
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 105
SELECTED MYCOTOXINS:
OCHRATOXINS, TRICHOTHECENES, ERGOT
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Organization
Geneva, 1990
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
Selected mycotoxins : ochratoxins, trichothecenes, ergot.
(Environmental health criteria ; 105)
1.Ochratoxins 2.Trichothecenes 3.Ergot alkaloids
I.Series
ISBN 92 4 157105 5 (NLM Classification: QW 630)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1990
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR SELECTED MYCOTOXINS: OCHRATOXINS,
TRICHOTHECENES, AND ERGOT
INTRODUCTION
SUMMARY AND RECOMMENDATIONS FOR FURTHER RESEARCH
1. Ochratoxin A
1.1. Natural occurrence
1.2. Analytical methods
1.3. Metabolism
1.4. Effects on animals
1.5. Effects on man
2. Trichothecenes
2.1. Natural occurrence
2.2. Analytical methods
2.3. Metabolism
2.4. Effects on animals
2.5. Effects on man
3. Ergot
3.1. Natural occurrence
3.2. Analytical methods
3.3. Effects on animals
3.4. Effects on man
4. Recommendations for further research
4.1. General recommendations
4.2. Ochratoxin A
4.3. Trichothecenes
4.4. Ergot
I. OCHRATOXINS
I.1 Properties and analytical methods
I.1.1 Chemical properties
I.1.2 Methods for the analysis of foodstuffs and
biological samples
I.2 Sources and occurrence
I.2.1 Fungal formation
I.2.2 Occurrence in foodstuffs
I.2.2.1 Plant products
I.2.2.2 Residues in food of animal origin
I.3 Metabolism
I.3.1 Absorption
I.3.2 Tissue distribution
I.3.2.1 Animal studies
I.3.2.2 Studies on man
I.3.3 Metabolic transformation
I.3.4 Excretion
I.4 Effects on animals
I.4.1 Field observations
I.4.1.1 Pigs
I.4.1.2 Poultry
I.4.2 Experimental animal studies
I.4.2.1 Acute and chronic effects
I.4.2.2 Teratogenicity
I.4.2.3 Mutagenicity
I.4.2.4 Carcinogenicity
I.4.2.5 Biochemical effects and mode of action
I.5 Effects on man
I.5.1 Ochratoxin A, Balkan endemic nephropathy, and
tumours of the urinary system
I.6 Evaluation of the human health risks
II. TRICHOTHECENES
II.1 Properties and analytical methods
II.1.1 Physical and chemical properties
II.1.1.1 Physical properties
II.1.1.2 Chemical properties
II.1.2 Analytical methods for trichothecenes
II.1.2.1 Chemical methods
II.1.2.2 Immunological methods
II.1.2.3 Biological methods
II.2 Sources and occurrence
II.2.1 Taxonomic considerations
II.2.2 Ecology of trichothecene-producing fungi
II.2.3 Natural occurrence
II.2.3.1 Agricultural products
II.2.3.2 Trichothecenes in human foodstuffs
II.3 Metabolism
II.3.1 Absorption and tissue distribution
II.3.1.1 Animal studies
II.3.2 Metabolic transformation
II.3.3 Excretion
II.3.3.1 Animal studies
II.3.3.2 Excretion in eggs and milk
II.4 Effects on animals
II.4.1 Field observations
II.4.2 Effects on experimental animals
II.4.2.1 General toxic effects
II.4.2.2 Haematological and haemostatic changes
II.4.2.3 Disturbances of the central nervous
system
II.4.2.4 Dermal toxicity
II.4.2.5 Impairment of immune response
II.4.2.6 Carcinogenicity
II.4.2.7 Mutagenicity
II.4.2.8 Teratogenicity and reproductive effects
II.4.3 Biochemical effects and mode of action
II.4.3.1 Cytotoxicity
II.4.3.2 Inhibition of protein synthesis
II.4.3.3 Inhibition of nucleic acid synthesis
II.4.3.4 Alterations of cellular membranes
II.4.3.5 Other biochemical effects
II.4.4 Structure-activity relationships
II.4.5 Prevention and therapy of trichothecene toxicosis
II.5 Effects on man
II.5.1 Contemporary episodes of human disease
II.5.2 Historical Fusarium-related diseases
II.5.3 Skin irritation
II.5.4 Studies of haemostasis
II.5.5 Airborne trichothecene-related diseases
II.5.6 Toxicological information on man, obtained from
therapeutic uses
II.6 Evaluation of the human health risks
III. ERGOT
III.1 Properties and analytical methods
III.1.1 Chemical properties
III.1.2 Analytical methods for ergot and ergot
alkaloids
III.1.2.1 Ergot
III.1.2.2 Ergot alkaloids
III.2 Sources and occurrence
III.2.1 Fungal producers
III.2.2 Biosynthesis
III.2.3 Occurrence in foodstuffs
III.2.4 Fate of ergolines during food processing
III.3 Metabolism
III.4 Effects on animals
III.4.1 Field studies
III.4.2 Experimental animal studies
III.4.2.1 Cattle
III.4.2.2 Sheep
III.4.2.3 Poultry
III.4.2.4 Swine
III.4.2.5 Primates
III.5 Effects on man
III.5.1 Ergometrine-related outbreaks
III.5.2 Clavine-related outbreaks
III.6 Evaluation of the human health risks
REFERENCES
RESUME
RESUMEN
WHO TASK GROUP ON SELECTED MYCOTOXINS: OCHRATOXINS, TRICHOTHECENES,
AND ERGOT
Members
Professor W.W. Carlton, Department of Veterinary Pathobiology,
School of Veterinary Medicine, Purdue University, West Lafayette,
Indiana, USA
Mr T. Demeke, Health Service Department, Ministry of Health, Addis
Ababa, Ethiopia
Dr J. Gilbert, Ministry of Agriculture, Fisheries and Food,
Norwich, United Kingdom
Professor P. Krogh, Department of Microbiology, Royal Dental
College, Copenhagen, Denmark (Co-Rapporteur)
Dr M. Nakadate, Section of Information and Investigation, Division
of Information on Chemical Safety, National Institute of Hygienic
Sciences, Tokyo, Japan
Dr J. Parizek, Institute of Nuclear Biology and Radiochemistry,
Czechoslovak Academy of Sciences, Prague, Czechoslovakia
Dr A.E. Pohland, Division of Chemical Contaminants, Center for Food
Safety and Applied Nutrition, Food and Drug Administration, US
Department of Health and Human Services, Washington DC, USA
Professor H.D.Tandon, ex-President, National Academy of Medical
Sciences, New Delhi, India (Chairman)
Professor Y. Ueno, Department of Toxicology and Microbial
Chemistry, Faculty of Pharmaceutical Sciences, Science University
of Tokyo, Tokyo, Japan (Co-Rapporteur)
Observers
Dr K. Ohtsubo, Department of Clinical Pathology, Tokyo Metropolitan
Institute of Gerontology, Tokyo, Japan
Dr T. Yoshizawa, Department of Bioresource Sciences, Faculty of
Agriculture, Kagawa University, Kagawa, Japan
Secretariat
Dr M. Gilbert, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr A. Prost, Division of Environmental Health, World Health
Organization, Geneva, Switzerland
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the
criteria documents as accurately as possible without unduly
delaying their publication. In the interest of all users of the
environmental health criteria documents, readers are kindly
requested to communicate any errors that may have occurred to the
Manager of the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland, in order that they may be
included in corrigenda, which will appear in subsequent volumes.
ENVIRONMENTAL HEALTH CRITERIA FOR SELECTED MYCOTOXINS:
OCHRATOXINS, TRICHOTHECENES, AND ERGOT
A WHO Task Group on Environmental Health Criteria for Selected
Mycotoxins met in London on 14-18 November, 1988. Dr Malcolm
HUTTON opened the meeting on behalf of the Director of the
Monitoring and Assessment Research Centre (MARC), King's College,
London, which hosted the meeting on behalf of the three cooperating
organizations of the International Programme on Chemical Safety
(WHO/ILO/UNEP). The Task Group reviewed and revised the draft
criteria document and made an evaluation of the health risks of
exposure to selected mycotoxins.
The draft documents for Ochratoxins and Ergot were prepared by
Professor P. KROGH. Those for Trichothecenes were prepared by Dr
M. NAKADATE AND HIS COLLEAGUES in the National Institute of
Hygienic Sciences and by Professor Y. UENO AND HIS COLLEAGUES in
Tokyo. During the task group meeting, several members of the Group
agreed to undertake a substantial revision of the draft. Dr A.
PROST was responsible for the overall scientific content of the
document and Mrs M. O. HEAD of Oxford, England, for the editing.
The Secretariat wishes to acknowledge the contributions of: Dr
J. GILBERT (Chemistry and analytical methods for trichothecenes);
Dr A.E. POHLAND (Sources and natural occurrence of trichothecenes);
and Professor W.W. CARLTON (Animal studies and metabolism of
trichothecenes).
The Secretariat also wishes to thank Professor Y. UENO, Co-
rapporteur of the Task Group, for his significant contributions and
revisions of the draft document during the meeting. Professor H.
TANDON, Chairman of the Task Group, and Professor P. KROGH and
Professor Y. UENO, Co-rapporteurs, met with members of the
Secretariat in Tokyo, 25-30 July, 1989, to review the final
document before its release.
The efforts of all who helped in the preparation and
finalization of the document, especially those of Dr H. KURATA and
Dr M. ICHINOE (National Institute of Hygienic Sciences, Tokyo) and
Dr K. OHTSUBO (Tokyo Metropolitan Institute of Gerontology), are
gratefully acknowledged.
* * *
Partial financial support for the publication of this criteria
document was kindly provided by the United States Department of
Health and Human Services, through a contract from the National
Institute of Environmental Health Sciences, Research Triangle Park,
North Carolina, USA -- a WHO Collaborating Centre for Environmental
Health Effects.
INTRODUCTION
A decade has passed since the publication of Environmental
Health Criteria 11: Mycotoxins (WHO, 1979), but this field of
research has expanded rapidly, and recent data indicate that the
health effects of several of the mycotoxins dealt with in the above
publication should be updated. More than 200 mycotoxins are now
known to exist. They are present in the environment and, in some
cases, human exposure has been documented, mainly through food
contamination or occasionally through inhalation. However,
information on adverse human health effects is often lacking and,
in many cases, the association between exposure to selected
mycotoxins and the occurrence of health disorders remains
hypothetical.
Ochratoxin A has been found as a contaminant in foods with a
frequency in the range of 2-30% in all countries where attempts to
perform food analysis have been made. Field cases of ochratoxin A-
associated nephropathy in farm animals have been encountered in
many countries, underlining the nephrotoxic potential of this
compound. More recently, ochratoxin A has been detected in the
blood of 6-18% of the human population in some areas where Balkan
endemic nephropathy is prevalent. Ochratoxin A has also been found
in human blood samples outside the Balkan peninsula. In some
studies, more than 50% of the samples analysed have been
contaminated. A high incidence of tumours of the urinary system is
strongly correlated with the prevalence of Balkan endemic
nephropathy. For these reasons, the human health effects of
ochratoxin A have been re-evaluated.
A considerable increase in trichothecenes research has been
seen over the last decade. The potential for the production of a
number of trichothecenes among Fusarium species is well documented,
and the toxicology of a few trichothecenes and the natural
occurrence of some of these compounds in food is fairly well
established. Thus, food-borne exposure of human beings to some
trichothecenes, in particular deoxynivalenol (vomitoxin) and
nivalenol, is likely to occur. Several reports have recently
associated outbreaks of human disease with the presence of
trichothecenes in food. For this reason, the health effects of
trichothecenes have been re-evaluated.
Ergotism is by far the oldest known mycotoxicosis in man and
animals. Recent episodes of Claviceps purpurea-associated
intoxication in Ethiopia, as well as episodes of Claviceps
fusiformis-associated intoxications in areas of India indicate that
ergotism is still a disease of public health importance,
particularly in developing countries. The evaluation of ergot as a
food contaminant has therefore been included in the present updated
environmental health criteria dealing with selected mycotoxins.
However, the review of available documentation has concentrated on
studies dealing with the naturally occurring ergot alkaloids.
Derivatives produced by the pharmaceutical industry have been
deliberately excluded. No attempt has been made to review the
literature on the pharmacology of derivatives of lysergic acid. The
section on ergot in this publication aims at alerting the
scientific community about the present status of ergotism as a
disease of our time, and about the differences in clinical symptoms
that are observed between Asia and Africa in relation to the
chemical differences in responsible toxins.
Studies on the etiology of hepatocellular carcinoma, in
particular those indicating that there is a consistent and specific
causal association between hepatitis B virus and hepatocellular
carcinoma, as well as the existence of other etiological factors
that may cause hepatocellular carcinoma independently, have
attracted much attention since the publication of the previous EHC
on mycotoxins (WHO, 1979). A report by WHO (1983) recognized the
value of available methods for the direct assessment of individual
exposure to aflatoxins, and the methods and their use in field
studies were considered in a later report by IARC (1984). Since the
evaluation of the carcinogenic risks of aflatoxins for human beings
has recently been reviewed by IARC (1987a), the present update on
mycotoxins will not assess the issue and readers should refer to
the IARC evaluation.
SUMMARY AND RECOMMENDATIONS FOR FURTHER RESEARCH
1. Ochratoxin A
1.1 Natural occurrence
Ochratoxins are produced by several species of the fungal
genera Aspergillus and Penicillium. These fungi are ubiquitous and
the potential for the contamination of foodstuffs and animal feed
is widespread. Ochratoxin A, the major compound has been found in
a number of countries in Australasia, Europe, and North America.
Ochratoxin formation by Aspergillus species appears to be limited
to conditions of high humidity and temperature, whereas at least
some Penicillium species may produce ochratoxin at temperatures as
low as 5 °C.
The highest incidences of ochratoxin A contamination have been
found in cereals, and to a lesser extent in some beans (coffee,
soya, cocoa). Ochratoxin B occurs extremely rarely.
1.2 Analytical methods
Analytical techniques have been developed for the
identification and quantitative determination of ochratoxin levels
in the µg/kg range.
1.3 Metabolism
Residues of unchanged ochratoxin A have been found in the
blood, kidney, liver, and muscle of pigs in slaughter houses and in
the muscle of hens and chickens. However, residues of ochratoxin
A have not generally been found in ruminants. The in vitro
binding of ochratoxin A to serum albumin is particularly strong in
cattle, pigs, and man. Experimental studies on pigs and hens have
shown that higher levels of ochratoxin A occur in the kidneys.
Microsomal hydroxylation might represent a detoxification reaction
in pigs, rats, and man. In experimental studies, residues could
still be identified in pig kidneys, one month after the termination
of exposure.
1.4 Effects on animals
Field cases of ochratoxicosis in farm animals (pigs, poultry)
have been reported from several European countries, the primary
manifestation being chronic nephropathy. The lesions include
tubular atrophy, interstitial fibrosis and, at later stages,
hyalinized glomeruli. Ochratoxin A has also been found in pig
blood collected at Canadian slaughterhouses. It has produced
nephrotoxic effects in all species of single-stomach animals
studied so far, even at the lowest level tested (200 µg/kg feed in
rats and pigs).
Teratogenic effects were observed in mice exposed orally to 3
mg/kg body weight. Fetal resorption was observed in rats given
doses from 0.75 mg/kg body weight orally. Teratogenic effects,
which in the rat were enhanced by a diet low in protein, have also
been observed in hamsters.
There is no evidence of ochratoxin A activity in short-term
tests for mutagenicity (bacteria and yeasts). Rats exposed orally
showed single-strand breaks in DNA in renal and hepatic tissues.
Ochratoxin A induced renal cell neoplasms in male mice and in both
sexes of rats dosed orally. Hepatic cell neoplasms were reported
in only one mouse strain and not in the rat.
Ochratoxin A is an inhibitor of protein synthesis and tRNA
synthetase in microorganisms, hepatoma cells, and in renal mRNA in
the rat.
Ochratoxin A can inhibit macrophage migration. In mice, a dose
of 0.005 µg/kg body weight suppressed the immune response to sheep
erythrocytes; however, contradictory results have also been
obtained.
Ochratoxin A has been shown to be carcinogenic to the renal
tubular epithelium in male mice and in both sexes in rats.
1.5 Effects on man
Human exposure, as demonstrated by the occurrence of ochratoxin
A in food, blood, and in human milk, has been observed in various
countries in Europe. Available epidemiological information
indicates that Balkan nephropathy may be associated with the
consumption of foodstuffs contaminated by this toxin.
A highly significant relationship has been observed between
Balkan nephropathy and tumours of the urinary tract, particularly
with tumours of the renal pelvis and ureters. However, no data
have been published that establish a direct causal role of
ochratoxin A in the etiology of such tumours.
2. Trichothecenes
2.1 Natural occurrence
To date, 148 trichothecenes, characterized chemically as having
the same basic tetracyclic scirpenol ring system, are known. These
compounds are produced primarily by moulds belonging to the genus
Fusarium, though other genera, including Trichoderma,
Trichothecium, Myrothecium, and Stachybotrys, are also known to
produce metabolites now characterized as trichothecenes. Only a
few of the known trichothecenes have been found to contaminate food
or animal feed including: deoxynivalenol (DON), nivalenol (NIV),
diacetoxyscirpenol (DAS), and T-2 toxin and, less frequently,
certain derivatives (3-Ac-DON, 15-Ac-DON, fusarenon-X and HT-2
toxin). Of these, by far the most commonly encountered in food and
animal feed is DON, with lesser amounts of NIV usually found as co-
contaminants. Some macrocyclic trichothecenes, such as satratoxins
G and H, and the verrucarins, occur occasionally in animal feed
(straw, hay) but there are no reports of their presence in foods.
Surveys for the presence of trichothecenes have revealed the
world-wide occurrence of DON, primarily in cereals, such as wheat
and corn, at levels occasionally as high as 92 mg/kg, though
average levels are considerably lower and vary with commodity.
There are isolated reports of the occurrence of DON in barley,
mixed feeds, potatoes, etc. NIV, though not normally reported in
cereals in Canada or the USA, is commonly found in conjunction with
DON in Asian and European grains; the highest concentration
recorded to date for NIV is 37.9 mg/kg. T-2 toxin and DAS have
been reported infrequently and at much lower concentrations.
Processing and milling studies have shown little reduction in
DON levels from the cereal to the finished product. Similarly,
baking is not effective in destroying DON. In general,
commercially available human foodstuffs rarely contain detectable
levels of DON and NIV.
2.2 Analytical methods
Analytical methods based on TLC, GC, HPLC, and immunological
techniques are available for the determination of the four most
frequently encountered toxins (DON, T-2 toxin, DAS, NIV) with
detection limits below 1 µg/g. Several of these methods have been
tested collaboratively. In addition, research methods, such as
GC/MS and LC/MS, are available for confirmation of identity.
2.3 Metabolism
Metabolic studies have been carried out on animals, principally
with T-2 toxin, but a few with DON. These trichothecenes are
rapidly absorbed from the alimentary tract, but quantitative data
are not available. The toxins are distributed fairly evenly
without marked accumulation in any specific organ or tissue.
Trichothecenes are metabolically transformed to less toxic
metabolites by such reactions as hydrolysis, hydroxylation,
de-epoxidation, and glucuronidation. Trichothecenes, such as T-2
toxin and DON, are rapidly eliminated in the faeces and urine. For
example, almost 100% of an oral dose of T-2 toxin in cattle was
eliminated within hours of dosing; in chickens, about 80% had been
eliminated 48 h after dosing. In the rat, 25% of DON was
eliminated in the urine and 65% in the faeces, 96 h after dosing.
The results of transmission of T-2 toxin in the laying hen and
lactating cow showed that less than 1% of the administered dose of
this toxin and its metabolites was present in eggs and milk. Tissue
residues of oral T-2 toxin and metabolites in chicken meat were
below 2% of the dose, 24 h after dosing.
2.4 Effects on animals
Ingestion of animal feed of plant origin is the main route of
exposure to trichothecenes. T-2 toxin and DAS, which are the most
potent for laboratory animals of the trichothecenes commonly
reported as feed contaminants (T-2 toxin, DAS, NIV, and DON),
induce a similar toxic response. NIV is less potent in some
systems than the previous two compounds and DON is the least toxic
of the four (examples of potency include the oral LD50s in the
mouse: T-2 toxin, 10.5 mg/kg body weight and DON, 46.0 mg/kg).
The more potent trichothecenes, such as T-2 toxin and DAS,
produce acute systemic effects when administered experimentally to
rodents, pigs, and cattle, via the oral, parenteral, or inhalation
(pig, mouse) route. Epithelionecrosis is a lesion produced by
contact exposure with potent trichothecenes, such as T-2 toxin and
DAS (dose of 0.2 µg per spot for T-2 toxin). Larger doses of other
trichothecenes (NIV, 10 µg per spot) are required to produce an
irritant effect. The cytotoxic trichothecenes, such as T-2 toxin,
produce necrosis of the intestinal crypt epithelium and of lymphoid
and haematopoietic tissues after oral, parenteral, or inhalation
exposure. Haematological and coagulopathic abnormalities follow
exposure to cytotoxic trichothecenes, such as T-2 toxin and DAS.
Severe toxicosis can result in pancytopenia. Suppression of cell-
mediated and humoral immunity has been demonstrated in studies with
T-2 toxin, DON, and DAS, and observations include effects such as
reduced concentrations of immunoglobulins and depressed phagocytic
activity of both macrophages and neutrophils. The results of
experimental animal studies have indicated that the immunodepressive
effect of such trichothecenes as T-2, DAS, and DON, results in
decreased resistance to secondary infection by bacteria
(Mycobacteria, Listeria monocytogenes), yeasts (Cryptococcus
neoformans), and viruses (Herpes simplex virus).
T-2 toxin has been reported to be teratogenic in the mouse,
when given by intraperitoneal injection (unusual route of
administration for teratogenic studies). DON was reported to be
teratogenic in mice after gastric intubation, but was not
teratogenic in rats when the toxin was provided in the feed. NIV
was not teratogenic in mice. T-2 toxin, DAS, and DON were not
mutagenic in an Ames-type assay. T-2 toxin had weak clastogenic
activity in some assays. There is no evidence from the published
long-term toxicity studies in animals to indicate that T-2 toxin,
fusarenon-X, and NIV are tumorigenic in animals. No long-term
studies of DON toxicity have been published.
Trichothecenes are toxic for actively dividing cells, such as
the intestinal crypt epithelium and the haematopoietic cells. The
cytotoxicity has been associated with either impairment of protein
synthesis by the binding of the compounds to the ribosomes of
eukaryotic cells, or the dysfunction of cellular membranes.
Inhibition of protein synthesis has been associated with the
induction of labile and regulatory proteins, such as IL-2 in
immunocytes. Transport of small molecules is impaired in cell
membranes by extremely low concentrations of trichothecenes.
2.5 Effects on man
Ingestion of contaminated foods of plant origin is the main
route of exposure to trichothecenes, but other routes have been
reported occasionally, such as accidental skin contact amongst
laboratory research workers, and airborne trichothecenes in dust.
Reported cases of illness associated with exposure to
trichothecenes are scarce and none has been established as being
due to trichothecenes. However, a causative role is suggested by
the two outbreaks referred to below.
One disease outbreak was reported from China and was associated
with the consumption of scabby wheat containing 1.0-40.0 mg DON/kg.
The disease was characterized by gastrointestinal symptoms. No
deaths occurred in human beings. Swine and chicks fed the leftover
cereals were also affected.
An analogous outbreak was reported from India and was
associated with consumption of baked bread made from contaminated
wheat. The disease was characterized by gastrointestinal symptoms
and throat irritation, which developed within 15 minutes to one
hour following ingestion of the bread. The following mycotoxins
were detected in samples of refined wheat flour used in the
preparation of the bread: DON (0.35-8.3 mg/kg), acetyldeoxynivalenol
(0.64-2.49 mg/kg), NIV (0.03-0.1 mg/kg) and T-2 toxin (0.5-0.8
mg/kg). However, there was no confirmation of the identity of the
detected trichothecenes. The concomitant occurrence of DON and NIV
with T-2 toxin is unusual.
Two diseases of historical interest, alimentary toxic aleukia
(ATA) in the USSR and scabby wheat toxicosis in Japan and Korea,
have been associated with the consumption of grain invaded by
Fusarium moulds. Some trichothecenes have since been identified
under laboratory conditions in fungal cultures of Fusarium moulds
isolated from grains involved in the incidents. Studies linking
ATA and scabby grain toxicosis to trichothecenes exposure could not
be made at the time that the disease occurred, because the toxins
were not known.
3. Ergot
3.1 Natural occurrence
Ergot is the name given to sclerotia of fungal species within
the genus Claviceps. Biologically active alkaloids contained in
the sclerotia cause the development of toxicoses when the sclerotia
are consumed by man and animals through contaminated food or animal
feed.
Ergot alkaloids produce two different patterns of diseases,
depending on the fungal organism involved (C. Purpurea, C.
fusiformis) and hence the alkaloids produced. Ergotism, induced by
ergotamine-ergocristine alkaloids produced by C. purpurea, is
characterized predominantly by gangrene of the extremities as well
as gastrointestinal symptoms. Intoxication induced by millet
contaminated with C. fusiformis is mainly characterized by
gastrointestinal symptoms, and is related to clavine alkaloids.
There are no signs or symptoms suggesting vaso-occlusion.
3.2 Analytical methods
Ergot alkaloids (ergolines) are derivatives of lysergic acid.
The individual alkaloids vary in the magnitude of their biological
activity. Determination of C. purpurea ergot alkaloids has been
carried out by HPLC with fluorescence detection. Concentrations of
0.2 µg ergoline per litre of human plasma can be measured.
Ergotamine and ergocristine can be determined very specifically by
radioimmunoassays at levels of 3.5 picomoles and 0.8 picomoles,
respectively.
3.3 Effects on animals
Ergolines, mainly ergotamine and ergotaminine, have been
associated with outbreaks of bovine abortion. Sheep, administered
ergotamine orally, rapidly became ill and intestinal inflammation
was observed. Orally exposed poultry, pigs, and primates
experienced slight effects. No data on the mutagenicity,
teratogenicity, and carcinogenicity of ergolines were available to
the task group.
3.4 Effects on man
Claviceps-infected grain is a source of human exposure to
ergolines. In most toxicological studies, identification of
specific alkaloids has not been undertaken. The published
information from only one survey of cereals and cereal products
indicates a total daily human intake of ergolines in Switzerland of
approximately 5.1 µg per person, the contents of certain
commodities being up to 140 µg/kg. Baking reduces the ergolines
present in contaminated flour by 25-100%.
An outbreak of ergotism in Ethiopia in 1978 resulted from
exposure to ergolines from C. purpurea sclerotia. The grain
contained up to 0.75% ergot; ergometrine was detected specifically.
Symptoms included dry gangrene with loss of one or more limb (29%
of cases), feeble or absent peripheral pulses (36%), and
desquamation of the skin. Gastrointestinal symptoms occurred in
only a few cases. Lower extremities were involved in 88% of
patients.
In India, several outbreaks have occurred since 1958 as a
result of ingesting pearl millet containing clavine-type ergot from
C. fusiformis. Symptoms included nausea, vomiting, and giddiness.
Pearl millet containing 15-26 mg ergoline/kg caused the toxic
symptoms.
Since autopsies were not performed in either of the episodes,
no information is on record of the pathological effects on human
viscera.
4. Recommendations for further research
4.1 General recommendations
A network of reference centres should be established to assist
Member States in confirming the identity of individual mycotoxins
found in human foods and tissues. These reference centres should
also provide mycotoxin reference samples, upon request, to
reinforce the intercomparability of analytical results obtained in
different parts of the world.
4.2 Ochratoxin A
(a) Extended retrospective, as well as focal prospective,
epidemiological studies on the association of ochratoxin A
with Balkan type endemic nephropathy and with urinary-tract
tumours should be conducted in the Balkan peninsula and the
Mediterranean region.
(b) Blood analysis for ochratoxin A should be performed on
patients with urinary-tract tumours, outside the Balkan
peninsula.
(c) The source of ochratoxin A exposure, as indicated by human
blood analysis, should be elucidated in countries outside the
Balkan peninsula.
(d) The mechanism of the sex differences in renal, neoplastic, and
non-neoplastic disease, caused by ochratoxin A in experimental
animals, should be elucidated.
(e) Extended surveys on the ochratoxin A contents of foods in
different parts of the world are required. Such surveys are
particularly important in regions of the world where high
incidence rates of urinary-tract tumours, renal tumours, or
nephropathy occur.
4.3 Trichothecenes
(a) Follow-up studies should be performed in the areas of India
and the People's Republic of China in which episodes of
trichothecenes intoxication in human beings have been
encountered recently. The unusual pattern of trichothecene
occurrence in these episodes should be further elucidated.
(b) The effects of long-term exposure of experimental animals to
DON, including the carcinogenic effects, should be studied.
Because the response to DON by different species varies
greatly, the test species must be chosen carefully.
(c) Secondary microbial infection in experimental animals
following trichothecenes exposure should be further
elucidated.
(d) The influence of environmental conditions, including the
presence of insecticides and other man-made chemicals, on the
fungal production of trichothecenes should be studied.
(e) The effects of food processing on trichothecenes should be
clarified.
(f) Agricultural plants, resistant to infection by trichothecene-
producing fungi, should be developed, using biotechnological
approaches.
(g) The possible synergistic effects in experimental animals of
combined exposure to trichothecenes, aflatoxins, ochratoxin A,
and other mycotoxins should be studied.
(h) Studies on the intake of trichothecenes by human beings should
be performed.
(i) Rapid and sensitive screening procedures for trichothecenes
should be developed, and surveys for trichothecenes in grain
and processed foods in temperate zones of the world should be
conducted.
4.4 Ergot
(a) Methods of analysis for agroclavines should be developed.
(b) Information should be made available to developing countries
on the use of pathological seed screening and milling
procedures for minimizing the problems of ergot.
(c) Epidemiological studies should be performed on the possible
effects of low levels of ergolines on the human population.
(d) Pharmacological and toxicological studies should be performed
using individual and combined ergolines on experimental
animals.
(e) The possible transmission of ergolines through the mother's
milk to the infant should be elucidated.
I. OCHRATOXINS
I.1 PROPERTIES AND ANALYTICAL METHODS
I.1.1 Chemical properties
The ochratoxins constitute a group of closely-related
derivatives of isocoumarin linked to L-phenylalanine (Fig. 1), and
classified according to biosynthetic origin as pentaketides within
the group of polyketides (Turner, 1971). The topic has been
reviewed by Scott (1977) and Steyn (1977, 1984).
 The first compound discovered, ochratoxin A, was isolated from
a culture of Aspergillus ochraceus, hence the name (Van der Merwe
et al., 1965). The acids, including 4-hydroxy-ochratoxin A, the
methyl and ethyl esters, and the isocoumarin part of ochratoxin B
(ochratoxin) have all been isolated from fungal cultures under
experimental conditions. On acid hydrolysis, ochratoxin A yields
phenylalanine, and the isocoumarin part, ochratoxin alpha, a
cleavage product also found in the intestines, faeces, urine, and
liver of rodents experimentally fed an ochratoxin A-containing diet
(Galtier & Alvinerie, 1976). Ochratoxin A and, very rarely,
ochratoxin B are the only compounds found as natural contaminants
in plant material, and most of the information available concerns
ochratoxin A. It can be stored in ethanol in the refrigerator for
more than a year without loss (Chu & Butz, 1970); however, such
solutions should be protected from light, since decomposition
occurs on exposure to fluorescent light for several days (Neely &
West, 1972).
Ochratoxin A is a colourless, crystalline compound, obtained by
crystallization from benzene, with a melting point of about 90 °C,
and containing approximately one mole of benzene. After drying for
1 h at 60 °C, it has a melting point in the range of 168-173 °C.
It is soluble in polar organic solvents, slightly soluble in water,
and soluble in dilute aqueous bicarbonate. Physical data on
ochratoxin A, based on a collaborative study, have been published
by IUPAC (Pohland et al., 1982). Ochratoxin A is optically active:
[alpha]21D: -46.8 °C (c = 2650 µmol/litre in chloroform). The
publication cited above includes information on the following
spectra of ochratoxin A: ultraviolet absorption spectrum; infrared
absorption spectrum; electron impact mass spectrum; nuclear
magnetic resonance spectrum.
I.1.2 Methods for the analysis of foodstuffs and biological
samples
Ochratoxin A in acidified commodities is readily soluble in
many organic solvents, and this characteristic has been used as the
principle of extraction in several methods.
A number of methods using thin-layer chromatography have been
published, one of the most commonly used being the official AOAC
method developed for barley (Nesheim et al., 1973). In this
method, ochratoxins A and B are extracted from ground samples with
chloroform, after acidification. The toxins are trapped in a
column containing diatomaceous earth impregnated with a basic
aqueous solution. After column clean-up, the toxins are eluted and
thin-layer chromatography is performed using long-wave UV
irradiation for visualization of the fluorescent ochratoxin spots
(limit of detection: 12 µg/kg). The method has been
collaboratively studied, revealing coefficients of variation
(between laboratories) in the range of 31-54% (Nesheim, 1973).
This method has been published as an IUPAC recommended procedure
(IUPAC, 1976). The sensitivity can be improved by exposing the
developed plate to ammonia fumes, resulting in a limit of detection
of a few µg/kg. A slightly modified method, developed for green
coffee (Levi, 1975), has a coefficient of variation in the range of
33-49%, based on a collaborative study.
The two methods have been combined into one procedure,
published as an IARC procedure (Nesheim, 1982). In an
international check sample survey on ochratoxin A in animal feed,
the AOAC procedure was used by 61% of the participants, and the
performance was slightly better than that of the other methods,
with a coefficient of variation of 69% compared to 79% for the
other methods combined (Freisen & Garren, 1983). Paulsch et al.
(1982) developed a procedure for the determination of ochratoxin A
in the kidneys of swine, using a liquid-liquid partitioning step
instead of column clean-up, followed by two-dimensional thin-layer
chromatography using an acidic and an alkaline developing solvent.
In addition, the procedure included a confirmatory test, based on
the formation of ochratoxin A methyl ester on the plate.
High-performance liquid chromatographic procedures have been
developed for the determination of ochratoxin A in food of plant
origin (Hunt et al., 1978; Josefsson & Moller, 1979), with limits
of detection in the range of 1-12 µg/kg, and with recoveries in the
55-92% range. A procedure for the determination of ochratoxin A
residues in renal tissue has been published, in which enzymic
digestion of the sample tissue is followed by dialysis and high
performance liquid chromatography (Hunt et al., 1979). The limit
of detection is about 1 µg/kg, and recoveries of 77-78% have been
observed. Rapid screening methods for ochratoxin A are available,
based on minicolumn chromatography, with limits of detection in
the 8-12 µg/kg range (Hald & Krogh, 1975; Holaday, 1976). By using
antisera to ochratoxin A, an enzyme-linked immunosorbent assay
(ELISA) has been constructed in which the toxin in barley can be
determined with only 0.5% cross-reaction for ochratoxin B, and with
a lower level of detection of 10 pg ochratoxin A/well of the ml
plate (Morgan et al., 1982). Immunological methodology has been
further improved by the use of monoclonal antibody (IgG) in a
radioimmunoassay with high specificity for ochratoxin A, which can
detect levels of this toxin as low as 0.2 µg/kg in swine kidney
tissue (Candlish et al., 1986; Rousseau et al., 1987). A method
has been described in which ochratoxin A is cleaved to ochratoxin
alpha and phenylalanine using the enzyme carboxypeptidase. The
quantification of ochratoxin A is based on the loss of fluorescence
intensity at 380 nm, the limit of detection being 4 µg/kg of
barley (Hult & Gatenbeck, 1976). The procedure has also been
applied to swine blood with a limit of detection of 2 µg/litre
(Hult et al., 1979).
The same procedure has been used in screening human blood for
the presence of ochratoxin A, with a limit of detection of 1-2
µg/litre serum for a 2-g sample. High-performance liquid
chromatography was used as confirmation (Hult et al., 1982). A
screening method involving flow injection has been developed by
which ochratoxin A concentrations of more than 10 µg/litre can be
determined, based on 50-µlitre samples of serum (Hult et al.,
1984). As the specificity of the method is limited, positive
results have to be confirmed by conventional methods requiring much
larger blood samples.
A method has been developed for the detection of ochratoxin
A (and aflatoxin B and citrinin) in human urine using hydrolysis
of urine, solid-phase extraction, and reversed-phase liquid
chromatography with fluorescence detection (Orti et al., 1986).
The detection limit for ochratoxin A was approximately 10 ng/ml in
samples of 10 ml urine.
I.2 SOURCES AND OCCURRENCE
I.2.1 Fungal formation
The ochratoxins were isolated in 1965 from a culture of
Aspergillus ochraceus, hence the name (Van der Merwe et al., 1965),
but subsequent investigations have revealed that a variety of
fungal organisms included in the genera Aspergillus and Penicillium
are able to produce ochratoxins (Table 1).
The effects of water activity (aw) and temperature, the main
factors controlling mycotoxin formation, have been elucidated in
relation to growth and ochratoxin production for 3 fungal
organisms: A. ochraceus, P. cyclopium, and P. viridicatum
(Northolt et al., 1979). The minimum aw values for ochratoxin
production ranged between 0.83 and 0.87, 0.87 and 0.90, and 0.83
and 0.86, respectively. At 24 °C, optimum aw values for A.
ochraceus and for both P. cyclopium and P. viridicatum were 0.99
and 0.95-0.99, respectively (Fig. 2).
Table 1. Ochratoxin-producing fungia
-------------------------------------------------------------------
Penicillium link
Monoverticillata:
P. frequentans series: P. purpurrescens Sopp
Asymmetrica lanata:
P. commune series: P. commune Thom
Asymmetrica fasciculata:
P. viridicatum series: P. viridicatum Westling
P. palitans Westling
P. cyclopium series: P. cyclopium Westling
Biverticillata symmetrica:
P. purpurogenum series: P. varibile Sopp
Aspergillus Micheli
Aspergillus ochraceus group: A. sulphureus (Fres.)
Thom & Church
A. sclerotiorum Huber
A. alliaceus Thom & Church
A. melleus Yukawa
A. ochraceus Wilhelm
A. ostianus Wehmer
A. petrakii Vörös
-------------------------------------------------------------------
a From: Krogh (1978).
The first compound discovered, ochratoxin A, was isolated from
a culture of Aspergillus ochraceus, hence the name (Van der Merwe
et al., 1965). The acids, including 4-hydroxy-ochratoxin A, the
methyl and ethyl esters, and the isocoumarin part of ochratoxin B
(ochratoxin) have all been isolated from fungal cultures under
experimental conditions. On acid hydrolysis, ochratoxin A yields
phenylalanine, and the isocoumarin part, ochratoxin alpha, a
cleavage product also found in the intestines, faeces, urine, and
liver of rodents experimentally fed an ochratoxin A-containing diet
(Galtier & Alvinerie, 1976). Ochratoxin A and, very rarely,
ochratoxin B are the only compounds found as natural contaminants
in plant material, and most of the information available concerns
ochratoxin A. It can be stored in ethanol in the refrigerator for
more than a year without loss (Chu & Butz, 1970); however, such
solutions should be protected from light, since decomposition
occurs on exposure to fluorescent light for several days (Neely &
West, 1972).
Ochratoxin A is a colourless, crystalline compound, obtained by
crystallization from benzene, with a melting point of about 90 °C,
and containing approximately one mole of benzene. After drying for
1 h at 60 °C, it has a melting point in the range of 168-173 °C.
It is soluble in polar organic solvents, slightly soluble in water,
and soluble in dilute aqueous bicarbonate. Physical data on
ochratoxin A, based on a collaborative study, have been published
by IUPAC (Pohland et al., 1982). Ochratoxin A is optically active:
[alpha]21D: -46.8 °C (c = 2650 µmol/litre in chloroform). The
publication cited above includes information on the following
spectra of ochratoxin A: ultraviolet absorption spectrum; infrared
absorption spectrum; electron impact mass spectrum; nuclear
magnetic resonance spectrum.
I.1.2 Methods for the analysis of foodstuffs and biological
samples
Ochratoxin A in acidified commodities is readily soluble in
many organic solvents, and this characteristic has been used as the
principle of extraction in several methods.
A number of methods using thin-layer chromatography have been
published, one of the most commonly used being the official AOAC
method developed for barley (Nesheim et al., 1973). In this
method, ochratoxins A and B are extracted from ground samples with
chloroform, after acidification. The toxins are trapped in a
column containing diatomaceous earth impregnated with a basic
aqueous solution. After column clean-up, the toxins are eluted and
thin-layer chromatography is performed using long-wave UV
irradiation for visualization of the fluorescent ochratoxin spots
(limit of detection: 12 µg/kg). The method has been
collaboratively studied, revealing coefficients of variation
(between laboratories) in the range of 31-54% (Nesheim, 1973).
This method has been published as an IUPAC recommended procedure
(IUPAC, 1976). The sensitivity can be improved by exposing the
developed plate to ammonia fumes, resulting in a limit of detection
of a few µg/kg. A slightly modified method, developed for green
coffee (Levi, 1975), has a coefficient of variation in the range of
33-49%, based on a collaborative study.
The two methods have been combined into one procedure,
published as an IARC procedure (Nesheim, 1982). In an
international check sample survey on ochratoxin A in animal feed,
the AOAC procedure was used by 61% of the participants, and the
performance was slightly better than that of the other methods,
with a coefficient of variation of 69% compared to 79% for the
other methods combined (Freisen & Garren, 1983). Paulsch et al.
(1982) developed a procedure for the determination of ochratoxin A
in the kidneys of swine, using a liquid-liquid partitioning step
instead of column clean-up, followed by two-dimensional thin-layer
chromatography using an acidic and an alkaline developing solvent.
In addition, the procedure included a confirmatory test, based on
the formation of ochratoxin A methyl ester on the plate.
High-performance liquid chromatographic procedures have been
developed for the determination of ochratoxin A in food of plant
origin (Hunt et al., 1978; Josefsson & Moller, 1979), with limits
of detection in the range of 1-12 µg/kg, and with recoveries in the
55-92% range. A procedure for the determination of ochratoxin A
residues in renal tissue has been published, in which enzymic
digestion of the sample tissue is followed by dialysis and high
performance liquid chromatography (Hunt et al., 1979). The limit
of detection is about 1 µg/kg, and recoveries of 77-78% have been
observed. Rapid screening methods for ochratoxin A are available,
based on minicolumn chromatography, with limits of detection in
the 8-12 µg/kg range (Hald & Krogh, 1975; Holaday, 1976). By using
antisera to ochratoxin A, an enzyme-linked immunosorbent assay
(ELISA) has been constructed in which the toxin in barley can be
determined with only 0.5% cross-reaction for ochratoxin B, and with
a lower level of detection of 10 pg ochratoxin A/well of the ml
plate (Morgan et al., 1982). Immunological methodology has been
further improved by the use of monoclonal antibody (IgG) in a
radioimmunoassay with high specificity for ochratoxin A, which can
detect levels of this toxin as low as 0.2 µg/kg in swine kidney
tissue (Candlish et al., 1986; Rousseau et al., 1987). A method
has been described in which ochratoxin A is cleaved to ochratoxin
alpha and phenylalanine using the enzyme carboxypeptidase. The
quantification of ochratoxin A is based on the loss of fluorescence
intensity at 380 nm, the limit of detection being 4 µg/kg of
barley (Hult & Gatenbeck, 1976). The procedure has also been
applied to swine blood with a limit of detection of 2 µg/litre
(Hult et al., 1979).
The same procedure has been used in screening human blood for
the presence of ochratoxin A, with a limit of detection of 1-2
µg/litre serum for a 2-g sample. High-performance liquid
chromatography was used as confirmation (Hult et al., 1982). A
screening method involving flow injection has been developed by
which ochratoxin A concentrations of more than 10 µg/litre can be
determined, based on 50-µlitre samples of serum (Hult et al.,
1984). As the specificity of the method is limited, positive
results have to be confirmed by conventional methods requiring much
larger blood samples.
A method has been developed for the detection of ochratoxin
A (and aflatoxin B and citrinin) in human urine using hydrolysis
of urine, solid-phase extraction, and reversed-phase liquid
chromatography with fluorescence detection (Orti et al., 1986).
The detection limit for ochratoxin A was approximately 10 ng/ml in
samples of 10 ml urine.
I.2 SOURCES AND OCCURRENCE
I.2.1 Fungal formation
The ochratoxins were isolated in 1965 from a culture of
Aspergillus ochraceus, hence the name (Van der Merwe et al., 1965),
but subsequent investigations have revealed that a variety of
fungal organisms included in the genera Aspergillus and Penicillium
are able to produce ochratoxins (Table 1).
The effects of water activity (aw) and temperature, the main
factors controlling mycotoxin formation, have been elucidated in
relation to growth and ochratoxin production for 3 fungal
organisms: A. ochraceus, P. cyclopium, and P. viridicatum
(Northolt et al., 1979). The minimum aw values for ochratoxin
production ranged between 0.83 and 0.87, 0.87 and 0.90, and 0.83
and 0.86, respectively. At 24 °C, optimum aw values for A.
ochraceus and for both P. cyclopium and P. viridicatum were 0.99
and 0.95-0.99, respectively (Fig. 2).
Table 1. Ochratoxin-producing fungia
-------------------------------------------------------------------
Penicillium link
Monoverticillata:
P. frequentans series: P. purpurrescens Sopp
Asymmetrica lanata:
P. commune series: P. commune Thom
Asymmetrica fasciculata:
P. viridicatum series: P. viridicatum Westling
P. palitans Westling
P. cyclopium series: P. cyclopium Westling
Biverticillata symmetrica:
P. purpurogenum series: P. varibile Sopp
Aspergillus Micheli
Aspergillus ochraceus group: A. sulphureus (Fres.)
Thom & Church
A. sclerotiorum Huber
A. alliaceus Thom & Church
A. melleus Yukawa
A. ochraceus Wilhelm
A. ostianus Wehmer
A. petrakii Vörös
-------------------------------------------------------------------
a From: Krogh (1978).
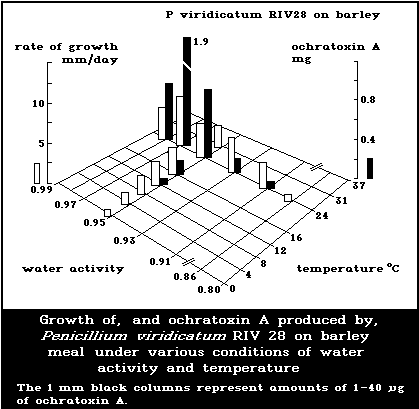 At optimum aw, the temperature range for ochratoxin production
by A. ochraceus was 12-37 °C, whereas that of P. cyclopium and P.
viridicatum was 4-31 °C. These laboratory data correspond with
those from field observations on ochratoxin contamination in crops.
Thus, the more frigophilic Penicillia, particularly P. viridicatum,
are the major ochratoxin producers in crops in colder climatic
zones, such as Scandinavia (Krogh, 1978; Rutqvist et al., 1978;
Häggblom, 1982), and in Canada (Scott et al., 1972).
In contrast, ochratoxin-producing fungal potential has been
found in 28-50% of A. ochraceus strains isolated from crops in
warmer climatic zones, such as Australia (Connole et al., 1981),
and Yugoslavia (Pepeljnjak & Cvetnic, 1981), and strains isolated
from coffee beans (Stack et al., 1982).
I.2.2 Occurrence in foodstuffs
I.2.2.1 Plant products
Ochratoxin A was first encountered as a natural contaminant in
maize (Shotwell et al., 1969), and subsequent surveys have
established that ochratoxin A is a contaminant of cereals and some
beans (coffee beans, soya beans, cocoa beans) in many areas of the
world (Table 2). Although the mean level of ochratoxin A in all
reported surveys up to 1979 was 1035 µg/kg (Fig. 3), 83% of the
samples contained less than 200 µg/kg (Krogh, 1980).
At optimum aw, the temperature range for ochratoxin production
by A. ochraceus was 12-37 °C, whereas that of P. cyclopium and P.
viridicatum was 4-31 °C. These laboratory data correspond with
those from field observations on ochratoxin contamination in crops.
Thus, the more frigophilic Penicillia, particularly P. viridicatum,
are the major ochratoxin producers in crops in colder climatic
zones, such as Scandinavia (Krogh, 1978; Rutqvist et al., 1978;
Häggblom, 1982), and in Canada (Scott et al., 1972).
In contrast, ochratoxin-producing fungal potential has been
found in 28-50% of A. ochraceus strains isolated from crops in
warmer climatic zones, such as Australia (Connole et al., 1981),
and Yugoslavia (Pepeljnjak & Cvetnic, 1981), and strains isolated
from coffee beans (Stack et al., 1982).
I.2.2 Occurrence in foodstuffs
I.2.2.1 Plant products
Ochratoxin A was first encountered as a natural contaminant in
maize (Shotwell et al., 1969), and subsequent surveys have
established that ochratoxin A is a contaminant of cereals and some
beans (coffee beans, soya beans, cocoa beans) in many areas of the
world (Table 2). Although the mean level of ochratoxin A in all
reported surveys up to 1979 was 1035 µg/kg (Fig. 3), 83% of the
samples contained less than 200 µg/kg (Krogh, 1980).
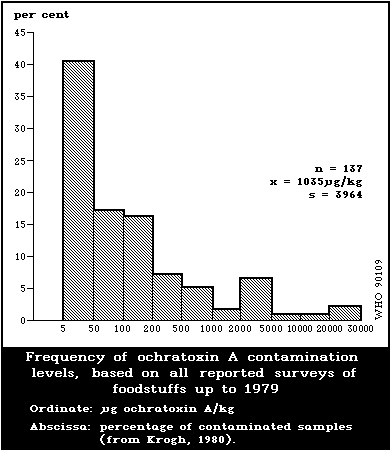 Table 2. Natural occurrence of ochratoxin A in foodstuffs and animal feed of plant origin
---------------------------------------------------------------------------------------------------------
Commodity Country Number of Percentage Range of Reference
samples contaminated ochratoxin A
analysed levels (µg/kg)
---------------------------------------------------------------------------------------------------------
FOOD
maize USA 293 1.0 83-166 Shottwell et al. (1971)
maize (1973) France 463 2.6 15-200 Galtier et al. (1977b)
maize (1974) France 461 1.3 20-200 Galtier et al. (1977b)
wheat (red winter) USA 291 1.0 5-115 Shottwell et al. (1976)
wheat (red spring) USA 286 2.8 5-115 Shottwell et al. (1976)
barley (malt) Denmark 50 6.0 9-189 Krogh (1978)
barley USA 127 14.2 10-40 Nesheim (1971)
coffee beans USA 267 7.1 20-360 Levi et al. (1974)
maize Yugoslaviaa 542 8.3b 6-140 Pavlovic et al. (1979)
wheat Yugoslaviaa 130 8.5b 14-135 Pavlovic et al. (1979)
wheat bread Yugoslaviaa 32 18.8b Pavlovic et al. (1979)
barley Yugoslaviaa 64 12.5b 14-27 Pavlovic et al. (1979)
barley Czechoslovakia 48 2.1 3800 Vesela et al. (1978)
bread United Kingdomc 50 2.0 210 Osborne (1980)
flour United Kingdomc 7 28.5 490-2900 Osborne (1980)
beans Sweden 71 8.5 10-442 Akerstrand &
peas Sweden 72 2.8 10 Josefsson (1979)
maize United Kingdom 29 37.9 50-500 Ministry of
cornflour United Kingdom 13 30.8 50-200 Agriculture (1980)
soya bean United Kingdom 25 36.0 50-500 Ministry of
soya flour United Kingdom 21 19.0 50-500 Agriculture (1980)
cocoa beans (raw) United Kingdom 56 17.9 100-500 Ministry of
(roasted) United Kingdom 19 15.8 100 Agriculture (1980)
grain German Democratic 49 4.1 18-22 Fritz et al.
Republic (1979)
grain (barley, Poland 296 6.8 20-470 Szebiotko et al. (1981)
wheat, rye)
grain (wheat, rye) Denmark 151 1.3 15-50 Pedersen &
bran (wheat) Denmark 57 10.5 5-20 Hansen (1981)
maize Bulgariaa 22 27.3 25-35 Petkova-Bocharova &
maize Bulgaria 22 9.0 10-25 Castegnaro (1985)
beans Bulgariaa 24 16.7 25-27 Petkova-Bocharova &
beans Bulgariaa 28 7.1 25-50 Castegnaro (1985)
---------------------------------------------------------------------------------------------------------
Table 2 (contd.)
---------------------------------------------------------------------------------------------------------
Commodity Country Number of Percentage Range of Reference
samples contaminated ochratoxin A
analysed levels (µg/kg)
---------------------------------------------------------------------------------------------------------
FOOD (continued)
barley, wheat, Poland 150 5.3 50-200 Juszkiewicz &
oats, rye, maize Piskorska-
Pliszczynska (1976)
FEED
mixed feed Poland 203 4.9 10-50 Juszkiewicz &
Piskorska-
Pliszczynska (1977)
maize Yugoslavia 191 25.7 45-5125 Balzer et al. (1977)
barley, oats Sweden 84 8.3 16-409 Krogh et al. (1974b)
wheat, hay Canada 95 7.4 30-6000 Prior (1976)
wheat, oats, Canadac 32 56.3 30-27 000 Scott et al. (1972)
barley, rye
barley, oats Denmarkc 33 57.6 28-27 500 Krogh et al. (1973)
mixed feed Canada 474 1.1 30-100 Prior (1981)
mixed feed Canadac 51 7.8 48-5900 Abramson et al. (1983)
mixed feed Australia 25 4.0 70 000 Connole et al. (1981)
mouldy bread Italyd 1 80 000 Visconti &
Bottalico (1983)
---------------------------------------------------------------------------------------------------------
a From an area with endemic nephropathy.
b Average values for a period of 2-5 years.
c All samples suspected of containing mycotoxins.
d This sample contained in addition 9600 µg ochratoxin B/kg.
Ochratoxin B has only been found in 3 samples and, thus, occurs
extremely rarely; the other ochratoxins have never been found in
plant products. Levels of ochratoxin A and the frequency of
contamination are generally higher in animal feed than in foodstuffs
(Table 2). Although the mean level of ochratoxin A in all reported
surveys up to 1979 was 1035 µg/kg (Fig. 3), 83% of the samples
contained less than 200 µg/kg (Krogh, 1980).
I.2.2.2 Residues in food of animal origin
Residues of ochratoxin A are not generally found in ruminants,
because ochratoxin A is cleaved in the forestomachs by protozoan and
bacterial enzymes (Galtier & Alvinerie, 1976; Hult et al., 1976;
Patterson et al., 1981). The non-toxic cleavage product, ochratoxin
alpha, has been found in the kidneys at levels below 10 µg/kg and in
the blood of calves fed a diet containing 300-500 µg ochratoxin A/kg
(Patterson et al., 1981). In half of the calves, the kidneys
contained low levels of ochratoxin A (up to 5 µg/kg), a feature that
may reflect that the calves were not yet functioning as ruminants.
When 2 milking cows were fed a ration containing 317-1125 µg
ochratoxin A/kg for 11 weeks, a residue of 5 µg ochratoxin A/kg was
found in the kidneys of one of the animals but not in any other
tissue or the milk. Ochratoxin alpha was not found in any tissue
(Shreeve et al., 1979). Residues of ochratoxin A have been detected
in a number of tissues in single-stomach food animals, such as pigs.
The carry-over of ochratoxin A from feed to animal tissues was
elucidated in a study in which groups of pigs were exposed for 3-4
months to dietary levels of ochratoxin A of 200, 1000, or 4000 µg/kg
(Krogh et al., 1974a). At termination (slaughter), the highest
levels of ochratoxin A residues were found in the kidneys (mean
levels 50 µg/kg at the 4000 µg/kg feed level), with lower levels
in the liver, muscle, and adipose tissue; other tissues, including
blood, were not analysed. There was a high correlation between the
feed level of ochratoxin A and the residue levels in the 4 tissues
investigated (Table 3).
Table 3. Correlation between feed level and tissue levels
(residues) of ochratoxin A in pigsa
-----------------------------------------------------------
Tissue Regression equation r
-----------------------------------------------------------
kidney Y = 2.15 + 0.0123X 0.86
liver Y = 0.35 + 0.0095X 0.82
adipose Y = 2.51 + 0.0099X 0.78
-----------------------------------------------------------
a Modified from Krogh et al. (1974a).
X = ochratoxin A in feed (µg/kg).
Y = ochratoxin A residue (µg/kg tissue).
r = correlation coefficient.
The regression is calculated on feed levels of ochratoxin A
in the range of 200-4000 µg/kg.
Ochratoxin A is present in the blood bound to serum-albumin
(Chu, 1971; Galtier, 1974a) and as free ochratoxin A; saturation (in
the rat) occurs at 70 mg ochratoxin A/litre plasma. The binding of
ochratoxin A to serum-albumin is particularly strong in cattle,
pigs, and man, based on in vitro studies (Table 4).
Table 4. In vitro binding of ochratoxin A to
serum-albumin in several speciesa
-----------------------------------------------
Animal Number of Intrinsic
species binding Association
sites constant (M-1)
-----------------------------------------------
Cattle 0.58 94 600
Pig 0.56 71 100
Man 0.58 63 500
Horse 0.57 57 400
Chicken 0.51 52 700
Rat 0.68 40 100
Sheep 0.88 22 600
-----------------------------------------------
a Adapted from: Galtier (1979).
The presence of ochratoxin A in the blood of pigs has been
elucidated in experimental animal studies as well as by surveys of
blood samples from pigs at farms (Hult et al., 1979, 1980). A
total of 1200 pig blood samples, collected from slaughterhouses
over various periods during 1986 in western Canada, was screened
for the presence of ochratoxin A using an HPLC procedure (Marqhardt
et al., 1988). It was shown that 3.6-4.2% of the blood samples
contained the toxin at concentrations higher than 20 ng/ml. It
appears that the concentration of ochratoxin A in the blood is
higher than that in any other tissue. In feeding studies, bacon
pigs were exposed for various periods to rations containing
ochratoxin A concentrations in the range of 58-1878 µg/kg; blood,
kidney, liver, muscle, and adipose tissues were analysed, at
slaughter, for residues of ochratoxin A (Mortensen et al., 1983).
The statistical association between residue levels in various
tissues is indicated by the regression analyses (Table 5).
Table 5. Regression between residues of ochratoxin A
in the serum and certain other tissuesa
------------------------------------------------------
Tissue Regression r sb
equation
------------------------------------------------------
kidney Y = 0.0651X 0.89 0.002
muscle Y = 0.0346X 0.88 0.001
liver Y = 0.0259X 0.89 0.001
adipose Y = 0.0191X 0.84 0.001
------------------------------------------------------
a Adapted from: Mortensen et al. (1983).
X = µg ochratoxin A/litre serum.
Y = µg ochratoxin A/kg in the other tissues.
r = correlation coefficient
sb = Standard error of slope
Epidemiological studies on the basis of data from meat
inspection in Danish slaughterhouses revealed prevalence rates of
porcine nephropathy ranging from 10 to 80 cases per 100 000
slaughtered pigs (Krogh, 1976a). Surveys in a number of European
countries for residues of ochratoxin A in kidneys from cases of
porcine nephropathy revealed that 25-39% of the cases contained
ochratoxin A levels in the range 2-100 µg/kg (Table 6).
Table 6. Surveys for ochratoxin A residues in kidneys from
cases of porcine nephropathy, based on meat inspection data
-------------------------------------------------------------------
Country No. of porcine Percentage Range of Reference
nephropathy containing ochratoxin
kidneys residues of A residues
investigated ochratoxin (µg/kg wet
A weight)
-------------------------------------------------------------------
Denmark 60 35 2-68 Krogh (1977)
Germany, 104 21 0.1-1.8 Bauer et al.
Federal (1984)
Republic of
Hungary 122 39 2-100 Sandor et
al. (1982)
Poland 113 24 1-23 Golinski et
al. (1984)
Sweden 129 25 2-104 Rutqvist et
al. (1977)
Sweden 90 27 2-88 Josefsson
(1979)
-------------------------------------------------------------------
Ochratoxin A levels of up to 29 µg/kg were found in the muscle
of hens and chickens collected at one slaughterhouse (Elling et
al., 1975). The birds had been condemned because of nephropathy.
In another study, groups of hens were exposed for 1-2 years to
dietary levels of ochratoxin A of 0.3 or 1 mg/kg (Krogh et al.,
1976b). The kidneys contained the highest residues with a mean
value of 19 µg/kg tissue in the group fed 1 mg ochratoxin A/kg; the
liver and muscle contained lower levels of residues and no
ochratoxin A was found in the eggs. These results are in
accordance with those of subsequent experimental animal studies.
For example, in a study in which 4 groups of hens were fed diets
containing 0, 0.5, 1, or 4 mg ochratoxin A/kg, the kidneys
contained the highest level of ochratoxin A residues (31 µg/kg) in
the highest dose group; eggs were not analysed (Prior & Sisodia,
1978).
In another study, hens were fed 1 mg ochratoxin A/kg feed for 8
weeks; the kidneys contained 3-10 µg ochratoxin A/kg and the liver,
1.5-2.5 µg/kg. The eggs were not analysed (Reichmann et al.,
1982). When groups of hens were fed diets containing 2.5 or 10
mg/kg, the kidneys contained 1-6 µg ochratoxin A/kg, lower levels
being found in the plasma, muscle, and liver (Juszkiewicz et al.,
1982). Eggs from the high-dose group contained 0.7-1.3 µg
ochratoxin A/kg, but none was detected in eggs from the low-dose
group.
White Leghorn hens (54 birds), divided into four groups, were
fed diets for one month containing the following concentrations of
ochratoxin A (mg/kg): 0, 1.3, 2.6, and 5.2 (Bauer et al., 1988).
At termination, three tissues were analysed for residues. The
following ranges of dose-dependent residues were found: serum,
4.7-11.7 ng/ml, liver, 9.1-18.0 ng/g, and yolk, 1.6-4.0 ng/g; very
little ochratoxin A was found in the egg white, and none was
detected in the tissues of the control group.
I.3 METABOLISM
I.3.1 Absorption
In a study on rats exposed by gavage to a single dose of
ochratoxin A at 10 mg/kg body weight, Galtier (1974b) found the
highest tissue level of unchanged ochratoxin A in the stomach wall
during the first 4 h following administration. The small and large
intestine and caecum contained small amounts of unchanged
ochratoxin A, and it was concluded that ochratoxin A was absorbed
mainly in the stomach. Small amounts (1-3% of the total dose) were
detected in the caecum and the large intestine, as the isocoumarin
moiety (ochratoxin alpha), most likely as the result of the
hydrolysing action of the intestinal microflora (Galtier &
Alvinerie, 1976; Hult et al., 1976).
In a study on intestinal absorption using the same animal
species, Kumagai & Aibara (1982) came to the conclusion that the
site of maximal absorption of ochratoxin A was the proximal
jejunum, and that the portal vein was the primary route of
transport from the intestinal tract, though part of the
transportation took place through the lymphatics.
Using a highly specific antibody against ochratoxin A, which is
used in a peroxidase-antiperoxidase detection method, Lee et al.
(1984) studied absorption and tissue distribution in Swiss mice
over a 48-h period, after the administration of a single dose of 25
mg ochratoxin A/kg body weight. Ochratoxin A was found in large
amounts, indicated by staining, in epithelial cells as well as in
macrophages of the lamina propria in the duodenum. Smaller amounts
were found in jejunal epithelial cells and lamina propria, and much
smaller amounts in the epithelial cells in the esophagus and
stomach; no toxin was found in the ileum. These findings suggest
that absorption mainly takes place in the duodenum and the jejunum.
I.3.2 Tissue distribution
I.3.2.1 Animal studies
In slaughterhouse cases of mycotoxic porcine nephropathy
studied by Hald & Krogh (1972), residues of unchanged ochratoxin A
were found in all tissues investigated (kidney, liver, and muscle),
the highest level (up to 67 µg/kg) occurring in the kidney. In
experimental studies on pigs ingesting feed containing ochratoxin
A, residues of the toxin were found in all 4 tissues in the
decreasing order of kidney, liver, muscle, adipose tissue (Krogh et
al., 1974a). A subsequent study revealed that the concentration of
ochratoxin A residues in the blood of the pig was higher than those
in the other tissues mentioned above (Mortensen et al., 1983).
When rats were exposed orally to an ochratoxin A dose of 10 mg/kg
body weight, Galtier (1974b) recovered 0.3% of the administered
dose in the whole kidneys, 0.9% in the whole liver, and 0.6% in the
total muscle tissue, 96 h after exposure. Chang & Chu (1977),
using a single intraperitoneal injection of 1 mg ochratoxin A per
rat (labelled with 14C in phenylalanine), found that the kidney
contained twice as much unchanged ochratoxin A as the liver after
30 minutes, amounting to 4-5% of the total dose.
In the study by Lee et al. (1984), the largest amounts of
ochratoxin A found in the kidney (as indicated by staining
intensity) were in the epithelium of the proximal convoluted
tubules, and to a lesser extent in the distal convoluted tubules,
the descending loop of Henle, and glomeruli and Bowman's capsule.
Small amounts were found in hepatocytes as well as in the lumina of
bile ducts, but not in biliary epithelium, indicating biliary
excretion.
Using pig renal cortical slices, it was found that ochratoxin A
enters the proximal tubule cells by the common organic anion
transport system (Friis et al., 1988). Ochratoxin A inhibited p-
aminohippurate (PAH) and phenolsulfophthalein uptake in a dose-
dependent manner.
I.3.2.2 Studies on man
In a study in Yugoslavia near Slavonski Brod, where endemic
nephropathy is prevalent, 639 samples of serum from the inhabitants
of 2 villages were screened for the presence of ochratoxin A; 42
(6.6%) were positive for the toxin "with concentrations in the
range of 1-57 ng ochratoxin A/g" (Hult et al., 1982). Detection
was carried out using the enzymic spectrofluorometric procedure,
and positives were confirmed by the esterification of ochratoxin A
in serum and of ochratoxin alpha obtained from the enzymetic
hydrolisates; the esters were measured by high performance liquid
chromatography.
In a screening of serum samples in Poland using the same method
of analysis, 77 out of 1065 samples (7.2%) contained ochratoxin A,
with a mean concentration of 0.27 ng/ml and a maximum value of 40
ng/ml (Colinski, 1987). In the Federal Republic of Germany, 173
out of 306 serum samples (56.6%) contained ochratoxin A, as
measured by an HPLC procedure, with a mean of 0.6 ng/g and a range
of 0.1-14.4 ng/g (Bauer & Gareis, 1987). Three out of 46 kidneys
(6.6%) contained ochratoxin A, with a mean of 0.2 ng/g and a range
of 0.1-0.3 ng/g. In a study of blood plasma in Denmark, 46 out of
96 samples (47.9%) contained ochratoxin A, as measured by an HPLC
procedure, with a mean of 1.7 ng/g and a range of 0.1-9.2 ng/g
(Hald, 1989). In Bulgaria, where endemic nephropathy also occurs
in some areas, 45 out of 312 blood samples contained ochratoxin A
(14.4%), with a mean of approximately 14 ng/g (Petkova-Bocharova et
al., 1988).
I.3.3 Metabolic transformation
It has been shown from in vitro studies that ochratoxin A binds
to serum-albumin (Chu, 1971, 1974b); this binding has also been
observed in in vivo studies on rats (Galtier, 1974a; Chang & Chu,
1977). Ochratoxin alpha has been detected in the urine and faeces
of rats injected intraperitoneally with ochratoxin A (Nel &
Purchase, 1968; Chang & Chu, 1977), indicating the cleavage of
ochratoxin A to ochratoxin alpha and phenylalanine, under these
conditions.
Studies with 14C-labelled ochratoxin A indicated that some
other, not yet identified, metabolites are formed in the body.
Less than half of the radioactivity excreted in rat urine within 24
h of a single intraperitoneal injection of 14C-phenylalanine-
labelled ochratoxin A was identified as ochratoxin A (Chang & Chu,
1977).
In both albino rats and brown rats given ochratoxin A orally or
intraperitoneally, 1-1.5% of the dose was excreted as (4R)-4-
hydroxyochratoxin A and 25-27% as ochratoxin alpha in the urine
(Storen et al., 1982). In in vitro studies using liver microsomes
from the pig, rat, and man, both (4R)-and (4S)-4-hydroxy-ochratoxin
A were produced in a hydroxylation process involving cytochrome
P-450 (Stormer & Pedersen, 1980; Stormer et al., 1981).
4-Hydroxyochratoxin A is non-toxic for rats in amounts up to 40 mg/kg
body weight (Hutchison et al., 1971); thus, it has been concluded
that the microsomal hydroxylation most likely represents a
detoxification reaction.
I.3.4 Excretion
The results of studies in which 14C-labelled ochratoxin A was
injected intraperitoneally in rats demonstrated that the toxin was
excreted primarily in the urine (Chang & Chu, 1977), though faecal
elimination also occurred to some extent (Galtier, 1974b; Chang &
Chu, 1977). In a study in which 14C-labelled ochratoxin A was
given orally to rats as a single dose (15 mg/kg body weight), the
cumulative elimination after 120 h was 11% ochratoxin A and 23%
ochratoxin alpha in the faeces; 11% ochratoxin A and 12% ochratoxin
alpha in the urine; and 33% ochratoxin A in the bile (Suzuki et
al., 1977). Absorption was influenced by enteritis, which was
caused by the high ochratoxin A doses given (75% of the LD50
value). Ochratoxin A injected intravenously as a single dose (4.1
mg/kg) in albumin-deficient and normal rats was excreted in the
bile and urine 20-70 times faster in the albumin-deficient rats
than in normal rats, indicating that the binding of ochratoxin A to
blood albumin delays the excretion of the compound through the
liver and kidney (Kumagai, 1985).
In rats given unlabelled ochratoxin A orally, Storen et al.
(1982) found 6% ochratoxin A, 1.5% (4R)-4-hydroxyochratoxin A, and
25-27% ochratoxin alpha in the urine; 12% ochratoxin A and 9%
ochratoxin alpha were found in the faeces.
The excretion of ochratoxin A in the milk was studied in
rabbits intravenously injected with 1-4 mg/kg body weight, as
single dose (Galtier et al., 1977a). At the highest dose injected,
the milk contained 1 mg ochratoxin A/litre; ochratoxin alpha and
4-hydroxy-ochratoxin A were not detected. Goats were given a
single dose of tritium-labelled ochratoxin A (0.5 mg/kg) and the
cumulative excretion (in terms of radioactivity) after 7 days
amounted to 53% in the faeces, 38% in the urine, 6% in the milk,
and 2% in the serum (Nip & Chu, 1979). Only a small fraction of
the radioactivity in milk was ochratoxin A, amounting to 0.026% of
the total ochratoxin A given.
In the Federal Republic of Germany, a study of human milk
obtained from women in two hospitals (patient category not stated)
revealed that 4 out of 36 samples (11.1%) contained ochratoxin A,
with a mean value of 0.024 ng/ml and a range of 0.017-0.030 ng/ml
(Bauer & Gareis, 1987; Gareis et al., 1988).
In mice given 14C-labelled ochratoxin A intravenously at
various stages of pregnancy, the toxin was shown to cross the
placental barrier on day 9 of pregnancy, at which time it is most
effective in producing fetal malformations (Appelgren & Arora,
1983). The highest toxin concentration was found in the bile,
which contained 5 times as much as the blood.
Ochratoxin A has been detected in the urine of bacon pigs
suffering from nephropathy (Krogh, unpublished information
communicated to the Task Group).
In a study on the disappearance rates for various tissues,
female bacon pigs were fed ochratoxin A at a level of 1 mg/kg feed
for one month and then kept on a toxin-free diet for another month,
during which animals were sacrificed at regular intervals (Krogh et
al., 1976a). Ochratoxin A disappeared exponentially (Table 7) from
the 4 tissues investigated (kidney, liver, muscle, and adipose
tissue) with residual life values (RL50)a in the range of 3.5-4.5
days; the toxin could still be detected in the kidneys one month
after termination of exposure.
Table 7. The rate of disappearance of ochratoxin A residues
from pig tissues after termination of a one-month exposure to
ochratoxin A at 1 mg/kg feeda
--------------------------------------------------------------
Tissue Ochratoxin A (µg/kg tissue) at time t (days)
after termination of exposure
---------------------------------------------------------------
kidney 28.22 exp (-0.1522 t)
liver 19.49 exp (-0.1598 t)
muscle 12.94 exp (-0.2096 t)
adipose 4.62 exp (-0.0565 t)
---------------------------------------------------------------
a From: Krogh et al. (1976a).
-------------------------------------------------------------------
a RL50 = half residual life, calculated from the exponential
equations shown in Table 7.
No data are available on ochratoxin levels in human urine or
faeces.
When the level in the serum is known, the ochratoxin A residues
in the four other tissues can be calculated (Table 5).
I.4 EFFECTS ON ANIMALS
I.4.1 Field observations
I.4.1.1 Pigs
The effects of ochratoxins on animals have been reviewed by
Krogh (1976a, 1978). Cases of mycotoxic porcine nephropathy have
been regularly encountered in studies in Denmark since the disease
was first discovered 50 years ago (Larsen, 1928). The disease is
endemic in all areas of the country, though unevenly distributed.
Prevalence rates in 1971 varied from 0.6 to 65.9 cases per 10 000
pigs, and epidemics encountered in 1963 and 1971 were associated
with a high moisture content in the grain caused by unusual
climatic conditions (Krogh, 1976b). On the basis of these studies,
Krogh (1978) concluded that ochratoxin A is the substance most
frequently associated with porcine nephropathy, though other
factors, such as citrinin, may also be involved.
A survey on porcine nephropathy was conducted at six
slaughterhouses in Sweden during the spring months of 1978
(Josefsson, 1979). A prevalence rate of 4.4 cases per 10 000 pigs
was encountered corresponding to the endemic level of prevalence
rates in Denmark; 26.7% of nephropathic kidneys contained residues
of ochratoxin A. In Hungary, an epidemiological study on porcine
nephropathy and the association with ochratoxin A was conducted in
1980-81 covering 4 areas in the country (Sandor et al., 1982). A
prevalence rate of 2.0 cases per 10 000 pigs was measured,
comparable to endemic prevalence rates in Scandinavia; 39% of
nephropathic kidneys contained residues of ochratoxin A. The
morphological changes in the kidneys in cases of mycotoxic porcine
nephropathy were characterized by degeneration of the proximal
tubules followed by atrophy of the tubular epithelium, interstitial
fibrosis in the renal cortex, and hyalinization of some glomeruli
(Elling & Moller, 1973).
In Poland, surveys for porcine nephropathy in 1983 and 1984
revealed prevalence rates of 4.7-5.7 cases per 10 000 pigs, with 5-
55% of the nephropathic kidneys containing detectable residues of
ochratoxin A, apparently depending on the season of the year
(Golinski et al., 1984, 1985). Porcine nephropathy has been
encountered in the Federal Republic of Germany (Bauer et al., 1984)
and in Belgium (Rousseau & van Peteghem, 1989), with respectively,
21% and 18% of the affected kidneys containing residues of
ochratoxin A, in the range of 0.1-12 ng/g. In Canada, 1200 samples
of pig blood, collected at a slaughterhouse, were screened for
ochratoxin A using HPLC (Marquardt et al., 1988). Levels exceeding
10 ng/ml (11.3%) were found in 136 samples; detection was confirmed
by derivative formation and spectrometry. No kidney examination
was conducted, but the ochratoxin A concentrations detected in the
blood suggest that nephropathy might have been present in some of
the pigs.
I.4.1.2 Poultry
In a preliminary study in Denmark on chickens condemned by meat
inspectors because of renal lesions, 4 out of 14 birds (29%) were
found to have nephropathy associated with the ingestion of
ochratoxin A, as revealed by the presence of residues of ochratoxin
A in tissues (Elling et al., 1975). The renal lesions were
characterized by degeneration of proximal and distal tubules of
both reptilian and mammalian nephrons and interstitial fibrosis.
I.4.2 Experimental animal studies
I.4.2.1 Acute and chronic effects
The acute and chronic effects of ochratoxins on experimental
animals have been reviewed by Chu (1974a), Harwig (1974), and Krogh
(1976a). Different species vary in their susceptibility to acute
poisoning by ochratoxin A with LD50 values ranging from 3.4 to 30.3
mg/kg (Table 8). When ochratoxin A was administered orally to rats
and guinea-pigs, the female was more sensitive than the male. In
rats, the kidney is the target organ, but necrosis of periportal
cells in the liver has also been noted during studies on acute
effects (Purchase & Theron, 1968).
Table 8. Acute toxicity of ochratoxin A
-------------------------------------------------------------------
Animal LD50 (mg/kg Route of Reference
body weight) administration
-------------------------------------------------------------------
mouse 22 intraperitoneal Sansing et
(female) al. (1976)
rat (male) 30.3 oral Galtier et
al. (1974)
rat 21.4 oral Galtier et
(female) al. (1974)
rat (male) 28 oral Kanizawa et
al. (1977)
rat (male) 12.6 intraperitoneal Galtier et
al. (1974)
rat 14.3 intraperitoneal Galtier et
(female) al. (1974)
guinea-pig 9.1 oral Thacker &
(male) Carlton
(1977)
-------------------------------------------------------------------
Table 8 (contd.)
-------------------------------------------------------------------
Animal LD50 (mg/kg Route of Reference
body weight) administration
-------------------------------------------------------------------
guinea-pig 8.1 oral Thacker &
(female) Carlton
(1977)
white 3.4 oral Prior et
leghorn al. (1976)
turkey 5.9 oral Prior et
al. (1976)
Japanese 16.5 oral Prior et
quail al. (1976)
rainbow 4.7 intraperitoneal Doster et
trout al. (1972)
beagle dog 9 orala Szczech et
(male) (total dose) al. (1973a)
pig 6 oralb Szczech et
(female) (total dose) al. (1973b)
-------------------------------------------------------------------
a All 3 dogs, dosed daily with 3 mg/kg body weight, died within
3 days.
b Both pigs receiving 2 mg/kg daily were moribund and killed
within 3 days, and both pigs receiving 1 mg/kg daily were
moribund and killed within 6 days.
The lesions observed in field cases of mycotoxic nephropathy
have been reproduced by feeding diets containing levels of
ochratoxin A identical to those encountered in the naturally
contaminated products. In a study by Krogh et al. (1974a), 39 pigs
fed rations containing ochratoxin A, at levels ranging from 200 to
4000 µg/kg, developed nephropathy after 4 months at all levels of
exposure. Changes in renal function were characterized by
impairment of tubular function, indicated particularly by a
decrease in TmPAH/Cin,a and reduced ability to produce concentrated
urine. These functional changes corresponded well with the changes
in renal structure observed at all exposure levels, including
atrophy of the proximal tubules and interstitial cortical fibrosis.
Sclerosed glomeruli were also observed in the group receiving the
highest dose of ochratoxin A of 4000 µg/kg feed. Changes were not
seen in any other organs or tissues.
-------------------------------------------------------------------
a TmPAH = transport maximum for para-aminohippuric acid.
Cin = clearance of insulin.
Kidney damage, identical to naturally occurring porcine
nephropathy, was produced in another study by feeding pigs (9
animals) with crystalline ochratoxin A in amounts corresponding to
a feed level of 1 mg/kg for 3 months. Significant renal tubular
functional impairment as measured by a decrease in TmPAH Cin was
detected after only 5 weeks of ochratoxin exposure (Krogh et al.,
1976b). The study was continued for a 2-year period during which
the renal impairment aggravated slightly without reaching a state
of terminal renal failure (Krogh et al., 1979).
In 2 pigs and 9 rats dosed orally with ochratoxin A (400 and
250 µg/kg body weight, respectively) for 5 days, ochratoxin A was
detected in the epithelial cells of the proximal convoluted tubules
of the nephron of all animals. The method of detection was
immunofluorescence microscopy using an antibody against ochratoxin
A that had been formed in rabbits after injection of an albumin-
ochratoxin A conjugate (Elling, 1977).
Groups of 80 F 344/N rats of each sex were administered 0, 21,
70, or 210 µg ochratoxin A/kg in corn oil by gavage, 5 days per
week for 103 weeks (Boorman, 1988). The administration of
ochratoxin A to male and female rats caused a spectrum of
degenerative and proliferative changes in the kidney. The
predominant non-neoplastic lesion in treated rats was degeneration
of the renal tubular epithelium in the inner cortex and the outer
stripe of the outer medulla (nephropathy).
In four pigs given 0.8 mg ochratoxin A/kg body weight orally
for 5 consecutive days, the activity of catalase and CN-insensitive
palmitoyl-CoA-dependent NAD (nicotinamide adenine di-nucleotide) in
renal homogenates decreased, but that in hepatic homogenates did
not, suggesting peroxisomal changes. This was confirmed by
ultrastructural observations of peroxisomes in the proximal
tubules in kidneys of ochratoxin A-treated animals (Elling et
al., 1985).
When 11 SPF pigs and 23 beagle dogs were given high oral doses
of ochratoxin A corresponding to feed levels of more than 5-10
mg/kg (levels rarely found in nature), pathological effects, mainly
necrosis, were observed in the liver, intestine, spleen, lymphoid
tissue, leukocytes, and kidney (Szczech et al., 1973a,b,c). Three
groups of Wistar rats, each consisting of 15 animals, were exposed
to feed levels of ochratoxin A ranging from 0.2 to 5 mg/kg for 3
months. Renal damage in the form of tubular degeneration was
observed at all dose levels (Munro et al., 1974). A decrease in
urinary osmolality, glycosuria, and proteinuria were observed in
an unstated number of Sprague-Dawley and Wistar rats administered
daily doses intraperitoneally of 0.75-2 mg ochratoxin A/kg body
weight (Berndt & Hayes, 1979).
In a study of coagulation factors, rats were given daily oral
doses of 4 mg/kg body weight over 4-10 days, resulting in decreases
in plasma-fibrinogen levels, at levels of factors II, VII, and X,
and in the platelet and megakaryocyte counts (Galtier et al.,
1979).
In 4 calves fed rations containing 0.1-2 mg ochratoxin A/kg
body weight for 30 days, the only signs observed were polyuria,
increased levels of glutamic oxalacetic transaminase (GOT) in
serum, and mild enteritis (Pier et al., 1976); there was mild
tubular degeneration in the kidneys. Cows given ochratoxin A
orally for 4 days at doses ranging from 0.2 to 1.66 mg/kg body
weight remained clinically normal (Ribelin et al., 1978). A cow
given a single dose of 13.3 mg/kg body weight (corresponding to 865
mg/kg feed) developed diarrhoea, anorexia, and cessation of milk
production, one day after.
Avian nephropathy, similar to that in spontaneously occurring
cases, developed in Leghorn hens (27 birds per group) exposed to
dietary levels of 0.3 or 1 mg ochratoxin A/kg for one year (Krogh
et al., 1976c). The renal changes included degeneration of the
tubular epithelium, mainly confined to the proximal and distal
tubules of both reptilian and mammalian nephrons; impairment of
glomerular and tubular function was also observed. "Acute
nephrosis" and "visceral gout" were observed in chickens exposed to
high levels of ochratoxin A (LD50 values) (Peckham et al., 1971).
The same authors reported that ochratoxin B, the other naturally
occurring ochratoxin, was not highly toxic for chickens (LD50, 54
mg/kg) or other animals.
Groups of chicks (40 birds per group) were fed diets containing
ochratoxin A levels in the range of 0-8 mg/kg for 3 weeks (Huff et
al., 1974). The group receiving the highest dose (8 mg/kg feed)
showed decreased packed blood cell volume, haemoglobin
concentration, and serum-iron and serum-transferrin saturation
percentages. In a similar study, Chang et al.(1979) observed that
lymphocytopenia developed at all dose levels. In the same study,
decreased bone strength, as measured physically by resistance to
fracturation, was observed at feed levels of 2-4 mg ochratoxin A/kg
(Huff et al., 1980). When groups of chicks were fed ochratoxin A
at levels of 0, 2, or 4 mg/kg for 20 days, concentrations of serum-
immunoglobulins (IgA, IgG, IgM) were reduced to 57-66% of normal
values in the toxin-exposed groups (Dwivedi & Burns, 1984).
In B6C3F1 female mice, groups of 6-7 animals were administered
a total of 0, 20, 40, or 80 mg ochratoxin A/kg body weight ip on
alternate days over an 8-day period. A dose-related decrease in
thymic mass was observed as well as myelotoxicity, indicated by
bone marrow hypocellularity, due to decreased marrow pluripotent
stem cells, and granulocyte-macrophage progenitors (Boorman et al.,
1984).
When determining the LD50 in mice following intraperitoneal
injection, synergistic effects were observed when ochratoxin A was
combined with citrinin as well as with penicillic acid (Sansing et
al., 1976). An additive effect was observed between ochratoxin A
and citrinin in terms of embryotoxicity in chicken embryos (Vesela
et al., 1983). In beagle dogs, when combined oral doses of
ochratoxin A (0.1-0.2 mg/kg body weight) and citrinin (5-10 mg/kg)
were injected intraperitoneally, synergism was observed with regard
to severity of clinical disease and mortality (Kitchen et al.,
1977a,b). Increased toxicity in terms of LD50 values and
pathological changes was observed in rats, when the ochratoxin A
was given orally combined with either of the drugs biscoumacetate
or phenylbutazone, apparently because of displacement of the toxin
from binding sites on plasma-proteins (Galtier et al., 1980). The
toxic effects of ochratoxin A on the renal epithelial cells of the
monkey were demonstrated in in vitro studies in the form of
abnormal mitotic cells (Steyn et al., 1975).
I.4.2.2 Teratogenicity
Intraperitoneal injection of pregnant mice with ochratoxin A at
5 mg/kg body weight on one of gestation days 7-12 resulted in
increased prenatal mortality, decreased fetal weight, and various
fetal malformations, including exencephaly and anomalies of the
eyes, face, digits, and tail (Hayes et al., 1974). When a
combination of ochratoxin A (2 or 4 mg/kg body weight) and T-2
toxin (0.5 mg/kg body weight) was injected intraperitoneally in
mice on gestation day 8 or 10, ochratoxin A exacerbated the
incidence of T-2-induced gross malformations (tail and limb
anomalies); increased fetocidal effects were also noted (Hood et
al., 1978). Mice were given 3-5 mg ochratoxin A/kg body weight
intraperitoneally or orally on gestation days 8, 9, and 10 or 15,
16, and 17 (Szczech & Hood, 1981). Cerebral necrosis was found in
most fetuses from dams treated on days 15-17, but no cerebral
necrosis developed after treatment on days 8-10, when ochratoxin A
is overtly teratogenic. Pups of mice given 1.25 and 2.25 mg
ochratoxin A/kg orally on gestation days 15, 16, and 17 were tested
for surface righting, swimming, and pivoting (Poppe et al., 1983).
The results of all 3 tests indicated that a delay in development
had occurred; no dose-related pathological alterations were found.
Pregnant mice were administered 1-2 mg ochratoxin A/kg body
weight, and/or 5-20 mg zearalenone/kg body weight or 0.125 mg
diethylstilboestrol/kg body weight, orally, on day 9 of pregnancy,
either individually or in combination, and the offspring were
examined on day 19 (Arora et al., 1983). Teratogenic effects
produced by ochratoxin A, such as exencephaly, open eyelids, and
microphthalmia, were reduced or absent when the toxin was given in
combination with one of the 2 non-steroidal estrogenic substances,
zearalenone or diethylstilboestrol.
Rats were treated orally with ochratoxin A at 0.25, 0.50, 0.75,
1, 2, 4, or 8 mg/kg body weight on gestation days 6-15 (Brown et
al., 1976). Maternal toxicity was not observed below 4 mg/kg body
weight, but an increased incidence of fetal resorptions was
observed from 0.75 mg/kg body weight. All fetuses from dams given
0.25-0.75 mg/kg body weight weighed significantly less than control
fetuses, and fetuses from dams given 0.75 or 1 mg/kg body weight
were stunted. Reduced litters and decreased fetal weight were
observed in rats administered 5 mg ochratoxin A/kg body weight,
orally, on gestation day 8 (Moré et al., 1978).
Subcutaneous administration of ochratoxin A to rats (1.75 mg/kg
body weight) on gestation days 5-7 resulted in the highest number
of malformations, including hydrocephaly, omphalocele, and
anophthalmia as well as a shift in position of the oesophagus
(Mayura et al., 1982); lower doses (0.5 and 1 mg ochratoxin A/kg
body weight) did not have any teratogenic effects, and higher doses
(5 mg/kg) caused all fetuses to be resorbed. In a subsequent study
by the same authors (Mayura et al., 1983), using the same
ochratoxin A exposure conditions, it was shown that a diet low in
protein (10% of protein concentration in normal rat feed) enhanced
the teratogenic action of ochratoxin A in the rat. The combined
action of ochratoxin A exposure and a low protein diet also
resulted in decreases in mating and fertilization rates (22% and
39%, respectively) compared with the control group.
When rats were exposed to ochratoxin A and citrinin (another
nephrotoxic mycotoxin produced by species of the Aspergillus and
Penicillium genera) either singly or combined, enhanced teratogenic
effects were observed in terms of gross malformations, visceral
anomalies, and skeletal defects following combined oral
administration of 1 mg ochratoxin A/kg body weight and 30 mg
citrinin/kg body weight (Mayura et al., 1984). Maternal deaths
(22-40%) occurred after the administration of the combined dose on
days 5, 6, 7, and 14 of gestation, whereas administration of
individual toxins did not cause any maternal deaths and only
minimal malformations no matter which gestation day they were
administered.
Increased prenatal mortality and malformations, including
hydrocephaly, micrognathia, and heart defects, were observed in
hamsters injected intraperitoneally with ochratoxin A at doses of
5-20 mg/kg body weight on one of gestation days 7-9 (Hood et al.,
1976).
I.4.2.3 Mutagenicity
Ochratoxin A did not have any effects in a Bacillus subtilis
Rec- assay, measuring DNA damage when tested at 20 and 100 µg/plate
(Ueno & Kubota, 1976). Ochratoxin A was not mutagenic to
Salmonella typhimurium TA 1535, TA 1537, TA 1538, TA 98, or TA 100
at doses of up to 500 µg/plate, with or without exogenous metabolic
activation (Kuczuk et al., 1978; Wehner et al., 1978b). No
increase was observed in genetic changes at the ade 2 locus of
Saccharomyces cerevisiae after treatment with 50 or 100 µg/plate
ochratoxin A, with or without exogenous metabolic activation
(Kuczuk et al., 1978). Ochratoxin A did not induce mutations to
8-azaguanine resistance in C3H mouse mammary carcinoma cells (FM3A)
treated with doses of 5 or 10 µg/litre (Umeda et al., 1977).
When rats were orally exposed to ochratoxin A every 48 h for 12
weeks (corresponding to a feed level of 4 mg/kg), single-strand
breaks of DNA in renal and hepatic tissue (the only tissues
investigated) were more pronounced than those in control animals
(Kane et al., 1986a,b).
Contradictory results have been obtained by testing ochratoxin
A in the Salmonella assay (SOS chromo test without metabolic
activation) (Ueno et al., in press).
I.4.2.4 Carcinogenicity
Kanizawa & Suzuki (1978) have indicated that ochratoxin A is a
hepatic and renal carcinogen in male mice. A group of 10 male ddY
mice were fed a diet containing 40 mg ochratoxin A/kg for 44 weeks.
A group of 10 untreated controls were fed the basal diet. All
survivors were killed after 49 weeks. Hepatic cell tumours were
found in 5 out of 9 treated mice; no tumours were found in the 10
controls. Solid renal cell tumours were found in 2 out of 9
treated mice and none in the 10 controls. Cystic renal adenomas
were found in 9 out of 10 treated mice compared with none in the 10
controls.
Two groups of 50 male and 2 groups of 50 female B6C3F1 mice
were fed diets containing 1 or 40 mg ochratoxin A/kg, respectively;
one group (control) was fed the basal diet. All survivors were
killed after 24 months. Eleven out of 49 male mice in the 40 mg/kg
group had renal carcinomas; 24 out of 49 male mice in the 40 mg/kg
groups showed renal adenomas. All male mice in the 40 mg/kg group
had microscopic evidence of nephropathy. A few females in the 40
mg/kg group exhibited nephropathic changes but no carcinomas or
adenomas. Compound-related lesions were absent in the controls
and the 1 mg/kg groups (Bendele et al., 1985). In a test for the
development of hyperplastic liver nodules in rats, ochratoxin A was
characterized as having both an initiating and a promoting activity
(Imaida et al., 1982).
Seven groups of 16 male ddY mice each were fed a diet
containing 50 mg ochratoxin A/kg for various periods ranging from 0
to 30 weeks followed by feeding of the basal diet until the end of
70 weeks (Kanizawa, 1984). After 15 weeks of ochratoxin A
exposure, 3 out of 15 animals had renal cell tumours; after 20
weeks, 1 out of 14 mice had renal cell tumours and 2 had hepatomas;
after 25 weeks of exposure, 2 out of 15 mice had renal cell tumours
and 5 had hepatomas; and after 30 weeks of exposure, 4 out of 17
mice had renal cell tumours and 6 had hepatomas; these tumours were
not observed in the controls. The nature of the renal cell tumours
was not further defined, but such tumours are mostly malignant. In
addition, pulmonary tumours were found in all groups, including the
controls, the incidence ranging from 20 to 73%. In a second study,
the effects of a combination of ochratoxin A and citrinin were
elucidated. Groups of 20 male ddY mice were fed diets containing
25 mg ochratoxin A/kg in combination with 100 or 200 mg
citrinin/kg for 70 weeks. Control groups were fed diets that did
not contain any toxins or the individual toxins at the
concentrations indicated above. In addition, one group was fed 25
mg ochratoxin A/kg feed for the first 25 weeks followed by 200 mg
citrinin/kg feed for the remaining period of time; another group
was exposed to the 2 toxins in the reverse order. Exposure to
citrinin alone did not produce any tumours. Exposure to ochratoxin
A alone resulted in renal cell tumours in 6 out of 20 mice, and in
hepatomas in 8 mice. Exposure to ochratoxin A and 100 mg
citrinin/kg feed did not result in any renal cell tumours, but 10
out of 19 mice had hepatomas. Exposure to ochratoxin A and 200 mg
citrinin/kg feed resulted in renal cell tumours in 10 out of 18
mice, and in hepatomas in 7 mice. Exposure to one toxin followed
by the other toxin did not produce any renal cell tumours, but
hepatomas were observed in less than 20% of the mice.
On the basis of these studies, IARC concluded that there was
limited evidence of carcinogenicity for animals, and inadequate
evidence of carcinogenicity for human beings (IARC, 1987a).
Groups of 80 F 344/N rats of each sex were administered 0, 21,
70 or 210 µg ochratoxin A/kg in corn oil by gavage, 5 days per
week, for 103 weeks (Boorman, 1988). In the male rats, renal
carcinomas were found in 16 out of 51 animals dosed with 70 µg/kg
and in 30 out of 50 animals dosed with 210 µg/kg; no carcinomas
were found in lower dose groups. In the female rats, renal
carcinomas were less common, as 1 out of 50 animals dosed with 70
µg/kg and 3 out of 50 animals dosed with 210 µg/kg had carcinomas;
no carcinomas were found in the lower dose groups. Renal adenomas
were found in all groups of male rats, with increasing frequencies
associated with increased doses. In the female groups, renal
adenomas were only found in the two highest dose groups. In the
female rats, fibroadenomas in the mammary gland were found in 45-
56% in the treated groups, a significantly higher percentage than
in the control group.
I.4.2.5 Biochemical effects and mode of action
Ochratoxin A is an inhibitor of tRNA synthetase and protein
synthesis in several microorganisms ( Bacillus subtilis, B.
stearothermophilus, Streptococcus faecalis, yeasts) as well as in
rat hepatoma cells (Konrad & Roschenthaler, 1977; Bunge et al.,
1978; Heller & Roschenthaler, 1978; Creppy et al., 1979a,b). The
competitive inhibitor effect of ochratoxin A on tRNA synthetase and
protein synthesis in rat hepatoma cells can be prevented by the
addition of phenylalanine in the cell culture medium at a molar
ratio of phenylalanine: ochratoxin A of 1.7:1 (Creppy et al.,
1979b). This observation suggested the possibility of preventive
measures for ochratoxin A-induced disease. Thus, the acute
intraperitoneal effect of ochratoxin A (LD100) in mice was
prevented by concomitant injection of phenylalanine (Creppy et al.,
1980; Moroi et al., 1985).
However, in the study on the reproduction of porcine
nephropathy previously mentioned (Krogh et al., 1974a), the molar
ratio of phenylalanine: ochratoxin A in the feed exceeded 4600:1,
implying that, in the field situation, phenylalanine does not
prevent ochratoxin A from inducing the development of nephropathy.
Ochratoxin A in the concentration range studied (20-1667 µmol/
litre) caused a 47-50% inhibition of macrophage migration (Klinkert
et al., 1981); this effect could be prevented by the simultaneous
addition of phenylalanine. In BALB/c mice, a dose of ochratoxin A
as low as 0.005 µg/kg body weight was able to suppress the immune
response to sheep erythrocytes (Haubeck et al., 1981); the effect
could be prevented by the simultaneous addition of phenylalanine.
Studying the same effect in Swiss mice, Prior & Sisodia (1982) were
unable to show any suppression of the immune response to sheep
erythrocytes, even after daily injection of 5 mg ochratoxin A/kg
for 50 days. (4R)-4-Hydroxyochratoxin A at a dose of 1 µg/kg body
weight caused an 80% reduction in the number of cells producing IgM
and a 93% reduction in cells producing IgG in BALB/c mice compared
with 90% and 92%, respectively, for ochratoxin A (Creppy et al.,
1983).
Female B6C3F1 mice (6 per group) were administered ochratoxin A
in amounts of 0.34, 6.7, or 13.4 mg/kg body weight or ochratoxin B
(13.4 mg/kg body weight) 6 times during 12 days. Ochratoxin A
inhibited the natural killer cell activity at all dose levels, and
increased the growth of transplantable tumour cells without
affecting T-cell or macrophage-mediated anti-tumour activity
(Luster et al., 1987). Ochratoxin B did not influence immune
function. The inhibition by ochratoxin A of natural killer cell
activity appeared to be caused by reduced production of basal
interferon.
Ochratoxin A affects the carbohydrate metabolism in rats.
Thus, a single oral dose of ochratoxin A at 15 mg/kg body weight
caused a decrease in the glycogen level in the liver and an
increase in the heart glycogen level, 4 h later (Suzuki & Satoh,
1973).
In a more extensive study on rats, the decrease in liver
glycogen level, 4 h after a single oral dose of ochratoxin A at 15
mg/kg body weight, was associated with an increase in serum glucose
levels and a decrease in liver glucose-6-phosphate (Suzuki et al.,
1975). At the same time, the liver glycogen synthetase (EC
2.7.1.37) activity decreased and the liver phosphorylase (EC
2.4.1.1) activity increased. Three daily oral doses of ochratoxin
A at 5 mg/kg body weight caused a decrease in the liver glycogen
concentration, which was measured on the fourth day. The decrease
was attributed to inhibition of the active transport of glucose
into the liver, suppression of glycogen synthesis from glucose, and
acceleration of glycogen decomposition.
During in vitro studies on rat liver mitochondria, it was
observed that ochratoxin A inhibited the respiration of whole
mitochondria by acting as a competitive inhibitor of transport
carrier proteins located in the inner mitochondrial membrane
(Meisner & Chan, 1974). Further studies with mitochondrial
preparations revealed that the mitochondrial uptake of ochratoxin A
was an energy-using process that resulted in depletion of
intramitochondrial adenosine triphosphate (ATP), and that
ochratoxin A inhibited intramitochondrial phosphate transport
resulting in deterioration of the mitochondria (Meisner, 1976).
This might explain the degeneration of liver mitochondria observed
by Purchase & Theron (1968) in rats exposed orally to a single dose
of ochratoxin A at 10 mg/kg body weight. These authors observed
accumulation of glycogen in the cytoplasm of the rat liver cells
microscopically. This was in contrast to the previously discussed
observations of Suzuki et al. (1975), who found a decrease in
glycogen levels.
In a study on mice, Sansing et al. (1976) found that ochratoxin
A, administered intraperitoneally at 6 mg/kg body weight, inhibited
orotic acid incorporation into both liver and kidney RNA, 6 h after
toxin injection. In this respect, ochratoxin A acted synergistically
with another nephrotoxic mycotoxin, citrinin.
When neonatal rats were exposed orally to a single dose of 1 mg
ochratoxin A/kg or of 25 mg citrinin/kg or both doses within 24 h
of birth, a synergistic effect of the 2 mycotoxins was observed on
cytochrome P-450, NADPH-dependent dehydrogenase, and NADPH-
cytochrome c reductase (Siraj et al., 1981).
In rats fed 2 mg ochratoxin A/kg body weight per day for 2
days, renal gluconeogenesis from pyruvate was decreased by 26%, and
renal phosphoenol-pyruvate carboxy kinase (PEPCK) (EC 4.1.1.32)
activity was reduced by 55%, whereas hepatic PEPCK was unchanged
(Meisner & Selanik, 1979). In a subsequent study on rats, it was
found that even lower dose levels (0.3-0.5 mg ochratoxin A/kg body
weight) caused a 50% reduction in PEPCK activity (Meisner &
Meisner, 1981). A number of other enzymes located in the proximal
tubule of the nephron were unaffected. When longer exposure
periods (8-12 weeks) were used, and 145 ng ochratoxin A/kg body
weight were administered orally to groups of 3 rats each, increased
urinary excretion and corresponding renal tubular depletion of the
following enzymes were observed: gamma-glutamyl transferase,
alkaline phosphatase, leucine aminopeptidase, lactate
dehydrogenase, and N-acetyl-beta- D-glucosaminidase (Kane et al.,
1986a). Renal PEPCK in pigs was also sensitive to ochratoxin A
with a feed level of 100 µg/kg causing a significant decrease in
PEPCK activity (Meisner & Krogh, 1982).
In a study on pigs fed ochratoxin A at 0, 0.2, or 1 mg/kg for 5
weeks, enzyme activities were measured in renal biopsies, collected
1, 3, and 5 weeks after initiation (Krogh et al., 1988). After one
week, the activities of renal PEPCK and gamma-glutamyltranspeptidase
were decreased by 40%. The dose-related decrease in the activity
of PEPCK and gamma-glutamyltranspeptidase was accompanied by a
dose-related increase in renal impairment, as measured by the
reduction of TmPAH/Cin, suggesting that these enzymes are sensitive
indicators of ochratoxin-induced porcine nephropathy. Thus, the
renal biopsy-based measurements of enzyme activities might prove
diagnostically useful in ochratoxin-induced disease in human
beings.
Ochratoxin A reduced the total renal mRNA concentration in male
Sprague-Dawley rats and certain mRNA species, notably PEPCK, were
reduced to a greater extent than the bulk of the RNA pool (Meisner
et al., 1983).
I.5 EFFECTS ON MAN
I.5.1 Ochratoxin A, Balkan endemic nephropathy, and tumours of the
urinary system
This topic has been reviewed by Krogh (1979, 1983) who called
attention to the striking similarities in the changes in renal
structure and function induced experimentally in animals by the
administration of ochratoxin A, and the clinical and pathological
features of a localized endemic disease known as Balkan endemic
nephropathy. So far, the disease has been observed only in rural
populations of Bulgaria, Romania, and Yugoslavia, but information
on the present magnitude of the problem was not available to the
Task Group.
The Balkan endemic nephropathy is a chronic disease that
predominantly affects women and progresses slowly up to death
(Hrabar et al., 1976, Chernozemsky et al., 1977). Age-specific
incidence rates are highest above the age of 40. Younger cases
occur in the 10-19-year-old age group, and the mean age of new
patients is in the early 50s (Stoyanov et al., 1978).
The disease is characterized by an extreme geographical
clustering with a tendency for familial aggregation of cases
(Nicolov et al., 1978). However, sporadic cases occur outside
endemic areas. Age-adjusted incidence rates of 555 per 100 000
population in females and 322 in males over a ten-year period have
been recorded from a population sample of 147 000 in an endemic
area of Bulgaria (Stoyanov et al., 1978). In one of several
endemic regions in Yugoslavia, the prevalence varied from 3% to 8%
(Hrabar et al., 1976).
Autopsy has shown that kidneys are notably reduced in size.
The histological lesions are interstitial fibrosis, tubular
degeneration, and hyalization of glomeruli in the more superficial
part of the cortex (Heptinstall, 1974). Impairment of tubular
function, indicated by a decrease in TmPAH, is a prominent and
early sign (Dotchev, 1973).
A high incidence of tumours of the urinary system is strongly
correlated with the prevalence of Balkan endemic nephropathy
(Ceovic et al., 1976; Chernozemsky et al., 1977; Nicolov et al.,
1978). In one instance in Bulgaria, 46.6% of patients with tumours
of the urinary system were also affected by endemic nephropathy.
Among the tumours of the urinary system, cancers of the renal
pelvis and ureters are more frequently associated with endemic
nephropathy than urinary bladder tumours.
The relative risk for developing cancer of the renal pelvis and
ureters is 88:1 in patients with nephropathy compared with controls
in non-endemic areas. The relative risk for the development of any
tumour of the urinary system is only 28:1 in the same sample
(Stoyanov et al., 1978).
Over the past 2 decades, investigations have been carried out
to verify a variety of etiological assumptions with unconvincing
results (review by Puchlev, 1973, 1974). However, the assumption
of the possible role of ochratoxin A, on the basis of similarities
with the animal disease, has received increasing epidemiological
support.
In Yugoslavia, surveys indicated that the contamination of
foodstuffs (grains, maize, pork meat) with ochratoxin A occurred in
12.8% of samples in an area where the prevalence of endemic
nephropathy was 7.3%, compared with only 1.6% of contaminated
samples in areas free of the disease (Krogh et al., 1977). The
concentration of ochratoxin A in maize was 5-90 µg/kg and that in
pork meat, 5 µg/kg (Krogh et al., 1977) with levels of up to 27
µg/kg in pig kidneys (Pepeljnjak et al., 1982). Similarly, studies
in Bulgaria have revealed that 16.7% of beans and 27.3% of maize
from an endemic area were contaminated with ochratoxin A compared
with 7.1% and 9%, respectively, from a non-endemic area (Petkova-
Bocharova & Castegnaro, 1985).
In a subsequent survey of home-produced foodstuffs (cereal and
bread) from the same endemic area in Yugoslavia over a 5-year
period, a mean contamination of 8.7% was found with pronounced
annual variations, which probably reflect climatic conditions
during the crop harvesting periods (Pavlovic et al., 1979).
Surveys on the presence of ochratoxin A in blood samples are
difficult to compare, as different analytical methods have been
used with different levels of sensitivity. Prevalences of 16.6%
and 5.9% with a one-year interval were reported in the same endemic
village in Yugoslavia (Hult et al., 1982). It is not possible to
determine whether this difference reflects annual variations in the
content of the blood, or the result of a less sensitive analytical
method in the second instance. However, higher prevalences of
ochratoxin A and higher concentrations in blood are generally
present in people from endemic areas, especially in persons
suffering from Balkan nephropathy.
A survey in Yugoslavia reported a prevalence in the blood of
16.6% in an endemic village and 6% in a non-affected one (Hult et
al., 1982). In Bulgaria, reported rates were 17.7% in an endemic
area and 7.7% in a non-endemic one (Petkova-Bocharova et al.,
1988). Table 9 illustrates the trend towards higher concentrations
in endemic situations and in patients. The similarity of results
in healthy families from affected villages and healthy persons from
unaffected villages (groups III and IV) suggests than an
environmental or a behavioural determinant plays a role at the
household level.
Thus, available epidemiological information seems to indicate
that Balkan endemic nephropathy is associated with consumption
patterns involving foodstuffs contaminated with ochratoxin A and
with a higher frequency of positive blood samples of ochratoxin A.
However, the association does not permit the establishment of a
causal relationship. Cross sectional surveys, such as those
reported in the literature so far, are probably not the appropriate
means to determine this relationship.
The results of experimental animal studies suggest that Balkan
nephropathy, a chronic condition, may require a long latency period
between exposure and the onset of symptoms, or more likely a
prolonged exposure or repeated exposure over a long period of time.
Investigations based on individual exposure time sequences
together with follow-up cohort surveys could provide a clue to the
causal role of ochratoxin A in Balkan endemic nephropathy. In view
of the lack of this information, such a causal relationship cannot
be established or rejected.
Table 9. Ochratoxin A in blood samples from people in endemic and
non-endemic areas in Bulgariaa
-------------------------------------------------------------------
Groupb No. of No. of Mean concentration
persons ochratoxin A ± S.D. (µg/kg)
assayed positive cases
(%)
-------------------------------------------------------------------
I. Persons with 61 16 (26.3) 20.3 ± 9.7
UST and/or EN
II. Healthy 63 10 (15.8) 14.5 ± 7.6
persons from
families with UST
and/or EN cases
III. Healthy 63 7 (11.1) 12.5 ± 3.5
persons from
families in
endemic villages
IV. Healthy 60 7 (11.6) 15.0 ± 4.2
persons from
unaffected villages
in endemic areas
V. Healthy 65 5 (7.7) 10.0
persons from
villages in
non-endemic areas
-------------------------------------------------------------------
a From: Petkova-Bocharova et al. (1988).
b UST: urinary system tumours; EN: endemic nephropathy.
The differences between group I and groups III and IV are
statistically significant ( P <0.002). Difference between
groups I and V is statistically significant ( P <0.001).
I.6 EVALUATION OF THE HUMAN HEALTH RISKS
Human exposure, as demonstrated by the occurrence of ochratoxin
A in food and in the blood, has been observed in various countries
in Europe. The Task Group was not aware of attempts to detect
ochratoxin A in human blood in other parts of the world.
The causal role of ochratoxin A in porcine nephropathy has been
established, based on studies of field cases as well as
reproduction of the disease with ochratoxin A. Using the porcine
model, it has been postulated that Balkan endemic nephropathy may
result from exposure to ochratoxin A. Available epidemiological
information indicates that Balkan nephropathy may be associated
with the consumption of foodstuffs contaminated by this toxin.
Since the publication of Environmental Health Criteria 11, in 1979,
epidemiological studies on the concentration of ochratoxin A in
human blood in affected and non-affected areas, have provided
additional support for the relationship between Balkan nephropathy
and exposure to ochratoxin A.
It has been shown that both the prevalence of ochratoxin A in
the blood and the blood concentrations are higher in residents in
endemic areas. However, a direct causal relationship cannot be
established on the basis of indirect evidence provided by the above
retrospective studies alone. Neither can it be excluded in view of
the long latency period between the exposure and the onset of
symptoms.
Ochratoxin A has been demonstrated to be carcinogenic to the
renal tubular epithelium in male mice and both sexes of rats. A
highly significant relationship has been observed between Balkan
nephropathy and tumours of the urinary tract, particularly with
tumours of the renal pelvis and ureters. However, there are no
published data to establish a direct causal role of ochratoxin A in
the etiology of such tumours.
II. TRICHOTHECENES
II.1 PROPERTIES AND ANALYTICAL METHODS
II.1.1 Physical and chemical properties
The sesquiterpenoid trichothecenes possess the tetracyclic
12,13-epoxytrichothecene skeleton. A total of 148 trichothecenes,
83 non-macrocyclic and 65 macrocyclic, have been isolated from
fungal cultures and plants (Drove, 1988). They can be conveniently
divided into 4 categories according to similarity of functional
groups (Ueno, 1977). The first class is characterized by a
functional group other than a ketone at C-8 (type A). This is the
largest category containing members such as T-2 toxin and
diacetoxyscirpenol (DAS). The second category of trichothecenes
has a carbonyl function at C-8 (type B) typified by 4-deoxynivalenol
(DON) and nivalenol (NIV). The third category is characterized by
a second epoxide group at C-7,8 or C-9,10 (type C), and the fourth
contains a macrocyclic ring system between C-4 and C-15 with two
ester linkages (type D). The structures of representative
trichothecenes of each category are illustrated below.
Table 2. Natural occurrence of ochratoxin A in foodstuffs and animal feed of plant origin
---------------------------------------------------------------------------------------------------------
Commodity Country Number of Percentage Range of Reference
samples contaminated ochratoxin A
analysed levels (µg/kg)
---------------------------------------------------------------------------------------------------------
FOOD
maize USA 293 1.0 83-166 Shottwell et al. (1971)
maize (1973) France 463 2.6 15-200 Galtier et al. (1977b)
maize (1974) France 461 1.3 20-200 Galtier et al. (1977b)
wheat (red winter) USA 291 1.0 5-115 Shottwell et al. (1976)
wheat (red spring) USA 286 2.8 5-115 Shottwell et al. (1976)
barley (malt) Denmark 50 6.0 9-189 Krogh (1978)
barley USA 127 14.2 10-40 Nesheim (1971)
coffee beans USA 267 7.1 20-360 Levi et al. (1974)
maize Yugoslaviaa 542 8.3b 6-140 Pavlovic et al. (1979)
wheat Yugoslaviaa 130 8.5b 14-135 Pavlovic et al. (1979)
wheat bread Yugoslaviaa 32 18.8b Pavlovic et al. (1979)
barley Yugoslaviaa 64 12.5b 14-27 Pavlovic et al. (1979)
barley Czechoslovakia 48 2.1 3800 Vesela et al. (1978)
bread United Kingdomc 50 2.0 210 Osborne (1980)
flour United Kingdomc 7 28.5 490-2900 Osborne (1980)
beans Sweden 71 8.5 10-442 Akerstrand &
peas Sweden 72 2.8 10 Josefsson (1979)
maize United Kingdom 29 37.9 50-500 Ministry of
cornflour United Kingdom 13 30.8 50-200 Agriculture (1980)
soya bean United Kingdom 25 36.0 50-500 Ministry of
soya flour United Kingdom 21 19.0 50-500 Agriculture (1980)
cocoa beans (raw) United Kingdom 56 17.9 100-500 Ministry of
(roasted) United Kingdom 19 15.8 100 Agriculture (1980)
grain German Democratic 49 4.1 18-22 Fritz et al.
Republic (1979)
grain (barley, Poland 296 6.8 20-470 Szebiotko et al. (1981)
wheat, rye)
grain (wheat, rye) Denmark 151 1.3 15-50 Pedersen &
bran (wheat) Denmark 57 10.5 5-20 Hansen (1981)
maize Bulgariaa 22 27.3 25-35 Petkova-Bocharova &
maize Bulgaria 22 9.0 10-25 Castegnaro (1985)
beans Bulgariaa 24 16.7 25-27 Petkova-Bocharova &
beans Bulgariaa 28 7.1 25-50 Castegnaro (1985)
---------------------------------------------------------------------------------------------------------
Table 2 (contd.)
---------------------------------------------------------------------------------------------------------
Commodity Country Number of Percentage Range of Reference
samples contaminated ochratoxin A
analysed levels (µg/kg)
---------------------------------------------------------------------------------------------------------
FOOD (continued)
barley, wheat, Poland 150 5.3 50-200 Juszkiewicz &
oats, rye, maize Piskorska-
Pliszczynska (1976)
FEED
mixed feed Poland 203 4.9 10-50 Juszkiewicz &
Piskorska-
Pliszczynska (1977)
maize Yugoslavia 191 25.7 45-5125 Balzer et al. (1977)
barley, oats Sweden 84 8.3 16-409 Krogh et al. (1974b)
wheat, hay Canada 95 7.4 30-6000 Prior (1976)
wheat, oats, Canadac 32 56.3 30-27 000 Scott et al. (1972)
barley, rye
barley, oats Denmarkc 33 57.6 28-27 500 Krogh et al. (1973)
mixed feed Canada 474 1.1 30-100 Prior (1981)
mixed feed Canadac 51 7.8 48-5900 Abramson et al. (1983)
mixed feed Australia 25 4.0 70 000 Connole et al. (1981)
mouldy bread Italyd 1 80 000 Visconti &
Bottalico (1983)
---------------------------------------------------------------------------------------------------------
a From an area with endemic nephropathy.
b Average values for a period of 2-5 years.
c All samples suspected of containing mycotoxins.
d This sample contained in addition 9600 µg ochratoxin B/kg.
Ochratoxin B has only been found in 3 samples and, thus, occurs
extremely rarely; the other ochratoxins have never been found in
plant products. Levels of ochratoxin A and the frequency of
contamination are generally higher in animal feed than in foodstuffs
(Table 2). Although the mean level of ochratoxin A in all reported
surveys up to 1979 was 1035 µg/kg (Fig. 3), 83% of the samples
contained less than 200 µg/kg (Krogh, 1980).
I.2.2.2 Residues in food of animal origin
Residues of ochratoxin A are not generally found in ruminants,
because ochratoxin A is cleaved in the forestomachs by protozoan and
bacterial enzymes (Galtier & Alvinerie, 1976; Hult et al., 1976;
Patterson et al., 1981). The non-toxic cleavage product, ochratoxin
alpha, has been found in the kidneys at levels below 10 µg/kg and in
the blood of calves fed a diet containing 300-500 µg ochratoxin A/kg
(Patterson et al., 1981). In half of the calves, the kidneys
contained low levels of ochratoxin A (up to 5 µg/kg), a feature that
may reflect that the calves were not yet functioning as ruminants.
When 2 milking cows were fed a ration containing 317-1125 µg
ochratoxin A/kg for 11 weeks, a residue of 5 µg ochratoxin A/kg was
found in the kidneys of one of the animals but not in any other
tissue or the milk. Ochratoxin alpha was not found in any tissue
(Shreeve et al., 1979). Residues of ochratoxin A have been detected
in a number of tissues in single-stomach food animals, such as pigs.
The carry-over of ochratoxin A from feed to animal tissues was
elucidated in a study in which groups of pigs were exposed for 3-4
months to dietary levels of ochratoxin A of 200, 1000, or 4000 µg/kg
(Krogh et al., 1974a). At termination (slaughter), the highest
levels of ochratoxin A residues were found in the kidneys (mean
levels 50 µg/kg at the 4000 µg/kg feed level), with lower levels
in the liver, muscle, and adipose tissue; other tissues, including
blood, were not analysed. There was a high correlation between the
feed level of ochratoxin A and the residue levels in the 4 tissues
investigated (Table 3).
Table 3. Correlation between feed level and tissue levels
(residues) of ochratoxin A in pigsa
-----------------------------------------------------------
Tissue Regression equation r
-----------------------------------------------------------
kidney Y = 2.15 + 0.0123X 0.86
liver Y = 0.35 + 0.0095X 0.82
adipose Y = 2.51 + 0.0099X 0.78
-----------------------------------------------------------
a Modified from Krogh et al. (1974a).
X = ochratoxin A in feed (µg/kg).
Y = ochratoxin A residue (µg/kg tissue).
r = correlation coefficient.
The regression is calculated on feed levels of ochratoxin A
in the range of 200-4000 µg/kg.
Ochratoxin A is present in the blood bound to serum-albumin
(Chu, 1971; Galtier, 1974a) and as free ochratoxin A; saturation (in
the rat) occurs at 70 mg ochratoxin A/litre plasma. The binding of
ochratoxin A to serum-albumin is particularly strong in cattle,
pigs, and man, based on in vitro studies (Table 4).
Table 4. In vitro binding of ochratoxin A to
serum-albumin in several speciesa
-----------------------------------------------
Animal Number of Intrinsic
species binding Association
sites constant (M-1)
-----------------------------------------------
Cattle 0.58 94 600
Pig 0.56 71 100
Man 0.58 63 500
Horse 0.57 57 400
Chicken 0.51 52 700
Rat 0.68 40 100
Sheep 0.88 22 600
-----------------------------------------------
a Adapted from: Galtier (1979).
The presence of ochratoxin A in the blood of pigs has been
elucidated in experimental animal studies as well as by surveys of
blood samples from pigs at farms (Hult et al., 1979, 1980). A
total of 1200 pig blood samples, collected from slaughterhouses
over various periods during 1986 in western Canada, was screened
for the presence of ochratoxin A using an HPLC procedure (Marqhardt
et al., 1988). It was shown that 3.6-4.2% of the blood samples
contained the toxin at concentrations higher than 20 ng/ml. It
appears that the concentration of ochratoxin A in the blood is
higher than that in any other tissue. In feeding studies, bacon
pigs were exposed for various periods to rations containing
ochratoxin A concentrations in the range of 58-1878 µg/kg; blood,
kidney, liver, muscle, and adipose tissues were analysed, at
slaughter, for residues of ochratoxin A (Mortensen et al., 1983).
The statistical association between residue levels in various
tissues is indicated by the regression analyses (Table 5).
Table 5. Regression between residues of ochratoxin A
in the serum and certain other tissuesa
------------------------------------------------------
Tissue Regression r sb
equation
------------------------------------------------------
kidney Y = 0.0651X 0.89 0.002
muscle Y = 0.0346X 0.88 0.001
liver Y = 0.0259X 0.89 0.001
adipose Y = 0.0191X 0.84 0.001
------------------------------------------------------
a Adapted from: Mortensen et al. (1983).
X = µg ochratoxin A/litre serum.
Y = µg ochratoxin A/kg in the other tissues.
r = correlation coefficient
sb = Standard error of slope
Epidemiological studies on the basis of data from meat
inspection in Danish slaughterhouses revealed prevalence rates of
porcine nephropathy ranging from 10 to 80 cases per 100 000
slaughtered pigs (Krogh, 1976a). Surveys in a number of European
countries for residues of ochratoxin A in kidneys from cases of
porcine nephropathy revealed that 25-39% of the cases contained
ochratoxin A levels in the range 2-100 µg/kg (Table 6).
Table 6. Surveys for ochratoxin A residues in kidneys from
cases of porcine nephropathy, based on meat inspection data
-------------------------------------------------------------------
Country No. of porcine Percentage Range of Reference
nephropathy containing ochratoxin
kidneys residues of A residues
investigated ochratoxin (µg/kg wet
A weight)
-------------------------------------------------------------------
Denmark 60 35 2-68 Krogh (1977)
Germany, 104 21 0.1-1.8 Bauer et al.
Federal (1984)
Republic of
Hungary 122 39 2-100 Sandor et
al. (1982)
Poland 113 24 1-23 Golinski et
al. (1984)
Sweden 129 25 2-104 Rutqvist et
al. (1977)
Sweden 90 27 2-88 Josefsson
(1979)
-------------------------------------------------------------------
Ochratoxin A levels of up to 29 µg/kg were found in the muscle
of hens and chickens collected at one slaughterhouse (Elling et
al., 1975). The birds had been condemned because of nephropathy.
In another study, groups of hens were exposed for 1-2 years to
dietary levels of ochratoxin A of 0.3 or 1 mg/kg (Krogh et al.,
1976b). The kidneys contained the highest residues with a mean
value of 19 µg/kg tissue in the group fed 1 mg ochratoxin A/kg; the
liver and muscle contained lower levels of residues and no
ochratoxin A was found in the eggs. These results are in
accordance with those of subsequent experimental animal studies.
For example, in a study in which 4 groups of hens were fed diets
containing 0, 0.5, 1, or 4 mg ochratoxin A/kg, the kidneys
contained the highest level of ochratoxin A residues (31 µg/kg) in
the highest dose group; eggs were not analysed (Prior & Sisodia,
1978).
In another study, hens were fed 1 mg ochratoxin A/kg feed for 8
weeks; the kidneys contained 3-10 µg ochratoxin A/kg and the liver,
1.5-2.5 µg/kg. The eggs were not analysed (Reichmann et al.,
1982). When groups of hens were fed diets containing 2.5 or 10
mg/kg, the kidneys contained 1-6 µg ochratoxin A/kg, lower levels
being found in the plasma, muscle, and liver (Juszkiewicz et al.,
1982). Eggs from the high-dose group contained 0.7-1.3 µg
ochratoxin A/kg, but none was detected in eggs from the low-dose
group.
White Leghorn hens (54 birds), divided into four groups, were
fed diets for one month containing the following concentrations of
ochratoxin A (mg/kg): 0, 1.3, 2.6, and 5.2 (Bauer et al., 1988).
At termination, three tissues were analysed for residues. The
following ranges of dose-dependent residues were found: serum,
4.7-11.7 ng/ml, liver, 9.1-18.0 ng/g, and yolk, 1.6-4.0 ng/g; very
little ochratoxin A was found in the egg white, and none was
detected in the tissues of the control group.
I.3 METABOLISM
I.3.1 Absorption
In a study on rats exposed by gavage to a single dose of
ochratoxin A at 10 mg/kg body weight, Galtier (1974b) found the
highest tissue level of unchanged ochratoxin A in the stomach wall
during the first 4 h following administration. The small and large
intestine and caecum contained small amounts of unchanged
ochratoxin A, and it was concluded that ochratoxin A was absorbed
mainly in the stomach. Small amounts (1-3% of the total dose) were
detected in the caecum and the large intestine, as the isocoumarin
moiety (ochratoxin alpha), most likely as the result of the
hydrolysing action of the intestinal microflora (Galtier &
Alvinerie, 1976; Hult et al., 1976).
In a study on intestinal absorption using the same animal
species, Kumagai & Aibara (1982) came to the conclusion that the
site of maximal absorption of ochratoxin A was the proximal
jejunum, and that the portal vein was the primary route of
transport from the intestinal tract, though part of the
transportation took place through the lymphatics.
Using a highly specific antibody against ochratoxin A, which is
used in a peroxidase-antiperoxidase detection method, Lee et al.
(1984) studied absorption and tissue distribution in Swiss mice
over a 48-h period, after the administration of a single dose of 25
mg ochratoxin A/kg body weight. Ochratoxin A was found in large
amounts, indicated by staining, in epithelial cells as well as in
macrophages of the lamina propria in the duodenum. Smaller amounts
were found in jejunal epithelial cells and lamina propria, and much
smaller amounts in the epithelial cells in the esophagus and
stomach; no toxin was found in the ileum. These findings suggest
that absorption mainly takes place in the duodenum and the jejunum.
I.3.2 Tissue distribution
I.3.2.1 Animal studies
In slaughterhouse cases of mycotoxic porcine nephropathy
studied by Hald & Krogh (1972), residues of unchanged ochratoxin A
were found in all tissues investigated (kidney, liver, and muscle),
the highest level (up to 67 µg/kg) occurring in the kidney. In
experimental studies on pigs ingesting feed containing ochratoxin
A, residues of the toxin were found in all 4 tissues in the
decreasing order of kidney, liver, muscle, adipose tissue (Krogh et
al., 1974a). A subsequent study revealed that the concentration of
ochratoxin A residues in the blood of the pig was higher than those
in the other tissues mentioned above (Mortensen et al., 1983).
When rats were exposed orally to an ochratoxin A dose of 10 mg/kg
body weight, Galtier (1974b) recovered 0.3% of the administered
dose in the whole kidneys, 0.9% in the whole liver, and 0.6% in the
total muscle tissue, 96 h after exposure. Chang & Chu (1977),
using a single intraperitoneal injection of 1 mg ochratoxin A per
rat (labelled with 14C in phenylalanine), found that the kidney
contained twice as much unchanged ochratoxin A as the liver after
30 minutes, amounting to 4-5% of the total dose.
In the study by Lee et al. (1984), the largest amounts of
ochratoxin A found in the kidney (as indicated by staining
intensity) were in the epithelium of the proximal convoluted
tubules, and to a lesser extent in the distal convoluted tubules,
the descending loop of Henle, and glomeruli and Bowman's capsule.
Small amounts were found in hepatocytes as well as in the lumina of
bile ducts, but not in biliary epithelium, indicating biliary
excretion.
Using pig renal cortical slices, it was found that ochratoxin A
enters the proximal tubule cells by the common organic anion
transport system (Friis et al., 1988). Ochratoxin A inhibited p-
aminohippurate (PAH) and phenolsulfophthalein uptake in a dose-
dependent manner.
I.3.2.2 Studies on man
In a study in Yugoslavia near Slavonski Brod, where endemic
nephropathy is prevalent, 639 samples of serum from the inhabitants
of 2 villages were screened for the presence of ochratoxin A; 42
(6.6%) were positive for the toxin "with concentrations in the
range of 1-57 ng ochratoxin A/g" (Hult et al., 1982). Detection
was carried out using the enzymic spectrofluorometric procedure,
and positives were confirmed by the esterification of ochratoxin A
in serum and of ochratoxin alpha obtained from the enzymetic
hydrolisates; the esters were measured by high performance liquid
chromatography.
In a screening of serum samples in Poland using the same method
of analysis, 77 out of 1065 samples (7.2%) contained ochratoxin A,
with a mean concentration of 0.27 ng/ml and a maximum value of 40
ng/ml (Colinski, 1987). In the Federal Republic of Germany, 173
out of 306 serum samples (56.6%) contained ochratoxin A, as
measured by an HPLC procedure, with a mean of 0.6 ng/g and a range
of 0.1-14.4 ng/g (Bauer & Gareis, 1987). Three out of 46 kidneys
(6.6%) contained ochratoxin A, with a mean of 0.2 ng/g and a range
of 0.1-0.3 ng/g. In a study of blood plasma in Denmark, 46 out of
96 samples (47.9%) contained ochratoxin A, as measured by an HPLC
procedure, with a mean of 1.7 ng/g and a range of 0.1-9.2 ng/g
(Hald, 1989). In Bulgaria, where endemic nephropathy also occurs
in some areas, 45 out of 312 blood samples contained ochratoxin A
(14.4%), with a mean of approximately 14 ng/g (Petkova-Bocharova et
al., 1988).
I.3.3 Metabolic transformation
It has been shown from in vitro studies that ochratoxin A binds
to serum-albumin (Chu, 1971, 1974b); this binding has also been
observed in in vivo studies on rats (Galtier, 1974a; Chang & Chu,
1977). Ochratoxin alpha has been detected in the urine and faeces
of rats injected intraperitoneally with ochratoxin A (Nel &
Purchase, 1968; Chang & Chu, 1977), indicating the cleavage of
ochratoxin A to ochratoxin alpha and phenylalanine, under these
conditions.
Studies with 14C-labelled ochratoxin A indicated that some
other, not yet identified, metabolites are formed in the body.
Less than half of the radioactivity excreted in rat urine within 24
h of a single intraperitoneal injection of 14C-phenylalanine-
labelled ochratoxin A was identified as ochratoxin A (Chang & Chu,
1977).
In both albino rats and brown rats given ochratoxin A orally or
intraperitoneally, 1-1.5% of the dose was excreted as (4R)-4-
hydroxyochratoxin A and 25-27% as ochratoxin alpha in the urine
(Storen et al., 1982). In in vitro studies using liver microsomes
from the pig, rat, and man, both (4R)-and (4S)-4-hydroxy-ochratoxin
A were produced in a hydroxylation process involving cytochrome
P-450 (Stormer & Pedersen, 1980; Stormer et al., 1981).
4-Hydroxyochratoxin A is non-toxic for rats in amounts up to 40 mg/kg
body weight (Hutchison et al., 1971); thus, it has been concluded
that the microsomal hydroxylation most likely represents a
detoxification reaction.
I.3.4 Excretion
The results of studies in which 14C-labelled ochratoxin A was
injected intraperitoneally in rats demonstrated that the toxin was
excreted primarily in the urine (Chang & Chu, 1977), though faecal
elimination also occurred to some extent (Galtier, 1974b; Chang &
Chu, 1977). In a study in which 14C-labelled ochratoxin A was
given orally to rats as a single dose (15 mg/kg body weight), the
cumulative elimination after 120 h was 11% ochratoxin A and 23%
ochratoxin alpha in the faeces; 11% ochratoxin A and 12% ochratoxin
alpha in the urine; and 33% ochratoxin A in the bile (Suzuki et
al., 1977). Absorption was influenced by enteritis, which was
caused by the high ochratoxin A doses given (75% of the LD50
value). Ochratoxin A injected intravenously as a single dose (4.1
mg/kg) in albumin-deficient and normal rats was excreted in the
bile and urine 20-70 times faster in the albumin-deficient rats
than in normal rats, indicating that the binding of ochratoxin A to
blood albumin delays the excretion of the compound through the
liver and kidney (Kumagai, 1985).
In rats given unlabelled ochratoxin A orally, Storen et al.
(1982) found 6% ochratoxin A, 1.5% (4R)-4-hydroxyochratoxin A, and
25-27% ochratoxin alpha in the urine; 12% ochratoxin A and 9%
ochratoxin alpha were found in the faeces.
The excretion of ochratoxin A in the milk was studied in
rabbits intravenously injected with 1-4 mg/kg body weight, as
single dose (Galtier et al., 1977a). At the highest dose injected,
the milk contained 1 mg ochratoxin A/litre; ochratoxin alpha and
4-hydroxy-ochratoxin A were not detected. Goats were given a
single dose of tritium-labelled ochratoxin A (0.5 mg/kg) and the
cumulative excretion (in terms of radioactivity) after 7 days
amounted to 53% in the faeces, 38% in the urine, 6% in the milk,
and 2% in the serum (Nip & Chu, 1979). Only a small fraction of
the radioactivity in milk was ochratoxin A, amounting to 0.026% of
the total ochratoxin A given.
In the Federal Republic of Germany, a study of human milk
obtained from women in two hospitals (patient category not stated)
revealed that 4 out of 36 samples (11.1%) contained ochratoxin A,
with a mean value of 0.024 ng/ml and a range of 0.017-0.030 ng/ml
(Bauer & Gareis, 1987; Gareis et al., 1988).
In mice given 14C-labelled ochratoxin A intravenously at
various stages of pregnancy, the toxin was shown to cross the
placental barrier on day 9 of pregnancy, at which time it is most
effective in producing fetal malformations (Appelgren & Arora,
1983). The highest toxin concentration was found in the bile,
which contained 5 times as much as the blood.
Ochratoxin A has been detected in the urine of bacon pigs
suffering from nephropathy (Krogh, unpublished information
communicated to the Task Group).
In a study on the disappearance rates for various tissues,
female bacon pigs were fed ochratoxin A at a level of 1 mg/kg feed
for one month and then kept on a toxin-free diet for another month,
during which animals were sacrificed at regular intervals (Krogh et
al., 1976a). Ochratoxin A disappeared exponentially (Table 7) from
the 4 tissues investigated (kidney, liver, muscle, and adipose
tissue) with residual life values (RL50)a in the range of 3.5-4.5
days; the toxin could still be detected in the kidneys one month
after termination of exposure.
Table 7. The rate of disappearance of ochratoxin A residues
from pig tissues after termination of a one-month exposure to
ochratoxin A at 1 mg/kg feeda
--------------------------------------------------------------
Tissue Ochratoxin A (µg/kg tissue) at time t (days)
after termination of exposure
---------------------------------------------------------------
kidney 28.22 exp (-0.1522 t)
liver 19.49 exp (-0.1598 t)
muscle 12.94 exp (-0.2096 t)
adipose 4.62 exp (-0.0565 t)
---------------------------------------------------------------
a From: Krogh et al. (1976a).
-------------------------------------------------------------------
a RL50 = half residual life, calculated from the exponential
equations shown in Table 7.
No data are available on ochratoxin levels in human urine or
faeces.
When the level in the serum is known, the ochratoxin A residues
in the four other tissues can be calculated (Table 5).
I.4 EFFECTS ON ANIMALS
I.4.1 Field observations
I.4.1.1 Pigs
The effects of ochratoxins on animals have been reviewed by
Krogh (1976a, 1978). Cases of mycotoxic porcine nephropathy have
been regularly encountered in studies in Denmark since the disease
was first discovered 50 years ago (Larsen, 1928). The disease is
endemic in all areas of the country, though unevenly distributed.
Prevalence rates in 1971 varied from 0.6 to 65.9 cases per 10 000
pigs, and epidemics encountered in 1963 and 1971 were associated
with a high moisture content in the grain caused by unusual
climatic conditions (Krogh, 1976b). On the basis of these studies,
Krogh (1978) concluded that ochratoxin A is the substance most
frequently associated with porcine nephropathy, though other
factors, such as citrinin, may also be involved.
A survey on porcine nephropathy was conducted at six
slaughterhouses in Sweden during the spring months of 1978
(Josefsson, 1979). A prevalence rate of 4.4 cases per 10 000 pigs
was encountered corresponding to the endemic level of prevalence
rates in Denmark; 26.7% of nephropathic kidneys contained residues
of ochratoxin A. In Hungary, an epidemiological study on porcine
nephropathy and the association with ochratoxin A was conducted in
1980-81 covering 4 areas in the country (Sandor et al., 1982). A
prevalence rate of 2.0 cases per 10 000 pigs was measured,
comparable to endemic prevalence rates in Scandinavia; 39% of
nephropathic kidneys contained residues of ochratoxin A. The
morphological changes in the kidneys in cases of mycotoxic porcine
nephropathy were characterized by degeneration of the proximal
tubules followed by atrophy of the tubular epithelium, interstitial
fibrosis in the renal cortex, and hyalinization of some glomeruli
(Elling & Moller, 1973).
In Poland, surveys for porcine nephropathy in 1983 and 1984
revealed prevalence rates of 4.7-5.7 cases per 10 000 pigs, with 5-
55% of the nephropathic kidneys containing detectable residues of
ochratoxin A, apparently depending on the season of the year
(Golinski et al., 1984, 1985). Porcine nephropathy has been
encountered in the Federal Republic of Germany (Bauer et al., 1984)
and in Belgium (Rousseau & van Peteghem, 1989), with respectively,
21% and 18% of the affected kidneys containing residues of
ochratoxin A, in the range of 0.1-12 ng/g. In Canada, 1200 samples
of pig blood, collected at a slaughterhouse, were screened for
ochratoxin A using HPLC (Marquardt et al., 1988). Levels exceeding
10 ng/ml (11.3%) were found in 136 samples; detection was confirmed
by derivative formation and spectrometry. No kidney examination
was conducted, but the ochratoxin A concentrations detected in the
blood suggest that nephropathy might have been present in some of
the pigs.
I.4.1.2 Poultry
In a preliminary study in Denmark on chickens condemned by meat
inspectors because of renal lesions, 4 out of 14 birds (29%) were
found to have nephropathy associated with the ingestion of
ochratoxin A, as revealed by the presence of residues of ochratoxin
A in tissues (Elling et al., 1975). The renal lesions were
characterized by degeneration of proximal and distal tubules of
both reptilian and mammalian nephrons and interstitial fibrosis.
I.4.2 Experimental animal studies
I.4.2.1 Acute and chronic effects
The acute and chronic effects of ochratoxins on experimental
animals have been reviewed by Chu (1974a), Harwig (1974), and Krogh
(1976a). Different species vary in their susceptibility to acute
poisoning by ochratoxin A with LD50 values ranging from 3.4 to 30.3
mg/kg (Table 8). When ochratoxin A was administered orally to rats
and guinea-pigs, the female was more sensitive than the male. In
rats, the kidney is the target organ, but necrosis of periportal
cells in the liver has also been noted during studies on acute
effects (Purchase & Theron, 1968).
Table 8. Acute toxicity of ochratoxin A
-------------------------------------------------------------------
Animal LD50 (mg/kg Route of Reference
body weight) administration
-------------------------------------------------------------------
mouse 22 intraperitoneal Sansing et
(female) al. (1976)
rat (male) 30.3 oral Galtier et
al. (1974)
rat 21.4 oral Galtier et
(female) al. (1974)
rat (male) 28 oral Kanizawa et
al. (1977)
rat (male) 12.6 intraperitoneal Galtier et
al. (1974)
rat 14.3 intraperitoneal Galtier et
(female) al. (1974)
guinea-pig 9.1 oral Thacker &
(male) Carlton
(1977)
-------------------------------------------------------------------
Table 8 (contd.)
-------------------------------------------------------------------
Animal LD50 (mg/kg Route of Reference
body weight) administration
-------------------------------------------------------------------
guinea-pig 8.1 oral Thacker &
(female) Carlton
(1977)
white 3.4 oral Prior et
leghorn al. (1976)
turkey 5.9 oral Prior et
al. (1976)
Japanese 16.5 oral Prior et
quail al. (1976)
rainbow 4.7 intraperitoneal Doster et
trout al. (1972)
beagle dog 9 orala Szczech et
(male) (total dose) al. (1973a)
pig 6 oralb Szczech et
(female) (total dose) al. (1973b)
-------------------------------------------------------------------
a All 3 dogs, dosed daily with 3 mg/kg body weight, died within
3 days.
b Both pigs receiving 2 mg/kg daily were moribund and killed
within 3 days, and both pigs receiving 1 mg/kg daily were
moribund and killed within 6 days.
The lesions observed in field cases of mycotoxic nephropathy
have been reproduced by feeding diets containing levels of
ochratoxin A identical to those encountered in the naturally
contaminated products. In a study by Krogh et al. (1974a), 39 pigs
fed rations containing ochratoxin A, at levels ranging from 200 to
4000 µg/kg, developed nephropathy after 4 months at all levels of
exposure. Changes in renal function were characterized by
impairment of tubular function, indicated particularly by a
decrease in TmPAH/Cin,a and reduced ability to produce concentrated
urine. These functional changes corresponded well with the changes
in renal structure observed at all exposure levels, including
atrophy of the proximal tubules and interstitial cortical fibrosis.
Sclerosed glomeruli were also observed in the group receiving the
highest dose of ochratoxin A of 4000 µg/kg feed. Changes were not
seen in any other organs or tissues.
-------------------------------------------------------------------
a TmPAH = transport maximum for para-aminohippuric acid.
Cin = clearance of insulin.
Kidney damage, identical to naturally occurring porcine
nephropathy, was produced in another study by feeding pigs (9
animals) with crystalline ochratoxin A in amounts corresponding to
a feed level of 1 mg/kg for 3 months. Significant renal tubular
functional impairment as measured by a decrease in TmPAH Cin was
detected after only 5 weeks of ochratoxin exposure (Krogh et al.,
1976b). The study was continued for a 2-year period during which
the renal impairment aggravated slightly without reaching a state
of terminal renal failure (Krogh et al., 1979).
In 2 pigs and 9 rats dosed orally with ochratoxin A (400 and
250 µg/kg body weight, respectively) for 5 days, ochratoxin A was
detected in the epithelial cells of the proximal convoluted tubules
of the nephron of all animals. The method of detection was
immunofluorescence microscopy using an antibody against ochratoxin
A that had been formed in rabbits after injection of an albumin-
ochratoxin A conjugate (Elling, 1977).
Groups of 80 F 344/N rats of each sex were administered 0, 21,
70, or 210 µg ochratoxin A/kg in corn oil by gavage, 5 days per
week for 103 weeks (Boorman, 1988). The administration of
ochratoxin A to male and female rats caused a spectrum of
degenerative and proliferative changes in the kidney. The
predominant non-neoplastic lesion in treated rats was degeneration
of the renal tubular epithelium in the inner cortex and the outer
stripe of the outer medulla (nephropathy).
In four pigs given 0.8 mg ochratoxin A/kg body weight orally
for 5 consecutive days, the activity of catalase and CN-insensitive
palmitoyl-CoA-dependent NAD (nicotinamide adenine di-nucleotide) in
renal homogenates decreased, but that in hepatic homogenates did
not, suggesting peroxisomal changes. This was confirmed by
ultrastructural observations of peroxisomes in the proximal
tubules in kidneys of ochratoxin A-treated animals (Elling et
al., 1985).
When 11 SPF pigs and 23 beagle dogs were given high oral doses
of ochratoxin A corresponding to feed levels of more than 5-10
mg/kg (levels rarely found in nature), pathological effects, mainly
necrosis, were observed in the liver, intestine, spleen, lymphoid
tissue, leukocytes, and kidney (Szczech et al., 1973a,b,c). Three
groups of Wistar rats, each consisting of 15 animals, were exposed
to feed levels of ochratoxin A ranging from 0.2 to 5 mg/kg for 3
months. Renal damage in the form of tubular degeneration was
observed at all dose levels (Munro et al., 1974). A decrease in
urinary osmolality, glycosuria, and proteinuria were observed in
an unstated number of Sprague-Dawley and Wistar rats administered
daily doses intraperitoneally of 0.75-2 mg ochratoxin A/kg body
weight (Berndt & Hayes, 1979).
In a study of coagulation factors, rats were given daily oral
doses of 4 mg/kg body weight over 4-10 days, resulting in decreases
in plasma-fibrinogen levels, at levels of factors II, VII, and X,
and in the platelet and megakaryocyte counts (Galtier et al.,
1979).
In 4 calves fed rations containing 0.1-2 mg ochratoxin A/kg
body weight for 30 days, the only signs observed were polyuria,
increased levels of glutamic oxalacetic transaminase (GOT) in
serum, and mild enteritis (Pier et al., 1976); there was mild
tubular degeneration in the kidneys. Cows given ochratoxin A
orally for 4 days at doses ranging from 0.2 to 1.66 mg/kg body
weight remained clinically normal (Ribelin et al., 1978). A cow
given a single dose of 13.3 mg/kg body weight (corresponding to 865
mg/kg feed) developed diarrhoea, anorexia, and cessation of milk
production, one day after.
Avian nephropathy, similar to that in spontaneously occurring
cases, developed in Leghorn hens (27 birds per group) exposed to
dietary levels of 0.3 or 1 mg ochratoxin A/kg for one year (Krogh
et al., 1976c). The renal changes included degeneration of the
tubular epithelium, mainly confined to the proximal and distal
tubules of both reptilian and mammalian nephrons; impairment of
glomerular and tubular function was also observed. "Acute
nephrosis" and "visceral gout" were observed in chickens exposed to
high levels of ochratoxin A (LD50 values) (Peckham et al., 1971).
The same authors reported that ochratoxin B, the other naturally
occurring ochratoxin, was not highly toxic for chickens (LD50, 54
mg/kg) or other animals.
Groups of chicks (40 birds per group) were fed diets containing
ochratoxin A levels in the range of 0-8 mg/kg for 3 weeks (Huff et
al., 1974). The group receiving the highest dose (8 mg/kg feed)
showed decreased packed blood cell volume, haemoglobin
concentration, and serum-iron and serum-transferrin saturation
percentages. In a similar study, Chang et al.(1979) observed that
lymphocytopenia developed at all dose levels. In the same study,
decreased bone strength, as measured physically by resistance to
fracturation, was observed at feed levels of 2-4 mg ochratoxin A/kg
(Huff et al., 1980). When groups of chicks were fed ochratoxin A
at levels of 0, 2, or 4 mg/kg for 20 days, concentrations of serum-
immunoglobulins (IgA, IgG, IgM) were reduced to 57-66% of normal
values in the toxin-exposed groups (Dwivedi & Burns, 1984).
In B6C3F1 female mice, groups of 6-7 animals were administered
a total of 0, 20, 40, or 80 mg ochratoxin A/kg body weight ip on
alternate days over an 8-day period. A dose-related decrease in
thymic mass was observed as well as myelotoxicity, indicated by
bone marrow hypocellularity, due to decreased marrow pluripotent
stem cells, and granulocyte-macrophage progenitors (Boorman et al.,
1984).
When determining the LD50 in mice following intraperitoneal
injection, synergistic effects were observed when ochratoxin A was
combined with citrinin as well as with penicillic acid (Sansing et
al., 1976). An additive effect was observed between ochratoxin A
and citrinin in terms of embryotoxicity in chicken embryos (Vesela
et al., 1983). In beagle dogs, when combined oral doses of
ochratoxin A (0.1-0.2 mg/kg body weight) and citrinin (5-10 mg/kg)
were injected intraperitoneally, synergism was observed with regard
to severity of clinical disease and mortality (Kitchen et al.,
1977a,b). Increased toxicity in terms of LD50 values and
pathological changes was observed in rats, when the ochratoxin A
was given orally combined with either of the drugs biscoumacetate
or phenylbutazone, apparently because of displacement of the toxin
from binding sites on plasma-proteins (Galtier et al., 1980). The
toxic effects of ochratoxin A on the renal epithelial cells of the
monkey were demonstrated in in vitro studies in the form of
abnormal mitotic cells (Steyn et al., 1975).
I.4.2.2 Teratogenicity
Intraperitoneal injection of pregnant mice with ochratoxin A at
5 mg/kg body weight on one of gestation days 7-12 resulted in
increased prenatal mortality, decreased fetal weight, and various
fetal malformations, including exencephaly and anomalies of the
eyes, face, digits, and tail (Hayes et al., 1974). When a
combination of ochratoxin A (2 or 4 mg/kg body weight) and T-2
toxin (0.5 mg/kg body weight) was injected intraperitoneally in
mice on gestation day 8 or 10, ochratoxin A exacerbated the
incidence of T-2-induced gross malformations (tail and limb
anomalies); increased fetocidal effects were also noted (Hood et
al., 1978). Mice were given 3-5 mg ochratoxin A/kg body weight
intraperitoneally or orally on gestation days 8, 9, and 10 or 15,
16, and 17 (Szczech & Hood, 1981). Cerebral necrosis was found in
most fetuses from dams treated on days 15-17, but no cerebral
necrosis developed after treatment on days 8-10, when ochratoxin A
is overtly teratogenic. Pups of mice given 1.25 and 2.25 mg
ochratoxin A/kg orally on gestation days 15, 16, and 17 were tested
for surface righting, swimming, and pivoting (Poppe et al., 1983).
The results of all 3 tests indicated that a delay in development
had occurred; no dose-related pathological alterations were found.
Pregnant mice were administered 1-2 mg ochratoxin A/kg body
weight, and/or 5-20 mg zearalenone/kg body weight or 0.125 mg
diethylstilboestrol/kg body weight, orally, on day 9 of pregnancy,
either individually or in combination, and the offspring were
examined on day 19 (Arora et al., 1983). Teratogenic effects
produced by ochratoxin A, such as exencephaly, open eyelids, and
microphthalmia, were reduced or absent when the toxin was given in
combination with one of the 2 non-steroidal estrogenic substances,
zearalenone or diethylstilboestrol.
Rats were treated orally with ochratoxin A at 0.25, 0.50, 0.75,
1, 2, 4, or 8 mg/kg body weight on gestation days 6-15 (Brown et
al., 1976). Maternal toxicity was not observed below 4 mg/kg body
weight, but an increased incidence of fetal resorptions was
observed from 0.75 mg/kg body weight. All fetuses from dams given
0.25-0.75 mg/kg body weight weighed significantly less than control
fetuses, and fetuses from dams given 0.75 or 1 mg/kg body weight
were stunted. Reduced litters and decreased fetal weight were
observed in rats administered 5 mg ochratoxin A/kg body weight,
orally, on gestation day 8 (Moré et al., 1978).
Subcutaneous administration of ochratoxin A to rats (1.75 mg/kg
body weight) on gestation days 5-7 resulted in the highest number
of malformations, including hydrocephaly, omphalocele, and
anophthalmia as well as a shift in position of the oesophagus
(Mayura et al., 1982); lower doses (0.5 and 1 mg ochratoxin A/kg
body weight) did not have any teratogenic effects, and higher doses
(5 mg/kg) caused all fetuses to be resorbed. In a subsequent study
by the same authors (Mayura et al., 1983), using the same
ochratoxin A exposure conditions, it was shown that a diet low in
protein (10% of protein concentration in normal rat feed) enhanced
the teratogenic action of ochratoxin A in the rat. The combined
action of ochratoxin A exposure and a low protein diet also
resulted in decreases in mating and fertilization rates (22% and
39%, respectively) compared with the control group.
When rats were exposed to ochratoxin A and citrinin (another
nephrotoxic mycotoxin produced by species of the Aspergillus and
Penicillium genera) either singly or combined, enhanced teratogenic
effects were observed in terms of gross malformations, visceral
anomalies, and skeletal defects following combined oral
administration of 1 mg ochratoxin A/kg body weight and 30 mg
citrinin/kg body weight (Mayura et al., 1984). Maternal deaths
(22-40%) occurred after the administration of the combined dose on
days 5, 6, 7, and 14 of gestation, whereas administration of
individual toxins did not cause any maternal deaths and only
minimal malformations no matter which gestation day they were
administered.
Increased prenatal mortality and malformations, including
hydrocephaly, micrognathia, and heart defects, were observed in
hamsters injected intraperitoneally with ochratoxin A at doses of
5-20 mg/kg body weight on one of gestation days 7-9 (Hood et al.,
1976).
I.4.2.3 Mutagenicity
Ochratoxin A did not have any effects in a Bacillus subtilis
Rec- assay, measuring DNA damage when tested at 20 and 100 µg/plate
(Ueno & Kubota, 1976). Ochratoxin A was not mutagenic to
Salmonella typhimurium TA 1535, TA 1537, TA 1538, TA 98, or TA 100
at doses of up to 500 µg/plate, with or without exogenous metabolic
activation (Kuczuk et al., 1978; Wehner et al., 1978b). No
increase was observed in genetic changes at the ade 2 locus of
Saccharomyces cerevisiae after treatment with 50 or 100 µg/plate
ochratoxin A, with or without exogenous metabolic activation
(Kuczuk et al., 1978). Ochratoxin A did not induce mutations to
8-azaguanine resistance in C3H mouse mammary carcinoma cells (FM3A)
treated with doses of 5 or 10 µg/litre (Umeda et al., 1977).
When rats were orally exposed to ochratoxin A every 48 h for 12
weeks (corresponding to a feed level of 4 mg/kg), single-strand
breaks of DNA in renal and hepatic tissue (the only tissues
investigated) were more pronounced than those in control animals
(Kane et al., 1986a,b).
Contradictory results have been obtained by testing ochratoxin
A in the Salmonella assay (SOS chromo test without metabolic
activation) (Ueno et al., in press).
I.4.2.4 Carcinogenicity
Kanizawa & Suzuki (1978) have indicated that ochratoxin A is a
hepatic and renal carcinogen in male mice. A group of 10 male ddY
mice were fed a diet containing 40 mg ochratoxin A/kg for 44 weeks.
A group of 10 untreated controls were fed the basal diet. All
survivors were killed after 49 weeks. Hepatic cell tumours were
found in 5 out of 9 treated mice; no tumours were found in the 10
controls. Solid renal cell tumours were found in 2 out of 9
treated mice and none in the 10 controls. Cystic renal adenomas
were found in 9 out of 10 treated mice compared with none in the 10
controls.
Two groups of 50 male and 2 groups of 50 female B6C3F1 mice
were fed diets containing 1 or 40 mg ochratoxin A/kg, respectively;
one group (control) was fed the basal diet. All survivors were
killed after 24 months. Eleven out of 49 male mice in the 40 mg/kg
group had renal carcinomas; 24 out of 49 male mice in the 40 mg/kg
groups showed renal adenomas. All male mice in the 40 mg/kg group
had microscopic evidence of nephropathy. A few females in the 40
mg/kg group exhibited nephropathic changes but no carcinomas or
adenomas. Compound-related lesions were absent in the controls
and the 1 mg/kg groups (Bendele et al., 1985). In a test for the
development of hyperplastic liver nodules in rats, ochratoxin A was
characterized as having both an initiating and a promoting activity
(Imaida et al., 1982).
Seven groups of 16 male ddY mice each were fed a diet
containing 50 mg ochratoxin A/kg for various periods ranging from 0
to 30 weeks followed by feeding of the basal diet until the end of
70 weeks (Kanizawa, 1984). After 15 weeks of ochratoxin A
exposure, 3 out of 15 animals had renal cell tumours; after 20
weeks, 1 out of 14 mice had renal cell tumours and 2 had hepatomas;
after 25 weeks of exposure, 2 out of 15 mice had renal cell tumours
and 5 had hepatomas; and after 30 weeks of exposure, 4 out of 17
mice had renal cell tumours and 6 had hepatomas; these tumours were
not observed in the controls. The nature of the renal cell tumours
was not further defined, but such tumours are mostly malignant. In
addition, pulmonary tumours were found in all groups, including the
controls, the incidence ranging from 20 to 73%. In a second study,
the effects of a combination of ochratoxin A and citrinin were
elucidated. Groups of 20 male ddY mice were fed diets containing
25 mg ochratoxin A/kg in combination with 100 or 200 mg
citrinin/kg for 70 weeks. Control groups were fed diets that did
not contain any toxins or the individual toxins at the
concentrations indicated above. In addition, one group was fed 25
mg ochratoxin A/kg feed for the first 25 weeks followed by 200 mg
citrinin/kg feed for the remaining period of time; another group
was exposed to the 2 toxins in the reverse order. Exposure to
citrinin alone did not produce any tumours. Exposure to ochratoxin
A alone resulted in renal cell tumours in 6 out of 20 mice, and in
hepatomas in 8 mice. Exposure to ochratoxin A and 100 mg
citrinin/kg feed did not result in any renal cell tumours, but 10
out of 19 mice had hepatomas. Exposure to ochratoxin A and 200 mg
citrinin/kg feed resulted in renal cell tumours in 10 out of 18
mice, and in hepatomas in 7 mice. Exposure to one toxin followed
by the other toxin did not produce any renal cell tumours, but
hepatomas were observed in less than 20% of the mice.
On the basis of these studies, IARC concluded that there was
limited evidence of carcinogenicity for animals, and inadequate
evidence of carcinogenicity for human beings (IARC, 1987a).
Groups of 80 F 344/N rats of each sex were administered 0, 21,
70 or 210 µg ochratoxin A/kg in corn oil by gavage, 5 days per
week, for 103 weeks (Boorman, 1988). In the male rats, renal
carcinomas were found in 16 out of 51 animals dosed with 70 µg/kg
and in 30 out of 50 animals dosed with 210 µg/kg; no carcinomas
were found in lower dose groups. In the female rats, renal
carcinomas were less common, as 1 out of 50 animals dosed with 70
µg/kg and 3 out of 50 animals dosed with 210 µg/kg had carcinomas;
no carcinomas were found in the lower dose groups. Renal adenomas
were found in all groups of male rats, with increasing frequencies
associated with increased doses. In the female groups, renal
adenomas were only found in the two highest dose groups. In the
female rats, fibroadenomas in the mammary gland were found in 45-
56% in the treated groups, a significantly higher percentage than
in the control group.
I.4.2.5 Biochemical effects and mode of action
Ochratoxin A is an inhibitor of tRNA synthetase and protein
synthesis in several microorganisms ( Bacillus subtilis, B.
stearothermophilus, Streptococcus faecalis, yeasts) as well as in
rat hepatoma cells (Konrad & Roschenthaler, 1977; Bunge et al.,
1978; Heller & Roschenthaler, 1978; Creppy et al., 1979a,b). The
competitive inhibitor effect of ochratoxin A on tRNA synthetase and
protein synthesis in rat hepatoma cells can be prevented by the
addition of phenylalanine in the cell culture medium at a molar
ratio of phenylalanine: ochratoxin A of 1.7:1 (Creppy et al.,
1979b). This observation suggested the possibility of preventive
measures for ochratoxin A-induced disease. Thus, the acute
intraperitoneal effect of ochratoxin A (LD100) in mice was
prevented by concomitant injection of phenylalanine (Creppy et al.,
1980; Moroi et al., 1985).
However, in the study on the reproduction of porcine
nephropathy previously mentioned (Krogh et al., 1974a), the molar
ratio of phenylalanine: ochratoxin A in the feed exceeded 4600:1,
implying that, in the field situation, phenylalanine does not
prevent ochratoxin A from inducing the development of nephropathy.
Ochratoxin A in the concentration range studied (20-1667 µmol/
litre) caused a 47-50% inhibition of macrophage migration (Klinkert
et al., 1981); this effect could be prevented by the simultaneous
addition of phenylalanine. In BALB/c mice, a dose of ochratoxin A
as low as 0.005 µg/kg body weight was able to suppress the immune
response to sheep erythrocytes (Haubeck et al., 1981); the effect
could be prevented by the simultaneous addition of phenylalanine.
Studying the same effect in Swiss mice, Prior & Sisodia (1982) were
unable to show any suppression of the immune response to sheep
erythrocytes, even after daily injection of 5 mg ochratoxin A/kg
for 50 days. (4R)-4-Hydroxyochratoxin A at a dose of 1 µg/kg body
weight caused an 80% reduction in the number of cells producing IgM
and a 93% reduction in cells producing IgG in BALB/c mice compared
with 90% and 92%, respectively, for ochratoxin A (Creppy et al.,
1983).
Female B6C3F1 mice (6 per group) were administered ochratoxin A
in amounts of 0.34, 6.7, or 13.4 mg/kg body weight or ochratoxin B
(13.4 mg/kg body weight) 6 times during 12 days. Ochratoxin A
inhibited the natural killer cell activity at all dose levels, and
increased the growth of transplantable tumour cells without
affecting T-cell or macrophage-mediated anti-tumour activity
(Luster et al., 1987). Ochratoxin B did not influence immune
function. The inhibition by ochratoxin A of natural killer cell
activity appeared to be caused by reduced production of basal
interferon.
Ochratoxin A affects the carbohydrate metabolism in rats.
Thus, a single oral dose of ochratoxin A at 15 mg/kg body weight
caused a decrease in the glycogen level in the liver and an
increase in the heart glycogen level, 4 h later (Suzuki & Satoh,
1973).
In a more extensive study on rats, the decrease in liver
glycogen level, 4 h after a single oral dose of ochratoxin A at 15
mg/kg body weight, was associated with an increase in serum glucose
levels and a decrease in liver glucose-6-phosphate (Suzuki et al.,
1975). At the same time, the liver glycogen synthetase (EC
2.7.1.37) activity decreased and the liver phosphorylase (EC
2.4.1.1) activity increased. Three daily oral doses of ochratoxin
A at 5 mg/kg body weight caused a decrease in the liver glycogen
concentration, which was measured on the fourth day. The decrease
was attributed to inhibition of the active transport of glucose
into the liver, suppression of glycogen synthesis from glucose, and
acceleration of glycogen decomposition.
During in vitro studies on rat liver mitochondria, it was
observed that ochratoxin A inhibited the respiration of whole
mitochondria by acting as a competitive inhibitor of transport
carrier proteins located in the inner mitochondrial membrane
(Meisner & Chan, 1974). Further studies with mitochondrial
preparations revealed that the mitochondrial uptake of ochratoxin A
was an energy-using process that resulted in depletion of
intramitochondrial adenosine triphosphate (ATP), and that
ochratoxin A inhibited intramitochondrial phosphate transport
resulting in deterioration of the mitochondria (Meisner, 1976).
This might explain the degeneration of liver mitochondria observed
by Purchase & Theron (1968) in rats exposed orally to a single dose
of ochratoxin A at 10 mg/kg body weight. These authors observed
accumulation of glycogen in the cytoplasm of the rat liver cells
microscopically. This was in contrast to the previously discussed
observations of Suzuki et al. (1975), who found a decrease in
glycogen levels.
In a study on mice, Sansing et al. (1976) found that ochratoxin
A, administered intraperitoneally at 6 mg/kg body weight, inhibited
orotic acid incorporation into both liver and kidney RNA, 6 h after
toxin injection. In this respect, ochratoxin A acted synergistically
with another nephrotoxic mycotoxin, citrinin.
When neonatal rats were exposed orally to a single dose of 1 mg
ochratoxin A/kg or of 25 mg citrinin/kg or both doses within 24 h
of birth, a synergistic effect of the 2 mycotoxins was observed on
cytochrome P-450, NADPH-dependent dehydrogenase, and NADPH-
cytochrome c reductase (Siraj et al., 1981).
In rats fed 2 mg ochratoxin A/kg body weight per day for 2
days, renal gluconeogenesis from pyruvate was decreased by 26%, and
renal phosphoenol-pyruvate carboxy kinase (PEPCK) (EC 4.1.1.32)
activity was reduced by 55%, whereas hepatic PEPCK was unchanged
(Meisner & Selanik, 1979). In a subsequent study on rats, it was
found that even lower dose levels (0.3-0.5 mg ochratoxin A/kg body
weight) caused a 50% reduction in PEPCK activity (Meisner &
Meisner, 1981). A number of other enzymes located in the proximal
tubule of the nephron were unaffected. When longer exposure
periods (8-12 weeks) were used, and 145 ng ochratoxin A/kg body
weight were administered orally to groups of 3 rats each, increased
urinary excretion and corresponding renal tubular depletion of the
following enzymes were observed: gamma-glutamyl transferase,
alkaline phosphatase, leucine aminopeptidase, lactate
dehydrogenase, and N-acetyl-beta- D-glucosaminidase (Kane et al.,
1986a). Renal PEPCK in pigs was also sensitive to ochratoxin A
with a feed level of 100 µg/kg causing a significant decrease in
PEPCK activity (Meisner & Krogh, 1982).
In a study on pigs fed ochratoxin A at 0, 0.2, or 1 mg/kg for 5
weeks, enzyme activities were measured in renal biopsies, collected
1, 3, and 5 weeks after initiation (Krogh et al., 1988). After one
week, the activities of renal PEPCK and gamma-glutamyltranspeptidase
were decreased by 40%. The dose-related decrease in the activity
of PEPCK and gamma-glutamyltranspeptidase was accompanied by a
dose-related increase in renal impairment, as measured by the
reduction of TmPAH/Cin, suggesting that these enzymes are sensitive
indicators of ochratoxin-induced porcine nephropathy. Thus, the
renal biopsy-based measurements of enzyme activities might prove
diagnostically useful in ochratoxin-induced disease in human
beings.
Ochratoxin A reduced the total renal mRNA concentration in male
Sprague-Dawley rats and certain mRNA species, notably PEPCK, were
reduced to a greater extent than the bulk of the RNA pool (Meisner
et al., 1983).
I.5 EFFECTS ON MAN
I.5.1 Ochratoxin A, Balkan endemic nephropathy, and tumours of the
urinary system
This topic has been reviewed by Krogh (1979, 1983) who called
attention to the striking similarities in the changes in renal
structure and function induced experimentally in animals by the
administration of ochratoxin A, and the clinical and pathological
features of a localized endemic disease known as Balkan endemic
nephropathy. So far, the disease has been observed only in rural
populations of Bulgaria, Romania, and Yugoslavia, but information
on the present magnitude of the problem was not available to the
Task Group.
The Balkan endemic nephropathy is a chronic disease that
predominantly affects women and progresses slowly up to death
(Hrabar et al., 1976, Chernozemsky et al., 1977). Age-specific
incidence rates are highest above the age of 40. Younger cases
occur in the 10-19-year-old age group, and the mean age of new
patients is in the early 50s (Stoyanov et al., 1978).
The disease is characterized by an extreme geographical
clustering with a tendency for familial aggregation of cases
(Nicolov et al., 1978). However, sporadic cases occur outside
endemic areas. Age-adjusted incidence rates of 555 per 100 000
population in females and 322 in males over a ten-year period have
been recorded from a population sample of 147 000 in an endemic
area of Bulgaria (Stoyanov et al., 1978). In one of several
endemic regions in Yugoslavia, the prevalence varied from 3% to 8%
(Hrabar et al., 1976).
Autopsy has shown that kidneys are notably reduced in size.
The histological lesions are interstitial fibrosis, tubular
degeneration, and hyalization of glomeruli in the more superficial
part of the cortex (Heptinstall, 1974). Impairment of tubular
function, indicated by a decrease in TmPAH, is a prominent and
early sign (Dotchev, 1973).
A high incidence of tumours of the urinary system is strongly
correlated with the prevalence of Balkan endemic nephropathy
(Ceovic et al., 1976; Chernozemsky et al., 1977; Nicolov et al.,
1978). In one instance in Bulgaria, 46.6% of patients with tumours
of the urinary system were also affected by endemic nephropathy.
Among the tumours of the urinary system, cancers of the renal
pelvis and ureters are more frequently associated with endemic
nephropathy than urinary bladder tumours.
The relative risk for developing cancer of the renal pelvis and
ureters is 88:1 in patients with nephropathy compared with controls
in non-endemic areas. The relative risk for the development of any
tumour of the urinary system is only 28:1 in the same sample
(Stoyanov et al., 1978).
Over the past 2 decades, investigations have been carried out
to verify a variety of etiological assumptions with unconvincing
results (review by Puchlev, 1973, 1974). However, the assumption
of the possible role of ochratoxin A, on the basis of similarities
with the animal disease, has received increasing epidemiological
support.
In Yugoslavia, surveys indicated that the contamination of
foodstuffs (grains, maize, pork meat) with ochratoxin A occurred in
12.8% of samples in an area where the prevalence of endemic
nephropathy was 7.3%, compared with only 1.6% of contaminated
samples in areas free of the disease (Krogh et al., 1977). The
concentration of ochratoxin A in maize was 5-90 µg/kg and that in
pork meat, 5 µg/kg (Krogh et al., 1977) with levels of up to 27
µg/kg in pig kidneys (Pepeljnjak et al., 1982). Similarly, studies
in Bulgaria have revealed that 16.7% of beans and 27.3% of maize
from an endemic area were contaminated with ochratoxin A compared
with 7.1% and 9%, respectively, from a non-endemic area (Petkova-
Bocharova & Castegnaro, 1985).
In a subsequent survey of home-produced foodstuffs (cereal and
bread) from the same endemic area in Yugoslavia over a 5-year
period, a mean contamination of 8.7% was found with pronounced
annual variations, which probably reflect climatic conditions
during the crop harvesting periods (Pavlovic et al., 1979).
Surveys on the presence of ochratoxin A in blood samples are
difficult to compare, as different analytical methods have been
used with different levels of sensitivity. Prevalences of 16.6%
and 5.9% with a one-year interval were reported in the same endemic
village in Yugoslavia (Hult et al., 1982). It is not possible to
determine whether this difference reflects annual variations in the
content of the blood, or the result of a less sensitive analytical
method in the second instance. However, higher prevalences of
ochratoxin A and higher concentrations in blood are generally
present in people from endemic areas, especially in persons
suffering from Balkan nephropathy.
A survey in Yugoslavia reported a prevalence in the blood of
16.6% in an endemic village and 6% in a non-affected one (Hult et
al., 1982). In Bulgaria, reported rates were 17.7% in an endemic
area and 7.7% in a non-endemic one (Petkova-Bocharova et al.,
1988). Table 9 illustrates the trend towards higher concentrations
in endemic situations and in patients. The similarity of results
in healthy families from affected villages and healthy persons from
unaffected villages (groups III and IV) suggests than an
environmental or a behavioural determinant plays a role at the
household level.
Thus, available epidemiological information seems to indicate
that Balkan endemic nephropathy is associated with consumption
patterns involving foodstuffs contaminated with ochratoxin A and
with a higher frequency of positive blood samples of ochratoxin A.
However, the association does not permit the establishment of a
causal relationship. Cross sectional surveys, such as those
reported in the literature so far, are probably not the appropriate
means to determine this relationship.
The results of experimental animal studies suggest that Balkan
nephropathy, a chronic condition, may require a long latency period
between exposure and the onset of symptoms, or more likely a
prolonged exposure or repeated exposure over a long period of time.
Investigations based on individual exposure time sequences
together with follow-up cohort surveys could provide a clue to the
causal role of ochratoxin A in Balkan endemic nephropathy. In view
of the lack of this information, such a causal relationship cannot
be established or rejected.
Table 9. Ochratoxin A in blood samples from people in endemic and
non-endemic areas in Bulgariaa
-------------------------------------------------------------------
Groupb No. of No. of Mean concentration
persons ochratoxin A ± S.D. (µg/kg)
assayed positive cases
(%)
-------------------------------------------------------------------
I. Persons with 61 16 (26.3) 20.3 ± 9.7
UST and/or EN
II. Healthy 63 10 (15.8) 14.5 ± 7.6
persons from
families with UST
and/or EN cases
III. Healthy 63 7 (11.1) 12.5 ± 3.5
persons from
families in
endemic villages
IV. Healthy 60 7 (11.6) 15.0 ± 4.2
persons from
unaffected villages
in endemic areas
V. Healthy 65 5 (7.7) 10.0
persons from
villages in
non-endemic areas
-------------------------------------------------------------------
a From: Petkova-Bocharova et al. (1988).
b UST: urinary system tumours; EN: endemic nephropathy.
The differences between group I and groups III and IV are
statistically significant ( P <0.002). Difference between
groups I and V is statistically significant ( P <0.001).
I.6 EVALUATION OF THE HUMAN HEALTH RISKS
Human exposure, as demonstrated by the occurrence of ochratoxin
A in food and in the blood, has been observed in various countries
in Europe. The Task Group was not aware of attempts to detect
ochratoxin A in human blood in other parts of the world.
The causal role of ochratoxin A in porcine nephropathy has been
established, based on studies of field cases as well as
reproduction of the disease with ochratoxin A. Using the porcine
model, it has been postulated that Balkan endemic nephropathy may
result from exposure to ochratoxin A. Available epidemiological
information indicates that Balkan nephropathy may be associated
with the consumption of foodstuffs contaminated by this toxin.
Since the publication of Environmental Health Criteria 11, in 1979,
epidemiological studies on the concentration of ochratoxin A in
human blood in affected and non-affected areas, have provided
additional support for the relationship between Balkan nephropathy
and exposure to ochratoxin A.
It has been shown that both the prevalence of ochratoxin A in
the blood and the blood concentrations are higher in residents in
endemic areas. However, a direct causal relationship cannot be
established on the basis of indirect evidence provided by the above
retrospective studies alone. Neither can it be excluded in view of
the long latency period between the exposure and the onset of
symptoms.
Ochratoxin A has been demonstrated to be carcinogenic to the
renal tubular epithelium in male mice and both sexes of rats. A
highly significant relationship has been observed between Balkan
nephropathy and tumours of the urinary tract, particularly with
tumours of the renal pelvis and ureters. However, there are no
published data to establish a direct causal role of ochratoxin A in
the etiology of such tumours.
II. TRICHOTHECENES
II.1 PROPERTIES AND ANALYTICAL METHODS
II.1.1 Physical and chemical properties
The sesquiterpenoid trichothecenes possess the tetracyclic
12,13-epoxytrichothecene skeleton. A total of 148 trichothecenes,
83 non-macrocyclic and 65 macrocyclic, have been isolated from
fungal cultures and plants (Drove, 1988). They can be conveniently
divided into 4 categories according to similarity of functional
groups (Ueno, 1977). The first class is characterized by a
functional group other than a ketone at C-8 (type A). This is the
largest category containing members such as T-2 toxin and
diacetoxyscirpenol (DAS). The second category of trichothecenes
has a carbonyl function at C-8 (type B) typified by 4-deoxynivalenol
(DON) and nivalenol (NIV). The third category is characterized by
a second epoxide group at C-7,8 or C-9,10 (type C), and the fourth
contains a macrocyclic ring system between C-4 and C-15 with two
ester linkages (type D). The structures of representative
trichothecenes of each category are illustrated below.
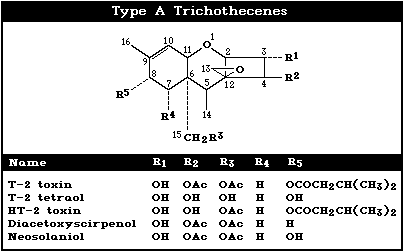
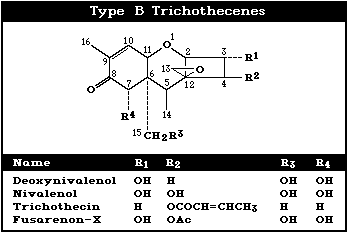
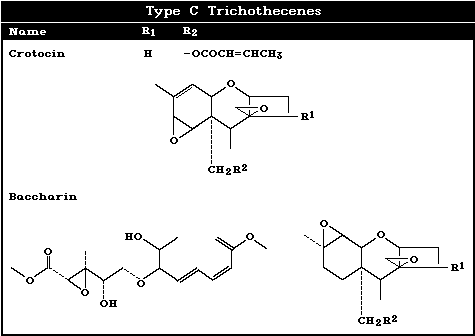
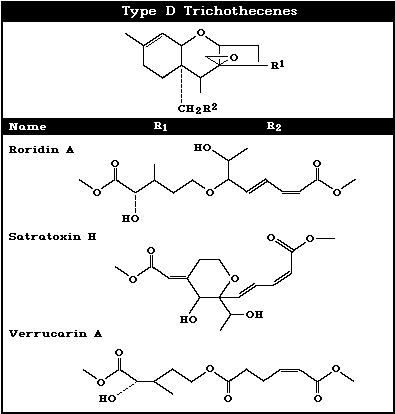 II.1.1.1 Physical properties
The trichothecenes are colourless, mostly crystalline solids
that have been well characterized by physical and spectroscopic
techniques (Cole & Cox, 1981). The type A trichothecenes are
soluble in moderately polar solvents, such as chloroform, diethyl
ether, ethyl acetate, and acetone, whereas the more polar type B
trichothecenes require higher polarity solvents, such as aqueous
methanol or aqueous acetonitrile. Some physical properties of the
main trichothecenes are summarized in Table 10.
Table 10. Some physical properties of main trichothecenes
-------------------------------------------------------------------------------
Trichothecenes Molecular Relative Melting (alpha)20D References
formula molecular point
mass (°C)
-------------------------------------------------------------------------------
T-2 toxin C24H34O9 466 151-152 +15 Bamburg et
al. (1968b)
HT-2 toxin C22H26O8 424 - - Bamburg &
Strong (1969)
Diacetoxyscirpenol C19H26O7 366 162-164 -27 Sigg et al.
(1965)
Neosolaniol C19H26O8 382 171-172 - Ishii et al.
(1971)
Deoxynivalenol C15H20O6 296 151-153 +6.35 Yoshizawa &
Morooka (1973)
Nivalenol C15H20O7 312 222-223 +21.54 Tatsuno et
al. (1968)
Trichothecin C19H24O5 332 118 +44 Freeman (1955)
Fusarenon-X C17H22O8 354 91-92 +58 Ueno et al.
(1969b)
Roridin A C29H40O9 532 198-204 +130 Harri et al.
(1962)
Satratoxin H C29H36O9 528 162-166 - Eppley &
Bailey (1973)
Verrucarin A C27H34O9 502 360 +20.6 Gutzwiller &
Tamm (1965)
-------------------------------------------------------------------------------
Most of the trichothecenes lack conjugated unsaturation in their
structures with a consequent absence of absorption in the ultraviolet
(UV) spectrum, except for end absorption due to unsaturation at C-9.
This lack of absorbance is a source of difficulty in achieving
sensitive and specific detection in HPLC analysis. In contrast, the
type D trichothecenes give characteristic ultraviolet spectra.
II.1.1.2 Chemical properties
When trichothecenes containing an ester group are treated with a
base, they are hydrolysed to their corresponding parent alcohol (Wei
et al., 1971). Free hydroxyl groups are readily acylated. The
12,13-epoxy group is itself extremely stable to nucleophilic attack.
However, prolonged boiling under highly acidic conditions causes an
intramolecular rearrangement of the trichothecene skeleton to the
apotrichothecene ring. Detailed discussion of the chemistry of
trichothecenes can be found in reviews by Bamburg & Strong (1971),
Bamburg (1976), and Tamm (1977).
The trichothecenes are generally stable; for example, DON can be
stored in organic solvents, such as ethyl acetate, for a long time
without any significant deterioration (Shepherd & Gilbert, 1988).
They remain unaffected when refluxed with various organic solvents
and also under mildly acidic conditions.
II.1.2 Analytical methods for trichothecenes
Analytical methods have been reviewed by Scott (1982) and Pohland
et al. (1986). Selected examples of recently published analytical
methods for type A trichothecenes and type B trichothecenes are
summarized in Tables 11 and 12 respectively. Although some of the
multi-trichothecene methods included in Table 11 may also be
applicable to certain of the type B compounds, the procedures in
Table 12 have been developed exclusively for the type B toxins.
II.1.2.1 Chemical methods
(a) Extraction
The most commonly used extraction solvents for trichothecenes are
chloroform, ethyl acetate, methanol, acetonitrile, aqueous methanol,
and aqueous acetonitrile. Chloroform, ethyl acetate, and
acetonitrile have been successfully used for the extraction of T-2
toxin, DAS, and some of their partially hydrolysed derivatives in
naturally contaminated cereals. Aqueous methanol and aqueous
acetonitrile are the solvents of choice for the extraction of several
trichothecenes of widely differing polarity as well as for the
extraction of type B toxins alone. Methods differ according to the
type of solvent used, whether samples are homogenised in a blender
with the solvent or agitated with a wrist action shaker, and in the
length of time of the extraction process. Spiking of samples with
standards is not an adequate way of demonstrating the efficiency of
extraction, and only methods validated with naturally contaminated
material can be regarded as having been rigorously tested.
Extraction procedures have been assessed for DON (Trenholm et al.,
1985) and it has been demonstrated that longer extraction times are
required for naturally contaminated samples than for those that have
been spiked (at least 120 min shaking). It has also been shown that
aqueous acetonitrile gives a cleaner extract than aqueous methanol.
(b) Clean-up procedures
The extent of sample clean-up required for a particular assay
depends on the specificity of the detection procedure and the nature
of the sample matrix. The less specific detection methods, such as
TLC, require extensive sample clean-up while more sophisticated
approaches, such as mass spectrometry and immunoassay, may only
require minimal sample preparation.
Table 11. Selected methods for the determination of T-2 toxin and diacetoxyscirpenol in
biological materials
------------------------------------------------------------------------------------------------
Matrix Extractiona Clean-upb Assayc Detection Toxins assayed References
limit -----------------
(µg/g) T-2 DAS Others
------------------------------------------------------------------------------------------------
Cereals MeOH/H2O XAD-4; flor. TLC 0.5 + + 7 Kamimura et
al. (1981)
Cereals EtOAC prep. TLC HPTLC 0.2 + - 2 Ilus et
al. (1981)
Foods MeCN/H2O char/alum TLC 1.0 + + 5 Romer
(1986)
Cereals MeCN/KCl Sep P HPLC(RI) 1.0 + - 1 Schmidt &
Dose (1984)
Wheat/rye MeCN Bond-Elut LC/MS (therm) 0.04 + + 2 Rajakyla et
al. (1987)
Plasma EtOAC Sep P/sil.g LC/MS (therm) 0.002 + + 2 Voyksner et
al. (1985)
Cereals EtOAC sil.g GC (FID)-TMS 0.1 + + 3 Bata et al.
(1983)
Cereals MeOH/H2O sil.g/cy GC (ECD)-HFB 0.05 + + 1 Cohen &
Lapointe
(1984)
Plasma benzene flor. GC (ECD)-HFB 0.02 + - - Swanson et
al. (1983)
Milk EtOAC prep. TLC GC/MS-TMS 0.003 + - - Collins &
Rosen
(1979)
Corn MeOH Sep P/sil.g GC/MS-TMS 0.02 + + 1 Rosen &
Rosen
(1984)
Foods MeOH flor. GC/MS-HFB 0.01 + + 11 Black et
al. (1987)
Urine EtOAC/MeOH Sep P GC/MS-HFB 0.005 + + 5 Black et
al. (1986)
Blood acetone Sep P GC/MS-PFP 0.005 + + 9 Begley et
al. (1986)
------------------------------------------------------------------------------------------------
Table 11 (contd.)
------------------------------------------------------------------------------------------------
Matrix Extractiona Clean-upb Assayc Detection Toxins assayed References
limit -----------------
(µg/g) T-2 DAS Others
------------------------------------------------------------------------------------------------
Milk/urine EtOAC Sep P RIA 0.002 + - - Lee & Chu
(1981a)
Corn/wheat MeOH Sep P RIA 0.001 + - - Lee & Chu
(1981b)
Urine none Sep P ELISA 0.0005 + - - Fan et al.
(1987)
------------------------------------------------------------------------------------------------
a Extraction: MeOH (methanol); H2O (water); EtOAC (ethyl acetate); MeCN (acetonitrile);
KCl (potassium chloride aqueous soln).
b Clean-up: XAD-4 (amberlite XAD-4 resin); flor. (florisil); char/alum (charcoal/alumina
columns); Sep P (C18-Sep Pak); sil.g (silica gel); cy (cyano extraction column).
c Assay: TLC (thin layer chromatography); HPTLC (high performance TLC); HPLC (high performance
liquid chromatography); RI (refractive index); LC/MS (combined HPLC/mass spectrometry);
GC (gas chromatography); FID (flame ionisation detector); ECD (electron capture detector);
TMS (trimethylsilyl derivative); HFB (hepafluorobutyrl ester derivative);
PFP (pentafluoropropionyl ester); RIA (radioimmunoassay); ELISA (enzyme linked
immunosorbent assay).
Table 12. Selected methods for the determination of deoxynivalenol and nivelenol in biological
materials
------------------------------------------------------------------------------------------------
Matrix Extractiona Clean-upb Assayc Detection Toxins assayed References
limit -----------------
(µg/g) T-2 DAS Others
------------------------------------------------------------------------------------------------
Wheat/corn MeCN/H2O char/alum TLC 0.04-0.1 + - - Trucksess
et al.
(1984)
Foods MeCn/H2O char/alum; TLC 0.5 + - - Trucksess
Sep P et al.
(1986a)
Cereals MeCN/H2O/ char/alum; HPTLC 0.05 + + 1 Trucksess
MeOH ppt et al.
(1987)
Corn/rice MeOH/H2O/ liq/liq extr HPLC (UV) O.005 + + 2 Visconti &
NaCl Bottalico
(1983a)
Corn/rice MeOH/H2O none HPLC (ED) 0.025 + - - Sylvia et
al. (1986)
Corn MeOH/H2O prep. TLC HPLC (UV) 0.01 + - - Ehrlich et
al. (1983)
Cereals MeCN/H2O ion/char/ HPLC (UV) 0.05 + + - Lauren &
alum Greenhalgh
(1987)
Cereals MeOH flor. LC/MS (micro) 0.01 + + - Tiebach et
al. (1985)
Cereals MeOH/H2O sil.g GC (ECD)-TMS 0.02 + + - Scott et
al. (1986)
Cereals MeCN/H2O flor/Sep P GC (ECD)-TMS 0.002 + + - Tanaka et
al. (1986)
Wheat chlor/EtOH sil.g GC (ECD)-HFB 0.1 + - - Ware et
al. (1986)
Cereals chlor/EtOH sil.g GC (ECD)-HFB 0.02 + - - Mulders &
Impelen-
Peek (1986)
Milk EtOAc Sep P GC (ECD)-TMS 0.001 + - - Swanson et
al. (1986)
------------------------------------------------------------------------------------------------
Table 12 (contd.)
------------------------------------------------------------------------------------------------
Matrix Extractiona Clean-upb Assayc Detection Toxins assayed References
limit -----------------
(µg/g) T-2 DAS Others
------------------------------------------------------------------------------------------------
Corn/barley MeOH/H2O sil.g GC/MS-TMS 0.01 + - - Gilbert et
al. (1983a)
Foods MeOH/H2O XAD-2/flor/ GC/MS-TMS 0.02 + + - Yoshizawa &
Sep P Hosokawa
(1983)
Corn/wheat MeCN/H2O none ELISA 0.01 + - - Xu et al.
(1988)
------------------------------------------------------------------------------------------------
a Extraction: MeCN (acetonitrile); H2O (water); MeOH (methanol); NaCl (sodium chloride soln);
chlor/EtOH (chloroform/ethanol); EtOAc (ethyl acetate).
b Clean-up: char/alum (charcoal/alumina column); Sep P (C18-Sep Pak); ppt (lead acetate
precipitation); liq/liq extr. (liquid/liquid extraction); ion (ion exchange resin);
flor. (florisil); sil.g (silica gel); XAD-2 (Amberlite XAD-2 resin).
c Assay: TLC (thin layer chromatography); HPTLC (high performance TLC); HPLC (high performance
liquid chromatography); UV (ultraviolet detection); ED (electrochemical detection);
LC/MS (combined microbore-HPLC/mass spectrometry); GC (gas chromatography); ECD (electron
capture detector); TMS (trimethylsilyl derivative); HFB (heptafluorobutyryl ester
derivative); ELISA (enzyme linked immunosorbent assay).
Early methods that may have involved the use of conventional
silica gel columns or preparative TLC for clean-up, have to a large
extent been superseded by methods using prepacked cartridges, such as
silica gel and C18-Sep Paks, which are both more reliable and more
convenient. Florisil columns are widely used for clean-up (e.g., see
Tanaka et al., 1985a), and the one step clean-up procedures using
alumina/charcoal (Romer, 1986) or alumina/charcoal/Celite columns
(Trucksess et al., 1984) have become widely adopted, particularly for
DON and NIV assays.
(c) Detection and quantification
(i) Thin-layer chromatography (TLC). The lack of native
fluorescence or UV absorbance of the trichothecenes means that TLC
detection relies on the use of spray reagents for visualisation.
Characteristic colours or fluorescence can be produced with sulfuric
acid or p-anisaldehyde followed by heating at 110-120 °C (Scott et
al., 1970; Ueno et al., 1973c). A general spray reagent for the
12,13-epoxy function is 4-( p-nitrobenzyl)pyridine, which produces a
blue coloration on heating and treatment with a base (Takitani et
al., 1979). A more sensitive, but somewhat elaborate, procedure,
which again is specific for the epoxide function, involves reaction
with nicotinamide and 2-acetylpyridine to produce fluorescent TLC
spots (Sano et al., 1982). Diphenylindenone sulfonyl esters of
trichothecenes can be formed prior to TLC and, subsequently, when
sprayed with sodium methoxide can yield fluorescent spots at high
sensitivity (Yagen et al., 1986).
Most of the above spray reagents, though frequently demonstrated
as useful for the determination of standards or of relatively high
concentrations of toxins in culture extracts, have not been well
developed in conjunction with clean-up procedures for the
determination of trichothecenes in naturally contaminated cereal
samples or other foods. However, the exception has been the use of
an aluminum chloride spray reagent for visualising the type B
trichothecenes (Kamimura et al., 1981). On spraying the plates,
heating for 10 min at 110 °C and then treating with a base, blue
fluorescent spots are produced for DON, NIV, and fusarenon X. Using
this approach, in conjunction with a clean-up utilising an alumina
charcoal Celite column, a quantitative TLC procedure was developed
using a fluorodensitometer for determining DON in wheat and corn
(Trucksess et al. 1984); DON in processed grain products including
breakfast cereals, corn syrup, and beer (Trucksess et al., 1986b);
and for simultaneously monitoring DON, NIV, and fusarenon X in
barley, corn, and wheat (Trucksess et al. 1987). This TLC procedure
was successfully collaboratively tested (Eppley et al., 1986) and was
accepted as the AOAC first action method for DON in wheat.
(ii) High performance liquid chromatography (HPLC). HPLC has not
proved particularly appropriate for the type A trichothecenes, which
lack any significant UV absorption, making sensitive detection
difficult. Refractive index detection has been employed, but at
relatively high toxin levels (Schmidt & Dose, 1984). UV detection at
short wavelength has been used for the determination of DON and NIV
in cereals, and a number of methods have been reported that offer an
advantage over alternative GC approaches in that they do not require
derivatization of the toxins. A post-column derivatization procedure
for DON and NIV has been developed (Sano et al., 1987) involving
alkaline decomposition of the trichothecenes to generate formaldehyde
and then reaction with methyl acetoacetate and ammonium acetate to
form a fluorescent derivative. Although more sensitive and specific
than UV detection, the approach does have the disadvantage of
requiring rather elaborate instrumentation, in order to carry out the
post column reaction. Electrochemical detection (Sylvia et al.,
1986) looks particularly promising for the determination of DON by
HPLC, offering both greater sensitivity and specificity than UV
detection, and the possibility of analysis of sample extracts that
have received minimal sample clean-up.
(iii) Gas chromatography (GC). A large number of GC methods have
been published that differ in the approach to sample extraction, in
sample clean-up, and in choice of derivative prior to GC analysis.
Trichothecenes containing a hydroxyl group require derivatization,
and the heptafluorobutyryl (HFB) ester and trimethylsilyl (TMS) ether
derivatives have been most frequently used in conjunction with
electron capture detection. Formation of the HFB derivatives is
relatively time-consuming, but complete derivatization is easily
achieved and the derivative once formed is stable for at least
several days. However, poor reproducibility has been noted with HFB
derivatives of DON (Mulders & Impelen-Peek, 1986) attributed to
adsorption on to glass surfaces. The relatively high mass of the HFB
derivatives can cause difficulties if GC/MS confirmation is required.
In contrast, TMS derivatives are easily prepared, and are of
suitable mass for GC/MS, but, for some type B toxins, they may
require optimization of conditions for complete derivatization
(Gilbert et al., 1985). Scott & Kanhere (1986) have compiled
retention data for 10 different trichothecenes as both TMS and HFB
derivatives on capillary columns of different stationary phases.
Trifluoroacetyl derivatives of trichothecenes are preferred by some
workers (Kientz & Verweij, 1986), and pentafluoropropionyl esters
have been used, particularly where detection has been by MS (Begley
et al., 1986; Krishnamurthy & Sarver, 1986; Rood et al., 1988a).
An area where improvement in quantification of trichothecenes is
still needed is in the selection of adequate internal standards.
Compounds, such as methoxychlor (Romer et al., 1978),
hexachlorobiphenyl (Blass et al., 1984), and alkanes (Ilus et al.,
1981), have been used, but these differ significantly in structure
from the trichothecenes. The deuterated TMS derivative of T-2 toxin
has been used as an internal standard in GC/MS analysis (Rosen &
Rosen, 1984), an isomer of T-2 toxin has been chemically synthesized
(Stahr et al., 1981) as have 4-deoxyverrucarol and 16-hydroxyver-
rucarol for use as internal stan-dards (Krishnamurthy et al., 1986).
Despite the many difficulties, a GC method using the HFB
derivative has been successfully collaboratively tested (Ware et al.,
1986) and subsequently adopted as an AOAC official first action
method.
Other related trichothecenes have been determined in foods by GC
methods, for example, the de-epoxidised metabolite of DON called
DOM-1 has been determined in milk as both its TMS and HFB derivative
(Swanson et al., 1986). Also, an isomer of DON was detected by
capillary GC, as its TMS derivative in bread and breakfast cereal
products prepared from flour naturally contaminated with DON
(Greenhalgh et al., 1984).
(iv) Mass spectrometry (MS). Mass spectrometry has been used for
the structural characterization of novel trichothecenes; for
identification and confirmation of trichothecenes in biological
materials, and as a sensitive and specific means of detection with
GC, LC, or supercritical fluid chromatography (SFC) sample
introduction.
The usefulness of negative ion chemical ionization (NICI) mass
spectrometry with hydroxide ion reagent gas has been demonstrated in
relation to producing relative molecular mass information, as well as
fragment ions indicative of structure for type A and B trichothecenes
(Brumley et al., 1982). The NICI technique has been applied to
confirmation of the presence of DON in cereals and snack foods by
rapid capillary GC introduction of the underivatized extract
obtaining full scan spectra (Brumley et al., 1985), and by selected
ion monitoring (Miles & Gurprasad, 1985).
NICI has also been used for selected ion monitoring GC/MS of the
HFB derivatives of 13 different trichothecenes of both type A and
type B in extracts from small samples of biological material
(Krishnamurthy et al., 1986). GC/MS is the preferred approach using
SIM in electron ionization (D'Agostino et al., 1986) or NICI (Begley
et al., 1986) modes for biological samples where only small sample
sizes are available but high sensitivity is required. For more
routine surveys of cereal and food samples, particularly for DON and
NIV, initial screening has normally been carried out using GC with an
ECD and only selected positive samples have been confirmed by GC/MS
(Tanaka et al., 1985a; Cohen & Lapointe, 1982).
Attempts have been made to determine the macrocyclic
trichothecenes as TMS derivatives on short fused silica GC columns
using GC/MS (Rosen et al., 1986). The approach has also been adopted
of alkaline hydrolysis of the ring system of the macrocyclic
compounds to yield verrucarols, which can then be determined as HFB
derivatives by NICI GC/MS (Krishnamurthy et al., 1987). A similar
approach using alkaline hydrolysis has been proposed (Rood et al.,
1988a,b) as a basis for a general method for the determination of
trichothecenes and metabolites by conversion to their corresponding
parent alcohols prior to derivatization and GC or GC/MS
determination.
Although LC/MS is really a research method, thermospray LC/MS is
becoming more widely available and shows promise for the
identification of low levels of trichothecenes in biological
materials (Voyksner et al., 1985; Rajakyla et al., 1987). Another
promising research method for the determination of both the simple
and the macrocyclic trichothecenes is supercritical fluid
chromatography (SFC) combined with MS (Smith et al., 1985).
Supercritical fluids can also be used for the on-line extraction from
biological materials and direct introduction into the MS to monitor a
number of different trichothecenes (Kalinoski et al., 1986).
II.1.2.2 Immunological methods
Chu et al. (1979) developed a radioimmunoassay (RIA) for T-2
toxin. The antibody preparation was obtained by immunizing rabbits
with bovine serum albumin T-2 toxin hemisuccinate conjugate. The
antibody had the greatest binding efficiency for T-2 toxin and less
efficiency for HT-2 toxin. Cross reaction with other trichothecenes
was either very slight or absent, and the limit of detection of the
assay ranged from 1 to 20 ng.
This RIA was applied to the determination of T-2 toxin in
agricultural commodities, biological fluids, and animal organs (Lee &
Chu, 1981a,b; Hewetson et al., 1987). Xu et al. (1988) also reported
an immunoassay using an antibody against triacetylated DON, for the
determination of DON in wheat and corn with a limit of detection of
about 0.02 µg/g.
A polyclonal enzyme-linked immunosorbent assay (ELISA) was
developed for the rapid quantification of T-2 toxin in food and
animal feed (Pestka et al., 1981). This assay, which could be
undertaken in 2 h, had a limit of detection of 2.5 pg/assay and was
used for the detection of T-2 toxin in Fusarium-infected corn
(Gendloff et al., 1984). More recently monoclonal ELISAs have been
developed for T-2 toxin and DON (Feuerstein et al., 1985; Hunter et
al., 1985; Chiba et al., 1988). Although these monoclonal assays are
of invariable specificity and the antibodies are available in
unlimited supply, competitive indirect ELISAs using monoclonals are
generally less sensitive than polyclonal-based ELISAs. However,
Chiba et al. (1988) developed a sensitive ELISA for the detection of
T-2 toxin with monoclonal antibodies at a limit of 2.5 pg/assay.
This assay has been applied to the detection of T-2 toxin in wheat
flour (Chiba et al., 1988) and in fungal cultures (Nagayama et
al., 1988).
Production of useful antibodies against DON has proved more
difficult than for other trichothecenes. Xu et al. (1988) adopted
the approach of raising antibodies against triacetyl-DON, which
requires acetylation of DON in an extract of the contaminated cereal
prior to carrying out a direct ELISA. This assay has the required
sensitivity but also the disadvantage of determining the total
concentration of DON plus any other acetylated derivatives that may
be present in the extract. A different approach to raising
monoclonal antibodies to DON has been carried out by forming the
hemisuccinyl derivative after protection of two of the available
adjacent hydroxyls with a cyclic boronate ester (Casale et al.,
1988). Some cross-reactivity to other trichothecenes and their
de-epoxy metabolites was observed, but the assay does show
considerable potential as a simple and rapid screening method for
contaminated cereals and for detection in biological samples.
II.1.2.3 Biological methods
Biological methods are essential for working with field cases
where the cause of an incident is unclear and evidence is sought to
associate an observed biological effect with the presence of fungally
contaminated material. Biological methods are also important as
monitoring procedures in the isolation and purification of new or
previously unrecognized toxins, prior to characterization by
spectroscopic and chemical methods.
The classical and commonly used bioassay for trichothecenes is
the skin necrotization test. Extracts prepared from samples (see
section II.1.2.1) are applied in a single dose to the shaved back of
a rabbit, rat, or guinea-pig. Toxic preparations applied in this way
produce erythema, oedema, intradermal haemorrhage, and necrosis. The
guinea-pig is the most sensitive of these animals to trichothecenes
(Ueno et al., 1970). As little as 0.2 µg of T-2 toxin and DAS were
detected on the skin of the rabbit and guinea-pig (Chung et al.,
1974; Balzer et al., 1977). Only 10 g of the corn sample are needed
for testing with an extraction and clean-up procedure recommended by
Eppley (1968). Macrocyclic trichothecenes are highly irritant to the
skin, but NIV and DON, which have a low skin-necrotizing potency,
could be routinely missed in this assay (Ueno et al., 1970).
Brine shrimp ( Artemia salina L.) larvae also provide a useful
biological system for monitoring trichothecenes, such as T-2 toxin,
DAS, and the macrocyclic trichothecenes. The limit of detection of
this test for these toxins is approximately 0.04-0.4 mg/litre
(Eppley, 1974).
The rabbit reticulocyte assay, based on the inhibition of uptake
of 14C-leucine in eukaryote cells, is highly specific for
trichothecenes; ID50 (50% inhibitory dose) values are 0.03 mg/litre
for T-2 toxin and DAS, and 2.0-3.0 mg/litre for DON and NIV (Ueno et
al., 1969a, 1973c). By combining chemical methods with this assay,
DON was successfully isolated from Fusarium-infected corn (Ishii et
al., 1975).
II.2 SOURCES AND OCCURRENCE
II.2.1 Taxonomic considerations
The vast majority of trichothecenes are produced by the Fusarium
species. Production of these metabolites depends, of course, on many
factors, including substrate, temperature, humidity, etc. In
general, the type A trichothecenes have been most frequently
associated with the following Fusarium species: F. tricinctum, F.
sporotrichioides, F. poe, F. equiseti. On the other hand, type B
trichothecenes are usually associated with F. graminearum (Gibberella
zeae), and F. culmorum. The type C trichothecenes, containing an
additional epoxide function at the 7,8- or 9,10-positions, are
produced by only a few species. The Type D trichothecenes, i.e.,
those containing the macrocyclic ring between the 4,15-positions, are
produced by several fungal genera, including Myrothecium and
Stachybotrys. Of these, the most important from an animal and human
health standpoint, is Stachybotrys atra. The relationships between
trichothecenes and fungal species are summarized in Table 13.
Table 13. The relationship between trichothecenes and fungal species
----------------------------------------------------------------------
Trichothecenes Fungal species References
----------------------------------------------------------------------
Type A
T-2, HT-2, DAS, NS Fusarium tricinctum Joffe & Yagen (1977)
F. sporotrichioides Scott et al. (1980)
F. poae Marasas et al. (1984)
F. acuminatum Ichinoe et al. (1985),
Rabie et al. (1986)
DAS F. equiseti Brian et al. (1961)
F. semitectum Suzuki et al. (1980),
Greenhalgh et al. (1984)
Type B
DON, 3-AcDON F. graminearum Yoshizawa & Morooka (1973)
NIV, F-X Gibberella zeae Marasas et al. (1984)
(anamorph) Ichinoe et al. (1985)
DON, 3-AcDON F. culmorum Marasas et al. (1979b)
Chelkowski et al. (1984)
Trichotecin Trichothecium Freeman & Morrison (1949)
roseum Ishii et al. (1986)
Type C
Baccharin Baccharis Kupchan et al. (1976)
megapotamica
(higher plant)
Type D
Roridin A, D, E Mycothecium roridum Bohner et al. (1965)
Verrucarin J M. verrucaria Harrach et al. (1981)
Satratoxin G, H Stachybotrys atra Jarvis et al. (1986)
----------------------------------------------------------------------
The taxonomy of the various fungal species is complex and has led
to some confusion, particularly regarding the Fusarium species
responsible for the production of the various trichothecene
metabolites. Several excellent descriptions are now available of the
Fusarium spp. and the metabolites produced by them (Nelson et al.,
1983; Ueno, 1983; Marasas et al., 1984; Ichinoe et al., 1985; Joffe,
1986).
II.2.2 Ecology of trichothecene-producing fungi
Fusarium species are widely distributed throughout the
environment. There are over 50 recognized species commonly occurring
in the soil (soil fungi), many of which are pathogenic to crop
plants. As a result, there are a number of frequently encountered
plant diseases (wilts, blights, rots), such as F. graminearum blight
of wheat and barley (Akakabibyo in Japanese), pink ear rot of corn,
pink scab (tombstone kernels) of wheat, Fusarium snow blight, etc.
Fusarium graminearum, which produces DON and NIV, is a most important
fungus.
II.2.3 Natural occurrence
It has become apparent in the past few years that whenever a
trichothecene-producing Fusarium species parasitizes a crop, food,
or animal feed, it is highly probable that the metabolites of the
trichothecene will be found as contaminants. The chance of detecting
the metabolite is then clearly a function of the efficiency of the
sampling procedure and the capabilities of the analytical methods
used (most importantly, the detection limit).
Because of its toxicity, analytical procedures for T-2 toxin were
developed first, and consequently early surveys for trichothecenes
tended to concentrate on T-2 toxin. However, it soon became apparent
that other trichothecenes, in particular, DON, NIV, and DAS, were
more frequent contaminants of food and animal feed than T-2 toxin.
As improved analytical procedures for these mycotoxins became
available, surveys were conducted and additional data on the
occurrence of these metabolites were published. In reviewing this
record, it should be recognized that the methodology used in
developing the data was quite variable, so that only broad
generalizations with respect to incidence/level may be drawn. In the
vast majority of cases, no evidence was included indicating that
methods used were rigorously tested, that quality assurance
programmes were in place, and that confirmation of identity was
adequately obtained. Much of the available survey data on the
natural occurrence of trichothecenes in raw agricultural commodities,
food, and animal feed is summarized in Tables 14-17. Reports in
which only a few samples were analysed have not been included.
II.2.3.1 Agricultural products
(a) T-2 Toxin
One of the first trichothecenes to be implicated in an episode of
mouldy corn toxicosis was T-2 toxin; in 1972, it was reported that
T-2 toxin at the level of 2 mg/kg was present in mouldy corn involved
in lethal toxicosis in dairy cattle (Hsu et al., 1972). This event,
along with increasing information regarding the acute toxicity of T-2
toxin, prompted considerable efforts to develop methods of analysis
for T-2 toxin and the analysis of a wide range of agricultural
commodities (Table 14). Only occasional samples were found to
contain T-2 toxin (incidence well below 10% in most cases), most
frequently at levels <0.1 mg/kg. Usually, other trichothecenes were
also found (Vesonder, 1983). On the other hand, there have been
isolated reports of the finding of rather high levels of T-2 toxin,
e.g., the finding of 25 mg T-2 toxin/kg in barley (Puls & Greenway,
1976), and 38.9 mg T-2 toxin/kg in peanuts (Bhavanishankar & Shantha,
1987). These findings, as well as reports from India of the presence
of T-2 toxin in safflower seed and sweet corn (Ghosal et al., 1977),
and sorghum (Ruckmini & Bhat, 1978), and from Italy of its presence
in barley, corn feed, oats, rice, and wheat (Cirilli, 1983) need to
be further investigated.
Table 14. Natural occurrence of T-2 toxin in raw agricultural
commodities
---------------------------------------------------------------------
Commodity Levels Incidence Country Reference
(mg/kg) (+ve/total)
---------------------------------------------------------------------
Corn 0.5-5.0 (5/150) Hungary Szathmary (1983)
0.080-0.65 (9/118) Taiwan Tseng et al. (1983)
0.01-0.2 (13/20) New Zealand Hussein et al.
(1989)
Feed 0.05-5.0 (28/464) Hungary Szathmary (1981)
Oats 0.01-0.05 Finland Ylimacki et al.
(1979)
Peanuts 0.63-38.89 (6/87) India Bhavanishankar &
Shantha (1987)
Rice (3/64) Egypt Abdel-Hafez et al.
(1987)
Sorghum 1.67-15.0 (4/84) India Bhavanishankar &
Shantha (1987)
Wheat 2.0-4.0 (3/12) India Bhat et al. (1989)
---------------------------------------------------------------------
DON/NIV: Deoxynivalenol (DON) and nivalenol (NIV) have been
found to be the most frequent trichothecene contaminants of
agricultural crops throughout the world (Table 15). Extensive
survey data indicate the common occurrence of these mycotoxins,
particularly in corn and wheat, at levels usually below 1 mg/kg
(Vesonder, 1983; Pohland & Wood, 1987; Jelinek et al., 1989).
Perhaps the most extensive survey, and the one giving the most
accurate picture of the global occurrence of DON and NIV, was
reported recently by Tanaka et al. (1988).
Table 15. Natural occurrence of DON and NIV in raw agricultural
commodities
---------------------------------------------------------------------------
Commodity Toxin Levels Incidence Country Reference
(mg/kg)a (+ve/total)
---------------------------------------------------------------------------
Barley DON t-40.4 (19/25) Japan Kamimura et
al. (1981)
NIV t-37.9 (19/23)
(unpolished) DON 0.004-0.508 (26/28) Korea Lee et al.
NIV 0.017-3.0 (28/28) Republic of (1985)
(polished) DON 0.008-0.043 (5/6)
NIV 0.085-0.328 (5/6)
DON 0.006-2.14 (34/49) Norway Sundheim et
NIV 0.013-1.56 (49/49) al. (1988)
DON 0.02-0.36 (34/87) United Gilbert et
Kingdom al. (1983b)
Corn DON 1-20 (19/60) Austria Lew et al.
(1979)
DON 0.15-0.82 (9/9) Canada Scott et
al. (1981)
---------------------------------------------------------------------------
Table 15 (contd.)
---------------------------------------------------------------------------
Commodity Toxin Levels Incidence Country Reference
(mg/kg)a (+ve/total)
---------------------------------------------------------------------------
Corn (contd.) DON 0.36-12.7 (100%) China Qiujie et
NIV 0.054-2.67 (100%) al. (1988)
DON 0.02-0.3 (11/20) New Zealand Hussein et
al. (1989)
DON t-15.8 (20/72) Transkei Thiel et
NIV t-1.41 (43/72) al. (1982)
DON 0.1-0.3 (37) United Gilbert et
Kingdom al. (1984)
DON 0.5-10.7 (24/52) USA Vesonder et
al. (1978)
DON 0.1-2.47 (93/198) USA Wood &
Carter
(1989)
Oats DON 20 Germany, Bauer et
Federal al. (1980)
Republic of
DON 0.02-0.1 (1/6) United Gilbert et
Kingdom al. (1984)
Rye DON 0.003 (1/5) Korea Lee et al.
NIV 0.046-0.114 (5/5) Republic of (1985)
Wheat DON 0.01-4.3 (51/52) Canada Scott et
al. (1981)
DON 0.06-8.53 (24/52) Canada Trenholm et
al. (1981)
DON 0.02-1.32 (55/199) Canada Osborne &
Willis
(1984)
DON 1.0 (1.36) Denmark Hald & Krogh
(1983)
DON t-4.7 (15/18) Germany, Bauer et
NIV t-7.8 Federal al. (1980)
Republic of
DON 0.008-3.19 (32/53) Norway Sundheim et
al. (1988)
NIV 0.015-0.887 (53/53)
DON 0.02 >0.5 (57/148) United Gilbert et
Kingdom al. (1984)
DON 0.12-5.5 (31/33) USA Hagler et
al. (1984)
DON 0.2-9 (54/57) USA Eppley et
al. (1984)
DON 0.1-2.65 (133/247) USA Wood &
Carter
(1989)
---------------------------------------------------------------------------
Table 15 (contd.)
---------------------------------------------------------------------------
Commodity Toxin Levels Incidence Country Reference
(mg/kg)a (+ve/total)
---------------------------------------------------------------------------
Animal DON 0.1-41.6 (274/342) USA Cote et
feed al. (1984)
DON <0.4-4.0 USA Vesonder
(1983)
---------------------------------------------------------------------------
a t = trace.
This survey involved the analysis of 500 samples of cereal grains
from 19 countries (Table 16) using a single analytical method.
Table 16. Natural occurrence of DON and NIV: worldwide survey
---------------------------------------------------------------------
Commodity DON NIV
(Mean, ng/g) (% + ve) (Mean, ng/g) (% + ve)
---------------------------------------------------------------------
Barley 149 75 401 76
Corn 402 20 766 16
Oats 115 22 438 26
Rice 0 22 22
Rye 183 33 47 33
Sorghum 0 0
Wheat 488a 39 127 50
Othersb 135 44 3 6
Total: 292 45 267 48
-----------------------------------------------------------------------
a A Beijing wheat sample containing 6 644 ng/g was excluded in
calculating this mean.
b Wheat flour, 7; rye flour, 1; spice, 3; sesame, 7.
It was found that 45-50% of random samples contained both DON and
NIV, barley being most frequently contaminated. Corn, although less
frequently contaminated, contained the highest average amounts of
NIV; of all the cereals examined, wheat was the most heavily
contaminated with DON. There were clear regional differences, not
totally predictable, in the relative quantities of DON and NIV. For
example, in Canada and the USA, NIV was only rarely encountered
whereas, in Japan, NIV was more frequently encountered than DON.
Even within a country there were clear differences between regions;
for example, in southern Japan, NIV was more frequently encountered
than DON, whereas in northern Japan the reverse was true. Similarly,
levels of DON were generally lower in western than in eastern Canada
(Jelinek et al., 1989).
The data indicate that crops parasitized by Fusarium species will
probably be contaminated with these mycotoxins. In wheat, for
example, there is an excellent correlation between the DON
contamination level and the percentage of mouldy kernels, the
percentage of total defects, and the degree of scab damage (Eppley et
al., 1984; Shotwell et al., 1985). It has been suggested that crop
rotation might be a factor, i.e., planting wheat after corn tends to
increase the DON levels in the resulting wheat crop (Teich &
Hamilton, 1985). Recently, it has been shown that the mean
concentration of DON in wheat declines quite significantly in the
2-week period immediately preceding harvest (Scott et al., 1984).
(b) Miscellaneous trichothecenes
There have been occasional reports of trichothecenes other than
those discussed above (T-2 toxin, DON, NIV) in agricultural products,
in particular, DAS, 15-acetyldeoxynivalenol (15-ADON), and 3-
acetyldeoxynivalenol (3-ADON) (Table 17). Most of these reports
involve corn. There is also an unconfirmed report of the finding of
T-2 toxin, DAS, and trichothecolone as well as the fatty acid esters
of trichothecolone, scirpenetriol, and T-2 tetraol in banana fruit,
which needs further investigation (Chakrabarti & Ghosal, 1986). In
1982, over 100 out of a flock of 1200 ewes on a Hungarian farm died
after ingestion of bedding straw highly infected with Stachybotrys
atra. TLC, HPLC, and MS analysis of the methanol extract of the
straw revealed the presence of the macrocyclic trichothecenes,
satratoxins G and H (Harrach et al., 1983).
Table 17. Natural occurrence: miscellanceous trichothecenes in
agricultural products
---------------------------------------------------------------------------
Commodity Toxin Level Incidence Country Reference
(mg/kg) (+ve/total)
---------------------------------------------------------------------------
Corn 15-ADON 0.113 China Qiujie et al.
ave. (1988)
3-ADON 0.495 China Qiujie et al.
ave. (1988)
DAS 31.5 Germany, Siegfried (1977)
Federal
Republic of
DAS 0.01- (6/20) New Zealand Hussein et al.
0.9 (1989)
15-ADON 0-7.9 USA Abbas et al.
(1986)
Peanuts DAS 0.41- (7/87) India Bhavanishankar &
2.03 Shantha (1987)
Wheat ADON 0.6-2.4 (4/12) India Bhat et al.
flour (1989)
Bedding satratoxin not Hungary Harrach et al.
straw G estimated (1983)
satratoxin not Hungary Harrach et al.
H estimated (1983)
---------------------------------------------------------------------------
The macrocyclic trichothecenes, such as satratoxin H, verrucarins
B and J, and trichoverrins A and B, were also detected in air dust
collected from a house in Chicago, where the occupants were subjected
to a variety of recurring maladies including colds, dermatitis, and
others (Croft et al., 1986). These data indicated a possible
airborne outbreak of macrocyclic trichothecene-induced toxicosis.
Undoubtedly, as good analytical methods become available, these
trichothecenes will be found more frequently.
II.2.3.2 Trichothecenes in human foodstuffs
Given the widespread occurrence of trichothecenes in agricultural
products, in particular DON and NIV, it is not surprising to find the
compounds in human foodstuffs (Table 18). The vast majority of the
confirmed cases of contamination of foodstuffs by trichothecenes
involve DON in wheat or wheat products. Overall, the finding of DON
in human foodstuffs at concentrations >1 mg/kg has been rare. NIV
has been detected in human foodstuffs, particularly in Japan, where
an effort was made to develop and apply analytical methods capable of
determining both DON and NIV.
Table 18. Natural occurrence of DON and NIV in commercial foods
---------------------------------------------------------------------------
Commodity Toxin Level Incidence Country Reference
(mg/kg)a (+ve/total)
---------------------------------------------------------------------------
Barley DON 0.027-0.085 (6/6) Japan Yoshizawa &
flour NIV 0.037-0.19 (6/6) Hosokawa
(parched) (1983)
Barley DON 0.008-0.039 (5/6) Tanaka et
flour NIV 0.013-0.041 (6/6) al. (1985b)
Barley DON 0.003-0.05 (10/14) Tanaka et
(pressed) NIV 0.008-0.033 (13/14) al. (1985a)
Barley DON t-0.26 (27/147) Kamimura et
products NIV 0.006-0.28 al. (1981)
Corn meal DON 0.025 (45/50) USA Trucksess et
al. (1986a)
Corn flour DON (0.18 ave.) (27) Canada Scott et al.
meal DON (0.1 ave.) (35) (1984)
products DON 0.011-1.25 (7/7)
Popcorn DON 0.012-0.25 (7/7) Japan Tanaka et al.
Job's tear DON 0.048-0.5 (2/12) (1985b)
NIV 0.003-0.92 (11/12)
Potatoes DON (4/17) Canada El Banna et
al. (1984)
Rye flour DON (0.12 ave.) (3) Canada Scott et al.
bread NIV (0.058 ave.) (4) (1984)
Wheat DON (0.4 ave.) (43)
flour DON 0.11-0.69 (5/5) China Ueno et al.
(1986)
DON 0.43-4.89 (9/12) India Bhat et al.
NIV 0.03-0.1 (2/12) (1989)
DON 0.002-0.239 (26/36) Japan Tanaka et
NIV 0.004-0.084 (12/36) al. (1985b)
DON 0-0.46 (44/50) USA Trucksess et
al. (1986a)
---------------------------------------------------------------------------
Table 18 (contd.)
---------------------------------------------------------------------------
Commodity Toxin Level Incidence Country Reference
(mg/kg)a (+ve/total)
---------------------------------------------------------------------------
Breakfast DON (0.086 ave.) (36) Canada Scott et al.
cereals (1984)
Bread DON (0.08 ave.) (21)
Baby cereal DON (0.043 ave.) (30)
Crackers DON (0.27 ave.) (20)
Cookies DON (0.12 ave.) (25)
Breakfast DON 0-0.53 (35/60) USA Trucksess et
cereals al. (1986a)
Bread DON 0-0.24 (20/25)
Baby foods DON 0-0.09 (14/39)
Snack foods DON 0-0.45 (25/44)
Bread DON 0.013-0.24 (39/45) USA Wood & Carter
(1989)
---------------------------------------------------------------------------
a t = trace.
The realization that trichothecene contamination of cereal grains
was not uncommon prompted a series of studies on the fate of these
compounds during the normal processing of such grains into consumer
food products. These studies can be summarized as follows:
In the case of corn, Collins & Rosen (1981) demonstrated that,
during wet milling, roughly 66% of the T-2 toxin originally present
was removed in the steeping water, about 4% remained in the starch,
and the remainder was distributed evenly in the germ, gluten, and
fibre fractions (a result clearly predictable in view of the
considerable water solubility of T-2 toxin). The same general
observation was made about the fate of DON during the wet milling of
corn (Scott, 1984b). During the dry milling of corn, the major
portion of contaminating DON was found in the germ meal, which is
used for animal feed. For any significant contamination of grits,
the corn used in the manufacture would have to be highly contaminated
(Gilbert et al., 1983a).
In the case of wheat, several studies have clearly demonstrated
that cleaning and milling are not effective in completely removing
DON (Hart & Baselton, 1983; Scott et al., 1983, 1984a; Young et al.,
1984; Seitz et al., 1985). The same was generally true for NIV (Lee
et al., 1987). Normal cleaning of wheat reduced average DON
contamination levels by 6-19% (Abbas et al., 1985). The remaining
DON, after milling, is distributed in all fractions (bran, shorts,
reduction flour, break flour), the greatest amounts being found in
the bran and the smallest amounts in the flour, depending on the
level of contamination of the wheat itself. In a typical study,
milling of whole wheat contaminated at the 2 µg/g level, resulted in
approximately 65% of the DON in the flour and 35% in the bran, red
dogs, and shorts; i.e., there was little reduction in the DON
concentration in the flour after milling (Hart & Baselton, 1983).
It has been found that the further processing of flour into baked
or cooked products results in variable DON losses. The overall range
of reduction in DON levels from uncleaned wheat to bread was 24-71%
(Abbas et al., 1985). No reduction in DON levels was found in the
preparation of Egyptian bread (El-Banna et al., 1983) or cookies
from hard wheat flour (Scott et al., 1983), illustrating the
importance played by the conditions of processing. Kamimura et al.
(1979) showed that bread and noodles prepared under conditions
simulating commercial processes retained about 50% of the
trichothecenes that had been previously added to the wheat flour.
The trichothecene content of Japanese noodles was reduced by 30% in
the boiling process.
Similarly, these authors found that 30% of NIV and DON was
extracted from naturally contaminated wheat by water, while there was
a 50% or more reduction in trichothecene levels in bread made from
the same flour. The preparation of Chinese noodles, where sodium
carbonate is used as an ingredient, results in an even greater loss
of DON than is observed in the preparation of Japanese noodles (Scott
et al., 1984b; Nowicki et al., 1988). Similarly, Young et al. (1984)
observed a decrease of up to 35% in the production of cookies from
biscuit and cake flours; in the process studied, the batter contained
ammonium carbonate. Finally, it has been found that, during the
baking of bread, 3-13% of DON is converted into an isomer, iso-DON
(Greenhalgh et al., 1984).
There have been some studies on possible detoxification
procedures for corn and wheat contaminated with trichothecenes;
agents studied include chlorine, ammonia (Young et al., 1984) and
aqueous bisulfite (Young et al., 1986). None of these processes are
currently commercially feasible.
There has been some concern about the transmission of the
trichothecenes, particularly DON, to milk. Studies have shown that
milk is only a minor excretion route in the lactating dairy cow;
thus, the possibility that DON might contaminate milk and milk
products is remote (Prelusky et al., 1984).
II.3 METABOLISM
II.3.1 Absorption and tissue distribution
II.3.1.1 Animal studies
In general, trichothecenes absorbed from the alimentary tract are
evenly distributed in many tissues and organs, without significant
accumulation in specific organs. However, at present, distribution
studies have been limited mainly to T-2 toxin, and the metabolism in
animals of other naturally contaminating trichothecenes, such as DON,
NIV, and DAS, remains to be elucidated.
In 6-week-old broiler chicks fed with a ration containing T-2
toxin at 2 mg/kg for 5 weeks and then intubated with a single dose of
3H-T-2 toxin at 0.5 mg/kg body weight, the radioactivity reached a
maximum concentration in most tissues, 4 h after dosing; exceptions
were the muscle, skin, and bile, in which the maximum level was
reached after 12 h (Chi et al., 1978b). After 48 h, chicks contained
the equivalent of 39 µg T-2 toxin and/or its metabolites/kg in the
muscle, and 40 µg/kg in the liver, as calculated on the basis of the
specific activity of the radiolabelled T-2 toxin administered.
In chicken organs, 18 h after intraperitoneal injection of T-2
toxin (3.5 mg/kg), considerable amounts of T-2 metabolites were found
in the liver (1370 µg 3'-hydroxy-H-T-2 toxin/kg). Smaller amounts of
H-T-2 toxin, T-2 triol, and other metabolites were detected in the
lungs (Visconti & Mirocha, 1985).
In weanling crossbred pigs (7.5-9.5 kg body weight) intubated
with 0.1 or 0.4 mg 3H-T-2 toxin/kg body weight, the percentage of
administered radioactivity (18 h after dosing) was 0.7% in the
muscle, 0.29-0.43% in the liver, 0.08% in the kidney, and 0.06-0.14%
in the bile (Robison et al., 1979b). In pigs intubated with 0.1 mg of
the toxin/kg body weight, the calculated residue levels for T-2 toxin
and/or its metabolites, based on the specific radioactivity of the
tissues, were as follows: muscle, 3.1 µg/kg; fat, 0.49 µg/kg; liver,
13.8 µg/kg; and kidney, 15.9 µg/kg. The corresponding residue levels
for T-2 toxin in the tissue of another pig intubated with 0.4 mg of
the toxin/kg body weight were 11.5 µg/kg in the muscle, 37.7 µg/kg in
the liver, and 61.4 µg/kg in the kidney.
Four hours after intravenous administration of T-2 toxin in
swine, the greatest amount of radioactivity was located in the
gastrointestinal tract (15-24% of the dose), and 4.7-5.2% of the dose
was found in the remaining tissues, among which the muscle and the
liver accounted for 2.9-3.2% and 0.7-1.7% of the dose, respectively
(Corley et al., 1986).
The fate and distribution of 3H-T-2 toxin was studied in guinea-
pigs (Pace et al., 1985). Except for the large intestine and bile,
the radioactivity had peaked by 30 min and rapidly declined, with no
measurable long-term accumulation. In general, the distribution
pattern in the guinea-pig during the first 12-24 h paralleled the
distribution found in the chicken and swine.
The metabolic fate of T-2 toxin was investigated in a lactating
Jersey cow weighing 375 kg, after daily oral administration, by
capsule, of unlabelled toxin at 180 mg/day for 3 days, followed by
administration of 3H-T-2 toxin at 156.9 mg (Yoshizawa et al., 1981).
Although almost all of the administered dose was eliminated in 72 h,
appreciable levels of tritium still remained in the bile, liver, and
kidney (equivalent, repectively, to 27.2, 18.5, and 13.9 µg/kg 3H-T-2
toxin) 3 days after dosing. These levels are higher than in whole
blood (13.3 µg/kg) and plasma (10.2 µg/kg) and in other tissues,
including the spleen (9.4 µg/kg) heart (10.1 µg/kg), mammary gland
(11.3 µg/kg), ovaries (10.7 µg/kg), muscle (8.8 µg/kg), and fat (4.7
µg/kg).
Experimentally derived relationships between the residue of
radioactivity in animal tissues or plasma and the toxin levels in the
feed, calculated on the basis of the amount of 3H-T-2 toxin
administered are summarized in Table 19 (Yoshizawa et al., 1981).
Table 19. The relationship between the level of 3H-T-2 toxin in the feed or the
tritium residues in plasma and tritium levels in the edible tissues of the cow, chick,
and piga
---------------------------------------------------------------------------------------
Tissue Animal Time after Feed level Tissue level Tissue/feed Tissue/plasma
dosing (h)b (mg/kg)c (µg/kg)d ratio ratioe
---------------------------------------------------------------------------------------
Muscle Cow 72 31.38 8.8 0.0003 0.863
Chick 24 1.26 17.3 0.0137 1.000
5.0 59.2 0.0118 0.938
18.95 228.6 0.0121 0.875
Pig 18 1.25 3.1 0.002 0.775
Heart Cow 72 31.38 10.1 0.0003 0.991
Chick 24 1.26 13.7 0.011 0.792
5.0 49.4 0.010 0.783
18.95 207.7 0.011 0.795
Pig 18 1.25 3.9 0.003 0.975
Liver Cow 72 31.38 18.5 0.0006 1.863
Chick 24 1.26 34.0 0.0270 1.965
5.0 107.3 0.0215 1.700
18.95 431.0 0.0227 1.649
Pig 18 1.25 13.8 0.011 3.450
Milk Cow 72 31.38 11.4 0.0004 1.118
---------------------------------------------------------------------------------------
a From: Yoshizawa et al. (1981).
b Animals were intubated with a single dose of 3H-T-2 toxin.
c Estimates of feed levels were based on the assumption that each animal would consume
the following amount of feed daily: cow, 5 kg; chick, 100 g; pig, 600 g.
d Values were calculated from residual tritium levels in the edible tissues of animals
given 3H-T-2 toxin and were expressed as equivalents of 3H-T-2 toxin (µg/kg).
e The plasma levels were 10.2 µg/kg equivalents of T-2 toxin in the cow, and 17.3,
63.1, and 261.3 µg/kg equivalents of T-2 toxin in the chick at feed levels of 1.26,
5, and 18.95 mg/kg, respectively. The whole-blood level of residual tritium in the
pig was 4 µg/kg equivalents of T-2 toxin.
The tissue/feed ratio of tritium in the edible tissues of the
cow, 72 h after dosing, ranges from 0.0003 to 0.0006. These figures
are 5-10% of those for swine, 18 h after dosing. The tissue/feed
ratio for chickens, is higher than those in the cow and pig, ranging
from 0.010 to 0.014 for the muscle and heart, and from 0.021 to 0.027
for the liver, regardless of T-2 toxin dosage. The tissue/plasma
ratios of T-2 toxin metabolites, ranging from 0.8 to 1 in the muscle
and heart, and from 1.6 to 3.5 in the liver, are independent of
animal species, of T-2 toxin dosage, and of time after dosing. The
milk/plasma ratio of radioactivity in a cow treated with 3H-T-2 toxin
increased linearly for 24 h and thereafter ranged from 1 to 1.3.
II.3.2 Metabolic transformation
The in vivo metabolic transformation of trichothecenes in animals
is reported in Table 20. The trichothecene metabolites produced are
less toxic than the corresponding parent toxins. Both de-epoxidation
and glucuronidation, in particular, are associated with remarkable
detoxification of trichothecenes.
Table 20. In vivo (metabolic) transformation of trichothecenes in animals
--------------------------------------------------------------------------------------------------
Animal Trichothecene Transformation reaction Reference
--------------------------------------------------------------------------------------------------
Chicken T-2 toxin hydrolysis, 3'-hydroxylation, Yoshizawa et al. (1980b);
acetylation Visconti & Mirocha (1985)
Cow T-2 toxin hydrolysis, 3'hydroxylation, Yoshizawa et al. (1981, 1982a,b)
7'-hydroxylation, de-epoxidation Pawlosky & Mirocha (1984)
Chatterjee et al. (1986)
DON de-epoxidation, Yoshizawa et al. (1986)
glucuronide conjugation Côté et al. (1986)
Pig T-2 toxin hydrolysis, 3'-hydroxylation, Corley et al. (1985, 1986)
de-epoxidation, glucuronide
conjugation
DAS hydrolysis Bauer et al. (1985)
Sheep DON glucuronide conjugation Prelusky et al. (1985)
Rat DON de-epoxidation Yoshizawa et al. (1983)
T-2 metabolites de-epoxidation Yoshizawa et al. (1985a,b)
DAS hydrolysis, de-epoxidation Sakamoto et al. (1986)
Guinea-pig T-2 toxin hydrolysis, 3'-hydroxylation Pace et al. (1985)
Dog T-2 toxin hydrolysis, glucuronide
conjugation Sintov et al. (1987)
--------------------------------------------------------------------------------------------------
In in vitro studies, HT-2 toxin was found to be the sole
metabolite of T-2 toxin in the microsomes of the liver, kidney, and
spleen of various animals (Ohta et al., 1977, Johnsen et al., 1986).
The reaction of hepatic microsomes of different species in nmol/mg
protein per 10 min were: rabbit, 3044; human, 331; mouse, 75;
chicken, 55; rat, 36; and guinea-pig, 14. Yoshizawa et al. (1984,
1986) have proposed an in vitro metabolic pathway for T-2 toxin that
includes the hydrolysates at the C-4, C-8, and C-15 positions, and
the hydroxylation at the C-3'position by liver homogenates from the
rat, mouse, or monkey. This hydrolytic transformation was also
observed in the hepatic homogenates of the rabbit, pig, and cow.
However, HT-2 toxin was the sole metabolite in the homogenate of the
chicken, suggesting species differences in the metabolic pathway of
T-2 toxin (Yoshizawa & Sakamoto, 1982). In addition to the
metabolites above, glucuronide conjugates were formed in a study
using isolated perfused rat livers and T-2 toxin and DAS (Gareis et
al., 1986). Various de-epoxidation metabolites were found by
incubating intestinal and rumen microbes with T-2 toxin and its
metabolites (Yoshizawa et al., 1985a; Swanson et al., 1987), DON
(King et al., 1984; Côté et al., 1986; Swanson et al., 1987), and DAS
(Swanson et al., 1987).
II.3.3 Excretion
II.3.3.1 Animal studies
The kinetics of T-2 toxin were determined in pigs and cattle
(Beasley et al., 1986), and dogs (Sintov et al., 1986). Mean
elimination phase half-lives were 13.8 and 17.4 min, and mean
apparent specific volumes of distribution were 0.366 and 0.375
litre/kg in intra-aortally dosed pigs and intravenously dosed calves,
respectively. In dogs, the following mean parameters were determined
after intravenous administration of T-2 toxin and HT-2 toxin,
respectively: half-life 5.3 and 19.6 min, clearance 0.107 and
0.167 litre/min per kg, and volumes of distribution 0.86 and 4.47
litre/kg.
The half-life of elimination of DON in sheep, ranged from 100 to
125 min following oral administration, and it took 20-30 h to clear
from the system. Glucuronidation after intravenous or oral
administration of DON appeared to occur quite efficiently (iv, 21%;
oral, 75%), with elimination half-lives of (150-200 min and 6.1-7.1
h, respectively). These were considerably longer than those of the
parent toxin (Prelusky et al., 1985).
II.3.3.2 Excretion in eggs and milk
Radioactivity was transmitted into the eggs from laying hens that
had been intubated gastrically with a single or several doses of 3H-
T-2 toxin (Chi et al., 1978a). In birds dosed singly with 0.25 mg
T-2 toxin/kg body weight, maximum residues in the eggs occurred 24 h
after dosing; the yolk contained 0.04% of the total dose and the
white contained 0.13%. In birds dosed with 0.1 mg T-2 toxin/kg body
weight per day for 8 consecutive days, the radioactivity in the egg
accumulated until the 5th day of dosing, remained unchanged until the
last day of dosing, and rapidly decreased thereafter. Assuming that
the birds weighing 1.6 kg consumed 100 g of the diet containing 1.6
mg toxin/kg daily, the residues (T-2 toxin and/or its metabolites) in
such contaminated eggs would be about 0.9 µg/egg.
Transmission of DON was studied in the eggs and meat of chickens
(El Banna et al., 1983; Prelusky et al., 1987). Following a single
oral dose of 14C DON, maximum radioactivity, which occurred in the
first eggs laid after dosing (within 24 h), amounted to 0.087% of the
dose: levels dropped rapidly in later eggs.
In a pregnant Holstein cow (third trimester) intubated daily with
180 mg T-2 toxin for consecutive days (the toxin level corresponded
to a concentration of 50 mg/kg in the feed), milk samples taken on
the 2nd, 5th, 10th, and 12th days of intubation contained T-2 toxin
concentrations ranging from 10 to 160 µg/kg (Robison et al., 1979a).
Transmission of DON-1 (de-epoxydeoxynivalenol) to milk was confirmed
in lactating dairy cows (Côté et al., 1986; Yoshizawa et al., 1986).
Fresh and conjugated DON were also present in cow's milk following
administration of a single oral dose of 920 mg DON, but only
extremely low amounts (<4 µg/litre) were detected (Prelusky et al.,
1984).
II.4 EFFECTS ON ANIMALS
II.4.1 Field observations
In Hungary and other central European countries, pyosepticaemia
has been reported sporadically, in the past, in horses after
ingestion of mouldy hay and straw. This disease was characterized by
haemorrhages of the intestine and muscles, severe diarrhoea, and
death. Bacterium pyosepticum viscosum was detected in 1929 in the
excreta, and the equine disease was diagnosed as a pyosepticaemia
(Forgacs, 1965; Danko & Szerafin, 1976). After the discovery of
toxigenic Stachybotrys atra and its metabolites, such as satratoxins
G and H (Eppley & Bailey, 1973), the disease was presumed to be the
same as stachybotryotoxicosis (Danko & Szerafin, 1976).
A field outbreak involving the death of 20% of a dairy herd was
associated with prolonged ingestion of a diet containing 60% mouldy
corn infested with F. tricinctum. The concentration of T-2 toxin in
the feed was approximately 2 mg/kg dry weight (Hsu et al., 1972).
The lesions in the cattle included extensive haemorrhages on the
serosal surface of the internal viscera. An outbreak of haemorrhagic
syndrome in cows was associated with commercial feed containing T-2
toxin or T-2-like toxin (concentration not determined). The affected
animals showed an extremely prolonged prothrombin time. Necropsy
findings in 2 adult cows were marked serosal, mucosal, and
subcutaneous haemorrhages (Hibbs et al., 1975).
An outbreak of a disease, observed in poultry (ducks, geese),
horses, and pigs, was associated with mouldy barley containing T-2
toxin at approximately 25 mg/kg (Greenway & Puls, 1976). Pigs fed
the suspect barley exhibited signs of feed refusal, vomiting, and
diarrhoea. The horses became depressed and salivated excessively.
The lesions in the geese included necrosis of the mucosa of the
oesophagus, proventriculus, and gizzard. No pathological lesions
were described in other animals.
DON was isolated from a batch of maize that had caused vomiting
in pigs (Vesonder et al., 1973).
Equine leukoencephalomalacia reported from South Africa
(Kellerman et al., 1972; Marasas et al., 1979a; Pienaar et al., 1981)
and bean-hull toxicosis reported in horses in Hokkaido, Japan
(Konishi & Ichijo, 1970) appear to be very similar diseases with
nervous signs and hepatopathy as the major components. The signs in
these diseases were quite different from those caused by
trichothecenes, though some fungal isolates from samples of bean-
hulls produced the trichothecenes, T-2 toxin, and neosolaniol (Ueno
et al., 1972). F. moniliforme was considered to be the causative
fungus (Haliburton et al., 1979), but none of its metabolites,
including moniliformin, have been established as the cause of the
disease (Kriek et al., 1977).
Reports of field outbreaks of animal toxicoses associated with
trichothecene-contaminated feed are summarized in Table 21.
II.4.2 Effects on experimental animals
II.4.2.1 General toxic effects
LD50 values for certain trichothecenes in several experimental
animal species are summarized in Table 22 (Ueno et al., 1983). The
oral LD50 for T-2 toxin was 10.5 mg/kg body weight in mice, 3.06
mg/kg in guinea-pigs, 5.2 mg/kg in rats, and 6.1 mg/kg in trout.
The LD50 values for T-2 toxin in different species vary, but not
greatly.
The LD50 values of fusarenon-X were compared using different
routes of administration. The LD50s (mg/kg) in mice were 3.4 (iv),
3.4 (ip), 4.2 (sc), and 4.5 (oral). These data indicate that the
acute toxicity estimated for a single administration did not differ
markedly when the toxin was administered by different routes (Table
20) (Ueno et al., 1971).
Similar data were obtained with DON and acetyl-DON. An
interesting finding was that the ratio of the maximum lethal dose
to the minimum lethal dose was approximately two, indicating a
sharp dose-response curve for lethality. No marked differences in
acute toxicity were observed between treated male and female
animals.
Newborn animals are more sensitive than adults to the
toxic effects of the trichothecenes. For example, the LD50 values
for toxin given sc (mg/kg body weight) in newborn mice were: T-2
toxin, 0.15; DAS, 0.17; and fusarenon-X 0.23 (Ueno et al., 1973a).
Table 21. Field observations on animal toxicosis caused by trichothecenes
----------------------------------------------------------------------------------------------------
Mycotoxin Sample Animal Signs & lesions Reference
(concentration in feed)
----------------------------------------------------------------------------------------------------
T-2 toxin (2 mg/kg) mouldy corn cattle extensive haemorrhages Hsu et al.
(20% died) (1972)
T-2 toxin (25 mg/kg) mouldy barley poultry, necrosis of mucosa in the Greenway &
horse, proventriculus and Puls (1976)
pig oesophagous of geese
T-2 toxin commercial feed cow haemorrhages Hibbs et
al. (1975)
DAS maize pig vomiting Vesonder et
al. (1973)
T-2 toxin corn meal horse oral lesions, haemorrhages, Szathmary
(6 out of 58 died) (1983)
T-2 toxin alfalfa horse inappetence, listlessness, Szathmary
(13 out of 31 died) (1983)
T-2 toxin poultry oral lesions, inappetence, Szathmary
death (1983)
DAS and T-2 toxin corn cattle death Szathmary
(1983)
DAS oat, sifting pigeon emesis, bloody stools Szathmary
(1983)
T-2 toxin (2.5 mg/kg) chicken feed broiler inflammations, "atrophies" Szathmary
chicken (1983)
DAS (150-300 mg/kg) cattle, pig haemorrhagic syndrome Cirilli
(1983)
T-2 toxin (50-150 mg/kg) pig, cattle blood stools (swine, cattle) Cirilli
ear necrosis (swine) (1983)
intestinal lesions (poultry)
hepatic lesions (swine)
----------------------------------------------------------------------------------------------------
Table 22. LD50 values (mg/kg) of trichothecenesa
----------------------------------------------------------------------------------------------------------
Type Trichothecenes Mouse Rat Guinea-pig
iv ip sc oral iv ip sc oral ip sc oral
----------------------------------------------------------------------------------------------------------
A T-2 toxin 5.2 10.5 5.2 3.06
HT-2 toxin 9.0
Diacetoxyscirpenol (DAS) 12 23.0 1.3 0.75 7.3
Neosolaniol 14.5
Monoacetoxyscirpenol 0.725
B Nivalenol (NIV) 7.3 7.4 7.2 38.9
Fusarenon-X 3.4 3.4 4.2 4.5 4.4 0.5 0.1
Diacetylnivalenol 9.6
Deoxynivalenol (DON) 70.0 46.0
3-Acetyldeoxynivalenol 49.0 34.0
Trichothecin 300 250
D Roridin A 1.0
Verrucarin A 1.5 0.5 0.87
Verrucarin B 7.0
Verrucarin J 0.5
----------------------------------------------------------------------------------------------------------
Table 22 (contd.)
-----------------------------------------------------------------------------------------------------
Type Trichothecenes Rabbit Cat Dog Pig Duckling Day-old Chick Trout
(iv) (sc) (iv) (iv) (sc) chick (oral) (oral)
(oral)
-----------------------------------------------------------------------------------------------------
A T-2 toxin 0.5 1.21 1.75 4.0 6.1
HT-2 toxin 6.25
DAS 1.0 ca 1.1 0.37
3'-OH HT-2 toxin 8.5 5.0
15-Acetyl-T-2 tetraol 10.0
T-2 tetraol 10.0
B Fusarenon-X 5.0 ca 2.0
DON 27.0
Acetyldeoxynivalenol 37.0
D Verrucarin A 0.54
-----------------------------------------------------------------------------------------------------
a Adapted from: Ueno et al. (1983) and Ryu et al. (1988).
The toxic potency of the trichothecenes varies depending on the
modification of side chains in the molecule. The acute lethal
toxicity of certain trichothecenes was investigated using a single ip
injection in mice and the LD50 values (mg/kg body weight) were:
verrucarin A and B, 0.5; fusarenon-X, 3.4; NIV, 4.1; T-2 toxin, 5.2;
HT-2 toxin, 9.0; diacetylnivalenol, 9.6; neosolaniol
(8-hydroxydiacetoxyscirpenol), 14.5; DAS, 23.0; acetyldeoxynivalenol,
49.0; DON, 70.0; and crotocin, 810.0.
Matsuoka et al. (1979) investigated the general effects of
fusarenon-X on mice and rats. Fusarenon-X induced hypothermia, but
did not induce appreciable behavioural changes in mice. In ether-
anaesthetized rats, it caused a rise in blood pressure and a decrease
in respiratory rate, but did not induce any significant effects on
cardiac rate, the muscle cell membrane, or nerve elements.
The administration of trichothecenes to some animals (rats, mice,
and guinea-pigs) induces diarrhoea. The mechanism of this sign was
investigated using fusarenon-X and rats (Matsuoka & Kubota, 1981).
The ip injection of fusarenon-X in rats caused watery diarrhoea
within 36-60 h. At necropsy, 24 h after injection of fusarenon-X,
the small intestine was distended, but no blood was found in the
lumen of the intestine. The mycotoxin increased the absorption rate
of D-xylose from the intestine in vitro (Matsuoka & Kubota, 1981).
The leakage of intravenously injected Evan's blue dye into the
intestine also increased, but the sodium level in the serum
decreased. The intestinal villi were shortened and there was
extravasation of erythrocytes in the intestinal lamina propria. The
diarrhoea induced by ip admin-istration of 1.0 mg fusarenon/kg in
male Wistar rats was not mediated by the cyclic nucleotide system as
the mycotoxin did not increase the cyclic GMP and AMP contents in the
intestinal mucosa (Matsuoka & Kubota, 1987). The permeability of
abdominal blood vessels was increased in a dose-dependent manner in
mice given an ip injection of fusarenon-X, and the peak was reached
about 8 h after injection. The increased permeability was not
mediated by serotonin, histamine, norepinephrine, prostaglandins,
leukotrienes, or thromboxanes (Matsuoka & Kubota, 1987).
The effects of fusarenon-X and T-2 toxin on intestinal absorption
of monosaccharides were studied in rats. The absorption of 3- O-
methyl-glucose was reduced 1-3 times after either toxin was injected
into the jejunal lumen. Absorption of 3- O-methyl-glucose was also
reduced after the toxins were given by intravenous injection. Both
toxins impaired jejunal function by causing specific damage in the
active transport and diffusional movement of monosaccharides (Kumagai
& Shimizu, 1988).
Vomiting was one of the most significant signs of trichothecene-
induced toxicosis in the cat, dog, pig, and duckling (Ueno, 1980a).
T-2 toxin and related trichothecene mycotoxins at doses of 0.1-10
mg/kg induced vomiting (Vesonder et al., 1973; Sato et al., 1975;
Yoshizawa & Morooka, 1977; Ueno, 1980b; Matsuoka & Kubota, 1981).
The presence of the causal factor, DON, in mouldy corn was
established using ducklings as the assay animal (Ueno et al., 1974).
In pigs given 0.5 mg/kg body weight by infusion, vomiting commenced
6-7 min after dosing. Emesis or retching occurred at intervals of 2-
15 min and lasted from 0.5 to 2 min (Coppock et al., 1985). It is
strongly suggested that the mechanism of the vomiting of
trichothecenes is their possible action on the chemoreceptor trigger
zone (CTZ) in the medulla oblongata (Matsuoka et al., 1979). The iv
administration of 0.3 mg fusarenon-X/kg body weight to dogs induced
emesis and vomiting, 5-15 min after injection. Vomiting after
injection of fusarenon-X was prevented by prior administration of 0.5
mg metoclopramide hydrochloride/kg body weight or 1 mg
chloropromazine hydrochloride/kg.
The cardiovascular effects of the trichothecenes have varied
according to such factors as species, dose, and duration of exposure.
In acute studies, T-2 toxin given intravenously at 1 mg/kg body
weight produced a decline in blood pressure several hours after
administration, the reduced blood pressure being accompanied by a
decrease in heart rate (Smalley et al., 1970). A single dose of T-2
toxin administered to guinea-pigs and rabbits resulted in a decrease
in systemic blood pressure and a decrease in heart rate (Parker et
al., 1984; Wilson, 1984).
Using the in vitro bovine ear perfusion system, it was determined
that T-2 toxin can cause a dose-dependent vasoconstrictor response in
peripheral vasculature, but that the toxin is a less potent
vasoactive agent than either histamine or norepinephrine. The
presence of known histamine or noradrenergic anatogonist did not
affect the response to the toxin (Wilson & Gentry, 1985). T-2 toxin
administered systemically produced a marked increase in peripheral
vascular resistance in the conscious rat. The cardiac output
gradually decreased eventually resulting in cardiovascular collapse
and death (Feuerstein et al., 1985).
(a) Swine
The acute and short-term toxicities of T-2 toxin, DAS, and DON
were investigated in pigs (Weaver et al., 1978a,b; Coppock et al.,
1985). A single dose LD50 of T-2 toxin dissolved in ethanol and
administered iv was 1.21 ± 0.15 mg/kg body weight in normal, healthy,
crossbred pigs weighing from 3 to 50 kg. Soon after administration,
emesis was followed by eager consumption of feed, moderate posterior
paresis, staggering gait, extreme listlessness, and frequent
defecation of normal stools. Between 1 and 6 h, severe posterior
paresis, knuckling-over of the rear feet, and extreme lethargy were
observed. These signs were followed by severe posterior paresis,
frequent falling because of hind-quarter weakness, and the dragging
of both rear legs while moving about. Twenty-four hours after
administration, the surviving pigs appeared normal. Similar clinical
signs were observed in pigs exposed to T-2 toxin through inhalation
(Pang et al., 1988). Pathologically, necrosis was present in the
epithelial cells of the mucosa and in the crypt cells of the jejunum
and ileum, the Peyer's patches of the ileum, the lymphoid elements of
the caecum, the lymphoid follicles in the spleen, and the germinal
centre of the mesenteric lymph node (Weaver et al., 1978a).
Young pigs were fed with T-2 toxin at 1, 2, 4, or 8 mg/kg
standard pig ration for 8 weeks. No statistically significant
differences in body weight gain and feed consumption were observed
between the treated animals and the controls. Young pigs refused a
ration containing 16 mg T-2 toxin/kg, but not a diet containing 10-12
mg/kg. The no-observed-effect level was estimated to be less than 1
mg/kg, based on differences in body weight gain (Weaver et al.,
1978b). In terms of clinical haematological changes, such as
haemorrhaging, blood cell counts, serum-enzyme activities, and serum-
protein levels, the no-observed-effect level could not be accurately
determined, but was higher than 12 mg/kg, based on the weight gain.
The intravenous administration of T-2 toxin to pigs at doses of 4
or 8 mg/kg resulted in a shock syndrome characterized by reductions
in cardiac output and blood pressure and increased plasma
concentrations of epinephrine, norepinephrine, thromboxane B2, 6-
keto-PGF1a and lactate (Lorenzana et al., 1985). The pigs in the
high-dose group produced such signs as persistent vomiting, watery
diarrhoea, abdominal straining, cold extremities, coma, and death.
Eighteen white cross-bred female pigs weighing 40-60 kg,
immunized against erysepelas, were administered purified T-2 toxin
dissolved in 70% ethanol, intravenously, in doses of 0 (5 pigs), 0.6
(5 pigs), 1.2 (1 pig), 4.8 (5 pigs) and 5.4 (2 pigs) mg/kg. The
animals administered doses of 4.8 and 5.4 mg/kg died between 5 and
10.5 h later and other groups were killed 12-24 h after treatment.
Gross lesions were observed in pigs given 1.2 mg/kg or more and these
consisted of oedema, congestion, and haemorrhages of the lymph nodes
and pancreas and congestion and haemorrhages of the gastrointestinal
mucosa, subendocardium, adrenal glands, and meninges. Histological
alterations confirmed the gross lesions. Other lesions were
widespread degeneration and necrosis of lymphoid tissue and the
surface and crypt epithelium of the intestines. Scattered foci of
necrosis were present in the pancreas, myocardium, bone marrow,
adrenal cortex, and the tubular epithelium of the renal medulla.
Most lesions were dose dependent. The T-2 toxin-induced lesions in
the lymphoid and gastrointestinal tract of pigs were similar to those
described in other species. The heart and pancreas were additional
target organs in pigs (Pang et al., 1987b).
Male, castrated, crossbred, specific pathogen-free pigs (17
controls and 17 treated), 9-11 weeks of age, were used in a study
to characterize the pulmonary and systemic responses to inhaled T-2
toxin (nebulized dose of 9 mg/kg given by endotracheal tube) (Pang et
al., 1987a). The animals were exposed to the aerosol in pairs, one
animal receiving the toxin, the other acting as a control. From 20
to 30% of the toxin was retained by the pigs (T-2 toxin was mixed
with 100-200 µCi technetium for measurement). Five pairs of animals
each were killed 1, 3, and 7 days after dosing. Two pairs were
designated a 0.33 day group, when one treated pig died and the
other was killed in a moribund state, 0-10 h after dosing.
Clinically, the T-2 toxin-treated pigs vomited after exposure
producing such signs as cyanosis, anorexia, and lethargy. The pigs
became laterally recumbent. Alveolar macrophages showed reduced
phagocytosis and the blastogenic responses to mitogen were reduced
for pulmonary lymphocytes, but not for lymphocytes of the peripheral
blood. The lesions in pigs that died included multifocal
interstitial pneumonia, necrosis of lymphoid tissue,
necrohaemorrhagic gastroenteritis, oedema of gall bladder mucosa, and
multifocal areas of necrosis in the heart and pancreas. Inhalation
exposure to T-2 toxin produced a clinical and morphological syndrome
resembling that produced by intravenously administered T-2 toxin, at
doses of 1.2 mg/kg (approximate LD50) or more, as well as death.
Furthermore, the lesions produced by the inhaled toxin were more
severe.
The acute effects of DAS were studied using single, intravenous
doses (range: 0.30-0.48 mg/kg body weight) in 13 crossbred pigs;
there were 2 control pigs (Weaver et al., 1978b). Seven of the
toxin-exposed pigs developed emesis, frequent defecation, lethargy,
staggering gait, and prostration by 10 h leading to death. Severe
haemorrhagic necrotizing lesions and mucosal congestion involved the
jejunum and ileum and large intestines, portions of which were blood-
filled at necropsy. Lymphoid follicular necrosis was present in the
lymph nodes and spleen. The LD50 value was found to be 0.376 ± 0.043
mg/kg body weight.
Two female crossbred pigs were administered DON by rapid
intravenous infusion at a dose of 0.5 mg/kg body weight; there were
two matching controls. Vomiting was observed within a few minutes
of dosing, the skin was flushed and the extremities became cold.
Pigs had signs of diarrhoea, muscular weakness, tremors, and coma.
Symptoms were progressive in severity reaching a maximum 6-7 h after
the injection; recovery occurred after 12 h. Necrosis of pancreatic
acinar and islet cells was observed (Coppock et al., 1985). Pigs can
ingest up to 2 mg DON/kg feed without suffering any serious toxic
effects (Trenholm et al., 1984). T-2 toxin added to the ration at 18
or 30 mg/kg caused the refusal of feed in pigs (Szathmary & Rafai,
1978). Feed refusal and emesis have been produced in other species
and by other toxic metabolites of Fusarium species (Kotsonis et al.,
1975; Vesonder et al., 1977).
The minimum emetic dose of DON in pigs weighing 9-10 kg was 0.05
mg/kg body weight, when administered intraperitoneally, and 0.1-0.2
mg/kg body weight, when given orally. When this toxin was added to
feed, the feed consumption of 20-45 kg pigs, was reduced by 20% at a
dose of 3.6 mg/kg and by a 90% at a dose of 40 mg/kg (Forsyth et al.,
1977). Pigs were about twice as sensitive to DON as rats (Vesonder
et al., 1979). DON in contaminated wheat reduced feed intake and
weight gain, when it was fed to the pigs. The intake of feed
decreased linearly with increasing dietary concentration of DON
(Friend et al., 1982). The emetic activity of 15-acetyl DON in pigs
was similar to that of DON, the minimum emetic doses being 75 and 50
µg/kg, respectively (Pestka et al., 1987a).
(b) Poultry
Chi et al. (1977b), reported that the single oral LD50 dose of
T-2 toxin for one-day-old broiler chicks was 5 mg/kg body weight. It
was 5 and 6.3 mg/kg body weight for 8-week-old broiler chicks and
laying hens, respectively. Death of the birds occurred within 48 h
of T-2 toxin administration. Within 4 h of receiving the toxin,
birds developed asthenia, inappetence, diarrhoea, and panting. The
abdominal cavities of birds given lethal doses contained a white
chalk-like material that covered much of the viscera.
In a study by Wyatt et al.(1972), chickens were fed a diet
containing 1-16 mg T-2 toxin/kg feed for 3 weeks. The birds with
reduced growth at 4, 8, and 16 mg/kg developed yellow-white lesions
in the mouthparts at all dietary concentrations. The lesions
consisted of a fibrinous surface layer and a heavy infiltration of
the underlying tissues by granular leukocytes. Escherichia coli and
Staphylococcus epidermis were isolated from the lesions.
Terao et al.(1978) observed the effects of T-2 toxin and related
trichothecenes on the bursa of Fabricius of one-day-old chicks.
After injection of 5 mg T-2 toxin/kg body weight into the residual
yolk sac, cellular toxic effects were observed on the follicle-
associated epithelium, resulting in necrosis, which spread to the
periphery. The lesions induced by fusarenon-X and NIV were similar
to those induced by T-2 toxin, but the toxins were less potent and
their activity was estimated to be more than 40 times less than that
of cyclophosphamide.
The acute toxicity of DAS and of T-2 toxin dissolved in
dimethylsulfoxide in 7-day-old male broiler chicks was described by
Hoerr et al. (1981). The 72-h single oral LD50 doses of T-2 toxin
and DAS were estimated to be 4 and 5 mg/kg body weight, respectively.
Combination of the 2 toxins caused increased mortality in both the
single-and multiple-dose tests. Lesions produced by crop gavage with
T-2 toxin and DAS were similar, but were more severe in chicks given
T-2 toxin. Necrosis of lymphoid tissue and bone marrow was observed
in tissue taken 1 h after treatment followed by rapid depletion.
Necrosis was observed in the liver, gall bladder, and gut.
Chi et al.(1977c), fed broiler chicks (36 per group), aged one
day to 9 weeks, a diet containing T-2 toxin at concentrations of 0.2,
0.4, 2, or 4 mg/kg. Birds fed 4 mg T-2 toxin/kg showed reduced body
weight gain and feed consumption and developed oral lesions
characterized by circumscribed proliferating yellow caseous
plaques at the margin of the beak, the mucosa of the hard
palate and the tongue, and the angle of the mouth. No lesions were
observed in the bone marrow or, to any significant extent, in the
peripheral blood. The no-observed-effect doses of T-2 toxin were 0.2
mg/kg for weight gain, and 0.2 mg/kg for oral lesions. When one-day-
old broiler chicks were fed a diet containing 1, 2, 4, 8, or 16 mg T-
2 toxin/kg feed for 3 weeks the no-effect doses were estimated as
follows: growth rate, weight of pancreas, and weight of spleen: 2
mg/kg; oral lesions: <1 mg/kg (Wyatt et al., 1973c).
T-2 toxin administered to laying hens at a concentration of 20
mg/kg feed reduced egg production and resulted in the production of a
thinner egg shell (Wyatt et al., 1975). Speers et al.(1977), also
observed cessation of egg production in hens fed diets containing
25-50 mg monoacetoxyscirpenol/kg or 16 mg T-2 toxin/kg. It was
reported by Chi et al. (1977a) that feed consumption, egg production,
and shell thickness were significantly decreased in hens fed 8 mg of
T-2 toxin/kg. Furthermore, the hatchability of fertile eggs of hens
fed 2 or 8 mg T-2 toxin/kg was lower than that of hens fed the
control diet (Chi et al., 1977a).
Three groups of 1-day-old chicks (10 chicks per group) were each
fed 0.5-15 mg T-2 toxin/kg for 3 weeks (Coffin & Combs, 1981).
Plasma-vitamin E activity and hepatic-vitamin A content were
measured. Dose-dependent depression of plasma-vitamin E activity was
observed, with a 65% decrease compared with controls in chicks fed a
diet containing 15 mg T-2 toxin/kg. This decrease was believed to be
caused by a reduction in the plasma level of lipoproteins, which are
required for the transport of vitamin E.
(c) Ruminants
In a study by Pier et al. (1976), 4 calves received 0.08-0.6 mg
T-2 toxin/kg body weight orally in capsules for 30 days. The high-
dose calf developed a hunched stance and died on day 20. At all
levels, some evidence of mild enteritis with loose faeces was
obtained. Clinically apparent signs were confirmed at doses of 0.16
mg/kg or more, and bloody faeces at doses of 0.32 mg/kg or more. At
necropsy, abomasal ulcers were present in the calf given 0.16 mg/kg
and ruminal ulcers in calves given the 2 higher doses. Prothrombin
times and levels of serum GOT activity were increased in calves given
the 2 higher doses.
Ten male Suffolk-Finn-Columbian lambs, in 2 groups of 5 animals
each, were fed T-2 toxin at 0.3 or 0.6 mg/kg body weight for 21 days.
There were 5 controls. Experimental lambs developed focal hyperaemia
and dermatitis at the mucocutaneous junction of the commissure of the
lips, diarrhoea, leukopenia, lymphopenia and lymphoid depletion of
the mesenteric lymph nodes and spleen (Friend et al., 1983b).
Sheep dosed with roridin A and verrucarin A (4 mg/kg) had severe
and extensive haemorrhagic gastroenteritis. Oedema was marked in the
abomasum; the small intestine of the roridin-treated lamb had casts
of clotted blood and necrotic debris. Both small and large
intestines contained grossly haemorrhagic areas and extensive mucosal
erosions. In the lamb given verrucarin A at 4 mg/kg body weight, the
lesions were sublobular haemorrhages in the liver, which had a nutmeg
appearance, mucosal erosions, haemorrhages in the small intestine,
and haemorrhages of the endocardium of the left ventricle of the
heart (Mortimer et al., 1971).
(d) Cats
Three studies have described the clinical and tissue alterations
produced by administration of T-2 toxin to cats (Sato et al., 1975;
Lutsky et al., 1978; Lutsky & Mor, 1981). Lutsky et al. (1978) used
20 cats in 4 groups of 4-6 animals each. There were 4 controls. The
toxin was administered orally in gelatin capsules on alternative days
at doses of 0.06, 0.08, or 0.10 mg/kg body weight, until death. The
survival time ranged from 6 to 40 days. The signs included emesis,
anorexia, bloody diarrhoea, and ataxia. The cats lost weight and
became emaciated. Gross lesions included multiple petechiae to
ecchymotic haemorrhages of the intestinal tract, lymph nodes, and
heart. The lumen of the gut contained copious amounts of dark red
contents. Microscopic lesions included haemorrhages in the gut,
lymph nodes, heart, and mininges, necrosis of gastrointestinal
epithelium and decreased cellularity of the bone marrow, lymph nodes,
and spleen.
(e) Rodents
The trichothecenes used in long-term studies were T-2 toxin and
fusarenon-X. Lesions were observed in the oesophageal region of the
stomach of DDD mice fed T-2 toxin at 10 or 15 mg/kg diet for 12
months. The alterations included hyperplasia, hyperkeratosis, and
acanthosis of the squamous epithelium. Such changes were found 13
weeks after the start of feeding the toxins and were consistently
observed during the 12-month feeding period. However, most had
subsided 3 months after cessation of feeding. Similar gastric
lesions were observed in Wistar rats fed T-2 toxin at concentrations
of 5, 10, or 15 mg/kg feed for 4 weeks. The lesions were diffuse and
severe in the rats fed 15 mg/kg, focal but definite in those fed 10
mg/kg, and negligible in the stomach of rats fed 5 mg/kg (Ohtsubo &
Saito, 1977).
Six female Holtzman albino rats were fed T-2 toxin at 5 or 15
mg/kg for 19 days and T-2 toxin at 10 mg/kg diet for 8 months. No
gastric lesions were observed in any of the animals in the
experimental groups (Marasas et al., 1969).
Three groups of 12, six-week-old female Swiss ICR mice (15-20 g
body weight) were administered T-2 toxin (by 10-minute aerosol
exposure) Two control groups contained 8 mice each using nose-only
exposure. The aerosol mass concentration varied between 225 and 275
µg T-2 toxin/litre of air. Tissues from mice were microscopically
examined 0.25, 1, 2, 4, 6, 8, 12, and 24 h after exposure. Lymphoid
necrosis was observed 1 h after exposure in the thymus, spleen, and
lyphoid nodules of the intestinal tract. Necrosis of intestinal
crypt epithelial cells was present 2 h after exposure and necrosis of
adrenal cortical cells 4 h after exposure (Thurman et al., 1988).
In male Sprague-Dawley rats, T-2 toxin, given intravenously,
produced reduced blood flow and increased vascular resistance in
hind-quarter, mesenteric, and renal vascular beds. Mean arterial
pressure and heart rate were not significantly altered. A maximum
drop in blood flow in mesenteric and renal vascular beds occurred 4 h
after the T-2 toxin was injected (Siren and Feuerstein, 1986).
II.4.2.2 Haematological and haemostatic changes
A haemorrhagic syndrome was reported to be the characteristic
feature of mouldy corn toxicosis in the cow (section II.4.1.).
However, this hemorrhagic syndrome could not be produced in other
studies.
In a study by Patterson et al. (1979), 2 calves were administered
0.2 mg T-2 toxin/kg body weight and one calf was given the same dose
of DAS; both compounds were given by stomach tube,daily for 11 days.
There were no controls. The T-2-treated animals developed clinical
signs of weakness, inappetence, and one died. Prothrombin time was
prolonged in both animals and one had marked neutrophilia. No
clinical signs or haematological changes were observed in the animal
administered DAS. No haemorrhagic syndrome was found in these
calves.
When pigs (9-10 weeks old, male, castrated, specific pathogen-
free) were exposed to a T-2 toxin aerosol (390 µg/litre, 15 µm mass
median aerodynamic diameter) for a period that allowed an amount
equivalent to 8 mg/kg to be nebulized, the haematological alterations
included a decrease in lymphocyte and neutrophil counts, and
decreased concentrations of serum-protein and haemoglobin (Pang et
al., 1988).
Haematological changes were observed in mice, rats, cats, and
guinea-pigs treated with T-2 toxin and related trichothecenes (Sato
et al., 1975, 1978; Sato & Ueno, 1977; DeNicola et al., 1978). In
cats, leukocytosis occurred early after the administration of T-2
toxin. A similar change was observed in mice treated with T-2 toxin,
neosolaniol, and fusarenon-X. Among the leukocytes, lymphocytes
showed the greatest increase followed by neutrophils; the
leukocytosis was followed by marked leukopenia after short-term
exposure to T-2 toxin. This leucopenic state was also induced by DAS
in mice (Conner et al., 1986), rats and dogs (Stahelin et al., 1968)
and by verrucarin A in rats, dogs, guinea-pigs, and monkeys (Rusch &
Stahelin, 1965). Pancytopenia was also reported in cats administered
T-2 toxin for 2 weeks (Lutsky et al., 1978; Lutsky & Mor, 1981). In
guinea-pigs treated with T-2 toxin (0.9 mg/kg body weight per day)
for 27 days, erythropenia, leukopenia, and absolute lymphopenia were
observed, with a marked decrease in the lymphocyte contents of the
bone marrow (DeNicola et al., 1978).
Hayes et al. (1980) studied the effects of T-2 toxin on the
haematopoiesis in mice. Twenty-four male weanling outbred Swiss mice
were fed a balanced semipurified diet, containing crystalline
purified T-2 toxin at a level of 20 mg/kg dry diet. One group of 20
animals received the toxin in the diet for 41 days and another group
of 4 animals, for 21 days, followed by control diet for 7 days.
Forty-eight animals in 3 groups served as controls and received the
semipurified diet with restricted intake (20 animals for 41 days),
and ad lib (8 animals for 28 days). In addition, 12 animals were
sacrificed at day 0. Haematological studies were made at weekly
intervals. During the first 3 weeks of exposure to T-2 toxin,
lymphoid tissues, bone marrow, and splenic red pulp became
hypoplastic resulting in anaemia, lymphopenia, and eosinopenia.
Subsequently, during continued exposure to T-2 toxin, there was
regeneration leading to hyperplasia of the haematopoietic cells by 6
weeks. All animals also developed perioral dermatitis and ulceration
of the gastric mucosa. The above results indicate both the irritant
and haematopoietic suppressive effects of the T-2 toxin. However,
the haematopoietic effects were transient at the dose administered
and did not lead to haematopoietic failure.
The haemostatic derangements produced by T-2 toxin have been
studied in the guinea-pig (Cosgriff et al., 1984), rabbit (Gentry,
1982), chicken (Doerr et al., 1981), and monkey (Cosgriff et al.,
1986). Guinea-pigs (Hartley strain, number not stated) admin-istered
T-2 toxin dissolved in ethanol, by intramuscular injection, at a dose
of 1 mg/kg body weight (LD50-24 h) developed decreased activities of
all coagulation factors except fibrinogen. Platelet aggregation in
whole blood response to ADP and collagen was depressed. The animals
also showed an initial rise followed by a fall in the haematocrit
level, leukocytosis, and a fall in platelet count. These changes,
which were found within a few hours of toxin administration, reached
a maximal at 24 h and returned to normal over the next 2 days.
Pretreatment with vitamin K1 did not prevent the effects of T-2 toxin
on coagulation. The addition of T-2 toxin to the plasma and blood of
untreated guinea-pigs at a concentration of 1 mg/litre did not have
any effect on clotting times or platelet aggregation, indicating that
the T-2 toxin itself did not have any direct effect on the activ-ity
of coagulation factors (Cosgriff et al., 1984).
Eight New Zealand White rabbits were administered T-2 toxin
dissolved in dimethyl sulfoxide (DMSO) by intravenous injection at
0.5 mg/kg body weight; 5 rabbits were given a single oral dose of 2.0
mg/kg body weight. In the rabbits treated intravenously, both the
packed cell volume and the total leukocyte counts were reduced.
However, no significant alterations occurred in the haematological
parameters of rabbits given the T-2 toxin orally (Gentry & Cooper,
1981).
In another study, 9 New Zealand White rabbits were given a single
intravenous injection of T-2 toxin dissolved in DMSO at a dose of 0.5
mg/kg body weight. A second group of 5 animals received daily
subcutaneous injections of vitamin K, at a dose of 0.5 mg/kg body
weight, for 5 days prior to administration of a similar dose of T-2
toxin and for a subsequent 4 days. A total of 16 rabbits in 2 groups
served as controls. Blood samples were examined from each animal
before toxin or DMSO treatment and 6-96 h later. Several coagulation
factors (VII, VIII, IX, X, XI) were decreased by about 40% within 6 h
of toxin administration in the group administered toxin alone.
Fibrinogen content was elevated at 24 h. However, the reduction
in the coagulation factors did not induce clinical haemorrhage and
administration of vitamin K did not alter the effects of T-2 toxin
administration, indicating that the mechanism of action of the toxin
on coagulation was not as a vitamin K antagonist.
In a study by Cosgriff et al. (1986), 9 Cynomolgus monkeys
received an intramuscular injection of an LD20 dose (0.65 mg/kg body
weight) of T-2 toxin dissolved in ethanol. Three monkeys served as
controls. Haematological studies were made before toxin injection
and at different intervals from 6 to 24 h and 2 to 7 days after
treatment. The animals were studied for signs of toxicity and
particularly for evidence of haemorrhage. Necropsy was performed on
animals that died during study. Leukocytosis levels in treated
animals were 4-5 times pretreatment levels. Prolongation of
prothrombin, activated thromboplastin times, and a decrease in
multiple coagulation factors were also observed. These changes were
detected within hours of toxin administration, reached a maximum at
24 h, and returned to normal over the next 3 days. Fibrin-fibrinogen
degradation products were not detected at any time. Platelet counts
which were unchanged in treated animals, were significantly raised in
control animals following repeated phlebotomies. None of the animals
developed the haemorrhagic syndrome. Five animals that died during
the study showed mild peticheal haemorrhages involving the colon and
heart, as well as necrosis of lymphoid tissues.
Rukmini et al. (1980) conducted a study on adult rhesus monkeys
in which 3 males and 2 females were administered pure T-2 toxin in
20 ml milk by stomach tube, daily, initially at 1 mg/kg body weight
for 4 days, and then at 0.5 mg/kg body weight from day 5 to day 15.
Three males and 3 females served as controls. All 3 males in the
treated group died of respiratory failure between days 0 and 15.
Subsequently, after 30 days recovery, the 2 treated female and 2
additional male monkeys received 0.1 mg T-2 toxin/kg body weight for
15 days. All monkeys given 1 mg T-2 toxin/kg per day showed signs of
toxicity similar to those of alimentary toxic aleukia in man, i.e.,
vomiting, apathy, and weakness of lower limbs. The signs were more
severe in males, and they also developed peticheal haemorrhage on the
face. All male animals developed severe leukocytopenia, follicular
atrophy of the spleen and lymph nodes, and pneumonia, suggesting
involvement of the immune system. At a dose of 0.1 mg/kg per day,
both male and female animals developed leukocytopenia and mild
anaemia after 15 days of treatment.
The effects of T-2 toxicosis on blood coagulation were studied in
groups of 40, day-old chickens fed diets containing the toxin at
concentrations of 1, 2, 4, 8, or 16 mg/kg. Forty animals served as
controls. Factor X, and prothrombin and fibrinogen activities were
reduced only at the highest dietary dose, whereas Factor VII was
reduced at dietary doses of 4, 8, and 16 mg/kg and was the most
sensitive of the clotting components to T-2 toxin toxicosis. Thus,
T-2 toxin toxicosis induced by high doses results in multiple-factor
coagulopathy and mild toxicosis results in a deficiency of Factor VII
(Doerr et al., 1981).
II.4.2.3 Disturbances of the central nervous system
Four-week-old male broiler chickens were intubated with a single
dose of T-2 toxin at 2.5 mg/kg body weight, and the brain
concentrations of dopamine, norepinephrine, and serotonin, and
selected blood components were determined 4-48 h after
administration. There was a significant elevation in the brain-
dopamine concentration and a reduction in the brain-norepinephrine
concentration. The brain-serotonin contents did not change (Chi et
al., 1981). Batches of 40 broiler chickens fed graded concentrations
of 1-16 mg T-2 toxin/kg feed for 3 weeks developed an abnormal
positioning of the wings, hysteroid seizures, and impaired
righting reflex. Neural toxicity, which occurred at levels above 4
mg T-2 toxin/kg diet, might have been related to alterations in brain
biogenic amines (Wyatt et al., 1973a,b; Chi et al., 1977a).
Signs of nervous system dysfunction (restlessness, dyspnoea,
ataxia) were observed in rats after subcutaneous or intracerebral
injection of T-2 toxin (10-20 µg toxin) or after intracerebral
implantation of toxin adsorbed on talc (Bergmann et al., 1985).
Weanling, male Wistar rats were administered T-2 toxin orally at 2.0
mg/kg body weight and the concentrations of neurotransmitters
determined. The toxin increased concentrations of tryptophan,
serotonin, and dopamine in the brain, but decreased concentrations of
3,4-dihydroxyphenylacetic acid (MacDonald et al., 1988). Male
Sprague-Dawley rats (180 g) were dosed orally with DON or T-2 toxin
at 21.5 mg/kg body weight. Both the toxins significantly increased
serotonin and 5-hydroxy-3-indoleacetic acid concentrations in all
regions of the brain examined, whereas norepinephrine and dopamine
concentrations were not altered (Fitzpatrick et al., 1988). Male
Sprague-Dawley rats received 1 mg T-2 toxin/kg body weight by
intravenous injection. Concentrations of vasopressin, oxytocin, and
leucine enkephalin decreased in the posterior pituitary and
concentrations of methionine enkephalin increased (Zamir et al.,
1985).
Male Sprague-Dawley rats (180 g) and 4-week-old White Leghorn
cockerels were dosed orally with DON at 2.5 mg/kg body weight. Whole
brain concentrations of monoamine neurotransmitters were not altered
in either species. The treatment produced elevated concentrations of
serotonin and 5-hydroxy-3-indoleacetic acid in the rat, but not in
the chicken (Fitzpatrick et al., 1988).
II.4.2.4 Dermal toxicity
After the discovery of the skin-necrotizing property of toxic
metabolites of F. sporotrichioides and related fungi (Joffe, 1962),
the skin irritation test was introduced for the screening of toxins
and metabolites of Fusarium species (see section II.1.2.3). T-2
toxin, HT-2 toxin, and DAS were isolated from cultures of F.
tricinctum using the skin test for selection of active fractions
(Gilgan et al., 1966; Bamburg et al., 1968b). Toxins, such as T-2
toxin, HT-2 toxin, and DAS are extremely potent irritants while NIV
and fusarenon-X are much less so (Bamburg et al., 1968b; Ueno et al.,
1970; Wei et al., 1972; Chung et al., 1974; Hayes & Schiefer, 1979;
Bhavanishankar et al., 1988).
The mechanism of the skin toxicity of trichothecenes has not been
established. Results of studies with fusarenon-X have indicated that
the vascular permeability of the skin of the back of the rabbit,
estimated by exudation of a vital dye (pontamine sky blue), was
biphasic reaching maxima 5 and 24 h after topical application. These
data indicate that increased vascular permeability is one of the
early responses of the skin to these toxins and that some chemical
mediators participate in the biphasic increase in vascular
permeability (Ueno, 1980a).
II.4.2.5 Impairment of immune response
Experimental animal studies show that some trichothecenes affect
the immune system and thereby modify the immune response. The
impairment comprises the following functions: antibody formation;
allograft rejection; delayed hypersensitivity; and blastogenic
response to lectins. As a consequence of the impairment, decreased
resistance to microbial infection has been experimentally
established. It is likely that the impairment of the immune system
is linked to the inhibitory effect of trichothecenes on macromolecule
synthesis.
(a) Antibody formation
Rosenstein et al. (1979) showed that T-2 toxin and DAS inhibited
responsiveness to sheep red blood cells in male Swiss IC and C5781/6
mice. In their first study, T-2 toxin or DAS was injected ip daily
for 7 days at 0.75 mg/kg body weight, in 6 groups of 4 mice each,
with matching controls. Mice were immunized with sheep erythrocytes
(SRBC) on day 3 after treatment and killed 5 days after immunization.
Both toxins produced a fall in anti-SRBC titres measured by
haemagglutination and reduced thymic weight. In a second study, 7
groups of 5 mice each were administered daily (ip) doses of T-2 toxin
ranging from 0 (solvent alone) to 2.5 mg/kg body weight over a 7-day
period. The same number of mice received DAS under similar
conditions. Mice were immunized on day 3 and killed 5 days later.
Antibody-producing cells from the spleen were counted by numbering
the plaque-forming cells (PFC) on sheep erythrocytes. A dose-
dependent inhibition of PFC was observed in T-2 toxin-treated mice,
with a total suppression of the immune response at 2.5 mg/kg. The
effects of DAS were less. A subsequent follow-up of the evolution of
the immune response in 36 mice administered T-2 toxin (ip) at 0.75
mg/kg body weight daily for 7 days indicated that the immunosuppressive
effect disappeared within 6 days followup cessation treatment.
The T-cell-independent-responses-production of anti-
polyvinylpyrrolidone and anti-dinitrophenol-ficoll-antibodies were
enhanced by T-2 toxin and DAS. T-2 toxin-treated mice produced 50%
fewer plaque-forming cells against SRBC. There was also a decreased
response to phytohaemagglutinin in splenic cells from treated mice.
However, Masuko et al. (1977), and Otokawa et al. (1979), reported
that a single dose of 3 mg T-2 toxin/kg body weight in mice caused
modification of delayed hypersensitivity responses without affecting
antibody response. This apparent contradiction was explained by a
difference in the timing of the administration of T-2 toxin, mice
receiving the toxin once, several days before or after antigen-
stimulation. Results of studies with fusarenon-X indicated that both
IgE and IgG anti-body responses to DNA-OVA were suppressed when male
BALB/c mice were repeatedly dosed with mycotoxin at doses exceeding
25 mg/day; the inhibition of antibody-formation was greater when
given 7 days before antigen-stimulation. In mice stimulated with
pokeweed mitogen and lipopolysaccharides, the in vitro antibody
production by splenic cells from fusarenon-X-treated mice was
suppressed (Masuda et al., 1982).
Sato et al. (1981), examined the effects of fusarenon-X on
serological responses in chicks inoculated with Newcastle disease
vaccines. When chicks were fed 8 mg fusarenon-X/kg for 6 weeks, no
significant reductions in body or organ weights were observed.
However, haemagglutinin inhibitory antibody titres were reduced when
chicks were immunized with live, but not with inactivated, vaccine.
Mann et al. (1983), reported alterations in the levels
of several serum proteins in calves orally administered T-2 toxin
(0.6 mg/kg per day over 43 days). Total protein, albumin, and
immunoglobulin fractions were decreased in toxin-treated calves,
including the alpha-beta1-and beta2-globulin fractions. IgA and IgM
values and complement proteins were lower in treated calves.
Sublethal doses of DON (0.25, 0.50, or 1.0 mg/kg feed) were fed
for 54 weeks, beginning at 21 days of age, to a total of 96 weanling
male Swiss Webster mice divided into 4 groups. There were 32
controls. The dose of 1.0 mg/kg reduced serum alpha1- and alpha2-
globulins, increased serum-albumin levels, and reduced feed
consumption and body weight gain. The dose of 0.5 mg/kg reduced
alpha2- and beta-globulins (Tryphonas et al., 1986).
T-2 toxin was fed to 6 weaned pigs at 5 mg/kg feed for 25 days
and the immune response evaluated by in vitro testing for blast
transformation, immune-rosette formation, and IF-detectable IgG-
positive cell counts. T-2 toxin produced a 40-50% reduction in
immune responsiveness and a decrease in total leukocyte count, but an
increase in adrenocortical activity. Neutralizing antibody titres to
vaccination with enteritic B vaccine were lower in the treated pigs.
It was concluded that T-2 toxin had a distinct immunosuppressive
effect during the early phase of immune induction by altering the
function of both T- and B-lymphocytes (Rafai & Tuboly, 1982).
Dietary DON (2, 5, or 25 mg/kg feed for 2 or 8 weeks) depressed
the plaque-forming response to sheep erythrocytes in splenic cultures
from B6C3F1 mice. Some effect on the plaque-forming response was
detectable with both the 2- and the 8-week period of feeding (Pestka
et al., 1987b). DON, given by gavage at 0.75, 2.5, or 7.5 mg/kg body
weight also reduced serum-IgM response to sheep erythrocytes and
plaque-forming cell numbers were lower in the treated groups
(Tryphonas et al., 1984).
(b) Allograft rejection
Observations on the inhibition of cellular immunity by
trichothecenes have included responses to grafting. According to
Rosenstein et al. (1979), the mean survival time of the skin grafted
from C57Bl/6 mice on to Swiss mice was 8.69 days in the control
recipients. However, when the recipients were treated with 0.75 mg
T-2 toxin/kg per day for 7 days before skin graft and then 3 times a
week for 20 days, the mean survival time of the graft was increased
to 12 days, indicating that T-2 toxin suppressed certain steps of
immunity resulting in allograft rejection. The areas of the graft in
T-2 toxin-treated mice lacked the typical cellular infiltrates of a
cell-mediated immune response of macrophages and lymphocytes.
(c) Delayed hypersensitivity
Delayed hypersensitivity (DH) is an immune response mediated by
sensitized T lymphocytes. The possible impairment of T lymphocytes
by T-2 toxin was studied in female BDF1 mice sensitized by the sc
injection of sheep erythrocytes (SRBC) followed by estimation of foot
pad swelling. When mice received 3 mg T-2 toxin/kg body weight,
before, or on the day of, sensitization, no appreciable effect on DH
was observed. However, when the toxin was administered 2 or 3 days
after sensitization, marked enhancement of the delayed
hypersensitivity response was seen (Otokawa et al., 1979). This
indicates that the timing of toxin exposure was critical for
enhancement of the delayed hypersensitivity response. Since the
life-time of the effective T-2 toxin dose in vivo was very short,
and the optimal timing of toxin injection corresponded with the time
of appearance of suppresser cells, it was presumed that trichothecenes
might interfere with the proliferation of suppresser T cells that
appear in DH-tolerant mice.
DON fed to B6C3F1 mice at 2, 5, or 25 mg/kg for 2 or 8 weeks
depressed the delayed hypersensitivity response to keyhole limpet
haemocyanin. The effects on hypersensitivity were detectable in mice
fed the mycotoxin for 2 weeks, but disappeared when the feeding
period was extended to 8 weeks (Pestka et al., 1987b).
(d) Blastogenic response to lectins
Certain mitogens, such as phytohaemagglutinin (PHA) and
concanavalin A, stimulate the proliferation of T cells in vitro;
lipopolysaccharide (LPS) causes the same phenomena in B cells.
Lafarge-Frayssinet et al. (1979) investigated the responses of
lymphocytes to PHA and LPS in mice treated with crude T-2 toxin and
DAS. Mice were treated with the mycotoxins at doses of one-quarter
of the LD50 or one-twelfth of the LD50 for 15 days and the response
of splenic or thymic cells to the mitogens was examined. The data
indicated that stimulation of both T and B cells was inhibited
reversibly, and that the ability to synthesize anti-SRBC antibodies
was suppressed. In vitro effects on lymphocytes and fibrosarcoma
cell cultures included a direct cytostatic action at high
concentrations and a stimulating action at low concentrations.
Histopathological observations included severe lymphoid damage in the
thymus and spleen. The results of these studies indicate that the
immune system appears sensitive to the trichothecenes and is impaired
at doses not inhibitory for other organs.
Swiss mice were fed a diet containing T-2 toxin at 5, 10, or 20
mg/kg for 1, 2, 3, 4, or 6 weeks. The ingestion of T-2 toxin (only
at 20 mg/kg at 3 weeks) depressed total splenic cell counts. T-2
toxin at 20 mg/kg for 1-4 weeks decreased splenic proliferative
responses to T-cell mitogen concanavalin A; however, the response to
a lipopolysaccharide (LPS), B-cell mitogen, was decreased in mice fed
T-2 toxin at 10 or 20 mg/kg for 1-4 weeks. (Friend et al., 1983a).
Lymphocytes from calves exposed to T-2 toxin at 0.6 mg/kg for as
long as 43 days had a decreased response to the mitogen, PHA, on days
1, 8, and 29 after toxin administration. Lymphocyte responses to
concanavalin A and pokeweed mitogen were also observed on day 29
after dosing (Buening et al., 1982).
In a study to determine the effects of T-2 toxin on the bovine
immune system, calves (5) were orally dosed with 0.3 mg/kg per day,
for 56 days. Neutrophil function was reduced by treatment with T-2
toxin as was the cutaneous reaction to injected phytohaemagglutinin.
In a second study, calves (6) were given T-2 toxin at a dose of 0.5
mg/kg per day, for 28 days. B-lymphocyte number and the response of
the B-cell-enriched fraction to phytohaemagglutinin both increased
after treatment. The in vitro exposure of mononuclear cells, B-cell-
enriched or T-cell enriched fraction, reduced the lymphoblastic
response to mitogens. A 50% reduction was induced by 1.4 ng T-2
toxin/ml (Mann et al., 1984).
The effects of T-2 toxin on in vitro mitogen response and
antibody production by human peripheral blood lymphocytes were
reported by Tomar et al. (1988). The toxin inhibited the mitogen
response to concanavalin A at a lower concentration (1.6 mg/ml)
compared with phytohaemagglutinin (2-4 mg/ml) and pokeweed mitogen.
In the presence of the toxin, inhibition reached a maximum during
first 8 h. The results indicate that various subpopulations of
lymphocytes have different susceptibilities to T-2 toxin.
Mitogen-induced blastogenesis in cultured human lymphocytes was
inhibited by T-2 toxin and its metabolites. The concentrations of T-
2 toxin, HT-2, 3'-OH T-2,3'-OH HT-2, T-2 triol, and T-2 tetraol
toxins that produced 50% inhibition of 3H-thymidine uptake in
mitogen-stimulated human peripheral lymphocytes were 1.5, 3.5, 4.0,
50.0, 150.0, and 150.0 ng/ml, respectively. The initial hydrolysis
of T-2 toxin to HT-2 toxin and the hydroxylation to 3'-OH T-2 did not
significantly decrease the immunotoxicity (Forsell et al., 1985).
Other trichothecenes were less toxic than T-2 toxin in this system.
The doses that produced 50% inhibition of 3H-thymidine uptake in
mitogen-stimulated human lymphocytes for fusarenon-X, NIV, DON, and
15-AcDON were 18, 72, 140, and 240 ng/ml, respectively. These
results indicate that the lymphotoxicity of trichothecenes is related
to the C-4 substituent (Forsell & Pestka, 1985).
T-2 toxin was examined for its effects on lymphocyte activation
and interleukin-2 production by splenic cultures from mice. Splenic
cells were taken from female BALB/c mice given 2 mg T-2 toxin/kg body
weight by stomach tube for 4 days or 4 mg T-2 toxin/kg by stomach
tube in single dose. Cells were incubated with 1 µg concanavalin A
and the synthesis of cellular protein and DNA determined. The single
dose of 4 mg/kg did not alter lymphocyte activation, but the dose of
2 mg/kg for 4 days produced a 50% reduction in activation. The
supernatant from these cells had 4 times greater interleukin-2
activity (Holt et al., 1988a).
DON and 3-AcDON were evaluated in vitro for their effects on
mitogen-induced lymphocytic blastogenesis using rat or human
peripheral blood lymphocytes. Both mycotoxins produced a dose-
dependent reduction in lymphocytic proliferation and DON produced a
greater inhibitory effect than the acetylated compound. Thus, the
concentrations of DON producing 50% inhibition of blastogenesis were
90 and 220 ng/ml for rat and human lymphocytes, respectively. The
values for 3-AcDON were 450 and 1060 ng/ml, respectively (Atkinson &
Miller 1984).
(e) Resistance to infection
The immunosuppressive effect of trichothecenes has resulted in an
increased incidence and severity of infection in animals in several
studies. According to Boonchuvit et al. (1975), an increased
mortality rate was recorded when 40 chickens were fed a diet
containing 16 mg T-2 toxin/kg for 1 week and were then inoculated
orally with 1 x 108 cells of Salmonella.
The depression of resistance to experimental tubeculosis by T-2
toxin was studied in mice by Kanai & Kondo, (1984). Groups of male
mice, strain ddY, 18-20 g body weight, with 10-14 animals per group,
were administered mycobacteria by intravenous injection in the tail
vein. In the first study, the doses injected consisted of 0.01 mg
culture of tubercle bacteria per animal (species not indicated). One
group was administered 0.1 mg T-2 toxin per animal a total of 12
times orally, starting on the day before the injection, 7 times with
one-day intervals, and then 5 times daily. For comparison, a second
group of mice was administered 5 mg cortisone acetate per animal ip
under a similar time schedule. A third group was injected with
tubercle control bacteria only. At the end of the 20-day observation
period, the mice in the first group had a lower spleen weight and a
higher tubercle bacteria count in the spleen than those in the other
2 groups, indicating a more pronounced depression of resistance by T-
2 toxin than by cortisone. In a second study, two groups of animals
were injected with 0.25 mg of a culture of Mycobacterium bovis; one
group was then administered 0.1 mg T-2 toxin per animal daily for 6
days, starting 8 days after injection. There were two groups of
controls. The average survival time in the T-2 toxin-treated group
was reduced to 19 days, compared with 35 days in the untreated group,
indicating decreased resistance.
Rats were injected ip with T-2 toxin in a single dose of 1
mg/kg body weight or in doses of 0.5 mg/kg daily for 5 days.
The rats were then inoculated with 0.1 ml of medium
containing 109 Staphylococcus aureus/ml. Rats given
multiple intramuscular injections of T-2 toxin showed more
oedema and myofibre necrosis at the injection site of the
bacteria; the cellular infiltrate was sparse and bacteria
were abundant. Bone marrow myeloid cells were markedly
decreased by multiple injections of T-2 toxin. In in vitro
studies, small, non-lethal doses of T-2 toxin inhibited
the chemotaxis of leukocytes and decreased phagocytosis of
the bacteria by leukocytes (Yarom et al., 1984b).
Seven male rhesus monkeys (Macaca mulatta) were dosed daily by
stomach tube for 4-5 weeks with 100 µg T-2 toxin/kg body weight.
This dose resulted in the death of 3 animals, 40% reduction in
leukocyte counts, reduction in the bactericidal activity of
neutrophils (phagocytosis of E. coli), reduction in the
transformation of lymphocytes by mitogens, and a reduction in numbers
of C-cell and T-cell lymphocytes (Jagadeesan et al., 1982).
The immunotoxic effects of T-2 toxin on cell-mediated resistance
were studied in female ICR mice infected with Listeria monocytogenes.
Mice in groups of 17 animals (10 animals in the control group) were
inoculated ip with 4 x 105 (LD50) or 4 x 104 (non-lethal) doses of L.
monocytogenes per animal, treated with a single oral dose of 4 mg T-2
toxin/kg body weight, and observed for 15 days. Bacterial
multiplication was rapid in the spleen after T-2 toxin treatment and
mortality was increased in both treated groups. Necrosis and
depletion of lymphoid tissue were observed in the thymus, the
periarterioler lymphoid sheaths and the lymphoid follicles of the
spleen. Cellular response to L. monocytogenes in the spleen and
liver was decreased by treatment with T-2 toxin and the lesions were
sparsely populated with mononuclear cells. The foci of necrosis were
larger with numerous colonies of bacteria. The influx and number
of lymphocytes and macrophages were greater in Listeria-elicited
peritoneal exudates. The immunotoxic effects of T-2 toxin were
comparable with those produced by cyclophosphamide and were
attributed to depletion of T lymphocytes and subsequent failure of T-
cell-dependent macrophages to clear the host of bacteria (Corrier &
Ziprin, 1986a). In a continuation of the previous study, female ICR
mice (17 animals per group) were inoculated ip on day 1 with 4 x 105
(LD50) or 4 x 104 (non-lethal) bacteria per animal, treated orally on
day 0, 1, 2, and 3 with 0, 1, or 2 mg T-2 toxin/kg, and observed for
15 days. The suppression of resistance by the mycotoxin was
indicated by rapid multiplication of Listeria in the spleen and
increased mortality in mice in both exposed groups treated with 2
mg/kg. The thymuses and spleens of toxin-treated mice showed
necrosis and depletion of lymphoid cells. Foci of necrosis induced
by Listeria infection in the spleen and liver were larger in treated
mice and the inflammatory reaction was sparse (Corrier & Ziprin,
1986a,b).
Increased resistance to L. monocytogenes infection was
surprisingly observed by the same group (Corrier & Ziprin, 1986b) in
mice administered T-2 toxin several days prior to the inoculation
with bacteria. Female ICR mice, 16 animals per group, were
administered T-2 toxin by stomach tube at dosage levels of 2, 1, 0.5,
or 0 mg toxin/kg body weight, on days -5, -4, -3, -2, -1, +1 and +3.
On day 0 the two treated groups were inoculated ip with 106 (LD100)
and 105 (LD50) L. monocytogenes, respectively. In addition, 20 mice
were given 2 mg T-2 toxin/kg on the same days as above, and used to
determine the effect of the toxin. Although the cytotoxic effect of
T-2 toxin on lymphoid tissue was marked, enhanced resistance to
Listeria infection was revealed by a decrease in mortality due to
listeriosis (in both bacteria-exposed groups) in a T-2 toxin dose-
dependent way. No specific cause for the increased resistance to
listeriosis by T-2 toxin treatment prior to bacterial infection was
identified by the authors.
ICR female mice were treated with the trichothecene mycotoxin DAS
and subsequently inoculated ip with Listeria monocytogenes. The
effect of the mycotoxin on the course of the infection was monitored
by observing the resultant mortality and the bacterial content of the
spleens from inoculated mice. Mice given 3 mg DAS/kg body weight
orally, on 2 and 1 days before inoculation, showed increased
mortality and splenic Listeria counts. In these mice, thymus weights
were reduced, and lymphocytes were depleted from the thymus cortex
and from splenic lymphoid follicles and periarteriolar lymphoid
sheaths. A single dose of 4 mg DAS/kg given on day 6 before
challenge exposure did not affect mortality compared with controls.
Mice treated with DAS and subsequently inoculated with Listeria had
significantly ( P = 0.006) higher levels of neutrophil populations
than Listeria-infected control mice (Ziprin & Corrier, 1987).
Dietary DON decreased resistance of B6C3F1 mice to infection with
L. monocytogenes. Resistance to the infection was similarly
decreased in control mice fed restricted diets, comparable to dietary
restriction caused by DON-induced feed refusal. Resistance to L.
monocytogenes was reduced to a greater extent by feed containing both
DON and zearalenone (Pestka et al., 1987b). DON in the diet at 0.50
or 1.0 mg/kg was fed for 5 weeks. Both doses resulted in a dose-
related decrease in time-to-death interval following challenge with
L. monocytogenes (Tryphonas et al., 1986).
T-2 toxin was fed to young male white Swiss mice at doses of 10
or 20 mg/kg diet for 2-3 weeks. The mice were then inoculated ip
with herpes simplex virus (HSV-1). Mice fed the high dose of T-2
toxin were highly susceptible to HSV-1 infection and about 75% died
with extensive hepatic and adrenal gland necrosis and with little or
no inflammatory cellular reaction in affected tissues, such as the
liver and adrenal glands, and the central nervous system. No
necrotizing encephalitis was found in treated mice. Mice fed 10 mg
T-2 toxin/kg had lesions of intermediate severity between those of
the high-dose group and the virus-infected controls (Friend et al.,
1983c). Feeding of T-2 toxin at 5, 10, or 20 mg/kg for 3-6 weeks did
not reactivate the virus in mice latently infected with HSV-1 (Friend
et al., 1983a).
Mice (male, Swiss strain weighing 25 g) received inoculations
(route not stated) of Cryptococcus neoformans (1 x 106 cells) and ip
doses (1/8 or 1/4 LD50) of DAS on days 5, 6, and 7 after inoculation
with the fungal cells. No deaths occurred in either the C.
neoformans-treated groups or the T-2-toxin group. A marked additive
effect on mortality was observed when mice received both C.
neoformans and T-2 toxin (Fromentin et al., 1981).
II.4.2.6 Carcinogenicity
The IARC (1983) studied the experimental data on the
carcinogenicity of T-2 toxin and concluded that no evaluation of the
carcinogenic role of T-2 trichothecene in experimental animals could
be made, because of the inadequacy of experimental data.
In a 16-month feeding study, groups of 50 male and 50 female
weanling CD-1 mice were fed a semi-synthetic diet containing 1.5 or
3.0 mg T-2 toxin/kg. Survival was lowest in the control group. No
statistically recognizable differences were found in feed consumption
or body weight gains among the groups. Statistically significant
differences were found in the incidence of pulmonary adenomas and
hepatic adenomas in the males of the 3.0 mg/kg group and the
controls. Other treatment-related findings were an increased
prevalence of epithelial cell hyperplasia and hyperkeratosis in the
stomach of animals fed the T-2 toxin diets (Schiefer et al., 1987).
In an attempt to determine the carcinogenicity of NIV, a study
was conducted in which mice were fed mouldy rice for 2 years. Groups
of 42, 7-week-old female C57BL/6CrSlc SPF mice were fed diets
containing 6, 12, or 30 mg NIV/kg for 2 years, and were assessed for
the effects on body weight gain, feed efficiency, terminal organ
weights, haematological values, and lesions. The mortality was
lowest in the highest dose group, followed by the 12 mg/kg group;
body weight gains and feed efficiency were dose-dependently reduced.
No particular neoplasms attributable to treatment were found. The
incidence of naturally occurring neoplasms, mostly lymphomas, was
similar in all groups. On gross and microscopic examination of the
liver, thymus, spleen, kidneys, stomach, small intestines with or
without Peyer's patches, no alteration related to treatment was
observed except for amyloidosis, which was lower in the two higher
dose groups (Ohtsubo et al., in press).
Two groups of 16 or 18 DDD male mice received either 10 or 20
weekly sc injections of 2.5 mg fusarenon-X/kg body weight. No
increase in tumour incidence was noted in treated animals, compared
with the controls (Saito & Ohtsubo, 1974).
In a feeding study, fusarenon-X, at a dose of 3.5 or 7 mg/kg diet
was fed to 151 male Donryu rats for 1-2 years. Treatment with the
mycotoxin reduced the growth rate, but did not induce any
carcinogenic effects (Saito et al., 1980).
In studies to investigate skin tumour induction using T-2 toxin
and DAS, the skin of the back of mice was painted with 0.1-1 mg of
trichothecenes, twice a week, for one year. A notable finding was
necrosis of the skin, but no tumours were detected. The skin tumour
induction test was also carried out using the initiator-promotor
procedure. T-2 toxin and fusarenon-X were not promoting agents of
dimethylbenz (a)anthracene-induced skin neoplasia. According to
Lindenfelser et al. (1974), neither T-2 toxin nor DAS served as
initiating agents.
II.4.2.7 Mutagenicity
In a Rec-assay using Bacillus subtilis, DNA damage was not
induced by 20 or 100 µg of either T-2 toxin or fusarenon-X (Ueno &
Kubota, 1976). Such trichothecenes as T-2 toxin, DAS, and DON were
not mutagenic to Salmonella typhimurium strains TA98, TA100, TA1535,
TA1537, and TA1538, with or without S-9 fraction from rat liver
(Kuczuk et al., 1978; Ueno et al., 1978; Wehner et al., 1978a).
T-2 toxin and DAS were not mutagenic in the D-3 mitotic
recombination test with Saccharomyces cerevisiae (Kuczuk et al.,
1978).
Neither DNA-strand breakage nor induction of 8-azaguanine-
resistant mutation were detected with 1-32 mg fusarenon-X/litre in
Hela cells and with the same toxin at 0.1-1.0 mg/litre in FM3A cells
derived from C3H mouse mammary carcinoma cell line (Umeda et al.,
1972, 1977). On the other hand, Lafarge-Frayssinet et al. (1981)
reported that T-2 toxin induced single-strand breaks in the DNA of
lymphoid cells in vivo (3 mg/kg body weight) and in vitro (0.7-5
ng/ml). Such DNA breakage was not observed in hepatic cells.
T-2 toxin, NIV, and fusarenon-X produced weak clastogenic effects
in Chinese hamster V79-E cells (Thust et al., 1983). Both T-2 toxin
and HT-2 toxin inhibited the incorporation of tritiated
thymidine into the DNA of human fibroblasts in culture in a dose-
related fashion. Non-toxic and toxic doses of DON (0.1-1000
mg/litre), did not significantly increase unscheduled DNA synthesis
in primary cultures of rat hepatocytes (Bradlaw et al., 1985).
Norppa et al. (1980) also reported weak induction of chromosomal
aberrations by T-2 toxin (1.7, 2.7, or 3.0 mg/kg body weight) in
Chinese hamster bone marrow cells. The bone marrow micronucleus test
was negative at a dose of 3 mg T-2 toxin/kg body weight resulting in
a significant decrease in polychromatic erythrocytes.
Hamsters fed T-2 toxin for 6 weeks (2.5 mg/kg body weight) did
not have more chromosomal aberrations than controls. The clastogenic
potential of T-2 toxin was very weak. In hamster V79 cells, DON at
levels of 2-3 µg/ml or more was cytotoxic, but was non-mutagenic at
the hypoxanthine-guanine phosphoribosyl transferase locus, with or
without hepatocytic mediated activation (Rogers & Heroux-Metcalf,
1983).
According to Reiss (1975), DAS induced various cytological
abnormalities including shortened chromosomes, enlarged nucleoli, and
a few chromosome breaks in the root tips of Allium cepa (common
onion) at concentration of 1000 and 100 µg/ml. T-2 toxin and
satratoxin H were C-mitotic poisons at concentrations exceeding 10
µg/g. Typical C-mitotic action on chromosomes morphology was
produced by both toxins and was comparable to that of colchicine. In
addition, T-2 toxin induced polyploidy (Linnainmaa et al., 1979).
T-2 toxin and satratoxin H were not mutagenic in a sex-linked
recessive lethal test in Drosophila (Sorsa et al., 1980). Lack of
potency to produce recessive lethal mutations in Drosophila was
consistent with negative results obtained in certain bacterial assays
in which trichothecenes were inactive as both base pair and frame
shift mutagens (Kuczuk et al., 1978; Ueno et al., 1978a).
II.4.2.8 Teratogenicity and reproductive effects
T-2 toxin was embryotoxic and teratogenic in mice. T-2 toxin
dissolved in propylene glycol was injected intraperitoneally into
pregnant mice on one of days 7-11 of gestation at doses of 0.5, 1, or
1.5 mg/kg body weight. T-2 toxin (doses of 1 or 1.5 mg/kg) caused
significant maternal mortality, fetal death, and fetal body weight
loss. Approximately 37% of the fetuses from dams given 1 (8 litters)
or 1.5 mg (4 litters) T-2 toxin/kg on day 10 were grossly malformed.
The most frequent anomalies were bent, shortened, or missing tails,
and limb malformations, including oligodactyly and syndactyly.
Exencephaly, open eyes, retarded jaw, and skeletal malformations of
the rib or vertebrae were also found in the fetuses (Stanford et al.,
1975).
Pregnant CD-1 mice (18 litters/treatment group) were administered
T-2 toxin dissolved in propylene glycol ip at 0.5 mg/kg body weight
on gestation days 8 or 10. The T-2 toxin produced grossly malformed
fetuses, principally with tail and limb anomalies. A higher
incidence of malformations was observed when a T-2 toxin dose of 0.5
mg/kg body weight was combined with an ochratoxin A dose of 4 mg/kg
body weight. An increase in fetocidal effects was found in offspring
of dams in groups treated with the high-dose combination, on either
day. Few skeletal and visceral malformations were noted (Hood et
al., 1978). T-2 toxin (0.5 mg/kg body weight) dissolved in 1:1
mixture of propylene glycol and 0.1N sodium bicarbonate was
administered ip alone or in combination with rubratoxin B (0.4 mg/kg
body weight) to pregnant CD-1 mice on day 1 of gestation. Only T-2
toxin resulted in gross malformations. The combination of toxins
increased the adverse effects on fetal body weight and mortality, but
not the incidence or severity of the gross malformations (Hood,
1986).
The teratogenicity of orally administered T-2 toxin dissolved in
propylene glycol was evaluated in a study using 350 female CD-1
mice and doses of 0.5, 1.0, 2.0, 3.0, 3.5, or 4.0 mg/kg body weight
given on day 9 of gestation with a single dose of 3.0 mg/kg on day 6,
7, 8, 10, 11, or 12 of gestation. In the first study, the doses of
3.5 and 4.0 mg/kg produced maternal deaths and toxicity; no fetuses
were produced by the dams in the 4.0 mg/kg dose group and
significantly fewer fetuses were produced by dams in the 3.5 mg/kg
dose group. More major and minor defects were seen in offspring in
the 3.0 mg/kg dose group. In the second study, the treated females
had greater fetal loss than controls and the greatest number of dead
fetuses occurred among litters treated on day 9 of gestation. Major
skeletal defects were more numerous in mice treated on day 7 of
gestation. The results indicated that a single oral dose of T-2
toxin in propylene glycol was primarily maternally toxic and
embryolethal; defective development was possibly secondary to
maternal toxicity (Rousseaux & Schiefer, 1987).
Fusarenon-X was embryotoxic, but not teratogenic (Ito et al.,
1980). Fusarenon-X dissolved in saline was given to pregnant DDD
mice by subcutaneous injection at doses of 0.63, 1.0, 1.6, 2.6, or
4.1 mg/kg body weight, or by feeding at concentrations of 5, 10, or
20 mg/kg diet during pregnancy. Two dams given a single sc dose of
4.1 mg/kg died within 24 h of injection. Abortion was induced in all
females by a single injection of 2.6 mg/kg on day 10 of gestation.
Smaller doses (0.63-1.6 mg/kg) produced a 16-20% abortion rate, when
given on day 10. Multiple doses (8-12 or 8-14 days of gestation) of
1.0 or 1.6 mg/kg produced 100% abortion. When the mice were fed a
diet containing 5, 10, or 20 mg fusarenon-X/kg throughout the
gestation period or in the early stages of gestation, the mycotoxin
inhibited embryonal implantation. Feeding fusarenon-X at 20 mg/kg
for 7 days during the middle stages of gestation induced abortion in
100% of dams. Fetal body weight was significantly reduced by the
administration of the mycotoxin, but no significant teratogenic
effects were observed in the fetuses of dams in either the
subcutaneous injection or feeding study (Ito et al., 1980).
The effects of NIV on fertilization, course of pregnancy, and
fetuses were examined in ICR mice. In a study by Ito et al. (1986),
pure NIV was injected ip in pregnant mice (groups of 10 animals
each), at dose levels of 0, 0.1, 0.5, or 1.5 mg/kg body weight per
day, on days 7-5 of gestation. The highest dose caused stillbirths
after vaginal haemorrhage in 6 out of 10 animals. High embryo
lethality was recorded in the 2 highest dose groups (88 and 48%). No
fetal malformations were observed in the treated groups. A single
administration of 3 mg/kg on day 7 affected the embryo within 10 h,
damaged the placenta within 24 h, and caused stillbirths at 48 h.
While NIV is embryotoxic, it is not teratogenic (Ito et al.,
1988). Thirty ICR mice in 3 batches of 10 animals each were fed
diets mixed with mouldy rice powder containing NIV at final levels of
6, 12, or 30 mg/kg feed per day throughout gestation. There were 11
controls. Purified NIV was also administered by gavage to 35 animals
in 4 groups at doses of 1-20 mg/kg body weight on days 7-15 of
gestation. There were 10 controls. Embryotoxicity associated
with maternal weight loss was observed in the groups receiving 30
mg/kg diet and 10 mg/ kg body weight per day, by gavage, whereas
lower levels, such as 5 mg/kg body weight per day, by gavage, did not
have any embryotoxic effects. Intrauterine growth retardation was
found at term in the fetuses of mice exposed to 12 mg/kg feed and 5
mg/kg by gavage. NIV did not have any significant adverse effects on
the incidence of gross skeletal and visceral malformations. As 5
mg/kg body weight per day given by gavage corresponds to a feed level
of approximately 35 mg/kg feed, the above data indicate that
exposure to 30 mg NIV/kg feed throughout the gestation period results
in embryotoxicity. However, exposure to approximately 35 mg/kg feed
during days 7-15 only of the gestation period does not induce
embryotoxic effects, which shows the significance of constant
exposure versus intermittent exposure to NIV.
DON was embryotoxic and teratogenic when dissolved in distilled
water and given for 4 consecutive days (days 8-11 of gestation), by
oesophageal intubation, to 15-19 pregnant Swiss-Webster mice (Khera
et al., 1982). The incidence of resorptions was 100% at doses of 10
or 15 mg/kg body weight, and 80% at 5 mg/kg body weight. The dose of
5 mg/kg reduced the number of live fetuses and reduced the average
fetal weight compared with the controls. Low incidences of skeletal
and visceral anomalies were found in the fetuses of the 1, 2.5, and 5
mg/kg groups. The skeletal malformations occurred in a dose-related
manner and included lumbar vertebrae with fused arches or partly
absent centra, and absent or fused ribs.
On the other hand, DON failed to produce embryotoxic and
teratogenic effects when fed ad lib at 0.5, 2.0, or 5.0 mg/kg to
Fisher 344 rats during the entire course of pregnancy (Morrissey,
1984). No overt signs of toxicity were observed in the dams and no
changes in maternal feed consumption were observed at any dose.
DON was fed to 71 adult female New Zealand White rabbits in 7
batches of 6-14 animals during the entire period of gestation at
doses of 0.3, 0.6, 1.0, 1.6, 1.8, or 2.0 mg/kg body weight. There
were 25 controls. The fetal effects consisted of 100% incidence of
fetal resorption in the females fed 1.8 and 2.0 mg/kg and reduced
average body weight of fetuses from dams fed 1.0 and 1.6 mg/kg.
These doses were not teratogenic (Khera et al., 1986).
Weanling F0 male and female mice were fed diets containing DON at
a dose of 2.0 mg/kg body weight (15 male and 15 female animals) and
0.375, 0.75, or 1.5 mg/kg body weight (7 males and 59 female
animals). There were 30 controls in the first study and 26 in the
second. After 30 days of dietary feeding, the mice were allowed to
mate and the pregnant females were allowed to litter normally. The
F1a progeny were examined up to 21 days of age and discarded. The F0
mice were rebred. The females bred to produce the F1b litters were
killed on day 19 of gestation and the fetuses were examined for
gross, visceral and skeletal malformations. Reductions were
observed in feed intake and in the body weight of F0 male and female
mice, the number of live pups and postnatal survivors, postnatal body
weight of F1a progeny, number of live fetuses, and fetal body weight
of F1b. No adverse effects on the fertility of F0 mice and no
major malformations in F1b fetuses were found. Results of cross-
fostering offspring between control dams and 1.5 mg/kg dams indicated
that both postnatal survival and body weight were adversely affected
by prenatal exposure as well as by combined pre- and post-natal
exposure (Khera et al., 1984).
Male and female Sprague-Dawley rats (3 groups of 15 male and 15
female animals each) were fed diets containing DON at levels of 0.25,
0.5, or 1.0 mg/kg body weight. Controls consisted of two groups of
15 males and 15 females. After 6 weeks of feeding, the rats were
bred. The mated females, maintained on their respective diets, were
killed on the last day of pregnancy and fetuses were evaluated for
effects on pre-natal development. No adverse effects were observed
except for dilatation of the renal pelvis and urinary bladder (Khera
et al., 1984).
Male (20/group) and female Sprague-Dawley rats (25/group) were
fed a diet containing 20 mg purified DON/kg for 60 and 15 days,
respectively, before mating. Rats consuming the DON-supplemental
diet throughout gestation and lactation did not show any clinical
signs of toxicity, but had reduced body weights. Only 50% of the
matings between toxin-fed rats resulted in pregnancy compared with
80% in the controls. No differences were detected among the groups
in sex ratio, survival rate, or average litter number and weight.
Pup weight gains in all groups were comparable up to post-natal day
14. From day 14 to 21, however, male and female pups of the control
group showed significantly improved weight gains compared with pups
from treated dams. No treatment-related histological abnormalities
were found in the testes or ovaries of treated pups (Morrissey &
Vesonder, 1985).
A 2-generation reproduction and teratology study was carried out
using 90 female CD-1 mice fed a semisynthetic diet containing the T-2
toxin at 1.5 or 3.0 mg/kg, concentrations considered possible under
field conditions. Results indicated that continuous feeding of T-2
toxin at low concentrations had minimal, if any, toxic effects on
female reproduction and fetal development and that T-2 toxin fed
continuously at 1.5 or 3.0 mg/kg was not teratogenic or fetocidal and
produced minimal effects on the growth rates of CD-1 mice (Rousseaux
et al., 1986).
II.4.3 Biochemical effects and mode of action
II.4.3.1 Cytotoxicity
In the early stages of research on trichothecenes, DAS was
isolated from F. scirpi as a phytotoxic principle (Brian et al.,
1961). Subsequent surveys confirmed the phytotoxic nature of the
trichothecenes using germination of seeds of Brassica oleracea L.
(Ueno et al., 1971c), germination of pea seedlings (Marasas et al.,
1971), growth of tobacco callus tissues (Helgeson et al., 1973), the
germination of tobacco plant (Nicotiana sylvestris) pollen
(Siriwardana & Lafont, 1978), and the auxin-promoted elongation of
soybean hypocotyl (Stahl et al., 1973).
Most of the trichothecenes tested have had fungistatic activity
in a wide range of species (Bamburg et al., 1968a; Bamburg & Strong,
1971; Reiss, 1973); DAS and verrucarin A were particularly potent in
inhibiting growth and sporulation at concentrations of 0.5-1
mg/litre.
Many of the trichothecenes have been cytotoxic to mammalian
cells, both in in vivo and in vitro. In animals administered
trichothecenes, the mucosa of the stomach and small and large
intestines had mucosal erosions, mucosal necrosis with ulceration,
and severe haemorrhages; radiomimetic effects included necrosis of
actively-dividing cells in the thymus, spleen, ovary, testis, and
lymph nodes (Saito & Ohtsubo, 1974). In a cell culture system, Grove
& Mortimer (1969) demonstrated the cytotoxicity of DAS and its
chemically modified compounds on hepatocytes of human origin and
hamster kidney cells. Ohtsubo et al. (1968), Ohtsubo & Saito (1970),
and Bodon & Zoldag (1974) described the cytotoxicity of NIV and
related trichothecenes for HeLa cells, and of T-2 toxin for
epithelial cells of pig kidney, respectively. The macrocyclic
trichothecenes, verrucarins and roridins, were highly cytotoxic for
0815 mouse tumour cells in the ng/ml range (Harri et al., 1962).
Tanaka et al. (1977), investigated the cytotoxicity of 20 types of
trichothecenes (11 type A, 6 type B, 1 type C, and 2 type D) for 3
cell lines, HeLa, HEK, and HL cells. The type D trichothecenes,
such as verrucarin A and roridin A had LC50s in the range of 0.003-
0.005 mg/litre; neosolaniol and NIV in the range of 0.1-1 mg/litre,
and deoxynivalenol in the range of 1-5 mg/litre. Trichodermol,
calonectrin, monoacetyldeoxynivalenol, and tetraacetylnivalenol were
weakly cytotoxic within the range of 5-10 mg/litre.
Chinese hamster ovary (CHO) and African green monkey kidney
(VERO) cell cultures were exposed to 0.01 or 1.0 ng T-2 toxin/ml for
1 or 12 h. The cells exhibited morphological changes considered to
be related to inhibition of protein synthesis. The alterations
included disassociation of polysomes and matrix density, ballooning
of intracristal space, and malalignment of cristae in mitochondria.
The CHO cells had bleb formations of plasma membrane, a change
produced by exposure to establish inhibition of protein synthesis
(Trusal, 1985).
II.4.3.2 Inhibition of protein synthesis
The initial observation that the trichothecene mycotoxins
inhibited protein synthesis in mammalian cells was made by Ueno et
al. (1968). They reported that NIV produced a dose-dependent
inhibition of incorporation of several amino acids into protein in
rabbit reticulocytes (Ueno et al., 1968) and Ehrlich ascite tumour
cells (Ueno & Fukushima, 1968). This inhibitory effect of the
trichothecene mycotoxins was observed in the whole animal (Ueno,
1970), a protozoan (Ueno & Yamakawa, 1970), HeLa cells (Liao et al.,
1976), cultured mammalian cells (Ohtsubo et al., 1968; Ohtsubo &
Saito, 1970), rat hepatocytes (Ueno et al., 1973b), hamster ovary
cells (Gupta & Siminovitch, 1978), human tonsil (Carrasco et al.,
1973), yeast spheroplasts (Stafford & McLaughlin, 1973; Cundliffe et
al., 1974; McLaughlin et al., 1977), rabbit reticulocytes (Ueno et
al., 1968, 1969a, 1973b,c; Ueno & Shimada, 1974; Wei & McLaughlin,
1974; Mizuno, 1975; Carter et al., 1976), and lymphocytes (Hartman et
al., 1978). No inhibitory effect on bacterial cells was observed
(Ueno et al., 1973b).
The effects of T-2 toxin on protein and DNA synthesis were
studied in Swiss mice and hepatoma cell cultures. T-2 toxin was
given as a single ip dose of 0.75 mg/kg and as a 3-day and a 7-day
daily treatment. The toxin inhibited protein synthesis after all 3
schedules of treatment and inhibition was present in cells obtained
from bone marrow, spleen, and thymus. Protein synthesis was
inhibited in vitro in hepatoma cell cultures and PHA-stimulated
lymphocytes (Rosenstein & Lafarge-Frayssinet, 1983).
The effects of T-2 toxin on rat hepatocytes were studied in
culture by the addition of several doses at either 1 or 12 h of
exposure. A dose of 0.01 ng T-2 toxin/ml produced a 75% inhibition
of protein synthesis within 1 h. At a higher dose of 1.0 ng/ml,
hepatocytes recovered from a 1-h but not a 12-h exposure. Cell
damage (release of lactate dehydrogenase) lagged behind inhibition of
protein synthesis, which was 90% at the 1 ng/ml dose. Ultrastructural
alterations were present in the endoplasmic reticulum and
mitochondria. Degranulation involved the rough endoplasmic
reticulum. Mitochondria had translucent foci and electron dense
cores (Trusal & O'Brien, 1986).
NIV inhibited poly-U, poly-A, and poly-C directed incorporation
of phenylalanyl-tRNA into phenylalanine without affecting the
activation of amino acids. The inhibition was presumably caused by
impairment of ribosomal function (Ueno et al., 1968). Fusarenon-X
caused breakdown of polysomes in rabbit reticulocytes (Ueno et al.,
1973b) and in mouse fibroblasts (L-cells), shortly after exposure
(Ohtsubo et al., 1972). The breakdown of polysomes was consistent
with the action of an inhibitor of initiation of protein synthesis.
T-2 toxin, DAS, and verrucarin A, but not trichodermin, also induced
the disaggregation of polysomes in HeLa cells (Liao et al., 1976).
These results were consistent with the effects of an inhibitor of
prolongation or termination of protein synthesis (Stafford &
McLaughlin, 1973). An important point was that the trichothecenes,
regardless of whether they were I-type or ET-type, interacted with
the peptidyl transferase centre on the 60S ribosomal subunit and
inhibited the transpeptidation of the peptide bond formation process.
Current research has focused on clarifying the molecular species
of ribosomal protein that regulates the binding of trichothecene
mycotoxins. A mutant of yeast (Schindler et al., 1974; Jimenez &
Vazquez, 1975; Jimenez et al., 1975; Grant et al., 1976; Carter et
al., 1980) and Chinese hamster ovary cells, resistant to the effects
of trichothecenes on protein synthesis (Gupta & Siminovitch, 1978),
have been isolated. Friend & Warner (1981) clearly demonstrated that
the gene for trichodermin resistance in yeast specifies ribosomal
protein L3, the largest of the yeast ribosomal proteins.
The ribosomal subunits of Myrothecium verrucaria, a producer of
macrocyclic trichothecenes, were resistant to T-2 toxin. It can be
assumed that the 60S subunits of eukaryotes are responsible for the
sensitivity to the trichothecenes. (Hobden & Cundliffe, 1980).
II.4.3.3 Inhibition of nucleic acid synthesis
The dose-dependent inhibition by NIV of DNA and RNA synthesis in
Ehrlich ascites tumour cells was first observed by Ueno & Fukushima
(1968). Inhibition (70%) of protein synthesis was induced by 1-10 mg
NIV/litre, and thymidine incorporation into DNA was inhibited by as
much as 60%; suppression (30%) of uracil incorporation into RNA was
slight. Similar results were obtained with several trichothecenes,
including trichodermin, diacetoxyscirpenol, and fusarenon-X, in other
cultured cells, such as KB cells (Ohtsubo et al., 1968), HeLa cells
(Liao et al., 1976), mouse L-cell fibroblasts (Ohtsubo et al., 1972),
hamster ovary cells (Gupta & Siminovitch, 1978), and lymphocytes
(Hartman et al., 1978).
The inhibition of DNA and RNA synthesis by trichothecenes
required higher concentrations of toxins than the inhibition of
protein synthesis and the extent of the inhibition was much less.
Thus, the observed inhibition of DNA and RNA synthesis in toxin-
treated animal cells was presumed a secondary effect of the
trichothecenes. This hypothesis was supported by the findings of
Tashiro et al. (1979) who reported that, in in vitro studies, a
concentration of 0.0236-1.889 mmol fusarenon-X/litre did not inhibit
DNA-dependent RNA hybridases of rat liver and Tetrahymena pyriformis.
Munsch & Mueller (1980) reported that the incorporation of 3H-
thymidine into DNA in cell lines from thymus was strongly inhibited
by over 10 ng T-2 toxin/ml and slightly inhibited by 0.1-10 ng T-2
toxin/ml. A low concentration of 0.1-1 ng/ml toxin caused a
transient increase in DNA polymerases, alpha- and beta-terminal
deoxynucleotidyl transferases. At a high dose of more than 1 ng T-2
toxin/ml, these enzymatic activities were strongly inhibited.
Rosenstein & Lafarge-Frayssinet (1983) described the depression of
DNA synthesis in vivo and in vitro. Three treatment schedules: a
single dose, 3 daily doses, or 7 daily doses of 0.75 mg T-2 toxin/kg
inhibited DNA synthesis in cell cultures from the spleen, thymus, and
bone marrow of treated mice. The mycotoxin also inhibited DNA
synthesis in vitro in cultures of hepatoma cells and in PHA-
stimulated lymphocytes (Rosenstein & Lafarge-Frayssinet, 1983).
Agrelo & Schoental (1980) found that hydroxyurea did not alter
unscheduled DNA synthesis in cells treated with T-2 toxin and HT-2
toxin (6 ng/ml). However, the combination of rat liver microsomes
and hydroxyurea resulted in an increase in unscheduled DNA synthesis
in cells exposed to 100 µg HT-2 toxin/ litre. The data of Agrelo &
Schoental (1980) suggest that the microsomal drug-metabolizing enzyme
system may participate in the induction of DNA damage by
trichothecenes.
The effects of T-2 toxin and DAS on DNA synthesis in phyto-
haemagglutinin, stimulated in the peripheral blood lymphocytes of
human beings, was assayed by incorporation of 3H-thymidine. Total
inhibition was obtained by 8 ng T-2 toxin and DAS and 80% was
obtained with 1.5 ng T-2 toxin and 2.7 ng of DAS (Cooray, 1984).
Fusarenon-X and related toxins inhibited protein and nucleic acid
synthesis in Tetrahymena pyriformis in a manner similar to that
observed in cultured mammalian cells (Ueno & Yamakawa, 1970). In
synchronously dividing Tetrahymena cells (Iwahashi et al., 1982), the
incorporation of radioactive amino acids, thymidine, and uracil into
protein, DNA, and RNA, respectively, was achieved by nearly the same
concentration of T-2 toxin. Inhibition of protein synthesis was
explained by the high affinity of T-2 toxin for 60S ribosomal
subunits of Tetrahymena, as is the case with cultured cells.
However, neither DNA and RNA synthesis nor RNA hybridase activity
were altered by T-2 toxin in an in vitro system using isolated nuclei
from normal cells or from cells pretreated with T-2 toxin. The
mechanism of inhibition of nucleic acid synthesis in the in vivo
system is not yet understood.
II.4.3.4 Alterations of cellular membranes
The trichothecenes as a group inhibit protein synthesis, but the
potency of the effect varies according to the trichothecene and the
system ( in vitro or in vivo) used to measure the inhibition. T-2
toxin was a potent inhibitor of protein synthesis both in vitro and
in vivo (Ueno et al., 1973b; Rosenstein & Lafarge-Frayssinet, 1983).
In vitro experiments were conducted to determine the effects of
T-2 toxin on the entry of sucrose into bovine erythrocytes,
entrapment of sucrose and inulin in carrier erythrocytes, entrapment
of T-2 and binding of T-2 toxin, and the measurement of cell
permeability to the entrapped T-2 toxin. At the highest
concentration of T-2 toxin (20 µg), no entry of 14C-sucrose or 3H-
inulin was observed. Very little 3H-T-2 toxin was bound to bovine
erythrocytes and binding was independent of T-2 toxin concentration.
The mycotoxin had no effect on the entrapment of sucrose or inulin.
Carrier erythrocytes retained 85% of 14C-sucrose and only 18% of
3H-T-2 toxin. Thus, T-2 toxin diffused from carrier cells much more
rapidly than sucrose. It was concluded that the interaction of T-2
toxin with bovine erythrocytes was minimal and intercalation with the
inner bilayer was not likely, because the increase in cell volume
that would have resulted did not occur (DeLoach et al., 1987).
The effects of T-2 toxin on membrane function were studied using
L-6 myoblasts. The minimal effective concentration (MEC) of T-2
toxin for reduction in uptake of calcium and glucose and for
reduction in uptake of leucine and tyrosine and their incorporation
into protein was 4 pg/ml. The uptake of rubidium was increased at
0.4 pg/ml and reduced at 4 pg/ml or more. Thymidine uptake and
incorporation into DNA had a biphasic response with an increase at
0.4 pg/ml and a reduction at 4 pg/ml for uptake and 40 pg/ml for
incorporation. Calcium efflux was reduced after 1, 5, and 15 min
exposure to T-2 toxin at a concentration of 40 pg/ml. These data
indicate that T-2 toxin has multiple effects on all membrane function
at very low concentrations and that these effects are independent of
inhibition of protein synthesis (Bunner & Morris, 1988).
II.4.3.5 Other biochemical effects
Fusarenon-X inhibited the uptake of phosphate by Tetrahymena
cells (Chiba et al., 1972). It caused a 50% inhibition of
incorporation of acetate into phospholipids and a ten-fold
stimulation of incorporation into triglyceride, effects that may be
secondary to inhibition of phosphate uptake. However, in rabbit
reticulocytes, fusarenon-X, T-2 toxin, and neosolaniol at
concentrations of 100 mg/litre did not inhibit Na+-dependent glycine
transport (Ueno et al., 1978b).
When SH-enzymes were pre-incubated with selected trichothecenes
in the absence of substrates, the activities of such enzymes as
creatine phosphokinase and lactate and alcohol dehydrogenases were
reduced (Ueno & Matsumoto, 1975). However, neither urease activity
(Reiss, 1977) nor rat liver lysosomal cathepsin activity (Farb et
al., 1976) was affected by diacetoxyscirpenol or T-2 toxin,
respectively, in vitro. Foster et al. (1975), reported the
reactivity of T-2 toxin with glutathione in the presence of epoxide-
S-GSH-transferase.
When mice were exposed to trichothecene mycotoxins, no detectable
alterations were observed in hepatic and renal functions. In chicks
fed a diet containing 10 mg T-2 toxin/kg for 3 weeks, a hepatic
microsomal aminopyrine, demethylase, was reduced by 29% compared with
the control, while another mixed function oxidase, aniline
hydroxylase, was not significantly affected (Coffin & Combs,
1981). In mice receiving a sublethal dose of fusarenon-X ip, a rapid
hypoglycemia was followed by depletion of hepatic glycogen (Shimizu
et al., 1979). The authors suggested that the toxin had induced a
malfunctioning of glucose absorption in the intestines and had
accelerated glycolysis. However, the trichothecene mycotoxins are
highly cytotoxic to the epithelia of the intestine, and impairment of
carbohydrate metabolism and absorption may be a secondary effect of
this cytotoxicity.
II.4.4 Structure-activity relationships
After the isolation and identification of numerous derivatives of
the trichothecenes, the structure-activity relationship was
investigated, on the basis of the information on lethal toxicity,
dermal toxicity, cytotoxicity, inhibition of protein synthesis, and
association with ribosomes.
The 12,13-epoxide of the trichothecenes is essential for their
biological activity. The de-epoxidation of DON and T-2 toxin by
rumen-microorganisms (King et al., 1984) and in mammalian systems
(Yoshizawa et al., 1983) results in loss of toxicity.
The macrocyclic ring contributes to the highly lipophylic
property of the trichothecenes, and the macrocyclic trichothecenes,
such as verrucarins, roridins, satratoxins, and baccharins, exhibit a
potent cytotoxicity towards cultured mammalian cells (Kupchan et al.,
1976, 1977; Jarvis et al., 1978, 1980).
In contrast, the polyhydroxylated alcohols, such as verrucarol,
NIV, and DON, are highly hydrophilic, resulting in a decrease in
cytotoxicity and dermal toxicity compared with their parent
trichothecenes (Ueno et al., 1970; Wei et al., 1974).
II.4.5 Prevention and therapy of trichothecene toxicosis
In studies to investigate effects of various dietary supplements
on T-2 toxin toxicity, weanling male Wistar rats in groups of 10,
with adequate controls, were fed diets containing 5% each of
bentonite, anion exchange resin, cation exchange resin, or
vermiculite-hydrobiotite with and without 3 µg T-2 toxin/g feed, for
2 weeks. Bentonite and anion exchange resin were most effective in
reducing the growth depression and feed refusal caused by T-2 toxin.
In a second study in which bentonite and anion exchange resin were
administered at levels of 2.5, 5.0, 7.5, or 10% of diet, 10%
bentonite in the diet was the most effective dietary supplement.
Dietary bentonite reduced the absorption of 3H-T-2 toxin and
increased faecal elimination (Carson & Smith, 1983).
Smectite, a clay composed of insoluble silicates of aluminum and
magnesium, incubated with T-2 toxin for 24 h before the toxin was
administered orally to mice (1 mg/kg per day), prevented the
acceleration of gastric emptying and transit time for a milk meal,
usually induced by administration of T-2 toxin. Smectite given
together with the toxin did not prevent the gastrointestinal effects
of T-2 toxin (Fioramonti et al., 1987). Male Sprague-Dawley rats
were given 15 or 5 mg/kg body weight of a platelet activating factor
antagonist, with and without T-2 toxin, by intravenous injection.
The drug prolonged the survival of conscious rats exposed to 0.65 mg
T-2 toxin/kg (Feuerstein et al., 1987).
The effects of certain drugs and metabolic inhibitors on the
toxicity of T-2 toxin have been studied in male ddY mice. The toxin
at a dose of 1.8 mg/kg was given subcutaneously and the drugs and
other chemicals were given ip. The lethal effects were reduced by
the steroid drugs, prednisolone and dexamethasone; survival times
were increased by the antihistaminic drug diphenhydramine, and an
opioid antagonist, naloxone. Prednisolone also reduced the
leukocytosis that developed after T-2 toxin treatment and decreased
the increase in ear weight caused by the mycotoxin. Other
antihistaminic and/or antiserotonic drugs used in the treatment were
found not to be effective in preventing the lethal effects of T-2
toxin (Ryu et al., 1987).
Male ddY mice and male Wistar rats in several groups of 10
animals each, pretreated with a radioprotective compound or with an
anti-inflammatory agent, were given T-2 toxin or fusarenon-X (1.5
mg/kg body weight). Out of the 20 compounds evaluated, only
prednisolone and hydrocortisone (100 mg/kg ip) were effective. They
reduced mortality from 90% in controls to less than 30% and they also
reduced the trichothecene-induced increase in intestinal fluid volume
(Mutoh et al., 1988).
Cutaneous irritation produced by T-2 toxin in 10 Porton female
rats was largely reduced by application, within 10 min, of an aqueous
soap solution when the dose was low (1.0 µg/cm2), but the solution was
largely ineffective over a time span of 60 min when the dose was
higher (100 µg T-2 toxin). Washing the site of application with
polyethylene glycol 300 was very effective in removing even large
doses (100 µg) of T-2 toxin from the skin (Fairhurst et al., 1987).
The median effective dose of oral superactive charcoal in
preventing deaths in batches of 5 female Sprague-Dawley rats was
0.175 g/kg. A dose of 1 g superactive charcoal/kg body weight
increased survival times and rates in rats given the lethal dose of 8
mg T-2 toxin/kg as long as 3 h after the toxin was administered by
gavage (Galey et al., 1987).
II.5 EFFECTS ON MAN
II.5.1 Contemporary episodes of human disease
Two outbreaks of trichothecene-related disease have been
reported, one in China in 1984/85 (Luo, 1988) and one in India in
1987 (Bhat et al., 1989). Each involved several hundred cases.
During the first incident, outbreaks of mouldy corn and scabby
wheat poisoning were reported. Out of approximately 600 persons who
consumed mouldy cereals, there were 463 cases of poisoning (77% of
the total). The latency period for the onset of symptoms was 5-30
min. These included nausea, vomiting, abdominal pain, diarrhoea,
dizziness, and headache. No deaths occurred. Pigs and chicks fed
the same mouldy cereals were also affected (Luo, 1988). GC-MS and
RIA were used in the analysis of 5 samples of the mouldy corn. DON
was detected within a range of 0.34-92.8 mg/kg and zearalenone within
a range of 0.004-0.587 mg/kg. T-2 toxin and NIV were not found. TLC
was used in the analysis of 19 samples of scabby wheat collected from
the affected and non-affected families. The DON content was 1.0-40.0
mg/kg, which was significantly higher than that in the non scabby
wheat samples. In addition to DON, zearalenone was detected in 2
samples, the contents of which were 0.25 and 0.5 mg/kg, respectively.
No T-2 toxin was found in the samples (Luo, 1988).
An analogous outbreak was reported in Kashmir, India, in 1987
(Bhat et al., 1987, 1989). It was ascribed to the consumption of
bread made from flour that had become mouldy in storage following
unseasonal rains in the wheat-harvesting season, from which Fusarium
sp. was grown, and which were found to contain mycotoxins. Of the
224 persons investigated on a random sample basis, 97 were affected
with symptoms including abdominal pain (100%), throat irritation
(63%), diarrhoea (39%), blood in stools (5%), and vomiting (7%).
Symptoms developed 15 min to one hour after consumption of locally
baked bread. In 12 out of 24 samples of refined wheat flour used in
the preparation of bread, the following mycotoxins were found: DON
(0.35-8.38 mg/kg), Ac-DON (0.64-2.49 mg/kg) (no details of estimation
of this derivative were available), NIV (0.03-0.1 mg/kg) and T-2
toxin (0.55-0.8 mg/kg). Quantitative estimation of DON, Ac-DON, and
NIV was obtained using HPLC, and that of T-2 toxin by TLC (Bhat et
al., 1987) but no rigorous confirmation of identity was undertaken.
Since no fatalities occurred in either of the above outbreaks, no
information is available on the pathological changes, if any, at
autopsy.
II.5.2 Historical Fusarium-related diseases
In the period 1931-47, a human disease known as alimentary toxic
aleukia (ATA) occurred in the USSR that was suggested to be related
to the presence of toxic Fusarium species in mouldy over-wintered
grain. Data have been reviewed by Sarkisov et al. (1944) and more
recently by Bilai (1977), Leonov (1977), and Joffe (1986). An
association was established with the ingestion of grain invaded by
some moulds, in particular Fusarium poae and F. sporotrichioides.
The dominant pathological changes were necrotic lesions of the oral
cavity, the oesophagus, and stomach and, in particular, a pronounced
leukopenia. The primary lesion was bone marrow hypoplasia and
aplasia. The disease was lethal in a high proportion of cases.
The clinical symptoms reported in ATA, as well as the identified
occurrence of Fusarium in foodstuffs, suggest that it might have been
associated with mycotoxins, identified years later in fungal cultures
of Fusarium species under laboratory conditions, such as T-2 toxin
(Mirocha & Pathre 1973) or wortmannin (Mirocha & Abbas 1989).
Scabby grain toxicosis is a disease of human beings as well as
farm animals, and was reported from Japan and Korea in the period
1946-63 (Hirayama & Yamamoto 1948, 1950; Nakamura et al., 1951;
Tsunoda et al., 1957; Cho 1964; Ogasawara 1965; Chung 1975). The
common clinical symptoms were nausea, vomiting, diarrhoea, and
abdominal pain. All cases were acute with recovery within a few
days and no lethal cases were encountered. Fusarium fungi, F.
graminearum in particular, were isolated from suspected cereals
(Tochinai, 1933; Tsunoda et al., 1957).
When compared with the symptoms observed in experimental
animals, features of both the above human diseases were similar to
trichothecene toxicosis, notably symptoms caused by DON and NIV,
DAS, and T-2 toxin. However, no epidemiological studies have been
reported that link ATA and scabby grain toxicosis to these
chemicals.
II.5.3 Skin irritation
The Task Group was aware of several reports describing various
effects on the skin of crude extracts of fungal cultures or
solutions containing T-2 toxin, as well as other possible
substances (Bamburg & Strong, 1971; Saito & Otshubo, 1974). These
cases, all of them accidental, involved a very limited number of
persons who developed severe irritation, loss of sensitivity, and
desquamation. Despite the presence of T-2 toxin in the contact
material, there is no evidence that the involvement of other
compounds can be ruled out.
II.5.4 Studies of haemostasis
Platelet function and electron microscopic morphological
changes following T-2 toxin administration were studied on
platelets isolated from 12 healthy human volunteers. When
platelets were incubated with T-2 toxin at doses of 5-500 µg/109
platelets for 20 min, there was a dose-related inhibition of
platelet aggregation with different activators, including
epinephrine, arachidonic acid, and collagen, and a release of dense
bodies consisting mainly of serotonin-containing granules. There
was also a change in membrane permeability, but no changes in
shape. No correlated inhibition of thromboxane synthesis, or
significant alterations in platelet calcium content were observed.
The microtubular system was unaffected. It was suggested that the
above observations , notably suppressed aggregation, played a
contradictory role in the haemorrhagic phenomena associated with
these toxins in man and animals (Yarom et al., 1984a).
II.5.5 Airborne trichothecene-related diseases
High concentrations of spores of Stachybotrys atra were
discovered in the air of living rooms of a suburban house in
Chicago, USA (Croft et al., 1986). Over a period of several years,
the 5 occupants of the house had suffered a variety of non-specific
symptoms including signs of colds, sore throats, diarrhoea,
headaches, dermatitis, intermittent focal alopaecia, and
generalized malaise. Chemical analysis of building materials
supporting the growth of Stachybotrys atra confirmed the presence
of the macrocyclic trichothecenes, verrucarin B and J, satratoxin
H, and trichoverrins A and B. Five weanling Sprague-Dawley rats
and 5 mice, administered extracts of contaminated materials orally,
died within 24 h of exposure, whereas control animals remained
unaffected. Histological lesions of the animals tissues were
degeneration, necrosis and haemorrhage of the brain, thymus,
spleen, intestine, lungs, heart, lymph nodes, liver, and kidneys.
Although the clinical symptoms could have been related to allergic
responses, the isolation of potent mycotoxins suggests that they
were the causal factor of the illness observed.
II.5.6 Toxicological information on man, obtained from
therapeutic uses
DAS (Anguidine) has been undergoing clinical trials as a
chemotherapeutic agent in cancer patients. During a weekly
schedule of DAS, using healthy volunteers as controls, a dose of 5
mg/m2 body surface infused over 3 h, produced nausea, vomiting,
hypotension, neurological symptoms (confusion, hallucinations and
psychomotor seizures), chills, fever, and diarrhoea (DeSimone et
al., 1979). A similar study carried out on 20 other cancer
patients revealed gastrointestinal and neurological toxic effects
(Belt et al., 1979). A bolus administration or rapid infusion of
the drug also caused gastrointestinal and neurological symptoms
(Thigpen et al., 1981). The human haematopoietic system appears to
be extremely sensitive to DAS. Myelosuppression was the dose
limiting adverse effect of prolonged infusions over 8 h (Thigpen et
al., 1981). A mean myelosuppressive dose level in the above
investigation was, 5 mg/m2 body surface by 8-h infusion or, roughly
calculated in terms of body weight, 0.2 mg/kg (0.025 mg/kg per h).
II.6 EVALUATION OF THE HUMAN HEALTH RISKS
On the basis of the data made available to the Task Group, there
is a possible association between trichothecene exposure and
episodes of human disease. According to the limited data
available, the most frequently reported trichothecenes involved in
episodes of human exposure are DON and NIV. In the episodes of
alimentary toxic aleukia and scabby grain toxicosis reported in the
past, a possible etiological role of trichothecenes cannot be
excluded. Exposure occurs through the ingestion of contaminated
food, mainly cereals. Processing, milling, and baking are
not effective in destroying DON, NIV, and T-2 toxin. There is very
limited evidence of exposure through inhalation, but such a
possibility cannot be ruled out.
Among the naturally occurring trichothecenes in foods, T-2
toxin is the most potent, followed by DAS and NIV; DON was the
least toxic in acute toxicity studies. In experimental animals,
T-2 toxin and DAS produce acute systemic effects, with necrosis of
epithelial tissues and suppression of haematopoiesis. In
contemporary outbreaks of disease, only gastrointestinal symptoms
have been reported.
DON was shown to be teratogenic in mice but not in rats.
According to published chronic toxicity studies, NIV and T-2 toxin
are not tumorogenic in animals. No long-term carcinogenicity
studies on DON have been published. Certain trichothecenes, such
as T-2 toxin and DON, have an immunosuppressive action in animals
and have produced alterations in both cell-mediated and humoral
immunity. There is no evidence of immunosuppressive action in man.
Reported cases of human disease associated with trichothecene
exposure are limited in number and information. Symptoms of
digestive disorders and throat irritation develop rapidly after
ingestion of food contaminated with trichothecenes. At present,
there is no evidence of human cancer caused by trichothecenes. No
reports were available to the Task Group on secondary infection, by
bacteria, fungi, or viruses, in human beings following trichothecene
exposure, as has been observed in experimental animal studies. It
appears that adequate studies elucidating such a sequence have not
been made.
III. ERGOT
III.1 PROPERTIES AND ANALYTICAL METHODS
III.1.1 Chemical properties
Ergot is the French word for a rooster's spur and is used as
the common term for sclerotia of fungal species within the genus
Claviceps, in particular C. purpurea. The fungi infect the florets
of grasses and cereal, replacing the florets with compact fungal
structures, sclerotia, 2-20 mm long, somewhat curved, tapering off
at the ends, and strongly coloured, often purple-black, thus
resembling rooster's spurs. The sclerotia contain a large number
of biologically active alkaloids, as well as amino acids,
carbohydrates, lipids, and pigments, and, when the sclerotia are
consumed by man and animals, toxicosis develops, called ergotism.
The topic has been reviewed by Bove (1970), Van Rensburg &
Altenkirk (1974), Lorenz (1979), and Berde & Schild (1978). This
section deals with ergot as a toxic food contaminant; ergot
compounds used as pharmaceutical drugs are excluded.
Ergot alkaloids (ergolines), of which more than 40 have been
isolated from Claviceps sclerotia, are derivatives of lysergic acid
(Fig. 4), and can be divided into 3 groups:
Group I: Derivatives of lysergic acid, e.g., ergotamine.
Group II: Derivatives of isolysergic acid, e.g., ergotaminine.
Group III: Derivatives of dimethylergoline (clavines), e.g.,
agroclavine.
II.1.1.1 Physical properties
The trichothecenes are colourless, mostly crystalline solids
that have been well characterized by physical and spectroscopic
techniques (Cole & Cox, 1981). The type A trichothecenes are
soluble in moderately polar solvents, such as chloroform, diethyl
ether, ethyl acetate, and acetone, whereas the more polar type B
trichothecenes require higher polarity solvents, such as aqueous
methanol or aqueous acetonitrile. Some physical properties of the
main trichothecenes are summarized in Table 10.
Table 10. Some physical properties of main trichothecenes
-------------------------------------------------------------------------------
Trichothecenes Molecular Relative Melting (alpha)20D References
formula molecular point
mass (°C)
-------------------------------------------------------------------------------
T-2 toxin C24H34O9 466 151-152 +15 Bamburg et
al. (1968b)
HT-2 toxin C22H26O8 424 - - Bamburg &
Strong (1969)
Diacetoxyscirpenol C19H26O7 366 162-164 -27 Sigg et al.
(1965)
Neosolaniol C19H26O8 382 171-172 - Ishii et al.
(1971)
Deoxynivalenol C15H20O6 296 151-153 +6.35 Yoshizawa &
Morooka (1973)
Nivalenol C15H20O7 312 222-223 +21.54 Tatsuno et
al. (1968)
Trichothecin C19H24O5 332 118 +44 Freeman (1955)
Fusarenon-X C17H22O8 354 91-92 +58 Ueno et al.
(1969b)
Roridin A C29H40O9 532 198-204 +130 Harri et al.
(1962)
Satratoxin H C29H36O9 528 162-166 - Eppley &
Bailey (1973)
Verrucarin A C27H34O9 502 360 +20.6 Gutzwiller &
Tamm (1965)
-------------------------------------------------------------------------------
Most of the trichothecenes lack conjugated unsaturation in their
structures with a consequent absence of absorption in the ultraviolet
(UV) spectrum, except for end absorption due to unsaturation at C-9.
This lack of absorbance is a source of difficulty in achieving
sensitive and specific detection in HPLC analysis. In contrast, the
type D trichothecenes give characteristic ultraviolet spectra.
II.1.1.2 Chemical properties
When trichothecenes containing an ester group are treated with a
base, they are hydrolysed to their corresponding parent alcohol (Wei
et al., 1971). Free hydroxyl groups are readily acylated. The
12,13-epoxy group is itself extremely stable to nucleophilic attack.
However, prolonged boiling under highly acidic conditions causes an
intramolecular rearrangement of the trichothecene skeleton to the
apotrichothecene ring. Detailed discussion of the chemistry of
trichothecenes can be found in reviews by Bamburg & Strong (1971),
Bamburg (1976), and Tamm (1977).
The trichothecenes are generally stable; for example, DON can be
stored in organic solvents, such as ethyl acetate, for a long time
without any significant deterioration (Shepherd & Gilbert, 1988).
They remain unaffected when refluxed with various organic solvents
and also under mildly acidic conditions.
II.1.2 Analytical methods for trichothecenes
Analytical methods have been reviewed by Scott (1982) and Pohland
et al. (1986). Selected examples of recently published analytical
methods for type A trichothecenes and type B trichothecenes are
summarized in Tables 11 and 12 respectively. Although some of the
multi-trichothecene methods included in Table 11 may also be
applicable to certain of the type B compounds, the procedures in
Table 12 have been developed exclusively for the type B toxins.
II.1.2.1 Chemical methods
(a) Extraction
The most commonly used extraction solvents for trichothecenes are
chloroform, ethyl acetate, methanol, acetonitrile, aqueous methanol,
and aqueous acetonitrile. Chloroform, ethyl acetate, and
acetonitrile have been successfully used for the extraction of T-2
toxin, DAS, and some of their partially hydrolysed derivatives in
naturally contaminated cereals. Aqueous methanol and aqueous
acetonitrile are the solvents of choice for the extraction of several
trichothecenes of widely differing polarity as well as for the
extraction of type B toxins alone. Methods differ according to the
type of solvent used, whether samples are homogenised in a blender
with the solvent or agitated with a wrist action shaker, and in the
length of time of the extraction process. Spiking of samples with
standards is not an adequate way of demonstrating the efficiency of
extraction, and only methods validated with naturally contaminated
material can be regarded as having been rigorously tested.
Extraction procedures have been assessed for DON (Trenholm et al.,
1985) and it has been demonstrated that longer extraction times are
required for naturally contaminated samples than for those that have
been spiked (at least 120 min shaking). It has also been shown that
aqueous acetonitrile gives a cleaner extract than aqueous methanol.
(b) Clean-up procedures
The extent of sample clean-up required for a particular assay
depends on the specificity of the detection procedure and the nature
of the sample matrix. The less specific detection methods, such as
TLC, require extensive sample clean-up while more sophisticated
approaches, such as mass spectrometry and immunoassay, may only
require minimal sample preparation.
Table 11. Selected methods for the determination of T-2 toxin and diacetoxyscirpenol in
biological materials
------------------------------------------------------------------------------------------------
Matrix Extractiona Clean-upb Assayc Detection Toxins assayed References
limit -----------------
(µg/g) T-2 DAS Others
------------------------------------------------------------------------------------------------
Cereals MeOH/H2O XAD-4; flor. TLC 0.5 + + 7 Kamimura et
al. (1981)
Cereals EtOAC prep. TLC HPTLC 0.2 + - 2 Ilus et
al. (1981)
Foods MeCN/H2O char/alum TLC 1.0 + + 5 Romer
(1986)
Cereals MeCN/KCl Sep P HPLC(RI) 1.0 + - 1 Schmidt &
Dose (1984)
Wheat/rye MeCN Bond-Elut LC/MS (therm) 0.04 + + 2 Rajakyla et
al. (1987)
Plasma EtOAC Sep P/sil.g LC/MS (therm) 0.002 + + 2 Voyksner et
al. (1985)
Cereals EtOAC sil.g GC (FID)-TMS 0.1 + + 3 Bata et al.
(1983)
Cereals MeOH/H2O sil.g/cy GC (ECD)-HFB 0.05 + + 1 Cohen &
Lapointe
(1984)
Plasma benzene flor. GC (ECD)-HFB 0.02 + - - Swanson et
al. (1983)
Milk EtOAC prep. TLC GC/MS-TMS 0.003 + - - Collins &
Rosen
(1979)
Corn MeOH Sep P/sil.g GC/MS-TMS 0.02 + + 1 Rosen &
Rosen
(1984)
Foods MeOH flor. GC/MS-HFB 0.01 + + 11 Black et
al. (1987)
Urine EtOAC/MeOH Sep P GC/MS-HFB 0.005 + + 5 Black et
al. (1986)
Blood acetone Sep P GC/MS-PFP 0.005 + + 9 Begley et
al. (1986)
------------------------------------------------------------------------------------------------
Table 11 (contd.)
------------------------------------------------------------------------------------------------
Matrix Extractiona Clean-upb Assayc Detection Toxins assayed References
limit -----------------
(µg/g) T-2 DAS Others
------------------------------------------------------------------------------------------------
Milk/urine EtOAC Sep P RIA 0.002 + - - Lee & Chu
(1981a)
Corn/wheat MeOH Sep P RIA 0.001 + - - Lee & Chu
(1981b)
Urine none Sep P ELISA 0.0005 + - - Fan et al.
(1987)
------------------------------------------------------------------------------------------------
a Extraction: MeOH (methanol); H2O (water); EtOAC (ethyl acetate); MeCN (acetonitrile);
KCl (potassium chloride aqueous soln).
b Clean-up: XAD-4 (amberlite XAD-4 resin); flor. (florisil); char/alum (charcoal/alumina
columns); Sep P (C18-Sep Pak); sil.g (silica gel); cy (cyano extraction column).
c Assay: TLC (thin layer chromatography); HPTLC (high performance TLC); HPLC (high performance
liquid chromatography); RI (refractive index); LC/MS (combined HPLC/mass spectrometry);
GC (gas chromatography); FID (flame ionisation detector); ECD (electron capture detector);
TMS (trimethylsilyl derivative); HFB (hepafluorobutyrl ester derivative);
PFP (pentafluoropropionyl ester); RIA (radioimmunoassay); ELISA (enzyme linked
immunosorbent assay).
Table 12. Selected methods for the determination of deoxynivalenol and nivelenol in biological
materials
------------------------------------------------------------------------------------------------
Matrix Extractiona Clean-upb Assayc Detection Toxins assayed References
limit -----------------
(µg/g) T-2 DAS Others
------------------------------------------------------------------------------------------------
Wheat/corn MeCN/H2O char/alum TLC 0.04-0.1 + - - Trucksess
et al.
(1984)
Foods MeCn/H2O char/alum; TLC 0.5 + - - Trucksess
Sep P et al.
(1986a)
Cereals MeCN/H2O/ char/alum; HPTLC 0.05 + + 1 Trucksess
MeOH ppt et al.
(1987)
Corn/rice MeOH/H2O/ liq/liq extr HPLC (UV) O.005 + + 2 Visconti &
NaCl Bottalico
(1983a)
Corn/rice MeOH/H2O none HPLC (ED) 0.025 + - - Sylvia et
al. (1986)
Corn MeOH/H2O prep. TLC HPLC (UV) 0.01 + - - Ehrlich et
al. (1983)
Cereals MeCN/H2O ion/char/ HPLC (UV) 0.05 + + - Lauren &
alum Greenhalgh
(1987)
Cereals MeOH flor. LC/MS (micro) 0.01 + + - Tiebach et
al. (1985)
Cereals MeOH/H2O sil.g GC (ECD)-TMS 0.02 + + - Scott et
al. (1986)
Cereals MeCN/H2O flor/Sep P GC (ECD)-TMS 0.002 + + - Tanaka et
al. (1986)
Wheat chlor/EtOH sil.g GC (ECD)-HFB 0.1 + - - Ware et
al. (1986)
Cereals chlor/EtOH sil.g GC (ECD)-HFB 0.02 + - - Mulders &
Impelen-
Peek (1986)
Milk EtOAc Sep P GC (ECD)-TMS 0.001 + - - Swanson et
al. (1986)
------------------------------------------------------------------------------------------------
Table 12 (contd.)
------------------------------------------------------------------------------------------------
Matrix Extractiona Clean-upb Assayc Detection Toxins assayed References
limit -----------------
(µg/g) T-2 DAS Others
------------------------------------------------------------------------------------------------
Corn/barley MeOH/H2O sil.g GC/MS-TMS 0.01 + - - Gilbert et
al. (1983a)
Foods MeOH/H2O XAD-2/flor/ GC/MS-TMS 0.02 + + - Yoshizawa &
Sep P Hosokawa
(1983)
Corn/wheat MeCN/H2O none ELISA 0.01 + - - Xu et al.
(1988)
------------------------------------------------------------------------------------------------
a Extraction: MeCN (acetonitrile); H2O (water); MeOH (methanol); NaCl (sodium chloride soln);
chlor/EtOH (chloroform/ethanol); EtOAc (ethyl acetate).
b Clean-up: char/alum (charcoal/alumina column); Sep P (C18-Sep Pak); ppt (lead acetate
precipitation); liq/liq extr. (liquid/liquid extraction); ion (ion exchange resin);
flor. (florisil); sil.g (silica gel); XAD-2 (Amberlite XAD-2 resin).
c Assay: TLC (thin layer chromatography); HPTLC (high performance TLC); HPLC (high performance
liquid chromatography); UV (ultraviolet detection); ED (electrochemical detection);
LC/MS (combined microbore-HPLC/mass spectrometry); GC (gas chromatography); ECD (electron
capture detector); TMS (trimethylsilyl derivative); HFB (heptafluorobutyryl ester
derivative); ELISA (enzyme linked immunosorbent assay).
Early methods that may have involved the use of conventional
silica gel columns or preparative TLC for clean-up, have to a large
extent been superseded by methods using prepacked cartridges, such as
silica gel and C18-Sep Paks, which are both more reliable and more
convenient. Florisil columns are widely used for clean-up (e.g., see
Tanaka et al., 1985a), and the one step clean-up procedures using
alumina/charcoal (Romer, 1986) or alumina/charcoal/Celite columns
(Trucksess et al., 1984) have become widely adopted, particularly for
DON and NIV assays.
(c) Detection and quantification
(i) Thin-layer chromatography (TLC). The lack of native
fluorescence or UV absorbance of the trichothecenes means that TLC
detection relies on the use of spray reagents for visualisation.
Characteristic colours or fluorescence can be produced with sulfuric
acid or p-anisaldehyde followed by heating at 110-120 °C (Scott et
al., 1970; Ueno et al., 1973c). A general spray reagent for the
12,13-epoxy function is 4-( p-nitrobenzyl)pyridine, which produces a
blue coloration on heating and treatment with a base (Takitani et
al., 1979). A more sensitive, but somewhat elaborate, procedure,
which again is specific for the epoxide function, involves reaction
with nicotinamide and 2-acetylpyridine to produce fluorescent TLC
spots (Sano et al., 1982). Diphenylindenone sulfonyl esters of
trichothecenes can be formed prior to TLC and, subsequently, when
sprayed with sodium methoxide can yield fluorescent spots at high
sensitivity (Yagen et al., 1986).
Most of the above spray reagents, though frequently demonstrated
as useful for the determination of standards or of relatively high
concentrations of toxins in culture extracts, have not been well
developed in conjunction with clean-up procedures for the
determination of trichothecenes in naturally contaminated cereal
samples or other foods. However, the exception has been the use of
an aluminum chloride spray reagent for visualising the type B
trichothecenes (Kamimura et al., 1981). On spraying the plates,
heating for 10 min at 110 °C and then treating with a base, blue
fluorescent spots are produced for DON, NIV, and fusarenon X. Using
this approach, in conjunction with a clean-up utilising an alumina
charcoal Celite column, a quantitative TLC procedure was developed
using a fluorodensitometer for determining DON in wheat and corn
(Trucksess et al. 1984); DON in processed grain products including
breakfast cereals, corn syrup, and beer (Trucksess et al., 1986b);
and for simultaneously monitoring DON, NIV, and fusarenon X in
barley, corn, and wheat (Trucksess et al. 1987). This TLC procedure
was successfully collaboratively tested (Eppley et al., 1986) and was
accepted as the AOAC first action method for DON in wheat.
(ii) High performance liquid chromatography (HPLC). HPLC has not
proved particularly appropriate for the type A trichothecenes, which
lack any significant UV absorption, making sensitive detection
difficult. Refractive index detection has been employed, but at
relatively high toxin levels (Schmidt & Dose, 1984). UV detection at
short wavelength has been used for the determination of DON and NIV
in cereals, and a number of methods have been reported that offer an
advantage over alternative GC approaches in that they do not require
derivatization of the toxins. A post-column derivatization procedure
for DON and NIV has been developed (Sano et al., 1987) involving
alkaline decomposition of the trichothecenes to generate formaldehyde
and then reaction with methyl acetoacetate and ammonium acetate to
form a fluorescent derivative. Although more sensitive and specific
than UV detection, the approach does have the disadvantage of
requiring rather elaborate instrumentation, in order to carry out the
post column reaction. Electrochemical detection (Sylvia et al.,
1986) looks particularly promising for the determination of DON by
HPLC, offering both greater sensitivity and specificity than UV
detection, and the possibility of analysis of sample extracts that
have received minimal sample clean-up.
(iii) Gas chromatography (GC). A large number of GC methods have
been published that differ in the approach to sample extraction, in
sample clean-up, and in choice of derivative prior to GC analysis.
Trichothecenes containing a hydroxyl group require derivatization,
and the heptafluorobutyryl (HFB) ester and trimethylsilyl (TMS) ether
derivatives have been most frequently used in conjunction with
electron capture detection. Formation of the HFB derivatives is
relatively time-consuming, but complete derivatization is easily
achieved and the derivative once formed is stable for at least
several days. However, poor reproducibility has been noted with HFB
derivatives of DON (Mulders & Impelen-Peek, 1986) attributed to
adsorption on to glass surfaces. The relatively high mass of the HFB
derivatives can cause difficulties if GC/MS confirmation is required.
In contrast, TMS derivatives are easily prepared, and are of
suitable mass for GC/MS, but, for some type B toxins, they may
require optimization of conditions for complete derivatization
(Gilbert et al., 1985). Scott & Kanhere (1986) have compiled
retention data for 10 different trichothecenes as both TMS and HFB
derivatives on capillary columns of different stationary phases.
Trifluoroacetyl derivatives of trichothecenes are preferred by some
workers (Kientz & Verweij, 1986), and pentafluoropropionyl esters
have been used, particularly where detection has been by MS (Begley
et al., 1986; Krishnamurthy & Sarver, 1986; Rood et al., 1988a).
An area where improvement in quantification of trichothecenes is
still needed is in the selection of adequate internal standards.
Compounds, such as methoxychlor (Romer et al., 1978),
hexachlorobiphenyl (Blass et al., 1984), and alkanes (Ilus et al.,
1981), have been used, but these differ significantly in structure
from the trichothecenes. The deuterated TMS derivative of T-2 toxin
has been used as an internal standard in GC/MS analysis (Rosen &
Rosen, 1984), an isomer of T-2 toxin has been chemically synthesized
(Stahr et al., 1981) as have 4-deoxyverrucarol and 16-hydroxyver-
rucarol for use as internal stan-dards (Krishnamurthy et al., 1986).
Despite the many difficulties, a GC method using the HFB
derivative has been successfully collaboratively tested (Ware et al.,
1986) and subsequently adopted as an AOAC official first action
method.
Other related trichothecenes have been determined in foods by GC
methods, for example, the de-epoxidised metabolite of DON called
DOM-1 has been determined in milk as both its TMS and HFB derivative
(Swanson et al., 1986). Also, an isomer of DON was detected by
capillary GC, as its TMS derivative in bread and breakfast cereal
products prepared from flour naturally contaminated with DON
(Greenhalgh et al., 1984).
(iv) Mass spectrometry (MS). Mass spectrometry has been used for
the structural characterization of novel trichothecenes; for
identification and confirmation of trichothecenes in biological
materials, and as a sensitive and specific means of detection with
GC, LC, or supercritical fluid chromatography (SFC) sample
introduction.
The usefulness of negative ion chemical ionization (NICI) mass
spectrometry with hydroxide ion reagent gas has been demonstrated in
relation to producing relative molecular mass information, as well as
fragment ions indicative of structure for type A and B trichothecenes
(Brumley et al., 1982). The NICI technique has been applied to
confirmation of the presence of DON in cereals and snack foods by
rapid capillary GC introduction of the underivatized extract
obtaining full scan spectra (Brumley et al., 1985), and by selected
ion monitoring (Miles & Gurprasad, 1985).
NICI has also been used for selected ion monitoring GC/MS of the
HFB derivatives of 13 different trichothecenes of both type A and
type B in extracts from small samples of biological material
(Krishnamurthy et al., 1986). GC/MS is the preferred approach using
SIM in electron ionization (D'Agostino et al., 1986) or NICI (Begley
et al., 1986) modes for biological samples where only small sample
sizes are available but high sensitivity is required. For more
routine surveys of cereal and food samples, particularly for DON and
NIV, initial screening has normally been carried out using GC with an
ECD and only selected positive samples have been confirmed by GC/MS
(Tanaka et al., 1985a; Cohen & Lapointe, 1982).
Attempts have been made to determine the macrocyclic
trichothecenes as TMS derivatives on short fused silica GC columns
using GC/MS (Rosen et al., 1986). The approach has also been adopted
of alkaline hydrolysis of the ring system of the macrocyclic
compounds to yield verrucarols, which can then be determined as HFB
derivatives by NICI GC/MS (Krishnamurthy et al., 1987). A similar
approach using alkaline hydrolysis has been proposed (Rood et al.,
1988a,b) as a basis for a general method for the determination of
trichothecenes and metabolites by conversion to their corresponding
parent alcohols prior to derivatization and GC or GC/MS
determination.
Although LC/MS is really a research method, thermospray LC/MS is
becoming more widely available and shows promise for the
identification of low levels of trichothecenes in biological
materials (Voyksner et al., 1985; Rajakyla et al., 1987). Another
promising research method for the determination of both the simple
and the macrocyclic trichothecenes is supercritical fluid
chromatography (SFC) combined with MS (Smith et al., 1985).
Supercritical fluids can also be used for the on-line extraction from
biological materials and direct introduction into the MS to monitor a
number of different trichothecenes (Kalinoski et al., 1986).
II.1.2.2 Immunological methods
Chu et al. (1979) developed a radioimmunoassay (RIA) for T-2
toxin. The antibody preparation was obtained by immunizing rabbits
with bovine serum albumin T-2 toxin hemisuccinate conjugate. The
antibody had the greatest binding efficiency for T-2 toxin and less
efficiency for HT-2 toxin. Cross reaction with other trichothecenes
was either very slight or absent, and the limit of detection of the
assay ranged from 1 to 20 ng.
This RIA was applied to the determination of T-2 toxin in
agricultural commodities, biological fluids, and animal organs (Lee &
Chu, 1981a,b; Hewetson et al., 1987). Xu et al. (1988) also reported
an immunoassay using an antibody against triacetylated DON, for the
determination of DON in wheat and corn with a limit of detection of
about 0.02 µg/g.
A polyclonal enzyme-linked immunosorbent assay (ELISA) was
developed for the rapid quantification of T-2 toxin in food and
animal feed (Pestka et al., 1981). This assay, which could be
undertaken in 2 h, had a limit of detection of 2.5 pg/assay and was
used for the detection of T-2 toxin in Fusarium-infected corn
(Gendloff et al., 1984). More recently monoclonal ELISAs have been
developed for T-2 toxin and DON (Feuerstein et al., 1985; Hunter et
al., 1985; Chiba et al., 1988). Although these monoclonal assays are
of invariable specificity and the antibodies are available in
unlimited supply, competitive indirect ELISAs using monoclonals are
generally less sensitive than polyclonal-based ELISAs. However,
Chiba et al. (1988) developed a sensitive ELISA for the detection of
T-2 toxin with monoclonal antibodies at a limit of 2.5 pg/assay.
This assay has been applied to the detection of T-2 toxin in wheat
flour (Chiba et al., 1988) and in fungal cultures (Nagayama et
al., 1988).
Production of useful antibodies against DON has proved more
difficult than for other trichothecenes. Xu et al. (1988) adopted
the approach of raising antibodies against triacetyl-DON, which
requires acetylation of DON in an extract of the contaminated cereal
prior to carrying out a direct ELISA. This assay has the required
sensitivity but also the disadvantage of determining the total
concentration of DON plus any other acetylated derivatives that may
be present in the extract. A different approach to raising
monoclonal antibodies to DON has been carried out by forming the
hemisuccinyl derivative after protection of two of the available
adjacent hydroxyls with a cyclic boronate ester (Casale et al.,
1988). Some cross-reactivity to other trichothecenes and their
de-epoxy metabolites was observed, but the assay does show
considerable potential as a simple and rapid screening method for
contaminated cereals and for detection in biological samples.
II.1.2.3 Biological methods
Biological methods are essential for working with field cases
where the cause of an incident is unclear and evidence is sought to
associate an observed biological effect with the presence of fungally
contaminated material. Biological methods are also important as
monitoring procedures in the isolation and purification of new or
previously unrecognized toxins, prior to characterization by
spectroscopic and chemical methods.
The classical and commonly used bioassay for trichothecenes is
the skin necrotization test. Extracts prepared from samples (see
section II.1.2.1) are applied in a single dose to the shaved back of
a rabbit, rat, or guinea-pig. Toxic preparations applied in this way
produce erythema, oedema, intradermal haemorrhage, and necrosis. The
guinea-pig is the most sensitive of these animals to trichothecenes
(Ueno et al., 1970). As little as 0.2 µg of T-2 toxin and DAS were
detected on the skin of the rabbit and guinea-pig (Chung et al.,
1974; Balzer et al., 1977). Only 10 g of the corn sample are needed
for testing with an extraction and clean-up procedure recommended by
Eppley (1968). Macrocyclic trichothecenes are highly irritant to the
skin, but NIV and DON, which have a low skin-necrotizing potency,
could be routinely missed in this assay (Ueno et al., 1970).
Brine shrimp ( Artemia salina L.) larvae also provide a useful
biological system for monitoring trichothecenes, such as T-2 toxin,
DAS, and the macrocyclic trichothecenes. The limit of detection of
this test for these toxins is approximately 0.04-0.4 mg/litre
(Eppley, 1974).
The rabbit reticulocyte assay, based on the inhibition of uptake
of 14C-leucine in eukaryote cells, is highly specific for
trichothecenes; ID50 (50% inhibitory dose) values are 0.03 mg/litre
for T-2 toxin and DAS, and 2.0-3.0 mg/litre for DON and NIV (Ueno et
al., 1969a, 1973c). By combining chemical methods with this assay,
DON was successfully isolated from Fusarium-infected corn (Ishii et
al., 1975).
II.2 SOURCES AND OCCURRENCE
II.2.1 Taxonomic considerations
The vast majority of trichothecenes are produced by the Fusarium
species. Production of these metabolites depends, of course, on many
factors, including substrate, temperature, humidity, etc. In
general, the type A trichothecenes have been most frequently
associated with the following Fusarium species: F. tricinctum, F.
sporotrichioides, F. poe, F. equiseti. On the other hand, type B
trichothecenes are usually associated with F. graminearum (Gibberella
zeae), and F. culmorum. The type C trichothecenes, containing an
additional epoxide function at the 7,8- or 9,10-positions, are
produced by only a few species. The Type D trichothecenes, i.e.,
those containing the macrocyclic ring between the 4,15-positions, are
produced by several fungal genera, including Myrothecium and
Stachybotrys. Of these, the most important from an animal and human
health standpoint, is Stachybotrys atra. The relationships between
trichothecenes and fungal species are summarized in Table 13.
Table 13. The relationship between trichothecenes and fungal species
----------------------------------------------------------------------
Trichothecenes Fungal species References
----------------------------------------------------------------------
Type A
T-2, HT-2, DAS, NS Fusarium tricinctum Joffe & Yagen (1977)
F. sporotrichioides Scott et al. (1980)
F. poae Marasas et al. (1984)
F. acuminatum Ichinoe et al. (1985),
Rabie et al. (1986)
DAS F. equiseti Brian et al. (1961)
F. semitectum Suzuki et al. (1980),
Greenhalgh et al. (1984)
Type B
DON, 3-AcDON F. graminearum Yoshizawa & Morooka (1973)
NIV, F-X Gibberella zeae Marasas et al. (1984)
(anamorph) Ichinoe et al. (1985)
DON, 3-AcDON F. culmorum Marasas et al. (1979b)
Chelkowski et al. (1984)
Trichotecin Trichothecium Freeman & Morrison (1949)
roseum Ishii et al. (1986)
Type C
Baccharin Baccharis Kupchan et al. (1976)
megapotamica
(higher plant)
Type D
Roridin A, D, E Mycothecium roridum Bohner et al. (1965)
Verrucarin J M. verrucaria Harrach et al. (1981)
Satratoxin G, H Stachybotrys atra Jarvis et al. (1986)
----------------------------------------------------------------------
The taxonomy of the various fungal species is complex and has led
to some confusion, particularly regarding the Fusarium species
responsible for the production of the various trichothecene
metabolites. Several excellent descriptions are now available of the
Fusarium spp. and the metabolites produced by them (Nelson et al.,
1983; Ueno, 1983; Marasas et al., 1984; Ichinoe et al., 1985; Joffe,
1986).
II.2.2 Ecology of trichothecene-producing fungi
Fusarium species are widely distributed throughout the
environment. There are over 50 recognized species commonly occurring
in the soil (soil fungi), many of which are pathogenic to crop
plants. As a result, there are a number of frequently encountered
plant diseases (wilts, blights, rots), such as F. graminearum blight
of wheat and barley (Akakabibyo in Japanese), pink ear rot of corn,
pink scab (tombstone kernels) of wheat, Fusarium snow blight, etc.
Fusarium graminearum, which produces DON and NIV, is a most important
fungus.
II.2.3 Natural occurrence
It has become apparent in the past few years that whenever a
trichothecene-producing Fusarium species parasitizes a crop, food,
or animal feed, it is highly probable that the metabolites of the
trichothecene will be found as contaminants. The chance of detecting
the metabolite is then clearly a function of the efficiency of the
sampling procedure and the capabilities of the analytical methods
used (most importantly, the detection limit).
Because of its toxicity, analytical procedures for T-2 toxin were
developed first, and consequently early surveys for trichothecenes
tended to concentrate on T-2 toxin. However, it soon became apparent
that other trichothecenes, in particular, DON, NIV, and DAS, were
more frequent contaminants of food and animal feed than T-2 toxin.
As improved analytical procedures for these mycotoxins became
available, surveys were conducted and additional data on the
occurrence of these metabolites were published. In reviewing this
record, it should be recognized that the methodology used in
developing the data was quite variable, so that only broad
generalizations with respect to incidence/level may be drawn. In the
vast majority of cases, no evidence was included indicating that
methods used were rigorously tested, that quality assurance
programmes were in place, and that confirmation of identity was
adequately obtained. Much of the available survey data on the
natural occurrence of trichothecenes in raw agricultural commodities,
food, and animal feed is summarized in Tables 14-17. Reports in
which only a few samples were analysed have not been included.
II.2.3.1 Agricultural products
(a) T-2 Toxin
One of the first trichothecenes to be implicated in an episode of
mouldy corn toxicosis was T-2 toxin; in 1972, it was reported that
T-2 toxin at the level of 2 mg/kg was present in mouldy corn involved
in lethal toxicosis in dairy cattle (Hsu et al., 1972). This event,
along with increasing information regarding the acute toxicity of T-2
toxin, prompted considerable efforts to develop methods of analysis
for T-2 toxin and the analysis of a wide range of agricultural
commodities (Table 14). Only occasional samples were found to
contain T-2 toxin (incidence well below 10% in most cases), most
frequently at levels <0.1 mg/kg. Usually, other trichothecenes were
also found (Vesonder, 1983). On the other hand, there have been
isolated reports of the finding of rather high levels of T-2 toxin,
e.g., the finding of 25 mg T-2 toxin/kg in barley (Puls & Greenway,
1976), and 38.9 mg T-2 toxin/kg in peanuts (Bhavanishankar & Shantha,
1987). These findings, as well as reports from India of the presence
of T-2 toxin in safflower seed and sweet corn (Ghosal et al., 1977),
and sorghum (Ruckmini & Bhat, 1978), and from Italy of its presence
in barley, corn feed, oats, rice, and wheat (Cirilli, 1983) need to
be further investigated.
Table 14. Natural occurrence of T-2 toxin in raw agricultural
commodities
---------------------------------------------------------------------
Commodity Levels Incidence Country Reference
(mg/kg) (+ve/total)
---------------------------------------------------------------------
Corn 0.5-5.0 (5/150) Hungary Szathmary (1983)
0.080-0.65 (9/118) Taiwan Tseng et al. (1983)
0.01-0.2 (13/20) New Zealand Hussein et al.
(1989)
Feed 0.05-5.0 (28/464) Hungary Szathmary (1981)
Oats 0.01-0.05 Finland Ylimacki et al.
(1979)
Peanuts 0.63-38.89 (6/87) India Bhavanishankar &
Shantha (1987)
Rice (3/64) Egypt Abdel-Hafez et al.
(1987)
Sorghum 1.67-15.0 (4/84) India Bhavanishankar &
Shantha (1987)
Wheat 2.0-4.0 (3/12) India Bhat et al. (1989)
---------------------------------------------------------------------
DON/NIV: Deoxynivalenol (DON) and nivalenol (NIV) have been
found to be the most frequent trichothecene contaminants of
agricultural crops throughout the world (Table 15). Extensive
survey data indicate the common occurrence of these mycotoxins,
particularly in corn and wheat, at levels usually below 1 mg/kg
(Vesonder, 1983; Pohland & Wood, 1987; Jelinek et al., 1989).
Perhaps the most extensive survey, and the one giving the most
accurate picture of the global occurrence of DON and NIV, was
reported recently by Tanaka et al. (1988).
Table 15. Natural occurrence of DON and NIV in raw agricultural
commodities
---------------------------------------------------------------------------
Commodity Toxin Levels Incidence Country Reference
(mg/kg)a (+ve/total)
---------------------------------------------------------------------------
Barley DON t-40.4 (19/25) Japan Kamimura et
al. (1981)
NIV t-37.9 (19/23)
(unpolished) DON 0.004-0.508 (26/28) Korea Lee et al.
NIV 0.017-3.0 (28/28) Republic of (1985)
(polished) DON 0.008-0.043 (5/6)
NIV 0.085-0.328 (5/6)
DON 0.006-2.14 (34/49) Norway Sundheim et
NIV 0.013-1.56 (49/49) al. (1988)
DON 0.02-0.36 (34/87) United Gilbert et
Kingdom al. (1983b)
Corn DON 1-20 (19/60) Austria Lew et al.
(1979)
DON 0.15-0.82 (9/9) Canada Scott et
al. (1981)
---------------------------------------------------------------------------
Table 15 (contd.)
---------------------------------------------------------------------------
Commodity Toxin Levels Incidence Country Reference
(mg/kg)a (+ve/total)
---------------------------------------------------------------------------
Corn (contd.) DON 0.36-12.7 (100%) China Qiujie et
NIV 0.054-2.67 (100%) al. (1988)
DON 0.02-0.3 (11/20) New Zealand Hussein et
al. (1989)
DON t-15.8 (20/72) Transkei Thiel et
NIV t-1.41 (43/72) al. (1982)
DON 0.1-0.3 (37) United Gilbert et
Kingdom al. (1984)
DON 0.5-10.7 (24/52) USA Vesonder et
al. (1978)
DON 0.1-2.47 (93/198) USA Wood &
Carter
(1989)
Oats DON 20 Germany, Bauer et
Federal al. (1980)
Republic of
DON 0.02-0.1 (1/6) United Gilbert et
Kingdom al. (1984)
Rye DON 0.003 (1/5) Korea Lee et al.
NIV 0.046-0.114 (5/5) Republic of (1985)
Wheat DON 0.01-4.3 (51/52) Canada Scott et
al. (1981)
DON 0.06-8.53 (24/52) Canada Trenholm et
al. (1981)
DON 0.02-1.32 (55/199) Canada Osborne &
Willis
(1984)
DON 1.0 (1.36) Denmark Hald & Krogh
(1983)
DON t-4.7 (15/18) Germany, Bauer et
NIV t-7.8 Federal al. (1980)
Republic of
DON 0.008-3.19 (32/53) Norway Sundheim et
al. (1988)
NIV 0.015-0.887 (53/53)
DON 0.02 >0.5 (57/148) United Gilbert et
Kingdom al. (1984)
DON 0.12-5.5 (31/33) USA Hagler et
al. (1984)
DON 0.2-9 (54/57) USA Eppley et
al. (1984)
DON 0.1-2.65 (133/247) USA Wood &
Carter
(1989)
---------------------------------------------------------------------------
Table 15 (contd.)
---------------------------------------------------------------------------
Commodity Toxin Levels Incidence Country Reference
(mg/kg)a (+ve/total)
---------------------------------------------------------------------------
Animal DON 0.1-41.6 (274/342) USA Cote et
feed al. (1984)
DON <0.4-4.0 USA Vesonder
(1983)
---------------------------------------------------------------------------
a t = trace.
This survey involved the analysis of 500 samples of cereal grains
from 19 countries (Table 16) using a single analytical method.
Table 16. Natural occurrence of DON and NIV: worldwide survey
---------------------------------------------------------------------
Commodity DON NIV
(Mean, ng/g) (% + ve) (Mean, ng/g) (% + ve)
---------------------------------------------------------------------
Barley 149 75 401 76
Corn 402 20 766 16
Oats 115 22 438 26
Rice 0 22 22
Rye 183 33 47 33
Sorghum 0 0
Wheat 488a 39 127 50
Othersb 135 44 3 6
Total: 292 45 267 48
-----------------------------------------------------------------------
a A Beijing wheat sample containing 6 644 ng/g was excluded in
calculating this mean.
b Wheat flour, 7; rye flour, 1; spice, 3; sesame, 7.
It was found that 45-50% of random samples contained both DON and
NIV, barley being most frequently contaminated. Corn, although less
frequently contaminated, contained the highest average amounts of
NIV; of all the cereals examined, wheat was the most heavily
contaminated with DON. There were clear regional differences, not
totally predictable, in the relative quantities of DON and NIV. For
example, in Canada and the USA, NIV was only rarely encountered
whereas, in Japan, NIV was more frequently encountered than DON.
Even within a country there were clear differences between regions;
for example, in southern Japan, NIV was more frequently encountered
than DON, whereas in northern Japan the reverse was true. Similarly,
levels of DON were generally lower in western than in eastern Canada
(Jelinek et al., 1989).
The data indicate that crops parasitized by Fusarium species will
probably be contaminated with these mycotoxins. In wheat, for
example, there is an excellent correlation between the DON
contamination level and the percentage of mouldy kernels, the
percentage of total defects, and the degree of scab damage (Eppley et
al., 1984; Shotwell et al., 1985). It has been suggested that crop
rotation might be a factor, i.e., planting wheat after corn tends to
increase the DON levels in the resulting wheat crop (Teich &
Hamilton, 1985). Recently, it has been shown that the mean
concentration of DON in wheat declines quite significantly in the
2-week period immediately preceding harvest (Scott et al., 1984).
(b) Miscellaneous trichothecenes
There have been occasional reports of trichothecenes other than
those discussed above (T-2 toxin, DON, NIV) in agricultural products,
in particular, DAS, 15-acetyldeoxynivalenol (15-ADON), and 3-
acetyldeoxynivalenol (3-ADON) (Table 17). Most of these reports
involve corn. There is also an unconfirmed report of the finding of
T-2 toxin, DAS, and trichothecolone as well as the fatty acid esters
of trichothecolone, scirpenetriol, and T-2 tetraol in banana fruit,
which needs further investigation (Chakrabarti & Ghosal, 1986). In
1982, over 100 out of a flock of 1200 ewes on a Hungarian farm died
after ingestion of bedding straw highly infected with Stachybotrys
atra. TLC, HPLC, and MS analysis of the methanol extract of the
straw revealed the presence of the macrocyclic trichothecenes,
satratoxins G and H (Harrach et al., 1983).
Table 17. Natural occurrence: miscellanceous trichothecenes in
agricultural products
---------------------------------------------------------------------------
Commodity Toxin Level Incidence Country Reference
(mg/kg) (+ve/total)
---------------------------------------------------------------------------
Corn 15-ADON 0.113 China Qiujie et al.
ave. (1988)
3-ADON 0.495 China Qiujie et al.
ave. (1988)
DAS 31.5 Germany, Siegfried (1977)
Federal
Republic of
DAS 0.01- (6/20) New Zealand Hussein et al.
0.9 (1989)
15-ADON 0-7.9 USA Abbas et al.
(1986)
Peanuts DAS 0.41- (7/87) India Bhavanishankar &
2.03 Shantha (1987)
Wheat ADON 0.6-2.4 (4/12) India Bhat et al.
flour (1989)
Bedding satratoxin not Hungary Harrach et al.
straw G estimated (1983)
satratoxin not Hungary Harrach et al.
H estimated (1983)
---------------------------------------------------------------------------
The macrocyclic trichothecenes, such as satratoxin H, verrucarins
B and J, and trichoverrins A and B, were also detected in air dust
collected from a house in Chicago, where the occupants were subjected
to a variety of recurring maladies including colds, dermatitis, and
others (Croft et al., 1986). These data indicated a possible
airborne outbreak of macrocyclic trichothecene-induced toxicosis.
Undoubtedly, as good analytical methods become available, these
trichothecenes will be found more frequently.
II.2.3.2 Trichothecenes in human foodstuffs
Given the widespread occurrence of trichothecenes in agricultural
products, in particular DON and NIV, it is not surprising to find the
compounds in human foodstuffs (Table 18). The vast majority of the
confirmed cases of contamination of foodstuffs by trichothecenes
involve DON in wheat or wheat products. Overall, the finding of DON
in human foodstuffs at concentrations >1 mg/kg has been rare. NIV
has been detected in human foodstuffs, particularly in Japan, where
an effort was made to develop and apply analytical methods capable of
determining both DON and NIV.
Table 18. Natural occurrence of DON and NIV in commercial foods
---------------------------------------------------------------------------
Commodity Toxin Level Incidence Country Reference
(mg/kg)a (+ve/total)
---------------------------------------------------------------------------
Barley DON 0.027-0.085 (6/6) Japan Yoshizawa &
flour NIV 0.037-0.19 (6/6) Hosokawa
(parched) (1983)
Barley DON 0.008-0.039 (5/6) Tanaka et
flour NIV 0.013-0.041 (6/6) al. (1985b)
Barley DON 0.003-0.05 (10/14) Tanaka et
(pressed) NIV 0.008-0.033 (13/14) al. (1985a)
Barley DON t-0.26 (27/147) Kamimura et
products NIV 0.006-0.28 al. (1981)
Corn meal DON 0.025 (45/50) USA Trucksess et
al. (1986a)
Corn flour DON (0.18 ave.) (27) Canada Scott et al.
meal DON (0.1 ave.) (35) (1984)
products DON 0.011-1.25 (7/7)
Popcorn DON 0.012-0.25 (7/7) Japan Tanaka et al.
Job's tear DON 0.048-0.5 (2/12) (1985b)
NIV 0.003-0.92 (11/12)
Potatoes DON (4/17) Canada El Banna et
al. (1984)
Rye flour DON (0.12 ave.) (3) Canada Scott et al.
bread NIV (0.058 ave.) (4) (1984)
Wheat DON (0.4 ave.) (43)
flour DON 0.11-0.69 (5/5) China Ueno et al.
(1986)
DON 0.43-4.89 (9/12) India Bhat et al.
NIV 0.03-0.1 (2/12) (1989)
DON 0.002-0.239 (26/36) Japan Tanaka et
NIV 0.004-0.084 (12/36) al. (1985b)
DON 0-0.46 (44/50) USA Trucksess et
al. (1986a)
---------------------------------------------------------------------------
Table 18 (contd.)
---------------------------------------------------------------------------
Commodity Toxin Level Incidence Country Reference
(mg/kg)a (+ve/total)
---------------------------------------------------------------------------
Breakfast DON (0.086 ave.) (36) Canada Scott et al.
cereals (1984)
Bread DON (0.08 ave.) (21)
Baby cereal DON (0.043 ave.) (30)
Crackers DON (0.27 ave.) (20)
Cookies DON (0.12 ave.) (25)
Breakfast DON 0-0.53 (35/60) USA Trucksess et
cereals al. (1986a)
Bread DON 0-0.24 (20/25)
Baby foods DON 0-0.09 (14/39)
Snack foods DON 0-0.45 (25/44)
Bread DON 0.013-0.24 (39/45) USA Wood & Carter
(1989)
---------------------------------------------------------------------------
a t = trace.
The realization that trichothecene contamination of cereal grains
was not uncommon prompted a series of studies on the fate of these
compounds during the normal processing of such grains into consumer
food products. These studies can be summarized as follows:
In the case of corn, Collins & Rosen (1981) demonstrated that,
during wet milling, roughly 66% of the T-2 toxin originally present
was removed in the steeping water, about 4% remained in the starch,
and the remainder was distributed evenly in the germ, gluten, and
fibre fractions (a result clearly predictable in view of the
considerable water solubility of T-2 toxin). The same general
observation was made about the fate of DON during the wet milling of
corn (Scott, 1984b). During the dry milling of corn, the major
portion of contaminating DON was found in the germ meal, which is
used for animal feed. For any significant contamination of grits,
the corn used in the manufacture would have to be highly contaminated
(Gilbert et al., 1983a).
In the case of wheat, several studies have clearly demonstrated
that cleaning and milling are not effective in completely removing
DON (Hart & Baselton, 1983; Scott et al., 1983, 1984a; Young et al.,
1984; Seitz et al., 1985). The same was generally true for NIV (Lee
et al., 1987). Normal cleaning of wheat reduced average DON
contamination levels by 6-19% (Abbas et al., 1985). The remaining
DON, after milling, is distributed in all fractions (bran, shorts,
reduction flour, break flour), the greatest amounts being found in
the bran and the smallest amounts in the flour, depending on the
level of contamination of the wheat itself. In a typical study,
milling of whole wheat contaminated at the 2 µg/g level, resulted in
approximately 65% of the DON in the flour and 35% in the bran, red
dogs, and shorts; i.e., there was little reduction in the DON
concentration in the flour after milling (Hart & Baselton, 1983).
It has been found that the further processing of flour into baked
or cooked products results in variable DON losses. The overall range
of reduction in DON levels from uncleaned wheat to bread was 24-71%
(Abbas et al., 1985). No reduction in DON levels was found in the
preparation of Egyptian bread (El-Banna et al., 1983) or cookies
from hard wheat flour (Scott et al., 1983), illustrating the
importance played by the conditions of processing. Kamimura et al.
(1979) showed that bread and noodles prepared under conditions
simulating commercial processes retained about 50% of the
trichothecenes that had been previously added to the wheat flour.
The trichothecene content of Japanese noodles was reduced by 30% in
the boiling process.
Similarly, these authors found that 30% of NIV and DON was
extracted from naturally contaminated wheat by water, while there was
a 50% or more reduction in trichothecene levels in bread made from
the same flour. The preparation of Chinese noodles, where sodium
carbonate is used as an ingredient, results in an even greater loss
of DON than is observed in the preparation of Japanese noodles (Scott
et al., 1984b; Nowicki et al., 1988). Similarly, Young et al. (1984)
observed a decrease of up to 35% in the production of cookies from
biscuit and cake flours; in the process studied, the batter contained
ammonium carbonate. Finally, it has been found that, during the
baking of bread, 3-13% of DON is converted into an isomer, iso-DON
(Greenhalgh et al., 1984).
There have been some studies on possible detoxification
procedures for corn and wheat contaminated with trichothecenes;
agents studied include chlorine, ammonia (Young et al., 1984) and
aqueous bisulfite (Young et al., 1986). None of these processes are
currently commercially feasible.
There has been some concern about the transmission of the
trichothecenes, particularly DON, to milk. Studies have shown that
milk is only a minor excretion route in the lactating dairy cow;
thus, the possibility that DON might contaminate milk and milk
products is remote (Prelusky et al., 1984).
II.3 METABOLISM
II.3.1 Absorption and tissue distribution
II.3.1.1 Animal studies
In general, trichothecenes absorbed from the alimentary tract are
evenly distributed in many tissues and organs, without significant
accumulation in specific organs. However, at present, distribution
studies have been limited mainly to T-2 toxin, and the metabolism in
animals of other naturally contaminating trichothecenes, such as DON,
NIV, and DAS, remains to be elucidated.
In 6-week-old broiler chicks fed with a ration containing T-2
toxin at 2 mg/kg for 5 weeks and then intubated with a single dose of
3H-T-2 toxin at 0.5 mg/kg body weight, the radioactivity reached a
maximum concentration in most tissues, 4 h after dosing; exceptions
were the muscle, skin, and bile, in which the maximum level was
reached after 12 h (Chi et al., 1978b). After 48 h, chicks contained
the equivalent of 39 µg T-2 toxin and/or its metabolites/kg in the
muscle, and 40 µg/kg in the liver, as calculated on the basis of the
specific activity of the radiolabelled T-2 toxin administered.
In chicken organs, 18 h after intraperitoneal injection of T-2
toxin (3.5 mg/kg), considerable amounts of T-2 metabolites were found
in the liver (1370 µg 3'-hydroxy-H-T-2 toxin/kg). Smaller amounts of
H-T-2 toxin, T-2 triol, and other metabolites were detected in the
lungs (Visconti & Mirocha, 1985).
In weanling crossbred pigs (7.5-9.5 kg body weight) intubated
with 0.1 or 0.4 mg 3H-T-2 toxin/kg body weight, the percentage of
administered radioactivity (18 h after dosing) was 0.7% in the
muscle, 0.29-0.43% in the liver, 0.08% in the kidney, and 0.06-0.14%
in the bile (Robison et al., 1979b). In pigs intubated with 0.1 mg of
the toxin/kg body weight, the calculated residue levels for T-2 toxin
and/or its metabolites, based on the specific radioactivity of the
tissues, were as follows: muscle, 3.1 µg/kg; fat, 0.49 µg/kg; liver,
13.8 µg/kg; and kidney, 15.9 µg/kg. The corresponding residue levels
for T-2 toxin in the tissue of another pig intubated with 0.4 mg of
the toxin/kg body weight were 11.5 µg/kg in the muscle, 37.7 µg/kg in
the liver, and 61.4 µg/kg in the kidney.
Four hours after intravenous administration of T-2 toxin in
swine, the greatest amount of radioactivity was located in the
gastrointestinal tract (15-24% of the dose), and 4.7-5.2% of the dose
was found in the remaining tissues, among which the muscle and the
liver accounted for 2.9-3.2% and 0.7-1.7% of the dose, respectively
(Corley et al., 1986).
The fate and distribution of 3H-T-2 toxin was studied in guinea-
pigs (Pace et al., 1985). Except for the large intestine and bile,
the radioactivity had peaked by 30 min and rapidly declined, with no
measurable long-term accumulation. In general, the distribution
pattern in the guinea-pig during the first 12-24 h paralleled the
distribution found in the chicken and swine.
The metabolic fate of T-2 toxin was investigated in a lactating
Jersey cow weighing 375 kg, after daily oral administration, by
capsule, of unlabelled toxin at 180 mg/day for 3 days, followed by
administration of 3H-T-2 toxin at 156.9 mg (Yoshizawa et al., 1981).
Although almost all of the administered dose was eliminated in 72 h,
appreciable levels of tritium still remained in the bile, liver, and
kidney (equivalent, repectively, to 27.2, 18.5, and 13.9 µg/kg 3H-T-2
toxin) 3 days after dosing. These levels are higher than in whole
blood (13.3 µg/kg) and plasma (10.2 µg/kg) and in other tissues,
including the spleen (9.4 µg/kg) heart (10.1 µg/kg), mammary gland
(11.3 µg/kg), ovaries (10.7 µg/kg), muscle (8.8 µg/kg), and fat (4.7
µg/kg).
Experimentally derived relationships between the residue of
radioactivity in animal tissues or plasma and the toxin levels in the
feed, calculated on the basis of the amount of 3H-T-2 toxin
administered are summarized in Table 19 (Yoshizawa et al., 1981).
Table 19. The relationship between the level of 3H-T-2 toxin in the feed or the
tritium residues in plasma and tritium levels in the edible tissues of the cow, chick,
and piga
---------------------------------------------------------------------------------------
Tissue Animal Time after Feed level Tissue level Tissue/feed Tissue/plasma
dosing (h)b (mg/kg)c (µg/kg)d ratio ratioe
---------------------------------------------------------------------------------------
Muscle Cow 72 31.38 8.8 0.0003 0.863
Chick 24 1.26 17.3 0.0137 1.000
5.0 59.2 0.0118 0.938
18.95 228.6 0.0121 0.875
Pig 18 1.25 3.1 0.002 0.775
Heart Cow 72 31.38 10.1 0.0003 0.991
Chick 24 1.26 13.7 0.011 0.792
5.0 49.4 0.010 0.783
18.95 207.7 0.011 0.795
Pig 18 1.25 3.9 0.003 0.975
Liver Cow 72 31.38 18.5 0.0006 1.863
Chick 24 1.26 34.0 0.0270 1.965
5.0 107.3 0.0215 1.700
18.95 431.0 0.0227 1.649
Pig 18 1.25 13.8 0.011 3.450
Milk Cow 72 31.38 11.4 0.0004 1.118
---------------------------------------------------------------------------------------
a From: Yoshizawa et al. (1981).
b Animals were intubated with a single dose of 3H-T-2 toxin.
c Estimates of feed levels were based on the assumption that each animal would consume
the following amount of feed daily: cow, 5 kg; chick, 100 g; pig, 600 g.
d Values were calculated from residual tritium levels in the edible tissues of animals
given 3H-T-2 toxin and were expressed as equivalents of 3H-T-2 toxin (µg/kg).
e The plasma levels were 10.2 µg/kg equivalents of T-2 toxin in the cow, and 17.3,
63.1, and 261.3 µg/kg equivalents of T-2 toxin in the chick at feed levels of 1.26,
5, and 18.95 mg/kg, respectively. The whole-blood level of residual tritium in the
pig was 4 µg/kg equivalents of T-2 toxin.
The tissue/feed ratio of tritium in the edible tissues of the
cow, 72 h after dosing, ranges from 0.0003 to 0.0006. These figures
are 5-10% of those for swine, 18 h after dosing. The tissue/feed
ratio for chickens, is higher than those in the cow and pig, ranging
from 0.010 to 0.014 for the muscle and heart, and from 0.021 to 0.027
for the liver, regardless of T-2 toxin dosage. The tissue/plasma
ratios of T-2 toxin metabolites, ranging from 0.8 to 1 in the muscle
and heart, and from 1.6 to 3.5 in the liver, are independent of
animal species, of T-2 toxin dosage, and of time after dosing. The
milk/plasma ratio of radioactivity in a cow treated with 3H-T-2 toxin
increased linearly for 24 h and thereafter ranged from 1 to 1.3.
II.3.2 Metabolic transformation
The in vivo metabolic transformation of trichothecenes in animals
is reported in Table 20. The trichothecene metabolites produced are
less toxic than the corresponding parent toxins. Both de-epoxidation
and glucuronidation, in particular, are associated with remarkable
detoxification of trichothecenes.
Table 20. In vivo (metabolic) transformation of trichothecenes in animals
--------------------------------------------------------------------------------------------------
Animal Trichothecene Transformation reaction Reference
--------------------------------------------------------------------------------------------------
Chicken T-2 toxin hydrolysis, 3'-hydroxylation, Yoshizawa et al. (1980b);
acetylation Visconti & Mirocha (1985)
Cow T-2 toxin hydrolysis, 3'hydroxylation, Yoshizawa et al. (1981, 1982a,b)
7'-hydroxylation, de-epoxidation Pawlosky & Mirocha (1984)
Chatterjee et al. (1986)
DON de-epoxidation, Yoshizawa et al. (1986)
glucuronide conjugation Côté et al. (1986)
Pig T-2 toxin hydrolysis, 3'-hydroxylation, Corley et al. (1985, 1986)
de-epoxidation, glucuronide
conjugation
DAS hydrolysis Bauer et al. (1985)
Sheep DON glucuronide conjugation Prelusky et al. (1985)
Rat DON de-epoxidation Yoshizawa et al. (1983)
T-2 metabolites de-epoxidation Yoshizawa et al. (1985a,b)
DAS hydrolysis, de-epoxidation Sakamoto et al. (1986)
Guinea-pig T-2 toxin hydrolysis, 3'-hydroxylation Pace et al. (1985)
Dog T-2 toxin hydrolysis, glucuronide
conjugation Sintov et al. (1987)
--------------------------------------------------------------------------------------------------
In in vitro studies, HT-2 toxin was found to be the sole
metabolite of T-2 toxin in the microsomes of the liver, kidney, and
spleen of various animals (Ohta et al., 1977, Johnsen et al., 1986).
The reaction of hepatic microsomes of different species in nmol/mg
protein per 10 min were: rabbit, 3044; human, 331; mouse, 75;
chicken, 55; rat, 36; and guinea-pig, 14. Yoshizawa et al. (1984,
1986) have proposed an in vitro metabolic pathway for T-2 toxin that
includes the hydrolysates at the C-4, C-8, and C-15 positions, and
the hydroxylation at the C-3'position by liver homogenates from the
rat, mouse, or monkey. This hydrolytic transformation was also
observed in the hepatic homogenates of the rabbit, pig, and cow.
However, HT-2 toxin was the sole metabolite in the homogenate of the
chicken, suggesting species differences in the metabolic pathway of
T-2 toxin (Yoshizawa & Sakamoto, 1982). In addition to the
metabolites above, glucuronide conjugates were formed in a study
using isolated perfused rat livers and T-2 toxin and DAS (Gareis et
al., 1986). Various de-epoxidation metabolites were found by
incubating intestinal and rumen microbes with T-2 toxin and its
metabolites (Yoshizawa et al., 1985a; Swanson et al., 1987), DON
(King et al., 1984; Côté et al., 1986; Swanson et al., 1987), and DAS
(Swanson et al., 1987).
II.3.3 Excretion
II.3.3.1 Animal studies
The kinetics of T-2 toxin were determined in pigs and cattle
(Beasley et al., 1986), and dogs (Sintov et al., 1986). Mean
elimination phase half-lives were 13.8 and 17.4 min, and mean
apparent specific volumes of distribution were 0.366 and 0.375
litre/kg in intra-aortally dosed pigs and intravenously dosed calves,
respectively. In dogs, the following mean parameters were determined
after intravenous administration of T-2 toxin and HT-2 toxin,
respectively: half-life 5.3 and 19.6 min, clearance 0.107 and
0.167 litre/min per kg, and volumes of distribution 0.86 and 4.47
litre/kg.
The half-life of elimination of DON in sheep, ranged from 100 to
125 min following oral administration, and it took 20-30 h to clear
from the system. Glucuronidation after intravenous or oral
administration of DON appeared to occur quite efficiently (iv, 21%;
oral, 75%), with elimination half-lives of (150-200 min and 6.1-7.1
h, respectively). These were considerably longer than those of the
parent toxin (Prelusky et al., 1985).
II.3.3.2 Excretion in eggs and milk
Radioactivity was transmitted into the eggs from laying hens that
had been intubated gastrically with a single or several doses of 3H-
T-2 toxin (Chi et al., 1978a). In birds dosed singly with 0.25 mg
T-2 toxin/kg body weight, maximum residues in the eggs occurred 24 h
after dosing; the yolk contained 0.04% of the total dose and the
white contained 0.13%. In birds dosed with 0.1 mg T-2 toxin/kg body
weight per day for 8 consecutive days, the radioactivity in the egg
accumulated until the 5th day of dosing, remained unchanged until the
last day of dosing, and rapidly decreased thereafter. Assuming that
the birds weighing 1.6 kg consumed 100 g of the diet containing 1.6
mg toxin/kg daily, the residues (T-2 toxin and/or its metabolites) in
such contaminated eggs would be about 0.9 µg/egg.
Transmission of DON was studied in the eggs and meat of chickens
(El Banna et al., 1983; Prelusky et al., 1987). Following a single
oral dose of 14C DON, maximum radioactivity, which occurred in the
first eggs laid after dosing (within 24 h), amounted to 0.087% of the
dose: levels dropped rapidly in later eggs.
In a pregnant Holstein cow (third trimester) intubated daily with
180 mg T-2 toxin for consecutive days (the toxin level corresponded
to a concentration of 50 mg/kg in the feed), milk samples taken on
the 2nd, 5th, 10th, and 12th days of intubation contained T-2 toxin
concentrations ranging from 10 to 160 µg/kg (Robison et al., 1979a).
Transmission of DON-1 (de-epoxydeoxynivalenol) to milk was confirmed
in lactating dairy cows (Côté et al., 1986; Yoshizawa et al., 1986).
Fresh and conjugated DON were also present in cow's milk following
administration of a single oral dose of 920 mg DON, but only
extremely low amounts (<4 µg/litre) were detected (Prelusky et al.,
1984).
II.4 EFFECTS ON ANIMALS
II.4.1 Field observations
In Hungary and other central European countries, pyosepticaemia
has been reported sporadically, in the past, in horses after
ingestion of mouldy hay and straw. This disease was characterized by
haemorrhages of the intestine and muscles, severe diarrhoea, and
death. Bacterium pyosepticum viscosum was detected in 1929 in the
excreta, and the equine disease was diagnosed as a pyosepticaemia
(Forgacs, 1965; Danko & Szerafin, 1976). After the discovery of
toxigenic Stachybotrys atra and its metabolites, such as satratoxins
G and H (Eppley & Bailey, 1973), the disease was presumed to be the
same as stachybotryotoxicosis (Danko & Szerafin, 1976).
A field outbreak involving the death of 20% of a dairy herd was
associated with prolonged ingestion of a diet containing 60% mouldy
corn infested with F. tricinctum. The concentration of T-2 toxin in
the feed was approximately 2 mg/kg dry weight (Hsu et al., 1972).
The lesions in the cattle included extensive haemorrhages on the
serosal surface of the internal viscera. An outbreak of haemorrhagic
syndrome in cows was associated with commercial feed containing T-2
toxin or T-2-like toxin (concentration not determined). The affected
animals showed an extremely prolonged prothrombin time. Necropsy
findings in 2 adult cows were marked serosal, mucosal, and
subcutaneous haemorrhages (Hibbs et al., 1975).
An outbreak of a disease, observed in poultry (ducks, geese),
horses, and pigs, was associated with mouldy barley containing T-2
toxin at approximately 25 mg/kg (Greenway & Puls, 1976). Pigs fed
the suspect barley exhibited signs of feed refusal, vomiting, and
diarrhoea. The horses became depressed and salivated excessively.
The lesions in the geese included necrosis of the mucosa of the
oesophagus, proventriculus, and gizzard. No pathological lesions
were described in other animals.
DON was isolated from a batch of maize that had caused vomiting
in pigs (Vesonder et al., 1973).
Equine leukoencephalomalacia reported from South Africa
(Kellerman et al., 1972; Marasas et al., 1979a; Pienaar et al., 1981)
and bean-hull toxicosis reported in horses in Hokkaido, Japan
(Konishi & Ichijo, 1970) appear to be very similar diseases with
nervous signs and hepatopathy as the major components. The signs in
these diseases were quite different from those caused by
trichothecenes, though some fungal isolates from samples of bean-
hulls produced the trichothecenes, T-2 toxin, and neosolaniol (Ueno
et al., 1972). F. moniliforme was considered to be the causative
fungus (Haliburton et al., 1979), but none of its metabolites,
including moniliformin, have been established as the cause of the
disease (Kriek et al., 1977).
Reports of field outbreaks of animal toxicoses associated with
trichothecene-contaminated feed are summarized in Table 21.
II.4.2 Effects on experimental animals
II.4.2.1 General toxic effects
LD50 values for certain trichothecenes in several experimental
animal species are summarized in Table 22 (Ueno et al., 1983). The
oral LD50 for T-2 toxin was 10.5 mg/kg body weight in mice, 3.06
mg/kg in guinea-pigs, 5.2 mg/kg in rats, and 6.1 mg/kg in trout.
The LD50 values for T-2 toxin in different species vary, but not
greatly.
The LD50 values of fusarenon-X were compared using different
routes of administration. The LD50s (mg/kg) in mice were 3.4 (iv),
3.4 (ip), 4.2 (sc), and 4.5 (oral). These data indicate that the
acute toxicity estimated for a single administration did not differ
markedly when the toxin was administered by different routes (Table
20) (Ueno et al., 1971).
Similar data were obtained with DON and acetyl-DON. An
interesting finding was that the ratio of the maximum lethal dose
to the minimum lethal dose was approximately two, indicating a
sharp dose-response curve for lethality. No marked differences in
acute toxicity were observed between treated male and female
animals.
Newborn animals are more sensitive than adults to the
toxic effects of the trichothecenes. For example, the LD50 values
for toxin given sc (mg/kg body weight) in newborn mice were: T-2
toxin, 0.15; DAS, 0.17; and fusarenon-X 0.23 (Ueno et al., 1973a).
Table 21. Field observations on animal toxicosis caused by trichothecenes
----------------------------------------------------------------------------------------------------
Mycotoxin Sample Animal Signs & lesions Reference
(concentration in feed)
----------------------------------------------------------------------------------------------------
T-2 toxin (2 mg/kg) mouldy corn cattle extensive haemorrhages Hsu et al.
(20% died) (1972)
T-2 toxin (25 mg/kg) mouldy barley poultry, necrosis of mucosa in the Greenway &
horse, proventriculus and Puls (1976)
pig oesophagous of geese
T-2 toxin commercial feed cow haemorrhages Hibbs et
al. (1975)
DAS maize pig vomiting Vesonder et
al. (1973)
T-2 toxin corn meal horse oral lesions, haemorrhages, Szathmary
(6 out of 58 died) (1983)
T-2 toxin alfalfa horse inappetence, listlessness, Szathmary
(13 out of 31 died) (1983)
T-2 toxin poultry oral lesions, inappetence, Szathmary
death (1983)
DAS and T-2 toxin corn cattle death Szathmary
(1983)
DAS oat, sifting pigeon emesis, bloody stools Szathmary
(1983)
T-2 toxin (2.5 mg/kg) chicken feed broiler inflammations, "atrophies" Szathmary
chicken (1983)
DAS (150-300 mg/kg) cattle, pig haemorrhagic syndrome Cirilli
(1983)
T-2 toxin (50-150 mg/kg) pig, cattle blood stools (swine, cattle) Cirilli
ear necrosis (swine) (1983)
intestinal lesions (poultry)
hepatic lesions (swine)
----------------------------------------------------------------------------------------------------
Table 22. LD50 values (mg/kg) of trichothecenesa
----------------------------------------------------------------------------------------------------------
Type Trichothecenes Mouse Rat Guinea-pig
iv ip sc oral iv ip sc oral ip sc oral
----------------------------------------------------------------------------------------------------------
A T-2 toxin 5.2 10.5 5.2 3.06
HT-2 toxin 9.0
Diacetoxyscirpenol (DAS) 12 23.0 1.3 0.75 7.3
Neosolaniol 14.5
Monoacetoxyscirpenol 0.725
B Nivalenol (NIV) 7.3 7.4 7.2 38.9
Fusarenon-X 3.4 3.4 4.2 4.5 4.4 0.5 0.1
Diacetylnivalenol 9.6
Deoxynivalenol (DON) 70.0 46.0
3-Acetyldeoxynivalenol 49.0 34.0
Trichothecin 300 250
D Roridin A 1.0
Verrucarin A 1.5 0.5 0.87
Verrucarin B 7.0
Verrucarin J 0.5
----------------------------------------------------------------------------------------------------------
Table 22 (contd.)
-----------------------------------------------------------------------------------------------------
Type Trichothecenes Rabbit Cat Dog Pig Duckling Day-old Chick Trout
(iv) (sc) (iv) (iv) (sc) chick (oral) (oral)
(oral)
-----------------------------------------------------------------------------------------------------
A T-2 toxin 0.5 1.21 1.75 4.0 6.1
HT-2 toxin 6.25
DAS 1.0 ca 1.1 0.37
3'-OH HT-2 toxin 8.5 5.0
15-Acetyl-T-2 tetraol 10.0
T-2 tetraol 10.0
B Fusarenon-X 5.0 ca 2.0
DON 27.0
Acetyldeoxynivalenol 37.0
D Verrucarin A 0.54
-----------------------------------------------------------------------------------------------------
a Adapted from: Ueno et al. (1983) and Ryu et al. (1988).
The toxic potency of the trichothecenes varies depending on the
modification of side chains in the molecule. The acute lethal
toxicity of certain trichothecenes was investigated using a single ip
injection in mice and the LD50 values (mg/kg body weight) were:
verrucarin A and B, 0.5; fusarenon-X, 3.4; NIV, 4.1; T-2 toxin, 5.2;
HT-2 toxin, 9.0; diacetylnivalenol, 9.6; neosolaniol
(8-hydroxydiacetoxyscirpenol), 14.5; DAS, 23.0; acetyldeoxynivalenol,
49.0; DON, 70.0; and crotocin, 810.0.
Matsuoka et al. (1979) investigated the general effects of
fusarenon-X on mice and rats. Fusarenon-X induced hypothermia, but
did not induce appreciable behavioural changes in mice. In ether-
anaesthetized rats, it caused a rise in blood pressure and a decrease
in respiratory rate, but did not induce any significant effects on
cardiac rate, the muscle cell membrane, or nerve elements.
The administration of trichothecenes to some animals (rats, mice,
and guinea-pigs) induces diarrhoea. The mechanism of this sign was
investigated using fusarenon-X and rats (Matsuoka & Kubota, 1981).
The ip injection of fusarenon-X in rats caused watery diarrhoea
within 36-60 h. At necropsy, 24 h after injection of fusarenon-X,
the small intestine was distended, but no blood was found in the
lumen of the intestine. The mycotoxin increased the absorption rate
of D-xylose from the intestine in vitro (Matsuoka & Kubota, 1981).
The leakage of intravenously injected Evan's blue dye into the
intestine also increased, but the sodium level in the serum
decreased. The intestinal villi were shortened and there was
extravasation of erythrocytes in the intestinal lamina propria. The
diarrhoea induced by ip admin-istration of 1.0 mg fusarenon/kg in
male Wistar rats was not mediated by the cyclic nucleotide system as
the mycotoxin did not increase the cyclic GMP and AMP contents in the
intestinal mucosa (Matsuoka & Kubota, 1987). The permeability of
abdominal blood vessels was increased in a dose-dependent manner in
mice given an ip injection of fusarenon-X, and the peak was reached
about 8 h after injection. The increased permeability was not
mediated by serotonin, histamine, norepinephrine, prostaglandins,
leukotrienes, or thromboxanes (Matsuoka & Kubota, 1987).
The effects of fusarenon-X and T-2 toxin on intestinal absorption
of monosaccharides were studied in rats. The absorption of 3- O-
methyl-glucose was reduced 1-3 times after either toxin was injected
into the jejunal lumen. Absorption of 3- O-methyl-glucose was also
reduced after the toxins were given by intravenous injection. Both
toxins impaired jejunal function by causing specific damage in the
active transport and diffusional movement of monosaccharides (Kumagai
& Shimizu, 1988).
Vomiting was one of the most significant signs of trichothecene-
induced toxicosis in the cat, dog, pig, and duckling (Ueno, 1980a).
T-2 toxin and related trichothecene mycotoxins at doses of 0.1-10
mg/kg induced vomiting (Vesonder et al., 1973; Sato et al., 1975;
Yoshizawa & Morooka, 1977; Ueno, 1980b; Matsuoka & Kubota, 1981).
The presence of the causal factor, DON, in mouldy corn was
established using ducklings as the assay animal (Ueno et al., 1974).
In pigs given 0.5 mg/kg body weight by infusion, vomiting commenced
6-7 min after dosing. Emesis or retching occurred at intervals of 2-
15 min and lasted from 0.5 to 2 min (Coppock et al., 1985). It is
strongly suggested that the mechanism of the vomiting of
trichothecenes is their possible action on the chemoreceptor trigger
zone (CTZ) in the medulla oblongata (Matsuoka et al., 1979). The iv
administration of 0.3 mg fusarenon-X/kg body weight to dogs induced
emesis and vomiting, 5-15 min after injection. Vomiting after
injection of fusarenon-X was prevented by prior administration of 0.5
mg metoclopramide hydrochloride/kg body weight or 1 mg
chloropromazine hydrochloride/kg.
The cardiovascular effects of the trichothecenes have varied
according to such factors as species, dose, and duration of exposure.
In acute studies, T-2 toxin given intravenously at 1 mg/kg body
weight produced a decline in blood pressure several hours after
administration, the reduced blood pressure being accompanied by a
decrease in heart rate (Smalley et al., 1970). A single dose of T-2
toxin administered to guinea-pigs and rabbits resulted in a decrease
in systemic blood pressure and a decrease in heart rate (Parker et
al., 1984; Wilson, 1984).
Using the in vitro bovine ear perfusion system, it was determined
that T-2 toxin can cause a dose-dependent vasoconstrictor response in
peripheral vasculature, but that the toxin is a less potent
vasoactive agent than either histamine or norepinephrine. The
presence of known histamine or noradrenergic anatogonist did not
affect the response to the toxin (Wilson & Gentry, 1985). T-2 toxin
administered systemically produced a marked increase in peripheral
vascular resistance in the conscious rat. The cardiac output
gradually decreased eventually resulting in cardiovascular collapse
and death (Feuerstein et al., 1985).
(a) Swine
The acute and short-term toxicities of T-2 toxin, DAS, and DON
were investigated in pigs (Weaver et al., 1978a,b; Coppock et al.,
1985). A single dose LD50 of T-2 toxin dissolved in ethanol and
administered iv was 1.21 ± 0.15 mg/kg body weight in normal, healthy,
crossbred pigs weighing from 3 to 50 kg. Soon after administration,
emesis was followed by eager consumption of feed, moderate posterior
paresis, staggering gait, extreme listlessness, and frequent
defecation of normal stools. Between 1 and 6 h, severe posterior
paresis, knuckling-over of the rear feet, and extreme lethargy were
observed. These signs were followed by severe posterior paresis,
frequent falling because of hind-quarter weakness, and the dragging
of both rear legs while moving about. Twenty-four hours after
administration, the surviving pigs appeared normal. Similar clinical
signs were observed in pigs exposed to T-2 toxin through inhalation
(Pang et al., 1988). Pathologically, necrosis was present in the
epithelial cells of the mucosa and in the crypt cells of the jejunum
and ileum, the Peyer's patches of the ileum, the lymphoid elements of
the caecum, the lymphoid follicles in the spleen, and the germinal
centre of the mesenteric lymph node (Weaver et al., 1978a).
Young pigs were fed with T-2 toxin at 1, 2, 4, or 8 mg/kg
standard pig ration for 8 weeks. No statistically significant
differences in body weight gain and feed consumption were observed
between the treated animals and the controls. Young pigs refused a
ration containing 16 mg T-2 toxin/kg, but not a diet containing 10-12
mg/kg. The no-observed-effect level was estimated to be less than 1
mg/kg, based on differences in body weight gain (Weaver et al.,
1978b). In terms of clinical haematological changes, such as
haemorrhaging, blood cell counts, serum-enzyme activities, and serum-
protein levels, the no-observed-effect level could not be accurately
determined, but was higher than 12 mg/kg, based on the weight gain.
The intravenous administration of T-2 toxin to pigs at doses of 4
or 8 mg/kg resulted in a shock syndrome characterized by reductions
in cardiac output and blood pressure and increased plasma
concentrations of epinephrine, norepinephrine, thromboxane B2, 6-
keto-PGF1a and lactate (Lorenzana et al., 1985). The pigs in the
high-dose group produced such signs as persistent vomiting, watery
diarrhoea, abdominal straining, cold extremities, coma, and death.
Eighteen white cross-bred female pigs weighing 40-60 kg,
immunized against erysepelas, were administered purified T-2 toxin
dissolved in 70% ethanol, intravenously, in doses of 0 (5 pigs), 0.6
(5 pigs), 1.2 (1 pig), 4.8 (5 pigs) and 5.4 (2 pigs) mg/kg. The
animals administered doses of 4.8 and 5.4 mg/kg died between 5 and
10.5 h later and other groups were killed 12-24 h after treatment.
Gross lesions were observed in pigs given 1.2 mg/kg or more and these
consisted of oedema, congestion, and haemorrhages of the lymph nodes
and pancreas and congestion and haemorrhages of the gastrointestinal
mucosa, subendocardium, adrenal glands, and meninges. Histological
alterations confirmed the gross lesions. Other lesions were
widespread degeneration and necrosis of lymphoid tissue and the
surface and crypt epithelium of the intestines. Scattered foci of
necrosis were present in the pancreas, myocardium, bone marrow,
adrenal cortex, and the tubular epithelium of the renal medulla.
Most lesions were dose dependent. The T-2 toxin-induced lesions in
the lymphoid and gastrointestinal tract of pigs were similar to those
described in other species. The heart and pancreas were additional
target organs in pigs (Pang et al., 1987b).
Male, castrated, crossbred, specific pathogen-free pigs (17
controls and 17 treated), 9-11 weeks of age, were used in a study
to characterize the pulmonary and systemic responses to inhaled T-2
toxin (nebulized dose of 9 mg/kg given by endotracheal tube) (Pang et
al., 1987a). The animals were exposed to the aerosol in pairs, one
animal receiving the toxin, the other acting as a control. From 20
to 30% of the toxin was retained by the pigs (T-2 toxin was mixed
with 100-200 µCi technetium for measurement). Five pairs of animals
each were killed 1, 3, and 7 days after dosing. Two pairs were
designated a 0.33 day group, when one treated pig died and the
other was killed in a moribund state, 0-10 h after dosing.
Clinically, the T-2 toxin-treated pigs vomited after exposure
producing such signs as cyanosis, anorexia, and lethargy. The pigs
became laterally recumbent. Alveolar macrophages showed reduced
phagocytosis and the blastogenic responses to mitogen were reduced
for pulmonary lymphocytes, but not for lymphocytes of the peripheral
blood. The lesions in pigs that died included multifocal
interstitial pneumonia, necrosis of lymphoid tissue,
necrohaemorrhagic gastroenteritis, oedema of gall bladder mucosa, and
multifocal areas of necrosis in the heart and pancreas. Inhalation
exposure to T-2 toxin produced a clinical and morphological syndrome
resembling that produced by intravenously administered T-2 toxin, at
doses of 1.2 mg/kg (approximate LD50) or more, as well as death.
Furthermore, the lesions produced by the inhaled toxin were more
severe.
The acute effects of DAS were studied using single, intravenous
doses (range: 0.30-0.48 mg/kg body weight) in 13 crossbred pigs;
there were 2 control pigs (Weaver et al., 1978b). Seven of the
toxin-exposed pigs developed emesis, frequent defecation, lethargy,
staggering gait, and prostration by 10 h leading to death. Severe
haemorrhagic necrotizing lesions and mucosal congestion involved the
jejunum and ileum and large intestines, portions of which were blood-
filled at necropsy. Lymphoid follicular necrosis was present in the
lymph nodes and spleen. The LD50 value was found to be 0.376 ± 0.043
mg/kg body weight.
Two female crossbred pigs were administered DON by rapid
intravenous infusion at a dose of 0.5 mg/kg body weight; there were
two matching controls. Vomiting was observed within a few minutes
of dosing, the skin was flushed and the extremities became cold.
Pigs had signs of diarrhoea, muscular weakness, tremors, and coma.
Symptoms were progressive in severity reaching a maximum 6-7 h after
the injection; recovery occurred after 12 h. Necrosis of pancreatic
acinar and islet cells was observed (Coppock et al., 1985). Pigs can
ingest up to 2 mg DON/kg feed without suffering any serious toxic
effects (Trenholm et al., 1984). T-2 toxin added to the ration at 18
or 30 mg/kg caused the refusal of feed in pigs (Szathmary & Rafai,
1978). Feed refusal and emesis have been produced in other species
and by other toxic metabolites of Fusarium species (Kotsonis et al.,
1975; Vesonder et al., 1977).
The minimum emetic dose of DON in pigs weighing 9-10 kg was 0.05
mg/kg body weight, when administered intraperitoneally, and 0.1-0.2
mg/kg body weight, when given orally. When this toxin was added to
feed, the feed consumption of 20-45 kg pigs, was reduced by 20% at a
dose of 3.6 mg/kg and by a 90% at a dose of 40 mg/kg (Forsyth et al.,
1977). Pigs were about twice as sensitive to DON as rats (Vesonder
et al., 1979). DON in contaminated wheat reduced feed intake and
weight gain, when it was fed to the pigs. The intake of feed
decreased linearly with increasing dietary concentration of DON
(Friend et al., 1982). The emetic activity of 15-acetyl DON in pigs
was similar to that of DON, the minimum emetic doses being 75 and 50
µg/kg, respectively (Pestka et al., 1987a).
(b) Poultry
Chi et al. (1977b), reported that the single oral LD50 dose of
T-2 toxin for one-day-old broiler chicks was 5 mg/kg body weight. It
was 5 and 6.3 mg/kg body weight for 8-week-old broiler chicks and
laying hens, respectively. Death of the birds occurred within 48 h
of T-2 toxin administration. Within 4 h of receiving the toxin,
birds developed asthenia, inappetence, diarrhoea, and panting. The
abdominal cavities of birds given lethal doses contained a white
chalk-like material that covered much of the viscera.
In a study by Wyatt et al.(1972), chickens were fed a diet
containing 1-16 mg T-2 toxin/kg feed for 3 weeks. The birds with
reduced growth at 4, 8, and 16 mg/kg developed yellow-white lesions
in the mouthparts at all dietary concentrations. The lesions
consisted of a fibrinous surface layer and a heavy infiltration of
the underlying tissues by granular leukocytes. Escherichia coli and
Staphylococcus epidermis were isolated from the lesions.
Terao et al.(1978) observed the effects of T-2 toxin and related
trichothecenes on the bursa of Fabricius of one-day-old chicks.
After injection of 5 mg T-2 toxin/kg body weight into the residual
yolk sac, cellular toxic effects were observed on the follicle-
associated epithelium, resulting in necrosis, which spread to the
periphery. The lesions induced by fusarenon-X and NIV were similar
to those induced by T-2 toxin, but the toxins were less potent and
their activity was estimated to be more than 40 times less than that
of cyclophosphamide.
The acute toxicity of DAS and of T-2 toxin dissolved in
dimethylsulfoxide in 7-day-old male broiler chicks was described by
Hoerr et al. (1981). The 72-h single oral LD50 doses of T-2 toxin
and DAS were estimated to be 4 and 5 mg/kg body weight, respectively.
Combination of the 2 toxins caused increased mortality in both the
single-and multiple-dose tests. Lesions produced by crop gavage with
T-2 toxin and DAS were similar, but were more severe in chicks given
T-2 toxin. Necrosis of lymphoid tissue and bone marrow was observed
in tissue taken 1 h after treatment followed by rapid depletion.
Necrosis was observed in the liver, gall bladder, and gut.
Chi et al.(1977c), fed broiler chicks (36 per group), aged one
day to 9 weeks, a diet containing T-2 toxin at concentrations of 0.2,
0.4, 2, or 4 mg/kg. Birds fed 4 mg T-2 toxin/kg showed reduced body
weight gain and feed consumption and developed oral lesions
characterized by circumscribed proliferating yellow caseous
plaques at the margin of the beak, the mucosa of the hard
palate and the tongue, and the angle of the mouth. No lesions were
observed in the bone marrow or, to any significant extent, in the
peripheral blood. The no-observed-effect doses of T-2 toxin were 0.2
mg/kg for weight gain, and 0.2 mg/kg for oral lesions. When one-day-
old broiler chicks were fed a diet containing 1, 2, 4, 8, or 16 mg T-
2 toxin/kg feed for 3 weeks the no-effect doses were estimated as
follows: growth rate, weight of pancreas, and weight of spleen: 2
mg/kg; oral lesions: <1 mg/kg (Wyatt et al., 1973c).
T-2 toxin administered to laying hens at a concentration of 20
mg/kg feed reduced egg production and resulted in the production of a
thinner egg shell (Wyatt et al., 1975). Speers et al.(1977), also
observed cessation of egg production in hens fed diets containing
25-50 mg monoacetoxyscirpenol/kg or 16 mg T-2 toxin/kg. It was
reported by Chi et al. (1977a) that feed consumption, egg production,
and shell thickness were significantly decreased in hens fed 8 mg of
T-2 toxin/kg. Furthermore, the hatchability of fertile eggs of hens
fed 2 or 8 mg T-2 toxin/kg was lower than that of hens fed the
control diet (Chi et al., 1977a).
Three groups of 1-day-old chicks (10 chicks per group) were each
fed 0.5-15 mg T-2 toxin/kg for 3 weeks (Coffin & Combs, 1981).
Plasma-vitamin E activity and hepatic-vitamin A content were
measured. Dose-dependent depression of plasma-vitamin E activity was
observed, with a 65% decrease compared with controls in chicks fed a
diet containing 15 mg T-2 toxin/kg. This decrease was believed to be
caused by a reduction in the plasma level of lipoproteins, which are
required for the transport of vitamin E.
(c) Ruminants
In a study by Pier et al. (1976), 4 calves received 0.08-0.6 mg
T-2 toxin/kg body weight orally in capsules for 30 days. The high-
dose calf developed a hunched stance and died on day 20. At all
levels, some evidence of mild enteritis with loose faeces was
obtained. Clinically apparent signs were confirmed at doses of 0.16
mg/kg or more, and bloody faeces at doses of 0.32 mg/kg or more. At
necropsy, abomasal ulcers were present in the calf given 0.16 mg/kg
and ruminal ulcers in calves given the 2 higher doses. Prothrombin
times and levels of serum GOT activity were increased in calves given
the 2 higher doses.
Ten male Suffolk-Finn-Columbian lambs, in 2 groups of 5 animals
each, were fed T-2 toxin at 0.3 or 0.6 mg/kg body weight for 21 days.
There were 5 controls. Experimental lambs developed focal hyperaemia
and dermatitis at the mucocutaneous junction of the commissure of the
lips, diarrhoea, leukopenia, lymphopenia and lymphoid depletion of
the mesenteric lymph nodes and spleen (Friend et al., 1983b).
Sheep dosed with roridin A and verrucarin A (4 mg/kg) had severe
and extensive haemorrhagic gastroenteritis. Oedema was marked in the
abomasum; the small intestine of the roridin-treated lamb had casts
of clotted blood and necrotic debris. Both small and large
intestines contained grossly haemorrhagic areas and extensive mucosal
erosions. In the lamb given verrucarin A at 4 mg/kg body weight, the
lesions were sublobular haemorrhages in the liver, which had a nutmeg
appearance, mucosal erosions, haemorrhages in the small intestine,
and haemorrhages of the endocardium of the left ventricle of the
heart (Mortimer et al., 1971).
(d) Cats
Three studies have described the clinical and tissue alterations
produced by administration of T-2 toxin to cats (Sato et al., 1975;
Lutsky et al., 1978; Lutsky & Mor, 1981). Lutsky et al. (1978) used
20 cats in 4 groups of 4-6 animals each. There were 4 controls. The
toxin was administered orally in gelatin capsules on alternative days
at doses of 0.06, 0.08, or 0.10 mg/kg body weight, until death. The
survival time ranged from 6 to 40 days. The signs included emesis,
anorexia, bloody diarrhoea, and ataxia. The cats lost weight and
became emaciated. Gross lesions included multiple petechiae to
ecchymotic haemorrhages of the intestinal tract, lymph nodes, and
heart. The lumen of the gut contained copious amounts of dark red
contents. Microscopic lesions included haemorrhages in the gut,
lymph nodes, heart, and mininges, necrosis of gastrointestinal
epithelium and decreased cellularity of the bone marrow, lymph nodes,
and spleen.
(e) Rodents
The trichothecenes used in long-term studies were T-2 toxin and
fusarenon-X. Lesions were observed in the oesophageal region of the
stomach of DDD mice fed T-2 toxin at 10 or 15 mg/kg diet for 12
months. The alterations included hyperplasia, hyperkeratosis, and
acanthosis of the squamous epithelium. Such changes were found 13
weeks after the start of feeding the toxins and were consistently
observed during the 12-month feeding period. However, most had
subsided 3 months after cessation of feeding. Similar gastric
lesions were observed in Wistar rats fed T-2 toxin at concentrations
of 5, 10, or 15 mg/kg feed for 4 weeks. The lesions were diffuse and
severe in the rats fed 15 mg/kg, focal but definite in those fed 10
mg/kg, and negligible in the stomach of rats fed 5 mg/kg (Ohtsubo &
Saito, 1977).
Six female Holtzman albino rats were fed T-2 toxin at 5 or 15
mg/kg for 19 days and T-2 toxin at 10 mg/kg diet for 8 months. No
gastric lesions were observed in any of the animals in the
experimental groups (Marasas et al., 1969).
Three groups of 12, six-week-old female Swiss ICR mice (15-20 g
body weight) were administered T-2 toxin (by 10-minute aerosol
exposure) Two control groups contained 8 mice each using nose-only
exposure. The aerosol mass concentration varied between 225 and 275
µg T-2 toxin/litre of air. Tissues from mice were microscopically
examined 0.25, 1, 2, 4, 6, 8, 12, and 24 h after exposure. Lymphoid
necrosis was observed 1 h after exposure in the thymus, spleen, and
lyphoid nodules of the intestinal tract. Necrosis of intestinal
crypt epithelial cells was present 2 h after exposure and necrosis of
adrenal cortical cells 4 h after exposure (Thurman et al., 1988).
In male Sprague-Dawley rats, T-2 toxin, given intravenously,
produced reduced blood flow and increased vascular resistance in
hind-quarter, mesenteric, and renal vascular beds. Mean arterial
pressure and heart rate were not significantly altered. A maximum
drop in blood flow in mesenteric and renal vascular beds occurred 4 h
after the T-2 toxin was injected (Siren and Feuerstein, 1986).
II.4.2.2 Haematological and haemostatic changes
A haemorrhagic syndrome was reported to be the characteristic
feature of mouldy corn toxicosis in the cow (section II.4.1.).
However, this hemorrhagic syndrome could not be produced in other
studies.
In a study by Patterson et al. (1979), 2 calves were administered
0.2 mg T-2 toxin/kg body weight and one calf was given the same dose
of DAS; both compounds were given by stomach tube,daily for 11 days.
There were no controls. The T-2-treated animals developed clinical
signs of weakness, inappetence, and one died. Prothrombin time was
prolonged in both animals and one had marked neutrophilia. No
clinical signs or haematological changes were observed in the animal
administered DAS. No haemorrhagic syndrome was found in these
calves.
When pigs (9-10 weeks old, male, castrated, specific pathogen-
free) were exposed to a T-2 toxin aerosol (390 µg/litre, 15 µm mass
median aerodynamic diameter) for a period that allowed an amount
equivalent to 8 mg/kg to be nebulized, the haematological alterations
included a decrease in lymphocyte and neutrophil counts, and
decreased concentrations of serum-protein and haemoglobin (Pang et
al., 1988).
Haematological changes were observed in mice, rats, cats, and
guinea-pigs treated with T-2 toxin and related trichothecenes (Sato
et al., 1975, 1978; Sato & Ueno, 1977; DeNicola et al., 1978). In
cats, leukocytosis occurred early after the administration of T-2
toxin. A similar change was observed in mice treated with T-2 toxin,
neosolaniol, and fusarenon-X. Among the leukocytes, lymphocytes
showed the greatest increase followed by neutrophils; the
leukocytosis was followed by marked leukopenia after short-term
exposure to T-2 toxin. This leucopenic state was also induced by DAS
in mice (Conner et al., 1986), rats and dogs (Stahelin et al., 1968)
and by verrucarin A in rats, dogs, guinea-pigs, and monkeys (Rusch &
Stahelin, 1965). Pancytopenia was also reported in cats administered
T-2 toxin for 2 weeks (Lutsky et al., 1978; Lutsky & Mor, 1981). In
guinea-pigs treated with T-2 toxin (0.9 mg/kg body weight per day)
for 27 days, erythropenia, leukopenia, and absolute lymphopenia were
observed, with a marked decrease in the lymphocyte contents of the
bone marrow (DeNicola et al., 1978).
Hayes et al. (1980) studied the effects of T-2 toxin on the
haematopoiesis in mice. Twenty-four male weanling outbred Swiss mice
were fed a balanced semipurified diet, containing crystalline
purified T-2 toxin at a level of 20 mg/kg dry diet. One group of 20
animals received the toxin in the diet for 41 days and another group
of 4 animals, for 21 days, followed by control diet for 7 days.
Forty-eight animals in 3 groups served as controls and received the
semipurified diet with restricted intake (20 animals for 41 days),
and ad lib (8 animals for 28 days). In addition, 12 animals were
sacrificed at day 0. Haematological studies were made at weekly
intervals. During the first 3 weeks of exposure to T-2 toxin,
lymphoid tissues, bone marrow, and splenic red pulp became
hypoplastic resulting in anaemia, lymphopenia, and eosinopenia.
Subsequently, during continued exposure to T-2 toxin, there was
regeneration leading to hyperplasia of the haematopoietic cells by 6
weeks. All animals also developed perioral dermatitis and ulceration
of the gastric mucosa. The above results indicate both the irritant
and haematopoietic suppressive effects of the T-2 toxin. However,
the haematopoietic effects were transient at the dose administered
and did not lead to haematopoietic failure.
The haemostatic derangements produced by T-2 toxin have been
studied in the guinea-pig (Cosgriff et al., 1984), rabbit (Gentry,
1982), chicken (Doerr et al., 1981), and monkey (Cosgriff et al.,
1986). Guinea-pigs (Hartley strain, number not stated) admin-istered
T-2 toxin dissolved in ethanol, by intramuscular injection, at a dose
of 1 mg/kg body weight (LD50-24 h) developed decreased activities of
all coagulation factors except fibrinogen. Platelet aggregation in
whole blood response to ADP and collagen was depressed. The animals
also showed an initial rise followed by a fall in the haematocrit
level, leukocytosis, and a fall in platelet count. These changes,
which were found within a few hours of toxin administration, reached
a maximal at 24 h and returned to normal over the next 2 days.
Pretreatment with vitamin K1 did not prevent the effects of T-2 toxin
on coagulation. The addition of T-2 toxin to the plasma and blood of
untreated guinea-pigs at a concentration of 1 mg/litre did not have
any effect on clotting times or platelet aggregation, indicating that
the T-2 toxin itself did not have any direct effect on the activ-ity
of coagulation factors (Cosgriff et al., 1984).
Eight New Zealand White rabbits were administered T-2 toxin
dissolved in dimethyl sulfoxide (DMSO) by intravenous injection at
0.5 mg/kg body weight; 5 rabbits were given a single oral dose of 2.0
mg/kg body weight. In the rabbits treated intravenously, both the
packed cell volume and the total leukocyte counts were reduced.
However, no significant alterations occurred in the haematological
parameters of rabbits given the T-2 toxin orally (Gentry & Cooper,
1981).
In another study, 9 New Zealand White rabbits were given a single
intravenous injection of T-2 toxin dissolved in DMSO at a dose of 0.5
mg/kg body weight. A second group of 5 animals received daily
subcutaneous injections of vitamin K, at a dose of 0.5 mg/kg body
weight, for 5 days prior to administration of a similar dose of T-2
toxin and for a subsequent 4 days. A total of 16 rabbits in 2 groups
served as controls. Blood samples were examined from each animal
before toxin or DMSO treatment and 6-96 h later. Several coagulation
factors (VII, VIII, IX, X, XI) were decreased by about 40% within 6 h
of toxin administration in the group administered toxin alone.
Fibrinogen content was elevated at 24 h. However, the reduction
in the coagulation factors did not induce clinical haemorrhage and
administration of vitamin K did not alter the effects of T-2 toxin
administration, indicating that the mechanism of action of the toxin
on coagulation was not as a vitamin K antagonist.
In a study by Cosgriff et al. (1986), 9 Cynomolgus monkeys
received an intramuscular injection of an LD20 dose (0.65 mg/kg body
weight) of T-2 toxin dissolved in ethanol. Three monkeys served as
controls. Haematological studies were made before toxin injection
and at different intervals from 6 to 24 h and 2 to 7 days after
treatment. The animals were studied for signs of toxicity and
particularly for evidence of haemorrhage. Necropsy was performed on
animals that died during study. Leukocytosis levels in treated
animals were 4-5 times pretreatment levels. Prolongation of
prothrombin, activated thromboplastin times, and a decrease in
multiple coagulation factors were also observed. These changes were
detected within hours of toxin administration, reached a maximum at
24 h, and returned to normal over the next 3 days. Fibrin-fibrinogen
degradation products were not detected at any time. Platelet counts
which were unchanged in treated animals, were significantly raised in
control animals following repeated phlebotomies. None of the animals
developed the haemorrhagic syndrome. Five animals that died during
the study showed mild peticheal haemorrhages involving the colon and
heart, as well as necrosis of lymphoid tissues.
Rukmini et al. (1980) conducted a study on adult rhesus monkeys
in which 3 males and 2 females were administered pure T-2 toxin in
20 ml milk by stomach tube, daily, initially at 1 mg/kg body weight
for 4 days, and then at 0.5 mg/kg body weight from day 5 to day 15.
Three males and 3 females served as controls. All 3 males in the
treated group died of respiratory failure between days 0 and 15.
Subsequently, after 30 days recovery, the 2 treated female and 2
additional male monkeys received 0.1 mg T-2 toxin/kg body weight for
15 days. All monkeys given 1 mg T-2 toxin/kg per day showed signs of
toxicity similar to those of alimentary toxic aleukia in man, i.e.,
vomiting, apathy, and weakness of lower limbs. The signs were more
severe in males, and they also developed peticheal haemorrhage on the
face. All male animals developed severe leukocytopenia, follicular
atrophy of the spleen and lymph nodes, and pneumonia, suggesting
involvement of the immune system. At a dose of 0.1 mg/kg per day,
both male and female animals developed leukocytopenia and mild
anaemia after 15 days of treatment.
The effects of T-2 toxicosis on blood coagulation were studied in
groups of 40, day-old chickens fed diets containing the toxin at
concentrations of 1, 2, 4, 8, or 16 mg/kg. Forty animals served as
controls. Factor X, and prothrombin and fibrinogen activities were
reduced only at the highest dietary dose, whereas Factor VII was
reduced at dietary doses of 4, 8, and 16 mg/kg and was the most
sensitive of the clotting components to T-2 toxin toxicosis. Thus,
T-2 toxin toxicosis induced by high doses results in multiple-factor
coagulopathy and mild toxicosis results in a deficiency of Factor VII
(Doerr et al., 1981).
II.4.2.3 Disturbances of the central nervous system
Four-week-old male broiler chickens were intubated with a single
dose of T-2 toxin at 2.5 mg/kg body weight, and the brain
concentrations of dopamine, norepinephrine, and serotonin, and
selected blood components were determined 4-48 h after
administration. There was a significant elevation in the brain-
dopamine concentration and a reduction in the brain-norepinephrine
concentration. The brain-serotonin contents did not change (Chi et
al., 1981). Batches of 40 broiler chickens fed graded concentrations
of 1-16 mg T-2 toxin/kg feed for 3 weeks developed an abnormal
positioning of the wings, hysteroid seizures, and impaired
righting reflex. Neural toxicity, which occurred at levels above 4
mg T-2 toxin/kg diet, might have been related to alterations in brain
biogenic amines (Wyatt et al., 1973a,b; Chi et al., 1977a).
Signs of nervous system dysfunction (restlessness, dyspnoea,
ataxia) were observed in rats after subcutaneous or intracerebral
injection of T-2 toxin (10-20 µg toxin) or after intracerebral
implantation of toxin adsorbed on talc (Bergmann et al., 1985).
Weanling, male Wistar rats were administered T-2 toxin orally at 2.0
mg/kg body weight and the concentrations of neurotransmitters
determined. The toxin increased concentrations of tryptophan,
serotonin, and dopamine in the brain, but decreased concentrations of
3,4-dihydroxyphenylacetic acid (MacDonald et al., 1988). Male
Sprague-Dawley rats (180 g) were dosed orally with DON or T-2 toxin
at 21.5 mg/kg body weight. Both the toxins significantly increased
serotonin and 5-hydroxy-3-indoleacetic acid concentrations in all
regions of the brain examined, whereas norepinephrine and dopamine
concentrations were not altered (Fitzpatrick et al., 1988). Male
Sprague-Dawley rats received 1 mg T-2 toxin/kg body weight by
intravenous injection. Concentrations of vasopressin, oxytocin, and
leucine enkephalin decreased in the posterior pituitary and
concentrations of methionine enkephalin increased (Zamir et al.,
1985).
Male Sprague-Dawley rats (180 g) and 4-week-old White Leghorn
cockerels were dosed orally with DON at 2.5 mg/kg body weight. Whole
brain concentrations of monoamine neurotransmitters were not altered
in either species. The treatment produced elevated concentrations of
serotonin and 5-hydroxy-3-indoleacetic acid in the rat, but not in
the chicken (Fitzpatrick et al., 1988).
II.4.2.4 Dermal toxicity
After the discovery of the skin-necrotizing property of toxic
metabolites of F. sporotrichioides and related fungi (Joffe, 1962),
the skin irritation test was introduced for the screening of toxins
and metabolites of Fusarium species (see section II.1.2.3). T-2
toxin, HT-2 toxin, and DAS were isolated from cultures of F.
tricinctum using the skin test for selection of active fractions
(Gilgan et al., 1966; Bamburg et al., 1968b). Toxins, such as T-2
toxin, HT-2 toxin, and DAS are extremely potent irritants while NIV
and fusarenon-X are much less so (Bamburg et al., 1968b; Ueno et al.,
1970; Wei et al., 1972; Chung et al., 1974; Hayes & Schiefer, 1979;
Bhavanishankar et al., 1988).
The mechanism of the skin toxicity of trichothecenes has not been
established. Results of studies with fusarenon-X have indicated that
the vascular permeability of the skin of the back of the rabbit,
estimated by exudation of a vital dye (pontamine sky blue), was
biphasic reaching maxima 5 and 24 h after topical application. These
data indicate that increased vascular permeability is one of the
early responses of the skin to these toxins and that some chemical
mediators participate in the biphasic increase in vascular
permeability (Ueno, 1980a).
II.4.2.5 Impairment of immune response
Experimental animal studies show that some trichothecenes affect
the immune system and thereby modify the immune response. The
impairment comprises the following functions: antibody formation;
allograft rejection; delayed hypersensitivity; and blastogenic
response to lectins. As a consequence of the impairment, decreased
resistance to microbial infection has been experimentally
established. It is likely that the impairment of the immune system
is linked to the inhibitory effect of trichothecenes on macromolecule
synthesis.
(a) Antibody formation
Rosenstein et al. (1979) showed that T-2 toxin and DAS inhibited
responsiveness to sheep red blood cells in male Swiss IC and C5781/6
mice. In their first study, T-2 toxin or DAS was injected ip daily
for 7 days at 0.75 mg/kg body weight, in 6 groups of 4 mice each,
with matching controls. Mice were immunized with sheep erythrocytes
(SRBC) on day 3 after treatment and killed 5 days after immunization.
Both toxins produced a fall in anti-SRBC titres measured by
haemagglutination and reduced thymic weight. In a second study, 7
groups of 5 mice each were administered daily (ip) doses of T-2 toxin
ranging from 0 (solvent alone) to 2.5 mg/kg body weight over a 7-day
period. The same number of mice received DAS under similar
conditions. Mice were immunized on day 3 and killed 5 days later.
Antibody-producing cells from the spleen were counted by numbering
the plaque-forming cells (PFC) on sheep erythrocytes. A dose-
dependent inhibition of PFC was observed in T-2 toxin-treated mice,
with a total suppression of the immune response at 2.5 mg/kg. The
effects of DAS were less. A subsequent follow-up of the evolution of
the immune response in 36 mice administered T-2 toxin (ip) at 0.75
mg/kg body weight daily for 7 days indicated that the immunosuppressive
effect disappeared within 6 days followup cessation treatment.
The T-cell-independent-responses-production of anti-
polyvinylpyrrolidone and anti-dinitrophenol-ficoll-antibodies were
enhanced by T-2 toxin and DAS. T-2 toxin-treated mice produced 50%
fewer plaque-forming cells against SRBC. There was also a decreased
response to phytohaemagglutinin in splenic cells from treated mice.
However, Masuko et al. (1977), and Otokawa et al. (1979), reported
that a single dose of 3 mg T-2 toxin/kg body weight in mice caused
modification of delayed hypersensitivity responses without affecting
antibody response. This apparent contradiction was explained by a
difference in the timing of the administration of T-2 toxin, mice
receiving the toxin once, several days before or after antigen-
stimulation. Results of studies with fusarenon-X indicated that both
IgE and IgG anti-body responses to DNA-OVA were suppressed when male
BALB/c mice were repeatedly dosed with mycotoxin at doses exceeding
25 mg/day; the inhibition of antibody-formation was greater when
given 7 days before antigen-stimulation. In mice stimulated with
pokeweed mitogen and lipopolysaccharides, the in vitro antibody
production by splenic cells from fusarenon-X-treated mice was
suppressed (Masuda et al., 1982).
Sato et al. (1981), examined the effects of fusarenon-X on
serological responses in chicks inoculated with Newcastle disease
vaccines. When chicks were fed 8 mg fusarenon-X/kg for 6 weeks, no
significant reductions in body or organ weights were observed.
However, haemagglutinin inhibitory antibody titres were reduced when
chicks were immunized with live, but not with inactivated, vaccine.
Mann et al. (1983), reported alterations in the levels
of several serum proteins in calves orally administered T-2 toxin
(0.6 mg/kg per day over 43 days). Total protein, albumin, and
immunoglobulin fractions were decreased in toxin-treated calves,
including the alpha-beta1-and beta2-globulin fractions. IgA and IgM
values and complement proteins were lower in treated calves.
Sublethal doses of DON (0.25, 0.50, or 1.0 mg/kg feed) were fed
for 54 weeks, beginning at 21 days of age, to a total of 96 weanling
male Swiss Webster mice divided into 4 groups. There were 32
controls. The dose of 1.0 mg/kg reduced serum alpha1- and alpha2-
globulins, increased serum-albumin levels, and reduced feed
consumption and body weight gain. The dose of 0.5 mg/kg reduced
alpha2- and beta-globulins (Tryphonas et al., 1986).
T-2 toxin was fed to 6 weaned pigs at 5 mg/kg feed for 25 days
and the immune response evaluated by in vitro testing for blast
transformation, immune-rosette formation, and IF-detectable IgG-
positive cell counts. T-2 toxin produced a 40-50% reduction in
immune responsiveness and a decrease in total leukocyte count, but an
increase in adrenocortical activity. Neutralizing antibody titres to
vaccination with enteritic B vaccine were lower in the treated pigs.
It was concluded that T-2 toxin had a distinct immunosuppressive
effect during the early phase of immune induction by altering the
function of both T- and B-lymphocytes (Rafai & Tuboly, 1982).
Dietary DON (2, 5, or 25 mg/kg feed for 2 or 8 weeks) depressed
the plaque-forming response to sheep erythrocytes in splenic cultures
from B6C3F1 mice. Some effect on the plaque-forming response was
detectable with both the 2- and the 8-week period of feeding (Pestka
et al., 1987b). DON, given by gavage at 0.75, 2.5, or 7.5 mg/kg body
weight also reduced serum-IgM response to sheep erythrocytes and
plaque-forming cell numbers were lower in the treated groups
(Tryphonas et al., 1984).
(b) Allograft rejection
Observations on the inhibition of cellular immunity by
trichothecenes have included responses to grafting. According to
Rosenstein et al. (1979), the mean survival time of the skin grafted
from C57Bl/6 mice on to Swiss mice was 8.69 days in the control
recipients. However, when the recipients were treated with 0.75 mg
T-2 toxin/kg per day for 7 days before skin graft and then 3 times a
week for 20 days, the mean survival time of the graft was increased
to 12 days, indicating that T-2 toxin suppressed certain steps of
immunity resulting in allograft rejection. The areas of the graft in
T-2 toxin-treated mice lacked the typical cellular infiltrates of a
cell-mediated immune response of macrophages and lymphocytes.
(c) Delayed hypersensitivity
Delayed hypersensitivity (DH) is an immune response mediated by
sensitized T lymphocytes. The possible impairment of T lymphocytes
by T-2 toxin was studied in female BDF1 mice sensitized by the sc
injection of sheep erythrocytes (SRBC) followed by estimation of foot
pad swelling. When mice received 3 mg T-2 toxin/kg body weight,
before, or on the day of, sensitization, no appreciable effect on DH
was observed. However, when the toxin was administered 2 or 3 days
after sensitization, marked enhancement of the delayed
hypersensitivity response was seen (Otokawa et al., 1979). This
indicates that the timing of toxin exposure was critical for
enhancement of the delayed hypersensitivity response. Since the
life-time of the effective T-2 toxin dose in vivo was very short,
and the optimal timing of toxin injection corresponded with the time
of appearance of suppresser cells, it was presumed that trichothecenes
might interfere with the proliferation of suppresser T cells that
appear in DH-tolerant mice.
DON fed to B6C3F1 mice at 2, 5, or 25 mg/kg for 2 or 8 weeks
depressed the delayed hypersensitivity response to keyhole limpet
haemocyanin. The effects on hypersensitivity were detectable in mice
fed the mycotoxin for 2 weeks, but disappeared when the feeding
period was extended to 8 weeks (Pestka et al., 1987b).
(d) Blastogenic response to lectins
Certain mitogens, such as phytohaemagglutinin (PHA) and
concanavalin A, stimulate the proliferation of T cells in vitro;
lipopolysaccharide (LPS) causes the same phenomena in B cells.
Lafarge-Frayssinet et al. (1979) investigated the responses of
lymphocytes to PHA and LPS in mice treated with crude T-2 toxin and
DAS. Mice were treated with the mycotoxins at doses of one-quarter
of the LD50 or one-twelfth of the LD50 for 15 days and the response
of splenic or thymic cells to the mitogens was examined. The data
indicated that stimulation of both T and B cells was inhibited
reversibly, and that the ability to synthesize anti-SRBC antibodies
was suppressed. In vitro effects on lymphocytes and fibrosarcoma
cell cultures included a direct cytostatic action at high
concentrations and a stimulating action at low concentrations.
Histopathological observations included severe lymphoid damage in the
thymus and spleen. The results of these studies indicate that the
immune system appears sensitive to the trichothecenes and is impaired
at doses not inhibitory for other organs.
Swiss mice were fed a diet containing T-2 toxin at 5, 10, or 20
mg/kg for 1, 2, 3, 4, or 6 weeks. The ingestion of T-2 toxin (only
at 20 mg/kg at 3 weeks) depressed total splenic cell counts. T-2
toxin at 20 mg/kg for 1-4 weeks decreased splenic proliferative
responses to T-cell mitogen concanavalin A; however, the response to
a lipopolysaccharide (LPS), B-cell mitogen, was decreased in mice fed
T-2 toxin at 10 or 20 mg/kg for 1-4 weeks. (Friend et al., 1983a).
Lymphocytes from calves exposed to T-2 toxin at 0.6 mg/kg for as
long as 43 days had a decreased response to the mitogen, PHA, on days
1, 8, and 29 after toxin administration. Lymphocyte responses to
concanavalin A and pokeweed mitogen were also observed on day 29
after dosing (Buening et al., 1982).
In a study to determine the effects of T-2 toxin on the bovine
immune system, calves (5) were orally dosed with 0.3 mg/kg per day,
for 56 days. Neutrophil function was reduced by treatment with T-2
toxin as was the cutaneous reaction to injected phytohaemagglutinin.
In a second study, calves (6) were given T-2 toxin at a dose of 0.5
mg/kg per day, for 28 days. B-lymphocyte number and the response of
the B-cell-enriched fraction to phytohaemagglutinin both increased
after treatment. The in vitro exposure of mononuclear cells, B-cell-
enriched or T-cell enriched fraction, reduced the lymphoblastic
response to mitogens. A 50% reduction was induced by 1.4 ng T-2
toxin/ml (Mann et al., 1984).
The effects of T-2 toxin on in vitro mitogen response and
antibody production by human peripheral blood lymphocytes were
reported by Tomar et al. (1988). The toxin inhibited the mitogen
response to concanavalin A at a lower concentration (1.6 mg/ml)
compared with phytohaemagglutinin (2-4 mg/ml) and pokeweed mitogen.
In the presence of the toxin, inhibition reached a maximum during
first 8 h. The results indicate that various subpopulations of
lymphocytes have different susceptibilities to T-2 toxin.
Mitogen-induced blastogenesis in cultured human lymphocytes was
inhibited by T-2 toxin and its metabolites. The concentrations of T-
2 toxin, HT-2, 3'-OH T-2,3'-OH HT-2, T-2 triol, and T-2 tetraol
toxins that produced 50% inhibition of 3H-thymidine uptake in
mitogen-stimulated human peripheral lymphocytes were 1.5, 3.5, 4.0,
50.0, 150.0, and 150.0 ng/ml, respectively. The initial hydrolysis
of T-2 toxin to HT-2 toxin and the hydroxylation to 3'-OH T-2 did not
significantly decrease the immunotoxicity (Forsell et al., 1985).
Other trichothecenes were less toxic than T-2 toxin in this system.
The doses that produced 50% inhibition of 3H-thymidine uptake in
mitogen-stimulated human lymphocytes for fusarenon-X, NIV, DON, and
15-AcDON were 18, 72, 140, and 240 ng/ml, respectively. These
results indicate that the lymphotoxicity of trichothecenes is related
to the C-4 substituent (Forsell & Pestka, 1985).
T-2 toxin was examined for its effects on lymphocyte activation
and interleukin-2 production by splenic cultures from mice. Splenic
cells were taken from female BALB/c mice given 2 mg T-2 toxin/kg body
weight by stomach tube for 4 days or 4 mg T-2 toxin/kg by stomach
tube in single dose. Cells were incubated with 1 µg concanavalin A
and the synthesis of cellular protein and DNA determined. The single
dose of 4 mg/kg did not alter lymphocyte activation, but the dose of
2 mg/kg for 4 days produced a 50% reduction in activation. The
supernatant from these cells had 4 times greater interleukin-2
activity (Holt et al., 1988a).
DON and 3-AcDON were evaluated in vitro for their effects on
mitogen-induced lymphocytic blastogenesis using rat or human
peripheral blood lymphocytes. Both mycotoxins produced a dose-
dependent reduction in lymphocytic proliferation and DON produced a
greater inhibitory effect than the acetylated compound. Thus, the
concentrations of DON producing 50% inhibition of blastogenesis were
90 and 220 ng/ml for rat and human lymphocytes, respectively. The
values for 3-AcDON were 450 and 1060 ng/ml, respectively (Atkinson &
Miller 1984).
(e) Resistance to infection
The immunosuppressive effect of trichothecenes has resulted in an
increased incidence and severity of infection in animals in several
studies. According to Boonchuvit et al. (1975), an increased
mortality rate was recorded when 40 chickens were fed a diet
containing 16 mg T-2 toxin/kg for 1 week and were then inoculated
orally with 1 x 108 cells of Salmonella.
The depression of resistance to experimental tubeculosis by T-2
toxin was studied in mice by Kanai & Kondo, (1984). Groups of male
mice, strain ddY, 18-20 g body weight, with 10-14 animals per group,
were administered mycobacteria by intravenous injection in the tail
vein. In the first study, the doses injected consisted of 0.01 mg
culture of tubercle bacteria per animal (species not indicated). One
group was administered 0.1 mg T-2 toxin per animal a total of 12
times orally, starting on the day before the injection, 7 times with
one-day intervals, and then 5 times daily. For comparison, a second
group of mice was administered 5 mg cortisone acetate per animal ip
under a similar time schedule. A third group was injected with
tubercle control bacteria only. At the end of the 20-day observation
period, the mice in the first group had a lower spleen weight and a
higher tubercle bacteria count in the spleen than those in the other
2 groups, indicating a more pronounced depression of resistance by T-
2 toxin than by cortisone. In a second study, two groups of animals
were injected with 0.25 mg of a culture of Mycobacterium bovis; one
group was then administered 0.1 mg T-2 toxin per animal daily for 6
days, starting 8 days after injection. There were two groups of
controls. The average survival time in the T-2 toxin-treated group
was reduced to 19 days, compared with 35 days in the untreated group,
indicating decreased resistance.
Rats were injected ip with T-2 toxin in a single dose of 1
mg/kg body weight or in doses of 0.5 mg/kg daily for 5 days.
The rats were then inoculated with 0.1 ml of medium
containing 109 Staphylococcus aureus/ml. Rats given
multiple intramuscular injections of T-2 toxin showed more
oedema and myofibre necrosis at the injection site of the
bacteria; the cellular infiltrate was sparse and bacteria
were abundant. Bone marrow myeloid cells were markedly
decreased by multiple injections of T-2 toxin. In in vitro
studies, small, non-lethal doses of T-2 toxin inhibited
the chemotaxis of leukocytes and decreased phagocytosis of
the bacteria by leukocytes (Yarom et al., 1984b).
Seven male rhesus monkeys (Macaca mulatta) were dosed daily by
stomach tube for 4-5 weeks with 100 µg T-2 toxin/kg body weight.
This dose resulted in the death of 3 animals, 40% reduction in
leukocyte counts, reduction in the bactericidal activity of
neutrophils (phagocytosis of E. coli), reduction in the
transformation of lymphocytes by mitogens, and a reduction in numbers
of C-cell and T-cell lymphocytes (Jagadeesan et al., 1982).
The immunotoxic effects of T-2 toxin on cell-mediated resistance
were studied in female ICR mice infected with Listeria monocytogenes.
Mice in groups of 17 animals (10 animals in the control group) were
inoculated ip with 4 x 105 (LD50) or 4 x 104 (non-lethal) doses of L.
monocytogenes per animal, treated with a single oral dose of 4 mg T-2
toxin/kg body weight, and observed for 15 days. Bacterial
multiplication was rapid in the spleen after T-2 toxin treatment and
mortality was increased in both treated groups. Necrosis and
depletion of lymphoid tissue were observed in the thymus, the
periarterioler lymphoid sheaths and the lymphoid follicles of the
spleen. Cellular response to L. monocytogenes in the spleen and
liver was decreased by treatment with T-2 toxin and the lesions were
sparsely populated with mononuclear cells. The foci of necrosis were
larger with numerous colonies of bacteria. The influx and number
of lymphocytes and macrophages were greater in Listeria-elicited
peritoneal exudates. The immunotoxic effects of T-2 toxin were
comparable with those produced by cyclophosphamide and were
attributed to depletion of T lymphocytes and subsequent failure of T-
cell-dependent macrophages to clear the host of bacteria (Corrier &
Ziprin, 1986a). In a continuation of the previous study, female ICR
mice (17 animals per group) were inoculated ip on day 1 with 4 x 105
(LD50) or 4 x 104 (non-lethal) bacteria per animal, treated orally on
day 0, 1, 2, and 3 with 0, 1, or 2 mg T-2 toxin/kg, and observed for
15 days. The suppression of resistance by the mycotoxin was
indicated by rapid multiplication of Listeria in the spleen and
increased mortality in mice in both exposed groups treated with 2
mg/kg. The thymuses and spleens of toxin-treated mice showed
necrosis and depletion of lymphoid cells. Foci of necrosis induced
by Listeria infection in the spleen and liver were larger in treated
mice and the inflammatory reaction was sparse (Corrier & Ziprin,
1986a,b).
Increased resistance to L. monocytogenes infection was
surprisingly observed by the same group (Corrier & Ziprin, 1986b) in
mice administered T-2 toxin several days prior to the inoculation
with bacteria. Female ICR mice, 16 animals per group, were
administered T-2 toxin by stomach tube at dosage levels of 2, 1, 0.5,
or 0 mg toxin/kg body weight, on days -5, -4, -3, -2, -1, +1 and +3.
On day 0 the two treated groups were inoculated ip with 106 (LD100)
and 105 (LD50) L. monocytogenes, respectively. In addition, 20 mice
were given 2 mg T-2 toxin/kg on the same days as above, and used to
determine the effect of the toxin. Although the cytotoxic effect of
T-2 toxin on lymphoid tissue was marked, enhanced resistance to
Listeria infection was revealed by a decrease in mortality due to
listeriosis (in both bacteria-exposed groups) in a T-2 toxin dose-
dependent way. No specific cause for the increased resistance to
listeriosis by T-2 toxin treatment prior to bacterial infection was
identified by the authors.
ICR female mice were treated with the trichothecene mycotoxin DAS
and subsequently inoculated ip with Listeria monocytogenes. The
effect of the mycotoxin on the course of the infection was monitored
by observing the resultant mortality and the bacterial content of the
spleens from inoculated mice. Mice given 3 mg DAS/kg body weight
orally, on 2 and 1 days before inoculation, showed increased
mortality and splenic Listeria counts. In these mice, thymus weights
were reduced, and lymphocytes were depleted from the thymus cortex
and from splenic lymphoid follicles and periarteriolar lymphoid
sheaths. A single dose of 4 mg DAS/kg given on day 6 before
challenge exposure did not affect mortality compared with controls.
Mice treated with DAS and subsequently inoculated with Listeria had
significantly ( P = 0.006) higher levels of neutrophil populations
than Listeria-infected control mice (Ziprin & Corrier, 1987).
Dietary DON decreased resistance of B6C3F1 mice to infection with
L. monocytogenes. Resistance to the infection was similarly
decreased in control mice fed restricted diets, comparable to dietary
restriction caused by DON-induced feed refusal. Resistance to L.
monocytogenes was reduced to a greater extent by feed containing both
DON and zearalenone (Pestka et al., 1987b). DON in the diet at 0.50
or 1.0 mg/kg was fed for 5 weeks. Both doses resulted in a dose-
related decrease in time-to-death interval following challenge with
L. monocytogenes (Tryphonas et al., 1986).
T-2 toxin was fed to young male white Swiss mice at doses of 10
or 20 mg/kg diet for 2-3 weeks. The mice were then inoculated ip
with herpes simplex virus (HSV-1). Mice fed the high dose of T-2
toxin were highly susceptible to HSV-1 infection and about 75% died
with extensive hepatic and adrenal gland necrosis and with little or
no inflammatory cellular reaction in affected tissues, such as the
liver and adrenal glands, and the central nervous system. No
necrotizing encephalitis was found in treated mice. Mice fed 10 mg
T-2 toxin/kg had lesions of intermediate severity between those of
the high-dose group and the virus-infected controls (Friend et al.,
1983c). Feeding of T-2 toxin at 5, 10, or 20 mg/kg for 3-6 weeks did
not reactivate the virus in mice latently infected with HSV-1 (Friend
et al., 1983a).
Mice (male, Swiss strain weighing 25 g) received inoculations
(route not stated) of Cryptococcus neoformans (1 x 106 cells) and ip
doses (1/8 or 1/4 LD50) of DAS on days 5, 6, and 7 after inoculation
with the fungal cells. No deaths occurred in either the C.
neoformans-treated groups or the T-2-toxin group. A marked additive
effect on mortality was observed when mice received both C.
neoformans and T-2 toxin (Fromentin et al., 1981).
II.4.2.6 Carcinogenicity
The IARC (1983) studied the experimental data on the
carcinogenicity of T-2 toxin and concluded that no evaluation of the
carcinogenic role of T-2 trichothecene in experimental animals could
be made, because of the inadequacy of experimental data.
In a 16-month feeding study, groups of 50 male and 50 female
weanling CD-1 mice were fed a semi-synthetic diet containing 1.5 or
3.0 mg T-2 toxin/kg. Survival was lowest in the control group. No
statistically recognizable differences were found in feed consumption
or body weight gains among the groups. Statistically significant
differences were found in the incidence of pulmonary adenomas and
hepatic adenomas in the males of the 3.0 mg/kg group and the
controls. Other treatment-related findings were an increased
prevalence of epithelial cell hyperplasia and hyperkeratosis in the
stomach of animals fed the T-2 toxin diets (Schiefer et al., 1987).
In an attempt to determine the carcinogenicity of NIV, a study
was conducted in which mice were fed mouldy rice for 2 years. Groups
of 42, 7-week-old female C57BL/6CrSlc SPF mice were fed diets
containing 6, 12, or 30 mg NIV/kg for 2 years, and were assessed for
the effects on body weight gain, feed efficiency, terminal organ
weights, haematological values, and lesions. The mortality was
lowest in the highest dose group, followed by the 12 mg/kg group;
body weight gains and feed efficiency were dose-dependently reduced.
No particular neoplasms attributable to treatment were found. The
incidence of naturally occurring neoplasms, mostly lymphomas, was
similar in all groups. On gross and microscopic examination of the
liver, thymus, spleen, kidneys, stomach, small intestines with or
without Peyer's patches, no alteration related to treatment was
observed except for amyloidosis, which was lower in the two higher
dose groups (Ohtsubo et al., in press).
Two groups of 16 or 18 DDD male mice received either 10 or 20
weekly sc injections of 2.5 mg fusarenon-X/kg body weight. No
increase in tumour incidence was noted in treated animals, compared
with the controls (Saito & Ohtsubo, 1974).
In a feeding study, fusarenon-X, at a dose of 3.5 or 7 mg/kg diet
was fed to 151 male Donryu rats for 1-2 years. Treatment with the
mycotoxin reduced the growth rate, but did not induce any
carcinogenic effects (Saito et al., 1980).
In studies to investigate skin tumour induction using T-2 toxin
and DAS, the skin of the back of mice was painted with 0.1-1 mg of
trichothecenes, twice a week, for one year. A notable finding was
necrosis of the skin, but no tumours were detected. The skin tumour
induction test was also carried out using the initiator-promotor
procedure. T-2 toxin and fusarenon-X were not promoting agents of
dimethylbenz (a)anthracene-induced skin neoplasia. According to
Lindenfelser et al. (1974), neither T-2 toxin nor DAS served as
initiating agents.
II.4.2.7 Mutagenicity
In a Rec-assay using Bacillus subtilis, DNA damage was not
induced by 20 or 100 µg of either T-2 toxin or fusarenon-X (Ueno &
Kubota, 1976). Such trichothecenes as T-2 toxin, DAS, and DON were
not mutagenic to Salmonella typhimurium strains TA98, TA100, TA1535,
TA1537, and TA1538, with or without S-9 fraction from rat liver
(Kuczuk et al., 1978; Ueno et al., 1978; Wehner et al., 1978a).
T-2 toxin and DAS were not mutagenic in the D-3 mitotic
recombination test with Saccharomyces cerevisiae (Kuczuk et al.,
1978).
Neither DNA-strand breakage nor induction of 8-azaguanine-
resistant mutation were detected with 1-32 mg fusarenon-X/litre in
Hela cells and with the same toxin at 0.1-1.0 mg/litre in FM3A cells
derived from C3H mouse mammary carcinoma cell line (Umeda et al.,
1972, 1977). On the other hand, Lafarge-Frayssinet et al. (1981)
reported that T-2 toxin induced single-strand breaks in the DNA of
lymphoid cells in vivo (3 mg/kg body weight) and in vitro (0.7-5
ng/ml). Such DNA breakage was not observed in hepatic cells.
T-2 toxin, NIV, and fusarenon-X produced weak clastogenic effects
in Chinese hamster V79-E cells (Thust et al., 1983). Both T-2 toxin
and HT-2 toxin inhibited the incorporation of tritiated
thymidine into the DNA of human fibroblasts in culture in a dose-
related fashion. Non-toxic and toxic doses of DON (0.1-1000
mg/litre), did not significantly increase unscheduled DNA synthesis
in primary cultures of rat hepatocytes (Bradlaw et al., 1985).
Norppa et al. (1980) also reported weak induction of chromosomal
aberrations by T-2 toxin (1.7, 2.7, or 3.0 mg/kg body weight) in
Chinese hamster bone marrow cells. The bone marrow micronucleus test
was negative at a dose of 3 mg T-2 toxin/kg body weight resulting in
a significant decrease in polychromatic erythrocytes.
Hamsters fed T-2 toxin for 6 weeks (2.5 mg/kg body weight) did
not have more chromosomal aberrations than controls. The clastogenic
potential of T-2 toxin was very weak. In hamster V79 cells, DON at
levels of 2-3 µg/ml or more was cytotoxic, but was non-mutagenic at
the hypoxanthine-guanine phosphoribosyl transferase locus, with or
without hepatocytic mediated activation (Rogers & Heroux-Metcalf,
1983).
According to Reiss (1975), DAS induced various cytological
abnormalities including shortened chromosomes, enlarged nucleoli, and
a few chromosome breaks in the root tips of Allium cepa (common
onion) at concentration of 1000 and 100 µg/ml. T-2 toxin and
satratoxin H were C-mitotic poisons at concentrations exceeding 10
µg/g. Typical C-mitotic action on chromosomes morphology was
produced by both toxins and was comparable to that of colchicine. In
addition, T-2 toxin induced polyploidy (Linnainmaa et al., 1979).
T-2 toxin and satratoxin H were not mutagenic in a sex-linked
recessive lethal test in Drosophila (Sorsa et al., 1980). Lack of
potency to produce recessive lethal mutations in Drosophila was
consistent with negative results obtained in certain bacterial assays
in which trichothecenes were inactive as both base pair and frame
shift mutagens (Kuczuk et al., 1978; Ueno et al., 1978a).
II.4.2.8 Teratogenicity and reproductive effects
T-2 toxin was embryotoxic and teratogenic in mice. T-2 toxin
dissolved in propylene glycol was injected intraperitoneally into
pregnant mice on one of days 7-11 of gestation at doses of 0.5, 1, or
1.5 mg/kg body weight. T-2 toxin (doses of 1 or 1.5 mg/kg) caused
significant maternal mortality, fetal death, and fetal body weight
loss. Approximately 37% of the fetuses from dams given 1 (8 litters)
or 1.5 mg (4 litters) T-2 toxin/kg on day 10 were grossly malformed.
The most frequent anomalies were bent, shortened, or missing tails,
and limb malformations, including oligodactyly and syndactyly.
Exencephaly, open eyes, retarded jaw, and skeletal malformations of
the rib or vertebrae were also found in the fetuses (Stanford et al.,
1975).
Pregnant CD-1 mice (18 litters/treatment group) were administered
T-2 toxin dissolved in propylene glycol ip at 0.5 mg/kg body weight
on gestation days 8 or 10. The T-2 toxin produced grossly malformed
fetuses, principally with tail and limb anomalies. A higher
incidence of malformations was observed when a T-2 toxin dose of 0.5
mg/kg body weight was combined with an ochratoxin A dose of 4 mg/kg
body weight. An increase in fetocidal effects was found in offspring
of dams in groups treated with the high-dose combination, on either
day. Few skeletal and visceral malformations were noted (Hood et
al., 1978). T-2 toxin (0.5 mg/kg body weight) dissolved in 1:1
mixture of propylene glycol and 0.1N sodium bicarbonate was
administered ip alone or in combination with rubratoxin B (0.4 mg/kg
body weight) to pregnant CD-1 mice on day 1 of gestation. Only T-2
toxin resulted in gross malformations. The combination of toxins
increased the adverse effects on fetal body weight and mortality, but
not the incidence or severity of the gross malformations (Hood,
1986).
The teratogenicity of orally administered T-2 toxin dissolved in
propylene glycol was evaluated in a study using 350 female CD-1
mice and doses of 0.5, 1.0, 2.0, 3.0, 3.5, or 4.0 mg/kg body weight
given on day 9 of gestation with a single dose of 3.0 mg/kg on day 6,
7, 8, 10, 11, or 12 of gestation. In the first study, the doses of
3.5 and 4.0 mg/kg produced maternal deaths and toxicity; no fetuses
were produced by the dams in the 4.0 mg/kg dose group and
significantly fewer fetuses were produced by dams in the 3.5 mg/kg
dose group. More major and minor defects were seen in offspring in
the 3.0 mg/kg dose group. In the second study, the treated females
had greater fetal loss than controls and the greatest number of dead
fetuses occurred among litters treated on day 9 of gestation. Major
skeletal defects were more numerous in mice treated on day 7 of
gestation. The results indicated that a single oral dose of T-2
toxin in propylene glycol was primarily maternally toxic and
embryolethal; defective development was possibly secondary to
maternal toxicity (Rousseaux & Schiefer, 1987).
Fusarenon-X was embryotoxic, but not teratogenic (Ito et al.,
1980). Fusarenon-X dissolved in saline was given to pregnant DDD
mice by subcutaneous injection at doses of 0.63, 1.0, 1.6, 2.6, or
4.1 mg/kg body weight, or by feeding at concentrations of 5, 10, or
20 mg/kg diet during pregnancy. Two dams given a single sc dose of
4.1 mg/kg died within 24 h of injection. Abortion was induced in all
females by a single injection of 2.6 mg/kg on day 10 of gestation.
Smaller doses (0.63-1.6 mg/kg) produced a 16-20% abortion rate, when
given on day 10. Multiple doses (8-12 or 8-14 days of gestation) of
1.0 or 1.6 mg/kg produced 100% abortion. When the mice were fed a
diet containing 5, 10, or 20 mg fusarenon-X/kg throughout the
gestation period or in the early stages of gestation, the mycotoxin
inhibited embryonal implantation. Feeding fusarenon-X at 20 mg/kg
for 7 days during the middle stages of gestation induced abortion in
100% of dams. Fetal body weight was significantly reduced by the
administration of the mycotoxin, but no significant teratogenic
effects were observed in the fetuses of dams in either the
subcutaneous injection or feeding study (Ito et al., 1980).
The effects of NIV on fertilization, course of pregnancy, and
fetuses were examined in ICR mice. In a study by Ito et al. (1986),
pure NIV was injected ip in pregnant mice (groups of 10 animals
each), at dose levels of 0, 0.1, 0.5, or 1.5 mg/kg body weight per
day, on days 7-5 of gestation. The highest dose caused stillbirths
after vaginal haemorrhage in 6 out of 10 animals. High embryo
lethality was recorded in the 2 highest dose groups (88 and 48%). No
fetal malformations were observed in the treated groups. A single
administration of 3 mg/kg on day 7 affected the embryo within 10 h,
damaged the placenta within 24 h, and caused stillbirths at 48 h.
While NIV is embryotoxic, it is not teratogenic (Ito et al.,
1988). Thirty ICR mice in 3 batches of 10 animals each were fed
diets mixed with mouldy rice powder containing NIV at final levels of
6, 12, or 30 mg/kg feed per day throughout gestation. There were 11
controls. Purified NIV was also administered by gavage to 35 animals
in 4 groups at doses of 1-20 mg/kg body weight on days 7-15 of
gestation. There were 10 controls. Embryotoxicity associated
with maternal weight loss was observed in the groups receiving 30
mg/kg diet and 10 mg/ kg body weight per day, by gavage, whereas
lower levels, such as 5 mg/kg body weight per day, by gavage, did not
have any embryotoxic effects. Intrauterine growth retardation was
found at term in the fetuses of mice exposed to 12 mg/kg feed and 5
mg/kg by gavage. NIV did not have any significant adverse effects on
the incidence of gross skeletal and visceral malformations. As 5
mg/kg body weight per day given by gavage corresponds to a feed level
of approximately 35 mg/kg feed, the above data indicate that
exposure to 30 mg NIV/kg feed throughout the gestation period results
in embryotoxicity. However, exposure to approximately 35 mg/kg feed
during days 7-15 only of the gestation period does not induce
embryotoxic effects, which shows the significance of constant
exposure versus intermittent exposure to NIV.
DON was embryotoxic and teratogenic when dissolved in distilled
water and given for 4 consecutive days (days 8-11 of gestation), by
oesophageal intubation, to 15-19 pregnant Swiss-Webster mice (Khera
et al., 1982). The incidence of resorptions was 100% at doses of 10
or 15 mg/kg body weight, and 80% at 5 mg/kg body weight. The dose of
5 mg/kg reduced the number of live fetuses and reduced the average
fetal weight compared with the controls. Low incidences of skeletal
and visceral anomalies were found in the fetuses of the 1, 2.5, and 5
mg/kg groups. The skeletal malformations occurred in a dose-related
manner and included lumbar vertebrae with fused arches or partly
absent centra, and absent or fused ribs.
On the other hand, DON failed to produce embryotoxic and
teratogenic effects when fed ad lib at 0.5, 2.0, or 5.0 mg/kg to
Fisher 344 rats during the entire course of pregnancy (Morrissey,
1984). No overt signs of toxicity were observed in the dams and no
changes in maternal feed consumption were observed at any dose.
DON was fed to 71 adult female New Zealand White rabbits in 7
batches of 6-14 animals during the entire period of gestation at
doses of 0.3, 0.6, 1.0, 1.6, 1.8, or 2.0 mg/kg body weight. There
were 25 controls. The fetal effects consisted of 100% incidence of
fetal resorption in the females fed 1.8 and 2.0 mg/kg and reduced
average body weight of fetuses from dams fed 1.0 and 1.6 mg/kg.
These doses were not teratogenic (Khera et al., 1986).
Weanling F0 male and female mice were fed diets containing DON at
a dose of 2.0 mg/kg body weight (15 male and 15 female animals) and
0.375, 0.75, or 1.5 mg/kg body weight (7 males and 59 female
animals). There were 30 controls in the first study and 26 in the
second. After 30 days of dietary feeding, the mice were allowed to
mate and the pregnant females were allowed to litter normally. The
F1a progeny were examined up to 21 days of age and discarded. The F0
mice were rebred. The females bred to produce the F1b litters were
killed on day 19 of gestation and the fetuses were examined for
gross, visceral and skeletal malformations. Reductions were
observed in feed intake and in the body weight of F0 male and female
mice, the number of live pups and postnatal survivors, postnatal body
weight of F1a progeny, number of live fetuses, and fetal body weight
of F1b. No adverse effects on the fertility of F0 mice and no
major malformations in F1b fetuses were found. Results of cross-
fostering offspring between control dams and 1.5 mg/kg dams indicated
that both postnatal survival and body weight were adversely affected
by prenatal exposure as well as by combined pre- and post-natal
exposure (Khera et al., 1984).
Male and female Sprague-Dawley rats (3 groups of 15 male and 15
female animals each) were fed diets containing DON at levels of 0.25,
0.5, or 1.0 mg/kg body weight. Controls consisted of two groups of
15 males and 15 females. After 6 weeks of feeding, the rats were
bred. The mated females, maintained on their respective diets, were
killed on the last day of pregnancy and fetuses were evaluated for
effects on pre-natal development. No adverse effects were observed
except for dilatation of the renal pelvis and urinary bladder (Khera
et al., 1984).
Male (20/group) and female Sprague-Dawley rats (25/group) were
fed a diet containing 20 mg purified DON/kg for 60 and 15 days,
respectively, before mating. Rats consuming the DON-supplemental
diet throughout gestation and lactation did not show any clinical
signs of toxicity, but had reduced body weights. Only 50% of the
matings between toxin-fed rats resulted in pregnancy compared with
80% in the controls. No differences were detected among the groups
in sex ratio, survival rate, or average litter number and weight.
Pup weight gains in all groups were comparable up to post-natal day
14. From day 14 to 21, however, male and female pups of the control
group showed significantly improved weight gains compared with pups
from treated dams. No treatment-related histological abnormalities
were found in the testes or ovaries of treated pups (Morrissey &
Vesonder, 1985).
A 2-generation reproduction and teratology study was carried out
using 90 female CD-1 mice fed a semisynthetic diet containing the T-2
toxin at 1.5 or 3.0 mg/kg, concentrations considered possible under
field conditions. Results indicated that continuous feeding of T-2
toxin at low concentrations had minimal, if any, toxic effects on
female reproduction and fetal development and that T-2 toxin fed
continuously at 1.5 or 3.0 mg/kg was not teratogenic or fetocidal and
produced minimal effects on the growth rates of CD-1 mice (Rousseaux
et al., 1986).
II.4.3 Biochemical effects and mode of action
II.4.3.1 Cytotoxicity
In the early stages of research on trichothecenes, DAS was
isolated from F. scirpi as a phytotoxic principle (Brian et al.,
1961). Subsequent surveys confirmed the phytotoxic nature of the
trichothecenes using germination of seeds of Brassica oleracea L.
(Ueno et al., 1971c), germination of pea seedlings (Marasas et al.,
1971), growth of tobacco callus tissues (Helgeson et al., 1973), the
germination of tobacco plant (Nicotiana sylvestris) pollen
(Siriwardana & Lafont, 1978), and the auxin-promoted elongation of
soybean hypocotyl (Stahl et al., 1973).
Most of the trichothecenes tested have had fungistatic activity
in a wide range of species (Bamburg et al., 1968a; Bamburg & Strong,
1971; Reiss, 1973); DAS and verrucarin A were particularly potent in
inhibiting growth and sporulation at concentrations of 0.5-1
mg/litre.
Many of the trichothecenes have been cytotoxic to mammalian
cells, both in in vivo and in vitro. In animals administered
trichothecenes, the mucosa of the stomach and small and large
intestines had mucosal erosions, mucosal necrosis with ulceration,
and severe haemorrhages; radiomimetic effects included necrosis of
actively-dividing cells in the thymus, spleen, ovary, testis, and
lymph nodes (Saito & Ohtsubo, 1974). In a cell culture system, Grove
& Mortimer (1969) demonstrated the cytotoxicity of DAS and its
chemically modified compounds on hepatocytes of human origin and
hamster kidney cells. Ohtsubo et al. (1968), Ohtsubo & Saito (1970),
and Bodon & Zoldag (1974) described the cytotoxicity of NIV and
related trichothecenes for HeLa cells, and of T-2 toxin for
epithelial cells of pig kidney, respectively. The macrocyclic
trichothecenes, verrucarins and roridins, were highly cytotoxic for
0815 mouse tumour cells in the ng/ml range (Harri et al., 1962).
Tanaka et al. (1977), investigated the cytotoxicity of 20 types of
trichothecenes (11 type A, 6 type B, 1 type C, and 2 type D) for 3
cell lines, HeLa, HEK, and HL cells. The type D trichothecenes,
such as verrucarin A and roridin A had LC50s in the range of 0.003-
0.005 mg/litre; neosolaniol and NIV in the range of 0.1-1 mg/litre,
and deoxynivalenol in the range of 1-5 mg/litre. Trichodermol,
calonectrin, monoacetyldeoxynivalenol, and tetraacetylnivalenol were
weakly cytotoxic within the range of 5-10 mg/litre.
Chinese hamster ovary (CHO) and African green monkey kidney
(VERO) cell cultures were exposed to 0.01 or 1.0 ng T-2 toxin/ml for
1 or 12 h. The cells exhibited morphological changes considered to
be related to inhibition of protein synthesis. The alterations
included disassociation of polysomes and matrix density, ballooning
of intracristal space, and malalignment of cristae in mitochondria.
The CHO cells had bleb formations of plasma membrane, a change
produced by exposure to establish inhibition of protein synthesis
(Trusal, 1985).
II.4.3.2 Inhibition of protein synthesis
The initial observation that the trichothecene mycotoxins
inhibited protein synthesis in mammalian cells was made by Ueno et
al. (1968). They reported that NIV produced a dose-dependent
inhibition of incorporation of several amino acids into protein in
rabbit reticulocytes (Ueno et al., 1968) and Ehrlich ascite tumour
cells (Ueno & Fukushima, 1968). This inhibitory effect of the
trichothecene mycotoxins was observed in the whole animal (Ueno,
1970), a protozoan (Ueno & Yamakawa, 1970), HeLa cells (Liao et al.,
1976), cultured mammalian cells (Ohtsubo et al., 1968; Ohtsubo &
Saito, 1970), rat hepatocytes (Ueno et al., 1973b), hamster ovary
cells (Gupta & Siminovitch, 1978), human tonsil (Carrasco et al.,
1973), yeast spheroplasts (Stafford & McLaughlin, 1973; Cundliffe et
al., 1974; McLaughlin et al., 1977), rabbit reticulocytes (Ueno et
al., 1968, 1969a, 1973b,c; Ueno & Shimada, 1974; Wei & McLaughlin,
1974; Mizuno, 1975; Carter et al., 1976), and lymphocytes (Hartman et
al., 1978). No inhibitory effect on bacterial cells was observed
(Ueno et al., 1973b).
The effects of T-2 toxin on protein and DNA synthesis were
studied in Swiss mice and hepatoma cell cultures. T-2 toxin was
given as a single ip dose of 0.75 mg/kg and as a 3-day and a 7-day
daily treatment. The toxin inhibited protein synthesis after all 3
schedules of treatment and inhibition was present in cells obtained
from bone marrow, spleen, and thymus. Protein synthesis was
inhibited in vitro in hepatoma cell cultures and PHA-stimulated
lymphocytes (Rosenstein & Lafarge-Frayssinet, 1983).
The effects of T-2 toxin on rat hepatocytes were studied in
culture by the addition of several doses at either 1 or 12 h of
exposure. A dose of 0.01 ng T-2 toxin/ml produced a 75% inhibition
of protein synthesis within 1 h. At a higher dose of 1.0 ng/ml,
hepatocytes recovered from a 1-h but not a 12-h exposure. Cell
damage (release of lactate dehydrogenase) lagged behind inhibition of
protein synthesis, which was 90% at the 1 ng/ml dose. Ultrastructural
alterations were present in the endoplasmic reticulum and
mitochondria. Degranulation involved the rough endoplasmic
reticulum. Mitochondria had translucent foci and electron dense
cores (Trusal & O'Brien, 1986).
NIV inhibited poly-U, poly-A, and poly-C directed incorporation
of phenylalanyl-tRNA into phenylalanine without affecting the
activation of amino acids. The inhibition was presumably caused by
impairment of ribosomal function (Ueno et al., 1968). Fusarenon-X
caused breakdown of polysomes in rabbit reticulocytes (Ueno et al.,
1973b) and in mouse fibroblasts (L-cells), shortly after exposure
(Ohtsubo et al., 1972). The breakdown of polysomes was consistent
with the action of an inhibitor of initiation of protein synthesis.
T-2 toxin, DAS, and verrucarin A, but not trichodermin, also induced
the disaggregation of polysomes in HeLa cells (Liao et al., 1976).
These results were consistent with the effects of an inhibitor of
prolongation or termination of protein synthesis (Stafford &
McLaughlin, 1973). An important point was that the trichothecenes,
regardless of whether they were I-type or ET-type, interacted with
the peptidyl transferase centre on the 60S ribosomal subunit and
inhibited the transpeptidation of the peptide bond formation process.
Current research has focused on clarifying the molecular species
of ribosomal protein that regulates the binding of trichothecene
mycotoxins. A mutant of yeast (Schindler et al., 1974; Jimenez &
Vazquez, 1975; Jimenez et al., 1975; Grant et al., 1976; Carter et
al., 1980) and Chinese hamster ovary cells, resistant to the effects
of trichothecenes on protein synthesis (Gupta & Siminovitch, 1978),
have been isolated. Friend & Warner (1981) clearly demonstrated that
the gene for trichodermin resistance in yeast specifies ribosomal
protein L3, the largest of the yeast ribosomal proteins.
The ribosomal subunits of Myrothecium verrucaria, a producer of
macrocyclic trichothecenes, were resistant to T-2 toxin. It can be
assumed that the 60S subunits of eukaryotes are responsible for the
sensitivity to the trichothecenes. (Hobden & Cundliffe, 1980).
II.4.3.3 Inhibition of nucleic acid synthesis
The dose-dependent inhibition by NIV of DNA and RNA synthesis in
Ehrlich ascites tumour cells was first observed by Ueno & Fukushima
(1968). Inhibition (70%) of protein synthesis was induced by 1-10 mg
NIV/litre, and thymidine incorporation into DNA was inhibited by as
much as 60%; suppression (30%) of uracil incorporation into RNA was
slight. Similar results were obtained with several trichothecenes,
including trichodermin, diacetoxyscirpenol, and fusarenon-X, in other
cultured cells, such as KB cells (Ohtsubo et al., 1968), HeLa cells
(Liao et al., 1976), mouse L-cell fibroblasts (Ohtsubo et al., 1972),
hamster ovary cells (Gupta & Siminovitch, 1978), and lymphocytes
(Hartman et al., 1978).
The inhibition of DNA and RNA synthesis by trichothecenes
required higher concentrations of toxins than the inhibition of
protein synthesis and the extent of the inhibition was much less.
Thus, the observed inhibition of DNA and RNA synthesis in toxin-
treated animal cells was presumed a secondary effect of the
trichothecenes. This hypothesis was supported by the findings of
Tashiro et al. (1979) who reported that, in in vitro studies, a
concentration of 0.0236-1.889 mmol fusarenon-X/litre did not inhibit
DNA-dependent RNA hybridases of rat liver and Tetrahymena pyriformis.
Munsch & Mueller (1980) reported that the incorporation of 3H-
thymidine into DNA in cell lines from thymus was strongly inhibited
by over 10 ng T-2 toxin/ml and slightly inhibited by 0.1-10 ng T-2
toxin/ml. A low concentration of 0.1-1 ng/ml toxin caused a
transient increase in DNA polymerases, alpha- and beta-terminal
deoxynucleotidyl transferases. At a high dose of more than 1 ng T-2
toxin/ml, these enzymatic activities were strongly inhibited.
Rosenstein & Lafarge-Frayssinet (1983) described the depression of
DNA synthesis in vivo and in vitro. Three treatment schedules: a
single dose, 3 daily doses, or 7 daily doses of 0.75 mg T-2 toxin/kg
inhibited DNA synthesis in cell cultures from the spleen, thymus, and
bone marrow of treated mice. The mycotoxin also inhibited DNA
synthesis in vitro in cultures of hepatoma cells and in PHA-
stimulated lymphocytes (Rosenstein & Lafarge-Frayssinet, 1983).
Agrelo & Schoental (1980) found that hydroxyurea did not alter
unscheduled DNA synthesis in cells treated with T-2 toxin and HT-2
toxin (6 ng/ml). However, the combination of rat liver microsomes
and hydroxyurea resulted in an increase in unscheduled DNA synthesis
in cells exposed to 100 µg HT-2 toxin/ litre. The data of Agrelo &
Schoental (1980) suggest that the microsomal drug-metabolizing enzyme
system may participate in the induction of DNA damage by
trichothecenes.
The effects of T-2 toxin and DAS on DNA synthesis in phyto-
haemagglutinin, stimulated in the peripheral blood lymphocytes of
human beings, was assayed by incorporation of 3H-thymidine. Total
inhibition was obtained by 8 ng T-2 toxin and DAS and 80% was
obtained with 1.5 ng T-2 toxin and 2.7 ng of DAS (Cooray, 1984).
Fusarenon-X and related toxins inhibited protein and nucleic acid
synthesis in Tetrahymena pyriformis in a manner similar to that
observed in cultured mammalian cells (Ueno & Yamakawa, 1970). In
synchronously dividing Tetrahymena cells (Iwahashi et al., 1982), the
incorporation of radioactive amino acids, thymidine, and uracil into
protein, DNA, and RNA, respectively, was achieved by nearly the same
concentration of T-2 toxin. Inhibition of protein synthesis was
explained by the high affinity of T-2 toxin for 60S ribosomal
subunits of Tetrahymena, as is the case with cultured cells.
However, neither DNA and RNA synthesis nor RNA hybridase activity
were altered by T-2 toxin in an in vitro system using isolated nuclei
from normal cells or from cells pretreated with T-2 toxin. The
mechanism of inhibition of nucleic acid synthesis in the in vivo
system is not yet understood.
II.4.3.4 Alterations of cellular membranes
The trichothecenes as a group inhibit protein synthesis, but the
potency of the effect varies according to the trichothecene and the
system ( in vitro or in vivo) used to measure the inhibition. T-2
toxin was a potent inhibitor of protein synthesis both in vitro and
in vivo (Ueno et al., 1973b; Rosenstein & Lafarge-Frayssinet, 1983).
In vitro experiments were conducted to determine the effects of
T-2 toxin on the entry of sucrose into bovine erythrocytes,
entrapment of sucrose and inulin in carrier erythrocytes, entrapment
of T-2 and binding of T-2 toxin, and the measurement of cell
permeability to the entrapped T-2 toxin. At the highest
concentration of T-2 toxin (20 µg), no entry of 14C-sucrose or 3H-
inulin was observed. Very little 3H-T-2 toxin was bound to bovine
erythrocytes and binding was independent of T-2 toxin concentration.
The mycotoxin had no effect on the entrapment of sucrose or inulin.
Carrier erythrocytes retained 85% of 14C-sucrose and only 18% of
3H-T-2 toxin. Thus, T-2 toxin diffused from carrier cells much more
rapidly than sucrose. It was concluded that the interaction of T-2
toxin with bovine erythrocytes was minimal and intercalation with the
inner bilayer was not likely, because the increase in cell volume
that would have resulted did not occur (DeLoach et al., 1987).
The effects of T-2 toxin on membrane function were studied using
L-6 myoblasts. The minimal effective concentration (MEC) of T-2
toxin for reduction in uptake of calcium and glucose and for
reduction in uptake of leucine and tyrosine and their incorporation
into protein was 4 pg/ml. The uptake of rubidium was increased at
0.4 pg/ml and reduced at 4 pg/ml or more. Thymidine uptake and
incorporation into DNA had a biphasic response with an increase at
0.4 pg/ml and a reduction at 4 pg/ml for uptake and 40 pg/ml for
incorporation. Calcium efflux was reduced after 1, 5, and 15 min
exposure to T-2 toxin at a concentration of 40 pg/ml. These data
indicate that T-2 toxin has multiple effects on all membrane function
at very low concentrations and that these effects are independent of
inhibition of protein synthesis (Bunner & Morris, 1988).
II.4.3.5 Other biochemical effects
Fusarenon-X inhibited the uptake of phosphate by Tetrahymena
cells (Chiba et al., 1972). It caused a 50% inhibition of
incorporation of acetate into phospholipids and a ten-fold
stimulation of incorporation into triglyceride, effects that may be
secondary to inhibition of phosphate uptake. However, in rabbit
reticulocytes, fusarenon-X, T-2 toxin, and neosolaniol at
concentrations of 100 mg/litre did not inhibit Na+-dependent glycine
transport (Ueno et al., 1978b).
When SH-enzymes were pre-incubated with selected trichothecenes
in the absence of substrates, the activities of such enzymes as
creatine phosphokinase and lactate and alcohol dehydrogenases were
reduced (Ueno & Matsumoto, 1975). However, neither urease activity
(Reiss, 1977) nor rat liver lysosomal cathepsin activity (Farb et
al., 1976) was affected by diacetoxyscirpenol or T-2 toxin,
respectively, in vitro. Foster et al. (1975), reported the
reactivity of T-2 toxin with glutathione in the presence of epoxide-
S-GSH-transferase.
When mice were exposed to trichothecene mycotoxins, no detectable
alterations were observed in hepatic and renal functions. In chicks
fed a diet containing 10 mg T-2 toxin/kg for 3 weeks, a hepatic
microsomal aminopyrine, demethylase, was reduced by 29% compared with
the control, while another mixed function oxidase, aniline
hydroxylase, was not significantly affected (Coffin & Combs,
1981). In mice receiving a sublethal dose of fusarenon-X ip, a rapid
hypoglycemia was followed by depletion of hepatic glycogen (Shimizu
et al., 1979). The authors suggested that the toxin had induced a
malfunctioning of glucose absorption in the intestines and had
accelerated glycolysis. However, the trichothecene mycotoxins are
highly cytotoxic to the epithelia of the intestine, and impairment of
carbohydrate metabolism and absorption may be a secondary effect of
this cytotoxicity.
II.4.4 Structure-activity relationships
After the isolation and identification of numerous derivatives of
the trichothecenes, the structure-activity relationship was
investigated, on the basis of the information on lethal toxicity,
dermal toxicity, cytotoxicity, inhibition of protein synthesis, and
association with ribosomes.
The 12,13-epoxide of the trichothecenes is essential for their
biological activity. The de-epoxidation of DON and T-2 toxin by
rumen-microorganisms (King et al., 1984) and in mammalian systems
(Yoshizawa et al., 1983) results in loss of toxicity.
The macrocyclic ring contributes to the highly lipophylic
property of the trichothecenes, and the macrocyclic trichothecenes,
such as verrucarins, roridins, satratoxins, and baccharins, exhibit a
potent cytotoxicity towards cultured mammalian cells (Kupchan et al.,
1976, 1977; Jarvis et al., 1978, 1980).
In contrast, the polyhydroxylated alcohols, such as verrucarol,
NIV, and DON, are highly hydrophilic, resulting in a decrease in
cytotoxicity and dermal toxicity compared with their parent
trichothecenes (Ueno et al., 1970; Wei et al., 1974).
II.4.5 Prevention and therapy of trichothecene toxicosis
In studies to investigate effects of various dietary supplements
on T-2 toxin toxicity, weanling male Wistar rats in groups of 10,
with adequate controls, were fed diets containing 5% each of
bentonite, anion exchange resin, cation exchange resin, or
vermiculite-hydrobiotite with and without 3 µg T-2 toxin/g feed, for
2 weeks. Bentonite and anion exchange resin were most effective in
reducing the growth depression and feed refusal caused by T-2 toxin.
In a second study in which bentonite and anion exchange resin were
administered at levels of 2.5, 5.0, 7.5, or 10% of diet, 10%
bentonite in the diet was the most effective dietary supplement.
Dietary bentonite reduced the absorption of 3H-T-2 toxin and
increased faecal elimination (Carson & Smith, 1983).
Smectite, a clay composed of insoluble silicates of aluminum and
magnesium, incubated with T-2 toxin for 24 h before the toxin was
administered orally to mice (1 mg/kg per day), prevented the
acceleration of gastric emptying and transit time for a milk meal,
usually induced by administration of T-2 toxin. Smectite given
together with the toxin did not prevent the gastrointestinal effects
of T-2 toxin (Fioramonti et al., 1987). Male Sprague-Dawley rats
were given 15 or 5 mg/kg body weight of a platelet activating factor
antagonist, with and without T-2 toxin, by intravenous injection.
The drug prolonged the survival of conscious rats exposed to 0.65 mg
T-2 toxin/kg (Feuerstein et al., 1987).
The effects of certain drugs and metabolic inhibitors on the
toxicity of T-2 toxin have been studied in male ddY mice. The toxin
at a dose of 1.8 mg/kg was given subcutaneously and the drugs and
other chemicals were given ip. The lethal effects were reduced by
the steroid drugs, prednisolone and dexamethasone; survival times
were increased by the antihistaminic drug diphenhydramine, and an
opioid antagonist, naloxone. Prednisolone also reduced the
leukocytosis that developed after T-2 toxin treatment and decreased
the increase in ear weight caused by the mycotoxin. Other
antihistaminic and/or antiserotonic drugs used in the treatment were
found not to be effective in preventing the lethal effects of T-2
toxin (Ryu et al., 1987).
Male ddY mice and male Wistar rats in several groups of 10
animals each, pretreated with a radioprotective compound or with an
anti-inflammatory agent, were given T-2 toxin or fusarenon-X (1.5
mg/kg body weight). Out of the 20 compounds evaluated, only
prednisolone and hydrocortisone (100 mg/kg ip) were effective. They
reduced mortality from 90% in controls to less than 30% and they also
reduced the trichothecene-induced increase in intestinal fluid volume
(Mutoh et al., 1988).
Cutaneous irritation produced by T-2 toxin in 10 Porton female
rats was largely reduced by application, within 10 min, of an aqueous
soap solution when the dose was low (1.0 µg/cm2), but the solution was
largely ineffective over a time span of 60 min when the dose was
higher (100 µg T-2 toxin). Washing the site of application with
polyethylene glycol 300 was very effective in removing even large
doses (100 µg) of T-2 toxin from the skin (Fairhurst et al., 1987).
The median effective dose of oral superactive charcoal in
preventing deaths in batches of 5 female Sprague-Dawley rats was
0.175 g/kg. A dose of 1 g superactive charcoal/kg body weight
increased survival times and rates in rats given the lethal dose of 8
mg T-2 toxin/kg as long as 3 h after the toxin was administered by
gavage (Galey et al., 1987).
II.5 EFFECTS ON MAN
II.5.1 Contemporary episodes of human disease
Two outbreaks of trichothecene-related disease have been
reported, one in China in 1984/85 (Luo, 1988) and one in India in
1987 (Bhat et al., 1989). Each involved several hundred cases.
During the first incident, outbreaks of mouldy corn and scabby
wheat poisoning were reported. Out of approximately 600 persons who
consumed mouldy cereals, there were 463 cases of poisoning (77% of
the total). The latency period for the onset of symptoms was 5-30
min. These included nausea, vomiting, abdominal pain, diarrhoea,
dizziness, and headache. No deaths occurred. Pigs and chicks fed
the same mouldy cereals were also affected (Luo, 1988). GC-MS and
RIA were used in the analysis of 5 samples of the mouldy corn. DON
was detected within a range of 0.34-92.8 mg/kg and zearalenone within
a range of 0.004-0.587 mg/kg. T-2 toxin and NIV were not found. TLC
was used in the analysis of 19 samples of scabby wheat collected from
the affected and non-affected families. The DON content was 1.0-40.0
mg/kg, which was significantly higher than that in the non scabby
wheat samples. In addition to DON, zearalenone was detected in 2
samples, the contents of which were 0.25 and 0.5 mg/kg, respectively.
No T-2 toxin was found in the samples (Luo, 1988).
An analogous outbreak was reported in Kashmir, India, in 1987
(Bhat et al., 1987, 1989). It was ascribed to the consumption of
bread made from flour that had become mouldy in storage following
unseasonal rains in the wheat-harvesting season, from which Fusarium
sp. was grown, and which were found to contain mycotoxins. Of the
224 persons investigated on a random sample basis, 97 were affected
with symptoms including abdominal pain (100%), throat irritation
(63%), diarrhoea (39%), blood in stools (5%), and vomiting (7%).
Symptoms developed 15 min to one hour after consumption of locally
baked bread. In 12 out of 24 samples of refined wheat flour used in
the preparation of bread, the following mycotoxins were found: DON
(0.35-8.38 mg/kg), Ac-DON (0.64-2.49 mg/kg) (no details of estimation
of this derivative were available), NIV (0.03-0.1 mg/kg) and T-2
toxin (0.55-0.8 mg/kg). Quantitative estimation of DON, Ac-DON, and
NIV was obtained using HPLC, and that of T-2 toxin by TLC (Bhat et
al., 1987) but no rigorous confirmation of identity was undertaken.
Since no fatalities occurred in either of the above outbreaks, no
information is available on the pathological changes, if any, at
autopsy.
II.5.2 Historical Fusarium-related diseases
In the period 1931-47, a human disease known as alimentary toxic
aleukia (ATA) occurred in the USSR that was suggested to be related
to the presence of toxic Fusarium species in mouldy over-wintered
grain. Data have been reviewed by Sarkisov et al. (1944) and more
recently by Bilai (1977), Leonov (1977), and Joffe (1986). An
association was established with the ingestion of grain invaded by
some moulds, in particular Fusarium poae and F. sporotrichioides.
The dominant pathological changes were necrotic lesions of the oral
cavity, the oesophagus, and stomach and, in particular, a pronounced
leukopenia. The primary lesion was bone marrow hypoplasia and
aplasia. The disease was lethal in a high proportion of cases.
The clinical symptoms reported in ATA, as well as the identified
occurrence of Fusarium in foodstuffs, suggest that it might have been
associated with mycotoxins, identified years later in fungal cultures
of Fusarium species under laboratory conditions, such as T-2 toxin
(Mirocha & Pathre 1973) or wortmannin (Mirocha & Abbas 1989).
Scabby grain toxicosis is a disease of human beings as well as
farm animals, and was reported from Japan and Korea in the period
1946-63 (Hirayama & Yamamoto 1948, 1950; Nakamura et al., 1951;
Tsunoda et al., 1957; Cho 1964; Ogasawara 1965; Chung 1975). The
common clinical symptoms were nausea, vomiting, diarrhoea, and
abdominal pain. All cases were acute with recovery within a few
days and no lethal cases were encountered. Fusarium fungi, F.
graminearum in particular, were isolated from suspected cereals
(Tochinai, 1933; Tsunoda et al., 1957).
When compared with the symptoms observed in experimental
animals, features of both the above human diseases were similar to
trichothecene toxicosis, notably symptoms caused by DON and NIV,
DAS, and T-2 toxin. However, no epidemiological studies have been
reported that link ATA and scabby grain toxicosis to these
chemicals.
II.5.3 Skin irritation
The Task Group was aware of several reports describing various
effects on the skin of crude extracts of fungal cultures or
solutions containing T-2 toxin, as well as other possible
substances (Bamburg & Strong, 1971; Saito & Otshubo, 1974). These
cases, all of them accidental, involved a very limited number of
persons who developed severe irritation, loss of sensitivity, and
desquamation. Despite the presence of T-2 toxin in the contact
material, there is no evidence that the involvement of other
compounds can be ruled out.
II.5.4 Studies of haemostasis
Platelet function and electron microscopic morphological
changes following T-2 toxin administration were studied on
platelets isolated from 12 healthy human volunteers. When
platelets were incubated with T-2 toxin at doses of 5-500 µg/109
platelets for 20 min, there was a dose-related inhibition of
platelet aggregation with different activators, including
epinephrine, arachidonic acid, and collagen, and a release of dense
bodies consisting mainly of serotonin-containing granules. There
was also a change in membrane permeability, but no changes in
shape. No correlated inhibition of thromboxane synthesis, or
significant alterations in platelet calcium content were observed.
The microtubular system was unaffected. It was suggested that the
above observations , notably suppressed aggregation, played a
contradictory role in the haemorrhagic phenomena associated with
these toxins in man and animals (Yarom et al., 1984a).
II.5.5 Airborne trichothecene-related diseases
High concentrations of spores of Stachybotrys atra were
discovered in the air of living rooms of a suburban house in
Chicago, USA (Croft et al., 1986). Over a period of several years,
the 5 occupants of the house had suffered a variety of non-specific
symptoms including signs of colds, sore throats, diarrhoea,
headaches, dermatitis, intermittent focal alopaecia, and
generalized malaise. Chemical analysis of building materials
supporting the growth of Stachybotrys atra confirmed the presence
of the macrocyclic trichothecenes, verrucarin B and J, satratoxin
H, and trichoverrins A and B. Five weanling Sprague-Dawley rats
and 5 mice, administered extracts of contaminated materials orally,
died within 24 h of exposure, whereas control animals remained
unaffected. Histological lesions of the animals tissues were
degeneration, necrosis and haemorrhage of the brain, thymus,
spleen, intestine, lungs, heart, lymph nodes, liver, and kidneys.
Although the clinical symptoms could have been related to allergic
responses, the isolation of potent mycotoxins suggests that they
were the causal factor of the illness observed.
II.5.6 Toxicological information on man, obtained from
therapeutic uses
DAS (Anguidine) has been undergoing clinical trials as a
chemotherapeutic agent in cancer patients. During a weekly
schedule of DAS, using healthy volunteers as controls, a dose of 5
mg/m2 body surface infused over 3 h, produced nausea, vomiting,
hypotension, neurological symptoms (confusion, hallucinations and
psychomotor seizures), chills, fever, and diarrhoea (DeSimone et
al., 1979). A similar study carried out on 20 other cancer
patients revealed gastrointestinal and neurological toxic effects
(Belt et al., 1979). A bolus administration or rapid infusion of
the drug also caused gastrointestinal and neurological symptoms
(Thigpen et al., 1981). The human haematopoietic system appears to
be extremely sensitive to DAS. Myelosuppression was the dose
limiting adverse effect of prolonged infusions over 8 h (Thigpen et
al., 1981). A mean myelosuppressive dose level in the above
investigation was, 5 mg/m2 body surface by 8-h infusion or, roughly
calculated in terms of body weight, 0.2 mg/kg (0.025 mg/kg per h).
II.6 EVALUATION OF THE HUMAN HEALTH RISKS
On the basis of the data made available to the Task Group, there
is a possible association between trichothecene exposure and
episodes of human disease. According to the limited data
available, the most frequently reported trichothecenes involved in
episodes of human exposure are DON and NIV. In the episodes of
alimentary toxic aleukia and scabby grain toxicosis reported in the
past, a possible etiological role of trichothecenes cannot be
excluded. Exposure occurs through the ingestion of contaminated
food, mainly cereals. Processing, milling, and baking are
not effective in destroying DON, NIV, and T-2 toxin. There is very
limited evidence of exposure through inhalation, but such a
possibility cannot be ruled out.
Among the naturally occurring trichothecenes in foods, T-2
toxin is the most potent, followed by DAS and NIV; DON was the
least toxic in acute toxicity studies. In experimental animals,
T-2 toxin and DAS produce acute systemic effects, with necrosis of
epithelial tissues and suppression of haematopoiesis. In
contemporary outbreaks of disease, only gastrointestinal symptoms
have been reported.
DON was shown to be teratogenic in mice but not in rats.
According to published chronic toxicity studies, NIV and T-2 toxin
are not tumorogenic in animals. No long-term carcinogenicity
studies on DON have been published. Certain trichothecenes, such
as T-2 toxin and DON, have an immunosuppressive action in animals
and have produced alterations in both cell-mediated and humoral
immunity. There is no evidence of immunosuppressive action in man.
Reported cases of human disease associated with trichothecene
exposure are limited in number and information. Symptoms of
digestive disorders and throat irritation develop rapidly after
ingestion of food contaminated with trichothecenes. At present,
there is no evidence of human cancer caused by trichothecenes. No
reports were available to the Task Group on secondary infection, by
bacteria, fungi, or viruses, in human beings following trichothecene
exposure, as has been observed in experimental animal studies. It
appears that adequate studies elucidating such a sequence have not
been made.
III. ERGOT
III.1 PROPERTIES AND ANALYTICAL METHODS
III.1.1 Chemical properties
Ergot is the French word for a rooster's spur and is used as
the common term for sclerotia of fungal species within the genus
Claviceps, in particular C. purpurea. The fungi infect the florets
of grasses and cereal, replacing the florets with compact fungal
structures, sclerotia, 2-20 mm long, somewhat curved, tapering off
at the ends, and strongly coloured, often purple-black, thus
resembling rooster's spurs. The sclerotia contain a large number
of biologically active alkaloids, as well as amino acids,
carbohydrates, lipids, and pigments, and, when the sclerotia are
consumed by man and animals, toxicosis develops, called ergotism.
The topic has been reviewed by Bove (1970), Van Rensburg &
Altenkirk (1974), Lorenz (1979), and Berde & Schild (1978). This
section deals with ergot as a toxic food contaminant; ergot
compounds used as pharmaceutical drugs are excluded.
Ergot alkaloids (ergolines), of which more than 40 have been
isolated from Claviceps sclerotia, are derivatives of lysergic acid
(Fig. 4), and can be divided into 3 groups:
Group I: Derivatives of lysergic acid, e.g., ergotamine.
Group II: Derivatives of isolysergic acid, e.g., ergotaminine.
Group III: Derivatives of dimethylergoline (clavines), e.g.,
agroclavine.
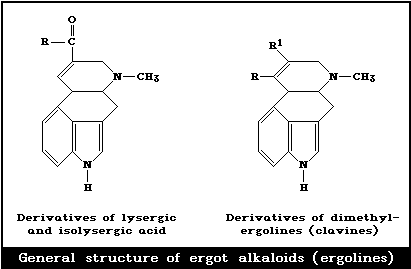 Physical and chemical properties of some ergolines are listed
in Table 23.
Table 23. Physical and chemical properties of selected
ergot alkaloids (ergolines)a
-----------------------------------------------------------
Ergoline Molecular Melting [alpha]20
formula point D
(°C)
-----------------------------------------------------------
Group I. Derivatives of lysergic acid
ergotamine C33H35O5N5 180 -160
alpha-ergocryptinine C32H41O5N5 212-214 -190
ergocristine C35H39O5N5 160-175 -183
ergosine C30H37O5N5 220-230 -183
ergocornine C31H39O5N5 182-184 -188
ergometrine C19H23O2N3 162 +41
Group II. Derivatives of isolysergic acid
ergocristinine C35H39O5N5 226 +366
ergometrinine C19H23O2N3 196 +414
ergosinine C30H37O5N5 228 +420
ergocorninine C31H39O5N5 228 +409
alpha-ergocryptinine C32H41O5N5 240-243 +480
ergotaminine C33H35O5N5 241-243 +369
Group III. Derivatives of dimethyloergolines (clavines)
agroclavine C16H18N2 206 -151
elymoclavine C16H18ON2 249 -109
chanoclavine C16H20ON2 222 -240
penniclavine C16H18O2N2 222 +153
setoclavine C16H18ON2 229-234 +174
-----------------------------------------------------------
a From: Van Rensberg & Altenkirk (1974) and Lorenz (1979).
III.1.2 Analytical methods for ergot and ergot alkaloids
III.1.2.1 Ergot
Sclerotia of Claviceps species are all rich in triglycerides,
but only a few species have a sufficiently distinctive fatty acid
composition in the sclerotia to provide a basis for identification.
Sclerotia of C. purpurea are unique among microorganisms in
containing a large (approximately 30%) triglyceride fraction in
which ricinoleic acid is the principal (30-40%) component. Thus,
the presence of ricinoleate in a foodstuff known to be free from
other sources of ricinoleic acid (e.g., castor oil) is diagnostic
for the presence of C. purpurea sclerotia. The sample is
saponified, the free fatty acids thereby released are methylated
with diazomethane, and the resulting methyl esters are analysed
using gas-liquid chromatography (Mantle, 1977a). As little as 0.3%
ergot in 1-2 g foodstuff has been detected by this procedure.
III.1.2.2 Ergot alkaloids
Sclerotia may contain up to 1% of total ergot alkaloids.
Lysergic acid derivatives can easily be epimerized at C-8 to give
isolysergic acid derivatives (Group III), thereby removing most of
their biological activity. As this may happen to a variable extent
during extraction, it is difficult to know whether the small
proportion of isolysergic acid derivatives, commonly found during
analysis of extracts from sclerotia, has been generated in whole or
in part during extraction (Mantle, 1977b).
A method for the determination of C. purpurea ergot alkaloids
in flour has been developed, based on liquid chromatography
following extraction of the flour by a mixture of methylene
chloride, ethyl acetate, methanol, and 28% ammonium hydroxide
solution (Scott & Lawrence, 1980). After filtration and
evaporation to dryness, the residue is dissolved in methanol-ether
and extracted with 0.5 N hydrochloric acid. The acid layer is
washed with hexane, made alkaline, re-extracted with methylene
chloride, evaporated, and the residue dissolved in methanol,
ready for liquid chromatographic analysis, with identification
based on fluorescence at an excitation wavelength of 235 nm. The
method has a recovery of 66-93%, based on analysis of flour spiked
with Group I alkaloids (ergotamine, ergocryptine, ergocristine,
ergosine, ergocornine, ergometrine).
A method using high-performance liquid chromatography for the
analysis of human blood has been reported (Zorz et al., 1985).
Samples (5 ml) of human plasma are extracted with benzene-toluene-
ethyl-acetate-diethylamine, the solvent layer separated and
evaporated to dryness. High-performance liquid chromatography is
performed with excitation wavelength at 285 nm for naturally
occurring ergot alkaloids. According to recovery studies,
concentrations as low as 0.2 mg/litre plasma can be detected.
A radioimmunoassay has been developed for determination of
ergotamine and ergocristine (Arens & Zenk, 1980). The alkaloids
were conjugated with bovine serum-albumin, and antibodies raised
in rabbits. Using 3H-labelled tracers, levels as low as 3.5 pmol
of ergotamine and 0.8 pmol of ergocristine could be measured. The
antibodies were highly specific, and simple lysergic acid
derivatives and clavines did not cross-react. The procedure has
been used in the detection of sclerotia of C. purpurea with a high
alkaloid concentration for industrial bioproduction.
A liquid chromatographic method for the detection of
ergotamine, ergotaminine, and ergocristine in human plasma has been
developed using extraction at pH 9, clean-up, and an ODS-hypersil
reverse-phase column (Edlund, 1981). Levels of ergolines as low as
0.1 mg/litre in 3 ml plasma samples can be detected, with a
recovery of 79-99% for the 3 ergolines.
Ergot contamination of pearl millet, due to infection by C.
fusiformis, is characterized by the presence of clavine alkaloids
(Group III). A procedure for its determination has been developed
using thin-layer chromatographic separation and spectrophotometric
detection following colour reaction using Van Urk's reagent (Bhat
et al., 1976; Krishnamachari & Bhat, 1976). The procedure includes
defatting of the grain sample, mixing of the defatted material with
ammonium hydroxide, extraction with diethyl-ether followed by
extraction of the diethyl-ether phase with 0.1 N sulfuric acid.
The extract is made alkaline and extracted with chloroform,
followed by thin-layer chromatographic separation.
III.2 SOURCES AND OCCURRENCE
III.2.1 Fungal producers
Sclerotia are compact hyphal structures that develop in the
colonies of many fungal genera. The sclerotia of species within
the genus Claviceps are unique in terms of size (length up to
several cm), pronounced colour, and because the sclerotia of
several species contain highly biologically active compounds, the
alkaloids. The sclerotia develop during the infection of plants;
host plants for the Claviceps species mainly belong to the grass
family (Gramineae), which comprises the true cereals. However, a
few plant species within the family Juncaceae and Cyperaceae can
also act as hosts. Most Claviceps species have a monogeneric host
range, but C. purpurea is unique in that it has a very wide host
range (Van Rensburg & Altenkirk, 1974; Lorenz, 1979). The eight
leading cereals produced in the world are wheat, rice, corn,
sorghum, rye, barley, oats, and millet, and they can all be hosts
for Claviceps species. Man and animals are exposed to toxic
sclerotia from two species, C. purpurea and C. fusiformis; farm
animals are also exposed to toxic sclerotia from C. paspali,
growing on grass. Sclerotia of C. purpurea have the dimension of
2-20 x 1-6 mm and are purple-black in colour, whereas C. fusiformis
has small (2 x 4 mm) purple-red coloured sclerotia (Loveless, 1967;
Siddiqui & Khan, 1973; Mantle, 1977b). C. purpurea primarily
attacks cross-pollinated species in which the florets tend to
remain open for a relatively long time and in which sterility
occurs.
The cereals most commonly contaminated with ergot from C.
purpurea are rye, wheat, triticale (the cross-breed between wheat
and rye), barley, oats, and sorghum. The ergot of C. purpurea
contain Group I and II ergolines as the principal ergot alkaloids.
C. fusiformis is a parasite of pearl millet (Pennisetum typhoideum)
in Africa and East Asia; in India, the cereal is called bajra.
Ergot of C. fusiformis contain predominantly Group III ergolines.
Ergot alkaloids have been isolated from fungi outside the genus
Claviceps (Aspergillus fumigatus, A. clavatus, A. nidulans,
Rhizopus nigricans, Penicillium chermesinum, P. concavo-rugulosum,
P. sizovae) and from higher plants (Rivea corymbosa, Ipomoea
violacea, I. argyrophylla, I. hildebrandtii, I. tricolor) (Van
Rensburg & Altenkirk, 1974; Kozlovsky & Reshetilova, 1984).
Whether these sources represent human exposure is not known at
present.
Successful attempts have been made to produce ergot alkaloids
using C. purpurea cultures in liquid media under laboratory
conditions, and new alkaloids have been identified (Bianchi et al.,
1982).
III.2.2 Biosynthesis
The ergoline ring system in ergot fungi is built up from L-
tryptophan and mevalonic acid (Fig. 5). The N-methyl group of
the ergot alkaloids is derived from methionine via a
transmethylation reaction. The precursors of tryptophan are
indole, anthranilic acid, and indolpyruvic acid (Van Rensburg &
Altenkirk, 1974; Mantle, 1977b). It appears that Group III
ergolines are intermediates in the production of Group I and II
ergolines by C. purpurea.
Physical and chemical properties of some ergolines are listed
in Table 23.
Table 23. Physical and chemical properties of selected
ergot alkaloids (ergolines)a
-----------------------------------------------------------
Ergoline Molecular Melting [alpha]20
formula point D
(°C)
-----------------------------------------------------------
Group I. Derivatives of lysergic acid
ergotamine C33H35O5N5 180 -160
alpha-ergocryptinine C32H41O5N5 212-214 -190
ergocristine C35H39O5N5 160-175 -183
ergosine C30H37O5N5 220-230 -183
ergocornine C31H39O5N5 182-184 -188
ergometrine C19H23O2N3 162 +41
Group II. Derivatives of isolysergic acid
ergocristinine C35H39O5N5 226 +366
ergometrinine C19H23O2N3 196 +414
ergosinine C30H37O5N5 228 +420
ergocorninine C31H39O5N5 228 +409
alpha-ergocryptinine C32H41O5N5 240-243 +480
ergotaminine C33H35O5N5 241-243 +369
Group III. Derivatives of dimethyloergolines (clavines)
agroclavine C16H18N2 206 -151
elymoclavine C16H18ON2 249 -109
chanoclavine C16H20ON2 222 -240
penniclavine C16H18O2N2 222 +153
setoclavine C16H18ON2 229-234 +174
-----------------------------------------------------------
a From: Van Rensberg & Altenkirk (1974) and Lorenz (1979).
III.1.2 Analytical methods for ergot and ergot alkaloids
III.1.2.1 Ergot
Sclerotia of Claviceps species are all rich in triglycerides,
but only a few species have a sufficiently distinctive fatty acid
composition in the sclerotia to provide a basis for identification.
Sclerotia of C. purpurea are unique among microorganisms in
containing a large (approximately 30%) triglyceride fraction in
which ricinoleic acid is the principal (30-40%) component. Thus,
the presence of ricinoleate in a foodstuff known to be free from
other sources of ricinoleic acid (e.g., castor oil) is diagnostic
for the presence of C. purpurea sclerotia. The sample is
saponified, the free fatty acids thereby released are methylated
with diazomethane, and the resulting methyl esters are analysed
using gas-liquid chromatography (Mantle, 1977a). As little as 0.3%
ergot in 1-2 g foodstuff has been detected by this procedure.
III.1.2.2 Ergot alkaloids
Sclerotia may contain up to 1% of total ergot alkaloids.
Lysergic acid derivatives can easily be epimerized at C-8 to give
isolysergic acid derivatives (Group III), thereby removing most of
their biological activity. As this may happen to a variable extent
during extraction, it is difficult to know whether the small
proportion of isolysergic acid derivatives, commonly found during
analysis of extracts from sclerotia, has been generated in whole or
in part during extraction (Mantle, 1977b).
A method for the determination of C. purpurea ergot alkaloids
in flour has been developed, based on liquid chromatography
following extraction of the flour by a mixture of methylene
chloride, ethyl acetate, methanol, and 28% ammonium hydroxide
solution (Scott & Lawrence, 1980). After filtration and
evaporation to dryness, the residue is dissolved in methanol-ether
and extracted with 0.5 N hydrochloric acid. The acid layer is
washed with hexane, made alkaline, re-extracted with methylene
chloride, evaporated, and the residue dissolved in methanol,
ready for liquid chromatographic analysis, with identification
based on fluorescence at an excitation wavelength of 235 nm. The
method has a recovery of 66-93%, based on analysis of flour spiked
with Group I alkaloids (ergotamine, ergocryptine, ergocristine,
ergosine, ergocornine, ergometrine).
A method using high-performance liquid chromatography for the
analysis of human blood has been reported (Zorz et al., 1985).
Samples (5 ml) of human plasma are extracted with benzene-toluene-
ethyl-acetate-diethylamine, the solvent layer separated and
evaporated to dryness. High-performance liquid chromatography is
performed with excitation wavelength at 285 nm for naturally
occurring ergot alkaloids. According to recovery studies,
concentrations as low as 0.2 mg/litre plasma can be detected.
A radioimmunoassay has been developed for determination of
ergotamine and ergocristine (Arens & Zenk, 1980). The alkaloids
were conjugated with bovine serum-albumin, and antibodies raised
in rabbits. Using 3H-labelled tracers, levels as low as 3.5 pmol
of ergotamine and 0.8 pmol of ergocristine could be measured. The
antibodies were highly specific, and simple lysergic acid
derivatives and clavines did not cross-react. The procedure has
been used in the detection of sclerotia of C. purpurea with a high
alkaloid concentration for industrial bioproduction.
A liquid chromatographic method for the detection of
ergotamine, ergotaminine, and ergocristine in human plasma has been
developed using extraction at pH 9, clean-up, and an ODS-hypersil
reverse-phase column (Edlund, 1981). Levels of ergolines as low as
0.1 mg/litre in 3 ml plasma samples can be detected, with a
recovery of 79-99% for the 3 ergolines.
Ergot contamination of pearl millet, due to infection by C.
fusiformis, is characterized by the presence of clavine alkaloids
(Group III). A procedure for its determination has been developed
using thin-layer chromatographic separation and spectrophotometric
detection following colour reaction using Van Urk's reagent (Bhat
et al., 1976; Krishnamachari & Bhat, 1976). The procedure includes
defatting of the grain sample, mixing of the defatted material with
ammonium hydroxide, extraction with diethyl-ether followed by
extraction of the diethyl-ether phase with 0.1 N sulfuric acid.
The extract is made alkaline and extracted with chloroform,
followed by thin-layer chromatographic separation.
III.2 SOURCES AND OCCURRENCE
III.2.1 Fungal producers
Sclerotia are compact hyphal structures that develop in the
colonies of many fungal genera. The sclerotia of species within
the genus Claviceps are unique in terms of size (length up to
several cm), pronounced colour, and because the sclerotia of
several species contain highly biologically active compounds, the
alkaloids. The sclerotia develop during the infection of plants;
host plants for the Claviceps species mainly belong to the grass
family (Gramineae), which comprises the true cereals. However, a
few plant species within the family Juncaceae and Cyperaceae can
also act as hosts. Most Claviceps species have a monogeneric host
range, but C. purpurea is unique in that it has a very wide host
range (Van Rensburg & Altenkirk, 1974; Lorenz, 1979). The eight
leading cereals produced in the world are wheat, rice, corn,
sorghum, rye, barley, oats, and millet, and they can all be hosts
for Claviceps species. Man and animals are exposed to toxic
sclerotia from two species, C. purpurea and C. fusiformis; farm
animals are also exposed to toxic sclerotia from C. paspali,
growing on grass. Sclerotia of C. purpurea have the dimension of
2-20 x 1-6 mm and are purple-black in colour, whereas C. fusiformis
has small (2 x 4 mm) purple-red coloured sclerotia (Loveless, 1967;
Siddiqui & Khan, 1973; Mantle, 1977b). C. purpurea primarily
attacks cross-pollinated species in which the florets tend to
remain open for a relatively long time and in which sterility
occurs.
The cereals most commonly contaminated with ergot from C.
purpurea are rye, wheat, triticale (the cross-breed between wheat
and rye), barley, oats, and sorghum. The ergot of C. purpurea
contain Group I and II ergolines as the principal ergot alkaloids.
C. fusiformis is a parasite of pearl millet (Pennisetum typhoideum)
in Africa and East Asia; in India, the cereal is called bajra.
Ergot of C. fusiformis contain predominantly Group III ergolines.
Ergot alkaloids have been isolated from fungi outside the genus
Claviceps (Aspergillus fumigatus, A. clavatus, A. nidulans,
Rhizopus nigricans, Penicillium chermesinum, P. concavo-rugulosum,
P. sizovae) and from higher plants (Rivea corymbosa, Ipomoea
violacea, I. argyrophylla, I. hildebrandtii, I. tricolor) (Van
Rensburg & Altenkirk, 1974; Kozlovsky & Reshetilova, 1984).
Whether these sources represent human exposure is not known at
present.
Successful attempts have been made to produce ergot alkaloids
using C. purpurea cultures in liquid media under laboratory
conditions, and new alkaloids have been identified (Bianchi et al.,
1982).
III.2.2 Biosynthesis
The ergoline ring system in ergot fungi is built up from L-
tryptophan and mevalonic acid (Fig. 5). The N-methyl group of
the ergot alkaloids is derived from methionine via a
transmethylation reaction. The precursors of tryptophan are
indole, anthranilic acid, and indolpyruvic acid (Van Rensburg &
Altenkirk, 1974; Mantle, 1977b). It appears that Group III
ergolines are intermediates in the production of Group I and II
ergolines by C. purpurea.
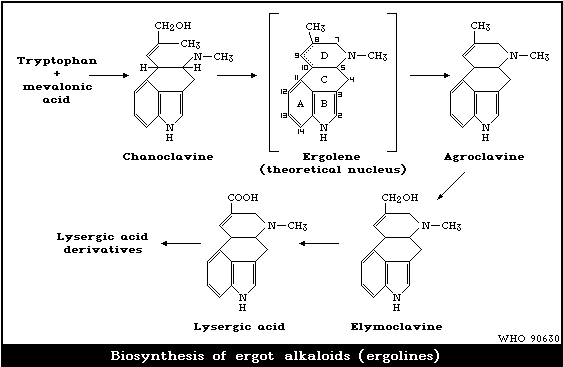 III.2.3 Occurrence in foodstuffs
Traditionally, contamination of grain with ergot has been
expressed as a percentage on a weight basis, without measurement of
total and individual amounts of ergolines. Thus, it is generally
recommended that feed containing more than 0.1% ergot should not be
given to animals (Young, 1981a,b). Quantitative analysis for total
and individual ergolines in 14 samples of rye and wheat flour
(Scott & Lawrence, 1980) indicated contamination with 6 Group I
ergolines (ergometrine, ergosine, ergotamine, ergocornine,
ergocryptine, ergocristine) at concentrations ranging from 0.3 to
62 µg/kg for individual ergolines. Ergocristine was the major
ergoline present in the flours, 62 µg/kg being the maximal
concentration found. In 17 samples of rye grain collected from
health shops, 6 contained ergot; ergoline concentration and
composition were not indicated (Akerstrand, 1980). In a survey of
ergolines in ergot-contaminated cereals, in North America, the
average total ergolines content was 0.24% (Young, 1981a,b; Young &
Chen, 1982). On average, the pooled ergoline composition in
sclerotia in rye, wheat, and triticale was as follows:
ergocristine (31%), ergocristinine (13%), ergotamine (17%),
ergotaminine (8%), ergocryptine (5%), ergocryptinine (3%),
ergometrine (5%), ergometrinine (2%), ergosine (4%), ergosinine
(2%), ergocornine (4%), and ergocorninine (2%). The individual
ergoline composition was uniform throughout a single sclerotium or
in different sclerotia from the same head, somewhat less uniform
between different fields throughout a region, and highly variable
from head-to-head in a given field. In contrast, the total
ergoline content was highly variable within-sclerotium, within-
head, head-to-head, and on a field-to-field basis. In a
comparative study of ergoline in ergots from rye and wheat (caused
by infection with C. purpurea) and in ergots from pearl millet
(caused by infection with C. fusiformis) in South-East Asia, it was
found that the total ergoline content in the sclerotia of pearl
millet was much lower (320 mg/kg) than that in the sclerotia of rye
(700 mg/kg) and wheat (920 mg/kg) (Bhat et al., 1976). The
ergolines in the sclerotia of pearl millet were reported to
comprise agroclavine, elymoclavine, chanoclavine, penniclavine, and
setoclavine.
In a survey of cereals and cereal products on the Swiss market,
using a high-performance liquid chromatography procedure, the
following average concentrations of total ergolines were found:
wheat flour, 4.2 µg/kg; wheat flour (coarse), 30.7 µg/kg; wheat
flour (more coarse), 103.4 µg/kg; rye flour, 139.7 µg/kg; and
"bioproducts", 10.2-22.7 µg/kg (Baumann et al., 1985). The daily
intake of total ergolines by human beings in Switzerland was
estimated to be 5.1 µg/person.
III.2.4 Fate of ergolines during food processing
Treatment of sclerotia from wheat with chlorine (1%) and heat
(150-200 °C) resulted in a 90% reduction in ergoline content within
4 h (Young et al., 1983). The reduction affected all the ergolines
(ergotamine, ergocornine, ergocryptine, ergosine, ergometrine) in
identical ways. Autoclaving sclerotia at 121 °C for 30 min
resulted in a 24.6% reduction in total ergoline content. In the
baking of bread and pancakes using grain that contained naturally
occurring ergot, a 59-100% reduction in the individual ergolines
(ergosine, ergocornine, ergometrine, ergotamine, alpha-
ergocryptine, ergocristine) was observed in whole wheat bread, a
50-86% reduction in all-rye flour bread, and a 25-74% reduction in
triticale pancakes (Scott & Lawrence, 1982).
When bread made of rye flour spiked with finely ground
sclerotia of C. purpurea, (continuing a total ergoline
concentration of 312.8 µg/kg), was baked, there was an overall
reduction of 50% in the ergoline concentration, as measured by high
performance liquid chromatography (Baumann et al., 1985).
III.3 METABOLISM
No published information is available on the metabolism in
animals or human beings of ergots containing ergolines and
combinations of individual ergolines.
III.4 EFFECTS ON ANIMALS
III.4.1 Field studies
An outbreak of bovine abortion associated with the ingestion of
ergot was reported by Appleyard (1986).
Eleven out of 36 suckler cows, all in late pregnancy, aborted
in 7-11 days following introduction to a rye grass pasture heavily
infested with ergot. At least 25% of the rye seed heads contained
sclerotia of C. purpurea, with up to 8 sclerotia present on any one
seed head. The sclerotia contained 1.57 mg total ergolines/g,
consisting of 67% ergotamine and 17% ergotaminine, and smaller
amounts of ergometrine. Ergocryptine and ergosine were also
present together with their corresponding -inine isomers.
Ten out of the 11 calves were delivered dead. None of the
aborting cows showed any premonitory signs of calving and, after
parturition, there was almost complete agalactia. The placenta was
retained in each case, but there was no other evidence of ill
health in the cows. Any other cause of abortion of bacterial,
viral, fungal, or toxic origin was ruled out, on the basis of
laboratory and field investigations.
III.4.2 Experimental animal studies
This topic has been reviewed by Ainsworth & Austwick (1959) and
Mantle (1977c). It appears that most reports of field cases and
experimental animal studies on ergotism do not contain any
information on the contents of individual ergolines in the ergot
associated with disease manifestations in animals.
III.4.2.1 Cattle
Lameness, sometimes leading to gangrene, is a common symptom
observed in cattle when the feed contains more than 10 g ergot/kg.
The symptoms are more pronounced when the animals are kept outside
under cold weather conditions (Mantle, 1977c). Four out of 6
animals administered ergotamine tartrate, orally, at 1 mg/kg body
weight per day, died within 10 days (Woods et al., 1966). The
animals became acutely ill within 1-2 days, the principal signs
being anorexia, hyperventilation, cold extremities, salivation,
and, occasionally, tongue necrosis. Post-mortem examination of the
most seriously affected animals revealed extensive intestinal
inflammation.
III.4.2.2 Sheep
Four lambs were administered aqueous suspensions of milled
ergot (from C. purpurea) through a stomach tube, over a 2-month
period (Loken, 1984). The doses ranged from 0.12 to 0.75 g
sclerotia/kg body weight. The sclerotia contained approximately 4
g ergolines/kg, composed of ergotamine (15%), ergosine (35%), and
ergocristine (5% each). One lamb, dosed with 0.12 g sclerotia/kg
body weight, was kept indoors at 15-17 °C and did not develop any
symptoms. The other 3 animals, given higher doses and kept
outdoors, became ill after 2-6 days, with signs that included
dullness, inappetence, high pulse rate, diarrhoea, edema of the
hind legs and tail, and lameness. Post-mortem findings included
inflammation and necrosis of the forestomach and intestinal mucosa.
III.4.2.3 Poultry
Leghorn chickens were fed diets containing ergotamine tartrate
at levels of up to 800 mg/kg in 7 to 10-day trials as well as in a
51-day trial (Young & Marquardt, 1982). In the short-term
trials, only the highest level (800 mg/kg) had an effect on
performance, in terms of a slight decrease in growth rate and a
slight increase in feed consumption. At the 250 mg/kg level, toe
necrosis was observed, as well as cardiomegaly. There were no
pathological effects on the brain, liver, or muscle tissues, even
at the highest level. In the long-term study (51 days), effects
were similar to those observed in the 7 to 10-day trials. No
residues of ergotamine were detected in tissues.
III.4.2.4 Swine
Ergot (from C. purpurea) containing 0.3% ergolines
(composition: ergotamine and ergosine) was used in a feeding study
on pigs (Mantle, 1977c). A diet containing ergot at 40 g/kg was
well tolerated, and the only effect at 100 g ergot/kg diet was
depression of the growth rate. Agalactia in the sow was observed
after feeding with rations containing ergot from C. purpurea as
well as from C. fusiformis.
III.4.2.5 Primates
Male rhesus monkeys in groups of 2-4 animals were dosed with
ergot from C. fusiformis, either as part of the diet, or as an
ergoline extract administered orally or intraperitoneally (Bhat &
Roy, 1976). The ergoline extract contained agroclavine (major
components: elymoclavine, chanoclavine, penniclavine, and
setoclavine). The period of treatment lasted from 2 days to one
month. There were no effects on the animals, with the exception of
those injected ip with 5.44-11.1 mg total ergoline/kg body weight,
who developed signs within 10 min including drowsiness,
hyperexcitation, redness of face, and loss of response to thermal
and tactile stimuli in the hind limbs and tail. The animals
recovered spontaneously in about 60 min. Animals administered a
total of 10 mg ergolines/kg body weight, orally, showed symptoms of
hyperexcitation, but these were far less severe than those after
intraperitoneal injection. It was concluded that the signs in
monkeys, in particular hyperexcitation, are different from those
observed in human beings after ingestion of ergot in pearl millet.
III.5 EFFECTS ON MAN
The history of ergotism in man, following ingestion of ergot
from C. purpurea, was reviewed by Barger (1931). Numerous
epidemics in Europe occurred between the 9th and the 18th century.
Two types of disease were noted: gangrenous and convulsive
ergotism. In the first type, the affected part (arm or leg)
shrank, became mummified and dry, and the gangrene gradually spread
upwards. In convulsive ergotism, the whole body was attacked by
general convulsion, which returned at intervals of a few days. The
latest outbreaks of ergotism in Europe occurred in 1926-28 in the
United Kingdom and the USSR. A suspected episode in France in 1951
turned out to be due to a different toxic substance (Gadiou, 1965).
III.5.1 Ergometrine-related outbreaks
In 1978, an epidemic was reported in Ethiopia (Demeke et al.,
1979; King, 1979; Pokrovskij & Tutelyan, 1982). The episode
occurred in the Wollo region, following two years of drought.
During this time, the locally grown barley, the staple food, had
become dominated by wild oats heavily contaminated with C. purpurea
sclerotia. The grain consisted of 70% wild oats, 12% barley, and
0.75% ergot; ergometrine was detected in the sclerotia by thin
layer chromatography. A total of 93 cases of ergotism was reported
during the spring of 1978. The male:female ratio was 2.5:1; more
than 80% of affected persons were between five and 34 years of age.
In addition to the 93 cases, 47 deaths were reported as having been
due to ergotism. Examination of 44 patients out of the 93
registered revealed ongoing dry gangrene of the whole or part of
one or more limbs (7.5%), feeble or absent peripheral pulses
(36.4%), swelling of limbs (11.2 %), desquamation of the skin
(12.8%), and loss of one or more limbs (21.5%). It was noted that
88% of patients had involvement of the lower extremities. The most
common general symptoms were weakness (78.5%), formication (15%),
burning sensation (14.3%), nausea (7.2%), vomiting (5.6%), and
diarrhoea (6.8%). In addition, 50-60 infants and young children
died from starvation due to failure of the mothers to lactate.
This may have been related to the effect of ergot on lactation
(Demeke et al., 1979). No autopsies were performed, and thus there
is no information on pathological changes in the viscera.
III.5.2 Clavine-related outbreaks
Intoxication following ingestion of ergot from C. fusiformis in
bajra or pearl millet has been reported from India. Symptoms
included nausea, vomiting, and giddiness. Several outbreaks have
been observed since 1958, when the first report was published; the
latest occurred in the autumn of 1975 in the state of Rajasthan
(Krishnamachari & Bhat, 1976). In 21 villages surveyed, 78 persons
belonging to 14 households developed symptoms, characterized by
nausea, repeated vomiting, and giddiness, followed by drowsiness
and prolonged sleepiness, extending sometimes to over 24-48 h.
There were no signs or symptoms suggesting vaso-occlusion. The
disease generally developed 1-2 h following a single meal.
Domestic camels, offered the contaminated grain as feed, also
developed sleepiness and signs suggesting abdominal discomfort.
The pearl millet from affected villages contained 15-174 g
ergot/kg, resulting in a contamination of the grain with 15-199 mg
total ergolines/kg. The individual ergolines were identified as
agroclavine, elymoclavine, chanoclavine, penniclavine, and
setoclavine. Pearl millet from villages with no cases of
intoxication contained 1-38 g ergot/kg with a total ergoline
content of 15-26 mg/kg. Since there were no deaths, no information
on pathological changes is available. The number of households
studied was too small for no-effect levels to be calculated, but
the authors suggested that the intake of 28 µg total ergolines/kg
body weight would be non-toxic.
III.6 EVALUATION OF THE HUMAN HEALTH RISKS
Human exposure to low levels of ergolines appears to be
widespread. Available data from the recent outbreaks in Ethiopia
and India indicate that the C. purpurea alkaloids (ergotamine
group) produced more severe effects, including gangrene of the legs
and death, than the alkaloids of C. fusiformis (clavine group),
which caused gastrointestinal symptoms without a fatal outcome.
It is not known whether such differences can be accounted for by
differences in the alkaloid content of the fungal species, in the
toxicological or toxicometric properties of the alkaloids, or in
the levels of intake by different types of populations.
Only low levels of ergolines remain in prepared foods as
cleaning and milling processes remove the sclerotia; baking or
other heat processing also destroys most alkaloids of the ergotamin
group.
REFERENCES
ABBAS, H.K., MIROCHA, C.J., PAWLOSKY, R.J., & PUCSH, D.J. (1985)
Effect of cleaning, milling, and baking on deoxynivalenol in wheat.
Appl. environ. Microbiol., 50(2): 482-486.
ABBAS, H.K., MIROCHA, C.J., & TUITE, J. (1986) Natural occurrence of
deoxynivalenol, 15-acetyl-deoxynivalenol and zearalenone in refusal
factor corn stored since 1972. Appl. environ. Microbiol., 51:841-843.
ABDEL-HAFEZ, S.I.I., EL-KADY, I.A., MAZEN, M.B., & EL-MAGHRABY,
O.M.O. (1987) Mycoflora and trichothecene toxins of paddy grains from
Egypt. Mycopathologia, 100: 103-112.
ABRAHAMSON, D., MILLS, J.T., & BOYCOTT, B.R. (1983) Mycotoxins and
mycoflora in animal feedstuffs in western Canada. Can. J. comp. Med.,
47: 23-26.
AGRELO, C.E. & SCHOENTAL, R. (1980) Synthesis of DNA in human
fibroblasts treated with T-2 toxin and HT-2 toxin (the trichothecene
metabolites of Fusarium species) and the effects of hydroxyurea.
Toxicol. Lett., 5(2): 155-160.
AINSWORTH, G.C. & AUSTWICK, P.K.C. (1959) Fungal diseases of
animals, Farnham Royal, Commonwealth Agricultural Bureaux, pp. 100-
111.
AKERSTRAND, K. (1980) [Ergots.] Var Föda, 32: 442-446 (in Swedish).
AKERSTRAND, K. & JOSEFSON, E. (1979) [Fungi and mycotoxins in beans
and peas.] Var Föda, 31: 405-414 (in Swedish).
APPELGREN, L.-E. & ARORA, R.G. (1983) Distribution of 14C-labelled
ochratoxin A in pregnant mice. Food chem. Toxicol., 21: 563-568.
APPLEYARD, W.T. (1986) Outbreak of bovine abortion attributed to
ergot poisoning. Vet. Res., 118: 48-49.
ARENS, H. & ZENK, M.H. (1980) [Radioimmunoassay for the
determination of the peptide alkaloids ergotamine and ergocristine in
Claviceps purpurea.] Planta med., 38: 214-226 (in German).
ARORA, R.G., FROLEN, H., & FELLNER-FELDEGG, H. (1983) Inhibition of
ochratoxin A teratogenesis by zearalenone and diethylstilboestrol.
Food chem. Toxicol., 21: 779-783.
ATKINSON, H.A.C. & MILLER, K. (1984) Inhibitory effect of
deoxynivalenol, 3-acetyldeoxynivalenol and zearalenone on induction
of rat and human erythrocyte proliferation. Toxicol. Lett, 23: 215-221.
BALZER, I., BOGDANIC, C., & MUZIC, S. (1977) Natural contamination
of corn (Zea mays) with mycotoxins in Yugoslavia. Ann. Nutr.
Aliment., 31(4-6): 425-430.
BAMBURG, J.R. (1976) Chemical and biochemical studies of the
trichothecene mycotoxins. In: Rodricks, J.V., ed. Mycotoxins and
other fungal related food problems, Washington, DC, American Chemical
Society, pp. 144-162 (Advances in Chemistry Series No. 149).
BAMBURG, J.R. & STRONG, F.M. (1969) Mycotoxins of the trichothecene
family produced by Fusarium tricinctum and Trichoderma lignorum.
Phytochemistry, 8(12): 2405-2410.
BAMBURG, J.R. & STRONG, F.M. (1971) 12,13-Epoxytrichothecenes. In:
Kadis, S., Ciegler, A., & Ajl, S.J. ed. Microbiological toxins. VII.
Algal and fungal toxins, New York, London, Academic Press, pp. 207-
292.
BAMBURG, J.R., MARASAS, W.F., RIGGS, N.V., SMALLEY, E.B., & STRONG,
F.M. (1968a) Toxic compounds from Fusaria and other Hyphomycetes.
Biotechnol. Bioeng., 10(4): 445-455.
BAMBURG, J.R., RIGGS, N.V., & STRONG, F.M. (1968b) The structures
of toxins from two strains of Fusarium tricinctum. Tetrahedron,
24(8): 3329-3336.
BARGER, G. (1931) Ergot and ergotism, London, Gurney & Jackson,
279 pp.
BATA, A., VANYI, A., & LASZTITY, R. (1983) Simultaneous detection of
some fusariotoxins by gas-liquid chromatography. J. Assoc. Off. Anal.
Chem., 66: 577-581.
BAUER, J. & GAREIS, M. (1987) [Ochratoxin A in the food chain.] J.
vet. Med., B34: 613-627 (in German).
BAUER, V.J., WERMTER, S., & GEDEK, B. (1980) Contamination of
feedstuffs with toxin-producing strains of Fusaria and their toxins.
Wiener Tieraerztl. Monatschrift., 67(10): 282-288.
BAUER, J., GAREIS, M., & GEDEK, B. (1984) [Detection and occurrence
of ochratoxin A in pigs for slaughter]. Berl. Muench. Tieraerztl.
Wschr. 97: 279-283 (in German).
BAUER, J., BOLLWAHN, W., GARCIS, M., GEDEK, B., & HEINRITZI, K.
(1985) Kinetic profiles of diacetoxyscirpenol and two of its
metabolites in blood serum of pigs. Appl. environ. Microbiol.,
49: 642-845.
BAUER, J., NIEMIEC, J. & SCHOLTYSSEK, S. (1988) [Ochratoxin A in feed
for laying hens. Second communication: Residues in serum, liver and
eggs.] Arch. Geflügelkd., 52: 71-75 (in German).
BAUMANN, U., HUNZIKER, H.R., & ZIMMERLI, B. (1985) [Ergot alkaloids
in Swiss grain products.] Mitt. Geb. Lebensm. Hyg., 76: 609-630 (in
German).
BEASLEY, V.R., SWANSON, S.P., CORLEY, R.A., BUCK, W.B., KORITZ, G.D.,
& BURMEISTER, H.R. (1986) Pharmacokinetics of the trichothecene
mycotoxin, T-2 toxin, in swine and cattle. Toxicon, 21: 13-23.
BEGLEY, P., FOULGER, B.E., JEFFERY, P.D., BLACK, R.M., & READ, R.W.
(1986) Detection of trace levels of trichothecenes in human blood
using capillary gas chromatography-electron-capture negative ion
chemical ionisation mass spectrometry. J. Chromatogr., 367: 87-101.
BELT, R.J., HAAS, C.D., JOSEPH, U., GOODWIN, W., MOORE, D., &
HOOGSTRATEN, B. (1979) Phase I study of anguidine administered
weekly. Cancer Treat. Rep., 63: 1993-1995.
BENDELE, S.A., CARLTON, W.W., KROGH, P., & LILLEHOJJ, E.B. (1985)
Ochratoxin A carcinogenesis in the (C57BL/6YxC3HF)1 mouse. J. Natl
Cancer Inst., 75: 733-739.
BERDE, B. & SCHILD, H.O. (1978) Ergot alkaloids and related
compounds, Berlin, Springer Verlag, pp. 1-1003.
BERGMANN, F., YAGEN, B., & SOFFER, D. (1985) Toxic and lethal effects
of T-2 toxin upon intracerebral administration to rats. Arch.
Toxicol., 58: 40-44.
BERNDT, W.O. & HAYES, A.W. (1979) In vivo and in vitro changes in
renal function caused by ochratoxin A in the rat. Toxicology, 12:
5-17.
BHAT, R.V. & ROY, D.N. (1976) Toxicity study of ergoty bajra (pearl
millet) in rhesus monkeys. Indian J. med. Res., 64: 1629-1633.
BHAT, R.V., ROY, D.N., & TULPULE, P.G. (1976) The nature of
alkaloids of ergoty pearl millet or bajra and its comparison with
alkaloids of ergoty rye and ergoty wheat. Toxicol. appl. Pharmacol.,
36: 11-17.
BHAT R.V., RAMAKRISHNA, Y., RAO, B.S., & NAHDI, S. (1987a)
Trichothecene mycotoxicosis, Hyderabad, India, Food and Drug
Toxicology Research Centre, National Institute of Nutrition.
BHAT, R.V., RAMAKRISHNA, Y., RAO, B.S,. & NAHDI, S. (1987b).
Trichothecene mycotoxicosis, Hyderabad, India, Food and Drug
Toxicology Research Centre, National Institute of Nutrition.
BHAT, R.V., BEEDU, S.R., RAMAKRISHNA, Y., & MUNSHI, K.L. (1989a)
Outbreak of trichothecene mycotoxicosis associated with consumption
of mould-damaged wheat products in Kashmir valley, India. Lancet, 7
January: 35-37.
BHAVANISHANKAR, T.R. & SHANTHA, T. (1987) Natural occurrence of
Fusarium toxins in peanuts, sorghum and maize from Mysore (India).
J. Sci. Food. Agric., 40: 327-332.
BHAVANISHANKAR, T.R., RAMESH, H.P., & SHANTHA, T. (1988) Dermal
toxicity of Fusarium toxins in combinations. Arch. Toxicol., 61:
241-244.
BIANCHI, M.L., PERELLINO, N.C., GIOIA, B., & MINGHETTI, A. (1982)
Production by Claviceps purpurea of two new peptide ergot alkaloids
belonging to a new series containing alpha-aminobutyric acid. J. nat.
Prod., 445: 191-196.
BILAI, V.I. (1977) [Fuzarii,] Kiev, USSR, Naukova Dumka, (in
Russian).
BLACK, R.M., CLARKE, R.J., & READ, R.W. (1986) Detection of trace
levels of trichothecene mycotoxins in human urine by gas
chromatography-mass spectrometry. J. Chromatogr., 367: 103-115.
BLACK, R.M., CLARKE, R.J., & READ, R.W. (1987) Detection of trace
levels of trichothecene mycotoxins in environmental residues and
foodstuffs using gas chromatography with mass spectrometric or
electron-capture detection. J. Chromatogr., 388: 365-378.
BLASS, W., KELLERT, M., STEINMEYER, S., TIEBACH, R., & WEBER, R.
(1984) Determination of deoxynivalenol and nivalenol in cereals at
the mg/kg concentrations range. Z. Lebensm. Unters. Forsch., 80:
104-108.
BODON, L. & ZOLDAG, L. (1974) Cytotoxicity studies on T-2
fusariotoxin. Acta vet. Acad. Sci. Hung., 24(3): 451-455.
BOHNER, F.E., FETZ, E., HARRI, E., SIGG, H.P., STOLL, CH., & TAMM,
CH. (1965) Verrucarine and roridin. VIII. Isolation of verrucarin
H, verrucarin J, roridin D, and roridin E from Myrothecium species.
Helv. Chim. Acta, 48(5): 1079-1089.
BOONCHUVIT, B., HAMILTON, P.B., & BURMEISTER, H.R. (1975)
Interaction of T-2 toxin with Salmonella infections of chickens.
Poult. Sci., 54(5): 1693-1696.
BOORMAN, G. (1988) Technical report on the toxicology and
carcinogenesis studies of ochratoxin A in F334/N rats. National
Toxicology Program, US Department of Health and Human Services,
Research Triangle Park, North Carolina (NIH Publication No. 88-28/3).
BOORMAN, G.A., HONG, H.L., DIETER, M.P., HAYES, H.T., POHLAND, A.E.,
STACK, M., & LUSTER, M.I. (1984) Myelotoxicity and macrophage
alteration in mice exposed to ochratoxin A. Toxicol. appl.
Pharmacol., 72: 304-312.
BOVE, F.J. (1970) The story of ergot, Basel, S. Karger, 297 pp.
BRADLAW, J.A., SWENTZEL, K.C., ALTERMAN, E., & HAUSWIRTH, J.W. (1985)
Evaluation of purified 4-deoxynivalenol (vomitoxin) for unscheduled
DNA synthesis in the primary rat hepatocyte-DNA repair assay. Food
chem. Toxicol., 23: 1063-1067.
BRIAN, P.W., DAWKINS, A.W., GROVE, J.F., HEMMING, H.G., LOWE, D., &
HORRIES, G.L.F. (1961) Phytotoxic compounds produced by Fusarium
equiseti. J. exp. Bot., 12: 1-21.
BROWN, M.H., SZCZECH, G.M., & PURMALIS, B.P. (1976) Teratogenic and
toxic effects of ochratoxin A in rats. Toxicol. appl. Pharmacol., 37:
331-338.
BRUMLEY, W.C., ANDRZEJEWSKI, D., TRUCKSESS, E.W., DREIFUSS, P.A.,
ROACH, J.A.G., EPPLEY, R.M., THOMAS, F.S., THORPE, C.W., & SPHON,
J.A. (1982) Negative ion chemical ionization mass spectrometry of
trichothecenes - Novel fragmentation under OH conditions. Biomed.
mass Spectrom., 9: 451-458.
BRUMLEY, W.C., TRUCKSESS, M.W., ADLER, S.H., COHEN, C.K., WHITE,
K.D., & SPHON, J.A. (1985) Negative ion chemical ionization mass
spectrometry of deoxynivalenol (DON): Application to identification
of DON in grains and snack foods after quantification/isolation by
thin-layer chromatography. J. agric. food Chem., 33:326-330.
BUENING, G.M., MANN, D.D., HOOK, B., & OSWEILER, G.D. (1982) The
effect of T-2 toxin on the bovine immune system: cellular factors.
Vet. Immunol. Immunopathol., 3: 411-417.
BUNGE, I., DIRHEIMER, G., & ROSCHENTHALER, R. (1978) in vivo and in
vitro inhibition of protein synthesis in Bacillus stearothermophilus
by ochratoxin A. Biochem. biophys. Res. Commun., 83: 398-405.
BUNNER, D.L. & MORRIS, E.R. (1988) Alteration of multiple cell
membrane functions in L-6 myoblasts by T-2 toxin: an important
mechanism of action. Toxicol. appl. Microbiol., 92: 113-121.
CANDLISH, A.A.G., STIMSON, W.H., & SMITH, J.E. (1986) A monoclonal
antibody to ochratoxin A. Lett. appl. Microbiol., 3: 9-11.
CARRASCO, L., BARBACID, M., & VAZQUEZ, D. (1973) The trichodermin
group of antibiotics inhibitors of peptide bond formation by
eukaryotic ribosomes. Biochim. Biophys. Acta, 312(2): 368-376.
CARSON, M.S. & SMITH, T.K. (1983) Role of bentonite in prevention of
T-2 toxicosis in rats. J. anim. Sci., 57: 1498-1506.
CARTER, C.J., CANNON, M., & SMITH, K.E. (1976) Inhibition of
protein synthesis in reticulocyte lysates by trichodermin. Biochem.
J., 154: 171-178.
CARTER, C.J., CANNON, M., & JIMENET, A. (1980) A trichodermin
resistant mutant of Saccharomyces cerevisiae with an abnormal
distribution of native ribosomal subunits. Eur. J. Biochem., 107
(1): 173-184.
CASALE, W.L., PESTKA, J.J., & HART, L.P. (1988) Enzyme-linked
immunosorbent assay employing monoclonal antibody specific for
deoxynivalenol (vomitoxin) and several analogues. J. agric. food
Chem., 36: 663-668.
CEOVIC, S., GRIMS, P., & MITAR, J. (1976) [The incidence of tumours
of the urinary organs in a region of endemic nephropathy and in a
control region.] Lijec. Vjesn., 98: 301-304 (in Croatian).
CHAKRABARTI, D.K. & HOSAL, S. (1986) Occurrence of free and
conjugated 12,13-epoxytrichothecenes and zearalenone in banana fruits
infected with Fusarium moniliforme. Appl. environ. Microbiol.,
51(1): 217-219.
CHANG, F.C. & CHU, F.S. (1977) The fate of ochratoxin A in rats.
Food Cosmet. Toxicol., 15: 199-204.
CHANG, K., KURTZ, J.J., & MIROCHA, C.J. (1979) Effects of the
mycotoxin zearalenone on swine reproduction. Am. J. vet. Res.,
40: 1260-1267.
CHATTERJEE, K., VISCONTI, A., & MIROCHA, C.J. (1986) Deepoxy T-2
tetraol: a metabolite of T-2 toxin found in cow urine. J. agric.
food Chem., 34: 695-697.
CHELKOWSKI, J., VISCONTI, A., & MANAKA, M. (1984) Production of
trichothecenes and zearaleneone by Fusarium species isolated from
wheat. Nahrung, 28: 493-496.
CHERNOZEMSKY, I.N., STOYANOV, I.S., PETKOV-ABOCHAROVA, T.K., NOCOLOV,
I.G., DRAGANOV, I.V., STOICHEV, I., TANCHEV, Y., NAIDENOV, D., &
KALCHEVA, N.D. (1977) Geographic correlation between the occurrence
of endemic nephropathy and urinary tract tumours in Vratza district,
Bulgaria. J. Cancer, 19: 1-11.
CHI, M.S., MIROCHA, C.J., KURTZ, H.J., WEAVER, G., BATES, F., &
SHIMODA, W. (1977a) Effects of T-2 toxin on reproductive
performance and health of laying hens. Poult. Sci., 56(2): 628-637.
CHI, M.S., MIROCHA, C.J., KURTZ, H.J., WEAVER, G., BATES, F.,
SHIMODA, W., & BURMEISTER, H.R. (1977b) Acute toxicity of T-2 toxin
in broiler chicks and laying hens. Poult Sci., 56(1): 103-116.
CHI, M.S., MIROCHA, C.J., KURTZ, H.J., WEAVER, G., BATES, F., &
SHIMODA, W. (1977c) Subacute toxicity of T-2 toxin in broiler
chicks. Poult. Sci., 56(1): 306-313.
CHI, M.S., ROBISON, T.S., MIROCHA, C.J., BEHRENS, J.C., & SHIMODA, W.
(1978a) Transmission of radioactivity into eggs from laying hens
(Gallus domesticus) administered tritium labeled T-2 toxin. Poult.
Sci., 57(5): 1234-1238.
CHI, M.S., ROBISON, T.S., MIROCHA, C.J., SWANSON, S.P., & SHIMODA, W.
(1978b) Excretion and tissue distribution of radioactivity from
tritium labeled T-2 toxin in chicks. Toxicol. appl. Pharmacol.,
45(2): 391-402.
CHI, M.S., EL-HALAWANI, M.E., WAIBEL, P.E., & MIROCHA, C.J. (1981)
Effects of T-2 toxin on brain catecholamines and selected blood
components in growing chickens. Poult. Sci., 60(1): 137-141.
CHIBA, J., NAKANO, N., MOROOKA, N., NAKAZAWA, S., & WATANABE, Y.
(1972) Inhibitory effects of fusarenon-X, a sesquiterpene mycotoxin
on lipid synthesis and phosphate uptake in Tetrahymena pyriformis.
Jpn. J. med. Sci. Biol., 25(5): 291-296.
CHO, B.R. (1964) Toxicity of water extracts of scabby barley to
suckling mice. Am. J. vet. Res., 25: 1267-1270.
CHU, F.S. (1971) Interaction of ochratoxin A with bovine serum
albumin. Arch. Biochem. Biophys., 147: 359-366.
CHU, F.S. (1974a) Studies on ochratoxins. CRC crit. Rev. Toxicol.,
2(4): 499-524.
CHU, F.S. (1974b) A comparative study of the interaction of
ochratoxins with bovine serum albumin. Biochem. Pharmacol., 23(6):
1105-1113.
CHU, F.S. & BUTZ, M.E. (1970) Spectrophotofluorodensitometric
measurement of ochratoxin A in cereal products. J. Assoc. Off. Anal.
Chem., 53(6): 1253-1257.
CHU, F.S., GROSSMAN, S., WEI, R.D., & MIROCHA, C.J. (1979)
Production of antibody against T-2 toxin. Appl. environ. Microbiol.,
37(1): 104-108.
CHUNG, C.W., TRUCKSESS, M.W., GILES, A.L., Jr, & FRIEDMAN, L. (1974)
Rabbit skin test for estimation of T-2 toxin and other skin
irritating toxins in contaminated corn. J. Assoc. Off. Anal. Chem.,
57(5): 1121-1127.
CHUNG, H.S. (1975) Cereal scab causing mycotoxicoses in Korea and
present status of mycotoxin researches. Korean J. Mycol., 3(1):
31-36.
CIRILLI, G. (1983) Trichothecene problems in Italy. In: Ueno, Y.,
ed. Developments in food science. IV. Trichothecenes, New York,
Elsevier, pp. 254-258.
COFFIN, J.L. & COMBS, G.F., Jr (1981) Impaired vitamin E status of
chicks fed T-2 toxin. Poult. Sci., 60(2): 385-392.
COHEN, H. & LAPOINTE, M. (1982) Capillary gas-liquid
chromatographic determination of vomitoxin in cereal grains. J.
Assoc. Off. Anal. Chem., 65(6): 1429-1434.
COHEN, H. & LAPOINTE, M. (1984) Capillary gas chromatographic
determination of T-2 toxin, HT-2 toxin and diacetoxyscirpenol in
cereal grains. J. Assoc. Off. Anal. Chem., 67: 1105-1107.
COLE, R.J. & COX, R.H. (1981) Handbook of toxic fungal metabolites,
New York, London, San Francisco, Academic Press, pp. 152-263.
COLINSKI, P. (1987) [Ochratoxin A in humans as a result of food and
feed contamination,] Poznan, Rocznalion Akademii Rolniczej (Doctoral
thesis) (in Polish).
COLLINS, G.J. & ROSEN, J.D. (1979) Gas-liquid chromatographic/mass
spectrometric screening method for T-2 toxin in milk. J. Assoc. Off.
Anal. Chem., 62(6): 1274-1280.
COLLINS, G.J. & ROSEN, J.D. (1981) Distribution of T-2 toxin in
wet-milled corn products. J. food Sci., 46(3): 877-879.
CONNOLE, M.D., BLANEY, B.J., & MCEWAN, T. (198l) Mycotoxins in
animal feeds and toxic fungi in Queensland 1971-1980. Aust. vet. J.,
57: 314-318.
CONNOR, M.W., CAMARGO, J.D., PANYANT, P., RIENGROPITOK, S., ROGERS,
A.E., & NEWBUNE, P.M. (1986) Toxicity of anguidine in mice. Fundam.
appl. Toxicol., 7: 153-164.
COORAY, R. (1984) Effects of some mycotoxins on mitogen-induced
blastogenesis and SCE frequency in human lymphocytes. Food Chem.
Toxicol., 22: 529-534.
COPPOCK, R.W., SWANSON, S.P., GELBERG, H.B., KORITZ, G.D., HOFFMAN,
W.E., BUCK, W.B., & VESONDER, R.F. (1985) Preliminary study of the
pharmacokinetics and toxicopathy of deoxynivalenol (vomitin) in
swine. Am. J. vet. Res., 46: 169-174.
CORLEY, R.A., SWANSON, S.P., & BUCK, W.B. (1985) Glucuronide
conjugates of T-2 toxin and metabolites in swine bile and urine. J.
agric. food Chem., 33: 1085-1089.
CORLEY, R.A., SWANSON, S.P., GULLO, G.J., JOHNSON, L., BEASLEY, V.R.,
& BUCK, W.B. (1986) Disposition of T-2 toxin, a trichothecene
mycotoxin, in intravascularly dosed swine. J. agric. food Chem.,
34: 868-875.
CORRIER, D.E. & ZIPRIN, R.L. (1986a) Immunotoxic effects of T-2 toxin
on cell-mediated immunity to listeriosis in mice: comparison with
cyclophosphamide. Am. J. vet. Res., 47: 1956-1960.
CORRIER, D.E. & ZIPRIN, R.L. (1986b) Enhanced resistance to
listeriosis induced in mice by preinoculation treatment with T-2
mycotoxin. Am. J. vet. Res., 47: 856-859.
COSGRIFF, T.M., BUNNER, D.P., WANNEMACHER, R.W., Jr., HODGSON, L.A.,
& DINTERMAN R.E. (1984) The hemostatic derangement produced by T-2
toxin in guinea pigs. Toxicol. appl. Pharmacol., 76: 454-463.
COSGRIFF, T.M., BUNNER, D.P., WANNEMACHER, R.W., Jr., HODGSON, L.A.,
& DINTERMAN, R.E. (1986) The hemostatic derangement produced by T-2
toxin in Cynomolgus monkeys. Toxicol. appl. Pharmacol., 82: 532-539.
COTE, L.M., REYNOLDS, J.D., VESONDER, R.F., BUCK, W.B., SWANSON,
S.P., COFFEY, R.T., & BROWN, D.C. (1984) Survey of vomitoxin-
contaminated feed grains in midwestern United States, and associated
health problems in swine. J. Am. Vet. Med. Assoc., 184(2): 189-192.
COTE, L.M., DAHLEM, A.M., YOSHIZAWA, T., SWANSON, S.P., & BUCK, W.B.
(1986) Excretion of deoxynivalenol and its metabolite in milk, urine
and feces of lactating dairy cows. Agric. biol. Chem., 50: 227-229.
CREPPY, E.E., LUGNIER, A.A.J., FASIOLO, F., HELLER, K.,
ROSCHENTHALER, R., & DIRHEIMER, G. (1979a) In vitro inhibition of
yeast phenylalanyl-tRNA synthetase by ochratoxin A. Chem.-biol.
Interact., 24: 257-261.
CREPPY, E.E., LUGNIER, A.A.J., BECK, G., ROSCHENTHALER, R., &
DIRHEIMER, G. (1979b) Action of ochratoxin A on cultured hepatoma
cells - reversion of inhibition by phenylalanine. FEBS Lett., 104:
287-290.
CREPPY, E.E., SCHLEGEL, M., ROSCHENTHALER, R., & DURHEINSER, G.
(1980) Phenylalamine prevents acute poisoning by ochratoxin A in
mice. Toxicol. Lett., 6: 77-80.
CREPPY, E.E., STORMER, F.O., ROSCHENTHALER, R., & DIRHEIMER, G.
(1983) Effects of two metabolites of ochratoxin A, (4R)-4-
hydroxyochratoxin A and ochratoxin alpha, on immune response in mice.
Infect. Immun., 39: 1015-1018.
CROFT, W.A., JARVIS, B.B., & YATAWARA, C.S. (1986) Airborne outbreak
of trichothecene toxicosis. Atmos. Environ., 20: 549-552.
CUNDLIFFE, E., CANNON, M., & DAVIS, J. (1974) Mechanism of
inhibition of eukaryotic protein synthesis by trichothecene fungal
toxins. Proc. Natl Acad. Sci. (USA), 71(1): 30-34.
D'AGOSTINO, P.A., PROVOST, L.R., & DROVER, D.R. (1986) Analysis of
trichothecene mycotoxins in human blood by capillary column gas
chromatography-ammonia chemical ionization mass spectrometry. J.
Chromatogr., 367: 77-86.
DANKO, G. & SZERAFIN, J. (1976) Experimental stachybotryotoxicosis
in horses. Magy. Allatorv. Lapja, 9: 597-600.
DE LOACH, J.R., ANDREWS, K., & NAGI, A. (1987) Interaction of T-2
toxin with bovine erythrocyte: effects on cell lysis, permeability
and entrapment. Toxicol. appl. Pharmacol., 88: 123-131.
DEMEKE, T., KIDANE, Y., & WUHIB, E. (1979) Ergotism: a report on an
epidemic. Ethiop. med. J., 17: 107-113.
DENICOLA, D.B., REBER, A.H., CARLTON, W.W., & YAGEN, B. (1978) T-2
toxin mycotoxicosis in the guinea-pig. Food Cosmet. Toxicol., 16
(6): 601-609.
DESIMONE, P.A., GRECO, F.A., & LESSNER, H.F. (1979) Phase I
evaluation of a weekly schedule of anguidine. Cancer Treat. Rep.,
63(11/12): 2015-2017.
DIVE, D., MOREAU, S., & CACAN, M. (1978) Use of a ciliate
protozoan for fungal toxins studies. Bull. environ. Contam. Toxicol.,
19(4): 489-495.
DOCHEV, D. (1973) [Endemic (Balkan) nephritis in Bulgaria.]
Münchener med. Wochenschr., 115(13): 537-541 (in German).
DOERR, J.A., HAMILTON, P.B., & BURMEISTER, H.R. (1981) T-2
toxicosis and blood coagulation in young chickens. Toxicol. appl.
Pharmacol., 60: 157-162.
DOSIK, G.M., BARLOGIE, B., JOHNSTON, D.A., MARPHY, W.K., & DREWINKO,
B. (1978) Lethal and cytokinetic effects of anguidine on a human
colon cancer cell line. Cancer Res., 38(10): 3304-3309.
DOSTER, R.C., SINHUBER, R.O., & WALES, J.H. (1972) Acute
intraperitoneal toxicity of ochratoxin A and B in rainbow trout
(Salmo gairdneri). Food. Cosmet. Toxicol., 10: 85-92.
DROVE, J.F. (1988) Non macrocyclic trichothecenes. Natural Products
Reports: 181-209.
DWIVEDI, P. & BURNS, R.B. (1984) Effect of ochratoxin A on
immunoglobulins in broiler chicks. Res. vet. Sci., 36: 117-121.
EDLUND, P.O. (1981) Determination of ergot alkaloids in plasma by
high-performance liquid chromatography and fluorescence detection. J.
Chromatogr., 226: 107-115.
EHRLICH, K.C., LEE, L.S., & CIEGLER, A. (1983) A simple, sensitive
method for detection of vomitoxin (deoxynivalenol) using reversed
phase, high performance liquid chromatography. J. liq. Chromatogr.,
6: 833-843.
EL-BANNA, A.A., LAU, P.-Y., & SCOTT, P.M. (1983) Fate of mycotoxins
during processing of foodstuffs II- Deoxynivalenol (vomitoxin) during
making of Egyptian bread. J. food Prot., 46: 484-486.
EL-BANNA, A.A., SCOTT, P.M., LAU, P.-Y., SAKUMA, T., PLOT, H., &
CAMPBELL, V. (1984) Formation of trichothecenes by F. solani var.
coeruleum and F. sambucinum in potatoes. Appl. environ. Microbiol.,
47: 1169-1171.
ELLING, F. (1977) Demonstration of ochratoxin A in kidneys of pigs
and rats by immunofluorescence microscopy. Acta pathol. microbiol.
Scand., A85: 151-156.
ELLING, F. & MOLLER, T. (1973) Mycotoxic nephropathy in pigs. Bull.
World Health Organ., 49: 411-418.
ELLING, F., HALD, B., JACOBSEN, C., & KROGH, P. (1975) Spontaneous
cases of toxic nephropathy in poultry associated with ochratoxin A.
Acta pathol. microbiol. Scand., A83: 739-741.
ELLING, F., NIELSEN, J.P., LILLEHOJ, E.B., THOMASSEN, M.S., &
STORMER, F.C. (1985) Ochratoxin A-induced porcine nephrotoxicity:
enzyme and ultrastructural changes after short-term exposure.
Toxicon, 23: 247-254.
EPPLEY, R.M. (1968) Screening method for zearalenone, aflatoxin, and
ochratoxin. J. Assoc. Off. Anal. Chem., 51(1): 74-78.
EPPLEY, R.M. (1974) Sensitivity of brine shrimp Artemia salina to
trichothecenes. J. Assoc. Off. Anal. Chem., 57(3): 618-620.
EPPLEY, R.M. & BAILEY, W.J. (1973) 12,13-epoxy-delta-trichothecenes
as the probable mycotoxins responsible for stachybotryotoxicosis.
Science, 181: 758-760.
EPPLEY, R.M., TRUCKSESS, M.W., NESHEIM, S., THORPE, C.W., WOOD, G.E.,
& POHLAND, A.E. (1984) Deoxynivalenol in winter wheat: Thin layer
chromatographic method and survey. J. Assoc. Off. Anal. Chem., 67
(1): 43-45.
EPPLEY, R.M., TRUCKSESS, M.W., NESHEIM, S., THORPE, C.W., & POHLAND,
A.E. (1986) Thin layer chromatographic method for determination of
deoxynivalenol in wheat: collaborative study. J. Assoc. Off. Anal.
Chem., 69: 37-40.
FAIRHURST, S., MAXWELL, S.A., SCAWIN, J.W., & SWANSTON, D.W. (1987)
Skin effects of trichothecenes and their amelioration by
decontamination. Toxicology, 46: 307-319.
FAN, T.S.L., XU, Y.-C., & CHU, F.S. (1987) Simultaneous analysis of
T-2 toxin and HT-2 toxin by indirect enzyme-linked immunosorbent
assay. J. Assoc. Off. Anal. Chem., 70: 657-661.
FARB, R.M., MEGO, J.L., & HAYES, A.W. (1976) Effect of mycotoxins
on uptake and degradation of iodine-125 albumin in mouse liver and
kidney lysosomes. J. Toxicol. environ. Health, 1(6): 985-990.
FEUERSTEIN, G., GOLDSTEIN, D.S., RAMWELL, P.W., ZERBE, R.L., LUX,
W.E. Jr, FODEN, A.I., & BAYORH, M.A. (1985) Cardiorespiratory
sympathetic and biochemical response to T-2 toxin in the guinea pig
and rat. J. Pharmacol. exp. Ther., 232: 786-794.
FEUERSTEIN, G., LEADER, P., SIREN, A.L., & BRAGNET, P. (1987)
Protective effect of a PAF-acether antagonist, BN52021 in
trichothecene toxicosis. Toxicol. Lett., 38: 271-274.
FIORAMONTI, J., FARGEAS, M.J., & BUENO, L. (1987) Action of T-2
toxin on gastrointestinal transit in mice: protective effect of an
argillaceous compound. Toxicol. Lett., 36: 227-232.
FITZPATRICK, D.W., BOYD, K.E., & WATTS, B.M. (1988) Comparison of the
trichothecenes deoxynivalenol and T-2 toxin for their effect on brain
biogenic monoamines in the rat. Toxicol. Lett., 40: 241-245.
FORGACS, J. (1965) In: Wogan, G., ed. Mycotoxins in foodstuffs,
Cambridge, Mass., MIT Press, p. 87.
FORSELL, J.H. & PESTKA, J.J. (1985) Relation of 8-ketotrichothecene
and zearalenone analog structure to inhibitions of mitogen-induced
human lymphocyte blastogenesis. Appl. environ. Microbiol., 50:
1304-1307.
FORSELL, J.H., KATEBY, J.R., YOSHIZAWA, T., & PESTKA, J.J. (1985)
Inhibition of mitogen-induced blastogenesis in human lymphocytes by
T-2 toxin and its metabolites. Appl. environ. Microbiol., 49:
1524-1526.
FORSYTH, D.M., YOSHIZAWA, T., MOROOKA, N., & TUITE, J. (1977)
Emetic and refusal activity of deoxynivalenol to swine. Appl.
environ. Microbiol., 34(5): 547-552.
FOSTER, P.M.D., SLATER, T.F., & PATTERSON, D.S.P. (1975) A possible
enzymic assay for trichothecene mycotoxins in animal feedstuffs.
Biochem. Soc. Trans., 3(6): 875-878.
FREEMAN, G.G. (1955) Further biological properties of trichotecin,
an antifungal substance from Trichothecium roseum Link, and its
derivatives. J. gen. Microbiol., 12: 213-221.
FREEMAN , G.G. & MORRISON, R.I. (1949) The isolation and chemical
properties of trichothecin, an antifungal substance from
Trichothecium roseum link. Biochem. J., 44: 1-5.
FRIED, H.M. & WARNER, J.R. (1981) Cloning of yeast gene for
trichodermin resistance and ribosomal protein L3. Proc. Natl Acad.
Sci. (USA), 78(1): 238-242.
FRIEND, S.C.E., TRENHOLM, H.L., ELLIOT, J.I., THOMPSON, B.K., &
HARTIN, K.E. (1982) Effect of feeding vomitoxin-contaminated wheat to
pigs. Can. J. anim. Sci., 62: 1211-1222.
FRIEND, S.C.E., BABUIK, L.A., & SCHIEFER, H.B. (1983a) The effects of
dietary T-2 toxin on the immunological function and Herpes simplex
reactivation in Swiss mice. Toxicol. appl. Pharmacol., 69:
234-244.
FRIEND, S.C.E., HANCOCK, D.S., & SCHIEFER, H.B. (1983b) Experimental
T-2 toxicosis in sheep. Can. J. comp. Med., 47: 291-297.
FRIEND, S.C.E., SCHIEFER, H.B,. & BABUIK, L.A. (1983c) The effects of
dietary T-2 toxin on acute Herpes simplex virus type 1 infection in
mice. Vet. Pathol., 20: 737-760.
FRIESEN, M.D. & GARREN, L. (1983) International mycotoxin check
sample survey programme. Part III. Report on performance of
participating laboratories for determining ochratoxin A in animal
feed. J. Assoc. Off. Anal. Chem., 66: 256-259.
FRIIS, Ch., BRINN, R., & HALD, B. (1988) Uptake of ochratoxin A by
slices of pig kidney cortex. Toxicology., 52: 209-217.
FRITZ, W., BUTHIG, CL., DONATH, R., & ENGST, R. (1979) [Studies on
the nutritionally significant formation of ochratoxin A in grain and
other foodstuffs.] Lebensm. Ernähr., 25: 929-932 (in German).
FROMENTIN, H., SALAZAR-MEJICANOS, S,. & MARIAT, F. (1981)
Experimental cryptococcosis in mice treated with diacetoxyscirpenol,
a mycotoxin of Fusarium. Sabouraudia, 19: 311-318.
GADIOU, J. (1965) Contribution à l'étude toxicologique du
dicyandiamide de methyl mercure, Université de Bordeaux, Faculté de
pharmacie. (Thèse de doctorat).
GALEY, F.D., LAMBERT, R.J., BUSSE, M., & BUCK, W.B. (1987)
Therapeutic efficacy of superactive charcoal in rats exposed to oral
lethal doses of T-2 toxins. Toxicon, 25: 493-499.
GALTIER, P. (1974a) Devenir de l'ochratoxine A dans l'organisme
animal. I. Transport sanguin de la toxine chez le rat. Ann. Rech.
vét., 5: 311-318.
GALTIER, P. (1974b) Devenir de l'ochratoxine A dans l'organisme
animal. II. Distribution tissulaire et élimination chez le rat.
Ann. Rech. vét., 5: 319-328.
GALTIER, P. (1979) Etude toxicologique et pharmacocinétique d'une
mycotoxine, l'ochratoxine A, Université de Toulouse, Thèse de
doctorat d'Etat en Sciences pharmaceutiques.
GALTIER, P. & ALVINERIE, M. (1976) In vitro transformation of
ochratoxin A by animal microbial floras. Ann. Rech. vét., 7:
91-98.
GALTIER, P., MORE, J., & BODIN, G. (1974) Toxines d' Aspergillus
ochraceus Wilhelm III. Toxicité aiguë de l'ochratoxine A chez le rat
et la souris adultes. Ann. Rech. vét., 5: 233-247.
GALTIER, P., BARADAT, C., & ALVINERIE, M. (1977a) De l'élimination
d'ochratoxine A par le lait chez la lapine. Ann. Nutr. Aliment.,
31: 911-918.
GALTIER, P., JEMMALI, M., & LARRIEU, G. (1977b) Enquete sur la
présence éventuelle d'aflatoxine et d'ochratoxine A dans des maïs
recolté en France en 1973 et 1974. Ann. Nutr. Aliment., 31: 38l-389.
GALTIER, P., BONEU, B., CHARPENTEAU, J.L., BODIN, G., ALVINERIE, M.,
& MORE, J. (1979) Physiopathology of haemorrhagic syndrome related
to ochratoxin A intoxication in rats. Food Cosmet. Toxicol., 17:
49-53.
GALTIER, P., CAMGUILHEM, R., & BODIN, G. (1980) Evidence for in
vitro and in vivo interaction between ochratoxin A and three acidic
drugs. Food Cosmet. Toxicol., 18: 493-496.
GAREIS, M., HASHEM, A., BAUER, J., & GEDEK, B. (1986) Identification
of glucuronide metabolites of T-2 toxin and diacetoxyscirpenol in the
bile of isolated perfused rat lever. Toxicol. appl. Pharmacol., 84:
168-172.
GAREIS, M., MARTLBAUER, E., BAUER, J., & GEDEK, B. (1988)
[Determination of ochratoxin A in breast milk.] Z. Lebensm. Unters.
Forsch., 186: 114-117 (in German).
GENDLOFF, E.H., PESTKA, J.J., SWANSON, S.P., & HART, L.P. (1984)
Detection of T-2 toxin in Fusarium sporotrichioides-infected corn by
enzyme-linked immunosorbent assay. Appl. environ. Microbiol., 47:
1161-1163.
GENTRY, P.A. (1982) The effect of administration of a single dose of
T-2 toxin on blood coagulation in the rabbit. Can. J. comp. Med.,
46: 414-419.
GENTRY, P.A. & COOPER, M.L. (1981) Effect of Fusarium T-2 toxin on
hematological and biochemical parameters in the rabbit. Can. J. comp.
Med., 45: 400-405.
GHOSAL, S., CHAKRABARTI, D.K., & BASU CHAUDHARY, K.C. (1977) The
occurrence of 12,13-epoxytrichothecenes in seeds of safflower
infected with Fusarium oxysporum f sp. carthami. Experientia (Basel),
33(5): 574-575.
GILBERT, J., SHEPHERD, M.J., & HOWELL, M.V. (1983a) Studies on the
fate of trichothecene mycotoxins during food processing. European
Conference on. Food Chemistry. II, Rome, Societa Italiana di Scienza
dell alimetazione. pp. 253-262.
GILBERT, J., SHEPHERD, M.J., & STARTIN, J.R. (1983b) A survey of the
occurrence of the trichothecene mycotoxin deoxynivalenol (vomitoxin)
in UK-grown barley and in imported maize by combined gas
chromatography-mass spectrometry. J. Sci. Food Agric., 34: 86-92.
GILBERT, J., SHEPHERD, M.J., & STARTIN, J.R. (1984) The analysis and
occurrence of Fusarium mycotoxins in the United Kingdom and their
fate during food processing. In: Kurata, H. & Ueno, Y., ed. Toxigenic
fungi - their toxins and health hazard, Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp. 209-216.
GILBERT, J., STARTIN, J.R,. & CREWS, C. (1985) Optimization of
conditions for trimethylsilylation of trichothecene mycotoxins. J.
Chromatogr., 319: 376-381.
GILGAN, M.W., SMALLEY, E.B., & STRONG, F.M. (1966) Isolation and
partial characterization of a toxin from Fusarium tricinctum on moldy
corn. Arch. Biochem. Biophys., 114: 1-3.
GOLINSKI, P., HULT, K., BRABARKIEWICZ-SZCZESNA, J., CHELKOWSKI, J.,
KNEBLEWSKI, P., & SZEBIOTKO, K. (1984) Mycotoxic porcine nephropathy
and spontaneous occurrence of ochratoxin A residues in kidneys and
blood of Polish swine. Appl. environ. Microbiol., 47: 1210-1212.
GOLINSKI, P., BRABARKIEWICZ-SZCZESNA, J., CHELKOWSKI, J., &
SZEBIOTKO, K. (1985) Spontaneous occurrence of ochratoxin A
residues in porcine kidney and serum samples in Poland. Appl.
environ. Microbiol., 49: 1014-1015.
GRANT, P.G., SCHINDLER, D., & DAVIES, J.E. (1976) Mapping of
trichodermin resistance in Saccharomyces cerevisiae: a genetic locus
for a component of the 60s ribosomal subunit. Genetics, 83(4):
667-673.
GREENHALGH, R., GILBERT, J., KING, R.R., BLACKWELL, B.A., STARTIN,
J.R., & SHEPHERD, M.J. (1984) Synthesis, characterization, and
occurrence in bread and cereal products of an isomer of 4-
deoxynivalenol (vomitoxin). J. agric. food Chem., 32: 1416-1420.
GREENWAY, J.A. & PULS, R. (1976) Fusariotoxicosis from barley in
British Colombia. I. Natural occurrence and diagnosis. Can. J. comp.
Med., 40: 12-15.
GROVE, J.F. & MORTIMER, P.H. (1969) The cytotoxicity of some
transformation products of diacetoxyscirpenol. Biochem. Pharmacol.,
18(6): 1473-1478.
GUPTA, R.S. & SIMINOVITCH, L. (1978) Genetic and biochemical
characterization of mutants of CHO cells resistant to the protein
synthesis inhibitor trichodermin. Somatic cell Genet., 4(3):
355-374.
GUTZWILLER, J. & TAMM, C. (1965) Verrucarin and roridin. V. The
structure of Verrucarin A. Helv. Chim. Acta, 48: 157-176.
HAGGBLOM, P. (1982) Production of ochratoxin A in barley by
Aspergillus ochraceus and Penicillium viridicatum: effect of fungal
growth, time, temperature, and inoculum size. Appl. environ.
Microbiol., 43: 1205-1207.
HAGLER, W.M., Jr, TYCZKOWDKA, K., & HAMILTON, P.B. (1984)
Simultaneous occurrence of deoxynivalenol, zearalenone, and aflatoxin
in 1982 scabby wheat from the midwestern United States. Appl.
environ. Microbiol., 47(1): 151-154.
HALD, B. (in press) Human exposure to ochratoxin A. In: Natori, S.,
Hashimoto, K., & Ueno, Y., ed. Mycotoxins and phycotoxins 1989,
Amsterdam, Oxford, New York, Elsevier Science Publishers.
HALD, B. & KROGH, P. (1972) Ochratoxin residues in bacon pigs.
Proceedings of the IUPAC Symposium: Control of Mycotoxins, Kungälv,
Sweden, page 18.
HALD, B. & KROGH, P. (1975) Detection of ochratoxin A. in barley,
using silica gel mini columns. J. Assoc. Off. Anal. Chem., 58:
156-158.
HALD, B. & KROGH, P. (1983) Toxicoses and natural occurrence in
Denmark. In: Ueno, Y., ed. Trichothecenes - chemical, biological
and toxicological aspects, Amsterdam, Oxford, New York, Elsevier,
Science Publishers, pp 251-253.
HALIBURTON, J.C., VESONDER, R.F., LOCK, T.F., & BUCK, W.B. (1979)
Equine leucoencephalomalacia (ELEM): a study of Fusarium moniliforme
as an etiologic agent. Vet. hum. Toxicol., 21(5): 348-351.
HARRACH, B., MIROCHA, C.J., PATHRE, S.V., & PALYUSIK, M. (1981)
Macrocyclic trichothecene toxins produced by a strain of Stachybotrys
atra from Hungary. Appl. environ. Microbiol., 41(6): 1428-1432.
HARRACH, B., BATA, A., BAJMOCY, E., & BENKO, M. (1983) Isolation of
satratoxins from the bedding straw of a sheep flock with fatal
stachybotryotoxicosis. Appl. environ. Microbiol., 45: 1419-1422.
HARRI, E., VON, LOEFFLER, W., SIGG, H.P., STAHELIN, H., STOLL, Ch.,
TAMM, Ch., & WIESINGER, D. (1962) [Verrucarins and roridins, a group
of antibiotics with high cytostatic effect from Myrothecium species.]
Helv. Chim. Acta, 45: 839-853 (in German).
HART, L.P. & BASELTON, W.E. (1983) Distribution of vomitoxin in dry
milled fractions of wheat infected with Gibberella. J. agric. food
Chem., 31: 657-659.
HARTMANN, G.R., RICHTER, H., WEINER, E.M., & ZIMMERMANN, W. (1978)
On the mechanism of action of the cytostatic drug anguidine and of
the immunosuppressive agent ovalicin, two sesquiterpenes from fungi.
Planta Med., 34(3): 231-252.
HARWIG, J. (1974) Ochratoxin A and related metabolites. In:
Purchase, I.F.H., ed. Mycotoxins, Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp. 345-368.
HAUBECK, H.D., LORKOWSKI, G., KULSCH, E., & ROSCHENTHALER, R. (1981)
Immunosuppression by ochratoxin A and its prevention by
phenylalanine. Appl. environ. Microbiol., 41: 1040-1042.
HAYES, A.W., HOOD, R.D., & LEE, H.L. (1974) Teratogenic effects of
ochratoxin A in mice. Teratology, 9(1): 93-97.
HAYES, M.A. & SCHIEFER, H.B. (1979) Quantitative and morphological
aspects of cutaneous irritation by trichothecene mycotoxins. Food
Cosmet. Toxicol., 17(6): 611-621.
HAYES, M.A., BELLAMY, J.E.C., & SCHIEFER, H.B. (1980) Subacute
toxicity of dietary T-2 toxin in mice: morphological and
hematological effects. Can. J. comp. Med., 44: 203-218.
HELGESON, J.P., HABERLACH, G.T., & VANDERHOEF, L.N. (1973) T-2
toxin decreases logarithmic growth rates of tobacco callus tissues.
Plant Physiol., 52(6): 660-662.
HELLER, K. & ROSCHENTHALER, R. (1978) Inhibition of protein
synthesis in Streptococcus faecalis by ochratoxin A. Can. J.
Microbiol., 24: 466-472.
HEPTINSTALL, R.H. (1974) Pathology of the kidney, Boston, Little
Brown and Co., Vol. 11, pp. 828-836.
HEWETSON, J.F., PACE, J.D., & BEHELER, J.E. (1987) Detection and
quantitation of T-2 mycotoxin in rat organs by radioimmunoassay. J.
Assoc. Off. Anal. Chem., 70: 654-657.
HIBBS, C.M., OSWEILER, G.D., BUCK, W.B., & MACFEE, G.P. (1975)
Bovine hemorrhagic syndrome related to T-2 mycotoxin. Proc. Ann.
Meet. Am. Assoc. Vet. Lab. Diagn., 17: 305-310.
HIRAYAMA, S. & YAMAMOTO, M. (1948) [Biological studies on the
poisonous wheat flour (I).] Eiseishikenjo Hokoku, 66: 85-98 (in
Japanese).
HIRAYAMA, S. & YAMAMOTO, M. (1950) [Biological studies on the
poisonous wheat flour (II).] Eiseishikenjo Hokoku, 67: 117-121 (in
Japanese).
HOBDEN, A.N. & CUNDLIFFE, E. (1980) Ribosomal resistance to the
12,13-epoxy-trichothecene antibiotics in the producing organism
Myrothecium verrucaria. Biochem. J., 190(3): 765-770.
HOERR, F.J., CARLTON, W.W., & YAGEN, B. (1981) Mycotoxicosis caused
by a single dose of T-2 toxin or diacetoxyscirpenol in broiler
chickens. Vet. Pathol., 18: 653-664.
HOLADAY, C.E. (1976) A rapid screening method for the aflatoxins
and ochratoxin A. J. Am. Assoc. Anal. Chem., 53: 603-605.
HOLT, P.S. & DE LOACH, J.R. (1988a) Cellular effects of T-2 mycotoxin
on two different lives. Biochem. Biophys. Acta, 971: 1-8.
HOOD, R.D. (1986) Effects of concurrent prenatal exposure to
rubratoxin B and T-2 toxin in the mouse. Drug chem. Toxicol., 9
(2): 185-190.
HOOD, R.D., NAUGHTON, M.J., & HAYES, A.W. (1976) Prenatal effects
of ochratoxin A in hamsters. Teratology, 13: 11-14.
HOOD, R.D., KUCZUK, M.H., & SZCZECK, G.M. (1978) Effects in mice of
simultaneous prenatal exposure to ochratoxin A and T-2 toxin.
Teratology, 17: 25-30.
HRABAR, A., SULJAGA, K., BORCIC, B., ALERAJ, B., CEOVIC, S., &
CVORISCEC, D. (1976) [Endemic nephropathy morbidity and mortality
in the village of Kaniza.] Arch. ind. Hyg. Toxicol., 27: 137-145
(in Croatian).
HSU, I.C., SMALIEY, E.B., STRONG, F.M., & RIBELIN, W.E. (1972)
Identification of T-2 toxin in moldy corn associated with a lethal
toxicosis in dairy cattle. Appl. Microbiol., 24(5): 684-690.
HUFF, W.E., WYATT, R.D., TUCKER, T.L., & HAMILTON, P.B. (1974)
Ochratoxicosis in the broiler chicken. Poult. Sci., 53: 1585-1591.
HUFF, W.E., DOERR, J.A., HAMILTON, P.B., HAMANN, D.D., PETERSON,
R.E., & CIEGLER, A. (1980) Evaluation of bone strength during
aflatoxicosis and ochratoxicosis. Appl. environ. Microbiol.,
40: 102-107.
HULT, K. & GATENBECK, S. (1976) A spectrophotometric procedure,
using carboxypeptidase A, for the quantitative measurement of
ochratoxin A. J. Assoc. Off. Anal. Chem., 59: l28-l29.
HULT, K., TEILING, A., & GATENBECK, S. (1976) Degradation of
ochratoxin A by a ruminant. Appl. environ. Microbiol., 32: 443-444.
HULT, K., HUKBY, E., HOGLUND, U., GATENBECK, S., RUTQVIST, L., &
SELLYEY, G. (1979) Ochratoxin A in pig blood: method of analysis
and use as a tool for feed studies. Appl. environ. Microbiol., 38:
772-776.
HULT, K., HUKBY, E., GATENBECK, S., & RUTQVIST, L. (1980).
Ochratoxin A in blood from slaughter pigs in Sweden: Use in
evaluation of toxin content of consumed feed. Appl. environ.
Microbiol., 39: 828-830.
HULT, K., PLESTINA, R., HABAZIN-NOVAK, V., RADIC, B., & CEOVIC, S.
(1982) Ochratoxin A in human blood and Balkan endemic nephropathy.
Arch. Toxicol., 51: 313-321.
HULT, K., FUCHS, R., PERAICA, M., PLESTINA, R., & CEOVIC, S. (1984)
Screening for ochratoxin A in blood by flow injection analysis. J.
appl. Toxicol., 4: 326-329.
HUNT, D.C., BOURDON, A.T., WILD, P.J., & CROSBY, N.T. (1978) Use of
high performance liquid chromatography combined with fluorescence
detection for the identification and estimation of aflatoxins and
ochratoxin in food. J. Sci. Food Agric., 29: 234-238.
HUNT, D.C., LESLYE, A.P., & CROSBY, N.T. (1979) Determination of
ochratoxin A in pig's kidney using enzymic digestion, dialysis and
high-performance liquid chromatography with post-column
derivatization. Analyst, 104: 1171-1175.
HUNTER, K.W., BRIMFIELD, A.A., MILLER, M., FINKELMAN, F.D., & CHU,
S.F. (1985) Preparation and characterization of monoclonal antibodies
to the trichothecene mycotoxin T-2. Appl. environ. Microbiol., 49:
168-172.
HUSSEIN, H.M., FRANICH, R.A., BAXTER, M,. & ANDREW, I.G. (1989)
Naturally occurring Fusarium toxins in New Zealand maize. Food Addit.
Contam., 6: 49-58.
HUTCHISON, R.D., STEYN, P.S., & THOMPSON, D.L. (1971) The isolation
and structure of 4-hydroxyochratoxin A and 7-carboxy-3,4-dihydro-8-
hydroxy-3-methylisocoumarin from Penicillium viridicatum.
Tetrahedron, 43: 4033-4036.
IARC (1987a) Overall evaluations of carcinogenicity: and updating of
IARC Monographs Vol. 1 to 42, Lyons, International Agency for
Research on Cancer, pp. 271-272. (IARC Monographs on the Evaluation
of Carcinogenic Risks to Humans, Supplement 7).
IARC (1987b) Biennial report 1986-1987, Lyons, International Agency
for Research on Cancer.
IARC (1983) Some food additives, feed additives and naturally
occurring substances: ochratoxin A, Lyons, International Agency for
Research on Cancer, pp. 191-206 (IARC Monographs on the Evaluation of
the Carcinogenic Risk of Chemicals to Humans, Vol. 31).
IARC (1984) Monitoring of aflatoxins in human body fluids and
application to field studies, Lyons, International Agency for
Research on Cancer (IARC International Technical Report No. 84/003).
ICHINOE, M., UCHIYAMA, S., AMANO, R., & KURATA, H. (1985)
Trichothecene-producing Fusarium in barley and wheat in Japan.
Trichothecenes and other Mycotoxins. Proceedings of the International
Mycotoxin Symposium, Sydney, Australia, New York, Chichester, John
Wiley & Sons, pp. 21-31.
ILUS, T., NIKU-PAAVOLA, M.L., & ENARI, T.M. (1981) Chromatographic
analysis of Fusarium toxins in grain samples. Eur. J. appl.
Microbiol. Biotechnol., 11(4): 244-247.
IMAIDA, K., HIROSE, M., OGISO, T., KURATA, Y., & ITO, N. (1982)
Quantitative analysis of initiating and promoting activities of five
mycotoxins in liver carcinogenesis in rats. Cancer Lett., 16:
137-143.
ISHII, K., SAKAI, K., UENO, Y., TSUNODA, H., & ENOMOTO, M. (1971)
Solaniol, a toxic metabolite of Fusarium solani. Appl. Microbiol.,
22(4): 718-720.
ISHII, K., ANDO, Y., & UENO, Y. (1975) Toxicological approaches to
the metabolites of Fusaria. Part 9. Isolation of vomiting factor from
moldy corn infected with Fusarium spp. Chem. pharm. Bull., 23(9):
2162-2164.
ISHII, K., KOBAYASHI, J., UENO, Y., & ICHINOE, M. (1986) Occurrence
of trichothecine in wheat. Appl. environ. Microbiol., 52: 331-333.
ITO, Y., OHTSUBO, K., & SAITO, M. (1980) Effects of fusarenon-X, a
trichothecene produced by Fusarium nivale, on pregnant mice and their
fetuses. Jpn. J. exp. Med., 50(3): 167-172.
ITO, Y., OHTSUBO, K., ISHII, K., & UENO, Y. (1986) Effects of
nivalenol on pregnancy and fetal development of mice. Mycotoxin
res., 2: 71-77.
ITO, Y., UENO, Y., TANAKA, T., NAKAMURA, K., & OHTSUBO, K. (1988)
[Embryotoxicity of oral nivalenol in mice.] Proc. Jpn. Assoc.
Mycotoxicol., 27: 33-36. (in Japanese).
IUPAC (1976) Recommended methods for ochratoxin A and B in barley,
Oxford, International Union of Pure and Applied Chemistry (IUPAC
Technical Report No.14).
IWAHASHI, T., TASHIRO, F., & UENO, Y. (1982) Mechanism of cytotoxic
effect of T-2 toxin on a protozoan, Tetrahymena pyriformis gl.
Maikotokishin, 15: 31-33.
JAGADEESAN, V., RUKMINI, C., VIJAYARAGHOVAN, M., & TULPULE, P.G.
(1982) Immune studies with T-2 toxin: effect of feeding and
withdrawal in monkeys. Food chem. Toxicol., 20: 83-87.
JARVIS, B.B., STAHLY, G.P., & CURTIS, C.R. (1978) Antitumor
activity of fungal metabolites: Verrucarin b-9,10-epoxides. Cancer
Treat. Rep., 62(10): 1585-1586.
JARVIS, B.B., STAHLY, G.P., PAVANASASIVAM, G., & MAZZOLA, E.P.
(1980) Antileukemic compounds derived from the chemical modification
of macrocyclic trichothecenes. 1. Derivatives of verrucarin A. J.
med. Chem., 23(9): 1054-1058.
JARVIS, B.B., LEE, Y.W., COMEZOGLU, S.N., & YATAWARA, C.S. (1986)
Trichothecenes produced by Stachybotrys atra from Eastern Europe.
Appl. environ. Microbiol., 51(5): 915-918.
JELINEK, C.F., POHLAND, A.E., & WOOD, G.E. (1989) Occurrence of
mycotoxins in foods and feeds - an update. J. Assoc. Off. Anal.
Chem., 72: 223-230.
JIMINEZ, A. & VASQUEZ, D. (1975) Quantitative binding of antibiotics
to ribosomes from a yeast mutant altered on the peptidyl transferase
centre. Eur. J. Biochem., 54(2): 483-492.
JIMENEZ, A., SANCHEZ, L., & VAZQUEZ, D. (1975) Simultaneous
ribosomal resistance to trichodermin and anisomycin in Saccharomyces
cerevisiae mutants. Biochim. Biophys. Acta, 383(4): 427-434.
JOFFE, A.Z. (1962) Biological properties of some toxic fungi
isolated from overwintered cereals. Mycopathol. Mycol. appl., 16:
201-221.
JOFFE, A.Z. (1986) Fusarium species, their biology and toxicology,
New York, Chichester, J. Wiley and Sons.
JOFFE, A.Z. & YAGEN, B. (1977) Comparative study of the yield of T-2
toxin produced by Fusarium poae, Fusarium sporotrichioides and
Fusarium sporotrichioides var. tricinctum strains from different
sources. Mycopathologia, 60(2): 93-97.
JOHNSEN, H., ODDEN, E., LIE, O., JOHNSEN, B.A., & FONNUM, F. (1986)
Metabolism of T-2 toxin by rat liver carboxylesterase. Biochem.
Pharmacol., 35: 1469-1473.
JOSEFSSON, E. (1979) [Study of ochratoxin A in pig kidneys.] Var
Föda, 31: 415-420 (in Swedish).
JOSEFSSON, E. & MOLLER, T. (1979) High pressure liquid
chromatographic determination of ochratoxin A and zearalenone in
cereals. J. Assoc. Off. Anal. Chem., 62: 1165-1168.
JUSZKIEWICZ, T. & PISKORSKA-PLISZCZYNSKA, J. (1976) [Occurrence of
aflatoxins B1, B2, G1, and G2, ochratoxins A and B, sterigmatocystin
and zearalenone in cereals]. Med. Weter., 32: 617-619 (in Polish).
JUSZKIEWICZ, T. & PISKORSKA-PLISZCZYNSKA, J. (1977) [Occurrence of
mycotoxins in mixed feeds and concentrates.] Med. Weter., 33: 193-196
(in Polish).
JUSZKIEWICZ, T., PISKORSKA-PLISZCZYNSKA, J., & WISNIEWSKA, H. (1982)
Ochratoxin A in laying hens: tissue deposition and passage into eggs.
In: Mycotoxins and Phycotoxins, Vienna, Technical University Vienna
Publ., pp. 122-125.
KALINOSKI, H.T., UDSETH, H.R., WRIGHT, B.W., & SMITH, R.D. (1986)
Supercritical fluid extraction and direct fluid injection mass
spectrometry for the determination of trichothecene mycotoxins in
wheat samples. Anal. Chem., 58: 2421-2425.
KAMIMURA, H., NISHIJIMA, M., SAITO, K., YASUDA, K., IBE, A.,
NAGAYAMA, T., USHIYAMA, H., & NAOI, Y. (1979) Studies on mycotoxins
in foods. XII. The decomposition of trichothecene mycotoxins during
food processing. J. Assoc. Off. Anal. Chem., 64(5): 1067-1073.
KANAI, K. & KONDO, E. (1984) Decreased resistance to myobacterial
infection in mice fed a trichothecene compound (T-2 toxin). Jpn. J.
med. Sci. Biol., 37: 97-104.
KANE, A., CREPPY, E.E., ROSCHENTHALER, R., & DIRHEIMER, G. (1986a)
Changes of urinary and renal tubular enzymes caused by subchronic
administration of ochratoxin A in rats. Toxicology, 61: 489-495.
KANE, A., CREPPY, E.E., ROTH, A., ROSCHENTHALER, R., & DIRHEIMER, G.
(1986b) Distribution of the [3]-label from low doses of radioactive
ochratoxin A ingested by rats, and evidence for DNA single-strand
breaks caused in liver and kidneys. Arch. Toxicol., 58: 219-224.
KANIZAWA, M. (1984) Synergistic effect of citrinin on hepatorenal
carcinogenesis of ochratoxin A in mice. In: Kurata, H. & Ueno, Y.,
ed. Toxigenic fungi - their toxins and health hazard, Amsterdam,
Oxford, New York, Elsevier Science Publishers, pp. 245-254
(Developments in Food Science No. 7).
KANIZAWA, M. & SUZUKI, S. (1978) Induction of renal and hepatic
tumours in mice by ochratoxin A, a mycotoxin. Gann, 69: 599-600.
KANIZAWA, M., SUZUKI, S., KOZUKA, Y., & YAMAZAKI, M. (1977)
Histopathological studies on the toxicity of ochratoxin A in rats. I.
Acute oral toxicity. Toxicol. appl. Pharmacol., 42: 55-64.
KELLERMAN, T.S., MARASAS, W.F.O., PIENAAR, J.G., & NAUDE, T.W.
(1972) A mycotoxicosis of equidae caused by Fusarium moniliforme
Sheldon. A preliminary communication. Onderstepoort J. vet. Res.,
39(4): 205-208.
KHERA, K.S., WHALEN, C., ANGERS, G., VESONDER, R.F., & KUIPER-
GOODMAN, I. (1982) Embryotoxicity of 4-deoxynivalenol (vomitoxin)
in mice. Bull. environ. Contam. Toxicol., 29: 487-491.
KHERA, K.S., ARNOLD, D.L., WHALEN, C., ANGERS, G., & SCOTT, P.M.
(1984) Vomitoxin (4-deoxynivalenol): Effects on reproduction of mice
and rats. Toxicol. appl. Pharmacol., 74: 345-356.
KHERA, K.S., WHALEN, C., & ANGERS, G. (1986) A teratology study on
vomitoxin (4-deoxynivalenol) in rabbits. Food chem. Toxicol., 24:
421-424.
KIENTZ, C.E. & VERWEIJ, A. (1986) Trimethylsilylation and
trifluoroacetylation of a number of trichothecenes followed by gas
chromatographic analysis on fused silica capillary columns. J.
Chromatogr., 355: 229-240.
KING, B. (1979) Outbreak of ergotism in Wollo, Ethiopia. Lancet,
1: 1411.
KING, R.R., MCQUEEN, R.E., LEVESQUE, D., & GREENHALGH, R. (1984)
Transformation of deoxynivalenol (vomitoxin) by rumen microorganisms.
J. agric. food Chem., 32: 1181-1183.
KITAGAWA, M., TASHIRO, F., & UENO, Y. (1982) Mutual interaction
between zearalenone, an estrogenic mycotoxin, and estrogen receptor
of rat brain. Proc. Jpn. Assoc. Mycotoxicol., 15: 28-30.
KITCHEN, D.N., CARLTON, W.W., & TUITE, J. (1977a) Ochratoxin A and
citrinin induced nephrosis in beagle dogs. I. Clinical and
clinicopathological features. Vet. Pathol., 14: 154-172.
KITCHEN, D.N., CARLTON, W.W., & TUITE, J. (1977b) Ochratoxin A and
citrinin induced nephrosis in beagle dogs. II. Pathology. Vet.
Pathol., 14: 261-272.
KLINKERT, W., LORKOWSKI, G., CREPPY, E.E., DIRHEIMER, G., &
ROSCHENTHALER, R. (1981) Inhibition of macrophage migration by
ochratoxin A and citrinin, and prevention by phenylalanine of the
ochratoxin A-induced inhibition. Toxicol. Eur. Res., 3: 186-189.
KONISHI, T. & ICHIJO, S. (1970) [Clinical studies on bean-hulls
poisoning of horse. I. Clinical and biochemical observations in
spontaneous cases.] Res. Bull. Obihiro Univ., 6: 242-257 (in
Japanese).
KONRAD, I. & ROSCHENTHALER, R. (1977) Inhibition of phenylalanine
tRNA synthetase from Bacillus subtilis by ochratoxin A. FEBS Lett.,
83: 341-347.
KOTSONIS, F.N., SMALLEY, E.B., ELLISON, R.A., & GALE, C.M. (1975)
Food refusal factors in pure cultures of Fusarium roseum graminearum.
Appl. Microbiol., 30(3): 362-368.
KOZLOVSKY, A.G. & RESHETILOVA, T.A. (1984) Regulation of
biosynthesis of ergot alkaloids by Penicillium sizovae. Folia
microbiol., 29: 301-305.
KRIEK, N.P.J., MARASAS, W.F.O., STEYN, P.S., VAN RENSBURG, S.J., &
STEYN, M. (1977) Toxicity of a moniliformin-producing strain of
Fusarium moniliforme var. subglutinans isolated from maize. Food
Cosmet. Toxicol., 15(6): 579-587.
KRISHNAMACHARI, K.A.V.R. & BHAT, R.V. (1976) Poisoning of ergoty
bajra (pearl millet) in man. Indian J. med. Res., 64: 1624-1628.
KRISHNAMURTHY, T. & SARVER, E.W. (1986) Mass spectral investigations
on trichothecene mycotoxins. III. Synthesis, characterization and
applications of pentafluoropropionyl and trifluoroacetyl esters of
simple trichothecenes. J. Chromatogr., 355: 253-264.
KRISHNAMURTHY, T., WASSERMAN, M.B., & SARVER, E.W. (1986) Mass
spectral investigations of trichothecene mycotoxins. I. Application
of negative ion chemical ionization techniques for the simultaneous
and accurate analysis of simple trichothecenes in picogram levels.
Biomed. environ. mass Spectrom., 13: 503-518.
KRISHNAMURTHY, T., SARVER, E.W., GREENE, S.L., & JARVIS, B.B. (1987)
Mass spectral investigations on trichothecene mycotoxins. II.
Detection and quantitation of macrocyclic trichothecenes by gas
chromatography/negative ion chemical ionization mass spectrometry. J.
Assoc. Off. Anal. Chem., 70: 132-140.
KROGH, P. (1976a) Epidemiology of mycotoxic porcine nephropathy.
Nord. vet. Med., 28: 452-458.
KROGH, P. (1976b) Mycotoxic nephropathy. In: Advances in veterinary
science and comparative medicine, New York, Academic Press, Vol. 20,
pp. 147-170.
KROGH, P. (1977) Ochratoxin A residues in tissues of slaughter pigs
with nephropathy. Nord. vet. Med., 29: 402-405.
KROGH, P. (1978) Causal associations of mycotoxic nephropathy. Acta
pathol. microbiol. Scand., A269: 1-28.
KROGH, P. (1979) Environmental ochratoxin A and Balkan (endemic)
nephropathy: Evidence for support of a causal relationship. In:
Strahinjic, S. & Stefanovic, V., ed. Endemic (Balkan) nephropathy,
Nis, Yugoslavia, Grafika, pp. 35-43.
KROGH, P. (1980) Ochratoxins: occurrence, biological effects and
causal role in disease. In: Eaker, D. & Wadstrom, T., ed. Natural
toxins, Oxford, Pergamon Press, pp. 673-680.
KROGH, P. (1983) Diagnostic criteria for ochratoxin-induced
nephropathy. In: Strahinjic, S. & Stefanovic, V., ed. Current
research in endemic (Balkan) nephropathy, Nis, Yugoslavia, Grafika,
pp. 11-14.
KROGH, P., HALD, B., & PEDERSEN, E.J. (1973) Occurrence of
ochratoxin A and citrinin in cereals associated with mycotoxic
porcine nephropathy. Acta pathol. microbiol. Scand., B81:
689-695.
KROGH, P., AXELSON, N.H., ELLING, F., GYRD-HANSEN, N., HALD, B.,
HYLDGAARD-JENSEN, J., LARSEN, A.E., MADSEN, A., MORTENSEN, H.P.,
MOLLER, T., PETERSEN, O.K., RAVNSKOV, U., ROSTGAARD, M., & AALUND, O.
(1974a) Experimental porcine nephropathy: changes of renal function
and structure induced by ochratoxin A-contaminated feed. Acta pathol.
microbiol. Scand., A246: 21.
KROGH, P., HALD, B., ENGLUND, P., RUTQVIST, L., & SWAHN, O. (1974b)
Contamination of Swedish cereals with ochratoxin A. Acta pathol.
microbiol. Scand., B82: 301-302.
KROGH, P., ELLING, F., HALD, B., LARSEN, A.E., LILLEHOJ, E.B.,
MADSEN, A., & MORTENSEN, H.P. (1976a) Time-dependent disappearance
of ochratoxin A residues of bacon pigs. Toxicology, 6: 235-242.
KROGH, P., ELLING, F., HALD, B., JYLLING, B., PETERSEN, V.E.,
SKADHAUGE, E., & SVENDSEN, C.K. (1976b) Experimental avian
nephropathy: changes of renal function and structure induced by
ochratoxin A-contaminated feed. Acta pathol. microbiol. Scand.,
A84: 215-221.
KROGH, P., ELLING, F., GYRD-HANSEN, N., HALD, B., LARSEN, A.E.,
LILLEHOJ, E. B., MADSEN, A., MORTENSEN, H.P., & RAVNSKOV, U. (1976c)
Experimental porcine nephropathy: changes of renal function and
structure perorally induced by crystalline ochratoxin A. Acta pathol.
microbiol. Scand., A84: 429-434.
KROGH, P., HALD, B., PLESTINA, R., & CEOVIC, S. (1977) Balkan
(endemic) nephropathy and food-borne ochratoxin A: Preliminary
results of a survey of foodstuff. Acta. Path. Microbiol. Scand.,
B85: 238-240.
KROGH, P., ELLING, F., FRIIS, CHR., MALD, B., LARSEN, A.E., LILLEHOJ,
E.B., MADSEN, A., MORTENSEN, H.P., RASMUSSEN, F., & RAVUSKOU, U.
(1979) Porcine nephropathy induced by long-term ingestion of
ochratoxin A. Vet. Pathol., 16: 466-475.
KROGH, P., GYRD-HANSEN, N., HALD, B., LARSEN, S., NIELSEN, J.P.,
SMITH, M., IVANOFF, C., & MEISNER, H. (1988). Renal enzyme
activities in experimental ochratoxin A-induced porcine nephropathy:
Diagnostic potential of phosphoenolpyruvate carboxykinase and
gammaglutamyl transpeptidase activity. J. Toxicol. environ. Health.,
23: 1-15.
KUCZUK, M.H., BENSON, P.M., HEATH, H., & HAYES, A.W. (1978)
Evaluation of the mutagenic potential of mycotoxins using Salmonella
typhimurium and Saccharomyces cerevisiae. Mutat. Res., 53(1): 11-20.
KUMAGAI, S. (1985) Ochratoxin A: Plasma concentration and excretion
into bile and urine in albumin-deficient rats. Food chem. Toxicol.,
23: 941-943.
KUMAGAI, S. & AIBARA, K. (1982) Intestinal absorption and secretion
of ochratoxin A in rat. Toxicol. appl. Pharmacol., 64: 94-102.
KUMAGAI, S. & SHIMIZU, T. (1988) Effects of Fusarenon-X and T-2 toxin
on intestinal absorption of monosaccharide in rats. Arch. Toxicol.,
61: 489-495.
KUPCHAN, S.M., JARVIS, B.B., DAILEY, R.G., Jr, BRIGHT, W., BRYAN,
R.F., & SHIZURI, Y. (1976) Baccharin, a novel potent antileukemic
trichothecene triepoxide from Baccharis megapotamica. J. Am. Chem.
Soc., 98(22): 7092-7093.
KUPCHAN, S.M., STREELMAN, D.R., JARVIS, B.B., DAILEY, R.G., Jr, &
SNEDEN, A.T. (1977) Isolation of potent new antileukemic
trichothecenes from Baccharis megapotamica. J. org. Chem., 42
(26): 4221-4225.
LAFARGE-FRAYSSINET, C., DECLOITRE, F., MOUSSET, S., MARTIN, M., &
FRAYSSINET, C. (1981) Induction of DNA single strand breaks by T-2
toxin, a trichothecene metabolites of Fusarium, effect on lymphoid
organs and liver. Mutat. Res., 88(2): 115-124.
LAFARGE-FRAYSSINET, C., LESPINATS, G., LAFONT, P., LOISILLIER, F.,
MOUSSET, S., ROSENSTEIN, Y., & FRAYSSINET, C. (1979)
Immunosuppressive effects of Fusarium cells to mitogens. Proc. Soc.
Exp. Biol. Med., 160(3): 302-311.
LARSEN, S. (1928) [On chronic degeneration of the kidneys caused by
mouldy rye.] Maanadsskr. Dyrl., 40: 259-284, 289-300 (in Danish).
LAUREN, D.R. & GREENHALGH, R. (1987) Simultaneous analysis of
nivalenol and deoxynivalenol in cereals by liquid chromatography. J.
Assoc. Off. Anal. Chem., 70: 479-483.
LEE, S. & CHU, F.S. (1981a) Radioimmunoassay of T-2 toxin in corn
and wheat. J. Assoc. Off. Anal. Chem., 64(1): 156-161.
LEE, S. & CHU, F.S. (1981b) Radioimmunoassay of T-2 toxin in
biological fluids. J. Assoc. Off. Anal. Chem., 64(3): 684-688.
LEE, S.C., BEERY, J.T., & CHU, F.S. (1984) Immunohistochemical fate
of ochratoxin A in mice. Toxicol. appl. Microbiol., 72: 218-227.
LEE, U., JANG, H., TANAKA, T., HASEGAWA, A., OH, Y., & UENO, Y.
(1985) The coexistence of the Fusarium mycotoxins nivalenol,
deoxynivalenol, and zearalenone in Korean cereals harvested in 1983.
Food Addit. Contam., 2(3): 185-192.
LEE, U., JANG, H., TANAKA, T., OH, Y., CHO, C., & UENO, Y. (1987)
Effect of milling on decontamination of Fusarium mycotoxins
nivalenol, deoxynivalenol, and zearalenone in Korean wheat. J. agric.
food Chem., 35: 126-129.
LEONOV, A.N. (1977) Current view of the chemical nature of factors
responsible for alimentary toxic aleukia. In: Rodricks, J.V.,
Hesseltine, C.W,. & Mehlman, M.A., ed. Mycotoxins in human and animal
health, Park Forest South, Illinois, Pathotox Publishers, pp. 323-
328.
LEVI, C.P. (1975) Collaborative study of a method for the
determination of ochratoxin A in green coffee. J. Assoc. Off. Anal.
Chem., 58(2): 258-262.
LEVI, C.P., TRENK, H.L., & MOHR, H.K. (1974) Study of the
occurrence of ochratoxin A in green coffee beans. J. Assoc. Off.
Anal. Chem., 57: 866-870.
LEW, V.H., MUELLNER, E., HAGER, R., & GREGOR, M. (1979) [Feed
refusal and emesis in fattening pigs caused by fusariotoxin-
contaminated corn.] Bodenkultur, 30(3): 309-316 (in German).
LIAO, L.L., GROLLMAN, A.P., & HORWITZ, S.B. (1976) Mechanism of
action of the 12,13-epoxytrichothecene anguidine: an inhibitor of
protein synthesis. Biochim. Biophys. Acta, 454(2): 273-284.
LINDENFELSER, L.A., LILLEHOJ, E.B., & BURMEISTER, H.R. (1974)
Aflatoxin and trichothecene toxins: skin tumor induction and
synergistic acute toxicity in white mice. J. Natl Cancer Inst.,
52(1): 113-116.
LINNAINMAA, K., SORSA, M., & ILUS, T. (1979) Epoxytrichothecene
mycotoxins as c-mitotic agents in Allium. Hereditas, 90(2):
151-156.
LOKEN, T. (1984) Ergot from meadow grass in Norway: chemical
composition and toxicological effects in sheep. Nord. vet. Med., 36:
259-265.
LORENZ, K. (1979) Ergot on cereal grains. In: Critical reviews in
food, science, and nutrition, Boca Raton, Florida, CRC Press, Vol.
11, pp. 311-354.
LORENZANA, R.M., BEASLEY, V.R., BUCK, W.B., GHENT, A.W., LUNDREN,
G.R., & POPPENGA, R.H. (1985) Experimental T-2 toxicosis in swine. I
Changes in cardiac output, aortic mean pressure, catecholamines, 6-
keto PGFa, thromboxane B2, and acid base parameters. Fundam. appl.
Toxicol., 5: 879-892.
LOVELESS, A.R. (1967) Claviceps fusiformis sp. nov.: the causal
agent of an agalactia of sows. Br. Mycol. Soc., 50: 15-18.
LUO, X. (1988) Food poisoning associated with Fusarium toxins.
Proceedings of the 7th International Symposium on Mycotoxins and
Phycotoxins, Tokyo, 16-19 August, 1988.
LUSTER, M.I., GERMOLEC, D.R., BURLESON, G.R., JAMESON, C.W.,
ACKERMANN, M.F., LAMM, K.R., & MAYER, H.T. (1987) Selective
immunosuppression in mice of natural killer cell activity by
ochratoxin A. Cancer Res., 47: 2259-2263.
LUTSKY, I. & MOR, N. (1981) Experimental alimentary toxic aleukia
in cats. Lab. anim. Sci., 31(1): 43-47.
LUTSKY, I., MOR, N., YAGEN, B., & JOFFE, A.Z. (1978) The role of
T-2 toxin in experimental alimentary aleukia: a toxicity study in
cats. Toxicol. appl. Pharmacol., 43(1): 111-124.
MACDONALD, E.J., COVAN, K.R. & SMITH, T.K. (1988) Effect of acute
oral doses of T-2 toxin on tissue concentrations of biogenic amines
in the rat. J. anim. Sci., 66: 434-441.
MCLAUGHLIN, C.S., VAUGHAMM, M.H., CAMPBELL, I.M., WEI, C.M.,
STAFFORD, M.E., & HANSEN, B.S. (1977) Inhibition of protein
synthesis by trichothecenes. In: Rodricks, J.V., Hesseltine, C.W., &
Mehlman, M.A., ed. Mycotoxins in human and animal health, Forest Park
South, Illinois, Pathotox Publishers, pp. 263-273.
MANN, D.D., BUENING, G.M., HOOK, B., & OSWEILER, G.D. (1983) Effects
of T-2 mycotoxin on bovine serum proteins. Am. J. vet. Res., 44:
1757-1759.
MANN, D.D., BUENING, G.M., OSWEILER, G.D., & HOOK, B. (1984) Effect
of subclinical levels of T-2 toxin on the bovine cellular immune
system. Can. J. comp. Med., 48: 308-312.
MANTLE, P.G. (1977a) The genus Claviceps. In: Wyllie, T.D. &
Morehouse, L.C., ed. Mycotoxic fungi, mycotoxins, mycotoxicoses: an
encyclopedia handbook, New York, Marcel Dekker, Vol. 1, pp. 83-89.
MANTLE, P.G. (1977b) Chemistry of Claviceps mycotoxins. In: Wyllie,
T.D. & Morehouse, L.C., ed. Mycotoxic fungi, mycotoxins,
mycotoxicoses: an encyclopedia handbook, New York, Marcel Dekker, Vol.
1, pp. 421-426.
MANTLE, P.G. (1977c) Ergotism in cattle, sheep, swine. In: Wyllie,
T.D. & Morehouse, L.C., ed. Mycotoxic fungi, mycotoxins,
mycotoxicoses: an encyclopedia handbook, New York, Marcel Dekker, Vol.
2, pp. 145-151, 207-213, 273-275.
MARASAS, W.F.O., BAMBURG, J.R., SMALLEY, E.B., STRONG, F.M., RAGLAND,
W.L., & DEGURSE, P.E. (1969) Toxic effects on trout, rats and mice
of T-2 toxin produced by the fungus Fusarium tricinctum. Toxicol.
appl. Pharmacol., 15(2): 471-482.
MARASAS, W.F.O., SMALLEY, E.B., BAMBURG, J.R., & STRONG, F.M. (1971)
Phytotoxicity of T-2 toxin produced by Fusarium tricinctum.
Phytopathology, 61(12): 1488-1491.
MARASAS, W.F.O., KRIEK, N.P.J., WIGGINS, V.M., STEYN, P.S., TOWERS,
D.K., & HASTIE, T.J. (1979a) Incidence, geographic distribution,
and toxigenicity of Fusarium species in South African corn.
Phytopathology, 69(11): 1181-1185.
MARASAS, W.F.O., LEISTNER, L., HOFMANN, G., & ECKARDT, C. (1979b)
Occurrence of toxigenic strains of Fusarium in maize and barley in
Germany. Appl. environ. Microbiol., 7: 289-305.
MARASAS, W.F.O., NELSON, P.E., & TOUSSON, T.A., ed. (1984) Toxigenic
Fusarium species - identity and mycotoxicology, University Park,
Pennsylvania, Pennsylvania State University Press.
MARQUARDT, R.R., FROHLICH, A.A., SREEMANNARAYANA, O., ABRAMSON, D., &
BERNATSKY, A. (1988) Ochratoxin A in blood from slaughter pigs in
Western Canada. Can. J. vet. Res., 52: 186-190.
MASUDA, E., TAKEMOTO, T., TATSUNO, T., & OBARA, T. (1982)
Immunosuppressive effect of a trichothecene mycotoxin, fusarenon-X in
mice. Immunology, 45: 743-749.
MASUKO, H., UENO, Y., OTOKAWA, M., & YAGINUMA, K. (1977) The
enhancing effect of T-2 toxin on delayed hyper-sensitivity in mice.
Jpn. J. med. Sci. Biol., 30(3): 159-164.
MATSUOKA, Y. & KUBOTA, K. (1981) Studies on mechanisms of diarrhea
induced by fusarenone-X, a trichothecene mycotoxin from Fusarium
species. Toxicol. appl. Pharmacol., 57(3): 293-301.
MATSUOKA, Y. & KUBOTA, K. (1987) Characteristics of inflammation
induced by Fusarenon-X, a trichothecene mycotoxin from Fusarium
species. Toxicol. appl. Pharmacol., 91: 333-340.
MATSUOKA, Y., KUBOTA, K., & UENO, Y. (1979) General pharmacological
studies of fusarenon-X, a trichothecene mycotoxin from Fusarium
spp. Toxicol. appl. Pharmacol., 50(1): 87-94
MAYURA, K., REDDY, R.V., HAYES, A.W., & BERNDT, W.O. (1982)
Embryocidal, fetotoxic and teratogenic effects of ochratoxin A in
rats. Toxicology, 25: 175-185.
MAYURA, K., HAYES, A.W., & BERNDT, W.O. (1983) Effects of dietary
protein on teratogenicity of ochratoxin A in rats. Toxicology, 27:
147-157.
MAYURA, K., PARKER, R., BERNDT, W.O., & PHILLIPS, T.D. (1984)
Effect of simultaneous prenatal exposure to ochratoxin A and citrinin
in the rat. J. Toxicol. environ. Health, 13: 553-561.
MEISNER, H. (1976) Energy-dependent uptake of ochratoxin A by
mitochondria. Arch. Biochem. Biophys., 173: 132-140.
MEISNER, H. & CHAN, S. (1974) Ochratoxin A, an inhibitor of
mitochondrial transport systems. Biochemistry, 13: 2795-2800.
MEISNER, H. & KROGH, P. (1982) Inhibition of renal
phosphoenolpyruvate carboxykinase in pigs by alimentary exposure to
ochratoxin A. In: Mycotoxins and Phycotoxins, Proceedings of the 5th
International IUPAC Symposium, Vienna, Technical University Vienna
publ., pp. 342-345.
MEISNER, H. & MEISNER, P. (1981) Ochratoxin A, an in vivo inhibitor
of renal phosphoenolpyruvate carboxykinase. Arch. Biochem. Biophys.,
208: 146-153.
MEISNER, H. & SELANIK, P. (1979) Inhibition of renal
gluconeogenesis in rats by ochratoxin. Biochem. J., 180: 681-684.
MEISNER, H., CIMBALA, M.A., & HANSON, R.W. (1983) Decrease of renal
phosphoenolpyruvate carboxykinase RNA and Poly(A)+ RNA level by
ochratoxin A. Arch. Biochem. Biophys., 223: 264-270.
MERWE, K.J.,VAN DER, J., STEYN, P.S., & FOURIE, L. (1965)
Mycotoxins. Part II. The constitution of ochratoxins A, B, and C,
metabolites of Aspergillus ochraceus Wilh. J. Chem. Soc., 1965:
7083-7088.
MILES, W.F. & GURPRASAD, N.P. (1985) Oxygen negative chemical
ionization mass spectrometry of trichothecenes. Biomed. mass
Spectrom., 12: 652-658.
MINISTRY OF AGRICULTURE, FISHERIES AND FOOD (1980) Survey of
mycotoxins in the United Kingdom, London, Ministry of Agriculture,
Fisheries and Food, 35 pp (Food Surveillance Paper No. 4).
MIROCHA, C.J. & ABBAS, H.K. (1989) Chemistry, occurrence and
toxicology of the haemorrhagic mycotoxin (wortmannin) produced by
Fusarium. In: Natori, S., Hashimoto, K., & Ueno, Y. ed. Mycotoxins
and phycotoxins, Science Publishers, Amsterdam, Oxford, New York,
Elsevier.
MIROCHA, C.J. & PATHRE, S. (1973) Identification of the toxic
principle in a sample of poefusarin. Appl. Microbiol., 26(5): 719-
724.
MIZUNO, S. (1975) Mechanism of inhibition of protein synthesis
initiation by diacetoxyscirpenol and fusarenon-X in the reticulocyte
lysate system. Biochim. Biophys. Acta, 383(2): 207-214.
MORE, J., GALTIER, P., & ALVINERIE, M. (1978) Toxicite de
l'ochratoxine A. III. Effets pendant les stades initiaux de la
gestation chez le rat. Ann. Rech. vet., 9(1): 169-173.
MORGAN, M.R.A., MATTHEW, J.A., MCNERNEY, R., & CHAN, H.W.-S. (1982)
The immunoassay for ochratoxin A. In: Mycotoxins and Phycotoxins,
Proceedings of the 5th International IUPAC Symposium, Vienna,
Technical University Vienna, pp. 32-35.
MOROI, K., SUZUKI, S., KUGA, T., YAMAZAKI, M., & KANISAWA, M. (1985)
Reduction of ochratoxin A toxicity in mice treated with phenylalanine
and phenobarbital. Toxicol. Lett., 25: 1-5.
MORRISSEY, R.E. (1984) Teratological study of Fisher rats fed diet
containing added vomitoxin. Food chem. Toxicol., 22(6): 453-457.
MORRISSEY, R.E. & VESONDER, R.F. (1985) Effect of deoxynivalenol
(vomitoxin) on fertility, pregnancy and postnatal development of
Sprague-Dawley rats. Appl. environ. Microbiol., 49: 1062-1066.
MORTENSEN, H.P., HALD, B., & MADSEN, A. (1983) Feeding experiments
with ochratoxin A contaminated barley for bacon pigs. V. Ochratoxin A
in blood. Acta agric. Scand., 33: 235-239.
MORTIMER, P.H., CAMPBELL, J., DI MEUNA, M.E., & WHITE, E.P. (1971)
Experimental myrotheciotoxicosis and poisoning in ruminants by
verrucarin A and roridin A. Res. vet. Sci., 12: 508-515.
MULDERS, E.J. & IMPELEN-PEEK, H.A.M. (1986) Gas chromatographic
determination of deoxynivalenol in cereals. Z. Lebensm. Unters.
Forsch., 183: 406-409.
MUNRO, I.C., MOODIE, C.A., KUIPER-GOODMAN, T., SCOTT, P.M., & GRICE,
H.C. (1974) Toxicologic changes in rats fed graded dietary levels
of ochratoxin A. Toxicol. appl. Pharmacol., 28(2): 180-188.
MUNSCH, N. & MUELLER, W.E.G. (1980) Effect of T-2 toxin on DNA
polymerases and terminal deoxynucleotidyl transferase of Molt-4 and
Nu-8 cell lines. Immunopharmacology, 2(4): 313-318.
MUTOH, A., ISHII, K., & UENO, Y. (1988) Effect of radioprotective
compounds and anti-inflammatory agents on the acute toxicity of
trichothecenes. Toxicol. Lett., 40: 165-174.
NAGAYAMA, S., KAWAMURA, O., OHTANI, K., RYU, J.C., LATUS, D.,
SUDHEIM, L., & UENO, Y. (1988) Application of an enzyme-linked
immunosorbent assay for screening of T-2 toxin-producing Fusarium
spp. Appl. environ. Microbiol., 54: 1302-1303.
NAKAMURA, Y., TAKEDA, S., OGASAWARA, K., KARASHIMADA, T., & ANDO K.
(1951) [A study on toxicosis caused by Akakabi poisoned wheat
flour.] Hokkaido doritsu Eiseikenkyujoho, 2: 35-46 (in Japanese).
NEELY, W.C. & WEST, A.D. (1972) Spectroanalytical parameters of
fungal metabolites. III. Ochratoxin A. J. Assoc. Off. Anal. Chem.,
55(6): 1305-1309.
NEL, W. & PURCHASE, I.F.H. (1968) The fate of ochratoxin A in rats.
J. S. Afr. Chem. Inst., 21: 87-88.
NELSON, P.E., TOUSSON, T.A., & MARASAS, W.F.O. (1983) Fusarium
species, an illustrated manual for identification, University Park,
Pennsylvania, Pennsylvania State University Press.
NESHEIM, S. (1971) Ochratoxins: occurrence, production, analysis,
and toxicity. Abstracts from the 85th Annual Meeting of the
Association of Official Analytical Chemists, Washington, 11-14
October, 1971, Washington, DC, Association of Official Analytical
Chemists.
NESHEIM, S. (1973) Analysis of ochratoxins A and B and their esters
in barley, using partition and thin-layer chromatography. II.
Collaborative study. J. Assoc. Off. Anal. Chem., 56: 822-826.
NESHEIM, S. (1982) Ochratoxin A- Analytical method 1: thin-layer
chromatographic determination of ochratoxin A in foodstuffs. In:
Egan, H., ed. Environmental carcinogens: selected methods of analysis
- some mycotoxins, Lyons, International Agency for Research on
Cancer, pp. 255-270.
NESHEIM, S., HARDIN, N.F., FRANCIS, O.J., & LANGHAM, W.S. (1973)
Analysis of ochratoxins A and B and their esters in barley, using
partition and thin-layer chromatography. I. Development of the
method. J. Assoc. Off. Anal. Chem., 56: 817-821.
NICOLOV, I.G., CHERNOZEMSKY, I.N., PETKOVA-BOCHAROVA, T., STOYANOV,
I.S., & STOICHEV, I.I. (1978) Epidemiologic characteristics of
urinary system tumours and Balkan nephropathy in an endemic region of
Bulgaria. Eur. J. Cancer, 14: 1237-1242.
NIP, W.K. & CHU, F.S. (1979) Fate of ochratoxin A in goats. J.
environ. Sci. Health, B14: 319-333.
NORPPA, H., PENTTILA, M., SORSA, M., HINTIKKA, E.L., & ILUS, T.
(1980) Mycotoxin T-2 of Fusarium tricinctum and chromosome changes
in Chinese hamster bone marrow. Hereditas, 93(2): 329-332.
NORTHOLT, M.D., VAN EGMOND, H.P., & PAULSCH, W.E. (1979) Ochratoxin
A production by some fungal species in relation to water activity and
temperature. J. food Prot., 42: 485-490.
NOWICKI, T.W., GABA, D.G., DEXTER, J.E., MATSUO, R.R., & CLEAR, R.M.
(1988) Retention of the Fusarium mycotoxin deoxynivalenol in wheat
during processing and cooking of spaghetti and noodles. J. Cereal
Sci., 8: 189-202.
OGASAWARA, K. (1965) [Akakabi-toxicosis.] J. Food Hyg. Soc. Jpn,
6: 81-82 (in Japanese).
OHTA, M., ISII, K., & UENO, Y. (1977) Metabolism of trichothecene
mycotoxins. Part 1. Microsomal deacetylation of T-2 toxin in animal
tissues. J. Biochem. (Tokyo), 82(6): 1591-1598.
OHTSUBO, K. & SAITO, M. (1970) Cytotoxic effects of scirpene
compounds, fusarenon-X produced by Fusarium nivale, dihydro-nivalenol
and dihydrofusarenon-X on Hela cells. Jpn. J. med. Sci. Biol., 23(4):
217-225.
OHTSUBO, K. & SAITO, M. (1977) Chronic effects of trichothecene
toxins. In Rodricks, J.V., Hesseltine, C.W., & Mehlman, M.A., ed.
Mycotoxins in human and animal health, Park Forest South, Illinois,
Pathotox Publishers, pp. 255-262.
OHTSUBO, K., YAMADA, M., & SAITO, M. (1968) Inhibitory effect of
nivalenol, a toxic metabolite of Fusarium nivale on the growth cycle
and biopolymer synthesis of Hela cells. Jpn. J. med. Sci. Biol.,
21(3): 185-194.
OHTSUBO, K., KADEN, P., & MITTERMAYER, C. (1972) Polyribosomal
breakdown in mouse fibroblasts L cells by fusarenon-X, a toxic
principle isolated from Fusarium nivale. Biochim. Biophys. Acta,
287(3): 520-525.
OHTSUBO, K., RYU, J.-C., NAKAMURA, K., IZUMIYAMA, N., TANAKA, T.,
YAMAMURA, H., KOBAYSAHI, T., & UENO, Y. (in press) Chronic toxicity
of nivalenol in mice: 2-year feeding trial with Fusarium nivale Fn
2B-moulded rice. Food Chem. Toxicol.
ORTI, D.L., HILL, R.H., LIDDLE, J.A., & NEEHAM, L.L. (1986) High-
performance liquid chromatography of mycotoxin metabolites in human
urine. J. anal. Toxicol., 10: 41-45.
OSBORNE, B.G. (1980) The occurrence of ochratoxin A in mouldy bread
and flour. Food Cosmet. Toxicol., 18: 615-617.
OSBORNE, B.G. & WILLIS, K. (1984) Studies into the occurrence of some
trichothecene mycotoxins in UK home-grown wheat and in imported
wheat. J. Sci. Food Agric., 35: 579-583.
OTOKAWA, M., SHIBAHARA, Y., & EGASHIRA, Y. (1979) The inhibitory
effect of T-2 toxin on tolerance induction of delayed-type
hypersensitivity in mice. Jpn. J. med. Sci. Biol., 32(1): 37-46.
PACE, J.G., WATTS, M.R., BURROWS, E.P., DINTERMAN, R.E., MATSON, C.,
HAUER, E.C., & WANNEMACHER, R.W., Jr (1985) Fate and distribution of
3H labeled T-2 mycotoxin in guinea pigs. Toxicol. appl. Pharmacol.,
80: 377-385.
PANG, V.F., LAMBERT, R.J., FELSBURG, P.J., BEASLEY, V.R., BUCK, W.B,.
& HASCHEK (1987a) Experimental T-2 toxicosis in swine following
inhalation exposure: effects on pulmonary and systemic immunity and
morphologic changes. Toxicol. Pathol., 15: 308-319.
PANG, V.F., LORENZANA, R.M., BEASLEY, V.R., BUCK, W.B., & HASCHEK,
W.M. (1987b) Experimental T-2 toxicosis in swine. III. Morphologic
changes following intravascular administration of T-2 toxic. Fundam.
appl. Toxicol., 8: 298-309.
PANG, V.F., LAMBERT, R.J., FELSBURG, P.J., BEASLEY, V.R., BUCK, W.B.,
& HASCHEK, W.M. (1988) Experimental T-2 toxicosis in swine following
inhalation exposure: clinical signs and effects on hematology, serum
biochemistry and immune response. Fundam. appl. Toxicol., 11:
100-109.
PARKER, G.W., WANNEMACHER, R.W., Jr, & GILMAN, F.G. (1984) The effect
of T-2 mycotoxin on the cardiovascular system in the guinea pig. Fed.
Proc., 43: 578.
PATTERSON, D.S.P., MATTHEWS, J.G., SHREEVE, B.J., ROBERTS, B.A.,
MCDONALD, S.M., & HAYES, A.W. (1979) The failure of trichothecene
mycotoxins and whole cultures of Fusarium tricinctum to cause
experimental haemorrhagic syndromes in calves and pigs. Vet. Rec.,
105(11): 252-253.
PATTERSON, D.S.P., SHREEVE, B.J., ROBERTS, B.A., BERRETT, S., BRUSH,
P.J., GLANCY, E.M., & KROGH, P. (1981) Effect on calves of barley
naturally contaminated with ochratoxin A and groundnut meal
contaminated with low concentrations of aflatoxin B1. Res. vet. Sci.,
31: 213-218.
PAULSCH, W.E., VAN EGMOND, H.P., & SCHULLER, P.L. (1982) Thin-layer
chromatographic method for analysis and chemical confirmation of
ochratoxin A in kidneys of pigs. In: Mycotoxins and Phycotoxins,
Proceedings of the 5th International IUPAC Symposium, Vienna,
Technical University Vienna publ., pp. 40-43.
PAVLOVIC, M., PLESTINA, R., & KROGH, P. (1979) Ochratoxin A
contamination of foodstuffs in an area with Balkan (endemic)
nephropathy. Acta pathol. microbiol. Scand. B87: 243-246.
PAWLOSKY, R.J. & MIROCHA, C.J. (1984) Structure of a metabolic
derivative of T-2 toxin (TC-6) based on mass spectrometry. J. agric.
food Chem., 32: 1420-1423.
PECKHAM, J.C., DOUPNIK, B., & JONES, O.H. (1971) Acute toxicity of
ochratoxins A and B in chicks. Appl. Microbiol., 21(3): 492-494.
PEDERSEN, E. & HANSEN, H.N. (1981) [Ochratoxin A in grain and grain
products.] Copenhagen, Stat. Levnedsm. Institute (Report F8l002) (in
Danish).
PEPELJNJAK, S. & CVETNIC, Z. (198l) [Ochratoxogenicity of
Aspergillus ochraceus strains from the territory of endemic
nephropathy.] Vet. Arch., 51: 101-103 (in Croatian).
PEPELJNJAK, S., BLAZEVIC, N., & CULJAK, N. (1982) Histopathological
changes and finding of ochratoxin A in organs of pig in the area of
endemic nephropathy in Yugoslavia. In: Mycotoxins and Phycotoxins,
Proceedings of the 5th International IUPAC Symposium, Vienna,
Technical University Vienna publ., pp. 346-348.
PESTKA, J.J., LEE, S.C., LAU, H.P., & CHU, F.S. (1981) Enzyme-
linked immunosorbent assay for T-2 toxin. J. Assoc. Off. Anal. Chem.,
58(12): 940A-944A.
PESTKA, J.J., LIN, W.S., & MITHS, E.R. (1987a) Emetic activity of the
trichothecene 15-acetyldeoxynivaleol in swine. Food chem. Toxicol.,
25: 855-858.
PESTKA, J.J., TAI, J.H., WITT, W.F., DIXON, D.E., & FORSELL, J.H.
(1987b) Suppression of immune response in the B6C3F1 mouse after
dietary exposure to the Fusarium mycotoxins deoxynivalenol
(vomitoxin) and zearalenone. Food chem. Toxicol., 25: 297-304.
PETKOVA-BOCHAROVA, T. & CASTEGNARO, M. (1985) Ochratoxin A
contamination of cereals in an area of high incidence of Balkan
endemic nephropathy in Bulgaria. Food Addit. Contam., 2: 267-270.
PETKOVA-BOCHAROVA, T., CHERNOZEMSKY, I.N., & CASTEGNARO, M. (1988)
Ochratoxin A in human blood in relation to Balkan endemic nephropathy
and urinary system tumours in Bulgaria. Food Addit. Contam., 5:
299, 301.
PIENAAR, J.G., KELLERMAN, T.S., & MARASAS, W.F.O. (1981) Field
outbreaks of leukoencephalomalacia in horses consuming maize infected
by Fusarium verticillioides (F. moniliforme) in South Africa. J. S.
Afr. Vet. Assoc., 52: 21-24.
PIER, A.C., CYSEWSKI, S.J., RICHARD, J.L., BAETZ, A.L., & MITCHELL,
L. (1976) Experimental mycotoxicoses in calves with aflatoxin,
ochratoxin, rubratoxin, and T-2 toxin. In: Proceedings of the 80th
Annual Meeting of the US Animal Health Association, Miami Beach,
Florida, 7-12 November, 1976, Richmond, Virginia, US Animal Health
Association, pp. 130-148.
POHLAND, A.E., SCHULLER, P.L., STEYN, P.S., & VAN EGMOND, H.P.
(1982) Physicochemical data for some selected mycotoxins. Pure appl.
Chem., 54: 2219-2284.
POHLAND, A.E., THORPE, C., TRUCKSESS, W., & EPPLEY, R. (1986) TLC and
HPLC methods for analysis of trichothecenes in commodities. In:
Richard, J. & Thurston, J., ed. Diagnosis of mycotoxicoses,
Dordrecht, Boston, Martinus Nijhoff Publishers, pp. 271-281.
POHLAND, A.E. & WOOD, G.E. (1987) Occurrence of mycotoxins in food.
In: Krogh, P., ed. Mycotoxins in foods, London, Academic Press, pp.
35-64.
POKROVSKIJ, V.I. & TUTELYAN, V.A. (1982) [Ergotism: a companion of
natural disaster.] Ter. Ark., 108-110 (in Russian).
POPPE, S.M., STUCKHARDT, J.L., & SZCZECH, G.M. (1983) Postnatal
behavioural effects of ochratoxin A in offspring of treated mice.
Teratology, 27: 293-300.
PRELUSKY, D.B., TRENHOLM, H.L., LAWRENCE, G.A., & SCOTT, P.M. (1984)
Nontransmission of deoxynivalenol (vomitoxin) to milk following oral
administration to dairy cows. J. environ. Sci. Health, B19(7): 593-
609.
PRELUSKY, D.B., VEIRA, D.M., & TRENHOLM, H.L. (1985) Plasma
pharmacokinetics of the mycotoxin deoxynivalenol following oral and
intravenous administration to sheep. J. environ. Sci. Health, B20:
603-624.
PRELUSKY, D.B., TRENHOLM, H.L., HAMILTON, R.M.G., & MILLER, J.D.
(1987) Transmission of [14C]deoxynivalenol to eggs following oral
administration to laying hens. J. agric. food Chem., 35: 182-186.
PRIOR, M.G. (1976) Mycotoxin in determinations on animal feedstuffs
and tissues in Western Canada. Can. J. comp. Med., 40: 75-79.
PRIOR, M.G. (198l) Mycotoxins in animal feedstuffs and tissues in
Western Canada 1975 to 1976. Can. J. comp. Med., 45: 116-119.
PRIOR, M.G. & SISODIA, C.S. (1978) Ochratoxicosis in white leghorn
hens. Poult. Sci., 57: 619-623.
PRIOR, M.G. & SISODIA, C.S. (1982) The effects of ochratoxin A on
the immune response of Swiss mice. Can. J. comp. Med., 46: 91-96.
PRIOR, M.G., SISODIA, C.S., & O'NEIL, J.B. (1976) Acute oral
ochratoxicosis in day-old white leghorns, turkeys and Japanese quail.
Poult. Sci., 55: 786-790.
PUCHLEV, A. (1973) La nephropathie endemique en Bulgarie. In:
Symposium sur la nephropathie endemique de l'Academie serbe des
sciences et des arts, Belgrade, pp. 15-27.
PUCHLEV, A., ed. (1974) Endemic nephropathy. In: Proceedings of the
2nd International Symposium on Endemic Nephropathy, Sofia, Bulgarian
Academy of Sciences, pp. 1-343.
PULS, R. & GREENWAY, J.A. (1976) Fusariotoxicosis from barley in
British Columbia. II. Analysis of suspected barley. Can. J. comp.
Med., 40(1): 16-19.
PURCHASE, I.F.H. & THERON, J.J. (1968) The acute toxicity of
ochratoxin A to rats. Food Cosmet. Toxicol., 6: 479-483.
QIUJIE, X., XIAOQIU, L., JIANLI, W., & YUNSIAN, L. (1988)
Trichothecenes in staple food from high incidence area of carcinoma
of esophagus and gastric cardia and their carcinogenic potential.
Zhonghua Zhongliu Zoshi, 10: 4-8.
RABIE, C.J., SYDENHAM, E.W., THEL, P.G., LUBBEN, A., & MARASAS,
W.F.O. (1986) T-2 toxin production by Fusarium acuminatum isolated
from oats and barley. Appl. environ. Microbiol., 52(3): 594-596.
RAFAI, P. & TUBOLY, S. (1982) Effect of T-2 toxin on adrenocortical
function and immune response in growing pigs. Zbl. Vet. Med., B29:
558-565.
RAJAKYLA, E., LAASASENAHO, K., & SAKKERS, P.J.D. (1987) Determination
of mycotoxins in grain by high-performance liquid chromatography and
thermospray liquid chromatography-mass spectrometry. J. Chromatogr.,
384: 391-402.
REICHMANN, K.G., BLANEY, B.J., CONNOR, J.K., & RUNGE, B.M. (1982)
The significance of aflatoxin and ochratoxin in the diet of
Australian chickens. Aust. vet. J., 58: 211-212.
REISS, J. (1973) Influence of the mycotoxins patulin and
diacetoxyscirpenol on fungi. J. gen. appl. Microbiol., 19(6):
415-420.
REISS, J. (1975) Mycotoxin poisoning of Allium cepa root tips. Part
2. Reduction of mitotic index and formation of chromosomal
aberrations and cytological abnormalities by patulin, rubratoxin B
and diacetoxyscirpenol. Cytologia, 39(4): 703-708.
REISS, J. (1977) Inhibition of urease by the mycotoxin patulin.
Naturwissenschaften, 64: 97.
RIBELIN, W.E., FUKUSHIMA, K., & STILL, P.E. (1978) The toxicity of
ochratoxin to ruminants. Can. J. comp. Med., 42: 172-176.
ROBISON, T.S., MIROCHA, C.J., KURTZ, H.J., BEHRENS, J.C., CHI, M.S.,
WEAVER, G.A., & NYSTROM, S.D. (1979a) Transmission of T-2 toxin
into bovine and porcine milk. J. dairy Sci., 62(4): 637-641.
ROBISON, T.S., MIROCHA, C.J., KURTZ, H.J., BEHRENS, J.C., WEAVER,
G.A., & CHI, M.S. (1979b) Distribution of tritium labeled T-2 toxin
in swine. J. agric. food Chem., 27(6): 1411-1413.
ROGERS, C.G. & HEROUX-METCALF, C. (1983) Cytotoxicity and absence of
mutagenic activity of vomitoxin (4-deoxynivalenol) in a hepatocyte-
mediated assay with V79 Chinese hamster lung cells. Cancer Lett.,
20: 29-35.
ROMER, T.R. (1986) Use of small charcoal/alumina cleanup columns in
determination of trichothecene mycotoxins in foods and feeds. J.
Assoc. Off. Anal. Chem., 69: 699-703.
ROMER, T.R., BOLING, T.M., & MACDONALD, J.L. (1978). Gas-liquid
chromatographic determination of T-2 toxin and diacetoxyscirpenol in
corn and mixed feeds. J. Assoc. Off. Anal. Chem., 61(4): 801-808.
ROOD, H.D., BUCK, W.B., & SWANSON, S.P. (1988a) Gas chromatographic
screening method for T-2 toxin, diacetoxyscirpenol, deoxynivalenol
and related trichothecenes in feeds. J. Assoc. Off. Anal. Chem., 71:
493-498.
ROOD, H.D., BUCK, W.B., & SWANSON, S.P. (1988b) Diagnostic screening
method for determination of trichothecene exposure in animals. J.
agric. food Chem., 36: 74-79.
ROSEN, J.D., ROSEN, R.T., & HARTMAN, T.G. (1986) Capillary gas
chromatography-mass spectrometry of several macrocyclic
trichothecenes. J. Chromatogr., 355: 241-251.
ROSEN, R.T. & ROSEN, J.D. (1984) Quantification and confirmation of
four Fusarium mycotoxins in corn by gas chromatography-mass
spectrometry. J. Chromatogr., 283: 223-230.
ROSENSTEIN, Y. & LAFARGE-FRAYSSINET, C. (1983) Inhibitory effect of
Fusarium T-2 toxin on lymphoid DNA and protein synthesis. Toxicol.
appl. Pharmacol., 70: 283-288.
ROSENSTEIN, Y., LAFARGE-FRAYSSINET, C., LESPINATS, G., LOISILLIER,
F., LAFONT, P., & FRAYSSINET, C. (1979) Immunosuppressive activity
of Fusarium toxins. Effects on antibody synthesis and skin grafts of
crude extracts of T-2 toxin and diacetoxyscirpenol. Immunology,
36(1): 111-118.
ROUSSEAUX, C.G. & SHEIFER, H.B. (1987) Maternal toxicity,
embryolethality and abnormal fetal development in CD-1 mice following
one oral dose of T-2 toxin. J. appl. Toxicol., 7: 281-288.
ROUSSEAUX, C.G., SHIEFER, H.B., & HANCOCK, D.S. (1986) Reproductive
and teratological effects of continuous low-level dietary T-2 toxin
in female CD-1 mice for two generations. J. appl. Toxicol., 6:
179-184.
ROUSSEAU, D.M. & VAN PETEGHEM, C.H. (1989) Spontaneous occurrence of
ochratoxin A residues in porcine kidneys in Belgium. Environ. Contam.
Toxicol., 42: 181-186.
ROUSSEAU, D.M., CANDLISH, A.A.G., SLEGERS, G.A., VAN PETEGHEM, C.H.,
STINSON, W.H., & SMITH, J.E. (1987) Detection of ochratoxin A in
porcine kidneys by a monoclonal antibody-based radioimmunoassay.
Appl. environ. Microbiol., 53: 514-518.
RUKMINI, C. & BHAT, R.V. (1978) Occurrence of T-2 toxin in Fusarium
incarnatum infested sorghum from India. J. agric. food Chem., 26
(3): 647-649.
RUKMINI, C., PRASAD, J.S., & RAO, K. (1980) Effect of feeding T-2
toxin to rats and monkeys. Food Cosmet. Toxicol., 18(3): 267-270.
RUSCH, M.E. & STAHELIN, H. (1965) [Some biological effects of the
cytostatic agent verrucarin A.] Arzneimittelforschung, 15: 893-897
(in German).
RUTQVIST, L., BJORKLUND, N.-E., HULT, K., & GATENBECK, S. (1977)
Spontaneous occurrence of ochratoxin residues in kidneys of fattening
pigs. Zbl. Veterinaermed., A24(5): 402-408.
RUTQVIST, L., BJORKLUND, N.-E., HULT, K., HOKBY, E., & CARLSSON, B.
(1978) Ochratoxin A as the cause of spontaneous nephropathy in
fattening pigs. Appl. environ. Microbiol., 36: 920-925.
RYU, J.C., SHIRAKI, N., & UENO, Y. (1987) Effects of drugs and
metabolic inhibitions on the acute toxicity of T-2 toxin in mice.
Toxicon, 25: 743-750.
RYU, J.-C., OHTSUBO, K., IZUMIYAMA, N., NAKAMURA, K., TANAKA, T.,
YAMAMURA, H., & UENO, Y. (1988) The acute and chronic toxicities of
nivalenol in mice. Fundam. appl. Toxicol., 11: 38-47.
SAITO, M. & OHTSUBO, K. (1974) Trichothecene toxins of Fusarium
species. In: Purchase, I.F.H., ed. Mycotoxins, Amsterdam, Oxford, New
York, Elsevier Science Publishers, pp. 263-281.
SAITO, M., HORIUCHI, T., OHTSUBO, K., HATANAKA, Y., & UENO, Y.
(1980) Low tumor incidence in rats with long-term feeding of
fusarenon-X a cytotoxic trichothecene produced by Fusarium nivale.
Jpn. J. exp. Med., 50(40): 293-302.
SAKAMOTO, T., SWANSON, S.P., YOSHIZAWA, T., & BUCK, W.B. (1986)
Structures of new metabolites of diacetoxyscirpenol in the excreta of
orally administered rats. J. agric. food Chem., 34: 698-701.
SANDOR, G., GLAVITS, R., VAJDA, L., VANYI, A., & KROGH, P. (1982)
Epidemiological study of ochratoxin A-associated porcine nephropathy
in Hungary. In: Mycotoxins and Phycotoxins, Proceedings of the 5th
International IUPAC Symposium, Technical University Vienna publ.,
Vienna, pp. 349-352.
SANO, A., ASABE, Y., TAKITANI, S., & UENO, Y. (1982).
Fluorodensitometric determination of trichothecene mycotoxins with
nicotinamide and 2-acetylpyridine on a silica gel layer. J.
Chromatogr., 235: 257-265.
SANO, A., MATSUTANI, S., SUZUKI, M., & TAKITANI, S. (1987) High-
performance liquid chromatographic method for determining
trichothecene mycotoxins by post-column fluorescence derivatization.
J. Chromatogr., 410: 427-436.
SANSING, G.A., LILLEHOJ, E.B., DETROY, R.W., & MILLER, M.A. (1976)
Synergistic toxic effects of citrinin, ochratoxin A, and penicillic
acid in mice. Toxicon, 14: 213-219.
SARKISOV, A.Ch., KVASCHINA, E.S., KORNEEV, N.E., KOROLEVA, V.P.,
GERASIMOVA, P.N., & AKILOVA, N.S. (1944) [Harmfulness of cereals
over-wintered in the field.] Veterinariya, 11-12: 39-41. (in
Russian).
SATO, N., UENO, Y., & ENOMOTO, M. (1975) Toxicological approaches
to the toxic metabolites of Fusaria. Part 8. Acute and subacute
toxicities of T-2 toxin in cats. Jpn. J. Pharmacol., 25(3): 263-270.
SATO, N. & UENO, Y. (1977) Comparative toxicities of
trichothecenes. In: Rodricks, J.V., Hesseltine, C.W., & Mehlman,
M.A., ed. Mycotoxins in human and animal health, Park Forest South,
Illinois, Pathotox Publishers, pp. 295-307.
SATO, N., ITO, T., KUMADA, H., UENO, Y., ASANO, K., SAITO, M.,
OHTSUBO, K., UENO, I., & HATANAKA, Y. (1978) Toxicological
approaches to the metabolites of Fusaria. XIII. Hematological changes
in mice by a single and repeated administrations of trichothecenes.
J. toxicol. Sci., 3(4): 335-356.
SATO, S., OHYA, T., HOMMA, S., & TATSUNO, T. (1981) [Effects of
fusarenon-X on serological responses of chicks inoculated with
newcastle disease vaccines.] Proc. Jpn. Assoc. Mycotoxicol., 13:
43-45 (in Japanese).
SCHIEFER, H.B., ROUSSEAUX, C.G., HANCOCK, D.S., & BLAKLEY, B.R.
(1987) Effects of low-level long-term oral exposure to T-2 toxin in
CD-1 mice. Food chem. Toxicol., 25: 593-601.
SCHMIDT, R. & DOSE, K. (1984) HPLC: A tool for the analysis of T-2
toxin and HT-2 toxin in cereals. J. anal. Toxicol., 8: 43-45.
SCHINDLER, D. (1974) Two classes of inhibitors of peptidyl
transferease activity in eukaryotes. Nature (Lond.), 249(5452):
38-41.
SCHINDLER, D., GRANT, P., & DAVIES, J. (1974) Trichodermin
resistance mutation affecting eukaryotic ribosomes. Nature (Lond.),
248(5448): 535-536.
SCOTT, P.M. (1977) Penicillium mycotoxins. In: Wyllie, T.D. &
Morehouse, L.G., ed. Mycotoxic fungi, mycotoxins, mycotoxicoses, New
York, Marcel Dekker, pp. 283-356.
SCOTT, P.M. (1982) Assessment of quantitative methods for
determination of trichothecenes in grains and grain products. J.
Assoc. Off. Anal. Chem., 65(4): 876-883.
SCOTT, P.M. (1984) Effects of food processing on mycotoxins. J. food
Prot., 47(6): 489-499.
SCOTT, P.M. & KANHERE, S.R. (1986) Comparison of column phases for
separation of derivatized trichothecenes by capillary gas
chromatography. J. Chromatogr., 368: 374-380.
SCOTT, P.M. & LAWRENCE, G.A. (1980) Analysis of ergot alkaloids in
flour. J. agric. food Chem., 28: 1258-1261.
SCOTT, P.M. & LAWRENCE, G.A. (1982) Losses of ergot alkaloids
during making of bread and pancakes. J. agric. food Chem., 30:
445-450.
SCOTT, P.M., LAWRENCE, J.W., & VAN WALBEEK, W. (1970) Detection of
mycotoxins by thin-layer chromatography: application to screening of
fungal extracts. Appl. Microbiol., 20(5): 839-842.
SCOTT, P.M., WALBEEK, W., VAN, KENNEDY, B., & ANYETI, D. (1972)
Mycotoxins (ochratoxin A, citrinin, and sterigmatocystin) and
toxigenic fungi in grains and other agricultural products. J. agric.
food Chem., 20: 1103-1109.
SCOTT, P.M., HARWIG, J., & BLANCHFIELD, B.J. (1980) Screening
Fusarium strains isolated from over-wintered Canadian grains for
trichothecenes. Mycopathologia, 72(3): 175-180.
SCOTT, P.M., LAU, P.Y., & KANHERE, S.R. (1981) Gas chromatography
with electron capture and mass spectrometric detection of
deoxynivalenol in wheat and other grains. J. Assoc. Off. Anal. Chem.,
64(6): 1364-1371.
SCOTT, P.M., KANHERE, S.R., LAU, P.-Y., DEXTER, J.E., & GREENHALGH,
R. (1983) Effects of experimental flour milling and bread baking on
retention of deoxynivalenol (vomitoxin) in hard red spring wheat.
Cereal Chem., 60: 421-424.
SCOTT, P.M., NELSON, K., KANHERE, S.R., KARPINSKI, K.F., HAYWARD, S.,
NIESH, G.A., & TEICH, A.H. (1984a) Decline in deoxynivalenol
(vomitoxin) concentrations in 1983 Ontario winter wheat before
harvest. Appl. environ. Microbiol., 48(6): 884-886.
SCOTT, P.M., KANHERE, S.R., DEXTER, J.E., BRENNAN, P.W., & TRENHOLM,
H.L. (1984b) Distribution of the trichothecene mycotoxin
deoxynivalenol (Vomitoxin) during the milling of naturally
contaminated hard red spring wheat and its fate in baked products.
Food Addit. Contam., 1(4): 313-323.
SCOTT, P.M., KANHERE, S.R., & TARTER, E.J. (1986) Determination of
nivalenol and deoxynivalenol in cereals by electron-capture gas
chromatography. J. Assoc. Off. Anal. Chem., 69: 889-893.
SEITZ, L.M., YAMAZAKI, W.T., CLEMENTS, R.L., MOHR, H.E., & ANDREWS,
L. (1985) Distribution of deoxynivalenol in soft wheat mill
streams. Cereal Chem., 62(6): 467-469.
SHEPHERD, M.J. & GILBERT, J. (1988) Long-term stability of
deoxynivalenol standard reference solutions. J. agric. food Chem.,
36: 305-308.
SHIMIZU, T., NAKANO, N., MATSUI, T., & AIBARA, K. (1979)
Hypoglycemia in mice administered fusarenon-X. Jpn. J. med. Sci.
Biol., 32(4): 189-198.
SHOTWELL, O.L., HESSELTINE, C.W., & GOULDEN, M.L. (1969) Ochratoxin
A: occurrence as natural contaminant of a corn sample. Appl.
Microbiol., 17(5): 765-766.
SHOTWELL, O.L., HESSELTINE, C.W., VANDEGRAFT, E.E., & GOULDEN, M.L.
(1971) Survey of corn from different regions for aflatoxin,
ochratoxin, and zearalenone. Cereal Sci. Today, 16(9): 266-273.
SHOTWELL, O.L., GOULDEN, M.L., & HESSELTINE, C.W. (1976) Survey of
US wheat for ochratoxin and aflatoxin. J. Assoc. Off. Anal. Chem.,
59: 122-124.
SHOTWELL, O.L., NENNETT, G.A., STUBBLEFIELD, R.D., SHANNON, G.M.,
KWOLEK, W.F., & PLATTNER, R.D. (1985) Deoxynivalenol in hard red
winter wheat: Relationship between toxin levels and factors that
could be used in grading. J. Assoc. Off. Anal. Chem., 68: 954-957.
SHREEVE, B.J., PATTERSON, D.S.P., & ROBERTS, B.A. (1979) The carry-
over of aflatoxin, ochratoxin and zearalenone from naturally
contaminated feed to tissues, urine and milk of dairy cows. Food
Cosmet. Toxicol., 17: 151-152.
SIDDIQUI, M.R. & KHAN, I.D. (1973) Renaming Claviceps microcephala
ergot fungus on Pennisetum typhoides in India as Claviceps
fusiformis. Trans. Mycol. Soc. Jpn, 144: 195-198.
SIEGFRIED, R. (1977) [ Fusarium toxin (trichothecene toxin) in feed
corn.] Landwirtsch. Forsch., Sonderh., 34(1): 37-43 (in German).
SIGG, H.P., MAULI, R., FLURY, E., & HANSER, D. (1965) The
constitution of diacetoxyscirpenol. Helv. Chim. Acta, 48: 962-988.
SINTOV, A., BIALER, M., & YAGEN, B. (1986) Pharmacokinetics of T-2
toxin and its metabolite HT-2 toxin, after intravenous administration
in dogs. Drug Metab. Disp., 14: 250-254.
SINTOV, A., BIALER, M., & YAGEN, B. (1987) Pharmacokinetics of T-2
tetraol, a urinary metabolite of the trichothecene mycotoxin, T-2
toxin, in dogs. Xenobiotica, 17: 941-950.
SIRAJ, M.Y., PHILLIPS, T.D., & HAYES, A.W. (1981) Effects of the
mycotoxins citrinin and ochratoxin A on hepatic mixed-function
oxidase and adenosinetriphosphatase in neonatal rats. J. Toxicol.
environ. Health, 8: 131-140.
SIREN, A.L. & FEUERSTEIN, G. (1986) Effect of T-2 toxin on regional
blood flow and vascular resistance in the conscious rat. Toxic. appl.
Pharmacol., 38: 438-444.
SIRIWARDANA, T.M.G. & LAFONT, P. (1978) New sensitive biological
assay for 12,13-epoxytrichothecenes. Appl. environ. Microbiol.,
35(1): 206-207.
SMALLEY, E.B., MARASAS, W.F.O., STRONG, F.M., BAMBURG, J.R., NICHOLS,
R.E., & KOSARI, N.R. (1970) Mycotoxicosis associated with moldy corn.
In: Herzberg, M., ed. Proceedings of the first US-Japan Conference on
Toxic Micro-organisms, Mycotoxins, Botulism, Honolulu, Hawaii, 7-10
October, 1968, Washington, DC, US Department of the Interior and UJNR
Joint Panels on Toxic Micro-organisms, pp. 163-173.
SMITH, R.D., UDSETH, H.R., & WRIGHT, B.W. (1985) Rapid and high
resolution capillary fluid chromatography (SFC) and SFC/MS of
trichothecene mycotoxins. J. chromatogr. Sci., 23: 192-199.
SORSA, M., LINSSAINMAA, K., PAITTELA, M., & ILUS, T. (1980)
Evaluation of the mutagenicity of epoxytrichothecene mycotoxins in
Drosophilia melanogaste. Hereditas, 92: 163-165.
SPEERS, G.M., MIROCHA, C.J., CHRISTENSEN, C.M., & BEHRENS, J.C.
(1977) Effects on laying hens of feeding corn invaded by 2 species
of Fusarium and pure T-2 mycotoxin. Poult. Sci., 56(1): 98-102.
STACK, M.E., MISLIVEC, P.B., GIBSON, R., POHLAND, A.E., & DENIZEL, T.
(1982) Mycotoxins produced by isolates of Aspergillus ochraceus
found in coffee. In: Mycotoxins and Phycotoxins, Proceedings of the
5th International IUPAC Symposium, Vienna, Technical University
Vienna, pp. 195-199.
STAFFORD, M.E. & MCLAUGHLIN, C.S. (1973) Trichodermin, a possible
inhibitor of the termination process of protein synthesis. J. cell.
Physiol., 82: 121-128.
STAHELIN, H., KALBERER-RUESCH, M.E., SIGNER, E., & LAZARY, S. (1968)
[Some biological effects of the cytostatic diacetoxyscirpenol.]
Arzneimittelforschung, 18: 989-994 (in German).
STAHL, C., VANDERHOEF, L.N., SIEGEL, N., & HELGESON, J.P. (1973)
Fusarium tricinctum T-2 toxin inhibits auxin promoted elongation in
soybean hypocotyl. Plant Physiol., 52(6): 663-666.
STAHR, H.M., HYDE, W., LEDERDAL, D., & PFEIFFER, R. (1981)
Trichothecene mycotoxin analysis for veterinary diagnostic
toxicology. Abstracts 95th Annual Meeting of AOAC., 191: 65.
STANFORD, G.K., HOOD, R.D., & HAYES, A.W. (1975) Effect of prenatal
administration of T-2 toxin to mice. Res. Commun. chem. Pathol.
Pharmacol., 10(4): 743-746.
STEYN, P.S. (1977) Mycotoxins, excluding aflatoxin, zearalenone and
the trichothecenes. In: Rodericks, J.V., Hesseltine, C.W., &
Mehlman, M.A., ed. Mycotoxins in human and animal health, Park Forest
South, Illinois, Pathotox Publishers, pp. 419-467.
STEYN, P.S. (1984) Ochratoxins and related dihydro-iso-coumarins.
In: Betina, V., ed. Mycotoxins: production, isolation, separation,
and purification, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 183-216.
STEYN, P.S., VLEGGAAR, R., DU PREEZ, N.P., BLYTH, A.A., & SEEGERS,
J.C. (1975) The in vitro toxicity of analogs of ochratoxin A in
monkey kidney epithelial cells. Toxicol. appl. Pharmacol., 32:
198-203.
STOREN, O., HOLM, H., & STORMER, F.C. (1982) Metabolism of
ochratoxin A by rats. Appl. environ. Microbiol., 44: 785-789.
STORMER, F.C. & PEDERSEN, J. (1980) Formation of 4-
hydroxyochratoxin A from ochratoxin A by rat liver microsomes.
Appl. environ. Microbiol., 39: 971-975.
STORMER, F.C., HANSEN, C.E., PEDERSEN, J.I., HVISTENDAHL, G., &
AASEN, A.J. (1981) Formation of (4R)- and (AS)-4-hydroxyochratoxin
A from ochratoxin A by liver microsomes from various species. Appl.
environ. Microbiol., 42: 1051-1056.
STOYANOV, I.S., CHERNOZEMSKY, I.N., NICOLOV, I.G., STOICHEV, I.I., &
PETKOVA-BOCHAROVA, T.K. (1978) Epidemiologic association between
endemic nephropathy and urinary system tumours in an endemic region.
J. chron. Dis., 31: 721-724.
SUNDHEIM, L., NAGAYAMA, S., KAWAMURA, O., TANAKA, T., BRODAL, G., &
UENO, Y. (1988) Trichothecenes and zearalenone in Norwegian barley
and wheat. Norw. J. agric. Sci., 2: 49-59.
SUZUKI, S. & SATOH, T. (1973) Effect of ochratoxin A on tissue
glycogen levels in rats. Jpn. J. Phamacol., 23: 415-419.
SUZUKI, S., SATOH, T., & YAMAZAKI, M. (1975) Effect of ochratoxin A
on carbohydrate metabolism in rat liver. Toxicol. appl. Pharmacol.,
32(1): 116-122.
SUZUKI, S., SATOH, T., & YAMAZAKI, M. (1977) The pharmacokinetics
of ochratoxin A in rats. Jpn. J. Pharmacol., 27: 735-744.
SUZUKI, T., KURISU, M., HOSHINO, Y., ICHINOE, M., NOSE, N., TOKUMARU,
Y., & WATANABE, Y. (1980) [Production of trichothecene mycotoxins of
Fusarium species in wheat and barley harvested in Saitama Prefecture,
Japan.] J. Food Hyg. Soc. Jpn, 21(1): 43-49 (in Japanese).
SWANSON, S.P., NICOLETTI, J., ROOD,.H.D., Jr, BUCK, W.B., COTE, L.M.,
& YOSHIZAWA, T. (1987) Metabolism of three trichothecene mycotoxins,
T-2 toxin, diacetoxyscirpenol and deoxynivalenol, by bovine rumen
microorganisms. J. agric. food Chem., 32: 1181-1183.
SWANSON, S.P., RAMASWAMY, V., BEASLEY, V.R., BUCK, W.B., &
BURMEISTER, H.H. (1983) Gas-liquid chromatographic determination of
T-2 toxin in plasma. J. Assoc. Off. Anal. Chem., 66: 909.912.
SWANSON, S.P., DAHLEM, A.M., ROOD, H.D., COTE, L.M., & YOSHIZAWA, T.
(1986) Gas chromatographic analysis of milk for deoxynivalenol and
its metabolite DOM-1. J. Assoc. Off. Anal. Chem., 69: 41-43.
SYLVIA, V.L., PHILLIPS, T.D., CLEMINT, B.A., GREEN, J.L., KUBENA,
L.F., & HEIDELBAUGH, N.D. (1986) Determination of deoxynivalenol
(vomitoxin) by high performance liquid chromatography with
electrochemical detection. J. Chromatogr., 362: 79-85.
SZATHMARY, C.I. (1983) Trichothecene toxicoses and natural
occurrence in Hungary. In: Ueno, Y., ed. Developments in food
science. IV. Trichothecenes, New York, Elsevier, pp. 229-250.
SZATHMARY, C.I. & RAFAI, P. (1978) Refusal of food contaminated
with T-2 fusariotoxin. Magy. Allatorv. Lapja, 33: 685-688.
SZCZECH, G.M. & HOOD, R.D. (1981) Brain necrosis in mouse fetuses
transplacentally exposed to the mycotoxin ochratoxin A. Toxicol.
appl. Pharmacol., 57: 127-137.
SZCZECH, G.M., CARLTON, W.W., & TUITE, J. (1973a) Ochratoxicosis in
Beagle dogs. I. Clinical and clinicopathological features. Vet.
Pathol., 10(2): 135-154.
SZCZECH, G.M., CARLTON, W.W., & TUITE, J. (1973b) Ochratoxicosis in
Beagle dogs. II. Pathology. Vet. Pathol., 10(3): 219-231.
SZCZECH, G.M., CARLTON, W.W., TUITE, J., & CALDWELL, R. (1973c)
Ochratoxin A toxicosis in swine. Vet. Pathol., 10(4): 347-364.
SZEBIOTKO, K., CHELKOWSKI, J., DOPIERALA, G., GODLEWSKA, B., &
RADOMYSKA, W. (1981) Mycotoxins in cereal grain. Part I.
Ochratoxin, critrinin, sterigmatocystin, penicillic acid and
toxigenic fungi in cereal grain. Nahrung, 25: 415-421.
TAKITANI, S., ASABE, Y., KATO, T., SUZUKI, M., & UENO, Y. (1979)
Spectrodensitometric determination of trichothecene mycotoxins with
4-( p-nitrobenzyl)pyridine on silica gel thin-layer chromatograms. J.
Chromatogr., 172: 335-342.
TAMM, C. (1977) Chemistry and biosynthesis of trichothecenes. In:
Rodricks, J.V., Hesseltine, C.W., & Mehlman, M.A., ed. Mycotoxins in
human and animal health, Park Forest South, Illinois, Pathotox
Publishers, pp. 209-228.
TANAKA, T., HASEGAWA, A., MATSUKI, Y., ISHII, K., & UENO, Y. (1985a)
Improved methodology for the simultaneous detection of the
trichothecene mycotoxins deoxynivalenol and nivalenol in cereals.
Food Addit. Contam., 2: 125-137.
TANAKA, T., HASEGAWA, A., MATSUKI, Y., & UENO, Y. (1985b) A survey
of the occurrence of nivalenol, deoxynivalenol and zearalenone in
foodstuffs and health foods in Japan. Food Addit. Contam., 2: 259-
265.
TANAKA, T., HASEGAWA, A., YAMAMOTO, S., LEE, U., SUGIURA, Y., & UENO,
Y. (1988) Worldwide contamination of cereals by the Fusarium
mycotoxins nivalenol, deoxynivalenol and zearalenone. 1. Survey of 19
countries. J. agric. food Chem., 36: 979-983.
TANAKA, T., HASEGAWA, A., YAMAMOTO, S., MATSUKI, Y., & UENO, Y.
(1986) Residues of Fusarium mycotoxins, nivalenol, deoxynivalenol
and zearalenone, in wheat and processed food after milling and
baking. J. Food Hyg. Soc. Jpn, 27(6): 653-655.
TANAKA, T., MATSUDA, Y., TOYASAKI, N., OGAWA, K., MATSUKI, Y., &
UENO, Y. (1977) Screening of trichothecene-producing Fusarium
species from river sediments by mammalian cell culture techniques.
Proc. Jpn. Assoc. Mycotoxicol., 5/6: 50-53.
TASHIRO, F., HIRAI, K., & UENO, Y. (1979) Inhibitory effects of
carcinogenic mycotoxins on deoxyribonucleic acid-dependent
ribonucleic acid polymerase and ribonulease H. Appl. environ.
Microbiol., 38(2): 191-196.
TATSUNO, T., SAITO, M., ENOMOTO, M., & TSUNODA, H. (1968) Nivalenol,
a toxic principle of Fusarium nivale. Chem. pharm. Bull., 16(12):
2519-2520.
TEICH, A.H. & HAMILTON, J.R. (1985) Effect of cultural practices,
soil phosphorus, potassium and pH on the incidence of Fusarium head
blight and deoxynivalenol levels in wheat. Appl. environ. Microbiol.,
49(6): 1429-1431.
TERAO, K., KERA, K., & YAZIMA, T. (1978) The effects of
trichothecene toxins on the bursa of Fabricius in day-old chicks.
Virchows Arch., B27(4): 359-370.
THACKER, H.L. & CARLTON, W.W. (1977) Ochratoxin A mycotoxicosis in
the guinea pig. Food. Cosmet. Toxicol., 15: 563-574.
THIEL, P.G., MEYER, C.J., & MARASAS, W.F.O. (1982) Natural
occurrence of moniliformin together with deoxynivalenol and
zearalenone in Transkeian corn. J. agric. food Chem., 30(2): 308-312.
THIGPEN, J.T., VAUGHN, C., & STUCKEY, W.J. (1981) Phase II trial of
anguidine in patients with sarcomas unresponsive to prior
chemotherapy: A southwest oncology group study. Cancer Treat. Rep.,
65(9-10): 881-882.
THUST, R., KNEIST, S., & HUEHNE, V. (1983) [Genotoxicity of
Fusarium mycotoxins (nivalenol, T-2 toxin and zearalenone) in Chinese
hamster V79-E cells in vitro.] Arch. Geschwulstforsch., 53: 9-15
(in German).
TIEBACH, R., BLAAS, W., KELLERT, M., STEINMEYER, S., & WEBER, R.
(1985) Confirmation of nivalenol and deoxynivalenol by on-line liquid
chromatography-mass spectrometry and gas-chromatography. Comparison
of methods. J. Chromatogr., 318: 103-111.
TOCHINAI, Y. (1933) [On scab of cereals and grains. I and II.]
Bochugai Zassi, 20: 106-114, 175-188 (in Japanese).
TOMAR, R.S., BLAKLEY, B.R., & COTEAU, W.E. (1988) In vitro effects of
T-2 toxin of the mitogen responsiveness and antibody-producing
ability of human lymphocytes. Toxicol. Lett., 40: 109-117.
TRENHOLM, H.L., COCHRANE, W.P., COHEN, H., ELLIOT, J.I., FARNWORTH,
E.R., FRIEND, D.W., HAMILTON, R.M.H., NEISH, G.A., & STANDISH, J.F.
(1981) Survey of vomitoxin contamination of the 1980 white winter
wheat crop in Ontario, Canada. J. Am. Oil Chem. Soc., 58: 992-994.
TRENHOLM, H.L., HAMILTON, R.M.G., FRIEND, D.W., THOMPSON, B.K., &
HARTIN, K.E. (1984) Feeding trials with vomitoxin (deoxynivalenol)-
contaminated wheat: effects on swine, poultry and dairy cattle. J.
Am. Vet. Med. Assoc., 185: 527-531.
TRENHOLM, H.L., WARNER, R.M., & PRELUSKY, D.B. (1985) Assessment of
extraction procedures in the analysis of naturally contaminated grain
products for deoxynivalenol (vomitoxin). J. Assoc. Off. Anal. Chem.,
68: 645-659.
TRUCKSESS, M.W., NESHEIM, S., & EPPLEY, R.M. (1984) Thin layer
chromatographic determination of deoxynivalenol in wheat and corn. J.
Assoc. Off. Anal. Chem., 67: 40-43.
TRUCKSESS, M.W., FLOOD, M.W., & PAGE, S.W. (1986a) Thin layer
chromatographic determination of deoxynivalenol in processed grain
products. J. Assoc. Off. Anal. Chem., 69: 35-36.
TRUCKSESS, M.W., WOOD, G.E., EPPLEY, R.M., YOUNG, K., COHEN, C.K.,
PAGE, S.W., & POHLAND, A.E. (1986b) Occurrence of deoxynivalenol in
grain products. In: Biodeterioration. VI, Aberystwyth, The Cambrian
News Ltd., pp. 243-247.
TRUCKSESS, M.W., FLOOD, M.T., MOSSOBA, M.M., & PAGE, S.W. (1987) High
performance thin-layer chromatographic determination of
deoxynivalenol, fusarenon-X and nivalenol in barley, corn and wheat.
J. agric. food Chem., 35: 445-448.
TRUSAL, L.R. (1985) Morphological changes in CHO and VERO cells
treated with T-2 mycotoxin. Correlation with inhibition of protein
synthesis. Cell Biochem. Funct. 3: 205-216.
TRUSAL, L.R. & O'BRIEN, J.C. (1986) Ultrastructural effects of T-2
mycotoxins on rat hepatocytes in vitro. Toxicon, 24: 481-488.
TRYPHONAS, H., O'GRADY, L., ARNOLD, D,L., MCGUIRE, F., KARPINSKY, K.,
& VESONDER, R.F. (1984) Effect of deoxynivalenol (vomitoxin) on the
humoral immunity of mice. Toxicol. Lett., 23: 17-24.
TRYPHONAS, H., IVERSON, F., SO, Y., NERA, E.A., MCGUIRE, P.F.,
O'GRADY, L., CLAYSON, D.B., & SCOTT, P.M. (1986) Effects of
deoxynivalenol (vomitoxin) on the humoral and cellular immunity of
mice. Toxicol. Lett., 30: 137-150.
TSENG, T., YUAN, G., TSENG, J., SHASO, E., & MIROCHA, C.J. (1983)
Natural occurrence of Fusarium mycotoxins in grains and feeds in
Taiwan. Proc. Int. Mycotoxins Symposium, Abstr. 1.5, Sydney,
Australia.
TSUNODA, H., TSURUTA, O., MATSUNAMI, S., & ISHII, S. (1957) [Studies
on the microorganisms which deteriorate the stored cereals and
grains. XIV.] Shokuryokenkyujo Hokoku, 12: 27-33 (in Japanese).
UENO, Y. (1970) Inhibition of protein synthesis in animal cells by
nivalenol and related metabolites: toxic principles of rice-M
infested with Fusarium nivale. In: Herzberg, M., ed. Proceedings of
the First US-Japan Conference on Toxic Microorganisms, Washington,
DC, US Department of the Interior, UNJR Joint Panel of Toxic
Microorganisms, pp.76-79.
UENO, Y. (1977) Trichothecenes: overview address. In: Rodricks,
J.V., Hesseltine, C.W., & Mehlman, M.A., ed. Mycotoxins in human and
animal health, Park Forest South, Illinois, Pathotox Publishers, pp.
189-228.
UENO, Y. (1980a) Toxicological evaluation of trichothecene
mycotoxins. In: Eaker, D. & Wadstrom, T., ed. Natural toxins.
Proceedings of the International Symposium on Animal and Plant
Microbial Toxins, Uppsala, 1979, Elmsford, New York, Pergamon Press,
Vol. 6, pp. 663-671.
UENO, Y. (1980b) Trichothecene mycotoxins: Mycology, chemistry, and
toxicology. In: Draper, H.H., ed. Advances in nutritional research,
New York, London, Plenum Press, Vol. 3, pp. 301-356.
UENO, Y., ed. (1983) General toxicology. In: Developments in food
science. IV. Trichothecenes, New York, Elsevier, pp. 135-146.
UENO, Y. & FUKUSHIMA, K. (1968) Inhibition of protein and DNA
synthesis in Ehrlich ascites tumor by nivalenol, a toxic principle of
Fusarium nivale growing rice-M. Experientia (Basel), 24(10): 1032-
1033.
UENO, Y. & KUBOTA, K. (1976) DAN-attacking ability of carcinogenic
mycotoxins in recombination-deficient mutant cells of Bacillus
subtilis. Cancer Res., 36: 445-451.
UENO, Y. & MATSUMOTO, H. (1975) Inactivation of some thiol enzymes
by trichothecene mycotoxins from Fusarium spp. Chem. pharm. Bull.,
23(10): 2439-2442.
UENO, Y. & SHIMADA, N. (1974) Reconfirmation of the specific nature
of reticulocytes bioassay system to trichothecene mycotoxins of
Fusarium spp. Chem. pharm. Bull., 22(11): 2744-2746.
UENO, Y. & YAMAKAWA, H. (1970) Antiprotozoal activity of scirpene
mycotoxins of Fusarium nivale FN 2B. Jpn. J. exp. Med., 40(5):
385-390.
UENO, Y., HOSOYA, M., MORITA, Y., UENO, I., & TATSUNO, T. (1968)
Inhibition of the protein synthesis in rabbit reticulocyte by
nivalenol, a toxic principle isolated from Fusarium nivale growing on
rice. J. Biochem. (Tokyo), 64(4): 479-485.
UENO, Y., HOSOYA, M., & ISHIKAWA, Y. (1969a) Inhibitory effects of
mycotoxins on protein synthesis in rabbit reticulocytes. J. Biochem.
(Tokyo), 66(3): 419-422.
UENO, Y., UENO, I., TATSUNO, T., OHOKUBO, K., & TSUNODA, H. (1969b)
Fusarenon-X, atopic principle of Fusarium nivale-culture filtrate.
Experientia (Basel), 25: 1062.
UENO, Y., ISHIKAWA, Y., AMAKAI, K., NAKAJIMA, M., SAITO, M.,
ENOMOTO, M., & OHTSUBO, K. (1970) Comparative study on skin-
necrotizing effect of scirpene metabolites of Fusaria. Jpn. J. exp.
Med., 40(1): 33-38.
UENO, Y., UENO, I., IITOI, Y., TSUNODA, H., ENOMOTO, M., & OHTSUBO,
K. (1971) Toxicological approaches to the metabolites of Fusaria.
Part 3. Acute toxicity of fusarenon-X. Jpn. J. exp. Med., 41(6): 512-
539.
UENO, Y., ISHII, K., SAKAI, K., KANAEDA, S., TSUNODA, H., TANAKA, T.,
& ENOMOTO, M. (1972) Toxicological approaches to the metabolites of
Fusarium. Part 4. Microbial survey on bean hulls poisoning of horses
with the isolation of toxic trichothecenes, neosolaniol and T-2 toxin
of Fusarium solani M-1-1. Jpn. J. exp. Med., 42(3): 187-203.
UENO, Y., ISHII, K., SATO, N., SHIMADA, N., TSUNODA, H., SAWANO, M.,
& ENOMOTO, M. (1973a) Screening of trichothecene producing fungi
and the comparative toxicity of isolated mycotoxins. Jpn. J.
Pharmacol., 23(Suppl.): 133.
UENO, Y., NAKAJIMA, M., SAKAI, K., ISHII, K., SATO, M., & SHIMADA, N.
(1973b) Comparative toxicology of trichothecene mycotoxins.
Inhibition of protein synthesis in animal cells. J. Biochem. (Tokyo),
74(2): 285-296.
UENO, Y., SATO, N., ISHII, K., SAKAI, K., TSUNODA, H., & ENOMOTO, M.
(1973c) Biological and chemical detection of trichothecene
mycotoxins of Fusarium spp. Appl. Microbiol., 25(4): 699-704.
UENO, Y., ISHII, K., SATO, N., & OHTSUBO, K. (1974) Toxicological
approaches to the metabolites of Fusaria. VI. Vomiting factor from
moldy corn infected with Fusarium spp. Jpn. J. exp. Med., 44(1):
123-127.
UENO, Y., KUBOTA, K., ITO, T., & NAKAMURA, Y. (1978a) Mutagenicity
and carcinogenic mycotoxins in Salmonella typhimurium. Cancer Res.,
38(3): 536-542.
UENO, Y., MATSUMOTO, H., ISHII, K., & KUKITA, K. (1978b) Inhibitory
effects of mycotoxins on Na+-dependent transport of glycine in rabbit
reticulocytes. Biochem. Pharmacol., 25(18): 2091-2095.
UENO, Y., TASHIRO, F., & KOBAYASHI, T. (1983) Species differences
in zearalenone-reductase activity. Food chem. Toxicol., 21(2):
167-173.
UENO, Y., LEE, U.S., TANAKA, T., HASAGAWA, A., & MATSUKI, Y. (1986)
Examination of Chinese and USSR cereals for the Fusarium mycotoxins
nivalenol, deoxynivalenol, and zearalenone. Toxicon, 24(6): 18-21.
UENO, Y., ABE, K., MORIMURA, S., SUGIURA, Y., & HORIE, Y. (in press)
Genotoxicity of mycotoxins evaluated by the SOS microplate assay.
Mutation Res.
UMEDA, M., YAMAMOTO, T., & SAITO, M. (1972) DNA-strand breakage of
HeLa cells by several mycotoxins. Jpn. J. exp. Med., 42: 527-535.
UMEDA, M., TSUTSUI, T., & SAITO, M. (1977) Mutagenicity and
inducibility of DNA single-strand breaks and chromosome aberrations
by various mycotoxins. Gann, 68(5): 619-625.
VAN RENSBURG, S.J. & ALTENKIRK, B. (1974) Claviceps purpurea:
ergotism. In: Purchase, I.F.H., ed. Mycotoxins, Amsterdam, New York,
Oxford, Elsevier Science Publishers, pp. 69-96.
VESELA, D., VESELY, D., JELINEK, R., & KUSAK, V. (1978) [Detection
of ochratoxin A in feed barley.] Vet. Med., 23: 431-436 (in Czech).
VESELA, D., VESELY, D., & JELINEK, R. (1983) Toxic effects of
ochratoxins A and citrinin, alone and in combination, on chicken
embryos. Appl. environ. Microbiol., 45: 91-93.
VESONDER, R.F. (1983) Natural occurrence of trichothecenes in North
America. Dev. food Sci., 4: 210-217.
VESONDER, R.F., CIEGLER, A., & JENSEN, A.H. (1973) Isolation of the
emetic principle from Fusarium-infected corn. Appl. Microbiol., 26:
1008-1010.
VESONDER, R.F., CIEGLER, A., & JENSEN, A.H. (1977) Production of
refusal factors by Fusarium strains on grains. Appl. environ.
Microbiol., 34(1): 105-106
VESONDER, R.F., CIEGLER, A., ROGERS, R.F., BURBRIDGE, K.A., BOTHAST,
R.J., & JENSEN, A.H. (1978) Survey of 1977 crop year preharvest
corn for vomitoxin. Appl. environ. Microbiol., 36(6): 885-888.
VESONDER, R.F., CIEGLER, A., BURMEISTER, H.R., & JENSEN, A.H. (1979)
Acceptance by swine and rats of corn amended with trichothecenes.
Appl. environ. Microbiol., 38(2): 344-346.
VISCONTI, A. & BOTTALICO, A. (1983a) Detection of Fusarium
trichothecenes (nivalenol, deoxynivalenol, fusarenone and
3-acetyldeoxynivalenol) by high-performance liquid chromatography.
Chromatographia, 17: 97-100.
VISCONTI, A. & BOTTALICO, A. (1983b) High levels of ochratoxin A
and B in mouldy bread responsible for mycotoxicosis in farm animals.
J. agric. food Chem., 31: 1122-1123.
VISCONTI, A. & MIROCHA, C.J. (1985) Identification of various T-2
toxin metabolites in chicken excreta and tissues. Appl. environ.
Microbiol., 49: 1246-1250.
VOYKSNER, R.D., HAGLER, W.M., TYCZKOWSKA, K., & HANEY, C.A. (1985)
Thermospray high-performance liquid chromatographic/mass
spectrometric analysis of some Fusarium mycotoxins. J. High Res.
Chrom. Chrom. Comm., 8: 119-125.
WARE, G.M., FRANCIS, O.J., CARMAN, A.S., & KUAN, S.S. (1986) Gas
chromatographic determination of deoxynivalenol in wheat with
electron capture detection: collaborative study. J. Assoc. Off. Anal.
Chem., 69: 899-901.
WEAVER, G.A., KURTZ, H.J., BATES, F.Y., CHI, M.S., MIROCHA, C.J.,
BEHRENS, J.C., & ROBISON, T.S. (1978a) Acute and chronic toxicity
of T-2 mycotoxin in swine. Vet. Rec., 103(24): 531-535.
WEAVER, G.A., KURTZ, H.J., MIROCHA, C.J., BATES, F.Y., & BEHRENS,
J.C. (1978b) Acute toxicity of the mycotoxin diacetoxyscirpenol in
swine. Can. vet. J., 19(10): 267-271.
WEHNER, F.C., THIEL, P.G., VAN RENSBURG, S.J., & DEMASIUS, I.P.C.
(1978a) Mutagenicity to Salmonella typhimurium of some Aspergillus
and Penicillium mycotoxins. Mutat. Res., 58: 193-203.
WEHNER, F.C., MARASAS, W.F.O., & THIEL, P.G. (1978b) Lack of
mutagenicity to Salmonella typhimurium of some Fusarium mycotoxins.
Appl. environ. Microbiol., 35(4): 659-662.
WEI, C.M. & MCLAUGHLIN, C.S. (1974) Structure function relationship
in the 12,13-epoxytrichothecenes novel inhibitors of protein
synthesis. Biochem. biophys. Res. Commun., 57(3): 838-844.
WEI, R.D., STRONG, F.M., SMALLEY, E.B., & SCHNOES, H.K. (1971)
Chemical interconversion of T-2 and HT-2 toxins and related
compounds. Biochem. biophys. Res. Commun., 45(2): 396-401.
WEI, R.D., SMALLEY, E.B., & STRONG, F.M. (1972) Improved skin test
of detection of T-2 toxin. Appl. Microbiol., 23(5): 1029-1030.
WEI, C.M., CAMPBELL, I.M., MCLAUGHLIN, C.S., & MAURICE, H. (1974)
Binding of trichodermin to mammalian ribosomes and its inhibition by
other 12,13-epoxytrichothecenes. Mol. cell Biochem., 3(3): 215-219.
WHO (1979) Environmental Health Criteria 11: Mycotoxins, Geneva,
World Health Organization, 127 pp.
WHO (1983) Prevention of liver cancer: Report of a WHO Meeting,
Geneva, World Health Organization, 30 pp. (Technical Report Series,
No. 691).
WILSON, D.J. (1984) Some effects of T-2 toxin on rabbit and bovine
musculature, Guelph, Ontario, Faculty of Graduate Studies of Guelph
(M. Sc. Thesis).
WILSON, D.J. & GENTRY, P.A. (1985) T-2 toxin can cause vasoconstriction
in an in vitro bovine ear perfusion system. Toxic. appl. Pharmacol.,
79: 159-165.
WOOD, G.E. & CARTER, L. (1989) Limited survey of deoxynivalenol in
wheat and corn in the United States. J. Assoc. Off. Anal. Chem., 72:
38-40.
WOODS, A.J., BRADLEY-JONES, J., & MANTLE, P.G. (1966) An outbreak of
gangrenous ergotism in cattle. Vet. Rec., 78: 742-749.
WYATT, R.D., HARRIS, J.R., HAMILTON, P.B., & BURMEISTER, H.R. (1972)
Possible outbreaks of furariotoxicosis in avians. Avian Dis., 16:
1123-1130.
WYATT, R.D., COLWELL, W.M., HAMILTON, P.B., & BURMEISTER, H.R.
(1973a) Neural disturbances in chickens caused by dietary T-2 toxin.
Appl. Microbiol., 26(5): 757-761.
WYATT, R.D., COLWELL, W.M., HAMILTON, P.B., & BURMEISTER, H.R.
(1973b) Neurotoxicity of T-2 toxin in broilers. Poult. Sci., 52(5):
2105.
WYATT, R.D., HAMILTON, P.B., & BURMEISTER, H.R. (1973c) Effects of
T-2 toxin in broiler chickens. Poult. Sci., 52(5): 1853-1859
WYATT, R.D., DOERR, J.A., HAMILTON, P.B., & BURMEISTER, H.R. (1975)
Egg production shell thickness and other physiological parameters of
laying hens affected by T-2 toxin. Appl. Microbiol., 29(5): 641-645.
XU, Y.C., ZANG, G.S., & CHU, F.S. (1988) Enzyme-linked immunosorbent
assay for deoxynivalenol in corn and wheat. J. Assoc. Off. Anal.
Chem., 71: 945-949.
YAGEN, B., SINTOV, A., & BIALER, M. (1986) New, sensitive thin-layer
chromatographic-high performance liquid chromatographic method for
detection of trichothecene mycotoxins. J. Chromatogr., 356: 195-201.
YAROM, R., MORE, R., ELDOR, A., & YAGEN, B. (1984a) The effect of T-2
toxin on human platelets. Toxicol. appl. Pharmacol., 73: 210-217.
YAROM, R., SHERMAN, Y., MORE, R., GINSBURG, I., BORINSKI, R., &
YAGEN, B. (1984b) T-2 toxin effect on bacterial infection and
leukocyte functions. Toxicol. appl. Pharmacol., 75: 60-68.
YLIMAEKI, A., KOPONEN, H., HINTIKKA, E.L., NUMMI, M., NIKU-PAAVOLA,
M.L., ILUS, T., & ENARI, T.M. (1979) Mycoflora and occurrence of
Fusarium toxins in Finnish grain, Espoo, Technical Research Center of
Finland, pp. 1-28, (Materials and Processing Technology, Publication
21).
YOSHIZAWA, T. & HOSOKAWA, H. (1983) Natural occurrence of
deoxynivalenol and nivalenol trichothecene mycotoxins in commercial
foods. J. Food Hyg. Soc. Jpn, 24: 413-415.
YOSHIZAWA, T. & MOROOKA, N. (1973) Deoxynivalenol and its
monoacetate new mycoatoxins from Fusarium roseum and mouldy barley.
Agric. biol. Chem., 37(12): 2933-2934.
YOSHIZAWA, T. & MOROOKA, N. (1977) Trichothecenes from mold-
infested cereals in Japan. In: Rodricks, J.V., Clifford, W.,
Hesseltine, C.W., & Myron, A.M., ed. Mycotoxins in human and animal
health, Park Forest South, Illinois, Pathotox Publishers, pp. 309-
321.
YOSHIZAWA, T. & SAKAMOTO, T. (1982) [ In vitro metabolism of T-2
toxin and its derivatives in animal livers.] Proc. Jpn. Assoc.
Mycotoxicol., 14: 26-28 (in Japanese).
YOSHIZAWA, T., SWANSON, S.P., & MIROCHA, C.J. (1980) T-2
metabolites in the excreta of broiler chickens administered 3H-
labeled T-2 toxin. Appl. environ. Microbiol., 39(6): 1172-1177.
YOSHIZAWA, T., MIROCHA, C.J., BEHRENS, J.C., & SWANSON, S.P. (1981)
Metabolic fate of T-2 toxin in a lactating cow. Food Cosmet.
Toxicol., 19(1): 31-39.
YOSHIZAWA, T., SAKAMOTO, T., AYANO, Y., & MIROCHA, C.J. (1982a)
[Chemical structures of new metabolites of T-2 toxin.] Proc. Jpn.
Assoc. Mycotoxiol., 15: 13-15 (in Japanese).
YOSHIZAWA, T., SAKAMOTO, T., AYANO, Y., & MIROCHA, C.J. (1982b)
3'-Hydroxy-T-2 and 3'-hydroxy HT-2 toxins: new metabolites of T-2 toxin,
a trichothecene mycotoxin, in animals. Agric. biol. Chem., 46(10):
2613-2615.
YOSHIZAWA, T., SAKAMOTO, T., & OKAMOTO, K. (1984) In vitro formation
of 3'-hydroxy T-2 and 3'-hydroxy HT-2 toxins form T-2 toxin by liver
homogenates from mice and monkeys. Appl. environ. Microbiol., 47:
130-134.
YOSHIZAWA, T., OKAMOTO, K., SAKAMOTO, T., & KUWAMURA, K. (1985a) In
vivo metabolism of T-2 toxin, a trichothecene mycotoxin. On the
formation of deepoxidation products. Proc. Jpn. Assoc. Mycotoxicol.,
21: 9-12.
YOSHIZAWA, T., SAKAMOTO, T., & KUWAMUAR, K. (1985b) Structures of
deepoxytrichthecene metabolites from 3'-hydroxy HT-2 and T-2 tetraol
in rats. Appl. environ. Microbiol., 50: 676-679.
YOSHIZAWA, T., COTE, L.M., SWANSON, S.P., & BUCK, W.B. (1986)
Confirmation of DOM-1, a deepoxydation metabolite of deoxynivalenol
in biological fluids of lactating cows. Agric. biol. Chem., 50:
227-229.
YOUNG, J.C. (1981a) Variability in the content and composition of
alkaloids found in Canadian ergot. I. Rye. J. environ. Sci. Health,
B16(4): 83-111.
YOUNG, J.C. (1981b) Variability in the content and composition of
alkaloids found in Canadian ergot. II. Wheat. J. environ. Sci.
Health, B16(1): 381-393.
YOUNG, J.C. & CHEN, Z. (1982) Variability in the content and
composition of alkaloids found in Canadian ergot. III. Triticale and
barley. J. environ. Sci. Health, B17(2): 93-107.
YOUNG, J.C. & MARQUARDT, R.R. (1982) Effects of ergotamine tartrate
in growing chicken. Can. J. anim. Sci., 62: 1181-1191
YOUNG, J.C., CHEN, Z., & MARQUARDT, R.R. (1983) Reduction in
alkaloid content of ergot sclerotia by chemical and physical
treatment. J. agric. food Chem., 31: 413.
YOUNG, J.C., FULCHER, R.G., HAYHOE, J.H., SCOTT, P.M., & DEXTER, J.E.
(1984) Effects of milling and baking on deoxynivalenol (Vomitoxin)
content of Eastern Canadian wheats. J. agric. food Chem., 32:
659-664.
YOUNG, J.C., SUBRYAN, L.M., POTTS, D., MCLAREN, M.E., & GOBRAN, F.H.
(1986) Reduction in levels of deoxynivalenol in contaminated wheat by
chemical and physical treatment. J. agric. food Chem., 34: 461-464.
ZAMIR, N., ZAMIR, D., EIDEN, L.E., PALKOVITS, M., BROWNSTEIN, M.J.,
ESHAY, R.L., WEBER, E., FRODEN, A.I., & FEUERSTEIN, G. (1985)
Metionine and leucine enkephalin in rat neurohypophysis: different
response to osmotic stimuli and T-2 toxin. Science, 228: 606-608.
ZIPRIN, R.L. & CORRIER, D.E. (1987) Listeriosis in diacetoxyscirpenol-
treated mice. Am. J. vet. Res., 48: 1516-1519.
ZORZ, M., CULIG, J., KOPITAR, Z., MILIVOJEVIC, D., MARUSIC, A., &
BANO, M. (1985) HPLC method for determination of ergot alkaloids
and some derivatives in human plasma. Hum. Toxicol., 4: 601-607.
RESUME ET RECOMMANDATIONS EN VUE DE RECHERCHES FUTURES
1. Ochratoxine A
1.1 Etat naturel
Les ochratoxines sont produites par plusieurs espèces de
champignons appartenant au genre Aspergillus et Penicillium. Il
s'agit d'espèces ubiquistes, aussi le risque de contamination des
denrées alimentaires destinées à l'homme et aux animaux est-il
omniprésent. Le principal composé, l'ochratoxine A se rencontre en
Australie ainsi que dans certains pays d'Europe et d'Amérique du
Nord. La production d'ochratoxine par les espèces du genre
Aspergillus semble limitée aux environnements très chauds et
humides alors que dans le cas de Penicillium, au moins certaines
espèces peuvent produire de l'ochratoxine à des températures
n'excédant pas 5 °C.
Ce sont les céréales qui sont le plus fréquemment contaminées
par l'ochratoxine A et à un moindre degré certaines fèves (café,
soja, cacao). L'ochratoxine B est très rare.
1.2 Méthodes d'analyse
On a mis au point des méthodes d'analyse permettant
l'identification et le dosage de l'ochratoxine à des concentrations
de l'ordre de 1 µg/kg.
1.3 Métabolisme
On trouve des résidus d'ochratoxine A intacte dans le sang, les
reins, le foie et les muscles de porcs à l'abattoir ainsi que dans
les muscles de poules et de poulets. En revanche on ne trouve
généralement pas de résidus chez les ruminants. In vitro,
l'ochratoxine A se fixe très solidement à l'albumine sérique
bovine, porcine et humaine. Des études expérimentales sur des
porcins et des poules ont montré que c'est au niveau des reins que
les concentrations d'ochratoxine A sont les plus élevées. Chez le
porc, le rat et l'homme, il pourrait y avoir détoxication par
hydroxylation au niveau des microsomes. L'expérience montre qu'on
peut encore identifier des résidus dans les reins de porc un mois
après la fin de l'exposition.
1.4 Effets sur les animaux
On a signalé des cas d'ochratoxicose chez des animaux d'élevage
(porcs et volaille) dans plusieurs pays d'Europe, la manifestation
essentielle étant une néphropathie chronique. Les lésions se
présentent sous la forme d'une atrophie tubulaire, d'une fibrose
interstitielle et à un stade plus avancé, d'une hyalinisation des
glomérules. Au Canada, on a également trouvé de l'ochratoxine A
dans du sang de porc recueilli à l'abattoir. Des effets
néphrotoxiques ont été relevés chez toutes les espèces d'animaux à
estomac simple étudiées jusqu'ici, même aux doses les plus faibles
qui aient été expérimentées (200 mg/kg de nourriture chez les rats
et les porcs).
Des effets tératogènes ont été observés chez des souris
exposées par voie orale à 3 mg d'ochratoxine A par kg de poids
corporel. On a observé une résorption des foetus chez des rats à
partir de 0,75 mg/kg de poids corporel, le produit étant administré
par voie orale. Ces effets tératogènes, qui chez le rat sont
accrus par un régime alimentaire pauvre en protéines, ont été
également observés chez des hamsters.
Les épreuves à court terme n'ont pas permis de mettre en
évidence d'activité mutagène (bactéries et levures). Administré
par voie orale à des rats, le produit a provoqué des ruptures
monocaténaires dans l'ADN des tissus du rein et du foie.
L'ochratoxine a provoqué l'apparition de cancers du rein chez des
souris mâles et des rats des deux sexes qui recevaient le produit
par voie orale. La formation de carcinomes hépatocellulaires n'a
été signalée que chez une souche de souris et pas du tout chez le
rat.
L'ochratoxine A est un inhibiteur de la synthèse protéique et
de la tRNA-synthétase chez les microorganismes et dans les cellules
hépatomateuses; elle inhibe également le mARN chez le rat.
L'ochratoxine A peut inhiber la migration des macrophages.
Chez la souris, une dose de 0,005 µg/kg de poids
corporel a aboli la réponse immunitaire aux érythrocytes de mouton;
toutefois d'autres résultats, contradictoires, ont été obtenus.
L'ochratoxine A s'est révélée cancérogène pour l'épithelium
tubulaire rénal chez des souris mâles et des rats des deux sexes.
1.5 Effets sur l'homme
L'exposition humaine, qui ressort de la présence d'ochratoxine
A dans les aliments, le sang et le lait humains, a été observée
dans divers pays d'Europe. Selon les données épidémiologiques
disponibles, la néphropathie des Balkans peut être attribuée à la
consommation de denrées alimentaires contaminées par cette toxine.
On a mis en évidence une relation tout à fait significative
entre la néphropathie des Balkans et les tumeurs des voies
urinaires, en particulier des tumeurs pyéliques et uretérales.
Toutefois on n'a pas publié de données qui établissent une
implication directe de l'ochratoxine A dans l'étiologie de ces
tumeurs.
2. Trichothécènes
2.1 Etat naturel
On connaît aujourd'hui 148 trichothécènes qui se caractérisent
sur le plan chimique par la présence d'une structure de base
commune, le système tétracyclique du scirpénol. Ces composés sont
principalement secrétés par des moisissures appartenant au genre
Fusarium, encore que d'autres genres notamment Trichoderma,
Trichothecium, Myrothecium et Stachybotrys produisent également des
métabolites considérés désormais comme des trichothécènes.
Quelques-uns seulement de ces trichothécènes contaminent les
denrées alimentaires destinées à l'homme ou aux animaux, en
particulier : le désoxyvalénol (DON), le nivalénol (NIV), le
disacétoxyscirpenol (DAS), ainsi que la toxine T-2 et, plus
rarement, certains dérivés (3-Ac-DON, 15-Ac-DON, fusarenone-X et
toxine HT-2). Parmi ces substances, c'est le DON qui est de loin
la plus fréquemment présente dans les denrées alimentaires
destinées à l'homme et aux animaux; à côté de quantités plus
faibles de NIV. Certains trichothécènes macrocycliques comme les
satratoxines G et H et les verrucarines, se rencontrent de temps à
autre dans les fourrages (paille, foin) mais il n'a pas été fait
état de leur présence dans les produits alimentaires.
Les enquêtes sur la présence de trichothécènes ont révélé que
le DON était présent dans le monde entier, essentiellement dans les
céréales telles que le froment et le maïs à des concentrations
pouvant parfois atteindre 92 mg/kg, encore que les teneurs moyennes
soient beaucoup plus faibles et varient selon la denrée. Des
rapports isolés font état de la présence de DON dans de l'orge, des
mélanges alimentaires pour aminaux, des pommes de terre, etc. Le
NIV, dont la présence n'est normalement pas signalée dans des
céréales au Canada ou aux Etats-Unis se rencontre en revanche
fréquemment au côté du DON dans les céréales originaires d'Asie ou
d'Europe; la concentration la plus forte enregistrée jusqu'ici est
de 37,9 mg/kg. On a signalé ça et là la présence de toxine T-2 et
de DAS à des concentrations beaucoup plus faibles.
Des études portant sur les divers traitements subis par les
produits alimentaires et notamment la mouture, montrent que, entre
la céréale brute et le produit définitif, il n'y a guère de
diminution des teneurs en DON. De même la panification ne parvient
pas à détruire le DON. En général, les aliments destinés à l'homme
que l'on trouve dans le commerce ne contiennent que rarement des
quantités décelables de DON et de NIV.
2.2 Methodes d'analyse
On dispose, pour le dosage des quatre toxines les plus
fréquemment rencontrées (DON, toxine T.2, DAS et NIV), des méthodes
d'analyse basées sur la chromatographie en couche mince, la
chromatographie en phase gazeuse ou la chromatographie liquide à
haute performance ainsi que sur des réactions immunologiques; les
limites de détection se situent en-dessous de 1 µg/g. Plusieurs de
ces méthodes ont fait l'objet d'une expérimentation collective. En
outre, certaines méthodes utilisées en recherche comme la
chromatographie gazeuse ou liquide associée à la spectrographie de
masse peuvent être utilisées pour confirmer l'identité des
substances.
2.3 Métabolisme
On a procédé à des études métaboliques, essentiellement de la
toxine T-2, mais dans quelques rares cas seulement du DON, chez
l'animal. Ces trichothécènes sont rapidement absorbés au niveau
des voies digestives mais l'on ne dispose pas de données
quantitatives. Les toxines se répartissent de façon uniforme sans
accumulation marquée au niveau d'un organe ou d'un tissu
particulier. Elles sont métabolisées en produits moins toxiques
par hydrolyse, hydroxylation, désépoxydation et glucuronidation.
La toxin T-2 et le DON sont rapidement éliminés par voie fécale et
urinaire.
Par exemple, une dose de toxine T-2 administrée par voie orale à
des bovins a été éliminée presque à hauteur de 100 % dans les heures
suivant l'administration; des poulets ont éliminé 80 % de la
substance dans les 48 heures suivant l'administration. Chez le rat,
25 % d'une dose de DON ont été éliminés dans les urines et 65 % dans
les matières fécales 96 heures après l'administration. Chez la poule
pondeuse et la vache laitière on a constaté que moins de 1 % de la
dose de toxine T-2 (et de ses métabolites) qui leur avait été
administrée se retrouvaient dans les oeufs et le lait. Après
administration par voie orale de toxine T-2 à des poulets, on a
constaté que les résidus présents dans la viande 24 heures plus tard
représentaient moins de 2 % de la dose initiale.
2.4 Effets sur les animaux
C'est principalement par l'ingestion de fourrage contaminé que
les animaux sont exposés aux trichothécènes. La toxine T-2 et le DAS
qui chez les animaux de laboratoire sont les plus actifs des
trichothécènes couramment cités comme contaminants des denrées
destinées aux animaux (toxine T-2, DAS, NIV et DON), provoquent des
réactions toxiques analogues. Le NIV est moins actif dans certains
systèmes que les deux précédents composés et le DON est le moins
toxique des quatre. (Cette activité toxique peut s'évaluer au moyen
de la DL50 pour la souris, par exemple dans le cas de la toxine T-2
elle est égale à 10,5 mg/kg de poids corporel et dans le cas du DON à
46,0 mg/kg).
Les trichothécènes les plus actifs, tels que la toxine T-2 et le
DAS produisent des effets généraux aigus lorsqu'ils sont administrés
expérimentalement à des rongeurs, des porcs et des bovins par voie
orale, parentérale ou respiratoire (porc, souris). Ces
trichothécènes produisent une épithélionécrose par contact (une dose
de 0,2 µg par touche dans le cas de la toxine T-2). Avec les autres
trichothécènes, il faut des doses plus élevées pour obtenir un effet
irritant (dans le cas du NIV, 10 ug par touche). Les trichothécènes
cytotoxiques comme la toxine T-2 ont une action nécrosante sur
l'épithélium des cryptes intestinales et sur les tissus lymphoïdes et
hematopoïétiques après exposition par voie orale, parentérale ou
respiratoire. Après exposition à la toxine T-2 et au DAS on observe
des anomalies hematologiques et des troubles de l'hémostase. Dans
les cas graves, la toxicose peut entraîner une pancytopénie. Des
études portant sur la toxine T-2, le DON et le DAS ont mis en
évidence la suppression de l'immunité à médiation cellulaire et de
l'immunité humorale et on a observé une réduction de la concentration
des immunoglobulines ainsi qu'une dépression de l'activité
phagocytaire des macrophages et des neutrophiles. Des études sur
animaux de laboratoire ont montré que l'effet immunodépresseur de
trichothécènes tels que la toxine T-2, le DAS et le DON entraînait
une moindre résistance aux infections secondaires par des bactéries
(Mycobactérie, Listeria monocytogenes) des levures ( Cryptococcus
neoformans) et des virus (virus de l'herpes simplex).
Il a été indiqué qu'après injection intrapéritonéale, la toxine
T-2 était tératogène pour la souris (cette voie d'administration
n'est pas courante dans les études de tératogénicité). Le DON est
tératogène pour la souris après intubation gastrique mais ne l'est
pas chez le rat lorsque la toxine est administrée dans la nourriture
de l'animal. Le NIV ne s'est pas révélé tératogène pour la souris.
La recherche du pouvoir mutagène de la toxine T-2, du DAS et du DON
par une épreuve du type Ames n'a pas donné de résultat positif. La
toxine T-2 présente dans certaines épreuves une faible activité
clastogène. D'après les études de toxicité à long terme qui ont été
publiées, rien n'indique que la toxine T-2, la fusarénone-X et le NIV
ne soient tumorigènes chez l'animal. Aucune étude de toxicité à long
terme n'a été publiée sur le DON.
Les trichothécènes sont toxiques pour les cellules à forte
activité mitotique telles que les cellules de l'épithélium des
cryptes intestinales et les cellules hematopoïétiques. Cette
cytotoxicité proviendrait, soit d'une perturbation de la synthèse des
protéines par une fixation des composés aux ribosomes des cellules
eucaryotes, soit d'une dysfonction des membranes cellulaires.
L'inhibition de la synthèse protéique serait due à l'induction de
protéines régulatrices labiles telles que l'IL-2 dans les
immunocytes. A concentrations extrêmement faibles, les
trichothécènes perturbent le transport des petites molécules à
travers les membranes cellulaires.
2.5 Effets sur l'homme
L'ingestion de produits alimentaires contaminés d'origine
végétale constitue la principale voie d'exposition aux tricho-
thécènes mais d'autres voies ont été signalées à l'occasion, par
exemple un contact cutané accidentel chez des chercheurs de
laboratoire ou l'inhalation de trichothécènes présents dans des
poussières aéroportées.
On n'a décrit que peu de cas de maladies attribuables à une
exposition aux trichothécènes, sans d'ailleurs que la responsa-
bilité de ces produits soit établie. Toutefois, dans les deux
flambées évoquées ci-dessous, il y a lieu de penser que leur
responsabilité est en cause.
Une des flambées, qui s'est produite en Chine, a été attribuée à
la consommation de blé moisi contenant 1,0 à 40,0 mg de DON par kg.
La maladie se caractérisait par des symptômes gastro-intestinaux.
Aucun décès n'a été à déplorer. Les porcs et les poulets qui avaient
mangé les restes de céréales ont également été affectés.
Une flambée du même genre a été signalée en Inde et attribuée à
la consommation de pain fabriqué à l'aide de blé contaminé. La
maladie se caractérisait par des symptômes gastro-intestinaux et une
irritation de la gorge qui apparaissaient 15 minutes à une heure
après l'ingestion du pain. On a décelé les mycotoxines suivantes
dans des échantillons de farine raffinée utilisée pour la préparation
du pain: DON (0,35-8,3 mg/kg), acétyldésoxynivalénol (0,64-2,49
mg/kg), NIV (0,03-0,1 mg/kg) et toxine T-2 (0,5-0,8 mg/kg).
Toutefois, l'identité de ces trichothécènes n'a pas été confirmée.
La présence de DON et de NIV à côté de la toxine T-2 est
inhabituelle.
Deux maladies d'intérêt historique, une aleucie alimentaire
d'origine toxique en URSS et une toxicose due à du blé moisi au
Japon et en Corée ont été attribuées à la consommation de céréales
contaminées par des moisissures du genre Fusarium. Depuis lors, on
a isolé en laboratoire, dans des cultures de Fusarium isolés des
céréales en cause, un certain nombre de trichothécènes. Il n'avait
pas été possible, au moment où ces maladies se sont déclarées,
d'effectuer des recherches pour tenter de corréler l'aleucie
toxique et la toxicose due au blé moisi à une exposition à des
trichothécènes car on ne connaissait pas les toxines en question.
3. Ergot
3.1 Etat naturel
Ergot est le nom que l'on donne aux sclérotes de certaines
espèces de champignons appartenant au genre Clavicepsa. Ces
sclérotes contiennent des alcaloïdes biologiquement actifs qui
peuvent être à l'origine de toxicoses chez les personnes ou les
animaux qui consomment des denrées contaminées.
Les alcaloïdes de l'ergot produisent deux types de maladies selon
leur nature, qui dépend du champignon en cause ( C. purpurea , C.
fusiformis ). L'ergotisme, dû à l'ergotamine et à l'ergocristine
produites par C. purpurea se caractérise principalement par une
gangrène des extrémités et des symptômes gastro-intestinaux. Quand à
l'intoxication provoquée par du millet contaminé par C. fusiformis,
elle se caractérise principalement par des symptômes digestifs et
elle est due aux clavines. Aucun des signes ou symptômes observés ne
sont révélateurs d'une oblitération vasculaire.
3.2 Méthodes d'analyse
Les alcaloïdes de l'ergot (ergolines) sont des dérivés de l'acide
lysergique. Ils ont une activité biologique variable selon leur
nature. Le dosage des alcaloïdes de C. purpurea a été effectué par
chromatographie liquide à haute performance avec détection par
fluorescence. On peut déterminer des concentrations de 0,2 µg
d'ergolines par litre de plasma humain. La détermination de
l'ergotamine et de l'ergocristine peut s'effectuer de façon très
spécifique par titrage radio-immunologique à des concentrations
respectives de 3,5 et 0,8 picomoles.
3.3 Effets sur les animaux
Des flambées d'avortements chez des bovins ont pu être attribuées
à l'ingestion d'ergoline, essentiellement de l'ergotamine et de
l'ergotaminine. Des moutons à qui l'on avait administré de
l'ergotamine par voie orale sont tombés rapidement malades et
présentaient une inflammation intestinale. Chez des volailles, des
porcs et des primates exposés par voie orale on a observé des effets
légers. Le groupe de travail ne disposait d'aucune donnée sur la
mutagénicité, la tératogénicité et la cancérogénicité des ergolines.
3.4 Effets sur l'homme
L'homme peut être exposé aux ergolines par la consommation de
céréales ergotées. Dans la plupart des études toxicologiques qui ont
été effectuées, on n'a pas procédé à l'identification précise des
alcaloïdes en cause. Les données qui ont été publiées au sujet d'une
seule enquête effectuée en Suisse sur des céréales et des produits
céréaliers indiquent que la consommation quotidienne totale
d'ergolines se situe à environ 1,5 ug par personne, certaines denrées
en contenant jusqu'à 140 µg/kg. La panification réduit de 25 à 100 %
la teneur en ergolines des farines contaminées.
En Ethiopie, une flambée d'ergotisme s'est produite en 1978 à la
suite de l'exposition à des ergolines provenant de sclérotes de ce C.
purpurea. Les céréales comptaient jusqu'à 0,75 % d'ergot; on a
relevé la présence d'ergométrine. Parmi les symptômes observés
figuraient une gangrène sèche ayant entraîné la perte d'un ou
plusieurs membres (29 % des cas), un pouls périphérique faible ou
absent (36 %) et une desquamation. Des symptômes digestifs n'ont été
observés que dans quelques cas. Chez 88 % des malades, les lésions
intéressaient les membres inférieurs.
En Inde, plusieurs flambées se sont déclarées depuis 1958 à la
suite de la consommation de millet contaminé par C. fusiformis. Ces
symptômes consistaient en nausées, vomissements et vertiges. Les
symptômes toxiques étaient dus à la présence d'ergolines à des
concentrations de 15 à 26 mg/kg. Aucune autopsie n'ayant été
effectuée, on ne dispose d'aucune information sur les effets
pathologiques au niveau des viscères.
4. Evaluation des risques pour la santé humaine
4.1 Ochratoxine A
L'exposition humaine, observée dans plusieurs pays d'Europe est
objectivée par la présence d'ochratoxine A dans les denrées
alimentaires et le sang. Le groupe de travail n'a pas connaissance
de tentatives qui ont effectuées dans d'autres régions du monde pour
déceler la présence d'ochratoxine A dans le sang humain.
En s'appuyant sur l'étude de la maladie-naturelle ou provoquée
par administration d'ochratoxine A-on a pu établir le rôle
étiologique de l'ochratoxine A dans la néphropathie porcine. A
partir de ce modèle, on a pu émettre l'hypothèse que la néphropathie
endémique des Balkans était due à une exposition à cette toxine. Les
données immunologiques disponibles montrent que cette affection
serait attribuable à la consommation de denrées alimentaires
contaminées par de l'ochratoxine A. Depuis la publication en 1980 du
No 11 des Critères d'hygiène de l'environnement, des études
épidémiologiques sur la concentration de l'ochratoxine A dans le sang
humain dans les régions touchées et non touchées ont conforté
l'hypothèse d'une relation entre la néphropathie balkanique et
l'exposition à l'ochratoxine A.
Ainsi, on a montré que les habitants des régions d'endémie
présentaient plus fréquemment de l'ochratoxine A dans leur sang et à
des concentrations plus élevées. Cependant, ces études
rétrospectives ne fournissent que des présomptions sur lesquelles on
ne peut établir l'existence d'une relation causale directe. On ne
peut cependant pas l'exclure du fait de la longue période de latence
entre l'exposition et l'apparition des symptômes.
On a montré que l'ochratoxine A exerçait, chez les souris mâles
et les rats des deux sexes, des effets cancérogènes sur l'épithélium
des tubules rénaux. Il existe une relation tout à fait significative
entre la néphropathie balkanique et la présence de tumeurs des voies
urinaires, notamment de tumeurs pyéliques et uretérales. Toutefois
aucune donnée n'a été publiée qui établissent la responsabilité
directe de l'ochratoxine A dans l'étiologie de ces tumeurs.
4.2 Trichothécènes
Sur la base des données dont disposait le groupe de travail, il
est possible d'établir une relation entre l'exposition aux
trichothécènes et certains épisodes toxiques chez l'homme. Si l'on
se réfère aux quelques données disponibles, ce sont le DON et le NIV
qui sont les plus fréquemment cités dans les différents cas
d'exposition humaine. Dans le cas des flambées d'aleucie toxique
alimentaire et de toxicose par ingestion de céréales moisies, on ne
peut exclure la responsabilité des trichothécènes. L'exposition se
produit par ingestion de denrées contaminées, principalement des
céréales. Les différents traitements qu'elles subissent, notamment
la mouture et la panification ne permettent pas d'éliminer le DON, le
NIV, ni la toxine T-2. L'existence d'une exposition par voie
respiratoire est très peu documentée mais c'est une possibilité qu'on
ne peut totalement écarter.
Parmi les tricothécènes qui se trouvent à l'état naturel dans les
aliments, c'est la toxine T-2 qui est la plus active, suivie du DAS
and du NIV; les études de toxicité aiguë ont montré que le DON était
la substance la moins toxique. Chez l'animal d'expérience, la toxine
T-2 et le DAS déterminent des symptômes généraux aigus, avec nécrose
des tissus épithéliaux et suppression de l'hematopoïèse. Lors des
récentes flambées d'intoxications, il n'a été fait état que des
symptômes digestifs.
Le DON est tératogène pour la souris mais pas pour le rat. Selon
les études de toxicité chroniques qui ont été publiées, le NIV et la
toxine T-2 ne sont pas tumorigènes chez l'animal. Aucune étude de
cancérogénicité à long terme n'a été publiée sur le DON. Certains
trichothécènes, tels que la toxine T-2 et le DON ont une action
immunosuppressive chez l'animal et produisent des modifications de
l'immunité à médiation cellulaire et de l'immunité humorale. En
revanche, rien n'indique l'existence de tels effets chez l'homme.
On est mal informé sur les cas d'intoxication humaine due à une
exposition aux trichothécènes, cas qui sont en nombre limité. Les
symptômes observés consistent en troubles digestifs et irritation de
la gorge; ils apparaissent peu après l'ingestion de denrées
alimentaires contaminées par des trichothécènes. A l'heure actuelle,
on ne connaît pas de cas de cancer humain attribuable aux
trichothécènes. Le groupe de travail ne disposait d'aucune
publication faisant état d'infections secondaires d'origine
bactérienne, fongique ou virale chez l'homme, à la suite d'une
exposition aux trichothécènes comme on en a observé chez l'animal
d'expérience. Il semble que l'on n'ait pas effectué d'études
appropriées sur ce point.
4.3 Ergot
Il semble que l'exposition humaine à de faibles concentrations
d'ergolines soit très répandue. Les données relatives aux flambées
qui se sont récemment déclarées en Ethiopie et en Inde montrent que
les alcaloïdes de C. purpurea (groupe de l'ergotamine) produisent
les effets les plus graves, notamment une gangrène des membres
inférieurs pouvant entraîner la mort, effets qui sont plus graves
que ceux des alcaloïdes de C. fusiformis (groupe de la clavine) qui
entraînent des symptômes digestifs sans issue fatale. On ignore si
ces différences s'expliquent par la teneur des différentes espèces
de champignons en alcaloïdes, par les propriétés toxicologiques de
ces substances ou par des différences dans les quantités ingérées
par les diverses populations.
Après nettoyage et mouture qui éliminent les sclérotes, il ne
subsiste dans les produits alimentaires que de faibles quantités
d'ergolines. La panification ou d'autres traitements thermiques
détruisent également la plupart des alcaloïdes du groupe de
l'ergotamine.
5. Recommandations en vue de recherches futures
5.1 Recommandations generales
Il conviendrait de créer un réseau de centres de référence pour
aider les Etats Membres à confirmer l'identité des mycotoxines
présentes dans les tissus humains et les denrées destinées à la
consommation humaine. Ces centres devraient également fournir sur
demande des échantillons de référence de mycotoxines afin de
faciliter la comparabilité des résultats d'analyse obtenus dans les
différentes régions du monde.
5.2 Ochratoxine A
a) Etudes épidémiologiques de grande ampleur à caractère
rétrospectif et études localisées à caractère prospectif sur
l'association entre l'ochratoxine A et la néphropathie
endémique des Balkans ainsi que les tumeurs des voies
urinaires : ces études devraient être menées dans la
péninsule de Balkans et la Région méditerranéenne.
b) Recherche et dosage de l'ochratoxine A dans le sang de
malades porteurs de tumeurs des voies urinaires, à
l'extérieur de la péninsule des Balkans.
c) Recherche de sources d'exposition à l'ochratoxine A, par
analyse du sang, dans les pays situés en dehors de la
péninsule des Balkans.
d) Elucidation du mécanisme à l'origine des différences entre
les sexes qui ont été relevées dans les anomalies rénales,
néoplasiques et non-néoplasiques produites par l'ochratoxine
A chez l'animal d'expérience.
e) Etudes de grande envergure sur la teneur en ochratoxine A des
denrées alimentaires dans les différentes régions du monde.
Ce type d'enquêtes est particulièrement important dans les
régions où l'on observe une forte incidence de tumeurs des
voies urinaires, notamment rénales, ou de néphropathies.
5.3 Trichothécènes
a) Il conviendrait d'effectuer des études longitudinales dans
les régions de l'Inde et de la République populaire de Chine
où ont été récemment observés des épisodes d'intoxication par
les trichothécènes. Il conviendrait de mieux éclaircir la
présence inhabituelle de certains trichothécènes dans ces
régions.
b) Il faudrait étudier les effets d'une exposition prolongée
d'animaux de laboratoire au DON, et notamment les effets
cancérogènes. Comme la réaction au DON varie beaucoup selon
les espèces, l'espèce qui sera retenue devra être choisie
avec soin.
c) Il faudrait étudier plus à fond les infections microbiennes
secondaires à une exposition aux trichothécènes chez l'animal
d'expérience.
d) Il convient d'étudier l'influence des conditions
environnementales, notamment la présence d'insecticides ou
autres produits chimiques industriels, sur la production de
trichothécènes par des champignons.
e) Il faudrait élucider l'effet des différents modes de
préparation des denrées alimentaires sur les trichothécènes.
f) Il conviendrait de mettre au point des espèces végétales qui
résistent aux champignons producteurs de trichothécènes, en
recourant aux biotechnologies.
g) Il faudrait étudier sur l'animal les effets synergistiques
éventuels d'une exposition simultanée aux trichothécènes, aux
aflatoxines, à l'ochratoxine A et à d'autres mycotoxines.
h) Il faudrait également déterminer l'apport de trichothécènes
d'origine alimentaire chez l'homme.
i) Il faudrait mettre au point des méthodes de criblage des
trichothécènes qui soient à la fois rapides et sensibles, et
mener des enquêtes dans les régions tempérées du monde pour
rechercher la présence de trichothécènes dans les céréales et
les préparations alimentaires.
5.4 Ergot
a) Il faudrait mettre au point des méthodes pour l'analyse des
agroclavines.
b) Il conviendrait de mettre à la disposition des pays en
développement des renseignements sur le repérage des semences
contaminées et sur les méthodes de mouture permettant de
réduire au minimum les problèmes posés par la présence de
l'ergot.
c) Il faudrait effectuer des études épidémiologiques sur les
effets éventuels que peut entraîner chez l'homme l'ingestion
de faible quantités d'ergolines.
d) Il faudrait effectuer des études pharmacologiques et
toxicologiques sur les ergolines, seules ou en association,
chez l'animal d'expérience.
e) Il conviendrait de déterminer si les ergolines peuvent se
transmettre de la mère à l'enfant par le lait maternel.
RESUMEN Y RECOMENDACIONES PARA ULTERIOR INVESTIGACION
1. Ocratoxina A
1.1 Distribución natural
Las ocratoxinas son producidas por varias especies de los
géneros de hongos Aspergillus y Penicillium. Esos hongos son
ubicuos y hay amplias posibilidades de contaminación de alimentos
para seres humanos y para animales. La ocratoxina A, la más
importante, se ha hallado en toda una serie de países de América
del Norte, Australia y Europa. La formación de ocratoxina por las
especies del género Aspergillus parece estar limitada a condiciones
de humedad y temperatura elevadas, mientras que algunas especies,
por lo menos, de Penicillium pueden producir ocratoxina a
temperaturas de sólo 5 °C.
Las mayores incidencias de contaminación con ocratoxina A se
han hallado en cereales y, en menor medida, en algunos granos
(café, soja, cacao). La ocratoxina B es muy poco frecuente.
1.2 Métodos de análisis
Se han desarrollado técnicas de análisis para identificar la
ocratoxina y determinar sus concentraciones en la gama de g/kg.
1.3 Metabolismo
Se han hallado residuos de ocratoxina A no modificada en la
sangre, los riñones, el hígado y los músculos de cerdos en los
mataderos y en los músculos de gallinas y pollos. Sin embargo, por
lo general no se han hallado residuos de ocratoxina A en los
rumiantes. El enlace in vitro de la ocratoxina A con la
seroalbúmina es especialmente importante en el ganado vacuno, el
ganado ovino y el ser humano. Estudios experimentales realizados
con cerdos y gallinas han demostrado que las concentraciones más
altas de ocratoxina A se hallan en los riñones. La hidroxilación
microsómica puede representar una reacción de detoxificación en los
cerdos, las ratas y el ser humano. En estudios experimentales, un
mes después de terminada la exposición se localizaron aún residuos
en riñones de cerdos.
1.4 Efectos en animales
Varios países europeos han notificado en animales de granja
(cerdos, aves de corral) casos de ocratoxicosis, cuya principal
manifestación era una nefropatía crónica. Entre las lesiones había
atrofia tubular, fibrosis intersticial y, en etapas ulteriores,
hialinización de los glomérulos. También se ha hallado ocratoxina
A en sangre de cerdo recogida en mataderos canadienses. La
ocratoxina A ha producido efectos nefrotóxicos en todas las
especies de animales provistas de un solo estómago que se han
estudiado hasta el momento, incluso con las dosis más bajas con que
se experimentó (200 µg/kg de alimentos en ratas y cerdos).
Se observaron efectos teratogénicos en ratones expuestos a
dosis de 3 mg/kg de peso corporal administradas por vía oral. En
ratas que recibieron por vía oral dosis de 0,75 mg/kg de peso
corporal se observó resorción fetal. Los efectos teratogénicos,
que en las ratas se acentuaron con una dieta baja en proteínas, se
han observado también en hámsters.
No hay datos que demuestren la actividad de la ocratoxina A en
pruebas a corto plazo de la mutagenicidad (bacterias y levaduras).
Ratas expuestas a ocratoxina A administrada por vía oral mostraron
roturas de una sola cadena del ADN en tejidos renales y hepáticos.
Ocratoxina A administrada por vía oral provocó neoplasias de
células renales en ratones machos y en ratas de uno y otro sexo.
Se registraron neoplasias de células hepáticas en sólo una estirpe
de ratones y no en la rata.
La ocratoxina A es inhibidora de la síntesis de proteínas y de
la sintetasa del ARNt en microorganismos, células de hepatoma y el
ARNm renal en la rata.
La ocratoxina A puede inhibir la migración de los macrófagos.
En ratones, una dosis de 0,005 µg/kg de peso corporal suprimió la
respuesta inmunitaria a eritrocitos de oveja; sin embargo, se han
obtenido también resultados contradictorios.
Se ha demostrado que la ocratoxina A es carcinógena para el
epitelio de los túbulos renales en ratones machos y de ratas de uno
y otro sexo.
1.5 Efectos en el ser humano
En varios países de Europa se ha observado exposición humana a
ocratoxina A, como lo demuestra la presencia de ésta en alimentos y
en la sangre y la leche humanas. Los datos epidemiológicos de que
se dispone indican que la nefropatía de los Balcanes podría estar
relacionada con el consumo de productos alimenticios contaminados
por esta toxina.
Se ha observado una relación muy significativa entre la
nefropatía de los Balcanes y tumores del tracto urinario, en
particular los de la pelvis renal y los uréteres. Sin embargo, no
se han publicado datos que demuestren el papel causal de la
ocratoxina A en la etiología de esos tumores.
2. Tricotecenos
2.1 Distribución natural
Hasta ahora se conocen 148 tricotecenos, caracterizados
químicamente por la presencia del mismo sistema básico de un anillo
tetracíclico de scirpenol. Son compuestos producidos
principalmente por hongos pertenecientes al género Fusarium,
aunque otros géneros, entre ellos Trichoderma, Trichothecium,
Myrothecium y Stachybotrys, producen también metabolitos
ahora reconocidos como tricotecenos. Se ha visto que sólo un
pequeño número de los tricotecenos conocidos contaminan los
alimentos para seres humanos o para animales, entre ellos el
deoxinivalenol (DON), el nivalenol (NIV), el diacetoxiscirpenol
(DAS) y la toxina T-2 y, con menos frecuencia, ciertos derivados
(3-Ac-DON,15-Ac-DON, fusarenon-X y toxina HT-2). De todos ellos,
el que se encuentra más a menudo en alimentos para seres humanos y
para animales es el DON, por lo general con cantidades más pequeñas
de NIV como co-contaminante. Algunos tricotecenos macrocíclicos
como las satratoxinas G y H y las verrucarinas se observan
ocasionalmente en alimentos para animales (paja, heno) pero no se
ha notificado su presencia en alimentos para seres humanos.
Estudios sobre la distribución de los tricotecenos han indicado
que el DON se encuentra en todo el mundo, sobre todo en cereales
como el trigo y el maíz, en concentraciones que pueden llegar hasta
92 mg/kg, aunque las concentraciones medias son considerablemente
inferiores y varían según el producto. Hay notificaciones aisladas
de la presencia de DON en la cebada, los piensos compuestos, las
patatas, etc. Habitualmente los cereales producidos en Canadá y
los Estados Unidos de América no contienen NIV pero éste se ha
encontrado en los cereales asiáticos y europeos, junto con DON; la
mayor concentración de NIV registrada hasta la fecha fue de 37,9
mg/kg. La presencia de toxina T-2 y DAS se ha notificado con escasa
frecuencia y en concentraciones muy inferiores.
Estudios sobre la elaboración y la molienda indican que las
concentraciones de DON apenas se reducen del cereal al producto
acabado. Tampoco se destruye cuando se cuece el cereal. En
general, los productos alimenticios para seres humanos que se
encuentran en el comercio rara vez contienen concentraciones
detectables de DON y NIV.
2.2 Métodos de análisis
Existen xistenmétodos de análisis basados en la cromatografía
de capa fina, la cromatografía de fase gaseosa, la cromatografía de
fase líquida de alto rendimiento y técnicas inmunológicas para la
determinación de las cuatro toxinas más frecuentes (DON, toxina T-
2, DAS y NIV) con límites de detección inferiores a 1 µg/g.
Algunos de esos métodos se han ensayado en colaboración. Además,
la identidad puede confirmarse mediante métodos de investigación
como cromatografía de fase gaseosa/espectografía de masas y
cromatografía de fase líquida/espectografía de masas.
2.3 Metabolismo
Se han realizado estudios metabólicos con animales, sobre todo
administrando toxina T-2 y en unos pocos casos DON. Estos
tricotecenos se absorben con rapidez por el tubo digestivo,
aunque no se dispone de datos cuantitativos. Las toxinas se
distribuyen bastante uniformemente, sin marcada acumulación en
ningún órgano o tejido determinado. Los tricotecenos se
transforman en metabolitos menos tóxicos por reacciones como
hidrólisis, hidroxilación, de-epoxidación y glucoronidación.
Tricotecenos como la toxina T-2 y el DON se eliminan rápidamente en
las heces y la orina. Por ejemplo, casi el 100% de una dosis de
toxina T-2 administrada a ganado por vía oral se eliminaba en unas
horas; en pollos, alrededor del 80% se había eliminado a las 48
horas. En la rata, 96 horas después de la administración, el 25%
del DON se había eliminado en la orina y el 65% en las heces. Los
resultados de la transmisión de toxina T-2 en gallinas ponedoras y
vacas lactantes indicaron que pasaba a los huevos y a la leche
menos del 1% de la dosis administrada de esa toxina y de sus
metabolitos. En la carne de pollo, 24 horas después de la
administración de toxina T-2 por vía oral, los residuos de ésta y
de sus metabolitos eran inferiores al 2% de la dosis.
2.4 Efectos en animales
La principal vía de exposición de los animales a tricotecenos
es la ingestión de alimentos de origen vegetal. La toxina T-2 y el
DAS, los más potentes en los experimentos de laboratorio con
animales, de todos los tricotecenos que habitualmente se consideran
contaminantes de alimentos para animales (toxina T-2, DAS, NIV y
DON), provocan una respuesta tóxica similar. El NIV es menos
potente en algunos sistemas que los dos compuestos anteriores y el
DON es el menos tóxico de los cuatro (un ejemplo de la potencia es
la DL50 por vía oral en el ratón: toxina T-2, 10,5 mg/kg de peso
corporal y DON, 46,0 mg/kg).
Los tricotecenos más potentes, como la toxina T-2 y el DAS,
producen efectos generales agudos cuando se administran
experimentalmente a roedores y ganado porcino y bovino por vía oral
o parenteral o por inhalación (cerdo, ratón). Una lesión producida
por el contacto con tricotecenos potentes como la toxina T-2 y el
DAS es la epitelionecrosis (dosis de 0,2 µg por zona en el caso de
la toxina T-2). En cuanto a otros tricotecenos, son necesarias
dosis mayores para producir un efecto irritante (NIV, 10 µg por
zona). Los tricotecenos citotóxicos, como la toxina T-2, producen
necrosis del epitelio de las criptas intestinales y de los tejidos
linfoides y hematopoyéticos, tras exposición oral o parenteral o
por inhalación. La exposición a tricotecenos citotóxicos como la
toxina T-2 y el DAS va seguida de alteraciones hematológicas y
coagulopáticas. Las toxicosis graves pueden originar
pancitopenia. En estudios con toxina T-2, DON y DAS se ha
demostrado la supresión de la inmunidad de base celular y humoral,
y entre los efectos observados figuran la reducción de las
concentraciones de inmunoglobulinas y la disminución de la
actividad fagocítaria de macrófagos y neutrófilos. Los resultados
de estudios experimentales con animales han indicado que el efecto
inmunodepresor de tricotecenos como la toxina T-2, el DAS y el DON
origina una disminución de la resistencia a la infección secundaria
por bacterias (micobacterias, Listeria monocytogenes), levaduras
(Cryptococcus neoformans) y virus (virus del herpes simplex).
Se ha comunicado que la toxina T-2 es teratogénica en el ratón,
cuando se administra por inyección intraperitoneal (vía de
administración poco corriente en los estudios de la
teratogenicidad). Se ha comunicado que el DON es teratogénico en
los ratones tras intubación gástrica, pero no en las ratas cuando
la toxina se administra con los alimentos. El NIV no es
teratogénico en el ratón. La toxina T-2, el DAS y el DON no
resultaron mutagénicos en una prueba de tipo Ames. En algunas
pruebas, se observó una débil actividad clastogénica de la toxina
T-2. En los estudios publicados sobre la toxicidad a largo plazo
en animales, no se obtuvieron datos que indiquen que la toxina T-2,
el fusarenón-X o el NIV sean oncogénicos en los animales. No se
han publicado estudios a largo plazo sobre la toxicidad del DON.
Los tricotecenos son tóxicos para las células que se dividen
activamente como las del epitelio de las criptas intestinales y las
hematopoyéticas. La citotoxicidad se ha asociado con el trastorno
de la síntesis de proteínas debido al enlace de los compuestos con
los ribosomas de las células eucarióticas o bien con la disfunción
de las membranas celulares. La inhibición de la síntesis de las
proteínas se ha relacionado con la inducción de proteínas lábiles y
reguladoras como la IL-2 en los inmunocitos. Concentraciones
extremadamente bajas de tricotecenos alteran el transporte de
pequeñas moléculas en las membranas celulares.
2.5 Efectos en el ser humano
La ingestión de alimentos contaminados de origen vegetal es la
principal vía de exposición a tricotecenos, pero ocasionalmente se
han notificado otras, por ejemplo el contacto accidental con la
piel entre los investigadores de laboratorio y la inhalación de
tricotecenos transportados por el polvo.
Los casos registrados de enfermedad asociados con la exposición
a tricotecenos son escasos y en ninguno de ellos se ha demostrado
la relación causal. No obstante, los dos brotes que se describen a
continuación parecen indicar su existencia.
En China se registró un brote de enfermedad asociado con el
consumo de trigo mohoso que contenía de 1,0 a 40,0 mg de DON/kg.
La enfermedad se caracterizó por síntomas gastro-intestinales. No
hubo defunciones. Los cerdos y los pollos alimentados con restos
de cereales también resultaron afectados.
Un brote análogo se registró en la India, asociado al consumo
de pan hecho con trigo contaminado. La enfermedad se caracterizó
por síntomas gastrointestinales e irritación de la garganta, que
aparecía de 15 minutos a una hora después de la ingestión del pan.
En muestras de la harina de trigo refinada utilizada para su
preparación se detectaron las siguientes micotoxinas: DON
(O,35-8,3 mg/kg), acetildeoxinivalenol (0,64-2,49 mg/kg),
NIV (0,03-0,1 mg/kg) y toxina T-2 (0,5-0,8 mg/kg). Sin
embargo, no hubo confirmación de la identidad de los tricotecenos
detectados. La presencia simultánea de DON y NIV y de toxina T-2
es insólita.
Dos enfermedades de interés histórico, la aleucia tóxica
alimentaria (ATA) de la URSS y la toxicosis del trigo mohoso del
Japón y Corea se han asociado con el consumo de cereales invadidos
por hongos Fusarium. Desde entonces se han identificado en
condiciones de laboratorio algunos tricotecenos en cultivos de
mohos Fusarium aislados en cereales encontrados en los incidentes.
En la época en que apareció la enfermedad, no pudieron realizarse
estudios que relacionaran la ATA o la toxicosis de los cereales
mohosos con la exposición a tricotecenos porque las toxinas no eran
conocidas.
3. Cornezuelo
3.1 Distribución natural
Cornezuelo es el nombre dado a los esclerocios de especies de
hongos pertenecientes al género Claviceps. Alcaloides biológi-
camente activos contenidos en el esclerocio provocan toxicosis
cuando éste es consumido por hombres o animales en alimentos
contaminados.
Los alcaloides del cornezuelo producen dos modalidades
diferentes de enfermedad, según el hongo de que se trate ( C.
purpurea, C. fusiformis) y, por lo tanto, los alcaloides
producidos. El ergotismo, provocado por los alcaloides ergotamina-
ergocristina producidos por C. purpurea, se caracteriza sobre
todo por gangrena de las extremidades, además de síntomas
gastrointestinales. La intoxicación resultante de la ingestión de
mijo contaminado por C. fusiformis se caracteriza principalmente
por síntomas gastrointestinales y está relacionada con los
alcaloides de tipo clavina. No hay signos ni síntomas que indiquen
la presencia de vaso-oclusión.
3.2 Métodos de análasis
Los alcaloides del cornezuelo (ergolinas) son derivados del
ácido lisérgico. Los diversos alcaloides difieren por la
importancia de su actividad biológica. La presencia de alcaloides
del cornezuelo producidos por C. purpurea se ha determinado por
cromatografía de fase líquida de alto rendimiento con detección por
fluorescencia. Pueden medirse concentraciones de 0,2 µg de
ergolina por litro de plasma humano. La presencia de ergotamina y
ergocristina puede determinarse con gran especificidad mediante
pruebas de radioinmunovaloración en concentraciones de 3,5
picomoles y 0,8 picomoles, respectivamente.
3.3 Efectos en animales
Las ergolinas, principalmente la ergotamina y la ergotaminina,
se han asociado con brotes de abortos en el ganado bovino. Ovejas
a las que se administró ergotamina por vía oral enfermaron
rápidamente y se observó en ellas inflamación intestinal. Aves de
corral, cerdos y primates expuestos por vía oral experimentaron
efectos leves. El Grupo Especial no dispuso de datos sobre la
mutagenicidad, la teratogenicidad y la carcinogenicidad de las
ergolinas.
3.4 Efectos en el ser humano
Los cereales infectados por Claviceps son fuente de exposición
humana a las ergolinas. En la mayor parte de los estudios
toxicológicos, no se han identificado los alcaloides específicos.
La información resultante de un solo estudio sobre cereales y
productos de cereales indica que en Suiza la ingesta total
de ergolinas por los seres humanos es de alrededor de 5,1 µg por
persona, siendo el contenido de ciertos productos de hasta 140
µg/kg. La cocción reduce las ergolinas presentes en la harina
contaminada un 25-100%.
En 1978 hubo en Etiopía un brote de ergotismo, resultante de
exposición a ergolinas procedentes de esclerocios de C.
purpurea. El cereal contenía hasta un 0,75% de cornezuelo; se
detectó específicamente ergometrina. Los síntomas fueron gangrena
seca, con pérdida de uno o varios miembros (29% de los casos),
pulsos periféricos débiles o ausentes (36%) y desescamación de la
piel. Sólo hubo síntomas gastrointestinales en unos pocos casos.
Trastornos de las extremidades inferiores se registraron en el 88%
de los pacientes.
En la India ha habido varios brotes desde 1958 a consecuencia
de la ingestión de mijo perlado que contenía cornezuelo de tipo
clavina producido por C. fusiformis. Los síntomas comprendían
náuseas, vómitos y mareos, y fueron causados por la ingestión de
mijo perlado con un contenido de 15 a 26 mg de ergolina/kg.
Como en ninguno de los dos episodios se practicaron autopsias,
no se dispone de información sobre los efectos anatomopatológicos
en las vísceras humanas.
4. Evaluación de los riesgos para la salud humana
4.1 Ocratoxina A
En diversos países de Europa se ha observado exposición humana
a la ocratoxina A, como lo demuestra la presencia de ésta en
alimentos y en la sangre. El Grupo Especial no conoce ningún
intento de detectar la ocratoxina A en la sangre humana en otras
partes del mundo.
El papel causal de la ocratoxina A en la nefropatía porcina se
ha demostrado sobre la base de estudios de casos sobre el terreno y
de la reproducción de la enfermedad con ocratoxina A. Utilizando
el modelo porcino, se ha supuesto que la nefropatía endémica de los
Balcanes podría obedecer a exposición a la ocratoxina A. Los datos
epidemiológicos de que se dispone indican que esa enfermedad podría
estar asociada al consumo de alimentos contaminados por dicha
toxina. Desde la publicación de Criterios de salud ambiental 11 en
1979, estudios epidemiológicos sobre concentración de ocratoxina A
en la sangre humana en zonas afectadas y no afectadas han
proporcionado un argumento más en favor de la relación entre la
nefropatía de los Balcanes y la exposición a la ocratoxina A.
Se ha demostrado que tanto la presencia de ocratoxina A en la
sangre como sus concentraciones son mayores en las personas que
residen en las zonas de endemicidad. No obstante, las pruebas
indirectas que proporcionan los mencionados estudios retrospectivos
no bastan para demostrar por sí solas la existencia de una relación
causal directa. Esta tampoco puede excluirse, dado el largo
periodo de latencia entre la exposición y el comienzo de los
síntomas.
Se ha demostrado que la ocratoxina A es carcinogénica para el
epitelio de los túbulos renales en los ratones machos y en las
ratas de uno y otro sexo. Se ha observado una relación muy
significativa entre la nefropatía de los Balcanes y los tumores del
tracto urinario, en particular de la pelvis renal y los ureteres.
Sin embargo, no hay datos publicados que demuestren un papel causal
directo de la ocratoxina A en la etiología de esos tumores.
4.2 Tricotecenos
Sobre la base de la información facilitada al Grupo Especial,
es posible asociar la exposición a tricotecenos con episodios de
enfermedad en los seres humanos. Según los limitados datos
disponibles, los tricotecenos que intervienen más frecuentemente en
episodios de exposición humana son el DON y el NIV. En los
episodios de aleucia tóxica alimentaria y toxicosis por ingestión
de cereales mohosos registrados en el pasado, no puede excluirse el
posible papel etiológico de los tricotecenos. La exposición se
produce por la ingestión de alimentos contaminados, principalmente
cereales. La elaboración, la molienda y la cocción no logran la
destrucción del DON, el NIV y la toxina T-2. Los datos en favor de
la exposición por inhalación son muy limitados, pero no puede
excluirse esa posibilidad.
Entre los tricotecenos naturalmente presentes en alimentos, el
más potente es la toxina T-2, seguida por el DAS y el NIV; el DON
resultó el menos tóxico en los estudios de la toxicidad aguda. En
los animales experimentales, la toxina T-2 y el DAS tienen efectos
generales agudos, con necrosis de tejidos epiteliales y supresión
de la hematopoyesis. En los brotes de enfermedad contemporáneos,
sólo se han registrado síntomas gastrointestinales.
Se ha demostrado que el DON es teratogénico en los ratones pero
no en las ratas. Según los estudios de la toxicidad crónica
publicados, el NIV y la toxina T-2 no son oncogénicos en los
animales. No se han publicado estudios sobre la carcino-genicidad
a largo plazo del DON. Ciertos tricotecenos, como la toxina T-2 y
el DON, tienen una acción inmunosupresora en los animales y han
producido alteraciones de la inmunidad, tanto de base celular como
humoral. No hay datos que demuestren la existencia de una acción
inmunosupresora en el hombre.
Los casos de enfermedad humana asociados con la exposición a
tricotecenos que se han notificado son limitados, tanto por su
número como por la información proporcionada. Tras la ingestión de
alimentos contaminados con tricotecenos aparecen rápidamente
síntomas de trastornos digestivos e irritación de la garganta. En
la actualidad no hay nada que demuestre que los tricotecenos
causen cáncer en el ser humano. El Grupo Especial no tuvo a su
disposición informes sobre infección secundaria por bacterias,
hongos o virus en los seres humanos tras la exposición a
tricotecenos, como las observadas en estudios experimentales con
animales. Al parecer, no se han realizado estudios suficientes
para aclarar esa sucesión.
4.3 Cornezuelo
La exposición humana a bajas concentraciones de ergolinas
parece estar muy extendida. Los datos de que se dispone sobre los
recientes brotes en Etiopía y en la India indican que los
alcaloides producidos por C. purpurea (grupo de la ergotamina)
tienen efectos más graves-entre ellos gangrena de las piernas y
muerte-que los alcaloides producidos por C. fusiformis (grupo de la
clavina), que provocaron síntomas gastrointestinales sin desenlace
mortal. No se sabe si esas diferencias corresponden a diferencias
en el contenido de alcaloides de las especies de hongos, en las
propiedades toxicológicas o toxicométricas de los alcaloides o en
las dosis ingeridas por diferentes tipos de poblaciones.
Cuando la limpieza y la molienda eliminan los esclerocios, sólo
quedan en los alimentos preparados bajas concentraciones de
ergolinas; la cocción y otras aplicaciones de calor destruyen
también la mayor parte de los alcaloides del grupo de la
ergotamina.
5. Recomendaciones para ulterior investigación
5.1 Recomendaciones generales
Debe establecerse una red de centros de referencia, que ayuden
a los Estados Miembros a confirmar la identidad de las mico-toxinas
halladas en alimentos y tejidos humanos. Esos centros deben
proporcionar también muestras de referencia de micotoxinas, cuando
se les solicite, a fin de aumentar las posibilidades de comparar
los resultados de análisis realizados en distintas partes del
mundo.
5.2 Ocratoxina A
a) En los Balcanes y en la región del Mediterráneo deben
realizarse estudios epidemiológicos retrospectivos extensos
y prospectivos focales sobre la asociación de la ocratoxina
A con la nefropatía endémica de tipo balcánico y con
tumores del tracto urinario.
b) Fuera de los Balcanes, deben realizarse, en enfermos con
tumores del tracto urinario, análisis de sangre para
detectar la presencia de ocratoxina A.
c) En países no situados en la zona de los Balcanes, debe
aclararse la fuente de la exposición a ocratoxina A, cuando
los análisis de sangre humana indiquen que la misma existe.
d) Debe aclararse la manera en que actúa la diferencia de sexo
en las enfermedades renales, neoplásicas y no neoplásicas,
causadas por la ocratoxina A en animales de
experimentación.
e) Son necesarios extensos estudios sobre el contenido de
ocra-toxina A de los alimentos en distintas partes del
mundo. Es especialmente importante realizar estudios de
ese tipo en las regiones con una elevada incidencia de
tumores del tracto urinario, tumores renales o nefropatías.
5.3 Tricotecenos
a) Deben realizarse estudios de seguimiento en las zonas de la
India y de la República Popular China en que ha habido
recientemente episodios de intoxicación de seres humanos
por tricotecenos. Debe aclararse más la modalidad no usual
de presencia de tricotecenos en esos episodios.
b) Deben estudiarse los efectos de la exposición a largo plazo
de animales de experimentación al DON, incluidos los
efectos carcinogénicos. Como la respuesta de distintas
especies al DON varía mucho, deben elegirse cuidadosamente
las especies utilizadas para el estudio.
c) Debe aclararse más la aparición de infecciones microbianas
secundarias en animales de experimentación tras la
exposición a tricotecenos.
d) Debe estudiarse la influencia de las condiciones
ambientales, incluida la presencia de insecticidas y de
otros productos químicos artificiales, en la producción de
tricotecenos por los hongos.
e) Deben aclararse los efectos de la elaboración de los
alimentos en los tricotecenos.
f) Deben desarrollarse, mediante métodos biotecnológicos,
cultivos agrícolas resistentes a la infección por hongos
productores de tricotecenos.
g) Deben estudiarse en animales de experimentación los
posibles efectos sinérgicos de la exposición combinada a
tricotecenos, aflatoxinas, ocratoxina A y otras
micotoxinas.
h) Deben realizarse estudios sobre la ingesta de tricotecenos
por seres humanos.
i) Deben elaborarse métodos rápidos y sensibles de detección
de los tricotecenos y deben realizarse estudios para
localizar los tricotecenos en cereales y alimentos tratados
en las zonas templadas.
5.4 Cornezuelo
a) Deben hallarse métodos de análisis que permitan detectar la
presencia de agroclavinas.
b) Debe proporcionarse a los países en desarrollo información
sobre el empleo de procedimientos de detección de semillas
patológicas y de molienda para reducir al mínimo los
problemas causados por el cornezuelo.
c) Deben realizarse estudios epidemiológicos sobre los
posibles efectos de bajas concentraciones de ergolinas en
la población humana.
d) Deben efectuarse estudios farmacológicos y toxicológicos en
los que se administren a animales de experimentación
distintas ergolinas, aisladas y en combinación.
e) Debe aclararse la posible trasmisión de ergolinas a los
lactantes a través de la leche materna.
III.2.3 Occurrence in foodstuffs
Traditionally, contamination of grain with ergot has been
expressed as a percentage on a weight basis, without measurement of
total and individual amounts of ergolines. Thus, it is generally
recommended that feed containing more than 0.1% ergot should not be
given to animals (Young, 1981a,b). Quantitative analysis for total
and individual ergolines in 14 samples of rye and wheat flour
(Scott & Lawrence, 1980) indicated contamination with 6 Group I
ergolines (ergometrine, ergosine, ergotamine, ergocornine,
ergocryptine, ergocristine) at concentrations ranging from 0.3 to
62 µg/kg for individual ergolines. Ergocristine was the major
ergoline present in the flours, 62 µg/kg being the maximal
concentration found. In 17 samples of rye grain collected from
health shops, 6 contained ergot; ergoline concentration and
composition were not indicated (Akerstrand, 1980). In a survey of
ergolines in ergot-contaminated cereals, in North America, the
average total ergolines content was 0.24% (Young, 1981a,b; Young &
Chen, 1982). On average, the pooled ergoline composition in
sclerotia in rye, wheat, and triticale was as follows:
ergocristine (31%), ergocristinine (13%), ergotamine (17%),
ergotaminine (8%), ergocryptine (5%), ergocryptinine (3%),
ergometrine (5%), ergometrinine (2%), ergosine (4%), ergosinine
(2%), ergocornine (4%), and ergocorninine (2%). The individual
ergoline composition was uniform throughout a single sclerotium or
in different sclerotia from the same head, somewhat less uniform
between different fields throughout a region, and highly variable
from head-to-head in a given field. In contrast, the total
ergoline content was highly variable within-sclerotium, within-
head, head-to-head, and on a field-to-field basis. In a
comparative study of ergoline in ergots from rye and wheat (caused
by infection with C. purpurea) and in ergots from pearl millet
(caused by infection with C. fusiformis) in South-East Asia, it was
found that the total ergoline content in the sclerotia of pearl
millet was much lower (320 mg/kg) than that in the sclerotia of rye
(700 mg/kg) and wheat (920 mg/kg) (Bhat et al., 1976). The
ergolines in the sclerotia of pearl millet were reported to
comprise agroclavine, elymoclavine, chanoclavine, penniclavine, and
setoclavine.
In a survey of cereals and cereal products on the Swiss market,
using a high-performance liquid chromatography procedure, the
following average concentrations of total ergolines were found:
wheat flour, 4.2 µg/kg; wheat flour (coarse), 30.7 µg/kg; wheat
flour (more coarse), 103.4 µg/kg; rye flour, 139.7 µg/kg; and
"bioproducts", 10.2-22.7 µg/kg (Baumann et al., 1985). The daily
intake of total ergolines by human beings in Switzerland was
estimated to be 5.1 µg/person.
III.2.4 Fate of ergolines during food processing
Treatment of sclerotia from wheat with chlorine (1%) and heat
(150-200 °C) resulted in a 90% reduction in ergoline content within
4 h (Young et al., 1983). The reduction affected all the ergolines
(ergotamine, ergocornine, ergocryptine, ergosine, ergometrine) in
identical ways. Autoclaving sclerotia at 121 °C for 30 min
resulted in a 24.6% reduction in total ergoline content. In the
baking of bread and pancakes using grain that contained naturally
occurring ergot, a 59-100% reduction in the individual ergolines
(ergosine, ergocornine, ergometrine, ergotamine, alpha-
ergocryptine, ergocristine) was observed in whole wheat bread, a
50-86% reduction in all-rye flour bread, and a 25-74% reduction in
triticale pancakes (Scott & Lawrence, 1982).
When bread made of rye flour spiked with finely ground
sclerotia of C. purpurea, (continuing a total ergoline
concentration of 312.8 µg/kg), was baked, there was an overall
reduction of 50% in the ergoline concentration, as measured by high
performance liquid chromatography (Baumann et al., 1985).
III.3 METABOLISM
No published information is available on the metabolism in
animals or human beings of ergots containing ergolines and
combinations of individual ergolines.
III.4 EFFECTS ON ANIMALS
III.4.1 Field studies
An outbreak of bovine abortion associated with the ingestion of
ergot was reported by Appleyard (1986).
Eleven out of 36 suckler cows, all in late pregnancy, aborted
in 7-11 days following introduction to a rye grass pasture heavily
infested with ergot. At least 25% of the rye seed heads contained
sclerotia of C. purpurea, with up to 8 sclerotia present on any one
seed head. The sclerotia contained 1.57 mg total ergolines/g,
consisting of 67% ergotamine and 17% ergotaminine, and smaller
amounts of ergometrine. Ergocryptine and ergosine were also
present together with their corresponding -inine isomers.
Ten out of the 11 calves were delivered dead. None of the
aborting cows showed any premonitory signs of calving and, after
parturition, there was almost complete agalactia. The placenta was
retained in each case, but there was no other evidence of ill
health in the cows. Any other cause of abortion of bacterial,
viral, fungal, or toxic origin was ruled out, on the basis of
laboratory and field investigations.
III.4.2 Experimental animal studies
This topic has been reviewed by Ainsworth & Austwick (1959) and
Mantle (1977c). It appears that most reports of field cases and
experimental animal studies on ergotism do not contain any
information on the contents of individual ergolines in the ergot
associated with disease manifestations in animals.
III.4.2.1 Cattle
Lameness, sometimes leading to gangrene, is a common symptom
observed in cattle when the feed contains more than 10 g ergot/kg.
The symptoms are more pronounced when the animals are kept outside
under cold weather conditions (Mantle, 1977c). Four out of 6
animals administered ergotamine tartrate, orally, at 1 mg/kg body
weight per day, died within 10 days (Woods et al., 1966). The
animals became acutely ill within 1-2 days, the principal signs
being anorexia, hyperventilation, cold extremities, salivation,
and, occasionally, tongue necrosis. Post-mortem examination of the
most seriously affected animals revealed extensive intestinal
inflammation.
III.4.2.2 Sheep
Four lambs were administered aqueous suspensions of milled
ergot (from C. purpurea) through a stomach tube, over a 2-month
period (Loken, 1984). The doses ranged from 0.12 to 0.75 g
sclerotia/kg body weight. The sclerotia contained approximately 4
g ergolines/kg, composed of ergotamine (15%), ergosine (35%), and
ergocristine (5% each). One lamb, dosed with 0.12 g sclerotia/kg
body weight, was kept indoors at 15-17 °C and did not develop any
symptoms. The other 3 animals, given higher doses and kept
outdoors, became ill after 2-6 days, with signs that included
dullness, inappetence, high pulse rate, diarrhoea, edema of the
hind legs and tail, and lameness. Post-mortem findings included
inflammation and necrosis of the forestomach and intestinal mucosa.
III.4.2.3 Poultry
Leghorn chickens were fed diets containing ergotamine tartrate
at levels of up to 800 mg/kg in 7 to 10-day trials as well as in a
51-day trial (Young & Marquardt, 1982). In the short-term
trials, only the highest level (800 mg/kg) had an effect on
performance, in terms of a slight decrease in growth rate and a
slight increase in feed consumption. At the 250 mg/kg level, toe
necrosis was observed, as well as cardiomegaly. There were no
pathological effects on the brain, liver, or muscle tissues, even
at the highest level. In the long-term study (51 days), effects
were similar to those observed in the 7 to 10-day trials. No
residues of ergotamine were detected in tissues.
III.4.2.4 Swine
Ergot (from C. purpurea) containing 0.3% ergolines
(composition: ergotamine and ergosine) was used in a feeding study
on pigs (Mantle, 1977c). A diet containing ergot at 40 g/kg was
well tolerated, and the only effect at 100 g ergot/kg diet was
depression of the growth rate. Agalactia in the sow was observed
after feeding with rations containing ergot from C. purpurea as
well as from C. fusiformis.
III.4.2.5 Primates
Male rhesus monkeys in groups of 2-4 animals were dosed with
ergot from C. fusiformis, either as part of the diet, or as an
ergoline extract administered orally or intraperitoneally (Bhat &
Roy, 1976). The ergoline extract contained agroclavine (major
components: elymoclavine, chanoclavine, penniclavine, and
setoclavine). The period of treatment lasted from 2 days to one
month. There were no effects on the animals, with the exception of
those injected ip with 5.44-11.1 mg total ergoline/kg body weight,
who developed signs within 10 min including drowsiness,
hyperexcitation, redness of face, and loss of response to thermal
and tactile stimuli in the hind limbs and tail. The animals
recovered spontaneously in about 60 min. Animals administered a
total of 10 mg ergolines/kg body weight, orally, showed symptoms of
hyperexcitation, but these were far less severe than those after
intraperitoneal injection. It was concluded that the signs in
monkeys, in particular hyperexcitation, are different from those
observed in human beings after ingestion of ergot in pearl millet.
III.5 EFFECTS ON MAN
The history of ergotism in man, following ingestion of ergot
from C. purpurea, was reviewed by Barger (1931). Numerous
epidemics in Europe occurred between the 9th and the 18th century.
Two types of disease were noted: gangrenous and convulsive
ergotism. In the first type, the affected part (arm or leg)
shrank, became mummified and dry, and the gangrene gradually spread
upwards. In convulsive ergotism, the whole body was attacked by
general convulsion, which returned at intervals of a few days. The
latest outbreaks of ergotism in Europe occurred in 1926-28 in the
United Kingdom and the USSR. A suspected episode in France in 1951
turned out to be due to a different toxic substance (Gadiou, 1965).
III.5.1 Ergometrine-related outbreaks
In 1978, an epidemic was reported in Ethiopia (Demeke et al.,
1979; King, 1979; Pokrovskij & Tutelyan, 1982). The episode
occurred in the Wollo region, following two years of drought.
During this time, the locally grown barley, the staple food, had
become dominated by wild oats heavily contaminated with C. purpurea
sclerotia. The grain consisted of 70% wild oats, 12% barley, and
0.75% ergot; ergometrine was detected in the sclerotia by thin
layer chromatography. A total of 93 cases of ergotism was reported
during the spring of 1978. The male:female ratio was 2.5:1; more
than 80% of affected persons were between five and 34 years of age.
In addition to the 93 cases, 47 deaths were reported as having been
due to ergotism. Examination of 44 patients out of the 93
registered revealed ongoing dry gangrene of the whole or part of
one or more limbs (7.5%), feeble or absent peripheral pulses
(36.4%), swelling of limbs (11.2 %), desquamation of the skin
(12.8%), and loss of one or more limbs (21.5%). It was noted that
88% of patients had involvement of the lower extremities. The most
common general symptoms were weakness (78.5%), formication (15%),
burning sensation (14.3%), nausea (7.2%), vomiting (5.6%), and
diarrhoea (6.8%). In addition, 50-60 infants and young children
died from starvation due to failure of the mothers to lactate.
This may have been related to the effect of ergot on lactation
(Demeke et al., 1979). No autopsies were performed, and thus there
is no information on pathological changes in the viscera.
III.5.2 Clavine-related outbreaks
Intoxication following ingestion of ergot from C. fusiformis in
bajra or pearl millet has been reported from India. Symptoms
included nausea, vomiting, and giddiness. Several outbreaks have
been observed since 1958, when the first report was published; the
latest occurred in the autumn of 1975 in the state of Rajasthan
(Krishnamachari & Bhat, 1976). In 21 villages surveyed, 78 persons
belonging to 14 households developed symptoms, characterized by
nausea, repeated vomiting, and giddiness, followed by drowsiness
and prolonged sleepiness, extending sometimes to over 24-48 h.
There were no signs or symptoms suggesting vaso-occlusion. The
disease generally developed 1-2 h following a single meal.
Domestic camels, offered the contaminated grain as feed, also
developed sleepiness and signs suggesting abdominal discomfort.
The pearl millet from affected villages contained 15-174 g
ergot/kg, resulting in a contamination of the grain with 15-199 mg
total ergolines/kg. The individual ergolines were identified as
agroclavine, elymoclavine, chanoclavine, penniclavine, and
setoclavine. Pearl millet from villages with no cases of
intoxication contained 1-38 g ergot/kg with a total ergoline
content of 15-26 mg/kg. Since there were no deaths, no information
on pathological changes is available. The number of households
studied was too small for no-effect levels to be calculated, but
the authors suggested that the intake of 28 µg total ergolines/kg
body weight would be non-toxic.
III.6 EVALUATION OF THE HUMAN HEALTH RISKS
Human exposure to low levels of ergolines appears to be
widespread. Available data from the recent outbreaks in Ethiopia
and India indicate that the C. purpurea alkaloids (ergotamine
group) produced more severe effects, including gangrene of the legs
and death, than the alkaloids of C. fusiformis (clavine group),
which caused gastrointestinal symptoms without a fatal outcome.
It is not known whether such differences can be accounted for by
differences in the alkaloid content of the fungal species, in the
toxicological or toxicometric properties of the alkaloids, or in
the levels of intake by different types of populations.
Only low levels of ergolines remain in prepared foods as
cleaning and milling processes remove the sclerotia; baking or
other heat processing also destroys most alkaloids of the ergotamin
group.
REFERENCES
ABBAS, H.K., MIROCHA, C.J., PAWLOSKY, R.J., & PUCSH, D.J. (1985)
Effect of cleaning, milling, and baking on deoxynivalenol in wheat.
Appl. environ. Microbiol., 50(2): 482-486.
ABBAS, H.K., MIROCHA, C.J., & TUITE, J. (1986) Natural occurrence of
deoxynivalenol, 15-acetyl-deoxynivalenol and zearalenone in refusal
factor corn stored since 1972. Appl. environ. Microbiol., 51:841-843.
ABDEL-HAFEZ, S.I.I., EL-KADY, I.A., MAZEN, M.B., & EL-MAGHRABY,
O.M.O. (1987) Mycoflora and trichothecene toxins of paddy grains from
Egypt. Mycopathologia, 100: 103-112.
ABRAHAMSON, D., MILLS, J.T., & BOYCOTT, B.R. (1983) Mycotoxins and
mycoflora in animal feedstuffs in western Canada. Can. J. comp. Med.,
47: 23-26.
AGRELO, C.E. & SCHOENTAL, R. (1980) Synthesis of DNA in human
fibroblasts treated with T-2 toxin and HT-2 toxin (the trichothecene
metabolites of Fusarium species) and the effects of hydroxyurea.
Toxicol. Lett., 5(2): 155-160.
AINSWORTH, G.C. & AUSTWICK, P.K.C. (1959) Fungal diseases of
animals, Farnham Royal, Commonwealth Agricultural Bureaux, pp. 100-
111.
AKERSTRAND, K. (1980) [Ergots.] Var Föda, 32: 442-446 (in Swedish).
AKERSTRAND, K. & JOSEFSON, E. (1979) [Fungi and mycotoxins in beans
and peas.] Var Föda, 31: 405-414 (in Swedish).
APPELGREN, L.-E. & ARORA, R.G. (1983) Distribution of 14C-labelled
ochratoxin A in pregnant mice. Food chem. Toxicol., 21: 563-568.
APPLEYARD, W.T. (1986) Outbreak of bovine abortion attributed to
ergot poisoning. Vet. Res., 118: 48-49.
ARENS, H. & ZENK, M.H. (1980) [Radioimmunoassay for the
determination of the peptide alkaloids ergotamine and ergocristine in
Claviceps purpurea.] Planta med., 38: 214-226 (in German).
ARORA, R.G., FROLEN, H., & FELLNER-FELDEGG, H. (1983) Inhibition of
ochratoxin A teratogenesis by zearalenone and diethylstilboestrol.
Food chem. Toxicol., 21: 779-783.
ATKINSON, H.A.C. & MILLER, K. (1984) Inhibitory effect of
deoxynivalenol, 3-acetyldeoxynivalenol and zearalenone on induction
of rat and human erythrocyte proliferation. Toxicol. Lett, 23: 215-221.
BALZER, I., BOGDANIC, C., & MUZIC, S. (1977) Natural contamination
of corn (Zea mays) with mycotoxins in Yugoslavia. Ann. Nutr.
Aliment., 31(4-6): 425-430.
BAMBURG, J.R. (1976) Chemical and biochemical studies of the
trichothecene mycotoxins. In: Rodricks, J.V., ed. Mycotoxins and
other fungal related food problems, Washington, DC, American Chemical
Society, pp. 144-162 (Advances in Chemistry Series No. 149).
BAMBURG, J.R. & STRONG, F.M. (1969) Mycotoxins of the trichothecene
family produced by Fusarium tricinctum and Trichoderma lignorum.
Phytochemistry, 8(12): 2405-2410.
BAMBURG, J.R. & STRONG, F.M. (1971) 12,13-Epoxytrichothecenes. In:
Kadis, S., Ciegler, A., & Ajl, S.J. ed. Microbiological toxins. VII.
Algal and fungal toxins, New York, London, Academic Press, pp. 207-
292.
BAMBURG, J.R., MARASAS, W.F., RIGGS, N.V., SMALLEY, E.B., & STRONG,
F.M. (1968a) Toxic compounds from Fusaria and other Hyphomycetes.
Biotechnol. Bioeng., 10(4): 445-455.
BAMBURG, J.R., RIGGS, N.V., & STRONG, F.M. (1968b) The structures
of toxins from two strains of Fusarium tricinctum. Tetrahedron,
24(8): 3329-3336.
BARGER, G. (1931) Ergot and ergotism, London, Gurney & Jackson,
279 pp.
BATA, A., VANYI, A., & LASZTITY, R. (1983) Simultaneous detection of
some fusariotoxins by gas-liquid chromatography. J. Assoc. Off. Anal.
Chem., 66: 577-581.
BAUER, J. & GAREIS, M. (1987) [Ochratoxin A in the food chain.] J.
vet. Med., B34: 613-627 (in German).
BAUER, V.J., WERMTER, S., & GEDEK, B. (1980) Contamination of
feedstuffs with toxin-producing strains of Fusaria and their toxins.
Wiener Tieraerztl. Monatschrift., 67(10): 282-288.
BAUER, J., GAREIS, M., & GEDEK, B. (1984) [Detection and occurrence
of ochratoxin A in pigs for slaughter]. Berl. Muench. Tieraerztl.
Wschr. 97: 279-283 (in German).
BAUER, J., BOLLWAHN, W., GARCIS, M., GEDEK, B., & HEINRITZI, K.
(1985) Kinetic profiles of diacetoxyscirpenol and two of its
metabolites in blood serum of pigs. Appl. environ. Microbiol.,
49: 642-845.
BAUER, J., NIEMIEC, J. & SCHOLTYSSEK, S. (1988) [Ochratoxin A in feed
for laying hens. Second communication: Residues in serum, liver and
eggs.] Arch. Geflügelkd., 52: 71-75 (in German).
BAUMANN, U., HUNZIKER, H.R., & ZIMMERLI, B. (1985) [Ergot alkaloids
in Swiss grain products.] Mitt. Geb. Lebensm. Hyg., 76: 609-630 (in
German).
BEASLEY, V.R., SWANSON, S.P., CORLEY, R.A., BUCK, W.B., KORITZ, G.D.,
& BURMEISTER, H.R. (1986) Pharmacokinetics of the trichothecene
mycotoxin, T-2 toxin, in swine and cattle. Toxicon, 21: 13-23.
BEGLEY, P., FOULGER, B.E., JEFFERY, P.D., BLACK, R.M., & READ, R.W.
(1986) Detection of trace levels of trichothecenes in human blood
using capillary gas chromatography-electron-capture negative ion
chemical ionisation mass spectrometry. J. Chromatogr., 367: 87-101.
BELT, R.J., HAAS, C.D., JOSEPH, U., GOODWIN, W., MOORE, D., &
HOOGSTRATEN, B. (1979) Phase I study of anguidine administered
weekly. Cancer Treat. Rep., 63: 1993-1995.
BENDELE, S.A., CARLTON, W.W., KROGH, P., & LILLEHOJJ, E.B. (1985)
Ochratoxin A carcinogenesis in the (C57BL/6YxC3HF)1 mouse. J. Natl
Cancer Inst., 75: 733-739.
BERDE, B. & SCHILD, H.O. (1978) Ergot alkaloids and related
compounds, Berlin, Springer Verlag, pp. 1-1003.
BERGMANN, F., YAGEN, B., & SOFFER, D. (1985) Toxic and lethal effects
of T-2 toxin upon intracerebral administration to rats. Arch.
Toxicol., 58: 40-44.
BERNDT, W.O. & HAYES, A.W. (1979) In vivo and in vitro changes in
renal function caused by ochratoxin A in the rat. Toxicology, 12:
5-17.
BHAT, R.V. & ROY, D.N. (1976) Toxicity study of ergoty bajra (pearl
millet) in rhesus monkeys. Indian J. med. Res., 64: 1629-1633.
BHAT, R.V., ROY, D.N., & TULPULE, P.G. (1976) The nature of
alkaloids of ergoty pearl millet or bajra and its comparison with
alkaloids of ergoty rye and ergoty wheat. Toxicol. appl. Pharmacol.,
36: 11-17.
BHAT R.V., RAMAKRISHNA, Y., RAO, B.S., & NAHDI, S. (1987a)
Trichothecene mycotoxicosis, Hyderabad, India, Food and Drug
Toxicology Research Centre, National Institute of Nutrition.
BHAT, R.V., RAMAKRISHNA, Y., RAO, B.S,. & NAHDI, S. (1987b).
Trichothecene mycotoxicosis, Hyderabad, India, Food and Drug
Toxicology Research Centre, National Institute of Nutrition.
BHAT, R.V., BEEDU, S.R., RAMAKRISHNA, Y., & MUNSHI, K.L. (1989a)
Outbreak of trichothecene mycotoxicosis associated with consumption
of mould-damaged wheat products in Kashmir valley, India. Lancet, 7
January: 35-37.
BHAVANISHANKAR, T.R. & SHANTHA, T. (1987) Natural occurrence of
Fusarium toxins in peanuts, sorghum and maize from Mysore (India).
J. Sci. Food. Agric., 40: 327-332.
BHAVANISHANKAR, T.R., RAMESH, H.P., & SHANTHA, T. (1988) Dermal
toxicity of Fusarium toxins in combinations. Arch. Toxicol., 61:
241-244.
BIANCHI, M.L., PERELLINO, N.C., GIOIA, B., & MINGHETTI, A. (1982)
Production by Claviceps purpurea of two new peptide ergot alkaloids
belonging to a new series containing alpha-aminobutyric acid. J. nat.
Prod., 445: 191-196.
BILAI, V.I. (1977) [Fuzarii,] Kiev, USSR, Naukova Dumka, (in
Russian).
BLACK, R.M., CLARKE, R.J., & READ, R.W. (1986) Detection of trace
levels of trichothecene mycotoxins in human urine by gas
chromatography-mass spectrometry. J. Chromatogr., 367: 103-115.
BLACK, R.M., CLARKE, R.J., & READ, R.W. (1987) Detection of trace
levels of trichothecene mycotoxins in environmental residues and
foodstuffs using gas chromatography with mass spectrometric or
electron-capture detection. J. Chromatogr., 388: 365-378.
BLASS, W., KELLERT, M., STEINMEYER, S., TIEBACH, R., & WEBER, R.
(1984) Determination of deoxynivalenol and nivalenol in cereals at
the mg/kg concentrations range. Z. Lebensm. Unters. Forsch., 80:
104-108.
BODON, L. & ZOLDAG, L. (1974) Cytotoxicity studies on T-2
fusariotoxin. Acta vet. Acad. Sci. Hung., 24(3): 451-455.
BOHNER, F.E., FETZ, E., HARRI, E., SIGG, H.P., STOLL, CH., & TAMM,
CH. (1965) Verrucarine and roridin. VIII. Isolation of verrucarin
H, verrucarin J, roridin D, and roridin E from Myrothecium species.
Helv. Chim. Acta, 48(5): 1079-1089.
BOONCHUVIT, B., HAMILTON, P.B., & BURMEISTER, H.R. (1975)
Interaction of T-2 toxin with Salmonella infections of chickens.
Poult. Sci., 54(5): 1693-1696.
BOORMAN, G. (1988) Technical report on the toxicology and
carcinogenesis studies of ochratoxin A in F334/N rats. National
Toxicology Program, US Department of Health and Human Services,
Research Triangle Park, North Carolina (NIH Publication No. 88-28/3).
BOORMAN, G.A., HONG, H.L., DIETER, M.P., HAYES, H.T., POHLAND, A.E.,
STACK, M., & LUSTER, M.I. (1984) Myelotoxicity and macrophage
alteration in mice exposed to ochratoxin A. Toxicol. appl.
Pharmacol., 72: 304-312.
BOVE, F.J. (1970) The story of ergot, Basel, S. Karger, 297 pp.
BRADLAW, J.A., SWENTZEL, K.C., ALTERMAN, E., & HAUSWIRTH, J.W. (1985)
Evaluation of purified 4-deoxynivalenol (vomitoxin) for unscheduled
DNA synthesis in the primary rat hepatocyte-DNA repair assay. Food
chem. Toxicol., 23: 1063-1067.
BRIAN, P.W., DAWKINS, A.W., GROVE, J.F., HEMMING, H.G., LOWE, D., &
HORRIES, G.L.F. (1961) Phytotoxic compounds produced by Fusarium
equiseti. J. exp. Bot., 12: 1-21.
BROWN, M.H., SZCZECH, G.M., & PURMALIS, B.P. (1976) Teratogenic and
toxic effects of ochratoxin A in rats. Toxicol. appl. Pharmacol., 37:
331-338.
BRUMLEY, W.C., ANDRZEJEWSKI, D., TRUCKSESS, E.W., DREIFUSS, P.A.,
ROACH, J.A.G., EPPLEY, R.M., THOMAS, F.S., THORPE, C.W., & SPHON,
J.A. (1982) Negative ion chemical ionization mass spectrometry of
trichothecenes - Novel fragmentation under OH conditions. Biomed.
mass Spectrom., 9: 451-458.
BRUMLEY, W.C., TRUCKSESS, M.W., ADLER, S.H., COHEN, C.K., WHITE,
K.D., & SPHON, J.A. (1985) Negative ion chemical ionization mass
spectrometry of deoxynivalenol (DON): Application to identification
of DON in grains and snack foods after quantification/isolation by
thin-layer chromatography. J. agric. food Chem., 33:326-330.
BUENING, G.M., MANN, D.D., HOOK, B., & OSWEILER, G.D. (1982) The
effect of T-2 toxin on the bovine immune system: cellular factors.
Vet. Immunol. Immunopathol., 3: 411-417.
BUNGE, I., DIRHEIMER, G., & ROSCHENTHALER, R. (1978) in vivo and in
vitro inhibition of protein synthesis in Bacillus stearothermophilus
by ochratoxin A. Biochem. biophys. Res. Commun., 83: 398-405.
BUNNER, D.L. & MORRIS, E.R. (1988) Alteration of multiple cell
membrane functions in L-6 myoblasts by T-2 toxin: an important
mechanism of action. Toxicol. appl. Microbiol., 92: 113-121.
CANDLISH, A.A.G., STIMSON, W.H., & SMITH, J.E. (1986) A monoclonal
antibody to ochratoxin A. Lett. appl. Microbiol., 3: 9-11.
CARRASCO, L., BARBACID, M., & VAZQUEZ, D. (1973) The trichodermin
group of antibiotics inhibitors of peptide bond formation by
eukaryotic ribosomes. Biochim. Biophys. Acta, 312(2): 368-376.
CARSON, M.S. & SMITH, T.K. (1983) Role of bentonite in prevention of
T-2 toxicosis in rats. J. anim. Sci., 57: 1498-1506.
CARTER, C.J., CANNON, M., & SMITH, K.E. (1976) Inhibition of
protein synthesis in reticulocyte lysates by trichodermin. Biochem.
J., 154: 171-178.
CARTER, C.J., CANNON, M., & JIMENET, A. (1980) A trichodermin
resistant mutant of Saccharomyces cerevisiae with an abnormal
distribution of native ribosomal subunits. Eur. J. Biochem., 107
(1): 173-184.
CASALE, W.L., PESTKA, J.J., & HART, L.P. (1988) Enzyme-linked
immunosorbent assay employing monoclonal antibody specific for
deoxynivalenol (vomitoxin) and several analogues. J. agric. food
Chem., 36: 663-668.
CEOVIC, S., GRIMS, P., & MITAR, J. (1976) [The incidence of tumours
of the urinary organs in a region of endemic nephropathy and in a
control region.] Lijec. Vjesn., 98: 301-304 (in Croatian).
CHAKRABARTI, D.K. & HOSAL, S. (1986) Occurrence of free and
conjugated 12,13-epoxytrichothecenes and zearalenone in banana fruits
infected with Fusarium moniliforme. Appl. environ. Microbiol.,
51(1): 217-219.
CHANG, F.C. & CHU, F.S. (1977) The fate of ochratoxin A in rats.
Food Cosmet. Toxicol., 15: 199-204.
CHANG, K., KURTZ, J.J., & MIROCHA, C.J. (1979) Effects of the
mycotoxin zearalenone on swine reproduction. Am. J. vet. Res.,
40: 1260-1267.
CHATTERJEE, K., VISCONTI, A., & MIROCHA, C.J. (1986) Deepoxy T-2
tetraol: a metabolite of T-2 toxin found in cow urine. J. agric.
food Chem., 34: 695-697.
CHELKOWSKI, J., VISCONTI, A., & MANAKA, M. (1984) Production of
trichothecenes and zearaleneone by Fusarium species isolated from
wheat. Nahrung, 28: 493-496.
CHERNOZEMSKY, I.N., STOYANOV, I.S., PETKOV-ABOCHAROVA, T.K., NOCOLOV,
I.G., DRAGANOV, I.V., STOICHEV, I., TANCHEV, Y., NAIDENOV, D., &
KALCHEVA, N.D. (1977) Geographic correlation between the occurrence
of endemic nephropathy and urinary tract tumours in Vratza district,
Bulgaria. J. Cancer, 19: 1-11.
CHI, M.S., MIROCHA, C.J., KURTZ, H.J., WEAVER, G., BATES, F., &
SHIMODA, W. (1977a) Effects of T-2 toxin on reproductive
performance and health of laying hens. Poult. Sci., 56(2): 628-637.
CHI, M.S., MIROCHA, C.J., KURTZ, H.J., WEAVER, G., BATES, F.,
SHIMODA, W., & BURMEISTER, H.R. (1977b) Acute toxicity of T-2 toxin
in broiler chicks and laying hens. Poult Sci., 56(1): 103-116.
CHI, M.S., MIROCHA, C.J., KURTZ, H.J., WEAVER, G., BATES, F., &
SHIMODA, W. (1977c) Subacute toxicity of T-2 toxin in broiler
chicks. Poult. Sci., 56(1): 306-313.
CHI, M.S., ROBISON, T.S., MIROCHA, C.J., BEHRENS, J.C., & SHIMODA, W.
(1978a) Transmission of radioactivity into eggs from laying hens
(Gallus domesticus) administered tritium labeled T-2 toxin. Poult.
Sci., 57(5): 1234-1238.
CHI, M.S., ROBISON, T.S., MIROCHA, C.J., SWANSON, S.P., & SHIMODA, W.
(1978b) Excretion and tissue distribution of radioactivity from
tritium labeled T-2 toxin in chicks. Toxicol. appl. Pharmacol.,
45(2): 391-402.
CHI, M.S., EL-HALAWANI, M.E., WAIBEL, P.E., & MIROCHA, C.J. (1981)
Effects of T-2 toxin on brain catecholamines and selected blood
components in growing chickens. Poult. Sci., 60(1): 137-141.
CHIBA, J., NAKANO, N., MOROOKA, N., NAKAZAWA, S., & WATANABE, Y.
(1972) Inhibitory effects of fusarenon-X, a sesquiterpene mycotoxin
on lipid synthesis and phosphate uptake in Tetrahymena pyriformis.
Jpn. J. med. Sci. Biol., 25(5): 291-296.
CHO, B.R. (1964) Toxicity of water extracts of scabby barley to
suckling mice. Am. J. vet. Res., 25: 1267-1270.
CHU, F.S. (1971) Interaction of ochratoxin A with bovine serum
albumin. Arch. Biochem. Biophys., 147: 359-366.
CHU, F.S. (1974a) Studies on ochratoxins. CRC crit. Rev. Toxicol.,
2(4): 499-524.
CHU, F.S. (1974b) A comparative study of the interaction of
ochratoxins with bovine serum albumin. Biochem. Pharmacol., 23(6):
1105-1113.
CHU, F.S. & BUTZ, M.E. (1970) Spectrophotofluorodensitometric
measurement of ochratoxin A in cereal products. J. Assoc. Off. Anal.
Chem., 53(6): 1253-1257.
CHU, F.S., GROSSMAN, S., WEI, R.D., & MIROCHA, C.J. (1979)
Production of antibody against T-2 toxin. Appl. environ. Microbiol.,
37(1): 104-108.
CHUNG, C.W., TRUCKSESS, M.W., GILES, A.L., Jr, & FRIEDMAN, L. (1974)
Rabbit skin test for estimation of T-2 toxin and other skin
irritating toxins in contaminated corn. J. Assoc. Off. Anal. Chem.,
57(5): 1121-1127.
CHUNG, H.S. (1975) Cereal scab causing mycotoxicoses in Korea and
present status of mycotoxin researches. Korean J. Mycol., 3(1):
31-36.
CIRILLI, G. (1983) Trichothecene problems in Italy. In: Ueno, Y.,
ed. Developments in food science. IV. Trichothecenes, New York,
Elsevier, pp. 254-258.
COFFIN, J.L. & COMBS, G.F., Jr (1981) Impaired vitamin E status of
chicks fed T-2 toxin. Poult. Sci., 60(2): 385-392.
COHEN, H. & LAPOINTE, M. (1982) Capillary gas-liquid
chromatographic determination of vomitoxin in cereal grains. J.
Assoc. Off. Anal. Chem., 65(6): 1429-1434.
COHEN, H. & LAPOINTE, M. (1984) Capillary gas chromatographic
determination of T-2 toxin, HT-2 toxin and diacetoxyscirpenol in
cereal grains. J. Assoc. Off. Anal. Chem., 67: 1105-1107.
COLE, R.J. & COX, R.H. (1981) Handbook of toxic fungal metabolites,
New York, London, San Francisco, Academic Press, pp. 152-263.
COLINSKI, P. (1987) [Ochratoxin A in humans as a result of food and
feed contamination,] Poznan, Rocznalion Akademii Rolniczej (Doctoral
thesis) (in Polish).
COLLINS, G.J. & ROSEN, J.D. (1979) Gas-liquid chromatographic/mass
spectrometric screening method for T-2 toxin in milk. J. Assoc. Off.
Anal. Chem., 62(6): 1274-1280.
COLLINS, G.J. & ROSEN, J.D. (1981) Distribution of T-2 toxin in
wet-milled corn products. J. food Sci., 46(3): 877-879.
CONNOLE, M.D., BLANEY, B.J., & MCEWAN, T. (198l) Mycotoxins in
animal feeds and toxic fungi in Queensland 1971-1980. Aust. vet. J.,
57: 314-318.
CONNOR, M.W., CAMARGO, J.D., PANYANT, P., RIENGROPITOK, S., ROGERS,
A.E., & NEWBUNE, P.M. (1986) Toxicity of anguidine in mice. Fundam.
appl. Toxicol., 7: 153-164.
COORAY, R. (1984) Effects of some mycotoxins on mitogen-induced
blastogenesis and SCE frequency in human lymphocytes. Food Chem.
Toxicol., 22: 529-534.
COPPOCK, R.W., SWANSON, S.P., GELBERG, H.B., KORITZ, G.D., HOFFMAN,
W.E., BUCK, W.B., & VESONDER, R.F. (1985) Preliminary study of the
pharmacokinetics and toxicopathy of deoxynivalenol (vomitin) in
swine. Am. J. vet. Res., 46: 169-174.
CORLEY, R.A., SWANSON, S.P., & BUCK, W.B. (1985) Glucuronide
conjugates of T-2 toxin and metabolites in swine bile and urine. J.
agric. food Chem., 33: 1085-1089.
CORLEY, R.A., SWANSON, S.P., GULLO, G.J., JOHNSON, L., BEASLEY, V.R.,
& BUCK, W.B. (1986) Disposition of T-2 toxin, a trichothecene
mycotoxin, in intravascularly dosed swine. J. agric. food Chem.,
34: 868-875.
CORRIER, D.E. & ZIPRIN, R.L. (1986a) Immunotoxic effects of T-2 toxin
on cell-mediated immunity to listeriosis in mice: comparison with
cyclophosphamide. Am. J. vet. Res., 47: 1956-1960.
CORRIER, D.E. & ZIPRIN, R.L. (1986b) Enhanced resistance to
listeriosis induced in mice by preinoculation treatment with T-2
mycotoxin. Am. J. vet. Res., 47: 856-859.
COSGRIFF, T.M., BUNNER, D.P., WANNEMACHER, R.W., Jr., HODGSON, L.A.,
& DINTERMAN R.E. (1984) The hemostatic derangement produced by T-2
toxin in guinea pigs. Toxicol. appl. Pharmacol., 76: 454-463.
COSGRIFF, T.M., BUNNER, D.P., WANNEMACHER, R.W., Jr., HODGSON, L.A.,
& DINTERMAN, R.E. (1986) The hemostatic derangement produced by T-2
toxin in Cynomolgus monkeys. Toxicol. appl. Pharmacol., 82: 532-539.
COTE, L.M., REYNOLDS, J.D., VESONDER, R.F., BUCK, W.B., SWANSON,
S.P., COFFEY, R.T., & BROWN, D.C. (1984) Survey of vomitoxin-
contaminated feed grains in midwestern United States, and associated
health problems in swine. J. Am. Vet. Med. Assoc., 184(2): 189-192.
COTE, L.M., DAHLEM, A.M., YOSHIZAWA, T., SWANSON, S.P., & BUCK, W.B.
(1986) Excretion of deoxynivalenol and its metabolite in milk, urine
and feces of lactating dairy cows. Agric. biol. Chem., 50: 227-229.
CREPPY, E.E., LUGNIER, A.A.J., FASIOLO, F., HELLER, K.,
ROSCHENTHALER, R., & DIRHEIMER, G. (1979a) In vitro inhibition of
yeast phenylalanyl-tRNA synthetase by ochratoxin A. Chem.-biol.
Interact., 24: 257-261.
CREPPY, E.E., LUGNIER, A.A.J., BECK, G., ROSCHENTHALER, R., &
DIRHEIMER, G. (1979b) Action of ochratoxin A on cultured hepatoma
cells - reversion of inhibition by phenylalanine. FEBS Lett., 104:
287-290.
CREPPY, E.E., SCHLEGEL, M., ROSCHENTHALER, R., & DURHEINSER, G.
(1980) Phenylalamine prevents acute poisoning by ochratoxin A in
mice. Toxicol. Lett., 6: 77-80.
CREPPY, E.E., STORMER, F.O., ROSCHENTHALER, R., & DIRHEIMER, G.
(1983) Effects of two metabolites of ochratoxin A, (4R)-4-
hydroxyochratoxin A and ochratoxin alpha, on immune response in mice.
Infect. Immun., 39: 1015-1018.
CROFT, W.A., JARVIS, B.B., & YATAWARA, C.S. (1986) Airborne outbreak
of trichothecene toxicosis. Atmos. Environ., 20: 549-552.
CUNDLIFFE, E., CANNON, M., & DAVIS, J. (1974) Mechanism of
inhibition of eukaryotic protein synthesis by trichothecene fungal
toxins. Proc. Natl Acad. Sci. (USA), 71(1): 30-34.
D'AGOSTINO, P.A., PROVOST, L.R., & DROVER, D.R. (1986) Analysis of
trichothecene mycotoxins in human blood by capillary column gas
chromatography-ammonia chemical ionization mass spectrometry. J.
Chromatogr., 367: 77-86.
DANKO, G. & SZERAFIN, J. (1976) Experimental stachybotryotoxicosis
in horses. Magy. Allatorv. Lapja, 9: 597-600.
DE LOACH, J.R., ANDREWS, K., & NAGI, A. (1987) Interaction of T-2
toxin with bovine erythrocyte: effects on cell lysis, permeability
and entrapment. Toxicol. appl. Pharmacol., 88: 123-131.
DEMEKE, T., KIDANE, Y., & WUHIB, E. (1979) Ergotism: a report on an
epidemic. Ethiop. med. J., 17: 107-113.
DENICOLA, D.B., REBER, A.H., CARLTON, W.W., & YAGEN, B. (1978) T-2
toxin mycotoxicosis in the guinea-pig. Food Cosmet. Toxicol., 16
(6): 601-609.
DESIMONE, P.A., GRECO, F.A., & LESSNER, H.F. (1979) Phase I
evaluation of a weekly schedule of anguidine. Cancer Treat. Rep.,
63(11/12): 2015-2017.
DIVE, D., MOREAU, S., & CACAN, M. (1978) Use of a ciliate
protozoan for fungal toxins studies. Bull. environ. Contam. Toxicol.,
19(4): 489-495.
DOCHEV, D. (1973) [Endemic (Balkan) nephritis in Bulgaria.]
Münchener med. Wochenschr., 115(13): 537-541 (in German).
DOERR, J.A., HAMILTON, P.B., & BURMEISTER, H.R. (1981) T-2
toxicosis and blood coagulation in young chickens. Toxicol. appl.
Pharmacol., 60: 157-162.
DOSIK, G.M., BARLOGIE, B., JOHNSTON, D.A., MARPHY, W.K., & DREWINKO,
B. (1978) Lethal and cytokinetic effects of anguidine on a human
colon cancer cell line. Cancer Res., 38(10): 3304-3309.
DOSTER, R.C., SINHUBER, R.O., & WALES, J.H. (1972) Acute
intraperitoneal toxicity of ochratoxin A and B in rainbow trout
(Salmo gairdneri). Food. Cosmet. Toxicol., 10: 85-92.
DROVE, J.F. (1988) Non macrocyclic trichothecenes. Natural Products
Reports: 181-209.
DWIVEDI, P. & BURNS, R.B. (1984) Effect of ochratoxin A on
immunoglobulins in broiler chicks. Res. vet. Sci., 36: 117-121.
EDLUND, P.O. (1981) Determination of ergot alkaloids in plasma by
high-performance liquid chromatography and fluorescence detection. J.
Chromatogr., 226: 107-115.
EHRLICH, K.C., LEE, L.S., & CIEGLER, A. (1983) A simple, sensitive
method for detection of vomitoxin (deoxynivalenol) using reversed
phase, high performance liquid chromatography. J. liq. Chromatogr.,
6: 833-843.
EL-BANNA, A.A., LAU, P.-Y., & SCOTT, P.M. (1983) Fate of mycotoxins
during processing of foodstuffs II- Deoxynivalenol (vomitoxin) during
making of Egyptian bread. J. food Prot., 46: 484-486.
EL-BANNA, A.A., SCOTT, P.M., LAU, P.-Y., SAKUMA, T., PLOT, H., &
CAMPBELL, V. (1984) Formation of trichothecenes by F. solani var.
coeruleum and F. sambucinum in potatoes. Appl. environ. Microbiol.,
47: 1169-1171.
ELLING, F. (1977) Demonstration of ochratoxin A in kidneys of pigs
and rats by immunofluorescence microscopy. Acta pathol. microbiol.
Scand., A85: 151-156.
ELLING, F. & MOLLER, T. (1973) Mycotoxic nephropathy in pigs. Bull.
World Health Organ., 49: 411-418.
ELLING, F., HALD, B., JACOBSEN, C., & KROGH, P. (1975) Spontaneous
cases of toxic nephropathy in poultry associated with ochratoxin A.
Acta pathol. microbiol. Scand., A83: 739-741.
ELLING, F., NIELSEN, J.P., LILLEHOJ, E.B., THOMASSEN, M.S., &
STORMER, F.C. (1985) Ochratoxin A-induced porcine nephrotoxicity:
enzyme and ultrastructural changes after short-term exposure.
Toxicon, 23: 247-254.
EPPLEY, R.M. (1968) Screening method for zearalenone, aflatoxin, and
ochratoxin. J. Assoc. Off. Anal. Chem., 51(1): 74-78.
EPPLEY, R.M. (1974) Sensitivity of brine shrimp Artemia salina to
trichothecenes. J. Assoc. Off. Anal. Chem., 57(3): 618-620.
EPPLEY, R.M. & BAILEY, W.J. (1973) 12,13-epoxy-delta-trichothecenes
as the probable mycotoxins responsible for stachybotryotoxicosis.
Science, 181: 758-760.
EPPLEY, R.M., TRUCKSESS, M.W., NESHEIM, S., THORPE, C.W., WOOD, G.E.,
& POHLAND, A.E. (1984) Deoxynivalenol in winter wheat: Thin layer
chromatographic method and survey. J. Assoc. Off. Anal. Chem., 67
(1): 43-45.
EPPLEY, R.M., TRUCKSESS, M.W., NESHEIM, S., THORPE, C.W., & POHLAND,
A.E. (1986) Thin layer chromatographic method for determination of
deoxynivalenol in wheat: collaborative study. J. Assoc. Off. Anal.
Chem., 69: 37-40.
FAIRHURST, S., MAXWELL, S.A., SCAWIN, J.W., & SWANSTON, D.W. (1987)
Skin effects of trichothecenes and their amelioration by
decontamination. Toxicology, 46: 307-319.
FAN, T.S.L., XU, Y.-C., & CHU, F.S. (1987) Simultaneous analysis of
T-2 toxin and HT-2 toxin by indirect enzyme-linked immunosorbent
assay. J. Assoc. Off. Anal. Chem., 70: 657-661.
FARB, R.M., MEGO, J.L., & HAYES, A.W. (1976) Effect of mycotoxins
on uptake and degradation of iodine-125 albumin in mouse liver and
kidney lysosomes. J. Toxicol. environ. Health, 1(6): 985-990.
FEUERSTEIN, G., GOLDSTEIN, D.S., RAMWELL, P.W., ZERBE, R.L., LUX,
W.E. Jr, FODEN, A.I., & BAYORH, M.A. (1985) Cardiorespiratory
sympathetic and biochemical response to T-2 toxin in the guinea pig
and rat. J. Pharmacol. exp. Ther., 232: 786-794.
FEUERSTEIN, G., LEADER, P., SIREN, A.L., & BRAGNET, P. (1987)
Protective effect of a PAF-acether antagonist, BN52021 in
trichothecene toxicosis. Toxicol. Lett., 38: 271-274.
FIORAMONTI, J., FARGEAS, M.J., & BUENO, L. (1987) Action of T-2
toxin on gastrointestinal transit in mice: protective effect of an
argillaceous compound. Toxicol. Lett., 36: 227-232.
FITZPATRICK, D.W., BOYD, K.E., & WATTS, B.M. (1988) Comparison of the
trichothecenes deoxynivalenol and T-2 toxin for their effect on brain
biogenic monoamines in the rat. Toxicol. Lett., 40: 241-245.
FORGACS, J. (1965) In: Wogan, G., ed. Mycotoxins in foodstuffs,
Cambridge, Mass., MIT Press, p. 87.
FORSELL, J.H. & PESTKA, J.J. (1985) Relation of 8-ketotrichothecene
and zearalenone analog structure to inhibitions of mitogen-induced
human lymphocyte blastogenesis. Appl. environ. Microbiol., 50:
1304-1307.
FORSELL, J.H., KATEBY, J.R., YOSHIZAWA, T., & PESTKA, J.J. (1985)
Inhibition of mitogen-induced blastogenesis in human lymphocytes by
T-2 toxin and its metabolites. Appl. environ. Microbiol., 49:
1524-1526.
FORSYTH, D.M., YOSHIZAWA, T., MOROOKA, N., & TUITE, J. (1977)
Emetic and refusal activity of deoxynivalenol to swine. Appl.
environ. Microbiol., 34(5): 547-552.
FOSTER, P.M.D., SLATER, T.F., & PATTERSON, D.S.P. (1975) A possible
enzymic assay for trichothecene mycotoxins in animal feedstuffs.
Biochem. Soc. Trans., 3(6): 875-878.
FREEMAN, G.G. (1955) Further biological properties of trichotecin,
an antifungal substance from Trichothecium roseum Link, and its
derivatives. J. gen. Microbiol., 12: 213-221.
FREEMAN , G.G. & MORRISON, R.I. (1949) The isolation and chemical
properties of trichothecin, an antifungal substance from
Trichothecium roseum link. Biochem. J., 44: 1-5.
FRIED, H.M. & WARNER, J.R. (1981) Cloning of yeast gene for
trichodermin resistance and ribosomal protein L3. Proc. Natl Acad.
Sci. (USA), 78(1): 238-242.
FRIEND, S.C.E., TRENHOLM, H.L., ELLIOT, J.I., THOMPSON, B.K., &
HARTIN, K.E. (1982) Effect of feeding vomitoxin-contaminated wheat to
pigs. Can. J. anim. Sci., 62: 1211-1222.
FRIEND, S.C.E., BABUIK, L.A., & SCHIEFER, H.B. (1983a) The effects of
dietary T-2 toxin on the immunological function and Herpes simplex
reactivation in Swiss mice. Toxicol. appl. Pharmacol., 69:
234-244.
FRIEND, S.C.E., HANCOCK, D.S., & SCHIEFER, H.B. (1983b) Experimental
T-2 toxicosis in sheep. Can. J. comp. Med., 47: 291-297.
FRIEND, S.C.E., SCHIEFER, H.B,. & BABUIK, L.A. (1983c) The effects of
dietary T-2 toxin on acute Herpes simplex virus type 1 infection in
mice. Vet. Pathol., 20: 737-760.
FRIESEN, M.D. & GARREN, L. (1983) International mycotoxin check
sample survey programme. Part III. Report on performance of
participating laboratories for determining ochratoxin A in animal
feed. J. Assoc. Off. Anal. Chem., 66: 256-259.
FRIIS, Ch., BRINN, R., & HALD, B. (1988) Uptake of ochratoxin A by
slices of pig kidney cortex. Toxicology., 52: 209-217.
FRITZ, W., BUTHIG, CL., DONATH, R., & ENGST, R. (1979) [Studies on
the nutritionally significant formation of ochratoxin A in grain and
other foodstuffs.] Lebensm. Ernähr., 25: 929-932 (in German).
FROMENTIN, H., SALAZAR-MEJICANOS, S,. & MARIAT, F. (1981)
Experimental cryptococcosis in mice treated with diacetoxyscirpenol,
a mycotoxin of Fusarium. Sabouraudia, 19: 311-318.
GADIOU, J. (1965) Contribution à l'étude toxicologique du
dicyandiamide de methyl mercure, Université de Bordeaux, Faculté de
pharmacie. (Thèse de doctorat).
GALEY, F.D., LAMBERT, R.J., BUSSE, M., & BUCK, W.B. (1987)
Therapeutic efficacy of superactive charcoal in rats exposed to oral
lethal doses of T-2 toxins. Toxicon, 25: 493-499.
GALTIER, P. (1974a) Devenir de l'ochratoxine A dans l'organisme
animal. I. Transport sanguin de la toxine chez le rat. Ann. Rech.
vét., 5: 311-318.
GALTIER, P. (1974b) Devenir de l'ochratoxine A dans l'organisme
animal. II. Distribution tissulaire et élimination chez le rat.
Ann. Rech. vét., 5: 319-328.
GALTIER, P. (1979) Etude toxicologique et pharmacocinétique d'une
mycotoxine, l'ochratoxine A, Université de Toulouse, Thèse de
doctorat d'Etat en Sciences pharmaceutiques.
GALTIER, P. & ALVINERIE, M. (1976) In vitro transformation of
ochratoxin A by animal microbial floras. Ann. Rech. vét., 7:
91-98.
GALTIER, P., MORE, J., & BODIN, G. (1974) Toxines d' Aspergillus
ochraceus Wilhelm III. Toxicité aiguë de l'ochratoxine A chez le rat
et la souris adultes. Ann. Rech. vét., 5: 233-247.
GALTIER, P., BARADAT, C., & ALVINERIE, M. (1977a) De l'élimination
d'ochratoxine A par le lait chez la lapine. Ann. Nutr. Aliment.,
31: 911-918.
GALTIER, P., JEMMALI, M., & LARRIEU, G. (1977b) Enquete sur la
présence éventuelle d'aflatoxine et d'ochratoxine A dans des maïs
recolté en France en 1973 et 1974. Ann. Nutr. Aliment., 31: 38l-389.
GALTIER, P., BONEU, B., CHARPENTEAU, J.L., BODIN, G., ALVINERIE, M.,
& MORE, J. (1979) Physiopathology of haemorrhagic syndrome related
to ochratoxin A intoxication in rats. Food Cosmet. Toxicol., 17:
49-53.
GALTIER, P., CAMGUILHEM, R., & BODIN, G. (1980) Evidence for in
vitro and in vivo interaction between ochratoxin A and three acidic
drugs. Food Cosmet. Toxicol., 18: 493-496.
GAREIS, M., HASHEM, A., BAUER, J., & GEDEK, B. (1986) Identification
of glucuronide metabolites of T-2 toxin and diacetoxyscirpenol in the
bile of isolated perfused rat lever. Toxicol. appl. Pharmacol., 84:
168-172.
GAREIS, M., MARTLBAUER, E., BAUER, J., & GEDEK, B. (1988)
[Determination of ochratoxin A in breast milk.] Z. Lebensm. Unters.
Forsch., 186: 114-117 (in German).
GENDLOFF, E.H., PESTKA, J.J., SWANSON, S.P., & HART, L.P. (1984)
Detection of T-2 toxin in Fusarium sporotrichioides-infected corn by
enzyme-linked immunosorbent assay. Appl. environ. Microbiol., 47:
1161-1163.
GENTRY, P.A. (1982) The effect of administration of a single dose of
T-2 toxin on blood coagulation in the rabbit. Can. J. comp. Med.,
46: 414-419.
GENTRY, P.A. & COOPER, M.L. (1981) Effect of Fusarium T-2 toxin on
hematological and biochemical parameters in the rabbit. Can. J. comp.
Med., 45: 400-405.
GHOSAL, S., CHAKRABARTI, D.K., & BASU CHAUDHARY, K.C. (1977) The
occurrence of 12,13-epoxytrichothecenes in seeds of safflower
infected with Fusarium oxysporum f sp. carthami. Experientia (Basel),
33(5): 574-575.
GILBERT, J., SHEPHERD, M.J., & HOWELL, M.V. (1983a) Studies on the
fate of trichothecene mycotoxins during food processing. European
Conference on. Food Chemistry. II, Rome, Societa Italiana di Scienza
dell alimetazione. pp. 253-262.
GILBERT, J., SHEPHERD, M.J., & STARTIN, J.R. (1983b) A survey of the
occurrence of the trichothecene mycotoxin deoxynivalenol (vomitoxin)
in UK-grown barley and in imported maize by combined gas
chromatography-mass spectrometry. J. Sci. Food Agric., 34: 86-92.
GILBERT, J., SHEPHERD, M.J., & STARTIN, J.R. (1984) The analysis and
occurrence of Fusarium mycotoxins in the United Kingdom and their
fate during food processing. In: Kurata, H. & Ueno, Y., ed. Toxigenic
fungi - their toxins and health hazard, Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp. 209-216.
GILBERT, J., STARTIN, J.R,. & CREWS, C. (1985) Optimization of
conditions for trimethylsilylation of trichothecene mycotoxins. J.
Chromatogr., 319: 376-381.
GILGAN, M.W., SMALLEY, E.B., & STRONG, F.M. (1966) Isolation and
partial characterization of a toxin from Fusarium tricinctum on moldy
corn. Arch. Biochem. Biophys., 114: 1-3.
GOLINSKI, P., HULT, K., BRABARKIEWICZ-SZCZESNA, J., CHELKOWSKI, J.,
KNEBLEWSKI, P., & SZEBIOTKO, K. (1984) Mycotoxic porcine nephropathy
and spontaneous occurrence of ochratoxin A residues in kidneys and
blood of Polish swine. Appl. environ. Microbiol., 47: 1210-1212.
GOLINSKI, P., BRABARKIEWICZ-SZCZESNA, J., CHELKOWSKI, J., &
SZEBIOTKO, K. (1985) Spontaneous occurrence of ochratoxin A
residues in porcine kidney and serum samples in Poland. Appl.
environ. Microbiol., 49: 1014-1015.
GRANT, P.G., SCHINDLER, D., & DAVIES, J.E. (1976) Mapping of
trichodermin resistance in Saccharomyces cerevisiae: a genetic locus
for a component of the 60s ribosomal subunit. Genetics, 83(4):
667-673.
GREENHALGH, R., GILBERT, J., KING, R.R., BLACKWELL, B.A., STARTIN,
J.R., & SHEPHERD, M.J. (1984) Synthesis, characterization, and
occurrence in bread and cereal products of an isomer of 4-
deoxynivalenol (vomitoxin). J. agric. food Chem., 32: 1416-1420.
GREENWAY, J.A. & PULS, R. (1976) Fusariotoxicosis from barley in
British Colombia. I. Natural occurrence and diagnosis. Can. J. comp.
Med., 40: 12-15.
GROVE, J.F. & MORTIMER, P.H. (1969) The cytotoxicity of some
transformation products of diacetoxyscirpenol. Biochem. Pharmacol.,
18(6): 1473-1478.
GUPTA, R.S. & SIMINOVITCH, L. (1978) Genetic and biochemical
characterization of mutants of CHO cells resistant to the protein
synthesis inhibitor trichodermin. Somatic cell Genet., 4(3):
355-374.
GUTZWILLER, J. & TAMM, C. (1965) Verrucarin and roridin. V. The
structure of Verrucarin A. Helv. Chim. Acta, 48: 157-176.
HAGGBLOM, P. (1982) Production of ochratoxin A in barley by
Aspergillus ochraceus and Penicillium viridicatum: effect of fungal
growth, time, temperature, and inoculum size. Appl. environ.
Microbiol., 43: 1205-1207.
HAGLER, W.M., Jr, TYCZKOWDKA, K., & HAMILTON, P.B. (1984)
Simultaneous occurrence of deoxynivalenol, zearalenone, and aflatoxin
in 1982 scabby wheat from the midwestern United States. Appl.
environ. Microbiol., 47(1): 151-154.
HALD, B. (in press) Human exposure to ochratoxin A. In: Natori, S.,
Hashimoto, K., & Ueno, Y., ed. Mycotoxins and phycotoxins 1989,
Amsterdam, Oxford, New York, Elsevier Science Publishers.
HALD, B. & KROGH, P. (1972) Ochratoxin residues in bacon pigs.
Proceedings of the IUPAC Symposium: Control of Mycotoxins, Kungälv,
Sweden, page 18.
HALD, B. & KROGH, P. (1975) Detection of ochratoxin A. in barley,
using silica gel mini columns. J. Assoc. Off. Anal. Chem., 58:
156-158.
HALD, B. & KROGH, P. (1983) Toxicoses and natural occurrence in
Denmark. In: Ueno, Y., ed. Trichothecenes - chemical, biological
and toxicological aspects, Amsterdam, Oxford, New York, Elsevier,
Science Publishers, pp 251-253.
HALIBURTON, J.C., VESONDER, R.F., LOCK, T.F., & BUCK, W.B. (1979)
Equine leucoencephalomalacia (ELEM): a study of Fusarium moniliforme
as an etiologic agent. Vet. hum. Toxicol., 21(5): 348-351.
HARRACH, B., MIROCHA, C.J., PATHRE, S.V., & PALYUSIK, M. (1981)
Macrocyclic trichothecene toxins produced by a strain of Stachybotrys
atra from Hungary. Appl. environ. Microbiol., 41(6): 1428-1432.
HARRACH, B., BATA, A., BAJMOCY, E., & BENKO, M. (1983) Isolation of
satratoxins from the bedding straw of a sheep flock with fatal
stachybotryotoxicosis. Appl. environ. Microbiol., 45: 1419-1422.
HARRI, E., VON, LOEFFLER, W., SIGG, H.P., STAHELIN, H., STOLL, Ch.,
TAMM, Ch., & WIESINGER, D. (1962) [Verrucarins and roridins, a group
of antibiotics with high cytostatic effect from Myrothecium species.]
Helv. Chim. Acta, 45: 839-853 (in German).
HART, L.P. & BASELTON, W.E. (1983) Distribution of vomitoxin in dry
milled fractions of wheat infected with Gibberella. J. agric. food
Chem., 31: 657-659.
HARTMANN, G.R., RICHTER, H., WEINER, E.M., & ZIMMERMANN, W. (1978)
On the mechanism of action of the cytostatic drug anguidine and of
the immunosuppressive agent ovalicin, two sesquiterpenes from fungi.
Planta Med., 34(3): 231-252.
HARWIG, J. (1974) Ochratoxin A and related metabolites. In:
Purchase, I.F.H., ed. Mycotoxins, Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp. 345-368.
HAUBECK, H.D., LORKOWSKI, G., KULSCH, E., & ROSCHENTHALER, R. (1981)
Immunosuppression by ochratoxin A and its prevention by
phenylalanine. Appl. environ. Microbiol., 41: 1040-1042.
HAYES, A.W., HOOD, R.D., & LEE, H.L. (1974) Teratogenic effects of
ochratoxin A in mice. Teratology, 9(1): 93-97.
HAYES, M.A. & SCHIEFER, H.B. (1979) Quantitative and morphological
aspects of cutaneous irritation by trichothecene mycotoxins. Food
Cosmet. Toxicol., 17(6): 611-621.
HAYES, M.A., BELLAMY, J.E.C., & SCHIEFER, H.B. (1980) Subacute
toxicity of dietary T-2 toxin in mice: morphological and
hematological effects. Can. J. comp. Med., 44: 203-218.
HELGESON, J.P., HABERLACH, G.T., & VANDERHOEF, L.N. (1973) T-2
toxin decreases logarithmic growth rates of tobacco callus tissues.
Plant Physiol., 52(6): 660-662.
HELLER, K. & ROSCHENTHALER, R. (1978) Inhibition of protein
synthesis in Streptococcus faecalis by ochratoxin A. Can. J.
Microbiol., 24: 466-472.
HEPTINSTALL, R.H. (1974) Pathology of the kidney, Boston, Little
Brown and Co., Vol. 11, pp. 828-836.
HEWETSON, J.F., PACE, J.D., & BEHELER, J.E. (1987) Detection and
quantitation of T-2 mycotoxin in rat organs by radioimmunoassay. J.
Assoc. Off. Anal. Chem., 70: 654-657.
HIBBS, C.M., OSWEILER, G.D., BUCK, W.B., & MACFEE, G.P. (1975)
Bovine hemorrhagic syndrome related to T-2 mycotoxin. Proc. Ann.
Meet. Am. Assoc. Vet. Lab. Diagn., 17: 305-310.
HIRAYAMA, S. & YAMAMOTO, M. (1948) [Biological studies on the
poisonous wheat flour (I).] Eiseishikenjo Hokoku, 66: 85-98 (in
Japanese).
HIRAYAMA, S. & YAMAMOTO, M. (1950) [Biological studies on the
poisonous wheat flour (II).] Eiseishikenjo Hokoku, 67: 117-121 (in
Japanese).
HOBDEN, A.N. & CUNDLIFFE, E. (1980) Ribosomal resistance to the
12,13-epoxy-trichothecene antibiotics in the producing organism
Myrothecium verrucaria. Biochem. J., 190(3): 765-770.
HOERR, F.J., CARLTON, W.W., & YAGEN, B. (1981) Mycotoxicosis caused
by a single dose of T-2 toxin or diacetoxyscirpenol in broiler
chickens. Vet. Pathol., 18: 653-664.
HOLADAY, C.E. (1976) A rapid screening method for the aflatoxins
and ochratoxin A. J. Am. Assoc. Anal. Chem., 53: 603-605.
HOLT, P.S. & DE LOACH, J.R. (1988a) Cellular effects of T-2 mycotoxin
on two different lives. Biochem. Biophys. Acta, 971: 1-8.
HOOD, R.D. (1986) Effects of concurrent prenatal exposure to
rubratoxin B and T-2 toxin in the mouse. Drug chem. Toxicol., 9
(2): 185-190.
HOOD, R.D., NAUGHTON, M.J., & HAYES, A.W. (1976) Prenatal effects
of ochratoxin A in hamsters. Teratology, 13: 11-14.
HOOD, R.D., KUCZUK, M.H., & SZCZECK, G.M. (1978) Effects in mice of
simultaneous prenatal exposure to ochratoxin A and T-2 toxin.
Teratology, 17: 25-30.
HRABAR, A., SULJAGA, K., BORCIC, B., ALERAJ, B., CEOVIC, S., &
CVORISCEC, D. (1976) [Endemic nephropathy morbidity and mortality
in the village of Kaniza.] Arch. ind. Hyg. Toxicol., 27: 137-145
(in Croatian).
HSU, I.C., SMALIEY, E.B., STRONG, F.M., & RIBELIN, W.E. (1972)
Identification of T-2 toxin in moldy corn associated with a lethal
toxicosis in dairy cattle. Appl. Microbiol., 24(5): 684-690.
HUFF, W.E., WYATT, R.D., TUCKER, T.L., & HAMILTON, P.B. (1974)
Ochratoxicosis in the broiler chicken. Poult. Sci., 53: 1585-1591.
HUFF, W.E., DOERR, J.A., HAMILTON, P.B., HAMANN, D.D., PETERSON,
R.E., & CIEGLER, A. (1980) Evaluation of bone strength during
aflatoxicosis and ochratoxicosis. Appl. environ. Microbiol.,
40: 102-107.
HULT, K. & GATENBECK, S. (1976) A spectrophotometric procedure,
using carboxypeptidase A, for the quantitative measurement of
ochratoxin A. J. Assoc. Off. Anal. Chem., 59: l28-l29.
HULT, K., TEILING, A., & GATENBECK, S. (1976) Degradation of
ochratoxin A by a ruminant. Appl. environ. Microbiol., 32: 443-444.
HULT, K., HUKBY, E., HOGLUND, U., GATENBECK, S., RUTQVIST, L., &
SELLYEY, G. (1979) Ochratoxin A in pig blood: method of analysis
and use as a tool for feed studies. Appl. environ. Microbiol., 38:
772-776.
HULT, K., HUKBY, E., GATENBECK, S., & RUTQVIST, L. (1980).
Ochratoxin A in blood from slaughter pigs in Sweden: Use in
evaluation of toxin content of consumed feed. Appl. environ.
Microbiol., 39: 828-830.
HULT, K., PLESTINA, R., HABAZIN-NOVAK, V., RADIC, B., & CEOVIC, S.
(1982) Ochratoxin A in human blood and Balkan endemic nephropathy.
Arch. Toxicol., 51: 313-321.
HULT, K., FUCHS, R., PERAICA, M., PLESTINA, R., & CEOVIC, S. (1984)
Screening for ochratoxin A in blood by flow injection analysis. J.
appl. Toxicol., 4: 326-329.
HUNT, D.C., BOURDON, A.T., WILD, P.J., & CROSBY, N.T. (1978) Use of
high performance liquid chromatography combined with fluorescence
detection for the identification and estimation of aflatoxins and
ochratoxin in food. J. Sci. Food Agric., 29: 234-238.
HUNT, D.C., LESLYE, A.P., & CROSBY, N.T. (1979) Determination of
ochratoxin A in pig's kidney using enzymic digestion, dialysis and
high-performance liquid chromatography with post-column
derivatization. Analyst, 104: 1171-1175.
HUNTER, K.W., BRIMFIELD, A.A., MILLER, M., FINKELMAN, F.D., & CHU,
S.F. (1985) Preparation and characterization of monoclonal antibodies
to the trichothecene mycotoxin T-2. Appl. environ. Microbiol., 49:
168-172.
HUSSEIN, H.M., FRANICH, R.A., BAXTER, M,. & ANDREW, I.G. (1989)
Naturally occurring Fusarium toxins in New Zealand maize. Food Addit.
Contam., 6: 49-58.
HUTCHISON, R.D., STEYN, P.S., & THOMPSON, D.L. (1971) The isolation
and structure of 4-hydroxyochratoxin A and 7-carboxy-3,4-dihydro-8-
hydroxy-3-methylisocoumarin from Penicillium viridicatum.
Tetrahedron, 43: 4033-4036.
IARC (1987a) Overall evaluations of carcinogenicity: and updating of
IARC Monographs Vol. 1 to 42, Lyons, International Agency for
Research on Cancer, pp. 271-272. (IARC Monographs on the Evaluation
of Carcinogenic Risks to Humans, Supplement 7).
IARC (1987b) Biennial report 1986-1987, Lyons, International Agency
for Research on Cancer.
IARC (1983) Some food additives, feed additives and naturally
occurring substances: ochratoxin A, Lyons, International Agency for
Research on Cancer, pp. 191-206 (IARC Monographs on the Evaluation of
the Carcinogenic Risk of Chemicals to Humans, Vol. 31).
IARC (1984) Monitoring of aflatoxins in human body fluids and
application to field studies, Lyons, International Agency for
Research on Cancer (IARC International Technical Report No. 84/003).
ICHINOE, M., UCHIYAMA, S., AMANO, R., & KURATA, H. (1985)
Trichothecene-producing Fusarium in barley and wheat in Japan.
Trichothecenes and other Mycotoxins. Proceedings of the International
Mycotoxin Symposium, Sydney, Australia, New York, Chichester, John
Wiley & Sons, pp. 21-31.
ILUS, T., NIKU-PAAVOLA, M.L., & ENARI, T.M. (1981) Chromatographic
analysis of Fusarium toxins in grain samples. Eur. J. appl.
Microbiol. Biotechnol., 11(4): 244-247.
IMAIDA, K., HIROSE, M., OGISO, T., KURATA, Y., & ITO, N. (1982)
Quantitative analysis of initiating and promoting activities of five
mycotoxins in liver carcinogenesis in rats. Cancer Lett., 16:
137-143.
ISHII, K., SAKAI, K., UENO, Y., TSUNODA, H., & ENOMOTO, M. (1971)
Solaniol, a toxic metabolite of Fusarium solani. Appl. Microbiol.,
22(4): 718-720.
ISHII, K., ANDO, Y., & UENO, Y. (1975) Toxicological approaches to
the metabolites of Fusaria. Part 9. Isolation of vomiting factor from
moldy corn infected with Fusarium spp. Chem. pharm. Bull., 23(9):
2162-2164.
ISHII, K., KOBAYASHI, J., UENO, Y., & ICHINOE, M. (1986) Occurrence
of trichothecine in wheat. Appl. environ. Microbiol., 52: 331-333.
ITO, Y., OHTSUBO, K., & SAITO, M. (1980) Effects of fusarenon-X, a
trichothecene produced by Fusarium nivale, on pregnant mice and their
fetuses. Jpn. J. exp. Med., 50(3): 167-172.
ITO, Y., OHTSUBO, K., ISHII, K., & UENO, Y. (1986) Effects of
nivalenol on pregnancy and fetal development of mice. Mycotoxin
res., 2: 71-77.
ITO, Y., UENO, Y., TANAKA, T., NAKAMURA, K., & OHTSUBO, K. (1988)
[Embryotoxicity of oral nivalenol in mice.] Proc. Jpn. Assoc.
Mycotoxicol., 27: 33-36. (in Japanese).
IUPAC (1976) Recommended methods for ochratoxin A and B in barley,
Oxford, International Union of Pure and Applied Chemistry (IUPAC
Technical Report No.14).
IWAHASHI, T., TASHIRO, F., & UENO, Y. (1982) Mechanism of cytotoxic
effect of T-2 toxin on a protozoan, Tetrahymena pyriformis gl.
Maikotokishin, 15: 31-33.
JAGADEESAN, V., RUKMINI, C., VIJAYARAGHOVAN, M., & TULPULE, P.G.
(1982) Immune studies with T-2 toxin: effect of feeding and
withdrawal in monkeys. Food chem. Toxicol., 20: 83-87.
JARVIS, B.B., STAHLY, G.P., & CURTIS, C.R. (1978) Antitumor
activity of fungal metabolites: Verrucarin b-9,10-epoxides. Cancer
Treat. Rep., 62(10): 1585-1586.
JARVIS, B.B., STAHLY, G.P., PAVANASASIVAM, G., & MAZZOLA, E.P.
(1980) Antileukemic compounds derived from the chemical modification
of macrocyclic trichothecenes. 1. Derivatives of verrucarin A. J.
med. Chem., 23(9): 1054-1058.
JARVIS, B.B., LEE, Y.W., COMEZOGLU, S.N., & YATAWARA, C.S. (1986)
Trichothecenes produced by Stachybotrys atra from Eastern Europe.
Appl. environ. Microbiol., 51(5): 915-918.
JELINEK, C.F., POHLAND, A.E., & WOOD, G.E. (1989) Occurrence of
mycotoxins in foods and feeds - an update. J. Assoc. Off. Anal.
Chem., 72: 223-230.
JIMINEZ, A. & VASQUEZ, D. (1975) Quantitative binding of antibiotics
to ribosomes from a yeast mutant altered on the peptidyl transferase
centre. Eur. J. Biochem., 54(2): 483-492.
JIMENEZ, A., SANCHEZ, L., & VAZQUEZ, D. (1975) Simultaneous
ribosomal resistance to trichodermin and anisomycin in Saccharomyces
cerevisiae mutants. Biochim. Biophys. Acta, 383(4): 427-434.
JOFFE, A.Z. (1962) Biological properties of some toxic fungi
isolated from overwintered cereals. Mycopathol. Mycol. appl., 16:
201-221.
JOFFE, A.Z. (1986) Fusarium species, their biology and toxicology,
New York, Chichester, J. Wiley and Sons.
JOFFE, A.Z. & YAGEN, B. (1977) Comparative study of the yield of T-2
toxin produced by Fusarium poae, Fusarium sporotrichioides and
Fusarium sporotrichioides var. tricinctum strains from different
sources. Mycopathologia, 60(2): 93-97.
JOHNSEN, H., ODDEN, E., LIE, O., JOHNSEN, B.A., & FONNUM, F. (1986)
Metabolism of T-2 toxin by rat liver carboxylesterase. Biochem.
Pharmacol., 35: 1469-1473.
JOSEFSSON, E. (1979) [Study of ochratoxin A in pig kidneys.] Var
Föda, 31: 415-420 (in Swedish).
JOSEFSSON, E. & MOLLER, T. (1979) High pressure liquid
chromatographic determination of ochratoxin A and zearalenone in
cereals. J. Assoc. Off. Anal. Chem., 62: 1165-1168.
JUSZKIEWICZ, T. & PISKORSKA-PLISZCZYNSKA, J. (1976) [Occurrence of
aflatoxins B1, B2, G1, and G2, ochratoxins A and B, sterigmatocystin
and zearalenone in cereals]. Med. Weter., 32: 617-619 (in Polish).
JUSZKIEWICZ, T. & PISKORSKA-PLISZCZYNSKA, J. (1977) [Occurrence of
mycotoxins in mixed feeds and concentrates.] Med. Weter., 33: 193-196
(in Polish).
JUSZKIEWICZ, T., PISKORSKA-PLISZCZYNSKA, J., & WISNIEWSKA, H. (1982)
Ochratoxin A in laying hens: tissue deposition and passage into eggs.
In: Mycotoxins and Phycotoxins, Vienna, Technical University Vienna
Publ., pp. 122-125.
KALINOSKI, H.T., UDSETH, H.R., WRIGHT, B.W., & SMITH, R.D. (1986)
Supercritical fluid extraction and direct fluid injection mass
spectrometry for the determination of trichothecene mycotoxins in
wheat samples. Anal. Chem., 58: 2421-2425.
KAMIMURA, H., NISHIJIMA, M., SAITO, K., YASUDA, K., IBE, A.,
NAGAYAMA, T., USHIYAMA, H., & NAOI, Y. (1979) Studies on mycotoxins
in foods. XII. The decomposition of trichothecene mycotoxins during
food processing. J. Assoc. Off. Anal. Chem., 64(5): 1067-1073.
KANAI, K. & KONDO, E. (1984) Decreased resistance to myobacterial
infection in mice fed a trichothecene compound (T-2 toxin). Jpn. J.
med. Sci. Biol., 37: 97-104.
KANE, A., CREPPY, E.E., ROSCHENTHALER, R., & DIRHEIMER, G. (1986a)
Changes of urinary and renal tubular enzymes caused by subchronic
administration of ochratoxin A in rats. Toxicology, 61: 489-495.
KANE, A., CREPPY, E.E., ROTH, A., ROSCHENTHALER, R., & DIRHEIMER, G.
(1986b) Distribution of the [3]-label from low doses of radioactive
ochratoxin A ingested by rats, and evidence for DNA single-strand
breaks caused in liver and kidneys. Arch. Toxicol., 58: 219-224.
KANIZAWA, M. (1984) Synergistic effect of citrinin on hepatorenal
carcinogenesis of ochratoxin A in mice. In: Kurata, H. & Ueno, Y.,
ed. Toxigenic fungi - their toxins and health hazard, Amsterdam,
Oxford, New York, Elsevier Science Publishers, pp. 245-254
(Developments in Food Science No. 7).
KANIZAWA, M. & SUZUKI, S. (1978) Induction of renal and hepatic
tumours in mice by ochratoxin A, a mycotoxin. Gann, 69: 599-600.
KANIZAWA, M., SUZUKI, S., KOZUKA, Y., & YAMAZAKI, M. (1977)
Histopathological studies on the toxicity of ochratoxin A in rats. I.
Acute oral toxicity. Toxicol. appl. Pharmacol., 42: 55-64.
KELLERMAN, T.S., MARASAS, W.F.O., PIENAAR, J.G., & NAUDE, T.W.
(1972) A mycotoxicosis of equidae caused by Fusarium moniliforme
Sheldon. A preliminary communication. Onderstepoort J. vet. Res.,
39(4): 205-208.
KHERA, K.S., WHALEN, C., ANGERS, G., VESONDER, R.F., & KUIPER-
GOODMAN, I. (1982) Embryotoxicity of 4-deoxynivalenol (vomitoxin)
in mice. Bull. environ. Contam. Toxicol., 29: 487-491.
KHERA, K.S., ARNOLD, D.L., WHALEN, C., ANGERS, G., & SCOTT, P.M.
(1984) Vomitoxin (4-deoxynivalenol): Effects on reproduction of mice
and rats. Toxicol. appl. Pharmacol., 74: 345-356.
KHERA, K.S., WHALEN, C., & ANGERS, G. (1986) A teratology study on
vomitoxin (4-deoxynivalenol) in rabbits. Food chem. Toxicol., 24:
421-424.
KIENTZ, C.E. & VERWEIJ, A. (1986) Trimethylsilylation and
trifluoroacetylation of a number of trichothecenes followed by gas
chromatographic analysis on fused silica capillary columns. J.
Chromatogr., 355: 229-240.
KING, B. (1979) Outbreak of ergotism in Wollo, Ethiopia. Lancet,
1: 1411.
KING, R.R., MCQUEEN, R.E., LEVESQUE, D., & GREENHALGH, R. (1984)
Transformation of deoxynivalenol (vomitoxin) by rumen microorganisms.
J. agric. food Chem., 32: 1181-1183.
KITAGAWA, M., TASHIRO, F., & UENO, Y. (1982) Mutual interaction
between zearalenone, an estrogenic mycotoxin, and estrogen receptor
of rat brain. Proc. Jpn. Assoc. Mycotoxicol., 15: 28-30.
KITCHEN, D.N., CARLTON, W.W., & TUITE, J. (1977a) Ochratoxin A and
citrinin induced nephrosis in beagle dogs. I. Clinical and
clinicopathological features. Vet. Pathol., 14: 154-172.
KITCHEN, D.N., CARLTON, W.W., & TUITE, J. (1977b) Ochratoxin A and
citrinin induced nephrosis in beagle dogs. II. Pathology. Vet.
Pathol., 14: 261-272.
KLINKERT, W., LORKOWSKI, G., CREPPY, E.E., DIRHEIMER, G., &
ROSCHENTHALER, R. (1981) Inhibition of macrophage migration by
ochratoxin A and citrinin, and prevention by phenylalanine of the
ochratoxin A-induced inhibition. Toxicol. Eur. Res., 3: 186-189.
KONISHI, T. & ICHIJO, S. (1970) [Clinical studies on bean-hulls
poisoning of horse. I. Clinical and biochemical observations in
spontaneous cases.] Res. Bull. Obihiro Univ., 6: 242-257 (in
Japanese).
KONRAD, I. & ROSCHENTHALER, R. (1977) Inhibition of phenylalanine
tRNA synthetase from Bacillus subtilis by ochratoxin A. FEBS Lett.,
83: 341-347.
KOTSONIS, F.N., SMALLEY, E.B., ELLISON, R.A., & GALE, C.M. (1975)
Food refusal factors in pure cultures of Fusarium roseum graminearum.
Appl. Microbiol., 30(3): 362-368.
KOZLOVSKY, A.G. & RESHETILOVA, T.A. (1984) Regulation of
biosynthesis of ergot alkaloids by Penicillium sizovae. Folia
microbiol., 29: 301-305.
KRIEK, N.P.J., MARASAS, W.F.O., STEYN, P.S., VAN RENSBURG, S.J., &
STEYN, M. (1977) Toxicity of a moniliformin-producing strain of
Fusarium moniliforme var. subglutinans isolated from maize. Food
Cosmet. Toxicol., 15(6): 579-587.
KRISHNAMACHARI, K.A.V.R. & BHAT, R.V. (1976) Poisoning of ergoty
bajra (pearl millet) in man. Indian J. med. Res., 64: 1624-1628.
KRISHNAMURTHY, T. & SARVER, E.W. (1986) Mass spectral investigations
on trichothecene mycotoxins. III. Synthesis, characterization and
applications of pentafluoropropionyl and trifluoroacetyl esters of
simple trichothecenes. J. Chromatogr., 355: 253-264.
KRISHNAMURTHY, T., WASSERMAN, M.B., & SARVER, E.W. (1986) Mass
spectral investigations of trichothecene mycotoxins. I. Application
of negative ion chemical ionization techniques for the simultaneous
and accurate analysis of simple trichothecenes in picogram levels.
Biomed. environ. mass Spectrom., 13: 503-518.
KRISHNAMURTHY, T., SARVER, E.W., GREENE, S.L., & JARVIS, B.B. (1987)
Mass spectral investigations on trichothecene mycotoxins. II.
Detection and quantitation of macrocyclic trichothecenes by gas
chromatography/negative ion chemical ionization mass spectrometry. J.
Assoc. Off. Anal. Chem., 70: 132-140.
KROGH, P. (1976a) Epidemiology of mycotoxic porcine nephropathy.
Nord. vet. Med., 28: 452-458.
KROGH, P. (1976b) Mycotoxic nephropathy. In: Advances in veterinary
science and comparative medicine, New York, Academic Press, Vol. 20,
pp. 147-170.
KROGH, P. (1977) Ochratoxin A residues in tissues of slaughter pigs
with nephropathy. Nord. vet. Med., 29: 402-405.
KROGH, P. (1978) Causal associations of mycotoxic nephropathy. Acta
pathol. microbiol. Scand., A269: 1-28.
KROGH, P. (1979) Environmental ochratoxin A and Balkan (endemic)
nephropathy: Evidence for support of a causal relationship. In:
Strahinjic, S. & Stefanovic, V., ed. Endemic (Balkan) nephropathy,
Nis, Yugoslavia, Grafika, pp. 35-43.
KROGH, P. (1980) Ochratoxins: occurrence, biological effects and
causal role in disease. In: Eaker, D. & Wadstrom, T., ed. Natural
toxins, Oxford, Pergamon Press, pp. 673-680.
KROGH, P. (1983) Diagnostic criteria for ochratoxin-induced
nephropathy. In: Strahinjic, S. & Stefanovic, V., ed. Current
research in endemic (Balkan) nephropathy, Nis, Yugoslavia, Grafika,
pp. 11-14.
KROGH, P., HALD, B., & PEDERSEN, E.J. (1973) Occurrence of
ochratoxin A and citrinin in cereals associated with mycotoxic
porcine nephropathy. Acta pathol. microbiol. Scand., B81:
689-695.
KROGH, P., AXELSON, N.H., ELLING, F., GYRD-HANSEN, N., HALD, B.,
HYLDGAARD-JENSEN, J., LARSEN, A.E., MADSEN, A., MORTENSEN, H.P.,
MOLLER, T., PETERSEN, O.K., RAVNSKOV, U., ROSTGAARD, M., & AALUND, O.
(1974a) Experimental porcine nephropathy: changes of renal function
and structure induced by ochratoxin A-contaminated feed. Acta pathol.
microbiol. Scand., A246: 21.
KROGH, P., HALD, B., ENGLUND, P., RUTQVIST, L., & SWAHN, O. (1974b)
Contamination of Swedish cereals with ochratoxin A. Acta pathol.
microbiol. Scand., B82: 301-302.
KROGH, P., ELLING, F., HALD, B., LARSEN, A.E., LILLEHOJ, E.B.,
MADSEN, A., & MORTENSEN, H.P. (1976a) Time-dependent disappearance
of ochratoxin A residues of bacon pigs. Toxicology, 6: 235-242.
KROGH, P., ELLING, F., HALD, B., JYLLING, B., PETERSEN, V.E.,
SKADHAUGE, E., & SVENDSEN, C.K. (1976b) Experimental avian
nephropathy: changes of renal function and structure induced by
ochratoxin A-contaminated feed. Acta pathol. microbiol. Scand.,
A84: 215-221.
KROGH, P., ELLING, F., GYRD-HANSEN, N., HALD, B., LARSEN, A.E.,
LILLEHOJ, E. B., MADSEN, A., MORTENSEN, H.P., & RAVNSKOV, U. (1976c)
Experimental porcine nephropathy: changes of renal function and
structure perorally induced by crystalline ochratoxin A. Acta pathol.
microbiol. Scand., A84: 429-434.
KROGH, P., HALD, B., PLESTINA, R., & CEOVIC, S. (1977) Balkan
(endemic) nephropathy and food-borne ochratoxin A: Preliminary
results of a survey of foodstuff. Acta. Path. Microbiol. Scand.,
B85: 238-240.
KROGH, P., ELLING, F., FRIIS, CHR., MALD, B., LARSEN, A.E., LILLEHOJ,
E.B., MADSEN, A., MORTENSEN, H.P., RASMUSSEN, F., & RAVUSKOU, U.
(1979) Porcine nephropathy induced by long-term ingestion of
ochratoxin A. Vet. Pathol., 16: 466-475.
KROGH, P., GYRD-HANSEN, N., HALD, B., LARSEN, S., NIELSEN, J.P.,
SMITH, M., IVANOFF, C., & MEISNER, H. (1988). Renal enzyme
activities in experimental ochratoxin A-induced porcine nephropathy:
Diagnostic potential of phosphoenolpyruvate carboxykinase and
gammaglutamyl transpeptidase activity. J. Toxicol. environ. Health.,
23: 1-15.
KUCZUK, M.H., BENSON, P.M., HEATH, H., & HAYES, A.W. (1978)
Evaluation of the mutagenic potential of mycotoxins using Salmonella
typhimurium and Saccharomyces cerevisiae. Mutat. Res., 53(1): 11-20.
KUMAGAI, S. (1985) Ochratoxin A: Plasma concentration and excretion
into bile and urine in albumin-deficient rats. Food chem. Toxicol.,
23: 941-943.
KUMAGAI, S. & AIBARA, K. (1982) Intestinal absorption and secretion
of ochratoxin A in rat. Toxicol. appl. Pharmacol., 64: 94-102.
KUMAGAI, S. & SHIMIZU, T. (1988) Effects of Fusarenon-X and T-2 toxin
on intestinal absorption of monosaccharide in rats. Arch. Toxicol.,
61: 489-495.
KUPCHAN, S.M., JARVIS, B.B., DAILEY, R.G., Jr, BRIGHT, W., BRYAN,
R.F., & SHIZURI, Y. (1976) Baccharin, a novel potent antileukemic
trichothecene triepoxide from Baccharis megapotamica. J. Am. Chem.
Soc., 98(22): 7092-7093.
KUPCHAN, S.M., STREELMAN, D.R., JARVIS, B.B., DAILEY, R.G., Jr, &
SNEDEN, A.T. (1977) Isolation of potent new antileukemic
trichothecenes from Baccharis megapotamica. J. org. Chem., 42
(26): 4221-4225.
LAFARGE-FRAYSSINET, C., DECLOITRE, F., MOUSSET, S., MARTIN, M., &
FRAYSSINET, C. (1981) Induction of DNA single strand breaks by T-2
toxin, a trichothecene metabolites of Fusarium, effect on lymphoid
organs and liver. Mutat. Res., 88(2): 115-124.
LAFARGE-FRAYSSINET, C., LESPINATS, G., LAFONT, P., LOISILLIER, F.,
MOUSSET, S., ROSENSTEIN, Y., & FRAYSSINET, C. (1979)
Immunosuppressive effects of Fusarium cells to mitogens. Proc. Soc.
Exp. Biol. Med., 160(3): 302-311.
LARSEN, S. (1928) [On chronic degeneration of the kidneys caused by
mouldy rye.] Maanadsskr. Dyrl., 40: 259-284, 289-300 (in Danish).
LAUREN, D.R. & GREENHALGH, R. (1987) Simultaneous analysis of
nivalenol and deoxynivalenol in cereals by liquid chromatography. J.
Assoc. Off. Anal. Chem., 70: 479-483.
LEE, S. & CHU, F.S. (1981a) Radioimmunoassay of T-2 toxin in corn
and wheat. J. Assoc. Off. Anal. Chem., 64(1): 156-161.
LEE, S. & CHU, F.S. (1981b) Radioimmunoassay of T-2 toxin in
biological fluids. J. Assoc. Off. Anal. Chem., 64(3): 684-688.
LEE, S.C., BEERY, J.T., & CHU, F.S. (1984) Immunohistochemical fate
of ochratoxin A in mice. Toxicol. appl. Microbiol., 72: 218-227.
LEE, U., JANG, H., TANAKA, T., HASEGAWA, A., OH, Y., & UENO, Y.
(1985) The coexistence of the Fusarium mycotoxins nivalenol,
deoxynivalenol, and zearalenone in Korean cereals harvested in 1983.
Food Addit. Contam., 2(3): 185-192.
LEE, U., JANG, H., TANAKA, T., OH, Y., CHO, C., & UENO, Y. (1987)
Effect of milling on decontamination of Fusarium mycotoxins
nivalenol, deoxynivalenol, and zearalenone in Korean wheat. J. agric.
food Chem., 35: 126-129.
LEONOV, A.N. (1977) Current view of the chemical nature of factors
responsible for alimentary toxic aleukia. In: Rodricks, J.V.,
Hesseltine, C.W,. & Mehlman, M.A., ed. Mycotoxins in human and animal
health, Park Forest South, Illinois, Pathotox Publishers, pp. 323-
328.
LEVI, C.P. (1975) Collaborative study of a method for the
determination of ochratoxin A in green coffee. J. Assoc. Off. Anal.
Chem., 58(2): 258-262.
LEVI, C.P., TRENK, H.L., & MOHR, H.K. (1974) Study of the
occurrence of ochratoxin A in green coffee beans. J. Assoc. Off.
Anal. Chem., 57: 866-870.
LEW, V.H., MUELLNER, E., HAGER, R., & GREGOR, M. (1979) [Feed
refusal and emesis in fattening pigs caused by fusariotoxin-
contaminated corn.] Bodenkultur, 30(3): 309-316 (in German).
LIAO, L.L., GROLLMAN, A.P., & HORWITZ, S.B. (1976) Mechanism of
action of the 12,13-epoxytrichothecene anguidine: an inhibitor of
protein synthesis. Biochim. Biophys. Acta, 454(2): 273-284.
LINDENFELSER, L.A., LILLEHOJ, E.B., & BURMEISTER, H.R. (1974)
Aflatoxin and trichothecene toxins: skin tumor induction and
synergistic acute toxicity in white mice. J. Natl Cancer Inst.,
52(1): 113-116.
LINNAINMAA, K., SORSA, M., & ILUS, T. (1979) Epoxytrichothecene
mycotoxins as c-mitotic agents in Allium. Hereditas, 90(2):
151-156.
LOKEN, T. (1984) Ergot from meadow grass in Norway: chemical
composition and toxicological effects in sheep. Nord. vet. Med., 36:
259-265.
LORENZ, K. (1979) Ergot on cereal grains. In: Critical reviews in
food, science, and nutrition, Boca Raton, Florida, CRC Press, Vol.
11, pp. 311-354.
LORENZANA, R.M., BEASLEY, V.R., BUCK, W.B., GHENT, A.W., LUNDREN,
G.R., & POPPENGA, R.H. (1985) Experimental T-2 toxicosis in swine. I
Changes in cardiac output, aortic mean pressure, catecholamines, 6-
keto PGFa, thromboxane B2, and acid base parameters. Fundam. appl.
Toxicol., 5: 879-892.
LOVELESS, A.R. (1967) Claviceps fusiformis sp. nov.: the causal
agent of an agalactia of sows. Br. Mycol. Soc., 50: 15-18.
LUO, X. (1988) Food poisoning associated with Fusarium toxins.
Proceedings of the 7th International Symposium on Mycotoxins and
Phycotoxins, Tokyo, 16-19 August, 1988.
LUSTER, M.I., GERMOLEC, D.R., BURLESON, G.R., JAMESON, C.W.,
ACKERMANN, M.F., LAMM, K.R., & MAYER, H.T. (1987) Selective
immunosuppression in mice of natural killer cell activity by
ochratoxin A. Cancer Res., 47: 2259-2263.
LUTSKY, I. & MOR, N. (1981) Experimental alimentary toxic aleukia
in cats. Lab. anim. Sci., 31(1): 43-47.
LUTSKY, I., MOR, N., YAGEN, B., & JOFFE, A.Z. (1978) The role of
T-2 toxin in experimental alimentary aleukia: a toxicity study in
cats. Toxicol. appl. Pharmacol., 43(1): 111-124.
MACDONALD, E.J., COVAN, K.R. & SMITH, T.K. (1988) Effect of acute
oral doses of T-2 toxin on tissue concentrations of biogenic amines
in the rat. J. anim. Sci., 66: 434-441.
MCLAUGHLIN, C.S., VAUGHAMM, M.H., CAMPBELL, I.M., WEI, C.M.,
STAFFORD, M.E., & HANSEN, B.S. (1977) Inhibition of protein
synthesis by trichothecenes. In: Rodricks, J.V., Hesseltine, C.W., &
Mehlman, M.A., ed. Mycotoxins in human and animal health, Forest Park
South, Illinois, Pathotox Publishers, pp. 263-273.
MANN, D.D., BUENING, G.M., HOOK, B., & OSWEILER, G.D. (1983) Effects
of T-2 mycotoxin on bovine serum proteins. Am. J. vet. Res., 44:
1757-1759.
MANN, D.D., BUENING, G.M., OSWEILER, G.D., & HOOK, B. (1984) Effect
of subclinical levels of T-2 toxin on the bovine cellular immune
system. Can. J. comp. Med., 48: 308-312.
MANTLE, P.G. (1977a) The genus Claviceps. In: Wyllie, T.D. &
Morehouse, L.C., ed. Mycotoxic fungi, mycotoxins, mycotoxicoses: an
encyclopedia handbook, New York, Marcel Dekker, Vol. 1, pp. 83-89.
MANTLE, P.G. (1977b) Chemistry of Claviceps mycotoxins. In: Wyllie,
T.D. & Morehouse, L.C., ed. Mycotoxic fungi, mycotoxins,
mycotoxicoses: an encyclopedia handbook, New York, Marcel Dekker, Vol.
1, pp. 421-426.
MANTLE, P.G. (1977c) Ergotism in cattle, sheep, swine. In: Wyllie,
T.D. & Morehouse, L.C., ed. Mycotoxic fungi, mycotoxins,
mycotoxicoses: an encyclopedia handbook, New York, Marcel Dekker, Vol.
2, pp. 145-151, 207-213, 273-275.
MARASAS, W.F.O., BAMBURG, J.R., SMALLEY, E.B., STRONG, F.M., RAGLAND,
W.L., & DEGURSE, P.E. (1969) Toxic effects on trout, rats and mice
of T-2 toxin produced by the fungus Fusarium tricinctum. Toxicol.
appl. Pharmacol., 15(2): 471-482.
MARASAS, W.F.O., SMALLEY, E.B., BAMBURG, J.R., & STRONG, F.M. (1971)
Phytotoxicity of T-2 toxin produced by Fusarium tricinctum.
Phytopathology, 61(12): 1488-1491.
MARASAS, W.F.O., KRIEK, N.P.J., WIGGINS, V.M., STEYN, P.S., TOWERS,
D.K., & HASTIE, T.J. (1979a) Incidence, geographic distribution,
and toxigenicity of Fusarium species in South African corn.
Phytopathology, 69(11): 1181-1185.
MARASAS, W.F.O., LEISTNER, L., HOFMANN, G., & ECKARDT, C. (1979b)
Occurrence of toxigenic strains of Fusarium in maize and barley in
Germany. Appl. environ. Microbiol., 7: 289-305.
MARASAS, W.F.O., NELSON, P.E., & TOUSSON, T.A., ed. (1984) Toxigenic
Fusarium species - identity and mycotoxicology, University Park,
Pennsylvania, Pennsylvania State University Press.
MARQUARDT, R.R., FROHLICH, A.A., SREEMANNARAYANA, O., ABRAMSON, D., &
BERNATSKY, A. (1988) Ochratoxin A in blood from slaughter pigs in
Western Canada. Can. J. vet. Res., 52: 186-190.
MASUDA, E., TAKEMOTO, T., TATSUNO, T., & OBARA, T. (1982)
Immunosuppressive effect of a trichothecene mycotoxin, fusarenon-X in
mice. Immunology, 45: 743-749.
MASUKO, H., UENO, Y., OTOKAWA, M., & YAGINUMA, K. (1977) The
enhancing effect of T-2 toxin on delayed hyper-sensitivity in mice.
Jpn. J. med. Sci. Biol., 30(3): 159-164.
MATSUOKA, Y. & KUBOTA, K. (1981) Studies on mechanisms of diarrhea
induced by fusarenone-X, a trichothecene mycotoxin from Fusarium
species. Toxicol. appl. Pharmacol., 57(3): 293-301.
MATSUOKA, Y. & KUBOTA, K. (1987) Characteristics of inflammation
induced by Fusarenon-X, a trichothecene mycotoxin from Fusarium
species. Toxicol. appl. Pharmacol., 91: 333-340.
MATSUOKA, Y., KUBOTA, K., & UENO, Y. (1979) General pharmacological
studies of fusarenon-X, a trichothecene mycotoxin from Fusarium
spp. Toxicol. appl. Pharmacol., 50(1): 87-94
MAYURA, K., REDDY, R.V., HAYES, A.W., & BERNDT, W.O. (1982)
Embryocidal, fetotoxic and teratogenic effects of ochratoxin A in
rats. Toxicology, 25: 175-185.
MAYURA, K., HAYES, A.W., & BERNDT, W.O. (1983) Effects of dietary
protein on teratogenicity of ochratoxin A in rats. Toxicology, 27:
147-157.
MAYURA, K., PARKER, R., BERNDT, W.O., & PHILLIPS, T.D. (1984)
Effect of simultaneous prenatal exposure to ochratoxin A and citrinin
in the rat. J. Toxicol. environ. Health, 13: 553-561.
MEISNER, H. (1976) Energy-dependent uptake of ochratoxin A by
mitochondria. Arch. Biochem. Biophys., 173: 132-140.
MEISNER, H. & CHAN, S. (1974) Ochratoxin A, an inhibitor of
mitochondrial transport systems. Biochemistry, 13: 2795-2800.
MEISNER, H. & KROGH, P. (1982) Inhibition of renal
phosphoenolpyruvate carboxykinase in pigs by alimentary exposure to
ochratoxin A. In: Mycotoxins and Phycotoxins, Proceedings of the 5th
International IUPAC Symposium, Vienna, Technical University Vienna
publ., pp. 342-345.
MEISNER, H. & MEISNER, P. (1981) Ochratoxin A, an in vivo inhibitor
of renal phosphoenolpyruvate carboxykinase. Arch. Biochem. Biophys.,
208: 146-153.
MEISNER, H. & SELANIK, P. (1979) Inhibition of renal
gluconeogenesis in rats by ochratoxin. Biochem. J., 180: 681-684.
MEISNER, H., CIMBALA, M.A., & HANSON, R.W. (1983) Decrease of renal
phosphoenolpyruvate carboxykinase RNA and Poly(A)+ RNA level by
ochratoxin A. Arch. Biochem. Biophys., 223: 264-270.
MERWE, K.J.,VAN DER, J., STEYN, P.S., & FOURIE, L. (1965)
Mycotoxins. Part II. The constitution of ochratoxins A, B, and C,
metabolites of Aspergillus ochraceus Wilh. J. Chem. Soc., 1965:
7083-7088.
MILES, W.F. & GURPRASAD, N.P. (1985) Oxygen negative chemical
ionization mass spectrometry of trichothecenes. Biomed. mass
Spectrom., 12: 652-658.
MINISTRY OF AGRICULTURE, FISHERIES AND FOOD (1980) Survey of
mycotoxins in the United Kingdom, London, Ministry of Agriculture,
Fisheries and Food, 35 pp (Food Surveillance Paper No. 4).
MIROCHA, C.J. & ABBAS, H.K. (1989) Chemistry, occurrence and
toxicology of the haemorrhagic mycotoxin (wortmannin) produced by
Fusarium. In: Natori, S., Hashimoto, K., & Ueno, Y. ed. Mycotoxins
and phycotoxins, Science Publishers, Amsterdam, Oxford, New York,
Elsevier.
MIROCHA, C.J. & PATHRE, S. (1973) Identification of the toxic
principle in a sample of poefusarin. Appl. Microbiol., 26(5): 719-
724.
MIZUNO, S. (1975) Mechanism of inhibition of protein synthesis
initiation by diacetoxyscirpenol and fusarenon-X in the reticulocyte
lysate system. Biochim. Biophys. Acta, 383(2): 207-214.
MORE, J., GALTIER, P., & ALVINERIE, M. (1978) Toxicite de
l'ochratoxine A. III. Effets pendant les stades initiaux de la
gestation chez le rat. Ann. Rech. vet., 9(1): 169-173.
MORGAN, M.R.A., MATTHEW, J.A., MCNERNEY, R., & CHAN, H.W.-S. (1982)
The immunoassay for ochratoxin A. In: Mycotoxins and Phycotoxins,
Proceedings of the 5th International IUPAC Symposium, Vienna,
Technical University Vienna, pp. 32-35.
MOROI, K., SUZUKI, S., KUGA, T., YAMAZAKI, M., & KANISAWA, M. (1985)
Reduction of ochratoxin A toxicity in mice treated with phenylalanine
and phenobarbital. Toxicol. Lett., 25: 1-5.
MORRISSEY, R.E. (1984) Teratological study of Fisher rats fed diet
containing added vomitoxin. Food chem. Toxicol., 22(6): 453-457.
MORRISSEY, R.E. & VESONDER, R.F. (1985) Effect of deoxynivalenol
(vomitoxin) on fertility, pregnancy and postnatal development of
Sprague-Dawley rats. Appl. environ. Microbiol., 49: 1062-1066.
MORTENSEN, H.P., HALD, B., & MADSEN, A. (1983) Feeding experiments
with ochratoxin A contaminated barley for bacon pigs. V. Ochratoxin A
in blood. Acta agric. Scand., 33: 235-239.
MORTIMER, P.H., CAMPBELL, J., DI MEUNA, M.E., & WHITE, E.P. (1971)
Experimental myrotheciotoxicosis and poisoning in ruminants by
verrucarin A and roridin A. Res. vet. Sci., 12: 508-515.
MULDERS, E.J. & IMPELEN-PEEK, H.A.M. (1986) Gas chromatographic
determination of deoxynivalenol in cereals. Z. Lebensm. Unters.
Forsch., 183: 406-409.
MUNRO, I.C., MOODIE, C.A., KUIPER-GOODMAN, T., SCOTT, P.M., & GRICE,
H.C. (1974) Toxicologic changes in rats fed graded dietary levels
of ochratoxin A. Toxicol. appl. Pharmacol., 28(2): 180-188.
MUNSCH, N. & MUELLER, W.E.G. (1980) Effect of T-2 toxin on DNA
polymerases and terminal deoxynucleotidyl transferase of Molt-4 and
Nu-8 cell lines. Immunopharmacology, 2(4): 313-318.
MUTOH, A., ISHII, K., & UENO, Y. (1988) Effect of radioprotective
compounds and anti-inflammatory agents on the acute toxicity of
trichothecenes. Toxicol. Lett., 40: 165-174.
NAGAYAMA, S., KAWAMURA, O., OHTANI, K., RYU, J.C., LATUS, D.,
SUDHEIM, L., & UENO, Y. (1988) Application of an enzyme-linked
immunosorbent assay for screening of T-2 toxin-producing Fusarium
spp. Appl. environ. Microbiol., 54: 1302-1303.
NAKAMURA, Y., TAKEDA, S., OGASAWARA, K., KARASHIMADA, T., & ANDO K.
(1951) [A study on toxicosis caused by Akakabi poisoned wheat
flour.] Hokkaido doritsu Eiseikenkyujoho, 2: 35-46 (in Japanese).
NEELY, W.C. & WEST, A.D. (1972) Spectroanalytical parameters of
fungal metabolites. III. Ochratoxin A. J. Assoc. Off. Anal. Chem.,
55(6): 1305-1309.
NEL, W. & PURCHASE, I.F.H. (1968) The fate of ochratoxin A in rats.
J. S. Afr. Chem. Inst., 21: 87-88.
NELSON, P.E., TOUSSON, T.A., & MARASAS, W.F.O. (1983) Fusarium
species, an illustrated manual for identification, University Park,
Pennsylvania, Pennsylvania State University Press.
NESHEIM, S. (1971) Ochratoxins: occurrence, production, analysis,
and toxicity. Abstracts from the 85th Annual Meeting of the
Association of Official Analytical Chemists, Washington, 11-14
October, 1971, Washington, DC, Association of Official Analytical
Chemists.
NESHEIM, S. (1973) Analysis of ochratoxins A and B and their esters
in barley, using partition and thin-layer chromatography. II.
Collaborative study. J. Assoc. Off. Anal. Chem., 56: 822-826.
NESHEIM, S. (1982) Ochratoxin A- Analytical method 1: thin-layer
chromatographic determination of ochratoxin A in foodstuffs. In:
Egan, H., ed. Environmental carcinogens: selected methods of analysis
- some mycotoxins, Lyons, International Agency for Research on
Cancer, pp. 255-270.
NESHEIM, S., HARDIN, N.F., FRANCIS, O.J., & LANGHAM, W.S. (1973)
Analysis of ochratoxins A and B and their esters in barley, using
partition and thin-layer chromatography. I. Development of the
method. J. Assoc. Off. Anal. Chem., 56: 817-821.
NICOLOV, I.G., CHERNOZEMSKY, I.N., PETKOVA-BOCHAROVA, T., STOYANOV,
I.S., & STOICHEV, I.I. (1978) Epidemiologic characteristics of
urinary system tumours and Balkan nephropathy in an endemic region of
Bulgaria. Eur. J. Cancer, 14: 1237-1242.
NIP, W.K. & CHU, F.S. (1979) Fate of ochratoxin A in goats. J.
environ. Sci. Health, B14: 319-333.
NORPPA, H., PENTTILA, M., SORSA, M., HINTIKKA, E.L., & ILUS, T.
(1980) Mycotoxin T-2 of Fusarium tricinctum and chromosome changes
in Chinese hamster bone marrow. Hereditas, 93(2): 329-332.
NORTHOLT, M.D., VAN EGMOND, H.P., & PAULSCH, W.E. (1979) Ochratoxin
A production by some fungal species in relation to water activity and
temperature. J. food Prot., 42: 485-490.
NOWICKI, T.W., GABA, D.G., DEXTER, J.E., MATSUO, R.R., & CLEAR, R.M.
(1988) Retention of the Fusarium mycotoxin deoxynivalenol in wheat
during processing and cooking of spaghetti and noodles. J. Cereal
Sci., 8: 189-202.
OGASAWARA, K. (1965) [Akakabi-toxicosis.] J. Food Hyg. Soc. Jpn,
6: 81-82 (in Japanese).
OHTA, M., ISII, K., & UENO, Y. (1977) Metabolism of trichothecene
mycotoxins. Part 1. Microsomal deacetylation of T-2 toxin in animal
tissues. J. Biochem. (Tokyo), 82(6): 1591-1598.
OHTSUBO, K. & SAITO, M. (1970) Cytotoxic effects of scirpene
compounds, fusarenon-X produced by Fusarium nivale, dihydro-nivalenol
and dihydrofusarenon-X on Hela cells. Jpn. J. med. Sci. Biol., 23(4):
217-225.
OHTSUBO, K. & SAITO, M. (1977) Chronic effects of trichothecene
toxins. In Rodricks, J.V., Hesseltine, C.W., & Mehlman, M.A., ed.
Mycotoxins in human and animal health, Park Forest South, Illinois,
Pathotox Publishers, pp. 255-262.
OHTSUBO, K., YAMADA, M., & SAITO, M. (1968) Inhibitory effect of
nivalenol, a toxic metabolite of Fusarium nivale on the growth cycle
and biopolymer synthesis of Hela cells. Jpn. J. med. Sci. Biol.,
21(3): 185-194.
OHTSUBO, K., KADEN, P., & MITTERMAYER, C. (1972) Polyribosomal
breakdown in mouse fibroblasts L cells by fusarenon-X, a toxic
principle isolated from Fusarium nivale. Biochim. Biophys. Acta,
287(3): 520-525.
OHTSUBO, K., RYU, J.-C., NAKAMURA, K., IZUMIYAMA, N., TANAKA, T.,
YAMAMURA, H., KOBAYSAHI, T., & UENO, Y. (in press) Chronic toxicity
of nivalenol in mice: 2-year feeding trial with Fusarium nivale Fn
2B-moulded rice. Food Chem. Toxicol.
ORTI, D.L., HILL, R.H., LIDDLE, J.A., & NEEHAM, L.L. (1986) High-
performance liquid chromatography of mycotoxin metabolites in human
urine. J. anal. Toxicol., 10: 41-45.
OSBORNE, B.G. (1980) The occurrence of ochratoxin A in mouldy bread
and flour. Food Cosmet. Toxicol., 18: 615-617.
OSBORNE, B.G. & WILLIS, K. (1984) Studies into the occurrence of some
trichothecene mycotoxins in UK home-grown wheat and in imported
wheat. J. Sci. Food Agric., 35: 579-583.
OTOKAWA, M., SHIBAHARA, Y., & EGASHIRA, Y. (1979) The inhibitory
effect of T-2 toxin on tolerance induction of delayed-type
hypersensitivity in mice. Jpn. J. med. Sci. Biol., 32(1): 37-46.
PACE, J.G., WATTS, M.R., BURROWS, E.P., DINTERMAN, R.E., MATSON, C.,
HAUER, E.C., & WANNEMACHER, R.W., Jr (1985) Fate and distribution of
3H labeled T-2 mycotoxin in guinea pigs. Toxicol. appl. Pharmacol.,
80: 377-385.
PANG, V.F., LAMBERT, R.J., FELSBURG, P.J., BEASLEY, V.R., BUCK, W.B,.
& HASCHEK (1987a) Experimental T-2 toxicosis in swine following
inhalation exposure: effects on pulmonary and systemic immunity and
morphologic changes. Toxicol. Pathol., 15: 308-319.
PANG, V.F., LORENZANA, R.M., BEASLEY, V.R., BUCK, W.B., & HASCHEK,
W.M. (1987b) Experimental T-2 toxicosis in swine. III. Morphologic
changes following intravascular administration of T-2 toxic. Fundam.
appl. Toxicol., 8: 298-309.
PANG, V.F., LAMBERT, R.J., FELSBURG, P.J., BEASLEY, V.R., BUCK, W.B.,
& HASCHEK, W.M. (1988) Experimental T-2 toxicosis in swine following
inhalation exposure: clinical signs and effects on hematology, serum
biochemistry and immune response. Fundam. appl. Toxicol., 11:
100-109.
PARKER, G.W., WANNEMACHER, R.W., Jr, & GILMAN, F.G. (1984) The effect
of T-2 mycotoxin on the cardiovascular system in the guinea pig. Fed.
Proc., 43: 578.
PATTERSON, D.S.P., MATTHEWS, J.G., SHREEVE, B.J., ROBERTS, B.A.,
MCDONALD, S.M., & HAYES, A.W. (1979) The failure of trichothecene
mycotoxins and whole cultures of Fusarium tricinctum to cause
experimental haemorrhagic syndromes in calves and pigs. Vet. Rec.,
105(11): 252-253.
PATTERSON, D.S.P., SHREEVE, B.J., ROBERTS, B.A., BERRETT, S., BRUSH,
P.J., GLANCY, E.M., & KROGH, P. (1981) Effect on calves of barley
naturally contaminated with ochratoxin A and groundnut meal
contaminated with low concentrations of aflatoxin B1. Res. vet. Sci.,
31: 213-218.
PAULSCH, W.E., VAN EGMOND, H.P., & SCHULLER, P.L. (1982) Thin-layer
chromatographic method for analysis and chemical confirmation of
ochratoxin A in kidneys of pigs. In: Mycotoxins and Phycotoxins,
Proceedings of the 5th International IUPAC Symposium, Vienna,
Technical University Vienna publ., pp. 40-43.
PAVLOVIC, M., PLESTINA, R., & KROGH, P. (1979) Ochratoxin A
contamination of foodstuffs in an area with Balkan (endemic)
nephropathy. Acta pathol. microbiol. Scand. B87: 243-246.
PAWLOSKY, R.J. & MIROCHA, C.J. (1984) Structure of a metabolic
derivative of T-2 toxin (TC-6) based on mass spectrometry. J. agric.
food Chem., 32: 1420-1423.
PECKHAM, J.C., DOUPNIK, B., & JONES, O.H. (1971) Acute toxicity of
ochratoxins A and B in chicks. Appl. Microbiol., 21(3): 492-494.
PEDERSEN, E. & HANSEN, H.N. (1981) [Ochratoxin A in grain and grain
products.] Copenhagen, Stat. Levnedsm. Institute (Report F8l002) (in
Danish).
PEPELJNJAK, S. & CVETNIC, Z. (198l) [Ochratoxogenicity of
Aspergillus ochraceus strains from the territory of endemic
nephropathy.] Vet. Arch., 51: 101-103 (in Croatian).
PEPELJNJAK, S., BLAZEVIC, N., & CULJAK, N. (1982) Histopathological
changes and finding of ochratoxin A in organs of pig in the area of
endemic nephropathy in Yugoslavia. In: Mycotoxins and Phycotoxins,
Proceedings of the 5th International IUPAC Symposium, Vienna,
Technical University Vienna publ., pp. 346-348.
PESTKA, J.J., LEE, S.C., LAU, H.P., & CHU, F.S. (1981) Enzyme-
linked immunosorbent assay for T-2 toxin. J. Assoc. Off. Anal. Chem.,
58(12): 940A-944A.
PESTKA, J.J., LIN, W.S., & MITHS, E.R. (1987a) Emetic activity of the
trichothecene 15-acetyldeoxynivaleol in swine. Food chem. Toxicol.,
25: 855-858.
PESTKA, J.J., TAI, J.H., WITT, W.F., DIXON, D.E., & FORSELL, J.H.
(1987b) Suppression of immune response in the B6C3F1 mouse after
dietary exposure to the Fusarium mycotoxins deoxynivalenol
(vomitoxin) and zearalenone. Food chem. Toxicol., 25: 297-304.
PETKOVA-BOCHAROVA, T. & CASTEGNARO, M. (1985) Ochratoxin A
contamination of cereals in an area of high incidence of Balkan
endemic nephropathy in Bulgaria. Food Addit. Contam., 2: 267-270.
PETKOVA-BOCHAROVA, T., CHERNOZEMSKY, I.N., & CASTEGNARO, M. (1988)
Ochratoxin A in human blood in relation to Balkan endemic nephropathy
and urinary system tumours in Bulgaria. Food Addit. Contam., 5:
299, 301.
PIENAAR, J.G., KELLERMAN, T.S., & MARASAS, W.F.O. (1981) Field
outbreaks of leukoencephalomalacia in horses consuming maize infected
by Fusarium verticillioides (F. moniliforme) in South Africa. J. S.
Afr. Vet. Assoc., 52: 21-24.
PIER, A.C., CYSEWSKI, S.J., RICHARD, J.L., BAETZ, A.L., & MITCHELL,
L. (1976) Experimental mycotoxicoses in calves with aflatoxin,
ochratoxin, rubratoxin, and T-2 toxin. In: Proceedings of the 80th
Annual Meeting of the US Animal Health Association, Miami Beach,
Florida, 7-12 November, 1976, Richmond, Virginia, US Animal Health
Association, pp. 130-148.
POHLAND, A.E., SCHULLER, P.L., STEYN, P.S., & VAN EGMOND, H.P.
(1982) Physicochemical data for some selected mycotoxins. Pure appl.
Chem., 54: 2219-2284.
POHLAND, A.E., THORPE, C., TRUCKSESS, W., & EPPLEY, R. (1986) TLC and
HPLC methods for analysis of trichothecenes in commodities. In:
Richard, J. & Thurston, J., ed. Diagnosis of mycotoxicoses,
Dordrecht, Boston, Martinus Nijhoff Publishers, pp. 271-281.
POHLAND, A.E. & WOOD, G.E. (1987) Occurrence of mycotoxins in food.
In: Krogh, P., ed. Mycotoxins in foods, London, Academic Press, pp.
35-64.
POKROVSKIJ, V.I. & TUTELYAN, V.A. (1982) [Ergotism: a companion of
natural disaster.] Ter. Ark., 108-110 (in Russian).
POPPE, S.M., STUCKHARDT, J.L., & SZCZECH, G.M. (1983) Postnatal
behavioural effects of ochratoxin A in offspring of treated mice.
Teratology, 27: 293-300.
PRELUSKY, D.B., TRENHOLM, H.L., LAWRENCE, G.A., & SCOTT, P.M. (1984)
Nontransmission of deoxynivalenol (vomitoxin) to milk following oral
administration to dairy cows. J. environ. Sci. Health, B19(7): 593-
609.
PRELUSKY, D.B., VEIRA, D.M., & TRENHOLM, H.L. (1985) Plasma
pharmacokinetics of the mycotoxin deoxynivalenol following oral and
intravenous administration to sheep. J. environ. Sci. Health, B20:
603-624.
PRELUSKY, D.B., TRENHOLM, H.L., HAMILTON, R.M.G., & MILLER, J.D.
(1987) Transmission of [14C]deoxynivalenol to eggs following oral
administration to laying hens. J. agric. food Chem., 35: 182-186.
PRIOR, M.G. (1976) Mycotoxin in determinations on animal feedstuffs
and tissues in Western Canada. Can. J. comp. Med., 40: 75-79.
PRIOR, M.G. (198l) Mycotoxins in animal feedstuffs and tissues in
Western Canada 1975 to 1976. Can. J. comp. Med., 45: 116-119.
PRIOR, M.G. & SISODIA, C.S. (1978) Ochratoxicosis in white leghorn
hens. Poult. Sci., 57: 619-623.
PRIOR, M.G. & SISODIA, C.S. (1982) The effects of ochratoxin A on
the immune response of Swiss mice. Can. J. comp. Med., 46: 91-96.
PRIOR, M.G., SISODIA, C.S., & O'NEIL, J.B. (1976) Acute oral
ochratoxicosis in day-old white leghorns, turkeys and Japanese quail.
Poult. Sci., 55: 786-790.
PUCHLEV, A. (1973) La nephropathie endemique en Bulgarie. In:
Symposium sur la nephropathie endemique de l'Academie serbe des
sciences et des arts, Belgrade, pp. 15-27.
PUCHLEV, A., ed. (1974) Endemic nephropathy. In: Proceedings of the
2nd International Symposium on Endemic Nephropathy, Sofia, Bulgarian
Academy of Sciences, pp. 1-343.
PULS, R. & GREENWAY, J.A. (1976) Fusariotoxicosis from barley in
British Columbia. II. Analysis of suspected barley. Can. J. comp.
Med., 40(1): 16-19.
PURCHASE, I.F.H. & THERON, J.J. (1968) The acute toxicity of
ochratoxin A to rats. Food Cosmet. Toxicol., 6: 479-483.
QIUJIE, X., XIAOQIU, L., JIANLI, W., & YUNSIAN, L. (1988)
Trichothecenes in staple food from high incidence area of carcinoma
of esophagus and gastric cardia and their carcinogenic potential.
Zhonghua Zhongliu Zoshi, 10: 4-8.
RABIE, C.J., SYDENHAM, E.W., THEL, P.G., LUBBEN, A., & MARASAS,
W.F.O. (1986) T-2 toxin production by Fusarium acuminatum isolated
from oats and barley. Appl. environ. Microbiol., 52(3): 594-596.
RAFAI, P. & TUBOLY, S. (1982) Effect of T-2 toxin on adrenocortical
function and immune response in growing pigs. Zbl. Vet. Med., B29:
558-565.
RAJAKYLA, E., LAASASENAHO, K., & SAKKERS, P.J.D. (1987) Determination
of mycotoxins in grain by high-performance liquid chromatography and
thermospray liquid chromatography-mass spectrometry. J. Chromatogr.,
384: 391-402.
REICHMANN, K.G., BLANEY, B.J., CONNOR, J.K., & RUNGE, B.M. (1982)
The significance of aflatoxin and ochratoxin in the diet of
Australian chickens. Aust. vet. J., 58: 211-212.
REISS, J. (1973) Influence of the mycotoxins patulin and
diacetoxyscirpenol on fungi. J. gen. appl. Microbiol., 19(6):
415-420.
REISS, J. (1975) Mycotoxin poisoning of Allium cepa root tips. Part
2. Reduction of mitotic index and formation of chromosomal
aberrations and cytological abnormalities by patulin, rubratoxin B
and diacetoxyscirpenol. Cytologia, 39(4): 703-708.
REISS, J. (1977) Inhibition of urease by the mycotoxin patulin.
Naturwissenschaften, 64: 97.
RIBELIN, W.E., FUKUSHIMA, K., & STILL, P.E. (1978) The toxicity of
ochratoxin to ruminants. Can. J. comp. Med., 42: 172-176.
ROBISON, T.S., MIROCHA, C.J., KURTZ, H.J., BEHRENS, J.C., CHI, M.S.,
WEAVER, G.A., & NYSTROM, S.D. (1979a) Transmission of T-2 toxin
into bovine and porcine milk. J. dairy Sci., 62(4): 637-641.
ROBISON, T.S., MIROCHA, C.J., KURTZ, H.J., BEHRENS, J.C., WEAVER,
G.A., & CHI, M.S. (1979b) Distribution of tritium labeled T-2 toxin
in swine. J. agric. food Chem., 27(6): 1411-1413.
ROGERS, C.G. & HEROUX-METCALF, C. (1983) Cytotoxicity and absence of
mutagenic activity of vomitoxin (4-deoxynivalenol) in a hepatocyte-
mediated assay with V79 Chinese hamster lung cells. Cancer Lett.,
20: 29-35.
ROMER, T.R. (1986) Use of small charcoal/alumina cleanup columns in
determination of trichothecene mycotoxins in foods and feeds. J.
Assoc. Off. Anal. Chem., 69: 699-703.
ROMER, T.R., BOLING, T.M., & MACDONALD, J.L. (1978). Gas-liquid
chromatographic determination of T-2 toxin and diacetoxyscirpenol in
corn and mixed feeds. J. Assoc. Off. Anal. Chem., 61(4): 801-808.
ROOD, H.D., BUCK, W.B., & SWANSON, S.P. (1988a) Gas chromatographic
screening method for T-2 toxin, diacetoxyscirpenol, deoxynivalenol
and related trichothecenes in feeds. J. Assoc. Off. Anal. Chem., 71:
493-498.
ROOD, H.D., BUCK, W.B., & SWANSON, S.P. (1988b) Diagnostic screening
method for determination of trichothecene exposure in animals. J.
agric. food Chem., 36: 74-79.
ROSEN, J.D., ROSEN, R.T., & HARTMAN, T.G. (1986) Capillary gas
chromatography-mass spectrometry of several macrocyclic
trichothecenes. J. Chromatogr., 355: 241-251.
ROSEN, R.T. & ROSEN, J.D. (1984) Quantification and confirmation of
four Fusarium mycotoxins in corn by gas chromatography-mass
spectrometry. J. Chromatogr., 283: 223-230.
ROSENSTEIN, Y. & LAFARGE-FRAYSSINET, C. (1983) Inhibitory effect of
Fusarium T-2 toxin on lymphoid DNA and protein synthesis. Toxicol.
appl. Pharmacol., 70: 283-288.
ROSENSTEIN, Y., LAFARGE-FRAYSSINET, C., LESPINATS, G., LOISILLIER,
F., LAFONT, P., & FRAYSSINET, C. (1979) Immunosuppressive activity
of Fusarium toxins. Effects on antibody synthesis and skin grafts of
crude extracts of T-2 toxin and diacetoxyscirpenol. Immunology,
36(1): 111-118.
ROUSSEAUX, C.G. & SHEIFER, H.B. (1987) Maternal toxicity,
embryolethality and abnormal fetal development in CD-1 mice following
one oral dose of T-2 toxin. J. appl. Toxicol., 7: 281-288.
ROUSSEAUX, C.G., SHIEFER, H.B., & HANCOCK, D.S. (1986) Reproductive
and teratological effects of continuous low-level dietary T-2 toxin
in female CD-1 mice for two generations. J. appl. Toxicol., 6:
179-184.
ROUSSEAU, D.M. & VAN PETEGHEM, C.H. (1989) Spontaneous occurrence of
ochratoxin A residues in porcine kidneys in Belgium. Environ. Contam.
Toxicol., 42: 181-186.
ROUSSEAU, D.M., CANDLISH, A.A.G., SLEGERS, G.A., VAN PETEGHEM, C.H.,
STINSON, W.H., & SMITH, J.E. (1987) Detection of ochratoxin A in
porcine kidneys by a monoclonal antibody-based radioimmunoassay.
Appl. environ. Microbiol., 53: 514-518.
RUKMINI, C. & BHAT, R.V. (1978) Occurrence of T-2 toxin in Fusarium
incarnatum infested sorghum from India. J. agric. food Chem., 26
(3): 647-649.
RUKMINI, C., PRASAD, J.S., & RAO, K. (1980) Effect of feeding T-2
toxin to rats and monkeys. Food Cosmet. Toxicol., 18(3): 267-270.
RUSCH, M.E. & STAHELIN, H. (1965) [Some biological effects of the
cytostatic agent verrucarin A.] Arzneimittelforschung, 15: 893-897
(in German).
RUTQVIST, L., BJORKLUND, N.-E., HULT, K., & GATENBECK, S. (1977)
Spontaneous occurrence of ochratoxin residues in kidneys of fattening
pigs. Zbl. Veterinaermed., A24(5): 402-408.
RUTQVIST, L., BJORKLUND, N.-E., HULT, K., HOKBY, E., & CARLSSON, B.
(1978) Ochratoxin A as the cause of spontaneous nephropathy in
fattening pigs. Appl. environ. Microbiol., 36: 920-925.
RYU, J.C., SHIRAKI, N., & UENO, Y. (1987) Effects of drugs and
metabolic inhibitions on the acute toxicity of T-2 toxin in mice.
Toxicon, 25: 743-750.
RYU, J.-C., OHTSUBO, K., IZUMIYAMA, N., NAKAMURA, K., TANAKA, T.,
YAMAMURA, H., & UENO, Y. (1988) The acute and chronic toxicities of
nivalenol in mice. Fundam. appl. Toxicol., 11: 38-47.
SAITO, M. & OHTSUBO, K. (1974) Trichothecene toxins of Fusarium
species. In: Purchase, I.F.H., ed. Mycotoxins, Amsterdam, Oxford, New
York, Elsevier Science Publishers, pp. 263-281.
SAITO, M., HORIUCHI, T., OHTSUBO, K., HATANAKA, Y., & UENO, Y.
(1980) Low tumor incidence in rats with long-term feeding of
fusarenon-X a cytotoxic trichothecene produced by Fusarium nivale.
Jpn. J. exp. Med., 50(40): 293-302.
SAKAMOTO, T., SWANSON, S.P., YOSHIZAWA, T., & BUCK, W.B. (1986)
Structures of new metabolites of diacetoxyscirpenol in the excreta of
orally administered rats. J. agric. food Chem., 34: 698-701.
SANDOR, G., GLAVITS, R., VAJDA, L., VANYI, A., & KROGH, P. (1982)
Epidemiological study of ochratoxin A-associated porcine nephropathy
in Hungary. In: Mycotoxins and Phycotoxins, Proceedings of the 5th
International IUPAC Symposium, Technical University Vienna publ.,
Vienna, pp. 349-352.
SANO, A., ASABE, Y., TAKITANI, S., & UENO, Y. (1982).
Fluorodensitometric determination of trichothecene mycotoxins with
nicotinamide and 2-acetylpyridine on a silica gel layer. J.
Chromatogr., 235: 257-265.
SANO, A., MATSUTANI, S., SUZUKI, M., & TAKITANI, S. (1987) High-
performance liquid chromatographic method for determining
trichothecene mycotoxins by post-column fluorescence derivatization.
J. Chromatogr., 410: 427-436.
SANSING, G.A., LILLEHOJ, E.B., DETROY, R.W., & MILLER, M.A. (1976)
Synergistic toxic effects of citrinin, ochratoxin A, and penicillic
acid in mice. Toxicon, 14: 213-219.
SARKISOV, A.Ch., KVASCHINA, E.S., KORNEEV, N.E., KOROLEVA, V.P.,
GERASIMOVA, P.N., & AKILOVA, N.S. (1944) [Harmfulness of cereals
over-wintered in the field.] Veterinariya, 11-12: 39-41. (in
Russian).
SATO, N., UENO, Y., & ENOMOTO, M. (1975) Toxicological approaches
to the toxic metabolites of Fusaria. Part 8. Acute and subacute
toxicities of T-2 toxin in cats. Jpn. J. Pharmacol., 25(3): 263-270.
SATO, N. & UENO, Y. (1977) Comparative toxicities of
trichothecenes. In: Rodricks, J.V., Hesseltine, C.W., & Mehlman,
M.A., ed. Mycotoxins in human and animal health, Park Forest South,
Illinois, Pathotox Publishers, pp. 295-307.
SATO, N., ITO, T., KUMADA, H., UENO, Y., ASANO, K., SAITO, M.,
OHTSUBO, K., UENO, I., & HATANAKA, Y. (1978) Toxicological
approaches to the metabolites of Fusaria. XIII. Hematological changes
in mice by a single and repeated administrations of trichothecenes.
J. toxicol. Sci., 3(4): 335-356.
SATO, S., OHYA, T., HOMMA, S., & TATSUNO, T. (1981) [Effects of
fusarenon-X on serological responses of chicks inoculated with
newcastle disease vaccines.] Proc. Jpn. Assoc. Mycotoxicol., 13:
43-45 (in Japanese).
SCHIEFER, H.B., ROUSSEAUX, C.G., HANCOCK, D.S., & BLAKLEY, B.R.
(1987) Effects of low-level long-term oral exposure to T-2 toxin in
CD-1 mice. Food chem. Toxicol., 25: 593-601.
SCHMIDT, R. & DOSE, K. (1984) HPLC: A tool for the analysis of T-2
toxin and HT-2 toxin in cereals. J. anal. Toxicol., 8: 43-45.
SCHINDLER, D. (1974) Two classes of inhibitors of peptidyl
transferease activity in eukaryotes. Nature (Lond.), 249(5452):
38-41.
SCHINDLER, D., GRANT, P., & DAVIES, J. (1974) Trichodermin
resistance mutation affecting eukaryotic ribosomes. Nature (Lond.),
248(5448): 535-536.
SCOTT, P.M. (1977) Penicillium mycotoxins. In: Wyllie, T.D. &
Morehouse, L.G., ed. Mycotoxic fungi, mycotoxins, mycotoxicoses, New
York, Marcel Dekker, pp. 283-356.
SCOTT, P.M. (1982) Assessment of quantitative methods for
determination of trichothecenes in grains and grain products. J.
Assoc. Off. Anal. Chem., 65(4): 876-883.
SCOTT, P.M. (1984) Effects of food processing on mycotoxins. J. food
Prot., 47(6): 489-499.
SCOTT, P.M. & KANHERE, S.R. (1986) Comparison of column phases for
separation of derivatized trichothecenes by capillary gas
chromatography. J. Chromatogr., 368: 374-380.
SCOTT, P.M. & LAWRENCE, G.A. (1980) Analysis of ergot alkaloids in
flour. J. agric. food Chem., 28: 1258-1261.
SCOTT, P.M. & LAWRENCE, G.A. (1982) Losses of ergot alkaloids
during making of bread and pancakes. J. agric. food Chem., 30:
445-450.
SCOTT, P.M., LAWRENCE, J.W., & VAN WALBEEK, W. (1970) Detection of
mycotoxins by thin-layer chromatography: application to screening of
fungal extracts. Appl. Microbiol., 20(5): 839-842.
SCOTT, P.M., WALBEEK, W., VAN, KENNEDY, B., & ANYETI, D. (1972)
Mycotoxins (ochratoxin A, citrinin, and sterigmatocystin) and
toxigenic fungi in grains and other agricultural products. J. agric.
food Chem., 20: 1103-1109.
SCOTT, P.M., HARWIG, J., & BLANCHFIELD, B.J. (1980) Screening
Fusarium strains isolated from over-wintered Canadian grains for
trichothecenes. Mycopathologia, 72(3): 175-180.
SCOTT, P.M., LAU, P.Y., & KANHERE, S.R. (1981) Gas chromatography
with electron capture and mass spectrometric detection of
deoxynivalenol in wheat and other grains. J. Assoc. Off. Anal. Chem.,
64(6): 1364-1371.
SCOTT, P.M., KANHERE, S.R., LAU, P.-Y., DEXTER, J.E., & GREENHALGH,
R. (1983) Effects of experimental flour milling and bread baking on
retention of deoxynivalenol (vomitoxin) in hard red spring wheat.
Cereal Chem., 60: 421-424.
SCOTT, P.M., NELSON, K., KANHERE, S.R., KARPINSKI, K.F., HAYWARD, S.,
NIESH, G.A., & TEICH, A.H. (1984a) Decline in deoxynivalenol
(vomitoxin) concentrations in 1983 Ontario winter wheat before
harvest. Appl. environ. Microbiol., 48(6): 884-886.
SCOTT, P.M., KANHERE, S.R., DEXTER, J.E., BRENNAN, P.W., & TRENHOLM,
H.L. (1984b) Distribution of the trichothecene mycotoxin
deoxynivalenol (Vomitoxin) during the milling of naturally
contaminated hard red spring wheat and its fate in baked products.
Food Addit. Contam., 1(4): 313-323.
SCOTT, P.M., KANHERE, S.R., & TARTER, E.J. (1986) Determination of
nivalenol and deoxynivalenol in cereals by electron-capture gas
chromatography. J. Assoc. Off. Anal. Chem., 69: 889-893.
SEITZ, L.M., YAMAZAKI, W.T., CLEMENTS, R.L., MOHR, H.E., & ANDREWS,
L. (1985) Distribution of deoxynivalenol in soft wheat mill
streams. Cereal Chem., 62(6): 467-469.
SHEPHERD, M.J. & GILBERT, J. (1988) Long-term stability of
deoxynivalenol standard reference solutions. J. agric. food Chem.,
36: 305-308.
SHIMIZU, T., NAKANO, N., MATSUI, T., & AIBARA, K. (1979)
Hypoglycemia in mice administered fusarenon-X. Jpn. J. med. Sci.
Biol., 32(4): 189-198.
SHOTWELL, O.L., HESSELTINE, C.W., & GOULDEN, M.L. (1969) Ochratoxin
A: occurrence as natural contaminant of a corn sample. Appl.
Microbiol., 17(5): 765-766.
SHOTWELL, O.L., HESSELTINE, C.W., VANDEGRAFT, E.E., & GOULDEN, M.L.
(1971) Survey of corn from different regions for aflatoxin,
ochratoxin, and zearalenone. Cereal Sci. Today, 16(9): 266-273.
SHOTWELL, O.L., GOULDEN, M.L., & HESSELTINE, C.W. (1976) Survey of
US wheat for ochratoxin and aflatoxin. J. Assoc. Off. Anal. Chem.,
59: 122-124.
SHOTWELL, O.L., NENNETT, G.A., STUBBLEFIELD, R.D., SHANNON, G.M.,
KWOLEK, W.F., & PLATTNER, R.D. (1985) Deoxynivalenol in hard red
winter wheat: Relationship between toxin levels and factors that
could be used in grading. J. Assoc. Off. Anal. Chem., 68: 954-957.
SHREEVE, B.J., PATTERSON, D.S.P., & ROBERTS, B.A. (1979) The carry-
over of aflatoxin, ochratoxin and zearalenone from naturally
contaminated feed to tissues, urine and milk of dairy cows. Food
Cosmet. Toxicol., 17: 151-152.
SIDDIQUI, M.R. & KHAN, I.D. (1973) Renaming Claviceps microcephala
ergot fungus on Pennisetum typhoides in India as Claviceps
fusiformis. Trans. Mycol. Soc. Jpn, 144: 195-198.
SIEGFRIED, R. (1977) [ Fusarium toxin (trichothecene toxin) in feed
corn.] Landwirtsch. Forsch., Sonderh., 34(1): 37-43 (in German).
SIGG, H.P., MAULI, R., FLURY, E., & HANSER, D. (1965) The
constitution of diacetoxyscirpenol. Helv. Chim. Acta, 48: 962-988.
SINTOV, A., BIALER, M., & YAGEN, B. (1986) Pharmacokinetics of T-2
toxin and its metabolite HT-2 toxin, after intravenous administration
in dogs. Drug Metab. Disp., 14: 250-254.
SINTOV, A., BIALER, M., & YAGEN, B. (1987) Pharmacokinetics of T-2
tetraol, a urinary metabolite of the trichothecene mycotoxin, T-2
toxin, in dogs. Xenobiotica, 17: 941-950.
SIRAJ, M.Y., PHILLIPS, T.D., & HAYES, A.W. (1981) Effects of the
mycotoxins citrinin and ochratoxin A on hepatic mixed-function
oxidase and adenosinetriphosphatase in neonatal rats. J. Toxicol.
environ. Health, 8: 131-140.
SIREN, A.L. & FEUERSTEIN, G. (1986) Effect of T-2 toxin on regional
blood flow and vascular resistance in the conscious rat. Toxic. appl.
Pharmacol., 38: 438-444.
SIRIWARDANA, T.M.G. & LAFONT, P. (1978) New sensitive biological
assay for 12,13-epoxytrichothecenes. Appl. environ. Microbiol.,
35(1): 206-207.
SMALLEY, E.B., MARASAS, W.F.O., STRONG, F.M., BAMBURG, J.R., NICHOLS,
R.E., & KOSARI, N.R. (1970) Mycotoxicosis associated with moldy corn.
In: Herzberg, M., ed. Proceedings of the first US-Japan Conference on
Toxic Micro-organisms, Mycotoxins, Botulism, Honolulu, Hawaii, 7-10
October, 1968, Washington, DC, US Department of the Interior and UJNR
Joint Panels on Toxic Micro-organisms, pp. 163-173.
SMITH, R.D., UDSETH, H.R., & WRIGHT, B.W. (1985) Rapid and high
resolution capillary fluid chromatography (SFC) and SFC/MS of
trichothecene mycotoxins. J. chromatogr. Sci., 23: 192-199.
SORSA, M., LINSSAINMAA, K., PAITTELA, M., & ILUS, T. (1980)
Evaluation of the mutagenicity of epoxytrichothecene mycotoxins in
Drosophilia melanogaste. Hereditas, 92: 163-165.
SPEERS, G.M., MIROCHA, C.J., CHRISTENSEN, C.M., & BEHRENS, J.C.
(1977) Effects on laying hens of feeding corn invaded by 2 species
of Fusarium and pure T-2 mycotoxin. Poult. Sci., 56(1): 98-102.
STACK, M.E., MISLIVEC, P.B., GIBSON, R., POHLAND, A.E., & DENIZEL, T.
(1982) Mycotoxins produced by isolates of Aspergillus ochraceus
found in coffee. In: Mycotoxins and Phycotoxins, Proceedings of the
5th International IUPAC Symposium, Vienna, Technical University
Vienna, pp. 195-199.
STAFFORD, M.E. & MCLAUGHLIN, C.S. (1973) Trichodermin, a possible
inhibitor of the termination process of protein synthesis. J. cell.
Physiol., 82: 121-128.
STAHELIN, H., KALBERER-RUESCH, M.E., SIGNER, E., & LAZARY, S. (1968)
[Some biological effects of the cytostatic diacetoxyscirpenol.]
Arzneimittelforschung, 18: 989-994 (in German).
STAHL, C., VANDERHOEF, L.N., SIEGEL, N., & HELGESON, J.P. (1973)
Fusarium tricinctum T-2 toxin inhibits auxin promoted elongation in
soybean hypocotyl. Plant Physiol., 52(6): 663-666.
STAHR, H.M., HYDE, W., LEDERDAL, D., & PFEIFFER, R. (1981)
Trichothecene mycotoxin analysis for veterinary diagnostic
toxicology. Abstracts 95th Annual Meeting of AOAC., 191: 65.
STANFORD, G.K., HOOD, R.D., & HAYES, A.W. (1975) Effect of prenatal
administration of T-2 toxin to mice. Res. Commun. chem. Pathol.
Pharmacol., 10(4): 743-746.
STEYN, P.S. (1977) Mycotoxins, excluding aflatoxin, zearalenone and
the trichothecenes. In: Rodericks, J.V., Hesseltine, C.W., &
Mehlman, M.A., ed. Mycotoxins in human and animal health, Park Forest
South, Illinois, Pathotox Publishers, pp. 419-467.
STEYN, P.S. (1984) Ochratoxins and related dihydro-iso-coumarins.
In: Betina, V., ed. Mycotoxins: production, isolation, separation,
and purification, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 183-216.
STEYN, P.S., VLEGGAAR, R., DU PREEZ, N.P., BLYTH, A.A., & SEEGERS,
J.C. (1975) The in vitro toxicity of analogs of ochratoxin A in
monkey kidney epithelial cells. Toxicol. appl. Pharmacol., 32:
198-203.
STOREN, O., HOLM, H., & STORMER, F.C. (1982) Metabolism of
ochratoxin A by rats. Appl. environ. Microbiol., 44: 785-789.
STORMER, F.C. & PEDERSEN, J. (1980) Formation of 4-
hydroxyochratoxin A from ochratoxin A by rat liver microsomes.
Appl. environ. Microbiol., 39: 971-975.
STORMER, F.C., HANSEN, C.E., PEDERSEN, J.I., HVISTENDAHL, G., &
AASEN, A.J. (1981) Formation of (4R)- and (AS)-4-hydroxyochratoxin
A from ochratoxin A by liver microsomes from various species. Appl.
environ. Microbiol., 42: 1051-1056.
STOYANOV, I.S., CHERNOZEMSKY, I.N., NICOLOV, I.G., STOICHEV, I.I., &
PETKOVA-BOCHAROVA, T.K. (1978) Epidemiologic association between
endemic nephropathy and urinary system tumours in an endemic region.
J. chron. Dis., 31: 721-724.
SUNDHEIM, L., NAGAYAMA, S., KAWAMURA, O., TANAKA, T., BRODAL, G., &
UENO, Y. (1988) Trichothecenes and zearalenone in Norwegian barley
and wheat. Norw. J. agric. Sci., 2: 49-59.
SUZUKI, S. & SATOH, T. (1973) Effect of ochratoxin A on tissue
glycogen levels in rats. Jpn. J. Phamacol., 23: 415-419.
SUZUKI, S., SATOH, T., & YAMAZAKI, M. (1975) Effect of ochratoxin A
on carbohydrate metabolism in rat liver. Toxicol. appl. Pharmacol.,
32(1): 116-122.
SUZUKI, S., SATOH, T., & YAMAZAKI, M. (1977) The pharmacokinetics
of ochratoxin A in rats. Jpn. J. Pharmacol., 27: 735-744.
SUZUKI, T., KURISU, M., HOSHINO, Y., ICHINOE, M., NOSE, N., TOKUMARU,
Y., & WATANABE, Y. (1980) [Production of trichothecene mycotoxins of
Fusarium species in wheat and barley harvested in Saitama Prefecture,
Japan.] J. Food Hyg. Soc. Jpn, 21(1): 43-49 (in Japanese).
SWANSON, S.P., NICOLETTI, J., ROOD,.H.D., Jr, BUCK, W.B., COTE, L.M.,
& YOSHIZAWA, T. (1987) Metabolism of three trichothecene mycotoxins,
T-2 toxin, diacetoxyscirpenol and deoxynivalenol, by bovine rumen
microorganisms. J. agric. food Chem., 32: 1181-1183.
SWANSON, S.P., RAMASWAMY, V., BEASLEY, V.R., BUCK, W.B., &
BURMEISTER, H.H. (1983) Gas-liquid chromatographic determination of
T-2 toxin in plasma. J. Assoc. Off. Anal. Chem., 66: 909.912.
SWANSON, S.P., DAHLEM, A.M., ROOD, H.D., COTE, L.M., & YOSHIZAWA, T.
(1986) Gas chromatographic analysis of milk for deoxynivalenol and
its metabolite DOM-1. J. Assoc. Off. Anal. Chem., 69: 41-43.
SYLVIA, V.L., PHILLIPS, T.D., CLEMINT, B.A., GREEN, J.L., KUBENA,
L.F., & HEIDELBAUGH, N.D. (1986) Determination of deoxynivalenol
(vomitoxin) by high performance liquid chromatography with
electrochemical detection. J. Chromatogr., 362: 79-85.
SZATHMARY, C.I. (1983) Trichothecene toxicoses and natural
occurrence in Hungary. In: Ueno, Y., ed. Developments in food
science. IV. Trichothecenes, New York, Elsevier, pp. 229-250.
SZATHMARY, C.I. & RAFAI, P. (1978) Refusal of food contaminated
with T-2 fusariotoxin. Magy. Allatorv. Lapja, 33: 685-688.
SZCZECH, G.M. & HOOD, R.D. (1981) Brain necrosis in mouse fetuses
transplacentally exposed to the mycotoxin ochratoxin A. Toxicol.
appl. Pharmacol., 57: 127-137.
SZCZECH, G.M., CARLTON, W.W., & TUITE, J. (1973a) Ochratoxicosis in
Beagle dogs. I. Clinical and clinicopathological features. Vet.
Pathol., 10(2): 135-154.
SZCZECH, G.M., CARLTON, W.W., & TUITE, J. (1973b) Ochratoxicosis in
Beagle dogs. II. Pathology. Vet. Pathol., 10(3): 219-231.
SZCZECH, G.M., CARLTON, W.W., TUITE, J., & CALDWELL, R. (1973c)
Ochratoxin A toxicosis in swine. Vet. Pathol., 10(4): 347-364.
SZEBIOTKO, K., CHELKOWSKI, J., DOPIERALA, G., GODLEWSKA, B., &
RADOMYSKA, W. (1981) Mycotoxins in cereal grain. Part I.
Ochratoxin, critrinin, sterigmatocystin, penicillic acid and
toxigenic fungi in cereal grain. Nahrung, 25: 415-421.
TAKITANI, S., ASABE, Y., KATO, T., SUZUKI, M., & UENO, Y. (1979)
Spectrodensitometric determination of trichothecene mycotoxins with
4-( p-nitrobenzyl)pyridine on silica gel thin-layer chromatograms. J.
Chromatogr., 172: 335-342.
TAMM, C. (1977) Chemistry and biosynthesis of trichothecenes. In:
Rodricks, J.V., Hesseltine, C.W., & Mehlman, M.A., ed. Mycotoxins in
human and animal health, Park Forest South, Illinois, Pathotox
Publishers, pp. 209-228.
TANAKA, T., HASEGAWA, A., MATSUKI, Y., ISHII, K., & UENO, Y. (1985a)
Improved methodology for the simultaneous detection of the
trichothecene mycotoxins deoxynivalenol and nivalenol in cereals.
Food Addit. Contam., 2: 125-137.
TANAKA, T., HASEGAWA, A., MATSUKI, Y., & UENO, Y. (1985b) A survey
of the occurrence of nivalenol, deoxynivalenol and zearalenone in
foodstuffs and health foods in Japan. Food Addit. Contam., 2: 259-
265.
TANAKA, T., HASEGAWA, A., YAMAMOTO, S., LEE, U., SUGIURA, Y., & UENO,
Y. (1988) Worldwide contamination of cereals by the Fusarium
mycotoxins nivalenol, deoxynivalenol and zearalenone. 1. Survey of 19
countries. J. agric. food Chem., 36: 979-983.
TANAKA, T., HASEGAWA, A., YAMAMOTO, S., MATSUKI, Y., & UENO, Y.
(1986) Residues of Fusarium mycotoxins, nivalenol, deoxynivalenol
and zearalenone, in wheat and processed food after milling and
baking. J. Food Hyg. Soc. Jpn, 27(6): 653-655.
TANAKA, T., MATSUDA, Y., TOYASAKI, N., OGAWA, K., MATSUKI, Y., &
UENO, Y. (1977) Screening of trichothecene-producing Fusarium
species from river sediments by mammalian cell culture techniques.
Proc. Jpn. Assoc. Mycotoxicol., 5/6: 50-53.
TASHIRO, F., HIRAI, K., & UENO, Y. (1979) Inhibitory effects of
carcinogenic mycotoxins on deoxyribonucleic acid-dependent
ribonucleic acid polymerase and ribonulease H. Appl. environ.
Microbiol., 38(2): 191-196.
TATSUNO, T., SAITO, M., ENOMOTO, M., & TSUNODA, H. (1968) Nivalenol,
a toxic principle of Fusarium nivale. Chem. pharm. Bull., 16(12):
2519-2520.
TEICH, A.H. & HAMILTON, J.R. (1985) Effect of cultural practices,
soil phosphorus, potassium and pH on the incidence of Fusarium head
blight and deoxynivalenol levels in wheat. Appl. environ. Microbiol.,
49(6): 1429-1431.
TERAO, K., KERA, K., & YAZIMA, T. (1978) The effects of
trichothecene toxins on the bursa of Fabricius in day-old chicks.
Virchows Arch., B27(4): 359-370.
THACKER, H.L. & CARLTON, W.W. (1977) Ochratoxin A mycotoxicosis in
the guinea pig. Food. Cosmet. Toxicol., 15: 563-574.
THIEL, P.G., MEYER, C.J., & MARASAS, W.F.O. (1982) Natural
occurrence of moniliformin together with deoxynivalenol and
zearalenone in Transkeian corn. J. agric. food Chem., 30(2): 308-312.
THIGPEN, J.T., VAUGHN, C., & STUCKEY, W.J. (1981) Phase II trial of
anguidine in patients with sarcomas unresponsive to prior
chemotherapy: A southwest oncology group study. Cancer Treat. Rep.,
65(9-10): 881-882.
THUST, R., KNEIST, S., & HUEHNE, V. (1983) [Genotoxicity of
Fusarium mycotoxins (nivalenol, T-2 toxin and zearalenone) in Chinese
hamster V79-E cells in vitro.] Arch. Geschwulstforsch., 53: 9-15
(in German).
TIEBACH, R., BLAAS, W., KELLERT, M., STEINMEYER, S., & WEBER, R.
(1985) Confirmation of nivalenol and deoxynivalenol by on-line liquid
chromatography-mass spectrometry and gas-chromatography. Comparison
of methods. J. Chromatogr., 318: 103-111.
TOCHINAI, Y. (1933) [On scab of cereals and grains. I and II.]
Bochugai Zassi, 20: 106-114, 175-188 (in Japanese).
TOMAR, R.S., BLAKLEY, B.R., & COTEAU, W.E. (1988) In vitro effects of
T-2 toxin of the mitogen responsiveness and antibody-producing
ability of human lymphocytes. Toxicol. Lett., 40: 109-117.
TRENHOLM, H.L., COCHRANE, W.P., COHEN, H., ELLIOT, J.I., FARNWORTH,
E.R., FRIEND, D.W., HAMILTON, R.M.H., NEISH, G.A., & STANDISH, J.F.
(1981) Survey of vomitoxin contamination of the 1980 white winter
wheat crop in Ontario, Canada. J. Am. Oil Chem. Soc., 58: 992-994.
TRENHOLM, H.L., HAMILTON, R.M.G., FRIEND, D.W., THOMPSON, B.K., &
HARTIN, K.E. (1984) Feeding trials with vomitoxin (deoxynivalenol)-
contaminated wheat: effects on swine, poultry and dairy cattle. J.
Am. Vet. Med. Assoc., 185: 527-531.
TRENHOLM, H.L., WARNER, R.M., & PRELUSKY, D.B. (1985) Assessment of
extraction procedures in the analysis of naturally contaminated grain
products for deoxynivalenol (vomitoxin). J. Assoc. Off. Anal. Chem.,
68: 645-659.
TRUCKSESS, M.W., NESHEIM, S., & EPPLEY, R.M. (1984) Thin layer
chromatographic determination of deoxynivalenol in wheat and corn. J.
Assoc. Off. Anal. Chem., 67: 40-43.
TRUCKSESS, M.W., FLOOD, M.W., & PAGE, S.W. (1986a) Thin layer
chromatographic determination of deoxynivalenol in processed grain
products. J. Assoc. Off. Anal. Chem., 69: 35-36.
TRUCKSESS, M.W., WOOD, G.E., EPPLEY, R.M., YOUNG, K., COHEN, C.K.,
PAGE, S.W., & POHLAND, A.E. (1986b) Occurrence of deoxynivalenol in
grain products. In: Biodeterioration. VI, Aberystwyth, The Cambrian
News Ltd., pp. 243-247.
TRUCKSESS, M.W., FLOOD, M.T., MOSSOBA, M.M., & PAGE, S.W. (1987) High
performance thin-layer chromatographic determination of
deoxynivalenol, fusarenon-X and nivalenol in barley, corn and wheat.
J. agric. food Chem., 35: 445-448.
TRUSAL, L.R. (1985) Morphological changes in CHO and VERO cells
treated with T-2 mycotoxin. Correlation with inhibition of protein
synthesis. Cell Biochem. Funct. 3: 205-216.
TRUSAL, L.R. & O'BRIEN, J.C. (1986) Ultrastructural effects of T-2
mycotoxins on rat hepatocytes in vitro. Toxicon, 24: 481-488.
TRYPHONAS, H., O'GRADY, L., ARNOLD, D,L., MCGUIRE, F., KARPINSKY, K.,
& VESONDER, R.F. (1984) Effect of deoxynivalenol (vomitoxin) on the
humoral immunity of mice. Toxicol. Lett., 23: 17-24.
TRYPHONAS, H., IVERSON, F., SO, Y., NERA, E.A., MCGUIRE, P.F.,
O'GRADY, L., CLAYSON, D.B., & SCOTT, P.M. (1986) Effects of
deoxynivalenol (vomitoxin) on the humoral and cellular immunity of
mice. Toxicol. Lett., 30: 137-150.
TSENG, T., YUAN, G., TSENG, J., SHASO, E., & MIROCHA, C.J. (1983)
Natural occurrence of Fusarium mycotoxins in grains and feeds in
Taiwan. Proc. Int. Mycotoxins Symposium, Abstr. 1.5, Sydney,
Australia.
TSUNODA, H., TSURUTA, O., MATSUNAMI, S., & ISHII, S. (1957) [Studies
on the microorganisms which deteriorate the stored cereals and
grains. XIV.] Shokuryokenkyujo Hokoku, 12: 27-33 (in Japanese).
UENO, Y. (1970) Inhibition of protein synthesis in animal cells by
nivalenol and related metabolites: toxic principles of rice-M
infested with Fusarium nivale. In: Herzberg, M., ed. Proceedings of
the First US-Japan Conference on Toxic Microorganisms, Washington,
DC, US Department of the Interior, UNJR Joint Panel of Toxic
Microorganisms, pp.76-79.
UENO, Y. (1977) Trichothecenes: overview address. In: Rodricks,
J.V., Hesseltine, C.W., & Mehlman, M.A., ed. Mycotoxins in human and
animal health, Park Forest South, Illinois, Pathotox Publishers, pp.
189-228.
UENO, Y. (1980a) Toxicological evaluation of trichothecene
mycotoxins. In: Eaker, D. & Wadstrom, T., ed. Natural toxins.
Proceedings of the International Symposium on Animal and Plant
Microbial Toxins, Uppsala, 1979, Elmsford, New York, Pergamon Press,
Vol. 6, pp. 663-671.
UENO, Y. (1980b) Trichothecene mycotoxins: Mycology, chemistry, and
toxicology. In: Draper, H.H., ed. Advances in nutritional research,
New York, London, Plenum Press, Vol. 3, pp. 301-356.
UENO, Y., ed. (1983) General toxicology. In: Developments in food
science. IV. Trichothecenes, New York, Elsevier, pp. 135-146.
UENO, Y. & FUKUSHIMA, K. (1968) Inhibition of protein and DNA
synthesis in Ehrlich ascites tumor by nivalenol, a toxic principle of
Fusarium nivale growing rice-M. Experientia (Basel), 24(10): 1032-
1033.
UENO, Y. & KUBOTA, K. (1976) DAN-attacking ability of carcinogenic
mycotoxins in recombination-deficient mutant cells of Bacillus
subtilis. Cancer Res., 36: 445-451.
UENO, Y. & MATSUMOTO, H. (1975) Inactivation of some thiol enzymes
by trichothecene mycotoxins from Fusarium spp. Chem. pharm. Bull.,
23(10): 2439-2442.
UENO, Y. & SHIMADA, N. (1974) Reconfirmation of the specific nature
of reticulocytes bioassay system to trichothecene mycotoxins of
Fusarium spp. Chem. pharm. Bull., 22(11): 2744-2746.
UENO, Y. & YAMAKAWA, H. (1970) Antiprotozoal activity of scirpene
mycotoxins of Fusarium nivale FN 2B. Jpn. J. exp. Med., 40(5):
385-390.
UENO, Y., HOSOYA, M., MORITA, Y., UENO, I., & TATSUNO, T. (1968)
Inhibition of the protein synthesis in rabbit reticulocyte by
nivalenol, a toxic principle isolated from Fusarium nivale growing on
rice. J. Biochem. (Tokyo), 64(4): 479-485.
UENO, Y., HOSOYA, M., & ISHIKAWA, Y. (1969a) Inhibitory effects of
mycotoxins on protein synthesis in rabbit reticulocytes. J. Biochem.
(Tokyo), 66(3): 419-422.
UENO, Y., UENO, I., TATSUNO, T., OHOKUBO, K., & TSUNODA, H. (1969b)
Fusarenon-X, atopic principle of Fusarium nivale-culture filtrate.
Experientia (Basel), 25: 1062.
UENO, Y., ISHIKAWA, Y., AMAKAI, K., NAKAJIMA, M., SAITO, M.,
ENOMOTO, M., & OHTSUBO, K. (1970) Comparative study on skin-
necrotizing effect of scirpene metabolites of Fusaria. Jpn. J. exp.
Med., 40(1): 33-38.
UENO, Y., UENO, I., IITOI, Y., TSUNODA, H., ENOMOTO, M., & OHTSUBO,
K. (1971) Toxicological approaches to the metabolites of Fusaria.
Part 3. Acute toxicity of fusarenon-X. Jpn. J. exp. Med., 41(6): 512-
539.
UENO, Y., ISHII, K., SAKAI, K., KANAEDA, S., TSUNODA, H., TANAKA, T.,
& ENOMOTO, M. (1972) Toxicological approaches to the metabolites of
Fusarium. Part 4. Microbial survey on bean hulls poisoning of horses
with the isolation of toxic trichothecenes, neosolaniol and T-2 toxin
of Fusarium solani M-1-1. Jpn. J. exp. Med., 42(3): 187-203.
UENO, Y., ISHII, K., SATO, N., SHIMADA, N., TSUNODA, H., SAWANO, M.,
& ENOMOTO, M. (1973a) Screening of trichothecene producing fungi
and the comparative toxicity of isolated mycotoxins. Jpn. J.
Pharmacol., 23(Suppl.): 133.
UENO, Y., NAKAJIMA, M., SAKAI, K., ISHII, K., SATO, M., & SHIMADA, N.
(1973b) Comparative toxicology of trichothecene mycotoxins.
Inhibition of protein synthesis in animal cells. J. Biochem. (Tokyo),
74(2): 285-296.
UENO, Y., SATO, N., ISHII, K., SAKAI, K., TSUNODA, H., & ENOMOTO, M.
(1973c) Biological and chemical detection of trichothecene
mycotoxins of Fusarium spp. Appl. Microbiol., 25(4): 699-704.
UENO, Y., ISHII, K., SATO, N., & OHTSUBO, K. (1974) Toxicological
approaches to the metabolites of Fusaria. VI. Vomiting factor from
moldy corn infected with Fusarium spp. Jpn. J. exp. Med., 44(1):
123-127.
UENO, Y., KUBOTA, K., ITO, T., & NAKAMURA, Y. (1978a) Mutagenicity
and carcinogenic mycotoxins in Salmonella typhimurium. Cancer Res.,
38(3): 536-542.
UENO, Y., MATSUMOTO, H., ISHII, K., & KUKITA, K. (1978b) Inhibitory
effects of mycotoxins on Na+-dependent transport of glycine in rabbit
reticulocytes. Biochem. Pharmacol., 25(18): 2091-2095.
UENO, Y., TASHIRO, F., & KOBAYASHI, T. (1983) Species differences
in zearalenone-reductase activity. Food chem. Toxicol., 21(2):
167-173.
UENO, Y., LEE, U.S., TANAKA, T., HASAGAWA, A., & MATSUKI, Y. (1986)
Examination of Chinese and USSR cereals for the Fusarium mycotoxins
nivalenol, deoxynivalenol, and zearalenone. Toxicon, 24(6): 18-21.
UENO, Y., ABE, K., MORIMURA, S., SUGIURA, Y., & HORIE, Y. (in press)
Genotoxicity of mycotoxins evaluated by the SOS microplate assay.
Mutation Res.
UMEDA, M., YAMAMOTO, T., & SAITO, M. (1972) DNA-strand breakage of
HeLa cells by several mycotoxins. Jpn. J. exp. Med., 42: 527-535.
UMEDA, M., TSUTSUI, T., & SAITO, M. (1977) Mutagenicity and
inducibility of DNA single-strand breaks and chromosome aberrations
by various mycotoxins. Gann, 68(5): 619-625.
VAN RENSBURG, S.J. & ALTENKIRK, B. (1974) Claviceps purpurea:
ergotism. In: Purchase, I.F.H., ed. Mycotoxins, Amsterdam, New York,
Oxford, Elsevier Science Publishers, pp. 69-96.
VESELA, D., VESELY, D., JELINEK, R., & KUSAK, V. (1978) [Detection
of ochratoxin A in feed barley.] Vet. Med., 23: 431-436 (in Czech).
VESELA, D., VESELY, D., & JELINEK, R. (1983) Toxic effects of
ochratoxins A and citrinin, alone and in combination, on chicken
embryos. Appl. environ. Microbiol., 45: 91-93.
VESONDER, R.F. (1983) Natural occurrence of trichothecenes in North
America. Dev. food Sci., 4: 210-217.
VESONDER, R.F., CIEGLER, A., & JENSEN, A.H. (1973) Isolation of the
emetic principle from Fusarium-infected corn. Appl. Microbiol., 26:
1008-1010.
VESONDER, R.F., CIEGLER, A., & JENSEN, A.H. (1977) Production of
refusal factors by Fusarium strains on grains. Appl. environ.
Microbiol., 34(1): 105-106
VESONDER, R.F., CIEGLER, A., ROGERS, R.F., BURBRIDGE, K.A., BOTHAST,
R.J., & JENSEN, A.H. (1978) Survey of 1977 crop year preharvest
corn for vomitoxin. Appl. environ. Microbiol., 36(6): 885-888.
VESONDER, R.F., CIEGLER, A., BURMEISTER, H.R., & JENSEN, A.H. (1979)
Acceptance by swine and rats of corn amended with trichothecenes.
Appl. environ. Microbiol., 38(2): 344-346.
VISCONTI, A. & BOTTALICO, A. (1983a) Detection of Fusarium
trichothecenes (nivalenol, deoxynivalenol, fusarenone and
3-acetyldeoxynivalenol) by high-performance liquid chromatography.
Chromatographia, 17: 97-100.
VISCONTI, A. & BOTTALICO, A. (1983b) High levels of ochratoxin A
and B in mouldy bread responsible for mycotoxicosis in farm animals.
J. agric. food Chem., 31: 1122-1123.
VISCONTI, A. & MIROCHA, C.J. (1985) Identification of various T-2
toxin metabolites in chicken excreta and tissues. Appl. environ.
Microbiol., 49: 1246-1250.
VOYKSNER, R.D., HAGLER, W.M., TYCZKOWSKA, K., & HANEY, C.A. (1985)
Thermospray high-performance liquid chromatographic/mass
spectrometric analysis of some Fusarium mycotoxins. J. High Res.
Chrom. Chrom. Comm., 8: 119-125.
WARE, G.M., FRANCIS, O.J., CARMAN, A.S., & KUAN, S.S. (1986) Gas
chromatographic determination of deoxynivalenol in wheat with
electron capture detection: collaborative study. J. Assoc. Off. Anal.
Chem., 69: 899-901.
WEAVER, G.A., KURTZ, H.J., BATES, F.Y., CHI, M.S., MIROCHA, C.J.,
BEHRENS, J.C., & ROBISON, T.S. (1978a) Acute and chronic toxicity
of T-2 mycotoxin in swine. Vet. Rec., 103(24): 531-535.
WEAVER, G.A., KURTZ, H.J., MIROCHA, C.J., BATES, F.Y., & BEHRENS,
J.C. (1978b) Acute toxicity of the mycotoxin diacetoxyscirpenol in
swine. Can. vet. J., 19(10): 267-271.
WEHNER, F.C., THIEL, P.G., VAN RENSBURG, S.J., & DEMASIUS, I.P.C.
(1978a) Mutagenicity to Salmonella typhimurium of some Aspergillus
and Penicillium mycotoxins. Mutat. Res., 58: 193-203.
WEHNER, F.C., MARASAS, W.F.O., & THIEL, P.G. (1978b) Lack of
mutagenicity to Salmonella typhimurium of some Fusarium mycotoxins.
Appl. environ. Microbiol., 35(4): 659-662.
WEI, C.M. & MCLAUGHLIN, C.S. (1974) Structure function relationship
in the 12,13-epoxytrichothecenes novel inhibitors of protein
synthesis. Biochem. biophys. Res. Commun., 57(3): 838-844.
WEI, R.D., STRONG, F.M., SMALLEY, E.B., & SCHNOES, H.K. (1971)
Chemical interconversion of T-2 and HT-2 toxins and related
compounds. Biochem. biophys. Res. Commun., 45(2): 396-401.
WEI, R.D., SMALLEY, E.B., & STRONG, F.M. (1972) Improved skin test
of detection of T-2 toxin. Appl. Microbiol., 23(5): 1029-1030.
WEI, C.M., CAMPBELL, I.M., MCLAUGHLIN, C.S., & MAURICE, H. (1974)
Binding of trichodermin to mammalian ribosomes and its inhibition by
other 12,13-epoxytrichothecenes. Mol. cell Biochem., 3(3): 215-219.
WHO (1979) Environmental Health Criteria 11: Mycotoxins, Geneva,
World Health Organization, 127 pp.
WHO (1983) Prevention of liver cancer: Report of a WHO Meeting,
Geneva, World Health Organization, 30 pp. (Technical Report Series,
No. 691).
WILSON, D.J. (1984) Some effects of T-2 toxin on rabbit and bovine
musculature, Guelph, Ontario, Faculty of Graduate Studies of Guelph
(M. Sc. Thesis).
WILSON, D.J. & GENTRY, P.A. (1985) T-2 toxin can cause vasoconstriction
in an in vitro bovine ear perfusion system. Toxic. appl. Pharmacol.,
79: 159-165.
WOOD, G.E. & CARTER, L. (1989) Limited survey of deoxynivalenol in
wheat and corn in the United States. J. Assoc. Off. Anal. Chem., 72:
38-40.
WOODS, A.J., BRADLEY-JONES, J., & MANTLE, P.G. (1966) An outbreak of
gangrenous ergotism in cattle. Vet. Rec., 78: 742-749.
WYATT, R.D., HARRIS, J.R., HAMILTON, P.B., & BURMEISTER, H.R. (1972)
Possible outbreaks of furariotoxicosis in avians. Avian Dis., 16:
1123-1130.
WYATT, R.D., COLWELL, W.M., HAMILTON, P.B., & BURMEISTER, H.R.
(1973a) Neural disturbances in chickens caused by dietary T-2 toxin.
Appl. Microbiol., 26(5): 757-761.
WYATT, R.D., COLWELL, W.M., HAMILTON, P.B., & BURMEISTER, H.R.
(1973b) Neurotoxicity of T-2 toxin in broilers. Poult. Sci., 52(5):
2105.
WYATT, R.D., HAMILTON, P.B., & BURMEISTER, H.R. (1973c) Effects of
T-2 toxin in broiler chickens. Poult. Sci., 52(5): 1853-1859
WYATT, R.D., DOERR, J.A., HAMILTON, P.B., & BURMEISTER, H.R. (1975)
Egg production shell thickness and other physiological parameters of
laying hens affected by T-2 toxin. Appl. Microbiol., 29(5): 641-645.
XU, Y.C., ZANG, G.S., & CHU, F.S. (1988) Enzyme-linked immunosorbent
assay for deoxynivalenol in corn and wheat. J. Assoc. Off. Anal.
Chem., 71: 945-949.
YAGEN, B., SINTOV, A., & BIALER, M. (1986) New, sensitive thin-layer
chromatographic-high performance liquid chromatographic method for
detection of trichothecene mycotoxins. J. Chromatogr., 356: 195-201.
YAROM, R., MORE, R., ELDOR, A., & YAGEN, B. (1984a) The effect of T-2
toxin on human platelets. Toxicol. appl. Pharmacol., 73: 210-217.
YAROM, R., SHERMAN, Y., MORE, R., GINSBURG, I., BORINSKI, R., &
YAGEN, B. (1984b) T-2 toxin effect on bacterial infection and
leukocyte functions. Toxicol. appl. Pharmacol., 75: 60-68.
YLIMAEKI, A., KOPONEN, H., HINTIKKA, E.L., NUMMI, M., NIKU-PAAVOLA,
M.L., ILUS, T., & ENARI, T.M. (1979) Mycoflora and occurrence of
Fusarium toxins in Finnish grain, Espoo, Technical Research Center of
Finland, pp. 1-28, (Materials and Processing Technology, Publication
21).
YOSHIZAWA, T. & HOSOKAWA, H. (1983) Natural occurrence of
deoxynivalenol and nivalenol trichothecene mycotoxins in commercial
foods. J. Food Hyg. Soc. Jpn, 24: 413-415.
YOSHIZAWA, T. & MOROOKA, N. (1973) Deoxynivalenol and its
monoacetate new mycoatoxins from Fusarium roseum and mouldy barley.
Agric. biol. Chem., 37(12): 2933-2934.
YOSHIZAWA, T. & MOROOKA, N. (1977) Trichothecenes from mold-
infested cereals in Japan. In: Rodricks, J.V., Clifford, W.,
Hesseltine, C.W., & Myron, A.M., ed. Mycotoxins in human and animal
health, Park Forest South, Illinois, Pathotox Publishers, pp. 309-
321.
YOSHIZAWA, T. & SAKAMOTO, T. (1982) [ In vitro metabolism of T-2
toxin and its derivatives in animal livers.] Proc. Jpn. Assoc.
Mycotoxicol., 14: 26-28 (in Japanese).
YOSHIZAWA, T., SWANSON, S.P., & MIROCHA, C.J. (1980) T-2
metabolites in the excreta of broiler chickens administered 3H-
labeled T-2 toxin. Appl. environ. Microbiol., 39(6): 1172-1177.
YOSHIZAWA, T., MIROCHA, C.J., BEHRENS, J.C., & SWANSON, S.P. (1981)
Metabolic fate of T-2 toxin in a lactating cow. Food Cosmet.
Toxicol., 19(1): 31-39.
YOSHIZAWA, T., SAKAMOTO, T., AYANO, Y., & MIROCHA, C.J. (1982a)
[Chemical structures of new metabolites of T-2 toxin.] Proc. Jpn.
Assoc. Mycotoxiol., 15: 13-15 (in Japanese).
YOSHIZAWA, T., SAKAMOTO, T., AYANO, Y., & MIROCHA, C.J. (1982b)
3'-Hydroxy-T-2 and 3'-hydroxy HT-2 toxins: new metabolites of T-2 toxin,
a trichothecene mycotoxin, in animals. Agric. biol. Chem., 46(10):
2613-2615.
YOSHIZAWA, T., SAKAMOTO, T., & OKAMOTO, K. (1984) In vitro formation
of 3'-hydroxy T-2 and 3'-hydroxy HT-2 toxins form T-2 toxin by liver
homogenates from mice and monkeys. Appl. environ. Microbiol., 47:
130-134.
YOSHIZAWA, T., OKAMOTO, K., SAKAMOTO, T., & KUWAMURA, K. (1985a) In
vivo metabolism of T-2 toxin, a trichothecene mycotoxin. On the
formation of deepoxidation products. Proc. Jpn. Assoc. Mycotoxicol.,
21: 9-12.
YOSHIZAWA, T., SAKAMOTO, T., & KUWAMUAR, K. (1985b) Structures of
deepoxytrichthecene metabolites from 3'-hydroxy HT-2 and T-2 tetraol
in rats. Appl. environ. Microbiol., 50: 676-679.
YOSHIZAWA, T., COTE, L.M., SWANSON, S.P., & BUCK, W.B. (1986)
Confirmation of DOM-1, a deepoxydation metabolite of deoxynivalenol
in biological fluids of lactating cows. Agric. biol. Chem., 50:
227-229.
YOUNG, J.C. (1981a) Variability in the content and composition of
alkaloids found in Canadian ergot. I. Rye. J. environ. Sci. Health,
B16(4): 83-111.
YOUNG, J.C. (1981b) Variability in the content and composition of
alkaloids found in Canadian ergot. II. Wheat. J. environ. Sci.
Health, B16(1): 381-393.
YOUNG, J.C. & CHEN, Z. (1982) Variability in the content and
composition of alkaloids found in Canadian ergot. III. Triticale and
barley. J. environ. Sci. Health, B17(2): 93-107.
YOUNG, J.C. & MARQUARDT, R.R. (1982) Effects of ergotamine tartrate
in growing chicken. Can. J. anim. Sci., 62: 1181-1191
YOUNG, J.C., CHEN, Z., & MARQUARDT, R.R. (1983) Reduction in
alkaloid content of ergot sclerotia by chemical and physical
treatment. J. agric. food Chem., 31: 413.
YOUNG, J.C., FULCHER, R.G., HAYHOE, J.H., SCOTT, P.M., & DEXTER, J.E.
(1984) Effects of milling and baking on deoxynivalenol (Vomitoxin)
content of Eastern Canadian wheats. J. agric. food Chem., 32:
659-664.
YOUNG, J.C., SUBRYAN, L.M., POTTS, D., MCLAREN, M.E., & GOBRAN, F.H.
(1986) Reduction in levels of deoxynivalenol in contaminated wheat by
chemical and physical treatment. J. agric. food Chem., 34: 461-464.
ZAMIR, N., ZAMIR, D., EIDEN, L.E., PALKOVITS, M., BROWNSTEIN, M.J.,
ESHAY, R.L., WEBER, E., FRODEN, A.I., & FEUERSTEIN, G. (1985)
Metionine and leucine enkephalin in rat neurohypophysis: different
response to osmotic stimuli and T-2 toxin. Science, 228: 606-608.
ZIPRIN, R.L. & CORRIER, D.E. (1987) Listeriosis in diacetoxyscirpenol-
treated mice. Am. J. vet. Res., 48: 1516-1519.
ZORZ, M., CULIG, J., KOPITAR, Z., MILIVOJEVIC, D., MARUSIC, A., &
BANO, M. (1985) HPLC method for determination of ergot alkaloids
and some derivatives in human plasma. Hum. Toxicol., 4: 601-607.
RESUME ET RECOMMANDATIONS EN VUE DE RECHERCHES FUTURES
1. Ochratoxine A
1.1 Etat naturel
Les ochratoxines sont produites par plusieurs espèces de
champignons appartenant au genre Aspergillus et Penicillium. Il
s'agit d'espèces ubiquistes, aussi le risque de contamination des
denrées alimentaires destinées à l'homme et aux animaux est-il
omniprésent. Le principal composé, l'ochratoxine A se rencontre en
Australie ainsi que dans certains pays d'Europe et d'Amérique du
Nord. La production d'ochratoxine par les espèces du genre
Aspergillus semble limitée aux environnements très chauds et
humides alors que dans le cas de Penicillium, au moins certaines
espèces peuvent produire de l'ochratoxine à des températures
n'excédant pas 5 °C.
Ce sont les céréales qui sont le plus fréquemment contaminées
par l'ochratoxine A et à un moindre degré certaines fèves (café,
soja, cacao). L'ochratoxine B est très rare.
1.2 Méthodes d'analyse
On a mis au point des méthodes d'analyse permettant
l'identification et le dosage de l'ochratoxine à des concentrations
de l'ordre de 1 µg/kg.
1.3 Métabolisme
On trouve des résidus d'ochratoxine A intacte dans le sang, les
reins, le foie et les muscles de porcs à l'abattoir ainsi que dans
les muscles de poules et de poulets. En revanche on ne trouve
généralement pas de résidus chez les ruminants. In vitro,
l'ochratoxine A se fixe très solidement à l'albumine sérique
bovine, porcine et humaine. Des études expérimentales sur des
porcins et des poules ont montré que c'est au niveau des reins que
les concentrations d'ochratoxine A sont les plus élevées. Chez le
porc, le rat et l'homme, il pourrait y avoir détoxication par
hydroxylation au niveau des microsomes. L'expérience montre qu'on
peut encore identifier des résidus dans les reins de porc un mois
après la fin de l'exposition.
1.4 Effets sur les animaux
On a signalé des cas d'ochratoxicose chez des animaux d'élevage
(porcs et volaille) dans plusieurs pays d'Europe, la manifestation
essentielle étant une néphropathie chronique. Les lésions se
présentent sous la forme d'une atrophie tubulaire, d'une fibrose
interstitielle et à un stade plus avancé, d'une hyalinisation des
glomérules. Au Canada, on a également trouvé de l'ochratoxine A
dans du sang de porc recueilli à l'abattoir. Des effets
néphrotoxiques ont été relevés chez toutes les espèces d'animaux à
estomac simple étudiées jusqu'ici, même aux doses les plus faibles
qui aient été expérimentées (200 mg/kg de nourriture chez les rats
et les porcs).
Des effets tératogènes ont été observés chez des souris
exposées par voie orale à 3 mg d'ochratoxine A par kg de poids
corporel. On a observé une résorption des foetus chez des rats à
partir de 0,75 mg/kg de poids corporel, le produit étant administré
par voie orale. Ces effets tératogènes, qui chez le rat sont
accrus par un régime alimentaire pauvre en protéines, ont été
également observés chez des hamsters.
Les épreuves à court terme n'ont pas permis de mettre en
évidence d'activité mutagène (bactéries et levures). Administré
par voie orale à des rats, le produit a provoqué des ruptures
monocaténaires dans l'ADN des tissus du rein et du foie.
L'ochratoxine a provoqué l'apparition de cancers du rein chez des
souris mâles et des rats des deux sexes qui recevaient le produit
par voie orale. La formation de carcinomes hépatocellulaires n'a
été signalée que chez une souche de souris et pas du tout chez le
rat.
L'ochratoxine A est un inhibiteur de la synthèse protéique et
de la tRNA-synthétase chez les microorganismes et dans les cellules
hépatomateuses; elle inhibe également le mARN chez le rat.
L'ochratoxine A peut inhiber la migration des macrophages.
Chez la souris, une dose de 0,005 µg/kg de poids
corporel a aboli la réponse immunitaire aux érythrocytes de mouton;
toutefois d'autres résultats, contradictoires, ont été obtenus.
L'ochratoxine A s'est révélée cancérogène pour l'épithelium
tubulaire rénal chez des souris mâles et des rats des deux sexes.
1.5 Effets sur l'homme
L'exposition humaine, qui ressort de la présence d'ochratoxine
A dans les aliments, le sang et le lait humains, a été observée
dans divers pays d'Europe. Selon les données épidémiologiques
disponibles, la néphropathie des Balkans peut être attribuée à la
consommation de denrées alimentaires contaminées par cette toxine.
On a mis en évidence une relation tout à fait significative
entre la néphropathie des Balkans et les tumeurs des voies
urinaires, en particulier des tumeurs pyéliques et uretérales.
Toutefois on n'a pas publié de données qui établissent une
implication directe de l'ochratoxine A dans l'étiologie de ces
tumeurs.
2. Trichothécènes
2.1 Etat naturel
On connaît aujourd'hui 148 trichothécènes qui se caractérisent
sur le plan chimique par la présence d'une structure de base
commune, le système tétracyclique du scirpénol. Ces composés sont
principalement secrétés par des moisissures appartenant au genre
Fusarium, encore que d'autres genres notamment Trichoderma,
Trichothecium, Myrothecium et Stachybotrys produisent également des
métabolites considérés désormais comme des trichothécènes.
Quelques-uns seulement de ces trichothécènes contaminent les
denrées alimentaires destinées à l'homme ou aux animaux, en
particulier : le désoxyvalénol (DON), le nivalénol (NIV), le
disacétoxyscirpenol (DAS), ainsi que la toxine T-2 et, plus
rarement, certains dérivés (3-Ac-DON, 15-Ac-DON, fusarenone-X et
toxine HT-2). Parmi ces substances, c'est le DON qui est de loin
la plus fréquemment présente dans les denrées alimentaires
destinées à l'homme et aux animaux; à côté de quantités plus
faibles de NIV. Certains trichothécènes macrocycliques comme les
satratoxines G et H et les verrucarines, se rencontrent de temps à
autre dans les fourrages (paille, foin) mais il n'a pas été fait
état de leur présence dans les produits alimentaires.
Les enquêtes sur la présence de trichothécènes ont révélé que
le DON était présent dans le monde entier, essentiellement dans les
céréales telles que le froment et le maïs à des concentrations
pouvant parfois atteindre 92 mg/kg, encore que les teneurs moyennes
soient beaucoup plus faibles et varient selon la denrée. Des
rapports isolés font état de la présence de DON dans de l'orge, des
mélanges alimentaires pour aminaux, des pommes de terre, etc. Le
NIV, dont la présence n'est normalement pas signalée dans des
céréales au Canada ou aux Etats-Unis se rencontre en revanche
fréquemment au côté du DON dans les céréales originaires d'Asie ou
d'Europe; la concentration la plus forte enregistrée jusqu'ici est
de 37,9 mg/kg. On a signalé ça et là la présence de toxine T-2 et
de DAS à des concentrations beaucoup plus faibles.
Des études portant sur les divers traitements subis par les
produits alimentaires et notamment la mouture, montrent que, entre
la céréale brute et le produit définitif, il n'y a guère de
diminution des teneurs en DON. De même la panification ne parvient
pas à détruire le DON. En général, les aliments destinés à l'homme
que l'on trouve dans le commerce ne contiennent que rarement des
quantités décelables de DON et de NIV.
2.2 Methodes d'analyse
On dispose, pour le dosage des quatre toxines les plus
fréquemment rencontrées (DON, toxine T.2, DAS et NIV), des méthodes
d'analyse basées sur la chromatographie en couche mince, la
chromatographie en phase gazeuse ou la chromatographie liquide à
haute performance ainsi que sur des réactions immunologiques; les
limites de détection se situent en-dessous de 1 µg/g. Plusieurs de
ces méthodes ont fait l'objet d'une expérimentation collective. En
outre, certaines méthodes utilisées en recherche comme la
chromatographie gazeuse ou liquide associée à la spectrographie de
masse peuvent être utilisées pour confirmer l'identité des
substances.
2.3 Métabolisme
On a procédé à des études métaboliques, essentiellement de la
toxine T-2, mais dans quelques rares cas seulement du DON, chez
l'animal. Ces trichothécènes sont rapidement absorbés au niveau
des voies digestives mais l'on ne dispose pas de données
quantitatives. Les toxines se répartissent de façon uniforme sans
accumulation marquée au niveau d'un organe ou d'un tissu
particulier. Elles sont métabolisées en produits moins toxiques
par hydrolyse, hydroxylation, désépoxydation et glucuronidation.
La toxin T-2 et le DON sont rapidement éliminés par voie fécale et
urinaire.
Par exemple, une dose de toxine T-2 administrée par voie orale à
des bovins a été éliminée presque à hauteur de 100 % dans les heures
suivant l'administration; des poulets ont éliminé 80 % de la
substance dans les 48 heures suivant l'administration. Chez le rat,
25 % d'une dose de DON ont été éliminés dans les urines et 65 % dans
les matières fécales 96 heures après l'administration. Chez la poule
pondeuse et la vache laitière on a constaté que moins de 1 % de la
dose de toxine T-2 (et de ses métabolites) qui leur avait été
administrée se retrouvaient dans les oeufs et le lait. Après
administration par voie orale de toxine T-2 à des poulets, on a
constaté que les résidus présents dans la viande 24 heures plus tard
représentaient moins de 2 % de la dose initiale.
2.4 Effets sur les animaux
C'est principalement par l'ingestion de fourrage contaminé que
les animaux sont exposés aux trichothécènes. La toxine T-2 et le DAS
qui chez les animaux de laboratoire sont les plus actifs des
trichothécènes couramment cités comme contaminants des denrées
destinées aux animaux (toxine T-2, DAS, NIV et DON), provoquent des
réactions toxiques analogues. Le NIV est moins actif dans certains
systèmes que les deux précédents composés et le DON est le moins
toxique des quatre. (Cette activité toxique peut s'évaluer au moyen
de la DL50 pour la souris, par exemple dans le cas de la toxine T-2
elle est égale à 10,5 mg/kg de poids corporel et dans le cas du DON à
46,0 mg/kg).
Les trichothécènes les plus actifs, tels que la toxine T-2 et le
DAS produisent des effets généraux aigus lorsqu'ils sont administrés
expérimentalement à des rongeurs, des porcs et des bovins par voie
orale, parentérale ou respiratoire (porc, souris). Ces
trichothécènes produisent une épithélionécrose par contact (une dose
de 0,2 µg par touche dans le cas de la toxine T-2). Avec les autres
trichothécènes, il faut des doses plus élevées pour obtenir un effet
irritant (dans le cas du NIV, 10 ug par touche). Les trichothécènes
cytotoxiques comme la toxine T-2 ont une action nécrosante sur
l'épithélium des cryptes intestinales et sur les tissus lymphoïdes et
hematopoïétiques après exposition par voie orale, parentérale ou
respiratoire. Après exposition à la toxine T-2 et au DAS on observe
des anomalies hematologiques et des troubles de l'hémostase. Dans
les cas graves, la toxicose peut entraîner une pancytopénie. Des
études portant sur la toxine T-2, le DON et le DAS ont mis en
évidence la suppression de l'immunité à médiation cellulaire et de
l'immunité humorale et on a observé une réduction de la concentration
des immunoglobulines ainsi qu'une dépression de l'activité
phagocytaire des macrophages et des neutrophiles. Des études sur
animaux de laboratoire ont montré que l'effet immunodépresseur de
trichothécènes tels que la toxine T-2, le DAS et le DON entraînait
une moindre résistance aux infections secondaires par des bactéries
(Mycobactérie, Listeria monocytogenes) des levures ( Cryptococcus
neoformans) et des virus (virus de l'herpes simplex).
Il a été indiqué qu'après injection intrapéritonéale, la toxine
T-2 était tératogène pour la souris (cette voie d'administration
n'est pas courante dans les études de tératogénicité). Le DON est
tératogène pour la souris après intubation gastrique mais ne l'est
pas chez le rat lorsque la toxine est administrée dans la nourriture
de l'animal. Le NIV ne s'est pas révélé tératogène pour la souris.
La recherche du pouvoir mutagène de la toxine T-2, du DAS et du DON
par une épreuve du type Ames n'a pas donné de résultat positif. La
toxine T-2 présente dans certaines épreuves une faible activité
clastogène. D'après les études de toxicité à long terme qui ont été
publiées, rien n'indique que la toxine T-2, la fusarénone-X et le NIV
ne soient tumorigènes chez l'animal. Aucune étude de toxicité à long
terme n'a été publiée sur le DON.
Les trichothécènes sont toxiques pour les cellules à forte
activité mitotique telles que les cellules de l'épithélium des
cryptes intestinales et les cellules hematopoïétiques. Cette
cytotoxicité proviendrait, soit d'une perturbation de la synthèse des
protéines par une fixation des composés aux ribosomes des cellules
eucaryotes, soit d'une dysfonction des membranes cellulaires.
L'inhibition de la synthèse protéique serait due à l'induction de
protéines régulatrices labiles telles que l'IL-2 dans les
immunocytes. A concentrations extrêmement faibles, les
trichothécènes perturbent le transport des petites molécules à
travers les membranes cellulaires.
2.5 Effets sur l'homme
L'ingestion de produits alimentaires contaminés d'origine
végétale constitue la principale voie d'exposition aux tricho-
thécènes mais d'autres voies ont été signalées à l'occasion, par
exemple un contact cutané accidentel chez des chercheurs de
laboratoire ou l'inhalation de trichothécènes présents dans des
poussières aéroportées.
On n'a décrit que peu de cas de maladies attribuables à une
exposition aux trichothécènes, sans d'ailleurs que la responsa-
bilité de ces produits soit établie. Toutefois, dans les deux
flambées évoquées ci-dessous, il y a lieu de penser que leur
responsabilité est en cause.
Une des flambées, qui s'est produite en Chine, a été attribuée à
la consommation de blé moisi contenant 1,0 à 40,0 mg de DON par kg.
La maladie se caractérisait par des symptômes gastro-intestinaux.
Aucun décès n'a été à déplorer. Les porcs et les poulets qui avaient
mangé les restes de céréales ont également été affectés.
Une flambée du même genre a été signalée en Inde et attribuée à
la consommation de pain fabriqué à l'aide de blé contaminé. La
maladie se caractérisait par des symptômes gastro-intestinaux et une
irritation de la gorge qui apparaissaient 15 minutes à une heure
après l'ingestion du pain. On a décelé les mycotoxines suivantes
dans des échantillons de farine raffinée utilisée pour la préparation
du pain: DON (0,35-8,3 mg/kg), acétyldésoxynivalénol (0,64-2,49
mg/kg), NIV (0,03-0,1 mg/kg) et toxine T-2 (0,5-0,8 mg/kg).
Toutefois, l'identité de ces trichothécènes n'a pas été confirmée.
La présence de DON et de NIV à côté de la toxine T-2 est
inhabituelle.
Deux maladies d'intérêt historique, une aleucie alimentaire
d'origine toxique en URSS et une toxicose due à du blé moisi au
Japon et en Corée ont été attribuées à la consommation de céréales
contaminées par des moisissures du genre Fusarium. Depuis lors, on
a isolé en laboratoire, dans des cultures de Fusarium isolés des
céréales en cause, un certain nombre de trichothécènes. Il n'avait
pas été possible, au moment où ces maladies se sont déclarées,
d'effectuer des recherches pour tenter de corréler l'aleucie
toxique et la toxicose due au blé moisi à une exposition à des
trichothécènes car on ne connaissait pas les toxines en question.
3. Ergot
3.1 Etat naturel
Ergot est le nom que l'on donne aux sclérotes de certaines
espèces de champignons appartenant au genre Clavicepsa. Ces
sclérotes contiennent des alcaloïdes biologiquement actifs qui
peuvent être à l'origine de toxicoses chez les personnes ou les
animaux qui consomment des denrées contaminées.
Les alcaloïdes de l'ergot produisent deux types de maladies selon
leur nature, qui dépend du champignon en cause ( C. purpurea , C.
fusiformis ). L'ergotisme, dû à l'ergotamine et à l'ergocristine
produites par C. purpurea se caractérise principalement par une
gangrène des extrémités et des symptômes gastro-intestinaux. Quand à
l'intoxication provoquée par du millet contaminé par C. fusiformis,
elle se caractérise principalement par des symptômes digestifs et
elle est due aux clavines. Aucun des signes ou symptômes observés ne
sont révélateurs d'une oblitération vasculaire.
3.2 Méthodes d'analyse
Les alcaloïdes de l'ergot (ergolines) sont des dérivés de l'acide
lysergique. Ils ont une activité biologique variable selon leur
nature. Le dosage des alcaloïdes de C. purpurea a été effectué par
chromatographie liquide à haute performance avec détection par
fluorescence. On peut déterminer des concentrations de 0,2 µg
d'ergolines par litre de plasma humain. La détermination de
l'ergotamine et de l'ergocristine peut s'effectuer de façon très
spécifique par titrage radio-immunologique à des concentrations
respectives de 3,5 et 0,8 picomoles.
3.3 Effets sur les animaux
Des flambées d'avortements chez des bovins ont pu être attribuées
à l'ingestion d'ergoline, essentiellement de l'ergotamine et de
l'ergotaminine. Des moutons à qui l'on avait administré de
l'ergotamine par voie orale sont tombés rapidement malades et
présentaient une inflammation intestinale. Chez des volailles, des
porcs et des primates exposés par voie orale on a observé des effets
légers. Le groupe de travail ne disposait d'aucune donnée sur la
mutagénicité, la tératogénicité et la cancérogénicité des ergolines.
3.4 Effets sur l'homme
L'homme peut être exposé aux ergolines par la consommation de
céréales ergotées. Dans la plupart des études toxicologiques qui ont
été effectuées, on n'a pas procédé à l'identification précise des
alcaloïdes en cause. Les données qui ont été publiées au sujet d'une
seule enquête effectuée en Suisse sur des céréales et des produits
céréaliers indiquent que la consommation quotidienne totale
d'ergolines se situe à environ 1,5 ug par personne, certaines denrées
en contenant jusqu'à 140 µg/kg. La panification réduit de 25 à 100 %
la teneur en ergolines des farines contaminées.
En Ethiopie, une flambée d'ergotisme s'est produite en 1978 à la
suite de l'exposition à des ergolines provenant de sclérotes de ce C.
purpurea. Les céréales comptaient jusqu'à 0,75 % d'ergot; on a
relevé la présence d'ergométrine. Parmi les symptômes observés
figuraient une gangrène sèche ayant entraîné la perte d'un ou
plusieurs membres (29 % des cas), un pouls périphérique faible ou
absent (36 %) et une desquamation. Des symptômes digestifs n'ont été
observés que dans quelques cas. Chez 88 % des malades, les lésions
intéressaient les membres inférieurs.
En Inde, plusieurs flambées se sont déclarées depuis 1958 à la
suite de la consommation de millet contaminé par C. fusiformis. Ces
symptômes consistaient en nausées, vomissements et vertiges. Les
symptômes toxiques étaient dus à la présence d'ergolines à des
concentrations de 15 à 26 mg/kg. Aucune autopsie n'ayant été
effectuée, on ne dispose d'aucune information sur les effets
pathologiques au niveau des viscères.
4. Evaluation des risques pour la santé humaine
4.1 Ochratoxine A
L'exposition humaine, observée dans plusieurs pays d'Europe est
objectivée par la présence d'ochratoxine A dans les denrées
alimentaires et le sang. Le groupe de travail n'a pas connaissance
de tentatives qui ont effectuées dans d'autres régions du monde pour
déceler la présence d'ochratoxine A dans le sang humain.
En s'appuyant sur l'étude de la maladie-naturelle ou provoquée
par administration d'ochratoxine A-on a pu établir le rôle
étiologique de l'ochratoxine A dans la néphropathie porcine. A
partir de ce modèle, on a pu émettre l'hypothèse que la néphropathie
endémique des Balkans était due à une exposition à cette toxine. Les
données immunologiques disponibles montrent que cette affection
serait attribuable à la consommation de denrées alimentaires
contaminées par de l'ochratoxine A. Depuis la publication en 1980 du
No 11 des Critères d'hygiène de l'environnement, des études
épidémiologiques sur la concentration de l'ochratoxine A dans le sang
humain dans les régions touchées et non touchées ont conforté
l'hypothèse d'une relation entre la néphropathie balkanique et
l'exposition à l'ochratoxine A.
Ainsi, on a montré que les habitants des régions d'endémie
présentaient plus fréquemment de l'ochratoxine A dans leur sang et à
des concentrations plus élevées. Cependant, ces études
rétrospectives ne fournissent que des présomptions sur lesquelles on
ne peut établir l'existence d'une relation causale directe. On ne
peut cependant pas l'exclure du fait de la longue période de latence
entre l'exposition et l'apparition des symptômes.
On a montré que l'ochratoxine A exerçait, chez les souris mâles
et les rats des deux sexes, des effets cancérogènes sur l'épithélium
des tubules rénaux. Il existe une relation tout à fait significative
entre la néphropathie balkanique et la présence de tumeurs des voies
urinaires, notamment de tumeurs pyéliques et uretérales. Toutefois
aucune donnée n'a été publiée qui établissent la responsabilité
directe de l'ochratoxine A dans l'étiologie de ces tumeurs.
4.2 Trichothécènes
Sur la base des données dont disposait le groupe de travail, il
est possible d'établir une relation entre l'exposition aux
trichothécènes et certains épisodes toxiques chez l'homme. Si l'on
se réfère aux quelques données disponibles, ce sont le DON et le NIV
qui sont les plus fréquemment cités dans les différents cas
d'exposition humaine. Dans le cas des flambées d'aleucie toxique
alimentaire et de toxicose par ingestion de céréales moisies, on ne
peut exclure la responsabilité des trichothécènes. L'exposition se
produit par ingestion de denrées contaminées, principalement des
céréales. Les différents traitements qu'elles subissent, notamment
la mouture et la panification ne permettent pas d'éliminer le DON, le
NIV, ni la toxine T-2. L'existence d'une exposition par voie
respiratoire est très peu documentée mais c'est une possibilité qu'on
ne peut totalement écarter.
Parmi les tricothécènes qui se trouvent à l'état naturel dans les
aliments, c'est la toxine T-2 qui est la plus active, suivie du DAS
and du NIV; les études de toxicité aiguë ont montré que le DON était
la substance la moins toxique. Chez l'animal d'expérience, la toxine
T-2 et le DAS déterminent des symptômes généraux aigus, avec nécrose
des tissus épithéliaux et suppression de l'hematopoïèse. Lors des
récentes flambées d'intoxications, il n'a été fait état que des
symptômes digestifs.
Le DON est tératogène pour la souris mais pas pour le rat. Selon
les études de toxicité chroniques qui ont été publiées, le NIV et la
toxine T-2 ne sont pas tumorigènes chez l'animal. Aucune étude de
cancérogénicité à long terme n'a été publiée sur le DON. Certains
trichothécènes, tels que la toxine T-2 et le DON ont une action
immunosuppressive chez l'animal et produisent des modifications de
l'immunité à médiation cellulaire et de l'immunité humorale. En
revanche, rien n'indique l'existence de tels effets chez l'homme.
On est mal informé sur les cas d'intoxication humaine due à une
exposition aux trichothécènes, cas qui sont en nombre limité. Les
symptômes observés consistent en troubles digestifs et irritation de
la gorge; ils apparaissent peu après l'ingestion de denrées
alimentaires contaminées par des trichothécènes. A l'heure actuelle,
on ne connaît pas de cas de cancer humain attribuable aux
trichothécènes. Le groupe de travail ne disposait d'aucune
publication faisant état d'infections secondaires d'origine
bactérienne, fongique ou virale chez l'homme, à la suite d'une
exposition aux trichothécènes comme on en a observé chez l'animal
d'expérience. Il semble que l'on n'ait pas effectué d'études
appropriées sur ce point.
4.3 Ergot
Il semble que l'exposition humaine à de faibles concentrations
d'ergolines soit très répandue. Les données relatives aux flambées
qui se sont récemment déclarées en Ethiopie et en Inde montrent que
les alcaloïdes de C. purpurea (groupe de l'ergotamine) produisent
les effets les plus graves, notamment une gangrène des membres
inférieurs pouvant entraîner la mort, effets qui sont plus graves
que ceux des alcaloïdes de C. fusiformis (groupe de la clavine) qui
entraînent des symptômes digestifs sans issue fatale. On ignore si
ces différences s'expliquent par la teneur des différentes espèces
de champignons en alcaloïdes, par les propriétés toxicologiques de
ces substances ou par des différences dans les quantités ingérées
par les diverses populations.
Après nettoyage et mouture qui éliminent les sclérotes, il ne
subsiste dans les produits alimentaires que de faibles quantités
d'ergolines. La panification ou d'autres traitements thermiques
détruisent également la plupart des alcaloïdes du groupe de
l'ergotamine.
5. Recommandations en vue de recherches futures
5.1 Recommandations generales
Il conviendrait de créer un réseau de centres de référence pour
aider les Etats Membres à confirmer l'identité des mycotoxines
présentes dans les tissus humains et les denrées destinées à la
consommation humaine. Ces centres devraient également fournir sur
demande des échantillons de référence de mycotoxines afin de
faciliter la comparabilité des résultats d'analyse obtenus dans les
différentes régions du monde.
5.2 Ochratoxine A
a) Etudes épidémiologiques de grande ampleur à caractère
rétrospectif et études localisées à caractère prospectif sur
l'association entre l'ochratoxine A et la néphropathie
endémique des Balkans ainsi que les tumeurs des voies
urinaires : ces études devraient être menées dans la
péninsule de Balkans et la Région méditerranéenne.
b) Recherche et dosage de l'ochratoxine A dans le sang de
malades porteurs de tumeurs des voies urinaires, à
l'extérieur de la péninsule des Balkans.
c) Recherche de sources d'exposition à l'ochratoxine A, par
analyse du sang, dans les pays situés en dehors de la
péninsule des Balkans.
d) Elucidation du mécanisme à l'origine des différences entre
les sexes qui ont été relevées dans les anomalies rénales,
néoplasiques et non-néoplasiques produites par l'ochratoxine
A chez l'animal d'expérience.
e) Etudes de grande envergure sur la teneur en ochratoxine A des
denrées alimentaires dans les différentes régions du monde.
Ce type d'enquêtes est particulièrement important dans les
régions où l'on observe une forte incidence de tumeurs des
voies urinaires, notamment rénales, ou de néphropathies.
5.3 Trichothécènes
a) Il conviendrait d'effectuer des études longitudinales dans
les régions de l'Inde et de la République populaire de Chine
où ont été récemment observés des épisodes d'intoxication par
les trichothécènes. Il conviendrait de mieux éclaircir la
présence inhabituelle de certains trichothécènes dans ces
régions.
b) Il faudrait étudier les effets d'une exposition prolongée
d'animaux de laboratoire au DON, et notamment les effets
cancérogènes. Comme la réaction au DON varie beaucoup selon
les espèces, l'espèce qui sera retenue devra être choisie
avec soin.
c) Il faudrait étudier plus à fond les infections microbiennes
secondaires à une exposition aux trichothécènes chez l'animal
d'expérience.
d) Il convient d'étudier l'influence des conditions
environnementales, notamment la présence d'insecticides ou
autres produits chimiques industriels, sur la production de
trichothécènes par des champignons.
e) Il faudrait élucider l'effet des différents modes de
préparation des denrées alimentaires sur les trichothécènes.
f) Il conviendrait de mettre au point des espèces végétales qui
résistent aux champignons producteurs de trichothécènes, en
recourant aux biotechnologies.
g) Il faudrait étudier sur l'animal les effets synergistiques
éventuels d'une exposition simultanée aux trichothécènes, aux
aflatoxines, à l'ochratoxine A et à d'autres mycotoxines.
h) Il faudrait également déterminer l'apport de trichothécènes
d'origine alimentaire chez l'homme.
i) Il faudrait mettre au point des méthodes de criblage des
trichothécènes qui soient à la fois rapides et sensibles, et
mener des enquêtes dans les régions tempérées du monde pour
rechercher la présence de trichothécènes dans les céréales et
les préparations alimentaires.
5.4 Ergot
a) Il faudrait mettre au point des méthodes pour l'analyse des
agroclavines.
b) Il conviendrait de mettre à la disposition des pays en
développement des renseignements sur le repérage des semences
contaminées et sur les méthodes de mouture permettant de
réduire au minimum les problèmes posés par la présence de
l'ergot.
c) Il faudrait effectuer des études épidémiologiques sur les
effets éventuels que peut entraîner chez l'homme l'ingestion
de faible quantités d'ergolines.
d) Il faudrait effectuer des études pharmacologiques et
toxicologiques sur les ergolines, seules ou en association,
chez l'animal d'expérience.
e) Il conviendrait de déterminer si les ergolines peuvent se
transmettre de la mère à l'enfant par le lait maternel.
RESUMEN Y RECOMENDACIONES PARA ULTERIOR INVESTIGACION
1. Ocratoxina A
1.1 Distribución natural
Las ocratoxinas son producidas por varias especies de los
géneros de hongos Aspergillus y Penicillium. Esos hongos son
ubicuos y hay amplias posibilidades de contaminación de alimentos
para seres humanos y para animales. La ocratoxina A, la más
importante, se ha hallado en toda una serie de países de América
del Norte, Australia y Europa. La formación de ocratoxina por las
especies del género Aspergillus parece estar limitada a condiciones
de humedad y temperatura elevadas, mientras que algunas especies,
por lo menos, de Penicillium pueden producir ocratoxina a
temperaturas de sólo 5 °C.
Las mayores incidencias de contaminación con ocratoxina A se
han hallado en cereales y, en menor medida, en algunos granos
(café, soja, cacao). La ocratoxina B es muy poco frecuente.
1.2 Métodos de análisis
Se han desarrollado técnicas de análisis para identificar la
ocratoxina y determinar sus concentraciones en la gama de g/kg.
1.3 Metabolismo
Se han hallado residuos de ocratoxina A no modificada en la
sangre, los riñones, el hígado y los músculos de cerdos en los
mataderos y en los músculos de gallinas y pollos. Sin embargo, por
lo general no se han hallado residuos de ocratoxina A en los
rumiantes. El enlace in vitro de la ocratoxina A con la
seroalbúmina es especialmente importante en el ganado vacuno, el
ganado ovino y el ser humano. Estudios experimentales realizados
con cerdos y gallinas han demostrado que las concentraciones más
altas de ocratoxina A se hallan en los riñones. La hidroxilación
microsómica puede representar una reacción de detoxificación en los
cerdos, las ratas y el ser humano. En estudios experimentales, un
mes después de terminada la exposición se localizaron aún residuos
en riñones de cerdos.
1.4 Efectos en animales
Varios países europeos han notificado en animales de granja
(cerdos, aves de corral) casos de ocratoxicosis, cuya principal
manifestación era una nefropatía crónica. Entre las lesiones había
atrofia tubular, fibrosis intersticial y, en etapas ulteriores,
hialinización de los glomérulos. También se ha hallado ocratoxina
A en sangre de cerdo recogida en mataderos canadienses. La
ocratoxina A ha producido efectos nefrotóxicos en todas las
especies de animales provistas de un solo estómago que se han
estudiado hasta el momento, incluso con las dosis más bajas con que
se experimentó (200 µg/kg de alimentos en ratas y cerdos).
Se observaron efectos teratogénicos en ratones expuestos a
dosis de 3 mg/kg de peso corporal administradas por vía oral. En
ratas que recibieron por vía oral dosis de 0,75 mg/kg de peso
corporal se observó resorción fetal. Los efectos teratogénicos,
que en las ratas se acentuaron con una dieta baja en proteínas, se
han observado también en hámsters.
No hay datos que demuestren la actividad de la ocratoxina A en
pruebas a corto plazo de la mutagenicidad (bacterias y levaduras).
Ratas expuestas a ocratoxina A administrada por vía oral mostraron
roturas de una sola cadena del ADN en tejidos renales y hepáticos.
Ocratoxina A administrada por vía oral provocó neoplasias de
células renales en ratones machos y en ratas de uno y otro sexo.
Se registraron neoplasias de células hepáticas en sólo una estirpe
de ratones y no en la rata.
La ocratoxina A es inhibidora de la síntesis de proteínas y de
la sintetasa del ARNt en microorganismos, células de hepatoma y el
ARNm renal en la rata.
La ocratoxina A puede inhibir la migración de los macrófagos.
En ratones, una dosis de 0,005 µg/kg de peso corporal suprimió la
respuesta inmunitaria a eritrocitos de oveja; sin embargo, se han
obtenido también resultados contradictorios.
Se ha demostrado que la ocratoxina A es carcinógena para el
epitelio de los túbulos renales en ratones machos y de ratas de uno
y otro sexo.
1.5 Efectos en el ser humano
En varios países de Europa se ha observado exposición humana a
ocratoxina A, como lo demuestra la presencia de ésta en alimentos y
en la sangre y la leche humanas. Los datos epidemiológicos de que
se dispone indican que la nefropatía de los Balcanes podría estar
relacionada con el consumo de productos alimenticios contaminados
por esta toxina.
Se ha observado una relación muy significativa entre la
nefropatía de los Balcanes y tumores del tracto urinario, en
particular los de la pelvis renal y los uréteres. Sin embargo, no
se han publicado datos que demuestren el papel causal de la
ocratoxina A en la etiología de esos tumores.
2. Tricotecenos
2.1 Distribución natural
Hasta ahora se conocen 148 tricotecenos, caracterizados
químicamente por la presencia del mismo sistema básico de un anillo
tetracíclico de scirpenol. Son compuestos producidos
principalmente por hongos pertenecientes al género Fusarium,
aunque otros géneros, entre ellos Trichoderma, Trichothecium,
Myrothecium y Stachybotrys, producen también metabolitos
ahora reconocidos como tricotecenos. Se ha visto que sólo un
pequeño número de los tricotecenos conocidos contaminan los
alimentos para seres humanos o para animales, entre ellos el
deoxinivalenol (DON), el nivalenol (NIV), el diacetoxiscirpenol
(DAS) y la toxina T-2 y, con menos frecuencia, ciertos derivados
(3-Ac-DON,15-Ac-DON, fusarenon-X y toxina HT-2). De todos ellos,
el que se encuentra más a menudo en alimentos para seres humanos y
para animales es el DON, por lo general con cantidades más pequeñas
de NIV como co-contaminante. Algunos tricotecenos macrocíclicos
como las satratoxinas G y H y las verrucarinas se observan
ocasionalmente en alimentos para animales (paja, heno) pero no se
ha notificado su presencia en alimentos para seres humanos.
Estudios sobre la distribución de los tricotecenos han indicado
que el DON se encuentra en todo el mundo, sobre todo en cereales
como el trigo y el maíz, en concentraciones que pueden llegar hasta
92 mg/kg, aunque las concentraciones medias son considerablemente
inferiores y varían según el producto. Hay notificaciones aisladas
de la presencia de DON en la cebada, los piensos compuestos, las
patatas, etc. Habitualmente los cereales producidos en Canadá y
los Estados Unidos de América no contienen NIV pero éste se ha
encontrado en los cereales asiáticos y europeos, junto con DON; la
mayor concentración de NIV registrada hasta la fecha fue de 37,9
mg/kg. La presencia de toxina T-2 y DAS se ha notificado con escasa
frecuencia y en concentraciones muy inferiores.
Estudios sobre la elaboración y la molienda indican que las
concentraciones de DON apenas se reducen del cereal al producto
acabado. Tampoco se destruye cuando se cuece el cereal. En
general, los productos alimenticios para seres humanos que se
encuentran en el comercio rara vez contienen concentraciones
detectables de DON y NIV.
2.2 Métodos de análisis
Existen xistenmétodos de análisis basados en la cromatografía
de capa fina, la cromatografía de fase gaseosa, la cromatografía de
fase líquida de alto rendimiento y técnicas inmunológicas para la
determinación de las cuatro toxinas más frecuentes (DON, toxina T-
2, DAS y NIV) con límites de detección inferiores a 1 µg/g.
Algunos de esos métodos se han ensayado en colaboración. Además,
la identidad puede confirmarse mediante métodos de investigación
como cromatografía de fase gaseosa/espectografía de masas y
cromatografía de fase líquida/espectografía de masas.
2.3 Metabolismo
Se han realizado estudios metabólicos con animales, sobre todo
administrando toxina T-2 y en unos pocos casos DON. Estos
tricotecenos se absorben con rapidez por el tubo digestivo,
aunque no se dispone de datos cuantitativos. Las toxinas se
distribuyen bastante uniformemente, sin marcada acumulación en
ningún órgano o tejido determinado. Los tricotecenos se
transforman en metabolitos menos tóxicos por reacciones como
hidrólisis, hidroxilación, de-epoxidación y glucoronidación.
Tricotecenos como la toxina T-2 y el DON se eliminan rápidamente en
las heces y la orina. Por ejemplo, casi el 100% de una dosis de
toxina T-2 administrada a ganado por vía oral se eliminaba en unas
horas; en pollos, alrededor del 80% se había eliminado a las 48
horas. En la rata, 96 horas después de la administración, el 25%
del DON se había eliminado en la orina y el 65% en las heces. Los
resultados de la transmisión de toxina T-2 en gallinas ponedoras y
vacas lactantes indicaron que pasaba a los huevos y a la leche
menos del 1% de la dosis administrada de esa toxina y de sus
metabolitos. En la carne de pollo, 24 horas después de la
administración de toxina T-2 por vía oral, los residuos de ésta y
de sus metabolitos eran inferiores al 2% de la dosis.
2.4 Efectos en animales
La principal vía de exposición de los animales a tricotecenos
es la ingestión de alimentos de origen vegetal. La toxina T-2 y el
DAS, los más potentes en los experimentos de laboratorio con
animales, de todos los tricotecenos que habitualmente se consideran
contaminantes de alimentos para animales (toxina T-2, DAS, NIV y
DON), provocan una respuesta tóxica similar. El NIV es menos
potente en algunos sistemas que los dos compuestos anteriores y el
DON es el menos tóxico de los cuatro (un ejemplo de la potencia es
la DL50 por vía oral en el ratón: toxina T-2, 10,5 mg/kg de peso
corporal y DON, 46,0 mg/kg).
Los tricotecenos más potentes, como la toxina T-2 y el DAS,
producen efectos generales agudos cuando se administran
experimentalmente a roedores y ganado porcino y bovino por vía oral
o parenteral o por inhalación (cerdo, ratón). Una lesión producida
por el contacto con tricotecenos potentes como la toxina T-2 y el
DAS es la epitelionecrosis (dosis de 0,2 µg por zona en el caso de
la toxina T-2). En cuanto a otros tricotecenos, son necesarias
dosis mayores para producir un efecto irritante (NIV, 10 µg por
zona). Los tricotecenos citotóxicos, como la toxina T-2, producen
necrosis del epitelio de las criptas intestinales y de los tejidos
linfoides y hematopoyéticos, tras exposición oral o parenteral o
por inhalación. La exposición a tricotecenos citotóxicos como la
toxina T-2 y el DAS va seguida de alteraciones hematológicas y
coagulopáticas. Las toxicosis graves pueden originar
pancitopenia. En estudios con toxina T-2, DON y DAS se ha
demostrado la supresión de la inmunidad de base celular y humoral,
y entre los efectos observados figuran la reducción de las
concentraciones de inmunoglobulinas y la disminución de la
actividad fagocítaria de macrófagos y neutrófilos. Los resultados
de estudios experimentales con animales han indicado que el efecto
inmunodepresor de tricotecenos como la toxina T-2, el DAS y el DON
origina una disminución de la resistencia a la infección secundaria
por bacterias (micobacterias, Listeria monocytogenes), levaduras
(Cryptococcus neoformans) y virus (virus del herpes simplex).
Se ha comunicado que la toxina T-2 es teratogénica en el ratón,
cuando se administra por inyección intraperitoneal (vía de
administración poco corriente en los estudios de la
teratogenicidad). Se ha comunicado que el DON es teratogénico en
los ratones tras intubación gástrica, pero no en las ratas cuando
la toxina se administra con los alimentos. El NIV no es
teratogénico en el ratón. La toxina T-2, el DAS y el DON no
resultaron mutagénicos en una prueba de tipo Ames. En algunas
pruebas, se observó una débil actividad clastogénica de la toxina
T-2. En los estudios publicados sobre la toxicidad a largo plazo
en animales, no se obtuvieron datos que indiquen que la toxina T-2,
el fusarenón-X o el NIV sean oncogénicos en los animales. No se
han publicado estudios a largo plazo sobre la toxicidad del DON.
Los tricotecenos son tóxicos para las células que se dividen
activamente como las del epitelio de las criptas intestinales y las
hematopoyéticas. La citotoxicidad se ha asociado con el trastorno
de la síntesis de proteínas debido al enlace de los compuestos con
los ribosomas de las células eucarióticas o bien con la disfunción
de las membranas celulares. La inhibición de la síntesis de las
proteínas se ha relacionado con la inducción de proteínas lábiles y
reguladoras como la IL-2 en los inmunocitos. Concentraciones
extremadamente bajas de tricotecenos alteran el transporte de
pequeñas moléculas en las membranas celulares.
2.5 Efectos en el ser humano
La ingestión de alimentos contaminados de origen vegetal es la
principal vía de exposición a tricotecenos, pero ocasionalmente se
han notificado otras, por ejemplo el contacto accidental con la
piel entre los investigadores de laboratorio y la inhalación de
tricotecenos transportados por el polvo.
Los casos registrados de enfermedad asociados con la exposición
a tricotecenos son escasos y en ninguno de ellos se ha demostrado
la relación causal. No obstante, los dos brotes que se describen a
continuación parecen indicar su existencia.
En China se registró un brote de enfermedad asociado con el
consumo de trigo mohoso que contenía de 1,0 a 40,0 mg de DON/kg.
La enfermedad se caracterizó por síntomas gastro-intestinales. No
hubo defunciones. Los cerdos y los pollos alimentados con restos
de cereales también resultaron afectados.
Un brote análogo se registró en la India, asociado al consumo
de pan hecho con trigo contaminado. La enfermedad se caracterizó
por síntomas gastrointestinales e irritación de la garganta, que
aparecía de 15 minutos a una hora después de la ingestión del pan.
En muestras de la harina de trigo refinada utilizada para su
preparación se detectaron las siguientes micotoxinas: DON
(O,35-8,3 mg/kg), acetildeoxinivalenol (0,64-2,49 mg/kg),
NIV (0,03-0,1 mg/kg) y toxina T-2 (0,5-0,8 mg/kg). Sin
embargo, no hubo confirmación de la identidad de los tricotecenos
detectados. La presencia simultánea de DON y NIV y de toxina T-2
es insólita.
Dos enfermedades de interés histórico, la aleucia tóxica
alimentaria (ATA) de la URSS y la toxicosis del trigo mohoso del
Japón y Corea se han asociado con el consumo de cereales invadidos
por hongos Fusarium. Desde entonces se han identificado en
condiciones de laboratorio algunos tricotecenos en cultivos de
mohos Fusarium aislados en cereales encontrados en los incidentes.
En la época en que apareció la enfermedad, no pudieron realizarse
estudios que relacionaran la ATA o la toxicosis de los cereales
mohosos con la exposición a tricotecenos porque las toxinas no eran
conocidas.
3. Cornezuelo
3.1 Distribución natural
Cornezuelo es el nombre dado a los esclerocios de especies de
hongos pertenecientes al género Claviceps. Alcaloides biológi-
camente activos contenidos en el esclerocio provocan toxicosis
cuando éste es consumido por hombres o animales en alimentos
contaminados.
Los alcaloides del cornezuelo producen dos modalidades
diferentes de enfermedad, según el hongo de que se trate ( C.
purpurea, C. fusiformis) y, por lo tanto, los alcaloides
producidos. El ergotismo, provocado por los alcaloides ergotamina-
ergocristina producidos por C. purpurea, se caracteriza sobre
todo por gangrena de las extremidades, además de síntomas
gastrointestinales. La intoxicación resultante de la ingestión de
mijo contaminado por C. fusiformis se caracteriza principalmente
por síntomas gastrointestinales y está relacionada con los
alcaloides de tipo clavina. No hay signos ni síntomas que indiquen
la presencia de vaso-oclusión.
3.2 Métodos de análasis
Los alcaloides del cornezuelo (ergolinas) son derivados del
ácido lisérgico. Los diversos alcaloides difieren por la
importancia de su actividad biológica. La presencia de alcaloides
del cornezuelo producidos por C. purpurea se ha determinado por
cromatografía de fase líquida de alto rendimiento con detección por
fluorescencia. Pueden medirse concentraciones de 0,2 µg de
ergolina por litro de plasma humano. La presencia de ergotamina y
ergocristina puede determinarse con gran especificidad mediante
pruebas de radioinmunovaloración en concentraciones de 3,5
picomoles y 0,8 picomoles, respectivamente.
3.3 Efectos en animales
Las ergolinas, principalmente la ergotamina y la ergotaminina,
se han asociado con brotes de abortos en el ganado bovino. Ovejas
a las que se administró ergotamina por vía oral enfermaron
rápidamente y se observó en ellas inflamación intestinal. Aves de
corral, cerdos y primates expuestos por vía oral experimentaron
efectos leves. El Grupo Especial no dispuso de datos sobre la
mutagenicidad, la teratogenicidad y la carcinogenicidad de las
ergolinas.
3.4 Efectos en el ser humano
Los cereales infectados por Claviceps son fuente de exposición
humana a las ergolinas. En la mayor parte de los estudios
toxicológicos, no se han identificado los alcaloides específicos.
La información resultante de un solo estudio sobre cereales y
productos de cereales indica que en Suiza la ingesta total
de ergolinas por los seres humanos es de alrededor de 5,1 µg por
persona, siendo el contenido de ciertos productos de hasta 140
µg/kg. La cocción reduce las ergolinas presentes en la harina
contaminada un 25-100%.
En 1978 hubo en Etiopía un brote de ergotismo, resultante de
exposición a ergolinas procedentes de esclerocios de C.
purpurea. El cereal contenía hasta un 0,75% de cornezuelo; se
detectó específicamente ergometrina. Los síntomas fueron gangrena
seca, con pérdida de uno o varios miembros (29% de los casos),
pulsos periféricos débiles o ausentes (36%) y desescamación de la
piel. Sólo hubo síntomas gastrointestinales en unos pocos casos.
Trastornos de las extremidades inferiores se registraron en el 88%
de los pacientes.
En la India ha habido varios brotes desde 1958 a consecuencia
de la ingestión de mijo perlado que contenía cornezuelo de tipo
clavina producido por C. fusiformis. Los síntomas comprendían
náuseas, vómitos y mareos, y fueron causados por la ingestión de
mijo perlado con un contenido de 15 a 26 mg de ergolina/kg.
Como en ninguno de los dos episodios se practicaron autopsias,
no se dispone de información sobre los efectos anatomopatológicos
en las vísceras humanas.
4. Evaluación de los riesgos para la salud humana
4.1 Ocratoxina A
En diversos países de Europa se ha observado exposición humana
a la ocratoxina A, como lo demuestra la presencia de ésta en
alimentos y en la sangre. El Grupo Especial no conoce ningún
intento de detectar la ocratoxina A en la sangre humana en otras
partes del mundo.
El papel causal de la ocratoxina A en la nefropatía porcina se
ha demostrado sobre la base de estudios de casos sobre el terreno y
de la reproducción de la enfermedad con ocratoxina A. Utilizando
el modelo porcino, se ha supuesto que la nefropatía endémica de los
Balcanes podría obedecer a exposición a la ocratoxina A. Los datos
epidemiológicos de que se dispone indican que esa enfermedad podría
estar asociada al consumo de alimentos contaminados por dicha
toxina. Desde la publicación de Criterios de salud ambiental 11 en
1979, estudios epidemiológicos sobre concentración de ocratoxina A
en la sangre humana en zonas afectadas y no afectadas han
proporcionado un argumento más en favor de la relación entre la
nefropatía de los Balcanes y la exposición a la ocratoxina A.
Se ha demostrado que tanto la presencia de ocratoxina A en la
sangre como sus concentraciones son mayores en las personas que
residen en las zonas de endemicidad. No obstante, las pruebas
indirectas que proporcionan los mencionados estudios retrospectivos
no bastan para demostrar por sí solas la existencia de una relación
causal directa. Esta tampoco puede excluirse, dado el largo
periodo de latencia entre la exposición y el comienzo de los
síntomas.
Se ha demostrado que la ocratoxina A es carcinogénica para el
epitelio de los túbulos renales en los ratones machos y en las
ratas de uno y otro sexo. Se ha observado una relación muy
significativa entre la nefropatía de los Balcanes y los tumores del
tracto urinario, en particular de la pelvis renal y los ureteres.
Sin embargo, no hay datos publicados que demuestren un papel causal
directo de la ocratoxina A en la etiología de esos tumores.
4.2 Tricotecenos
Sobre la base de la información facilitada al Grupo Especial,
es posible asociar la exposición a tricotecenos con episodios de
enfermedad en los seres humanos. Según los limitados datos
disponibles, los tricotecenos que intervienen más frecuentemente en
episodios de exposición humana son el DON y el NIV. En los
episodios de aleucia tóxica alimentaria y toxicosis por ingestión
de cereales mohosos registrados en el pasado, no puede excluirse el
posible papel etiológico de los tricotecenos. La exposición se
produce por la ingestión de alimentos contaminados, principalmente
cereales. La elaboración, la molienda y la cocción no logran la
destrucción del DON, el NIV y la toxina T-2. Los datos en favor de
la exposición por inhalación son muy limitados, pero no puede
excluirse esa posibilidad.
Entre los tricotecenos naturalmente presentes en alimentos, el
más potente es la toxina T-2, seguida por el DAS y el NIV; el DON
resultó el menos tóxico en los estudios de la toxicidad aguda. En
los animales experimentales, la toxina T-2 y el DAS tienen efectos
generales agudos, con necrosis de tejidos epiteliales y supresión
de la hematopoyesis. En los brotes de enfermedad contemporáneos,
sólo se han registrado síntomas gastrointestinales.
Se ha demostrado que el DON es teratogénico en los ratones pero
no en las ratas. Según los estudios de la toxicidad crónica
publicados, el NIV y la toxina T-2 no son oncogénicos en los
animales. No se han publicado estudios sobre la carcino-genicidad
a largo plazo del DON. Ciertos tricotecenos, como la toxina T-2 y
el DON, tienen una acción inmunosupresora en los animales y han
producido alteraciones de la inmunidad, tanto de base celular como
humoral. No hay datos que demuestren la existencia de una acción
inmunosupresora en el hombre.
Los casos de enfermedad humana asociados con la exposición a
tricotecenos que se han notificado son limitados, tanto por su
número como por la información proporcionada. Tras la ingestión de
alimentos contaminados con tricotecenos aparecen rápidamente
síntomas de trastornos digestivos e irritación de la garganta. En
la actualidad no hay nada que demuestre que los tricotecenos
causen cáncer en el ser humano. El Grupo Especial no tuvo a su
disposición informes sobre infección secundaria por bacterias,
hongos o virus en los seres humanos tras la exposición a
tricotecenos, como las observadas en estudios experimentales con
animales. Al parecer, no se han realizado estudios suficientes
para aclarar esa sucesión.
4.3 Cornezuelo
La exposición humana a bajas concentraciones de ergolinas
parece estar muy extendida. Los datos de que se dispone sobre los
recientes brotes en Etiopía y en la India indican que los
alcaloides producidos por C. purpurea (grupo de la ergotamina)
tienen efectos más graves-entre ellos gangrena de las piernas y
muerte-que los alcaloides producidos por C. fusiformis (grupo de la
clavina), que provocaron síntomas gastrointestinales sin desenlace
mortal. No se sabe si esas diferencias corresponden a diferencias
en el contenido de alcaloides de las especies de hongos, en las
propiedades toxicológicas o toxicométricas de los alcaloides o en
las dosis ingeridas por diferentes tipos de poblaciones.
Cuando la limpieza y la molienda eliminan los esclerocios, sólo
quedan en los alimentos preparados bajas concentraciones de
ergolinas; la cocción y otras aplicaciones de calor destruyen
también la mayor parte de los alcaloides del grupo de la
ergotamina.
5. Recomendaciones para ulterior investigación
5.1 Recomendaciones generales
Debe establecerse una red de centros de referencia, que ayuden
a los Estados Miembros a confirmar la identidad de las mico-toxinas
halladas en alimentos y tejidos humanos. Esos centros deben
proporcionar también muestras de referencia de micotoxinas, cuando
se les solicite, a fin de aumentar las posibilidades de comparar
los resultados de análisis realizados en distintas partes del
mundo.
5.2 Ocratoxina A
a) En los Balcanes y en la región del Mediterráneo deben
realizarse estudios epidemiológicos retrospectivos extensos
y prospectivos focales sobre la asociación de la ocratoxina
A con la nefropatía endémica de tipo balcánico y con
tumores del tracto urinario.
b) Fuera de los Balcanes, deben realizarse, en enfermos con
tumores del tracto urinario, análisis de sangre para
detectar la presencia de ocratoxina A.
c) En países no situados en la zona de los Balcanes, debe
aclararse la fuente de la exposición a ocratoxina A, cuando
los análisis de sangre humana indiquen que la misma existe.
d) Debe aclararse la manera en que actúa la diferencia de sexo
en las enfermedades renales, neoplásicas y no neoplásicas,
causadas por la ocratoxina A en animales de
experimentación.
e) Son necesarios extensos estudios sobre el contenido de
ocra-toxina A de los alimentos en distintas partes del
mundo. Es especialmente importante realizar estudios de
ese tipo en las regiones con una elevada incidencia de
tumores del tracto urinario, tumores renales o nefropatías.
5.3 Tricotecenos
a) Deben realizarse estudios de seguimiento en las zonas de la
India y de la República Popular China en que ha habido
recientemente episodios de intoxicación de seres humanos
por tricotecenos. Debe aclararse más la modalidad no usual
de presencia de tricotecenos en esos episodios.
b) Deben estudiarse los efectos de la exposición a largo plazo
de animales de experimentación al DON, incluidos los
efectos carcinogénicos. Como la respuesta de distintas
especies al DON varía mucho, deben elegirse cuidadosamente
las especies utilizadas para el estudio.
c) Debe aclararse más la aparición de infecciones microbianas
secundarias en animales de experimentación tras la
exposición a tricotecenos.
d) Debe estudiarse la influencia de las condiciones
ambientales, incluida la presencia de insecticidas y de
otros productos químicos artificiales, en la producción de
tricotecenos por los hongos.
e) Deben aclararse los efectos de la elaboración de los
alimentos en los tricotecenos.
f) Deben desarrollarse, mediante métodos biotecnológicos,
cultivos agrícolas resistentes a la infección por hongos
productores de tricotecenos.
g) Deben estudiarse en animales de experimentación los
posibles efectos sinérgicos de la exposición combinada a
tricotecenos, aflatoxinas, ocratoxina A y otras
micotoxinas.
h) Deben realizarse estudios sobre la ingesta de tricotecenos
por seres humanos.
i) Deben elaborarse métodos rápidos y sensibles de detección
de los tricotecenos y deben realizarse estudios para
localizar los tricotecenos en cereales y alimentos tratados
en las zonas templadas.
5.4 Cornezuelo
a) Deben hallarse métodos de análisis que permitan detectar la
presencia de agroclavinas.
b) Debe proporcionarse a los países en desarrollo información
sobre el empleo de procedimientos de detección de semillas
patológicas y de molienda para reducir al mínimo los
problemas causados por el cornezuelo.
c) Deben realizarse estudios epidemiológicos sobre los
posibles efectos de bajas concentraciones de ergolinas en
la población humana.
d) Deben efectuarse estudios farmacológicos y toxicológicos en
los que se administren a animales de experimentación
distintas ergolinas, aisladas y en combinación.
e) Debe aclararse la posible trasmisión de ergolinas a los
lactantes a través de la leche materna.
 The first compound discovered, ochratoxin A, was isolated from
a culture of Aspergillus ochraceus, hence the name (Van der Merwe
et al., 1965). The acids, including 4-hydroxy-ochratoxin A, the
methyl and ethyl esters, and the isocoumarin part of ochratoxin B
(ochratoxin) have all been isolated from fungal cultures under
experimental conditions. On acid hydrolysis, ochratoxin A yields
phenylalanine, and the isocoumarin part, ochratoxin alpha, a
cleavage product also found in the intestines, faeces, urine, and
liver of rodents experimentally fed an ochratoxin A-containing diet
(Galtier & Alvinerie, 1976). Ochratoxin A and, very rarely,
ochratoxin B are the only compounds found as natural contaminants
in plant material, and most of the information available concerns
ochratoxin A. It can be stored in ethanol in the refrigerator for
more than a year without loss (Chu & Butz, 1970); however, such
solutions should be protected from light, since decomposition
occurs on exposure to fluorescent light for several days (Neely &
West, 1972).
Ochratoxin A is a colourless, crystalline compound, obtained by
crystallization from benzene, with a melting point of about 90 °C,
and containing approximately one mole of benzene. After drying for
1 h at 60 °C, it has a melting point in the range of 168-173 °C.
It is soluble in polar organic solvents, slightly soluble in water,
and soluble in dilute aqueous bicarbonate. Physical data on
ochratoxin A, based on a collaborative study, have been published
by IUPAC (Pohland et al., 1982). Ochratoxin A is optically active:
[alpha]21D: -46.8 °C (c = 2650 µmol/litre in chloroform). The
publication cited above includes information on the following
spectra of ochratoxin A: ultraviolet absorption spectrum; infrared
absorption spectrum; electron impact mass spectrum; nuclear
magnetic resonance spectrum.
I.1.2 Methods for the analysis of foodstuffs and biological
samples
Ochratoxin A in acidified commodities is readily soluble in
many organic solvents, and this characteristic has been used as the
principle of extraction in several methods.
A number of methods using thin-layer chromatography have been
published, one of the most commonly used being the official AOAC
method developed for barley (Nesheim et al., 1973). In this
method, ochratoxins A and B are extracted from ground samples with
chloroform, after acidification. The toxins are trapped in a
column containing diatomaceous earth impregnated with a basic
aqueous solution. After column clean-up, the toxins are eluted and
thin-layer chromatography is performed using long-wave UV
irradiation for visualization of the fluorescent ochratoxin spots
(limit of detection: 12 µg/kg). The method has been
collaboratively studied, revealing coefficients of variation
(between laboratories) in the range of 31-54% (Nesheim, 1973).
This method has been published as an IUPAC recommended procedure
(IUPAC, 1976). The sensitivity can be improved by exposing the
developed plate to ammonia fumes, resulting in a limit of detection
of a few µg/kg. A slightly modified method, developed for green
coffee (Levi, 1975), has a coefficient of variation in the range of
33-49%, based on a collaborative study.
The two methods have been combined into one procedure,
published as an IARC procedure (Nesheim, 1982). In an
international check sample survey on ochratoxin A in animal feed,
the AOAC procedure was used by 61% of the participants, and the
performance was slightly better than that of the other methods,
with a coefficient of variation of 69% compared to 79% for the
other methods combined (Freisen & Garren, 1983). Paulsch et al.
(1982) developed a procedure for the determination of ochratoxin A
in the kidneys of swine, using a liquid-liquid partitioning step
instead of column clean-up, followed by two-dimensional thin-layer
chromatography using an acidic and an alkaline developing solvent.
In addition, the procedure included a confirmatory test, based on
the formation of ochratoxin A methyl ester on the plate.
High-performance liquid chromatographic procedures have been
developed for the determination of ochratoxin A in food of plant
origin (Hunt et al., 1978; Josefsson & Moller, 1979), with limits
of detection in the range of 1-12 µg/kg, and with recoveries in the
55-92% range. A procedure for the determination of ochratoxin A
residues in renal tissue has been published, in which enzymic
digestion of the sample tissue is followed by dialysis and high
performance liquid chromatography (Hunt et al., 1979). The limit
of detection is about 1 µg/kg, and recoveries of 77-78% have been
observed. Rapid screening methods for ochratoxin A are available,
based on minicolumn chromatography, with limits of detection in
the 8-12 µg/kg range (Hald & Krogh, 1975; Holaday, 1976). By using
antisera to ochratoxin A, an enzyme-linked immunosorbent assay
(ELISA) has been constructed in which the toxin in barley can be
determined with only 0.5% cross-reaction for ochratoxin B, and with
a lower level of detection of 10 pg ochratoxin A/well of the ml
plate (Morgan et al., 1982). Immunological methodology has been
further improved by the use of monoclonal antibody (IgG) in a
radioimmunoassay with high specificity for ochratoxin A, which can
detect levels of this toxin as low as 0.2 µg/kg in swine kidney
tissue (Candlish et al., 1986; Rousseau et al., 1987). A method
has been described in which ochratoxin A is cleaved to ochratoxin
alpha and phenylalanine using the enzyme carboxypeptidase. The
quantification of ochratoxin A is based on the loss of fluorescence
intensity at 380 nm, the limit of detection being 4 µg/kg of
barley (Hult & Gatenbeck, 1976). The procedure has also been
applied to swine blood with a limit of detection of 2 µg/litre
(Hult et al., 1979).
The same procedure has been used in screening human blood for
the presence of ochratoxin A, with a limit of detection of 1-2
µg/litre serum for a 2-g sample. High-performance liquid
chromatography was used as confirmation (Hult et al., 1982). A
screening method involving flow injection has been developed by
which ochratoxin A concentrations of more than 10 µg/litre can be
determined, based on 50-µlitre samples of serum (Hult et al.,
1984). As the specificity of the method is limited, positive
results have to be confirmed by conventional methods requiring much
larger blood samples.
A method has been developed for the detection of ochratoxin
A (and aflatoxin B and citrinin) in human urine using hydrolysis
of urine, solid-phase extraction, and reversed-phase liquid
chromatography with fluorescence detection (Orti et al., 1986).
The detection limit for ochratoxin A was approximately 10 ng/ml in
samples of 10 ml urine.
I.2 SOURCES AND OCCURRENCE
I.2.1 Fungal formation
The ochratoxins were isolated in 1965 from a culture of
Aspergillus ochraceus, hence the name (Van der Merwe et al., 1965),
but subsequent investigations have revealed that a variety of
fungal organisms included in the genera Aspergillus and Penicillium
are able to produce ochratoxins (Table 1).
The effects of water activity (aw) and temperature, the main
factors controlling mycotoxin formation, have been elucidated in
relation to growth and ochratoxin production for 3 fungal
organisms: A. ochraceus, P. cyclopium, and P. viridicatum
(Northolt et al., 1979). The minimum aw values for ochratoxin
production ranged between 0.83 and 0.87, 0.87 and 0.90, and 0.83
and 0.86, respectively. At 24 °C, optimum aw values for A.
ochraceus and for both P. cyclopium and P. viridicatum were 0.99
and 0.95-0.99, respectively (Fig. 2).
Table 1. Ochratoxin-producing fungia
-------------------------------------------------------------------
Penicillium link
Monoverticillata:
P. frequentans series: P. purpurrescens Sopp
Asymmetrica lanata:
P. commune series: P. commune Thom
Asymmetrica fasciculata:
P. viridicatum series: P. viridicatum Westling
P. palitans Westling
P. cyclopium series: P. cyclopium Westling
Biverticillata symmetrica:
P. purpurogenum series: P. varibile Sopp
Aspergillus Micheli
Aspergillus ochraceus group: A. sulphureus (Fres.)
Thom & Church
A. sclerotiorum Huber
A. alliaceus Thom & Church
A. melleus Yukawa
A. ochraceus Wilhelm
A. ostianus Wehmer
A. petrakii Vörös
-------------------------------------------------------------------
a From: Krogh (1978).
The first compound discovered, ochratoxin A, was isolated from
a culture of Aspergillus ochraceus, hence the name (Van der Merwe
et al., 1965). The acids, including 4-hydroxy-ochratoxin A, the
methyl and ethyl esters, and the isocoumarin part of ochratoxin B
(ochratoxin) have all been isolated from fungal cultures under
experimental conditions. On acid hydrolysis, ochratoxin A yields
phenylalanine, and the isocoumarin part, ochratoxin alpha, a
cleavage product also found in the intestines, faeces, urine, and
liver of rodents experimentally fed an ochratoxin A-containing diet
(Galtier & Alvinerie, 1976). Ochratoxin A and, very rarely,
ochratoxin B are the only compounds found as natural contaminants
in plant material, and most of the information available concerns
ochratoxin A. It can be stored in ethanol in the refrigerator for
more than a year without loss (Chu & Butz, 1970); however, such
solutions should be protected from light, since decomposition
occurs on exposure to fluorescent light for several days (Neely &
West, 1972).
Ochratoxin A is a colourless, crystalline compound, obtained by
crystallization from benzene, with a melting point of about 90 °C,
and containing approximately one mole of benzene. After drying for
1 h at 60 °C, it has a melting point in the range of 168-173 °C.
It is soluble in polar organic solvents, slightly soluble in water,
and soluble in dilute aqueous bicarbonate. Physical data on
ochratoxin A, based on a collaborative study, have been published
by IUPAC (Pohland et al., 1982). Ochratoxin A is optically active:
[alpha]21D: -46.8 °C (c = 2650 µmol/litre in chloroform). The
publication cited above includes information on the following
spectra of ochratoxin A: ultraviolet absorption spectrum; infrared
absorption spectrum; electron impact mass spectrum; nuclear
magnetic resonance spectrum.
I.1.2 Methods for the analysis of foodstuffs and biological
samples
Ochratoxin A in acidified commodities is readily soluble in
many organic solvents, and this characteristic has been used as the
principle of extraction in several methods.
A number of methods using thin-layer chromatography have been
published, one of the most commonly used being the official AOAC
method developed for barley (Nesheim et al., 1973). In this
method, ochratoxins A and B are extracted from ground samples with
chloroform, after acidification. The toxins are trapped in a
column containing diatomaceous earth impregnated with a basic
aqueous solution. After column clean-up, the toxins are eluted and
thin-layer chromatography is performed using long-wave UV
irradiation for visualization of the fluorescent ochratoxin spots
(limit of detection: 12 µg/kg). The method has been
collaboratively studied, revealing coefficients of variation
(between laboratories) in the range of 31-54% (Nesheim, 1973).
This method has been published as an IUPAC recommended procedure
(IUPAC, 1976). The sensitivity can be improved by exposing the
developed plate to ammonia fumes, resulting in a limit of detection
of a few µg/kg. A slightly modified method, developed for green
coffee (Levi, 1975), has a coefficient of variation in the range of
33-49%, based on a collaborative study.
The two methods have been combined into one procedure,
published as an IARC procedure (Nesheim, 1982). In an
international check sample survey on ochratoxin A in animal feed,
the AOAC procedure was used by 61% of the participants, and the
performance was slightly better than that of the other methods,
with a coefficient of variation of 69% compared to 79% for the
other methods combined (Freisen & Garren, 1983). Paulsch et al.
(1982) developed a procedure for the determination of ochratoxin A
in the kidneys of swine, using a liquid-liquid partitioning step
instead of column clean-up, followed by two-dimensional thin-layer
chromatography using an acidic and an alkaline developing solvent.
In addition, the procedure included a confirmatory test, based on
the formation of ochratoxin A methyl ester on the plate.
High-performance liquid chromatographic procedures have been
developed for the determination of ochratoxin A in food of plant
origin (Hunt et al., 1978; Josefsson & Moller, 1979), with limits
of detection in the range of 1-12 µg/kg, and with recoveries in the
55-92% range. A procedure for the determination of ochratoxin A
residues in renal tissue has been published, in which enzymic
digestion of the sample tissue is followed by dialysis and high
performance liquid chromatography (Hunt et al., 1979). The limit
of detection is about 1 µg/kg, and recoveries of 77-78% have been
observed. Rapid screening methods for ochratoxin A are available,
based on minicolumn chromatography, with limits of detection in
the 8-12 µg/kg range (Hald & Krogh, 1975; Holaday, 1976). By using
antisera to ochratoxin A, an enzyme-linked immunosorbent assay
(ELISA) has been constructed in which the toxin in barley can be
determined with only 0.5% cross-reaction for ochratoxin B, and with
a lower level of detection of 10 pg ochratoxin A/well of the ml
plate (Morgan et al., 1982). Immunological methodology has been
further improved by the use of monoclonal antibody (IgG) in a
radioimmunoassay with high specificity for ochratoxin A, which can
detect levels of this toxin as low as 0.2 µg/kg in swine kidney
tissue (Candlish et al., 1986; Rousseau et al., 1987). A method
has been described in which ochratoxin A is cleaved to ochratoxin
alpha and phenylalanine using the enzyme carboxypeptidase. The
quantification of ochratoxin A is based on the loss of fluorescence
intensity at 380 nm, the limit of detection being 4 µg/kg of
barley (Hult & Gatenbeck, 1976). The procedure has also been
applied to swine blood with a limit of detection of 2 µg/litre
(Hult et al., 1979).
The same procedure has been used in screening human blood for
the presence of ochratoxin A, with a limit of detection of 1-2
µg/litre serum for a 2-g sample. High-performance liquid
chromatography was used as confirmation (Hult et al., 1982). A
screening method involving flow injection has been developed by
which ochratoxin A concentrations of more than 10 µg/litre can be
determined, based on 50-µlitre samples of serum (Hult et al.,
1984). As the specificity of the method is limited, positive
results have to be confirmed by conventional methods requiring much
larger blood samples.
A method has been developed for the detection of ochratoxin
A (and aflatoxin B and citrinin) in human urine using hydrolysis
of urine, solid-phase extraction, and reversed-phase liquid
chromatography with fluorescence detection (Orti et al., 1986).
The detection limit for ochratoxin A was approximately 10 ng/ml in
samples of 10 ml urine.
I.2 SOURCES AND OCCURRENCE
I.2.1 Fungal formation
The ochratoxins were isolated in 1965 from a culture of
Aspergillus ochraceus, hence the name (Van der Merwe et al., 1965),
but subsequent investigations have revealed that a variety of
fungal organisms included in the genera Aspergillus and Penicillium
are able to produce ochratoxins (Table 1).
The effects of water activity (aw) and temperature, the main
factors controlling mycotoxin formation, have been elucidated in
relation to growth and ochratoxin production for 3 fungal
organisms: A. ochraceus, P. cyclopium, and P. viridicatum
(Northolt et al., 1979). The minimum aw values for ochratoxin
production ranged between 0.83 and 0.87, 0.87 and 0.90, and 0.83
and 0.86, respectively. At 24 °C, optimum aw values for A.
ochraceus and for both P. cyclopium and P. viridicatum were 0.99
and 0.95-0.99, respectively (Fig. 2).
Table 1. Ochratoxin-producing fungia
-------------------------------------------------------------------
Penicillium link
Monoverticillata:
P. frequentans series: P. purpurrescens Sopp
Asymmetrica lanata:
P. commune series: P. commune Thom
Asymmetrica fasciculata:
P. viridicatum series: P. viridicatum Westling
P. palitans Westling
P. cyclopium series: P. cyclopium Westling
Biverticillata symmetrica:
P. purpurogenum series: P. varibile Sopp
Aspergillus Micheli
Aspergillus ochraceus group: A. sulphureus (Fres.)
Thom & Church
A. sclerotiorum Huber
A. alliaceus Thom & Church
A. melleus Yukawa
A. ochraceus Wilhelm
A. ostianus Wehmer
A. petrakii Vörös
-------------------------------------------------------------------
a From: Krogh (1978).
 At optimum aw, the temperature range for ochratoxin production
by A. ochraceus was 12-37 °C, whereas that of P. cyclopium and P.
viridicatum was 4-31 °C. These laboratory data correspond with
those from field observations on ochratoxin contamination in crops.
Thus, the more frigophilic Penicillia, particularly P. viridicatum,
are the major ochratoxin producers in crops in colder climatic
zones, such as Scandinavia (Krogh, 1978; Rutqvist et al., 1978;
Häggblom, 1982), and in Canada (Scott et al., 1972).
In contrast, ochratoxin-producing fungal potential has been
found in 28-50% of A. ochraceus strains isolated from crops in
warmer climatic zones, such as Australia (Connole et al., 1981),
and Yugoslavia (Pepeljnjak & Cvetnic, 1981), and strains isolated
from coffee beans (Stack et al., 1982).
I.2.2 Occurrence in foodstuffs
I.2.2.1 Plant products
Ochratoxin A was first encountered as a natural contaminant in
maize (Shotwell et al., 1969), and subsequent surveys have
established that ochratoxin A is a contaminant of cereals and some
beans (coffee beans, soya beans, cocoa beans) in many areas of the
world (Table 2). Although the mean level of ochratoxin A in all
reported surveys up to 1979 was 1035 µg/kg (Fig. 3), 83% of the
samples contained less than 200 µg/kg (Krogh, 1980).
At optimum aw, the temperature range for ochratoxin production
by A. ochraceus was 12-37 °C, whereas that of P. cyclopium and P.
viridicatum was 4-31 °C. These laboratory data correspond with
those from field observations on ochratoxin contamination in crops.
Thus, the more frigophilic Penicillia, particularly P. viridicatum,
are the major ochratoxin producers in crops in colder climatic
zones, such as Scandinavia (Krogh, 1978; Rutqvist et al., 1978;
Häggblom, 1982), and in Canada (Scott et al., 1972).
In contrast, ochratoxin-producing fungal potential has been
found in 28-50% of A. ochraceus strains isolated from crops in
warmer climatic zones, such as Australia (Connole et al., 1981),
and Yugoslavia (Pepeljnjak & Cvetnic, 1981), and strains isolated
from coffee beans (Stack et al., 1982).
I.2.2 Occurrence in foodstuffs
I.2.2.1 Plant products
Ochratoxin A was first encountered as a natural contaminant in
maize (Shotwell et al., 1969), and subsequent surveys have
established that ochratoxin A is a contaminant of cereals and some
beans (coffee beans, soya beans, cocoa beans) in many areas of the
world (Table 2). Although the mean level of ochratoxin A in all
reported surveys up to 1979 was 1035 µg/kg (Fig. 3), 83% of the
samples contained less than 200 µg/kg (Krogh, 1980).
 Table 2. Natural occurrence of ochratoxin A in foodstuffs and animal feed of plant origin
---------------------------------------------------------------------------------------------------------
Commodity Country Number of Percentage Range of Reference
samples contaminated ochratoxin A
analysed levels (µg/kg)
---------------------------------------------------------------------------------------------------------
FOOD
maize USA 293 1.0 83-166 Shottwell et al. (1971)
maize (1973) France 463 2.6 15-200 Galtier et al. (1977b)
maize (1974) France 461 1.3 20-200 Galtier et al. (1977b)
wheat (red winter) USA 291 1.0 5-115 Shottwell et al. (1976)
wheat (red spring) USA 286 2.8 5-115 Shottwell et al. (1976)
barley (malt) Denmark 50 6.0 9-189 Krogh (1978)
barley USA 127 14.2 10-40 Nesheim (1971)
coffee beans USA 267 7.1 20-360 Levi et al. (1974)
maize Yugoslaviaa 542 8.3b 6-140 Pavlovic et al. (1979)
wheat Yugoslaviaa 130 8.5b 14-135 Pavlovic et al. (1979)
wheat bread Yugoslaviaa 32 18.8b Pavlovic et al. (1979)
barley Yugoslaviaa 64 12.5b 14-27 Pavlovic et al. (1979)
barley Czechoslovakia 48 2.1 3800 Vesela et al. (1978)
bread United Kingdomc 50 2.0 210 Osborne (1980)
flour United Kingdomc 7 28.5 490-2900 Osborne (1980)
beans Sweden 71 8.5 10-442 Akerstrand &
peas Sweden 72 2.8 10 Josefsson (1979)
maize United Kingdom 29 37.9 50-500 Ministry of
cornflour United Kingdom 13 30.8 50-200 Agriculture (1980)
soya bean United Kingdom 25 36.0 50-500 Ministry of
soya flour United Kingdom 21 19.0 50-500 Agriculture (1980)
cocoa beans (raw) United Kingdom 56 17.9 100-500 Ministry of
(roasted) United Kingdom 19 15.8 100 Agriculture (1980)
grain German Democratic 49 4.1 18-22 Fritz et al.
Republic (1979)
grain (barley, Poland 296 6.8 20-470 Szebiotko et al. (1981)
wheat, rye)
grain (wheat, rye) Denmark 151 1.3 15-50 Pedersen &
bran (wheat) Denmark 57 10.5 5-20 Hansen (1981)
maize Bulgariaa 22 27.3 25-35 Petkova-Bocharova &
maize Bulgaria 22 9.0 10-25 Castegnaro (1985)
beans Bulgariaa 24 16.7 25-27 Petkova-Bocharova &
beans Bulgariaa 28 7.1 25-50 Castegnaro (1985)
---------------------------------------------------------------------------------------------------------
Table 2 (contd.)
---------------------------------------------------------------------------------------------------------
Commodity Country Number of Percentage Range of Reference
samples contaminated ochratoxin A
analysed levels (µg/kg)
---------------------------------------------------------------------------------------------------------
FOOD (continued)
barley, wheat, Poland 150 5.3 50-200 Juszkiewicz &
oats, rye, maize Piskorska-
Pliszczynska (1976)
FEED
mixed feed Poland 203 4.9 10-50 Juszkiewicz &
Piskorska-
Pliszczynska (1977)
maize Yugoslavia 191 25.7 45-5125 Balzer et al. (1977)
barley, oats Sweden 84 8.3 16-409 Krogh et al. (1974b)
wheat, hay Canada 95 7.4 30-6000 Prior (1976)
wheat, oats, Canadac 32 56.3 30-27 000 Scott et al. (1972)
barley, rye
barley, oats Denmarkc 33 57.6 28-27 500 Krogh et al. (1973)
mixed feed Canada 474 1.1 30-100 Prior (1981)
mixed feed Canadac 51 7.8 48-5900 Abramson et al. (1983)
mixed feed Australia 25 4.0 70 000 Connole et al. (1981)
mouldy bread Italyd 1 80 000 Visconti &
Bottalico (1983)
---------------------------------------------------------------------------------------------------------
a From an area with endemic nephropathy.
b Average values for a period of 2-5 years.
c All samples suspected of containing mycotoxins.
d This sample contained in addition 9600 µg ochratoxin B/kg.
Ochratoxin B has only been found in 3 samples and, thus, occurs
extremely rarely; the other ochratoxins have never been found in
plant products. Levels of ochratoxin A and the frequency of
contamination are generally higher in animal feed than in foodstuffs
(Table 2). Although the mean level of ochratoxin A in all reported
surveys up to 1979 was 1035 µg/kg (Fig. 3), 83% of the samples
contained less than 200 µg/kg (Krogh, 1980).
I.2.2.2 Residues in food of animal origin
Residues of ochratoxin A are not generally found in ruminants,
because ochratoxin A is cleaved in the forestomachs by protozoan and
bacterial enzymes (Galtier & Alvinerie, 1976; Hult et al., 1976;
Patterson et al., 1981). The non-toxic cleavage product, ochratoxin
alpha, has been found in the kidneys at levels below 10 µg/kg and in
the blood of calves fed a diet containing 300-500 µg ochratoxin A/kg
(Patterson et al., 1981). In half of the calves, the kidneys
contained low levels of ochratoxin A (up to 5 µg/kg), a feature that
may reflect that the calves were not yet functioning as ruminants.
When 2 milking cows were fed a ration containing 317-1125 µg
ochratoxin A/kg for 11 weeks, a residue of 5 µg ochratoxin A/kg was
found in the kidneys of one of the animals but not in any other
tissue or the milk. Ochratoxin alpha was not found in any tissue
(Shreeve et al., 1979). Residues of ochratoxin A have been detected
in a number of tissues in single-stomach food animals, such as pigs.
The carry-over of ochratoxin A from feed to animal tissues was
elucidated in a study in which groups of pigs were exposed for 3-4
months to dietary levels of ochratoxin A of 200, 1000, or 4000 µg/kg
(Krogh et al., 1974a). At termination (slaughter), the highest
levels of ochratoxin A residues were found in the kidneys (mean
levels 50 µg/kg at the 4000 µg/kg feed level), with lower levels
in the liver, muscle, and adipose tissue; other tissues, including
blood, were not analysed. There was a high correlation between the
feed level of ochratoxin A and the residue levels in the 4 tissues
investigated (Table 3).
Table 3. Correlation between feed level and tissue levels
(residues) of ochratoxin A in pigsa
-----------------------------------------------------------
Tissue Regression equation r
-----------------------------------------------------------
kidney Y = 2.15 + 0.0123X 0.86
liver Y = 0.35 + 0.0095X 0.82
adipose Y = 2.51 + 0.0099X 0.78
-----------------------------------------------------------
a Modified from Krogh et al. (1974a).
X = ochratoxin A in feed (µg/kg).
Y = ochratoxin A residue (µg/kg tissue).
r = correlation coefficient.
The regression is calculated on feed levels of ochratoxin A
in the range of 200-4000 µg/kg.
Ochratoxin A is present in the blood bound to serum-albumin
(Chu, 1971; Galtier, 1974a) and as free ochratoxin A; saturation (in
the rat) occurs at 70 mg ochratoxin A/litre plasma. The binding of
ochratoxin A to serum-albumin is particularly strong in cattle,
pigs, and man, based on in vitro studies (Table 4).
Table 4. In vitro binding of ochratoxin A to
serum-albumin in several speciesa
-----------------------------------------------
Animal Number of Intrinsic
species binding Association
sites constant (M-1)
-----------------------------------------------
Cattle 0.58 94 600
Pig 0.56 71 100
Man 0.58 63 500
Horse 0.57 57 400
Chicken 0.51 52 700
Rat 0.68 40 100
Sheep 0.88 22 600
-----------------------------------------------
a Adapted from: Galtier (1979).
The presence of ochratoxin A in the blood of pigs has been
elucidated in experimental animal studies as well as by surveys of
blood samples from pigs at farms (Hult et al., 1979, 1980). A
total of 1200 pig blood samples, collected from slaughterhouses
over various periods during 1986 in western Canada, was screened
for the presence of ochratoxin A using an HPLC procedure (Marqhardt
et al., 1988). It was shown that 3.6-4.2% of the blood samples
contained the toxin at concentrations higher than 20 ng/ml. It
appears that the concentration of ochratoxin A in the blood is
higher than that in any other tissue. In feeding studies, bacon
pigs were exposed for various periods to rations containing
ochratoxin A concentrations in the range of 58-1878 µg/kg; blood,
kidney, liver, muscle, and adipose tissues were analysed, at
slaughter, for residues of ochratoxin A (Mortensen et al., 1983).
The statistical association between residue levels in various
tissues is indicated by the regression analyses (Table 5).
Table 5. Regression between residues of ochratoxin A
in the serum and certain other tissuesa
------------------------------------------------------
Tissue Regression r sb
equation
------------------------------------------------------
kidney Y = 0.0651X 0.89 0.002
muscle Y = 0.0346X 0.88 0.001
liver Y = 0.0259X 0.89 0.001
adipose Y = 0.0191X 0.84 0.001
------------------------------------------------------
a Adapted from: Mortensen et al. (1983).
X = µg ochratoxin A/litre serum.
Y = µg ochratoxin A/kg in the other tissues.
r = correlation coefficient
sb = Standard error of slope
Epidemiological studies on the basis of data from meat
inspection in Danish slaughterhouses revealed prevalence rates of
porcine nephropathy ranging from 10 to 80 cases per 100 000
slaughtered pigs (Krogh, 1976a). Surveys in a number of European
countries for residues of ochratoxin A in kidneys from cases of
porcine nephropathy revealed that 25-39% of the cases contained
ochratoxin A levels in the range 2-100 µg/kg (Table 6).
Table 6. Surveys for ochratoxin A residues in kidneys from
cases of porcine nephropathy, based on meat inspection data
-------------------------------------------------------------------
Country No. of porcine Percentage Range of Reference
nephropathy containing ochratoxin
kidneys residues of A residues
investigated ochratoxin (µg/kg wet
A weight)
-------------------------------------------------------------------
Denmark 60 35 2-68 Krogh (1977)
Germany, 104 21 0.1-1.8 Bauer et al.
Federal (1984)
Republic of
Hungary 122 39 2-100 Sandor et
al. (1982)
Poland 113 24 1-23 Golinski et
al. (1984)
Sweden 129 25 2-104 Rutqvist et
al. (1977)
Sweden 90 27 2-88 Josefsson
(1979)
-------------------------------------------------------------------
Ochratoxin A levels of up to 29 µg/kg were found in the muscle
of hens and chickens collected at one slaughterhouse (Elling et
al., 1975). The birds had been condemned because of nephropathy.
In another study, groups of hens were exposed for 1-2 years to
dietary levels of ochratoxin A of 0.3 or 1 mg/kg (Krogh et al.,
1976b). The kidneys contained the highest residues with a mean
value of 19 µg/kg tissue in the group fed 1 mg ochratoxin A/kg; the
liver and muscle contained lower levels of residues and no
ochratoxin A was found in the eggs. These results are in
accordance with those of subsequent experimental animal studies.
For example, in a study in which 4 groups of hens were fed diets
containing 0, 0.5, 1, or 4 mg ochratoxin A/kg, the kidneys
contained the highest level of ochratoxin A residues (31 µg/kg) in
the highest dose group; eggs were not analysed (Prior & Sisodia,
1978).
In another study, hens were fed 1 mg ochratoxin A/kg feed for 8
weeks; the kidneys contained 3-10 µg ochratoxin A/kg and the liver,
1.5-2.5 µg/kg. The eggs were not analysed (Reichmann et al.,
1982). When groups of hens were fed diets containing 2.5 or 10
mg/kg, the kidneys contained 1-6 µg ochratoxin A/kg, lower levels
being found in the plasma, muscle, and liver (Juszkiewicz et al.,
1982). Eggs from the high-dose group contained 0.7-1.3 µg
ochratoxin A/kg, but none was detected in eggs from the low-dose
group.
White Leghorn hens (54 birds), divided into four groups, were
fed diets for one month containing the following concentrations of
ochratoxin A (mg/kg): 0, 1.3, 2.6, and 5.2 (Bauer et al., 1988).
At termination, three tissues were analysed for residues. The
following ranges of dose-dependent residues were found: serum,
4.7-11.7 ng/ml, liver, 9.1-18.0 ng/g, and yolk, 1.6-4.0 ng/g; very
little ochratoxin A was found in the egg white, and none was
detected in the tissues of the control group.
I.3 METABOLISM
I.3.1 Absorption
In a study on rats exposed by gavage to a single dose of
ochratoxin A at 10 mg/kg body weight, Galtier (1974b) found the
highest tissue level of unchanged ochratoxin A in the stomach wall
during the first 4 h following administration. The small and large
intestine and caecum contained small amounts of unchanged
ochratoxin A, and it was concluded that ochratoxin A was absorbed
mainly in the stomach. Small amounts (1-3% of the total dose) were
detected in the caecum and the large intestine, as the isocoumarin
moiety (ochratoxin alpha), most likely as the result of the
hydrolysing action of the intestinal microflora (Galtier &
Alvinerie, 1976; Hult et al., 1976).
In a study on intestinal absorption using the same animal
species, Kumagai & Aibara (1982) came to the conclusion that the
site of maximal absorption of ochratoxin A was the proximal
jejunum, and that the portal vein was the primary route of
transport from the intestinal tract, though part of the
transportation took place through the lymphatics.
Using a highly specific antibody against ochratoxin A, which is
used in a peroxidase-antiperoxidase detection method, Lee et al.
(1984) studied absorption and tissue distribution in Swiss mice
over a 48-h period, after the administration of a single dose of 25
mg ochratoxin A/kg body weight. Ochratoxin A was found in large
amounts, indicated by staining, in epithelial cells as well as in
macrophages of the lamina propria in the duodenum. Smaller amounts
were found in jejunal epithelial cells and lamina propria, and much
smaller amounts in the epithelial cells in the esophagus and
stomach; no toxin was found in the ileum. These findings suggest
that absorption mainly takes place in the duodenum and the jejunum.
I.3.2 Tissue distribution
I.3.2.1 Animal studies
In slaughterhouse cases of mycotoxic porcine nephropathy
studied by Hald & Krogh (1972), residues of unchanged ochratoxin A
were found in all tissues investigated (kidney, liver, and muscle),
the highest level (up to 67 µg/kg) occurring in the kidney. In
experimental studies on pigs ingesting feed containing ochratoxin
A, residues of the toxin were found in all 4 tissues in the
decreasing order of kidney, liver, muscle, adipose tissue (Krogh et
al., 1974a). A subsequent study revealed that the concentration of
ochratoxin A residues in the blood of the pig was higher than those
in the other tissues mentioned above (Mortensen et al., 1983).
When rats were exposed orally to an ochratoxin A dose of 10 mg/kg
body weight, Galtier (1974b) recovered 0.3% of the administered
dose in the whole kidneys, 0.9% in the whole liver, and 0.6% in the
total muscle tissue, 96 h after exposure. Chang & Chu (1977),
using a single intraperitoneal injection of 1 mg ochratoxin A per
rat (labelled with 14C in phenylalanine), found that the kidney
contained twice as much unchanged ochratoxin A as the liver after
30 minutes, amounting to 4-5% of the total dose.
In the study by Lee et al. (1984), the largest amounts of
ochratoxin A found in the kidney (as indicated by staining
intensity) were in the epithelium of the proximal convoluted
tubules, and to a lesser extent in the distal convoluted tubules,
the descending loop of Henle, and glomeruli and Bowman's capsule.
Small amounts were found in hepatocytes as well as in the lumina of
bile ducts, but not in biliary epithelium, indicating biliary
excretion.
Using pig renal cortical slices, it was found that ochratoxin A
enters the proximal tubule cells by the common organic anion
transport system (Friis et al., 1988). Ochratoxin A inhibited p-
aminohippurate (PAH) and phenolsulfophthalein uptake in a dose-
dependent manner.
I.3.2.2 Studies on man
In a study in Yugoslavia near Slavonski Brod, where endemic
nephropathy is prevalent, 639 samples of serum from the inhabitants
of 2 villages were screened for the presence of ochratoxin A; 42
(6.6%) were positive for the toxin "with concentrations in the
range of 1-57 ng ochratoxin A/g" (Hult et al., 1982). Detection
was carried out using the enzymic spectrofluorometric procedure,
and positives were confirmed by the esterification of ochratoxin A
in serum and of ochratoxin alpha obtained from the enzymetic
hydrolisates; the esters were measured by high performance liquid
chromatography.
In a screening of serum samples in Poland using the same method
of analysis, 77 out of 1065 samples (7.2%) contained ochratoxin A,
with a mean concentration of 0.27 ng/ml and a maximum value of 40
ng/ml (Colinski, 1987). In the Federal Republic of Germany, 173
out of 306 serum samples (56.6%) contained ochratoxin A, as
measured by an HPLC procedure, with a mean of 0.6 ng/g and a range
of 0.1-14.4 ng/g (Bauer & Gareis, 1987). Three out of 46 kidneys
(6.6%) contained ochratoxin A, with a mean of 0.2 ng/g and a range
of 0.1-0.3 ng/g. In a study of blood plasma in Denmark, 46 out of
96 samples (47.9%) contained ochratoxin A, as measured by an HPLC
procedure, with a mean of 1.7 ng/g and a range of 0.1-9.2 ng/g
(Hald, 1989). In Bulgaria, where endemic nephropathy also occurs
in some areas, 45 out of 312 blood samples contained ochratoxin A
(14.4%), with a mean of approximately 14 ng/g (Petkova-Bocharova et
al., 1988).
I.3.3 Metabolic transformation
It has been shown from in vitro studies that ochratoxin A binds
to serum-albumin (Chu, 1971, 1974b); this binding has also been
observed in in vivo studies on rats (Galtier, 1974a; Chang & Chu,
1977). Ochratoxin alpha has been detected in the urine and faeces
of rats injected intraperitoneally with ochratoxin A (Nel &
Purchase, 1968; Chang & Chu, 1977), indicating the cleavage of
ochratoxin A to ochratoxin alpha and phenylalanine, under these
conditions.
Studies with 14C-labelled ochratoxin A indicated that some
other, not yet identified, metabolites are formed in the body.
Less than half of the radioactivity excreted in rat urine within 24
h of a single intraperitoneal injection of 14C-phenylalanine-
labelled ochratoxin A was identified as ochratoxin A (Chang & Chu,
1977).
In both albino rats and brown rats given ochratoxin A orally or
intraperitoneally, 1-1.5% of the dose was excreted as (4R)-4-
hydroxyochratoxin A and 25-27% as ochratoxin alpha in the urine
(Storen et al., 1982). In in vitro studies using liver microsomes
from the pig, rat, and man, both (4R)-and (4S)-4-hydroxy-ochratoxin
A were produced in a hydroxylation process involving cytochrome
P-450 (Stormer & Pedersen, 1980; Stormer et al., 1981).
4-Hydroxyochratoxin A is non-toxic for rats in amounts up to 40 mg/kg
body weight (Hutchison et al., 1971); thus, it has been concluded
that the microsomal hydroxylation most likely represents a
detoxification reaction.
I.3.4 Excretion
The results of studies in which 14C-labelled ochratoxin A was
injected intraperitoneally in rats demonstrated that the toxin was
excreted primarily in the urine (Chang & Chu, 1977), though faecal
elimination also occurred to some extent (Galtier, 1974b; Chang &
Chu, 1977). In a study in which 14C-labelled ochratoxin A was
given orally to rats as a single dose (15 mg/kg body weight), the
cumulative elimination after 120 h was 11% ochratoxin A and 23%
ochratoxin alpha in the faeces; 11% ochratoxin A and 12% ochratoxin
alpha in the urine; and 33% ochratoxin A in the bile (Suzuki et
al., 1977). Absorption was influenced by enteritis, which was
caused by the high ochratoxin A doses given (75% of the LD50
value). Ochratoxin A injected intravenously as a single dose (4.1
mg/kg) in albumin-deficient and normal rats was excreted in the
bile and urine 20-70 times faster in the albumin-deficient rats
than in normal rats, indicating that the binding of ochratoxin A to
blood albumin delays the excretion of the compound through the
liver and kidney (Kumagai, 1985).
In rats given unlabelled ochratoxin A orally, Storen et al.
(1982) found 6% ochratoxin A, 1.5% (4R)-4-hydroxyochratoxin A, and
25-27% ochratoxin alpha in the urine; 12% ochratoxin A and 9%
ochratoxin alpha were found in the faeces.
The excretion of ochratoxin A in the milk was studied in
rabbits intravenously injected with 1-4 mg/kg body weight, as
single dose (Galtier et al., 1977a). At the highest dose injected,
the milk contained 1 mg ochratoxin A/litre; ochratoxin alpha and
4-hydroxy-ochratoxin A were not detected. Goats were given a
single dose of tritium-labelled ochratoxin A (0.5 mg/kg) and the
cumulative excretion (in terms of radioactivity) after 7 days
amounted to 53% in the faeces, 38% in the urine, 6% in the milk,
and 2% in the serum (Nip & Chu, 1979). Only a small fraction of
the radioactivity in milk was ochratoxin A, amounting to 0.026% of
the total ochratoxin A given.
In the Federal Republic of Germany, a study of human milk
obtained from women in two hospitals (patient category not stated)
revealed that 4 out of 36 samples (11.1%) contained ochratoxin A,
with a mean value of 0.024 ng/ml and a range of 0.017-0.030 ng/ml
(Bauer & Gareis, 1987; Gareis et al., 1988).
In mice given 14C-labelled ochratoxin A intravenously at
various stages of pregnancy, the toxin was shown to cross the
placental barrier on day 9 of pregnancy, at which time it is most
effective in producing fetal malformations (Appelgren & Arora,
1983). The highest toxin concentration was found in the bile,
which contained 5 times as much as the blood.
Ochratoxin A has been detected in the urine of bacon pigs
suffering from nephropathy (Krogh, unpublished information
communicated to the Task Group).
In a study on the disappearance rates for various tissues,
female bacon pigs were fed ochratoxin A at a level of 1 mg/kg feed
for one month and then kept on a toxin-free diet for another month,
during which animals were sacrificed at regular intervals (Krogh et
al., 1976a). Ochratoxin A disappeared exponentially (Table 7) from
the 4 tissues investigated (kidney, liver, muscle, and adipose
tissue) with residual life values (RL50)a in the range of 3.5-4.5
days; the toxin could still be detected in the kidneys one month
after termination of exposure.
Table 7. The rate of disappearance of ochratoxin A residues
from pig tissues after termination of a one-month exposure to
ochratoxin A at 1 mg/kg feeda
--------------------------------------------------------------
Tissue Ochratoxin A (µg/kg tissue) at time t (days)
after termination of exposure
---------------------------------------------------------------
kidney 28.22 exp (-0.1522 t)
liver 19.49 exp (-0.1598 t)
muscle 12.94 exp (-0.2096 t)
adipose 4.62 exp (-0.0565 t)
---------------------------------------------------------------
a From: Krogh et al. (1976a).
-------------------------------------------------------------------
a RL50 = half residual life, calculated from the exponential
equations shown in Table 7.
No data are available on ochratoxin levels in human urine or
faeces.
When the level in the serum is known, the ochratoxin A residues
in the four other tissues can be calculated (Table 5).
I.4 EFFECTS ON ANIMALS
I.4.1 Field observations
I.4.1.1 Pigs
The effects of ochratoxins on animals have been reviewed by
Krogh (1976a, 1978). Cases of mycotoxic porcine nephropathy have
been regularly encountered in studies in Denmark since the disease
was first discovered 50 years ago (Larsen, 1928). The disease is
endemic in all areas of the country, though unevenly distributed.
Prevalence rates in 1971 varied from 0.6 to 65.9 cases per 10 000
pigs, and epidemics encountered in 1963 and 1971 were associated
with a high moisture content in the grain caused by unusual
climatic conditions (Krogh, 1976b). On the basis of these studies,
Krogh (1978) concluded that ochratoxin A is the substance most
frequently associated with porcine nephropathy, though other
factors, such as citrinin, may also be involved.
A survey on porcine nephropathy was conducted at six
slaughterhouses in Sweden during the spring months of 1978
(Josefsson, 1979). A prevalence rate of 4.4 cases per 10 000 pigs
was encountered corresponding to the endemic level of prevalence
rates in Denmark; 26.7% of nephropathic kidneys contained residues
of ochratoxin A. In Hungary, an epidemiological study on porcine
nephropathy and the association with ochratoxin A was conducted in
1980-81 covering 4 areas in the country (Sandor et al., 1982). A
prevalence rate of 2.0 cases per 10 000 pigs was measured,
comparable to endemic prevalence rates in Scandinavia; 39% of
nephropathic kidneys contained residues of ochratoxin A. The
morphological changes in the kidneys in cases of mycotoxic porcine
nephropathy were characterized by degeneration of the proximal
tubules followed by atrophy of the tubular epithelium, interstitial
fibrosis in the renal cortex, and hyalinization of some glomeruli
(Elling & Moller, 1973).
In Poland, surveys for porcine nephropathy in 1983 and 1984
revealed prevalence rates of 4.7-5.7 cases per 10 000 pigs, with 5-
55% of the nephropathic kidneys containing detectable residues of
ochratoxin A, apparently depending on the season of the year
(Golinski et al., 1984, 1985). Porcine nephropathy has been
encountered in the Federal Republic of Germany (Bauer et al., 1984)
and in Belgium (Rousseau & van Peteghem, 1989), with respectively,
21% and 18% of the affected kidneys containing residues of
ochratoxin A, in the range of 0.1-12 ng/g. In Canada, 1200 samples
of pig blood, collected at a slaughterhouse, were screened for
ochratoxin A using HPLC (Marquardt et al., 1988). Levels exceeding
10 ng/ml (11.3%) were found in 136 samples; detection was confirmed
by derivative formation and spectrometry. No kidney examination
was conducted, but the ochratoxin A concentrations detected in the
blood suggest that nephropathy might have been present in some of
the pigs.
I.4.1.2 Poultry
In a preliminary study in Denmark on chickens condemned by meat
inspectors because of renal lesions, 4 out of 14 birds (29%) were
found to have nephropathy associated with the ingestion of
ochratoxin A, as revealed by the presence of residues of ochratoxin
A in tissues (Elling et al., 1975). The renal lesions were
characterized by degeneration of proximal and distal tubules of
both reptilian and mammalian nephrons and interstitial fibrosis.
I.4.2 Experimental animal studies
I.4.2.1 Acute and chronic effects
The acute and chronic effects of ochratoxins on experimental
animals have been reviewed by Chu (1974a), Harwig (1974), and Krogh
(1976a). Different species vary in their susceptibility to acute
poisoning by ochratoxin A with LD50 values ranging from 3.4 to 30.3
mg/kg (Table 8). When ochratoxin A was administered orally to rats
and guinea-pigs, the female was more sensitive than the male. In
rats, the kidney is the target organ, but necrosis of periportal
cells in the liver has also been noted during studies on acute
effects (Purchase & Theron, 1968).
Table 8. Acute toxicity of ochratoxin A
-------------------------------------------------------------------
Animal LD50 (mg/kg Route of Reference
body weight) administration
-------------------------------------------------------------------
mouse 22 intraperitoneal Sansing et
(female) al. (1976)
rat (male) 30.3 oral Galtier et
al. (1974)
rat 21.4 oral Galtier et
(female) al. (1974)
rat (male) 28 oral Kanizawa et
al. (1977)
rat (male) 12.6 intraperitoneal Galtier et
al. (1974)
rat 14.3 intraperitoneal Galtier et
(female) al. (1974)
guinea-pig 9.1 oral Thacker &
(male) Carlton
(1977)
-------------------------------------------------------------------
Table 8 (contd.)
-------------------------------------------------------------------
Animal LD50 (mg/kg Route of Reference
body weight) administration
-------------------------------------------------------------------
guinea-pig 8.1 oral Thacker &
(female) Carlton
(1977)
white 3.4 oral Prior et
leghorn al. (1976)
turkey 5.9 oral Prior et
al. (1976)
Japanese 16.5 oral Prior et
quail al. (1976)
rainbow 4.7 intraperitoneal Doster et
trout al. (1972)
beagle dog 9 orala Szczech et
(male) (total dose) al. (1973a)
pig 6 oralb Szczech et
(female) (total dose) al. (1973b)
-------------------------------------------------------------------
a All 3 dogs, dosed daily with 3 mg/kg body weight, died within
3 days.
b Both pigs receiving 2 mg/kg daily were moribund and killed
within 3 days, and both pigs receiving 1 mg/kg daily were
moribund and killed within 6 days.
The lesions observed in field cases of mycotoxic nephropathy
have been reproduced by feeding diets containing levels of
ochratoxin A identical to those encountered in the naturally
contaminated products. In a study by Krogh et al. (1974a), 39 pigs
fed rations containing ochratoxin A, at levels ranging from 200 to
4000 µg/kg, developed nephropathy after 4 months at all levels of
exposure. Changes in renal function were characterized by
impairment of tubular function, indicated particularly by a
decrease in TmPAH/Cin,a and reduced ability to produce concentrated
urine. These functional changes corresponded well with the changes
in renal structure observed at all exposure levels, including
atrophy of the proximal tubules and interstitial cortical fibrosis.
Sclerosed glomeruli were also observed in the group receiving the
highest dose of ochratoxin A of 4000 µg/kg feed. Changes were not
seen in any other organs or tissues.
-------------------------------------------------------------------
a TmPAH = transport maximum for para-aminohippuric acid.
Cin = clearance of insulin.
Kidney damage, identical to naturally occurring porcine
nephropathy, was produced in another study by feeding pigs (9
animals) with crystalline ochratoxin A in amounts corresponding to
a feed level of 1 mg/kg for 3 months. Significant renal tubular
functional impairment as measured by a decrease in TmPAH Cin was
detected after only 5 weeks of ochratoxin exposure (Krogh et al.,
1976b). The study was continued for a 2-year period during which
the renal impairment aggravated slightly without reaching a state
of terminal renal failure (Krogh et al., 1979).
In 2 pigs and 9 rats dosed orally with ochratoxin A (400 and
250 µg/kg body weight, respectively) for 5 days, ochratoxin A was
detected in the epithelial cells of the proximal convoluted tubules
of the nephron of all animals. The method of detection was
immunofluorescence microscopy using an antibody against ochratoxin
A that had been formed in rabbits after injection of an albumin-
ochratoxin A conjugate (Elling, 1977).
Groups of 80 F 344/N rats of each sex were administered 0, 21,
70, or 210 µg ochratoxin A/kg in corn oil by gavage, 5 days per
week for 103 weeks (Boorman, 1988). The administration of
ochratoxin A to male and female rats caused a spectrum of
degenerative and proliferative changes in the kidney. The
predominant non-neoplastic lesion in treated rats was degeneration
of the renal tubular epithelium in the inner cortex and the outer
stripe of the outer medulla (nephropathy).
In four pigs given 0.8 mg ochratoxin A/kg body weight orally
for 5 consecutive days, the activity of catalase and CN-insensitive
palmitoyl-CoA-dependent NAD (nicotinamide adenine di-nucleotide) in
renal homogenates decreased, but that in hepatic homogenates did
not, suggesting peroxisomal changes. This was confirmed by
ultrastructural observations of peroxisomes in the proximal
tubules in kidneys of ochratoxin A-treated animals (Elling et
al., 1985).
When 11 SPF pigs and 23 beagle dogs were given high oral doses
of ochratoxin A corresponding to feed levels of more than 5-10
mg/kg (levels rarely found in nature), pathological effects, mainly
necrosis, were observed in the liver, intestine, spleen, lymphoid
tissue, leukocytes, and kidney (Szczech et al., 1973a,b,c). Three
groups of Wistar rats, each consisting of 15 animals, were exposed
to feed levels of ochratoxin A ranging from 0.2 to 5 mg/kg for 3
months. Renal damage in the form of tubular degeneration was
observed at all dose levels (Munro et al., 1974). A decrease in
urinary osmolality, glycosuria, and proteinuria were observed in
an unstated number of Sprague-Dawley and Wistar rats administered
daily doses intraperitoneally of 0.75-2 mg ochratoxin A/kg body
weight (Berndt & Hayes, 1979).
In a study of coagulation factors, rats were given daily oral
doses of 4 mg/kg body weight over 4-10 days, resulting in decreases
in plasma-fibrinogen levels, at levels of factors II, VII, and X,
and in the platelet and megakaryocyte counts (Galtier et al.,
1979).
In 4 calves fed rations containing 0.1-2 mg ochratoxin A/kg
body weight for 30 days, the only signs observed were polyuria,
increased levels of glutamic oxalacetic transaminase (GOT) in
serum, and mild enteritis (Pier et al., 1976); there was mild
tubular degeneration in the kidneys. Cows given ochratoxin A
orally for 4 days at doses ranging from 0.2 to 1.66 mg/kg body
weight remained clinically normal (Ribelin et al., 1978). A cow
given a single dose of 13.3 mg/kg body weight (corresponding to 865
mg/kg feed) developed diarrhoea, anorexia, and cessation of milk
production, one day after.
Avian nephropathy, similar to that in spontaneously occurring
cases, developed in Leghorn hens (27 birds per group) exposed to
dietary levels of 0.3 or 1 mg ochratoxin A/kg for one year (Krogh
et al., 1976c). The renal changes included degeneration of the
tubular epithelium, mainly confined to the proximal and distal
tubules of both reptilian and mammalian nephrons; impairment of
glomerular and tubular function was also observed. "Acute
nephrosis" and "visceral gout" were observed in chickens exposed to
high levels of ochratoxin A (LD50 values) (Peckham et al., 1971).
The same authors reported that ochratoxin B, the other naturally
occurring ochratoxin, was not highly toxic for chickens (LD50, 54
mg/kg) or other animals.
Groups of chicks (40 birds per group) were fed diets containing
ochratoxin A levels in the range of 0-8 mg/kg for 3 weeks (Huff et
al., 1974). The group receiving the highest dose (8 mg/kg feed)
showed decreased packed blood cell volume, haemoglobin
concentration, and serum-iron and serum-transferrin saturation
percentages. In a similar study, Chang et al.(1979) observed that
lymphocytopenia developed at all dose levels. In the same study,
decreased bone strength, as measured physically by resistance to
fracturation, was observed at feed levels of 2-4 mg ochratoxin A/kg
(Huff et al., 1980). When groups of chicks were fed ochratoxin A
at levels of 0, 2, or 4 mg/kg for 20 days, concentrations of serum-
immunoglobulins (IgA, IgG, IgM) were reduced to 57-66% of normal
values in the toxin-exposed groups (Dwivedi & Burns, 1984).
In B6C3F1 female mice, groups of 6-7 animals were administered
a total of 0, 20, 40, or 80 mg ochratoxin A/kg body weight ip on
alternate days over an 8-day period. A dose-related decrease in
thymic mass was observed as well as myelotoxicity, indicated by
bone marrow hypocellularity, due to decreased marrow pluripotent
stem cells, and granulocyte-macrophage progenitors (Boorman et al.,
1984).
When determining the LD50 in mice following intraperitoneal
injection, synergistic effects were observed when ochratoxin A was
combined with citrinin as well as with penicillic acid (Sansing et
al., 1976). An additive effect was observed between ochratoxin A
and citrinin in terms of embryotoxicity in chicken embryos (Vesela
et al., 1983). In beagle dogs, when combined oral doses of
ochratoxin A (0.1-0.2 mg/kg body weight) and citrinin (5-10 mg/kg)
were injected intraperitoneally, synergism was observed with regard
to severity of clinical disease and mortality (Kitchen et al.,
1977a,b). Increased toxicity in terms of LD50 values and
pathological changes was observed in rats, when the ochratoxin A
was given orally combined with either of the drugs biscoumacetate
or phenylbutazone, apparently because of displacement of the toxin
from binding sites on plasma-proteins (Galtier et al., 1980). The
toxic effects of ochratoxin A on the renal epithelial cells of the
monkey were demonstrated in in vitro studies in the form of
abnormal mitotic cells (Steyn et al., 1975).
I.4.2.2 Teratogenicity
Intraperitoneal injection of pregnant mice with ochratoxin A at
5 mg/kg body weight on one of gestation days 7-12 resulted in
increased prenatal mortality, decreased fetal weight, and various
fetal malformations, including exencephaly and anomalies of the
eyes, face, digits, and tail (Hayes et al., 1974). When a
combination of ochratoxin A (2 or 4 mg/kg body weight) and T-2
toxin (0.5 mg/kg body weight) was injected intraperitoneally in
mice on gestation day 8 or 10, ochratoxin A exacerbated the
incidence of T-2-induced gross malformations (tail and limb
anomalies); increased fetocidal effects were also noted (Hood et
al., 1978). Mice were given 3-5 mg ochratoxin A/kg body weight
intraperitoneally or orally on gestation days 8, 9, and 10 or 15,
16, and 17 (Szczech & Hood, 1981). Cerebral necrosis was found in
most fetuses from dams treated on days 15-17, but no cerebral
necrosis developed after treatment on days 8-10, when ochratoxin A
is overtly teratogenic. Pups of mice given 1.25 and 2.25 mg
ochratoxin A/kg orally on gestation days 15, 16, and 17 were tested
for surface righting, swimming, and pivoting (Poppe et al., 1983).
The results of all 3 tests indicated that a delay in development
had occurred; no dose-related pathological alterations were found.
Pregnant mice were administered 1-2 mg ochratoxin A/kg body
weight, and/or 5-20 mg zearalenone/kg body weight or 0.125 mg
diethylstilboestrol/kg body weight, orally, on day 9 of pregnancy,
either individually or in combination, and the offspring were
examined on day 19 (Arora et al., 1983). Teratogenic effects
produced by ochratoxin A, such as exencephaly, open eyelids, and
microphthalmia, were reduced or absent when the toxin was given in
combination with one of the 2 non-steroidal estrogenic substances,
zearalenone or diethylstilboestrol.
Rats were treated orally with ochratoxin A at 0.25, 0.50, 0.75,
1, 2, 4, or 8 mg/kg body weight on gestation days 6-15 (Brown et
al., 1976). Maternal toxicity was not observed below 4 mg/kg body
weight, but an increased incidence of fetal resorptions was
observed from 0.75 mg/kg body weight. All fetuses from dams given
0.25-0.75 mg/kg body weight weighed significantly less than control
fetuses, and fetuses from dams given 0.75 or 1 mg/kg body weight
were stunted. Reduced litters and decreased fetal weight were
observed in rats administered 5 mg ochratoxin A/kg body weight,
orally, on gestation day 8 (Moré et al., 1978).
Subcutaneous administration of ochratoxin A to rats (1.75 mg/kg
body weight) on gestation days 5-7 resulted in the highest number
of malformations, including hydrocephaly, omphalocele, and
anophthalmia as well as a shift in position of the oesophagus
(Mayura et al., 1982); lower doses (0.5 and 1 mg ochratoxin A/kg
body weight) did not have any teratogenic effects, and higher doses
(5 mg/kg) caused all fetuses to be resorbed. In a subsequent study
by the same authors (Mayura et al., 1983), using the same
ochratoxin A exposure conditions, it was shown that a diet low in
protein (10% of protein concentration in normal rat feed) enhanced
the teratogenic action of ochratoxin A in the rat. The combined
action of ochratoxin A exposure and a low protein diet also
resulted in decreases in mating and fertilization rates (22% and
39%, respectively) compared with the control group.
When rats were exposed to ochratoxin A and citrinin (another
nephrotoxic mycotoxin produced by species of the Aspergillus and
Penicillium genera) either singly or combined, enhanced teratogenic
effects were observed in terms of gross malformations, visceral
anomalies, and skeletal defects following combined oral
administration of 1 mg ochratoxin A/kg body weight and 30 mg
citrinin/kg body weight (Mayura et al., 1984). Maternal deaths
(22-40%) occurred after the administration of the combined dose on
days 5, 6, 7, and 14 of gestation, whereas administration of
individual toxins did not cause any maternal deaths and only
minimal malformations no matter which gestation day they were
administered.
Increased prenatal mortality and malformations, including
hydrocephaly, micrognathia, and heart defects, were observed in
hamsters injected intraperitoneally with ochratoxin A at doses of
5-20 mg/kg body weight on one of gestation days 7-9 (Hood et al.,
1976).
I.4.2.3 Mutagenicity
Ochratoxin A did not have any effects in a Bacillus subtilis
Rec- assay, measuring DNA damage when tested at 20 and 100 µg/plate
(Ueno & Kubota, 1976). Ochratoxin A was not mutagenic to
Salmonella typhimurium TA 1535, TA 1537, TA 1538, TA 98, or TA 100
at doses of up to 500 µg/plate, with or without exogenous metabolic
activation (Kuczuk et al., 1978; Wehner et al., 1978b). No
increase was observed in genetic changes at the ade 2 locus of
Saccharomyces cerevisiae after treatment with 50 or 100 µg/plate
ochratoxin A, with or without exogenous metabolic activation
(Kuczuk et al., 1978). Ochratoxin A did not induce mutations to
8-azaguanine resistance in C3H mouse mammary carcinoma cells (FM3A)
treated with doses of 5 or 10 µg/litre (Umeda et al., 1977).
When rats were orally exposed to ochratoxin A every 48 h for 12
weeks (corresponding to a feed level of 4 mg/kg), single-strand
breaks of DNA in renal and hepatic tissue (the only tissues
investigated) were more pronounced than those in control animals
(Kane et al., 1986a,b).
Contradictory results have been obtained by testing ochratoxin
A in the Salmonella assay (SOS chromo test without metabolic
activation) (Ueno et al., in press).
I.4.2.4 Carcinogenicity
Kanizawa & Suzuki (1978) have indicated that ochratoxin A is a
hepatic and renal carcinogen in male mice. A group of 10 male ddY
mice were fed a diet containing 40 mg ochratoxin A/kg for 44 weeks.
A group of 10 untreated controls were fed the basal diet. All
survivors were killed after 49 weeks. Hepatic cell tumours were
found in 5 out of 9 treated mice; no tumours were found in the 10
controls. Solid renal cell tumours were found in 2 out of 9
treated mice and none in the 10 controls. Cystic renal adenomas
were found in 9 out of 10 treated mice compared with none in the 10
controls.
Two groups of 50 male and 2 groups of 50 female B6C3F1 mice
were fed diets containing 1 or 40 mg ochratoxin A/kg, respectively;
one group (control) was fed the basal diet. All survivors were
killed after 24 months. Eleven out of 49 male mice in the 40 mg/kg
group had renal carcinomas; 24 out of 49 male mice in the 40 mg/kg
groups showed renal adenomas. All male mice in the 40 mg/kg group
had microscopic evidence of nephropathy. A few females in the 40
mg/kg group exhibited nephropathic changes but no carcinomas or
adenomas. Compound-related lesions were absent in the controls
and the 1 mg/kg groups (Bendele et al., 1985). In a test for the
development of hyperplastic liver nodules in rats, ochratoxin A was
characterized as having both an initiating and a promoting activity
(Imaida et al., 1982).
Seven groups of 16 male ddY mice each were fed a diet
containing 50 mg ochratoxin A/kg for various periods ranging from 0
to 30 weeks followed by feeding of the basal diet until the end of
70 weeks (Kanizawa, 1984). After 15 weeks of ochratoxin A
exposure, 3 out of 15 animals had renal cell tumours; after 20
weeks, 1 out of 14 mice had renal cell tumours and 2 had hepatomas;
after 25 weeks of exposure, 2 out of 15 mice had renal cell tumours
and 5 had hepatomas; and after 30 weeks of exposure, 4 out of 17
mice had renal cell tumours and 6 had hepatomas; these tumours were
not observed in the controls. The nature of the renal cell tumours
was not further defined, but such tumours are mostly malignant. In
addition, pulmonary tumours were found in all groups, including the
controls, the incidence ranging from 20 to 73%. In a second study,
the effects of a combination of ochratoxin A and citrinin were
elucidated. Groups of 20 male ddY mice were fed diets containing
25 mg ochratoxin A/kg in combination with 100 or 200 mg
citrinin/kg for 70 weeks. Control groups were fed diets that did
not contain any toxins or the individual toxins at the
concentrations indicated above. In addition, one group was fed 25
mg ochratoxin A/kg feed for the first 25 weeks followed by 200 mg
citrinin/kg feed for the remaining period of time; another group
was exposed to the 2 toxins in the reverse order. Exposure to
citrinin alone did not produce any tumours. Exposure to ochratoxin
A alone resulted in renal cell tumours in 6 out of 20 mice, and in
hepatomas in 8 mice. Exposure to ochratoxin A and 100 mg
citrinin/kg feed did not result in any renal cell tumours, but 10
out of 19 mice had hepatomas. Exposure to ochratoxin A and 200 mg
citrinin/kg feed resulted in renal cell tumours in 10 out of 18
mice, and in hepatomas in 7 mice. Exposure to one toxin followed
by the other toxin did not produce any renal cell tumours, but
hepatomas were observed in less than 20% of the mice.
On the basis of these studies, IARC concluded that there was
limited evidence of carcinogenicity for animals, and inadequate
evidence of carcinogenicity for human beings (IARC, 1987a).
Groups of 80 F 344/N rats of each sex were administered 0, 21,
70 or 210 µg ochratoxin A/kg in corn oil by gavage, 5 days per
week, for 103 weeks (Boorman, 1988). In the male rats, renal
carcinomas were found in 16 out of 51 animals dosed with 70 µg/kg
and in 30 out of 50 animals dosed with 210 µg/kg; no carcinomas
were found in lower dose groups. In the female rats, renal
carcinomas were less common, as 1 out of 50 animals dosed with 70
µg/kg and 3 out of 50 animals dosed with 210 µg/kg had carcinomas;
no carcinomas were found in the lower dose groups. Renal adenomas
were found in all groups of male rats, with increasing frequencies
associated with increased doses. In the female groups, renal
adenomas were only found in the two highest dose groups. In the
female rats, fibroadenomas in the mammary gland were found in 45-
56% in the treated groups, a significantly higher percentage than
in the control group.
I.4.2.5 Biochemical effects and mode of action
Ochratoxin A is an inhibitor of tRNA synthetase and protein
synthesis in several microorganisms ( Bacillus subtilis, B.
stearothermophilus, Streptococcus faecalis, yeasts) as well as in
rat hepatoma cells (Konrad & Roschenthaler, 1977; Bunge et al.,
1978; Heller & Roschenthaler, 1978; Creppy et al., 1979a,b). The
competitive inhibitor effect of ochratoxin A on tRNA synthetase and
protein synthesis in rat hepatoma cells can be prevented by the
addition of phenylalanine in the cell culture medium at a molar
ratio of phenylalanine: ochratoxin A of 1.7:1 (Creppy et al.,
1979b). This observation suggested the possibility of preventive
measures for ochratoxin A-induced disease. Thus, the acute
intraperitoneal effect of ochratoxin A (LD100) in mice was
prevented by concomitant injection of phenylalanine (Creppy et al.,
1980; Moroi et al., 1985).
However, in the study on the reproduction of porcine
nephropathy previously mentioned (Krogh et al., 1974a), the molar
ratio of phenylalanine: ochratoxin A in the feed exceeded 4600:1,
implying that, in the field situation, phenylalanine does not
prevent ochratoxin A from inducing the development of nephropathy.
Ochratoxin A in the concentration range studied (20-1667 µmol/
litre) caused a 47-50% inhibition of macrophage migration (Klinkert
et al., 1981); this effect could be prevented by the simultaneous
addition of phenylalanine. In BALB/c mice, a dose of ochratoxin A
as low as 0.005 µg/kg body weight was able to suppress the immune
response to sheep erythrocytes (Haubeck et al., 1981); the effect
could be prevented by the simultaneous addition of phenylalanine.
Studying the same effect in Swiss mice, Prior & Sisodia (1982) were
unable to show any suppression of the immune response to sheep
erythrocytes, even after daily injection of 5 mg ochratoxin A/kg
for 50 days. (4R)-4-Hydroxyochratoxin A at a dose of 1 µg/kg body
weight caused an 80% reduction in the number of cells producing IgM
and a 93% reduction in cells producing IgG in BALB/c mice compared
with 90% and 92%, respectively, for ochratoxin A (Creppy et al.,
1983).
Female B6C3F1 mice (6 per group) were administered ochratoxin A
in amounts of 0.34, 6.7, or 13.4 mg/kg body weight or ochratoxin B
(13.4 mg/kg body weight) 6 times during 12 days. Ochratoxin A
inhibited the natural killer cell activity at all dose levels, and
increased the growth of transplantable tumour cells without
affecting T-cell or macrophage-mediated anti-tumour activity
(Luster et al., 1987). Ochratoxin B did not influence immune
function. The inhibition by ochratoxin A of natural killer cell
activity appeared to be caused by reduced production of basal
interferon.
Ochratoxin A affects the carbohydrate metabolism in rats.
Thus, a single oral dose of ochratoxin A at 15 mg/kg body weight
caused a decrease in the glycogen level in the liver and an
increase in the heart glycogen level, 4 h later (Suzuki & Satoh,
1973).
In a more extensive study on rats, the decrease in liver
glycogen level, 4 h after a single oral dose of ochratoxin A at 15
mg/kg body weight, was associated with an increase in serum glucose
levels and a decrease in liver glucose-6-phosphate (Suzuki et al.,
1975). At the same time, the liver glycogen synthetase (EC
2.7.1.37) activity decreased and the liver phosphorylase (EC
2.4.1.1) activity increased. Three daily oral doses of ochratoxin
A at 5 mg/kg body weight caused a decrease in the liver glycogen
concentration, which was measured on the fourth day. The decrease
was attributed to inhibition of the active transport of glucose
into the liver, suppression of glycogen synthesis from glucose, and
acceleration of glycogen decomposition.
During in vitro studies on rat liver mitochondria, it was
observed that ochratoxin A inhibited the respiration of whole
mitochondria by acting as a competitive inhibitor of transport
carrier proteins located in the inner mitochondrial membrane
(Meisner & Chan, 1974). Further studies with mitochondrial
preparations revealed that the mitochondrial uptake of ochratoxin A
was an energy-using process that resulted in depletion of
intramitochondrial adenosine triphosphate (ATP), and that
ochratoxin A inhibited intramitochondrial phosphate transport
resulting in deterioration of the mitochondria (Meisner, 1976).
This might explain the degeneration of liver mitochondria observed
by Purchase & Theron (1968) in rats exposed orally to a single dose
of ochratoxin A at 10 mg/kg body weight. These authors observed
accumulation of glycogen in the cytoplasm of the rat liver cells
microscopically. This was in contrast to the previously discussed
observations of Suzuki et al. (1975), who found a decrease in
glycogen levels.
In a study on mice, Sansing et al. (1976) found that ochratoxin
A, administered intraperitoneally at 6 mg/kg body weight, inhibited
orotic acid incorporation into both liver and kidney RNA, 6 h after
toxin injection. In this respect, ochratoxin A acted synergistically
with another nephrotoxic mycotoxin, citrinin.
When neonatal rats were exposed orally to a single dose of 1 mg
ochratoxin A/kg or of 25 mg citrinin/kg or both doses within 24 h
of birth, a synergistic effect of the 2 mycotoxins was observed on
cytochrome P-450, NADPH-dependent dehydrogenase, and NADPH-
cytochrome c reductase (Siraj et al., 1981).
In rats fed 2 mg ochratoxin A/kg body weight per day for 2
days, renal gluconeogenesis from pyruvate was decreased by 26%, and
renal phosphoenol-pyruvate carboxy kinase (PEPCK) (EC 4.1.1.32)
activity was reduced by 55%, whereas hepatic PEPCK was unchanged
(Meisner & Selanik, 1979). In a subsequent study on rats, it was
found that even lower dose levels (0.3-0.5 mg ochratoxin A/kg body
weight) caused a 50% reduction in PEPCK activity (Meisner &
Meisner, 1981). A number of other enzymes located in the proximal
tubule of the nephron were unaffected. When longer exposure
periods (8-12 weeks) were used, and 145 ng ochratoxin A/kg body
weight were administered orally to groups of 3 rats each, increased
urinary excretion and corresponding renal tubular depletion of the
following enzymes were observed: gamma-glutamyl transferase,
alkaline phosphatase, leucine aminopeptidase, lactate
dehydrogenase, and N-acetyl-beta- D-glucosaminidase (Kane et al.,
1986a). Renal PEPCK in pigs was also sensitive to ochratoxin A
with a feed level of 100 µg/kg causing a significant decrease in
PEPCK activity (Meisner & Krogh, 1982).
In a study on pigs fed ochratoxin A at 0, 0.2, or 1 mg/kg for 5
weeks, enzyme activities were measured in renal biopsies, collected
1, 3, and 5 weeks after initiation (Krogh et al., 1988). After one
week, the activities of renal PEPCK and gamma-glutamyltranspeptidase
were decreased by 40%. The dose-related decrease in the activity
of PEPCK and gamma-glutamyltranspeptidase was accompanied by a
dose-related increase in renal impairment, as measured by the
reduction of TmPAH/Cin, suggesting that these enzymes are sensitive
indicators of ochratoxin-induced porcine nephropathy. Thus, the
renal biopsy-based measurements of enzyme activities might prove
diagnostically useful in ochratoxin-induced disease in human
beings.
Ochratoxin A reduced the total renal mRNA concentration in male
Sprague-Dawley rats and certain mRNA species, notably PEPCK, were
reduced to a greater extent than the bulk of the RNA pool (Meisner
et al., 1983).
I.5 EFFECTS ON MAN
I.5.1 Ochratoxin A, Balkan endemic nephropathy, and tumours of the
urinary system
This topic has been reviewed by Krogh (1979, 1983) who called
attention to the striking similarities in the changes in renal
structure and function induced experimentally in animals by the
administration of ochratoxin A, and the clinical and pathological
features of a localized endemic disease known as Balkan endemic
nephropathy. So far, the disease has been observed only in rural
populations of Bulgaria, Romania, and Yugoslavia, but information
on the present magnitude of the problem was not available to the
Task Group.
The Balkan endemic nephropathy is a chronic disease that
predominantly affects women and progresses slowly up to death
(Hrabar et al., 1976, Chernozemsky et al., 1977). Age-specific
incidence rates are highest above the age of 40. Younger cases
occur in the 10-19-year-old age group, and the mean age of new
patients is in the early 50s (Stoyanov et al., 1978).
The disease is characterized by an extreme geographical
clustering with a tendency for familial aggregation of cases
(Nicolov et al., 1978). However, sporadic cases occur outside
endemic areas. Age-adjusted incidence rates of 555 per 100 000
population in females and 322 in males over a ten-year period have
been recorded from a population sample of 147 000 in an endemic
area of Bulgaria (Stoyanov et al., 1978). In one of several
endemic regions in Yugoslavia, the prevalence varied from 3% to 8%
(Hrabar et al., 1976).
Autopsy has shown that kidneys are notably reduced in size.
The histological lesions are interstitial fibrosis, tubular
degeneration, and hyalization of glomeruli in the more superficial
part of the cortex (Heptinstall, 1974). Impairment of tubular
function, indicated by a decrease in TmPAH, is a prominent and
early sign (Dotchev, 1973).
A high incidence of tumours of the urinary system is strongly
correlated with the prevalence of Balkan endemic nephropathy
(Ceovic et al., 1976; Chernozemsky et al., 1977; Nicolov et al.,
1978). In one instance in Bulgaria, 46.6% of patients with tumours
of the urinary system were also affected by endemic nephropathy.
Among the tumours of the urinary system, cancers of the renal
pelvis and ureters are more frequently associated with endemic
nephropathy than urinary bladder tumours.
The relative risk for developing cancer of the renal pelvis and
ureters is 88:1 in patients with nephropathy compared with controls
in non-endemic areas. The relative risk for the development of any
tumour of the urinary system is only 28:1 in the same sample
(Stoyanov et al., 1978).
Over the past 2 decades, investigations have been carried out
to verify a variety of etiological assumptions with unconvincing
results (review by Puchlev, 1973, 1974). However, the assumption
of the possible role of ochratoxin A, on the basis of similarities
with the animal disease, has received increasing epidemiological
support.
In Yugoslavia, surveys indicated that the contamination of
foodstuffs (grains, maize, pork meat) with ochratoxin A occurred in
12.8% of samples in an area where the prevalence of endemic
nephropathy was 7.3%, compared with only 1.6% of contaminated
samples in areas free of the disease (Krogh et al., 1977). The
concentration of ochratoxin A in maize was 5-90 µg/kg and that in
pork meat, 5 µg/kg (Krogh et al., 1977) with levels of up to 27
µg/kg in pig kidneys (Pepeljnjak et al., 1982). Similarly, studies
in Bulgaria have revealed that 16.7% of beans and 27.3% of maize
from an endemic area were contaminated with ochratoxin A compared
with 7.1% and 9%, respectively, from a non-endemic area (Petkova-
Bocharova & Castegnaro, 1985).
In a subsequent survey of home-produced foodstuffs (cereal and
bread) from the same endemic area in Yugoslavia over a 5-year
period, a mean contamination of 8.7% was found with pronounced
annual variations, which probably reflect climatic conditions
during the crop harvesting periods (Pavlovic et al., 1979).
Surveys on the presence of ochratoxin A in blood samples are
difficult to compare, as different analytical methods have been
used with different levels of sensitivity. Prevalences of 16.6%
and 5.9% with a one-year interval were reported in the same endemic
village in Yugoslavia (Hult et al., 1982). It is not possible to
determine whether this difference reflects annual variations in the
content of the blood, or the result of a less sensitive analytical
method in the second instance. However, higher prevalences of
ochratoxin A and higher concentrations in blood are generally
present in people from endemic areas, especially in persons
suffering from Balkan nephropathy.
A survey in Yugoslavia reported a prevalence in the blood of
16.6% in an endemic village and 6% in a non-affected one (Hult et
al., 1982). In Bulgaria, reported rates were 17.7% in an endemic
area and 7.7% in a non-endemic one (Petkova-Bocharova et al.,
1988). Table 9 illustrates the trend towards higher concentrations
in endemic situations and in patients. The similarity of results
in healthy families from affected villages and healthy persons from
unaffected villages (groups III and IV) suggests than an
environmental or a behavioural determinant plays a role at the
household level.
Thus, available epidemiological information seems to indicate
that Balkan endemic nephropathy is associated with consumption
patterns involving foodstuffs contaminated with ochratoxin A and
with a higher frequency of positive blood samples of ochratoxin A.
However, the association does not permit the establishment of a
causal relationship. Cross sectional surveys, such as those
reported in the literature so far, are probably not the appropriate
means to determine this relationship.
The results of experimental animal studies suggest that Balkan
nephropathy, a chronic condition, may require a long latency period
between exposure and the onset of symptoms, or more likely a
prolonged exposure or repeated exposure over a long period of time.
Investigations based on individual exposure time sequences
together with follow-up cohort surveys could provide a clue to the
causal role of ochratoxin A in Balkan endemic nephropathy. In view
of the lack of this information, such a causal relationship cannot
be established or rejected.
Table 9. Ochratoxin A in blood samples from people in endemic and
non-endemic areas in Bulgariaa
-------------------------------------------------------------------
Groupb No. of No. of Mean concentration
persons ochratoxin A ± S.D. (µg/kg)
assayed positive cases
(%)
-------------------------------------------------------------------
I. Persons with 61 16 (26.3) 20.3 ± 9.7
UST and/or EN
II. Healthy 63 10 (15.8) 14.5 ± 7.6
persons from
families with UST
and/or EN cases
III. Healthy 63 7 (11.1) 12.5 ± 3.5
persons from
families in
endemic villages
IV. Healthy 60 7 (11.6) 15.0 ± 4.2
persons from
unaffected villages
in endemic areas
V. Healthy 65 5 (7.7) 10.0
persons from
villages in
non-endemic areas
-------------------------------------------------------------------
a From: Petkova-Bocharova et al. (1988).
b UST: urinary system tumours; EN: endemic nephropathy.
The differences between group I and groups III and IV are
statistically significant ( P <0.002). Difference between
groups I and V is statistically significant ( P <0.001).
I.6 EVALUATION OF THE HUMAN HEALTH RISKS
Human exposure, as demonstrated by the occurrence of ochratoxin
A in food and in the blood, has been observed in various countries
in Europe. The Task Group was not aware of attempts to detect
ochratoxin A in human blood in other parts of the world.
The causal role of ochratoxin A in porcine nephropathy has been
established, based on studies of field cases as well as
reproduction of the disease with ochratoxin A. Using the porcine
model, it has been postulated that Balkan endemic nephropathy may
result from exposure to ochratoxin A. Available epidemiological
information indicates that Balkan nephropathy may be associated
with the consumption of foodstuffs contaminated by this toxin.
Since the publication of Environmental Health Criteria 11, in 1979,
epidemiological studies on the concentration of ochratoxin A in
human blood in affected and non-affected areas, have provided
additional support for the relationship between Balkan nephropathy
and exposure to ochratoxin A.
It has been shown that both the prevalence of ochratoxin A in
the blood and the blood concentrations are higher in residents in
endemic areas. However, a direct causal relationship cannot be
established on the basis of indirect evidence provided by the above
retrospective studies alone. Neither can it be excluded in view of
the long latency period between the exposure and the onset of
symptoms.
Ochratoxin A has been demonstrated to be carcinogenic to the
renal tubular epithelium in male mice and both sexes of rats. A
highly significant relationship has been observed between Balkan
nephropathy and tumours of the urinary tract, particularly with
tumours of the renal pelvis and ureters. However, there are no
published data to establish a direct causal role of ochratoxin A in
the etiology of such tumours.
II. TRICHOTHECENES
II.1 PROPERTIES AND ANALYTICAL METHODS
II.1.1 Physical and chemical properties
The sesquiterpenoid trichothecenes possess the tetracyclic
12,13-epoxytrichothecene skeleton. A total of 148 trichothecenes,
83 non-macrocyclic and 65 macrocyclic, have been isolated from
fungal cultures and plants (Drove, 1988). They can be conveniently
divided into 4 categories according to similarity of functional
groups (Ueno, 1977). The first class is characterized by a
functional group other than a ketone at C-8 (type A). This is the
largest category containing members such as T-2 toxin and
diacetoxyscirpenol (DAS). The second category of trichothecenes
has a carbonyl function at C-8 (type B) typified by 4-deoxynivalenol
(DON) and nivalenol (NIV). The third category is characterized by
a second epoxide group at C-7,8 or C-9,10 (type C), and the fourth
contains a macrocyclic ring system between C-4 and C-15 with two
ester linkages (type D). The structures of representative
trichothecenes of each category are illustrated below.
Table 2. Natural occurrence of ochratoxin A in foodstuffs and animal feed of plant origin
---------------------------------------------------------------------------------------------------------
Commodity Country Number of Percentage Range of Reference
samples contaminated ochratoxin A
analysed levels (µg/kg)
---------------------------------------------------------------------------------------------------------
FOOD
maize USA 293 1.0 83-166 Shottwell et al. (1971)
maize (1973) France 463 2.6 15-200 Galtier et al. (1977b)
maize (1974) France 461 1.3 20-200 Galtier et al. (1977b)
wheat (red winter) USA 291 1.0 5-115 Shottwell et al. (1976)
wheat (red spring) USA 286 2.8 5-115 Shottwell et al. (1976)
barley (malt) Denmark 50 6.0 9-189 Krogh (1978)
barley USA 127 14.2 10-40 Nesheim (1971)
coffee beans USA 267 7.1 20-360 Levi et al. (1974)
maize Yugoslaviaa 542 8.3b 6-140 Pavlovic et al. (1979)
wheat Yugoslaviaa 130 8.5b 14-135 Pavlovic et al. (1979)
wheat bread Yugoslaviaa 32 18.8b Pavlovic et al. (1979)
barley Yugoslaviaa 64 12.5b 14-27 Pavlovic et al. (1979)
barley Czechoslovakia 48 2.1 3800 Vesela et al. (1978)
bread United Kingdomc 50 2.0 210 Osborne (1980)
flour United Kingdomc 7 28.5 490-2900 Osborne (1980)
beans Sweden 71 8.5 10-442 Akerstrand &
peas Sweden 72 2.8 10 Josefsson (1979)
maize United Kingdom 29 37.9 50-500 Ministry of
cornflour United Kingdom 13 30.8 50-200 Agriculture (1980)
soya bean United Kingdom 25 36.0 50-500 Ministry of
soya flour United Kingdom 21 19.0 50-500 Agriculture (1980)
cocoa beans (raw) United Kingdom 56 17.9 100-500 Ministry of
(roasted) United Kingdom 19 15.8 100 Agriculture (1980)
grain German Democratic 49 4.1 18-22 Fritz et al.
Republic (1979)
grain (barley, Poland 296 6.8 20-470 Szebiotko et al. (1981)
wheat, rye)
grain (wheat, rye) Denmark 151 1.3 15-50 Pedersen &
bran (wheat) Denmark 57 10.5 5-20 Hansen (1981)
maize Bulgariaa 22 27.3 25-35 Petkova-Bocharova &
maize Bulgaria 22 9.0 10-25 Castegnaro (1985)
beans Bulgariaa 24 16.7 25-27 Petkova-Bocharova &
beans Bulgariaa 28 7.1 25-50 Castegnaro (1985)
---------------------------------------------------------------------------------------------------------
Table 2 (contd.)
---------------------------------------------------------------------------------------------------------
Commodity Country Number of Percentage Range of Reference
samples contaminated ochratoxin A
analysed levels (µg/kg)
---------------------------------------------------------------------------------------------------------
FOOD (continued)
barley, wheat, Poland 150 5.3 50-200 Juszkiewicz &
oats, rye, maize Piskorska-
Pliszczynska (1976)
FEED
mixed feed Poland 203 4.9 10-50 Juszkiewicz &
Piskorska-
Pliszczynska (1977)
maize Yugoslavia 191 25.7 45-5125 Balzer et al. (1977)
barley, oats Sweden 84 8.3 16-409 Krogh et al. (1974b)
wheat, hay Canada 95 7.4 30-6000 Prior (1976)
wheat, oats, Canadac 32 56.3 30-27 000 Scott et al. (1972)
barley, rye
barley, oats Denmarkc 33 57.6 28-27 500 Krogh et al. (1973)
mixed feed Canada 474 1.1 30-100 Prior (1981)
mixed feed Canadac 51 7.8 48-5900 Abramson et al. (1983)
mixed feed Australia 25 4.0 70 000 Connole et al. (1981)
mouldy bread Italyd 1 80 000 Visconti &
Bottalico (1983)
---------------------------------------------------------------------------------------------------------
a From an area with endemic nephropathy.
b Average values for a period of 2-5 years.
c All samples suspected of containing mycotoxins.
d This sample contained in addition 9600 µg ochratoxin B/kg.
Ochratoxin B has only been found in 3 samples and, thus, occurs
extremely rarely; the other ochratoxins have never been found in
plant products. Levels of ochratoxin A and the frequency of
contamination are generally higher in animal feed than in foodstuffs
(Table 2). Although the mean level of ochratoxin A in all reported
surveys up to 1979 was 1035 µg/kg (Fig. 3), 83% of the samples
contained less than 200 µg/kg (Krogh, 1980).
I.2.2.2 Residues in food of animal origin
Residues of ochratoxin A are not generally found in ruminants,
because ochratoxin A is cleaved in the forestomachs by protozoan and
bacterial enzymes (Galtier & Alvinerie, 1976; Hult et al., 1976;
Patterson et al., 1981). The non-toxic cleavage product, ochratoxin
alpha, has been found in the kidneys at levels below 10 µg/kg and in
the blood of calves fed a diet containing 300-500 µg ochratoxin A/kg
(Patterson et al., 1981). In half of the calves, the kidneys
contained low levels of ochratoxin A (up to 5 µg/kg), a feature that
may reflect that the calves were not yet functioning as ruminants.
When 2 milking cows were fed a ration containing 317-1125 µg
ochratoxin A/kg for 11 weeks, a residue of 5 µg ochratoxin A/kg was
found in the kidneys of one of the animals but not in any other
tissue or the milk. Ochratoxin alpha was not found in any tissue
(Shreeve et al., 1979). Residues of ochratoxin A have been detected
in a number of tissues in single-stomach food animals, such as pigs.
The carry-over of ochratoxin A from feed to animal tissues was
elucidated in a study in which groups of pigs were exposed for 3-4
months to dietary levels of ochratoxin A of 200, 1000, or 4000 µg/kg
(Krogh et al., 1974a). At termination (slaughter), the highest
levels of ochratoxin A residues were found in the kidneys (mean
levels 50 µg/kg at the 4000 µg/kg feed level), with lower levels
in the liver, muscle, and adipose tissue; other tissues, including
blood, were not analysed. There was a high correlation between the
feed level of ochratoxin A and the residue levels in the 4 tissues
investigated (Table 3).
Table 3. Correlation between feed level and tissue levels
(residues) of ochratoxin A in pigsa
-----------------------------------------------------------
Tissue Regression equation r
-----------------------------------------------------------
kidney Y = 2.15 + 0.0123X 0.86
liver Y = 0.35 + 0.0095X 0.82
adipose Y = 2.51 + 0.0099X 0.78
-----------------------------------------------------------
a Modified from Krogh et al. (1974a).
X = ochratoxin A in feed (µg/kg).
Y = ochratoxin A residue (µg/kg tissue).
r = correlation coefficient.
The regression is calculated on feed levels of ochratoxin A
in the range of 200-4000 µg/kg.
Ochratoxin A is present in the blood bound to serum-albumin
(Chu, 1971; Galtier, 1974a) and as free ochratoxin A; saturation (in
the rat) occurs at 70 mg ochratoxin A/litre plasma. The binding of
ochratoxin A to serum-albumin is particularly strong in cattle,
pigs, and man, based on in vitro studies (Table 4).
Table 4. In vitro binding of ochratoxin A to
serum-albumin in several speciesa
-----------------------------------------------
Animal Number of Intrinsic
species binding Association
sites constant (M-1)
-----------------------------------------------
Cattle 0.58 94 600
Pig 0.56 71 100
Man 0.58 63 500
Horse 0.57 57 400
Chicken 0.51 52 700
Rat 0.68 40 100
Sheep 0.88 22 600
-----------------------------------------------
a Adapted from: Galtier (1979).
The presence of ochratoxin A in the blood of pigs has been
elucidated in experimental animal studies as well as by surveys of
blood samples from pigs at farms (Hult et al., 1979, 1980). A
total of 1200 pig blood samples, collected from slaughterhouses
over various periods during 1986 in western Canada, was screened
for the presence of ochratoxin A using an HPLC procedure (Marqhardt
et al., 1988). It was shown that 3.6-4.2% of the blood samples
contained the toxin at concentrations higher than 20 ng/ml. It
appears that the concentration of ochratoxin A in the blood is
higher than that in any other tissue. In feeding studies, bacon
pigs were exposed for various periods to rations containing
ochratoxin A concentrations in the range of 58-1878 µg/kg; blood,
kidney, liver, muscle, and adipose tissues were analysed, at
slaughter, for residues of ochratoxin A (Mortensen et al., 1983).
The statistical association between residue levels in various
tissues is indicated by the regression analyses (Table 5).
Table 5. Regression between residues of ochratoxin A
in the serum and certain other tissuesa
------------------------------------------------------
Tissue Regression r sb
equation
------------------------------------------------------
kidney Y = 0.0651X 0.89 0.002
muscle Y = 0.0346X 0.88 0.001
liver Y = 0.0259X 0.89 0.001
adipose Y = 0.0191X 0.84 0.001
------------------------------------------------------
a Adapted from: Mortensen et al. (1983).
X = µg ochratoxin A/litre serum.
Y = µg ochratoxin A/kg in the other tissues.
r = correlation coefficient
sb = Standard error of slope
Epidemiological studies on the basis of data from meat
inspection in Danish slaughterhouses revealed prevalence rates of
porcine nephropathy ranging from 10 to 80 cases per 100 000
slaughtered pigs (Krogh, 1976a). Surveys in a number of European
countries for residues of ochratoxin A in kidneys from cases of
porcine nephropathy revealed that 25-39% of the cases contained
ochratoxin A levels in the range 2-100 µg/kg (Table 6).
Table 6. Surveys for ochratoxin A residues in kidneys from
cases of porcine nephropathy, based on meat inspection data
-------------------------------------------------------------------
Country No. of porcine Percentage Range of Reference
nephropathy containing ochratoxin
kidneys residues of A residues
investigated ochratoxin (µg/kg wet
A weight)
-------------------------------------------------------------------
Denmark 60 35 2-68 Krogh (1977)
Germany, 104 21 0.1-1.8 Bauer et al.
Federal (1984)
Republic of
Hungary 122 39 2-100 Sandor et
al. (1982)
Poland 113 24 1-23 Golinski et
al. (1984)
Sweden 129 25 2-104 Rutqvist et
al. (1977)
Sweden 90 27 2-88 Josefsson
(1979)
-------------------------------------------------------------------
Ochratoxin A levels of up to 29 µg/kg were found in the muscle
of hens and chickens collected at one slaughterhouse (Elling et
al., 1975). The birds had been condemned because of nephropathy.
In another study, groups of hens were exposed for 1-2 years to
dietary levels of ochratoxin A of 0.3 or 1 mg/kg (Krogh et al.,
1976b). The kidneys contained the highest residues with a mean
value of 19 µg/kg tissue in the group fed 1 mg ochratoxin A/kg; the
liver and muscle contained lower levels of residues and no
ochratoxin A was found in the eggs. These results are in
accordance with those of subsequent experimental animal studies.
For example, in a study in which 4 groups of hens were fed diets
containing 0, 0.5, 1, or 4 mg ochratoxin A/kg, the kidneys
contained the highest level of ochratoxin A residues (31 µg/kg) in
the highest dose group; eggs were not analysed (Prior & Sisodia,
1978).
In another study, hens were fed 1 mg ochratoxin A/kg feed for 8
weeks; the kidneys contained 3-10 µg ochratoxin A/kg and the liver,
1.5-2.5 µg/kg. The eggs were not analysed (Reichmann et al.,
1982). When groups of hens were fed diets containing 2.5 or 10
mg/kg, the kidneys contained 1-6 µg ochratoxin A/kg, lower levels
being found in the plasma, muscle, and liver (Juszkiewicz et al.,
1982). Eggs from the high-dose group contained 0.7-1.3 µg
ochratoxin A/kg, but none was detected in eggs from the low-dose
group.
White Leghorn hens (54 birds), divided into four groups, were
fed diets for one month containing the following concentrations of
ochratoxin A (mg/kg): 0, 1.3, 2.6, and 5.2 (Bauer et al., 1988).
At termination, three tissues were analysed for residues. The
following ranges of dose-dependent residues were found: serum,
4.7-11.7 ng/ml, liver, 9.1-18.0 ng/g, and yolk, 1.6-4.0 ng/g; very
little ochratoxin A was found in the egg white, and none was
detected in the tissues of the control group.
I.3 METABOLISM
I.3.1 Absorption
In a study on rats exposed by gavage to a single dose of
ochratoxin A at 10 mg/kg body weight, Galtier (1974b) found the
highest tissue level of unchanged ochratoxin A in the stomach wall
during the first 4 h following administration. The small and large
intestine and caecum contained small amounts of unchanged
ochratoxin A, and it was concluded that ochratoxin A was absorbed
mainly in the stomach. Small amounts (1-3% of the total dose) were
detected in the caecum and the large intestine, as the isocoumarin
moiety (ochratoxin alpha), most likely as the result of the
hydrolysing action of the intestinal microflora (Galtier &
Alvinerie, 1976; Hult et al., 1976).
In a study on intestinal absorption using the same animal
species, Kumagai & Aibara (1982) came to the conclusion that the
site of maximal absorption of ochratoxin A was the proximal
jejunum, and that the portal vein was the primary route of
transport from the intestinal tract, though part of the
transportation took place through the lymphatics.
Using a highly specific antibody against ochratoxin A, which is
used in a peroxidase-antiperoxidase detection method, Lee et al.
(1984) studied absorption and tissue distribution in Swiss mice
over a 48-h period, after the administration of a single dose of 25
mg ochratoxin A/kg body weight. Ochratoxin A was found in large
amounts, indicated by staining, in epithelial cells as well as in
macrophages of the lamina propria in the duodenum. Smaller amounts
were found in jejunal epithelial cells and lamina propria, and much
smaller amounts in the epithelial cells in the esophagus and
stomach; no toxin was found in the ileum. These findings suggest
that absorption mainly takes place in the duodenum and the jejunum.
I.3.2 Tissue distribution
I.3.2.1 Animal studies
In slaughterhouse cases of mycotoxic porcine nephropathy
studied by Hald & Krogh (1972), residues of unchanged ochratoxin A
were found in all tissues investigated (kidney, liver, and muscle),
the highest level (up to 67 µg/kg) occurring in the kidney. In
experimental studies on pigs ingesting feed containing ochratoxin
A, residues of the toxin were found in all 4 tissues in the
decreasing order of kidney, liver, muscle, adipose tissue (Krogh et
al., 1974a). A subsequent study revealed that the concentration of
ochratoxin A residues in the blood of the pig was higher than those
in the other tissues mentioned above (Mortensen et al., 1983).
When rats were exposed orally to an ochratoxin A dose of 10 mg/kg
body weight, Galtier (1974b) recovered 0.3% of the administered
dose in the whole kidneys, 0.9% in the whole liver, and 0.6% in the
total muscle tissue, 96 h after exposure. Chang & Chu (1977),
using a single intraperitoneal injection of 1 mg ochratoxin A per
rat (labelled with 14C in phenylalanine), found that the kidney
contained twice as much unchanged ochratoxin A as the liver after
30 minutes, amounting to 4-5% of the total dose.
In the study by Lee et al. (1984), the largest amounts of
ochratoxin A found in the kidney (as indicated by staining
intensity) were in the epithelium of the proximal convoluted
tubules, and to a lesser extent in the distal convoluted tubules,
the descending loop of Henle, and glomeruli and Bowman's capsule.
Small amounts were found in hepatocytes as well as in the lumina of
bile ducts, but not in biliary epithelium, indicating biliary
excretion.
Using pig renal cortical slices, it was found that ochratoxin A
enters the proximal tubule cells by the common organic anion
transport system (Friis et al., 1988). Ochratoxin A inhibited p-
aminohippurate (PAH) and phenolsulfophthalein uptake in a dose-
dependent manner.
I.3.2.2 Studies on man
In a study in Yugoslavia near Slavonski Brod, where endemic
nephropathy is prevalent, 639 samples of serum from the inhabitants
of 2 villages were screened for the presence of ochratoxin A; 42
(6.6%) were positive for the toxin "with concentrations in the
range of 1-57 ng ochratoxin A/g" (Hult et al., 1982). Detection
was carried out using the enzymic spectrofluorometric procedure,
and positives were confirmed by the esterification of ochratoxin A
in serum and of ochratoxin alpha obtained from the enzymetic
hydrolisates; the esters were measured by high performance liquid
chromatography.
In a screening of serum samples in Poland using the same method
of analysis, 77 out of 1065 samples (7.2%) contained ochratoxin A,
with a mean concentration of 0.27 ng/ml and a maximum value of 40
ng/ml (Colinski, 1987). In the Federal Republic of Germany, 173
out of 306 serum samples (56.6%) contained ochratoxin A, as
measured by an HPLC procedure, with a mean of 0.6 ng/g and a range
of 0.1-14.4 ng/g (Bauer & Gareis, 1987). Three out of 46 kidneys
(6.6%) contained ochratoxin A, with a mean of 0.2 ng/g and a range
of 0.1-0.3 ng/g. In a study of blood plasma in Denmark, 46 out of
96 samples (47.9%) contained ochratoxin A, as measured by an HPLC
procedure, with a mean of 1.7 ng/g and a range of 0.1-9.2 ng/g
(Hald, 1989). In Bulgaria, where endemic nephropathy also occurs
in some areas, 45 out of 312 blood samples contained ochratoxin A
(14.4%), with a mean of approximately 14 ng/g (Petkova-Bocharova et
al., 1988).
I.3.3 Metabolic transformation
It has been shown from in vitro studies that ochratoxin A binds
to serum-albumin (Chu, 1971, 1974b); this binding has also been
observed in in vivo studies on rats (Galtier, 1974a; Chang & Chu,
1977). Ochratoxin alpha has been detected in the urine and faeces
of rats injected intraperitoneally with ochratoxin A (Nel &
Purchase, 1968; Chang & Chu, 1977), indicating the cleavage of
ochratoxin A to ochratoxin alpha and phenylalanine, under these
conditions.
Studies with 14C-labelled ochratoxin A indicated that some
other, not yet identified, metabolites are formed in the body.
Less than half of the radioactivity excreted in rat urine within 24
h of a single intraperitoneal injection of 14C-phenylalanine-
labelled ochratoxin A was identified as ochratoxin A (Chang & Chu,
1977).
In both albino rats and brown rats given ochratoxin A orally or
intraperitoneally, 1-1.5% of the dose was excreted as (4R)-4-
hydroxyochratoxin A and 25-27% as ochratoxin alpha in the urine
(Storen et al., 1982). In in vitro studies using liver microsomes
from the pig, rat, and man, both (4R)-and (4S)-4-hydroxy-ochratoxin
A were produced in a hydroxylation process involving cytochrome
P-450 (Stormer & Pedersen, 1980; Stormer et al., 1981).
4-Hydroxyochratoxin A is non-toxic for rats in amounts up to 40 mg/kg
body weight (Hutchison et al., 1971); thus, it has been concluded
that the microsomal hydroxylation most likely represents a
detoxification reaction.
I.3.4 Excretion
The results of studies in which 14C-labelled ochratoxin A was
injected intraperitoneally in rats demonstrated that the toxin was
excreted primarily in the urine (Chang & Chu, 1977), though faecal
elimination also occurred to some extent (Galtier, 1974b; Chang &
Chu, 1977). In a study in which 14C-labelled ochratoxin A was
given orally to rats as a single dose (15 mg/kg body weight), the
cumulative elimination after 120 h was 11% ochratoxin A and 23%
ochratoxin alpha in the faeces; 11% ochratoxin A and 12% ochratoxin
alpha in the urine; and 33% ochratoxin A in the bile (Suzuki et
al., 1977). Absorption was influenced by enteritis, which was
caused by the high ochratoxin A doses given (75% of the LD50
value). Ochratoxin A injected intravenously as a single dose (4.1
mg/kg) in albumin-deficient and normal rats was excreted in the
bile and urine 20-70 times faster in the albumin-deficient rats
than in normal rats, indicating that the binding of ochratoxin A to
blood albumin delays the excretion of the compound through the
liver and kidney (Kumagai, 1985).
In rats given unlabelled ochratoxin A orally, Storen et al.
(1982) found 6% ochratoxin A, 1.5% (4R)-4-hydroxyochratoxin A, and
25-27% ochratoxin alpha in the urine; 12% ochratoxin A and 9%
ochratoxin alpha were found in the faeces.
The excretion of ochratoxin A in the milk was studied in
rabbits intravenously injected with 1-4 mg/kg body weight, as
single dose (Galtier et al., 1977a). At the highest dose injected,
the milk contained 1 mg ochratoxin A/litre; ochratoxin alpha and
4-hydroxy-ochratoxin A were not detected. Goats were given a
single dose of tritium-labelled ochratoxin A (0.5 mg/kg) and the
cumulative excretion (in terms of radioactivity) after 7 days
amounted to 53% in the faeces, 38% in the urine, 6% in the milk,
and 2% in the serum (Nip & Chu, 1979). Only a small fraction of
the radioactivity in milk was ochratoxin A, amounting to 0.026% of
the total ochratoxin A given.
In the Federal Republic of Germany, a study of human milk
obtained from women in two hospitals (patient category not stated)
revealed that 4 out of 36 samples (11.1%) contained ochratoxin A,
with a mean value of 0.024 ng/ml and a range of 0.017-0.030 ng/ml
(Bauer & Gareis, 1987; Gareis et al., 1988).
In mice given 14C-labelled ochratoxin A intravenously at
various stages of pregnancy, the toxin was shown to cross the
placental barrier on day 9 of pregnancy, at which time it is most
effective in producing fetal malformations (Appelgren & Arora,
1983). The highest toxin concentration was found in the bile,
which contained 5 times as much as the blood.
Ochratoxin A has been detected in the urine of bacon pigs
suffering from nephropathy (Krogh, unpublished information
communicated to the Task Group).
In a study on the disappearance rates for various tissues,
female bacon pigs were fed ochratoxin A at a level of 1 mg/kg feed
for one month and then kept on a toxin-free diet for another month,
during which animals were sacrificed at regular intervals (Krogh et
al., 1976a). Ochratoxin A disappeared exponentially (Table 7) from
the 4 tissues investigated (kidney, liver, muscle, and adipose
tissue) with residual life values (RL50)a in the range of 3.5-4.5
days; the toxin could still be detected in the kidneys one month
after termination of exposure.
Table 7. The rate of disappearance of ochratoxin A residues
from pig tissues after termination of a one-month exposure to
ochratoxin A at 1 mg/kg feeda
--------------------------------------------------------------
Tissue Ochratoxin A (µg/kg tissue) at time t (days)
after termination of exposure
---------------------------------------------------------------
kidney 28.22 exp (-0.1522 t)
liver 19.49 exp (-0.1598 t)
muscle 12.94 exp (-0.2096 t)
adipose 4.62 exp (-0.0565 t)
---------------------------------------------------------------
a From: Krogh et al. (1976a).
-------------------------------------------------------------------
a RL50 = half residual life, calculated from the exponential
equations shown in Table 7.
No data are available on ochratoxin levels in human urine or
faeces.
When the level in the serum is known, the ochratoxin A residues
in the four other tissues can be calculated (Table 5).
I.4 EFFECTS ON ANIMALS
I.4.1 Field observations
I.4.1.1 Pigs
The effects of ochratoxins on animals have been reviewed by
Krogh (1976a, 1978). Cases of mycotoxic porcine nephropathy have
been regularly encountered in studies in Denmark since the disease
was first discovered 50 years ago (Larsen, 1928). The disease is
endemic in all areas of the country, though unevenly distributed.
Prevalence rates in 1971 varied from 0.6 to 65.9 cases per 10 000
pigs, and epidemics encountered in 1963 and 1971 were associated
with a high moisture content in the grain caused by unusual
climatic conditions (Krogh, 1976b). On the basis of these studies,
Krogh (1978) concluded that ochratoxin A is the substance most
frequently associated with porcine nephropathy, though other
factors, such as citrinin, may also be involved.
A survey on porcine nephropathy was conducted at six
slaughterhouses in Sweden during the spring months of 1978
(Josefsson, 1979). A prevalence rate of 4.4 cases per 10 000 pigs
was encountered corresponding to the endemic level of prevalence
rates in Denmark; 26.7% of nephropathic kidneys contained residues
of ochratoxin A. In Hungary, an epidemiological study on porcine
nephropathy and the association with ochratoxin A was conducted in
1980-81 covering 4 areas in the country (Sandor et al., 1982). A
prevalence rate of 2.0 cases per 10 000 pigs was measured,
comparable to endemic prevalence rates in Scandinavia; 39% of
nephropathic kidneys contained residues of ochratoxin A. The
morphological changes in the kidneys in cases of mycotoxic porcine
nephropathy were characterized by degeneration of the proximal
tubules followed by atrophy of the tubular epithelium, interstitial
fibrosis in the renal cortex, and hyalinization of some glomeruli
(Elling & Moller, 1973).
In Poland, surveys for porcine nephropathy in 1983 and 1984
revealed prevalence rates of 4.7-5.7 cases per 10 000 pigs, with 5-
55% of the nephropathic kidneys containing detectable residues of
ochratoxin A, apparently depending on the season of the year
(Golinski et al., 1984, 1985). Porcine nephropathy has been
encountered in the Federal Republic of Germany (Bauer et al., 1984)
and in Belgium (Rousseau & van Peteghem, 1989), with respectively,
21% and 18% of the affected kidneys containing residues of
ochratoxin A, in the range of 0.1-12 ng/g. In Canada, 1200 samples
of pig blood, collected at a slaughterhouse, were screened for
ochratoxin A using HPLC (Marquardt et al., 1988). Levels exceeding
10 ng/ml (11.3%) were found in 136 samples; detection was confirmed
by derivative formation and spectrometry. No kidney examination
was conducted, but the ochratoxin A concentrations detected in the
blood suggest that nephropathy might have been present in some of
the pigs.
I.4.1.2 Poultry
In a preliminary study in Denmark on chickens condemned by meat
inspectors because of renal lesions, 4 out of 14 birds (29%) were
found to have nephropathy associated with the ingestion of
ochratoxin A, as revealed by the presence of residues of ochratoxin
A in tissues (Elling et al., 1975). The renal lesions were
characterized by degeneration of proximal and distal tubules of
both reptilian and mammalian nephrons and interstitial fibrosis.
I.4.2 Experimental animal studies
I.4.2.1 Acute and chronic effects
The acute and chronic effects of ochratoxins on experimental
animals have been reviewed by Chu (1974a), Harwig (1974), and Krogh
(1976a). Different species vary in their susceptibility to acute
poisoning by ochratoxin A with LD50 values ranging from 3.4 to 30.3
mg/kg (Table 8). When ochratoxin A was administered orally to rats
and guinea-pigs, the female was more sensitive than the male. In
rats, the kidney is the target organ, but necrosis of periportal
cells in the liver has also been noted during studies on acute
effects (Purchase & Theron, 1968).
Table 8. Acute toxicity of ochratoxin A
-------------------------------------------------------------------
Animal LD50 (mg/kg Route of Reference
body weight) administration
-------------------------------------------------------------------
mouse 22 intraperitoneal Sansing et
(female) al. (1976)
rat (male) 30.3 oral Galtier et
al. (1974)
rat 21.4 oral Galtier et
(female) al. (1974)
rat (male) 28 oral Kanizawa et
al. (1977)
rat (male) 12.6 intraperitoneal Galtier et
al. (1974)
rat 14.3 intraperitoneal Galtier et
(female) al. (1974)
guinea-pig 9.1 oral Thacker &
(male) Carlton
(1977)
-------------------------------------------------------------------
Table 8 (contd.)
-------------------------------------------------------------------
Animal LD50 (mg/kg Route of Reference
body weight) administration
-------------------------------------------------------------------
guinea-pig 8.1 oral Thacker &
(female) Carlton
(1977)
white 3.4 oral Prior et
leghorn al. (1976)
turkey 5.9 oral Prior et
al. (1976)
Japanese 16.5 oral Prior et
quail al. (1976)
rainbow 4.7 intraperitoneal Doster et
trout al. (1972)
beagle dog 9 orala Szczech et
(male) (total dose) al. (1973a)
pig 6 oralb Szczech et
(female) (total dose) al. (1973b)
-------------------------------------------------------------------
a All 3 dogs, dosed daily with 3 mg/kg body weight, died within
3 days.
b Both pigs receiving 2 mg/kg daily were moribund and killed
within 3 days, and both pigs receiving 1 mg/kg daily were
moribund and killed within 6 days.
The lesions observed in field cases of mycotoxic nephropathy
have been reproduced by feeding diets containing levels of
ochratoxin A identical to those encountered in the naturally
contaminated products. In a study by Krogh et al. (1974a), 39 pigs
fed rations containing ochratoxin A, at levels ranging from 200 to
4000 µg/kg, developed nephropathy after 4 months at all levels of
exposure. Changes in renal function were characterized by
impairment of tubular function, indicated particularly by a
decrease in TmPAH/Cin,a and reduced ability to produce concentrated
urine. These functional changes corresponded well with the changes
in renal structure observed at all exposure levels, including
atrophy of the proximal tubules and interstitial cortical fibrosis.
Sclerosed glomeruli were also observed in the group receiving the
highest dose of ochratoxin A of 4000 µg/kg feed. Changes were not
seen in any other organs or tissues.
-------------------------------------------------------------------
a TmPAH = transport maximum for para-aminohippuric acid.
Cin = clearance of insulin.
Kidney damage, identical to naturally occurring porcine
nephropathy, was produced in another study by feeding pigs (9
animals) with crystalline ochratoxin A in amounts corresponding to
a feed level of 1 mg/kg for 3 months. Significant renal tubular
functional impairment as measured by a decrease in TmPAH Cin was
detected after only 5 weeks of ochratoxin exposure (Krogh et al.,
1976b). The study was continued for a 2-year period during which
the renal impairment aggravated slightly without reaching a state
of terminal renal failure (Krogh et al., 1979).
In 2 pigs and 9 rats dosed orally with ochratoxin A (400 and
250 µg/kg body weight, respectively) for 5 days, ochratoxin A was
detected in the epithelial cells of the proximal convoluted tubules
of the nephron of all animals. The method of detection was
immunofluorescence microscopy using an antibody against ochratoxin
A that had been formed in rabbits after injection of an albumin-
ochratoxin A conjugate (Elling, 1977).
Groups of 80 F 344/N rats of each sex were administered 0, 21,
70, or 210 µg ochratoxin A/kg in corn oil by gavage, 5 days per
week for 103 weeks (Boorman, 1988). The administration of
ochratoxin A to male and female rats caused a spectrum of
degenerative and proliferative changes in the kidney. The
predominant non-neoplastic lesion in treated rats was degeneration
of the renal tubular epithelium in the inner cortex and the outer
stripe of the outer medulla (nephropathy).
In four pigs given 0.8 mg ochratoxin A/kg body weight orally
for 5 consecutive days, the activity of catalase and CN-insensitive
palmitoyl-CoA-dependent NAD (nicotinamide adenine di-nucleotide) in
renal homogenates decreased, but that in hepatic homogenates did
not, suggesting peroxisomal changes. This was confirmed by
ultrastructural observations of peroxisomes in the proximal
tubules in kidneys of ochratoxin A-treated animals (Elling et
al., 1985).
When 11 SPF pigs and 23 beagle dogs were given high oral doses
of ochratoxin A corresponding to feed levels of more than 5-10
mg/kg (levels rarely found in nature), pathological effects, mainly
necrosis, were observed in the liver, intestine, spleen, lymphoid
tissue, leukocytes, and kidney (Szczech et al., 1973a,b,c). Three
groups of Wistar rats, each consisting of 15 animals, were exposed
to feed levels of ochratoxin A ranging from 0.2 to 5 mg/kg for 3
months. Renal damage in the form of tubular degeneration was
observed at all dose levels (Munro et al., 1974). A decrease in
urinary osmolality, glycosuria, and proteinuria were observed in
an unstated number of Sprague-Dawley and Wistar rats administered
daily doses intraperitoneally of 0.75-2 mg ochratoxin A/kg body
weight (Berndt & Hayes, 1979).
In a study of coagulation factors, rats were given daily oral
doses of 4 mg/kg body weight over 4-10 days, resulting in decreases
in plasma-fibrinogen levels, at levels of factors II, VII, and X,
and in the platelet and megakaryocyte counts (Galtier et al.,
1979).
In 4 calves fed rations containing 0.1-2 mg ochratoxin A/kg
body weight for 30 days, the only signs observed were polyuria,
increased levels of glutamic oxalacetic transaminase (GOT) in
serum, and mild enteritis (Pier et al., 1976); there was mild
tubular degeneration in the kidneys. Cows given ochratoxin A
orally for 4 days at doses ranging from 0.2 to 1.66 mg/kg body
weight remained clinically normal (Ribelin et al., 1978). A cow
given a single dose of 13.3 mg/kg body weight (corresponding to 865
mg/kg feed) developed diarrhoea, anorexia, and cessation of milk
production, one day after.
Avian nephropathy, similar to that in spontaneously occurring
cases, developed in Leghorn hens (27 birds per group) exposed to
dietary levels of 0.3 or 1 mg ochratoxin A/kg for one year (Krogh
et al., 1976c). The renal changes included degeneration of the
tubular epithelium, mainly confined to the proximal and distal
tubules of both reptilian and mammalian nephrons; impairment of
glomerular and tubular function was also observed. "Acute
nephrosis" and "visceral gout" were observed in chickens exposed to
high levels of ochratoxin A (LD50 values) (Peckham et al., 1971).
The same authors reported that ochratoxin B, the other naturally
occurring ochratoxin, was not highly toxic for chickens (LD50, 54
mg/kg) or other animals.
Groups of chicks (40 birds per group) were fed diets containing
ochratoxin A levels in the range of 0-8 mg/kg for 3 weeks (Huff et
al., 1974). The group receiving the highest dose (8 mg/kg feed)
showed decreased packed blood cell volume, haemoglobin
concentration, and serum-iron and serum-transferrin saturation
percentages. In a similar study, Chang et al.(1979) observed that
lymphocytopenia developed at all dose levels. In the same study,
decreased bone strength, as measured physically by resistance to
fracturation, was observed at feed levels of 2-4 mg ochratoxin A/kg
(Huff et al., 1980). When groups of chicks were fed ochratoxin A
at levels of 0, 2, or 4 mg/kg for 20 days, concentrations of serum-
immunoglobulins (IgA, IgG, IgM) were reduced to 57-66% of normal
values in the toxin-exposed groups (Dwivedi & Burns, 1984).
In B6C3F1 female mice, groups of 6-7 animals were administered
a total of 0, 20, 40, or 80 mg ochratoxin A/kg body weight ip on
alternate days over an 8-day period. A dose-related decrease in
thymic mass was observed as well as myelotoxicity, indicated by
bone marrow hypocellularity, due to decreased marrow pluripotent
stem cells, and granulocyte-macrophage progenitors (Boorman et al.,
1984).
When determining the LD50 in mice following intraperitoneal
injection, synergistic effects were observed when ochratoxin A was
combined with citrinin as well as with penicillic acid (Sansing et
al., 1976). An additive effect was observed between ochratoxin A
and citrinin in terms of embryotoxicity in chicken embryos (Vesela
et al., 1983). In beagle dogs, when combined oral doses of
ochratoxin A (0.1-0.2 mg/kg body weight) and citrinin (5-10 mg/kg)
were injected intraperitoneally, synergism was observed with regard
to severity of clinical disease and mortality (Kitchen et al.,
1977a,b). Increased toxicity in terms of LD50 values and
pathological changes was observed in rats, when the ochratoxin A
was given orally combined with either of the drugs biscoumacetate
or phenylbutazone, apparently because of displacement of the toxin
from binding sites on plasma-proteins (Galtier et al., 1980). The
toxic effects of ochratoxin A on the renal epithelial cells of the
monkey were demonstrated in in vitro studies in the form of
abnormal mitotic cells (Steyn et al., 1975).
I.4.2.2 Teratogenicity
Intraperitoneal injection of pregnant mice with ochratoxin A at
5 mg/kg body weight on one of gestation days 7-12 resulted in
increased prenatal mortality, decreased fetal weight, and various
fetal malformations, including exencephaly and anomalies of the
eyes, face, digits, and tail (Hayes et al., 1974). When a
combination of ochratoxin A (2 or 4 mg/kg body weight) and T-2
toxin (0.5 mg/kg body weight) was injected intraperitoneally in
mice on gestation day 8 or 10, ochratoxin A exacerbated the
incidence of T-2-induced gross malformations (tail and limb
anomalies); increased fetocidal effects were also noted (Hood et
al., 1978). Mice were given 3-5 mg ochratoxin A/kg body weight
intraperitoneally or orally on gestation days 8, 9, and 10 or 15,
16, and 17 (Szczech & Hood, 1981). Cerebral necrosis was found in
most fetuses from dams treated on days 15-17, but no cerebral
necrosis developed after treatment on days 8-10, when ochratoxin A
is overtly teratogenic. Pups of mice given 1.25 and 2.25 mg
ochratoxin A/kg orally on gestation days 15, 16, and 17 were tested
for surface righting, swimming, and pivoting (Poppe et al., 1983).
The results of all 3 tests indicated that a delay in development
had occurred; no dose-related pathological alterations were found.
Pregnant mice were administered 1-2 mg ochratoxin A/kg body
weight, and/or 5-20 mg zearalenone/kg body weight or 0.125 mg
diethylstilboestrol/kg body weight, orally, on day 9 of pregnancy,
either individually or in combination, and the offspring were
examined on day 19 (Arora et al., 1983). Teratogenic effects
produced by ochratoxin A, such as exencephaly, open eyelids, and
microphthalmia, were reduced or absent when the toxin was given in
combination with one of the 2 non-steroidal estrogenic substances,
zearalenone or diethylstilboestrol.
Rats were treated orally with ochratoxin A at 0.25, 0.50, 0.75,
1, 2, 4, or 8 mg/kg body weight on gestation days 6-15 (Brown et
al., 1976). Maternal toxicity was not observed below 4 mg/kg body
weight, but an increased incidence of fetal resorptions was
observed from 0.75 mg/kg body weight. All fetuses from dams given
0.25-0.75 mg/kg body weight weighed significantly less than control
fetuses, and fetuses from dams given 0.75 or 1 mg/kg body weight
were stunted. Reduced litters and decreased fetal weight were
observed in rats administered 5 mg ochratoxin A/kg body weight,
orally, on gestation day 8 (Moré et al., 1978).
Subcutaneous administration of ochratoxin A to rats (1.75 mg/kg
body weight) on gestation days 5-7 resulted in the highest number
of malformations, including hydrocephaly, omphalocele, and
anophthalmia as well as a shift in position of the oesophagus
(Mayura et al., 1982); lower doses (0.5 and 1 mg ochratoxin A/kg
body weight) did not have any teratogenic effects, and higher doses
(5 mg/kg) caused all fetuses to be resorbed. In a subsequent study
by the same authors (Mayura et al., 1983), using the same
ochratoxin A exposure conditions, it was shown that a diet low in
protein (10% of protein concentration in normal rat feed) enhanced
the teratogenic action of ochratoxin A in the rat. The combined
action of ochratoxin A exposure and a low protein diet also
resulted in decreases in mating and fertilization rates (22% and
39%, respectively) compared with the control group.
When rats were exposed to ochratoxin A and citrinin (another
nephrotoxic mycotoxin produced by species of the Aspergillus and
Penicillium genera) either singly or combined, enhanced teratogenic
effects were observed in terms of gross malformations, visceral
anomalies, and skeletal defects following combined oral
administration of 1 mg ochratoxin A/kg body weight and 30 mg
citrinin/kg body weight (Mayura et al., 1984). Maternal deaths
(22-40%) occurred after the administration of the combined dose on
days 5, 6, 7, and 14 of gestation, whereas administration of
individual toxins did not cause any maternal deaths and only
minimal malformations no matter which gestation day they were
administered.
Increased prenatal mortality and malformations, including
hydrocephaly, micrognathia, and heart defects, were observed in
hamsters injected intraperitoneally with ochratoxin A at doses of
5-20 mg/kg body weight on one of gestation days 7-9 (Hood et al.,
1976).
I.4.2.3 Mutagenicity
Ochratoxin A did not have any effects in a Bacillus subtilis
Rec- assay, measuring DNA damage when tested at 20 and 100 µg/plate
(Ueno & Kubota, 1976). Ochratoxin A was not mutagenic to
Salmonella typhimurium TA 1535, TA 1537, TA 1538, TA 98, or TA 100
at doses of up to 500 µg/plate, with or without exogenous metabolic
activation (Kuczuk et al., 1978; Wehner et al., 1978b). No
increase was observed in genetic changes at the ade 2 locus of
Saccharomyces cerevisiae after treatment with 50 or 100 µg/plate
ochratoxin A, with or without exogenous metabolic activation
(Kuczuk et al., 1978). Ochratoxin A did not induce mutations to
8-azaguanine resistance in C3H mouse mammary carcinoma cells (FM3A)
treated with doses of 5 or 10 µg/litre (Umeda et al., 1977).
When rats were orally exposed to ochratoxin A every 48 h for 12
weeks (corresponding to a feed level of 4 mg/kg), single-strand
breaks of DNA in renal and hepatic tissue (the only tissues
investigated) were more pronounced than those in control animals
(Kane et al., 1986a,b).
Contradictory results have been obtained by testing ochratoxin
A in the Salmonella assay (SOS chromo test without metabolic
activation) (Ueno et al., in press).
I.4.2.4 Carcinogenicity
Kanizawa & Suzuki (1978) have indicated that ochratoxin A is a
hepatic and renal carcinogen in male mice. A group of 10 male ddY
mice were fed a diet containing 40 mg ochratoxin A/kg for 44 weeks.
A group of 10 untreated controls were fed the basal diet. All
survivors were killed after 49 weeks. Hepatic cell tumours were
found in 5 out of 9 treated mice; no tumours were found in the 10
controls. Solid renal cell tumours were found in 2 out of 9
treated mice and none in the 10 controls. Cystic renal adenomas
were found in 9 out of 10 treated mice compared with none in the 10
controls.
Two groups of 50 male and 2 groups of 50 female B6C3F1 mice
were fed diets containing 1 or 40 mg ochratoxin A/kg, respectively;
one group (control) was fed the basal diet. All survivors were
killed after 24 months. Eleven out of 49 male mice in the 40 mg/kg
group had renal carcinomas; 24 out of 49 male mice in the 40 mg/kg
groups showed renal adenomas. All male mice in the 40 mg/kg group
had microscopic evidence of nephropathy. A few females in the 40
mg/kg group exhibited nephropathic changes but no carcinomas or
adenomas. Compound-related lesions were absent in the controls
and the 1 mg/kg groups (Bendele et al., 1985). In a test for the
development of hyperplastic liver nodules in rats, ochratoxin A was
characterized as having both an initiating and a promoting activity
(Imaida et al., 1982).
Seven groups of 16 male ddY mice each were fed a diet
containing 50 mg ochratoxin A/kg for various periods ranging from 0
to 30 weeks followed by feeding of the basal diet until the end of
70 weeks (Kanizawa, 1984). After 15 weeks of ochratoxin A
exposure, 3 out of 15 animals had renal cell tumours; after 20
weeks, 1 out of 14 mice had renal cell tumours and 2 had hepatomas;
after 25 weeks of exposure, 2 out of 15 mice had renal cell tumours
and 5 had hepatomas; and after 30 weeks of exposure, 4 out of 17
mice had renal cell tumours and 6 had hepatomas; these tumours were
not observed in the controls. The nature of the renal cell tumours
was not further defined, but such tumours are mostly malignant. In
addition, pulmonary tumours were found in all groups, including the
controls, the incidence ranging from 20 to 73%. In a second study,
the effects of a combination of ochratoxin A and citrinin were
elucidated. Groups of 20 male ddY mice were fed diets containing
25 mg ochratoxin A/kg in combination with 100 or 200 mg
citrinin/kg for 70 weeks. Control groups were fed diets that did
not contain any toxins or the individual toxins at the
concentrations indicated above. In addition, one group was fed 25
mg ochratoxin A/kg feed for the first 25 weeks followed by 200 mg
citrinin/kg feed for the remaining period of time; another group
was exposed to the 2 toxins in the reverse order. Exposure to
citrinin alone did not produce any tumours. Exposure to ochratoxin
A alone resulted in renal cell tumours in 6 out of 20 mice, and in
hepatomas in 8 mice. Exposure to ochratoxin A and 100 mg
citrinin/kg feed did not result in any renal cell tumours, but 10
out of 19 mice had hepatomas. Exposure to ochratoxin A and 200 mg
citrinin/kg feed resulted in renal cell tumours in 10 out of 18
mice, and in hepatomas in 7 mice. Exposure to one toxin followed
by the other toxin did not produce any renal cell tumours, but
hepatomas were observed in less than 20% of the mice.
On the basis of these studies, IARC concluded that there was
limited evidence of carcinogenicity for animals, and inadequate
evidence of carcinogenicity for human beings (IARC, 1987a).
Groups of 80 F 344/N rats of each sex were administered 0, 21,
70 or 210 µg ochratoxin A/kg in corn oil by gavage, 5 days per
week, for 103 weeks (Boorman, 1988). In the male rats, renal
carcinomas were found in 16 out of 51 animals dosed with 70 µg/kg
and in 30 out of 50 animals dosed with 210 µg/kg; no carcinomas
were found in lower dose groups. In the female rats, renal
carcinomas were less common, as 1 out of 50 animals dosed with 70
µg/kg and 3 out of 50 animals dosed with 210 µg/kg had carcinomas;
no carcinomas were found in the lower dose groups. Renal adenomas
were found in all groups of male rats, with increasing frequencies
associated with increased doses. In the female groups, renal
adenomas were only found in the two highest dose groups. In the
female rats, fibroadenomas in the mammary gland were found in 45-
56% in the treated groups, a significantly higher percentage than
in the control group.
I.4.2.5 Biochemical effects and mode of action
Ochratoxin A is an inhibitor of tRNA synthetase and protein
synthesis in several microorganisms ( Bacillus subtilis, B.
stearothermophilus, Streptococcus faecalis, yeasts) as well as in
rat hepatoma cells (Konrad & Roschenthaler, 1977; Bunge et al.,
1978; Heller & Roschenthaler, 1978; Creppy et al., 1979a,b). The
competitive inhibitor effect of ochratoxin A on tRNA synthetase and
protein synthesis in rat hepatoma cells can be prevented by the
addition of phenylalanine in the cell culture medium at a molar
ratio of phenylalanine: ochratoxin A of 1.7:1 (Creppy et al.,
1979b). This observation suggested the possibility of preventive
measures for ochratoxin A-induced disease. Thus, the acute
intraperitoneal effect of ochratoxin A (LD100) in mice was
prevented by concomitant injection of phenylalanine (Creppy et al.,
1980; Moroi et al., 1985).
However, in the study on the reproduction of porcine
nephropathy previously mentioned (Krogh et al., 1974a), the molar
ratio of phenylalanine: ochratoxin A in the feed exceeded 4600:1,
implying that, in the field situation, phenylalanine does not
prevent ochratoxin A from inducing the development of nephropathy.
Ochratoxin A in the concentration range studied (20-1667 µmol/
litre) caused a 47-50% inhibition of macrophage migration (Klinkert
et al., 1981); this effect could be prevented by the simultaneous
addition of phenylalanine. In BALB/c mice, a dose of ochratoxin A
as low as 0.005 µg/kg body weight was able to suppress the immune
response to sheep erythrocytes (Haubeck et al., 1981); the effect
could be prevented by the simultaneous addition of phenylalanine.
Studying the same effect in Swiss mice, Prior & Sisodia (1982) were
unable to show any suppression of the immune response to sheep
erythrocytes, even after daily injection of 5 mg ochratoxin A/kg
for 50 days. (4R)-4-Hydroxyochratoxin A at a dose of 1 µg/kg body
weight caused an 80% reduction in the number of cells producing IgM
and a 93% reduction in cells producing IgG in BALB/c mice compared
with 90% and 92%, respectively, for ochratoxin A (Creppy et al.,
1983).
Female B6C3F1 mice (6 per group) were administered ochratoxin A
in amounts of 0.34, 6.7, or 13.4 mg/kg body weight or ochratoxin B
(13.4 mg/kg body weight) 6 times during 12 days. Ochratoxin A
inhibited the natural killer cell activity at all dose levels, and
increased the growth of transplantable tumour cells without
affecting T-cell or macrophage-mediated anti-tumour activity
(Luster et al., 1987). Ochratoxin B did not influence immune
function. The inhibition by ochratoxin A of natural killer cell
activity appeared to be caused by reduced production of basal
interferon.
Ochratoxin A affects the carbohydrate metabolism in rats.
Thus, a single oral dose of ochratoxin A at 15 mg/kg body weight
caused a decrease in the glycogen level in the liver and an
increase in the heart glycogen level, 4 h later (Suzuki & Satoh,
1973).
In a more extensive study on rats, the decrease in liver
glycogen level, 4 h after a single oral dose of ochratoxin A at 15
mg/kg body weight, was associated with an increase in serum glucose
levels and a decrease in liver glucose-6-phosphate (Suzuki et al.,
1975). At the same time, the liver glycogen synthetase (EC
2.7.1.37) activity decreased and the liver phosphorylase (EC
2.4.1.1) activity increased. Three daily oral doses of ochratoxin
A at 5 mg/kg body weight caused a decrease in the liver glycogen
concentration, which was measured on the fourth day. The decrease
was attributed to inhibition of the active transport of glucose
into the liver, suppression of glycogen synthesis from glucose, and
acceleration of glycogen decomposition.
During in vitro studies on rat liver mitochondria, it was
observed that ochratoxin A inhibited the respiration of whole
mitochondria by acting as a competitive inhibitor of transport
carrier proteins located in the inner mitochondrial membrane
(Meisner & Chan, 1974). Further studies with mitochondrial
preparations revealed that the mitochondrial uptake of ochratoxin A
was an energy-using process that resulted in depletion of
intramitochondrial adenosine triphosphate (ATP), and that
ochratoxin A inhibited intramitochondrial phosphate transport
resulting in deterioration of the mitochondria (Meisner, 1976).
This might explain the degeneration of liver mitochondria observed
by Purchase & Theron (1968) in rats exposed orally to a single dose
of ochratoxin A at 10 mg/kg body weight. These authors observed
accumulation of glycogen in the cytoplasm of the rat liver cells
microscopically. This was in contrast to the previously discussed
observations of Suzuki et al. (1975), who found a decrease in
glycogen levels.
In a study on mice, Sansing et al. (1976) found that ochratoxin
A, administered intraperitoneally at 6 mg/kg body weight, inhibited
orotic acid incorporation into both liver and kidney RNA, 6 h after
toxin injection. In this respect, ochratoxin A acted synergistically
with another nephrotoxic mycotoxin, citrinin.
When neonatal rats were exposed orally to a single dose of 1 mg
ochratoxin A/kg or of 25 mg citrinin/kg or both doses within 24 h
of birth, a synergistic effect of the 2 mycotoxins was observed on
cytochrome P-450, NADPH-dependent dehydrogenase, and NADPH-
cytochrome c reductase (Siraj et al., 1981).
In rats fed 2 mg ochratoxin A/kg body weight per day for 2
days, renal gluconeogenesis from pyruvate was decreased by 26%, and
renal phosphoenol-pyruvate carboxy kinase (PEPCK) (EC 4.1.1.32)
activity was reduced by 55%, whereas hepatic PEPCK was unchanged
(Meisner & Selanik, 1979). In a subsequent study on rats, it was
found that even lower dose levels (0.3-0.5 mg ochratoxin A/kg body
weight) caused a 50% reduction in PEPCK activity (Meisner &
Meisner, 1981). A number of other enzymes located in the proximal
tubule of the nephron were unaffected. When longer exposure
periods (8-12 weeks) were used, and 145 ng ochratoxin A/kg body
weight were administered orally to groups of 3 rats each, increased
urinary excretion and corresponding renal tubular depletion of the
following enzymes were observed: gamma-glutamyl transferase,
alkaline phosphatase, leucine aminopeptidase, lactate
dehydrogenase, and N-acetyl-beta- D-glucosaminidase (Kane et al.,
1986a). Renal PEPCK in pigs was also sensitive to ochratoxin A
with a feed level of 100 µg/kg causing a significant decrease in
PEPCK activity (Meisner & Krogh, 1982).
In a study on pigs fed ochratoxin A at 0, 0.2, or 1 mg/kg for 5
weeks, enzyme activities were measured in renal biopsies, collected
1, 3, and 5 weeks after initiation (Krogh et al., 1988). After one
week, the activities of renal PEPCK and gamma-glutamyltranspeptidase
were decreased by 40%. The dose-related decrease in the activity
of PEPCK and gamma-glutamyltranspeptidase was accompanied by a
dose-related increase in renal impairment, as measured by the
reduction of TmPAH/Cin, suggesting that these enzymes are sensitive
indicators of ochratoxin-induced porcine nephropathy. Thus, the
renal biopsy-based measurements of enzyme activities might prove
diagnostically useful in ochratoxin-induced disease in human
beings.
Ochratoxin A reduced the total renal mRNA concentration in male
Sprague-Dawley rats and certain mRNA species, notably PEPCK, were
reduced to a greater extent than the bulk of the RNA pool (Meisner
et al., 1983).
I.5 EFFECTS ON MAN
I.5.1 Ochratoxin A, Balkan endemic nephropathy, and tumours of the
urinary system
This topic has been reviewed by Krogh (1979, 1983) who called
attention to the striking similarities in the changes in renal
structure and function induced experimentally in animals by the
administration of ochratoxin A, and the clinical and pathological
features of a localized endemic disease known as Balkan endemic
nephropathy. So far, the disease has been observed only in rural
populations of Bulgaria, Romania, and Yugoslavia, but information
on the present magnitude of the problem was not available to the
Task Group.
The Balkan endemic nephropathy is a chronic disease that
predominantly affects women and progresses slowly up to death
(Hrabar et al., 1976, Chernozemsky et al., 1977). Age-specific
incidence rates are highest above the age of 40. Younger cases
occur in the 10-19-year-old age group, and the mean age of new
patients is in the early 50s (Stoyanov et al., 1978).
The disease is characterized by an extreme geographical
clustering with a tendency for familial aggregation of cases
(Nicolov et al., 1978). However, sporadic cases occur outside
endemic areas. Age-adjusted incidence rates of 555 per 100 000
population in females and 322 in males over a ten-year period have
been recorded from a population sample of 147 000 in an endemic
area of Bulgaria (Stoyanov et al., 1978). In one of several
endemic regions in Yugoslavia, the prevalence varied from 3% to 8%
(Hrabar et al., 1976).
Autopsy has shown that kidneys are notably reduced in size.
The histological lesions are interstitial fibrosis, tubular
degeneration, and hyalization of glomeruli in the more superficial
part of the cortex (Heptinstall, 1974). Impairment of tubular
function, indicated by a decrease in TmPAH, is a prominent and
early sign (Dotchev, 1973).
A high incidence of tumours of the urinary system is strongly
correlated with the prevalence of Balkan endemic nephropathy
(Ceovic et al., 1976; Chernozemsky et al., 1977; Nicolov et al.,
1978). In one instance in Bulgaria, 46.6% of patients with tumours
of the urinary system were also affected by endemic nephropathy.
Among the tumours of the urinary system, cancers of the renal
pelvis and ureters are more frequently associated with endemic
nephropathy than urinary bladder tumours.
The relative risk for developing cancer of the renal pelvis and
ureters is 88:1 in patients with nephropathy compared with controls
in non-endemic areas. The relative risk for the development of any
tumour of the urinary system is only 28:1 in the same sample
(Stoyanov et al., 1978).
Over the past 2 decades, investigations have been carried out
to verify a variety of etiological assumptions with unconvincing
results (review by Puchlev, 1973, 1974). However, the assumption
of the possible role of ochratoxin A, on the basis of similarities
with the animal disease, has received increasing epidemiological
support.
In Yugoslavia, surveys indicated that the contamination of
foodstuffs (grains, maize, pork meat) with ochratoxin A occurred in
12.8% of samples in an area where the prevalence of endemic
nephropathy was 7.3%, compared with only 1.6% of contaminated
samples in areas free of the disease (Krogh et al., 1977). The
concentration of ochratoxin A in maize was 5-90 µg/kg and that in
pork meat, 5 µg/kg (Krogh et al., 1977) with levels of up to 27
µg/kg in pig kidneys (Pepeljnjak et al., 1982). Similarly, studies
in Bulgaria have revealed that 16.7% of beans and 27.3% of maize
from an endemic area were contaminated with ochratoxin A compared
with 7.1% and 9%, respectively, from a non-endemic area (Petkova-
Bocharova & Castegnaro, 1985).
In a subsequent survey of home-produced foodstuffs (cereal and
bread) from the same endemic area in Yugoslavia over a 5-year
period, a mean contamination of 8.7% was found with pronounced
annual variations, which probably reflect climatic conditions
during the crop harvesting periods (Pavlovic et al., 1979).
Surveys on the presence of ochratoxin A in blood samples are
difficult to compare, as different analytical methods have been
used with different levels of sensitivity. Prevalences of 16.6%
and 5.9% with a one-year interval were reported in the same endemic
village in Yugoslavia (Hult et al., 1982). It is not possible to
determine whether this difference reflects annual variations in the
content of the blood, or the result of a less sensitive analytical
method in the second instance. However, higher prevalences of
ochratoxin A and higher concentrations in blood are generally
present in people from endemic areas, especially in persons
suffering from Balkan nephropathy.
A survey in Yugoslavia reported a prevalence in the blood of
16.6% in an endemic village and 6% in a non-affected one (Hult et
al., 1982). In Bulgaria, reported rates were 17.7% in an endemic
area and 7.7% in a non-endemic one (Petkova-Bocharova et al.,
1988). Table 9 illustrates the trend towards higher concentrations
in endemic situations and in patients. The similarity of results
in healthy families from affected villages and healthy persons from
unaffected villages (groups III and IV) suggests than an
environmental or a behavioural determinant plays a role at the
household level.
Thus, available epidemiological information seems to indicate
that Balkan endemic nephropathy is associated with consumption
patterns involving foodstuffs contaminated with ochratoxin A and
with a higher frequency of positive blood samples of ochratoxin A.
However, the association does not permit the establishment of a
causal relationship. Cross sectional surveys, such as those
reported in the literature so far, are probably not the appropriate
means to determine this relationship.
The results of experimental animal studies suggest that Balkan
nephropathy, a chronic condition, may require a long latency period
between exposure and the onset of symptoms, or more likely a
prolonged exposure or repeated exposure over a long period of time.
Investigations based on individual exposure time sequences
together with follow-up cohort surveys could provide a clue to the
causal role of ochratoxin A in Balkan endemic nephropathy. In view
of the lack of this information, such a causal relationship cannot
be established or rejected.
Table 9. Ochratoxin A in blood samples from people in endemic and
non-endemic areas in Bulgariaa
-------------------------------------------------------------------
Groupb No. of No. of Mean concentration
persons ochratoxin A ± S.D. (µg/kg)
assayed positive cases
(%)
-------------------------------------------------------------------
I. Persons with 61 16 (26.3) 20.3 ± 9.7
UST and/or EN
II. Healthy 63 10 (15.8) 14.5 ± 7.6
persons from
families with UST
and/or EN cases
III. Healthy 63 7 (11.1) 12.5 ± 3.5
persons from
families in
endemic villages
IV. Healthy 60 7 (11.6) 15.0 ± 4.2
persons from
unaffected villages
in endemic areas
V. Healthy 65 5 (7.7) 10.0
persons from
villages in
non-endemic areas
-------------------------------------------------------------------
a From: Petkova-Bocharova et al. (1988).
b UST: urinary system tumours; EN: endemic nephropathy.
The differences between group I and groups III and IV are
statistically significant ( P <0.002). Difference between
groups I and V is statistically significant ( P <0.001).
I.6 EVALUATION OF THE HUMAN HEALTH RISKS
Human exposure, as demonstrated by the occurrence of ochratoxin
A in food and in the blood, has been observed in various countries
in Europe. The Task Group was not aware of attempts to detect
ochratoxin A in human blood in other parts of the world.
The causal role of ochratoxin A in porcine nephropathy has been
established, based on studies of field cases as well as
reproduction of the disease with ochratoxin A. Using the porcine
model, it has been postulated that Balkan endemic nephropathy may
result from exposure to ochratoxin A. Available epidemiological
information indicates that Balkan nephropathy may be associated
with the consumption of foodstuffs contaminated by this toxin.
Since the publication of Environmental Health Criteria 11, in 1979,
epidemiological studies on the concentration of ochratoxin A in
human blood in affected and non-affected areas, have provided
additional support for the relationship between Balkan nephropathy
and exposure to ochratoxin A.
It has been shown that both the prevalence of ochratoxin A in
the blood and the blood concentrations are higher in residents in
endemic areas. However, a direct causal relationship cannot be
established on the basis of indirect evidence provided by the above
retrospective studies alone. Neither can it be excluded in view of
the long latency period between the exposure and the onset of
symptoms.
Ochratoxin A has been demonstrated to be carcinogenic to the
renal tubular epithelium in male mice and both sexes of rats. A
highly significant relationship has been observed between Balkan
nephropathy and tumours of the urinary tract, particularly with
tumours of the renal pelvis and ureters. However, there are no
published data to establish a direct causal role of ochratoxin A in
the etiology of such tumours.
II. TRICHOTHECENES
II.1 PROPERTIES AND ANALYTICAL METHODS
II.1.1 Physical and chemical properties
The sesquiterpenoid trichothecenes possess the tetracyclic
12,13-epoxytrichothecene skeleton. A total of 148 trichothecenes,
83 non-macrocyclic and 65 macrocyclic, have been isolated from
fungal cultures and plants (Drove, 1988). They can be conveniently
divided into 4 categories according to similarity of functional
groups (Ueno, 1977). The first class is characterized by a
functional group other than a ketone at C-8 (type A). This is the
largest category containing members such as T-2 toxin and
diacetoxyscirpenol (DAS). The second category of trichothecenes
has a carbonyl function at C-8 (type B) typified by 4-deoxynivalenol
(DON) and nivalenol (NIV). The third category is characterized by
a second epoxide group at C-7,8 or C-9,10 (type C), and the fourth
contains a macrocyclic ring system between C-4 and C-15 with two
ester linkages (type D). The structures of representative
trichothecenes of each category are illustrated below.



 II.1.1.1 Physical properties
The trichothecenes are colourless, mostly crystalline solids
that have been well characterized by physical and spectroscopic
techniques (Cole & Cox, 1981). The type A trichothecenes are
soluble in moderately polar solvents, such as chloroform, diethyl
ether, ethyl acetate, and acetone, whereas the more polar type B
trichothecenes require higher polarity solvents, such as aqueous
methanol or aqueous acetonitrile. Some physical properties of the
main trichothecenes are summarized in Table 10.
Table 10. Some physical properties of main trichothecenes
-------------------------------------------------------------------------------
Trichothecenes Molecular Relative Melting (alpha)20D References
formula molecular point
mass (°C)
-------------------------------------------------------------------------------
T-2 toxin C24H34O9 466 151-152 +15 Bamburg et
al. (1968b)
HT-2 toxin C22H26O8 424 - - Bamburg &
Strong (1969)
Diacetoxyscirpenol C19H26O7 366 162-164 -27 Sigg et al.
(1965)
Neosolaniol C19H26O8 382 171-172 - Ishii et al.
(1971)
Deoxynivalenol C15H20O6 296 151-153 +6.35 Yoshizawa &
Morooka (1973)
Nivalenol C15H20O7 312 222-223 +21.54 Tatsuno et
al. (1968)
Trichothecin C19H24O5 332 118 +44 Freeman (1955)
Fusarenon-X C17H22O8 354 91-92 +58 Ueno et al.
(1969b)
Roridin A C29H40O9 532 198-204 +130 Harri et al.
(1962)
Satratoxin H C29H36O9 528 162-166 - Eppley &
Bailey (1973)
Verrucarin A C27H34O9 502 360 +20.6 Gutzwiller &
Tamm (1965)
-------------------------------------------------------------------------------
Most of the trichothecenes lack conjugated unsaturation in their
structures with a consequent absence of absorption in the ultraviolet
(UV) spectrum, except for end absorption due to unsaturation at C-9.
This lack of absorbance is a source of difficulty in achieving
sensitive and specific detection in HPLC analysis. In contrast, the
type D trichothecenes give characteristic ultraviolet spectra.
II.1.1.2 Chemical properties
When trichothecenes containing an ester group are treated with a
base, they are hydrolysed to their corresponding parent alcohol (Wei
et al., 1971). Free hydroxyl groups are readily acylated. The
12,13-epoxy group is itself extremely stable to nucleophilic attack.
However, prolonged boiling under highly acidic conditions causes an
intramolecular rearrangement of the trichothecene skeleton to the
apotrichothecene ring. Detailed discussion of the chemistry of
trichothecenes can be found in reviews by Bamburg & Strong (1971),
Bamburg (1976), and Tamm (1977).
The trichothecenes are generally stable; for example, DON can be
stored in organic solvents, such as ethyl acetate, for a long time
without any significant deterioration (Shepherd & Gilbert, 1988).
They remain unaffected when refluxed with various organic solvents
and also under mildly acidic conditions.
II.1.2 Analytical methods for trichothecenes
Analytical methods have been reviewed by Scott (1982) and Pohland
et al. (1986). Selected examples of recently published analytical
methods for type A trichothecenes and type B trichothecenes are
summarized in Tables 11 and 12 respectively. Although some of the
multi-trichothecene methods included in Table 11 may also be
applicable to certain of the type B compounds, the procedures in
Table 12 have been developed exclusively for the type B toxins.
II.1.2.1 Chemical methods
(a) Extraction
The most commonly used extraction solvents for trichothecenes are
chloroform, ethyl acetate, methanol, acetonitrile, aqueous methanol,
and aqueous acetonitrile. Chloroform, ethyl acetate, and
acetonitrile have been successfully used for the extraction of T-2
toxin, DAS, and some of their partially hydrolysed derivatives in
naturally contaminated cereals. Aqueous methanol and aqueous
acetonitrile are the solvents of choice for the extraction of several
trichothecenes of widely differing polarity as well as for the
extraction of type B toxins alone. Methods differ according to the
type of solvent used, whether samples are homogenised in a blender
with the solvent or agitated with a wrist action shaker, and in the
length of time of the extraction process. Spiking of samples with
standards is not an adequate way of demonstrating the efficiency of
extraction, and only methods validated with naturally contaminated
material can be regarded as having been rigorously tested.
Extraction procedures have been assessed for DON (Trenholm et al.,
1985) and it has been demonstrated that longer extraction times are
required for naturally contaminated samples than for those that have
been spiked (at least 120 min shaking). It has also been shown that
aqueous acetonitrile gives a cleaner extract than aqueous methanol.
(b) Clean-up procedures
The extent of sample clean-up required for a particular assay
depends on the specificity of the detection procedure and the nature
of the sample matrix. The less specific detection methods, such as
TLC, require extensive sample clean-up while more sophisticated
approaches, such as mass spectrometry and immunoassay, may only
require minimal sample preparation.
Table 11. Selected methods for the determination of T-2 toxin and diacetoxyscirpenol in
biological materials
------------------------------------------------------------------------------------------------
Matrix Extractiona Clean-upb Assayc Detection Toxins assayed References
limit -----------------
(µg/g) T-2 DAS Others
------------------------------------------------------------------------------------------------
Cereals MeOH/H2O XAD-4; flor. TLC 0.5 + + 7 Kamimura et
al. (1981)
Cereals EtOAC prep. TLC HPTLC 0.2 + - 2 Ilus et
al. (1981)
Foods MeCN/H2O char/alum TLC 1.0 + + 5 Romer
(1986)
Cereals MeCN/KCl Sep P HPLC(RI) 1.0 + - 1 Schmidt &
Dose (1984)
Wheat/rye MeCN Bond-Elut LC/MS (therm) 0.04 + + 2 Rajakyla et
al. (1987)
Plasma EtOAC Sep P/sil.g LC/MS (therm) 0.002 + + 2 Voyksner et
al. (1985)
Cereals EtOAC sil.g GC (FID)-TMS 0.1 + + 3 Bata et al.
(1983)
Cereals MeOH/H2O sil.g/cy GC (ECD)-HFB 0.05 + + 1 Cohen &
Lapointe
(1984)
Plasma benzene flor. GC (ECD)-HFB 0.02 + - - Swanson et
al. (1983)
Milk EtOAC prep. TLC GC/MS-TMS 0.003 + - - Collins &
Rosen
(1979)
Corn MeOH Sep P/sil.g GC/MS-TMS 0.02 + + 1 Rosen &
Rosen
(1984)
Foods MeOH flor. GC/MS-HFB 0.01 + + 11 Black et
al. (1987)
Urine EtOAC/MeOH Sep P GC/MS-HFB 0.005 + + 5 Black et
al. (1986)
Blood acetone Sep P GC/MS-PFP 0.005 + + 9 Begley et
al. (1986)
------------------------------------------------------------------------------------------------
Table 11 (contd.)
------------------------------------------------------------------------------------------------
Matrix Extractiona Clean-upb Assayc Detection Toxins assayed References
limit -----------------
(µg/g) T-2 DAS Others
------------------------------------------------------------------------------------------------
Milk/urine EtOAC Sep P RIA 0.002 + - - Lee & Chu
(1981a)
Corn/wheat MeOH Sep P RIA 0.001 + - - Lee & Chu
(1981b)
Urine none Sep P ELISA 0.0005 + - - Fan et al.
(1987)
------------------------------------------------------------------------------------------------
a Extraction: MeOH (methanol); H2O (water); EtOAC (ethyl acetate); MeCN (acetonitrile);
KCl (potassium chloride aqueous soln).
b Clean-up: XAD-4 (amberlite XAD-4 resin); flor. (florisil); char/alum (charcoal/alumina
columns); Sep P (C18-Sep Pak); sil.g (silica gel); cy (cyano extraction column).
c Assay: TLC (thin layer chromatography); HPTLC (high performance TLC); HPLC (high performance
liquid chromatography); RI (refractive index); LC/MS (combined HPLC/mass spectrometry);
GC (gas chromatography); FID (flame ionisation detector); ECD (electron capture detector);
TMS (trimethylsilyl derivative); HFB (hepafluorobutyrl ester derivative);
PFP (pentafluoropropionyl ester); RIA (radioimmunoassay); ELISA (enzyme linked
immunosorbent assay).
Table 12. Selected methods for the determination of deoxynivalenol and nivelenol in biological
materials
------------------------------------------------------------------------------------------------
Matrix Extractiona Clean-upb Assayc Detection Toxins assayed References
limit -----------------
(µg/g) T-2 DAS Others
------------------------------------------------------------------------------------------------
Wheat/corn MeCN/H2O char/alum TLC 0.04-0.1 + - - Trucksess
et al.
(1984)
Foods MeCn/H2O char/alum; TLC 0.5 + - - Trucksess
Sep P et al.
(1986a)
Cereals MeCN/H2O/ char/alum; HPTLC 0.05 + + 1 Trucksess
MeOH ppt et al.
(1987)
Corn/rice MeOH/H2O/ liq/liq extr HPLC (UV) O.005 + + 2 Visconti &
NaCl Bottalico
(1983a)
Corn/rice MeOH/H2O none HPLC (ED) 0.025 + - - Sylvia et
al. (1986)
Corn MeOH/H2O prep. TLC HPLC (UV) 0.01 + - - Ehrlich et
al. (1983)
Cereals MeCN/H2O ion/char/ HPLC (UV) 0.05 + + - Lauren &
alum Greenhalgh
(1987)
Cereals MeOH flor. LC/MS (micro) 0.01 + + - Tiebach et
al. (1985)
Cereals MeOH/H2O sil.g GC (ECD)-TMS 0.02 + + - Scott et
al. (1986)
Cereals MeCN/H2O flor/Sep P GC (ECD)-TMS 0.002 + + - Tanaka et
al. (1986)
Wheat chlor/EtOH sil.g GC (ECD)-HFB 0.1 + - - Ware et
al. (1986)
Cereals chlor/EtOH sil.g GC (ECD)-HFB 0.02 + - - Mulders &
Impelen-
Peek (1986)
Milk EtOAc Sep P GC (ECD)-TMS 0.001 + - - Swanson et
al. (1986)
------------------------------------------------------------------------------------------------
Table 12 (contd.)
------------------------------------------------------------------------------------------------
Matrix Extractiona Clean-upb Assayc Detection Toxins assayed References
limit -----------------
(µg/g) T-2 DAS Others
------------------------------------------------------------------------------------------------
Corn/barley MeOH/H2O sil.g GC/MS-TMS 0.01 + - - Gilbert et
al. (1983a)
Foods MeOH/H2O XAD-2/flor/ GC/MS-TMS 0.02 + + - Yoshizawa &
Sep P Hosokawa
(1983)
Corn/wheat MeCN/H2O none ELISA 0.01 + - - Xu et al.
(1988)
------------------------------------------------------------------------------------------------
a Extraction: MeCN (acetonitrile); H2O (water); MeOH (methanol); NaCl (sodium chloride soln);
chlor/EtOH (chloroform/ethanol); EtOAc (ethyl acetate).
b Clean-up: char/alum (charcoal/alumina column); Sep P (C18-Sep Pak); ppt (lead acetate
precipitation); liq/liq extr. (liquid/liquid extraction); ion (ion exchange resin);
flor. (florisil); sil.g (silica gel); XAD-2 (Amberlite XAD-2 resin).
c Assay: TLC (thin layer chromatography); HPTLC (high performance TLC); HPLC (high performance
liquid chromatography); UV (ultraviolet detection); ED (electrochemical detection);
LC/MS (combined microbore-HPLC/mass spectrometry); GC (gas chromatography); ECD (electron
capture detector); TMS (trimethylsilyl derivative); HFB (heptafluorobutyryl ester
derivative); ELISA (enzyme linked immunosorbent assay).
Early methods that may have involved the use of conventional
silica gel columns or preparative TLC for clean-up, have to a large
extent been superseded by methods using prepacked cartridges, such as
silica gel and C18-Sep Paks, which are both more reliable and more
convenient. Florisil columns are widely used for clean-up (e.g., see
Tanaka et al., 1985a), and the one step clean-up procedures using
alumina/charcoal (Romer, 1986) or alumina/charcoal/Celite columns
(Trucksess et al., 1984) have become widely adopted, particularly for
DON and NIV assays.
(c) Detection and quantification
(i) Thin-layer chromatography (TLC). The lack of native
fluorescence or UV absorbance of the trichothecenes means that TLC
detection relies on the use of spray reagents for visualisation.
Characteristic colours or fluorescence can be produced with sulfuric
acid or p-anisaldehyde followed by heating at 110-120 °C (Scott et
al., 1970; Ueno et al., 1973c). A general spray reagent for the
12,13-epoxy function is 4-( p-nitrobenzyl)pyridine, which produces a
blue coloration on heating and treatment with a base (Takitani et
al., 1979). A more sensitive, but somewhat elaborate, procedure,
which again is specific for the epoxide function, involves reaction
with nicotinamide and 2-acetylpyridine to produce fluorescent TLC
spots (Sano et al., 1982). Diphenylindenone sulfonyl esters of
trichothecenes can be formed prior to TLC and, subsequently, when
sprayed with sodium methoxide can yield fluorescent spots at high
sensitivity (Yagen et al., 1986).
Most of the above spray reagents, though frequently demonstrated
as useful for the determination of standards or of relatively high
concentrations of toxins in culture extracts, have not been well
developed in conjunction with clean-up procedures for the
determination of trichothecenes in naturally contaminated cereal
samples or other foods. However, the exception has been the use of
an aluminum chloride spray reagent for visualising the type B
trichothecenes (Kamimura et al., 1981). On spraying the plates,
heating for 10 min at 110 °C and then treating with a base, blue
fluorescent spots are produced for DON, NIV, and fusarenon X. Using
this approach, in conjunction with a clean-up utilising an alumina
charcoal Celite column, a quantitative TLC procedure was developed
using a fluorodensitometer for determining DON in wheat and corn
(Trucksess et al. 1984); DON in processed grain products including
breakfast cereals, corn syrup, and beer (Trucksess et al., 1986b);
and for simultaneously monitoring DON, NIV, and fusarenon X in
barley, corn, and wheat (Trucksess et al. 1987). This TLC procedure
was successfully collaboratively tested (Eppley et al., 1986) and was
accepted as the AOAC first action method for DON in wheat.
(ii) High performance liquid chromatography (HPLC). HPLC has not
proved particularly appropriate for the type A trichothecenes, which
lack any significant UV absorption, making sensitive detection
difficult. Refractive index detection has been employed, but at
relatively high toxin levels (Schmidt & Dose, 1984). UV detection at
short wavelength has been used for the determination of DON and NIV
in cereals, and a number of methods have been reported that offer an
advantage over alternative GC approaches in that they do not require
derivatization of the toxins. A post-column derivatization procedure
for DON and NIV has been developed (Sano et al., 1987) involving
alkaline decomposition of the trichothecenes to generate formaldehyde
and then reaction with methyl acetoacetate and ammonium acetate to
form a fluorescent derivative. Although more sensitive and specific
than UV detection, the approach does have the disadvantage of
requiring rather elaborate instrumentation, in order to carry out the
post column reaction. Electrochemical detection (Sylvia et al.,
1986) looks particularly promising for the determination of DON by
HPLC, offering both greater sensitivity and specificity than UV
detection, and the possibility of analysis of sample extracts that
have received minimal sample clean-up.
(iii) Gas chromatography (GC). A large number of GC methods have
been published that differ in the approach to sample extraction, in
sample clean-up, and in choice of derivative prior to GC analysis.
Trichothecenes containing a hydroxyl group require derivatization,
and the heptafluorobutyryl (HFB) ester and trimethylsilyl (TMS) ether
derivatives have been most frequently used in conjunction with
electron capture detection. Formation of the HFB derivatives is
relatively time-consuming, but complete derivatization is easily
achieved and the derivative once formed is stable for at least
several days. However, poor reproducibility has been noted with HFB
derivatives of DON (Mulders & Impelen-Peek, 1986) attributed to
adsorption on to glass surfaces. The relatively high mass of the HFB
derivatives can cause difficulties if GC/MS confirmation is required.
In contrast, TMS derivatives are easily prepared, and are of
suitable mass for GC/MS, but, for some type B toxins, they may
require optimization of conditions for complete derivatization
(Gilbert et al., 1985). Scott & Kanhere (1986) have compiled
retention data for 10 different trichothecenes as both TMS and HFB
derivatives on capillary columns of different stationary phases.
Trifluoroacetyl derivatives of trichothecenes are preferred by some
workers (Kientz & Verweij, 1986), and pentafluoropropionyl esters
have been used, particularly where detection has been by MS (Begley
et al., 1986; Krishnamurthy & Sarver, 1986; Rood et al., 1988a).
An area where improvement in quantification of trichothecenes is
still needed is in the selection of adequate internal standards.
Compounds, such as methoxychlor (Romer et al., 1978),
hexachlorobiphenyl (Blass et al., 1984), and alkanes (Ilus et al.,
1981), have been used, but these differ significantly in structure
from the trichothecenes. The deuterated TMS derivative of T-2 toxin
has been used as an internal standard in GC/MS analysis (Rosen &
Rosen, 1984), an isomer of T-2 toxin has been chemically synthesized
(Stahr et al., 1981) as have 4-deoxyverrucarol and 16-hydroxyver-
rucarol for use as internal stan-dards (Krishnamurthy et al., 1986).
Despite the many difficulties, a GC method using the HFB
derivative has been successfully collaboratively tested (Ware et al.,
1986) and subsequently adopted as an AOAC official first action
method.
Other related trichothecenes have been determined in foods by GC
methods, for example, the de-epoxidised metabolite of DON called
DOM-1 has been determined in milk as both its TMS and HFB derivative
(Swanson et al., 1986). Also, an isomer of DON was detected by
capillary GC, as its TMS derivative in bread and breakfast cereal
products prepared from flour naturally contaminated with DON
(Greenhalgh et al., 1984).
(iv) Mass spectrometry (MS). Mass spectrometry has been used for
the structural characterization of novel trichothecenes; for
identification and confirmation of trichothecenes in biological
materials, and as a sensitive and specific means of detection with
GC, LC, or supercritical fluid chromatography (SFC) sample
introduction.
The usefulness of negative ion chemical ionization (NICI) mass
spectrometry with hydroxide ion reagent gas has been demonstrated in
relation to producing relative molecular mass information, as well as
fragment ions indicative of structure for type A and B trichothecenes
(Brumley et al., 1982). The NICI technique has been applied to
confirmation of the presence of DON in cereals and snack foods by
rapid capillary GC introduction of the underivatized extract
obtaining full scan spectra (Brumley et al., 1985), and by selected
ion monitoring (Miles & Gurprasad, 1985).
NICI has also been used for selected ion monitoring GC/MS of the
HFB derivatives of 13 different trichothecenes of both type A and
type B in extracts from small samples of biological material
(Krishnamurthy et al., 1986). GC/MS is the preferred approach using
SIM in electron ionization (D'Agostino et al., 1986) or NICI (Begley
et al., 1986) modes for biological samples where only small sample
sizes are available but high sensitivity is required. For more
routine surveys of cereal and food samples, particularly for DON and
NIV, initial screening has normally been carried out using GC with an
ECD and only selected positive samples have been confirmed by GC/MS
(Tanaka et al., 1985a; Cohen & Lapointe, 1982).
Attempts have been made to determine the macrocyclic
trichothecenes as TMS derivatives on short fused silica GC columns
using GC/MS (Rosen et al., 1986). The approach has also been adopted
of alkaline hydrolysis of the ring system of the macrocyclic
compounds to yield verrucarols, which can then be determined as HFB
derivatives by NICI GC/MS (Krishnamurthy et al., 1987). A similar
approach using alkaline hydrolysis has been proposed (Rood et al.,
1988a,b) as a basis for a general method for the determination of
trichothecenes and metabolites by conversion to their corresponding
parent alcohols prior to derivatization and GC or GC/MS
determination.
Although LC/MS is really a research method, thermospray LC/MS is
becoming more widely available and shows promise for the
identification of low levels of trichothecenes in biological
materials (Voyksner et al., 1985; Rajakyla et al., 1987). Another
promising research method for the determination of both the simple
and the macrocyclic trichothecenes is supercritical fluid
chromatography (SFC) combined with MS (Smith et al., 1985).
Supercritical fluids can also be used for the on-line extraction from
biological materials and direct introduction into the MS to monitor a
number of different trichothecenes (Kalinoski et al., 1986).
II.1.2.2 Immunological methods
Chu et al. (1979) developed a radioimmunoassay (RIA) for T-2
toxin. The antibody preparation was obtained by immunizing rabbits
with bovine serum albumin T-2 toxin hemisuccinate conjugate. The
antibody had the greatest binding efficiency for T-2 toxin and less
efficiency for HT-2 toxin. Cross reaction with other trichothecenes
was either very slight or absent, and the limit of detection of the
assay ranged from 1 to 20 ng.
This RIA was applied to the determination of T-2 toxin in
agricultural commodities, biological fluids, and animal organs (Lee &
Chu, 1981a,b; Hewetson et al., 1987). Xu et al. (1988) also reported
an immunoassay using an antibody against triacetylated DON, for the
determination of DON in wheat and corn with a limit of detection of
about 0.02 µg/g.
A polyclonal enzyme-linked immunosorbent assay (ELISA) was
developed for the rapid quantification of T-2 toxin in food and
animal feed (Pestka et al., 1981). This assay, which could be
undertaken in 2 h, had a limit of detection of 2.5 pg/assay and was
used for the detection of T-2 toxin in Fusarium-infected corn
(Gendloff et al., 1984). More recently monoclonal ELISAs have been
developed for T-2 toxin and DON (Feuerstein et al., 1985; Hunter et
al., 1985; Chiba et al., 1988). Although these monoclonal assays are
of invariable specificity and the antibodies are available in
unlimited supply, competitive indirect ELISAs using monoclonals are
generally less sensitive than polyclonal-based ELISAs. However,
Chiba et al. (1988) developed a sensitive ELISA for the detection of
T-2 toxin with monoclonal antibodies at a limit of 2.5 pg/assay.
This assay has been applied to the detection of T-2 toxin in wheat
flour (Chiba et al., 1988) and in fungal cultures (Nagayama et
al., 1988).
Production of useful antibodies against DON has proved more
difficult than for other trichothecenes. Xu et al. (1988) adopted
the approach of raising antibodies against triacetyl-DON, which
requires acetylation of DON in an extract of the contaminated cereal
prior to carrying out a direct ELISA. This assay has the required
sensitivity but also the disadvantage of determining the total
concentration of DON plus any other acetylated derivatives that may
be present in the extract. A different approach to raising
monoclonal antibodies to DON has been carried out by forming the
hemisuccinyl derivative after protection of two of the available
adjacent hydroxyls with a cyclic boronate ester (Casale et al.,
1988). Some cross-reactivity to other trichothecenes and their
de-epoxy metabolites was observed, but the assay does show
considerable potential as a simple and rapid screening method for
contaminated cereals and for detection in biological samples.
II.1.2.3 Biological methods
Biological methods are essential for working with field cases
where the cause of an incident is unclear and evidence is sought to
associate an observed biological effect with the presence of fungally
contaminated material. Biological methods are also important as
monitoring procedures in the isolation and purification of new or
previously unrecognized toxins, prior to characterization by
spectroscopic and chemical methods.
The classical and commonly used bioassay for trichothecenes is
the skin necrotization test. Extracts prepared from samples (see
section II.1.2.1) are applied in a single dose to the shaved back of
a rabbit, rat, or guinea-pig. Toxic preparations applied in this way
produce erythema, oedema, intradermal haemorrhage, and necrosis. The
guinea-pig is the most sensitive of these animals to trichothecenes
(Ueno et al., 1970). As little as 0.2 µg of T-2 toxin and DAS were
detected on the skin of the rabbit and guinea-pig (Chung et al.,
1974; Balzer et al., 1977). Only 10 g of the corn sample are needed
for testing with an extraction and clean-up procedure recommended by
Eppley (1968). Macrocyclic trichothecenes are highly irritant to the
skin, but NIV and DON, which have a low skin-necrotizing potency,
could be routinely missed in this assay (Ueno et al., 1970).
Brine shrimp ( Artemia salina L.) larvae also provide a useful
biological system for monitoring trichothecenes, such as T-2 toxin,
DAS, and the macrocyclic trichothecenes. The limit of detection of
this test for these toxins is approximately 0.04-0.4 mg/litre
(Eppley, 1974).
The rabbit reticulocyte assay, based on the inhibition of uptake
of 14C-leucine in eukaryote cells, is highly specific for
trichothecenes; ID50 (50% inhibitory dose) values are 0.03 mg/litre
for T-2 toxin and DAS, and 2.0-3.0 mg/litre for DON and NIV (Ueno et
al., 1969a, 1973c). By combining chemical methods with this assay,
DON was successfully isolated from Fusarium-infected corn (Ishii et
al., 1975).
II.2 SOURCES AND OCCURRENCE
II.2.1 Taxonomic considerations
The vast majority of trichothecenes are produced by the Fusarium
species. Production of these metabolites depends, of course, on many
factors, including substrate, temperature, humidity, etc. In
general, the type A trichothecenes have been most frequently
associated with the following Fusarium species: F. tricinctum, F.
sporotrichioides, F. poe, F. equiseti. On the other hand, type B
trichothecenes are usually associated with F. graminearum (Gibberella
zeae), and F. culmorum. The type C trichothecenes, containing an
additional epoxide function at the 7,8- or 9,10-positions, are
produced by only a few species. The Type D trichothecenes, i.e.,
those containing the macrocyclic ring between the 4,15-positions, are
produced by several fungal genera, including Myrothecium and
Stachybotrys. Of these, the most important from an animal and human
health standpoint, is Stachybotrys atra. The relationships between
trichothecenes and fungal species are summarized in Table 13.
Table 13. The relationship between trichothecenes and fungal species
----------------------------------------------------------------------
Trichothecenes Fungal species References
----------------------------------------------------------------------
Type A
T-2, HT-2, DAS, NS Fusarium tricinctum Joffe & Yagen (1977)
F. sporotrichioides Scott et al. (1980)
F. poae Marasas et al. (1984)
F. acuminatum Ichinoe et al. (1985),
Rabie et al. (1986)
DAS F. equiseti Brian et al. (1961)
F. semitectum Suzuki et al. (1980),
Greenhalgh et al. (1984)
Type B
DON, 3-AcDON F. graminearum Yoshizawa & Morooka (1973)
NIV, F-X Gibberella zeae Marasas et al. (1984)
(anamorph) Ichinoe et al. (1985)
DON, 3-AcDON F. culmorum Marasas et al. (1979b)
Chelkowski et al. (1984)
Trichotecin Trichothecium Freeman & Morrison (1949)
roseum Ishii et al. (1986)
Type C
Baccharin Baccharis Kupchan et al. (1976)
megapotamica
(higher plant)
Type D
Roridin A, D, E Mycothecium roridum Bohner et al. (1965)
Verrucarin J M. verrucaria Harrach et al. (1981)
Satratoxin G, H Stachybotrys atra Jarvis et al. (1986)
----------------------------------------------------------------------
The taxonomy of the various fungal species is complex and has led
to some confusion, particularly regarding the Fusarium species
responsible for the production of the various trichothecene
metabolites. Several excellent descriptions are now available of the
Fusarium spp. and the metabolites produced by them (Nelson et al.,
1983; Ueno, 1983; Marasas et al., 1984; Ichinoe et al., 1985; Joffe,
1986).
II.2.2 Ecology of trichothecene-producing fungi
Fusarium species are widely distributed throughout the
environment. There are over 50 recognized species commonly occurring
in the soil (soil fungi), many of which are pathogenic to crop
plants. As a result, there are a number of frequently encountered
plant diseases (wilts, blights, rots), such as F. graminearum blight
of wheat and barley (Akakabibyo in Japanese), pink ear rot of corn,
pink scab (tombstone kernels) of wheat, Fusarium snow blight, etc.
Fusarium graminearum, which produces DON and NIV, is a most important
fungus.
II.2.3 Natural occurrence
It has become apparent in the past few years that whenever a
trichothecene-producing Fusarium species parasitizes a crop, food,
or animal feed, it is highly probable that the metabolites of the
trichothecene will be found as contaminants. The chance of detecting
the metabolite is then clearly a function of the efficiency of the
sampling procedure and the capabilities of the analytical methods
used (most importantly, the detection limit).
Because of its toxicity, analytical procedures for T-2 toxin were
developed first, and consequently early surveys for trichothecenes
tended to concentrate on T-2 toxin. However, it soon became apparent
that other trichothecenes, in particular, DON, NIV, and DAS, were
more frequent contaminants of food and animal feed than T-2 toxin.
As improved analytical procedures for these mycotoxins became
available, surveys were conducted and additional data on the
occurrence of these metabolites were published. In reviewing this
record, it should be recognized that the methodology used in
developing the data was quite variable, so that only broad
generalizations with respect to incidence/level may be drawn. In the
vast majority of cases, no evidence was included indicating that
methods used were rigorously tested, that quality assurance
programmes were in place, and that confirmation of identity was
adequately obtained. Much of the available survey data on the
natural occurrence of trichothecenes in raw agricultural commodities,
food, and animal feed is summarized in Tables 14-17. Reports in
which only a few samples were analysed have not been included.
II.2.3.1 Agricultural products
(a) T-2 Toxin
One of the first trichothecenes to be implicated in an episode of
mouldy corn toxicosis was T-2 toxin; in 1972, it was reported that
T-2 toxin at the level of 2 mg/kg was present in mouldy corn involved
in lethal toxicosis in dairy cattle (Hsu et al., 1972). This event,
along with increasing information regarding the acute toxicity of T-2
toxin, prompted considerable efforts to develop methods of analysis
for T-2 toxin and the analysis of a wide range of agricultural
commodities (Table 14). Only occasional samples were found to
contain T-2 toxin (incidence well below 10% in most cases), most
frequently at levels <0.1 mg/kg. Usually, other trichothecenes were
also found (Vesonder, 1983). On the other hand, there have been
isolated reports of the finding of rather high levels of T-2 toxin,
e.g., the finding of 25 mg T-2 toxin/kg in barley (Puls & Greenway,
1976), and 38.9 mg T-2 toxin/kg in peanuts (Bhavanishankar & Shantha,
1987). These findings, as well as reports from India of the presence
of T-2 toxin in safflower seed and sweet corn (Ghosal et al., 1977),
and sorghum (Ruckmini & Bhat, 1978), and from Italy of its presence
in barley, corn feed, oats, rice, and wheat (Cirilli, 1983) need to
be further investigated.
Table 14. Natural occurrence of T-2 toxin in raw agricultural
commodities
---------------------------------------------------------------------
Commodity Levels Incidence Country Reference
(mg/kg) (+ve/total)
---------------------------------------------------------------------
Corn 0.5-5.0 (5/150) Hungary Szathmary (1983)
0.080-0.65 (9/118) Taiwan Tseng et al. (1983)
0.01-0.2 (13/20) New Zealand Hussein et al.
(1989)
Feed 0.05-5.0 (28/464) Hungary Szathmary (1981)
Oats 0.01-0.05 Finland Ylimacki et al.
(1979)
Peanuts 0.63-38.89 (6/87) India Bhavanishankar &
Shantha (1987)
Rice (3/64) Egypt Abdel-Hafez et al.
(1987)
Sorghum 1.67-15.0 (4/84) India Bhavanishankar &
Shantha (1987)
Wheat 2.0-4.0 (3/12) India Bhat et al. (1989)
---------------------------------------------------------------------
DON/NIV: Deoxynivalenol (DON) and nivalenol (NIV) have been
found to be the most frequent trichothecene contaminants of
agricultural crops throughout the world (Table 15). Extensive
survey data indicate the common occurrence of these mycotoxins,
particularly in corn and wheat, at levels usually below 1 mg/kg
(Vesonder, 1983; Pohland & Wood, 1987; Jelinek et al., 1989).
Perhaps the most extensive survey, and the one giving the most
accurate picture of the global occurrence of DON and NIV, was
reported recently by Tanaka et al. (1988).
Table 15. Natural occurrence of DON and NIV in raw agricultural
commodities
---------------------------------------------------------------------------
Commodity Toxin Levels Incidence Country Reference
(mg/kg)a (+ve/total)
---------------------------------------------------------------------------
Barley DON t-40.4 (19/25) Japan Kamimura et
al. (1981)
NIV t-37.9 (19/23)
(unpolished) DON 0.004-0.508 (26/28) Korea Lee et al.
NIV 0.017-3.0 (28/28) Republic of (1985)
(polished) DON 0.008-0.043 (5/6)
NIV 0.085-0.328 (5/6)
DON 0.006-2.14 (34/49) Norway Sundheim et
NIV 0.013-1.56 (49/49) al. (1988)
DON 0.02-0.36 (34/87) United Gilbert et
Kingdom al. (1983b)
Corn DON 1-20 (19/60) Austria Lew et al.
(1979)
DON 0.15-0.82 (9/9) Canada Scott et
al. (1981)
---------------------------------------------------------------------------
Table 15 (contd.)
---------------------------------------------------------------------------
Commodity Toxin Levels Incidence Country Reference
(mg/kg)a (+ve/total)
---------------------------------------------------------------------------
Corn (contd.) DON 0.36-12.7 (100%) China Qiujie et
NIV 0.054-2.67 (100%) al. (1988)
DON 0.02-0.3 (11/20) New Zealand Hussein et
al. (1989)
DON t-15.8 (20/72) Transkei Thiel et
NIV t-1.41 (43/72) al. (1982)
DON 0.1-0.3 (37) United Gilbert et
Kingdom al. (1984)
DON 0.5-10.7 (24/52) USA Vesonder et
al. (1978)
DON 0.1-2.47 (93/198) USA Wood &
Carter
(1989)
Oats DON 20 Germany, Bauer et
Federal al. (1980)
Republic of
DON 0.02-0.1 (1/6) United Gilbert et
Kingdom al. (1984)
Rye DON 0.003 (1/5) Korea Lee et al.
NIV 0.046-0.114 (5/5) Republic of (1985)
Wheat DON 0.01-4.3 (51/52) Canada Scott et
al. (1981)
DON 0.06-8.53 (24/52) Canada Trenholm et
al. (1981)
DON 0.02-1.32 (55/199) Canada Osborne &
Willis
(1984)
DON 1.0 (1.36) Denmark Hald & Krogh
(1983)
DON t-4.7 (15/18) Germany, Bauer et
NIV t-7.8 Federal al. (1980)
Republic of
DON 0.008-3.19 (32/53) Norway Sundheim et
al. (1988)
NIV 0.015-0.887 (53/53)
DON 0.02 >0.5 (57/148) United Gilbert et
Kingdom al. (1984)
DON 0.12-5.5 (31/33) USA Hagler et
al. (1984)
DON 0.2-9 (54/57) USA Eppley et
al. (1984)
DON 0.1-2.65 (133/247) USA Wood &
Carter
(1989)
---------------------------------------------------------------------------
Table 15 (contd.)
---------------------------------------------------------------------------
Commodity Toxin Levels Incidence Country Reference
(mg/kg)a (+ve/total)
---------------------------------------------------------------------------
Animal DON 0.1-41.6 (274/342) USA Cote et
feed al. (1984)
DON <0.4-4.0 USA Vesonder
(1983)
---------------------------------------------------------------------------
a t = trace.
This survey involved the analysis of 500 samples of cereal grains
from 19 countries (Table 16) using a single analytical method.
Table 16. Natural occurrence of DON and NIV: worldwide survey
---------------------------------------------------------------------
Commodity DON NIV
(Mean, ng/g) (% + ve) (Mean, ng/g) (% + ve)
---------------------------------------------------------------------
Barley 149 75 401 76
Corn 402 20 766 16
Oats 115 22 438 26
Rice 0 22 22
Rye 183 33 47 33
Sorghum 0 0
Wheat 488a 39 127 50
Othersb 135 44 3 6
Total: 292 45 267 48
-----------------------------------------------------------------------
a A Beijing wheat sample containing 6 644 ng/g was excluded in
calculating this mean.
b Wheat flour, 7; rye flour, 1; spice, 3; sesame, 7.
It was found that 45-50% of random samples contained both DON and
NIV, barley being most frequently contaminated. Corn, although less
frequently contaminated, contained the highest average amounts of
NIV; of all the cereals examined, wheat was the most heavily
contaminated with DON. There were clear regional differences, not
totally predictable, in the relative quantities of DON and NIV. For
example, in Canada and the USA, NIV was only rarely encountered
whereas, in Japan, NIV was more frequently encountered than DON.
Even within a country there were clear differences between regions;
for example, in southern Japan, NIV was more frequently encountered
than DON, whereas in northern Japan the reverse was true. Similarly,
levels of DON were generally lower in western than in eastern Canada
(Jelinek et al., 1989).
The data indicate that crops parasitized by Fusarium species will
probably be contaminated with these mycotoxins. In wheat, for
example, there is an excellent correlation between the DON
contamination level and the percentage of mouldy kernels, the
percentage of total defects, and the degree of scab damage (Eppley et
al., 1984; Shotwell et al., 1985). It has been suggested that crop
rotation might be a factor, i.e., planting wheat after corn tends to
increase the DON levels in the resulting wheat crop (Teich &
Hamilton, 1985). Recently, it has been shown that the mean
concentration of DON in wheat declines quite significantly in the
2-week period immediately preceding harvest (Scott et al., 1984).
(b) Miscellaneous trichothecenes
There have been occasional reports of trichothecenes other than
those discussed above (T-2 toxin, DON, NIV) in agricultural products,
in particular, DAS, 15-acetyldeoxynivalenol (15-ADON), and 3-
acetyldeoxynivalenol (3-ADON) (Table 17). Most of these reports
involve corn. There is also an unconfirmed report of the finding of
T-2 toxin, DAS, and trichothecolone as well as the fatty acid esters
of trichothecolone, scirpenetriol, and T-2 tetraol in banana fruit,
which needs further investigation (Chakrabarti & Ghosal, 1986). In
1982, over 100 out of a flock of 1200 ewes on a Hungarian farm died
after ingestion of bedding straw highly infected with Stachybotrys
atra. TLC, HPLC, and MS analysis of the methanol extract of the
straw revealed the presence of the macrocyclic trichothecenes,
satratoxins G and H (Harrach et al., 1983).
Table 17. Natural occurrence: miscellanceous trichothecenes in
agricultural products
---------------------------------------------------------------------------
Commodity Toxin Level Incidence Country Reference
(mg/kg) (+ve/total)
---------------------------------------------------------------------------
Corn 15-ADON 0.113 China Qiujie et al.
ave. (1988)
3-ADON 0.495 China Qiujie et al.
ave. (1988)
DAS 31.5 Germany, Siegfried (1977)
Federal
Republic of
DAS 0.01- (6/20) New Zealand Hussein et al.
0.9 (1989)
15-ADON 0-7.9 USA Abbas et al.
(1986)
Peanuts DAS 0.41- (7/87) India Bhavanishankar &
2.03 Shantha (1987)
Wheat ADON 0.6-2.4 (4/12) India Bhat et al.
flour (1989)
Bedding satratoxin not Hungary Harrach et al.
straw G estimated (1983)
satratoxin not Hungary Harrach et al.
H estimated (1983)
---------------------------------------------------------------------------
The macrocyclic trichothecenes, such as satratoxin H, verrucarins
B and J, and trichoverrins A and B, were also detected in air dust
collected from a house in Chicago, where the occupants were subjected
to a variety of recurring maladies including colds, dermatitis, and
others (Croft et al., 1986). These data indicated a possible
airborne outbreak of macrocyclic trichothecene-induced toxicosis.
Undoubtedly, as good analytical methods become available, these
trichothecenes will be found more frequently.
II.2.3.2 Trichothecenes in human foodstuffs
Given the widespread occurrence of trichothecenes in agricultural
products, in particular DON and NIV, it is not surprising to find the
compounds in human foodstuffs (Table 18). The vast majority of the
confirmed cases of contamination of foodstuffs by trichothecenes
involve DON in wheat or wheat products. Overall, the finding of DON
in human foodstuffs at concentrations >1 mg/kg has been rare. NIV
has been detected in human foodstuffs, particularly in Japan, where
an effort was made to develop and apply analytical methods capable of
determining both DON and NIV.
Table 18. Natural occurrence of DON and NIV in commercial foods
---------------------------------------------------------------------------
Commodity Toxin Level Incidence Country Reference
(mg/kg)a (+ve/total)
---------------------------------------------------------------------------
Barley DON 0.027-0.085 (6/6) Japan Yoshizawa &
flour NIV 0.037-0.19 (6/6) Hosokawa
(parched) (1983)
Barley DON 0.008-0.039 (5/6) Tanaka et
flour NIV 0.013-0.041 (6/6) al. (1985b)
Barley DON 0.003-0.05 (10/14) Tanaka et
(pressed) NIV 0.008-0.033 (13/14) al. (1985a)
Barley DON t-0.26 (27/147) Kamimura et
products NIV 0.006-0.28 al. (1981)
Corn meal DON 0.025 (45/50) USA Trucksess et
al. (1986a)
Corn flour DON (0.18 ave.) (27) Canada Scott et al.
meal DON (0.1 ave.) (35) (1984)
products DON 0.011-1.25 (7/7)
Popcorn DON 0.012-0.25 (7/7) Japan Tanaka et al.
Job's tear DON 0.048-0.5 (2/12) (1985b)
NIV 0.003-0.92 (11/12)
Potatoes DON (4/17) Canada El Banna et
al. (1984)
Rye flour DON (0.12 ave.) (3) Canada Scott et al.
bread NIV (0.058 ave.) (4) (1984)
Wheat DON (0.4 ave.) (43)
flour DON 0.11-0.69 (5/5) China Ueno et al.
(1986)
DON 0.43-4.89 (9/12) India Bhat et al.
NIV 0.03-0.1 (2/12) (1989)
DON 0.002-0.239 (26/36) Japan Tanaka et
NIV 0.004-0.084 (12/36) al. (1985b)
DON 0-0.46 (44/50) USA Trucksess et
al. (1986a)
---------------------------------------------------------------------------
Table 18 (contd.)
---------------------------------------------------------------------------
Commodity Toxin Level Incidence Country Reference
(mg/kg)a (+ve/total)
---------------------------------------------------------------------------
Breakfast DON (0.086 ave.) (36) Canada Scott et al.
cereals (1984)
Bread DON (0.08 ave.) (21)
Baby cereal DON (0.043 ave.) (30)
Crackers DON (0.27 ave.) (20)
Cookies DON (0.12 ave.) (25)
Breakfast DON 0-0.53 (35/60) USA Trucksess et
cereals al. (1986a)
Bread DON 0-0.24 (20/25)
Baby foods DON 0-0.09 (14/39)
Snack foods DON 0-0.45 (25/44)
Bread DON 0.013-0.24 (39/45) USA Wood & Carter
(1989)
---------------------------------------------------------------------------
a t = trace.
The realization that trichothecene contamination of cereal grains
was not uncommon prompted a series of studies on the fate of these
compounds during the normal processing of such grains into consumer
food products. These studies can be summarized as follows:
In the case of corn, Collins & Rosen (1981) demonstrated that,
during wet milling, roughly 66% of the T-2 toxin originally present
was removed in the steeping water, about 4% remained in the starch,
and the remainder was distributed evenly in the germ, gluten, and
fibre fractions (a result clearly predictable in view of the
considerable water solubility of T-2 toxin). The same general
observation was made about the fate of DON during the wet milling of
corn (Scott, 1984b). During the dry milling of corn, the major
portion of contaminating DON was found in the germ meal, which is
used for animal feed. For any significant contamination of grits,
the corn used in the manufacture would have to be highly contaminated
(Gilbert et al., 1983a).
In the case of wheat, several studies have clearly demonstrated
that cleaning and milling are not effective in completely removing
DON (Hart & Baselton, 1983; Scott et al., 1983, 1984a; Young et al.,
1984; Seitz et al., 1985). The same was generally true for NIV (Lee
et al., 1987). Normal cleaning of wheat reduced average DON
contamination levels by 6-19% (Abbas et al., 1985). The remaining
DON, after milling, is distributed in all fractions (bran, shorts,
reduction flour, break flour), the greatest amounts being found in
the bran and the smallest amounts in the flour, depending on the
level of contamination of the wheat itself. In a typical study,
milling of whole wheat contaminated at the 2 µg/g level, resulted in
approximately 65% of the DON in the flour and 35% in the bran, red
dogs, and shorts; i.e., there was little reduction in the DON
concentration in the flour after milling (Hart & Baselton, 1983).
It has been found that the further processing of flour into baked
or cooked products results in variable DON losses. The overall range
of reduction in DON levels from uncleaned wheat to bread was 24-71%
(Abbas et al., 1985). No reduction in DON levels was found in the
preparation of Egyptian bread (El-Banna et al., 1983) or cookies
from hard wheat flour (Scott et al., 1983), illustrating the
importance played by the conditions of processing. Kamimura et al.
(1979) showed that bread and noodles prepared under conditions
simulating commercial processes retained about 50% of the
trichothecenes that had been previously added to the wheat flour.
The trichothecene content of Japanese noodles was reduced by 30% in
the boiling process.
Similarly, these authors found that 30% of NIV and DON was
extracted from naturally contaminated wheat by water, while there was
a 50% or more reduction in trichothecene levels in bread made from
the same flour. The preparation of Chinese noodles, where sodium
carbonate is used as an ingredient, results in an even greater loss
of DON than is observed in the preparation of Japanese noodles (Scott
et al., 1984b; Nowicki et al., 1988). Similarly, Young et al. (1984)
observed a decrease of up to 35% in the production of cookies from
biscuit and cake flours; in the process studied, the batter contained
ammonium carbonate. Finally, it has been found that, during the
baking of bread, 3-13% of DON is converted into an isomer, iso-DON
(Greenhalgh et al., 1984).
There have been some studies on possible detoxification
procedures for corn and wheat contaminated with trichothecenes;
agents studied include chlorine, ammonia (Young et al., 1984) and
aqueous bisulfite (Young et al., 1986). None of these processes are
currently commercially feasible.
There has been some concern about the transmission of the
trichothecenes, particularly DON, to milk. Studies have shown that
milk is only a minor excretion route in the lactating dairy cow;
thus, the possibility that DON might contaminate milk and milk
products is remote (Prelusky et al., 1984).
II.3 METABOLISM
II.3.1 Absorption and tissue distribution
II.3.1.1 Animal studies
In general, trichothecenes absorbed from the alimentary tract are
evenly distributed in many tissues and organs, without significant
accumulation in specific organs. However, at present, distribution
studies have been limited mainly to T-2 toxin, and the metabolism in
animals of other naturally contaminating trichothecenes, such as DON,
NIV, and DAS, remains to be elucidated.
In 6-week-old broiler chicks fed with a ration containing T-2
toxin at 2 mg/kg for 5 weeks and then intubated with a single dose of
3H-T-2 toxin at 0.5 mg/kg body weight, the radioactivity reached a
maximum concentration in most tissues, 4 h after dosing; exceptions
were the muscle, skin, and bile, in which the maximum level was
reached after 12 h (Chi et al., 1978b). After 48 h, chicks contained
the equivalent of 39 µg T-2 toxin and/or its metabolites/kg in the
muscle, and 40 µg/kg in the liver, as calculated on the basis of the
specific activity of the radiolabelled T-2 toxin administered.
In chicken organs, 18 h after intraperitoneal injection of T-2
toxin (3.5 mg/kg), considerable amounts of T-2 metabolites were found
in the liver (1370 µg 3'-hydroxy-H-T-2 toxin/kg). Smaller amounts of
H-T-2 toxin, T-2 triol, and other metabolites were detected in the
lungs (Visconti & Mirocha, 1985).
In weanling crossbred pigs (7.5-9.5 kg body weight) intubated
with 0.1 or 0.4 mg 3H-T-2 toxin/kg body weight, the percentage of
administered radioactivity (18 h after dosing) was 0.7% in the
muscle, 0.29-0.43% in the liver, 0.08% in the kidney, and 0.06-0.14%
in the bile (Robison et al., 1979b). In pigs intubated with 0.1 mg of
the toxin/kg body weight, the calculated residue levels for T-2 toxin
and/or its metabolites, based on the specific radioactivity of the
tissues, were as follows: muscle, 3.1 µg/kg; fat, 0.49 µg/kg; liver,
13.8 µg/kg; and kidney, 15.9 µg/kg. The corresponding residue levels
for T-2 toxin in the tissue of another pig intubated with 0.4 mg of
the toxin/kg body weight were 11.5 µg/kg in the muscle, 37.7 µg/kg in
the liver, and 61.4 µg/kg in the kidney.
Four hours after intravenous administration of T-2 toxin in
swine, the greatest amount of radioactivity was located in the
gastrointestinal tract (15-24% of the dose), and 4.7-5.2% of the dose
was found in the remaining tissues, among which the muscle and the
liver accounted for 2.9-3.2% and 0.7-1.7% of the dose, respectively
(Corley et al., 1986).
The fate and distribution of 3H-T-2 toxin was studied in guinea-
pigs (Pace et al., 1985). Except for the large intestine and bile,
the radioactivity had peaked by 30 min and rapidly declined, with no
measurable long-term accumulation. In general, the distribution
pattern in the guinea-pig during the first 12-24 h paralleled the
distribution found in the chicken and swine.
The metabolic fate of T-2 toxin was investigated in a lactating
Jersey cow weighing 375 kg, after daily oral administration, by
capsule, of unlabelled toxin at 180 mg/day for 3 days, followed by
administration of 3H-T-2 toxin at 156.9 mg (Yoshizawa et al., 1981).
Although almost all of the administered dose was eliminated in 72 h,
appreciable levels of tritium still remained in the bile, liver, and
kidney (equivalent, repectively, to 27.2, 18.5, and 13.9 µg/kg 3H-T-2
toxin) 3 days after dosing. These levels are higher than in whole
blood (13.3 µg/kg) and plasma (10.2 µg/kg) and in other tissues,
including the spleen (9.4 µg/kg) heart (10.1 µg/kg), mammary gland
(11.3 µg/kg), ovaries (10.7 µg/kg), muscle (8.8 µg/kg), and fat (4.7
µg/kg).
Experimentally derived relationships between the residue of
radioactivity in animal tissues or plasma and the toxin levels in the
feed, calculated on the basis of the amount of 3H-T-2 toxin
administered are summarized in Table 19 (Yoshizawa et al., 1981).
Table 19. The relationship between the level of 3H-T-2 toxin in the feed or the
tritium residues in plasma and tritium levels in the edible tissues of the cow, chick,
and piga
---------------------------------------------------------------------------------------
Tissue Animal Time after Feed level Tissue level Tissue/feed Tissue/plasma
dosing (h)b (mg/kg)c (µg/kg)d ratio ratioe
---------------------------------------------------------------------------------------
Muscle Cow 72 31.38 8.8 0.0003 0.863
Chick 24 1.26 17.3 0.0137 1.000
5.0 59.2 0.0118 0.938
18.95 228.6 0.0121 0.875
Pig 18 1.25 3.1 0.002 0.775
Heart Cow 72 31.38 10.1 0.0003 0.991
Chick 24 1.26 13.7 0.011 0.792
5.0 49.4 0.010 0.783
18.95 207.7 0.011 0.795
Pig 18 1.25 3.9 0.003 0.975
Liver Cow 72 31.38 18.5 0.0006 1.863
Chick 24 1.26 34.0 0.0270 1.965
5.0 107.3 0.0215 1.700
18.95 431.0 0.0227 1.649
Pig 18 1.25 13.8 0.011 3.450
Milk Cow 72 31.38 11.4 0.0004 1.118
---------------------------------------------------------------------------------------
a From: Yoshizawa et al. (1981).
b Animals were intubated with a single dose of 3H-T-2 toxin.
c Estimates of feed levels were based on the assumption that each animal would consume
the following amount of feed daily: cow, 5 kg; chick, 100 g; pig, 600 g.
d Values were calculated from residual tritium levels in the edible tissues of animals
given 3H-T-2 toxin and were expressed as equivalents of 3H-T-2 toxin (µg/kg).
e The plasma levels were 10.2 µg/kg equivalents of T-2 toxin in the cow, and 17.3,
63.1, and 261.3 µg/kg equivalents of T-2 toxin in the chick at feed levels of 1.26,
5, and 18.95 mg/kg, respectively. The whole-blood level of residual tritium in the
pig was 4 µg/kg equivalents of T-2 toxin.
The tissue/feed ratio of tritium in the edible tissues of the
cow, 72 h after dosing, ranges from 0.0003 to 0.0006. These figures
are 5-10% of those for swine, 18 h after dosing. The tissue/feed
ratio for chickens, is higher than those in the cow and pig, ranging
from 0.010 to 0.014 for the muscle and heart, and from 0.021 to 0.027
for the liver, regardless of T-2 toxin dosage. The tissue/plasma
ratios of T-2 toxin metabolites, ranging from 0.8 to 1 in the muscle
and heart, and from 1.6 to 3.5 in the liver, are independent of
animal species, of T-2 toxin dosage, and of time after dosing. The
milk/plasma ratio of radioactivity in a cow treated with 3H-T-2 toxin
increased linearly for 24 h and thereafter ranged from 1 to 1.3.
II.3.2 Metabolic transformation
The in vivo metabolic transformation of trichothecenes in animals
is reported in Table 20. The trichothecene metabolites produced are
less toxic than the corresponding parent toxins. Both de-epoxidation
and glucuronidation, in particular, are associated with remarkable
detoxification of trichothecenes.
Table 20. In vivo (metabolic) transformation of trichothecenes in animals
--------------------------------------------------------------------------------------------------
Animal Trichothecene Transformation reaction Reference
--------------------------------------------------------------------------------------------------
Chicken T-2 toxin hydrolysis, 3'-hydroxylation, Yoshizawa et al. (1980b);
acetylation Visconti & Mirocha (1985)
Cow T-2 toxin hydrolysis, 3'hydroxylation, Yoshizawa et al. (1981, 1982a,b)
7'-hydroxylation, de-epoxidation Pawlosky & Mirocha (1984)
Chatterjee et al. (1986)
DON de-epoxidation, Yoshizawa et al. (1986)
glucuronide conjugation Côté et al. (1986)
Pig T-2 toxin hydrolysis, 3'-hydroxylation, Corley et al. (1985, 1986)
de-epoxidation, glucuronide
conjugation
DAS hydrolysis Bauer et al. (1985)
Sheep DON glucuronide conjugation Prelusky et al. (1985)
Rat DON de-epoxidation Yoshizawa et al. (1983)
T-2 metabolites de-epoxidation Yoshizawa et al. (1985a,b)
DAS hydrolysis, de-epoxidation Sakamoto et al. (1986)
Guinea-pig T-2 toxin hydrolysis, 3'-hydroxylation Pace et al. (1985)
Dog T-2 toxin hydrolysis, glucuronide
conjugation Sintov et al. (1987)
--------------------------------------------------------------------------------------------------
In in vitro studies, HT-2 toxin was found to be the sole
metabolite of T-2 toxin in the microsomes of the liver, kidney, and
spleen of various animals (Ohta et al., 1977, Johnsen et al., 1986).
The reaction of hepatic microsomes of different species in nmol/mg
protein per 10 min were: rabbit, 3044; human, 331; mouse, 75;
chicken, 55; rat, 36; and guinea-pig, 14. Yoshizawa et al. (1984,
1986) have proposed an in vitro metabolic pathway for T-2 toxin that
includes the hydrolysates at the C-4, C-8, and C-15 positions, and
the hydroxylation at the C-3'position by liver homogenates from the
rat, mouse, or monkey. This hydrolytic transformation was also
observed in the hepatic homogenates of the rabbit, pig, and cow.
However, HT-2 toxin was the sole metabolite in the homogenate of the
chicken, suggesting species differences in the metabolic pathway of
T-2 toxin (Yoshizawa & Sakamoto, 1982). In addition to the
metabolites above, glucuronide conjugates were formed in a study
using isolated perfused rat livers and T-2 toxin and DAS (Gareis et
al., 1986). Various de-epoxidation metabolites were found by
incubating intestinal and rumen microbes with T-2 toxin and its
metabolites (Yoshizawa et al., 1985a; Swanson et al., 1987), DON
(King et al., 1984; Côté et al., 1986; Swanson et al., 1987), and DAS
(Swanson et al., 1987).
II.3.3 Excretion
II.3.3.1 Animal studies
The kinetics of T-2 toxin were determined in pigs and cattle
(Beasley et al., 1986), and dogs (Sintov et al., 1986). Mean
elimination phase half-lives were 13.8 and 17.4 min, and mean
apparent specific volumes of distribution were 0.366 and 0.375
litre/kg in intra-aortally dosed pigs and intravenously dosed calves,
respectively. In dogs, the following mean parameters were determined
after intravenous administration of T-2 toxin and HT-2 toxin,
respectively: half-life 5.3 and 19.6 min, clearance 0.107 and
0.167 litre/min per kg, and volumes of distribution 0.86 and 4.47
litre/kg.
The half-life of elimination of DON in sheep, ranged from 100 to
125 min following oral administration, and it took 20-30 h to clear
from the system. Glucuronidation after intravenous or oral
administration of DON appeared to occur quite efficiently (iv, 21%;
oral, 75%), with elimination half-lives of (150-200 min and 6.1-7.1
h, respectively). These were considerably longer than those of the
parent toxin (Prelusky et al., 1985).
II.3.3.2 Excretion in eggs and milk
Radioactivity was transmitted into the eggs from laying hens that
had been intubated gastrically with a single or several doses of 3H-
T-2 toxin (Chi et al., 1978a). In birds dosed singly with 0.25 mg
T-2 toxin/kg body weight, maximum residues in the eggs occurred 24 h
after dosing; the yolk contained 0.04% of the total dose and the
white contained 0.13%. In birds dosed with 0.1 mg T-2 toxin/kg body
weight per day for 8 consecutive days, the radioactivity in the egg
accumulated until the 5th day of dosing, remained unchanged until the
last day of dosing, and rapidly decreased thereafter. Assuming that
the birds weighing 1.6 kg consumed 100 g of the diet containing 1.6
mg toxin/kg daily, the residues (T-2 toxin and/or its metabolites) in
such contaminated eggs would be about 0.9 µg/egg.
Transmission of DON was studied in the eggs and meat of chickens
(El Banna et al., 1983; Prelusky et al., 1987). Following a single
oral dose of 14C DON, maximum radioactivity, which occurred in the
first eggs laid after dosing (within 24 h), amounted to 0.087% of the
dose: levels dropped rapidly in later eggs.
In a pregnant Holstein cow (third trimester) intubated daily with
180 mg T-2 toxin for consecutive days (the toxin level corresponded
to a concentration of 50 mg/kg in the feed), milk samples taken on
the 2nd, 5th, 10th, and 12th days of intubation contained T-2 toxin
concentrations ranging from 10 to 160 µg/kg (Robison et al., 1979a).
Transmission of DON-1 (de-epoxydeoxynivalenol) to milk was confirmed
in lactating dairy cows (Côté et al., 1986; Yoshizawa et al., 1986).
Fresh and conjugated DON were also present in cow's milk following
administration of a single oral dose of 920 mg DON, but only
extremely low amounts (<4 µg/litre) were detected (Prelusky et al.,
1984).
II.4 EFFECTS ON ANIMALS
II.4.1 Field observations
In Hungary and other central European countries, pyosepticaemia
has been reported sporadically, in the past, in horses after
ingestion of mouldy hay and straw. This disease was characterized by
haemorrhages of the intestine and muscles, severe diarrhoea, and
death. Bacterium pyosepticum viscosum was detected in 1929 in the
excreta, and the equine disease was diagnosed as a pyosepticaemia
(Forgacs, 1965; Danko & Szerafin, 1976). After the discovery of
toxigenic Stachybotrys atra and its metabolites, such as satratoxins
G and H (Eppley & Bailey, 1973), the disease was presumed to be the
same as stachybotryotoxicosis (Danko & Szerafin, 1976).
A field outbreak involving the death of 20% of a dairy herd was
associated with prolonged ingestion of a diet containing 60% mouldy
corn infested with F. tricinctum. The concentration of T-2 toxin in
the feed was approximately 2 mg/kg dry weight (Hsu et al., 1972).
The lesions in the cattle included extensive haemorrhages on the
serosal surface of the internal viscera. An outbreak of haemorrhagic
syndrome in cows was associated with commercial feed containing T-2
toxin or T-2-like toxin (concentration not determined). The affected
animals showed an extremely prolonged prothrombin time. Necropsy
findings in 2 adult cows were marked serosal, mucosal, and
subcutaneous haemorrhages (Hibbs et al., 1975).
An outbreak of a disease, observed in poultry (ducks, geese),
horses, and pigs, was associated with mouldy barley containing T-2
toxin at approximately 25 mg/kg (Greenway & Puls, 1976). Pigs fed
the suspect barley exhibited signs of feed refusal, vomiting, and
diarrhoea. The horses became depressed and salivated excessively.
The lesions in the geese included necrosis of the mucosa of the
oesophagus, proventriculus, and gizzard. No pathological lesions
were described in other animals.
DON was isolated from a batch of maize that had caused vomiting
in pigs (Vesonder et al., 1973).
Equine leukoencephalomalacia reported from South Africa
(Kellerman et al., 1972; Marasas et al., 1979a; Pienaar et al., 1981)
and bean-hull toxicosis reported in horses in Hokkaido, Japan
(Konishi & Ichijo, 1970) appear to be very similar diseases with
nervous signs and hepatopathy as the major components. The signs in
these diseases were quite different from those caused by
trichothecenes, though some fungal isolates from samples of bean-
hulls produced the trichothecenes, T-2 toxin, and neosolaniol (Ueno
et al., 1972). F. moniliforme was considered to be the causative
fungus (Haliburton et al., 1979), but none of its metabolites,
including moniliformin, have been established as the cause of the
disease (Kriek et al., 1977).
Reports of field outbreaks of animal toxicoses associated with
trichothecene-contaminated feed are summarized in Table 21.
II.4.2 Effects on experimental animals
II.4.2.1 General toxic effects
LD50 values for certain trichothecenes in several experimental
animal species are summarized in Table 22 (Ueno et al., 1983). The
oral LD50 for T-2 toxin was 10.5 mg/kg body weight in mice, 3.06
mg/kg in guinea-pigs, 5.2 mg/kg in rats, and 6.1 mg/kg in trout.
The LD50 values for T-2 toxin in different species vary, but not
greatly.
The LD50 values of fusarenon-X were compared using different
routes of administration. The LD50s (mg/kg) in mice were 3.4 (iv),
3.4 (ip), 4.2 (sc), and 4.5 (oral). These data indicate that the
acute toxicity estimated for a single administration did not differ
markedly when the toxin was administered by different routes (Table
20) (Ueno et al., 1971).
Similar data were obtained with DON and acetyl-DON. An
interesting finding was that the ratio of the maximum lethal dose
to the minimum lethal dose was approximately two, indicating a
sharp dose-response curve for lethality. No marked differences in
acute toxicity were observed between treated male and female
animals.
Newborn animals are more sensitive than adults to the
toxic effects of the trichothecenes. For example, the LD50 values
for toxin given sc (mg/kg body weight) in newborn mice were: T-2
toxin, 0.15; DAS, 0.17; and fusarenon-X 0.23 (Ueno et al., 1973a).
Table 21. Field observations on animal toxicosis caused by trichothecenes
----------------------------------------------------------------------------------------------------
Mycotoxin Sample Animal Signs & lesions Reference
(concentration in feed)
----------------------------------------------------------------------------------------------------
T-2 toxin (2 mg/kg) mouldy corn cattle extensive haemorrhages Hsu et al.
(20% died) (1972)
T-2 toxin (25 mg/kg) mouldy barley poultry, necrosis of mucosa in the Greenway &
horse, proventriculus and Puls (1976)
pig oesophagous of geese
T-2 toxin commercial feed cow haemorrhages Hibbs et
al. (1975)
DAS maize pig vomiting Vesonder et
al. (1973)
T-2 toxin corn meal horse oral lesions, haemorrhages, Szathmary
(6 out of 58 died) (1983)
T-2 toxin alfalfa horse inappetence, listlessness, Szathmary
(13 out of 31 died) (1983)
T-2 toxin poultry oral lesions, inappetence, Szathmary
death (1983)
DAS and T-2 toxin corn cattle death Szathmary
(1983)
DAS oat, sifting pigeon emesis, bloody stools Szathmary
(1983)
T-2 toxin (2.5 mg/kg) chicken feed broiler inflammations, "atrophies" Szathmary
chicken (1983)
DAS (150-300 mg/kg) cattle, pig haemorrhagic syndrome Cirilli
(1983)
T-2 toxin (50-150 mg/kg) pig, cattle blood stools (swine, cattle) Cirilli
ear necrosis (swine) (1983)
intestinal lesions (poultry)
hepatic lesions (swine)
----------------------------------------------------------------------------------------------------
Table 22. LD50 values (mg/kg) of trichothecenesa
----------------------------------------------------------------------------------------------------------
Type Trichothecenes Mouse Rat Guinea-pig
iv ip sc oral iv ip sc oral ip sc oral
----------------------------------------------------------------------------------------------------------
A T-2 toxin 5.2 10.5 5.2 3.06
HT-2 toxin 9.0
Diacetoxyscirpenol (DAS) 12 23.0 1.3 0.75 7.3
Neosolaniol 14.5
Monoacetoxyscirpenol 0.725
B Nivalenol (NIV) 7.3 7.4 7.2 38.9
Fusarenon-X 3.4 3.4 4.2 4.5 4.4 0.5 0.1
Diacetylnivalenol 9.6
Deoxynivalenol (DON) 70.0 46.0
3-Acetyldeoxynivalenol 49.0 34.0
Trichothecin 300 250
D Roridin A 1.0
Verrucarin A 1.5 0.5 0.87
Verrucarin B 7.0
Verrucarin J 0.5
----------------------------------------------------------------------------------------------------------
Table 22 (contd.)
-----------------------------------------------------------------------------------------------------
Type Trichothecenes Rabbit Cat Dog Pig Duckling Day-old Chick Trout
(iv) (sc) (iv) (iv) (sc) chick (oral) (oral)
(oral)
-----------------------------------------------------------------------------------------------------
A T-2 toxin 0.5 1.21 1.75 4.0 6.1
HT-2 toxin 6.25
DAS 1.0 ca 1.1 0.37
3'-OH HT-2 toxin 8.5 5.0
15-Acetyl-T-2 tetraol 10.0
T-2 tetraol 10.0
B Fusarenon-X 5.0 ca 2.0
DON 27.0
Acetyldeoxynivalenol 37.0
D Verrucarin A 0.54
-----------------------------------------------------------------------------------------------------
a Adapted from: Ueno et al. (1983) and Ryu et al. (1988).
The toxic potency of the trichothecenes varies depending on the
modification of side chains in the molecule. The acute lethal
toxicity of certain trichothecenes was investigated using a single ip
injection in mice and the LD50 values (mg/kg body weight) were:
verrucarin A and B, 0.5; fusarenon-X, 3.4; NIV, 4.1; T-2 toxin, 5.2;
HT-2 toxin, 9.0; diacetylnivalenol, 9.6; neosolaniol
(8-hydroxydiacetoxyscirpenol), 14.5; DAS, 23.0; acetyldeoxynivalenol,
49.0; DON, 70.0; and crotocin, 810.0.
Matsuoka et al. (1979) investigated the general effects of
fusarenon-X on mice and rats. Fusarenon-X induced hypothermia, but
did not induce appreciable behavioural changes in mice. In ether-
anaesthetized rats, it caused a rise in blood pressure and a decrease
in respiratory rate, but did not induce any significant effects on
cardiac rate, the muscle cell membrane, or nerve elements.
The administration of trichothecenes to some animals (rats, mice,
and guinea-pigs) induces diarrhoea. The mechanism of this sign was
investigated using fusarenon-X and rats (Matsuoka & Kubota, 1981).
The ip injection of fusarenon-X in rats caused watery diarrhoea
within 36-60 h. At necropsy, 24 h after injection of fusarenon-X,
the small intestine was distended, but no blood was found in the
lumen of the intestine. The mycotoxin increased the absorption rate
of D-xylose from the intestine in vitro (Matsuoka & Kubota, 1981).
The leakage of intravenously injected Evan's blue dye into the
intestine also increased, but the sodium level in the serum
decreased. The intestinal villi were shortened and there was
extravasation of erythrocytes in the intestinal lamina propria. The
diarrhoea induced by ip admin-istration of 1.0 mg fusarenon/kg in
male Wistar rats was not mediated by the cyclic nucleotide system as
the mycotoxin did not increase the cyclic GMP and AMP contents in the
intestinal mucosa (Matsuoka & Kubota, 1987). The permeability of
abdominal blood vessels was increased in a dose-dependent manner in
mice given an ip injection of fusarenon-X, and the peak was reached
about 8 h after injection. The increased permeability was not
mediated by serotonin, histamine, norepinephrine, prostaglandins,
leukotrienes, or thromboxanes (Matsuoka & Kubota, 1987).
The effects of fusarenon-X and T-2 toxin on intestinal absorption
of monosaccharides were studied in rats. The absorption of 3- O-
methyl-glucose was reduced 1-3 times after either toxin was injected
into the jejunal lumen. Absorption of 3- O-methyl-glucose was also
reduced after the toxins were given by intravenous injection. Both
toxins impaired jejunal function by causing specific damage in the
active transport and diffusional movement of monosaccharides (Kumagai
& Shimizu, 1988).
Vomiting was one of the most significant signs of trichothecene-
induced toxicosis in the cat, dog, pig, and duckling (Ueno, 1980a).
T-2 toxin and related trichothecene mycotoxins at doses of 0.1-10
mg/kg induced vomiting (Vesonder et al., 1973; Sato et al., 1975;
Yoshizawa & Morooka, 1977; Ueno, 1980b; Matsuoka & Kubota, 1981).
The presence of the causal factor, DON, in mouldy corn was
established using ducklings as the assay animal (Ueno et al., 1974).
In pigs given 0.5 mg/kg body weight by infusion, vomiting commenced
6-7 min after dosing. Emesis or retching occurred at intervals of 2-
15 min and lasted from 0.5 to 2 min (Coppock et al., 1985). It is
strongly suggested that the mechanism of the vomiting of
trichothecenes is their possible action on the chemoreceptor trigger
zone (CTZ) in the medulla oblongata (Matsuoka et al., 1979). The iv
administration of 0.3 mg fusarenon-X/kg body weight to dogs induced
emesis and vomiting, 5-15 min after injection. Vomiting after
injection of fusarenon-X was prevented by prior administration of 0.5
mg metoclopramide hydrochloride/kg body weight or 1 mg
chloropromazine hydrochloride/kg.
The cardiovascular effects of the trichothecenes have varied
according to such factors as species, dose, and duration of exposure.
In acute studies, T-2 toxin given intravenously at 1 mg/kg body
weight produced a decline in blood pressure several hours after
administration, the reduced blood pressure being accompanied by a
decrease in heart rate (Smalley et al., 1970). A single dose of T-2
toxin administered to guinea-pigs and rabbits resulted in a decrease
in systemic blood pressure and a decrease in heart rate (Parker et
al., 1984; Wilson, 1984).
Using the in vitro bovine ear perfusion system, it was determined
that T-2 toxin can cause a dose-dependent vasoconstrictor response in
peripheral vasculature, but that the toxin is a less potent
vasoactive agent than either histamine or norepinephrine. The
presence of known histamine or noradrenergic anatogonist did not
affect the response to the toxin (Wilson & Gentry, 1985). T-2 toxin
administered systemically produced a marked increase in peripheral
vascular resistance in the conscious rat. The cardiac output
gradually decreased eventually resulting in cardiovascular collapse
and death (Feuerstein et al., 1985).
(a) Swine
The acute and short-term toxicities of T-2 toxin, DAS, and DON
were investigated in pigs (Weaver et al., 1978a,b; Coppock et al.,
1985). A single dose LD50 of T-2 toxin dissolved in ethanol and
administered iv was 1.21 ± 0.15 mg/kg body weight in normal, healthy,
crossbred pigs weighing from 3 to 50 kg. Soon after administration,
emesis was followed by eager consumption of feed, moderate posterior
paresis, staggering gait, extreme listlessness, and frequent
defecation of normal stools. Between 1 and 6 h, severe posterior
paresis, knuckling-over of the rear feet, and extreme lethargy were
observed. These signs were followed by severe posterior paresis,
frequent falling because of hind-quarter weakness, and the dragging
of both rear legs while moving about. Twenty-four hours after
administration, the surviving pigs appeared normal. Similar clinical
signs were observed in pigs exposed to T-2 toxin through inhalation
(Pang et al., 1988). Pathologically, necrosis was present in the
epithelial cells of the mucosa and in the crypt cells of the jejunum
and ileum, the Peyer's patches of the ileum, the lymphoid elements of
the caecum, the lymphoid follicles in the spleen, and the germinal
centre of the mesenteric lymph node (Weaver et al., 1978a).
Young pigs were fed with T-2 toxin at 1, 2, 4, or 8 mg/kg
standard pig ration for 8 weeks. No statistically significant
differences in body weight gain and feed consumption were observed
between the treated animals and the controls. Young pigs refused a
ration containing 16 mg T-2 toxin/kg, but not a diet containing 10-12
mg/kg. The no-observed-effect level was estimated to be less than 1
mg/kg, based on differences in body weight gain (Weaver et al.,
1978b). In terms of clinical haematological changes, such as
haemorrhaging, blood cell counts, serum-enzyme activities, and serum-
protein levels, the no-observed-effect level could not be accurately
determined, but was higher than 12 mg/kg, based on the weight gain.
The intravenous administration of T-2 toxin to pigs at doses of 4
or 8 mg/kg resulted in a shock syndrome characterized by reductions
in cardiac output and blood pressure and increased plasma
concentrations of epinephrine, norepinephrine, thromboxane B2, 6-
keto-PGF1a and lactate (Lorenzana et al., 1985). The pigs in the
high-dose group produced such signs as persistent vomiting, watery
diarrhoea, abdominal straining, cold extremities, coma, and death.
Eighteen white cross-bred female pigs weighing 40-60 kg,
immunized against erysepelas, were administered purified T-2 toxin
dissolved in 70% ethanol, intravenously, in doses of 0 (5 pigs), 0.6
(5 pigs), 1.2 (1 pig), 4.8 (5 pigs) and 5.4 (2 pigs) mg/kg. The
animals administered doses of 4.8 and 5.4 mg/kg died between 5 and
10.5 h later and other groups were killed 12-24 h after treatment.
Gross lesions were observed in pigs given 1.2 mg/kg or more and these
consisted of oedema, congestion, and haemorrhages of the lymph nodes
and pancreas and congestion and haemorrhages of the gastrointestinal
mucosa, subendocardium, adrenal glands, and meninges. Histological
alterations confirmed the gross lesions. Other lesions were
widespread degeneration and necrosis of lymphoid tissue and the
surface and crypt epithelium of the intestines. Scattered foci of
necrosis were present in the pancreas, myocardium, bone marrow,
adrenal cortex, and the tubular epithelium of the renal medulla.
Most lesions were dose dependent. The T-2 toxin-induced lesions in
the lymphoid and gastrointestinal tract of pigs were similar to those
described in other species. The heart and pancreas were additional
target organs in pigs (Pang et al., 1987b).
Male, castrated, crossbred, specific pathogen-free pigs (17
controls and 17 treated), 9-11 weeks of age, were used in a study
to characterize the pulmonary and systemic responses to inhaled T-2
toxin (nebulized dose of 9 mg/kg given by endotracheal tube) (Pang et
al., 1987a). The animals were exposed to the aerosol in pairs, one
animal receiving the toxin, the other acting as a control. From 20
to 30% of the toxin was retained by the pigs (T-2 toxin was mixed
with 100-200 µCi technetium for measurement). Five pairs of animals
each were killed 1, 3, and 7 days after dosing. Two pairs were
designated a 0.33 day group, when one treated pig died and the
other was killed in a moribund state, 0-10 h after dosing.
Clinically, the T-2 toxin-treated pigs vomited after exposure
producing such signs as cyanosis, anorexia, and lethargy. The pigs
became laterally recumbent. Alveolar macrophages showed reduced
phagocytosis and the blastogenic responses to mitogen were reduced
for pulmonary lymphocytes, but not for lymphocytes of the peripheral
blood. The lesions in pigs that died included multifocal
interstitial pneumonia, necrosis of lymphoid tissue,
necrohaemorrhagic gastroenteritis, oedema of gall bladder mucosa, and
multifocal areas of necrosis in the heart and pancreas. Inhalation
exposure to T-2 toxin produced a clinical and morphological syndrome
resembling that produced by intravenously administered T-2 toxin, at
doses of 1.2 mg/kg (approximate LD50) or more, as well as death.
Furthermore, the lesions produced by the inhaled toxin were more
severe.
The acute effects of DAS were studied using single, intravenous
doses (range: 0.30-0.48 mg/kg body weight) in 13 crossbred pigs;
there were 2 control pigs (Weaver et al., 1978b). Seven of the
toxin-exposed pigs developed emesis, frequent defecation, lethargy,
staggering gait, and prostration by 10 h leading to death. Severe
haemorrhagic necrotizing lesions and mucosal congestion involved the
jejunum and ileum and large intestines, portions of which were blood-
filled at necropsy. Lymphoid follicular necrosis was present in the
lymph nodes and spleen. The LD50 value was found to be 0.376 ± 0.043
mg/kg body weight.
Two female crossbred pigs were administered DON by rapid
intravenous infusion at a dose of 0.5 mg/kg body weight; there were
two matching controls. Vomiting was observed within a few minutes
of dosing, the skin was flushed and the extremities became cold.
Pigs had signs of diarrhoea, muscular weakness, tremors, and coma.
Symptoms were progressive in severity reaching a maximum 6-7 h after
the injection; recovery occurred after 12 h. Necrosis of pancreatic
acinar and islet cells was observed (Coppock et al., 1985). Pigs can
ingest up to 2 mg DON/kg feed without suffering any serious toxic
effects (Trenholm et al., 1984). T-2 toxin added to the ration at 18
or 30 mg/kg caused the refusal of feed in pigs (Szathmary & Rafai,
1978). Feed refusal and emesis have been produced in other species
and by other toxic metabolites of Fusarium species (Kotsonis et al.,
1975; Vesonder et al., 1977).
The minimum emetic dose of DON in pigs weighing 9-10 kg was 0.05
mg/kg body weight, when administered intraperitoneally, and 0.1-0.2
mg/kg body weight, when given orally. When this toxin was added to
feed, the feed consumption of 20-45 kg pigs, was reduced by 20% at a
dose of 3.6 mg/kg and by a 90% at a dose of 40 mg/kg (Forsyth et al.,
1977). Pigs were about twice as sensitive to DON as rats (Vesonder
et al., 1979). DON in contaminated wheat reduced feed intake and
weight gain, when it was fed to the pigs. The intake of feed
decreased linearly with increasing dietary concentration of DON
(Friend et al., 1982). The emetic activity of 15-acetyl DON in pigs
was similar to that of DON, the minimum emetic doses being 75 and 50
µg/kg, respectively (Pestka et al., 1987a).
(b) Poultry
Chi et al. (1977b), reported that the single oral LD50 dose of
T-2 toxin for one-day-old broiler chicks was 5 mg/kg body weight. It
was 5 and 6.3 mg/kg body weight for 8-week-old broiler chicks and
laying hens, respectively. Death of the birds occurred within 48 h
of T-2 toxin administration. Within 4 h of receiving the toxin,
birds developed asthenia, inappetence, diarrhoea, and panting. The
abdominal cavities of birds given lethal doses contained a white
chalk-like material that covered much of the viscera.
In a study by Wyatt et al.(1972), chickens were fed a diet
containing 1-16 mg T-2 toxin/kg feed for 3 weeks. The birds with
reduced growth at 4, 8, and 16 mg/kg developed yellow-white lesions
in the mouthparts at all dietary concentrations. The lesions
consisted of a fibrinous surface layer and a heavy infiltration of
the underlying tissues by granular leukocytes. Escherichia coli and
Staphylococcus epidermis were isolated from the lesions.
Terao et al.(1978) observed the effects of T-2 toxin and related
trichothecenes on the bursa of Fabricius of one-day-old chicks.
After injection of 5 mg T-2 toxin/kg body weight into the residual
yolk sac, cellular toxic effects were observed on the follicle-
associated epithelium, resulting in necrosis, which spread to the
periphery. The lesions induced by fusarenon-X and NIV were similar
to those induced by T-2 toxin, but the toxins were less potent and
their activity was estimated to be more than 40 times less than that
of cyclophosphamide.
The acute toxicity of DAS and of T-2 toxin dissolved in
dimethylsulfoxide in 7-day-old male broiler chicks was described by
Hoerr et al. (1981). The 72-h single oral LD50 doses of T-2 toxin
and DAS were estimated to be 4 and 5 mg/kg body weight, respectively.
Combination of the 2 toxins caused increased mortality in both the
single-and multiple-dose tests. Lesions produced by crop gavage with
T-2 toxin and DAS were similar, but were more severe in chicks given
T-2 toxin. Necrosis of lymphoid tissue and bone marrow was observed
in tissue taken 1 h after treatment followed by rapid depletion.
Necrosis was observed in the liver, gall bladder, and gut.
Chi et al.(1977c), fed broiler chicks (36 per group), aged one
day to 9 weeks, a diet containing T-2 toxin at concentrations of 0.2,
0.4, 2, or 4 mg/kg. Birds fed 4 mg T-2 toxin/kg showed reduced body
weight gain and feed consumption and developed oral lesions
characterized by circumscribed proliferating yellow caseous
plaques at the margin of the beak, the mucosa of the hard
palate and the tongue, and the angle of the mouth. No lesions were
observed in the bone marrow or, to any significant extent, in the
peripheral blood. The no-observed-effect doses of T-2 toxin were 0.2
mg/kg for weight gain, and 0.2 mg/kg for oral lesions. When one-day-
old broiler chicks were fed a diet containing 1, 2, 4, 8, or 16 mg T-
2 toxin/kg feed for 3 weeks the no-effect doses were estimated as
follows: growth rate, weight of pancreas, and weight of spleen: 2
mg/kg; oral lesions: <1 mg/kg (Wyatt et al., 1973c).
T-2 toxin administered to laying hens at a concentration of 20
mg/kg feed reduced egg production and resulted in the production of a
thinner egg shell (Wyatt et al., 1975). Speers et al.(1977), also
observed cessation of egg production in hens fed diets containing
25-50 mg monoacetoxyscirpenol/kg or 16 mg T-2 toxin/kg. It was
reported by Chi et al. (1977a) that feed consumption, egg production,
and shell thickness were significantly decreased in hens fed 8 mg of
T-2 toxin/kg. Furthermore, the hatchability of fertile eggs of hens
fed 2 or 8 mg T-2 toxin/kg was lower than that of hens fed the
control diet (Chi et al., 1977a).
Three groups of 1-day-old chicks (10 chicks per group) were each
fed 0.5-15 mg T-2 toxin/kg for 3 weeks (Coffin & Combs, 1981).
Plasma-vitamin E activity and hepatic-vitamin A content were
measured. Dose-dependent depression of plasma-vitamin E activity was
observed, with a 65% decrease compared with controls in chicks fed a
diet containing 15 mg T-2 toxin/kg. This decrease was believed to be
caused by a reduction in the plasma level of lipoproteins, which are
required for the transport of vitamin E.
(c) Ruminants
In a study by Pier et al. (1976), 4 calves received 0.08-0.6 mg
T-2 toxin/kg body weight orally in capsules for 30 days. The high-
dose calf developed a hunched stance and died on day 20. At all
levels, some evidence of mild enteritis with loose faeces was
obtained. Clinically apparent signs were confirmed at doses of 0.16
mg/kg or more, and bloody faeces at doses of 0.32 mg/kg or more. At
necropsy, abomasal ulcers were present in the calf given 0.16 mg/kg
and ruminal ulcers in calves given the 2 higher doses. Prothrombin
times and levels of serum GOT activity were increased in calves given
the 2 higher doses.
Ten male Suffolk-Finn-Columbian lambs, in 2 groups of 5 animals
each, were fed T-2 toxin at 0.3 or 0.6 mg/kg body weight for 21 days.
There were 5 controls. Experimental lambs developed focal hyperaemia
and dermatitis at the mucocutaneous junction of the commissure of the
lips, diarrhoea, leukopenia, lymphopenia and lymphoid depletion of
the mesenteric lymph nodes and spleen (Friend et al., 1983b).
Sheep dosed with roridin A and verrucarin A (4 mg/kg) had severe
and extensive haemorrhagic gastroenteritis. Oedema was marked in the
abomasum; the small intestine of the roridin-treated lamb had casts
of clotted blood and necrotic debris. Both small and large
intestines contained grossly haemorrhagic areas and extensive mucosal
erosions. In the lamb given verrucarin A at 4 mg/kg body weight, the
lesions were sublobular haemorrhages in the liver, which had a nutmeg
appearance, mucosal erosions, haemorrhages in the small intestine,
and haemorrhages of the endocardium of the left ventricle of the
heart (Mortimer et al., 1971).
(d) Cats
Three studies have described the clinical and tissue alterations
produced by administration of T-2 toxin to cats (Sato et al., 1975;
Lutsky et al., 1978; Lutsky & Mor, 1981). Lutsky et al. (1978) used
20 cats in 4 groups of 4-6 animals each. There were 4 controls. The
toxin was administered orally in gelatin capsules on alternative days
at doses of 0.06, 0.08, or 0.10 mg/kg body weight, until death. The
survival time ranged from 6 to 40 days. The signs included emesis,
anorexia, bloody diarrhoea, and ataxia. The cats lost weight and
became emaciated. Gross lesions included multiple petechiae to
ecchymotic haemorrhages of the intestinal tract, lymph nodes, and
heart. The lumen of the gut contained copious amounts of dark red
contents. Microscopic lesions included haemorrhages in the gut,
lymph nodes, heart, and mininges, necrosis of gastrointestinal
epithelium and decreased cellularity of the bone marrow, lymph nodes,
and spleen.
(e) Rodents
The trichothecenes used in long-term studies were T-2 toxin and
fusarenon-X. Lesions were observed in the oesophageal region of the
stomach of DDD mice fed T-2 toxin at 10 or 15 mg/kg diet for 12
months. The alterations included hyperplasia, hyperkeratosis, and
acanthosis of the squamous epithelium. Such changes were found 13
weeks after the start of feeding the toxins and were consistently
observed during the 12-month feeding period. However, most had
subsided 3 months after cessation of feeding. Similar gastric
lesions were observed in Wistar rats fed T-2 toxin at concentrations
of 5, 10, or 15 mg/kg feed for 4 weeks. The lesions were diffuse and
severe in the rats fed 15 mg/kg, focal but definite in those fed 10
mg/kg, and negligible in the stomach of rats fed 5 mg/kg (Ohtsubo &
Saito, 1977).
Six female Holtzman albino rats were fed T-2 toxin at 5 or 15
mg/kg for 19 days and T-2 toxin at 10 mg/kg diet for 8 months. No
gastric lesions were observed in any of the animals in the
experimental groups (Marasas et al., 1969).
Three groups of 12, six-week-old female Swiss ICR mice (15-20 g
body weight) were administered T-2 toxin (by 10-minute aerosol
exposure) Two control groups contained 8 mice each using nose-only
exposure. The aerosol mass concentration varied between 225 and 275
µg T-2 toxin/litre of air. Tissues from mice were microscopically
examined 0.25, 1, 2, 4, 6, 8, 12, and 24 h after exposure. Lymphoid
necrosis was observed 1 h after exposure in the thymus, spleen, and
lyphoid nodules of the intestinal tract. Necrosis of intestinal
crypt epithelial cells was present 2 h after exposure and necrosis of
adrenal cortical cells 4 h after exposure (Thurman et al., 1988).
In male Sprague-Dawley rats, T-2 toxin, given intravenously,
produced reduced blood flow and increased vascular resistance in
hind-quarter, mesenteric, and renal vascular beds. Mean arterial
pressure and heart rate were not significantly altered. A maximum
drop in blood flow in mesenteric and renal vascular beds occurred 4 h
after the T-2 toxin was injected (Siren and Feuerstein, 1986).
II.4.2.2 Haematological and haemostatic changes
A haemorrhagic syndrome was reported to be the characteristic
feature of mouldy corn toxicosis in the cow (section II.4.1.).
However, this hemorrhagic syndrome could not be produced in other
studies.
In a study by Patterson et al. (1979), 2 calves were administered
0.2 mg T-2 toxin/kg body weight and one calf was given the same dose
of DAS; both compounds were given by stomach tube,daily for 11 days.
There were no controls. The T-2-treated animals developed clinical
signs of weakness, inappetence, and one died. Prothrombin time was
prolonged in both animals and one had marked neutrophilia. No
clinical signs or haematological changes were observed in the animal
administered DAS. No haemorrhagic syndrome was found in these
calves.
When pigs (9-10 weeks old, male, castrated, specific pathogen-
free) were exposed to a T-2 toxin aerosol (390 µg/litre, 15 µm mass
median aerodynamic diameter) for a period that allowed an amount
equivalent to 8 mg/kg to be nebulized, the haematological alterations
included a decrease in lymphocyte and neutrophil counts, and
decreased concentrations of serum-protein and haemoglobin (Pang et
al., 1988).
Haematological changes were observed in mice, rats, cats, and
guinea-pigs treated with T-2 toxin and related trichothecenes (Sato
et al., 1975, 1978; Sato & Ueno, 1977; DeNicola et al., 1978). In
cats, leukocytosis occurred early after the administration of T-2
toxin. A similar change was observed in mice treated with T-2 toxin,
neosolaniol, and fusarenon-X. Among the leukocytes, lymphocytes
showed the greatest increase followed by neutrophils; the
leukocytosis was followed by marked leukopenia after short-term
exposure to T-2 toxin. This leucopenic state was also induced by DAS
in mice (Conner et al., 1986), rats and dogs (Stahelin et al., 1968)
and by verrucarin A in rats, dogs, guinea-pigs, and monkeys (Rusch &
Stahelin, 1965). Pancytopenia was also reported in cats administered
T-2 toxin for 2 weeks (Lutsky et al., 1978; Lutsky & Mor, 1981). In
guinea-pigs treated with T-2 toxin (0.9 mg/kg body weight per day)
for 27 days, erythropenia, leukopenia, and absolute lymphopenia were
observed, with a marked decrease in the lymphocyte contents of the
bone marrow (DeNicola et al., 1978).
Hayes et al. (1980) studied the effects of T-2 toxin on the
haematopoiesis in mice. Twenty-four male weanling outbred Swiss mice
were fed a balanced semipurified diet, containing crystalline
purified T-2 toxin at a level of 20 mg/kg dry diet. One group of 20
animals received the toxin in the diet for 41 days and another group
of 4 animals, for 21 days, followed by control diet for 7 days.
Forty-eight animals in 3 groups served as controls and received the
semipurified diet with restricted intake (20 animals for 41 days),
and ad lib (8 animals for 28 days). In addition, 12 animals were
sacrificed at day 0. Haematological studies were made at weekly
intervals. During the first 3 weeks of exposure to T-2 toxin,
lymphoid tissues, bone marrow, and splenic red pulp became
hypoplastic resulting in anaemia, lymphopenia, and eosinopenia.
Subsequently, during continued exposure to T-2 toxin, there was
regeneration leading to hyperplasia of the haematopoietic cells by 6
weeks. All animals also developed perioral dermatitis and ulceration
of the gastric mucosa. The above results indicate both the irritant
and haematopoietic suppressive effects of the T-2 toxin. However,
the haematopoietic effects were transient at the dose administered
and did not lead to haematopoietic failure.
The haemostatic derangements produced by T-2 toxin have been
studied in the guinea-pig (Cosgriff et al., 1984), rabbit (Gentry,
1982), chicken (Doerr et al., 1981), and monkey (Cosgriff et al.,
1986). Guinea-pigs (Hartley strain, number not stated) admin-istered
T-2 toxin dissolved in ethanol, by intramuscular injection, at a dose
of 1 mg/kg body weight (LD50-24 h) developed decreased activities of
all coagulation factors except fibrinogen. Platelet aggregation in
whole blood response to ADP and collagen was depressed. The animals
also showed an initial rise followed by a fall in the haematocrit
level, leukocytosis, and a fall in platelet count. These changes,
which were found within a few hours of toxin administration, reached
a maximal at 24 h and returned to normal over the next 2 days.
Pretreatment with vitamin K1 did not prevent the effects of T-2 toxin
on coagulation. The addition of T-2 toxin to the plasma and blood of
untreated guinea-pigs at a concentration of 1 mg/litre did not have
any effect on clotting times or platelet aggregation, indicating that
the T-2 toxin itself did not have any direct effect on the activ-ity
of coagulation factors (Cosgriff et al., 1984).
Eight New Zealand White rabbits were administered T-2 toxin
dissolved in dimethyl sulfoxide (DMSO) by intravenous injection at
0.5 mg/kg body weight; 5 rabbits were given a single oral dose of 2.0
mg/kg body weight. In the rabbits treated intravenously, both the
packed cell volume and the total leukocyte counts were reduced.
However, no significant alterations occurred in the haematological
parameters of rabbits given the T-2 toxin orally (Gentry & Cooper,
1981).
In another study, 9 New Zealand White rabbits were given a single
intravenous injection of T-2 toxin dissolved in DMSO at a dose of 0.5
mg/kg body weight. A second group of 5 animals received daily
subcutaneous injections of vitamin K, at a dose of 0.5 mg/kg body
weight, for 5 days prior to administration of a similar dose of T-2
toxin and for a subsequent 4 days. A total of 16 rabbits in 2 groups
served as controls. Blood samples were examined from each animal
before toxin or DMSO treatment and 6-96 h later. Several coagulation
factors (VII, VIII, IX, X, XI) were decreased by about 40% within 6 h
of toxin administration in the group administered toxin alone.
Fibrinogen content was elevated at 24 h. However, the reduction
in the coagulation factors did not induce clinical haemorrhage and
administration of vitamin K did not alter the effects of T-2 toxin
administration, indicating that the mechanism of action of the toxin
on coagulation was not as a vitamin K antagonist.
In a study by Cosgriff et al. (1986), 9 Cynomolgus monkeys
received an intramuscular injection of an LD20 dose (0.65 mg/kg body
weight) of T-2 toxin dissolved in ethanol. Three monkeys served as
controls. Haematological studies were made before toxin injection
and at different intervals from 6 to 24 h and 2 to 7 days after
treatment. The animals were studied for signs of toxicity and
particularly for evidence of haemorrhage. Necropsy was performed on
animals that died during study. Leukocytosis levels in treated
animals were 4-5 times pretreatment levels. Prolongation of
prothrombin, activated thromboplastin times, and a decrease in
multiple coagulation factors were also observed. These changes were
detected within hours of toxin administration, reached a maximum at
24 h, and returned to normal over the next 3 days. Fibrin-fibrinogen
degradation products were not detected at any time. Platelet counts
which were unchanged in treated animals, were significantly raised in
control animals following repeated phlebotomies. None of the animals
developed the haemorrhagic syndrome. Five animals that died during
the study showed mild peticheal haemorrhages involving the colon and
heart, as well as necrosis of lymphoid tissues.
Rukmini et al. (1980) conducted a study on adult rhesus monkeys
in which 3 males and 2 females were administered pure T-2 toxin in
20 ml milk by stomach tube, daily, initially at 1 mg/kg body weight
for 4 days, and then at 0.5 mg/kg body weight from day 5 to day 15.
Three males and 3 females served as controls. All 3 males in the
treated group died of respiratory failure between days 0 and 15.
Subsequently, after 30 days recovery, the 2 treated female and 2
additional male monkeys received 0.1 mg T-2 toxin/kg body weight for
15 days. All monkeys given 1 mg T-2 toxin/kg per day showed signs of
toxicity similar to those of alimentary toxic aleukia in man, i.e.,
vomiting, apathy, and weakness of lower limbs. The signs were more
severe in males, and they also developed peticheal haemorrhage on the
face. All male animals developed severe leukocytopenia, follicular
atrophy of the spleen and lymph nodes, and pneumonia, suggesting
involvement of the immune system. At a dose of 0.1 mg/kg per day,
both male and female animals developed leukocytopenia and mild
anaemia after 15 days of treatment.
The effects of T-2 toxicosis on blood coagulation were studied in
groups of 40, day-old chickens fed diets containing the toxin at
concentrations of 1, 2, 4, 8, or 16 mg/kg. Forty animals served as
controls. Factor X, and prothrombin and fibrinogen activities were
reduced only at the highest dietary dose, whereas Factor VII was
reduced at dietary doses of 4, 8, and 16 mg/kg and was the most
sensitive of the clotting components to T-2 toxin toxicosis. Thus,
T-2 toxin toxicosis induced by high doses results in multiple-factor
coagulopathy and mild toxicosis results in a deficiency of Factor VII
(Doerr et al., 1981).
II.4.2.3 Disturbances of the central nervous system
Four-week-old male broiler chickens were intubated with a single
dose of T-2 toxin at 2.5 mg/kg body weight, and the brain
concentrations of dopamine, norepinephrine, and serotonin, and
selected blood components were determined 4-48 h after
administration. There was a significant elevation in the brain-
dopamine concentration and a reduction in the brain-norepinephrine
concentration. The brain-serotonin contents did not change (Chi et
al., 1981). Batches of 40 broiler chickens fed graded concentrations
of 1-16 mg T-2 toxin/kg feed for 3 weeks developed an abnormal
positioning of the wings, hysteroid seizures, and impaired
righting reflex. Neural toxicity, which occurred at levels above 4
mg T-2 toxin/kg diet, might have been related to alterations in brain
biogenic amines (Wyatt et al., 1973a,b; Chi et al., 1977a).
Signs of nervous system dysfunction (restlessness, dyspnoea,
ataxia) were observed in rats after subcutaneous or intracerebral
injection of T-2 toxin (10-20 µg toxin) or after intracerebral
implantation of toxin adsorbed on talc (Bergmann et al., 1985).
Weanling, male Wistar rats were administered T-2 toxin orally at 2.0
mg/kg body weight and the concentrations of neurotransmitters
determined. The toxin increased concentrations of tryptophan,
serotonin, and dopamine in the brain, but decreased concentrations of
3,4-dihydroxyphenylacetic acid (MacDonald et al., 1988). Male
Sprague-Dawley rats (180 g) were dosed orally with DON or T-2 toxin
at 21.5 mg/kg body weight. Both the toxins significantly increased
serotonin and 5-hydroxy-3-indoleacetic acid concentrations in all
regions of the brain examined, whereas norepinephrine and dopamine
concentrations were not altered (Fitzpatrick et al., 1988). Male
Sprague-Dawley rats received 1 mg T-2 toxin/kg body weight by
intravenous injection. Concentrations of vasopressin, oxytocin, and
leucine enkephalin decreased in the posterior pituitary and
concentrations of methionine enkephalin increased (Zamir et al.,
1985).
Male Sprague-Dawley rats (180 g) and 4-week-old White Leghorn
cockerels were dosed orally with DON at 2.5 mg/kg body weight. Whole
brain concentrations of monoamine neurotransmitters were not altered
in either species. The treatment produced elevated concentrations of
serotonin and 5-hydroxy-3-indoleacetic acid in the rat, but not in
the chicken (Fitzpatrick et al., 1988).
II.4.2.4 Dermal toxicity
After the discovery of the skin-necrotizing property of toxic
metabolites of F. sporotrichioides and related fungi (Joffe, 1962),
the skin irritation test was introduced for the screening of toxins
and metabolites of Fusarium species (see section II.1.2.3). T-2
toxin, HT-2 toxin, and DAS were isolated from cultures of F.
tricinctum using the skin test for selection of active fractions
(Gilgan et al., 1966; Bamburg et al., 1968b). Toxins, such as T-2
toxin, HT-2 toxin, and DAS are extremely potent irritants while NIV
and fusarenon-X are much less so (Bamburg et al., 1968b; Ueno et al.,
1970; Wei et al., 1972; Chung et al., 1974; Hayes & Schiefer, 1979;
Bhavanishankar et al., 1988).
The mechanism of the skin toxicity of trichothecenes has not been
established. Results of studies with fusarenon-X have indicated that
the vascular permeability of the skin of the back of the rabbit,
estimated by exudation of a vital dye (pontamine sky blue), was
biphasic reaching maxima 5 and 24 h after topical application. These
data indicate that increased vascular permeability is one of the
early responses of the skin to these toxins and that some chemical
mediators participate in the biphasic increase in vascular
permeability (Ueno, 1980a).
II.4.2.5 Impairment of immune response
Experimental animal studies show that some trichothecenes affect
the immune system and thereby modify the immune response. The
impairment comprises the following functions: antibody formation;
allograft rejection; delayed hypersensitivity; and blastogenic
response to lectins. As a consequence of the impairment, decreased
resistance to microbial infection has been experimentally
established. It is likely that the impairment of the immune system
is linked to the inhibitory effect of trichothecenes on macromolecule
synthesis.
(a) Antibody formation
Rosenstein et al. (1979) showed that T-2 toxin and DAS inhibited
responsiveness to sheep red blood cells in male Swiss IC and C5781/6
mice. In their first study, T-2 toxin or DAS was injected ip daily
for 7 days at 0.75 mg/kg body weight, in 6 groups of 4 mice each,
with matching controls. Mice were immunized with sheep erythrocytes
(SRBC) on day 3 after treatment and killed 5 days after immunization.
Both toxins produced a fall in anti-SRBC titres measured by
haemagglutination and reduced thymic weight. In a second study, 7
groups of 5 mice each were administered daily (ip) doses of T-2 toxin
ranging from 0 (solvent alone) to 2.5 mg/kg body weight over a 7-day
period. The same number of mice received DAS under similar
conditions. Mice were immunized on day 3 and killed 5 days later.
Antibody-producing cells from the spleen were counted by numbering
the plaque-forming cells (PFC) on sheep erythrocytes. A dose-
dependent inhibition of PFC was observed in T-2 toxin-treated mice,
with a total suppression of the immune response at 2.5 mg/kg. The
effects of DAS were less. A subsequent follow-up of the evolution of
the immune response in 36 mice administered T-2 toxin (ip) at 0.75
mg/kg body weight daily for 7 days indicated that the immunosuppressive
effect disappeared within 6 days followup cessation treatment.
The T-cell-independent-responses-production of anti-
polyvinylpyrrolidone and anti-dinitrophenol-ficoll-antibodies were
enhanced by T-2 toxin and DAS. T-2 toxin-treated mice produced 50%
fewer plaque-forming cells against SRBC. There was also a decreased
response to phytohaemagglutinin in splenic cells from treated mice.
However, Masuko et al. (1977), and Otokawa et al. (1979), reported
that a single dose of 3 mg T-2 toxin/kg body weight in mice caused
modification of delayed hypersensitivity responses without affecting
antibody response. This apparent contradiction was explained by a
difference in the timing of the administration of T-2 toxin, mice
receiving the toxin once, several days before or after antigen-
stimulation. Results of studies with fusarenon-X indicated that both
IgE and IgG anti-body responses to DNA-OVA were suppressed when male
BALB/c mice were repeatedly dosed with mycotoxin at doses exceeding
25 mg/day; the inhibition of antibody-formation was greater when
given 7 days before antigen-stimulation. In mice stimulated with
pokeweed mitogen and lipopolysaccharides, the in vitro antibody
production by splenic cells from fusarenon-X-treated mice was
suppressed (Masuda et al., 1982).
Sato et al. (1981), examined the effects of fusarenon-X on
serological responses in chicks inoculated with Newcastle disease
vaccines. When chicks were fed 8 mg fusarenon-X/kg for 6 weeks, no
significant reductions in body or organ weights were observed.
However, haemagglutinin inhibitory antibody titres were reduced when
chicks were immunized with live, but not with inactivated, vaccine.
Mann et al. (1983), reported alterations in the levels
of several serum proteins in calves orally administered T-2 toxin
(0.6 mg/kg per day over 43 days). Total protein, albumin, and
immunoglobulin fractions were decreased in toxin-treated calves,
including the alpha-beta1-and beta2-globulin fractions. IgA and IgM
values and complement proteins were lower in treated calves.
Sublethal doses of DON (0.25, 0.50, or 1.0 mg/kg feed) were fed
for 54 weeks, beginning at 21 days of age, to a total of 96 weanling
male Swiss Webster mice divided into 4 groups. There were 32
controls. The dose of 1.0 mg/kg reduced serum alpha1- and alpha2-
globulins, increased serum-albumin levels, and reduced feed
consumption and body weight gain. The dose of 0.5 mg/kg reduced
alpha2- and beta-globulins (Tryphonas et al., 1986).
T-2 toxin was fed to 6 weaned pigs at 5 mg/kg feed for 25 days
and the immune response evaluated by in vitro testing for blast
transformation, immune-rosette formation, and IF-detectable IgG-
positive cell counts. T-2 toxin produced a 40-50% reduction in
immune responsiveness and a decrease in total leukocyte count, but an
increase in adrenocortical activity. Neutralizing antibody titres to
vaccination with enteritic B vaccine were lower in the treated pigs.
It was concluded that T-2 toxin had a distinct immunosuppressive
effect during the early phase of immune induction by altering the
function of both T- and B-lymphocytes (Rafai & Tuboly, 1982).
Dietary DON (2, 5, or 25 mg/kg feed for 2 or 8 weeks) depressed
the plaque-forming response to sheep erythrocytes in splenic cultures
from B6C3F1 mice. Some effect on the plaque-forming response was
detectable with both the 2- and the 8-week period of feeding (Pestka
et al., 1987b). DON, given by gavage at 0.75, 2.5, or 7.5 mg/kg body
weight also reduced serum-IgM response to sheep erythrocytes and
plaque-forming cell numbers were lower in the treated groups
(Tryphonas et al., 1984).
(b) Allograft rejection
Observations on the inhibition of cellular immunity by
trichothecenes have included responses to grafting. According to
Rosenstein et al. (1979), the mean survival time of the skin grafted
from C57Bl/6 mice on to Swiss mice was 8.69 days in the control
recipients. However, when the recipients were treated with 0.75 mg
T-2 toxin/kg per day for 7 days before skin graft and then 3 times a
week for 20 days, the mean survival time of the graft was increased
to 12 days, indicating that T-2 toxin suppressed certain steps of
immunity resulting in allograft rejection. The areas of the graft in
T-2 toxin-treated mice lacked the typical cellular infiltrates of a
cell-mediated immune response of macrophages and lymphocytes.
(c) Delayed hypersensitivity
Delayed hypersensitivity (DH) is an immune response mediated by
sensitized T lymphocytes. The possible impairment of T lymphocytes
by T-2 toxin was studied in female BDF1 mice sensitized by the sc
injection of sheep erythrocytes (SRBC) followed by estimation of foot
pad swelling. When mice received 3 mg T-2 toxin/kg body weight,
before, or on the day of, sensitization, no appreciable effect on DH
was observed. However, when the toxin was administered 2 or 3 days
after sensitization, marked enhancement of the delayed
hypersensitivity response was seen (Otokawa et al., 1979). This
indicates that the timing of toxin exposure was critical for
enhancement of the delayed hypersensitivity response. Since the
life-time of the effective T-2 toxin dose in vivo was very short,
and the optimal timing of toxin injection corresponded with the time
of appearance of suppresser cells, it was presumed that trichothecenes
might interfere with the proliferation of suppresser T cells that
appear in DH-tolerant mice.
DON fed to B6C3F1 mice at 2, 5, or 25 mg/kg for 2 or 8 weeks
depressed the delayed hypersensitivity response to keyhole limpet
haemocyanin. The effects on hypersensitivity were detectable in mice
fed the mycotoxin for 2 weeks, but disappeared when the feeding
period was extended to 8 weeks (Pestka et al., 1987b).
(d) Blastogenic response to lectins
Certain mitogens, such as phytohaemagglutinin (PHA) and
concanavalin A, stimulate the proliferation of T cells in vitro;
lipopolysaccharide (LPS) causes the same phenomena in B cells.
Lafarge-Frayssinet et al. (1979) investigated the responses of
lymphocytes to PHA and LPS in mice treated with crude T-2 toxin and
DAS. Mice were treated with the mycotoxins at doses of one-quarter
of the LD50 or one-twelfth of the LD50 for 15 days and the response
of splenic or thymic cells to the mitogens was examined. The data
indicated that stimulation of both T and B cells was inhibited
reversibly, and that the ability to synthesize anti-SRBC antibodies
was suppressed. In vitro effects on lymphocytes and fibrosarcoma
cell cultures included a direct cytostatic action at high
concentrations and a stimulating action at low concentrations.
Histopathological observations included severe lymphoid damage in the
thymus and spleen. The results of these studies indicate that the
immune system appears sensitive to the trichothecenes and is impaired
at doses not inhibitory for other organs.
Swiss mice were fed a diet containing T-2 toxin at 5, 10, or 20
mg/kg for 1, 2, 3, 4, or 6 weeks. The ingestion of T-2 toxin (only
at 20 mg/kg at 3 weeks) depressed total splenic cell counts. T-2
toxin at 20 mg/kg for 1-4 weeks decreased splenic proliferative
responses to T-cell mitogen concanavalin A; however, the response to
a lipopolysaccharide (LPS), B-cell mitogen, was decreased in mice fed
T-2 toxin at 10 or 20 mg/kg for 1-4 weeks. (Friend et al., 1983a).
Lymphocytes from calves exposed to T-2 toxin at 0.6 mg/kg for as
long as 43 days had a decreased response to the mitogen, PHA, on days
1, 8, and 29 after toxin administration. Lymphocyte responses to
concanavalin A and pokeweed mitogen were also observed on day 29
after dosing (Buening et al., 1982).
In a study to determine the effects of T-2 toxin on the bovine
immune system, calves (5) were orally dosed with 0.3 mg/kg per day,
for 56 days. Neutrophil function was reduced by treatment with T-2
toxin as was the cutaneous reaction to injected phytohaemagglutinin.
In a second study, calves (6) were given T-2 toxin at a dose of 0.5
mg/kg per day, for 28 days. B-lymphocyte number and the response of
the B-cell-enriched fraction to phytohaemagglutinin both increased
after treatment. The in vitro exposure of mononuclear cells, B-cell-
enriched or T-cell enriched fraction, reduced the lymphoblastic
response to mitogens. A 50% reduction was induced by 1.4 ng T-2
toxin/ml (Mann et al., 1984).
The effects of T-2 toxin on in vitro mitogen response and
antibody production by human peripheral blood lymphocytes were
reported by Tomar et al. (1988). The toxin inhibited the mitogen
response to concanavalin A at a lower concentration (1.6 mg/ml)
compared with phytohaemagglutinin (2-4 mg/ml) and pokeweed mitogen.
In the presence of the toxin, inhibition reached a maximum during
first 8 h. The results indicate that various subpopulations of
lymphocytes have different susceptibilities to T-2 toxin.
Mitogen-induced blastogenesis in cultured human lymphocytes was
inhibited by T-2 toxin and its metabolites. The concentrations of T-
2 toxin, HT-2, 3'-OH T-2,3'-OH HT-2, T-2 triol, and T-2 tetraol
toxins that produced 50% inhibition of 3H-thymidine uptake in
mitogen-stimulated human peripheral lymphocytes were 1.5, 3.5, 4.0,
50.0, 150.0, and 150.0 ng/ml, respectively. The initial hydrolysis
of T-2 toxin to HT-2 toxin and the hydroxylation to 3'-OH T-2 did not
significantly decrease the immunotoxicity (Forsell et al., 1985).
Other trichothecenes were less toxic than T-2 toxin in this system.
The doses that produced 50% inhibition of 3H-thymidine uptake in
mitogen-stimulated human lymphocytes for fusarenon-X, NIV, DON, and
15-AcDON were 18, 72, 140, and 240 ng/ml, respectively. These
results indicate that the lymphotoxicity of trichothecenes is related
to the C-4 substituent (Forsell & Pestka, 1985).
T-2 toxin was examined for its effects on lymphocyte activation
and interleukin-2 production by splenic cultures from mice. Splenic
cells were taken from female BALB/c mice given 2 mg T-2 toxin/kg body
weight by stomach tube for 4 days or 4 mg T-2 toxin/kg by stomach
tube in single dose. Cells were incubated with 1 µg concanavalin A
and the synthesis of cellular protein and DNA determined. The single
dose of 4 mg/kg did not alter lymphocyte activation, but the dose of
2 mg/kg for 4 days produced a 50% reduction in activation. The
supernatant from these cells had 4 times greater interleukin-2
activity (Holt et al., 1988a).
DON and 3-AcDON were evaluated in vitro for their effects on
mitogen-induced lymphocytic blastogenesis using rat or human
peripheral blood lymphocytes. Both mycotoxins produced a dose-
dependent reduction in lymphocytic proliferation and DON produced a
greater inhibitory effect than the acetylated compound. Thus, the
concentrations of DON producing 50% inhibition of blastogenesis were
90 and 220 ng/ml for rat and human lymphocytes, respectively. The
values for 3-AcDON were 450 and 1060 ng/ml, respectively (Atkinson &
Miller 1984).
(e) Resistance to infection
The immunosuppressive effect of trichothecenes has resulted in an
increased incidence and severity of infection in animals in several
studies. According to Boonchuvit et al. (1975), an increased
mortality rate was recorded when 40 chickens were fed a diet
containing 16 mg T-2 toxin/kg for 1 week and were then inoculated
orally with 1 x 108 cells of Salmonella.
The depression of resistance to experimental tubeculosis by T-2
toxin was studied in mice by Kanai & Kondo, (1984). Groups of male
mice, strain ddY, 18-20 g body weight, with 10-14 animals per group,
were administered mycobacteria by intravenous injection in the tail
vein. In the first study, the doses injected consisted of 0.01 mg
culture of tubercle bacteria per animal (species not indicated). One
group was administered 0.1 mg T-2 toxin per animal a total of 12
times orally, starting on the day before the injection, 7 times with
one-day intervals, and then 5 times daily. For comparison, a second
group of mice was administered 5 mg cortisone acetate per animal ip
under a similar time schedule. A third group was injected with
tubercle control bacteria only. At the end of the 20-day observation
period, the mice in the first group had a lower spleen weight and a
higher tubercle bacteria count in the spleen than those in the other
2 groups, indicating a more pronounced depression of resistance by T-
2 toxin than by cortisone. In a second study, two groups of animals
were injected with 0.25 mg of a culture of Mycobacterium bovis; one
group was then administered 0.1 mg T-2 toxin per animal daily for 6
days, starting 8 days after injection. There were two groups of
controls. The average survival time in the T-2 toxin-treated group
was reduced to 19 days, compared with 35 days in the untreated group,
indicating decreased resistance.
Rats were injected ip with T-2 toxin in a single dose of 1
mg/kg body weight or in doses of 0.5 mg/kg daily for 5 days.
The rats were then inoculated with 0.1 ml of medium
containing 109 Staphylococcus aureus/ml. Rats given
multiple intramuscular injections of T-2 toxin showed more
oedema and myofibre necrosis at the injection site of the
bacteria; the cellular infiltrate was sparse and bacteria
were abundant. Bone marrow myeloid cells were markedly
decreased by multiple injections of T-2 toxin. In in vitro
studies, small, non-lethal doses of T-2 toxin inhibited
the chemotaxis of leukocytes and decreased phagocytosis of
the bacteria by leukocytes (Yarom et al., 1984b).
Seven male rhesus monkeys (Macaca mulatta) were dosed daily by
stomach tube for 4-5 weeks with 100 µg T-2 toxin/kg body weight.
This dose resulted in the death of 3 animals, 40% reduction in
leukocyte counts, reduction in the bactericidal activity of
neutrophils (phagocytosis of E. coli), reduction in the
transformation of lymphocytes by mitogens, and a reduction in numbers
of C-cell and T-cell lymphocytes (Jagadeesan et al., 1982).
The immunotoxic effects of T-2 toxin on cell-mediated resistance
were studied in female ICR mice infected with Listeria monocytogenes.
Mice in groups of 17 animals (10 animals in the control group) were
inoculated ip with 4 x 105 (LD50) or 4 x 104 (non-lethal) doses of L.
monocytogenes per animal, treated with a single oral dose of 4 mg T-2
toxin/kg body weight, and observed for 15 days. Bacterial
multiplication was rapid in the spleen after T-2 toxin treatment and
mortality was increased in both treated groups. Necrosis and
depletion of lymphoid tissue were observed in the thymus, the
periarterioler lymphoid sheaths and the lymphoid follicles of the
spleen. Cellular response to L. monocytogenes in the spleen and
liver was decreased by treatment with T-2 toxin and the lesions were
sparsely populated with mononuclear cells. The foci of necrosis were
larger with numerous colonies of bacteria. The influx and number
of lymphocytes and macrophages were greater in Listeria-elicited
peritoneal exudates. The immunotoxic effects of T-2 toxin were
comparable with those produced by cyclophosphamide and were
attributed to depletion of T lymphocytes and subsequent failure of T-
cell-dependent macrophages to clear the host of bacteria (Corrier &
Ziprin, 1986a). In a continuation of the previous study, female ICR
mice (17 animals per group) were inoculated ip on day 1 with 4 x 105
(LD50) or 4 x 104 (non-lethal) bacteria per animal, treated orally on
day 0, 1, 2, and 3 with 0, 1, or 2 mg T-2 toxin/kg, and observed for
15 days. The suppression of resistance by the mycotoxin was
indicated by rapid multiplication of Listeria in the spleen and
increased mortality in mice in both exposed groups treated with 2
mg/kg. The thymuses and spleens of toxin-treated mice showed
necrosis and depletion of lymphoid cells. Foci of necrosis induced
by Listeria infection in the spleen and liver were larger in treated
mice and the inflammatory reaction was sparse (Corrier & Ziprin,
1986a,b).
Increased resistance to L. monocytogenes infection was
surprisingly observed by the same group (Corrier & Ziprin, 1986b) in
mice administered T-2 toxin several days prior to the inoculation
with bacteria. Female ICR mice, 16 animals per group, were
administered T-2 toxin by stomach tube at dosage levels of 2, 1, 0.5,
or 0 mg toxin/kg body weight, on days -5, -4, -3, -2, -1, +1 and +3.
On day 0 the two treated groups were inoculated ip with 106 (LD100)
and 105 (LD50) L. monocytogenes, respectively. In addition, 20 mice
were given 2 mg T-2 toxin/kg on the same days as above, and used to
determine the effect of the toxin. Although the cytotoxic effect of
T-2 toxin on lymphoid tissue was marked, enhanced resistance to
Listeria infection was revealed by a decrease in mortality due to
listeriosis (in both bacteria-exposed groups) in a T-2 toxin dose-
dependent way. No specific cause for the increased resistance to
listeriosis by T-2 toxin treatment prior to bacterial infection was
identified by the authors.
ICR female mice were treated with the trichothecene mycotoxin DAS
and subsequently inoculated ip with Listeria monocytogenes. The
effect of the mycotoxin on the course of the infection was monitored
by observing the resultant mortality and the bacterial content of the
spleens from inoculated mice. Mice given 3 mg DAS/kg body weight
orally, on 2 and 1 days before inoculation, showed increased
mortality and splenic Listeria counts. In these mice, thymus weights
were reduced, and lymphocytes were depleted from the thymus cortex
and from splenic lymphoid follicles and periarteriolar lymphoid
sheaths. A single dose of 4 mg DAS/kg given on day 6 before
challenge exposure did not affect mortality compared with controls.
Mice treated with DAS and subsequently inoculated with Listeria had
significantly ( P = 0.006) higher levels of neutrophil populations
than Listeria-infected control mice (Ziprin & Corrier, 1987).
Dietary DON decreased resistance of B6C3F1 mice to infection with
L. monocytogenes. Resistance to the infection was similarly
decreased in control mice fed restricted diets, comparable to dietary
restriction caused by DON-induced feed refusal. Resistance to L.
monocytogenes was reduced to a greater extent by feed containing both
DON and zearalenone (Pestka et al., 1987b). DON in the diet at 0.50
or 1.0 mg/kg was fed for 5 weeks. Both doses resulted in a dose-
related decrease in time-to-death interval following challenge with
L. monocytogenes (Tryphonas et al., 1986).
T-2 toxin was fed to young male white Swiss mice at doses of 10
or 20 mg/kg diet for 2-3 weeks. The mice were then inoculated ip
with herpes simplex virus (HSV-1). Mice fed the high dose of T-2
toxin were highly susceptible to HSV-1 infection and about 75% died
with extensive hepatic and adrenal gland necrosis and with little or
no inflammatory cellular reaction in affected tissues, such as the
liver and adrenal glands, and the central nervous system. No
necrotizing encephalitis was found in treated mice. Mice fed 10 mg
T-2 toxin/kg had lesions of intermediate severity between those of
the high-dose group and the virus-infected controls (Friend et al.,
1983c). Feeding of T-2 toxin at 5, 10, or 20 mg/kg for 3-6 weeks did
not reactivate the virus in mice latently infected with HSV-1 (Friend
et al., 1983a).
Mice (male, Swiss strain weighing 25 g) received inoculations
(route not stated) of Cryptococcus neoformans (1 x 106 cells) and ip
doses (1/8 or 1/4 LD50) of DAS on days 5, 6, and 7 after inoculation
with the fungal cells. No deaths occurred in either the C.
neoformans-treated groups or the T-2-toxin group. A marked additive
effect on mortality was observed when mice received both C.
neoformans and T-2 toxin (Fromentin et al., 1981).
II.4.2.6 Carcinogenicity
The IARC (1983) studied the experimental data on the
carcinogenicity of T-2 toxin and concluded that no evaluation of the
carcinogenic role of T-2 trichothecene in experimental animals could
be made, because of the inadequacy of experimental data.
In a 16-month feeding study, groups of 50 male and 50 female
weanling CD-1 mice were fed a semi-synthetic diet containing 1.5 or
3.0 mg T-2 toxin/kg. Survival was lowest in the control group. No
statistically recognizable differences were found in feed consumption
or body weight gains among the groups. Statistically significant
differences were found in the incidence of pulmonary adenomas and
hepatic adenomas in the males of the 3.0 mg/kg group and the
controls. Other treatment-related findings were an increased
prevalence of epithelial cell hyperplasia and hyperkeratosis in the
stomach of animals fed the T-2 toxin diets (Schiefer et al., 1987).
In an attempt to determine the carcinogenicity of NIV, a study
was conducted in which mice were fed mouldy rice for 2 years. Groups
of 42, 7-week-old female C57BL/6CrSlc SPF mice were fed diets
containing 6, 12, or 30 mg NIV/kg for 2 years, and were assessed for
the effects on body weight gain, feed efficiency, terminal organ
weights, haematological values, and lesions. The mortality was
lowest in the highest dose group, followed by the 12 mg/kg group;
body weight gains and feed efficiency were dose-dependently reduced.
No particular neoplasms attributable to treatment were found. The
incidence of naturally occurring neoplasms, mostly lymphomas, was
similar in all groups. On gross and microscopic examination of the
liver, thymus, spleen, kidneys, stomach, small intestines with or
without Peyer's patches, no alteration related to treatment was
observed except for amyloidosis, which was lower in the two higher
dose groups (Ohtsubo et al., in press).
Two groups of 16 or 18 DDD male mice received either 10 or 20
weekly sc injections of 2.5 mg fusarenon-X/kg body weight. No
increase in tumour incidence was noted in treated animals, compared
with the controls (Saito & Ohtsubo, 1974).
In a feeding study, fusarenon-X, at a dose of 3.5 or 7 mg/kg diet
was fed to 151 male Donryu rats for 1-2 years. Treatment with the
mycotoxin reduced the growth rate, but did not induce any
carcinogenic effects (Saito et al., 1980).
In studies to investigate skin tumour induction using T-2 toxin
and DAS, the skin of the back of mice was painted with 0.1-1 mg of
trichothecenes, twice a week, for one year. A notable finding was
necrosis of the skin, but no tumours were detected. The skin tumour
induction test was also carried out using the initiator-promotor
procedure. T-2 toxin and fusarenon-X were not promoting agents of
dimethylbenz (a)anthracene-induced skin neoplasia. According to
Lindenfelser et al. (1974), neither T-2 toxin nor DAS served as
initiating agents.
II.4.2.7 Mutagenicity
In a Rec-assay using Bacillus subtilis, DNA damage was not
induced by 20 or 100 µg of either T-2 toxin or fusarenon-X (Ueno &
Kubota, 1976). Such trichothecenes as T-2 toxin, DAS, and DON were
not mutagenic to Salmonella typhimurium strains TA98, TA100, TA1535,
TA1537, and TA1538, with or without S-9 fraction from rat liver
(Kuczuk et al., 1978; Ueno et al., 1978; Wehner et al., 1978a).
T-2 toxin and DAS were not mutagenic in the D-3 mitotic
recombination test with Saccharomyces cerevisiae (Kuczuk et al.,
1978).
Neither DNA-strand breakage nor induction of 8-azaguanine-
resistant mutation were detected with 1-32 mg fusarenon-X/litre in
Hela cells and with the same toxin at 0.1-1.0 mg/litre in FM3A cells
derived from C3H mouse mammary carcinoma cell line (Umeda et al.,
1972, 1977). On the other hand, Lafarge-Frayssinet et al. (1981)
reported that T-2 toxin induced single-strand breaks in the DNA of
lymphoid cells in vivo (3 mg/kg body weight) and in vitro (0.7-5
ng/ml). Such DNA breakage was not observed in hepatic cells.
T-2 toxin, NIV, and fusarenon-X produced weak clastogenic effects
in Chinese hamster V79-E cells (Thust et al., 1983). Both T-2 toxin
and HT-2 toxin inhibited the incorporation of tritiated
thymidine into the DNA of human fibroblasts in culture in a dose-
related fashion. Non-toxic and toxic doses of DON (0.1-1000
mg/litre), did not significantly increase unscheduled DNA synthesis
in primary cultures of rat hepatocytes (Bradlaw et al., 1985).
Norppa et al. (1980) also reported weak induction of chromosomal
aberrations by T-2 toxin (1.7, 2.7, or 3.0 mg/kg body weight) in
Chinese hamster bone marrow cells. The bone marrow micronucleus test
was negative at a dose of 3 mg T-2 toxin/kg body weight resulting in
a significant decrease in polychromatic erythrocytes.
Hamsters fed T-2 toxin for 6 weeks (2.5 mg/kg body weight) did
not have more chromosomal aberrations than controls. The clastogenic
potential of T-2 toxin was very weak. In hamster V79 cells, DON at
levels of 2-3 µg/ml or more was cytotoxic, but was non-mutagenic at
the hypoxanthine-guanine phosphoribosyl transferase locus, with or
without hepatocytic mediated activation (Rogers & Heroux-Metcalf,
1983).
According to Reiss (1975), DAS induced various cytological
abnormalities including shortened chromosomes, enlarged nucleoli, and
a few chromosome breaks in the root tips of Allium cepa (common
onion) at concentration of 1000 and 100 µg/ml. T-2 toxin and
satratoxin H were C-mitotic poisons at concentrations exceeding 10
µg/g. Typical C-mitotic action on chromosomes morphology was
produced by both toxins and was comparable to that of colchicine. In
addition, T-2 toxin induced polyploidy (Linnainmaa et al., 1979).
T-2 toxin and satratoxin H were not mutagenic in a sex-linked
recessive lethal test in Drosophila (Sorsa et al., 1980). Lack of
potency to produce recessive lethal mutations in Drosophila was
consistent with negative results obtained in certain bacterial assays
in which trichothecenes were inactive as both base pair and frame
shift mutagens (Kuczuk et al., 1978; Ueno et al., 1978a).
II.4.2.8 Teratogenicity and reproductive effects
T-2 toxin was embryotoxic and teratogenic in mice. T-2 toxin
dissolved in propylene glycol was injected intraperitoneally into
pregnant mice on one of days 7-11 of gestation at doses of 0.5, 1, or
1.5 mg/kg body weight. T-2 toxin (doses of 1 or 1.5 mg/kg) caused
significant maternal mortality, fetal death, and fetal body weight
loss. Approximately 37% of the fetuses from dams given 1 (8 litters)
or 1.5 mg (4 litters) T-2 toxin/kg on day 10 were grossly malformed.
The most frequent anomalies were bent, shortened, or missing tails,
and limb malformations, including oligodactyly and syndactyly.
Exencephaly, open eyes, retarded jaw, and skeletal malformations of
the rib or vertebrae were also found in the fetuses (Stanford et al.,
1975).
Pregnant CD-1 mice (18 litters/treatment group) were administered
T-2 toxin dissolved in propylene glycol ip at 0.5 mg/kg body weight
on gestation days 8 or 10. The T-2 toxin produced grossly malformed
fetuses, principally with tail and limb anomalies. A higher
incidence of malformations was observed when a T-2 toxin dose of 0.5
mg/kg body weight was combined with an ochratoxin A dose of 4 mg/kg
body weight. An increase in fetocidal effects was found in offspring
of dams in groups treated with the high-dose combination, on either
day. Few skeletal and visceral malformations were noted (Hood et
al., 1978). T-2 toxin (0.5 mg/kg body weight) dissolved in 1:1
mixture of propylene glycol and 0.1N sodium bicarbonate was
administered ip alone or in combination with rubratoxin B (0.4 mg/kg
body weight) to pregnant CD-1 mice on day 1 of gestation. Only T-2
toxin resulted in gross malformations. The combination of toxins
increased the adverse effects on fetal body weight and mortality, but
not the incidence or severity of the gross malformations (Hood,
1986).
The teratogenicity of orally administered T-2 toxin dissolved in
propylene glycol was evaluated in a study using 350 female CD-1
mice and doses of 0.5, 1.0, 2.0, 3.0, 3.5, or 4.0 mg/kg body weight
given on day 9 of gestation with a single dose of 3.0 mg/kg on day 6,
7, 8, 10, 11, or 12 of gestation. In the first study, the doses of
3.5 and 4.0 mg/kg produced maternal deaths and toxicity; no fetuses
were produced by the dams in the 4.0 mg/kg dose group and
significantly fewer fetuses were produced by dams in the 3.5 mg/kg
dose group. More major and minor defects were seen in offspring in
the 3.0 mg/kg dose group. In the second study, the treated females
had greater fetal loss than controls and the greatest number of dead
fetuses occurred among litters treated on day 9 of gestation. Major
skeletal defects were more numerous in mice treated on day 7 of
gestation. The results indicated that a single oral dose of T-2
toxin in propylene glycol was primarily maternally toxic and
embryolethal; defective development was possibly secondary to
maternal toxicity (Rousseaux & Schiefer, 1987).
Fusarenon-X was embryotoxic, but not teratogenic (Ito et al.,
1980). Fusarenon-X dissolved in saline was given to pregnant DDD
mice by subcutaneous injection at doses of 0.63, 1.0, 1.6, 2.6, or
4.1 mg/kg body weight, or by feeding at concentrations of 5, 10, or
20 mg/kg diet during pregnancy. Two dams given a single sc dose of
4.1 mg/kg died within 24 h of injection. Abortion was induced in all
females by a single injection of 2.6 mg/kg on day 10 of gestation.
Smaller doses (0.63-1.6 mg/kg) produced a 16-20% abortion rate, when
given on day 10. Multiple doses (8-12 or 8-14 days of gestation) of
1.0 or 1.6 mg/kg produced 100% abortion. When the mice were fed a
diet containing 5, 10, or 20 mg fusarenon-X/kg throughout the
gestation period or in the early stages of gestation, the mycotoxin
inhibited embryonal implantation. Feeding fusarenon-X at 20 mg/kg
for 7 days during the middle stages of gestation induced abortion in
100% of dams. Fetal body weight was significantly reduced by the
administration of the mycotoxin, but no significant teratogenic
effects were observed in the fetuses of dams in either the
subcutaneous injection or feeding study (Ito et al., 1980).
The effects of NIV on fertilization, course of pregnancy, and
fetuses were examined in ICR mice. In a study by Ito et al. (1986),
pure NIV was injected ip in pregnant mice (groups of 10 animals
each), at dose levels of 0, 0.1, 0.5, or 1.5 mg/kg body weight per
day, on days 7-5 of gestation. The highest dose caused stillbirths
after vaginal haemorrhage in 6 out of 10 animals. High embryo
lethality was recorded in the 2 highest dose groups (88 and 48%). No
fetal malformations were observed in the treated groups. A single
administration of 3 mg/kg on day 7 affected the embryo within 10 h,
damaged the placenta within 24 h, and caused stillbirths at 48 h.
While NIV is embryotoxic, it is not teratogenic (Ito et al.,
1988). Thirty ICR mice in 3 batches of 10 animals each were fed
diets mixed with mouldy rice powder containing NIV at final levels of
6, 12, or 30 mg/kg feed per day throughout gestation. There were 11
controls. Purified NIV was also administered by gavage to 35 animals
in 4 groups at doses of 1-20 mg/kg body weight on days 7-15 of
gestation. There were 10 controls. Embryotoxicity associated
with maternal weight loss was observed in the groups receiving 30
mg/kg diet and 10 mg/ kg body weight per day, by gavage, whereas
lower levels, such as 5 mg/kg body weight per day, by gavage, did not
have any embryotoxic effects. Intrauterine growth retardation was
found at term in the fetuses of mice exposed to 12 mg/kg feed and 5
mg/kg by gavage. NIV did not have any significant adverse effects on
the incidence of gross skeletal and visceral malformations. As 5
mg/kg body weight per day given by gavage corresponds to a feed level
of approximately 35 mg/kg feed, the above data indicate that
exposure to 30 mg NIV/kg feed throughout the gestation period results
in embryotoxicity. However, exposure to approximately 35 mg/kg feed
during days 7-15 only of the gestation period does not induce
embryotoxic effects, which shows the significance of constant
exposure versus intermittent exposure to NIV.
DON was embryotoxic and teratogenic when dissolved in distilled
water and given for 4 consecutive days (days 8-11 of gestation), by
oesophageal intubation, to 15-19 pregnant Swiss-Webster mice (Khera
et al., 1982). The incidence of resorptions was 100% at doses of 10
or 15 mg/kg body weight, and 80% at 5 mg/kg body weight. The dose of
5 mg/kg reduced the number of live fetuses and reduced the average
fetal weight compared with the controls. Low incidences of skeletal
and visceral anomalies were found in the fetuses of the 1, 2.5, and 5
mg/kg groups. The skeletal malformations occurred in a dose-related
manner and included lumbar vertebrae with fused arches or partly
absent centra, and absent or fused ribs.
On the other hand, DON failed to produce embryotoxic and
teratogenic effects when fed ad lib at 0.5, 2.0, or 5.0 mg/kg to
Fisher 344 rats during the entire course of pregnancy (Morrissey,
1984). No overt signs of toxicity were observed in the dams and no
changes in maternal feed consumption were observed at any dose.
DON was fed to 71 adult female New Zealand White rabbits in 7
batches of 6-14 animals during the entire period of gestation at
doses of 0.3, 0.6, 1.0, 1.6, 1.8, or 2.0 mg/kg body weight. There
were 25 controls. The fetal effects consisted of 100% incidence of
fetal resorption in the females fed 1.8 and 2.0 mg/kg and reduced
average body weight of fetuses from dams fed 1.0 and 1.6 mg/kg.
These doses were not teratogenic (Khera et al., 1986).
Weanling F0 male and female mice were fed diets containing DON at
a dose of 2.0 mg/kg body weight (15 male and 15 female animals) and
0.375, 0.75, or 1.5 mg/kg body weight (7 males and 59 female
animals). There were 30 controls in the first study and 26 in the
second. After 30 days of dietary feeding, the mice were allowed to
mate and the pregnant females were allowed to litter normally. The
F1a progeny were examined up to 21 days of age and discarded. The F0
mice were rebred. The females bred to produce the F1b litters were
killed on day 19 of gestation and the fetuses were examined for
gross, visceral and skeletal malformations. Reductions were
observed in feed intake and in the body weight of F0 male and female
mice, the number of live pups and postnatal survivors, postnatal body
weight of F1a progeny, number of live fetuses, and fetal body weight
of F1b. No adverse effects on the fertility of F0 mice and no
major malformations in F1b fetuses were found. Results of cross-
fostering offspring between control dams and 1.5 mg/kg dams indicated
that both postnatal survival and body weight were adversely affected
by prenatal exposure as well as by combined pre- and post-natal
exposure (Khera et al., 1984).
Male and female Sprague-Dawley rats (3 groups of 15 male and 15
female animals each) were fed diets containing DON at levels of 0.25,
0.5, or 1.0 mg/kg body weight. Controls consisted of two groups of
15 males and 15 females. After 6 weeks of feeding, the rats were
bred. The mated females, maintained on their respective diets, were
killed on the last day of pregnancy and fetuses were evaluated for
effects on pre-natal development. No adverse effects were observed
except for dilatation of the renal pelvis and urinary bladder (Khera
et al., 1984).
Male (20/group) and female Sprague-Dawley rats (25/group) were
fed a diet containing 20 mg purified DON/kg for 60 and 15 days,
respectively, before mating. Rats consuming the DON-supplemental
diet throughout gestation and lactation did not show any clinical
signs of toxicity, but had reduced body weights. Only 50% of the
matings between toxin-fed rats resulted in pregnancy compared with
80% in the controls. No differences were detected among the groups
in sex ratio, survival rate, or average litter number and weight.
Pup weight gains in all groups were comparable up to post-natal day
14. From day 14 to 21, however, male and female pups of the control
group showed significantly improved weight gains compared with pups
from treated dams. No treatment-related histological abnormalities
were found in the testes or ovaries of treated pups (Morrissey &
Vesonder, 1985).
A 2-generation reproduction and teratology study was carried out
using 90 female CD-1 mice fed a semisynthetic diet containing the T-2
toxin at 1.5 or 3.0 mg/kg, concentrations considered possible under
field conditions. Results indicated that continuous feeding of T-2
toxin at low concentrations had minimal, if any, toxic effects on
female reproduction and fetal development and that T-2 toxin fed
continuously at 1.5 or 3.0 mg/kg was not teratogenic or fetocidal and
produced minimal effects on the growth rates of CD-1 mice (Rousseaux
et al., 1986).
II.4.3 Biochemical effects and mode of action
II.4.3.1 Cytotoxicity
In the early stages of research on trichothecenes, DAS was
isolated from F. scirpi as a phytotoxic principle (Brian et al.,
1961). Subsequent surveys confirmed the phytotoxic nature of the
trichothecenes using germination of seeds of Brassica oleracea L.
(Ueno et al., 1971c), germination of pea seedlings (Marasas et al.,
1971), growth of tobacco callus tissues (Helgeson et al., 1973), the
germination of tobacco plant (Nicotiana sylvestris) pollen
(Siriwardana & Lafont, 1978), and the auxin-promoted elongation of
soybean hypocotyl (Stahl et al., 1973).
Most of the trichothecenes tested have had fungistatic activity
in a wide range of species (Bamburg et al., 1968a; Bamburg & Strong,
1971; Reiss, 1973); DAS and verrucarin A were particularly potent in
inhibiting growth and sporulation at concentrations of 0.5-1
mg/litre.
Many of the trichothecenes have been cytotoxic to mammalian
cells, both in in vivo and in vitro. In animals administered
trichothecenes, the mucosa of the stomach and small and large
intestines had mucosal erosions, mucosal necrosis with ulceration,
and severe haemorrhages; radiomimetic effects included necrosis of
actively-dividing cells in the thymus, spleen, ovary, testis, and
lymph nodes (Saito & Ohtsubo, 1974). In a cell culture system, Grove
& Mortimer (1969) demonstrated the cytotoxicity of DAS and its
chemically modified compounds on hepatocytes of human origin and
hamster kidney cells. Ohtsubo et al. (1968), Ohtsubo & Saito (1970),
and Bodon & Zoldag (1974) described the cytotoxicity of NIV and
related trichothecenes for HeLa cells, and of T-2 toxin for
epithelial cells of pig kidney, respectively. The macrocyclic
trichothecenes, verrucarins and roridins, were highly cytotoxic for
0815 mouse tumour cells in the ng/ml range (Harri et al., 1962).
Tanaka et al. (1977), investigated the cytotoxicity of 20 types of
trichothecenes (11 type A, 6 type B, 1 type C, and 2 type D) for 3
cell lines, HeLa, HEK, and HL cells. The type D trichothecenes,
such as verrucarin A and roridin A had LC50s in the range of 0.003-
0.005 mg/litre; neosolaniol and NIV in the range of 0.1-1 mg/litre,
and deoxynivalenol in the range of 1-5 mg/litre. Trichodermol,
calonectrin, monoacetyldeoxynivalenol, and tetraacetylnivalenol were
weakly cytotoxic within the range of 5-10 mg/litre.
Chinese hamster ovary (CHO) and African green monkey kidney
(VERO) cell cultures were exposed to 0.01 or 1.0 ng T-2 toxin/ml for
1 or 12 h. The cells exhibited morphological changes considered to
be related to inhibition of protein synthesis. The alterations
included disassociation of polysomes and matrix density, ballooning
of intracristal space, and malalignment of cristae in mitochondria.
The CHO cells had bleb formations of plasma membrane, a change
produced by exposure to establish inhibition of protein synthesis
(Trusal, 1985).
II.4.3.2 Inhibition of protein synthesis
The initial observation that the trichothecene mycotoxins
inhibited protein synthesis in mammalian cells was made by Ueno et
al. (1968). They reported that NIV produced a dose-dependent
inhibition of incorporation of several amino acids into protein in
rabbit reticulocytes (Ueno et al., 1968) and Ehrlich ascite tumour
cells (Ueno & Fukushima, 1968). This inhibitory effect of the
trichothecene mycotoxins was observed in the whole animal (Ueno,
1970), a protozoan (Ueno & Yamakawa, 1970), HeLa cells (Liao et al.,
1976), cultured mammalian cells (Ohtsubo et al., 1968; Ohtsubo &
Saito, 1970), rat hepatocytes (Ueno et al., 1973b), hamster ovary
cells (Gupta & Siminovitch, 1978), human tonsil (Carrasco et al.,
1973), yeast spheroplasts (Stafford & McLaughlin, 1973; Cundliffe et
al., 1974; McLaughlin et al., 1977), rabbit reticulocytes (Ueno et
al., 1968, 1969a, 1973b,c; Ueno & Shimada, 1974; Wei & McLaughlin,
1974; Mizuno, 1975; Carter et al., 1976), and lymphocytes (Hartman et
al., 1978). No inhibitory effect on bacterial cells was observed
(Ueno et al., 1973b).
The effects of T-2 toxin on protein and DNA synthesis were
studied in Swiss mice and hepatoma cell cultures. T-2 toxin was
given as a single ip dose of 0.75 mg/kg and as a 3-day and a 7-day
daily treatment. The toxin inhibited protein synthesis after all 3
schedules of treatment and inhibition was present in cells obtained
from bone marrow, spleen, and thymus. Protein synthesis was
inhibited in vitro in hepatoma cell cultures and PHA-stimulated
lymphocytes (Rosenstein & Lafarge-Frayssinet, 1983).
The effects of T-2 toxin on rat hepatocytes were studied in
culture by the addition of several doses at either 1 or 12 h of
exposure. A dose of 0.01 ng T-2 toxin/ml produced a 75% inhibition
of protein synthesis within 1 h. At a higher dose of 1.0 ng/ml,
hepatocytes recovered from a 1-h but not a 12-h exposure. Cell
damage (release of lactate dehydrogenase) lagged behind inhibition of
protein synthesis, which was 90% at the 1 ng/ml dose. Ultrastructural
alterations were present in the endoplasmic reticulum and
mitochondria. Degranulation involved the rough endoplasmic
reticulum. Mitochondria had translucent foci and electron dense
cores (Trusal & O'Brien, 1986).
NIV inhibited poly-U, poly-A, and poly-C directed incorporation
of phenylalanyl-tRNA into phenylalanine without affecting the
activation of amino acids. The inhibition was presumably caused by
impairment of ribosomal function (Ueno et al., 1968). Fusarenon-X
caused breakdown of polysomes in rabbit reticulocytes (Ueno et al.,
1973b) and in mouse fibroblasts (L-cells), shortly after exposure
(Ohtsubo et al., 1972). The breakdown of polysomes was consistent
with the action of an inhibitor of initiation of protein synthesis.
T-2 toxin, DAS, and verrucarin A, but not trichodermin, also induced
the disaggregation of polysomes in HeLa cells (Liao et al., 1976).
These results were consistent with the effects of an inhibitor of
prolongation or termination of protein synthesis (Stafford &
McLaughlin, 1973). An important point was that the trichothecenes,
regardless of whether they were I-type or ET-type, interacted with
the peptidyl transferase centre on the 60S ribosomal subunit and
inhibited the transpeptidation of the peptide bond formation process.
Current research has focused on clarifying the molecular species
of ribosomal protein that regulates the binding of trichothecene
mycotoxins. A mutant of yeast (Schindler et al., 1974; Jimenez &
Vazquez, 1975; Jimenez et al., 1975; Grant et al., 1976; Carter et
al., 1980) and Chinese hamster ovary cells, resistant to the effects
of trichothecenes on protein synthesis (Gupta & Siminovitch, 1978),
have been isolated. Friend & Warner (1981) clearly demonstrated that
the gene for trichodermin resistance in yeast specifies ribosomal
protein L3, the largest of the yeast ribosomal proteins.
The ribosomal subunits of Myrothecium verrucaria, a producer of
macrocyclic trichothecenes, were resistant to T-2 toxin. It can be
assumed that the 60S subunits of eukaryotes are responsible for the
sensitivity to the trichothecenes. (Hobden & Cundliffe, 1980).
II.4.3.3 Inhibition of nucleic acid synthesis
The dose-dependent inhibition by NIV of DNA and RNA synthesis in
Ehrlich ascites tumour cells was first observed by Ueno & Fukushima
(1968). Inhibition (70%) of protein synthesis was induced by 1-10 mg
NIV/litre, and thymidine incorporation into DNA was inhibited by as
much as 60%; suppression (30%) of uracil incorporation into RNA was
slight. Similar results were obtained with several trichothecenes,
including trichodermin, diacetoxyscirpenol, and fusarenon-X, in other
cultured cells, such as KB cells (Ohtsubo et al., 1968), HeLa cells
(Liao et al., 1976), mouse L-cell fibroblasts (Ohtsubo et al., 1972),
hamster ovary cells (Gupta & Siminovitch, 1978), and lymphocytes
(Hartman et al., 1978).
The inhibition of DNA and RNA synthesis by trichothecenes
required higher concentrations of toxins than the inhibition of
protein synthesis and the extent of the inhibition was much less.
Thus, the observed inhibition of DNA and RNA synthesis in toxin-
treated animal cells was presumed a secondary effect of the
trichothecenes. This hypothesis was supported by the findings of
Tashiro et al. (1979) who reported that, in in vitro studies, a
concentration of 0.0236-1.889 mmol fusarenon-X/litre did not inhibit
DNA-dependent RNA hybridases of rat liver and Tetrahymena pyriformis.
Munsch & Mueller (1980) reported that the incorporation of 3H-
thymidine into DNA in cell lines from thymus was strongly inhibited
by over 10 ng T-2 toxin/ml and slightly inhibited by 0.1-10 ng T-2
toxin/ml. A low concentration of 0.1-1 ng/ml toxin caused a
transient increase in DNA polymerases, alpha- and beta-terminal
deoxynucleotidyl transferases. At a high dose of more than 1 ng T-2
toxin/ml, these enzymatic activities were strongly inhibited.
Rosenstein & Lafarge-Frayssinet (1983) described the depression of
DNA synthesis in vivo and in vitro. Three treatment schedules: a
single dose, 3 daily doses, or 7 daily doses of 0.75 mg T-2 toxin/kg
inhibited DNA synthesis in cell cultures from the spleen, thymus, and
bone marrow of treated mice. The mycotoxin also inhibited DNA
synthesis in vitro in cultures of hepatoma cells and in PHA-
stimulated lymphocytes (Rosenstein & Lafarge-Frayssinet, 1983).
Agrelo & Schoental (1980) found that hydroxyurea did not alter
unscheduled DNA synthesis in cells treated with T-2 toxin and HT-2
toxin (6 ng/ml). However, the combination of rat liver microsomes
and hydroxyurea resulted in an increase in unscheduled DNA synthesis
in cells exposed to 100 µg HT-2 toxin/ litre. The data of Agrelo &
Schoental (1980) suggest that the microsomal drug-metabolizing enzyme
system may participate in the induction of DNA damage by
trichothecenes.
The effects of T-2 toxin and DAS on DNA synthesis in phyto-
haemagglutinin, stimulated in the peripheral blood lymphocytes of
human beings, was assayed by incorporation of 3H-thymidine. Total
inhibition was obtained by 8 ng T-2 toxin and DAS and 80% was
obtained with 1.5 ng T-2 toxin and 2.7 ng of DAS (Cooray, 1984).
Fusarenon-X and related toxins inhibited protein and nucleic acid
synthesis in Tetrahymena pyriformis in a manner similar to that
observed in cultured mammalian cells (Ueno & Yamakawa, 1970). In
synchronously dividing Tetrahymena cells (Iwahashi et al., 1982), the
incorporation of radioactive amino acids, thymidine, and uracil into
protein, DNA, and RNA, respectively, was achieved by nearly the same
concentration of T-2 toxin. Inhibition of protein synthesis was
explained by the high affinity of T-2 toxin for 60S ribosomal
subunits of Tetrahymena, as is the case with cultured cells.
However, neither DNA and RNA synthesis nor RNA hybridase activity
were altered by T-2 toxin in an in vitro system using isolated nuclei
from normal cells or from cells pretreated with T-2 toxin. The
mechanism of inhibition of nucleic acid synthesis in the in vivo
system is not yet understood.
II.4.3.4 Alterations of cellular membranes
The trichothecenes as a group inhibit protein synthesis, but the
potency of the effect varies according to the trichothecene and the
system ( in vitro or in vivo) used to measure the inhibition. T-2
toxin was a potent inhibitor of protein synthesis both in vitro and
in vivo (Ueno et al., 1973b; Rosenstein & Lafarge-Frayssinet, 1983).
In vitro experiments were conducted to determine the effects of
T-2 toxin on the entry of sucrose into bovine erythrocytes,
entrapment of sucrose and inulin in carrier erythrocytes, entrapment
of T-2 and binding of T-2 toxin, and the measurement of cell
permeability to the entrapped T-2 toxin. At the highest
concentration of T-2 toxin (20 µg), no entry of 14C-sucrose or 3H-
inulin was observed. Very little 3H-T-2 toxin was bound to bovine
erythrocytes and binding was independent of T-2 toxin concentration.
The mycotoxin had no effect on the entrapment of sucrose or inulin.
Carrier erythrocytes retained 85% of 14C-sucrose and only 18% of
3H-T-2 toxin. Thus, T-2 toxin diffused from carrier cells much more
rapidly than sucrose. It was concluded that the interaction of T-2
toxin with bovine erythrocytes was minimal and intercalation with the
inner bilayer was not likely, because the increase in cell volume
that would have resulted did not occur (DeLoach et al., 1987).
The effects of T-2 toxin on membrane function were studied using
L-6 myoblasts. The minimal effective concentration (MEC) of T-2
toxin for reduction in uptake of calcium and glucose and for
reduction in uptake of leucine and tyrosine and their incorporation
into protein was 4 pg/ml. The uptake of rubidium was increased at
0.4 pg/ml and reduced at 4 pg/ml or more. Thymidine uptake and
incorporation into DNA had a biphasic response with an increase at
0.4 pg/ml and a reduction at 4 pg/ml for uptake and 40 pg/ml for
incorporation. Calcium efflux was reduced after 1, 5, and 15 min
exposure to T-2 toxin at a concentration of 40 pg/ml. These data
indicate that T-2 toxin has multiple effects on all membrane function
at very low concentrations and that these effects are independent of
inhibition of protein synthesis (Bunner & Morris, 1988).
II.4.3.5 Other biochemical effects
Fusarenon-X inhibited the uptake of phosphate by Tetrahymena
cells (Chiba et al., 1972). It caused a 50% inhibition of
incorporation of acetate into phospholipids and a ten-fold
stimulation of incorporation into triglyceride, effects that may be
secondary to inhibition of phosphate uptake. However, in rabbit
reticulocytes, fusarenon-X, T-2 toxin, and neosolaniol at
concentrations of 100 mg/litre did not inhibit Na+-dependent glycine
transport (Ueno et al., 1978b).
When SH-enzymes were pre-incubated with selected trichothecenes
in the absence of substrates, the activities of such enzymes as
creatine phosphokinase and lactate and alcohol dehydrogenases were
reduced (Ueno & Matsumoto, 1975). However, neither urease activity
(Reiss, 1977) nor rat liver lysosomal cathepsin activity (Farb et
al., 1976) was affected by diacetoxyscirpenol or T-2 toxin,
respectively, in vitro. Foster et al. (1975), reported the
reactivity of T-2 toxin with glutathione in the presence of epoxide-
S-GSH-transferase.
When mice were exposed to trichothecene mycotoxins, no detectable
alterations were observed in hepatic and renal functions. In chicks
fed a diet containing 10 mg T-2 toxin/kg for 3 weeks, a hepatic
microsomal aminopyrine, demethylase, was reduced by 29% compared with
the control, while another mixed function oxidase, aniline
hydroxylase, was not significantly affected (Coffin & Combs,
1981). In mice receiving a sublethal dose of fusarenon-X ip, a rapid
hypoglycemia was followed by depletion of hepatic glycogen (Shimizu
et al., 1979). The authors suggested that the toxin had induced a
malfunctioning of glucose absorption in the intestines and had
accelerated glycolysis. However, the trichothecene mycotoxins are
highly cytotoxic to the epithelia of the intestine, and impairment of
carbohydrate metabolism and absorption may be a secondary effect of
this cytotoxicity.
II.4.4 Structure-activity relationships
After the isolation and identification of numerous derivatives of
the trichothecenes, the structure-activity relationship was
investigated, on the basis of the information on lethal toxicity,
dermal toxicity, cytotoxicity, inhibition of protein synthesis, and
association with ribosomes.
The 12,13-epoxide of the trichothecenes is essential for their
biological activity. The de-epoxidation of DON and T-2 toxin by
rumen-microorganisms (King et al., 1984) and in mammalian systems
(Yoshizawa et al., 1983) results in loss of toxicity.
The macrocyclic ring contributes to the highly lipophylic
property of the trichothecenes, and the macrocyclic trichothecenes,
such as verrucarins, roridins, satratoxins, and baccharins, exhibit a
potent cytotoxicity towards cultured mammalian cells (Kupchan et al.,
1976, 1977; Jarvis et al., 1978, 1980).
In contrast, the polyhydroxylated alcohols, such as verrucarol,
NIV, and DON, are highly hydrophilic, resulting in a decrease in
cytotoxicity and dermal toxicity compared with their parent
trichothecenes (Ueno et al., 1970; Wei et al., 1974).
II.4.5 Prevention and therapy of trichothecene toxicosis
In studies to investigate effects of various dietary supplements
on T-2 toxin toxicity, weanling male Wistar rats in groups of 10,
with adequate controls, were fed diets containing 5% each of
bentonite, anion exchange resin, cation exchange resin, or
vermiculite-hydrobiotite with and without 3 µg T-2 toxin/g feed, for
2 weeks. Bentonite and anion exchange resin were most effective in
reducing the growth depression and feed refusal caused by T-2 toxin.
In a second study in which bentonite and anion exchange resin were
administered at levels of 2.5, 5.0, 7.5, or 10% of diet, 10%
bentonite in the diet was the most effective dietary supplement.
Dietary bentonite reduced the absorption of 3H-T-2 toxin and
increased faecal elimination (Carson & Smith, 1983).
Smectite, a clay composed of insoluble silicates of aluminum and
magnesium, incubated with T-2 toxin for 24 h before the toxin was
administered orally to mice (1 mg/kg per day), prevented the
acceleration of gastric emptying and transit time for a milk meal,
usually induced by administration of T-2 toxin. Smectite given
together with the toxin did not prevent the gastrointestinal effects
of T-2 toxin (Fioramonti et al., 1987). Male Sprague-Dawley rats
were given 15 or 5 mg/kg body weight of a platelet activating factor
antagonist, with and without T-2 toxin, by intravenous injection.
The drug prolonged the survival of conscious rats exposed to 0.65 mg
T-2 toxin/kg (Feuerstein et al., 1987).
The effects of certain drugs and metabolic inhibitors on the
toxicity of T-2 toxin have been studied in male ddY mice. The toxin
at a dose of 1.8 mg/kg was given subcutaneously and the drugs and
other chemicals were given ip. The lethal effects were reduced by
the steroid drugs, prednisolone and dexamethasone; survival times
were increased by the antihistaminic drug diphenhydramine, and an
opioid antagonist, naloxone. Prednisolone also reduced the
leukocytosis that developed after T-2 toxin treatment and decreased
the increase in ear weight caused by the mycotoxin. Other
antihistaminic and/or antiserotonic drugs used in the treatment were
found not to be effective in preventing the lethal effects of T-2
toxin (Ryu et al., 1987).
Male ddY mice and male Wistar rats in several groups of 10
animals each, pretreated with a radioprotective compound or with an
anti-inflammatory agent, were given T-2 toxin or fusarenon-X (1.5
mg/kg body weight). Out of the 20 compounds evaluated, only
prednisolone and hydrocortisone (100 mg/kg ip) were effective. They
reduced mortality from 90% in controls to less than 30% and they also
reduced the trichothecene-induced increase in intestinal fluid volume
(Mutoh et al., 1988).
Cutaneous irritation produced by T-2 toxin in 10 Porton female
rats was largely reduced by application, within 10 min, of an aqueous
soap solution when the dose was low (1.0 µg/cm2), but the solution was
largely ineffective over a time span of 60 min when the dose was
higher (100 µg T-2 toxin). Washing the site of application with
polyethylene glycol 300 was very effective in removing even large
doses (100 µg) of T-2 toxin from the skin (Fairhurst et al., 1987).
The median effective dose of oral superactive charcoal in
preventing deaths in batches of 5 female Sprague-Dawley rats was
0.175 g/kg. A dose of 1 g superactive charcoal/kg body weight
increased survival times and rates in rats given the lethal dose of 8
mg T-2 toxin/kg as long as 3 h after the toxin was administered by
gavage (Galey et al., 1987).
II.5 EFFECTS ON MAN
II.5.1 Contemporary episodes of human disease
Two outbreaks of trichothecene-related disease have been
reported, one in China in 1984/85 (Luo, 1988) and one in India in
1987 (Bhat et al., 1989). Each involved several hundred cases.
During the first incident, outbreaks of mouldy corn and scabby
wheat poisoning were reported. Out of approximately 600 persons who
consumed mouldy cereals, there were 463 cases of poisoning (77% of
the total). The latency period for the onset of symptoms was 5-30
min. These included nausea, vomiting, abdominal pain, diarrhoea,
dizziness, and headache. No deaths occurred. Pigs and chicks fed
the same mouldy cereals were also affected (Luo, 1988). GC-MS and
RIA were used in the analysis of 5 samples of the mouldy corn. DON
was detected within a range of 0.34-92.8 mg/kg and zearalenone within
a range of 0.004-0.587 mg/kg. T-2 toxin and NIV were not found. TLC
was used in the analysis of 19 samples of scabby wheat collected from
the affected and non-affected families. The DON content was 1.0-40.0
mg/kg, which was significantly higher than that in the non scabby
wheat samples. In addition to DON, zearalenone was detected in 2
samples, the contents of which were 0.25 and 0.5 mg/kg, respectively.
No T-2 toxin was found in the samples (Luo, 1988).
An analogous outbreak was reported in Kashmir, India, in 1987
(Bhat et al., 1987, 1989). It was ascribed to the consumption of
bread made from flour that had become mouldy in storage following
unseasonal rains in the wheat-harvesting season, from which Fusarium
sp. was grown, and which were found to contain mycotoxins. Of the
224 persons investigated on a random sample basis, 97 were affected
with symptoms including abdominal pain (100%), throat irritation
(63%), diarrhoea (39%), blood in stools (5%), and vomiting (7%).
Symptoms developed 15 min to one hour after consumption of locally
baked bread. In 12 out of 24 samples of refined wheat flour used in
the preparation of bread, the following mycotoxins were found: DON
(0.35-8.38 mg/kg), Ac-DON (0.64-2.49 mg/kg) (no details of estimation
of this derivative were available), NIV (0.03-0.1 mg/kg) and T-2
toxin (0.55-0.8 mg/kg). Quantitative estimation of DON, Ac-DON, and
NIV was obtained using HPLC, and that of T-2 toxin by TLC (Bhat et
al., 1987) but no rigorous confirmation of identity was undertaken.
Since no fatalities occurred in either of the above outbreaks, no
information is available on the pathological changes, if any, at
autopsy.
II.5.2 Historical Fusarium-related diseases
In the period 1931-47, a human disease known as alimentary toxic
aleukia (ATA) occurred in the USSR that was suggested to be related
to the presence of toxic Fusarium species in mouldy over-wintered
grain. Data have been reviewed by Sarkisov et al. (1944) and more
recently by Bilai (1977), Leonov (1977), and Joffe (1986). An
association was established with the ingestion of grain invaded by
some moulds, in particular Fusarium poae and F. sporotrichioides.
The dominant pathological changes were necrotic lesions of the oral
cavity, the oesophagus, and stomach and, in particular, a pronounced
leukopenia. The primary lesion was bone marrow hypoplasia and
aplasia. The disease was lethal in a high proportion of cases.
The clinical symptoms reported in ATA, as well as the identified
occurrence of Fusarium in foodstuffs, suggest that it might have been
associated with mycotoxins, identified years later in fungal cultures
of Fusarium species under laboratory conditions, such as T-2 toxin
(Mirocha & Pathre 1973) or wortmannin (Mirocha & Abbas 1989).
Scabby grain toxicosis is a disease of human beings as well as
farm animals, and was reported from Japan and Korea in the period
1946-63 (Hirayama & Yamamoto 1948, 1950; Nakamura et al., 1951;
Tsunoda et al., 1957; Cho 1964; Ogasawara 1965; Chung 1975). The
common clinical symptoms were nausea, vomiting, diarrhoea, and
abdominal pain. All cases were acute with recovery within a few
days and no lethal cases were encountered. Fusarium fungi, F.
graminearum in particular, were isolated from suspected cereals
(Tochinai, 1933; Tsunoda et al., 1957).
When compared with the symptoms observed in experimental
animals, features of both the above human diseases were similar to
trichothecene toxicosis, notably symptoms caused by DON and NIV,
DAS, and T-2 toxin. However, no epidemiological studies have been
reported that link ATA and scabby grain toxicosis to these
chemicals.
II.5.3 Skin irritation
The Task Group was aware of several reports describing various
effects on the skin of crude extracts of fungal cultures or
solutions containing T-2 toxin, as well as other possible
substances (Bamburg & Strong, 1971; Saito & Otshubo, 1974). These
cases, all of them accidental, involved a very limited number of
persons who developed severe irritation, loss of sensitivity, and
desquamation. Despite the presence of T-2 toxin in the contact
material, there is no evidence that the involvement of other
compounds can be ruled out.
II.5.4 Studies of haemostasis
Platelet function and electron microscopic morphological
changes following T-2 toxin administration were studied on
platelets isolated from 12 healthy human volunteers. When
platelets were incubated with T-2 toxin at doses of 5-500 µg/109
platelets for 20 min, there was a dose-related inhibition of
platelet aggregation with different activators, including
epinephrine, arachidonic acid, and collagen, and a release of dense
bodies consisting mainly of serotonin-containing granules. There
was also a change in membrane permeability, but no changes in
shape. No correlated inhibition of thromboxane synthesis, or
significant alterations in platelet calcium content were observed.
The microtubular system was unaffected. It was suggested that the
above observations , notably suppressed aggregation, played a
contradictory role in the haemorrhagic phenomena associated with
these toxins in man and animals (Yarom et al., 1984a).
II.5.5 Airborne trichothecene-related diseases
High concentrations of spores of Stachybotrys atra were
discovered in the air of living rooms of a suburban house in
Chicago, USA (Croft et al., 1986). Over a period of several years,
the 5 occupants of the house had suffered a variety of non-specific
symptoms including signs of colds, sore throats, diarrhoea,
headaches, dermatitis, intermittent focal alopaecia, and
generalized malaise. Chemical analysis of building materials
supporting the growth of Stachybotrys atra confirmed the presence
of the macrocyclic trichothecenes, verrucarin B and J, satratoxin
H, and trichoverrins A and B. Five weanling Sprague-Dawley rats
and 5 mice, administered extracts of contaminated materials orally,
died within 24 h of exposure, whereas control animals remained
unaffected. Histological lesions of the animals tissues were
degeneration, necrosis and haemorrhage of the brain, thymus,
spleen, intestine, lungs, heart, lymph nodes, liver, and kidneys.
Although the clinical symptoms could have been related to allergic
responses, the isolation of potent mycotoxins suggests that they
were the causal factor of the illness observed.
II.5.6 Toxicological information on man, obtained from
therapeutic uses
DAS (Anguidine) has been undergoing clinical trials as a
chemotherapeutic agent in cancer patients. During a weekly
schedule of DAS, using healthy volunteers as controls, a dose of 5
mg/m2 body surface infused over 3 h, produced nausea, vomiting,
hypotension, neurological symptoms (confusion, hallucinations and
psychomotor seizures), chills, fever, and diarrhoea (DeSimone et
al., 1979). A similar study carried out on 20 other cancer
patients revealed gastrointestinal and neurological toxic effects
(Belt et al., 1979). A bolus administration or rapid infusion of
the drug also caused gastrointestinal and neurological symptoms
(Thigpen et al., 1981). The human haematopoietic system appears to
be extremely sensitive to DAS. Myelosuppression was the dose
limiting adverse effect of prolonged infusions over 8 h (Thigpen et
al., 1981). A mean myelosuppressive dose level in the above
investigation was, 5 mg/m2 body surface by 8-h infusion or, roughly
calculated in terms of body weight, 0.2 mg/kg (0.025 mg/kg per h).
II.6 EVALUATION OF THE HUMAN HEALTH RISKS
On the basis of the data made available to the Task Group, there
is a possible association between trichothecene exposure and
episodes of human disease. According to the limited data
available, the most frequently reported trichothecenes involved in
episodes of human exposure are DON and NIV. In the episodes of
alimentary toxic aleukia and scabby grain toxicosis reported in the
past, a possible etiological role of trichothecenes cannot be
excluded. Exposure occurs through the ingestion of contaminated
food, mainly cereals. Processing, milling, and baking are
not effective in destroying DON, NIV, and T-2 toxin. There is very
limited evidence of exposure through inhalation, but such a
possibility cannot be ruled out.
Among the naturally occurring trichothecenes in foods, T-2
toxin is the most potent, followed by DAS and NIV; DON was the
least toxic in acute toxicity studies. In experimental animals,
T-2 toxin and DAS produce acute systemic effects, with necrosis of
epithelial tissues and suppression of haematopoiesis. In
contemporary outbreaks of disease, only gastrointestinal symptoms
have been reported.
DON was shown to be teratogenic in mice but not in rats.
According to published chronic toxicity studies, NIV and T-2 toxin
are not tumorogenic in animals. No long-term carcinogenicity
studies on DON have been published. Certain trichothecenes, such
as T-2 toxin and DON, have an immunosuppressive action in animals
and have produced alterations in both cell-mediated and humoral
immunity. There is no evidence of immunosuppressive action in man.
Reported cases of human disease associated with trichothecene
exposure are limited in number and information. Symptoms of
digestive disorders and throat irritation develop rapidly after
ingestion of food contaminated with trichothecenes. At present,
there is no evidence of human cancer caused by trichothecenes. No
reports were available to the Task Group on secondary infection, by
bacteria, fungi, or viruses, in human beings following trichothecene
exposure, as has been observed in experimental animal studies. It
appears that adequate studies elucidating such a sequence have not
been made.
III. ERGOT
III.1 PROPERTIES AND ANALYTICAL METHODS
III.1.1 Chemical properties
Ergot is the French word for a rooster's spur and is used as
the common term for sclerotia of fungal species within the genus
Claviceps, in particular C. purpurea. The fungi infect the florets
of grasses and cereal, replacing the florets with compact fungal
structures, sclerotia, 2-20 mm long, somewhat curved, tapering off
at the ends, and strongly coloured, often purple-black, thus
resembling rooster's spurs. The sclerotia contain a large number
of biologically active alkaloids, as well as amino acids,
carbohydrates, lipids, and pigments, and, when the sclerotia are
consumed by man and animals, toxicosis develops, called ergotism.
The topic has been reviewed by Bove (1970), Van Rensburg &
Altenkirk (1974), Lorenz (1979), and Berde & Schild (1978). This
section deals with ergot as a toxic food contaminant; ergot
compounds used as pharmaceutical drugs are excluded.
Ergot alkaloids (ergolines), of which more than 40 have been
isolated from Claviceps sclerotia, are derivatives of lysergic acid
(Fig. 4), and can be divided into 3 groups:
Group I: Derivatives of lysergic acid, e.g., ergotamine.
Group II: Derivatives of isolysergic acid, e.g., ergotaminine.
Group III: Derivatives of dimethylergoline (clavines), e.g.,
agroclavine.
II.1.1.1 Physical properties
The trichothecenes are colourless, mostly crystalline solids
that have been well characterized by physical and spectroscopic
techniques (Cole & Cox, 1981). The type A trichothecenes are
soluble in moderately polar solvents, such as chloroform, diethyl
ether, ethyl acetate, and acetone, whereas the more polar type B
trichothecenes require higher polarity solvents, such as aqueous
methanol or aqueous acetonitrile. Some physical properties of the
main trichothecenes are summarized in Table 10.
Table 10. Some physical properties of main trichothecenes
-------------------------------------------------------------------------------
Trichothecenes Molecular Relative Melting (alpha)20D References
formula molecular point
mass (°C)
-------------------------------------------------------------------------------
T-2 toxin C24H34O9 466 151-152 +15 Bamburg et
al. (1968b)
HT-2 toxin C22H26O8 424 - - Bamburg &
Strong (1969)
Diacetoxyscirpenol C19H26O7 366 162-164 -27 Sigg et al.
(1965)
Neosolaniol C19H26O8 382 171-172 - Ishii et al.
(1971)
Deoxynivalenol C15H20O6 296 151-153 +6.35 Yoshizawa &
Morooka (1973)
Nivalenol C15H20O7 312 222-223 +21.54 Tatsuno et
al. (1968)
Trichothecin C19H24O5 332 118 +44 Freeman (1955)
Fusarenon-X C17H22O8 354 91-92 +58 Ueno et al.
(1969b)
Roridin A C29H40O9 532 198-204 +130 Harri et al.
(1962)
Satratoxin H C29H36O9 528 162-166 - Eppley &
Bailey (1973)
Verrucarin A C27H34O9 502 360 +20.6 Gutzwiller &
Tamm (1965)
-------------------------------------------------------------------------------
Most of the trichothecenes lack conjugated unsaturation in their
structures with a consequent absence of absorption in the ultraviolet
(UV) spectrum, except for end absorption due to unsaturation at C-9.
This lack of absorbance is a source of difficulty in achieving
sensitive and specific detection in HPLC analysis. In contrast, the
type D trichothecenes give characteristic ultraviolet spectra.
II.1.1.2 Chemical properties
When trichothecenes containing an ester group are treated with a
base, they are hydrolysed to their corresponding parent alcohol (Wei
et al., 1971). Free hydroxyl groups are readily acylated. The
12,13-epoxy group is itself extremely stable to nucleophilic attack.
However, prolonged boiling under highly acidic conditions causes an
intramolecular rearrangement of the trichothecene skeleton to the
apotrichothecene ring. Detailed discussion of the chemistry of
trichothecenes can be found in reviews by Bamburg & Strong (1971),
Bamburg (1976), and Tamm (1977).
The trichothecenes are generally stable; for example, DON can be
stored in organic solvents, such as ethyl acetate, for a long time
without any significant deterioration (Shepherd & Gilbert, 1988).
They remain unaffected when refluxed with various organic solvents
and also under mildly acidic conditions.
II.1.2 Analytical methods for trichothecenes
Analytical methods have been reviewed by Scott (1982) and Pohland
et al. (1986). Selected examples of recently published analytical
methods for type A trichothecenes and type B trichothecenes are
summarized in Tables 11 and 12 respectively. Although some of the
multi-trichothecene methods included in Table 11 may also be
applicable to certain of the type B compounds, the procedures in
Table 12 have been developed exclusively for the type B toxins.
II.1.2.1 Chemical methods
(a) Extraction
The most commonly used extraction solvents for trichothecenes are
chloroform, ethyl acetate, methanol, acetonitrile, aqueous methanol,
and aqueous acetonitrile. Chloroform, ethyl acetate, and
acetonitrile have been successfully used for the extraction of T-2
toxin, DAS, and some of their partially hydrolysed derivatives in
naturally contaminated cereals. Aqueous methanol and aqueous
acetonitrile are the solvents of choice for the extraction of several
trichothecenes of widely differing polarity as well as for the
extraction of type B toxins alone. Methods differ according to the
type of solvent used, whether samples are homogenised in a blender
with the solvent or agitated with a wrist action shaker, and in the
length of time of the extraction process. Spiking of samples with
standards is not an adequate way of demonstrating the efficiency of
extraction, and only methods validated with naturally contaminated
material can be regarded as having been rigorously tested.
Extraction procedures have been assessed for DON (Trenholm et al.,
1985) and it has been demonstrated that longer extraction times are
required for naturally contaminated samples than for those that have
been spiked (at least 120 min shaking). It has also been shown that
aqueous acetonitrile gives a cleaner extract than aqueous methanol.
(b) Clean-up procedures
The extent of sample clean-up required for a particular assay
depends on the specificity of the detection procedure and the nature
of the sample matrix. The less specific detection methods, such as
TLC, require extensive sample clean-up while more sophisticated
approaches, such as mass spectrometry and immunoassay, may only
require minimal sample preparation.
Table 11. Selected methods for the determination of T-2 toxin and diacetoxyscirpenol in
biological materials
------------------------------------------------------------------------------------------------
Matrix Extractiona Clean-upb Assayc Detection Toxins assayed References
limit -----------------
(µg/g) T-2 DAS Others
------------------------------------------------------------------------------------------------
Cereals MeOH/H2O XAD-4; flor. TLC 0.5 + + 7 Kamimura et
al. (1981)
Cereals EtOAC prep. TLC HPTLC 0.2 + - 2 Ilus et
al. (1981)
Foods MeCN/H2O char/alum TLC 1.0 + + 5 Romer
(1986)
Cereals MeCN/KCl Sep P HPLC(RI) 1.0 + - 1 Schmidt &
Dose (1984)
Wheat/rye MeCN Bond-Elut LC/MS (therm) 0.04 + + 2 Rajakyla et
al. (1987)
Plasma EtOAC Sep P/sil.g LC/MS (therm) 0.002 + + 2 Voyksner et
al. (1985)
Cereals EtOAC sil.g GC (FID)-TMS 0.1 + + 3 Bata et al.
(1983)
Cereals MeOH/H2O sil.g/cy GC (ECD)-HFB 0.05 + + 1 Cohen &
Lapointe
(1984)
Plasma benzene flor. GC (ECD)-HFB 0.02 + - - Swanson et
al. (1983)
Milk EtOAC prep. TLC GC/MS-TMS 0.003 + - - Collins &
Rosen
(1979)
Corn MeOH Sep P/sil.g GC/MS-TMS 0.02 + + 1 Rosen &
Rosen
(1984)
Foods MeOH flor. GC/MS-HFB 0.01 + + 11 Black et
al. (1987)
Urine EtOAC/MeOH Sep P GC/MS-HFB 0.005 + + 5 Black et
al. (1986)
Blood acetone Sep P GC/MS-PFP 0.005 + + 9 Begley et
al. (1986)
------------------------------------------------------------------------------------------------
Table 11 (contd.)
------------------------------------------------------------------------------------------------
Matrix Extractiona Clean-upb Assayc Detection Toxins assayed References
limit -----------------
(µg/g) T-2 DAS Others
------------------------------------------------------------------------------------------------
Milk/urine EtOAC Sep P RIA 0.002 + - - Lee & Chu
(1981a)
Corn/wheat MeOH Sep P RIA 0.001 + - - Lee & Chu
(1981b)
Urine none Sep P ELISA 0.0005 + - - Fan et al.
(1987)
------------------------------------------------------------------------------------------------
a Extraction: MeOH (methanol); H2O (water); EtOAC (ethyl acetate); MeCN (acetonitrile);
KCl (potassium chloride aqueous soln).
b Clean-up: XAD-4 (amberlite XAD-4 resin); flor. (florisil); char/alum (charcoal/alumina
columns); Sep P (C18-Sep Pak); sil.g (silica gel); cy (cyano extraction column).
c Assay: TLC (thin layer chromatography); HPTLC (high performance TLC); HPLC (high performance
liquid chromatography); RI (refractive index); LC/MS (combined HPLC/mass spectrometry);
GC (gas chromatography); FID (flame ionisation detector); ECD (electron capture detector);
TMS (trimethylsilyl derivative); HFB (hepafluorobutyrl ester derivative);
PFP (pentafluoropropionyl ester); RIA (radioimmunoassay); ELISA (enzyme linked
immunosorbent assay).
Table 12. Selected methods for the determination of deoxynivalenol and nivelenol in biological
materials
------------------------------------------------------------------------------------------------
Matrix Extractiona Clean-upb Assayc Detection Toxins assayed References
limit -----------------
(µg/g) T-2 DAS Others
------------------------------------------------------------------------------------------------
Wheat/corn MeCN/H2O char/alum TLC 0.04-0.1 + - - Trucksess
et al.
(1984)
Foods MeCn/H2O char/alum; TLC 0.5 + - - Trucksess
Sep P et al.
(1986a)
Cereals MeCN/H2O/ char/alum; HPTLC 0.05 + + 1 Trucksess
MeOH ppt et al.
(1987)
Corn/rice MeOH/H2O/ liq/liq extr HPLC (UV) O.005 + + 2 Visconti &
NaCl Bottalico
(1983a)
Corn/rice MeOH/H2O none HPLC (ED) 0.025 + - - Sylvia et
al. (1986)
Corn MeOH/H2O prep. TLC HPLC (UV) 0.01 + - - Ehrlich et
al. (1983)
Cereals MeCN/H2O ion/char/ HPLC (UV) 0.05 + + - Lauren &
alum Greenhalgh
(1987)
Cereals MeOH flor. LC/MS (micro) 0.01 + + - Tiebach et
al. (1985)
Cereals MeOH/H2O sil.g GC (ECD)-TMS 0.02 + + - Scott et
al. (1986)
Cereals MeCN/H2O flor/Sep P GC (ECD)-TMS 0.002 + + - Tanaka et
al. (1986)
Wheat chlor/EtOH sil.g GC (ECD)-HFB 0.1 + - - Ware et
al. (1986)
Cereals chlor/EtOH sil.g GC (ECD)-HFB 0.02 + - - Mulders &
Impelen-
Peek (1986)
Milk EtOAc Sep P GC (ECD)-TMS 0.001 + - - Swanson et
al. (1986)
------------------------------------------------------------------------------------------------
Table 12 (contd.)
------------------------------------------------------------------------------------------------
Matrix Extractiona Clean-upb Assayc Detection Toxins assayed References
limit -----------------
(µg/g) T-2 DAS Others
------------------------------------------------------------------------------------------------
Corn/barley MeOH/H2O sil.g GC/MS-TMS 0.01 + - - Gilbert et
al. (1983a)
Foods MeOH/H2O XAD-2/flor/ GC/MS-TMS 0.02 + + - Yoshizawa &
Sep P Hosokawa
(1983)
Corn/wheat MeCN/H2O none ELISA 0.01 + - - Xu et al.
(1988)
------------------------------------------------------------------------------------------------
a Extraction: MeCN (acetonitrile); H2O (water); MeOH (methanol); NaCl (sodium chloride soln);
chlor/EtOH (chloroform/ethanol); EtOAc (ethyl acetate).
b Clean-up: char/alum (charcoal/alumina column); Sep P (C18-Sep Pak); ppt (lead acetate
precipitation); liq/liq extr. (liquid/liquid extraction); ion (ion exchange resin);
flor. (florisil); sil.g (silica gel); XAD-2 (Amberlite XAD-2 resin).
c Assay: TLC (thin layer chromatography); HPTLC (high performance TLC); HPLC (high performance
liquid chromatography); UV (ultraviolet detection); ED (electrochemical detection);
LC/MS (combined microbore-HPLC/mass spectrometry); GC (gas chromatography); ECD (electron
capture detector); TMS (trimethylsilyl derivative); HFB (heptafluorobutyryl ester
derivative); ELISA (enzyme linked immunosorbent assay).
Early methods that may have involved the use of conventional
silica gel columns or preparative TLC for clean-up, have to a large
extent been superseded by methods using prepacked cartridges, such as
silica gel and C18-Sep Paks, which are both more reliable and more
convenient. Florisil columns are widely used for clean-up (e.g., see
Tanaka et al., 1985a), and the one step clean-up procedures using
alumina/charcoal (Romer, 1986) or alumina/charcoal/Celite columns
(Trucksess et al., 1984) have become widely adopted, particularly for
DON and NIV assays.
(c) Detection and quantification
(i) Thin-layer chromatography (TLC). The lack of native
fluorescence or UV absorbance of the trichothecenes means that TLC
detection relies on the use of spray reagents for visualisation.
Characteristic colours or fluorescence can be produced with sulfuric
acid or p-anisaldehyde followed by heating at 110-120 °C (Scott et
al., 1970; Ueno et al., 1973c). A general spray reagent for the
12,13-epoxy function is 4-( p-nitrobenzyl)pyridine, which produces a
blue coloration on heating and treatment with a base (Takitani et
al., 1979). A more sensitive, but somewhat elaborate, procedure,
which again is specific for the epoxide function, involves reaction
with nicotinamide and 2-acetylpyridine to produce fluorescent TLC
spots (Sano et al., 1982). Diphenylindenone sulfonyl esters of
trichothecenes can be formed prior to TLC and, subsequently, when
sprayed with sodium methoxide can yield fluorescent spots at high
sensitivity (Yagen et al., 1986).
Most of the above spray reagents, though frequently demonstrated
as useful for the determination of standards or of relatively high
concentrations of toxins in culture extracts, have not been well
developed in conjunction with clean-up procedures for the
determination of trichothecenes in naturally contaminated cereal
samples or other foods. However, the exception has been the use of
an aluminum chloride spray reagent for visualising the type B
trichothecenes (Kamimura et al., 1981). On spraying the plates,
heating for 10 min at 110 °C and then treating with a base, blue
fluorescent spots are produced for DON, NIV, and fusarenon X. Using
this approach, in conjunction with a clean-up utilising an alumina
charcoal Celite column, a quantitative TLC procedure was developed
using a fluorodensitometer for determining DON in wheat and corn
(Trucksess et al. 1984); DON in processed grain products including
breakfast cereals, corn syrup, and beer (Trucksess et al., 1986b);
and for simultaneously monitoring DON, NIV, and fusarenon X in
barley, corn, and wheat (Trucksess et al. 1987). This TLC procedure
was successfully collaboratively tested (Eppley et al., 1986) and was
accepted as the AOAC first action method for DON in wheat.
(ii) High performance liquid chromatography (HPLC). HPLC has not
proved particularly appropriate for the type A trichothecenes, which
lack any significant UV absorption, making sensitive detection
difficult. Refractive index detection has been employed, but at
relatively high toxin levels (Schmidt & Dose, 1984). UV detection at
short wavelength has been used for the determination of DON and NIV
in cereals, and a number of methods have been reported that offer an
advantage over alternative GC approaches in that they do not require
derivatization of the toxins. A post-column derivatization procedure
for DON and NIV has been developed (Sano et al., 1987) involving
alkaline decomposition of the trichothecenes to generate formaldehyde
and then reaction with methyl acetoacetate and ammonium acetate to
form a fluorescent derivative. Although more sensitive and specific
than UV detection, the approach does have the disadvantage of
requiring rather elaborate instrumentation, in order to carry out the
post column reaction. Electrochemical detection (Sylvia et al.,
1986) looks particularly promising for the determination of DON by
HPLC, offering both greater sensitivity and specificity than UV
detection, and the possibility of analysis of sample extracts that
have received minimal sample clean-up.
(iii) Gas chromatography (GC). A large number of GC methods have
been published that differ in the approach to sample extraction, in
sample clean-up, and in choice of derivative prior to GC analysis.
Trichothecenes containing a hydroxyl group require derivatization,
and the heptafluorobutyryl (HFB) ester and trimethylsilyl (TMS) ether
derivatives have been most frequently used in conjunction with
electron capture detection. Formation of the HFB derivatives is
relatively time-consuming, but complete derivatization is easily
achieved and the derivative once formed is stable for at least
several days. However, poor reproducibility has been noted with HFB
derivatives of DON (Mulders & Impelen-Peek, 1986) attributed to
adsorption on to glass surfaces. The relatively high mass of the HFB
derivatives can cause difficulties if GC/MS confirmation is required.
In contrast, TMS derivatives are easily prepared, and are of
suitable mass for GC/MS, but, for some type B toxins, they may
require optimization of conditions for complete derivatization
(Gilbert et al., 1985). Scott & Kanhere (1986) have compiled
retention data for 10 different trichothecenes as both TMS and HFB
derivatives on capillary columns of different stationary phases.
Trifluoroacetyl derivatives of trichothecenes are preferred by some
workers (Kientz & Verweij, 1986), and pentafluoropropionyl esters
have been used, particularly where detection has been by MS (Begley
et al., 1986; Krishnamurthy & Sarver, 1986; Rood et al., 1988a).
An area where improvement in quantification of trichothecenes is
still needed is in the selection of adequate internal standards.
Compounds, such as methoxychlor (Romer et al., 1978),
hexachlorobiphenyl (Blass et al., 1984), and alkanes (Ilus et al.,
1981), have been used, but these differ significantly in structure
from the trichothecenes. The deuterated TMS derivative of T-2 toxin
has been used as an internal standard in GC/MS analysis (Rosen &
Rosen, 1984), an isomer of T-2 toxin has been chemically synthesized
(Stahr et al., 1981) as have 4-deoxyverrucarol and 16-hydroxyver-
rucarol for use as internal stan-dards (Krishnamurthy et al., 1986).
Despite the many difficulties, a GC method using the HFB
derivative has been successfully collaboratively tested (Ware et al.,
1986) and subsequently adopted as an AOAC official first action
method.
Other related trichothecenes have been determined in foods by GC
methods, for example, the de-epoxidised metabolite of DON called
DOM-1 has been determined in milk as both its TMS and HFB derivative
(Swanson et al., 1986). Also, an isomer of DON was detected by
capillary GC, as its TMS derivative in bread and breakfast cereal
products prepared from flour naturally contaminated with DON
(Greenhalgh et al., 1984).
(iv) Mass spectrometry (MS). Mass spectrometry has been used for
the structural characterization of novel trichothecenes; for
identification and confirmation of trichothecenes in biological
materials, and as a sensitive and specific means of detection with
GC, LC, or supercritical fluid chromatography (SFC) sample
introduction.
The usefulness of negative ion chemical ionization (NICI) mass
spectrometry with hydroxide ion reagent gas has been demonstrated in
relation to producing relative molecular mass information, as well as
fragment ions indicative of structure for type A and B trichothecenes
(Brumley et al., 1982). The NICI technique has been applied to
confirmation of the presence of DON in cereals and snack foods by
rapid capillary GC introduction of the underivatized extract
obtaining full scan spectra (Brumley et al., 1985), and by selected
ion monitoring (Miles & Gurprasad, 1985).
NICI has also been used for selected ion monitoring GC/MS of the
HFB derivatives of 13 different trichothecenes of both type A and
type B in extracts from small samples of biological material
(Krishnamurthy et al., 1986). GC/MS is the preferred approach using
SIM in electron ionization (D'Agostino et al., 1986) or NICI (Begley
et al., 1986) modes for biological samples where only small sample
sizes are available but high sensitivity is required. For more
routine surveys of cereal and food samples, particularly for DON and
NIV, initial screening has normally been carried out using GC with an
ECD and only selected positive samples have been confirmed by GC/MS
(Tanaka et al., 1985a; Cohen & Lapointe, 1982).
Attempts have been made to determine the macrocyclic
trichothecenes as TMS derivatives on short fused silica GC columns
using GC/MS (Rosen et al., 1986). The approach has also been adopted
of alkaline hydrolysis of the ring system of the macrocyclic
compounds to yield verrucarols, which can then be determined as HFB
derivatives by NICI GC/MS (Krishnamurthy et al., 1987). A similar
approach using alkaline hydrolysis has been proposed (Rood et al.,
1988a,b) as a basis for a general method for the determination of
trichothecenes and metabolites by conversion to their corresponding
parent alcohols prior to derivatization and GC or GC/MS
determination.
Although LC/MS is really a research method, thermospray LC/MS is
becoming more widely available and shows promise for the
identification of low levels of trichothecenes in biological
materials (Voyksner et al., 1985; Rajakyla et al., 1987). Another
promising research method for the determination of both the simple
and the macrocyclic trichothecenes is supercritical fluid
chromatography (SFC) combined with MS (Smith et al., 1985).
Supercritical fluids can also be used for the on-line extraction from
biological materials and direct introduction into the MS to monitor a
number of different trichothecenes (Kalinoski et al., 1986).
II.1.2.2 Immunological methods
Chu et al. (1979) developed a radioimmunoassay (RIA) for T-2
toxin. The antibody preparation was obtained by immunizing rabbits
with bovine serum albumin T-2 toxin hemisuccinate conjugate. The
antibody had the greatest binding efficiency for T-2 toxin and less
efficiency for HT-2 toxin. Cross reaction with other trichothecenes
was either very slight or absent, and the limit of detection of the
assay ranged from 1 to 20 ng.
This RIA was applied to the determination of T-2 toxin in
agricultural commodities, biological fluids, and animal organs (Lee &
Chu, 1981a,b; Hewetson et al., 1987). Xu et al. (1988) also reported
an immunoassay using an antibody against triacetylated DON, for the
determination of DON in wheat and corn with a limit of detection of
about 0.02 µg/g.
A polyclonal enzyme-linked immunosorbent assay (ELISA) was
developed for the rapid quantification of T-2 toxin in food and
animal feed (Pestka et al., 1981). This assay, which could be
undertaken in 2 h, had a limit of detection of 2.5 pg/assay and was
used for the detection of T-2 toxin in Fusarium-infected corn
(Gendloff et al., 1984). More recently monoclonal ELISAs have been
developed for T-2 toxin and DON (Feuerstein et al., 1985; Hunter et
al., 1985; Chiba et al., 1988). Although these monoclonal assays are
of invariable specificity and the antibodies are available in
unlimited supply, competitive indirect ELISAs using monoclonals are
generally less sensitive than polyclonal-based ELISAs. However,
Chiba et al. (1988) developed a sensitive ELISA for the detection of
T-2 toxin with monoclonal antibodies at a limit of 2.5 pg/assay.
This assay has been applied to the detection of T-2 toxin in wheat
flour (Chiba et al., 1988) and in fungal cultures (Nagayama et
al., 1988).
Production of useful antibodies against DON has proved more
difficult than for other trichothecenes. Xu et al. (1988) adopted
the approach of raising antibodies against triacetyl-DON, which
requires acetylation of DON in an extract of the contaminated cereal
prior to carrying out a direct ELISA. This assay has the required
sensitivity but also the disadvantage of determining the total
concentration of DON plus any other acetylated derivatives that may
be present in the extract. A different approach to raising
monoclonal antibodies to DON has been carried out by forming the
hemisuccinyl derivative after protection of two of the available
adjacent hydroxyls with a cyclic boronate ester (Casale et al.,
1988). Some cross-reactivity to other trichothecenes and their
de-epoxy metabolites was observed, but the assay does show
considerable potential as a simple and rapid screening method for
contaminated cereals and for detection in biological samples.
II.1.2.3 Biological methods
Biological methods are essential for working with field cases
where the cause of an incident is unclear and evidence is sought to
associate an observed biological effect with the presence of fungally
contaminated material. Biological methods are also important as
monitoring procedures in the isolation and purification of new or
previously unrecognized toxins, prior to characterization by
spectroscopic and chemical methods.
The classical and commonly used bioassay for trichothecenes is
the skin necrotization test. Extracts prepared from samples (see
section II.1.2.1) are applied in a single dose to the shaved back of
a rabbit, rat, or guinea-pig. Toxic preparations applied in this way
produce erythema, oedema, intradermal haemorrhage, and necrosis. The
guinea-pig is the most sensitive of these animals to trichothecenes
(Ueno et al., 1970). As little as 0.2 µg of T-2 toxin and DAS were
detected on the skin of the rabbit and guinea-pig (Chung et al.,
1974; Balzer et al., 1977). Only 10 g of the corn sample are needed
for testing with an extraction and clean-up procedure recommended by
Eppley (1968). Macrocyclic trichothecenes are highly irritant to the
skin, but NIV and DON, which have a low skin-necrotizing potency,
could be routinely missed in this assay (Ueno et al., 1970).
Brine shrimp ( Artemia salina L.) larvae also provide a useful
biological system for monitoring trichothecenes, such as T-2 toxin,
DAS, and the macrocyclic trichothecenes. The limit of detection of
this test for these toxins is approximately 0.04-0.4 mg/litre
(Eppley, 1974).
The rabbit reticulocyte assay, based on the inhibition of uptake
of 14C-leucine in eukaryote cells, is highly specific for
trichothecenes; ID50 (50% inhibitory dose) values are 0.03 mg/litre
for T-2 toxin and DAS, and 2.0-3.0 mg/litre for DON and NIV (Ueno et
al., 1969a, 1973c). By combining chemical methods with this assay,
DON was successfully isolated from Fusarium-infected corn (Ishii et
al., 1975).
II.2 SOURCES AND OCCURRENCE
II.2.1 Taxonomic considerations
The vast majority of trichothecenes are produced by the Fusarium
species. Production of these metabolites depends, of course, on many
factors, including substrate, temperature, humidity, etc. In
general, the type A trichothecenes have been most frequently
associated with the following Fusarium species: F. tricinctum, F.
sporotrichioides, F. poe, F. equiseti. On the other hand, type B
trichothecenes are usually associated with F. graminearum (Gibberella
zeae), and F. culmorum. The type C trichothecenes, containing an
additional epoxide function at the 7,8- or 9,10-positions, are
produced by only a few species. The Type D trichothecenes, i.e.,
those containing the macrocyclic ring between the 4,15-positions, are
produced by several fungal genera, including Myrothecium and
Stachybotrys. Of these, the most important from an animal and human
health standpoint, is Stachybotrys atra. The relationships between
trichothecenes and fungal species are summarized in Table 13.
Table 13. The relationship between trichothecenes and fungal species
----------------------------------------------------------------------
Trichothecenes Fungal species References
----------------------------------------------------------------------
Type A
T-2, HT-2, DAS, NS Fusarium tricinctum Joffe & Yagen (1977)
F. sporotrichioides Scott et al. (1980)
F. poae Marasas et al. (1984)
F. acuminatum Ichinoe et al. (1985),
Rabie et al. (1986)
DAS F. equiseti Brian et al. (1961)
F. semitectum Suzuki et al. (1980),
Greenhalgh et al. (1984)
Type B
DON, 3-AcDON F. graminearum Yoshizawa & Morooka (1973)
NIV, F-X Gibberella zeae Marasas et al. (1984)
(anamorph) Ichinoe et al. (1985)
DON, 3-AcDON F. culmorum Marasas et al. (1979b)
Chelkowski et al. (1984)
Trichotecin Trichothecium Freeman & Morrison (1949)
roseum Ishii et al. (1986)
Type C
Baccharin Baccharis Kupchan et al. (1976)
megapotamica
(higher plant)
Type D
Roridin A, D, E Mycothecium roridum Bohner et al. (1965)
Verrucarin J M. verrucaria Harrach et al. (1981)
Satratoxin G, H Stachybotrys atra Jarvis et al. (1986)
----------------------------------------------------------------------
The taxonomy of the various fungal species is complex and has led
to some confusion, particularly regarding the Fusarium species
responsible for the production of the various trichothecene
metabolites. Several excellent descriptions are now available of the
Fusarium spp. and the metabolites produced by them (Nelson et al.,
1983; Ueno, 1983; Marasas et al., 1984; Ichinoe et al., 1985; Joffe,
1986).
II.2.2 Ecology of trichothecene-producing fungi
Fusarium species are widely distributed throughout the
environment. There are over 50 recognized species commonly occurring
in the soil (soil fungi), many of which are pathogenic to crop
plants. As a result, there are a number of frequently encountered
plant diseases (wilts, blights, rots), such as F. graminearum blight
of wheat and barley (Akakabibyo in Japanese), pink ear rot of corn,
pink scab (tombstone kernels) of wheat, Fusarium snow blight, etc.
Fusarium graminearum, which produces DON and NIV, is a most important
fungus.
II.2.3 Natural occurrence
It has become apparent in the past few years that whenever a
trichothecene-producing Fusarium species parasitizes a crop, food,
or animal feed, it is highly probable that the metabolites of the
trichothecene will be found as contaminants. The chance of detecting
the metabolite is then clearly a function of the efficiency of the
sampling procedure and the capabilities of the analytical methods
used (most importantly, the detection limit).
Because of its toxicity, analytical procedures for T-2 toxin were
developed first, and consequently early surveys for trichothecenes
tended to concentrate on T-2 toxin. However, it soon became apparent
that other trichothecenes, in particular, DON, NIV, and DAS, were
more frequent contaminants of food and animal feed than T-2 toxin.
As improved analytical procedures for these mycotoxins became
available, surveys were conducted and additional data on the
occurrence of these metabolites were published. In reviewing this
record, it should be recognized that the methodology used in
developing the data was quite variable, so that only broad
generalizations with respect to incidence/level may be drawn. In the
vast majority of cases, no evidence was included indicating that
methods used were rigorously tested, that quality assurance
programmes were in place, and that confirmation of identity was
adequately obtained. Much of the available survey data on the
natural occurrence of trichothecenes in raw agricultural commodities,
food, and animal feed is summarized in Tables 14-17. Reports in
which only a few samples were analysed have not been included.
II.2.3.1 Agricultural products
(a) T-2 Toxin
One of the first trichothecenes to be implicated in an episode of
mouldy corn toxicosis was T-2 toxin; in 1972, it was reported that
T-2 toxin at the level of 2 mg/kg was present in mouldy corn involved
in lethal toxicosis in dairy cattle (Hsu et al., 1972). This event,
along with increasing information regarding the acute toxicity of T-2
toxin, prompted considerable efforts to develop methods of analysis
for T-2 toxin and the analysis of a wide range of agricultural
commodities (Table 14). Only occasional samples were found to
contain T-2 toxin (incidence well below 10% in most cases), most
frequently at levels <0.1 mg/kg. Usually, other trichothecenes were
also found (Vesonder, 1983). On the other hand, there have been
isolated reports of the finding of rather high levels of T-2 toxin,
e.g., the finding of 25 mg T-2 toxin/kg in barley (Puls & Greenway,
1976), and 38.9 mg T-2 toxin/kg in peanuts (Bhavanishankar & Shantha,
1987). These findings, as well as reports from India of the presence
of T-2 toxin in safflower seed and sweet corn (Ghosal et al., 1977),
and sorghum (Ruckmini & Bhat, 1978), and from Italy of its presence
in barley, corn feed, oats, rice, and wheat (Cirilli, 1983) need to
be further investigated.
Table 14. Natural occurrence of T-2 toxin in raw agricultural
commodities
---------------------------------------------------------------------
Commodity Levels Incidence Country Reference
(mg/kg) (+ve/total)
---------------------------------------------------------------------
Corn 0.5-5.0 (5/150) Hungary Szathmary (1983)
0.080-0.65 (9/118) Taiwan Tseng et al. (1983)
0.01-0.2 (13/20) New Zealand Hussein et al.
(1989)
Feed 0.05-5.0 (28/464) Hungary Szathmary (1981)
Oats 0.01-0.05 Finland Ylimacki et al.
(1979)
Peanuts 0.63-38.89 (6/87) India Bhavanishankar &
Shantha (1987)
Rice (3/64) Egypt Abdel-Hafez et al.
(1987)
Sorghum 1.67-15.0 (4/84) India Bhavanishankar &
Shantha (1987)
Wheat 2.0-4.0 (3/12) India Bhat et al. (1989)
---------------------------------------------------------------------
DON/NIV: Deoxynivalenol (DON) and nivalenol (NIV) have been
found to be the most frequent trichothecene contaminants of
agricultural crops throughout the world (Table 15). Extensive
survey data indicate the common occurrence of these mycotoxins,
particularly in corn and wheat, at levels usually below 1 mg/kg
(Vesonder, 1983; Pohland & Wood, 1987; Jelinek et al., 1989).
Perhaps the most extensive survey, and the one giving the most
accurate picture of the global occurrence of DON and NIV, was
reported recently by Tanaka et al. (1988).
Table 15. Natural occurrence of DON and NIV in raw agricultural
commodities
---------------------------------------------------------------------------
Commodity Toxin Levels Incidence Country Reference
(mg/kg)a (+ve/total)
---------------------------------------------------------------------------
Barley DON t-40.4 (19/25) Japan Kamimura et
al. (1981)
NIV t-37.9 (19/23)
(unpolished) DON 0.004-0.508 (26/28) Korea Lee et al.
NIV 0.017-3.0 (28/28) Republic of (1985)
(polished) DON 0.008-0.043 (5/6)
NIV 0.085-0.328 (5/6)
DON 0.006-2.14 (34/49) Norway Sundheim et
NIV 0.013-1.56 (49/49) al. (1988)
DON 0.02-0.36 (34/87) United Gilbert et
Kingdom al. (1983b)
Corn DON 1-20 (19/60) Austria Lew et al.
(1979)
DON 0.15-0.82 (9/9) Canada Scott et
al. (1981)
---------------------------------------------------------------------------
Table 15 (contd.)
---------------------------------------------------------------------------
Commodity Toxin Levels Incidence Country Reference
(mg/kg)a (+ve/total)
---------------------------------------------------------------------------
Corn (contd.) DON 0.36-12.7 (100%) China Qiujie et
NIV 0.054-2.67 (100%) al. (1988)
DON 0.02-0.3 (11/20) New Zealand Hussein et
al. (1989)
DON t-15.8 (20/72) Transkei Thiel et
NIV t-1.41 (43/72) al. (1982)
DON 0.1-0.3 (37) United Gilbert et
Kingdom al. (1984)
DON 0.5-10.7 (24/52) USA Vesonder et
al. (1978)
DON 0.1-2.47 (93/198) USA Wood &
Carter
(1989)
Oats DON 20 Germany, Bauer et
Federal al. (1980)
Republic of
DON 0.02-0.1 (1/6) United Gilbert et
Kingdom al. (1984)
Rye DON 0.003 (1/5) Korea Lee et al.
NIV 0.046-0.114 (5/5) Republic of (1985)
Wheat DON 0.01-4.3 (51/52) Canada Scott et
al. (1981)
DON 0.06-8.53 (24/52) Canada Trenholm et
al. (1981)
DON 0.02-1.32 (55/199) Canada Osborne &
Willis
(1984)
DON 1.0 (1.36) Denmark Hald & Krogh
(1983)
DON t-4.7 (15/18) Germany, Bauer et
NIV t-7.8 Federal al. (1980)
Republic of
DON 0.008-3.19 (32/53) Norway Sundheim et
al. (1988)
NIV 0.015-0.887 (53/53)
DON 0.02 >0.5 (57/148) United Gilbert et
Kingdom al. (1984)
DON 0.12-5.5 (31/33) USA Hagler et
al. (1984)
DON 0.2-9 (54/57) USA Eppley et
al. (1984)
DON 0.1-2.65 (133/247) USA Wood &
Carter
(1989)
---------------------------------------------------------------------------
Table 15 (contd.)
---------------------------------------------------------------------------
Commodity Toxin Levels Incidence Country Reference
(mg/kg)a (+ve/total)
---------------------------------------------------------------------------
Animal DON 0.1-41.6 (274/342) USA Cote et
feed al. (1984)
DON <0.4-4.0 USA Vesonder
(1983)
---------------------------------------------------------------------------
a t = trace.
This survey involved the analysis of 500 samples of cereal grains
from 19 countries (Table 16) using a single analytical method.
Table 16. Natural occurrence of DON and NIV: worldwide survey
---------------------------------------------------------------------
Commodity DON NIV
(Mean, ng/g) (% + ve) (Mean, ng/g) (% + ve)
---------------------------------------------------------------------
Barley 149 75 401 76
Corn 402 20 766 16
Oats 115 22 438 26
Rice 0 22 22
Rye 183 33 47 33
Sorghum 0 0
Wheat 488a 39 127 50
Othersb 135 44 3 6
Total: 292 45 267 48
-----------------------------------------------------------------------
a A Beijing wheat sample containing 6 644 ng/g was excluded in
calculating this mean.
b Wheat flour, 7; rye flour, 1; spice, 3; sesame, 7.
It was found that 45-50% of random samples contained both DON and
NIV, barley being most frequently contaminated. Corn, although less
frequently contaminated, contained the highest average amounts of
NIV; of all the cereals examined, wheat was the most heavily
contaminated with DON. There were clear regional differences, not
totally predictable, in the relative quantities of DON and NIV. For
example, in Canada and the USA, NIV was only rarely encountered
whereas, in Japan, NIV was more frequently encountered than DON.
Even within a country there were clear differences between regions;
for example, in southern Japan, NIV was more frequently encountered
than DON, whereas in northern Japan the reverse was true. Similarly,
levels of DON were generally lower in western than in eastern Canada
(Jelinek et al., 1989).
The data indicate that crops parasitized by Fusarium species will
probably be contaminated with these mycotoxins. In wheat, for
example, there is an excellent correlation between the DON
contamination level and the percentage of mouldy kernels, the
percentage of total defects, and the degree of scab damage (Eppley et
al., 1984; Shotwell et al., 1985). It has been suggested that crop
rotation might be a factor, i.e., planting wheat after corn tends to
increase the DON levels in the resulting wheat crop (Teich &
Hamilton, 1985). Recently, it has been shown that the mean
concentration of DON in wheat declines quite significantly in the
2-week period immediately preceding harvest (Scott et al., 1984).
(b) Miscellaneous trichothecenes
There have been occasional reports of trichothecenes other than
those discussed above (T-2 toxin, DON, NIV) in agricultural products,
in particular, DAS, 15-acetyldeoxynivalenol (15-ADON), and 3-
acetyldeoxynivalenol (3-ADON) (Table 17). Most of these reports
involve corn. There is also an unconfirmed report of the finding of
T-2 toxin, DAS, and trichothecolone as well as the fatty acid esters
of trichothecolone, scirpenetriol, and T-2 tetraol in banana fruit,
which needs further investigation (Chakrabarti & Ghosal, 1986). In
1982, over 100 out of a flock of 1200 ewes on a Hungarian farm died
after ingestion of bedding straw highly infected with Stachybotrys
atra. TLC, HPLC, and MS analysis of the methanol extract of the
straw revealed the presence of the macrocyclic trichothecenes,
satratoxins G and H (Harrach et al., 1983).
Table 17. Natural occurrence: miscellanceous trichothecenes in
agricultural products
---------------------------------------------------------------------------
Commodity Toxin Level Incidence Country Reference
(mg/kg) (+ve/total)
---------------------------------------------------------------------------
Corn 15-ADON 0.113 China Qiujie et al.
ave. (1988)
3-ADON 0.495 China Qiujie et al.
ave. (1988)
DAS 31.5 Germany, Siegfried (1977)
Federal
Republic of
DAS 0.01- (6/20) New Zealand Hussein et al.
0.9 (1989)
15-ADON 0-7.9 USA Abbas et al.
(1986)
Peanuts DAS 0.41- (7/87) India Bhavanishankar &
2.03 Shantha (1987)
Wheat ADON 0.6-2.4 (4/12) India Bhat et al.
flour (1989)
Bedding satratoxin not Hungary Harrach et al.
straw G estimated (1983)
satratoxin not Hungary Harrach et al.
H estimated (1983)
---------------------------------------------------------------------------
The macrocyclic trichothecenes, such as satratoxin H, verrucarins
B and J, and trichoverrins A and B, were also detected in air dust
collected from a house in Chicago, where the occupants were subjected
to a variety of recurring maladies including colds, dermatitis, and
others (Croft et al., 1986). These data indicated a possible
airborne outbreak of macrocyclic trichothecene-induced toxicosis.
Undoubtedly, as good analytical methods become available, these
trichothecenes will be found more frequently.
II.2.3.2 Trichothecenes in human foodstuffs
Given the widespread occurrence of trichothecenes in agricultural
products, in particular DON and NIV, it is not surprising to find the
compounds in human foodstuffs (Table 18). The vast majority of the
confirmed cases of contamination of foodstuffs by trichothecenes
involve DON in wheat or wheat products. Overall, the finding of DON
in human foodstuffs at concentrations >1 mg/kg has been rare. NIV
has been detected in human foodstuffs, particularly in Japan, where
an effort was made to develop and apply analytical methods capable of
determining both DON and NIV.
Table 18. Natural occurrence of DON and NIV in commercial foods
---------------------------------------------------------------------------
Commodity Toxin Level Incidence Country Reference
(mg/kg)a (+ve/total)
---------------------------------------------------------------------------
Barley DON 0.027-0.085 (6/6) Japan Yoshizawa &
flour NIV 0.037-0.19 (6/6) Hosokawa
(parched) (1983)
Barley DON 0.008-0.039 (5/6) Tanaka et
flour NIV 0.013-0.041 (6/6) al. (1985b)
Barley DON 0.003-0.05 (10/14) Tanaka et
(pressed) NIV 0.008-0.033 (13/14) al. (1985a)
Barley DON t-0.26 (27/147) Kamimura et
products NIV 0.006-0.28 al. (1981)
Corn meal DON 0.025 (45/50) USA Trucksess et
al. (1986a)
Corn flour DON (0.18 ave.) (27) Canada Scott et al.
meal DON (0.1 ave.) (35) (1984)
products DON 0.011-1.25 (7/7)
Popcorn DON 0.012-0.25 (7/7) Japan Tanaka et al.
Job's tear DON 0.048-0.5 (2/12) (1985b)
NIV 0.003-0.92 (11/12)
Potatoes DON (4/17) Canada El Banna et
al. (1984)
Rye flour DON (0.12 ave.) (3) Canada Scott et al.
bread NIV (0.058 ave.) (4) (1984)
Wheat DON (0.4 ave.) (43)
flour DON 0.11-0.69 (5/5) China Ueno et al.
(1986)
DON 0.43-4.89 (9/12) India Bhat et al.
NIV 0.03-0.1 (2/12) (1989)
DON 0.002-0.239 (26/36) Japan Tanaka et
NIV 0.004-0.084 (12/36) al. (1985b)
DON 0-0.46 (44/50) USA Trucksess et
al. (1986a)
---------------------------------------------------------------------------
Table 18 (contd.)
---------------------------------------------------------------------------
Commodity Toxin Level Incidence Country Reference
(mg/kg)a (+ve/total)
---------------------------------------------------------------------------
Breakfast DON (0.086 ave.) (36) Canada Scott et al.
cereals (1984)
Bread DON (0.08 ave.) (21)
Baby cereal DON (0.043 ave.) (30)
Crackers DON (0.27 ave.) (20)
Cookies DON (0.12 ave.) (25)
Breakfast DON 0-0.53 (35/60) USA Trucksess et
cereals al. (1986a)
Bread DON 0-0.24 (20/25)
Baby foods DON 0-0.09 (14/39)
Snack foods DON 0-0.45 (25/44)
Bread DON 0.013-0.24 (39/45) USA Wood & Carter
(1989)
---------------------------------------------------------------------------
a t = trace.
The realization that trichothecene contamination of cereal grains
was not uncommon prompted a series of studies on the fate of these
compounds during the normal processing of such grains into consumer
food products. These studies can be summarized as follows:
In the case of corn, Collins & Rosen (1981) demonstrated that,
during wet milling, roughly 66% of the T-2 toxin originally present
was removed in the steeping water, about 4% remained in the starch,
and the remainder was distributed evenly in the germ, gluten, and
fibre fractions (a result clearly predictable in view of the
considerable water solubility of T-2 toxin). The same general
observation was made about the fate of DON during the wet milling of
corn (Scott, 1984b). During the dry milling of corn, the major
portion of contaminating DON was found in the germ meal, which is
used for animal feed. For any significant contamination of grits,
the corn used in the manufacture would have to be highly contaminated
(Gilbert et al., 1983a).
In the case of wheat, several studies have clearly demonstrated
that cleaning and milling are not effective in completely removing
DON (Hart & Baselton, 1983; Scott et al., 1983, 1984a; Young et al.,
1984; Seitz et al., 1985). The same was generally true for NIV (Lee
et al., 1987). Normal cleaning of wheat reduced average DON
contamination levels by 6-19% (Abbas et al., 1985). The remaining
DON, after milling, is distributed in all fractions (bran, shorts,
reduction flour, break flour), the greatest amounts being found in
the bran and the smallest amounts in the flour, depending on the
level of contamination of the wheat itself. In a typical study,
milling of whole wheat contaminated at the 2 µg/g level, resulted in
approximately 65% of the DON in the flour and 35% in the bran, red
dogs, and shorts; i.e., there was little reduction in the DON
concentration in the flour after milling (Hart & Baselton, 1983).
It has been found that the further processing of flour into baked
or cooked products results in variable DON losses. The overall range
of reduction in DON levels from uncleaned wheat to bread was 24-71%
(Abbas et al., 1985). No reduction in DON levels was found in the
preparation of Egyptian bread (El-Banna et al., 1983) or cookies
from hard wheat flour (Scott et al., 1983), illustrating the
importance played by the conditions of processing. Kamimura et al.
(1979) showed that bread and noodles prepared under conditions
simulating commercial processes retained about 50% of the
trichothecenes that had been previously added to the wheat flour.
The trichothecene content of Japanese noodles was reduced by 30% in
the boiling process.
Similarly, these authors found that 30% of NIV and DON was
extracted from naturally contaminated wheat by water, while there was
a 50% or more reduction in trichothecene levels in bread made from
the same flour. The preparation of Chinese noodles, where sodium
carbonate is used as an ingredient, results in an even greater loss
of DON than is observed in the preparation of Japanese noodles (Scott
et al., 1984b; Nowicki et al., 1988). Similarly, Young et al. (1984)
observed a decrease of up to 35% in the production of cookies from
biscuit and cake flours; in the process studied, the batter contained
ammonium carbonate. Finally, it has been found that, during the
baking of bread, 3-13% of DON is converted into an isomer, iso-DON
(Greenhalgh et al., 1984).
There have been some studies on possible detoxification
procedures for corn and wheat contaminated with trichothecenes;
agents studied include chlorine, ammonia (Young et al., 1984) and
aqueous bisulfite (Young et al., 1986). None of these processes are
currently commercially feasible.
There has been some concern about the transmission of the
trichothecenes, particularly DON, to milk. Studies have shown that
milk is only a minor excretion route in the lactating dairy cow;
thus, the possibility that DON might contaminate milk and milk
products is remote (Prelusky et al., 1984).
II.3 METABOLISM
II.3.1 Absorption and tissue distribution
II.3.1.1 Animal studies
In general, trichothecenes absorbed from the alimentary tract are
evenly distributed in many tissues and organs, without significant
accumulation in specific organs. However, at present, distribution
studies have been limited mainly to T-2 toxin, and the metabolism in
animals of other naturally contaminating trichothecenes, such as DON,
NIV, and DAS, remains to be elucidated.
In 6-week-old broiler chicks fed with a ration containing T-2
toxin at 2 mg/kg for 5 weeks and then intubated with a single dose of
3H-T-2 toxin at 0.5 mg/kg body weight, the radioactivity reached a
maximum concentration in most tissues, 4 h after dosing; exceptions
were the muscle, skin, and bile, in which the maximum level was
reached after 12 h (Chi et al., 1978b). After 48 h, chicks contained
the equivalent of 39 µg T-2 toxin and/or its metabolites/kg in the
muscle, and 40 µg/kg in the liver, as calculated on the basis of the
specific activity of the radiolabelled T-2 toxin administered.
In chicken organs, 18 h after intraperitoneal injection of T-2
toxin (3.5 mg/kg), considerable amounts of T-2 metabolites were found
in the liver (1370 µg 3'-hydroxy-H-T-2 toxin/kg). Smaller amounts of
H-T-2 toxin, T-2 triol, and other metabolites were detected in the
lungs (Visconti & Mirocha, 1985).
In weanling crossbred pigs (7.5-9.5 kg body weight) intubated
with 0.1 or 0.4 mg 3H-T-2 toxin/kg body weight, the percentage of
administered radioactivity (18 h after dosing) was 0.7% in the
muscle, 0.29-0.43% in the liver, 0.08% in the kidney, and 0.06-0.14%
in the bile (Robison et al., 1979b). In pigs intubated with 0.1 mg of
the toxin/kg body weight, the calculated residue levels for T-2 toxin
and/or its metabolites, based on the specific radioactivity of the
tissues, were as follows: muscle, 3.1 µg/kg; fat, 0.49 µg/kg; liver,
13.8 µg/kg; and kidney, 15.9 µg/kg. The corresponding residue levels
for T-2 toxin in the tissue of another pig intubated with 0.4 mg of
the toxin/kg body weight were 11.5 µg/kg in the muscle, 37.7 µg/kg in
the liver, and 61.4 µg/kg in the kidney.
Four hours after intravenous administration of T-2 toxin in
swine, the greatest amount of radioactivity was located in the
gastrointestinal tract (15-24% of the dose), and 4.7-5.2% of the dose
was found in the remaining tissues, among which the muscle and the
liver accounted for 2.9-3.2% and 0.7-1.7% of the dose, respectively
(Corley et al., 1986).
The fate and distribution of 3H-T-2 toxin was studied in guinea-
pigs (Pace et al., 1985). Except for the large intestine and bile,
the radioactivity had peaked by 30 min and rapidly declined, with no
measurable long-term accumulation. In general, the distribution
pattern in the guinea-pig during the first 12-24 h paralleled the
distribution found in the chicken and swine.
The metabolic fate of T-2 toxin was investigated in a lactating
Jersey cow weighing 375 kg, after daily oral administration, by
capsule, of unlabelled toxin at 180 mg/day for 3 days, followed by
administration of 3H-T-2 toxin at 156.9 mg (Yoshizawa et al., 1981).
Although almost all of the administered dose was eliminated in 72 h,
appreciable levels of tritium still remained in the bile, liver, and
kidney (equivalent, repectively, to 27.2, 18.5, and 13.9 µg/kg 3H-T-2
toxin) 3 days after dosing. These levels are higher than in whole
blood (13.3 µg/kg) and plasma (10.2 µg/kg) and in other tissues,
including the spleen (9.4 µg/kg) heart (10.1 µg/kg), mammary gland
(11.3 µg/kg), ovaries (10.7 µg/kg), muscle (8.8 µg/kg), and fat (4.7
µg/kg).
Experimentally derived relationships between the residue of
radioactivity in animal tissues or plasma and the toxin levels in the
feed, calculated on the basis of the amount of 3H-T-2 toxin
administered are summarized in Table 19 (Yoshizawa et al., 1981).
Table 19. The relationship between the level of 3H-T-2 toxin in the feed or the
tritium residues in plasma and tritium levels in the edible tissues of the cow, chick,
and piga
---------------------------------------------------------------------------------------
Tissue Animal Time after Feed level Tissue level Tissue/feed Tissue/plasma
dosing (h)b (mg/kg)c (µg/kg)d ratio ratioe
---------------------------------------------------------------------------------------
Muscle Cow 72 31.38 8.8 0.0003 0.863
Chick 24 1.26 17.3 0.0137 1.000
5.0 59.2 0.0118 0.938
18.95 228.6 0.0121 0.875
Pig 18 1.25 3.1 0.002 0.775
Heart Cow 72 31.38 10.1 0.0003 0.991
Chick 24 1.26 13.7 0.011 0.792
5.0 49.4 0.010 0.783
18.95 207.7 0.011 0.795
Pig 18 1.25 3.9 0.003 0.975
Liver Cow 72 31.38 18.5 0.0006 1.863
Chick 24 1.26 34.0 0.0270 1.965
5.0 107.3 0.0215 1.700
18.95 431.0 0.0227 1.649
Pig 18 1.25 13.8 0.011 3.450
Milk Cow 72 31.38 11.4 0.0004 1.118
---------------------------------------------------------------------------------------
a From: Yoshizawa et al. (1981).
b Animals were intubated with a single dose of 3H-T-2 toxin.
c Estimates of feed levels were based on the assumption that each animal would consume
the following amount of feed daily: cow, 5 kg; chick, 100 g; pig, 600 g.
d Values were calculated from residual tritium levels in the edible tissues of animals
given 3H-T-2 toxin and were expressed as equivalents of 3H-T-2 toxin (µg/kg).
e The plasma levels were 10.2 µg/kg equivalents of T-2 toxin in the cow, and 17.3,
63.1, and 261.3 µg/kg equivalents of T-2 toxin in the chick at feed levels of 1.26,
5, and 18.95 mg/kg, respectively. The whole-blood level of residual tritium in the
pig was 4 µg/kg equivalents of T-2 toxin.
The tissue/feed ratio of tritium in the edible tissues of the
cow, 72 h after dosing, ranges from 0.0003 to 0.0006. These figures
are 5-10% of those for swine, 18 h after dosing. The tissue/feed
ratio for chickens, is higher than those in the cow and pig, ranging
from 0.010 to 0.014 for the muscle and heart, and from 0.021 to 0.027
for the liver, regardless of T-2 toxin dosage. The tissue/plasma
ratios of T-2 toxin metabolites, ranging from 0.8 to 1 in the muscle
and heart, and from 1.6 to 3.5 in the liver, are independent of
animal species, of T-2 toxin dosage, and of time after dosing. The
milk/plasma ratio of radioactivity in a cow treated with 3H-T-2 toxin
increased linearly for 24 h and thereafter ranged from 1 to 1.3.
II.3.2 Metabolic transformation
The in vivo metabolic transformation of trichothecenes in animals
is reported in Table 20. The trichothecene metabolites produced are
less toxic than the corresponding parent toxins. Both de-epoxidation
and glucuronidation, in particular, are associated with remarkable
detoxification of trichothecenes.
Table 20. In vivo (metabolic) transformation of trichothecenes in animals
--------------------------------------------------------------------------------------------------
Animal Trichothecene Transformation reaction Reference
--------------------------------------------------------------------------------------------------
Chicken T-2 toxin hydrolysis, 3'-hydroxylation, Yoshizawa et al. (1980b);
acetylation Visconti & Mirocha (1985)
Cow T-2 toxin hydrolysis, 3'hydroxylation, Yoshizawa et al. (1981, 1982a,b)
7'-hydroxylation, de-epoxidation Pawlosky & Mirocha (1984)
Chatterjee et al. (1986)
DON de-epoxidation, Yoshizawa et al. (1986)
glucuronide conjugation Côté et al. (1986)
Pig T-2 toxin hydrolysis, 3'-hydroxylation, Corley et al. (1985, 1986)
de-epoxidation, glucuronide
conjugation
DAS hydrolysis Bauer et al. (1985)
Sheep DON glucuronide conjugation Prelusky et al. (1985)
Rat DON de-epoxidation Yoshizawa et al. (1983)
T-2 metabolites de-epoxidation Yoshizawa et al. (1985a,b)
DAS hydrolysis, de-epoxidation Sakamoto et al. (1986)
Guinea-pig T-2 toxin hydrolysis, 3'-hydroxylation Pace et al. (1985)
Dog T-2 toxin hydrolysis, glucuronide
conjugation Sintov et al. (1987)
--------------------------------------------------------------------------------------------------
In in vitro studies, HT-2 toxin was found to be the sole
metabolite of T-2 toxin in the microsomes of the liver, kidney, and
spleen of various animals (Ohta et al., 1977, Johnsen et al., 1986).
The reaction of hepatic microsomes of different species in nmol/mg
protein per 10 min were: rabbit, 3044; human, 331; mouse, 75;
chicken, 55; rat, 36; and guinea-pig, 14. Yoshizawa et al. (1984,
1986) have proposed an in vitro metabolic pathway for T-2 toxin that
includes the hydrolysates at the C-4, C-8, and C-15 positions, and
the hydroxylation at the C-3'position by liver homogenates from the
rat, mouse, or monkey. This hydrolytic transformation was also
observed in the hepatic homogenates of the rabbit, pig, and cow.
However, HT-2 toxin was the sole metabolite in the homogenate of the
chicken, suggesting species differences in the metabolic pathway of
T-2 toxin (Yoshizawa & Sakamoto, 1982). In addition to the
metabolites above, glucuronide conjugates were formed in a study
using isolated perfused rat livers and T-2 toxin and DAS (Gareis et
al., 1986). Various de-epoxidation metabolites were found by
incubating intestinal and rumen microbes with T-2 toxin and its
metabolites (Yoshizawa et al., 1985a; Swanson et al., 1987), DON
(King et al., 1984; Côté et al., 1986; Swanson et al., 1987), and DAS
(Swanson et al., 1987).
II.3.3 Excretion
II.3.3.1 Animal studies
The kinetics of T-2 toxin were determined in pigs and cattle
(Beasley et al., 1986), and dogs (Sintov et al., 1986). Mean
elimination phase half-lives were 13.8 and 17.4 min, and mean
apparent specific volumes of distribution were 0.366 and 0.375
litre/kg in intra-aortally dosed pigs and intravenously dosed calves,
respectively. In dogs, the following mean parameters were determined
after intravenous administration of T-2 toxin and HT-2 toxin,
respectively: half-life 5.3 and 19.6 min, clearance 0.107 and
0.167 litre/min per kg, and volumes of distribution 0.86 and 4.47
litre/kg.
The half-life of elimination of DON in sheep, ranged from 100 to
125 min following oral administration, and it took 20-30 h to clear
from the system. Glucuronidation after intravenous or oral
administration of DON appeared to occur quite efficiently (iv, 21%;
oral, 75%), with elimination half-lives of (150-200 min and 6.1-7.1
h, respectively). These were considerably longer than those of the
parent toxin (Prelusky et al., 1985).
II.3.3.2 Excretion in eggs and milk
Radioactivity was transmitted into the eggs from laying hens that
had been intubated gastrically with a single or several doses of 3H-
T-2 toxin (Chi et al., 1978a). In birds dosed singly with 0.25 mg
T-2 toxin/kg body weight, maximum residues in the eggs occurred 24 h
after dosing; the yolk contained 0.04% of the total dose and the
white contained 0.13%. In birds dosed with 0.1 mg T-2 toxin/kg body
weight per day for 8 consecutive days, the radioactivity in the egg
accumulated until the 5th day of dosing, remained unchanged until the
last day of dosing, and rapidly decreased thereafter. Assuming that
the birds weighing 1.6 kg consumed 100 g of the diet containing 1.6
mg toxin/kg daily, the residues (T-2 toxin and/or its metabolites) in
such contaminated eggs would be about 0.9 µg/egg.
Transmission of DON was studied in the eggs and meat of chickens
(El Banna et al., 1983; Prelusky et al., 1987). Following a single
oral dose of 14C DON, maximum radioactivity, which occurred in the
first eggs laid after dosing (within 24 h), amounted to 0.087% of the
dose: levels dropped rapidly in later eggs.
In a pregnant Holstein cow (third trimester) intubated daily with
180 mg T-2 toxin for consecutive days (the toxin level corresponded
to a concentration of 50 mg/kg in the feed), milk samples taken on
the 2nd, 5th, 10th, and 12th days of intubation contained T-2 toxin
concentrations ranging from 10 to 160 µg/kg (Robison et al., 1979a).
Transmission of DON-1 (de-epoxydeoxynivalenol) to milk was confirmed
in lactating dairy cows (Côté et al., 1986; Yoshizawa et al., 1986).
Fresh and conjugated DON were also present in cow's milk following
administration of a single oral dose of 920 mg DON, but only
extremely low amounts (<4 µg/litre) were detected (Prelusky et al.,
1984).
II.4 EFFECTS ON ANIMALS
II.4.1 Field observations
In Hungary and other central European countries, pyosepticaemia
has been reported sporadically, in the past, in horses after
ingestion of mouldy hay and straw. This disease was characterized by
haemorrhages of the intestine and muscles, severe diarrhoea, and
death. Bacterium pyosepticum viscosum was detected in 1929 in the
excreta, and the equine disease was diagnosed as a pyosepticaemia
(Forgacs, 1965; Danko & Szerafin, 1976). After the discovery of
toxigenic Stachybotrys atra and its metabolites, such as satratoxins
G and H (Eppley & Bailey, 1973), the disease was presumed to be the
same as stachybotryotoxicosis (Danko & Szerafin, 1976).
A field outbreak involving the death of 20% of a dairy herd was
associated with prolonged ingestion of a diet containing 60% mouldy
corn infested with F. tricinctum. The concentration of T-2 toxin in
the feed was approximately 2 mg/kg dry weight (Hsu et al., 1972).
The lesions in the cattle included extensive haemorrhages on the
serosal surface of the internal viscera. An outbreak of haemorrhagic
syndrome in cows was associated with commercial feed containing T-2
toxin or T-2-like toxin (concentration not determined). The affected
animals showed an extremely prolonged prothrombin time. Necropsy
findings in 2 adult cows were marked serosal, mucosal, and
subcutaneous haemorrhages (Hibbs et al., 1975).
An outbreak of a disease, observed in poultry (ducks, geese),
horses, and pigs, was associated with mouldy barley containing T-2
toxin at approximately 25 mg/kg (Greenway & Puls, 1976). Pigs fed
the suspect barley exhibited signs of feed refusal, vomiting, and
diarrhoea. The horses became depressed and salivated excessively.
The lesions in the geese included necrosis of the mucosa of the
oesophagus, proventriculus, and gizzard. No pathological lesions
were described in other animals.
DON was isolated from a batch of maize that had caused vomiting
in pigs (Vesonder et al., 1973).
Equine leukoencephalomalacia reported from South Africa
(Kellerman et al., 1972; Marasas et al., 1979a; Pienaar et al., 1981)
and bean-hull toxicosis reported in horses in Hokkaido, Japan
(Konishi & Ichijo, 1970) appear to be very similar diseases with
nervous signs and hepatopathy as the major components. The signs in
these diseases were quite different from those caused by
trichothecenes, though some fungal isolates from samples of bean-
hulls produced the trichothecenes, T-2 toxin, and neosolaniol (Ueno
et al., 1972). F. moniliforme was considered to be the causative
fungus (Haliburton et al., 1979), but none of its metabolites,
including moniliformin, have been established as the cause of the
disease (Kriek et al., 1977).
Reports of field outbreaks of animal toxicoses associated with
trichothecene-contaminated feed are summarized in Table 21.
II.4.2 Effects on experimental animals
II.4.2.1 General toxic effects
LD50 values for certain trichothecenes in several experimental
animal species are summarized in Table 22 (Ueno et al., 1983). The
oral LD50 for T-2 toxin was 10.5 mg/kg body weight in mice, 3.06
mg/kg in guinea-pigs, 5.2 mg/kg in rats, and 6.1 mg/kg in trout.
The LD50 values for T-2 toxin in different species vary, but not
greatly.
The LD50 values of fusarenon-X were compared using different
routes of administration. The LD50s (mg/kg) in mice were 3.4 (iv),
3.4 (ip), 4.2 (sc), and 4.5 (oral). These data indicate that the
acute toxicity estimated for a single administration did not differ
markedly when the toxin was administered by different routes (Table
20) (Ueno et al., 1971).
Similar data were obtained with DON and acetyl-DON. An
interesting finding was that the ratio of the maximum lethal dose
to the minimum lethal dose was approximately two, indicating a
sharp dose-response curve for lethality. No marked differences in
acute toxicity were observed between treated male and female
animals.
Newborn animals are more sensitive than adults to the
toxic effects of the trichothecenes. For example, the LD50 values
for toxin given sc (mg/kg body weight) in newborn mice were: T-2
toxin, 0.15; DAS, 0.17; and fusarenon-X 0.23 (Ueno et al., 1973a).
Table 21. Field observations on animal toxicosis caused by trichothecenes
----------------------------------------------------------------------------------------------------
Mycotoxin Sample Animal Signs & lesions Reference
(concentration in feed)
----------------------------------------------------------------------------------------------------
T-2 toxin (2 mg/kg) mouldy corn cattle extensive haemorrhages Hsu et al.
(20% died) (1972)
T-2 toxin (25 mg/kg) mouldy barley poultry, necrosis of mucosa in the Greenway &
horse, proventriculus and Puls (1976)
pig oesophagous of geese
T-2 toxin commercial feed cow haemorrhages Hibbs et
al. (1975)
DAS maize pig vomiting Vesonder et
al. (1973)
T-2 toxin corn meal horse oral lesions, haemorrhages, Szathmary
(6 out of 58 died) (1983)
T-2 toxin alfalfa horse inappetence, listlessness, Szathmary
(13 out of 31 died) (1983)
T-2 toxin poultry oral lesions, inappetence, Szathmary
death (1983)
DAS and T-2 toxin corn cattle death Szathmary
(1983)
DAS oat, sifting pigeon emesis, bloody stools Szathmary
(1983)
T-2 toxin (2.5 mg/kg) chicken feed broiler inflammations, "atrophies" Szathmary
chicken (1983)
DAS (150-300 mg/kg) cattle, pig haemorrhagic syndrome Cirilli
(1983)
T-2 toxin (50-150 mg/kg) pig, cattle blood stools (swine, cattle) Cirilli
ear necrosis (swine) (1983)
intestinal lesions (poultry)
hepatic lesions (swine)
----------------------------------------------------------------------------------------------------
Table 22. LD50 values (mg/kg) of trichothecenesa
----------------------------------------------------------------------------------------------------------
Type Trichothecenes Mouse Rat Guinea-pig
iv ip sc oral iv ip sc oral ip sc oral
----------------------------------------------------------------------------------------------------------
A T-2 toxin 5.2 10.5 5.2 3.06
HT-2 toxin 9.0
Diacetoxyscirpenol (DAS) 12 23.0 1.3 0.75 7.3
Neosolaniol 14.5
Monoacetoxyscirpenol 0.725
B Nivalenol (NIV) 7.3 7.4 7.2 38.9
Fusarenon-X 3.4 3.4 4.2 4.5 4.4 0.5 0.1
Diacetylnivalenol 9.6
Deoxynivalenol (DON) 70.0 46.0
3-Acetyldeoxynivalenol 49.0 34.0
Trichothecin 300 250
D Roridin A 1.0
Verrucarin A 1.5 0.5 0.87
Verrucarin B 7.0
Verrucarin J 0.5
----------------------------------------------------------------------------------------------------------
Table 22 (contd.)
-----------------------------------------------------------------------------------------------------
Type Trichothecenes Rabbit Cat Dog Pig Duckling Day-old Chick Trout
(iv) (sc) (iv) (iv) (sc) chick (oral) (oral)
(oral)
-----------------------------------------------------------------------------------------------------
A T-2 toxin 0.5 1.21 1.75 4.0 6.1
HT-2 toxin 6.25
DAS 1.0 ca 1.1 0.37
3'-OH HT-2 toxin 8.5 5.0
15-Acetyl-T-2 tetraol 10.0
T-2 tetraol 10.0
B Fusarenon-X 5.0 ca 2.0
DON 27.0
Acetyldeoxynivalenol 37.0
D Verrucarin A 0.54
-----------------------------------------------------------------------------------------------------
a Adapted from: Ueno et al. (1983) and Ryu et al. (1988).
The toxic potency of the trichothecenes varies depending on the
modification of side chains in the molecule. The acute lethal
toxicity of certain trichothecenes was investigated using a single ip
injection in mice and the LD50 values (mg/kg body weight) were:
verrucarin A and B, 0.5; fusarenon-X, 3.4; NIV, 4.1; T-2 toxin, 5.2;
HT-2 toxin, 9.0; diacetylnivalenol, 9.6; neosolaniol
(8-hydroxydiacetoxyscirpenol), 14.5; DAS, 23.0; acetyldeoxynivalenol,
49.0; DON, 70.0; and crotocin, 810.0.
Matsuoka et al. (1979) investigated the general effects of
fusarenon-X on mice and rats. Fusarenon-X induced hypothermia, but
did not induce appreciable behavioural changes in mice. In ether-
anaesthetized rats, it caused a rise in blood pressure and a decrease
in respiratory rate, but did not induce any significant effects on
cardiac rate, the muscle cell membrane, or nerve elements.
The administration of trichothecenes to some animals (rats, mice,
and guinea-pigs) induces diarrhoea. The mechanism of this sign was
investigated using fusarenon-X and rats (Matsuoka & Kubota, 1981).
The ip injection of fusarenon-X in rats caused watery diarrhoea
within 36-60 h. At necropsy, 24 h after injection of fusarenon-X,
the small intestine was distended, but no blood was found in the
lumen of the intestine. The mycotoxin increased the absorption rate
of D-xylose from the intestine in vitro (Matsuoka & Kubota, 1981).
The leakage of intravenously injected Evan's blue dye into the
intestine also increased, but the sodium level in the serum
decreased. The intestinal villi were shortened and there was
extravasation of erythrocytes in the intestinal lamina propria. The
diarrhoea induced by ip admin-istration of 1.0 mg fusarenon/kg in
male Wistar rats was not mediated by the cyclic nucleotide system as
the mycotoxin did not increase the cyclic GMP and AMP contents in the
intestinal mucosa (Matsuoka & Kubota, 1987). The permeability of
abdominal blood vessels was increased in a dose-dependent manner in
mice given an ip injection of fusarenon-X, and the peak was reached
about 8 h after injection. The increased permeability was not
mediated by serotonin, histamine, norepinephrine, prostaglandins,
leukotrienes, or thromboxanes (Matsuoka & Kubota, 1987).
The effects of fusarenon-X and T-2 toxin on intestinal absorption
of monosaccharides were studied in rats. The absorption of 3- O-
methyl-glucose was reduced 1-3 times after either toxin was injected
into the jejunal lumen. Absorption of 3- O-methyl-glucose was also
reduced after the toxins were given by intravenous injection. Both
toxins impaired jejunal function by causing specific damage in the
active transport and diffusional movement of monosaccharides (Kumagai
& Shimizu, 1988).
Vomiting was one of the most significant signs of trichothecene-
induced toxicosis in the cat, dog, pig, and duckling (Ueno, 1980a).
T-2 toxin and related trichothecene mycotoxins at doses of 0.1-10
mg/kg induced vomiting (Vesonder et al., 1973; Sato et al., 1975;
Yoshizawa & Morooka, 1977; Ueno, 1980b; Matsuoka & Kubota, 1981).
The presence of the causal factor, DON, in mouldy corn was
established using ducklings as the assay animal (Ueno et al., 1974).
In pigs given 0.5 mg/kg body weight by infusion, vomiting commenced
6-7 min after dosing. Emesis or retching occurred at intervals of 2-
15 min and lasted from 0.5 to 2 min (Coppock et al., 1985). It is
strongly suggested that the mechanism of the vomiting of
trichothecenes is their possible action on the chemoreceptor trigger
zone (CTZ) in the medulla oblongata (Matsuoka et al., 1979). The iv
administration of 0.3 mg fusarenon-X/kg body weight to dogs induced
emesis and vomiting, 5-15 min after injection. Vomiting after
injection of fusarenon-X was prevented by prior administration of 0.5
mg metoclopramide hydrochloride/kg body weight or 1 mg
chloropromazine hydrochloride/kg.
The cardiovascular effects of the trichothecenes have varied
according to such factors as species, dose, and duration of exposure.
In acute studies, T-2 toxin given intravenously at 1 mg/kg body
weight produced a decline in blood pressure several hours after
administration, the reduced blood pressure being accompanied by a
decrease in heart rate (Smalley et al., 1970). A single dose of T-2
toxin administered to guinea-pigs and rabbits resulted in a decrease
in systemic blood pressure and a decrease in heart rate (Parker et
al., 1984; Wilson, 1984).
Using the in vitro bovine ear perfusion system, it was determined
that T-2 toxin can cause a dose-dependent vasoconstrictor response in
peripheral vasculature, but that the toxin is a less potent
vasoactive agent than either histamine or norepinephrine. The
presence of known histamine or noradrenergic anatogonist did not
affect the response to the toxin (Wilson & Gentry, 1985). T-2 toxin
administered systemically produced a marked increase in peripheral
vascular resistance in the conscious rat. The cardiac output
gradually decreased eventually resulting in cardiovascular collapse
and death (Feuerstein et al., 1985).
(a) Swine
The acute and short-term toxicities of T-2 toxin, DAS, and DON
were investigated in pigs (Weaver et al., 1978a,b; Coppock et al.,
1985). A single dose LD50 of T-2 toxin dissolved in ethanol and
administered iv was 1.21 ± 0.15 mg/kg body weight in normal, healthy,
crossbred pigs weighing from 3 to 50 kg. Soon after administration,
emesis was followed by eager consumption of feed, moderate posterior
paresis, staggering gait, extreme listlessness, and frequent
defecation of normal stools. Between 1 and 6 h, severe posterior
paresis, knuckling-over of the rear feet, and extreme lethargy were
observed. These signs were followed by severe posterior paresis,
frequent falling because of hind-quarter weakness, and the dragging
of both rear legs while moving about. Twenty-four hours after
administration, the surviving pigs appeared normal. Similar clinical
signs were observed in pigs exposed to T-2 toxin through inhalation
(Pang et al., 1988). Pathologically, necrosis was present in the
epithelial cells of the mucosa and in the crypt cells of the jejunum
and ileum, the Peyer's patches of the ileum, the lymphoid elements of
the caecum, the lymphoid follicles in the spleen, and the germinal
centre of the mesenteric lymph node (Weaver et al., 1978a).
Young pigs were fed with T-2 toxin at 1, 2, 4, or 8 mg/kg
standard pig ration for 8 weeks. No statistically significant
differences in body weight gain and feed consumption were observed
between the treated animals and the controls. Young pigs refused a
ration containing 16 mg T-2 toxin/kg, but not a diet containing 10-12
mg/kg. The no-observed-effect level was estimated to be less than 1
mg/kg, based on differences in body weight gain (Weaver et al.,
1978b). In terms of clinical haematological changes, such as
haemorrhaging, blood cell counts, serum-enzyme activities, and serum-
protein levels, the no-observed-effect level could not be accurately
determined, but was higher than 12 mg/kg, based on the weight gain.
The intravenous administration of T-2 toxin to pigs at doses of 4
or 8 mg/kg resulted in a shock syndrome characterized by reductions
in cardiac output and blood pressure and increased plasma
concentrations of epinephrine, norepinephrine, thromboxane B2, 6-
keto-PGF1a and lactate (Lorenzana et al., 1985). The pigs in the
high-dose group produced such signs as persistent vomiting, watery
diarrhoea, abdominal straining, cold extremities, coma, and death.
Eighteen white cross-bred female pigs weighing 40-60 kg,
immunized against erysepelas, were administered purified T-2 toxin
dissolved in 70% ethanol, intravenously, in doses of 0 (5 pigs), 0.6
(5 pigs), 1.2 (1 pig), 4.8 (5 pigs) and 5.4 (2 pigs) mg/kg. The
animals administered doses of 4.8 and 5.4 mg/kg died between 5 and
10.5 h later and other groups were killed 12-24 h after treatment.
Gross lesions were observed in pigs given 1.2 mg/kg or more and these
consisted of oedema, congestion, and haemorrhages of the lymph nodes
and pancreas and congestion and haemorrhages of the gastrointestinal
mucosa, subendocardium, adrenal glands, and meninges. Histological
alterations confirmed the gross lesions. Other lesions were
widespread degeneration and necrosis of lymphoid tissue and the
surface and crypt epithelium of the intestines. Scattered foci of
necrosis were present in the pancreas, myocardium, bone marrow,
adrenal cortex, and the tubular epithelium of the renal medulla.
Most lesions were dose dependent. The T-2 toxin-induced lesions in
the lymphoid and gastrointestinal tract of pigs were similar to those
described in other species. The heart and pancreas were additional
target organs in pigs (Pang et al., 1987b).
Male, castrated, crossbred, specific pathogen-free pigs (17
controls and 17 treated), 9-11 weeks of age, were used in a study
to characterize the pulmonary and systemic responses to inhaled T-2
toxin (nebulized dose of 9 mg/kg given by endotracheal tube) (Pang et
al., 1987a). The animals were exposed to the aerosol in pairs, one
animal receiving the toxin, the other acting as a control. From 20
to 30% of the toxin was retained by the pigs (T-2 toxin was mixed
with 100-200 µCi technetium for measurement). Five pairs of animals
each were killed 1, 3, and 7 days after dosing. Two pairs were
designated a 0.33 day group, when one treated pig died and the
other was killed in a moribund state, 0-10 h after dosing.
Clinically, the T-2 toxin-treated pigs vomited after exposure
producing such signs as cyanosis, anorexia, and lethargy. The pigs
became laterally recumbent. Alveolar macrophages showed reduced
phagocytosis and the blastogenic responses to mitogen were reduced
for pulmonary lymphocytes, but not for lymphocytes of the peripheral
blood. The lesions in pigs that died included multifocal
interstitial pneumonia, necrosis of lymphoid tissue,
necrohaemorrhagic gastroenteritis, oedema of gall bladder mucosa, and
multifocal areas of necrosis in the heart and pancreas. Inhalation
exposure to T-2 toxin produced a clinical and morphological syndrome
resembling that produced by intravenously administered T-2 toxin, at
doses of 1.2 mg/kg (approximate LD50) or more, as well as death.
Furthermore, the lesions produced by the inhaled toxin were more
severe.
The acute effects of DAS were studied using single, intravenous
doses (range: 0.30-0.48 mg/kg body weight) in 13 crossbred pigs;
there were 2 control pigs (Weaver et al., 1978b). Seven of the
toxin-exposed pigs developed emesis, frequent defecation, lethargy,
staggering gait, and prostration by 10 h leading to death. Severe
haemorrhagic necrotizing lesions and mucosal congestion involved the
jejunum and ileum and large intestines, portions of which were blood-
filled at necropsy. Lymphoid follicular necrosis was present in the
lymph nodes and spleen. The LD50 value was found to be 0.376 ± 0.043
mg/kg body weight.
Two female crossbred pigs were administered DON by rapid
intravenous infusion at a dose of 0.5 mg/kg body weight; there were
two matching controls. Vomiting was observed within a few minutes
of dosing, the skin was flushed and the extremities became cold.
Pigs had signs of diarrhoea, muscular weakness, tremors, and coma.
Symptoms were progressive in severity reaching a maximum 6-7 h after
the injection; recovery occurred after 12 h. Necrosis of pancreatic
acinar and islet cells was observed (Coppock et al., 1985). Pigs can
ingest up to 2 mg DON/kg feed without suffering any serious toxic
effects (Trenholm et al., 1984). T-2 toxin added to the ration at 18
or 30 mg/kg caused the refusal of feed in pigs (Szathmary & Rafai,
1978). Feed refusal and emesis have been produced in other species
and by other toxic metabolites of Fusarium species (Kotsonis et al.,
1975; Vesonder et al., 1977).
The minimum emetic dose of DON in pigs weighing 9-10 kg was 0.05
mg/kg body weight, when administered intraperitoneally, and 0.1-0.2
mg/kg body weight, when given orally. When this toxin was added to
feed, the feed consumption of 20-45 kg pigs, was reduced by 20% at a
dose of 3.6 mg/kg and by a 90% at a dose of 40 mg/kg (Forsyth et al.,
1977). Pigs were about twice as sensitive to DON as rats (Vesonder
et al., 1979). DON in contaminated wheat reduced feed intake and
weight gain, when it was fed to the pigs. The intake of feed
decreased linearly with increasing dietary concentration of DON
(Friend et al., 1982). The emetic activity of 15-acetyl DON in pigs
was similar to that of DON, the minimum emetic doses being 75 and 50
µg/kg, respectively (Pestka et al., 1987a).
(b) Poultry
Chi et al. (1977b), reported that the single oral LD50 dose of
T-2 toxin for one-day-old broiler chicks was 5 mg/kg body weight. It
was 5 and 6.3 mg/kg body weight for 8-week-old broiler chicks and
laying hens, respectively. Death of the birds occurred within 48 h
of T-2 toxin administration. Within 4 h of receiving the toxin,
birds developed asthenia, inappetence, diarrhoea, and panting. The
abdominal cavities of birds given lethal doses contained a white
chalk-like material that covered much of the viscera.
In a study by Wyatt et al.(1972), chickens were fed a diet
containing 1-16 mg T-2 toxin/kg feed for 3 weeks. The birds with
reduced growth at 4, 8, and 16 mg/kg developed yellow-white lesions
in the mouthparts at all dietary concentrations. The lesions
consisted of a fibrinous surface layer and a heavy infiltration of
the underlying tissues by granular leukocytes. Escherichia coli and
Staphylococcus epidermis were isolated from the lesions.
Terao et al.(1978) observed the effects of T-2 toxin and related
trichothecenes on the bursa of Fabricius of one-day-old chicks.
After injection of 5 mg T-2 toxin/kg body weight into the residual
yolk sac, cellular toxic effects were observed on the follicle-
associated epithelium, resulting in necrosis, which spread to the
periphery. The lesions induced by fusarenon-X and NIV were similar
to those induced by T-2 toxin, but the toxins were less potent and
their activity was estimated to be more than 40 times less than that
of cyclophosphamide.
The acute toxicity of DAS and of T-2 toxin dissolved in
dimethylsulfoxide in 7-day-old male broiler chicks was described by
Hoerr et al. (1981). The 72-h single oral LD50 doses of T-2 toxin
and DAS were estimated to be 4 and 5 mg/kg body weight, respectively.
Combination of the 2 toxins caused increased mortality in both the
single-and multiple-dose tests. Lesions produced by crop gavage with
T-2 toxin and DAS were similar, but were more severe in chicks given
T-2 toxin. Necrosis of lymphoid tissue and bone marrow was observed
in tissue taken 1 h after treatment followed by rapid depletion.
Necrosis was observed in the liver, gall bladder, and gut.
Chi et al.(1977c), fed broiler chicks (36 per group), aged one
day to 9 weeks, a diet containing T-2 toxin at concentrations of 0.2,
0.4, 2, or 4 mg/kg. Birds fed 4 mg T-2 toxin/kg showed reduced body
weight gain and feed consumption and developed oral lesions
characterized by circumscribed proliferating yellow caseous
plaques at the margin of the beak, the mucosa of the hard
palate and the tongue, and the angle of the mouth. No lesions were
observed in the bone marrow or, to any significant extent, in the
peripheral blood. The no-observed-effect doses of T-2 toxin were 0.2
mg/kg for weight gain, and 0.2 mg/kg for oral lesions. When one-day-
old broiler chicks were fed a diet containing 1, 2, 4, 8, or 16 mg T-
2 toxin/kg feed for 3 weeks the no-effect doses were estimated as
follows: growth rate, weight of pancreas, and weight of spleen: 2
mg/kg; oral lesions: <1 mg/kg (Wyatt et al., 1973c).
T-2 toxin administered to laying hens at a concentration of 20
mg/kg feed reduced egg production and resulted in the production of a
thinner egg shell (Wyatt et al., 1975). Speers et al.(1977), also
observed cessation of egg production in hens fed diets containing
25-50 mg monoacetoxyscirpenol/kg or 16 mg T-2 toxin/kg. It was
reported by Chi et al. (1977a) that feed consumption, egg production,
and shell thickness were significantly decreased in hens fed 8 mg of
T-2 toxin/kg. Furthermore, the hatchability of fertile eggs of hens
fed 2 or 8 mg T-2 toxin/kg was lower than that of hens fed the
control diet (Chi et al., 1977a).
Three groups of 1-day-old chicks (10 chicks per group) were each
fed 0.5-15 mg T-2 toxin/kg for 3 weeks (Coffin & Combs, 1981).
Plasma-vitamin E activity and hepatic-vitamin A content were
measured. Dose-dependent depression of plasma-vitamin E activity was
observed, with a 65% decrease compared with controls in chicks fed a
diet containing 15 mg T-2 toxin/kg. This decrease was believed to be
caused by a reduction in the plasma level of lipoproteins, which are
required for the transport of vitamin E.
(c) Ruminants
In a study by Pier et al. (1976), 4 calves received 0.08-0.6 mg
T-2 toxin/kg body weight orally in capsules for 30 days. The high-
dose calf developed a hunched stance and died on day 20. At all
levels, some evidence of mild enteritis with loose faeces was
obtained. Clinically apparent signs were confirmed at doses of 0.16
mg/kg or more, and bloody faeces at doses of 0.32 mg/kg or more. At
necropsy, abomasal ulcers were present in the calf given 0.16 mg/kg
and ruminal ulcers in calves given the 2 higher doses. Prothrombin
times and levels of serum GOT activity were increased in calves given
the 2 higher doses.
Ten male Suffolk-Finn-Columbian lambs, in 2 groups of 5 animals
each, were fed T-2 toxin at 0.3 or 0.6 mg/kg body weight for 21 days.
There were 5 controls. Experimental lambs developed focal hyperaemia
and dermatitis at the mucocutaneous junction of the commissure of the
lips, diarrhoea, leukopenia, lymphopenia and lymphoid depletion of
the mesenteric lymph nodes and spleen (Friend et al., 1983b).
Sheep dosed with roridin A and verrucarin A (4 mg/kg) had severe
and extensive haemorrhagic gastroenteritis. Oedema was marked in the
abomasum; the small intestine of the roridin-treated lamb had casts
of clotted blood and necrotic debris. Both small and large
intestines contained grossly haemorrhagic areas and extensive mucosal
erosions. In the lamb given verrucarin A at 4 mg/kg body weight, the
lesions were sublobular haemorrhages in the liver, which had a nutmeg
appearance, mucosal erosions, haemorrhages in the small intestine,
and haemorrhages of the endocardium of the left ventricle of the
heart (Mortimer et al., 1971).
(d) Cats
Three studies have described the clinical and tissue alterations
produced by administration of T-2 toxin to cats (Sato et al., 1975;
Lutsky et al., 1978; Lutsky & Mor, 1981). Lutsky et al. (1978) used
20 cats in 4 groups of 4-6 animals each. There were 4 controls. The
toxin was administered orally in gelatin capsules on alternative days
at doses of 0.06, 0.08, or 0.10 mg/kg body weight, until death. The
survival time ranged from 6 to 40 days. The signs included emesis,
anorexia, bloody diarrhoea, and ataxia. The cats lost weight and
became emaciated. Gross lesions included multiple petechiae to
ecchymotic haemorrhages of the intestinal tract, lymph nodes, and
heart. The lumen of the gut contained copious amounts of dark red
contents. Microscopic lesions included haemorrhages in the gut,
lymph nodes, heart, and mininges, necrosis of gastrointestinal
epithelium and decreased cellularity of the bone marrow, lymph nodes,
and spleen.
(e) Rodents
The trichothecenes used in long-term studies were T-2 toxin and
fusarenon-X. Lesions were observed in the oesophageal region of the
stomach of DDD mice fed T-2 toxin at 10 or 15 mg/kg diet for 12
months. The alterations included hyperplasia, hyperkeratosis, and
acanthosis of the squamous epithelium. Such changes were found 13
weeks after the start of feeding the toxins and were consistently
observed during the 12-month feeding period. However, most had
subsided 3 months after cessation of feeding. Similar gastric
lesions were observed in Wistar rats fed T-2 toxin at concentrations
of 5, 10, or 15 mg/kg feed for 4 weeks. The lesions were diffuse and
severe in the rats fed 15 mg/kg, focal but definite in those fed 10
mg/kg, and negligible in the stomach of rats fed 5 mg/kg (Ohtsubo &
Saito, 1977).
Six female Holtzman albino rats were fed T-2 toxin at 5 or 15
mg/kg for 19 days and T-2 toxin at 10 mg/kg diet for 8 months. No
gastric lesions were observed in any of the animals in the
experimental groups (Marasas et al., 1969).
Three groups of 12, six-week-old female Swiss ICR mice (15-20 g
body weight) were administered T-2 toxin (by 10-minute aerosol
exposure) Two control groups contained 8 mice each using nose-only
exposure. The aerosol mass concentration varied between 225 and 275
µg T-2 toxin/litre of air. Tissues from mice were microscopically
examined 0.25, 1, 2, 4, 6, 8, 12, and 24 h after exposure. Lymphoid
necrosis was observed 1 h after exposure in the thymus, spleen, and
lyphoid nodules of the intestinal tract. Necrosis of intestinal
crypt epithelial cells was present 2 h after exposure and necrosis of
adrenal cortical cells 4 h after exposure (Thurman et al., 1988).
In male Sprague-Dawley rats, T-2 toxin, given intravenously,
produced reduced blood flow and increased vascular resistance in
hind-quarter, mesenteric, and renal vascular beds. Mean arterial
pressure and heart rate were not significantly altered. A maximum
drop in blood flow in mesenteric and renal vascular beds occurred 4 h
after the T-2 toxin was injected (Siren and Feuerstein, 1986).
II.4.2.2 Haematological and haemostatic changes
A haemorrhagic syndrome was reported to be the characteristic
feature of mouldy corn toxicosis in the cow (section II.4.1.).
However, this hemorrhagic syndrome could not be produced in other
studies.
In a study by Patterson et al. (1979), 2 calves were administered
0.2 mg T-2 toxin/kg body weight and one calf was given the same dose
of DAS; both compounds were given by stomach tube,daily for 11 days.
There were no controls. The T-2-treated animals developed clinical
signs of weakness, inappetence, and one died. Prothrombin time was
prolonged in both animals and one had marked neutrophilia. No
clinical signs or haematological changes were observed in the animal
administered DAS. No haemorrhagic syndrome was found in these
calves.
When pigs (9-10 weeks old, male, castrated, specific pathogen-
free) were exposed to a T-2 toxin aerosol (390 µg/litre, 15 µm mass
median aerodynamic diameter) for a period that allowed an amount
equivalent to 8 mg/kg to be nebulized, the haematological alterations
included a decrease in lymphocyte and neutrophil counts, and
decreased concentrations of serum-protein and haemoglobin (Pang et
al., 1988).
Haematological changes were observed in mice, rats, cats, and
guinea-pigs treated with T-2 toxin and related trichothecenes (Sato
et al., 1975, 1978; Sato & Ueno, 1977; DeNicola et al., 1978). In
cats, leukocytosis occurred early after the administration of T-2
toxin. A similar change was observed in mice treated with T-2 toxin,
neosolaniol, and fusarenon-X. Among the leukocytes, lymphocytes
showed the greatest increase followed by neutrophils; the
leukocytosis was followed by marked leukopenia after short-term
exposure to T-2 toxin. This leucopenic state was also induced by DAS
in mice (Conner et al., 1986), rats and dogs (Stahelin et al., 1968)
and by verrucarin A in rats, dogs, guinea-pigs, and monkeys (Rusch &
Stahelin, 1965). Pancytopenia was also reported in cats administered
T-2 toxin for 2 weeks (Lutsky et al., 1978; Lutsky & Mor, 1981). In
guinea-pigs treated with T-2 toxin (0.9 mg/kg body weight per day)
for 27 days, erythropenia, leukopenia, and absolute lymphopenia were
observed, with a marked decrease in the lymphocyte contents of the
bone marrow (DeNicola et al., 1978).
Hayes et al. (1980) studied the effects of T-2 toxin on the
haematopoiesis in mice. Twenty-four male weanling outbred Swiss mice
were fed a balanced semipurified diet, containing crystalline
purified T-2 toxin at a level of 20 mg/kg dry diet. One group of 20
animals received the toxin in the diet for 41 days and another group
of 4 animals, for 21 days, followed by control diet for 7 days.
Forty-eight animals in 3 groups served as controls and received the
semipurified diet with restricted intake (20 animals for 41 days),
and ad lib (8 animals for 28 days). In addition, 12 animals were
sacrificed at day 0. Haematological studies were made at weekly
intervals. During the first 3 weeks of exposure to T-2 toxin,
lymphoid tissues, bone marrow, and splenic red pulp became
hypoplastic resulting in anaemia, lymphopenia, and eosinopenia.
Subsequently, during continued exposure to T-2 toxin, there was
regeneration leading to hyperplasia of the haematopoietic cells by 6
weeks. All animals also developed perioral dermatitis and ulceration
of the gastric mucosa. The above results indicate both the irritant
and haematopoietic suppressive effects of the T-2 toxin. However,
the haematopoietic effects were transient at the dose administered
and did not lead to haematopoietic failure.
The haemostatic derangements produced by T-2 toxin have been
studied in the guinea-pig (Cosgriff et al., 1984), rabbit (Gentry,
1982), chicken (Doerr et al., 1981), and monkey (Cosgriff et al.,
1986). Guinea-pigs (Hartley strain, number not stated) admin-istered
T-2 toxin dissolved in ethanol, by intramuscular injection, at a dose
of 1 mg/kg body weight (LD50-24 h) developed decreased activities of
all coagulation factors except fibrinogen. Platelet aggregation in
whole blood response to ADP and collagen was depressed. The animals
also showed an initial rise followed by a fall in the haematocrit
level, leukocytosis, and a fall in platelet count. These changes,
which were found within a few hours of toxin administration, reached
a maximal at 24 h and returned to normal over the next 2 days.
Pretreatment with vitamin K1 did not prevent the effects of T-2 toxin
on coagulation. The addition of T-2 toxin to the plasma and blood of
untreated guinea-pigs at a concentration of 1 mg/litre did not have
any effect on clotting times or platelet aggregation, indicating that
the T-2 toxin itself did not have any direct effect on the activ-ity
of coagulation factors (Cosgriff et al., 1984).
Eight New Zealand White rabbits were administered T-2 toxin
dissolved in dimethyl sulfoxide (DMSO) by intravenous injection at
0.5 mg/kg body weight; 5 rabbits were given a single oral dose of 2.0
mg/kg body weight. In the rabbits treated intravenously, both the
packed cell volume and the total leukocyte counts were reduced.
However, no significant alterations occurred in the haematological
parameters of rabbits given the T-2 toxin orally (Gentry & Cooper,
1981).
In another study, 9 New Zealand White rabbits were given a single
intravenous injection of T-2 toxin dissolved in DMSO at a dose of 0.5
mg/kg body weight. A second group of 5 animals received daily
subcutaneous injections of vitamin K, at a dose of 0.5 mg/kg body
weight, for 5 days prior to administration of a similar dose of T-2
toxin and for a subsequent 4 days. A total of 16 rabbits in 2 groups
served as controls. Blood samples were examined from each animal
before toxin or DMSO treatment and 6-96 h later. Several coagulation
factors (VII, VIII, IX, X, XI) were decreased by about 40% within 6 h
of toxin administration in the group administered toxin alone.
Fibrinogen content was elevated at 24 h. However, the reduction
in the coagulation factors did not induce clinical haemorrhage and
administration of vitamin K did not alter the effects of T-2 toxin
administration, indicating that the mechanism of action of the toxin
on coagulation was not as a vitamin K antagonist.
In a study by Cosgriff et al. (1986), 9 Cynomolgus monkeys
received an intramuscular injection of an LD20 dose (0.65 mg/kg body
weight) of T-2 toxin dissolved in ethanol. Three monkeys served as
controls. Haematological studies were made before toxin injection
and at different intervals from 6 to 24 h and 2 to 7 days after
treatment. The animals were studied for signs of toxicity and
particularly for evidence of haemorrhage. Necropsy was performed on
animals that died during study. Leukocytosis levels in treated
animals were 4-5 times pretreatment levels. Prolongation of
prothrombin, activated thromboplastin times, and a decrease in
multiple coagulation factors were also observed. These changes were
detected within hours of toxin administration, reached a maximum at
24 h, and returned to normal over the next 3 days. Fibrin-fibrinogen
degradation products were not detected at any time. Platelet counts
which were unchanged in treated animals, were significantly raised in
control animals following repeated phlebotomies. None of the animals
developed the haemorrhagic syndrome. Five animals that died during
the study showed mild peticheal haemorrhages involving the colon and
heart, as well as necrosis of lymphoid tissues.
Rukmini et al. (1980) conducted a study on adult rhesus monkeys
in which 3 males and 2 females were administered pure T-2 toxin in
20 ml milk by stomach tube, daily, initially at 1 mg/kg body weight
for 4 days, and then at 0.5 mg/kg body weight from day 5 to day 15.
Three males and 3 females served as controls. All 3 males in the
treated group died of respiratory failure between days 0 and 15.
Subsequently, after 30 days recovery, the 2 treated female and 2
additional male monkeys received 0.1 mg T-2 toxin/kg body weight for
15 days. All monkeys given 1 mg T-2 toxin/kg per day showed signs of
toxicity similar to those of alimentary toxic aleukia in man, i.e.,
vomiting, apathy, and weakness of lower limbs. The signs were more
severe in males, and they also developed peticheal haemorrhage on the
face. All male animals developed severe leukocytopenia, follicular
atrophy of the spleen and lymph nodes, and pneumonia, suggesting
involvement of the immune system. At a dose of 0.1 mg/kg per day,
both male and female animals developed leukocytopenia and mild
anaemia after 15 days of treatment.
The effects of T-2 toxicosis on blood coagulation were studied in
groups of 40, day-old chickens fed diets containing the toxin at
concentrations of 1, 2, 4, 8, or 16 mg/kg. Forty animals served as
controls. Factor X, and prothrombin and fibrinogen activities were
reduced only at the highest dietary dose, whereas Factor VII was
reduced at dietary doses of 4, 8, and 16 mg/kg and was the most
sensitive of the clotting components to T-2 toxin toxicosis. Thus,
T-2 toxin toxicosis induced by high doses results in multiple-factor
coagulopathy and mild toxicosis results in a deficiency of Factor VII
(Doerr et al., 1981).
II.4.2.3 Disturbances of the central nervous system
Four-week-old male broiler chickens were intubated with a single
dose of T-2 toxin at 2.5 mg/kg body weight, and the brain
concentrations of dopamine, norepinephrine, and serotonin, and
selected blood components were determined 4-48 h after
administration. There was a significant elevation in the brain-
dopamine concentration and a reduction in the brain-norepinephrine
concentration. The brain-serotonin contents did not change (Chi et
al., 1981). Batches of 40 broiler chickens fed graded concentrations
of 1-16 mg T-2 toxin/kg feed for 3 weeks developed an abnormal
positioning of the wings, hysteroid seizures, and impaired
righting reflex. Neural toxicity, which occurred at levels above 4
mg T-2 toxin/kg diet, might have been related to alterations in brain
biogenic amines (Wyatt et al., 1973a,b; Chi et al., 1977a).
Signs of nervous system dysfunction (restlessness, dyspnoea,
ataxia) were observed in rats after subcutaneous or intracerebral
injection of T-2 toxin (10-20 µg toxin) or after intracerebral
implantation of toxin adsorbed on talc (Bergmann et al., 1985).
Weanling, male Wistar rats were administered T-2 toxin orally at 2.0
mg/kg body weight and the concentrations of neurotransmitters
determined. The toxin increased concentrations of tryptophan,
serotonin, and dopamine in the brain, but decreased concentrations of
3,4-dihydroxyphenylacetic acid (MacDonald et al., 1988). Male
Sprague-Dawley rats (180 g) were dosed orally with DON or T-2 toxin
at 21.5 mg/kg body weight. Both the toxins significantly increased
serotonin and 5-hydroxy-3-indoleacetic acid concentrations in all
regions of the brain examined, whereas norepinephrine and dopamine
concentrations were not altered (Fitzpatrick et al., 1988). Male
Sprague-Dawley rats received 1 mg T-2 toxin/kg body weight by
intravenous injection. Concentrations of vasopressin, oxytocin, and
leucine enkephalin decreased in the posterior pituitary and
concentrations of methionine enkephalin increased (Zamir et al.,
1985).
Male Sprague-Dawley rats (180 g) and 4-week-old White Leghorn
cockerels were dosed orally with DON at 2.5 mg/kg body weight. Whole
brain concentrations of monoamine neurotransmitters were not altered
in either species. The treatment produced elevated concentrations of
serotonin and 5-hydroxy-3-indoleacetic acid in the rat, but not in
the chicken (Fitzpatrick et al., 1988).
II.4.2.4 Dermal toxicity
After the discovery of the skin-necrotizing property of toxic
metabolites of F. sporotrichioides and related fungi (Joffe, 1962),
the skin irritation test was introduced for the screening of toxins
and metabolites of Fusarium species (see section II.1.2.3). T-2
toxin, HT-2 toxin, and DAS were isolated from cultures of F.
tricinctum using the skin test for selection of active fractions
(Gilgan et al., 1966; Bamburg et al., 1968b). Toxins, such as T-2
toxin, HT-2 toxin, and DAS are extremely potent irritants while NIV
and fusarenon-X are much less so (Bamburg et al., 1968b; Ueno et al.,
1970; Wei et al., 1972; Chung et al., 1974; Hayes & Schiefer, 1979;
Bhavanishankar et al., 1988).
The mechanism of the skin toxicity of trichothecenes has not been
established. Results of studies with fusarenon-X have indicated that
the vascular permeability of the skin of the back of the rabbit,
estimated by exudation of a vital dye (pontamine sky blue), was
biphasic reaching maxima 5 and 24 h after topical application. These
data indicate that increased vascular permeability is one of the
early responses of the skin to these toxins and that some chemical
mediators participate in the biphasic increase in vascular
permeability (Ueno, 1980a).
II.4.2.5 Impairment of immune response
Experimental animal studies show that some trichothecenes affect
the immune system and thereby modify the immune response. The
impairment comprises the following functions: antibody formation;
allograft rejection; delayed hypersensitivity; and blastogenic
response to lectins. As a consequence of the impairment, decreased
resistance to microbial infection has been experimentally
established. It is likely that the impairment of the immune system
is linked to the inhibitory effect of trichothecenes on macromolecule
synthesis.
(a) Antibody formation
Rosenstein et al. (1979) showed that T-2 toxin and DAS inhibited
responsiveness to sheep red blood cells in male Swiss IC and C5781/6
mice. In their first study, T-2 toxin or DAS was injected ip daily
for 7 days at 0.75 mg/kg body weight, in 6 groups of 4 mice each,
with matching controls. Mice were immunized with sheep erythrocytes
(SRBC) on day 3 after treatment and killed 5 days after immunization.
Both toxins produced a fall in anti-SRBC titres measured by
haemagglutination and reduced thymic weight. In a second study, 7
groups of 5 mice each were administered daily (ip) doses of T-2 toxin
ranging from 0 (solvent alone) to 2.5 mg/kg body weight over a 7-day
period. The same number of mice received DAS under similar
conditions. Mice were immunized on day 3 and killed 5 days later.
Antibody-producing cells from the spleen were counted by numbering
the plaque-forming cells (PFC) on sheep erythrocytes. A dose-
dependent inhibition of PFC was observed in T-2 toxin-treated mice,
with a total suppression of the immune response at 2.5 mg/kg. The
effects of DAS were less. A subsequent follow-up of the evolution of
the immune response in 36 mice administered T-2 toxin (ip) at 0.75
mg/kg body weight daily for 7 days indicated that the immunosuppressive
effect disappeared within 6 days followup cessation treatment.
The T-cell-independent-responses-production of anti-
polyvinylpyrrolidone and anti-dinitrophenol-ficoll-antibodies were
enhanced by T-2 toxin and DAS. T-2 toxin-treated mice produced 50%
fewer plaque-forming cells against SRBC. There was also a decreased
response to phytohaemagglutinin in splenic cells from treated mice.
However, Masuko et al. (1977), and Otokawa et al. (1979), reported
that a single dose of 3 mg T-2 toxin/kg body weight in mice caused
modification of delayed hypersensitivity responses without affecting
antibody response. This apparent contradiction was explained by a
difference in the timing of the administration of T-2 toxin, mice
receiving the toxin once, several days before or after antigen-
stimulation. Results of studies with fusarenon-X indicated that both
IgE and IgG anti-body responses to DNA-OVA were suppressed when male
BALB/c mice were repeatedly dosed with mycotoxin at doses exceeding
25 mg/day; the inhibition of antibody-formation was greater when
given 7 days before antigen-stimulation. In mice stimulated with
pokeweed mitogen and lipopolysaccharides, the in vitro antibody
production by splenic cells from fusarenon-X-treated mice was
suppressed (Masuda et al., 1982).
Sato et al. (1981), examined the effects of fusarenon-X on
serological responses in chicks inoculated with Newcastle disease
vaccines. When chicks were fed 8 mg fusarenon-X/kg for 6 weeks, no
significant reductions in body or organ weights were observed.
However, haemagglutinin inhibitory antibody titres were reduced when
chicks were immunized with live, but not with inactivated, vaccine.
Mann et al. (1983), reported alterations in the levels
of several serum proteins in calves orally administered T-2 toxin
(0.6 mg/kg per day over 43 days). Total protein, albumin, and
immunoglobulin fractions were decreased in toxin-treated calves,
including the alpha-beta1-and beta2-globulin fractions. IgA and IgM
values and complement proteins were lower in treated calves.
Sublethal doses of DON (0.25, 0.50, or 1.0 mg/kg feed) were fed
for 54 weeks, beginning at 21 days of age, to a total of 96 weanling
male Swiss Webster mice divided into 4 groups. There were 32
controls. The dose of 1.0 mg/kg reduced serum alpha1- and alpha2-
globulins, increased serum-albumin levels, and reduced feed
consumption and body weight gain. The dose of 0.5 mg/kg reduced
alpha2- and beta-globulins (Tryphonas et al., 1986).
T-2 toxin was fed to 6 weaned pigs at 5 mg/kg feed for 25 days
and the immune response evaluated by in vitro testing for blast
transformation, immune-rosette formation, and IF-detectable IgG-
positive cell counts. T-2 toxin produced a 40-50% reduction in
immune responsiveness and a decrease in total leukocyte count, but an
increase in adrenocortical activity. Neutralizing antibody titres to
vaccination with enteritic B vaccine were lower in the treated pigs.
It was concluded that T-2 toxin had a distinct immunosuppressive
effect during the early phase of immune induction by altering the
function of both T- and B-lymphocytes (Rafai & Tuboly, 1982).
Dietary DON (2, 5, or 25 mg/kg feed for 2 or 8 weeks) depressed
the plaque-forming response to sheep erythrocytes in splenic cultures
from B6C3F1 mice. Some effect on the plaque-forming response was
detectable with both the 2- and the 8-week period of feeding (Pestka
et al., 1987b). DON, given by gavage at 0.75, 2.5, or 7.5 mg/kg body
weight also reduced serum-IgM response to sheep erythrocytes and
plaque-forming cell numbers were lower in the treated groups
(Tryphonas et al., 1984).
(b) Allograft rejection
Observations on the inhibition of cellular immunity by
trichothecenes have included responses to grafting. According to
Rosenstein et al. (1979), the mean survival time of the skin grafted
from C57Bl/6 mice on to Swiss mice was 8.69 days in the control
recipients. However, when the recipients were treated with 0.75 mg
T-2 toxin/kg per day for 7 days before skin graft and then 3 times a
week for 20 days, the mean survival time of the graft was increased
to 12 days, indicating that T-2 toxin suppressed certain steps of
immunity resulting in allograft rejection. The areas of the graft in
T-2 toxin-treated mice lacked the typical cellular infiltrates of a
cell-mediated immune response of macrophages and lymphocytes.
(c) Delayed hypersensitivity
Delayed hypersensitivity (DH) is an immune response mediated by
sensitized T lymphocytes. The possible impairment of T lymphocytes
by T-2 toxin was studied in female BDF1 mice sensitized by the sc
injection of sheep erythrocytes (SRBC) followed by estimation of foot
pad swelling. When mice received 3 mg T-2 toxin/kg body weight,
before, or on the day of, sensitization, no appreciable effect on DH
was observed. However, when the toxin was administered 2 or 3 days
after sensitization, marked enhancement of the delayed
hypersensitivity response was seen (Otokawa et al., 1979). This
indicates that the timing of toxin exposure was critical for
enhancement of the delayed hypersensitivity response. Since the
life-time of the effective T-2 toxin dose in vivo was very short,
and the optimal timing of toxin injection corresponded with the time
of appearance of suppresser cells, it was presumed that trichothecenes
might interfere with the proliferation of suppresser T cells that
appear in DH-tolerant mice.
DON fed to B6C3F1 mice at 2, 5, or 25 mg/kg for 2 or 8 weeks
depressed the delayed hypersensitivity response to keyhole limpet
haemocyanin. The effects on hypersensitivity were detectable in mice
fed the mycotoxin for 2 weeks, but disappeared when the feeding
period was extended to 8 weeks (Pestka et al., 1987b).
(d) Blastogenic response to lectins
Certain mitogens, such as phytohaemagglutinin (PHA) and
concanavalin A, stimulate the proliferation of T cells in vitro;
lipopolysaccharide (LPS) causes the same phenomena in B cells.
Lafarge-Frayssinet et al. (1979) investigated the responses of
lymphocytes to PHA and LPS in mice treated with crude T-2 toxin and
DAS. Mice were treated with the mycotoxins at doses of one-quarter
of the LD50 or one-twelfth of the LD50 for 15 days and the response
of splenic or thymic cells to the mitogens was examined. The data
indicated that stimulation of both T and B cells was inhibited
reversibly, and that the ability to synthesize anti-SRBC antibodies
was suppressed. In vitro effects on lymphocytes and fibrosarcoma
cell cultures included a direct cytostatic action at high
concentrations and a stimulating action at low concentrations.
Histopathological observations included severe lymphoid damage in the
thymus and spleen. The results of these studies indicate that the
immune system appears sensitive to the trichothecenes and is impaired
at doses not inhibitory for other organs.
Swiss mice were fed a diet containing T-2 toxin at 5, 10, or 20
mg/kg for 1, 2, 3, 4, or 6 weeks. The ingestion of T-2 toxin (only
at 20 mg/kg at 3 weeks) depressed total splenic cell counts. T-2
toxin at 20 mg/kg for 1-4 weeks decreased splenic proliferative
responses to T-cell mitogen concanavalin A; however, the response to
a lipopolysaccharide (LPS), B-cell mitogen, was decreased in mice fed
T-2 toxin at 10 or 20 mg/kg for 1-4 weeks. (Friend et al., 1983a).
Lymphocytes from calves exposed to T-2 toxin at 0.6 mg/kg for as
long as 43 days had a decreased response to the mitogen, PHA, on days
1, 8, and 29 after toxin administration. Lymphocyte responses to
concanavalin A and pokeweed mitogen were also observed on day 29
after dosing (Buening et al., 1982).
In a study to determine the effects of T-2 toxin on the bovine
immune system, calves (5) were orally dosed with 0.3 mg/kg per day,
for 56 days. Neutrophil function was reduced by treatment with T-2
toxin as was the cutaneous reaction to injected phytohaemagglutinin.
In a second study, calves (6) were given T-2 toxin at a dose of 0.5
mg/kg per day, for 28 days. B-lymphocyte number and the response of
the B-cell-enriched fraction to phytohaemagglutinin both increased
after treatment. The in vitro exposure of mononuclear cells, B-cell-
enriched or T-cell enriched fraction, reduced the lymphoblastic
response to mitogens. A 50% reduction was induced by 1.4 ng T-2
toxin/ml (Mann et al., 1984).
The effects of T-2 toxin on in vitro mitogen response and
antibody production by human peripheral blood lymphocytes were
reported by Tomar et al. (1988). The toxin inhibited the mitogen
response to concanavalin A at a lower concentration (1.6 mg/ml)
compared with phytohaemagglutinin (2-4 mg/ml) and pokeweed mitogen.
In the presence of the toxin, inhibition reached a maximum during
first 8 h. The results indicate that various subpopulations of
lymphocytes have different susceptibilities to T-2 toxin.
Mitogen-induced blastogenesis in cultured human lymphocytes was
inhibited by T-2 toxin and its metabolites. The concentrations of T-
2 toxin, HT-2, 3'-OH T-2,3'-OH HT-2, T-2 triol, and T-2 tetraol
toxins that produced 50% inhibition of 3H-thymidine uptake in
mitogen-stimulated human peripheral lymphocytes were 1.5, 3.5, 4.0,
50.0, 150.0, and 150.0 ng/ml, respectively. The initial hydrolysis
of T-2 toxin to HT-2 toxin and the hydroxylation to 3'-OH T-2 did not
significantly decrease the immunotoxicity (Forsell et al., 1985).
Other trichothecenes were less toxic than T-2 toxin in this system.
The doses that produced 50% inhibition of 3H-thymidine uptake in
mitogen-stimulated human lymphocytes for fusarenon-X, NIV, DON, and
15-AcDON were 18, 72, 140, and 240 ng/ml, respectively. These
results indicate that the lymphotoxicity of trichothecenes is related
to the C-4 substituent (Forsell & Pestka, 1985).
T-2 toxin was examined for its effects on lymphocyte activation
and interleukin-2 production by splenic cultures from mice. Splenic
cells were taken from female BALB/c mice given 2 mg T-2 toxin/kg body
weight by stomach tube for 4 days or 4 mg T-2 toxin/kg by stomach
tube in single dose. Cells were incubated with 1 µg concanavalin A
and the synthesis of cellular protein and DNA determined. The single
dose of 4 mg/kg did not alter lymphocyte activation, but the dose of
2 mg/kg for 4 days produced a 50% reduction in activation. The
supernatant from these cells had 4 times greater interleukin-2
activity (Holt et al., 1988a).
DON and 3-AcDON were evaluated in vitro for their effects on
mitogen-induced lymphocytic blastogenesis using rat or human
peripheral blood lymphocytes. Both mycotoxins produced a dose-
dependent reduction in lymphocytic proliferation and DON produced a
greater inhibitory effect than the acetylated compound. Thus, the
concentrations of DON producing 50% inhibition of blastogenesis were
90 and 220 ng/ml for rat and human lymphocytes, respectively. The
values for 3-AcDON were 450 and 1060 ng/ml, respectively (Atkinson &
Miller 1984).
(e) Resistance to infection
The immunosuppressive effect of trichothecenes has resulted in an
increased incidence and severity of infection in animals in several
studies. According to Boonchuvit et al. (1975), an increased
mortality rate was recorded when 40 chickens were fed a diet
containing 16 mg T-2 toxin/kg for 1 week and were then inoculated
orally with 1 x 108 cells of Salmonella.
The depression of resistance to experimental tubeculosis by T-2
toxin was studied in mice by Kanai & Kondo, (1984). Groups of male
mice, strain ddY, 18-20 g body weight, with 10-14 animals per group,
were administered mycobacteria by intravenous injection in the tail
vein. In the first study, the doses injected consisted of 0.01 mg
culture of tubercle bacteria per animal (species not indicated). One
group was administered 0.1 mg T-2 toxin per animal a total of 12
times orally, starting on the day before the injection, 7 times with
one-day intervals, and then 5 times daily. For comparison, a second
group of mice was administered 5 mg cortisone acetate per animal ip
under a similar time schedule. A third group was injected with
tubercle control bacteria only. At the end of the 20-day observation
period, the mice in the first group had a lower spleen weight and a
higher tubercle bacteria count in the spleen than those in the other
2 groups, indicating a more pronounced depression of resistance by T-
2 toxin than by cortisone. In a second study, two groups of animals
were injected with 0.25 mg of a culture of Mycobacterium bovis; one
group was then administered 0.1 mg T-2 toxin per animal daily for 6
days, starting 8 days after injection. There were two groups of
controls. The average survival time in the T-2 toxin-treated group
was reduced to 19 days, compared with 35 days in the untreated group,
indicating decreased resistance.
Rats were injected ip with T-2 toxin in a single dose of 1
mg/kg body weight or in doses of 0.5 mg/kg daily for 5 days.
The rats were then inoculated with 0.1 ml of medium
containing 109 Staphylococcus aureus/ml. Rats given
multiple intramuscular injections of T-2 toxin showed more
oedema and myofibre necrosis at the injection site of the
bacteria; the cellular infiltrate was sparse and bacteria
were abundant. Bone marrow myeloid cells were markedly
decreased by multiple injections of T-2 toxin. In in vitro
studies, small, non-lethal doses of T-2 toxin inhibited
the chemotaxis of leukocytes and decreased phagocytosis of
the bacteria by leukocytes (Yarom et al., 1984b).
Seven male rhesus monkeys (Macaca mulatta) were dosed daily by
stomach tube for 4-5 weeks with 100 µg T-2 toxin/kg body weight.
This dose resulted in the death of 3 animals, 40% reduction in
leukocyte counts, reduction in the bactericidal activity of
neutrophils (phagocytosis of E. coli), reduction in the
transformation of lymphocytes by mitogens, and a reduction in numbers
of C-cell and T-cell lymphocytes (Jagadeesan et al., 1982).
The immunotoxic effects of T-2 toxin on cell-mediated resistance
were studied in female ICR mice infected with Listeria monocytogenes.
Mice in groups of 17 animals (10 animals in the control group) were
inoculated ip with 4 x 105 (LD50) or 4 x 104 (non-lethal) doses of L.
monocytogenes per animal, treated with a single oral dose of 4 mg T-2
toxin/kg body weight, and observed for 15 days. Bacterial
multiplication was rapid in the spleen after T-2 toxin treatment and
mortality was increased in both treated groups. Necrosis and
depletion of lymphoid tissue were observed in the thymus, the
periarterioler lymphoid sheaths and the lymphoid follicles of the
spleen. Cellular response to L. monocytogenes in the spleen and
liver was decreased by treatment with T-2 toxin and the lesions were
sparsely populated with mononuclear cells. The foci of necrosis were
larger with numerous colonies of bacteria. The influx and number
of lymphocytes and macrophages were greater in Listeria-elicited
peritoneal exudates. The immunotoxic effects of T-2 toxin were
comparable with those produced by cyclophosphamide and were
attributed to depletion of T lymphocytes and subsequent failure of T-
cell-dependent macrophages to clear the host of bacteria (Corrier &
Ziprin, 1986a). In a continuation of the previous study, female ICR
mice (17 animals per group) were inoculated ip on day 1 with 4 x 105
(LD50) or 4 x 104 (non-lethal) bacteria per animal, treated orally on
day 0, 1, 2, and 3 with 0, 1, or 2 mg T-2 toxin/kg, and observed for
15 days. The suppression of resistance by the mycotoxin was
indicated by rapid multiplication of Listeria in the spleen and
increased mortality in mice in both exposed groups treated with 2
mg/kg. The thymuses and spleens of toxin-treated mice showed
necrosis and depletion of lymphoid cells. Foci of necrosis induced
by Listeria infection in the spleen and liver were larger in treated
mice and the inflammatory reaction was sparse (Corrier & Ziprin,
1986a,b).
Increased resistance to L. monocytogenes infection was
surprisingly observed by the same group (Corrier & Ziprin, 1986b) in
mice administered T-2 toxin several days prior to the inoculation
with bacteria. Female ICR mice, 16 animals per group, were
administered T-2 toxin by stomach tube at dosage levels of 2, 1, 0.5,
or 0 mg toxin/kg body weight, on days -5, -4, -3, -2, -1, +1 and +3.
On day 0 the two treated groups were inoculated ip with 106 (LD100)
and 105 (LD50) L. monocytogenes, respectively. In addition, 20 mice
were given 2 mg T-2 toxin/kg on the same days as above, and used to
determine the effect of the toxin. Although the cytotoxic effect of
T-2 toxin on lymphoid tissue was marked, enhanced resistance to
Listeria infection was revealed by a decrease in mortality due to
listeriosis (in both bacteria-exposed groups) in a T-2 toxin dose-
dependent way. No specific cause for the increased resistance to
listeriosis by T-2 toxin treatment prior to bacterial infection was
identified by the authors.
ICR female mice were treated with the trichothecene mycotoxin DAS
and subsequently inoculated ip with Listeria monocytogenes. The
effect of the mycotoxin on the course of the infection was monitored
by observing the resultant mortality and the bacterial content of the
spleens from inoculated mice. Mice given 3 mg DAS/kg body weight
orally, on 2 and 1 days before inoculation, showed increased
mortality and splenic Listeria counts. In these mice, thymus weights
were reduced, and lymphocytes were depleted from the thymus cortex
and from splenic lymphoid follicles and periarteriolar lymphoid
sheaths. A single dose of 4 mg DAS/kg given on day 6 before
challenge exposure did not affect mortality compared with controls.
Mice treated with DAS and subsequently inoculated with Listeria had
significantly ( P = 0.006) higher levels of neutrophil populations
than Listeria-infected control mice (Ziprin & Corrier, 1987).
Dietary DON decreased resistance of B6C3F1 mice to infection with
L. monocytogenes. Resistance to the infection was similarly
decreased in control mice fed restricted diets, comparable to dietary
restriction caused by DON-induced feed refusal. Resistance to L.
monocytogenes was reduced to a greater extent by feed containing both
DON and zearalenone (Pestka et al., 1987b). DON in the diet at 0.50
or 1.0 mg/kg was fed for 5 weeks. Both doses resulted in a dose-
related decrease in time-to-death interval following challenge with
L. monocytogenes (Tryphonas et al., 1986).
T-2 toxin was fed to young male white Swiss mice at doses of 10
or 20 mg/kg diet for 2-3 weeks. The mice were then inoculated ip
with herpes simplex virus (HSV-1). Mice fed the high dose of T-2
toxin were highly susceptible to HSV-1 infection and about 75% died
with extensive hepatic and adrenal gland necrosis and with little or
no inflammatory cellular reaction in affected tissues, such as the
liver and adrenal glands, and the central nervous system. No
necrotizing encephalitis was found in treated mice. Mice fed 10 mg
T-2 toxin/kg had lesions of intermediate severity between those of
the high-dose group and the virus-infected controls (Friend et al.,
1983c). Feeding of T-2 toxin at 5, 10, or 20 mg/kg for 3-6 weeks did
not reactivate the virus in mice latently infected with HSV-1 (Friend
et al., 1983a).
Mice (male, Swiss strain weighing 25 g) received inoculations
(route not stated) of Cryptococcus neoformans (1 x 106 cells) and ip
doses (1/8 or 1/4 LD50) of DAS on days 5, 6, and 7 after inoculation
with the fungal cells. No deaths occurred in either the C.
neoformans-treated groups or the T-2-toxin group. A marked additive
effect on mortality was observed when mice received both C.
neoformans and T-2 toxin (Fromentin et al., 1981).
II.4.2.6 Carcinogenicity
The IARC (1983) studied the experimental data on the
carcinogenicity of T-2 toxin and concluded that no evaluation of the
carcinogenic role of T-2 trichothecene in experimental animals could
be made, because of the inadequacy of experimental data.
In a 16-month feeding study, groups of 50 male and 50 female
weanling CD-1 mice were fed a semi-synthetic diet containing 1.5 or
3.0 mg T-2 toxin/kg. Survival was lowest in the control group. No
statistically recognizable differences were found in feed consumption
or body weight gains among the groups. Statistically significant
differences were found in the incidence of pulmonary adenomas and
hepatic adenomas in the males of the 3.0 mg/kg group and the
controls. Other treatment-related findings were an increased
prevalence of epithelial cell hyperplasia and hyperkeratosis in the
stomach of animals fed the T-2 toxin diets (Schiefer et al., 1987).
In an attempt to determine the carcinogenicity of NIV, a study
was conducted in which mice were fed mouldy rice for 2 years. Groups
of 42, 7-week-old female C57BL/6CrSlc SPF mice were fed diets
containing 6, 12, or 30 mg NIV/kg for 2 years, and were assessed for
the effects on body weight gain, feed efficiency, terminal organ
weights, haematological values, and lesions. The mortality was
lowest in the highest dose group, followed by the 12 mg/kg group;
body weight gains and feed efficiency were dose-dependently reduced.
No particular neoplasms attributable to treatment were found. The
incidence of naturally occurring neoplasms, mostly lymphomas, was
similar in all groups. On gross and microscopic examination of the
liver, thymus, spleen, kidneys, stomach, small intestines with or
without Peyer's patches, no alteration related to treatment was
observed except for amyloidosis, which was lower in the two higher
dose groups (Ohtsubo et al., in press).
Two groups of 16 or 18 DDD male mice received either 10 or 20
weekly sc injections of 2.5 mg fusarenon-X/kg body weight. No
increase in tumour incidence was noted in treated animals, compared
with the controls (Saito & Ohtsubo, 1974).
In a feeding study, fusarenon-X, at a dose of 3.5 or 7 mg/kg diet
was fed to 151 male Donryu rats for 1-2 years. Treatment with the
mycotoxin reduced the growth rate, but did not induce any
carcinogenic effects (Saito et al., 1980).
In studies to investigate skin tumour induction using T-2 toxin
and DAS, the skin of the back of mice was painted with 0.1-1 mg of
trichothecenes, twice a week, for one year. A notable finding was
necrosis of the skin, but no tumours were detected. The skin tumour
induction test was also carried out using the initiator-promotor
procedure. T-2 toxin and fusarenon-X were not promoting agents of
dimethylbenz (a)anthracene-induced skin neoplasia. According to
Lindenfelser et al. (1974), neither T-2 toxin nor DAS served as
initiating agents.
II.4.2.7 Mutagenicity
In a Rec-assay using Bacillus subtilis, DNA damage was not
induced by 20 or 100 µg of either T-2 toxin or fusarenon-X (Ueno &
Kubota, 1976). Such trichothecenes as T-2 toxin, DAS, and DON were
not mutagenic to Salmonella typhimurium strains TA98, TA100, TA1535,
TA1537, and TA1538, with or without S-9 fraction from rat liver
(Kuczuk et al., 1978; Ueno et al., 1978; Wehner et al., 1978a).
T-2 toxin and DAS were not mutagenic in the D-3 mitotic
recombination test with Saccharomyces cerevisiae (Kuczuk et al.,
1978).
Neither DNA-strand breakage nor induction of 8-azaguanine-
resistant mutation were detected with 1-32 mg fusarenon-X/litre in
Hela cells and with the same toxin at 0.1-1.0 mg/litre in FM3A cells
derived from C3H mouse mammary carcinoma cell line (Umeda et al.,
1972, 1977). On the other hand, Lafarge-Frayssinet et al. (1981)
reported that T-2 toxin induced single-strand breaks in the DNA of
lymphoid cells in vivo (3 mg/kg body weight) and in vitro (0.7-5
ng/ml). Such DNA breakage was not observed in hepatic cells.
T-2 toxin, NIV, and fusarenon-X produced weak clastogenic effects
in Chinese hamster V79-E cells (Thust et al., 1983). Both T-2 toxin
and HT-2 toxin inhibited the incorporation of tritiated
thymidine into the DNA of human fibroblasts in culture in a dose-
related fashion. Non-toxic and toxic doses of DON (0.1-1000
mg/litre), did not significantly increase unscheduled DNA synthesis
in primary cultures of rat hepatocytes (Bradlaw et al., 1985).
Norppa et al. (1980) also reported weak induction of chromosomal
aberrations by T-2 toxin (1.7, 2.7, or 3.0 mg/kg body weight) in
Chinese hamster bone marrow cells. The bone marrow micronucleus test
was negative at a dose of 3 mg T-2 toxin/kg body weight resulting in
a significant decrease in polychromatic erythrocytes.
Hamsters fed T-2 toxin for 6 weeks (2.5 mg/kg body weight) did
not have more chromosomal aberrations than controls. The clastogenic
potential of T-2 toxin was very weak. In hamster V79 cells, DON at
levels of 2-3 µg/ml or more was cytotoxic, but was non-mutagenic at
the hypoxanthine-guanine phosphoribosyl transferase locus, with or
without hepatocytic mediated activation (Rogers & Heroux-Metcalf,
1983).
According to Reiss (1975), DAS induced various cytological
abnormalities including shortened chromosomes, enlarged nucleoli, and
a few chromosome breaks in the root tips of Allium cepa (common
onion) at concentration of 1000 and 100 µg/ml. T-2 toxin and
satratoxin H were C-mitotic poisons at concentrations exceeding 10
µg/g. Typical C-mitotic action on chromosomes morphology was
produced by both toxins and was comparable to that of colchicine. In
addition, T-2 toxin induced polyploidy (Linnainmaa et al., 1979).
T-2 toxin and satratoxin H were not mutagenic in a sex-linked
recessive lethal test in Drosophila (Sorsa et al., 1980). Lack of
potency to produce recessive lethal mutations in Drosophila was
consistent with negative results obtained in certain bacterial assays
in which trichothecenes were inactive as both base pair and frame
shift mutagens (Kuczuk et al., 1978; Ueno et al., 1978a).
II.4.2.8 Teratogenicity and reproductive effects
T-2 toxin was embryotoxic and teratogenic in mice. T-2 toxin
dissolved in propylene glycol was injected intraperitoneally into
pregnant mice on one of days 7-11 of gestation at doses of 0.5, 1, or
1.5 mg/kg body weight. T-2 toxin (doses of 1 or 1.5 mg/kg) caused
significant maternal mortality, fetal death, and fetal body weight
loss. Approximately 37% of the fetuses from dams given 1 (8 litters)
or 1.5 mg (4 litters) T-2 toxin/kg on day 10 were grossly malformed.
The most frequent anomalies were bent, shortened, or missing tails,
and limb malformations, including oligodactyly and syndactyly.
Exencephaly, open eyes, retarded jaw, and skeletal malformations of
the rib or vertebrae were also found in the fetuses (Stanford et al.,
1975).
Pregnant CD-1 mice (18 litters/treatment group) were administered
T-2 toxin dissolved in propylene glycol ip at 0.5 mg/kg body weight
on gestation days 8 or 10. The T-2 toxin produced grossly malformed
fetuses, principally with tail and limb anomalies. A higher
incidence of malformations was observed when a T-2 toxin dose of 0.5
mg/kg body weight was combined with an ochratoxin A dose of 4 mg/kg
body weight. An increase in fetocidal effects was found in offspring
of dams in groups treated with the high-dose combination, on either
day. Few skeletal and visceral malformations were noted (Hood et
al., 1978). T-2 toxin (0.5 mg/kg body weight) dissolved in 1:1
mixture of propylene glycol and 0.1N sodium bicarbonate was
administered ip alone or in combination with rubratoxin B (0.4 mg/kg
body weight) to pregnant CD-1 mice on day 1 of gestation. Only T-2
toxin resulted in gross malformations. The combination of toxins
increased the adverse effects on fetal body weight and mortality, but
not the incidence or severity of the gross malformations (Hood,
1986).
The teratogenicity of orally administered T-2 toxin dissolved in
propylene glycol was evaluated in a study using 350 female CD-1
mice and doses of 0.5, 1.0, 2.0, 3.0, 3.5, or 4.0 mg/kg body weight
given on day 9 of gestation with a single dose of 3.0 mg/kg on day 6,
7, 8, 10, 11, or 12 of gestation. In the first study, the doses of
3.5 and 4.0 mg/kg produced maternal deaths and toxicity; no fetuses
were produced by the dams in the 4.0 mg/kg dose group and
significantly fewer fetuses were produced by dams in the 3.5 mg/kg
dose group. More major and minor defects were seen in offspring in
the 3.0 mg/kg dose group. In the second study, the treated females
had greater fetal loss than controls and the greatest number of dead
fetuses occurred among litters treated on day 9 of gestation. Major
skeletal defects were more numerous in mice treated on day 7 of
gestation. The results indicated that a single oral dose of T-2
toxin in propylene glycol was primarily maternally toxic and
embryolethal; defective development was possibly secondary to
maternal toxicity (Rousseaux & Schiefer, 1987).
Fusarenon-X was embryotoxic, but not teratogenic (Ito et al.,
1980). Fusarenon-X dissolved in saline was given to pregnant DDD
mice by subcutaneous injection at doses of 0.63, 1.0, 1.6, 2.6, or
4.1 mg/kg body weight, or by feeding at concentrations of 5, 10, or
20 mg/kg diet during pregnancy. Two dams given a single sc dose of
4.1 mg/kg died within 24 h of injection. Abortion was induced in all
females by a single injection of 2.6 mg/kg on day 10 of gestation.
Smaller doses (0.63-1.6 mg/kg) produced a 16-20% abortion rate, when
given on day 10. Multiple doses (8-12 or 8-14 days of gestation) of
1.0 or 1.6 mg/kg produced 100% abortion. When the mice were fed a
diet containing 5, 10, or 20 mg fusarenon-X/kg throughout the
gestation period or in the early stages of gestation, the mycotoxin
inhibited embryonal implantation. Feeding fusarenon-X at 20 mg/kg
for 7 days during the middle stages of gestation induced abortion in
100% of dams. Fetal body weight was significantly reduced by the
administration of the mycotoxin, but no significant teratogenic
effects were observed in the fetuses of dams in either the
subcutaneous injection or feeding study (Ito et al., 1980).
The effects of NIV on fertilization, course of pregnancy, and
fetuses were examined in ICR mice. In a study by Ito et al. (1986),
pure NIV was injected ip in pregnant mice (groups of 10 animals
each), at dose levels of 0, 0.1, 0.5, or 1.5 mg/kg body weight per
day, on days 7-5 of gestation. The highest dose caused stillbirths
after vaginal haemorrhage in 6 out of 10 animals. High embryo
lethality was recorded in the 2 highest dose groups (88 and 48%). No
fetal malformations were observed in the treated groups. A single
administration of 3 mg/kg on day 7 affected the embryo within 10 h,
damaged the placenta within 24 h, and caused stillbirths at 48 h.
While NIV is embryotoxic, it is not teratogenic (Ito et al.,
1988). Thirty ICR mice in 3 batches of 10 animals each were fed
diets mixed with mouldy rice powder containing NIV at final levels of
6, 12, or 30 mg/kg feed per day throughout gestation. There were 11
controls. Purified NIV was also administered by gavage to 35 animals
in 4 groups at doses of 1-20 mg/kg body weight on days 7-15 of
gestation. There were 10 controls. Embryotoxicity associated
with maternal weight loss was observed in the groups receiving 30
mg/kg diet and 10 mg/ kg body weight per day, by gavage, whereas
lower levels, such as 5 mg/kg body weight per day, by gavage, did not
have any embryotoxic effects. Intrauterine growth retardation was
found at term in the fetuses of mice exposed to 12 mg/kg feed and 5
mg/kg by gavage. NIV did not have any significant adverse effects on
the incidence of gross skeletal and visceral malformations. As 5
mg/kg body weight per day given by gavage corresponds to a feed level
of approximately 35 mg/kg feed, the above data indicate that
exposure to 30 mg NIV/kg feed throughout the gestation period results
in embryotoxicity. However, exposure to approximately 35 mg/kg feed
during days 7-15 only of the gestation period does not induce
embryotoxic effects, which shows the significance of constant
exposure versus intermittent exposure to NIV.
DON was embryotoxic and teratogenic when dissolved in distilled
water and given for 4 consecutive days (days 8-11 of gestation), by
oesophageal intubation, to 15-19 pregnant Swiss-Webster mice (Khera
et al., 1982). The incidence of resorptions was 100% at doses of 10
or 15 mg/kg body weight, and 80% at 5 mg/kg body weight. The dose of
5 mg/kg reduced the number of live fetuses and reduced the average
fetal weight compared with the controls. Low incidences of skeletal
and visceral anomalies were found in the fetuses of the 1, 2.5, and 5
mg/kg groups. The skeletal malformations occurred in a dose-related
manner and included lumbar vertebrae with fused arches or partly
absent centra, and absent or fused ribs.
On the other hand, DON failed to produce embryotoxic and
teratogenic effects when fed ad lib at 0.5, 2.0, or 5.0 mg/kg to
Fisher 344 rats during the entire course of pregnancy (Morrissey,
1984). No overt signs of toxicity were observed in the dams and no
changes in maternal feed consumption were observed at any dose.
DON was fed to 71 adult female New Zealand White rabbits in 7
batches of 6-14 animals during the entire period of gestation at
doses of 0.3, 0.6, 1.0, 1.6, 1.8, or 2.0 mg/kg body weight. There
were 25 controls. The fetal effects consisted of 100% incidence of
fetal resorption in the females fed 1.8 and 2.0 mg/kg and reduced
average body weight of fetuses from dams fed 1.0 and 1.6 mg/kg.
These doses were not teratogenic (Khera et al., 1986).
Weanling F0 male and female mice were fed diets containing DON at
a dose of 2.0 mg/kg body weight (15 male and 15 female animals) and
0.375, 0.75, or 1.5 mg/kg body weight (7 males and 59 female
animals). There were 30 controls in the first study and 26 in the
second. After 30 days of dietary feeding, the mice were allowed to
mate and the pregnant females were allowed to litter normally. The
F1a progeny were examined up to 21 days of age and discarded. The F0
mice were rebred. The females bred to produce the F1b litters were
killed on day 19 of gestation and the fetuses were examined for
gross, visceral and skeletal malformations. Reductions were
observed in feed intake and in the body weight of F0 male and female
mice, the number of live pups and postnatal survivors, postnatal body
weight of F1a progeny, number of live fetuses, and fetal body weight
of F1b. No adverse effects on the fertility of F0 mice and no
major malformations in F1b fetuses were found. Results of cross-
fostering offspring between control dams and 1.5 mg/kg dams indicated
that both postnatal survival and body weight were adversely affected
by prenatal exposure as well as by combined pre- and post-natal
exposure (Khera et al., 1984).
Male and female Sprague-Dawley rats (3 groups of 15 male and 15
female animals each) were fed diets containing DON at levels of 0.25,
0.5, or 1.0 mg/kg body weight. Controls consisted of two groups of
15 males and 15 females. After 6 weeks of feeding, the rats were
bred. The mated females, maintained on their respective diets, were
killed on the last day of pregnancy and fetuses were evaluated for
effects on pre-natal development. No adverse effects were observed
except for dilatation of the renal pelvis and urinary bladder (Khera
et al., 1984).
Male (20/group) and female Sprague-Dawley rats (25/group) were
fed a diet containing 20 mg purified DON/kg for 60 and 15 days,
respectively, before mating. Rats consuming the DON-supplemental
diet throughout gestation and lactation did not show any clinical
signs of toxicity, but had reduced body weights. Only 50% of the
matings between toxin-fed rats resulted in pregnancy compared with
80% in the controls. No differences were detected among the groups
in sex ratio, survival rate, or average litter number and weight.
Pup weight gains in all groups were comparable up to post-natal day
14. From day 14 to 21, however, male and female pups of the control
group showed significantly improved weight gains compared with pups
from treated dams. No treatment-related histological abnormalities
were found in the testes or ovaries of treated pups (Morrissey &
Vesonder, 1985).
A 2-generation reproduction and teratology study was carried out
using 90 female CD-1 mice fed a semisynthetic diet containing the T-2
toxin at 1.5 or 3.0 mg/kg, concentrations considered possible under
field conditions. Results indicated that continuous feeding of T-2
toxin at low concentrations had minimal, if any, toxic effects on
female reproduction and fetal development and that T-2 toxin fed
continuously at 1.5 or 3.0 mg/kg was not teratogenic or fetocidal and
produced minimal effects on the growth rates of CD-1 mice (Rousseaux
et al., 1986).
II.4.3 Biochemical effects and mode of action
II.4.3.1 Cytotoxicity
In the early stages of research on trichothecenes, DAS was
isolated from F. scirpi as a phytotoxic principle (Brian et al.,
1961). Subsequent surveys confirmed the phytotoxic nature of the
trichothecenes using germination of seeds of Brassica oleracea L.
(Ueno et al., 1971c), germination of pea seedlings (Marasas et al.,
1971), growth of tobacco callus tissues (Helgeson et al., 1973), the
germination of tobacco plant (Nicotiana sylvestris) pollen
(Siriwardana & Lafont, 1978), and the auxin-promoted elongation of
soybean hypocotyl (Stahl et al., 1973).
Most of the trichothecenes tested have had fungistatic activity
in a wide range of species (Bamburg et al., 1968a; Bamburg & Strong,
1971; Reiss, 1973); DAS and verrucarin A were particularly potent in
inhibiting growth and sporulation at concentrations of 0.5-1
mg/litre.
Many of the trichothecenes have been cytotoxic to mammalian
cells, both in in vivo and in vitro. In animals administered
trichothecenes, the mucosa of the stomach and small and large
intestines had mucosal erosions, mucosal necrosis with ulceration,
and severe haemorrhages; radiomimetic effects included necrosis of
actively-dividing cells in the thymus, spleen, ovary, testis, and
lymph nodes (Saito & Ohtsubo, 1974). In a cell culture system, Grove
& Mortimer (1969) demonstrated the cytotoxicity of DAS and its
chemically modified compounds on hepatocytes of human origin and
hamster kidney cells. Ohtsubo et al. (1968), Ohtsubo & Saito (1970),
and Bodon & Zoldag (1974) described the cytotoxicity of NIV and
related trichothecenes for HeLa cells, and of T-2 toxin for
epithelial cells of pig kidney, respectively. The macrocyclic
trichothecenes, verrucarins and roridins, were highly cytotoxic for
0815 mouse tumour cells in the ng/ml range (Harri et al., 1962).
Tanaka et al. (1977), investigated the cytotoxicity of 20 types of
trichothecenes (11 type A, 6 type B, 1 type C, and 2 type D) for 3
cell lines, HeLa, HEK, and HL cells. The type D trichothecenes,
such as verrucarin A and roridin A had LC50s in the range of 0.003-
0.005 mg/litre; neosolaniol and NIV in the range of 0.1-1 mg/litre,
and deoxynivalenol in the range of 1-5 mg/litre. Trichodermol,
calonectrin, monoacetyldeoxynivalenol, and tetraacetylnivalenol were
weakly cytotoxic within the range of 5-10 mg/litre.
Chinese hamster ovary (CHO) and African green monkey kidney
(VERO) cell cultures were exposed to 0.01 or 1.0 ng T-2 toxin/ml for
1 or 12 h. The cells exhibited morphological changes considered to
be related to inhibition of protein synthesis. The alterations
included disassociation of polysomes and matrix density, ballooning
of intracristal space, and malalignment of cristae in mitochondria.
The CHO cells had bleb formations of plasma membrane, a change
produced by exposure to establish inhibition of protein synthesis
(Trusal, 1985).
II.4.3.2 Inhibition of protein synthesis
The initial observation that the trichothecene mycotoxins
inhibited protein synthesis in mammalian cells was made by Ueno et
al. (1968). They reported that NIV produced a dose-dependent
inhibition of incorporation of several amino acids into protein in
rabbit reticulocytes (Ueno et al., 1968) and Ehrlich ascite tumour
cells (Ueno & Fukushima, 1968). This inhibitory effect of the
trichothecene mycotoxins was observed in the whole animal (Ueno,
1970), a protozoan (Ueno & Yamakawa, 1970), HeLa cells (Liao et al.,
1976), cultured mammalian cells (Ohtsubo et al., 1968; Ohtsubo &
Saito, 1970), rat hepatocytes (Ueno et al., 1973b), hamster ovary
cells (Gupta & Siminovitch, 1978), human tonsil (Carrasco et al.,
1973), yeast spheroplasts (Stafford & McLaughlin, 1973; Cundliffe et
al., 1974; McLaughlin et al., 1977), rabbit reticulocytes (Ueno et
al., 1968, 1969a, 1973b,c; Ueno & Shimada, 1974; Wei & McLaughlin,
1974; Mizuno, 1975; Carter et al., 1976), and lymphocytes (Hartman et
al., 1978). No inhibitory effect on bacterial cells was observed
(Ueno et al., 1973b).
The effects of T-2 toxin on protein and DNA synthesis were
studied in Swiss mice and hepatoma cell cultures. T-2 toxin was
given as a single ip dose of 0.75 mg/kg and as a 3-day and a 7-day
daily treatment. The toxin inhibited protein synthesis after all 3
schedules of treatment and inhibition was present in cells obtained
from bone marrow, spleen, and thymus. Protein synthesis was
inhibited in vitro in hepatoma cell cultures and PHA-stimulated
lymphocytes (Rosenstein & Lafarge-Frayssinet, 1983).
The effects of T-2 toxin on rat hepatocytes were studied in
culture by the addition of several doses at either 1 or 12 h of
exposure. A dose of 0.01 ng T-2 toxin/ml produced a 75% inhibition
of protein synthesis within 1 h. At a higher dose of 1.0 ng/ml,
hepatocytes recovered from a 1-h but not a 12-h exposure. Cell
damage (release of lactate dehydrogenase) lagged behind inhibition of
protein synthesis, which was 90% at the 1 ng/ml dose. Ultrastructural
alterations were present in the endoplasmic reticulum and
mitochondria. Degranulation involved the rough endoplasmic
reticulum. Mitochondria had translucent foci and electron dense
cores (Trusal & O'Brien, 1986).
NIV inhibited poly-U, poly-A, and poly-C directed incorporation
of phenylalanyl-tRNA into phenylalanine without affecting the
activation of amino acids. The inhibition was presumably caused by
impairment of ribosomal function (Ueno et al., 1968). Fusarenon-X
caused breakdown of polysomes in rabbit reticulocytes (Ueno et al.,
1973b) and in mouse fibroblasts (L-cells), shortly after exposure
(Ohtsubo et al., 1972). The breakdown of polysomes was consistent
with the action of an inhibitor of initiation of protein synthesis.
T-2 toxin, DAS, and verrucarin A, but not trichodermin, also induced
the disaggregation of polysomes in HeLa cells (Liao et al., 1976).
These results were consistent with the effects of an inhibitor of
prolongation or termination of protein synthesis (Stafford &
McLaughlin, 1973). An important point was that the trichothecenes,
regardless of whether they were I-type or ET-type, interacted with
the peptidyl transferase centre on the 60S ribosomal subunit and
inhibited the transpeptidation of the peptide bond formation process.
Current research has focused on clarifying the molecular species
of ribosomal protein that regulates the binding of trichothecene
mycotoxins. A mutant of yeast (Schindler et al., 1974; Jimenez &
Vazquez, 1975; Jimenez et al., 1975; Grant et al., 1976; Carter et
al., 1980) and Chinese hamster ovary cells, resistant to the effects
of trichothecenes on protein synthesis (Gupta & Siminovitch, 1978),
have been isolated. Friend & Warner (1981) clearly demonstrated that
the gene for trichodermin resistance in yeast specifies ribosomal
protein L3, the largest of the yeast ribosomal proteins.
The ribosomal subunits of Myrothecium verrucaria, a producer of
macrocyclic trichothecenes, were resistant to T-2 toxin. It can be
assumed that the 60S subunits of eukaryotes are responsible for the
sensitivity to the trichothecenes. (Hobden & Cundliffe, 1980).
II.4.3.3 Inhibition of nucleic acid synthesis
The dose-dependent inhibition by NIV of DNA and RNA synthesis in
Ehrlich ascites tumour cells was first observed by Ueno & Fukushima
(1968). Inhibition (70%) of protein synthesis was induced by 1-10 mg
NIV/litre, and thymidine incorporation into DNA was inhibited by as
much as 60%; suppression (30%) of uracil incorporation into RNA was
slight. Similar results were obtained with several trichothecenes,
including trichodermin, diacetoxyscirpenol, and fusarenon-X, in other
cultured cells, such as KB cells (Ohtsubo et al., 1968), HeLa cells
(Liao et al., 1976), mouse L-cell fibroblasts (Ohtsubo et al., 1972),
hamster ovary cells (Gupta & Siminovitch, 1978), and lymphocytes
(Hartman et al., 1978).
The inhibition of DNA and RNA synthesis by trichothecenes
required higher concentrations of toxins than the inhibition of
protein synthesis and the extent of the inhibition was much less.
Thus, the observed inhibition of DNA and RNA synthesis in toxin-
treated animal cells was presumed a secondary effect of the
trichothecenes. This hypothesis was supported by the findings of
Tashiro et al. (1979) who reported that, in in vitro studies, a
concentration of 0.0236-1.889 mmol fusarenon-X/litre did not inhibit
DNA-dependent RNA hybridases of rat liver and Tetrahymena pyriformis.
Munsch & Mueller (1980) reported that the incorporation of 3H-
thymidine into DNA in cell lines from thymus was strongly inhibited
by over 10 ng T-2 toxin/ml and slightly inhibited by 0.1-10 ng T-2
toxin/ml. A low concentration of 0.1-1 ng/ml toxin caused a
transient increase in DNA polymerases, alpha- and beta-terminal
deoxynucleotidyl transferases. At a high dose of more than 1 ng T-2
toxin/ml, these enzymatic activities were strongly inhibited.
Rosenstein & Lafarge-Frayssinet (1983) described the depression of
DNA synthesis in vivo and in vitro. Three treatment schedules: a
single dose, 3 daily doses, or 7 daily doses of 0.75 mg T-2 toxin/kg
inhibited DNA synthesis in cell cultures from the spleen, thymus, and
bone marrow of treated mice. The mycotoxin also inhibited DNA
synthesis in vitro in cultures of hepatoma cells and in PHA-
stimulated lymphocytes (Rosenstein & Lafarge-Frayssinet, 1983).
Agrelo & Schoental (1980) found that hydroxyurea did not alter
unscheduled DNA synthesis in cells treated with T-2 toxin and HT-2
toxin (6 ng/ml). However, the combination of rat liver microsomes
and hydroxyurea resulted in an increase in unscheduled DNA synthesis
in cells exposed to 100 µg HT-2 toxin/ litre. The data of Agrelo &
Schoental (1980) suggest that the microsomal drug-metabolizing enzyme
system may participate in the induction of DNA damage by
trichothecenes.
The effects of T-2 toxin and DAS on DNA synthesis in phyto-
haemagglutinin, stimulated in the peripheral blood lymphocytes of
human beings, was assayed by incorporation of 3H-thymidine. Total
inhibition was obtained by 8 ng T-2 toxin and DAS and 80% was
obtained with 1.5 ng T-2 toxin and 2.7 ng of DAS (Cooray, 1984).
Fusarenon-X and related toxins inhibited protein and nucleic acid
synthesis in Tetrahymena pyriformis in a manner similar to that
observed in cultured mammalian cells (Ueno & Yamakawa, 1970). In
synchronously dividing Tetrahymena cells (Iwahashi et al., 1982), the
incorporation of radioactive amino acids, thymidine, and uracil into
protein, DNA, and RNA, respectively, was achieved by nearly the same
concentration of T-2 toxin. Inhibition of protein synthesis was
explained by the high affinity of T-2 toxin for 60S ribosomal
subunits of Tetrahymena, as is the case with cultured cells.
However, neither DNA and RNA synthesis nor RNA hybridase activity
were altered by T-2 toxin in an in vitro system using isolated nuclei
from normal cells or from cells pretreated with T-2 toxin. The
mechanism of inhibition of nucleic acid synthesis in the in vivo
system is not yet understood.
II.4.3.4 Alterations of cellular membranes
The trichothecenes as a group inhibit protein synthesis, but the
potency of the effect varies according to the trichothecene and the
system ( in vitro or in vivo) used to measure the inhibition. T-2
toxin was a potent inhibitor of protein synthesis both in vitro and
in vivo (Ueno et al., 1973b; Rosenstein & Lafarge-Frayssinet, 1983).
In vitro experiments were conducted to determine the effects of
T-2 toxin on the entry of sucrose into bovine erythrocytes,
entrapment of sucrose and inulin in carrier erythrocytes, entrapment
of T-2 and binding of T-2 toxin, and the measurement of cell
permeability to the entrapped T-2 toxin. At the highest
concentration of T-2 toxin (20 µg), no entry of 14C-sucrose or 3H-
inulin was observed. Very little 3H-T-2 toxin was bound to bovine
erythrocytes and binding was independent of T-2 toxin concentration.
The mycotoxin had no effect on the entrapment of sucrose or inulin.
Carrier erythrocytes retained 85% of 14C-sucrose and only 18% of
3H-T-2 toxin. Thus, T-2 toxin diffused from carrier cells much more
rapidly than sucrose. It was concluded that the interaction of T-2
toxin with bovine erythrocytes was minimal and intercalation with the
inner bilayer was not likely, because the increase in cell volume
that would have resulted did not occur (DeLoach et al., 1987).
The effects of T-2 toxin on membrane function were studied using
L-6 myoblasts. The minimal effective concentration (MEC) of T-2
toxin for reduction in uptake of calcium and glucose and for
reduction in uptake of leucine and tyrosine and their incorporation
into protein was 4 pg/ml. The uptake of rubidium was increased at
0.4 pg/ml and reduced at 4 pg/ml or more. Thymidine uptake and
incorporation into DNA had a biphasic response with an increase at
0.4 pg/ml and a reduction at 4 pg/ml for uptake and 40 pg/ml for
incorporation. Calcium efflux was reduced after 1, 5, and 15 min
exposure to T-2 toxin at a concentration of 40 pg/ml. These data
indicate that T-2 toxin has multiple effects on all membrane function
at very low concentrations and that these effects are independent of
inhibition of protein synthesis (Bunner & Morris, 1988).
II.4.3.5 Other biochemical effects
Fusarenon-X inhibited the uptake of phosphate by Tetrahymena
cells (Chiba et al., 1972). It caused a 50% inhibition of
incorporation of acetate into phospholipids and a ten-fold
stimulation of incorporation into triglyceride, effects that may be
secondary to inhibition of phosphate uptake. However, in rabbit
reticulocytes, fusarenon-X, T-2 toxin, and neosolaniol at
concentrations of 100 mg/litre did not inhibit Na+-dependent glycine
transport (Ueno et al., 1978b).
When SH-enzymes were pre-incubated with selected trichothecenes
in the absence of substrates, the activities of such enzymes as
creatine phosphokinase and lactate and alcohol dehydrogenases were
reduced (Ueno & Matsumoto, 1975). However, neither urease activity
(Reiss, 1977) nor rat liver lysosomal cathepsin activity (Farb et
al., 1976) was affected by diacetoxyscirpenol or T-2 toxin,
respectively, in vitro. Foster et al. (1975), reported the
reactivity of T-2 toxin with glutathione in the presence of epoxide-
S-GSH-transferase.
When mice were exposed to trichothecene mycotoxins, no detectable
alterations were observed in hepatic and renal functions. In chicks
fed a diet containing 10 mg T-2 toxin/kg for 3 weeks, a hepatic
microsomal aminopyrine, demethylase, was reduced by 29% compared with
the control, while another mixed function oxidase, aniline
hydroxylase, was not significantly affected (Coffin & Combs,
1981). In mice receiving a sublethal dose of fusarenon-X ip, a rapid
hypoglycemia was followed by depletion of hepatic glycogen (Shimizu
et al., 1979). The authors suggested that the toxin had induced a
malfunctioning of glucose absorption in the intestines and had
accelerated glycolysis. However, the trichothecene mycotoxins are
highly cytotoxic to the epithelia of the intestine, and impairment of
carbohydrate metabolism and absorption may be a secondary effect of
this cytotoxicity.
II.4.4 Structure-activity relationships
After the isolation and identification of numerous derivatives of
the trichothecenes, the structure-activity relationship was
investigated, on the basis of the information on lethal toxicity,
dermal toxicity, cytotoxicity, inhibition of protein synthesis, and
association with ribosomes.
The 12,13-epoxide of the trichothecenes is essential for their
biological activity. The de-epoxidation of DON and T-2 toxin by
rumen-microorganisms (King et al., 1984) and in mammalian systems
(Yoshizawa et al., 1983) results in loss of toxicity.
The macrocyclic ring contributes to the highly lipophylic
property of the trichothecenes, and the macrocyclic trichothecenes,
such as verrucarins, roridins, satratoxins, and baccharins, exhibit a
potent cytotoxicity towards cultured mammalian cells (Kupchan et al.,
1976, 1977; Jarvis et al., 1978, 1980).
In contrast, the polyhydroxylated alcohols, such as verrucarol,
NIV, and DON, are highly hydrophilic, resulting in a decrease in
cytotoxicity and dermal toxicity compared with their parent
trichothecenes (Ueno et al., 1970; Wei et al., 1974).
II.4.5 Prevention and therapy of trichothecene toxicosis
In studies to investigate effects of various dietary supplements
on T-2 toxin toxicity, weanling male Wistar rats in groups of 10,
with adequate controls, were fed diets containing 5% each of
bentonite, anion exchange resin, cation exchange resin, or
vermiculite-hydrobiotite with and without 3 µg T-2 toxin/g feed, for
2 weeks. Bentonite and anion exchange resin were most effective in
reducing the growth depression and feed refusal caused by T-2 toxin.
In a second study in which bentonite and anion exchange resin were
administered at levels of 2.5, 5.0, 7.5, or 10% of diet, 10%
bentonite in the diet was the most effective dietary supplement.
Dietary bentonite reduced the absorption of 3H-T-2 toxin and
increased faecal elimination (Carson & Smith, 1983).
Smectite, a clay composed of insoluble silicates of aluminum and
magnesium, incubated with T-2 toxin for 24 h before the toxin was
administered orally to mice (1 mg/kg per day), prevented the
acceleration of gastric emptying and transit time for a milk meal,
usually induced by administration of T-2 toxin. Smectite given
together with the toxin did not prevent the gastrointestinal effects
of T-2 toxin (Fioramonti et al., 1987). Male Sprague-Dawley rats
were given 15 or 5 mg/kg body weight of a platelet activating factor
antagonist, with and without T-2 toxin, by intravenous injection.
The drug prolonged the survival of conscious rats exposed to 0.65 mg
T-2 toxin/kg (Feuerstein et al., 1987).
The effects of certain drugs and metabolic inhibitors on the
toxicity of T-2 toxin have been studied in male ddY mice. The toxin
at a dose of 1.8 mg/kg was given subcutaneously and the drugs and
other chemicals were given ip. The lethal effects were reduced by
the steroid drugs, prednisolone and dexamethasone; survival times
were increased by the antihistaminic drug diphenhydramine, and an
opioid antagonist, naloxone. Prednisolone also reduced the
leukocytosis that developed after T-2 toxin treatment and decreased
the increase in ear weight caused by the mycotoxin. Other
antihistaminic and/or antiserotonic drugs used in the treatment were
found not to be effective in preventing the lethal effects of T-2
toxin (Ryu et al., 1987).
Male ddY mice and male Wistar rats in several groups of 10
animals each, pretreated with a radioprotective compound or with an
anti-inflammatory agent, were given T-2 toxin or fusarenon-X (1.5
mg/kg body weight). Out of the 20 compounds evaluated, only
prednisolone and hydrocortisone (100 mg/kg ip) were effective. They
reduced mortality from 90% in controls to less than 30% and they also
reduced the trichothecene-induced increase in intestinal fluid volume
(Mutoh et al., 1988).
Cutaneous irritation produced by T-2 toxin in 10 Porton female
rats was largely reduced by application, within 10 min, of an aqueous
soap solution when the dose was low (1.0 µg/cm2), but the solution was
largely ineffective over a time span of 60 min when the dose was
higher (100 µg T-2 toxin). Washing the site of application with
polyethylene glycol 300 was very effective in removing even large
doses (100 µg) of T-2 toxin from the skin (Fairhurst et al., 1987).
The median effective dose of oral superactive charcoal in
preventing deaths in batches of 5 female Sprague-Dawley rats was
0.175 g/kg. A dose of 1 g superactive charcoal/kg body weight
increased survival times and rates in rats given the lethal dose of 8
mg T-2 toxin/kg as long as 3 h after the toxin was administered by
gavage (Galey et al., 1987).
II.5 EFFECTS ON MAN
II.5.1 Contemporary episodes of human disease
Two outbreaks of trichothecene-related disease have been
reported, one in China in 1984/85 (Luo, 1988) and one in India in
1987 (Bhat et al., 1989). Each involved several hundred cases.
During the first incident, outbreaks of mouldy corn and scabby
wheat poisoning were reported. Out of approximately 600 persons who
consumed mouldy cereals, there were 463 cases of poisoning (77% of
the total). The latency period for the onset of symptoms was 5-30
min. These included nausea, vomiting, abdominal pain, diarrhoea,
dizziness, and headache. No deaths occurred. Pigs and chicks fed
the same mouldy cereals were also affected (Luo, 1988). GC-MS and
RIA were used in the analysis of 5 samples of the mouldy corn. DON
was detected within a range of 0.34-92.8 mg/kg and zearalenone within
a range of 0.004-0.587 mg/kg. T-2 toxin and NIV were not found. TLC
was used in the analysis of 19 samples of scabby wheat collected from
the affected and non-affected families. The DON content was 1.0-40.0
mg/kg, which was significantly higher than that in the non scabby
wheat samples. In addition to DON, zearalenone was detected in 2
samples, the contents of which were 0.25 and 0.5 mg/kg, respectively.
No T-2 toxin was found in the samples (Luo, 1988).
An analogous outbreak was reported in Kashmir, India, in 1987
(Bhat et al., 1987, 1989). It was ascribed to the consumption of
bread made from flour that had become mouldy in storage following
unseasonal rains in the wheat-harvesting season, from which Fusarium
sp. was grown, and which were found to contain mycotoxins. Of the
224 persons investigated on a random sample basis, 97 were affected
with symptoms including abdominal pain (100%), throat irritation
(63%), diarrhoea (39%), blood in stools (5%), and vomiting (7%).
Symptoms developed 15 min to one hour after consumption of locally
baked bread. In 12 out of 24 samples of refined wheat flour used in
the preparation of bread, the following mycotoxins were found: DON
(0.35-8.38 mg/kg), Ac-DON (0.64-2.49 mg/kg) (no details of estimation
of this derivative were available), NIV (0.03-0.1 mg/kg) and T-2
toxin (0.55-0.8 mg/kg). Quantitative estimation of DON, Ac-DON, and
NIV was obtained using HPLC, and that of T-2 toxin by TLC (Bhat et
al., 1987) but no rigorous confirmation of identity was undertaken.
Since no fatalities occurred in either of the above outbreaks, no
information is available on the pathological changes, if any, at
autopsy.
II.5.2 Historical Fusarium-related diseases
In the period 1931-47, a human disease known as alimentary toxic
aleukia (ATA) occurred in the USSR that was suggested to be related
to the presence of toxic Fusarium species in mouldy over-wintered
grain. Data have been reviewed by Sarkisov et al. (1944) and more
recently by Bilai (1977), Leonov (1977), and Joffe (1986). An
association was established with the ingestion of grain invaded by
some moulds, in particular Fusarium poae and F. sporotrichioides.
The dominant pathological changes were necrotic lesions of the oral
cavity, the oesophagus, and stomach and, in particular, a pronounced
leukopenia. The primary lesion was bone marrow hypoplasia and
aplasia. The disease was lethal in a high proportion of cases.
The clinical symptoms reported in ATA, as well as the identified
occurrence of Fusarium in foodstuffs, suggest that it might have been
associated with mycotoxins, identified years later in fungal cultures
of Fusarium species under laboratory conditions, such as T-2 toxin
(Mirocha & Pathre 1973) or wortmannin (Mirocha & Abbas 1989).
Scabby grain toxicosis is a disease of human beings as well as
farm animals, and was reported from Japan and Korea in the period
1946-63 (Hirayama & Yamamoto 1948, 1950; Nakamura et al., 1951;
Tsunoda et al., 1957; Cho 1964; Ogasawara 1965; Chung 1975). The
common clinical symptoms were nausea, vomiting, diarrhoea, and
abdominal pain. All cases were acute with recovery within a few
days and no lethal cases were encountered. Fusarium fungi, F.
graminearum in particular, were isolated from suspected cereals
(Tochinai, 1933; Tsunoda et al., 1957).
When compared with the symptoms observed in experimental
animals, features of both the above human diseases were similar to
trichothecene toxicosis, notably symptoms caused by DON and NIV,
DAS, and T-2 toxin. However, no epidemiological studies have been
reported that link ATA and scabby grain toxicosis to these
chemicals.
II.5.3 Skin irritation
The Task Group was aware of several reports describing various
effects on the skin of crude extracts of fungal cultures or
solutions containing T-2 toxin, as well as other possible
substances (Bamburg & Strong, 1971; Saito & Otshubo, 1974). These
cases, all of them accidental, involved a very limited number of
persons who developed severe irritation, loss of sensitivity, and
desquamation. Despite the presence of T-2 toxin in the contact
material, there is no evidence that the involvement of other
compounds can be ruled out.
II.5.4 Studies of haemostasis
Platelet function and electron microscopic morphological
changes following T-2 toxin administration were studied on
platelets isolated from 12 healthy human volunteers. When
platelets were incubated with T-2 toxin at doses of 5-500 µg/109
platelets for 20 min, there was a dose-related inhibition of
platelet aggregation with different activators, including
epinephrine, arachidonic acid, and collagen, and a release of dense
bodies consisting mainly of serotonin-containing granules. There
was also a change in membrane permeability, but no changes in
shape. No correlated inhibition of thromboxane synthesis, or
significant alterations in platelet calcium content were observed.
The microtubular system was unaffected. It was suggested that the
above observations , notably suppressed aggregation, played a
contradictory role in the haemorrhagic phenomena associated with
these toxins in man and animals (Yarom et al., 1984a).
II.5.5 Airborne trichothecene-related diseases
High concentrations of spores of Stachybotrys atra were
discovered in the air of living rooms of a suburban house in
Chicago, USA (Croft et al., 1986). Over a period of several years,
the 5 occupants of the house had suffered a variety of non-specific
symptoms including signs of colds, sore throats, diarrhoea,
headaches, dermatitis, intermittent focal alopaecia, and
generalized malaise. Chemical analysis of building materials
supporting the growth of Stachybotrys atra confirmed the presence
of the macrocyclic trichothecenes, verrucarin B and J, satratoxin
H, and trichoverrins A and B. Five weanling Sprague-Dawley rats
and 5 mice, administered extracts of contaminated materials orally,
died within 24 h of exposure, whereas control animals remained
unaffected. Histological lesions of the animals tissues were
degeneration, necrosis and haemorrhage of the brain, thymus,
spleen, intestine, lungs, heart, lymph nodes, liver, and kidneys.
Although the clinical symptoms could have been related to allergic
responses, the isolation of potent mycotoxins suggests that they
were the causal factor of the illness observed.
II.5.6 Toxicological information on man, obtained from
therapeutic uses
DAS (Anguidine) has been undergoing clinical trials as a
chemotherapeutic agent in cancer patients. During a weekly
schedule of DAS, using healthy volunteers as controls, a dose of 5
mg/m2 body surface infused over 3 h, produced nausea, vomiting,
hypotension, neurological symptoms (confusion, hallucinations and
psychomotor seizures), chills, fever, and diarrhoea (DeSimone et
al., 1979). A similar study carried out on 20 other cancer
patients revealed gastrointestinal and neurological toxic effects
(Belt et al., 1979). A bolus administration or rapid infusion of
the drug also caused gastrointestinal and neurological symptoms
(Thigpen et al., 1981). The human haematopoietic system appears to
be extremely sensitive to DAS. Myelosuppression was the dose
limiting adverse effect of prolonged infusions over 8 h (Thigpen et
al., 1981). A mean myelosuppressive dose level in the above
investigation was, 5 mg/m2 body surface by 8-h infusion or, roughly
calculated in terms of body weight, 0.2 mg/kg (0.025 mg/kg per h).
II.6 EVALUATION OF THE HUMAN HEALTH RISKS
On the basis of the data made available to the Task Group, there
is a possible association between trichothecene exposure and
episodes of human disease. According to the limited data
available, the most frequently reported trichothecenes involved in
episodes of human exposure are DON and NIV. In the episodes of
alimentary toxic aleukia and scabby grain toxicosis reported in the
past, a possible etiological role of trichothecenes cannot be
excluded. Exposure occurs through the ingestion of contaminated
food, mainly cereals. Processing, milling, and baking are
not effective in destroying DON, NIV, and T-2 toxin. There is very
limited evidence of exposure through inhalation, but such a
possibility cannot be ruled out.
Among the naturally occurring trichothecenes in foods, T-2
toxin is the most potent, followed by DAS and NIV; DON was the
least toxic in acute toxicity studies. In experimental animals,
T-2 toxin and DAS produce acute systemic effects, with necrosis of
epithelial tissues and suppression of haematopoiesis. In
contemporary outbreaks of disease, only gastrointestinal symptoms
have been reported.
DON was shown to be teratogenic in mice but not in rats.
According to published chronic toxicity studies, NIV and T-2 toxin
are not tumorogenic in animals. No long-term carcinogenicity
studies on DON have been published. Certain trichothecenes, such
as T-2 toxin and DON, have an immunosuppressive action in animals
and have produced alterations in both cell-mediated and humoral
immunity. There is no evidence of immunosuppressive action in man.
Reported cases of human disease associated with trichothecene
exposure are limited in number and information. Symptoms of
digestive disorders and throat irritation develop rapidly after
ingestion of food contaminated with trichothecenes. At present,
there is no evidence of human cancer caused by trichothecenes. No
reports were available to the Task Group on secondary infection, by
bacteria, fungi, or viruses, in human beings following trichothecene
exposure, as has been observed in experimental animal studies. It
appears that adequate studies elucidating such a sequence have not
been made.
III. ERGOT
III.1 PROPERTIES AND ANALYTICAL METHODS
III.1.1 Chemical properties
Ergot is the French word for a rooster's spur and is used as
the common term for sclerotia of fungal species within the genus
Claviceps, in particular C. purpurea. The fungi infect the florets
of grasses and cereal, replacing the florets with compact fungal
structures, sclerotia, 2-20 mm long, somewhat curved, tapering off
at the ends, and strongly coloured, often purple-black, thus
resembling rooster's spurs. The sclerotia contain a large number
of biologically active alkaloids, as well as amino acids,
carbohydrates, lipids, and pigments, and, when the sclerotia are
consumed by man and animals, toxicosis develops, called ergotism.
The topic has been reviewed by Bove (1970), Van Rensburg &
Altenkirk (1974), Lorenz (1979), and Berde & Schild (1978). This
section deals with ergot as a toxic food contaminant; ergot
compounds used as pharmaceutical drugs are excluded.
Ergot alkaloids (ergolines), of which more than 40 have been
isolated from Claviceps sclerotia, are derivatives of lysergic acid
(Fig. 4), and can be divided into 3 groups:
Group I: Derivatives of lysergic acid, e.g., ergotamine.
Group II: Derivatives of isolysergic acid, e.g., ergotaminine.
Group III: Derivatives of dimethylergoline (clavines), e.g.,
agroclavine.
 Physical and chemical properties of some ergolines are listed
in Table 23.
Table 23. Physical and chemical properties of selected
ergot alkaloids (ergolines)a
-----------------------------------------------------------
Ergoline Molecular Melting [alpha]20
formula point D
(°C)
-----------------------------------------------------------
Group I. Derivatives of lysergic acid
ergotamine C33H35O5N5 180 -160
alpha-ergocryptinine C32H41O5N5 212-214 -190
ergocristine C35H39O5N5 160-175 -183
ergosine C30H37O5N5 220-230 -183
ergocornine C31H39O5N5 182-184 -188
ergometrine C19H23O2N3 162 +41
Group II. Derivatives of isolysergic acid
ergocristinine C35H39O5N5 226 +366
ergometrinine C19H23O2N3 196 +414
ergosinine C30H37O5N5 228 +420
ergocorninine C31H39O5N5 228 +409
alpha-ergocryptinine C32H41O5N5 240-243 +480
ergotaminine C33H35O5N5 241-243 +369
Group III. Derivatives of dimethyloergolines (clavines)
agroclavine C16H18N2 206 -151
elymoclavine C16H18ON2 249 -109
chanoclavine C16H20ON2 222 -240
penniclavine C16H18O2N2 222 +153
setoclavine C16H18ON2 229-234 +174
-----------------------------------------------------------
a From: Van Rensberg & Altenkirk (1974) and Lorenz (1979).
III.1.2 Analytical methods for ergot and ergot alkaloids
III.1.2.1 Ergot
Sclerotia of Claviceps species are all rich in triglycerides,
but only a few species have a sufficiently distinctive fatty acid
composition in the sclerotia to provide a basis for identification.
Sclerotia of C. purpurea are unique among microorganisms in
containing a large (approximately 30%) triglyceride fraction in
which ricinoleic acid is the principal (30-40%) component. Thus,
the presence of ricinoleate in a foodstuff known to be free from
other sources of ricinoleic acid (e.g., castor oil) is diagnostic
for the presence of C. purpurea sclerotia. The sample is
saponified, the free fatty acids thereby released are methylated
with diazomethane, and the resulting methyl esters are analysed
using gas-liquid chromatography (Mantle, 1977a). As little as 0.3%
ergot in 1-2 g foodstuff has been detected by this procedure.
III.1.2.2 Ergot alkaloids
Sclerotia may contain up to 1% of total ergot alkaloids.
Lysergic acid derivatives can easily be epimerized at C-8 to give
isolysergic acid derivatives (Group III), thereby removing most of
their biological activity. As this may happen to a variable extent
during extraction, it is difficult to know whether the small
proportion of isolysergic acid derivatives, commonly found during
analysis of extracts from sclerotia, has been generated in whole or
in part during extraction (Mantle, 1977b).
A method for the determination of C. purpurea ergot alkaloids
in flour has been developed, based on liquid chromatography
following extraction of the flour by a mixture of methylene
chloride, ethyl acetate, methanol, and 28% ammonium hydroxide
solution (Scott & Lawrence, 1980). After filtration and
evaporation to dryness, the residue is dissolved in methanol-ether
and extracted with 0.5 N hydrochloric acid. The acid layer is
washed with hexane, made alkaline, re-extracted with methylene
chloride, evaporated, and the residue dissolved in methanol,
ready for liquid chromatographic analysis, with identification
based on fluorescence at an excitation wavelength of 235 nm. The
method has a recovery of 66-93%, based on analysis of flour spiked
with Group I alkaloids (ergotamine, ergocryptine, ergocristine,
ergosine, ergocornine, ergometrine).
A method using high-performance liquid chromatography for the
analysis of human blood has been reported (Zorz et al., 1985).
Samples (5 ml) of human plasma are extracted with benzene-toluene-
ethyl-acetate-diethylamine, the solvent layer separated and
evaporated to dryness. High-performance liquid chromatography is
performed with excitation wavelength at 285 nm for naturally
occurring ergot alkaloids. According to recovery studies,
concentrations as low as 0.2 mg/litre plasma can be detected.
A radioimmunoassay has been developed for determination of
ergotamine and ergocristine (Arens & Zenk, 1980). The alkaloids
were conjugated with bovine serum-albumin, and antibodies raised
in rabbits. Using 3H-labelled tracers, levels as low as 3.5 pmol
of ergotamine and 0.8 pmol of ergocristine could be measured. The
antibodies were highly specific, and simple lysergic acid
derivatives and clavines did not cross-react. The procedure has
been used in the detection of sclerotia of C. purpurea with a high
alkaloid concentration for industrial bioproduction.
A liquid chromatographic method for the detection of
ergotamine, ergotaminine, and ergocristine in human plasma has been
developed using extraction at pH 9, clean-up, and an ODS-hypersil
reverse-phase column (Edlund, 1981). Levels of ergolines as low as
0.1 mg/litre in 3 ml plasma samples can be detected, with a
recovery of 79-99% for the 3 ergolines.
Ergot contamination of pearl millet, due to infection by C.
fusiformis, is characterized by the presence of clavine alkaloids
(Group III). A procedure for its determination has been developed
using thin-layer chromatographic separation and spectrophotometric
detection following colour reaction using Van Urk's reagent (Bhat
et al., 1976; Krishnamachari & Bhat, 1976). The procedure includes
defatting of the grain sample, mixing of the defatted material with
ammonium hydroxide, extraction with diethyl-ether followed by
extraction of the diethyl-ether phase with 0.1 N sulfuric acid.
The extract is made alkaline and extracted with chloroform,
followed by thin-layer chromatographic separation.
III.2 SOURCES AND OCCURRENCE
III.2.1 Fungal producers
Sclerotia are compact hyphal structures that develop in the
colonies of many fungal genera. The sclerotia of species within
the genus Claviceps are unique in terms of size (length up to
several cm), pronounced colour, and because the sclerotia of
several species contain highly biologically active compounds, the
alkaloids. The sclerotia develop during the infection of plants;
host plants for the Claviceps species mainly belong to the grass
family (Gramineae), which comprises the true cereals. However, a
few plant species within the family Juncaceae and Cyperaceae can
also act as hosts. Most Claviceps species have a monogeneric host
range, but C. purpurea is unique in that it has a very wide host
range (Van Rensburg & Altenkirk, 1974; Lorenz, 1979). The eight
leading cereals produced in the world are wheat, rice, corn,
sorghum, rye, barley, oats, and millet, and they can all be hosts
for Claviceps species. Man and animals are exposed to toxic
sclerotia from two species, C. purpurea and C. fusiformis; farm
animals are also exposed to toxic sclerotia from C. paspali,
growing on grass. Sclerotia of C. purpurea have the dimension of
2-20 x 1-6 mm and are purple-black in colour, whereas C. fusiformis
has small (2 x 4 mm) purple-red coloured sclerotia (Loveless, 1967;
Siddiqui & Khan, 1973; Mantle, 1977b). C. purpurea primarily
attacks cross-pollinated species in which the florets tend to
remain open for a relatively long time and in which sterility
occurs.
The cereals most commonly contaminated with ergot from C.
purpurea are rye, wheat, triticale (the cross-breed between wheat
and rye), barley, oats, and sorghum. The ergot of C. purpurea
contain Group I and II ergolines as the principal ergot alkaloids.
C. fusiformis is a parasite of pearl millet (Pennisetum typhoideum)
in Africa and East Asia; in India, the cereal is called bajra.
Ergot of C. fusiformis contain predominantly Group III ergolines.
Ergot alkaloids have been isolated from fungi outside the genus
Claviceps (Aspergillus fumigatus, A. clavatus, A. nidulans,
Rhizopus nigricans, Penicillium chermesinum, P. concavo-rugulosum,
P. sizovae) and from higher plants (Rivea corymbosa, Ipomoea
violacea, I. argyrophylla, I. hildebrandtii, I. tricolor) (Van
Rensburg & Altenkirk, 1974; Kozlovsky & Reshetilova, 1984).
Whether these sources represent human exposure is not known at
present.
Successful attempts have been made to produce ergot alkaloids
using C. purpurea cultures in liquid media under laboratory
conditions, and new alkaloids have been identified (Bianchi et al.,
1982).
III.2.2 Biosynthesis
The ergoline ring system in ergot fungi is built up from L-
tryptophan and mevalonic acid (Fig. 5). The N-methyl group of
the ergot alkaloids is derived from methionine via a
transmethylation reaction. The precursors of tryptophan are
indole, anthranilic acid, and indolpyruvic acid (Van Rensburg &
Altenkirk, 1974; Mantle, 1977b). It appears that Group III
ergolines are intermediates in the production of Group I and II
ergolines by C. purpurea.
Physical and chemical properties of some ergolines are listed
in Table 23.
Table 23. Physical and chemical properties of selected
ergot alkaloids (ergolines)a
-----------------------------------------------------------
Ergoline Molecular Melting [alpha]20
formula point D
(°C)
-----------------------------------------------------------
Group I. Derivatives of lysergic acid
ergotamine C33H35O5N5 180 -160
alpha-ergocryptinine C32H41O5N5 212-214 -190
ergocristine C35H39O5N5 160-175 -183
ergosine C30H37O5N5 220-230 -183
ergocornine C31H39O5N5 182-184 -188
ergometrine C19H23O2N3 162 +41
Group II. Derivatives of isolysergic acid
ergocristinine C35H39O5N5 226 +366
ergometrinine C19H23O2N3 196 +414
ergosinine C30H37O5N5 228 +420
ergocorninine C31H39O5N5 228 +409
alpha-ergocryptinine C32H41O5N5 240-243 +480
ergotaminine C33H35O5N5 241-243 +369
Group III. Derivatives of dimethyloergolines (clavines)
agroclavine C16H18N2 206 -151
elymoclavine C16H18ON2 249 -109
chanoclavine C16H20ON2 222 -240
penniclavine C16H18O2N2 222 +153
setoclavine C16H18ON2 229-234 +174
-----------------------------------------------------------
a From: Van Rensberg & Altenkirk (1974) and Lorenz (1979).
III.1.2 Analytical methods for ergot and ergot alkaloids
III.1.2.1 Ergot
Sclerotia of Claviceps species are all rich in triglycerides,
but only a few species have a sufficiently distinctive fatty acid
composition in the sclerotia to provide a basis for identification.
Sclerotia of C. purpurea are unique among microorganisms in
containing a large (approximately 30%) triglyceride fraction in
which ricinoleic acid is the principal (30-40%) component. Thus,
the presence of ricinoleate in a foodstuff known to be free from
other sources of ricinoleic acid (e.g., castor oil) is diagnostic
for the presence of C. purpurea sclerotia. The sample is
saponified, the free fatty acids thereby released are methylated
with diazomethane, and the resulting methyl esters are analysed
using gas-liquid chromatography (Mantle, 1977a). As little as 0.3%
ergot in 1-2 g foodstuff has been detected by this procedure.
III.1.2.2 Ergot alkaloids
Sclerotia may contain up to 1% of total ergot alkaloids.
Lysergic acid derivatives can easily be epimerized at C-8 to give
isolysergic acid derivatives (Group III), thereby removing most of
their biological activity. As this may happen to a variable extent
during extraction, it is difficult to know whether the small
proportion of isolysergic acid derivatives, commonly found during
analysis of extracts from sclerotia, has been generated in whole or
in part during extraction (Mantle, 1977b).
A method for the determination of C. purpurea ergot alkaloids
in flour has been developed, based on liquid chromatography
following extraction of the flour by a mixture of methylene
chloride, ethyl acetate, methanol, and 28% ammonium hydroxide
solution (Scott & Lawrence, 1980). After filtration and
evaporation to dryness, the residue is dissolved in methanol-ether
and extracted with 0.5 N hydrochloric acid. The acid layer is
washed with hexane, made alkaline, re-extracted with methylene
chloride, evaporated, and the residue dissolved in methanol,
ready for liquid chromatographic analysis, with identification
based on fluorescence at an excitation wavelength of 235 nm. The
method has a recovery of 66-93%, based on analysis of flour spiked
with Group I alkaloids (ergotamine, ergocryptine, ergocristine,
ergosine, ergocornine, ergometrine).
A method using high-performance liquid chromatography for the
analysis of human blood has been reported (Zorz et al., 1985).
Samples (5 ml) of human plasma are extracted with benzene-toluene-
ethyl-acetate-diethylamine, the solvent layer separated and
evaporated to dryness. High-performance liquid chromatography is
performed with excitation wavelength at 285 nm for naturally
occurring ergot alkaloids. According to recovery studies,
concentrations as low as 0.2 mg/litre plasma can be detected.
A radioimmunoassay has been developed for determination of
ergotamine and ergocristine (Arens & Zenk, 1980). The alkaloids
were conjugated with bovine serum-albumin, and antibodies raised
in rabbits. Using 3H-labelled tracers, levels as low as 3.5 pmol
of ergotamine and 0.8 pmol of ergocristine could be measured. The
antibodies were highly specific, and simple lysergic acid
derivatives and clavines did not cross-react. The procedure has
been used in the detection of sclerotia of C. purpurea with a high
alkaloid concentration for industrial bioproduction.
A liquid chromatographic method for the detection of
ergotamine, ergotaminine, and ergocristine in human plasma has been
developed using extraction at pH 9, clean-up, and an ODS-hypersil
reverse-phase column (Edlund, 1981). Levels of ergolines as low as
0.1 mg/litre in 3 ml plasma samples can be detected, with a
recovery of 79-99% for the 3 ergolines.
Ergot contamination of pearl millet, due to infection by C.
fusiformis, is characterized by the presence of clavine alkaloids
(Group III). A procedure for its determination has been developed
using thin-layer chromatographic separation and spectrophotometric
detection following colour reaction using Van Urk's reagent (Bhat
et al., 1976; Krishnamachari & Bhat, 1976). The procedure includes
defatting of the grain sample, mixing of the defatted material with
ammonium hydroxide, extraction with diethyl-ether followed by
extraction of the diethyl-ether phase with 0.1 N sulfuric acid.
The extract is made alkaline and extracted with chloroform,
followed by thin-layer chromatographic separation.
III.2 SOURCES AND OCCURRENCE
III.2.1 Fungal producers
Sclerotia are compact hyphal structures that develop in the
colonies of many fungal genera. The sclerotia of species within
the genus Claviceps are unique in terms of size (length up to
several cm), pronounced colour, and because the sclerotia of
several species contain highly biologically active compounds, the
alkaloids. The sclerotia develop during the infection of plants;
host plants for the Claviceps species mainly belong to the grass
family (Gramineae), which comprises the true cereals. However, a
few plant species within the family Juncaceae and Cyperaceae can
also act as hosts. Most Claviceps species have a monogeneric host
range, but C. purpurea is unique in that it has a very wide host
range (Van Rensburg & Altenkirk, 1974; Lorenz, 1979). The eight
leading cereals produced in the world are wheat, rice, corn,
sorghum, rye, barley, oats, and millet, and they can all be hosts
for Claviceps species. Man and animals are exposed to toxic
sclerotia from two species, C. purpurea and C. fusiformis; farm
animals are also exposed to toxic sclerotia from C. paspali,
growing on grass. Sclerotia of C. purpurea have the dimension of
2-20 x 1-6 mm and are purple-black in colour, whereas C. fusiformis
has small (2 x 4 mm) purple-red coloured sclerotia (Loveless, 1967;
Siddiqui & Khan, 1973; Mantle, 1977b). C. purpurea primarily
attacks cross-pollinated species in which the florets tend to
remain open for a relatively long time and in which sterility
occurs.
The cereals most commonly contaminated with ergot from C.
purpurea are rye, wheat, triticale (the cross-breed between wheat
and rye), barley, oats, and sorghum. The ergot of C. purpurea
contain Group I and II ergolines as the principal ergot alkaloids.
C. fusiformis is a parasite of pearl millet (Pennisetum typhoideum)
in Africa and East Asia; in India, the cereal is called bajra.
Ergot of C. fusiformis contain predominantly Group III ergolines.
Ergot alkaloids have been isolated from fungi outside the genus
Claviceps (Aspergillus fumigatus, A. clavatus, A. nidulans,
Rhizopus nigricans, Penicillium chermesinum, P. concavo-rugulosum,
P. sizovae) and from higher plants (Rivea corymbosa, Ipomoea
violacea, I. argyrophylla, I. hildebrandtii, I. tricolor) (Van
Rensburg & Altenkirk, 1974; Kozlovsky & Reshetilova, 1984).
Whether these sources represent human exposure is not known at
present.
Successful attempts have been made to produce ergot alkaloids
using C. purpurea cultures in liquid media under laboratory
conditions, and new alkaloids have been identified (Bianchi et al.,
1982).
III.2.2 Biosynthesis
The ergoline ring system in ergot fungi is built up from L-
tryptophan and mevalonic acid (Fig. 5). The N-methyl group of
the ergot alkaloids is derived from methionine via a
transmethylation reaction. The precursors of tryptophan are
indole, anthranilic acid, and indolpyruvic acid (Van Rensburg &
Altenkirk, 1974; Mantle, 1977b). It appears that Group III
ergolines are intermediates in the production of Group I and II
ergolines by C. purpurea.
 III.2.3 Occurrence in foodstuffs
Traditionally, contamination of grain with ergot has been
expressed as a percentage on a weight basis, without measurement of
total and individual amounts of ergolines. Thus, it is generally
recommended that feed containing more than 0.1% ergot should not be
given to animals (Young, 1981a,b). Quantitative analysis for total
and individual ergolines in 14 samples of rye and wheat flour
(Scott & Lawrence, 1980) indicated contamination with 6 Group I
ergolines (ergometrine, ergosine, ergotamine, ergocornine,
ergocryptine, ergocristine) at concentrations ranging from 0.3 to
62 µg/kg for individual ergolines. Ergocristine was the major
ergoline present in the flours, 62 µg/kg being the maximal
concentration found. In 17 samples of rye grain collected from
health shops, 6 contained ergot; ergoline concentration and
composition were not indicated (Akerstrand, 1980). In a survey of
ergolines in ergot-contaminated cereals, in North America, the
average total ergolines content was 0.24% (Young, 1981a,b; Young &
Chen, 1982). On average, the pooled ergoline composition in
sclerotia in rye, wheat, and triticale was as follows:
ergocristine (31%), ergocristinine (13%), ergotamine (17%),
ergotaminine (8%), ergocryptine (5%), ergocryptinine (3%),
ergometrine (5%), ergometrinine (2%), ergosine (4%), ergosinine
(2%), ergocornine (4%), and ergocorninine (2%). The individual
ergoline composition was uniform throughout a single sclerotium or
in different sclerotia from the same head, somewhat less uniform
between different fields throughout a region, and highly variable
from head-to-head in a given field. In contrast, the total
ergoline content was highly variable within-sclerotium, within-
head, head-to-head, and on a field-to-field basis. In a
comparative study of ergoline in ergots from rye and wheat (caused
by infection with C. purpurea) and in ergots from pearl millet
(caused by infection with C. fusiformis) in South-East Asia, it was
found that the total ergoline content in the sclerotia of pearl
millet was much lower (320 mg/kg) than that in the sclerotia of rye
(700 mg/kg) and wheat (920 mg/kg) (Bhat et al., 1976). The
ergolines in the sclerotia of pearl millet were reported to
comprise agroclavine, elymoclavine, chanoclavine, penniclavine, and
setoclavine.
In a survey of cereals and cereal products on the Swiss market,
using a high-performance liquid chromatography procedure, the
following average concentrations of total ergolines were found:
wheat flour, 4.2 µg/kg; wheat flour (coarse), 30.7 µg/kg; wheat
flour (more coarse), 103.4 µg/kg; rye flour, 139.7 µg/kg; and
"bioproducts", 10.2-22.7 µg/kg (Baumann et al., 1985). The daily
intake of total ergolines by human beings in Switzerland was
estimated to be 5.1 µg/person.
III.2.4 Fate of ergolines during food processing
Treatment of sclerotia from wheat with chlorine (1%) and heat
(150-200 °C) resulted in a 90% reduction in ergoline content within
4 h (Young et al., 1983). The reduction affected all the ergolines
(ergotamine, ergocornine, ergocryptine, ergosine, ergometrine) in
identical ways. Autoclaving sclerotia at 121 °C for 30 min
resulted in a 24.6% reduction in total ergoline content. In the
baking of bread and pancakes using grain that contained naturally
occurring ergot, a 59-100% reduction in the individual ergolines
(ergosine, ergocornine, ergometrine, ergotamine, alpha-
ergocryptine, ergocristine) was observed in whole wheat bread, a
50-86% reduction in all-rye flour bread, and a 25-74% reduction in
triticale pancakes (Scott & Lawrence, 1982).
When bread made of rye flour spiked with finely ground
sclerotia of C. purpurea, (continuing a total ergoline
concentration of 312.8 µg/kg), was baked, there was an overall
reduction of 50% in the ergoline concentration, as measured by high
performance liquid chromatography (Baumann et al., 1985).
III.3 METABOLISM
No published information is available on the metabolism in
animals or human beings of ergots containing ergolines and
combinations of individual ergolines.
III.4 EFFECTS ON ANIMALS
III.4.1 Field studies
An outbreak of bovine abortion associated with the ingestion of
ergot was reported by Appleyard (1986).
Eleven out of 36 suckler cows, all in late pregnancy, aborted
in 7-11 days following introduction to a rye grass pasture heavily
infested with ergot. At least 25% of the rye seed heads contained
sclerotia of C. purpurea, with up to 8 sclerotia present on any one
seed head. The sclerotia contained 1.57 mg total ergolines/g,
consisting of 67% ergotamine and 17% ergotaminine, and smaller
amounts of ergometrine. Ergocryptine and ergosine were also
present together with their corresponding -inine isomers.
Ten out of the 11 calves were delivered dead. None of the
aborting cows showed any premonitory signs of calving and, after
parturition, there was almost complete agalactia. The placenta was
retained in each case, but there was no other evidence of ill
health in the cows. Any other cause of abortion of bacterial,
viral, fungal, or toxic origin was ruled out, on the basis of
laboratory and field investigations.
III.4.2 Experimental animal studies
This topic has been reviewed by Ainsworth & Austwick (1959) and
Mantle (1977c). It appears that most reports of field cases and
experimental animal studies on ergotism do not contain any
information on the contents of individual ergolines in the ergot
associated with disease manifestations in animals.
III.4.2.1 Cattle
Lameness, sometimes leading to gangrene, is a common symptom
observed in cattle when the feed contains more than 10 g ergot/kg.
The symptoms are more pronounced when the animals are kept outside
under cold weather conditions (Mantle, 1977c). Four out of 6
animals administered ergotamine tartrate, orally, at 1 mg/kg body
weight per day, died within 10 days (Woods et al., 1966). The
animals became acutely ill within 1-2 days, the principal signs
being anorexia, hyperventilation, cold extremities, salivation,
and, occasionally, tongue necrosis. Post-mortem examination of the
most seriously affected animals revealed extensive intestinal
inflammation.
III.4.2.2 Sheep
Four lambs were administered aqueous suspensions of milled
ergot (from C. purpurea) through a stomach tube, over a 2-month
period (Loken, 1984). The doses ranged from 0.12 to 0.75 g
sclerotia/kg body weight. The sclerotia contained approximately 4
g ergolines/kg, composed of ergotamine (15%), ergosine (35%), and
ergocristine (5% each). One lamb, dosed with 0.12 g sclerotia/kg
body weight, was kept indoors at 15-17 °C and did not develop any
symptoms. The other 3 animals, given higher doses and kept
outdoors, became ill after 2-6 days, with signs that included
dullness, inappetence, high pulse rate, diarrhoea, edema of the
hind legs and tail, and lameness. Post-mortem findings included
inflammation and necrosis of the forestomach and intestinal mucosa.
III.4.2.3 Poultry
Leghorn chickens were fed diets containing ergotamine tartrate
at levels of up to 800 mg/kg in 7 to 10-day trials as well as in a
51-day trial (Young & Marquardt, 1982). In the short-term
trials, only the highest level (800 mg/kg) had an effect on
performance, in terms of a slight decrease in growth rate and a
slight increase in feed consumption. At the 250 mg/kg level, toe
necrosis was observed, as well as cardiomegaly. There were no
pathological effects on the brain, liver, or muscle tissues, even
at the highest level. In the long-term study (51 days), effects
were similar to those observed in the 7 to 10-day trials. No
residues of ergotamine were detected in tissues.
III.4.2.4 Swine
Ergot (from C. purpurea) containing 0.3% ergolines
(composition: ergotamine and ergosine) was used in a feeding study
on pigs (Mantle, 1977c). A diet containing ergot at 40 g/kg was
well tolerated, and the only effect at 100 g ergot/kg diet was
depression of the growth rate. Agalactia in the sow was observed
after feeding with rations containing ergot from C. purpurea as
well as from C. fusiformis.
III.4.2.5 Primates
Male rhesus monkeys in groups of 2-4 animals were dosed with
ergot from C. fusiformis, either as part of the diet, or as an
ergoline extract administered orally or intraperitoneally (Bhat &
Roy, 1976). The ergoline extract contained agroclavine (major
components: elymoclavine, chanoclavine, penniclavine, and
setoclavine). The period of treatment lasted from 2 days to one
month. There were no effects on the animals, with the exception of
those injected ip with 5.44-11.1 mg total ergoline/kg body weight,
who developed signs within 10 min including drowsiness,
hyperexcitation, redness of face, and loss of response to thermal
and tactile stimuli in the hind limbs and tail. The animals
recovered spontaneously in about 60 min. Animals administered a
total of 10 mg ergolines/kg body weight, orally, showed symptoms of
hyperexcitation, but these were far less severe than those after
intraperitoneal injection. It was concluded that the signs in
monkeys, in particular hyperexcitation, are different from those
observed in human beings after ingestion of ergot in pearl millet.
III.5 EFFECTS ON MAN
The history of ergotism in man, following ingestion of ergot
from C. purpurea, was reviewed by Barger (1931). Numerous
epidemics in Europe occurred between the 9th and the 18th century.
Two types of disease were noted: gangrenous and convulsive
ergotism. In the first type, the affected part (arm or leg)
shrank, became mummified and dry, and the gangrene gradually spread
upwards. In convulsive ergotism, the whole body was attacked by
general convulsion, which returned at intervals of a few days. The
latest outbreaks of ergotism in Europe occurred in 1926-28 in the
United Kingdom and the USSR. A suspected episode in France in 1951
turned out to be due to a different toxic substance (Gadiou, 1965).
III.5.1 Ergometrine-related outbreaks
In 1978, an epidemic was reported in Ethiopia (Demeke et al.,
1979; King, 1979; Pokrovskij & Tutelyan, 1982). The episode
occurred in the Wollo region, following two years of drought.
During this time, the locally grown barley, the staple food, had
become dominated by wild oats heavily contaminated with C. purpurea
sclerotia. The grain consisted of 70% wild oats, 12% barley, and
0.75% ergot; ergometrine was detected in the sclerotia by thin
layer chromatography. A total of 93 cases of ergotism was reported
during the spring of 1978. The male:female ratio was 2.5:1; more
than 80% of affected persons were between five and 34 years of age.
In addition to the 93 cases, 47 deaths were reported as having been
due to ergotism. Examination of 44 patients out of the 93
registered revealed ongoing dry gangrene of the whole or part of
one or more limbs (7.5%), feeble or absent peripheral pulses
(36.4%), swelling of limbs (11.2 %), desquamation of the skin
(12.8%), and loss of one or more limbs (21.5%). It was noted that
88% of patients had involvement of the lower extremities. The most
common general symptoms were weakness (78.5%), formication (15%),
burning sensation (14.3%), nausea (7.2%), vomiting (5.6%), and
diarrhoea (6.8%). In addition, 50-60 infants and young children
died from starvation due to failure of the mothers to lactate.
This may have been related to the effect of ergot on lactation
(Demeke et al., 1979). No autopsies were performed, and thus there
is no information on pathological changes in the viscera.
III.5.2 Clavine-related outbreaks
Intoxication following ingestion of ergot from C. fusiformis in
bajra or pearl millet has been reported from India. Symptoms
included nausea, vomiting, and giddiness. Several outbreaks have
been observed since 1958, when the first report was published; the
latest occurred in the autumn of 1975 in the state of Rajasthan
(Krishnamachari & Bhat, 1976). In 21 villages surveyed, 78 persons
belonging to 14 households developed symptoms, characterized by
nausea, repeated vomiting, and giddiness, followed by drowsiness
and prolonged sleepiness, extending sometimes to over 24-48 h.
There were no signs or symptoms suggesting vaso-occlusion. The
disease generally developed 1-2 h following a single meal.
Domestic camels, offered the contaminated grain as feed, also
developed sleepiness and signs suggesting abdominal discomfort.
The pearl millet from affected villages contained 15-174 g
ergot/kg, resulting in a contamination of the grain with 15-199 mg
total ergolines/kg. The individual ergolines were identified as
agroclavine, elymoclavine, chanoclavine, penniclavine, and
setoclavine. Pearl millet from villages with no cases of
intoxication contained 1-38 g ergot/kg with a total ergoline
content of 15-26 mg/kg. Since there were no deaths, no information
on pathological changes is available. The number of households
studied was too small for no-effect levels to be calculated, but
the authors suggested that the intake of 28 µg total ergolines/kg
body weight would be non-toxic.
III.6 EVALUATION OF THE HUMAN HEALTH RISKS
Human exposure to low levels of ergolines appears to be
widespread. Available data from the recent outbreaks in Ethiopia
and India indicate that the C. purpurea alkaloids (ergotamine
group) produced more severe effects, including gangrene of the legs
and death, than the alkaloids of C. fusiformis (clavine group),
which caused gastrointestinal symptoms without a fatal outcome.
It is not known whether such differences can be accounted for by
differences in the alkaloid content of the fungal species, in the
toxicological or toxicometric properties of the alkaloids, or in
the levels of intake by different types of populations.
Only low levels of ergolines remain in prepared foods as
cleaning and milling processes remove the sclerotia; baking or
other heat processing also destroys most alkaloids of the ergotamin
group.
REFERENCES
ABBAS, H.K., MIROCHA, C.J., PAWLOSKY, R.J., & PUCSH, D.J. (1985)
Effect of cleaning, milling, and baking on deoxynivalenol in wheat.
Appl. environ. Microbiol., 50(2): 482-486.
ABBAS, H.K., MIROCHA, C.J., & TUITE, J. (1986) Natural occurrence of
deoxynivalenol, 15-acetyl-deoxynivalenol and zearalenone in refusal
factor corn stored since 1972. Appl. environ. Microbiol., 51:841-843.
ABDEL-HAFEZ, S.I.I., EL-KADY, I.A., MAZEN, M.B., & EL-MAGHRABY,
O.M.O. (1987) Mycoflora and trichothecene toxins of paddy grains from
Egypt. Mycopathologia, 100: 103-112.
ABRAHAMSON, D., MILLS, J.T., & BOYCOTT, B.R. (1983) Mycotoxins and
mycoflora in animal feedstuffs in western Canada. Can. J. comp. Med.,
47: 23-26.
AGRELO, C.E. & SCHOENTAL, R. (1980) Synthesis of DNA in human
fibroblasts treated with T-2 toxin and HT-2 toxin (the trichothecene
metabolites of Fusarium species) and the effects of hydroxyurea.
Toxicol. Lett., 5(2): 155-160.
AINSWORTH, G.C. & AUSTWICK, P.K.C. (1959) Fungal diseases of
animals, Farnham Royal, Commonwealth Agricultural Bureaux, pp. 100-
111.
AKERSTRAND, K. (1980) [Ergots.] Var Föda, 32: 442-446 (in Swedish).
AKERSTRAND, K. & JOSEFSON, E. (1979) [Fungi and mycotoxins in beans
and peas.] Var Föda, 31: 405-414 (in Swedish).
APPELGREN, L.-E. & ARORA, R.G. (1983) Distribution of 14C-labelled
ochratoxin A in pregnant mice. Food chem. Toxicol., 21: 563-568.
APPLEYARD, W.T. (1986) Outbreak of bovine abortion attributed to
ergot poisoning. Vet. Res., 118: 48-49.
ARENS, H. & ZENK, M.H. (1980) [Radioimmunoassay for the
determination of the peptide alkaloids ergotamine and ergocristine in
Claviceps purpurea.] Planta med., 38: 214-226 (in German).
ARORA, R.G., FROLEN, H., & FELLNER-FELDEGG, H. (1983) Inhibition of
ochratoxin A teratogenesis by zearalenone and diethylstilboestrol.
Food chem. Toxicol., 21: 779-783.
ATKINSON, H.A.C. & MILLER, K. (1984) Inhibitory effect of
deoxynivalenol, 3-acetyldeoxynivalenol and zearalenone on induction
of rat and human erythrocyte proliferation. Toxicol. Lett, 23: 215-221.
BALZER, I., BOGDANIC, C., & MUZIC, S. (1977) Natural contamination
of corn (Zea mays) with mycotoxins in Yugoslavia. Ann. Nutr.
Aliment., 31(4-6): 425-430.
BAMBURG, J.R. (1976) Chemical and biochemical studies of the
trichothecene mycotoxins. In: Rodricks, J.V., ed. Mycotoxins and
other fungal related food problems, Washington, DC, American Chemical
Society, pp. 144-162 (Advances in Chemistry Series No. 149).
BAMBURG, J.R. & STRONG, F.M. (1969) Mycotoxins of the trichothecene
family produced by Fusarium tricinctum and Trichoderma lignorum.
Phytochemistry, 8(12): 2405-2410.
BAMBURG, J.R. & STRONG, F.M. (1971) 12,13-Epoxytrichothecenes. In:
Kadis, S., Ciegler, A., & Ajl, S.J. ed. Microbiological toxins. VII.
Algal and fungal toxins, New York, London, Academic Press, pp. 207-
292.
BAMBURG, J.R., MARASAS, W.F., RIGGS, N.V., SMALLEY, E.B., & STRONG,
F.M. (1968a) Toxic compounds from Fusaria and other Hyphomycetes.
Biotechnol. Bioeng., 10(4): 445-455.
BAMBURG, J.R., RIGGS, N.V., & STRONG, F.M. (1968b) The structures
of toxins from two strains of Fusarium tricinctum. Tetrahedron,
24(8): 3329-3336.
BARGER, G. (1931) Ergot and ergotism, London, Gurney & Jackson,
279 pp.
BATA, A., VANYI, A., & LASZTITY, R. (1983) Simultaneous detection of
some fusariotoxins by gas-liquid chromatography. J. Assoc. Off. Anal.
Chem., 66: 577-581.
BAUER, J. & GAREIS, M. (1987) [Ochratoxin A in the food chain.] J.
vet. Med., B34: 613-627 (in German).
BAUER, V.J., WERMTER, S., & GEDEK, B. (1980) Contamination of
feedstuffs with toxin-producing strains of Fusaria and their toxins.
Wiener Tieraerztl. Monatschrift., 67(10): 282-288.
BAUER, J., GAREIS, M., & GEDEK, B. (1984) [Detection and occurrence
of ochratoxin A in pigs for slaughter]. Berl. Muench. Tieraerztl.
Wschr. 97: 279-283 (in German).
BAUER, J., BOLLWAHN, W., GARCIS, M., GEDEK, B., & HEINRITZI, K.
(1985) Kinetic profiles of diacetoxyscirpenol and two of its
metabolites in blood serum of pigs. Appl. environ. Microbiol.,
49: 642-845.
BAUER, J., NIEMIEC, J. & SCHOLTYSSEK, S. (1988) [Ochratoxin A in feed
for laying hens. Second communication: Residues in serum, liver and
eggs.] Arch. Geflügelkd., 52: 71-75 (in German).
BAUMANN, U., HUNZIKER, H.R., & ZIMMERLI, B. (1985) [Ergot alkaloids
in Swiss grain products.] Mitt. Geb. Lebensm. Hyg., 76: 609-630 (in
German).
BEASLEY, V.R., SWANSON, S.P., CORLEY, R.A., BUCK, W.B., KORITZ, G.D.,
& BURMEISTER, H.R. (1986) Pharmacokinetics of the trichothecene
mycotoxin, T-2 toxin, in swine and cattle. Toxicon, 21: 13-23.
BEGLEY, P., FOULGER, B.E., JEFFERY, P.D., BLACK, R.M., & READ, R.W.
(1986) Detection of trace levels of trichothecenes in human blood
using capillary gas chromatography-electron-capture negative ion
chemical ionisation mass spectrometry. J. Chromatogr., 367: 87-101.
BELT, R.J., HAAS, C.D., JOSEPH, U., GOODWIN, W., MOORE, D., &
HOOGSTRATEN, B. (1979) Phase I study of anguidine administered
weekly. Cancer Treat. Rep., 63: 1993-1995.
BENDELE, S.A., CARLTON, W.W., KROGH, P., & LILLEHOJJ, E.B. (1985)
Ochratoxin A carcinogenesis in the (C57BL/6YxC3HF)1 mouse. J. Natl
Cancer Inst., 75: 733-739.
BERDE, B. & SCHILD, H.O. (1978) Ergot alkaloids and related
compounds, Berlin, Springer Verlag, pp. 1-1003.
BERGMANN, F., YAGEN, B., & SOFFER, D. (1985) Toxic and lethal effects
of T-2 toxin upon intracerebral administration to rats. Arch.
Toxicol., 58: 40-44.
BERNDT, W.O. & HAYES, A.W. (1979) In vivo and in vitro changes in
renal function caused by ochratoxin A in the rat. Toxicology, 12:
5-17.
BHAT, R.V. & ROY, D.N. (1976) Toxicity study of ergoty bajra (pearl
millet) in rhesus monkeys. Indian J. med. Res., 64: 1629-1633.
BHAT, R.V., ROY, D.N., & TULPULE, P.G. (1976) The nature of
alkaloids of ergoty pearl millet or bajra and its comparison with
alkaloids of ergoty rye and ergoty wheat. Toxicol. appl. Pharmacol.,
36: 11-17.
BHAT R.V., RAMAKRISHNA, Y., RAO, B.S., & NAHDI, S. (1987a)
Trichothecene mycotoxicosis, Hyderabad, India, Food and Drug
Toxicology Research Centre, National Institute of Nutrition.
BHAT, R.V., RAMAKRISHNA, Y., RAO, B.S,. & NAHDI, S. (1987b).
Trichothecene mycotoxicosis, Hyderabad, India, Food and Drug
Toxicology Research Centre, National Institute of Nutrition.
BHAT, R.V., BEEDU, S.R., RAMAKRISHNA, Y., & MUNSHI, K.L. (1989a)
Outbreak of trichothecene mycotoxicosis associated with consumption
of mould-damaged wheat products in Kashmir valley, India. Lancet, 7
January: 35-37.
BHAVANISHANKAR, T.R. & SHANTHA, T. (1987) Natural occurrence of
Fusarium toxins in peanuts, sorghum and maize from Mysore (India).
J. Sci. Food. Agric., 40: 327-332.
BHAVANISHANKAR, T.R., RAMESH, H.P., & SHANTHA, T. (1988) Dermal
toxicity of Fusarium toxins in combinations. Arch. Toxicol., 61:
241-244.
BIANCHI, M.L., PERELLINO, N.C., GIOIA, B., & MINGHETTI, A. (1982)
Production by Claviceps purpurea of two new peptide ergot alkaloids
belonging to a new series containing alpha-aminobutyric acid. J. nat.
Prod., 445: 191-196.
BILAI, V.I. (1977) [Fuzarii,] Kiev, USSR, Naukova Dumka, (in
Russian).
BLACK, R.M., CLARKE, R.J., & READ, R.W. (1986) Detection of trace
levels of trichothecene mycotoxins in human urine by gas
chromatography-mass spectrometry. J. Chromatogr., 367: 103-115.
BLACK, R.M., CLARKE, R.J., & READ, R.W. (1987) Detection of trace
levels of trichothecene mycotoxins in environmental residues and
foodstuffs using gas chromatography with mass spectrometric or
electron-capture detection. J. Chromatogr., 388: 365-378.
BLASS, W., KELLERT, M., STEINMEYER, S., TIEBACH, R., & WEBER, R.
(1984) Determination of deoxynivalenol and nivalenol in cereals at
the mg/kg concentrations range. Z. Lebensm. Unters. Forsch., 80:
104-108.
BODON, L. & ZOLDAG, L. (1974) Cytotoxicity studies on T-2
fusariotoxin. Acta vet. Acad. Sci. Hung., 24(3): 451-455.
BOHNER, F.E., FETZ, E., HARRI, E., SIGG, H.P., STOLL, CH., & TAMM,
CH. (1965) Verrucarine and roridin. VIII. Isolation of verrucarin
H, verrucarin J, roridin D, and roridin E from Myrothecium species.
Helv. Chim. Acta, 48(5): 1079-1089.
BOONCHUVIT, B., HAMILTON, P.B., & BURMEISTER, H.R. (1975)
Interaction of T-2 toxin with Salmonella infections of chickens.
Poult. Sci., 54(5): 1693-1696.
BOORMAN, G. (1988) Technical report on the toxicology and
carcinogenesis studies of ochratoxin A in F334/N rats. National
Toxicology Program, US Department of Health and Human Services,
Research Triangle Park, North Carolina (NIH Publication No. 88-28/3).
BOORMAN, G.A., HONG, H.L., DIETER, M.P., HAYES, H.T., POHLAND, A.E.,
STACK, M., & LUSTER, M.I. (1984) Myelotoxicity and macrophage
alteration in mice exposed to ochratoxin A. Toxicol. appl.
Pharmacol., 72: 304-312.
BOVE, F.J. (1970) The story of ergot, Basel, S. Karger, 297 pp.
BRADLAW, J.A., SWENTZEL, K.C., ALTERMAN, E., & HAUSWIRTH, J.W. (1985)
Evaluation of purified 4-deoxynivalenol (vomitoxin) for unscheduled
DNA synthesis in the primary rat hepatocyte-DNA repair assay. Food
chem. Toxicol., 23: 1063-1067.
BRIAN, P.W., DAWKINS, A.W., GROVE, J.F., HEMMING, H.G., LOWE, D., &
HORRIES, G.L.F. (1961) Phytotoxic compounds produced by Fusarium
equiseti. J. exp. Bot., 12: 1-21.
BROWN, M.H., SZCZECH, G.M., & PURMALIS, B.P. (1976) Teratogenic and
toxic effects of ochratoxin A in rats. Toxicol. appl. Pharmacol., 37:
331-338.
BRUMLEY, W.C., ANDRZEJEWSKI, D., TRUCKSESS, E.W., DREIFUSS, P.A.,
ROACH, J.A.G., EPPLEY, R.M., THOMAS, F.S., THORPE, C.W., & SPHON,
J.A. (1982) Negative ion chemical ionization mass spectrometry of
trichothecenes - Novel fragmentation under OH conditions. Biomed.
mass Spectrom., 9: 451-458.
BRUMLEY, W.C., TRUCKSESS, M.W., ADLER, S.H., COHEN, C.K., WHITE,
K.D., & SPHON, J.A. (1985) Negative ion chemical ionization mass
spectrometry of deoxynivalenol (DON): Application to identification
of DON in grains and snack foods after quantification/isolation by
thin-layer chromatography. J. agric. food Chem., 33:326-330.
BUENING, G.M., MANN, D.D., HOOK, B., & OSWEILER, G.D. (1982) The
effect of T-2 toxin on the bovine immune system: cellular factors.
Vet. Immunol. Immunopathol., 3: 411-417.
BUNGE, I., DIRHEIMER, G., & ROSCHENTHALER, R. (1978) in vivo and in
vitro inhibition of protein synthesis in Bacillus stearothermophilus
by ochratoxin A. Biochem. biophys. Res. Commun., 83: 398-405.
BUNNER, D.L. & MORRIS, E.R. (1988) Alteration of multiple cell
membrane functions in L-6 myoblasts by T-2 toxin: an important
mechanism of action. Toxicol. appl. Microbiol., 92: 113-121.
CANDLISH, A.A.G., STIMSON, W.H., & SMITH, J.E. (1986) A monoclonal
antibody to ochratoxin A. Lett. appl. Microbiol., 3: 9-11.
CARRASCO, L., BARBACID, M., & VAZQUEZ, D. (1973) The trichodermin
group of antibiotics inhibitors of peptide bond formation by
eukaryotic ribosomes. Biochim. Biophys. Acta, 312(2): 368-376.
CARSON, M.S. & SMITH, T.K. (1983) Role of bentonite in prevention of
T-2 toxicosis in rats. J. anim. Sci., 57: 1498-1506.
CARTER, C.J., CANNON, M., & SMITH, K.E. (1976) Inhibition of
protein synthesis in reticulocyte lysates by trichodermin. Biochem.
J., 154: 171-178.
CARTER, C.J., CANNON, M., & JIMENET, A. (1980) A trichodermin
resistant mutant of Saccharomyces cerevisiae with an abnormal
distribution of native ribosomal subunits. Eur. J. Biochem., 107
(1): 173-184.
CASALE, W.L., PESTKA, J.J., & HART, L.P. (1988) Enzyme-linked
immunosorbent assay employing monoclonal antibody specific for
deoxynivalenol (vomitoxin) and several analogues. J. agric. food
Chem., 36: 663-668.
CEOVIC, S., GRIMS, P., & MITAR, J. (1976) [The incidence of tumours
of the urinary organs in a region of endemic nephropathy and in a
control region.] Lijec. Vjesn., 98: 301-304 (in Croatian).
CHAKRABARTI, D.K. & HOSAL, S. (1986) Occurrence of free and
conjugated 12,13-epoxytrichothecenes and zearalenone in banana fruits
infected with Fusarium moniliforme. Appl. environ. Microbiol.,
51(1): 217-219.
CHANG, F.C. & CHU, F.S. (1977) The fate of ochratoxin A in rats.
Food Cosmet. Toxicol., 15: 199-204.
CHANG, K., KURTZ, J.J., & MIROCHA, C.J. (1979) Effects of the
mycotoxin zearalenone on swine reproduction. Am. J. vet. Res.,
40: 1260-1267.
CHATTERJEE, K., VISCONTI, A., & MIROCHA, C.J. (1986) Deepoxy T-2
tetraol: a metabolite of T-2 toxin found in cow urine. J. agric.
food Chem., 34: 695-697.
CHELKOWSKI, J., VISCONTI, A., & MANAKA, M. (1984) Production of
trichothecenes and zearaleneone by Fusarium species isolated from
wheat. Nahrung, 28: 493-496.
CHERNOZEMSKY, I.N., STOYANOV, I.S., PETKOV-ABOCHAROVA, T.K., NOCOLOV,
I.G., DRAGANOV, I.V., STOICHEV, I., TANCHEV, Y., NAIDENOV, D., &
KALCHEVA, N.D. (1977) Geographic correlation between the occurrence
of endemic nephropathy and urinary tract tumours in Vratza district,
Bulgaria. J. Cancer, 19: 1-11.
CHI, M.S., MIROCHA, C.J., KURTZ, H.J., WEAVER, G., BATES, F., &
SHIMODA, W. (1977a) Effects of T-2 toxin on reproductive
performance and health of laying hens. Poult. Sci., 56(2): 628-637.
CHI, M.S., MIROCHA, C.J., KURTZ, H.J., WEAVER, G., BATES, F.,
SHIMODA, W., & BURMEISTER, H.R. (1977b) Acute toxicity of T-2 toxin
in broiler chicks and laying hens. Poult Sci., 56(1): 103-116.
CHI, M.S., MIROCHA, C.J., KURTZ, H.J., WEAVER, G., BATES, F., &
SHIMODA, W. (1977c) Subacute toxicity of T-2 toxin in broiler
chicks. Poult. Sci., 56(1): 306-313.
CHI, M.S., ROBISON, T.S., MIROCHA, C.J., BEHRENS, J.C., & SHIMODA, W.
(1978a) Transmission of radioactivity into eggs from laying hens
(Gallus domesticus) administered tritium labeled T-2 toxin. Poult.
Sci., 57(5): 1234-1238.
CHI, M.S., ROBISON, T.S., MIROCHA, C.J., SWANSON, S.P., & SHIMODA, W.
(1978b) Excretion and tissue distribution of radioactivity from
tritium labeled T-2 toxin in chicks. Toxicol. appl. Pharmacol.,
45(2): 391-402.
CHI, M.S., EL-HALAWANI, M.E., WAIBEL, P.E., & MIROCHA, C.J. (1981)
Effects of T-2 toxin on brain catecholamines and selected blood
components in growing chickens. Poult. Sci., 60(1): 137-141.
CHIBA, J., NAKANO, N., MOROOKA, N., NAKAZAWA, S., & WATANABE, Y.
(1972) Inhibitory effects of fusarenon-X, a sesquiterpene mycotoxin
on lipid synthesis and phosphate uptake in Tetrahymena pyriformis.
Jpn. J. med. Sci. Biol., 25(5): 291-296.
CHO, B.R. (1964) Toxicity of water extracts of scabby barley to
suckling mice. Am. J. vet. Res., 25: 1267-1270.
CHU, F.S. (1971) Interaction of ochratoxin A with bovine serum
albumin. Arch. Biochem. Biophys., 147: 359-366.
CHU, F.S. (1974a) Studies on ochratoxins. CRC crit. Rev. Toxicol.,
2(4): 499-524.
CHU, F.S. (1974b) A comparative study of the interaction of
ochratoxins with bovine serum albumin. Biochem. Pharmacol., 23(6):
1105-1113.
CHU, F.S. & BUTZ, M.E. (1970) Spectrophotofluorodensitometric
measurement of ochratoxin A in cereal products. J. Assoc. Off. Anal.
Chem., 53(6): 1253-1257.
CHU, F.S., GROSSMAN, S., WEI, R.D., & MIROCHA, C.J. (1979)
Production of antibody against T-2 toxin. Appl. environ. Microbiol.,
37(1): 104-108.
CHUNG, C.W., TRUCKSESS, M.W., GILES, A.L., Jr, & FRIEDMAN, L. (1974)
Rabbit skin test for estimation of T-2 toxin and other skin
irritating toxins in contaminated corn. J. Assoc. Off. Anal. Chem.,
57(5): 1121-1127.
CHUNG, H.S. (1975) Cereal scab causing mycotoxicoses in Korea and
present status of mycotoxin researches. Korean J. Mycol., 3(1):
31-36.
CIRILLI, G. (1983) Trichothecene problems in Italy. In: Ueno, Y.,
ed. Developments in food science. IV. Trichothecenes, New York,
Elsevier, pp. 254-258.
COFFIN, J.L. & COMBS, G.F., Jr (1981) Impaired vitamin E status of
chicks fed T-2 toxin. Poult. Sci., 60(2): 385-392.
COHEN, H. & LAPOINTE, M. (1982) Capillary gas-liquid
chromatographic determination of vomitoxin in cereal grains. J.
Assoc. Off. Anal. Chem., 65(6): 1429-1434.
COHEN, H. & LAPOINTE, M. (1984) Capillary gas chromatographic
determination of T-2 toxin, HT-2 toxin and diacetoxyscirpenol in
cereal grains. J. Assoc. Off. Anal. Chem., 67: 1105-1107.
COLE, R.J. & COX, R.H. (1981) Handbook of toxic fungal metabolites,
New York, London, San Francisco, Academic Press, pp. 152-263.
COLINSKI, P. (1987) [Ochratoxin A in humans as a result of food and
feed contamination,] Poznan, Rocznalion Akademii Rolniczej (Doctoral
thesis) (in Polish).
COLLINS, G.J. & ROSEN, J.D. (1979) Gas-liquid chromatographic/mass
spectrometric screening method for T-2 toxin in milk. J. Assoc. Off.
Anal. Chem., 62(6): 1274-1280.
COLLINS, G.J. & ROSEN, J.D. (1981) Distribution of T-2 toxin in
wet-milled corn products. J. food Sci., 46(3): 877-879.
CONNOLE, M.D., BLANEY, B.J., & MCEWAN, T. (198l) Mycotoxins in
animal feeds and toxic fungi in Queensland 1971-1980. Aust. vet. J.,
57: 314-318.
CONNOR, M.W., CAMARGO, J.D., PANYANT, P., RIENGROPITOK, S., ROGERS,
A.E., & NEWBUNE, P.M. (1986) Toxicity of anguidine in mice. Fundam.
appl. Toxicol., 7: 153-164.
COORAY, R. (1984) Effects of some mycotoxins on mitogen-induced
blastogenesis and SCE frequency in human lymphocytes. Food Chem.
Toxicol., 22: 529-534.
COPPOCK, R.W., SWANSON, S.P., GELBERG, H.B., KORITZ, G.D., HOFFMAN,
W.E., BUCK, W.B., & VESONDER, R.F. (1985) Preliminary study of the
pharmacokinetics and toxicopathy of deoxynivalenol (vomitin) in
swine. Am. J. vet. Res., 46: 169-174.
CORLEY, R.A., SWANSON, S.P., & BUCK, W.B. (1985) Glucuronide
conjugates of T-2 toxin and metabolites in swine bile and urine. J.
agric. food Chem., 33: 1085-1089.
CORLEY, R.A., SWANSON, S.P., GULLO, G.J., JOHNSON, L., BEASLEY, V.R.,
& BUCK, W.B. (1986) Disposition of T-2 toxin, a trichothecene
mycotoxin, in intravascularly dosed swine. J. agric. food Chem.,
34: 868-875.
CORRIER, D.E. & ZIPRIN, R.L. (1986a) Immunotoxic effects of T-2 toxin
on cell-mediated immunity to listeriosis in mice: comparison with
cyclophosphamide. Am. J. vet. Res., 47: 1956-1960.
CORRIER, D.E. & ZIPRIN, R.L. (1986b) Enhanced resistance to
listeriosis induced in mice by preinoculation treatment with T-2
mycotoxin. Am. J. vet. Res., 47: 856-859.
COSGRIFF, T.M., BUNNER, D.P., WANNEMACHER, R.W., Jr., HODGSON, L.A.,
& DINTERMAN R.E. (1984) The hemostatic derangement produced by T-2
toxin in guinea pigs. Toxicol. appl. Pharmacol., 76: 454-463.
COSGRIFF, T.M., BUNNER, D.P., WANNEMACHER, R.W., Jr., HODGSON, L.A.,
& DINTERMAN, R.E. (1986) The hemostatic derangement produced by T-2
toxin in Cynomolgus monkeys. Toxicol. appl. Pharmacol., 82: 532-539.
COTE, L.M., REYNOLDS, J.D., VESONDER, R.F., BUCK, W.B., SWANSON,
S.P., COFFEY, R.T., & BROWN, D.C. (1984) Survey of vomitoxin-
contaminated feed grains in midwestern United States, and associated
health problems in swine. J. Am. Vet. Med. Assoc., 184(2): 189-192.
COTE, L.M., DAHLEM, A.M., YOSHIZAWA, T., SWANSON, S.P., & BUCK, W.B.
(1986) Excretion of deoxynivalenol and its metabolite in milk, urine
and feces of lactating dairy cows. Agric. biol. Chem., 50: 227-229.
CREPPY, E.E., LUGNIER, A.A.J., FASIOLO, F., HELLER, K.,
ROSCHENTHALER, R., & DIRHEIMER, G. (1979a) In vitro inhibition of
yeast phenylalanyl-tRNA synthetase by ochratoxin A. Chem.-biol.
Interact., 24: 257-261.
CREPPY, E.E., LUGNIER, A.A.J., BECK, G., ROSCHENTHALER, R., &
DIRHEIMER, G. (1979b) Action of ochratoxin A on cultured hepatoma
cells - reversion of inhibition by phenylalanine. FEBS Lett., 104:
287-290.
CREPPY, E.E., SCHLEGEL, M., ROSCHENTHALER, R., & DURHEINSER, G.
(1980) Phenylalamine prevents acute poisoning by ochratoxin A in
mice. Toxicol. Lett., 6: 77-80.
CREPPY, E.E., STORMER, F.O., ROSCHENTHALER, R., & DIRHEIMER, G.
(1983) Effects of two metabolites of ochratoxin A, (4R)-4-
hydroxyochratoxin A and ochratoxin alpha, on immune response in mice.
Infect. Immun., 39: 1015-1018.
CROFT, W.A., JARVIS, B.B., & YATAWARA, C.S. (1986) Airborne outbreak
of trichothecene toxicosis. Atmos. Environ., 20: 549-552.
CUNDLIFFE, E., CANNON, M., & DAVIS, J. (1974) Mechanism of
inhibition of eukaryotic protein synthesis by trichothecene fungal
toxins. Proc. Natl Acad. Sci. (USA), 71(1): 30-34.
D'AGOSTINO, P.A., PROVOST, L.R., & DROVER, D.R. (1986) Analysis of
trichothecene mycotoxins in human blood by capillary column gas
chromatography-ammonia chemical ionization mass spectrometry. J.
Chromatogr., 367: 77-86.
DANKO, G. & SZERAFIN, J. (1976) Experimental stachybotryotoxicosis
in horses. Magy. Allatorv. Lapja, 9: 597-600.
DE LOACH, J.R., ANDREWS, K., & NAGI, A. (1987) Interaction of T-2
toxin with bovine erythrocyte: effects on cell lysis, permeability
and entrapment. Toxicol. appl. Pharmacol., 88: 123-131.
DEMEKE, T., KIDANE, Y., & WUHIB, E. (1979) Ergotism: a report on an
epidemic. Ethiop. med. J., 17: 107-113.
DENICOLA, D.B., REBER, A.H., CARLTON, W.W., & YAGEN, B. (1978) T-2
toxin mycotoxicosis in the guinea-pig. Food Cosmet. Toxicol., 16
(6): 601-609.
DESIMONE, P.A., GRECO, F.A., & LESSNER, H.F. (1979) Phase I
evaluation of a weekly schedule of anguidine. Cancer Treat. Rep.,
63(11/12): 2015-2017.
DIVE, D., MOREAU, S., & CACAN, M. (1978) Use of a ciliate
protozoan for fungal toxins studies. Bull. environ. Contam. Toxicol.,
19(4): 489-495.
DOCHEV, D. (1973) [Endemic (Balkan) nephritis in Bulgaria.]
Münchener med. Wochenschr., 115(13): 537-541 (in German).
DOERR, J.A., HAMILTON, P.B., & BURMEISTER, H.R. (1981) T-2
toxicosis and blood coagulation in young chickens. Toxicol. appl.
Pharmacol., 60: 157-162.
DOSIK, G.M., BARLOGIE, B., JOHNSTON, D.A., MARPHY, W.K., & DREWINKO,
B. (1978) Lethal and cytokinetic effects of anguidine on a human
colon cancer cell line. Cancer Res., 38(10): 3304-3309.
DOSTER, R.C., SINHUBER, R.O., & WALES, J.H. (1972) Acute
intraperitoneal toxicity of ochratoxin A and B in rainbow trout
(Salmo gairdneri). Food. Cosmet. Toxicol., 10: 85-92.
DROVE, J.F. (1988) Non macrocyclic trichothecenes. Natural Products
Reports: 181-209.
DWIVEDI, P. & BURNS, R.B. (1984) Effect of ochratoxin A on
immunoglobulins in broiler chicks. Res. vet. Sci., 36: 117-121.
EDLUND, P.O. (1981) Determination of ergot alkaloids in plasma by
high-performance liquid chromatography and fluorescence detection. J.
Chromatogr., 226: 107-115.
EHRLICH, K.C., LEE, L.S., & CIEGLER, A. (1983) A simple, sensitive
method for detection of vomitoxin (deoxynivalenol) using reversed
phase, high performance liquid chromatography. J. liq. Chromatogr.,
6: 833-843.
EL-BANNA, A.A., LAU, P.-Y., & SCOTT, P.M. (1983) Fate of mycotoxins
during processing of foodstuffs II- Deoxynivalenol (vomitoxin) during
making of Egyptian bread. J. food Prot., 46: 484-486.
EL-BANNA, A.A., SCOTT, P.M., LAU, P.-Y., SAKUMA, T., PLOT, H., &
CAMPBELL, V. (1984) Formation of trichothecenes by F. solani var.
coeruleum and F. sambucinum in potatoes. Appl. environ. Microbiol.,
47: 1169-1171.
ELLING, F. (1977) Demonstration of ochratoxin A in kidneys of pigs
and rats by immunofluorescence microscopy. Acta pathol. microbiol.
Scand., A85: 151-156.
ELLING, F. & MOLLER, T. (1973) Mycotoxic nephropathy in pigs. Bull.
World Health Organ., 49: 411-418.
ELLING, F., HALD, B., JACOBSEN, C., & KROGH, P. (1975) Spontaneous
cases of toxic nephropathy in poultry associated with ochratoxin A.
Acta pathol. microbiol. Scand., A83: 739-741.
ELLING, F., NIELSEN, J.P., LILLEHOJ, E.B., THOMASSEN, M.S., &
STORMER, F.C. (1985) Ochratoxin A-induced porcine nephrotoxicity:
enzyme and ultrastructural changes after short-term exposure.
Toxicon, 23: 247-254.
EPPLEY, R.M. (1968) Screening method for zearalenone, aflatoxin, and
ochratoxin. J. Assoc. Off. Anal. Chem., 51(1): 74-78.
EPPLEY, R.M. (1974) Sensitivity of brine shrimp Artemia salina to
trichothecenes. J. Assoc. Off. Anal. Chem., 57(3): 618-620.
EPPLEY, R.M. & BAILEY, W.J. (1973) 12,13-epoxy-delta-trichothecenes
as the probable mycotoxins responsible for stachybotryotoxicosis.
Science, 181: 758-760.
EPPLEY, R.M., TRUCKSESS, M.W., NESHEIM, S., THORPE, C.W., WOOD, G.E.,
& POHLAND, A.E. (1984) Deoxynivalenol in winter wheat: Thin layer
chromatographic method and survey. J. Assoc. Off. Anal. Chem., 67
(1): 43-45.
EPPLEY, R.M., TRUCKSESS, M.W., NESHEIM, S., THORPE, C.W., & POHLAND,
A.E. (1986) Thin layer chromatographic method for determination of
deoxynivalenol in wheat: collaborative study. J. Assoc. Off. Anal.
Chem., 69: 37-40.
FAIRHURST, S., MAXWELL, S.A., SCAWIN, J.W., & SWANSTON, D.W. (1987)
Skin effects of trichothecenes and their amelioration by
decontamination. Toxicology, 46: 307-319.
FAN, T.S.L., XU, Y.-C., & CHU, F.S. (1987) Simultaneous analysis of
T-2 toxin and HT-2 toxin by indirect enzyme-linked immunosorbent
assay. J. Assoc. Off. Anal. Chem., 70: 657-661.
FARB, R.M., MEGO, J.L., & HAYES, A.W. (1976) Effect of mycotoxins
on uptake and degradation of iodine-125 albumin in mouse liver and
kidney lysosomes. J. Toxicol. environ. Health, 1(6): 985-990.
FEUERSTEIN, G., GOLDSTEIN, D.S., RAMWELL, P.W., ZERBE, R.L., LUX,
W.E. Jr, FODEN, A.I., & BAYORH, M.A. (1985) Cardiorespiratory
sympathetic and biochemical response to T-2 toxin in the guinea pig
and rat. J. Pharmacol. exp. Ther., 232: 786-794.
FEUERSTEIN, G., LEADER, P., SIREN, A.L., & BRAGNET, P. (1987)
Protective effect of a PAF-acether antagonist, BN52021 in
trichothecene toxicosis. Toxicol. Lett., 38: 271-274.
FIORAMONTI, J., FARGEAS, M.J., & BUENO, L. (1987) Action of T-2
toxin on gastrointestinal transit in mice: protective effect of an
argillaceous compound. Toxicol. Lett., 36: 227-232.
FITZPATRICK, D.W., BOYD, K.E., & WATTS, B.M. (1988) Comparison of the
trichothecenes deoxynivalenol and T-2 toxin for their effect on brain
biogenic monoamines in the rat. Toxicol. Lett., 40: 241-245.
FORGACS, J. (1965) In: Wogan, G., ed. Mycotoxins in foodstuffs,
Cambridge, Mass., MIT Press, p. 87.
FORSELL, J.H. & PESTKA, J.J. (1985) Relation of 8-ketotrichothecene
and zearalenone analog structure to inhibitions of mitogen-induced
human lymphocyte blastogenesis. Appl. environ. Microbiol., 50:
1304-1307.
FORSELL, J.H., KATEBY, J.R., YOSHIZAWA, T., & PESTKA, J.J. (1985)
Inhibition of mitogen-induced blastogenesis in human lymphocytes by
T-2 toxin and its metabolites. Appl. environ. Microbiol., 49:
1524-1526.
FORSYTH, D.M., YOSHIZAWA, T., MOROOKA, N., & TUITE, J. (1977)
Emetic and refusal activity of deoxynivalenol to swine. Appl.
environ. Microbiol., 34(5): 547-552.
FOSTER, P.M.D., SLATER, T.F., & PATTERSON, D.S.P. (1975) A possible
enzymic assay for trichothecene mycotoxins in animal feedstuffs.
Biochem. Soc. Trans., 3(6): 875-878.
FREEMAN, G.G. (1955) Further biological properties of trichotecin,
an antifungal substance from Trichothecium roseum Link, and its
derivatives. J. gen. Microbiol., 12: 213-221.
FREEMAN , G.G. & MORRISON, R.I. (1949) The isolation and chemical
properties of trichothecin, an antifungal substance from
Trichothecium roseum link. Biochem. J., 44: 1-5.
FRIED, H.M. & WARNER, J.R. (1981) Cloning of yeast gene for
trichodermin resistance and ribosomal protein L3. Proc. Natl Acad.
Sci. (USA), 78(1): 238-242.
FRIEND, S.C.E., TRENHOLM, H.L., ELLIOT, J.I., THOMPSON, B.K., &
HARTIN, K.E. (1982) Effect of feeding vomitoxin-contaminated wheat to
pigs. Can. J. anim. Sci., 62: 1211-1222.
FRIEND, S.C.E., BABUIK, L.A., & SCHIEFER, H.B. (1983a) The effects of
dietary T-2 toxin on the immunological function and Herpes simplex
reactivation in Swiss mice. Toxicol. appl. Pharmacol., 69:
234-244.
FRIEND, S.C.E., HANCOCK, D.S., & SCHIEFER, H.B. (1983b) Experimental
T-2 toxicosis in sheep. Can. J. comp. Med., 47: 291-297.
FRIEND, S.C.E., SCHIEFER, H.B,. & BABUIK, L.A. (1983c) The effects of
dietary T-2 toxin on acute Herpes simplex virus type 1 infection in
mice. Vet. Pathol., 20: 737-760.
FRIESEN, M.D. & GARREN, L. (1983) International mycotoxin check
sample survey programme. Part III. Report on performance of
participating laboratories for determining ochratoxin A in animal
feed. J. Assoc. Off. Anal. Chem., 66: 256-259.
FRIIS, Ch., BRINN, R., & HALD, B. (1988) Uptake of ochratoxin A by
slices of pig kidney cortex. Toxicology., 52: 209-217.
FRITZ, W., BUTHIG, CL., DONATH, R., & ENGST, R. (1979) [Studies on
the nutritionally significant formation of ochratoxin A in grain and
other foodstuffs.] Lebensm. Ernähr., 25: 929-932 (in German).
FROMENTIN, H., SALAZAR-MEJICANOS, S,. & MARIAT, F. (1981)
Experimental cryptococcosis in mice treated with diacetoxyscirpenol,
a mycotoxin of Fusarium. Sabouraudia, 19: 311-318.
GADIOU, J. (1965) Contribution à l'étude toxicologique du
dicyandiamide de methyl mercure, Université de Bordeaux, Faculté de
pharmacie. (Thèse de doctorat).
GALEY, F.D., LAMBERT, R.J., BUSSE, M., & BUCK, W.B. (1987)
Therapeutic efficacy of superactive charcoal in rats exposed to oral
lethal doses of T-2 toxins. Toxicon, 25: 493-499.
GALTIER, P. (1974a) Devenir de l'ochratoxine A dans l'organisme
animal. I. Transport sanguin de la toxine chez le rat. Ann. Rech.
vét., 5: 311-318.
GALTIER, P. (1974b) Devenir de l'ochratoxine A dans l'organisme
animal. II. Distribution tissulaire et élimination chez le rat.
Ann. Rech. vét., 5: 319-328.
GALTIER, P. (1979) Etude toxicologique et pharmacocinétique d'une
mycotoxine, l'ochratoxine A, Université de Toulouse, Thèse de
doctorat d'Etat en Sciences pharmaceutiques.
GALTIER, P. & ALVINERIE, M. (1976) In vitro transformation of
ochratoxin A by animal microbial floras. Ann. Rech. vét., 7:
91-98.
GALTIER, P., MORE, J., & BODIN, G. (1974) Toxines d' Aspergillus
ochraceus Wilhelm III. Toxicité aiguë de l'ochratoxine A chez le rat
et la souris adultes. Ann. Rech. vét., 5: 233-247.
GALTIER, P., BARADAT, C., & ALVINERIE, M. (1977a) De l'élimination
d'ochratoxine A par le lait chez la lapine. Ann. Nutr. Aliment.,
31: 911-918.
GALTIER, P., JEMMALI, M., & LARRIEU, G. (1977b) Enquete sur la
présence éventuelle d'aflatoxine et d'ochratoxine A dans des maïs
recolté en France en 1973 et 1974. Ann. Nutr. Aliment., 31: 38l-389.
GALTIER, P., BONEU, B., CHARPENTEAU, J.L., BODIN, G., ALVINERIE, M.,
& MORE, J. (1979) Physiopathology of haemorrhagic syndrome related
to ochratoxin A intoxication in rats. Food Cosmet. Toxicol., 17:
49-53.
GALTIER, P., CAMGUILHEM, R., & BODIN, G. (1980) Evidence for in
vitro and in vivo interaction between ochratoxin A and three acidic
drugs. Food Cosmet. Toxicol., 18: 493-496.
GAREIS, M., HASHEM, A., BAUER, J., & GEDEK, B. (1986) Identification
of glucuronide metabolites of T-2 toxin and diacetoxyscirpenol in the
bile of isolated perfused rat lever. Toxicol. appl. Pharmacol., 84:
168-172.
GAREIS, M., MARTLBAUER, E., BAUER, J., & GEDEK, B. (1988)
[Determination of ochratoxin A in breast milk.] Z. Lebensm. Unters.
Forsch., 186: 114-117 (in German).
GENDLOFF, E.H., PESTKA, J.J., SWANSON, S.P., & HART, L.P. (1984)
Detection of T-2 toxin in Fusarium sporotrichioides-infected corn by
enzyme-linked immunosorbent assay. Appl. environ. Microbiol., 47:
1161-1163.
GENTRY, P.A. (1982) The effect of administration of a single dose of
T-2 toxin on blood coagulation in the rabbit. Can. J. comp. Med.,
46: 414-419.
GENTRY, P.A. & COOPER, M.L. (1981) Effect of Fusarium T-2 toxin on
hematological and biochemical parameters in the rabbit. Can. J. comp.
Med., 45: 400-405.
GHOSAL, S., CHAKRABARTI, D.K., & BASU CHAUDHARY, K.C. (1977) The
occurrence of 12,13-epoxytrichothecenes in seeds of safflower
infected with Fusarium oxysporum f sp. carthami. Experientia (Basel),
33(5): 574-575.
GILBERT, J., SHEPHERD, M.J., & HOWELL, M.V. (1983a) Studies on the
fate of trichothecene mycotoxins during food processing. European
Conference on. Food Chemistry. II, Rome, Societa Italiana di Scienza
dell alimetazione. pp. 253-262.
GILBERT, J., SHEPHERD, M.J., & STARTIN, J.R. (1983b) A survey of the
occurrence of the trichothecene mycotoxin deoxynivalenol (vomitoxin)
in UK-grown barley and in imported maize by combined gas
chromatography-mass spectrometry. J. Sci. Food Agric., 34: 86-92.
GILBERT, J., SHEPHERD, M.J., & STARTIN, J.R. (1984) The analysis and
occurrence of Fusarium mycotoxins in the United Kingdom and their
fate during food processing. In: Kurata, H. & Ueno, Y., ed. Toxigenic
fungi - their toxins and health hazard, Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp. 209-216.
GILBERT, J., STARTIN, J.R,. & CREWS, C. (1985) Optimization of
conditions for trimethylsilylation of trichothecene mycotoxins. J.
Chromatogr., 319: 376-381.
GILGAN, M.W., SMALLEY, E.B., & STRONG, F.M. (1966) Isolation and
partial characterization of a toxin from Fusarium tricinctum on moldy
corn. Arch. Biochem. Biophys., 114: 1-3.
GOLINSKI, P., HULT, K., BRABARKIEWICZ-SZCZESNA, J., CHELKOWSKI, J.,
KNEBLEWSKI, P., & SZEBIOTKO, K. (1984) Mycotoxic porcine nephropathy
and spontaneous occurrence of ochratoxin A residues in kidneys and
blood of Polish swine. Appl. environ. Microbiol., 47: 1210-1212.
GOLINSKI, P., BRABARKIEWICZ-SZCZESNA, J., CHELKOWSKI, J., &
SZEBIOTKO, K. (1985) Spontaneous occurrence of ochratoxin A
residues in porcine kidney and serum samples in Poland. Appl.
environ. Microbiol., 49: 1014-1015.
GRANT, P.G., SCHINDLER, D., & DAVIES, J.E. (1976) Mapping of
trichodermin resistance in Saccharomyces cerevisiae: a genetic locus
for a component of the 60s ribosomal subunit. Genetics, 83(4):
667-673.
GREENHALGH, R., GILBERT, J., KING, R.R., BLACKWELL, B.A., STARTIN,
J.R., & SHEPHERD, M.J. (1984) Synthesis, characterization, and
occurrence in bread and cereal products of an isomer of 4-
deoxynivalenol (vomitoxin). J. agric. food Chem., 32: 1416-1420.
GREENWAY, J.A. & PULS, R. (1976) Fusariotoxicosis from barley in
British Colombia. I. Natural occurrence and diagnosis. Can. J. comp.
Med., 40: 12-15.
GROVE, J.F. & MORTIMER, P.H. (1969) The cytotoxicity of some
transformation products of diacetoxyscirpenol. Biochem. Pharmacol.,
18(6): 1473-1478.
GUPTA, R.S. & SIMINOVITCH, L. (1978) Genetic and biochemical
characterization of mutants of CHO cells resistant to the protein
synthesis inhibitor trichodermin. Somatic cell Genet., 4(3):
355-374.
GUTZWILLER, J. & TAMM, C. (1965) Verrucarin and roridin. V. The
structure of Verrucarin A. Helv. Chim. Acta, 48: 157-176.
HAGGBLOM, P. (1982) Production of ochratoxin A in barley by
Aspergillus ochraceus and Penicillium viridicatum: effect of fungal
growth, time, temperature, and inoculum size. Appl. environ.
Microbiol., 43: 1205-1207.
HAGLER, W.M., Jr, TYCZKOWDKA, K., & HAMILTON, P.B. (1984)
Simultaneous occurrence of deoxynivalenol, zearalenone, and aflatoxin
in 1982 scabby wheat from the midwestern United States. Appl.
environ. Microbiol., 47(1): 151-154.
HALD, B. (in press) Human exposure to ochratoxin A. In: Natori, S.,
Hashimoto, K., & Ueno, Y., ed. Mycotoxins and phycotoxins 1989,
Amsterdam, Oxford, New York, Elsevier Science Publishers.
HALD, B. & KROGH, P. (1972) Ochratoxin residues in bacon pigs.
Proceedings of the IUPAC Symposium: Control of Mycotoxins, Kungälv,
Sweden, page 18.
HALD, B. & KROGH, P. (1975) Detection of ochratoxin A. in barley,
using silica gel mini columns. J. Assoc. Off. Anal. Chem., 58:
156-158.
HALD, B. & KROGH, P. (1983) Toxicoses and natural occurrence in
Denmark. In: Ueno, Y., ed. Trichothecenes - chemical, biological
and toxicological aspects, Amsterdam, Oxford, New York, Elsevier,
Science Publishers, pp 251-253.
HALIBURTON, J.C., VESONDER, R.F., LOCK, T.F., & BUCK, W.B. (1979)
Equine leucoencephalomalacia (ELEM): a study of Fusarium moniliforme
as an etiologic agent. Vet. hum. Toxicol., 21(5): 348-351.
HARRACH, B., MIROCHA, C.J., PATHRE, S.V., & PALYUSIK, M. (1981)
Macrocyclic trichothecene toxins produced by a strain of Stachybotrys
atra from Hungary. Appl. environ. Microbiol., 41(6): 1428-1432.
HARRACH, B., BATA, A., BAJMOCY, E., & BENKO, M. (1983) Isolation of
satratoxins from the bedding straw of a sheep flock with fatal
stachybotryotoxicosis. Appl. environ. Microbiol., 45: 1419-1422.
HARRI, E., VON, LOEFFLER, W., SIGG, H.P., STAHELIN, H., STOLL, Ch.,
TAMM, Ch., & WIESINGER, D. (1962) [Verrucarins and roridins, a group
of antibiotics with high cytostatic effect from Myrothecium species.]
Helv. Chim. Acta, 45: 839-853 (in German).
HART, L.P. & BASELTON, W.E. (1983) Distribution of vomitoxin in dry
milled fractions of wheat infected with Gibberella. J. agric. food
Chem., 31: 657-659.
HARTMANN, G.R., RICHTER, H., WEINER, E.M., & ZIMMERMANN, W. (1978)
On the mechanism of action of the cytostatic drug anguidine and of
the immunosuppressive agent ovalicin, two sesquiterpenes from fungi.
Planta Med., 34(3): 231-252.
HARWIG, J. (1974) Ochratoxin A and related metabolites. In:
Purchase, I.F.H., ed. Mycotoxins, Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp. 345-368.
HAUBECK, H.D., LORKOWSKI, G., KULSCH, E., & ROSCHENTHALER, R. (1981)
Immunosuppression by ochratoxin A and its prevention by
phenylalanine. Appl. environ. Microbiol., 41: 1040-1042.
HAYES, A.W., HOOD, R.D., & LEE, H.L. (1974) Teratogenic effects of
ochratoxin A in mice. Teratology, 9(1): 93-97.
HAYES, M.A. & SCHIEFER, H.B. (1979) Quantitative and morphological
aspects of cutaneous irritation by trichothecene mycotoxins. Food
Cosmet. Toxicol., 17(6): 611-621.
HAYES, M.A., BELLAMY, J.E.C., & SCHIEFER, H.B. (1980) Subacute
toxicity of dietary T-2 toxin in mice: morphological and
hematological effects. Can. J. comp. Med., 44: 203-218.
HELGESON, J.P., HABERLACH, G.T., & VANDERHOEF, L.N. (1973) T-2
toxin decreases logarithmic growth rates of tobacco callus tissues.
Plant Physiol., 52(6): 660-662.
HELLER, K. & ROSCHENTHALER, R. (1978) Inhibition of protein
synthesis in Streptococcus faecalis by ochratoxin A. Can. J.
Microbiol., 24: 466-472.
HEPTINSTALL, R.H. (1974) Pathology of the kidney, Boston, Little
Brown and Co., Vol. 11, pp. 828-836.
HEWETSON, J.F., PACE, J.D., & BEHELER, J.E. (1987) Detection and
quantitation of T-2 mycotoxin in rat organs by radioimmunoassay. J.
Assoc. Off. Anal. Chem., 70: 654-657.
HIBBS, C.M., OSWEILER, G.D., BUCK, W.B., & MACFEE, G.P. (1975)
Bovine hemorrhagic syndrome related to T-2 mycotoxin. Proc. Ann.
Meet. Am. Assoc. Vet. Lab. Diagn., 17: 305-310.
HIRAYAMA, S. & YAMAMOTO, M. (1948) [Biological studies on the
poisonous wheat flour (I).] Eiseishikenjo Hokoku, 66: 85-98 (in
Japanese).
HIRAYAMA, S. & YAMAMOTO, M. (1950) [Biological studies on the
poisonous wheat flour (II).] Eiseishikenjo Hokoku, 67: 117-121 (in
Japanese).
HOBDEN, A.N. & CUNDLIFFE, E. (1980) Ribosomal resistance to the
12,13-epoxy-trichothecene antibiotics in the producing organism
Myrothecium verrucaria. Biochem. J., 190(3): 765-770.
HOERR, F.J., CARLTON, W.W., & YAGEN, B. (1981) Mycotoxicosis caused
by a single dose of T-2 toxin or diacetoxyscirpenol in broiler
chickens. Vet. Pathol., 18: 653-664.
HOLADAY, C.E. (1976) A rapid screening method for the aflatoxins
and ochratoxin A. J. Am. Assoc. Anal. Chem., 53: 603-605.
HOLT, P.S. & DE LOACH, J.R. (1988a) Cellular effects of T-2 mycotoxin
on two different lives. Biochem. Biophys. Acta, 971: 1-8.
HOOD, R.D. (1986) Effects of concurrent prenatal exposure to
rubratoxin B and T-2 toxin in the mouse. Drug chem. Toxicol., 9
(2): 185-190.
HOOD, R.D., NAUGHTON, M.J., & HAYES, A.W. (1976) Prenatal effects
of ochratoxin A in hamsters. Teratology, 13: 11-14.
HOOD, R.D., KUCZUK, M.H., & SZCZECK, G.M. (1978) Effects in mice of
simultaneous prenatal exposure to ochratoxin A and T-2 toxin.
Teratology, 17: 25-30.
HRABAR, A., SULJAGA, K., BORCIC, B., ALERAJ, B., CEOVIC, S., &
CVORISCEC, D. (1976) [Endemic nephropathy morbidity and mortality
in the village of Kaniza.] Arch. ind. Hyg. Toxicol., 27: 137-145
(in Croatian).
HSU, I.C., SMALIEY, E.B., STRONG, F.M., & RIBELIN, W.E. (1972)
Identification of T-2 toxin in moldy corn associated with a lethal
toxicosis in dairy cattle. Appl. Microbiol., 24(5): 684-690.
HUFF, W.E., WYATT, R.D., TUCKER, T.L., & HAMILTON, P.B. (1974)
Ochratoxicosis in the broiler chicken. Poult. Sci., 53: 1585-1591.
HUFF, W.E., DOERR, J.A., HAMILTON, P.B., HAMANN, D.D., PETERSON,
R.E., & CIEGLER, A. (1980) Evaluation of bone strength during
aflatoxicosis and ochratoxicosis. Appl. environ. Microbiol.,
40: 102-107.
HULT, K. & GATENBECK, S. (1976) A spectrophotometric procedure,
using carboxypeptidase A, for the quantitative measurement of
ochratoxin A. J. Assoc. Off. Anal. Chem., 59: l28-l29.
HULT, K., TEILING, A., & GATENBECK, S. (1976) Degradation of
ochratoxin A by a ruminant. Appl. environ. Microbiol., 32: 443-444.
HULT, K., HUKBY, E., HOGLUND, U., GATENBECK, S., RUTQVIST, L., &
SELLYEY, G. (1979) Ochratoxin A in pig blood: method of analysis
and use as a tool for feed studies. Appl. environ. Microbiol., 38:
772-776.
HULT, K., HUKBY, E., GATENBECK, S., & RUTQVIST, L. (1980).
Ochratoxin A in blood from slaughter pigs in Sweden: Use in
evaluation of toxin content of consumed feed. Appl. environ.
Microbiol., 39: 828-830.
HULT, K., PLESTINA, R., HABAZIN-NOVAK, V., RADIC, B., & CEOVIC, S.
(1982) Ochratoxin A in human blood and Balkan endemic nephropathy.
Arch. Toxicol., 51: 313-321.
HULT, K., FUCHS, R., PERAICA, M., PLESTINA, R., & CEOVIC, S. (1984)
Screening for ochratoxin A in blood by flow injection analysis. J.
appl. Toxicol., 4: 326-329.
HUNT, D.C., BOURDON, A.T., WILD, P.J., & CROSBY, N.T. (1978) Use of
high performance liquid chromatography combined with fluorescence
detection for the identification and estimation of aflatoxins and
ochratoxin in food. J. Sci. Food Agric., 29: 234-238.
HUNT, D.C., LESLYE, A.P., & CROSBY, N.T. (1979) Determination of
ochratoxin A in pig's kidney using enzymic digestion, dialysis and
high-performance liquid chromatography with post-column
derivatization. Analyst, 104: 1171-1175.
HUNTER, K.W., BRIMFIELD, A.A., MILLER, M., FINKELMAN, F.D., & CHU,
S.F. (1985) Preparation and characterization of monoclonal antibodies
to the trichothecene mycotoxin T-2. Appl. environ. Microbiol., 49:
168-172.
HUSSEIN, H.M., FRANICH, R.A., BAXTER, M,. & ANDREW, I.G. (1989)
Naturally occurring Fusarium toxins in New Zealand maize. Food Addit.
Contam., 6: 49-58.
HUTCHISON, R.D., STEYN, P.S., & THOMPSON, D.L. (1971) The isolation
and structure of 4-hydroxyochratoxin A and 7-carboxy-3,4-dihydro-8-
hydroxy-3-methylisocoumarin from Penicillium viridicatum.
Tetrahedron, 43: 4033-4036.
IARC (1987a) Overall evaluations of carcinogenicity: and updating of
IARC Monographs Vol. 1 to 42, Lyons, International Agency for
Research on Cancer, pp. 271-272. (IARC Monographs on the Evaluation
of Carcinogenic Risks to Humans, Supplement 7).
IARC (1987b) Biennial report 1986-1987, Lyons, International Agency
for Research on Cancer.
IARC (1983) Some food additives, feed additives and naturally
occurring substances: ochratoxin A, Lyons, International Agency for
Research on Cancer, pp. 191-206 (IARC Monographs on the Evaluation of
the Carcinogenic Risk of Chemicals to Humans, Vol. 31).
IARC (1984) Monitoring of aflatoxins in human body fluids and
application to field studies, Lyons, International Agency for
Research on Cancer (IARC International Technical Report No. 84/003).
ICHINOE, M., UCHIYAMA, S., AMANO, R., & KURATA, H. (1985)
Trichothecene-producing Fusarium in barley and wheat in Japan.
Trichothecenes and other Mycotoxins. Proceedings of the International
Mycotoxin Symposium, Sydney, Australia, New York, Chichester, John
Wiley & Sons, pp. 21-31.
ILUS, T., NIKU-PAAVOLA, M.L., & ENARI, T.M. (1981) Chromatographic
analysis of Fusarium toxins in grain samples. Eur. J. appl.
Microbiol. Biotechnol., 11(4): 244-247.
IMAIDA, K., HIROSE, M., OGISO, T., KURATA, Y., & ITO, N. (1982)
Quantitative analysis of initiating and promoting activities of five
mycotoxins in liver carcinogenesis in rats. Cancer Lett., 16:
137-143.
ISHII, K., SAKAI, K., UENO, Y., TSUNODA, H., & ENOMOTO, M. (1971)
Solaniol, a toxic metabolite of Fusarium solani. Appl. Microbiol.,
22(4): 718-720.
ISHII, K., ANDO, Y., & UENO, Y. (1975) Toxicological approaches to
the metabolites of Fusaria. Part 9. Isolation of vomiting factor from
moldy corn infected with Fusarium spp. Chem. pharm. Bull., 23(9):
2162-2164.
ISHII, K., KOBAYASHI, J., UENO, Y., & ICHINOE, M. (1986) Occurrence
of trichothecine in wheat. Appl. environ. Microbiol., 52: 331-333.
ITO, Y., OHTSUBO, K., & SAITO, M. (1980) Effects of fusarenon-X, a
trichothecene produced by Fusarium nivale, on pregnant mice and their
fetuses. Jpn. J. exp. Med., 50(3): 167-172.
ITO, Y., OHTSUBO, K., ISHII, K., & UENO, Y. (1986) Effects of
nivalenol on pregnancy and fetal development of mice. Mycotoxin
res., 2: 71-77.
ITO, Y., UENO, Y., TANAKA, T., NAKAMURA, K., & OHTSUBO, K. (1988)
[Embryotoxicity of oral nivalenol in mice.] Proc. Jpn. Assoc.
Mycotoxicol., 27: 33-36. (in Japanese).
IUPAC (1976) Recommended methods for ochratoxin A and B in barley,
Oxford, International Union of Pure and Applied Chemistry (IUPAC
Technical Report No.14).
IWAHASHI, T., TASHIRO, F., & UENO, Y. (1982) Mechanism of cytotoxic
effect of T-2 toxin on a protozoan, Tetrahymena pyriformis gl.
Maikotokishin, 15: 31-33.
JAGADEESAN, V., RUKMINI, C., VIJAYARAGHOVAN, M., & TULPULE, P.G.
(1982) Immune studies with T-2 toxin: effect of feeding and
withdrawal in monkeys. Food chem. Toxicol., 20: 83-87.
JARVIS, B.B., STAHLY, G.P., & CURTIS, C.R. (1978) Antitumor
activity of fungal metabolites: Verrucarin b-9,10-epoxides. Cancer
Treat. Rep., 62(10): 1585-1586.
JARVIS, B.B., STAHLY, G.P., PAVANASASIVAM, G., & MAZZOLA, E.P.
(1980) Antileukemic compounds derived from the chemical modification
of macrocyclic trichothecenes. 1. Derivatives of verrucarin A. J.
med. Chem., 23(9): 1054-1058.
JARVIS, B.B., LEE, Y.W., COMEZOGLU, S.N., & YATAWARA, C.S. (1986)
Trichothecenes produced by Stachybotrys atra from Eastern Europe.
Appl. environ. Microbiol., 51(5): 915-918.
JELINEK, C.F., POHLAND, A.E., & WOOD, G.E. (1989) Occurrence of
mycotoxins in foods and feeds - an update. J. Assoc. Off. Anal.
Chem., 72: 223-230.
JIMINEZ, A. & VASQUEZ, D. (1975) Quantitative binding of antibiotics
to ribosomes from a yeast mutant altered on the peptidyl transferase
centre. Eur. J. Biochem., 54(2): 483-492.
JIMENEZ, A., SANCHEZ, L., & VAZQUEZ, D. (1975) Simultaneous
ribosomal resistance to trichodermin and anisomycin in Saccharomyces
cerevisiae mutants. Biochim. Biophys. Acta, 383(4): 427-434.
JOFFE, A.Z. (1962) Biological properties of some toxic fungi
isolated from overwintered cereals. Mycopathol. Mycol. appl., 16:
201-221.
JOFFE, A.Z. (1986) Fusarium species, their biology and toxicology,
New York, Chichester, J. Wiley and Sons.
JOFFE, A.Z. & YAGEN, B. (1977) Comparative study of the yield of T-2
toxin produced by Fusarium poae, Fusarium sporotrichioides and
Fusarium sporotrichioides var. tricinctum strains from different
sources. Mycopathologia, 60(2): 93-97.
JOHNSEN, H., ODDEN, E., LIE, O., JOHNSEN, B.A., & FONNUM, F. (1986)
Metabolism of T-2 toxin by rat liver carboxylesterase. Biochem.
Pharmacol., 35: 1469-1473.
JOSEFSSON, E. (1979) [Study of ochratoxin A in pig kidneys.] Var
Föda, 31: 415-420 (in Swedish).
JOSEFSSON, E. & MOLLER, T. (1979) High pressure liquid
chromatographic determination of ochratoxin A and zearalenone in
cereals. J. Assoc. Off. Anal. Chem., 62: 1165-1168.
JUSZKIEWICZ, T. & PISKORSKA-PLISZCZYNSKA, J. (1976) [Occurrence of
aflatoxins B1, B2, G1, and G2, ochratoxins A and B, sterigmatocystin
and zearalenone in cereals]. Med. Weter., 32: 617-619 (in Polish).
JUSZKIEWICZ, T. & PISKORSKA-PLISZCZYNSKA, J. (1977) [Occurrence of
mycotoxins in mixed feeds and concentrates.] Med. Weter., 33: 193-196
(in Polish).
JUSZKIEWICZ, T., PISKORSKA-PLISZCZYNSKA, J., & WISNIEWSKA, H. (1982)
Ochratoxin A in laying hens: tissue deposition and passage into eggs.
In: Mycotoxins and Phycotoxins, Vienna, Technical University Vienna
Publ., pp. 122-125.
KALINOSKI, H.T., UDSETH, H.R., WRIGHT, B.W., & SMITH, R.D. (1986)
Supercritical fluid extraction and direct fluid injection mass
spectrometry for the determination of trichothecene mycotoxins in
wheat samples. Anal. Chem., 58: 2421-2425.
KAMIMURA, H., NISHIJIMA, M., SAITO, K., YASUDA, K., IBE, A.,
NAGAYAMA, T., USHIYAMA, H., & NAOI, Y. (1979) Studies on mycotoxins
in foods. XII. The decomposition of trichothecene mycotoxins during
food processing. J. Assoc. Off. Anal. Chem., 64(5): 1067-1073.
KANAI, K. & KONDO, E. (1984) Decreased resistance to myobacterial
infection in mice fed a trichothecene compound (T-2 toxin). Jpn. J.
med. Sci. Biol., 37: 97-104.
KANE, A., CREPPY, E.E., ROSCHENTHALER, R., & DIRHEIMER, G. (1986a)
Changes of urinary and renal tubular enzymes caused by subchronic
administration of ochratoxin A in rats. Toxicology, 61: 489-495.
KANE, A., CREPPY, E.E., ROTH, A., ROSCHENTHALER, R., & DIRHEIMER, G.
(1986b) Distribution of the [3]-label from low doses of radioactive
ochratoxin A ingested by rats, and evidence for DNA single-strand
breaks caused in liver and kidneys. Arch. Toxicol., 58: 219-224.
KANIZAWA, M. (1984) Synergistic effect of citrinin on hepatorenal
carcinogenesis of ochratoxin A in mice. In: Kurata, H. & Ueno, Y.,
ed. Toxigenic fungi - their toxins and health hazard, Amsterdam,
Oxford, New York, Elsevier Science Publishers, pp. 245-254
(Developments in Food Science No. 7).
KANIZAWA, M. & SUZUKI, S. (1978) Induction of renal and hepatic
tumours in mice by ochratoxin A, a mycotoxin. Gann, 69: 599-600.
KANIZAWA, M., SUZUKI, S., KOZUKA, Y., & YAMAZAKI, M. (1977)
Histopathological studies on the toxicity of ochratoxin A in rats. I.
Acute oral toxicity. Toxicol. appl. Pharmacol., 42: 55-64.
KELLERMAN, T.S., MARASAS, W.F.O., PIENAAR, J.G., & NAUDE, T.W.
(1972) A mycotoxicosis of equidae caused by Fusarium moniliforme
Sheldon. A preliminary communication. Onderstepoort J. vet. Res.,
39(4): 205-208.
KHERA, K.S., WHALEN, C., ANGERS, G., VESONDER, R.F., & KUIPER-
GOODMAN, I. (1982) Embryotoxicity of 4-deoxynivalenol (vomitoxin)
in mice. Bull. environ. Contam. Toxicol., 29: 487-491.
KHERA, K.S., ARNOLD, D.L., WHALEN, C., ANGERS, G., & SCOTT, P.M.
(1984) Vomitoxin (4-deoxynivalenol): Effects on reproduction of mice
and rats. Toxicol. appl. Pharmacol., 74: 345-356.
KHERA, K.S., WHALEN, C., & ANGERS, G. (1986) A teratology study on
vomitoxin (4-deoxynivalenol) in rabbits. Food chem. Toxicol., 24:
421-424.
KIENTZ, C.E. & VERWEIJ, A. (1986) Trimethylsilylation and
trifluoroacetylation of a number of trichothecenes followed by gas
chromatographic analysis on fused silica capillary columns. J.
Chromatogr., 355: 229-240.
KING, B. (1979) Outbreak of ergotism in Wollo, Ethiopia. Lancet,
1: 1411.
KING, R.R., MCQUEEN, R.E., LEVESQUE, D., & GREENHALGH, R. (1984)
Transformation of deoxynivalenol (vomitoxin) by rumen microorganisms.
J. agric. food Chem., 32: 1181-1183.
KITAGAWA, M., TASHIRO, F., & UENO, Y. (1982) Mutual interaction
between zearalenone, an estrogenic mycotoxin, and estrogen receptor
of rat brain. Proc. Jpn. Assoc. Mycotoxicol., 15: 28-30.
KITCHEN, D.N., CARLTON, W.W., & TUITE, J. (1977a) Ochratoxin A and
citrinin induced nephrosis in beagle dogs. I. Clinical and
clinicopathological features. Vet. Pathol., 14: 154-172.
KITCHEN, D.N., CARLTON, W.W., & TUITE, J. (1977b) Ochratoxin A and
citrinin induced nephrosis in beagle dogs. II. Pathology. Vet.
Pathol., 14: 261-272.
KLINKERT, W., LORKOWSKI, G., CREPPY, E.E., DIRHEIMER, G., &
ROSCHENTHALER, R. (1981) Inhibition of macrophage migration by
ochratoxin A and citrinin, and prevention by phenylalanine of the
ochratoxin A-induced inhibition. Toxicol. Eur. Res., 3: 186-189.
KONISHI, T. & ICHIJO, S. (1970) [Clinical studies on bean-hulls
poisoning of horse. I. Clinical and biochemical observations in
spontaneous cases.] Res. Bull. Obihiro Univ., 6: 242-257 (in
Japanese).
KONRAD, I. & ROSCHENTHALER, R. (1977) Inhibition of phenylalanine
tRNA synthetase from Bacillus subtilis by ochratoxin A. FEBS Lett.,
83: 341-347.
KOTSONIS, F.N., SMALLEY, E.B., ELLISON, R.A., & GALE, C.M. (1975)
Food refusal factors in pure cultures of Fusarium roseum graminearum.
Appl. Microbiol., 30(3): 362-368.
KOZLOVSKY, A.G. & RESHETILOVA, T.A. (1984) Regulation of
biosynthesis of ergot alkaloids by Penicillium sizovae. Folia
microbiol., 29: 301-305.
KRIEK, N.P.J., MARASAS, W.F.O., STEYN, P.S., VAN RENSBURG, S.J., &
STEYN, M. (1977) Toxicity of a moniliformin-producing strain of
Fusarium moniliforme var. subglutinans isolated from maize. Food
Cosmet. Toxicol., 15(6): 579-587.
KRISHNAMACHARI, K.A.V.R. & BHAT, R.V. (1976) Poisoning of ergoty
bajra (pearl millet) in man. Indian J. med. Res., 64: 1624-1628.
KRISHNAMURTHY, T. & SARVER, E.W. (1986) Mass spectral investigations
on trichothecene mycotoxins. III. Synthesis, characterization and
applications of pentafluoropropionyl and trifluoroacetyl esters of
simple trichothecenes. J. Chromatogr., 355: 253-264.
KRISHNAMURTHY, T., WASSERMAN, M.B., & SARVER, E.W. (1986) Mass
spectral investigations of trichothecene mycotoxins. I. Application
of negative ion chemical ionization techniques for the simultaneous
and accurate analysis of simple trichothecenes in picogram levels.
Biomed. environ. mass Spectrom., 13: 503-518.
KRISHNAMURTHY, T., SARVER, E.W., GREENE, S.L., & JARVIS, B.B. (1987)
Mass spectral investigations on trichothecene mycotoxins. II.
Detection and quantitation of macrocyclic trichothecenes by gas
chromatography/negative ion chemical ionization mass spectrometry. J.
Assoc. Off. Anal. Chem., 70: 132-140.
KROGH, P. (1976a) Epidemiology of mycotoxic porcine nephropathy.
Nord. vet. Med., 28: 452-458.
KROGH, P. (1976b) Mycotoxic nephropathy. In: Advances in veterinary
science and comparative medicine, New York, Academic Press, Vol. 20,
pp. 147-170.
KROGH, P. (1977) Ochratoxin A residues in tissues of slaughter pigs
with nephropathy. Nord. vet. Med., 29: 402-405.
KROGH, P. (1978) Causal associations of mycotoxic nephropathy. Acta
pathol. microbiol. Scand., A269: 1-28.
KROGH, P. (1979) Environmental ochratoxin A and Balkan (endemic)
nephropathy: Evidence for support of a causal relationship. In:
Strahinjic, S. & Stefanovic, V., ed. Endemic (Balkan) nephropathy,
Nis, Yugoslavia, Grafika, pp. 35-43.
KROGH, P. (1980) Ochratoxins: occurrence, biological effects and
causal role in disease. In: Eaker, D. & Wadstrom, T., ed. Natural
toxins, Oxford, Pergamon Press, pp. 673-680.
KROGH, P. (1983) Diagnostic criteria for ochratoxin-induced
nephropathy. In: Strahinjic, S. & Stefanovic, V., ed. Current
research in endemic (Balkan) nephropathy, Nis, Yugoslavia, Grafika,
pp. 11-14.
KROGH, P., HALD, B., & PEDERSEN, E.J. (1973) Occurrence of
ochratoxin A and citrinin in cereals associated with mycotoxic
porcine nephropathy. Acta pathol. microbiol. Scand., B81:
689-695.
KROGH, P., AXELSON, N.H., ELLING, F., GYRD-HANSEN, N., HALD, B.,
HYLDGAARD-JENSEN, J., LARSEN, A.E., MADSEN, A., MORTENSEN, H.P.,
MOLLER, T., PETERSEN, O.K., RAVNSKOV, U., ROSTGAARD, M., & AALUND, O.
(1974a) Experimental porcine nephropathy: changes of renal function
and structure induced by ochratoxin A-contaminated feed. Acta pathol.
microbiol. Scand., A246: 21.
KROGH, P., HALD, B., ENGLUND, P., RUTQVIST, L., & SWAHN, O. (1974b)
Contamination of Swedish cereals with ochratoxin A. Acta pathol.
microbiol. Scand., B82: 301-302.
KROGH, P., ELLING, F., HALD, B., LARSEN, A.E., LILLEHOJ, E.B.,
MADSEN, A., & MORTENSEN, H.P. (1976a) Time-dependent disappearance
of ochratoxin A residues of bacon pigs. Toxicology, 6: 235-242.
KROGH, P., ELLING, F., HALD, B., JYLLING, B., PETERSEN, V.E.,
SKADHAUGE, E., & SVENDSEN, C.K. (1976b) Experimental avian
nephropathy: changes of renal function and structure induced by
ochratoxin A-contaminated feed. Acta pathol. microbiol. Scand.,
A84: 215-221.
KROGH, P., ELLING, F., GYRD-HANSEN, N., HALD, B., LARSEN, A.E.,
LILLEHOJ, E. B., MADSEN, A., MORTENSEN, H.P., & RAVNSKOV, U. (1976c)
Experimental porcine nephropathy: changes of renal function and
structure perorally induced by crystalline ochratoxin A. Acta pathol.
microbiol. Scand., A84: 429-434.
KROGH, P., HALD, B., PLESTINA, R., & CEOVIC, S. (1977) Balkan
(endemic) nephropathy and food-borne ochratoxin A: Preliminary
results of a survey of foodstuff. Acta. Path. Microbiol. Scand.,
B85: 238-240.
KROGH, P., ELLING, F., FRIIS, CHR., MALD, B., LARSEN, A.E., LILLEHOJ,
E.B., MADSEN, A., MORTENSEN, H.P., RASMUSSEN, F., & RAVUSKOU, U.
(1979) Porcine nephropathy induced by long-term ingestion of
ochratoxin A. Vet. Pathol., 16: 466-475.
KROGH, P., GYRD-HANSEN, N., HALD, B., LARSEN, S., NIELSEN, J.P.,
SMITH, M., IVANOFF, C., & MEISNER, H. (1988). Renal enzyme
activities in experimental ochratoxin A-induced porcine nephropathy:
Diagnostic potential of phosphoenolpyruvate carboxykinase and
gammaglutamyl transpeptidase activity. J. Toxicol. environ. Health.,
23: 1-15.
KUCZUK, M.H., BENSON, P.M., HEATH, H., & HAYES, A.W. (1978)
Evaluation of the mutagenic potential of mycotoxins using Salmonella
typhimurium and Saccharomyces cerevisiae. Mutat. Res., 53(1): 11-20.
KUMAGAI, S. (1985) Ochratoxin A: Plasma concentration and excretion
into bile and urine in albumin-deficient rats. Food chem. Toxicol.,
23: 941-943.
KUMAGAI, S. & AIBARA, K. (1982) Intestinal absorption and secretion
of ochratoxin A in rat. Toxicol. appl. Pharmacol., 64: 94-102.
KUMAGAI, S. & SHIMIZU, T. (1988) Effects of Fusarenon-X and T-2 toxin
on intestinal absorption of monosaccharide in rats. Arch. Toxicol.,
61: 489-495.
KUPCHAN, S.M., JARVIS, B.B., DAILEY, R.G., Jr, BRIGHT, W., BRYAN,
R.F., & SHIZURI, Y. (1976) Baccharin, a novel potent antileukemic
trichothecene triepoxide from Baccharis megapotamica. J. Am. Chem.
Soc., 98(22): 7092-7093.
KUPCHAN, S.M., STREELMAN, D.R., JARVIS, B.B., DAILEY, R.G., Jr, &
SNEDEN, A.T. (1977) Isolation of potent new antileukemic
trichothecenes from Baccharis megapotamica. J. org. Chem., 42
(26): 4221-4225.
LAFARGE-FRAYSSINET, C., DECLOITRE, F., MOUSSET, S., MARTIN, M., &
FRAYSSINET, C. (1981) Induction of DNA single strand breaks by T-2
toxin, a trichothecene metabolites of Fusarium, effect on lymphoid
organs and liver. Mutat. Res., 88(2): 115-124.
LAFARGE-FRAYSSINET, C., LESPINATS, G., LAFONT, P., LOISILLIER, F.,
MOUSSET, S., ROSENSTEIN, Y., & FRAYSSINET, C. (1979)
Immunosuppressive effects of Fusarium cells to mitogens. Proc. Soc.
Exp. Biol. Med., 160(3): 302-311.
LARSEN, S. (1928) [On chronic degeneration of the kidneys caused by
mouldy rye.] Maanadsskr. Dyrl., 40: 259-284, 289-300 (in Danish).
LAUREN, D.R. & GREENHALGH, R. (1987) Simultaneous analysis of
nivalenol and deoxynivalenol in cereals by liquid chromatography. J.
Assoc. Off. Anal. Chem., 70: 479-483.
LEE, S. & CHU, F.S. (1981a) Radioimmunoassay of T-2 toxin in corn
and wheat. J. Assoc. Off. Anal. Chem., 64(1): 156-161.
LEE, S. & CHU, F.S. (1981b) Radioimmunoassay of T-2 toxin in
biological fluids. J. Assoc. Off. Anal. Chem., 64(3): 684-688.
LEE, S.C., BEERY, J.T., & CHU, F.S. (1984) Immunohistochemical fate
of ochratoxin A in mice. Toxicol. appl. Microbiol., 72: 218-227.
LEE, U., JANG, H., TANAKA, T., HASEGAWA, A., OH, Y., & UENO, Y.
(1985) The coexistence of the Fusarium mycotoxins nivalenol,
deoxynivalenol, and zearalenone in Korean cereals harvested in 1983.
Food Addit. Contam., 2(3): 185-192.
LEE, U., JANG, H., TANAKA, T., OH, Y., CHO, C., & UENO, Y. (1987)
Effect of milling on decontamination of Fusarium mycotoxins
nivalenol, deoxynivalenol, and zearalenone in Korean wheat. J. agric.
food Chem., 35: 126-129.
LEONOV, A.N. (1977) Current view of the chemical nature of factors
responsible for alimentary toxic aleukia. In: Rodricks, J.V.,
Hesseltine, C.W,. & Mehlman, M.A., ed. Mycotoxins in human and animal
health, Park Forest South, Illinois, Pathotox Publishers, pp. 323-
328.
LEVI, C.P. (1975) Collaborative study of a method for the
determination of ochratoxin A in green coffee. J. Assoc. Off. Anal.
Chem., 58(2): 258-262.
LEVI, C.P., TRENK, H.L., & MOHR, H.K. (1974) Study of the
occurrence of ochratoxin A in green coffee beans. J. Assoc. Off.
Anal. Chem., 57: 866-870.
LEW, V.H., MUELLNER, E., HAGER, R., & GREGOR, M. (1979) [Feed
refusal and emesis in fattening pigs caused by fusariotoxin-
contaminated corn.] Bodenkultur, 30(3): 309-316 (in German).
LIAO, L.L., GROLLMAN, A.P., & HORWITZ, S.B. (1976) Mechanism of
action of the 12,13-epoxytrichothecene anguidine: an inhibitor of
protein synthesis. Biochim. Biophys. Acta, 454(2): 273-284.
LINDENFELSER, L.A., LILLEHOJ, E.B., & BURMEISTER, H.R. (1974)
Aflatoxin and trichothecene toxins: skin tumor induction and
synergistic acute toxicity in white mice. J. Natl Cancer Inst.,
52(1): 113-116.
LINNAINMAA, K., SORSA, M., & ILUS, T. (1979) Epoxytrichothecene
mycotoxins as c-mitotic agents in Allium. Hereditas, 90(2):
151-156.
LOKEN, T. (1984) Ergot from meadow grass in Norway: chemical
composition and toxicological effects in sheep. Nord. vet. Med., 36:
259-265.
LORENZ, K. (1979) Ergot on cereal grains. In: Critical reviews in
food, science, and nutrition, Boca Raton, Florida, CRC Press, Vol.
11, pp. 311-354.
LORENZANA, R.M., BEASLEY, V.R., BUCK, W.B., GHENT, A.W., LUNDREN,
G.R., & POPPENGA, R.H. (1985) Experimental T-2 toxicosis in swine. I
Changes in cardiac output, aortic mean pressure, catecholamines, 6-
keto PGFa, thromboxane B2, and acid base parameters. Fundam. appl.
Toxicol., 5: 879-892.
LOVELESS, A.R. (1967) Claviceps fusiformis sp. nov.: the causal
agent of an agalactia of sows. Br. Mycol. Soc., 50: 15-18.
LUO, X. (1988) Food poisoning associated with Fusarium toxins.
Proceedings of the 7th International Symposium on Mycotoxins and
Phycotoxins, Tokyo, 16-19 August, 1988.
LUSTER, M.I., GERMOLEC, D.R., BURLESON, G.R., JAMESON, C.W.,
ACKERMANN, M.F., LAMM, K.R., & MAYER, H.T. (1987) Selective
immunosuppression in mice of natural killer cell activity by
ochratoxin A. Cancer Res., 47: 2259-2263.
LUTSKY, I. & MOR, N. (1981) Experimental alimentary toxic aleukia
in cats. Lab. anim. Sci., 31(1): 43-47.
LUTSKY, I., MOR, N., YAGEN, B., & JOFFE, A.Z. (1978) The role of
T-2 toxin in experimental alimentary aleukia: a toxicity study in
cats. Toxicol. appl. Pharmacol., 43(1): 111-124.
MACDONALD, E.J., COVAN, K.R. & SMITH, T.K. (1988) Effect of acute
oral doses of T-2 toxin on tissue concentrations of biogenic amines
in the rat. J. anim. Sci., 66: 434-441.
MCLAUGHLIN, C.S., VAUGHAMM, M.H., CAMPBELL, I.M., WEI, C.M.,
STAFFORD, M.E., & HANSEN, B.S. (1977) Inhibition of protein
synthesis by trichothecenes. In: Rodricks, J.V., Hesseltine, C.W., &
Mehlman, M.A., ed. Mycotoxins in human and animal health, Forest Park
South, Illinois, Pathotox Publishers, pp. 263-273.
MANN, D.D., BUENING, G.M., HOOK, B., & OSWEILER, G.D. (1983) Effects
of T-2 mycotoxin on bovine serum proteins. Am. J. vet. Res., 44:
1757-1759.
MANN, D.D., BUENING, G.M., OSWEILER, G.D., & HOOK, B. (1984) Effect
of subclinical levels of T-2 toxin on the bovine cellular immune
system. Can. J. comp. Med., 48: 308-312.
MANTLE, P.G. (1977a) The genus Claviceps. In: Wyllie, T.D. &
Morehouse, L.C., ed. Mycotoxic fungi, mycotoxins, mycotoxicoses: an
encyclopedia handbook, New York, Marcel Dekker, Vol. 1, pp. 83-89.
MANTLE, P.G. (1977b) Chemistry of Claviceps mycotoxins. In: Wyllie,
T.D. & Morehouse, L.C., ed. Mycotoxic fungi, mycotoxins,
mycotoxicoses: an encyclopedia handbook, New York, Marcel Dekker, Vol.
1, pp. 421-426.
MANTLE, P.G. (1977c) Ergotism in cattle, sheep, swine. In: Wyllie,
T.D. & Morehouse, L.C., ed. Mycotoxic fungi, mycotoxins,
mycotoxicoses: an encyclopedia handbook, New York, Marcel Dekker, Vol.
2, pp. 145-151, 207-213, 273-275.
MARASAS, W.F.O., BAMBURG, J.R., SMALLEY, E.B., STRONG, F.M., RAGLAND,
W.L., & DEGURSE, P.E. (1969) Toxic effects on trout, rats and mice
of T-2 toxin produced by the fungus Fusarium tricinctum. Toxicol.
appl. Pharmacol., 15(2): 471-482.
MARASAS, W.F.O., SMALLEY, E.B., BAMBURG, J.R., & STRONG, F.M. (1971)
Phytotoxicity of T-2 toxin produced by Fusarium tricinctum.
Phytopathology, 61(12): 1488-1491.
MARASAS, W.F.O., KRIEK, N.P.J., WIGGINS, V.M., STEYN, P.S., TOWERS,
D.K., & HASTIE, T.J. (1979a) Incidence, geographic distribution,
and toxigenicity of Fusarium species in South African corn.
Phytopathology, 69(11): 1181-1185.
MARASAS, W.F.O., LEISTNER, L., HOFMANN, G., & ECKARDT, C. (1979b)
Occurrence of toxigenic strains of Fusarium in maize and barley in
Germany. Appl. environ. Microbiol., 7: 289-305.
MARASAS, W.F.O., NELSON, P.E., & TOUSSON, T.A., ed. (1984) Toxigenic
Fusarium species - identity and mycotoxicology, University Park,
Pennsylvania, Pennsylvania State University Press.
MARQUARDT, R.R., FROHLICH, A.A., SREEMANNARAYANA, O., ABRAMSON, D., &
BERNATSKY, A. (1988) Ochratoxin A in blood from slaughter pigs in
Western Canada. Can. J. vet. Res., 52: 186-190.
MASUDA, E., TAKEMOTO, T., TATSUNO, T., & OBARA, T. (1982)
Immunosuppressive effect of a trichothecene mycotoxin, fusarenon-X in
mice. Immunology, 45: 743-749.
MASUKO, H., UENO, Y., OTOKAWA, M., & YAGINUMA, K. (1977) The
enhancing effect of T-2 toxin on delayed hyper-sensitivity in mice.
Jpn. J. med. Sci. Biol., 30(3): 159-164.
MATSUOKA, Y. & KUBOTA, K. (1981) Studies on mechanisms of diarrhea
induced by fusarenone-X, a trichothecene mycotoxin from Fusarium
species. Toxicol. appl. Pharmacol., 57(3): 293-301.
MATSUOKA, Y. & KUBOTA, K. (1987) Characteristics of inflammation
induced by Fusarenon-X, a trichothecene mycotoxin from Fusarium
species. Toxicol. appl. Pharmacol., 91: 333-340.
MATSUOKA, Y., KUBOTA, K., & UENO, Y. (1979) General pharmacological
studies of fusarenon-X, a trichothecene mycotoxin from Fusarium
spp. Toxicol. appl. Pharmacol., 50(1): 87-94
MAYURA, K., REDDY, R.V., HAYES, A.W., & BERNDT, W.O. (1982)
Embryocidal, fetotoxic and teratogenic effects of ochratoxin A in
rats. Toxicology, 25: 175-185.
MAYURA, K., HAYES, A.W., & BERNDT, W.O. (1983) Effects of dietary
protein on teratogenicity of ochratoxin A in rats. Toxicology, 27:
147-157.
MAYURA, K., PARKER, R., BERNDT, W.O., & PHILLIPS, T.D. (1984)
Effect of simultaneous prenatal exposure to ochratoxin A and citrinin
in the rat. J. Toxicol. environ. Health, 13: 553-561.
MEISNER, H. (1976) Energy-dependent uptake of ochratoxin A by
mitochondria. Arch. Biochem. Biophys., 173: 132-140.
MEISNER, H. & CHAN, S. (1974) Ochratoxin A, an inhibitor of
mitochondrial transport systems. Biochemistry, 13: 2795-2800.
MEISNER, H. & KROGH, P. (1982) Inhibition of renal
phosphoenolpyruvate carboxykinase in pigs by alimentary exposure to
ochratoxin A. In: Mycotoxins and Phycotoxins, Proceedings of the 5th
International IUPAC Symposium, Vienna, Technical University Vienna
publ., pp. 342-345.
MEISNER, H. & MEISNER, P. (1981) Ochratoxin A, an in vivo inhibitor
of renal phosphoenolpyruvate carboxykinase. Arch. Biochem. Biophys.,
208: 146-153.
MEISNER, H. & SELANIK, P. (1979) Inhibition of renal
gluconeogenesis in rats by ochratoxin. Biochem. J., 180: 681-684.
MEISNER, H., CIMBALA, M.A., & HANSON, R.W. (1983) Decrease of renal
phosphoenolpyruvate carboxykinase RNA and Poly(A)+ RNA level by
ochratoxin A. Arch. Biochem. Biophys., 223: 264-270.
MERWE, K.J.,VAN DER, J., STEYN, P.S., & FOURIE, L. (1965)
Mycotoxins. Part II. The constitution of ochratoxins A, B, and C,
metabolites of Aspergillus ochraceus Wilh. J. Chem. Soc., 1965:
7083-7088.
MILES, W.F. & GURPRASAD, N.P. (1985) Oxygen negative chemical
ionization mass spectrometry of trichothecenes. Biomed. mass
Spectrom., 12: 652-658.
MINISTRY OF AGRICULTURE, FISHERIES AND FOOD (1980) Survey of
mycotoxins in the United Kingdom, London, Ministry of Agriculture,
Fisheries and Food, 35 pp (Food Surveillance Paper No. 4).
MIROCHA, C.J. & ABBAS, H.K. (1989) Chemistry, occurrence and
toxicology of the haemorrhagic mycotoxin (wortmannin) produced by
Fusarium. In: Natori, S., Hashimoto, K., & Ueno, Y. ed. Mycotoxins
and phycotoxins, Science Publishers, Amsterdam, Oxford, New York,
Elsevier.
MIROCHA, C.J. & PATHRE, S. (1973) Identification of the toxic
principle in a sample of poefusarin. Appl. Microbiol., 26(5): 719-
724.
MIZUNO, S. (1975) Mechanism of inhibition of protein synthesis
initiation by diacetoxyscirpenol and fusarenon-X in the reticulocyte
lysate system. Biochim. Biophys. Acta, 383(2): 207-214.
MORE, J., GALTIER, P., & ALVINERIE, M. (1978) Toxicite de
l'ochratoxine A. III. Effets pendant les stades initiaux de la
gestation chez le rat. Ann. Rech. vet., 9(1): 169-173.
MORGAN, M.R.A., MATTHEW, J.A., MCNERNEY, R., & CHAN, H.W.-S. (1982)
The immunoassay for ochratoxin A. In: Mycotoxins and Phycotoxins,
Proceedings of the 5th International IUPAC Symposium, Vienna,
Technical University Vienna, pp. 32-35.
MOROI, K., SUZUKI, S., KUGA, T., YAMAZAKI, M., & KANISAWA, M. (1985)
Reduction of ochratoxin A toxicity in mice treated with phenylalanine
and phenobarbital. Toxicol. Lett., 25: 1-5.
MORRISSEY, R.E. (1984) Teratological study of Fisher rats fed diet
containing added vomitoxin. Food chem. Toxicol., 22(6): 453-457.
MORRISSEY, R.E. & VESONDER, R.F. (1985) Effect of deoxynivalenol
(vomitoxin) on fertility, pregnancy and postnatal development of
Sprague-Dawley rats. Appl. environ. Microbiol., 49: 1062-1066.
MORTENSEN, H.P., HALD, B., & MADSEN, A. (1983) Feeding experiments
with ochratoxin A contaminated barley for bacon pigs. V. Ochratoxin A
in blood. Acta agric. Scand., 33: 235-239.
MORTIMER, P.H., CAMPBELL, J., DI MEUNA, M.E., & WHITE, E.P. (1971)
Experimental myrotheciotoxicosis and poisoning in ruminants by
verrucarin A and roridin A. Res. vet. Sci., 12: 508-515.
MULDERS, E.J. & IMPELEN-PEEK, H.A.M. (1986) Gas chromatographic
determination of deoxynivalenol in cereals. Z. Lebensm. Unters.
Forsch., 183: 406-409.
MUNRO, I.C., MOODIE, C.A., KUIPER-GOODMAN, T., SCOTT, P.M., & GRICE,
H.C. (1974) Toxicologic changes in rats fed graded dietary levels
of ochratoxin A. Toxicol. appl. Pharmacol., 28(2): 180-188.
MUNSCH, N. & MUELLER, W.E.G. (1980) Effect of T-2 toxin on DNA
polymerases and terminal deoxynucleotidyl transferase of Molt-4 and
Nu-8 cell lines. Immunopharmacology, 2(4): 313-318.
MUTOH, A., ISHII, K., & UENO, Y. (1988) Effect of radioprotective
compounds and anti-inflammatory agents on the acute toxicity of
trichothecenes. Toxicol. Lett., 40: 165-174.
NAGAYAMA, S., KAWAMURA, O., OHTANI, K., RYU, J.C., LATUS, D.,
SUDHEIM, L., & UENO, Y. (1988) Application of an enzyme-linked
immunosorbent assay for screening of T-2 toxin-producing Fusarium
spp. Appl. environ. Microbiol., 54: 1302-1303.
NAKAMURA, Y., TAKEDA, S., OGASAWARA, K., KARASHIMADA, T., & ANDO K.
(1951) [A study on toxicosis caused by Akakabi poisoned wheat
flour.] Hokkaido doritsu Eiseikenkyujoho, 2: 35-46 (in Japanese).
NEELY, W.C. & WEST, A.D. (1972) Spectroanalytical parameters of
fungal metabolites. III. Ochratoxin A. J. Assoc. Off. Anal. Chem.,
55(6): 1305-1309.
NEL, W. & PURCHASE, I.F.H. (1968) The fate of ochratoxin A in rats.
J. S. Afr. Chem. Inst., 21: 87-88.
NELSON, P.E., TOUSSON, T.A., & MARASAS, W.F.O. (1983) Fusarium
species, an illustrated manual for identification, University Park,
Pennsylvania, Pennsylvania State University Press.
NESHEIM, S. (1971) Ochratoxins: occurrence, production, analysis,
and toxicity. Abstracts from the 85th Annual Meeting of the
Association of Official Analytical Chemists, Washington, 11-14
October, 1971, Washington, DC, Association of Official Analytical
Chemists.
NESHEIM, S. (1973) Analysis of ochratoxins A and B and their esters
in barley, using partition and thin-layer chromatography. II.
Collaborative study. J. Assoc. Off. Anal. Chem., 56: 822-826.
NESHEIM, S. (1982) Ochratoxin A- Analytical method 1: thin-layer
chromatographic determination of ochratoxin A in foodstuffs. In:
Egan, H., ed. Environmental carcinogens: selected methods of analysis
- some mycotoxins, Lyons, International Agency for Research on
Cancer, pp. 255-270.
NESHEIM, S., HARDIN, N.F., FRANCIS, O.J., & LANGHAM, W.S. (1973)
Analysis of ochratoxins A and B and their esters in barley, using
partition and thin-layer chromatography. I. Development of the
method. J. Assoc. Off. Anal. Chem., 56: 817-821.
NICOLOV, I.G., CHERNOZEMSKY, I.N., PETKOVA-BOCHAROVA, T., STOYANOV,
I.S., & STOICHEV, I.I. (1978) Epidemiologic characteristics of
urinary system tumours and Balkan nephropathy in an endemic region of
Bulgaria. Eur. J. Cancer, 14: 1237-1242.
NIP, W.K. & CHU, F.S. (1979) Fate of ochratoxin A in goats. J.
environ. Sci. Health, B14: 319-333.
NORPPA, H., PENTTILA, M., SORSA, M., HINTIKKA, E.L., & ILUS, T.
(1980) Mycotoxin T-2 of Fusarium tricinctum and chromosome changes
in Chinese hamster bone marrow. Hereditas, 93(2): 329-332.
NORTHOLT, M.D., VAN EGMOND, H.P., & PAULSCH, W.E. (1979) Ochratoxin
A production by some fungal species in relation to water activity and
temperature. J. food Prot., 42: 485-490.
NOWICKI, T.W., GABA, D.G., DEXTER, J.E., MATSUO, R.R., & CLEAR, R.M.
(1988) Retention of the Fusarium mycotoxin deoxynivalenol in wheat
during processing and cooking of spaghetti and noodles. J. Cereal
Sci., 8: 189-202.
OGASAWARA, K. (1965) [Akakabi-toxicosis.] J. Food Hyg. Soc. Jpn,
6: 81-82 (in Japanese).
OHTA, M., ISII, K., & UENO, Y. (1977) Metabolism of trichothecene
mycotoxins. Part 1. Microsomal deacetylation of T-2 toxin in animal
tissues. J. Biochem. (Tokyo), 82(6): 1591-1598.
OHTSUBO, K. & SAITO, M. (1970) Cytotoxic effects of scirpene
compounds, fusarenon-X produced by Fusarium nivale, dihydro-nivalenol
and dihydrofusarenon-X on Hela cells. Jpn. J. med. Sci. Biol., 23(4):
217-225.
OHTSUBO, K. & SAITO, M. (1977) Chronic effects of trichothecene
toxins. In Rodricks, J.V., Hesseltine, C.W., & Mehlman, M.A., ed.
Mycotoxins in human and animal health, Park Forest South, Illinois,
Pathotox Publishers, pp. 255-262.
OHTSUBO, K., YAMADA, M., & SAITO, M. (1968) Inhibitory effect of
nivalenol, a toxic metabolite of Fusarium nivale on the growth cycle
and biopolymer synthesis of Hela cells. Jpn. J. med. Sci. Biol.,
21(3): 185-194.
OHTSUBO, K., KADEN, P., & MITTERMAYER, C. (1972) Polyribosomal
breakdown in mouse fibroblasts L cells by fusarenon-X, a toxic
principle isolated from Fusarium nivale. Biochim. Biophys. Acta,
287(3): 520-525.
OHTSUBO, K., RYU, J.-C., NAKAMURA, K., IZUMIYAMA, N., TANAKA, T.,
YAMAMURA, H., KOBAYSAHI, T., & UENO, Y. (in press) Chronic toxicity
of nivalenol in mice: 2-year feeding trial with Fusarium nivale Fn
2B-moulded rice. Food Chem. Toxicol.
ORTI, D.L., HILL, R.H., LIDDLE, J.A., & NEEHAM, L.L. (1986) High-
performance liquid chromatography of mycotoxin metabolites in human
urine. J. anal. Toxicol., 10: 41-45.
OSBORNE, B.G. (1980) The occurrence of ochratoxin A in mouldy bread
and flour. Food Cosmet. Toxicol., 18: 615-617.
OSBORNE, B.G. & WILLIS, K. (1984) Studies into the occurrence of some
trichothecene mycotoxins in UK home-grown wheat and in imported
wheat. J. Sci. Food Agric., 35: 579-583.
OTOKAWA, M., SHIBAHARA, Y., & EGASHIRA, Y. (1979) The inhibitory
effect of T-2 toxin on tolerance induction of delayed-type
hypersensitivity in mice. Jpn. J. med. Sci. Biol., 32(1): 37-46.
PACE, J.G., WATTS, M.R., BURROWS, E.P., DINTERMAN, R.E., MATSON, C.,
HAUER, E.C., & WANNEMACHER, R.W., Jr (1985) Fate and distribution of
3H labeled T-2 mycotoxin in guinea pigs. Toxicol. appl. Pharmacol.,
80: 377-385.
PANG, V.F., LAMBERT, R.J., FELSBURG, P.J., BEASLEY, V.R., BUCK, W.B,.
& HASCHEK (1987a) Experimental T-2 toxicosis in swine following
inhalation exposure: effects on pulmonary and systemic immunity and
morphologic changes. Toxicol. Pathol., 15: 308-319.
PANG, V.F., LORENZANA, R.M., BEASLEY, V.R., BUCK, W.B., & HASCHEK,
W.M. (1987b) Experimental T-2 toxicosis in swine. III. Morphologic
changes following intravascular administration of T-2 toxic. Fundam.
appl. Toxicol., 8: 298-309.
PANG, V.F., LAMBERT, R.J., FELSBURG, P.J., BEASLEY, V.R., BUCK, W.B.,
& HASCHEK, W.M. (1988) Experimental T-2 toxicosis in swine following
inhalation exposure: clinical signs and effects on hematology, serum
biochemistry and immune response. Fundam. appl. Toxicol., 11:
100-109.
PARKER, G.W., WANNEMACHER, R.W., Jr, & GILMAN, F.G. (1984) The effect
of T-2 mycotoxin on the cardiovascular system in the guinea pig. Fed.
Proc., 43: 578.
PATTERSON, D.S.P., MATTHEWS, J.G., SHREEVE, B.J., ROBERTS, B.A.,
MCDONALD, S.M., & HAYES, A.W. (1979) The failure of trichothecene
mycotoxins and whole cultures of Fusarium tricinctum to cause
experimental haemorrhagic syndromes in calves and pigs. Vet. Rec.,
105(11): 252-253.
PATTERSON, D.S.P., SHREEVE, B.J., ROBERTS, B.A., BERRETT, S., BRUSH,
P.J., GLANCY, E.M., & KROGH, P. (1981) Effect on calves of barley
naturally contaminated with ochratoxin A and groundnut meal
contaminated with low concentrations of aflatoxin B1. Res. vet. Sci.,
31: 213-218.
PAULSCH, W.E., VAN EGMOND, H.P., & SCHULLER, P.L. (1982) Thin-layer
chromatographic method for analysis and chemical confirmation of
ochratoxin A in kidneys of pigs. In: Mycotoxins and Phycotoxins,
Proceedings of the 5th International IUPAC Symposium, Vienna,
Technical University Vienna publ., pp. 40-43.
PAVLOVIC, M., PLESTINA, R., & KROGH, P. (1979) Ochratoxin A
contamination of foodstuffs in an area with Balkan (endemic)
nephropathy. Acta pathol. microbiol. Scand. B87: 243-246.
PAWLOSKY, R.J. & MIROCHA, C.J. (1984) Structure of a metabolic
derivative of T-2 toxin (TC-6) based on mass spectrometry. J. agric.
food Chem., 32: 1420-1423.
PECKHAM, J.C., DOUPNIK, B., & JONES, O.H. (1971) Acute toxicity of
ochratoxins A and B in chicks. Appl. Microbiol., 21(3): 492-494.
PEDERSEN, E. & HANSEN, H.N. (1981) [Ochratoxin A in grain and grain
products.] Copenhagen, Stat. Levnedsm. Institute (Report F8l002) (in
Danish).
PEPELJNJAK, S. & CVETNIC, Z. (198l) [Ochratoxogenicity of
Aspergillus ochraceus strains from the territory of endemic
nephropathy.] Vet. Arch., 51: 101-103 (in Croatian).
PEPELJNJAK, S., BLAZEVIC, N., & CULJAK, N. (1982) Histopathological
changes and finding of ochratoxin A in organs of pig in the area of
endemic nephropathy in Yugoslavia. In: Mycotoxins and Phycotoxins,
Proceedings of the 5th International IUPAC Symposium, Vienna,
Technical University Vienna publ., pp. 346-348.
PESTKA, J.J., LEE, S.C., LAU, H.P., & CHU, F.S. (1981) Enzyme-
linked immunosorbent assay for T-2 toxin. J. Assoc. Off. Anal. Chem.,
58(12): 940A-944A.
PESTKA, J.J., LIN, W.S., & MITHS, E.R. (1987a) Emetic activity of the
trichothecene 15-acetyldeoxynivaleol in swine. Food chem. Toxicol.,
25: 855-858.
PESTKA, J.J., TAI, J.H., WITT, W.F., DIXON, D.E., & FORSELL, J.H.
(1987b) Suppression of immune response in the B6C3F1 mouse after
dietary exposure to the Fusarium mycotoxins deoxynivalenol
(vomitoxin) and zearalenone. Food chem. Toxicol., 25: 297-304.
PETKOVA-BOCHAROVA, T. & CASTEGNARO, M. (1985) Ochratoxin A
contamination of cereals in an area of high incidence of Balkan
endemic nephropathy in Bulgaria. Food Addit. Contam., 2: 267-270.
PETKOVA-BOCHAROVA, T., CHERNOZEMSKY, I.N., & CASTEGNARO, M. (1988)
Ochratoxin A in human blood in relation to Balkan endemic nephropathy
and urinary system tumours in Bulgaria. Food Addit. Contam., 5:
299, 301.
PIENAAR, J.G., KELLERMAN, T.S., & MARASAS, W.F.O. (1981) Field
outbreaks of leukoencephalomalacia in horses consuming maize infected
by Fusarium verticillioides (F. moniliforme) in South Africa. J. S.
Afr. Vet. Assoc., 52: 21-24.
PIER, A.C., CYSEWSKI, S.J., RICHARD, J.L., BAETZ, A.L., & MITCHELL,
L. (1976) Experimental mycotoxicoses in calves with aflatoxin,
ochratoxin, rubratoxin, and T-2 toxin. In: Proceedings of the 80th
Annual Meeting of the US Animal Health Association, Miami Beach,
Florida, 7-12 November, 1976, Richmond, Virginia, US Animal Health
Association, pp. 130-148.
POHLAND, A.E., SCHULLER, P.L., STEYN, P.S., & VAN EGMOND, H.P.
(1982) Physicochemical data for some selected mycotoxins. Pure appl.
Chem., 54: 2219-2284.
POHLAND, A.E., THORPE, C., TRUCKSESS, W., & EPPLEY, R. (1986) TLC and
HPLC methods for analysis of trichothecenes in commodities. In:
Richard, J. & Thurston, J., ed. Diagnosis of mycotoxicoses,
Dordrecht, Boston, Martinus Nijhoff Publishers, pp. 271-281.
POHLAND, A.E. & WOOD, G.E. (1987) Occurrence of mycotoxins in food.
In: Krogh, P., ed. Mycotoxins in foods, London, Academic Press, pp.
35-64.
POKROVSKIJ, V.I. & TUTELYAN, V.A. (1982) [Ergotism: a companion of
natural disaster.] Ter. Ark., 108-110 (in Russian).
POPPE, S.M., STUCKHARDT, J.L., & SZCZECH, G.M. (1983) Postnatal
behavioural effects of ochratoxin A in offspring of treated mice.
Teratology, 27: 293-300.
PRELUSKY, D.B., TRENHOLM, H.L., LAWRENCE, G.A., & SCOTT, P.M. (1984)
Nontransmission of deoxynivalenol (vomitoxin) to milk following oral
administration to dairy cows. J. environ. Sci. Health, B19(7): 593-
609.
PRELUSKY, D.B., VEIRA, D.M., & TRENHOLM, H.L. (1985) Plasma
pharmacokinetics of the mycotoxin deoxynivalenol following oral and
intravenous administration to sheep. J. environ. Sci. Health, B20:
603-624.
PRELUSKY, D.B., TRENHOLM, H.L., HAMILTON, R.M.G., & MILLER, J.D.
(1987) Transmission of [14C]deoxynivalenol to eggs following oral
administration to laying hens. J. agric. food Chem., 35: 182-186.
PRIOR, M.G. (1976) Mycotoxin in determinations on animal feedstuffs
and tissues in Western Canada. Can. J. comp. Med., 40: 75-79.
PRIOR, M.G. (198l) Mycotoxins in animal feedstuffs and tissues in
Western Canada 1975 to 1976. Can. J. comp. Med., 45: 116-119.
PRIOR, M.G. & SISODIA, C.S. (1978) Ochratoxicosis in white leghorn
hens. Poult. Sci., 57: 619-623.
PRIOR, M.G. & SISODIA, C.S. (1982) The effects of ochratoxin A on
the immune response of Swiss mice. Can. J. comp. Med., 46: 91-96.
PRIOR, M.G., SISODIA, C.S., & O'NEIL, J.B. (1976) Acute oral
ochratoxicosis in day-old white leghorns, turkeys and Japanese quail.
Poult. Sci., 55: 786-790.
PUCHLEV, A. (1973) La nephropathie endemique en Bulgarie. In:
Symposium sur la nephropathie endemique de l'Academie serbe des
sciences et des arts, Belgrade, pp. 15-27.
PUCHLEV, A., ed. (1974) Endemic nephropathy. In: Proceedings of the
2nd International Symposium on Endemic Nephropathy, Sofia, Bulgarian
Academy of Sciences, pp. 1-343.
PULS, R. & GREENWAY, J.A. (1976) Fusariotoxicosis from barley in
British Columbia. II. Analysis of suspected barley. Can. J. comp.
Med., 40(1): 16-19.
PURCHASE, I.F.H. & THERON, J.J. (1968) The acute toxicity of
ochratoxin A to rats. Food Cosmet. Toxicol., 6: 479-483.
QIUJIE, X., XIAOQIU, L., JIANLI, W., & YUNSIAN, L. (1988)
Trichothecenes in staple food from high incidence area of carcinoma
of esophagus and gastric cardia and their carcinogenic potential.
Zhonghua Zhongliu Zoshi, 10: 4-8.
RABIE, C.J., SYDENHAM, E.W., THEL, P.G., LUBBEN, A., & MARASAS,
W.F.O. (1986) T-2 toxin production by Fusarium acuminatum isolated
from oats and barley. Appl. environ. Microbiol., 52(3): 594-596.
RAFAI, P. & TUBOLY, S. (1982) Effect of T-2 toxin on adrenocortical
function and immune response in growing pigs. Zbl. Vet. Med., B29:
558-565.
RAJAKYLA, E., LAASASENAHO, K., & SAKKERS, P.J.D. (1987) Determination
of mycotoxins in grain by high-performance liquid chromatography and
thermospray liquid chromatography-mass spectrometry. J. Chromatogr.,
384: 391-402.
REICHMANN, K.G., BLANEY, B.J., CONNOR, J.K., & RUNGE, B.M. (1982)
The significance of aflatoxin and ochratoxin in the diet of
Australian chickens. Aust. vet. J., 58: 211-212.
REISS, J. (1973) Influence of the mycotoxins patulin and
diacetoxyscirpenol on fungi. J. gen. appl. Microbiol., 19(6):
415-420.
REISS, J. (1975) Mycotoxin poisoning of Allium cepa root tips. Part
2. Reduction of mitotic index and formation of chromosomal
aberrations and cytological abnormalities by patulin, rubratoxin B
and diacetoxyscirpenol. Cytologia, 39(4): 703-708.
REISS, J. (1977) Inhibition of urease by the mycotoxin patulin.
Naturwissenschaften, 64: 97.
RIBELIN, W.E., FUKUSHIMA, K., & STILL, P.E. (1978) The toxicity of
ochratoxin to ruminants. Can. J. comp. Med., 42: 172-176.
ROBISON, T.S., MIROCHA, C.J., KURTZ, H.J., BEHRENS, J.C., CHI, M.S.,
WEAVER, G.A., & NYSTROM, S.D. (1979a) Transmission of T-2 toxin
into bovine and porcine milk. J. dairy Sci., 62(4): 637-641.
ROBISON, T.S., MIROCHA, C.J., KURTZ, H.J., BEHRENS, J.C., WEAVER,
G.A., & CHI, M.S. (1979b) Distribution of tritium labeled T-2 toxin
in swine. J. agric. food Chem., 27(6): 1411-1413.
ROGERS, C.G. & HEROUX-METCALF, C. (1983) Cytotoxicity and absence of
mutagenic activity of vomitoxin (4-deoxynivalenol) in a hepatocyte-
mediated assay with V79 Chinese hamster lung cells. Cancer Lett.,
20: 29-35.
ROMER, T.R. (1986) Use of small charcoal/alumina cleanup columns in
determination of trichothecene mycotoxins in foods and feeds. J.
Assoc. Off. Anal. Chem., 69: 699-703.
ROMER, T.R., BOLING, T.M., & MACDONALD, J.L. (1978). Gas-liquid
chromatographic determination of T-2 toxin and diacetoxyscirpenol in
corn and mixed feeds. J. Assoc. Off. Anal. Chem., 61(4): 801-808.
ROOD, H.D., BUCK, W.B., & SWANSON, S.P. (1988a) Gas chromatographic
screening method for T-2 toxin, diacetoxyscirpenol, deoxynivalenol
and related trichothecenes in feeds. J. Assoc. Off. Anal. Chem., 71:
493-498.
ROOD, H.D., BUCK, W.B., & SWANSON, S.P. (1988b) Diagnostic screening
method for determination of trichothecene exposure in animals. J.
agric. food Chem., 36: 74-79.
ROSEN, J.D., ROSEN, R.T., & HARTMAN, T.G. (1986) Capillary gas
chromatography-mass spectrometry of several macrocyclic
trichothecenes. J. Chromatogr., 355: 241-251.
ROSEN, R.T. & ROSEN, J.D. (1984) Quantification and confirmation of
four Fusarium mycotoxins in corn by gas chromatography-mass
spectrometry. J. Chromatogr., 283: 223-230.
ROSENSTEIN, Y. & LAFARGE-FRAYSSINET, C. (1983) Inhibitory effect of
Fusarium T-2 toxin on lymphoid DNA and protein synthesis. Toxicol.
appl. Pharmacol., 70: 283-288.
ROSENSTEIN, Y., LAFARGE-FRAYSSINET, C., LESPINATS, G., LOISILLIER,
F., LAFONT, P., & FRAYSSINET, C. (1979) Immunosuppressive activity
of Fusarium toxins. Effects on antibody synthesis and skin grafts of
crude extracts of T-2 toxin and diacetoxyscirpenol. Immunology,
36(1): 111-118.
ROUSSEAUX, C.G. & SHEIFER, H.B. (1987) Maternal toxicity,
embryolethality and abnormal fetal development in CD-1 mice following
one oral dose of T-2 toxin. J. appl. Toxicol., 7: 281-288.
ROUSSEAUX, C.G., SHIEFER, H.B., & HANCOCK, D.S. (1986) Reproductive
and teratological effects of continuous low-level dietary T-2 toxin
in female CD-1 mice for two generations. J. appl. Toxicol., 6:
179-184.
ROUSSEAU, D.M. & VAN PETEGHEM, C.H. (1989) Spontaneous occurrence of
ochratoxin A residues in porcine kidneys in Belgium. Environ. Contam.
Toxicol., 42: 181-186.
ROUSSEAU, D.M., CANDLISH, A.A.G., SLEGERS, G.A., VAN PETEGHEM, C.H.,
STINSON, W.H., & SMITH, J.E. (1987) Detection of ochratoxin A in
porcine kidneys by a monoclonal antibody-based radioimmunoassay.
Appl. environ. Microbiol., 53: 514-518.
RUKMINI, C. & BHAT, R.V. (1978) Occurrence of T-2 toxin in Fusarium
incarnatum infested sorghum from India. J. agric. food Chem., 26
(3): 647-649.
RUKMINI, C., PRASAD, J.S., & RAO, K. (1980) Effect of feeding T-2
toxin to rats and monkeys. Food Cosmet. Toxicol., 18(3): 267-270.
RUSCH, M.E. & STAHELIN, H. (1965) [Some biological effects of the
cytostatic agent verrucarin A.] Arzneimittelforschung, 15: 893-897
(in German).
RUTQVIST, L., BJORKLUND, N.-E., HULT, K., & GATENBECK, S. (1977)
Spontaneous occurrence of ochratoxin residues in kidneys of fattening
pigs. Zbl. Veterinaermed., A24(5): 402-408.
RUTQVIST, L., BJORKLUND, N.-E., HULT, K., HOKBY, E., & CARLSSON, B.
(1978) Ochratoxin A as the cause of spontaneous nephropathy in
fattening pigs. Appl. environ. Microbiol., 36: 920-925.
RYU, J.C., SHIRAKI, N., & UENO, Y. (1987) Effects of drugs and
metabolic inhibitions on the acute toxicity of T-2 toxin in mice.
Toxicon, 25: 743-750.
RYU, J.-C., OHTSUBO, K., IZUMIYAMA, N., NAKAMURA, K., TANAKA, T.,
YAMAMURA, H., & UENO, Y. (1988) The acute and chronic toxicities of
nivalenol in mice. Fundam. appl. Toxicol., 11: 38-47.
SAITO, M. & OHTSUBO, K. (1974) Trichothecene toxins of Fusarium
species. In: Purchase, I.F.H., ed. Mycotoxins, Amsterdam, Oxford, New
York, Elsevier Science Publishers, pp. 263-281.
SAITO, M., HORIUCHI, T., OHTSUBO, K., HATANAKA, Y., & UENO, Y.
(1980) Low tumor incidence in rats with long-term feeding of
fusarenon-X a cytotoxic trichothecene produced by Fusarium nivale.
Jpn. J. exp. Med., 50(40): 293-302.
SAKAMOTO, T., SWANSON, S.P., YOSHIZAWA, T., & BUCK, W.B. (1986)
Structures of new metabolites of diacetoxyscirpenol in the excreta of
orally administered rats. J. agric. food Chem., 34: 698-701.
SANDOR, G., GLAVITS, R., VAJDA, L., VANYI, A., & KROGH, P. (1982)
Epidemiological study of ochratoxin A-associated porcine nephropathy
in Hungary. In: Mycotoxins and Phycotoxins, Proceedings of the 5th
International IUPAC Symposium, Technical University Vienna publ.,
Vienna, pp. 349-352.
SANO, A., ASABE, Y., TAKITANI, S., & UENO, Y. (1982).
Fluorodensitometric determination of trichothecene mycotoxins with
nicotinamide and 2-acetylpyridine on a silica gel layer. J.
Chromatogr., 235: 257-265.
SANO, A., MATSUTANI, S., SUZUKI, M., & TAKITANI, S. (1987) High-
performance liquid chromatographic method for determining
trichothecene mycotoxins by post-column fluorescence derivatization.
J. Chromatogr., 410: 427-436.
SANSING, G.A., LILLEHOJ, E.B., DETROY, R.W., & MILLER, M.A. (1976)
Synergistic toxic effects of citrinin, ochratoxin A, and penicillic
acid in mice. Toxicon, 14: 213-219.
SARKISOV, A.Ch., KVASCHINA, E.S., KORNEEV, N.E., KOROLEVA, V.P.,
GERASIMOVA, P.N., & AKILOVA, N.S. (1944) [Harmfulness of cereals
over-wintered in the field.] Veterinariya, 11-12: 39-41. (in
Russian).
SATO, N., UENO, Y., & ENOMOTO, M. (1975) Toxicological approaches
to the toxic metabolites of Fusaria. Part 8. Acute and subacute
toxicities of T-2 toxin in cats. Jpn. J. Pharmacol., 25(3): 263-270.
SATO, N. & UENO, Y. (1977) Comparative toxicities of
trichothecenes. In: Rodricks, J.V., Hesseltine, C.W., & Mehlman,
M.A., ed. Mycotoxins in human and animal health, Park Forest South,
Illinois, Pathotox Publishers, pp. 295-307.
SATO, N., ITO, T., KUMADA, H., UENO, Y., ASANO, K., SAITO, M.,
OHTSUBO, K., UENO, I., & HATANAKA, Y. (1978) Toxicological
approaches to the metabolites of Fusaria. XIII. Hematological changes
in mice by a single and repeated administrations of trichothecenes.
J. toxicol. Sci., 3(4): 335-356.
SATO, S., OHYA, T., HOMMA, S., & TATSUNO, T. (1981) [Effects of
fusarenon-X on serological responses of chicks inoculated with
newcastle disease vaccines.] Proc. Jpn. Assoc. Mycotoxicol., 13:
43-45 (in Japanese).
SCHIEFER, H.B., ROUSSEAUX, C.G., HANCOCK, D.S., & BLAKLEY, B.R.
(1987) Effects of low-level long-term oral exposure to T-2 toxin in
CD-1 mice. Food chem. Toxicol., 25: 593-601.
SCHMIDT, R. & DOSE, K. (1984) HPLC: A tool for the analysis of T-2
toxin and HT-2 toxin in cereals. J. anal. Toxicol., 8: 43-45.
SCHINDLER, D. (1974) Two classes of inhibitors of peptidyl
transferease activity in eukaryotes. Nature (Lond.), 249(5452):
38-41.
SCHINDLER, D., GRANT, P., & DAVIES, J. (1974) Trichodermin
resistance mutation affecting eukaryotic ribosomes. Nature (Lond.),
248(5448): 535-536.
SCOTT, P.M. (1977) Penicillium mycotoxins. In: Wyllie, T.D. &
Morehouse, L.G., ed. Mycotoxic fungi, mycotoxins, mycotoxicoses, New
York, Marcel Dekker, pp. 283-356.
SCOTT, P.M. (1982) Assessment of quantitative methods for
determination of trichothecenes in grains and grain products. J.
Assoc. Off. Anal. Chem., 65(4): 876-883.
SCOTT, P.M. (1984) Effects of food processing on mycotoxins. J. food
Prot., 47(6): 489-499.
SCOTT, P.M. & KANHERE, S.R. (1986) Comparison of column phases for
separation of derivatized trichothecenes by capillary gas
chromatography. J. Chromatogr., 368: 374-380.
SCOTT, P.M. & LAWRENCE, G.A. (1980) Analysis of ergot alkaloids in
flour. J. agric. food Chem., 28: 1258-1261.
SCOTT, P.M. & LAWRENCE, G.A. (1982) Losses of ergot alkaloids
during making of bread and pancakes. J. agric. food Chem., 30:
445-450.
SCOTT, P.M., LAWRENCE, J.W., & VAN WALBEEK, W. (1970) Detection of
mycotoxins by thin-layer chromatography: application to screening of
fungal extracts. Appl. Microbiol., 20(5): 839-842.
SCOTT, P.M., WALBEEK, W., VAN, KENNEDY, B., & ANYETI, D. (1972)
Mycotoxins (ochratoxin A, citrinin, and sterigmatocystin) and
toxigenic fungi in grains and other agricultural products. J. agric.
food Chem., 20: 1103-1109.
SCOTT, P.M., HARWIG, J., & BLANCHFIELD, B.J. (1980) Screening
Fusarium strains isolated from over-wintered Canadian grains for
trichothecenes. Mycopathologia, 72(3): 175-180.
SCOTT, P.M., LAU, P.Y., & KANHERE, S.R. (1981) Gas chromatography
with electron capture and mass spectrometric detection of
deoxynivalenol in wheat and other grains. J. Assoc. Off. Anal. Chem.,
64(6): 1364-1371.
SCOTT, P.M., KANHERE, S.R., LAU, P.-Y., DEXTER, J.E., & GREENHALGH,
R. (1983) Effects of experimental flour milling and bread baking on
retention of deoxynivalenol (vomitoxin) in hard red spring wheat.
Cereal Chem., 60: 421-424.
SCOTT, P.M., NELSON, K., KANHERE, S.R., KARPINSKI, K.F., HAYWARD, S.,
NIESH, G.A., & TEICH, A.H. (1984a) Decline in deoxynivalenol
(vomitoxin) concentrations in 1983 Ontario winter wheat before
harvest. Appl. environ. Microbiol., 48(6): 884-886.
SCOTT, P.M., KANHERE, S.R., DEXTER, J.E., BRENNAN, P.W., & TRENHOLM,
H.L. (1984b) Distribution of the trichothecene mycotoxin
deoxynivalenol (Vomitoxin) during the milling of naturally
contaminated hard red spring wheat and its fate in baked products.
Food Addit. Contam., 1(4): 313-323.
SCOTT, P.M., KANHERE, S.R., & TARTER, E.J. (1986) Determination of
nivalenol and deoxynivalenol in cereals by electron-capture gas
chromatography. J. Assoc. Off. Anal. Chem., 69: 889-893.
SEITZ, L.M., YAMAZAKI, W.T., CLEMENTS, R.L., MOHR, H.E., & ANDREWS,
L. (1985) Distribution of deoxynivalenol in soft wheat mill
streams. Cereal Chem., 62(6): 467-469.
SHEPHERD, M.J. & GILBERT, J. (1988) Long-term stability of
deoxynivalenol standard reference solutions. J. agric. food Chem.,
36: 305-308.
SHIMIZU, T., NAKANO, N., MATSUI, T., & AIBARA, K. (1979)
Hypoglycemia in mice administered fusarenon-X. Jpn. J. med. Sci.
Biol., 32(4): 189-198.
SHOTWELL, O.L., HESSELTINE, C.W., & GOULDEN, M.L. (1969) Ochratoxin
A: occurrence as natural contaminant of a corn sample. Appl.
Microbiol., 17(5): 765-766.
SHOTWELL, O.L., HESSELTINE, C.W., VANDEGRAFT, E.E., & GOULDEN, M.L.
(1971) Survey of corn from different regions for aflatoxin,
ochratoxin, and zearalenone. Cereal Sci. Today, 16(9): 266-273.
SHOTWELL, O.L., GOULDEN, M.L., & HESSELTINE, C.W. (1976) Survey of
US wheat for ochratoxin and aflatoxin. J. Assoc. Off. Anal. Chem.,
59: 122-124.
SHOTWELL, O.L., NENNETT, G.A., STUBBLEFIELD, R.D., SHANNON, G.M.,
KWOLEK, W.F., & PLATTNER, R.D. (1985) Deoxynivalenol in hard red
winter wheat: Relationship between toxin levels and factors that
could be used in grading. J. Assoc. Off. Anal. Chem., 68: 954-957.
SHREEVE, B.J., PATTERSON, D.S.P., & ROBERTS, B.A. (1979) The carry-
over of aflatoxin, ochratoxin and zearalenone from naturally
contaminated feed to tissues, urine and milk of dairy cows. Food
Cosmet. Toxicol., 17: 151-152.
SIDDIQUI, M.R. & KHAN, I.D. (1973) Renaming Claviceps microcephala
ergot fungus on Pennisetum typhoides in India as Claviceps
fusiformis. Trans. Mycol. Soc. Jpn, 144: 195-198.
SIEGFRIED, R. (1977) [ Fusarium toxin (trichothecene toxin) in feed
corn.] Landwirtsch. Forsch., Sonderh., 34(1): 37-43 (in German).
SIGG, H.P., MAULI, R., FLURY, E., & HANSER, D. (1965) The
constitution of diacetoxyscirpenol. Helv. Chim. Acta, 48: 962-988.
SINTOV, A., BIALER, M., & YAGEN, B. (1986) Pharmacokinetics of T-2
toxin and its metabolite HT-2 toxin, after intravenous administration
in dogs. Drug Metab. Disp., 14: 250-254.
SINTOV, A., BIALER, M., & YAGEN, B. (1987) Pharmacokinetics of T-2
tetraol, a urinary metabolite of the trichothecene mycotoxin, T-2
toxin, in dogs. Xenobiotica, 17: 941-950.
SIRAJ, M.Y., PHILLIPS, T.D., & HAYES, A.W. (1981) Effects of the
mycotoxins citrinin and ochratoxin A on hepatic mixed-function
oxidase and adenosinetriphosphatase in neonatal rats. J. Toxicol.
environ. Health, 8: 131-140.
SIREN, A.L. & FEUERSTEIN, G. (1986) Effect of T-2 toxin on regional
blood flow and vascular resistance in the conscious rat. Toxic. appl.
Pharmacol., 38: 438-444.
SIRIWARDANA, T.M.G. & LAFONT, P. (1978) New sensitive biological
assay for 12,13-epoxytrichothecenes. Appl. environ. Microbiol.,
35(1): 206-207.
SMALLEY, E.B., MARASAS, W.F.O., STRONG, F.M., BAMBURG, J.R., NICHOLS,
R.E., & KOSARI, N.R. (1970) Mycotoxicosis associated with moldy corn.
In: Herzberg, M., ed. Proceedings of the first US-Japan Conference on
Toxic Micro-organisms, Mycotoxins, Botulism, Honolulu, Hawaii, 7-10
October, 1968, Washington, DC, US Department of the Interior and UJNR
Joint Panels on Toxic Micro-organisms, pp. 163-173.
SMITH, R.D., UDSETH, H.R., & WRIGHT, B.W. (1985) Rapid and high
resolution capillary fluid chromatography (SFC) and SFC/MS of
trichothecene mycotoxins. J. chromatogr. Sci., 23: 192-199.
SORSA, M., LINSSAINMAA, K., PAITTELA, M., & ILUS, T. (1980)
Evaluation of the mutagenicity of epoxytrichothecene mycotoxins in
Drosophilia melanogaste. Hereditas, 92: 163-165.
SPEERS, G.M., MIROCHA, C.J., CHRISTENSEN, C.M., & BEHRENS, J.C.
(1977) Effects on laying hens of feeding corn invaded by 2 species
of Fusarium and pure T-2 mycotoxin. Poult. Sci., 56(1): 98-102.
STACK, M.E., MISLIVEC, P.B., GIBSON, R., POHLAND, A.E., & DENIZEL, T.
(1982) Mycotoxins produced by isolates of Aspergillus ochraceus
found in coffee. In: Mycotoxins and Phycotoxins, Proceedings of the
5th International IUPAC Symposium, Vienna, Technical University
Vienna, pp. 195-199.
STAFFORD, M.E. & MCLAUGHLIN, C.S. (1973) Trichodermin, a possible
inhibitor of the termination process of protein synthesis. J. cell.
Physiol., 82: 121-128.
STAHELIN, H., KALBERER-RUESCH, M.E., SIGNER, E., & LAZARY, S. (1968)
[Some biological effects of the cytostatic diacetoxyscirpenol.]
Arzneimittelforschung, 18: 989-994 (in German).
STAHL, C., VANDERHOEF, L.N., SIEGEL, N., & HELGESON, J.P. (1973)
Fusarium tricinctum T-2 toxin inhibits auxin promoted elongation in
soybean hypocotyl. Plant Physiol., 52(6): 663-666.
STAHR, H.M., HYDE, W., LEDERDAL, D., & PFEIFFER, R. (1981)
Trichothecene mycotoxin analysis for veterinary diagnostic
toxicology. Abstracts 95th Annual Meeting of AOAC., 191: 65.
STANFORD, G.K., HOOD, R.D., & HAYES, A.W. (1975) Effect of prenatal
administration of T-2 toxin to mice. Res. Commun. chem. Pathol.
Pharmacol., 10(4): 743-746.
STEYN, P.S. (1977) Mycotoxins, excluding aflatoxin, zearalenone and
the trichothecenes. In: Rodericks, J.V., Hesseltine, C.W., &
Mehlman, M.A., ed. Mycotoxins in human and animal health, Park Forest
South, Illinois, Pathotox Publishers, pp. 419-467.
STEYN, P.S. (1984) Ochratoxins and related dihydro-iso-coumarins.
In: Betina, V., ed. Mycotoxins: production, isolation, separation,
and purification, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 183-216.
STEYN, P.S., VLEGGAAR, R., DU PREEZ, N.P., BLYTH, A.A., & SEEGERS,
J.C. (1975) The in vitro toxicity of analogs of ochratoxin A in
monkey kidney epithelial cells. Toxicol. appl. Pharmacol., 32:
198-203.
STOREN, O., HOLM, H., & STORMER, F.C. (1982) Metabolism of
ochratoxin A by rats. Appl. environ. Microbiol., 44: 785-789.
STORMER, F.C. & PEDERSEN, J. (1980) Formation of 4-
hydroxyochratoxin A from ochratoxin A by rat liver microsomes.
Appl. environ. Microbiol., 39: 971-975.
STORMER, F.C., HANSEN, C.E., PEDERSEN, J.I., HVISTENDAHL, G., &
AASEN, A.J. (1981) Formation of (4R)- and (AS)-4-hydroxyochratoxin
A from ochratoxin A by liver microsomes from various species. Appl.
environ. Microbiol., 42: 1051-1056.
STOYANOV, I.S., CHERNOZEMSKY, I.N., NICOLOV, I.G., STOICHEV, I.I., &
PETKOVA-BOCHAROVA, T.K. (1978) Epidemiologic association between
endemic nephropathy and urinary system tumours in an endemic region.
J. chron. Dis., 31: 721-724.
SUNDHEIM, L., NAGAYAMA, S., KAWAMURA, O., TANAKA, T., BRODAL, G., &
UENO, Y. (1988) Trichothecenes and zearalenone in Norwegian barley
and wheat. Norw. J. agric. Sci., 2: 49-59.
SUZUKI, S. & SATOH, T. (1973) Effect of ochratoxin A on tissue
glycogen levels in rats. Jpn. J. Phamacol., 23: 415-419.
SUZUKI, S., SATOH, T., & YAMAZAKI, M. (1975) Effect of ochratoxin A
on carbohydrate metabolism in rat liver. Toxicol. appl. Pharmacol.,
32(1): 116-122.
SUZUKI, S., SATOH, T., & YAMAZAKI, M. (1977) The pharmacokinetics
of ochratoxin A in rats. Jpn. J. Pharmacol., 27: 735-744.
SUZUKI, T., KURISU, M., HOSHINO, Y., ICHINOE, M., NOSE, N., TOKUMARU,
Y., & WATANABE, Y. (1980) [Production of trichothecene mycotoxins of
Fusarium species in wheat and barley harvested in Saitama Prefecture,
Japan.] J. Food Hyg. Soc. Jpn, 21(1): 43-49 (in Japanese).
SWANSON, S.P., NICOLETTI, J., ROOD,.H.D., Jr, BUCK, W.B., COTE, L.M.,
& YOSHIZAWA, T. (1987) Metabolism of three trichothecene mycotoxins,
T-2 toxin, diacetoxyscirpenol and deoxynivalenol, by bovine rumen
microorganisms. J. agric. food Chem., 32: 1181-1183.
SWANSON, S.P., RAMASWAMY, V., BEASLEY, V.R., BUCK, W.B., &
BURMEISTER, H.H. (1983) Gas-liquid chromatographic determination of
T-2 toxin in plasma. J. Assoc. Off. Anal. Chem., 66: 909.912.
SWANSON, S.P., DAHLEM, A.M., ROOD, H.D., COTE, L.M., & YOSHIZAWA, T.
(1986) Gas chromatographic analysis of milk for deoxynivalenol and
its metabolite DOM-1. J. Assoc. Off. Anal. Chem., 69: 41-43.
SYLVIA, V.L., PHILLIPS, T.D., CLEMINT, B.A., GREEN, J.L., KUBENA,
L.F., & HEIDELBAUGH, N.D. (1986) Determination of deoxynivalenol
(vomitoxin) by high performance liquid chromatography with
electrochemical detection. J. Chromatogr., 362: 79-85.
SZATHMARY, C.I. (1983) Trichothecene toxicoses and natural
occurrence in Hungary. In: Ueno, Y., ed. Developments in food
science. IV. Trichothecenes, New York, Elsevier, pp. 229-250.
SZATHMARY, C.I. & RAFAI, P. (1978) Refusal of food contaminated
with T-2 fusariotoxin. Magy. Allatorv. Lapja, 33: 685-688.
SZCZECH, G.M. & HOOD, R.D. (1981) Brain necrosis in mouse fetuses
transplacentally exposed to the mycotoxin ochratoxin A. Toxicol.
appl. Pharmacol., 57: 127-137.
SZCZECH, G.M., CARLTON, W.W., & TUITE, J. (1973a) Ochratoxicosis in
Beagle dogs. I. Clinical and clinicopathological features. Vet.
Pathol., 10(2): 135-154.
SZCZECH, G.M., CARLTON, W.W., & TUITE, J. (1973b) Ochratoxicosis in
Beagle dogs. II. Pathology. Vet. Pathol., 10(3): 219-231.
SZCZECH, G.M., CARLTON, W.W., TUITE, J., & CALDWELL, R. (1973c)
Ochratoxin A toxicosis in swine. Vet. Pathol., 10(4): 347-364.
SZEBIOTKO, K., CHELKOWSKI, J., DOPIERALA, G., GODLEWSKA, B., &
RADOMYSKA, W. (1981) Mycotoxins in cereal grain. Part I.
Ochratoxin, critrinin, sterigmatocystin, penicillic acid and
toxigenic fungi in cereal grain. Nahrung, 25: 415-421.
TAKITANI, S., ASABE, Y., KATO, T., SUZUKI, M., & UENO, Y. (1979)
Spectrodensitometric determination of trichothecene mycotoxins with
4-( p-nitrobenzyl)pyridine on silica gel thin-layer chromatograms. J.
Chromatogr., 172: 335-342.
TAMM, C. (1977) Chemistry and biosynthesis of trichothecenes. In:
Rodricks, J.V., Hesseltine, C.W., & Mehlman, M.A., ed. Mycotoxins in
human and animal health, Park Forest South, Illinois, Pathotox
Publishers, pp. 209-228.
TANAKA, T., HASEGAWA, A., MATSUKI, Y., ISHII, K., & UENO, Y. (1985a)
Improved methodology for the simultaneous detection of the
trichothecene mycotoxins deoxynivalenol and nivalenol in cereals.
Food Addit. Contam., 2: 125-137.
TANAKA, T., HASEGAWA, A., MATSUKI, Y., & UENO, Y. (1985b) A survey
of the occurrence of nivalenol, deoxynivalenol and zearalenone in
foodstuffs and health foods in Japan. Food Addit. Contam., 2: 259-
265.
TANAKA, T., HASEGAWA, A., YAMAMOTO, S., LEE, U., SUGIURA, Y., & UENO,
Y. (1988) Worldwide contamination of cereals by the Fusarium
mycotoxins nivalenol, deoxynivalenol and zearalenone. 1. Survey of 19
countries. J. agric. food Chem., 36: 979-983.
TANAKA, T., HASEGAWA, A., YAMAMOTO, S., MATSUKI, Y., & UENO, Y.
(1986) Residues of Fusarium mycotoxins, nivalenol, deoxynivalenol
and zearalenone, in wheat and processed food after milling and
baking. J. Food Hyg. Soc. Jpn, 27(6): 653-655.
TANAKA, T., MATSUDA, Y., TOYASAKI, N., OGAWA, K., MATSUKI, Y., &
UENO, Y. (1977) Screening of trichothecene-producing Fusarium
species from river sediments by mammalian cell culture techniques.
Proc. Jpn. Assoc. Mycotoxicol., 5/6: 50-53.
TASHIRO, F., HIRAI, K., & UENO, Y. (1979) Inhibitory effects of
carcinogenic mycotoxins on deoxyribonucleic acid-dependent
ribonucleic acid polymerase and ribonulease H. Appl. environ.
Microbiol., 38(2): 191-196.
TATSUNO, T., SAITO, M., ENOMOTO, M., & TSUNODA, H. (1968) Nivalenol,
a toxic principle of Fusarium nivale. Chem. pharm. Bull., 16(12):
2519-2520.
TEICH, A.H. & HAMILTON, J.R. (1985) Effect of cultural practices,
soil phosphorus, potassium and pH on the incidence of Fusarium head
blight and deoxynivalenol levels in wheat. Appl. environ. Microbiol.,
49(6): 1429-1431.
TERAO, K., KERA, K., & YAZIMA, T. (1978) The effects of
trichothecene toxins on the bursa of Fabricius in day-old chicks.
Virchows Arch., B27(4): 359-370.
THACKER, H.L. & CARLTON, W.W. (1977) Ochratoxin A mycotoxicosis in
the guinea pig. Food. Cosmet. Toxicol., 15: 563-574.
THIEL, P.G., MEYER, C.J., & MARASAS, W.F.O. (1982) Natural
occurrence of moniliformin together with deoxynivalenol and
zearalenone in Transkeian corn. J. agric. food Chem., 30(2): 308-312.
THIGPEN, J.T., VAUGHN, C., & STUCKEY, W.J. (1981) Phase II trial of
anguidine in patients with sarcomas unresponsive to prior
chemotherapy: A southwest oncology group study. Cancer Treat. Rep.,
65(9-10): 881-882.
THUST, R., KNEIST, S., & HUEHNE, V. (1983) [Genotoxicity of
Fusarium mycotoxins (nivalenol, T-2 toxin and zearalenone) in Chinese
hamster V79-E cells in vitro.] Arch. Geschwulstforsch., 53: 9-15
(in German).
TIEBACH, R., BLAAS, W., KELLERT, M., STEINMEYER, S., & WEBER, R.
(1985) Confirmation of nivalenol and deoxynivalenol by on-line liquid
chromatography-mass spectrometry and gas-chromatography. Comparison
of methods. J. Chromatogr., 318: 103-111.
TOCHINAI, Y. (1933) [On scab of cereals and grains. I and II.]
Bochugai Zassi, 20: 106-114, 175-188 (in Japanese).
TOMAR, R.S., BLAKLEY, B.R., & COTEAU, W.E. (1988) In vitro effects of
T-2 toxin of the mitogen responsiveness and antibody-producing
ability of human lymphocytes. Toxicol. Lett., 40: 109-117.
TRENHOLM, H.L., COCHRANE, W.P., COHEN, H., ELLIOT, J.I., FARNWORTH,
E.R., FRIEND, D.W., HAMILTON, R.M.H., NEISH, G.A., & STANDISH, J.F.
(1981) Survey of vomitoxin contamination of the 1980 white winter
wheat crop in Ontario, Canada. J. Am. Oil Chem. Soc., 58: 992-994.
TRENHOLM, H.L., HAMILTON, R.M.G., FRIEND, D.W., THOMPSON, B.K., &
HARTIN, K.E. (1984) Feeding trials with vomitoxin (deoxynivalenol)-
contaminated wheat: effects on swine, poultry and dairy cattle. J.
Am. Vet. Med. Assoc., 185: 527-531.
TRENHOLM, H.L., WARNER, R.M., & PRELUSKY, D.B. (1985) Assessment of
extraction procedures in the analysis of naturally contaminated grain
products for deoxynivalenol (vomitoxin). J. Assoc. Off. Anal. Chem.,
68: 645-659.
TRUCKSESS, M.W., NESHEIM, S., & EPPLEY, R.M. (1984) Thin layer
chromatographic determination of deoxynivalenol in wheat and corn. J.
Assoc. Off. Anal. Chem., 67: 40-43.
TRUCKSESS, M.W., FLOOD, M.W., & PAGE, S.W. (1986a) Thin layer
chromatographic determination of deoxynivalenol in processed grain
products. J. Assoc. Off. Anal. Chem., 69: 35-36.
TRUCKSESS, M.W., WOOD, G.E., EPPLEY, R.M., YOUNG, K., COHEN, C.K.,
PAGE, S.W., & POHLAND, A.E. (1986b) Occurrence of deoxynivalenol in
grain products. In: Biodeterioration. VI, Aberystwyth, The Cambrian
News Ltd., pp. 243-247.
TRUCKSESS, M.W., FLOOD, M.T., MOSSOBA, M.M., & PAGE, S.W. (1987) High
performance thin-layer chromatographic determination of
deoxynivalenol, fusarenon-X and nivalenol in barley, corn and wheat.
J. agric. food Chem., 35: 445-448.
TRUSAL, L.R. (1985) Morphological changes in CHO and VERO cells
treated with T-2 mycotoxin. Correlation with inhibition of protein
synthesis. Cell Biochem. Funct. 3: 205-216.
TRUSAL, L.R. & O'BRIEN, J.C. (1986) Ultrastructural effects of T-2
mycotoxins on rat hepatocytes in vitro. Toxicon, 24: 481-488.
TRYPHONAS, H., O'GRADY, L., ARNOLD, D,L., MCGUIRE, F., KARPINSKY, K.,
& VESONDER, R.F. (1984) Effect of deoxynivalenol (vomitoxin) on the
humoral immunity of mice. Toxicol. Lett., 23: 17-24.
TRYPHONAS, H., IVERSON, F., SO, Y., NERA, E.A., MCGUIRE, P.F.,
O'GRADY, L., CLAYSON, D.B., & SCOTT, P.M. (1986) Effects of
deoxynivalenol (vomitoxin) on the humoral and cellular immunity of
mice. Toxicol. Lett., 30: 137-150.
TSENG, T., YUAN, G., TSENG, J., SHASO, E., & MIROCHA, C.J. (1983)
Natural occurrence of Fusarium mycotoxins in grains and feeds in
Taiwan. Proc. Int. Mycotoxins Symposium, Abstr. 1.5, Sydney,
Australia.
TSUNODA, H., TSURUTA, O., MATSUNAMI, S., & ISHII, S. (1957) [Studies
on the microorganisms which deteriorate the stored cereals and
grains. XIV.] Shokuryokenkyujo Hokoku, 12: 27-33 (in Japanese).
UENO, Y. (1970) Inhibition of protein synthesis in animal cells by
nivalenol and related metabolites: toxic principles of rice-M
infested with Fusarium nivale. In: Herzberg, M., ed. Proceedings of
the First US-Japan Conference on Toxic Microorganisms, Washington,
DC, US Department of the Interior, UNJR Joint Panel of Toxic
Microorganisms, pp.76-79.
UENO, Y. (1977) Trichothecenes: overview address. In: Rodricks,
J.V., Hesseltine, C.W., & Mehlman, M.A., ed. Mycotoxins in human and
animal health, Park Forest South, Illinois, Pathotox Publishers, pp.
189-228.
UENO, Y. (1980a) Toxicological evaluation of trichothecene
mycotoxins. In: Eaker, D. & Wadstrom, T., ed. Natural toxins.
Proceedings of the International Symposium on Animal and Plant
Microbial Toxins, Uppsala, 1979, Elmsford, New York, Pergamon Press,
Vol. 6, pp. 663-671.
UENO, Y. (1980b) Trichothecene mycotoxins: Mycology, chemistry, and
toxicology. In: Draper, H.H., ed. Advances in nutritional research,
New York, London, Plenum Press, Vol. 3, pp. 301-356.
UENO, Y., ed. (1983) General toxicology. In: Developments in food
science. IV. Trichothecenes, New York, Elsevier, pp. 135-146.
UENO, Y. & FUKUSHIMA, K. (1968) Inhibition of protein and DNA
synthesis in Ehrlich ascites tumor by nivalenol, a toxic principle of
Fusarium nivale growing rice-M. Experientia (Basel), 24(10): 1032-
1033.
UENO, Y. & KUBOTA, K. (1976) DAN-attacking ability of carcinogenic
mycotoxins in recombination-deficient mutant cells of Bacillus
subtilis. Cancer Res., 36: 445-451.
UENO, Y. & MATSUMOTO, H. (1975) Inactivation of some thiol enzymes
by trichothecene mycotoxins from Fusarium spp. Chem. pharm. Bull.,
23(10): 2439-2442.
UENO, Y. & SHIMADA, N. (1974) Reconfirmation of the specific nature
of reticulocytes bioassay system to trichothecene mycotoxins of
Fusarium spp. Chem. pharm. Bull., 22(11): 2744-2746.
UENO, Y. & YAMAKAWA, H. (1970) Antiprotozoal activity of scirpene
mycotoxins of Fusarium nivale FN 2B. Jpn. J. exp. Med., 40(5):
385-390.
UENO, Y., HOSOYA, M., MORITA, Y., UENO, I., & TATSUNO, T. (1968)
Inhibition of the protein synthesis in rabbit reticulocyte by
nivalenol, a toxic principle isolated from Fusarium nivale growing on
rice. J. Biochem. (Tokyo), 64(4): 479-485.
UENO, Y., HOSOYA, M., & ISHIKAWA, Y. (1969a) Inhibitory effects of
mycotoxins on protein synthesis in rabbit reticulocytes. J. Biochem.
(Tokyo), 66(3): 419-422.
UENO, Y., UENO, I., TATSUNO, T., OHOKUBO, K., & TSUNODA, H. (1969b)
Fusarenon-X, atopic principle of Fusarium nivale-culture filtrate.
Experientia (Basel), 25: 1062.
UENO, Y., ISHIKAWA, Y., AMAKAI, K., NAKAJIMA, M., SAITO, M.,
ENOMOTO, M., & OHTSUBO, K. (1970) Comparative study on skin-
necrotizing effect of scirpene metabolites of Fusaria. Jpn. J. exp.
Med., 40(1): 33-38.
UENO, Y., UENO, I., IITOI, Y., TSUNODA, H., ENOMOTO, M., & OHTSUBO,
K. (1971) Toxicological approaches to the metabolites of Fusaria.
Part 3. Acute toxicity of fusarenon-X. Jpn. J. exp. Med., 41(6): 512-
539.
UENO, Y., ISHII, K., SAKAI, K., KANAEDA, S., TSUNODA, H., TANAKA, T.,
& ENOMOTO, M. (1972) Toxicological approaches to the metabolites of
Fusarium. Part 4. Microbial survey on bean hulls poisoning of horses
with the isolation of toxic trichothecenes, neosolaniol and T-2 toxin
of Fusarium solani M-1-1. Jpn. J. exp. Med., 42(3): 187-203.
UENO, Y., ISHII, K., SATO, N., SHIMADA, N., TSUNODA, H., SAWANO, M.,
& ENOMOTO, M. (1973a) Screening of trichothecene producing fungi
and the comparative toxicity of isolated mycotoxins. Jpn. J.
Pharmacol., 23(Suppl.): 133.
UENO, Y., NAKAJIMA, M., SAKAI, K., ISHII, K., SATO, M., & SHIMADA, N.
(1973b) Comparative toxicology of trichothecene mycotoxins.
Inhibition of protein synthesis in animal cells. J. Biochem. (Tokyo),
74(2): 285-296.
UENO, Y., SATO, N., ISHII, K., SAKAI, K., TSUNODA, H., & ENOMOTO, M.
(1973c) Biological and chemical detection of trichothecene
mycotoxins of Fusarium spp. Appl. Microbiol., 25(4): 699-704.
UENO, Y., ISHII, K., SATO, N., & OHTSUBO, K. (1974) Toxicological
approaches to the metabolites of Fusaria. VI. Vomiting factor from
moldy corn infected with Fusarium spp. Jpn. J. exp. Med., 44(1):
123-127.
UENO, Y., KUBOTA, K., ITO, T., & NAKAMURA, Y. (1978a) Mutagenicity
and carcinogenic mycotoxins in Salmonella typhimurium. Cancer Res.,
38(3): 536-542.
UENO, Y., MATSUMOTO, H., ISHII, K., & KUKITA, K. (1978b) Inhibitory
effects of mycotoxins on Na+-dependent transport of glycine in rabbit
reticulocytes. Biochem. Pharmacol., 25(18): 2091-2095.
UENO, Y., TASHIRO, F., & KOBAYASHI, T. (1983) Species differences
in zearalenone-reductase activity. Food chem. Toxicol., 21(2):
167-173.
UENO, Y., LEE, U.S., TANAKA, T., HASAGAWA, A., & MATSUKI, Y. (1986)
Examination of Chinese and USSR cereals for the Fusarium mycotoxins
nivalenol, deoxynivalenol, and zearalenone. Toxicon, 24(6): 18-21.
UENO, Y., ABE, K., MORIMURA, S., SUGIURA, Y., & HORIE, Y. (in press)
Genotoxicity of mycotoxins evaluated by the SOS microplate assay.
Mutation Res.
UMEDA, M., YAMAMOTO, T., & SAITO, M. (1972) DNA-strand breakage of
HeLa cells by several mycotoxins. Jpn. J. exp. Med., 42: 527-535.
UMEDA, M., TSUTSUI, T., & SAITO, M. (1977) Mutagenicity and
inducibility of DNA single-strand breaks and chromosome aberrations
by various mycotoxins. Gann, 68(5): 619-625.
VAN RENSBURG, S.J. & ALTENKIRK, B. (1974) Claviceps purpurea:
ergotism. In: Purchase, I.F.H., ed. Mycotoxins, Amsterdam, New York,
Oxford, Elsevier Science Publishers, pp. 69-96.
VESELA, D., VESELY, D., JELINEK, R., & KUSAK, V. (1978) [Detection
of ochratoxin A in feed barley.] Vet. Med., 23: 431-436 (in Czech).
VESELA, D., VESELY, D., & JELINEK, R. (1983) Toxic effects of
ochratoxins A and citrinin, alone and in combination, on chicken
embryos. Appl. environ. Microbiol., 45: 91-93.
VESONDER, R.F. (1983) Natural occurrence of trichothecenes in North
America. Dev. food Sci., 4: 210-217.
VESONDER, R.F., CIEGLER, A., & JENSEN, A.H. (1973) Isolation of the
emetic principle from Fusarium-infected corn. Appl. Microbiol., 26:
1008-1010.
VESONDER, R.F., CIEGLER, A., & JENSEN, A.H. (1977) Production of
refusal factors by Fusarium strains on grains. Appl. environ.
Microbiol., 34(1): 105-106
VESONDER, R.F., CIEGLER, A., ROGERS, R.F., BURBRIDGE, K.A., BOTHAST,
R.J., & JENSEN, A.H. (1978) Survey of 1977 crop year preharvest
corn for vomitoxin. Appl. environ. Microbiol., 36(6): 885-888.
VESONDER, R.F., CIEGLER, A., BURMEISTER, H.R., & JENSEN, A.H. (1979)
Acceptance by swine and rats of corn amended with trichothecenes.
Appl. environ. Microbiol., 38(2): 344-346.
VISCONTI, A. & BOTTALICO, A. (1983a) Detection of Fusarium
trichothecenes (nivalenol, deoxynivalenol, fusarenone and
3-acetyldeoxynivalenol) by high-performance liquid chromatography.
Chromatographia, 17: 97-100.
VISCONTI, A. & BOTTALICO, A. (1983b) High levels of ochratoxin A
and B in mouldy bread responsible for mycotoxicosis in farm animals.
J. agric. food Chem., 31: 1122-1123.
VISCONTI, A. & MIROCHA, C.J. (1985) Identification of various T-2
toxin metabolites in chicken excreta and tissues. Appl. environ.
Microbiol., 49: 1246-1250.
VOYKSNER, R.D., HAGLER, W.M., TYCZKOWSKA, K., & HANEY, C.A. (1985)
Thermospray high-performance liquid chromatographic/mass
spectrometric analysis of some Fusarium mycotoxins. J. High Res.
Chrom. Chrom. Comm., 8: 119-125.
WARE, G.M., FRANCIS, O.J., CARMAN, A.S., & KUAN, S.S. (1986) Gas
chromatographic determination of deoxynivalenol in wheat with
electron capture detection: collaborative study. J. Assoc. Off. Anal.
Chem., 69: 899-901.
WEAVER, G.A., KURTZ, H.J., BATES, F.Y., CHI, M.S., MIROCHA, C.J.,
BEHRENS, J.C., & ROBISON, T.S. (1978a) Acute and chronic toxicity
of T-2 mycotoxin in swine. Vet. Rec., 103(24): 531-535.
WEAVER, G.A., KURTZ, H.J., MIROCHA, C.J., BATES, F.Y., & BEHRENS,
J.C. (1978b) Acute toxicity of the mycotoxin diacetoxyscirpenol in
swine. Can. vet. J., 19(10): 267-271.
WEHNER, F.C., THIEL, P.G., VAN RENSBURG, S.J., & DEMASIUS, I.P.C.
(1978a) Mutagenicity to Salmonella typhimurium of some Aspergillus
and Penicillium mycotoxins. Mutat. Res., 58: 193-203.
WEHNER, F.C., MARASAS, W.F.O., & THIEL, P.G. (1978b) Lack of
mutagenicity to Salmonella typhimurium of some Fusarium mycotoxins.
Appl. environ. Microbiol., 35(4): 659-662.
WEI, C.M. & MCLAUGHLIN, C.S. (1974) Structure function relationship
in the 12,13-epoxytrichothecenes novel inhibitors of protein
synthesis. Biochem. biophys. Res. Commun., 57(3): 838-844.
WEI, R.D., STRONG, F.M., SMALLEY, E.B., & SCHNOES, H.K. (1971)
Chemical interconversion of T-2 and HT-2 toxins and related
compounds. Biochem. biophys. Res. Commun., 45(2): 396-401.
WEI, R.D., SMALLEY, E.B., & STRONG, F.M. (1972) Improved skin test
of detection of T-2 toxin. Appl. Microbiol., 23(5): 1029-1030.
WEI, C.M., CAMPBELL, I.M., MCLAUGHLIN, C.S., & MAURICE, H. (1974)
Binding of trichodermin to mammalian ribosomes and its inhibition by
other 12,13-epoxytrichothecenes. Mol. cell Biochem., 3(3): 215-219.
WHO (1979) Environmental Health Criteria 11: Mycotoxins, Geneva,
World Health Organization, 127 pp.
WHO (1983) Prevention of liver cancer: Report of a WHO Meeting,
Geneva, World Health Organization, 30 pp. (Technical Report Series,
No. 691).
WILSON, D.J. (1984) Some effects of T-2 toxin on rabbit and bovine
musculature, Guelph, Ontario, Faculty of Graduate Studies of Guelph
(M. Sc. Thesis).
WILSON, D.J. & GENTRY, P.A. (1985) T-2 toxin can cause vasoconstriction
in an in vitro bovine ear perfusion system. Toxic. appl. Pharmacol.,
79: 159-165.
WOOD, G.E. & CARTER, L. (1989) Limited survey of deoxynivalenol in
wheat and corn in the United States. J. Assoc. Off. Anal. Chem., 72:
38-40.
WOODS, A.J., BRADLEY-JONES, J., & MANTLE, P.G. (1966) An outbreak of
gangrenous ergotism in cattle. Vet. Rec., 78: 742-749.
WYATT, R.D., HARRIS, J.R., HAMILTON, P.B., & BURMEISTER, H.R. (1972)
Possible outbreaks of furariotoxicosis in avians. Avian Dis., 16:
1123-1130.
WYATT, R.D., COLWELL, W.M., HAMILTON, P.B., & BURMEISTER, H.R.
(1973a) Neural disturbances in chickens caused by dietary T-2 toxin.
Appl. Microbiol., 26(5): 757-761.
WYATT, R.D., COLWELL, W.M., HAMILTON, P.B., & BURMEISTER, H.R.
(1973b) Neurotoxicity of T-2 toxin in broilers. Poult. Sci., 52(5):
2105.
WYATT, R.D., HAMILTON, P.B., & BURMEISTER, H.R. (1973c) Effects of
T-2 toxin in broiler chickens. Poult. Sci., 52(5): 1853-1859
WYATT, R.D., DOERR, J.A., HAMILTON, P.B., & BURMEISTER, H.R. (1975)
Egg production shell thickness and other physiological parameters of
laying hens affected by T-2 toxin. Appl. Microbiol., 29(5): 641-645.
XU, Y.C., ZANG, G.S., & CHU, F.S. (1988) Enzyme-linked immunosorbent
assay for deoxynivalenol in corn and wheat. J. Assoc. Off. Anal.
Chem., 71: 945-949.
YAGEN, B., SINTOV, A., & BIALER, M. (1986) New, sensitive thin-layer
chromatographic-high performance liquid chromatographic method for
detection of trichothecene mycotoxins. J. Chromatogr., 356: 195-201.
YAROM, R., MORE, R., ELDOR, A., & YAGEN, B. (1984a) The effect of T-2
toxin on human platelets. Toxicol. appl. Pharmacol., 73: 210-217.
YAROM, R., SHERMAN, Y., MORE, R., GINSBURG, I., BORINSKI, R., &
YAGEN, B. (1984b) T-2 toxin effect on bacterial infection and
leukocyte functions. Toxicol. appl. Pharmacol., 75: 60-68.
YLIMAEKI, A., KOPONEN, H., HINTIKKA, E.L., NUMMI, M., NIKU-PAAVOLA,
M.L., ILUS, T., & ENARI, T.M. (1979) Mycoflora and occurrence of
Fusarium toxins in Finnish grain, Espoo, Technical Research Center of
Finland, pp. 1-28, (Materials and Processing Technology, Publication
21).
YOSHIZAWA, T. & HOSOKAWA, H. (1983) Natural occurrence of
deoxynivalenol and nivalenol trichothecene mycotoxins in commercial
foods. J. Food Hyg. Soc. Jpn, 24: 413-415.
YOSHIZAWA, T. & MOROOKA, N. (1973) Deoxynivalenol and its
monoacetate new mycoatoxins from Fusarium roseum and mouldy barley.
Agric. biol. Chem., 37(12): 2933-2934.
YOSHIZAWA, T. & MOROOKA, N. (1977) Trichothecenes from mold-
infested cereals in Japan. In: Rodricks, J.V., Clifford, W.,
Hesseltine, C.W., & Myron, A.M., ed. Mycotoxins in human and animal
health, Park Forest South, Illinois, Pathotox Publishers, pp. 309-
321.
YOSHIZAWA, T. & SAKAMOTO, T. (1982) [ In vitro metabolism of T-2
toxin and its derivatives in animal livers.] Proc. Jpn. Assoc.
Mycotoxicol., 14: 26-28 (in Japanese).
YOSHIZAWA, T., SWANSON, S.P., & MIROCHA, C.J. (1980) T-2
metabolites in the excreta of broiler chickens administered 3H-
labeled T-2 toxin. Appl. environ. Microbiol., 39(6): 1172-1177.
YOSHIZAWA, T., MIROCHA, C.J., BEHRENS, J.C., & SWANSON, S.P. (1981)
Metabolic fate of T-2 toxin in a lactating cow. Food Cosmet.
Toxicol., 19(1): 31-39.
YOSHIZAWA, T., SAKAMOTO, T., AYANO, Y., & MIROCHA, C.J. (1982a)
[Chemical structures of new metabolites of T-2 toxin.] Proc. Jpn.
Assoc. Mycotoxiol., 15: 13-15 (in Japanese).
YOSHIZAWA, T., SAKAMOTO, T., AYANO, Y., & MIROCHA, C.J. (1982b)
3'-Hydroxy-T-2 and 3'-hydroxy HT-2 toxins: new metabolites of T-2 toxin,
a trichothecene mycotoxin, in animals. Agric. biol. Chem., 46(10):
2613-2615.
YOSHIZAWA, T., SAKAMOTO, T., & OKAMOTO, K. (1984) In vitro formation
of 3'-hydroxy T-2 and 3'-hydroxy HT-2 toxins form T-2 toxin by liver
homogenates from mice and monkeys. Appl. environ. Microbiol., 47:
130-134.
YOSHIZAWA, T., OKAMOTO, K., SAKAMOTO, T., & KUWAMURA, K. (1985a) In
vivo metabolism of T-2 toxin, a trichothecene mycotoxin. On the
formation of deepoxidation products. Proc. Jpn. Assoc. Mycotoxicol.,
21: 9-12.
YOSHIZAWA, T., SAKAMOTO, T., & KUWAMUAR, K. (1985b) Structures of
deepoxytrichthecene metabolites from 3'-hydroxy HT-2 and T-2 tetraol
in rats. Appl. environ. Microbiol., 50: 676-679.
YOSHIZAWA, T., COTE, L.M., SWANSON, S.P., & BUCK, W.B. (1986)
Confirmation of DOM-1, a deepoxydation metabolite of deoxynivalenol
in biological fluids of lactating cows. Agric. biol. Chem., 50:
227-229.
YOUNG, J.C. (1981a) Variability in the content and composition of
alkaloids found in Canadian ergot. I. Rye. J. environ. Sci. Health,
B16(4): 83-111.
YOUNG, J.C. (1981b) Variability in the content and composition of
alkaloids found in Canadian ergot. II. Wheat. J. environ. Sci.
Health, B16(1): 381-393.
YOUNG, J.C. & CHEN, Z. (1982) Variability in the content and
composition of alkaloids found in Canadian ergot. III. Triticale and
barley. J. environ. Sci. Health, B17(2): 93-107.
YOUNG, J.C. & MARQUARDT, R.R. (1982) Effects of ergotamine tartrate
in growing chicken. Can. J. anim. Sci., 62: 1181-1191
YOUNG, J.C., CHEN, Z., & MARQUARDT, R.R. (1983) Reduction in
alkaloid content of ergot sclerotia by chemical and physical
treatment. J. agric. food Chem., 31: 413.
YOUNG, J.C., FULCHER, R.G., HAYHOE, J.H., SCOTT, P.M., & DEXTER, J.E.
(1984) Effects of milling and baking on deoxynivalenol (Vomitoxin)
content of Eastern Canadian wheats. J. agric. food Chem., 32:
659-664.
YOUNG, J.C., SUBRYAN, L.M., POTTS, D., MCLAREN, M.E., & GOBRAN, F.H.
(1986) Reduction in levels of deoxynivalenol in contaminated wheat by
chemical and physical treatment. J. agric. food Chem., 34: 461-464.
ZAMIR, N., ZAMIR, D., EIDEN, L.E., PALKOVITS, M., BROWNSTEIN, M.J.,
ESHAY, R.L., WEBER, E., FRODEN, A.I., & FEUERSTEIN, G. (1985)
Metionine and leucine enkephalin in rat neurohypophysis: different
response to osmotic stimuli and T-2 toxin. Science, 228: 606-608.
ZIPRIN, R.L. & CORRIER, D.E. (1987) Listeriosis in diacetoxyscirpenol-
treated mice. Am. J. vet. Res., 48: 1516-1519.
ZORZ, M., CULIG, J., KOPITAR, Z., MILIVOJEVIC, D., MARUSIC, A., &
BANO, M. (1985) HPLC method for determination of ergot alkaloids
and some derivatives in human plasma. Hum. Toxicol., 4: 601-607.
RESUME ET RECOMMANDATIONS EN VUE DE RECHERCHES FUTURES
1. Ochratoxine A
1.1 Etat naturel
Les ochratoxines sont produites par plusieurs espèces de
champignons appartenant au genre Aspergillus et Penicillium. Il
s'agit d'espèces ubiquistes, aussi le risque de contamination des
denrées alimentaires destinées à l'homme et aux animaux est-il
omniprésent. Le principal composé, l'ochratoxine A se rencontre en
Australie ainsi que dans certains pays d'Europe et d'Amérique du
Nord. La production d'ochratoxine par les espèces du genre
Aspergillus semble limitée aux environnements très chauds et
humides alors que dans le cas de Penicillium, au moins certaines
espèces peuvent produire de l'ochratoxine à des températures
n'excédant pas 5 °C.
Ce sont les céréales qui sont le plus fréquemment contaminées
par l'ochratoxine A et à un moindre degré certaines fèves (café,
soja, cacao). L'ochratoxine B est très rare.
1.2 Méthodes d'analyse
On a mis au point des méthodes d'analyse permettant
l'identification et le dosage de l'ochratoxine à des concentrations
de l'ordre de 1 µg/kg.
1.3 Métabolisme
On trouve des résidus d'ochratoxine A intacte dans le sang, les
reins, le foie et les muscles de porcs à l'abattoir ainsi que dans
les muscles de poules et de poulets. En revanche on ne trouve
généralement pas de résidus chez les ruminants. In vitro,
l'ochratoxine A se fixe très solidement à l'albumine sérique
bovine, porcine et humaine. Des études expérimentales sur des
porcins et des poules ont montré que c'est au niveau des reins que
les concentrations d'ochratoxine A sont les plus élevées. Chez le
porc, le rat et l'homme, il pourrait y avoir détoxication par
hydroxylation au niveau des microsomes. L'expérience montre qu'on
peut encore identifier des résidus dans les reins de porc un mois
après la fin de l'exposition.
1.4 Effets sur les animaux
On a signalé des cas d'ochratoxicose chez des animaux d'élevage
(porcs et volaille) dans plusieurs pays d'Europe, la manifestation
essentielle étant une néphropathie chronique. Les lésions se
présentent sous la forme d'une atrophie tubulaire, d'une fibrose
interstitielle et à un stade plus avancé, d'une hyalinisation des
glomérules. Au Canada, on a également trouvé de l'ochratoxine A
dans du sang de porc recueilli à l'abattoir. Des effets
néphrotoxiques ont été relevés chez toutes les espèces d'animaux à
estomac simple étudiées jusqu'ici, même aux doses les plus faibles
qui aient été expérimentées (200 mg/kg de nourriture chez les rats
et les porcs).
Des effets tératogènes ont été observés chez des souris
exposées par voie orale à 3 mg d'ochratoxine A par kg de poids
corporel. On a observé une résorption des foetus chez des rats à
partir de 0,75 mg/kg de poids corporel, le produit étant administré
par voie orale. Ces effets tératogènes, qui chez le rat sont
accrus par un régime alimentaire pauvre en protéines, ont été
également observés chez des hamsters.
Les épreuves à court terme n'ont pas permis de mettre en
évidence d'activité mutagène (bactéries et levures). Administré
par voie orale à des rats, le produit a provoqué des ruptures
monocaténaires dans l'ADN des tissus du rein et du foie.
L'ochratoxine a provoqué l'apparition de cancers du rein chez des
souris mâles et des rats des deux sexes qui recevaient le produit
par voie orale. La formation de carcinomes hépatocellulaires n'a
été signalée que chez une souche de souris et pas du tout chez le
rat.
L'ochratoxine A est un inhibiteur de la synthèse protéique et
de la tRNA-synthétase chez les microorganismes et dans les cellules
hépatomateuses; elle inhibe également le mARN chez le rat.
L'ochratoxine A peut inhiber la migration des macrophages.
Chez la souris, une dose de 0,005 µg/kg de poids
corporel a aboli la réponse immunitaire aux érythrocytes de mouton;
toutefois d'autres résultats, contradictoires, ont été obtenus.
L'ochratoxine A s'est révélée cancérogène pour l'épithelium
tubulaire rénal chez des souris mâles et des rats des deux sexes.
1.5 Effets sur l'homme
L'exposition humaine, qui ressort de la présence d'ochratoxine
A dans les aliments, le sang et le lait humains, a été observée
dans divers pays d'Europe. Selon les données épidémiologiques
disponibles, la néphropathie des Balkans peut être attribuée à la
consommation de denrées alimentaires contaminées par cette toxine.
On a mis en évidence une relation tout à fait significative
entre la néphropathie des Balkans et les tumeurs des voies
urinaires, en particulier des tumeurs pyéliques et uretérales.
Toutefois on n'a pas publié de données qui établissent une
implication directe de l'ochratoxine A dans l'étiologie de ces
tumeurs.
2. Trichothécènes
2.1 Etat naturel
On connaît aujourd'hui 148 trichothécènes qui se caractérisent
sur le plan chimique par la présence d'une structure de base
commune, le système tétracyclique du scirpénol. Ces composés sont
principalement secrétés par des moisissures appartenant au genre
Fusarium, encore que d'autres genres notamment Trichoderma,
Trichothecium, Myrothecium et Stachybotrys produisent également des
métabolites considérés désormais comme des trichothécènes.
Quelques-uns seulement de ces trichothécènes contaminent les
denrées alimentaires destinées à l'homme ou aux animaux, en
particulier : le désoxyvalénol (DON), le nivalénol (NIV), le
disacétoxyscirpenol (DAS), ainsi que la toxine T-2 et, plus
rarement, certains dérivés (3-Ac-DON, 15-Ac-DON, fusarenone-X et
toxine HT-2). Parmi ces substances, c'est le DON qui est de loin
la plus fréquemment présente dans les denrées alimentaires
destinées à l'homme et aux animaux; à côté de quantités plus
faibles de NIV. Certains trichothécènes macrocycliques comme les
satratoxines G et H et les verrucarines, se rencontrent de temps à
autre dans les fourrages (paille, foin) mais il n'a pas été fait
état de leur présence dans les produits alimentaires.
Les enquêtes sur la présence de trichothécènes ont révélé que
le DON était présent dans le monde entier, essentiellement dans les
céréales telles que le froment et le maïs à des concentrations
pouvant parfois atteindre 92 mg/kg, encore que les teneurs moyennes
soient beaucoup plus faibles et varient selon la denrée. Des
rapports isolés font état de la présence de DON dans de l'orge, des
mélanges alimentaires pour aminaux, des pommes de terre, etc. Le
NIV, dont la présence n'est normalement pas signalée dans des
céréales au Canada ou aux Etats-Unis se rencontre en revanche
fréquemment au côté du DON dans les céréales originaires d'Asie ou
d'Europe; la concentration la plus forte enregistrée jusqu'ici est
de 37,9 mg/kg. On a signalé ça et là la présence de toxine T-2 et
de DAS à des concentrations beaucoup plus faibles.
Des études portant sur les divers traitements subis par les
produits alimentaires et notamment la mouture, montrent que, entre
la céréale brute et le produit définitif, il n'y a guère de
diminution des teneurs en DON. De même la panification ne parvient
pas à détruire le DON. En général, les aliments destinés à l'homme
que l'on trouve dans le commerce ne contiennent que rarement des
quantités décelables de DON et de NIV.
2.2 Methodes d'analyse
On dispose, pour le dosage des quatre toxines les plus
fréquemment rencontrées (DON, toxine T.2, DAS et NIV), des méthodes
d'analyse basées sur la chromatographie en couche mince, la
chromatographie en phase gazeuse ou la chromatographie liquide à
haute performance ainsi que sur des réactions immunologiques; les
limites de détection se situent en-dessous de 1 µg/g. Plusieurs de
ces méthodes ont fait l'objet d'une expérimentation collective. En
outre, certaines méthodes utilisées en recherche comme la
chromatographie gazeuse ou liquide associée à la spectrographie de
masse peuvent être utilisées pour confirmer l'identité des
substances.
2.3 Métabolisme
On a procédé à des études métaboliques, essentiellement de la
toxine T-2, mais dans quelques rares cas seulement du DON, chez
l'animal. Ces trichothécènes sont rapidement absorbés au niveau
des voies digestives mais l'on ne dispose pas de données
quantitatives. Les toxines se répartissent de façon uniforme sans
accumulation marquée au niveau d'un organe ou d'un tissu
particulier. Elles sont métabolisées en produits moins toxiques
par hydrolyse, hydroxylation, désépoxydation et glucuronidation.
La toxin T-2 et le DON sont rapidement éliminés par voie fécale et
urinaire.
Par exemple, une dose de toxine T-2 administrée par voie orale à
des bovins a été éliminée presque à hauteur de 100 % dans les heures
suivant l'administration; des poulets ont éliminé 80 % de la
substance dans les 48 heures suivant l'administration. Chez le rat,
25 % d'une dose de DON ont été éliminés dans les urines et 65 % dans
les matières fécales 96 heures après l'administration. Chez la poule
pondeuse et la vache laitière on a constaté que moins de 1 % de la
dose de toxine T-2 (et de ses métabolites) qui leur avait été
administrée se retrouvaient dans les oeufs et le lait. Après
administration par voie orale de toxine T-2 à des poulets, on a
constaté que les résidus présents dans la viande 24 heures plus tard
représentaient moins de 2 % de la dose initiale.
2.4 Effets sur les animaux
C'est principalement par l'ingestion de fourrage contaminé que
les animaux sont exposés aux trichothécènes. La toxine T-2 et le DAS
qui chez les animaux de laboratoire sont les plus actifs des
trichothécènes couramment cités comme contaminants des denrées
destinées aux animaux (toxine T-2, DAS, NIV et DON), provoquent des
réactions toxiques analogues. Le NIV est moins actif dans certains
systèmes que les deux précédents composés et le DON est le moins
toxique des quatre. (Cette activité toxique peut s'évaluer au moyen
de la DL50 pour la souris, par exemple dans le cas de la toxine T-2
elle est égale à 10,5 mg/kg de poids corporel et dans le cas du DON à
46,0 mg/kg).
Les trichothécènes les plus actifs, tels que la toxine T-2 et le
DAS produisent des effets généraux aigus lorsqu'ils sont administrés
expérimentalement à des rongeurs, des porcs et des bovins par voie
orale, parentérale ou respiratoire (porc, souris). Ces
trichothécènes produisent une épithélionécrose par contact (une dose
de 0,2 µg par touche dans le cas de la toxine T-2). Avec les autres
trichothécènes, il faut des doses plus élevées pour obtenir un effet
irritant (dans le cas du NIV, 10 ug par touche). Les trichothécènes
cytotoxiques comme la toxine T-2 ont une action nécrosante sur
l'épithélium des cryptes intestinales et sur les tissus lymphoïdes et
hematopoïétiques après exposition par voie orale, parentérale ou
respiratoire. Après exposition à la toxine T-2 et au DAS on observe
des anomalies hematologiques et des troubles de l'hémostase. Dans
les cas graves, la toxicose peut entraîner une pancytopénie. Des
études portant sur la toxine T-2, le DON et le DAS ont mis en
évidence la suppression de l'immunité à médiation cellulaire et de
l'immunité humorale et on a observé une réduction de la concentration
des immunoglobulines ainsi qu'une dépression de l'activité
phagocytaire des macrophages et des neutrophiles. Des études sur
animaux de laboratoire ont montré que l'effet immunodépresseur de
trichothécènes tels que la toxine T-2, le DAS et le DON entraînait
une moindre résistance aux infections secondaires par des bactéries
(Mycobactérie, Listeria monocytogenes) des levures ( Cryptococcus
neoformans) et des virus (virus de l'herpes simplex).
Il a été indiqué qu'après injection intrapéritonéale, la toxine
T-2 était tératogène pour la souris (cette voie d'administration
n'est pas courante dans les études de tératogénicité). Le DON est
tératogène pour la souris après intubation gastrique mais ne l'est
pas chez le rat lorsque la toxine est administrée dans la nourriture
de l'animal. Le NIV ne s'est pas révélé tératogène pour la souris.
La recherche du pouvoir mutagène de la toxine T-2, du DAS et du DON
par une épreuve du type Ames n'a pas donné de résultat positif. La
toxine T-2 présente dans certaines épreuves une faible activité
clastogène. D'après les études de toxicité à long terme qui ont été
publiées, rien n'indique que la toxine T-2, la fusarénone-X et le NIV
ne soient tumorigènes chez l'animal. Aucune étude de toxicité à long
terme n'a été publiée sur le DON.
Les trichothécènes sont toxiques pour les cellules à forte
activité mitotique telles que les cellules de l'épithélium des
cryptes intestinales et les cellules hematopoïétiques. Cette
cytotoxicité proviendrait, soit d'une perturbation de la synthèse des
protéines par une fixation des composés aux ribosomes des cellules
eucaryotes, soit d'une dysfonction des membranes cellulaires.
L'inhibition de la synthèse protéique serait due à l'induction de
protéines régulatrices labiles telles que l'IL-2 dans les
immunocytes. A concentrations extrêmement faibles, les
trichothécènes perturbent le transport des petites molécules à
travers les membranes cellulaires.
2.5 Effets sur l'homme
L'ingestion de produits alimentaires contaminés d'origine
végétale constitue la principale voie d'exposition aux tricho-
thécènes mais d'autres voies ont été signalées à l'occasion, par
exemple un contact cutané accidentel chez des chercheurs de
laboratoire ou l'inhalation de trichothécènes présents dans des
poussières aéroportées.
On n'a décrit que peu de cas de maladies attribuables à une
exposition aux trichothécènes, sans d'ailleurs que la responsa-
bilité de ces produits soit établie. Toutefois, dans les deux
flambées évoquées ci-dessous, il y a lieu de penser que leur
responsabilité est en cause.
Une des flambées, qui s'est produite en Chine, a été attribuée à
la consommation de blé moisi contenant 1,0 à 40,0 mg de DON par kg.
La maladie se caractérisait par des symptômes gastro-intestinaux.
Aucun décès n'a été à déplorer. Les porcs et les poulets qui avaient
mangé les restes de céréales ont également été affectés.
Une flambée du même genre a été signalée en Inde et attribuée à
la consommation de pain fabriqué à l'aide de blé contaminé. La
maladie se caractérisait par des symptômes gastro-intestinaux et une
irritation de la gorge qui apparaissaient 15 minutes à une heure
après l'ingestion du pain. On a décelé les mycotoxines suivantes
dans des échantillons de farine raffinée utilisée pour la préparation
du pain: DON (0,35-8,3 mg/kg), acétyldésoxynivalénol (0,64-2,49
mg/kg), NIV (0,03-0,1 mg/kg) et toxine T-2 (0,5-0,8 mg/kg).
Toutefois, l'identité de ces trichothécènes n'a pas été confirmée.
La présence de DON et de NIV à côté de la toxine T-2 est
inhabituelle.
Deux maladies d'intérêt historique, une aleucie alimentaire
d'origine toxique en URSS et une toxicose due à du blé moisi au
Japon et en Corée ont été attribuées à la consommation de céréales
contaminées par des moisissures du genre Fusarium. Depuis lors, on
a isolé en laboratoire, dans des cultures de Fusarium isolés des
céréales en cause, un certain nombre de trichothécènes. Il n'avait
pas été possible, au moment où ces maladies se sont déclarées,
d'effectuer des recherches pour tenter de corréler l'aleucie
toxique et la toxicose due au blé moisi à une exposition à des
trichothécènes car on ne connaissait pas les toxines en question.
3. Ergot
3.1 Etat naturel
Ergot est le nom que l'on donne aux sclérotes de certaines
espèces de champignons appartenant au genre Clavicepsa. Ces
sclérotes contiennent des alcaloïdes biologiquement actifs qui
peuvent être à l'origine de toxicoses chez les personnes ou les
animaux qui consomment des denrées contaminées.
Les alcaloïdes de l'ergot produisent deux types de maladies selon
leur nature, qui dépend du champignon en cause ( C. purpurea , C.
fusiformis ). L'ergotisme, dû à l'ergotamine et à l'ergocristine
produites par C. purpurea se caractérise principalement par une
gangrène des extrémités et des symptômes gastro-intestinaux. Quand à
l'intoxication provoquée par du millet contaminé par C. fusiformis,
elle se caractérise principalement par des symptômes digestifs et
elle est due aux clavines. Aucun des signes ou symptômes observés ne
sont révélateurs d'une oblitération vasculaire.
3.2 Méthodes d'analyse
Les alcaloïdes de l'ergot (ergolines) sont des dérivés de l'acide
lysergique. Ils ont une activité biologique variable selon leur
nature. Le dosage des alcaloïdes de C. purpurea a été effectué par
chromatographie liquide à haute performance avec détection par
fluorescence. On peut déterminer des concentrations de 0,2 µg
d'ergolines par litre de plasma humain. La détermination de
l'ergotamine et de l'ergocristine peut s'effectuer de façon très
spécifique par titrage radio-immunologique à des concentrations
respectives de 3,5 et 0,8 picomoles.
3.3 Effets sur les animaux
Des flambées d'avortements chez des bovins ont pu être attribuées
à l'ingestion d'ergoline, essentiellement de l'ergotamine et de
l'ergotaminine. Des moutons à qui l'on avait administré de
l'ergotamine par voie orale sont tombés rapidement malades et
présentaient une inflammation intestinale. Chez des volailles, des
porcs et des primates exposés par voie orale on a observé des effets
légers. Le groupe de travail ne disposait d'aucune donnée sur la
mutagénicité, la tératogénicité et la cancérogénicité des ergolines.
3.4 Effets sur l'homme
L'homme peut être exposé aux ergolines par la consommation de
céréales ergotées. Dans la plupart des études toxicologiques qui ont
été effectuées, on n'a pas procédé à l'identification précise des
alcaloïdes en cause. Les données qui ont été publiées au sujet d'une
seule enquête effectuée en Suisse sur des céréales et des produits
céréaliers indiquent que la consommation quotidienne totale
d'ergolines se situe à environ 1,5 ug par personne, certaines denrées
en contenant jusqu'à 140 µg/kg. La panification réduit de 25 à 100 %
la teneur en ergolines des farines contaminées.
En Ethiopie, une flambée d'ergotisme s'est produite en 1978 à la
suite de l'exposition à des ergolines provenant de sclérotes de ce C.
purpurea. Les céréales comptaient jusqu'à 0,75 % d'ergot; on a
relevé la présence d'ergométrine. Parmi les symptômes observés
figuraient une gangrène sèche ayant entraîné la perte d'un ou
plusieurs membres (29 % des cas), un pouls périphérique faible ou
absent (36 %) et une desquamation. Des symptômes digestifs n'ont été
observés que dans quelques cas. Chez 88 % des malades, les lésions
intéressaient les membres inférieurs.
En Inde, plusieurs flambées se sont déclarées depuis 1958 à la
suite de la consommation de millet contaminé par C. fusiformis. Ces
symptômes consistaient en nausées, vomissements et vertiges. Les
symptômes toxiques étaient dus à la présence d'ergolines à des
concentrations de 15 à 26 mg/kg. Aucune autopsie n'ayant été
effectuée, on ne dispose d'aucune information sur les effets
pathologiques au niveau des viscères.
4. Evaluation des risques pour la santé humaine
4.1 Ochratoxine A
L'exposition humaine, observée dans plusieurs pays d'Europe est
objectivée par la présence d'ochratoxine A dans les denrées
alimentaires et le sang. Le groupe de travail n'a pas connaissance
de tentatives qui ont effectuées dans d'autres régions du monde pour
déceler la présence d'ochratoxine A dans le sang humain.
En s'appuyant sur l'étude de la maladie-naturelle ou provoquée
par administration d'ochratoxine A-on a pu établir le rôle
étiologique de l'ochratoxine A dans la néphropathie porcine. A
partir de ce modèle, on a pu émettre l'hypothèse que la néphropathie
endémique des Balkans était due à une exposition à cette toxine. Les
données immunologiques disponibles montrent que cette affection
serait attribuable à la consommation de denrées alimentaires
contaminées par de l'ochratoxine A. Depuis la publication en 1980 du
No 11 des Critères d'hygiène de l'environnement, des études
épidémiologiques sur la concentration de l'ochratoxine A dans le sang
humain dans les régions touchées et non touchées ont conforté
l'hypothèse d'une relation entre la néphropathie balkanique et
l'exposition à l'ochratoxine A.
Ainsi, on a montré que les habitants des régions d'endémie
présentaient plus fréquemment de l'ochratoxine A dans leur sang et à
des concentrations plus élevées. Cependant, ces études
rétrospectives ne fournissent que des présomptions sur lesquelles on
ne peut établir l'existence d'une relation causale directe. On ne
peut cependant pas l'exclure du fait de la longue période de latence
entre l'exposition et l'apparition des symptômes.
On a montré que l'ochratoxine A exerçait, chez les souris mâles
et les rats des deux sexes, des effets cancérogènes sur l'épithélium
des tubules rénaux. Il existe une relation tout à fait significative
entre la néphropathie balkanique et la présence de tumeurs des voies
urinaires, notamment de tumeurs pyéliques et uretérales. Toutefois
aucune donnée n'a été publiée qui établissent la responsabilité
directe de l'ochratoxine A dans l'étiologie de ces tumeurs.
4.2 Trichothécènes
Sur la base des données dont disposait le groupe de travail, il
est possible d'établir une relation entre l'exposition aux
trichothécènes et certains épisodes toxiques chez l'homme. Si l'on
se réfère aux quelques données disponibles, ce sont le DON et le NIV
qui sont les plus fréquemment cités dans les différents cas
d'exposition humaine. Dans le cas des flambées d'aleucie toxique
alimentaire et de toxicose par ingestion de céréales moisies, on ne
peut exclure la responsabilité des trichothécènes. L'exposition se
produit par ingestion de denrées contaminées, principalement des
céréales. Les différents traitements qu'elles subissent, notamment
la mouture et la panification ne permettent pas d'éliminer le DON, le
NIV, ni la toxine T-2. L'existence d'une exposition par voie
respiratoire est très peu documentée mais c'est une possibilité qu'on
ne peut totalement écarter.
Parmi les tricothécènes qui se trouvent à l'état naturel dans les
aliments, c'est la toxine T-2 qui est la plus active, suivie du DAS
and du NIV; les études de toxicité aiguë ont montré que le DON était
la substance la moins toxique. Chez l'animal d'expérience, la toxine
T-2 et le DAS déterminent des symptômes généraux aigus, avec nécrose
des tissus épithéliaux et suppression de l'hematopoïèse. Lors des
récentes flambées d'intoxications, il n'a été fait état que des
symptômes digestifs.
Le DON est tératogène pour la souris mais pas pour le rat. Selon
les études de toxicité chroniques qui ont été publiées, le NIV et la
toxine T-2 ne sont pas tumorigènes chez l'animal. Aucune étude de
cancérogénicité à long terme n'a été publiée sur le DON. Certains
trichothécènes, tels que la toxine T-2 et le DON ont une action
immunosuppressive chez l'animal et produisent des modifications de
l'immunité à médiation cellulaire et de l'immunité humorale. En
revanche, rien n'indique l'existence de tels effets chez l'homme.
On est mal informé sur les cas d'intoxication humaine due à une
exposition aux trichothécènes, cas qui sont en nombre limité. Les
symptômes observés consistent en troubles digestifs et irritation de
la gorge; ils apparaissent peu après l'ingestion de denrées
alimentaires contaminées par des trichothécènes. A l'heure actuelle,
on ne connaît pas de cas de cancer humain attribuable aux
trichothécènes. Le groupe de travail ne disposait d'aucune
publication faisant état d'infections secondaires d'origine
bactérienne, fongique ou virale chez l'homme, à la suite d'une
exposition aux trichothécènes comme on en a observé chez l'animal
d'expérience. Il semble que l'on n'ait pas effectué d'études
appropriées sur ce point.
4.3 Ergot
Il semble que l'exposition humaine à de faibles concentrations
d'ergolines soit très répandue. Les données relatives aux flambées
qui se sont récemment déclarées en Ethiopie et en Inde montrent que
les alcaloïdes de C. purpurea (groupe de l'ergotamine) produisent
les effets les plus graves, notamment une gangrène des membres
inférieurs pouvant entraîner la mort, effets qui sont plus graves
que ceux des alcaloïdes de C. fusiformis (groupe de la clavine) qui
entraînent des symptômes digestifs sans issue fatale. On ignore si
ces différences s'expliquent par la teneur des différentes espèces
de champignons en alcaloïdes, par les propriétés toxicologiques de
ces substances ou par des différences dans les quantités ingérées
par les diverses populations.
Après nettoyage et mouture qui éliminent les sclérotes, il ne
subsiste dans les produits alimentaires que de faibles quantités
d'ergolines. La panification ou d'autres traitements thermiques
détruisent également la plupart des alcaloïdes du groupe de
l'ergotamine.
5. Recommandations en vue de recherches futures
5.1 Recommandations generales
Il conviendrait de créer un réseau de centres de référence pour
aider les Etats Membres à confirmer l'identité des mycotoxines
présentes dans les tissus humains et les denrées destinées à la
consommation humaine. Ces centres devraient également fournir sur
demande des échantillons de référence de mycotoxines afin de
faciliter la comparabilité des résultats d'analyse obtenus dans les
différentes régions du monde.
5.2 Ochratoxine A
a) Etudes épidémiologiques de grande ampleur à caractère
rétrospectif et études localisées à caractère prospectif sur
l'association entre l'ochratoxine A et la néphropathie
endémique des Balkans ainsi que les tumeurs des voies
urinaires : ces études devraient être menées dans la
péninsule de Balkans et la Région méditerranéenne.
b) Recherche et dosage de l'ochratoxine A dans le sang de
malades porteurs de tumeurs des voies urinaires, à
l'extérieur de la péninsule des Balkans.
c) Recherche de sources d'exposition à l'ochratoxine A, par
analyse du sang, dans les pays situés en dehors de la
péninsule des Balkans.
d) Elucidation du mécanisme à l'origine des différences entre
les sexes qui ont été relevées dans les anomalies rénales,
néoplasiques et non-néoplasiques produites par l'ochratoxine
A chez l'animal d'expérience.
e) Etudes de grande envergure sur la teneur en ochratoxine A des
denrées alimentaires dans les différentes régions du monde.
Ce type d'enquêtes est particulièrement important dans les
régions où l'on observe une forte incidence de tumeurs des
voies urinaires, notamment rénales, ou de néphropathies.
5.3 Trichothécènes
a) Il conviendrait d'effectuer des études longitudinales dans
les régions de l'Inde et de la République populaire de Chine
où ont été récemment observés des épisodes d'intoxication par
les trichothécènes. Il conviendrait de mieux éclaircir la
présence inhabituelle de certains trichothécènes dans ces
régions.
b) Il faudrait étudier les effets d'une exposition prolongée
d'animaux de laboratoire au DON, et notamment les effets
cancérogènes. Comme la réaction au DON varie beaucoup selon
les espèces, l'espèce qui sera retenue devra être choisie
avec soin.
c) Il faudrait étudier plus à fond les infections microbiennes
secondaires à une exposition aux trichothécènes chez l'animal
d'expérience.
d) Il convient d'étudier l'influence des conditions
environnementales, notamment la présence d'insecticides ou
autres produits chimiques industriels, sur la production de
trichothécènes par des champignons.
e) Il faudrait élucider l'effet des différents modes de
préparation des denrées alimentaires sur les trichothécènes.
f) Il conviendrait de mettre au point des espèces végétales qui
résistent aux champignons producteurs de trichothécènes, en
recourant aux biotechnologies.
g) Il faudrait étudier sur l'animal les effets synergistiques
éventuels d'une exposition simultanée aux trichothécènes, aux
aflatoxines, à l'ochratoxine A et à d'autres mycotoxines.
h) Il faudrait également déterminer l'apport de trichothécènes
d'origine alimentaire chez l'homme.
i) Il faudrait mettre au point des méthodes de criblage des
trichothécènes qui soient à la fois rapides et sensibles, et
mener des enquêtes dans les régions tempérées du monde pour
rechercher la présence de trichothécènes dans les céréales et
les préparations alimentaires.
5.4 Ergot
a) Il faudrait mettre au point des méthodes pour l'analyse des
agroclavines.
b) Il conviendrait de mettre à la disposition des pays en
développement des renseignements sur le repérage des semences
contaminées et sur les méthodes de mouture permettant de
réduire au minimum les problèmes posés par la présence de
l'ergot.
c) Il faudrait effectuer des études épidémiologiques sur les
effets éventuels que peut entraîner chez l'homme l'ingestion
de faible quantités d'ergolines.
d) Il faudrait effectuer des études pharmacologiques et
toxicologiques sur les ergolines, seules ou en association,
chez l'animal d'expérience.
e) Il conviendrait de déterminer si les ergolines peuvent se
transmettre de la mère à l'enfant par le lait maternel.
RESUMEN Y RECOMENDACIONES PARA ULTERIOR INVESTIGACION
1. Ocratoxina A
1.1 Distribución natural
Las ocratoxinas son producidas por varias especies de los
géneros de hongos Aspergillus y Penicillium. Esos hongos son
ubicuos y hay amplias posibilidades de contaminación de alimentos
para seres humanos y para animales. La ocratoxina A, la más
importante, se ha hallado en toda una serie de países de América
del Norte, Australia y Europa. La formación de ocratoxina por las
especies del género Aspergillus parece estar limitada a condiciones
de humedad y temperatura elevadas, mientras que algunas especies,
por lo menos, de Penicillium pueden producir ocratoxina a
temperaturas de sólo 5 °C.
Las mayores incidencias de contaminación con ocratoxina A se
han hallado en cereales y, en menor medida, en algunos granos
(café, soja, cacao). La ocratoxina B es muy poco frecuente.
1.2 Métodos de análisis
Se han desarrollado técnicas de análisis para identificar la
ocratoxina y determinar sus concentraciones en la gama de g/kg.
1.3 Metabolismo
Se han hallado residuos de ocratoxina A no modificada en la
sangre, los riñones, el hígado y los músculos de cerdos en los
mataderos y en los músculos de gallinas y pollos. Sin embargo, por
lo general no se han hallado residuos de ocratoxina A en los
rumiantes. El enlace in vitro de la ocratoxina A con la
seroalbúmina es especialmente importante en el ganado vacuno, el
ganado ovino y el ser humano. Estudios experimentales realizados
con cerdos y gallinas han demostrado que las concentraciones más
altas de ocratoxina A se hallan en los riñones. La hidroxilación
microsómica puede representar una reacción de detoxificación en los
cerdos, las ratas y el ser humano. En estudios experimentales, un
mes después de terminada la exposición se localizaron aún residuos
en riñones de cerdos.
1.4 Efectos en animales
Varios países europeos han notificado en animales de granja
(cerdos, aves de corral) casos de ocratoxicosis, cuya principal
manifestación era una nefropatía crónica. Entre las lesiones había
atrofia tubular, fibrosis intersticial y, en etapas ulteriores,
hialinización de los glomérulos. También se ha hallado ocratoxina
A en sangre de cerdo recogida en mataderos canadienses. La
ocratoxina A ha producido efectos nefrotóxicos en todas las
especies de animales provistas de un solo estómago que se han
estudiado hasta el momento, incluso con las dosis más bajas con que
se experimentó (200 µg/kg de alimentos en ratas y cerdos).
Se observaron efectos teratogénicos en ratones expuestos a
dosis de 3 mg/kg de peso corporal administradas por vía oral. En
ratas que recibieron por vía oral dosis de 0,75 mg/kg de peso
corporal se observó resorción fetal. Los efectos teratogénicos,
que en las ratas se acentuaron con una dieta baja en proteínas, se
han observado también en hámsters.
No hay datos que demuestren la actividad de la ocratoxina A en
pruebas a corto plazo de la mutagenicidad (bacterias y levaduras).
Ratas expuestas a ocratoxina A administrada por vía oral mostraron
roturas de una sola cadena del ADN en tejidos renales y hepáticos.
Ocratoxina A administrada por vía oral provocó neoplasias de
células renales en ratones machos y en ratas de uno y otro sexo.
Se registraron neoplasias de células hepáticas en sólo una estirpe
de ratones y no en la rata.
La ocratoxina A es inhibidora de la síntesis de proteínas y de
la sintetasa del ARNt en microorganismos, células de hepatoma y el
ARNm renal en la rata.
La ocratoxina A puede inhibir la migración de los macrófagos.
En ratones, una dosis de 0,005 µg/kg de peso corporal suprimió la
respuesta inmunitaria a eritrocitos de oveja; sin embargo, se han
obtenido también resultados contradictorios.
Se ha demostrado que la ocratoxina A es carcinógena para el
epitelio de los túbulos renales en ratones machos y de ratas de uno
y otro sexo.
1.5 Efectos en el ser humano
En varios países de Europa se ha observado exposición humana a
ocratoxina A, como lo demuestra la presencia de ésta en alimentos y
en la sangre y la leche humanas. Los datos epidemiológicos de que
se dispone indican que la nefropatía de los Balcanes podría estar
relacionada con el consumo de productos alimenticios contaminados
por esta toxina.
Se ha observado una relación muy significativa entre la
nefropatía de los Balcanes y tumores del tracto urinario, en
particular los de la pelvis renal y los uréteres. Sin embargo, no
se han publicado datos que demuestren el papel causal de la
ocratoxina A en la etiología de esos tumores.
2. Tricotecenos
2.1 Distribución natural
Hasta ahora se conocen 148 tricotecenos, caracterizados
químicamente por la presencia del mismo sistema básico de un anillo
tetracíclico de scirpenol. Son compuestos producidos
principalmente por hongos pertenecientes al género Fusarium,
aunque otros géneros, entre ellos Trichoderma, Trichothecium,
Myrothecium y Stachybotrys, producen también metabolitos
ahora reconocidos como tricotecenos. Se ha visto que sólo un
pequeño número de los tricotecenos conocidos contaminan los
alimentos para seres humanos o para animales, entre ellos el
deoxinivalenol (DON), el nivalenol (NIV), el diacetoxiscirpenol
(DAS) y la toxina T-2 y, con menos frecuencia, ciertos derivados
(3-Ac-DON,15-Ac-DON, fusarenon-X y toxina HT-2). De todos ellos,
el que se encuentra más a menudo en alimentos para seres humanos y
para animales es el DON, por lo general con cantidades más pequeñas
de NIV como co-contaminante. Algunos tricotecenos macrocíclicos
como las satratoxinas G y H y las verrucarinas se observan
ocasionalmente en alimentos para animales (paja, heno) pero no se
ha notificado su presencia en alimentos para seres humanos.
Estudios sobre la distribución de los tricotecenos han indicado
que el DON se encuentra en todo el mundo, sobre todo en cereales
como el trigo y el maíz, en concentraciones que pueden llegar hasta
92 mg/kg, aunque las concentraciones medias son considerablemente
inferiores y varían según el producto. Hay notificaciones aisladas
de la presencia de DON en la cebada, los piensos compuestos, las
patatas, etc. Habitualmente los cereales producidos en Canadá y
los Estados Unidos de América no contienen NIV pero éste se ha
encontrado en los cereales asiáticos y europeos, junto con DON; la
mayor concentración de NIV registrada hasta la fecha fue de 37,9
mg/kg. La presencia de toxina T-2 y DAS se ha notificado con escasa
frecuencia y en concentraciones muy inferiores.
Estudios sobre la elaboración y la molienda indican que las
concentraciones de DON apenas se reducen del cereal al producto
acabado. Tampoco se destruye cuando se cuece el cereal. En
general, los productos alimenticios para seres humanos que se
encuentran en el comercio rara vez contienen concentraciones
detectables de DON y NIV.
2.2 Métodos de análisis
Existen xistenmétodos de análisis basados en la cromatografía
de capa fina, la cromatografía de fase gaseosa, la cromatografía de
fase líquida de alto rendimiento y técnicas inmunológicas para la
determinación de las cuatro toxinas más frecuentes (DON, toxina T-
2, DAS y NIV) con límites de detección inferiores a 1 µg/g.
Algunos de esos métodos se han ensayado en colaboración. Además,
la identidad puede confirmarse mediante métodos de investigación
como cromatografía de fase gaseosa/espectografía de masas y
cromatografía de fase líquida/espectografía de masas.
2.3 Metabolismo
Se han realizado estudios metabólicos con animales, sobre todo
administrando toxina T-2 y en unos pocos casos DON. Estos
tricotecenos se absorben con rapidez por el tubo digestivo,
aunque no se dispone de datos cuantitativos. Las toxinas se
distribuyen bastante uniformemente, sin marcada acumulación en
ningún órgano o tejido determinado. Los tricotecenos se
transforman en metabolitos menos tóxicos por reacciones como
hidrólisis, hidroxilación, de-epoxidación y glucoronidación.
Tricotecenos como la toxina T-2 y el DON se eliminan rápidamente en
las heces y la orina. Por ejemplo, casi el 100% de una dosis de
toxina T-2 administrada a ganado por vía oral se eliminaba en unas
horas; en pollos, alrededor del 80% se había eliminado a las 48
horas. En la rata, 96 horas después de la administración, el 25%
del DON se había eliminado en la orina y el 65% en las heces. Los
resultados de la transmisión de toxina T-2 en gallinas ponedoras y
vacas lactantes indicaron que pasaba a los huevos y a la leche
menos del 1% de la dosis administrada de esa toxina y de sus
metabolitos. En la carne de pollo, 24 horas después de la
administración de toxina T-2 por vía oral, los residuos de ésta y
de sus metabolitos eran inferiores al 2% de la dosis.
2.4 Efectos en animales
La principal vía de exposición de los animales a tricotecenos
es la ingestión de alimentos de origen vegetal. La toxina T-2 y el
DAS, los más potentes en los experimentos de laboratorio con
animales, de todos los tricotecenos que habitualmente se consideran
contaminantes de alimentos para animales (toxina T-2, DAS, NIV y
DON), provocan una respuesta tóxica similar. El NIV es menos
potente en algunos sistemas que los dos compuestos anteriores y el
DON es el menos tóxico de los cuatro (un ejemplo de la potencia es
la DL50 por vía oral en el ratón: toxina T-2, 10,5 mg/kg de peso
corporal y DON, 46,0 mg/kg).
Los tricotecenos más potentes, como la toxina T-2 y el DAS,
producen efectos generales agudos cuando se administran
experimentalmente a roedores y ganado porcino y bovino por vía oral
o parenteral o por inhalación (cerdo, ratón). Una lesión producida
por el contacto con tricotecenos potentes como la toxina T-2 y el
DAS es la epitelionecrosis (dosis de 0,2 µg por zona en el caso de
la toxina T-2). En cuanto a otros tricotecenos, son necesarias
dosis mayores para producir un efecto irritante (NIV, 10 µg por
zona). Los tricotecenos citotóxicos, como la toxina T-2, producen
necrosis del epitelio de las criptas intestinales y de los tejidos
linfoides y hematopoyéticos, tras exposición oral o parenteral o
por inhalación. La exposición a tricotecenos citotóxicos como la
toxina T-2 y el DAS va seguida de alteraciones hematológicas y
coagulopáticas. Las toxicosis graves pueden originar
pancitopenia. En estudios con toxina T-2, DON y DAS se ha
demostrado la supresión de la inmunidad de base celular y humoral,
y entre los efectos observados figuran la reducción de las
concentraciones de inmunoglobulinas y la disminución de la
actividad fagocítaria de macrófagos y neutrófilos. Los resultados
de estudios experimentales con animales han indicado que el efecto
inmunodepresor de tricotecenos como la toxina T-2, el DAS y el DON
origina una disminución de la resistencia a la infección secundaria
por bacterias (micobacterias, Listeria monocytogenes), levaduras
(Cryptococcus neoformans) y virus (virus del herpes simplex).
Se ha comunicado que la toxina T-2 es teratogénica en el ratón,
cuando se administra por inyección intraperitoneal (vía de
administración poco corriente en los estudios de la
teratogenicidad). Se ha comunicado que el DON es teratogénico en
los ratones tras intubación gástrica, pero no en las ratas cuando
la toxina se administra con los alimentos. El NIV no es
teratogénico en el ratón. La toxina T-2, el DAS y el DON no
resultaron mutagénicos en una prueba de tipo Ames. En algunas
pruebas, se observó una débil actividad clastogénica de la toxina
T-2. En los estudios publicados sobre la toxicidad a largo plazo
en animales, no se obtuvieron datos que indiquen que la toxina T-2,
el fusarenón-X o el NIV sean oncogénicos en los animales. No se
han publicado estudios a largo plazo sobre la toxicidad del DON.
Los tricotecenos son tóxicos para las células que se dividen
activamente como las del epitelio de las criptas intestinales y las
hematopoyéticas. La citotoxicidad se ha asociado con el trastorno
de la síntesis de proteínas debido al enlace de los compuestos con
los ribosomas de las células eucarióticas o bien con la disfunción
de las membranas celulares. La inhibición de la síntesis de las
proteínas se ha relacionado con la inducción de proteínas lábiles y
reguladoras como la IL-2 en los inmunocitos. Concentraciones
extremadamente bajas de tricotecenos alteran el transporte de
pequeñas moléculas en las membranas celulares.
2.5 Efectos en el ser humano
La ingestión de alimentos contaminados de origen vegetal es la
principal vía de exposición a tricotecenos, pero ocasionalmente se
han notificado otras, por ejemplo el contacto accidental con la
piel entre los investigadores de laboratorio y la inhalación de
tricotecenos transportados por el polvo.
Los casos registrados de enfermedad asociados con la exposición
a tricotecenos son escasos y en ninguno de ellos se ha demostrado
la relación causal. No obstante, los dos brotes que se describen a
continuación parecen indicar su existencia.
En China se registró un brote de enfermedad asociado con el
consumo de trigo mohoso que contenía de 1,0 a 40,0 mg de DON/kg.
La enfermedad se caracterizó por síntomas gastro-intestinales. No
hubo defunciones. Los cerdos y los pollos alimentados con restos
de cereales también resultaron afectados.
Un brote análogo se registró en la India, asociado al consumo
de pan hecho con trigo contaminado. La enfermedad se caracterizó
por síntomas gastrointestinales e irritación de la garganta, que
aparecía de 15 minutos a una hora después de la ingestión del pan.
En muestras de la harina de trigo refinada utilizada para su
preparación se detectaron las siguientes micotoxinas: DON
(O,35-8,3 mg/kg), acetildeoxinivalenol (0,64-2,49 mg/kg),
NIV (0,03-0,1 mg/kg) y toxina T-2 (0,5-0,8 mg/kg). Sin
embargo, no hubo confirmación de la identidad de los tricotecenos
detectados. La presencia simultánea de DON y NIV y de toxina T-2
es insólita.
Dos enfermedades de interés histórico, la aleucia tóxica
alimentaria (ATA) de la URSS y la toxicosis del trigo mohoso del
Japón y Corea se han asociado con el consumo de cereales invadidos
por hongos Fusarium. Desde entonces se han identificado en
condiciones de laboratorio algunos tricotecenos en cultivos de
mohos Fusarium aislados en cereales encontrados en los incidentes.
En la época en que apareció la enfermedad, no pudieron realizarse
estudios que relacionaran la ATA o la toxicosis de los cereales
mohosos con la exposición a tricotecenos porque las toxinas no eran
conocidas.
3. Cornezuelo
3.1 Distribución natural
Cornezuelo es el nombre dado a los esclerocios de especies de
hongos pertenecientes al género Claviceps. Alcaloides biológi-
camente activos contenidos en el esclerocio provocan toxicosis
cuando éste es consumido por hombres o animales en alimentos
contaminados.
Los alcaloides del cornezuelo producen dos modalidades
diferentes de enfermedad, según el hongo de que se trate ( C.
purpurea, C. fusiformis) y, por lo tanto, los alcaloides
producidos. El ergotismo, provocado por los alcaloides ergotamina-
ergocristina producidos por C. purpurea, se caracteriza sobre
todo por gangrena de las extremidades, además de síntomas
gastrointestinales. La intoxicación resultante de la ingestión de
mijo contaminado por C. fusiformis se caracteriza principalmente
por síntomas gastrointestinales y está relacionada con los
alcaloides de tipo clavina. No hay signos ni síntomas que indiquen
la presencia de vaso-oclusión.
3.2 Métodos de análasis
Los alcaloides del cornezuelo (ergolinas) son derivados del
ácido lisérgico. Los diversos alcaloides difieren por la
importancia de su actividad biológica. La presencia de alcaloides
del cornezuelo producidos por C. purpurea se ha determinado por
cromatografía de fase líquida de alto rendimiento con detección por
fluorescencia. Pueden medirse concentraciones de 0,2 µg de
ergolina por litro de plasma humano. La presencia de ergotamina y
ergocristina puede determinarse con gran especificidad mediante
pruebas de radioinmunovaloración en concentraciones de 3,5
picomoles y 0,8 picomoles, respectivamente.
3.3 Efectos en animales
Las ergolinas, principalmente la ergotamina y la ergotaminina,
se han asociado con brotes de abortos en el ganado bovino. Ovejas
a las que se administró ergotamina por vía oral enfermaron
rápidamente y se observó en ellas inflamación intestinal. Aves de
corral, cerdos y primates expuestos por vía oral experimentaron
efectos leves. El Grupo Especial no dispuso de datos sobre la
mutagenicidad, la teratogenicidad y la carcinogenicidad de las
ergolinas.
3.4 Efectos en el ser humano
Los cereales infectados por Claviceps son fuente de exposición
humana a las ergolinas. En la mayor parte de los estudios
toxicológicos, no se han identificado los alcaloides específicos.
La información resultante de un solo estudio sobre cereales y
productos de cereales indica que en Suiza la ingesta total
de ergolinas por los seres humanos es de alrededor de 5,1 µg por
persona, siendo el contenido de ciertos productos de hasta 140
µg/kg. La cocción reduce las ergolinas presentes en la harina
contaminada un 25-100%.
En 1978 hubo en Etiopía un brote de ergotismo, resultante de
exposición a ergolinas procedentes de esclerocios de C.
purpurea. El cereal contenía hasta un 0,75% de cornezuelo; se
detectó específicamente ergometrina. Los síntomas fueron gangrena
seca, con pérdida de uno o varios miembros (29% de los casos),
pulsos periféricos débiles o ausentes (36%) y desescamación de la
piel. Sólo hubo síntomas gastrointestinales en unos pocos casos.
Trastornos de las extremidades inferiores se registraron en el 88%
de los pacientes.
En la India ha habido varios brotes desde 1958 a consecuencia
de la ingestión de mijo perlado que contenía cornezuelo de tipo
clavina producido por C. fusiformis. Los síntomas comprendían
náuseas, vómitos y mareos, y fueron causados por la ingestión de
mijo perlado con un contenido de 15 a 26 mg de ergolina/kg.
Como en ninguno de los dos episodios se practicaron autopsias,
no se dispone de información sobre los efectos anatomopatológicos
en las vísceras humanas.
4. Evaluación de los riesgos para la salud humana
4.1 Ocratoxina A
En diversos países de Europa se ha observado exposición humana
a la ocratoxina A, como lo demuestra la presencia de ésta en
alimentos y en la sangre. El Grupo Especial no conoce ningún
intento de detectar la ocratoxina A en la sangre humana en otras
partes del mundo.
El papel causal de la ocratoxina A en la nefropatía porcina se
ha demostrado sobre la base de estudios de casos sobre el terreno y
de la reproducción de la enfermedad con ocratoxina A. Utilizando
el modelo porcino, se ha supuesto que la nefropatía endémica de los
Balcanes podría obedecer a exposición a la ocratoxina A. Los datos
epidemiológicos de que se dispone indican que esa enfermedad podría
estar asociada al consumo de alimentos contaminados por dicha
toxina. Desde la publicación de Criterios de salud ambiental 11 en
1979, estudios epidemiológicos sobre concentración de ocratoxina A
en la sangre humana en zonas afectadas y no afectadas han
proporcionado un argumento más en favor de la relación entre la
nefropatía de los Balcanes y la exposición a la ocratoxina A.
Se ha demostrado que tanto la presencia de ocratoxina A en la
sangre como sus concentraciones son mayores en las personas que
residen en las zonas de endemicidad. No obstante, las pruebas
indirectas que proporcionan los mencionados estudios retrospectivos
no bastan para demostrar por sí solas la existencia de una relación
causal directa. Esta tampoco puede excluirse, dado el largo
periodo de latencia entre la exposición y el comienzo de los
síntomas.
Se ha demostrado que la ocratoxina A es carcinogénica para el
epitelio de los túbulos renales en los ratones machos y en las
ratas de uno y otro sexo. Se ha observado una relación muy
significativa entre la nefropatía de los Balcanes y los tumores del
tracto urinario, en particular de la pelvis renal y los ureteres.
Sin embargo, no hay datos publicados que demuestren un papel causal
directo de la ocratoxina A en la etiología de esos tumores.
4.2 Tricotecenos
Sobre la base de la información facilitada al Grupo Especial,
es posible asociar la exposición a tricotecenos con episodios de
enfermedad en los seres humanos. Según los limitados datos
disponibles, los tricotecenos que intervienen más frecuentemente en
episodios de exposición humana son el DON y el NIV. En los
episodios de aleucia tóxica alimentaria y toxicosis por ingestión
de cereales mohosos registrados en el pasado, no puede excluirse el
posible papel etiológico de los tricotecenos. La exposición se
produce por la ingestión de alimentos contaminados, principalmente
cereales. La elaboración, la molienda y la cocción no logran la
destrucción del DON, el NIV y la toxina T-2. Los datos en favor de
la exposición por inhalación son muy limitados, pero no puede
excluirse esa posibilidad.
Entre los tricotecenos naturalmente presentes en alimentos, el
más potente es la toxina T-2, seguida por el DAS y el NIV; el DON
resultó el menos tóxico en los estudios de la toxicidad aguda. En
los animales experimentales, la toxina T-2 y el DAS tienen efectos
generales agudos, con necrosis de tejidos epiteliales y supresión
de la hematopoyesis. En los brotes de enfermedad contemporáneos,
sólo se han registrado síntomas gastrointestinales.
Se ha demostrado que el DON es teratogénico en los ratones pero
no en las ratas. Según los estudios de la toxicidad crónica
publicados, el NIV y la toxina T-2 no son oncogénicos en los
animales. No se han publicado estudios sobre la carcino-genicidad
a largo plazo del DON. Ciertos tricotecenos, como la toxina T-2 y
el DON, tienen una acción inmunosupresora en los animales y han
producido alteraciones de la inmunidad, tanto de base celular como
humoral. No hay datos que demuestren la existencia de una acción
inmunosupresora en el hombre.
Los casos de enfermedad humana asociados con la exposición a
tricotecenos que se han notificado son limitados, tanto por su
número como por la información proporcionada. Tras la ingestión de
alimentos contaminados con tricotecenos aparecen rápidamente
síntomas de trastornos digestivos e irritación de la garganta. En
la actualidad no hay nada que demuestre que los tricotecenos
causen cáncer en el ser humano. El Grupo Especial no tuvo a su
disposición informes sobre infección secundaria por bacterias,
hongos o virus en los seres humanos tras la exposición a
tricotecenos, como las observadas en estudios experimentales con
animales. Al parecer, no se han realizado estudios suficientes
para aclarar esa sucesión.
4.3 Cornezuelo
La exposición humana a bajas concentraciones de ergolinas
parece estar muy extendida. Los datos de que se dispone sobre los
recientes brotes en Etiopía y en la India indican que los
alcaloides producidos por C. purpurea (grupo de la ergotamina)
tienen efectos más graves-entre ellos gangrena de las piernas y
muerte-que los alcaloides producidos por C. fusiformis (grupo de la
clavina), que provocaron síntomas gastrointestinales sin desenlace
mortal. No se sabe si esas diferencias corresponden a diferencias
en el contenido de alcaloides de las especies de hongos, en las
propiedades toxicológicas o toxicométricas de los alcaloides o en
las dosis ingeridas por diferentes tipos de poblaciones.
Cuando la limpieza y la molienda eliminan los esclerocios, sólo
quedan en los alimentos preparados bajas concentraciones de
ergolinas; la cocción y otras aplicaciones de calor destruyen
también la mayor parte de los alcaloides del grupo de la
ergotamina.
5. Recomendaciones para ulterior investigación
5.1 Recomendaciones generales
Debe establecerse una red de centros de referencia, que ayuden
a los Estados Miembros a confirmar la identidad de las mico-toxinas
halladas en alimentos y tejidos humanos. Esos centros deben
proporcionar también muestras de referencia de micotoxinas, cuando
se les solicite, a fin de aumentar las posibilidades de comparar
los resultados de análisis realizados en distintas partes del
mundo.
5.2 Ocratoxina A
a) En los Balcanes y en la región del Mediterráneo deben
realizarse estudios epidemiológicos retrospectivos extensos
y prospectivos focales sobre la asociación de la ocratoxina
A con la nefropatía endémica de tipo balcánico y con
tumores del tracto urinario.
b) Fuera de los Balcanes, deben realizarse, en enfermos con
tumores del tracto urinario, análisis de sangre para
detectar la presencia de ocratoxina A.
c) En países no situados en la zona de los Balcanes, debe
aclararse la fuente de la exposición a ocratoxina A, cuando
los análisis de sangre humana indiquen que la misma existe.
d) Debe aclararse la manera en que actúa la diferencia de sexo
en las enfermedades renales, neoplásicas y no neoplásicas,
causadas por la ocratoxina A en animales de
experimentación.
e) Son necesarios extensos estudios sobre el contenido de
ocra-toxina A de los alimentos en distintas partes del
mundo. Es especialmente importante realizar estudios de
ese tipo en las regiones con una elevada incidencia de
tumores del tracto urinario, tumores renales o nefropatías.
5.3 Tricotecenos
a) Deben realizarse estudios de seguimiento en las zonas de la
India y de la República Popular China en que ha habido
recientemente episodios de intoxicación de seres humanos
por tricotecenos. Debe aclararse más la modalidad no usual
de presencia de tricotecenos en esos episodios.
b) Deben estudiarse los efectos de la exposición a largo plazo
de animales de experimentación al DON, incluidos los
efectos carcinogénicos. Como la respuesta de distintas
especies al DON varía mucho, deben elegirse cuidadosamente
las especies utilizadas para el estudio.
c) Debe aclararse más la aparición de infecciones microbianas
secundarias en animales de experimentación tras la
exposición a tricotecenos.
d) Debe estudiarse la influencia de las condiciones
ambientales, incluida la presencia de insecticidas y de
otros productos químicos artificiales, en la producción de
tricotecenos por los hongos.
e) Deben aclararse los efectos de la elaboración de los
alimentos en los tricotecenos.
f) Deben desarrollarse, mediante métodos biotecnológicos,
cultivos agrícolas resistentes a la infección por hongos
productores de tricotecenos.
g) Deben estudiarse en animales de experimentación los
posibles efectos sinérgicos de la exposición combinada a
tricotecenos, aflatoxinas, ocratoxina A y otras
micotoxinas.
h) Deben realizarse estudios sobre la ingesta de tricotecenos
por seres humanos.
i) Deben elaborarse métodos rápidos y sensibles de detección
de los tricotecenos y deben realizarse estudios para
localizar los tricotecenos en cereales y alimentos tratados
en las zonas templadas.
5.4 Cornezuelo
a) Deben hallarse métodos de análisis que permitan detectar la
presencia de agroclavinas.
b) Debe proporcionarse a los países en desarrollo información
sobre el empleo de procedimientos de detección de semillas
patológicas y de molienda para reducir al mínimo los
problemas causados por el cornezuelo.
c) Deben realizarse estudios epidemiológicos sobre los
posibles efectos de bajas concentraciones de ergolinas en
la población humana.
d) Deben efectuarse estudios farmacológicos y toxicológicos en
los que se administren a animales de experimentación
distintas ergolinas, aisladas y en combinación.
e) Debe aclararse la posible trasmisión de ergolinas a los
lactantes a través de la leche materna.
III.2.3 Occurrence in foodstuffs
Traditionally, contamination of grain with ergot has been
expressed as a percentage on a weight basis, without measurement of
total and individual amounts of ergolines. Thus, it is generally
recommended that feed containing more than 0.1% ergot should not be
given to animals (Young, 1981a,b). Quantitative analysis for total
and individual ergolines in 14 samples of rye and wheat flour
(Scott & Lawrence, 1980) indicated contamination with 6 Group I
ergolines (ergometrine, ergosine, ergotamine, ergocornine,
ergocryptine, ergocristine) at concentrations ranging from 0.3 to
62 µg/kg for individual ergolines. Ergocristine was the major
ergoline present in the flours, 62 µg/kg being the maximal
concentration found. In 17 samples of rye grain collected from
health shops, 6 contained ergot; ergoline concentration and
composition were not indicated (Akerstrand, 1980). In a survey of
ergolines in ergot-contaminated cereals, in North America, the
average total ergolines content was 0.24% (Young, 1981a,b; Young &
Chen, 1982). On average, the pooled ergoline composition in
sclerotia in rye, wheat, and triticale was as follows:
ergocristine (31%), ergocristinine (13%), ergotamine (17%),
ergotaminine (8%), ergocryptine (5%), ergocryptinine (3%),
ergometrine (5%), ergometrinine (2%), ergosine (4%), ergosinine
(2%), ergocornine (4%), and ergocorninine (2%). The individual
ergoline composition was uniform throughout a single sclerotium or
in different sclerotia from the same head, somewhat less uniform
between different fields throughout a region, and highly variable
from head-to-head in a given field. In contrast, the total
ergoline content was highly variable within-sclerotium, within-
head, head-to-head, and on a field-to-field basis. In a
comparative study of ergoline in ergots from rye and wheat (caused
by infection with C. purpurea) and in ergots from pearl millet
(caused by infection with C. fusiformis) in South-East Asia, it was
found that the total ergoline content in the sclerotia of pearl
millet was much lower (320 mg/kg) than that in the sclerotia of rye
(700 mg/kg) and wheat (920 mg/kg) (Bhat et al., 1976). The
ergolines in the sclerotia of pearl millet were reported to
comprise agroclavine, elymoclavine, chanoclavine, penniclavine, and
setoclavine.
In a survey of cereals and cereal products on the Swiss market,
using a high-performance liquid chromatography procedure, the
following average concentrations of total ergolines were found:
wheat flour, 4.2 µg/kg; wheat flour (coarse), 30.7 µg/kg; wheat
flour (more coarse), 103.4 µg/kg; rye flour, 139.7 µg/kg; and
"bioproducts", 10.2-22.7 µg/kg (Baumann et al., 1985). The daily
intake of total ergolines by human beings in Switzerland was
estimated to be 5.1 µg/person.
III.2.4 Fate of ergolines during food processing
Treatment of sclerotia from wheat with chlorine (1%) and heat
(150-200 °C) resulted in a 90% reduction in ergoline content within
4 h (Young et al., 1983). The reduction affected all the ergolines
(ergotamine, ergocornine, ergocryptine, ergosine, ergometrine) in
identical ways. Autoclaving sclerotia at 121 °C for 30 min
resulted in a 24.6% reduction in total ergoline content. In the
baking of bread and pancakes using grain that contained naturally
occurring ergot, a 59-100% reduction in the individual ergolines
(ergosine, ergocornine, ergometrine, ergotamine, alpha-
ergocryptine, ergocristine) was observed in whole wheat bread, a
50-86% reduction in all-rye flour bread, and a 25-74% reduction in
triticale pancakes (Scott & Lawrence, 1982).
When bread made of rye flour spiked with finely ground
sclerotia of C. purpurea, (continuing a total ergoline
concentration of 312.8 µg/kg), was baked, there was an overall
reduction of 50% in the ergoline concentration, as measured by high
performance liquid chromatography (Baumann et al., 1985).
III.3 METABOLISM
No published information is available on the metabolism in
animals or human beings of ergots containing ergolines and
combinations of individual ergolines.
III.4 EFFECTS ON ANIMALS
III.4.1 Field studies
An outbreak of bovine abortion associated with the ingestion of
ergot was reported by Appleyard (1986).
Eleven out of 36 suckler cows, all in late pregnancy, aborted
in 7-11 days following introduction to a rye grass pasture heavily
infested with ergot. At least 25% of the rye seed heads contained
sclerotia of C. purpurea, with up to 8 sclerotia present on any one
seed head. The sclerotia contained 1.57 mg total ergolines/g,
consisting of 67% ergotamine and 17% ergotaminine, and smaller
amounts of ergometrine. Ergocryptine and ergosine were also
present together with their corresponding -inine isomers.
Ten out of the 11 calves were delivered dead. None of the
aborting cows showed any premonitory signs of calving and, after
parturition, there was almost complete agalactia. The placenta was
retained in each case, but there was no other evidence of ill
health in the cows. Any other cause of abortion of bacterial,
viral, fungal, or toxic origin was ruled out, on the basis of
laboratory and field investigations.
III.4.2 Experimental animal studies
This topic has been reviewed by Ainsworth & Austwick (1959) and
Mantle (1977c). It appears that most reports of field cases and
experimental animal studies on ergotism do not contain any
information on the contents of individual ergolines in the ergot
associated with disease manifestations in animals.
III.4.2.1 Cattle
Lameness, sometimes leading to gangrene, is a common symptom
observed in cattle when the feed contains more than 10 g ergot/kg.
The symptoms are more pronounced when the animals are kept outside
under cold weather conditions (Mantle, 1977c). Four out of 6
animals administered ergotamine tartrate, orally, at 1 mg/kg body
weight per day, died within 10 days (Woods et al., 1966). The
animals became acutely ill within 1-2 days, the principal signs
being anorexia, hyperventilation, cold extremities, salivation,
and, occasionally, tongue necrosis. Post-mortem examination of the
most seriously affected animals revealed extensive intestinal
inflammation.
III.4.2.2 Sheep
Four lambs were administered aqueous suspensions of milled
ergot (from C. purpurea) through a stomach tube, over a 2-month
period (Loken, 1984). The doses ranged from 0.12 to 0.75 g
sclerotia/kg body weight. The sclerotia contained approximately 4
g ergolines/kg, composed of ergotamine (15%), ergosine (35%), and
ergocristine (5% each). One lamb, dosed with 0.12 g sclerotia/kg
body weight, was kept indoors at 15-17 °C and did not develop any
symptoms. The other 3 animals, given higher doses and kept
outdoors, became ill after 2-6 days, with signs that included
dullness, inappetence, high pulse rate, diarrhoea, edema of the
hind legs and tail, and lameness. Post-mortem findings included
inflammation and necrosis of the forestomach and intestinal mucosa.
III.4.2.3 Poultry
Leghorn chickens were fed diets containing ergotamine tartrate
at levels of up to 800 mg/kg in 7 to 10-day trials as well as in a
51-day trial (Young & Marquardt, 1982). In the short-term
trials, only the highest level (800 mg/kg) had an effect on
performance, in terms of a slight decrease in growth rate and a
slight increase in feed consumption. At the 250 mg/kg level, toe
necrosis was observed, as well as cardiomegaly. There were no
pathological effects on the brain, liver, or muscle tissues, even
at the highest level. In the long-term study (51 days), effects
were similar to those observed in the 7 to 10-day trials. No
residues of ergotamine were detected in tissues.
III.4.2.4 Swine
Ergot (from C. purpurea) containing 0.3% ergolines
(composition: ergotamine and ergosine) was used in a feeding study
on pigs (Mantle, 1977c). A diet containing ergot at 40 g/kg was
well tolerated, and the only effect at 100 g ergot/kg diet was
depression of the growth rate. Agalactia in the sow was observed
after feeding with rations containing ergot from C. purpurea as
well as from C. fusiformis.
III.4.2.5 Primates
Male rhesus monkeys in groups of 2-4 animals were dosed with
ergot from C. fusiformis, either as part of the diet, or as an
ergoline extract administered orally or intraperitoneally (Bhat &
Roy, 1976). The ergoline extract contained agroclavine (major
components: elymoclavine, chanoclavine, penniclavine, and
setoclavine). The period of treatment lasted from 2 days to one
month. There were no effects on the animals, with the exception of
those injected ip with 5.44-11.1 mg total ergoline/kg body weight,
who developed signs within 10 min including drowsiness,
hyperexcitation, redness of face, and loss of response to thermal
and tactile stimuli in the hind limbs and tail. The animals
recovered spontaneously in about 60 min. Animals administered a
total of 10 mg ergolines/kg body weight, orally, showed symptoms of
hyperexcitation, but these were far less severe than those after
intraperitoneal injection. It was concluded that the signs in
monkeys, in particular hyperexcitation, are different from those
observed in human beings after ingestion of ergot in pearl millet.
III.5 EFFECTS ON MAN
The history of ergotism in man, following ingestion of ergot
from C. purpurea, was reviewed by Barger (1931). Numerous
epidemics in Europe occurred between the 9th and the 18th century.
Two types of disease were noted: gangrenous and convulsive
ergotism. In the first type, the affected part (arm or leg)
shrank, became mummified and dry, and the gangrene gradually spread
upwards. In convulsive ergotism, the whole body was attacked by
general convulsion, which returned at intervals of a few days. The
latest outbreaks of ergotism in Europe occurred in 1926-28 in the
United Kingdom and the USSR. A suspected episode in France in 1951
turned out to be due to a different toxic substance (Gadiou, 1965).
III.5.1 Ergometrine-related outbreaks
In 1978, an epidemic was reported in Ethiopia (Demeke et al.,
1979; King, 1979; Pokrovskij & Tutelyan, 1982). The episode
occurred in the Wollo region, following two years of drought.
During this time, the locally grown barley, the staple food, had
become dominated by wild oats heavily contaminated with C. purpurea
sclerotia. The grain consisted of 70% wild oats, 12% barley, and
0.75% ergot; ergometrine was detected in the sclerotia by thin
layer chromatography. A total of 93 cases of ergotism was reported
during the spring of 1978. The male:female ratio was 2.5:1; more
than 80% of affected persons were between five and 34 years of age.
In addition to the 93 cases, 47 deaths were reported as having been
due to ergotism. Examination of 44 patients out of the 93
registered revealed ongoing dry gangrene of the whole or part of
one or more limbs (7.5%), feeble or absent peripheral pulses
(36.4%), swelling of limbs (11.2 %), desquamation of the skin
(12.8%), and loss of one or more limbs (21.5%). It was noted that
88% of patients had involvement of the lower extremities. The most
common general symptoms were weakness (78.5%), formication (15%),
burning sensation (14.3%), nausea (7.2%), vomiting (5.6%), and
diarrhoea (6.8%). In addition, 50-60 infants and young children
died from starvation due to failure of the mothers to lactate.
This may have been related to the effect of ergot on lactation
(Demeke et al., 1979). No autopsies were performed, and thus there
is no information on pathological changes in the viscera.
III.5.2 Clavine-related outbreaks
Intoxication following ingestion of ergot from C. fusiformis in
bajra or pearl millet has been reported from India. Symptoms
included nausea, vomiting, and giddiness. Several outbreaks have
been observed since 1958, when the first report was published; the
latest occurred in the autumn of 1975 in the state of Rajasthan
(Krishnamachari & Bhat, 1976). In 21 villages surveyed, 78 persons
belonging to 14 households developed symptoms, characterized by
nausea, repeated vomiting, and giddiness, followed by drowsiness
and prolonged sleepiness, extending sometimes to over 24-48 h.
There were no signs or symptoms suggesting vaso-occlusion. The
disease generally developed 1-2 h following a single meal.
Domestic camels, offered the contaminated grain as feed, also
developed sleepiness and signs suggesting abdominal discomfort.
The pearl millet from affected villages contained 15-174 g
ergot/kg, resulting in a contamination of the grain with 15-199 mg
total ergolines/kg. The individual ergolines were identified as
agroclavine, elymoclavine, chanoclavine, penniclavine, and
setoclavine. Pearl millet from villages with no cases of
intoxication contained 1-38 g ergot/kg with a total ergoline
content of 15-26 mg/kg. Since there were no deaths, no information
on pathological changes is available. The number of households
studied was too small for no-effect levels to be calculated, but
the authors suggested that the intake of 28 µg total ergolines/kg
body weight would be non-toxic.
III.6 EVALUATION OF THE HUMAN HEALTH RISKS
Human exposure to low levels of ergolines appears to be
widespread. Available data from the recent outbreaks in Ethiopia
and India indicate that the C. purpurea alkaloids (ergotamine
group) produced more severe effects, including gangrene of the legs
and death, than the alkaloids of C. fusiformis (clavine group),
which caused gastrointestinal symptoms without a fatal outcome.
It is not known whether such differences can be accounted for by
differences in the alkaloid content of the fungal species, in the
toxicological or toxicometric properties of the alkaloids, or in
the levels of intake by different types of populations.
Only low levels of ergolines remain in prepared foods as
cleaning and milling processes remove the sclerotia; baking or
other heat processing also destroys most alkaloids of the ergotamin
group.
REFERENCES
ABBAS, H.K., MIROCHA, C.J., PAWLOSKY, R.J., & PUCSH, D.J. (1985)
Effect of cleaning, milling, and baking on deoxynivalenol in wheat.
Appl. environ. Microbiol., 50(2): 482-486.
ABBAS, H.K., MIROCHA, C.J., & TUITE, J. (1986) Natural occurrence of
deoxynivalenol, 15-acetyl-deoxynivalenol and zearalenone in refusal
factor corn stored since 1972. Appl. environ. Microbiol., 51:841-843.
ABDEL-HAFEZ, S.I.I., EL-KADY, I.A., MAZEN, M.B., & EL-MAGHRABY,
O.M.O. (1987) Mycoflora and trichothecene toxins of paddy grains from
Egypt. Mycopathologia, 100: 103-112.
ABRAHAMSON, D., MILLS, J.T., & BOYCOTT, B.R. (1983) Mycotoxins and
mycoflora in animal feedstuffs in western Canada. Can. J. comp. Med.,
47: 23-26.
AGRELO, C.E. & SCHOENTAL, R. (1980) Synthesis of DNA in human
fibroblasts treated with T-2 toxin and HT-2 toxin (the trichothecene
metabolites of Fusarium species) and the effects of hydroxyurea.
Toxicol. Lett., 5(2): 155-160.
AINSWORTH, G.C. & AUSTWICK, P.K.C. (1959) Fungal diseases of
animals, Farnham Royal, Commonwealth Agricultural Bureaux, pp. 100-
111.
AKERSTRAND, K. (1980) [Ergots.] Var Föda, 32: 442-446 (in Swedish).
AKERSTRAND, K. & JOSEFSON, E. (1979) [Fungi and mycotoxins in beans
and peas.] Var Föda, 31: 405-414 (in Swedish).
APPELGREN, L.-E. & ARORA, R.G. (1983) Distribution of 14C-labelled
ochratoxin A in pregnant mice. Food chem. Toxicol., 21: 563-568.
APPLEYARD, W.T. (1986) Outbreak of bovine abortion attributed to
ergot poisoning. Vet. Res., 118: 48-49.
ARENS, H. & ZENK, M.H. (1980) [Radioimmunoassay for the
determination of the peptide alkaloids ergotamine and ergocristine in
Claviceps purpurea.] Planta med., 38: 214-226 (in German).
ARORA, R.G., FROLEN, H., & FELLNER-FELDEGG, H. (1983) Inhibition of
ochratoxin A teratogenesis by zearalenone and diethylstilboestrol.
Food chem. Toxicol., 21: 779-783.
ATKINSON, H.A.C. & MILLER, K. (1984) Inhibitory effect of
deoxynivalenol, 3-acetyldeoxynivalenol and zearalenone on induction
of rat and human erythrocyte proliferation. Toxicol. Lett, 23: 215-221.
BALZER, I., BOGDANIC, C., & MUZIC, S. (1977) Natural contamination
of corn (Zea mays) with mycotoxins in Yugoslavia. Ann. Nutr.
Aliment., 31(4-6): 425-430.
BAMBURG, J.R. (1976) Chemical and biochemical studies of the
trichothecene mycotoxins. In: Rodricks, J.V., ed. Mycotoxins and
other fungal related food problems, Washington, DC, American Chemical
Society, pp. 144-162 (Advances in Chemistry Series No. 149).
BAMBURG, J.R. & STRONG, F.M. (1969) Mycotoxins of the trichothecene
family produced by Fusarium tricinctum and Trichoderma lignorum.
Phytochemistry, 8(12): 2405-2410.
BAMBURG, J.R. & STRONG, F.M. (1971) 12,13-Epoxytrichothecenes. In:
Kadis, S., Ciegler, A., & Ajl, S.J. ed. Microbiological toxins. VII.
Algal and fungal toxins, New York, London, Academic Press, pp. 207-
292.
BAMBURG, J.R., MARASAS, W.F., RIGGS, N.V., SMALLEY, E.B., & STRONG,
F.M. (1968a) Toxic compounds from Fusaria and other Hyphomycetes.
Biotechnol. Bioeng., 10(4): 445-455.
BAMBURG, J.R., RIGGS, N.V., & STRONG, F.M. (1968b) The structures
of toxins from two strains of Fusarium tricinctum. Tetrahedron,
24(8): 3329-3336.
BARGER, G. (1931) Ergot and ergotism, London, Gurney & Jackson,
279 pp.
BATA, A., VANYI, A., & LASZTITY, R. (1983) Simultaneous detection of
some fusariotoxins by gas-liquid chromatography. J. Assoc. Off. Anal.
Chem., 66: 577-581.
BAUER, J. & GAREIS, M. (1987) [Ochratoxin A in the food chain.] J.
vet. Med., B34: 613-627 (in German).
BAUER, V.J., WERMTER, S., & GEDEK, B. (1980) Contamination of
feedstuffs with toxin-producing strains of Fusaria and their toxins.
Wiener Tieraerztl. Monatschrift., 67(10): 282-288.
BAUER, J., GAREIS, M., & GEDEK, B. (1984) [Detection and occurrence
of ochratoxin A in pigs for slaughter]. Berl. Muench. Tieraerztl.
Wschr. 97: 279-283 (in German).
BAUER, J., BOLLWAHN, W., GARCIS, M., GEDEK, B., & HEINRITZI, K.
(1985) Kinetic profiles of diacetoxyscirpenol and two of its
metabolites in blood serum of pigs. Appl. environ. Microbiol.,
49: 642-845.
BAUER, J., NIEMIEC, J. & SCHOLTYSSEK, S. (1988) [Ochratoxin A in feed
for laying hens. Second communication: Residues in serum, liver and
eggs.] Arch. Geflügelkd., 52: 71-75 (in German).
BAUMANN, U., HUNZIKER, H.R., & ZIMMERLI, B. (1985) [Ergot alkaloids
in Swiss grain products.] Mitt. Geb. Lebensm. Hyg., 76: 609-630 (in
German).
BEASLEY, V.R., SWANSON, S.P., CORLEY, R.A., BUCK, W.B., KORITZ, G.D.,
& BURMEISTER, H.R. (1986) Pharmacokinetics of the trichothecene
mycotoxin, T-2 toxin, in swine and cattle. Toxicon, 21: 13-23.
BEGLEY, P., FOULGER, B.E., JEFFERY, P.D., BLACK, R.M., & READ, R.W.
(1986) Detection of trace levels of trichothecenes in human blood
using capillary gas chromatography-electron-capture negative ion
chemical ionisation mass spectrometry. J. Chromatogr., 367: 87-101.
BELT, R.J., HAAS, C.D., JOSEPH, U., GOODWIN, W., MOORE, D., &
HOOGSTRATEN, B. (1979) Phase I study of anguidine administered
weekly. Cancer Treat. Rep., 63: 1993-1995.
BENDELE, S.A., CARLTON, W.W., KROGH, P., & LILLEHOJJ, E.B. (1985)
Ochratoxin A carcinogenesis in the (C57BL/6YxC3HF)1 mouse. J. Natl
Cancer Inst., 75: 733-739.
BERDE, B. & SCHILD, H.O. (1978) Ergot alkaloids and related
compounds, Berlin, Springer Verlag, pp. 1-1003.
BERGMANN, F., YAGEN, B., & SOFFER, D. (1985) Toxic and lethal effects
of T-2 toxin upon intracerebral administration to rats. Arch.
Toxicol., 58: 40-44.
BERNDT, W.O. & HAYES, A.W. (1979) In vivo and in vitro changes in
renal function caused by ochratoxin A in the rat. Toxicology, 12:
5-17.
BHAT, R.V. & ROY, D.N. (1976) Toxicity study of ergoty bajra (pearl
millet) in rhesus monkeys. Indian J. med. Res., 64: 1629-1633.
BHAT, R.V., ROY, D.N., & TULPULE, P.G. (1976) The nature of
alkaloids of ergoty pearl millet or bajra and its comparison with
alkaloids of ergoty rye and ergoty wheat. Toxicol. appl. Pharmacol.,
36: 11-17.
BHAT R.V., RAMAKRISHNA, Y., RAO, B.S., & NAHDI, S. (1987a)
Trichothecene mycotoxicosis, Hyderabad, India, Food and Drug
Toxicology Research Centre, National Institute of Nutrition.
BHAT, R.V., RAMAKRISHNA, Y., RAO, B.S,. & NAHDI, S. (1987b).
Trichothecene mycotoxicosis, Hyderabad, India, Food and Drug
Toxicology Research Centre, National Institute of Nutrition.
BHAT, R.V., BEEDU, S.R., RAMAKRISHNA, Y., & MUNSHI, K.L. (1989a)
Outbreak of trichothecene mycotoxicosis associated with consumption
of mould-damaged wheat products in Kashmir valley, India. Lancet, 7
January: 35-37.
BHAVANISHANKAR, T.R. & SHANTHA, T. (1987) Natural occurrence of
Fusarium toxins in peanuts, sorghum and maize from Mysore (India).
J. Sci. Food. Agric., 40: 327-332.
BHAVANISHANKAR, T.R., RAMESH, H.P., & SHANTHA, T. (1988) Dermal
toxicity of Fusarium toxins in combinations. Arch. Toxicol., 61:
241-244.
BIANCHI, M.L., PERELLINO, N.C., GIOIA, B., & MINGHETTI, A. (1982)
Production by Claviceps purpurea of two new peptide ergot alkaloids
belonging to a new series containing alpha-aminobutyric acid. J. nat.
Prod., 445: 191-196.
BILAI, V.I. (1977) [Fuzarii,] Kiev, USSR, Naukova Dumka, (in
Russian).
BLACK, R.M., CLARKE, R.J., & READ, R.W. (1986) Detection of trace
levels of trichothecene mycotoxins in human urine by gas
chromatography-mass spectrometry. J. Chromatogr., 367: 103-115.
BLACK, R.M., CLARKE, R.J., & READ, R.W. (1987) Detection of trace
levels of trichothecene mycotoxins in environmental residues and
foodstuffs using gas chromatography with mass spectrometric or
electron-capture detection. J. Chromatogr., 388: 365-378.
BLASS, W., KELLERT, M., STEINMEYER, S., TIEBACH, R., & WEBER, R.
(1984) Determination of deoxynivalenol and nivalenol in cereals at
the mg/kg concentrations range. Z. Lebensm. Unters. Forsch., 80:
104-108.
BODON, L. & ZOLDAG, L. (1974) Cytotoxicity studies on T-2
fusariotoxin. Acta vet. Acad. Sci. Hung., 24(3): 451-455.
BOHNER, F.E., FETZ, E., HARRI, E., SIGG, H.P., STOLL, CH., & TAMM,
CH. (1965) Verrucarine and roridin. VIII. Isolation of verrucarin
H, verrucarin J, roridin D, and roridin E from Myrothecium species.
Helv. Chim. Acta, 48(5): 1079-1089.
BOONCHUVIT, B., HAMILTON, P.B., & BURMEISTER, H.R. (1975)
Interaction of T-2 toxin with Salmonella infections of chickens.
Poult. Sci., 54(5): 1693-1696.
BOORMAN, G. (1988) Technical report on the toxicology and
carcinogenesis studies of ochratoxin A in F334/N rats. National
Toxicology Program, US Department of Health and Human Services,
Research Triangle Park, North Carolina (NIH Publication No. 88-28/3).
BOORMAN, G.A., HONG, H.L., DIETER, M.P., HAYES, H.T., POHLAND, A.E.,
STACK, M., & LUSTER, M.I. (1984) Myelotoxicity and macrophage
alteration in mice exposed to ochratoxin A. Toxicol. appl.
Pharmacol., 72: 304-312.
BOVE, F.J. (1970) The story of ergot, Basel, S. Karger, 297 pp.
BRADLAW, J.A., SWENTZEL, K.C., ALTERMAN, E., & HAUSWIRTH, J.W. (1985)
Evaluation of purified 4-deoxynivalenol (vomitoxin) for unscheduled
DNA synthesis in the primary rat hepatocyte-DNA repair assay. Food
chem. Toxicol., 23: 1063-1067.
BRIAN, P.W., DAWKINS, A.W., GROVE, J.F., HEMMING, H.G., LOWE, D., &
HORRIES, G.L.F. (1961) Phytotoxic compounds produced by Fusarium
equiseti. J. exp. Bot., 12: 1-21.
BROWN, M.H., SZCZECH, G.M., & PURMALIS, B.P. (1976) Teratogenic and
toxic effects of ochratoxin A in rats. Toxicol. appl. Pharmacol., 37:
331-338.
BRUMLEY, W.C., ANDRZEJEWSKI, D., TRUCKSESS, E.W., DREIFUSS, P.A.,
ROACH, J.A.G., EPPLEY, R.M., THOMAS, F.S., THORPE, C.W., & SPHON,
J.A. (1982) Negative ion chemical ionization mass spectrometry of
trichothecenes - Novel fragmentation under OH conditions. Biomed.
mass Spectrom., 9: 451-458.
BRUMLEY, W.C., TRUCKSESS, M.W., ADLER, S.H., COHEN, C.K., WHITE,
K.D., & SPHON, J.A. (1985) Negative ion chemical ionization mass
spectrometry of deoxynivalenol (DON): Application to identification
of DON in grains and snack foods after quantification/isolation by
thin-layer chromatography. J. agric. food Chem., 33:326-330.
BUENING, G.M., MANN, D.D., HOOK, B., & OSWEILER, G.D. (1982) The
effect of T-2 toxin on the bovine immune system: cellular factors.
Vet. Immunol. Immunopathol., 3: 411-417.
BUNGE, I., DIRHEIMER, G., & ROSCHENTHALER, R. (1978) in vivo and in
vitro inhibition of protein synthesis in Bacillus stearothermophilus
by ochratoxin A. Biochem. biophys. Res. Commun., 83: 398-405.
BUNNER, D.L. & MORRIS, E.R. (1988) Alteration of multiple cell
membrane functions in L-6 myoblasts by T-2 toxin: an important
mechanism of action. Toxicol. appl. Microbiol., 92: 113-121.
CANDLISH, A.A.G., STIMSON, W.H., & SMITH, J.E. (1986) A monoclonal
antibody to ochratoxin A. Lett. appl. Microbiol., 3: 9-11.
CARRASCO, L., BARBACID, M., & VAZQUEZ, D. (1973) The trichodermin
group of antibiotics inhibitors of peptide bond formation by
eukaryotic ribosomes. Biochim. Biophys. Acta, 312(2): 368-376.
CARSON, M.S. & SMITH, T.K. (1983) Role of bentonite in prevention of
T-2 toxicosis in rats. J. anim. Sci., 57: 1498-1506.
CARTER, C.J., CANNON, M., & SMITH, K.E. (1976) Inhibition of
protein synthesis in reticulocyte lysates by trichodermin. Biochem.
J., 154: 171-178.
CARTER, C.J., CANNON, M., & JIMENET, A. (1980) A trichodermin
resistant mutant of Saccharomyces cerevisiae with an abnormal
distribution of native ribosomal subunits. Eur. J. Biochem., 107
(1): 173-184.
CASALE, W.L., PESTKA, J.J., & HART, L.P. (1988) Enzyme-linked
immunosorbent assay employing monoclonal antibody specific for
deoxynivalenol (vomitoxin) and several analogues. J. agric. food
Chem., 36: 663-668.
CEOVIC, S., GRIMS, P., & MITAR, J. (1976) [The incidence of tumours
of the urinary organs in a region of endemic nephropathy and in a
control region.] Lijec. Vjesn., 98: 301-304 (in Croatian).
CHAKRABARTI, D.K. & HOSAL, S. (1986) Occurrence of free and
conjugated 12,13-epoxytrichothecenes and zearalenone in banana fruits
infected with Fusarium moniliforme. Appl. environ. Microbiol.,
51(1): 217-219.
CHANG, F.C. & CHU, F.S. (1977) The fate of ochratoxin A in rats.
Food Cosmet. Toxicol., 15: 199-204.
CHANG, K., KURTZ, J.J., & MIROCHA, C.J. (1979) Effects of the
mycotoxin zearalenone on swine reproduction. Am. J. vet. Res.,
40: 1260-1267.
CHATTERJEE, K., VISCONTI, A., & MIROCHA, C.J. (1986) Deepoxy T-2
tetraol: a metabolite of T-2 toxin found in cow urine. J. agric.
food Chem., 34: 695-697.
CHELKOWSKI, J., VISCONTI, A., & MANAKA, M. (1984) Production of
trichothecenes and zearaleneone by Fusarium species isolated from
wheat. Nahrung, 28: 493-496.
CHERNOZEMSKY, I.N., STOYANOV, I.S., PETKOV-ABOCHAROVA, T.K., NOCOLOV,
I.G., DRAGANOV, I.V., STOICHEV, I., TANCHEV, Y., NAIDENOV, D., &
KALCHEVA, N.D. (1977) Geographic correlation between the occurrence
of endemic nephropathy and urinary tract tumours in Vratza district,
Bulgaria. J. Cancer, 19: 1-11.
CHI, M.S., MIROCHA, C.J., KURTZ, H.J., WEAVER, G., BATES, F., &
SHIMODA, W. (1977a) Effects of T-2 toxin on reproductive
performance and health of laying hens. Poult. Sci., 56(2): 628-637.
CHI, M.S., MIROCHA, C.J., KURTZ, H.J., WEAVER, G., BATES, F.,
SHIMODA, W., & BURMEISTER, H.R. (1977b) Acute toxicity of T-2 toxin
in broiler chicks and laying hens. Poult Sci., 56(1): 103-116.
CHI, M.S., MIROCHA, C.J., KURTZ, H.J., WEAVER, G., BATES, F., &
SHIMODA, W. (1977c) Subacute toxicity of T-2 toxin in broiler
chicks. Poult. Sci., 56(1): 306-313.
CHI, M.S., ROBISON, T.S., MIROCHA, C.J., BEHRENS, J.C., & SHIMODA, W.
(1978a) Transmission of radioactivity into eggs from laying hens
(Gallus domesticus) administered tritium labeled T-2 toxin. Poult.
Sci., 57(5): 1234-1238.
CHI, M.S., ROBISON, T.S., MIROCHA, C.J., SWANSON, S.P., & SHIMODA, W.
(1978b) Excretion and tissue distribution of radioactivity from
tritium labeled T-2 toxin in chicks. Toxicol. appl. Pharmacol.,
45(2): 391-402.
CHI, M.S., EL-HALAWANI, M.E., WAIBEL, P.E., & MIROCHA, C.J. (1981)
Effects of T-2 toxin on brain catecholamines and selected blood
components in growing chickens. Poult. Sci., 60(1): 137-141.
CHIBA, J., NAKANO, N., MOROOKA, N., NAKAZAWA, S., & WATANABE, Y.
(1972) Inhibitory effects of fusarenon-X, a sesquiterpene mycotoxin
on lipid synthesis and phosphate uptake in Tetrahymena pyriformis.
Jpn. J. med. Sci. Biol., 25(5): 291-296.
CHO, B.R. (1964) Toxicity of water extracts of scabby barley to
suckling mice. Am. J. vet. Res., 25: 1267-1270.
CHU, F.S. (1971) Interaction of ochratoxin A with bovine serum
albumin. Arch. Biochem. Biophys., 147: 359-366.
CHU, F.S. (1974a) Studies on ochratoxins. CRC crit. Rev. Toxicol.,
2(4): 499-524.
CHU, F.S. (1974b) A comparative study of the interaction of
ochratoxins with bovine serum albumin. Biochem. Pharmacol., 23(6):
1105-1113.
CHU, F.S. & BUTZ, M.E. (1970) Spectrophotofluorodensitometric
measurement of ochratoxin A in cereal products. J. Assoc. Off. Anal.
Chem., 53(6): 1253-1257.
CHU, F.S., GROSSMAN, S., WEI, R.D., & MIROCHA, C.J. (1979)
Production of antibody against T-2 toxin. Appl. environ. Microbiol.,
37(1): 104-108.
CHUNG, C.W., TRUCKSESS, M.W., GILES, A.L., Jr, & FRIEDMAN, L. (1974)
Rabbit skin test for estimation of T-2 toxin and other skin
irritating toxins in contaminated corn. J. Assoc. Off. Anal. Chem.,
57(5): 1121-1127.
CHUNG, H.S. (1975) Cereal scab causing mycotoxicoses in Korea and
present status of mycotoxin researches. Korean J. Mycol., 3(1):
31-36.
CIRILLI, G. (1983) Trichothecene problems in Italy. In: Ueno, Y.,
ed. Developments in food science. IV. Trichothecenes, New York,
Elsevier, pp. 254-258.
COFFIN, J.L. & COMBS, G.F., Jr (1981) Impaired vitamin E status of
chicks fed T-2 toxin. Poult. Sci., 60(2): 385-392.
COHEN, H. & LAPOINTE, M. (1982) Capillary gas-liquid
chromatographic determination of vomitoxin in cereal grains. J.
Assoc. Off. Anal. Chem., 65(6): 1429-1434.
COHEN, H. & LAPOINTE, M. (1984) Capillary gas chromatographic
determination of T-2 toxin, HT-2 toxin and diacetoxyscirpenol in
cereal grains. J. Assoc. Off. Anal. Chem., 67: 1105-1107.
COLE, R.J. & COX, R.H. (1981) Handbook of toxic fungal metabolites,
New York, London, San Francisco, Academic Press, pp. 152-263.
COLINSKI, P. (1987) [Ochratoxin A in humans as a result of food and
feed contamination,] Poznan, Rocznalion Akademii Rolniczej (Doctoral
thesis) (in Polish).
COLLINS, G.J. & ROSEN, J.D. (1979) Gas-liquid chromatographic/mass
spectrometric screening method for T-2 toxin in milk. J. Assoc. Off.
Anal. Chem., 62(6): 1274-1280.
COLLINS, G.J. & ROSEN, J.D. (1981) Distribution of T-2 toxin in
wet-milled corn products. J. food Sci., 46(3): 877-879.
CONNOLE, M.D., BLANEY, B.J., & MCEWAN, T. (198l) Mycotoxins in
animal feeds and toxic fungi in Queensland 1971-1980. Aust. vet. J.,
57: 314-318.
CONNOR, M.W., CAMARGO, J.D., PANYANT, P., RIENGROPITOK, S., ROGERS,
A.E., & NEWBUNE, P.M. (1986) Toxicity of anguidine in mice. Fundam.
appl. Toxicol., 7: 153-164.
COORAY, R. (1984) Effects of some mycotoxins on mitogen-induced
blastogenesis and SCE frequency in human lymphocytes. Food Chem.
Toxicol., 22: 529-534.
COPPOCK, R.W., SWANSON, S.P., GELBERG, H.B., KORITZ, G.D., HOFFMAN,
W.E., BUCK, W.B., & VESONDER, R.F. (1985) Preliminary study of the
pharmacokinetics and toxicopathy of deoxynivalenol (vomitin) in
swine. Am. J. vet. Res., 46: 169-174.
CORLEY, R.A., SWANSON, S.P., & BUCK, W.B. (1985) Glucuronide
conjugates of T-2 toxin and metabolites in swine bile and urine. J.
agric. food Chem., 33: 1085-1089.
CORLEY, R.A., SWANSON, S.P., GULLO, G.J., JOHNSON, L., BEASLEY, V.R.,
& BUCK, W.B. (1986) Disposition of T-2 toxin, a trichothecene
mycotoxin, in intravascularly dosed swine. J. agric. food Chem.,
34: 868-875.
CORRIER, D.E. & ZIPRIN, R.L. (1986a) Immunotoxic effects of T-2 toxin
on cell-mediated immunity to listeriosis in mice: comparison with
cyclophosphamide. Am. J. vet. Res., 47: 1956-1960.
CORRIER, D.E. & ZIPRIN, R.L. (1986b) Enhanced resistance to
listeriosis induced in mice by preinoculation treatment with T-2
mycotoxin. Am. J. vet. Res., 47: 856-859.
COSGRIFF, T.M., BUNNER, D.P., WANNEMACHER, R.W., Jr., HODGSON, L.A.,
& DINTERMAN R.E. (1984) The hemostatic derangement produced by T-2
toxin in guinea pigs. Toxicol. appl. Pharmacol., 76: 454-463.
COSGRIFF, T.M., BUNNER, D.P., WANNEMACHER, R.W., Jr., HODGSON, L.A.,
& DINTERMAN, R.E. (1986) The hemostatic derangement produced by T-2
toxin in Cynomolgus monkeys. Toxicol. appl. Pharmacol., 82: 532-539.
COTE, L.M., REYNOLDS, J.D., VESONDER, R.F., BUCK, W.B., SWANSON,
S.P., COFFEY, R.T., & BROWN, D.C. (1984) Survey of vomitoxin-
contaminated feed grains in midwestern United States, and associated
health problems in swine. J. Am. Vet. Med. Assoc., 184(2): 189-192.
COTE, L.M., DAHLEM, A.M., YOSHIZAWA, T., SWANSON, S.P., & BUCK, W.B.
(1986) Excretion of deoxynivalenol and its metabolite in milk, urine
and feces of lactating dairy cows. Agric. biol. Chem., 50: 227-229.
CREPPY, E.E., LUGNIER, A.A.J., FASIOLO, F., HELLER, K.,
ROSCHENTHALER, R., & DIRHEIMER, G. (1979a) In vitro inhibition of
yeast phenylalanyl-tRNA synthetase by ochratoxin A. Chem.-biol.
Interact., 24: 257-261.
CREPPY, E.E., LUGNIER, A.A.J., BECK, G., ROSCHENTHALER, R., &
DIRHEIMER, G. (1979b) Action of ochratoxin A on cultured hepatoma
cells - reversion of inhibition by phenylalanine. FEBS Lett., 104:
287-290.
CREPPY, E.E., SCHLEGEL, M., ROSCHENTHALER, R., & DURHEINSER, G.
(1980) Phenylalamine prevents acute poisoning by ochratoxin A in
mice. Toxicol. Lett., 6: 77-80.
CREPPY, E.E., STORMER, F.O., ROSCHENTHALER, R., & DIRHEIMER, G.
(1983) Effects of two metabolites of ochratoxin A, (4R)-4-
hydroxyochratoxin A and ochratoxin alpha, on immune response in mice.
Infect. Immun., 39: 1015-1018.
CROFT, W.A., JARVIS, B.B., & YATAWARA, C.S. (1986) Airborne outbreak
of trichothecene toxicosis. Atmos. Environ., 20: 549-552.
CUNDLIFFE, E., CANNON, M., & DAVIS, J. (1974) Mechanism of
inhibition of eukaryotic protein synthesis by trichothecene fungal
toxins. Proc. Natl Acad. Sci. (USA), 71(1): 30-34.
D'AGOSTINO, P.A., PROVOST, L.R., & DROVER, D.R. (1986) Analysis of
trichothecene mycotoxins in human blood by capillary column gas
chromatography-ammonia chemical ionization mass spectrometry. J.
Chromatogr., 367: 77-86.
DANKO, G. & SZERAFIN, J. (1976) Experimental stachybotryotoxicosis
in horses. Magy. Allatorv. Lapja, 9: 597-600.
DE LOACH, J.R., ANDREWS, K., & NAGI, A. (1987) Interaction of T-2
toxin with bovine erythrocyte: effects on cell lysis, permeability
and entrapment. Toxicol. appl. Pharmacol., 88: 123-131.
DEMEKE, T., KIDANE, Y., & WUHIB, E. (1979) Ergotism: a report on an
epidemic. Ethiop. med. J., 17: 107-113.
DENICOLA, D.B., REBER, A.H., CARLTON, W.W., & YAGEN, B. (1978) T-2
toxin mycotoxicosis in the guinea-pig. Food Cosmet. Toxicol., 16
(6): 601-609.
DESIMONE, P.A., GRECO, F.A., & LESSNER, H.F. (1979) Phase I
evaluation of a weekly schedule of anguidine. Cancer Treat. Rep.,
63(11/12): 2015-2017.
DIVE, D., MOREAU, S., & CACAN, M. (1978) Use of a ciliate
protozoan for fungal toxins studies. Bull. environ. Contam. Toxicol.,
19(4): 489-495.
DOCHEV, D. (1973) [Endemic (Balkan) nephritis in Bulgaria.]
Münchener med. Wochenschr., 115(13): 537-541 (in German).
DOERR, J.A., HAMILTON, P.B., & BURMEISTER, H.R. (1981) T-2
toxicosis and blood coagulation in young chickens. Toxicol. appl.
Pharmacol., 60: 157-162.
DOSIK, G.M., BARLOGIE, B., JOHNSTON, D.A., MARPHY, W.K., & DREWINKO,
B. (1978) Lethal and cytokinetic effects of anguidine on a human
colon cancer cell line. Cancer Res., 38(10): 3304-3309.
DOSTER, R.C., SINHUBER, R.O., & WALES, J.H. (1972) Acute
intraperitoneal toxicity of ochratoxin A and B in rainbow trout
(Salmo gairdneri). Food. Cosmet. Toxicol., 10: 85-92.
DROVE, J.F. (1988) Non macrocyclic trichothecenes. Natural Products
Reports: 181-209.
DWIVEDI, P. & BURNS, R.B. (1984) Effect of ochratoxin A on
immunoglobulins in broiler chicks. Res. vet. Sci., 36: 117-121.
EDLUND, P.O. (1981) Determination of ergot alkaloids in plasma by
high-performance liquid chromatography and fluorescence detection. J.
Chromatogr., 226: 107-115.
EHRLICH, K.C., LEE, L.S., & CIEGLER, A. (1983) A simple, sensitive
method for detection of vomitoxin (deoxynivalenol) using reversed
phase, high performance liquid chromatography. J. liq. Chromatogr.,
6: 833-843.
EL-BANNA, A.A., LAU, P.-Y., & SCOTT, P.M. (1983) Fate of mycotoxins
during processing of foodstuffs II- Deoxynivalenol (vomitoxin) during
making of Egyptian bread. J. food Prot., 46: 484-486.
EL-BANNA, A.A., SCOTT, P.M., LAU, P.-Y., SAKUMA, T., PLOT, H., &
CAMPBELL, V. (1984) Formation of trichothecenes by F. solani var.
coeruleum and F. sambucinum in potatoes. Appl. environ. Microbiol.,
47: 1169-1171.
ELLING, F. (1977) Demonstration of ochratoxin A in kidneys of pigs
and rats by immunofluorescence microscopy. Acta pathol. microbiol.
Scand., A85: 151-156.
ELLING, F. & MOLLER, T. (1973) Mycotoxic nephropathy in pigs. Bull.
World Health Organ., 49: 411-418.
ELLING, F., HALD, B., JACOBSEN, C., & KROGH, P. (1975) Spontaneous
cases of toxic nephropathy in poultry associated with ochratoxin A.
Acta pathol. microbiol. Scand., A83: 739-741.
ELLING, F., NIELSEN, J.P., LILLEHOJ, E.B., THOMASSEN, M.S., &
STORMER, F.C. (1985) Ochratoxin A-induced porcine nephrotoxicity:
enzyme and ultrastructural changes after short-term exposure.
Toxicon, 23: 247-254.
EPPLEY, R.M. (1968) Screening method for zearalenone, aflatoxin, and
ochratoxin. J. Assoc. Off. Anal. Chem., 51(1): 74-78.
EPPLEY, R.M. (1974) Sensitivity of brine shrimp Artemia salina to
trichothecenes. J. Assoc. Off. Anal. Chem., 57(3): 618-620.
EPPLEY, R.M. & BAILEY, W.J. (1973) 12,13-epoxy-delta-trichothecenes
as the probable mycotoxins responsible for stachybotryotoxicosis.
Science, 181: 758-760.
EPPLEY, R.M., TRUCKSESS, M.W., NESHEIM, S., THORPE, C.W., WOOD, G.E.,
& POHLAND, A.E. (1984) Deoxynivalenol in winter wheat: Thin layer
chromatographic method and survey. J. Assoc. Off. Anal. Chem., 67
(1): 43-45.
EPPLEY, R.M., TRUCKSESS, M.W., NESHEIM, S., THORPE, C.W., & POHLAND,
A.E. (1986) Thin layer chromatographic method for determination of
deoxynivalenol in wheat: collaborative study. J. Assoc. Off. Anal.
Chem., 69: 37-40.
FAIRHURST, S., MAXWELL, S.A., SCAWIN, J.W., & SWANSTON, D.W. (1987)
Skin effects of trichothecenes and their amelioration by
decontamination. Toxicology, 46: 307-319.
FAN, T.S.L., XU, Y.-C., & CHU, F.S. (1987) Simultaneous analysis of
T-2 toxin and HT-2 toxin by indirect enzyme-linked immunosorbent
assay. J. Assoc. Off. Anal. Chem., 70: 657-661.
FARB, R.M., MEGO, J.L., & HAYES, A.W. (1976) Effect of mycotoxins
on uptake and degradation of iodine-125 albumin in mouse liver and
kidney lysosomes. J. Toxicol. environ. Health, 1(6): 985-990.
FEUERSTEIN, G., GOLDSTEIN, D.S., RAMWELL, P.W., ZERBE, R.L., LUX,
W.E. Jr, FODEN, A.I., & BAYORH, M.A. (1985) Cardiorespiratory
sympathetic and biochemical response to T-2 toxin in the guinea pig
and rat. J. Pharmacol. exp. Ther., 232: 786-794.
FEUERSTEIN, G., LEADER, P., SIREN, A.L., & BRAGNET, P. (1987)
Protective effect of a PAF-acether antagonist, BN52021 in
trichothecene toxicosis. Toxicol. Lett., 38: 271-274.
FIORAMONTI, J., FARGEAS, M.J., & BUENO, L. (1987) Action of T-2
toxin on gastrointestinal transit in mice: protective effect of an
argillaceous compound. Toxicol. Lett., 36: 227-232.
FITZPATRICK, D.W., BOYD, K.E., & WATTS, B.M. (1988) Comparison of the
trichothecenes deoxynivalenol and T-2 toxin for their effect on brain
biogenic monoamines in the rat. Toxicol. Lett., 40: 241-245.
FORGACS, J. (1965) In: Wogan, G., ed. Mycotoxins in foodstuffs,
Cambridge, Mass., MIT Press, p. 87.
FORSELL, J.H. & PESTKA, J.J. (1985) Relation of 8-ketotrichothecene
and zearalenone analog structure to inhibitions of mitogen-induced
human lymphocyte blastogenesis. Appl. environ. Microbiol., 50:
1304-1307.
FORSELL, J.H., KATEBY, J.R., YOSHIZAWA, T., & PESTKA, J.J. (1985)
Inhibition of mitogen-induced blastogenesis in human lymphocytes by
T-2 toxin and its metabolites. Appl. environ. Microbiol., 49:
1524-1526.
FORSYTH, D.M., YOSHIZAWA, T., MOROOKA, N., & TUITE, J. (1977)
Emetic and refusal activity of deoxynivalenol to swine. Appl.
environ. Microbiol., 34(5): 547-552.
FOSTER, P.M.D., SLATER, T.F., & PATTERSON, D.S.P. (1975) A possible
enzymic assay for trichothecene mycotoxins in animal feedstuffs.
Biochem. Soc. Trans., 3(6): 875-878.
FREEMAN, G.G. (1955) Further biological properties of trichotecin,
an antifungal substance from Trichothecium roseum Link, and its
derivatives. J. gen. Microbiol., 12: 213-221.
FREEMAN , G.G. & MORRISON, R.I. (1949) The isolation and chemical
properties of trichothecin, an antifungal substance from
Trichothecium roseum link. Biochem. J., 44: 1-5.
FRIED, H.M. & WARNER, J.R. (1981) Cloning of yeast gene for
trichodermin resistance and ribosomal protein L3. Proc. Natl Acad.
Sci. (USA), 78(1): 238-242.
FRIEND, S.C.E., TRENHOLM, H.L., ELLIOT, J.I., THOMPSON, B.K., &
HARTIN, K.E. (1982) Effect of feeding vomitoxin-contaminated wheat to
pigs. Can. J. anim. Sci., 62: 1211-1222.
FRIEND, S.C.E., BABUIK, L.A., & SCHIEFER, H.B. (1983a) The effects of
dietary T-2 toxin on the immunological function and Herpes simplex
reactivation in Swiss mice. Toxicol. appl. Pharmacol., 69:
234-244.
FRIEND, S.C.E., HANCOCK, D.S., & SCHIEFER, H.B. (1983b) Experimental
T-2 toxicosis in sheep. Can. J. comp. Med., 47: 291-297.
FRIEND, S.C.E., SCHIEFER, H.B,. & BABUIK, L.A. (1983c) The effects of
dietary T-2 toxin on acute Herpes simplex virus type 1 infection in
mice. Vet. Pathol., 20: 737-760.
FRIESEN, M.D. & GARREN, L. (1983) International mycotoxin check
sample survey programme. Part III. Report on performance of
participating laboratories for determining ochratoxin A in animal
feed. J. Assoc. Off. Anal. Chem., 66: 256-259.
FRIIS, Ch., BRINN, R., & HALD, B. (1988) Uptake of ochratoxin A by
slices of pig kidney cortex. Toxicology., 52: 209-217.
FRITZ, W., BUTHIG, CL., DONATH, R., & ENGST, R. (1979) [Studies on
the nutritionally significant formation of ochratoxin A in grain and
other foodstuffs.] Lebensm. Ernähr., 25: 929-932 (in German).
FROMENTIN, H., SALAZAR-MEJICANOS, S,. & MARIAT, F. (1981)
Experimental cryptococcosis in mice treated with diacetoxyscirpenol,
a mycotoxin of Fusarium. Sabouraudia, 19: 311-318.
GADIOU, J. (1965) Contribution à l'étude toxicologique du
dicyandiamide de methyl mercure, Université de Bordeaux, Faculté de
pharmacie. (Thèse de doctorat).
GALEY, F.D., LAMBERT, R.J., BUSSE, M., & BUCK, W.B. (1987)
Therapeutic efficacy of superactive charcoal in rats exposed to oral
lethal doses of T-2 toxins. Toxicon, 25: 493-499.
GALTIER, P. (1974a) Devenir de l'ochratoxine A dans l'organisme
animal. I. Transport sanguin de la toxine chez le rat. Ann. Rech.
vét., 5: 311-318.
GALTIER, P. (1974b) Devenir de l'ochratoxine A dans l'organisme
animal. II. Distribution tissulaire et élimination chez le rat.
Ann. Rech. vét., 5: 319-328.
GALTIER, P. (1979) Etude toxicologique et pharmacocinétique d'une
mycotoxine, l'ochratoxine A, Université de Toulouse, Thèse de
doctorat d'Etat en Sciences pharmaceutiques.
GALTIER, P. & ALVINERIE, M. (1976) In vitro transformation of
ochratoxin A by animal microbial floras. Ann. Rech. vét., 7:
91-98.
GALTIER, P., MORE, J., & BODIN, G. (1974) Toxines d' Aspergillus
ochraceus Wilhelm III. Toxicité aiguë de l'ochratoxine A chez le rat
et la souris adultes. Ann. Rech. vét., 5: 233-247.
GALTIER, P., BARADAT, C., & ALVINERIE, M. (1977a) De l'élimination
d'ochratoxine A par le lait chez la lapine. Ann. Nutr. Aliment.,
31: 911-918.
GALTIER, P., JEMMALI, M., & LARRIEU, G. (1977b) Enquete sur la
présence éventuelle d'aflatoxine et d'ochratoxine A dans des maïs
recolté en France en 1973 et 1974. Ann. Nutr. Aliment., 31: 38l-389.
GALTIER, P., BONEU, B., CHARPENTEAU, J.L., BODIN, G., ALVINERIE, M.,
& MORE, J. (1979) Physiopathology of haemorrhagic syndrome related
to ochratoxin A intoxication in rats. Food Cosmet. Toxicol., 17:
49-53.
GALTIER, P., CAMGUILHEM, R., & BODIN, G. (1980) Evidence for in
vitro and in vivo interaction between ochratoxin A and three acidic
drugs. Food Cosmet. Toxicol., 18: 493-496.
GAREIS, M., HASHEM, A., BAUER, J., & GEDEK, B. (1986) Identification
of glucuronide metabolites of T-2 toxin and diacetoxyscirpenol in the
bile of isolated perfused rat lever. Toxicol. appl. Pharmacol., 84:
168-172.
GAREIS, M., MARTLBAUER, E., BAUER, J., & GEDEK, B. (1988)
[Determination of ochratoxin A in breast milk.] Z. Lebensm. Unters.
Forsch., 186: 114-117 (in German).
GENDLOFF, E.H., PESTKA, J.J., SWANSON, S.P., & HART, L.P. (1984)
Detection of T-2 toxin in Fusarium sporotrichioides-infected corn by
enzyme-linked immunosorbent assay. Appl. environ. Microbiol., 47:
1161-1163.
GENTRY, P.A. (1982) The effect of administration of a single dose of
T-2 toxin on blood coagulation in the rabbit. Can. J. comp. Med.,
46: 414-419.
GENTRY, P.A. & COOPER, M.L. (1981) Effect of Fusarium T-2 toxin on
hematological and biochemical parameters in the rabbit. Can. J. comp.
Med., 45: 400-405.
GHOSAL, S., CHAKRABARTI, D.K., & BASU CHAUDHARY, K.C. (1977) The
occurrence of 12,13-epoxytrichothecenes in seeds of safflower
infected with Fusarium oxysporum f sp. carthami. Experientia (Basel),
33(5): 574-575.
GILBERT, J., SHEPHERD, M.J., & HOWELL, M.V. (1983a) Studies on the
fate of trichothecene mycotoxins during food processing. European
Conference on. Food Chemistry. II, Rome, Societa Italiana di Scienza
dell alimetazione. pp. 253-262.
GILBERT, J., SHEPHERD, M.J., & STARTIN, J.R. (1983b) A survey of the
occurrence of the trichothecene mycotoxin deoxynivalenol (vomitoxin)
in UK-grown barley and in imported maize by combined gas
chromatography-mass spectrometry. J. Sci. Food Agric., 34: 86-92.
GILBERT, J., SHEPHERD, M.J., & STARTIN, J.R. (1984) The analysis and
occurrence of Fusarium mycotoxins in the United Kingdom and their
fate during food processing. In: Kurata, H. & Ueno, Y., ed. Toxigenic
fungi - their toxins and health hazard, Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp. 209-216.
GILBERT, J., STARTIN, J.R,. & CREWS, C. (1985) Optimization of
conditions for trimethylsilylation of trichothecene mycotoxins. J.
Chromatogr., 319: 376-381.
GILGAN, M.W., SMALLEY, E.B., & STRONG, F.M. (1966) Isolation and
partial characterization of a toxin from Fusarium tricinctum on moldy
corn. Arch. Biochem. Biophys., 114: 1-3.
GOLINSKI, P., HULT, K., BRABARKIEWICZ-SZCZESNA, J., CHELKOWSKI, J.,
KNEBLEWSKI, P., & SZEBIOTKO, K. (1984) Mycotoxic porcine nephropathy
and spontaneous occurrence of ochratoxin A residues in kidneys and
blood of Polish swine. Appl. environ. Microbiol., 47: 1210-1212.
GOLINSKI, P., BRABARKIEWICZ-SZCZESNA, J., CHELKOWSKI, J., &
SZEBIOTKO, K. (1985) Spontaneous occurrence of ochratoxin A
residues in porcine kidney and serum samples in Poland. Appl.
environ. Microbiol., 49: 1014-1015.
GRANT, P.G., SCHINDLER, D., & DAVIES, J.E. (1976) Mapping of
trichodermin resistance in Saccharomyces cerevisiae: a genetic locus
for a component of the 60s ribosomal subunit. Genetics, 83(4):
667-673.
GREENHALGH, R., GILBERT, J., KING, R.R., BLACKWELL, B.A., STARTIN,
J.R., & SHEPHERD, M.J. (1984) Synthesis, characterization, and
occurrence in bread and cereal products of an isomer of 4-
deoxynivalenol (vomitoxin). J. agric. food Chem., 32: 1416-1420.
GREENWAY, J.A. & PULS, R. (1976) Fusariotoxicosis from barley in
British Colombia. I. Natural occurrence and diagnosis. Can. J. comp.
Med., 40: 12-15.
GROVE, J.F. & MORTIMER, P.H. (1969) The cytotoxicity of some
transformation products of diacetoxyscirpenol. Biochem. Pharmacol.,
18(6): 1473-1478.
GUPTA, R.S. & SIMINOVITCH, L. (1978) Genetic and biochemical
characterization of mutants of CHO cells resistant to the protein
synthesis inhibitor trichodermin. Somatic cell Genet., 4(3):
355-374.
GUTZWILLER, J. & TAMM, C. (1965) Verrucarin and roridin. V. The
structure of Verrucarin A. Helv. Chim. Acta, 48: 157-176.
HAGGBLOM, P. (1982) Production of ochratoxin A in barley by
Aspergillus ochraceus and Penicillium viridicatum: effect of fungal
growth, time, temperature, and inoculum size. Appl. environ.
Microbiol., 43: 1205-1207.
HAGLER, W.M., Jr, TYCZKOWDKA, K., & HAMILTON, P.B. (1984)
Simultaneous occurrence of deoxynivalenol, zearalenone, and aflatoxin
in 1982 scabby wheat from the midwestern United States. Appl.
environ. Microbiol., 47(1): 151-154.
HALD, B. (in press) Human exposure to ochratoxin A. In: Natori, S.,
Hashimoto, K., & Ueno, Y., ed. Mycotoxins and phycotoxins 1989,
Amsterdam, Oxford, New York, Elsevier Science Publishers.
HALD, B. & KROGH, P. (1972) Ochratoxin residues in bacon pigs.
Proceedings of the IUPAC Symposium: Control of Mycotoxins, Kungälv,
Sweden, page 18.
HALD, B. & KROGH, P. (1975) Detection of ochratoxin A. in barley,
using silica gel mini columns. J. Assoc. Off. Anal. Chem., 58:
156-158.
HALD, B. & KROGH, P. (1983) Toxicoses and natural occurrence in
Denmark. In: Ueno, Y., ed. Trichothecenes - chemical, biological
and toxicological aspects, Amsterdam, Oxford, New York, Elsevier,
Science Publishers, pp 251-253.
HALIBURTON, J.C., VESONDER, R.F., LOCK, T.F., & BUCK, W.B. (1979)
Equine leucoencephalomalacia (ELEM): a study of Fusarium moniliforme
as an etiologic agent. Vet. hum. Toxicol., 21(5): 348-351.
HARRACH, B., MIROCHA, C.J., PATHRE, S.V., & PALYUSIK, M. (1981)
Macrocyclic trichothecene toxins produced by a strain of Stachybotrys
atra from Hungary. Appl. environ. Microbiol., 41(6): 1428-1432.
HARRACH, B., BATA, A., BAJMOCY, E., & BENKO, M. (1983) Isolation of
satratoxins from the bedding straw of a sheep flock with fatal
stachybotryotoxicosis. Appl. environ. Microbiol., 45: 1419-1422.
HARRI, E., VON, LOEFFLER, W., SIGG, H.P., STAHELIN, H., STOLL, Ch.,
TAMM, Ch., & WIESINGER, D. (1962) [Verrucarins and roridins, a group
of antibiotics with high cytostatic effect from Myrothecium species.]
Helv. Chim. Acta, 45: 839-853 (in German).
HART, L.P. & BASELTON, W.E. (1983) Distribution of vomitoxin in dry
milled fractions of wheat infected with Gibberella. J. agric. food
Chem., 31: 657-659.
HARTMANN, G.R., RICHTER, H., WEINER, E.M., & ZIMMERMANN, W. (1978)
On the mechanism of action of the cytostatic drug anguidine and of
the immunosuppressive agent ovalicin, two sesquiterpenes from fungi.
Planta Med., 34(3): 231-252.
HARWIG, J. (1974) Ochratoxin A and related metabolites. In:
Purchase, I.F.H., ed. Mycotoxins, Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp. 345-368.
HAUBECK, H.D., LORKOWSKI, G., KULSCH, E., & ROSCHENTHALER, R. (1981)
Immunosuppression by ochratoxin A and its prevention by
phenylalanine. Appl. environ. Microbiol., 41: 1040-1042.
HAYES, A.W., HOOD, R.D., & LEE, H.L. (1974) Teratogenic effects of
ochratoxin A in mice. Teratology, 9(1): 93-97.
HAYES, M.A. & SCHIEFER, H.B. (1979) Quantitative and morphological
aspects of cutaneous irritation by trichothecene mycotoxins. Food
Cosmet. Toxicol., 17(6): 611-621.
HAYES, M.A., BELLAMY, J.E.C., & SCHIEFER, H.B. (1980) Subacute
toxicity of dietary T-2 toxin in mice: morphological and
hematological effects. Can. J. comp. Med., 44: 203-218.
HELGESON, J.P., HABERLACH, G.T., & VANDERHOEF, L.N. (1973) T-2
toxin decreases logarithmic growth rates of tobacco callus tissues.
Plant Physiol., 52(6): 660-662.
HELLER, K. & ROSCHENTHALER, R. (1978) Inhibition of protein
synthesis in Streptococcus faecalis by ochratoxin A. Can. J.
Microbiol., 24: 466-472.
HEPTINSTALL, R.H. (1974) Pathology of the kidney, Boston, Little
Brown and Co., Vol. 11, pp. 828-836.
HEWETSON, J.F., PACE, J.D., & BEHELER, J.E. (1987) Detection and
quantitation of T-2 mycotoxin in rat organs by radioimmunoassay. J.
Assoc. Off. Anal. Chem., 70: 654-657.
HIBBS, C.M., OSWEILER, G.D., BUCK, W.B., & MACFEE, G.P. (1975)
Bovine hemorrhagic syndrome related to T-2 mycotoxin. Proc. Ann.
Meet. Am. Assoc. Vet. Lab. Diagn., 17: 305-310.
HIRAYAMA, S. & YAMAMOTO, M. (1948) [Biological studies on the
poisonous wheat flour (I).] Eiseishikenjo Hokoku, 66: 85-98 (in
Japanese).
HIRAYAMA, S. & YAMAMOTO, M. (1950) [Biological studies on the
poisonous wheat flour (II).] Eiseishikenjo Hokoku, 67: 117-121 (in
Japanese).
HOBDEN, A.N. & CUNDLIFFE, E. (1980) Ribosomal resistance to the
12,13-epoxy-trichothecene antibiotics in the producing organism
Myrothecium verrucaria. Biochem. J., 190(3): 765-770.
HOERR, F.J., CARLTON, W.W., & YAGEN, B. (1981) Mycotoxicosis caused
by a single dose of T-2 toxin or diacetoxyscirpenol in broiler
chickens. Vet. Pathol., 18: 653-664.
HOLADAY, C.E. (1976) A rapid screening method for the aflatoxins
and ochratoxin A. J. Am. Assoc. Anal. Chem., 53: 603-605.
HOLT, P.S. & DE LOACH, J.R. (1988a) Cellular effects of T-2 mycotoxin
on two different lives. Biochem. Biophys. Acta, 971: 1-8.
HOOD, R.D. (1986) Effects of concurrent prenatal exposure to
rubratoxin B and T-2 toxin in the mouse. Drug chem. Toxicol., 9
(2): 185-190.
HOOD, R.D., NAUGHTON, M.J., & HAYES, A.W. (1976) Prenatal effects
of ochratoxin A in hamsters. Teratology, 13: 11-14.
HOOD, R.D., KUCZUK, M.H., & SZCZECK, G.M. (1978) Effects in mice of
simultaneous prenatal exposure to ochratoxin A and T-2 toxin.
Teratology, 17: 25-30.
HRABAR, A., SULJAGA, K., BORCIC, B., ALERAJ, B., CEOVIC, S., &
CVORISCEC, D. (1976) [Endemic nephropathy morbidity and mortality
in the village of Kaniza.] Arch. ind. Hyg. Toxicol., 27: 137-145
(in Croatian).
HSU, I.C., SMALIEY, E.B., STRONG, F.M., & RIBELIN, W.E. (1972)
Identification of T-2 toxin in moldy corn associated with a lethal
toxicosis in dairy cattle. Appl. Microbiol., 24(5): 684-690.
HUFF, W.E., WYATT, R.D., TUCKER, T.L., & HAMILTON, P.B. (1974)
Ochratoxicosis in the broiler chicken. Poult. Sci., 53: 1585-1591.
HUFF, W.E., DOERR, J.A., HAMILTON, P.B., HAMANN, D.D., PETERSON,
R.E., & CIEGLER, A. (1980) Evaluation of bone strength during
aflatoxicosis and ochratoxicosis. Appl. environ. Microbiol.,
40: 102-107.
HULT, K. & GATENBECK, S. (1976) A spectrophotometric procedure,
using carboxypeptidase A, for the quantitative measurement of
ochratoxin A. J. Assoc. Off. Anal. Chem., 59: l28-l29.
HULT, K., TEILING, A., & GATENBECK, S. (1976) Degradation of
ochratoxin A by a ruminant. Appl. environ. Microbiol., 32: 443-444.
HULT, K., HUKBY, E., HOGLUND, U., GATENBECK, S., RUTQVIST, L., &
SELLYEY, G. (1979) Ochratoxin A in pig blood: method of analysis
and use as a tool for feed studies. Appl. environ. Microbiol., 38:
772-776.
HULT, K., HUKBY, E., GATENBECK, S., & RUTQVIST, L. (1980).
Ochratoxin A in blood from slaughter pigs in Sweden: Use in
evaluation of toxin content of consumed feed. Appl. environ.
Microbiol., 39: 828-830.
HULT, K., PLESTINA, R., HABAZIN-NOVAK, V., RADIC, B., & CEOVIC, S.
(1982) Ochratoxin A in human blood and Balkan endemic nephropathy.
Arch. Toxicol., 51: 313-321.
HULT, K., FUCHS, R., PERAICA, M., PLESTINA, R., & CEOVIC, S. (1984)
Screening for ochratoxin A in blood by flow injection analysis. J.
appl. Toxicol., 4: 326-329.
HUNT, D.C., BOURDON, A.T., WILD, P.J., & CROSBY, N.T. (1978) Use of
high performance liquid chromatography combined with fluorescence
detection for the identification and estimation of aflatoxins and
ochratoxin in food. J. Sci. Food Agric., 29: 234-238.
HUNT, D.C., LESLYE, A.P., & CROSBY, N.T. (1979) Determination of
ochratoxin A in pig's kidney using enzymic digestion, dialysis and
high-performance liquid chromatography with post-column
derivatization. Analyst, 104: 1171-1175.
HUNTER, K.W., BRIMFIELD, A.A., MILLER, M., FINKELMAN, F.D., & CHU,
S.F. (1985) Preparation and characterization of monoclonal antibodies
to the trichothecene mycotoxin T-2. Appl. environ. Microbiol., 49:
168-172.
HUSSEIN, H.M., FRANICH, R.A., BAXTER, M,. & ANDREW, I.G. (1989)
Naturally occurring Fusarium toxins in New Zealand maize. Food Addit.
Contam., 6: 49-58.
HUTCHISON, R.D., STEYN, P.S., & THOMPSON, D.L. (1971) The isolation
and structure of 4-hydroxyochratoxin A and 7-carboxy-3,4-dihydro-8-
hydroxy-3-methylisocoumarin from Penicillium viridicatum.
Tetrahedron, 43: 4033-4036.
IARC (1987a) Overall evaluations of carcinogenicity: and updating of
IARC Monographs Vol. 1 to 42, Lyons, International Agency for
Research on Cancer, pp. 271-272. (IARC Monographs on the Evaluation
of Carcinogenic Risks to Humans, Supplement 7).
IARC (1987b) Biennial report 1986-1987, Lyons, International Agency
for Research on Cancer.
IARC (1983) Some food additives, feed additives and naturally
occurring substances: ochratoxin A, Lyons, International Agency for
Research on Cancer, pp. 191-206 (IARC Monographs on the Evaluation of
the Carcinogenic Risk of Chemicals to Humans, Vol. 31).
IARC (1984) Monitoring of aflatoxins in human body fluids and
application to field studies, Lyons, International Agency for
Research on Cancer (IARC International Technical Report No. 84/003).
ICHINOE, M., UCHIYAMA, S., AMANO, R., & KURATA, H. (1985)
Trichothecene-producing Fusarium in barley and wheat in Japan.
Trichothecenes and other Mycotoxins. Proceedings of the International
Mycotoxin Symposium, Sydney, Australia, New York, Chichester, John
Wiley & Sons, pp. 21-31.
ILUS, T., NIKU-PAAVOLA, M.L., & ENARI, T.M. (1981) Chromatographic
analysis of Fusarium toxins in grain samples. Eur. J. appl.
Microbiol. Biotechnol., 11(4): 244-247.
IMAIDA, K., HIROSE, M., OGISO, T., KURATA, Y., & ITO, N. (1982)
Quantitative analysis of initiating and promoting activities of five
mycotoxins in liver carcinogenesis in rats. Cancer Lett., 16:
137-143.
ISHII, K., SAKAI, K., UENO, Y., TSUNODA, H., & ENOMOTO, M. (1971)
Solaniol, a toxic metabolite of Fusarium solani. Appl. Microbiol.,
22(4): 718-720.
ISHII, K., ANDO, Y., & UENO, Y. (1975) Toxicological approaches to
the metabolites of Fusaria. Part 9. Isolation of vomiting factor from
moldy corn infected with Fusarium spp. Chem. pharm. Bull., 23(9):
2162-2164.
ISHII, K., KOBAYASHI, J., UENO, Y., & ICHINOE, M. (1986) Occurrence
of trichothecine in wheat. Appl. environ. Microbiol., 52: 331-333.
ITO, Y., OHTSUBO, K., & SAITO, M. (1980) Effects of fusarenon-X, a
trichothecene produced by Fusarium nivale, on pregnant mice and their
fetuses. Jpn. J. exp. Med., 50(3): 167-172.
ITO, Y., OHTSUBO, K., ISHII, K., & UENO, Y. (1986) Effects of
nivalenol on pregnancy and fetal development of mice. Mycotoxin
res., 2: 71-77.
ITO, Y., UENO, Y., TANAKA, T., NAKAMURA, K., & OHTSUBO, K. (1988)
[Embryotoxicity of oral nivalenol in mice.] Proc. Jpn. Assoc.
Mycotoxicol., 27: 33-36. (in Japanese).
IUPAC (1976) Recommended methods for ochratoxin A and B in barley,
Oxford, International Union of Pure and Applied Chemistry (IUPAC
Technical Report No.14).
IWAHASHI, T., TASHIRO, F., & UENO, Y. (1982) Mechanism of cytotoxic
effect of T-2 toxin on a protozoan, Tetrahymena pyriformis gl.
Maikotokishin, 15: 31-33.
JAGADEESAN, V., RUKMINI, C., VIJAYARAGHOVAN, M., & TULPULE, P.G.
(1982) Immune studies with T-2 toxin: effect of feeding and
withdrawal in monkeys. Food chem. Toxicol., 20: 83-87.
JARVIS, B.B., STAHLY, G.P., & CURTIS, C.R. (1978) Antitumor
activity of fungal metabolites: Verrucarin b-9,10-epoxides. Cancer
Treat. Rep., 62(10): 1585-1586.
JARVIS, B.B., STAHLY, G.P., PAVANASASIVAM, G., & MAZZOLA, E.P.
(1980) Antileukemic compounds derived from the chemical modification
of macrocyclic trichothecenes. 1. Derivatives of verrucarin A. J.
med. Chem., 23(9): 1054-1058.
JARVIS, B.B., LEE, Y.W., COMEZOGLU, S.N., & YATAWARA, C.S. (1986)
Trichothecenes produced by Stachybotrys atra from Eastern Europe.
Appl. environ. Microbiol., 51(5): 915-918.
JELINEK, C.F., POHLAND, A.E., & WOOD, G.E. (1989) Occurrence of
mycotoxins in foods and feeds - an update. J. Assoc. Off. Anal.
Chem., 72: 223-230.
JIMINEZ, A. & VASQUEZ, D. (1975) Quantitative binding of antibiotics
to ribosomes from a yeast mutant altered on the peptidyl transferase
centre. Eur. J. Biochem., 54(2): 483-492.
JIMENEZ, A., SANCHEZ, L., & VAZQUEZ, D. (1975) Simultaneous
ribosomal resistance to trichodermin and anisomycin in Saccharomyces
cerevisiae mutants. Biochim. Biophys. Acta, 383(4): 427-434.
JOFFE, A.Z. (1962) Biological properties of some toxic fungi
isolated from overwintered cereals. Mycopathol. Mycol. appl., 16:
201-221.
JOFFE, A.Z. (1986) Fusarium species, their biology and toxicology,
New York, Chichester, J. Wiley and Sons.
JOFFE, A.Z. & YAGEN, B. (1977) Comparative study of the yield of T-2
toxin produced by Fusarium poae, Fusarium sporotrichioides and
Fusarium sporotrichioides var. tricinctum strains from different
sources. Mycopathologia, 60(2): 93-97.
JOHNSEN, H., ODDEN, E., LIE, O., JOHNSEN, B.A., & FONNUM, F. (1986)
Metabolism of T-2 toxin by rat liver carboxylesterase. Biochem.
Pharmacol., 35: 1469-1473.
JOSEFSSON, E. (1979) [Study of ochratoxin A in pig kidneys.] Var
Föda, 31: 415-420 (in Swedish).
JOSEFSSON, E. & MOLLER, T. (1979) High pressure liquid
chromatographic determination of ochratoxin A and zearalenone in
cereals. J. Assoc. Off. Anal. Chem., 62: 1165-1168.
JUSZKIEWICZ, T. & PISKORSKA-PLISZCZYNSKA, J. (1976) [Occurrence of
aflatoxins B1, B2, G1, and G2, ochratoxins A and B, sterigmatocystin
and zearalenone in cereals]. Med. Weter., 32: 617-619 (in Polish).
JUSZKIEWICZ, T. & PISKORSKA-PLISZCZYNSKA, J. (1977) [Occurrence of
mycotoxins in mixed feeds and concentrates.] Med. Weter., 33: 193-196
(in Polish).
JUSZKIEWICZ, T., PISKORSKA-PLISZCZYNSKA, J., & WISNIEWSKA, H. (1982)
Ochratoxin A in laying hens: tissue deposition and passage into eggs.
In: Mycotoxins and Phycotoxins, Vienna, Technical University Vienna
Publ., pp. 122-125.
KALINOSKI, H.T., UDSETH, H.R., WRIGHT, B.W., & SMITH, R.D. (1986)
Supercritical fluid extraction and direct fluid injection mass
spectrometry for the determination of trichothecene mycotoxins in
wheat samples. Anal. Chem., 58: 2421-2425.
KAMIMURA, H., NISHIJIMA, M., SAITO, K., YASUDA, K., IBE, A.,
NAGAYAMA, T., USHIYAMA, H., & NAOI, Y. (1979) Studies on mycotoxins
in foods. XII. The decomposition of trichothecene mycotoxins during
food processing. J. Assoc. Off. Anal. Chem., 64(5): 1067-1073.
KANAI, K. & KONDO, E. (1984) Decreased resistance to myobacterial
infection in mice fed a trichothecene compound (T-2 toxin). Jpn. J.
med. Sci. Biol., 37: 97-104.
KANE, A., CREPPY, E.E., ROSCHENTHALER, R., & DIRHEIMER, G. (1986a)
Changes of urinary and renal tubular enzymes caused by subchronic
administration of ochratoxin A in rats. Toxicology, 61: 489-495.
KANE, A., CREPPY, E.E., ROTH, A., ROSCHENTHALER, R., & DIRHEIMER, G.
(1986b) Distribution of the [3]-label from low doses of radioactive
ochratoxin A ingested by rats, and evidence for DNA single-strand
breaks caused in liver and kidneys. Arch. Toxicol., 58: 219-224.
KANIZAWA, M. (1984) Synergistic effect of citrinin on hepatorenal
carcinogenesis of ochratoxin A in mice. In: Kurata, H. & Ueno, Y.,
ed. Toxigenic fungi - their toxins and health hazard, Amsterdam,
Oxford, New York, Elsevier Science Publishers, pp. 245-254
(Developments in Food Science No. 7).
KANIZAWA, M. & SUZUKI, S. (1978) Induction of renal and hepatic
tumours in mice by ochratoxin A, a mycotoxin. Gann, 69: 599-600.
KANIZAWA, M., SUZUKI, S., KOZUKA, Y., & YAMAZAKI, M. (1977)
Histopathological studies on the toxicity of ochratoxin A in rats. I.
Acute oral toxicity. Toxicol. appl. Pharmacol., 42: 55-64.
KELLERMAN, T.S., MARASAS, W.F.O., PIENAAR, J.G., & NAUDE, T.W.
(1972) A mycotoxicosis of equidae caused by Fusarium moniliforme
Sheldon. A preliminary communication. Onderstepoort J. vet. Res.,
39(4): 205-208.
KHERA, K.S., WHALEN, C., ANGERS, G., VESONDER, R.F., & KUIPER-
GOODMAN, I. (1982) Embryotoxicity of 4-deoxynivalenol (vomitoxin)
in mice. Bull. environ. Contam. Toxicol., 29: 487-491.
KHERA, K.S., ARNOLD, D.L., WHALEN, C., ANGERS, G., & SCOTT, P.M.
(1984) Vomitoxin (4-deoxynivalenol): Effects on reproduction of mice
and rats. Toxicol. appl. Pharmacol., 74: 345-356.
KHERA, K.S., WHALEN, C., & ANGERS, G. (1986) A teratology study on
vomitoxin (4-deoxynivalenol) in rabbits. Food chem. Toxicol., 24:
421-424.
KIENTZ, C.E. & VERWEIJ, A. (1986) Trimethylsilylation and
trifluoroacetylation of a number of trichothecenes followed by gas
chromatographic analysis on fused silica capillary columns. J.
Chromatogr., 355: 229-240.
KING, B. (1979) Outbreak of ergotism in Wollo, Ethiopia. Lancet,
1: 1411.
KING, R.R., MCQUEEN, R.E., LEVESQUE, D., & GREENHALGH, R. (1984)
Transformation of deoxynivalenol (vomitoxin) by rumen microorganisms.
J. agric. food Chem., 32: 1181-1183.
KITAGAWA, M., TASHIRO, F., & UENO, Y. (1982) Mutual interaction
between zearalenone, an estrogenic mycotoxin, and estrogen receptor
of rat brain. Proc. Jpn. Assoc. Mycotoxicol., 15: 28-30.
KITCHEN, D.N., CARLTON, W.W., & TUITE, J. (1977a) Ochratoxin A and
citrinin induced nephrosis in beagle dogs. I. Clinical and
clinicopathological features. Vet. Pathol., 14: 154-172.
KITCHEN, D.N., CARLTON, W.W., & TUITE, J. (1977b) Ochratoxin A and
citrinin induced nephrosis in beagle dogs. II. Pathology. Vet.
Pathol., 14: 261-272.
KLINKERT, W., LORKOWSKI, G., CREPPY, E.E., DIRHEIMER, G., &
ROSCHENTHALER, R. (1981) Inhibition of macrophage migration by
ochratoxin A and citrinin, and prevention by phenylalanine of the
ochratoxin A-induced inhibition. Toxicol. Eur. Res., 3: 186-189.
KONISHI, T. & ICHIJO, S. (1970) [Clinical studies on bean-hulls
poisoning of horse. I. Clinical and biochemical observations in
spontaneous cases.] Res. Bull. Obihiro Univ., 6: 242-257 (in
Japanese).
KONRAD, I. & ROSCHENTHALER, R. (1977) Inhibition of phenylalanine
tRNA synthetase from Bacillus subtilis by ochratoxin A. FEBS Lett.,
83: 341-347.
KOTSONIS, F.N., SMALLEY, E.B., ELLISON, R.A., & GALE, C.M. (1975)
Food refusal factors in pure cultures of Fusarium roseum graminearum.
Appl. Microbiol., 30(3): 362-368.
KOZLOVSKY, A.G. & RESHETILOVA, T.A. (1984) Regulation of
biosynthesis of ergot alkaloids by Penicillium sizovae. Folia
microbiol., 29: 301-305.
KRIEK, N.P.J., MARASAS, W.F.O., STEYN, P.S., VAN RENSBURG, S.J., &
STEYN, M. (1977) Toxicity of a moniliformin-producing strain of
Fusarium moniliforme var. subglutinans isolated from maize. Food
Cosmet. Toxicol., 15(6): 579-587.
KRISHNAMACHARI, K.A.V.R. & BHAT, R.V. (1976) Poisoning of ergoty
bajra (pearl millet) in man. Indian J. med. Res., 64: 1624-1628.
KRISHNAMURTHY, T. & SARVER, E.W. (1986) Mass spectral investigations
on trichothecene mycotoxins. III. Synthesis, characterization and
applications of pentafluoropropionyl and trifluoroacetyl esters of
simple trichothecenes. J. Chromatogr., 355: 253-264.
KRISHNAMURTHY, T., WASSERMAN, M.B., & SARVER, E.W. (1986) Mass
spectral investigations of trichothecene mycotoxins. I. Application
of negative ion chemical ionization techniques for the simultaneous
and accurate analysis of simple trichothecenes in picogram levels.
Biomed. environ. mass Spectrom., 13: 503-518.
KRISHNAMURTHY, T., SARVER, E.W., GREENE, S.L., & JARVIS, B.B. (1987)
Mass spectral investigations on trichothecene mycotoxins. II.
Detection and quantitation of macrocyclic trichothecenes by gas
chromatography/negative ion chemical ionization mass spectrometry. J.
Assoc. Off. Anal. Chem., 70: 132-140.
KROGH, P. (1976a) Epidemiology of mycotoxic porcine nephropathy.
Nord. vet. Med., 28: 452-458.
KROGH, P. (1976b) Mycotoxic nephropathy. In: Advances in veterinary
science and comparative medicine, New York, Academic Press, Vol. 20,
pp. 147-170.
KROGH, P. (1977) Ochratoxin A residues in tissues of slaughter pigs
with nephropathy. Nord. vet. Med., 29: 402-405.
KROGH, P. (1978) Causal associations of mycotoxic nephropathy. Acta
pathol. microbiol. Scand., A269: 1-28.
KROGH, P. (1979) Environmental ochratoxin A and Balkan (endemic)
nephropathy: Evidence for support of a causal relationship. In:
Strahinjic, S. & Stefanovic, V., ed. Endemic (Balkan) nephropathy,
Nis, Yugoslavia, Grafika, pp. 35-43.
KROGH, P. (1980) Ochratoxins: occurrence, biological effects and
causal role in disease. In: Eaker, D. & Wadstrom, T., ed. Natural
toxins, Oxford, Pergamon Press, pp. 673-680.
KROGH, P. (1983) Diagnostic criteria for ochratoxin-induced
nephropathy. In: Strahinjic, S. & Stefanovic, V., ed. Current
research in endemic (Balkan) nephropathy, Nis, Yugoslavia, Grafika,
pp. 11-14.
KROGH, P., HALD, B., & PEDERSEN, E.J. (1973) Occurrence of
ochratoxin A and citrinin in cereals associated with mycotoxic
porcine nephropathy. Acta pathol. microbiol. Scand., B81:
689-695.
KROGH, P., AXELSON, N.H., ELLING, F., GYRD-HANSEN, N., HALD, B.,
HYLDGAARD-JENSEN, J., LARSEN, A.E., MADSEN, A., MORTENSEN, H.P.,
MOLLER, T., PETERSEN, O.K., RAVNSKOV, U., ROSTGAARD, M., & AALUND, O.
(1974a) Experimental porcine nephropathy: changes of renal function
and structure induced by ochratoxin A-contaminated feed. Acta pathol.
microbiol. Scand., A246: 21.
KROGH, P., HALD, B., ENGLUND, P., RUTQVIST, L., & SWAHN, O. (1974b)
Contamination of Swedish cereals with ochratoxin A. Acta pathol.
microbiol. Scand., B82: 301-302.
KROGH, P., ELLING, F., HALD, B., LARSEN, A.E., LILLEHOJ, E.B.,
MADSEN, A., & MORTENSEN, H.P. (1976a) Time-dependent disappearance
of ochratoxin A residues of bacon pigs. Toxicology, 6: 235-242.
KROGH, P., ELLING, F., HALD, B., JYLLING, B., PETERSEN, V.E.,
SKADHAUGE, E., & SVENDSEN, C.K. (1976b) Experimental avian
nephropathy: changes of renal function and structure induced by
ochratoxin A-contaminated feed. Acta pathol. microbiol. Scand.,
A84: 215-221.
KROGH, P., ELLING, F., GYRD-HANSEN, N., HALD, B., LARSEN, A.E.,
LILLEHOJ, E. B., MADSEN, A., MORTENSEN, H.P., & RAVNSKOV, U. (1976c)
Experimental porcine nephropathy: changes of renal function and
structure perorally induced by crystalline ochratoxin A. Acta pathol.
microbiol. Scand., A84: 429-434.
KROGH, P., HALD, B., PLESTINA, R., & CEOVIC, S. (1977) Balkan
(endemic) nephropathy and food-borne ochratoxin A: Preliminary
results of a survey of foodstuff. Acta. Path. Microbiol. Scand.,
B85: 238-240.
KROGH, P., ELLING, F., FRIIS, CHR., MALD, B., LARSEN, A.E., LILLEHOJ,
E.B., MADSEN, A., MORTENSEN, H.P., RASMUSSEN, F., & RAVUSKOU, U.
(1979) Porcine nephropathy induced by long-term ingestion of
ochratoxin A. Vet. Pathol., 16: 466-475.
KROGH, P., GYRD-HANSEN, N., HALD, B., LARSEN, S., NIELSEN, J.P.,
SMITH, M., IVANOFF, C., & MEISNER, H. (1988). Renal enzyme
activities in experimental ochratoxin A-induced porcine nephropathy:
Diagnostic potential of phosphoenolpyruvate carboxykinase and
gammaglutamyl transpeptidase activity. J. Toxicol. environ. Health.,
23: 1-15.
KUCZUK, M.H., BENSON, P.M., HEATH, H., & HAYES, A.W. (1978)
Evaluation of the mutagenic potential of mycotoxins using Salmonella
typhimurium and Saccharomyces cerevisiae. Mutat. Res., 53(1): 11-20.
KUMAGAI, S. (1985) Ochratoxin A: Plasma concentration and excretion
into bile and urine in albumin-deficient rats. Food chem. Toxicol.,
23: 941-943.
KUMAGAI, S. & AIBARA, K. (1982) Intestinal absorption and secretion
of ochratoxin A in rat. Toxicol. appl. Pharmacol., 64: 94-102.
KUMAGAI, S. & SHIMIZU, T. (1988) Effects of Fusarenon-X and T-2 toxin
on intestinal absorption of monosaccharide in rats. Arch. Toxicol.,
61: 489-495.
KUPCHAN, S.M., JARVIS, B.B., DAILEY, R.G., Jr, BRIGHT, W., BRYAN,
R.F., & SHIZURI, Y. (1976) Baccharin, a novel potent antileukemic
trichothecene triepoxide from Baccharis megapotamica. J. Am. Chem.
Soc., 98(22): 7092-7093.
KUPCHAN, S.M., STREELMAN, D.R., JARVIS, B.B., DAILEY, R.G., Jr, &
SNEDEN, A.T. (1977) Isolation of potent new antileukemic
trichothecenes from Baccharis megapotamica. J. org. Chem., 42
(26): 4221-4225.
LAFARGE-FRAYSSINET, C., DECLOITRE, F., MOUSSET, S., MARTIN, M., &
FRAYSSINET, C. (1981) Induction of DNA single strand breaks by T-2
toxin, a trichothecene metabolites of Fusarium, effect on lymphoid
organs and liver. Mutat. Res., 88(2): 115-124.
LAFARGE-FRAYSSINET, C., LESPINATS, G., LAFONT, P., LOISILLIER, F.,
MOUSSET, S., ROSENSTEIN, Y., & FRAYSSINET, C. (1979)
Immunosuppressive effects of Fusarium cells to mitogens. Proc. Soc.
Exp. Biol. Med., 160(3): 302-311.
LARSEN, S. (1928) [On chronic degeneration of the kidneys caused by
mouldy rye.] Maanadsskr. Dyrl., 40: 259-284, 289-300 (in Danish).
LAUREN, D.R. & GREENHALGH, R. (1987) Simultaneous analysis of
nivalenol and deoxynivalenol in cereals by liquid chromatography. J.
Assoc. Off. Anal. Chem., 70: 479-483.
LEE, S. & CHU, F.S. (1981a) Radioimmunoassay of T-2 toxin in corn
and wheat. J. Assoc. Off. Anal. Chem., 64(1): 156-161.
LEE, S. & CHU, F.S. (1981b) Radioimmunoassay of T-2 toxin in
biological fluids. J. Assoc. Off. Anal. Chem., 64(3): 684-688.
LEE, S.C., BEERY, J.T., & CHU, F.S. (1984) Immunohistochemical fate
of ochratoxin A in mice. Toxicol. appl. Microbiol., 72: 218-227.
LEE, U., JANG, H., TANAKA, T., HASEGAWA, A., OH, Y., & UENO, Y.
(1985) The coexistence of the Fusarium mycotoxins nivalenol,
deoxynivalenol, and zearalenone in Korean cereals harvested in 1983.
Food Addit. Contam., 2(3): 185-192.
LEE, U., JANG, H., TANAKA, T., OH, Y., CHO, C., & UENO, Y. (1987)
Effect of milling on decontamination of Fusarium mycotoxins
nivalenol, deoxynivalenol, and zearalenone in Korean wheat. J. agric.
food Chem., 35: 126-129.
LEONOV, A.N. (1977) Current view of the chemical nature of factors
responsible for alimentary toxic aleukia. In: Rodricks, J.V.,
Hesseltine, C.W,. & Mehlman, M.A., ed. Mycotoxins in human and animal
health, Park Forest South, Illinois, Pathotox Publishers, pp. 323-
328.
LEVI, C.P. (1975) Collaborative study of a method for the
determination of ochratoxin A in green coffee. J. Assoc. Off. Anal.
Chem., 58(2): 258-262.
LEVI, C.P., TRENK, H.L., & MOHR, H.K. (1974) Study of the
occurrence of ochratoxin A in green coffee beans. J. Assoc. Off.
Anal. Chem., 57: 866-870.
LEW, V.H., MUELLNER, E., HAGER, R., & GREGOR, M. (1979) [Feed
refusal and emesis in fattening pigs caused by fusariotoxin-
contaminated corn.] Bodenkultur, 30(3): 309-316 (in German).
LIAO, L.L., GROLLMAN, A.P., & HORWITZ, S.B. (1976) Mechanism of
action of the 12,13-epoxytrichothecene anguidine: an inhibitor of
protein synthesis. Biochim. Biophys. Acta, 454(2): 273-284.
LINDENFELSER, L.A., LILLEHOJ, E.B., & BURMEISTER, H.R. (1974)
Aflatoxin and trichothecene toxins: skin tumor induction and
synergistic acute toxicity in white mice. J. Natl Cancer Inst.,
52(1): 113-116.
LINNAINMAA, K., SORSA, M., & ILUS, T. (1979) Epoxytrichothecene
mycotoxins as c-mitotic agents in Allium. Hereditas, 90(2):
151-156.
LOKEN, T. (1984) Ergot from meadow grass in Norway: chemical
composition and toxicological effects in sheep. Nord. vet. Med., 36:
259-265.
LORENZ, K. (1979) Ergot on cereal grains. In: Critical reviews in
food, science, and nutrition, Boca Raton, Florida, CRC Press, Vol.
11, pp. 311-354.
LORENZANA, R.M., BEASLEY, V.R., BUCK, W.B., GHENT, A.W., LUNDREN,
G.R., & POPPENGA, R.H. (1985) Experimental T-2 toxicosis in swine. I
Changes in cardiac output, aortic mean pressure, catecholamines, 6-
keto PGFa, thromboxane B2, and acid base parameters. Fundam. appl.
Toxicol., 5: 879-892.
LOVELESS, A.R. (1967) Claviceps fusiformis sp. nov.: the causal
agent of an agalactia of sows. Br. Mycol. Soc., 50: 15-18.
LUO, X. (1988) Food poisoning associated with Fusarium toxins.
Proceedings of the 7th International Symposium on Mycotoxins and
Phycotoxins, Tokyo, 16-19 August, 1988.
LUSTER, M.I., GERMOLEC, D.R., BURLESON, G.R., JAMESON, C.W.,
ACKERMANN, M.F., LAMM, K.R., & MAYER, H.T. (1987) Selective
immunosuppression in mice of natural killer cell activity by
ochratoxin A. Cancer Res., 47: 2259-2263.
LUTSKY, I. & MOR, N. (1981) Experimental alimentary toxic aleukia
in cats. Lab. anim. Sci., 31(1): 43-47.
LUTSKY, I., MOR, N., YAGEN, B., & JOFFE, A.Z. (1978) The role of
T-2 toxin in experimental alimentary aleukia: a toxicity study in
cats. Toxicol. appl. Pharmacol., 43(1): 111-124.
MACDONALD, E.J., COVAN, K.R. & SMITH, T.K. (1988) Effect of acute
oral doses of T-2 toxin on tissue concentrations of biogenic amines
in the rat. J. anim. Sci., 66: 434-441.
MCLAUGHLIN, C.S., VAUGHAMM, M.H., CAMPBELL, I.M., WEI, C.M.,
STAFFORD, M.E., & HANSEN, B.S. (1977) Inhibition of protein
synthesis by trichothecenes. In: Rodricks, J.V., Hesseltine, C.W., &
Mehlman, M.A., ed. Mycotoxins in human and animal health, Forest Park
South, Illinois, Pathotox Publishers, pp. 263-273.
MANN, D.D., BUENING, G.M., HOOK, B., & OSWEILER, G.D. (1983) Effects
of T-2 mycotoxin on bovine serum proteins. Am. J. vet. Res., 44:
1757-1759.
MANN, D.D., BUENING, G.M., OSWEILER, G.D., & HOOK, B. (1984) Effect
of subclinical levels of T-2 toxin on the bovine cellular immune
system. Can. J. comp. Med., 48: 308-312.
MANTLE, P.G. (1977a) The genus Claviceps. In: Wyllie, T.D. &
Morehouse, L.C., ed. Mycotoxic fungi, mycotoxins, mycotoxicoses: an
encyclopedia handbook, New York, Marcel Dekker, Vol. 1, pp. 83-89.
MANTLE, P.G. (1977b) Chemistry of Claviceps mycotoxins. In: Wyllie,
T.D. & Morehouse, L.C., ed. Mycotoxic fungi, mycotoxins,
mycotoxicoses: an encyclopedia handbook, New York, Marcel Dekker, Vol.
1, pp. 421-426.
MANTLE, P.G. (1977c) Ergotism in cattle, sheep, swine. In: Wyllie,
T.D. & Morehouse, L.C., ed. Mycotoxic fungi, mycotoxins,
mycotoxicoses: an encyclopedia handbook, New York, Marcel Dekker, Vol.
2, pp. 145-151, 207-213, 273-275.
MARASAS, W.F.O., BAMBURG, J.R., SMALLEY, E.B., STRONG, F.M., RAGLAND,
W.L., & DEGURSE, P.E. (1969) Toxic effects on trout, rats and mice
of T-2 toxin produced by the fungus Fusarium tricinctum. Toxicol.
appl. Pharmacol., 15(2): 471-482.
MARASAS, W.F.O., SMALLEY, E.B., BAMBURG, J.R., & STRONG, F.M. (1971)
Phytotoxicity of T-2 toxin produced by Fusarium tricinctum.
Phytopathology, 61(12): 1488-1491.
MARASAS, W.F.O., KRIEK, N.P.J., WIGGINS, V.M., STEYN, P.S., TOWERS,
D.K., & HASTIE, T.J. (1979a) Incidence, geographic distribution,
and toxigenicity of Fusarium species in South African corn.
Phytopathology, 69(11): 1181-1185.
MARASAS, W.F.O., LEISTNER, L., HOFMANN, G., & ECKARDT, C. (1979b)
Occurrence of toxigenic strains of Fusarium in maize and barley in
Germany. Appl. environ. Microbiol., 7: 289-305.
MARASAS, W.F.O., NELSON, P.E., & TOUSSON, T.A., ed. (1984) Toxigenic
Fusarium species - identity and mycotoxicology, University Park,
Pennsylvania, Pennsylvania State University Press.
MARQUARDT, R.R., FROHLICH, A.A., SREEMANNARAYANA, O., ABRAMSON, D., &
BERNATSKY, A. (1988) Ochratoxin A in blood from slaughter pigs in
Western Canada. Can. J. vet. Res., 52: 186-190.
MASUDA, E., TAKEMOTO, T., TATSUNO, T., & OBARA, T. (1982)
Immunosuppressive effect of a trichothecene mycotoxin, fusarenon-X in
mice. Immunology, 45: 743-749.
MASUKO, H., UENO, Y., OTOKAWA, M., & YAGINUMA, K. (1977) The
enhancing effect of T-2 toxin on delayed hyper-sensitivity in mice.
Jpn. J. med. Sci. Biol., 30(3): 159-164.
MATSUOKA, Y. & KUBOTA, K. (1981) Studies on mechanisms of diarrhea
induced by fusarenone-X, a trichothecene mycotoxin from Fusarium
species. Toxicol. appl. Pharmacol., 57(3): 293-301.
MATSUOKA, Y. & KUBOTA, K. (1987) Characteristics of inflammation
induced by Fusarenon-X, a trichothecene mycotoxin from Fusarium
species. Toxicol. appl. Pharmacol., 91: 333-340.
MATSUOKA, Y., KUBOTA, K., & UENO, Y. (1979) General pharmacological
studies of fusarenon-X, a trichothecene mycotoxin from Fusarium
spp. Toxicol. appl. Pharmacol., 50(1): 87-94
MAYURA, K., REDDY, R.V., HAYES, A.W., & BERNDT, W.O. (1982)
Embryocidal, fetotoxic and teratogenic effects of ochratoxin A in
rats. Toxicology, 25: 175-185.
MAYURA, K., HAYES, A.W., & BERNDT, W.O. (1983) Effects of dietary
protein on teratogenicity of ochratoxin A in rats. Toxicology, 27:
147-157.
MAYURA, K., PARKER, R., BERNDT, W.O., & PHILLIPS, T.D. (1984)
Effect of simultaneous prenatal exposure to ochratoxin A and citrinin
in the rat. J. Toxicol. environ. Health, 13: 553-561.
MEISNER, H. (1976) Energy-dependent uptake of ochratoxin A by
mitochondria. Arch. Biochem. Biophys., 173: 132-140.
MEISNER, H. & CHAN, S. (1974) Ochratoxin A, an inhibitor of
mitochondrial transport systems. Biochemistry, 13: 2795-2800.
MEISNER, H. & KROGH, P. (1982) Inhibition of renal
phosphoenolpyruvate carboxykinase in pigs by alimentary exposure to
ochratoxin A. In: Mycotoxins and Phycotoxins, Proceedings of the 5th
International IUPAC Symposium, Vienna, Technical University Vienna
publ., pp. 342-345.
MEISNER, H. & MEISNER, P. (1981) Ochratoxin A, an in vivo inhibitor
of renal phosphoenolpyruvate carboxykinase. Arch. Biochem. Biophys.,
208: 146-153.
MEISNER, H. & SELANIK, P. (1979) Inhibition of renal
gluconeogenesis in rats by ochratoxin. Biochem. J., 180: 681-684.
MEISNER, H., CIMBALA, M.A., & HANSON, R.W. (1983) Decrease of renal
phosphoenolpyruvate carboxykinase RNA and Poly(A)+ RNA level by
ochratoxin A. Arch. Biochem. Biophys., 223: 264-270.
MERWE, K.J.,VAN DER, J., STEYN, P.S., & FOURIE, L. (1965)
Mycotoxins. Part II. The constitution of ochratoxins A, B, and C,
metabolites of Aspergillus ochraceus Wilh. J. Chem. Soc., 1965:
7083-7088.
MILES, W.F. & GURPRASAD, N.P. (1985) Oxygen negative chemical
ionization mass spectrometry of trichothecenes. Biomed. mass
Spectrom., 12: 652-658.
MINISTRY OF AGRICULTURE, FISHERIES AND FOOD (1980) Survey of
mycotoxins in the United Kingdom, London, Ministry of Agriculture,
Fisheries and Food, 35 pp (Food Surveillance Paper No. 4).
MIROCHA, C.J. & ABBAS, H.K. (1989) Chemistry, occurrence and
toxicology of the haemorrhagic mycotoxin (wortmannin) produced by
Fusarium. In: Natori, S., Hashimoto, K., & Ueno, Y. ed. Mycotoxins
and phycotoxins, Science Publishers, Amsterdam, Oxford, New York,
Elsevier.
MIROCHA, C.J. & PATHRE, S. (1973) Identification of the toxic
principle in a sample of poefusarin. Appl. Microbiol., 26(5): 719-
724.
MIZUNO, S. (1975) Mechanism of inhibition of protein synthesis
initiation by diacetoxyscirpenol and fusarenon-X in the reticulocyte
lysate system. Biochim. Biophys. Acta, 383(2): 207-214.
MORE, J., GALTIER, P., & ALVINERIE, M. (1978) Toxicite de
l'ochratoxine A. III. Effets pendant les stades initiaux de la
gestation chez le rat. Ann. Rech. vet., 9(1): 169-173.
MORGAN, M.R.A., MATTHEW, J.A., MCNERNEY, R., & CHAN, H.W.-S. (1982)
The immunoassay for ochratoxin A. In: Mycotoxins and Phycotoxins,
Proceedings of the 5th International IUPAC Symposium, Vienna,
Technical University Vienna, pp. 32-35.
MOROI, K., SUZUKI, S., KUGA, T., YAMAZAKI, M., & KANISAWA, M. (1985)
Reduction of ochratoxin A toxicity in mice treated with phenylalanine
and phenobarbital. Toxicol. Lett., 25: 1-5.
MORRISSEY, R.E. (1984) Teratological study of Fisher rats fed diet
containing added vomitoxin. Food chem. Toxicol., 22(6): 453-457.
MORRISSEY, R.E. & VESONDER, R.F. (1985) Effect of deoxynivalenol
(vomitoxin) on fertility, pregnancy and postnatal development of
Sprague-Dawley rats. Appl. environ. Microbiol., 49: 1062-1066.
MORTENSEN, H.P., HALD, B., & MADSEN, A. (1983) Feeding experiments
with ochratoxin A contaminated barley for bacon pigs. V. Ochratoxin A
in blood. Acta agric. Scand., 33: 235-239.
MORTIMER, P.H., CAMPBELL, J., DI MEUNA, M.E., & WHITE, E.P. (1971)
Experimental myrotheciotoxicosis and poisoning in ruminants by
verrucarin A and roridin A. Res. vet. Sci., 12: 508-515.
MULDERS, E.J. & IMPELEN-PEEK, H.A.M. (1986) Gas chromatographic
determination of deoxynivalenol in cereals. Z. Lebensm. Unters.
Forsch., 183: 406-409.
MUNRO, I.C., MOODIE, C.A., KUIPER-GOODMAN, T., SCOTT, P.M., & GRICE,
H.C. (1974) Toxicologic changes in rats fed graded dietary levels
of ochratoxin A. Toxicol. appl. Pharmacol., 28(2): 180-188.
MUNSCH, N. & MUELLER, W.E.G. (1980) Effect of T-2 toxin on DNA
polymerases and terminal deoxynucleotidyl transferase of Molt-4 and
Nu-8 cell lines. Immunopharmacology, 2(4): 313-318.
MUTOH, A., ISHII, K., & UENO, Y. (1988) Effect of radioprotective
compounds and anti-inflammatory agents on the acute toxicity of
trichothecenes. Toxicol. Lett., 40: 165-174.
NAGAYAMA, S., KAWAMURA, O., OHTANI, K., RYU, J.C., LATUS, D.,
SUDHEIM, L., & UENO, Y. (1988) Application of an enzyme-linked
immunosorbent assay for screening of T-2 toxin-producing Fusarium
spp. Appl. environ. Microbiol., 54: 1302-1303.
NAKAMURA, Y., TAKEDA, S., OGASAWARA, K., KARASHIMADA, T., & ANDO K.
(1951) [A study on toxicosis caused by Akakabi poisoned wheat
flour.] Hokkaido doritsu Eiseikenkyujoho, 2: 35-46 (in Japanese).
NEELY, W.C. & WEST, A.D. (1972) Spectroanalytical parameters of
fungal metabolites. III. Ochratoxin A. J. Assoc. Off. Anal. Chem.,
55(6): 1305-1309.
NEL, W. & PURCHASE, I.F.H. (1968) The fate of ochratoxin A in rats.
J. S. Afr. Chem. Inst., 21: 87-88.
NELSON, P.E., TOUSSON, T.A., & MARASAS, W.F.O. (1983) Fusarium
species, an illustrated manual for identification, University Park,
Pennsylvania, Pennsylvania State University Press.
NESHEIM, S. (1971) Ochratoxins: occurrence, production, analysis,
and toxicity. Abstracts from the 85th Annual Meeting of the
Association of Official Analytical Chemists, Washington, 11-14
October, 1971, Washington, DC, Association of Official Analytical
Chemists.
NESHEIM, S. (1973) Analysis of ochratoxins A and B and their esters
in barley, using partition and thin-layer chromatography. II.
Collaborative study. J. Assoc. Off. Anal. Chem., 56: 822-826.
NESHEIM, S. (1982) Ochratoxin A- Analytical method 1: thin-layer
chromatographic determination of ochratoxin A in foodstuffs. In:
Egan, H., ed. Environmental carcinogens: selected methods of analysis
- some mycotoxins, Lyons, International Agency for Research on
Cancer, pp. 255-270.
NESHEIM, S., HARDIN, N.F., FRANCIS, O.J., & LANGHAM, W.S. (1973)
Analysis of ochratoxins A and B and their esters in barley, using
partition and thin-layer chromatography. I. Development of the
method. J. Assoc. Off. Anal. Chem., 56: 817-821.
NICOLOV, I.G., CHERNOZEMSKY, I.N., PETKOVA-BOCHAROVA, T., STOYANOV,
I.S., & STOICHEV, I.I. (1978) Epidemiologic characteristics of
urinary system tumours and Balkan nephropathy in an endemic region of
Bulgaria. Eur. J. Cancer, 14: 1237-1242.
NIP, W.K. & CHU, F.S. (1979) Fate of ochratoxin A in goats. J.
environ. Sci. Health, B14: 319-333.
NORPPA, H., PENTTILA, M., SORSA, M., HINTIKKA, E.L., & ILUS, T.
(1980) Mycotoxin T-2 of Fusarium tricinctum and chromosome changes
in Chinese hamster bone marrow. Hereditas, 93(2): 329-332.
NORTHOLT, M.D., VAN EGMOND, H.P., & PAULSCH, W.E. (1979) Ochratoxin
A production by some fungal species in relation to water activity and
temperature. J. food Prot., 42: 485-490.
NOWICKI, T.W., GABA, D.G., DEXTER, J.E., MATSUO, R.R., & CLEAR, R.M.
(1988) Retention of the Fusarium mycotoxin deoxynivalenol in wheat
during processing and cooking of spaghetti and noodles. J. Cereal
Sci., 8: 189-202.
OGASAWARA, K. (1965) [Akakabi-toxicosis.] J. Food Hyg. Soc. Jpn,
6: 81-82 (in Japanese).
OHTA, M., ISII, K., & UENO, Y. (1977) Metabolism of trichothecene
mycotoxins. Part 1. Microsomal deacetylation of T-2 toxin in animal
tissues. J. Biochem. (Tokyo), 82(6): 1591-1598.
OHTSUBO, K. & SAITO, M. (1970) Cytotoxic effects of scirpene
compounds, fusarenon-X produced by Fusarium nivale, dihydro-nivalenol
and dihydrofusarenon-X on Hela cells. Jpn. J. med. Sci. Biol., 23(4):
217-225.
OHTSUBO, K. & SAITO, M. (1977) Chronic effects of trichothecene
toxins. In Rodricks, J.V., Hesseltine, C.W., & Mehlman, M.A., ed.
Mycotoxins in human and animal health, Park Forest South, Illinois,
Pathotox Publishers, pp. 255-262.
OHTSUBO, K., YAMADA, M., & SAITO, M. (1968) Inhibitory effect of
nivalenol, a toxic metabolite of Fusarium nivale on the growth cycle
and biopolymer synthesis of Hela cells. Jpn. J. med. Sci. Biol.,
21(3): 185-194.
OHTSUBO, K., KADEN, P., & MITTERMAYER, C. (1972) Polyribosomal
breakdown in mouse fibroblasts L cells by fusarenon-X, a toxic
principle isolated from Fusarium nivale. Biochim. Biophys. Acta,
287(3): 520-525.
OHTSUBO, K., RYU, J.-C., NAKAMURA, K., IZUMIYAMA, N., TANAKA, T.,
YAMAMURA, H., KOBAYSAHI, T., & UENO, Y. (in press) Chronic toxicity
of nivalenol in mice: 2-year feeding trial with Fusarium nivale Fn
2B-moulded rice. Food Chem. Toxicol.
ORTI, D.L., HILL, R.H., LIDDLE, J.A., & NEEHAM, L.L. (1986) High-
performance liquid chromatography of mycotoxin metabolites in human
urine. J. anal. Toxicol., 10: 41-45.
OSBORNE, B.G. (1980) The occurrence of ochratoxin A in mouldy bread
and flour. Food Cosmet. Toxicol., 18: 615-617.
OSBORNE, B.G. & WILLIS, K. (1984) Studies into the occurrence of some
trichothecene mycotoxins in UK home-grown wheat and in imported
wheat. J. Sci. Food Agric., 35: 579-583.
OTOKAWA, M., SHIBAHARA, Y., & EGASHIRA, Y. (1979) The inhibitory
effect of T-2 toxin on tolerance induction of delayed-type
hypersensitivity in mice. Jpn. J. med. Sci. Biol., 32(1): 37-46.
PACE, J.G., WATTS, M.R., BURROWS, E.P., DINTERMAN, R.E., MATSON, C.,
HAUER, E.C., & WANNEMACHER, R.W., Jr (1985) Fate and distribution of
3H labeled T-2 mycotoxin in guinea pigs. Toxicol. appl. Pharmacol.,
80: 377-385.
PANG, V.F., LAMBERT, R.J., FELSBURG, P.J., BEASLEY, V.R., BUCK, W.B,.
& HASCHEK (1987a) Experimental T-2 toxicosis in swine following
inhalation exposure: effects on pulmonary and systemic immunity and
morphologic changes. Toxicol. Pathol., 15: 308-319.
PANG, V.F., LORENZANA, R.M., BEASLEY, V.R., BUCK, W.B., & HASCHEK,
W.M. (1987b) Experimental T-2 toxicosis in swine. III. Morphologic
changes following intravascular administration of T-2 toxic. Fundam.
appl. Toxicol., 8: 298-309.
PANG, V.F., LAMBERT, R.J., FELSBURG, P.J., BEASLEY, V.R., BUCK, W.B.,
& HASCHEK, W.M. (1988) Experimental T-2 toxicosis in swine following
inhalation exposure: clinical signs and effects on hematology, serum
biochemistry and immune response. Fundam. appl. Toxicol., 11:
100-109.
PARKER, G.W., WANNEMACHER, R.W., Jr, & GILMAN, F.G. (1984) The effect
of T-2 mycotoxin on the cardiovascular system in the guinea pig. Fed.
Proc., 43: 578.
PATTERSON, D.S.P., MATTHEWS, J.G., SHREEVE, B.J., ROBERTS, B.A.,
MCDONALD, S.M., & HAYES, A.W. (1979) The failure of trichothecene
mycotoxins and whole cultures of Fusarium tricinctum to cause
experimental haemorrhagic syndromes in calves and pigs. Vet. Rec.,
105(11): 252-253.
PATTERSON, D.S.P., SHREEVE, B.J., ROBERTS, B.A., BERRETT, S., BRUSH,
P.J., GLANCY, E.M., & KROGH, P. (1981) Effect on calves of barley
naturally contaminated with ochratoxin A and groundnut meal
contaminated with low concentrations of aflatoxin B1. Res. vet. Sci.,
31: 213-218.
PAULSCH, W.E., VAN EGMOND, H.P., & SCHULLER, P.L. (1982) Thin-layer
chromatographic method for analysis and chemical confirmation of
ochratoxin A in kidneys of pigs. In: Mycotoxins and Phycotoxins,
Proceedings of the 5th International IUPAC Symposium, Vienna,
Technical University Vienna publ., pp. 40-43.
PAVLOVIC, M., PLESTINA, R., & KROGH, P. (1979) Ochratoxin A
contamination of foodstuffs in an area with Balkan (endemic)
nephropathy. Acta pathol. microbiol. Scand. B87: 243-246.
PAWLOSKY, R.J. & MIROCHA, C.J. (1984) Structure of a metabolic
derivative of T-2 toxin (TC-6) based on mass spectrometry. J. agric.
food Chem., 32: 1420-1423.
PECKHAM, J.C., DOUPNIK, B., & JONES, O.H. (1971) Acute toxicity of
ochratoxins A and B in chicks. Appl. Microbiol., 21(3): 492-494.
PEDERSEN, E. & HANSEN, H.N. (1981) [Ochratoxin A in grain and grain
products.] Copenhagen, Stat. Levnedsm. Institute (Report F8l002) (in
Danish).
PEPELJNJAK, S. & CVETNIC, Z. (198l) [Ochratoxogenicity of
Aspergillus ochraceus strains from the territory of endemic
nephropathy.] Vet. Arch., 51: 101-103 (in Croatian).
PEPELJNJAK, S., BLAZEVIC, N., & CULJAK, N. (1982) Histopathological
changes and finding of ochratoxin A in organs of pig in the area of
endemic nephropathy in Yugoslavia. In: Mycotoxins and Phycotoxins,
Proceedings of the 5th International IUPAC Symposium, Vienna,
Technical University Vienna publ., pp. 346-348.
PESTKA, J.J., LEE, S.C., LAU, H.P., & CHU, F.S. (1981) Enzyme-
linked immunosorbent assay for T-2 toxin. J. Assoc. Off. Anal. Chem.,
58(12): 940A-944A.
PESTKA, J.J., LIN, W.S., & MITHS, E.R. (1987a) Emetic activity of the
trichothecene 15-acetyldeoxynivaleol in swine. Food chem. Toxicol.,
25: 855-858.
PESTKA, J.J., TAI, J.H., WITT, W.F., DIXON, D.E., & FORSELL, J.H.
(1987b) Suppression of immune response in the B6C3F1 mouse after
dietary exposure to the Fusarium mycotoxins deoxynivalenol
(vomitoxin) and zearalenone. Food chem. Toxicol., 25: 297-304.
PETKOVA-BOCHAROVA, T. & CASTEGNARO, M. (1985) Ochratoxin A
contamination of cereals in an area of high incidence of Balkan
endemic nephropathy in Bulgaria. Food Addit. Contam., 2: 267-270.
PETKOVA-BOCHAROVA, T., CHERNOZEMSKY, I.N., & CASTEGNARO, M. (1988)
Ochratoxin A in human blood in relation to Balkan endemic nephropathy
and urinary system tumours in Bulgaria. Food Addit. Contam., 5:
299, 301.
PIENAAR, J.G., KELLERMAN, T.S., & MARASAS, W.F.O. (1981) Field
outbreaks of leukoencephalomalacia in horses consuming maize infected
by Fusarium verticillioides (F. moniliforme) in South Africa. J. S.
Afr. Vet. Assoc., 52: 21-24.
PIER, A.C., CYSEWSKI, S.J., RICHARD, J.L., BAETZ, A.L., & MITCHELL,
L. (1976) Experimental mycotoxicoses in calves with aflatoxin,
ochratoxin, rubratoxin, and T-2 toxin. In: Proceedings of the 80th
Annual Meeting of the US Animal Health Association, Miami Beach,
Florida, 7-12 November, 1976, Richmond, Virginia, US Animal Health
Association, pp. 130-148.
POHLAND, A.E., SCHULLER, P.L., STEYN, P.S., & VAN EGMOND, H.P.
(1982) Physicochemical data for some selected mycotoxins. Pure appl.
Chem., 54: 2219-2284.
POHLAND, A.E., THORPE, C., TRUCKSESS, W., & EPPLEY, R. (1986) TLC and
HPLC methods for analysis of trichothecenes in commodities. In:
Richard, J. & Thurston, J., ed. Diagnosis of mycotoxicoses,
Dordrecht, Boston, Martinus Nijhoff Publishers, pp. 271-281.
POHLAND, A.E. & WOOD, G.E. (1987) Occurrence of mycotoxins in food.
In: Krogh, P., ed. Mycotoxins in foods, London, Academic Press, pp.
35-64.
POKROVSKIJ, V.I. & TUTELYAN, V.A. (1982) [Ergotism: a companion of
natural disaster.] Ter. Ark., 108-110 (in Russian).
POPPE, S.M., STUCKHARDT, J.L., & SZCZECH, G.M. (1983) Postnatal
behavioural effects of ochratoxin A in offspring of treated mice.
Teratology, 27: 293-300.
PRELUSKY, D.B., TRENHOLM, H.L., LAWRENCE, G.A., & SCOTT, P.M. (1984)
Nontransmission of deoxynivalenol (vomitoxin) to milk following oral
administration to dairy cows. J. environ. Sci. Health, B19(7): 593-
609.
PRELUSKY, D.B., VEIRA, D.M., & TRENHOLM, H.L. (1985) Plasma
pharmacokinetics of the mycotoxin deoxynivalenol following oral and
intravenous administration to sheep. J. environ. Sci. Health, B20:
603-624.
PRELUSKY, D.B., TRENHOLM, H.L., HAMILTON, R.M.G., & MILLER, J.D.
(1987) Transmission of [14C]deoxynivalenol to eggs following oral
administration to laying hens. J. agric. food Chem., 35: 182-186.
PRIOR, M.G. (1976) Mycotoxin in determinations on animal feedstuffs
and tissues in Western Canada. Can. J. comp. Med., 40: 75-79.
PRIOR, M.G. (198l) Mycotoxins in animal feedstuffs and tissues in
Western Canada 1975 to 1976. Can. J. comp. Med., 45: 116-119.
PRIOR, M.G. & SISODIA, C.S. (1978) Ochratoxicosis in white leghorn
hens. Poult. Sci., 57: 619-623.
PRIOR, M.G. & SISODIA, C.S. (1982) The effects of ochratoxin A on
the immune response of Swiss mice. Can. J. comp. Med., 46: 91-96.
PRIOR, M.G., SISODIA, C.S., & O'NEIL, J.B. (1976) Acute oral
ochratoxicosis in day-old white leghorns, turkeys and Japanese quail.
Poult. Sci., 55: 786-790.
PUCHLEV, A. (1973) La nephropathie endemique en Bulgarie. In:
Symposium sur la nephropathie endemique de l'Academie serbe des
sciences et des arts, Belgrade, pp. 15-27.
PUCHLEV, A., ed. (1974) Endemic nephropathy. In: Proceedings of the
2nd International Symposium on Endemic Nephropathy, Sofia, Bulgarian
Academy of Sciences, pp. 1-343.
PULS, R. & GREENWAY, J.A. (1976) Fusariotoxicosis from barley in
British Columbia. II. Analysis of suspected barley. Can. J. comp.
Med., 40(1): 16-19.
PURCHASE, I.F.H. & THERON, J.J. (1968) The acute toxicity of
ochratoxin A to rats. Food Cosmet. Toxicol., 6: 479-483.
QIUJIE, X., XIAOQIU, L., JIANLI, W., & YUNSIAN, L. (1988)
Trichothecenes in staple food from high incidence area of carcinoma
of esophagus and gastric cardia and their carcinogenic potential.
Zhonghua Zhongliu Zoshi, 10: 4-8.
RABIE, C.J., SYDENHAM, E.W., THEL, P.G., LUBBEN, A., & MARASAS,
W.F.O. (1986) T-2 toxin production by Fusarium acuminatum isolated
from oats and barley. Appl. environ. Microbiol., 52(3): 594-596.
RAFAI, P. & TUBOLY, S. (1982) Effect of T-2 toxin on adrenocortical
function and immune response in growing pigs. Zbl. Vet. Med., B29:
558-565.
RAJAKYLA, E., LAASASENAHO, K., & SAKKERS, P.J.D. (1987) Determination
of mycotoxins in grain by high-performance liquid chromatography and
thermospray liquid chromatography-mass spectrometry. J. Chromatogr.,
384: 391-402.
REICHMANN, K.G., BLANEY, B.J., CONNOR, J.K., & RUNGE, B.M. (1982)
The significance of aflatoxin and ochratoxin in the diet of
Australian chickens. Aust. vet. J., 58: 211-212.
REISS, J. (1973) Influence of the mycotoxins patulin and
diacetoxyscirpenol on fungi. J. gen. appl. Microbiol., 19(6):
415-420.
REISS, J. (1975) Mycotoxin poisoning of Allium cepa root tips. Part
2. Reduction of mitotic index and formation of chromosomal
aberrations and cytological abnormalities by patulin, rubratoxin B
and diacetoxyscirpenol. Cytologia, 39(4): 703-708.
REISS, J. (1977) Inhibition of urease by the mycotoxin patulin.
Naturwissenschaften, 64: 97.
RIBELIN, W.E., FUKUSHIMA, K., & STILL, P.E. (1978) The toxicity of
ochratoxin to ruminants. Can. J. comp. Med., 42: 172-176.
ROBISON, T.S., MIROCHA, C.J., KURTZ, H.J., BEHRENS, J.C., CHI, M.S.,
WEAVER, G.A., & NYSTROM, S.D. (1979a) Transmission of T-2 toxin
into bovine and porcine milk. J. dairy Sci., 62(4): 637-641.
ROBISON, T.S., MIROCHA, C.J., KURTZ, H.J., BEHRENS, J.C., WEAVER,
G.A., & CHI, M.S. (1979b) Distribution of tritium labeled T-2 toxin
in swine. J. agric. food Chem., 27(6): 1411-1413.
ROGERS, C.G. & HEROUX-METCALF, C. (1983) Cytotoxicity and absence of
mutagenic activity of vomitoxin (4-deoxynivalenol) in a hepatocyte-
mediated assay with V79 Chinese hamster lung cells. Cancer Lett.,
20: 29-35.
ROMER, T.R. (1986) Use of small charcoal/alumina cleanup columns in
determination of trichothecene mycotoxins in foods and feeds. J.
Assoc. Off. Anal. Chem., 69: 699-703.
ROMER, T.R., BOLING, T.M., & MACDONALD, J.L. (1978). Gas-liquid
chromatographic determination of T-2 toxin and diacetoxyscirpenol in
corn and mixed feeds. J. Assoc. Off. Anal. Chem., 61(4): 801-808.
ROOD, H.D., BUCK, W.B., & SWANSON, S.P. (1988a) Gas chromatographic
screening method for T-2 toxin, diacetoxyscirpenol, deoxynivalenol
and related trichothecenes in feeds. J. Assoc. Off. Anal. Chem., 71:
493-498.
ROOD, H.D., BUCK, W.B., & SWANSON, S.P. (1988b) Diagnostic screening
method for determination of trichothecene exposure in animals. J.
agric. food Chem., 36: 74-79.
ROSEN, J.D., ROSEN, R.T., & HARTMAN, T.G. (1986) Capillary gas
chromatography-mass spectrometry of several macrocyclic
trichothecenes. J. Chromatogr., 355: 241-251.
ROSEN, R.T. & ROSEN, J.D. (1984) Quantification and confirmation of
four Fusarium mycotoxins in corn by gas chromatography-mass
spectrometry. J. Chromatogr., 283: 223-230.
ROSENSTEIN, Y. & LAFARGE-FRAYSSINET, C. (1983) Inhibitory effect of
Fusarium T-2 toxin on lymphoid DNA and protein synthesis. Toxicol.
appl. Pharmacol., 70: 283-288.
ROSENSTEIN, Y., LAFARGE-FRAYSSINET, C., LESPINATS, G., LOISILLIER,
F., LAFONT, P., & FRAYSSINET, C. (1979) Immunosuppressive activity
of Fusarium toxins. Effects on antibody synthesis and skin grafts of
crude extracts of T-2 toxin and diacetoxyscirpenol. Immunology,
36(1): 111-118.
ROUSSEAUX, C.G. & SHEIFER, H.B. (1987) Maternal toxicity,
embryolethality and abnormal fetal development in CD-1 mice following
one oral dose of T-2 toxin. J. appl. Toxicol., 7: 281-288.
ROUSSEAUX, C.G., SHIEFER, H.B., & HANCOCK, D.S. (1986) Reproductive
and teratological effects of continuous low-level dietary T-2 toxin
in female CD-1 mice for two generations. J. appl. Toxicol., 6:
179-184.
ROUSSEAU, D.M. & VAN PETEGHEM, C.H. (1989) Spontaneous occurrence of
ochratoxin A residues in porcine kidneys in Belgium. Environ. Contam.
Toxicol., 42: 181-186.
ROUSSEAU, D.M., CANDLISH, A.A.G., SLEGERS, G.A., VAN PETEGHEM, C.H.,
STINSON, W.H., & SMITH, J.E. (1987) Detection of ochratoxin A in
porcine kidneys by a monoclonal antibody-based radioimmunoassay.
Appl. environ. Microbiol., 53: 514-518.
RUKMINI, C. & BHAT, R.V. (1978) Occurrence of T-2 toxin in Fusarium
incarnatum infested sorghum from India. J. agric. food Chem., 26
(3): 647-649.
RUKMINI, C., PRASAD, J.S., & RAO, K. (1980) Effect of feeding T-2
toxin to rats and monkeys. Food Cosmet. Toxicol., 18(3): 267-270.
RUSCH, M.E. & STAHELIN, H. (1965) [Some biological effects of the
cytostatic agent verrucarin A.] Arzneimittelforschung, 15: 893-897
(in German).
RUTQVIST, L., BJORKLUND, N.-E., HULT, K., & GATENBECK, S. (1977)
Spontaneous occurrence of ochratoxin residues in kidneys of fattening
pigs. Zbl. Veterinaermed., A24(5): 402-408.
RUTQVIST, L., BJORKLUND, N.-E., HULT, K., HOKBY, E., & CARLSSON, B.
(1978) Ochratoxin A as the cause of spontaneous nephropathy in
fattening pigs. Appl. environ. Microbiol., 36: 920-925.
RYU, J.C., SHIRAKI, N., & UENO, Y. (1987) Effects of drugs and
metabolic inhibitions on the acute toxicity of T-2 toxin in mice.
Toxicon, 25: 743-750.
RYU, J.-C., OHTSUBO, K., IZUMIYAMA, N., NAKAMURA, K., TANAKA, T.,
YAMAMURA, H., & UENO, Y. (1988) The acute and chronic toxicities of
nivalenol in mice. Fundam. appl. Toxicol., 11: 38-47.
SAITO, M. & OHTSUBO, K. (1974) Trichothecene toxins of Fusarium
species. In: Purchase, I.F.H., ed. Mycotoxins, Amsterdam, Oxford, New
York, Elsevier Science Publishers, pp. 263-281.
SAITO, M., HORIUCHI, T., OHTSUBO, K., HATANAKA, Y., & UENO, Y.
(1980) Low tumor incidence in rats with long-term feeding of
fusarenon-X a cytotoxic trichothecene produced by Fusarium nivale.
Jpn. J. exp. Med., 50(40): 293-302.
SAKAMOTO, T., SWANSON, S.P., YOSHIZAWA, T., & BUCK, W.B. (1986)
Structures of new metabolites of diacetoxyscirpenol in the excreta of
orally administered rats. J. agric. food Chem., 34: 698-701.
SANDOR, G., GLAVITS, R., VAJDA, L., VANYI, A., & KROGH, P. (1982)
Epidemiological study of ochratoxin A-associated porcine nephropathy
in Hungary. In: Mycotoxins and Phycotoxins, Proceedings of the 5th
International IUPAC Symposium, Technical University Vienna publ.,
Vienna, pp. 349-352.
SANO, A., ASABE, Y., TAKITANI, S., & UENO, Y. (1982).
Fluorodensitometric determination of trichothecene mycotoxins with
nicotinamide and 2-acetylpyridine on a silica gel layer. J.
Chromatogr., 235: 257-265.
SANO, A., MATSUTANI, S., SUZUKI, M., & TAKITANI, S. (1987) High-
performance liquid chromatographic method for determining
trichothecene mycotoxins by post-column fluorescence derivatization.
J. Chromatogr., 410: 427-436.
SANSING, G.A., LILLEHOJ, E.B., DETROY, R.W., & MILLER, M.A. (1976)
Synergistic toxic effects of citrinin, ochratoxin A, and penicillic
acid in mice. Toxicon, 14: 213-219.
SARKISOV, A.Ch., KVASCHINA, E.S., KORNEEV, N.E., KOROLEVA, V.P.,
GERASIMOVA, P.N., & AKILOVA, N.S. (1944) [Harmfulness of cereals
over-wintered in the field.] Veterinariya, 11-12: 39-41. (in
Russian).
SATO, N., UENO, Y., & ENOMOTO, M. (1975) Toxicological approaches
to the toxic metabolites of Fusaria. Part 8. Acute and subacute
toxicities of T-2 toxin in cats. Jpn. J. Pharmacol., 25(3): 263-270.
SATO, N. & UENO, Y. (1977) Comparative toxicities of
trichothecenes. In: Rodricks, J.V., Hesseltine, C.W., & Mehlman,
M.A., ed. Mycotoxins in human and animal health, Park Forest South,
Illinois, Pathotox Publishers, pp. 295-307.
SATO, N., ITO, T., KUMADA, H., UENO, Y., ASANO, K., SAITO, M.,
OHTSUBO, K., UENO, I., & HATANAKA, Y. (1978) Toxicological
approaches to the metabolites of Fusaria. XIII. Hematological changes
in mice by a single and repeated administrations of trichothecenes.
J. toxicol. Sci., 3(4): 335-356.
SATO, S., OHYA, T., HOMMA, S., & TATSUNO, T. (1981) [Effects of
fusarenon-X on serological responses of chicks inoculated with
newcastle disease vaccines.] Proc. Jpn. Assoc. Mycotoxicol., 13:
43-45 (in Japanese).
SCHIEFER, H.B., ROUSSEAUX, C.G., HANCOCK, D.S., & BLAKLEY, B.R.
(1987) Effects of low-level long-term oral exposure to T-2 toxin in
CD-1 mice. Food chem. Toxicol., 25: 593-601.
SCHMIDT, R. & DOSE, K. (1984) HPLC: A tool for the analysis of T-2
toxin and HT-2 toxin in cereals. J. anal. Toxicol., 8: 43-45.
SCHINDLER, D. (1974) Two classes of inhibitors of peptidyl
transferease activity in eukaryotes. Nature (Lond.), 249(5452):
38-41.
SCHINDLER, D., GRANT, P., & DAVIES, J. (1974) Trichodermin
resistance mutation affecting eukaryotic ribosomes. Nature (Lond.),
248(5448): 535-536.
SCOTT, P.M. (1977) Penicillium mycotoxins. In: Wyllie, T.D. &
Morehouse, L.G., ed. Mycotoxic fungi, mycotoxins, mycotoxicoses, New
York, Marcel Dekker, pp. 283-356.
SCOTT, P.M. (1982) Assessment of quantitative methods for
determination of trichothecenes in grains and grain products. J.
Assoc. Off. Anal. Chem., 65(4): 876-883.
SCOTT, P.M. (1984) Effects of food processing on mycotoxins. J. food
Prot., 47(6): 489-499.
SCOTT, P.M. & KANHERE, S.R. (1986) Comparison of column phases for
separation of derivatized trichothecenes by capillary gas
chromatography. J. Chromatogr., 368: 374-380.
SCOTT, P.M. & LAWRENCE, G.A. (1980) Analysis of ergot alkaloids in
flour. J. agric. food Chem., 28: 1258-1261.
SCOTT, P.M. & LAWRENCE, G.A. (1982) Losses of ergot alkaloids
during making of bread and pancakes. J. agric. food Chem., 30:
445-450.
SCOTT, P.M., LAWRENCE, J.W., & VAN WALBEEK, W. (1970) Detection of
mycotoxins by thin-layer chromatography: application to screening of
fungal extracts. Appl. Microbiol., 20(5): 839-842.
SCOTT, P.M., WALBEEK, W., VAN, KENNEDY, B., & ANYETI, D. (1972)
Mycotoxins (ochratoxin A, citrinin, and sterigmatocystin) and
toxigenic fungi in grains and other agricultural products. J. agric.
food Chem., 20: 1103-1109.
SCOTT, P.M., HARWIG, J., & BLANCHFIELD, B.J. (1980) Screening
Fusarium strains isolated from over-wintered Canadian grains for
trichothecenes. Mycopathologia, 72(3): 175-180.
SCOTT, P.M., LAU, P.Y., & KANHERE, S.R. (1981) Gas chromatography
with electron capture and mass spectrometric detection of
deoxynivalenol in wheat and other grains. J. Assoc. Off. Anal. Chem.,
64(6): 1364-1371.
SCOTT, P.M., KANHERE, S.R., LAU, P.-Y., DEXTER, J.E., & GREENHALGH,
R. (1983) Effects of experimental flour milling and bread baking on
retention of deoxynivalenol (vomitoxin) in hard red spring wheat.
Cereal Chem., 60: 421-424.
SCOTT, P.M., NELSON, K., KANHERE, S.R., KARPINSKI, K.F., HAYWARD, S.,
NIESH, G.A., & TEICH, A.H. (1984a) Decline in deoxynivalenol
(vomitoxin) concentrations in 1983 Ontario winter wheat before
harvest. Appl. environ. Microbiol., 48(6): 884-886.
SCOTT, P.M., KANHERE, S.R., DEXTER, J.E., BRENNAN, P.W., & TRENHOLM,
H.L. (1984b) Distribution of the trichothecene mycotoxin
deoxynivalenol (Vomitoxin) during the milling of naturally
contaminated hard red spring wheat and its fate in baked products.
Food Addit. Contam., 1(4): 313-323.
SCOTT, P.M., KANHERE, S.R., & TARTER, E.J. (1986) Determination of
nivalenol and deoxynivalenol in cereals by electron-capture gas
chromatography. J. Assoc. Off. Anal. Chem., 69: 889-893.
SEITZ, L.M., YAMAZAKI, W.T., CLEMENTS, R.L., MOHR, H.E., & ANDREWS,
L. (1985) Distribution of deoxynivalenol in soft wheat mill
streams. Cereal Chem., 62(6): 467-469.
SHEPHERD, M.J. & GILBERT, J. (1988) Long-term stability of
deoxynivalenol standard reference solutions. J. agric. food Chem.,
36: 305-308.
SHIMIZU, T., NAKANO, N., MATSUI, T., & AIBARA, K. (1979)
Hypoglycemia in mice administered fusarenon-X. Jpn. J. med. Sci.
Biol., 32(4): 189-198.
SHOTWELL, O.L., HESSELTINE, C.W., & GOULDEN, M.L. (1969) Ochratoxin
A: occurrence as natural contaminant of a corn sample. Appl.
Microbiol., 17(5): 765-766.
SHOTWELL, O.L., HESSELTINE, C.W., VANDEGRAFT, E.E., & GOULDEN, M.L.
(1971) Survey of corn from different regions for aflatoxin,
ochratoxin, and zearalenone. Cereal Sci. Today, 16(9): 266-273.
SHOTWELL, O.L., GOULDEN, M.L., & HESSELTINE, C.W. (1976) Survey of
US wheat for ochratoxin and aflatoxin. J. Assoc. Off. Anal. Chem.,
59: 122-124.
SHOTWELL, O.L., NENNETT, G.A., STUBBLEFIELD, R.D., SHANNON, G.M.,
KWOLEK, W.F., & PLATTNER, R.D. (1985) Deoxynivalenol in hard red
winter wheat: Relationship between toxin levels and factors that
could be used in grading. J. Assoc. Off. Anal. Chem., 68: 954-957.
SHREEVE, B.J., PATTERSON, D.S.P., & ROBERTS, B.A. (1979) The carry-
over of aflatoxin, ochratoxin and zearalenone from naturally
contaminated feed to tissues, urine and milk of dairy cows. Food
Cosmet. Toxicol., 17: 151-152.
SIDDIQUI, M.R. & KHAN, I.D. (1973) Renaming Claviceps microcephala
ergot fungus on Pennisetum typhoides in India as Claviceps
fusiformis. Trans. Mycol. Soc. Jpn, 144: 195-198.
SIEGFRIED, R. (1977) [ Fusarium toxin (trichothecene toxin) in feed
corn.] Landwirtsch. Forsch., Sonderh., 34(1): 37-43 (in German).
SIGG, H.P., MAULI, R., FLURY, E., & HANSER, D. (1965) The
constitution of diacetoxyscirpenol. Helv. Chim. Acta, 48: 962-988.
SINTOV, A., BIALER, M., & YAGEN, B. (1986) Pharmacokinetics of T-2
toxin and its metabolite HT-2 toxin, after intravenous administration
in dogs. Drug Metab. Disp., 14: 250-254.
SINTOV, A., BIALER, M., & YAGEN, B. (1987) Pharmacokinetics of T-2
tetraol, a urinary metabolite of the trichothecene mycotoxin, T-2
toxin, in dogs. Xenobiotica, 17: 941-950.
SIRAJ, M.Y., PHILLIPS, T.D., & HAYES, A.W. (1981) Effects of the
mycotoxins citrinin and ochratoxin A on hepatic mixed-function
oxidase and adenosinetriphosphatase in neonatal rats. J. Toxicol.
environ. Health, 8: 131-140.
SIREN, A.L. & FEUERSTEIN, G. (1986) Effect of T-2 toxin on regional
blood flow and vascular resistance in the conscious rat. Toxic. appl.
Pharmacol., 38: 438-444.
SIRIWARDANA, T.M.G. & LAFONT, P. (1978) New sensitive biological
assay for 12,13-epoxytrichothecenes. Appl. environ. Microbiol.,
35(1): 206-207.
SMALLEY, E.B., MARASAS, W.F.O., STRONG, F.M., BAMBURG, J.R., NICHOLS,
R.E., & KOSARI, N.R. (1970) Mycotoxicosis associated with moldy corn.
In: Herzberg, M., ed. Proceedings of the first US-Japan Conference on
Toxic Micro-organisms, Mycotoxins, Botulism, Honolulu, Hawaii, 7-10
October, 1968, Washington, DC, US Department of the Interior and UJNR
Joint Panels on Toxic Micro-organisms, pp. 163-173.
SMITH, R.D., UDSETH, H.R., & WRIGHT, B.W. (1985) Rapid and high
resolution capillary fluid chromatography (SFC) and SFC/MS of
trichothecene mycotoxins. J. chromatogr. Sci., 23: 192-199.
SORSA, M., LINSSAINMAA, K., PAITTELA, M., & ILUS, T. (1980)
Evaluation of the mutagenicity of epoxytrichothecene mycotoxins in
Drosophilia melanogaste. Hereditas, 92: 163-165.
SPEERS, G.M., MIROCHA, C.J., CHRISTENSEN, C.M., & BEHRENS, J.C.
(1977) Effects on laying hens of feeding corn invaded by 2 species
of Fusarium and pure T-2 mycotoxin. Poult. Sci., 56(1): 98-102.
STACK, M.E., MISLIVEC, P.B., GIBSON, R., POHLAND, A.E., & DENIZEL, T.
(1982) Mycotoxins produced by isolates of Aspergillus ochraceus
found in coffee. In: Mycotoxins and Phycotoxins, Proceedings of the
5th International IUPAC Symposium, Vienna, Technical University
Vienna, pp. 195-199.
STAFFORD, M.E. & MCLAUGHLIN, C.S. (1973) Trichodermin, a possible
inhibitor of the termination process of protein synthesis. J. cell.
Physiol., 82: 121-128.
STAHELIN, H., KALBERER-RUESCH, M.E., SIGNER, E., & LAZARY, S. (1968)
[Some biological effects of the cytostatic diacetoxyscirpenol.]
Arzneimittelforschung, 18: 989-994 (in German).
STAHL, C., VANDERHOEF, L.N., SIEGEL, N., & HELGESON, J.P. (1973)
Fusarium tricinctum T-2 toxin inhibits auxin promoted elongation in
soybean hypocotyl. Plant Physiol., 52(6): 663-666.
STAHR, H.M., HYDE, W., LEDERDAL, D., & PFEIFFER, R. (1981)
Trichothecene mycotoxin analysis for veterinary diagnostic
toxicology. Abstracts 95th Annual Meeting of AOAC., 191: 65.
STANFORD, G.K., HOOD, R.D., & HAYES, A.W. (1975) Effect of prenatal
administration of T-2 toxin to mice. Res. Commun. chem. Pathol.
Pharmacol., 10(4): 743-746.
STEYN, P.S. (1977) Mycotoxins, excluding aflatoxin, zearalenone and
the trichothecenes. In: Rodericks, J.V., Hesseltine, C.W., &
Mehlman, M.A., ed. Mycotoxins in human and animal health, Park Forest
South, Illinois, Pathotox Publishers, pp. 419-467.
STEYN, P.S. (1984) Ochratoxins and related dihydro-iso-coumarins.
In: Betina, V., ed. Mycotoxins: production, isolation, separation,
and purification, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 183-216.
STEYN, P.S., VLEGGAAR, R., DU PREEZ, N.P., BLYTH, A.A., & SEEGERS,
J.C. (1975) The in vitro toxicity of analogs of ochratoxin A in
monkey kidney epithelial cells. Toxicol. appl. Pharmacol., 32:
198-203.
STOREN, O., HOLM, H., & STORMER, F.C. (1982) Metabolism of
ochratoxin A by rats. Appl. environ. Microbiol., 44: 785-789.
STORMER, F.C. & PEDERSEN, J. (1980) Formation of 4-
hydroxyochratoxin A from ochratoxin A by rat liver microsomes.
Appl. environ. Microbiol., 39: 971-975.
STORMER, F.C., HANSEN, C.E., PEDERSEN, J.I., HVISTENDAHL, G., &
AASEN, A.J. (1981) Formation of (4R)- and (AS)-4-hydroxyochratoxin
A from ochratoxin A by liver microsomes from various species. Appl.
environ. Microbiol., 42: 1051-1056.
STOYANOV, I.S., CHERNOZEMSKY, I.N., NICOLOV, I.G., STOICHEV, I.I., &
PETKOVA-BOCHAROVA, T.K. (1978) Epidemiologic association between
endemic nephropathy and urinary system tumours in an endemic region.
J. chron. Dis., 31: 721-724.
SUNDHEIM, L., NAGAYAMA, S., KAWAMURA, O., TANAKA, T., BRODAL, G., &
UENO, Y. (1988) Trichothecenes and zearalenone in Norwegian barley
and wheat. Norw. J. agric. Sci., 2: 49-59.
SUZUKI, S. & SATOH, T. (1973) Effect of ochratoxin A on tissue
glycogen levels in rats. Jpn. J. Phamacol., 23: 415-419.
SUZUKI, S., SATOH, T., & YAMAZAKI, M. (1975) Effect of ochratoxin A
on carbohydrate metabolism in rat liver. Toxicol. appl. Pharmacol.,
32(1): 116-122.
SUZUKI, S., SATOH, T., & YAMAZAKI, M. (1977) The pharmacokinetics
of ochratoxin A in rats. Jpn. J. Pharmacol., 27: 735-744.
SUZUKI, T., KURISU, M., HOSHINO, Y., ICHINOE, M., NOSE, N., TOKUMARU,
Y., & WATANABE, Y. (1980) [Production of trichothecene mycotoxins of
Fusarium species in wheat and barley harvested in Saitama Prefecture,
Japan.] J. Food Hyg. Soc. Jpn, 21(1): 43-49 (in Japanese).
SWANSON, S.P., NICOLETTI, J., ROOD,.H.D., Jr, BUCK, W.B., COTE, L.M.,
& YOSHIZAWA, T. (1987) Metabolism of three trichothecene mycotoxins,
T-2 toxin, diacetoxyscirpenol and deoxynivalenol, by bovine rumen
microorganisms. J. agric. food Chem., 32: 1181-1183.
SWANSON, S.P., RAMASWAMY, V., BEASLEY, V.R., BUCK, W.B., &
BURMEISTER, H.H. (1983) Gas-liquid chromatographic determination of
T-2 toxin in plasma. J. Assoc. Off. Anal. Chem., 66: 909.912.
SWANSON, S.P., DAHLEM, A.M., ROOD, H.D., COTE, L.M., & YOSHIZAWA, T.
(1986) Gas chromatographic analysis of milk for deoxynivalenol and
its metabolite DOM-1. J. Assoc. Off. Anal. Chem., 69: 41-43.
SYLVIA, V.L., PHILLIPS, T.D., CLEMINT, B.A., GREEN, J.L., KUBENA,
L.F., & HEIDELBAUGH, N.D. (1986) Determination of deoxynivalenol
(vomitoxin) by high performance liquid chromatography with
electrochemical detection. J. Chromatogr., 362: 79-85.
SZATHMARY, C.I. (1983) Trichothecene toxicoses and natural
occurrence in Hungary. In: Ueno, Y., ed. Developments in food
science. IV. Trichothecenes, New York, Elsevier, pp. 229-250.
SZATHMARY, C.I. & RAFAI, P. (1978) Refusal of food contaminated
with T-2 fusariotoxin. Magy. Allatorv. Lapja, 33: 685-688.
SZCZECH, G.M. & HOOD, R.D. (1981) Brain necrosis in mouse fetuses
transplacentally exposed to the mycotoxin ochratoxin A. Toxicol.
appl. Pharmacol., 57: 127-137.
SZCZECH, G.M., CARLTON, W.W., & TUITE, J. (1973a) Ochratoxicosis in
Beagle dogs. I. Clinical and clinicopathological features. Vet.
Pathol., 10(2): 135-154.
SZCZECH, G.M., CARLTON, W.W., & TUITE, J. (1973b) Ochratoxicosis in
Beagle dogs. II. Pathology. Vet. Pathol., 10(3): 219-231.
SZCZECH, G.M., CARLTON, W.W., TUITE, J., & CALDWELL, R. (1973c)
Ochratoxin A toxicosis in swine. Vet. Pathol., 10(4): 347-364.
SZEBIOTKO, K., CHELKOWSKI, J., DOPIERALA, G., GODLEWSKA, B., &
RADOMYSKA, W. (1981) Mycotoxins in cereal grain. Part I.
Ochratoxin, critrinin, sterigmatocystin, penicillic acid and
toxigenic fungi in cereal grain. Nahrung, 25: 415-421.
TAKITANI, S., ASABE, Y., KATO, T., SUZUKI, M., & UENO, Y. (1979)
Spectrodensitometric determination of trichothecene mycotoxins with
4-( p-nitrobenzyl)pyridine on silica gel thin-layer chromatograms. J.
Chromatogr., 172: 335-342.
TAMM, C. (1977) Chemistry and biosynthesis of trichothecenes. In:
Rodricks, J.V., Hesseltine, C.W., & Mehlman, M.A., ed. Mycotoxins in
human and animal health, Park Forest South, Illinois, Pathotox
Publishers, pp. 209-228.
TANAKA, T., HASEGAWA, A., MATSUKI, Y., ISHII, K., & UENO, Y. (1985a)
Improved methodology for the simultaneous detection of the
trichothecene mycotoxins deoxynivalenol and nivalenol in cereals.
Food Addit. Contam., 2: 125-137.
TANAKA, T., HASEGAWA, A., MATSUKI, Y., & UENO, Y. (1985b) A survey
of the occurrence of nivalenol, deoxynivalenol and zearalenone in
foodstuffs and health foods in Japan. Food Addit. Contam., 2: 259-
265.
TANAKA, T., HASEGAWA, A., YAMAMOTO, S., LEE, U., SUGIURA, Y., & UENO,
Y. (1988) Worldwide contamination of cereals by the Fusarium
mycotoxins nivalenol, deoxynivalenol and zearalenone. 1. Survey of 19
countries. J. agric. food Chem., 36: 979-983.
TANAKA, T., HASEGAWA, A., YAMAMOTO, S., MATSUKI, Y., & UENO, Y.
(1986) Residues of Fusarium mycotoxins, nivalenol, deoxynivalenol
and zearalenone, in wheat and processed food after milling and
baking. J. Food Hyg. Soc. Jpn, 27(6): 653-655.
TANAKA, T., MATSUDA, Y., TOYASAKI, N., OGAWA, K., MATSUKI, Y., &
UENO, Y. (1977) Screening of trichothecene-producing Fusarium
species from river sediments by mammalian cell culture techniques.
Proc. Jpn. Assoc. Mycotoxicol., 5/6: 50-53.
TASHIRO, F., HIRAI, K., & UENO, Y. (1979) Inhibitory effects of
carcinogenic mycotoxins on deoxyribonucleic acid-dependent
ribonucleic acid polymerase and ribonulease H. Appl. environ.
Microbiol., 38(2): 191-196.
TATSUNO, T., SAITO, M., ENOMOTO, M., & TSUNODA, H. (1968) Nivalenol,
a toxic principle of Fusarium nivale. Chem. pharm. Bull., 16(12):
2519-2520.
TEICH, A.H. & HAMILTON, J.R. (1985) Effect of cultural practices,
soil phosphorus, potassium and pH on the incidence of Fusarium head
blight and deoxynivalenol levels in wheat. Appl. environ. Microbiol.,
49(6): 1429-1431.
TERAO, K., KERA, K., & YAZIMA, T. (1978) The effects of
trichothecene toxins on the bursa of Fabricius in day-old chicks.
Virchows Arch., B27(4): 359-370.
THACKER, H.L. & CARLTON, W.W. (1977) Ochratoxin A mycotoxicosis in
the guinea pig. Food. Cosmet. Toxicol., 15: 563-574.
THIEL, P.G., MEYER, C.J., & MARASAS, W.F.O. (1982) Natural
occurrence of moniliformin together with deoxynivalenol and
zearalenone in Transkeian corn. J. agric. food Chem., 30(2): 308-312.
THIGPEN, J.T., VAUGHN, C., & STUCKEY, W.J. (1981) Phase II trial of
anguidine in patients with sarcomas unresponsive to prior
chemotherapy: A southwest oncology group study. Cancer Treat. Rep.,
65(9-10): 881-882.
THUST, R., KNEIST, S., & HUEHNE, V. (1983) [Genotoxicity of
Fusarium mycotoxins (nivalenol, T-2 toxin and zearalenone) in Chinese
hamster V79-E cells in vitro.] Arch. Geschwulstforsch., 53: 9-15
(in German).
TIEBACH, R., BLAAS, W., KELLERT, M., STEINMEYER, S., & WEBER, R.
(1985) Confirmation of nivalenol and deoxynivalenol by on-line liquid
chromatography-mass spectrometry and gas-chromatography. Comparison
of methods. J. Chromatogr., 318: 103-111.
TOCHINAI, Y. (1933) [On scab of cereals and grains. I and II.]
Bochugai Zassi, 20: 106-114, 175-188 (in Japanese).
TOMAR, R.S., BLAKLEY, B.R., & COTEAU, W.E. (1988) In vitro effects of
T-2 toxin of the mitogen responsiveness and antibody-producing
ability of human lymphocytes. Toxicol. Lett., 40: 109-117.
TRENHOLM, H.L., COCHRANE, W.P., COHEN, H., ELLIOT, J.I., FARNWORTH,
E.R., FRIEND, D.W., HAMILTON, R.M.H., NEISH, G.A., & STANDISH, J.F.
(1981) Survey of vomitoxin contamination of the 1980 white winter
wheat crop in Ontario, Canada. J. Am. Oil Chem. Soc., 58: 992-994.
TRENHOLM, H.L., HAMILTON, R.M.G., FRIEND, D.W., THOMPSON, B.K., &
HARTIN, K.E. (1984) Feeding trials with vomitoxin (deoxynivalenol)-
contaminated wheat: effects on swine, poultry and dairy cattle. J.
Am. Vet. Med. Assoc., 185: 527-531.
TRENHOLM, H.L., WARNER, R.M., & PRELUSKY, D.B. (1985) Assessment of
extraction procedures in the analysis of naturally contaminated grain
products for deoxynivalenol (vomitoxin). J. Assoc. Off. Anal. Chem.,
68: 645-659.
TRUCKSESS, M.W., NESHEIM, S., & EPPLEY, R.M. (1984) Thin layer
chromatographic determination of deoxynivalenol in wheat and corn. J.
Assoc. Off. Anal. Chem., 67: 40-43.
TRUCKSESS, M.W., FLOOD, M.W., & PAGE, S.W. (1986a) Thin layer
chromatographic determination of deoxynivalenol in processed grain
products. J. Assoc. Off. Anal. Chem., 69: 35-36.
TRUCKSESS, M.W., WOOD, G.E., EPPLEY, R.M., YOUNG, K., COHEN, C.K.,
PAGE, S.W., & POHLAND, A.E. (1986b) Occurrence of deoxynivalenol in
grain products. In: Biodeterioration. VI, Aberystwyth, The Cambrian
News Ltd., pp. 243-247.
TRUCKSESS, M.W., FLOOD, M.T., MOSSOBA, M.M., & PAGE, S.W. (1987) High
performance thin-layer chromatographic determination of
deoxynivalenol, fusarenon-X and nivalenol in barley, corn and wheat.
J. agric. food Chem., 35: 445-448.
TRUSAL, L.R. (1985) Morphological changes in CHO and VERO cells
treated with T-2 mycotoxin. Correlation with inhibition of protein
synthesis. Cell Biochem. Funct. 3: 205-216.
TRUSAL, L.R. & O'BRIEN, J.C. (1986) Ultrastructural effects of T-2
mycotoxins on rat hepatocytes in vitro. Toxicon, 24: 481-488.
TRYPHONAS, H., O'GRADY, L., ARNOLD, D,L., MCGUIRE, F., KARPINSKY, K.,
& VESONDER, R.F. (1984) Effect of deoxynivalenol (vomitoxin) on the
humoral immunity of mice. Toxicol. Lett., 23: 17-24.
TRYPHONAS, H., IVERSON, F., SO, Y., NERA, E.A., MCGUIRE, P.F.,
O'GRADY, L., CLAYSON, D.B., & SCOTT, P.M. (1986) Effects of
deoxynivalenol (vomitoxin) on the humoral and cellular immunity of
mice. Toxicol. Lett., 30: 137-150.
TSENG, T., YUAN, G., TSENG, J., SHASO, E., & MIROCHA, C.J. (1983)
Natural occurrence of Fusarium mycotoxins in grains and feeds in
Taiwan. Proc. Int. Mycotoxins Symposium, Abstr. 1.5, Sydney,
Australia.
TSUNODA, H., TSURUTA, O., MATSUNAMI, S., & ISHII, S. (1957) [Studies
on the microorganisms which deteriorate the stored cereals and
grains. XIV.] Shokuryokenkyujo Hokoku, 12: 27-33 (in Japanese).
UENO, Y. (1970) Inhibition of protein synthesis in animal cells by
nivalenol and related metabolites: toxic principles of rice-M
infested with Fusarium nivale. In: Herzberg, M., ed. Proceedings of
the First US-Japan Conference on Toxic Microorganisms, Washington,
DC, US Department of the Interior, UNJR Joint Panel of Toxic
Microorganisms, pp.76-79.
UENO, Y. (1977) Trichothecenes: overview address. In: Rodricks,
J.V., Hesseltine, C.W., & Mehlman, M.A., ed. Mycotoxins in human and
animal health, Park Forest South, Illinois, Pathotox Publishers, pp.
189-228.
UENO, Y. (1980a) Toxicological evaluation of trichothecene
mycotoxins. In: Eaker, D. & Wadstrom, T., ed. Natural toxins.
Proceedings of the International Symposium on Animal and Plant
Microbial Toxins, Uppsala, 1979, Elmsford, New York, Pergamon Press,
Vol. 6, pp. 663-671.
UENO, Y. (1980b) Trichothecene mycotoxins: Mycology, chemistry, and
toxicology. In: Draper, H.H., ed. Advances in nutritional research,
New York, London, Plenum Press, Vol. 3, pp. 301-356.
UENO, Y., ed. (1983) General toxicology. In: Developments in food
science. IV. Trichothecenes, New York, Elsevier, pp. 135-146.
UENO, Y. & FUKUSHIMA, K. (1968) Inhibition of protein and DNA
synthesis in Ehrlich ascites tumor by nivalenol, a toxic principle of
Fusarium nivale growing rice-M. Experientia (Basel), 24(10): 1032-
1033.
UENO, Y. & KUBOTA, K. (1976) DAN-attacking ability of carcinogenic
mycotoxins in recombination-deficient mutant cells of Bacillus
subtilis. Cancer Res., 36: 445-451.
UENO, Y. & MATSUMOTO, H. (1975) Inactivation of some thiol enzymes
by trichothecene mycotoxins from Fusarium spp. Chem. pharm. Bull.,
23(10): 2439-2442.
UENO, Y. & SHIMADA, N. (1974) Reconfirmation of the specific nature
of reticulocytes bioassay system to trichothecene mycotoxins of
Fusarium spp. Chem. pharm. Bull., 22(11): 2744-2746.
UENO, Y. & YAMAKAWA, H. (1970) Antiprotozoal activity of scirpene
mycotoxins of Fusarium nivale FN 2B. Jpn. J. exp. Med., 40(5):
385-390.
UENO, Y., HOSOYA, M., MORITA, Y., UENO, I., & TATSUNO, T. (1968)
Inhibition of the protein synthesis in rabbit reticulocyte by
nivalenol, a toxic principle isolated from Fusarium nivale growing on
rice. J. Biochem. (Tokyo), 64(4): 479-485.
UENO, Y., HOSOYA, M., & ISHIKAWA, Y. (1969a) Inhibitory effects of
mycotoxins on protein synthesis in rabbit reticulocytes. J. Biochem.
(Tokyo), 66(3): 419-422.
UENO, Y., UENO, I., TATSUNO, T., OHOKUBO, K., & TSUNODA, H. (1969b)
Fusarenon-X, atopic principle of Fusarium nivale-culture filtrate.
Experientia (Basel), 25: 1062.
UENO, Y., ISHIKAWA, Y., AMAKAI, K., NAKAJIMA, M., SAITO, M.,
ENOMOTO, M., & OHTSUBO, K. (1970) Comparative study on skin-
necrotizing effect of scirpene metabolites of Fusaria. Jpn. J. exp.
Med., 40(1): 33-38.
UENO, Y., UENO, I., IITOI, Y., TSUNODA, H., ENOMOTO, M., & OHTSUBO,
K. (1971) Toxicological approaches to the metabolites of Fusaria.
Part 3. Acute toxicity of fusarenon-X. Jpn. J. exp. Med., 41(6): 512-
539.
UENO, Y., ISHII, K., SAKAI, K., KANAEDA, S., TSUNODA, H., TANAKA, T.,
& ENOMOTO, M. (1972) Toxicological approaches to the metabolites of
Fusarium. Part 4. Microbial survey on bean hulls poisoning of horses
with the isolation of toxic trichothecenes, neosolaniol and T-2 toxin
of Fusarium solani M-1-1. Jpn. J. exp. Med., 42(3): 187-203.
UENO, Y., ISHII, K., SATO, N., SHIMADA, N., TSUNODA, H., SAWANO, M.,
& ENOMOTO, M. (1973a) Screening of trichothecene producing fungi
and the comparative toxicity of isolated mycotoxins. Jpn. J.
Pharmacol., 23(Suppl.): 133.
UENO, Y., NAKAJIMA, M., SAKAI, K., ISHII, K., SATO, M., & SHIMADA, N.
(1973b) Comparative toxicology of trichothecene mycotoxins.
Inhibition of protein synthesis in animal cells. J. Biochem. (Tokyo),
74(2): 285-296.
UENO, Y., SATO, N., ISHII, K., SAKAI, K., TSUNODA, H., & ENOMOTO, M.
(1973c) Biological and chemical detection of trichothecene
mycotoxins of Fusarium spp. Appl. Microbiol., 25(4): 699-704.
UENO, Y., ISHII, K., SATO, N., & OHTSUBO, K. (1974) Toxicological
approaches to the metabolites of Fusaria. VI. Vomiting factor from
moldy corn infected with Fusarium spp. Jpn. J. exp. Med., 44(1):
123-127.
UENO, Y., KUBOTA, K., ITO, T., & NAKAMURA, Y. (1978a) Mutagenicity
and carcinogenic mycotoxins in Salmonella typhimurium. Cancer Res.,
38(3): 536-542.
UENO, Y., MATSUMOTO, H., ISHII, K., & KUKITA, K. (1978b) Inhibitory
effects of mycotoxins on Na+-dependent transport of glycine in rabbit
reticulocytes. Biochem. Pharmacol., 25(18): 2091-2095.
UENO, Y., TASHIRO, F., & KOBAYASHI, T. (1983) Species differences
in zearalenone-reductase activity. Food chem. Toxicol., 21(2):
167-173.
UENO, Y., LEE, U.S., TANAKA, T., HASAGAWA, A., & MATSUKI, Y. (1986)
Examination of Chinese and USSR cereals for the Fusarium mycotoxins
nivalenol, deoxynivalenol, and zearalenone. Toxicon, 24(6): 18-21.
UENO, Y., ABE, K., MORIMURA, S., SUGIURA, Y., & HORIE, Y. (in press)
Genotoxicity of mycotoxins evaluated by the SOS microplate assay.
Mutation Res.
UMEDA, M., YAMAMOTO, T., & SAITO, M. (1972) DNA-strand breakage of
HeLa cells by several mycotoxins. Jpn. J. exp. Med., 42: 527-535.
UMEDA, M., TSUTSUI, T., & SAITO, M. (1977) Mutagenicity and
inducibility of DNA single-strand breaks and chromosome aberrations
by various mycotoxins. Gann, 68(5): 619-625.
VAN RENSBURG, S.J. & ALTENKIRK, B. (1974) Claviceps purpurea:
ergotism. In: Purchase, I.F.H., ed. Mycotoxins, Amsterdam, New York,
Oxford, Elsevier Science Publishers, pp. 69-96.
VESELA, D., VESELY, D., JELINEK, R., & KUSAK, V. (1978) [Detection
of ochratoxin A in feed barley.] Vet. Med., 23: 431-436 (in Czech).
VESELA, D., VESELY, D., & JELINEK, R. (1983) Toxic effects of
ochratoxins A and citrinin, alone and in combination, on chicken
embryos. Appl. environ. Microbiol., 45: 91-93.
VESONDER, R.F. (1983) Natural occurrence of trichothecenes in North
America. Dev. food Sci., 4: 210-217.
VESONDER, R.F., CIEGLER, A., & JENSEN, A.H. (1973) Isolation of the
emetic principle from Fusarium-infected corn. Appl. Microbiol., 26:
1008-1010.
VESONDER, R.F., CIEGLER, A., & JENSEN, A.H. (1977) Production of
refusal factors by Fusarium strains on grains. Appl. environ.
Microbiol., 34(1): 105-106
VESONDER, R.F., CIEGLER, A., ROGERS, R.F., BURBRIDGE, K.A., BOTHAST,
R.J., & JENSEN, A.H. (1978) Survey of 1977 crop year preharvest
corn for vomitoxin. Appl. environ. Microbiol., 36(6): 885-888.
VESONDER, R.F., CIEGLER, A., BURMEISTER, H.R., & JENSEN, A.H. (1979)
Acceptance by swine and rats of corn amended with trichothecenes.
Appl. environ. Microbiol., 38(2): 344-346.
VISCONTI, A. & BOTTALICO, A. (1983a) Detection of Fusarium
trichothecenes (nivalenol, deoxynivalenol, fusarenone and
3-acetyldeoxynivalenol) by high-performance liquid chromatography.
Chromatographia, 17: 97-100.
VISCONTI, A. & BOTTALICO, A. (1983b) High levels of ochratoxin A
and B in mouldy bread responsible for mycotoxicosis in farm animals.
J. agric. food Chem., 31: 1122-1123.
VISCONTI, A. & MIROCHA, C.J. (1985) Identification of various T-2
toxin metabolites in chicken excreta and tissues. Appl. environ.
Microbiol., 49: 1246-1250.
VOYKSNER, R.D., HAGLER, W.M., TYCZKOWSKA, K., & HANEY, C.A. (1985)
Thermospray high-performance liquid chromatographic/mass
spectrometric analysis of some Fusarium mycotoxins. J. High Res.
Chrom. Chrom. Comm., 8: 119-125.
WARE, G.M., FRANCIS, O.J., CARMAN, A.S., & KUAN, S.S. (1986) Gas
chromatographic determination of deoxynivalenol in wheat with
electron capture detection: collaborative study. J. Assoc. Off. Anal.
Chem., 69: 899-901.
WEAVER, G.A., KURTZ, H.J., BATES, F.Y., CHI, M.S., MIROCHA, C.J.,
BEHRENS, J.C., & ROBISON, T.S. (1978a) Acute and chronic toxicity
of T-2 mycotoxin in swine. Vet. Rec., 103(24): 531-535.
WEAVER, G.A., KURTZ, H.J., MIROCHA, C.J., BATES, F.Y., & BEHRENS,
J.C. (1978b) Acute toxicity of the mycotoxin diacetoxyscirpenol in
swine. Can. vet. J., 19(10): 267-271.
WEHNER, F.C., THIEL, P.G., VAN RENSBURG, S.J., & DEMASIUS, I.P.C.
(1978a) Mutagenicity to Salmonella typhimurium of some Aspergillus
and Penicillium mycotoxins. Mutat. Res., 58: 193-203.
WEHNER, F.C., MARASAS, W.F.O., & THIEL, P.G. (1978b) Lack of
mutagenicity to Salmonella typhimurium of some Fusarium mycotoxins.
Appl. environ. Microbiol., 35(4): 659-662.
WEI, C.M. & MCLAUGHLIN, C.S. (1974) Structure function relationship
in the 12,13-epoxytrichothecenes novel inhibitors of protein
synthesis. Biochem. biophys. Res. Commun., 57(3): 838-844.
WEI, R.D., STRONG, F.M., SMALLEY, E.B., & SCHNOES, H.K. (1971)
Chemical interconversion of T-2 and HT-2 toxins and related
compounds. Biochem. biophys. Res. Commun., 45(2): 396-401.
WEI, R.D., SMALLEY, E.B., & STRONG, F.M. (1972) Improved skin test
of detection of T-2 toxin. Appl. Microbiol., 23(5): 1029-1030.
WEI, C.M., CAMPBELL, I.M., MCLAUGHLIN, C.S., & MAURICE, H. (1974)
Binding of trichodermin to mammalian ribosomes and its inhibition by
other 12,13-epoxytrichothecenes. Mol. cell Biochem., 3(3): 215-219.
WHO (1979) Environmental Health Criteria 11: Mycotoxins, Geneva,
World Health Organization, 127 pp.
WHO (1983) Prevention of liver cancer: Report of a WHO Meeting,
Geneva, World Health Organization, 30 pp. (Technical Report Series,
No. 691).
WILSON, D.J. (1984) Some effects of T-2 toxin on rabbit and bovine
musculature, Guelph, Ontario, Faculty of Graduate Studies of Guelph
(M. Sc. Thesis).
WILSON, D.J. & GENTRY, P.A. (1985) T-2 toxin can cause vasoconstriction
in an in vitro bovine ear perfusion system. Toxic. appl. Pharmacol.,
79: 159-165.
WOOD, G.E. & CARTER, L. (1989) Limited survey of deoxynivalenol in
wheat and corn in the United States. J. Assoc. Off. Anal. Chem., 72:
38-40.
WOODS, A.J., BRADLEY-JONES, J., & MANTLE, P.G. (1966) An outbreak of
gangrenous ergotism in cattle. Vet. Rec., 78: 742-749.
WYATT, R.D., HARRIS, J.R., HAMILTON, P.B., & BURMEISTER, H.R. (1972)
Possible outbreaks of furariotoxicosis in avians. Avian Dis., 16:
1123-1130.
WYATT, R.D., COLWELL, W.M., HAMILTON, P.B., & BURMEISTER, H.R.
(1973a) Neural disturbances in chickens caused by dietary T-2 toxin.
Appl. Microbiol., 26(5): 757-761.
WYATT, R.D., COLWELL, W.M., HAMILTON, P.B., & BURMEISTER, H.R.
(1973b) Neurotoxicity of T-2 toxin in broilers. Poult. Sci., 52(5):
2105.
WYATT, R.D., HAMILTON, P.B., & BURMEISTER, H.R. (1973c) Effects of
T-2 toxin in broiler chickens. Poult. Sci., 52(5): 1853-1859
WYATT, R.D., DOERR, J.A., HAMILTON, P.B., & BURMEISTER, H.R. (1975)
Egg production shell thickness and other physiological parameters of
laying hens affected by T-2 toxin. Appl. Microbiol., 29(5): 641-645.
XU, Y.C., ZANG, G.S., & CHU, F.S. (1988) Enzyme-linked immunosorbent
assay for deoxynivalenol in corn and wheat. J. Assoc. Off. Anal.
Chem., 71: 945-949.
YAGEN, B., SINTOV, A., & BIALER, M. (1986) New, sensitive thin-layer
chromatographic-high performance liquid chromatographic method for
detection of trichothecene mycotoxins. J. Chromatogr., 356: 195-201.
YAROM, R., MORE, R., ELDOR, A., & YAGEN, B. (1984a) The effect of T-2
toxin on human platelets. Toxicol. appl. Pharmacol., 73: 210-217.
YAROM, R., SHERMAN, Y., MORE, R., GINSBURG, I., BORINSKI, R., &
YAGEN, B. (1984b) T-2 toxin effect on bacterial infection and
leukocyte functions. Toxicol. appl. Pharmacol., 75: 60-68.
YLIMAEKI, A., KOPONEN, H., HINTIKKA, E.L., NUMMI, M., NIKU-PAAVOLA,
M.L., ILUS, T., & ENARI, T.M. (1979) Mycoflora and occurrence of
Fusarium toxins in Finnish grain, Espoo, Technical Research Center of
Finland, pp. 1-28, (Materials and Processing Technology, Publication
21).
YOSHIZAWA, T. & HOSOKAWA, H. (1983) Natural occurrence of
deoxynivalenol and nivalenol trichothecene mycotoxins in commercial
foods. J. Food Hyg. Soc. Jpn, 24: 413-415.
YOSHIZAWA, T. & MOROOKA, N. (1973) Deoxynivalenol and its
monoacetate new mycoatoxins from Fusarium roseum and mouldy barley.
Agric. biol. Chem., 37(12): 2933-2934.
YOSHIZAWA, T. & MOROOKA, N. (1977) Trichothecenes from mold-
infested cereals in Japan. In: Rodricks, J.V., Clifford, W.,
Hesseltine, C.W., & Myron, A.M., ed. Mycotoxins in human and animal
health, Park Forest South, Illinois, Pathotox Publishers, pp. 309-
321.
YOSHIZAWA, T. & SAKAMOTO, T. (1982) [ In vitro metabolism of T-2
toxin and its derivatives in animal livers.] Proc. Jpn. Assoc.
Mycotoxicol., 14: 26-28 (in Japanese).
YOSHIZAWA, T., SWANSON, S.P., & MIROCHA, C.J. (1980) T-2
metabolites in the excreta of broiler chickens administered 3H-
labeled T-2 toxin. Appl. environ. Microbiol., 39(6): 1172-1177.
YOSHIZAWA, T., MIROCHA, C.J., BEHRENS, J.C., & SWANSON, S.P. (1981)
Metabolic fate of T-2 toxin in a lactating cow. Food Cosmet.
Toxicol., 19(1): 31-39.
YOSHIZAWA, T., SAKAMOTO, T., AYANO, Y., & MIROCHA, C.J. (1982a)
[Chemical structures of new metabolites of T-2 toxin.] Proc. Jpn.
Assoc. Mycotoxiol., 15: 13-15 (in Japanese).
YOSHIZAWA, T., SAKAMOTO, T., AYANO, Y., & MIROCHA, C.J. (1982b)
3'-Hydroxy-T-2 and 3'-hydroxy HT-2 toxins: new metabolites of T-2 toxin,
a trichothecene mycotoxin, in animals. Agric. biol. Chem., 46(10):
2613-2615.
YOSHIZAWA, T., SAKAMOTO, T., & OKAMOTO, K. (1984) In vitro formation
of 3'-hydroxy T-2 and 3'-hydroxy HT-2 toxins form T-2 toxin by liver
homogenates from mice and monkeys. Appl. environ. Microbiol., 47:
130-134.
YOSHIZAWA, T., OKAMOTO, K., SAKAMOTO, T., & KUWAMURA, K. (1985a) In
vivo metabolism of T-2 toxin, a trichothecene mycotoxin. On the
formation of deepoxidation products. Proc. Jpn. Assoc. Mycotoxicol.,
21: 9-12.
YOSHIZAWA, T., SAKAMOTO, T., & KUWAMUAR, K. (1985b) Structures of
deepoxytrichthecene metabolites from 3'-hydroxy HT-2 and T-2 tetraol
in rats. Appl. environ. Microbiol., 50: 676-679.
YOSHIZAWA, T., COTE, L.M., SWANSON, S.P., & BUCK, W.B. (1986)
Confirmation of DOM-1, a deepoxydation metabolite of deoxynivalenol
in biological fluids of lactating cows. Agric. biol. Chem., 50:
227-229.
YOUNG, J.C. (1981a) Variability in the content and composition of
alkaloids found in Canadian ergot. I. Rye. J. environ. Sci. Health,
B16(4): 83-111.
YOUNG, J.C. (1981b) Variability in the content and composition of
alkaloids found in Canadian ergot. II. Wheat. J. environ. Sci.
Health, B16(1): 381-393.
YOUNG, J.C. & CHEN, Z. (1982) Variability in the content and
composition of alkaloids found in Canadian ergot. III. Triticale and
barley. J. environ. Sci. Health, B17(2): 93-107.
YOUNG, J.C. & MARQUARDT, R.R. (1982) Effects of ergotamine tartrate
in growing chicken. Can. J. anim. Sci., 62: 1181-1191
YOUNG, J.C., CHEN, Z., & MARQUARDT, R.R. (1983) Reduction in
alkaloid content of ergot sclerotia by chemical and physical
treatment. J. agric. food Chem., 31: 413.
YOUNG, J.C., FULCHER, R.G., HAYHOE, J.H., SCOTT, P.M., & DEXTER, J.E.
(1984) Effects of milling and baking on deoxynivalenol (Vomitoxin)
content of Eastern Canadian wheats. J. agric. food Chem., 32:
659-664.
YOUNG, J.C., SUBRYAN, L.M., POTTS, D., MCLAREN, M.E., & GOBRAN, F.H.
(1986) Reduction in levels of deoxynivalenol in contaminated wheat by
chemical and physical treatment. J. agric. food Chem., 34: 461-464.
ZAMIR, N., ZAMIR, D., EIDEN, L.E., PALKOVITS, M., BROWNSTEIN, M.J.,
ESHAY, R.L., WEBER, E., FRODEN, A.I., & FEUERSTEIN, G. (1985)
Metionine and leucine enkephalin in rat neurohypophysis: different
response to osmotic stimuli and T-2 toxin. Science, 228: 606-608.
ZIPRIN, R.L. & CORRIER, D.E. (1987) Listeriosis in diacetoxyscirpenol-
treated mice. Am. J. vet. Res., 48: 1516-1519.
ZORZ, M., CULIG, J., KOPITAR, Z., MILIVOJEVIC, D., MARUSIC, A., &
BANO, M. (1985) HPLC method for determination of ergot alkaloids
and some derivatives in human plasma. Hum. Toxicol., 4: 601-607.
RESUME ET RECOMMANDATIONS EN VUE DE RECHERCHES FUTURES
1. Ochratoxine A
1.1 Etat naturel
Les ochratoxines sont produites par plusieurs espèces de
champignons appartenant au genre Aspergillus et Penicillium. Il
s'agit d'espèces ubiquistes, aussi le risque de contamination des
denrées alimentaires destinées à l'homme et aux animaux est-il
omniprésent. Le principal composé, l'ochratoxine A se rencontre en
Australie ainsi que dans certains pays d'Europe et d'Amérique du
Nord. La production d'ochratoxine par les espèces du genre
Aspergillus semble limitée aux environnements très chauds et
humides alors que dans le cas de Penicillium, au moins certaines
espèces peuvent produire de l'ochratoxine à des températures
n'excédant pas 5 °C.
Ce sont les céréales qui sont le plus fréquemment contaminées
par l'ochratoxine A et à un moindre degré certaines fèves (café,
soja, cacao). L'ochratoxine B est très rare.
1.2 Méthodes d'analyse
On a mis au point des méthodes d'analyse permettant
l'identification et le dosage de l'ochratoxine à des concentrations
de l'ordre de 1 µg/kg.
1.3 Métabolisme
On trouve des résidus d'ochratoxine A intacte dans le sang, les
reins, le foie et les muscles de porcs à l'abattoir ainsi que dans
les muscles de poules et de poulets. En revanche on ne trouve
généralement pas de résidus chez les ruminants. In vitro,
l'ochratoxine A se fixe très solidement à l'albumine sérique
bovine, porcine et humaine. Des études expérimentales sur des
porcins et des poules ont montré que c'est au niveau des reins que
les concentrations d'ochratoxine A sont les plus élevées. Chez le
porc, le rat et l'homme, il pourrait y avoir détoxication par
hydroxylation au niveau des microsomes. L'expérience montre qu'on
peut encore identifier des résidus dans les reins de porc un mois
après la fin de l'exposition.
1.4 Effets sur les animaux
On a signalé des cas d'ochratoxicose chez des animaux d'élevage
(porcs et volaille) dans plusieurs pays d'Europe, la manifestation
essentielle étant une néphropathie chronique. Les lésions se
présentent sous la forme d'une atrophie tubulaire, d'une fibrose
interstitielle et à un stade plus avancé, d'une hyalinisation des
glomérules. Au Canada, on a également trouvé de l'ochratoxine A
dans du sang de porc recueilli à l'abattoir. Des effets
néphrotoxiques ont été relevés chez toutes les espèces d'animaux à
estomac simple étudiées jusqu'ici, même aux doses les plus faibles
qui aient été expérimentées (200 mg/kg de nourriture chez les rats
et les porcs).
Des effets tératogènes ont été observés chez des souris
exposées par voie orale à 3 mg d'ochratoxine A par kg de poids
corporel. On a observé une résorption des foetus chez des rats à
partir de 0,75 mg/kg de poids corporel, le produit étant administré
par voie orale. Ces effets tératogènes, qui chez le rat sont
accrus par un régime alimentaire pauvre en protéines, ont été
également observés chez des hamsters.
Les épreuves à court terme n'ont pas permis de mettre en
évidence d'activité mutagène (bactéries et levures). Administré
par voie orale à des rats, le produit a provoqué des ruptures
monocaténaires dans l'ADN des tissus du rein et du foie.
L'ochratoxine a provoqué l'apparition de cancers du rein chez des
souris mâles et des rats des deux sexes qui recevaient le produit
par voie orale. La formation de carcinomes hépatocellulaires n'a
été signalée que chez une souche de souris et pas du tout chez le
rat.
L'ochratoxine A est un inhibiteur de la synthèse protéique et
de la tRNA-synthétase chez les microorganismes et dans les cellules
hépatomateuses; elle inhibe également le mARN chez le rat.
L'ochratoxine A peut inhiber la migration des macrophages.
Chez la souris, une dose de 0,005 µg/kg de poids
corporel a aboli la réponse immunitaire aux érythrocytes de mouton;
toutefois d'autres résultats, contradictoires, ont été obtenus.
L'ochratoxine A s'est révélée cancérogène pour l'épithelium
tubulaire rénal chez des souris mâles et des rats des deux sexes.
1.5 Effets sur l'homme
L'exposition humaine, qui ressort de la présence d'ochratoxine
A dans les aliments, le sang et le lait humains, a été observée
dans divers pays d'Europe. Selon les données épidémiologiques
disponibles, la néphropathie des Balkans peut être attribuée à la
consommation de denrées alimentaires contaminées par cette toxine.
On a mis en évidence une relation tout à fait significative
entre la néphropathie des Balkans et les tumeurs des voies
urinaires, en particulier des tumeurs pyéliques et uretérales.
Toutefois on n'a pas publié de données qui établissent une
implication directe de l'ochratoxine A dans l'étiologie de ces
tumeurs.
2. Trichothécènes
2.1 Etat naturel
On connaît aujourd'hui 148 trichothécènes qui se caractérisent
sur le plan chimique par la présence d'une structure de base
commune, le système tétracyclique du scirpénol. Ces composés sont
principalement secrétés par des moisissures appartenant au genre
Fusarium, encore que d'autres genres notamment Trichoderma,
Trichothecium, Myrothecium et Stachybotrys produisent également des
métabolites considérés désormais comme des trichothécènes.
Quelques-uns seulement de ces trichothécènes contaminent les
denrées alimentaires destinées à l'homme ou aux animaux, en
particulier : le désoxyvalénol (DON), le nivalénol (NIV), le
disacétoxyscirpenol (DAS), ainsi que la toxine T-2 et, plus
rarement, certains dérivés (3-Ac-DON, 15-Ac-DON, fusarenone-X et
toxine HT-2). Parmi ces substances, c'est le DON qui est de loin
la plus fréquemment présente dans les denrées alimentaires
destinées à l'homme et aux animaux; à côté de quantités plus
faibles de NIV. Certains trichothécènes macrocycliques comme les
satratoxines G et H et les verrucarines, se rencontrent de temps à
autre dans les fourrages (paille, foin) mais il n'a pas été fait
état de leur présence dans les produits alimentaires.
Les enquêtes sur la présence de trichothécènes ont révélé que
le DON était présent dans le monde entier, essentiellement dans les
céréales telles que le froment et le maïs à des concentrations
pouvant parfois atteindre 92 mg/kg, encore que les teneurs moyennes
soient beaucoup plus faibles et varient selon la denrée. Des
rapports isolés font état de la présence de DON dans de l'orge, des
mélanges alimentaires pour aminaux, des pommes de terre, etc. Le
NIV, dont la présence n'est normalement pas signalée dans des
céréales au Canada ou aux Etats-Unis se rencontre en revanche
fréquemment au côté du DON dans les céréales originaires d'Asie ou
d'Europe; la concentration la plus forte enregistrée jusqu'ici est
de 37,9 mg/kg. On a signalé ça et là la présence de toxine T-2 et
de DAS à des concentrations beaucoup plus faibles.
Des études portant sur les divers traitements subis par les
produits alimentaires et notamment la mouture, montrent que, entre
la céréale brute et le produit définitif, il n'y a guère de
diminution des teneurs en DON. De même la panification ne parvient
pas à détruire le DON. En général, les aliments destinés à l'homme
que l'on trouve dans le commerce ne contiennent que rarement des
quantités décelables de DON et de NIV.
2.2 Methodes d'analyse
On dispose, pour le dosage des quatre toxines les plus
fréquemment rencontrées (DON, toxine T.2, DAS et NIV), des méthodes
d'analyse basées sur la chromatographie en couche mince, la
chromatographie en phase gazeuse ou la chromatographie liquide à
haute performance ainsi que sur des réactions immunologiques; les
limites de détection se situent en-dessous de 1 µg/g. Plusieurs de
ces méthodes ont fait l'objet d'une expérimentation collective. En
outre, certaines méthodes utilisées en recherche comme la
chromatographie gazeuse ou liquide associée à la spectrographie de
masse peuvent être utilisées pour confirmer l'identité des
substances.
2.3 Métabolisme
On a procédé à des études métaboliques, essentiellement de la
toxine T-2, mais dans quelques rares cas seulement du DON, chez
l'animal. Ces trichothécènes sont rapidement absorbés au niveau
des voies digestives mais l'on ne dispose pas de données
quantitatives. Les toxines se répartissent de façon uniforme sans
accumulation marquée au niveau d'un organe ou d'un tissu
particulier. Elles sont métabolisées en produits moins toxiques
par hydrolyse, hydroxylation, désépoxydation et glucuronidation.
La toxin T-2 et le DON sont rapidement éliminés par voie fécale et
urinaire.
Par exemple, une dose de toxine T-2 administrée par voie orale à
des bovins a été éliminée presque à hauteur de 100 % dans les heures
suivant l'administration; des poulets ont éliminé 80 % de la
substance dans les 48 heures suivant l'administration. Chez le rat,
25 % d'une dose de DON ont été éliminés dans les urines et 65 % dans
les matières fécales 96 heures après l'administration. Chez la poule
pondeuse et la vache laitière on a constaté que moins de 1 % de la
dose de toxine T-2 (et de ses métabolites) qui leur avait été
administrée se retrouvaient dans les oeufs et le lait. Après
administration par voie orale de toxine T-2 à des poulets, on a
constaté que les résidus présents dans la viande 24 heures plus tard
représentaient moins de 2 % de la dose initiale.
2.4 Effets sur les animaux
C'est principalement par l'ingestion de fourrage contaminé que
les animaux sont exposés aux trichothécènes. La toxine T-2 et le DAS
qui chez les animaux de laboratoire sont les plus actifs des
trichothécènes couramment cités comme contaminants des denrées
destinées aux animaux (toxine T-2, DAS, NIV et DON), provoquent des
réactions toxiques analogues. Le NIV est moins actif dans certains
systèmes que les deux précédents composés et le DON est le moins
toxique des quatre. (Cette activité toxique peut s'évaluer au moyen
de la DL50 pour la souris, par exemple dans le cas de la toxine T-2
elle est égale à 10,5 mg/kg de poids corporel et dans le cas du DON à
46,0 mg/kg).
Les trichothécènes les plus actifs, tels que la toxine T-2 et le
DAS produisent des effets généraux aigus lorsqu'ils sont administrés
expérimentalement à des rongeurs, des porcs et des bovins par voie
orale, parentérale ou respiratoire (porc, souris). Ces
trichothécènes produisent une épithélionécrose par contact (une dose
de 0,2 µg par touche dans le cas de la toxine T-2). Avec les autres
trichothécènes, il faut des doses plus élevées pour obtenir un effet
irritant (dans le cas du NIV, 10 ug par touche). Les trichothécènes
cytotoxiques comme la toxine T-2 ont une action nécrosante sur
l'épithélium des cryptes intestinales et sur les tissus lymphoïdes et
hematopoïétiques après exposition par voie orale, parentérale ou
respiratoire. Après exposition à la toxine T-2 et au DAS on observe
des anomalies hematologiques et des troubles de l'hémostase. Dans
les cas graves, la toxicose peut entraîner une pancytopénie. Des
études portant sur la toxine T-2, le DON et le DAS ont mis en
évidence la suppression de l'immunité à médiation cellulaire et de
l'immunité humorale et on a observé une réduction de la concentration
des immunoglobulines ainsi qu'une dépression de l'activité
phagocytaire des macrophages et des neutrophiles. Des études sur
animaux de laboratoire ont montré que l'effet immunodépresseur de
trichothécènes tels que la toxine T-2, le DAS et le DON entraînait
une moindre résistance aux infections secondaires par des bactéries
(Mycobactérie, Listeria monocytogenes) des levures ( Cryptococcus
neoformans) et des virus (virus de l'herpes simplex).
Il a été indiqué qu'après injection intrapéritonéale, la toxine
T-2 était tératogène pour la souris (cette voie d'administration
n'est pas courante dans les études de tératogénicité). Le DON est
tératogène pour la souris après intubation gastrique mais ne l'est
pas chez le rat lorsque la toxine est administrée dans la nourriture
de l'animal. Le NIV ne s'est pas révélé tératogène pour la souris.
La recherche du pouvoir mutagène de la toxine T-2, du DAS et du DON
par une épreuve du type Ames n'a pas donné de résultat positif. La
toxine T-2 présente dans certaines épreuves une faible activité
clastogène. D'après les études de toxicité à long terme qui ont été
publiées, rien n'indique que la toxine T-2, la fusarénone-X et le NIV
ne soient tumorigènes chez l'animal. Aucune étude de toxicité à long
terme n'a été publiée sur le DON.
Les trichothécènes sont toxiques pour les cellules à forte
activité mitotique telles que les cellules de l'épithélium des
cryptes intestinales et les cellules hematopoïétiques. Cette
cytotoxicité proviendrait, soit d'une perturbation de la synthèse des
protéines par une fixation des composés aux ribosomes des cellules
eucaryotes, soit d'une dysfonction des membranes cellulaires.
L'inhibition de la synthèse protéique serait due à l'induction de
protéines régulatrices labiles telles que l'IL-2 dans les
immunocytes. A concentrations extrêmement faibles, les
trichothécènes perturbent le transport des petites molécules à
travers les membranes cellulaires.
2.5 Effets sur l'homme
L'ingestion de produits alimentaires contaminés d'origine
végétale constitue la principale voie d'exposition aux tricho-
thécènes mais d'autres voies ont été signalées à l'occasion, par
exemple un contact cutané accidentel chez des chercheurs de
laboratoire ou l'inhalation de trichothécènes présents dans des
poussières aéroportées.
On n'a décrit que peu de cas de maladies attribuables à une
exposition aux trichothécènes, sans d'ailleurs que la responsa-
bilité de ces produits soit établie. Toutefois, dans les deux
flambées évoquées ci-dessous, il y a lieu de penser que leur
responsabilité est en cause.
Une des flambées, qui s'est produite en Chine, a été attribuée à
la consommation de blé moisi contenant 1,0 à 40,0 mg de DON par kg.
La maladie se caractérisait par des symptômes gastro-intestinaux.
Aucun décès n'a été à déplorer. Les porcs et les poulets qui avaient
mangé les restes de céréales ont également été affectés.
Une flambée du même genre a été signalée en Inde et attribuée à
la consommation de pain fabriqué à l'aide de blé contaminé. La
maladie se caractérisait par des symptômes gastro-intestinaux et une
irritation de la gorge qui apparaissaient 15 minutes à une heure
après l'ingestion du pain. On a décelé les mycotoxines suivantes
dans des échantillons de farine raffinée utilisée pour la préparation
du pain: DON (0,35-8,3 mg/kg), acétyldésoxynivalénol (0,64-2,49
mg/kg), NIV (0,03-0,1 mg/kg) et toxine T-2 (0,5-0,8 mg/kg).
Toutefois, l'identité de ces trichothécènes n'a pas été confirmée.
La présence de DON et de NIV à côté de la toxine T-2 est
inhabituelle.
Deux maladies d'intérêt historique, une aleucie alimentaire
d'origine toxique en URSS et une toxicose due à du blé moisi au
Japon et en Corée ont été attribuées à la consommation de céréales
contaminées par des moisissures du genre Fusarium. Depuis lors, on
a isolé en laboratoire, dans des cultures de Fusarium isolés des
céréales en cause, un certain nombre de trichothécènes. Il n'avait
pas été possible, au moment où ces maladies se sont déclarées,
d'effectuer des recherches pour tenter de corréler l'aleucie
toxique et la toxicose due au blé moisi à une exposition à des
trichothécènes car on ne connaissait pas les toxines en question.
3. Ergot
3.1 Etat naturel
Ergot est le nom que l'on donne aux sclérotes de certaines
espèces de champignons appartenant au genre Clavicepsa. Ces
sclérotes contiennent des alcaloïdes biologiquement actifs qui
peuvent être à l'origine de toxicoses chez les personnes ou les
animaux qui consomment des denrées contaminées.
Les alcaloïdes de l'ergot produisent deux types de maladies selon
leur nature, qui dépend du champignon en cause ( C. purpurea , C.
fusiformis ). L'ergotisme, dû à l'ergotamine et à l'ergocristine
produites par C. purpurea se caractérise principalement par une
gangrène des extrémités et des symptômes gastro-intestinaux. Quand à
l'intoxication provoquée par du millet contaminé par C. fusiformis,
elle se caractérise principalement par des symptômes digestifs et
elle est due aux clavines. Aucun des signes ou symptômes observés ne
sont révélateurs d'une oblitération vasculaire.
3.2 Méthodes d'analyse
Les alcaloïdes de l'ergot (ergolines) sont des dérivés de l'acide
lysergique. Ils ont une activité biologique variable selon leur
nature. Le dosage des alcaloïdes de C. purpurea a été effectué par
chromatographie liquide à haute performance avec détection par
fluorescence. On peut déterminer des concentrations de 0,2 µg
d'ergolines par litre de plasma humain. La détermination de
l'ergotamine et de l'ergocristine peut s'effectuer de façon très
spécifique par titrage radio-immunologique à des concentrations
respectives de 3,5 et 0,8 picomoles.
3.3 Effets sur les animaux
Des flambées d'avortements chez des bovins ont pu être attribuées
à l'ingestion d'ergoline, essentiellement de l'ergotamine et de
l'ergotaminine. Des moutons à qui l'on avait administré de
l'ergotamine par voie orale sont tombés rapidement malades et
présentaient une inflammation intestinale. Chez des volailles, des
porcs et des primates exposés par voie orale on a observé des effets
légers. Le groupe de travail ne disposait d'aucune donnée sur la
mutagénicité, la tératogénicité et la cancérogénicité des ergolines.
3.4 Effets sur l'homme
L'homme peut être exposé aux ergolines par la consommation de
céréales ergotées. Dans la plupart des études toxicologiques qui ont
été effectuées, on n'a pas procédé à l'identification précise des
alcaloïdes en cause. Les données qui ont été publiées au sujet d'une
seule enquête effectuée en Suisse sur des céréales et des produits
céréaliers indiquent que la consommation quotidienne totale
d'ergolines se situe à environ 1,5 ug par personne, certaines denrées
en contenant jusqu'à 140 µg/kg. La panification réduit de 25 à 100 %
la teneur en ergolines des farines contaminées.
En Ethiopie, une flambée d'ergotisme s'est produite en 1978 à la
suite de l'exposition à des ergolines provenant de sclérotes de ce C.
purpurea. Les céréales comptaient jusqu'à 0,75 % d'ergot; on a
relevé la présence d'ergométrine. Parmi les symptômes observés
figuraient une gangrène sèche ayant entraîné la perte d'un ou
plusieurs membres (29 % des cas), un pouls périphérique faible ou
absent (36 %) et une desquamation. Des symptômes digestifs n'ont été
observés que dans quelques cas. Chez 88 % des malades, les lésions
intéressaient les membres inférieurs.
En Inde, plusieurs flambées se sont déclarées depuis 1958 à la
suite de la consommation de millet contaminé par C. fusiformis. Ces
symptômes consistaient en nausées, vomissements et vertiges. Les
symptômes toxiques étaient dus à la présence d'ergolines à des
concentrations de 15 à 26 mg/kg. Aucune autopsie n'ayant été
effectuée, on ne dispose d'aucune information sur les effets
pathologiques au niveau des viscères.
4. Evaluation des risques pour la santé humaine
4.1 Ochratoxine A
L'exposition humaine, observée dans plusieurs pays d'Europe est
objectivée par la présence d'ochratoxine A dans les denrées
alimentaires et le sang. Le groupe de travail n'a pas connaissance
de tentatives qui ont effectuées dans d'autres régions du monde pour
déceler la présence d'ochratoxine A dans le sang humain.
En s'appuyant sur l'étude de la maladie-naturelle ou provoquée
par administration d'ochratoxine A-on a pu établir le rôle
étiologique de l'ochratoxine A dans la néphropathie porcine. A
partir de ce modèle, on a pu émettre l'hypothèse que la néphropathie
endémique des Balkans était due à une exposition à cette toxine. Les
données immunologiques disponibles montrent que cette affection
serait attribuable à la consommation de denrées alimentaires
contaminées par de l'ochratoxine A. Depuis la publication en 1980 du
No 11 des Critères d'hygiène de l'environnement, des études
épidémiologiques sur la concentration de l'ochratoxine A dans le sang
humain dans les régions touchées et non touchées ont conforté
l'hypothèse d'une relation entre la néphropathie balkanique et
l'exposition à l'ochratoxine A.
Ainsi, on a montré que les habitants des régions d'endémie
présentaient plus fréquemment de l'ochratoxine A dans leur sang et à
des concentrations plus élevées. Cependant, ces études
rétrospectives ne fournissent que des présomptions sur lesquelles on
ne peut établir l'existence d'une relation causale directe. On ne
peut cependant pas l'exclure du fait de la longue période de latence
entre l'exposition et l'apparition des symptômes.
On a montré que l'ochratoxine A exerçait, chez les souris mâles
et les rats des deux sexes, des effets cancérogènes sur l'épithélium
des tubules rénaux. Il existe une relation tout à fait significative
entre la néphropathie balkanique et la présence de tumeurs des voies
urinaires, notamment de tumeurs pyéliques et uretérales. Toutefois
aucune donnée n'a été publiée qui établissent la responsabilité
directe de l'ochratoxine A dans l'étiologie de ces tumeurs.
4.2 Trichothécènes
Sur la base des données dont disposait le groupe de travail, il
est possible d'établir une relation entre l'exposition aux
trichothécènes et certains épisodes toxiques chez l'homme. Si l'on
se réfère aux quelques données disponibles, ce sont le DON et le NIV
qui sont les plus fréquemment cités dans les différents cas
d'exposition humaine. Dans le cas des flambées d'aleucie toxique
alimentaire et de toxicose par ingestion de céréales moisies, on ne
peut exclure la responsabilité des trichothécènes. L'exposition se
produit par ingestion de denrées contaminées, principalement des
céréales. Les différents traitements qu'elles subissent, notamment
la mouture et la panification ne permettent pas d'éliminer le DON, le
NIV, ni la toxine T-2. L'existence d'une exposition par voie
respiratoire est très peu documentée mais c'est une possibilité qu'on
ne peut totalement écarter.
Parmi les tricothécènes qui se trouvent à l'état naturel dans les
aliments, c'est la toxine T-2 qui est la plus active, suivie du DAS
and du NIV; les études de toxicité aiguë ont montré que le DON était
la substance la moins toxique. Chez l'animal d'expérience, la toxine
T-2 et le DAS déterminent des symptômes généraux aigus, avec nécrose
des tissus épithéliaux et suppression de l'hematopoïèse. Lors des
récentes flambées d'intoxications, il n'a été fait état que des
symptômes digestifs.
Le DON est tératogène pour la souris mais pas pour le rat. Selon
les études de toxicité chroniques qui ont été publiées, le NIV et la
toxine T-2 ne sont pas tumorigènes chez l'animal. Aucune étude de
cancérogénicité à long terme n'a été publiée sur le DON. Certains
trichothécènes, tels que la toxine T-2 et le DON ont une action
immunosuppressive chez l'animal et produisent des modifications de
l'immunité à médiation cellulaire et de l'immunité humorale. En
revanche, rien n'indique l'existence de tels effets chez l'homme.
On est mal informé sur les cas d'intoxication humaine due à une
exposition aux trichothécènes, cas qui sont en nombre limité. Les
symptômes observés consistent en troubles digestifs et irritation de
la gorge; ils apparaissent peu après l'ingestion de denrées
alimentaires contaminées par des trichothécènes. A l'heure actuelle,
on ne connaît pas de cas de cancer humain attribuable aux
trichothécènes. Le groupe de travail ne disposait d'aucune
publication faisant état d'infections secondaires d'origine
bactérienne, fongique ou virale chez l'homme, à la suite d'une
exposition aux trichothécènes comme on en a observé chez l'animal
d'expérience. Il semble que l'on n'ait pas effectué d'études
appropriées sur ce point.
4.3 Ergot
Il semble que l'exposition humaine à de faibles concentrations
d'ergolines soit très répandue. Les données relatives aux flambées
qui se sont récemment déclarées en Ethiopie et en Inde montrent que
les alcaloïdes de C. purpurea (groupe de l'ergotamine) produisent
les effets les plus graves, notamment une gangrène des membres
inférieurs pouvant entraîner la mort, effets qui sont plus graves
que ceux des alcaloïdes de C. fusiformis (groupe de la clavine) qui
entraînent des symptômes digestifs sans issue fatale. On ignore si
ces différences s'expliquent par la teneur des différentes espèces
de champignons en alcaloïdes, par les propriétés toxicologiques de
ces substances ou par des différences dans les quantités ingérées
par les diverses populations.
Après nettoyage et mouture qui éliminent les sclérotes, il ne
subsiste dans les produits alimentaires que de faibles quantités
d'ergolines. La panification ou d'autres traitements thermiques
détruisent également la plupart des alcaloïdes du groupe de
l'ergotamine.
5. Recommandations en vue de recherches futures
5.1 Recommandations generales
Il conviendrait de créer un réseau de centres de référence pour
aider les Etats Membres à confirmer l'identité des mycotoxines
présentes dans les tissus humains et les denrées destinées à la
consommation humaine. Ces centres devraient également fournir sur
demande des échantillons de référence de mycotoxines afin de
faciliter la comparabilité des résultats d'analyse obtenus dans les
différentes régions du monde.
5.2 Ochratoxine A
a) Etudes épidémiologiques de grande ampleur à caractère
rétrospectif et études localisées à caractère prospectif sur
l'association entre l'ochratoxine A et la néphropathie
endémique des Balkans ainsi que les tumeurs des voies
urinaires : ces études devraient être menées dans la
péninsule de Balkans et la Région méditerranéenne.
b) Recherche et dosage de l'ochratoxine A dans le sang de
malades porteurs de tumeurs des voies urinaires, à
l'extérieur de la péninsule des Balkans.
c) Recherche de sources d'exposition à l'ochratoxine A, par
analyse du sang, dans les pays situés en dehors de la
péninsule des Balkans.
d) Elucidation du mécanisme à l'origine des différences entre
les sexes qui ont été relevées dans les anomalies rénales,
néoplasiques et non-néoplasiques produites par l'ochratoxine
A chez l'animal d'expérience.
e) Etudes de grande envergure sur la teneur en ochratoxine A des
denrées alimentaires dans les différentes régions du monde.
Ce type d'enquêtes est particulièrement important dans les
régions où l'on observe une forte incidence de tumeurs des
voies urinaires, notamment rénales, ou de néphropathies.
5.3 Trichothécènes
a) Il conviendrait d'effectuer des études longitudinales dans
les régions de l'Inde et de la République populaire de Chine
où ont été récemment observés des épisodes d'intoxication par
les trichothécènes. Il conviendrait de mieux éclaircir la
présence inhabituelle de certains trichothécènes dans ces
régions.
b) Il faudrait étudier les effets d'une exposition prolongée
d'animaux de laboratoire au DON, et notamment les effets
cancérogènes. Comme la réaction au DON varie beaucoup selon
les espèces, l'espèce qui sera retenue devra être choisie
avec soin.
c) Il faudrait étudier plus à fond les infections microbiennes
secondaires à une exposition aux trichothécènes chez l'animal
d'expérience.
d) Il convient d'étudier l'influence des conditions
environnementales, notamment la présence d'insecticides ou
autres produits chimiques industriels, sur la production de
trichothécènes par des champignons.
e) Il faudrait élucider l'effet des différents modes de
préparation des denrées alimentaires sur les trichothécènes.
f) Il conviendrait de mettre au point des espèces végétales qui
résistent aux champignons producteurs de trichothécènes, en
recourant aux biotechnologies.
g) Il faudrait étudier sur l'animal les effets synergistiques
éventuels d'une exposition simultanée aux trichothécènes, aux
aflatoxines, à l'ochratoxine A et à d'autres mycotoxines.
h) Il faudrait également déterminer l'apport de trichothécènes
d'origine alimentaire chez l'homme.
i) Il faudrait mettre au point des méthodes de criblage des
trichothécènes qui soient à la fois rapides et sensibles, et
mener des enquêtes dans les régions tempérées du monde pour
rechercher la présence de trichothécènes dans les céréales et
les préparations alimentaires.
5.4 Ergot
a) Il faudrait mettre au point des méthodes pour l'analyse des
agroclavines.
b) Il conviendrait de mettre à la disposition des pays en
développement des renseignements sur le repérage des semences
contaminées et sur les méthodes de mouture permettant de
réduire au minimum les problèmes posés par la présence de
l'ergot.
c) Il faudrait effectuer des études épidémiologiques sur les
effets éventuels que peut entraîner chez l'homme l'ingestion
de faible quantités d'ergolines.
d) Il faudrait effectuer des études pharmacologiques et
toxicologiques sur les ergolines, seules ou en association,
chez l'animal d'expérience.
e) Il conviendrait de déterminer si les ergolines peuvent se
transmettre de la mère à l'enfant par le lait maternel.
RESUMEN Y RECOMENDACIONES PARA ULTERIOR INVESTIGACION
1. Ocratoxina A
1.1 Distribución natural
Las ocratoxinas son producidas por varias especies de los
géneros de hongos Aspergillus y Penicillium. Esos hongos son
ubicuos y hay amplias posibilidades de contaminación de alimentos
para seres humanos y para animales. La ocratoxina A, la más
importante, se ha hallado en toda una serie de países de América
del Norte, Australia y Europa. La formación de ocratoxina por las
especies del género Aspergillus parece estar limitada a condiciones
de humedad y temperatura elevadas, mientras que algunas especies,
por lo menos, de Penicillium pueden producir ocratoxina a
temperaturas de sólo 5 °C.
Las mayores incidencias de contaminación con ocratoxina A se
han hallado en cereales y, en menor medida, en algunos granos
(café, soja, cacao). La ocratoxina B es muy poco frecuente.
1.2 Métodos de análisis
Se han desarrollado técnicas de análisis para identificar la
ocratoxina y determinar sus concentraciones en la gama de g/kg.
1.3 Metabolismo
Se han hallado residuos de ocratoxina A no modificada en la
sangre, los riñones, el hígado y los músculos de cerdos en los
mataderos y en los músculos de gallinas y pollos. Sin embargo, por
lo general no se han hallado residuos de ocratoxina A en los
rumiantes. El enlace in vitro de la ocratoxina A con la
seroalbúmina es especialmente importante en el ganado vacuno, el
ganado ovino y el ser humano. Estudios experimentales realizados
con cerdos y gallinas han demostrado que las concentraciones más
altas de ocratoxina A se hallan en los riñones. La hidroxilación
microsómica puede representar una reacción de detoxificación en los
cerdos, las ratas y el ser humano. En estudios experimentales, un
mes después de terminada la exposición se localizaron aún residuos
en riñones de cerdos.
1.4 Efectos en animales
Varios países europeos han notificado en animales de granja
(cerdos, aves de corral) casos de ocratoxicosis, cuya principal
manifestación era una nefropatía crónica. Entre las lesiones había
atrofia tubular, fibrosis intersticial y, en etapas ulteriores,
hialinización de los glomérulos. También se ha hallado ocratoxina
A en sangre de cerdo recogida en mataderos canadienses. La
ocratoxina A ha producido efectos nefrotóxicos en todas las
especies de animales provistas de un solo estómago que se han
estudiado hasta el momento, incluso con las dosis más bajas con que
se experimentó (200 µg/kg de alimentos en ratas y cerdos).
Se observaron efectos teratogénicos en ratones expuestos a
dosis de 3 mg/kg de peso corporal administradas por vía oral. En
ratas que recibieron por vía oral dosis de 0,75 mg/kg de peso
corporal se observó resorción fetal. Los efectos teratogénicos,
que en las ratas se acentuaron con una dieta baja en proteínas, se
han observado también en hámsters.
No hay datos que demuestren la actividad de la ocratoxina A en
pruebas a corto plazo de la mutagenicidad (bacterias y levaduras).
Ratas expuestas a ocratoxina A administrada por vía oral mostraron
roturas de una sola cadena del ADN en tejidos renales y hepáticos.
Ocratoxina A administrada por vía oral provocó neoplasias de
células renales en ratones machos y en ratas de uno y otro sexo.
Se registraron neoplasias de células hepáticas en sólo una estirpe
de ratones y no en la rata.
La ocratoxina A es inhibidora de la síntesis de proteínas y de
la sintetasa del ARNt en microorganismos, células de hepatoma y el
ARNm renal en la rata.
La ocratoxina A puede inhibir la migración de los macrófagos.
En ratones, una dosis de 0,005 µg/kg de peso corporal suprimió la
respuesta inmunitaria a eritrocitos de oveja; sin embargo, se han
obtenido también resultados contradictorios.
Se ha demostrado que la ocratoxina A es carcinógena para el
epitelio de los túbulos renales en ratones machos y de ratas de uno
y otro sexo.
1.5 Efectos en el ser humano
En varios países de Europa se ha observado exposición humana a
ocratoxina A, como lo demuestra la presencia de ésta en alimentos y
en la sangre y la leche humanas. Los datos epidemiológicos de que
se dispone indican que la nefropatía de los Balcanes podría estar
relacionada con el consumo de productos alimenticios contaminados
por esta toxina.
Se ha observado una relación muy significativa entre la
nefropatía de los Balcanes y tumores del tracto urinario, en
particular los de la pelvis renal y los uréteres. Sin embargo, no
se han publicado datos que demuestren el papel causal de la
ocratoxina A en la etiología de esos tumores.
2. Tricotecenos
2.1 Distribución natural
Hasta ahora se conocen 148 tricotecenos, caracterizados
químicamente por la presencia del mismo sistema básico de un anillo
tetracíclico de scirpenol. Son compuestos producidos
principalmente por hongos pertenecientes al género Fusarium,
aunque otros géneros, entre ellos Trichoderma, Trichothecium,
Myrothecium y Stachybotrys, producen también metabolitos
ahora reconocidos como tricotecenos. Se ha visto que sólo un
pequeño número de los tricotecenos conocidos contaminan los
alimentos para seres humanos o para animales, entre ellos el
deoxinivalenol (DON), el nivalenol (NIV), el diacetoxiscirpenol
(DAS) y la toxina T-2 y, con menos frecuencia, ciertos derivados
(3-Ac-DON,15-Ac-DON, fusarenon-X y toxina HT-2). De todos ellos,
el que se encuentra más a menudo en alimentos para seres humanos y
para animales es el DON, por lo general con cantidades más pequeñas
de NIV como co-contaminante. Algunos tricotecenos macrocíclicos
como las satratoxinas G y H y las verrucarinas se observan
ocasionalmente en alimentos para animales (paja, heno) pero no se
ha notificado su presencia en alimentos para seres humanos.
Estudios sobre la distribución de los tricotecenos han indicado
que el DON se encuentra en todo el mundo, sobre todo en cereales
como el trigo y el maíz, en concentraciones que pueden llegar hasta
92 mg/kg, aunque las concentraciones medias son considerablemente
inferiores y varían según el producto. Hay notificaciones aisladas
de la presencia de DON en la cebada, los piensos compuestos, las
patatas, etc. Habitualmente los cereales producidos en Canadá y
los Estados Unidos de América no contienen NIV pero éste se ha
encontrado en los cereales asiáticos y europeos, junto con DON; la
mayor concentración de NIV registrada hasta la fecha fue de 37,9
mg/kg. La presencia de toxina T-2 y DAS se ha notificado con escasa
frecuencia y en concentraciones muy inferiores.
Estudios sobre la elaboración y la molienda indican que las
concentraciones de DON apenas se reducen del cereal al producto
acabado. Tampoco se destruye cuando se cuece el cereal. En
general, los productos alimenticios para seres humanos que se
encuentran en el comercio rara vez contienen concentraciones
detectables de DON y NIV.
2.2 Métodos de análisis
Existen xistenmétodos de análisis basados en la cromatografía
de capa fina, la cromatografía de fase gaseosa, la cromatografía de
fase líquida de alto rendimiento y técnicas inmunológicas para la
determinación de las cuatro toxinas más frecuentes (DON, toxina T-
2, DAS y NIV) con límites de detección inferiores a 1 µg/g.
Algunos de esos métodos se han ensayado en colaboración. Además,
la identidad puede confirmarse mediante métodos de investigación
como cromatografía de fase gaseosa/espectografía de masas y
cromatografía de fase líquida/espectografía de masas.
2.3 Metabolismo
Se han realizado estudios metabólicos con animales, sobre todo
administrando toxina T-2 y en unos pocos casos DON. Estos
tricotecenos se absorben con rapidez por el tubo digestivo,
aunque no se dispone de datos cuantitativos. Las toxinas se
distribuyen bastante uniformemente, sin marcada acumulación en
ningún órgano o tejido determinado. Los tricotecenos se
transforman en metabolitos menos tóxicos por reacciones como
hidrólisis, hidroxilación, de-epoxidación y glucoronidación.
Tricotecenos como la toxina T-2 y el DON se eliminan rápidamente en
las heces y la orina. Por ejemplo, casi el 100% de una dosis de
toxina T-2 administrada a ganado por vía oral se eliminaba en unas
horas; en pollos, alrededor del 80% se había eliminado a las 48
horas. En la rata, 96 horas después de la administración, el 25%
del DON se había eliminado en la orina y el 65% en las heces. Los
resultados de la transmisión de toxina T-2 en gallinas ponedoras y
vacas lactantes indicaron que pasaba a los huevos y a la leche
menos del 1% de la dosis administrada de esa toxina y de sus
metabolitos. En la carne de pollo, 24 horas después de la
administración de toxina T-2 por vía oral, los residuos de ésta y
de sus metabolitos eran inferiores al 2% de la dosis.
2.4 Efectos en animales
La principal vía de exposición de los animales a tricotecenos
es la ingestión de alimentos de origen vegetal. La toxina T-2 y el
DAS, los más potentes en los experimentos de laboratorio con
animales, de todos los tricotecenos que habitualmente se consideran
contaminantes de alimentos para animales (toxina T-2, DAS, NIV y
DON), provocan una respuesta tóxica similar. El NIV es menos
potente en algunos sistemas que los dos compuestos anteriores y el
DON es el menos tóxico de los cuatro (un ejemplo de la potencia es
la DL50 por vía oral en el ratón: toxina T-2, 10,5 mg/kg de peso
corporal y DON, 46,0 mg/kg).
Los tricotecenos más potentes, como la toxina T-2 y el DAS,
producen efectos generales agudos cuando se administran
experimentalmente a roedores y ganado porcino y bovino por vía oral
o parenteral o por inhalación (cerdo, ratón). Una lesión producida
por el contacto con tricotecenos potentes como la toxina T-2 y el
DAS es la epitelionecrosis (dosis de 0,2 µg por zona en el caso de
la toxina T-2). En cuanto a otros tricotecenos, son necesarias
dosis mayores para producir un efecto irritante (NIV, 10 µg por
zona). Los tricotecenos citotóxicos, como la toxina T-2, producen
necrosis del epitelio de las criptas intestinales y de los tejidos
linfoides y hematopoyéticos, tras exposición oral o parenteral o
por inhalación. La exposición a tricotecenos citotóxicos como la
toxina T-2 y el DAS va seguida de alteraciones hematológicas y
coagulopáticas. Las toxicosis graves pueden originar
pancitopenia. En estudios con toxina T-2, DON y DAS se ha
demostrado la supresión de la inmunidad de base celular y humoral,
y entre los efectos observados figuran la reducción de las
concentraciones de inmunoglobulinas y la disminución de la
actividad fagocítaria de macrófagos y neutrófilos. Los resultados
de estudios experimentales con animales han indicado que el efecto
inmunodepresor de tricotecenos como la toxina T-2, el DAS y el DON
origina una disminución de la resistencia a la infección secundaria
por bacterias (micobacterias, Listeria monocytogenes), levaduras
(Cryptococcus neoformans) y virus (virus del herpes simplex).
Se ha comunicado que la toxina T-2 es teratogénica en el ratón,
cuando se administra por inyección intraperitoneal (vía de
administración poco corriente en los estudios de la
teratogenicidad). Se ha comunicado que el DON es teratogénico en
los ratones tras intubación gástrica, pero no en las ratas cuando
la toxina se administra con los alimentos. El NIV no es
teratogénico en el ratón. La toxina T-2, el DAS y el DON no
resultaron mutagénicos en una prueba de tipo Ames. En algunas
pruebas, se observó una débil actividad clastogénica de la toxina
T-2. En los estudios publicados sobre la toxicidad a largo plazo
en animales, no se obtuvieron datos que indiquen que la toxina T-2,
el fusarenón-X o el NIV sean oncogénicos en los animales. No se
han publicado estudios a largo plazo sobre la toxicidad del DON.
Los tricotecenos son tóxicos para las células que se dividen
activamente como las del epitelio de las criptas intestinales y las
hematopoyéticas. La citotoxicidad se ha asociado con el trastorno
de la síntesis de proteínas debido al enlace de los compuestos con
los ribosomas de las células eucarióticas o bien con la disfunción
de las membranas celulares. La inhibición de la síntesis de las
proteínas se ha relacionado con la inducción de proteínas lábiles y
reguladoras como la IL-2 en los inmunocitos. Concentraciones
extremadamente bajas de tricotecenos alteran el transporte de
pequeñas moléculas en las membranas celulares.
2.5 Efectos en el ser humano
La ingestión de alimentos contaminados de origen vegetal es la
principal vía de exposición a tricotecenos, pero ocasionalmente se
han notificado otras, por ejemplo el contacto accidental con la
piel entre los investigadores de laboratorio y la inhalación de
tricotecenos transportados por el polvo.
Los casos registrados de enfermedad asociados con la exposición
a tricotecenos son escasos y en ninguno de ellos se ha demostrado
la relación causal. No obstante, los dos brotes que se describen a
continuación parecen indicar su existencia.
En China se registró un brote de enfermedad asociado con el
consumo de trigo mohoso que contenía de 1,0 a 40,0 mg de DON/kg.
La enfermedad se caracterizó por síntomas gastro-intestinales. No
hubo defunciones. Los cerdos y los pollos alimentados con restos
de cereales también resultaron afectados.
Un brote análogo se registró en la India, asociado al consumo
de pan hecho con trigo contaminado. La enfermedad se caracterizó
por síntomas gastrointestinales e irritación de la garganta, que
aparecía de 15 minutos a una hora después de la ingestión del pan.
En muestras de la harina de trigo refinada utilizada para su
preparación se detectaron las siguientes micotoxinas: DON
(O,35-8,3 mg/kg), acetildeoxinivalenol (0,64-2,49 mg/kg),
NIV (0,03-0,1 mg/kg) y toxina T-2 (0,5-0,8 mg/kg). Sin
embargo, no hubo confirmación de la identidad de los tricotecenos
detectados. La presencia simultánea de DON y NIV y de toxina T-2
es insólita.
Dos enfermedades de interés histórico, la aleucia tóxica
alimentaria (ATA) de la URSS y la toxicosis del trigo mohoso del
Japón y Corea se han asociado con el consumo de cereales invadidos
por hongos Fusarium. Desde entonces se han identificado en
condiciones de laboratorio algunos tricotecenos en cultivos de
mohos Fusarium aislados en cereales encontrados en los incidentes.
En la época en que apareció la enfermedad, no pudieron realizarse
estudios que relacionaran la ATA o la toxicosis de los cereales
mohosos con la exposición a tricotecenos porque las toxinas no eran
conocidas.
3. Cornezuelo
3.1 Distribución natural
Cornezuelo es el nombre dado a los esclerocios de especies de
hongos pertenecientes al género Claviceps. Alcaloides biológi-
camente activos contenidos en el esclerocio provocan toxicosis
cuando éste es consumido por hombres o animales en alimentos
contaminados.
Los alcaloides del cornezuelo producen dos modalidades
diferentes de enfermedad, según el hongo de que se trate ( C.
purpurea, C. fusiformis) y, por lo tanto, los alcaloides
producidos. El ergotismo, provocado por los alcaloides ergotamina-
ergocristina producidos por C. purpurea, se caracteriza sobre
todo por gangrena de las extremidades, además de síntomas
gastrointestinales. La intoxicación resultante de la ingestión de
mijo contaminado por C. fusiformis se caracteriza principalmente
por síntomas gastrointestinales y está relacionada con los
alcaloides de tipo clavina. No hay signos ni síntomas que indiquen
la presencia de vaso-oclusión.
3.2 Métodos de análasis
Los alcaloides del cornezuelo (ergolinas) son derivados del
ácido lisérgico. Los diversos alcaloides difieren por la
importancia de su actividad biológica. La presencia de alcaloides
del cornezuelo producidos por C. purpurea se ha determinado por
cromatografía de fase líquida de alto rendimiento con detección por
fluorescencia. Pueden medirse concentraciones de 0,2 µg de
ergolina por litro de plasma humano. La presencia de ergotamina y
ergocristina puede determinarse con gran especificidad mediante
pruebas de radioinmunovaloración en concentraciones de 3,5
picomoles y 0,8 picomoles, respectivamente.
3.3 Efectos en animales
Las ergolinas, principalmente la ergotamina y la ergotaminina,
se han asociado con brotes de abortos en el ganado bovino. Ovejas
a las que se administró ergotamina por vía oral enfermaron
rápidamente y se observó en ellas inflamación intestinal. Aves de
corral, cerdos y primates expuestos por vía oral experimentaron
efectos leves. El Grupo Especial no dispuso de datos sobre la
mutagenicidad, la teratogenicidad y la carcinogenicidad de las
ergolinas.
3.4 Efectos en el ser humano
Los cereales infectados por Claviceps son fuente de exposición
humana a las ergolinas. En la mayor parte de los estudios
toxicológicos, no se han identificado los alcaloides específicos.
La información resultante de un solo estudio sobre cereales y
productos de cereales indica que en Suiza la ingesta total
de ergolinas por los seres humanos es de alrededor de 5,1 µg por
persona, siendo el contenido de ciertos productos de hasta 140
µg/kg. La cocción reduce las ergolinas presentes en la harina
contaminada un 25-100%.
En 1978 hubo en Etiopía un brote de ergotismo, resultante de
exposición a ergolinas procedentes de esclerocios de C.
purpurea. El cereal contenía hasta un 0,75% de cornezuelo; se
detectó específicamente ergometrina. Los síntomas fueron gangrena
seca, con pérdida de uno o varios miembros (29% de los casos),
pulsos periféricos débiles o ausentes (36%) y desescamación de la
piel. Sólo hubo síntomas gastrointestinales en unos pocos casos.
Trastornos de las extremidades inferiores se registraron en el 88%
de los pacientes.
En la India ha habido varios brotes desde 1958 a consecuencia
de la ingestión de mijo perlado que contenía cornezuelo de tipo
clavina producido por C. fusiformis. Los síntomas comprendían
náuseas, vómitos y mareos, y fueron causados por la ingestión de
mijo perlado con un contenido de 15 a 26 mg de ergolina/kg.
Como en ninguno de los dos episodios se practicaron autopsias,
no se dispone de información sobre los efectos anatomopatológicos
en las vísceras humanas.
4. Evaluación de los riesgos para la salud humana
4.1 Ocratoxina A
En diversos países de Europa se ha observado exposición humana
a la ocratoxina A, como lo demuestra la presencia de ésta en
alimentos y en la sangre. El Grupo Especial no conoce ningún
intento de detectar la ocratoxina A en la sangre humana en otras
partes del mundo.
El papel causal de la ocratoxina A en la nefropatía porcina se
ha demostrado sobre la base de estudios de casos sobre el terreno y
de la reproducción de la enfermedad con ocratoxina A. Utilizando
el modelo porcino, se ha supuesto que la nefropatía endémica de los
Balcanes podría obedecer a exposición a la ocratoxina A. Los datos
epidemiológicos de que se dispone indican que esa enfermedad podría
estar asociada al consumo de alimentos contaminados por dicha
toxina. Desde la publicación de Criterios de salud ambiental 11 en
1979, estudios epidemiológicos sobre concentración de ocratoxina A
en la sangre humana en zonas afectadas y no afectadas han
proporcionado un argumento más en favor de la relación entre la
nefropatía de los Balcanes y la exposición a la ocratoxina A.
Se ha demostrado que tanto la presencia de ocratoxina A en la
sangre como sus concentraciones son mayores en las personas que
residen en las zonas de endemicidad. No obstante, las pruebas
indirectas que proporcionan los mencionados estudios retrospectivos
no bastan para demostrar por sí solas la existencia de una relación
causal directa. Esta tampoco puede excluirse, dado el largo
periodo de latencia entre la exposición y el comienzo de los
síntomas.
Se ha demostrado que la ocratoxina A es carcinogénica para el
epitelio de los túbulos renales en los ratones machos y en las
ratas de uno y otro sexo. Se ha observado una relación muy
significativa entre la nefropatía de los Balcanes y los tumores del
tracto urinario, en particular de la pelvis renal y los ureteres.
Sin embargo, no hay datos publicados que demuestren un papel causal
directo de la ocratoxina A en la etiología de esos tumores.
4.2 Tricotecenos
Sobre la base de la información facilitada al Grupo Especial,
es posible asociar la exposición a tricotecenos con episodios de
enfermedad en los seres humanos. Según los limitados datos
disponibles, los tricotecenos que intervienen más frecuentemente en
episodios de exposición humana son el DON y el NIV. En los
episodios de aleucia tóxica alimentaria y toxicosis por ingestión
de cereales mohosos registrados en el pasado, no puede excluirse el
posible papel etiológico de los tricotecenos. La exposición se
produce por la ingestión de alimentos contaminados, principalmente
cereales. La elaboración, la molienda y la cocción no logran la
destrucción del DON, el NIV y la toxina T-2. Los datos en favor de
la exposición por inhalación son muy limitados, pero no puede
excluirse esa posibilidad.
Entre los tricotecenos naturalmente presentes en alimentos, el
más potente es la toxina T-2, seguida por el DAS y el NIV; el DON
resultó el menos tóxico en los estudios de la toxicidad aguda. En
los animales experimentales, la toxina T-2 y el DAS tienen efectos
generales agudos, con necrosis de tejidos epiteliales y supresión
de la hematopoyesis. En los brotes de enfermedad contemporáneos,
sólo se han registrado síntomas gastrointestinales.
Se ha demostrado que el DON es teratogénico en los ratones pero
no en las ratas. Según los estudios de la toxicidad crónica
publicados, el NIV y la toxina T-2 no son oncogénicos en los
animales. No se han publicado estudios sobre la carcino-genicidad
a largo plazo del DON. Ciertos tricotecenos, como la toxina T-2 y
el DON, tienen una acción inmunosupresora en los animales y han
producido alteraciones de la inmunidad, tanto de base celular como
humoral. No hay datos que demuestren la existencia de una acción
inmunosupresora en el hombre.
Los casos de enfermedad humana asociados con la exposición a
tricotecenos que se han notificado son limitados, tanto por su
número como por la información proporcionada. Tras la ingestión de
alimentos contaminados con tricotecenos aparecen rápidamente
síntomas de trastornos digestivos e irritación de la garganta. En
la actualidad no hay nada que demuestre que los tricotecenos
causen cáncer en el ser humano. El Grupo Especial no tuvo a su
disposición informes sobre infección secundaria por bacterias,
hongos o virus en los seres humanos tras la exposición a
tricotecenos, como las observadas en estudios experimentales con
animales. Al parecer, no se han realizado estudios suficientes
para aclarar esa sucesión.
4.3 Cornezuelo
La exposición humana a bajas concentraciones de ergolinas
parece estar muy extendida. Los datos de que se dispone sobre los
recientes brotes en Etiopía y en la India indican que los
alcaloides producidos por C. purpurea (grupo de la ergotamina)
tienen efectos más graves-entre ellos gangrena de las piernas y
muerte-que los alcaloides producidos por C. fusiformis (grupo de la
clavina), que provocaron síntomas gastrointestinales sin desenlace
mortal. No se sabe si esas diferencias corresponden a diferencias
en el contenido de alcaloides de las especies de hongos, en las
propiedades toxicológicas o toxicométricas de los alcaloides o en
las dosis ingeridas por diferentes tipos de poblaciones.
Cuando la limpieza y la molienda eliminan los esclerocios, sólo
quedan en los alimentos preparados bajas concentraciones de
ergolinas; la cocción y otras aplicaciones de calor destruyen
también la mayor parte de los alcaloides del grupo de la
ergotamina.
5. Recomendaciones para ulterior investigación
5.1 Recomendaciones generales
Debe establecerse una red de centros de referencia, que ayuden
a los Estados Miembros a confirmar la identidad de las mico-toxinas
halladas en alimentos y tejidos humanos. Esos centros deben
proporcionar también muestras de referencia de micotoxinas, cuando
se les solicite, a fin de aumentar las posibilidades de comparar
los resultados de análisis realizados en distintas partes del
mundo.
5.2 Ocratoxina A
a) En los Balcanes y en la región del Mediterráneo deben
realizarse estudios epidemiológicos retrospectivos extensos
y prospectivos focales sobre la asociación de la ocratoxina
A con la nefropatía endémica de tipo balcánico y con
tumores del tracto urinario.
b) Fuera de los Balcanes, deben realizarse, en enfermos con
tumores del tracto urinario, análisis de sangre para
detectar la presencia de ocratoxina A.
c) En países no situados en la zona de los Balcanes, debe
aclararse la fuente de la exposición a ocratoxina A, cuando
los análisis de sangre humana indiquen que la misma existe.
d) Debe aclararse la manera en que actúa la diferencia de sexo
en las enfermedades renales, neoplásicas y no neoplásicas,
causadas por la ocratoxina A en animales de
experimentación.
e) Son necesarios extensos estudios sobre el contenido de
ocra-toxina A de los alimentos en distintas partes del
mundo. Es especialmente importante realizar estudios de
ese tipo en las regiones con una elevada incidencia de
tumores del tracto urinario, tumores renales o nefropatías.
5.3 Tricotecenos
a) Deben realizarse estudios de seguimiento en las zonas de la
India y de la República Popular China en que ha habido
recientemente episodios de intoxicación de seres humanos
por tricotecenos. Debe aclararse más la modalidad no usual
de presencia de tricotecenos en esos episodios.
b) Deben estudiarse los efectos de la exposición a largo plazo
de animales de experimentación al DON, incluidos los
efectos carcinogénicos. Como la respuesta de distintas
especies al DON varía mucho, deben elegirse cuidadosamente
las especies utilizadas para el estudio.
c) Debe aclararse más la aparición de infecciones microbianas
secundarias en animales de experimentación tras la
exposición a tricotecenos.
d) Debe estudiarse la influencia de las condiciones
ambientales, incluida la presencia de insecticidas y de
otros productos químicos artificiales, en la producción de
tricotecenos por los hongos.
e) Deben aclararse los efectos de la elaboración de los
alimentos en los tricotecenos.
f) Deben desarrollarse, mediante métodos biotecnológicos,
cultivos agrícolas resistentes a la infección por hongos
productores de tricotecenos.
g) Deben estudiarse en animales de experimentación los
posibles efectos sinérgicos de la exposición combinada a
tricotecenos, aflatoxinas, ocratoxina A y otras
micotoxinas.
h) Deben realizarse estudios sobre la ingesta de tricotecenos
por seres humanos.
i) Deben elaborarse métodos rápidos y sensibles de detección
de los tricotecenos y deben realizarse estudios para
localizar los tricotecenos en cereales y alimentos tratados
en las zonas templadas.
5.4 Cornezuelo
a) Deben hallarse métodos de análisis que permitan detectar la
presencia de agroclavinas.
b) Debe proporcionarse a los países en desarrollo información
sobre el empleo de procedimientos de detección de semillas
patológicas y de molienda para reducir al mínimo los
problemas causados por el cornezuelo.
c) Deben realizarse estudios epidemiológicos sobre los
posibles efectos de bajas concentraciones de ergolinas en
la población humana.
d) Deben efectuarse estudios farmacológicos y toxicológicos en
los que se administren a animales de experimentación
distintas ergolinas, aisladas y en combinación.
e) Debe aclararse la posible trasmisión de ergolinas a los
lactantes a través de la leche materna.
