects at drinking-
water doses as high as 1470 mg/kg per day. In a teratology study,
drinking-water doses of 252 and 1008 mg/kg per day produced
developmental toxicity, but this was not related to maternal
toxicity. Inhalation exposure of pregnant rats to 2-propanol
provided a LOEL of 18 327 mg/m3 (7450 ppm) and a NOEL of 9001 mg/m3
(3659 ppm) for maternal toxicity. In the same study, 9001 mg/m3
(3659 ppm) was a LOEL for developmental toxicity, with no
demonstration of a NOEL (section 8.6). These concentrations are
higher than those likely to be encountered under conditions of
human exposure.
2-Propanol was negative in a bacterial spot test and in a test
for sister chromatid exchange in mammalian cells in vitro. It
induced mitotic aberrations in the bone marrow of rats. Although
these findings suggest that the substance has no genotoxic
potential, no adequate assessment of mutagenicity can be made on
the basis of the limited data available. An in vitro test said to
predict promotional activity was negative (section 8.7).
The data available are inadequate to assess the carcinogenicity
of 2-propanol in experimental animals (section 8.8). There are no
data to assess the carcinogenicity of 2-propanol itself in human
beings. There are adequate data to indicate that the strong acid
process for the production of 2-propanol is causally associated
with the induction of paranasal sinus cancer in human beings,
probably due to exposure to the intermediate, di-2-propyl sulfate,
an alkylating agent and not to 2-propanol itself (section 9.2.1).
2-Propanol is shown to potentiate the hepatic toxicity of
halocarbons, such as carbon tetrachloride. Therefore simultaneous
exposure to 2-propanol and halocarbons should be avoided (section
9.2.2).
The Task Group considers it unlikely that 2-propanol will pose
a serious health risk to the general population under exposure
conditions likely to be encountered.
10.2 Evaluation of Effects on the Environment
By reacting with hydroxyl radicals and through rain-out,
2-propanol will disappear rapidly from the atmosphere, with a
residence time of less than 2.5 days (section 4.2). Hydrolysis and
photolysis are not important in the removal of 2-propanol from
water and soil but removal occurs quite rapidly by aerobic and
anaerobic biodegradation, especially after adaptation of initially
seeded microorganisms (section 4.3.1). Adsorption of 2-propanol on
soil particles is poor but it should be mobile in soil and it has
been shown to increase the permeability of soil to some aromatic
hydrocarbons. In view of the physical properties of 2-propanol,
its potential for bioaccumulation is low (section 4.3.2).
Toxicity in aquatic organisms was observed at levels ranging
from 104 mg/litre for one protozoan to over 50 000 mg/litre for
Tubifex worms. Insects and plant seeds were only affected at
concentrations above 2000 mg/litre (section 7).
On the basis of these data, it can be concluded that, except in
cases of accident and inappropriate disposal, 2-propanol does not
present a risk to naturally occurring organisms at concentrations
that usually occur in the environment.
11. RECOMMENDATIONS
1. 2-Propanol has not shown mutagenic potential in the small number
of assays performed. A full array of modern genotoxicity tests
should be completed.
2. Several studies of the carcinogenic activity of 2-propanol have
been published but all are seriously flawed and cannot be used
to evaluate the potential carcinogenicity of 2-propanol. The
desirability of a carcinogenesis bioassay of 2-propanol should
be considered based on the outcome of genotoxicity tests.
3. Inhalation exposure to overtly toxic concentrations of
2-propanol produced reproductive and developmental toxicity.
Additionally, the data available from drinking-water studies
are conflicting. In view of the potential for environmental
and drinking-water contamination, reproductive and
developmental toxicity should be conducted using oral dosing.
4. Epidemiological studies including precise exposure data would
assist in an assessment of the occupational hazards from
2-propanol.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
An evaluation of the carcinogenicity of 2-Propanol by the
International Agency for Research on Cancera was reported as
follows:
"A. Evidence for carcinogenicity to humans (sufficient for the
manufacture of isopropyl alcohol by the strong-acid process;
inadequate for isopropyl alcohol and isopropyl oils).
An increased incidence of cancer of the paranasal sinuses was
observed in workers at factories where isopropyl alcohol was
manufactured by the strong-acid process. The risk for
laryngeal cancer may also have been elevated in these workers.
It is unclear whether the cancer risk is due to the presence of
diisopropyl sulfate, which is an intermediate in the process,
to isopropyl oils, which are formed as by-products, or to other
factors, such as sulfuric acid. Epidemiological data
concerning the manufacture of isopropyl alcohol by the weak-
acid process are insufficient for an evaluation of
carcinogenicity."
"B. Evidence for carcinogenicity to animals (inadequate for
isopropyl alcohol and isopropyl oils).
Isopropyl oils, formed during the manufacture of isopropyl
alcohol by both the strong-acid and weak-acid processes, were
tested inadequately in mice by inhalation, skin application and
subcutaneous administration. Isopropyl oils formed during the
strong-acid process were also tested inadequately in dogs by
inhalation and instillation into the sinuses.
The available data on isopropyl alcohol were inadequate for
evaluation."
"C. Other relevant data
No data were available to the Working Group."
-------------------------------------------------------------------
a International Agency for Research on Cancer, Overall Evaluations
of Carcinogenicity: An updating of IARC Monographs volumes 1 to
42. Lyon, France, 1987 (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Supplement 7)
REFERENCES
1. ABSHAGEN, U. & RIETBROCK, N. (1969) [Elimination of
2-propanol in dogs and rats.] Naunyn-Schmiedebergs Arch.
Pharmakol. exp. Pathol., 264: 110-118 (in German).
2. ABSHAGEN, U. & RIETBROCK, N. (1970) [The mechanism of the
2-propanol oxidation.] Naunyn-Schmiedebergs Arch. Pharmakol.
exp. Pathol., 265: 411-424 (in German).
3. ACKMAN, R.G., HINLEY, H.J., & POWER, H.E. (1967)
Determination of isopropyl alcohol in fish protein
concentrate by solvent extraction and gas-liquid
chromatography. J. Fish. Res. Board Can., 24: 1521-1529.
4. ADELSON, L. (1962) Fatal intoxication with isopropyl
alcohol (rubbing alcohol). Am J. clin. Pathol., 38: 144-151.
5. AGARWAL, S.K. (1979) Non-acidotic acetonemia: a syndrome
due to isopropyl alcohol intoxication. J. Med. Soc. New
Jersey, 76: 914-916.
6. AGOSTINI, C. (1982) Effects of various inhibitors on lipid
peroxidation by homogenates of normal, regenerating and
hepatomous rat liver, by liver slices and by hepatoma cells.
Med. Biol. Environ., 10: 3-15.
7. AHAMED, A. & MATCHES, J.R. (1983) Alcohol production by
fish spoilage bacteria. J. food Prot., 46: 1055-1059.
8. AKHREM, A.A., POPOVA, E.M., & METELITSA, D.I. (1978)
[Interaction of aliphatic alcohols with cytochrome P-450
from rat liver microsomes.] Biokhimiya (Mosk), 43: 1485-1491
(in Russian).
9. ALDERSON, M.R. & RATTAN, N.S. (1980) Mortality of workers
on an isopropyl alcohol plant and two MEK dewaxing plants.
Br. J. ind. Med., 37: 85-89.
10. ALEKPEROV, I.I. & GUSEINOV, V.G. (1980) Toxicological
characteristics of isopropanol as an industrial poison, All
Union Foundation Conference on Toxicology, Moscow, 25-27
November 1980, p. 33.
11. ALEXANDER, C.B., MCBAY, A.J., & HUDSON, R.P. (1982)
Isopropanol and isopropanol deaths: ten years' experience.
J. forensic Sci., 27: 541-548.
12. ANDERS, M.W. & HARRIS, R.N. (1981) Effect of 2-propanol
treatment on carbon tetrachloride metabolism and toxicity.
Adv. exp. Med. Biol., 136A: 591-602.
13. ANDERSON, S.M. & MCDONALD, J.F. (1981) Effect of
environmental alcohol on in vivo properties of Drosophila
alcohol dehydrogenase. Biochem. Genet., 19: 421-430.
14. ANON. (1987) Chemical market profile: isopropanol. Chem.
Market. Rep., 31 August: 46.
15. ANTONOVA, V.I. & SALMINA, Z.A. (1978) [The maximum
permissible concentration of isopropyl alcohol in water
bodies with due regard for its action on the gonads and the
progeny.] Gig. i Sanit., 43: 8-11 (in Russian).
16. ARDITI, M. & KILLMER, M.S. (1987) Coma following use of
rubbing alcohol for fever control. Am. J. DC, 141: 237-238.
17. ARISTOV, V.N. (1982) [Combined effect of toluene, isopropanol,
and sulfur dioxide in conditions of petrochemical production.]
Gig. Tr. Prof. Zabol., ISS9: 5-9 (in Russian).
18. ARISTOV, V.N., REDKIN, Y.V., BRUSKIN, Z.Z., & OGLEZNEV, G.A.
(1981) [Experimental data on the mutagenous effects of
toluene, isopropanol, and sulfur dioxide.] Gig. Tr. Prof.
Zabol., 25: 33-36 (in Russian).
19. BAIKOV, B.K., GORLOVA, O.E., GUSEV, M.I., NOVIKOV, Y.V.,
YUDINA, T.V., & SERGEEV, A.N. (1974) [Hygienic
standardization of the daily average maximal permissible
concentrations of propyl and isopropyl alcohols in the
atmosphere.] Gig. i Sanit., 4: 6-13 (in Russian).
20. BALD, E. & MAZURKIEWICZ, B. (1980) Analytical utility of
2-halopyridinium salts. Part III. Paper electrophoretic
characterization of alcohols as 2-alkoxy-1-methylpyridinium
p-toluenesulfonates. Chromatographia, 13: 295-297.
21. BEAUGE, F., CLEMENT, M., RENAUD, G., NORDMANN, R., &
NORDMANN, J. (1975) Action de l'isopropanol sur le
métabolisme lipidique chez le rat: études complémentaires
sur les méchanismes impliqués dans l'accumulation hépatique
des triacylglycérides. Arch. int. Physiol. Biochim., 83:
573-591.
22. BEAUGE, F., CLEMENT, M., NORDMANN, J., & NORDMANN, R.
(1977) Etudes comparatives des actions de l'acétone et de
l'isopropanol sur le métabolisme lipidique chez le rat.
Arch. int. Physiol. Biochim., 85: 931-940.
23. BEAUGE, F., CLEMENT, M., NORDMANN, J., & NORDMANN, R.
(1979) Comparative effects of ethanol, n-propanol and
isopropanol on lipid disposal by rat liver. Chem.-biol.
Interact., 26: 155-166.
24. BEAUGE, F., FLEURET, C., BARIN, F., & NORDMANN, R. (1984)
Brain membrane disordering after acute in vivo administration
of ethanol, isopropanol or t-butanol in rats. Biochem.
Pharmacol., 33: 3591-3595.
25. BESS, F.D. & CONWAY, R.A. (1966) Aerated stabilization of
synthetic organic chemical wastes. J. Water Pollut. Control
Fed., 38: 939-956.
26. BLACKMAN, R.A.A. (1974) Toxicity of oil-sinking agents.
Mar. Pollut. Bull., 5: 116-118.
27. BONTE, W., RUDELL, E., SPRUNG, R., FRAUENRATH, C., BLANKE,
E., KUPILAS, G., WOCHNIK, J. & ZAH, G. (1981a)
[Experimental investigations concerning the analytical
detection of small doses of higher aliphatic alcohols in
blood in man.] Blutalkohol, 18: 399-411 (in German).
28. BONTE, W., SPRUNG, R., RUDELL, E., FRAUENRATH, C., BLANKE,
E., KUPILAS, G., WOCHNIK, J., & ZAH, G. (1981b)
[Experimental investigations concerning the analytical
detection of small doses of higher aliphatic alcohols in
human urine.] Blutalkohol, 18: 412-426 (in German).
29. BORENFREUND, E. & BORRERO, O. (1984) In vitro cytotoxicity
assays. Potential alternatives to the Draize ocular allergy
test. Cell Biol. Toxicol., 1: 55-65.
30. BORENFREUND, E. & SHOPSIS, C. (1985) Toxicity monitored
with a correlated set of cell-culture assays. Xenobiotica,
15: 705-711.
31. BOSSET, J.O. & LIARDON, R. (1984) The aroma composition of
Swiss Gruyere cheese. II. The neutral volatile components.
Lebensm.-Wiss. Technol., 17: 359-362.
32. BOUGHTON, L.L. (1944) The relative toxicity of ethyl and
isopropyl alcohols as determined by long term rat feeding
and external application. Am. pharm. Assoc., 33: 111-113.
33. BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979a) BOD and
COD of some petrochemicals. Water Res., 13: 627-630.
34. BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979b) The acute
toxicity of some petrochemicals to goldfish. Water Res., 13:
623-626.
35. BRINGMANN, G. (1975) [Determination of the harmful
biological action of water-endangering substances through
inhibition of cell multiplication in the blue alga
Microcystis.] Ges.-Ing., 96: 238-241 (in German).
36. BRINGMANN, G. (1978) [Determination of the harmful
biological action of water-endangering substances on
protozoa. I. Bacteria fed flagellates.] Z. Wasser-Abwasser
Forsch., 11: 210-215 (in German).
37. BRINGMANN, G. & KUHN, R. (1977) [Limiting values of the
harmful action of water-endangering substances on bacteria
(Pseudomonas putida) and green algae (Scenedesmus
quadricauda) in the cell multiplication inhibition test.] Z.
Wasser-Abwasser Forsch., 10: 87-98 (in German).
38. BRINGMANN, G. & KUHN, R. (1980) [Determination of the
harmful biological action of water-endangering substances on
protozoa. II. Bacteria fed ciliates.] Z. Wasser-Abwasser
Forsch., 13: 26-31 (in German).
39. BRINGMANN, G. & KUHN, R. (1982) [Findings regarding the
harmful action of water-endangering substances on Daphnia
magna in a further developed standardised test.] Z. Wasser-
Abwasser Forsch., 15: 1-16 (in German).
40. BRINGMANN, G., KUHN, R. & WINTER, A. (1980) [Determination
of the harmful biological action of water-endangering
substances on protozoa. III. Saprozoic flagellates.] Z.
Wasser-Abwasser Forsch., 13: 170-173 (in German).
41. BRUGNONE, F., PERBELLINI, L., APOSTOLI, P., BELLOMI, M., &
CARETTA, D. (1983) Isopropanol exposure: environmental and
biological monitoring in a printing works. Br. J. ind. Med.,
40: 160-168.
42. BUFFONI, F., SANTONI, G., ALBANESE, V., & DOLARA, P. (1983)
Urinary mercapturic acid in chemical workers and in control
subjects. J. appl. Toxicol., 3: 63-65.
43. BULICH, A.A. (1979) Use of luminescent bacteria for
determining toxicity in aquatic environments. In: Marking,
L.L. & Kimerle, R.A., ed. Aquatic toxicology, American
Society for Testing and Materials, pp. 98-106 (ASTM STP 667).
44. BULICH, A.A., GREENE, M.W., & ISENBERG, D.L. (1981)
Reliability of the bacterial luminescence assay for
determination of the toxicity of pure compounds and complex
effluents. In: Branson, D.R. & Dickson, K.L., ed. Aquatic
toxicology and hazard assessment. Fourth Conference,
American Society for Testing and Materials, pp. 338-347
(ASTM STP 737).
45. CALHOUN, M.J. & DELLAMONICA, E.S. (1974) Determination of
2-propanol residue in some fruits dewaxed with alcohol
vapors. J. Assoc. Off. Anal. Chem., 57: 1342-1345.
46. CARTER, W.P.L., DARNALL, K.R., GRAHAM, R.A., WINER, A.M., &
PITTS, J.N. (1979) Reactions of C2 and C4 alpha-hydroxy
radicals with oxygen. J. phys. Chem., 83: 2305-2311.
47. CEC (1982) Propan-2-ol: chemico-physical data, toxicity
data, environmental occurrence, and permissible levels. In:
Report of the Scientific Committee for Food on Extraction
Solvents, Brussels, Belgium, Commission of the European
Communities, Directorate General for Internal Market and
Industrial Affairs, pp. 46-72.
48. CEDERBAUM, A.I., QURESHI, A., & MESSENGER, P. (1981)
Oxidation of isopropanol by rat liver microsomes. Possible
role of hydroxyl radicals. Biochem. Pharmacol., 30: 825-831.
49. CHADOEUF-HANNEL, R. & TAYLORSON, R.B. (1985) Anaesthetic
stimulation of Amaranthus albus seed germination:
interaction with phytochrome. Physiol. Plant, 65: 451-454.
50. CHARBONNEAU, M., IJIMA, M., COTE, M.G., & PLAA, G.L. (1985)
Temporal analysis of rat liver injury following potentiation
of carbon tetrachloride hepatotoxicity with ketonic or
ketogenic compounds. Toxicology, 35: 95-112.
51. CHARRETON, M. (1981) Estimation des rejets de vapeurs de
solvants au voisinage d'une usine de peintures. Double
Liaison - Chimie des Peintures, 28: 233-241.
52. CHECKOWAY, H., WILCOSKY, T., WOLF, P., & TYROLER, H. (1984)
An evaluation of associations of leukemia and rubber
industry solvent exposures. Am. J. ind. Med., 5: 239-249.
53. CHEN, T.-H., KAVANAGH, T.J., CHANG, C.C., & TROSKO, J.E.
(1984) Inhibition of metabolic cooperation in Chinese
hamster V79 cells by various organic solvents and simple
compounds. Cell Biol. Toxicol., 1: 155-171.
54. CHEN, W.-S. & PLAPP, B.V. (1980) Kinetics and control of
alcohol oxidation in rats. Adv. exp. Med. Biol., 132:
543-549.
55. CHOU, W.L., SPEECE, R.E., & SIDDIQI, R.H. (1978)
Acclimation and degradation of petrochemical wastewater
components by methane fermentation. Biotechnol. Bioeng.
Symp., 8: 391-414.
56. CHVAPIL, M., ZAHRADNIK, R., & CMUCHALOVA, B. (1962)
Influence of alcohols and potassium salts of xanthogenic
acids on various biological objects. Arch. int. Pharmacodyn.
Ther., 135: 330-343.
57. COHEN, G.M. & MANNERING, G.J. (1973) Involvement of a
hydrophobic site in the inhibition of the microsomal
p-hydroxylation of aniline by alcohols. Mol. Pharmacol., 9:
383-397.
58. COLEMAN, R.L., LUND, E.D., & SHAW, P.E. (1972) Analysis of
grapefruit essence and aroma oils. J. agric. food Chem., 20:
100-103.
59. CORBETT, J. & MEIER, G. (1968) Suicide attempted by rectal
administration of drug. J. Am. Med. Assoc., 206: 2320-2321.
60. CORNISH, H.H. & ADEFUIN, J. (1967) Potentiation of carbon
tetrachloride toxicity by aliphatic alcohols. Arch. environ.
Health, 14: 447-449.
61. CUPITT, L.T. (1980) Fate of toxic and hazardous materials
in the air environment, Research Triangle Park, North
Carolina, US Environmental Protection Agency, Environmental
Sciences Laboratory, Office of Research and Development (EPA
600/3-80-084, PB 80-221948).
62. CURTIS, C., LIMA, A., LOZANO, S.J., & VEITH, G.D. (1982)
Evaluation of a bacterial bioluminiscence bioassay as a
method for predicting acute toxicity of organic chemicals to
fish. In: Pearson, J.G., Foster, R.B., & Bishop, W.E., ed.
Aquatic Toxicology and Hazard Assessment. Fifth Conference,
American Society for Testing and Materials, pp. 170-178
(ASTM STP 766).
63. DAINTY, R.H., EDWARDS, R.A., & HIBBARD, C.M. (1984)
Volatile compounds associated with the aerobic growth of
some Pseudomonas species on beef. J. appl. Bacteriol., 57:
75-81.
64. DALZIEL, K. & DICKINSON, F.M. (1966) The kinetics and
mechanism of liver alcohol dehydrogenase with primary and
secondary alcohols as substrates. Biochem. J., 100: 34-46.
65. DANIEL, D.R., MCANALLEY, B.H., & GARRIOTT, J.C. (1981)
Isopropyl alcohol metabolism after acute intoxication in
humans. J. anal. Toxicol., 5: 110-112.
66. DAVID, J. & BOCQUET, C. (1976) Compared toxicities of
different alcohols for two Drosophila sibling species: D.
melanogaster and D. simulans. Comp. Biochem. Physiol., 54C:
71-74.
67. DAVIS, D.G., WERGIN, W.P., & DUSBABEK, K.E. (1978) Effects
of organic solvents on growth and ultrastructure of plant
cell suspensions. Pestic. Biochem. Physiol., 8: 84-97.
68. DAVIS, P.L., DAL CORTIVO, L.A., & MATURO, J. (1984)
Endogenous isopropanol: forensic and biochemical
implications. J. anal. Toxicol., 8: 209-212.
69. DE CEAURRIZ, J.C., MICILLINO, J.C., BONNET, P., & GUENIER,
J.P. (1981) Sensory irritation caused by various industrial
airborne chemicals. Toxicol. Lett., 9: 137-143.
70. DEL ROSARIO, R., DE LUMEN, B.O., HABU, T., FLATH, R.A., MON,
T.R., & TERANISHI, R. (1984) Comparison of headspace
volatiles from winged beans and soybeans. J. agric. food
Chem., 32: 1011-1015.
71. DENG, J.-F., WANG, J.-D., SHIH, T.-S., & LAN, F.-L. (1987)
Outbreak of carbon tetrachloride poisoning in a color
printing factory related to the use of isopropyl alcohol and
an air conditioning system in Taiwan. Am. J. ind. Med., 12:
11-19.
72. DGEP (1987) Review of literature data on 2-propanol,
Leidschendam, Netherlands, Directorate-General of
Environmental Protection, Ministry of Housing, Physical
Planning and Environment.
73. DIVINCENZO, G.D. & KRASAVAGE, W.J. (1974) Serum ornithine
carbamyl transferase as a liver response test for exposure
to organic solvents. Am. Ind. Hyg. Assoc. J., 35: 21-29.
74. DORIGAN, J., FULLER, B., & DUFFY, R. (1976) Scoring of
organic air pollutants. Chemistry, production and toxicity
of selected synthetic organic chemicals, The MITRE
Corporation (MITRE Technical Report MTR-7248, Rev. 1,
Appendix III).
75. DUA, S.L. (1974) Peritoneal dialysis for isopropyl alcohol
poisoning. J. Am. Med. Assoc., 230: 35.
76. DUVEL, W.A. & HELFGOTT, T. (1975) Removal of wastewater
organics by reverse osmosis. J. Water Pollut. Control Fed.,
47: 57-65.
77. EGBERT, A.M., REED, J.S., POWELL, B.J., LISKOW, B.I., &
LIESE, B.S. (1985) Alcoholics who drink mouthwash: the
spectrum of nonbeverage alcohol use. J. Stud. Alcohol, 46:
473-481.
78. EGOROV, Y.L. (1966) Vision of workers engaged in the
production of synthetic fatty acids and issues relative to
setting hygienic standards for the alcohol content in the
air. Gig. Tr. Prof. Zabol., 7: 33-38.
79. ENTERLINE, P.E. (1982) Importance of sequential exposure in
the production of epichlorohydrin and isopropanol. Ann. N.Y.
Acad. Sci., 381: 344-349.
80. FANG, H.H.P & CHIAN, E.S.K. (1976) Reverse osmosis
separation of polar organic compounds in aqueous solution.
Environ. Sci. Technol., 10: 364-369.
81. FERNANDEZ, F. & QUIGLEY, R.M. (1985) Hydraulic conductivity
of natural clays permeated with simple liquid hydrocarbons.
Can. Geotech. J., 22: 205-214.
82. FLORIN, I., RUTBERG, L., CURVALL, M., & ENZELL, C.R. (1980)
Screening of tobacco smoke constituents for mutagenicity
using the Ames test. Toxicology, 18: 219-232.
83. FOLLAND, D.S., SCHAFFNER, W., GINN, E., CROFFORD, O.B., &
MCMURRAY, D.R. (1976) Carbon tetrachloride toxicity
potentiated by isopropyl alcohol. Investigation of an
industrial outbreak. J. Am. Med. Assoc., 236: 1853-1856.
84. FORE, S.P., RAYNER, E.T., & DUPUY, H.P. (1971)
Determination of residual solvent in oilseed meals and
flours. III. Isopropanol. J. Am. Oil Chem. Soc., 48:
140-142.
85. FORSANDER, O.A. (1967) Influence of some aliphatic
alcohols on the metabolism of rat liver slices. Biochem. J.,
105: 93-97.
86. FREGERT, S., GROTH, O., HJORTH, N., MAGNUSSON, B., RORSMAN,
H., & OVRUM, P. (1969) Alcohol dermatitis. Acta
dermatovenereol., 49: 493-497.
87. FREGERT, S., GROTH, O., GRUVBERGER, B., MAGNUSSON, B.,
MOBACKEN, H., & RORSMAN, H. (1971) Hypersensitivity to
secondary alcohols. Acta dermatovenereol., 51: 271-272.
88. FREIREICH, A.W., CINQUE, T.J., XANTHAKY, G., & LANDAU, D.
(1967) Hemodialysis for isopropanol poisoning. New Engl. J.
Med., 277: 699-700.
89. GADSDEN, R.H., MELETTE, R.R., & MILLER, W.C. (1958) Scrap
iron intoxication. J. Am. Med. Assoc., 168: 1220-1224.
90. GAILLARD, D. & DERACHE, R. (1966) Action de quelques
alcools aliphatiques sur la mobilisation de différentes
fractions lipidiques chez la rate. Food cosmet. Toxicol., 4:
515-520.
91. GARRISON, R.F. (1953) Acute poisoning from use of isopropyl
alcohol in tepid sponging. J. Am. Med. Assoc., 152: 317-318.
92. GEORGE, H.A., JOHNSON, J.L., MOORE, W.E.C., HOLDEMAN, L.V.,
& CHEN, J.S. (1983) Acetone, isopropanol, and butanol
production by Clostridium beijerinckii (syn. Clostridium
butylicum) and Clostridium aurantibutyricum. Appl. environ.
Microbiol., 45: 1160-1163.
93. GEORGE, V., SHARMA, S.D., TRIPATHI, A.K., & ABRAHAM, S.P.
(1985) Flavour components of some edible fungi from
Kashmir. I. Pafai J., 7: 27-30.
94. GERARDE, H.W., AHLSTROM, D.B., & LINDEN, N.J. (1966) The
aspiration hazard and toxicity of a homologous series of
alcohols. Arch. environ. Health, 13: 457-461.
95. GERHOLD, R.M. & MALANEY, G.W. (1966) Structural
determinants in the oxidation of aliphatic compounds by
activated sludge. J. Water Pollut. Control Fed., 38:
562-579.
96. GILLETTE, L.A., MILLER, D.L., & REDMAN, H.E. (1952)
Appraisal of a chemical waste problem by fish toxicity
tests. Sewage ind. Waste, 24: 1397-1401.
97. GIUSTI, D.M., CONWAY, R.A., & LAWSON, C.T. (1974) Activated
carbon adsorption of petrochemicals. J. Water Pollut.
Control Fed., 46: 947-965.
98. GORLOVA, O.E. (1970) [Hygienic assessment of isopropyl
alcohol as an atmospheric pollutant.] Gig. i Sanit., 35:
9-14 (in Russian).
99. GOTZ-SCHMIDT, E.-M. & SCHREIER, P. (1986) Neutral volatiles
from blended endive ( Cichorium endivia L.). J. agric. food
Chem., 34: 212-215.
100. GRIFFITH, J.F., NIXON, G.A., BRUCE, R.D., REER, P.J., &
BANNAN, E.A. (1980) Dose-response studies with chemical
irritants in the albino rabbit eye as a basis for selecting
optimum testing conditions for predicting hazard to the human
eye. Toxicol. appl. Pharmacol., 55: 501-513.
101. GUNTER, B. (1982) Health hazard evaluation: Jeppesen
Sanderson Inc., Cincinnati, Ohio, US National Institute for
Occupational Safety and Health (HETA 81-261-1085, PB 83-
201749).
102. GUNTER, B.J., LIGO, R.L., & RUHE, R.L. (1976) Health hazard
evaluation determination, Steiger Tractor Inc., Cincinnati,
Ohio, US National Institute for Occupational Safety and
Health (NIOSH-TR-HHE-75-30-266, PB 273711).
103. GUSEINOV, V.G. (1985) [Toxicological hygienic characteristics
of isopropyl alcohol.] Gig. Tr. Prof. Zabol., (7): 60-62
(in Russian).
104. HABU, T., FLATH, R.A., MON, T.R., & MORTON, J.F. (1985)
Volatile components of Rooibos tea (Asphalathus linearis). J.
agric. food Chem., 33: 249-254.
105. HADDOCK, N.F. & WILKIN J.K. (1982) Cutaneous reactions to
lower aliphatic alcohols before and during disulfiram
therapy. Arch. Dermatol., 118: 157-159.
106. HALLIDAY, M.M. & CARTER, K.B. (1978) A chemical adsorption
system for the sampling of gaseous organic pollutants in
operating theatre atmospheres. Br. J. Anaesth., 50:
1013-1018.
107. HANSSEN, H.-P., SPRECHER, E., & KLINGENBERG, A. (1984)
Accumulation of volatile flavour compounds in liquid cultures
of Kluyveromyces lactis strains. Z. Naturforsch., 39c:
1030-1033.
108. HAWLEY, P.C. & FALKO, J.M. (1982) "Pseudo" renal failure
after isopropyl alcohol intoxication. South. med. J., 75:
630-631.
109. HELLMAN, T.M. & SMALL, F.H. (1974) Characterization of the
odor properties of 101 petrochemicals using sensory methods.
J. Air Pollut. Control Assoc., 24: 979-982.
110. HERMENS, J. CANTON, H., JANSSEN, P., & DE JONG, R. (1984)
Quantitative structure-activity relationships and toxicity
studies of mixtures of chemicals with anaesthetic potency:
acute lethal and sublethal toxicity to Daphnia magna. Aquat.
Toxicol., 5: 143-154.
111. HERMENS, J., BROEKHUYZEN, E., CANTON, H., & WEGMAN, R.
(1985) Quantitative structure-activity relationships and
mixture toxicity studies of mixtures of alcohols and
chlorohydrocarbons: effects on growth of Daphnia magna.
Aquat. Toxicol., 6: 209-217.
112. HO, Y.H., SCHWARZE, I., & SOEHRING, K. (1970) [The influence
of low aliphatic alcohols on the chloral hydrate metabolism
in rat liver sections.] Arzneim.-Forsch., 20: 1507-1509 (in
German).
113. HODGE, H.C. & STERNER, J.H. (1943) Am. Ind. Hyg. Assoc. J.,
10: 93.
114. HOIGNE, J. & BADER, H. (1983) Rate constants of reactions of
ozone with organic and inorganic compounds in water. I. Non-
dissociating organic compounds. Water Res., 17: 173-183.
115. HORWITZ, W., ed. (1975) Official methods of analysis of the
Association of Official Analytical Chemists, 12th ed.,
Washington DC, Association of Official Analytical Chemists,
pp. 328, 337-338, 343, 656-658.
116. HORWOOD, J.F., LLOYD, G.T., & STARK, W. (1981) Some flavour
components of feta cheese. Aust. J. dairy Technol., 36:
34-37.
117. HOU, C.T., PATEL, R.N., LASKIN, A.I., MARCZAK, I., & BARNABE,
N. (1981) Microbial oxidation of gaseous hydrocarbons:
production of alcohols and methyl ketones from their
corresponding n-alkanes by methylotrophic bacteria. Can. J.
Microbiol., 27: 107-115.
118. HOVIOUS, J.C., CONWAY, R.A., & GANZE, C.W. (1973) Anaerobic
lagoon pretreatment of petrochemical wastes. J. Water Pollut.
Control Fed., 45: 71-84.
119. HUEPER, W.C. (1966) Occupational and environmental cancers
of the respiratory system. Recent results. Cancer Res., 3:
105-107.
120. IARC (1977) Some fumigants, the herbicides 2,4-D and 2,4,5-
T, chlorinated dibenzodioxins and miscellaneous industrial
chemicals, Lyons, International Agency for Research on
Cancer, pp. 223-243 (Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Man, 15).
121. IDOTA, S. (1985) [Studies on isopropanol metabolism and
poisoning.] J. Nihon Univ. Med. Assoc., 44: 39-47 (in
Japanese).
122. IRPTC (1987) Data profile on 2-propanol, Geneva,
Switzerland, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
123. JARKE, F.H., DRAVNIEKS, A., & GORDON, S.M. (1981) Organic
contaminants in indoor air and their relation to outdoor
contaminants. ASHRAE Trans., 87: 153-166.
124. JENSEN, O. (1981) Contact allergy to propylene oxide and
isopropyl alcohol in a skin disinfectant swab. Contact
Dermat., 7: 148-150.
125. JONES, C.J. & MCGUGAN, P.J. (1977/1978) An investigation of
the evaporation of some volatile solvents from domestic
waste. J. hazard. Mater., 2: 235-251.
126. JONSSON, A., PERSSON, K.A. & GRIGORIADIS, V. (1985)
Measurements of some low molecular-weight oxygenated,
aromatic, and chlorinated hydrocarbons in ambient air and in
vehicle emissions. Environ. Int., 11: 383-392.
127. JUHNKE, I. & LUDEMANN, D. (1978) [Results of examination of
200 chemical compounds for acute toxicity towards fish by
means of the golden orfe test.] Z. Wasser-Abwasser Forsch.,
11: 161-164 (in German).
128. JUNCOS, L. & TAGUCHI, J.T. (1968) Isopropyl alcohol
intoxication. Report of a case associated with myopathy,
renal failure, and hemolytic anemia. J. Am. Med. Assoc., 204:
186-188.
129. KAMIL, I.A., SMITH, J.N., & WILLIAMS, R.T. (1953) The
metabolism of aliphatic alcohols. The glucuronic acid
conjugation of acyclic aliphatic alcohols. Biochem. J., 53:
129-136.
130. KANE, L.E., DOMBROSKE, R., & ALARIE, Y. (1980) Evaluation of
sensory irritation from some common industrial solvents. Am.
Ind. Hyg. Assoc. J., 41: 451-455.
131. KELNER, M. & BAILEY, D.N. (1983) Isopropanol ingestion:
interpretation of blood concentrations and clinical findings.
J. Toxicol.-clin. Toxicol., 20: 497-507.
132. KEMAL, H. (1927) [Contribution to investigations into the
fate of isopropyl alcohol in the human body.] Biochem. Z.,
187: 461-466 (in German).
133. KHAN, J.S., WILSON, M.C., & TAYLOR, T.V. (1979) A case of
dettol addiction. Br. med. J., 1: 791-792.
134. KIMURA, E.T., EBERT, D.M., & DODGE, P.W. (1971) Acute
toxicity and limits of solvent residue for sixteen organic
solvents. Toxicol. appl. Pharmacol., 19: 699-703.
135. KING, L.H., BRADLEY, K.P., & SHIRES, D.L. (1970)
Hemodialysis for isopropyl alcohol poisoning. J. Am. Med.
Assoc., 211: 1855.
136. KINLIN, T.E., MURALIDHARA, R., PITTET, A.O., SANDERSON, A., &
WALRADT, J.P. (1972) Volatile components of roasted
filberts. J. agric. food Chem., 20: 1021-1028.
137. KIRK, R.E. & OTHMER, D.F., ed. (1978-1984) Encyclopedia of
chemical technology, 3rd ed., New York, Wiley Interscience.
138. KLECKA, G.M. & LANDI, L.P. (1985) Evaluation of the OECD
activated sludge, respiration inhibition test. Chemosphere,
14: 1239-1251.
139. KNUTH, M.L. & HOGLUND, M.D. (1984) Quantitative analysis of
68 polar compounds from ten chemical classes by direct
aqueous injection gas chromatography. J. Chromatogr., 285:
153-160.
140. KOMINSKY, J., LOVE, J., & ANDERSON, K. (1982) Health hazard
Evaluation, Tweddle Litho Company,Cincinnati, Ohio, US
National Institute for Occupational Safety and Health (HETA
81-117-1087, PB 83-202390).
141. KONEMANN, H. (1981) Quantitative structure-activity
relationships in fish toxicity studies. Toxicology, 19:
209-221.
142. KONIG, H. & HERMES, M. (1981) [Separation, identification
and estimation of propellant gases and solvents in aerosol
products by gas chromatography.] Chromatographia, 14: 351-354
(in German).
143. KRAMER, V.C., SCHNELL, D.J., & NICKERSON, K.W. (1983)
Relative toxicity of organic solvents to Aedes aegyptilarvae.
J. Invertebr. Pathol., 42: 285-287.
144. KRING, E.V., ANSUL, G.R., HENRY, T.J., MORELLO, J.A., DIXON,
S.W., VASTA, J.F., & HEMINGWAY, R.E. (1984) Evaluation of
the standard NIOSH type charcoal tube sampling method for
organic vapors in air. Am. Ind. Hyg. Assoc. J., 45: 250-259.
145. KRULL, I.S., SWARTZ, M., & DRISCOLL, J.N. (1984)
Derivatizations for improved detection of alcohols by gas
chromatography and photoionization detection. Anal. Lett.,
17(A20): 2369-2384.
146. KUMAI, M., KOIZUMI, A., SAITO, K., SAKURAI, H., INOUE, T.,
TAKEUCHI, Y., HARA, I., OGATA, M., MATSUSHITA, T., & IKEDA,
M. (1983) A nationwide survey on organic solvent components
in various solvent products. Part 2. Heterogeneous products
such as paints, inks, and adhesives. Ind. Health, 21:
185-197.
147. KUMKE, G.W., HALL, J.F., & OEBEN, R.W. (1968) Conversion to
activated sludge at Union Carbide's institute plant. J. Water
Pollut. Control Fed., 40: 1408-1422.
148. KURPPA, K. & HUSMAN, K. (1982) Car painters' exposure to a
mixture of organic solvents. Serum activities of liver
enzymes. Scand. J. Work environ. Health, 8: 137-140.
149. LACOUTURE, P.G., WASON, S., ABRAMS, A., & LOVEJOY, F.H.
(1983) Acute isopropyl alcohol intoxication. Diagnosis and
management. Am. J. Med., 75: 680-686.
150. LAHAM, S., POTVIN, M., & SCHRADER, K. (1979) Microméthode de
dosage simultané de l'alcool isopropylique et de son
metabolite l'acétone. Chemosphere, 2: 79-87.
151. LAHAM, S., POTVIN, M., SCHRADER, K., & MARINO, I. (1980)
Studies on inhalation toxicity of 2-propanol. Drug Chem.
Toxicol., 3: 343-360.
152. LANGVARDT, P.W. & MELCHER, R. (1979) Simultaneous
determination of polar and non-polar solvents in air using a
two-phase desorption from charcoal. Am. Ind. Hyg. Assoc. J.,
40: 1006-1012.
153. LAREGINA, J., BOZZELLI, J.W., HARKOV, R., & GIANTI, S.
(1986) Volatile organic compounds at hazardous waste sites
and a sanitary landfill in New Jersey. An up-to-date review
of the present situation. Environ. Progr., 5: 18-27.
154. LEASURE, C.S., FLEISCHER, M.E., ANDERSON, G.K., & EICEMAN,
G.A. (1986) Photoionization in air with ion mobility
spectrometry using a hydrogen discharge lamp. Anal. Chem.,
58: 2142-2147.
155. LEFFINGWELL, S.S., WAXWEILER, R., ALEXANDER, V., LUDWIG, H.R.
& HALPERIN, W. (1983) Case-control study of gliomas of the
brain among workers employed by a Texas City, Texas chemical
plant. Neuroepidemiology, 2: 179-195.
156. LEGENDRE, M.G. & DUPUY, H.P. (1981) Flavor volatiles as
measured by rapid instrumental techniques, American Chemical
Society, pp. 41-49 (ACS Symposium Series No. 147).
157. LEHMAN, A.J. & CHASE, H.F. (1944) The acute and chronic
toxicity of isopropyl alcohol. J. Lab. clin. Med., 29:
561-567.
158. LEHMAN, A.J., SCHWERMA, H., & RICKARDS, E. (1944) Isopropyl
alcohol: rate of disappearance from the blood stream of dogs
after intravenous and oral administration. J. Pharmacol. exp.
Ther., 82: 196-201.
159. LEHMAN, A.J., SCHWERMA, H., & RICKARDS, E. (1945) Acquired
tolerance in dogs, rate of disappearance from the blood
stream in various species, and effects on successive
generation of rats. J. Pharmacol. exp. Ther., 85: 61-69.
160. LEWIN, G.A., OPPENHEIMER, P.R., & WINGERT, W.A. (1977) Coma
from alcohol sponging. J.A.C.E.P., 6: 165-167.
161. LEWIS, G.D., LAUFMAN, A.K., MCANALLY, B.H., & GARRIOTT, J.C.
(1984) Metabolism of acetone to isopropyl alcohol in rats
and humans. J. forensic Sci., 29: 541-549.
162. LINDSTROM, T.D. & ANDERS, M.W. (1978) Effect of agents known
to alter carbon tetrachloride hepatotoxicity and cytochrome
P-450 levels on carbon tetrachloride-stimulated lipid
peroxidation and ethane expiration in the intact rat.
Biochem. Pharmacol., 27: 563-567.
163. LITOVITZ, T. (1986) The alcohols: ethanol, methanol, iso-
propanol, ethylene glycol. Pediatr. Clin. N. Am., 33:
311-323.
164. LLOYD, A.C., DARNALL, K.R. WINER, A.M., & PITTS, J.N. (1976)
Relative rate constants for the reactions of OH radicals with
isopropyl alcohol, diethyl and di- n-propyl ether at 305 ±
20 °K. Chem. Phys. Lett., 42: 205-209.
165. LORR, N.A., MILLER, K.W., CHUNG, H.R., & YANG, C.S. (1984)
Potentiation of the hepatotoxicity of N-nitrosodimethylamine
by fasting, diabetes, acetone, and isopropanol. Toxicol.
appl. Pharmacol., 73: 423-431.
166. LOVEGREN, N.V., VINNETT, C.H., & ANGELO, A.J.S. (1982) Gas
chromatographic profile of good quality raw peanuts. Peanut
Sci., 9: 93-96,
167. LUDWIG, E. & HAUSEN, B.M. (1977) Sensitivity to isopropyl
alcohol. Contact Dermat., 3: 240-244.
168. LUNDBERG, I. & HAKANSSON, M. (1985) Normal serum activities
of liver enzymes in Swedish paint industry workers with heavy
exposure to organic solvents. Br. J. ind. Med., 42: 596-600.
169. LYON, R.C., MCCOMB, J.A., SCHREURS, J., & GOLDSTEIN, D.B.
(1981) A relationship between alcohol intoxication and the
disordering of brain membranes by a series of short-chain
alcohols. J. Pharmacol. exp. Ther., 218: 669-675.
170. MCCORD, W.M., SWITZER, P.K., & BRILL, H.H. (1948) Isopropyl
alcohol intoxication. South med. J., 41: 639-642.
171. MCCREERY, M.J. & HUNT, W.A. (1978) Physico-chemical
correlates of alcohol intoxication. Neuropharmacology, 17:
451-461.
172. MCFADDEN, S.W. & HADDOW, J.E. (1969) Coma produced by
topical application of isopropanol. Pediatrics, 43: 622-623.
173. MACHYULITE, N.I. (1978) [Hygienic characterization of
conditions of work in production of levomycetin.] Gig. Tr.
Prof. Zabol., 12: 8-12 (in Russian).
174. MCINNES, A. (1973) Skin reaction to isopropyl alcohol. Br.
med. J., 1: 357.
175. MACK, K. (1973) The problem of waste water purification in
the chemical pharmaceutical industry. Prog. Water Technol.,
3: 239-249.
176. MACK, R.B. (1985) Pervasive procrustianism-isopropyl alcohol
intoxication. N.C. med. J., 46: 101-102.
177. MAIZLISH, N.A., LANGOLF, G.D., WHITEHEAD, L.W., FINE, L.J.,
ALBERS, J.W., GOLDBERG, J. & SMITH, P. (1985) Behavioural
evaluation of workers exposed to mixtures of organic
solvents. Br. J. ind. Med., 42: 579-590.
178. MALILA, A. (1978) Intoxicating effects of three aliphatic
alcohols and barbital on two rat strains genetically selected
for their ethanol intake. Pharmacol. Biochem. Behav., 8:
197-201.
179. MARKEL, H. (1982) Health hazard evaluation, Federal
Correctional Institution, Cincinnati, Ohio, US National
Institute for Occupational Safety and Health (HETA 80-119-
1066, PB 83-199398).
180. MARTIN, M. & HAERDI, W. (1982) Determination de composes
volatils toxiques dans le sang et dans l'urine par
chromatographie en phase gazeuse, methode de l'espace de tete
(head-space). Trav. Chim. aliment. Hyg., 73: 212-217.
181. MARTINEZ, T.T., JAEGER, R.W., DECASTRO, F.J., THOMPSON, M.W.,
& HAMILTON, M.F. (1986) A comparison of the absorption and
metabolism of isopropyl alcohol by oral, dermal, and
inhalation routes. Vet. human Toxicol., 28: 233-236.
182. MARZULLI, F.N. & RUGGLES, D.I. (1973) Rabbit eye irritation
test: collaborative study. J. Assoc. Off. Anal. Chem., 56:
905-914.
183. MATSUI, F., LOVERING, E.G., WATSON, J.R., BLACK, D.B., &
SEARS, R.W. (1984) Gas chromatographic method for solvent
residues in drug raw materials. J. pharm. Sci., 73:
1664-1666.
184. MATTSON, V.R., ARTHUR, J.W., & WALBRIDGE, C.T. (1976) Acute
toxicity of selected organic compounds to fathead minnows,
Duluth, Minnesota, US Environmental Protection Agency,
Environmental Research Laboratory (EPA 600/3-76-097; PB
262897).
185. MAY, J. (1966) [Odour thresholds of solvents for the
judgement of solvent odour in air.] Staub Reinhalt. Luft, 26:
385-389 (in German).
186. MECIKALSKI, M.B. & DEPNER, T.A. (1982) Peritoneal dialysis
for isopropanol poisoning. West. J. Med., 137: 322-324.
187. MENDELSON, J., WEXLER, D., LEIDERMAN, P.H., & SOLOMON, P.
(1957) A study of addiction to nonethyl alcohols and other
poisonous compounds. Quart. J. Stud. Alcohol, 18: 561-580.
188. MORGAN, R.L., SORENSON, S.S., & CASTLES, T.R. (1987)
Prediction of ocular irritation by corneal pachymetry. Food
chem. Toxicol., 25: 609-613.
189. MOSHONAS, M.G. & SHAW, P.E. (1972) Analysis of flavor
constituents from lemon and lime essence. J. agric. food
Chem., 20: 1029-1030.
190. MUNCH, J.C. (1972) Aliphatic alcohols and alkyl esters:
narcotic and lethal potencies to tadpoles and to rabbits.
Ind. Med., 41: 31-33.
191. NATOWICZ, M., DONAHUE, J., GORMAN, L., KANE, M., MCKISSICK,
J. & SHAW, L. (1985) Pharmacokinetic analysis of a case of
isopropanol intoxication. Clin. Med., 31: 326-328.
192. NELSON, K.W., EGE, J.F., ROSS, M., WOODMAN, L.E., &
SILVERMAN, L. (1943) Sensory response to certain industrial
solvent vapors. J. ind. Hyg. Toxicol., 25: 282-285.
193. NELSON, B.K., BRIGHTWELL, W.S., MACKENZIE-TAYLOR, D.R., KHAN,
A., BURG, J.R., WEIGEL, W.W., & GOAD, P.T. (1988)
Teratogenicity of n-propanol and isopropanol administered at
high inhalation concentrations to rats. Food Chem. Toxicol.,
26: 247-254.
194. NIXON, G.A., TYSON, C.A., & WERTZ, W.C. (1975) Interspecies
comparisons of skin irritancy. Toxicol. appl. Pharmacol., 31:
481-490.
195. NORDMANN, R., RIBIERE, C., ROUACH, H., BEAUGE, F.,
GIUDICELLI, Y., & NORDMANN, J. (1973) Metabolic pathways
involved in the oxidation of isopropanol into acetone by the
intact rat. Life Sci., 13: 919-932.
196. OELERT, H.H. & FLORIAN, T. (1972) [Recording and valuation
of the inconvenience caused by odours from diesel exhaust.]
Staub Reinhalt. Luft, 32: 400-407 (in German).
197. OHASHI, Y., NAKAI, Y., IKEOKA, H., KOSHIMO, H., ESAKI, Y.,
HORIGUCHI, S., TERAMOTO, K., & NAKASEKO, H. (1987a)
Recovery process of tracheal mucosa of guinea-pigs exposed to
isopropyl alcohol. Arch. Toxicol., 61: 12-20.
198. OHASHI, Y., NAKAI, Y., IKEOKA, H., KOSHIMO, H., ESAKI, Y.,
HORIGUCHI, S., TERAMOTO, K., & NAKASEKO, H. (1987b) Acute
effects of isopropyl alcohol exposure on the middle ear
mucosa. J. appl. Toxicol., 7: 205-211.
199. OLSON, B.A. (1982) Effects of organic solvents on
behavioural performance of workers in the paint industry.
Neurobehav. Toxicol. Teratol., 4: 703-708.
200. OVEREND, R. & PARASKEVOPOULOS, G. (1978) Rates of OH radical
reactions. IV. Reactions with methanol, ethanol, 1-propanol,
and 2-propanol at 296 ° K. J. phys. Chem., 82: 1329-1333.
201. PALO, V. & ILKOVA, H. (1970) Direct gas chromatographic
estimation of lower alcohols, acetaldehyde, acetone and
diacetyl in milk products. J. Chromatogr., 53: 363-367.
202. PARKER, W.A. (1982-1983) Alcohol-containing pharmaceuticals.
Am. J. drug alcohol Abuse, 9: 195-209.
203. PITTER, P. (1976) Determination of biological degradability
of organic substances. Water Res., 10: 231-235.
204. POHL, J. (1922) [Investigations into the fate of methyl and
isopropyl alcohol.] Biochem. Z., 127: 66-71 (in German).
205. POSO, H. & POSO, A.R. (1980) Inhibition by aliphatic
alcohols of the stimulated activity of ornithine
decarboxylase and tyrosine amino-transferase occurring in
regenerating rat liver. Biochem. Pharmacol., 29: 2799-2803.
206. POWIS, G. (1975) Effect of a single oral dose of methanol,
ethanol and propan-2-ol on the hepatic microsomal metabolism
of foreign compounds in the rat. Biochem. J., 148: 269-277.
207. POWIS, G. & GRANT, L. (1976) The effect of inhibitors of
alcohol metabolism upon the changes in the hepatic microsomal
metabolism of foreign compounds produced by the acute
administration of some alcohols to the rat. Biochem.
Pharmacol., 25: 2197-2201.
208. PRICE, K.S., WAGGY, G.T., & CONWAY, R.A. (1974) Brine shrimp
bioassay and seawater BOD of petrochemicals. J. Water Pollut.
Control Fed., 46: 63-77.
209. RAICHLE, M.E., EICHLING, J.O., STRAATMANN, M.G., WELCH, M.J.,
LARSON, K.B., & TER-POGOSSIAN, M.M. (1976) Blood-brain
barrier permeability of 11C-labeled alcohols and 15U-labeled
water. Am. J. Physiol., 230: 543-552.
210. RAILE, A., HAMMAN, K.P., SCHEINER, O., SCHULTZ, T., ERDEI,
A., & DIERICH, M.P. (1982) Differential effect of low
molecular weight alcohols on the Con A stimulation of mouse
spleen cells. Immunol. Lett., 4: 305-309.
211. RAMSEY, J.D. & FLANAGAN, R.J. (1982) Detection and
identification of volatile organic compounds in blood by
headspace gas chromatograpy as an aid to the diagnosis of
solvent abuse. J. Chromatogr., 240: 423-444.
212. REINDERS, M.E. (1980) Handbook of emission factors. Part I.
Non-industrial sources, The Hague, Netherlands, Ministry of
Health and Environmental Protection.
213. REINHARDT, C.A., PELLI, D.A., & ZBINDEN, G. (1985)
Interpretation of cell toxicity data for the estimation of
potential irritation. Food chem. Toxicol., 23: 247-252.
214. REQUENA, J., VELAZ, M.E., GUERRERO, J.R., & MEDINA, J.D.
(1985) Isomers of long-chain alkane derivatives and nervous
impulse blockage. J. Membr. Biol., 84: 229-238.
215. REYNOLDS, E.S., MOSLEN, M.T., & TREINEN, R.J. (1982)
Isopropanol enhancement of carbon tetrachloride metabolism in
vivo. Life Sci., 31: 661-669.
216. REYNOLDS, T. (1977) An anomalous effect of isopropanol on
lettuce germination. Plant Sci. Lett., 15: 25-28.
217. RICHARDSON, D.R., CARAVATI, C.M., PEYTON, E., & WEARY, P.E.
(1969) Allergic contact dermatitis to "alcohol" swabs.
Cutis, 5: 1115-1118.
218. RIETBROCK, N. & ABSHAGEN, U. (1971) [Pharmacokinetics and
metabolism of aliphatic alcohols.] Arzneim.-Forsch.,
21: 1309-1319 (in German).
219. RISTOW, S.S., STARKEY, J.R., & HASS, G.M. (1982) Inhibition
of natural killer cell activity in vitro by alcohols.
Biochem. Biophys. Res. Commun., 105: 1315-1321.
220. ROSANSKY, S.J. (1982) Isopropyl alcohol poisoning treated
with haemodialysis: kinetics of isopropyl alcohol and acetone
removal. J. Toxicol.-clin. Toxicol., 19: 265-271.
221. ROSS, D.H. (1976) Selective action of alcohols on cerebral
calcium levels. Ann. N.Y. Acad. Sci., 273: 280-294.
222. SABLJIC, A. & PROTIC-SABLIC, M. (1983) Quantitative
structure-activity study on the mechanism of inhibition of
microsomal p-hydroxylation of aniline by alcohols. Mol.
Pharmacol., 23: 213-218.
223. SANTODONATO, J. (1985) Monograph on human exposure to
chemicals in the workplace: isopropyl alcohol, Syracuse, New
York, Center for Chemical Hazard Assessment, Syracuse
Research Corporation (SRC-TR-84-1043, PB 86-143401).
224. SAVOLAINEN, H., PEKARI, K., & HELOJOKI, H. (1979)
Neurochemical and behavioural effects of extended exposure to
isopropanol vapour with simultaneous ethanol intake. Chem.-
biol. Interact., 28: 237-248.
225. SAWHNEY, B.L. & KOZLOSKI, R.P. (1984) Organic pollutants in
leachates from landfill sites. J. environ. Qual., 13:
349-352.
226. SCHICK, J.B. & MILSTEIN, J.M. (1981) Burn hazard of
isopropyl alcohol in the neonate. Pediatrics, 68: 587-588.
227. SEILER, H., BLAIM, H., & BUSSE, M. (1984) [Antibacterial
effects on predominant taxa in the activated sludge system of
a chemical combine.] Z. Wasser-Abwasser Forsch., 17: 127-133
(in German).
228. SENZ, E.H. & GOLDFARB, D.L. (1958) Coma in a child following
use of isopropyl alcohol in sponging. J. Pediatr., 53:
322-323.
229. SHAW, G.J., ALLEN, J.M., & VISSER, F.R. (1985) Volatile
flavor components of Babaco fruit ( Carica pentagona,
Heilborn). J. agric. food Chem., 33: 795-797.
230. SHEHAB, A.S. (1980) Comparative cytological studies of the
effect of some aliphatic alcohols and the fatty alcohols from
Euphorbia granulata and Pulicaria crispa on mitosis of Allium
cepa. Cytologia, 45: 507-513.
231. SHOFSTAHL, J.H. & HARDY, J.K. (1986) Determination of C1-C4
alcohols in gasoline using multiple ion detection. Anal.
Chem., 58: 2412-2414.
232. SIEBERT, H., SIEBERT, G., & BOHN, G. (1972) [Animal
experimental investigations into the metabolism of
propan-2-ol.] D tsch. Apoth.-ZTG., 112: 1040-1041 (in German).
233. SINGH, K.V. & AGRAWAL, S.C. (1981) Growth responses of
keratinophilic fungi to some volatile substances. Mykosen,
24: 630-634.
234. SIPES, I.G., STRIPP, B., KRISHNA, G., MALING, H., & GILLETTE,
J.R. (1973) Enhanced hepatic microsomal activity by
pretreatment of rats with acetone or isopropanol. Proc. Soc.
Exp. Biol. Med., 142: 237-240.
235. SMEA (1982) [Problems in industrial toxicology.] (in
Russian).
236. SMITH, N.B. (1984) Determination of volatile alcohols and
acetone in serum by non-polar capillary gas chromatography
after direct sample injection. Clin. Chem., 30: 1672-1674.
237. SMITH, P. & BROWN, N.L. (1969) Determination of isopropyl
alcohol in solid fish protein concentrate by gas-liquid
chromatography. J. agric. food Chem., 17: 34-37.
238. SMITH, R.P. (1959) Poisoning with Old Spice shaving lotion.
A case report. Bull. Suppl. Mat. clin. Toxicol. comm. Prod.,
2(11): 12.
239. SMYTH, H.F. & CARPENTER, C.P. (1948) Further experience with
the range finding test in the industrial toxicology
laboratory. J. ind. Hyg. Toxicol., 30: 63-70.
240. STEELE, R.H. & WILHELM, D.L. (1966) The inflammatory
reaction in chemical injury. I. Increased vascular
permeability and erythema induced by various chemicals. Br.
J. exp. Pathol., 47: 612-623.
241. STENSTROM, S., ENLOE, L., PFENNING, M., & RICHELSON, E.
(1986) Acute effects of ethanol and other short-chain
alcohols on the guanylate cyclase system of murine
neuroblastoma cells (clone N1E-115). J. Pharmacol. exp.
Ther., 236: 458-463.
242. STOFBERG, J. & GRUNDSCHOBER, F. (1984) Consumption ratio and
food predominance of flavoring materials-second cumulative
series. Perfumer & Flavorist, 9: 53-83.
243. STRANGE, A.W., SCHNEIDER, C.W., & GOLDBORT, R. (1976)
Selection of C3 alcohols by high and low ethanol selecting
mouse strains and the effects on open field activity.
Pharmacol. Biochem. Behav., 4: 527-530.
244. SWERDEL, M.R. & COUSINS, R.J. (1984) Changes in rat liver
metallothionein and metallothionein mRNA induced by
isopropanol. Proc. Soc. Exp. Biol. Med., 175: 522-529.
245. TAYLOR, C.D., COWART, C.O., & RYAN, N.T. (1985) Isopropanol
intoxication: managing the coma. Hosp. Pract., 20: 173-175.
246. TESTA, B. (1981) Structural and electronic factors
influencing the inhibition of aniline hydroxylation by
alcohols and their binding to cytochrome P-450. Chem.-biol.
Interact., 34: 287-300.
247. TICHY, M., TRCKA, V. ROTH, Z., & KRIVUCOVA, M. (1985) QSAR
analysis and data extrapolation among mammals in a series of
aliphatic alcohols. Environ. Health Perspect., 61: 321-328.
248. TIESS, D. & HAMMER, U. (1985) [On endogenous acetone
(propane-2-on) and isopropanol (propane-2-ol) levels in the
human body after ketoacidic states.] Z. gesamte Hyg., 31:
527-529 (in German).
249. TIMMER, R., TER HEIDE, R., DE VALOIS, P.J., & WOBBEN, H.J.
(1971) Qualitative analysis of the most volatile neutral
components of Reunion geranium oil ( Pelargonium roseum
Bourbon). J. agric. food Chem., 19: 1066-1068.
250. TOMITA, M. & NISHIMURA, M. (1982) Using saliva to estimate
human exposure to organic solvents. Bull. Tokyo dent. Coll.,
23: 175-188.
251. TOOBY, T.E., HURSEY, P.A., & ALABASTER, J.S. (1975) The
acute toxicity of 102 pesticides and miscellaneous substances
to fish. Chem. Ind., 12: 523-526.
252. TRAIGER, G.J. & PLAA, G.L. (1972) Relationship of alcohol
metabolism to the potentiation of CCl4 hepatotoxicity induced
by aliphatic alcohols. J. Pharmacol. exp. Ther., 183:
481-488.
253. TRAIGER, G.J. & PLAA, G.L. (1974) Chlorinated hydrocarbon
toxicity. Potentiation by isopropyl alcohol and acetone.
Arch. environ. Health, 28: 276-278.
254. TU, Y.Y., PENG, R., CHANG, Z.-F., & YANG, C.S. (1983)
Induction of a high affinity nitrosamine demethylase in rat
liver microsomes by acetone and isopropanol. Chem.-biol.
Interact., 44: 247-260.
255. UENG, T.-H., MOORE, L., ELVES, R.G., & ALVARES, A.P. (1983)
Isopropanol enhancement of cytochrome P-450-dependent
monooxygenase activities and its effects on carbon
tetrachloride intoxication. Toxicol. appl. Pharmacol., 71:
204-214.
256. URANO, K., OGURA, K., & WADA, H. (1981) Direct analytical
method for aliphatic compounds in water by steam carrier gas
chromatography. Water Res., 15: 225-231.
257. US NIOSH (1976) Criteria for a recommended standard:
occupational exposure to isopropyl alcohol, Cincinnati, Ohio,
US National Institute of Occupational Safety and Health, US
Department of Health, Education, and Welfare, Public Health
Services, Center for Disease Control (DHEW Publication No.
(NIOSH)76-142).
258. US NIOSH (1977) Manual of analytical methods, 2nd ed.,
Cincinnati, Ohio, US National Institute for Occupational
Safety and Health, US Department of Health, Education, and
Welfare, Vol. 2, pp. S185.
259. US NIOSH (1984) Method 1400. In: Eller, P.M., ed. NIOSH
Manual of analytical methods, 3rd ed., Cincinnati, Ohio,
National Institute for Occupational Safety and Health, Vol.
1, pp. 1400-1-1400-5.
260. VAN RILLAER, W.G. & BEERNAERT, H. (1983) Determination of
residual isopropanol and propylene glycol in soft drinks by
glass capillary gas chromatography. Z. Lebensm. Unters.
Forsch., 177: 196-199.
261. VASILIADES, J., POLLOCK, J., & ROBINSON, C.A. (1978)
Pitfalls of the alcohol dehydrogenase procedure for the
emergency assay of alcohol: a case study of isopropanol
overdose. Clin. Chem., 24: 383-385.
262. VEITH, G.D. & KOSIAN, P. (1983) Estimating bioconcentration
potential from octanol/water partition coefficients. In:
Mackay et al., ed. Physical behaviour of PCBs in the Great
Lakes, Ann Arbor, Michigan, Ann Arbor Science, pp. 269-282.
263. VEITH, G.D., CALL, D.J., & BROOKE, L.T. (1983) Structure-
toxicity relationships for the fathead minnow, Pimephales
promelas: narcotic industrial chemicals. Can. J. Fish. aquat.
Sci., 40: 743-748.
264. VIDELA, L.A., FERNANDEZ, V., & DE MARINIS, A. (1982) Liver
peroxidative pressure and glutathione status following
acetaldehyde and aliphatic alcohols pretreatment in the rat.
Biochem. Biophys. Res. Commun., 104: 965-970.
265. VILAGELIU ARQUES, L. & GONZALEZ DUARTE, R. (1980) Effect of
ethanol and isopropanol on the activity of alcohol
dehydrogenase, viability and life-span in Drosophila
melanogaster and Drosophila funebris. Experientia, 36:
828-830.
266. VISUDHIPAN, P. & KAUFMAN, H. (1971) Increased cerebrospinal
fluid protein following isopropyl alcohol intoxication. N.Y.
State J. Med., 71: 887-880.
267. VON DER HUDE, W., SCHEUTWINKEL, M., GRAMLICH, U., FISSLER,
B., & BUSLER, A. (1987) Genotoxicity of three carbon
compounds evaluated in the SCE test in vitro. Environ.
Mutagen., 9: 401-410.
268. WAGNER, R. (1974) [Investigations into the degradation
behaviour of organic compounds using the respirometric
dilution method. I. Monohydric alcohols.] Vom Wasser, 42:
271-305 (in German).
269. WAGNER, R. (1976) [Investigations into the degradation
behaviour of organic compounds using the respirometric
dilution method. II. The degradation kinetics of the test
compounds.] Vom Wasser, 47: 241-265 (in German).
270. WALLGREN, H. (1960) Relative intoxicating effects on rats of
ethyl, propyl and butyl alcohols. Acta pharmacol. toxicol.,
16: 217-222.
271. WALLINGFORD, K.M. (1983) Health hazard evaluation, Xomox
Corporation, Cincinnati, Ohio, US National Institute for
Occupational Safety and Health (HETA 83-170-1346, PB 85-
163434).
272. WASILEWSKI, C. (1968) Allergic contact dermatitis from
isopropyl alcohol. Arch. Dermatol., 98: 502-504.
273. WATERER, D.R. & PRITCHARD, M.K. (1984) Monitoring of
volatiles: a technique for detection of soft rot (Erwinia
carotovora) in potato tubers. Can. J. Plant. Pathol., 6:
165-171.
274. WAX, J., ELLIS, F.W., & LEHMAN, A.J. (1949) Absorption and
distribution of isopropyl alcohol. J. Pathol. exp. Ther., 97:
229-237.
275. WEBBER, D. (1984) Basic chemical output returns to growth.
Top 50 chemical products. Chem. eng. News, May 7: 8-10.
276. WEIL, C.S., SMYTH, H.F., & NALE, T.W. (1952) Quest for a
suspected industrial carcinogen. Arch. ind. Hyg. occup. Med.,
5: 535-547.
277. WEINTRAUB, Z. & IANCU, T.C. (1982) Isopropyl alcohol burns.
Pediatrics, 69: 506.
278. WHITE, G.A., EMMETT, E.A., KOMINSKY, J.R., & SINGAL, M.
(1983) Health hazard evaluation, Inland Division, GMC,
Cincinnati, Ohio, US National Instituue for Occupational
Safety and Health (HETA 77-011-1338, PB 85-101319).
279. WHITEHEAD, L.W., BALL, G.L., FINE, L.J., & LANGOLF, G.D.
(1984) Solvent vapor exposures in booth spray painting and
spray glueing, and associated operations. Am. ind. Hyg.
Assoc. J., 45: 767-772.
280. WILKINSON, C. & IGLEWICZ, R. (1982) Health hazard
evaluation, Syntrex Corporation, Cincinnati, Ohio, US
National Institute for Occupational Safety and Health (HETA
81-370-1050, PB 83-198424).
281. WILKINSON, T. & HAMER, G. (1979) The microbial oxidation of
mixtures of methanol, phenol, acetone, and isopropanol with
reference to effluent purification. J. chem. Technol.
Biotechnol., 29: 56-67.
282. WILLIAMS, T.M., HICKEY, J.L.S., & SHY,C.M. (1982) Health
hazard evaluation, Dittler Brothers, Inc., Cincinnati, Ohio,
US National Institute for Occupational Safety and Health
(HETA 81-173-1051, PB 83-198473).
283. WILLS, J.H., JAMESON, E.M., & COULSTON, F. (1969) Effects on
man of daily ingestion of small doses of isopropyl alcohol.
Toxicol. appl. Pharmacol., 15: 560-565.
284. WINEK, C.L. & JANSSEN, J.K. (1982) Blood versus bone marrow
isopropanol concentrations in rabbits. Forensic Sci. Int.,
20: 11-20.
285. WOLFF, T. (1978) In vitro inhibition of monooxygenase
dependent reactions by organic solvents. Int. Congr. Ser.
Excerpta Med., 440: 196-199.
286. WRIGHT, U. (1979) The hidden carcinogen in the manufacture
of isopropyl alcohol. Dev. Toxicol. environ. Sci., 4: 93-98.
287. YASHUDA, Y., CABRAL, A.M., & ANTONIO, A. (1976) Inhibitory
action of aliphatic alcohols on smooth muscle contraction.
Pharmacology, 14: 473-478.
288. YOUNG, P.J. & PARKER, A. (1983) The identification and
possible environmental impact of trace gases and vapours in
landfill gas. Waste Management Res., 1: 213-226.
289. YOUNG, R.H.F., RYCKMAN, D.W., & BUZZELL, J.C. (1968) An
improved tool for measuring biodegradability. J. Water
Pollut. Control Fed., 40: R354-R368.
290. ZAHLSEN, K., AARSTAD, K., & NILSEN, O.G. (1985) Inhalation
of isopropanol: induction of activating and deactivating
enzymes in rat kidney and liver, increased microsomal
metabolism of n-hexane. Toxicology, 34: 57-66.
291. ZAKHARI, S. (1977) Isopropanol and ketones in the
environment, Oxford, England, CRC Press.
292. ZINBO, M. (1984) Determination of one-carbon to three-carbon
alcohols and water in gasoline/alcohol blends by liquid
chromatography. Anal. Chem., 56: 244-247.
RESUME
1. Identité, propriétés physiques et chimiques, méthodes d'analyse
Le propanol-2 est un liquide incolore, très inflammable dont
l'odeur rappelle celle d'un mélange d'éthanol et d'acétone. Il est
entièrement miscible à l'eau, à l'éthanol, à l'acétone, au
chloroforme et au benzène. Il existe des méthodes d'analyse pour
la recherche du propanol-2 dans divers milieux (air, eau, sang,
sérum et urine), avec des limites de détection dans l'air, l'eau et
le sang respectivement égales à 2 x 10-5 mg/m3, 0,04 mg/litre et 1
mg/litre. La chromatographie en phase gazeuse (essentiellement
avec détection par ionisation de flamme) ainsi que l'électrophorèse
sur papier et la spectrométrie de mobilité ionique par
photoionisation permettent de doser le propanol-2 dans divers
mileux.
2. Sources d'exposition humaine et environnementale
On estime qu'en 1975, la production mondiale de propanol-2
dépassait 1100 kilotonnes, la capacité mondiale de production étant
supérieure à 2000 kilotonnes en 1984. Le propanol-2 est couramment
produit à partir du propène. Les procédés antérieurs de
fabrication qui reposaient sur l'utilisation d'acides forts ou
d'acides faibles, et donnaient naissance à des produits
intermédiaires et à des sous-produits potentiellement dangereux,
sont désormais largement supplantés par le procédé d'hydratation
catalytique. On peut également procéder par réduction catalytique
de l'acétone.
Le propanol-2 est un produit du métabolisme de divers micro-
organismes.
Il a de nombreuses applications comme solvant et il entre dans
la composition de différents produits ménagers et produits de soins
personnels, sous forme d'aérosols et d'excipients pour produits
pharmaceutiques à usage externe et pour cosmétiques. Le propanol-2
est également utilisé pour la production de l'acétone et autres
produits chimiques, comme agent de dégivrage, comme conservateur et
il entre dans la composition des concentrés pour le nettoyage des
pare-brise et de certains aromatisants alimentaires.
Le propanol-2 peut pénétrer dans l'atmosphère, dans l'eau et
dans le sol lors du rejet de déchets et on en a trouvé dans l'air
et dans les eaux de lessivage de décharges mal protégées. Présent
dans les gaz et eaux résiduaires industriels, on peut l'en éliminer
par oxydation biologique ou osmose inverse. Il peut être dissipé
dans l'atmosphère lors de l'utilisation de produits de consommation
qui en contiennent.
3. Transport et distribution dans l'environnement
C'est principalement lors d'opérations telles que la
production, la transformation, le stockage, le transport,
l'utilisation et le rejet de déchets que le propanol-2 pénètre dans
l'environnement atmosphérique. Il peut être également déchargé
dans le sol et l'eau. Il est difficile d'évaluer la part qui
revient à chaque compartiment du milieu. Toutefois on estimait en
1976 que plus de 50 % du propanol-2 produit finissait par être
libéré dans l'atmosphère.
Le propanol-2 est rapidement éliminé de l'atmosphère par
réaction sur les radicaux hydroxyles et entraînement par les
précipitations. Ce sont ces dernières qui sont responsables du
transport de ce composé de l'atmosphère dans le sol ou l'eau. Une
fois dans le sol, il doit y être très mobile et augmente la
perméabilité du sol à certains hydrocarbures aromatiques. Le
propanol-2 est facilement biodégradable par voie aérobie ou
anaérobie.
Comme il est biodégradable et complètement miscible à l'eau,
avec un coefficient de partition octanol/eau logarithmique de 0,14
et un facteur de bioconcentration de 0,5, il est peu probable qu'il
donne lieu à une bioaccumulation.
4. Niveaux dans l'environnement et exposition humaine
L'exposition de la population en général peut se produire par
ingestion accidentelle ou volontaire, par absorption de nourriture
contenant du propanol-2 d'origine naturelle, ou sous forme
d'aromatisant volatil ou de résidus de solvant, ou encore par
inhalation lors de l'utilisation de produits qui en contiennent.
On en a trouvé aux concentrations de 0,2 à 325 mg/litre dans des
boissons non alcoolisées et aux concentrations de 50 à 3000 mg/kg
dans des denrées alimentaires pour la production desquelles on
l'avait utilisé comme solvant. L'exposition de la population en
général par inhalation de l'air ambiant est faible, du fait de
l'élimination et de la dégradation rapides de ce produit. En
procédant à des contrôles en divers lieux et, en particulier, dans
des sites urbains, on a obtenu des concentrations moyennes
pondérées par rapport au temps allant jusqu'à 35 mg/m3.
Les travailleurs peuvent être exposés au propanol-2 au cours de
la production du composé lui-même, lors de la fabrication de
l'acétone ou d'autres dérivés et également lorsqu'on utilise ce
produit comme solvant. Aux Etats-Unis, une enquête (National
Occupational Exposure Survey) effectuée en 1980-83 a montré que
plus de 1,8 million de travailleurs pouvaient être exposés. On a
mesuré sur les lieux de travail des concentrations atteignant 1350
mg/m3 avec des moyennes pondérées par rapport au temps allant
jusqu'á 500 mg/m3.
5. Cinétique et métabolisme
Le propanol-2 est rapidement absorbé et se répartit dans tout
l'organisme par inhalation et ingestion. A forte dose,
l'absorption dans les voies digestives est retardée. Les taux
sanguins de propanol-2 (décelables lorsqu'il y a ingestion
simultanée d'éthanol) ou de son métabolite, l'acétone, sont
corrélés avec l'intensité de l'exposition. Des volontaires qui
avaient ingéré une dose de 3,75 mg/kg de propanol-2 (avec 1200 mg
d'éthanol/kg) dans du jus d'orange, présentaient une concentration
sanguine maximale de propanol-2 libre de 0,8 ± 0,3 mg/litre, et de
2,3 ± 1,4 mg/litre après incubation en présence d'arylsulfatase, ce
qui témoigne d'une sulfatation. Chez les ouvriers exposés à des
vapeurs de propanol-2 (8 - 647 mg/m3), on a observé des
concentrations de 3 - 270 mg/m3 dans l'air alvéolaire, mais dans ce
cas, c'est de l'acétone et non du propanol-2 que l'on a trouvé dans
le sang et les urines. Chez des animaux de laboratoire exposés au
propanol-2, on a retrouvé celui-ci non seulement dans le sang mais
également dans le liquide céphalo-rachidien, dans le foie, les
reins et le cerveau. Le propanol-2 traverse la barrière
hémoméningée deux fois plus facilement que l'éthanol. Le propanol-
2 est excrété en partie tel quel et en partie sous forme d'acétone,
essentiellement au niveau des poumons mais également dans la salive
et le suc gastrique. Il peut y avoir réabsorption après excrétion
par ces deux dernières voies. La métabolisation en acétone en
présence d'alcool-déshydrogénase (ADH) hépatique est assez lente,
du fait que l'ADH a une moindre affinité pour le propanol-2 que
pour l'éthanol. In vitro, l'activité de l'ADH humaine vis-à-vis du
propanol-2 correspond à 9 - 10 % de l'activité de cette enzyme
lorsque le substrat est de l'éthanol. Les oxydases microsomiques
du foie de rat sont également capables d'oxyder le propanol-2
in vitro. Chez l'homme, l'acétone est excrétée telle quelle,
essentiellement au niveau des poumons et en quantité minime au
niveau des reins. Plus l'exposition au propanol-2 se prolonge,
plus la concentration d'acétone dans l'air alvéolaire, dans le sang
et dans les urines est élevée. Le propanol-2 et l'acétone sont
éliminés de l'organisme selon une cinétique du premier ordre et
leur demi-vie chez l'homme est de 2,5 - 6,4 heures et 22 heures,
respectivement.
6. Effets sur les êtres vivants dans leur milieu naturel
Le propanol-2 est peu toxique pour la faune et la flore
aquatiques, les insectes et les plantes. Son seuil d'inhibition de
la multiplication cellulaire, mesuré chez une espèce sensible de
protozoaire, varie de 104 à 4930 mg/litre selon les conditions
expérimentales. Si l'on s'élève dans l'arbre phylogénétique, on
constate que diverses espèces de crustacés, notamment Daphnia
magna, présentent des CE50 allant de 2285 à 9714 mg/litre. Pour
des poissons d'eau douce, on a obtenu des CL50 à 96 heures allant
de 4200 à 11 130 mg/litre. Chez la drosophile, les CL50 vont de
10 200 à 13 340 mg/litre de milieu nutritif. Pour le troisième
stade larvaire du moustique Aedes aegypti, on a obtenu, lors d'une
épreuve statique de 4 heures, des valeurs de la CL50 allant de 25 à
120 mg/litre.
L'exposition de végétaux à du propanol-2 à des concentrations
comprises entre 2100 mg/litre et plus de 36 000 mg/litre, a
provoqué toute une gamme d'effets allant de l'absence totale
d'anomalies à une inhibition complète de la germination.
7. Effets sur les animaux d'expérience et les systèmes d'épreuves
in vitro
A en juger d'après la mortalité qu'il provoque, le propanol-2
présente une faible toxicité aiguë pour les mammifères, que
l'exposition ait lieu par voie orale, percutanée ou respiratoire.
Chez plusieurs espèces animales on a obtenu, après administration
par voie orale, des valeurs de la DL50 allant de 4475 à 7990 mg par
kg de poids corporel; la CL50 pour une inhalation de 8 heures
allait de 46 000 à 56 000 mg/m3 d'air chez le rat. A ces doses
mortelles, les rats présentaient une forte irritation des muqueuses
et une grave dépression du système nerveux central. La mort est
survenue par arrêt cardiaque ou respiratoire. Entre autres lésions
histopathologiques, on notait une congestion et un oedème du poumon
ainsi qu' une dégénérescence des hépatocytes.
Administré en dose unique par voie orale à des rats à raison de
3000 ou 6000 mg par kg de poids corporel, le propanol-2 a provoqué
une accumulation réversible de triglycérides dans le foie. On a
également observé chez ces rats une induction des enzymes
microsomiques à la dose de 390 mg/kg.
Non dilué, le propanol-2 n'est pas irritant en applications de
4 heures sur la peau, intacte ou abrasée, de lapins tondus.
Toutefois, en instillations oculaires de 0,1 ml, le propanol-2 non
dilué a provoqué une irritation chez le lapin. De fortes
concentrations de vapeurs de propanol-2 ont provoqué une irritation
respiratoire chez la souris et l'on a noté une réduction de 50 % du
rythme respiratoire à des concentrations allant de 12 300 à 43 525
mg/m3 d'air.
Les études conscrées aux effets sur l'animal d'exposition
répétées au propanol-2 sont plutôt limitées. Après inhalation par
des rats de 500 mg/m3 de propanol-2, cinq jours par semaine et
quatre heures par jour pendant quatre mois, on a noté une
irritation des voies respiratoires, des anomalies haématologiques
et des altérations histopathologiques au niveau du foie et de la
rate. Un autre groupe expérimental composé de cinq rats de chaque
sexe a reçu pendant 27 semaines du propanol-2 dans son eau de
boisson. En comparant les animaux qui recevaient environ 600 ou
2300 mg/kg par jour (mâles) et 1000 ou 3900 mg/kg par jour
(femelles) de propanol-2 à des témoins non traités, on a constaté
un retard de croissance, mais uniquement chez les deux groupes de
femelles traitées. Aucun autre effet indésirable n'a été constaté.
Les données disponibles donnent à penser que le propanol-2
produit sur le système nerveux central (SNC) des effets analogues à
ceux de l'éthanol. La DE50 d'anésthésie par voie orale est de 2280
mg/kg chez le lapin, la DE50 par voie intrapéritonéale
correspondant à la perte du réflexe de redressement chez la souris
est égale à 165 mg/kg et le seuil d'induction de l'ataxie par voie
intrapéritonéale est de 1106 mg/kg chez le rat. Ces valeurs sont
sont environ deux fois plus faibles que pour l'éthanol. Lors d'une
expérience menée à l'air libre, on a constaté que l'inhalation de
propanol-2 à la dose de 739 mg/m3, dix heures par jour, et cinq
jours par semaine pendant 15 semaines ne produisait aucun effet
indésirable.
Le propanol-2 a été soumis à une étude portant sur deux
générations de rats qui ont reçu dans leur eau de boisson des doses
quotidiennes de 1290, 1380 ou 1479 mg de ce produit par kg de poids
corporel. Les seuls effets indésirables qui ont été notés
consistaient dans une réduction passagère du taux de croissance
dans la génération F0. En revanche, d'autres chercheurs ont
constaté une augmentation des malformations lors d'une étude
tératogènicité, au cours de laquelle on avait administré à des
rates gravides, des doses orales quotidiennes de 252 ou 1008 mg de
propanol-2 par kg de poids corporel (toxicité maternelle non
étudiée). On a également indiqué que ces deux doses, administrées
pendant 45 jours dans l'eau de boisson, faisaient passer le cycle
oestral à cinq jours (contre quatre chez les témoins). Chez des
rates ayant reçu pendant six mois dans leur eau de boisson, des
doses quotidiennes de propanol-2 de 1800 mg/kg avant de mettre bas,
on a constaté un accroissement de la mortalité embryonnaire totale;
divers effets ont également été signalés concernant la survie
intra-utérine et postnatale à des doses aussi basses que 0,8 mg/kg,
sans qu'on puisse toutefois dégager une tendance précise. Des
rates gravides ont été exposées à de l'air contenant du propanol-2
aux concentrations respectives de 9001, 18 327 et 23 210 mg/m3
(3659, 7450 ou 9435 ppm). Les deux concentrations les plus fortes
se sont révélées toxiques pour les mères, la concentration de 9001
mg/m3 ne produisant aucun effet. A toutes les concentrations on a
constaté un effet nocif sur le développement.
Une épreuve de recherche des mutations ponctuelles utilisant
S. typhimurium a donné des résultats négatifs avec du propanol-2 à
la dose de 0,18 mg par boîte; la recherche d'échanges entre
chromatides soeurs sur fibroblastes de hamster chinois a également
été négative. Le propanol-2 a provoqué des anomalies de la mitose
dans des cellules médullaires de rat ainsi que des cellules de
l'extrêmité radiculaire d'oignons in vitro. On ne dispose d'aucune
autre donnée sur la mutagénicité de ce produit.
Un certain nombre d'études restreintes ont été consacrées au
pouvoir cancérogène du propanol-2, au cours desquelles des souris
ont été exposées à cette substance par voie percutanée (3 fois par
semaine pendant un an), par voie respiratoire (7700 mg/m3, 3 à 7
heures par jour, cinq jours par semaine pendant 5 à 8 mois) et par
voie sous-cutanée (20 mg de propanol non dilué par semaine pendant
20 à 40 semaines). Au cours de ces trois études, on a recherché la
présence de tumeurs sur la peau, dans les poumons et au point
d'injection. Aucun signe d'effets cancérogènes n'a été observé.
On ne dispose pas de données épidémiologiques suffisantes pour
évaluer la cancérogénicité du propanol-2 chez l'homme. A la
lumière des données disponibles, on peut penser que le sulfate de
dipropyl-2, un produit intermédiaire de la fabrication du
propanol-2 par le procédé aux acides forts et faibles, pourrait
être à l'origine de cancers du sinus maxillaire chez l'homme.
8. Effets sur la santé de l'homme
On a signalé plusieurs cas d'intoxication consécutifs à
l'ingestion de propanol-2 ou à l'utilisation de lotions à base de
ce produit pour rafraîchir des enfants fébriles. Les principaux
signes d'intoxication rappellent ceux de l'intoxication alcoolique:
nausées, vomissements, douleurs abdominales, gastrite, hypotension
et hypothermie. La dépression du SNC par le propanol-2 est deux
fois plus intense qu'avec l'éthanol et entraîne l'inconscience puis
un coma profond; la mort peut survenir par dépression respiratoire.
Parmi les autres effets on peut noter l'hyperglycémie, un taux
élevé de protéines dans le liquide céphalorachidien et une
atélectasie. Il semblerait que l'absorption percutanée soit
négligeable mais on connaît le cas d'un enfant qui a été intoxiqué
après avoir été lotionné avec du propanol-2, ce qui donne à penser
qu'il ne faut pas négliger l'absorption percutanée, notamment chez
les enfants. Aucun effet indésirable n'a été observé chez des
volontaires en bonne santé qui avaient bu tous les jours pendant
six semaines un sirop contenant du propanol-2 à des doses
corrrespondant respectivement à 2,6 et 6,4 mg de propanol-2 par kg
de poids corporel. Des volontaires du sexe masculin, exposés 3 à 5
minutes à des vapeurs de propanol-2 correspondant à des
concentrations de 490, 980 ou 1970 mg/m3 d'air, ont estimé qu'ils
ressentaient une irritation légère à 980 mg/m3 et que la situation
était "satisfaisante" pendant les 8 heures correspondant à leur
propre exposition professionnelle.
Des enfants prématurés ayant subi un contact prolongé avec du
propanol-2 ont présenté une irritation cutanée prenant la forme
d'érythèmes, voire de brûlures du 2ème et du 3ème degré et de
phlyctènes. On a également signalé ça et là des cas de dermatites
allergiques de contact.
Il n'existe de peu d'études épidémiologiques consacrées à la
mortalité par cancers ou autres maladies provoquées par le
propanol-2. Parmi un groupe de 61 travailleurs, employés pendant
plus de cinq ans dans un atelier de fabrication de propanol-2 par
le procédé à l'acide fort, on a observé sept cas de cancer dont
quatre de cancer des sinus maxillaires. Une étude portant sur une
cohorte de 779 travailleurs d'un atelier analogue a révélé que
l'incidence des cancers du sinus et du larynx, corrigée de
l'influence de l'âge et du sexe, était 21 fois plus forte que
prévue. La période minimale de latence était de dix ans. Dans une
autre étude rétrospective de cohorte portant sur le personnel d'une
autre usine où on utilisait le procédé à l'acide fort, on a
constitué une cohorte représentant plus de 4000 années-hommes
exposés au risque. Les résultats de cette étude ont montré que les
taux de mortalité pour toutes causes et les taux de mortalité par
cancer n'étaient pas sensiblement plus élevés que les taux
prévisibles. Une autre étude rétrospective a été menée dans une
usine qui fabriquait du propanol-2 par le procédé à l'acide faible.
Cette fois, on comptait plus de 11 000 années-hommes exposés au
risque. Dans ce cas, le taux de mortalité pour toutes causes était
inférieur aux prévisions et l'on ne constatait pas de surmortalité
attribuable aux cancers en général. Toutefois, l'incidence des
cancers de la bouche et du pharynx était 4 fois supérieure à la
normale. Dans l'ensemble, ces études de cohorte donnent à penser
qu'il existe un risque de cancer imputable au procédé à l'acide
fort; toutefois lors de deux petites études castémoins, on n'a noté
aucune association entre l'exposition au propanol-2 et l'incidence
des gliomes ou de la leucémie lymphatique.
Certaines études font état d'une potentialisation de la
toxicité du tétrachlorure de carbone chez des ouvriers
simultanément exposés au propanol-2.
9. Résumé de l'évaluation
L'homme peut être exposé au propanol-2 par inhalation lors de
la fabrication, de la transformation ou de l'utilisation de cette
substance dans le cadre professionnel ou domestique. En ce qui
concerne la population en général, l'exposition à des doses
potentiellement mortelles peut se produire par suite d'ingestion
accidentelle ou volontaire de cette substance et les enfants
peuvent être exposés par application de lotions à base de propanol-
2.
Le propanol-2 est vite absorbé et se répartit rapidement dans
l'ensemble de l'organisme, en partie sous forme d'acétone. Les
données relatives aux effets aigus consécutifs à l'exposition
d'êtres humains à des doses excessives sont rares et contrastées.
Les principaux effets consistent en gastrite, dépression du système
nerveux central, hypothermie, dépression respiratoire et
hypotension. Les données de mortalité aiguë obtenues sur des
animaux de laboratoire indiquent que le propanol-2 est peu toxique,
les valeurs de la DL50 par voie orale chez diverses espèces vont de
4475 à 7990 mg/kg, et les valeurs de la CL50 par inhalation se
situent aux alentours de 50 000 mg/m3 chez le rat. Chez le lapin,
le propanol-2 ne provoque pas d'irritation cutanée, toutefois
l'instillation de 0,1 ml de cette substance non diluée dans les
yeux a provoqué une irritation.
Chez l'homme, les effets aigus les plus probables d'une
exposition de fortes concentrations de propanol-2 par ingestion ou
inhalation, consistent en une intoxication de type alcoolique
aboutissant à la narcose.
Les études sur l'animal sont insuffisantes pour qu'on puisse
évaluer les risques encourus par l'homme à la suite d'expositions
répétées au propanol-2. Toutefois les résultats de deux études à
court terme chez le rat, au cours desquelles on a fait a) inhaler
500 mg/m3 de cette substance, 4 heures par jour, 5 heures par
semaine pendant 4 mois et b) ingérer le même produit à raison de
600 à 3900 mg/kg dans l'eau de boisson, donnent à penser qu'il
serait préférable d'éviter de s'exposer aux fortes concentrations
en propanol-2 signalées dans le cadre de certaines activités
professionnelles.
En faisant inhaler du propanol-2 à des rattes gravides on a
constaté que le seuil d'apparition d'un effet se situait à 18 327
mg/m3 (7450 ppm), la dose sans effet observable était de 9001 mg/m3
(3659 ppm), la toxicité maternelle étant prise comme critère. Au
cours de la même étude, le seuil d'apparition d'effets s'est situé
à 9001 mg/m3 (3659 ppm) pour les anomalies du développement et
aucune dose sans effet observable n'a pu être mise en évidence.
Ces concentrations sont plus élevées que celles auxquelles l'homme
est susceptible d'être exposé.
Les épreuves de génotoxicité ont donné des résultats négatifs
dans le cas du propanol-2, cependant on a observé des anomalies de
la mitose dans des cellules médullaires de rats. Ces résultats
indiquent que le propanol-2 n'est pas du tout génétoxique mais les
données sont trop limitées pour qu'on puisse se prononcer
véritablement sur le pouvoir mutagène de cette substance.
Les données existantes sont insuffisantes pour permettre une
évaluation de la cancérogénicité du propanol-2 chez l'animal
d'expérience. On ne dispose pas de données permettant d'évaluer
cette cancérogénicité chez l'homme.
Le propanol-2 ne fait probablement pas courir de risque
important à la population dans son ensemble dans les conditions
d'exposition qui sont susceptibles de se produire.
Le propanol-2 disparaît rapidement (demi-vie, 5 jours) de
l'atmosphère et s'élimine à bref délai de l'eau et du sol par
biodégradation aérobie ou anaérobie, en particulier une fois que
les microorganismes préalablement ensemencés se sont adaptés.
Compte tenu de ses propriétés physiques, le propanol-2 n'a qu'une
faible tendance à la bioaccumulation. Il ne présente pas de risque
pour la faune et la flore aux concentrations auxquelles il est
habituellement présent dans l'environnement.
RESUMEN
1. Identidad, propiedades físicas y químicas, métodos analíticos
El 2-propanol es un líquido incoloro, sumamente inflamable, con
un olor que recuerda al de la mezcla de etanol y acetona. El
compuesto es completamente miscible con agua, etanol, acetona,
cloroformo y benceno. Se dispone de métodos analíticos para
detectar el 2-propanol en diversos medios (aire, agua, sangre,
suero y orina) con límites de detección de 2 x 10-5 mg/m3, 0,04
mg/litro y 1 mg/litro en el aire, el agua y la sangre,
respectivamente. Existen métodos de cromatografía de gases (que se
sirven principalmente de la detección de ionización de llama) así
como métodos de electroforesis en papel y de espectrometría de
movilidad iónica inducida por fotoionización para determinar el
2-propanol en los distintos medios.
2. Fuentes de exposición humana y ambiental
La producción mundial estimada de 2-propanol en 1975 fue
superior a 1100 kilotoneladas y la capacidad de producción mundial
en 1984 se cifró en más de 2000 kilotoneladas. El 2-propanol se
fabrica comúnmente a partir del propeno. Los antiguos procesos
basados en ácidos fuertes y débiles, en los que se generaban
productos intermedios y desechos potencialmente peligrosos, se han
sustituido actualmente en gran medida por el proceso de hidratación
catalítica. La reducción catalítica de la acetona es otro proceso
posible.
El 2-propanol se ha identificado como producto metabólico de
diversos microorganismos.
El compuesto tiene amplias aplicaciones como disolvente y se
utiliza como componente de productos domésticos y personales, entre
ellos vaporizadores de aerosoles, productos farmacéuticos de
aplicación tópica y cosméticos. El 2-propanol se utiliza también
para producir acetona y otras sustancias químicas, como agente
descongelante, como conservante, en concentrados para
limpiaparabrisas y como aromatizante volátil en alimentos.
El 2-propanol puede ingresar en la atmósfera, el agua o el
suelo por la evacuación de desechos y se ha aislado en el aire y en
el líquido que rezuma de basureros y terraplenados. Se encuentra
en los gases y las aguas residuales que emiten algunas industrias,
y puede extraerse de esas aguas por oxidación biológica o por
ósmosis inversa. Durante el uso de 2-propanol en productos de
consumo se producen emisiones dispersas a la atmósfera.
3. Transporte, distribución y transformación en el medio ambiente
La vía principal de entrada del 2-propanol en el medio ambiente
es su emisión a la atmósfera durante la producción, el tratamiento,
el almacenamiento, el transporte, el uso y la evacuación. También
se producen emisiones al suelo y al agua. Es difícil calcular el
volumen que ingresa en cada compartimiento ambiental. No obstante,
se calculó que en 1976 la liberación total de este compuesto en la
atmósfera fue superior al 50% del 2-propanol producido.
El 2-propanol desaparece rápidamente de la atmósfera por
reacción con radicales hidroxilo y arrastrado por la lluvia. A
este último proceso se debe el transporte del 2-propanol desde la
atmósfera hasta el suelo o el agua. Una vez que está en el suelo,
se cree que es muy móvil y que aumenta la permeabilidad del suelo a
algunos hidrocarburos aromáticos. El 2-propanol es fácilmente
biodegradable, en condiciones tanto aerobias como anaerobias.
La bioacumulación del compuesto no es probable, dados su
carácter biodegradable y su miscibilidad total con el agua; su
coeficiente de reparto log n-octanol/agua es de 0,14 y su factor de
bioconcentración de 0,5.
4. Niveles ambientales y exposición humana
La exposición de la población general se produce por ingestión
accidental o intencionada, por la ingestión de alimentos que lo
contengan como aromatizante volátil natural o añadido o como
residuo de disolvente, y por inhalación durante su uso. Se han
encontrado concentraciones de 0,2 a 325 mg por litro en bebidas no
alcohólicas y de 50 a 3000 mg por kg en alimentos tras el uso de
2-propanol como disolvente en su producción. La exposición de la
población general por inhalación de aire ambiental es baja a causa
de su rápida desaparición y degradación. Se han estudiado diversas
localizaciones y se han medido concentraciones medias ponderadas en
función del tiempo de hasta 35 mg/m3 en emplazamientos urbanos.
Los trabajadores se ven expuestos al 2-propanol durante la
producción del propio compuesto y de acetona y otros derivados, así
como durante su uso como disolvente. En la encuesta nacional de
exposición ocupacional (1980 - 83) realizada en los Estados Unidos,
se estimó que más de 1,8 millones de trabajadores estaban
potencialmente expuestos. En ciertos lugares de trabajo se han
medido concentraciones de hasta 1350 mg/m3, con promedios
ponderados en función del tiempo de hasta 500 mg/m3.
5. Cinética y metabolismo
El 2-propanol se absorbe y distribuye rápidamente por todo el
organismo tras su inhalación o ingestión. A dosis elevadas se
retrasa la absorción gastrointestinal. Las concentraciones
sanguíneas de 2-propanol (detectables cuando se ingiere etanol
simultáneamente) o de su metabolito, la acetona, guardan relación
con los niveles de exposición. En voluntarios que ingirieron una
dosis de 3,75 mg/kg (con 1200 mg de etanol/kg) en zumo de naranja,
se observó un nivel máximo de 0,8 ± 0,3 mg de 2-propanol libre por
litro en la sangre, y de 2,3 ± 1,4 mg por litro tras la incubación
con arilsulfatasa, lo que es un signo de sulfatación. Los
trabajadores expuestos a vapores (8 - 647 mg/m3) mostraron
concentraciones de 3 - 270 mg/m3 en el aire alveolar, pero en este
caso se encontró acetona y no 2-propanol en la sangre y la orina.
En animales de laboratorio tratados, el 2-propanol se detectó no
sólo en la sangre sino también en el líquido espinal, el hígado,
los riñones y el cerebro. Atraviesa la barrera hematocerebral dos
veces mejor que el etanol. El 2-propanol se excreta en parte como
tal y en parte como acetona, principalmente por vía pulmonar, pero
también en la saliva y el jugo gástrico. La reabsorción puede
producirse después de la excreción por las últimas dos vías. La
transformación en acetona por medio de la deshidrogenasa alcohólica
del hígado es más bien lenta, porque la afinidad relativa de la
deshidrogenasa por el 2-propanol es más baja que por el etanol.
In vitro, la actividad enzimática de la deshidrogenasa humana con
2-propanol fue del 9 - 10% de la actividad que exhibe cuando el
sustrato es el etanol. In vitro las oxidasas microsómicas de
hígado de rata también son capaces de oxidar el 2-propanol. En el
hombre, la acetona se excreta sin cambios, principalmente por los
pulmones y en cantidad mínima por los riñones. La concentración de
acetona en el aire alveolar, la sangre y la orina aumenta con la
intensidad y la duración de la exposición al 2-propanol. La
eliminación de 2-propanol y de acetona del organismo es de primer
orden, y los periodos de semieliminación en el hombre son de
2,5 - 6,4 horas y 22 horas, respectivamente.
6. Efectos en los organismos en el medio ambiente
La toxicidad del 2-propanol para organismos acuáticos, insectos
y plantas es baja. El umbral inhibitorio para la multiplicación
celular de una especie de protozoo sensible varió de 104 a 4930 mg
por litro en diversas condiciones experimentales. Avanzando en la
cadena filogenética, varias especies de crustáceos, incluida
Daphnia magna, mostraron CE50 a concentraciones que iban desde 2285
hasta 9714 mg por litro. Las CL50 (96 h) para peces de agua dulce
variaron desde 4200 hasta 11 130 mg por litro. Los datos obtenidos
para especies de moscas de la fruta mostraron CL50 comprendidas
entre 10 200 y 13 340 mg por litro de medio nutritivo. La CL50
para larvas de mosquito ( Aedes aegypti) en la tercera etapa de
desarrollo fue de 25 - 120 mg/litro en un ensayo estático de 4
horas.
Los efectos que tiene en las plantas la exposición a 2-propanol
en concentraciones entre 2100 mg/litro y más de 36 000 mg/litro
variaron entre la ausencia de efecto y la inhibición total de la
germinación.
7. Efectos en animales de experimentación y en sistemas de ensayo
in vitro
La toxicidad aguda del 2-propanol para los mamíferos, a juzgar
por la mortalidad, es baja, sea cual sea la vía de exposición oral,
cutánea o respiratoria. Los valores de la DL50 para varias
especies animales tras la administración oral variaron entre 4475 y
7990 mg por kg de peso corporal; La CL50 de inhalación durante 8
horas en la rata varió de 46 000 a 55 000 mg por m3 de aire. A
estos niveles letales, las ratas mostraron grave irritación de las
mucosas y depresión profunda del sistema nervioso central. La
muerte fue provocada por paro respiratorio o cardiaco. Entre las
lesiones histopatológicas figuraron la congestión y el edema
pulmonar, así como la degeneración celular en el hígado.
Con dosis orales únicas de 3000 ó 6000 mg de 2-propanol por kg
de peso corporal se produjo una acumulación reversible de
triglicéridos en el hígado de la rata. En la rata se observó
inducción de enzimas microsómicas a dosis orales de 390 mg/kg.
El 2-propanol sin diluir no produjo irritaciones cuando se
aplicó a la piel cortada o raspada del conejo durante 4 horas. En
cambio, se observó irritación cuando se aplicó 0,1 ml de compuesto
sin diluir en el ojo del conejo. Con concentraciones de vapor
elevadas de 2-propanol se produjo irritación del tracto
respiratorio en el ratón, y el ritmo respiratorio disminuyó en un
50% a concentraciones de 12 300 - 43 525 mg/m3 de aire.
Se han hecho escasos estudios de exposición repetida sobre los
efectos del 2-propanol en animales. Tras la inhalación de 500 mg
de 2-propanol/m3 durante 5 días a la semana y 4 horas al día
durante más de 4 meses, se observaron irritación del tracto
respiratorio, cambios hematológicos y alteraciones histopatológicas
en el hígado y el bazo de la rata. En otro grupo de estudio, se
administró a 5 ratas de cada sexo agua de bebida que contenía
2-propanol durante 27 semanas. La comparación de animales que
recibían aproximadamente 600 ó 2300 mg por kg al día (machos) y
1000 ó 3900 mg por kg al día (hembras) con grupos de control no
tratados reveló un retraso del crecimiento sólo en ambos grupos de
hembras expuestas. No se observaron otros efectos adversos.
Los datos disponibles indican que los efectos del 2-propanol en
el sistema nervioso central son semejantes a los del etanol. La
DE50 por vía oral para la narcosis en conejos es de 2280 mg/kg; la
DE50 intraperitoneal correspondiente a la pérdida del reflejo de
enderezamiento en el ratón es de 165 mg/kg, y el umbral
intraperitoneal de inducción de ataxia en la rata es de 1106 mg/kg.
Estos valores son aproximadamente dos veces más bajos que los
correspondientes al etanol. La inhalación de 2-propanol a una
concentración de 739 mg/m3 durante 6 horas al día y 5 días a la
semana durante 15 semanas no originó ningún resultado adverso en un
ensayo en campo abierto.
El 2-propanol se evaluó en un estudio de 2 generaciones de
ratas mediante la administración de 1290, 1380 ó 1470 mg por kg al
día en el agua de bebida a ambas generaciones. El único efecto
adverso observado fue una reducción transitoria del ritmo de
crecimiento en la generación Fo. En cambio, otros investigadores
observaron un aumento de las malformaciones en un estudio de la
teratogénesis después de administrar por vía oral a ratas gestantes
252 ó 1008 mg de 2-propanol por kg al día (no se formularon
observaciones sobre la toxicidad materna). Ambas dosis,
administradas en el agua de bebida durante 45 días, también
aumentaron la duración del ciclo estrual hasta 5 días (frente a 4
días en los sujetos de control). Se observó una mortalidad
embrionaria total mayor cuando se administraban a la rata hembra
dosis de 1800 mg/kg en el agua de bebida al día durante 6 meses
antes de criar; se notificaron diversos efectos en la supervivencia
intrauterina y puerperal a dosis tan bajas como 0,18 mg/kg al día,
pero no se observó ninguna pauta coherente. Se expusieron ratas
gestantes a 2-propanol atmosférico a concentraciones de 9001,
18 327 ó 23 210 mg por m3 (3659, 7450 ó 9435 ppm). Las dos
concentraciones más elevadas fueron tóxicas para las madres, pero
no así la de 9001 mg/m3. Se observó toxicidad en el desarrollo con
las tres concentraciones.
El 2-propanol dio resultados negativos en una prueba con 0,18
mg por placa para detectar mutuaciones puntuales en S.
typhimurium y en una prueba de intercambio de cromátidas hermanas
en fibroblastos pulmonares de hámster chino. Indujo anomalías
mitóticas en células de médula ósea de rata y en células de ápice
radicular de cebolla in vitro. No se dispone de otros datos sobre
mutagenicidad.
El 2-propanol se ensayó en varios estudios limitados de
carcinogenicidad en el ratón utilizando las vías de exposición
cutánea (3 veces a la semana durante un año), inhalación (7700
mg/m3 durante 3 - 7 h/día, 5 días/semana, durante 5 - 8 meses) y
subcutánea (20 mg sin diluir, semanalmente durante 20 - 40
semanas). La aparición de tumores se investigó en los tres
estudios en la piel, el pulmón y el lugar de inyección,
respectivamente. No se observaron efectos carcinogénicos. No se
dispone de datos epidemiológicos adecuados con los que evaluar la
carcinogenicidad del 2-propanol para el ser humano. Los datos
disponibles indican que el di-2-propilsulfato, un producto
intermedio en los procesos de ácidos fuertes y débiles para
producir 2-propanol, puede estar asociado causalmente con la
inducción de cáncer del seno paranasal en el ser humano.
8. Efectos en la salud humana
Se han notificado varios casos de intoxicación tras la
ingestión oral y también en niños con fiebre a los que se refrescó
con esponjas impregnadas con preparaciones con 2-propanol. En
casos de envenenamiento, los principales signos son los de la
intoxicación alcohólica, en particular náuseas, vómitos, dolores
abdominales, gastritis, hipotensión e hipotermia. El 2-propanol
deprime el sistema nervioso central unas dos veces más que el
etanol, provocando una inconsciencia que termina en coma profundo;
puede sobrevenir la muerte por depresión respiratoria. Otros
efectos relacionados con el compuesto son la hiperglucemia,
elevados niveles de proteínas en el líquido cefalorraquídeo y
atelectasia. Aunque se considera que la absorción por la piel es
insignificante, en un informe sobre un caso de un niño intoxicado
tras refrescársele con una esponja impregnada con 2-propanol, se
indicaba que no conviene subestimar el riesgo de absorción dérmica,
especialmente en los niños. No se observaron efectos adversos en
voluntarios sanos que bebieron diariamente durante 6 semanas un
jarabe que contenía 2,6 ó 6,4 mg de 2-propanol/kg. Un grupo de
varones voluntarios, cuando se expusieron a vapores de 2-propanol
en concentraciones de 490, 980 ó 1970 mg por m3 de aire durante
3 - 5 minutos juzgaron que la irritación era "leve" a 980 mg/m3 y
"satisactoria" para su propia exposición ocupacional de 8 horas.
Las irritaciones de la piel en forma de eritema, quemaduras de
segundo y tercer grado y ampollas se notificaron en niños
prematuros tras un contacto prolongado con 2-propanol. En
ocasiones también se han notificado casos de dermatitis alérgica
por contacto.
Se dispone de pocos estudios epidemiológicos sobre mortalidad
por cáncer o por otras causas. En un grupo de 71 trabajadores
empleados durante más de 5 años en una fábrica de 2-propanol por el
proceso del ácido fuerte, se notificaron 7 casos de cáncer, entre
ellos 4 de cáncer del seno paranasal. En un estudio de cohortes
realizado sobre 779 trabajadores en una fábrica similar, las
incidencias reajustadas en función de la edad y del sexo de cáncer
del seno y de la laringe fueron 21 veces mayores de lo esperado.
El periodo mínimo de latencia fue de 10 años. En otro estudio
retrospectivo de cohortes realizado en otra fábrica que utilizaba
el proceso del ácido fuerte, había más de 4000 personas-años
expuestas. Los resultados mostraron que las tasas de mortalidad
por todas las causas y por neoplasmas no eran significativamente
mayores de lo previsto. Se llevó a cabo un estudio retrospectivo
de cohortes en una planta que fabricaba 2-propanol por el proceso
del ácido débil. Había más de 11 000 personas-años expuestas. La
tasa de mortalidad debida a todas las causas fue más baja de lo
esperado. No se observó mortalidad excesiva por todos los
cánceres. Sin embargo, la incidencia del cáncer de la boca y de la
faringe fue 4 veces más elevada de lo previsto. Los estudios de
cohortes indican en conjunto un riesgo de cáncer relacionado con el
proceso de fabricación con ácido fuerte, pero, en dos pequeños
estudios controlados de casos, no se observó correlación alguna
entre la exposición a 2-propanol y la incidencia de gliomas o de
leucemia linfática.
Algunos informes parecen indicar que la exposición combinada a
tetracloruro de carbono y 2-propanol en los trabajadores potencia
la toxicidad del primero.
9. Resumen de la evaluación
La exposición del hombre al 2-propanol puede producirse por
inhalación durante la fabricación, el tratamiento y el uso tanto
ocupacional como doméstico. La exposición a un nivel
potencialmente letal en la población general puede producirse por
ingestión accidental o intencionada y los niños pueden estar
expuestos cuando se les refresca con esponjas impregnadas con
preparaciones a base de 2-propanol (alcohol para friegas).
El 2-propanol se absorbe y distribuye rápidamente por todo el
organismo, en parte en forma de acetona. Los datos sobre
exposición-efecto en el hombre en condiciones de sobreexposición
aguda son escasos y muestran grandes variaciones. Los principales
efectos son la gastritis, la depresión del sistema nervioso central
con hipotermia y depresión respiratoria, y la hipotensión. Los
datos de mortalidad aguda en animales de experimentación indican
que la toxicidad del 2-propanol es baja, siendo los valores de DL50
orales en diversas especies entre 4475 y 7990 mg/kg, y los valores
de CL50 de inhalación en ratas alrededor de 50 000 mg/m3. En el
conejo, el 2-propanol no produjo irritaciones cutáneas, pero la
aplicación de 0,1 ml de 2-propanol sin diluir produjo irritación en
los ojos.
En el hombre, los efectos agudos más probables de la exposición
a concentraciones elevadas de 2-propanol por ingestión o inhalación
son la intoxicación alcohólica y la narcosis.
No se han hecho suficientes estudios en animales como para
evaluar los riesgos que entraña para la salud humana la exposición
repetida al 2-propanol. No obstante, los resultados de dos
estudios a corto plazo en la rata, incluida la exposición por
inhalación (500 mg/m3 durante 4 horas al día y 5 días a la semana
durante 4 meses) y la exposición oral (600 - 3900 mg/kg en el agua
de bebida) indican que debe evitarse la exposición al 2-propanol en
algunos de los muy elevados niveles de exposición ocupacional que
se han notificado.
La exposición por inhalación de ratas gestantes a 2-propanol
dio un nivel mínimo de observación de efectos de 18 327 mg/m3 (7450
ppm) y un nivel sin efectos observados de 9001 mg/m3 (3659 ppm)
respecto a la toxicidad materna. En el mismo estudio, 9001 mg/m3
(3659 ppm) fue el nivel más bajo de observación de efectos en lo
que respecta a la toxicidad de desarrollo; no se indicó nivel sin
efectos observados. Estas concentraciones son más elevadas que las
que normalmente se registran en condiciones de exposición humana.
El 2-propanol dio resultado negativo en las pruebas de
genotoxicidad, pero indujo aberraciones mitóticas en la médula ósea
de la rata. Aunque estos resultados indican que la sustancia no
tiene potencial genotóxico, no puede hacerse una evaluación
correcta de la mutagenicidad basándose en datos tan limitados.
Los datos disponibles no bastan para evaluar la carcinogenicidad
del 2-propanol en animales de experimentación. No se dispone de
datos para evaluar la carcinogenicidad del 2-propanol en el ser
humano.
Es poco probable que el 2-propanol plantee un riesgo grave para
la salud de la población general en las condiciones de exposición
que se producen normalmente.
El 2-propanol desaparece rápidamente (periodo de semieliminación
2,5 días) de la atmósfera y su desaparición del agua y del suelo se
produce rápidamente por biodegradación aerobia y anaerobia,
especialmente tras la adaptación de microorganismos inicialmente
sembrados. En vista de las propiedades físicas del 2-propanol, su
potencial de bioacumulación es bajo. No representa un riesgo para
los organismos naturales en las concentraciones en que suele
encontrarse en el medio ambiente.
