
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 123
ALPHA- and BETA-HEXACHLOROCYCLOHEXANES
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by Dr. G.J. van Esch,
Bilthoven, The Netherlands
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization
World Health Organization
Geneva, 1992
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme, the International
Labour Organisation, and the World Health Organization. The main
objective of the IPCS is to carry out and disseminate evaluations of
the effects of chemicals on human health and the quality of the
environment. Supporting activities include the development of
epidemiological, experimental laboratory, and risk-assessment methods
that could produce internationally comparable results, and the
development of manpower in the field of toxicology. Other activities
carried out by the IPCS include the development of know-how for coping
with chemical accidents, coordination of laboratory testing and
epidemiological studies, and promotion of research on the mechanisms
of the biological action of chemicals.
WHO Library Cataloguing in Publication Data
Alpha- and Beta-hexachlorocyclohexanes.
(Environmental health criteria ; 123)
1.Benzene hexachloride - adverse effects 2.Benzene hexachloride -
toxicity 3.Environmental exposure 4.Environmental pollutants
I.Series
ISBN 92 4 157123 3 (NLM Classification: QV 633)
ISSN 0250-863X
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1991
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved. The designations
employed and the presentation of the material in this publication do
not imply the expression of any opinion whatsoever on the part of the
Secretariat of the World Health Organization concerning the legal
status of any country, territory, city or area or of its authorities,
or concerning the delimitation of its frontiers or boundaries. The
mention of specific companies or of certain manufacturers' products
does not imply that they are endorsed or recommended by the World
Health Organization in preference to others of a similar nature that
are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR ALPHA- AND BETA-HEXACHLOROCYCLOHEXANES
A. ALPHA-HEXACHLOROCYCLOHEXANE
B. BETA-HEXACHLOROCYCLOHEXANE
CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH AND THE
ENVIRONMENT (ALPHA- AND BETA-HEXACHLOROCYCLOHEXANES)
FURTHER RESEARCH (ALPHA- AND BETA-HEXACHLOROCYCLOHEXANES)
PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
APPENDIX 1. CHEMICAL STRUCTURE
RESUME ET EVALUATION
1. Alpha-hexachlorocyclohexane
2. Béta-hexachlorocyclohexane
CONCLUSIONS ET RECOMMANDATIONS
RECHERCHES A EFFECTUER (ALPHA- ET BETA-HEXACHLOROCYCLOHEXANES)
RESUMEN Y EVALUACION
1. Alpha-hexaclorociclohexano
2. Beta-hexaclorociclohexano
CONCLUSIONES Y RECOMENDACIONES
OTRAS INVESTIGACIONES (ALPHA- Y BETA-HEXACLOROCICLOHEXANOS)
WHO TASK GROUP MEETING ON ENVIRONMENTAL HEALTH CRITERIA FOR ALPHA-
AND BETA-HEXACHLOROCYCLOHEXANES
Members
Dr S. Dobson, Institute of Terrestrial Ecology, Monkswood Experimental
Station, Abbots Ripton, Huntingdon, United Kingdom
Dr M. Herbst, ASTA Pharma A.G., Frankfurt, Germany (Joint Rapporteur)
Professor J.S. Kagan, Department of General Toxicology and
Experimental Pathology, All-Union Scientific Research Institute of
Hygiene and Toxicology of Pesticides, Polymers, and Plastics, Kiev,
USSR (Vice-Chairman)
Dr S.G.A. Magwood, Pesticides Division, Environmental Health Centre,
Health & Welfare Canada, Tunney's Pasture, Ottawa, Ontario, Canada
Professor Wai-On Phoon, National Institute of Occupational Health and
Safety, University of Sydney, Sydney, Australia (Chairman)
Dr J.F. Risher, US Environmental Protection Agency, Environmental
Criteria and Assessment Office, Cincinnati, Ohio, USA
Dr Y. Saito, Division of Foods, National Institute of Hygienic
Sciences, Setagaya-ku, Tokyo, Japan
Dr V. Turusov, Laboratory of Carcinogenic Substances, All-Union Cancer
Research Centre, Moscow, USSR
Dr G.J. van Esch, Bilthoven, The Netherlands (Joint Rapporteur)
Representatives of Non-Governmental Organizations
Dr P.G. Pontal, International Group of National Associations of
Manufacturers of Agrochemical Products (GIFAP), Rhône-Poulenc Agro,
Lyon, France
Observers
Dr A.V. Bolotny, All-Union Scientific Research Institute of Hygiene
and Toxicology of Pesticides, Polymers, and Plastics, Kiev, USSR
Dr D. Demozay, International Centre for Study on Lindane (CIEL),
Rhône-Poulenc Agro, Lyon, France
Secretariat
Dr G.J. Burin, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr K.W. Jager, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Dr V.A. Rezepov, Centre for International Projects, USSR State
Committee for Environmental Protection, Moscow, USSR
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the environmental health
criteria monographs, readers are kindly requested to communicate any
errors that may have occurred to the Manager of the International
Programme on Chemical Safety, World Health Organization, Geneva,
Switzerland, in order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Palais des
Nations, 1211 Geneva 10, Switzerland (Telephone No. 7988400 or
7985850).
ENVIRONMENTAL HEALTH CRITERIA FOR ALPHA- AND BETA-HEXACHLOROCYCLOHEXANES
A WHO Task Group on Environmental Health Criteria for Alpha- and
Beta-hexachlorocyclohexanes met in Moscow from 20 to 24 November 1989.
The meeting was convened with the financial assistance of the United
Nations Environment Programme (UNEP) and was hosted by the Centre for
International Projects (CIP), USSR State Committee for Environmental
Protection. Dr V.A. Rezepov opened the meeting on behalf of the CIP
and welcomed the participants. Dr K.W. Jager welcomed the participants
on behalf of the three IPCS cooperating organizations (UNEP/ILO/WHO).
The Task Group reviewed and revised the draft criteria monograph and
made an evaluation of the risks for human health and the environment
from exposure to alpha- and beta-hexa-chlorocyclohexanes.
The first and second drafts of this monograph were prepared by
Dr G.J. van Esch (on behalf of the IPCS). Dr K.W. Jager and Dr P.G.
Jenkins, both members of the IPCS Central Unit, were responsible for
the overall scientific content and technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the document are gratefully acknowledged.
ABBREVIATIONS
cGMP cyclic guanosine monophosphate
CNS central nervous system
EEG electroencephalogram
EMG electromyogram
FDA Food and Drug Administration (USA)
FSH follicle-stimulating hormone
GABA gamma-aminobutyric acid
GGT gamma-glutamyltransferase
GLC gas-liquid chromatography
HCB hexachlorobenzene
HCCH hexachlorocyclohexene
HCH hexachlorocyclohexane
ip intraperitoneal
LH luteinizing hormone
MTD maximum tolerated dose
nd not detected
NOEL no-observed-effect level
PCB polychlorinated biphenyl
PCCH pentachlorocyclohexane
PIC picrotoxin
PTZ pentylenetetrazole
SEM smooth endoplasmic reticulum
PART A
ENVIRONMENTAL HEALTH CRITERIA FOR
ALPHA-HEXACHLOROCYCLOHEXANE
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR ALPHA-HEXACHLOROCYCLOHEXANE
1. SUMMARY AND EVALUATION
1.1. General properties
1.2. Environmental transport, distribution, and
transformation
1.3. Environmental levels and human exposure
1.4. Kinetics and metabolism
1.5. Effects on organisms in the environment
1.6. Effects on experimental animals and
in vitro test systems
1.7. Effects on humans
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity of primary constituent
2.2. Physical and chemical properties
2.3. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and distribution between media
4.2. Biotransformation
4.2.1. Biodegradation
4.2.2. Abiotic degradation
4.2.3. Bioaccumulation/biomagnification
4.2.3.1 Algae
4.2.3.2 Invertebrates
4.2.3.3 Fish
4.2.3.4 Bioconcentration in humans
4.3. Isomerization
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
5.1.2. Water
5.1.2.1 Rain water
5.1.2.2 Fresh water
5.1.2.3 Sea water
5.1.3. Soil/sediment
5.1.3.1 Dumping grounds
5.1.4. Food and feed
5.1.5. Terrestrial and aquatic organisms
5.1.5.1 Plants
5.1.5.2 Fish and mussels
5.1.5.3 Birds
5.1.5.4 Mammals
5.2. General population exposure
5.2.1. Total-diet studies
5.2.2. Air
5.2.3. Concentrations in human samples
5.2.3.1 Blood
5.2.3.2 Adipose tissue
5.2.3.3 Breast milk
6. KINETICS AND METABOLISM
6.1. Absorption and elimination
6.2. Distribution
6.3. Metabolic transformation
6.3.1. Rat
6.3.2. Bird
6.3.3. Human
6.4. Retention and biological half-life
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1. Single exposure
7.1.1. Acute toxicity
7.2. Short-term exposure
7.2.1. Oral
7.2.2. Other routes
7.2.2.1 Intravenous
7.2.2.2 Subcutaneous
7.3. Skin and eye irritation; sensitization
7.4. Long-term exposure
7.4.1. Rat oral study
7.5. Reproduction, embryotoxicity, and teratogenicity
7.6. Mutagenicity and related end-points
7.7. Carcinogenicity
7.7.1. Mouse
7.7.2. Rat
7.7.3. Initiation-promotion
7.7.4. Mode of action
7.8. Special studies
7.8.1. Effect on liver enzymes
7.8.2. Neurotoxicity
8. EFFECTS ON HUMANS
8.1. Acute toxicity - poisoning incidents
8.2. General population
8.3. Occupational exposure
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1. Algae
9.2. Protozoa
9.3. Invertebrates
9.3.1. Acute toxicity
9.3.2. Short- and long-term toxicity
9.3.2.1 Crustaceae
9.3.2.2 Molluscs
9.4. Fish
9.4.1. Acute toxicity
9.4.2. Short- and long-term toxicity
9.5. Terrestrial organisms
1. SUMMARY AND EVALUATION
1.1 General properties
Alpha-hexachlorocyclohexane (alpha-HCH) is a major by-product
(65-70%) in the manufacture of lindane (> 99% gamma-HCH). Its
solubility in water is low, but it is very soluble in organic solvents
such as acetone, chloroform, and xylene. It is a solid with a low
vapour pressure. The n-octanol/water partition coefficient (log
Pow) is 3.82. It is an environmental pollutant.
Alpha-HCH can be determined separately from the other isomers by
gas chromatography with electron capture detection and other methods
after extraction by liquid/liquid partition and purification by column
chromatography.
1.2 Environmental transport, distribution, and transformation
Biodegradation and abiotic degradation (dechlorination) by
ultraviolet irradiation occur in the environment and produce,
respectively, delta-3,4,5,6-tetrachloro-hexene and
pentachlorocyclohexene. This breakdown process is slower than in the
case of lindane. The persistence of alpha-HCH in soil is determined by
environmental factors such as the action of microorganisms, organic
matter content, and co-distillation and evaporation from soils. No
isomerization occurs from lindane to alpha-HCH.
Rapid bioconcentration takes place in microorganisms (the
bioconcentration factor equals 1500-2700 on a dry-weight basis, or
approximately 12 000 on a lipid basis within 30 min), invertebrates
(60-2750 (dry weight basis) or > 8000 (lipid basis) within 24-72 h),
and fish (313-1216 within 4-28 days; up to 50 000 in the River Elbe).
However, biotransformation and elimination is also fairly rapid in
these organisms (15 min to 72 h).
1.3 Environmental levels and human exposure
Alpha-HCH is found in air over the oceans at a concentration of
0.02-1.5 ng/m3. In Canada, it was found to be present in rain water
at a concentration of 1-40 ng/litre, but only traces were present in
snow.
During the period 1969-1974, the River Rhine and its tributaries
contained alpha-HCH levels of 0.01-2.7 µg per litre, but more recently
the levels have been below 0.1 µg/litre. In the River Elbe, levels
decreased from a mean of 0.023 µg/litre in 1981 to below 0.012 µg per
litre in 1988. Selected rivers in the United Kingdom were found in
1966 to contain 0.001-0.43 µg/litre. Alpha-HCH has been found in North
Frisian Wadden Sea sediment at concentrations of between 0.3 and
1.4 µg/kg (0.002 µg per litre in water).
Alpha-HCH levels in different plant species from various
countries varied from 0.5-2140 µg/kg on a dry-weight basis, but were
much higher in polluted areas. Even in Antarctica, levels ranging from
0.2-1.15 µg/kg have been found.
Alpha-HCH is regularly detected in fish and aquatic
invertebrates, as well as in ducks, herons, and barn-owls. In
reindeer and Idaho moose, living in areas with negligible use of
pesticides, average amounts of alpha-HCH of approximately 70-80 µg/kg
were found in the subcutaneous fat. The adipose tissue of Canadian
polar bears contained 0.3-0.87 mg alpha-HCH/kg (on a fat basis).
In a number of countries, important food items have been analysed
for the presence of alpha-HCH. The levels, mainly in fat-containing
food products, ranged up to 0.05 mg/kg product, except in milk and
milk products (up to 0.22 mg/kg) and in fish and processed meat
products (up to 0.5 mg/kg on a fat basis). A slow decrease over the
years has been noted.
Food is the main source for general population exposure to
alpha-HCH. In total-diet studies in the Netherlands and the United
Kingdom, mean concentrations of 0.01 and 0.002-0.003 mg/kg food,
respectively, were found. The United Kingdom data indicate a downward
trend since 1967. In the USA, the average daily intake of alpha-HCH
was 0.009-0.025 µg/kg body weight during the period 1977-1979, and
0.003-0.016 µg/kg body weight during the period 1982-1984.
In a few countries, the concentration of alpha-HCH has been
determined in human blood, serum, or plasma. The mean (in some cases
median) concentration was < 0.1 µg per litre (ranging from
undetectable levels to 0.6 µg per litre). In one country, however, a
mean concentration of 3.5 (range 0.1-15.0) µg/litre was reported.
Alpha-HCH was detected in approximately one third of the blood
samples.
The concentrations in human adipose tissue and breast milk are
reported to be low (respectively < 0.01-0.1 and < 0.001-0.04 mg/kg
on a fat basis). Total-diet studies have shown daily intake levels
of the order of 0.01 µg/kg body weight per day or lower. These
concentrations are decreasing slowly over the years.
Alpha-HCH appears to be a universal environmental contaminant.
Concentrations are only decreasing slowly, in spite of measures taken
to prevent its spread into the environment.
1.4 Kinetics and metabolism
In rats, alpha-HCH is rapidly and almost completely absorbed from
the gastrointestinal tract. After intraperitoneal injection,
approximately 40-80% of the alpha-HCH was excreted via the urine and
5-20% via the faeces. In rats, the highest concentrations have been
found in liver, kidneys, body fat, brain and muscles, and substantial
deposition occurs in fatty tissue. The alpha-HCH concentrations in the
liver of sucklings were twice as high as those observed in the liver
of the mothers. In rats, the brain to blood and depot fat to blood
ratios were 120:1 and 397:1, respectively.
The biotransformation of alpha-HCH in rats involves
dechlorination. The major urinary metabolite is 2,4,6-tri-
chlorophenol; other identified metabolites include 1,2,4-, 2,3,4-, and
2,4,5-trichlorophenol and 2,3,4,5- and 2,3,4,6-tetrachlorophenol.
1,3,4,5,6-Pentachlorocyclohex-1-ene has been found in rat kidneys and
also in in vitro studies on chicken liver. A glutathione conjugate
is formed in the liver.
The half-life for clearance from the fat depot is 6.9 days in
female rats and 1.6 days in males.
1.5 Effects on organisms in the environment
Alpha-HCH has low toxicity for algae, 2 mg/litre generally being
the no-observed-effect level.
In a long-term study, Daphnia magna showed a no-observed-effect
level of 0.05 mg/litre. Alpha-HCH is moderately toxic for
invertebrates and fish. The acute L(E)C50 values for these
organisms are in the order of 1 mg/litre. In short-term studies with
guppies and Oryzia latipes, 0.8 mg/litre was without effect.
In three-month studies with Salmo gairdneriat dose levels of
10-1250 mg/kg diet, there were no effects on mortality, behaviour,
growth, or enzyme activities in liver and brain.
Short- and long-term studies with a snail (Lymnea stagnalis)
showed an EC50 (based on mortality and immobilization) of
1200 µg/litre. Inhibition of egg production occurred at a
concentration of 250 µg/litre. A 50% reduction in the overall
reproductivity was found at 65 µg/litre.
No data are available on effects on populations and ecosystems.
1.6 Effects on experimental animals and in vitro test systems
The acute oral LD50 values for mice lie between 1000-4000 and
for rats between 500-4670 mg/kg body weight. The poisoning signs are
mainly those of stimulation of the central nervous system.
A 90-day study with rats showed growth depression at a
concentration of 250 mg/kg diet. Histological and enzyme level
changes in the liver indicated enzyme induction at 50 mg/kg or more.
At these dose levels there were also indications of immunosuppression.
Liver weights were already increased at 10 mg/kg diet (equivalent to
0.5 mg/kg body weight). The no-observed-adverse-effect level in this
study appeared to be 2 mg/kg diet (equivalent to 0.1 mg/kg body weight
per day).
No adequate long-term toxicity studies or reproduction and
teratogenicity studies have been reported.
Studies with various strains of Salmonella typhimurium yielded
no evidence of mutagenicity either with or without metabolic
activation. Tests with Saccharomyces cerevisiae were also negative,
but a test for unscheduled DNA synthesis in rat hepatocytes in vitro
gave an equivocal result.
Studies to determine carcinogenic potential have been carried out
with mice and rats at dose levels from 100 to 600 mg/kg diet.
Hyperplastic nodules and/or hepatocellular adenomas were found in
studies on mice. In one study the dose levels exceeded the maximum
tolerated dose. Two mice studies and one rat study, using dose levels
of up to 160 mg/kg diet in mice and 640 mg/kg diet in rats, did not
show any increase in the incidence of tumours.
The results of the studies on initiation-promotion and mode of
action and the mutagenicity studies indicate that the
alpha-HCH-induced tumorigenicity observed in mice has a non-genetic
mechanism.
Alpha-HCH has been shown to cause a clear increase in the
activity of liver enzymes even at 5 mg/kg diet (equivalent to
0.25 mg/kg body weight). A dose of 2 mg/kg body weight did not affect
aminopyrine demethylation or the DNA content of the liver.
1.7 Effects on humans
When workers at a lindane-producing factory, with a geometric
mean exposure of 7.2 years (1-30), were investigated, it was concluded
that occupational HCH exposure did not induce signs of neurological
impairment or perturbation of "neuromuscular function".
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity of primary constituent
Common name Alpha-hexachlorocyclohexane (alpha-HCH)
Chemical formula C6H6Cl6
Chemical Alpha-HCH is a stereoisomer of gamma-
structure HCH, the active ingredient of lindane
(see Appendix 1) (> 99% gamma-HCH). It differs in the
spatial orientation of the hydrogen and
chlorine atoms on the carbon atoms:
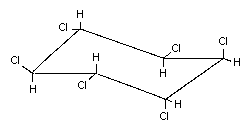 Relative
molecular mass 290.9
CAS chemical 1alpha,2alpha,3ß,4alpha,5ß,6ß-hexachloro-
name cyclohexane
Common
synonyms Alpha-benzenehexachloride (alpha-BHC)
CAS registry
number 319-84-6
RTECS registry
number GV3500000
2.2 Physical and chemical properties
Some physical and chemical properties are summarized in Table 1.
Table 1. Some physical and chemical properties of alpha-
hexachlorocyclohexane
Melting point 158°C
Boiling point 288°C
Vapour pressure (20°C) 2.67 Pa (0.02 mmHg)
Relative density (20°C) 1.87 g/cm3
Solubility
water (28°C) 2 mg/litre
organic solvents (20°C) acetone 139 g/litre
chloroform 63 g/litre
ethanol 18 g/litre
petroleum ether 7-13 g/litre
xylene 85 g/litre
Stability considerable stability in acids,
unstable in alkaline conditions
n-Octanol/water partition
coefficient (log Pow) 3.82
2.3 Analytical methods
Hildebrandt et al. (1986) and Wittlinger & Ballschmiter (1987)
described in detail the appropriate analytical methods, i.e. air
sampling by adsorption, extraction, purification, and determination
using high resolution gas chromatography. Sampling was conducted by
pumping air first through a glass fiber filter and then a layer of
silica gel. An internal standard was used. The extraction was
carried out with dichloromethane, and the extract was evaporated.
Preseparation was on silica gel and elution with a mixture of hexane
and dichloromethane. For the determination, use was made of high
resolution capillary gas chromatography with electron capture
detection and a mass selective detector.
Eder et al. (1987) described in detail three different analytical
methods for the determination of HCHs in sediments. Sediments are
extracted with a solvent or mixture of solvents and are concentrated
or fractionated. The alpha-HCH is determined by gas chromatography
with electron capture detection or other methods.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Alpha-HCH does not occur naturally. It is released to the
environment as a result of the use of technical-grade HCH and the
inappropriate disposal of the residue resulting from the purification
of lindane.
Alpha-HCH is basically a by-product (and impurity) in the
manufacturing of lindane (> 99% gamma-HCH). Technical-grade HCH,
which is synthesized from benzene and chlorine in the presence of
ultraviolet light, consists of:
65-70% alpha-HCH
7-10% beta-HCH
14-15% gamma-HCH (lindane)
approx. 7% delta-HCH
approx. 1-2% epsilon-HCH
approx. 1-2% other components
Purification of lindane produces a residue, consisting almost
entirely of non-insecticidal HCH isomers (mainly alpha- and beta-),
which can be used as an intermediate for the production of
trichlorobenzene and other chemicals.
Alpha- and beta-HCH have been used in mixtures with gamma-HCH (as
"HCH" or "fortified HCH") in agriculture and in wood protection.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
MacRae et al. (1967) studied the persistence and
bio-degradability of alpha-HCH in two clay soils. The rate of
treatment was 15 mg/kg soil, and incubation periods of 0, 15, 30, 50,
70, and 90 days were used. Only very small amounts of alpha-HCH could
be detected in non-sterilized soils after 70 days, indicating a low
level of persistence and biodegradation. However, the losses were
much slower in sterilized soils, and were probably due to
volatilization.
Tsukano (1973) studied the factors affecting the disappearance of
alpha-HCH from rice field soil after granular application
(0.05 mg/litre) to the surface water. The surface water and soil were
analysed at intervals, and alpha-HCH was found to disappear rapidly
with a half-life of about 5 days. Following translocation of
alpha-HCH (1 mg/litre) onto flooded levelled soil, a decrease in the
level in water and steady increase in the level in soil occurred.
After 7 days the concentration in soil reached a maximum. Data from a
soil column study showed that alpha-HCH moved downwards with the
percolating water.
Suzuki et al. (1975) studied the persistence of alpha-HCH in
three different types of soil. The persistence was found to be
determined by environmental factors such as the action of
microorganisms, co-distillation, evaporation from soil, and the
contents of water and organic matter in the soil.
In a study by Wahid & Sethunathan (1979), the sorption and
desorption of alpha-HCH by 12 soils from rice-growing areas in India
were studied using 14C label. The soils showed striking differences
in their ability to adsorb alpha-HCH, the sorption values ranging from
40 to 95% of total added alpha-HCH. After oxidation of the soil with
hydrogen peroxide, the sorption was lower (5-46%). Organic matter was
the most important factor governing the sorption and desorption, but
pH, exchange acidity, exchangeable sodium and magnesium, and
electrical conductivity also affected the results.
Korte (1980) summarized the behaviour of alpha-HCH in the
environment, especially in soil and plants.
4.2 Biotransformation
4.2.1 Biodegradation
Heritage & MacRae (1977, 1979) investigated the degradation of
alpha-HCH (final concentration 5 mg/litre) by a washed suspension of
Clostridium sphenoidesin the absence of oxygen at 30°C. The
alpha-isomer was no longer detectable after 4 h. Apparently the
degradation proceeded via delta-3,4,5,6-tetrachlorocyclohexene
(delta-TCCH). Aerobically grown facultative anaerobes actively
dechlorinated 36Cl-alpha-HCH during anaerobic incubation with
glucose, pyruvate or formate as substrates, but this dechlorination
was slower than in the case of lindane.
When incubation studies were performed under anaerobic or aerobic
conditions, the dechlorination of 36Cl-labelled alpha-HCH by mixed
soil flora and by pure cultures of Citrobacter freundii, C.
butyricum, and C. pasteurianumwas 6.5%, 13.9%, 97.4%, and 53.2%,
respectively, within 6 days of incubation. Again, alpha-HCH degraded
more slowly than lindane (Jagnow et al., 1977).
Screening experiments to study the possible isomerization of
lindane to alpha-HCH, using C. freundii, Serratia marcescens,
Pseudomonas putida, and other bacterial species, gave negative
results (Haider, 1979).
Doelman et al. (1985) carried out laboratory studies on the
degradation of alpha-HCH, at a concentration of approximately
5300 mg/kg, in a polluted Dutch sandy loam soil with 6.5% organic
matter. They found during 20 weeks constant degradation rates of
10 mg/kg per day under anaerobic conditions and 14 mg/kg per day under
aerobic conditions. At a lower concentration (approximately
3900 mg/kg) the average degradation rate appeared to be higher
(24 mg/kg per day) under both aerobic and anaerobic conditions. The
degradation was ascribed to microbial processes.
Studies in 1986 on HCH-polluted soil (personal communication by
P. Doelman and A. Zehnder to the IPCS) indicate that alpha-HCH
degrades considerably better in aerobic conditions (aerated slurry)
than in anaerobic conditions (non-aerated slurry) both in the
laboratory and in soil in greenhouses (Slooff & Matthijsen, 1988).
Assuming the degradation process to be a first-order reaction, MacRae
et al. (1984) calculated from laboratory studies (soil with 4.0%
organic carbon) half-lives of 125 and 48 days under aerobic and
anaerobic conditions, respectively.
In a study by Doelman et al. (1988a), microbial soil sanitation
was applied to calcareous alkaline sandy loam soil that was polluted
with a mixture of HCH isomers. Under anaerobic conditions, microbial
degradation in the Dutch climate (soil temperature of 5-17°C) did not
occur, and even the low concentration of the easily degradable
gamma-HCH did not decrease.
Microbial soil sanitation of alpha-HCH-polluted calcareous sandy
loam soil systems has been investigated. The soil systems involved
were aerated moist soil and continuously aerated and intermittently
aerated soil slurries. Degradation of alpha-HCH appeared to proceed
according to a first-order reaction. It was fastest during the first
4 weeks, even though soil temperatures were lowest during this period.
The percentage degradation during the first 4 weeks was 40, 80, and
37%, respectively, for the three soil systems. The degradation rate
gradually decreased with time even if the temperature increased.
Addition of microbial biomass did not significantly affect the
alpha-HCH degradation. In a continuously aerated thick slurry system,
the alpha-HCH concentration was reduced from approximately 420 to
15 mg/kg. Thus, alpha-HCH degradation will occur in regions with a
temperate climate, provided that the soil is aerobic (Doelman et al.,
1988b).
A field investigation into the distribution of HCHs was carried
out by Chessells et al. (1988) using soil from an agricultural area
treated with BHC-20 (HCH composition: 70% alpha-HCH, 6.5% beta-HCH,
13.5% gamma-HCH, and 5% delta-HCH. Although the concentration of
alpha-HCH was the highest of the HCHs, the alpha-isomer disappeared
more rapidly than beta-HCH. Furthermore, soil organic carbon content
was found to be of primary importance. A significant decrease in
isomer concentration was observed when soil moisture content was high
and was attributed to microbial degradation favoured by these
conditions.
4.2.2 Abiotic degradation
Alpha-HCH is broken down by ultraviolet light but at a slower
rate than lindane. Ultraviolet irradiation, using a 15-watt low
pressure mercury lamp, of alpha-HCH in 2-propanol solution for 10 h
resulted in the production of an isomer of pentachlorocyclohexene.
This substance may be produced by hydrogen abstraction of the
radiation-induced pentachlorocyclohexyl radicals (Hamada et al.,
1982).
4.2.3 Bioaccumulation/Biomagnification
4.2.3.1 Algae
A study was carried out to determine the bioconcentration of
alpha-HCH by an alga (Cladophora) during a period of 48 h. At
concentrations of alpha-HCH in water of 4.4 and 31 µg/litre, the
bioconcentration factors were 341 and 180, respectively (Bauer, 1972).
In a study by Canton et al. (1975), Chlorella pyrenoidosacells
taken from a log-phase culture were exposed for 96 h to alpha-HCH
(> 95%) concentrations of 10, 50 or 800 µg/litre, and after 15, 30, and
180 min the cells were analysed. At all dosage levels the average
bioconcentration from water was about 200-fold (153-267). There
seemed to be a tendency for alpha-HCH to accumulate in the cytoplasm
rather than the cell wall. When the cells were subsequently placed in
clean water, the elimination was rapid (15 min).
When Canton et al. (1977) investigated the accumulation and
elimination of alpha-HCH (> 95%) in marine algae (Chlamydomonas and
Dunaliella) in studies lasting a few days, both processes were found
to take place rapidly, (i.e. in less than 30 min). The average
concentration factor was 2700 in Chlamydomonas and 1500 in
Dunaliella (on a dry weight basis) and was 12 000 and 13 000,
respectively, on a lipid basis. The accumulated alpha-HCH was found
primarily in the lipophyllic parts of the cells.
4.2.3.2 Invertebrates
In a study by Canton et al. (1978), Artemia was exposed to
alpha-HCH (> 95%) levels of 0.01, 0.05 or 0.25 mg/litre and sampled
after 0.5, 3, 24, 48, 72, and 96 h. Once equilibrium was reached, the
animals were transferred to alpha-HCH-free water and were sampled
after 0, 3, 24, 48, 96, and 144 h. The bioconcentration factor was
about 60-90 (8000-11 000 on a lipid basis), and equilibrium was
reached within 24 h. The elimination half-life was 48-72 h.
Ernst (1979) measured alpha-HCH bioconcentration factors in two
marine invertebrates, the mussel (Mytilus edulis) and the polychaete
(Lanice conchilega), of 105 and 2750, respectively, at 10°C and an
alpha-HCH concentration of 2-5 µg/litre. Species differences and the
lipid content of the animals appeared clearly to affect the
bioconcentration factor, whereas the effect of temperature seemed to
be minimal.
In a study by Yamato et al. (1983), the short-necked clam
(Venerupis japonica) rapidly absorbed alpha-HCH and the
concentration reached a plateau on the third day. The
bioconcentration factor was 161 at an alpha-HCH concentration of
1 µg/litre water. The alpha-HCH concentrations on day 6 in organs and
tissues were 0.060 and 0.029 mg/kg, respectively. After a 3-day
elimination period, the levels were 0.033 and 0.024 mg/kg,
respectively.
Mouvet et al. (1985) investigated the presence of alpha-HCH in
the aquatic moss Cinclidotus danubicus to examine the potential use
of this species as an indicator of chlorinated pollutants in fresh
water. The moss was sampled 0, 13, 24, and 51 days after having been
transplanted in a polluted river, and levels of 0.20-1.33 µg per litre
water were found 4 km downstream of an area of industrial discharge.
The levels of alpha-HCH in the moss were < 0.025, 0.04-0.57,
0.08-2.37, and 0.81 mg/kg dry weight, respectively, at the time
intervals indicated above.
4.2.3.3 Fish
Canton et al. (1975) studied the accumulation and elimination of
alpha-HCH by Chlorella, Daphnia, and Poecilia reticulata, and in
Chlorella-Daphnia and Daphnia-Poecilia reticulata systems. In this
food-chain study, the following concentration ratios were measured:
Relative
molecular mass 290.9
CAS chemical 1alpha,2alpha,3ß,4alpha,5ß,6ß-hexachloro-
name cyclohexane
Common
synonyms Alpha-benzenehexachloride (alpha-BHC)
CAS registry
number 319-84-6
RTECS registry
number GV3500000
2.2 Physical and chemical properties
Some physical and chemical properties are summarized in Table 1.
Table 1. Some physical and chemical properties of alpha-
hexachlorocyclohexane
Melting point 158°C
Boiling point 288°C
Vapour pressure (20°C) 2.67 Pa (0.02 mmHg)
Relative density (20°C) 1.87 g/cm3
Solubility
water (28°C) 2 mg/litre
organic solvents (20°C) acetone 139 g/litre
chloroform 63 g/litre
ethanol 18 g/litre
petroleum ether 7-13 g/litre
xylene 85 g/litre
Stability considerable stability in acids,
unstable in alkaline conditions
n-Octanol/water partition
coefficient (log Pow) 3.82
2.3 Analytical methods
Hildebrandt et al. (1986) and Wittlinger & Ballschmiter (1987)
described in detail the appropriate analytical methods, i.e. air
sampling by adsorption, extraction, purification, and determination
using high resolution gas chromatography. Sampling was conducted by
pumping air first through a glass fiber filter and then a layer of
silica gel. An internal standard was used. The extraction was
carried out with dichloromethane, and the extract was evaporated.
Preseparation was on silica gel and elution with a mixture of hexane
and dichloromethane. For the determination, use was made of high
resolution capillary gas chromatography with electron capture
detection and a mass selective detector.
Eder et al. (1987) described in detail three different analytical
methods for the determination of HCHs in sediments. Sediments are
extracted with a solvent or mixture of solvents and are concentrated
or fractionated. The alpha-HCH is determined by gas chromatography
with electron capture detection or other methods.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Alpha-HCH does not occur naturally. It is released to the
environment as a result of the use of technical-grade HCH and the
inappropriate disposal of the residue resulting from the purification
of lindane.
Alpha-HCH is basically a by-product (and impurity) in the
manufacturing of lindane (> 99% gamma-HCH). Technical-grade HCH,
which is synthesized from benzene and chlorine in the presence of
ultraviolet light, consists of:
65-70% alpha-HCH
7-10% beta-HCH
14-15% gamma-HCH (lindane)
approx. 7% delta-HCH
approx. 1-2% epsilon-HCH
approx. 1-2% other components
Purification of lindane produces a residue, consisting almost
entirely of non-insecticidal HCH isomers (mainly alpha- and beta-),
which can be used as an intermediate for the production of
trichlorobenzene and other chemicals.
Alpha- and beta-HCH have been used in mixtures with gamma-HCH (as
"HCH" or "fortified HCH") in agriculture and in wood protection.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
MacRae et al. (1967) studied the persistence and
bio-degradability of alpha-HCH in two clay soils. The rate of
treatment was 15 mg/kg soil, and incubation periods of 0, 15, 30, 50,
70, and 90 days were used. Only very small amounts of alpha-HCH could
be detected in non-sterilized soils after 70 days, indicating a low
level of persistence and biodegradation. However, the losses were
much slower in sterilized soils, and were probably due to
volatilization.
Tsukano (1973) studied the factors affecting the disappearance of
alpha-HCH from rice field soil after granular application
(0.05 mg/litre) to the surface water. The surface water and soil were
analysed at intervals, and alpha-HCH was found to disappear rapidly
with a half-life of about 5 days. Following translocation of
alpha-HCH (1 mg/litre) onto flooded levelled soil, a decrease in the
level in water and steady increase in the level in soil occurred.
After 7 days the concentration in soil reached a maximum. Data from a
soil column study showed that alpha-HCH moved downwards with the
percolating water.
Suzuki et al. (1975) studied the persistence of alpha-HCH in
three different types of soil. The persistence was found to be
determined by environmental factors such as the action of
microorganisms, co-distillation, evaporation from soil, and the
contents of water and organic matter in the soil.
In a study by Wahid & Sethunathan (1979), the sorption and
desorption of alpha-HCH by 12 soils from rice-growing areas in India
were studied using 14C label. The soils showed striking differences
in their ability to adsorb alpha-HCH, the sorption values ranging from
40 to 95% of total added alpha-HCH. After oxidation of the soil with
hydrogen peroxide, the sorption was lower (5-46%). Organic matter was
the most important factor governing the sorption and desorption, but
pH, exchange acidity, exchangeable sodium and magnesium, and
electrical conductivity also affected the results.
Korte (1980) summarized the behaviour of alpha-HCH in the
environment, especially in soil and plants.
4.2 Biotransformation
4.2.1 Biodegradation
Heritage & MacRae (1977, 1979) investigated the degradation of
alpha-HCH (final concentration 5 mg/litre) by a washed suspension of
Clostridium sphenoidesin the absence of oxygen at 30°C. The
alpha-isomer was no longer detectable after 4 h. Apparently the
degradation proceeded via delta-3,4,5,6-tetrachlorocyclohexene
(delta-TCCH). Aerobically grown facultative anaerobes actively
dechlorinated 36Cl-alpha-HCH during anaerobic incubation with
glucose, pyruvate or formate as substrates, but this dechlorination
was slower than in the case of lindane.
When incubation studies were performed under anaerobic or aerobic
conditions, the dechlorination of 36Cl-labelled alpha-HCH by mixed
soil flora and by pure cultures of Citrobacter freundii, C.
butyricum, and C. pasteurianumwas 6.5%, 13.9%, 97.4%, and 53.2%,
respectively, within 6 days of incubation. Again, alpha-HCH degraded
more slowly than lindane (Jagnow et al., 1977).
Screening experiments to study the possible isomerization of
lindane to alpha-HCH, using C. freundii, Serratia marcescens,
Pseudomonas putida, and other bacterial species, gave negative
results (Haider, 1979).
Doelman et al. (1985) carried out laboratory studies on the
degradation of alpha-HCH, at a concentration of approximately
5300 mg/kg, in a polluted Dutch sandy loam soil with 6.5% organic
matter. They found during 20 weeks constant degradation rates of
10 mg/kg per day under anaerobic conditions and 14 mg/kg per day under
aerobic conditions. At a lower concentration (approximately
3900 mg/kg) the average degradation rate appeared to be higher
(24 mg/kg per day) under both aerobic and anaerobic conditions. The
degradation was ascribed to microbial processes.
Studies in 1986 on HCH-polluted soil (personal communication by
P. Doelman and A. Zehnder to the IPCS) indicate that alpha-HCH
degrades considerably better in aerobic conditions (aerated slurry)
than in anaerobic conditions (non-aerated slurry) both in the
laboratory and in soil in greenhouses (Slooff & Matthijsen, 1988).
Assuming the degradation process to be a first-order reaction, MacRae
et al. (1984) calculated from laboratory studies (soil with 4.0%
organic carbon) half-lives of 125 and 48 days under aerobic and
anaerobic conditions, respectively.
In a study by Doelman et al. (1988a), microbial soil sanitation
was applied to calcareous alkaline sandy loam soil that was polluted
with a mixture of HCH isomers. Under anaerobic conditions, microbial
degradation in the Dutch climate (soil temperature of 5-17°C) did not
occur, and even the low concentration of the easily degradable
gamma-HCH did not decrease.
Microbial soil sanitation of alpha-HCH-polluted calcareous sandy
loam soil systems has been investigated. The soil systems involved
were aerated moist soil and continuously aerated and intermittently
aerated soil slurries. Degradation of alpha-HCH appeared to proceed
according to a first-order reaction. It was fastest during the first
4 weeks, even though soil temperatures were lowest during this period.
The percentage degradation during the first 4 weeks was 40, 80, and
37%, respectively, for the three soil systems. The degradation rate
gradually decreased with time even if the temperature increased.
Addition of microbial biomass did not significantly affect the
alpha-HCH degradation. In a continuously aerated thick slurry system,
the alpha-HCH concentration was reduced from approximately 420 to
15 mg/kg. Thus, alpha-HCH degradation will occur in regions with a
temperate climate, provided that the soil is aerobic (Doelman et al.,
1988b).
A field investigation into the distribution of HCHs was carried
out by Chessells et al. (1988) using soil from an agricultural area
treated with BHC-20 (HCH composition: 70% alpha-HCH, 6.5% beta-HCH,
13.5% gamma-HCH, and 5% delta-HCH. Although the concentration of
alpha-HCH was the highest of the HCHs, the alpha-isomer disappeared
more rapidly than beta-HCH. Furthermore, soil organic carbon content
was found to be of primary importance. A significant decrease in
isomer concentration was observed when soil moisture content was high
and was attributed to microbial degradation favoured by these
conditions.
4.2.2 Abiotic degradation
Alpha-HCH is broken down by ultraviolet light but at a slower
rate than lindane. Ultraviolet irradiation, using a 15-watt low
pressure mercury lamp, of alpha-HCH in 2-propanol solution for 10 h
resulted in the production of an isomer of pentachlorocyclohexene.
This substance may be produced by hydrogen abstraction of the
radiation-induced pentachlorocyclohexyl radicals (Hamada et al.,
1982).
4.2.3 Bioaccumulation/Biomagnification
4.2.3.1 Algae
A study was carried out to determine the bioconcentration of
alpha-HCH by an alga (Cladophora) during a period of 48 h. At
concentrations of alpha-HCH in water of 4.4 and 31 µg/litre, the
bioconcentration factors were 341 and 180, respectively (Bauer, 1972).
In a study by Canton et al. (1975), Chlorella pyrenoidosacells
taken from a log-phase culture were exposed for 96 h to alpha-HCH
(> 95%) concentrations of 10, 50 or 800 µg/litre, and after 15, 30, and
180 min the cells were analysed. At all dosage levels the average
bioconcentration from water was about 200-fold (153-267). There
seemed to be a tendency for alpha-HCH to accumulate in the cytoplasm
rather than the cell wall. When the cells were subsequently placed in
clean water, the elimination was rapid (15 min).
When Canton et al. (1977) investigated the accumulation and
elimination of alpha-HCH (> 95%) in marine algae (Chlamydomonas and
Dunaliella) in studies lasting a few days, both processes were found
to take place rapidly, (i.e. in less than 30 min). The average
concentration factor was 2700 in Chlamydomonas and 1500 in
Dunaliella (on a dry weight basis) and was 12 000 and 13 000,
respectively, on a lipid basis. The accumulated alpha-HCH was found
primarily in the lipophyllic parts of the cells.
4.2.3.2 Invertebrates
In a study by Canton et al. (1978), Artemia was exposed to
alpha-HCH (> 95%) levels of 0.01, 0.05 or 0.25 mg/litre and sampled
after 0.5, 3, 24, 48, 72, and 96 h. Once equilibrium was reached, the
animals were transferred to alpha-HCH-free water and were sampled
after 0, 3, 24, 48, 96, and 144 h. The bioconcentration factor was
about 60-90 (8000-11 000 on a lipid basis), and equilibrium was
reached within 24 h. The elimination half-life was 48-72 h.
Ernst (1979) measured alpha-HCH bioconcentration factors in two
marine invertebrates, the mussel (Mytilus edulis) and the polychaete
(Lanice conchilega), of 105 and 2750, respectively, at 10°C and an
alpha-HCH concentration of 2-5 µg/litre. Species differences and the
lipid content of the animals appeared clearly to affect the
bioconcentration factor, whereas the effect of temperature seemed to
be minimal.
In a study by Yamato et al. (1983), the short-necked clam
(Venerupis japonica) rapidly absorbed alpha-HCH and the
concentration reached a plateau on the third day. The
bioconcentration factor was 161 at an alpha-HCH concentration of
1 µg/litre water. The alpha-HCH concentrations on day 6 in organs and
tissues were 0.060 and 0.029 mg/kg, respectively. After a 3-day
elimination period, the levels were 0.033 and 0.024 mg/kg,
respectively.
Mouvet et al. (1985) investigated the presence of alpha-HCH in
the aquatic moss Cinclidotus danubicus to examine the potential use
of this species as an indicator of chlorinated pollutants in fresh
water. The moss was sampled 0, 13, 24, and 51 days after having been
transplanted in a polluted river, and levels of 0.20-1.33 µg per litre
water were found 4 km downstream of an area of industrial discharge.
The levels of alpha-HCH in the moss were < 0.025, 0.04-0.57,
0.08-2.37, and 0.81 mg/kg dry weight, respectively, at the time
intervals indicated above.
4.2.3.3 Fish
Canton et al. (1975) studied the accumulation and elimination of
alpha-HCH by Chlorella, Daphnia, and Poecilia reticulata, and in
Chlorella-Daphnia and Daphnia-Poecilia reticulata systems. In this
food-chain study, the following concentration ratios were measured:
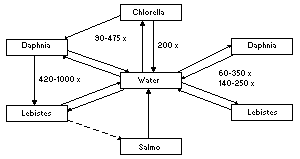 The direct uptake of alpha-HCH from contaminated water appeared to be
much greater than the uptake from contaminated food.
In a study with Salmo gairdneri, pellets containing alpha-HCH
(> 95%) levels of 0, 10, 50, 250, or 1250 mg/kg were fed to the fish,
and organs and tissues were analysed after 2, 4, 8, and 12 weeks.
There was a dose-related increase in the concentration of alpha-HCH
in the organs and tissues. After about 4-8 weeks (depending on the
type of tissue and dose level) a maximum concentration was reached,
which then slowly decreased. This suggests that after a few weeks a
balance is reached between the accumulation process (absorption of
alpha-HCH by the intestinal wall) and the elimination process (via the
gills and faeces). There is probably also a dilution effect resulting
from growth and biotransformation (Canton et al., 1975).
Ernst (1977) concluded from kinetic studies that biomagnification
of alpha-HCH does not occur. Compared with bioaccumulation from water
alone, the entry of alpha-HCH into the food chain Chlorella ->
Daphnia -> Poecilia (guppy) caused only a slight increase in
biomagnification in daphnids (factor 1.5), although in the case of the
guppies a greater increase in concentration ratio (3-4) was noted.
In a study by Canton et al. (1978), guppies (3-4 weeks old) were
exposed to alpha-HCH (> 95%) concentrations of 0.01, 0.05, or
0.14 mg/litre. When after 0.5, 3, 24, 48, 72, 96, and 120 h the
animals were analysed, the average concentration factor was about 500
for all alpha-HCH concentrations (about 17 000 on a lipid basis).
Equilibrium was reached within 24 h for the lower concentrations and
within 48 h at the highest concentrations. The elimination was rapid,
the initial concentration being halved in 10 h.
Sugiura et al. (1979) studied bioaccumulation in the carp
(Cyprinus carpio), brown trout (Salmo trutta fario), golden orfe
(Leuciscus idus melanotus), and guppy (Poecilia reticulata).
Alpha-HCH was dissolved in water to a concentration of 1 mg/litre
under steady-state conditions (time period not specified), and the
equilibrium bioconcentration factors for the four types of fish were
330, 605, 1216, and 588, respectively.
Based on the data given in section 5.1.5.2 concerning the
concentration of alpha-HCH in the muscle and fat of bream collected in
the River Elbe, the bioconcentration factor is between 10 000 and
50 000 (Arbeitsgemeinschaft für die Reinhaltung der Elbe, 1982).
In a study by Yamato et al. (1983), guppies (Poecilia
reticulata) rapidly bioaccumulated HCH isomers and the tissue
concentration reached a plateau on the fourth day (the alpha-HCH
concentration in the water was 1 µg per litre). The bioconcentration
factor (concentration in fish/concentration in water) was 706. The
concentration in the guppies decreased on the first day after the fish
were transferred to HCH-free water.
4.2.3.4 Bioconcentration in humans
Geyer et al. (1986) found that in industrialized countries more
than 90% of the exposure to HCHs derives from food. The mean
concentration of alpha-HCH in human adipose tissue (on a fat basis)
was found to be 0.03 mg/kg in the Federal Republic of Germany and
0.02 mg/kg in the Netherlands. The mean bioconcentration factor (on a
lipid basis), calculated on the basis of the concentration in the diet
(1.3 and 0.3 µg/kg, respectively) and levels in adipose tissue, was
20.0 ± 8 (range 11.5-32.5).
4.3 Isomerization
Deo et al. (1981) studied the isomerization of alpha-HCH in
sterile aqueous solution over a period of 4 weeks and found a slow
conversion of alpha-HCH to other HCH isomers.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Tanabe et al. (1982) found alpha-HCH in 24 samples of air over
the Western Pacific, Eastern Indian, and Antarctic Oceans at an
average concentration of 0.29 ng/m3 (0.022-1.4 ng/m3).
In a study by Strachan et al. (1980), samples of atmospheric
precipitation in the form of snow (1976; 17 samples) and rain (1976
and 1977; 81 samples) collected around the Canadian side of the Great
Lakes, as well as inland, were analysed. Alpha-HCH was found in the
snow samples as a trace (1 ng/litre) and in the rain samples at levels
of 1-40 ng/litre.
Air samples were taken near a road with heavy traffic, as well as
in a suburban residential area, near Ulm, in Germany. The alpha-HCH
levels were 0.22-1.3 ng/m3 in the location with heavy traffic and
0.11-1.1 ng/m3 in the rural area. It was concluded that the
concentrations in the lower troposphere under various meteorological
conditions reflect regional input and long-range transport (Wittlinger
& Ballschmiter, 1987).
In 1972, alpha-HCH air concentrations of 0.28 ng per m3 in
non-polluted areas of Germany, and 2.15 ng/m3 in the polluted Ruhr
area were determined (Hildebrandt et al., 1986).
The average concentration of alpha-HCH in 55 air samples
collected in Delft, the Netherlands, in 1979-1980 was 0.25 ng/m3
(maximum concentration: 1.2 ng/m3) (Slooff & Matthijsen, 1988).
5.1.2 Water
5.1.2.1 Rain water
Rain water sampled in 1983 in Bilt, the Netherlands, contained an
average alpha-HCH concentration of 0.01 (< 0.01-0.02) µg/litre
(Slooff & Matthijsen, 1988).
5.1.2.2 Fresh water
During the period 1969-1977, 1826 water samples were taken at 99
sampling sites in the Netherlands. The highest concentrations of
alpha-HCH were found in the River Rhine and its tributaries. The
concentrations varied between 0.01-0.3 µg/litre during the period
1969-1974, but in 1974 there was a sudden decrease and the subsequent
concentrations were all below 0.1 µg/litre. A sampling trip by boat
made along the River Rhine from Rheinfelden in Switzerland to
Rotterdam in the Netherlands proved that the source of alpha-, beta-,
and gamma-HCH was located in the upper Rhine. In the River Meuse, the
levels were all below 0.1 µg/litre during the period 1969-1977 (Wegman
& Greve, 1980).
Since 1969, alpha-, beta-, and gamma-HCH concentrations have been
measured regularly in the Rivers Rhine, Meuse, and West-Scheldt and in
other surface waters in the Netherlands. Alpha-HCH levels have been
below 0.05 µg per litre in the River Rhine since 1974/1975, and were
of the order of 0.02 µg/litre or less in the West-Scheldt during the
period 1973-1985. In the River Meuse, the concentration of alpha-HCH
was between 0.01-0.02 µg/litre. In other areas, for instance
agricultural and greenhouse horticulture areas, the levels of the
individual HCHs ranged from 0.01-1.0 µg/litre with incidental higher
peaks (up to 0.5 µg/litre) probably resulting from the use of lindane
(Slooff & Matthijsen, 1988).
Concentrations of HCH isomers in solution and in suspension
(particle-bound) in the Meuse and Rhine estuary were determined in
1974. The average concentrations of dissolved and suspended alpha-HCH
were 20 and 0-6 ng per litre, respectively. In 1981, the
concentration of dissolved alpha-HCH in coastal waters of the
Netherlands was 0.9-1.6 ng/litre, whereas that of suspended alpha-HCH
(only one measurement) was 5.3 ng/litre (Slooff & Matthijsen, 1988).
In 1970-1971, the levels of alpha-HCH were 0.66-1.5 µg/litre in
the surface water of the River Elbe near Hamburg, Germany, and
0.155-2.4 µg/litre in the River Rhine near Karlsruhe. However, a
significant decrease was observed in the mid-1970s. In 1974, 2.7 µg
per litre was found in the upper Rhine, but by 1976-1977 the levels
had decreased to 1-9 ng/litre (Hildebrandt et al., 1986).
The Arbeitsgemeinschaft der Elbe (the Elbe Study Group)
investigated the presence of alpha-HCH in the River Elbe from
Schnackenburg to the North Sea in 1981-1982 and found a mean
concentration of 0.023 (< 0.001-0.15) µg per litre. During the period
February to November 1988, the alpha-HCH concentration was
0.001-0.022 µg/litre (Arbeitsgemeinschaft der Elbe, 1988).
When certain rivers in Yorkshire, England, were analysed for
alpha-HCH in 1966, the concentration varied from 0.001 to
0.43 µg/litre. In 1968, the highest value was 0.543 µg/litre, and
the water from six other rivers contained an average of
0.001-0.004 µg/litre (highest level: 0.34 µg/litre) (Lowden et al.,
1969).
In Japan, 60 water samples were examined in 1974 and 0.1 µg
alpha-HCH/litre was detected in three of the samples (personal
communications by A. Hamada and by T. Onishi to the IPCS, July 1989).
5.1.2.3 Sea water
Atlas & Gias (1981); Bidleman & Leonard (1982); Oehme & Stray
(1982); and Oehme & Mano (1984) analysed sea water from areas such as
the North Pacific, Arabic Sea, Persian Gulf, Red Sea, Lillestrum, Bear
Island, and Spitsbergen. The alpha-HCH concentrations varied from
0.03 to 1.8 ngper litre (Slooff & Matthijsen, 1988).
In June-July 1986, the alpha-HCH in the surface water (5 m) of
the North Sea ranged from 1-2 ng/litre (Umweltbundesamt, 1989).
5.1.3 Soil/Sediment
Herrmann et al. (1984) studied the presence of alpha-HCH in
sediment along the Husum estuary and in the adjacent North Frisian
Wadden Sea. The mean concentrations varied in the different sampling
stations from 0.33 to 1.40 µg/kg sediment, while the concentrations in
bladder wrack(Fucus vesiculosus)varied from 0.7-1.2 µg/kg.
Edelman (1984) analysed 96 samples of the upper 10 cm of the soil
from 38 natural reserves in the Netherlands for alpha-HCH and
gamma-HCH. In 94 of the samples alpha-HCH was detected at levels
below 1 µg/kg (Slooff & Matthijsen, 1988).
When sediment from eight different rivers, harbours, and sites
close to dumping areas in the Netherlands were analysed for the
presence of alpha-, beta-, and gamma-HCH, the median alpha-HCH levels
were between 4 and 213 µg per kg dry matter (Slooff & Matthijsen,
1988).
In 1974, 60 sediment samples were analysed in Japan and 10 µg
alpha-HCH/kg was detected in five of the samples (personal
communications by A. Hamada and by T. Onishi to the IPCS, July 1989).
5.1.3.1 Dumping grounds
In the Netherlands, soil has been polluted with HCHs at various
locations as a result of their manufacture during the 1950s (spillage
during production, storage, and handling), and concentrations up to a
few grams of HCHs/kg dry soil have been found. Further pollution has
been caused by the dumping of chemical waste and its use in the
levelling of certain areas. From these dumping areas dispersal of the
chemical waste can occur by leaching or wind erosion from open storage
depots. In certain polluted areas, high concentrations of HCHs, mainly
alpha- and beta-HCH, have been found more than 2 m below ground level.
In 18 locations in the Netherlands, the average concentration of
alpha-HCH in sewage sludge in 1981 was between 5 and 70 µg/kg dry
matter. Pollution of ground water was also detected, but this was
restricted to the vicinity of the production areas. Horizontal
transportation of HCHs in ground water appeared to be limited (Slooff
& Matthijsen, 1988).
5.1.4 Food and feed
The presence of alpha-HCH in a number of important food items has
been determined in France by Laugel (1981). In milk and milk products
(2688 samples) the average level was 0.05 mg/kg (ranging from
undetectable to 0.22 mg/kg), in meat (37 samples) it was 0.01 mg/kg
(ranging from undetectable to 0.02 mg/kg), and in animal fat (67
samples) it was 0.02 mg/kg product (ranging from undetectable to
0.06 mg/kg. In other food items alpha-HCH was not detectable
(< 0.005 mg/kg).
Table 2 gives the mean alpha-HCH levels in a large number of
samples of various food items from the Federal Republic of Germany
reported by Hildebrandt et al. (1986).
Table 2. Alpha-hexachlorocyclohexane concentrations (mg/kg)
in various food itemsa
Food items 1973-78 1979-83 1973-83
Meatb 0.003-0.02
Meat productsb 0.007-0.037
(0.26)e
Animal fatb 0.003-0.008
(0.09)e
Gameb 0.019-0.367
Poultryb 0.003-0.004 0.003-0.016
(0.17)e
Chicken eggs < 0.001-0.003
Fish 0.002-0.011
Milk and milk
productsb 0.015e 0.01-0.03
Cow's milkb,c 0.004
Butterb,d 0.02-0.03
Table 2 (contd)
Food items 1973-78 1979-83 1973-83
Vegetable oil and
margarineb 0.01
Oil seeds, nuts,
pulses 0.001-0.042
Fruit, vegetables, < 0.0001
potatoes
Cereals 0.0002-0.007
Cereal products up to 0.14
a From: Hildebrandt et al. (1986).
b Determinations made on a fat basis
c WHO (1986).
d Anon (1984).
e Maximum value
In six samples of cows milk collected from six locations in
Switzerland, the levels of alpha-HCH were 9.5-27 mg/kg on a fat basis
(Rappe et al., 1987).
Skaftason & Johannesson (1979) analysed 35 samples of butter from
Iceland during 1968-1970 and found a level of mean alpha-HCH of 87 ±
38 µg/kg. In 1974-1978, 32 samples were studied and all contained
alpha-HCH, the mean concentration being 58 ± 21 µg/kg.
In a total-diet study in the United Kingdom, 24 samples of each
food group were analysed for alpha-HCH. The following concentrations
(mean and range) were found: bread, not detected (nd); other cereal
products, < 0.0005 (nd-0.002); carcass meat, < 0.0005 (nd-0.006);
offal, < 0.0005 (nd-0.007); meat products, eggs, green vegetables,
potatoes, fresh fruit, nd; poultry, 0.003 (nd-0.025); fish, 0.0005
(nd-0.008); oil and fats, 0.0005 (nd-0.003); milk, 0.0005 (nd-0.002);
dairy products, 0.006 (nd-0.02) mg/kg product. Imported meat products
were also analysed during the period 1981-1983, and concentrations of
up to 0.5 mg/kg were measured. Imported retail cereal products
collected in 1982 contained alpha-HCH levels of up to 0.03 mg/kg and
animal feed stuffs collected in 1984 had levels of up to 0.02 mg/kg
(HMSO, 1986).
Various types of pulses were analysed during the period
1986-1987, and 31 out of 142 samples contained alpha-HCH residues at
levels of up to 0.03 mg/kg. Processed pork and poultry, sampled
during the period 1985-1987, contained alpha-HCH at levels of up to
3.2 (mean 0.2) and 0.1-2.0 (mean 0.8) mg/kg product, respectively (26
out of the 86 samples were positive). Of other processed meat
products, 631 samples were negative. Retail milk and dairy products
were analysed during the period 1984-1987, and 499 of the 849
samples contained alpha-HCH residues at a mean concentration of
0.01-0.03 mg/kg (highest level, 0.06 mg/kg). Samples of eel muscle
(1124 eels from 62 sites) were analysed during the period 1986-1987,
and mean concentrations were 0.001-0.03 mg/kg (highest level,
0.4 mg/kg). Peanut butter and vegetable oils were analysed during the
period 1985-1987, and 95 samples showed mean concentrations of <
0.01-0.03 mg/kg product (16 of the samples were positive) (HMSO,
1989).
The mean residue level of alpha-HCH in milk samples collected
during spring 1983 from 359 bulk transporters representing 16
counties, municipalities, and districts of Ontario was 5.3 µg/kg
butter fat. Alpha-HCH was found in over 90% of the samples (Frank et
al., 1985).
5.1.5 Terrestrial and aquatic organisms
5.1.5.1 Plants
Samples of three types of mosses and four types of lichens (in
total 13 samples) were collected in the Antarctic Peninsula (Graham
Land) in 1985, and alpha-HCH was detected in most of them at a mean
concentration of 0.4 (0.20-1.15) µg/kg (Bacci et al., 1986).
In a study by Gaggi et al. (1986), fallen leaves (at the end of
their natural life-cycle) and lichens were collected in 1984 at sites
near Florence and Siena, Italy, in a woodland hilly area away from
primary pollution sources. The leaves were from ten different species
of trees and two different lichen species were involved. The average
levels of alpha-HCH in leaves and lichen were 37 (16-61) µg/kg dry
weight and 27 (25-29) µg/kg dry weight, respectively. The same
authors reported that the levels of alpha-HCH in various plant species
collected in 14 countries were 0.5-2140 µg/kg dry weight.
5.1.5.2 Fish and mussels
Martin & Hartman (1985) analysed 60 fish samples from nine
locations in the north-central part of the USA and found
concentrations of 5-27 µg alpha-HCH/kg (wet weight) in 36% of the
samples. The frequency with which alpha-HCH was detected in fish from
the different rivers varied between 17 and 100%.
In a study by Saiki & Schmitt (1986), samples of three to five
adult bluegills (Lepomis macrochirus) and common carp (Cyprinus
carpio) were collected at eight sites in three rivers in California,
USA, in 1981. Alpha-HCH concentrations in carp of up to 0.036 mg/kg on
fat basis were reported, but the concentrations in bluegill were
lower.
Cowan (1981) studied the extent of pollution of Scottish coastal
waters by HCHs using Mytilus edulis as biological indicator. The
levels of alpha-HCH at the 118 sites sampled ranged from < 6 to
23 µg/kg dry weight.
The fish and shellfish sampling programme carried out by the
United Kingdom Ministry of Agriculture, Fisheries, and Food between
1977-1984 was directed mainly to areas around the coasts of England
and Wales. The levels of alpha-HCH, which varied between the
different fish and shellfish species and also between the collection
sites, ranged from < 0.001 (nd) to 0.06 mg/kg wet weight. The
concentration in fish muscle was < 0.001 mg/kg wet weight (Franklin,
1987).
The mean alpha-HCH concentration in the muscle of flounders
collected off the North Sea coast of Germany in 1986 was 2.5 µg/kg
(nd-5.0 µg/kg) (Umweltbundesamt, 1989). Bream collected from different
locations in the River Elbe (between Schnackenburg and the North Sea)
contained 0.007-0.066 mg alpha-HCH/kg in muscle tissue and
0.9-2.2 mg/kg in adipose tissue (Arbeitsgemeinschaft für die
Reinhaltung der Elbe, 1982), while the same species collected from 15
rivers and lakes in the Federal Republic of Germany contained (on a
fat basis) up to 468 µg per kg (Umweltbundesamt, 1989).
Freshwater fish from different rivers in the Federal Republic of
Germany were analysed during the period 1973-1981, and in the first
3-4 years the alpha-HCH levels were mainly between 0.01 and 0.02 mg/kg
fresh weight. However, a clear decrease then took place and most of
the samples were below 0.01 mg/kg fresh weight, with the exception of
certain types of fish such as the eel and fish from industrially
contaminated areas (Hildebrandt et al., 1986).
In 1981-1983, shellfish and molluscs collected in the Federal
Republic of Germany contained < 0.001-0.20 mg alpha-HCH/kg fresh
weight. Eels collected in the North Sea and Baltic Sea contained
alpha-HCH levels of 0.011 mg per kg and 0.033 mg/kg fresh weight,
respectively. Flounders and herrings caught in the North Sea contained
0.002 and 0.008 mg/kg fresh weight, respectively, but in the Baltic
Sea the levels were about twice as high (Hildebrandt et al., 1986).
5.1.5.3 Birds
An average alpha-HCH residue level of 0.05 mg/kg was found in 17
adult herons in 1964 (HMSO, 1969).
In a study by Sierra & Santiago (1987), alpha-HCH concentrations
were determined in 23 barn owls (Tyto alba Scop.) from Leon, Spain.
The mean levels (and range) in muscle, liver, fat, brain, and
kidneys (in total 91 samples) were 0.242 (0.019-0.591), 0.323
(0.009-0.830), 1.073 (0.691-1.499), 0.238 (0.007-0.676), and 0.710
(0.051-2.381) mg/kg (wet weight), respectively.
Faladysz & Szefer (1982) analysed adipose fat from seven species
of diving ducks at their winter quarters in the Southern Baltic.
Residues of alpha-HCH were found in all of the 37 specimens of
long-tailed duck at mean concentrations (on a fat basis) of 3.4
(0.17-18) and 1.5 (0.23-6) mg/kg for female and male ducks,
respectively.
5.1.5.4 Mammals
Skaftason & Johannesson (1979) analysed 24 samples of the fat of
reindeer living in an area of the eastern and south-eastern parts of
Iceland where the use of pesticides is negligible. Alpha-HCH was
found in all samples at a mean level of 70 ± 22 µg/kg. These results
are in agreement with those of Benson et al. (1973), who found an
average of 77.5 µg/kg in the subcutaneous fat of wild Idaho moose
living in a forest area where pesticides were used very restrictively.
Skaftason & Johannesson (1979) analysed samples of body fat from
10-year-old sheep in 1974 and found an average of 51 ± 12 µg/kg.
Norström et al. (1988) investigated the contamination by
organochlorine compounds of Canadian arctic and subarctic marine
ecosystems by analysing the adipose tissue and liver of polar bears
( Ursus maritimus; 6-20 animals per area) collected from 12 areas
between 1982 and 1984. There was a difference in tissue distribution;
liver contained only alpha-HCH, but 29% of the HCH in adipose tissue
was beta-HCH. Adipose tissue contained 0.3-0.87 mg alpha-HCH per kg on
a fat basis.
The mean concentrations of alpha-HCH in the kidney fat of roe
(86 samples) collected in five areas of Germany in 1985-1986 were
about 7-12 µg/kg fat, the maximum value being about 50 µg/kg fat
(Umweltbundesamt, 1989).
5.2 General population exposure
From the data presented in section 5.1 it is evident that food is
the main source of exposure of the general population to alpha-HCH.
5.2.1 Total-diet studies
In a total-diet study carried out in the United Kingdom during
1966-1985, food purchased in 21 towns throughout the country was
prepared by cooking. The study covered 20 to 24 food groups, and the
number of total-diet samples examined varied from 22 to 25 samples.
The calculated mean alpha-HCH residue levels in the total diet for the
periods 1966-1967, 1970-1971, 1974-1975, 1975-1977, 1979-1980, 1981,
and 1984-1985 were 0.003, 0.002, 0.002, 0.0015, 0.001, < 0.0005, and
< 0.0005 mg/kg, respectively (Egan & Hubbard, 1975; HMSO, 1982, 1986,
1989).
Gartrell et al. (1985a) conducted a study to determine the
dietary intake of pesticides in the USA in 1978-1979. The samples,
purchased from retail outlets, were representative of the diets of
adults in 20 cities, and consisted of about 120 individual food items.
The daily intake of alpha-HCH in 1977, 1978, and 1979 was 0.011,
0.009, and 0.010 µg/kg body weight, respectively. In a similar way,
samples were collected in 10 cities in 1978-1979 consisting of about
50 items of infant food and 110 items of toddler food. The daily
intake of alpha-HCH in 1977, 1978, and 1979 was, respectively, 0.031,
0.034, 0.033 µg/kg for infants and 0.025, 0.029, and 0.029 µg/kg body
weight for toddlers, respectively (Gartrell et al., 1985b).
Total-diet studies conducted in the USA by the FDA before 1982
were based on a "composite sample approach" regardless of the diet
involved. Later on they were based on dietary survey information and
allowed the "total diet" of the population to be represented by a
relatively small number of food items for a greater number of age-sex
groups. The daily intakes of alpha-HCH during 1982-1984 for the age
groups 6-11 months, 2 years, 14-16-year-old females, 14-16-year-old
males, 25-30-year-old females, 25-30-year-old males, 60-65-year-old
females, and 60-65-year-old males were 7.2, 16.1, 6.1, 7.3, 4.5, 5.9,
3.3, and 3.7 ng/kg body weight, respectively (Gunderson, 1988).
In a total-diet study in the Netherlands in 1977, the average
concentration of alpha-HCH in 100 samples was 0.01 mg/kg on a fat
basis. The highest level was 0.05 mg/kg (Greve & van Hulst, 1977).
5.2.2 Air
Guicherit & Schulting (1985) measured the atmospheric
concentration of alpha-HCH in the Netherlands and calculated an
average daily intake by inhalation for a 70-kg person of 5 ng. The
equivalent value for the Federal Republic of Germany was calculated to
be 32 ng, which is about 1% of the total daily intake via the various
routes (Hildebrandt et al., 1986).
5.2.3 Concentrations in human samples
Alpha-HCH concentrations in human samples are a good indication
of the total exposure of the general population.
5.2.3.1 Blood
Blood samples of Dutch citizens analysed in 1978, 1980, 1981, and
1982 (70, 48, 127, and 54 samples, respectively), contained less than
0.1 µg alpha-HCH/litre (Greve & Wegman, 1985). Blok et al. (1984)
analysed the blood of 65 healthy volunteers in the Netherlands (34
female and 31 male) and detected alpha-HCH in less than one third of
the samples. The median concentration for both groups was below the
detection limit (0.1 µg per litre), but levels of up to 0.4 µg/litre
were measured.
Polishuk et al. (1970) studied the presence of alpha-HCH in the
blood of 24 pregnant women and 23 infants living in Israel. The
mean concentration was 0.6 ± 0.3 µg per litre in the women and 0.5 ±
0.3 µg/litre in the infants.
In 1975, Reiner et al. (1977) analysed the serum and plasma of 82
women and 65 men (with an average age of 42) living in a town in
Yugoslavia. In 57 of the 147 samples, alpha-HCH was found at a mean
concentration of 3.3 ± 0.5 µg/litre (range, 0.1-15.0 µg/litre).
Similar values were found in other parts of the country in 1976-1979
(Krauthacker et al., 1980).
The median concentration of alpha-HCH in whole blood of 118
people in the Federal Republic of Germany was reported to be
0.98 µg/litre (range, nd-2.06) (Bertram et al., 1980).
5.2.3.2 Adipose tissue
The alpha-HCH concentrations of 567 samples of adipose tissues of
Dutch citizens analysed during 1968-1983 varied from < 0.01 to
0.1 mg/kg (on a fat basis). The highest levels occurred during the
period 1968-1976 (Greve & van Harten, 1983; Greve & Wegman, 1985).
In a study by Niessen et al. (1984), specimens of subcutaneous
adipose tissue from 48 infants (34 under the age of 1 year, 14 in
their second year of life) were examined during 1982-1983 in the
Federal Republic of Germany. The average concentration of alpha-HCH
was 0.01 mg/kg fat (range, nd-0.02 mg/kg). The average concentration
was highest (0.02 mg/kg fat) for the age-range 0-6 weeks. Bertram et
al. (1980) found a median concentration of 0.03 mg/kg fat (range,
nd-0.35) in 72 samples of adipose tissue from people in the Federal
Republic of Germany. Hildebrandt et al. (1986) summarized the results
of nine studies carried out in the Federal Republic of Germany during
1969-1983. The mean alpha-HCH concentrations (568 samples) ranged
from 0.01 to 0.03 mg/kg fat.
Mes et al. (1982) analysed 99 samples of adipose tissue from
autopsies of accident victims from different areas of Canada. Nearly
all the samples (97%) contained alpha-HCH, the average concentration
of which was 0.004 mg/kg wet weight (range, 0.001-0.043 mg/kg).
In 1974, 360 samples of adipose tissue were collected in eight
regions of Japan and the mean level of alpha-HCH was 0.031 mg/kg
tissue (Takabatake, 1978).
Twenty-nine samples of adipose tissue were taken at necropsy and
24 at surgery in the Poznan district of Poland and compared with 100
samples from residents of the Warsaw area. In Poznan the mean
concentration of alpha-HCH was 0.013 ± 0.033 mg/kg, while in Warsaw it
was 0.008 ± 0.001 mg/kg (Szymczynski et al., 1986).
5.2.3.3 Breast milk
Breast milk is a major route for the elimination of
organochlorine pesticides in women. In a Swedish study, the levels of
alpha-HCH in breast milk were found to be related to the dietary
habit. Levels in lacto-vegetarians were lower than those in women
eating a mixed diet, and these were lower than those found in mothers
using a mixed diet that regularly included fatty fish from the Baltic
(Noren, 1983).
In a study by Fooken & Butte (1987), the variation of residue
levels in breast milk during lactation was investigated in five women
(aged 23-36) in the Federal Republic of Germany. Alpha-HCH
concentrations of up to 0.009 mg/kg fat were measured, and no
essential changes in residue level occurred over the lactation period.
Residues of alpha-HCH in breast milk during the periods,
1974-1975 and 1979-1980 in the Federal Republic of Germany were
reported to be 0.03 and 0.02 mg/kg milk on a fat basis, respectively
(Anon., 1984).
In the Federal Republic of Germany, more than 7100 samples of
breast milk were analysed from 1969-1984. These studies were carried
out by 20 authors, and the results were summarized by Hildebrandt et
al. (1986). The mean concentrations of alpha-HCH ranged from
0.01-0.04 mg/kg on a fat basis. In one case a mean concentration of
0.21 mg/kg was found in 320 samples. During the period investigated, a
slow decrease in the mean concentration of alpha-HCH was observed. The
average concentration in breast milk in the same country (2709
samples) in 1979-1981 was 0.024 mg/kg on a fat basis (Fooken & Butte,
1987). In 1981-1983, 132 samples of breast milk were analysed and the
average level was 0.001 mg alpha-HCH/kg milk fat (Cetinkaya et al.,
1984).
Tuinstra (1971) analysed 36 individual samples of breast milk,
collected in 1969, from young mothers (18-32 years of age) living in
the Netherlands. A median alpha-HCH concentration of 0.01 mg/kg milk
(on a fat basis) was found (range, nd-0.04). When 278 samples of
breast milk, collected in 11 maternity centres in the Netherlands,
were analysed for the presence of alpha-HCH, the median alpha-HCH
concentration was < 0.01 mg/kg (on a fat basis) (Greve & Wegman,
1985).
Vukavic et al. (1986) measured the alpha-HCH concentration in 59
samples of colostrum collected during autumn 1982 (26 samples) and
spring 1983 (33 samples) in Yugoslavia from healthy nursing mothers on
the third day after delivery. The alpha-HCH levels were significantly
lower in the autumn than in the spring (mean concentrations of 0.49 ±
0.09 and 1.50 ± 0.26 µg/litre whole colostrum, respectively).
Mes et al. (1986) studied 210 breast milk samples from five
different regions of Canada and measured a mean alpha-HCH
concentration of 7 µg/kg (on a fat basis). Davies & Mes (1987)
studied 18 breast milk samples from Canadian, Indian, and Inuit
mothers in Canada, whose fish consumption was comparable to the
national level. The level of alpha-HCH in milk fat of the indigenous
population was 5 µg/kg, which was the same value as that obtained from
a national survey.
6. KINETICS AND METABOLISM
6.1 Absorption and elimination
The intestinal absorption rate for alpha-HCH was 97.4% after the
administration of an HCH mixture to male rats (Albro & Thomas, 1974).
The total excretion in rats after a single intraperitoneal (ip)
36Cl-labelled alpha-HCH dose of 200 mg/kg body weight was 80% of the
dose in the urine and 20% in the faeces (Koransky et al., 1963;
Koransky et al., 1964; Noack et al., 1975). In a study in rats with
36Cl-labelled alpha-HCH, a low excretion rate was found. 36Cl was
detected in the excreta up to 40 days after a single ip dose (Koransky
et al., 1963). During continued dosing alpha-HCH was observed to
stimulate its own degradation (Noack et al., 1975). The decrease in
rat liver alpha-HCH levels after an initial increase, observed by
Eichler et al. (1983), was assumed to be due to this effect.
When 14C-labelled alpha-HCH was administered intraperitoneally
to male mice (ddY-strain, 4 weeks old) at a dose level of 22 µg, the
average percentage of urinary excretion of radioactivity in 3 days was
37% (Kurihara & Nakajima, 1974).
6.2 Distribution
One day after an ip injection of a mixture of 14C- and
36Cl-labelled alpha-HCH into rats (200 mg/kg body weight in rapeseed
oil), the highest level of radioactivity was found in fat, skin, and
bones plus muscles (18.2, 13.1, and 11.9%, respectively, after 4
days). Much lower levels were found in other organs or tissues (up to
1% in liver and kidneys and 0.28% in the central nervous system. In
the faeces and urine, 3.9 and 7.9%, respectively, were found after 4
days (Koransky et al., 1963). In other studies with rats, higher
concentrations were found in liver, kidneys, body fat, brain, and
muscle (Portig & Vohland, 1983; Kuiper et al., 1985). In a 90-day
study in rats, marked deposition of alpha-HCH was found in renal fat;
the concentrations exceeded those obtained in a similar study on
beta-HCH (Greve & van Hulst, 1980; Kuiper et al., 1985). In lactating
rats given a single oral dose 5 days after birth, the alpha-HCH
concentrations in the livers of the sucklings were twice as high as
those observed in the livers of the mothers (Wittich &
Schulte-Hermann, 1977).
Vohland et al. (1981) studied the distribution of alpha-HCH in
the brain and depot fat of rats after the administration of 200 mg/kg
body weight by gavage. With an average blood concentration of
1.5 µg/litre, the brain to blood, and depot fat to blood ratios were
120:1 and 397:1, respectively, whereas with a blood concentration of
17.7 mg/litre the ratios were 5:1 and 82:1, respectively.
Nagasaki (1973) orally administered alpha-HCH to male mice at
concentrations of 100, 250 or 500 mg/kg for 24 weeks, and found high
residual levels of this isomer in liver and adipose tissue. Similarly,
Macholz et al. (1986) reported that a 30-day administration of
alpha-HCH to rats resulted in high residues of this isomer in fat,
kidneys, and adrenal tissue.
In the brain, alpha-HCH is stored preferentially in the white
matter (Stein et al., 1980; Portig et al., 1989).
6.3 Metabolic transformation
6.3.1 Rat
When Sprague-Dawley weanling female rats were administered 2 mg
alpha-HCH/rat per day in peanut oil for 7 days, the alpha-HCH was
metabolized to 2,4,6- and 2,4,5-trichlorophenol, with an excretion
ratio of 2,4,6- to 2,4,5-trichlorophenol of 1.3:1. This study also
indicated that pre-treatment with alpha-HCH alters the metabolism of
lindane in rats (Freal & Chadwick, 1973).
The biotransformation of alpha-HCH in rats involves
dechlorination (Kraus, 1975). The dose-dependent decrease in liver
glutathione concentrations indicates the formation of a glutathione
conjugate in this organ (Noack & Portig, 1973; Portig et al., 1973;
Kraus, 1975). Such a decrease does not occur in the brain or kidneys
(Noack & Portig, 1973).
The major urinary metabolite in rats is 2,4,6-trichlorophenol, a
compound reported by IARC (1987) to be carcinogenic for animals
(Portig et al., 1973; Stein & Portig, 1976; Stein et al., 1977). Other
metabolites that have been identified are 1,2,4-trichlorophenol,
2,3,4-trichlorophenol, 2,4,5-trichlorophenol, 2,3,4,5-
tetrachlorophenol, and 2,3,4,6-tetrachlorophenol (Noack et al., 1975;
Stein et al., 1977; Macholz et al., 1982). In addition,
chlorothiophenols (not specified) have been detected, and
1,3,4,5,6-pentachlorocyclohex-1-ene has been identified in the kidneys
of rats (Macholz et al., 1983).
Artigas et al. (1988) have identified several lindane metabolites
(tetra-, penta-, and hexachlorocyclohexenes, and tetra- and
pentachlorobenzenes) in rat brain homogenates by gas
chromatography-mass spectrometry. Male Wistar rats were orally
administered 30 mg alpha-HCH/kg and were sacrificed 5 h later. The
cerebella of the animals were analysed and the following metabolites
were found: 3.6/4.5-PCCH, 3.5/4.6-PCCH, HCCH, pentachlorobenzene, and
HCB. HCCH was the major metabolite (about 100 µg per kg) while levels
of the other metabolites were mainly below 5 µg/kg. Alpha-HCH was
present at 17.2 mg/kg tissue. This study showed that the HCH isomers
are cleared from the brain via different metabolic pathways.
Isomerization of alpha-HCH to lindane did not occur after
repeated dosage (Eichler et al., 1983).
6.3.2 Bird
In a model 4-week feeding study on poultry using four HCH
isomers, the rate of degradation of the individual HCH isomers in
broilers followed the order: delta > gamma > alpha > beta.
Biotransformation (to one or more of the other HCH isomers) was not
detected (Szokolay et al., 1977b).
In a study by Foster & Saha (1978) on the in vitro metabolism
of alpha-HCH in chicken livers, the first metabolite was identified as
an isomer of pentachlorocyclohexane.
6.3.3 Human
When Engst et al. (1978) analysed the urine of occupationally
exposed workers (apparently to technical-grade HCH in manufacturing
processes), they found, apart from alpha-, beta-, gamma-, and
delta-HCH, traces of hexa- and pentachlorobenzene, gamma- and
delta-pentachlorocyclohexane, pentachlorophenol, 2,3,4,5-, 2,3,4,6-,
and 2,3,5,6-tetrachlorophenol, and several trichlorophenols, as well
as the glucuronides of several of these metabolites. The
pentachlorocyclohexenes, tetrachlorophenol, hexachlorobenzene, and
pentachlorophenol were also identified in the blood.
6.4 Retention and biological half-life
The half-life for the clearance of alpha-HCH from depot fat was
found to be 6.9 days in female rats and 1.6 days in male rats (Stein
et al., 1980; Portig, 1983).
Vohland et al. (1981) and Portig & Vohland (1983) studied the
kinetics of alpha-HCH in Wistar rats, and observed that, after a
single oral dose of 200 mg/kg body weight, the approximate half-life
in females for the elimination from brain was 6 days.
The retention of alpha-HCH in rat brain after a single dose is
greater than that of beta- and gamma-HCH (Stein et al., 1980).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Acute toxicity
In mice oral LD50 values have been found to range from 1000 to
4000 mg/kg body weight, depending on the vehicule, while in rats
values of 500-4674 mg/kg body weight have been obtained. Riemschneider
(1949) determined a LD50 (oral intubation in olive oil) for rats of
1500 mg/kg body weight. The signs of poisoning were those of nervous
system stimulation: excitation, hunched posture, rough fur, dyspnoea,
anorexia, tremors, convulsions, and cramps (Hoffmann, 1983; WHO,
1986).
7.2 Short-term exposure
7.2.1 Oral
In a 90-day study on rats carried out with dose levels of 0, 2,
10, 50, or 250 mg alpha-HCH/kg diet, reductions in white blood cell
count were noted in several groups of animals. Growth was decreased
at 250 mg/kg diet, and at this dose level the number of erythrocytes
and protein excretion in the urine were elevated in female animals. At
levels of 50 and 250 mg/kg, the activities of liver
amino-pyrine- N-demethylase and aniline hydroxylase were increased
while those of blood aspartate aminotransferase (ASAT) and creatine
phosphokinase were decreased. Liver weights were increased at dose
levels of 10, 50, and 250 mg/kg. Enlargement of liver parenchyma cells
(with a foamy/hyaline appearance of the cytoplasm) and accentuation of
the plasmalemma, indicative of proliferation of smooth endoplasmatic
reticulum (SER), occurred at levels of 50 and 250 mg/kg. At the
250-mg/kg level, there were increases in the relative weights of
heart, kidneys, and adrenals. In addition, serum levels of
immunoglobulins G and M showed a decrease at 50 and 250 mg/kg diet
(Kuiper et al., 1985).
Macholz et al. (1986) reported that the administration of 1000 mg
alpha-HCH/kg to rats for 30 days resulted in growth retardation and
liver mass increase. High residue levels of alpha-HCH were identified
in fat, kidneys, and adrenal tissue.
7.2.2 Other routes
7.2.2.1 Intravenous
In a study by van Asperen (1954), groups of 12-15 male and female
albino mice (8-10 weeks of age) were given an intravenous injection of
alpha-HCH (in peanut oil). The dose levels were 480 or 960 µg/mouse
(equivalent to approximately 32 and 64 mg/kg body weight,
respectively). No deaths occurred within 7 days.
7.2.2.2 Subcutaneous
Groups of 13-21 male and female albino mice (8-10 weeks of age)
were given a subcutaneous injection of alpha-HCH at dose levels
ranging from 3 to 20 mg/animal (equivalent to approximately 200 to
1330 mg/kg body weight, respectively). With doses of up to 4 mg, no
death occurred within 7 days, but with 4.5 mg, 8 mg, and 20 mg, 8, 25,
and 90%, respectively, of the animals died (van Asperen, 1954).
7.3 Skin and eye irritation; sensitization
No data on skin and eye irritation or sensitization have been
reported.
7.4 Long-term exposure
7.4.1 Rat oral study
When groups of 10 female and 10 male weanling Wistar rats were
administered diets containing 0, 10, 50, 100, or 800 mg alpha-HCH/kg
diet (in corn oil) for 107 weeks, the highest dose level resulted in
growth retardation, increased mortality, and slight kidney damage.
With dose levels of 100 or 800 mg/kg, liver enlargement and
histo-pathological changes in the liver were found. However, there
were no liver changes at 50 mg/kg diet (Fitzhugh et al., 1950).
7.5 Reproduction, embyrotoxicity, and teratogenicity
No information on reproduction, embryotoxicity, or teratogenicity
is available.
7.6 Mutagenicity and related end-points
Alpha-HCH did not induce mutations in Salmonella typhimurium
test strains TA98, TA100, TA1535 or TA1537 either with or without rat
liver metabolic activation (Lawlor & Haworth, 1979). A test for point
mutations in Saccharomyces cerevisiae XV 185 14 C was also negative
(Shahin & von Borstel, 1977). In addition, the compound produced no
mutations in Allium cepa roots (Nybom & Knutsson, 1947). A test for
unscheduled DNA synthesis in rat hepatocytes in vitro produced an
equivocal result (Althaus et al., 1982).
A mutagen test strain of Bacillus subtilis (TKJ5211) showed a
higher sensitivity for hisw+ reversion than the parental strain
HA101 when treated with UV and UV-mimetic chemicals. However, a
negative result was obtained when alpha-HCH dissolved in DMSO was used
at a dose level of 5 mg/ml (Tanooka, 1977).
A DNA repair test was carried out with stationary-phase cultures
of B. subtilis HLL3g and HJ-15 strains in which the size of growth
inhibition zones of repair-proficient and repair-deficient cells for
vegetative cells and spores was determined. There was no effect at a
dose level of 5 mg alpha-HCH (in benzene) per ml (Tanooka, 1977).
The available data are inadequate to make an assessment of the
mutagenic potential.
7.7 Carcinogenicity
Appraisal
The reported studies on the carcinogen effects of alpha-HCH on
mice and rats have some short-comings. In most cases, very high dose
levels were tested. Nevertheless, it is clear from the results that
alpha-HCH, at high dose levels, produces nodular hyperplasia and
hepatocellular carcinomas in mice (the incidence varying according to
the strain) and also in rats (low incidence), but only at higher dose
levels.
The results of the studies on initiation-promotion and mode of
action indicate that the neoplastic response observed with alpha-HCH
is most likely due to a non-genotoxic mechanism.
7.7.1 Mouse
When 20 male ICR/JCL mice (aged 5 weeks) were administered a diet
containing 600 mg alpha-HCH/kg diet for 26 weeks, increased liver
weight was observed. In all treated mice there were liver tumours,
which were characterized histologically as benign tumours and
malignant tumours with atypical liver cells. Unfortunately,
insufficient details were reported (Goto et al., 1972a,b).
In a study by Hanada et al. (1973), 6-week-old DD mice (10-11 of
each sex per group) were given diets containing 0, 100, 300, or 600 mg
alpha-HCH/kg diet for 32 weeks, followed by a control diet for 5-6
weeks. The control group consisted of 20 female and 21 male animals.
During the experiment several animals died. The numbers of hepatomas
in the four groups surviving for 36-38 weeks were 0/29 (control), 1/16
(100 mg/kg), 9/10 (300 mg/kg), and 13/15 (600 mg/kg).
Alpha-fetoprotein was not detected in the serum of animals with
hepatomas.
When 8-week-old male DD mice, divided into groups of 20 or 38
animals, were fed a diet containing 0, 100, 250, or 500 mg
alpha-HCH/kg for 24 weeks, the two highest dose levels induced an
increase in liver weight. At the four respective dose levels, the
incidence of nodules classified as nodular hyperplasia was 0/20, 0/20,
30/38 (79%), and 20/20 (100%) and that of hepatocellular carcinoma was
0/20, 0/20, 10/38 (26%), and 17/20 (85%) (Ito et al., 1973b).
Following the oral administration of 100, 250 or 500 mg
alpha-HCH/kg to male DD mice for 24 weeks, hepatocellular tumours were
found in all mice treated with 500 mg/kg and in 17 of the 20 mice that
received 250 mg/kg (Nagasaki, 1973).
Nagasaki et al. (1975) studied the tumorigenic effects of a diet
containing 0 or 500 mg alpha-HCH/kg, fed for 24 weeks to groups of
male and female DDY, ICR, DBA/2, C57BL/6, and C3H/He mice (13-29 of
each sex per group), male Wistar rats, and male golden Syrian
hamsters. It was found that alpha-HCH induced liver tumours in male
and female mice but not in rats and hamsters. The histological changes
in the liver of mice were much greater than those induced in rats and
hamsters. Male animals were more susceptible to the tumorigenic
action (i.e. liver nodules) than females. Among the different strains
of mice, a difference in susceptibility was observed. The occurrence
of liver nodules varied from 16.7 to 100% and the incidence of
hepatocellular carcinomas varied from none to 65%. The DDY mouse
strain was the most sensitive and the C57BL/6 the least sensitive
strain.
Ito et al. (1976) studied the reversibility of liver tumours
induced by alpha-HCH (99.0%). Male 8-week-old DDY mice were fed a
diet containing 0 or 500 mg/kg for 16, 20, 24, and 35 weeks and then
fed a basal diet without alpha-HCH for 4, 8, and 12, or 4, 8, 12, 16,
24, and 36 weeks. In total 341 mice were used, of which 21 were fed
the compound for 16 weeks. A total of 300 mice were fed the diet with
alpha-HCH for 20 or more weeks and 20 control mice were fed the basal
diet for 72 weeks. At the various intervals indicated, 12-20 mice were
killed. The incidence of liver tumours increased progressively during
continuous administration of alpha-HCH, but when its administration
was discontinued some tumours disappeared. After 24 weeks of
administration most tumours were nodular hyperplasias with only a few
well-differentiated hepatocellular carcinomas. However, 60 or 72
weeks after the beginning of the study most of the liver tumours were
hepatocellular carcinomas. The findings suggested that nodular
hyperplasia was usually reversible.
Two groups of male HPBC57B1 black mice (6-9 weeks old) were fed
a diet containing 500 mg/kg alpha-HCH (99.8%) per diet, 48 mice being
used as controls and 75 mice being administered alpha-HCH. From each
group, 4-9 mice were killed at 1, 3, 4, 8, 14, 21, 30, 33, 44, and 50
weeks after the initiation of treatment. Progressive liver
enlargement was first noticed at 3 weeks, hepatic nodules at 21 weeks,
and emaciation at 30 weeks. Histopathological liver alterations
included hypertrophy of centrolobular hepatocytes first seen at 1 week
and the merging of adjacent megalocytic zones at 3 weeks. At 21
weeks, adenomas were seen in two out of seven mice, at 30 weeks in
seven out of eight mice, and at 33, 44, and 50 weeks in all the mice
studied. Under the condition of this study, neither hepatocellular
carcinomas nor metastases in the lungs were detected (Tryphonas &
Iverson, 1983).
7.7.2 Rat
When groups of 10 male and 10 female weanling Wistar rats were
fed throughout their life on diets containing 10, 50, 100, or 800 mg
alpha-HCH (> 98% pure) per kg, no increase in tumour incidence was
found. However, only a limited number of organs were examined
microscopically (Fitzhugh et al., 1950).
In a study by Ito et al. (1975), male Wistar rats (5-8-weeks old)
were divided into seven groups and administered alpha-HCH diets
containing 0, 500 (two groups), 1000 (three groups), or 1500 mg
alpha-HCH/kg diet. The duration of the treatment for the different
groups was 72 weeks for the controls, 24 or 48 weeks at 500 mg/kg, 24,
48, or 72 weeks at 1000 mg/kg, and 72 weeks at 1500 mg/kg. In the
liver, oval cells and bile duct cell proliferation were found in the
groups fed 1000 or 1500 mg/kg after 48 and 72 weeks. Cell hypertrophy
was found in all the groups, the increase in severity depending on the
dose level and the duration of administration. In the two groups fed
500 mg/kg and the group fed 1000 mg/kg for 24 weeks no nodular
hyperplasia or hepatocellular carcinomas were found. Nodular
hyperplasia developed in the groups fed 1000 mg/kg (48 and 72 weeks)
or 1500 mg/kg (72 weeks) in 42, 76, and 77% of the animals,
respectively. Hepatocellular carcinomas were found only in the groups
fed 1000 or 1500 mg/kg for 72 weeks (1/16 and 3/13 animals,
respectively).
In a series of studies, an oral dose of 20 mg/kg body weight was
administered daily to female rats during periods of 4.5, 13.5, or 23.5
months. Liver enzyme induction was found at all intervals, white foci
and nodules were present after 13.5 months, and one animal had a
hepatocellular carcinoma after 23.5 months (Schulte-Hermann &
Parzefall, 1981). The value of this study was reduced by the very low
number of animals (4-6 per group) used at each interval.
7.7.3 Initiation-promotion
In a study on 8-week-old white male mice (25-30 per group) of
strain DD, the influence of alpha-HCH on tumour induction by
polychlorinated biphenyls (PCBs) was tested and vice versa. Whereas
500 mg PCB/kg diet induced nodular hyperplasia and hepatocellular
carcinomas in the liver of male mice after 32 weeks, exposure to
alpha-HCH at dose levels of 50, 100, or 250 mg/kg diet, only resulted
in both type of tumours at the highest dose level. The incidence of
nodular hyperplasia was 23/30 (77%) and that of hepatocellular
carcinoma was 8/30 (27%). However, 50 or 100 mg alpha-HCH/kg diet, in
combination with 250 mg PCB per kg diet (PCB alone did not induce
tumours), induced nodular hyperplasia (approximately 30%) and
hepatocellular carcinoma (approximately 5%). It seems that PCBs
promote the induction of liver tumours by alpha-HCH (Ito et al.,
1973a).
In studies on rats, alpha-HCH showed a tumour-promoting action
towards the hepatocarcinogenic effects of aflatoxin B1,
diethylnitrosamine, and nitrosomorpholine (Schulte-Hermann &
Parzefall, 1981; Schulte-Hermann et al., 1981; Angsubhakorn et al.,
1981). In one test, alpha-HCH produced only a slight liver
tumour-promoting effect in rats after initiation with
N-nitrosodiethylamine (Ito et al., 1983). However, in another study
on the same species the compound had an inhibitory effect on the
hepa-tocarcinogenic action of 3-methyl-4-dimethylaminoazobenzene and
DL-ethionine (Thamavit et al., 1974).
Nagasaki et al. (1975) studied the influence of
3-methylcholanthrene, 1-naphthyl isothiocyanate, and
p-hydroxypropiophenone on the induction of liver tumours by
alpha-HCH. Eight groups of 24 mice received a diet containing either
500 mg alpha-HCH/kg diet in combination with 67 mg
methylcholanthrene/kg, 600 mg 1-naphthyl isothiocyanate/kg or 1000 mg
p-hydroxypropiophenone/kg or just one of the four compounds. A
control group with the basal diet was also used. The induction of
mouse liver tumours by alpha-HCH was not inhibited by the concomitant
feeding of 1-naphthyl isothiocyanate or p-hydroxypropiophenone.
However, 3-methylcholanthrene slightly inhibited their induction by
alpha-HCH.
In a study by Schröter et al. (1987), the tumour-initiating
activity of alpha-HCH was studied by examining for phenotypically
altered foci in female Wistar rats. Groups of three to eight rats
were used and, after removing the median and right liver lobes, 200 mg
alpha-HCH/kg body weight was administered followed by phenobarbital at
50 mg/kg body weight per day for 15 weeks. Liver foci were identified
by means of the gamma-glutamyltransferase (GGT) reaction and by
morphological alterations. No evidence of initiating activity was
found. In another part of the study, the promoting activity was
investigated. A single dose of N-nitrosomorpholine (250 mg/kg body
weight by gavage) was followed by the administration of 0.1, 0.5, 2.0,
7.0, or 20.0 mg alpha-HCH/kg body weight per day for 4, 15, and 20
weeks. The criteria used were growth and phenotypic changes of foci
as end-points. It was concluded from the study that alpha-HCH is a
tumour promotor. Both the number and size of altered foci were
enhanced by alpha-HCH doses of 2 mg/kg or more. The tumour-promoting
action was generally associated with liver enlargement and induction
of monooxygenases or other specific enzymes.
Schulte-Hermann et al. (1983) carried out three experiments with
Han-Wistar rats using, in experiment 1, 39 female rats (8-24 months
old) and, in experiments 2 and 3, 41 male (2 years old) rats.
Alpha-HCH (200 mg/kg in corn oil) was administered orally as a single
dose, while the control group received only corn oil. Beginning 25 h
after the dosing, 3H-thymidine was injected intravenously five times
at intervals of 6 h (experiment 2) or 8 h (experiment 3) , and the
animals were killed 18 (experiment 2) or 3 h (experiment 3) after the
last dose of 3H-thymidine. The effect of age on the incidence of
spontaneous foci was studied in experiment 1. Foci of putative
preneoplastic cells were detected in the livers of untreated rats of
both sexes, especially at 1 and 2 years of age. These foci exhibited
markers similar to those of their counterparts in carcinogen-treated
rats, such as cytoplasmic basophilia, clearness of cytoplasm, or
expression of gamma-glutamyl transferase. Rates of DNA synthesis in
foci were higher than in normal liver cells and were increased by
single doses of liver mitogens assumed to promote liver tumour
development. Thus cells in the spontaneous foci appeared to possess a
defect in the growth control, rendering them more susceptible to
endogenous and exogenous growth stimuli.
The incorporation of orally administered radiolabelled thymidine
into liver DNA was determined in SIV-50-SD rats 24 h after a single
oral gavage dose of 2.9, 29.1, 58.2, or 291 mg alpha-HCH/kg. Alpha-HCH
was found to stimulate liver DNA synthesis at 58.2 mg/kg (Büsser &
Lutz, 1987).
7.7.4 Mode of action
Sagelsdorff et al. (1983) studied the relevance to the
carcinogenic action of alpha-HCH of covalent binding to mouse liver
DNA. Three strains of mice were used (NMRI, CF1, and C6B3F1), and
alpha-HCH was administered by oral gavage and 14C-thymidine by the
intraperitoneal route. In all three strains, a similar low covalent
binding index or DNA damage/dose (values ranging from 0.17-0.28) was
found. There was no quantitative correlation with the carcinogenicity
potency of alpha-HCH.
Iverson et al. (1984) studied the ability of alpha-HCH to bind to
macromolecules from male HPB black mouse liver. In vivo and in
vitro binding studies with 14C-alpha-HCH and hepatic microsomes
from untreated and phenobarbital-pretreated mice showed no
preferential binding of alpha-HCH to protein or DNA. The results
suggest that the neo-plastic response observed with alpha-HCH results
from a non-genotoxic mechanism.
7.8 Special studies
7.8.1 Effect on liver enzymes
After a single oral administration to female rats of 5 mg
alpha-HCH/kg body weight or more the rate of aminopyrine demethylation
and the liver DNA content were both increased, but at 2 mg/kg body
weight these effects did not occur (Schulte-Hermann et al., 1974). In
a further study, the liver cytochrome P450 concentration in male rats
after a single oral administration was elevated at all tested dose
levels, 25 mg/kg body weight being the lowest (Seifart & Buchar,
1978). After alpha-HCH was given to male rats at dose levels of 5, 10,
20, 50, or 200 mg/kg feed for 2 weeks, aniline hydroxylase and
aminopyrine demethylase activities were increased at all dose levels
(den Tonkelaar et al., 1981).
7.8.2 Neurotoxicity
Appraisal
Alpha-HCH has been shown to have no effect on motor nerve
conduction velocity or the fronto-occipital EEG in rats fed 1000 mg
alpha-HCH/kg diet for 30 days. This isomer is a mild antagonist of
pentylenetetrazol-induced convulsions but increases the tonic/clonic
activity and the lethality of picro-toxin when administered
intraperitoneally to mice. It decreases the accumulation of
cerebellar cyclic GMP and prohibits the increase of cGMP caused by
gamma-HCH in mouse brain. Alpha-HCH has been demonstrated to inhibit
GABA-mediated chloride ion uptake in mouse brain, and this effect is
believed to play a primary role in the CNS action of this isomer.
In a study by Vohland et al. (1981), alpha-HCH did not give rise
in brain tissue to appreciable quantities of hydrophobic metabolites
such as 2,4,6-trichlorophenol. It had a weak protecting action
against convulsions induced by pentylenetetrazole (PTZ). The intensity
and duration of the PTZ-antagonistic effects after a single oral dose
were related to the alpha-HCH content of the brain.
In a 30-day study on groups of 15 male Wistar rats fed alpha-HCH
at levels of up to 1000 mg/kg diet, there was no effect on the
fronto-occipital electroencephalogram or on the motor conduction
velocity of the tail nerve (Müller et al., 1981).
The effect of alpha-HCH on body temperature, food intake, and
body weight was studied in Wistar rats (eight males and eight females)
given a single 30-mg/kg oral dose of alpha-HCH in olive oil. Controls
received only olive oil. Alpha-HCH treatment induced no significant
decrease in core temperature 5 h after treatment, and no decrease in
food intake or growth was observed (Camon et al., 1988).
Fishman & Gianutsos (1987) studied the effects of an
intraperitoneal injection of alpha-HCH (99.0%) in corn oil
(80-480 mg/kg body weight) on the accumulation of cerebellar cyclic
GMP in male CD-1 mice. Alpha-HCH decreased the accumulation of
cerebellar cyclic GMP and also prevented the increase in cyclic GMP
resulting from lindane treatment. Furthermore, alpha-HCH inhibited
the binding of 3H-TBOB (a ligand for the GABA-A-receptor-linked
chloride channel) in mouse cerebellum.
Fishman & Gianutsos (1988) compared the CNS-related
pharmacological and biochemical effects of gamma-HCH and the
non-convulsant isomer alpha-HCH. The studies were carried out on male
CD-1 mice injected intraperitoneally with a single alpha-HCH (in corn
oil) dose of 80-400 mg/kg body weight. Alpha-HCH inhibited the
myoclonic jerk and tonic/clonic activity of PTZ but increased the
tonic/clonic activity and lethality of picrotoxin (PIC) (PTZ and PIC
were given as a single ip injection of 50 mg/kg and 20 mg/kg body
weight, respectively). The highest dose of alpha-HCH caused a
significant decrease in motor activity. Gamma-HCH inhibited the
binding of 3H-TBOB to mouse whole brain membranes. Furthermore, this
isomer is a weak inhibitor of GABA-stimulated uptake of 36wCl into
mouse brain neurosynaptosome preparations in vitro. The
non-seizure-inducing alpha-HCH has biochemical and pharmacological
effects in the CNS which differ from those of the gamma-HCH.
Matsumoto et al. (1988) provided evidence that all HCH isomers
are capable of inhibiting GABA-A-mediated chloride channels in the
brain, the relative potency being alpha = gamma > delta > beta.
Alpha-HCH was also found to be a potent inhibitor of the
batrachotoxin-stimulated action potential flux of sodium ions in N18
neuroblastoma cell cultures (Shain et al., 1987).
8. EFFECTS ON HUMANS
8.1 Acute toxicity - poisoning incidents
Several cases of acute poisoning by technical-grade HCH,
resulting either from accidents or occupational exposure, have been
described (WHO, 1991). Although alpha-HCH constitutes 65-70% of the
technical product, it is likely that the most acutely toxic component,
i.e. gamma-HCH, played the major role in these incidents. These cases
cannot, therefore, assist in the evaluation of alpha-HCH.
8.2 General population
No specific studies relating to alpha-HCH are available.
A study comparing liver cancer deaths in the USA and the
"domestic disappearance" of organochlorine pesticides revealed that in
1962, 18 and 15 years after the introduction of DDT and
technical-grade HCH, respectively (when an increase in primary liver
cancer due to the organochlorines would be manifest), the number of
cases of primary liver cancer as a percentage of the total number of
liver cancer deaths began a gradual and steady decline (from 61.3% in
1962 to 56.9% in 1972). The death rate (per 100 000 per year) due to
primary liver cancer declined from 3.46 to 3.18 during this period
(Deichmann & MacDonald, 1977).
8.3 Occupational exposure
The evaluation of the effects of alpha-HCH on occupationally
exposed workers is seriously hampered by the fact that most of the
relevant studies concern workers who were exposed during the
manufacture and handling of lindane or the handling and spraying of
technical-grade HCH among other pesticides, and were thus exposed to
all HCH isomers plus impurities and other (process) chemicals.
Therefore, it is difficult, if not impossible, to relate the observed
effects to individual substances. Consequently these studies have only
been described in this monograph where they aid the evaluation.
Behrbohm & Brandt (1959) described 26 cases of allergic and toxic
dermatitis that arose during the manufacture of technical-grade HCH.
Patch testing with pure alpha-, beta-, gamma-, and delta-HCH yielded
negative results, but positive reactions were obtained with the
residual fractions.
The level of alpha-HCH in 57 healthy workers (with normal liver
function, EMG and EEG) at a lindane-manufacturing plant ranged from 10
to 273 µg/litre, whereas it was below the detection limit in control
workers. The concentration in the adipose tissue of eight of the
exposed workers ranged from 1 to 15 mg alpha-HCH/kg (in extractable
lipids) (Baumann et al., 1980, 1981; Brassow et al., 1981; Tomczak et
al., 1981).
The mean serum alpha-HCH level of malaria-control workers that
sprayed technical-grade HCH for 16 weeks increased from 10 to
78 µg/litre in previously non-exposed workers and from 18 to
77 µg/litre in those that had been exposed during three previous
spraying seasons (Gupta et al., 1982).
Nigam et al. (1986) studied 64 employees from a plant
manufacturing HCH who were directly or indirectly associated with the
production of this insecticide and thus also exposed to chemicals such
as benzene and chlorine. The exposed group was composed of 19
"handlers" (who handled and packed the insecticide), 26 "non-handlers"
(plant operators and supervisors exposed indirectly to HCH), and 19
maintenance staff (who visited the plant frequently). The control
group consisted of 14 workers who had no occupational contact with the
insecticide. The exposure period varied up to 30 years. The mean
serum alpha-HCH concentrations in the four groups were 21.1 µg/litre
(controls), 21.8 µg/litre (maintenance staff), 41.2 µg per litre
(non-handlers), and 100 µg/litre (handlers). Lindane and beta- and
delta-HCH were also present. The total HCH concentrations were 51.4,
143.6, 265.6, and 604 µg per litre, respectively. Clinical examination
revealed that the majority of the workers from the "handler" and
"non-handler" groups exhibited paraesthesia of the face and
extremities, headache, and giddiness, and some of them also showed
symptoms of malaise, vomiting, tremors, apprehension, confusion, loss
of sleep, impaired memory, and loss of libido. The same symptoms were
found among the maintenance staff but were less severe and less
frequent.
Chattopadhyay et al. (1988) studied 45 male workers exposed to
HCH during its manufacture and compared them with 22 matched controls.
Exposure was mainly via the skin. Paraesthesia of face and
extremities, headache, giddiness, vomiting, apprehension, and loss of
sleep, as well as some changes in liver function tests, were reported
and were found to be related more to the intensity of exposure (as
measured by the HCH levels in blood serum) than to the duration of
exposure. The measured exposures to total HCH were 13 to 20 times
higher than those in the control groups (no detailed figures were
reported). Of the total serum HCH, 60-80% was beta-HCH.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Algae
Palmer & Maloney (1955) used alpha-HCH in a preliminary screening
test with two cyanobacterium (blue-green alga), two green alga, and
two diatom species. The test concentration was 2 mg/litre of water,
and the incubation period was 3-21 days. Alpha-HCH was not toxic at
this concentration.
When Canton et al. (1975) exposed Chlorella pyrenoidosa to
alpha-HCH for 96 h at 28°C (static system), the EC50 (growth
inhibition) was > 10 mg/litre (maximum solubility in the medium).
In a study by Krishnakumari (1977), cultures of the green alga
Scenedesmus acutus of 1, 3, or 5 days of age were tested for
sensitivity to alpha-HCH at 28°C, the growth rate being used as a
parameter. Alpha-HCH dissolved in ethanol was added at nominal
concentrations of 0.5-100 mg/litre water. The alpha-HCH concentrations
that caused a reduction in growth in 1-, 3-, and 5-day-old cultures
were 10 (or more), 5, and 0.5 mg/kg, respectively.
When Chlamydomonas sp. was exposed at a temperature of 20-25°C
in a static system, the no-observed-effect level (based on the growth
in 48 h) was > 1.4 mg/litre. A similar result was obtained with
Dunaliella sp. at 15°C and a study duration of 48 and 96 h, the
NOEL for growth being 1.4 mg/litre (maximum solubility) (Canton et
al., 1978).
9.2 Protozoa
The EC50 for Tetrahymena pyriformis (3 days in closed system
at 27°C) was reported to be 0.75 mg/litre (Mathur et al., 1984).
9.3 Invertebrates
9.3.1 Acute toxicity
The result of acute or short-term toxicity studies lasting a few
days on Artemia salina, Daphnia magna, and Lymnaea stagnalis are
summarized in Table 3.
Table 3. Acute or short-term toxicity of alpha-hexachlorocyclohexane for invertebrates
Species Age Temperature Parameter Concentration References
(°C) (mg/litre)
Artemia salina 3 weeks 24 LC50a,b 0.5 Canton et al. (1978)
Daphnia magna < 1 day 20 LC50c,d 0.8 Canton et al. (1975)
Lymnaea stagnalis 6 months 22 EC50c,e 1.2 Canton & Slooff (1977)
a synthetic saltwater
b 35 days (but exposure time was 4 days)
c 48 h
d closed system
e growth inhibition/mortality or immobilization
9.3.2 Short- and long-term toxicity
9.3.2.1 Crustaceae
In a study by Canton et al. (1975), Daphnia magna was exposed
to 0, 10, 50, 200, 1000, or 2000 µg alpha-HCH (> 95%) per litre for
25 days. The daphnids were fed Chlorella pyrenoidosa. The
sensitivity of daphnids to alpha-HCH markedly increased with exposure
time. A concentration of approximately 50 µg/litre or less did not
lead to death at any time during the whole life cycle of 2 months.
Only with 2000 µg/litre was there an influence on reproduction, the
EC50 for reproduction inhibition being 100 (54-186) µg/litre.
The EC50 based on mortality and immobilization was 800
(600-1000) µg/litre (see Table 4).
9.3.2.2 Molluscs
In a short-term (2-day) study, groups of five adult snails
(Lymnaea stagnalis L.) (6 months of age) were exposed to various
dose levels. Based on mortality and immobility, the EC50 was
estimated to be 1200 (600-2300) µg alpha-HCH (> 95%) per litre
(Canton & Slooff, 1977).
In a long-term (70-day) study, groups of 10 snails (5 months of
age) were exposed to 20, 100, 300, or 600 µg per litre. The study was
divided into a pre-exposure period (14 days) during which all egg
capsules and the number of eggs per capsule were counted, an exposure
period of 40 days during which four groups of adults and five capsules
of each group were exposed to alpha-HCH, and a post-exposure period
(16 days) during which snails were placed in water to recover. Based
on egg production inhibition, the 40-day EC50 was 250 µg/litre. The
percentage of fertilized eggs per capsule was not affected, and no
morphological abnormalities were noticed during embryonic development.
Based on the number of eggs that did not hatch, an EC50 of
230 µg/litre was determined. Considering a combination of the
inhibition of egg production and the mortality of the young during
their development, a 50% reduction of the overall reproductivity was
found at 65 µg alpha-HCH/litre. These effects did not disappear during
the recovery period of 16 days (Canton & Slooff, 1977) (see Table 4).
Table 4. Long-term toxicity of alpha-hexachlorocyclohexane for invertebrates
Species Age Temperature Duration Criteria Concentration References
(°C) (days) (mg/litre)
Daphnia magna 19 21 no mortality; 0.27a Janssen et al.
no effects on behaviour, 0.09 (1987)
appearance or growth;
no influence on reproduction 0.27
(4 groups of offspring)
Lymnaea stagnalis adults 22 40 EC50 (egg production inhibition) 0.25 Canton & Slooff
eggs and 22 40 hatching, overall productivity 0.065 (1977)
adults
a water renewal system
9.4 Fish
9.4.1 Acute toxicity
LC50 and EC50 (mortality and immobilization) values for fish
are summarized in Table 5.
9.4.2 Short- and long-term toxicity
During a 3-month study, rainbow trout (Salmo gairdneri)
(200-250 g) were fed pellets containing 0, 10, 50, 250, or 1250 mg
alpha-HCH (purity > 95%) per kg diet. After 2, 4, 8, and 12 weeks,
the fish were examined. Growth, microsomal liver enzymes (aniline
hydroxylase and aminopyrine demethylase), brain cholinesterase, serum
alkaline phosphatase, and the histopathology of the brain, liver, and
kidneys were all investigated but no effects were found (Canton et
al., 1975).
When guppies (Poecilia reticulata) aged 3-4 weeks were exposed
to 0, 200, 800, or 2000 µg alpha-HCH (> 95%) per litre in a 50-day
study, the EC50, based on mortality and immobilization, was 800
(600-1200) µg/litre (Canton et al., 1975).
In a study by Janssen et al. (1987), fertilized eggs of Oryzia
latipes were exposed for 35 days (up to 28 days after hatching) to
alpha-HCH. No influence on growth, mortality or behaviour was seen at
800 µg/litre.
9.5 Terrestrial organisms
No data on terrestrial organisms are available.
Table 5. Acute toxicity (48 h) of alpha-hexachlorocyclohexane for fish (growth inhibition/mortality or immobilization)
Species Age Temperature Parameter Concentration References
(°C) (mg/litre)
Freshwater
Poecilia reticulata 3-4 weeks 24 EC50a 0.8 (0.6-1.2) Canton et al. (1975)
Salmo gairdneri 4 weeks 12 EC50a 1.05 (0.9-1.2) Canton et al. (1975)
Saltwatera
Poecilia reticulata 3 weeks 24 EC50b 1.38 (1.35-1.42) Canton et al. (1978)
Poecilia reticulata LC50 3.5 Boulekbache (1980
a closed system
b water renewal system
PART B
ENVIRONMENTAL HEALTH CRITERIA FOR BETA-HEXACHLOROCYCLOHEXANE
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR BETA-HEXACHLOROCYCLOHEXANE
1. SUMMARY AND EVALUATION
1.1. General properties
1.2. Environmental transport, distribution, and
transformation
1.3. Environmental levels and human exposure
1.4. Kinetics and metabolism
1.5. Effects on organisms in the environment
1.6. Effects on experimental animals and
in vitro test systems
1.7. Effects on humans
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity of primary constituent
2.2. Physical and chemical properties
2.3. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and distribution between media
4.2. Biotransformation and bioaccumulation
4.2.1. Biodegradation
4.2.2. Abiotic degradation
4.2.3. Bioaccumulation
4.2.3.1 Aquatic invertebrates
4.2.3.2 Fish
4.2.3.3 Birds
4.2.3.4 Bioaccumulation in humans
4.3. Isomerization
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
5.1.2. Water
5.1.2.1 Fresh water
5.1.2.2 Sea water
5.1.3. Soil/sediment
5.1.3.1 Dumping grounds
5.1.4. Food and feed
5.1.5. Terrestrial and aquatic organisms
5.1.5.1 Aquatic organism
5.1.5.2 Birds
5.1.5.3 Mammals
5.2. General population exposure
5.2.1. Total-diet studies
5.2.2. Concentrations in human samples
5.2.2.1 Blood
5.2.2.2 Adipose tissue
5.2.2.3 Breast milk
6. KINETICS AND METABOLISM
6.1. Absorption and elimination
6.2. Distribution
6.3. Transplacental transfer and transfer via lactation
6.4. Metabolic transformation
6.4.1. Rat
6.4.2. Mouse
6.4.3. Human
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1. Acute toxicity data
7.1.1. Oral
7.1.2. Intraperitoneal
7.2. Short-term exposure
7.2.1. Mouse oral studies
7.2.2. Rat oral studies
7.3. Skin and eye irritation; sensitization
7.4. Long-term exposure
7.4.1. Rat oral studies
7.5. Reproduction, embryotoxicity, and
teratogenicity
7.5.1. Reproduction
7.5.2. Teratogenicity
7.6. Mutagenicity and related end-points
7.7. Carcinogenicity
7.7.1. Mouse
7.7.2. Rat
7.7.3. Initiation-promotion
7.7.4. Mode of action
7.8. Special studies
7.8.1. Effects on endocrine organs
7.8.2. Neurotoxicity
7.8.3. Effect on liver enzymes
7.8.4. Immunosuppression
8. EFFECTS ON HUMANS
8.1. Acute toxicity - poisoning incidents
8.2. General population
8.3. Occupational exposure
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1. Algae
9.2. Protozoa
9.3. Invertebrates
9.4. Fish
9.4.1. Acute toxicity
9.4.2. Longer-term toxicity
9.5. Terrestrial organisms
9.5.1. Birds
9.6. Model ecosystem studies
1. SUMMARY AND EVALUATION
1.1 General properties
Beta-hexachlorocyclohexane (beta-HCH) is a by-product (7-10%) in
the manufacture of lindane (> 99% gamma-HCH). Its solubility in water
is low, but it is very soluble in organic solvents such as acetone,
cyclohexane, and xylene. It is a solid with a low vapour pressure.
The n-octanol/water partition coefficient (log Pow) is 3.80. It
is an environmental pollutant.
Beta-HCH can be determined separately from the other isomers by
gas chromatography with electron capture detection and other methods
after extraction by liquid/liquid partition and purification by column
chromatography.
1.2 Environmental transport, distribution, and transformation
Biodegradation and abiotic degradation (dechlorination) by
ultraviolet irradiation occur in the environment and produce
pentachlorocyclohexane, but at a much slower rate than in the case of
lindane (gamma-HCH).
Beta-HCH is the most persistent HCH isomer. Its persistence in
soil is determined by environmental factors such as the action of
microorganisms, content of organic matter and water, and
co-distillation and evaporation from soil.
Owing to the persistence of beta-HCH, rapid bioconcentration takes
place in invertebrates (the bioconcentration factor is approximately
125 within 3 days), fish (250-1500 on a dry weight basis or
approximately 500 000 times on a lipid basis within 3-10 days), birds
and man (approximately 525). The bioconcentration is higher and the
elimination is slower for beta-HCH than for the other HCH isomers.
1.3 Environmental levels and human exposure
Beta-HCH is found in air over the oceans at a concentration of
0.004-0.13 ng/m3.
Until 1974, the River Rhine and its tributaries contained beta-HCH
levels of 0.14-0.22 µg/litre, but thereafter the levels were
consistently below 0.1 µg per litre. Samples from the River Meuse
also contained < 0.1 µg/litre. In the River Elbe, levels decreased
from an average of 0.009 to 0.004 µg/litre between 1981 and 1988.
Beta-HCH has been measured in birds such as sparrowhawks,
kestrels, owls, herons, and grebe over a number of years and the
concentrations ranged from 0.1 to 0.3 mg/kg. Up to 0.87 mg/kg (on a
fat basis) has been found in the liver and adipose tissue of the polar
bear.
Important food items have been analysed in a few countries for the
presence of beta-HCH. The mean concentrations, mainly in
fat-containing food products, ranged up to 0.03 mg/kg (on a fat
basis), but in milk products levels up to 4 mg/kg (on a fat basis)
were found. In non-fatty food items, the levels were < 0.005 mg/kg
product. In general, levels are slowly decreasing.
Food is the main source for general population exposure to
beta-HCH. In total-diet studies in the United Kingdom, 0.003, 0.0005,
and < 0.0005 mg/kg were found for the years 1966-1967, 1975-1977, and
1981, respectively. In the USA, the average daily intake of beta-HCH
in 1982-1984 ranged from < 0.1-0.4 ng/kg body weight for various age
groups.
In a number of countries, the concentration of beta-HCH has been
determined in the blood, serum, or plasma of the general population.
The concentrations varied between the different countries and ranged
up to 25 µg/litre.
Many studies have been carried out to determine the presence of
beta-HCH in human adipose tissues. The concentrations found in Canada,
Germany, Kenya, the Netherlands, and the United Kingdom ranged up to
4.4 mg/kg (on a fat basis). A gradual increase with age was found up
to approximately 50 years; thereafter levels decreased. Beta-HCH
concentrations in adipose tissues are higher than those of the other
HCH isomers, a phenomenon that reflects the accumulative properties of
beta-HCH. There is, in general, no clear trend for a decrease in
beta-HCH concentrations over the period that studies have been made.
There is a relationship between the concentrations in adipose tissue
and breast milk and the consumption of meat products, animal fat, and
fatty fish.
In a few countries (Canada, Germany, the Netherlands, and the
United Kingdom), breast milk has been analysed and beta-HCH levels of
between 0.1 and 0.69 mg/kg (on a fat basis) have been found. The
levels in the milk of women living in rural areas appears to be higher
than in urban areas.
The high beta-HCH levels that have been found in breast milk
exceed permissible concentrations temporarily and locally. The
beta-HCH concentrations in the blood of babies lie within the same
range as those in the mothers.
Beta-HCH appears to be a universal environmental contaminant.
Concentrations are only decreasing very slowly in spite of measures
taken to prevent its spread into the environment.
1.4 Kinetics and metabolism
Up to 95% of beta-HCH in the mouse gastrointestinal tract is
absorbed, most of it being subsequently accumulated in adipose tissue.
The elimination follows a 2-stage mechanism, the half-life for the
first stage being 2.5 days and for the second stage 18 days.
After absorption, beta-HCH is rapidly distributed to the liver,
brain, kidneys, and adipose tissues. The maximum concentration in the
liver is reached in rats after 4 days. At an average blood
concentration of 92 µg/litre (but also with concentrations of 540 and
2100 µg per litre), the brain to blood and adipose tissue to blood
ratios were 2:1 and 170:1, respectively. After lethal acute human
poisoning with HCH isomers, the beta-HCH concentration, relative to
that of blood, was 363 in fat, 3 in the brain, and 15 in the liver.
Beta-HCH passes the blood-brain barrier much less readily than the
other HCH isomers.
Transplacental transfer from pregnant mice to their fetuses was
about 2% of the dose, but in rats a transfer of 40% was found.
Lactational transfer in rats from dams to sucklings via milk was about
60% of the dose.
In rats 70% of beta-HCH is eliminated during 28 days, one third of
this being excreted in the urine. No unchanged beta-HCH is present in
the urine. The major metabolite resulting from cis-dehydrochlorination
is 2,4,6-trichlorophenol in a conjugated form.
Pretreatment with beta-HCH alters the metabolism of lindane in
rats. From intraperitoneal studies with mice, it seems that beta-HCH
is metabolized more slowly than lindane.
1.5 Effects on organisms in the environment
Beta-HCH generally has moderate toxicity for algae, invertebrates,
and fish. The acute LD50 values for these organisms are of
the order of 1 mg/litre, but the EC50 values are lower
(0.05-0.5 mg/litre). The no-observed-effect level for Oryzia latipes
and Poecilia reticulata, two freshwater fish exposed for 1 or 3
months, was 0.03 mg/litre.
No data are available on effects on populations and ecosystems.
1.6 Effects on experimental animals and in vitro test systems
The acute oral LD50 values for mice and rats were reported in
1968 to lie between 1500 and 2000 mg/kg body weight. However, more
recent studies yielded values of 16 g/kg body weight for mice and
8 g/kg body weight for rats. Signs of intoxication were mainly of
neurological origin.
Two short-term mouse studies, with dose levels of up to 600 mg/kg
diet for 26-32 weeks, showed increased liver weight and nodular
hyperplasia and atypical proliferations in the liver. In a third
study, dose levels of up to 500 mg/kg diet for 24 weeks did not result
in liver tumours or nodular hyperplasia.
A 90-day study with rats fed 50 or 250 mg/kg diet revealed liver
changes, i.e. hypertrophy and proliferation of smooth endoplasmic
reticulum and increased activity of microsomal enzymes. Changes in
the gonads occurred at the highest dose levels but these were
associated with severe effects on body weight. Hormonal changes
associated with the gonadal atrophy showed no consistent endocrine
effect. There were no adverse effects at a dose level of 2 mg/kg diet
(equivalent to 0.1 mg/kg body weight).
In a long-term rat study (reported in 1950), doses of 10 mg/kg
diet (equivalent to 0.5 mg/kg body weight) or more led to liver
enlargement and histological changes.
In a two-generation reproduction study on rats, the same effects
were found as in the 90-day study. There were no effects at 2 mg/kg
diet (equivalent to 0.1 mg/kg body weight), but a dose level of
10 mg/kg diet resulted in increased mortality and infertility. No
compound-related teratogenic effects were found in an extension to
this study.
A weak "estrogenic" effect has been described. The uterus was the
target organ for this effect; there were no clear effects on endocrine
control systems. The mechanism and significance of this effect are
uncertain.
The mutagenicity studies reported did not show any increase in
mutation frequency in Salmonella typhimurium strains. An in vivo
bone marrow metaphase analysis in rats yielded positive results.
Two studies have been carried out on mice to determine
carcinogenic potential. In one study, 200 mg/kg diet was given for
110 weeks, and liver enlargement, hyperplastic changes, and an
increase in benign and malignant tumours were reported. In the other
study, where 500 mg/kg diet was administered for 24 weeks, no tumours
were observed.
Studies in which rats were fed combinations of beta-HCH with
polychlorinated biphenyls suggested a promoting effect of beta-HCH.
At 300 mg/kg diet, beta-HCH caused significant changes in several
immune functions in mice within one month.
1.7 Effects on humans
When workers at a lindane-producing factory, with a geometric mean
exposure of 7.2 years (1-30), were investigated, it was concluded that
occupational HCH exposure did not induce signs of neurological
impairment or perturbation of "neuromuscular function".
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity of primary constituent
Common name Beta-hexachlorocyclohexane (beta-HCH)
Chemical formula C6H6Cl6
Chemical Beta-HCH is a stereoisomer of gamma-HCH,
structure the active ingredient of lindane (> 99%
(see Annex I) gamma-HCH). It differs in the spatial
orientation of the hydrogen and chlorine
atoms on the carbon atoms:
The direct uptake of alpha-HCH from contaminated water appeared to be
much greater than the uptake from contaminated food.
In a study with Salmo gairdneri, pellets containing alpha-HCH
(> 95%) levels of 0, 10, 50, 250, or 1250 mg/kg were fed to the fish,
and organs and tissues were analysed after 2, 4, 8, and 12 weeks.
There was a dose-related increase in the concentration of alpha-HCH
in the organs and tissues. After about 4-8 weeks (depending on the
type of tissue and dose level) a maximum concentration was reached,
which then slowly decreased. This suggests that after a few weeks a
balance is reached between the accumulation process (absorption of
alpha-HCH by the intestinal wall) and the elimination process (via the
gills and faeces). There is probably also a dilution effect resulting
from growth and biotransformation (Canton et al., 1975).
Ernst (1977) concluded from kinetic studies that biomagnification
of alpha-HCH does not occur. Compared with bioaccumulation from water
alone, the entry of alpha-HCH into the food chain Chlorella ->
Daphnia -> Poecilia (guppy) caused only a slight increase in
biomagnification in daphnids (factor 1.5), although in the case of the
guppies a greater increase in concentration ratio (3-4) was noted.
In a study by Canton et al. (1978), guppies (3-4 weeks old) were
exposed to alpha-HCH (> 95%) concentrations of 0.01, 0.05, or
0.14 mg/litre. When after 0.5, 3, 24, 48, 72, 96, and 120 h the
animals were analysed, the average concentration factor was about 500
for all alpha-HCH concentrations (about 17 000 on a lipid basis).
Equilibrium was reached within 24 h for the lower concentrations and
within 48 h at the highest concentrations. The elimination was rapid,
the initial concentration being halved in 10 h.
Sugiura et al. (1979) studied bioaccumulation in the carp
(Cyprinus carpio), brown trout (Salmo trutta fario), golden orfe
(Leuciscus idus melanotus), and guppy (Poecilia reticulata).
Alpha-HCH was dissolved in water to a concentration of 1 mg/litre
under steady-state conditions (time period not specified), and the
equilibrium bioconcentration factors for the four types of fish were
330, 605, 1216, and 588, respectively.
Based on the data given in section 5.1.5.2 concerning the
concentration of alpha-HCH in the muscle and fat of bream collected in
the River Elbe, the bioconcentration factor is between 10 000 and
50 000 (Arbeitsgemeinschaft für die Reinhaltung der Elbe, 1982).
In a study by Yamato et al. (1983), guppies (Poecilia
reticulata) rapidly bioaccumulated HCH isomers and the tissue
concentration reached a plateau on the fourth day (the alpha-HCH
concentration in the water was 1 µg per litre). The bioconcentration
factor (concentration in fish/concentration in water) was 706. The
concentration in the guppies decreased on the first day after the fish
were transferred to HCH-free water.
4.2.3.4 Bioconcentration in humans
Geyer et al. (1986) found that in industrialized countries more
than 90% of the exposure to HCHs derives from food. The mean
concentration of alpha-HCH in human adipose tissue (on a fat basis)
was found to be 0.03 mg/kg in the Federal Republic of Germany and
0.02 mg/kg in the Netherlands. The mean bioconcentration factor (on a
lipid basis), calculated on the basis of the concentration in the diet
(1.3 and 0.3 µg/kg, respectively) and levels in adipose tissue, was
20.0 ± 8 (range 11.5-32.5).
4.3 Isomerization
Deo et al. (1981) studied the isomerization of alpha-HCH in
sterile aqueous solution over a period of 4 weeks and found a slow
conversion of alpha-HCH to other HCH isomers.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Tanabe et al. (1982) found alpha-HCH in 24 samples of air over
the Western Pacific, Eastern Indian, and Antarctic Oceans at an
average concentration of 0.29 ng/m3 (0.022-1.4 ng/m3).
In a study by Strachan et al. (1980), samples of atmospheric
precipitation in the form of snow (1976; 17 samples) and rain (1976
and 1977; 81 samples) collected around the Canadian side of the Great
Lakes, as well as inland, were analysed. Alpha-HCH was found in the
snow samples as a trace (1 ng/litre) and in the rain samples at levels
of 1-40 ng/litre.
Air samples were taken near a road with heavy traffic, as well as
in a suburban residential area, near Ulm, in Germany. The alpha-HCH
levels were 0.22-1.3 ng/m3 in the location with heavy traffic and
0.11-1.1 ng/m3 in the rural area. It was concluded that the
concentrations in the lower troposphere under various meteorological
conditions reflect regional input and long-range transport (Wittlinger
& Ballschmiter, 1987).
In 1972, alpha-HCH air concentrations of 0.28 ng per m3 in
non-polluted areas of Germany, and 2.15 ng/m3 in the polluted Ruhr
area were determined (Hildebrandt et al., 1986).
The average concentration of alpha-HCH in 55 air samples
collected in Delft, the Netherlands, in 1979-1980 was 0.25 ng/m3
(maximum concentration: 1.2 ng/m3) (Slooff & Matthijsen, 1988).
5.1.2 Water
5.1.2.1 Rain water
Rain water sampled in 1983 in Bilt, the Netherlands, contained an
average alpha-HCH concentration of 0.01 (< 0.01-0.02) µg/litre
(Slooff & Matthijsen, 1988).
5.1.2.2 Fresh water
During the period 1969-1977, 1826 water samples were taken at 99
sampling sites in the Netherlands. The highest concentrations of
alpha-HCH were found in the River Rhine and its tributaries. The
concentrations varied between 0.01-0.3 µg/litre during the period
1969-1974, but in 1974 there was a sudden decrease and the subsequent
concentrations were all below 0.1 µg/litre. A sampling trip by boat
made along the River Rhine from Rheinfelden in Switzerland to
Rotterdam in the Netherlands proved that the source of alpha-, beta-,
and gamma-HCH was located in the upper Rhine. In the River Meuse, the
levels were all below 0.1 µg/litre during the period 1969-1977 (Wegman
& Greve, 1980).
Since 1969, alpha-, beta-, and gamma-HCH concentrations have been
measured regularly in the Rivers Rhine, Meuse, and West-Scheldt and in
other surface waters in the Netherlands. Alpha-HCH levels have been
below 0.05 µg per litre in the River Rhine since 1974/1975, and were
of the order of 0.02 µg/litre or less in the West-Scheldt during the
period 1973-1985. In the River Meuse, the concentration of alpha-HCH
was between 0.01-0.02 µg/litre. In other areas, for instance
agricultural and greenhouse horticulture areas, the levels of the
individual HCHs ranged from 0.01-1.0 µg/litre with incidental higher
peaks (up to 0.5 µg/litre) probably resulting from the use of lindane
(Slooff & Matthijsen, 1988).
Concentrations of HCH isomers in solution and in suspension
(particle-bound) in the Meuse and Rhine estuary were determined in
1974. The average concentrations of dissolved and suspended alpha-HCH
were 20 and 0-6 ng per litre, respectively. In 1981, the
concentration of dissolved alpha-HCH in coastal waters of the
Netherlands was 0.9-1.6 ng/litre, whereas that of suspended alpha-HCH
(only one measurement) was 5.3 ng/litre (Slooff & Matthijsen, 1988).
In 1970-1971, the levels of alpha-HCH were 0.66-1.5 µg/litre in
the surface water of the River Elbe near Hamburg, Germany, and
0.155-2.4 µg/litre in the River Rhine near Karlsruhe. However, a
significant decrease was observed in the mid-1970s. In 1974, 2.7 µg
per litre was found in the upper Rhine, but by 1976-1977 the levels
had decreased to 1-9 ng/litre (Hildebrandt et al., 1986).
The Arbeitsgemeinschaft der Elbe (the Elbe Study Group)
investigated the presence of alpha-HCH in the River Elbe from
Schnackenburg to the North Sea in 1981-1982 and found a mean
concentration of 0.023 (< 0.001-0.15) µg per litre. During the period
February to November 1988, the alpha-HCH concentration was
0.001-0.022 µg/litre (Arbeitsgemeinschaft der Elbe, 1988).
When certain rivers in Yorkshire, England, were analysed for
alpha-HCH in 1966, the concentration varied from 0.001 to
0.43 µg/litre. In 1968, the highest value was 0.543 µg/litre, and
the water from six other rivers contained an average of
0.001-0.004 µg/litre (highest level: 0.34 µg/litre) (Lowden et al.,
1969).
In Japan, 60 water samples were examined in 1974 and 0.1 µg
alpha-HCH/litre was detected in three of the samples (personal
communications by A. Hamada and by T. Onishi to the IPCS, July 1989).
5.1.2.3 Sea water
Atlas & Gias (1981); Bidleman & Leonard (1982); Oehme & Stray
(1982); and Oehme & Mano (1984) analysed sea water from areas such as
the North Pacific, Arabic Sea, Persian Gulf, Red Sea, Lillestrum, Bear
Island, and Spitsbergen. The alpha-HCH concentrations varied from
0.03 to 1.8 ngper litre (Slooff & Matthijsen, 1988).
In June-July 1986, the alpha-HCH in the surface water (5 m) of
the North Sea ranged from 1-2 ng/litre (Umweltbundesamt, 1989).
5.1.3 Soil/Sediment
Herrmann et al. (1984) studied the presence of alpha-HCH in
sediment along the Husum estuary and in the adjacent North Frisian
Wadden Sea. The mean concentrations varied in the different sampling
stations from 0.33 to 1.40 µg/kg sediment, while the concentrations in
bladder wrack(Fucus vesiculosus)varied from 0.7-1.2 µg/kg.
Edelman (1984) analysed 96 samples of the upper 10 cm of the soil
from 38 natural reserves in the Netherlands for alpha-HCH and
gamma-HCH. In 94 of the samples alpha-HCH was detected at levels
below 1 µg/kg (Slooff & Matthijsen, 1988).
When sediment from eight different rivers, harbours, and sites
close to dumping areas in the Netherlands were analysed for the
presence of alpha-, beta-, and gamma-HCH, the median alpha-HCH levels
were between 4 and 213 µg per kg dry matter (Slooff & Matthijsen,
1988).
In 1974, 60 sediment samples were analysed in Japan and 10 µg
alpha-HCH/kg was detected in five of the samples (personal
communications by A. Hamada and by T. Onishi to the IPCS, July 1989).
5.1.3.1 Dumping grounds
In the Netherlands, soil has been polluted with HCHs at various
locations as a result of their manufacture during the 1950s (spillage
during production, storage, and handling), and concentrations up to a
few grams of HCHs/kg dry soil have been found. Further pollution has
been caused by the dumping of chemical waste and its use in the
levelling of certain areas. From these dumping areas dispersal of the
chemical waste can occur by leaching or wind erosion from open storage
depots. In certain polluted areas, high concentrations of HCHs, mainly
alpha- and beta-HCH, have been found more than 2 m below ground level.
In 18 locations in the Netherlands, the average concentration of
alpha-HCH in sewage sludge in 1981 was between 5 and 70 µg/kg dry
matter. Pollution of ground water was also detected, but this was
restricted to the vicinity of the production areas. Horizontal
transportation of HCHs in ground water appeared to be limited (Slooff
& Matthijsen, 1988).
5.1.4 Food and feed
The presence of alpha-HCH in a number of important food items has
been determined in France by Laugel (1981). In milk and milk products
(2688 samples) the average level was 0.05 mg/kg (ranging from
undetectable to 0.22 mg/kg), in meat (37 samples) it was 0.01 mg/kg
(ranging from undetectable to 0.02 mg/kg), and in animal fat (67
samples) it was 0.02 mg/kg product (ranging from undetectable to
0.06 mg/kg. In other food items alpha-HCH was not detectable
(< 0.005 mg/kg).
Table 2 gives the mean alpha-HCH levels in a large number of
samples of various food items from the Federal Republic of Germany
reported by Hildebrandt et al. (1986).
Table 2. Alpha-hexachlorocyclohexane concentrations (mg/kg)
in various food itemsa
Food items 1973-78 1979-83 1973-83
Meatb 0.003-0.02
Meat productsb 0.007-0.037
(0.26)e
Animal fatb 0.003-0.008
(0.09)e
Gameb 0.019-0.367
Poultryb 0.003-0.004 0.003-0.016
(0.17)e
Chicken eggs < 0.001-0.003
Fish 0.002-0.011
Milk and milk
productsb 0.015e 0.01-0.03
Cow's milkb,c 0.004
Butterb,d 0.02-0.03
Table 2 (contd)
Food items 1973-78 1979-83 1973-83
Vegetable oil and
margarineb 0.01
Oil seeds, nuts,
pulses 0.001-0.042
Fruit, vegetables, < 0.0001
potatoes
Cereals 0.0002-0.007
Cereal products up to 0.14
a From: Hildebrandt et al. (1986).
b Determinations made on a fat basis
c WHO (1986).
d Anon (1984).
e Maximum value
In six samples of cows milk collected from six locations in
Switzerland, the levels of alpha-HCH were 9.5-27 mg/kg on a fat basis
(Rappe et al., 1987).
Skaftason & Johannesson (1979) analysed 35 samples of butter from
Iceland during 1968-1970 and found a level of mean alpha-HCH of 87 ±
38 µg/kg. In 1974-1978, 32 samples were studied and all contained
alpha-HCH, the mean concentration being 58 ± 21 µg/kg.
In a total-diet study in the United Kingdom, 24 samples of each
food group were analysed for alpha-HCH. The following concentrations
(mean and range) were found: bread, not detected (nd); other cereal
products, < 0.0005 (nd-0.002); carcass meat, < 0.0005 (nd-0.006);
offal, < 0.0005 (nd-0.007); meat products, eggs, green vegetables,
potatoes, fresh fruit, nd; poultry, 0.003 (nd-0.025); fish, 0.0005
(nd-0.008); oil and fats, 0.0005 (nd-0.003); milk, 0.0005 (nd-0.002);
dairy products, 0.006 (nd-0.02) mg/kg product. Imported meat products
were also analysed during the period 1981-1983, and concentrations of
up to 0.5 mg/kg were measured. Imported retail cereal products
collected in 1982 contained alpha-HCH levels of up to 0.03 mg/kg and
animal feed stuffs collected in 1984 had levels of up to 0.02 mg/kg
(HMSO, 1986).
Various types of pulses were analysed during the period
1986-1987, and 31 out of 142 samples contained alpha-HCH residues at
levels of up to 0.03 mg/kg. Processed pork and poultry, sampled
during the period 1985-1987, contained alpha-HCH at levels of up to
3.2 (mean 0.2) and 0.1-2.0 (mean 0.8) mg/kg product, respectively (26
out of the 86 samples were positive). Of other processed meat
products, 631 samples were negative. Retail milk and dairy products
were analysed during the period 1984-1987, and 499 of the 849
samples contained alpha-HCH residues at a mean concentration of
0.01-0.03 mg/kg (highest level, 0.06 mg/kg). Samples of eel muscle
(1124 eels from 62 sites) were analysed during the period 1986-1987,
and mean concentrations were 0.001-0.03 mg/kg (highest level,
0.4 mg/kg). Peanut butter and vegetable oils were analysed during the
period 1985-1987, and 95 samples showed mean concentrations of <
0.01-0.03 mg/kg product (16 of the samples were positive) (HMSO,
1989).
The mean residue level of alpha-HCH in milk samples collected
during spring 1983 from 359 bulk transporters representing 16
counties, municipalities, and districts of Ontario was 5.3 µg/kg
butter fat. Alpha-HCH was found in over 90% of the samples (Frank et
al., 1985).
5.1.5 Terrestrial and aquatic organisms
5.1.5.1 Plants
Samples of three types of mosses and four types of lichens (in
total 13 samples) were collected in the Antarctic Peninsula (Graham
Land) in 1985, and alpha-HCH was detected in most of them at a mean
concentration of 0.4 (0.20-1.15) µg/kg (Bacci et al., 1986).
In a study by Gaggi et al. (1986), fallen leaves (at the end of
their natural life-cycle) and lichens were collected in 1984 at sites
near Florence and Siena, Italy, in a woodland hilly area away from
primary pollution sources. The leaves were from ten different species
of trees and two different lichen species were involved. The average
levels of alpha-HCH in leaves and lichen were 37 (16-61) µg/kg dry
weight and 27 (25-29) µg/kg dry weight, respectively. The same
authors reported that the levels of alpha-HCH in various plant species
collected in 14 countries were 0.5-2140 µg/kg dry weight.
5.1.5.2 Fish and mussels
Martin & Hartman (1985) analysed 60 fish samples from nine
locations in the north-central part of the USA and found
concentrations of 5-27 µg alpha-HCH/kg (wet weight) in 36% of the
samples. The frequency with which alpha-HCH was detected in fish from
the different rivers varied between 17 and 100%.
In a study by Saiki & Schmitt (1986), samples of three to five
adult bluegills (Lepomis macrochirus) and common carp (Cyprinus
carpio) were collected at eight sites in three rivers in California,
USA, in 1981. Alpha-HCH concentrations in carp of up to 0.036 mg/kg on
fat basis were reported, but the concentrations in bluegill were
lower.
Cowan (1981) studied the extent of pollution of Scottish coastal
waters by HCHs using Mytilus edulis as biological indicator. The
levels of alpha-HCH at the 118 sites sampled ranged from < 6 to
23 µg/kg dry weight.
The fish and shellfish sampling programme carried out by the
United Kingdom Ministry of Agriculture, Fisheries, and Food between
1977-1984 was directed mainly to areas around the coasts of England
and Wales. The levels of alpha-HCH, which varied between the
different fish and shellfish species and also between the collection
sites, ranged from < 0.001 (nd) to 0.06 mg/kg wet weight. The
concentration in fish muscle was < 0.001 mg/kg wet weight (Franklin,
1987).
The mean alpha-HCH concentration in the muscle of flounders
collected off the North Sea coast of Germany in 1986 was 2.5 µg/kg
(nd-5.0 µg/kg) (Umweltbundesamt, 1989). Bream collected from different
locations in the River Elbe (between Schnackenburg and the North Sea)
contained 0.007-0.066 mg alpha-HCH/kg in muscle tissue and
0.9-2.2 mg/kg in adipose tissue (Arbeitsgemeinschaft für die
Reinhaltung der Elbe, 1982), while the same species collected from 15
rivers and lakes in the Federal Republic of Germany contained (on a
fat basis) up to 468 µg per kg (Umweltbundesamt, 1989).
Freshwater fish from different rivers in the Federal Republic of
Germany were analysed during the period 1973-1981, and in the first
3-4 years the alpha-HCH levels were mainly between 0.01 and 0.02 mg/kg
fresh weight. However, a clear decrease then took place and most of
the samples were below 0.01 mg/kg fresh weight, with the exception of
certain types of fish such as the eel and fish from industrially
contaminated areas (Hildebrandt et al., 1986).
In 1981-1983, shellfish and molluscs collected in the Federal
Republic of Germany contained < 0.001-0.20 mg alpha-HCH/kg fresh
weight. Eels collected in the North Sea and Baltic Sea contained
alpha-HCH levels of 0.011 mg per kg and 0.033 mg/kg fresh weight,
respectively. Flounders and herrings caught in the North Sea contained
0.002 and 0.008 mg/kg fresh weight, respectively, but in the Baltic
Sea the levels were about twice as high (Hildebrandt et al., 1986).
5.1.5.3 Birds
An average alpha-HCH residue level of 0.05 mg/kg was found in 17
adult herons in 1964 (HMSO, 1969).
In a study by Sierra & Santiago (1987), alpha-HCH concentrations
were determined in 23 barn owls (Tyto alba Scop.) from Leon, Spain.
The mean levels (and range) in muscle, liver, fat, brain, and
kidneys (in total 91 samples) were 0.242 (0.019-0.591), 0.323
(0.009-0.830), 1.073 (0.691-1.499), 0.238 (0.007-0.676), and 0.710
(0.051-2.381) mg/kg (wet weight), respectively.
Faladysz & Szefer (1982) analysed adipose fat from seven species
of diving ducks at their winter quarters in the Southern Baltic.
Residues of alpha-HCH were found in all of the 37 specimens of
long-tailed duck at mean concentrations (on a fat basis) of 3.4
(0.17-18) and 1.5 (0.23-6) mg/kg for female and male ducks,
respectively.
5.1.5.4 Mammals
Skaftason & Johannesson (1979) analysed 24 samples of the fat of
reindeer living in an area of the eastern and south-eastern parts of
Iceland where the use of pesticides is negligible. Alpha-HCH was
found in all samples at a mean level of 70 ± 22 µg/kg. These results
are in agreement with those of Benson et al. (1973), who found an
average of 77.5 µg/kg in the subcutaneous fat of wild Idaho moose
living in a forest area where pesticides were used very restrictively.
Skaftason & Johannesson (1979) analysed samples of body fat from
10-year-old sheep in 1974 and found an average of 51 ± 12 µg/kg.
Norström et al. (1988) investigated the contamination by
organochlorine compounds of Canadian arctic and subarctic marine
ecosystems by analysing the adipose tissue and liver of polar bears
( Ursus maritimus; 6-20 animals per area) collected from 12 areas
between 1982 and 1984. There was a difference in tissue distribution;
liver contained only alpha-HCH, but 29% of the HCH in adipose tissue
was beta-HCH. Adipose tissue contained 0.3-0.87 mg alpha-HCH per kg on
a fat basis.
The mean concentrations of alpha-HCH in the kidney fat of roe
(86 samples) collected in five areas of Germany in 1985-1986 were
about 7-12 µg/kg fat, the maximum value being about 50 µg/kg fat
(Umweltbundesamt, 1989).
5.2 General population exposure
From the data presented in section 5.1 it is evident that food is
the main source of exposure of the general population to alpha-HCH.
5.2.1 Total-diet studies
In a total-diet study carried out in the United Kingdom during
1966-1985, food purchased in 21 towns throughout the country was
prepared by cooking. The study covered 20 to 24 food groups, and the
number of total-diet samples examined varied from 22 to 25 samples.
The calculated mean alpha-HCH residue levels in the total diet for the
periods 1966-1967, 1970-1971, 1974-1975, 1975-1977, 1979-1980, 1981,
and 1984-1985 were 0.003, 0.002, 0.002, 0.0015, 0.001, < 0.0005, and
< 0.0005 mg/kg, respectively (Egan & Hubbard, 1975; HMSO, 1982, 1986,
1989).
Gartrell et al. (1985a) conducted a study to determine the
dietary intake of pesticides in the USA in 1978-1979. The samples,
purchased from retail outlets, were representative of the diets of
adults in 20 cities, and consisted of about 120 individual food items.
The daily intake of alpha-HCH in 1977, 1978, and 1979 was 0.011,
0.009, and 0.010 µg/kg body weight, respectively. In a similar way,
samples were collected in 10 cities in 1978-1979 consisting of about
50 items of infant food and 110 items of toddler food. The daily
intake of alpha-HCH in 1977, 1978, and 1979 was, respectively, 0.031,
0.034, 0.033 µg/kg for infants and 0.025, 0.029, and 0.029 µg/kg body
weight for toddlers, respectively (Gartrell et al., 1985b).
Total-diet studies conducted in the USA by the FDA before 1982
were based on a "composite sample approach" regardless of the diet
involved. Later on they were based on dietary survey information and
allowed the "total diet" of the population to be represented by a
relatively small number of food items for a greater number of age-sex
groups. The daily intakes of alpha-HCH during 1982-1984 for the age
groups 6-11 months, 2 years, 14-16-year-old females, 14-16-year-old
males, 25-30-year-old females, 25-30-year-old males, 60-65-year-old
females, and 60-65-year-old males were 7.2, 16.1, 6.1, 7.3, 4.5, 5.9,
3.3, and 3.7 ng/kg body weight, respectively (Gunderson, 1988).
In a total-diet study in the Netherlands in 1977, the average
concentration of alpha-HCH in 100 samples was 0.01 mg/kg on a fat
basis. The highest level was 0.05 mg/kg (Greve & van Hulst, 1977).
5.2.2 Air
Guicherit & Schulting (1985) measured the atmospheric
concentration of alpha-HCH in the Netherlands and calculated an
average daily intake by inhalation for a 70-kg person of 5 ng. The
equivalent value for the Federal Republic of Germany was calculated to
be 32 ng, which is about 1% of the total daily intake via the various
routes (Hildebrandt et al., 1986).
5.2.3 Concentrations in human samples
Alpha-HCH concentrations in human samples are a good indication
of the total exposure of the general population.
5.2.3.1 Blood
Blood samples of Dutch citizens analysed in 1978, 1980, 1981, and
1982 (70, 48, 127, and 54 samples, respectively), contained less than
0.1 µg alpha-HCH/litre (Greve & Wegman, 1985). Blok et al. (1984)
analysed the blood of 65 healthy volunteers in the Netherlands (34
female and 31 male) and detected alpha-HCH in less than one third of
the samples. The median concentration for both groups was below the
detection limit (0.1 µg per litre), but levels of up to 0.4 µg/litre
were measured.
Polishuk et al. (1970) studied the presence of alpha-HCH in the
blood of 24 pregnant women and 23 infants living in Israel. The
mean concentration was 0.6 ± 0.3 µg per litre in the women and 0.5 ±
0.3 µg/litre in the infants.
In 1975, Reiner et al. (1977) analysed the serum and plasma of 82
women and 65 men (with an average age of 42) living in a town in
Yugoslavia. In 57 of the 147 samples, alpha-HCH was found at a mean
concentration of 3.3 ± 0.5 µg/litre (range, 0.1-15.0 µg/litre).
Similar values were found in other parts of the country in 1976-1979
(Krauthacker et al., 1980).
The median concentration of alpha-HCH in whole blood of 118
people in the Federal Republic of Germany was reported to be
0.98 µg/litre (range, nd-2.06) (Bertram et al., 1980).
5.2.3.2 Adipose tissue
The alpha-HCH concentrations of 567 samples of adipose tissues of
Dutch citizens analysed during 1968-1983 varied from < 0.01 to
0.1 mg/kg (on a fat basis). The highest levels occurred during the
period 1968-1976 (Greve & van Harten, 1983; Greve & Wegman, 1985).
In a study by Niessen et al. (1984), specimens of subcutaneous
adipose tissue from 48 infants (34 under the age of 1 year, 14 in
their second year of life) were examined during 1982-1983 in the
Federal Republic of Germany. The average concentration of alpha-HCH
was 0.01 mg/kg fat (range, nd-0.02 mg/kg). The average concentration
was highest (0.02 mg/kg fat) for the age-range 0-6 weeks. Bertram et
al. (1980) found a median concentration of 0.03 mg/kg fat (range,
nd-0.35) in 72 samples of adipose tissue from people in the Federal
Republic of Germany. Hildebrandt et al. (1986) summarized the results
of nine studies carried out in the Federal Republic of Germany during
1969-1983. The mean alpha-HCH concentrations (568 samples) ranged
from 0.01 to 0.03 mg/kg fat.
Mes et al. (1982) analysed 99 samples of adipose tissue from
autopsies of accident victims from different areas of Canada. Nearly
all the samples (97%) contained alpha-HCH, the average concentration
of which was 0.004 mg/kg wet weight (range, 0.001-0.043 mg/kg).
In 1974, 360 samples of adipose tissue were collected in eight
regions of Japan and the mean level of alpha-HCH was 0.031 mg/kg
tissue (Takabatake, 1978).
Twenty-nine samples of adipose tissue were taken at necropsy and
24 at surgery in the Poznan district of Poland and compared with 100
samples from residents of the Warsaw area. In Poznan the mean
concentration of alpha-HCH was 0.013 ± 0.033 mg/kg, while in Warsaw it
was 0.008 ± 0.001 mg/kg (Szymczynski et al., 1986).
5.2.3.3 Breast milk
Breast milk is a major route for the elimination of
organochlorine pesticides in women. In a Swedish study, the levels of
alpha-HCH in breast milk were found to be related to the dietary
habit. Levels in lacto-vegetarians were lower than those in women
eating a mixed diet, and these were lower than those found in mothers
using a mixed diet that regularly included fatty fish from the Baltic
(Noren, 1983).
In a study by Fooken & Butte (1987), the variation of residue
levels in breast milk during lactation was investigated in five women
(aged 23-36) in the Federal Republic of Germany. Alpha-HCH
concentrations of up to 0.009 mg/kg fat were measured, and no
essential changes in residue level occurred over the lactation period.
Residues of alpha-HCH in breast milk during the periods,
1974-1975 and 1979-1980 in the Federal Republic of Germany were
reported to be 0.03 and 0.02 mg/kg milk on a fat basis, respectively
(Anon., 1984).
In the Federal Republic of Germany, more than 7100 samples of
breast milk were analysed from 1969-1984. These studies were carried
out by 20 authors, and the results were summarized by Hildebrandt et
al. (1986). The mean concentrations of alpha-HCH ranged from
0.01-0.04 mg/kg on a fat basis. In one case a mean concentration of
0.21 mg/kg was found in 320 samples. During the period investigated, a
slow decrease in the mean concentration of alpha-HCH was observed. The
average concentration in breast milk in the same country (2709
samples) in 1979-1981 was 0.024 mg/kg on a fat basis (Fooken & Butte,
1987). In 1981-1983, 132 samples of breast milk were analysed and the
average level was 0.001 mg alpha-HCH/kg milk fat (Cetinkaya et al.,
1984).
Tuinstra (1971) analysed 36 individual samples of breast milk,
collected in 1969, from young mothers (18-32 years of age) living in
the Netherlands. A median alpha-HCH concentration of 0.01 mg/kg milk
(on a fat basis) was found (range, nd-0.04). When 278 samples of
breast milk, collected in 11 maternity centres in the Netherlands,
were analysed for the presence of alpha-HCH, the median alpha-HCH
concentration was < 0.01 mg/kg (on a fat basis) (Greve & Wegman,
1985).
Vukavic et al. (1986) measured the alpha-HCH concentration in 59
samples of colostrum collected during autumn 1982 (26 samples) and
spring 1983 (33 samples) in Yugoslavia from healthy nursing mothers on
the third day after delivery. The alpha-HCH levels were significantly
lower in the autumn than in the spring (mean concentrations of 0.49 ±
0.09 and 1.50 ± 0.26 µg/litre whole colostrum, respectively).
Mes et al. (1986) studied 210 breast milk samples from five
different regions of Canada and measured a mean alpha-HCH
concentration of 7 µg/kg (on a fat basis). Davies & Mes (1987)
studied 18 breast milk samples from Canadian, Indian, and Inuit
mothers in Canada, whose fish consumption was comparable to the
national level. The level of alpha-HCH in milk fat of the indigenous
population was 5 µg/kg, which was the same value as that obtained from
a national survey.
6. KINETICS AND METABOLISM
6.1 Absorption and elimination
The intestinal absorption rate for alpha-HCH was 97.4% after the
administration of an HCH mixture to male rats (Albro & Thomas, 1974).
The total excretion in rats after a single intraperitoneal (ip)
36Cl-labelled alpha-HCH dose of 200 mg/kg body weight was 80% of the
dose in the urine and 20% in the faeces (Koransky et al., 1963;
Koransky et al., 1964; Noack et al., 1975). In a study in rats with
36Cl-labelled alpha-HCH, a low excretion rate was found. 36Cl was
detected in the excreta up to 40 days after a single ip dose (Koransky
et al., 1963). During continued dosing alpha-HCH was observed to
stimulate its own degradation (Noack et al., 1975). The decrease in
rat liver alpha-HCH levels after an initial increase, observed by
Eichler et al. (1983), was assumed to be due to this effect.
When 14C-labelled alpha-HCH was administered intraperitoneally
to male mice (ddY-strain, 4 weeks old) at a dose level of 22 µg, the
average percentage of urinary excretion of radioactivity in 3 days was
37% (Kurihara & Nakajima, 1974).
6.2 Distribution
One day after an ip injection of a mixture of 14C- and
36Cl-labelled alpha-HCH into rats (200 mg/kg body weight in rapeseed
oil), the highest level of radioactivity was found in fat, skin, and
bones plus muscles (18.2, 13.1, and 11.9%, respectively, after 4
days). Much lower levels were found in other organs or tissues (up to
1% in liver and kidneys and 0.28% in the central nervous system. In
the faeces and urine, 3.9 and 7.9%, respectively, were found after 4
days (Koransky et al., 1963). In other studies with rats, higher
concentrations were found in liver, kidneys, body fat, brain, and
muscle (Portig & Vohland, 1983; Kuiper et al., 1985). In a 90-day
study in rats, marked deposition of alpha-HCH was found in renal fat;
the concentrations exceeded those obtained in a similar study on
beta-HCH (Greve & van Hulst, 1980; Kuiper et al., 1985). In lactating
rats given a single oral dose 5 days after birth, the alpha-HCH
concentrations in the livers of the sucklings were twice as high as
those observed in the livers of the mothers (Wittich &
Schulte-Hermann, 1977).
Vohland et al. (1981) studied the distribution of alpha-HCH in
the brain and depot fat of rats after the administration of 200 mg/kg
body weight by gavage. With an average blood concentration of
1.5 µg/litre, the brain to blood, and depot fat to blood ratios were
120:1 and 397:1, respectively, whereas with a blood concentration of
17.7 mg/litre the ratios were 5:1 and 82:1, respectively.
Nagasaki (1973) orally administered alpha-HCH to male mice at
concentrations of 100, 250 or 500 mg/kg for 24 weeks, and found high
residual levels of this isomer in liver and adipose tissue. Similarly,
Macholz et al. (1986) reported that a 30-day administration of
alpha-HCH to rats resulted in high residues of this isomer in fat,
kidneys, and adrenal tissue.
In the brain, alpha-HCH is stored preferentially in the white
matter (Stein et al., 1980; Portig et al., 1989).
6.3 Metabolic transformation
6.3.1 Rat
When Sprague-Dawley weanling female rats were administered 2 mg
alpha-HCH/rat per day in peanut oil for 7 days, the alpha-HCH was
metabolized to 2,4,6- and 2,4,5-trichlorophenol, with an excretion
ratio of 2,4,6- to 2,4,5-trichlorophenol of 1.3:1. This study also
indicated that pre-treatment with alpha-HCH alters the metabolism of
lindane in rats (Freal & Chadwick, 1973).
The biotransformation of alpha-HCH in rats involves
dechlorination (Kraus, 1975). The dose-dependent decrease in liver
glutathione concentrations indicates the formation of a glutathione
conjugate in this organ (Noack & Portig, 1973; Portig et al., 1973;
Kraus, 1975). Such a decrease does not occur in the brain or kidneys
(Noack & Portig, 1973).
The major urinary metabolite in rats is 2,4,6-trichlorophenol, a
compound reported by IARC (1987) to be carcinogenic for animals
(Portig et al., 1973; Stein & Portig, 1976; Stein et al., 1977). Other
metabolites that have been identified are 1,2,4-trichlorophenol,
2,3,4-trichlorophenol, 2,4,5-trichlorophenol, 2,3,4,5-
tetrachlorophenol, and 2,3,4,6-tetrachlorophenol (Noack et al., 1975;
Stein et al., 1977; Macholz et al., 1982). In addition,
chlorothiophenols (not specified) have been detected, and
1,3,4,5,6-pentachlorocyclohex-1-ene has been identified in the kidneys
of rats (Macholz et al., 1983).
Artigas et al. (1988) have identified several lindane metabolites
(tetra-, penta-, and hexachlorocyclohexenes, and tetra- and
pentachlorobenzenes) in rat brain homogenates by gas
chromatography-mass spectrometry. Male Wistar rats were orally
administered 30 mg alpha-HCH/kg and were sacrificed 5 h later. The
cerebella of the animals were analysed and the following metabolites
were found: 3.6/4.5-PCCH, 3.5/4.6-PCCH, HCCH, pentachlorobenzene, and
HCB. HCCH was the major metabolite (about 100 µg per kg) while levels
of the other metabolites were mainly below 5 µg/kg. Alpha-HCH was
present at 17.2 mg/kg tissue. This study showed that the HCH isomers
are cleared from the brain via different metabolic pathways.
Isomerization of alpha-HCH to lindane did not occur after
repeated dosage (Eichler et al., 1983).
6.3.2 Bird
In a model 4-week feeding study on poultry using four HCH
isomers, the rate of degradation of the individual HCH isomers in
broilers followed the order: delta > gamma > alpha > beta.
Biotransformation (to one or more of the other HCH isomers) was not
detected (Szokolay et al., 1977b).
In a study by Foster & Saha (1978) on the in vitro metabolism
of alpha-HCH in chicken livers, the first metabolite was identified as
an isomer of pentachlorocyclohexane.
6.3.3 Human
When Engst et al. (1978) analysed the urine of occupationally
exposed workers (apparently to technical-grade HCH in manufacturing
processes), they found, apart from alpha-, beta-, gamma-, and
delta-HCH, traces of hexa- and pentachlorobenzene, gamma- and
delta-pentachlorocyclohexane, pentachlorophenol, 2,3,4,5-, 2,3,4,6-,
and 2,3,5,6-tetrachlorophenol, and several trichlorophenols, as well
as the glucuronides of several of these metabolites. The
pentachlorocyclohexenes, tetrachlorophenol, hexachlorobenzene, and
pentachlorophenol were also identified in the blood.
6.4 Retention and biological half-life
The half-life for the clearance of alpha-HCH from depot fat was
found to be 6.9 days in female rats and 1.6 days in male rats (Stein
et al., 1980; Portig, 1983).
Vohland et al. (1981) and Portig & Vohland (1983) studied the
kinetics of alpha-HCH in Wistar rats, and observed that, after a
single oral dose of 200 mg/kg body weight, the approximate half-life
in females for the elimination from brain was 6 days.
The retention of alpha-HCH in rat brain after a single dose is
greater than that of beta- and gamma-HCH (Stein et al., 1980).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Acute toxicity
In mice oral LD50 values have been found to range from 1000 to
4000 mg/kg body weight, depending on the vehicule, while in rats
values of 500-4674 mg/kg body weight have been obtained. Riemschneider
(1949) determined a LD50 (oral intubation in olive oil) for rats of
1500 mg/kg body weight. The signs of poisoning were those of nervous
system stimulation: excitation, hunched posture, rough fur, dyspnoea,
anorexia, tremors, convulsions, and cramps (Hoffmann, 1983; WHO,
1986).
7.2 Short-term exposure
7.2.1 Oral
In a 90-day study on rats carried out with dose levels of 0, 2,
10, 50, or 250 mg alpha-HCH/kg diet, reductions in white blood cell
count were noted in several groups of animals. Growth was decreased
at 250 mg/kg diet, and at this dose level the number of erythrocytes
and protein excretion in the urine were elevated in female animals. At
levels of 50 and 250 mg/kg, the activities of liver
amino-pyrine- N-demethylase and aniline hydroxylase were increased
while those of blood aspartate aminotransferase (ASAT) and creatine
phosphokinase were decreased. Liver weights were increased at dose
levels of 10, 50, and 250 mg/kg. Enlargement of liver parenchyma cells
(with a foamy/hyaline appearance of the cytoplasm) and accentuation of
the plasmalemma, indicative of proliferation of smooth endoplasmatic
reticulum (SER), occurred at levels of 50 and 250 mg/kg. At the
250-mg/kg level, there were increases in the relative weights of
heart, kidneys, and adrenals. In addition, serum levels of
immunoglobulins G and M showed a decrease at 50 and 250 mg/kg diet
(Kuiper et al., 1985).
Macholz et al. (1986) reported that the administration of 1000 mg
alpha-HCH/kg to rats for 30 days resulted in growth retardation and
liver mass increase. High residue levels of alpha-HCH were identified
in fat, kidneys, and adrenal tissue.
7.2.2 Other routes
7.2.2.1 Intravenous
In a study by van Asperen (1954), groups of 12-15 male and female
albino mice (8-10 weeks of age) were given an intravenous injection of
alpha-HCH (in peanut oil). The dose levels were 480 or 960 µg/mouse
(equivalent to approximately 32 and 64 mg/kg body weight,
respectively). No deaths occurred within 7 days.
7.2.2.2 Subcutaneous
Groups of 13-21 male and female albino mice (8-10 weeks of age)
were given a subcutaneous injection of alpha-HCH at dose levels
ranging from 3 to 20 mg/animal (equivalent to approximately 200 to
1330 mg/kg body weight, respectively). With doses of up to 4 mg, no
death occurred within 7 days, but with 4.5 mg, 8 mg, and 20 mg, 8, 25,
and 90%, respectively, of the animals died (van Asperen, 1954).
7.3 Skin and eye irritation; sensitization
No data on skin and eye irritation or sensitization have been
reported.
7.4 Long-term exposure
7.4.1 Rat oral study
When groups of 10 female and 10 male weanling Wistar rats were
administered diets containing 0, 10, 50, 100, or 800 mg alpha-HCH/kg
diet (in corn oil) for 107 weeks, the highest dose level resulted in
growth retardation, increased mortality, and slight kidney damage.
With dose levels of 100 or 800 mg/kg, liver enlargement and
histo-pathological changes in the liver were found. However, there
were no liver changes at 50 mg/kg diet (Fitzhugh et al., 1950).
7.5 Reproduction, embyrotoxicity, and teratogenicity
No information on reproduction, embryotoxicity, or teratogenicity
is available.
7.6 Mutagenicity and related end-points
Alpha-HCH did not induce mutations in Salmonella typhimurium
test strains TA98, TA100, TA1535 or TA1537 either with or without rat
liver metabolic activation (Lawlor & Haworth, 1979). A test for point
mutations in Saccharomyces cerevisiae XV 185 14 C was also negative
(Shahin & von Borstel, 1977). In addition, the compound produced no
mutations in Allium cepa roots (Nybom & Knutsson, 1947). A test for
unscheduled DNA synthesis in rat hepatocytes in vitro produced an
equivocal result (Althaus et al., 1982).
A mutagen test strain of Bacillus subtilis (TKJ5211) showed a
higher sensitivity for hisw+ reversion than the parental strain
HA101 when treated with UV and UV-mimetic chemicals. However, a
negative result was obtained when alpha-HCH dissolved in DMSO was used
at a dose level of 5 mg/ml (Tanooka, 1977).
A DNA repair test was carried out with stationary-phase cultures
of B. subtilis HLL3g and HJ-15 strains in which the size of growth
inhibition zones of repair-proficient and repair-deficient cells for
vegetative cells and spores was determined. There was no effect at a
dose level of 5 mg alpha-HCH (in benzene) per ml (Tanooka, 1977).
The available data are inadequate to make an assessment of the
mutagenic potential.
7.7 Carcinogenicity
Appraisal
The reported studies on the carcinogen effects of alpha-HCH on
mice and rats have some short-comings. In most cases, very high dose
levels were tested. Nevertheless, it is clear from the results that
alpha-HCH, at high dose levels, produces nodular hyperplasia and
hepatocellular carcinomas in mice (the incidence varying according to
the strain) and also in rats (low incidence), but only at higher dose
levels.
The results of the studies on initiation-promotion and mode of
action indicate that the neoplastic response observed with alpha-HCH
is most likely due to a non-genotoxic mechanism.
7.7.1 Mouse
When 20 male ICR/JCL mice (aged 5 weeks) were administered a diet
containing 600 mg alpha-HCH/kg diet for 26 weeks, increased liver
weight was observed. In all treated mice there were liver tumours,
which were characterized histologically as benign tumours and
malignant tumours with atypical liver cells. Unfortunately,
insufficient details were reported (Goto et al., 1972a,b).
In a study by Hanada et al. (1973), 6-week-old DD mice (10-11 of
each sex per group) were given diets containing 0, 100, 300, or 600 mg
alpha-HCH/kg diet for 32 weeks, followed by a control diet for 5-6
weeks. The control group consisted of 20 female and 21 male animals.
During the experiment several animals died. The numbers of hepatomas
in the four groups surviving for 36-38 weeks were 0/29 (control), 1/16
(100 mg/kg), 9/10 (300 mg/kg), and 13/15 (600 mg/kg).
Alpha-fetoprotein was not detected in the serum of animals with
hepatomas.
When 8-week-old male DD mice, divided into groups of 20 or 38
animals, were fed a diet containing 0, 100, 250, or 500 mg
alpha-HCH/kg for 24 weeks, the two highest dose levels induced an
increase in liver weight. At the four respective dose levels, the
incidence of nodules classified as nodular hyperplasia was 0/20, 0/20,
30/38 (79%), and 20/20 (100%) and that of hepatocellular carcinoma was
0/20, 0/20, 10/38 (26%), and 17/20 (85%) (Ito et al., 1973b).
Following the oral administration of 100, 250 or 500 mg
alpha-HCH/kg to male DD mice for 24 weeks, hepatocellular tumours were
found in all mice treated with 500 mg/kg and in 17 of the 20 mice that
received 250 mg/kg (Nagasaki, 1973).
Nagasaki et al. (1975) studied the tumorigenic effects of a diet
containing 0 or 500 mg alpha-HCH/kg, fed for 24 weeks to groups of
male and female DDY, ICR, DBA/2, C57BL/6, and C3H/He mice (13-29 of
each sex per group), male Wistar rats, and male golden Syrian
hamsters. It was found that alpha-HCH induced liver tumours in male
and female mice but not in rats and hamsters. The histological changes
in the liver of mice were much greater than those induced in rats and
hamsters. Male animals were more susceptible to the tumorigenic
action (i.e. liver nodules) than females. Among the different strains
of mice, a difference in susceptibility was observed. The occurrence
of liver nodules varied from 16.7 to 100% and the incidence of
hepatocellular carcinomas varied from none to 65%. The DDY mouse
strain was the most sensitive and the C57BL/6 the least sensitive
strain.
Ito et al. (1976) studied the reversibility of liver tumours
induced by alpha-HCH (99.0%). Male 8-week-old DDY mice were fed a
diet containing 0 or 500 mg/kg for 16, 20, 24, and 35 weeks and then
fed a basal diet without alpha-HCH for 4, 8, and 12, or 4, 8, 12, 16,
24, and 36 weeks. In total 341 mice were used, of which 21 were fed
the compound for 16 weeks. A total of 300 mice were fed the diet with
alpha-HCH for 20 or more weeks and 20 control mice were fed the basal
diet for 72 weeks. At the various intervals indicated, 12-20 mice were
killed. The incidence of liver tumours increased progressively during
continuous administration of alpha-HCH, but when its administration
was discontinued some tumours disappeared. After 24 weeks of
administration most tumours were nodular hyperplasias with only a few
well-differentiated hepatocellular carcinomas. However, 60 or 72
weeks after the beginning of the study most of the liver tumours were
hepatocellular carcinomas. The findings suggested that nodular
hyperplasia was usually reversible.
Two groups of male HPBC57B1 black mice (6-9 weeks old) were fed
a diet containing 500 mg/kg alpha-HCH (99.8%) per diet, 48 mice being
used as controls and 75 mice being administered alpha-HCH. From each
group, 4-9 mice were killed at 1, 3, 4, 8, 14, 21, 30, 33, 44, and 50
weeks after the initiation of treatment. Progressive liver
enlargement was first noticed at 3 weeks, hepatic nodules at 21 weeks,
and emaciation at 30 weeks. Histopathological liver alterations
included hypertrophy of centrolobular hepatocytes first seen at 1 week
and the merging of adjacent megalocytic zones at 3 weeks. At 21
weeks, adenomas were seen in two out of seven mice, at 30 weeks in
seven out of eight mice, and at 33, 44, and 50 weeks in all the mice
studied. Under the condition of this study, neither hepatocellular
carcinomas nor metastases in the lungs were detected (Tryphonas &
Iverson, 1983).
7.7.2 Rat
When groups of 10 male and 10 female weanling Wistar rats were
fed throughout their life on diets containing 10, 50, 100, or 800 mg
alpha-HCH (> 98% pure) per kg, no increase in tumour incidence was
found. However, only a limited number of organs were examined
microscopically (Fitzhugh et al., 1950).
In a study by Ito et al. (1975), male Wistar rats (5-8-weeks old)
were divided into seven groups and administered alpha-HCH diets
containing 0, 500 (two groups), 1000 (three groups), or 1500 mg
alpha-HCH/kg diet. The duration of the treatment for the different
groups was 72 weeks for the controls, 24 or 48 weeks at 500 mg/kg, 24,
48, or 72 weeks at 1000 mg/kg, and 72 weeks at 1500 mg/kg. In the
liver, oval cells and bile duct cell proliferation were found in the
groups fed 1000 or 1500 mg/kg after 48 and 72 weeks. Cell hypertrophy
was found in all the groups, the increase in severity depending on the
dose level and the duration of administration. In the two groups fed
500 mg/kg and the group fed 1000 mg/kg for 24 weeks no nodular
hyperplasia or hepatocellular carcinomas were found. Nodular
hyperplasia developed in the groups fed 1000 mg/kg (48 and 72 weeks)
or 1500 mg/kg (72 weeks) in 42, 76, and 77% of the animals,
respectively. Hepatocellular carcinomas were found only in the groups
fed 1000 or 1500 mg/kg for 72 weeks (1/16 and 3/13 animals,
respectively).
In a series of studies, an oral dose of 20 mg/kg body weight was
administered daily to female rats during periods of 4.5, 13.5, or 23.5
months. Liver enzyme induction was found at all intervals, white foci
and nodules were present after 13.5 months, and one animal had a
hepatocellular carcinoma after 23.5 months (Schulte-Hermann &
Parzefall, 1981). The value of this study was reduced by the very low
number of animals (4-6 per group) used at each interval.
7.7.3 Initiation-promotion
In a study on 8-week-old white male mice (25-30 per group) of
strain DD, the influence of alpha-HCH on tumour induction by
polychlorinated biphenyls (PCBs) was tested and vice versa. Whereas
500 mg PCB/kg diet induced nodular hyperplasia and hepatocellular
carcinomas in the liver of male mice after 32 weeks, exposure to
alpha-HCH at dose levels of 50, 100, or 250 mg/kg diet, only resulted
in both type of tumours at the highest dose level. The incidence of
nodular hyperplasia was 23/30 (77%) and that of hepatocellular
carcinoma was 8/30 (27%). However, 50 or 100 mg alpha-HCH/kg diet, in
combination with 250 mg PCB per kg diet (PCB alone did not induce
tumours), induced nodular hyperplasia (approximately 30%) and
hepatocellular carcinoma (approximately 5%). It seems that PCBs
promote the induction of liver tumours by alpha-HCH (Ito et al.,
1973a).
In studies on rats, alpha-HCH showed a tumour-promoting action
towards the hepatocarcinogenic effects of aflatoxin B1,
diethylnitrosamine, and nitrosomorpholine (Schulte-Hermann &
Parzefall, 1981; Schulte-Hermann et al., 1981; Angsubhakorn et al.,
1981). In one test, alpha-HCH produced only a slight liver
tumour-promoting effect in rats after initiation with
N-nitrosodiethylamine (Ito et al., 1983). However, in another study
on the same species the compound had an inhibitory effect on the
hepa-tocarcinogenic action of 3-methyl-4-dimethylaminoazobenzene and
DL-ethionine (Thamavit et al., 1974).
Nagasaki et al. (1975) studied the influence of
3-methylcholanthrene, 1-naphthyl isothiocyanate, and
p-hydroxypropiophenone on the induction of liver tumours by
alpha-HCH. Eight groups of 24 mice received a diet containing either
500 mg alpha-HCH/kg diet in combination with 67 mg
methylcholanthrene/kg, 600 mg 1-naphthyl isothiocyanate/kg or 1000 mg
p-hydroxypropiophenone/kg or just one of the four compounds. A
control group with the basal diet was also used. The induction of
mouse liver tumours by alpha-HCH was not inhibited by the concomitant
feeding of 1-naphthyl isothiocyanate or p-hydroxypropiophenone.
However, 3-methylcholanthrene slightly inhibited their induction by
alpha-HCH.
In a study by Schröter et al. (1987), the tumour-initiating
activity of alpha-HCH was studied by examining for phenotypically
altered foci in female Wistar rats. Groups of three to eight rats
were used and, after removing the median and right liver lobes, 200 mg
alpha-HCH/kg body weight was administered followed by phenobarbital at
50 mg/kg body weight per day for 15 weeks. Liver foci were identified
by means of the gamma-glutamyltransferase (GGT) reaction and by
morphological alterations. No evidence of initiating activity was
found. In another part of the study, the promoting activity was
investigated. A single dose of N-nitrosomorpholine (250 mg/kg body
weight by gavage) was followed by the administration of 0.1, 0.5, 2.0,
7.0, or 20.0 mg alpha-HCH/kg body weight per day for 4, 15, and 20
weeks. The criteria used were growth and phenotypic changes of foci
as end-points. It was concluded from the study that alpha-HCH is a
tumour promotor. Both the number and size of altered foci were
enhanced by alpha-HCH doses of 2 mg/kg or more. The tumour-promoting
action was generally associated with liver enlargement and induction
of monooxygenases or other specific enzymes.
Schulte-Hermann et al. (1983) carried out three experiments with
Han-Wistar rats using, in experiment 1, 39 female rats (8-24 months
old) and, in experiments 2 and 3, 41 male (2 years old) rats.
Alpha-HCH (200 mg/kg in corn oil) was administered orally as a single
dose, while the control group received only corn oil. Beginning 25 h
after the dosing, 3H-thymidine was injected intravenously five times
at intervals of 6 h (experiment 2) or 8 h (experiment 3) , and the
animals were killed 18 (experiment 2) or 3 h (experiment 3) after the
last dose of 3H-thymidine. The effect of age on the incidence of
spontaneous foci was studied in experiment 1. Foci of putative
preneoplastic cells were detected in the livers of untreated rats of
both sexes, especially at 1 and 2 years of age. These foci exhibited
markers similar to those of their counterparts in carcinogen-treated
rats, such as cytoplasmic basophilia, clearness of cytoplasm, or
expression of gamma-glutamyl transferase. Rates of DNA synthesis in
foci were higher than in normal liver cells and were increased by
single doses of liver mitogens assumed to promote liver tumour
development. Thus cells in the spontaneous foci appeared to possess a
defect in the growth control, rendering them more susceptible to
endogenous and exogenous growth stimuli.
The incorporation of orally administered radiolabelled thymidine
into liver DNA was determined in SIV-50-SD rats 24 h after a single
oral gavage dose of 2.9, 29.1, 58.2, or 291 mg alpha-HCH/kg. Alpha-HCH
was found to stimulate liver DNA synthesis at 58.2 mg/kg (Büsser &
Lutz, 1987).
7.7.4 Mode of action
Sagelsdorff et al. (1983) studied the relevance to the
carcinogenic action of alpha-HCH of covalent binding to mouse liver
DNA. Three strains of mice were used (NMRI, CF1, and C6B3F1), and
alpha-HCH was administered by oral gavage and 14C-thymidine by the
intraperitoneal route. In all three strains, a similar low covalent
binding index or DNA damage/dose (values ranging from 0.17-0.28) was
found. There was no quantitative correlation with the carcinogenicity
potency of alpha-HCH.
Iverson et al. (1984) studied the ability of alpha-HCH to bind to
macromolecules from male HPB black mouse liver. In vivo and in
vitro binding studies with 14C-alpha-HCH and hepatic microsomes
from untreated and phenobarbital-pretreated mice showed no
preferential binding of alpha-HCH to protein or DNA. The results
suggest that the neo-plastic response observed with alpha-HCH results
from a non-genotoxic mechanism.
7.8 Special studies
7.8.1 Effect on liver enzymes
After a single oral administration to female rats of 5 mg
alpha-HCH/kg body weight or more the rate of aminopyrine demethylation
and the liver DNA content were both increased, but at 2 mg/kg body
weight these effects did not occur (Schulte-Hermann et al., 1974). In
a further study, the liver cytochrome P450 concentration in male rats
after a single oral administration was elevated at all tested dose
levels, 25 mg/kg body weight being the lowest (Seifart & Buchar,
1978). After alpha-HCH was given to male rats at dose levels of 5, 10,
20, 50, or 200 mg/kg feed for 2 weeks, aniline hydroxylase and
aminopyrine demethylase activities were increased at all dose levels
(den Tonkelaar et al., 1981).
7.8.2 Neurotoxicity
Appraisal
Alpha-HCH has been shown to have no effect on motor nerve
conduction velocity or the fronto-occipital EEG in rats fed 1000 mg
alpha-HCH/kg diet for 30 days. This isomer is a mild antagonist of
pentylenetetrazol-induced convulsions but increases the tonic/clonic
activity and the lethality of picro-toxin when administered
intraperitoneally to mice. It decreases the accumulation of
cerebellar cyclic GMP and prohibits the increase of cGMP caused by
gamma-HCH in mouse brain. Alpha-HCH has been demonstrated to inhibit
GABA-mediated chloride ion uptake in mouse brain, and this effect is
believed to play a primary role in the CNS action of this isomer.
In a study by Vohland et al. (1981), alpha-HCH did not give rise
in brain tissue to appreciable quantities of hydrophobic metabolites
such as 2,4,6-trichlorophenol. It had a weak protecting action
against convulsions induced by pentylenetetrazole (PTZ). The intensity
and duration of the PTZ-antagonistic effects after a single oral dose
were related to the alpha-HCH content of the brain.
In a 30-day study on groups of 15 male Wistar rats fed alpha-HCH
at levels of up to 1000 mg/kg diet, there was no effect on the
fronto-occipital electroencephalogram or on the motor conduction
velocity of the tail nerve (Müller et al., 1981).
The effect of alpha-HCH on body temperature, food intake, and
body weight was studied in Wistar rats (eight males and eight females)
given a single 30-mg/kg oral dose of alpha-HCH in olive oil. Controls
received only olive oil. Alpha-HCH treatment induced no significant
decrease in core temperature 5 h after treatment, and no decrease in
food intake or growth was observed (Camon et al., 1988).
Fishman & Gianutsos (1987) studied the effects of an
intraperitoneal injection of alpha-HCH (99.0%) in corn oil
(80-480 mg/kg body weight) on the accumulation of cerebellar cyclic
GMP in male CD-1 mice. Alpha-HCH decreased the accumulation of
cerebellar cyclic GMP and also prevented the increase in cyclic GMP
resulting from lindane treatment. Furthermore, alpha-HCH inhibited
the binding of 3H-TBOB (a ligand for the GABA-A-receptor-linked
chloride channel) in mouse cerebellum.
Fishman & Gianutsos (1988) compared the CNS-related
pharmacological and biochemical effects of gamma-HCH and the
non-convulsant isomer alpha-HCH. The studies were carried out on male
CD-1 mice injected intraperitoneally with a single alpha-HCH (in corn
oil) dose of 80-400 mg/kg body weight. Alpha-HCH inhibited the
myoclonic jerk and tonic/clonic activity of PTZ but increased the
tonic/clonic activity and lethality of picrotoxin (PIC) (PTZ and PIC
were given as a single ip injection of 50 mg/kg and 20 mg/kg body
weight, respectively). The highest dose of alpha-HCH caused a
significant decrease in motor activity. Gamma-HCH inhibited the
binding of 3H-TBOB to mouse whole brain membranes. Furthermore, this
isomer is a weak inhibitor of GABA-stimulated uptake of 36wCl into
mouse brain neurosynaptosome preparations in vitro. The
non-seizure-inducing alpha-HCH has biochemical and pharmacological
effects in the CNS which differ from those of the gamma-HCH.
Matsumoto et al. (1988) provided evidence that all HCH isomers
are capable of inhibiting GABA-A-mediated chloride channels in the
brain, the relative potency being alpha = gamma > delta > beta.
Alpha-HCH was also found to be a potent inhibitor of the
batrachotoxin-stimulated action potential flux of sodium ions in N18
neuroblastoma cell cultures (Shain et al., 1987).
8. EFFECTS ON HUMANS
8.1 Acute toxicity - poisoning incidents
Several cases of acute poisoning by technical-grade HCH,
resulting either from accidents or occupational exposure, have been
described (WHO, 1991). Although alpha-HCH constitutes 65-70% of the
technical product, it is likely that the most acutely toxic component,
i.e. gamma-HCH, played the major role in these incidents. These cases
cannot, therefore, assist in the evaluation of alpha-HCH.
8.2 General population
No specific studies relating to alpha-HCH are available.
A study comparing liver cancer deaths in the USA and the
"domestic disappearance" of organochlorine pesticides revealed that in
1962, 18 and 15 years after the introduction of DDT and
technical-grade HCH, respectively (when an increase in primary liver
cancer due to the organochlorines would be manifest), the number of
cases of primary liver cancer as a percentage of the total number of
liver cancer deaths began a gradual and steady decline (from 61.3% in
1962 to 56.9% in 1972). The death rate (per 100 000 per year) due to
primary liver cancer declined from 3.46 to 3.18 during this period
(Deichmann & MacDonald, 1977).
8.3 Occupational exposure
The evaluation of the effects of alpha-HCH on occupationally
exposed workers is seriously hampered by the fact that most of the
relevant studies concern workers who were exposed during the
manufacture and handling of lindane or the handling and spraying of
technical-grade HCH among other pesticides, and were thus exposed to
all HCH isomers plus impurities and other (process) chemicals.
Therefore, it is difficult, if not impossible, to relate the observed
effects to individual substances. Consequently these studies have only
been described in this monograph where they aid the evaluation.
Behrbohm & Brandt (1959) described 26 cases of allergic and toxic
dermatitis that arose during the manufacture of technical-grade HCH.
Patch testing with pure alpha-, beta-, gamma-, and delta-HCH yielded
negative results, but positive reactions were obtained with the
residual fractions.
The level of alpha-HCH in 57 healthy workers (with normal liver
function, EMG and EEG) at a lindane-manufacturing plant ranged from 10
to 273 µg/litre, whereas it was below the detection limit in control
workers. The concentration in the adipose tissue of eight of the
exposed workers ranged from 1 to 15 mg alpha-HCH/kg (in extractable
lipids) (Baumann et al., 1980, 1981; Brassow et al., 1981; Tomczak et
al., 1981).
The mean serum alpha-HCH level of malaria-control workers that
sprayed technical-grade HCH for 16 weeks increased from 10 to
78 µg/litre in previously non-exposed workers and from 18 to
77 µg/litre in those that had been exposed during three previous
spraying seasons (Gupta et al., 1982).
Nigam et al. (1986) studied 64 employees from a plant
manufacturing HCH who were directly or indirectly associated with the
production of this insecticide and thus also exposed to chemicals such
as benzene and chlorine. The exposed group was composed of 19
"handlers" (who handled and packed the insecticide), 26 "non-handlers"
(plant operators and supervisors exposed indirectly to HCH), and 19
maintenance staff (who visited the plant frequently). The control
group consisted of 14 workers who had no occupational contact with the
insecticide. The exposure period varied up to 30 years. The mean
serum alpha-HCH concentrations in the four groups were 21.1 µg/litre
(controls), 21.8 µg/litre (maintenance staff), 41.2 µg per litre
(non-handlers), and 100 µg/litre (handlers). Lindane and beta- and
delta-HCH were also present. The total HCH concentrations were 51.4,
143.6, 265.6, and 604 µg per litre, respectively. Clinical examination
revealed that the majority of the workers from the "handler" and
"non-handler" groups exhibited paraesthesia of the face and
extremities, headache, and giddiness, and some of them also showed
symptoms of malaise, vomiting, tremors, apprehension, confusion, loss
of sleep, impaired memory, and loss of libido. The same symptoms were
found among the maintenance staff but were less severe and less
frequent.
Chattopadhyay et al. (1988) studied 45 male workers exposed to
HCH during its manufacture and compared them with 22 matched controls.
Exposure was mainly via the skin. Paraesthesia of face and
extremities, headache, giddiness, vomiting, apprehension, and loss of
sleep, as well as some changes in liver function tests, were reported
and were found to be related more to the intensity of exposure (as
measured by the HCH levels in blood serum) than to the duration of
exposure. The measured exposures to total HCH were 13 to 20 times
higher than those in the control groups (no detailed figures were
reported). Of the total serum HCH, 60-80% was beta-HCH.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Algae
Palmer & Maloney (1955) used alpha-HCH in a preliminary screening
test with two cyanobacterium (blue-green alga), two green alga, and
two diatom species. The test concentration was 2 mg/litre of water,
and the incubation period was 3-21 days. Alpha-HCH was not toxic at
this concentration.
When Canton et al. (1975) exposed Chlorella pyrenoidosa to
alpha-HCH for 96 h at 28°C (static system), the EC50 (growth
inhibition) was > 10 mg/litre (maximum solubility in the medium).
In a study by Krishnakumari (1977), cultures of the green alga
Scenedesmus acutus of 1, 3, or 5 days of age were tested for
sensitivity to alpha-HCH at 28°C, the growth rate being used as a
parameter. Alpha-HCH dissolved in ethanol was added at nominal
concentrations of 0.5-100 mg/litre water. The alpha-HCH concentrations
that caused a reduction in growth in 1-, 3-, and 5-day-old cultures
were 10 (or more), 5, and 0.5 mg/kg, respectively.
When Chlamydomonas sp. was exposed at a temperature of 20-25°C
in a static system, the no-observed-effect level (based on the growth
in 48 h) was > 1.4 mg/litre. A similar result was obtained with
Dunaliella sp. at 15°C and a study duration of 48 and 96 h, the
NOEL for growth being 1.4 mg/litre (maximum solubility) (Canton et
al., 1978).
9.2 Protozoa
The EC50 for Tetrahymena pyriformis (3 days in closed system
at 27°C) was reported to be 0.75 mg/litre (Mathur et al., 1984).
9.3 Invertebrates
9.3.1 Acute toxicity
The result of acute or short-term toxicity studies lasting a few
days on Artemia salina, Daphnia magna, and Lymnaea stagnalis are
summarized in Table 3.
Table 3. Acute or short-term toxicity of alpha-hexachlorocyclohexane for invertebrates
Species Age Temperature Parameter Concentration References
(°C) (mg/litre)
Artemia salina 3 weeks 24 LC50a,b 0.5 Canton et al. (1978)
Daphnia magna < 1 day 20 LC50c,d 0.8 Canton et al. (1975)
Lymnaea stagnalis 6 months 22 EC50c,e 1.2 Canton & Slooff (1977)
a synthetic saltwater
b 35 days (but exposure time was 4 days)
c 48 h
d closed system
e growth inhibition/mortality or immobilization
9.3.2 Short- and long-term toxicity
9.3.2.1 Crustaceae
In a study by Canton et al. (1975), Daphnia magna was exposed
to 0, 10, 50, 200, 1000, or 2000 µg alpha-HCH (> 95%) per litre for
25 days. The daphnids were fed Chlorella pyrenoidosa. The
sensitivity of daphnids to alpha-HCH markedly increased with exposure
time. A concentration of approximately 50 µg/litre or less did not
lead to death at any time during the whole life cycle of 2 months.
Only with 2000 µg/litre was there an influence on reproduction, the
EC50 for reproduction inhibition being 100 (54-186) µg/litre.
The EC50 based on mortality and immobilization was 800
(600-1000) µg/litre (see Table 4).
9.3.2.2 Molluscs
In a short-term (2-day) study, groups of five adult snails
(Lymnaea stagnalis L.) (6 months of age) were exposed to various
dose levels. Based on mortality and immobility, the EC50 was
estimated to be 1200 (600-2300) µg alpha-HCH (> 95%) per litre
(Canton & Slooff, 1977).
In a long-term (70-day) study, groups of 10 snails (5 months of
age) were exposed to 20, 100, 300, or 600 µg per litre. The study was
divided into a pre-exposure period (14 days) during which all egg
capsules and the number of eggs per capsule were counted, an exposure
period of 40 days during which four groups of adults and five capsules
of each group were exposed to alpha-HCH, and a post-exposure period
(16 days) during which snails were placed in water to recover. Based
on egg production inhibition, the 40-day EC50 was 250 µg/litre. The
percentage of fertilized eggs per capsule was not affected, and no
morphological abnormalities were noticed during embryonic development.
Based on the number of eggs that did not hatch, an EC50 of
230 µg/litre was determined. Considering a combination of the
inhibition of egg production and the mortality of the young during
their development, a 50% reduction of the overall reproductivity was
found at 65 µg alpha-HCH/litre. These effects did not disappear during
the recovery period of 16 days (Canton & Slooff, 1977) (see Table 4).
Table 4. Long-term toxicity of alpha-hexachlorocyclohexane for invertebrates
Species Age Temperature Duration Criteria Concentration References
(°C) (days) (mg/litre)
Daphnia magna 19 21 no mortality; 0.27a Janssen et al.
no effects on behaviour, 0.09 (1987)
appearance or growth;
no influence on reproduction 0.27
(4 groups of offspring)
Lymnaea stagnalis adults 22 40 EC50 (egg production inhibition) 0.25 Canton & Slooff
eggs and 22 40 hatching, overall productivity 0.065 (1977)
adults
a water renewal system
9.4 Fish
9.4.1 Acute toxicity
LC50 and EC50 (mortality and immobilization) values for fish
are summarized in Table 5.
9.4.2 Short- and long-term toxicity
During a 3-month study, rainbow trout (Salmo gairdneri)
(200-250 g) were fed pellets containing 0, 10, 50, 250, or 1250 mg
alpha-HCH (purity > 95%) per kg diet. After 2, 4, 8, and 12 weeks,
the fish were examined. Growth, microsomal liver enzymes (aniline
hydroxylase and aminopyrine demethylase), brain cholinesterase, serum
alkaline phosphatase, and the histopathology of the brain, liver, and
kidneys were all investigated but no effects were found (Canton et
al., 1975).
When guppies (Poecilia reticulata) aged 3-4 weeks were exposed
to 0, 200, 800, or 2000 µg alpha-HCH (> 95%) per litre in a 50-day
study, the EC50, based on mortality and immobilization, was 800
(600-1200) µg/litre (Canton et al., 1975).
In a study by Janssen et al. (1987), fertilized eggs of Oryzia
latipes were exposed for 35 days (up to 28 days after hatching) to
alpha-HCH. No influence on growth, mortality or behaviour was seen at
800 µg/litre.
9.5 Terrestrial organisms
No data on terrestrial organisms are available.
Table 5. Acute toxicity (48 h) of alpha-hexachlorocyclohexane for fish (growth inhibition/mortality or immobilization)
Species Age Temperature Parameter Concentration References
(°C) (mg/litre)
Freshwater
Poecilia reticulata 3-4 weeks 24 EC50a 0.8 (0.6-1.2) Canton et al. (1975)
Salmo gairdneri 4 weeks 12 EC50a 1.05 (0.9-1.2) Canton et al. (1975)
Saltwatera
Poecilia reticulata 3 weeks 24 EC50b 1.38 (1.35-1.42) Canton et al. (1978)
Poecilia reticulata LC50 3.5 Boulekbache (1980
a closed system
b water renewal system
PART B
ENVIRONMENTAL HEALTH CRITERIA FOR BETA-HEXACHLOROCYCLOHEXANE
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR BETA-HEXACHLOROCYCLOHEXANE
1. SUMMARY AND EVALUATION
1.1. General properties
1.2. Environmental transport, distribution, and
transformation
1.3. Environmental levels and human exposure
1.4. Kinetics and metabolism
1.5. Effects on organisms in the environment
1.6. Effects on experimental animals and
in vitro test systems
1.7. Effects on humans
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity of primary constituent
2.2. Physical and chemical properties
2.3. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and distribution between media
4.2. Biotransformation and bioaccumulation
4.2.1. Biodegradation
4.2.2. Abiotic degradation
4.2.3. Bioaccumulation
4.2.3.1 Aquatic invertebrates
4.2.3.2 Fish
4.2.3.3 Birds
4.2.3.4 Bioaccumulation in humans
4.3. Isomerization
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
5.1.2. Water
5.1.2.1 Fresh water
5.1.2.2 Sea water
5.1.3. Soil/sediment
5.1.3.1 Dumping grounds
5.1.4. Food and feed
5.1.5. Terrestrial and aquatic organisms
5.1.5.1 Aquatic organism
5.1.5.2 Birds
5.1.5.3 Mammals
5.2. General population exposure
5.2.1. Total-diet studies
5.2.2. Concentrations in human samples
5.2.2.1 Blood
5.2.2.2 Adipose tissue
5.2.2.3 Breast milk
6. KINETICS AND METABOLISM
6.1. Absorption and elimination
6.2. Distribution
6.3. Transplacental transfer and transfer via lactation
6.4. Metabolic transformation
6.4.1. Rat
6.4.2. Mouse
6.4.3. Human
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1. Acute toxicity data
7.1.1. Oral
7.1.2. Intraperitoneal
7.2. Short-term exposure
7.2.1. Mouse oral studies
7.2.2. Rat oral studies
7.3. Skin and eye irritation; sensitization
7.4. Long-term exposure
7.4.1. Rat oral studies
7.5. Reproduction, embryotoxicity, and
teratogenicity
7.5.1. Reproduction
7.5.2. Teratogenicity
7.6. Mutagenicity and related end-points
7.7. Carcinogenicity
7.7.1. Mouse
7.7.2. Rat
7.7.3. Initiation-promotion
7.7.4. Mode of action
7.8. Special studies
7.8.1. Effects on endocrine organs
7.8.2. Neurotoxicity
7.8.3. Effect on liver enzymes
7.8.4. Immunosuppression
8. EFFECTS ON HUMANS
8.1. Acute toxicity - poisoning incidents
8.2. General population
8.3. Occupational exposure
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1. Algae
9.2. Protozoa
9.3. Invertebrates
9.4. Fish
9.4.1. Acute toxicity
9.4.2. Longer-term toxicity
9.5. Terrestrial organisms
9.5.1. Birds
9.6. Model ecosystem studies
1. SUMMARY AND EVALUATION
1.1 General properties
Beta-hexachlorocyclohexane (beta-HCH) is a by-product (7-10%) in
the manufacture of lindane (> 99% gamma-HCH). Its solubility in water
is low, but it is very soluble in organic solvents such as acetone,
cyclohexane, and xylene. It is a solid with a low vapour pressure.
The n-octanol/water partition coefficient (log Pow) is 3.80. It
is an environmental pollutant.
Beta-HCH can be determined separately from the other isomers by
gas chromatography with electron capture detection and other methods
after extraction by liquid/liquid partition and purification by column
chromatography.
1.2 Environmental transport, distribution, and transformation
Biodegradation and abiotic degradation (dechlorination) by
ultraviolet irradiation occur in the environment and produce
pentachlorocyclohexane, but at a much slower rate than in the case of
lindane (gamma-HCH).
Beta-HCH is the most persistent HCH isomer. Its persistence in
soil is determined by environmental factors such as the action of
microorganisms, content of organic matter and water, and
co-distillation and evaporation from soil.
Owing to the persistence of beta-HCH, rapid bioconcentration takes
place in invertebrates (the bioconcentration factor is approximately
125 within 3 days), fish (250-1500 on a dry weight basis or
approximately 500 000 times on a lipid basis within 3-10 days), birds
and man (approximately 525). The bioconcentration is higher and the
elimination is slower for beta-HCH than for the other HCH isomers.
1.3 Environmental levels and human exposure
Beta-HCH is found in air over the oceans at a concentration of
0.004-0.13 ng/m3.
Until 1974, the River Rhine and its tributaries contained beta-HCH
levels of 0.14-0.22 µg/litre, but thereafter the levels were
consistently below 0.1 µg per litre. Samples from the River Meuse
also contained < 0.1 µg/litre. In the River Elbe, levels decreased
from an average of 0.009 to 0.004 µg/litre between 1981 and 1988.
Beta-HCH has been measured in birds such as sparrowhawks,
kestrels, owls, herons, and grebe over a number of years and the
concentrations ranged from 0.1 to 0.3 mg/kg. Up to 0.87 mg/kg (on a
fat basis) has been found in the liver and adipose tissue of the polar
bear.
Important food items have been analysed in a few countries for the
presence of beta-HCH. The mean concentrations, mainly in
fat-containing food products, ranged up to 0.03 mg/kg (on a fat
basis), but in milk products levels up to 4 mg/kg (on a fat basis)
were found. In non-fatty food items, the levels were < 0.005 mg/kg
product. In general, levels are slowly decreasing.
Food is the main source for general population exposure to
beta-HCH. In total-diet studies in the United Kingdom, 0.003, 0.0005,
and < 0.0005 mg/kg were found for the years 1966-1967, 1975-1977, and
1981, respectively. In the USA, the average daily intake of beta-HCH
in 1982-1984 ranged from < 0.1-0.4 ng/kg body weight for various age
groups.
In a number of countries, the concentration of beta-HCH has been
determined in the blood, serum, or plasma of the general population.
The concentrations varied between the different countries and ranged
up to 25 µg/litre.
Many studies have been carried out to determine the presence of
beta-HCH in human adipose tissues. The concentrations found in Canada,
Germany, Kenya, the Netherlands, and the United Kingdom ranged up to
4.4 mg/kg (on a fat basis). A gradual increase with age was found up
to approximately 50 years; thereafter levels decreased. Beta-HCH
concentrations in adipose tissues are higher than those of the other
HCH isomers, a phenomenon that reflects the accumulative properties of
beta-HCH. There is, in general, no clear trend for a decrease in
beta-HCH concentrations over the period that studies have been made.
There is a relationship between the concentrations in adipose tissue
and breast milk and the consumption of meat products, animal fat, and
fatty fish.
In a few countries (Canada, Germany, the Netherlands, and the
United Kingdom), breast milk has been analysed and beta-HCH levels of
between 0.1 and 0.69 mg/kg (on a fat basis) have been found. The
levels in the milk of women living in rural areas appears to be higher
than in urban areas.
The high beta-HCH levels that have been found in breast milk
exceed permissible concentrations temporarily and locally. The
beta-HCH concentrations in the blood of babies lie within the same
range as those in the mothers.
Beta-HCH appears to be a universal environmental contaminant.
Concentrations are only decreasing very slowly in spite of measures
taken to prevent its spread into the environment.
1.4 Kinetics and metabolism
Up to 95% of beta-HCH in the mouse gastrointestinal tract is
absorbed, most of it being subsequently accumulated in adipose tissue.
The elimination follows a 2-stage mechanism, the half-life for the
first stage being 2.5 days and for the second stage 18 days.
After absorption, beta-HCH is rapidly distributed to the liver,
brain, kidneys, and adipose tissues. The maximum concentration in the
liver is reached in rats after 4 days. At an average blood
concentration of 92 µg/litre (but also with concentrations of 540 and
2100 µg per litre), the brain to blood and adipose tissue to blood
ratios were 2:1 and 170:1, respectively. After lethal acute human
poisoning with HCH isomers, the beta-HCH concentration, relative to
that of blood, was 363 in fat, 3 in the brain, and 15 in the liver.
Beta-HCH passes the blood-brain barrier much less readily than the
other HCH isomers.
Transplacental transfer from pregnant mice to their fetuses was
about 2% of the dose, but in rats a transfer of 40% was found.
Lactational transfer in rats from dams to sucklings via milk was about
60% of the dose.
In rats 70% of beta-HCH is eliminated during 28 days, one third of
this being excreted in the urine. No unchanged beta-HCH is present in
the urine. The major metabolite resulting from cis-dehydrochlorination
is 2,4,6-trichlorophenol in a conjugated form.
Pretreatment with beta-HCH alters the metabolism of lindane in
rats. From intraperitoneal studies with mice, it seems that beta-HCH
is metabolized more slowly than lindane.
1.5 Effects on organisms in the environment
Beta-HCH generally has moderate toxicity for algae, invertebrates,
and fish. The acute LD50 values for these organisms are of
the order of 1 mg/litre, but the EC50 values are lower
(0.05-0.5 mg/litre). The no-observed-effect level for Oryzia latipes
and Poecilia reticulata, two freshwater fish exposed for 1 or 3
months, was 0.03 mg/litre.
No data are available on effects on populations and ecosystems.
1.6 Effects on experimental animals and in vitro test systems
The acute oral LD50 values for mice and rats were reported in
1968 to lie between 1500 and 2000 mg/kg body weight. However, more
recent studies yielded values of 16 g/kg body weight for mice and
8 g/kg body weight for rats. Signs of intoxication were mainly of
neurological origin.
Two short-term mouse studies, with dose levels of up to 600 mg/kg
diet for 26-32 weeks, showed increased liver weight and nodular
hyperplasia and atypical proliferations in the liver. In a third
study, dose levels of up to 500 mg/kg diet for 24 weeks did not result
in liver tumours or nodular hyperplasia.
A 90-day study with rats fed 50 or 250 mg/kg diet revealed liver
changes, i.e. hypertrophy and proliferation of smooth endoplasmic
reticulum and increased activity of microsomal enzymes. Changes in
the gonads occurred at the highest dose levels but these were
associated with severe effects on body weight. Hormonal changes
associated with the gonadal atrophy showed no consistent endocrine
effect. There were no adverse effects at a dose level of 2 mg/kg diet
(equivalent to 0.1 mg/kg body weight).
In a long-term rat study (reported in 1950), doses of 10 mg/kg
diet (equivalent to 0.5 mg/kg body weight) or more led to liver
enlargement and histological changes.
In a two-generation reproduction study on rats, the same effects
were found as in the 90-day study. There were no effects at 2 mg/kg
diet (equivalent to 0.1 mg/kg body weight), but a dose level of
10 mg/kg diet resulted in increased mortality and infertility. No
compound-related teratogenic effects were found in an extension to
this study.
A weak "estrogenic" effect has been described. The uterus was the
target organ for this effect; there were no clear effects on endocrine
control systems. The mechanism and significance of this effect are
uncertain.
The mutagenicity studies reported did not show any increase in
mutation frequency in Salmonella typhimurium strains. An in vivo
bone marrow metaphase analysis in rats yielded positive results.
Two studies have been carried out on mice to determine
carcinogenic potential. In one study, 200 mg/kg diet was given for
110 weeks, and liver enlargement, hyperplastic changes, and an
increase in benign and malignant tumours were reported. In the other
study, where 500 mg/kg diet was administered for 24 weeks, no tumours
were observed.
Studies in which rats were fed combinations of beta-HCH with
polychlorinated biphenyls suggested a promoting effect of beta-HCH.
At 300 mg/kg diet, beta-HCH caused significant changes in several
immune functions in mice within one month.
1.7 Effects on humans
When workers at a lindane-producing factory, with a geometric mean
exposure of 7.2 years (1-30), were investigated, it was concluded that
occupational HCH exposure did not induce signs of neurological
impairment or perturbation of "neuromuscular function".
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity of primary constituent
Common name Beta-hexachlorocyclohexane (beta-HCH)
Chemical formula C6H6Cl6
Chemical Beta-HCH is a stereoisomer of gamma-HCH,
structure the active ingredient of lindane (> 99%
(see Annex I) gamma-HCH). It differs in the spatial
orientation of the hydrogen and chlorine
atoms on the carbon atoms:
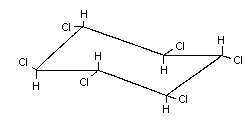 Relative
molecular mass 290.9
CAS chemical 1alpha,2ß,3alpha,4ß,5alpha,6ß-hexachloro-
name cyclohexane
Common
synonym Beta-benzenehexachloride (beta-BHC)
CAS registry
number 319-85-7
RTECS registry
number GV4375000
2.2 Physical and chemical properties
Some physical and chemical properties are summarized in Table 6.
Table 6. Some physical and chemical properties of beta-
hexachlorocyclohexane
Melting point 309°C
Vapour pressure (20°C) 0.67 Pa (0.005 mmHg)
Relative density (20°C) 1.89 g/cm3
Solubility
water (20°C) 1.5 mg/litre
water (28°C) 0.2 mg/litre
organic solvents (20°C)
acetone 103.9 g/litre
chloroform 3 g/litre
ethanol 11 g/litre
petroleum ether 1-2 g/litre
xylene 33 g/litre
cyclohexane 121 g/litre
Stability considerable stability in acids,
unstable in alkaline conditions
n-Octanol/water partition
coefficient (log Pow) 3.80
2.3 Analytical methods
The same methods can be used as for alpha-HCH (see section 2.3
alpha-HCH).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Beta-HCH does not occur naturally. It is released to the
environment as a result of the use of technical-grade HCH and the
inappropriate disposal of the residue resulting from the purification
of lindane.
Beta-HCH is basically a by-product (and impurity) in the
manufacturing of lindane (> 99% gamma-HCH) (van Velsen, 1986).
Technical-grade HCH, which is synthesized from benzene and chlorine in
the presence of ultraviolet light, consists of:
65-70% alpha-HCH
7-10% beta-HCH
14-15% gamma-HCH (lindane)
approx.7% delta-HCH
approx.1-2% epsilon-HCH
approx.1-2% other components
Purification of lindane produces a residue, consisting almost
entirely of non-insecticidal HCH isomers (mainly alpha- and beta-),
which can be used as an intermediate for the production of
trichlorobenzene and other chemicals.
Alpha- and beta-HCH have been used in mixtures with gamma-HCH (as
"HCH" or "fortified HCH") in agriculture and in wood protection.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
Tsukano (1973) studied the factors affecting the disappearance of
beta-HCH from rice field soil. After granular application of
technical-grade HCH (0.05 mg/litre) into surface water, beta-HCH
disappeared very slowly (half-life > 28 days). After the
translocation of beta-HCH (1 mg/litre) onto flooded levelled soil, the
surface water and soil was analysed at intervals. A decrease in the
beta-HCH concentration in water and a steady increase in soil was
found. After 7 days a maximum concentration in soil was reached.
From a soil column study it was found that beta-HCH did not move
through the soil.
Suzuki et al. (1975) studied the persistence of beta-HCH in three
different types of soils. Beta-HCH is the most persistent isomer of
HCH, the persistence being dependent on environmental factors such as
the action of soil microorganisms, co-distillation, and evaporation
from soil. Furthermore, the water content and the content of organic
matter in the soil are of importance.
Siddaramappa & Sethunathan (1975) studied the persistence of
14C-labelled beta-HCH in five Indian rice soils under flooded
conditions, using incubation times of 0, 20, and 41 days. The
degradation of beta-HCH was much slower than that of lindane. However,
there was a great difference in the degradation rates between the
soils. In two types of soils (sandy and kari soils) both isomers
persisted even after 41 days of flooding.
Sorption and desorption of beta-HCH by 12 soils from rice-growing
areas in India were studied using a 14C-label. The soils showed
striking differences in their ability to adsorb beta-HCH, the sorption
values ranging from 46 to 96% of total added beta-HCH. After oxidation
of the soil with hydrogen peroxide, the sorption was lower (14-58%).
Organic matter was the most important factor governing the sorption
and desorption, but pH, exchange acidity, exchangeable sodium and
magnesium, and electrical conductivity also affected the results
(Wahid & Sethunathan, 1979).
Kampe (1980) concluded from experimental data that the transport
of beta-HCH to ground water is unlikely, owing to low water solubility
and anaerobic degradation.
Korte (1980) summarized the behaviour of beta-HCH in the
environment, especially in soil and plants.
4.2 Biotransformation and bioaccumulation
4.2.1 Biodegradation
MacRae et al. (1967) studied the persistence and biodegradability
of beta-HCH in two clay soils. Beta-HCH was applied at a level of
15 mg/kg soil and the incubation periods were 0, 15, 30, 50, 70, and
90 days. In non-sterilized soils only very small amounts could be
detected after 70 days, indicating biodegradation, whereas in
sterilized soils the losses were much slower and probably due to
volatilization.
In studies using either mixed or pure bacterial cultures under
anaerobic or aerobic conditions, the dechlorination of 36Cl-labelled
beta-HCH by mixed soil flora and by pure cultures of Citrobacter
freundii, C. butyricum, and C. pasteurianum was 7.4, 15.3, 23.8,
and 10.1%, respectively, within 6 days of incubation. Aerobically
grown facultative anaerobes dechlorinated actively. Beta-HCH degraded
more slowly than lindane (Jagnow et al., 1977; Haider, 1979).
Cell suspensions of Clostridium sphenoides cultured under
anaerobic conditions did not degrade beta-HCH (10 mg/litre) within
24 h. Even with more concentrated cell suspensions of the organism
and conditions most conducive to lindane degradation (pH 8.0, 40°C),
there was no indication of any degradation (Heritage & MacRae, 1979).
MacRae et al. (1984) carried out laboratory studies on the
transformation of beta-HCH (dosage: 20 mg beta-HCH/g of soil) in a
Japanese soil containing 4% organic carbon under both aerobic and
anaerobic conditions. From the transformation rates, half-life values
of 91 and 122 days, respectively, were calculated.
In a study by Doelman et al. (1988a), microbial soil sanitation
was applied to calcareous alkaline sandy loam soil that was polluted
with a mixture of HCH isomers. Under anaerobic conditions, microbial
degradation in the Dutch climate (soil temperature of 5-17°C) did not
occur, and even the low concentration of the easily degradable
gamma-HCH did not decrease. Microbial soil sanitation of
beta-HCH-polluted sandy loam soil systems have been investigated. The
soil systems involved were aerated moist soil and continuously aerated
and intermittently aerated thick soil slurry. Degradation of beta-HCH
did not take place during a 40-week incubation period (Doelman et al.,
1988b).
A field investigation into the distribution of HCHs was carried
out by Chessells et al. (1988) using soil from an agricultural area
treated with BHC-20 (HCH composition: 70% alpha-HCH, 6.5% beta-HCH,
13.5% gamma-HCH, and 5% delta-HCH. The beta-HCH concentration
decreased only very slowly, probably owing to its comparatively high
stability and low water solubility. Furthermore, soil organic carbon
content was found to be of primary importance. A significant decrease
in isomer concentration was observed when soil moisture content was
high and was attributed to microbial degradation favoured by these
conditions.
4.2.2 Abiotic degradation
Ultraviolet irradiation, using a 15-watt low pressure mercury
lamp, of beta-HCH in 2-propanol solution for 16 h resulted in the
production of an isomer of pentachloro-cyclohexene. This substance
seems to be formed by the migration of an equatorial chlorine atom to
the vicinal axial position at the intermediate pentachlorocyclohexyl
radical (Hamada et al., 1982).
4.2.3 Bioaccumulation
4.2.3.1 Aquatic invertebrates
In a study by Yamato et al. (1983), short-necked clam (Venerupis
japonica) rapidly absorbed beta-HCH and the concentration reached a
plateau on the third day. The bioconcentration factor was 127 at a
beta-HCH concentration in water of 2 µg/litre. The beta-HCH
concentrations on day 6 in internal organs and tissues were 0.194 and
0.076 mg/kg, respectively. After a 3-day elimination period, the
levels were 0.115 and 0.075 mg/kg, respectively.
4.2.3.2 Fish
Sugiura et al. (1979) studied bioaccumulation in the carp
(Cyprinus carpio), brown trout (Salmo trutta fario), golden orfe
(Leuciscus idus melanotus), and guppy (Poecilia reticulata).
Beta-HCH was dissolved in water to a concentration of 1 mg/litre under
steady-state conditions (time period not specified), and the
equilibrium bioconcentration factors for the four types of fish were
273, 658, 973, and 1485, respectively.
In a study by Yamato et al. (1983), guppies (Poecilia
reticulata) rapidly bioaccumulated beta-HCH and the tissue
concentration reached a plateau on the fourth day. The beta-HCH
concentration in the water was 2 µg/litre and the bioconcentration
factor 1043. The concentration in the guppy slowly decreased on the
first day after the fish were transferred to HCH-free water.
In general, the equilibrium levels were reached within 3-10 days.
A bioconcentration factor of 100 000 to 500 000 has been calculated
using data on the concentration of beta-HCH in the muscle and fat of
bream collected in the River Elbe (Arbeitsgemeinschaft für die
Reinhaltung der Elbe, 1982).
4.2.3.3 Birds
When low levels of HCH were administered together with other
organochloropesticides in the feed to broilers for 6-16 weeks, of the
three HCH isomers tested (alpha, beta, and gamma), beta-HCH showed the
greatest bioaccumulation (the mean bioconcentration factors for eggs
and fat were 13 and 15, respectively). The half-life (after
administration of uncontaminated food for 12 weeks) was about 6-8
weeks (Kan & Jonker-den Rooyen, 1978a,b; Kan et al., 1978).
This relatively higher accumulation of beta-HCH was also observed
in chickens after feeding diets fortified with 1 mg beta-HCH/kg for 4
weeks. The order of the degradation rate for the four HCH isomers was
delta > gamma > alpha > beta. Biotransformation to one or more of
the other HCH isomers did not occur (Szokolay et al., 1977a).
4.2.3.4 Bioaccumulation in humans
Geyer et al. (1986) found that in industrialized countries more
than 90% of the non-occupation exposure to HCHs derives from food.
The mean concentration (on a fat basis) of beta-HCH in human adipose
tissue was found to be 0.33-0.38, 0.40, 0.31, 0.90, 0.27, and
0.31 mg/kg in the Federal Republic of Germany, the Netherlands, USSR,
Switzerland, USA, and United Kingdom, respectively. The mean
bioconcentration factor (on a lipid basis), calculated on the basis of
the concentration in the diet (0.68, 0.62, 1.0, 1.21, 0.56, and
0.67 µg/kg, respectively) and the levels in adipose tissue, was 527.0
± 140 (range 310-744).
4.3 Isomerization
Deo et al. (1980, 1981) studied the isomerization of beta-HCH by
shaking it with distilled water at 25°C for various time intervals
(5 min to 4 days). The results of GLC analysis of the extracts
indicated that a small portion of the beta-HCH isomerized into alpha-,
gamma-, and delta-HCH. There were indications that other compounds
were also formed by reactions such as dehydrochlorination. Toxicity
studies with mosquito larvae, flour beetle larvae, and houseflies
exposed to the extracts demonstrated that the resulting aqueous
solution contained substances that were more toxic than beta-HCH.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Tanabe et al. (1982) found an average of 0.03 ng beta-HCH/m3
(0.004-0.13 ng/m3) in 24 samples of air over the Western Pacific,
Eastern Indian, and Antarctic Oceans.
The concentrations of beta-HCH measured in the air of Delft,
the Netherlands, in 1979-1980 were below the limit of detection
(2-3 pg/m3) (Slooff & Matthijsen, 1988).
5.1.2 Water
5.1.2.1 Fresh water
During the period 1969-1977, 1826 water samples were taken at 99
sampling sites in the Netherlands. The highest concentrations of
beta-HCH were found in the River Rhine and its tributaries. The
concentrations during the period 1969-1974 were 0.14-0.22 µg/litre,
but from 1974 on, the concentrations were all below 0.1 µg/litre. A
sampling trip by boat made along the River Rhine from Rheinfelden in
Switzerland to Rotterdam in the Netherlands proved that the source of
alpha-, beta-, and gamma-HCH was located in the upper Rhine. In the
River Meuse, the levels were all below 0.1 µg/litre during the period
1969-1977 (Wegman & Greve, 1980). Since 1983 the contents of beta-HCH
in the Rivers Rhine, Meuse, and West-Scheldt and in other surface
waters in the Netherlands have generally been below 0.001 µg/litre
(Slooff & Matthijsen, 1988). The average concentration of dissolved
beta-HCH in the Meuse-Rhine estuary in 1974 was 6 ng/litre whereas
that of suspended beta-HCH was < 1 to 3 ng/litre (Slooff &
Matthijsen, 1988).
The Arbeitsgemeinschaft der Elbe (the Elbe Study Group)
investigated the presence of beta-HCH in the River Elbe from
Schnackenburg to the North Sea in 1981-1982 and found a mean
concentration of 0.009 (< 0.001-0.072) µg per litre. During the
period February to November 1988 the concentrations varied from 0.001
to 0.009 µg per litre (Arbeitsgemeinschaft der Elbe, 1988).
In the surface water of the Upper Rhine, the beta-HCH
concentration was 200 ng/litre in 1974 but decreased in 1976-1977 to
2-25 ng/litre (Hildebrandt et al., 1986). LWA (1987) failed to detect
beta-HCH in the River Rhine (three locations) and in six tributaries.
5.1.2.2 Sea water
Beta-HCH was detected in the North Sea at a level of 1.4 µg/litre
in 1972 (Mestres, 1974), but in June-July 1986 the levels in surface
water (5 m) were < 0.03-0.2 ng/litre (Umweltbundesamt, 1989).
The concentration of beta-HCH in the Japan Sea and the Pacific
Ocean around Japan was below the detection limit of 0.1 µg/litre in
1974 (personal communications by A. Hamada and by T. Onishi to the
IPCS, July 1989).
5.1.3 Soil/sediment
In 1974 beta-HCH was found in 9 out of 60 sediment samples
collected in Japan, the range of concentration being 30-50 µg/kg
(personal communications by A. Hamada and by T. Onishi to the IPCS,
July 1989).
Slooff & Matthijsen (1988) analysed sediments from eight
different locations close to dumping places in the Netherlands for the
presence of alpha-, beta-, and gamma-HCH and obtained median values
for beta-HCH of 9-214 µg per kg dry matter.
5.1.3.1 Dumping grounds
In the Netherlands soil has been polluted with HCHs at various
location as the result of their manufacture in the 1950s (spillage
during production, storage, and handling), and concentrations up to a
few grams of HCHs/kg dry soil have been found. Further pollution has
been caused by both the dumping of chemical waste and its use in the
levelling of certain areas. From these dumping areas dispersal of the
chemical waste can occur by leaching or wind erosion from open storage
depots. In certain polluted areas, high concentrations of HCHs,
mainly the alpha and beta-isomers, have been found more than 2 m below
ground level. In 18 locations in the Netherlands, the average
concentrations of beta-HCH in sewage sludge in 1981 were between 30
and 150 µg/kg dry matter. Pollution of ground water also occurred,
but this was restricted to the vicinity of the production areas.
Horizontal transportation of HCHs in ground water appeared to be
limited (Slooff & Matthijsen, 1988).
5.1.4 Food and feed
The concentration of beta-HCH has been determined in a number of
important food items in France. The mean concentration was 0.03
(nd-0.25) mg/kg in milk and milk products (2688 samples), 0.02
(nd-0.04) mg/kg in meat (27 samples), 0.01 (nd-0.03) mg/kg in meat
products (34 samples), and 0.01 (nd-0.1) mg/kg in animal fat
(67 samples). In other food items, beta-HCH was not detected
(<0.005 mg/kg) (Laugel, 1981).
In a survey of milk contamination carried out in various areas of
Japan in 1970, the average beta-HCH content in cow's milk ranged from
0.009 mg/litre in the Hokkaido area to 1.288 mg/litre in the Nagasaki
area (Matsushima, 1972).
Table 7 gives the mean beta-HCH levels in a large number of
samples of various food items from the Federal Republic of Germany
reported by Hildebrandt et al. (1986).
Skaftason & Johannesson (1979) analysed a total of 32 samples of
butter from Iceland between 1974 and 1978 and found beta-HCH, at a
mean concentration of 23 ± 16 µg per kg, in 31 out of 32 samples.
In six samples of cow's milk collected from six locations in
Switzerland, the levels of beta-HCH were 1.0-4.0 mg/kg on a fat basis
(Rappe et al., 1987).
In a study carried out in the United Kingdom, 24 samples of each
food group were analysed. Bread, other cereal products, meat products,
fish, oils and fats, eggs, green vegetables, potatoes, other
vegetables, and fresh fruit contained no detectable amounts of
beta-HCH. Carcass meat contained < 0.0005 (nd-0.003) mg/kg, offals <
0.0005 (nd-0.003) mg/kg, poultry 0.008 (nd-0.08) mg/kg, milk < 0.0005
(nd-0.001) mg/kg, and dairy products 0.001 (nd-0.008) mg/kg. Imported
meat products collected in 1981-1983 contained up to 1.4 mg/kg
product, imported retail cereal products collected in 1982 contained
up to 0.03 mg/kg and animal feed collected in 1984 contained up to
0.08 mg/kg (HMSO, 1986).
No beta-HCH was found in meat and poultry products including
eggs (976 samples) collected during 1984-1986. Peanut butter and
vegetable oils (in total 95 samples) showed mean beta-HCH levels of
0.01-0.02 mg/kg product, whereas processed pork and poultry products
collected in 1985-1987 contained mean levels of 0.2 and 1.9 mg/kg,
respectively. Twenty-six out of 86 samples were positive and the
highest level that was found was 6.3 mg/kg. Other processed meat
products (631 samples) contained up to 0.01 mg/kg product. In
1984-1987, retail milk and dairy products were analysed, and 499 out
of 849 samples contained beta-HCH at a mean concentration of
0.01-0.03 mg/kg product (the highest level was 0.08 mg/kg). Samples of
eel muscle (1124 eels from 62 sites) collected during 1986-1987 showed
mean beta-HCH concentration of up to 0.02 mg/kg. The highest level
found was 0.05 mg/kg (HMSO, 1989).
Table 7. Beta-hexachlorocyclohexane concentrations (mg/kg) in various
food itemsa
Food items 1973-78 1979-83 1973-83
Meatb 0.01-0.083
(0.26)d
Meat productsb 0.003-0.055
(0.15)d
Animal fatb 0.003-0.024
(0.075)d
Gameb 0.025-0.285
Poultryb 0.001-0.016
(0.42)d
Chicken eggs 0.001
Fish 0.001-0.007
Milk and milk
productsb 0.05 < 0.01
Butterb,c < 0.01-0.02
Cereals up to 0.001
Cereal products up to 0.01
a From: Hildebrandt et al. (1986).
b Determinations made on a fat basis
c Anon (1984)
d maximum value
5.1.5 Terrestrial and aquatic organisms
5.1.5.1 Aquatic organism
Mouvet et al. (1985) measured the presence of beta-HCH in the
aquatic mossCinclidotus danubicusin order to examine its potential use
as an indicator of chlorinated pollutants in fresh water. The level in
water 4 km down-stream of an industrial discharge was 0.5-2.6 µg per
litre, while the levels in moss 0, 13, 24, and 51 days after
transplant to the polluted river were < 0.025, 0.025-0.33,
0.025-1.29, and 0.4 mg/kg dry weight, respectively.
Bream collected from various locations in the River Elbe (between
Schnackenburg and the North Sea) contained beta-HCH levels of
0.008-0.063 mg/kg in muscle tissue and 0.7-2.8 mg/kg in adipose tissue
(Arbeitsgemeinschaft für die Reinhaltung der Elbe, 1982).
Freshwater fish from different rivers in the Federal Republic of
Germany were analysed during the period 1973-1981. In the first 3-4
years the beta-HCH levels were mainly between 0.01-0.02 mg/kg fresh
weight. However, a decrease then took place and most of the samples
were below 0.01 mg/kg fresh weight, with the exception of certain
types of fish such as the eel and fish from industrially contaminated
areas. In 1981-1983, shell-fish and molluscs were analysed in the
Federal Republic of Germany, and the beta-HCH concentration ranged
from < 0.001 to 0.011 mg/kg fresh weight (Hildebrandt et al., 1986).
5.1.5.2 Birds
Organochlorine pesticides were determined in the livers of
predatory birds in the United Kingdom during 1963-1966. The average
residues (arithmetic means) of beta-HCH found are given in Table 8.
5.1.5.3 Mammals
Skaftason & Johannesson (1979) analysed samples of body fat from
10-year-old sheep, collected in 1974 in Iceland, and found an average
of 79 ± 48 µg beta-HCH/kg.
Norström et al. (1988) determined the contamination of Canadian
arctic and subarctic marine ecosystems by analysing the adipose tissue
and liver of polar bears ( Ursus maritimus; 6-20 animals per area)
collected from 12 areas between 1982-1984. Of the total HCH in adipose
tissue, 29% was beta-HCH (0.3-0.87 mg/kg on a fat basis).
Table 8. Residues of beta-hexachlorocyclohexane in the livers of
predatory birdsa
Species Year Number of Concentration
samples (mg/kg)
Sparrowhawk 1963 11 0.3
1964 8 0.23
1965 9 0.25
Kestrel 1964 28 0.1
1965 60 0.01
Tawny owl 1963 12 0.02
1965 29 0.01
Barn owl 1964 23 0.07
Heron (adults) 1964 17 0.1
Heron (nestlings) 1965 20 0.005
Great crested grebe 1963/1966 15 0.1
a From: HMSO (1969)
5.2 General population exposure
From the data presented in section 5.1 it is evident that food is
the main source of exposure of the general population to beta-HCH.
5.2.1 Total-diet studies
In a total-diet study carried out in the United Kingdom during
1966-1985, food purchased in 21 towns throughout the country was
prepared by cooking. The study covered 20 to 24 food groups, and the
number of total-diet samples examined varied from 22 to 25 samples.
The calculated mean beta-HCH residue levels in the total diet for the
periods 1966-1967, 1970-1971, 1974-1975, 1975-1977, 1979-1980, 1981,
and 1984-1985 were 0.003, 0.001, 0.0005, 0.005, 0.001, < 0.0005, and
< 0.0006 mg/kg, respectively (Egan & Hubbard, 1975; HMSO, 1982, 1986,
1989).
Samples consisting of 50 items of infant food and 110 items of
toddler food were collected in 1978-1979 in 10 USA cities. The daily
intake of beta-HCH in 1977, 1978, and 1979 in infant food was below
the limit of detection. In toddler food beta-HCH was only detectable
in 1977, the daily intake being 0.002 µg/kg body weight per day
(Gartrell et al., 1985b).
Total-diet studies conducted by the FDA in the USA before 1982
were based on a "composite sample approach" regardless of the diet
involved. Later on they were based on dietary survey information and
allowed the "total diet" of the population to be represented by a
relatively small number of food items for a greater number of age-sex
groups. The daily intakes of beta-HCH during 1982-1984 for the age
groups 6-11 months, 2 years, 14-16-year-old females, 14-16-year-old
males, 25-30-year-old females, 25-30-year-old males, 60-65-year-old
females and 60-65-year-old males were < 0.1, 0.3, 0.2, 0.2, 0.2,
0.4, 0.2, and 0.2 ng/kg body weight, respectively (Gunderson, 1988).
Matsushima (1972) reported that the total diet of an average
citizen of Matsuyama City, Japan, contained about 0.177 mg HCH/day,
the major portion being the beta isomer. Of the beta-HCH intake 90%
was identified as originating from meat and dairy products.
In a total-diet study in the Netherlands in 1977, the average
concentration of beta-HCH in 100 samples was < 0.02 mg/kg on a fat
basis. The highest level was 0.19 mg/kg (Greve & van Hulst, 1977).
5.2.2 Concentrations in human samples
Beta-HCH concentrations in human samples are a good indication of
the total exposure of the general population. Concentrations in human
tissues are markedly higher than those of the other HCH isomers, a
phenomenon which reflects the cumulative properties of the beta
isomer. There has been a trend, but only a very slow one, towards
lower values in recent data.
Greve (1985) was unable to detect any correlation between life
style, such as type of food, and the beta-HCH concentrations in
tissues of Dutch citizens. However, in a Swedish study the levels of
beta-HCH (and of other organochlorine contaminants) in breast milk
were found to be related to dietary habit. Levels in lacto-vegetarians
were lower than those in women eating a mixed diet, and the latter
were in turn lower than those in women using a mixed diet that
regularly included fatty fish from the Baltic (Noren, 1983).
5.2.2.1 Blood
Eckenhausen et al. (1981) detected beta-HCH at a concentration of
< 0.5 to 25 µg/litre in the blood of 19 out of 47 pregnant women in
the Netherlands. A concentration range of < 1.0 to 12 µg/litre was
found in 30 out of 69 women after they had given birth and beta-HCH
was detected in the blood of 17 out of 46 babies (< 1.0 to
6.0 µg/litre).
When Blok et al. (1984) studied the presence of beta-HCH in the
blood of 65 healthy Dutch volunteers (34 females and 31 males),
beta-HCH was found in approximately half of the volunteers. The median
concentration was 0.4 µg/litre (range nd-1.4 µg/litre) in both males
and females.
Blood samples of Dutch citizens analysed in 1978, 1980, 1981, and
1982 (70, 48, 127, and 54 samples, respectively), contained 0.3-1.4 µg
beta-HCH/litre (Greve & Wegman, 1985).
Polishuk et al. (1970) found beta-HCH in the blood of 24 pregnant
women and 23 infants living in Israel. The mean concentrations was 0.5
± 0.6 µg/litre in the women and 0.3 ± 0.5 µg/litre in the infants.
The average concentrations of beta-HCH in the plasma of five
subjects in the USA, who were not occupationally exposed, were
0.83-0.94 µg/litre, and were remarkably consistent throughout the 5
days during which samples were taken (Radomski et al., 1971a).
Starr et al. (1974) analysed the blood of 187 men and 171 women
living in Colorado, USA. In men, a mean concentration of 4.9 µg
beta-HCH/litre was found in 15 samples, while in women the mean
concentration in 7 samples was 10.9 µg/litre (range, 9.0-15.0).
In a 4-year study to assess the exposure of the general
population, 6252 blood samples were collected from people (12-74 years
of age) living in 64 locations across the USA. Beta-HCH was detected
in 13.9% of the samples at a mean level of 1.7 µg/litre (range,
1-28 µg/litre). The percentage of positive samples increased from the
youngest to oldest age group (from 3.2 to 26.8%) (Murphy & Harvey,
1985).
Bertram et al. (1980) found a median concentration of 1.32 µg
beta-HCH/litre (range, nd-4.81) in whole blood (18 samples) of
citizens of the Federal Republic of Germany.
5.2.2.2 Adipose tissue
In fifteen samples of adipose tissue from the general population
of the Federal Republic of Germany, the median concentration of
beta-HCH was 0.33 mg/kg on a fat basis (range, nd-0.66) (Bertram et
al., 1980).
Specimens of subcutaneous adipose tissue from 48 children (34
under the age of 1 year, 14 in the second year of life) were analysed
during the period 1982-1983 in the Federal Republic of Germany. The
average concentration of beta-HCH was 0.15 (range, nd-1.02) mg/kg fat.
The average concentration was highest, 0.17 (nd-0.38) mg/kg fat, at
the age of 0-6 weeks (Niessen et al., 1984).
Hildebrandt et al. (1986) summarized the results of nine studies
carried out in the Federal Republic of Germany in 1969-1983. The mean
beta-HCH concentrations (636 samples) ranged from 0.01 to 1.30 mg/kg
on a fat basis.
Mes et al. (1982) analysed 99 samples of adipose tissue from
autopsies of accident victims from different areas of Canada. All
samples contained beta-HCH, the average concentration of which was
0.151 ± 0.459 (range, 0.016-4.413) mg/kg wet weight. In males (53)
the average value was 0.183 ± 0.612 mg/kg, whereas in women (45) it
was 0.116 ± 0.166 mg/kg. The influence of age was evident. The
average concentration in 33 people aged up to 25 was 0.067 ±
0.051 mg/kg, in 41 people aged 26-50 it was 0.260 ± 0.698 mg/kg, and
in 24 people aged 51 or more it was 0.082 ± 0.037 mg/kg.
Human adipose tissue was analysed during the periods 1965-1967,
1969-1971, and 1976-1977 (male and female) in the United Kingdom, the
number of samples being 66, 248, 201 (male), and 236 (female),
respectively. The arithmetic means of the beta-HCH concentrations were
0.28, 0.27, 0.30 (male), and 0.33 (female) mg/kg, respectively (HMSO,
1982). During 1982-1983, the beta-HCH concentrations were 0.24 (male)
and 0.31 (female) with a range of 0.01-0.81 mg/kg (HMSO, 1986).
Kutz et al. (1979) studied the presence of organochlorine
pesticides in human adipose tissue in 48 states of the USA in
1970-1975. Beta-HCH was widely distributed at low levels (geometric
means of between 0.2 and 0.4 mg/kg tissue on a fat basis) during this
period, and there was a slow downward trend. Residues of alpha-HCH and
gamma-HCH were found at a very low frequency.
In 1980, eight adipose tissue samples were taken as part of the
US EPA National Human Monitoring Program in North East Louisiana and
in 1984 10 samples were collected. The average beta-HCH concentration
(on a lipid basis) was 0.77 mg/kg (nd-2.31) in 1980 and 0.62 mg/kg
(0.31-1.03) in 1984 (Holt et al., 1986).
In a study by Szymczynski et al. (1986), 29 samples of adipose
tissue were taken at necropsy and 24 at surgery in the Poznan region
of Poland and were compared with 100 samples from residents of the
Warsaw region. In Poznan, the mean concentration of beta-HCH was 0.211
± 0.154 mg/kg, while in Warsaw it was 0.184 ± 0.017 mg/kg.
In Kenya, Wassermann et al. (1972) analysed 83 adipose tissue
samples collected during autopsy in 1969-1970 from people without
occupational exposure to insecticides. The mean concentration of
beta-HCH in the age group 5-24 (32 samples) was 0.0686 ± 0.064 mg/kg,
in the age group 25-44 (28 samples) it was 0.263 ± 0.266 mg/kg, and in
people aged 45 or more (23 samples) it was 0.186 ± 0.228 mg/kg.
In 1974, 360 adipose tissue samples were collected in 8 regions
of Japan, and the mean concentration of beta-HCH was 6.55 mg/kg on a
fat basis (Takabatake, 1978).
The beta-HCH concentration of 567 samples of adipose tissues of
Dutch citizens analysed during 1968-1983 ranged from 0.21 to 0.6 mg/kg
(on a fat basis). The highest levels were found in 1968-1976 (Greve &
van Harten, 1983; Greve & Wegman, 1985).
5.2.2.3 Breast milk
Breast milk is a major route for the elimination of
organochlorine pesticides and PCBs in women. In a study by Cetinkaya
et al. (1984), a significant correlation was found between the
concentration of beta-HCH in breast milk and the level of consumption
of meat products and animal fat. In addition, concentrations of
beta-HCH in breast milk in rural areas appeared to be higher than
those in urban areas.
The variation during lactation of residue levels in breast milk
was investigated in five women aged 23-36 in the Federal Republic of
Germany. The beta-HCH concentrations were between 0.04 and 0.20 mg/kg
fat, and no significant changes in residue level occurred during the
lactation period (Fooken & Butte, 1987).
The residues of beta-HCH in breast milk during the periods
1974-1975 and 1979-1980 in the Federal Republic of Germany were 0.6
and 0.3 mg/kg fat, respectively (Anon., 1984).
More than 7100 samples of breast milk were analysed in the
Federal Republic of Germany from 1969 to 1984. These studies were
carried out by 20 authors, and the results were summarized by
Hildebrandt et al. (1986). The mean concentrations of beta-HCH ranged
from 0.02 to 0.56 mg/kg fat. There was no clear decrease in the mean
concentrations during the period 1969-1979, but thereafter a slow
decrease was observed. A further study carried out in the Federal
Republic of Germany (2709 samples in 1979-1981) yielded an average
concentration of 0.37 mg/kg fat (Fooken & Butte, 1987). In 1981-1983,
132 samples of breast milk were analysed and the average level was
0.209 mg beta-HCH/kg milk fat (Cetinkaya et al., 1984).
Tuinstra (1971) analysed 42 individual samples of breast milk
collected in 1969 from young mothers (18-32 years of age) living in
the Netherlands and determined a median beta-HCH concentration of
0.28 mg/kg milk fat (range, 0.1-0.69 mg/kg). When 278 samples of
breast milk, collected in 11 maternity centres in the Netherlands,
were analysed for the presence of beta-HCH, the median beta-HCH
concentration was 0.1 mg/kg (on a fat basis). The maximum value was
0.3 mg/kg (Greve & Wegman, 1985).
Samples of maternal blood, milk, and umbilical cord blood were
collected from 43 mothers and their infants during 1981-1982 in Oslo,
Norway. The residue levels in maternal and umbilical cord serum were
below < 1 µg per kg. In colostrum and milk, concentrations ranging
from 0.05 to 0.45 mg/kg fat were found (Skaare et al., 1988).
Vukavic et al. (1986) measured beta-HCH in 59 samples of
colostrum collected during autumn 1982 (26 samples) and spring 1983
(33 samples) from healthy nursing mothers on the third day after
delivery. The beta-HCH concentrations in the autumn and spring were
not significantly different (mean concentrations of 0.95 ± 0.21 and
0.88 ± 0.16 µg/litre whole colostrum, respectively).
Mes et al. (1986) studied 210 breast milk samples from five
different regions of Canada and measured a mean beta-HCH concentration
of 0.214 mg/kg (on a fat basis). Davies & Mes (1987) studied 18
breast milk samples from Canadian, Indian, and Inuit mothers in
Canada, whose fish consumption was comparable to the national
consumption. The level of beta-HCH in milk fat of the indigenous
population was 0.022 mg/kg, compared with a value of 0.206 mg/kg from
a national survey.
In Japan, the average beta-HCH content in breast milk was found
to be 0.120 mg/kg (Matsushima, 1972).
In Japan, 378, 328, 87, and 77 samples of breast milk,
respectively, were analysed in 1980, 1981, 1982, and 1983. The mean
concentrations were 0.031, 0.034, 0.043, and 0.020 mg beta-HCH/kg
whole milk, respectively (WHO, 1986).
Breast milk was analysed for the presence of beta-HCH during
1979-1980 and 1983-1984 in the United Kingdom. In these two
periods, 30 and 40 samples, respectively, were collected in Scotland.
The mean concentrations were 0.008 (< 0.001-0.14) and 0.005
(< 0.001-0.032) mg/kg milk, respectively (HMSO, 1986).
6. KINETICS AND METABOLISM
6.1 Absorption and elimination
Shibata (1978) reported that the absorption of beta-HCH from the
gastrointestinal tract in mice was 80-95%, most of this being
accumulated in adipose tissue. The elimination followed a 2-stage
mechanism, the half-life for the first stage being 2.5 days and that
for the second stage being 18 days. The half-life for clearance from
blood in rats (sex not specified) was 1 month (Altmann et al., 1980),
and the half-life for clearance from fat was 14 days in male rats and
28 days in female rats (Portig, 1983). Vohland & Koransky (1983)
reported a half-life for clearance from "internal organs" of 22 days
in female rats. A half-life of 20 days for the clearance from the
brain of female rats was reported by Portig & Vohland (1983) and
Vohland et al. (1981). In cows the half-life for clearance from fat
was 4.2-22.0 weeks (Wolf, 1983). The elimination in humans was slow
after continuous exposure ceased, the concentration in fatty tissues
decreasing only slightly over several years (Vohland & Koransky,
1983).
6.2 Distribution
Oshiba (1972) fed groups of six rats a diet containing 10 mg
beta-HCH/kg for up to 56 days. The beta-HCH level in adipose tissue
was 60 mg/kg tissue and in the liver was 45 mg/kg tissue after 56
days. The maximum concentration in the liver was reached after 4
weeks. During subsequent starvation, beta-HCH was mobilized from
adipose tissue by an enhanced lipid metabolism. Furthermore, there
was a tendency for deposition in other organs and tissues.
Vohland et al. (1981) and Portig & Vohland (1983) studied the
distribution of beta-HCH in the brain and depot fat of rats. With an
average concentration in blood of 92 µg beta-HCH/litre, a brain to
blood ratio of 2:1 and depot fat to blood ratio of 170:1 were found,
whereas with blood concentrations of 540 µg/litre and 2100 µg per
litre the ratios were 2:1 and 177:1 and 2:1 and 168:1, respectively.
After lethal acute intoxication of humans by HCH, the HCH
concentration, relative to that in blood, was in the ratio of 363:1
for fat, 3:1 for brain, and 15:1 for liver (Vohland & Koransky, 1983).
When beta-HCH is applied repeatedly to rats, mice, and mini-pigs
there is marked storage in fat, especially in females, and the fat
levels increase continuously as dosing progresses (Nakajima et al.,
1970; Oshiba & Kawakita, 1972; Altmann et al., 1980; van Velsen et
al., 1982; Srinivasan & Radhakrishnamurty, 1983). Data on
concentrations in organs are contradictory: according to one report
the levels in the kidneys, brain, and liver of rats reached a plateau
after 4 weeks (Oshiba & Kawakita, 1972), whereas other sources
reported steady increases in these organs throughout a 12-week dosing
period (van Velsen et al., 1982).
6.3 Transplacental transfer and transfer via lactation
Hori & Kashimoto (1974) found, after oral dosing of pregnant
mice, a carry-over from dam to fetus of approximately 2% of the dose.
Shibata (1978), however, reported a placental transfer of beta-HCH in
rats of approximately 40%.
During lactation the transfer of beta-HCH to milk was 85% from
adipose tissue and 64% from administered beta-HCH (Shibata, 1978).
Carry-over from dam to suckling via the milk was about 60% of the dose
during lactation.
In a study by Hapke & Hollmann (1985), significant carry-over via
milk was found after female rats were dosed for a period of 8 weeks
that terminated 3 weeks before mating. At both the dose levels tested
(i.e. 1 and 5 mg/kg body weight) beta-HCH concentrations in milk were
elevated and signs of liver enzyme induction were found in the pups.
As significant excretion in milk was shown to occur in cows after
oral dosing (the carry-over was 30-37%) (Heeschen, 1985).
6.4 Metabolic transformation
6.4.1 Rat
In a study by Freal & Chadwick (1973), Sprague-Dawley weanling
female rats were administered 2 mg beta-HCH/rat per day orally in
peanut oil for 7 days. Since beta-HCH was metabolized to
2,4,6-trichlorophenol but to no other chlorophenols, it appears that
cis-dehydrochlorination may lead exclusively to this metabolite.
This study also indicated that pre-treatment with beta-HCH alters the
metabolism of lindane in rats.
Rats fed 1.5 mg 14C-labelled beta-HCH/kg diet for 7 days
excreted 70% of the dose during these 7 days and the following 28
days. One third of the eliminated radiolabel was found in the urine.
There was no unchanged beta-HCH in urine; the major urinary metabolite
was 2,4,6-trichlorophenol and small amounts of trichlorohydroxy-
methoxybenzene, a dichlorophenol, and a trace of a tetrachloro-
cyclohexane isomer were found. In faeces, only 2,4,6-trichlorophenol
was identified (Lay et al., 1981).
Artigas et al. (1988) applied a new method of gas
chromatographymass spectrometry (GC-MS) to identify several lindane
metabolites (tetra-, penta-, and hexachlorocyclohexenes, and tetra-
and pentachlorobenzene) in rat brain homogenates. Male Wistar rats
were administered orally 30 mg beta-HCH/kg and were sacrificed 5 h
later. The cerebella of the animals were analysed and 3.6/4.5-PCCH,
3.5/4.6-PCCH, HCCH, pentachlorobenzene, HCB, and beta-HCH were found
at levels below 5 µg/kg. Beta-HCH was present at a concentration of
4.2 mg/kg tissue. This study revealed that the various HCH isomers are
cleared from the brain via different metabolic pathways.
6.4.2 Mouse
When 14C-labelled beta-HCH was administered intraperitoneally
to male mice (strain ddY, 4 weeks old) as a single dose of 32 µg, the
average urinary excretion of radioactivity within 3 days was 10%.
Beta-HCH seemed to be metabolized more slowly than lindane. The
principal metabolite of beta-HCH in the urine was
2,4,6-trichlorophenol (25%), but 2,4-dichlorophenol (up to 5%) was
also found. These metabolites were mainly conjugated with glucuronide
or sulfate (Kurihara & Nakajima, 1974; Kurihara, 1975).
6.4.3 Human
When Engst et al. (1978) analysed the urine of occupationally
exposed workers (apparently to technical-grade HCH in manufacturing
processes), they found, apart from alpha-, beta-, gamma-, and
delta-HCH, traces of hexa- and pentachlorobenzene, gamma- and
delta-pentachlorocyclohexene, pentachlorophenol, 2,3,4,5-, 2,3,4,6-,
and 2,3,5,6,-tetrachlorophenol, and several trichlorophenols, as well
as the glucuronides of several of these metabolites. The
pentachlorocyclohexenes, tetrachlorophenol, hexachlorobenzene, and
pentachlorophenol were also identified in the blood.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Acute toxicity data
7.1.1 Oral
Coper et al. (1951) reported the death of three out of five rats
that received a oral dose of 150 mg beta-HCH/kg body weight. Oral
LD50 values of 1500 and 2000 mg/kg body weight for mice and rats,
respectively, have been reported (WHO, 1986). However, a more recent
study reported the LD50 to be > 16 000 mg/kg body weight in mice
and > 8000 mg/kg body weight in rats (Hoffmann, 1983). Symptoms of
poisoning were decreased activity, ataxia, tremors, dyspnoea,
anorexia, convulsions, and rough fur. Portig (1983) reported a rat
LD50 of 9000 mg/kg body weight.
7.1.2 Intraperitoneal
In a study by Coper et al. (1951), no deaths occurred among 6
rats given an intraperitoneal dose of 160 mg beta-HCH/kg body weight.
7.2 Short-term exposure
7.2.1 Mouse oral studies
Groups of 10-11 DD mice of each sex (6 weeks of age) received
diets containing 0, 100, 300, or 600 mg beta-HCH per kg diet for 32
weeks, followed by a control diet for 5-6 weeks. The control group
consisted of 20 animals. During the experiment a number of animals
died. The frequency of atypical proliferation in the liver was 0/29 in
the control group, 0/18 at 100 mg/kg, 6/16 at 300 mg/kg, and 11/12 at
600 mg/kg. No tumours were found (Hanada et al., 1973).
Ito et al. (1973b) fed DD mice 0, 50, 100, 200, or 500 mg
beta-HCH/kg diet for 24 weeks but found no liver tumours or nodular
hyperplasia.
7.2.2 Rat oral studies
In a study by Doisy & Bocklage (1950), all rats (20 weanling
male) administered 0.6 g beta-HCH/kg diet for 4 weeks died within 3
weeks.
Rats administered beta-HCH at a dietary concentration of
600 mg/kg were reported to undergo growth retardation, liver mass
enlargement, and a decrease in absolute brain mass, beta-HCH residues
being found primarily in the fat and adrenals (Macholz et al., 1986).
A 13-week oral toxicity study with beta-HCH (> 98%) in
SPF-derived Wistar RIV:Tox rats (10 male and 10 female per group) has
been carried out. The levels were 0 (< 0.01), 2, 10, 50, or
250 mg/kg diet, clinical signs, growth, food intake, and organ weights
were checked, and biochemical, haematological, and extensive
histopathological investigations were carried out. At the highest
concentration, half of the animals died following ataxia, progressive
inactivity, and coma. Growth inhibition was only observed in this
concentration, and red and white blood cell concentrations were
decreased. The liver glycogen level was significantly higher in the
group fed 250 mg/kg than in control rats. Furthermore, a significantly
higher activity of aniline hydroxylase (AH) and aminopyrin-
N-demethylase (APDM) microsomal enzymes and a higher concentration
of cytochrome P450 were observed. In the group fed 50 mg/kg only APDM
activity was increased. In all groups of treated rats, liver effects
were observed (increase in organ weight, centrilobular hepatocytic
hypertrophy, and proliferation of smooth endoplasmic reticulum or
increased activity of microsomal enzymes). The thymus weight was
significantly decreased at 50 and 250 mg/kg and the testes weight at
250 mg. At 2 mg/kg the only effect found was liver enzyme induction
(van Velsen, 1986; van Velsen et al., 1986).
When young male Wistar rats were administered 800 mg beta-HCH/kg
diet for 2 weeks, liver weight was increased but no differences in the
content of water, nitrogen, protein, or glycogen were found. Liver
fat content was increased and the DNA content per kg tissue was
decreased, but the whole liver DNA content was increased. The most
predominant change in the liver was hypertrophy of the liver cells.
The testes weight was not different from that of the control animals
but the protein content was higher. The testicular DNA content was
lower than in control animals. The histological changes reported were
testicular tubular atrophy, interstitial oedema, and spermatogenic
arrest (Srinivasan et al., 1988).
In a study by Srinivasan et al. (1984), eight young male Wistar
rats were fed 800 mg beta-HCH/kg diet for two weeks, and a control
group of five rats was used. Special attention was given to the
urinary excretion of body constituents reflecting renal function.
Glucosuria, increased excretion of creatinine and urea, and
hypertrophy and degeneration of the renal tubular epithelia were
observed.
In another study with male Wistar rats fed 0 or 800 mg
beta-HCH/kg diet for two weeks, special attention was given to liver
function. The control group consisted of 8 rats and the treated group
of 12 animals. The HCH isomer produced effects on various enzyme
systems: serum aminotransferase, hepatic glucose-6-phosphate
dehydrogenase, and aldolase activities were increased, while liver
glucose-6-phosphatase activity was decreased. The activities of liver
mitochondrial DNP/Mg2±/Ca2±-activated ATPase and liver microsomal
Na±/K±-ATPase were lower in the treated animals (Srinivasan &
Radhakrishnamurty, 1988).
Two 13-week feeding studies (Loeber & van Velsen, 1985; van
Velsen et al., 1986) showed similar atrophy of the testes, with
reduced tubular area, lack of mature spermatozoa, and oedema of the
interstitial spaces. In both studies, only the highest beta-HCH
concentration (150 mg/kg diet in one study and 250 mg/kg diet in the
other) produced these effects and resulted in a significantly reduced
weight gain in the affected animals. The measurement of circulating
hormone levels (testosterone, FSH, LH) showed no dose-related effects.
No clear endocrine effects on the testes or on male reproductive
hormones were demonstrated (van Velsen, 1986).
7.3 Skin and eye irritation; sensitization
No data are available on skin and eye irritation or
sensitization.
7.4 Long-term exposure
7.4.1 Rat oral studies
In a study by Fitzhugh et al. (1950), groups of 10 female and 10
male weanling Wistar rats were administered diets containing 0, 10,
100, or 800 mg beta-HCH/kg (in corn oil) for 107 weeks. With
concentrations of 100 mg/kg diet or more, there was growth depression,
and with 800 mg/kg increased mortality was found. Concentrations of
10 mg/kg or more led to liver enlargement and slight or moderate
histopathological changes in the liver.
7.5 Reproduction, embryotoxicity, and teratogenicity
7.5.1 Reproduction
A 2-generation study with SPF-derived Wistar RIV:Tox rats (13
males and 26 females per group) was carried out to study fertility,
reproduction, and development of the offspring. The parents (F0)
were fed beta-HCH (> 98%) from weaning at levels of 0 (< 0.01), 2,
10, or 50 mg/kg, and, following a 12-week premating period, F1a and
F1b litters were produced. The F1b generation was used to produce
F2a, F2b, and F2c litters, the last-mentioned being used for
teratological investigations (see section 7.5.2). In the highest-dose
group, the F1a litter size was reduced and there was almost complete
infertility when mating for the Fw1bgeneration took place. All pups in
this group died before weaning. At a concentration of 10 mg/kg diet,
increased mortality in F1a and F1b litters was observed. In this
group, precocious vaginal opening and complete infertility in the
second generation were observed. There were no effects in the 2-mg/kg
group (van Velsen, 1986).
The parental animals (F0) from the above study were used to
investigate the influence of beta-HCH on the endocrine organs after 40
weeks of exposure. In females, mean autopsy body weight and ovary
weight were decreased, but adrenal gland and uterus weight increased.
The organ weight changes in males were less clear, except for an
increase in the weight of pituitary and adrenal glands. The proportion
of animals without corpora lutea in the ovaries was greater in the
group fed 50 mg/kg than it was in control rats. The testes in the
animals fed at this level showed a reduced number of Leydig cells.
Atrophy of the dorsolateral prostate, seminal vesicles, and
coagulation glands was found in one animal. The pituitary glands of
the treated animals revealed no differences in the immunoreactivity
for prolactin. The pregnancy index (number of females with
litters/number of females mated) was less than 0.5 in all groups of
rats. Normally this strain produced pregnancy indices of nearly 1.0.
The study was performed using a semi-synthetic purified feed as
compared to the conventional feed used in the breeding colony. The age
of females at first delivery was 20 weeks, much higher than normal.
These factors cast some doubt on the results (van Velsen, 1986).
7.5.2 Teratogenicity
The F2c litter from the study described in 7.5.1 was used to
investigate the teratogenic effects of beta-HCH. The females were
killed 20 days following sperm detection or 20 days after the last
mating of the F1b generation, and fetuses were inspected for
internal and skeletal abnormalities. No compound-related increase in
teratogenic effects was found (van Velsen, 1986).
7.6 Mutagenicity and related end-points
The available data are limited. Beta-HCH did not induce mutations
in Salmonella typhimurium strains TA98, TA100, TA1535, or TA1537
(Lawlor & Haworth, 1979; Nishimura, 1982). The result of an in vivo
bone marrow metaphase analysis in rats was reported to be positive
(Shimazu et al., 1976; IARC, 1979). Beta-HCH did not induce mutations
inAllium ceparoots (Nybom & Knutsson, 1947).
A mutagen test strain of Bacillus subtilis (TKJ5211) showed a
higher sensitivity for his± reversion than the parental strain
(HA101) when treated with UV and UV-mimetic chemicals. However, a
negative result was obtained at a level of beta-HCH (dissolved in
DMSO) of 5 mg/ml (Tanooka, 1977).
In a repair test using stationary phase cultures of HLL3g and
HJ-15 strains, in which the size of growth inhibition zones of
repair-proficient and repair-deficient cells (for vegetative cells and
spores) was determined, a level of 5 mg beta-HCH (in benzene) per ml
was without effect (Tanooka, 1977).
A thorough evaluation of the mutagenic potency of beta-HCH
requires additional tests.
7.7 Carcinogenicity
Appraisal
In one study on mice, a beta-HCH dose exceeding the MTD produced
an increased incidence of benign and malignant liver tumours. All
other reported studies on mice were inadequate for the evaluation of
beta-HCH carcinogenicity due to the very short duration of treatment
and/or observation.
Two studies on rats were inadequate for evaluation due to the
small number of animals in one and the short duration of treatment in
the other.
The results of the studies on initiation-promotion and mode of
action and the mutagenicity studies suggest that the neo-plastic
response observed with beta-HCH is most likely due to a non-genotoxic
mechanism.
7.7.1 Mouse
In a study by Goto et al. (1972a), 20 male ICR/JCL mice (5-week
old) were fed 0 or 600 mg beta-HCH/kg diet for 26 weeks. Increased
liver weights were reported and hepatomas described as benign liver
tumours were induced. However, insufficient details were reported.
Nagasaki (1973) administered beta-HCH orally to male DD mice at
concentrations of 100, 250, or 500 mg/kg for 24 weeks. There were no
signs of tumour development at any treatment level.
Groups of 10-11 DD mice of both sexes (6-weeks old) received
diets containing 0, 100, 300, or 600 mg beta-HCH per kg for 32 weeks,
followed by a control diet for 5-6 weeks. The control group consisted
of 20 animals. During the experiment a number of animals died. In the
treated mice, atypical proliferation was found in the liver in the two
highest dose levels, but no hepatomas were observed. Alpha-fetoprotein
was not detected in the serum of animals with hepatomas (Hanada et
al., 1973).
Thorpe & Walker (1973) performed a 110-week study, using a
dietary concentration of 200 mg beta-HCH (> 99%) per kg, on CF1 mice
(groups of 30 males and 30 females). During the first 3 months of the
study 12% of the males and 25% of the females died. Liver enlargement
was detected by week 50 in both females and males. Hyperplastic
changes were found in the liver (hyperplastic nodules and
hepatocellular carcinomas), sometimes with lung metastases. The
combined incidence of benign and malignant liver tumours for males and
females was 24 and 23%, respectively, in the control group and 73 and
43%, respectively, in the treated group.
When male DD mice (8 weeks old), in groups of 20 or 29 animals,
were fed a diet containing 0, 100, 250, or 500 mg beta-HCH per kg for
24 weeks, there was a moderate increase in liver weight at the two
highest levels. No nodules classified as nodular hyperplasia or
hepatocellular carcinoma were detected (Ito et al., 1973b).
7.7.2 Rat
In a study by Fitzhugh et al. (1950), groups of 10 male and 10
female weanling Wistar rats were fed throughout their lives on a diet
containing beta-HCH > 98% pure (10, 100, or 800 mg/kg diet). No
increase in tumour incidence was reported in the treated animals, but
only a limited number of organs were examined microscopically.
Male W rats (5-8 weeks old, 18-24 animals per group) were
administered diets containing beta-HCH at a concentration of 500 mg/kg
(for 24 or 48 weeks) or 1000 mg/kg (for 24 weeks). Only slight cell
hypertrophy was found, but no nodular hyperplasia, bile duct
proliferation or hepatocellular carcinomas were detected (Ito et al.,
1975).
7.7.3 Initiation-promotion
The influence of beta-HCH on tumour induction by PCBs (and vice
versa) was tested with male DD mice (26-30 animals per group).
Whereas 500 mg PCBs/kg diet induced nodular hyperplasia and
hepatocellular carcinomas in the liver of male mice after 32 weeks,
exposure to beta-HCH at levels of 50, 100, or 250 mg/kg diet or to
PCBs at a level of 250 mg/kg diet did not. However, the combination of
100 mg beta-HCH/kg and 250 mg PCB/kg caused the induction of nodular
hyperplasia in 17% (5/30) of the mice and hepatocellular carcinoma in
3.3% (1/30). The corresponding values for the combination of 250 mg
beta-HCH/kg and 250 mg PCB/kg were 55% (16/29) and 21% (6/29),
respectively. These results showed that PCBs promoted the
hepatocarcinogenic action of beta-HCH (Ito et al., 1973a).
The tumour-initiating activity of beta-HCH has been studied by
examining for phenotypically altered foci in female Wistar rats.
Groups of three to eight rats were used and, after the median and
right liver lobes had been removed, the rats were administered 100 mg
beta-HCH/kg body weight followed by phenobarbital at 50 mg/kg body
weight per day for 15 weeks. Liver foci were identified by means of
the gamma-glutamyltransferase (GGT) reaction and by morphological
alterations. No evidence of initiating activity was found. In another
part of the study, the promoting activity was investigated. A single
dose of N-nitrosomorpholine (250 mg/kg body weight by gavage) was
followed by the administration of beta-HCH (0.03, 0.2, 1.0, 3.0, or
10.0 mg/kg body weight per day) for 4, 15, and 20 weeks. The criteria
used were growth and phenotypic changes of foci as end-points. It was
concluded from the study that beta-HCH is a tumour promotor. Both the
number and size of altered foci were enhanced by a beta-HCH dose of
3 mg/kg. The tumour-promoting action was generally associated with
liver growth and induction of monooxygenases or other specific enzymes
(Schröter et al., 1987).
7.7.4 Mode of action
Sagelsdorff et al. (1983) studied the relevance to the
carcinogenic action of HCH isomers of covalent binding to mouse liver
DNA. NMRI mice were given 7.3-7.7 mg beta-HCH per kg body weight
orally and 14C-thymidine intraperitoneally. A very low covalent
binding index (CBI) of < 0.08 was found.
7.8 Special studies
7.8.1 Effects on endocrine organs
Juvenile female Swiss mice and SPF-derived Wistar rats (RIV:Tox)
were used to study the uterotropic effect of beta-HCH, in comparison
with that of 17-alpha-ethynyl-estradiol, using the method of Tiecco
(1961). Beta-HCH levels of up to 500 mg/kg diet were fed for 5 days,
and in both animal species there were clear uterotropic effects at
50 mg/kg or more. In quantitative terms, the estrogenic potency of
beta-HCH was, however, minimal in comparison to that of
17-alpha-ethynylestradiol (Loeber & van Velsen, 1985).
Other parameters of estrogenic potency have also been studied.
Beta-HCH has been shown to increase the uterine concentration of
progesterone receptors and the immunoreactivity of the adenohypophysis
for prolactin in rats, and to cause the redistribution of human tumour
cell receptors for progesterone. Adrenalectomy and ovariectomy did
not counteract the uterotropic effect of beta-HCH in rats. However,
the principal metabolite of beta-HCH, 2,4,6-tri-chlorophenol, had no
estrogenic effect, and beta-HCH did not displace 17-beta-estradiol
from its receptors (van Velsen, 1986). Both the significance of these
observations and the possible mechanisms of action are unclear.
7.8.2 Neurotoxicity
Beta-HCH may raise the threshold for electrically induced
seizures in rats. The ratio of the beta-HCH concentration in the
brain to that in blood indicates that this isomer passes the
blood-brain barrier less readily than the other HCH isomers.
Vohland et al. (1981) studied the neuropharmacological effects of
beta-HCH in Wistar rats. The kinetics of beta-HCH concentrations in
the brain were established after the administration of a single oral
dose of 200 mg/kg body weight. The approximate half-life for
elimination from the brain was 20 days in females. Beta-HCH did not
give rise to appreciable quantities of hydrophobic metabolites in the
brain. In rats 4-5 mg beta-HCH/kg in the brain had an anti-convulsive
effect (i.e. there was protection against the action of pentylene
tetrazole). Neurotoxic effects (ataxia and adynamia) occurred at
brain levels of 15-20 mg beta-HCH/kg (Vohland et al., 1981; Portig &
Vohland, 1983).
Beta-HCH has been demonstrated to cause a decrease in peripheral
nerve conduction velocity in rats fed 600 mg beta-HCH/kg diet for 30
days, but did not cause a change in the fronto-occipital
electroencephalogram at 3000 mg/kg diet (Müller et al. 1981). A
decrease in absolute brain mass was reported in one study in which
rats were fed beta-HCH for 30 days at a dietary concentration of
600 mg/kg (Macholz et al. 1986). Beta-HCH has been reported to raise
the seizure threshold for pentylenetetrazol, a known convulsant
(Vohland et al., 1981; Portig & Vohland, 1983). It has been shown
that beta-HCH blocks the binding of tert-butylbicyclo-
phosphorothionate (TBPS), a ligand known to bind to the GABA receptors
in chloride channels in the brain, but was the least effective of the
various HCH isomers in this respect (Fishman, 1987; Matsumoto et al.,
1988).
7.8.3 Effect on liver enzymes
Several short-term studies on enzyme induction have been
performed in rats, using levels ranging from 0.4 to 800 mg/kg feed.
The highest dose level without effect in one study was 10 mg/kg body
weight (van Hoof et al., 1982). However, in other studies, in which
the same parameters were determined, the levels without effects
included 50 mg/kg body weight. Histopathological changes in the liver
correlated with the induction of microsomal enzymes (den Tonkelaar et
al., 1981; van Hoof et al., 1982; van Giersbergen et al., 1984).
Weanling male rats fed diets of 800 mg beta-HCH/kg for 14-18 days
showed significant increases in hepatic alanine aminotransferase (80%)
and glucose-6-phosphate dehydrogenase (130%) and statistically
significant decreases in hepatic aspartate aminotransferase (130%),
alkaline phosphatase (45%), and acid phosphatase (40%) (Srinivasan &
Radhakrishnamurty, 1977). Similarly, the dietary administration of
800 mg/kg to albino rats for two weeks resulted in noticeable
hepatocellular damage, as indicated by elevations in the activity of
serum aminotransferases and decreases in that of hepatic soluble
enzymes. An increase in glucose-6-phosphate dehydrogenase and aldolase
activities was reported to suggest a higher rate of glucose oxidation,
while a decrease in liver glucose-6-phosphatase activity was
attributed to an inactivation of hepatic gluconeogenesis (Srinivasan &
Radhakrishnamurty, 1988).
7.8.4 Immunosuppression
To investigate potential effects on the reproductive and immune
systems, beta-HCH (0, 100, or 300 mg/kg diet) was fed to groups of six
female B6C3F1 mice for 30 days. Investigations were conducted on
changes in ovarian and uterine histology, body weight, lymphoid organ
weight and histology, splenic cellularity, antigen-specific IgM and
IgG plaque-forming cells (PFC), proliferative responses to mitogens,
natural killer cell activity, and induction of cytosolic T
lymphocytes. Significant changes in several immune functions were
only found at a beta-HCH concentration of 300 mg/kg. The proliferation
of splenocytes to the mitogens lipopolysaccharide (LPS), phytohaemag-
glutinin (PHA), and concanavalin A and T-lymphocyte-mediated cytolysis
of tumour targets were decreased, and a concurrent reduction in natural
killer activity was found. These data indicate that beta-HCH causes
non-estrogenic immune function changes without significant changes in
lymphoid organ weight, histology or cellularity (Cornacoff et al., 1988).
8. EFFECTS ON HUMANS
8.1 Acute toxicity - poisoning incidents
Several cases of acute poisoning by technical-grade HCH,
resulting either from accident or occupational exposure have been
described (WHO, 1991). It is likely that gamma-HCH, the most acutely
toxic component, played the major role in these incidents. These
cases cannot, therefore, assist in the evaluation of beta-HCH.
8.2 General population
No specific studies relating to beta-HCH are available.
A study comparing liver cancer deaths in the USA and the
"domestic disappearance" of organochlorine pesticides revealed that in
1962, 18 and 15 years after the introduction of DDT and
technical-grade HCH, respectively (when an increase in primary liver
cancer due to the organochlorines would be manifest), the number of
cases of primary liver cancer as a percentage of the total number of
liver cancer deaths began a gradual and steady decline (from 61.3% in
1962 to 56.9% in 1972). The death rate (per 100 000 per year) due to
primary liver cancer declined from 3.46 to 3.18 during this period
(Deichmann & MacDonald, 1977).
8.3 Occupational exposure
The evaluation of the effects of beta-HCH on occupationally
exposed workers is seriously hampered by the fact that most of the
relevant studies relate to workers who were exposed during the
manufacture and handling of lindane, or the handling and spraying of
technical-grade HCH among other pesticides, and were thus exposed to
all HCH isomers plus impurities and other (process) chemicals.
Therefore, it is difficult, if not impossible, to relate the observed
effects to individual substances. Consequently these studies have only
been described in this monograph where they aid the evaluation.
Behrbohm & Brandt (1959) described 26 cases of allergic and toxic
dermatitis that arose during the manufacture of technical-grade HCH.
Patch testing with pure alpha-, beta-, gamma-, and delta-HCH yielded
negative results, but positive reactions were obtained with the
residual fractions.
The level of beta-HCH was determined in the serum of 57 workers
at a lindane-manufacturing plant. No beta-HCH was detected in
controls, but the levels in exposed workers ranged from 17 to
760 µg/litre and increased with the duration of exposure. The beta-HCH
levels found in the adipose tissue of eight of these workers was
18-103 mg/kg (in extractable lipids). There were no clinical signs or
symptoms and no significant changes were found in extensive
biochemical, haematological, and neurophysiological tests, or in the
EMG or EEG. Serum leutinizing hormone levels were higher than in the
controls, but FSH and testosterone levels showed only insignificant
and inconclusive changes (Baumann et al., 1980, 1981; Brassow et al.,
1981; Tomczak et al., 1981).
The serum beta-HCH level of malaria-control workers who sprayed
technical-grade HCH for 16 weeks increased from 58 to 250 µg/litre in
previously non-exposed workers and from 294 to 385 µg/litre in those
that had been exposed during three previous spraying seasons (Gupta et
al., 1982). Although beta-HCH is only a minor component of
technical-grade HCH (7-10%), it reached higher levels and persisted
longer in the serum than either alpha- or gamma-HCH.
Nigam et al. (1986) studied 64 employees from a HCH-manufacturing
plant who were directly or indirectly associated with the production
of this insecticide. The exposed group was composed of 19 "handlers"
(who handled and packed the insecticide), 26 "non-handlers" (plant
operators and supervisors exposed indirectly to HCH), and 19
maintenance staff (who visited the plant frequently). The control
group consisted of 14 workers who had no occupational contact with the
insecticide. The exposure period varied up to 30 years. The mean
serum beta-HCH concentrations in the four groups were 28.5 µg/litre
(controls), 97.2 µg/litre (maintenance staff), 206.7 µg per litre
(non-handlers), and 413.1 µg/litre (handlers). Alpha-, gamma-, and
delta-HCH were also present. The total HCH concentrations were 51.4,
143.6, 265.6, and 604 µg/litre, respectively. Clinical examination
revealed that the majority of the workers from the "handler" and
"non-handler" groups exhibited paraesthesia of the face and
extremities, headache, and giddiness, and some of them also showed
symptoms of malaise, vomiting, tremors, apprehension, confusion, loss
of sleep, impaired memory, and loss of libido. The same symptoms were
found among the maintenance staff but were less severe and less
frequent.
Chattopadhyay et al. (1988) studied 45 male workers exposed to
HCH during its manufacture and compared them with 22 matched controls.
Exposure was mainly via the skin. Paraesthesia of face and
extremities, headache, giddiness, vomiting, apprehension, and loss of
sleep, as well as some changes in liver function tests, were reported
and were found to be related more to the intensity of exposure (as
measured by the HCH levels in blood serum) than to the duration of
exposure. The measured exposures to total HCH were 13 to 20 times
higher than those in the control groups (no detailed figures were
reported). Of the total HCH, 60-80% was beta-HCH.
Fitzhugh et al. (1950) drew attention to the importance of
beta-HCH for the long-term toxicity of HCH. The slower metabolism of
the beta isomer and its consequent longer persistence in the body are
significant factors.
A significant correlation between the beta-HCH levels in human
blood and adipose tissue has been described by Radomski et al. (1971a)
and Baumann et al. (1980).
In a group of workers that were no longer exposed to HCH for at
least 5 years, mean beta-HCH levels of 50 µg/litre were found, i.e.
twice as much as in the general population of that area at that time
(Radomski et al., 1971b).
Similar findings were reported by Morgan & Lin (1978), who found
20-348 µg/litre in the serum of 38 healthy workers whose last
occupational exposure to HCH was 10-15 years previously. Liver
function was normal and there were no indications of bone-marrow
damage.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Algae
Palmer & Maloney (1955) used beta-HCH in a preliminary screening
test with two cyanobacterium (blue alga), two green alga, and two
diatom species. The test concentration was 2 mg/litre water and the
incubation period was 3-21 days. Beta-HCH was not toxic at this
concentration.
Zhou et al. (1986) studied the effects of alpha-, beta-, gamma-,
and delta-HCH on the photosynthetic evolution of oxygen by the green
algae Chlorella vulgaris and Scenedesmus obliquus, and reported
that the beta- and gamma-isomers showed low toxicity, compared to the
alpha- and delta-isomers, in this respect.
In a study by Krishnakumari (1977), cultures of the green alga
Scenedesmus acutus (1, 3, or 5 days of age) were tested for
sensitivity to beta-HCH at 28°C, growth rate being used as parameter.
The nominal concentrations of beta-HCH (dissolved in ethanol) were
0.5-100 mg/litre. A decrease in growth rate was observed in the 1, 3,
and 5 day cultures exposed to 100, 10, and 5 mg/litre, respectively.
9.2 Protozoa
In short-term tests on Tetrahymena, the EC50 (growth) was
1.2 mg/litre (Mathur et al., 1984).
9.3 Invertebrates
In short-term tests on daphnids no effects were found at
concentrations up to the limit of solubility of beta-HCH in water
(about 1 mg/litre) (Janssen et al., 1987).
Canton et al. (1982) investigated long-term toxicity using
Daphnia and obtained a no-observed-effect level (NOEL) for
reproduction of 0.32 mg beta-HCH/litre.
9.4 Fish
9.4.1 Acute toxicity
In 4-day tests, beta-HCH affected the behaviour of fish. The NOEL
and EC50 values for Oryzias (as well as for Poecilia) were 0.026
and 0.047 mg/litre, respectively (Wester et al., 1985; Wester &
Canton, 1986).
In a study by Boulekbache (1980), the 48-h LC50 of beta-HCH
(98.9%) for the guppy (Poecilia reticulata) was 0.9 mg/litre. Female
fish were less sensitive than males.
9.4.2 Longer-term toxicity
Two tests were carried out with beta-HCH (98.9%) in Japanese
ricefish (medaka, Oryzias latipes) using fertilized eggs (40
eggs/group) or 25 young fish (1 month after hatching). The exposure
levels ranged from 0.032-1.0 mg beta-HCH/litre water and
histopathological examinations were performed after 1 and 3 months. In
the experiment on eggs, decreased growth was noted at 0.56 mg/litre;
with young fish this occurred at 0.1 mg/litre. The NOELs for abnormal
behaviour (loss of buoyancy and balance, uncoordinated movements) were
0.056 mg/litre and 0.032 mg/litre, respectively, for the two
experiments. Histopathological lesions indicating an estrogenic
activity were detected. Furthermore, lesions were observed in the
liver (vacuolation), kidneys (glomerular hyalinosis), and thyroid
(hypertrophy) (Wester & Canton, 1986).
In a study by Wester et al. (1985), groups of 35 young guppies
(Poecilia reticulata) (3-4 weeks old) were exposed to various
concentrations of beta-HCH (98.9%) ranging from 0.0032 to
1.0 mg/litre. After 1 and 3 months of exposure, toxicological and
histopathological parameters were studied. The gross NOEL was
0.032 mg/litre after both 1 and 3 months. Changes in the liver
(hypertrophy of rough endoplasmic reticulum) and kidneys (accumulation
of hyaline droplets in epithelium) were detected. Hypertrophy of the
endocardial lining cells (attributed to the accumulation of hyaline
droplets within lysosomes) was also reported.
After 3 months dysvitellogenesis was noted in the females. In
males the pituitary gland cells producing gonadotrophic hormone
appeared to be stimulated and testicular development was retarded at
0.32 mg/litre or more. It was suggested that all the observed effects
were attributable to an excessive production of the yolk precursor
vitellogenin by the liver as a result of an estrogen-like activity of
beta-HCH or its metabolites.
9.5 Terrestrial organisms
9.5.1 Birds
No toxic effects (e.g., effects on body weight, food consumption,
growth, egg production, egg weight, shell quality, mortality) were
observed in chickens fed diets containing 1-625 mg beta-HCH/kg for 12
weeks (Kan et al., 1979).
9.6 Model ecosystem studies
Sugiura et al. (1976) studied the effects of 0.01, 0.1, 1, 3, and
5 mg beta-HCH/litre on an aquatic microcosm consisting of bacteria,
ciliata, rotifera, oligochaeta, green algae, and blue-green algae.
The specific growth rates of protozoans and a rotifer were increased
by 0.01 and 0.1 mg/litre. No effects were found on the total
community respiration, although the gross primary production
increased.
CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT (ALPHA- AND BETA-HEXACHLOROCYCLOHEXANES)
1. Conclusions
The potential adverse effects of alpha- and beta-
hexachlorocyclohexanes (HCHs) on humans and the environment cannot be
balanced against benefits, since these isomers have no insecticidal
action. Their presence in the environment is thus of serious concern.
Consequently, the use of technical-grade HCH products containing high
concentrations of alpha- and beta-HCH is never justified.
1.1 General population
Alpha- and beta-HCH are circulating in the environment and
present in food chains. Thus there is a continuous potential for human
exposure. This exposure is low and is expected to decrease slowly in
the coming years. Therefore, there is no serious health concern for
the general population.
1.2 Sub-populations at special risk
Alpha-HCH concentrations in breast milk are low.
The exposure of babies resulting from present beta-HCH
concentrations in breast milk is a matter of concern but is no reason
for not promoting the use of breast-feeding.
However, every possible effort should be made to decrease dietary
and all other exposure to these isomers. Decreased dietary exposure is
expected to result in decreased levels of alpha- and beta-HCH in
breast milk.
1.3 Occupational exposure
As long as recommended precautions to minimize the exposure of
workers involved in lindane manufacturing are observed, alpha- and
beta-HCH pose no health risk to process operators.
1.4 Environmental effects
Apart from spills into the aquatic environment, there is no
evidence to suggest that the presence of alpha- and beta-HCH in the
environment poses a significant hazard to populations of organisms.
2. Recommendations for protection of human health and
the environment
a) In order to minimize environmental pollution with alpha- and
beta-HCH, lindane (> 99% gamma-HCH) must be used instead of
technical-grade HCH.
b) In order to avoid environmental pollution with alpha- and
beta-HCH, by-products and effluents from the manufacturing of lindane
must be disposed of in an appropriate way, and contamination of
natural waters and soil must be avoided.
c) Monitoring of alpha- and beta-HCH in food should continue. It is
essential that a mechanism for setting internationally acceptable
levels of alpha- and beta-HCH in food be initiated.
d) Monitoring of the daily intake of the general population and the
levels of alpha- and beta-HCH in breast milk should continue.
FURTHER RESEARCH (ALPHA- AND BETA-HEXACHLOROCYCLOHEXANES)
The following experimental studies are needed to allow a better
evaluation of the hazards of alpha- and beta-HCH:
* mutagenicity studies, especially with chromosome mutagenic
end-points;
* reproduction and fetotoxicity/teratogenicity studies;
* pharmacokinetic and toxicokinetic studies;
* carcinogenicity studies;
* neurotoxicity studies;
* surveillance studies on populations at risk.
PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer (IARC, 1987)
evaluated the hexachlorocyclohexanes and concluded that for the
technical grade and the alpha isomer there is sufficient evidence for
carcinogenicity to animals, whereas this evidence is limited for the
beta and gamma isomers. There is inadequate evidence for their
carcinogenicity to human beings. The hexachlorocyclohexanes were
classified in group 2B.
REFERENCES
ALBRO, P.W. & THOMAS, R. (1974) Intestinal absorption of
hexachlorobenzene and hexachlorocyclohexane isomers in rats. Bull.
environ. Contam. Toxicol., 12(3): 289-294.
ALTHAUS, F.R., LAWRENCE, S.D., SATTLER, G.L., LONGFELLOW, D.G., &
PITOT, H.C. (1982) Chemical quantification of unscheduled DNA
synthesis in cultured hepatocytes as an assay for the rapid screening
of potential chemical carcinogens. Cancer Res., 42: 3010-3015.
ALTMANN, H.J., BÖHME, Chr., & UEHLEKE, H. (1980) Long-term kinetics
of beta-hexachlorocyclohexane (beta-HCH) in rats and mini-pigs. Arch.
Pharmacol., 31 (Suppl.): R20 (Abstract No. 78).
ANGSUBHAKORN, S., BHAMARAPRAVATI, N., ROMRUEN, K., SAHAPHONG, S.,
THAMAVIT, W., & MIYAMOTO, M. (1981) Further study of
alpha-benzenehexachloride inhibition of aflatoxin B1 carcinogenesis in
rats. Br. J. Cancer, 13: 881-883.
ANON. (1984) [1984 Nutrition report], Frankfurt am Main, German
Nutrition Association (in German).
ARBEITSGEMEINSCHAFT FÜR DIE REINHALTUNG DER ELBE (1982) [Chlorinated
hydrocarbons; data for the river Elbe. Report on the results of the
programme for the measurement of chlorinated hydrocarbons in the
section of the Elbe between Schnackenburg and the North Sea,
1980-1982], Hamburg, Study Group for Keeping the Elbe Clean, pp.
64-65, 84, 86-94 (in German).
ARBEITSGEMEINSCHAFT FÜR DIE REINHALTUNG DER ELBE (1988) [Water
quality data for the Elbe from Schnackenburg to the sea. Numerical
table, 1988], Hamburg, Study Group for Keeping the Elbe Clean, p. 158
(in German).
ARTIGAS, F., MARTINEZ, E., CAMON, L., GELPI, E., & RODRIGUEZ-FARRE, E.
(1988) Brain metabolites of lindane and related isomers:
Identification by negative ion mass spectrometry. Toxicology, 49:
57-63.
ATLAS, E. & GIAS, C.S. (1981) Global transport of organic pollutants:
Ambient concentrations in the remote marine atmosphere. Science, 211:
163-165.
BACCI, E., CALAMARI, D., GAGGI, C., FANELLI, R., FOCARDI, S., &
MOROSINI, M. (1986) Chlorinated hydrocarbons in lichen and moss
samples from the Antarctic Peninsular. Chemosphere, 15(6): 747-754.
BAUER, U. (1972) [Concentration of insecticides, chlorinated
hydrocarbons and PCB in algae.] Schr. Reihe Ver.
Wasser-Boden-Lufthyg., 37: 211-219 (in German).
BAUMANN, K., ANGERER, J., HEINRICH, R., & LEHNERT, G. (1980)
Occupational exposure to hexachlorocyclohexane. 1. Body burden of
HCH-isomers. Int. Arch. occup. environ. Health, 47: 119-127.
BAUMANN, K., BEHLING, K., BRASSOW, H.L., & STAPEL, K. (1981)
Occupational exposure to hexachlorocyclohexane. III.
Neurophysiological findings and neuromuscular function in chronically
exposed workers. Int. Arch. occup. environ. Health, 48: 165-172.
BEHRBOHM, P. & BRANDT, B. (1959) [Allergic and toxic dermatitis in
the manufacturing and processing of hexachlorocyclohexane.] Arch.
Gewerbepathol. Gewerbehyg., 17: 365-383 (in German).
BENSON, W.W., WATSON, M., & WYLLIE, J. (1973) Organochlorine
residues in wild moose, Idaho-1972. Pestic. monit. J., 7: 97-99.
BERTRAM, H.P., KEMPER, F.H., & ZENZEN, C. (1980) [Occurrence of HCH
isomers in man.] In: [Hexachlorocyclohexane as a harmful substance
in foods. Papers from two symposia of the Senate Committee on the
Testing of Residues in Foods, held on 28-29 November 1979 and 6 March
1980], Weinheim, Verlag Chemie, pp. 155-163 (in German).
BIDLEMAN, T.F. & LEONARD, R. (1982) Aerial transport of pesticides
over the Northern Indian Ocean and adjacent seas. Atmos. Environ., 16:
1099-1107.
BLOK, S.M.G., GREVE, P.A., SANGSTER, B., SAVELKOUL, T.J.F., & WEGMAN,
R.C.C. (1984) [Investigation of normally occurring values of a
number of organochlorine pesticides and related compounds and their
metabolites, of polychlorobiphenyls and of chlorophenols in the blood
or plasma of healthy volunteers], Bilthoven, National Institute of
Public Health and Environmental Hygiene (Report No. 638101001) (in
Dutch).
BOULEKBACHE, H. (1980) Study of the toxicity of the alpha-, beta-,
and gammaisomers of hexachlorocyclohexane to fish, using the guppy as
the test species, Paris, Université Paris VII, Laboratoire d'Anatomie
comparée (Unpublished report).
BRASSOW, H.L., BAUMANN, K., & LEHNERT, G. (1981) Occupational
exposure to hexachlorocyclohexane. II. Health conditions of
chronically exposed workers. Int. Arch. occup. environ. Health, 48:
81-87.
BÜSSER, M.T. & LUTZ, W.K. (1987) Stimulation of DNA synthesis in rat
and mouse liver by various tumour promoters. Carcinogenesis, 8(10):
1433-1437.
CAMON, L., MARTINEZ, E., ARTIGAS, F., SOLA, C., & RODRIGUEZ-FARRE, E.
(1988) The effect of non-convulsant doses of lindane on temperature
and body weight. Toxicology, 49: 389-394.
CANTON, J.H. & SLOOFF, W. (1977) The usefulness of Lymnea stagnalis L.
as a biological indicator in toxicological bioassays (model substance
alpha-HCH). Water Res., 11: 117-121.
CANTON, J.H., GREVE, P.A., SLOOFF, W., & VAN ESCH, G.J. (1975)
Toxicity, accumulation, and elimination studies of
alpha-hexachlorocyclohexane (alpha-HCH) with freshwater organisms of
different trophic levels. Water Res., 9: 1163-1169.
CANTON, J.H., VAN ESCH, G.J., GREVE, P.A., & VAN HELLEMOND, A.B.A.M
(1977) Accumulation and elimination of alpha-hexachlorocyclohexane
(alpha-HCH) by the marine algae Chlamydomonas and Dunaliella.
Water Res., 11: 111-115.
CANTON, J.H., WEGMAN, R.C.C., VULTO, Th.J.A., VERHOEF, C.H., & VAN
ESCH, G.J. (1978) Toxicity-, accumulation-, and elimination-studies
of alpha-hexachlorocyclohexane (alpha-HCH) with saltwater organisms of
different trophic levels. Water Res., 12: 687-690.
CANTON, J.H., MENNES, W.C., MATHIJSSEN-SPIEKMAN, E.A.M., WEGMAN,
R.C.C., HOFSTEE, A.W.M., & WESTER, P.W. (1982) [Study of the toxicity
of beta-HCH for freshwater organisms], Bilthoven, National Institute
of Public Health and Environmental Hygiene (Unpublished report No.
62797001) (in Dutch).
CETINKAYA, M., GABEL, B., PODBIELSKI, A., & THIEMANN, W. (1984) [On
the correlation of nutrition and living habits of feeding mothers and
contamination of human milk with non-volatile chlorinated organic
chemicals.] Akt. Ernähr., 9(4): 157-162 (in German).
CHATTOPADHYAY, P., KARNIK, A.B., THAKORE, K.N., LAKKAD, B.C., NIGAM,
S.K., & KASHYAP, S.K. (1988) Health effects among workers involved
in the manufacture of hexachlorocyclohexane. J. Soc. Occup. Med., 38:
77-81.
CHESSELLS, M.J., HAWKER, D.W., CONNELL, D.W., & PAPAJCSIK, I.A. (1988)
Factors influencing the distribution of lindane and isomers in soil
of an agricultural environment. Chemosphere, 17(9): 1741-1749.
COPER, H., HERKEN, H., & KLEMPAU, I. (1951) [Antagonism and
synergism of ß- and gamma-hexachlorocyclohexanes.] Klin. Wochenschr.,
29(13/14): 264-265 (in German).
CORNACOFF, J.B., LAUER, L.D., HOUSE, R.V., TUCKER, A.N., THURMOND,
L.M., VOS, J.G., WORKING, P.K., & DEAN, J.H. (1988) Evaluation of
the immunotoxicity of ß-Hexachlorocyclohexane (ß-HCH). Fundam. appl.
Toxicol., 11: 293-299.
COWAN, A.A. (1981) Organochlorine compounds in mussels from Scottish
coastal waters. Environ. Pollut., B2: 129-143.
DAVIES, D. & MES, J. (1987) Comparison of the residue levels of some
organochlorine compounds in breastmilk of the general and indigenous
Canadian populations. Bull. environ. Contam. Toxicol., 39: 743-749.
DEICHMANN, W.B. & MACDONALD, W.E. (1977) Organochlorine pesticides and
liver cancer deaths in the United States, 1930-1972. Ecotoxicol.
environ. Saf., 1: 89-110.
DEMOZAY, D. & MARECHAL, G. (1972) Physical and chemical properties.
In: Ulman, E., ed. Lindane: Monograph of an insecticide, Freiburg im
Breisgau, Verlag K. Schillinger, pp. 14-21.
DEN TONKELAAR, E.M., VAN NIMWEGEN, J.M., & SEKHUIS, V.M. (1981)
[Induction of microsomal liver enzymes following subacute
administration of alpha-, beta-, and gamma-HCH to the rat], Bilthoven,
National Institute of Public Health and Environmental Hygiene
(Unpublished report No. 618111001) (in Dutch).
DEO, P.G., HASAN, S.B., & MAJUMDER, S.K. (1980) Isomerization of ß-HCH
in aqueous solution. J. environ. Sci. Health, B15(2): 147-164.
DEO, P.G., HASAN, S.B., & MAJUMDER, S.K. (1981) Interconversions and
toxicity changes in hexachlorocyclohexane isomers on dispersion in
water. J. environ. Sci. Health, B16(6): 691-701.
DFG (1978) [Residues in breast milk: situation and assessment],
Boppard, Germany, Harald Boldt Verlag (in German).
DOELMAN, P., HAANSTRA, L., DE RUITER, E., & SLANGE, J., (1985) Rate
of microbial degradation of high concentrations of
delta-hexachlorocyclohexane in soil under aerobic and anaerobic
conditions. Chemosphere, 14(5): 565-570.
DOELMAN, P., HAANSTRA, L., & VOS, A. (1988a) Microbial degradation
by the autochthonous soil population of alpha and beta-HCH under
anaerobic field conditions in temperate regions. Chemosphere, 17(2):
481-487.
DOELMAN, P., HAANSTRA, L., & VOS, A. (1988b) Microbial sanitation of
soil with alpha- and beta-HCH under aerobic glass house conditions.
Chemosphere, 17(2): 489-492.
DOISY, E.A., Jr & BOCKLAGE, B.C. (1950) Inositol and the toxicity of
four isomers of benzenehexachloride for the rat. Proc. Soc. Exp. Biol.
Med., 74: 613-616.
ECKENHAUSEN, F.W., BENNETT, D., BEYNON, K.I., & ELGAR, K.E. (1981)
Organochlorine pesticide concentrations in perinatal samples from
mothers and babies. Arch. environ. Health, 36(2): 81-92.
EDELMAN, Th. (1984) [Background values of a number of anorganic and
organic substances in the soil of the Netherlands; an initial
reconnaissance], The Hague, State Publishing House (Soil protection,
report VROM 34) (in Dutch).
EDER, G., STURM, R., & ERNST, W. (1987) Chlorinated hydrocarbons in
sediments of the Elbe River and the estuary. Chemosphere, 16(10/12):
2487-2496.
EGAN, H. & HUBBARD, A.W. (1975) Analytical surveys of food. Br. med.
Bull., 31(3): 201-208.
EICHLER, D. (1977) Experiments on the decomposition of lindane when
exposed to UV-light (Unpublished report Celamerck No. 111AC-143-004,
submitted to WHO by CIEL).
EICHLER, D., HEUPT, W., & PAUL, W. (1983) Comparative study on the
distribution of alpha- and gamma-hexachlorocyclohexane in the rat with
particular reference to the problem of isomerization. Xenobiotica,
13(11): 639-647.
ENGST, R., MACHOLZ, R.M., & KUJAWA, M. (1978) [Metabolites of
HCH-isomers in human blood.] Pharmazie, 33(2/3): 109-111 (in German).
ERNST, W. (1977) Determination of the bioconcentration of marine
organisms. A steady state approach. I. Bioconcentration data for
seven chlorinated pesticides in mussels (Mytilus edulis) and their
relation to solubility data. Chemosphere, 6(11): 731-340.
ERNST, W. (1979) Factors affecting the evaluation of chemicals in
laboratory experiments using marine organisms. Ecotoxicol. environ.
Saf., 3: 90-98.
FALADYSZ, J. & SZEFER, D. (1982) Chlorinated hydrocarbons in diving
ducks wintering in Gdansk Bay, Baltic Sea. Sci. total Environ., 24:
119-127.
FISHMAN, B.E. (1987) The neurotoxicity of the isomers of
hexachlorocyclohexane: actions at the GABA-A-receptor linked chloride
channel. Diss. Abstr. Int., B48: 3257-3258.
FISHMAN, B.E. & GIANUTSOS, G. (1987) Opposite effects of different
hexachlorocyclohexane (lindane) isomers on cerebellar cyclic GMP:
relation of cyclic GMP accumulation to seizure activity. Life Sci.,
41: 1703-1709.
FISHMAN, B.E. & GIANUTSOS, G. (1988) CNS biochemical and
pharmacological effects of the isomers of hexachlorocyclohexane
(lindane) in the mouse. Toxicol. appl. Pharmacol., 93: 146-153.
FITZHUGH, O.G., NELSON, A.A., & FRAWLEY, J.P. (1950) The chronic
toxicities of technical benzene hexachloride and its alpha-, beta-,
and gamma-isomers. J. Pharmacol. exp. Ther., 100(1): 59-66.
FOOKEN, C. & BUTTE, W. (1987) Organochlorine pesticides and
polychlorinated biphenyls in human milk during lactation. Chemosphere,
16(6): 1301-1309.
FOSTER, T.S. & SAHA, J.G. (1978) The in vitro metabolisation of
lindane by an enzyme preparation from chicken liver. J. environ. Sci.
Health, B13(1): 25-45.
FRANK, R., BRAUN, H.E., SIRONS, G.H., RASPER, J., & WARD, G.G. (1985)
Organochlorine and organophosphorus insecticides and industrial
pollutants in the milk supplies of Ontario, 1983. J. Food Prod.,
48(6): 499-504.
FRANKLIN, A. (1987) The concentration of metals, organochlorine
pesticide and PCB residues in marine fish and shell fish: results from
MAFF fish and shell fish monitoring programmes, 1977-1984, Lowestoft,
Ministry of Agriculture, Fisheries, and Food, Directorate of Fisheries
Research (Aquatic Environment Monitoring Report No. 16).
FREAL, J.J. & CHADWICK, R.W. (1973) Metabolism of
hexachlorocyclohexane to chlorophenols and effect of isomer
pretreatment on lindane metabolism in rat. J. agric. food Chem.,
21(3): 424-427.
GAGGI, C., BACCI, E., CALAMARI, D., & FANELLI, R. (1986) Chlorinated
hydrocarbons in plant foliage: An indication of the tropospheric
contamination level. Chemosphere, 14(11-12): 1673-1686.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1985a) Pesticides, selected elements and other chemicals in adult
total diet samples, October 1978-September 1979. J. Assoc. Off. Anal.
Chem., 68(5): 862-875.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1985b) Pesticides, selected elements and other chemicals in Infant
and Toddler total diet samples, October 1978-September 1979. J. Assoc.
Off. Anal. Chem., 68(5): 842-861.
GEYER, H., SCHEUNERT, I., & KORTE, F. (1986) Bioconcentration
potential of organic environmental chemicals in humans. Regul.
Toxicol. Pharmacol., 6: 313-347.
GOTO, M., HATTORI, M., & MIYAGAWA, T. (1972a) [Contributions to
ecological chemistry. II. Hepatoma formation in mice after
administration of high doses of HCH isomers.] Chemosphere, 6: 279-282
(in German).
GOTO, M., HATTORI, M., & MIYAGAWA, T. (1972b) [Contributions to
ecological chemistry. Toxicity of alpha-BHC in mice.] Chemosphere, 4:
153-154 (in German).
GREVE, P.A. (1985) Organochlorine pesticides and PCBs in human
tissues from Dutch citizens (1968-1983), Bilthoven, National Institute
of Public Health and Environmental Hygiene (Unpublished concept
report).
GREVE, P.A. & VAN HARTEN, D.C. (1983) [Relationship between levels
of organochlorine pesticides and PCBs in fat and blood in human
subjects], Bilthoven, National Institute of Public Health and
Environmental Hygiene (Unpublished report No. 638219001) (in Dutch).
GREVE, P.A. & VAN HULST, Sj. (1977) [Organochlorine pesticides and
PCBs in duplicate 24-hour feeds], Bilthoven, National Institute of
Public Health and Environmental Hygiene (Unpublished report No. 192/77
Tox/ROB) (in Dutch).
GREVE, P.A. & VAN HULST, Sj. (1980) [Content of beta- and gamma-HCH
and some of their metabolites in rat tissues derived from the
semi-chronic toxicity tests], Bilthoven, National Institute of Public
Health and Environmental Hygiene (Unpublished report No. 144/80,
RA/617908001) (in Dutch).
GREVE, P.A. & WEGMAN, R.C.C. (1985) Organochlorine compounds in
human milk; data from a recent investigation in the Netherlands.
Working paper for WHO Consultation on Organochlorine Compounds in
Human Milk and Related Hazards, Bilthoven, 9-11 January, 1985,
Bilthoven, National Institute of Public Health and Environmental
Hygiene.
GUICHERIT, R. & SCHULTING, F.L. (1985) The occurrence of organic
chemicals in the atmosphere of the Netherlands. Sci. total Environ.,
43: 193-219.
GUNDERSON, E.L. (1988) FDA total diet study, April 1982-April 1984.
Dietary intakes of pesticides, selected elements and other chemicals.
J. Assoc. Off. Anal. Chem., 71(6): 1200-1209.
GUPTA, S.K., PARIKH, J.R., SHAH, M.P., CHATTERJEE, S.K., & KASHYAP,
S.K. (1982) Changes in serum HCH residues in malaria spraymen after
short-term occupational exposure. Arch. environ. Health, 37(1): 41-44.
HAIDER, K. (1979) Degradation and metabolization of lindane and
other hexachlorocyclohexane isomers by anaerobic and aerobic soil
microorganisms. Z. Naturforsch., 34C: 1066-1069.
HAMADA, M., KAWANO, E., KAWAMURA, S., & SHIRO, M. (1982) A new
isomer of 1,2,3,4,5-pentachlorocyclohexane from UV-irradiation
products of alpha-, ß-, and delta-isomers of 1,2,3,4,5,6-
hexachlorocyclohexane. Agric. biol. Chem., 46(1): 153-157.
HANADA, M., YUTANI, C., & MIYAJI, T. (1973) Induction of hepatoma in
mice by benzene hexachloride. Gann., 64(5): 511-513.
HAPKE, H.J. & HOLLMANN, A.C. (1985) [Effects of
beta-hexachlorocyclohexane on suckling rats.] Dtsch. Tierärztl.
Wschr., 92(1): 125-164 (in German).
HEESCHEN, W. (1985) Studies on the carry-over of beta-
hexachlorocyclohexane in the milk of cows. Kieler Milchwirtsch.
Forschungsber., 37(1): 29-43.
HERITAGE, A.D. & MACRAE, I.C. (1977) Identification of intermediates
formed during the degradation of hexachlorocyclohexanes by
Clostridium sphenoïdes. Appl. environ. Microbiol., 33(6): 1295-1297.
HERITAGE, A.D. & MACRAE, I.C. (1979) Degradation of
hexachlorocyclohexanes and structurally related substances by
Clostridium sphenoïdes. Aust. J. biol. Sci., 32(4/5): 493-500.
HERRMANN, R., THOMAS, W., & HÜBNER, D. (1984) [Behaviour of organic
micropollutants (PAH, PCB and BHC) and of a fecal sterol in the Husum
estuary and in the adjacent North Frisian Wadden sea.] Dtsch.
Gewässerkd. Mitt., 4: 101-107 (in German).
HILDEBRANDT, G., JEEP, H., HURKA, H., STUKE, T., HEITMAN, M., &
BOISELLE, C. (1986) [Breast milk as a biological indicator: Study on
the organic halogen burden in breast milk and foodstuffs], Berlin,
Schmidt E. Verlag (Report 5/86 of the Federal Office for the
Environment) (in German).
HMSO (1969) Further review of certain persistent organochlorine
pesticides used in Great Britain. Report by the Advisory Committee on
Pesticides and other Toxic Chemicals, London, Her Majesty's Stationery
Office.
HMSO (1982) Report of the Working Party on Pesticide Residues
(1977-1981): Ninth report of the Steering Group on Food Surveillance,
London, Ministry of Agriculture, Fisheries, and Food, Her Majesty's
Stationery Office (Food Surveillance Paper No. 9).
HMSO (1986) Report of the Working Party Pesticide on Residues
(1982-1985): Sixteenth report of the Steering Group on Food
Surveillance, London, Ministry of Agriculture, Fisheries, and Food,
Her Majesty's Stationery Office (Food Surveillance Paper No. 16).
HMSO (1989) Report of the Working Party on Pesticide Residues
(1985-1988): Twenty-fifth report of the Steering Group on Food
Surveillance, London, Ministry of Agriculture, Fisheries, and Food,
Her Majesty's Stationery Office (Food Surveillance Paper No. 25).
HOFFMANN, A. (1983) [Toxicity of alpha- and beta-HCH.] In:
[Hexachlorocyclohexane as a harmful substance in foodstuffs. Papers
from two seminars of the Senate Committee on the Testing of Residues
in Foodstuffs held on 28-29 November 1979 and 6 March 1980], Weinheim,
Chemie Verlag, pp. 201-214 (in German).
HOLT, R.L., CRUSE, S., & GREER, E.S. (1986) Pesticide and
polychlorinated biphenyl residues in human adipose tissue from North
East Louisiana. Bull. environ. Contam. Toxicol., 36: 651-655.
HORI, S. & KASHIMOTO, T. (1974) Transfer of beta-HCH from mother to
suckling mouse. Shokuhin Eiseigaku Zasshi, 15(6): 446-450.
IARC (1979) Hexachlorocyclohexane (technical HCH and lindane). In:
Some halogenated hydrocarbons, Lyon, International Agency for Research
on Cancer, pp. 195-239 (IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Vol. 20).
IARC (1987) Hexachlorocyclohexanes (Group 2B). In: Overall
evaluations of carcinogenicity: An updating of IARC Monographs Vol. 1
to 42, Lyon, International Agency for Research on Cancer (IARC
Monographs on the Evaluation of Carcinogenic Risks to Humans, Suppl.
7).
ITO, N., NAGASAKI, H., ARAI, M., MAKIURA, S., SUGIHARA, S., & HIRAO,
K. (1973a) Histopathologic studies on liver tumorigenesis induced in
mice by technical polychlorinated biphenyls and its promoting effect
on liver tumours induced by benzene hexachloride. J. Natl Cancer
Inst., 51(5): 1637-1646.
ITO, N., NAGASAKI, H., ARAI, M., SUGIHARA, S., & MAKIURA, S. (1973b)
Histologic and ultrastructural studies on the hepatocarcinogenicity of
benzene hexachloride in mice. J. Natl Cancer Inst., 51(3): 817-826.
ITO, N., NAGASAKI, H., AOE, H., SUGIHARA, S., MIYATA, Y., ARAI, M., &
SHIRAI, T. (1975) Brief Communication: Development of hepatocellular
carcinomas in rats treated with benzene hexachloride. J. Natl Cancer
Inst., 54(3): 801-905.
ITO, N., HANAOUCHI, M., SUGIHARA, S., SHIRAI, T., TSUDA, H.,
FUKUSHIMA, S., & NAGASAKI, H. (1976) Reversibility and
irreversibility of liver tumours in mice induced by the alpha-isomer
of 1,2,3,4,5,6,-hexachlorocyclohexane. Cancer Res., 36: 2227-2234.
ITO, N., TSUDA, H., HASEGAWA, R., & IMAIDA, K. (1983) Comparison of
the promoting effects of various agents in induction of preneoplastic
lesions in rat liver. Environ. Health Perspect., 50: 131-138.
IVERSON, F., RYAN, J.J., LIZOTTE, R., & HIERLIHY, S.L. (1984) In vivo
and in vitro binding of alpha- and gamma-hexachlorocyclohexane to
mouse liver macromolecules. Toxicol. Lett., 20(3): 331-335.
JAGNOW, G., HAIDER, K., & ELLWARDT, P.Chr. (1977) Anaerobic
dechlorination and degradation of hexachlorocyclohexane isomers by
anaerobic and facultative anaerobic bacteria. Arch. Microbiol., 115:
285-292.
JANSSEN, P.J.C.M., VAN KOTEN-VERMEULEN, J.E.M., KRAJNC, E.I., CANTON,
J.H., VAN GESTEL, C.A.M., VAN DER HEIJDEN, C.A., & HEIJNA-MERKENS, E.
(1987) [Hexachlorocyclohexane (Basic document)], Bilthoven, National
Institute of Public Health and Environmental Hygiene (Project No.
840820) (in Dutch).
KAMPE, W. (1980) [Occurrence of hexachlorocyclohexane in the soil.]
In: [Hexachlorocyclohexane as a harmful substance in foodstuffs],
Weinheim, Verlag Chemie, pp. 18-23 (Research report) (in German).
KAN, C.A. & JONKER-DEN ROOYEN, J.C. (1978a) Accumulation and depletion
of some organochlorine pesticides in broiler breeder hens during the
second laying cycle. J. agric. food Chem., 26(2): 465-470.
KAN, C.A. & JONKER-DEN ROOYEN, J.C. (1978b) Accumulation and depletion
of some organochlorine pesticides in high producing laying hens. J.
agric. food Chem., 26(4): 935-940.
KAN, C.A., JONKER-DEN ROOYEN, J.C., TUINSTRA, L.G.M.Th., ROOS, A.H.,
& TRAUG, W. (1978) Possible influence of sex and embryonic content on
accumulation of some organochlorine pesticides in broilers. J. agric.
food Chem., 26(3): 618-621.
KAN, C.A., STRIK, J.J.T.W.A., & KOEMAN, J.H. (1979) Semi-chronic
toxicity of beta-HCH in laying hens. Meded. Fac. Landbouwwet.
Rijksuniv. Gent, 44(2): 965-973.
KORANSKY, W., PORTIG, J., & MÜNCH, G. (1963) [Absorption,
distribution and excretion of alpha- and gamma-hexachlorocyclohexane.]
Naunyn-Schmiedebergs Arch. exp. Pathol. Pharmakol. 244: 564-575 (in
German).
KORANSKY, W., PORTIG, J., VOHLAND, H.W., & KLEMPAU, I. (1964)
[Elimination of alpha- and gamma-hexachlorocyclohexane and the way it
is influenced by enzymes of liver microsomes.] Naunyn-Schmiedebergs
Arch. exp. Pathol. Pharmakol., 247: 49-60 (in German).
KORTE, F. (1980) [Occurrence of HCH isomers in man.] In:
[Hexachlorocyclohexane as a harmful substance in foodstuffs. Papers
from two seminars of the Senate Committee on the Testing of Residues
in Foodstuffs held on 28-29 November 1979 and 6 March 1980], Weinheim,
Verlag Chemie, pp. 46-64 (in German).
KRAUS, D. (1975) Biodegradation of alpha-hexachlorocyclohexane by
microsomes and cytosol of rat liver. Naunyn-Schmiedebergs Arch.
Pharmakol., 291: 79-87.
KRAUTHACKER, B., ALEBIC-KOLBAH, T., KRALJ, M., TKALCEVIC, B., &
REINER, E. (1980) Organochlorine pesticides in blood serum of the
general Yugoslav population and in occupationally exposed workers.
Int. Arch. occup. environ. Health, 45: 217-220.
KRISHNAKUMARI, M.K. (1977) Sensitivity of the alga Scenedesmus
acutusto some pesticides. Life Sci., 20: 1525-1532.
KUIPER, H.A., DEN TONKELAAR, E.M., VAN LEEUWEN, F.X.R., FRANKEN,
M.A.M., & DORMANS, J.A.M.A. (1985) [Semi-chronic toxicity test with
alpha-HCH], Bilthoven, National Institute of Public Health and
Environmental Hygiene (Report No. 617917002) (in Dutch).
KURIHARA, N. (1975) Urinary metabolites from ß- and gamma-HCH in the
mouse: chlorophenol conjugates. Environ. Qual. Saf., 4: 56-73.
KURIHARA, N. & NAKAJIMA, M. (1974) Studies on BHC isomers and
related compounds VIII. Urinary metabolites produced from gamma- and
ß-BHC in the mouse: Chlorophenol conjugates. Pestic. Biochem.
Physiol., 4: 220-231.
KUTZ, F.W., STRASSMAN, S.C., & SPERLING, J.F. (1979) Survey of
selected organochlorine pesticides in the general population of the
United States: Fiscal years 1970-1975. Ann. NY Acad. Sci., 320: 60-68.
LAUGEL, P. (1981) [The residue situation in France and ways of
overcoming it.] Lebensmittelchem. gerichtl. Chem., 35: 29-32 (in
German).
LAWLOR, T. & HAWORTH, S.R. (1979) Evaluation of the genetic activity
of nine chlorinated phenols, seven chlorinated benzenes and three
chlorinated hexanes. Environ. Mutagen., 1(2): 143.
LAY, J.P., KLEIN, W., KORTE, F., & RICHTER, E. (1981) Metabolism
of ß-hexachlorocyclohexane-[14]C in rats following low dosing in the
daily diet. J. environ. Sci. Health, B16: 227-238.
LOEBER, J.G. & VAN VELSEN, F.L. (1985) Uterotropic effect of ß-HCH.
A food chain contaminant. Food Addit. Contam., 1: 63-66.
LOWDEN, G.F., SAUNDERS, C.L., & EDWARDS, R.W. (1969) Organochlorine
insecticides in water (Part II). J. Water Treat. Exam., 18: 275-287.
LWA (1987) [Water quality report], Düsseldorf, North-Rhine Westphalia
Office for Water and Refuse, 24 pp (in German).
MACHOLZ, R.M., KNOLL, R., LEWERENZ, H.J., PETRZIKA, M., & ENGST, R.
(1982) Metabolism of alpha-hexachlorocyclohexane. Free metabolites in
urine and organs of rats. Xenobiotica, 12(4): 227-231.
MACHOLZ, R.M., KNOLL, R., LEWERENZ, H.J., PLASS, R., & SCHULZE, J.
(1983) [Metabolism of gamma-hexachlorocyclohexane (HCH) in germ-free
and conventionalized rats.] Zentralbl. Pharm., 122(2): 221 (in
German).
MACHOLZ, R.M., BLEYL, D.W., KLEPEL, H., KNOLL, R., KUJAWA, M.,
LEWERENZ, H.J., MUELLER, D., & PLASS, R. (1986) Comparison of
distribution and toxicity of alpha-, beta-, and gamma-BHC after
application to rats for 30 days. Nahrung, 30: 701-708.
MACRAE, I.C., RAGHU, K., & CASTRO, T.F. (1967) Persistence and
biodegradation of four common isomers of benzenehexachloride in
submerged soils. J. agric. food Chem., 15(5): 911-914.
MACRAE, J.C., YAMAYA, Y., & YOSHIDA, T. (1984) Persistence of
HCH-isomers in soil suspensions. Soil Biol. Biochem., 16: 285-286.
MARTIN, D.B. & HARTMANN, W.A. (1985) Organochlorine pesticides and
polychlorinated biphenyls in sediment and fish from wetlands in the
North-Central United States. J. Assoc. Off. Anal. Chem., 69(4):
712-717.
MATHUR, R., SAXENA, D.M., & AGARWAL, H.C. (1984) Growth of a ciliate
protozoan. Tetrahymena pyriformis in the presence of different
isomers of hexachlorocyclohexane (HCH). Acta. Protozool., 23(3):
165-174.
MATSUMOTO, K., ELDERFRAWI, M.E., & ELDEFRAWI, A.T. (1988) Action of
polychlorocycloalkane insecticides in binding of [355]
t-butyldicyclophosphorothionate to torpedo electric organ membranes
and stereospecificity of the binding site. Toxicol. appl. Pharmacol.,
95: 220-229.
MATSUSHIMA, S. (1972) [Pollution of food by agricultural chemicals and
the diet.] Rinsho. Eiyo., 40: 555-563 (in Japanese).
MES, J., DAVIES, D.J., & TURTON, D. (1982) Polychlorinated biphenyl
and other chlorinated hydrocarbon residues in adipose tissue of
Canadians. Bull. environ. Contam. Toxicol., 28: 97-104.
MES, J., DAVIES, D.J., TURTON, D., & SUN, W.-F. (1986) Levels and
trends of chlorinated hydrocarbon contaminants in the breast milk of
Canadian women. Food Addit. Contam., 3(4): 313-322.
MESTRES, R. (1974) Report of a group of experts on the content of
organohalogen compounds detected between 1968 and 1972 in water, air
and foodstuffs and the methods of analysis used in the nine Member
States of the European Communities. Presented at the European
Colloquium on Problems raised by the Contamination of Man and his
Environment by Persistent Pesticides and Organohalogenated Compounds,
Luxembourg, 14-16 May, 1974, Luxembourg, Commission of the European
Communities (Paper No. 1).
MORGAN, D.P. & LIN, L.I. (1978) Blood-organochlorine pesticide
concentrations, clinical hematology and biochemistry in workers
occupationally exposed to pesticides. Arch. environ. Contam. Toxicol.,
7: 423-447.
MOUVET, C., GALOUX, M., & BERNES, A. (1985) Monitoring of
polychlorinated biphenyls (PCBs) and hexachlorocyclohexanes (HCH) in
freshwater using the aquatic mossCinclidotus danubicus.Sci. total
Environ., 44: 253-267.
MÜLLER, D., KLEPEL, H., MACHOLZ, R.M., LEWERENZ, H.J., & ENGST, R.
(1981) Electroneurophysiological studies on neurotoxic effects of
hexachlorocyclohexane-isomers and gamma-pentachlorocyclohexane. Bull.
environ. Contam. Toxicol., 27: 704-706.
MURPHY, R. & HARVEY, C. (1985) Residues and metabolites of selected
persistent halogenated hydrocarbons in blood specimens from a general
population survey. Environ. Health Perspect., 60: 115-120.
NAGASAKI, H. (1973) Chronic toxicity of benzene hexachloride (BHC).
Nara Igaku Zasshi, 24: 1-26.
NAGASAKI, H., KAWABATA, H., MIYATA, Y., INOUE, K., HIRAO, K., AOE, H.,
& ITO, N. (1975) Effect of various factors on induction of liver
tumours in animals by the isomer of benzene hexachloride. Gann., 66:
185-191.
NAKAJIMA, E., SHINDO, H., & KURIHARA, N. (1970) [Whole body
autoradiographic studies on the distribution of alpha-, beta-, and
gamma-benzene hexachloride in mice.] Radio-isotopes, 19: 538 (in
Japanese).
NIESSEN, K.H., RAMOLLAA, J., BINDER, M., BRUGMANN, G., & HOFMANN, U.
(1984) Chlorinated hydrocarbons in adipose tissue of infants and
toddlers: inventory and studies on their association with intake of
mother's milk. Eur. J. Pediatr., 142: 238-243.
NIGAM, S.K., KARNIK, A.B., MAJUMDER, S.K., VISWESWARRIAH, K.,
SURYANARAYANA RAJU, G., MUKTHA BAI, K., LAKKAD, B.C., THAKORE, K.N., &
CHATTERJEE, B.B. (1986) Serum hexachlorocyclohexane residues in
workers engaged at a HCH manufacturing plant. Int. Arch. occup.
environ. Health, 57: 315-320.
NISHIMURA, N. (1982) Survey on the mutagenicity of pesticides by a
Salmonella microsome test. Aichi Ika Daigaku Igakkai Zasshi, 10(4):
305-312.
NOACK, G. & PORTIG, J. (1973) Biodegradation of alpha-hexa-
chlorocyclohexane. III. Decrease in liver non-protein thiol after
intragastric application of the drug. Naunyn-Schmiedebergs Arch.
Pharmakol., 280: 183-189.
NOACK, G., PORTIG, J., & WIRSCHING, M. (1975) Biodegradation of
alpha-hexachlorocyclohexane. IV. The extent of degradation of single
doses in vivo. Naunyn-Schmiedebergs Arch. Pharmakol., 288: 57-64.
NOREN, K. (1983) Levels of organochlorine contaminants in human milk
in relation to the dietary habits of the mothers. Acta paediatr.
Scand., 72: 811-816.
NORSTROM, R.J., SIMON, M., MUIR, D.C.G., & SCHWEINSBURG, R.E. (1988)
Organochlorine contaminants in Arctic marine food chains:
Identification, geographical distribution and temporal trends in polar
bears. Environ. Sci. Technol., 22: 1063-1071.
NYBOM, N. & KNUTSSON, B. (1947) Investigations on c-mitosis in
Allium cepa. I. The cytological effect of hexachlorocyclohexane.
Hereditas, 33: 220-234.
OEHME, M. & MANO (1984) The long-range transport of organic
pollutants to the Arctic. Fresenius Z. anal. Chem., 319: 141-146.
OEHME, M. & STRAY, H. (1982) Quantitative determination of
ultra-traces of chlorinated compounds in high-volume air samples from
the Arctic using polyurethane foam as collecting medium. Fresenius Z.
anal. Chem., 311: 665-673.
OSHIBA, K. (1972) Experimental studies on the fate of ß- and
gamma-BHC in vivo following daily administration. Osaka Shiritsu
Daigaku Igaku Zasshi, 21(1-3): 1-19.
OSHIBA, K. & KAWAKITA, H. (1972) Interaction between toxicant and
nutrition. IV. Effect of lipid metabolism on reduction of the BHC
deposition in rat tissues. Chem. Abstr., 77(8): 148231.
PALMER, C.M. & MALONEY, T.E. (1955) Preliminary screening for
potential algicides. Ohio J. Sci., 55(1): 1-8.
POLISHUK, Z.W., WASSERMANN, M., WASSERMANN, D., GRONER, Y.,
LAZAROVICI, S., & TOMATIS, L. (1970) Effects of pregnancy on storage
of organochlorine insecticides. Arch. environ. Health, 20: 215-217.
PORTIG, J. (1983) [Elimination of hexachlorocyclohexane (HCH) by
mammals.] In: [Hexachlorocyclohexane as a harmful substance in
foodstuffs. Papers from two seminars of the Senate Committee on the
Testing of Residues in Foodstuffs held on 28-29 November 1979 and 6
March 1980], Weinheim, Chemie Verlag, pp. 108-118 (in German).
PORTIG, J. & VOHLAND, H.W. (1983) [Neuropharmacological and
neurotoxic effects of hexachlorocyclohexane in man.] In:
[Hexachlorocyclohexane as a harmful substance in foodstuffs. Papers
from two seminars of the Senate Committee on the Testing of Residues
in Foodstuffs held on 28-29 November 1979 and 6 March 1980], Weinheim,
Chemie Verlag, pp. 215-226 (in German).
PORTIG, J., KRAUS, P., SODOMANN, S., & NOACK, G. (1973)
Glutathion-dependent biotransformation in rats of isomers of
hexachlorocyclohexane. Naunyn-Schmiedebergs Arch. Pharmakol., 279:
185-198.
PORTIG, J., STEIN, K., & VOHLAND, H.W. (1989) Preferential
distribution of delta-hexachlorocyclohexane into cerebral white
matter. Xenobiotica, 19(1): 123-130.
RADOMSKI, J.L., DEICHMANN, W.B., ALBERTO, A., REY, A., & MERKIN, T.
(1971a) Human pesticide blood levels as a measure of body burden and
pesticide exposure. Toxicol. appl. Pharmacol., 20: 175-185.
RADOMSKI, J.L., ASTOLFI, E., DEICHMANN, W.B., & REY, A.A. (1971b)
Blood levels of organochlorine pesticides in Argentina. Occupationally
and non-occupationally exposed adults, children, and new-born infants.
Toxicol. appl. Pharmacol., 20: 186-193.
RAPPE, C., NYGREN, M., & LINDSTRÖM, G. (1987) Polychlorinated
dibenzofurans and dibenzo-p-dioxins and other chlorinated
contaminants in cow milk from various locations in Switzerland.
Environ. Sci. Technol., 21: 964-970.
REINER, E., KRAUTHACKER, B., STIPCEVIC, M., & STEFANAC, Z. (1977)
Blood levels of chlorinated hydrocarbon residues in the population of
a continental town in Croatia (Yugoslavia). Pestic. monit. J., 11:
54-55.
RIEMSCHNEIDER, R. (1949) [A contribution to the toxicity of contact
insecticides.] Anz. Schädlingskd., 22(1): 1-3 (in German).
SAGELSDORFF, P., LUTZ, W.K., & SCHLATTER, C. (1983) The relevance of
covalent binding to mouse liver DNA to the carcinogenic action of
hexachlorocyclohexane isomers. Carcinogenesis, 4(10): 1267-1273.
SAIKI, M.K. & SCHMITT, C.J. (1986) Organochlorine chemical residues
in bluegills and common carp from the irrigated San Joaquin Valley
Floor, California. Arch. environ. Contam. Toxicol., 15: 357-366.
SCHRÖTER, C., PARZEFALL, W., SCHRÖTER, H., & SCHULTE-HERMANN, R.
(1987) Dose-response studies on the effects of alpha-, ß-, and
gamma-hexachlorocyclohexane on putative preneoplastic foci,
monooxygenases, and growth in rat liver. Cancer Res., 47: 80-88.
SCHULTE-HERMANN, R. & PARZEFALL, W. (1981) Failure to discriminate
initiation from promotion of liver tumours in a long term study with
the phenobarbital-type inducer alpha-hexachlorocyclohexane and the
role of sustained stimulation of hepatic growth and monooxygenases.
Cancer Res., 41: 4140-4146.
SCHULTE-HERMANN, R., LEBERL, C., LANDGRAF, H., & KORANSKY, W. (1974)
Liver growth and mixed function oxidase activity: dose dependent
stimulatory and inhibitory effects of alpha-hexachlorocyclohexane.
Naunyn-Schmiedebergs Arch. exp. Pathol. Pharmakol., 285: 355-366.
SCHULTE-HERMANN, R., OHDE, G., SCHUPPLER, J., & TIMMERMANN-TROSIENER,
I. (1981) Enhanced proliferation of putative preneoplastic cells in
rat liver following treatment with the tumour promotors phenobarbital,
hexachlorocyclohexane, steroid compounds and nafenopin. Cancer Res.,
41(6): 2556-2562.
SCHULTE-HERMANN, R., TIMMERMANN-TROSIENER, I., & SCHUPPLER, J., (1983)
Promotion of spontaneous preneoplastic cells in rat liver as a
possible explanation of tumour production by non-mutagenic compounds.
Cancer Res., 43: 839-844.
SEIFART, J. & BUCHAR, E. (1978) The biosynthesis of pyrimidine
nucleotides and cytochrome P-450 content in rat liver microsomes after
the administration of alpha-hexachlorocyclohexane ( in vivo study).
Toxicology, 11: 37-44.
SHAHIN, M. & VON BORSTEL, R. (1977) Mutagenic and lethal effects of
alphabenzene hexachloride, dibutyl phthalate and trichloroethylene in
Saccharomyces cerevisiae. Mutat. Res., 48: 173-180.
SHAIN, W., NARANG, R., & SEEGAL, R. (1987) Neurotoxicity testing of
chlorinated hydrocarbons by measuring specific neuronal and glial cell
functions. Prog. clin. biol. Res., 253: 207-216.
SHIBATA, T. (1978) Experimental studies on the dynamic aspects of
BHC and PCB in vivo. Jpn. J. Hyg., 32: 736-746.
SHIMAZU, H., SHIRAISHI, N., AKEMATSU, T., UEDA, N., & SUGIYAMA, T.
(1976) Carcinogenicity screening tests on induction of chromosomal
aberrations in rat bone marrow cells in vivo. Mutat. Res., 38: 347
(Abstract No. 20).
SIDDARAMAPPA, R. & SETHUNATHAN, N. (1975) Persistence of gamma-BHC
and beta-BHC in Indian rice soils under flooded conditions. Pestic.
Sci., 6: 395-403.
SIERRA, M. & SANTIAGO, D. (1987) Organochlorine pesticide levels in
barn owls collected in Leon, Spain. Bull. environ. Contam. Toxicol.,
38: 261-265.
SKAARE, J.U., TUVENG, J.M., & SANDE, H.A. (1988) Organochlorine
pesticides and polychlorinated biphenyls in maternal adipose tissue,
blood, milk, & cord blood from mothers and their infants living in
Norway. Arch. environ. Toxicol., 17: 55-63.
SKAFTASON, J.F. & JOHANNESSON, T. (1979) Organochlorine compounds
(DDT, hexachlorocyclohexane, hexachlorobenzene) in Icelandic animal
body fat and butter fat: Local and global sources of contamination.
Acta pharmacol. toxicol., 44: 156-157.
SLOOFF, W. & MATTHIJSEN, A.J.C.M. (1988) Integrated criteria
document: Hexachlorocyclohexanes, Bilthoven, National Institute of
Public Health and Environmental Protection (Report No. 758473011).
SRINIVASAN, K. & RADHAKRISHNAMURTY, R. (1977) Effect of beta- and
gamma-isomers of hexachlorocyclohexane on some liver and kidney
enzymes in albino rats. Curr. Sci., 46: 598-600.
SRINIVASAN, K. & RADHAKRISHNAMURTY, R. (1983) Studies on the
distribution of the beta- and gamma-isomers of hexachlorocyclohexane
in rat tissues. J. environ. Sci. Health, B18(3): 401-418.
SRINIVASAN, K. & RADHAKRISHNAMURTY, R. (1988) Biochemical changes
produced by beta- and gamma-Hexachlorocyclohexane isomers in albino
rats. J. environ. Sci. Health, B23(4): 367-386.
SRINIVASAN, K., RAMESH, H.P., & RADHAKRISHNAMURTY, R. (1984) Renal
tubular dysfunction caused by dietary hexachlorocyclohexane (HCH)
isomers. J. environ. Sci. Health, B19(45): 453-466.
SRINIVASAN, K., RAMESH, H.P., & RADHAKRISHNAMURTY, R. (1988) Changes
induced by hexachlorocyclohexane isomers in rat livers and testes.
Bull. environ. Contam. Toxicol., 41: 531-539.
STARR, H.G., Jr, ALDRICH, F.D., MCDOUGALL, W.D., III, & MOUNCE, L.M.
(1974) Contribution of household dust to the human exposure to
pesticides. Pestic. monit. J., 8: 209-212.
STEIN, K. & PORTIG, J. (1976) Oxidative transformation of
hexachlorocyclohexane in the rat. Naunyn-Schmiedebergs Arch.
Pharmacol., 293: R51.
STEIN, K., PORTIG, J., & KORANSKY, W. (1977) Oxidative
transformation of hexachlorocyclohexane in rats and with rat liver
microsomes. Naunyn-Schmiedebergs Arch. Pharmakol., 298: 115-128.
STEIN, K., PORTIG, J., FUHRMANN, H., KORANSKY, W., & NOACK, G. (1980)
Steric factors in the pharmacokinetics of lindane and
alpha-hexachlorocyclohexane in rats. Xenobiotica, 10(1): 65-77.
STRACHAN, W.M.J., HUNEAULT, H., SCHERTZER, W.M., & ELDER, F.C. (1980)
Organochlorines in precipitation in the Great Lakes Region. In:
Afghan, B.K. & MacKay, D., ed. Hydrocarbons and halogenated
hydrocarbons in the aquatic environment, New York, London, Plenum
Press.
SUGIURA, K., SATO, S., GOTO, M., & KURIHARA, Y. (1976) Effects of
beta-HCH on aquatic microcosm. Chemosphere, 1: 39-44.
SUGIURA, K., WASHINO, T., HATTORI, M., SATO, E., & GOTO, M. (1979)
Accumulation of organochlorine compounds in fishes. Difference of
accumulation factors by fishes. Chemosphere, 6: 359-364.
SUZUKI, M., YAMATO, Y., & WATANABE, T. (1975) Persistence of BHC
(1,2,3,4,5,6-hexachlorocyclohexane) and dieldrin residues in field
soils. Bull. environ. Contam. Toxicol., 14(5): 520-529.
SZOKOLAY, A., ROSIVAL, L., UHNAK, J., & MADARIC, A. (1977a) Dynamics
of benzene hexachloride (BHC) isomers and other chlorinated pesticides
in the food chain and human fat. Ecotoxicol. environ. Saf., 1(1):
349-359.
SZOKOLAY, A., MADARIC, A., & UHNAK, J. (1977b) Relative cumulation
of beta-HCH in ecological and biological systems. J. environ. Sci.
Health, B12(3): 193-212.
SZYMCZYNSKI, G.A., WALISZEWSKI, S.M., TUSZEWSKI, M., & PYDA, P. (1986)
Chlorinated pesticides levels in human adipose tissue in the district
of Poznan. J. environ. Sci. Health, A21(1): 5-14.
TAKABATAKE, E. (1978) Levels of organochlorine compounds and heavy
metals in tissues of the general population in Japan. Geogr. med., 8:
1-23.
TANABE, S., TATSUKAWA, R., KAWANO, M., & HIDAKA, H. (1982) Global
distribution and atmospheric transport of chlorinated hydrocarbons:
HCH(BHC) isomers and DDT compounds in the Western Pacific, Eastern
Indian- and Antarctic Oceans. J. Oceanogr. Soc. Jpn, 38(3): 137-148.
TANOOKA, H. (1977) Development and applications ofBacillus
subtilistest systems for mutagens, involving DNA-repair deficiency and
suppressible auxotrophic mutations. Mutat. Res., 42: 19-32.
THAMAVIT, W., HIASA, Y., ITO, N., & BHAMARA-PRAVATI, N. (1974) The
inhibitory effects of alpha-benzene hexachloride on
3'-methyl-4-dimethyl aminobenzene and DL-ethionine carcinogenesis in
rats. Cancer Res., 34: 337-340.
THORPE, E. & WALKER, A.I.T. (1973) The toxicology of dieldrin
(HEOD). II Comparative long-term oral toxicity studies in mice with
dieldrin, DDT, phenobarbitone, ß-BHC and gamma-BHC. Food Cosmet.
Toxicol., 11: 433-442.
TIECCO, G. (1961) [Testing for synthetic oestrogens in foodstuffs.]
Vet. Ital., 12: 447-460 (in Italian).
TOMCZAK, S., BAUMANN, K., & LEHNERT, G. (1981) Occupational exposure
to hexachlorocyclohexane. IV. Sex hormone alterations in HCH-exposed
workers. Int. Arch. occup. environ. Health, 48: 283-287.
TRYPHONAS, L. & IVERSON, F. (1983) Sequential histopathologic
analysis of alpha-hexachlorocyclohexane induced hepatic megalocytosis
and adenoma formation in the HPB mouse. J. Natl Cancer Inst., 71(6):
1307-1318.
TSUKANO, Y. (1973) Factors affecting disappearance of BHC-isomers
from rice field soil. Jpn. agric. Res. Q., 7(2): 93-97.
TUINSTRA, L.G.M.Th. (1971) Organochlorine insecticide residues in
human milk in the Leiden region. Neth. Milk Dairy J., 25: 24-32.
UMWELTBUNDESAMT (1989) [Data on the environment, 1988/89], Berlin,
Schmidt E. Verlag, pp. 403-405, 410-412, 416-417, 521-522, 524, 526
(in German).
VAN ASPEREN, K. (1954) Interaction of the isomers of
benzenehexachloride in mice. Arch. Int. Pharmacodyn., 99(3/4):
368-377.
VAN GIERSBERGEN, P.L.M., VAN VELSEN, F.L., VAN LEEUWEN, F.X.R., &
DANSE, L.H.J.C. (1984) [Toxicity of beta-HCH: relationship between
the biochemistry and pathology of the liver in a 6-week feeding test],
Bilthoven, National Institute of Public Health and Environmental
Hygiene (Unpublished report No. 618303001) (in Dutch).
VAN HOOF, M., VAN VELSEN, F.L., DANSE, L.H.J.C., & VAN LEEUWEN, F.X.R.
(1982) [Toxicity of beta-HCH: relationship between the biochemistry
and pathology of the liver in a 3-week feeding test], Bilthoven,
National Institute of Public Health and Environmental Hygiene (Report
No. 618115001) (in Dutch).
VAN VELSEN, F.L. (1986) The oestrogenicity of the environmental
contaminant ß-hexachlorocyclohexane, University of Utrecht (Thesis).
VAN VELSEN, F.L., VAN LEEUWEN, F.X.R., & DANSE, L.H.J.C. (1982)
[Investigation into the toxicity, kinetics and metabolism of beta- and
gamma-HCH], Bilthoven, National Institute of Public Health and
Environmental Hygiene (Unpublished report No. 617908003) (in Dutch).
VAN VELSEN, F.L., DANSE, L.H.J.C., VAN LEEUWEN, F.X.R., DORMANS,
J.A.M.A., & VAN LOGTEN, M.J. (1986) The subchronic oral toxicity of
the ß-isomer of hexachlorocyclohexane in rats. Fundam. appl. Toxicol.,
6: 697-712.
VOHLAND, H.W., PORTIG, J., & STEIN, K. (1981) Neuropharmacological
effects of isomers of hexachlorocyclohexane. 1. Protection against
pentylenetetrazolinduced convulsions. Toxicol. appl. Pharmacol.,
57(3): 425-438.
VOHLAND, H.W. & KORANSKY, W. (1983) [The behaviour and effects of
hexachlorocyclohexane in man.] In: [Hexachlorocyclohexane as a harmful
substance in foodstuffs. Paper from two seminars of the Senate
Committee on the Testing of Residues in Foodstuffs held on 28-29
November 1979 and 6 March 1980], Weinheim, Chemie Verlag, pp. 246-262
(in German).
VUKAVIC, T., PAVKOV, S., CUSIC, S., RONCEVIC, N., VOJINOVIC, M., &
TOKOVIC, B. (1986) Pesticide residues in human colostrum: seasonal
variations, Yugoslavia. Arch. environ. Contam. Toxicol., 15: 525-528.
WAHID, P.A. & SETHUNATHAN, N. (1979) Sorption-desorption of alpha, ß,
and gamma-isomers of hexachlorocyclohexane in soils. J. agric. food
Chem., 27(5): 1050-1053.
WASSERMANN, M., ROGOFF, M.G., TOMATIS, L., DAY, N.E., WASSERMANN, D.,
DJAVAHERIAN, M., & GUTTEL, C. (1972) Storage of organochlorine
insecticides in the adipose tissue of people in Kenya. Ann. Soc. Belge
Méd. Trop., 52(6): 509-514.
WEGMAN, R.C.C. & GREVE, P.A. (1980) Halogenated hydrocarbons in
Dutch water samples over the years 1969-1977. In: Afghan, B.K. &
MacKay, D., ed. Hydrocarbons and halogenated hydrocarbons in the
aquatic environment, New York, London, Plenum Press, pp. 405-415.
WESTER, P.W. & CANTON, J.H. (1986) Histopathological study of
Oryzias latipes (medaka) after long-term ß-hexachlorocyclohexane
exposure. Aquat. Toxicol., 9: 21-45.
WESTER, P.W., CANTON, J.H., & BISSCHOP, A. (1985) Histopathological
study of Poecilia reticulata (guppy) after long-term
ß-hexachlorocyclohexane exposure. Aquat. Toxicol., 6: 271-296.
WHO (1986) Joint FAO/WHO Food Contamination Monitoring Programme.
Summary of 1980-1983 monitoring data, Geneva, World Health
Organization (GEMS report WHO/EHE/FOS/86.2).
WHO (1991) Environmental Health Criteria 124: Lindane, Geneva, World
Health Organization.
WITTICH, U. & SCHULTE-HERMANN, R. (1977) Liver growth and hepatic
monooxygenase activity in suckling rats following administration of
alpha-hexachlorocyclohexane to the mother. Arch. Pharmacol.,
297(suppl. II): R15.
WITTLINGER, R. & BALLSCHMITER, K. (1987) Global baseline pollution
studies XI: Congener specific determination of polychlorinated
biphenyls (PCBs) and occurrence of alpha- and gamma-
hexachlorocyclohexane (HCH), 4.4'-DDE and 4.4'-DDT in continental air.
Chemosphere, 16(10/12): 2497-2513.
WOLF, H.P. (1983) [Implementation of results of a trial with dairy
cows contaminated with 12-HCH.] In: [Hexachlorocyclohexane as a
harmful substance in foodstuffs. Papers from two seminars of the
Senate Committee on the Testing of Residues in Foodstuffs held on
28-29 November 1979 and 6 March 1980], Weinheim, Chemie Verlag, pp.
143-154 (in German).
YAMATO, Y., KIYONAGA, M., & WATANABE, T. (1983) Comparative
bioaccumulation and elimination of HCH-isomers in short-necked clam
(Venerupis japonica) and guppy (Poecilia reticulata). Bull.
environ. Contam. Toxicol., 31: 352-359.
ZHOU, X., LIU, R., ZHANG, W., XUE, X., & LIU, H. (1986) The effect
of BHC isomers on photosynthetic formation of oxygen by green algae.
Zhiwu Shenglixue Tongxun, 6: 32-34.
APPENDIX 1. CHEMICAL STRUCTURE
The basic structure of HCH is a closed chain of six carbon atoms.
The structure can have two spatial forms, i.e. cis and trans
configurations. Every carbon atom is bound to a hydrogen and a
chlorine atom. One of these substituents forms a plane with the two
connecting carbon atoms. Since this plane parallels the "equator" of
the molecule, this atom is said to be in the equatorial position. The
binding with the other atom parallels the "axis" of the molecule.
Therefore, this one is in the axial position. Due to the size of the
chlorine atom the carbon atoms are not free to rotate. Hence the
positions of the chlorine and hydrogen atoms are fixed, one being
always in the equatorial position and the other in the axial position.
The various combinations of the spatial orientation of the
hydrogen and chlorine atoms on each of the carbon atoms of cyclohexane
results in different isomeric compounds. Theoretically, 17 isomers of
HCH are possible, but, due to spatial incompatibilities and
thermodynamic instability, only nine isomers have in fact been
detected. They all have the trans configuration.
In the beta-isomer, all chlorine atoms are in the equatorial
position.
The positions of the chlorine atoms in the major isomers of HCH
are presented in Table 9 (Demozay & Marechal, 1972; Van Velsen, 1986).
Table 9. Positions of chlorine atoms in the major HCH isomers
Isomer Chlorine positionsb Physical structure
Alphaa A A E E E E monoclinic prisms
Beta E E E E E E octahedral cubic crystals
Gamma A A A E E E monoclinic crystals
Delta A E E E E E crystals/fine platelets
Epsilon A E E A E E monoclinic needles or hexagonal mono-
clinic crystals
a racemate of two optical isomers
b A = axial position; E = equatorial position From: van Velsen (1986).
RESUME ET EVALUATION
1. Alpha-hexachlorocyclohexane
1.1 Propriétés générales
L'Alpha-hexachlorocyclohexane (alpha-HCH) est un important
sous-produit (60-70%) de la fabrication du lindane (> 99% de
gamma-HCH). Il est peu soluble dans l'eau mais très soluble dans les
solvants organiques comme l'acétone, le chloroforme et le xylène.
C'est un solide de faible tension de vapeur. Son coefficient de
partage entre le n-octanol et l'eau (log Pow) est de 3,82. C'est
un polluant de l'environnement.
L'alpha-HCH peut être dosé séparément des autres isomères par
chromatographie en phase gazeuse avec détection par capture
d'électrons ou par d'autres méthodes après extraction par partage
liquide/liquide et purification sur colonne chromatographique.
1.2 Transport, distribution et transformation dans l'environnement
Il se produit dans l'environnement une biodégradation ainsi
qu'une dégradation abiotique (déchloration) sous l'action du
rayonnement ultraviolet, qui aboutissent respectivement, au
delta-3,4,5,6-tétrachlohéxène et au pentachlorocyclohéxène Ce
processus de décomposition est plus lent que dans le cas du lindane.
La persistance de l'alpha-HCH dans le sol dépend de facteurs
environnementaux comme l'action des microorganismes, la teneur en
matières organiques, la co-distillation et l'évaporation. Il n'y a pas
d'isomérisation du lindane en alpha-HCH.
La bioconcentration est rapide chez les microorganismes (facteur
de bioconcentration allant de 1500 à 2700, calculé en poids à sec ou
environ 12 000 calculé par rapport aux lipides en l'espace de 30
minutes): chez les invertébrés, il est de 60 à 2750 (poids à sec) ou
> 8000 (par rapport aux lipides) sur une durée de 24 à 72 heures.
Dans le cas des poissons ces chiffres vont de 313 à 1216 sur 4 à 28
jours, les valeurs pouvant atteindre 50 000 pour les poissons de
l'Elbe. Toutefois la biotransformation et l'élimination sont également
assez rapide chez ces organismes (15 minutes à 72 heures).
1.3 Concentrations dans l'environnement et exposition humaine
L'alpha-HCH est présent dans l'air des océans à la concentration
de 0,02 à 1,5 mg/m3. Au Canada, on en a trouvé dans l'eau de pluie
à des teneurs de 1 à 40 ng par litre, mais la neige n'en contenait que
des traces.
Au cours de la période 1969-1974, on a constaté dans le Rhin et
ses affluents des concentrations d'alpha-HCH comprises entre 0,01 et
2,7 µg/litre; toutefois, plus récemment, ces concentrations sont
descendues en-dessous de 0,01 µg/litre. Dans l'Elbe, les
concentrations sont passées de 0,023 µg/litre en moyenne en 1981, à
moins de 0,012 µg/litre en 1988. Dans un certain nombre de cours d'eau
du Royaume-Uni on a trouvé en 1966 des concentrations allant de 0,001 à
0,43 µg/litre. En Frise, dans le nord de la Mer des Wadden on a trouvé
dans les sédiments des concentrations d'alpha-HCH allant de 0,3 à
1,4 µg/kg (0,002 µg/litre d'eau).
Les teneurs en alpha-HCH de différentes espèces végétales en
provenance de divers pays vont de 0,5 à 2140 µg/kg de poids sec mais
elles peuvent être encore beaucoup plus élevées dans les régions
polluées. Même dans l'Antarctique, on a rencontré des concentrations
allant de 0,2 à 1,15 µg/kg. On trouve régulièrement du alpha-HCH
dans les poissons et les invertébrés aquatiques ainsi que chez des
canards, des hérons et des effraies. On a trouvé dans la graisse
sous-cutanée de rennes et d'orignaux vivant dans des régions où
l'épandage de pesticides est négligeable, des quantités d'alpha-HCH
égales en moyenne à 7-80 µg/kg. Dans les tissus adipeux d'ours
polaires canadiens on a trouvé des teneurs d'alpha-HCH allant de
0,3 à 0,87 mg/kg (calculées par rapport au tissu adipeux).
Dans un certain nombre de pays on a procédé à l'analyse de
denrées alimentaires importantes à la recherche d'alpha-HCH. Les
teneurs mesurées, présentes essentiellement dans des produits
contenant des graisses, allaient jusqu'à 0,05 mg/kg de produit,
sauf dans le lait et les produits laitiers (jusqu'à 0,22 mg/kg)
et dans le poisson et les produits carnés industriels (jusqu'à
0,5 mg/kg par rapport aux graisses). On a constaté un léger recul
des teneurs au cours des années.
C'est principalement par les aliments que la population dans
son ensemble est exposée à l'alpha-HCH. Des études de ration totale
effectuées aux Pays-Bas et au Royaume-Uni ont révélé des
concentrations moyennes respectivement égales à 0,01 et
0,002-0,003 mg/kg. Les données concernant le Royaume-Uni montrent
qu'il existe une tendance à la baisse depuis 1967. Aux Etats-Unis
d'Amérique, l'apport alimentaire moyen d'alpha-HCH a été de 0,09 à
0,025 mg/kg de poids corporel par jour, au cours de la période
1977-1979 et de 0,003 à 0,016 µg/kg de poids corporel au cours de
la période 1982-1984.
Dans quelques pays, on a mesuré la concentration d'alpha-HCH
dans le sang, le sérum et le plasma humain. La concentration moyenne
(dans certains cas, la médiane) était inférieure à 0,1 µg/litre
(intervalle de variation: de non décelable à 0,6 µg/litre). Dans un
des pays cependant on a relevé une concentration moyenne de
3,5 µg/litre (intervalle de variation 0,1 à 15,0) dans le tiers
environ des échantillons de sang.
Dans le tissu adipeux humain et le lait maternel, les
concentrations d'alpha-HCH qui ont été relevées sont faibles
(respectivement moins de 0,01-0,1 et moins de 0,001-0,04 mg/kg
calculées par rapport aux graisses). Des études de ration totale
ont montré que l'apport alimentaire quotidien était de l'ordre de
0,01 µg/kg de poids corporel ou moins. Ces concentrations ont
tendance à diminuer avec le temps.
L'alpha-HCH se présente comme un contaminant universel de
l'environnement. Les teneurs ne diminuent que lentement malgré les
mesures prises en vue d'en éviter la propagation dans le milieu.
1.4 Cinétique et métabolisme
Chez le rat, l'alpha-HCH est rapidement et presque complètement
résorbé au niveau des voies digestives. Après injection intra-
péritonéale, environ 40 à 80% de l'alpha-HCH administré a été
excrété dans les urines et 50 à 20% dans Les matières fécales. Chez
le rat également, les concentrations les plus élevées se rencontrent
dans le foie, les reins, les tissus adipeux, l'encéphale et les
muscles, avec une accumulation importante dans la fraction Lipidique.
On a constaté que chez des rats à la mamelle, la concentration
hépatique d'alpha-HCH était deux fois plus élevée que chez les mères.
Chez le rat également, le rapport de la concentration dans l'encéphale
à la concentration sanguine et de la concentration dans la masse
grasse à la concentration sanguine était respectivement de 120:1 et
397:1.
Chez le rat, la biotransformation de l'alpha-HCH comporte une
déchloration. Le principal métabolite urinaire est le 2,4,6-
trichlorophénol; on a également identifié d'autres métabolites tels
que le 1,2,4, le 2,3,4 et le 2,4,5-trichlorophénol ainsi que le 2,3,4,5-
et le 2,3,4,6-tétrachlorophénol. On a trouvé dans les reins de rats du
1,3,4,5,6-pentachlorocyhex-1-ène, substance dont la présence a été
également observée dans le foie de poulets lors d'études in vitro.
Dans le foie, il y a conjugaison avec le glutathion.
Le demi-vie de libération à partir de la masse grasse est de 6,9
jours chez la ratte et de 1,6 jour chez le rat.
1.5 Effets sur les êtres vivants dans leur milieu naturel
L'alpha-HCH est faiblement toxique pour les algues, la dose sans
effet observable se situant en général à 2 mg/litre.
Une étude de longue durée sur Daphnia magna a montré que la
dose sans effet observable était de 0,05 mg/litre pour cette espèce.
L'alpha-HCH est modérément toxique pour les invertébrés et les
poissons. Pour ces organismes, les valeurs de la CL(E)50 sont de
l'ordre de 1 mg/litre. Lors d'études de courte durée effectuées sur
des guppies et sur Oryzia latipes, on a constaté qu'une dose de
0,8 mg/litre était sans effet.
Lors d'études de trois mois sur Salmo gairdneri soumis à des
doses allant de 10 à 1250 mg/kg de nourriture, on n'a observé aucun
effet sur la mortalité, le comportement, la croissance ou l'activité
enzymatique du foie et du cerveau.
Des études de courte et de longue durée sur un mollus-que
(Lymnea stagnalis) ont montré que la CE50 était dans ce cas de
1200 µg/litre (déterminée d'après la mortalité et l'immobilisation des
mollusques). A la concentration de 250 µg/litre il y a eu inhibition
de la ponte. Une réduction de 50% a été notée dans le taux global de
reproduction à la concentration de 65 µg/litre.
On ne dispose d'aucune donnée concernant les effets sur les
populations et les écosystèmes.
1.6 Effets sur les animaux d'expérience et les systèmes
d'épreuve in vitro
La DL50 se situe entre 1000 et 4000 mg/kg pour la souris et
entre 500 et 4670 mg/kg de poids corporel pour le rat. Les signes
d'intoxication sont essentiellement ceux d'une stimulation du système
nerveux central.
Lors d'une étude de 90 jours sur des rats, on a constaté une
baisse de la croissance à la concentration de 250 mg/kg de nourriture.
A partir de 50 mg/kg, des modifications au niveau histologique et
enzymatique témoignaient d'une induction des enzymes. A ces doses on a
également noté des signes d'immunodépression. Il y avait déjà
accroissement du poids du foie à partir de 10 mg/kg de nourriture
(soit l'équivalent de 0,5 mg/kg de poids corporel). La dose sans effet
nocif observé se situait à 2 mg/kg de nourriture (soit l'équivalent de
0,1 mg/kg de poids corporel par jour).
Il n'y a pas eu d'études convenables de toxicité à long terme ni
d'études sur la reproduction et le pouvoir tératogène.
Des études effectuées sur diverses souches de Salmonella
typhimurium n'ont révélé aucun signe de mutagénicité, que ce soit en
présence ou en l'absence d'une activation métabolique. Les tests sur
Saccharomyces cerevisiae ont également été négatifs, toutefois la
recherche d'une synthèse non programmée de l'ADN sur des hépatocytes
de rat in vitro a donné un résultat équivoque.
On a effectué des travaux en vue de déterminer le pouvoir
cancérogène de l'alpha-HCH sur des rats et des souris à des doses
allant de 100 à 600 mg/kg de nourriture. Chez des souris, on a observé
des nodules hyperplastiques et/ou des adénomes hépatocellulaires. Dans
une des études, les doses dépassaient la dose maximale tolérable. Lors
de trois autres études, deux sur des souris et une sur des rats, on
n'a observé aucune augmentation dans l'incidence des tumeurs à des
doses allant jusqu'à 160 mg/kg de nourriture (souris) et 640 mg/kg de
nourriture (rats).
Les résultats des études sur le pouvoir d'initiation et de
promotion ainsi que sur le mode d'action de l'alpha-HCH, de même que
les tests de mutagénicité, montrent que les tumeurs induites par
l'alpha-HCH chez la souris ne sont pas d'origine génétique.
On a montré que l'alpha-HCH provoquait une nette augmentation de
l'activité des enzymes hépatiques, même à des doses de 5 mg/kg de
nourriture (soit l'équivalent de 0,25 mg/kg de poids corporel). A la
dose de 2 mg/kg de poids corporel, l'alpha-HCH n'a eu aucun effet sur
la déméthylation de l'aminopyrine ni sur la teneur du foie en ADN.
1.7 Effets sur l'homme
L'examen de travailleurs d'une usine produisant du lindane, qui
avaient été exposés pendant 7,2 années (en moyenne géométrique, avec
des limites de 1 à 30 ans), a permis de conclure qu'une exposition
professionnelle au HCH ne produit pas de signes de troubles
neurologiques ni de perturbation de la fonction neuromusculaire.
RESUME ET EVALUATION
2. Béta-hexachlorocyclohexane
2.1 Propriétés générales
Le béta-hexachlorocyclohexane (béta-HCH) est un sous-produit
(7-10%) de la fabrication du lindane (> de 99% de gamma-HCH). Peu
soluble dans l'eau, il est très soluble dans les solvants organiques
tels que l'acétone, le cyclohexane et xylène. C'est un solide de
faible tension de vapeur. Son coefficient de partage entre le
n-octanol et l'eau (log Pow) est égal à 3,80. C'est un polluant
de l'environnement.
On peut doser le béta-HCH séparément des autres isomères par
chromatographie en phase gazeuse avec détection par capture
d'électrons ainsi que par d'autres méthodes après extraction par
partage liquide/liquide et purification sur colonne chromatographique.
2.2 Transport, distribution et transformation dans l'environnement
La biodégradation et la dégradation abiotique (déchloration) sous
l'effet du rayonnement ultraviolet, produisent du
pentachlorocyclohexane, mais beaucoup plus lentement que dans le cas
du lindane (gamma-HCH).
Le béta-HCH est l'isomère le plus persistant de l'HCH. Sa
persistance dans le sol dépend de facteurs environnementaux tels que
l'action des microorganismes, la teneur en matières organiques et en
eau ainsi que la co-distillation et l'évaporation.
En raison de sa persistance, le béta-HCH subit une
bioconcentration rapide chez les invertébrés (le facteur de
bioconcentration est d'environ 125 en l'espace de trois jours), chez
les poissons (250-1500 calculé à partir du poids à sec ou environ
500 000 fois calculé sur la base des lipides en l'espace de 3 à
10 minutes), ainsi que chez les oiseaux et l'homme (environ 525). Le
béta-HCH se concentre davantage et s'élimine plus lentement que les
autres isomères de l'HCH.
2.3 Concentrations dans l'environnement et exposition humaine
On rencontre le béta-HCH dans l'air des océans à des
concentrations de 0,004 à 0,13 ng/m3.
Jusqu'en 1974, le Rhin et ses affluents présentaient des teneurs
en béta-HCH allant de 0,14 à 0,22 µg par litre, mais depuis on
constate que ces valeurs sont systématiquement inférieures à
0,12 µg/litre. Des échantillons prélevés dans la Meuse présentaient
des teneurs inférieures à 0,12 µg/litre. Dans l'Elbe, les
concentrations sont passées en moyenne de 0,009 à 0,004 µg/litre entre
1981 et 1988.
On a dosé le béta-HCH chez des oiseaux tels que les éperviers,
les faucons crécerelles, les hiboux, les hérons et les grèbes pendant
un certain nombre d'années et l'on a observé des concentrations allant
de 0,1 à 0,3 mg/kg. Chez les ours polaires on a mesuré des
concentrations allant jusqu'à 0,87 mg/kg (par rapport au tissu
adipeux) dans le foie et les graisses.
Dans quelques pays on a procédé à l'analyse de denrées
alimentaires importantes en vue d'y rechercher la présence de
béta-HCH. Les concentrations moyennes, mesurées essentiellement dans
des denrées contenant des graisses, allaient jusqu'à 0.03 mg/kg (par
rapport au contenu lipidique), mais on en a trouvé jusqu'à 4 mg/kg
(par rapport au contenu lipidique) dans des produits laitiers. Dans
les denrées non grasses, les teneurs étaient inférieures à 0,05 mg/kg
de produit. En général, ces teneurs sont en lent recul.
C'est principalement par les aliments que la population dans son
ensemble est exposée au béta-HCH. Lors d'études de ration totale
effectuées au Royaume-Uni, on a mesuré des concentrations de 0,003,
0,0005 et moins de 0,0005 mg/kg respectivement en 1966/67, 1975/77 et
1981. Aux Etats-Unis d'Amérique, l'apport moyen quotidien d'origine
alimentaire allait en 1982-84 de moins de 0,1 à 0,4 ng/kg de poids
corporel dans les différents groupes d'âge.
Dans un certain nombre de pays, on a procédé au dosage du
béta-HCH dans le sang, le sérum ou le plasma au sein de la population
générale. Les concentrations varient d'un pays à l'autre, atteignant
parfois 25 µg/litre.
De nombreuses études ont été menées afin de rechercher la
présence de béta-HCH dans les tissus adipeux humains. Les
concentrations relevées au Canada, en République fédérale d'Allemagne,
au Kenya, aux Pays-Bas et au Royaume-Uni atteignaient jusqu'à
4,4 mg/kg (par rapport au contenu lipidique). On a constaté une
augmentation progressive avec l'âge jusqu'à environ 50 ans, après quoi
les teneurs déclinaient. Dans les tissus adipeux, les concentrations
de béta-HCH sont plus élevées que celles des autres isomères,
phénomène qui traduit la tendance à l'accumulation de cette substance.
Il n'y a pas de tendance claire à la baisse des concentrations de
béta-HCH sur la période au cours de laquelle ces études ont été
effectuées. On a constaté l'existence d'une relation entre les
concentrations de béta-HCH dans les tissus adipeux et le lait maternel
d'une part, et la consommation de produits carnés, de graisses
animales et de poissons gras, d'autre part.
Dans quelques pays (Canada, République fédérale d'Allemagne,
Pays-Bas et Royaume-Uni) on a procédé au dosage du béta-HCH dans le
lait maternel et obtenu des concentrations allant de 0,1 à 0,69 mg/kg
(par rapport au contenu lipidique). Il ressort de ces analyses que la
concentration du béta-HCH est plus élevée dans le lait des femmes des
zones rurales que dans celui des femmes des zones urbaines.
Le béta-HCH apparaît comme un contaminant universel de
l'environnement. Les concentrations n'accusent qu'une très lente
tendance à la baisse malgré les mesures prises en vue d'en empêcher la
propagation dans l'environnement.
2.4 Cinétique et métabolisme
Le béta-HCH est absorbé jusqu'à 95% dans les voies digestives de
la souris, et s'accumule ensuite en majeure partie dans les tissus
adipeux. L'élimination s'effectue selon un mécanisme en deux étapes,
la demi-vie étant de 2,5 jours pour la première et de 18 jours pour la
seconde.
Une fois résorbé, le béta-HCH se répartit rapidement dans les
divers organes et tissus: foie, encéphale, reins et tissus adipeux.
Chez le rat, la concentration maximale dans le foie est atteinte en
quatre jours. Pour une concentration sanguine moyenne de 92 µg/litre
(mais également pour des concentrations de 540 et 2100 µg/litre), le
rapport des concentrations dans le cerveau et le sang d'une part et
dans le tissu adipeux et le sang d'autre part était respectivement de
2:1 et de 170:1. Après une intoxication mortelle chez l'homme par des
isomères de l'HCH, on a constaté que la concentration en béta-HCH
mesurée par rapport à la teneur du sang était de 363 dans les tissus
adipeux, de 3 dans le cerveau et de 15 dans le foie. Le béta-HCH
franchit la barrière hémo-méningée beaucoup moins facilement que les
autres isomères de la l'HCH.
Chez des souris gravides, le passage transplacentaire du béta-HCH
au foetus était d'environ 2% de la dose, mais atteignait 40% chez des
rattes gravides. Chez le rat, le passage de la mère aux ratons à la
mamelle par l'intermédiaire du lait correspondait à environ 60% de la
dose.
Chez le rat, 70% du béta-HCH est éliminé dans les 28 premiers
jours, dont un tiers par la voie urinaire. On ne retrouve pas de
béta-HCH inchangé dans l'urine. Le principal métabolite résultant de
la cis-déshydrochloration est le 2,4,6-trichlorophénol sous forme
conjuguée.
Un prétraitement au moyen de béta-HCH modifie le métabolisme du
lindane chez le rat. D'après des études comportant l'administration
de béta-HCH par voie intrapéritonéale à des souris, il semble que
celui-ci soit métabolisé plus lentement que le lindane.
2.5 Effets sur les êtres vivants dans leur milieu naturel
En général le béta-HCH est modérément toxique pour les algues,
les invertébrés et les poissons. La DL50 aiguë pour ces organismes
est de l'ordre de 1 mg/litre mais les valeurs de la CE50 sont plus
faibles (0,05-0,5 mg/litre). Dans le cas de deux espèces de poisson
d'eau douce, Oryzia latipes et Poecilia reticulata, la dose sans
effet observable a été fixée à 0,03 mg/litre sur une durée de un et
trois mois respectivement.
On ne dispose d'aucune donnée concernant les effets sur les
populations et les écosystèmes.
2.6 Effets sur les animaux d'expérience et les systèmes
d'épreuve in vitro
Les valeurs de la DL50 aiguë par voie orale pour les souris et
les rats publiées en 1968, se situaient entre 1500 et 2000 mg/kg de
poids corporel. Toutefois des études plus récentes ont fourni des
valeurs de 16 g/kg de poids corporel pour les souris et de 8 g/kg de
poids corporel pour les rats. Les signes d'intoxication sont
essentiellement neurologiques.
Des études de courte durée sur des souris avec des doses allant
jusqu'à 600 mg/kg de nourriture pendant 26 à 32 semaines, ont révélé
la présence d'une hyperplasie nodulaire et de proliférations atypiques
au niveau du foie ainsi qu'une augmentation du poids de cet organe.
Lors d'une troisième étude, consistant dans l'administration de doses
allant jusqu'à 500 mg/kg de nourriture pendant 24 semaines, on a
observé ni tumeurs hépatiques ni hyperplasie nodulaire.
Une étude de 90 jours au cours de laquelle des rats ont reçu soit
50 soit 250 mg de béta-HCH par kg de nourriture, a révélé des
altérations au niveau du foie, notamment une hypertrophie et une
prolifération du réticulum endoplasmique lisse ainsi qu'un
accroissement de l'activité des enzymes microsomiques. Aux doses les
plus élevées on a également observé des altérations au niveau des
gonades qui s'accompagnaient également d'effets graves sur le poids du
corps. Les modifications hormonales accompagnant l'atrophie des
gonades ne correspondaient pas à un effet endocrinien systématique.
Aucun effet nocif n'a été constaté à la dose de 2 mg/kg de nourriture
(soit l'équivalent de 0,1 mg/kg de poids corporel).
Une étude de longue durée sur des rats (publiée en 1950) au cours
de laquelle on a administré des doses de 10 mg/kg de nourriture (soit
l'équivalent de 0,5 mg/kg de poids corporel) ou davantage, a révélé
que ce régime conduisait à une hypertrophie du foie et à des
modifications histologiques.
Lors d'une étude de reproduction portant sur deux générations de
rats, on a observé les mêmes effets que dans l'étude de 90 jours citée
plus haut. Aucun effet n'a été observé à la dose de 2 mg/kg de
nourriture (soit l'équivalent de 0,1 mg/kg de poids corporel), mais à
la dose de 10 mg/kg, il y avait accroissement de la mortalité et de la
stérilité. L'étude a également porté sur les effets tératogènes
éventuels du béta-HCH mais aucun effet de ce genre imputable au
produit n'a été observé.
On a décrit un effet "oestrogénique" faible dont l'organe cible
serait l'utérus. En fait il n'y a pas d'effet bien net sur le système
de régulation endocrinienne. Le mécanisme et la portée de cet effet
demeurent incertains.
Les études de mutagénicité publiées ne font état d'aucune
augmentation dans la fréquence des mutations chez les souches de
Salmonella typhimurium. Une étude in vivo chez le rat sur des
cellules de moelle osseuse en métaphase a donné des résultats
positifs.
Deux études ont été effectuées sur des souris afin de déterminer
le pouvoir cancérogène du béta-HCH. Dans l'une d'elles, on a
administré pendant 110 semaines une dose de 100 mg/kg de nourriture et
l'on a observé une hyperplasie du tissu hépatique et une hypertrophie
de cet organe. Il y avait également augmentation des tumeurs bénignes
et malignes. Dans une autre étude, où la dose administrée était de
500 mg/kg de nourriture pendant une période de 24 semaines, aucune
tumeur n'a été observée.
Des études au cours desquelles des rats ont reçu des mélanges de
béta-HCH et de biphényles polychlorés donnent à penser que le béta-HCH
aurait un effet promoteur.
A la dose de 300 mg/kg de nourriture, le béta-HCH a provoqué une
altération sensible de plusieurs des fonctions du système immunitaire
en l'espace d'un mois chez la souris.
2.7 Effets sur l'homme
L'examen de travailleurs d'une usine produisant du lindane,
exposés à cette substance pendant 7,2 années (en moyenne géométrique,
avec des limites de 1 à 30 ans), a permis de conclure que l'exposition
professionelle au HCH ne produisait pas de signes d'une atteinte
neurologique ni d'une perturbation des fonctions neuromusculaires.
CONCLUSIONS ET RECOMMANDATIONS EN VUE DE LA PROTECTION DE LA SANTE
HUMAINE ET DE L'ENVIRONNEMENT (ALPHA- ET BETA-HEXACHLOROCYCLOHEXANES)
1. Conclusions
On ne peut pas comparer les effets nocifs potentiels de l'alpha-
et du béta-hexachlorocyclohexane sur l'homme et l'environnement à
leurs avantages éventuels, étant donné que ces produits n'ont aucune
action insecticide. Leur présence dans l'environnement est donc fort
préoccupante. Dans ces conditions, l'usage de produits à base d'HCH
technique contenant de fortes concentrations d'alpha- et de béta-HCH
n'est en aucun cas justifié.
1.1 Population générale
L'alpha- et le béta-HCH circulent dans l'environnement et sont
présents dans les chaînes alimentaires. Il existe donc un risque
permanent d'exposition humaine. Cette exposition est faible et devrait
lentement diminuer dans les années à venir. Dans ces conditions, il
n'y a pas lieu de s'inquiéter sérieusement pour la santé de la
population dans son ensemble.
1.2 Sous-groupes de population exposés à un risque particulier
La concentration de l'alpha-HCH dans le lait maternel est faible.
On peut se préoccuper de l'exposition des nourrissons au béta-HCH
actuellement présent dans le lait maternel mais il faut malgré tout
continuer à encourager l'allaitement maternel.
Il faut cependant faire un maximum d'efforts pour réduire
l'exposition par voie alimentaire ou autre à ces isomères. Une
moindre exposition d'origine alimentaire à ces substances devrait
entraîner une diminution de la teneur du lait maternel en alpha- et
béta-HCH.
1.3 Exposition professionnelle
Dans la mesure où elles observent les précautions recommandées en
vue réduire au minimum l'exposition à l'alpha- et au béta-HCH, les
personnes employées à la fabrication du lindane ne courent pas de
risque particulier.
1.4 Effets sur l'environnement
A part le cas de décharge dans le milieu aquatique, rien
n'indique que la présence d'alpha- et de béta-HCH dans l'environnement
constitue une menace particulière pour la faune et la flore.
2. Recommandations pour la protection de la santé humaine et de
l'environnement
a) Afin de réduire au minimum la pollution de l'environnement par
l'alpha- et le béta-HCH, il faut utiliser du lindane (> de 99% de
gamma-HCH) à la place de l'HCH technique.
b) Pour éviter la pollution de l'environnement par l'alpha- et le
béta-HCH, les sous-produits et les effluents issus de la fabrication
du lindane doivent être évacués de façon convenable et il faut en
particulier éviter la contamination des eaux et du sol.
c) Il faut poursuivre la surveillance de l'alpha- et du béta-HCH
dans les denrées alimentaires. Il est essentiel d'instituer un
mécanisme par lequel seront fixées des doses limites acceptables sur
le plan international pour l'alpha- et le béta-HCH.
d) Il faut poursuivre la surveillance de l'apport quoti-dien
d'alpha- et de béta-HCH et continuer à en contrôler les concentrations
dans le lait maternel.
RECHERCHES A EFFECTUER (ALPHA- ET BETA-HEXACHLOROCYCLOHEXANES)
Les études expérimentales suivantes sont nécessaires pour
permettre une meilleure évaluation des dangers que représentent
l'alpha- et le béta-HCH:
* études de mutagénicité portant en particulier sur les
chromosomes;
* études de reproduction, études de foetotoxicité et de
tératogénicité;
* études pharmacocinétiques et toxicocinétiques;
* études de cancérogénicité;
* études de neurotoxicité;
* surveillance des populations à risque.
RESUMEN Y EVALUACION
1. Alpha-hexaclorociclohexano
1.1 Propiedades generales
El alpha-hexaclorociclohexano (alpha-HCH) es uno de los
principales subproductos (65-70%) de la fabricación del lindano (>
99% gamma-HCH). Su solubilidad en agua es baja, pero es muy soluble
en disolventes orgánicos como la acetona, el cloroformo y el xileno.
Es una sustancia sólida con baja presión de vapor. El coeficiente de
partición n-octanol/agua (log Poa) es 3,82. Se trata de un
contaminante ambiental.
El alpha-HCH puede determinarse por separado de los otros
isómeros mediante cromatografía de gases con detección de captura
electrónica y otros métodos, tras la extracción por partición
líquido/líquido y la purificación en cromatografía de columna.
1.2 Transporte, distribución y transformación en el medio ambiente
La biodegradación y la degradación abiótica (decloración) por
irradiación ultravioleta tienen lugar en el medio ambiente y producen,
respectivamente, delta-3,4,5,6-tetraclorohexeno y pentacloro-
ciclohexeno. Este proceso de degradación es más lento que en el caso
del lindano. La persistencia del alpha-HCH en el suelo depende de
factores ambientales como la acción de los microorganismos, el
contenido de materia orgánica y la codestilación y la evaporación a
partir de los suelos. No se produce isomerización del lindano a
alpha-HCH.
En los microorganismos se produce una bioconcentración rápida (el
factor de bioconcentración es igual a 1500-2700 en peso seco, o
aproximadamente 12 000 en lípidos al cabo de 30 minutos),
invertebrados (60-2750 (peso seco) o > 8000 (lípidos) al cabo de
24-72 h), y peces (313-1216 al cabo de 4-28 días; hasta 50 000 en el
río Elba). No obstante, la biotransformación y la eliminación también
son relativamente rápidas en esos organismos (de 15 minutos a 72 h).
1.3 Niveles en el medio ambiente y exposición humana
El alpha-HCH se encuentra en el aire oceánico con una
concentración de 0,02-1,5 ng/m3. En el Canadá, se encontró en el
agua de lluvia con una concentración de 1-40 ng/litro, pero sólo se
detectaron indicios en la nieve.
Durante el periodo 1969-1974, se encontraron en el río Rin y sus
afluentes niveles de alpha-HCH de 0,01-2,7 µg por litro, pero
últimamente los niveles han sido inferiores a 0,1 µg/litro. En el río
Elba, los niveles disminuyeron desde un promedio de 0,023 mg/litro en
1981 hasta menos de 0,012 µg/litro en 1988. En 1966 se encontró que
ciertos ríos del Reino Unido contenían 0,001-0,43 µg por litro. Se ha
encontrado alpha-HCH en sedimentos de la región norte del mar de
Wadden en concentraciones de 0,3 a 1,4 µg/kg (0,002 µg/litro en el
agua).
Las concentraciones de alpha-HCH en diferentes especies vegetales
de distintos países variaron entre 0,5-2140 µg/kg en peso seco, pero
fueron mucho más altos en zonas contaminadas. Incluso en la Antártida
se han encontrado niveles que varían entre 0,2 y 1,15 µg/kg.
El alpha-HCH se detecta con regularidad en peces e invertebrados
acuáticos, así como en patos, garzas y lechuzas. En renos y alces de
Idaho, que viven en zonas en las que el uso de plaguicidas es
prácticamente insignificante, se encontraron niveles medios de
alpha-HCH de aproximadamente 70-80 µg/kg en la grasa subcutánea. El
tejido adiposo de los osos polares del Canadá contenía 0,3-0,87 mg de
alpha-HCH/kg (en grasa).
En varios países se han analizado importantes alimentos en busca
de alpha-HCH. Las concentraciones, principalmente en alimentos que
contienen grasas, variaron hasta un máximo de 0,05 mg/kg de producto,
salvo en la leche y los productos lácteos (hasta 0,22 mg/kg) y en el
pescado y en preparaciones de carne (hasta 0,5 mg/kg en grasa). Se ha
observado una ligera disminución con los años.
Los alimentos son la principal fuente de exposición de la
población general al alpha-HCH. En estudios de la dieta total
realizados en los Países Bajos y en el Reino Unido, se encontraron
concentraciones medias de 0,01 y 0,002-0,003 mg/kg de alimento,
respectivamente. Los datos procedentes del Reino Unido indican una
tendencia decreciente desde 1967. En los EE.UU., la ingesta diaria
media de alpha-HCH fue de 0,009-0,025 µg/kg de peso corporal durante
el periodo 1977-1979, y de 0,003-0,016 µg/kg de peso corporal durante
el periodo 1982-1984.
En unos pocos países, se ha determinado la concentración de
alpha-HCH en la sangre, el suero o el plasma humanos. La
concentración promedio (mediana en algunos casos) fue < 0,1 µg/litro
(desde niveles no detectables hasta 0,6 µg/litro). En un país, no
obstante, se notificó una concentración media de 3,5 (margen
0,1-15,0) µg por litro. Se detectó alpha-HCH en aproximadamente la
tercera parte de las muestras de sangre.
En el ser humano las concentraciones en el tejido adiposo y la
leche que se han comunicado son bajas (respectivamente < 0,01-0,1 y
< 0,001-0,04 mg/kg en grasa). Los estudios de la dieta total han
revelado niveles diarios de ingesta del orden de 0,01 µg/kg de peso
corporal por día o menos. Esas concentraciones están disminuyendo
poco a poco con los años.
El alpha-HCH parece ser un contaminante ambiental universal. Las
concentraciones están disminuyendo muy despacio, a pesar de las
medidas adoptadas para impedir su dispersión en el medio ambiente.
1.4 Cinética y metabolismo
En las ratas, el alpha-HCH se absorbe rápida y casi completamente
a partir del tracto gastrointestinal. Después de una inyección
intraperitoneal, aproximadamente el 40-80% del alpha-HCH se excretó en
la orina y el 5-20% en las heces. En la rata, las concentraciones más
elevadas se han encontrado en el hígado, los riñones, la grasa, el
cerebro y los músculos; el tejido adiposo constituye un importante
depósito. Las concentraciones de alpha-HCH en el hígado de las crías
lactantes duplicaron las observadas en el hígado de las madres. En la
rata, los cocientes cerebro-sangre y grasa de depósito-sangre fueron
de 120:1 y 397:1, respectivamente.
La biotransformación del alpha-HCH en la rata entraña la
decloración. El principal metabolito urinario es el
2,4,6-triclorofenol; entre otros metabolitos identificados figuran el
1,2,4-, 2,3,4-, y 2,4,5-triclorofenol y el 2,3,4,5- y
2,3,4,6-tetraclorofenol. En el riñón de rata y también en estudios
in vitro en hígado de pollo se ha encontrado 1,3,4,5,6-
pentaclorociclohex-1-eno. En el hígado se forma un conjugado de
glutatión.
En la rata, la semivida de eliminación de la sustancia presente
en del depósito graso es de 6,9 días en la hembra y 1,6 días en el
macho.
1.5 Efectos en los organismos del medio ambiente
El alpha-HCH tiene baja toxicidad para las algas, siendo por lo
general 2 mg/litro el nivel sin efectos observados.
En un estudio a largo plazo, Daphnia magna mostró un nivel sin
efectos observados de 0,05 mg/litro. El alpha-HCH es moderadamente
tóxico para los invertebrados y los peces. Los valores de la
C(E)L50 aguda para esos organismos son del orden de 1 mg/litro. En
estudios a corto plazo con Lebistes reticulatus y Oryzia latipes se
observó que 0,8 mg/litro no ejercían efecto alguno.
En estudios de tres meses de duración con Salmo gairdneri con
dosis de 10-1250 mg/kg de dieta no se observaron efectos en la
mortalidad, la conducta, el crecimiento ni las actividades enzimáticas
del hígado y el cerebro.
En estudios a corto y a largo plazo con un gasterópodo (Lymnea
stagnalis) se observó una CE50 (basada en la mortalidad y la
inmovilización) de 1200 µg/litro. La inhibición de la producción de
huevos se produjo con una concentración de 250 µg/litro. Con
65 µg/litro se observó una reducción del 50% en la reproductividad
general.
No se dispone de datos sobre los efectos en las poblaciones y los
ecosistemas.
1.6 Efectos en animales de experimentación y sistemas de ensayo
in vitro
Los valores de la DL50 aguda por vía oral en ratones se
encuentran entre 1000-4000 y en ratas entre 500 y 4670 mg/kg de peso
corporal. Los signos de envenenamiento coinciden principalmente con
los de la estimulación del sistema nervioso central.
En un estudio de 90 días de duración en ratas se observó
depresión del crecimiento con una concentración de 250 mg/kg de dieta.
Los cambios histológicos y enzimáticos en el hígado indicaron
inducción enzimática con 50 mg/kg o más. Con esas dosis se observaron
también signos de inmunosupresión. Ya se observó aumento del peso
hepático con 10 mg/kg de dieta (equivalente a 0,5 mg/kg de peso
corporal). El nivel sin efectos adversos observados resultó en este
estudio ser 2 mg/kg de dieta (equivalente a 0,1 mg/kg de peso corporal
al día).
No se han comunicado estudios adecuados a largo plazo de
toxicidad ni estudios de reproducción y teratogenicidad.
Los estudios realizados con diversas cepas de Salmonella
typhimurium no dieron prueba alguna de mutagenicidad ni con
activación metabólica ni sin ella. Los ensayos realizados con
Saccharomyces cerevisiae también dieron resultado negativo, pero un
ensayo de síntesis no programada de ADN en hepatocitos de rata in
vitro dio resultados ambiguos.
Se ha intentado determinar el potencial carcinogénico en ratones
y ratas con dosis de 100 a 600 mg/kg de dieta. En estudios realizados
en ratones se encontraron nódulos hiperplásicos y/o adenomas
hepatocelulares. En un estudio los niveles de administración
excedieron la dosis máxima tolerada. En dos estudios en ratones y uno
en ratas, en los que se administraron hasta 160 mg/kg de dieta a
ratones y 640 mg/kg de dieta a ratas, no se observó aumento alguno en
la incidencia de tumores.
Los resultados de los estudios sobre la iniciación-promoción y el
modo de acción, y los estudios de mutagenicidad indican que la
tumorigenicidad inducida por el alpha-HCH observada en ratones tiene
un mecanismo no genético.
Se ha demostrado que el alpha-HCH provoca un aumento neto de la
actividad de los enzimas hepáticos incluso con 5 mg/kg de dieta
(equivalente a 0,25 mg/kg de peso corporal). Una dosis de 2 mg/kg de
peso corporal no afectó la desmetilación de la aminopirina ni el
contenido de ADN en el hígado.
1.7 Efectos en el ser humano
Cuando se examinó a trabajadores de una fábrica de producción de
lindano, con una exposición media geométrica de 7,2 años (1-30), se
concluyó que la exposición profesional al HCH no induce síntomas de
trastornos neurales ni perturbaciones de la "función neuromuscular".
RESUMEN Y EVALUACION
2. Beta-hexaclorociclohexano
2.1 Propiedades generales
El beta-hexaclorociclohexano (beta-HCH) es un subproducto (7-10%)
de la fabricación del lindano (> 99% gamma-HCH). Su solubilidad en
agua es baja, pero es muy soluble en disolventes orgánicos como la
acetona, el ciclohexano y el xileno. Es un sólido con una baja
presión de vapor. El coeficiente de partición n-octanol/agua (log
Poa) es 3,80. Es un contaminante ambiental.
El beta-HCH puede determinarse por separado de los otros isómeros
mediante cromatografía de gases con detección de captura electrónica y
otros métodos tras la extracción por partición líquido/líquido y la
purificación en cromatografía de columna.
2.2 Transporte, distribución y transformación en el medio ambiente
La biodegradación y la degradación abiótica (decloración) por
irradiación ultravioleta tienen lugar en el medio ambiente y producen
pentaclorociclohexano, pero a una velocidad mucho menor que en el caso
del lindano (gamma-HCH).
El beta-HCH es el isómero más persistente del HCH. Su
persistencia en el suelo depende de factores ambientales como la
acción de los microorganismos, el contenido de materia orgánica y de
agua, y la codestilación y la evaporación a partir del suelo.
Dada la persistencia del beta-HCH, tiene lugar una rápida
bioconcentración en invertebrados (el factor de bioconcentración es de
aproximadamente 125 al cabo de tres días), peces (250-1500 en peso
seco o aproximadamente 500 000 veces en lípidos al cabo de 3-10 días),
aves y el hombre (aproximadamente 525). La bioconcentración es más
elevada y la eliminación más lenta en el caso del beta-HCH que en los
otros isómeros del HCH.
2.3 Niveles ambientales y exposición humana
El beta-HCH se encuentra en el aire oceánico con una
concentración de 0,004-0,13 ng/m3.
Hasta 1974, el río Rin y sus afluentes contenían niveles de
beta-HCH de 0,14-0,22 µg/litro, pero después los niveles estuvieron
siempre por debajo de 0,1 µg por litro. Las muestras tomadas en el
río Mosa también contenían < 0,1 µg/litro. En el río Elba, los
niveles descendieron desde un promedio de 0,009 hasta 0,004 µg por
litro entre 1981 y 1988.
El beta-HCH se ha medido en aves como el gavilán, el cernícalo,
el búho, la garza y el colimbo durante varios años y las
concentraciones variaron entre 0,1 y 0,3 mg/kg. Se han encontrado
hasta 0,87 mg/kg (en grasa) en el hígado y el tejido adiposo del oso
polar.
Se han analizado importantes alimentos en algunos países en busca
de beta-HCH. Las concentraciones medias, principalmente en alimentos
que contienen grasas, variaron entre 0,03 mg/kg (en grasa), pero en
los productos lácteos se encontraron niveles de hasta 4 mg/kg (en
grasa). En alimentos no grasos, los niveles fueron < 0,005 mg/kg de
producto. En general, los niveles están descendiendo lentamente.
Los alimentos son la principal fuente de exposición de la
población general al beta-HCH. En estudios de la dieta total en el
Reino Unido, se encontraron 0,003, 0,0005, y < 0,0005 mg/kg durante
los años 1966/67, 1975/77 y 1981, respectivamente. En los EE.UU., la
ingesta diaria media de beta-HCH en 1982-1984 varió entre <
0,1-0,4 ng/kg de peso corporal en distintos grupos de edad.
En varios países, la concentración de beta-HCH se ha determinado
en la sangre, el suero o el plasma de la población general. Las
concentraciones variaron entre los distintos países y el máximo
encontrado fue de 25 µg por litro.
Se han llevado a cabo numerosos estudios para determinar la
presencia de beta-HCH en los tejidos adiposos humanos. Las
concentraciones encontradas en el Canadá, Kenya, los Países Bajos, el
Reino Unido, y la República Federal de Alemania, variaron hasta
4,4 mg/kg (en grasa). Se encontró que hasta los 50 años se produce un
aumento gradual con la edad; en adelante, los niveles disminuyen. Las
concentraciones de beta-HCH en los tejidos adiposos son más altas que
las de los otros isómeros del HCH, fenómeno que refleja las
propiedades acumulativas del beta-HCH. En general no se ha observado
una tendencia clara de disminución de las concentraciones de beta-HCH
durante el periodo en que se han hecho los estudios. Existe una
relación entre las concentraciones en el tejido adiposo y la leche
materna y el consumo de productos cárnicos, grasas animales y pescados
grasos.
En unos pocos países (Canadá, Países Bajos, Reino Unido y
República Federal de Alemania), se ha analizado la leche humana y se
han encontrado niveles de beta-HCH entre 0,1 y 0,69 mg/kg (en grasa).
Los niveles medidos en la leche de mujeres de zonas rurales parece ser
más elevado que en las de zonas urbanas.
Los elevados niveles de beta-HCH que se han encontrado en la
leche materna exceden las concentraciones permisibles a título
temporal y local. Las concentraciones de beta-HCH en la sangre de
lactantes se encuentran entre los mismos límites que las medidas en
las madres.
El beta-HCH parece ser un contaminante ambiental universal. Las
concentraciones están disminuyendo muy despacio a pesar de las medidas
adoptadas para evitar su dispersión en el medio ambiente.
2.4 Cinética y metabolismo
Hasta el 95% del beta-HCH en el tracto gastrointestinal del ratón
es absorbido y a continuación se acumula en su mayor parte en el
tejido adiposo. La eliminación sigue un mecanismo de dos etapas;
durante la primera, la semivida es de 2,5 días y durante la segunda,
18 días.
Después de la absorción, el beta-HCH se distribuye rápidamente al
hígado, el cerebro, los riñones y los tejidos adiposos. En la rata,
la concentración máxima en el hígado se alcanza al cabo de cuatro
días. Con una concentración sanguínea media de 92 µg/litro (pero
también con concentraciones de 540 y 2100 µg/litro), los cocientes
cerebro-sangre y tejido adiposo-sangre fueron 2:1 y 170:1,
respectivamente. Tras el envenenamiento agudo y mortal de un hombre
con isómeros de HCH, la concentración de beta-HCH, en relación con la
de la sangre, fue de 363 en la grasa, 3 en el cerebro y 15 en el
hígado. El beta-HCH atraviesa la barrera hematoencefálica con mucha
menos facilidad que los demás isómeros del HCH.
En el ratón, el paso transplacentario de la hembra gestante al
feto fue de aproximadamente el 2% de la dosis, pero en la rata se
observó un paso del 40%. En la rata, la transferencia de la madre al
lactante en la leche fue de aproximadamente el 60% de la dosis.
En la rata, el 70% del beta-HCH se elimina durante 28 días; un
tercio de esa cantidad se excreta en la orina. No aparece beta-HCH
sin modificar en la orina. El principal metabolito procedente de la
cis-deshidrocloración es el 2,4,6-triclorofenol en forma conjugada.
El pretratamiento con beta-HCH altera el metabolismo del lindano
en las ratas. Según estudios intraperitoneales realizados en ratones,
parece que el beta-HCH se metaboliza con más lentitud que el lindano.
2.5 Efectos en los organismos del medio ambiente
En general, el beta-HCH tiene una toxicidad moderada para las
algas, los invertebrados y los peces. Los valores de la DL50 aguda
para esos organismos son del orden de 1 mg/litro, pero los valores de
la CE50 son más bajos (0,05-0,5 mg/litro). El nivel sin efectos
observados en Oryzia latipes y Poecilia reticulata, dos peces de
agua dulce expuestos durante 1 ó 3 meses, fue de 0,03 mg por litro.
No se dispone de datos sobre los efectos en las poblaciones y los
ecosistemas.
2.6 Efectos en animales de experimentación y sistemas de ensayo
in vitro
Los valores de DL50 aguda por vía oral en ratones y ratas
comunicados en 1968 se encontraban entre 1500 y 2000 mg/kg de peso
corporal. No obstante, en estudios más recientes se han obtenido
valores de 16 g/kg de peso cor poral en ratones y 8 g/kg de peso
corporal en ratas. Los signos de intoxicación fueron principalmente
de origen neural.
En dos estudios en ratones a corto plazo, con dosis de hasta
600 mg/kg de dieta durante 26-32 semanas, se observó un aumento del
peso hepático, así como hiperplasia nodular y proliferaciones atípicas
en el hígado. En un tercer estudio, la administración de hasta
500 mg/kg de dieta durante 24 semanas no produjo tumores hepáticos ni
hiperplasia nodular.
En un estudio a 90 días con ratas a las que se administraron 50 ó
250 mg/kg de dieta se observaron cambios hepáticos, a saber,
hipertrofia y proliferación del retículo endoplásmico liso y mayor
actividad de los enzimas microsómicas. Con las dosis más altas se
produjeron cambios en las gónadas pero éstos estuvieron asociados a
modificaciones muy acusadas del peso corporal. Los cambios hormonales
asociados a la atrofia gonadal no mostraron un efecto endocrino
consecuente. No se observaron efectos adversos con una dosis de
2 mg/kg de dieta (equivalente a 0,1 mg/kg de peso corporal).
En un estudio en ratas a largo plazo (comunicado en 1950), la
administración de dosis de 10 mg/kg de dieta (equivalente a 0,5 mg/kg
de peso corporal) o superiores produjo dilatación y cambios
histológicos en el hígado.
En un estudio de reproducción de ratas en dos generaciones, se
observaron los mismos efectos que en el estudio de 90 días. No se
observaron efectos con 2 mg/kg de dieta (equivalente a 0,1 mg/kg de
peso corporal), pero con una dosis de 10 mg/kg de dieta aumentaron la
mortalidad y la infecundidad. En una ampliación de este estudio no se
observaron efectos teratogénicos relacionados con el compuesto.
Se ha descrito un ligero efecto "estrogénico". El órgano diana
de este efecto era el útero; no se apreciaron efectos claros en los
sistemas de control endocrino. No se sabe con seguridad cuál es el
mecanismo ni el significado de este efecto.
Los estudios de mutagenicidad comunicados no mostraron aumento
alguno en la frecuencia de mutaciones en cepas de Salmonella
typhimurium. En un análisis in vivo de la metafase en médula ósea
de ratas se obtuvieron resultados positivos.
Se han llevado a cabo dos estudios en el ratón para determinar el
potencial carcinogénico. En uno de los estudios, se administraron
200 mg/kg de dieta durante 110 semanas, y se notificaron dilatación
del hígado, cambios hiperplásicos y aumento de tumores tanto benignos
como malignos. En el otro estudio, en el que se administraron
500 mg/kg de dieta durante 24 semanas, no se observaron tumores.
En estudios en los que se administró a ratas combinaciones de
beta-HCH con bifenilos policlorados se sugirió que el beta-HCH tenía
un efecto de promoción.
Con 300 mg/kg de dieta, el beta-HCH provocó cambios
significativos en varias funciones inmunitarias en el ratón al cabo de
un mes.
2.7 Efectos en el ser humano
Cuando se examinó a trabajadores de una fábrica de producción de
lindano, con una exposición media geométrica de 7,2 años (1-30), se
concluyó que la exposición profesional al HCH no induce signos de
trastornos neurales ni de perturbación de la "función neuromuscular".
CONCLUSIONES Y RECOMENDACIONES PARA LA PROTECCION DE LA SALUD
HUMANA Y DEL MEDIO AMBIENTE (ALPHA- Y BETA-HEXACLOROCICLOHEXANOS)
1. Conclusiones
Los efectos adversos potenciales del alpha- y el beta-
hexaclorociclohexano (HCH) en el ser humano y el medio ambiente
no pueden sopesarse frente a sus beneficios, puesto que estos isómeros
no tienen acción insecticida. Su presencia en el medio ambiente es
por tanto causa de gran inquietud. En consecuencia, en ningún caso se
justifica el uso de productos técnicos del HCH que contengan elevadas
concentraciones de alpha- y beta-HCH.
1.1 Población general
El alpha- y el beta-HCH circulan en el medio ambiente y están
presentes en las cadenas alimentarias. Así pues, existe un potencial
continuo de exposición humana. Esta exposición es baja y se espera
que disminuya lentamente en los años por venir. Así pues, no hay
motivos de gran inquietud en cuanto a la salud de la población
general.
1.2 Subpoblaciones especialmente expuestas
Las concentraciones de alpha-HCH en la leche humana son bajas.
La exposición de lactantes debida a las actuales concentraciones
de beta-HCH en la leche materna es preocupante, pero no suficiente
para dejar de fomentar la lactancia natural.
No obstante, debe hacerse todo lo posible para disminuir la
exposición a esos isómeros por la vía alimentaria y por toda otra vía.
Se espera que la menor exposición por la dieta dé como resultado
menores niveles de alpha- y beta-HCH en la leche humana.
1.3 Exposición profesional
Mientras se observen las precauciones recomendadas para reducir
al mínimo la exposición del personal que tra-baja en la fabricación
del lindano, el alpha- y el beta-HCH no plantean riesgos para la salud
de los operarios.
1.4 Efectos en el medio ambiente
Aparte de los vertidos en el medio acuático, no hay pruebas que
sugieran que la presencia de alpha- y beta-HCH en el medio ambiente
plantee un riesgo significativo para las poblaciones de seres vivos.
2. Recomendaciones para la protección de la salud humana y el medio
ambiente
a) A fin de reducir al mínimo la contaminación ambiental con alpha-
y beta-HCH, debe usarse lindano (< 99% gamma-HCH) en lugar de HCH
técnico.
b) A fin de evitar la contaminación ambiental con alpha- y beta-HCH,
los subproductos y los efluentes de la fabricación del lindano deben
evacuarse de modo apropiado, y debe evitarse la contaminación de aguas
naturales y del suelo.
c) Debe proseguir la vigilancia del alpha- y del beta-HCH en los
alimentos. Es imprescindible poner en marcha un mecanismo para
establecer niveles internacionalmente aceptables de alpha- y beta-HCH
en los alimentos.
d) Debe proseguir la vigilancia de la ingesta diaria de la población
general y de los niveles de alpha- y beta-HCH en la leche materna.
OTRAS INVESTIGACIONES (ALPHA- Y BETA-HEXACLOROCICLOHEXANOS)
Deben hacerse los estudios siguientes para evaluar mejor los
riesgos del alpha- y el beta-HCH:
* oestudios de mutagenicidad, especialmente con puntos
terminales mutagénicos en los cromosomas;
* oestudios de reproducción y fetotoxicidad/teratogenicidad;
* oestudios farmacocinéticos y toxicocinéticos;
* oestudios de carcinogenicidad;
* oestudios de neurotoxicidad;
* oestudios de vigilancia de poblaciones en riesgo.
Relative
molecular mass 290.9
CAS chemical 1alpha,2ß,3alpha,4ß,5alpha,6ß-hexachloro-
name cyclohexane
Common
synonym Beta-benzenehexachloride (beta-BHC)
CAS registry
number 319-85-7
RTECS registry
number GV4375000
2.2 Physical and chemical properties
Some physical and chemical properties are summarized in Table 6.
Table 6. Some physical and chemical properties of beta-
hexachlorocyclohexane
Melting point 309°C
Vapour pressure (20°C) 0.67 Pa (0.005 mmHg)
Relative density (20°C) 1.89 g/cm3
Solubility
water (20°C) 1.5 mg/litre
water (28°C) 0.2 mg/litre
organic solvents (20°C)
acetone 103.9 g/litre
chloroform 3 g/litre
ethanol 11 g/litre
petroleum ether 1-2 g/litre
xylene 33 g/litre
cyclohexane 121 g/litre
Stability considerable stability in acids,
unstable in alkaline conditions
n-Octanol/water partition
coefficient (log Pow) 3.80
2.3 Analytical methods
The same methods can be used as for alpha-HCH (see section 2.3
alpha-HCH).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Beta-HCH does not occur naturally. It is released to the
environment as a result of the use of technical-grade HCH and the
inappropriate disposal of the residue resulting from the purification
of lindane.
Beta-HCH is basically a by-product (and impurity) in the
manufacturing of lindane (> 99% gamma-HCH) (van Velsen, 1986).
Technical-grade HCH, which is synthesized from benzene and chlorine in
the presence of ultraviolet light, consists of:
65-70% alpha-HCH
7-10% beta-HCH
14-15% gamma-HCH (lindane)
approx.7% delta-HCH
approx.1-2% epsilon-HCH
approx.1-2% other components
Purification of lindane produces a residue, consisting almost
entirely of non-insecticidal HCH isomers (mainly alpha- and beta-),
which can be used as an intermediate for the production of
trichlorobenzene and other chemicals.
Alpha- and beta-HCH have been used in mixtures with gamma-HCH (as
"HCH" or "fortified HCH") in agriculture and in wood protection.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
Tsukano (1973) studied the factors affecting the disappearance of
beta-HCH from rice field soil. After granular application of
technical-grade HCH (0.05 mg/litre) into surface water, beta-HCH
disappeared very slowly (half-life > 28 days). After the
translocation of beta-HCH (1 mg/litre) onto flooded levelled soil, the
surface water and soil was analysed at intervals. A decrease in the
beta-HCH concentration in water and a steady increase in soil was
found. After 7 days a maximum concentration in soil was reached.
From a soil column study it was found that beta-HCH did not move
through the soil.
Suzuki et al. (1975) studied the persistence of beta-HCH in three
different types of soils. Beta-HCH is the most persistent isomer of
HCH, the persistence being dependent on environmental factors such as
the action of soil microorganisms, co-distillation, and evaporation
from soil. Furthermore, the water content and the content of organic
matter in the soil are of importance.
Siddaramappa & Sethunathan (1975) studied the persistence of
14C-labelled beta-HCH in five Indian rice soils under flooded
conditions, using incubation times of 0, 20, and 41 days. The
degradation of beta-HCH was much slower than that of lindane. However,
there was a great difference in the degradation rates between the
soils. In two types of soils (sandy and kari soils) both isomers
persisted even after 41 days of flooding.
Sorption and desorption of beta-HCH by 12 soils from rice-growing
areas in India were studied using a 14C-label. The soils showed
striking differences in their ability to adsorb beta-HCH, the sorption
values ranging from 46 to 96% of total added beta-HCH. After oxidation
of the soil with hydrogen peroxide, the sorption was lower (14-58%).
Organic matter was the most important factor governing the sorption
and desorption, but pH, exchange acidity, exchangeable sodium and
magnesium, and electrical conductivity also affected the results
(Wahid & Sethunathan, 1979).
Kampe (1980) concluded from experimental data that the transport
of beta-HCH to ground water is unlikely, owing to low water solubility
and anaerobic degradation.
Korte (1980) summarized the behaviour of beta-HCH in the
environment, especially in soil and plants.
4.2 Biotransformation and bioaccumulation
4.2.1 Biodegradation
MacRae et al. (1967) studied the persistence and biodegradability
of beta-HCH in two clay soils. Beta-HCH was applied at a level of
15 mg/kg soil and the incubation periods were 0, 15, 30, 50, 70, and
90 days. In non-sterilized soils only very small amounts could be
detected after 70 days, indicating biodegradation, whereas in
sterilized soils the losses were much slower and probably due to
volatilization.
In studies using either mixed or pure bacterial cultures under
anaerobic or aerobic conditions, the dechlorination of 36Cl-labelled
beta-HCH by mixed soil flora and by pure cultures of Citrobacter
freundii, C. butyricum, and C. pasteurianum was 7.4, 15.3, 23.8,
and 10.1%, respectively, within 6 days of incubation. Aerobically
grown facultative anaerobes dechlorinated actively. Beta-HCH degraded
more slowly than lindane (Jagnow et al., 1977; Haider, 1979).
Cell suspensions of Clostridium sphenoides cultured under
anaerobic conditions did not degrade beta-HCH (10 mg/litre) within
24 h. Even with more concentrated cell suspensions of the organism
and conditions most conducive to lindane degradation (pH 8.0, 40°C),
there was no indication of any degradation (Heritage & MacRae, 1979).
MacRae et al. (1984) carried out laboratory studies on the
transformation of beta-HCH (dosage: 20 mg beta-HCH/g of soil) in a
Japanese soil containing 4% organic carbon under both aerobic and
anaerobic conditions. From the transformation rates, half-life values
of 91 and 122 days, respectively, were calculated.
In a study by Doelman et al. (1988a), microbial soil sanitation
was applied to calcareous alkaline sandy loam soil that was polluted
with a mixture of HCH isomers. Under anaerobic conditions, microbial
degradation in the Dutch climate (soil temperature of 5-17°C) did not
occur, and even the low concentration of the easily degradable
gamma-HCH did not decrease. Microbial soil sanitation of
beta-HCH-polluted sandy loam soil systems have been investigated. The
soil systems involved were aerated moist soil and continuously aerated
and intermittently aerated thick soil slurry. Degradation of beta-HCH
did not take place during a 40-week incubation period (Doelman et al.,
1988b).
A field investigation into the distribution of HCHs was carried
out by Chessells et al. (1988) using soil from an agricultural area
treated with BHC-20 (HCH composition: 70% alpha-HCH, 6.5% beta-HCH,
13.5% gamma-HCH, and 5% delta-HCH. The beta-HCH concentration
decreased only very slowly, probably owing to its comparatively high
stability and low water solubility. Furthermore, soil organic carbon
content was found to be of primary importance. A significant decrease
in isomer concentration was observed when soil moisture content was
high and was attributed to microbial degradation favoured by these
conditions.
4.2.2 Abiotic degradation
Ultraviolet irradiation, using a 15-watt low pressure mercury
lamp, of beta-HCH in 2-propanol solution for 16 h resulted in the
production of an isomer of pentachloro-cyclohexene. This substance
seems to be formed by the migration of an equatorial chlorine atom to
the vicinal axial position at the intermediate pentachlorocyclohexyl
radical (Hamada et al., 1982).
4.2.3 Bioaccumulation
4.2.3.1 Aquatic invertebrates
In a study by Yamato et al. (1983), short-necked clam (Venerupis
japonica) rapidly absorbed beta-HCH and the concentration reached a
plateau on the third day. The bioconcentration factor was 127 at a
beta-HCH concentration in water of 2 µg/litre. The beta-HCH
concentrations on day 6 in internal organs and tissues were 0.194 and
0.076 mg/kg, respectively. After a 3-day elimination period, the
levels were 0.115 and 0.075 mg/kg, respectively.
4.2.3.2 Fish
Sugiura et al. (1979) studied bioaccumulation in the carp
(Cyprinus carpio), brown trout (Salmo trutta fario), golden orfe
(Leuciscus idus melanotus), and guppy (Poecilia reticulata).
Beta-HCH was dissolved in water to a concentration of 1 mg/litre under
steady-state conditions (time period not specified), and the
equilibrium bioconcentration factors for the four types of fish were
273, 658, 973, and 1485, respectively.
In a study by Yamato et al. (1983), guppies (Poecilia
reticulata) rapidly bioaccumulated beta-HCH and the tissue
concentration reached a plateau on the fourth day. The beta-HCH
concentration in the water was 2 µg/litre and the bioconcentration
factor 1043. The concentration in the guppy slowly decreased on the
first day after the fish were transferred to HCH-free water.
In general, the equilibrium levels were reached within 3-10 days.
A bioconcentration factor of 100 000 to 500 000 has been calculated
using data on the concentration of beta-HCH in the muscle and fat of
bream collected in the River Elbe (Arbeitsgemeinschaft für die
Reinhaltung der Elbe, 1982).
4.2.3.3 Birds
When low levels of HCH were administered together with other
organochloropesticides in the feed to broilers for 6-16 weeks, of the
three HCH isomers tested (alpha, beta, and gamma), beta-HCH showed the
greatest bioaccumulation (the mean bioconcentration factors for eggs
and fat were 13 and 15, respectively). The half-life (after
administration of uncontaminated food for 12 weeks) was about 6-8
weeks (Kan & Jonker-den Rooyen, 1978a,b; Kan et al., 1978).
This relatively higher accumulation of beta-HCH was also observed
in chickens after feeding diets fortified with 1 mg beta-HCH/kg for 4
weeks. The order of the degradation rate for the four HCH isomers was
delta > gamma > alpha > beta. Biotransformation to one or more of
the other HCH isomers did not occur (Szokolay et al., 1977a).
4.2.3.4 Bioaccumulation in humans
Geyer et al. (1986) found that in industrialized countries more
than 90% of the non-occupation exposure to HCHs derives from food.
The mean concentration (on a fat basis) of beta-HCH in human adipose
tissue was found to be 0.33-0.38, 0.40, 0.31, 0.90, 0.27, and
0.31 mg/kg in the Federal Republic of Germany, the Netherlands, USSR,
Switzerland, USA, and United Kingdom, respectively. The mean
bioconcentration factor (on a lipid basis), calculated on the basis of
the concentration in the diet (0.68, 0.62, 1.0, 1.21, 0.56, and
0.67 µg/kg, respectively) and the levels in adipose tissue, was 527.0
± 140 (range 310-744).
4.3 Isomerization
Deo et al. (1980, 1981) studied the isomerization of beta-HCH by
shaking it with distilled water at 25°C for various time intervals
(5 min to 4 days). The results of GLC analysis of the extracts
indicated that a small portion of the beta-HCH isomerized into alpha-,
gamma-, and delta-HCH. There were indications that other compounds
were also formed by reactions such as dehydrochlorination. Toxicity
studies with mosquito larvae, flour beetle larvae, and houseflies
exposed to the extracts demonstrated that the resulting aqueous
solution contained substances that were more toxic than beta-HCH.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Tanabe et al. (1982) found an average of 0.03 ng beta-HCH/m3
(0.004-0.13 ng/m3) in 24 samples of air over the Western Pacific,
Eastern Indian, and Antarctic Oceans.
The concentrations of beta-HCH measured in the air of Delft,
the Netherlands, in 1979-1980 were below the limit of detection
(2-3 pg/m3) (Slooff & Matthijsen, 1988).
5.1.2 Water
5.1.2.1 Fresh water
During the period 1969-1977, 1826 water samples were taken at 99
sampling sites in the Netherlands. The highest concentrations of
beta-HCH were found in the River Rhine and its tributaries. The
concentrations during the period 1969-1974 were 0.14-0.22 µg/litre,
but from 1974 on, the concentrations were all below 0.1 µg/litre. A
sampling trip by boat made along the River Rhine from Rheinfelden in
Switzerland to Rotterdam in the Netherlands proved that the source of
alpha-, beta-, and gamma-HCH was located in the upper Rhine. In the
River Meuse, the levels were all below 0.1 µg/litre during the period
1969-1977 (Wegman & Greve, 1980). Since 1983 the contents of beta-HCH
in the Rivers Rhine, Meuse, and West-Scheldt and in other surface
waters in the Netherlands have generally been below 0.001 µg/litre
(Slooff & Matthijsen, 1988). The average concentration of dissolved
beta-HCH in the Meuse-Rhine estuary in 1974 was 6 ng/litre whereas
that of suspended beta-HCH was < 1 to 3 ng/litre (Slooff &
Matthijsen, 1988).
The Arbeitsgemeinschaft der Elbe (the Elbe Study Group)
investigated the presence of beta-HCH in the River Elbe from
Schnackenburg to the North Sea in 1981-1982 and found a mean
concentration of 0.009 (< 0.001-0.072) µg per litre. During the
period February to November 1988 the concentrations varied from 0.001
to 0.009 µg per litre (Arbeitsgemeinschaft der Elbe, 1988).
In the surface water of the Upper Rhine, the beta-HCH
concentration was 200 ng/litre in 1974 but decreased in 1976-1977 to
2-25 ng/litre (Hildebrandt et al., 1986). LWA (1987) failed to detect
beta-HCH in the River Rhine (three locations) and in six tributaries.
5.1.2.2 Sea water
Beta-HCH was detected in the North Sea at a level of 1.4 µg/litre
in 1972 (Mestres, 1974), but in June-July 1986 the levels in surface
water (5 m) were < 0.03-0.2 ng/litre (Umweltbundesamt, 1989).
The concentration of beta-HCH in the Japan Sea and the Pacific
Ocean around Japan was below the detection limit of 0.1 µg/litre in
1974 (personal communications by A. Hamada and by T. Onishi to the
IPCS, July 1989).
5.1.3 Soil/sediment
In 1974 beta-HCH was found in 9 out of 60 sediment samples
collected in Japan, the range of concentration being 30-50 µg/kg
(personal communications by A. Hamada and by T. Onishi to the IPCS,
July 1989).
Slooff & Matthijsen (1988) analysed sediments from eight
different locations close to dumping places in the Netherlands for the
presence of alpha-, beta-, and gamma-HCH and obtained median values
for beta-HCH of 9-214 µg per kg dry matter.
5.1.3.1 Dumping grounds
In the Netherlands soil has been polluted with HCHs at various
location as the result of their manufacture in the 1950s (spillage
during production, storage, and handling), and concentrations up to a
few grams of HCHs/kg dry soil have been found. Further pollution has
been caused by both the dumping of chemical waste and its use in the
levelling of certain areas. From these dumping areas dispersal of the
chemical waste can occur by leaching or wind erosion from open storage
depots. In certain polluted areas, high concentrations of HCHs,
mainly the alpha and beta-isomers, have been found more than 2 m below
ground level. In 18 locations in the Netherlands, the average
concentrations of beta-HCH in sewage sludge in 1981 were between 30
and 150 µg/kg dry matter. Pollution of ground water also occurred,
but this was restricted to the vicinity of the production areas.
Horizontal transportation of HCHs in ground water appeared to be
limited (Slooff & Matthijsen, 1988).
5.1.4 Food and feed
The concentration of beta-HCH has been determined in a number of
important food items in France. The mean concentration was 0.03
(nd-0.25) mg/kg in milk and milk products (2688 samples), 0.02
(nd-0.04) mg/kg in meat (27 samples), 0.01 (nd-0.03) mg/kg in meat
products (34 samples), and 0.01 (nd-0.1) mg/kg in animal fat
(67 samples). In other food items, beta-HCH was not detected
(<0.005 mg/kg) (Laugel, 1981).
In a survey of milk contamination carried out in various areas of
Japan in 1970, the average beta-HCH content in cow's milk ranged from
0.009 mg/litre in the Hokkaido area to 1.288 mg/litre in the Nagasaki
area (Matsushima, 1972).
Table 7 gives the mean beta-HCH levels in a large number of
samples of various food items from the Federal Republic of Germany
reported by Hildebrandt et al. (1986).
Skaftason & Johannesson (1979) analysed a total of 32 samples of
butter from Iceland between 1974 and 1978 and found beta-HCH, at a
mean concentration of 23 ± 16 µg per kg, in 31 out of 32 samples.
In six samples of cow's milk collected from six locations in
Switzerland, the levels of beta-HCH were 1.0-4.0 mg/kg on a fat basis
(Rappe et al., 1987).
In a study carried out in the United Kingdom, 24 samples of each
food group were analysed. Bread, other cereal products, meat products,
fish, oils and fats, eggs, green vegetables, potatoes, other
vegetables, and fresh fruit contained no detectable amounts of
beta-HCH. Carcass meat contained < 0.0005 (nd-0.003) mg/kg, offals <
0.0005 (nd-0.003) mg/kg, poultry 0.008 (nd-0.08) mg/kg, milk < 0.0005
(nd-0.001) mg/kg, and dairy products 0.001 (nd-0.008) mg/kg. Imported
meat products collected in 1981-1983 contained up to 1.4 mg/kg
product, imported retail cereal products collected in 1982 contained
up to 0.03 mg/kg and animal feed collected in 1984 contained up to
0.08 mg/kg (HMSO, 1986).
No beta-HCH was found in meat and poultry products including
eggs (976 samples) collected during 1984-1986. Peanut butter and
vegetable oils (in total 95 samples) showed mean beta-HCH levels of
0.01-0.02 mg/kg product, whereas processed pork and poultry products
collected in 1985-1987 contained mean levels of 0.2 and 1.9 mg/kg,
respectively. Twenty-six out of 86 samples were positive and the
highest level that was found was 6.3 mg/kg. Other processed meat
products (631 samples) contained up to 0.01 mg/kg product. In
1984-1987, retail milk and dairy products were analysed, and 499 out
of 849 samples contained beta-HCH at a mean concentration of
0.01-0.03 mg/kg product (the highest level was 0.08 mg/kg). Samples of
eel muscle (1124 eels from 62 sites) collected during 1986-1987 showed
mean beta-HCH concentration of up to 0.02 mg/kg. The highest level
found was 0.05 mg/kg (HMSO, 1989).
Table 7. Beta-hexachlorocyclohexane concentrations (mg/kg) in various
food itemsa
Food items 1973-78 1979-83 1973-83
Meatb 0.01-0.083
(0.26)d
Meat productsb 0.003-0.055
(0.15)d
Animal fatb 0.003-0.024
(0.075)d
Gameb 0.025-0.285
Poultryb 0.001-0.016
(0.42)d
Chicken eggs 0.001
Fish 0.001-0.007
Milk and milk
productsb 0.05 < 0.01
Butterb,c < 0.01-0.02
Cereals up to 0.001
Cereal products up to 0.01
a From: Hildebrandt et al. (1986).
b Determinations made on a fat basis
c Anon (1984)
d maximum value
5.1.5 Terrestrial and aquatic organisms
5.1.5.1 Aquatic organism
Mouvet et al. (1985) measured the presence of beta-HCH in the
aquatic mossCinclidotus danubicusin order to examine its potential use
as an indicator of chlorinated pollutants in fresh water. The level in
water 4 km down-stream of an industrial discharge was 0.5-2.6 µg per
litre, while the levels in moss 0, 13, 24, and 51 days after
transplant to the polluted river were < 0.025, 0.025-0.33,
0.025-1.29, and 0.4 mg/kg dry weight, respectively.
Bream collected from various locations in the River Elbe (between
Schnackenburg and the North Sea) contained beta-HCH levels of
0.008-0.063 mg/kg in muscle tissue and 0.7-2.8 mg/kg in adipose tissue
(Arbeitsgemeinschaft für die Reinhaltung der Elbe, 1982).
Freshwater fish from different rivers in the Federal Republic of
Germany were analysed during the period 1973-1981. In the first 3-4
years the beta-HCH levels were mainly between 0.01-0.02 mg/kg fresh
weight. However, a decrease then took place and most of the samples
were below 0.01 mg/kg fresh weight, with the exception of certain
types of fish such as the eel and fish from industrially contaminated
areas. In 1981-1983, shell-fish and molluscs were analysed in the
Federal Republic of Germany, and the beta-HCH concentration ranged
from < 0.001 to 0.011 mg/kg fresh weight (Hildebrandt et al., 1986).
5.1.5.2 Birds
Organochlorine pesticides were determined in the livers of
predatory birds in the United Kingdom during 1963-1966. The average
residues (arithmetic means) of beta-HCH found are given in Table 8.
5.1.5.3 Mammals
Skaftason & Johannesson (1979) analysed samples of body fat from
10-year-old sheep, collected in 1974 in Iceland, and found an average
of 79 ± 48 µg beta-HCH/kg.
Norström et al. (1988) determined the contamination of Canadian
arctic and subarctic marine ecosystems by analysing the adipose tissue
and liver of polar bears ( Ursus maritimus; 6-20 animals per area)
collected from 12 areas between 1982-1984. Of the total HCH in adipose
tissue, 29% was beta-HCH (0.3-0.87 mg/kg on a fat basis).
Table 8. Residues of beta-hexachlorocyclohexane in the livers of
predatory birdsa
Species Year Number of Concentration
samples (mg/kg)
Sparrowhawk 1963 11 0.3
1964 8 0.23
1965 9 0.25
Kestrel 1964 28 0.1
1965 60 0.01
Tawny owl 1963 12 0.02
1965 29 0.01
Barn owl 1964 23 0.07
Heron (adults) 1964 17 0.1
Heron (nestlings) 1965 20 0.005
Great crested grebe 1963/1966 15 0.1
a From: HMSO (1969)
5.2 General population exposure
From the data presented in section 5.1 it is evident that food is
the main source of exposure of the general population to beta-HCH.
5.2.1 Total-diet studies
In a total-diet study carried out in the United Kingdom during
1966-1985, food purchased in 21 towns throughout the country was
prepared by cooking. The study covered 20 to 24 food groups, and the
number of total-diet samples examined varied from 22 to 25 samples.
The calculated mean beta-HCH residue levels in the total diet for the
periods 1966-1967, 1970-1971, 1974-1975, 1975-1977, 1979-1980, 1981,
and 1984-1985 were 0.003, 0.001, 0.0005, 0.005, 0.001, < 0.0005, and
< 0.0006 mg/kg, respectively (Egan & Hubbard, 1975; HMSO, 1982, 1986,
1989).
Samples consisting of 50 items of infant food and 110 items of
toddler food were collected in 1978-1979 in 10 USA cities. The daily
intake of beta-HCH in 1977, 1978, and 1979 in infant food was below
the limit of detection. In toddler food beta-HCH was only detectable
in 1977, the daily intake being 0.002 µg/kg body weight per day
(Gartrell et al., 1985b).
Total-diet studies conducted by the FDA in the USA before 1982
were based on a "composite sample approach" regardless of the diet
involved. Later on they were based on dietary survey information and
allowed the "total diet" of the population to be represented by a
relatively small number of food items for a greater number of age-sex
groups. The daily intakes of beta-HCH during 1982-1984 for the age
groups 6-11 months, 2 years, 14-16-year-old females, 14-16-year-old
males, 25-30-year-old females, 25-30-year-old males, 60-65-year-old
females and 60-65-year-old males were < 0.1, 0.3, 0.2, 0.2, 0.2,
0.4, 0.2, and 0.2 ng/kg body weight, respectively (Gunderson, 1988).
Matsushima (1972) reported that the total diet of an average
citizen of Matsuyama City, Japan, contained about 0.177 mg HCH/day,
the major portion being the beta isomer. Of the beta-HCH intake 90%
was identified as originating from meat and dairy products.
In a total-diet study in the Netherlands in 1977, the average
concentration of beta-HCH in 100 samples was < 0.02 mg/kg on a fat
basis. The highest level was 0.19 mg/kg (Greve & van Hulst, 1977).
5.2.2 Concentrations in human samples
Beta-HCH concentrations in human samples are a good indication of
the total exposure of the general population. Concentrations in human
tissues are markedly higher than those of the other HCH isomers, a
phenomenon which reflects the cumulative properties of the beta
isomer. There has been a trend, but only a very slow one, towards
lower values in recent data.
Greve (1985) was unable to detect any correlation between life
style, such as type of food, and the beta-HCH concentrations in
tissues of Dutch citizens. However, in a Swedish study the levels of
beta-HCH (and of other organochlorine contaminants) in breast milk
were found to be related to dietary habit. Levels in lacto-vegetarians
were lower than those in women eating a mixed diet, and the latter
were in turn lower than those in women using a mixed diet that
regularly included fatty fish from the Baltic (Noren, 1983).
5.2.2.1 Blood
Eckenhausen et al. (1981) detected beta-HCH at a concentration of
< 0.5 to 25 µg/litre in the blood of 19 out of 47 pregnant women in
the Netherlands. A concentration range of < 1.0 to 12 µg/litre was
found in 30 out of 69 women after they had given birth and beta-HCH
was detected in the blood of 17 out of 46 babies (< 1.0 to
6.0 µg/litre).
When Blok et al. (1984) studied the presence of beta-HCH in the
blood of 65 healthy Dutch volunteers (34 females and 31 males),
beta-HCH was found in approximately half of the volunteers. The median
concentration was 0.4 µg/litre (range nd-1.4 µg/litre) in both males
and females.
Blood samples of Dutch citizens analysed in 1978, 1980, 1981, and
1982 (70, 48, 127, and 54 samples, respectively), contained 0.3-1.4 µg
beta-HCH/litre (Greve & Wegman, 1985).
Polishuk et al. (1970) found beta-HCH in the blood of 24 pregnant
women and 23 infants living in Israel. The mean concentrations was 0.5
± 0.6 µg/litre in the women and 0.3 ± 0.5 µg/litre in the infants.
The average concentrations of beta-HCH in the plasma of five
subjects in the USA, who were not occupationally exposed, were
0.83-0.94 µg/litre, and were remarkably consistent throughout the 5
days during which samples were taken (Radomski et al., 1971a).
Starr et al. (1974) analysed the blood of 187 men and 171 women
living in Colorado, USA. In men, a mean concentration of 4.9 µg
beta-HCH/litre was found in 15 samples, while in women the mean
concentration in 7 samples was 10.9 µg/litre (range, 9.0-15.0).
In a 4-year study to assess the exposure of the general
population, 6252 blood samples were collected from people (12-74 years
of age) living in 64 locations across the USA. Beta-HCH was detected
in 13.9% of the samples at a mean level of 1.7 µg/litre (range,
1-28 µg/litre). The percentage of positive samples increased from the
youngest to oldest age group (from 3.2 to 26.8%) (Murphy & Harvey,
1985).
Bertram et al. (1980) found a median concentration of 1.32 µg
beta-HCH/litre (range, nd-4.81) in whole blood (18 samples) of
citizens of the Federal Republic of Germany.
5.2.2.2 Adipose tissue
In fifteen samples of adipose tissue from the general population
of the Federal Republic of Germany, the median concentration of
beta-HCH was 0.33 mg/kg on a fat basis (range, nd-0.66) (Bertram et
al., 1980).
Specimens of subcutaneous adipose tissue from 48 children (34
under the age of 1 year, 14 in the second year of life) were analysed
during the period 1982-1983 in the Federal Republic of Germany. The
average concentration of beta-HCH was 0.15 (range, nd-1.02) mg/kg fat.
The average concentration was highest, 0.17 (nd-0.38) mg/kg fat, at
the age of 0-6 weeks (Niessen et al., 1984).
Hildebrandt et al. (1986) summarized the results of nine studies
carried out in the Federal Republic of Germany in 1969-1983. The mean
beta-HCH concentrations (636 samples) ranged from 0.01 to 1.30 mg/kg
on a fat basis.
Mes et al. (1982) analysed 99 samples of adipose tissue from
autopsies of accident victims from different areas of Canada. All
samples contained beta-HCH, the average concentration of which was
0.151 ± 0.459 (range, 0.016-4.413) mg/kg wet weight. In males (53)
the average value was 0.183 ± 0.612 mg/kg, whereas in women (45) it
was 0.116 ± 0.166 mg/kg. The influence of age was evident. The
average concentration in 33 people aged up to 25 was 0.067 ±
0.051 mg/kg, in 41 people aged 26-50 it was 0.260 ± 0.698 mg/kg, and
in 24 people aged 51 or more it was 0.082 ± 0.037 mg/kg.
Human adipose tissue was analysed during the periods 1965-1967,
1969-1971, and 1976-1977 (male and female) in the United Kingdom, the
number of samples being 66, 248, 201 (male), and 236 (female),
respectively. The arithmetic means of the beta-HCH concentrations were
0.28, 0.27, 0.30 (male), and 0.33 (female) mg/kg, respectively (HMSO,
1982). During 1982-1983, the beta-HCH concentrations were 0.24 (male)
and 0.31 (female) with a range of 0.01-0.81 mg/kg (HMSO, 1986).
Kutz et al. (1979) studied the presence of organochlorine
pesticides in human adipose tissue in 48 states of the USA in
1970-1975. Beta-HCH was widely distributed at low levels (geometric
means of between 0.2 and 0.4 mg/kg tissue on a fat basis) during this
period, and there was a slow downward trend. Residues of alpha-HCH and
gamma-HCH were found at a very low frequency.
In 1980, eight adipose tissue samples were taken as part of the
US EPA National Human Monitoring Program in North East Louisiana and
in 1984 10 samples were collected. The average beta-HCH concentration
(on a lipid basis) was 0.77 mg/kg (nd-2.31) in 1980 and 0.62 mg/kg
(0.31-1.03) in 1984 (Holt et al., 1986).
In a study by Szymczynski et al. (1986), 29 samples of adipose
tissue were taken at necropsy and 24 at surgery in the Poznan region
of Poland and were compared with 100 samples from residents of the
Warsaw region. In Poznan, the mean concentration of beta-HCH was 0.211
± 0.154 mg/kg, while in Warsaw it was 0.184 ± 0.017 mg/kg.
In Kenya, Wassermann et al. (1972) analysed 83 adipose tissue
samples collected during autopsy in 1969-1970 from people without
occupational exposure to insecticides. The mean concentration of
beta-HCH in the age group 5-24 (32 samples) was 0.0686 ± 0.064 mg/kg,
in the age group 25-44 (28 samples) it was 0.263 ± 0.266 mg/kg, and in
people aged 45 or more (23 samples) it was 0.186 ± 0.228 mg/kg.
In 1974, 360 adipose tissue samples were collected in 8 regions
of Japan, and the mean concentration of beta-HCH was 6.55 mg/kg on a
fat basis (Takabatake, 1978).
The beta-HCH concentration of 567 samples of adipose tissues of
Dutch citizens analysed during 1968-1983 ranged from 0.21 to 0.6 mg/kg
(on a fat basis). The highest levels were found in 1968-1976 (Greve &
van Harten, 1983; Greve & Wegman, 1985).
5.2.2.3 Breast milk
Breast milk is a major route for the elimination of
organochlorine pesticides and PCBs in women. In a study by Cetinkaya
et al. (1984), a significant correlation was found between the
concentration of beta-HCH in breast milk and the level of consumption
of meat products and animal fat. In addition, concentrations of
beta-HCH in breast milk in rural areas appeared to be higher than
those in urban areas.
The variation during lactation of residue levels in breast milk
was investigated in five women aged 23-36 in the Federal Republic of
Germany. The beta-HCH concentrations were between 0.04 and 0.20 mg/kg
fat, and no significant changes in residue level occurred during the
lactation period (Fooken & Butte, 1987).
The residues of beta-HCH in breast milk during the periods
1974-1975 and 1979-1980 in the Federal Republic of Germany were 0.6
and 0.3 mg/kg fat, respectively (Anon., 1984).
More than 7100 samples of breast milk were analysed in the
Federal Republic of Germany from 1969 to 1984. These studies were
carried out by 20 authors, and the results were summarized by
Hildebrandt et al. (1986). The mean concentrations of beta-HCH ranged
from 0.02 to 0.56 mg/kg fat. There was no clear decrease in the mean
concentrations during the period 1969-1979, but thereafter a slow
decrease was observed. A further study carried out in the Federal
Republic of Germany (2709 samples in 1979-1981) yielded an average
concentration of 0.37 mg/kg fat (Fooken & Butte, 1987). In 1981-1983,
132 samples of breast milk were analysed and the average level was
0.209 mg beta-HCH/kg milk fat (Cetinkaya et al., 1984).
Tuinstra (1971) analysed 42 individual samples of breast milk
collected in 1969 from young mothers (18-32 years of age) living in
the Netherlands and determined a median beta-HCH concentration of
0.28 mg/kg milk fat (range, 0.1-0.69 mg/kg). When 278 samples of
breast milk, collected in 11 maternity centres in the Netherlands,
were analysed for the presence of beta-HCH, the median beta-HCH
concentration was 0.1 mg/kg (on a fat basis). The maximum value was
0.3 mg/kg (Greve & Wegman, 1985).
Samples of maternal blood, milk, and umbilical cord blood were
collected from 43 mothers and their infants during 1981-1982 in Oslo,
Norway. The residue levels in maternal and umbilical cord serum were
below < 1 µg per kg. In colostrum and milk, concentrations ranging
from 0.05 to 0.45 mg/kg fat were found (Skaare et al., 1988).
Vukavic et al. (1986) measured beta-HCH in 59 samples of
colostrum collected during autumn 1982 (26 samples) and spring 1983
(33 samples) from healthy nursing mothers on the third day after
delivery. The beta-HCH concentrations in the autumn and spring were
not significantly different (mean concentrations of 0.95 ± 0.21 and
0.88 ± 0.16 µg/litre whole colostrum, respectively).
Mes et al. (1986) studied 210 breast milk samples from five
different regions of Canada and measured a mean beta-HCH concentration
of 0.214 mg/kg (on a fat basis). Davies & Mes (1987) studied 18
breast milk samples from Canadian, Indian, and Inuit mothers in
Canada, whose fish consumption was comparable to the national
consumption. The level of beta-HCH in milk fat of the indigenous
population was 0.022 mg/kg, compared with a value of 0.206 mg/kg from
a national survey.
In Japan, the average beta-HCH content in breast milk was found
to be 0.120 mg/kg (Matsushima, 1972).
In Japan, 378, 328, 87, and 77 samples of breast milk,
respectively, were analysed in 1980, 1981, 1982, and 1983. The mean
concentrations were 0.031, 0.034, 0.043, and 0.020 mg beta-HCH/kg
whole milk, respectively (WHO, 1986).
Breast milk was analysed for the presence of beta-HCH during
1979-1980 and 1983-1984 in the United Kingdom. In these two
periods, 30 and 40 samples, respectively, were collected in Scotland.
The mean concentrations were 0.008 (< 0.001-0.14) and 0.005
(< 0.001-0.032) mg/kg milk, respectively (HMSO, 1986).
6. KINETICS AND METABOLISM
6.1 Absorption and elimination
Shibata (1978) reported that the absorption of beta-HCH from the
gastrointestinal tract in mice was 80-95%, most of this being
accumulated in adipose tissue. The elimination followed a 2-stage
mechanism, the half-life for the first stage being 2.5 days and that
for the second stage being 18 days. The half-life for clearance from
blood in rats (sex not specified) was 1 month (Altmann et al., 1980),
and the half-life for clearance from fat was 14 days in male rats and
28 days in female rats (Portig, 1983). Vohland & Koransky (1983)
reported a half-life for clearance from "internal organs" of 22 days
in female rats. A half-life of 20 days for the clearance from the
brain of female rats was reported by Portig & Vohland (1983) and
Vohland et al. (1981). In cows the half-life for clearance from fat
was 4.2-22.0 weeks (Wolf, 1983). The elimination in humans was slow
after continuous exposure ceased, the concentration in fatty tissues
decreasing only slightly over several years (Vohland & Koransky,
1983).
6.2 Distribution
Oshiba (1972) fed groups of six rats a diet containing 10 mg
beta-HCH/kg for up to 56 days. The beta-HCH level in adipose tissue
was 60 mg/kg tissue and in the liver was 45 mg/kg tissue after 56
days. The maximum concentration in the liver was reached after 4
weeks. During subsequent starvation, beta-HCH was mobilized from
adipose tissue by an enhanced lipid metabolism. Furthermore, there
was a tendency for deposition in other organs and tissues.
Vohland et al. (1981) and Portig & Vohland (1983) studied the
distribution of beta-HCH in the brain and depot fat of rats. With an
average concentration in blood of 92 µg beta-HCH/litre, a brain to
blood ratio of 2:1 and depot fat to blood ratio of 170:1 were found,
whereas with blood concentrations of 540 µg/litre and 2100 µg per
litre the ratios were 2:1 and 177:1 and 2:1 and 168:1, respectively.
After lethal acute intoxication of humans by HCH, the HCH
concentration, relative to that in blood, was in the ratio of 363:1
for fat, 3:1 for brain, and 15:1 for liver (Vohland & Koransky, 1983).
When beta-HCH is applied repeatedly to rats, mice, and mini-pigs
there is marked storage in fat, especially in females, and the fat
levels increase continuously as dosing progresses (Nakajima et al.,
1970; Oshiba & Kawakita, 1972; Altmann et al., 1980; van Velsen et
al., 1982; Srinivasan & Radhakrishnamurty, 1983). Data on
concentrations in organs are contradictory: according to one report
the levels in the kidneys, brain, and liver of rats reached a plateau
after 4 weeks (Oshiba & Kawakita, 1972), whereas other sources
reported steady increases in these organs throughout a 12-week dosing
period (van Velsen et al., 1982).
6.3 Transplacental transfer and transfer via lactation
Hori & Kashimoto (1974) found, after oral dosing of pregnant
mice, a carry-over from dam to fetus of approximately 2% of the dose.
Shibata (1978), however, reported a placental transfer of beta-HCH in
rats of approximately 40%.
During lactation the transfer of beta-HCH to milk was 85% from
adipose tissue and 64% from administered beta-HCH (Shibata, 1978).
Carry-over from dam to suckling via the milk was about 60% of the dose
during lactation.
In a study by Hapke & Hollmann (1985), significant carry-over via
milk was found after female rats were dosed for a period of 8 weeks
that terminated 3 weeks before mating. At both the dose levels tested
(i.e. 1 and 5 mg/kg body weight) beta-HCH concentrations in milk were
elevated and signs of liver enzyme induction were found in the pups.
As significant excretion in milk was shown to occur in cows after
oral dosing (the carry-over was 30-37%) (Heeschen, 1985).
6.4 Metabolic transformation
6.4.1 Rat
In a study by Freal & Chadwick (1973), Sprague-Dawley weanling
female rats were administered 2 mg beta-HCH/rat per day orally in
peanut oil for 7 days. Since beta-HCH was metabolized to
2,4,6-trichlorophenol but to no other chlorophenols, it appears that
cis-dehydrochlorination may lead exclusively to this metabolite.
This study also indicated that pre-treatment with beta-HCH alters the
metabolism of lindane in rats.
Rats fed 1.5 mg 14C-labelled beta-HCH/kg diet for 7 days
excreted 70% of the dose during these 7 days and the following 28
days. One third of the eliminated radiolabel was found in the urine.
There was no unchanged beta-HCH in urine; the major urinary metabolite
was 2,4,6-trichlorophenol and small amounts of trichlorohydroxy-
methoxybenzene, a dichlorophenol, and a trace of a tetrachloro-
cyclohexane isomer were found. In faeces, only 2,4,6-trichlorophenol
was identified (Lay et al., 1981).
Artigas et al. (1988) applied a new method of gas
chromatographymass spectrometry (GC-MS) to identify several lindane
metabolites (tetra-, penta-, and hexachlorocyclohexenes, and tetra-
and pentachlorobenzene) in rat brain homogenates. Male Wistar rats
were administered orally 30 mg beta-HCH/kg and were sacrificed 5 h
later. The cerebella of the animals were analysed and 3.6/4.5-PCCH,
3.5/4.6-PCCH, HCCH, pentachlorobenzene, HCB, and beta-HCH were found
at levels below 5 µg/kg. Beta-HCH was present at a concentration of
4.2 mg/kg tissue. This study revealed that the various HCH isomers are
cleared from the brain via different metabolic pathways.
6.4.2 Mouse
When 14C-labelled beta-HCH was administered intraperitoneally
to male mice (strain ddY, 4 weeks old) as a single dose of 32 µg, the
average urinary excretion of radioactivity within 3 days was 10%.
Beta-HCH seemed to be metabolized more slowly than lindane. The
principal metabolite of beta-HCH in the urine was
2,4,6-trichlorophenol (25%), but 2,4-dichlorophenol (up to 5%) was
also found. These metabolites were mainly conjugated with glucuronide
or sulfate (Kurihara & Nakajima, 1974; Kurihara, 1975).
6.4.3 Human
When Engst et al. (1978) analysed the urine of occupationally
exposed workers (apparently to technical-grade HCH in manufacturing
processes), they found, apart from alpha-, beta-, gamma-, and
delta-HCH, traces of hexa- and pentachlorobenzene, gamma- and
delta-pentachlorocyclohexene, pentachlorophenol, 2,3,4,5-, 2,3,4,6-,
and 2,3,5,6,-tetrachlorophenol, and several trichlorophenols, as well
as the glucuronides of several of these metabolites. The
pentachlorocyclohexenes, tetrachlorophenol, hexachlorobenzene, and
pentachlorophenol were also identified in the blood.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Acute toxicity data
7.1.1 Oral
Coper et al. (1951) reported the death of three out of five rats
that received a oral dose of 150 mg beta-HCH/kg body weight. Oral
LD50 values of 1500 and 2000 mg/kg body weight for mice and rats,
respectively, have been reported (WHO, 1986). However, a more recent
study reported the LD50 to be > 16 000 mg/kg body weight in mice
and > 8000 mg/kg body weight in rats (Hoffmann, 1983). Symptoms of
poisoning were decreased activity, ataxia, tremors, dyspnoea,
anorexia, convulsions, and rough fur. Portig (1983) reported a rat
LD50 of 9000 mg/kg body weight.
7.1.2 Intraperitoneal
In a study by Coper et al. (1951), no deaths occurred among 6
rats given an intraperitoneal dose of 160 mg beta-HCH/kg body weight.
7.2 Short-term exposure
7.2.1 Mouse oral studies
Groups of 10-11 DD mice of each sex (6 weeks of age) received
diets containing 0, 100, 300, or 600 mg beta-HCH per kg diet for 32
weeks, followed by a control diet for 5-6 weeks. The control group
consisted of 20 animals. During the experiment a number of animals
died. The frequency of atypical proliferation in the liver was 0/29 in
the control group, 0/18 at 100 mg/kg, 6/16 at 300 mg/kg, and 11/12 at
600 mg/kg. No tumours were found (Hanada et al., 1973).
Ito et al. (1973b) fed DD mice 0, 50, 100, 200, or 500 mg
beta-HCH/kg diet for 24 weeks but found no liver tumours or nodular
hyperplasia.
7.2.2 Rat oral studies
In a study by Doisy & Bocklage (1950), all rats (20 weanling
male) administered 0.6 g beta-HCH/kg diet for 4 weeks died within 3
weeks.
Rats administered beta-HCH at a dietary concentration of
600 mg/kg were reported to undergo growth retardation, liver mass
enlargement, and a decrease in absolute brain mass, beta-HCH residues
being found primarily in the fat and adrenals (Macholz et al., 1986).
A 13-week oral toxicity study with beta-HCH (> 98%) in
SPF-derived Wistar RIV:Tox rats (10 male and 10 female per group) has
been carried out. The levels were 0 (< 0.01), 2, 10, 50, or
250 mg/kg diet, clinical signs, growth, food intake, and organ weights
were checked, and biochemical, haematological, and extensive
histopathological investigations were carried out. At the highest
concentration, half of the animals died following ataxia, progressive
inactivity, and coma. Growth inhibition was only observed in this
concentration, and red and white blood cell concentrations were
decreased. The liver glycogen level was significantly higher in the
group fed 250 mg/kg than in control rats. Furthermore, a significantly
higher activity of aniline hydroxylase (AH) and aminopyrin-
N-demethylase (APDM) microsomal enzymes and a higher concentration
of cytochrome P450 were observed. In the group fed 50 mg/kg only APDM
activity was increased. In all groups of treated rats, liver effects
were observed (increase in organ weight, centrilobular hepatocytic
hypertrophy, and proliferation of smooth endoplasmic reticulum or
increased activity of microsomal enzymes). The thymus weight was
significantly decreased at 50 and 250 mg/kg and the testes weight at
250 mg. At 2 mg/kg the only effect found was liver enzyme induction
(van Velsen, 1986; van Velsen et al., 1986).
When young male Wistar rats were administered 800 mg beta-HCH/kg
diet for 2 weeks, liver weight was increased but no differences in the
content of water, nitrogen, protein, or glycogen were found. Liver
fat content was increased and the DNA content per kg tissue was
decreased, but the whole liver DNA content was increased. The most
predominant change in the liver was hypertrophy of the liver cells.
The testes weight was not different from that of the control animals
but the protein content was higher. The testicular DNA content was
lower than in control animals. The histological changes reported were
testicular tubular atrophy, interstitial oedema, and spermatogenic
arrest (Srinivasan et al., 1988).
In a study by Srinivasan et al. (1984), eight young male Wistar
rats were fed 800 mg beta-HCH/kg diet for two weeks, and a control
group of five rats was used. Special attention was given to the
urinary excretion of body constituents reflecting renal function.
Glucosuria, increased excretion of creatinine and urea, and
hypertrophy and degeneration of the renal tubular epithelia were
observed.
In another study with male Wistar rats fed 0 or 800 mg
beta-HCH/kg diet for two weeks, special attention was given to liver
function. The control group consisted of 8 rats and the treated group
of 12 animals. The HCH isomer produced effects on various enzyme
systems: serum aminotransferase, hepatic glucose-6-phosphate
dehydrogenase, and aldolase activities were increased, while liver
glucose-6-phosphatase activity was decreased. The activities of liver
mitochondrial DNP/Mg2±/Ca2±-activated ATPase and liver microsomal
Na±/K±-ATPase were lower in the treated animals (Srinivasan &
Radhakrishnamurty, 1988).
Two 13-week feeding studies (Loeber & van Velsen, 1985; van
Velsen et al., 1986) showed similar atrophy of the testes, with
reduced tubular area, lack of mature spermatozoa, and oedema of the
interstitial spaces. In both studies, only the highest beta-HCH
concentration (150 mg/kg diet in one study and 250 mg/kg diet in the
other) produced these effects and resulted in a significantly reduced
weight gain in the affected animals. The measurement of circulating
hormone levels (testosterone, FSH, LH) showed no dose-related effects.
No clear endocrine effects on the testes or on male reproductive
hormones were demonstrated (van Velsen, 1986).
7.3 Skin and eye irritation; sensitization
No data are available on skin and eye irritation or
sensitization.
7.4 Long-term exposure
7.4.1 Rat oral studies
In a study by Fitzhugh et al. (1950), groups of 10 female and 10
male weanling Wistar rats were administered diets containing 0, 10,
100, or 800 mg beta-HCH/kg (in corn oil) for 107 weeks. With
concentrations of 100 mg/kg diet or more, there was growth depression,
and with 800 mg/kg increased mortality was found. Concentrations of
10 mg/kg or more led to liver enlargement and slight or moderate
histopathological changes in the liver.
7.5 Reproduction, embryotoxicity, and teratogenicity
7.5.1 Reproduction
A 2-generation study with SPF-derived Wistar RIV:Tox rats (13
males and 26 females per group) was carried out to study fertility,
reproduction, and development of the offspring. The parents (F0)
were fed beta-HCH (> 98%) from weaning at levels of 0 (< 0.01), 2,
10, or 50 mg/kg, and, following a 12-week premating period, F1a and
F1b litters were produced. The F1b generation was used to produce
F2a, F2b, and F2c litters, the last-mentioned being used for
teratological investigations (see section 7.5.2). In the highest-dose
group, the F1a litter size was reduced and there was almost complete
infertility when mating for the Fw1bgeneration took place. All pups in
this group died before weaning. At a concentration of 10 mg/kg diet,
increased mortality in F1a and F1b litters was observed. In this
group, precocious vaginal opening and complete infertility in the
second generation were observed. There were no effects in the 2-mg/kg
group (van Velsen, 1986).
The parental animals (F0) from the above study were used to
investigate the influence of beta-HCH on the endocrine organs after 40
weeks of exposure. In females, mean autopsy body weight and ovary
weight were decreased, but adrenal gland and uterus weight increased.
The organ weight changes in males were less clear, except for an
increase in the weight of pituitary and adrenal glands. The proportion
of animals without corpora lutea in the ovaries was greater in the
group fed 50 mg/kg than it was in control rats. The testes in the
animals fed at this level showed a reduced number of Leydig cells.
Atrophy of the dorsolateral prostate, seminal vesicles, and
coagulation glands was found in one animal. The pituitary glands of
the treated animals revealed no differences in the immunoreactivity
for prolactin. The pregnancy index (number of females with
litters/number of females mated) was less than 0.5 in all groups of
rats. Normally this strain produced pregnancy indices of nearly 1.0.
The study was performed using a semi-synthetic purified feed as
compared to the conventional feed used in the breeding colony. The age
of females at first delivery was 20 weeks, much higher than normal.
These factors cast some doubt on the results (van Velsen, 1986).
7.5.2 Teratogenicity
The F2c litter from the study described in 7.5.1 was used to
investigate the teratogenic effects of beta-HCH. The females were
killed 20 days following sperm detection or 20 days after the last
mating of the F1b generation, and fetuses were inspected for
internal and skeletal abnormalities. No compound-related increase in
teratogenic effects was found (van Velsen, 1986).
7.6 Mutagenicity and related end-points
The available data are limited. Beta-HCH did not induce mutations
in Salmonella typhimurium strains TA98, TA100, TA1535, or TA1537
(Lawlor & Haworth, 1979; Nishimura, 1982). The result of an in vivo
bone marrow metaphase analysis in rats was reported to be positive
(Shimazu et al., 1976; IARC, 1979). Beta-HCH did not induce mutations
inAllium ceparoots (Nybom & Knutsson, 1947).
A mutagen test strain of Bacillus subtilis (TKJ5211) showed a
higher sensitivity for his± reversion than the parental strain
(HA101) when treated with UV and UV-mimetic chemicals. However, a
negative result was obtained at a level of beta-HCH (dissolved in
DMSO) of 5 mg/ml (Tanooka, 1977).
In a repair test using stationary phase cultures of HLL3g and
HJ-15 strains, in which the size of growth inhibition zones of
repair-proficient and repair-deficient cells (for vegetative cells and
spores) was determined, a level of 5 mg beta-HCH (in benzene) per ml
was without effect (Tanooka, 1977).
A thorough evaluation of the mutagenic potency of beta-HCH
requires additional tests.
7.7 Carcinogenicity
Appraisal
In one study on mice, a beta-HCH dose exceeding the MTD produced
an increased incidence of benign and malignant liver tumours. All
other reported studies on mice were inadequate for the evaluation of
beta-HCH carcinogenicity due to the very short duration of treatment
and/or observation.
Two studies on rats were inadequate for evaluation due to the
small number of animals in one and the short duration of treatment in
the other.
The results of the studies on initiation-promotion and mode of
action and the mutagenicity studies suggest that the neo-plastic
response observed with beta-HCH is most likely due to a non-genotoxic
mechanism.
7.7.1 Mouse
In a study by Goto et al. (1972a), 20 male ICR/JCL mice (5-week
old) were fed 0 or 600 mg beta-HCH/kg diet for 26 weeks. Increased
liver weights were reported and hepatomas described as benign liver
tumours were induced. However, insufficient details were reported.
Nagasaki (1973) administered beta-HCH orally to male DD mice at
concentrations of 100, 250, or 500 mg/kg for 24 weeks. There were no
signs of tumour development at any treatment level.
Groups of 10-11 DD mice of both sexes (6-weeks old) received
diets containing 0, 100, 300, or 600 mg beta-HCH per kg for 32 weeks,
followed by a control diet for 5-6 weeks. The control group consisted
of 20 animals. During the experiment a number of animals died. In the
treated mice, atypical proliferation was found in the liver in the two
highest dose levels, but no hepatomas were observed. Alpha-fetoprotein
was not detected in the serum of animals with hepatomas (Hanada et
al., 1973).
Thorpe & Walker (1973) performed a 110-week study, using a
dietary concentration of 200 mg beta-HCH (> 99%) per kg, on CF1 mice
(groups of 30 males and 30 females). During the first 3 months of the
study 12% of the males and 25% of the females died. Liver enlargement
was detected by week 50 in both females and males. Hyperplastic
changes were found in the liver (hyperplastic nodules and
hepatocellular carcinomas), sometimes with lung metastases. The
combined incidence of benign and malignant liver tumours for males and
females was 24 and 23%, respectively, in the control group and 73 and
43%, respectively, in the treated group.
When male DD mice (8 weeks old), in groups of 20 or 29 animals,
were fed a diet containing 0, 100, 250, or 500 mg beta-HCH per kg for
24 weeks, there was a moderate increase in liver weight at the two
highest levels. No nodules classified as nodular hyperplasia or
hepatocellular carcinoma were detected (Ito et al., 1973b).
7.7.2 Rat
In a study by Fitzhugh et al. (1950), groups of 10 male and 10
female weanling Wistar rats were fed throughout their lives on a diet
containing beta-HCH > 98% pure (10, 100, or 800 mg/kg diet). No
increase in tumour incidence was reported in the treated animals, but
only a limited number of organs were examined microscopically.
Male W rats (5-8 weeks old, 18-24 animals per group) were
administered diets containing beta-HCH at a concentration of 500 mg/kg
(for 24 or 48 weeks) or 1000 mg/kg (for 24 weeks). Only slight cell
hypertrophy was found, but no nodular hyperplasia, bile duct
proliferation or hepatocellular carcinomas were detected (Ito et al.,
1975).
7.7.3 Initiation-promotion
The influence of beta-HCH on tumour induction by PCBs (and vice
versa) was tested with male DD mice (26-30 animals per group).
Whereas 500 mg PCBs/kg diet induced nodular hyperplasia and
hepatocellular carcinomas in the liver of male mice after 32 weeks,
exposure to beta-HCH at levels of 50, 100, or 250 mg/kg diet or to
PCBs at a level of 250 mg/kg diet did not. However, the combination of
100 mg beta-HCH/kg and 250 mg PCB/kg caused the induction of nodular
hyperplasia in 17% (5/30) of the mice and hepatocellular carcinoma in
3.3% (1/30). The corresponding values for the combination of 250 mg
beta-HCH/kg and 250 mg PCB/kg were 55% (16/29) and 21% (6/29),
respectively. These results showed that PCBs promoted the
hepatocarcinogenic action of beta-HCH (Ito et al., 1973a).
The tumour-initiating activity of beta-HCH has been studied by
examining for phenotypically altered foci in female Wistar rats.
Groups of three to eight rats were used and, after the median and
right liver lobes had been removed, the rats were administered 100 mg
beta-HCH/kg body weight followed by phenobarbital at 50 mg/kg body
weight per day for 15 weeks. Liver foci were identified by means of
the gamma-glutamyltransferase (GGT) reaction and by morphological
alterations. No evidence of initiating activity was found. In another
part of the study, the promoting activity was investigated. A single
dose of N-nitrosomorpholine (250 mg/kg body weight by gavage) was
followed by the administration of beta-HCH (0.03, 0.2, 1.0, 3.0, or
10.0 mg/kg body weight per day) for 4, 15, and 20 weeks. The criteria
used were growth and phenotypic changes of foci as end-points. It was
concluded from the study that beta-HCH is a tumour promotor. Both the
number and size of altered foci were enhanced by a beta-HCH dose of
3 mg/kg. The tumour-promoting action was generally associated with
liver growth and induction of monooxygenases or other specific enzymes
(Schröter et al., 1987).
7.7.4 Mode of action
Sagelsdorff et al. (1983) studied the relevance to the
carcinogenic action of HCH isomers of covalent binding to mouse liver
DNA. NMRI mice were given 7.3-7.7 mg beta-HCH per kg body weight
orally and 14C-thymidine intraperitoneally. A very low covalent
binding index (CBI) of < 0.08 was found.
7.8 Special studies
7.8.1 Effects on endocrine organs
Juvenile female Swiss mice and SPF-derived Wistar rats (RIV:Tox)
were used to study the uterotropic effect of beta-HCH, in comparison
with that of 17-alpha-ethynyl-estradiol, using the method of Tiecco
(1961). Beta-HCH levels of up to 500 mg/kg diet were fed for 5 days,
and in both animal species there were clear uterotropic effects at
50 mg/kg or more. In quantitative terms, the estrogenic potency of
beta-HCH was, however, minimal in comparison to that of
17-alpha-ethynylestradiol (Loeber & van Velsen, 1985).
Other parameters of estrogenic potency have also been studied.
Beta-HCH has been shown to increase the uterine concentration of
progesterone receptors and the immunoreactivity of the adenohypophysis
for prolactin in rats, and to cause the redistribution of human tumour
cell receptors for progesterone. Adrenalectomy and ovariectomy did
not counteract the uterotropic effect of beta-HCH in rats. However,
the principal metabolite of beta-HCH, 2,4,6-tri-chlorophenol, had no
estrogenic effect, and beta-HCH did not displace 17-beta-estradiol
from its receptors (van Velsen, 1986). Both the significance of these
observations and the possible mechanisms of action are unclear.
7.8.2 Neurotoxicity
Beta-HCH may raise the threshold for electrically induced
seizures in rats. The ratio of the beta-HCH concentration in the
brain to that in blood indicates that this isomer passes the
blood-brain barrier less readily than the other HCH isomers.
Vohland et al. (1981) studied the neuropharmacological effects of
beta-HCH in Wistar rats. The kinetics of beta-HCH concentrations in
the brain were established after the administration of a single oral
dose of 200 mg/kg body weight. The approximate half-life for
elimination from the brain was 20 days in females. Beta-HCH did not
give rise to appreciable quantities of hydrophobic metabolites in the
brain. In rats 4-5 mg beta-HCH/kg in the brain had an anti-convulsive
effect (i.e. there was protection against the action of pentylene
tetrazole). Neurotoxic effects (ataxia and adynamia) occurred at
brain levels of 15-20 mg beta-HCH/kg (Vohland et al., 1981; Portig &
Vohland, 1983).
Beta-HCH has been demonstrated to cause a decrease in peripheral
nerve conduction velocity in rats fed 600 mg beta-HCH/kg diet for 30
days, but did not cause a change in the fronto-occipital
electroencephalogram at 3000 mg/kg diet (Müller et al. 1981). A
decrease in absolute brain mass was reported in one study in which
rats were fed beta-HCH for 30 days at a dietary concentration of
600 mg/kg (Macholz et al. 1986). Beta-HCH has been reported to raise
the seizure threshold for pentylenetetrazol, a known convulsant
(Vohland et al., 1981; Portig & Vohland, 1983). It has been shown
that beta-HCH blocks the binding of tert-butylbicyclo-
phosphorothionate (TBPS), a ligand known to bind to the GABA receptors
in chloride channels in the brain, but was the least effective of the
various HCH isomers in this respect (Fishman, 1987; Matsumoto et al.,
1988).
7.8.3 Effect on liver enzymes
Several short-term studies on enzyme induction have been
performed in rats, using levels ranging from 0.4 to 800 mg/kg feed.
The highest dose level without effect in one study was 10 mg/kg body
weight (van Hoof et al., 1982). However, in other studies, in which
the same parameters were determined, the levels without effects
included 50 mg/kg body weight. Histopathological changes in the liver
correlated with the induction of microsomal enzymes (den Tonkelaar et
al., 1981; van Hoof et al., 1982; van Giersbergen et al., 1984).
Weanling male rats fed diets of 800 mg beta-HCH/kg for 14-18 days
showed significant increases in hepatic alanine aminotransferase (80%)
and glucose-6-phosphate dehydrogenase (130%) and statistically
significant decreases in hepatic aspartate aminotransferase (130%),
alkaline phosphatase (45%), and acid phosphatase (40%) (Srinivasan &
Radhakrishnamurty, 1977). Similarly, the dietary administration of
800 mg/kg to albino rats for two weeks resulted in noticeable
hepatocellular damage, as indicated by elevations in the activity of
serum aminotransferases and decreases in that of hepatic soluble
enzymes. An increase in glucose-6-phosphate dehydrogenase and aldolase
activities was reported to suggest a higher rate of glucose oxidation,
while a decrease in liver glucose-6-phosphatase activity was
attributed to an inactivation of hepatic gluconeogenesis (Srinivasan &
Radhakrishnamurty, 1988).
7.8.4 Immunosuppression
To investigate potential effects on the reproductive and immune
systems, beta-HCH (0, 100, or 300 mg/kg diet) was fed to groups of six
female B6C3F1 mice for 30 days. Investigations were conducted on
changes in ovarian and uterine histology, body weight, lymphoid organ
weight and histology, splenic cellularity, antigen-specific IgM and
IgG plaque-forming cells (PFC), proliferative responses to mitogens,
natural killer cell activity, and induction of cytosolic T
lymphocytes. Significant changes in several immune functions were
only found at a beta-HCH concentration of 300 mg/kg. The proliferation
of splenocytes to the mitogens lipopolysaccharide (LPS), phytohaemag-
glutinin (PHA), and concanavalin A and T-lymphocyte-mediated cytolysis
of tumour targets were decreased, and a concurrent reduction in natural
killer activity was found. These data indicate that beta-HCH causes
non-estrogenic immune function changes without significant changes in
lymphoid organ weight, histology or cellularity (Cornacoff et al., 1988).
8. EFFECTS ON HUMANS
8.1 Acute toxicity - poisoning incidents
Several cases of acute poisoning by technical-grade HCH,
resulting either from accident or occupational exposure have been
described (WHO, 1991). It is likely that gamma-HCH, the most acutely
toxic component, played the major role in these incidents. These
cases cannot, therefore, assist in the evaluation of beta-HCH.
8.2 General population
No specific studies relating to beta-HCH are available.
A study comparing liver cancer deaths in the USA and the
"domestic disappearance" of organochlorine pesticides revealed that in
1962, 18 and 15 years after the introduction of DDT and
technical-grade HCH, respectively (when an increase in primary liver
cancer due to the organochlorines would be manifest), the number of
cases of primary liver cancer as a percentage of the total number of
liver cancer deaths began a gradual and steady decline (from 61.3% in
1962 to 56.9% in 1972). The death rate (per 100 000 per year) due to
primary liver cancer declined from 3.46 to 3.18 during this period
(Deichmann & MacDonald, 1977).
8.3 Occupational exposure
The evaluation of the effects of beta-HCH on occupationally
exposed workers is seriously hampered by the fact that most of the
relevant studies relate to workers who were exposed during the
manufacture and handling of lindane, or the handling and spraying of
technical-grade HCH among other pesticides, and were thus exposed to
all HCH isomers plus impurities and other (process) chemicals.
Therefore, it is difficult, if not impossible, to relate the observed
effects to individual substances. Consequently these studies have only
been described in this monograph where they aid the evaluation.
Behrbohm & Brandt (1959) described 26 cases of allergic and toxic
dermatitis that arose during the manufacture of technical-grade HCH.
Patch testing with pure alpha-, beta-, gamma-, and delta-HCH yielded
negative results, but positive reactions were obtained with the
residual fractions.
The level of beta-HCH was determined in the serum of 57 workers
at a lindane-manufacturing plant. No beta-HCH was detected in
controls, but the levels in exposed workers ranged from 17 to
760 µg/litre and increased with the duration of exposure. The beta-HCH
levels found in the adipose tissue of eight of these workers was
18-103 mg/kg (in extractable lipids). There were no clinical signs or
symptoms and no significant changes were found in extensive
biochemical, haematological, and neurophysiological tests, or in the
EMG or EEG. Serum leutinizing hormone levels were higher than in the
controls, but FSH and testosterone levels showed only insignificant
and inconclusive changes (Baumann et al., 1980, 1981; Brassow et al.,
1981; Tomczak et al., 1981).
The serum beta-HCH level of malaria-control workers who sprayed
technical-grade HCH for 16 weeks increased from 58 to 250 µg/litre in
previously non-exposed workers and from 294 to 385 µg/litre in those
that had been exposed during three previous spraying seasons (Gupta et
al., 1982). Although beta-HCH is only a minor component of
technical-grade HCH (7-10%), it reached higher levels and persisted
longer in the serum than either alpha- or gamma-HCH.
Nigam et al. (1986) studied 64 employees from a HCH-manufacturing
plant who were directly or indirectly associated with the production
of this insecticide. The exposed group was composed of 19 "handlers"
(who handled and packed the insecticide), 26 "non-handlers" (plant
operators and supervisors exposed indirectly to HCH), and 19
maintenance staff (who visited the plant frequently). The control
group consisted of 14 workers who had no occupational contact with the
insecticide. The exposure period varied up to 30 years. The mean
serum beta-HCH concentrations in the four groups were 28.5 µg/litre
(controls), 97.2 µg/litre (maintenance staff), 206.7 µg per litre
(non-handlers), and 413.1 µg/litre (handlers). Alpha-, gamma-, and
delta-HCH were also present. The total HCH concentrations were 51.4,
143.6, 265.6, and 604 µg/litre, respectively. Clinical examination
revealed that the majority of the workers from the "handler" and
"non-handler" groups exhibited paraesthesia of the face and
extremities, headache, and giddiness, and some of them also showed
symptoms of malaise, vomiting, tremors, apprehension, confusion, loss
of sleep, impaired memory, and loss of libido. The same symptoms were
found among the maintenance staff but were less severe and less
frequent.
Chattopadhyay et al. (1988) studied 45 male workers exposed to
HCH during its manufacture and compared them with 22 matched controls.
Exposure was mainly via the skin. Paraesthesia of face and
extremities, headache, giddiness, vomiting, apprehension, and loss of
sleep, as well as some changes in liver function tests, were reported
and were found to be related more to the intensity of exposure (as
measured by the HCH levels in blood serum) than to the duration of
exposure. The measured exposures to total HCH were 13 to 20 times
higher than those in the control groups (no detailed figures were
reported). Of the total HCH, 60-80% was beta-HCH.
Fitzhugh et al. (1950) drew attention to the importance of
beta-HCH for the long-term toxicity of HCH. The slower metabolism of
the beta isomer and its consequent longer persistence in the body are
significant factors.
A significant correlation between the beta-HCH levels in human
blood and adipose tissue has been described by Radomski et al. (1971a)
and Baumann et al. (1980).
In a group of workers that were no longer exposed to HCH for at
least 5 years, mean beta-HCH levels of 50 µg/litre were found, i.e.
twice as much as in the general population of that area at that time
(Radomski et al., 1971b).
Similar findings were reported by Morgan & Lin (1978), who found
20-348 µg/litre in the serum of 38 healthy workers whose last
occupational exposure to HCH was 10-15 years previously. Liver
function was normal and there were no indications of bone-marrow
damage.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Algae
Palmer & Maloney (1955) used beta-HCH in a preliminary screening
test with two cyanobacterium (blue alga), two green alga, and two
diatom species. The test concentration was 2 mg/litre water and the
incubation period was 3-21 days. Beta-HCH was not toxic at this
concentration.
Zhou et al. (1986) studied the effects of alpha-, beta-, gamma-,
and delta-HCH on the photosynthetic evolution of oxygen by the green
algae Chlorella vulgaris and Scenedesmus obliquus, and reported
that the beta- and gamma-isomers showed low toxicity, compared to the
alpha- and delta-isomers, in this respect.
In a study by Krishnakumari (1977), cultures of the green alga
Scenedesmus acutus (1, 3, or 5 days of age) were tested for
sensitivity to beta-HCH at 28°C, growth rate being used as parameter.
The nominal concentrations of beta-HCH (dissolved in ethanol) were
0.5-100 mg/litre. A decrease in growth rate was observed in the 1, 3,
and 5 day cultures exposed to 100, 10, and 5 mg/litre, respectively.
9.2 Protozoa
In short-term tests on Tetrahymena, the EC50 (growth) was
1.2 mg/litre (Mathur et al., 1984).
9.3 Invertebrates
In short-term tests on daphnids no effects were found at
concentrations up to the limit of solubility of beta-HCH in water
(about 1 mg/litre) (Janssen et al., 1987).
Canton et al. (1982) investigated long-term toxicity using
Daphnia and obtained a no-observed-effect level (NOEL) for
reproduction of 0.32 mg beta-HCH/litre.
9.4 Fish
9.4.1 Acute toxicity
In 4-day tests, beta-HCH affected the behaviour of fish. The NOEL
and EC50 values for Oryzias (as well as for Poecilia) were 0.026
and 0.047 mg/litre, respectively (Wester et al., 1985; Wester &
Canton, 1986).
In a study by Boulekbache (1980), the 48-h LC50 of beta-HCH
(98.9%) for the guppy (Poecilia reticulata) was 0.9 mg/litre. Female
fish were less sensitive than males.
9.4.2 Longer-term toxicity
Two tests were carried out with beta-HCH (98.9%) in Japanese
ricefish (medaka, Oryzias latipes) using fertilized eggs (40
eggs/group) or 25 young fish (1 month after hatching). The exposure
levels ranged from 0.032-1.0 mg beta-HCH/litre water and
histopathological examinations were performed after 1 and 3 months. In
the experiment on eggs, decreased growth was noted at 0.56 mg/litre;
with young fish this occurred at 0.1 mg/litre. The NOELs for abnormal
behaviour (loss of buoyancy and balance, uncoordinated movements) were
0.056 mg/litre and 0.032 mg/litre, respectively, for the two
experiments. Histopathological lesions indicating an estrogenic
activity were detected. Furthermore, lesions were observed in the
liver (vacuolation), kidneys (glomerular hyalinosis), and thyroid
(hypertrophy) (Wester & Canton, 1986).
In a study by Wester et al. (1985), groups of 35 young guppies
(Poecilia reticulata) (3-4 weeks old) were exposed to various
concentrations of beta-HCH (98.9%) ranging from 0.0032 to
1.0 mg/litre. After 1 and 3 months of exposure, toxicological and
histopathological parameters were studied. The gross NOEL was
0.032 mg/litre after both 1 and 3 months. Changes in the liver
(hypertrophy of rough endoplasmic reticulum) and kidneys (accumulation
of hyaline droplets in epithelium) were detected. Hypertrophy of the
endocardial lining cells (attributed to the accumulation of hyaline
droplets within lysosomes) was also reported.
After 3 months dysvitellogenesis was noted in the females. In
males the pituitary gland cells producing gonadotrophic hormone
appeared to be stimulated and testicular development was retarded at
0.32 mg/litre or more. It was suggested that all the observed effects
were attributable to an excessive production of the yolk precursor
vitellogenin by the liver as a result of an estrogen-like activity of
beta-HCH or its metabolites.
9.5 Terrestrial organisms
9.5.1 Birds
No toxic effects (e.g., effects on body weight, food consumption,
growth, egg production, egg weight, shell quality, mortality) were
observed in chickens fed diets containing 1-625 mg beta-HCH/kg for 12
weeks (Kan et al., 1979).
9.6 Model ecosystem studies
Sugiura et al. (1976) studied the effects of 0.01, 0.1, 1, 3, and
5 mg beta-HCH/litre on an aquatic microcosm consisting of bacteria,
ciliata, rotifera, oligochaeta, green algae, and blue-green algae.
The specific growth rates of protozoans and a rotifer were increased
by 0.01 and 0.1 mg/litre. No effects were found on the total
community respiration, although the gross primary production
increased.
CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT (ALPHA- AND BETA-HEXACHLOROCYCLOHEXANES)
1. Conclusions
The potential adverse effects of alpha- and beta-
hexachlorocyclohexanes (HCHs) on humans and the environment cannot be
balanced against benefits, since these isomers have no insecticidal
action. Their presence in the environment is thus of serious concern.
Consequently, the use of technical-grade HCH products containing high
concentrations of alpha- and beta-HCH is never justified.
1.1 General population
Alpha- and beta-HCH are circulating in the environment and
present in food chains. Thus there is a continuous potential for human
exposure. This exposure is low and is expected to decrease slowly in
the coming years. Therefore, there is no serious health concern for
the general population.
1.2 Sub-populations at special risk
Alpha-HCH concentrations in breast milk are low.
The exposure of babies resulting from present beta-HCH
concentrations in breast milk is a matter of concern but is no reason
for not promoting the use of breast-feeding.
However, every possible effort should be made to decrease dietary
and all other exposure to these isomers. Decreased dietary exposure is
expected to result in decreased levels of alpha- and beta-HCH in
breast milk.
1.3 Occupational exposure
As long as recommended precautions to minimize the exposure of
workers involved in lindane manufacturing are observed, alpha- and
beta-HCH pose no health risk to process operators.
1.4 Environmental effects
Apart from spills into the aquatic environment, there is no
evidence to suggest that the presence of alpha- and beta-HCH in the
environment poses a significant hazard to populations of organisms.
2. Recommendations for protection of human health and
the environment
a) In order to minimize environmental pollution with alpha- and
beta-HCH, lindane (> 99% gamma-HCH) must be used instead of
technical-grade HCH.
b) In order to avoid environmental pollution with alpha- and
beta-HCH, by-products and effluents from the manufacturing of lindane
must be disposed of in an appropriate way, and contamination of
natural waters and soil must be avoided.
c) Monitoring of alpha- and beta-HCH in food should continue. It is
essential that a mechanism for setting internationally acceptable
levels of alpha- and beta-HCH in food be initiated.
d) Monitoring of the daily intake of the general population and the
levels of alpha- and beta-HCH in breast milk should continue.
FURTHER RESEARCH (ALPHA- AND BETA-HEXACHLOROCYCLOHEXANES)
The following experimental studies are needed to allow a better
evaluation of the hazards of alpha- and beta-HCH:
* mutagenicity studies, especially with chromosome mutagenic
end-points;
* reproduction and fetotoxicity/teratogenicity studies;
* pharmacokinetic and toxicokinetic studies;
* carcinogenicity studies;
* neurotoxicity studies;
* surveillance studies on populations at risk.
PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer (IARC, 1987)
evaluated the hexachlorocyclohexanes and concluded that for the
technical grade and the alpha isomer there is sufficient evidence for
carcinogenicity to animals, whereas this evidence is limited for the
beta and gamma isomers. There is inadequate evidence for their
carcinogenicity to human beings. The hexachlorocyclohexanes were
classified in group 2B.
REFERENCES
ALBRO, P.W. & THOMAS, R. (1974) Intestinal absorption of
hexachlorobenzene and hexachlorocyclohexane isomers in rats. Bull.
environ. Contam. Toxicol., 12(3): 289-294.
ALTHAUS, F.R., LAWRENCE, S.D., SATTLER, G.L., LONGFELLOW, D.G., &
PITOT, H.C. (1982) Chemical quantification of unscheduled DNA
synthesis in cultured hepatocytes as an assay for the rapid screening
of potential chemical carcinogens. Cancer Res., 42: 3010-3015.
ALTMANN, H.J., BÖHME, Chr., & UEHLEKE, H. (1980) Long-term kinetics
of beta-hexachlorocyclohexane (beta-HCH) in rats and mini-pigs. Arch.
Pharmacol., 31 (Suppl.): R20 (Abstract No. 78).
ANGSUBHAKORN, S., BHAMARAPRAVATI, N., ROMRUEN, K., SAHAPHONG, S.,
THAMAVIT, W., & MIYAMOTO, M. (1981) Further study of
alpha-benzenehexachloride inhibition of aflatoxin B1 carcinogenesis in
rats. Br. J. Cancer, 13: 881-883.
ANON. (1984) [1984 Nutrition report], Frankfurt am Main, German
Nutrition Association (in German).
ARBEITSGEMEINSCHAFT FÜR DIE REINHALTUNG DER ELBE (1982) [Chlorinated
hydrocarbons; data for the river Elbe. Report on the results of the
programme for the measurement of chlorinated hydrocarbons in the
section of the Elbe between Schnackenburg and the North Sea,
1980-1982], Hamburg, Study Group for Keeping the Elbe Clean, pp.
64-65, 84, 86-94 (in German).
ARBEITSGEMEINSCHAFT FÜR DIE REINHALTUNG DER ELBE (1988) [Water
quality data for the Elbe from Schnackenburg to the sea. Numerical
table, 1988], Hamburg, Study Group for Keeping the Elbe Clean, p. 158
(in German).
ARTIGAS, F., MARTINEZ, E., CAMON, L., GELPI, E., & RODRIGUEZ-FARRE, E.
(1988) Brain metabolites of lindane and related isomers:
Identification by negative ion mass spectrometry. Toxicology, 49:
57-63.
ATLAS, E. & GIAS, C.S. (1981) Global transport of organic pollutants:
Ambient concentrations in the remote marine atmosphere. Science, 211:
163-165.
BACCI, E., CALAMARI, D., GAGGI, C., FANELLI, R., FOCARDI, S., &
MOROSINI, M. (1986) Chlorinated hydrocarbons in lichen and moss
samples from the Antarctic Peninsular. Chemosphere, 15(6): 747-754.
BAUER, U. (1972) [Concentration of insecticides, chlorinated
hydrocarbons and PCB in algae.] Schr. Reihe Ver.
Wasser-Boden-Lufthyg., 37: 211-219 (in German).
BAUMANN, K., ANGERER, J., HEINRICH, R., & LEHNERT, G. (1980)
Occupational exposure to hexachlorocyclohexane. 1. Body burden of
HCH-isomers. Int. Arch. occup. environ. Health, 47: 119-127.
BAUMANN, K., BEHLING, K., BRASSOW, H.L., & STAPEL, K. (1981)
Occupational exposure to hexachlorocyclohexane. III.
Neurophysiological findings and neuromuscular function in chronically
exposed workers. Int. Arch. occup. environ. Health, 48: 165-172.
BEHRBOHM, P. & BRANDT, B. (1959) [Allergic and toxic dermatitis in
the manufacturing and processing of hexachlorocyclohexane.] Arch.
Gewerbepathol. Gewerbehyg., 17: 365-383 (in German).
BENSON, W.W., WATSON, M., & WYLLIE, J. (1973) Organochlorine
residues in wild moose, Idaho-1972. Pestic. monit. J., 7: 97-99.
BERTRAM, H.P., KEMPER, F.H., & ZENZEN, C. (1980) [Occurrence of HCH
isomers in man.] In: [Hexachlorocyclohexane as a harmful substance
in foods. Papers from two symposia of the Senate Committee on the
Testing of Residues in Foods, held on 28-29 November 1979 and 6 March
1980], Weinheim, Verlag Chemie, pp. 155-163 (in German).
BIDLEMAN, T.F. & LEONARD, R. (1982) Aerial transport of pesticides
over the Northern Indian Ocean and adjacent seas. Atmos. Environ., 16:
1099-1107.
BLOK, S.M.G., GREVE, P.A., SANGSTER, B., SAVELKOUL, T.J.F., & WEGMAN,
R.C.C. (1984) [Investigation of normally occurring values of a
number of organochlorine pesticides and related compounds and their
metabolites, of polychlorobiphenyls and of chlorophenols in the blood
or plasma of healthy volunteers], Bilthoven, National Institute of
Public Health and Environmental Hygiene (Report No. 638101001) (in
Dutch).
BOULEKBACHE, H. (1980) Study of the toxicity of the alpha-, beta-,
and gammaisomers of hexachlorocyclohexane to fish, using the guppy as
the test species, Paris, Université Paris VII, Laboratoire d'Anatomie
comparée (Unpublished report).
BRASSOW, H.L., BAUMANN, K., & LEHNERT, G. (1981) Occupational
exposure to hexachlorocyclohexane. II. Health conditions of
chronically exposed workers. Int. Arch. occup. environ. Health, 48:
81-87.
BÜSSER, M.T. & LUTZ, W.K. (1987) Stimulation of DNA synthesis in rat
and mouse liver by various tumour promoters. Carcinogenesis, 8(10):
1433-1437.
CAMON, L., MARTINEZ, E., ARTIGAS, F., SOLA, C., & RODRIGUEZ-FARRE, E.
(1988) The effect of non-convulsant doses of lindane on temperature
and body weight. Toxicology, 49: 389-394.
CANTON, J.H. & SLOOFF, W. (1977) The usefulness of Lymnea stagnalis L.
as a biological indicator in toxicological bioassays (model substance
alpha-HCH). Water Res., 11: 117-121.
CANTON, J.H., GREVE, P.A., SLOOFF, W., & VAN ESCH, G.J. (1975)
Toxicity, accumulation, and elimination studies of
alpha-hexachlorocyclohexane (alpha-HCH) with freshwater organisms of
different trophic levels. Water Res., 9: 1163-1169.
CANTON, J.H., VAN ESCH, G.J., GREVE, P.A., & VAN HELLEMOND, A.B.A.M
(1977) Accumulation and elimination of alpha-hexachlorocyclohexane
(alpha-HCH) by the marine algae Chlamydomonas and Dunaliella.
Water Res., 11: 111-115.
CANTON, J.H., WEGMAN, R.C.C., VULTO, Th.J.A., VERHOEF, C.H., & VAN
ESCH, G.J. (1978) Toxicity-, accumulation-, and elimination-studies
of alpha-hexachlorocyclohexane (alpha-HCH) with saltwater organisms of
different trophic levels. Water Res., 12: 687-690.
CANTON, J.H., MENNES, W.C., MATHIJSSEN-SPIEKMAN, E.A.M., WEGMAN,
R.C.C., HOFSTEE, A.W.M., & WESTER, P.W. (1982) [Study of the toxicity
of beta-HCH for freshwater organisms], Bilthoven, National Institute
of Public Health and Environmental Hygiene (Unpublished report No.
62797001) (in Dutch).
CETINKAYA, M., GABEL, B., PODBIELSKI, A., & THIEMANN, W. (1984) [On
the correlation of nutrition and living habits of feeding mothers and
contamination of human milk with non-volatile chlorinated organic
chemicals.] Akt. Ernähr., 9(4): 157-162 (in German).
CHATTOPADHYAY, P., KARNIK, A.B., THAKORE, K.N., LAKKAD, B.C., NIGAM,
S.K., & KASHYAP, S.K. (1988) Health effects among workers involved
in the manufacture of hexachlorocyclohexane. J. Soc. Occup. Med., 38:
77-81.
CHESSELLS, M.J., HAWKER, D.W., CONNELL, D.W., & PAPAJCSIK, I.A. (1988)
Factors influencing the distribution of lindane and isomers in soil
of an agricultural environment. Chemosphere, 17(9): 1741-1749.
COPER, H., HERKEN, H., & KLEMPAU, I. (1951) [Antagonism and
synergism of ß- and gamma-hexachlorocyclohexanes.] Klin. Wochenschr.,
29(13/14): 264-265 (in German).
CORNACOFF, J.B., LAUER, L.D., HOUSE, R.V., TUCKER, A.N., THURMOND,
L.M., VOS, J.G., WORKING, P.K., & DEAN, J.H. (1988) Evaluation of
the immunotoxicity of ß-Hexachlorocyclohexane (ß-HCH). Fundam. appl.
Toxicol., 11: 293-299.
COWAN, A.A. (1981) Organochlorine compounds in mussels from Scottish
coastal waters. Environ. Pollut., B2: 129-143.
DAVIES, D. & MES, J. (1987) Comparison of the residue levels of some
organochlorine compounds in breastmilk of the general and indigenous
Canadian populations. Bull. environ. Contam. Toxicol., 39: 743-749.
DEICHMANN, W.B. & MACDONALD, W.E. (1977) Organochlorine pesticides and
liver cancer deaths in the United States, 1930-1972. Ecotoxicol.
environ. Saf., 1: 89-110.
DEMOZAY, D. & MARECHAL, G. (1972) Physical and chemical properties.
In: Ulman, E., ed. Lindane: Monograph of an insecticide, Freiburg im
Breisgau, Verlag K. Schillinger, pp. 14-21.
DEN TONKELAAR, E.M., VAN NIMWEGEN, J.M., & SEKHUIS, V.M. (1981)
[Induction of microsomal liver enzymes following subacute
administration of alpha-, beta-, and gamma-HCH to the rat], Bilthoven,
National Institute of Public Health and Environmental Hygiene
(Unpublished report No. 618111001) (in Dutch).
DEO, P.G., HASAN, S.B., & MAJUMDER, S.K. (1980) Isomerization of ß-HCH
in aqueous solution. J. environ. Sci. Health, B15(2): 147-164.
DEO, P.G., HASAN, S.B., & MAJUMDER, S.K. (1981) Interconversions and
toxicity changes in hexachlorocyclohexane isomers on dispersion in
water. J. environ. Sci. Health, B16(6): 691-701.
DFG (1978) [Residues in breast milk: situation and assessment],
Boppard, Germany, Harald Boldt Verlag (in German).
DOELMAN, P., HAANSTRA, L., DE RUITER, E., & SLANGE, J., (1985) Rate
of microbial degradation of high concentrations of
delta-hexachlorocyclohexane in soil under aerobic and anaerobic
conditions. Chemosphere, 14(5): 565-570.
DOELMAN, P., HAANSTRA, L., & VOS, A. (1988a) Microbial degradation
by the autochthonous soil population of alpha and beta-HCH under
anaerobic field conditions in temperate regions. Chemosphere, 17(2):
481-487.
DOELMAN, P., HAANSTRA, L., & VOS, A. (1988b) Microbial sanitation of
soil with alpha- and beta-HCH under aerobic glass house conditions.
Chemosphere, 17(2): 489-492.
DOISY, E.A., Jr & BOCKLAGE, B.C. (1950) Inositol and the toxicity of
four isomers of benzenehexachloride for the rat. Proc. Soc. Exp. Biol.
Med., 74: 613-616.
ECKENHAUSEN, F.W., BENNETT, D., BEYNON, K.I., & ELGAR, K.E. (1981)
Organochlorine pesticide concentrations in perinatal samples from
mothers and babies. Arch. environ. Health, 36(2): 81-92.
EDELMAN, Th. (1984) [Background values of a number of anorganic and
organic substances in the soil of the Netherlands; an initial
reconnaissance], The Hague, State Publishing House (Soil protection,
report VROM 34) (in Dutch).
EDER, G., STURM, R., & ERNST, W. (1987) Chlorinated hydrocarbons in
sediments of the Elbe River and the estuary. Chemosphere, 16(10/12):
2487-2496.
EGAN, H. & HUBBARD, A.W. (1975) Analytical surveys of food. Br. med.
Bull., 31(3): 201-208.
EICHLER, D. (1977) Experiments on the decomposition of lindane when
exposed to UV-light (Unpublished report Celamerck No. 111AC-143-004,
submitted to WHO by CIEL).
EICHLER, D., HEUPT, W., & PAUL, W. (1983) Comparative study on the
distribution of alpha- and gamma-hexachlorocyclohexane in the rat with
particular reference to the problem of isomerization. Xenobiotica,
13(11): 639-647.
ENGST, R., MACHOLZ, R.M., & KUJAWA, M. (1978) [Metabolites of
HCH-isomers in human blood.] Pharmazie, 33(2/3): 109-111 (in German).
ERNST, W. (1977) Determination of the bioconcentration of marine
organisms. A steady state approach. I. Bioconcentration data for
seven chlorinated pesticides in mussels (Mytilus edulis) and their
relation to solubility data. Chemosphere, 6(11): 731-340.
ERNST, W. (1979) Factors affecting the evaluation of chemicals in
laboratory experiments using marine organisms. Ecotoxicol. environ.
Saf., 3: 90-98.
FALADYSZ, J. & SZEFER, D. (1982) Chlorinated hydrocarbons in diving
ducks wintering in Gdansk Bay, Baltic Sea. Sci. total Environ., 24:
119-127.
FISHMAN, B.E. (1987) The neurotoxicity of the isomers of
hexachlorocyclohexane: actions at the GABA-A-receptor linked chloride
channel. Diss. Abstr. Int., B48: 3257-3258.
FISHMAN, B.E. & GIANUTSOS, G. (1987) Opposite effects of different
hexachlorocyclohexane (lindane) isomers on cerebellar cyclic GMP:
relation of cyclic GMP accumulation to seizure activity. Life Sci.,
41: 1703-1709.
FISHMAN, B.E. & GIANUTSOS, G. (1988) CNS biochemical and
pharmacological effects of the isomers of hexachlorocyclohexane
(lindane) in the mouse. Toxicol. appl. Pharmacol., 93: 146-153.
FITZHUGH, O.G., NELSON, A.A., & FRAWLEY, J.P. (1950) The chronic
toxicities of technical benzene hexachloride and its alpha-, beta-,
and gamma-isomers. J. Pharmacol. exp. Ther., 100(1): 59-66.
FOOKEN, C. & BUTTE, W. (1987) Organochlorine pesticides and
polychlorinated biphenyls in human milk during lactation. Chemosphere,
16(6): 1301-1309.
FOSTER, T.S. & SAHA, J.G. (1978) The in vitro metabolisation of
lindane by an enzyme preparation from chicken liver. J. environ. Sci.
Health, B13(1): 25-45.
FRANK, R., BRAUN, H.E., SIRONS, G.H., RASPER, J., & WARD, G.G. (1985)
Organochlorine and organophosphorus insecticides and industrial
pollutants in the milk supplies of Ontario, 1983. J. Food Prod.,
48(6): 499-504.
FRANKLIN, A. (1987) The concentration of metals, organochlorine
pesticide and PCB residues in marine fish and shell fish: results from
MAFF fish and shell fish monitoring programmes, 1977-1984, Lowestoft,
Ministry of Agriculture, Fisheries, and Food, Directorate of Fisheries
Research (Aquatic Environment Monitoring Report No. 16).
FREAL, J.J. & CHADWICK, R.W. (1973) Metabolism of
hexachlorocyclohexane to chlorophenols and effect of isomer
pretreatment on lindane metabolism in rat. J. agric. food Chem.,
21(3): 424-427.
GAGGI, C., BACCI, E., CALAMARI, D., & FANELLI, R. (1986) Chlorinated
hydrocarbons in plant foliage: An indication of the tropospheric
contamination level. Chemosphere, 14(11-12): 1673-1686.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1985a) Pesticides, selected elements and other chemicals in adult
total diet samples, October 1978-September 1979. J. Assoc. Off. Anal.
Chem., 68(5): 862-875.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1985b) Pesticides, selected elements and other chemicals in Infant
and Toddler total diet samples, October 1978-September 1979. J. Assoc.
Off. Anal. Chem., 68(5): 842-861.
GEYER, H., SCHEUNERT, I., & KORTE, F. (1986) Bioconcentration
potential of organic environmental chemicals in humans. Regul.
Toxicol. Pharmacol., 6: 313-347.
GOTO, M., HATTORI, M., & MIYAGAWA, T. (1972a) [Contributions to
ecological chemistry. II. Hepatoma formation in mice after
administration of high doses of HCH isomers.] Chemosphere, 6: 279-282
(in German).
GOTO, M., HATTORI, M., & MIYAGAWA, T. (1972b) [Contributions to
ecological chemistry. Toxicity of alpha-BHC in mice.] Chemosphere, 4:
153-154 (in German).
GREVE, P.A. (1985) Organochlorine pesticides and PCBs in human
tissues from Dutch citizens (1968-1983), Bilthoven, National Institute
of Public Health and Environmental Hygiene (Unpublished concept
report).
GREVE, P.A. & VAN HARTEN, D.C. (1983) [Relationship between levels
of organochlorine pesticides and PCBs in fat and blood in human
subjects], Bilthoven, National Institute of Public Health and
Environmental Hygiene (Unpublished report No. 638219001) (in Dutch).
GREVE, P.A. & VAN HULST, Sj. (1977) [Organochlorine pesticides and
PCBs in duplicate 24-hour feeds], Bilthoven, National Institute of
Public Health and Environmental Hygiene (Unpublished report No. 192/77
Tox/ROB) (in Dutch).
GREVE, P.A. & VAN HULST, Sj. (1980) [Content of beta- and gamma-HCH
and some of their metabolites in rat tissues derived from the
semi-chronic toxicity tests], Bilthoven, National Institute of Public
Health and Environmental Hygiene (Unpublished report No. 144/80,
RA/617908001) (in Dutch).
GREVE, P.A. & WEGMAN, R.C.C. (1985) Organochlorine compounds in
human milk; data from a recent investigation in the Netherlands.
Working paper for WHO Consultation on Organochlorine Compounds in
Human Milk and Related Hazards, Bilthoven, 9-11 January, 1985,
Bilthoven, National Institute of Public Health and Environmental
Hygiene.
GUICHERIT, R. & SCHULTING, F.L. (1985) The occurrence of organic
chemicals in the atmosphere of the Netherlands. Sci. total Environ.,
43: 193-219.
GUNDERSON, E.L. (1988) FDA total diet study, April 1982-April 1984.
Dietary intakes of pesticides, selected elements and other chemicals.
J. Assoc. Off. Anal. Chem., 71(6): 1200-1209.
GUPTA, S.K., PARIKH, J.R., SHAH, M.P., CHATTERJEE, S.K., & KASHYAP,
S.K. (1982) Changes in serum HCH residues in malaria spraymen after
short-term occupational exposure. Arch. environ. Health, 37(1): 41-44.
HAIDER, K. (1979) Degradation and metabolization of lindane and
other hexachlorocyclohexane isomers by anaerobic and aerobic soil
microorganisms. Z. Naturforsch., 34C: 1066-1069.
HAMADA, M., KAWANO, E., KAWAMURA, S., & SHIRO, M. (1982) A new
isomer of 1,2,3,4,5-pentachlorocyclohexane from UV-irradiation
products of alpha-, ß-, and delta-isomers of 1,2,3,4,5,6-
hexachlorocyclohexane. Agric. biol. Chem., 46(1): 153-157.
HANADA, M., YUTANI, C., & MIYAJI, T. (1973) Induction of hepatoma in
mice by benzene hexachloride. Gann., 64(5): 511-513.
HAPKE, H.J. & HOLLMANN, A.C. (1985) [Effects of
beta-hexachlorocyclohexane on suckling rats.] Dtsch. Tierärztl.
Wschr., 92(1): 125-164 (in German).
HEESCHEN, W. (1985) Studies on the carry-over of beta-
hexachlorocyclohexane in the milk of cows. Kieler Milchwirtsch.
Forschungsber., 37(1): 29-43.
HERITAGE, A.D. & MACRAE, I.C. (1977) Identification of intermediates
formed during the degradation of hexachlorocyclohexanes by
Clostridium sphenoïdes. Appl. environ. Microbiol., 33(6): 1295-1297.
HERITAGE, A.D. & MACRAE, I.C. (1979) Degradation of
hexachlorocyclohexanes and structurally related substances by
Clostridium sphenoïdes. Aust. J. biol. Sci., 32(4/5): 493-500.
HERRMANN, R., THOMAS, W., & HÜBNER, D. (1984) [Behaviour of organic
micropollutants (PAH, PCB and BHC) and of a fecal sterol in the Husum
estuary and in the adjacent North Frisian Wadden sea.] Dtsch.
Gewässerkd. Mitt., 4: 101-107 (in German).
HILDEBRANDT, G., JEEP, H., HURKA, H., STUKE, T., HEITMAN, M., &
BOISELLE, C. (1986) [Breast milk as a biological indicator: Study on
the organic halogen burden in breast milk and foodstuffs], Berlin,
Schmidt E. Verlag (Report 5/86 of the Federal Office for the
Environment) (in German).
HMSO (1969) Further review of certain persistent organochlorine
pesticides used in Great Britain. Report by the Advisory Committee on
Pesticides and other Toxic Chemicals, London, Her Majesty's Stationery
Office.
HMSO (1982) Report of the Working Party on Pesticide Residues
(1977-1981): Ninth report of the Steering Group on Food Surveillance,
London, Ministry of Agriculture, Fisheries, and Food, Her Majesty's
Stationery Office (Food Surveillance Paper No. 9).
HMSO (1986) Report of the Working Party Pesticide on Residues
(1982-1985): Sixteenth report of the Steering Group on Food
Surveillance, London, Ministry of Agriculture, Fisheries, and Food,
Her Majesty's Stationery Office (Food Surveillance Paper No. 16).
HMSO (1989) Report of the Working Party on Pesticide Residues
(1985-1988): Twenty-fifth report of the Steering Group on Food
Surveillance, London, Ministry of Agriculture, Fisheries, and Food,
Her Majesty's Stationery Office (Food Surveillance Paper No. 25).
HOFFMANN, A. (1983) [Toxicity of alpha- and beta-HCH.] In:
[Hexachlorocyclohexane as a harmful substance in foodstuffs. Papers
from two seminars of the Senate Committee on the Testing of Residues
in Foodstuffs held on 28-29 November 1979 and 6 March 1980], Weinheim,
Chemie Verlag, pp. 201-214 (in German).
HOLT, R.L., CRUSE, S., & GREER, E.S. (1986) Pesticide and
polychlorinated biphenyl residues in human adipose tissue from North
East Louisiana. Bull. environ. Contam. Toxicol., 36: 651-655.
HORI, S. & KASHIMOTO, T. (1974) Transfer of beta-HCH from mother to
suckling mouse. Shokuhin Eiseigaku Zasshi, 15(6): 446-450.
IARC (1979) Hexachlorocyclohexane (technical HCH and lindane). In:
Some halogenated hydrocarbons, Lyon, International Agency for Research
on Cancer, pp. 195-239 (IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Vol. 20).
IARC (1987) Hexachlorocyclohexanes (Group 2B). In: Overall
evaluations of carcinogenicity: An updating of IARC Monographs Vol. 1
to 42, Lyon, International Agency for Research on Cancer (IARC
Monographs on the Evaluation of Carcinogenic Risks to Humans, Suppl.
7).
ITO, N., NAGASAKI, H., ARAI, M., MAKIURA, S., SUGIHARA, S., & HIRAO,
K. (1973a) Histopathologic studies on liver tumorigenesis induced in
mice by technical polychlorinated biphenyls and its promoting effect
on liver tumours induced by benzene hexachloride. J. Natl Cancer
Inst., 51(5): 1637-1646.
ITO, N., NAGASAKI, H., ARAI, M., SUGIHARA, S., & MAKIURA, S. (1973b)
Histologic and ultrastructural studies on the hepatocarcinogenicity of
benzene hexachloride in mice. J. Natl Cancer Inst., 51(3): 817-826.
ITO, N., NAGASAKI, H., AOE, H., SUGIHARA, S., MIYATA, Y., ARAI, M., &
SHIRAI, T. (1975) Brief Communication: Development of hepatocellular
carcinomas in rats treated with benzene hexachloride. J. Natl Cancer
Inst., 54(3): 801-905.
ITO, N., HANAOUCHI, M., SUGIHARA, S., SHIRAI, T., TSUDA, H.,
FUKUSHIMA, S., & NAGASAKI, H. (1976) Reversibility and
irreversibility of liver tumours in mice induced by the alpha-isomer
of 1,2,3,4,5,6,-hexachlorocyclohexane. Cancer Res., 36: 2227-2234.
ITO, N., TSUDA, H., HASEGAWA, R., & IMAIDA, K. (1983) Comparison of
the promoting effects of various agents in induction of preneoplastic
lesions in rat liver. Environ. Health Perspect., 50: 131-138.
IVERSON, F., RYAN, J.J., LIZOTTE, R., & HIERLIHY, S.L. (1984) In vivo
and in vitro binding of alpha- and gamma-hexachlorocyclohexane to
mouse liver macromolecules. Toxicol. Lett., 20(3): 331-335.
JAGNOW, G., HAIDER, K., & ELLWARDT, P.Chr. (1977) Anaerobic
dechlorination and degradation of hexachlorocyclohexane isomers by
anaerobic and facultative anaerobic bacteria. Arch. Microbiol., 115:
285-292.
JANSSEN, P.J.C.M., VAN KOTEN-VERMEULEN, J.E.M., KRAJNC, E.I., CANTON,
J.H., VAN GESTEL, C.A.M., VAN DER HEIJDEN, C.A., & HEIJNA-MERKENS, E.
(1987) [Hexachlorocyclohexane (Basic document)], Bilthoven, National
Institute of Public Health and Environmental Hygiene (Project No.
840820) (in Dutch).
KAMPE, W. (1980) [Occurrence of hexachlorocyclohexane in the soil.]
In: [Hexachlorocyclohexane as a harmful substance in foodstuffs],
Weinheim, Verlag Chemie, pp. 18-23 (Research report) (in German).
KAN, C.A. & JONKER-DEN ROOYEN, J.C. (1978a) Accumulation and depletion
of some organochlorine pesticides in broiler breeder hens during the
second laying cycle. J. agric. food Chem., 26(2): 465-470.
KAN, C.A. & JONKER-DEN ROOYEN, J.C. (1978b) Accumulation and depletion
of some organochlorine pesticides in high producing laying hens. J.
agric. food Chem., 26(4): 935-940.
KAN, C.A., JONKER-DEN ROOYEN, J.C., TUINSTRA, L.G.M.Th., ROOS, A.H.,
& TRAUG, W. (1978) Possible influence of sex and embryonic content on
accumulation of some organochlorine pesticides in broilers. J. agric.
food Chem., 26(3): 618-621.
KAN, C.A., STRIK, J.J.T.W.A., & KOEMAN, J.H. (1979) Semi-chronic
toxicity of beta-HCH in laying hens. Meded. Fac. Landbouwwet.
Rijksuniv. Gent, 44(2): 965-973.
KORANSKY, W., PORTIG, J., & MÜNCH, G. (1963) [Absorption,
distribution and excretion of alpha- and gamma-hexachlorocyclohexane.]
Naunyn-Schmiedebergs Arch. exp. Pathol. Pharmakol. 244: 564-575 (in
German).
KORANSKY, W., PORTIG, J., VOHLAND, H.W., & KLEMPAU, I. (1964)
[Elimination of alpha- and gamma-hexachlorocyclohexane and the way it
is influenced by enzymes of liver microsomes.] Naunyn-Schmiedebergs
Arch. exp. Pathol. Pharmakol., 247: 49-60 (in German).
KORTE, F. (1980) [Occurrence of HCH isomers in man.] In:
[Hexachlorocyclohexane as a harmful substance in foodstuffs. Papers
from two seminars of the Senate Committee on the Testing of Residues
in Foodstuffs held on 28-29 November 1979 and 6 March 1980], Weinheim,
Verlag Chemie, pp. 46-64 (in German).
KRAUS, D. (1975) Biodegradation of alpha-hexachlorocyclohexane by
microsomes and cytosol of rat liver. Naunyn-Schmiedebergs Arch.
Pharmakol., 291: 79-87.
KRAUTHACKER, B., ALEBIC-KOLBAH, T., KRALJ, M., TKALCEVIC, B., &
REINER, E. (1980) Organochlorine pesticides in blood serum of the
general Yugoslav population and in occupationally exposed workers.
Int. Arch. occup. environ. Health, 45: 217-220.
KRISHNAKUMARI, M.K. (1977) Sensitivity of the alga Scenedesmus
acutusto some pesticides. Life Sci., 20: 1525-1532.
KUIPER, H.A., DEN TONKELAAR, E.M., VAN LEEUWEN, F.X.R., FRANKEN,
M.A.M., & DORMANS, J.A.M.A. (1985) [Semi-chronic toxicity test with
alpha-HCH], Bilthoven, National Institute of Public Health and
Environmental Hygiene (Report No. 617917002) (in Dutch).
KURIHARA, N. (1975) Urinary metabolites from ß- and gamma-HCH in the
mouse: chlorophenol conjugates. Environ. Qual. Saf., 4: 56-73.
KURIHARA, N. & NAKAJIMA, M. (1974) Studies on BHC isomers and
related compounds VIII. Urinary metabolites produced from gamma- and
ß-BHC in the mouse: Chlorophenol conjugates. Pestic. Biochem.
Physiol., 4: 220-231.
KUTZ, F.W., STRASSMAN, S.C., & SPERLING, J.F. (1979) Survey of
selected organochlorine pesticides in the general population of the
United States: Fiscal years 1970-1975. Ann. NY Acad. Sci., 320: 60-68.
LAUGEL, P. (1981) [The residue situation in France and ways of
overcoming it.] Lebensmittelchem. gerichtl. Chem., 35: 29-32 (in
German).
LAWLOR, T. & HAWORTH, S.R. (1979) Evaluation of the genetic activity
of nine chlorinated phenols, seven chlorinated benzenes and three
chlorinated hexanes. Environ. Mutagen., 1(2): 143.
LAY, J.P., KLEIN, W., KORTE, F., & RICHTER, E. (1981) Metabolism
of ß-hexachlorocyclohexane-[14]C in rats following low dosing in the
daily diet. J. environ. Sci. Health, B16: 227-238.
LOEBER, J.G. & VAN VELSEN, F.L. (1985) Uterotropic effect of ß-HCH.
A food chain contaminant. Food Addit. Contam., 1: 63-66.
LOWDEN, G.F., SAUNDERS, C.L., & EDWARDS, R.W. (1969) Organochlorine
insecticides in water (Part II). J. Water Treat. Exam., 18: 275-287.
LWA (1987) [Water quality report], Düsseldorf, North-Rhine Westphalia
Office for Water and Refuse, 24 pp (in German).
MACHOLZ, R.M., KNOLL, R., LEWERENZ, H.J., PETRZIKA, M., & ENGST, R.
(1982) Metabolism of alpha-hexachlorocyclohexane. Free metabolites in
urine and organs of rats. Xenobiotica, 12(4): 227-231.
MACHOLZ, R.M., KNOLL, R., LEWERENZ, H.J., PLASS, R., & SCHULZE, J.
(1983) [Metabolism of gamma-hexachlorocyclohexane (HCH) in germ-free
and conventionalized rats.] Zentralbl. Pharm., 122(2): 221 (in
German).
MACHOLZ, R.M., BLEYL, D.W., KLEPEL, H., KNOLL, R., KUJAWA, M.,
LEWERENZ, H.J., MUELLER, D., & PLASS, R. (1986) Comparison of
distribution and toxicity of alpha-, beta-, and gamma-BHC after
application to rats for 30 days. Nahrung, 30: 701-708.
MACRAE, I.C., RAGHU, K., & CASTRO, T.F. (1967) Persistence and
biodegradation of four common isomers of benzenehexachloride in
submerged soils. J. agric. food Chem., 15(5): 911-914.
MACRAE, J.C., YAMAYA, Y., & YOSHIDA, T. (1984) Persistence of
HCH-isomers in soil suspensions. Soil Biol. Biochem., 16: 285-286.
MARTIN, D.B. & HARTMANN, W.A. (1985) Organochlorine pesticides and
polychlorinated biphenyls in sediment and fish from wetlands in the
North-Central United States. J. Assoc. Off. Anal. Chem., 69(4):
712-717.
MATHUR, R., SAXENA, D.M., & AGARWAL, H.C. (1984) Growth of a ciliate
protozoan. Tetrahymena pyriformis in the presence of different
isomers of hexachlorocyclohexane (HCH). Acta. Protozool., 23(3):
165-174.
MATSUMOTO, K., ELDERFRAWI, M.E., & ELDEFRAWI, A.T. (1988) Action of
polychlorocycloalkane insecticides in binding of [355]
t-butyldicyclophosphorothionate to torpedo electric organ membranes
and stereospecificity of the binding site. Toxicol. appl. Pharmacol.,
95: 220-229.
MATSUSHIMA, S. (1972) [Pollution of food by agricultural chemicals and
the diet.] Rinsho. Eiyo., 40: 555-563 (in Japanese).
MES, J., DAVIES, D.J., & TURTON, D. (1982) Polychlorinated biphenyl
and other chlorinated hydrocarbon residues in adipose tissue of
Canadians. Bull. environ. Contam. Toxicol., 28: 97-104.
MES, J., DAVIES, D.J., TURTON, D., & SUN, W.-F. (1986) Levels and
trends of chlorinated hydrocarbon contaminants in the breast milk of
Canadian women. Food Addit. Contam., 3(4): 313-322.
MESTRES, R. (1974) Report of a group of experts on the content of
organohalogen compounds detected between 1968 and 1972 in water, air
and foodstuffs and the methods of analysis used in the nine Member
States of the European Communities. Presented at the European
Colloquium on Problems raised by the Contamination of Man and his
Environment by Persistent Pesticides and Organohalogenated Compounds,
Luxembourg, 14-16 May, 1974, Luxembourg, Commission of the European
Communities (Paper No. 1).
MORGAN, D.P. & LIN, L.I. (1978) Blood-organochlorine pesticide
concentrations, clinical hematology and biochemistry in workers
occupationally exposed to pesticides. Arch. environ. Contam. Toxicol.,
7: 423-447.
MOUVET, C., GALOUX, M., & BERNES, A. (1985) Monitoring of
polychlorinated biphenyls (PCBs) and hexachlorocyclohexanes (HCH) in
freshwater using the aquatic mossCinclidotus danubicus.Sci. total
Environ., 44: 253-267.
MÜLLER, D., KLEPEL, H., MACHOLZ, R.M., LEWERENZ, H.J., & ENGST, R.
(1981) Electroneurophysiological studies on neurotoxic effects of
hexachlorocyclohexane-isomers and gamma-pentachlorocyclohexane. Bull.
environ. Contam. Toxicol., 27: 704-706.
MURPHY, R. & HARVEY, C. (1985) Residues and metabolites of selected
persistent halogenated hydrocarbons in blood specimens from a general
population survey. Environ. Health Perspect., 60: 115-120.
NAGASAKI, H. (1973) Chronic toxicity of benzene hexachloride (BHC).
Nara Igaku Zasshi, 24: 1-26.
NAGASAKI, H., KAWABATA, H., MIYATA, Y., INOUE, K., HIRAO, K., AOE, H.,
& ITO, N. (1975) Effect of various factors on induction of liver
tumours in animals by the isomer of benzene hexachloride. Gann., 66:
185-191.
NAKAJIMA, E., SHINDO, H., & KURIHARA, N. (1970) [Whole body
autoradiographic studies on the distribution of alpha-, beta-, and
gamma-benzene hexachloride in mice.] Radio-isotopes, 19: 538 (in
Japanese).
NIESSEN, K.H., RAMOLLAA, J., BINDER, M., BRUGMANN, G., & HOFMANN, U.
(1984) Chlorinated hydrocarbons in adipose tissue of infants and
toddlers: inventory and studies on their association with intake of
mother's milk. Eur. J. Pediatr., 142: 238-243.
NIGAM, S.K., KARNIK, A.B., MAJUMDER, S.K., VISWESWARRIAH, K.,
SURYANARAYANA RAJU, G., MUKTHA BAI, K., LAKKAD, B.C., THAKORE, K.N., &
CHATTERJEE, B.B. (1986) Serum hexachlorocyclohexane residues in
workers engaged at a HCH manufacturing plant. Int. Arch. occup.
environ. Health, 57: 315-320.
NISHIMURA, N. (1982) Survey on the mutagenicity of pesticides by a
Salmonella microsome test. Aichi Ika Daigaku Igakkai Zasshi, 10(4):
305-312.
NOACK, G. & PORTIG, J. (1973) Biodegradation of alpha-hexa-
chlorocyclohexane. III. Decrease in liver non-protein thiol after
intragastric application of the drug. Naunyn-Schmiedebergs Arch.
Pharmakol., 280: 183-189.
NOACK, G., PORTIG, J., & WIRSCHING, M. (1975) Biodegradation of
alpha-hexachlorocyclohexane. IV. The extent of degradation of single
doses in vivo. Naunyn-Schmiedebergs Arch. Pharmakol., 288: 57-64.
NOREN, K. (1983) Levels of organochlorine contaminants in human milk
in relation to the dietary habits of the mothers. Acta paediatr.
Scand., 72: 811-816.
NORSTROM, R.J., SIMON, M., MUIR, D.C.G., & SCHWEINSBURG, R.E. (1988)
Organochlorine contaminants in Arctic marine food chains:
Identification, geographical distribution and temporal trends in polar
bears. Environ. Sci. Technol., 22: 1063-1071.
NYBOM, N. & KNUTSSON, B. (1947) Investigations on c-mitosis in
Allium cepa. I. The cytological effect of hexachlorocyclohexane.
Hereditas, 33: 220-234.
OEHME, M. & MANO (1984) The long-range transport of organic
pollutants to the Arctic. Fresenius Z. anal. Chem., 319: 141-146.
OEHME, M. & STRAY, H. (1982) Quantitative determination of
ultra-traces of chlorinated compounds in high-volume air samples from
the Arctic using polyurethane foam as collecting medium. Fresenius Z.
anal. Chem., 311: 665-673.
OSHIBA, K. (1972) Experimental studies on the fate of ß- and
gamma-BHC in vivo following daily administration. Osaka Shiritsu
Daigaku Igaku Zasshi, 21(1-3): 1-19.
OSHIBA, K. & KAWAKITA, H. (1972) Interaction between toxicant and
nutrition. IV. Effect of lipid metabolism on reduction of the BHC
deposition in rat tissues. Chem. Abstr., 77(8): 148231.
PALMER, C.M. & MALONEY, T.E. (1955) Preliminary screening for
potential algicides. Ohio J. Sci., 55(1): 1-8.
POLISHUK, Z.W., WASSERMANN, M., WASSERMANN, D., GRONER, Y.,
LAZAROVICI, S., & TOMATIS, L. (1970) Effects of pregnancy on storage
of organochlorine insecticides. Arch. environ. Health, 20: 215-217.
PORTIG, J. (1983) [Elimination of hexachlorocyclohexane (HCH) by
mammals.] In: [Hexachlorocyclohexane as a harmful substance in
foodstuffs. Papers from two seminars of the Senate Committee on the
Testing of Residues in Foodstuffs held on 28-29 November 1979 and 6
March 1980], Weinheim, Chemie Verlag, pp. 108-118 (in German).
PORTIG, J. & VOHLAND, H.W. (1983) [Neuropharmacological and
neurotoxic effects of hexachlorocyclohexane in man.] In:
[Hexachlorocyclohexane as a harmful substance in foodstuffs. Papers
from two seminars of the Senate Committee on the Testing of Residues
in Foodstuffs held on 28-29 November 1979 and 6 March 1980], Weinheim,
Chemie Verlag, pp. 215-226 (in German).
PORTIG, J., KRAUS, P., SODOMANN, S., & NOACK, G. (1973)
Glutathion-dependent biotransformation in rats of isomers of
hexachlorocyclohexane. Naunyn-Schmiedebergs Arch. Pharmakol., 279:
185-198.
PORTIG, J., STEIN, K., & VOHLAND, H.W. (1989) Preferential
distribution of delta-hexachlorocyclohexane into cerebral white
matter. Xenobiotica, 19(1): 123-130.
RADOMSKI, J.L., DEICHMANN, W.B., ALBERTO, A., REY, A., & MERKIN, T.
(1971a) Human pesticide blood levels as a measure of body burden and
pesticide exposure. Toxicol. appl. Pharmacol., 20: 175-185.
RADOMSKI, J.L., ASTOLFI, E., DEICHMANN, W.B., & REY, A.A. (1971b)
Blood levels of organochlorine pesticides in Argentina. Occupationally
and non-occupationally exposed adults, children, and new-born infants.
Toxicol. appl. Pharmacol., 20: 186-193.
RAPPE, C., NYGREN, M., & LINDSTRÖM, G. (1987) Polychlorinated
dibenzofurans and dibenzo-p-dioxins and other chlorinated
contaminants in cow milk from various locations in Switzerland.
Environ. Sci. Technol., 21: 964-970.
REINER, E., KRAUTHACKER, B., STIPCEVIC, M., & STEFANAC, Z. (1977)
Blood levels of chlorinated hydrocarbon residues in the population of
a continental town in Croatia (Yugoslavia). Pestic. monit. J., 11:
54-55.
RIEMSCHNEIDER, R. (1949) [A contribution to the toxicity of contact
insecticides.] Anz. Schädlingskd., 22(1): 1-3 (in German).
SAGELSDORFF, P., LUTZ, W.K., & SCHLATTER, C. (1983) The relevance of
covalent binding to mouse liver DNA to the carcinogenic action of
hexachlorocyclohexane isomers. Carcinogenesis, 4(10): 1267-1273.
SAIKI, M.K. & SCHMITT, C.J. (1986) Organochlorine chemical residues
in bluegills and common carp from the irrigated San Joaquin Valley
Floor, California. Arch. environ. Contam. Toxicol., 15: 357-366.
SCHRÖTER, C., PARZEFALL, W., SCHRÖTER, H., & SCHULTE-HERMANN, R.
(1987) Dose-response studies on the effects of alpha-, ß-, and
gamma-hexachlorocyclohexane on putative preneoplastic foci,
monooxygenases, and growth in rat liver. Cancer Res., 47: 80-88.
SCHULTE-HERMANN, R. & PARZEFALL, W. (1981) Failure to discriminate
initiation from promotion of liver tumours in a long term study with
the phenobarbital-type inducer alpha-hexachlorocyclohexane and the
role of sustained stimulation of hepatic growth and monooxygenases.
Cancer Res., 41: 4140-4146.
SCHULTE-HERMANN, R., LEBERL, C., LANDGRAF, H., & KORANSKY, W. (1974)
Liver growth and mixed function oxidase activity: dose dependent
stimulatory and inhibitory effects of alpha-hexachlorocyclohexane.
Naunyn-Schmiedebergs Arch. exp. Pathol. Pharmakol., 285: 355-366.
SCHULTE-HERMANN, R., OHDE, G., SCHUPPLER, J., & TIMMERMANN-TROSIENER,
I. (1981) Enhanced proliferation of putative preneoplastic cells in
rat liver following treatment with the tumour promotors phenobarbital,
hexachlorocyclohexane, steroid compounds and nafenopin. Cancer Res.,
41(6): 2556-2562.
SCHULTE-HERMANN, R., TIMMERMANN-TROSIENER, I., & SCHUPPLER, J., (1983)
Promotion of spontaneous preneoplastic cells in rat liver as a
possible explanation of tumour production by non-mutagenic compounds.
Cancer Res., 43: 839-844.
SEIFART, J. & BUCHAR, E. (1978) The biosynthesis of pyrimidine
nucleotides and cytochrome P-450 content in rat liver microsomes after
the administration of alpha-hexachlorocyclohexane ( in vivo study).
Toxicology, 11: 37-44.
SHAHIN, M. & VON BORSTEL, R. (1977) Mutagenic and lethal effects of
alphabenzene hexachloride, dibutyl phthalate and trichloroethylene in
Saccharomyces cerevisiae. Mutat. Res., 48: 173-180.
SHAIN, W., NARANG, R., & SEEGAL, R. (1987) Neurotoxicity testing of
chlorinated hydrocarbons by measuring specific neuronal and glial cell
functions. Prog. clin. biol. Res., 253: 207-216.
SHIBATA, T. (1978) Experimental studies on the dynamic aspects of
BHC and PCB in vivo. Jpn. J. Hyg., 32: 736-746.
SHIMAZU, H., SHIRAISHI, N., AKEMATSU, T., UEDA, N., & SUGIYAMA, T.
(1976) Carcinogenicity screening tests on induction of chromosomal
aberrations in rat bone marrow cells in vivo. Mutat. Res., 38: 347
(Abstract No. 20).
SIDDARAMAPPA, R. & SETHUNATHAN, N. (1975) Persistence of gamma-BHC
and beta-BHC in Indian rice soils under flooded conditions. Pestic.
Sci., 6: 395-403.
SIERRA, M. & SANTIAGO, D. (1987) Organochlorine pesticide levels in
barn owls collected in Leon, Spain. Bull. environ. Contam. Toxicol.,
38: 261-265.
SKAARE, J.U., TUVENG, J.M., & SANDE, H.A. (1988) Organochlorine
pesticides and polychlorinated biphenyls in maternal adipose tissue,
blood, milk, & cord blood from mothers and their infants living in
Norway. Arch. environ. Toxicol., 17: 55-63.
SKAFTASON, J.F. & JOHANNESSON, T. (1979) Organochlorine compounds
(DDT, hexachlorocyclohexane, hexachlorobenzene) in Icelandic animal
body fat and butter fat: Local and global sources of contamination.
Acta pharmacol. toxicol., 44: 156-157.
SLOOFF, W. & MATTHIJSEN, A.J.C.M. (1988) Integrated criteria
document: Hexachlorocyclohexanes, Bilthoven, National Institute of
Public Health and Environmental Protection (Report No. 758473011).
SRINIVASAN, K. & RADHAKRISHNAMURTY, R. (1977) Effect of beta- and
gamma-isomers of hexachlorocyclohexane on some liver and kidney
enzymes in albino rats. Curr. Sci., 46: 598-600.
SRINIVASAN, K. & RADHAKRISHNAMURTY, R. (1983) Studies on the
distribution of the beta- and gamma-isomers of hexachlorocyclohexane
in rat tissues. J. environ. Sci. Health, B18(3): 401-418.
SRINIVASAN, K. & RADHAKRISHNAMURTY, R. (1988) Biochemical changes
produced by beta- and gamma-Hexachlorocyclohexane isomers in albino
rats. J. environ. Sci. Health, B23(4): 367-386.
SRINIVASAN, K., RAMESH, H.P., & RADHAKRISHNAMURTY, R. (1984) Renal
tubular dysfunction caused by dietary hexachlorocyclohexane (HCH)
isomers. J. environ. Sci. Health, B19(45): 453-466.
SRINIVASAN, K., RAMESH, H.P., & RADHAKRISHNAMURTY, R. (1988) Changes
induced by hexachlorocyclohexane isomers in rat livers and testes.
Bull. environ. Contam. Toxicol., 41: 531-539.
STARR, H.G., Jr, ALDRICH, F.D., MCDOUGALL, W.D., III, & MOUNCE, L.M.
(1974) Contribution of household dust to the human exposure to
pesticides. Pestic. monit. J., 8: 209-212.
STEIN, K. & PORTIG, J. (1976) Oxidative transformation of
hexachlorocyclohexane in the rat. Naunyn-Schmiedebergs Arch.
Pharmacol., 293: R51.
STEIN, K., PORTIG, J., & KORANSKY, W. (1977) Oxidative
transformation of hexachlorocyclohexane in rats and with rat liver
microsomes. Naunyn-Schmiedebergs Arch. Pharmakol., 298: 115-128.
STEIN, K., PORTIG, J., FUHRMANN, H., KORANSKY, W., & NOACK, G. (1980)
Steric factors in the pharmacokinetics of lindane and
alpha-hexachlorocyclohexane in rats. Xenobiotica, 10(1): 65-77.
STRACHAN, W.M.J., HUNEAULT, H., SCHERTZER, W.M., & ELDER, F.C. (1980)
Organochlorines in precipitation in the Great Lakes Region. In:
Afghan, B.K. & MacKay, D., ed. Hydrocarbons and halogenated
hydrocarbons in the aquatic environment, New York, London, Plenum
Press.
SUGIURA, K., SATO, S., GOTO, M., & KURIHARA, Y. (1976) Effects of
beta-HCH on aquatic microcosm. Chemosphere, 1: 39-44.
SUGIURA, K., WASHINO, T., HATTORI, M., SATO, E., & GOTO, M. (1979)
Accumulation of organochlorine compounds in fishes. Difference of
accumulation factors by fishes. Chemosphere, 6: 359-364.
SUZUKI, M., YAMATO, Y., & WATANABE, T. (1975) Persistence of BHC
(1,2,3,4,5,6-hexachlorocyclohexane) and dieldrin residues in field
soils. Bull. environ. Contam. Toxicol., 14(5): 520-529.
SZOKOLAY, A., ROSIVAL, L., UHNAK, J., & MADARIC, A. (1977a) Dynamics
of benzene hexachloride (BHC) isomers and other chlorinated pesticides
in the food chain and human fat. Ecotoxicol. environ. Saf., 1(1):
349-359.
SZOKOLAY, A., MADARIC, A., & UHNAK, J. (1977b) Relative cumulation
of beta-HCH in ecological and biological systems. J. environ. Sci.
Health, B12(3): 193-212.
SZYMCZYNSKI, G.A., WALISZEWSKI, S.M., TUSZEWSKI, M., & PYDA, P. (1986)
Chlorinated pesticides levels in human adipose tissue in the district
of Poznan. J. environ. Sci. Health, A21(1): 5-14.
TAKABATAKE, E. (1978) Levels of organochlorine compounds and heavy
metals in tissues of the general population in Japan. Geogr. med., 8:
1-23.
TANABE, S., TATSUKAWA, R., KAWANO, M., & HIDAKA, H. (1982) Global
distribution and atmospheric transport of chlorinated hydrocarbons:
HCH(BHC) isomers and DDT compounds in the Western Pacific, Eastern
Indian- and Antarctic Oceans. J. Oceanogr. Soc. Jpn, 38(3): 137-148.
TANOOKA, H. (1977) Development and applications ofBacillus
subtilistest systems for mutagens, involving DNA-repair deficiency and
suppressible auxotrophic mutations. Mutat. Res., 42: 19-32.
THAMAVIT, W., HIASA, Y., ITO, N., & BHAMARA-PRAVATI, N. (1974) The
inhibitory effects of alpha-benzene hexachloride on
3'-methyl-4-dimethyl aminobenzene and DL-ethionine carcinogenesis in
rats. Cancer Res., 34: 337-340.
THORPE, E. & WALKER, A.I.T. (1973) The toxicology of dieldrin
(HEOD). II Comparative long-term oral toxicity studies in mice with
dieldrin, DDT, phenobarbitone, ß-BHC and gamma-BHC. Food Cosmet.
Toxicol., 11: 433-442.
TIECCO, G. (1961) [Testing for synthetic oestrogens in foodstuffs.]
Vet. Ital., 12: 447-460 (in Italian).
TOMCZAK, S., BAUMANN, K., & LEHNERT, G. (1981) Occupational exposure
to hexachlorocyclohexane. IV. Sex hormone alterations in HCH-exposed
workers. Int. Arch. occup. environ. Health, 48: 283-287.
TRYPHONAS, L. & IVERSON, F. (1983) Sequential histopathologic
analysis of alpha-hexachlorocyclohexane induced hepatic megalocytosis
and adenoma formation in the HPB mouse. J. Natl Cancer Inst., 71(6):
1307-1318.
TSUKANO, Y. (1973) Factors affecting disappearance of BHC-isomers
from rice field soil. Jpn. agric. Res. Q., 7(2): 93-97.
TUINSTRA, L.G.M.Th. (1971) Organochlorine insecticide residues in
human milk in the Leiden region. Neth. Milk Dairy J., 25: 24-32.
UMWELTBUNDESAMT (1989) [Data on the environment, 1988/89], Berlin,
Schmidt E. Verlag, pp. 403-405, 410-412, 416-417, 521-522, 524, 526
(in German).
VAN ASPEREN, K. (1954) Interaction of the isomers of
benzenehexachloride in mice. Arch. Int. Pharmacodyn., 99(3/4):
368-377.
VAN GIERSBERGEN, P.L.M., VAN VELSEN, F.L., VAN LEEUWEN, F.X.R., &
DANSE, L.H.J.C. (1984) [Toxicity of beta-HCH: relationship between
the biochemistry and pathology of the liver in a 6-week feeding test],
Bilthoven, National Institute of Public Health and Environmental
Hygiene (Unpublished report No. 618303001) (in Dutch).
VAN HOOF, M., VAN VELSEN, F.L., DANSE, L.H.J.C., & VAN LEEUWEN, F.X.R.
(1982) [Toxicity of beta-HCH: relationship between the biochemistry
and pathology of the liver in a 3-week feeding test], Bilthoven,
National Institute of Public Health and Environmental Hygiene (Report
No. 618115001) (in Dutch).
VAN VELSEN, F.L. (1986) The oestrogenicity of the environmental
contaminant ß-hexachlorocyclohexane, University of Utrecht (Thesis).
VAN VELSEN, F.L., VAN LEEUWEN, F.X.R., & DANSE, L.H.J.C. (1982)
[Investigation into the toxicity, kinetics and metabolism of beta- and
gamma-HCH], Bilthoven, National Institute of Public Health and
Environmental Hygiene (Unpublished report No. 617908003) (in Dutch).
VAN VELSEN, F.L., DANSE, L.H.J.C., VAN LEEUWEN, F.X.R., DORMANS,
J.A.M.A., & VAN LOGTEN, M.J. (1986) The subchronic oral toxicity of
the ß-isomer of hexachlorocyclohexane in rats. Fundam. appl. Toxicol.,
6: 697-712.
VOHLAND, H.W., PORTIG, J., & STEIN, K. (1981) Neuropharmacological
effects of isomers of hexachlorocyclohexane. 1. Protection against
pentylenetetrazolinduced convulsions. Toxicol. appl. Pharmacol.,
57(3): 425-438.
VOHLAND, H.W. & KORANSKY, W. (1983) [The behaviour and effects of
hexachlorocyclohexane in man.] In: [Hexachlorocyclohexane as a harmful
substance in foodstuffs. Paper from two seminars of the Senate
Committee on the Testing of Residues in Foodstuffs held on 28-29
November 1979 and 6 March 1980], Weinheim, Chemie Verlag, pp. 246-262
(in German).
VUKAVIC, T., PAVKOV, S., CUSIC, S., RONCEVIC, N., VOJINOVIC, M., &
TOKOVIC, B. (1986) Pesticide residues in human colostrum: seasonal
variations, Yugoslavia. Arch. environ. Contam. Toxicol., 15: 525-528.
WAHID, P.A. & SETHUNATHAN, N. (1979) Sorption-desorption of alpha, ß,
and gamma-isomers of hexachlorocyclohexane in soils. J. agric. food
Chem., 27(5): 1050-1053.
WASSERMANN, M., ROGOFF, M.G., TOMATIS, L., DAY, N.E., WASSERMANN, D.,
DJAVAHERIAN, M., & GUTTEL, C. (1972) Storage of organochlorine
insecticides in the adipose tissue of people in Kenya. Ann. Soc. Belge
Méd. Trop., 52(6): 509-514.
WEGMAN, R.C.C. & GREVE, P.A. (1980) Halogenated hydrocarbons in
Dutch water samples over the years 1969-1977. In: Afghan, B.K. &
MacKay, D., ed. Hydrocarbons and halogenated hydrocarbons in the
aquatic environment, New York, London, Plenum Press, pp. 405-415.
WESTER, P.W. & CANTON, J.H. (1986) Histopathological study of
Oryzias latipes (medaka) after long-term ß-hexachlorocyclohexane
exposure. Aquat. Toxicol., 9: 21-45.
WESTER, P.W., CANTON, J.H., & BISSCHOP, A. (1985) Histopathological
study of Poecilia reticulata (guppy) after long-term
ß-hexachlorocyclohexane exposure. Aquat. Toxicol., 6: 271-296.
WHO (1986) Joint FAO/WHO Food Contamination Monitoring Programme.
Summary of 1980-1983 monitoring data, Geneva, World Health
Organization (GEMS report WHO/EHE/FOS/86.2).
WHO (1991) Environmental Health Criteria 124: Lindane, Geneva, World
Health Organization.
WITTICH, U. & SCHULTE-HERMANN, R. (1977) Liver growth and hepatic
monooxygenase activity in suckling rats following administration of
alpha-hexachlorocyclohexane to the mother. Arch. Pharmacol.,
297(suppl. II): R15.
WITTLINGER, R. & BALLSCHMITER, K. (1987) Global baseline pollution
studies XI: Congener specific determination of polychlorinated
biphenyls (PCBs) and occurrence of alpha- and gamma-
hexachlorocyclohexane (HCH), 4.4'-DDE and 4.4'-DDT in continental air.
Chemosphere, 16(10/12): 2497-2513.
WOLF, H.P. (1983) [Implementation of results of a trial with dairy
cows contaminated with 12-HCH.] In: [Hexachlorocyclohexane as a
harmful substance in foodstuffs. Papers from two seminars of the
Senate Committee on the Testing of Residues in Foodstuffs held on
28-29 November 1979 and 6 March 1980], Weinheim, Chemie Verlag, pp.
143-154 (in German).
YAMATO, Y., KIYONAGA, M., & WATANABE, T. (1983) Comparative
bioaccumulation and elimination of HCH-isomers in short-necked clam
(Venerupis japonica) and guppy (Poecilia reticulata). Bull.
environ. Contam. Toxicol., 31: 352-359.
ZHOU, X., LIU, R., ZHANG, W., XUE, X., & LIU, H. (1986) The effect
of BHC isomers on photosynthetic formation of oxygen by green algae.
Zhiwu Shenglixue Tongxun, 6: 32-34.
APPENDIX 1. CHEMICAL STRUCTURE
The basic structure of HCH is a closed chain of six carbon atoms.
The structure can have two spatial forms, i.e. cis and trans
configurations. Every carbon atom is bound to a hydrogen and a
chlorine atom. One of these substituents forms a plane with the two
connecting carbon atoms. Since this plane parallels the "equator" of
the molecule, this atom is said to be in the equatorial position. The
binding with the other atom parallels the "axis" of the molecule.
Therefore, this one is in the axial position. Due to the size of the
chlorine atom the carbon atoms are not free to rotate. Hence the
positions of the chlorine and hydrogen atoms are fixed, one being
always in the equatorial position and the other in the axial position.
The various combinations of the spatial orientation of the
hydrogen and chlorine atoms on each of the carbon atoms of cyclohexane
results in different isomeric compounds. Theoretically, 17 isomers of
HCH are possible, but, due to spatial incompatibilities and
thermodynamic instability, only nine isomers have in fact been
detected. They all have the trans configuration.
In the beta-isomer, all chlorine atoms are in the equatorial
position.
The positions of the chlorine atoms in the major isomers of HCH
are presented in Table 9 (Demozay & Marechal, 1972; Van Velsen, 1986).
Table 9. Positions of chlorine atoms in the major HCH isomers
Isomer Chlorine positionsb Physical structure
Alphaa A A E E E E monoclinic prisms
Beta E E E E E E octahedral cubic crystals
Gamma A A A E E E monoclinic crystals
Delta A E E E E E crystals/fine platelets
Epsilon A E E A E E monoclinic needles or hexagonal mono-
clinic crystals
a racemate of two optical isomers
b A = axial position; E = equatorial position From: van Velsen (1986).
RESUME ET EVALUATION
1. Alpha-hexachlorocyclohexane
1.1 Propriétés générales
L'Alpha-hexachlorocyclohexane (alpha-HCH) est un important
sous-produit (60-70%) de la fabrication du lindane (> 99% de
gamma-HCH). Il est peu soluble dans l'eau mais très soluble dans les
solvants organiques comme l'acétone, le chloroforme et le xylène.
C'est un solide de faible tension de vapeur. Son coefficient de
partage entre le n-octanol et l'eau (log Pow) est de 3,82. C'est
un polluant de l'environnement.
L'alpha-HCH peut être dosé séparément des autres isomères par
chromatographie en phase gazeuse avec détection par capture
d'électrons ou par d'autres méthodes après extraction par partage
liquide/liquide et purification sur colonne chromatographique.
1.2 Transport, distribution et transformation dans l'environnement
Il se produit dans l'environnement une biodégradation ainsi
qu'une dégradation abiotique (déchloration) sous l'action du
rayonnement ultraviolet, qui aboutissent respectivement, au
delta-3,4,5,6-tétrachlohéxène et au pentachlorocyclohéxène Ce
processus de décomposition est plus lent que dans le cas du lindane.
La persistance de l'alpha-HCH dans le sol dépend de facteurs
environnementaux comme l'action des microorganismes, la teneur en
matières organiques, la co-distillation et l'évaporation. Il n'y a pas
d'isomérisation du lindane en alpha-HCH.
La bioconcentration est rapide chez les microorganismes (facteur
de bioconcentration allant de 1500 à 2700, calculé en poids à sec ou
environ 12 000 calculé par rapport aux lipides en l'espace de 30
minutes): chez les invertébrés, il est de 60 à 2750 (poids à sec) ou
> 8000 (par rapport aux lipides) sur une durée de 24 à 72 heures.
Dans le cas des poissons ces chiffres vont de 313 à 1216 sur 4 à 28
jours, les valeurs pouvant atteindre 50 000 pour les poissons de
l'Elbe. Toutefois la biotransformation et l'élimination sont également
assez rapide chez ces organismes (15 minutes à 72 heures).
1.3 Concentrations dans l'environnement et exposition humaine
L'alpha-HCH est présent dans l'air des océans à la concentration
de 0,02 à 1,5 mg/m3. Au Canada, on en a trouvé dans l'eau de pluie
à des teneurs de 1 à 40 ng par litre, mais la neige n'en contenait que
des traces.
Au cours de la période 1969-1974, on a constaté dans le Rhin et
ses affluents des concentrations d'alpha-HCH comprises entre 0,01 et
2,7 µg/litre; toutefois, plus récemment, ces concentrations sont
descendues en-dessous de 0,01 µg/litre. Dans l'Elbe, les
concentrations sont passées de 0,023 µg/litre en moyenne en 1981, à
moins de 0,012 µg/litre en 1988. Dans un certain nombre de cours d'eau
du Royaume-Uni on a trouvé en 1966 des concentrations allant de 0,001 à
0,43 µg/litre. En Frise, dans le nord de la Mer des Wadden on a trouvé
dans les sédiments des concentrations d'alpha-HCH allant de 0,3 à
1,4 µg/kg (0,002 µg/litre d'eau).
Les teneurs en alpha-HCH de différentes espèces végétales en
provenance de divers pays vont de 0,5 à 2140 µg/kg de poids sec mais
elles peuvent être encore beaucoup plus élevées dans les régions
polluées. Même dans l'Antarctique, on a rencontré des concentrations
allant de 0,2 à 1,15 µg/kg. On trouve régulièrement du alpha-HCH
dans les poissons et les invertébrés aquatiques ainsi que chez des
canards, des hérons et des effraies. On a trouvé dans la graisse
sous-cutanée de rennes et d'orignaux vivant dans des régions où
l'épandage de pesticides est négligeable, des quantités d'alpha-HCH
égales en moyenne à 7-80 µg/kg. Dans les tissus adipeux d'ours
polaires canadiens on a trouvé des teneurs d'alpha-HCH allant de
0,3 à 0,87 mg/kg (calculées par rapport au tissu adipeux).
Dans un certain nombre de pays on a procédé à l'analyse de
denrées alimentaires importantes à la recherche d'alpha-HCH. Les
teneurs mesurées, présentes essentiellement dans des produits
contenant des graisses, allaient jusqu'à 0,05 mg/kg de produit,
sauf dans le lait et les produits laitiers (jusqu'à 0,22 mg/kg)
et dans le poisson et les produits carnés industriels (jusqu'à
0,5 mg/kg par rapport aux graisses). On a constaté un léger recul
des teneurs au cours des années.
C'est principalement par les aliments que la population dans
son ensemble est exposée à l'alpha-HCH. Des études de ration totale
effectuées aux Pays-Bas et au Royaume-Uni ont révélé des
concentrations moyennes respectivement égales à 0,01 et
0,002-0,003 mg/kg. Les données concernant le Royaume-Uni montrent
qu'il existe une tendance à la baisse depuis 1967. Aux Etats-Unis
d'Amérique, l'apport alimentaire moyen d'alpha-HCH a été de 0,09 à
0,025 mg/kg de poids corporel par jour, au cours de la période
1977-1979 et de 0,003 à 0,016 µg/kg de poids corporel au cours de
la période 1982-1984.
Dans quelques pays, on a mesuré la concentration d'alpha-HCH
dans le sang, le sérum et le plasma humain. La concentration moyenne
(dans certains cas, la médiane) était inférieure à 0,1 µg/litre
(intervalle de variation: de non décelable à 0,6 µg/litre). Dans un
des pays cependant on a relevé une concentration moyenne de
3,5 µg/litre (intervalle de variation 0,1 à 15,0) dans le tiers
environ des échantillons de sang.
Dans le tissu adipeux humain et le lait maternel, les
concentrations d'alpha-HCH qui ont été relevées sont faibles
(respectivement moins de 0,01-0,1 et moins de 0,001-0,04 mg/kg
calculées par rapport aux graisses). Des études de ration totale
ont montré que l'apport alimentaire quotidien était de l'ordre de
0,01 µg/kg de poids corporel ou moins. Ces concentrations ont
tendance à diminuer avec le temps.
L'alpha-HCH se présente comme un contaminant universel de
l'environnement. Les teneurs ne diminuent que lentement malgré les
mesures prises en vue d'en éviter la propagation dans le milieu.
1.4 Cinétique et métabolisme
Chez le rat, l'alpha-HCH est rapidement et presque complètement
résorbé au niveau des voies digestives. Après injection intra-
péritonéale, environ 40 à 80% de l'alpha-HCH administré a été
excrété dans les urines et 50 à 20% dans Les matières fécales. Chez
le rat également, les concentrations les plus élevées se rencontrent
dans le foie, les reins, les tissus adipeux, l'encéphale et les
muscles, avec une accumulation importante dans la fraction Lipidique.
On a constaté que chez des rats à la mamelle, la concentration
hépatique d'alpha-HCH était deux fois plus élevée que chez les mères.
Chez le rat également, le rapport de la concentration dans l'encéphale
à la concentration sanguine et de la concentration dans la masse
grasse à la concentration sanguine était respectivement de 120:1 et
397:1.
Chez le rat, la biotransformation de l'alpha-HCH comporte une
déchloration. Le principal métabolite urinaire est le 2,4,6-
trichlorophénol; on a également identifié d'autres métabolites tels
que le 1,2,4, le 2,3,4 et le 2,4,5-trichlorophénol ainsi que le 2,3,4,5-
et le 2,3,4,6-tétrachlorophénol. On a trouvé dans les reins de rats du
1,3,4,5,6-pentachlorocyhex-1-ène, substance dont la présence a été
également observée dans le foie de poulets lors d'études in vitro.
Dans le foie, il y a conjugaison avec le glutathion.
Le demi-vie de libération à partir de la masse grasse est de 6,9
jours chez la ratte et de 1,6 jour chez le rat.
1.5 Effets sur les êtres vivants dans leur milieu naturel
L'alpha-HCH est faiblement toxique pour les algues, la dose sans
effet observable se situant en général à 2 mg/litre.
Une étude de longue durée sur Daphnia magna a montré que la
dose sans effet observable était de 0,05 mg/litre pour cette espèce.
L'alpha-HCH est modérément toxique pour les invertébrés et les
poissons. Pour ces organismes, les valeurs de la CL(E)50 sont de
l'ordre de 1 mg/litre. Lors d'études de courte durée effectuées sur
des guppies et sur Oryzia latipes, on a constaté qu'une dose de
0,8 mg/litre était sans effet.
Lors d'études de trois mois sur Salmo gairdneri soumis à des
doses allant de 10 à 1250 mg/kg de nourriture, on n'a observé aucun
effet sur la mortalité, le comportement, la croissance ou l'activité
enzymatique du foie et du cerveau.
Des études de courte et de longue durée sur un mollus-que
(Lymnea stagnalis) ont montré que la CE50 était dans ce cas de
1200 µg/litre (déterminée d'après la mortalité et l'immobilisation des
mollusques). A la concentration de 250 µg/litre il y a eu inhibition
de la ponte. Une réduction de 50% a été notée dans le taux global de
reproduction à la concentration de 65 µg/litre.
On ne dispose d'aucune donnée concernant les effets sur les
populations et les écosystèmes.
1.6 Effets sur les animaux d'expérience et les systèmes
d'épreuve in vitro
La DL50 se situe entre 1000 et 4000 mg/kg pour la souris et
entre 500 et 4670 mg/kg de poids corporel pour le rat. Les signes
d'intoxication sont essentiellement ceux d'une stimulation du système
nerveux central.
Lors d'une étude de 90 jours sur des rats, on a constaté une
baisse de la croissance à la concentration de 250 mg/kg de nourriture.
A partir de 50 mg/kg, des modifications au niveau histologique et
enzymatique témoignaient d'une induction des enzymes. A ces doses on a
également noté des signes d'immunodépression. Il y avait déjà
accroissement du poids du foie à partir de 10 mg/kg de nourriture
(soit l'équivalent de 0,5 mg/kg de poids corporel). La dose sans effet
nocif observé se situait à 2 mg/kg de nourriture (soit l'équivalent de
0,1 mg/kg de poids corporel par jour).
Il n'y a pas eu d'études convenables de toxicité à long terme ni
d'études sur la reproduction et le pouvoir tératogène.
Des études effectuées sur diverses souches de Salmonella
typhimurium n'ont révélé aucun signe de mutagénicité, que ce soit en
présence ou en l'absence d'une activation métabolique. Les tests sur
Saccharomyces cerevisiae ont également été négatifs, toutefois la
recherche d'une synthèse non programmée de l'ADN sur des hépatocytes
de rat in vitro a donné un résultat équivoque.
On a effectué des travaux en vue de déterminer le pouvoir
cancérogène de l'alpha-HCH sur des rats et des souris à des doses
allant de 100 à 600 mg/kg de nourriture. Chez des souris, on a observé
des nodules hyperplastiques et/ou des adénomes hépatocellulaires. Dans
une des études, les doses dépassaient la dose maximale tolérable. Lors
de trois autres études, deux sur des souris et une sur des rats, on
n'a observé aucune augmentation dans l'incidence des tumeurs à des
doses allant jusqu'à 160 mg/kg de nourriture (souris) et 640 mg/kg de
nourriture (rats).
Les résultats des études sur le pouvoir d'initiation et de
promotion ainsi que sur le mode d'action de l'alpha-HCH, de même que
les tests de mutagénicité, montrent que les tumeurs induites par
l'alpha-HCH chez la souris ne sont pas d'origine génétique.
On a montré que l'alpha-HCH provoquait une nette augmentation de
l'activité des enzymes hépatiques, même à des doses de 5 mg/kg de
nourriture (soit l'équivalent de 0,25 mg/kg de poids corporel). A la
dose de 2 mg/kg de poids corporel, l'alpha-HCH n'a eu aucun effet sur
la déméthylation de l'aminopyrine ni sur la teneur du foie en ADN.
1.7 Effets sur l'homme
L'examen de travailleurs d'une usine produisant du lindane, qui
avaient été exposés pendant 7,2 années (en moyenne géométrique, avec
des limites de 1 à 30 ans), a permis de conclure qu'une exposition
professionnelle au HCH ne produit pas de signes de troubles
neurologiques ni de perturbation de la fonction neuromusculaire.
RESUME ET EVALUATION
2. Béta-hexachlorocyclohexane
2.1 Propriétés générales
Le béta-hexachlorocyclohexane (béta-HCH) est un sous-produit
(7-10%) de la fabrication du lindane (> de 99% de gamma-HCH). Peu
soluble dans l'eau, il est très soluble dans les solvants organiques
tels que l'acétone, le cyclohexane et xylène. C'est un solide de
faible tension de vapeur. Son coefficient de partage entre le
n-octanol et l'eau (log Pow) est égal à 3,80. C'est un polluant
de l'environnement.
On peut doser le béta-HCH séparément des autres isomères par
chromatographie en phase gazeuse avec détection par capture
d'électrons ainsi que par d'autres méthodes après extraction par
partage liquide/liquide et purification sur colonne chromatographique.
2.2 Transport, distribution et transformation dans l'environnement
La biodégradation et la dégradation abiotique (déchloration) sous
l'effet du rayonnement ultraviolet, produisent du
pentachlorocyclohexane, mais beaucoup plus lentement que dans le cas
du lindane (gamma-HCH).
Le béta-HCH est l'isomère le plus persistant de l'HCH. Sa
persistance dans le sol dépend de facteurs environnementaux tels que
l'action des microorganismes, la teneur en matières organiques et en
eau ainsi que la co-distillation et l'évaporation.
En raison de sa persistance, le béta-HCH subit une
bioconcentration rapide chez les invertébrés (le facteur de
bioconcentration est d'environ 125 en l'espace de trois jours), chez
les poissons (250-1500 calculé à partir du poids à sec ou environ
500 000 fois calculé sur la base des lipides en l'espace de 3 à
10 minutes), ainsi que chez les oiseaux et l'homme (environ 525). Le
béta-HCH se concentre davantage et s'élimine plus lentement que les
autres isomères de l'HCH.
2.3 Concentrations dans l'environnement et exposition humaine
On rencontre le béta-HCH dans l'air des océans à des
concentrations de 0,004 à 0,13 ng/m3.
Jusqu'en 1974, le Rhin et ses affluents présentaient des teneurs
en béta-HCH allant de 0,14 à 0,22 µg par litre, mais depuis on
constate que ces valeurs sont systématiquement inférieures à
0,12 µg/litre. Des échantillons prélevés dans la Meuse présentaient
des teneurs inférieures à 0,12 µg/litre. Dans l'Elbe, les
concentrations sont passées en moyenne de 0,009 à 0,004 µg/litre entre
1981 et 1988.
On a dosé le béta-HCH chez des oiseaux tels que les éperviers,
les faucons crécerelles, les hiboux, les hérons et les grèbes pendant
un certain nombre d'années et l'on a observé des concentrations allant
de 0,1 à 0,3 mg/kg. Chez les ours polaires on a mesuré des
concentrations allant jusqu'à 0,87 mg/kg (par rapport au tissu
adipeux) dans le foie et les graisses.
Dans quelques pays on a procédé à l'analyse de denrées
alimentaires importantes en vue d'y rechercher la présence de
béta-HCH. Les concentrations moyennes, mesurées essentiellement dans
des denrées contenant des graisses, allaient jusqu'à 0.03 mg/kg (par
rapport au contenu lipidique), mais on en a trouvé jusqu'à 4 mg/kg
(par rapport au contenu lipidique) dans des produits laitiers. Dans
les denrées non grasses, les teneurs étaient inférieures à 0,05 mg/kg
de produit. En général, ces teneurs sont en lent recul.
C'est principalement par les aliments que la population dans son
ensemble est exposée au béta-HCH. Lors d'études de ration totale
effectuées au Royaume-Uni, on a mesuré des concentrations de 0,003,
0,0005 et moins de 0,0005 mg/kg respectivement en 1966/67, 1975/77 et
1981. Aux Etats-Unis d'Amérique, l'apport moyen quotidien d'origine
alimentaire allait en 1982-84 de moins de 0,1 à 0,4 ng/kg de poids
corporel dans les différents groupes d'âge.
Dans un certain nombre de pays, on a procédé au dosage du
béta-HCH dans le sang, le sérum ou le plasma au sein de la population
générale. Les concentrations varient d'un pays à l'autre, atteignant
parfois 25 µg/litre.
De nombreuses études ont été menées afin de rechercher la
présence de béta-HCH dans les tissus adipeux humains. Les
concentrations relevées au Canada, en République fédérale d'Allemagne,
au Kenya, aux Pays-Bas et au Royaume-Uni atteignaient jusqu'à
4,4 mg/kg (par rapport au contenu lipidique). On a constaté une
augmentation progressive avec l'âge jusqu'à environ 50 ans, après quoi
les teneurs déclinaient. Dans les tissus adipeux, les concentrations
de béta-HCH sont plus élevées que celles des autres isomères,
phénomène qui traduit la tendance à l'accumulation de cette substance.
Il n'y a pas de tendance claire à la baisse des concentrations de
béta-HCH sur la période au cours de laquelle ces études ont été
effectuées. On a constaté l'existence d'une relation entre les
concentrations de béta-HCH dans les tissus adipeux et le lait maternel
d'une part, et la consommation de produits carnés, de graisses
animales et de poissons gras, d'autre part.
Dans quelques pays (Canada, République fédérale d'Allemagne,
Pays-Bas et Royaume-Uni) on a procédé au dosage du béta-HCH dans le
lait maternel et obtenu des concentrations allant de 0,1 à 0,69 mg/kg
(par rapport au contenu lipidique). Il ressort de ces analyses que la
concentration du béta-HCH est plus élevée dans le lait des femmes des
zones rurales que dans celui des femmes des zones urbaines.
Le béta-HCH apparaît comme un contaminant universel de
l'environnement. Les concentrations n'accusent qu'une très lente
tendance à la baisse malgré les mesures prises en vue d'en empêcher la
propagation dans l'environnement.
2.4 Cinétique et métabolisme
Le béta-HCH est absorbé jusqu'à 95% dans les voies digestives de
la souris, et s'accumule ensuite en majeure partie dans les tissus
adipeux. L'élimination s'effectue selon un mécanisme en deux étapes,
la demi-vie étant de 2,5 jours pour la première et de 18 jours pour la
seconde.
Une fois résorbé, le béta-HCH se répartit rapidement dans les
divers organes et tissus: foie, encéphale, reins et tissus adipeux.
Chez le rat, la concentration maximale dans le foie est atteinte en
quatre jours. Pour une concentration sanguine moyenne de 92 µg/litre
(mais également pour des concentrations de 540 et 2100 µg/litre), le
rapport des concentrations dans le cerveau et le sang d'une part et
dans le tissu adipeux et le sang d'autre part était respectivement de
2:1 et de 170:1. Après une intoxication mortelle chez l'homme par des
isomères de l'HCH, on a constaté que la concentration en béta-HCH
mesurée par rapport à la teneur du sang était de 363 dans les tissus
adipeux, de 3 dans le cerveau et de 15 dans le foie. Le béta-HCH
franchit la barrière hémo-méningée beaucoup moins facilement que les
autres isomères de la l'HCH.
Chez des souris gravides, le passage transplacentaire du béta-HCH
au foetus était d'environ 2% de la dose, mais atteignait 40% chez des
rattes gravides. Chez le rat, le passage de la mère aux ratons à la
mamelle par l'intermédiaire du lait correspondait à environ 60% de la
dose.
Chez le rat, 70% du béta-HCH est éliminé dans les 28 premiers
jours, dont un tiers par la voie urinaire. On ne retrouve pas de
béta-HCH inchangé dans l'urine. Le principal métabolite résultant de
la cis-déshydrochloration est le 2,4,6-trichlorophénol sous forme
conjuguée.
Un prétraitement au moyen de béta-HCH modifie le métabolisme du
lindane chez le rat. D'après des études comportant l'administration
de béta-HCH par voie intrapéritonéale à des souris, il semble que
celui-ci soit métabolisé plus lentement que le lindane.
2.5 Effets sur les êtres vivants dans leur milieu naturel
En général le béta-HCH est modérément toxique pour les algues,
les invertébrés et les poissons. La DL50 aiguë pour ces organismes
est de l'ordre de 1 mg/litre mais les valeurs de la CE50 sont plus
faibles (0,05-0,5 mg/litre). Dans le cas de deux espèces de poisson
d'eau douce, Oryzia latipes et Poecilia reticulata, la dose sans
effet observable a été fixée à 0,03 mg/litre sur une durée de un et
trois mois respectivement.
On ne dispose d'aucune donnée concernant les effets sur les
populations et les écosystèmes.
2.6 Effets sur les animaux d'expérience et les systèmes
d'épreuve in vitro
Les valeurs de la DL50 aiguë par voie orale pour les souris et
les rats publiées en 1968, se situaient entre 1500 et 2000 mg/kg de
poids corporel. Toutefois des études plus récentes ont fourni des
valeurs de 16 g/kg de poids corporel pour les souris et de 8 g/kg de
poids corporel pour les rats. Les signes d'intoxication sont
essentiellement neurologiques.
Des études de courte durée sur des souris avec des doses allant
jusqu'à 600 mg/kg de nourriture pendant 26 à 32 semaines, ont révélé
la présence d'une hyperplasie nodulaire et de proliférations atypiques
au niveau du foie ainsi qu'une augmentation du poids de cet organe.
Lors d'une troisième étude, consistant dans l'administration de doses
allant jusqu'à 500 mg/kg de nourriture pendant 24 semaines, on a
observé ni tumeurs hépatiques ni hyperplasie nodulaire.
Une étude de 90 jours au cours de laquelle des rats ont reçu soit
50 soit 250 mg de béta-HCH par kg de nourriture, a révélé des
altérations au niveau du foie, notamment une hypertrophie et une
prolifération du réticulum endoplasmique lisse ainsi qu'un
accroissement de l'activité des enzymes microsomiques. Aux doses les
plus élevées on a également observé des altérations au niveau des
gonades qui s'accompagnaient également d'effets graves sur le poids du
corps. Les modifications hormonales accompagnant l'atrophie des
gonades ne correspondaient pas à un effet endocrinien systématique.
Aucun effet nocif n'a été constaté à la dose de 2 mg/kg de nourriture
(soit l'équivalent de 0,1 mg/kg de poids corporel).
Une étude de longue durée sur des rats (publiée en 1950) au cours
de laquelle on a administré des doses de 10 mg/kg de nourriture (soit
l'équivalent de 0,5 mg/kg de poids corporel) ou davantage, a révélé
que ce régime conduisait à une hypertrophie du foie et à des
modifications histologiques.
Lors d'une étude de reproduction portant sur deux générations de
rats, on a observé les mêmes effets que dans l'étude de 90 jours citée
plus haut. Aucun effet n'a été observé à la dose de 2 mg/kg de
nourriture (soit l'équivalent de 0,1 mg/kg de poids corporel), mais à
la dose de 10 mg/kg, il y avait accroissement de la mortalité et de la
stérilité. L'étude a également porté sur les effets tératogènes
éventuels du béta-HCH mais aucun effet de ce genre imputable au
produit n'a été observé.
On a décrit un effet "oestrogénique" faible dont l'organe cible
serait l'utérus. En fait il n'y a pas d'effet bien net sur le système
de régulation endocrinienne. Le mécanisme et la portée de cet effet
demeurent incertains.
Les études de mutagénicité publiées ne font état d'aucune
augmentation dans la fréquence des mutations chez les souches de
Salmonella typhimurium. Une étude in vivo chez le rat sur des
cellules de moelle osseuse en métaphase a donné des résultats
positifs.
Deux études ont été effectuées sur des souris afin de déterminer
le pouvoir cancérogène du béta-HCH. Dans l'une d'elles, on a
administré pendant 110 semaines une dose de 100 mg/kg de nourriture et
l'on a observé une hyperplasie du tissu hépatique et une hypertrophie
de cet organe. Il y avait également augmentation des tumeurs bénignes
et malignes. Dans une autre étude, où la dose administrée était de
500 mg/kg de nourriture pendant une période de 24 semaines, aucune
tumeur n'a été observée.
Des études au cours desquelles des rats ont reçu des mélanges de
béta-HCH et de biphényles polychlorés donnent à penser que le béta-HCH
aurait un effet promoteur.
A la dose de 300 mg/kg de nourriture, le béta-HCH a provoqué une
altération sensible de plusieurs des fonctions du système immunitaire
en l'espace d'un mois chez la souris.
2.7 Effets sur l'homme
L'examen de travailleurs d'une usine produisant du lindane,
exposés à cette substance pendant 7,2 années (en moyenne géométrique,
avec des limites de 1 à 30 ans), a permis de conclure que l'exposition
professionelle au HCH ne produisait pas de signes d'une atteinte
neurologique ni d'une perturbation des fonctions neuromusculaires.
CONCLUSIONS ET RECOMMANDATIONS EN VUE DE LA PROTECTION DE LA SANTE
HUMAINE ET DE L'ENVIRONNEMENT (ALPHA- ET BETA-HEXACHLOROCYCLOHEXANES)
1. Conclusions
On ne peut pas comparer les effets nocifs potentiels de l'alpha-
et du béta-hexachlorocyclohexane sur l'homme et l'environnement à
leurs avantages éventuels, étant donné que ces produits n'ont aucune
action insecticide. Leur présence dans l'environnement est donc fort
préoccupante. Dans ces conditions, l'usage de produits à base d'HCH
technique contenant de fortes concentrations d'alpha- et de béta-HCH
n'est en aucun cas justifié.
1.1 Population générale
L'alpha- et le béta-HCH circulent dans l'environnement et sont
présents dans les chaînes alimentaires. Il existe donc un risque
permanent d'exposition humaine. Cette exposition est faible et devrait
lentement diminuer dans les années à venir. Dans ces conditions, il
n'y a pas lieu de s'inquiéter sérieusement pour la santé de la
population dans son ensemble.
1.2 Sous-groupes de population exposés à un risque particulier
La concentration de l'alpha-HCH dans le lait maternel est faible.
On peut se préoccuper de l'exposition des nourrissons au béta-HCH
actuellement présent dans le lait maternel mais il faut malgré tout
continuer à encourager l'allaitement maternel.
Il faut cependant faire un maximum d'efforts pour réduire
l'exposition par voie alimentaire ou autre à ces isomères. Une
moindre exposition d'origine alimentaire à ces substances devrait
entraîner une diminution de la teneur du lait maternel en alpha- et
béta-HCH.
1.3 Exposition professionnelle
Dans la mesure où elles observent les précautions recommandées en
vue réduire au minimum l'exposition à l'alpha- et au béta-HCH, les
personnes employées à la fabrication du lindane ne courent pas de
risque particulier.
1.4 Effets sur l'environnement
A part le cas de décharge dans le milieu aquatique, rien
n'indique que la présence d'alpha- et de béta-HCH dans l'environnement
constitue une menace particulière pour la faune et la flore.
2. Recommandations pour la protection de la santé humaine et de
l'environnement
a) Afin de réduire au minimum la pollution de l'environnement par
l'alpha- et le béta-HCH, il faut utiliser du lindane (> de 99% de
gamma-HCH) à la place de l'HCH technique.
b) Pour éviter la pollution de l'environnement par l'alpha- et le
béta-HCH, les sous-produits et les effluents issus de la fabrication
du lindane doivent être évacués de façon convenable et il faut en
particulier éviter la contamination des eaux et du sol.
c) Il faut poursuivre la surveillance de l'alpha- et du béta-HCH
dans les denrées alimentaires. Il est essentiel d'instituer un
mécanisme par lequel seront fixées des doses limites acceptables sur
le plan international pour l'alpha- et le béta-HCH.
d) Il faut poursuivre la surveillance de l'apport quoti-dien
d'alpha- et de béta-HCH et continuer à en contrôler les concentrations
dans le lait maternel.
RECHERCHES A EFFECTUER (ALPHA- ET BETA-HEXACHLOROCYCLOHEXANES)
Les études expérimentales suivantes sont nécessaires pour
permettre une meilleure évaluation des dangers que représentent
l'alpha- et le béta-HCH:
* études de mutagénicité portant en particulier sur les
chromosomes;
* études de reproduction, études de foetotoxicité et de
tératogénicité;
* études pharmacocinétiques et toxicocinétiques;
* études de cancérogénicité;
* études de neurotoxicité;
* surveillance des populations à risque.
RESUMEN Y EVALUACION
1. Alpha-hexaclorociclohexano
1.1 Propiedades generales
El alpha-hexaclorociclohexano (alpha-HCH) es uno de los
principales subproductos (65-70%) de la fabricación del lindano (>
99% gamma-HCH). Su solubilidad en agua es baja, pero es muy soluble
en disolventes orgánicos como la acetona, el cloroformo y el xileno.
Es una sustancia sólida con baja presión de vapor. El coeficiente de
partición n-octanol/agua (log Poa) es 3,82. Se trata de un
contaminante ambiental.
El alpha-HCH puede determinarse por separado de los otros
isómeros mediante cromatografía de gases con detección de captura
electrónica y otros métodos, tras la extracción por partición
líquido/líquido y la purificación en cromatografía de columna.
1.2 Transporte, distribución y transformación en el medio ambiente
La biodegradación y la degradación abiótica (decloración) por
irradiación ultravioleta tienen lugar en el medio ambiente y producen,
respectivamente, delta-3,4,5,6-tetraclorohexeno y pentacloro-
ciclohexeno. Este proceso de degradación es más lento que en el caso
del lindano. La persistencia del alpha-HCH en el suelo depende de
factores ambientales como la acción de los microorganismos, el
contenido de materia orgánica y la codestilación y la evaporación a
partir de los suelos. No se produce isomerización del lindano a
alpha-HCH.
En los microorganismos se produce una bioconcentración rápida (el
factor de bioconcentración es igual a 1500-2700 en peso seco, o
aproximadamente 12 000 en lípidos al cabo de 30 minutos),
invertebrados (60-2750 (peso seco) o > 8000 (lípidos) al cabo de
24-72 h), y peces (313-1216 al cabo de 4-28 días; hasta 50 000 en el
río Elba). No obstante, la biotransformación y la eliminación también
son relativamente rápidas en esos organismos (de 15 minutos a 72 h).
1.3 Niveles en el medio ambiente y exposición humana
El alpha-HCH se encuentra en el aire oceánico con una
concentración de 0,02-1,5 ng/m3. En el Canadá, se encontró en el
agua de lluvia con una concentración de 1-40 ng/litro, pero sólo se
detectaron indicios en la nieve.
Durante el periodo 1969-1974, se encontraron en el río Rin y sus
afluentes niveles de alpha-HCH de 0,01-2,7 µg por litro, pero
últimamente los niveles han sido inferiores a 0,1 µg/litro. En el río
Elba, los niveles disminuyeron desde un promedio de 0,023 mg/litro en
1981 hasta menos de 0,012 µg/litro en 1988. En 1966 se encontró que
ciertos ríos del Reino Unido contenían 0,001-0,43 µg por litro. Se ha
encontrado alpha-HCH en sedimentos de la región norte del mar de
Wadden en concentraciones de 0,3 a 1,4 µg/kg (0,002 µg/litro en el
agua).
Las concentraciones de alpha-HCH en diferentes especies vegetales
de distintos países variaron entre 0,5-2140 µg/kg en peso seco, pero
fueron mucho más altos en zonas contaminadas. Incluso en la Antártida
se han encontrado niveles que varían entre 0,2 y 1,15 µg/kg.
El alpha-HCH se detecta con regularidad en peces e invertebrados
acuáticos, así como en patos, garzas y lechuzas. En renos y alces de
Idaho, que viven en zonas en las que el uso de plaguicidas es
prácticamente insignificante, se encontraron niveles medios de
alpha-HCH de aproximadamente 70-80 µg/kg en la grasa subcutánea. El
tejido adiposo de los osos polares del Canadá contenía 0,3-0,87 mg de
alpha-HCH/kg (en grasa).
En varios países se han analizado importantes alimentos en busca
de alpha-HCH. Las concentraciones, principalmente en alimentos que
contienen grasas, variaron hasta un máximo de 0,05 mg/kg de producto,
salvo en la leche y los productos lácteos (hasta 0,22 mg/kg) y en el
pescado y en preparaciones de carne (hasta 0,5 mg/kg en grasa). Se ha
observado una ligera disminución con los años.
Los alimentos son la principal fuente de exposición de la
población general al alpha-HCH. En estudios de la dieta total
realizados en los Países Bajos y en el Reino Unido, se encontraron
concentraciones medias de 0,01 y 0,002-0,003 mg/kg de alimento,
respectivamente. Los datos procedentes del Reino Unido indican una
tendencia decreciente desde 1967. En los EE.UU., la ingesta diaria
media de alpha-HCH fue de 0,009-0,025 µg/kg de peso corporal durante
el periodo 1977-1979, y de 0,003-0,016 µg/kg de peso corporal durante
el periodo 1982-1984.
En unos pocos países, se ha determinado la concentración de
alpha-HCH en la sangre, el suero o el plasma humanos. La
concentración promedio (mediana en algunos casos) fue < 0,1 µg/litro
(desde niveles no detectables hasta 0,6 µg/litro). En un país, no
obstante, se notificó una concentración media de 3,5 (margen
0,1-15,0) µg por litro. Se detectó alpha-HCH en aproximadamente la
tercera parte de las muestras de sangre.
En el ser humano las concentraciones en el tejido adiposo y la
leche que se han comunicado son bajas (respectivamente < 0,01-0,1 y
< 0,001-0,04 mg/kg en grasa). Los estudios de la dieta total han
revelado niveles diarios de ingesta del orden de 0,01 µg/kg de peso
corporal por día o menos. Esas concentraciones están disminuyendo
poco a poco con los años.
El alpha-HCH parece ser un contaminante ambiental universal. Las
concentraciones están disminuyendo muy despacio, a pesar de las
medidas adoptadas para impedir su dispersión en el medio ambiente.
1.4 Cinética y metabolismo
En las ratas, el alpha-HCH se absorbe rápida y casi completamente
a partir del tracto gastrointestinal. Después de una inyección
intraperitoneal, aproximadamente el 40-80% del alpha-HCH se excretó en
la orina y el 5-20% en las heces. En la rata, las concentraciones más
elevadas se han encontrado en el hígado, los riñones, la grasa, el
cerebro y los músculos; el tejido adiposo constituye un importante
depósito. Las concentraciones de alpha-HCH en el hígado de las crías
lactantes duplicaron las observadas en el hígado de las madres. En la
rata, los cocientes cerebro-sangre y grasa de depósito-sangre fueron
de 120:1 y 397:1, respectivamente.
La biotransformación del alpha-HCH en la rata entraña la
decloración. El principal metabolito urinario es el
2,4,6-triclorofenol; entre otros metabolitos identificados figuran el
1,2,4-, 2,3,4-, y 2,4,5-triclorofenol y el 2,3,4,5- y
2,3,4,6-tetraclorofenol. En el riñón de rata y también en estudios
in vitro en hígado de pollo se ha encontrado 1,3,4,5,6-
pentaclorociclohex-1-eno. En el hígado se forma un conjugado de
glutatión.
En la rata, la semivida de eliminación de la sustancia presente
en del depósito graso es de 6,9 días en la hembra y 1,6 días en el
macho.
1.5 Efectos en los organismos del medio ambiente
El alpha-HCH tiene baja toxicidad para las algas, siendo por lo
general 2 mg/litro el nivel sin efectos observados.
En un estudio a largo plazo, Daphnia magna mostró un nivel sin
efectos observados de 0,05 mg/litro. El alpha-HCH es moderadamente
tóxico para los invertebrados y los peces. Los valores de la
C(E)L50 aguda para esos organismos son del orden de 1 mg/litro. En
estudios a corto plazo con Lebistes reticulatus y Oryzia latipes se
observó que 0,8 mg/litro no ejercían efecto alguno.
En estudios de tres meses de duración con Salmo gairdneri con
dosis de 10-1250 mg/kg de dieta no se observaron efectos en la
mortalidad, la conducta, el crecimiento ni las actividades enzimáticas
del hígado y el cerebro.
En estudios a corto y a largo plazo con un gasterópodo (Lymnea
stagnalis) se observó una CE50 (basada en la mortalidad y la
inmovilización) de 1200 µg/litro. La inhibición de la producción de
huevos se produjo con una concentración de 250 µg/litro. Con
65 µg/litro se observó una reducción del 50% en la reproductividad
general.
No se dispone de datos sobre los efectos en las poblaciones y los
ecosistemas.
1.6 Efectos en animales de experimentación y sistemas de ensayo
in vitro
Los valores de la DL50 aguda por vía oral en ratones se
encuentran entre 1000-4000 y en ratas entre 500 y 4670 mg/kg de peso
corporal. Los signos de envenenamiento coinciden principalmente con
los de la estimulación del sistema nervioso central.
En un estudio de 90 días de duración en ratas se observó
depresión del crecimiento con una concentración de 250 mg/kg de dieta.
Los cambios histológicos y enzimáticos en el hígado indicaron
inducción enzimática con 50 mg/kg o más. Con esas dosis se observaron
también signos de inmunosupresión. Ya se observó aumento del peso
hepático con 10 mg/kg de dieta (equivalente a 0,5 mg/kg de peso
corporal). El nivel sin efectos adversos observados resultó en este
estudio ser 2 mg/kg de dieta (equivalente a 0,1 mg/kg de peso corporal
al día).
No se han comunicado estudios adecuados a largo plazo de
toxicidad ni estudios de reproducción y teratogenicidad.
Los estudios realizados con diversas cepas de Salmonella
typhimurium no dieron prueba alguna de mutagenicidad ni con
activación metabólica ni sin ella. Los ensayos realizados con
Saccharomyces cerevisiae también dieron resultado negativo, pero un
ensayo de síntesis no programada de ADN en hepatocitos de rata in
vitro dio resultados ambiguos.
Se ha intentado determinar el potencial carcinogénico en ratones
y ratas con dosis de 100 a 600 mg/kg de dieta. En estudios realizados
en ratones se encontraron nódulos hiperplásicos y/o adenomas
hepatocelulares. En un estudio los niveles de administración
excedieron la dosis máxima tolerada. En dos estudios en ratones y uno
en ratas, en los que se administraron hasta 160 mg/kg de dieta a
ratones y 640 mg/kg de dieta a ratas, no se observó aumento alguno en
la incidencia de tumores.
Los resultados de los estudios sobre la iniciación-promoción y el
modo de acción, y los estudios de mutagenicidad indican que la
tumorigenicidad inducida por el alpha-HCH observada en ratones tiene
un mecanismo no genético.
Se ha demostrado que el alpha-HCH provoca un aumento neto de la
actividad de los enzimas hepáticos incluso con 5 mg/kg de dieta
(equivalente a 0,25 mg/kg de peso corporal). Una dosis de 2 mg/kg de
peso corporal no afectó la desmetilación de la aminopirina ni el
contenido de ADN en el hígado.
1.7 Efectos en el ser humano
Cuando se examinó a trabajadores de una fábrica de producción de
lindano, con una exposición media geométrica de 7,2 años (1-30), se
concluyó que la exposición profesional al HCH no induce síntomas de
trastornos neurales ni perturbaciones de la "función neuromuscular".
RESUMEN Y EVALUACION
2. Beta-hexaclorociclohexano
2.1 Propiedades generales
El beta-hexaclorociclohexano (beta-HCH) es un subproducto (7-10%)
de la fabricación del lindano (> 99% gamma-HCH). Su solubilidad en
agua es baja, pero es muy soluble en disolventes orgánicos como la
acetona, el ciclohexano y el xileno. Es un sólido con una baja
presión de vapor. El coeficiente de partición n-octanol/agua (log
Poa) es 3,80. Es un contaminante ambiental.
El beta-HCH puede determinarse por separado de los otros isómeros
mediante cromatografía de gases con detección de captura electrónica y
otros métodos tras la extracción por partición líquido/líquido y la
purificación en cromatografía de columna.
2.2 Transporte, distribución y transformación en el medio ambiente
La biodegradación y la degradación abiótica (decloración) por
irradiación ultravioleta tienen lugar en el medio ambiente y producen
pentaclorociclohexano, pero a una velocidad mucho menor que en el caso
del lindano (gamma-HCH).
El beta-HCH es el isómero más persistente del HCH. Su
persistencia en el suelo depende de factores ambientales como la
acción de los microorganismos, el contenido de materia orgánica y de
agua, y la codestilación y la evaporación a partir del suelo.
Dada la persistencia del beta-HCH, tiene lugar una rápida
bioconcentración en invertebrados (el factor de bioconcentración es de
aproximadamente 125 al cabo de tres días), peces (250-1500 en peso
seco o aproximadamente 500 000 veces en lípidos al cabo de 3-10 días),
aves y el hombre (aproximadamente 525). La bioconcentración es más
elevada y la eliminación más lenta en el caso del beta-HCH que en los
otros isómeros del HCH.
2.3 Niveles ambientales y exposición humana
El beta-HCH se encuentra en el aire oceánico con una
concentración de 0,004-0,13 ng/m3.
Hasta 1974, el río Rin y sus afluentes contenían niveles de
beta-HCH de 0,14-0,22 µg/litro, pero después los niveles estuvieron
siempre por debajo de 0,1 µg por litro. Las muestras tomadas en el
río Mosa también contenían < 0,1 µg/litro. En el río Elba, los
niveles descendieron desde un promedio de 0,009 hasta 0,004 µg por
litro entre 1981 y 1988.
El beta-HCH se ha medido en aves como el gavilán, el cernícalo,
el búho, la garza y el colimbo durante varios años y las
concentraciones variaron entre 0,1 y 0,3 mg/kg. Se han encontrado
hasta 0,87 mg/kg (en grasa) en el hígado y el tejido adiposo del oso
polar.
Se han analizado importantes alimentos en algunos países en busca
de beta-HCH. Las concentraciones medias, principalmente en alimentos
que contienen grasas, variaron entre 0,03 mg/kg (en grasa), pero en
los productos lácteos se encontraron niveles de hasta 4 mg/kg (en
grasa). En alimentos no grasos, los niveles fueron < 0,005 mg/kg de
producto. En general, los niveles están descendiendo lentamente.
Los alimentos son la principal fuente de exposición de la
población general al beta-HCH. En estudios de la dieta total en el
Reino Unido, se encontraron 0,003, 0,0005, y < 0,0005 mg/kg durante
los años 1966/67, 1975/77 y 1981, respectivamente. En los EE.UU., la
ingesta diaria media de beta-HCH en 1982-1984 varió entre <
0,1-0,4 ng/kg de peso corporal en distintos grupos de edad.
En varios países, la concentración de beta-HCH se ha determinado
en la sangre, el suero o el plasma de la población general. Las
concentraciones variaron entre los distintos países y el máximo
encontrado fue de 25 µg por litro.
Se han llevado a cabo numerosos estudios para determinar la
presencia de beta-HCH en los tejidos adiposos humanos. Las
concentraciones encontradas en el Canadá, Kenya, los Países Bajos, el
Reino Unido, y la República Federal de Alemania, variaron hasta
4,4 mg/kg (en grasa). Se encontró que hasta los 50 años se produce un
aumento gradual con la edad; en adelante, los niveles disminuyen. Las
concentraciones de beta-HCH en los tejidos adiposos son más altas que
las de los otros isómeros del HCH, fenómeno que refleja las
propiedades acumulativas del beta-HCH. En general no se ha observado
una tendencia clara de disminución de las concentraciones de beta-HCH
durante el periodo en que se han hecho los estudios. Existe una
relación entre las concentraciones en el tejido adiposo y la leche
materna y el consumo de productos cárnicos, grasas animales y pescados
grasos.
En unos pocos países (Canadá, Países Bajos, Reino Unido y
República Federal de Alemania), se ha analizado la leche humana y se
han encontrado niveles de beta-HCH entre 0,1 y 0,69 mg/kg (en grasa).
Los niveles medidos en la leche de mujeres de zonas rurales parece ser
más elevado que en las de zonas urbanas.
Los elevados niveles de beta-HCH que se han encontrado en la
leche materna exceden las concentraciones permisibles a título
temporal y local. Las concentraciones de beta-HCH en la sangre de
lactantes se encuentran entre los mismos límites que las medidas en
las madres.
El beta-HCH parece ser un contaminante ambiental universal. Las
concentraciones están disminuyendo muy despacio a pesar de las medidas
adoptadas para evitar su dispersión en el medio ambiente.
2.4 Cinética y metabolismo
Hasta el 95% del beta-HCH en el tracto gastrointestinal del ratón
es absorbido y a continuación se acumula en su mayor parte en el
tejido adiposo. La eliminación sigue un mecanismo de dos etapas;
durante la primera, la semivida es de 2,5 días y durante la segunda,
18 días.
Después de la absorción, el beta-HCH se distribuye rápidamente al
hígado, el cerebro, los riñones y los tejidos adiposos. En la rata,
la concentración máxima en el hígado se alcanza al cabo de cuatro
días. Con una concentración sanguínea media de 92 µg/litro (pero
también con concentraciones de 540 y 2100 µg/litro), los cocientes
cerebro-sangre y tejido adiposo-sangre fueron 2:1 y 170:1,
respectivamente. Tras el envenenamiento agudo y mortal de un hombre
con isómeros de HCH, la concentración de beta-HCH, en relación con la
de la sangre, fue de 363 en la grasa, 3 en el cerebro y 15 en el
hígado. El beta-HCH atraviesa la barrera hematoencefálica con mucha
menos facilidad que los demás isómeros del HCH.
En el ratón, el paso transplacentario de la hembra gestante al
feto fue de aproximadamente el 2% de la dosis, pero en la rata se
observó un paso del 40%. En la rata, la transferencia de la madre al
lactante en la leche fue de aproximadamente el 60% de la dosis.
En la rata, el 70% del beta-HCH se elimina durante 28 días; un
tercio de esa cantidad se excreta en la orina. No aparece beta-HCH
sin modificar en la orina. El principal metabolito procedente de la
cis-deshidrocloración es el 2,4,6-triclorofenol en forma conjugada.
El pretratamiento con beta-HCH altera el metabolismo del lindano
en las ratas. Según estudios intraperitoneales realizados en ratones,
parece que el beta-HCH se metaboliza con más lentitud que el lindano.
2.5 Efectos en los organismos del medio ambiente
En general, el beta-HCH tiene una toxicidad moderada para las
algas, los invertebrados y los peces. Los valores de la DL50 aguda
para esos organismos son del orden de 1 mg/litro, pero los valores de
la CE50 son más bajos (0,05-0,5 mg/litro). El nivel sin efectos
observados en Oryzia latipes y Poecilia reticulata, dos peces de
agua dulce expuestos durante 1 ó 3 meses, fue de 0,03 mg por litro.
No se dispone de datos sobre los efectos en las poblaciones y los
ecosistemas.
2.6 Efectos en animales de experimentación y sistemas de ensayo
in vitro
Los valores de DL50 aguda por vía oral en ratones y ratas
comunicados en 1968 se encontraban entre 1500 y 2000 mg/kg de peso
corporal. No obstante, en estudios más recientes se han obtenido
valores de 16 g/kg de peso cor poral en ratones y 8 g/kg de peso
corporal en ratas. Los signos de intoxicación fueron principalmente
de origen neural.
En dos estudios en ratones a corto plazo, con dosis de hasta
600 mg/kg de dieta durante 26-32 semanas, se observó un aumento del
peso hepático, así como hiperplasia nodular y proliferaciones atípicas
en el hígado. En un tercer estudio, la administración de hasta
500 mg/kg de dieta durante 24 semanas no produjo tumores hepáticos ni
hiperplasia nodular.
En un estudio a 90 días con ratas a las que se administraron 50 ó
250 mg/kg de dieta se observaron cambios hepáticos, a saber,
hipertrofia y proliferación del retículo endoplásmico liso y mayor
actividad de los enzimas microsómicas. Con las dosis más altas se
produjeron cambios en las gónadas pero éstos estuvieron asociados a
modificaciones muy acusadas del peso corporal. Los cambios hormonales
asociados a la atrofia gonadal no mostraron un efecto endocrino
consecuente. No se observaron efectos adversos con una dosis de
2 mg/kg de dieta (equivalente a 0,1 mg/kg de peso corporal).
En un estudio en ratas a largo plazo (comunicado en 1950), la
administración de dosis de 10 mg/kg de dieta (equivalente a 0,5 mg/kg
de peso corporal) o superiores produjo dilatación y cambios
histológicos en el hígado.
En un estudio de reproducción de ratas en dos generaciones, se
observaron los mismos efectos que en el estudio de 90 días. No se
observaron efectos con 2 mg/kg de dieta (equivalente a 0,1 mg/kg de
peso corporal), pero con una dosis de 10 mg/kg de dieta aumentaron la
mortalidad y la infecundidad. En una ampliación de este estudio no se
observaron efectos teratogénicos relacionados con el compuesto.
Se ha descrito un ligero efecto "estrogénico". El órgano diana
de este efecto era el útero; no se apreciaron efectos claros en los
sistemas de control endocrino. No se sabe con seguridad cuál es el
mecanismo ni el significado de este efecto.
Los estudios de mutagenicidad comunicados no mostraron aumento
alguno en la frecuencia de mutaciones en cepas de Salmonella
typhimurium. En un análisis in vivo de la metafase en médula ósea
de ratas se obtuvieron resultados positivos.
Se han llevado a cabo dos estudios en el ratón para determinar el
potencial carcinogénico. En uno de los estudios, se administraron
200 mg/kg de dieta durante 110 semanas, y se notificaron dilatación
del hígado, cambios hiperplásicos y aumento de tumores tanto benignos
como malignos. En el otro estudio, en el que se administraron
500 mg/kg de dieta durante 24 semanas, no se observaron tumores.
En estudios en los que se administró a ratas combinaciones de
beta-HCH con bifenilos policlorados se sugirió que el beta-HCH tenía
un efecto de promoción.
Con 300 mg/kg de dieta, el beta-HCH provocó cambios
significativos en varias funciones inmunitarias en el ratón al cabo de
un mes.
2.7 Efectos en el ser humano
Cuando se examinó a trabajadores de una fábrica de producción de
lindano, con una exposición media geométrica de 7,2 años (1-30), se
concluyó que la exposición profesional al HCH no induce signos de
trastornos neurales ni de perturbación de la "función neuromuscular".
CONCLUSIONES Y RECOMENDACIONES PARA LA PROTECCION DE LA SALUD
HUMANA Y DEL MEDIO AMBIENTE (ALPHA- Y BETA-HEXACLOROCICLOHEXANOS)
1. Conclusiones
Los efectos adversos potenciales del alpha- y el beta-
hexaclorociclohexano (HCH) en el ser humano y el medio ambiente
no pueden sopesarse frente a sus beneficios, puesto que estos isómeros
no tienen acción insecticida. Su presencia en el medio ambiente es
por tanto causa de gran inquietud. En consecuencia, en ningún caso se
justifica el uso de productos técnicos del HCH que contengan elevadas
concentraciones de alpha- y beta-HCH.
1.1 Población general
El alpha- y el beta-HCH circulan en el medio ambiente y están
presentes en las cadenas alimentarias. Así pues, existe un potencial
continuo de exposición humana. Esta exposición es baja y se espera
que disminuya lentamente en los años por venir. Así pues, no hay
motivos de gran inquietud en cuanto a la salud de la población
general.
1.2 Subpoblaciones especialmente expuestas
Las concentraciones de alpha-HCH en la leche humana son bajas.
La exposición de lactantes debida a las actuales concentraciones
de beta-HCH en la leche materna es preocupante, pero no suficiente
para dejar de fomentar la lactancia natural.
No obstante, debe hacerse todo lo posible para disminuir la
exposición a esos isómeros por la vía alimentaria y por toda otra vía.
Se espera que la menor exposición por la dieta dé como resultado
menores niveles de alpha- y beta-HCH en la leche humana.
1.3 Exposición profesional
Mientras se observen las precauciones recomendadas para reducir
al mínimo la exposición del personal que tra-baja en la fabricación
del lindano, el alpha- y el beta-HCH no plantean riesgos para la salud
de los operarios.
1.4 Efectos en el medio ambiente
Aparte de los vertidos en el medio acuático, no hay pruebas que
sugieran que la presencia de alpha- y beta-HCH en el medio ambiente
plantee un riesgo significativo para las poblaciones de seres vivos.
2. Recomendaciones para la protección de la salud humana y el medio
ambiente
a) A fin de reducir al mínimo la contaminación ambiental con alpha-
y beta-HCH, debe usarse lindano (< 99% gamma-HCH) en lugar de HCH
técnico.
b) A fin de evitar la contaminación ambiental con alpha- y beta-HCH,
los subproductos y los efluentes de la fabricación del lindano deben
evacuarse de modo apropiado, y debe evitarse la contaminación de aguas
naturales y del suelo.
c) Debe proseguir la vigilancia del alpha- y del beta-HCH en los
alimentos. Es imprescindible poner en marcha un mecanismo para
establecer niveles internacionalmente aceptables de alpha- y beta-HCH
en los alimentos.
d) Debe proseguir la vigilancia de la ingesta diaria de la población
general y de los niveles de alpha- y beta-HCH en la leche materna.
OTRAS INVESTIGACIONES (ALPHA- Y BETA-HEXACLOROCICLOHEXANOS)
Deben hacerse los estudios siguientes para evaluar mejor los
riesgos del alpha- y el beta-HCH:
* oestudios de mutagenicidad, especialmente con puntos
terminales mutagénicos en los cromosomas;
* oestudios de reproducción y fetotoxicidad/teratogenicidad;
* oestudios farmacocinéticos y toxicocinéticos;
* oestudios de carcinogenicidad;
* oestudios de neurotoxicidad;
* oestudios de vigilancia de poblaciones en riesgo.
 Relative
molecular mass 290.9
CAS chemical 1alpha,2alpha,3ß,4alpha,5ß,6ß-hexachloro-
name cyclohexane
Common
synonyms Alpha-benzenehexachloride (alpha-BHC)
CAS registry
number
Relative
molecular mass 290.9
CAS chemical 1alpha,2alpha,3ß,4alpha,5ß,6ß-hexachloro-
name cyclohexane
Common
synonyms Alpha-benzenehexachloride (alpha-BHC)
CAS registry
number  The direct uptake of alpha-HCH from contaminated water appeared to be
much greater than the uptake from contaminated food.
In a study with Salmo gairdneri, pellets containing alpha-HCH
(> 95%) levels of 0, 10, 50, 250, or 1250 mg/kg were fed to the fish,
and organs and tissues were analysed after 2, 4, 8, and 12 weeks.
There was a dose-related increase in the concentration of alpha-HCH
in the organs and tissues. After about 4-8 weeks (depending on the
type of tissue and dose level) a maximum concentration was reached,
which then slowly decreased. This suggests that after a few weeks a
balance is reached between the accumulation process (absorption of
alpha-HCH by the intestinal wall) and the elimination process (via the
gills and faeces). There is probably also a dilution effect resulting
from growth and biotransformation (Canton et al., 1975).
Ernst (1977) concluded from kinetic studies that biomagnification
of alpha-HCH does not occur. Compared with bioaccumulation from water
alone, the entry of alpha-HCH into the food chain Chlorella ->
Daphnia -> Poecilia (guppy) caused only a slight increase in
biomagnification in daphnids (factor 1.5), although in the case of the
guppies a greater increase in concentration ratio (3-4) was noted.
In a study by Canton et al. (1978), guppies (3-4 weeks old) were
exposed to alpha-HCH (> 95%) concentrations of 0.01, 0.05, or
0.14 mg/litre. When after 0.5, 3, 24, 48, 72, 96, and 120 h the
animals were analysed, the average concentration factor was about 500
for all alpha-HCH concentrations (about 17 000 on a lipid basis).
Equilibrium was reached within 24 h for the lower concentrations and
within 48 h at the highest concentrations. The elimination was rapid,
the initial concentration being halved in 10 h.
Sugiura et al. (1979) studied bioaccumulation in the carp
(Cyprinus carpio), brown trout (Salmo trutta fario), golden orfe
(Leuciscus idus melanotus), and guppy (Poecilia reticulata).
Alpha-HCH was dissolved in water to a concentration of 1 mg/litre
under steady-state conditions (time period not specified), and the
equilibrium bioconcentration factors for the four types of fish were
330, 605, 1216, and 588, respectively.
Based on the data given in section 5.1.5.2 concerning the
concentration of alpha-HCH in the muscle and fat of bream collected in
the River Elbe, the bioconcentration factor is between 10 000 and
50 000 (Arbeitsgemeinschaft für die Reinhaltung der Elbe, 1982).
In a study by Yamato et al. (1983), guppies (Poecilia
reticulata) rapidly bioaccumulated HCH isomers and the tissue
concentration reached a plateau on the fourth day (the alpha-HCH
concentration in the water was 1 µg per litre). The bioconcentration
factor (concentration in fish/concentration in water) was 706. The
concentration in the guppies decreased on the first day after the fish
were transferred to HCH-free water.
4.2.3.4 Bioconcentration in humans
Geyer et al. (1986) found that in industrialized countries more
than 90% of the exposure to HCHs derives from food. The mean
concentration of alpha-HCH in human adipose tissue (on a fat basis)
was found to be 0.03 mg/kg in the Federal Republic of Germany and
0.02 mg/kg in the Netherlands. The mean bioconcentration factor (on a
lipid basis), calculated on the basis of the concentration in the diet
(1.3 and 0.3 µg/kg, respectively) and levels in adipose tissue, was
20.0 ± 8 (range 11.5-32.5).
4.3 Isomerization
Deo et al. (1981) studied the isomerization of alpha-HCH in
sterile aqueous solution over a period of 4 weeks and found a slow
conversion of alpha-HCH to other HCH isomers.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Tanabe et al. (1982) found alpha-HCH in 24 samples of air over
the Western Pacific, Eastern Indian, and Antarctic Oceans at an
average concentration of 0.29 ng/m3 (0.022-1.4 ng/m3).
In a study by Strachan et al. (1980), samples of atmospheric
precipitation in the form of snow (1976; 17 samples) and rain (1976
and 1977; 81 samples) collected around the Canadian side of the Great
Lakes, as well as inland, were analysed. Alpha-HCH was found in the
snow samples as a trace (1 ng/litre) and in the rain samples at levels
of 1-40 ng/litre.
Air samples were taken near a road with heavy traffic, as well as
in a suburban residential area, near Ulm, in Germany. The alpha-HCH
levels were 0.22-1.3 ng/m3 in the location with heavy traffic and
0.11-1.1 ng/m3 in the rural area. It was concluded that the
concentrations in the lower troposphere under various meteorological
conditions reflect regional input and long-range transport (Wittlinger
& Ballschmiter, 1987).
In 1972, alpha-HCH air concentrations of 0.28 ng per m3 in
non-polluted areas of Germany, and 2.15 ng/m3 in the polluted Ruhr
area were determined (Hildebrandt et al., 1986).
The average concentration of alpha-HCH in 55 air samples
collected in Delft, the Netherlands, in 1979-1980 was 0.25 ng/m3
(maximum concentration: 1.2 ng/m3) (Slooff & Matthijsen, 1988).
5.1.2 Water
5.1.2.1 Rain water
Rain water sampled in 1983 in Bilt, the Netherlands, contained an
average alpha-HCH concentration of 0.01 (< 0.01-0.02) µg/litre
(Slooff & Matthijsen, 1988).
5.1.2.2 Fresh water
During the period 1969-1977, 1826 water samples were taken at 99
sampling sites in the Netherlands. The highest concentrations of
alpha-HCH were found in the River Rhine and its tributaries. The
concentrations varied between 0.01-0.3 µg/litre during the period
1969-1974, but in 1974 there was a sudden decrease and the subsequent
concentrations were all below 0.1 µg/litre. A sampling trip by boat
made along the River Rhine from Rheinfelden in Switzerland to
Rotterdam in the Netherlands proved that the source of alpha-, beta-,
and gamma-HCH was located in the upper Rhine. In the River Meuse, the
levels were all below 0.1 µg/litre during the period 1969-1977 (Wegman
& Greve, 1980).
Since 1969, alpha-, beta-, and gamma-HCH concentrations have been
measured regularly in the Rivers Rhine, Meuse, and West-Scheldt and in
other surface waters in the Netherlands. Alpha-HCH levels have been
below 0.05 µg per litre in the River Rhine since 1974/1975, and were
of the order of 0.02 µg/litre or less in the West-Scheldt during the
period 1973-1985. In the River Meuse, the concentration of alpha-HCH
was between 0.01-0.02 µg/litre. In other areas, for instance
agricultural and greenhouse horticulture areas, the levels of the
individual HCHs ranged from 0.01-1.0 µg/litre with incidental higher
peaks (up to 0.5 µg/litre) probably resulting from the use of lindane
(Slooff & Matthijsen, 1988).
Concentrations of HCH isomers in solution and in suspension
(particle-bound) in the Meuse and Rhine estuary were determined in
1974. The average concentrations of dissolved and suspended alpha-HCH
were 20 and 0-6 ng per litre, respectively. In 1981, the
concentration of dissolved alpha-HCH in coastal waters of the
Netherlands was 0.9-1.6 ng/litre, whereas that of suspended alpha-HCH
(only one measurement) was 5.3 ng/litre (Slooff & Matthijsen, 1988).
In 1970-1971, the levels of alpha-HCH were 0.66-1.5 µg/litre in
the surface water of the River Elbe near Hamburg, Germany, and
0.155-2.4 µg/litre in the River Rhine near Karlsruhe. However, a
significant decrease was observed in the mid-1970s. In 1974, 2.7 µg
per litre was found in the upper Rhine, but by 1976-1977 the levels
had decreased to 1-9 ng/litre (Hildebrandt et al., 1986).
The Arbeitsgemeinschaft der Elbe (the Elbe Study Group)
investigated the presence of alpha-HCH in the River Elbe from
Schnackenburg to the North Sea in 1981-1982 and found a mean
concentration of 0.023 (< 0.001-0.15) µg per litre. During the period
February to November 1988, the alpha-HCH concentration was
0.001-0.022 µg/litre (Arbeitsgemeinschaft der Elbe, 1988).
When certain rivers in Yorkshire, England, were analysed for
alpha-HCH in 1966, the concentration varied from 0.001 to
0.43 µg/litre. In 1968, the highest value was 0.543 µg/litre, and
the water from six other rivers contained an average of
0.001-0.004 µg/litre (highest level: 0.34 µg/litre) (Lowden et al.,
1969).
In Japan, 60 water samples were examined in 1974 and 0.1 µg
alpha-HCH/litre was detected in three of the samples (personal
communications by A. Hamada and by T. Onishi to the IPCS, July 1989).
5.1.2.3 Sea water
Atlas & Gias (1981); Bidleman & Leonard (1982); Oehme & Stray
(1982); and Oehme & Mano (1984) analysed sea water from areas such as
the North Pacific, Arabic Sea, Persian Gulf, Red Sea, Lillestrum, Bear
Island, and Spitsbergen. The alpha-HCH concentrations varied from
0.03 to 1.8 ngper litre (Slooff & Matthijsen, 1988).
In June-July 1986, the alpha-HCH in the surface water (5 m) of
the North Sea ranged from 1-2 ng/litre (Umweltbundesamt, 1989).
5.1.3 Soil/Sediment
Herrmann et al. (1984) studied the presence of alpha-HCH in
sediment along the Husum estuary and in the adjacent North Frisian
Wadden Sea. The mean concentrations varied in the different sampling
stations from 0.33 to 1.40 µg/kg sediment, while the concentrations in
bladder wrack(Fucus vesiculosus)varied from 0.7-1.2 µg/kg.
Edelman (1984) analysed 96 samples of the upper 10 cm of the soil
from 38 natural reserves in the Netherlands for alpha-HCH and
gamma-HCH. In 94 of the samples alpha-HCH was detected at levels
below 1 µg/kg (Slooff & Matthijsen, 1988).
When sediment from eight different rivers, harbours, and sites
close to dumping areas in the Netherlands were analysed for the
presence of alpha-, beta-, and gamma-HCH, the median alpha-HCH levels
were between 4 and 213 µg per kg dry matter (Slooff & Matthijsen,
1988).
In 1974, 60 sediment samples were analysed in Japan and 10 µg
alpha-HCH/kg was detected in five of the samples (personal
communications by A. Hamada and by T. Onishi to the IPCS, July 1989).
5.1.3.1 Dumping grounds
In the Netherlands, soil has been polluted with HCHs at various
locations as a result of their manufacture during the 1950s (spillage
during production, storage, and handling), and concentrations up to a
few grams of HCHs/kg dry soil have been found. Further pollution has
been caused by the dumping of chemical waste and its use in the
levelling of certain areas. From these dumping areas dispersal of the
chemical waste can occur by leaching or wind erosion from open storage
depots. In certain polluted areas, high concentrations of HCHs, mainly
alpha- and beta-HCH, have been found more than 2 m below ground level.
In 18 locations in the Netherlands, the average concentration of
alpha-HCH in sewage sludge in 1981 was between 5 and 70 µg/kg dry
matter. Pollution of ground water was also detected, but this was
restricted to the vicinity of the production areas. Horizontal
transportation of HCHs in ground water appeared to be limited (Slooff
& Matthijsen, 1988).
5.1.4 Food and feed
The presence of alpha-HCH in a number of important food items has
been determined in France by Laugel (1981). In milk and milk products
(2688 samples) the average level was 0.05 mg/kg (ranging from
undetectable to 0.22 mg/kg), in meat (37 samples) it was 0.01 mg/kg
(ranging from undetectable to 0.02 mg/kg), and in animal fat (67
samples) it was 0.02 mg/kg product (ranging from undetectable to
0.06 mg/kg. In other food items alpha-HCH was not detectable
(< 0.005 mg/kg).
Table 2 gives the mean alpha-HCH levels in a large number of
samples of various food items from the Federal Republic of Germany
reported by Hildebrandt et al. (1986).
Table 2. Alpha-hexachlorocyclohexane concentrations (mg/kg)
in various food itemsa
Food items 1973-78 1979-83 1973-83
Meatb 0.003-0.02
Meat productsb 0.007-0.037
(0.26)e
Animal fatb 0.003-0.008
(0.09)e
Gameb 0.019-0.367
Poultryb 0.003-0.004 0.003-0.016
(0.17)e
Chicken eggs < 0.001-0.003
Fish 0.002-0.011
Milk and milk
productsb 0.015e 0.01-0.03
Cow's milkb,c 0.004
Butterb,d 0.02-0.03
Table 2 (contd)
Food items 1973-78 1979-83 1973-83
Vegetable oil and
margarineb 0.01
Oil seeds, nuts,
pulses 0.001-0.042
Fruit, vegetables, < 0.0001
potatoes
Cereals 0.0002-0.007
Cereal products up to 0.14
a From: Hildebrandt et al. (1986).
b Determinations made on a fat basis
c WHO (1986).
d Anon (1984).
e Maximum value
In six samples of cows milk collected from six locations in
Switzerland, the levels of alpha-HCH were 9.5-27 mg/kg on a fat basis
(Rappe et al., 1987).
Skaftason & Johannesson (1979) analysed 35 samples of butter from
Iceland during 1968-1970 and found a level of mean alpha-HCH of 87 ±
38 µg/kg. In 1974-1978, 32 samples were studied and all contained
alpha-HCH, the mean concentration being 58 ± 21 µg/kg.
In a total-diet study in the United Kingdom, 24 samples of each
food group were analysed for alpha-HCH. The following concentrations
(mean and range) were found: bread, not detected (nd); other cereal
products, < 0.0005 (nd-0.002); carcass meat, < 0.0005 (nd-0.006);
offal, < 0.0005 (nd-0.007); meat products, eggs, green vegetables,
potatoes, fresh fruit, nd; poultry, 0.003 (nd-0.025); fish, 0.0005
(nd-0.008); oil and fats, 0.0005 (nd-0.003); milk, 0.0005 (nd-0.002);
dairy products, 0.006 (nd-0.02) mg/kg product. Imported meat products
were also analysed during the period 1981-1983, and concentrations of
up to 0.5 mg/kg were measured. Imported retail cereal products
collected in 1982 contained alpha-HCH levels of up to 0.03 mg/kg and
animal feed stuffs collected in 1984 had levels of up to 0.02 mg/kg
(HMSO, 1986).
Various types of pulses were analysed during the period
1986-1987, and 31 out of 142 samples contained alpha-HCH residues at
levels of up to 0.03 mg/kg. Processed pork and poultry, sampled
during the period 1985-1987, contained alpha-HCH at levels of up to
3.2 (mean 0.2) and 0.1-2.0 (mean 0.8) mg/kg product, respectively (26
out of the 86 samples were positive). Of other processed meat
products, 631 samples were negative. Retail milk and dairy products
were analysed during the period 1984-1987, and 499 of the 849
samples contained alpha-HCH residues at a mean concentration of
0.01-0.03 mg/kg (highest level, 0.06 mg/kg). Samples of eel muscle
(1124 eels from 62 sites) were analysed during the period 1986-1987,
and mean concentrations were 0.001-0.03 mg/kg (highest level,
0.4 mg/kg). Peanut butter and vegetable oils were analysed during the
period 1985-1987, and 95 samples showed mean concentrations of <
0.01-0.03 mg/kg product (16 of the samples were positive) (HMSO,
1989).
The mean residue level of alpha-HCH in milk samples collected
during spring 1983 from 359 bulk transporters representing 16
counties, municipalities, and districts of Ontario was 5.3 µg/kg
butter fat. Alpha-HCH was found in over 90% of the samples (Frank et
al., 1985).
5.1.5 Terrestrial and aquatic organisms
5.1.5.1 Plants
Samples of three types of mosses and four types of lichens (in
total 13 samples) were collected in the Antarctic Peninsula (Graham
Land) in 1985, and alpha-HCH was detected in most of them at a mean
concentration of 0.4 (0.20-1.15) µg/kg (Bacci et al., 1986).
In a study by Gaggi et al. (1986), fallen leaves (at the end of
their natural life-cycle) and lichens were collected in 1984 at sites
near Florence and Siena, Italy, in a woodland hilly area away from
primary pollution sources. The leaves were from ten different species
of trees and two different lichen species were involved. The average
levels of alpha-HCH in leaves and lichen were 37 (16-61) µg/kg dry
weight and 27 (25-29) µg/kg dry weight, respectively. The same
authors reported that the levels of alpha-HCH in various plant species
collected in 14 countries were 0.5-2140 µg/kg dry weight.
5.1.5.2 Fish and mussels
Martin & Hartman (1985) analysed 60 fish samples from nine
locations in the north-central part of the USA and found
concentrations of 5-27 µg alpha-HCH/kg (wet weight) in 36% of the
samples. The frequency with which alpha-HCH was detected in fish from
the different rivers varied between 17 and 100%.
In a study by Saiki & Schmitt (1986), samples of three to five
adult bluegills (Lepomis macrochirus) and common carp (Cyprinus
carpio) were collected at eight sites in three rivers in California,
USA, in 1981. Alpha-HCH concentrations in carp of up to 0.036 mg/kg on
fat basis were reported, but the concentrations in bluegill were
lower.
Cowan (1981) studied the extent of pollution of Scottish coastal
waters by HCHs using Mytilus edulis as biological indicator. The
levels of alpha-HCH at the 118 sites sampled ranged from < 6 to
23 µg/kg dry weight.
The fish and shellfish sampling programme carried out by the
United Kingdom Ministry of Agriculture, Fisheries, and Food between
1977-1984 was directed mainly to areas around the coasts of England
and Wales. The levels of alpha-HCH, which varied between the
different fish and shellfish species and also between the collection
sites, ranged from < 0.001 (nd) to 0.06 mg/kg wet weight. The
concentration in fish muscle was < 0.001 mg/kg wet weight (Franklin,
1987).
The mean alpha-HCH concentration in the muscle of flounders
collected off the North Sea coast of Germany in 1986 was 2.5 µg/kg
(nd-5.0 µg/kg) (Umweltbundesamt, 1989). Bream collected from different
locations in the River Elbe (between Schnackenburg and the North Sea)
contained 0.007-0.066 mg alpha-HCH/kg in muscle tissue and
0.9-2.2 mg/kg in adipose tissue (Arbeitsgemeinschaft für die
Reinhaltung der Elbe, 1982), while the same species collected from 15
rivers and lakes in the Federal Republic of Germany contained (on a
fat basis) up to 468 µg per kg (Umweltbundesamt, 1989).
Freshwater fish from different rivers in the Federal Republic of
Germany were analysed during the period 1973-1981, and in the first
3-4 years the alpha-HCH levels were mainly between 0.01 and 0.02 mg/kg
fresh weight. However, a clear decrease then took place and most of
the samples were below 0.01 mg/kg fresh weight, with the exception of
certain types of fish such as the eel and fish from industrially
contaminated areas (Hildebrandt et al., 1986).
In 1981-1983, shellfish and molluscs collected in the Federal
Republic of Germany contained < 0.001-0.20 mg alpha-HCH/kg fresh
weight. Eels collected in the North Sea and Baltic Sea contained
alpha-HCH levels of 0.011 mg per kg and 0.033 mg/kg fresh weight,
respectively. Flounders and herrings caught in the North Sea contained
0.002 and 0.008 mg/kg fresh weight, respectively, but in the Baltic
Sea the levels were about twice as high (Hildebrandt et al., 1986).
5.1.5.3 Birds
An average alpha-HCH residue level of 0.05 mg/kg was found in 17
adult herons in 1964 (HMSO, 1969).
In a study by Sierra & Santiago (1987), alpha-HCH concentrations
were determined in 23 barn owls (Tyto alba Scop.) from Leon, Spain.
The mean levels (and range) in muscle, liver, fat, brain, and
kidneys (in total 91 samples) were 0.242 (0.019-0.591), 0.323
(0.009-0.830), 1.073 (0.691-1.499), 0.238 (0.007-0.676), and 0.710
(0.051-2.381) mg/kg (wet weight), respectively.
Faladysz & Szefer (1982) analysed adipose fat from seven species
of diving ducks at their winter quarters in the Southern Baltic.
Residues of alpha-HCH were found in all of the 37 specimens of
long-tailed duck at mean concentrations (on a fat basis) of 3.4
(0.17-18) and 1.5 (0.23-6) mg/kg for female and male ducks,
respectively.
5.1.5.4 Mammals
Skaftason & Johannesson (1979) analysed 24 samples of the fat of
reindeer living in an area of the eastern and south-eastern parts of
Iceland where the use of pesticides is negligible. Alpha-HCH was
found in all samples at a mean level of 70 ± 22 µg/kg. These results
are in agreement with those of Benson et al. (1973), who found an
average of 77.5 µg/kg in the subcutaneous fat of wild Idaho moose
living in a forest area where pesticides were used very restrictively.
Skaftason & Johannesson (1979) analysed samples of body fat from
10-year-old sheep in 1974 and found an average of 51 ± 12 µg/kg.
Norström et al. (1988) investigated the contamination by
organochlorine compounds of Canadian arctic and subarctic marine
ecosystems by analysing the adipose tissue and liver of polar bears
( Ursus maritimus; 6-20 animals per area) collected from 12 areas
between 1982 and 1984. There was a difference in tissue distribution;
liver contained only alpha-HCH, but 29% of the HCH in adipose tissue
was beta-HCH. Adipose tissue contained 0.3-0.87 mg alpha-HCH per kg on
a fat basis.
The mean concentrations of alpha-HCH in the kidney fat of roe
(86 samples) collected in five areas of Germany in 1985-1986 were
about 7-12 µg/kg fat, the maximum value being about 50 µg/kg fat
(Umweltbundesamt, 1989).
5.2 General population exposure
From the data presented in section 5.1 it is evident that food is
the main source of exposure of the general population to alpha-HCH.
5.2.1 Total-diet studies
In a total-diet study carried out in the United Kingdom during
1966-1985, food purchased in 21 towns throughout the country was
prepared by cooking. The study covered 20 to 24 food groups, and the
number of total-diet samples examined varied from 22 to 25 samples.
The calculated mean alpha-HCH residue levels in the total diet for the
periods 1966-1967, 1970-1971, 1974-1975, 1975-1977, 1979-1980, 1981,
and 1984-1985 were 0.003, 0.002, 0.002, 0.0015, 0.001, < 0.0005, and
< 0.0005 mg/kg, respectively (Egan & Hubbard, 1975; HMSO, 1982, 1986,
1989).
Gartrell et al. (1985a) conducted a study to determine the
dietary intake of pesticides in the USA in 1978-1979. The samples,
purchased from retail outlets, were representative of the diets of
adults in 20 cities, and consisted of about 120 individual food items.
The daily intake of alpha-HCH in 1977, 1978, and 1979 was 0.011,
0.009, and 0.010 µg/kg body weight, respectively. In a similar way,
samples were collected in 10 cities in 1978-1979 consisting of about
50 items of infant food and 110 items of toddler food. The daily
intake of alpha-HCH in 1977, 1978, and 1979 was, respectively, 0.031,
0.034, 0.033 µg/kg for infants and 0.025, 0.029, and 0.029 µg/kg body
weight for toddlers, respectively (Gartrell et al., 1985b).
Total-diet studies conducted in the USA by the FDA before 1982
were based on a "composite sample approach" regardless of the diet
involved. Later on they were based on dietary survey information and
allowed the "total diet" of the population to be represented by a
relatively small number of food items for a greater number of age-sex
groups. The daily intakes of alpha-HCH during 1982-1984 for the age
groups 6-11 months, 2 years, 14-16-year-old females, 14-16-year-old
males, 25-30-year-old females, 25-30-year-old males, 60-65-year-old
females, and 60-65-year-old males were 7.2, 16.1, 6.1, 7.3, 4.5, 5.9,
3.3, and 3.7 ng/kg body weight, respectively (Gunderson, 1988).
In a total-diet study in the Netherlands in 1977, the average
concentration of alpha-HCH in 100 samples was 0.01 mg/kg on a fat
basis. The highest level was 0.05 mg/kg (Greve & van Hulst, 1977).
5.2.2 Air
Guicherit & Schulting (1985) measured the atmospheric
concentration of alpha-HCH in the Netherlands and calculated an
average daily intake by inhalation for a 70-kg person of 5 ng. The
equivalent value for the Federal Republic of Germany was calculated to
be 32 ng, which is about 1% of the total daily intake via the various
routes (Hildebrandt et al., 1986).
5.2.3 Concentrations in human samples
Alpha-HCH concentrations in human samples are a good indication
of the total exposure of the general population.
5.2.3.1 Blood
Blood samples of Dutch citizens analysed in 1978, 1980, 1981, and
1982 (70, 48, 127, and 54 samples, respectively), contained less than
0.1 µg alpha-HCH/litre (Greve & Wegman, 1985). Blok et al. (1984)
analysed the blood of 65 healthy volunteers in the Netherlands (34
female and 31 male) and detected alpha-HCH in less than one third of
the samples. The median concentration for both groups was below the
detection limit (0.1 µg per litre), but levels of up to 0.4 µg/litre
were measured.
Polishuk et al. (1970) studied the presence of alpha-HCH in the
blood of 24 pregnant women and 23 infants living in Israel. The
mean concentration was 0.6 ± 0.3 µg per litre in the women and 0.5 ±
0.3 µg/litre in the infants.
In 1975, Reiner et al. (1977) analysed the serum and plasma of 82
women and 65 men (with an average age of 42) living in a town in
Yugoslavia. In 57 of the 147 samples, alpha-HCH was found at a mean
concentration of 3.3 ± 0.5 µg/litre (range, 0.1-15.0 µg/litre).
Similar values were found in other parts of the country in 1976-1979
(Krauthacker et al., 1980).
The median concentration of alpha-HCH in whole blood of 118
people in the Federal Republic of Germany was reported to be
0.98 µg/litre (range, nd-2.06) (Bertram et al., 1980).
5.2.3.2 Adipose tissue
The alpha-HCH concentrations of 567 samples of adipose tissues of
Dutch citizens analysed during 1968-1983 varied from < 0.01 to
0.1 mg/kg (on a fat basis). The highest levels occurred during the
period 1968-1976 (Greve & van Harten, 1983; Greve & Wegman, 1985).
In a study by Niessen et al. (1984), specimens of subcutaneous
adipose tissue from 48 infants (34 under the age of 1 year, 14 in
their second year of life) were examined during 1982-1983 in the
Federal Republic of Germany. The average concentration of alpha-HCH
was 0.01 mg/kg fat (range, nd-0.02 mg/kg). The average concentration
was highest (0.02 mg/kg fat) for the age-range 0-6 weeks. Bertram et
al. (1980) found a median concentration of 0.03 mg/kg fat (range,
nd-0.35) in 72 samples of adipose tissue from people in the Federal
Republic of Germany. Hildebrandt et al. (1986) summarized the results
of nine studies carried out in the Federal Republic of Germany during
1969-1983. The mean alpha-HCH concentrations (568 samples) ranged
from 0.01 to 0.03 mg/kg fat.
Mes et al. (1982) analysed 99 samples of adipose tissue from
autopsies of accident victims from different areas of Canada. Nearly
all the samples (97%) contained alpha-HCH, the average concentration
of which was 0.004 mg/kg wet weight (range, 0.001-0.043 mg/kg).
In 1974, 360 samples of adipose tissue were collected in eight
regions of Japan and the mean level of alpha-HCH was 0.031 mg/kg
tissue (Takabatake, 1978).
Twenty-nine samples of adipose tissue were taken at necropsy and
24 at surgery in the Poznan district of Poland and compared with 100
samples from residents of the Warsaw area. In Poznan the mean
concentration of alpha-HCH was 0.013 ± 0.033 mg/kg, while in Warsaw it
was 0.008 ± 0.001 mg/kg (Szymczynski et al., 1986).
5.2.3.3 Breast milk
Breast milk is a major route for the elimination of
organochlorine pesticides in women. In a Swedish study, the levels of
alpha-HCH in breast milk were found to be related to the dietary
habit. Levels in lacto-vegetarians were lower than those in women
eating a mixed diet, and these were lower than those found in mothers
using a mixed diet that regularly included fatty fish from the Baltic
(Noren, 1983).
In a study by Fooken & Butte (1987), the variation of residue
levels in breast milk during lactation was investigated in five women
(aged 23-36) in the Federal Republic of Germany. Alpha-HCH
concentrations of up to 0.009 mg/kg fat were measured, and no
essential changes in residue level occurred over the lactation period.
Residues of alpha-HCH in breast milk during the periods,
1974-1975 and 1979-1980 in the Federal Republic of Germany were
reported to be 0.03 and 0.02 mg/kg milk on a fat basis, respectively
(Anon., 1984).
In the Federal Republic of Germany, more than 7100 samples of
breast milk were analysed from 1969-1984. These studies were carried
out by 20 authors, and the results were summarized by Hildebrandt et
al. (1986). The mean concentrations of alpha-HCH ranged from
0.01-0.04 mg/kg on a fat basis. In one case a mean concentration of
0.21 mg/kg was found in 320 samples. During the period investigated, a
slow decrease in the mean concentration of alpha-HCH was observed. The
average concentration in breast milk in the same country (2709
samples) in 1979-1981 was 0.024 mg/kg on a fat basis (Fooken & Butte,
1987). In 1981-1983, 132 samples of breast milk were analysed and the
average level was 0.001 mg alpha-HCH/kg milk fat (Cetinkaya et al.,
1984).
Tuinstra (1971) analysed 36 individual samples of breast milk,
collected in 1969, from young mothers (18-32 years of age) living in
the Netherlands. A median alpha-HCH concentration of 0.01 mg/kg milk
(on a fat basis) was found (range, nd-0.04). When 278 samples of
breast milk, collected in 11 maternity centres in the Netherlands,
were analysed for the presence of alpha-HCH, the median alpha-HCH
concentration was < 0.01 mg/kg (on a fat basis) (Greve & Wegman,
1985).
Vukavic et al. (1986) measured the alpha-HCH concentration in 59
samples of colostrum collected during autumn 1982 (26 samples) and
spring 1983 (33 samples) in Yugoslavia from healthy nursing mothers on
the third day after delivery. The alpha-HCH levels were significantly
lower in the autumn than in the spring (mean concentrations of 0.49 ±
0.09 and 1.50 ± 0.26 µg/litre whole colostrum, respectively).
Mes et al. (1986) studied 210 breast milk samples from five
different regions of Canada and measured a mean alpha-HCH
concentration of 7 µg/kg (on a fat basis). Davies & Mes (1987)
studied 18 breast milk samples from Canadian, Indian, and Inuit
mothers in Canada, whose fish consumption was comparable to the
national level. The level of alpha-HCH in milk fat of the indigenous
population was 5 µg/kg, which was the same value as that obtained from
a national survey.
6. KINETICS AND METABOLISM
6.1 Absorption and elimination
The intestinal absorption rate for alpha-HCH was 97.4% after the
administration of an HCH mixture to male rats (Albro & Thomas, 1974).
The total excretion in rats after a single intraperitoneal (ip)
36Cl-labelled alpha-HCH dose of 200 mg/kg body weight was 80% of the
dose in the urine and 20% in the faeces (Koransky et al., 1963;
Koransky et al., 1964; Noack et al., 1975). In a study in rats with
36Cl-labelled alpha-HCH, a low excretion rate was found. 36Cl was
detected in the excreta up to 40 days after a single ip dose (Koransky
et al., 1963). During continued dosing alpha-HCH was observed to
stimulate its own degradation (Noack et al., 1975). The decrease in
rat liver alpha-HCH levels after an initial increase, observed by
Eichler et al. (1983), was assumed to be due to this effect.
When 14C-labelled alpha-HCH was administered intraperitoneally
to male mice (ddY-strain, 4 weeks old) at a dose level of 22 µg, the
average percentage of urinary excretion of radioactivity in 3 days was
37% (Kurihara & Nakajima, 1974).
6.2 Distribution
One day after an ip injection of a mixture of 14C- and
36Cl-labelled alpha-HCH into rats (200 mg/kg body weight in rapeseed
oil), the highest level of radioactivity was found in fat, skin, and
bones plus muscles (18.2, 13.1, and 11.9%, respectively, after 4
days). Much lower levels were found in other organs or tissues (up to
1% in liver and kidneys and 0.28% in the central nervous system. In
the faeces and urine, 3.9 and 7.9%, respectively, were found after 4
days (Koransky et al., 1963). In other studies with rats, higher
concentrations were found in liver, kidneys, body fat, brain, and
muscle (Portig & Vohland, 1983; Kuiper et al., 1985). In a 90-day
study in rats, marked deposition of alpha-HCH was found in renal fat;
the concentrations exceeded those obtained in a similar study on
beta-HCH (Greve & van Hulst, 1980; Kuiper et al., 1985). In lactating
rats given a single oral dose 5 days after birth, the alpha-HCH
concentrations in the livers of the sucklings were twice as high as
those observed in the livers of the mothers (Wittich &
Schulte-Hermann, 1977).
Vohland et al. (1981) studied the distribution of alpha-HCH in
the brain and depot fat of rats after the administration of 200 mg/kg
body weight by gavage. With an average blood concentration of
1.5 µg/litre, the brain to blood, and depot fat to blood ratios were
120:1 and 397:1, respectively, whereas with a blood concentration of
17.7 mg/litre the ratios were 5:1 and 82:1, respectively.
Nagasaki (1973) orally administered alpha-HCH to male mice at
concentrations of 100, 250 or 500 mg/kg for 24 weeks, and found high
residual levels of this isomer in liver and adipose tissue. Similarly,
Macholz et al. (1986) reported that a 30-day administration of
alpha-HCH to rats resulted in high residues of this isomer in fat,
kidneys, and adrenal tissue.
In the brain, alpha-HCH is stored preferentially in the white
matter (Stein et al., 1980; Portig et al., 1989).
6.3 Metabolic transformation
6.3.1 Rat
When Sprague-Dawley weanling female rats were administered 2 mg
alpha-HCH/rat per day in peanut oil for 7 days, the alpha-HCH was
metabolized to 2,4,6- and 2,4,5-trichlorophenol, with an excretion
ratio of 2,4,6- to 2,4,5-trichlorophenol of 1.3:1. This study also
indicated that pre-treatment with alpha-HCH alters the metabolism of
lindane in rats (Freal & Chadwick, 1973).
The biotransformation of alpha-HCH in rats involves
dechlorination (Kraus, 1975). The dose-dependent decrease in liver
glutathione concentrations indicates the formation of a glutathione
conjugate in this organ (Noack & Portig, 1973; Portig et al., 1973;
Kraus, 1975). Such a decrease does not occur in the brain or kidneys
(Noack & Portig, 1973).
The major urinary metabolite in rats is 2,4,6-trichlorophenol, a
compound reported by IARC (1987) to be carcinogenic for animals
(Portig et al., 1973; Stein & Portig, 1976; Stein et al., 1977). Other
metabolites that have been identified are 1,2,4-trichlorophenol,
2,3,4-trichlorophenol, 2,4,5-trichlorophenol, 2,3,4,5-
tetrachlorophenol, and 2,3,4,6-tetrachlorophenol (Noack et al., 1975;
Stein et al., 1977; Macholz et al., 1982). In addition,
chlorothiophenols (not specified) have been detected, and
1,3,4,5,6-pentachlorocyclohex-1-ene has been identified in the kidneys
of rats (Macholz et al., 1983).
Artigas et al. (1988) have identified several lindane metabolites
(tetra-, penta-, and hexachlorocyclohexenes, and tetra- and
pentachlorobenzenes) in rat brain homogenates by gas
chromatography-mass spectrometry. Male Wistar rats were orally
administered 30 mg alpha-HCH/kg and were sacrificed 5 h later. The
cerebella of the animals were analysed and the following metabolites
were found: 3.6/4.5-PCCH, 3.5/4.6-PCCH, HCCH, pentachlorobenzene, and
HCB. HCCH was the major metabolite (about 100 µg per kg) while levels
of the other metabolites were mainly below 5 µg/kg. Alpha-HCH was
present at 17.2 mg/kg tissue. This study showed that the HCH isomers
are cleared from the brain via different metabolic pathways.
Isomerization of alpha-HCH to lindane did not occur after
repeated dosage (Eichler et al., 1983).
6.3.2 Bird
In a model 4-week feeding study on poultry using four HCH
isomers, the rate of degradation of the individual HCH isomers in
broilers followed the order: delta > gamma > alpha > beta.
Biotransformation (to one or more of the other HCH isomers) was not
detected (Szokolay et al., 1977b).
In a study by Foster & Saha (1978) on the in vitro metabolism
of alpha-HCH in chicken livers, the first metabolite was identified as
an isomer of pentachlorocyclohexane.
6.3.3 Human
When Engst et al. (1978) analysed the urine of occupationally
exposed workers (apparently to technical-grade HCH in manufacturing
processes), they found, apart from alpha-, beta-, gamma-, and
delta-HCH, traces of hexa- and pentachlorobenzene, gamma- and
delta-pentachlorocyclohexane, pentachlorophenol, 2,3,4,5-, 2,3,4,6-,
and 2,3,5,6-tetrachlorophenol, and several trichlorophenols, as well
as the glucuronides of several of these metabolites. The
pentachlorocyclohexenes, tetrachlorophenol, hexachlorobenzene, and
pentachlorophenol were also identified in the blood.
6.4 Retention and biological half-life
The half-life for the clearance of alpha-HCH from depot fat was
found to be 6.9 days in female rats and 1.6 days in male rats (Stein
et al., 1980; Portig, 1983).
Vohland et al. (1981) and Portig & Vohland (1983) studied the
kinetics of alpha-HCH in Wistar rats, and observed that, after a
single oral dose of 200 mg/kg body weight, the approximate half-life
in females for the elimination from brain was 6 days.
The retention of alpha-HCH in rat brain after a single dose is
greater than that of beta- and gamma-HCH (Stein et al., 1980).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Acute toxicity
In mice oral LD50 values have been found to range from 1000 to
4000 mg/kg body weight, depending on the vehicule, while in rats
values of 500-4674 mg/kg body weight have been obtained. Riemschneider
(1949) determined a LD50 (oral intubation in olive oil) for rats of
1500 mg/kg body weight. The signs of poisoning were those of nervous
system stimulation: excitation, hunched posture, rough fur, dyspnoea,
anorexia, tremors, convulsions, and cramps (Hoffmann, 1983; WHO,
1986).
7.2 Short-term exposure
7.2.1 Oral
In a 90-day study on rats carried out with dose levels of 0, 2,
10, 50, or 250 mg alpha-HCH/kg diet, reductions in white blood cell
count were noted in several groups of animals. Growth was decreased
at 250 mg/kg diet, and at this dose level the number of erythrocytes
and protein excretion in the urine were elevated in female animals. At
levels of 50 and 250 mg/kg, the activities of liver
amino-pyrine- N-demethylase and aniline hydroxylase were increased
while those of blood aspartate aminotransferase (ASAT) and creatine
phosphokinase were decreased. Liver weights were increased at dose
levels of 10, 50, and 250 mg/kg. Enlargement of liver parenchyma cells
(with a foamy/hyaline appearance of the cytoplasm) and accentuation of
the plasmalemma, indicative of proliferation of smooth endoplasmatic
reticulum (SER), occurred at levels of 50 and 250 mg/kg. At the
250-mg/kg level, there were increases in the relative weights of
heart, kidneys, and adrenals. In addition, serum levels of
immunoglobulins G and M showed a decrease at 50 and 250 mg/kg diet
(Kuiper et al., 1985).
Macholz et al. (1986) reported that the administration of 1000 mg
alpha-HCH/kg to rats for 30 days resulted in growth retardation and
liver mass increase. High residue levels of alpha-HCH were identified
in fat, kidneys, and adrenal tissue.
7.2.2 Other routes
7.2.2.1 Intravenous
In a study by van Asperen (1954), groups of 12-15 male and female
albino mice (8-10 weeks of age) were given an intravenous injection of
alpha-HCH (in peanut oil). The dose levels were 480 or 960 µg/mouse
(equivalent to approximately 32 and 64 mg/kg body weight,
respectively). No deaths occurred within 7 days.
7.2.2.2 Subcutaneous
Groups of 13-21 male and female albino mice (8-10 weeks of age)
were given a subcutaneous injection of alpha-HCH at dose levels
ranging from 3 to 20 mg/animal (equivalent to approximately 200 to
1330 mg/kg body weight, respectively). With doses of up to 4 mg, no
death occurred within 7 days, but with 4.5 mg, 8 mg, and 20 mg, 8, 25,
and 90%, respectively, of the animals died (van Asperen, 1954).
7.3 Skin and eye irritation; sensitization
No data on skin and eye irritation or sensitization have been
reported.
7.4 Long-term exposure
7.4.1 Rat oral study
When groups of 10 female and 10 male weanling Wistar rats were
administered diets containing 0, 10, 50, 100, or 800 mg alpha-HCH/kg
diet (in corn oil) for 107 weeks, the highest dose level resulted in
growth retardation, increased mortality, and slight kidney damage.
With dose levels of 100 or 800 mg/kg, liver enlargement and
histo-pathological changes in the liver were found. However, there
were no liver changes at 50 mg/kg diet (Fitzhugh et al., 1950).
7.5 Reproduction, embyrotoxicity, and teratogenicity
No information on reproduction, embryotoxicity, or teratogenicity
is available.
7.6 Mutagenicity and related end-points
Alpha-HCH did not induce mutations in Salmonella typhimurium
test strains TA98, TA100, TA1535 or TA1537 either with or without rat
liver metabolic activation (Lawlor & Haworth, 1979). A test for point
mutations in Saccharomyces cerevisiae XV 185 14 C was also negative
(Shahin & von Borstel, 1977). In addition, the compound produced no
mutations in Allium cepa roots (Nybom & Knutsson, 1947). A test for
unscheduled DNA synthesis in rat hepatocytes in vitro produced an
equivocal result (Althaus et al., 1982).
A mutagen test strain of Bacillus subtilis (TKJ5211) showed a
higher sensitivity for hisw+ reversion than the parental strain
HA101 when treated with UV and UV-mimetic chemicals. However, a
negative result was obtained when alpha-HCH dissolved in DMSO was used
at a dose level of 5 mg/ml (Tanooka, 1977).
A DNA repair test was carried out with stationary-phase cultures
of B. subtilis HLL3g and HJ-15 strains in which the size of growth
inhibition zones of repair-proficient and repair-deficient cells for
vegetative cells and spores was determined. There was no effect at a
dose level of 5 mg alpha-HCH (in benzene) per ml (Tanooka, 1977).
The available data are inadequate to make an assessment of the
mutagenic potential.
7.7 Carcinogenicity
Appraisal
The reported studies on the carcinogen effects of alpha-HCH on
mice and rats have some short-comings. In most cases, very high dose
levels were tested. Nevertheless, it is clear from the results that
alpha-HCH, at high dose levels, produces nodular hyperplasia and
hepatocellular carcinomas in mice (the incidence varying according to
the strain) and also in rats (low incidence), but only at higher dose
levels.
The results of the studies on initiation-promotion and mode of
action indicate that the neoplastic response observed with alpha-HCH
is most likely due to a non-genotoxic mechanism.
7.7.1 Mouse
When 20 male ICR/JCL mice (aged 5 weeks) were administered a diet
containing 600 mg alpha-HCH/kg diet for 26 weeks, increased liver
weight was observed. In all treated mice there were liver tumours,
which were characterized histologically as benign tumours and
malignant tumours with atypical liver cells. Unfortunately,
insufficient details were reported (Goto et al., 1972a,b).
In a study by Hanada et al. (1973), 6-week-old DD mice (10-11 of
each sex per group) were given diets containing 0, 100, 300, or 600 mg
alpha-HCH/kg diet for 32 weeks, followed by a control diet for 5-6
weeks. The control group consisted of 20 female and 21 male animals.
During the experiment several animals died. The numbers of hepatomas
in the four groups surviving for 36-38 weeks were 0/29 (control), 1/16
(100 mg/kg), 9/10 (300 mg/kg), and 13/15 (600 mg/kg).
Alpha-fetoprotein was not detected in the serum of animals with
hepatomas.
When 8-week-old male DD mice, divided into groups of 20 or 38
animals, were fed a diet containing 0, 100, 250, or 500 mg
alpha-HCH/kg for 24 weeks, the two highest dose levels induced an
increase in liver weight. At the four respective dose levels, the
incidence of nodules classified as nodular hyperplasia was 0/20, 0/20,
30/38 (79%), and 20/20 (100%) and that of hepatocellular carcinoma was
0/20, 0/20, 10/38 (26%), and 17/20 (85%) (Ito et al., 1973b).
Following the oral administration of 100, 250 or 500 mg
alpha-HCH/kg to male DD mice for 24 weeks, hepatocellular tumours were
found in all mice treated with 500 mg/kg and in 17 of the 20 mice that
received 250 mg/kg (Nagasaki, 1973).
Nagasaki et al. (1975) studied the tumorigenic effects of a diet
containing 0 or 500 mg alpha-HCH/kg, fed for 24 weeks to groups of
male and female DDY, ICR, DBA/2, C57BL/6, and C3H/He mice (13-29 of
each sex per group), male Wistar rats, and male golden Syrian
hamsters. It was found that alpha-HCH induced liver tumours in male
and female mice but not in rats and hamsters. The histological changes
in the liver of mice were much greater than those induced in rats and
hamsters. Male animals were more susceptible to the tumorigenic
action (i.e. liver nodules) than females. Among the different strains
of mice, a difference in susceptibility was observed. The occurrence
of liver nodules varied from 16.7 to 100% and the incidence of
hepatocellular carcinomas varied from none to 65%. The DDY mouse
strain was the most sensitive and the C57BL/6 the least sensitive
strain.
Ito et al. (1976) studied the reversibility of liver tumours
induced by alpha-HCH (99.0%). Male 8-week-old DDY mice were fed a
diet containing 0 or 500 mg/kg for 16, 20, 24, and 35 weeks and then
fed a basal diet without alpha-HCH for 4, 8, and 12, or 4, 8, 12, 16,
24, and 36 weeks. In total 341 mice were used, of which 21 were fed
the compound for 16 weeks. A total of 300 mice were fed the diet with
alpha-HCH for 20 or more weeks and 20 control mice were fed the basal
diet for 72 weeks. At the various intervals indicated, 12-20 mice were
killed. The incidence of liver tumours increased progressively during
continuous administration of alpha-HCH, but when its administration
was discontinued some tumours disappeared. After 24 weeks of
administration most tumours were nodular hyperplasias with only a few
well-differentiated hepatocellular carcinomas. However, 60 or 72
weeks after the beginning of the study most of the liver tumours were
hepatocellular carcinomas. The findings suggested that nodular
hyperplasia was usually reversible.
Two groups of male HPBC57B1 black mice (6-9 weeks old) were fed
a diet containing 500 mg/kg alpha-HCH (99.8%) per diet, 48 mice being
used as controls and 75 mice being administered alpha-HCH. From each
group, 4-9 mice were killed at 1, 3, 4, 8, 14, 21, 30, 33, 44, and 50
weeks after the initiation of treatment. Progressive liver
enlargement was first noticed at 3 weeks, hepatic nodules at 21 weeks,
and emaciation at 30 weeks. Histopathological liver alterations
included hypertrophy of centrolobular hepatocytes first seen at 1 week
and the merging of adjacent megalocytic zones at 3 weeks. At 21
weeks, adenomas were seen in two out of seven mice, at 30 weeks in
seven out of eight mice, and at 33, 44, and 50 weeks in all the mice
studied. Under the condition of this study, neither hepatocellular
carcinomas nor metastases in the lungs were detected (Tryphonas &
Iverson, 1983).
7.7.2 Rat
When groups of 10 male and 10 female weanling Wistar rats were
fed throughout their life on diets containing 10, 50, 100, or 800 mg
alpha-HCH (> 98% pure) per kg, no increase in tumour incidence was
found. However, only a limited number of organs were examined
microscopically (Fitzhugh et al., 1950).
In a study by Ito et al. (1975), male Wistar rats (5-8-weeks old)
were divided into seven groups and administered alpha-HCH diets
containing 0, 500 (two groups), 1000 (three groups), or 1500 mg
alpha-HCH/kg diet. The duration of the treatment for the different
groups was 72 weeks for the controls, 24 or 48 weeks at 500 mg/kg, 24,
48, or 72 weeks at 1000 mg/kg, and 72 weeks at 1500 mg/kg. In the
liver, oval cells and bile duct cell proliferation were found in the
groups fed 1000 or 1500 mg/kg after 48 and 72 weeks. Cell hypertrophy
was found in all the groups, the increase in severity depending on the
dose level and the duration of administration. In the two groups fed
500 mg/kg and the group fed 1000 mg/kg for 24 weeks no nodular
hyperplasia or hepatocellular carcinomas were found. Nodular
hyperplasia developed in the groups fed 1000 mg/kg (48 and 72 weeks)
or 1500 mg/kg (72 weeks) in 42, 76, and 77% of the animals,
respectively. Hepatocellular carcinomas were found only in the groups
fed 1000 or 1500 mg/kg for 72 weeks (1/16 and 3/13 animals,
respectively).
In a series of studies, an oral dose of 20 mg/kg body weight was
administered daily to female rats during periods of 4.5, 13.5, or 23.5
months. Liver enzyme induction was found at all intervals, white foci
and nodules were present after 13.5 months, and one animal had a
hepatocellular carcinoma after 23.5 months (Schulte-Hermann &
Parzefall, 1981). The value of this study was reduced by the very low
number of animals (4-6 per group) used at each interval.
7.7.3 Initiation-promotion
In a study on 8-week-old white male mice (25-30 per group) of
strain DD, the influence of alpha-HCH on tumour induction by
polychlorinated biphenyls (PCBs) was tested and vice versa. Whereas
500 mg PCB/kg diet induced nodular hyperplasia and hepatocellular
carcinomas in the liver of male mice after 32 weeks, exposure to
alpha-HCH at dose levels of 50, 100, or 250 mg/kg diet, only resulted
in both type of tumours at the highest dose level. The incidence of
nodular hyperplasia was 23/30 (77%) and that of hepatocellular
carcinoma was 8/30 (27%). However, 50 or 100 mg alpha-HCH/kg diet, in
combination with 250 mg PCB per kg diet (PCB alone did not induce
tumours), induced nodular hyperplasia (approximately 30%) and
hepatocellular carcinoma (approximately 5%). It seems that PCBs
promote the induction of liver tumours by alpha-HCH (Ito et al.,
1973a).
In studies on rats, alpha-HCH showed a tumour-promoting action
towards the hepatocarcinogenic effects of aflatoxin B1,
diethylnitrosamine, and nitrosomorpholine (Schulte-Hermann &
Parzefall, 1981; Schulte-Hermann et al., 1981; Angsubhakorn et al.,
1981). In one test, alpha-HCH produced only a slight liver
tumour-promoting effect in rats after initiation with
N-nitrosodiethylamine (Ito et al., 1983). However, in another study
on the same species the compound had an inhibitory effect on the
hepa-tocarcinogenic action of 3-methyl-4-dimethylaminoazobenzene and
DL-ethionine (Thamavit et al., 1974).
Nagasaki et al. (1975) studied the influence of
3-methylcholanthrene, 1-naphthyl isothiocyanate, and
p-hydroxypropiophenone on the induction of liver tumours by
alpha-HCH. Eight groups of 24 mice received a diet containing either
500 mg alpha-HCH/kg diet in combination with 67 mg
methylcholanthrene/kg, 600 mg 1-naphthyl isothiocyanate/kg or 1000 mg
p-hydroxypropiophenone/kg or just one of the four compounds. A
control group with the basal diet was also used. The induction of
mouse liver tumours by alpha-HCH was not inhibited by the concomitant
feeding of 1-naphthyl isothiocyanate or p-hydroxypropiophenone.
However, 3-methylcholanthrene slightly inhibited their induction by
alpha-HCH.
In a study by Schröter et al. (1987), the tumour-initiating
activity of alpha-HCH was studied by examining for phenotypically
altered foci in female Wistar rats. Groups of three to eight rats
were used and, after removing the median and right liver lobes, 200 mg
alpha-HCH/kg body weight was administered followed by phenobarbital at
50 mg/kg body weight per day for 15 weeks. Liver foci were identified
by means of the gamma-glutamyltransferase (GGT) reaction and by
morphological alterations. No evidence of initiating activity was
found. In another part of the study, the promoting activity was
investigated. A single dose of N-nitrosomorpholine (250 mg/kg body
weight by gavage) was followed by the administration of 0.1, 0.5, 2.0,
7.0, or 20.0 mg alpha-HCH/kg body weight per day for 4, 15, and 20
weeks. The criteria used were growth and phenotypic changes of foci
as end-points. It was concluded from the study that alpha-HCH is a
tumour promotor. Both the number and size of altered foci were
enhanced by alpha-HCH doses of 2 mg/kg or more. The tumour-promoting
action was generally associated with liver enlargement and induction
of monooxygenases or other specific enzymes.
Schulte-Hermann et al. (1983) carried out three experiments with
Han-Wistar rats using, in experiment 1, 39 female rats (8-24 months
old) and, in experiments 2 and 3, 41 male (2 years old) rats.
Alpha-HCH (200 mg/kg in corn oil) was administered orally as a single
dose, while the control group received only corn oil. Beginning 25 h
after the dosing, 3H-thymidine was injected intravenously five times
at intervals of 6 h (experiment 2) or 8 h (experiment 3) , and the
animals were killed 18 (experiment 2) or 3 h (experiment 3) after the
last dose of 3H-thymidine. The effect of age on the incidence of
spontaneous foci was studied in experiment 1. Foci of putative
preneoplastic cells were detected in the livers of untreated rats of
both sexes, especially at 1 and 2 years of age. These foci exhibited
markers similar to those of their counterparts in carcinogen-treated
rats, such as cytoplasmic basophilia, clearness of cytoplasm, or
expression of gamma-glutamyl transferase. Rates of DNA synthesis in
foci were higher than in normal liver cells and were increased by
single doses of liver mitogens assumed to promote liver tumour
development. Thus cells in the spontaneous foci appeared to possess a
defect in the growth control, rendering them more susceptible to
endogenous and exogenous growth stimuli.
The incorporation of orally administered radiolabelled thymidine
into liver DNA was determined in SIV-50-SD rats 24 h after a single
oral gavage dose of 2.9, 29.1, 58.2, or 291 mg alpha-HCH/kg. Alpha-HCH
was found to stimulate liver DNA synthesis at 58.2 mg/kg (Büsser &
Lutz, 1987).
7.7.4 Mode of action
Sagelsdorff et al. (1983) studied the relevance to the
carcinogenic action of alpha-HCH of covalent binding to mouse liver
DNA. Three strains of mice were used (NMRI, CF1, and C6B3F1), and
alpha-HCH was administered by oral gavage and 14C-thymidine by the
intraperitoneal route. In all three strains, a similar low covalent
binding index or DNA damage/dose (values ranging from 0.17-0.28) was
found. There was no quantitative correlation with the carcinogenicity
potency of alpha-HCH.
Iverson et al. (1984) studied the ability of alpha-HCH to bind to
macromolecules from male HPB black mouse liver. In vivo and in
vitro binding studies with 14C-alpha-HCH and hepatic microsomes
from untreated and phenobarbital-pretreated mice showed no
preferential binding of alpha-HCH to protein or DNA. The results
suggest that the neo-plastic response observed with alpha-HCH results
from a non-genotoxic mechanism.
7.8 Special studies
7.8.1 Effect on liver enzymes
After a single oral administration to female rats of 5 mg
alpha-HCH/kg body weight or more the rate of aminopyrine demethylation
and the liver DNA content were both increased, but at 2 mg/kg body
weight these effects did not occur (Schulte-Hermann et al., 1974). In
a further study, the liver cytochrome P450 concentration in male rats
after a single oral administration was elevated at all tested dose
levels, 25 mg/kg body weight being the lowest (Seifart & Buchar,
1978). After alpha-HCH was given to male rats at dose levels of 5, 10,
20, 50, or 200 mg/kg feed for 2 weeks, aniline hydroxylase and
aminopyrine demethylase activities were increased at all dose levels
(den Tonkelaar et al., 1981).
7.8.2 Neurotoxicity
Appraisal
Alpha-HCH has been shown to have no effect on motor nerve
conduction velocity or the fronto-occipital EEG in rats fed 1000 mg
alpha-HCH/kg diet for 30 days. This isomer is a mild antagonist of
pentylenetetrazol-induced convulsions but increases the tonic/clonic
activity and the lethality of picro-toxin when administered
intraperitoneally to mice. It decreases the accumulation of
cerebellar cyclic GMP and prohibits the increase of cGMP caused by
gamma-HCH in mouse brain. Alpha-HCH has been demonstrated to inhibit
GABA-mediated chloride ion uptake in mouse brain, and this effect is
believed to play a primary role in the CNS action of this isomer.
In a study by Vohland et al. (1981), alpha-HCH did not give rise
in brain tissue to appreciable quantities of hydrophobic metabolites
such as 2,4,6-trichlorophenol. It had a weak protecting action
against convulsions induced by pentylenetetrazole (PTZ). The intensity
and duration of the PTZ-antagonistic effects after a single oral dose
were related to the alpha-HCH content of the brain.
In a 30-day study on groups of 15 male Wistar rats fed alpha-HCH
at levels of up to 1000 mg/kg diet, there was no effect on the
fronto-occipital electroencephalogram or on the motor conduction
velocity of the tail nerve (Müller et al., 1981).
The effect of alpha-HCH on body temperature, food intake, and
body weight was studied in Wistar rats (eight males and eight females)
given a single 30-mg/kg oral dose of alpha-HCH in olive oil. Controls
received only olive oil. Alpha-HCH treatment induced no significant
decrease in core temperature 5 h after treatment, and no decrease in
food intake or growth was observed (Camon et al., 1988).
Fishman & Gianutsos (1987) studied the effects of an
intraperitoneal injection of alpha-HCH (99.0%) in corn oil
(80-480 mg/kg body weight) on the accumulation of cerebellar cyclic
GMP in male CD-1 mice. Alpha-HCH decreased the accumulation of
cerebellar cyclic GMP and also prevented the increase in cyclic GMP
resulting from lindane treatment. Furthermore, alpha-HCH inhibited
the binding of 3H-TBOB (a ligand for the GABA-A-receptor-linked
chloride channel) in mouse cerebellum.
Fishman & Gianutsos (1988) compared the CNS-related
pharmacological and biochemical effects of gamma-HCH and the
non-convulsant isomer alpha-HCH. The studies were carried out on male
CD-1 mice injected intraperitoneally with a single alpha-HCH (in corn
oil) dose of 80-400 mg/kg body weight. Alpha-HCH inhibited the
myoclonic jerk and tonic/clonic activity of PTZ but increased the
tonic/clonic activity and lethality of picrotoxin (PIC) (PTZ and PIC
were given as a single ip injection of 50 mg/kg and 20 mg/kg body
weight, respectively). The highest dose of alpha-HCH caused a
significant decrease in motor activity. Gamma-HCH inhibited the
binding of 3H-TBOB to mouse whole brain membranes. Furthermore, this
isomer is a weak inhibitor of GABA-stimulated uptake of 36wCl into
mouse brain neurosynaptosome preparations in vitro. The
non-seizure-inducing alpha-HCH has biochemical and pharmacological
effects in the CNS which differ from those of the gamma-HCH.
Matsumoto et al. (1988) provided evidence that all HCH isomers
are capable of inhibiting GABA-A-mediated chloride channels in the
brain, the relative potency being alpha = gamma > delta > beta.
Alpha-HCH was also found to be a potent inhibitor of the
batrachotoxin-stimulated action potential flux of sodium ions in N18
neuroblastoma cell cultures (Shain et al., 1987).
8. EFFECTS ON HUMANS
8.1 Acute toxicity - poisoning incidents
Several cases of acute poisoning by technical-grade HCH,
resulting either from accidents or occupational exposure, have been
described (WHO, 1991). Although alpha-HCH constitutes 65-70% of the
technical product, it is likely that the most acutely toxic component,
i.e. gamma-HCH, played the major role in these incidents. These cases
cannot, therefore, assist in the evaluation of alpha-HCH.
8.2 General population
No specific studies relating to alpha-HCH are available.
A study comparing liver cancer deaths in the USA and the
"domestic disappearance" of organochlorine pesticides revealed that in
1962, 18 and 15 years after the introduction of DDT and
technical-grade HCH, respectively (when an increase in primary liver
cancer due to the organochlorines would be manifest), the number of
cases of primary liver cancer as a percentage of the total number of
liver cancer deaths began a gradual and steady decline (from 61.3% in
1962 to 56.9% in 1972). The death rate (per 100 000 per year) due to
primary liver cancer declined from 3.46 to 3.18 during this period
(Deichmann & MacDonald, 1977).
8.3 Occupational exposure
The evaluation of the effects of alpha-HCH on occupationally
exposed workers is seriously hampered by the fact that most of the
relevant studies concern workers who were exposed during the
manufacture and handling of lindane or the handling and spraying of
technical-grade HCH among other pesticides, and were thus exposed to
all HCH isomers plus impurities and other (process) chemicals.
Therefore, it is difficult, if not impossible, to relate the observed
effects to individual substances. Consequently these studies have only
been described in this monograph where they aid the evaluation.
Behrbohm & Brandt (1959) described 26 cases of allergic and toxic
dermatitis that arose during the manufacture of technical-grade HCH.
Patch testing with pure alpha-, beta-, gamma-, and delta-HCH yielded
negative results, but positive reactions were obtained with the
residual fractions.
The level of alpha-HCH in 57 healthy workers (with normal liver
function, EMG and EEG) at a lindane-manufacturing plant ranged from 10
to 273 µg/litre, whereas it was below the detection limit in control
workers. The concentration in the adipose tissue of eight of the
exposed workers ranged from 1 to 15 mg alpha-HCH/kg (in extractable
lipids) (Baumann et al., 1980, 1981; Brassow et al., 1981; Tomczak et
al., 1981).
The mean serum alpha-HCH level of malaria-control workers that
sprayed technical-grade HCH for 16 weeks increased from 10 to
78 µg/litre in previously non-exposed workers and from 18 to
77 µg/litre in those that had been exposed during three previous
spraying seasons (Gupta et al., 1982).
Nigam et al. (1986) studied 64 employees from a plant
manufacturing HCH who were directly or indirectly associated with the
production of this insecticide and thus also exposed to chemicals such
as benzene and chlorine. The exposed group was composed of 19
"handlers" (who handled and packed the insecticide), 26 "non-handlers"
(plant operators and supervisors exposed indirectly to HCH), and 19
maintenance staff (who visited the plant frequently). The control
group consisted of 14 workers who had no occupational contact with the
insecticide. The exposure period varied up to 30 years. The mean
serum alpha-HCH concentrations in the four groups were 21.1 µg/litre
(controls), 21.8 µg/litre (maintenance staff), 41.2 µg per litre
(non-handlers), and 100 µg/litre (handlers). Lindane and beta- and
delta-HCH were also present. The total HCH concentrations were 51.4,
143.6, 265.6, and 604 µg per litre, respectively. Clinical examination
revealed that the majority of the workers from the "handler" and
"non-handler" groups exhibited paraesthesia of the face and
extremities, headache, and giddiness, and some of them also showed
symptoms of malaise, vomiting, tremors, apprehension, confusion, loss
of sleep, impaired memory, and loss of libido. The same symptoms were
found among the maintenance staff but were less severe and less
frequent.
Chattopadhyay et al. (1988) studied 45 male workers exposed to
HCH during its manufacture and compared them with 22 matched controls.
Exposure was mainly via the skin. Paraesthesia of face and
extremities, headache, giddiness, vomiting, apprehension, and loss of
sleep, as well as some changes in liver function tests, were reported
and were found to be related more to the intensity of exposure (as
measured by the HCH levels in blood serum) than to the duration of
exposure. The measured exposures to total HCH were 13 to 20 times
higher than those in the control groups (no detailed figures were
reported). Of the total serum HCH, 60-80% was beta-HCH.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Algae
Palmer & Maloney (1955) used alpha-HCH in a preliminary screening
test with two cyanobacterium (blue-green alga), two green alga, and
two diatom species. The test concentration was 2 mg/litre of water,
and the incubation period was 3-21 days. Alpha-HCH was not toxic at
this concentration.
When Canton et al. (1975) exposed Chlorella pyrenoidosa to
alpha-HCH for 96 h at 28°C (static system), the EC50 (growth
inhibition) was > 10 mg/litre (maximum solubility in the medium).
In a study by Krishnakumari (1977), cultures of the green alga
Scenedesmus acutus of 1, 3, or 5 days of age were tested for
sensitivity to alpha-HCH at 28°C, the growth rate being used as a
parameter. Alpha-HCH dissolved in ethanol was added at nominal
concentrations of 0.5-100 mg/litre water. The alpha-HCH concentrations
that caused a reduction in growth in 1-, 3-, and 5-day-old cultures
were 10 (or more), 5, and 0.5 mg/kg, respectively.
When Chlamydomonas sp. was exposed at a temperature of 20-25°C
in a static system, the no-observed-effect level (based on the growth
in 48 h) was > 1.4 mg/litre. A similar result was obtained with
Dunaliella sp. at 15°C and a study duration of 48 and 96 h, the
NOEL for growth being 1.4 mg/litre (maximum solubility) (Canton et
al., 1978).
9.2 Protozoa
The EC50 for Tetrahymena pyriformis (3 days in closed system
at 27°C) was reported to be 0.75 mg/litre (Mathur et al., 1984).
9.3 Invertebrates
9.3.1 Acute toxicity
The result of acute or short-term toxicity studies lasting a few
days on Artemia salina, Daphnia magna, and Lymnaea stagnalis are
summarized in Table 3.
Table 3. Acute or short-term toxicity of alpha-hexachlorocyclohexane for invertebrates
Species Age Temperature Parameter Concentration References
(°C) (mg/litre)
Artemia salina 3 weeks 24 LC50a,b 0.5 Canton et al. (1978)
Daphnia magna < 1 day 20 LC50c,d 0.8 Canton et al. (1975)
Lymnaea stagnalis 6 months 22 EC50c,e 1.2 Canton & Slooff (1977)
a synthetic saltwater
b 35 days (but exposure time was 4 days)
c 48 h
d closed system
e growth inhibition/mortality or immobilization
9.3.2 Short- and long-term toxicity
9.3.2.1 Crustaceae
In a study by Canton et al. (1975), Daphnia magna was exposed
to 0, 10, 50, 200, 1000, or 2000 µg alpha-HCH (> 95%) per litre for
25 days. The daphnids were fed Chlorella pyrenoidosa. The
sensitivity of daphnids to alpha-HCH markedly increased with exposure
time. A concentration of approximately 50 µg/litre or less did not
lead to death at any time during the whole life cycle of 2 months.
Only with 2000 µg/litre was there an influence on reproduction, the
EC50 for reproduction inhibition being 100 (54-186) µg/litre.
The EC50 based on mortality and immobilization was 800
(600-1000) µg/litre (see Table 4).
9.3.2.2 Molluscs
In a short-term (2-day) study, groups of five adult snails
(Lymnaea stagnalis L.) (6 months of age) were exposed to various
dose levels. Based on mortality and immobility, the EC50 was
estimated to be 1200 (600-2300) µg alpha-HCH (> 95%) per litre
(Canton & Slooff, 1977).
In a long-term (70-day) study, groups of 10 snails (5 months of
age) were exposed to 20, 100, 300, or 600 µg per litre. The study was
divided into a pre-exposure period (14 days) during which all egg
capsules and the number of eggs per capsule were counted, an exposure
period of 40 days during which four groups of adults and five capsules
of each group were exposed to alpha-HCH, and a post-exposure period
(16 days) during which snails were placed in water to recover. Based
on egg production inhibition, the 40-day EC50 was 250 µg/litre. The
percentage of fertilized eggs per capsule was not affected, and no
morphological abnormalities were noticed during embryonic development.
Based on the number of eggs that did not hatch, an EC50 of
230 µg/litre was determined. Considering a combination of the
inhibition of egg production and the mortality of the young during
their development, a 50% reduction of the overall reproductivity was
found at 65 µg alpha-HCH/litre. These effects did not disappear during
the recovery period of 16 days (Canton & Slooff, 1977) (see Table 4).
Table 4. Long-term toxicity of alpha-hexachlorocyclohexane for invertebrates
Species Age Temperature Duration Criteria Concentration References
(°C) (days) (mg/litre)
Daphnia magna 19 21 no mortality; 0.27a Janssen et al.
no effects on behaviour, 0.09 (1987)
appearance or growth;
no influence on reproduction 0.27
(4 groups of offspring)
Lymnaea stagnalis adults 22 40 EC50 (egg production inhibition) 0.25 Canton & Slooff
eggs and 22 40 hatching, overall productivity 0.065 (1977)
adults
a water renewal system
9.4 Fish
9.4.1 Acute toxicity
LC50 and EC50 (mortality and immobilization) values for fish
are summarized in Table 5.
9.4.2 Short- and long-term toxicity
During a 3-month study, rainbow trout (Salmo gairdneri)
(200-250 g) were fed pellets containing 0, 10, 50, 250, or 1250 mg
alpha-HCH (purity > 95%) per kg diet. After 2, 4, 8, and 12 weeks,
the fish were examined. Growth, microsomal liver enzymes (aniline
hydroxylase and aminopyrine demethylase), brain cholinesterase, serum
alkaline phosphatase, and the histopathology of the brain, liver, and
kidneys were all investigated but no effects were found (Canton et
al., 1975).
When guppies (Poecilia reticulata) aged 3-4 weeks were exposed
to 0, 200, 800, or 2000 µg alpha-HCH (> 95%) per litre in a 50-day
study, the EC50, based on mortality and immobilization, was 800
(600-1200) µg/litre (Canton et al., 1975).
In a study by Janssen et al. (1987), fertilized eggs of Oryzia
latipes were exposed for 35 days (up to 28 days after hatching) to
alpha-HCH. No influence on growth, mortality or behaviour was seen at
800 µg/litre.
9.5 Terrestrial organisms
No data on terrestrial organisms are available.
Table 5. Acute toxicity (48 h) of alpha-hexachlorocyclohexane for fish (growth inhibition/mortality or immobilization)
Species Age Temperature Parameter Concentration References
(°C) (mg/litre)
Freshwater
Poecilia reticulata 3-4 weeks 24 EC50a 0.8 (0.6-1.2) Canton et al. (1975)
Salmo gairdneri 4 weeks 12 EC50a 1.05 (0.9-1.2) Canton et al. (1975)
Saltwatera
Poecilia reticulata 3 weeks 24 EC50b 1.38 (1.35-1.42) Canton et al. (1978)
Poecilia reticulata LC50 3.5 Boulekbache (1980
a closed system
b water renewal system
PART B
ENVIRONMENTAL HEALTH CRITERIA FOR BETA-HEXACHLOROCYCLOHEXANE
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR BETA-HEXACHLOROCYCLOHEXANE
1. SUMMARY AND EVALUATION
1.1. General properties
1.2. Environmental transport, distribution, and
transformation
1.3. Environmental levels and human exposure
1.4. Kinetics and metabolism
1.5. Effects on organisms in the environment
1.6. Effects on experimental animals and
in vitro test systems
1.7. Effects on humans
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity of primary constituent
2.2. Physical and chemical properties
2.3. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and distribution between media
4.2. Biotransformation and bioaccumulation
4.2.1. Biodegradation
4.2.2. Abiotic degradation
4.2.3. Bioaccumulation
4.2.3.1 Aquatic invertebrates
4.2.3.2 Fish
4.2.3.3 Birds
4.2.3.4 Bioaccumulation in humans
4.3. Isomerization
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
5.1.2. Water
5.1.2.1 Fresh water
5.1.2.2 Sea water
5.1.3. Soil/sediment
5.1.3.1 Dumping grounds
5.1.4. Food and feed
5.1.5. Terrestrial and aquatic organisms
5.1.5.1 Aquatic organism
5.1.5.2 Birds
5.1.5.3 Mammals
5.2. General population exposure
5.2.1. Total-diet studies
5.2.2. Concentrations in human samples
5.2.2.1 Blood
5.2.2.2 Adipose tissue
5.2.2.3 Breast milk
6. KINETICS AND METABOLISM
6.1. Absorption and elimination
6.2. Distribution
6.3. Transplacental transfer and transfer via lactation
6.4. Metabolic transformation
6.4.1. Rat
6.4.2. Mouse
6.4.3. Human
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1. Acute toxicity data
7.1.1. Oral
7.1.2. Intraperitoneal
7.2. Short-term exposure
7.2.1. Mouse oral studies
7.2.2. Rat oral studies
7.3. Skin and eye irritation; sensitization
7.4. Long-term exposure
7.4.1. Rat oral studies
7.5. Reproduction, embryotoxicity, and
teratogenicity
7.5.1. Reproduction
7.5.2. Teratogenicity
7.6. Mutagenicity and related end-points
7.7. Carcinogenicity
7.7.1. Mouse
7.7.2. Rat
7.7.3. Initiation-promotion
7.7.4. Mode of action
7.8. Special studies
7.8.1. Effects on endocrine organs
7.8.2. Neurotoxicity
7.8.3. Effect on liver enzymes
7.8.4. Immunosuppression
8. EFFECTS ON HUMANS
8.1. Acute toxicity - poisoning incidents
8.2. General population
8.3. Occupational exposure
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1. Algae
9.2. Protozoa
9.3. Invertebrates
9.4. Fish
9.4.1. Acute toxicity
9.4.2. Longer-term toxicity
9.5. Terrestrial organisms
9.5.1. Birds
9.6. Model ecosystem studies
1. SUMMARY AND EVALUATION
1.1 General properties
Beta-hexachlorocyclohexane (beta-HCH) is a by-product (7-10%) in
the manufacture of lindane (> 99% gamma-HCH). Its solubility in water
is low, but it is very soluble in organic solvents such as acetone,
cyclohexane, and xylene. It is a solid with a low vapour pressure.
The n-octanol/water partition coefficient (log Pow) is 3.80. It
is an environmental pollutant.
Beta-HCH can be determined separately from the other isomers by
gas chromatography with electron capture detection and other methods
after extraction by liquid/liquid partition and purification by column
chromatography.
1.2 Environmental transport, distribution, and transformation
Biodegradation and abiotic degradation (dechlorination) by
ultraviolet irradiation occur in the environment and produce
pentachlorocyclohexane, but at a much slower rate than in the case of
lindane (gamma-HCH).
Beta-HCH is the most persistent HCH isomer. Its persistence in
soil is determined by environmental factors such as the action of
microorganisms, content of organic matter and water, and
co-distillation and evaporation from soil.
Owing to the persistence of beta-HCH, rapid bioconcentration takes
place in invertebrates (the bioconcentration factor is approximately
125 within 3 days), fish (250-1500 on a dry weight basis or
approximately 500 000 times on a lipid basis within 3-10 days), birds
and man (approximately 525). The bioconcentration is higher and the
elimination is slower for beta-HCH than for the other HCH isomers.
1.3 Environmental levels and human exposure
Beta-HCH is found in air over the oceans at a concentration of
0.004-0.13 ng/m3.
Until 1974, the River Rhine and its tributaries contained beta-HCH
levels of 0.14-0.22 µg/litre, but thereafter the levels were
consistently below 0.1 µg per litre. Samples from the River Meuse
also contained < 0.1 µg/litre. In the River Elbe, levels decreased
from an average of 0.009 to 0.004 µg/litre between 1981 and 1988.
Beta-HCH has been measured in birds such as sparrowhawks,
kestrels, owls, herons, and grebe over a number of years and the
concentrations ranged from 0.1 to 0.3 mg/kg. Up to 0.87 mg/kg (on a
fat basis) has been found in the liver and adipose tissue of the polar
bear.
Important food items have been analysed in a few countries for the
presence of beta-HCH. The mean concentrations, mainly in
fat-containing food products, ranged up to 0.03 mg/kg (on a fat
basis), but in milk products levels up to 4 mg/kg (on a fat basis)
were found. In non-fatty food items, the levels were < 0.005 mg/kg
product. In general, levels are slowly decreasing.
Food is the main source for general population exposure to
beta-HCH. In total-diet studies in the United Kingdom, 0.003, 0.0005,
and < 0.0005 mg/kg were found for the years 1966-1967, 1975-1977, and
1981, respectively. In the USA, the average daily intake of beta-HCH
in 1982-1984 ranged from < 0.1-0.4 ng/kg body weight for various age
groups.
In a number of countries, the concentration of beta-HCH has been
determined in the blood, serum, or plasma of the general population.
The concentrations varied between the different countries and ranged
up to 25 µg/litre.
Many studies have been carried out to determine the presence of
beta-HCH in human adipose tissues. The concentrations found in Canada,
Germany, Kenya, the Netherlands, and the United Kingdom ranged up to
4.4 mg/kg (on a fat basis). A gradual increase with age was found up
to approximately 50 years; thereafter levels decreased. Beta-HCH
concentrations in adipose tissues are higher than those of the other
HCH isomers, a phenomenon that reflects the accumulative properties of
beta-HCH. There is, in general, no clear trend for a decrease in
beta-HCH concentrations over the period that studies have been made.
There is a relationship between the concentrations in adipose tissue
and breast milk and the consumption of meat products, animal fat, and
fatty fish.
In a few countries (Canada, Germany, the Netherlands, and the
United Kingdom), breast milk has been analysed and beta-HCH levels of
between 0.1 and 0.69 mg/kg (on a fat basis) have been found. The
levels in the milk of women living in rural areas appears to be higher
than in urban areas.
The high beta-HCH levels that have been found in breast milk
exceed permissible concentrations temporarily and locally. The
beta-HCH concentrations in the blood of babies lie within the same
range as those in the mothers.
Beta-HCH appears to be a universal environmental contaminant.
Concentrations are only decreasing very slowly in spite of measures
taken to prevent its spread into the environment.
1.4 Kinetics and metabolism
Up to 95% of beta-HCH in the mouse gastrointestinal tract is
absorbed, most of it being subsequently accumulated in adipose tissue.
The elimination follows a 2-stage mechanism, the half-life for the
first stage being 2.5 days and for the second stage 18 days.
After absorption, beta-HCH is rapidly distributed to the liver,
brain, kidneys, and adipose tissues. The maximum concentration in the
liver is reached in rats after 4 days. At an average blood
concentration of 92 µg/litre (but also with concentrations of 540 and
2100 µg per litre), the brain to blood and adipose tissue to blood
ratios were 2:1 and 170:1, respectively. After lethal acute human
poisoning with HCH isomers, the beta-HCH concentration, relative to
that of blood, was 363 in fat, 3 in the brain, and 15 in the liver.
Beta-HCH passes the blood-brain barrier much less readily than the
other HCH isomers.
Transplacental transfer from pregnant mice to their fetuses was
about 2% of the dose, but in rats a transfer of 40% was found.
Lactational transfer in rats from dams to sucklings via milk was about
60% of the dose.
In rats 70% of beta-HCH is eliminated during 28 days, one third of
this being excreted in the urine. No unchanged beta-HCH is present in
the urine. The major metabolite resulting from cis-dehydrochlorination
is 2,4,6-trichlorophenol in a conjugated form.
Pretreatment with beta-HCH alters the metabolism of lindane in
rats. From intraperitoneal studies with mice, it seems that beta-HCH
is metabolized more slowly than lindane.
1.5 Effects on organisms in the environment
Beta-HCH generally has moderate toxicity for algae, invertebrates,
and fish. The acute LD50 values for these organisms are of
the order of 1 mg/litre, but the EC50 values are lower
(0.05-0.5 mg/litre). The no-observed-effect level for Oryzia latipes
and Poecilia reticulata, two freshwater fish exposed for 1 or 3
months, was 0.03 mg/litre.
No data are available on effects on populations and ecosystems.
1.6 Effects on experimental animals and in vitro test systems
The acute oral LD50 values for mice and rats were reported in
1968 to lie between 1500 and 2000 mg/kg body weight. However, more
recent studies yielded values of 16 g/kg body weight for mice and
8 g/kg body weight for rats. Signs of intoxication were mainly of
neurological origin.
Two short-term mouse studies, with dose levels of up to 600 mg/kg
diet for 26-32 weeks, showed increased liver weight and nodular
hyperplasia and atypical proliferations in the liver. In a third
study, dose levels of up to 500 mg/kg diet for 24 weeks did not result
in liver tumours or nodular hyperplasia.
A 90-day study with rats fed 50 or 250 mg/kg diet revealed liver
changes, i.e. hypertrophy and proliferation of smooth endoplasmic
reticulum and increased activity of microsomal enzymes. Changes in
the gonads occurred at the highest dose levels but these were
associated with severe effects on body weight. Hormonal changes
associated with the gonadal atrophy showed no consistent endocrine
effect. There were no adverse effects at a dose level of 2 mg/kg diet
(equivalent to 0.1 mg/kg body weight).
In a long-term rat study (reported in 1950), doses of 10 mg/kg
diet (equivalent to 0.5 mg/kg body weight) or more led to liver
enlargement and histological changes.
In a two-generation reproduction study on rats, the same effects
were found as in the 90-day study. There were no effects at 2 mg/kg
diet (equivalent to 0.1 mg/kg body weight), but a dose level of
10 mg/kg diet resulted in increased mortality and infertility. No
compound-related teratogenic effects were found in an extension to
this study.
A weak "estrogenic" effect has been described. The uterus was the
target organ for this effect; there were no clear effects on endocrine
control systems. The mechanism and significance of this effect are
uncertain.
The mutagenicity studies reported did not show any increase in
mutation frequency in Salmonella typhimurium strains. An in vivo
bone marrow metaphase analysis in rats yielded positive results.
Two studies have been carried out on mice to determine
carcinogenic potential. In one study, 200 mg/kg diet was given for
110 weeks, and liver enlargement, hyperplastic changes, and an
increase in benign and malignant tumours were reported. In the other
study, where 500 mg/kg diet was administered for 24 weeks, no tumours
were observed.
Studies in which rats were fed combinations of beta-HCH with
polychlorinated biphenyls suggested a promoting effect of beta-HCH.
At 300 mg/kg diet, beta-HCH caused significant changes in several
immune functions in mice within one month.
1.7 Effects on humans
When workers at a lindane-producing factory, with a geometric mean
exposure of 7.2 years (1-30), were investigated, it was concluded that
occupational HCH exposure did not induce signs of neurological
impairment or perturbation of "neuromuscular function".
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity of primary constituent
Common name Beta-hexachlorocyclohexane (beta-HCH)
Chemical formula C6H6Cl6
Chemical Beta-HCH is a stereoisomer of gamma-HCH,
structure the active ingredient of lindane (> 99%
(see Annex I) gamma-HCH). It differs in the spatial
orientation of the hydrogen and chlorine
atoms on the carbon atoms:
The direct uptake of alpha-HCH from contaminated water appeared to be
much greater than the uptake from contaminated food.
In a study with Salmo gairdneri, pellets containing alpha-HCH
(> 95%) levels of 0, 10, 50, 250, or 1250 mg/kg were fed to the fish,
and organs and tissues were analysed after 2, 4, 8, and 12 weeks.
There was a dose-related increase in the concentration of alpha-HCH
in the organs and tissues. After about 4-8 weeks (depending on the
type of tissue and dose level) a maximum concentration was reached,
which then slowly decreased. This suggests that after a few weeks a
balance is reached between the accumulation process (absorption of
alpha-HCH by the intestinal wall) and the elimination process (via the
gills and faeces). There is probably also a dilution effect resulting
from growth and biotransformation (Canton et al., 1975).
Ernst (1977) concluded from kinetic studies that biomagnification
of alpha-HCH does not occur. Compared with bioaccumulation from water
alone, the entry of alpha-HCH into the food chain Chlorella ->
Daphnia -> Poecilia (guppy) caused only a slight increase in
biomagnification in daphnids (factor 1.5), although in the case of the
guppies a greater increase in concentration ratio (3-4) was noted.
In a study by Canton et al. (1978), guppies (3-4 weeks old) were
exposed to alpha-HCH (> 95%) concentrations of 0.01, 0.05, or
0.14 mg/litre. When after 0.5, 3, 24, 48, 72, 96, and 120 h the
animals were analysed, the average concentration factor was about 500
for all alpha-HCH concentrations (about 17 000 on a lipid basis).
Equilibrium was reached within 24 h for the lower concentrations and
within 48 h at the highest concentrations. The elimination was rapid,
the initial concentration being halved in 10 h.
Sugiura et al. (1979) studied bioaccumulation in the carp
(Cyprinus carpio), brown trout (Salmo trutta fario), golden orfe
(Leuciscus idus melanotus), and guppy (Poecilia reticulata).
Alpha-HCH was dissolved in water to a concentration of 1 mg/litre
under steady-state conditions (time period not specified), and the
equilibrium bioconcentration factors for the four types of fish were
330, 605, 1216, and 588, respectively.
Based on the data given in section 5.1.5.2 concerning the
concentration of alpha-HCH in the muscle and fat of bream collected in
the River Elbe, the bioconcentration factor is between 10 000 and
50 000 (Arbeitsgemeinschaft für die Reinhaltung der Elbe, 1982).
In a study by Yamato et al. (1983), guppies (Poecilia
reticulata) rapidly bioaccumulated HCH isomers and the tissue
concentration reached a plateau on the fourth day (the alpha-HCH
concentration in the water was 1 µg per litre). The bioconcentration
factor (concentration in fish/concentration in water) was 706. The
concentration in the guppies decreased on the first day after the fish
were transferred to HCH-free water.
4.2.3.4 Bioconcentration in humans
Geyer et al. (1986) found that in industrialized countries more
than 90% of the exposure to HCHs derives from food. The mean
concentration of alpha-HCH in human adipose tissue (on a fat basis)
was found to be 0.03 mg/kg in the Federal Republic of Germany and
0.02 mg/kg in the Netherlands. The mean bioconcentration factor (on a
lipid basis), calculated on the basis of the concentration in the diet
(1.3 and 0.3 µg/kg, respectively) and levels in adipose tissue, was
20.0 ± 8 (range 11.5-32.5).
4.3 Isomerization
Deo et al. (1981) studied the isomerization of alpha-HCH in
sterile aqueous solution over a period of 4 weeks and found a slow
conversion of alpha-HCH to other HCH isomers.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Tanabe et al. (1982) found alpha-HCH in 24 samples of air over
the Western Pacific, Eastern Indian, and Antarctic Oceans at an
average concentration of 0.29 ng/m3 (0.022-1.4 ng/m3).
In a study by Strachan et al. (1980), samples of atmospheric
precipitation in the form of snow (1976; 17 samples) and rain (1976
and 1977; 81 samples) collected around the Canadian side of the Great
Lakes, as well as inland, were analysed. Alpha-HCH was found in the
snow samples as a trace (1 ng/litre) and in the rain samples at levels
of 1-40 ng/litre.
Air samples were taken near a road with heavy traffic, as well as
in a suburban residential area, near Ulm, in Germany. The alpha-HCH
levels were 0.22-1.3 ng/m3 in the location with heavy traffic and
0.11-1.1 ng/m3 in the rural area. It was concluded that the
concentrations in the lower troposphere under various meteorological
conditions reflect regional input and long-range transport (Wittlinger
& Ballschmiter, 1987).
In 1972, alpha-HCH air concentrations of 0.28 ng per m3 in
non-polluted areas of Germany, and 2.15 ng/m3 in the polluted Ruhr
area were determined (Hildebrandt et al., 1986).
The average concentration of alpha-HCH in 55 air samples
collected in Delft, the Netherlands, in 1979-1980 was 0.25 ng/m3
(maximum concentration: 1.2 ng/m3) (Slooff & Matthijsen, 1988).
5.1.2 Water
5.1.2.1 Rain water
Rain water sampled in 1983 in Bilt, the Netherlands, contained an
average alpha-HCH concentration of 0.01 (< 0.01-0.02) µg/litre
(Slooff & Matthijsen, 1988).
5.1.2.2 Fresh water
During the period 1969-1977, 1826 water samples were taken at 99
sampling sites in the Netherlands. The highest concentrations of
alpha-HCH were found in the River Rhine and its tributaries. The
concentrations varied between 0.01-0.3 µg/litre during the period
1969-1974, but in 1974 there was a sudden decrease and the subsequent
concentrations were all below 0.1 µg/litre. A sampling trip by boat
made along the River Rhine from Rheinfelden in Switzerland to
Rotterdam in the Netherlands proved that the source of alpha-, beta-,
and gamma-HCH was located in the upper Rhine. In the River Meuse, the
levels were all below 0.1 µg/litre during the period 1969-1977 (Wegman
& Greve, 1980).
Since 1969, alpha-, beta-, and gamma-HCH concentrations have been
measured regularly in the Rivers Rhine, Meuse, and West-Scheldt and in
other surface waters in the Netherlands. Alpha-HCH levels have been
below 0.05 µg per litre in the River Rhine since 1974/1975, and were
of the order of 0.02 µg/litre or less in the West-Scheldt during the
period 1973-1985. In the River Meuse, the concentration of alpha-HCH
was between 0.01-0.02 µg/litre. In other areas, for instance
agricultural and greenhouse horticulture areas, the levels of the
individual HCHs ranged from 0.01-1.0 µg/litre with incidental higher
peaks (up to 0.5 µg/litre) probably resulting from the use of lindane
(Slooff & Matthijsen, 1988).
Concentrations of HCH isomers in solution and in suspension
(particle-bound) in the Meuse and Rhine estuary were determined in
1974. The average concentrations of dissolved and suspended alpha-HCH
were 20 and 0-6 ng per litre, respectively. In 1981, the
concentration of dissolved alpha-HCH in coastal waters of the
Netherlands was 0.9-1.6 ng/litre, whereas that of suspended alpha-HCH
(only one measurement) was 5.3 ng/litre (Slooff & Matthijsen, 1988).
In 1970-1971, the levels of alpha-HCH were 0.66-1.5 µg/litre in
the surface water of the River Elbe near Hamburg, Germany, and
0.155-2.4 µg/litre in the River Rhine near Karlsruhe. However, a
significant decrease was observed in the mid-1970s. In 1974, 2.7 µg
per litre was found in the upper Rhine, but by 1976-1977 the levels
had decreased to 1-9 ng/litre (Hildebrandt et al., 1986).
The Arbeitsgemeinschaft der Elbe (the Elbe Study Group)
investigated the presence of alpha-HCH in the River Elbe from
Schnackenburg to the North Sea in 1981-1982 and found a mean
concentration of 0.023 (< 0.001-0.15) µg per litre. During the period
February to November 1988, the alpha-HCH concentration was
0.001-0.022 µg/litre (Arbeitsgemeinschaft der Elbe, 1988).
When certain rivers in Yorkshire, England, were analysed for
alpha-HCH in 1966, the concentration varied from 0.001 to
0.43 µg/litre. In 1968, the highest value was 0.543 µg/litre, and
the water from six other rivers contained an average of
0.001-0.004 µg/litre (highest level: 0.34 µg/litre) (Lowden et al.,
1969).
In Japan, 60 water samples were examined in 1974 and 0.1 µg
alpha-HCH/litre was detected in three of the samples (personal
communications by A. Hamada and by T. Onishi to the IPCS, July 1989).
5.1.2.3 Sea water
Atlas & Gias (1981); Bidleman & Leonard (1982); Oehme & Stray
(1982); and Oehme & Mano (1984) analysed sea water from areas such as
the North Pacific, Arabic Sea, Persian Gulf, Red Sea, Lillestrum, Bear
Island, and Spitsbergen. The alpha-HCH concentrations varied from
0.03 to 1.8 ngper litre (Slooff & Matthijsen, 1988).
In June-July 1986, the alpha-HCH in the surface water (5 m) of
the North Sea ranged from 1-2 ng/litre (Umweltbundesamt, 1989).
5.1.3 Soil/Sediment
Herrmann et al. (1984) studied the presence of alpha-HCH in
sediment along the Husum estuary and in the adjacent North Frisian
Wadden Sea. The mean concentrations varied in the different sampling
stations from 0.33 to 1.40 µg/kg sediment, while the concentrations in
bladder wrack(Fucus vesiculosus)varied from 0.7-1.2 µg/kg.
Edelman (1984) analysed 96 samples of the upper 10 cm of the soil
from 38 natural reserves in the Netherlands for alpha-HCH and
gamma-HCH. In 94 of the samples alpha-HCH was detected at levels
below 1 µg/kg (Slooff & Matthijsen, 1988).
When sediment from eight different rivers, harbours, and sites
close to dumping areas in the Netherlands were analysed for the
presence of alpha-, beta-, and gamma-HCH, the median alpha-HCH levels
were between 4 and 213 µg per kg dry matter (Slooff & Matthijsen,
1988).
In 1974, 60 sediment samples were analysed in Japan and 10 µg
alpha-HCH/kg was detected in five of the samples (personal
communications by A. Hamada and by T. Onishi to the IPCS, July 1989).
5.1.3.1 Dumping grounds
In the Netherlands, soil has been polluted with HCHs at various
locations as a result of their manufacture during the 1950s (spillage
during production, storage, and handling), and concentrations up to a
few grams of HCHs/kg dry soil have been found. Further pollution has
been caused by the dumping of chemical waste and its use in the
levelling of certain areas. From these dumping areas dispersal of the
chemical waste can occur by leaching or wind erosion from open storage
depots. In certain polluted areas, high concentrations of HCHs, mainly
alpha- and beta-HCH, have been found more than 2 m below ground level.
In 18 locations in the Netherlands, the average concentration of
alpha-HCH in sewage sludge in 1981 was between 5 and 70 µg/kg dry
matter. Pollution of ground water was also detected, but this was
restricted to the vicinity of the production areas. Horizontal
transportation of HCHs in ground water appeared to be limited (Slooff
& Matthijsen, 1988).
5.1.4 Food and feed
The presence of alpha-HCH in a number of important food items has
been determined in France by Laugel (1981). In milk and milk products
(2688 samples) the average level was 0.05 mg/kg (ranging from
undetectable to 0.22 mg/kg), in meat (37 samples) it was 0.01 mg/kg
(ranging from undetectable to 0.02 mg/kg), and in animal fat (67
samples) it was 0.02 mg/kg product (ranging from undetectable to
0.06 mg/kg. In other food items alpha-HCH was not detectable
(< 0.005 mg/kg).
Table 2 gives the mean alpha-HCH levels in a large number of
samples of various food items from the Federal Republic of Germany
reported by Hildebrandt et al. (1986).
Table 2. Alpha-hexachlorocyclohexane concentrations (mg/kg)
in various food itemsa
Food items 1973-78 1979-83 1973-83
Meatb 0.003-0.02
Meat productsb 0.007-0.037
(0.26)e
Animal fatb 0.003-0.008
(0.09)e
Gameb 0.019-0.367
Poultryb 0.003-0.004 0.003-0.016
(0.17)e
Chicken eggs < 0.001-0.003
Fish 0.002-0.011
Milk and milk
productsb 0.015e 0.01-0.03
Cow's milkb,c 0.004
Butterb,d 0.02-0.03
Table 2 (contd)
Food items 1973-78 1979-83 1973-83
Vegetable oil and
margarineb 0.01
Oil seeds, nuts,
pulses 0.001-0.042
Fruit, vegetables, < 0.0001
potatoes
Cereals 0.0002-0.007
Cereal products up to 0.14
a From: Hildebrandt et al. (1986).
b Determinations made on a fat basis
c WHO (1986).
d Anon (1984).
e Maximum value
In six samples of cows milk collected from six locations in
Switzerland, the levels of alpha-HCH were 9.5-27 mg/kg on a fat basis
(Rappe et al., 1987).
Skaftason & Johannesson (1979) analysed 35 samples of butter from
Iceland during 1968-1970 and found a level of mean alpha-HCH of 87 ±
38 µg/kg. In 1974-1978, 32 samples were studied and all contained
alpha-HCH, the mean concentration being 58 ± 21 µg/kg.
In a total-diet study in the United Kingdom, 24 samples of each
food group were analysed for alpha-HCH. The following concentrations
(mean and range) were found: bread, not detected (nd); other cereal
products, < 0.0005 (nd-0.002); carcass meat, < 0.0005 (nd-0.006);
offal, < 0.0005 (nd-0.007); meat products, eggs, green vegetables,
potatoes, fresh fruit, nd; poultry, 0.003 (nd-0.025); fish, 0.0005
(nd-0.008); oil and fats, 0.0005 (nd-0.003); milk, 0.0005 (nd-0.002);
dairy products, 0.006 (nd-0.02) mg/kg product. Imported meat products
were also analysed during the period 1981-1983, and concentrations of
up to 0.5 mg/kg were measured. Imported retail cereal products
collected in 1982 contained alpha-HCH levels of up to 0.03 mg/kg and
animal feed stuffs collected in 1984 had levels of up to 0.02 mg/kg
(HMSO, 1986).
Various types of pulses were analysed during the period
1986-1987, and 31 out of 142 samples contained alpha-HCH residues at
levels of up to 0.03 mg/kg. Processed pork and poultry, sampled
during the period 1985-1987, contained alpha-HCH at levels of up to
3.2 (mean 0.2) and 0.1-2.0 (mean 0.8) mg/kg product, respectively (26
out of the 86 samples were positive). Of other processed meat
products, 631 samples were negative. Retail milk and dairy products
were analysed during the period 1984-1987, and 499 of the 849
samples contained alpha-HCH residues at a mean concentration of
0.01-0.03 mg/kg (highest level, 0.06 mg/kg). Samples of eel muscle
(1124 eels from 62 sites) were analysed during the period 1986-1987,
and mean concentrations were 0.001-0.03 mg/kg (highest level,
0.4 mg/kg). Peanut butter and vegetable oils were analysed during the
period 1985-1987, and 95 samples showed mean concentrations of <
0.01-0.03 mg/kg product (16 of the samples were positive) (HMSO,
1989).
The mean residue level of alpha-HCH in milk samples collected
during spring 1983 from 359 bulk transporters representing 16
counties, municipalities, and districts of Ontario was 5.3 µg/kg
butter fat. Alpha-HCH was found in over 90% of the samples (Frank et
al., 1985).
5.1.5 Terrestrial and aquatic organisms
5.1.5.1 Plants
Samples of three types of mosses and four types of lichens (in
total 13 samples) were collected in the Antarctic Peninsula (Graham
Land) in 1985, and alpha-HCH was detected in most of them at a mean
concentration of 0.4 (0.20-1.15) µg/kg (Bacci et al., 1986).
In a study by Gaggi et al. (1986), fallen leaves (at the end of
their natural life-cycle) and lichens were collected in 1984 at sites
near Florence and Siena, Italy, in a woodland hilly area away from
primary pollution sources. The leaves were from ten different species
of trees and two different lichen species were involved. The average
levels of alpha-HCH in leaves and lichen were 37 (16-61) µg/kg dry
weight and 27 (25-29) µg/kg dry weight, respectively. The same
authors reported that the levels of alpha-HCH in various plant species
collected in 14 countries were 0.5-2140 µg/kg dry weight.
5.1.5.2 Fish and mussels
Martin & Hartman (1985) analysed 60 fish samples from nine
locations in the north-central part of the USA and found
concentrations of 5-27 µg alpha-HCH/kg (wet weight) in 36% of the
samples. The frequency with which alpha-HCH was detected in fish from
the different rivers varied between 17 and 100%.
In a study by Saiki & Schmitt (1986), samples of three to five
adult bluegills (Lepomis macrochirus) and common carp (Cyprinus
carpio) were collected at eight sites in three rivers in California,
USA, in 1981. Alpha-HCH concentrations in carp of up to 0.036 mg/kg on
fat basis were reported, but the concentrations in bluegill were
lower.
Cowan (1981) studied the extent of pollution of Scottish coastal
waters by HCHs using Mytilus edulis as biological indicator. The
levels of alpha-HCH at the 118 sites sampled ranged from < 6 to
23 µg/kg dry weight.
The fish and shellfish sampling programme carried out by the
United Kingdom Ministry of Agriculture, Fisheries, and Food between
1977-1984 was directed mainly to areas around the coasts of England
and Wales. The levels of alpha-HCH, which varied between the
different fish and shellfish species and also between the collection
sites, ranged from < 0.001 (nd) to 0.06 mg/kg wet weight. The
concentration in fish muscle was < 0.001 mg/kg wet weight (Franklin,
1987).
The mean alpha-HCH concentration in the muscle of flounders
collected off the North Sea coast of Germany in 1986 was 2.5 µg/kg
(nd-5.0 µg/kg) (Umweltbundesamt, 1989). Bream collected from different
locations in the River Elbe (between Schnackenburg and the North Sea)
contained 0.007-0.066 mg alpha-HCH/kg in muscle tissue and
0.9-2.2 mg/kg in adipose tissue (Arbeitsgemeinschaft für die
Reinhaltung der Elbe, 1982), while the same species collected from 15
rivers and lakes in the Federal Republic of Germany contained (on a
fat basis) up to 468 µg per kg (Umweltbundesamt, 1989).
Freshwater fish from different rivers in the Federal Republic of
Germany were analysed during the period 1973-1981, and in the first
3-4 years the alpha-HCH levels were mainly between 0.01 and 0.02 mg/kg
fresh weight. However, a clear decrease then took place and most of
the samples were below 0.01 mg/kg fresh weight, with the exception of
certain types of fish such as the eel and fish from industrially
contaminated areas (Hildebrandt et al., 1986).
In 1981-1983, shellfish and molluscs collected in the Federal
Republic of Germany contained < 0.001-0.20 mg alpha-HCH/kg fresh
weight. Eels collected in the North Sea and Baltic Sea contained
alpha-HCH levels of 0.011 mg per kg and 0.033 mg/kg fresh weight,
respectively. Flounders and herrings caught in the North Sea contained
0.002 and 0.008 mg/kg fresh weight, respectively, but in the Baltic
Sea the levels were about twice as high (Hildebrandt et al., 1986).
5.1.5.3 Birds
An average alpha-HCH residue level of 0.05 mg/kg was found in 17
adult herons in 1964 (HMSO, 1969).
In a study by Sierra & Santiago (1987), alpha-HCH concentrations
were determined in 23 barn owls (Tyto alba Scop.) from Leon, Spain.
The mean levels (and range) in muscle, liver, fat, brain, and
kidneys (in total 91 samples) were 0.242 (0.019-0.591), 0.323
(0.009-0.830), 1.073 (0.691-1.499), 0.238 (0.007-0.676), and 0.710
(0.051-2.381) mg/kg (wet weight), respectively.
Faladysz & Szefer (1982) analysed adipose fat from seven species
of diving ducks at their winter quarters in the Southern Baltic.
Residues of alpha-HCH were found in all of the 37 specimens of
long-tailed duck at mean concentrations (on a fat basis) of 3.4
(0.17-18) and 1.5 (0.23-6) mg/kg for female and male ducks,
respectively.
5.1.5.4 Mammals
Skaftason & Johannesson (1979) analysed 24 samples of the fat of
reindeer living in an area of the eastern and south-eastern parts of
Iceland where the use of pesticides is negligible. Alpha-HCH was
found in all samples at a mean level of 70 ± 22 µg/kg. These results
are in agreement with those of Benson et al. (1973), who found an
average of 77.5 µg/kg in the subcutaneous fat of wild Idaho moose
living in a forest area where pesticides were used very restrictively.
Skaftason & Johannesson (1979) analysed samples of body fat from
10-year-old sheep in 1974 and found an average of 51 ± 12 µg/kg.
Norström et al. (1988) investigated the contamination by
organochlorine compounds of Canadian arctic and subarctic marine
ecosystems by analysing the adipose tissue and liver of polar bears
( Ursus maritimus; 6-20 animals per area) collected from 12 areas
between 1982 and 1984. There was a difference in tissue distribution;
liver contained only alpha-HCH, but 29% of the HCH in adipose tissue
was beta-HCH. Adipose tissue contained 0.3-0.87 mg alpha-HCH per kg on
a fat basis.
The mean concentrations of alpha-HCH in the kidney fat of roe
(86 samples) collected in five areas of Germany in 1985-1986 were
about 7-12 µg/kg fat, the maximum value being about 50 µg/kg fat
(Umweltbundesamt, 1989).
5.2 General population exposure
From the data presented in section 5.1 it is evident that food is
the main source of exposure of the general population to alpha-HCH.
5.2.1 Total-diet studies
In a total-diet study carried out in the United Kingdom during
1966-1985, food purchased in 21 towns throughout the country was
prepared by cooking. The study covered 20 to 24 food groups, and the
number of total-diet samples examined varied from 22 to 25 samples.
The calculated mean alpha-HCH residue levels in the total diet for the
periods 1966-1967, 1970-1971, 1974-1975, 1975-1977, 1979-1980, 1981,
and 1984-1985 were 0.003, 0.002, 0.002, 0.0015, 0.001, < 0.0005, and
< 0.0005 mg/kg, respectively (Egan & Hubbard, 1975; HMSO, 1982, 1986,
1989).
Gartrell et al. (1985a) conducted a study to determine the
dietary intake of pesticides in the USA in 1978-1979. The samples,
purchased from retail outlets, were representative of the diets of
adults in 20 cities, and consisted of about 120 individual food items.
The daily intake of alpha-HCH in 1977, 1978, and 1979 was 0.011,
0.009, and 0.010 µg/kg body weight, respectively. In a similar way,
samples were collected in 10 cities in 1978-1979 consisting of about
50 items of infant food and 110 items of toddler food. The daily
intake of alpha-HCH in 1977, 1978, and 1979 was, respectively, 0.031,
0.034, 0.033 µg/kg for infants and 0.025, 0.029, and 0.029 µg/kg body
weight for toddlers, respectively (Gartrell et al., 1985b).
Total-diet studies conducted in the USA by the FDA before 1982
were based on a "composite sample approach" regardless of the diet
involved. Later on they were based on dietary survey information and
allowed the "total diet" of the population to be represented by a
relatively small number of food items for a greater number of age-sex
groups. The daily intakes of alpha-HCH during 1982-1984 for the age
groups 6-11 months, 2 years, 14-16-year-old females, 14-16-year-old
males, 25-30-year-old females, 25-30-year-old males, 60-65-year-old
females, and 60-65-year-old males were 7.2, 16.1, 6.1, 7.3, 4.5, 5.9,
3.3, and 3.7 ng/kg body weight, respectively (Gunderson, 1988).
In a total-diet study in the Netherlands in 1977, the average
concentration of alpha-HCH in 100 samples was 0.01 mg/kg on a fat
basis. The highest level was 0.05 mg/kg (Greve & van Hulst, 1977).
5.2.2 Air
Guicherit & Schulting (1985) measured the atmospheric
concentration of alpha-HCH in the Netherlands and calculated an
average daily intake by inhalation for a 70-kg person of 5 ng. The
equivalent value for the Federal Republic of Germany was calculated to
be 32 ng, which is about 1% of the total daily intake via the various
routes (Hildebrandt et al., 1986).
5.2.3 Concentrations in human samples
Alpha-HCH concentrations in human samples are a good indication
of the total exposure of the general population.
5.2.3.1 Blood
Blood samples of Dutch citizens analysed in 1978, 1980, 1981, and
1982 (70, 48, 127, and 54 samples, respectively), contained less than
0.1 µg alpha-HCH/litre (Greve & Wegman, 1985). Blok et al. (1984)
analysed the blood of 65 healthy volunteers in the Netherlands (34
female and 31 male) and detected alpha-HCH in less than one third of
the samples. The median concentration for both groups was below the
detection limit (0.1 µg per litre), but levels of up to 0.4 µg/litre
were measured.
Polishuk et al. (1970) studied the presence of alpha-HCH in the
blood of 24 pregnant women and 23 infants living in Israel. The
mean concentration was 0.6 ± 0.3 µg per litre in the women and 0.5 ±
0.3 µg/litre in the infants.
In 1975, Reiner et al. (1977) analysed the serum and plasma of 82
women and 65 men (with an average age of 42) living in a town in
Yugoslavia. In 57 of the 147 samples, alpha-HCH was found at a mean
concentration of 3.3 ± 0.5 µg/litre (range, 0.1-15.0 µg/litre).
Similar values were found in other parts of the country in 1976-1979
(Krauthacker et al., 1980).
The median concentration of alpha-HCH in whole blood of 118
people in the Federal Republic of Germany was reported to be
0.98 µg/litre (range, nd-2.06) (Bertram et al., 1980).
5.2.3.2 Adipose tissue
The alpha-HCH concentrations of 567 samples of adipose tissues of
Dutch citizens analysed during 1968-1983 varied from < 0.01 to
0.1 mg/kg (on a fat basis). The highest levels occurred during the
period 1968-1976 (Greve & van Harten, 1983; Greve & Wegman, 1985).
In a study by Niessen et al. (1984), specimens of subcutaneous
adipose tissue from 48 infants (34 under the age of 1 year, 14 in
their second year of life) were examined during 1982-1983 in the
Federal Republic of Germany. The average concentration of alpha-HCH
was 0.01 mg/kg fat (range, nd-0.02 mg/kg). The average concentration
was highest (0.02 mg/kg fat) for the age-range 0-6 weeks. Bertram et
al. (1980) found a median concentration of 0.03 mg/kg fat (range,
nd-0.35) in 72 samples of adipose tissue from people in the Federal
Republic of Germany. Hildebrandt et al. (1986) summarized the results
of nine studies carried out in the Federal Republic of Germany during
1969-1983. The mean alpha-HCH concentrations (568 samples) ranged
from 0.01 to 0.03 mg/kg fat.
Mes et al. (1982) analysed 99 samples of adipose tissue from
autopsies of accident victims from different areas of Canada. Nearly
all the samples (97%) contained alpha-HCH, the average concentration
of which was 0.004 mg/kg wet weight (range, 0.001-0.043 mg/kg).
In 1974, 360 samples of adipose tissue were collected in eight
regions of Japan and the mean level of alpha-HCH was 0.031 mg/kg
tissue (Takabatake, 1978).
Twenty-nine samples of adipose tissue were taken at necropsy and
24 at surgery in the Poznan district of Poland and compared with 100
samples from residents of the Warsaw area. In Poznan the mean
concentration of alpha-HCH was 0.013 ± 0.033 mg/kg, while in Warsaw it
was 0.008 ± 0.001 mg/kg (Szymczynski et al., 1986).
5.2.3.3 Breast milk
Breast milk is a major route for the elimination of
organochlorine pesticides in women. In a Swedish study, the levels of
alpha-HCH in breast milk were found to be related to the dietary
habit. Levels in lacto-vegetarians were lower than those in women
eating a mixed diet, and these were lower than those found in mothers
using a mixed diet that regularly included fatty fish from the Baltic
(Noren, 1983).
In a study by Fooken & Butte (1987), the variation of residue
levels in breast milk during lactation was investigated in five women
(aged 23-36) in the Federal Republic of Germany. Alpha-HCH
concentrations of up to 0.009 mg/kg fat were measured, and no
essential changes in residue level occurred over the lactation period.
Residues of alpha-HCH in breast milk during the periods,
1974-1975 and 1979-1980 in the Federal Republic of Germany were
reported to be 0.03 and 0.02 mg/kg milk on a fat basis, respectively
(Anon., 1984).
In the Federal Republic of Germany, more than 7100 samples of
breast milk were analysed from 1969-1984. These studies were carried
out by 20 authors, and the results were summarized by Hildebrandt et
al. (1986). The mean concentrations of alpha-HCH ranged from
0.01-0.04 mg/kg on a fat basis. In one case a mean concentration of
0.21 mg/kg was found in 320 samples. During the period investigated, a
slow decrease in the mean concentration of alpha-HCH was observed. The
average concentration in breast milk in the same country (2709
samples) in 1979-1981 was 0.024 mg/kg on a fat basis (Fooken & Butte,
1987). In 1981-1983, 132 samples of breast milk were analysed and the
average level was 0.001 mg alpha-HCH/kg milk fat (Cetinkaya et al.,
1984).
Tuinstra (1971) analysed 36 individual samples of breast milk,
collected in 1969, from young mothers (18-32 years of age) living in
the Netherlands. A median alpha-HCH concentration of 0.01 mg/kg milk
(on a fat basis) was found (range, nd-0.04). When 278 samples of
breast milk, collected in 11 maternity centres in the Netherlands,
were analysed for the presence of alpha-HCH, the median alpha-HCH
concentration was < 0.01 mg/kg (on a fat basis) (Greve & Wegman,
1985).
Vukavic et al. (1986) measured the alpha-HCH concentration in 59
samples of colostrum collected during autumn 1982 (26 samples) and
spring 1983 (33 samples) in Yugoslavia from healthy nursing mothers on
the third day after delivery. The alpha-HCH levels were significantly
lower in the autumn than in the spring (mean concentrations of 0.49 ±
0.09 and 1.50 ± 0.26 µg/litre whole colostrum, respectively).
Mes et al. (1986) studied 210 breast milk samples from five
different regions of Canada and measured a mean alpha-HCH
concentration of 7 µg/kg (on a fat basis). Davies & Mes (1987)
studied 18 breast milk samples from Canadian, Indian, and Inuit
mothers in Canada, whose fish consumption was comparable to the
national level. The level of alpha-HCH in milk fat of the indigenous
population was 5 µg/kg, which was the same value as that obtained from
a national survey.
6. KINETICS AND METABOLISM
6.1 Absorption and elimination
The intestinal absorption rate for alpha-HCH was 97.4% after the
administration of an HCH mixture to male rats (Albro & Thomas, 1974).
The total excretion in rats after a single intraperitoneal (ip)
36Cl-labelled alpha-HCH dose of 200 mg/kg body weight was 80% of the
dose in the urine and 20% in the faeces (Koransky et al., 1963;
Koransky et al., 1964; Noack et al., 1975). In a study in rats with
36Cl-labelled alpha-HCH, a low excretion rate was found. 36Cl was
detected in the excreta up to 40 days after a single ip dose (Koransky
et al., 1963). During continued dosing alpha-HCH was observed to
stimulate its own degradation (Noack et al., 1975). The decrease in
rat liver alpha-HCH levels after an initial increase, observed by
Eichler et al. (1983), was assumed to be due to this effect.
When 14C-labelled alpha-HCH was administered intraperitoneally
to male mice (ddY-strain, 4 weeks old) at a dose level of 22 µg, the
average percentage of urinary excretion of radioactivity in 3 days was
37% (Kurihara & Nakajima, 1974).
6.2 Distribution
One day after an ip injection of a mixture of 14C- and
36Cl-labelled alpha-HCH into rats (200 mg/kg body weight in rapeseed
oil), the highest level of radioactivity was found in fat, skin, and
bones plus muscles (18.2, 13.1, and 11.9%, respectively, after 4
days). Much lower levels were found in other organs or tissues (up to
1% in liver and kidneys and 0.28% in the central nervous system. In
the faeces and urine, 3.9 and 7.9%, respectively, were found after 4
days (Koransky et al., 1963). In other studies with rats, higher
concentrations were found in liver, kidneys, body fat, brain, and
muscle (Portig & Vohland, 1983; Kuiper et al., 1985). In a 90-day
study in rats, marked deposition of alpha-HCH was found in renal fat;
the concentrations exceeded those obtained in a similar study on
beta-HCH (Greve & van Hulst, 1980; Kuiper et al., 1985). In lactating
rats given a single oral dose 5 days after birth, the alpha-HCH
concentrations in the livers of the sucklings were twice as high as
those observed in the livers of the mothers (Wittich &
Schulte-Hermann, 1977).
Vohland et al. (1981) studied the distribution of alpha-HCH in
the brain and depot fat of rats after the administration of 200 mg/kg
body weight by gavage. With an average blood concentration of
1.5 µg/litre, the brain to blood, and depot fat to blood ratios were
120:1 and 397:1, respectively, whereas with a blood concentration of
17.7 mg/litre the ratios were 5:1 and 82:1, respectively.
Nagasaki (1973) orally administered alpha-HCH to male mice at
concentrations of 100, 250 or 500 mg/kg for 24 weeks, and found high
residual levels of this isomer in liver and adipose tissue. Similarly,
Macholz et al. (1986) reported that a 30-day administration of
alpha-HCH to rats resulted in high residues of this isomer in fat,
kidneys, and adrenal tissue.
In the brain, alpha-HCH is stored preferentially in the white
matter (Stein et al., 1980; Portig et al., 1989).
6.3 Metabolic transformation
6.3.1 Rat
When Sprague-Dawley weanling female rats were administered 2 mg
alpha-HCH/rat per day in peanut oil for 7 days, the alpha-HCH was
metabolized to 2,4,6- and 2,4,5-trichlorophenol, with an excretion
ratio of 2,4,6- to 2,4,5-trichlorophenol of 1.3:1. This study also
indicated that pre-treatment with alpha-HCH alters the metabolism of
lindane in rats (Freal & Chadwick, 1973).
The biotransformation of alpha-HCH in rats involves
dechlorination (Kraus, 1975). The dose-dependent decrease in liver
glutathione concentrations indicates the formation of a glutathione
conjugate in this organ (Noack & Portig, 1973; Portig et al., 1973;
Kraus, 1975). Such a decrease does not occur in the brain or kidneys
(Noack & Portig, 1973).
The major urinary metabolite in rats is 2,4,6-trichlorophenol, a
compound reported by IARC (1987) to be carcinogenic for animals
(Portig et al., 1973; Stein & Portig, 1976; Stein et al., 1977). Other
metabolites that have been identified are 1,2,4-trichlorophenol,
2,3,4-trichlorophenol, 2,4,5-trichlorophenol, 2,3,4,5-
tetrachlorophenol, and 2,3,4,6-tetrachlorophenol (Noack et al., 1975;
Stein et al., 1977; Macholz et al., 1982). In addition,
chlorothiophenols (not specified) have been detected, and
1,3,4,5,6-pentachlorocyclohex-1-ene has been identified in the kidneys
of rats (Macholz et al., 1983).
Artigas et al. (1988) have identified several lindane metabolites
(tetra-, penta-, and hexachlorocyclohexenes, and tetra- and
pentachlorobenzenes) in rat brain homogenates by gas
chromatography-mass spectrometry. Male Wistar rats were orally
administered 30 mg alpha-HCH/kg and were sacrificed 5 h later. The
cerebella of the animals were analysed and the following metabolites
were found: 3.6/4.5-PCCH, 3.5/4.6-PCCH, HCCH, pentachlorobenzene, and
HCB. HCCH was the major metabolite (about 100 µg per kg) while levels
of the other metabolites were mainly below 5 µg/kg. Alpha-HCH was
present at 17.2 mg/kg tissue. This study showed that the HCH isomers
are cleared from the brain via different metabolic pathways.
Isomerization of alpha-HCH to lindane did not occur after
repeated dosage (Eichler et al., 1983).
6.3.2 Bird
In a model 4-week feeding study on poultry using four HCH
isomers, the rate of degradation of the individual HCH isomers in
broilers followed the order: delta > gamma > alpha > beta.
Biotransformation (to one or more of the other HCH isomers) was not
detected (Szokolay et al., 1977b).
In a study by Foster & Saha (1978) on the in vitro metabolism
of alpha-HCH in chicken livers, the first metabolite was identified as
an isomer of pentachlorocyclohexane.
6.3.3 Human
When Engst et al. (1978) analysed the urine of occupationally
exposed workers (apparently to technical-grade HCH in manufacturing
processes), they found, apart from alpha-, beta-, gamma-, and
delta-HCH, traces of hexa- and pentachlorobenzene, gamma- and
delta-pentachlorocyclohexane, pentachlorophenol, 2,3,4,5-, 2,3,4,6-,
and 2,3,5,6-tetrachlorophenol, and several trichlorophenols, as well
as the glucuronides of several of these metabolites. The
pentachlorocyclohexenes, tetrachlorophenol, hexachlorobenzene, and
pentachlorophenol were also identified in the blood.
6.4 Retention and biological half-life
The half-life for the clearance of alpha-HCH from depot fat was
found to be 6.9 days in female rats and 1.6 days in male rats (Stein
et al., 1980; Portig, 1983).
Vohland et al. (1981) and Portig & Vohland (1983) studied the
kinetics of alpha-HCH in Wistar rats, and observed that, after a
single oral dose of 200 mg/kg body weight, the approximate half-life
in females for the elimination from brain was 6 days.
The retention of alpha-HCH in rat brain after a single dose is
greater than that of beta- and gamma-HCH (Stein et al., 1980).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Acute toxicity
In mice oral LD50 values have been found to range from 1000 to
4000 mg/kg body weight, depending on the vehicule, while in rats
values of 500-4674 mg/kg body weight have been obtained. Riemschneider
(1949) determined a LD50 (oral intubation in olive oil) for rats of
1500 mg/kg body weight. The signs of poisoning were those of nervous
system stimulation: excitation, hunched posture, rough fur, dyspnoea,
anorexia, tremors, convulsions, and cramps (Hoffmann, 1983; WHO,
1986).
7.2 Short-term exposure
7.2.1 Oral
In a 90-day study on rats carried out with dose levels of 0, 2,
10, 50, or 250 mg alpha-HCH/kg diet, reductions in white blood cell
count were noted in several groups of animals. Growth was decreased
at 250 mg/kg diet, and at this dose level the number of erythrocytes
and protein excretion in the urine were elevated in female animals. At
levels of 50 and 250 mg/kg, the activities of liver
amino-pyrine- N-demethylase and aniline hydroxylase were increased
while those of blood aspartate aminotransferase (ASAT) and creatine
phosphokinase were decreased. Liver weights were increased at dose
levels of 10, 50, and 250 mg/kg. Enlargement of liver parenchyma cells
(with a foamy/hyaline appearance of the cytoplasm) and accentuation of
the plasmalemma, indicative of proliferation of smooth endoplasmatic
reticulum (SER), occurred at levels of 50 and 250 mg/kg. At the
250-mg/kg level, there were increases in the relative weights of
heart, kidneys, and adrenals. In addition, serum levels of
immunoglobulins G and M showed a decrease at 50 and 250 mg/kg diet
(Kuiper et al., 1985).
Macholz et al. (1986) reported that the administration of 1000 mg
alpha-HCH/kg to rats for 30 days resulted in growth retardation and
liver mass increase. High residue levels of alpha-HCH were identified
in fat, kidneys, and adrenal tissue.
7.2.2 Other routes
7.2.2.1 Intravenous
In a study by van Asperen (1954), groups of 12-15 male and female
albino mice (8-10 weeks of age) were given an intravenous injection of
alpha-HCH (in peanut oil). The dose levels were 480 or 960 µg/mouse
(equivalent to approximately 32 and 64 mg/kg body weight,
respectively). No deaths occurred within 7 days.
7.2.2.2 Subcutaneous
Groups of 13-21 male and female albino mice (8-10 weeks of age)
were given a subcutaneous injection of alpha-HCH at dose levels
ranging from 3 to 20 mg/animal (equivalent to approximately 200 to
1330 mg/kg body weight, respectively). With doses of up to 4 mg, no
death occurred within 7 days, but with 4.5 mg, 8 mg, and 20 mg, 8, 25,
and 90%, respectively, of the animals died (van Asperen, 1954).
7.3 Skin and eye irritation; sensitization
No data on skin and eye irritation or sensitization have been
reported.
7.4 Long-term exposure
7.4.1 Rat oral study
When groups of 10 female and 10 male weanling Wistar rats were
administered diets containing 0, 10, 50, 100, or 800 mg alpha-HCH/kg
diet (in corn oil) for 107 weeks, the highest dose level resulted in
growth retardation, increased mortality, and slight kidney damage.
With dose levels of 100 or 800 mg/kg, liver enlargement and
histo-pathological changes in the liver were found. However, there
were no liver changes at 50 mg/kg diet (Fitzhugh et al., 1950).
7.5 Reproduction, embyrotoxicity, and teratogenicity
No information on reproduction, embryotoxicity, or teratogenicity
is available.
7.6 Mutagenicity and related end-points
Alpha-HCH did not induce mutations in Salmonella typhimurium
test strains TA98, TA100, TA1535 or TA1537 either with or without rat
liver metabolic activation (Lawlor & Haworth, 1979). A test for point
mutations in Saccharomyces cerevisiae XV 185 14 C was also negative
(Shahin & von Borstel, 1977). In addition, the compound produced no
mutations in Allium cepa roots (Nybom & Knutsson, 1947). A test for
unscheduled DNA synthesis in rat hepatocytes in vitro produced an
equivocal result (Althaus et al., 1982).
A mutagen test strain of Bacillus subtilis (TKJ5211) showed a
higher sensitivity for hisw+ reversion than the parental strain
HA101 when treated with UV and UV-mimetic chemicals. However, a
negative result was obtained when alpha-HCH dissolved in DMSO was used
at a dose level of 5 mg/ml (Tanooka, 1977).
A DNA repair test was carried out with stationary-phase cultures
of B. subtilis HLL3g and HJ-15 strains in which the size of growth
inhibition zones of repair-proficient and repair-deficient cells for
vegetative cells and spores was determined. There was no effect at a
dose level of 5 mg alpha-HCH (in benzene) per ml (Tanooka, 1977).
The available data are inadequate to make an assessment of the
mutagenic potential.
7.7 Carcinogenicity
Appraisal
The reported studies on the carcinogen effects of alpha-HCH on
mice and rats have some short-comings. In most cases, very high dose
levels were tested. Nevertheless, it is clear from the results that
alpha-HCH, at high dose levels, produces nodular hyperplasia and
hepatocellular carcinomas in mice (the incidence varying according to
the strain) and also in rats (low incidence), but only at higher dose
levels.
The results of the studies on initiation-promotion and mode of
action indicate that the neoplastic response observed with alpha-HCH
is most likely due to a non-genotoxic mechanism.
7.7.1 Mouse
When 20 male ICR/JCL mice (aged 5 weeks) were administered a diet
containing 600 mg alpha-HCH/kg diet for 26 weeks, increased liver
weight was observed. In all treated mice there were liver tumours,
which were characterized histologically as benign tumours and
malignant tumours with atypical liver cells. Unfortunately,
insufficient details were reported (Goto et al., 1972a,b).
In a study by Hanada et al. (1973), 6-week-old DD mice (10-11 of
each sex per group) were given diets containing 0, 100, 300, or 600 mg
alpha-HCH/kg diet for 32 weeks, followed by a control diet for 5-6
weeks. The control group consisted of 20 female and 21 male animals.
During the experiment several animals died. The numbers of hepatomas
in the four groups surviving for 36-38 weeks were 0/29 (control), 1/16
(100 mg/kg), 9/10 (300 mg/kg), and 13/15 (600 mg/kg).
Alpha-fetoprotein was not detected in the serum of animals with
hepatomas.
When 8-week-old male DD mice, divided into groups of 20 or 38
animals, were fed a diet containing 0, 100, 250, or 500 mg
alpha-HCH/kg for 24 weeks, the two highest dose levels induced an
increase in liver weight. At the four respective dose levels, the
incidence of nodules classified as nodular hyperplasia was 0/20, 0/20,
30/38 (79%), and 20/20 (100%) and that of hepatocellular carcinoma was
0/20, 0/20, 10/38 (26%), and 17/20 (85%) (Ito et al., 1973b).
Following the oral administration of 100, 250 or 500 mg
alpha-HCH/kg to male DD mice for 24 weeks, hepatocellular tumours were
found in all mice treated with 500 mg/kg and in 17 of the 20 mice that
received 250 mg/kg (Nagasaki, 1973).
Nagasaki et al. (1975) studied the tumorigenic effects of a diet
containing 0 or 500 mg alpha-HCH/kg, fed for 24 weeks to groups of
male and female DDY, ICR, DBA/2, C57BL/6, and C3H/He mice (13-29 of
each sex per group), male Wistar rats, and male golden Syrian
hamsters. It was found that alpha-HCH induced liver tumours in male
and female mice but not in rats and hamsters. The histological changes
in the liver of mice were much greater than those induced in rats and
hamsters. Male animals were more susceptible to the tumorigenic
action (i.e. liver nodules) than females. Among the different strains
of mice, a difference in susceptibility was observed. The occurrence
of liver nodules varied from 16.7 to 100% and the incidence of
hepatocellular carcinomas varied from none to 65%. The DDY mouse
strain was the most sensitive and the C57BL/6 the least sensitive
strain.
Ito et al. (1976) studied the reversibility of liver tumours
induced by alpha-HCH (99.0%). Male 8-week-old DDY mice were fed a
diet containing 0 or 500 mg/kg for 16, 20, 24, and 35 weeks and then
fed a basal diet without alpha-HCH for 4, 8, and 12, or 4, 8, 12, 16,
24, and 36 weeks. In total 341 mice were used, of which 21 were fed
the compound for 16 weeks. A total of 300 mice were fed the diet with
alpha-HCH for 20 or more weeks and 20 control mice were fed the basal
diet for 72 weeks. At the various intervals indicated, 12-20 mice were
killed. The incidence of liver tumours increased progressively during
continuous administration of alpha-HCH, but when its administration
was discontinued some tumours disappeared. After 24 weeks of
administration most tumours were nodular hyperplasias with only a few
well-differentiated hepatocellular carcinomas. However, 60 or 72
weeks after the beginning of the study most of the liver tumours were
hepatocellular carcinomas. The findings suggested that nodular
hyperplasia was usually reversible.
Two groups of male HPBC57B1 black mice (6-9 weeks old) were fed
a diet containing 500 mg/kg alpha-HCH (99.8%) per diet, 48 mice being
used as controls and 75 mice being administered alpha-HCH. From each
group, 4-9 mice were killed at 1, 3, 4, 8, 14, 21, 30, 33, 44, and 50
weeks after the initiation of treatment. Progressive liver
enlargement was first noticed at 3 weeks, hepatic nodules at 21 weeks,
and emaciation at 30 weeks. Histopathological liver alterations
included hypertrophy of centrolobular hepatocytes first seen at 1 week
and the merging of adjacent megalocytic zones at 3 weeks. At 21
weeks, adenomas were seen in two out of seven mice, at 30 weeks in
seven out of eight mice, and at 33, 44, and 50 weeks in all the mice
studied. Under the condition of this study, neither hepatocellular
carcinomas nor metastases in the lungs were detected (Tryphonas &
Iverson, 1983).
7.7.2 Rat
When groups of 10 male and 10 female weanling Wistar rats were
fed throughout their life on diets containing 10, 50, 100, or 800 mg
alpha-HCH (> 98% pure) per kg, no increase in tumour incidence was
found. However, only a limited number of organs were examined
microscopically (Fitzhugh et al., 1950).
In a study by Ito et al. (1975), male Wistar rats (5-8-weeks old)
were divided into seven groups and administered alpha-HCH diets
containing 0, 500 (two groups), 1000 (three groups), or 1500 mg
alpha-HCH/kg diet. The duration of the treatment for the different
groups was 72 weeks for the controls, 24 or 48 weeks at 500 mg/kg, 24,
48, or 72 weeks at 1000 mg/kg, and 72 weeks at 1500 mg/kg. In the
liver, oval cells and bile duct cell proliferation were found in the
groups fed 1000 or 1500 mg/kg after 48 and 72 weeks. Cell hypertrophy
was found in all the groups, the increase in severity depending on the
dose level and the duration of administration. In the two groups fed
500 mg/kg and the group fed 1000 mg/kg for 24 weeks no nodular
hyperplasia or hepatocellular carcinomas were found. Nodular
hyperplasia developed in the groups fed 1000 mg/kg (48 and 72 weeks)
or 1500 mg/kg (72 weeks) in 42, 76, and 77% of the animals,
respectively. Hepatocellular carcinomas were found only in the groups
fed 1000 or 1500 mg/kg for 72 weeks (1/16 and 3/13 animals,
respectively).
In a series of studies, an oral dose of 20 mg/kg body weight was
administered daily to female rats during periods of 4.5, 13.5, or 23.5
months. Liver enzyme induction was found at all intervals, white foci
and nodules were present after 13.5 months, and one animal had a
hepatocellular carcinoma after 23.5 months (Schulte-Hermann &
Parzefall, 1981). The value of this study was reduced by the very low
number of animals (4-6 per group) used at each interval.
7.7.3 Initiation-promotion
In a study on 8-week-old white male mice (25-30 per group) of
strain DD, the influence of alpha-HCH on tumour induction by
polychlorinated biphenyls (PCBs) was tested and vice versa. Whereas
500 mg PCB/kg diet induced nodular hyperplasia and hepatocellular
carcinomas in the liver of male mice after 32 weeks, exposure to
alpha-HCH at dose levels of 50, 100, or 250 mg/kg diet, only resulted
in both type of tumours at the highest dose level. The incidence of
nodular hyperplasia was 23/30 (77%) and that of hepatocellular
carcinoma was 8/30 (27%). However, 50 or 100 mg alpha-HCH/kg diet, in
combination with 250 mg PCB per kg diet (PCB alone did not induce
tumours), induced nodular hyperplasia (approximately 30%) and
hepatocellular carcinoma (approximately 5%). It seems that PCBs
promote the induction of liver tumours by alpha-HCH (Ito et al.,
1973a).
In studies on rats, alpha-HCH showed a tumour-promoting action
towards the hepatocarcinogenic effects of aflatoxin B1,
diethylnitrosamine, and nitrosomorpholine (Schulte-Hermann &
Parzefall, 1981; Schulte-Hermann et al., 1981; Angsubhakorn et al.,
1981). In one test, alpha-HCH produced only a slight liver
tumour-promoting effect in rats after initiation with
N-nitrosodiethylamine (Ito et al., 1983). However, in another study
on the same species the compound had an inhibitory effect on the
hepa-tocarcinogenic action of 3-methyl-4-dimethylaminoazobenzene and
DL-ethionine (Thamavit et al., 1974).
Nagasaki et al. (1975) studied the influence of
3-methylcholanthrene, 1-naphthyl isothiocyanate, and
p-hydroxypropiophenone on the induction of liver tumours by
alpha-HCH. Eight groups of 24 mice received a diet containing either
500 mg alpha-HCH/kg diet in combination with 67 mg
methylcholanthrene/kg, 600 mg 1-naphthyl isothiocyanate/kg or 1000 mg
p-hydroxypropiophenone/kg or just one of the four compounds. A
control group with the basal diet was also used. The induction of
mouse liver tumours by alpha-HCH was not inhibited by the concomitant
feeding of 1-naphthyl isothiocyanate or p-hydroxypropiophenone.
However, 3-methylcholanthrene slightly inhibited their induction by
alpha-HCH.
In a study by Schröter et al. (1987), the tumour-initiating
activity of alpha-HCH was studied by examining for phenotypically
altered foci in female Wistar rats. Groups of three to eight rats
were used and, after removing the median and right liver lobes, 200 mg
alpha-HCH/kg body weight was administered followed by phenobarbital at
50 mg/kg body weight per day for 15 weeks. Liver foci were identified
by means of the gamma-glutamyltransferase (GGT) reaction and by
morphological alterations. No evidence of initiating activity was
found. In another part of the study, the promoting activity was
investigated. A single dose of N-nitrosomorpholine (250 mg/kg body
weight by gavage) was followed by the administration of 0.1, 0.5, 2.0,
7.0, or 20.0 mg alpha-HCH/kg body weight per day for 4, 15, and 20
weeks. The criteria used were growth and phenotypic changes of foci
as end-points. It was concluded from the study that alpha-HCH is a
tumour promotor. Both the number and size of altered foci were
enhanced by alpha-HCH doses of 2 mg/kg or more. The tumour-promoting
action was generally associated with liver enlargement and induction
of monooxygenases or other specific enzymes.
Schulte-Hermann et al. (1983) carried out three experiments with
Han-Wistar rats using, in experiment 1, 39 female rats (8-24 months
old) and, in experiments 2 and 3, 41 male (2 years old) rats.
Alpha-HCH (200 mg/kg in corn oil) was administered orally as a single
dose, while the control group received only corn oil. Beginning 25 h
after the dosing, 3H-thymidine was injected intravenously five times
at intervals of 6 h (experiment 2) or 8 h (experiment 3) , and the
animals were killed 18 (experiment 2) or 3 h (experiment 3) after the
last dose of 3H-thymidine. The effect of age on the incidence of
spontaneous foci was studied in experiment 1. Foci of putative
preneoplastic cells were detected in the livers of untreated rats of
both sexes, especially at 1 and 2 years of age. These foci exhibited
markers similar to those of their counterparts in carcinogen-treated
rats, such as cytoplasmic basophilia, clearness of cytoplasm, or
expression of gamma-glutamyl transferase. Rates of DNA synthesis in
foci were higher than in normal liver cells and were increased by
single doses of liver mitogens assumed to promote liver tumour
development. Thus cells in the spontaneous foci appeared to possess a
defect in the growth control, rendering them more susceptible to
endogenous and exogenous growth stimuli.
The incorporation of orally administered radiolabelled thymidine
into liver DNA was determined in SIV-50-SD rats 24 h after a single
oral gavage dose of 2.9, 29.1, 58.2, or 291 mg alpha-HCH/kg. Alpha-HCH
was found to stimulate liver DNA synthesis at 58.2 mg/kg (Büsser &
Lutz, 1987).
7.7.4 Mode of action
Sagelsdorff et al. (1983) studied the relevance to the
carcinogenic action of alpha-HCH of covalent binding to mouse liver
DNA. Three strains of mice were used (NMRI, CF1, and C6B3F1), and
alpha-HCH was administered by oral gavage and 14C-thymidine by the
intraperitoneal route. In all three strains, a similar low covalent
binding index or DNA damage/dose (values ranging from 0.17-0.28) was
found. There was no quantitative correlation with the carcinogenicity
potency of alpha-HCH.
Iverson et al. (1984) studied the ability of alpha-HCH to bind to
macromolecules from male HPB black mouse liver. In vivo and in
vitro binding studies with 14C-alpha-HCH and hepatic microsomes
from untreated and phenobarbital-pretreated mice showed no
preferential binding of alpha-HCH to protein or DNA. The results
suggest that the neo-plastic response observed with alpha-HCH results
from a non-genotoxic mechanism.
7.8 Special studies
7.8.1 Effect on liver enzymes
After a single oral administration to female rats of 5 mg
alpha-HCH/kg body weight or more the rate of aminopyrine demethylation
and the liver DNA content were both increased, but at 2 mg/kg body
weight these effects did not occur (Schulte-Hermann et al., 1974). In
a further study, the liver cytochrome P450 concentration in male rats
after a single oral administration was elevated at all tested dose
levels, 25 mg/kg body weight being the lowest (Seifart & Buchar,
1978). After alpha-HCH was given to male rats at dose levels of 5, 10,
20, 50, or 200 mg/kg feed for 2 weeks, aniline hydroxylase and
aminopyrine demethylase activities were increased at all dose levels
(den Tonkelaar et al., 1981).
7.8.2 Neurotoxicity
Appraisal
Alpha-HCH has been shown to have no effect on motor nerve
conduction velocity or the fronto-occipital EEG in rats fed 1000 mg
alpha-HCH/kg diet for 30 days. This isomer is a mild antagonist of
pentylenetetrazol-induced convulsions but increases the tonic/clonic
activity and the lethality of picro-toxin when administered
intraperitoneally to mice. It decreases the accumulation of
cerebellar cyclic GMP and prohibits the increase of cGMP caused by
gamma-HCH in mouse brain. Alpha-HCH has been demonstrated to inhibit
GABA-mediated chloride ion uptake in mouse brain, and this effect is
believed to play a primary role in the CNS action of this isomer.
In a study by Vohland et al. (1981), alpha-HCH did not give rise
in brain tissue to appreciable quantities of hydrophobic metabolites
such as 2,4,6-trichlorophenol. It had a weak protecting action
against convulsions induced by pentylenetetrazole (PTZ). The intensity
and duration of the PTZ-antagonistic effects after a single oral dose
were related to the alpha-HCH content of the brain.
In a 30-day study on groups of 15 male Wistar rats fed alpha-HCH
at levels of up to 1000 mg/kg diet, there was no effect on the
fronto-occipital electroencephalogram or on the motor conduction
velocity of the tail nerve (Müller et al., 1981).
The effect of alpha-HCH on body temperature, food intake, and
body weight was studied in Wistar rats (eight males and eight females)
given a single 30-mg/kg oral dose of alpha-HCH in olive oil. Controls
received only olive oil. Alpha-HCH treatment induced no significant
decrease in core temperature 5 h after treatment, and no decrease in
food intake or growth was observed (Camon et al., 1988).
Fishman & Gianutsos (1987) studied the effects of an
intraperitoneal injection of alpha-HCH (99.0%) in corn oil
(80-480 mg/kg body weight) on the accumulation of cerebellar cyclic
GMP in male CD-1 mice. Alpha-HCH decreased the accumulation of
cerebellar cyclic GMP and also prevented the increase in cyclic GMP
resulting from lindane treatment. Furthermore, alpha-HCH inhibited
the binding of 3H-TBOB (a ligand for the GABA-A-receptor-linked
chloride channel) in mouse cerebellum.
Fishman & Gianutsos (1988) compared the CNS-related
pharmacological and biochemical effects of gamma-HCH and the
non-convulsant isomer alpha-HCH. The studies were carried out on male
CD-1 mice injected intraperitoneally with a single alpha-HCH (in corn
oil) dose of 80-400 mg/kg body weight. Alpha-HCH inhibited the
myoclonic jerk and tonic/clonic activity of PTZ but increased the
tonic/clonic activity and lethality of picrotoxin (PIC) (PTZ and PIC
were given as a single ip injection of 50 mg/kg and 20 mg/kg body
weight, respectively). The highest dose of alpha-HCH caused a
significant decrease in motor activity. Gamma-HCH inhibited the
binding of 3H-TBOB to mouse whole brain membranes. Furthermore, this
isomer is a weak inhibitor of GABA-stimulated uptake of 36wCl into
mouse brain neurosynaptosome preparations in vitro. The
non-seizure-inducing alpha-HCH has biochemical and pharmacological
effects in the CNS which differ from those of the gamma-HCH.
Matsumoto et al. (1988) provided evidence that all HCH isomers
are capable of inhibiting GABA-A-mediated chloride channels in the
brain, the relative potency being alpha = gamma > delta > beta.
Alpha-HCH was also found to be a potent inhibitor of the
batrachotoxin-stimulated action potential flux of sodium ions in N18
neuroblastoma cell cultures (Shain et al., 1987).
8. EFFECTS ON HUMANS
8.1 Acute toxicity - poisoning incidents
Several cases of acute poisoning by technical-grade HCH,
resulting either from accidents or occupational exposure, have been
described (WHO, 1991). Although alpha-HCH constitutes 65-70% of the
technical product, it is likely that the most acutely toxic component,
i.e. gamma-HCH, played the major role in these incidents. These cases
cannot, therefore, assist in the evaluation of alpha-HCH.
8.2 General population
No specific studies relating to alpha-HCH are available.
A study comparing liver cancer deaths in the USA and the
"domestic disappearance" of organochlorine pesticides revealed that in
1962, 18 and 15 years after the introduction of DDT and
technical-grade HCH, respectively (when an increase in primary liver
cancer due to the organochlorines would be manifest), the number of
cases of primary liver cancer as a percentage of the total number of
liver cancer deaths began a gradual and steady decline (from 61.3% in
1962 to 56.9% in 1972). The death rate (per 100 000 per year) due to
primary liver cancer declined from 3.46 to 3.18 during this period
(Deichmann & MacDonald, 1977).
8.3 Occupational exposure
The evaluation of the effects of alpha-HCH on occupationally
exposed workers is seriously hampered by the fact that most of the
relevant studies concern workers who were exposed during the
manufacture and handling of lindane or the handling and spraying of
technical-grade HCH among other pesticides, and were thus exposed to
all HCH isomers plus impurities and other (process) chemicals.
Therefore, it is difficult, if not impossible, to relate the observed
effects to individual substances. Consequently these studies have only
been described in this monograph where they aid the evaluation.
Behrbohm & Brandt (1959) described 26 cases of allergic and toxic
dermatitis that arose during the manufacture of technical-grade HCH.
Patch testing with pure alpha-, beta-, gamma-, and delta-HCH yielded
negative results, but positive reactions were obtained with the
residual fractions.
The level of alpha-HCH in 57 healthy workers (with normal liver
function, EMG and EEG) at a lindane-manufacturing plant ranged from 10
to 273 µg/litre, whereas it was below the detection limit in control
workers. The concentration in the adipose tissue of eight of the
exposed workers ranged from 1 to 15 mg alpha-HCH/kg (in extractable
lipids) (Baumann et al., 1980, 1981; Brassow et al., 1981; Tomczak et
al., 1981).
The mean serum alpha-HCH level of malaria-control workers that
sprayed technical-grade HCH for 16 weeks increased from 10 to
78 µg/litre in previously non-exposed workers and from 18 to
77 µg/litre in those that had been exposed during three previous
spraying seasons (Gupta et al., 1982).
Nigam et al. (1986) studied 64 employees from a plant
manufacturing HCH who were directly or indirectly associated with the
production of this insecticide and thus also exposed to chemicals such
as benzene and chlorine. The exposed group was composed of 19
"handlers" (who handled and packed the insecticide), 26 "non-handlers"
(plant operators and supervisors exposed indirectly to HCH), and 19
maintenance staff (who visited the plant frequently). The control
group consisted of 14 workers who had no occupational contact with the
insecticide. The exposure period varied up to 30 years. The mean
serum alpha-HCH concentrations in the four groups were 21.1 µg/litre
(controls), 21.8 µg/litre (maintenance staff), 41.2 µg per litre
(non-handlers), and 100 µg/litre (handlers). Lindane and beta- and
delta-HCH were also present. The total HCH concentrations were 51.4,
143.6, 265.6, and 604 µg per litre, respectively. Clinical examination
revealed that the majority of the workers from the "handler" and
"non-handler" groups exhibited paraesthesia of the face and
extremities, headache, and giddiness, and some of them also showed
symptoms of malaise, vomiting, tremors, apprehension, confusion, loss
of sleep, impaired memory, and loss of libido. The same symptoms were
found among the maintenance staff but were less severe and less
frequent.
Chattopadhyay et al. (1988) studied 45 male workers exposed to
HCH during its manufacture and compared them with 22 matched controls.
Exposure was mainly via the skin. Paraesthesia of face and
extremities, headache, giddiness, vomiting, apprehension, and loss of
sleep, as well as some changes in liver function tests, were reported
and were found to be related more to the intensity of exposure (as
measured by the HCH levels in blood serum) than to the duration of
exposure. The measured exposures to total HCH were 13 to 20 times
higher than those in the control groups (no detailed figures were
reported). Of the total serum HCH, 60-80% was beta-HCH.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Algae
Palmer & Maloney (1955) used alpha-HCH in a preliminary screening
test with two cyanobacterium (blue-green alga), two green alga, and
two diatom species. The test concentration was 2 mg/litre of water,
and the incubation period was 3-21 days. Alpha-HCH was not toxic at
this concentration.
When Canton et al. (1975) exposed Chlorella pyrenoidosa to
alpha-HCH for 96 h at 28°C (static system), the EC50 (growth
inhibition) was > 10 mg/litre (maximum solubility in the medium).
In a study by Krishnakumari (1977), cultures of the green alga
Scenedesmus acutus of 1, 3, or 5 days of age were tested for
sensitivity to alpha-HCH at 28°C, the growth rate being used as a
parameter. Alpha-HCH dissolved in ethanol was added at nominal
concentrations of 0.5-100 mg/litre water. The alpha-HCH concentrations
that caused a reduction in growth in 1-, 3-, and 5-day-old cultures
were 10 (or more), 5, and 0.5 mg/kg, respectively.
When Chlamydomonas sp. was exposed at a temperature of 20-25°C
in a static system, the no-observed-effect level (based on the growth
in 48 h) was > 1.4 mg/litre. A similar result was obtained with
Dunaliella sp. at 15°C and a study duration of 48 and 96 h, the
NOEL for growth being 1.4 mg/litre (maximum solubility) (Canton et
al., 1978).
9.2 Protozoa
The EC50 for Tetrahymena pyriformis (3 days in closed system
at 27°C) was reported to be 0.75 mg/litre (Mathur et al., 1984).
9.3 Invertebrates
9.3.1 Acute toxicity
The result of acute or short-term toxicity studies lasting a few
days on Artemia salina, Daphnia magna, and Lymnaea stagnalis are
summarized in Table 3.
Table 3. Acute or short-term toxicity of alpha-hexachlorocyclohexane for invertebrates
Species Age Temperature Parameter Concentration References
(°C) (mg/litre)
Artemia salina 3 weeks 24 LC50a,b 0.5 Canton et al. (1978)
Daphnia magna < 1 day 20 LC50c,d 0.8 Canton et al. (1975)
Lymnaea stagnalis 6 months 22 EC50c,e 1.2 Canton & Slooff (1977)
a synthetic saltwater
b 35 days (but exposure time was 4 days)
c 48 h
d closed system
e growth inhibition/mortality or immobilization
9.3.2 Short- and long-term toxicity
9.3.2.1 Crustaceae
In a study by Canton et al. (1975), Daphnia magna was exposed
to 0, 10, 50, 200, 1000, or 2000 µg alpha-HCH (> 95%) per litre for
25 days. The daphnids were fed Chlorella pyrenoidosa. The
sensitivity of daphnids to alpha-HCH markedly increased with exposure
time. A concentration of approximately 50 µg/litre or less did not
lead to death at any time during the whole life cycle of 2 months.
Only with 2000 µg/litre was there an influence on reproduction, the
EC50 for reproduction inhibition being 100 (54-186) µg/litre.
The EC50 based on mortality and immobilization was 800
(600-1000) µg/litre (see Table 4).
9.3.2.2 Molluscs
In a short-term (2-day) study, groups of five adult snails
(Lymnaea stagnalis L.) (6 months of age) were exposed to various
dose levels. Based on mortality and immobility, the EC50 was
estimated to be 1200 (600-2300) µg alpha-HCH (> 95%) per litre
(Canton & Slooff, 1977).
In a long-term (70-day) study, groups of 10 snails (5 months of
age) were exposed to 20, 100, 300, or 600 µg per litre. The study was
divided into a pre-exposure period (14 days) during which all egg
capsules and the number of eggs per capsule were counted, an exposure
period of 40 days during which four groups of adults and five capsules
of each group were exposed to alpha-HCH, and a post-exposure period
(16 days) during which snails were placed in water to recover. Based
on egg production inhibition, the 40-day EC50 was 250 µg/litre. The
percentage of fertilized eggs per capsule was not affected, and no
morphological abnormalities were noticed during embryonic development.
Based on the number of eggs that did not hatch, an EC50 of
230 µg/litre was determined. Considering a combination of the
inhibition of egg production and the mortality of the young during
their development, a 50% reduction of the overall reproductivity was
found at 65 µg alpha-HCH/litre. These effects did not disappear during
the recovery period of 16 days (Canton & Slooff, 1977) (see Table 4).
Table 4. Long-term toxicity of alpha-hexachlorocyclohexane for invertebrates
Species Age Temperature Duration Criteria Concentration References
(°C) (days) (mg/litre)
Daphnia magna 19 21 no mortality; 0.27a Janssen et al.
no effects on behaviour, 0.09 (1987)
appearance or growth;
no influence on reproduction 0.27
(4 groups of offspring)
Lymnaea stagnalis adults 22 40 EC50 (egg production inhibition) 0.25 Canton & Slooff
eggs and 22 40 hatching, overall productivity 0.065 (1977)
adults
a water renewal system
9.4 Fish
9.4.1 Acute toxicity
LC50 and EC50 (mortality and immobilization) values for fish
are summarized in Table 5.
9.4.2 Short- and long-term toxicity
During a 3-month study, rainbow trout (Salmo gairdneri)
(200-250 g) were fed pellets containing 0, 10, 50, 250, or 1250 mg
alpha-HCH (purity > 95%) per kg diet. After 2, 4, 8, and 12 weeks,
the fish were examined. Growth, microsomal liver enzymes (aniline
hydroxylase and aminopyrine demethylase), brain cholinesterase, serum
alkaline phosphatase, and the histopathology of the brain, liver, and
kidneys were all investigated but no effects were found (Canton et
al., 1975).
When guppies (Poecilia reticulata) aged 3-4 weeks were exposed
to 0, 200, 800, or 2000 µg alpha-HCH (> 95%) per litre in a 50-day
study, the EC50, based on mortality and immobilization, was 800
(600-1200) µg/litre (Canton et al., 1975).
In a study by Janssen et al. (1987), fertilized eggs of Oryzia
latipes were exposed for 35 days (up to 28 days after hatching) to
alpha-HCH. No influence on growth, mortality or behaviour was seen at
800 µg/litre.
9.5 Terrestrial organisms
No data on terrestrial organisms are available.
Table 5. Acute toxicity (48 h) of alpha-hexachlorocyclohexane for fish (growth inhibition/mortality or immobilization)
Species Age Temperature Parameter Concentration References
(°C) (mg/litre)
Freshwater
Poecilia reticulata 3-4 weeks 24 EC50a 0.8 (0.6-1.2) Canton et al. (1975)
Salmo gairdneri 4 weeks 12 EC50a 1.05 (0.9-1.2) Canton et al. (1975)
Saltwatera
Poecilia reticulata 3 weeks 24 EC50b 1.38 (1.35-1.42) Canton et al. (1978)
Poecilia reticulata LC50 3.5 Boulekbache (1980
a closed system
b water renewal system
PART B
ENVIRONMENTAL HEALTH CRITERIA FOR BETA-HEXACHLOROCYCLOHEXANE
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR BETA-HEXACHLOROCYCLOHEXANE
1. SUMMARY AND EVALUATION
1.1. General properties
1.2. Environmental transport, distribution, and
transformation
1.3. Environmental levels and human exposure
1.4. Kinetics and metabolism
1.5. Effects on organisms in the environment
1.6. Effects on experimental animals and
in vitro test systems
1.7. Effects on humans
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity of primary constituent
2.2. Physical and chemical properties
2.3. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and distribution between media
4.2. Biotransformation and bioaccumulation
4.2.1. Biodegradation
4.2.2. Abiotic degradation
4.2.3. Bioaccumulation
4.2.3.1 Aquatic invertebrates
4.2.3.2 Fish
4.2.3.3 Birds
4.2.3.4 Bioaccumulation in humans
4.3. Isomerization
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
5.1.2. Water
5.1.2.1 Fresh water
5.1.2.2 Sea water
5.1.3. Soil/sediment
5.1.3.1 Dumping grounds
5.1.4. Food and feed
5.1.5. Terrestrial and aquatic organisms
5.1.5.1 Aquatic organism
5.1.5.2 Birds
5.1.5.3 Mammals
5.2. General population exposure
5.2.1. Total-diet studies
5.2.2. Concentrations in human samples
5.2.2.1 Blood
5.2.2.2 Adipose tissue
5.2.2.3 Breast milk
6. KINETICS AND METABOLISM
6.1. Absorption and elimination
6.2. Distribution
6.3. Transplacental transfer and transfer via lactation
6.4. Metabolic transformation
6.4.1. Rat
6.4.2. Mouse
6.4.3. Human
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1. Acute toxicity data
7.1.1. Oral
7.1.2. Intraperitoneal
7.2. Short-term exposure
7.2.1. Mouse oral studies
7.2.2. Rat oral studies
7.3. Skin and eye irritation; sensitization
7.4. Long-term exposure
7.4.1. Rat oral studies
7.5. Reproduction, embryotoxicity, and
teratogenicity
7.5.1. Reproduction
7.5.2. Teratogenicity
7.6. Mutagenicity and related end-points
7.7. Carcinogenicity
7.7.1. Mouse
7.7.2. Rat
7.7.3. Initiation-promotion
7.7.4. Mode of action
7.8. Special studies
7.8.1. Effects on endocrine organs
7.8.2. Neurotoxicity
7.8.3. Effect on liver enzymes
7.8.4. Immunosuppression
8. EFFECTS ON HUMANS
8.1. Acute toxicity - poisoning incidents
8.2. General population
8.3. Occupational exposure
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1. Algae
9.2. Protozoa
9.3. Invertebrates
9.4. Fish
9.4.1. Acute toxicity
9.4.2. Longer-term toxicity
9.5. Terrestrial organisms
9.5.1. Birds
9.6. Model ecosystem studies
1. SUMMARY AND EVALUATION
1.1 General properties
Beta-hexachlorocyclohexane (beta-HCH) is a by-product (7-10%) in
the manufacture of lindane (> 99% gamma-HCH). Its solubility in water
is low, but it is very soluble in organic solvents such as acetone,
cyclohexane, and xylene. It is a solid with a low vapour pressure.
The n-octanol/water partition coefficient (log Pow) is 3.80. It
is an environmental pollutant.
Beta-HCH can be determined separately from the other isomers by
gas chromatography with electron capture detection and other methods
after extraction by liquid/liquid partition and purification by column
chromatography.
1.2 Environmental transport, distribution, and transformation
Biodegradation and abiotic degradation (dechlorination) by
ultraviolet irradiation occur in the environment and produce
pentachlorocyclohexane, but at a much slower rate than in the case of
lindane (gamma-HCH).
Beta-HCH is the most persistent HCH isomer. Its persistence in
soil is determined by environmental factors such as the action of
microorganisms, content of organic matter and water, and
co-distillation and evaporation from soil.
Owing to the persistence of beta-HCH, rapid bioconcentration takes
place in invertebrates (the bioconcentration factor is approximately
125 within 3 days), fish (250-1500 on a dry weight basis or
approximately 500 000 times on a lipid basis within 3-10 days), birds
and man (approximately 525). The bioconcentration is higher and the
elimination is slower for beta-HCH than for the other HCH isomers.
1.3 Environmental levels and human exposure
Beta-HCH is found in air over the oceans at a concentration of
0.004-0.13 ng/m3.
Until 1974, the River Rhine and its tributaries contained beta-HCH
levels of 0.14-0.22 µg/litre, but thereafter the levels were
consistently below 0.1 µg per litre. Samples from the River Meuse
also contained < 0.1 µg/litre. In the River Elbe, levels decreased
from an average of 0.009 to 0.004 µg/litre between 1981 and 1988.
Beta-HCH has been measured in birds such as sparrowhawks,
kestrels, owls, herons, and grebe over a number of years and the
concentrations ranged from 0.1 to 0.3 mg/kg. Up to 0.87 mg/kg (on a
fat basis) has been found in the liver and adipose tissue of the polar
bear.
Important food items have been analysed in a few countries for the
presence of beta-HCH. The mean concentrations, mainly in
fat-containing food products, ranged up to 0.03 mg/kg (on a fat
basis), but in milk products levels up to 4 mg/kg (on a fat basis)
were found. In non-fatty food items, the levels were < 0.005 mg/kg
product. In general, levels are slowly decreasing.
Food is the main source for general population exposure to
beta-HCH. In total-diet studies in the United Kingdom, 0.003, 0.0005,
and < 0.0005 mg/kg were found for the years 1966-1967, 1975-1977, and
1981, respectively. In the USA, the average daily intake of beta-HCH
in 1982-1984 ranged from < 0.1-0.4 ng/kg body weight for various age
groups.
In a number of countries, the concentration of beta-HCH has been
determined in the blood, serum, or plasma of the general population.
The concentrations varied between the different countries and ranged
up to 25 µg/litre.
Many studies have been carried out to determine the presence of
beta-HCH in human adipose tissues. The concentrations found in Canada,
Germany, Kenya, the Netherlands, and the United Kingdom ranged up to
4.4 mg/kg (on a fat basis). A gradual increase with age was found up
to approximately 50 years; thereafter levels decreased. Beta-HCH
concentrations in adipose tissues are higher than those of the other
HCH isomers, a phenomenon that reflects the accumulative properties of
beta-HCH. There is, in general, no clear trend for a decrease in
beta-HCH concentrations over the period that studies have been made.
There is a relationship between the concentrations in adipose tissue
and breast milk and the consumption of meat products, animal fat, and
fatty fish.
In a few countries (Canada, Germany, the Netherlands, and the
United Kingdom), breast milk has been analysed and beta-HCH levels of
between 0.1 and 0.69 mg/kg (on a fat basis) have been found. The
levels in the milk of women living in rural areas appears to be higher
than in urban areas.
The high beta-HCH levels that have been found in breast milk
exceed permissible concentrations temporarily and locally. The
beta-HCH concentrations in the blood of babies lie within the same
range as those in the mothers.
Beta-HCH appears to be a universal environmental contaminant.
Concentrations are only decreasing very slowly in spite of measures
taken to prevent its spread into the environment.
1.4 Kinetics and metabolism
Up to 95% of beta-HCH in the mouse gastrointestinal tract is
absorbed, most of it being subsequently accumulated in adipose tissue.
The elimination follows a 2-stage mechanism, the half-life for the
first stage being 2.5 days and for the second stage 18 days.
After absorption, beta-HCH is rapidly distributed to the liver,
brain, kidneys, and adipose tissues. The maximum concentration in the
liver is reached in rats after 4 days. At an average blood
concentration of 92 µg/litre (but also with concentrations of 540 and
2100 µg per litre), the brain to blood and adipose tissue to blood
ratios were 2:1 and 170:1, respectively. After lethal acute human
poisoning with HCH isomers, the beta-HCH concentration, relative to
that of blood, was 363 in fat, 3 in the brain, and 15 in the liver.
Beta-HCH passes the blood-brain barrier much less readily than the
other HCH isomers.
Transplacental transfer from pregnant mice to their fetuses was
about 2% of the dose, but in rats a transfer of 40% was found.
Lactational transfer in rats from dams to sucklings via milk was about
60% of the dose.
In rats 70% of beta-HCH is eliminated during 28 days, one third of
this being excreted in the urine. No unchanged beta-HCH is present in
the urine. The major metabolite resulting from cis-dehydrochlorination
is 2,4,6-trichlorophenol in a conjugated form.
Pretreatment with beta-HCH alters the metabolism of lindane in
rats. From intraperitoneal studies with mice, it seems that beta-HCH
is metabolized more slowly than lindane.
1.5 Effects on organisms in the environment
Beta-HCH generally has moderate toxicity for algae, invertebrates,
and fish. The acute LD50 values for these organisms are of
the order of 1 mg/litre, but the EC50 values are lower
(0.05-0.5 mg/litre). The no-observed-effect level for Oryzia latipes
and Poecilia reticulata, two freshwater fish exposed for 1 or 3
months, was 0.03 mg/litre.
No data are available on effects on populations and ecosystems.
1.6 Effects on experimental animals and in vitro test systems
The acute oral LD50 values for mice and rats were reported in
1968 to lie between 1500 and 2000 mg/kg body weight. However, more
recent studies yielded values of 16 g/kg body weight for mice and
8 g/kg body weight for rats. Signs of intoxication were mainly of
neurological origin.
Two short-term mouse studies, with dose levels of up to 600 mg/kg
diet for 26-32 weeks, showed increased liver weight and nodular
hyperplasia and atypical proliferations in the liver. In a third
study, dose levels of up to 500 mg/kg diet for 24 weeks did not result
in liver tumours or nodular hyperplasia.
A 90-day study with rats fed 50 or 250 mg/kg diet revealed liver
changes, i.e. hypertrophy and proliferation of smooth endoplasmic
reticulum and increased activity of microsomal enzymes. Changes in
the gonads occurred at the highest dose levels but these were
associated with severe effects on body weight. Hormonal changes
associated with the gonadal atrophy showed no consistent endocrine
effect. There were no adverse effects at a dose level of 2 mg/kg diet
(equivalent to 0.1 mg/kg body weight).
In a long-term rat study (reported in 1950), doses of 10 mg/kg
diet (equivalent to 0.5 mg/kg body weight) or more led to liver
enlargement and histological changes.
In a two-generation reproduction study on rats, the same effects
were found as in the 90-day study. There were no effects at 2 mg/kg
diet (equivalent to 0.1 mg/kg body weight), but a dose level of
10 mg/kg diet resulted in increased mortality and infertility. No
compound-related teratogenic effects were found in an extension to
this study.
A weak "estrogenic" effect has been described. The uterus was the
target organ for this effect; there were no clear effects on endocrine
control systems. The mechanism and significance of this effect are
uncertain.
The mutagenicity studies reported did not show any increase in
mutation frequency in Salmonella typhimurium strains. An in vivo
bone marrow metaphase analysis in rats yielded positive results.
Two studies have been carried out on mice to determine
carcinogenic potential. In one study, 200 mg/kg diet was given for
110 weeks, and liver enlargement, hyperplastic changes, and an
increase in benign and malignant tumours were reported. In the other
study, where 500 mg/kg diet was administered for 24 weeks, no tumours
were observed.
Studies in which rats were fed combinations of beta-HCH with
polychlorinated biphenyls suggested a promoting effect of beta-HCH.
At 300 mg/kg diet, beta-HCH caused significant changes in several
immune functions in mice within one month.
1.7 Effects on humans
When workers at a lindane-producing factory, with a geometric mean
exposure of 7.2 years (1-30), were investigated, it was concluded that
occupational HCH exposure did not induce signs of neurological
impairment or perturbation of "neuromuscular function".
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity of primary constituent
Common name Beta-hexachlorocyclohexane (beta-HCH)
Chemical formula C6H6Cl6
Chemical Beta-HCH is a stereoisomer of gamma-HCH,
structure the active ingredient of lindane (> 99%
(see Annex I) gamma-HCH). It differs in the spatial
orientation of the hydrogen and chlorine
atoms on the carbon atoms:
 Relative
molecular mass 290.9
CAS chemical 1alpha,2ß,3alpha,4ß,5alpha,6ß-hexachloro-
name cyclohexane
Common
synonym Beta-benzenehexachloride (beta-BHC)
CAS registry
number
Relative
molecular mass 290.9
CAS chemical 1alpha,2ß,3alpha,4ß,5alpha,6ß-hexachloro-
name cyclohexane
Common
synonym Beta-benzenehexachloride (beta-BHC)
CAS registry
number 