
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 142
ALPHA - CYPERMETHRIN
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
First draft prepared by Dr E.A.H. van
Heemstra-Lequin and Dr G.T. van Esch,
Netherlands
World Health Orgnization
Geneva, 1992
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
Alpha-cypermethrin.
(Environmental health criteria ; 142)
1.Environmental exposure 2.Pyrethrins - adverse effects
3.Pyrethrins - toxicity I.Series
ISBN 92 4 157142 X (NLM Classification: WA 240)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1992
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR ALPHA-CYPERMETHRIN
INTRODUCTION
1. SUMMARY AND EVALUATION; CONCLUSIONS AND RECOMMENDATIONS
1.1. Summary and evaluation
1.1.1. Identity, use, environmental fate and
environmental levels
1.1.2. Kinetics and metabolism
1.1.3. Effects on laboratory mammals and
in vitro test systems
1.1.4. Effects on humans
1.1.5. Effects on other organisms in the
laboratory and field
1.2. Conclusions
1.2.1. General population
1.2.2. Occupational exposure
1.2.3. Environment
1.3. Recommendations
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
2.1. Identity
2.1.1. Primary constituent
2.1.2. Technical product
2.2. Physical and chemical properties
2.3. Formulations
2.4. Conversion factors
2.5. Analytical methods
2.5.1. Sampling
2.5.1.1 Air
2.5.1.2 Surface-wipe
2.5.2. Methods for determination
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Anthropogenic sources
3.2.1. Production levels and processes
3.2.2. Use
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1. Transport and distribution between media
4.1.1. Air
4.1.2. Water
4.1.3. Soil
4.2. Biotransformation
4.2.1. Biodegradation
4.2.2. Bioaccumulation
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Soil
5.2. Food
5.2.1. Crops
5.2.2. Fish
5.2.3. Milk
5.3. Human exposure
6. KINETICS AND METABOLISM
6.1. Absorption, elimination, retention and turnover
6.1.1. Rats
6.1.2. Domestic animals
6.1.3. Humans
6.2. Metabolic transformation
6.3. In vitro metabolic transformation
6.4. Plants
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1. Single exposure
7.1.1. Oral (technical product)
7.1.2. Oral (formulations)
7.1.3. Dermal
7.1.4. Inhalation
7.1.5. Other routes
7.2. Short-term exposure
7.2.1. Oral
7.2.1.1 Rat
7.2.1.2 Dog
7.3. Skin and eye irritation; sensitization
7.3.1. Skin irritation
7.3.2. Eye irritation
7.3.3. Sensitization
7.4. Long-term and carcinogenicity studies
7.5. Reproduction, embryotoxicity and teratogenicity
7.6. Mutagenicity and related end-points
7.6.1. Mutation
7.6.2. Chromosomal effects
7.6.3. DNA damage
7.6.4. Conclusion
7.7. Special studies
7.7.1. Skin sensation
7.7.2. Neurotoxicity
7.7.3. Immunosuppressive action
7.8. Mechanism of toxicity - mode of action
8. EFFECTS ON HUMANS
8.1. General population exposure
8.2. Occupational exposure
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1. Microorganisms
9.1.1. Algae
9.1.2. Bacteria
9.2. Aquatic organisms
9.2.1. Invertebrates
9.2.1.1 Laboratory studies
9.2.1.2 Field studies
9.2.2. Fish
9.2.2.1 Laboratory studies
9.2.2.2 Small scale field or outdoor tank
studies
9.3. Terrestrial organisms
9.3.1. Earthworms
9.3.2. Invertebrates - field studies
9.3.3. Honey-bees
9.3.3.1 Laboratory studies
9.3.3.2 Field studies
9.3.4. Leaf-cutting bees
9.3.5. Birds
10. COMPARISON BETWEEN ALPHA-CYPERMETHRIN AND CYPERMETHRIN
10.1. Use and residue levels
10.2. Environmental impact
10.3. Mammalian toxicity
11. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
APPENDIX I
RESUME ET EVALUATION; CONCLUSIONS ET RECOMMANDATIONS
RESUMEN Y EVALUACION; CONCLUSIONES Y RECOMENDACIONES
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ALPHA-CYPERMETHRIN
Members
Dr V. Benes, Department of Toxicology and Reference Laboratory,
Institute of Hygiene and Epidemiology, Prague, Czechoslovakia
Dr R. Drew, Key Centre for Toxicology, Department of Applied Biology,
Royal Melbourne Institute for Technology, Melbourne, Australia
(Chairman)
Dr S.K. Kashyap, National Institute of Occupational Health, Meghani
Nagar, Ahmedabad, India
Dr J.I. Kundiev, Research Institute of Labour, Hygiene and
Occupational Diseases, Ul. Saksaganskogo, Kiev, USSR
(Vice-Chairman)
Dr K. Mitsumori, Division of Pathology, Biological Safety Research
Center, National Institute of Hygienic Sciences, Setagaya-ku,
Tokyo, Japan
Dr R.F. Shore, Ecotoxicology and Pollution Section, Institute of
Terrestrial Ecology, Monks Wood Experimental Station, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr G.J. van Esch, Bilthoven, Netherlands (Joint Rapporteur)
Dr E.A.H. van Heemstra-Lequin, Laren, Netherlands (Joint Rapporteur)
Dr S. Wong, Bureau of Chemical Hazards, Environmental Health
Directorate, Department of National Health and Welfare, Tunney's
Pasture, Ottawa, Ontario, Canada
Observers
Dr W.H. Gross, Fraunhofer Institute of Toxicology and Aerosol
Research, Hanover, Germany
Dr J.R. Kielhorn, Fraunhofer Institute of Toxicology and Aerosol
Research, Hanover, Germany
Dr C.M. Melber, Fraunhofer Institute of Toxicology and Aerosol
Research, Hanover, Germany
Dr D.E. Owen, Shell Internationale Petroleum Maatschappij BV, The
Hague, Netherlands
Secretariat
Dr R.F. Hertel, Fraunhofer Institute of Toxicology and Aerosol
Research, Hanover, Germany
Dr K.W. Jager, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Mrs C. Partensky, Unit of Carcinogen Identification and Evaluation,
International Agency for Research on Cancer, Lyon, France
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are kindly requested to communicate any
errors that may have occurred to the Director of the International
Programme on Chemical Safety, World Health Organization, Geneva,
Switzerland, in order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Palais des
Nations, 1211 Geneva 10, Switzerland (Telephone No. 7988400 or
7985850).
* * *
The proprietary information contained in this monograph cannot
replace documentation for registration purposes, because the latter
has to be closely linked to the source, the manufacturing route, and
the purity/impurities of the substance to be registered. The data
should be used in accordance with paragraphs 82-84 and recommendations
paragraph 90 of the Second FAO Government Consultation (1982).
ENVIRONMENTAL HEALTH CRITERIA FOR ALPHA-CYPERMETHRIN
A WHO Task Group on Environmental Health Criteria for
Alpha-cypermethrin met at the Fraunhofer Institute of Toxicology and
Aerosol Research, Hanover, Germany, from 16 to 20 September 1990, and
was sponsored by the German Ministry of the Environment. Dr R.F.
Hertel welcomed the participants on behalf of the host institute. Dr
K.W. Jager, IPCS, welcomed the participants on behalf of Dr M.
Mercier, Director of the IPCS, and the three IPCS cooperating
organizations (UNEP/ILO/WHO). The Group reviewed and revised the
draft document and made an evaluation of the risks for human health
and the environment from exposure to alpha-cypermethrin
The first draft was prepared by Dr E.A.H. van Heemstra-Lequin and
Dr G.J. van Esch of the Netherlands. Dr van Esch prepared the second
draft, incorporating the comments received following circulation of
the first draft to the IPCS contact points for Environmental Health
Criteria monographs.
Dr K.W. Jager and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the technical development and
editing, respectively.
The assistance of Shell in making available to the IPCS and the
Task Group its proprietary toxicological information on
alpha-cypermethrin is gratefully acknowledged. This allowed the Task
Group to make its evaluation on the basis of more complete data.
* * *
Partial financial support for the publication of this monograph
was kindly provided by the United States Department of Health and
Human Services through a contract from the National Institute of
Environmental Health Sciences, Research Triangle Park, North Carolina,
USA - a WHO Collaborating Centre for Environmental Health Effects.
ABBREVIATIONS
CPA cyclopropane carboxylic acid
EC emulsifiable concentrate
EEC European Economic Community
GC gas chromatography
MRL maximum residue level
MS mass spectrophotometry
NOEL no-observed-effect level
OECD Organisation for Economic Co-operation and Development
OSC oil-enhanced suspension concentrate
PBA phenoxybenzoic acid
SC suspension concentrate
ULV ultra-low volume
WP wettable powder
INTRODUCTION
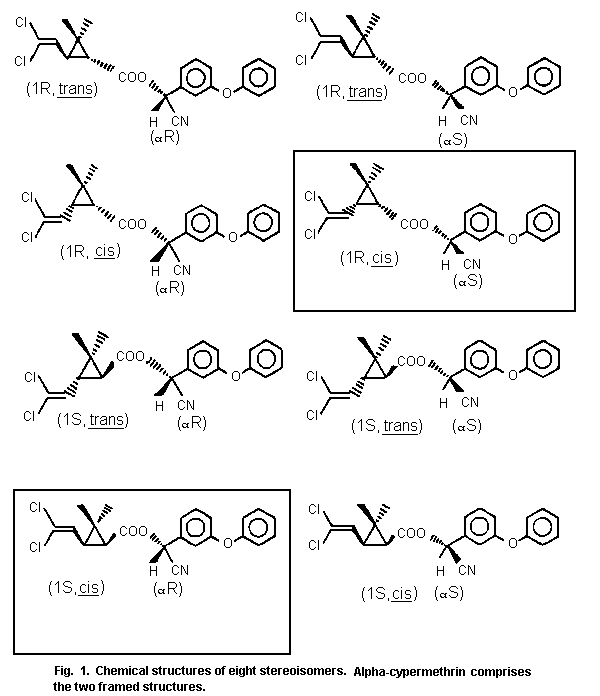
Cypermethrin (alpha-cyano-3-phenoxybenzyl-3-(2,2-dichloro-vinyl)
-2,2-dimethylcyclopropanecarboxylate) is a racemic mixture of eight
isomers. These eight isomers consist of two groups, those with a cis
orientation across the cyclopropyl ring of the dichlorovinyl and ester
groups and those with a trans orientation.
Alpha-cypermethrin is a mixture of two of the four cis isomers
present to approximately 25% in cypermethrin, i.e. the (1R, cis)S and
the (1S, cis)R isomers. The structure of the eight isomers is
summarized in Fig. 1.
In this monograph the toxicological information specifically
related to alpha-cypermethrin is summarized and compared with the data
on cypermethrin. An evaluation of the full data on cypermethrin, which
is also relevant for alpha-cypermethrin, is given in Environmental
Health Criteria 82: Cypermethrin (WHO, 1989). The summary, evaluation,
conclusions and recommendations of that monograph are added here as
Appendix I.
1. SUMMARY AND EVALUATION; CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Identity, use, environmental fate and environmental levels
Alpha-cypermethrin contains more than 90% of the insecticidally
most active enantiomer pair of the four cis isomers of cypermethrin as
a racemic mixture.
It is a highly active pyrethroid insecticide, effective against
a wide range of pests encountered in agriculture and animal husbandry.
It is supplied as emulsifiable concentrate, ultra-low-volume
formulation, suspension concentrate and in mixtures with other
insecticides.
The technical product is a crystalline powder with good
solubility in acetone, cyclohexanone and xylene, but its solubility in
water is low. It is stable under acidic and neutral conditions but
hydrolyses at pH 12-13. It decomposes above 220 °C.
No information on levels of alpha-cypermethrin in air is
available.
In water, alpha-cypermethrin is likely to be degraded by
photochemical and biological processes. Surface and sub-surface water
in a pond oversprayed with 15 g/ha active ingredient contained 5% and
19% of the applied dose one day after spraying and 0.1% and 2% of the
applied dose seven days later. About 5% of the applied dose was
present in sediment 16 days after application.
Alpha-cypermethrin is likely to be absorbed strongly onto soil
particles. Residues in soil were below 0.1 mg/kg one year after
treatment with 0.5 kg active ingredient per ha.
The n-octanol/water partition coefficient of alpha-cypermethrin
is 1.4 x 105 (log Pow = 5.16).
The recommended application rates of alpha-cypermethrin are lower
than those of cypermethrin because the former is biologically more
active. As a result, residues on crops are low, and following the use
of recommended application rates the residues in crops are between
0.05 and 1 mg/kg. Residues in marine catfish treated at between 0.001
and 0.05% w/w active ingredient were 0.3-30 mg/kg one week after
storage and 0.22-4.0 mg/kg after 15 weeks of storage.
1.1.2 Kinetics and metabolism
Alpha-cypermethrin administered orally to rats is eliminated, in
the urine, as the sulfate conjugate of 3-(4-hydroxyphenoxy) benzoic
acid and, in the faeces, partly as unchanged compound. Approximately
90% of a single oral dose is eliminated from the body over a 4-day
period, 78% within the first day. Residues in tissues are low except
in fat tissue. The concentration in fat 3 days after a single oral
dose of 2 mg/kg was 0.4 mg/kg. Elimination from the fat is biphasic;
the half-life for the initial phase is 2.5 days and for the second
phase 17-26 days.
Alpha-cypermethrin is metabolized by cleavage of its ester bond.
In the rat, the phenoxybenzyl alcohol portion of the molecule is
hydroxylated and conjugated with sulfate; the cyclopropane carboxylic
acid portion is also conjugated (probably as a glucuronide) prior to
urinary excretion. Studies with liver microsomes from rats, rabbits
and man have demonstrated that esteric hydrolysis and oxidative
pathways can occur in all three species but esteric hydrolysis is the
more prominent pathway for liver preparations from rabbit and man.
In humans, 43% of an oral dose (0.25-0.75 mg) was excreted within
24 h in the urine as free or conjugated cis-cyclopropane carboxylic
acid. The urinary excretion was not increased after five successive
daily doses.
High concentrations (up to 1156 mg/kg) of alpha-cypermethrin were
found in the wool of sheep 14 days after the application of a dip or
pour-on formulation. Low levels were found in subcutaneous fat (up to
0.04 mg/kg). After treating calves along the mid-dorsal line with 10
ml of a 1.6% formulation, no alpha-cypermethrin was found in muscle
and liver. The maximum concentration in perirenal fat over a 14-day
period was 0.26 mg/kg.
After treating lactating cows along the mid-dorsal line with up
to 0.2 g active ingredient, alpha-cypermethrin residues of 0.003 to
0.005 mg/litre were found in the milk from 3 out of 15 treated
animals.
1.1.3 Effects on laboratory mammals and in vitro test systems
Alpha-cypermethrin has moderate to high acute oral toxicity to
rodents. The LD50 values in mice and rats are highly variable and
depend on the concentration of the compound and vehicle. For practical
purposes an LD50 value of 80 mg/kg body weight is considered
representative. However, some reported acute oral LD50 values are
higher. Acute oral exposure results in clinical signs associated with
central nervous system activity.
Single dermal applications of alpha-cypermethrin to mice and rats
at 100 and 500 mg/kg body weight, respectively, did not cause
mortality or signs of intoxication. Similarly, a 4-h inhalation
exposure of rats to an atmospheric concentration of 400 mg/m3 did
not result in mortality or clinical signs.
Technical alpha-cypermethrin has been reported to be minimally
irritating to rabbit skin. Some alpha-cypermethrin formulations cause
severe eye irritation. Technical alpha-cypermethrin is not a skin
sensitizer. In guinea-pigs, alpha-cypermethrin caused stimulation of
sensory nerve-endings in the skin.
Short-term exposure of rats to alpha-cypermethrin at
concentrations up to 200 mg/kg diet per day for 5 weeks or up to 180
mg/kg diet per day for 13 weeks did not cause toxic effects. At higher
dose levels, rats exhibited signs of intoxication associated with
pathology of the nervous system, decreased growth, or increased liver
and kidney weights. No clear haematological, clinical chemistry or
histopathological effects were evident.
In a 13-week oral dog study, the highest dose of 270 mg/kg diet
caused signs of intoxication, but all other parameters examined
(including haematology, clinical chemistry, urinalysis, organ weights,
gross pathology and histopathology) were unaffected. The
no-observed-effect level (NOEL) was 90 mg/kg diet (equivalent to 2.25
mg/kg body weight per day).
An oral study in rats demonstrated that alpha-cypermethrin
induces neurotoxicity due to histopathological alterations of the
tibial and sciatic nerves, axonal degeneration and increased
beta-galactosidase activity.
No data are available on long-term toxicity, reproductive
toxicity, teratogenicity or immunotoxicity.
From the available data on alpha-cypermethrin, it can be
concluded that this compound is non-mutagenic in tests with
Salmonella typhimurium, Escherichia coli and Saccharomyces
cerevisiae, and in vivo and in vitro tests with rat liver cells
for the induction of chromosome aberration and production of DNA
single-strand damage. No increase in chromosomal aberrations was seen
in rat bone marrow cells.
No data are available on the carcinogenicity of alpha-
cypermethrin.
1.1.4 Effects on humans
Exposure of the general population to alpha-cypermethrin is
negligible, provided its use follows good agricultural practice.
Occupational dermal exposure in operators during mixing/loading,
during spraying and washing of the equipment was found to be up to
2.94 mg, 0.61 mg and 0.73 mg, respectively.
In a study of exposure to alpha-cypermethrin during formulation,
exposure levels were assessed by personal and static monitoring of
atmospheric concentrations and measurement of urinary
alpha-cypermethrin metabolites. The group mean personal exposure
levels on the two days while formulating technical concentrates were
2.8 and 4.9 mg/m3, whereas the group mean personal exposure to
technical material on day 3 was 54.1 mg/m3. No metabolites could be
detected in urine (limit of detection, 0.02 mg/litre). During
formulation, skin sensations were reported but these were only mild.
No poisoning incidents have been reported.
1.1.5 Effects on other organisms in the laboratory and field
The 48 and 96-h EC50 (growth) value for the freshwater alga
Selenastrum capricornutum is above 100 µg/litre.
Alpha-cypermethrin is highly toxic to aquatic invertebrates. The
24- and 48-h EC50 (immobilization) values for Daphnia magna are
1.0 and 0.3 µg/litre, respectively, and the 24-h LC50 value for
Gammarus pulex is 0.05 µg/litre. Alpha-cypermethrin is highly toxic
to a number of aquatic arthropod taxa, but is of lower toxicity to
molluscs. The short-term toxicity of the compound can be reduced by
formulation of the product as an oil-enhanced suspension. Although
spray drift may result in toxic effects on aquatic invertebrates, the
rapid loss of alpha-cypermethrin from the water gives potential for
recovery.
Alpha-cypermethrin is highly toxic to fish. The 96-h LC50
values range between 0.7 and 350 µg/litre depending upon the
formulation. Emulsifiable concentrate formulations are much more toxic
than suspension concentrate, wettable powder and micro-encapsulated
formulations. The hazard of alpha-cypermethrin to aquatic
invertebrates and fish lies in its acute toxicity. There is no
evidence for the occurrence of cumulative effects as a result of
long-term exposure.
No data are available concerning the effects of alpha-
cypermethrin on soil microbes. Sewage bacteria were not affected by a
concentration of 3 mg/litre in a closed system.
The toxicity of alpha-cypermethrin to certain Carabid beetles and
neuropteran larvae is relatively low, and there is limited hazard to
pre-adult stages of parasitoid Hymenoptera. Small-plot and large-scale
field studies have demonstrated a low hazard of alpha-cypermethrin to
Carabid and Staphylinid beetles but a relatively high hazard to
Linyphiid spiders. The effects on populations were limited to a single
growing season. Furthermore, alpha-cypermethrin has a low hazard to
Syrphid larvae but has a significant effect on Coccinellids. However,
the rapid dissipation of the residues on foliage gives the potential
for these animals to recolonize rapidly.
Field application of alpha-cypermethrin had no adverse effects on
the relative abundance of entomophages within the arthropod
communities. Its use in small grain cereals would not be associated
with pest "resurgence" or the development of secondary pest
infestations.
In laboratory tests, the toxicity of alpha-cypermethrin to
earthworms is low. No mortality was recorded after 14 days for worms
exposed to up to 100 mg/kg of artificial soil.
In laboratory acute toxicity tests, alpha-cypermethrin was found
to be highly toxic to bees. Oral administration of an emulsifiable
concentrate formulation gave a 24-h LD50 of 0.13 µg/bee, whereas the
corresponding value for topical administration was 0.03 µg/bee
(technical product) or 0.11 µg/bee (EC). The high toxicity of
alpha-cypermethrin to bees did not manifest itself in field trials,
probably as a result of the short-lived repellent effect of
alpha-cypermethrin which causes a decline in bee foraging behaviour
and, thus, in exposure.
No data for the toxicity of alpha-cypermethrin to birds are
available.
1.2 Conclusions
1.2.1 General population
When applied according to good agricultural practice, exposure of
the general population to alpha-cypermethrin is low and is unlikely to
present a hazard.
1.2.2 Occupational exposure
With good work practices, hygiene measures, and safety
precautions, the use of alpha-cypermethrin is unlikely to present a
hazard to those occupationally exposed to it. The occurrence of
"facial sensations" is an indication of exposure. Under these
circumstances work practices should be reviewed.
1.2.3 Environment
With recommended application rates, it is unlikely that
alpha-cypermethrin will attain levels of environmental significance.
It is highly toxic to aquatic arthropods, fish and honey-bees under
laboratory conditions. Significant toxic effects on non-target
invertebrates and fish are only likely to occur in cases of spillage,
overspraying and misuse.
1.3 Recommendations
* Contamination of surface waters with alpha-cypermethrin should be
avoided.
* Alpha-cypermethrin binds strongly to particles. Further
ecotoxicological studies on the effects of alpha-cypermethrin on
sediment-dwelling organisms should be carried out, since this
subject seems to have received little attention.
* The gastrointestinal absorption of alpha-cypermethrin should be
investigated under various conditions.
* The fate of dermally applied alpha-cypermethrin should be
investigated.
* Further information on the long-term toxicity/carcinogenicity and
immunotoxicity of alpha-cypermethrin should be obtained.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
2.1.1 Primary constituent
Chemical structure: racemic mixture of the two stereoisomers
indicated by boxes in Fig. 1
Empirical formula: C22H19NO3Cl2
Relative molecular
mass: 416.3
Chemical name:
(IUPAC) a racemate comprising (S)-alpha-cyano-3-
phenoxybenzyl (1R,3R)-3-(2,2-dichloro-
vinyl)-2,2-dimethylcyclopropanecarboxy-late
and (R)-alpha-cyano-3-phenoxybenzyl
(1S,3S)-3-(2,2-dichlorovinyl)-2,2-dimethyl-
cyclopropanecarboxylate; a racemate
comprising (S)-alpha-cyano-3-phenoxybenzyl
(1R)- cis-3(2,2-dichloro-vinyl)-2,2-
dimethylcyclopropanecarboxylate and (R)-
alpha-cyano-3-phenoxybenzyl (1S)- cis-3-
(2,2-dichlorovinyl)-2,2-dimethylcyclopro-
panecarboxylate
(Chemical [1alpha(S*),3alpha}-(±)-cyano(3-phenoxy-
Abstracts) phenyl)methyl 3-(2,2-dichloroethenyl)-2,2-
dimethylcyclopropanecarboxylate (9CI)
(From: Worthing & Hance, 1991)
Common name: alpha-cypermethrin (alphamethrin and
alfoxylate are non-official names)
Code numbers: WL 85 871; OMS 3004
CAS registry
number: [67375-30-8] correct stereochemistry;
[52315-07-8] (formerly [69865-74-0],
[86752-99-0], [86753-92-6] cypermethrin (no
stereochemistry stated) were sometimes used
in Chemical Abstracts)
 2.1.2 Technical product
Common trade
names: Fastac, Concord, Fendona, Renegade
Purity: technical grade: > 90% pure (m/m)
Impurities: no data.
2.2 Physical and chemical properties
Alpha-cypermethrin is a racemic mixture of the two stereo-isomers
(1:1) indicated by boxes in Fig. 1 and is a crystalline powder. Some
physical and chemical properties of alpha-cypermethrin are given in
Table 1.
Table 1. Physical and chemical properties of alpha-cypermethrin (pure
enantiomeric pair; purity > 99%)
Boiling point 200 °C at 9.3 N/m2
Melting point 80.5 °C
Vapour pressure (20 °C) 170 nPa (1.7 x 107 N/m2)
Density 1.12 g/cm3 at 20 °C
1.28 g/cm3 at 22 °C
Solubility (25 °C) 0.005-0.01 mg/litre water; 620 g/litre
acetone; 515 g/litre cyclohexanone; 7 g/kg
hexane; 351 g/litre xylene
Stability It is stable under acidic or neutral
conditions (pH 3-7) but hydrolyses in
strongly alkaline media (pH 12-13). It
decomposes above 220 °C. Field data indicate
that in practice it is stable to air and
light.
Partition coefficient
n-octanol/water log Pow 5.16 (Pow = 1.4 x 105)
From: Langner (1980); Shell (1983a); Worthing & Hance (1991).
The water solubility of alpha-cypermethrin (98.0%), calculated as
the sum of the cis-1 and the cis-2 isomer (ratio 2.6:97.4)
concentrations, at 20 °C in 0.01 M buffers at pH values of
approximately 4 to 9, ranges from 4.59 to 7.87 µg/litre, as measured
by the OECD and EEC microcolumn techniques. In distilled water alone
the solubility is slightly less, i.e. 2.06 µg/litre. The solubility is
not strongly dependent on pH values within the range of 4 to 9. It is
likely that ionic strength differences account for differences in
solubility between values in pure water and in the buffer solutions
(Baldwin, 1990).
2.3 Formulations
The following formulations exist:
* "Fastac", EC (20-100 g/litre), WP (50 g/kg), SC (15-250
g/litre), ULV (6 to 15 g/litre);
* "Fendona" and "Renegade", EC (50 or 100 g/litre), SC (250
g/litre), WP (50 g/kg).
Combination with other active ingredients also exist, e.g.,
"Azofas" (alpha-cypermethrin and monocrotophos) and combinations of
alpha-cypermethrin with methomyl or Fenobucarb (Worthing & Hance,
1991).
2.4 Conversion factors
1 ppm = 17.02 mg/m3
1 mg/m3 = 0.059 ppm
2.5 Analytical methods
2.5.1 Sampling
2.5.1.1 Air
Samples are collected by drawing a measured volume of air through
a 37-mm diameter silver membrane filter with a glass fibre pre-filter.
They are analysed for total pyrethroid content ( cis- and
trans-cypermethrin isomers) by gas chromatography with electron
capture detection (ECD). The limit of determination is 0.01 µg/filter
(see Table 2) (Armitage, 1984).
2.5.1.2 Surface-wipe
Surface-wipe samples are collected using a filter paper wetted
with diethyl ether. These samples are analysed for total pyrethroid
content ( cis- and trans-cypermethrin isomers) by gas
chromatography with flame ionization detection (FID). The limit of
determination is 0.03 mg/filter (see Table 2) (Armitage, 1984).
2.5.2 Methods for determination
A method for the determination of alpha-cypermethrin in technical
material and formulated products, excluding suspension concentrates,
was described by Shell (1987a). This method is also used to determine
the ratio of the enantiomer pairs cis 1 to cis 2.
The alpha-cypermethrin content is determined by means of
high-performance liquid chromatography (HPLC), using a column packed
with Zorbax SIL, together with ultraviolet detection at 230 nm
(Shell, 1987a).
Methods have been described for the determination of
alpha-cypermethrin in water, soil, crops, and animal tissues and
fluids (see Table 2).
Table 2. Analytical methods for alpha-cypermethrin in air, soil, water and biological mediaa
Sample Extraction Clean-up Detection and Recovery Limit of References
quantification determination
Air 20% ethyl acetate column chromatography gas chromatography with - 0.01 µg/filter Armitage (1984)
in hexane chromosorb W.HP. electron capture detection
Surface 20% ethyl acetate column chromatography gas chromatography with - 30 µg/filter Armitage (1984)
wipe in hexane chromosorb W.HP. flame ionization detection
Soil anhydrous sodium liquid-solid packed column gas 95-100%b 10 µg/kg Shell (1990b)
sulfate with chromatography chromatography, electron capture
acetone/hexane using Florisil detection; confirmation by
capillary GC and packed column
GC-MS
Water solvent partition Florisil disposable capillary gas-liquid 80-100%c 0.01 µg/litre Shell (1990a)
with hexane cartridge chromatography, electron-capture
detection; confirmation by GC-MS
Crops anhydrous sodium partition between packed column gas 90-100%b 10 µg/kg Shell (1989a)
sulfate with hexane and water/ chromatography, electron capture
acetone/hexane acetonitrile; liquid- detection; confirmation by
solid chromatography capillary GC and packed
using Florisil column GC-MS
Animal acetone/hexane partition with gas-liquid chromatography, 80-100%d 10 µg/kg Shell (1988a)
tissues mixture acetonitrile or electron capture detection;
hexane-acetonitrile; confirmation by GC-MS
liquid-solid
chromatography on
Florisil
Milk diethyl ether/ cyano Bond Elut gas-liquid chromatography, 90-100%e 1 µg/litre Shell (1988b)
hexane; Extrelut cartridge electron capture detection;
extraction column confirmation by GC-MS
Table 2 (continued)
Sample Extraction Clean-up Detection and Recovery Limit of References
quantification determination
Blood acetone partition with hexane packed column gas - 10 µg/litre Shell (1986)
(rat) (washed with water); chromatography, electron capture
dried with sodium detection; confirmation by
sulfate; liquid-solid capillary GC and GC-MS
chromatography on
Florisil
a Details of the analytical methods are available from Shell International Chemical Company, London. These methods differentiate
between alpha-cypermethrin and the other isomers.
b Over the concentration range 0.05-0.5 mg/kg
c Over the concentration range 0.05-0.5 µg/litre
d Concentrations 0.1-0.2 mg/kg
e Over the concentration range 0.005-0.02 mg/litre
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Alpha-cypermethrin does not occur in nature.
3.2 Anthropogenic sources
3.2.1 Production levels and processes
Alpha-cypermethrin is manufactured from cis-2,2,dimethyl-3-
(2",2"-dichlorovinyl)-cyclopropane carboxylate ( cis-DVO), 3-phenoxy
benzaldehyde (POAL) and sodium cyanide.
After removal of the solvent, the cis-cypermethrin is
epimerized into alpha-cypermethrin. Solid alpha-cypermethrin crystals
separate and are filtered, washed and dried under vacuum before
drumming-off1.
No data are available on production levels.
3.2.2 Use
Alpha-cypermethrin has been available commercially since late
1983. It is a potent insecticide effective against a wide range of
pests, particularly Lepidoptera and Coleoptera in citrus, cotton,
forestry, fruit, rice, soybeans, tomatoes, vegetables, grapes and
other crops, at a concentration of 5-30 g active ingredient per ha.
Good control of plant-sucking Hemiptera can also be obtained if the
insecticide is applied before populations have become established. It
also controls soil-dwelling Lepidoptera.
Alpha-cypermethrin can be used in most crops for either curative
or preventive treatment. It can replace conventional insecticides in
short-interval spray programmes, or the longer residual performance
may be exploited to reduce the number of sprays per season. Either
option may be chosen since no reports of phytotoxicity have been
received even when sensitive crops have been involved in repeated
applications. It controls ectoparasites ( Boophilus microplus at a
concentration of 50 mg/litre), including strains resistant to
organophosphorus pesticides, as well as sheep lice and Melophagus
ovinus.
1 Manufacturing process of alpha-cypermethrin; Shell International
Chemical Company; letter dated 10 January 1989 (ref. CTMAR/4)
Rapid knockdown and residual control of biting flies in and
around animal housing have been obtained following direct spray
application to animals or structural surfaces. Furthermore,
alpha-cypermethrin controls Blattellidae, Culicidae, flies and other
nuisance or disease-carrying insects, at a level of 10-30 mg/m2,
with good persistence on most surfaces (Fisher et al., 1983; Worthing
& Hance, 1991).
Alpha-cypermethrin is available as an emulsifiable concentrate,
ultra-low-volume formulation and suspension concentrate (flowable
formulations). Mixtures with organophosphorus and carbamate
insecticides have also been developed. Details of formulations are
given in section 2.3.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
Data relevant to alpha-cypermethrin can be found in Environmental
Health Criteria 82: Cypermethrin (WHO, 1989).
4.1.1 Air
No information on the transport of alpha-cypermethrin in air is
available, but its volatility is very low.
4.1.2 Water
Alpha-cypermethrin as an emulsifiable concentrate (EC) was
sprayed from the air (15 g active ingredient/ha) to a field along one
side of which ran a freshwater ditch. The fate and biological effects
of spray drift in the ditch were monitored for 7 weeks after the
application (see sections 9.2.1.2 and 9.2.2.2). Deposition on the
surface of the ditch was around 5 g active ingredient/ha (30% of the
nominal application rate). Alpha-cypermethrin concentrations in the
sub-surface water were 0.6 µg/litre shortly after the application and
decreased to < 0.02 µg/litre within 2 to 4 days. No contamination of
the water was found 200 m beyond either end of the treated field
(Garforth & Woodbridge, 1984).
Two freshwater ponds were treated with an EC formulation of
alpha-cypermethrin in 1987. One pond was oversprayed with 15 g active
ingredient/ha, while the other was treated with the same amount of
alpha-cypermethrin but by direct incorporation into the water. A third
pond served as a control. One day after the treatment, 5% of the
applied substance was found in the surface film of the oversprayed
pond and 19% in the sub-surface water. Residue levels in both
compartments subsequently declined rapidly so that one week later only
0.1 and 2% were still present, respectively. In the pond that received
direct treatment, 37% of the applied alpha-cypermethrin was found in
the sub-surface water one day after treatment. The concentration
subsequently declined more rapidly than in the oversprayed pond so
that one week later only 2% was present. The concentration of alpha-
cypermethrin found in the sediment samples from both ponds 16 days
after treatment indicated that approximately 5% of the
alpha-cypermethrin applied was present at that time. Thereafter the
concentration decreased and was less than 3% in the sediment 33 days
after application. In a bioassay test, the water in both ponds was
found to be acutely toxic to Gammarus pulex for at least 4 days
after application. After a further 29 days, the water was no longer
acutely toxic. The sediment was not toxic to Gammarus pulex
(Pearson, 1990).
4.1.3 Soil
A trial in the United Kingdom (Reculver) investigated the decay
of alpha-cypermethrin in sandy-clay soil treated with a diluted EC
formulation at a dosage rate of 0.5 kg active ingredient per ha.
Samples of soil were taken from the 0-15 cm layer of each plot at
various intervals over a period of one year. Once a year a sample was
also taken from the 15-30 cm layer. The residue immediately after the
application was 0.07 mg/kg soil in the 0-15 cm layer, and within 2
weeks this had declined by 50%. The residues of alpha-cypermethrin in
samples from the 0-15 cm layer and 15-30 cm layer taken 40 weeks and
52 weeks after application were below the limit of determination, i.e.
0.01 mg/kg (Forbes & Knight, 1983).
After one year, a second application to the bare soil was made
and again a diluted EC formulation was applied at a dosage rate of 0.5
kg active ingredient/ha. Samples were taken at various intervals
during this second year. Residues of alpha-cypermethrin in the 0-15 cm
soil layer declined from 0.19 mg/kg immediately after treatment to
0.11 mg/kg after 2.1 weeks and < 0.01 mg/kg after 49 weeks. In
samples from the 15-30 cm layer no residues (< 0.01 mg/kg) were
detectable 23 and 49 weeks after application (Forbes & Burden, 1984).
In the third year of the trial, another application to the same plots
was made with the EC formulation at a dosage rate of 0.5 kg active
ingredient/ha. Residues of alpha-cypermethrin in the 0-15 cm layer
declined from 0.20 mg/kg immediately after treatment to 0.08 mg/kg
after 18 weeks and 0.01 mg/kg after 52 weeks. Residues were not
detectable in the 15-30 cm layer sampled after 32 and 52 weeks. Over
the three years of the trial there was no indication of a build-up of
alpha-cypermethrin residues in the surface soil layer or any evidence
to suggest leaching of the compound into sub-surface soil layers
(Forbes & Wales, 1985a).
A further trial was carried out in the United Kingdom (Coates) to
study the decay of alpha-cypermethrin applied to a peat type soil as
a diluted EC formulation at a dosage rate of 0.5 kg active
ingredient/ha. As in the Reculver study, residues were determined in
the 0-15 cm layer at various intervals and in the 15-30 cm layer 32
weeks after application. At the beginning of the second and third
year, one application was made as at the beginning of the first year.
The residue in the 0-15 cm layer immediately after the first
application was 0.65 mg/kg declining to 0.36 mg/kg within 2 weeks and
to 0.30 mg/kg after 8 weeks. After 16 weeks, the residue was 0.05
mg/kg or less. In the 15-30 cm layer, no residues were found after 32
weeks (Forbes & Mackay, 1983). Immediately after the second
application, the residue in the 0-15 cm layer was 0.65 mg/kg; after
two weeks the level was 0.36 mg/kg and declined to 0.07 mg/kg by 48
weeks after application. No residues were found in the 15-30 cm layer
(Forbes & Wales, 1985b).
In the third year, a residue level of 0.55 mg/kg was found in the
0-15 cm layer immediately after treatment, declining to 0.20 mg/kg
within 8 weeks and to 0.09 mg/kg after 50 weeks. In the 15-30 cm
layer, residues of 0.01 and 0.03 mg/kg were found after 40 and 50
weeks respectively. In this 3-year trial there was no indication of a
build-up of alpha-cypermethrin residues in the surface soil layers, or
any evidence to suggest significant leaching into sub-surface soil
layers (Coveney & Forbes, 1986).
4.2 Biotransformation
4.2.1 Biodegradation
Alpha-cypermethrin has been tested for "ready biodegradability"
in two tests: a) the closed bottle and modified Sturm test, and b)
growth inhibition in a Pseudomonas fluorescens growth test. In these
tests, mineralization of alpha-cypermethrin was not detected. It was
not degraded in these two tests and hence is not considered to be
readily biodegradable (Stone & Watkinson, 1983).
Maloney et al. (1988) studied the microbial transformation of
technical alpha-cypermethrin (96.3% pure) in aerobic batch enrichment
cultures. These microbial enrichments, which contained Pseudomonas
fluorescens (SM-1), Achromobacter sp. and Bacillus cereus, were
able to transform alpha-cypermethrin with a half-life of 7 to 14 days
at a concentration of 50 mg/litre in the presence of 0.05% Tween 80
(v/v). One of the major transformation products was 3-phenoxybenzoic
acid, which was further transformed to 4-hydroxy-3-phenoxybenzoic
acid.
McMinn (1983a) investigated the degradation under aerobic
conditions of alpha-cypermethrin, labelled with 14C in the benzyl
ring, in two types of soil, i.e. sandy clay loam and clay loam. The
soils were treated with 1 mg of the labelled material and gently
agitated to distribute the insecticide. Soils samples were removed for
analysis 2.5, 6, 10, 20 and 42 weeks after treatment. The initial
degradation half-lives were 27 and 13 weeks for sandy clay loam and
clay loam, respectively. However, after 42 weeks the percentage of
applied radioactivity remaining unchanged was 28.9 and 21.6%,
respectively, for the two soils. The formation of total organo-soluble
products after 42 weeks was 32.2 and 24.3% for sandy clay loam and
clay loam, respectively. Total extractable and total non-extractable
radioactivity for sandy clay loam was 32.5 and 18.0% and for clay loam
25.3 and 32.0%, respectively. Metabolites were found in both cases at
levels of 2 to 3%. Unchanged alpha-cypermethrin was present, and the
degradation products had similar chromatographic mobilities to the
previously identified major products of cypermethrin (McMinn, 1983b).
4.2.2 Bioaccumulation
The n-octanol/water partition coefficient of alpha-cypermethrin
is 1.4 x 105 (log Pow = 5.16), compared to a value for
cypermethrin of 2 x 106 (log Pow = 6.3). The actual
bioaccumulation in fish found experimentally for cypermethrin is lower
than might be expected from the partition coefficient. This should
also apply to alpha-cypermethrin, because the pathway and rate of
metabolism are comparable with those of cypermethrin (Shell, 1983b;
WHO, 1989).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
Information relevant to alpha-cypermethrin was given in
Environmental Health Criteria 82: Cypermethrin (WHO, 1989).
5.1.1 Soil
In a study on the deposition of alpha-cypermethrin on the orchard
floor following commercial application to apple trees,
alpha-cypermethrin (100 g EC/litre) was applied at a nominal dose rate
of 26 g/ha using a tractor-driven "Kinkelder" mist-blower. Following
normal practice, spray runs were made between each row of trees and
then around the perimeter of the orchard. One hour after spraying,
pesticide deposits were collected in foil-lined trays positioned on
the orchard floor and analysed. Deposition was found to be variable,
ranging from 10 to 76% of the nominal application rate (Hillaby,
1988).
5.2 Food
5.2.1 Crops
Residue data on cypermethrin have been evaluated by the Joint
FAO/WHO Meeting on Pesticide Residues (FAO/WHO, l980, 1982).
Alpha-cypermethrin application rates to crops range from 5 to 30
g active ingredient/ha. Residue data have been obtained from
supervised trials in many countries. The residue concentrations of
alpha-cypermethrin derived from recommended application rates vary
from 0.05 to 1.0 mg/kg product (Shell, 1984).
A study was carried out to determine whether there was
significant isomerization of alpha-cypermethrin after treatment of
certain crops. Grapes were treated with 10% EC applied at a rate of 18
g active ingredient/ha, and apples and lettuce with 10% EC at 15 g
active ingredient/ha. Samples were taken 3 and 7 days (grapes), 7 days
(apples) and 10 days (lettuce) after treatment. Residues of 0.17 and
0.09 mg/kg were found on grapes, 0.05 mg/kg on apples and 0.17 mg/kg
on lettuce, but none of the samples showed any significant isomer
conversion of alpha-cypermethrin (Bosio, 1982).
In 1983 two trials were carried out in Canada in which
alpha-cypermethrin was applied with a knapsack sprayer to maize
(sweetcorn). Five applications with diluted 10% EC formulations at a
dosage rate of 20 g active ingredient/ha were made, samples were
harvested 7 days after the last application, and the husks, grain and
cobs were analysed separately. Alpha-cypermethrin residues of 0.38
mg/kg were found in the husks, but no residues (limit of
determination, 0.01 mg/kg) were found in the grain or the cobs (Forbes
& Cole, 1986).
5.2.2 Fish
To reduce blow-fly infestations during the curing of marine
catfish, the fish were dipped in EC solutions (15 g/litre) at various
concentrations (0.001-0.05% active ingredient w/v) between the salting
and drying stages of the curing process. Dipping after the salting
stage in a 0.001% solution of the EC proved to be effective. The
levels of residues in treated fish were dependent on the season (wet
and dry season), storage time, concentration of the dip solution and
the size of the fish. In the wet season, the range was from 0.9 to 2.8
mg/kg, whereas in the dry season it was from 0.26 to 30.0 after one
week of storage and 0.22 to 4.0 mg/kg (wet weight of homogenized fish)
after 15 weeks of storage (Forbes, 1985).
5.2.3 Milk
A trial was carried out in 1987 in the United Kingdom where
lactating cows were treated with pour-on formulations of
alpha-cypermethrin. Two formulations were used containing either 10
g/litre or 15 g/litre (see also section 6.1.2). Either 10 ml or 20 ml
of formulation containing 0.1, 0.15 or 0.2 g active ingredient was
applied along the mid-dorsal line of five cows for each treatment.
Milk samples were taken 1, 2, 3, 4, 7, 14 and 21 days after treatment
for the analysis of alpha-cypermethrin. The residues of
alpha-cypermethrin in milk were at a maximum from 2 to 4 days after
treatment. Generally, residues were highest in the 0.2 g group, the
maximum concentration being 0.005 mg/litre (in two samples only). By
day 21 the residues in the milk from all treated cows were < 0.002
mg/litre (the limit of determination) (Sherren, 1988b).
5.3 Human exposure
In a study to quantify the maximum potential dermal exposure of
operators to crop protection products, 13 exposure pads were mounted
on each of three operators and chemicals adhering to gloves were
analysed. The operation involved three distinct stages: mixing product
and loading the tractor; spraying; and washing-up the equipment and
tractor after the exercise. The total dermal exposure for the three
operators was: mixing/loading, 2.45, 0.57 and 2.94 mg/operation;
spraying, 0.38, 0.61 and 0.40 mg/h; and washing-up, 0.12, 0.29 and
0.73 mg/operation (Senior & Lavers, 1990a,b).
6. KINETICS AND METABOLISM
Both cis and trans isomers of cypermethrin are metabolized via
cleavage of the ester bond to phenoxybenzoic acid (PBA) and
cyclopropane carboxylic acid (CPA). The PBA moiety is mainly excreted
as a conjugate. The type of conjugate differs in a number of animal
species. PBA is further metabolized to a hydroxy derivative and
conjugated as a glucuronate or sulfate. The CPA moiety is mainly
excreted as a glucuronate. Consistent with the lipophilic nature of
cypermethrin, the highest tissue concentrations are found in body fat,
skin, liver, kidneys, adrenals and ovaries. The elimination from fat
is approximately 3 to 4 times slower for the cis isomers than for the
trans isomers (WHO, 1989).
6.1 Absorption, elimination, retention and turnover
6.1.1 Rats
Alpha-cypermethrin labelled in the 14C-benzyl moiety has been
studied in Wistar rats at a concentration of approximately 2 mg/kg
body weight in corn oil. The compound, which was given by stomach
tube, was rapidly broken down and the radioactivity was mainly
eliminated in the urine as the sulfate conjugate of
3-(4-hydroxyphenoxy)benzoic acid (40-45% of the dose). Approximately
35% of the dose was eliminated in the faeces, 20% of which was
unchanged alpha-cypermethrin. The proportion of the dose excreted in
the urine and faeces within the first 24 h was approximately 78% and
within 4 days was 90%. Residues in major organs and tissues of rats 4
days after a single oral dose were in general low: liver, 0.03 and
0.05; skin, 0.04 and 0.02; adrenals, 0.03 and 0.06; and kidneys, 0.02
and 0.02 (values are expressed as mg equivalent of
alpha-cypermethrin/kg tissue for females and males, respectively).
However, in body fat, higher residues were found (0.22 and 0.42
mg/kg). The release from skin and fat was biphasic in nature. The
half-life of elimination of radioactivity from fat was approximately
2.5 days for the initial phase and 17-26 days for the slower phase
(the half-life of elimination from fat for cis-cypermethrin was 18.9
days). The half-life values for skin were 2 days for the initial phase
and 40 days for the slower phase. The radioactivity in liver and
kidneys was eliminated apparently by a monophasic process. More than
95% of the residue in fat was present as unchanged alpha-cypermethrin
(Hutson, 1982; Logan, 1983; Hutson & Logan, 1986).
6.1.2 Domestic animals
In a study by Francis & Gill (1991), a formulation containing a
mixture of flufenoxuron and alpha-cypermethrin was applied to groups
of three sheep. The formulation was applied once, either as a dip
diluted at 1:1000 to give a solution of 80 mg flufenoxuron per litre
and 60 mg alpha-cypermethrin/litre or as a pour-on solution applied
directly to the backs of the sheep giving a dose of 0.15 g active
ingredient flufenoxuron and 0.2 g alpha-cypermethrin per sheep. The
sheep were killed at 3, 7 and 14 days after application and samples of
subcutaneous fat, fleece and sheep skin were analysed. The residues of
alpha-cypermethrin in fat ranged from < 0.01 to 0.04 mg/kg and in
skin from 0.02 to 1.4 mg/kg over the three sampling periods, and they
were lower for pour-on formulations than for the dip. Highest tissue
residues were found in wool (sampled from the back); these were (for
the 3, 7 and 14 day sampling periods, respectively) 730, 1020 and 360
mg/kg for dip application and 360, 440 and 360 mg/kg for pour-on
application. Wool sampled from the side of sheep treated with pour-on
formulation were 10 to 30 times lower than wool sampled from the back
region; with the dip solution, however, wool from the side region
contained higher residues than that from the back. Pour-on application
gave lower residues than after a dip.
A trial was carried out during 1987 in the United Kingdom in
which Friesian/Hereford calves (in total 17 female animals) were
treated with an alpha-cypermethrin pour-on formulation. Ten ml of a 16
g/litre formulation was applied to calves along the middorsal line
from shoulder to tail. At 3, 7 and 14 days following treatment,
animals were sacrificed for analysis of tissues, i.e. perirenal and
subcutaneous fat, muscle, kidneys and liver. No residues were detected
in muscle and liver samples at any time (limit of determination, 0.01
mg/kg). In the kidneys a maximum of 0.03 mg/kg was found on day 7 but
by day 14 the residues had decreased to 0.01 mg/kg or less. The fat
tissues contained maximum levels on day 7, i.e. mean concentrations of
0.26 mg/kg (perirenal fat) and 0.08 mg/kg (subcutaneous fat). By day
14 these concentrations had decreased by about two and a half times
(Sherren, 1988a) (see also section 5.2.3).
6.1.3 Humans
Six volunteers (two per dose level) received a single oral dose
of 0.25, 0.5 or 0.75 mg alpha-cypermethrin and, after a period of 2-3
weeks, five successive daily doses of 0.25, 0.5 or 0.75 mg to study
the urinary excretion and bioaccumulation of alpha-cypermethrin. A
parallel study with cypermethrin itself was carried out for comparison
purposes. The metabolism and rate of excretion of a single oral dose
of alpha-cypermethrin were similar to those of cypermethrin itself.
The rate of excretion was dose-related, approximately 43% of the dose
of alpha-cypermethrin being excreted in the urine as free or
conjugated cis-cyclopropane carboxylic acid ( cis-CPA) during the
first 24 h. Urinary excretion did not increase with repeated oral
dosing; an average of 49% of alpha-cypermethrin was excreted in the
urine as free or conjugated cis-CPA within 24 h (van Sittert et al.,
1985; Eadsforth et al., 1988).
6.2 Metabolic transformation
In a study on Wistar rats using alpha-cypermethrin,
14C-labelled in the benzyl moiety, (see section 6.1.1) no evidence
was found for any racemization of the chiral centres of
alpha-cypermethrin in the residues in intestines, faeces or fat. The
major urinary metabolite was the sulfate conjugate of
3-(4-hydroxyphenoxy)benzoic acid, and smaller amounts of
3-phenoxybenzoic acid (II) and 3-(4-hydroxyphenoxy)benzoic acid (III)
were identified. In the faeces, 75% of the radioactivity in the
extract was unchanged alpha-cypermethrin; minor metabolites included
a dihydroxy metabolite (V), 3-(4-hydroxyphenoxy)benzoic acid (III),
3-phenoxybenzoic acid (II) and the 4-hydroxyphenoxy metabolite (IV).
In the adipose tissue, the 14C label was mainly associated with
unchanged alpha-cypermethrin, but a lipophilic metabolite of either
alpha-cypermethrin or 3-phenoxybenzoic acid, probably a mixture of
3-phenoxybenzoyl diacylglycerols, was also present (Hutson, 1982;
Logan, 1983; Hutson & Logan, 1986) (see Fig. 2).
6.3 In vitro metabolic transformation
Creedy & Logan (1984) studied the in vitro metabolism of
cypermethrin and alpha-cypermethrin using liver microsomal
preparations from rats, rabbits and humans. In order to obtain
information on the relative importance of the oxidative and esteric
pathways of degradation of these compounds, incubations were carried
out both in the presence and absence of an NADPH-generating system.
Both cypermethrin and alpha-cypermethrin were broken down via esteric
and oxidative pathways by the liver preparations from the three
species. For rabbit and human liver microsomes, oxidation was a minor
metabolic route compared to esteric hydrolysis in the case of both
compounds. Human liver microsomes were able to carry out the esteric
hydrolysis of alpha-cypermethrin slightly faster than cypermethrin. In
the liver preparations of all three species, cyclopropane carboxylic
acid (mainly produced via the esteric pathway) was the main metabolite
for both compounds (to the extent of approximately 90-99%). Via the
oxidative route, mono-hydroxycypermethrins, dihydroxy-cypermethrin and
small amounts of hydroxycyclopropane carboxylic acid (rat only) were
also produced.
2.1.2 Technical product
Common trade
names: Fastac, Concord, Fendona, Renegade
Purity: technical grade: > 90% pure (m/m)
Impurities: no data.
2.2 Physical and chemical properties
Alpha-cypermethrin is a racemic mixture of the two stereo-isomers
(1:1) indicated by boxes in Fig. 1 and is a crystalline powder. Some
physical and chemical properties of alpha-cypermethrin are given in
Table 1.
Table 1. Physical and chemical properties of alpha-cypermethrin (pure
enantiomeric pair; purity > 99%)
Boiling point 200 °C at 9.3 N/m2
Melting point 80.5 °C
Vapour pressure (20 °C) 170 nPa (1.7 x 107 N/m2)
Density 1.12 g/cm3 at 20 °C
1.28 g/cm3 at 22 °C
Solubility (25 °C) 0.005-0.01 mg/litre water; 620 g/litre
acetone; 515 g/litre cyclohexanone; 7 g/kg
hexane; 351 g/litre xylene
Stability It is stable under acidic or neutral
conditions (pH 3-7) but hydrolyses in
strongly alkaline media (pH 12-13). It
decomposes above 220 °C. Field data indicate
that in practice it is stable to air and
light.
Partition coefficient
n-octanol/water log Pow 5.16 (Pow = 1.4 x 105)
From: Langner (1980); Shell (1983a); Worthing & Hance (1991).
The water solubility of alpha-cypermethrin (98.0%), calculated as
the sum of the cis-1 and the cis-2 isomer (ratio 2.6:97.4)
concentrations, at 20 °C in 0.01 M buffers at pH values of
approximately 4 to 9, ranges from 4.59 to 7.87 µg/litre, as measured
by the OECD and EEC microcolumn techniques. In distilled water alone
the solubility is slightly less, i.e. 2.06 µg/litre. The solubility is
not strongly dependent on pH values within the range of 4 to 9. It is
likely that ionic strength differences account for differences in
solubility between values in pure water and in the buffer solutions
(Baldwin, 1990).
2.3 Formulations
The following formulations exist:
* "Fastac", EC (20-100 g/litre), WP (50 g/kg), SC (15-250
g/litre), ULV (6 to 15 g/litre);
* "Fendona" and "Renegade", EC (50 or 100 g/litre), SC (250
g/litre), WP (50 g/kg).
Combination with other active ingredients also exist, e.g.,
"Azofas" (alpha-cypermethrin and monocrotophos) and combinations of
alpha-cypermethrin with methomyl or Fenobucarb (Worthing & Hance,
1991).
2.4 Conversion factors
1 ppm = 17.02 mg/m3
1 mg/m3 = 0.059 ppm
2.5 Analytical methods
2.5.1 Sampling
2.5.1.1 Air
Samples are collected by drawing a measured volume of air through
a 37-mm diameter silver membrane filter with a glass fibre pre-filter.
They are analysed for total pyrethroid content ( cis- and
trans-cypermethrin isomers) by gas chromatography with electron
capture detection (ECD). The limit of determination is 0.01 µg/filter
(see Table 2) (Armitage, 1984).
2.5.1.2 Surface-wipe
Surface-wipe samples are collected using a filter paper wetted
with diethyl ether. These samples are analysed for total pyrethroid
content ( cis- and trans-cypermethrin isomers) by gas
chromatography with flame ionization detection (FID). The limit of
determination is 0.03 mg/filter (see Table 2) (Armitage, 1984).
2.5.2 Methods for determination
A method for the determination of alpha-cypermethrin in technical
material and formulated products, excluding suspension concentrates,
was described by Shell (1987a). This method is also used to determine
the ratio of the enantiomer pairs cis 1 to cis 2.
The alpha-cypermethrin content is determined by means of
high-performance liquid chromatography (HPLC), using a column packed
with Zorbax SIL, together with ultraviolet detection at 230 nm
(Shell, 1987a).
Methods have been described for the determination of
alpha-cypermethrin in water, soil, crops, and animal tissues and
fluids (see Table 2).
Table 2. Analytical methods for alpha-cypermethrin in air, soil, water and biological mediaa
Sample Extraction Clean-up Detection and Recovery Limit of References
quantification determination
Air 20% ethyl acetate column chromatography gas chromatography with - 0.01 µg/filter Armitage (1984)
in hexane chromosorb W.HP. electron capture detection
Surface 20% ethyl acetate column chromatography gas chromatography with - 30 µg/filter Armitage (1984)
wipe in hexane chromosorb W.HP. flame ionization detection
Soil anhydrous sodium liquid-solid packed column gas 95-100%b 10 µg/kg Shell (1990b)
sulfate with chromatography chromatography, electron capture
acetone/hexane using Florisil detection; confirmation by
capillary GC and packed column
GC-MS
Water solvent partition Florisil disposable capillary gas-liquid 80-100%c 0.01 µg/litre Shell (1990a)
with hexane cartridge chromatography, electron-capture
detection; confirmation by GC-MS
Crops anhydrous sodium partition between packed column gas 90-100%b 10 µg/kg Shell (1989a)
sulfate with hexane and water/ chromatography, electron capture
acetone/hexane acetonitrile; liquid- detection; confirmation by
solid chromatography capillary GC and packed
using Florisil column GC-MS
Animal acetone/hexane partition with gas-liquid chromatography, 80-100%d 10 µg/kg Shell (1988a)
tissues mixture acetonitrile or electron capture detection;
hexane-acetonitrile; confirmation by GC-MS
liquid-solid
chromatography on
Florisil
Milk diethyl ether/ cyano Bond Elut gas-liquid chromatography, 90-100%e 1 µg/litre Shell (1988b)
hexane; Extrelut cartridge electron capture detection;
extraction column confirmation by GC-MS
Table 2 (continued)
Sample Extraction Clean-up Detection and Recovery Limit of References
quantification determination
Blood acetone partition with hexane packed column gas - 10 µg/litre Shell (1986)
(rat) (washed with water); chromatography, electron capture
dried with sodium detection; confirmation by
sulfate; liquid-solid capillary GC and GC-MS
chromatography on
Florisil
a Details of the analytical methods are available from Shell International Chemical Company, London. These methods differentiate
between alpha-cypermethrin and the other isomers.
b Over the concentration range 0.05-0.5 mg/kg
c Over the concentration range 0.05-0.5 µg/litre
d Concentrations 0.1-0.2 mg/kg
e Over the concentration range 0.005-0.02 mg/litre
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Alpha-cypermethrin does not occur in nature.
3.2 Anthropogenic sources
3.2.1 Production levels and processes
Alpha-cypermethrin is manufactured from cis-2,2,dimethyl-3-
(2",2"-dichlorovinyl)-cyclopropane carboxylate ( cis-DVO), 3-phenoxy
benzaldehyde (POAL) and sodium cyanide.
After removal of the solvent, the cis-cypermethrin is
epimerized into alpha-cypermethrin. Solid alpha-cypermethrin crystals
separate and are filtered, washed and dried under vacuum before
drumming-off1.
No data are available on production levels.
3.2.2 Use
Alpha-cypermethrin has been available commercially since late
1983. It is a potent insecticide effective against a wide range of
pests, particularly Lepidoptera and Coleoptera in citrus, cotton,
forestry, fruit, rice, soybeans, tomatoes, vegetables, grapes and
other crops, at a concentration of 5-30 g active ingredient per ha.
Good control of plant-sucking Hemiptera can also be obtained if the
insecticide is applied before populations have become established. It
also controls soil-dwelling Lepidoptera.
Alpha-cypermethrin can be used in most crops for either curative
or preventive treatment. It can replace conventional insecticides in
short-interval spray programmes, or the longer residual performance
may be exploited to reduce the number of sprays per season. Either
option may be chosen since no reports of phytotoxicity have been
received even when sensitive crops have been involved in repeated
applications. It controls ectoparasites ( Boophilus microplus at a
concentration of 50 mg/litre), including strains resistant to
organophosphorus pesticides, as well as sheep lice and Melophagus
ovinus.
1 Manufacturing process of alpha-cypermethrin; Shell International
Chemical Company; letter dated 10 January 1989 (ref. CTMAR/4)
Rapid knockdown and residual control of biting flies in and
around animal housing have been obtained following direct spray
application to animals or structural surfaces. Furthermore,
alpha-cypermethrin controls Blattellidae, Culicidae, flies and other
nuisance or disease-carrying insects, at a level of 10-30 mg/m2,
with good persistence on most surfaces (Fisher et al., 1983; Worthing
& Hance, 1991).
Alpha-cypermethrin is available as an emulsifiable concentrate,
ultra-low-volume formulation and suspension concentrate (flowable
formulations). Mixtures with organophosphorus and carbamate
insecticides have also been developed. Details of formulations are
given in section 2.3.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
Data relevant to alpha-cypermethrin can be found in Environmental
Health Criteria 82: Cypermethrin (WHO, 1989).
4.1.1 Air
No information on the transport of alpha-cypermethrin in air is
available, but its volatility is very low.
4.1.2 Water
Alpha-cypermethrin as an emulsifiable concentrate (EC) was
sprayed from the air (15 g active ingredient/ha) to a field along one
side of which ran a freshwater ditch. The fate and biological effects
of spray drift in the ditch were monitored for 7 weeks after the
application (see sections 9.2.1.2 and 9.2.2.2). Deposition on the
surface of the ditch was around 5 g active ingredient/ha (30% of the
nominal application rate). Alpha-cypermethrin concentrations in the
sub-surface water were 0.6 µg/litre shortly after the application and
decreased to < 0.02 µg/litre within 2 to 4 days. No contamination of
the water was found 200 m beyond either end of the treated field
(Garforth & Woodbridge, 1984).
Two freshwater ponds were treated with an EC formulation of
alpha-cypermethrin in 1987. One pond was oversprayed with 15 g active
ingredient/ha, while the other was treated with the same amount of
alpha-cypermethrin but by direct incorporation into the water. A third
pond served as a control. One day after the treatment, 5% of the
applied substance was found in the surface film of the oversprayed
pond and 19% in the sub-surface water. Residue levels in both
compartments subsequently declined rapidly so that one week later only
0.1 and 2% were still present, respectively. In the pond that received
direct treatment, 37% of the applied alpha-cypermethrin was found in
the sub-surface water one day after treatment. The concentration
subsequently declined more rapidly than in the oversprayed pond so
that one week later only 2% was present. The concentration of alpha-
cypermethrin found in the sediment samples from both ponds 16 days
after treatment indicated that approximately 5% of the
alpha-cypermethrin applied was present at that time. Thereafter the
concentration decreased and was less than 3% in the sediment 33 days
after application. In a bioassay test, the water in both ponds was
found to be acutely toxic to Gammarus pulex for at least 4 days
after application. After a further 29 days, the water was no longer
acutely toxic. The sediment was not toxic to Gammarus pulex
(Pearson, 1990).
4.1.3 Soil
A trial in the United Kingdom (Reculver) investigated the decay
of alpha-cypermethrin in sandy-clay soil treated with a diluted EC
formulation at a dosage rate of 0.5 kg active ingredient per ha.
Samples of soil were taken from the 0-15 cm layer of each plot at
various intervals over a period of one year. Once a year a sample was
also taken from the 15-30 cm layer. The residue immediately after the
application was 0.07 mg/kg soil in the 0-15 cm layer, and within 2
weeks this had declined by 50%. The residues of alpha-cypermethrin in
samples from the 0-15 cm layer and 15-30 cm layer taken 40 weeks and
52 weeks after application were below the limit of determination, i.e.
0.01 mg/kg (Forbes & Knight, 1983).
After one year, a second application to the bare soil was made
and again a diluted EC formulation was applied at a dosage rate of 0.5
kg active ingredient/ha. Samples were taken at various intervals
during this second year. Residues of alpha-cypermethrin in the 0-15 cm
soil layer declined from 0.19 mg/kg immediately after treatment to
0.11 mg/kg after 2.1 weeks and < 0.01 mg/kg after 49 weeks. In
samples from the 15-30 cm layer no residues (< 0.01 mg/kg) were
detectable 23 and 49 weeks after application (Forbes & Burden, 1984).
In the third year of the trial, another application to the same plots
was made with the EC formulation at a dosage rate of 0.5 kg active
ingredient/ha. Residues of alpha-cypermethrin in the 0-15 cm layer
declined from 0.20 mg/kg immediately after treatment to 0.08 mg/kg
after 18 weeks and 0.01 mg/kg after 52 weeks. Residues were not
detectable in the 15-30 cm layer sampled after 32 and 52 weeks. Over
the three years of the trial there was no indication of a build-up of
alpha-cypermethrin residues in the surface soil layer or any evidence
to suggest leaching of the compound into sub-surface soil layers
(Forbes & Wales, 1985a).
A further trial was carried out in the United Kingdom (Coates) to
study the decay of alpha-cypermethrin applied to a peat type soil as
a diluted EC formulation at a dosage rate of 0.5 kg active
ingredient/ha. As in the Reculver study, residues were determined in
the 0-15 cm layer at various intervals and in the 15-30 cm layer 32
weeks after application. At the beginning of the second and third
year, one application was made as at the beginning of the first year.
The residue in the 0-15 cm layer immediately after the first
application was 0.65 mg/kg declining to 0.36 mg/kg within 2 weeks and
to 0.30 mg/kg after 8 weeks. After 16 weeks, the residue was 0.05
mg/kg or less. In the 15-30 cm layer, no residues were found after 32
weeks (Forbes & Mackay, 1983). Immediately after the second
application, the residue in the 0-15 cm layer was 0.65 mg/kg; after
two weeks the level was 0.36 mg/kg and declined to 0.07 mg/kg by 48
weeks after application. No residues were found in the 15-30 cm layer
(Forbes & Wales, 1985b).
In the third year, a residue level of 0.55 mg/kg was found in the
0-15 cm layer immediately after treatment, declining to 0.20 mg/kg
within 8 weeks and to 0.09 mg/kg after 50 weeks. In the 15-30 cm
layer, residues of 0.01 and 0.03 mg/kg were found after 40 and 50
weeks respectively. In this 3-year trial there was no indication of a
build-up of alpha-cypermethrin residues in the surface soil layers, or
any evidence to suggest significant leaching into sub-surface soil
layers (Coveney & Forbes, 1986).
4.2 Biotransformation
4.2.1 Biodegradation
Alpha-cypermethrin has been tested for "ready biodegradability"
in two tests: a) the closed bottle and modified Sturm test, and b)
growth inhibition in a Pseudomonas fluorescens growth test. In these
tests, mineralization of alpha-cypermethrin was not detected. It was
not degraded in these two tests and hence is not considered to be
readily biodegradable (Stone & Watkinson, 1983).
Maloney et al. (1988) studied the microbial transformation of
technical alpha-cypermethrin (96.3% pure) in aerobic batch enrichment
cultures. These microbial enrichments, which contained Pseudomonas
fluorescens (SM-1), Achromobacter sp. and Bacillus cereus, were
able to transform alpha-cypermethrin with a half-life of 7 to 14 days
at a concentration of 50 mg/litre in the presence of 0.05% Tween 80
(v/v). One of the major transformation products was 3-phenoxybenzoic
acid, which was further transformed to 4-hydroxy-3-phenoxybenzoic
acid.
McMinn (1983a) investigated the degradation under aerobic
conditions of alpha-cypermethrin, labelled with 14C in the benzyl
ring, in two types of soil, i.e. sandy clay loam and clay loam. The
soils were treated with 1 mg of the labelled material and gently
agitated to distribute the insecticide. Soils samples were removed for
analysis 2.5, 6, 10, 20 and 42 weeks after treatment. The initial
degradation half-lives were 27 and 13 weeks for sandy clay loam and
clay loam, respectively. However, after 42 weeks the percentage of
applied radioactivity remaining unchanged was 28.9 and 21.6%,
respectively, for the two soils. The formation of total organo-soluble
products after 42 weeks was 32.2 and 24.3% for sandy clay loam and
clay loam, respectively. Total extractable and total non-extractable
radioactivity for sandy clay loam was 32.5 and 18.0% and for clay loam
25.3 and 32.0%, respectively. Metabolites were found in both cases at
levels of 2 to 3%. Unchanged alpha-cypermethrin was present, and the
degradation products had similar chromatographic mobilities to the
previously identified major products of cypermethrin (McMinn, 1983b).
4.2.2 Bioaccumulation
The n-octanol/water partition coefficient of alpha-cypermethrin
is 1.4 x 105 (log Pow = 5.16), compared to a value for
cypermethrin of 2 x 106 (log Pow = 6.3). The actual
bioaccumulation in fish found experimentally for cypermethrin is lower
than might be expected from the partition coefficient. This should
also apply to alpha-cypermethrin, because the pathway and rate of
metabolism are comparable with those of cypermethrin (Shell, 1983b;
WHO, 1989).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
Information relevant to alpha-cypermethrin was given in
Environmental Health Criteria 82: Cypermethrin (WHO, 1989).
5.1.1 Soil
In a study on the deposition of alpha-cypermethrin on the orchard
floor following commercial application to apple trees,
alpha-cypermethrin (100 g EC/litre) was applied at a nominal dose rate
of 26 g/ha using a tractor-driven "Kinkelder" mist-blower. Following
normal practice, spray runs were made between each row of trees and
then around the perimeter of the orchard. One hour after spraying,
pesticide deposits were collected in foil-lined trays positioned on
the orchard floor and analysed. Deposition was found to be variable,
ranging from 10 to 76% of the nominal application rate (Hillaby,
1988).
5.2 Food
5.2.1 Crops
Residue data on cypermethrin have been evaluated by the Joint
FAO/WHO Meeting on Pesticide Residues (FAO/WHO, l980, 1982).
Alpha-cypermethrin application rates to crops range from 5 to 30
g active ingredient/ha. Residue data have been obtained from
supervised trials in many countries. The residue concentrations of
alpha-cypermethrin derived from recommended application rates vary
from 0.05 to 1.0 mg/kg product (Shell, 1984).
A study was carried out to determine whether there was
significant isomerization of alpha-cypermethrin after treatment of
certain crops. Grapes were treated with 10% EC applied at a rate of 18
g active ingredient/ha, and apples and lettuce with 10% EC at 15 g
active ingredient/ha. Samples were taken 3 and 7 days (grapes), 7 days
(apples) and 10 days (lettuce) after treatment. Residues of 0.17 and
0.09 mg/kg were found on grapes, 0.05 mg/kg on apples and 0.17 mg/kg
on lettuce, but none of the samples showed any significant isomer
conversion of alpha-cypermethrin (Bosio, 1982).
In 1983 two trials were carried out in Canada in which
alpha-cypermethrin was applied with a knapsack sprayer to maize
(sweetcorn). Five applications with diluted 10% EC formulations at a
dosage rate of 20 g active ingredient/ha were made, samples were
harvested 7 days after the last application, and the husks, grain and
cobs were analysed separately. Alpha-cypermethrin residues of 0.38
mg/kg were found in the husks, but no residues (limit of
determination, 0.01 mg/kg) were found in the grain or the cobs (Forbes
& Cole, 1986).
5.2.2 Fish
To reduce blow-fly infestations during the curing of marine
catfish, the fish were dipped in EC solutions (15 g/litre) at various
concentrations (0.001-0.05% active ingredient w/v) between the salting
and drying stages of the curing process. Dipping after the salting
stage in a 0.001% solution of the EC proved to be effective. The
levels of residues in treated fish were dependent on the season (wet
and dry season), storage time, concentration of the dip solution and
the size of the fish. In the wet season, the range was from 0.9 to 2.8
mg/kg, whereas in the dry season it was from 0.26 to 30.0 after one
week of storage and 0.22 to 4.0 mg/kg (wet weight of homogenized fish)
after 15 weeks of storage (Forbes, 1985).
5.2.3 Milk
A trial was carried out in 1987 in the United Kingdom where
lactating cows were treated with pour-on formulations of
alpha-cypermethrin. Two formulations were used containing either 10
g/litre or 15 g/litre (see also section 6.1.2). Either 10 ml or 20 ml
of formulation containing 0.1, 0.15 or 0.2 g active ingredient was
applied along the mid-dorsal line of five cows for each treatment.
Milk samples were taken 1, 2, 3, 4, 7, 14 and 21 days after treatment
for the analysis of alpha-cypermethrin. The residues of
alpha-cypermethrin in milk were at a maximum from 2 to 4 days after
treatment. Generally, residues were highest in the 0.2 g group, the
maximum concentration being 0.005 mg/litre (in two samples only). By
day 21 the residues in the milk from all treated cows were < 0.002
mg/litre (the limit of determination) (Sherren, 1988b).
5.3 Human exposure
In a study to quantify the maximum potential dermal exposure of
operators to crop protection products, 13 exposure pads were mounted
on each of three operators and chemicals adhering to gloves were
analysed. The operation involved three distinct stages: mixing product
and loading the tractor; spraying; and washing-up the equipment and
tractor after the exercise. The total dermal exposure for the three
operators was: mixing/loading, 2.45, 0.57 and 2.94 mg/operation;
spraying, 0.38, 0.61 and 0.40 mg/h; and washing-up, 0.12, 0.29 and
0.73 mg/operation (Senior & Lavers, 1990a,b).
6. KINETICS AND METABOLISM
Both cis and trans isomers of cypermethrin are metabolized via
cleavage of the ester bond to phenoxybenzoic acid (PBA) and
cyclopropane carboxylic acid (CPA). The PBA moiety is mainly excreted
as a conjugate. The type of conjugate differs in a number of animal
species. PBA is further metabolized to a hydroxy derivative and
conjugated as a glucuronate or sulfate. The CPA moiety is mainly
excreted as a glucuronate. Consistent with the lipophilic nature of
cypermethrin, the highest tissue concentrations are found in body fat,
skin, liver, kidneys, adrenals and ovaries. The elimination from fat
is approximately 3 to 4 times slower for the cis isomers than for the
trans isomers (WHO, 1989).
6.1 Absorption, elimination, retention and turnover
6.1.1 Rats
Alpha-cypermethrin labelled in the 14C-benzyl moiety has been
studied in Wistar rats at a concentration of approximately 2 mg/kg
body weight in corn oil. The compound, which was given by stomach
tube, was rapidly broken down and the radioactivity was mainly
eliminated in the urine as the sulfate conjugate of
3-(4-hydroxyphenoxy)benzoic acid (40-45% of the dose). Approximately
35% of the dose was eliminated in the faeces, 20% of which was
unchanged alpha-cypermethrin. The proportion of the dose excreted in
the urine and faeces within the first 24 h was approximately 78% and
within 4 days was 90%. Residues in major organs and tissues of rats 4
days after a single oral dose were in general low: liver, 0.03 and
0.05; skin, 0.04 and 0.02; adrenals, 0.03 and 0.06; and kidneys, 0.02
and 0.02 (values are expressed as mg equivalent of
alpha-cypermethrin/kg tissue for females and males, respectively).
However, in body fat, higher residues were found (0.22 and 0.42
mg/kg). The release from skin and fat was biphasic in nature. The
half-life of elimination of radioactivity from fat was approximately
2.5 days for the initial phase and 17-26 days for the slower phase
(the half-life of elimination from fat for cis-cypermethrin was 18.9
days). The half-life values for skin were 2 days for the initial phase
and 40 days for the slower phase. The radioactivity in liver and
kidneys was eliminated apparently by a monophasic process. More than
95% of the residue in fat was present as unchanged alpha-cypermethrin
(Hutson, 1982; Logan, 1983; Hutson & Logan, 1986).
6.1.2 Domestic animals
In a study by Francis & Gill (1991), a formulation containing a
mixture of flufenoxuron and alpha-cypermethrin was applied to groups
of three sheep. The formulation was applied once, either as a dip
diluted at 1:1000 to give a solution of 80 mg flufenoxuron per litre
and 60 mg alpha-cypermethrin/litre or as a pour-on solution applied
directly to the backs of the sheep giving a dose of 0.15 g active
ingredient flufenoxuron and 0.2 g alpha-cypermethrin per sheep. The
sheep were killed at 3, 7 and 14 days after application and samples of
subcutaneous fat, fleece and sheep skin were analysed. The residues of
alpha-cypermethrin in fat ranged from < 0.01 to 0.04 mg/kg and in
skin from 0.02 to 1.4 mg/kg over the three sampling periods, and they
were lower for pour-on formulations than for the dip. Highest tissue
residues were found in wool (sampled from the back); these were (for
the 3, 7 and 14 day sampling periods, respectively) 730, 1020 and 360
mg/kg for dip application and 360, 440 and 360 mg/kg for pour-on
application. Wool sampled from the side of sheep treated with pour-on
formulation were 10 to 30 times lower than wool sampled from the back
region; with the dip solution, however, wool from the side region
contained higher residues than that from the back. Pour-on application
gave lower residues than after a dip.
A trial was carried out during 1987 in the United Kingdom in
which Friesian/Hereford calves (in total 17 female animals) were
treated with an alpha-cypermethrin pour-on formulation. Ten ml of a 16
g/litre formulation was applied to calves along the middorsal line
from shoulder to tail. At 3, 7 and 14 days following treatment,
animals were sacrificed for analysis of tissues, i.e. perirenal and
subcutaneous fat, muscle, kidneys and liver. No residues were detected
in muscle and liver samples at any time (limit of determination, 0.01
mg/kg). In the kidneys a maximum of 0.03 mg/kg was found on day 7 but
by day 14 the residues had decreased to 0.01 mg/kg or less. The fat
tissues contained maximum levels on day 7, i.e. mean concentrations of
0.26 mg/kg (perirenal fat) and 0.08 mg/kg (subcutaneous fat). By day
14 these concentrations had decreased by about two and a half times
(Sherren, 1988a) (see also section 5.2.3).
6.1.3 Humans
Six volunteers (two per dose level) received a single oral dose
of 0.25, 0.5 or 0.75 mg alpha-cypermethrin and, after a period of 2-3
weeks, five successive daily doses of 0.25, 0.5 or 0.75 mg to study
the urinary excretion and bioaccumulation of alpha-cypermethrin. A
parallel study with cypermethrin itself was carried out for comparison
purposes. The metabolism and rate of excretion of a single oral dose
of alpha-cypermethrin were similar to those of cypermethrin itself.
The rate of excretion was dose-related, approximately 43% of the dose
of alpha-cypermethrin being excreted in the urine as free or
conjugated cis-cyclopropane carboxylic acid ( cis-CPA) during the
first 24 h. Urinary excretion did not increase with repeated oral
dosing; an average of 49% of alpha-cypermethrin was excreted in the
urine as free or conjugated cis-CPA within 24 h (van Sittert et al.,
1985; Eadsforth et al., 1988).
6.2 Metabolic transformation
In a study on Wistar rats using alpha-cypermethrin,
14C-labelled in the benzyl moiety, (see section 6.1.1) no evidence
was found for any racemization of the chiral centres of
alpha-cypermethrin in the residues in intestines, faeces or fat. The
major urinary metabolite was the sulfate conjugate of
3-(4-hydroxyphenoxy)benzoic acid, and smaller amounts of
3-phenoxybenzoic acid (II) and 3-(4-hydroxyphenoxy)benzoic acid (III)
were identified. In the faeces, 75% of the radioactivity in the
extract was unchanged alpha-cypermethrin; minor metabolites included
a dihydroxy metabolite (V), 3-(4-hydroxyphenoxy)benzoic acid (III),
3-phenoxybenzoic acid (II) and the 4-hydroxyphenoxy metabolite (IV).
In the adipose tissue, the 14C label was mainly associated with
unchanged alpha-cypermethrin, but a lipophilic metabolite of either
alpha-cypermethrin or 3-phenoxybenzoic acid, probably a mixture of
3-phenoxybenzoyl diacylglycerols, was also present (Hutson, 1982;
Logan, 1983; Hutson & Logan, 1986) (see Fig. 2).
6.3 In vitro metabolic transformation
Creedy & Logan (1984) studied the in vitro metabolism of
cypermethrin and alpha-cypermethrin using liver microsomal
preparations from rats, rabbits and humans. In order to obtain
information on the relative importance of the oxidative and esteric
pathways of degradation of these compounds, incubations were carried
out both in the presence and absence of an NADPH-generating system.
Both cypermethrin and alpha-cypermethrin were broken down via esteric
and oxidative pathways by the liver preparations from the three
species. For rabbit and human liver microsomes, oxidation was a minor
metabolic route compared to esteric hydrolysis in the case of both
compounds. Human liver microsomes were able to carry out the esteric
hydrolysis of alpha-cypermethrin slightly faster than cypermethrin. In
the liver preparations of all three species, cyclopropane carboxylic
acid (mainly produced via the esteric pathway) was the main metabolite
for both compounds (to the extent of approximately 90-99%). Via the
oxidative route, mono-hydroxycypermethrins, dihydroxy-cypermethrin and
small amounts of hydroxycyclopropane carboxylic acid (rat only) were
also produced.
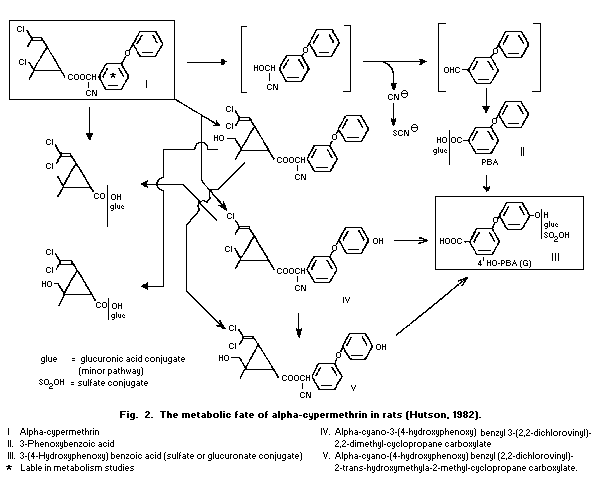 6.4 Plants
The metabolism of cypermethrin in plants is described in WHO
(1989).
The degradation of alpha-cypermethrin and cypermethrin in
cabbages grown to maturity outdoors has been studied. Eighteen days
after transplanting, the cabbages were treated three times with
14C-labelled alpha-cypermethrin or cypermethrin as an EC
formulation. Each treatment consisted of 1.8 mg equivalent with a
spray concentration of 36 g/litre. This treatment was repeated after
11 and 27 days. Each box of cabbages received a total application of
5.4 mg at a dose rate equivalent to 50 g active ingredient/ha. At
harvest (3 months later) the plants were separated into old and new
outer leaves, heart, stalk and roots. No major differences between the
two compounds in distribution of radioactivity throughout the plants
or in the metabolic profile were observed. The highest radioactive
residues were present in the old outer leaves (23% for
alpha-cypermethrin and 27% for cypermethrin), lower levels being found
in new outer leaves, stalk, roots and heart. Very low levels (< 0.05
mg/kg) of both compounds were found in the soil. The major radioactive
residue at harvest was shown to be the pesticide, which was either in
the unchanged form or had undergone cis/trans-isomerization,
presumably photochemically. The profiles of the organosoluble
metabolites were similar, and the major products of alpha-cypermethrin
had chromatographic mobility similar to previously identified products
of cypermethrin metabolism, such as 3-phenoxybenzoic acid and
3-phenoxybenzyl alcohol, partly hydroxylated and/or conjugated. These
compounds were found in minor quantities (McMinn, 1983a; WHO, 1989).
Appraisal
A wide range of studies in mice, rats, dogs, sheep, cows and
humans has shown that cypermethrin is rapidly absorbed, distributed
to a variety of organs and tissues, metabolized and rapidly excreted
from the body (WHO, 1989). There are no major differences in the
absorption, distribution, retention or excretion between the species.
Differences, where they do occur, are related to the rate rather than
the nature of the metabolites formed and the conjugation reactions.
Cypermethrin, both the cis and trans isomers, and
alpha-cypermethrin are primarily metabolized by cleavage of the ester
bond. The metabolites PBA and CPA are mainly excreted as conjugates.
The type of conjugate differs in a number of animal species dosed
with cypermethrin, but humans and rats have the same pathway. Minor
quantities of hydroxylated PBA (conjugated) may also be found. The
terminal half-life of elimination of alpha-cypermethrin from the fat
of rats is 17-26 days, compared to 18.9 days for cis-cypermethrin.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Oral (technical product)
Alpha-cypermethrin is moderately to highly toxic and 3-4 times
more toxic than cypermethrin.
The clinical signs of toxicity observed in the various acute
toxicity studies on experimental animals with alpha-cypermethrin are
typical for a cyano-containing pyrethroid intoxication. They included
ataxia, abasia, gait abnormalities, choreoathetosis, "tip-toe" walk,
and increased salivation, lacrimation, piloerection, tremor and clonic
convulsions. The majority of the mortalities occurred within the first
3 h and surviving animals recovered within 7 days (Rose, 1982, 1983a).
In the study with alpha-cypermethrin administered in corn-oil
(Rose, 1983a), clonic convulsions, piloerection, salivation and
splayed hind-leg gait were found. The oral LD50 values for
alpha-cypermethrin are summarized in Table 3.
Table 3. Oral LD50 values for technical alpha-cypermethrin
Species Concentration and LD50 in mg/kg body Reference
(strain) vehicle weight (with 95%
confidence limits)
Mouse 5% in corn oil 35 (26-48) Rose (1982)
(CD) 40% in DMSO 762 (514-912) Rose (1982)
50% aqueous suspension 798 (568-1074) Rose (1982)
Rat 5% in corn oil 79 (63-98) Dewar (1981)
(Wistar) 40% in DMSO approximately 4000 Rose (1982)
50% aqueous suspension > 5000 Rose (1982)
Rat 10% in corn oil 40-80 Rose (1983a)
(Wistar) 20% in corn oil 368 (282-487) Rose (1983a)
Woollen et al. (1991) noted a higher degree of absorption of
cypermethrin when it was applied in corn oil. This could be the
explanation for the higher toxicity of alpha-cypermethrin administered
in corn oil.
7.1.2 Oral (formulations)
Formulations of alpha-cypermethrin have moderate acute oral
toxicity (Table 4). The clinical signs observed after oral
administration to rats are characteristic of cyano-containing
pyrethroid intoxication (see section 7.1.1.). The majority of the
mortalities occurred within 3 days of dosing. The degree of acute oral
toxicity of formulations containing mixtures with other active
ingredients depended on the toxicity of the latter ingredients.
7.1.3 Dermal
Alpha-cypermethrin has low dermal toxicity. No deaths or signs of
intoxication were observed in rats (Dewar, 1981; Shell, 1983a) and
mice (Rose, 1982; Shell, 1983a) receiving a single 24-h dermal
exposure of 500 mg/kg body weight (25% in DMSO) and 100 mg/kg body
weight (5% in corn oil), respectively.
The dermal LD50 values in rats of formulations of
alpha-cypermethrin and of alpha-cypermethrin mixed with another active
ingredient are summarized in Table 4. In all cases, the maximum dose
that could be applied was tested.
With the pour-on formulations, no clinical signs were observed.
Blood around the nose and eyes was the only sign seen in the case of
SC formulations. Clinical signs observed after the application of EC
or ULV formulations of alpha-cypermethrin included increased
lacrimation, chromodacryorrhoea and unkempt appearance, aggressiveness
and diarrhoea. The Fastac/BPMC formulation caused the same signs of
intoxication and also oedema at the application site. With the
Fastac/methomyl formulation, fasciculation, lethargy, salivation,
piloerection, hunched back, chromodacryorrhoea and cyanosis were
observed. Animals treated with Fastac/Azodrin formulation showed the
above-mentioned symptoms, and some additionally showed ataxia, abasia,
hypothermia, eye pallor and prostration/coma.
7.1.4 Inhalation
Groups of five male and five female albino Fischer-344 rats were
exposed for 4 h to a dust atmosphere containing 30% (m/m)
alpha-cypermethrin on silica powder at an average concentration of 1.3
g/m3 (equivalent to 0.4 g active ingredient/m3). The mass media
diameter of the dust particles was 4.2 µm (geometric standard
deviation 6.4). The animals were observed for 14 days after the
exposure but there were no signs of intoxication. Macroscopic
examination of the lungs did not reveal any effects. Thus, the acute
LC50 was > 1.3 g 30% silica powder dust/m3 or > 0.4 g active
ingredient/m3 (Blair, 1984).
Table 4. Oral and dermal LD50 values for formulated alpha-cypermethrin in rats (Fischer-344)
Formulationa LD50 in mg total formulation per kg Reference
body weight (with 95% confidence limits)
Oral Dermal
100 g/litre EC 101 (82-119) > 1800 Rose (1984d)
100 g/litre EC 136 (98-186) > 1800 Rose (1984e)
100 g/litre EC 174 (125-327) > 2000 Price (1985a)
30 g/litre EC 229 (178-292) > 2000 Rose (1984f)
30 g/litre EC 673 (597-753) > 2000 Rose (1985)
15 g/litre pour-on > 2000 > 2000 Price (1988)
10 g/litre pour-on > 2000 > 2000 Price (1988)
100 g/litre SC 1804 (1507-2168) > 2000 Price (1985b)
60 g/litre SC > 5000 > 2000 Gardner (1991)
15 g/litre SC > 5000 > 2000 Price (1986)
15 g/litre ULV 5838 (5130-6665) > 2000 Rose (1984c)
Mixtures with other active ingredients
Fastac/methomyl EC 58-97 (males) > 1900 Rose (1984h)
15/120 g/litre 73 (51-89) (females) > 1900
Fastac/BPMCb EC 310 (215-462) > 2000 Price (1987)
10/400 g/litre
Fastac/Azodrin EC 25 (18-34) > 2000 Gardner (1989)
20/400 g/litre
a EC = emulsifiable concentrate; SC = suspension concentrate; ULV = ultra-low volume
b BPMC = 2 sec-butylphenyl methylcarbamate (fenobucarb)
7.1.5 Other routes
The acute intraperitoneal LD50 for rats of a 10% solution of
alpha-cypermethrin in corn oil was 3.39 (3.03-3.83) ml/kg body weight,
or 339 mg active ingredient per kg body weight. The surviving animals
showed characteristic pyrethroid signs of intoxication, e.g., ataxia,
abasia, choreoathetosis, gait abnormalities, "tip-toe" walk and
salivation (Rose, 1984a).
7.2 Short-term exposure
7.2.1 Oral
7.2.1.1 Rat
Groups of Wistar rats (10 of each sex at each dose level and 20
of each sex as controls) were fed 0, 25, 100, 200, 400 or 800 mg
alpha-cypermethrin/kg diet (equivalent to 0, 1.25, 5, 10, 20 or 40
mg/kg body weight) for 5 weeks. At 400 and 800 mg/kg diet, signs of
intoxication, such as abnormal gait and hypersensitivity were
observed, and food intake and body weight were decreased in both sexes
compared with the control group. Changes in blood chemistry, e.g.,
decreases in protein and increases in urea levels, were observed in
both sexes of rats fed 800 mg/kg diet and in males fed 400 mg/kg. The
weights of livers and kidneys of both sexes of rats fed 800 mg/kg diet
and livers of male rats fed 400 mg/kg were increased. No
histopathological changes were observed except in the case of one
severely intoxicated male animal fed 800 mg/kg diet, which showed
sparse axonal degeneration in the sciatic nerve. No effects were seen
in the animals fed 200 mg/kg diet for five weeks (Pickering, 1982).
In a 13-week study, Wistar rats (30 males and 30 females per test
group, and a control group consisting of 60 males and 60 females),
which were initially 5 weeks old, were fed 0, 20, 60, 180 or 540 mg
alpha-cypermethrin/kg diet (equivalent to 0, 1, 3, 9 or 27 mg/kg body
weight). After six weeks of feeding, one third of the animals were
killed for interim haematological, clinical chemical and gross
post-mortem examination. The remaining animals were killed after 13
weeks. Signs of intoxication, such as abnormal gait with splayed hind
limbs, were found in 3 out of 20 males fed 540 mg/kg diet. Several
instances of transient skin sores and fur loss were observed
particularly in the rats fed 540 mg/kg. There was decreased growth,
which correlated with decreased food intake, in both sexes fed 540
mg/kg from the first week onwards. No clear effects on the
haematological and clinical chemical parameters were found. Organ
weights were comparable with those of the control animals. No
histopathological abnormalities were found except sparse axonal
degeneration in the sciatic nerve, without clinical signs of toxicity,
in two males fed 540 mg/kg. No axonopathy was observed in the three
animals with abnormal gait. There were marginal effects (decreased
growth during week one and from week 10 onwards) in the males fed 180
mg/kg diet. No effects were found in the 60-mg/kg group (Clark, 1982).
7.2.1.2 Dog
Beagle dogs (one male and one female) were fed alpha-cypermethrin
in the diet at the following concentrations: 200 mg/kg diet for 7
days, 400 mg/kg diet for 2 days, and 300 mg/kg diet for 7 days. With
200 mg/kg no signs of intoxication were observed, whereas dosing with
300 mg/kg or more caused weight loss, ataxia, subdued behaviour, head
nodding, food regurgitation, inflammation of gums and tongue, body
tremors and diminished response to stimuli. Haematological, clinical
chemical and gross pathological examination showed no effects
(Greenough & Goburdhun, 1984).
In a further study, Beagle dogs (one male and one female)
received 300 mg/kg diet for 3 days (male dog) or 4 days (female dog)
and 250 mg/kg diet for 7 days. Both animals showed the above-mentioned
signs of intoxication, the only difference being that when it was
being fed the 250-mg/kg diet the female animal showed these signs more
frequently than the male animal. There were no effects on haematology,
clinical chemical parameters, urinalysis, faecal occult blood test or
gross pathology (Greenough & Goburdhun, 1984).
In a study by Greenough et al. (1984), 36 pure bred Beagle dogs
(18 males and 18 females) received a diet containing
alpha-cypermethrin at 0, 30, 90 or 270 mg/kg diet for 13 weeks. The
group dosed at the highest concentration comprised six males and six
females while the other groups consisted of four males and four
females. All animals fed 270 mg/kg diet exhibited signs of
intoxication, such as whole body tremors, head nodding, "lip-licking",
subduedness, ataxia, agitation and a high-stepping gait. These signs
increased in both intensity and duration as the study progressed. Food
consumption, body weight gain, organ weights, ophthalmoscopy,
haematological and clinical chemical parameters, urinalysis, gross
pathology and microscopy of 18 organs and tissues of all test groups
showed no dose-related effects. In this study, the no-observed-effect
level was considered to be 90 mg/kg diet (equivalent to 2.25 mg/kg
body weight).
7.3 Skin and eye irritation; sensitization
7.3.1 Skin irritation
Undiluted technical alpha-cypermethrin was minimally irritating
when applied as a single occluded dose for 24 h to intact and abraded
rabbit skin (Dewar, 1981).
New Zealand white rabbits were used to study the primary skin
irritation of a number of alpha-cypermethrin formulations. The test
duration was 4 h, the observation period was 7-21 days, and the
formulations tested were 30 and 100 g/litre EC, 15 g/litre ULV, 10 and
15 g/litre pour-on formulation, 15 and 100 g/litre SC, and
Fastac/methomyl (15/120) EC. The EC formulations caused mild to
moderate skin irritation. Superficial necrosis was observed in one or
two animals treated with 100 g/litre EC, but there was no permanent
in-depth skin damage. The effects persisted for up to 7 days. The EC
formulations and the 100 g/litre SC formulation were classified as
mildly irritating. All other formulations tested were either
non-irritating or only slightly irritating (Rose, 1984c,d,e,f,h, 1985;
Price, 1985a,b, 1986, 1988).
7.3.2 Eye irritation
Undiluted formulations were tested for their eye irritancy
potential in groups of six rabbits using the Draize test. All the EC
formulations tested (30 or 100 g/litre) caused severe eye irritation,
including corneal opacity and damage to the iris (Rose, 1984d,e,f,
1985).
When an EC formulation (100 g/litre) and its components, both in
the undiluted form and at typical in-use dilutions (1 in 400 and 1 in
1333 aqueous dilution), were tested for eye irritancy potential, the
undiluted formulation was severely irritating, with or without
irrigation, while the undiluted blank formulations were mildly to
severely irritating. The diluted test formulations, with or without
alpha-cypermethrin or with emulsifier, were non-irritating. It was
concluded that the eye irritation resulted from the combined
formulation ingredients (especially the emulsifier) and that
alpha-cypermethrin per se gave only slight irritation, if any
(Dewar, 1981; Rose, 1984b). In-use dilutions (0.0075%) of another 100
g/litre EC formulation and its blank formulation were non-irritating
(Rose, 1984g).
Two pour-on formulations (10 and 15 g/litre) caused moderate and
slight conjunctival inflammation, respectively. The 10 g/litre
formulation was considered to be an eye irritant (Price, 1988). Two SC
formulations (15 and 100 g/litre) were mildly irritating, causing
slight conjunctival redness and chemosis (Price, 1985b, 1986). A 15
g/litre ULV formulation was mildly irritating to rabbit eyes and there
was a moderate initial pain response (Rose, 1984c). An EC formulation
containing Fastac/methomyl (15:120 g/litre) was a severe eye irritant.
The vascularization of the cornea and iritis were considered to be
irreversible (Rose, 1984h).
7.3.3 Sensitization
Technical alpha-cypermethrin was tested in the guinea-pig
maximization test of Magnusson and Kligman using groups of 10 male and
10 female guinea-pigs and a control group of 55 animals of each sex.
The following concentrations were used: intradermal injection, 0.05%
(v/v) in corn oil; topical application and challenge, 50% (m/m) in
vaseline. On the basis of the negative results it was concluded that
alpha-cypermethrin is not a skin sensitizer in guinea-pigs (Dewar,
1981).
An EC formulation (100 g/litre) and its corresponding blank were
tested, as a 50% solution in corn oil, in the Buehler guinea-pig
sensitization test. The topical challenge was carried out with a 30%
solution in corn oil. None of the animals showed positive responses at
24 or 48 h after the challenge (Rose, 1984g).
7.4 Long-term and carcinogenicity studies
No long-term or carcinogenicity studies have been conducted with
alpha-cypermethrin.
7.5 Reproduction, embryotoxicity and teratogenicity
Alpha-cypermethrin has not been tested for reproductive effects
or teratogenicity.
From the available reproduction and teratogenicity studies with
cypermethrin it is clear that no influence on reproduction performance
occurs at a level of 100 mg/kg diet, nor are there any teratogenic
effects even with dose levels high enough to cause maternal toxicity
(WHO, 1989). Furthermore, the no-observed-effect level of cypermethrin
for reproduction and teratogenicity is comparable with the
no-observed-effect levels based on other parameters of toxicity. In
consequence, there is no reason to believe that alpha-cypermethrin,
consisting of two cis isomers also present in cypermethrin, would
behave differently.
7.6 Mutagenicity and related end points
7.6.1 Mutation
The results of the various mutagenicity studies with
alpha-cypermethrin are summarized in Table 5.
Alpha-cypermethrin (in DMSO) at concentrations of 31.25, 62.5,
125, 250, 500, 1000, 2000 or 4000 µg/ml did not increase reverse gene
mutation (at the arg 4-17, trp 5-48 or hom 3-10 markers) in log- or
stationary-phase cultures or forward mutation (to cyclo-heximide
-resistance) in log-phase cultures of Saccharomyces cerevisiae XV
185-14C, either in the presence or absence of rat-liver S9 fraction.
Concentrations of 10 and 50 µg/ml 4-nitroquinoline- N-oxide and 1250
and 5000 µg/ml cyclophosphamide were used as positive controls
(Brooks, 1984).
Alpha-cypermethrin (in DMSO) at concentrations of 31.25, 62.5,
125, 250, 500, 1000, 2000 or 4000 µg/plate, both with and without
microsomal activation, did not increase reverse mutation rates in
Salmonella typhimurium TA98, TA100, TA1535, TA1537 and TA1538 or in
Escherichia coli WP2 and WP2 uvr A. Mitotic gene conversion was not
induced in liquid suspension cultures of log-phase cells of
Saccharomyces cerevisiae JD 1, dosed with solutions of
alpha-cypermethrin at concentrations of 10, 100, 500, 1000 or 5000
µg/ml, both in the presence or absence of a rat liver S9 fraction.
These studies were carried out in comparison with four positive
control compounds (Brooks, 1982).
7.6.2 Chromosomal effects
In a study by Clare & Wiggins (1984), groups of five male and
five female Wistar rats were administered a single oral dose of 2, 4
or 8 mg alpha-cypermethrin in 5% corn oil/kg body weight and killed 24
h after dosing. The control group received corn oil alone.
Cyclophosphamide was used as a positive control. Alpha-cypermethrin
caused no increase in the incidence of chromatid or chromosome
aberrations or polyploidy in bone marrow cells.
Alpha-cypermethrin in aqueous carboxymethylcellulose at
concentrations of up to 40 µg/ml did not increase the frequency of
chromatid gaps, chromatid breaks or total chromatid aberrations in rat
liver (RL4) cell cultures (Brooks, 1982).
7.6.3 DNA damage
Alpha-cypermethrin in DMSO (20%) was administered to Wistar rats
as a single oral dose of 40 mg/kg body weight. The exposure time was
6 h. Alpha-cypermethrin failed to produce any detectable DNA
single-strand damage using alkaline elution profiles of liver DNA.
Methylmethane sulfonate was used as a positive control and DMSO as the
solvent control (Wooder, 1982).
7.6.4 Conclusion
From the available data on alpha-cypermethrin, it can be
concluded that this compound is non-mutagenic in tests with
Salmonella typhimurium, Saccharomyces cerevisiae, and in vivo and
in vitro tests with rat liver cells for the induction of chromosome
aberration and production of DNA single-strand damage.
Table 5. Mutagenicity tests on microorganisms
Organism/strain Dose Type of test Metabolic Result Reference
activation
Salmonella typhimurium up to 4000 µg/plate plate with or negative Brooks (1982)
TA98, TA100, TA1535, without
TA1537, TA1538
Escherichia coli up to 4000 µg/plate plate with or negative Brooks (1982)
WP2, WP2 uvrA without
Saccharomyces cerevisiae up to 5000 µg/ml liquid suspension with or negative Brooks (1982)
JDI culture without
Saccharomyces cerevisiae up to 4000 µg/ml liquid suspension with or negative Brooks (1984)
XV 185-14C culture without
Rat liver cells (RL4) up to 40 µg/ml negative Brooks (1982)
(chromatid gaps, breaks or
aberrations)
Rat liver DNA one oral dose of negative Wooder (1982)
(DNA single strand damage) 40 mg/kg body weight
Rat bone marrow one oral dose of negative Clare & Wiggins
chromosome study up to 8 mg/kg body (1984)
weight
7.7 Special studies
7.7.1 Skin sensation
It is known that exposure to certain types of pyrethroids can
result in a transient skin sensation in humans (Le Quesne et al.,
1980).
Guinea-pigs received 0.1 ml of a 0.01, 0.1 or 1.0% solution of
alpha-cypermethrin in ethanol or a 1, 10 or 20% solution of
alpha-cypermethrin (w/v) in corn oil on the skin. Sensory stimulation
was quantified by counting the number of times each animal turned to
lick or bite its treated flank in preference to the non-treated flank.
Skin stimulation was observed during a 2-h period at all dose levels
except the lowest. In the groups of guinea-pigs treated with 10% and
20%, some animals exhibited an exaggerated hopping movement and a
repeated head shaking activity at the time of maximum skin stimulation
(40-60 min after treatment). This behaviour was not seen with the 1%
solution or more dilute ones (Hend, 1983).
7.7.2 Neurotoxicity
Large oral doses of alpha-cypermethrin and other synthetic
pyrethroids (WHO, 1989) have been shown to produce minor
histopathological lesions in the sciatic nerve of rats, described as
sparse axonopathy in peripheral nerves.
Rose (1983b) conducted a two-phase study. In the first phase, the
time-course for development and recovery from pyrethroid-induced nerve
lesions was investigated by measuring biochemical correlates of
neuropathological change (the enzymes beta-glucuronidase and
beta-galactosidase) in groups of Wistar rats (five of each sex per
group) at periods of 2-12 weeks after the start of dosing. The daily
doses of alpha-cypermethrin (96.6%), administered by stomach tube,
were 37.5 mg/kg body weight for the first 11 doses and 25.0 mg/kg body
weight for the subsequent 9 doses over a 4-week period (5 times/week).
DMSO was used as the solvent for 10 doses and then arachis oil. In
all, 21% of the animals died and more than 80% of the treated animals
showed clinical signs of intoxication. Maximum enzyme activities in
the sciatic posterior tibial nerves were found 5 weeks after the start
of the experiment and had returned to control values by 12 weeks. In
the trigeminal nerve and trigeminal ganglia, a slight but not
significant increase in enzyme activities was found.
In the second phase of the study, 10 male and 10 female Wistar
rats were given 20 oral doses of alpha-cypermethrin in DMSO (0, 10, 20
or 40 mg/kg body weight per day) over a period of 4 weeks (5
times/week). Only two animals died, one given 10 mg/kg and one given
20 mg/kg. In the 40-mg/kg group, 75% of the animals developed clinical
signs, whereas in the 20-mg/kg group only 25% of the animals showed
these clinical effects. In the 10-mg/kg group, the animals showed no
differences from the controls. No clear influence of
alpha-cypermethrin on growth was found. Five weeks after the initial
dose, which corresponded to the period of maximal enzyme changes,
biochemical changes (increases of up to 60%) indicative of a mild
axonal degeneration were found in both the distal and proximal
sections of the sciatic posterior tibial nerve in animals administered
40 mg/kg body weight. In the 20-mg/kg group, only a small (up to 20%)
increase in beta-galactosidase activity was found in the proximal
sections of the sciatic posterior nerve. The same trends were found in
the trigeminal nerve and ganglia. No changes were found in the
10-mg/kg group (Rose, 1983b).
7.7.3 Immunosuppressive action
No data on the immunosuppressive action of alpha-cypermethrin are
available.
7.8 Mechanism of toxicity - mode of action
The mechanism of toxicity and mode of action of cypermethrin (and
other pyrethroids) are extensively described in section 8.8 of the
Environmental Health Criteria 82: Cypermethrin (WHO, 1989). Recently
Vijverberg & van den Bercken (1990) and Aldridge (1990) summarized
current knowledge of the neurotoxicity and mode of action of the
different pyrethroids (see also Appendix 1).
Pyrethroids induce toxic signs that are characteristic of a
strong excitatory action on the nervous system. Toxic doses generally
cause hypersensitivity to sensory stimuli, and a number of compounds
may induce tingling sensations in the skin. Two distinct toxic
syndromes have been described in mammals. The T-syndrome is induced by
pyrethrins and non-cyano pyrethroids, and the CS-syndrome, induced by
cyano-pyrethroids such as cypermethrin and alpha-cypermethrin, is
characterized by choreoathetosis and salivation.
The available data strongly suggest that the primary target site
of pyrethroid insecticides in the vertebrate nervous system is the
sodium channel in the nerve membrane. Pyrethroids without an
alpha-cyano group cause a moderate prolongation of the transient
increase in sodium permeability of the nerve membrane during
excitation. This results in relatively short trains of repetitive
nerve impulses in sense organs, sensory (afferent) nerve fibres and,
in effect, nerve terminals. On the other hand, the alpha-cyano
pyrethroids (for instance cypermethrin and alpha-cypermethrin) cause
a long-lasting prolongation of the transient increase in sodium
permeability of the nerve membrane during excitation. This results in
long-lasting trains of repetitive impulses in sense organs and a
frequency-dependent depression of the nerve impulse in nerve fibres.
The difference in effects between permethrin (with no alpha-cyano
group) and the two insecticides cypermethrin and alpha-cypermethrin,
which have identical molecular structures except for the presence of
an alpha-cyano group on the phenoxybenzyl alcohol, indicates that it
is this alpha-cyano group that is responsible for the long-lasting
prolongation of the sodium permeability.
Since the mechanisms responsible for nerve impulse generation and
conduction are basically the same throughout the entire nervous
system, pyrethroids may also induce repetitive activity in various
parts of the brain. The difference between the symptoms of poisoning
by alpha-cyano pyrethroids and those of the classical pyrethroids is
not necessarily due to an exclusive central site of action. It may be
related to the long-lasting repetitive activity in sense organs and
possibly in other parts of the nervous system, which, in a more
advance state of poisoning, may be accompanied by a
frequency-dependent depression of the nervous impulse.
Pyrethroids also cause pronounced repetitive activity and a
prolongation of the transient increase in sodium permeability of the
nerve membrane in insects and other invertebrates. Available
information indicates that the sodium channel in the nerve membrane is
also the most important target site of pyrethroids in the invertebrate
nervous system.
Because of the universal character of the processes underlying
nerve excitability, the action of pyrethroids should not be considered
to be restricted to particular animal species or to a certain region
of the nervous system.
Although it has been established that sense organs and nerve
endings are most vulnerable to the action of pyrethroids, the ultimate
lesion that causes death will depend on the animal species,
environmental conditions, and on the chemical structure and physical
characteristics of the pyrethroid molecule.
8. EFFECTS ON HUMANS
8.1 General population exposure
No data concerning the exposure of the general population to
alpha-cypermethrin are available.
8.2 Occupational exposure
A study of alpha-cypermethrin exposures was carried out during
formulation using both technical concentrate and technical material at
Durban, South Africa. The oil-damped solid, crystalline, dry technical
concentrate contained a minimum of 90% (m/m) alpha-cypermethrin.
Exposures were assessed by personal and static monitoring of
atmospheric alpha-cypermethrin concentrations, urinary
alpha-cypermethrin metabolite concentrations and by medical
examination. Four individuals were exposed during 3 days of operation.
The group mean personal exposures for the two days whilst formulating
technical concentrate were 2.8 and 4.9 µg/m3 and the group mean
personal exposure to technical material on day 3 was 54.1 µg/m3.
Urinary alpha-cypermethrin metabolites could not be identified (limit
of detection, 0.02 mg/litre). Formulation was successfully completed,
only minor skin sensations being reported by two of the
non-operational personnel, possibly resulting from particles of
alpha-cypermethrin settling directly on the skin, face and neck. Dust
concentrations were up to 30 times greater during handling the
technical material compared with oil-damped technical concentrate, and
local exhaust dust extraction reduced dust emission by a factor of up
to 17 (Western, 1984).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
Appraisal
The acute toxicity of alpha-cypermethrin to Daphnia magna and
Gammarus pulex is similar to that of cypermethrin. However, in a
reproduction study with Daphnia magna, alpha-cypermethrin seemed to
be slightly more toxic than cypermethrin. Comparison of the results
of a reproduction study in Daphnia magna with the results of acute
tests shows that the hazard of alpha-cypermethrin lies in its acute
toxicity. There is no significant potential for cumulative effects
occurring as a result of long-term exposure to lower concentrations.
Alpha-cypermethrin is highly toxic to a number of aquatic
arthropod taxa but of low toxicity to molluscs. The short-term
toxicity of the compound can be reduced by formulating the product as
an OSC. Contamination through spray drift from commercial
applications will generally be low, and so the effects on susceptible
taxa will be limited. The rapid loss of alpha-cypermethrin from the
water gives the potential for complete recovery of affected
populations.
Laboratory studies show that values for the acute toxicity and
the toxicity to the early-life stages of fish are similar for
alpha-cypermethrin and cypermethrin. A comparison of the results from
both alpha-cypermethrin studies reveals that the hazard of the
compound results from its acute toxicity, and there is no significant
potential for additional effects occurring as a result of long-term
exposure to lower concentrations. Laboratory and field studies have
shown that the toxicity of alpha-cypermethrin to fish is greatly
influenced by the formulation, particulate formulations showing
significantly less toxicity than emulsifiable concentrates.
Field studies demonstrate that the high toxicity of
alpha-cypermethrin to fish observed in laboratory studies is not
realized under field conditions. Contamination of water bodies by
inadvertent overspraying or spray drift does not present a hazard to
fish.
9.1 Microorganisms
9.1.1 Algae
The acute toxicity of alpha-cypermethrin to a single-celled green
alga, Selenastrum capricornutum, at 24 °C has been determined. The
pesticide was dispersed using acetone, and the 2- to 4-day EC50 for
growth was above 100 µg/litre (Stephenson, 1982).
9.1.2 Bacteria
The effects of cypermethrin on microbial activity in the soil
have been investigated in a series of studies. Cypermethrin was
applied to sandy loam at concentrations of 2.5 and 250 mg/kg. No
effects on the rates of carbon dioxide evolution or oxygen uptake were
observed at the lower rate, but significant inhibition of carbon
dioxide evolution and a decrease in oxygen uptake were observed at the
higher rate of application. There were no effects at either
concentration on nitrogen fixation, ammonification, nitrification or
glucose utilization (WHO, 1989).
Sewage bacteria were unaffected by the presence of
alpha-cypermethrin (3 mg/litre) in a closed system test, while the
growth of Pseudomonas fluorescens was unaffected at 100 mg/litre
(Stone & Watkinson, 1983).
9.2 Aquatic organisms
9.2.1 Invertebrates
9.2.1.1 Laboratory studies
Acute toxicity studies with Daphnia magna (aged < 24 h) showed
that at 20 °C technical alpha-cypermethrin, dispersed in acetone under
static conditions (daily renewal), has effects at concentrations below
1 µg/litre. The 24-h and 48-h EC50 values (immobilization) were 1.1
and 0.3 µg/litre, respectively (Stephenson, 1982).
Water samples taken from field enclosures 24 h after treatment
with an EC formulation were analysed for alpha-cypermethrin and
bioassayed with Gammarus pulex. The 24-h LC50 value for this
organism was 0.05 µg/litre (Garforth, 1982a; Shires, 1982).
The effect of technical alpha-cypermethrin on survival, growth
and reproduction of Daphnia magna was studied over a period of 21
days by Garforth (1982b). The test solution was renewed daily and the
temperature ranged between 18.5 and 20.2 °C. The results are
summarized in Table 6.
Table 6. Effects of alpha-cypermethrin on the reproductive cycle of
Daphnia magnaa
Effect Nominal concentration (µg/litre)
LOEL NOEL
Survival of parent generation 0.3 0.1
Growth of parent generation 0.1 0.03
Production of young 0.1 0.03
a From: Garforth (1982b)
LOEL = Lowest-observed-effect level; NOEL = No-observed-effect
level
9.2.1.2 Field studies
Garforth (1982a) studied the effects of alpha-cypermethrin on a
range of aquatic invertebrates in metal enclosures, each containing
about 1 m3 water, placed in an outdoor experimental pond. A diluted
EC formulation was sprayed onto the water surface at concentrations of
1, 3, 10, 30 and 100 g active ingredient/ha, and samples were taken up
to 7 days after application. The concentration of alpha-cypermethrin
was around 50% of the nominal level 24 h after application and
decreased to 10 to 20% of the nominal level 7 days after application.
The lowest concentration was toxic to Asellidae. Thirteen families
of aquatic arthropods were tested; most were killed at 1 g/ha except
one species of Coenagriidae, which tolerated about 3 g/ha. Three
families of molluscs were tested and were found to be unaffected at
100 g/ha.
Studies to investigate the relative toxicity of two formulations
of alpha-cypermethrin (a 100-g/litre EC and a 100-g/litre oil-enhanced
suspension concentrate (OSC) with and without anti-evaporant agents)
to aquatic invertebrates have been carried out. Water samples were
taken from field enclosures treated with a range of doses (0.1-5 g/ha
for the EC and 0.1-10 g/ha for the two OSC formulations) and
bioassayed with Gammarus pulex. The 24-h LD50 values (in g
alpha-cypermethrin/ha equivalents) of water samples taken 24 h after
application were 0.9 for the EC, 6.3 for the OSC without
anti-evaporant, and 2.8 for the OSC plus anti-evaporant formulation.
Thus, one day after treatment, both OSC formulations were less toxic
to Gammarus pulex than the EC formulation. However, the EC
formulation lost its toxicity more rapidly than either of the OSC
formulations (see also section 4.1.2). The effects on other aquatic
invertebrate communities could not be accurately assessed, since the
results for macro-arthropods and zooplankton were inconclusive.
Coenagriidae, Chironomidae and zooplankton seemed to be relatively
tolerant. The residues in water and sediment, 22 days after treatment
with the EC at 2 g alpha-cypermethrin/ha or with both OSC formulations
at 5 g/ha, were < 0.004 µg/litre and < 0.01 mg/kg, respectively, for
all three formulations (Inglesfield, 1985b).
The hazard to aquatic invertebrates resulting from spray drift
from the aerial application of an EC (15 g alpha-cypermethrin/ha) has
been investigated by Garforth & Woodbridge (1984). Details of the
study are described in section 9.2.2.2. The sub-surface water
concentration was 0.6 µg alpha-cypermethrin/litre shortly after
application and decreased to < 0.02 µg/litre within 2 to 4 days. The
contamination initially caused a significant reduction in the
abundance of several groups of aquatic arthropods, including beetles,
chironomids, corixids, mites and zooplankton. However, within 4 to 7
weeks the affected fauna had completely recovered.
In a study by Pearson (1990), two freshwater ponds were treated
with alpha-cypermethrin as an emulsifiable concentrate in 1987. One
pond was oversprayed at 15 g active ingredient/ha, while the other was
treated with the same amount of alpha-cypermethrin but by direct
incorporation into the water (see section 4.1.2). Indigenous
populations of zooplankton nauplii and of copepods ( Cyclops spp.)
were significantly reduced in both ponds by the treatment but
recovered within 26-45 days. Other zooplankton were not plentiful but
appeared to be less severely affected in the oversprayed pond than in
the pond treated by incorporation. Populations of phantom midge larvae
( Chaoborus spp) were killed by both treatments. However, within 47
days after treatment, populations of young larvae had developed in the
oversprayed pond to almost pre-treatment numbers, and to a lesser
extent in the pond with direct incorporation.
9.2.2 Fish
9.2.2.1 Laboratory studies
The available acute 96-h LC50 values for two fish species are
summarized in Table 7.
The effects of the type of formulation on the acute toxicity to
fish are summarized in Table 8. Suspension concentrate, wettable
powder and micro-encapsulated formulations were 10 to 70 times less
acutely toxic to rainbow trout than the EC formulation (Shires,
1983b).
Table 7. Acute toxicity of technical alpha-cypermethrin in fish
Species Mean weight Vehicle Test system Temperature 96-h LC50 Reference
(g) (°C) (µg/litre) (95%
confidence limits)
Rainbow trout 3.3 dispersed via static water; 15 2.8 Stephenson (1982)
(Oncorhynchus mykiss) acetone 12 h renewal of (2.1-3.5)
test solutions
Fathead minnow 0.76 adsorbed onto continuous 23-25 0.93 Stephenson (1983)
(Pimephales promelas) pumice flow-through (0.78-1.2)
Table 8. Effect of type of formulation on the toxicity of alpha-cypermethrin to fish in laboratory studies
Species Weight Tempera- Formulation 96-h LC50 Reference
(g) ture (°C) (µg/litre)
Rainbow trout 0.8-2.5 15 100 g/litre EC 5.6 Shires (1983b)
(Oncorhynchus 250 g/litre SC 350
mykiss) 100 g/kg WP 120
50 g/kg WP 220
50 g/kg ME > 100
50 g/kg CD 65
Rainbow trout 0.11-0.25 15 15 g/litre OSC 10a Pearson (1986)
(Oncorhynchus 15 g/litre OSC 71
mykiss) 100 g/litre OSC 16a
100 g/litre OSC 56
Common carp 3.5-4 24-30 100 g/litre OSC 11 Stephenson
(Cyprinus carpio) 15 g/litre EC 0.8 (1986)
50 g/kg WP 60
Puntius 0.3-0.5 24-30 100 g/litre OSC 3.2 Stephenson
gonionotus 15 g/litre EC 0.7 (1986)
50 g/kg WP 22
a Denotes daily renewal of test solutions. All other tests were carried out without renewal of the test solutions.
EC = emulsifiable concentrate; SC = suspension concentrate; OSC = oil-enhanced suspension concentrate; WP= wettable powder;
ME = micro-encapsulated;
CD = ß-cyclodextrin.
The toxicity of alpha-cypermethrin to the early-life stages of
fish has been studied in a 34-day continuous-flow embryo-larval test
with the fathead minnow (Pimephales promelas). Eggs less than 24 h
old were exposed to nominal concentrations of 0.03 to 1.0 µg/litre.
Pre-hatch, post-hatch and overall mortality and final body weight were
recorded. On the basis of the most sensitive parameter (overall
survival) and measured exposure concentrations, the lowest
concentration of alpha-cypermethrin producing an adverse effect was
0.09 µg/litre and the highest concentration producing no effect (NOEL)
was 0.03 µg/litre (Stephenson, 1983).
9.2.2.2 Small scale field or outdoor tank studies
The acute effect of formulation on the toxicity of
alpha-cypermethrin to fish under field conditions was investigated in
studies using stainless steel enclosures placed in an experimental
pond. In the first study (Shires, 1983b), the rainbow trout (13-32 g)
was the test species and the results are summarized in Table 9. The EC
formulation was at least 30 times more toxic than any of the other
formulations. The number of fish used was not reported.
Table 9. Effect of formulation on the toxicity of alpha-cypermethrin
to rainbow trout in field studies
Formulation 14-day LD50 (g active
ingredient/ha equivalent)
100 g/litre emulsifiable concentrate 29
250 g/litre suspension concentrate > 1000
100 g/kg wettable powder > 1000
50 g/kg micro-encapsulated > 1000
In the second study, Stephenson (1987c) tested an 1.5%
oil-enriched SC formulation of alpha-cypermethrin in outdoor tanks
containing carp ( Cyprinus carpio; 6.3 g). The application rate was
approximately 4, 8 and 15-16 g active ingredient/ha applied by hand
sprayer. The water temperature ranged from 23-26 °C, the water was
aerated and the duration of the experiment was 7 days. There was 20%
mortality at all treatment rates, which was comparable with the
control group. The number of fish used was not reported.
In the third study (Shires, 1985b), rainbow trout ( Oncorhynchus
mykiss; 2-5 g) were introduced into open-ended stainless steel
enclosures placed in a mature experimental pond. Different volumes of
alpha-cypermethrin (as a diluted EC containing 100 g active
ingredient/litre) were applied with a hand-held sprayer onto the water
surface. The dose rates were equivalent to 5-500 g active
ingredient/ha. The concentrations in the water samples were dependent
on the dose applied and the time after the treatment that the samples
were taken. For instance, with a dose of 5 g alpha-cypermethrin/ha the
concentration in the water was 0.4 µg/litre or less after 96 h,
whereas with a dose of 500 g/ha residues of up to 30 µg/litre were
found. Alpha-cypermethrin was toxic to the rainbow trout at a
concentration of about 2-5 µg/litre water. In terms of nominal
application rate, the no-observed-effect level for alpha-cypermethrin
lies between 50 and 100 g alpha-cypermethrin/ha. These dose rates are
much higher than those used for crop protection purposes.
In a further study, 20 rainbow trout were placed in stainless
steel enclosures in a shallow pond. The diluted EC and SC formulations
were sprayed onto the surface of the water and the fish were monitored
for mortality over 8 days. The SC formulation did not cause mortality,
even at an application rate equivalent to 300 g active ingredient/ha.
However, high mortality (90%) occurred with the EC formulation at 30
g/ha alpha-cypermethrin, but application of 10 g active ingredient/ha
did not cause mortality. From these results it is clear that the SC
formulation has a lower toxicity than the EC formulation (Stephenson,
1987b).
The hazard to fish caused by spray drift from aerial applications
of alpha-cypermethrin to agricultural land has been investigated by
Garforth & Woodbridge (1984). The trial site was a cereal field
bordered on one side by a freshwater ditch. The water's edge was
generally less than 2 m from the crop margin. Four days prior to
application, two cages, each containing 15 common carp ( Cyprinus
carpio; 30-50 cm) were placed in the ditch. The ditch was known to
contain a good stock of different types of fish, macroinvert-ebrates
and zooplankton. An alpha-cypermethrin EC containing 100 g/litre was
applied by air at 15 g active ingredient/ha to the crop when a gentle
breeze was blowing from the crop over the ditch. The
alpha-cypermethrin concentration in the sub-surface water was 0.6
µg/litre shortly after the application and decreased to < 0.02
µg/litre within 2 to 4 days (see section 4.1.2.). None of the fish
died and no adverse effects were observed either on the carp placed in
the cages or on the indigenous species. It was concluded that
inadvertent contamination of water bodies by spray drift resulting
from the normal commercial use of alpha-cypermethrin does not present
a hazard to fish.
Several field tests in rice paddies have examined the toxicity of
alpha-cypermethrin to fish.
Stephenson (1986, 1987b) investigated the acute toxicity of 15
g/litre EC, 100 g/litre OSC and 50 g/kg WP formulations to fish in
plots of rice paddy in West Java. The application rates were 7.5, 15,
and 30 g alpha-cypermethrin/ha for the EC and OSC formulations and 30
g alpha-cypermethrin/ha for the WP formulation. Each of the
insecticide treatments was applied to the same plot on two separate
occasions; once 12 days after transplantation of the rice seedlings
and again 57 days after the transplantation. Prior to application, two
cages each containing 20 carp (Cyprinus carpio) (2.5-5 g) and two
cages each containing 20 specimens of Puntius gonionotus (1.4-5 g)
were placed in trenches cut in each plot. In addition, before the
first experiment, 30 fish of each of these two species were released
to swim freely in each plot. Survival of the fish was monitored daily
up to 7 days after each treatment. The water temperature was between
25 and 36 °C. Following the first application, only the EC formulation
at 15 and 30 g/ha resulted in significant mortality of caged and
free-swimming carp and Puntius gonionotus. The same was true for
carp following the second application, but no results were available
for Puntius gonionotus following the second application due to an
outbreak of disease among these fish.
Two other field studies were carried out in West Java to examine
the effects of an OSC formulation on the growth and survival of
free-swimming carp (6-8 g) in rice paddies. Experimental conditions
were similar to those described above for the acute studies. The water
temperature was 23-32 °C. In the first experiment, treatments with 15
g active ingredient/ha were carried out 21 and 33 days after
transplantation of the rice seedlings. In the second experiment, the
same dose rate was applied 51 and 64 days after transplanting. The
fish were monitored over periods of 3 to 4 weeks in each experiment.
No adverse effects on survival and growth of the carp were observed
(Stephenson, 1987a,b).
9.3 Terrestrial organisms
9.3.1 Earthworms
The toxicity of technical alpha-cypermethrin to the red
earthworm, Eisenia foetida, has been assessed in laboratory tests.
In the filter paper contact toxicity test, 50% mortality occurred
within 48 h at a dose of about 0.01 mg/cm2 of filter paper. However,
increases in the dose up to 1 mg/cm2 did not result in a significant
increase in mortality. In the artificial soil test no significant
mortality occurred within a period of 14 days in earthworms exposed to
up to 100 mg alpha-cypermethrin/kg soil (Inglesfield & Sherwood,
1983).
9.3.2 Invertebrates - field studies
In a study designed to investigate the effects of
alpha-cypermethrin on non-target arthropod fauna in Italian vineyards,
two formulations of alpha-cypermethrin were tested, i.e. an
emulsifiable concentrate (100 g active ingredient/litre) and a
suspension concentrate (250 g active ingredient/litre). Both were
diluted to 1.25 g active ingredient/ha and sprayed to run-off. The
effect on predators and parasites (crop foliage fauna) such as
parasitoid Hymenoptera, Heteroptera and Chrysopa carnea, soil
surface predators such as Coleoptera and Araneae, phytophagous
arthropods such as Homoptera, Thysanoptera and Acari, and other
invertebrates such as nematocerous, Diptera and epigeal fauna was
inconclusive but in most cases negative. Spiders were the only group
significantly affected by alpha-cypermethrin. The abundance of major
phytophagous insect taxa (Homoptera, Thysanoptera and Acari) was
markedly reduced by both treatments although neither treatment had any
long-term adverse effects (Inglesfield, 1984).
An emulsifiable concentrate (EC) of alpha-cypermethrin was tested
on the non-target arthropod fauna of maize in France at 20 and 30 g
active ingredient/ha and applied using tractor-mounted boom and nozzle
equipment at different stages of crop development. Organisms from the
following taxa were collected: phytophagous arthropods (Aphidoidea),
Thysanoptera (Thripidae), Cicadellidae and entomophagous arthropods;
predatory Coleoptera (Coccinellidae, Cantharidae, Carabidae and
Staphylinidae); parasitoid Hymenoptera (Braconidae, Chalcidoidea);
and predatory Diptera, Neuroptera and Aranea. In addition, the
effects on community structure were studied. The two treatments
reduced transiently the numbers of some of the entomophagous taxa,
especially Coccinellidae (Coleoptera), Carabidae (Coleoptera),
Neuroptera and Araneae. Alpha-cypermethrin at a concentration of
20 g/ha promoted some late-season resurgence of aphid populations
(Inglesfield, 1985a).
A large-scale replicated field experiment was carried out in
Indonesia to investigate the effects of an EC (10 g and 20 g
alpha-cypermethrin/ha) and an OSC (10 g and 20 g alpha-cypermethrin
per ha), applied at 17 and 62 days after transplanting using a single
fan-jet nozzle knapsack sprayer, on rice pests and their natural
enemies. Alpha-cypermethrin was applied at two crop stages, and
populations of both pests and beneficial arthropods were monitored
throughout the season. Alpha-cypermethrin produced good control of
stemborers, grasshoppers, leafhoppers and stinkbugs, the most numerous
pests found during the trial. It had a significant but short-lived
effect on spiders and, generally, no effect on either dragon-flies or
other beneficial arthropods (Shires & Inglesfield, 1986).
A study was initiated in England in 1982 to compare the effects
on entomophagous arthropods of annual applications of an EC of
alpha-cypermethrin (at a rate of 10 g active ingredient/ha in the
first year and 15 g active ingredient/ha in subsequent years) with
those resulting from the use of non-pyrethroid products. The study was
carried out on a range of crops (oilseed rape, wheat and barley) over
the duration of a 5-year arable crop rotation. A field of 8 ha was
divided into two equal areas, and each year alpha-cypermethrin was
applied to one of the areas and other products to the other area. All
treatments were carried out with standard tractor-driven boom and
nozzle spraying equipment. The crop rotation was as follows: winter
rape in 1982, winter wheat in 1983 and 1984, and winter barley in
1985. Organisms from a variety of taxa were collected during the
study, such as parasitoid Hymenoptera, predatory Diptera,
predatory Coleoptera, Araneae, phytophagous insects and other
arthropods. The results indicated that any difference between the
effects of alpha-cypermethrin and the reference compounds on
entomophagous arthropods was generally short-lived. There is no
evidence that alpha-cypermethrin treatments had any long-term effects
on any of the taxa studied. In addition, there appeared to be no
long-term effects on the relative abundance of entomophages within the
arthropod communities or on the structure and integrity of the
arthropod communities of which they form a part (Inglesfield, 1985c,
1988, 1989).
The results of these studies have been reviewed by Inglesfield
(1991). They showed that field application of alpha-cypermethrin and
cypermethrin had no adverse effects on the relative abundance of
entomophages within the arthropod communities and that the use of
these two pesticides in small grain cereals would not be associated
with pest "resurgence" or the development of secondary pest
infestations.
9.3.3 Honey-bees
9.3.3.1 Laboratory studies
The oral administration of alpha-cypermethrin in acetone produced
a 24-h LD50 of 0.06 µg/bee, whereas an EC formulation (100 g/litre)
of the pesticide yielded a 24-h LD50 of 0.13 µg formulation/bee.
After topical application, 24-h LD50 values of 0.03 µg (technical)
and 0.11 µg (EC) per bee were obtained (Murray, 1985).
The mortality of honey-bees (Apis mellifera) exposed to
Phacelia flowers 30 min after they had been treated with an EC
formulation (15 g active ingredient/ha) in a residual test was low
(15%) within 48 h. However, the foraging activity was reduced (Murray,
1985).
The residual toxicity to the honey-bee of alpha-cypermethrin as
EC, OSC, and EC/fungicide mixtures has been investigated. Bees were
exposed for 48 h to flowering Phacelia campanularia plants that had
been sprayed with each formulation at a rate of 10 or 20 g active
ingredient/ha, and the mortality was assessed 24 and 48 h after
initial exposure to the treated plants. Although there was great
variation in the results, there appeared to be no difference in the
residual toxicity of the EC and OSC formulations at a rate of 10 g
active ingredient/ha. However, at 20 g active ingredient/ha the OSC
appeared to be more toxic than the EC. Application of the EC in
admixtures did not appear to significantly increase bee mortality
(Hillaby & Inglesfield, 1986).
9.3.3.2 Field studies
Alpha-cypermethrin, applied as an EC (10 and 20 g active
ingredient/ha) by tractor-mounted boom and nozzle equipment to small
plots of flowering mustard in France, caused a sharp decline in
foraging activity of bees immediately after application, but there was
a return to normal activity within a few hours. No effect on bee
survival or hive development was observed (Shires, 1983a; Shires et
al., 1984b; Shires, 1985a).
In a further study, the same EC formulation was applied at the
same concentration on large isolated fields of flowering oilseed rape
during peak foraging activity of honey-bees in France. No increase in
bee mortality was found, but foraging activity declined for a few
hours after application at a rate of 10 g active ingredient/ha. With
a rate of 20 g active ingredient/ha a more prolonged decline in
foraging activity occurred. No effects on the overall condition of the
hives were seen at the end of the season and very low or undetectable
residues were found in dead bees, pollen, honey and wax (Shires et
al., 1984c; Shires, 1985a).
Two large and two small plots of winter wheat were enclosed
beneath large mesh-covered tunnels. A small bee-hive was placed in
each tunnel and sucrose solution was sprayed onto all of the wheat in
order to simulate aphid honey dew. Alpha-cypermethrin (an EC at 10, 15
or 30 g active ingredient/ha) was applied to the larger plots of wheat
when the bees were actively foraging the sugar deposits. No increase
in bee mortality, compared with that in the pre-treatment period, was
observed. Foraging activity in the plots declined sharply after
treatment and remained at a reduced level, probably because of its
repellent effect. Examination of the hives about 2 weeks after the
trial showed that the hives, both the control and the
alpha-cypermethrin-treated, were in excellent condition with strong
adult populations and large areas of developing brood. In
post-treatment samples, alpha-cypermethrin residues of 0.03 mg/kg of
honey and 0.01 mg/kg of wax were found. In live and dead bees
collected from the tunnel treated with 15 g/ha, a concentration of
0.026 µg/bee was found (Shires et al., 1984a; Le Blanc, 1985).
In a study by Inglesfield & Forbes (1986), alpha-cypermethrin was
applied as an OSC (10 g active ingredient/ha) and an EC (10 g active
ingredient/ha) to three fields of flowering winter-sown oilseed rape
in Germany while bees were actively foraging the crops. None of the
treatments had any significant effect on adult bee survival or on the
longer-term development of the experimental colonies. The number of
bees actively foraging in the crops declined following application.
This reduction in activity can probably be partly attributed to the
repellent effects of these treatments. Both the OSC and EC
formulations of alpha-cypermethrin had similar effects on bee
behaviour. Residues of alpha-cypermethrin in post-treatment samples of
pollen, honey and wax were below 0.01 mg/kg. In dead bees, the residue
on the day of application with the OSC was 1.8 mg/kg and in the case
of the EC was 0.12 mg/kg.
A diluted EC formulation of alpha-cypermethrin (mixed with the
fungicide vinclozolin, 100 g active ingredient/ha) was applied by a
tractor-driven boom and nozzle sprayer, at a rate of 20 g active
ingredient/ha, to a 206-ha block of flowering oilseed rape situated in
Kent, England. Shortly before spraying, which took place over a 3-day
period, five beehives were positioned at each of two sites adjacent to
the crop. At each site the hives were either fitted with pollen traps
or with traps to collect dead bees as they were removed from the hive.
The hives were observed daily before and for ten days after spraying.
Hive activity was recorded at intervals each day, and, on days when
bees were flying, pollen traps were set and samples of pollen
collected. Following the application of alpha-cypermethrin the bees
foraged normally. At least 90% of the pollen returned to the hives
during the immediate post-treatment period was found to be from
oilseed rape, showing that the bees had continued to forage on the
treated crop. The number of dead bees collected remained low
throughout the study and did not increase after application of the
alpha-cypermethrin. After the study, all hives were in good condition
and subsequently yielded a good crop of honey. A local beekeeper with
many hives adjacent to the same block of oilseed rape reported no
effects amongst his hives. It was concluded that the application of
alpha-cypermethrin at 20 g active ingredient/ha to flowering oilseed
rape had no direct effects on honey-bee survival and hive development
(Brown, 1989).
9.3.4 Leaf-cutting bees
Laboratory trials using 15 male adult alfalfa leaf-cutting bees
( Megachile rotundata F.) per group, exposed to treated filter papers
for 4 h, showed that alpha-cypermethrin EC (100 g/litre) at the rate
of 10 or 15 g active ingredient/ha caused 12 and 30% mortality,
respectively, and 42 and 100% mortality after 24 h of contact (Tasei
et al., 1987).
In a study by Tasei et al. (1987), populations of about 400
female alfalfa leaf-cutting bees were reared in three flowering
lucerne fields. One field was left untreated, while the two others
were treated with alpha-cypermethrin as an EC (10 or 15 g active
ingredient/ha), applied by a tractor-mounted fan-jet sprayer. Two days
after the treatments, counts of live females in artificial nesting
sites showed the losses due to 10 and 15 g active ingredient/ha to be
21 and 12%, respectively. After hibernation and incubation of the
progeny larvae, little effect of the treatments could be observed. The
maximum residue in leaves collected from nests was 1 mg
alpha-cypermethrin/kg. The mean values after 5, 10 and 27 days in leaf
pieces capping the nests of the bees were 0.75, 0.48, and 0.19 mg/kg
at the lower application rate and 0.59, 0.53, and 0.10 mg/kg at the
higher rate. No residues (limit of determination, 0.01 mg/kg) were
detected in live larvae but 0.07 mg/kg was found in pollen provisions
(Tasei et al., 1987).
9.3.5 Birds
Cypermethrin is practically non-toxic to birds; acute oral LD50
values are greater than 2000 mg/kg body weight. The dietary LC50
value is above 10 000 mg/kg diet (see WHO, 1989). However, studies
with alpha-cypermethrin have not been carried out.
10. COMPARISON BETWEEN ALPHA-CYPERMETHRIN AND CYPERMETHRIN
Alpha-cypermethrin comprises one quarter of the racemic mixture
cypermethrin, with which an extensive agricultural, ecological and
(eco)toxicological programme has been carried out (WHO, 1989). It
contains more than 90% of the insecticidally most active enantiomer
pair of the four cis isomers of cypermethrin, i.e. the two cis isomers
(IRcis)S and (IScis)R (see Fig. 1).
10.1 Use and residue levels
Alpha-cypermethrin is used to control the same pests in
agriculture as cypermethrin. Its rate of application to crops (5-30 g
active ingredient/ha) is lower than that of cypermethrin (10-200 g
active ingredient/ha) since alpha-cypermethrin is biologically more
active than cypermethrin.
Residue data for alpha-cypermethrin have been obtained from a
large number of supervised trials carried out worldwide. These trials
cover the most important crop groupings for which alpha-cypermethrin
is recommended, including oilseeds, pome fruits, peaches, fruiting
vegetables, berries, leafy vegetables, maize and speciality crops such
as hops and tobacco (Shell, 1984). Residues in a variety of these
crops resulting from application of alpha-cypermethrin at the
recommended rate ranged between 0.05 and 1.0 mg/kg (Shell, 1984). In
comparison, residues of cypermethrin in crops were higher and ranged
between 0.05 and 2.0 mg/kg. In comparative trials where
alpha-cypermethrin was applied at half the dose rate of cypermethrin,
alpha-cypermethrin residues in general averaged 40% (20-50%) of those
in cypermethrin-treated samples (Shell, 1984; WHO, 1989).
The residue data on cypermethrin have been evaluated by the Joint
FAO/WHO Meeting on Pesticide Residues and recommended MRLs (1979/1981)
have been published (FAO/WHO, 1980, 1982) (see Table 10). From the
available residue data obtained with good agricultural practice and
using the MRLs set for cypermethrin, suggested MRLs for
alpha-cypermethrin can be extrapolated (Table 10).
It can be concluded that alpha-cypermethrin, since it is
biologically more active, is always used at a lower application rate
than cypermethrin and, as a result, the residues on crops are
approximately half those of cypermethrin.
Table 10. Comparison of current MRLs in mg/kg product for cypermethrin
recommended by the FAO/WHO Joint Meeting on Pesticides Residues (JMPR)
with those which may be proposed for alpha-cypermethrin
Commodity Cypermethrin Alpha-cypermethrin
(JMPR)a (extrapolated)
Cotton seed 0.2 0.05
Rapeseed 0.2 0.05
Pome fruits 2 0.5
Peaches 2 0.5
Grapes 1 0.5
Citrus fruits 2 1
Tomatoes 0.5 0.1
Brassica, leafy vegetables 1 1
Lettuce 2 1
Peas, kidney beans (less pod) 0.05 0.05
Soyabeans (less pod) 0.05 0.05
Potatoes 0.05 0.05
Sugarbeet (roots) 0.05 0.05
Maize grain 0.05 0.05
a From: FAO/WHO (1980, 1982)
For analytical reasons, 0.05 mg/kg is considered the minimum practical
MRL.
10.2 Environmental impact
Because its physico-chemical properties are similar to those of
cypermethrin, alpha-cypermethrin is expected to have a similar
environmental fate to that of cypermethrin. The potential of
alpha-cypermethrin to bioaccumulate may therefore be estimated from
experimental data on the bioaccumulation of cypermethrin. This is
because the octanol/water partition coefficients of alpha-cypermethrin
(1.4 x 105; log Pow = 5.16) and cypermethrin (2 x 106; log Pow
= 6.3) are relatively similar. In fish, the bioaccumulation of
cypermethrin determined experimentally was lower than might have been
expected from its partition coefficient, presumably because it was
rapidly metabolized. This would also be expected for
alpha-cypermethrin because both the route of metabolism and its rate
are similar to those of cypermethrin (Shell, 1983b; WHO, 1989).
In the area of environmental toxicology, results available for
green algae, aquatic invertebrates, fish and bees show that the acute
toxicity of alpha-cypermethrin is slightly higher than, but broadly
similar to that of cypermethrin (Table 11). This is because the
toxicity of cypermethrin results largely from its alpha-cypermethrin
component. No toxicity data are available concerning the effects of
alpha-cypermethrin on soil microbes, but little or no effect on carbon
dioxide evolution, oxygen uptake and nitrogen fixation would be
expected if alpha-cypermethrin acts on soil microbes in a similar way
to cypermethrin (WHO, 1989). There are also no toxicity data for
alpha-cypermethrin in birds. However, cypermethrin has a low toxicity
to birds. Since alpha-cypermethrin constitutes 25% of the active
ingredients of cypermethrin and is used at lower application rates, it
is expected that alpha-cypermethrin will also have a low toxicity to
birds (Shell, 1983b).
Overall, in the natural environment, the more biologically active
alpha-cypermethrin is likely to have a similar toxicity to that of
cypermethrin. This is because alpha-cypermethrin is used at a lower
application rate than cypermethrin.
10.3 Mammalian toxicity
In acute oral toxicity studies, alpha-cypermethrin is either
equally toxic or two to three times more toxic than cypermethrin (WHO,
1989), depending on the vehicle and the concentrations used.
In Table 12, the no-observed-effect levels and
lowest-observed-effect levels of the various mouse, rat and dog
studies are compared for cypermethrin, cis-cypermethrin and
alpha-cypermethrin. Short- and long-term studies with the racemic
mixture cypermethrin (containing four cis and four trans isomers),
cis-cypermethrin (four cis isomers) and alpha-cypermethrin (two cis
isomers) have shown similar toxicological effects.
The data from the short-term toxicity studies indicate that
alpha-cypermethrin is approximately 2 to 3 times more toxic than
cypermethrin in rats and dogs. This reflects the amount of
alpha-cypermethrin present in cypermethrin.
The signs of intoxication, the effects on target organs and
tissues, and the metabolic pathway of alpha-cypermethrin are similar
to those of cis-cypermethrin. The mode of action of
alpha-cypermethrin is similar to that of cypermethrin.
Cypermethrin has been tested in a rodent multigeneration
reproduction study and for embryotoxicity and teratogenicity in two
species. There were no effects on either reproductive performance or
on fetal development, even at doses producing systemic toxicity (WHO,
1989). Alpha-cypermethrin has not been tested for reproductive
toxicity or teratogenicity, but there is no indication that it would
have effects on these parameters since it is a component of
cypermethrin.
Table 11. Comparison of environmental toxicology of cypermethrin and alpha-cypermethrin
Species Life stage/ Water Administration Measured Values for Reference
age temper- route or vehicle end-point cypermethrin alpha-
ature (°C) cypermethrin
Green alga
(Selenastrum - 24 dispersal 2- to 4-day > 100 µg/litre > 100 µg/litre Stephenson
capricornutum) via acetone EC50 (growth) (1982)
Aquatic invertebrates
Water flea up to 24 h 20 dispersal EC50 Stephenson
(Daphnia magna) old via acetone (immobilization) (1982)
24 h 1.2 µg/litre 1.1 µg/litre
48 h 0.3 µg/litre 0.3 µg/litre
Fish
Rainbow trout 3.3 g 15 dispersal 96-h LC50 2.8 µg/litre 2.8 µg/litre Stephenson
(Oncorhynchus mykiss) via acetone (1982)
Fathead minnow 0.74-0.76 g 23-25 absorbed on 96-h LC50 1.2 µg/litre 0.93 µg/litre Stephenson
(Pimephales promelas) (juvenile) to pumice (1983)
Earthworm
(Eisenia foetida) - - filter paper 48-h LD50 26.1 µg/cm2 10 µg/cm2 Rob
contact & Dorough
toxicity (1984);
Inglesfield
& Sherwood
(1983)
Table 11 (continued)
Species Life stage/ Water Administration Measured Values for Reference
age temper- route or vehicle end-point cypermethrin alpha-
ature (°C) cypermethrin
Bees
(Apis mellifera) worker - oral 24-h LD50 0.035 µg/bee 0.06 µg/bee Badmin
adminis- & Twydell
tration (1976);
Murray
(1985)
Table 12. Comparison between short- and long-term oral studies with
cypermethrin, cis-cypermethrin and alpha-cypermethrin
Animal Number of Duration of Dose level in mg/kg diet
species studies experiment No-observed- Lowest-observed-
effect level effect level
Cypermethrin (4 cis and 4 trans isomers)a
Mouse 1 2 years 400 1600
Rat 1 5 weeks 750 1500
2 13 weeks 100/150 400
1 2 years 100 1000
Dog 1 13 weeks 500 1500
1 2 years 300 750/600
cis-cypermethrin (4 cis isomers)a
Rat 1 5 weeks 100 300
Alpha-cypermethrin (2 cis isomers)
Rat 1 5 weeks 200 400
1 13 weeks 60 180
Dog 2 2-7 days 200 250
1 13 weeks 90 270
a See WHO (1989)
The WHO Task Group on Environmental Health Criteria for
Cypermethrin, which met in 1986, concluded that cypermethrin was
without mutagenic activity. Available data on alpha-cypermethrin
indicate that this compound also is non-mutagenic in tests with
Salmonella typhimurium, Saccharomyces cerevisiae, and in vivo and
in vitro tests with rat liver cells for the induction of chromosome
aberration and production of DNA single-strand damage.
Alpha-cypermethrin has not been tested for carcinogenicity, but
the long-term toxicity studies in mice and rats did not indicate any
carcinogenic potential for cypermethrin. Because cypermethrin contains
the two cis isomers present in alpha-cypermethrin, it is unlikely that
these two isomers would have a carcinogenic potential. This
supposition is supported by the fact that all the mutagenicity studies
on alpha-cypermethrin have yielded negative results.
Near lethal doses of alpha-cypermethrin and cypermethrin produce
sparse axonopathy in the peripheral nerves of rats (see section
7.7.2). Similar signs of intoxication were observed with both
compounds, and increases in beta-glucuronidase and beta-galactosidase
activities, consistent with an axonal degeneration, could only be
detected in severely intoxicated animals. The magnitude of the enzyme
changes was substantially less than for other known neurotoxic
compounds, thereby confirming the minor nature of the lesion. The
short time period needed to produce ataxia and/or abnormal gait rules
out a causal relationship between ataxia and axonopathy. The signs of
intoxication are consistent with a pharmacologically-mediated effect.
No biochemical changes indicative of axonopathy were detected in rats
given 20 oral doses of 20 mg alpha-cypermethrin/kg body weight per day
or 75 mg cypermethrin/kg body weight per day. From these comparative
studies, it is clear that the neurotoxic potential of
alpha-cypermethrin and cypermethrin is qualitatively similar, but
alpha-cypermethrin is 3 to 4 times more potent. This reflects the fact
that alpha-cypermethrin constitutes the active ingredient and 25% of
cypermethrin.
Appraisal
The effects of alpha-cypermethrin on vertebrates and
invertebrates are qualitatively and, in a number of cases, even
quantitatively similar to those of cypermethrin, reflecting the fact
that alpha-cypermethrin is composed of two of the four cis isomers
present in cypermethrin (these two being the most active components
of cypermethrin). Therefore, the toxicological information of
cypermethrin can be used to evaluate the effects of
alpha-cypermethrin where certain important toxicity studies for
alpha-cypermethrin are lacking.
Alpha-cypermethrin has a higher toxicity (lower
no-observed-effect level) than cypermethrin but its application rate
is at most only half that of cypermethrin (5-30 g active
ingredient/ha for alpha-cypermethrin and 10-200 g active
ingredient/ha for cypermethrin). Therefore, residue levels of
alpha-cypermethrin in crops are less than half those of cypermethrin.
11. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The toxicological and residue data on cypermethrin have been
evaluated by the Joint FAO/WHO Meeting on Pesticide Residues (JMPR)
(FAO/WHO, 1980, 1982), but alpha-cypermethrin has not yet been
evaluated by the JMPR.
REFERENCES
Aldridge WN (1990) An assessment of the toxicological properties of
pyrethroids and their neurotoxicity. Crit Rev Toxicol, 21(2): 89-104.
Armitage GD (1984) Fastac exposure study. Analysis of atmospheric and
surface wipe samples ex. Durban, South Africa. Sittingbourne, Shell
Research (SBRN 84.091).
Badmin JS & Twydell RS (1976) Evaluation of the insecticide WL 43467
against the honey bee Apis mellifera. Sittingbourne, Shell Research
(WKSR.0021.76).
Baldwin MK (1990) Alpha-cypermethrin (Fastac): Water solubility at
various pH values. London, Shell International Chemical Company, Ltd
(Internal report SBGR 90.158).
Blair D (1984) The toxicology of pyrethroids; the acute 4-h inhalation
toxicity of a 30% m/m Fastac on silica powder formulation.
Sittingbourne, Shell Research (SBGR 84.066).
Bosio PG (1982) Study of isomer conversion of WL85871 in various crops
from 1981 treatments. Berre, France, Shell Chimie (BEGR 82.031).
Brooks TM (1982) Toxicity studies with pyrethroids; in vitro
genotoxicity studies with WL85871. Sittingbourne, Shell Research (SBGR
81.273).
Brooks TM (1984) Genotoxicity studies with Fastac; the induction of
gene mutation in the yeast Saccharomyces cerevisiaee XV 185-14-C.
Sittingbourne, Shell Research (SBGR 84.117).
Brown K (1989) A study of the effects on honey-bees of a large scale
commercial application of "Fastac" to oilseed rape. London, Shell
International Chemical Company, Ltd (Internal report SBGR 89.198).
Clare MG & Wiggins DE (1984) Genotoxicity studies with Fastac; in
vivo cytogenetic test using rat bone marrow. Sittingbourne, Shell
Research (SBGR 84.120).
Clark DG (1982) WL85871; a 90-day feeding study in rats.
Sittingbourne, Shell Research, vol 1 and 2 (SBGR 81.293).
Coveney PC & Forbes S (1986) Analysis of soil from UK (Coates) for
residues of "Fastac" (WL85871) - soil persistence trial - third year.
Sittingbourne, Shell Research (SBGR 86.201).
a The proprietary Shell Research reports in the following list were
submitted to the IPCS by Shell.
Creedy CL & Logan CJ (1984) In vitro metabolism of cypermethrin
isomers by rat, rabbit, and human liver preparations. Sittingbourne,
Shell Research (SBGR 84.108).
Dewar AJ (1981) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity, skin and eye irritancy and skin sensitizing
potential of WL85871 including a comparison with the acute toxicity of
WL 43467 (Ripcord). Sittingbourne, Shell Research (TLGR 80.148).
Eadsforth CV, Bragt PC, & Van Sittert NJ (1988) Human dose-excretion
studies with pyrethroid insecticides cypermethrin and
alpha-cypermethrin; relevance for biological monitoring. Xenobiotica,
18(5): 603-614.
FAO (1982) Second Government Consultation on International
Harmonization of Pesticide Registration Requirements, Rome, 11-15
October 1982. Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1980) 1979 Evaluations of some pesticide residues in food.
Rome, Food and Agriculture Organization of the United Nations (FAO
Plant Production and Protection Paper 20 sup).
FAO/WHO (1982) 1981 Evaluations of some pesticide residues in food.
Rome, Food and Agriculture Organization of the United Nations (FAO
Plant Production and Protection Paper 42).
Fisher JR, Robinson J, & Debray PH (1983) A new multipurpose
insecticide. Proceedings of 10th International Congress of Plant
Protection, Brighton, England, 20-25 November 1983. Vol. 1, pp
452-459.
Forbes S (1985) Residues of Fastac (WL85871) in cured marine catfish
( Arius sp.) from Indonesia. Sittingbourne, Shell Research (SBGR
85.200).
Forbes S & Burden AN (1984) Analysis of soil from UK (Reculver) for
residues of WL85871 (Fastac) - soil persistence trial - second year.
Sittingbourne, Shell Research (SBGR 84.005).
Forbes S & Cole DA (1986) Residues of Fastac (WL85871) in sweetcorn
from Canada. Sittingbourne, Shell Research (SBGR 86.217).
Forbes S & Knight CJ (1983) Analysis of soil from UK (Reculver) for
residues of WL85871 - soil persistence trial - first year.
Sittingbourne, Shell Research (SBGR.83.162).
Forbes S & Mackay CE (1983) Analysis of soil from UK (Coates) for
residues of WL85871 (Fastac) - soil persistence trial - first year.
Sittingbourne, Shell Research (SBGR 83.418).
Forbes S & Wales GH (1985a) Analysis of soil from UK (Reculver) for
residues of Fastac (WL85871) - soil persistence trial - third year.
Sittingbourne, Shell Research (SBGR 85.070).
Forbes S & Wales GH (1985b) Analysis of soil from UK (Coates) for
residues of Fastac (WL85871) - soil persistence trial - second year.
Sittingbourne, Shell Research (SBGR 85.071).
Francis WP & Gill JP (1991) Flufenoxuron/alpha-cypermethrin - residues
in sheep tissues following treatment with PAMPASS/RENEGADE mixtures by
dip or pour-on. London, Shell International Chemical Company, Ltd
(Internal report SBGR 89.254).
Gardner JR (1989) "Fastac/Azodrin" 20/400 g/litre emulsifiable
concentrate: Acute oral and dermal toxicity. London, Shell
International Chemical Company, Ltd (Internal report SBGR 89.026).
Gardner JR (1991) Fastac 60 G/l SC (SF07396); acute oral and dermal
toxicity in rat. Sittingbourne, Shell Research (SBGR 91.020).
Garforth BM (1982a) A comparison of the toxicities of WL85871 and
Ripcord to freshwater invertebrates in small field enclosures.
Sittingbourne, Shell Research (SBGR 82.015).
Garforth BM (1982b) WL85871 and cypermethrin; chronic toxicity to
Daphnia magna. Sittingbourne, Shell Research (SBGR 82.119).
Garforth BM & Woodbridge AP (1984) Spray drift from an aerial
application of Fastac; fate and biological effects in an adjacent
freshwater ditch. Sittingbourne, Shell Research (SBGR 84.055).
Greenough RJ & Goburdhun R (1984) WL85871; Oral (dietary) maximum
tolerated dose study in dogs. Musselburgh, Inveresk Research
International (Unpublished report No. 3107, submitted to WHO by Shell
Research).
Greenough RJ, Cockrill JB, & Goburdhun R (1984) WL85871; 13-week oral
(dietary) toxicity study in dogs. Musselburgh, Inveresk Research
International (Unpublished report No. 3197, submitted to WHO by Shell
Research).
Hend RW (1983) Toxicology of pyrethroids; skin stimulation studies
using guinea-pigs. Sittingbourne, Shell Research (SBGR 83.197).
Hillaby JM (1988) Deposition of pesticide on the orchard floor
following a commercial mistblower application to apples. London, Shell
International Chemical Company, Ltd (Internal report SBGR 88.106).
Hillaby JM & Inglesfield C (1986) The residual toxicity of "Fastac"
formulations and "Fastac"/fungicide mixtures to the honey bee, Apis
mellifera L. Sittingbourne, Shell Research (SBGR 86.018).
Hutson DH (1982) WL85871; metabolism of a single oral dose in the rat.
Sittingbourne, Shell Research (SBGR 82.205).
Hutson DH & Logan CJ (1986) The metabolic fate in rats of the
pyrethroid insecticide WL85871, a mixture of two isomers of
cypermethrin. Pestic Sci, 17: 548-558.
Inglesfield C (1984) The effects of two formulations of Fastac on the
beneficial arthropod fauna of grape vines. Sittingbourne, Shell
Research (SBGR 84.039).
Inglesfield C (1985a) A field study on the effects of Fastac on the
beneficial arthropod fauna of maize in France. Sittingbourne, Shell
Research (SBGR 85.069).
Inglesfield C (1985b) A pond enclosure study of the effects of Fastac
formulations on aquatic invertebrates. Sittingbourne, Shell Research
(SBGR 85.083).
Inglesfield C (1985c) The effects of the pyrethroid insecticide
WL85871 on non-target arthropods: Field studies. Pestic Sci, 16(2):
211.
Inglesfield C (1988) Effects of "Fastac" on non-target arthropods; an
overview of a five-year arable crop rotation study. Sittingbourne,
Shell Research (SBGR 87.149).
Inglesfield C (1989) A long-term field study to investigate the
effects of alpha-cypermethrin on predatory and parasitic arthropods.
Meded Fac Landbouwwet Rijksuniv Gent, 54(3a): 895-904.
Inglesfield C (1991) Effects of alpha-cypermethrin (Fastac) on
entomophagous organisms and game-bird chick-food insects in summer
cereals. The Hague, Shell Internationale Petroleum Maatschappij B.V.
(Report Series HSE 91.008).
Inglesfield C & Forbes S (1986) A field trial to assess the effects of
"Fastac" 100 g/litre OSC on foraging honey bees in oilseed rape.
Sittingbourne, Shell Research (SBGR 86.087).
Inglesfield C & Sherwood CM (1983) Toxicity of cypermethrin and
WL85871 to the earthworm, Eisenia foetida L.
(Oligochaeta:Lumbriculidae) in laboratory tests. Sittingbourne,
Shell Research (SBGR 83.071).
Langner EJ (1980) Determination of the vapour pressure of WL 43467 and
WL 85871 at 20 °C. Sittingbourne Shell Research (Research Note No.
FCDN 80.137).
Le Blanc J (1985) Field experiments on the effects of a new pyrethroid
insecticide WL85871 on bees foraging artificial aphid honey-dew on
winter wheat. Pestic Sci, 16(2): 206.
Le Quesne PM, Maxwell IC, & Butterworth STG (1980) Transient facial
sensory symptoms following exposure to synthetic pyrethroids; a
clinical and electrophysiological assessment. Neurotoxicology, 2:
1-11.
Logan CJ (1983) WL85871; depletion from tissues of female rats after
a single oral dose. Sittingbourne, Shell Research (SBGR 83.075).
McMinn AL (1983a) The degradation of the pyrethroid insecticides
WL85871 (Fastac) and WL43481 in cabbage. Sittingbourne, Shell Research
(SBGR 83.396).
McMinn AL (1983b) The degradation of the pyrethroid insecticides
WL85871 and WL43481 in soil. Sittingbourne, Shell Research (SBGR
83.395).
Maloney SE, Maule A, & Smith ARW (1988) Microbial transformation of
the pyrethroid insecticides: Permethrin, deltamethrin, fastac,
fenvalerate and fluvalinate. Appl Environ Microbiol, 54(11):
2874-2876.
Murray A (1985) Acute and residual toxicity of a new pyrethroid
insecticide WL85871, to honey-bees. Bull Environ Contam Toxicol, 34:
560-564.
Pearson N (1986) Fastac oil-enriched suspension concentrates; acute
toxicities of two formulations to the rainbow trout, Salmo gairdneri.
Sittingbourne, Shell Research (SBGR 85.290).
Pearson N (1990) The fate of "Fastac" in experimental ponds.
Sittingbourne, Shell Research (SBGR 88.177).
Pickering RG (1982) A 5-week feeding study with WL85871 in rats.
Sittingbourne, Shell Research, vol 1 and 2 (SBGR 81.212).
Price JB (1985a) Toxicology of Fastac (WL85871); the acute oral and
percutaneous toxicity and skin irritancy of a Fastac 10% EC (SF
06510). Sittingbourne, Shell Research (SBGR 85.087).
Price JB (1985b) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity, skin and eye irritancy of a Fastac 100 g/litre
suspension concentrate (SF 06378). Sittingbourne, Shell Research (SBGR
84.298).
Price JB (1986) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity, skin and eye irritancy of SF 06615, a 15
g/litre suspension concentrate of WL85871 ("Fastac", "Fendona").
Sittingbourne, Shell Research (SBGR 85.224).
Price JB (1987) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity of Fastac/BPMC 10/400 g/litre EC (SF 06717).
Sittingbourne, Shell Research (SBGR 86.253).
Price JB (1988) Toxicology of animal health products; the acute oral
and percutaneous toxicity, skin and eye irritancy of two pour-on
formulations of Renegade, SF 06954 (10 g/litre) and SF 06977 (15
g/litre). Sittingbourne, Shell Research (SBGR 87.161).
Roberts BL & Dorough HW (1984) Relative toxicities of chemicals to the
earthworm Eisenia foedita. Environ Toxicol Chem, 3(1): 67-68.
Rose GP (1982) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity of WL85871 ( cis-2-Ripcord) comparison with
Ripcord. Sittingbourne, Shell Research (SBGR 82.130).
Rose GP (1983a) Toxicology of pyrethroids; the acute oral toxicity of
WL85871 in comparison with WL43467. Sittingbourne, Shell Research
(SBGR 83.101).
Rose GP (1983b) Neurotoxicity of WL85871 in comparison with WL43467;
the effect of twenty oral doses of WL85871 or WL43467 over a period of
4 weeks on the rat sciatic/posterior tibial nerve, trigeminal nerve
and trigeminal ganglion. Sittingbourne, Shell Research (SBGR 83.185).
Rose GP (1984a) Toxicology of pyrethroids; the acute intraperitoneal
toxicity of technical Fastac. Sittingbourne, Shell Research (SBGR
84.085).
Rose GP (1984b) Toxicology of Fastac; the eye irritancy potential of
the Fastac 100 g/litre emulsifiable concentrate formulation DF 05898
and its formulation components. Sittingbourne, Shell Research (SBGR
84.053).
Rose GP (1984c) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity, skin and eye irritancy of the Fastac 15 g/litre
ULV formulation SF 06363. Sittingbourne, Shell Research (SBGR 84.145).
Rose GP (1984d) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity, skin and eye irritancy of the Fastac 10 EC
formulation, 5835 B. Sittingbourne, Shell Research (SBGR 84.077).
Rose GP (1984e) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity, skin and eye irritancy of the Fastac 10 EC
formulation 5898 B. Sittingbourne, Shell Research (SBGR 84.078).
Rose GP (1984f) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity, skin and eye irritancy of the Fastac 3 EC
formulation, DF 06353. Sittingbourne, Shell Research (SBGR 84.084).
Rose GP (1984g) Toxicology of pyrethroids; the eye irritancy and skin
sensitizing potential of the 100 g/litre Fastac emulsifiable
concentrate formulation EF 5835. Sittingbourne, Shell Research (SBGR
84.086).
Rose GP (1984h) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity, skin and eye irritancy of the 15:120 g/litre
Fastac/methomyl emulsifiable concentrate formulation, FD 9148.
Sittingbourne, Shell Research (SBGR 84.104).
Rose GP (1985) Toxicology of insecticides; the acute oral and
percutaneous toxicity, skin and eye irritancy of the 30 g/litre Fastac
EC formulation SF 06446. Sittingbourne, Shell Research (SBGR 84.209).
Senior PL & Lavers A (1990a) Fastac - Potential dermal exposure. The
Hague, Shell Internationale Petroleum Maatschappij B.V. (Report Series
HSE 90.016).
Senior PL & Lavers A (1990b) A field study of operator exposure to
Fastac during crop spraying at Shell Research Ltd, Sittingbourne
Research Centre. The Hague, Shell Internationale Petroleum
Maatschappij B.V. (Report Series HSE 90.014).
SHELL (1983a) Review of mammalian and human toxicology; Fastac. The
Hague, Shell Internationale Petroleum Maatschappij B.V. (Review Series
MDT 83.001).
SHELL (1983b) Review of environmental toxicology; Fastac. The Hague,
Shell Internationale Petroleum Maatschappij B.V. (Review Series MDT
83.005).
SHELL (1984) Review of residue information; Fastac. London, Shell
International Chemical Company Ltd.
SHELL (1986) Determination of residues of alpha-cypermethrin in rat
blood - Gas chromatographic method. Sittingbourne, Shell Research
(Analytical Method Series, No. SAMS 436-1).
SHELL (1987a) Determination of WL 85871 and the ratio of the
enantiomer pairs in technical material and formulated products -
liquid chromatographic method. Sittingbourne, Shell Research
(Analytical Methods Series, No. SAMS 346-4).
SHELL (1987b) Intereg residues section: Ripcord residues in crops.
London, International Chemical Company, Ltd (Internal report).
SHELL (1987c) Intereg residues section: Fastac residues in crops.
London, International Chemical Company, Ltd (Internal report).
SHELL (1988a) Determination of residues of alpha-cypermethrin in
animal tissues - Gas-liquid chromatographic method. Sittingbourne,
Shell Research (Analytical Method Series, No. SAMS 461-1).
SHELL (1988b) Determination of residues of alpha-cypermethrin in milk
- Gas-liquid chromatographic method. Sittingbourne, Shell Research
(Analytical Method Series, No. SAMS 456-1).
SHELL (1989a) Determination of residues of alpha-cypermethrin in crops
- Gas chromatographic method. Sittingbourne, Shell Research
(Analytical Method Series, No. SAMS 351-2).
SHELL (1989b) Review of environmental toxicology; Fastac. The Hague,
Shell Internationale Petroleum Maatschappij B.V. (Review Series HSE
89.008).
SHELL (1990a) Determination of residues of alpha-cypermethrin in water
- Gas chromatographic method. Sittingbourne, Shell Research
(Analytical Method Series, No. SAMS 469-2).
SHELL (1990b) Determination of residues of alpha-cypermethrin in soils
- Gas chromatographic method. Sittingbourne, Shell Research
(Analytical Method Series, No. SAMS 354-2).
Sherren AJ (1988a) Residues of alpha-cypermethrin in cattle tissues
following topical treatment of calves with "Renegade" pour-on in the
UK. London, Shell International Chemical Company, Ltd (Internal report
SBGR 88.036).
Sherren AJ (1988b) Residues of alpha-cypermethrin in milk following
topical treatment of cows with "Renegade" pour-on in the UK. London,
Shell International Chemical Company, Ltd (Internal report SBGR
88.037).
Shires SW (1982) A comparison of the toxicity of WL85871 and Ripcord
to rainbow trout ( Salmo gairdneri, Richardson) in small field
enclosures. Sittingbourne, Shell Research (SBGR 82.089).
Shires SW (1983a) Pesticides and honey bees; case studies with Ripcord
and Fastac. Span, 26(3): 118-120.
Shires SW (1983b) Effect of formulation type on the toxicity of
insecticides to fish. Sittingbourne, Shell Research (SBGR 83.015).
Shires SW (1985a) A step-wise evaluation of the effects of a new
pyrethroid insecticide WL85871 on honey bees. Pestic Sci, 16(2):
205-216.
Shires SW (1985b) Toxicity of a new pyrethroid insecticide WL85871 to
rainbow trout. Bull Environ Contam Toxicol, 34: 134-137.
Shires SW & Inglesfield C (1986) A field study of the effects of
"Fastac" (WL85871) on rice pests and their natural enemies.
Sittingbourne, Shell Research (SBGR 86.003).
Shires SW, Le Blanc J, Debray P, Forbes S, & Louveaux J (1984a) Field
experiments on the effects of a new pyrethroid insecticide WL85871 on
bees foraging artificial aphid honey-dew on winter wheat. Pestic Sci,
15: 543-552.
Shires SW, Murray A, Debray P, & Le Blanc J (1984b) The effects of a
new pyrethroid insecticide WL85871 on foraging honey bees ( Apis
mellifera L.) Pestic Sci, 15: 491-499.
Shires SW, Le Blanc J, Murray A, Forbes S, & Debray P (1984c) A field
trial to assess the effects of a new pyrethroid insecticide WL85871 on
foraging honey bees in oilseed rape. J Agric Res, 23(4): 217-226.
Stephenson RR (1982) WL85871 and cypermethrin; a comparison of their
acute toxicity to Salmo gairdneri, Daphnia magna and Selenastrum
capricornutum. Sittingbourne, Shell Research (SBGR 81.277).
Stephenson RR (1983) WL85871 and cypermethrin; a comparative study of
their toxicity to the fathead minnow, Pimephales promelas
(Rafinesque). Sittingbourne, Shell Research (SBGR 82.298).
Stephenson RR (1986) "Fastac"; the acute toxicity of different
formulations to fish in rice paddies. Sittingbourne, Shell Research
(SBGR 85.201).
Stephenson RR (1987a) The effects of "Fastac" (OSC) on fish survival
and growth following its application to paddy rice. Sittingbourne,
Shell Research (SBGR 87.054).
Stephenson RR (1987b) An insecticide formulation that spares fish.
Span, 30(2): 75-77.
Stephenson RR (1987c) The effects of "Fastac" (OSC) on fish following
its application to outdoor tanks. Sittingbourne, Shell Research (SBGR
86.227).
Stone CM & Watkinson RJ (1983) WL85871; an assessment of ready
biodegradability. Sittingbourne, Shell Research (SBGR 83.206).
Tasei J-N, Carre S, Bosio PG, Debray P, & Hariot J (1987) Effects of
the pyrethroid insecticide WL85871 and Phosalone on adults and progeny
of the leaf-cutting bee, Megachile rotundata F., pollinator of
lucerne. Pestic Sci, 21: 119-128.
Van Sittert NJ, Eadsforth CV, & Bragt P (1985) Human oral
dose-excretion study with Fastac. The Hague, Shell Internationale
Petroleum Maatschappij B.V. (Report Series HSE 85.010).
Vijverberg HPM & Van Den Bercken J (1990) Neurotoxicological effects
and the mode of action of pyrethroid insecticides. Crit Rev Toxicol,
21(2): 105-126.
Western NJ (1984) Report on the assessment of Fastac exposures during
formulation of technical concentrate (TC) and technical material (TM)
at Durban, South Africa, September 1983. The Hague, Shell
Internationale Petroleum Maatschappij B.V. (Report Series HSE 84.003).
WHO (1989) Environmental Health Criteria 82: Cypermethrin. Geneva,
World Health Organization, 154 pp.
Wooder MF (1982) Studies on the effect of WL85871 on the integrity of
rat liver DNA in vivo. Sittingbourne, Shell Research (SBGR 81.225).
Woollen BH, Marsh JR, & Chester G (1991) Metabolite profiles of a
pyrethroid insecticide following oral and dermal absorption in man.
In: Proceedings of a Conference on Percutaneous Penetration,
Southampton, England, 10-12 April 1991.
Worthing CR & Hance RJ (1991) Fastac. In: The pesticides manual: a
world compendium, 9th ed. Croydon, British Crop Protection Council, pp
210-211.
APPENDIX I
Cypermethrin: Summary, Evaluation, Conclusions, and
Recommendations (Reprint from EHC 82 on Cypermethrin, WHO/IPCS, 1989)
1. Summary
1.1 General
Cypermethrin was initially synthesized in 1974 and first marketed
in 1977 as a highly active synthetic pyrethroid insecticide, effective
against a wide range of pests in agriculture, public health, and
animal husbandry. In agriculture, its main use is against foliage
pests and certain surface soil pests, such as cutworms, but because of
its rapid breakdown in soil, it is not recommended for use against
soil-borne pests below the surface.
In 1980, 92.5% of all the cypermethrin produced in the world was
used on cotton; in 1982, world production was 340 tonnes of the active
material. It is mainly used in the form of an emulsifiable
concentrate, but ultra-low volume concentrates, wettable powders, and
combined formulations with other pesticides are also available.
Chemically, cypermethrin is the alpha-cyano-3-phenoxybenzyl ester
of the dichloro analogue of chrysanthemic acid,
2,2-dimethyl-3-(2,2-dichlorovinyl) cyclopropanecarboxylic acid. The
molecule embodies three chiral centres, two in the cyclopropane ring
and one on the alpha cyano carbon. These isomers are commonly grouped
into four cis and four trans isomers, the cis group being the more
powerful insecticide. The ratio of cis to trans isomers varies from
50:50 to 40:60. Cypermethrin is the racemic mixture of all eight
isomers and, in this appraisal, cypermethrin refers exclusively to the
racemic mixture (ratio 50:50) unless otherwise stated.
Most technical grades of cypermethrin contain more than 90% of
the active material. The material varies in physical form from a
brown-yellow viscous liquid to a semi-solid.
Cypermethrin has a very low vapour pressure and solubility in
water, but it is highly soluble in a wide range of organic solvents.
Analytical methods are available for the determination of cypermethrin
in commercially available preparations. In addition, methods for the
determination of residues of cypermethrin in foods and in the
environment are well established. In most substrates, the practical
limit of determination is 0.01 mg/kg.
1.2 Environmental transport, distribution and transformation
Unlike the natural pyrethrins, cypermethrin is relatively stable
to sunlight and, though it is probable that photodegradation plays a
significant role in the degradation of the product on leaf surfaces
and in surface waters, its effects in soils are limited. The most
important photodegradation products, 2,2-dimethyl-3-
(2,2-dichlorovinyl) cyclopropane carboxylic acid (CPA),
3-phenoxy-benzoic acid (PBA) and, to some extent, the amide of the
intact ester, do not differ greatly from those resulting from
biological degradation.
Degradation in the soil occurs primarily through cleavage of the
ester linkage to give CPA, PBA, and carbon dioxide. Some of the carbon
dioxide is formed through the cleavage of both the cyclopropyl and
phenyl rings under oxidative conditions. The half-life of cypermethrin
in a typical fertile soil is between 2 and 4 weeks.
Cypermethrin is adsorbed very strongly on soil particles,
especially in soils containing large amounts of clay or organic
matter. Movement in the soil is therefore extremely limited and
downward leaching of the parent molecule through the soil does not
occur to an appreciable extent under normal conditions of use. The two
principal degradation products show, on the scale of Helling,
"intermediate mobility".
Cypermethrin is also relatively immobile in surface waters and,
when applied to the surface of a body of water at rates typical of
those used in agriculture applications, it is largely confined to the
surface film and does not reach deeper levels or the sediment in
appreciable concentrations. Cypermethrin also degrades readily in
natural waters with a typical half-life of about 2 weeks. It is
probable that both photochemical and biological processes play a part.
It has been shown that spray drift reaching surface waters adjacent to
sprayed fields does not result in long-term residues in such waters.
Accumulation studies have shown that cypermethrin is rapidly
taken up by fish (accumulation factor approximately 1000); the
half-life of residues in rainbow trout was 8 days. In view of the low
concentrations of cypermethrin that are likely to arise in water
bodies and their rapid decline, it has been concluded that, under
practical conditions, residues in fish will not reach measurable
levels.
The results of field studies have shown that, when applied at
recommended rates, the levels of cypermethrin and its degradation
products in soil and surface waters are very low. Thus, it is unlikely
that the recommended use of cypermethrin will have any effects on the
environment.
1.3 Environmental levels and human exposure
Cypermethrin is used in a wide range of crops. In general, the
maximum residue limits are low, ranging from 0.05 to 2.0 mg/kg in the
different food commodities. The residues will be further reduced
during food processing. In food of animal origin, residues may range
between 0.01 and 0.2 mg/kg product. Residues in non-food commodities
are generally higher, ranging up to 20 mg/kg product.
Total dietary intake values for man are not available, but it can
be expected that the oral exposure of the general population is low to
negligible.
1.4 Kinetics and metabolism
Absorption of cypermethrin from the gastrointestinal tract and
its elimination are quite rapid. The major metabolic reaction is
cleavage of the ester bond. Elimination of the cyclopropane moiety in
the rat, over a 7-day period, ranged from 40 to 60% in the urine and
from 30 to 50% in the faeces; elimination of the phenoxybenzyl moiety
was about 30% in the urine and 55 to 60% in the faeces. Biliary
excretion is a minor route of elimination for the cyclopropane moiety
and small amounts are exhaled as carbon dioxide. In principle, these
absorption and elimination rates and metabolic pathways hold for all
animal species studied, including domestic animals. In cows fed 100 mg
cypermethrin/day, the highest level found in milk was 0.03 mg/litre;
levels of up to 0.1 mg/kg tissue were found in subcutaneous fat. Under
practical conditions, the oral intake of cypermethrin with feed will
be much lower. Cypermethrin used as a spray or dip to combat
parasites, may give rise to maximum residues of 0.05 mg/kg tissue and
0.01 mg/litre milk.
Laying hens exposed orally to 10 mg cypermethrin/kg diet for 2
weeks, showed cypermethrin levels of up to 0.1 mg/kg in the fat, and
up to 0.09 mg/kg in the eggs (predominantly in the yolk).
Consistent with the lipophilic nature of cypermethrin, the
highest mean tissue concentrations are found in body fat, skin, liver,
kidneys, adrenals, and ovaries. Only negligible concentrations are
found in the brain. The half-life of cis-cypermethrin in the fat of
the rat ranges from 12 to 19 days and that of the trans isomer, from
3 to 4 days. In mice, these half-lives are 13 days and 1 day,
respectively.
Overall, the metabolic transformation has been similar in the
different animals studied, including man. Differences that occur have
been related to the rate of formation rather than to the nature of the
metabolites formed and to conjugation reactions. Cypermethrin (both
the cis and trans isomers) is metabolized via the cleavage of the
ester bond to phenoxybenzoic acid and cyclopropane carbolic acid. The
fact that thiocyanate has been identified in in vivo studies,
indicates that the cyanide moiety is further metabolized. The
3-phenoxybenzoic acid is mainly excreted as a conjugate. The type of
conjugate differs in a number of animal species. Phenoxybenzoic acid
is further metabolized to a hydroxy derivative and conjugated with
glucuronic acid or sulfate. The cyclopropyl moiety is mainly excreted
as a glucuronide conjugate, hydroxylation of the methyl group only
occurring to a limited extent.
Ester cleavage is much slower in certain fish species than in
other animal species, the main metabolic pathway being hydroxylation
of the phenoxybenzoic and the cyclopropyl moieties.
Ester cleavage also takes place in plants. The phenoxybenzyl and
cyclopropyl moieties are readily converted into glucoside conjugates.
In mammals, these conjugates are hydrolysed into the original acids
and metabolized.
1.5 Effects on organisms in the environment
High doses of cypermethrin may exert transient minor effects on
microflora activity in the soil. However, no influence on
ammonification and nitrification has been found.
Cypermethrin is very toxic for fish (in laboratory tests 96-h
LC50s were generally within the range of 0.4-2.8 µg/litre), and
aquatic invertebrates (LC50s in the range of 0.01 - > 5 µg/litre).
The presence of suspended solids decreases the toxicity by at least a
factor of 2, because of adsorption of cypermethrin to the solids.
Cypermethrin is not very toxic for birds. Signs of cypermethrin
intoxication were seen at dose levels of 3000 mg/kg body weight or
more. Administration of 1000 mg cypermethrin/kg body weight to laying
hens over a 5-day period did not cause signs of intoxication. However,
cypermethrin was highly toxic for honey bees in laboratory tests, the
oral LD50 ranging from 0.03 to 0.12 µg/bee. Under field conditions,
the hazard is considerably lower, because of the repellent effect of
cypermethrin on worker honey bees, which lasts for at least 6 h after
spraying.
Earthworms are not sensitive to cypermethrin. No deaths occurred
in worms exposed to levels of 100 mg/kg soil for 14 days.
In studies involving deliberate overspraying of experimental
ponds under field conditions, peak concentrations of 2.6 µg
cypermethrin/litre were measured in the water. Fish were not affected,
but populations of crustaceae, mites, and surface-breathing insects
were severely reduced. Most of these populations returned to normal
levels after 15 weeks. Free-swimming dipterous larvae and
bottom-dwelling invertebrates, snails, flatworms, etc., were not
affected. Under normal agricultural conditions (during which drifts
may reach adjacent ditches or streams), the only effects seen in
surface-breathing or surface-dwelling insects were hyperactivity or
immobilization. The relative toxicity of cypermethrin for pests and
their parasites and predators is such that the balance between
host/prey and parasites/predator may not be adversely affected in the
field. However, care should be taken where predatory mites are
important in pest management.
1.6 Effects on experimental animals and in vitro test systems
The acute oral toxicity of cypermethrin is moderate. While LD50
values differed considerably among animal species depending on the
vehicle used and the cis/trans isomeric ratios, the toxic responses in
all species were found to be very similar. The acute toxicity of the
trans isomer in the rat (LD50 > 2000 mg/kg body weight) was lower
than that of the cis isomer (LD50, 160-300 mg/kg body weight). The
onset of toxic signs of poisoning was rapid and they disappeared
within several days in survivors. The toxic signs are characterized by
salivation, tremors, increased startle response, sinuous writhing of
the whole body (choreoathetosis), and clonic seizures. Myelin and axon
degeneration were noted in the sciatic nerve at near lethal dose
levels.
Cypermethrin was moderately to severely irritating, when applied
to the skin or the eye of the rabbit. The severity was partly
dependent on the vehicle used. In guinea-pigs, a mild skin sensitizing
potential was found using the maximization test.
No toxic effects were observed in rats, fed cypermethrin at 100
mg/kg diet for 3 months. Furthermore, prolonged feeding of
cypermethrin (2 years) to dogs at a level of 300 mg/kg feed did not
produce any toxicological effects. A level of 600 mg/kg diet resulted
in reduced body weight gain, but no gross pathological or
histopathological effects were seen.
Two long-term studies on rats and one on mice were carried out.
The dose levels in the rat studies ranged up to 1500 mg/kg diet,
equivalent to 75 mg/kg body weight. No effects were seen at 150 mg/kg
diet. At the highest dose level, reduced body weight gain, increased
liver weights (accompanied by increased smooth endoplasmatic
reticulum), and some haematological and biochemical changes were
observed. No increase in tumour incidence was noted. The same type of
effects were seen in the mouse study at 1600 mg cypermethrin/kg diet.
No effects were seen in the 400 mg/kg diet group.
The effect of cypermethrin on the immune system was studied in
rats. The results showed the possibility of immune suppression by
pyrethroids. More attention should be paid to this aspect, but, at
present, no opinion can be given about its relevance in the
extrapolation of these data for man.
Repeated oral administration of cypermethrin to rats and other
animal species at levels sufficiently high to produce significant
mortality in one group of animals, produced biochemical changes in the
peripheral nerves, consistent with sparse axonal degeneration.
Histopathological changes (swelling and/or disintegration of axons of
the sciatic nerve) were observed. There was no cumulative effect. The
magnitude of the change was substantially less than that encountered
with established neurotoxic agents. The neurotoxic effects seem to be
reversible; presumably the clinical signs are not related to the
induction of neuropathological lesions.
Further evidence to support the minor nature of the nerve lesions
has been afforded by electrophysiological studies on rats.
Measurements of the maximal motor conduction velocities of the sciatic
and tail nerves of rats were made before, and at intervals of up to 5
weeks after, exposure to a single dose or repeated high doses of
cypermethrin. It was concluded from the results that, even at
near-lethal doses, cypermethrin did not cause any effects on maximal
motor conduction velocities and conduction velocities of the slower
motor fibres in rat peripheral nerves. No delayed neurotoxicity was
observed in domestic hens.
The ability of the major metabolite of cypermethrin,
3-phenoxybenzoic acid, to produce axonal changes has been investigated
and found to be negative.
In a multigeneration reproduction study on rats, dose levels up
to 500 mg/kg feed were tested. The parent animals at the highest dose
level showed decreased food intake and reduction in body weight gain.
No influence on reproductive performance or on survival of the
offspring was found. However, at the highest dose level, reductions in
litter size and total litter weights were seen. The pooled body
weights of weaning pups of the 500 mg/kg group were decreased over 3
generations. No effect was found with 100 mg cypermethrin/kg diet.
Embryotoxic and teratogenic effects were not found in rats
administered dose levels of up to 70 mg/kg body weight and clear
teratogenic effects were not observed in rabbits given dose levels of
up to 30 mg/kg body weight during days 6-18 of gestation.
Cypermethrin did not show any mutagenic activity in bacteria or
in yeast, with or without metabolic activation, or in V79 Chinese
hamster cells. Furthermore, cypermethrin gave negative results in an
in vivo chromosomal aberration test with Chinese hamsters and in
dominant lethal studies on mice. In a host-mediated assay with mice,
no increase in the rate of mitotic gene conversion in Saccharomyces
cerevisae was found. In a chromosome study using the bone marrow
cells of Chinese hamsters, cypermethrin did not increase the number of
chromosome abnormalities. However, in a micronucleus test with mouse
bone marrow cells, an increase in the frequency of polychromatic
erythrocytes with micronuclei was found after oral and dermal
applications of cypermethrin. Intraperitoneal application gave a
negative result. A sister chromatid exchange study using bone marrow
cells of mice showed a dose-response related increase in sister
chromatid exchanges of dividing cells.
In long-term/carcinogenicity studies, oral administration of
cypermethrin to rats did not induce an increase in the incidence of
tumours. In a mouse study, dose levels of up to 1600 mg
cypermethrin/kg diet did not produce any increase in tumours of types
not commonly associated with the mouse strain employed. The incidence
of tumours was similar in all groups with the exception of a slight
increase in the incidence of benign alveolar lung tumours in the
females in the 1600 mg/kg diet group. However, the increased
incidence, when compared with concurrent and historical control
incidence, was not sufficient to warrant concern. There was no
suggestion of increased malignancy and no evidence of a decrease in
the latency of the tumours. Furthermore, there was no evidence of a
carcinogenic response in the male mice in this study and, as the
results of mutagenicity studies on cypermethrin have been mainly
negative, it is concluded that there is no evidence for the
carcinogenic potential of cypermethrin.
1.7 Mechanism of toxicity
Extensive studies have been carried out to explain the mechanism
of toxicity of cypermethrin, especially with regard to the effects on
the nervous system. The results strongly suggest that the primary
target site of cypermethrin (and of pyrethroid insecticides in
general) in the vertebrate nervous system is the sodium channel in the
nerve membrane. The alpha-cyano pyrethroids, such as cypermethrin,
cause a long-lasting prolongation of the normally transient increase
in sodium permeability of the nerve membrane during excitation,
resulting in long-lasting trains of repetitive impulses in sense
organs and a frequency-dependent depression of the nerve impulse in
nerve fibres. Since the mechanisms responsible for nerve impulse
generation and conduction are basically the same throughout the entire
nervous system, pyrethroids may well act in a similar way in various
parts of the central nervous system. It is suggested that the facial
skin sensations that may be experienced by people handling
cypermethrin are brought about by repetitive firing of sensory nerve
terminals in the skin, and may be considered as an early warning
signal that exposure has occurred.
1.8 Effects on man
No cases of accidental poisoning have been reported as a result
of occupational exposure.
Skin sensations, reported by a number of authors to have occurred
during field studies, generally lasted only a few hours and did not
persist for more than one day after exposure. Neurological signs were
not observed. General medical and extensive clinical blood-chemistry
studies, and electrophysiological studies on selected motor and
sensory nerves in the legs and arms did not show any abnormalities.
2. Evaluation
Cypermethrin, an alpha-cyano pyrethroid consisting of a mixture
of 8 stereoisomers, is a highly active insecticide effective against
a wide range of pests in many food and non-food commodities.
It is stable to light and heat, it has a low vapour pressure and
is more stable in acidic than in alkaline media. Sensitive analytical
methods for the determination of residues in food and the environment
are available.
When cypermethrin is applied to crops, residues may occur in
soils and surface waters, but biological degradation is fairly rapid
and residues do not accumulate in the environment. Photo-degradation
is unlikely to play an important role. The main route of degradation
is cleavage of the ester linkage to give 2 main degradation products
containing the cyclopropane, and the phenoxybenzyl moiety. The
half-lives in the soil are determined by many factors, but are in the
range of 2-4 weeks. Cypermethrin is strongly adsorbed by soil and
downward leaching is negligible. Because of its rather fast breakdown
forming less toxic products, and the low dose rates used in good
agricultural practice, it is unlikely that cypermethrin will attain
significant levels in the environment.
Bioaccumulation in certain organisms, such as fish, took place
under laboratory conditions, but levels declined on cessation of
exposure and there are indications that, under natural conditions,
fish will not contain measurable residues.
When applied according to good agricultural practice, the levels
of cypermethrin residues in food commodities are generally low. Total
diet studies are not available, but from the available residue
information, it can be inferred that the oral intake by man is well
below the ADI.
High dose levels of cypermethrin may exert transient effects on
the soil microflora. Earthworms and other soil organisms are generally
rather resistant to cypermethrin, while fish and other aquatic
invertebrates are very sensitive. Because of its strong adsorption on
soil, only low levels of cypermethrin may leak into surface water.
These may have transient effects, mainly on surface breathing insects.
The toxicity of cypermethrin for birds is low. Bees appear to be
very sensitive in laboratory tests. Under field conditions, the effect
on bees is minimal, because cypermethrin seems to have a repellent
effect for bees. Absorption and elimination of cypermethrin has been
rapid in the different mammalian species tested. The major metabolic
reaction is cleavage of the ester bond followed by hydroxylation and
conjugation of the cyclopropane and phenoxybenzyl moiety. The highest
levels are found in body fat, which is consistent with the lipophilic
nature of cypermethrin. The half-life in the fat of the rat is about
12-19 days for the cis isomer and 3-4 days for the trans isomer.
Breakdown products in plants are bound as glucosides.
The acute toxicity of cypermethrin for mammals is of a moderate
order. The oral LD50 for the rat ranges from 200 to 4000 mg/kg body
weight. Short-term and long-term toxicity studies on rats, mice, and
dogs have shown effects on growth, on the liver and kidneys, and the
nervous system, and on haematology. A no-observed-adverse-effect level
of 7.5 mg/kg body weight has been adopted by the Task Group.
Cypermethrin was not carcinogenic for mice or rats fed diets
containing high levels of the material over a 2-year period.
Cypermethrin did not induce teratogenic effects in either rats at 70
mg/kg body weight or rabbits at 30 mg/kg body weight. It was also
shown not to have any effects on reproductive performance during a
3-generation reproduction study in rats administered 100 mg/kg diet.
In a variety of mutagenicity studies, cypermethrin was shown to be
mainly without mutagenic activity.
The mechanism of the action on the nervous system has been
extensively studied. From these studies and the occupational studies
available, it seems that the skin sensation seen in workers handling
cypermethrin, generally lasts only a few hours and does not persist
for more than one day after exposure. Other neurological signs were
not observed. These skin sensations can be considered to be an early
warning that exposure has occurred and that work practice should be
reviewed. Cypermethrin may cause eye irritation and may be a
sensitizer for certain persons.
3. Conclusions
It can be concluded that:
General population: When applied according to good agricultural
practice, exposure of the general population to cypermethrin is
negligible and is unlikely to present a hazard.
Occupational exposure: With reasonable work practices, hygiene
measures, and safety precautions, the use of cypermethrin is unlikely
to present a hazard to those occupationally exposed to it. The
occurrence of "facial sensations" is an indication of exposure. Under
these circumstances work practice should be reviewed.
Environment: With recommended application rates it is unlikely
that cypermethrin or its degradation products will attain levels of
environmental significance. Notwithstanding its high toxicity for fish
and honey bees, this is only likely to cause a problem in the case of
spillage and overspraying.
4. Recommendations
- Cypermethrin should be included among the residues looked for in
surveillance, market-basket, or total diet studies.
- Attention should be paid to the implications for the welfare of
human beings of animal studies indicating immune suppression.
- Further follow-up studies into the facial effects in human beings
should be conducted, in order to better understand this
phenomenon.
RESUME ET EVALUATION; CONCLUSIONS ET RECOMMANDATIONS
1. Résumé et évaluation
1.1 Identité, emploi, destinée et concentrations dans l'environnement
L'alpha-cyperméthrine est constituée à plus de 90% de
l'énantiomère le plus actif du point de vue insecticide parmi les
quatre isomères cis qui entrent dans la composition de la
cyperméthrine racémique.
C'est un pyréthrinoïde extrêmement actif, qui agit contre de
nombreux parasites auxquels on a affaire dans l'agriculture et
l'élevage. Il est commercialisé sous forme de concentrés
émulsionnables, de formulations à très bas volume, de concentrés pour
suspension ou en mélange avec d'autres insecticides.
Le produit technique se présente sous la forme d'une poudre
cristalline facilement soluble dans l'acétone, la cyclohexanone et le
xylène mais peu soluble dans l'eau. Il est stable en milieu acide ou
neutre mais s'hydrolyse à pH 12-13. Il se décompose au-dessus de 120
°C.
On ne dispose d'aucune donnée sur la concentration de
l'alpha-cyperméthrine dans l'air.
Dans l'eau, il est probable que l'alpha-cyperméthrine est
décomposée par voie photochimique et biologique. On a constaté que,
après avoir traité un étang à raison de 15 g de matière active par
hectare, les eaux de surface et les couches plus profondes contenaient
respectivement 5% et 19% de la dose appliquée une journée après
l'épandage et 0,1% et 2% respectivement de cette dose sept jours plus
tard. Environ 5% de la dose appliquée se retrouvaient dans les
sédiments 16 jours après l'épandage.
Il est probable que l'alpha-cyperméthrine est fortement adsorbée
aux particules du sol. Une année après un épandage à raison de 0,5 kg
de matière active par hectare, on retrouvait dans le sol des résidus
inférieurs à 0,1 mg/kg.
Le coefficient de partage de l'alpha-cyperméthrine entre le
n-octanol et l'eau est égal à 1,4 x 105 (log de Pow = 5,16).
La dose d'emploi recommandée pour l'alpha-cyperméthrine est
moindre que pour la cyperméthrine car elle est biologiquement plus
active. Il en résulte moins de résidus sur les récoltes et si l'on se
conforme aux doses recommandées, ils se situent entre 0,05 et 1 mg/kg.
Chez des poissons-chats d'eau de mer traités à raison de 0,01 à 0,05%
p/p de matière active, on a mesuré des résidus de 0,3 à 30 mg/kg une
semaine après l'entreposage et entre 0,22 et 4,0 mg/kg 15 semaines
plus tard.
1.2 Cinétique et métabolisme
Après avoir été administrée par voie orale à des rats,
l'alpha-cyperméthrine est éliminée dans les urines sous forme du
sulfo-conjugué de l'acide 3-(4-hydroxyphénoxy)benzoïque ainsi que dans
les matières fécales, en partie sans modification. Environ 90% d'une
dose orale unique sont éliminées de l'organisme en quatre jours, dont
78% au cours du premier jour. Les résidus sont faibles dans les
tissus, sauf dans les tissus lipidiques. Trois jours après
administration d'une dose orale unique de 2 mg/kg, la concentration
dans les graisses était de 0,4 mg/kg. L'élimination à partir des
graisses est biphasique; la demi-vie relative à la phase initiale est
de 2,5 jours, et de 17 à 26 jours pour la deuxième phase.
L'alpha-cyperméthrine est métabolisée par coupure de la liaison
ester. Chez le rat, la fraction alcool phénoxybenzylique est
hydroxylée et transformée en sulfo-conjugué; la fraction acide
cyclopropane-carboxylique subit également une conjugaison,
probablement sous forme de glucuronide, avant d'être excrétée par la
voie urinaire. Des études portant sur des microsomes hépatiques de
rats, de lapins et d'origine humaine ont montré que chez ces trois
espèces, il pouvait y avoir hydrolyse de l'ester et métabolisation
oxydative mais que l'hydrolyse de l'ester prédominait dans le cas des
préparations de foie provenant de lapins ou de sujets humains.
Chez l'homme, 43% d'une dose orale (0,25-0,75 mg/litre) ont été
excrétés dans les 24 heures par la voie urinaire sous forme d'acide
cis-cyclopropane-carboxylique libre ou conjugué. Après
l'administration de cinq doses quotidiennes successives, l'excrétion
urinaire n'a pas augmenté.
On a trouvé de fortes concentrations (jusqu'à 1156 mg/kg)
d'alpha-cyperméthrine dans la laine de mouton, 14 jours après avoir
traité les animaux par des bains ou des aspersions. De faibles
quantités ont été retrouvées dans la graisse sous-cutanée (jusqu'à
0,04 mg/kg). Après avoir traité des veaux le long de l'épine dorsale
avec 10 ml d'une formulation à 1,6%, on n'a pas retrouvé
d'alpha-cyperméthrine dans les muscles ni le foie. La concentration
maximale dans la graisse périrénale était de 0,25 mg/kg au bout de 14
jours.
Après avoir traité des vaches en lactation le long de l'épine
dorsale avec des formulations contenant jusqu'à 0,2 g de matière
active on a retrouvé dans le lait de 3 des 15 animaux traités, des
résidus d'alpha-cyperméthrine compris entre 0,003 et 0,005 mg/litre.
1.3 Effets sur les mammifères de laboratoire et les systèmes
d'épreuves in vitro
L'alpha-cyperméthrine présente une toxicité orale aiguë modérée
à forte pour les rongeurs. La DL50 varie beaucoup chez la souris et
le rat et dépend de la concentration du composé et du véhicule
utilisé. En pratique, on considère qu'une DL50 de 80 mg/kg de poids
corporel est représentative. Toutefois, on a fait état de valeurs plus
élevées pour la DL50 par voie orale dans des conditions
d'intoxication aiguë. Une intoxication aiguë par voie orale entraîne
des signes cliniques qui traduisent une action au niveau du système
nerveux central.
Une application cutanée d'alpha-cyperméthrine à des rats et des
souris aux doses respectives de 100 et 500 mg/kg de poids corporel,
n'a entraîné ni mortalité ni signes d'intoxication. De même, des rats
à qui l'on avait fait inhaler pendant quatre heures de
l'alpha-cyperméthrine à une concentration de 400 mg/m3, n'ont
présenté aucune mortalité ni signes cliniques d'intoxication.
L'alpha-cyperméthrine technique ne provoquerait qu'une irritation
minimale de la peau chez le lapin. En revanche, certaines formulations
de ce composé peuvent déterminer une très forte irritation oculaire.
L'alpha-cyperméthrine technique n'a pas d'effet sensibilisant cutané.
Chez le cobaye, elle provoque une stimulation des terminaisons
nerveuses sensorielles de l'épiderme.
Exposés pendant une courte période à de l'alpha-cyperméthrine à
des concentrations quotidiennes allant jusqu'à 200 mg/kg de nourriture
pendant cinq semaines ou jusqu'à 100 mg/kg de nourriture pendant 13
semaines, des rats n'ont pas présenté d'effets toxiques. Aux doses les
plus élevées, on notait des signes d'intoxication traduisant une
atteinte du système nerveux, ainsi qu'une réduction de la croissance
et une augmentation du poids du foie et des reins. Aucun effet bien
net n'a été observé en ce qui concerne les paramètres hématologiques
ou histopathologiques.
Lors d'une étude de 13 semaines au cours de laquelle des chiens
ont reçu de l'alpha-cyperméthrine par voie orale, on a observé que la
dose la plus forte (270 mg/kg de nourriture) produisait des signes
d'intoxication; en revanche tous les autres paramètres étudiés (NFS,
biochimie du sang, urines, poids des organes, anatomopathologie et
histopathologie) sont restés normaux. La dose sans effet observable
était de 90 mg/kg de nourriture (soit l'équivalent de 2,25 mg/kg de
poids corporel par jour).
Le même genre d'étude menée sur des rats a montré que
l'alpha-cyperméthrine provoquait des effets neurotoxiques imputables
à des lésions histopathologiques des nerfs tibial et sciatique, une
dégénérescence des axones et un accroissement de l'activité de la
bêta-galactosidase.
On ne dispose d'aucune donnée sur la toxicité à long terme, la
toxicité pour la fonction de reproduction, la tératogénicité ou
l'immunotoxicité.
En se basant sur les données disponibles, on peut conclure que
l'alpha-cyperméthrine n'est pas mutagène, comme le montrent les tests
effectués sur Salmonella typhimurium, Escherichia coli et
Saccharomyces cerevisiae ainsi que les épreuves in vivo et in
vitro sur des cellules de foie de rat, qui n'ont révélé ni
aberration chromosomique ni lésion de l'ADN monobrin. On n'a pas non
plus observé d'augmentation du nombre d'aberrations chromosomiques
dans des cellules de moelle osseuse de rat.
On ne dispose d'aucune donnée sur la cancérogénicité de
l'alpha-cyperméthrine.
1.4 Effets sur l'homme
Dans la mesure où l'alpha-cyperméthrine est utilisée conformément
aux règles de bonne pratique agricole, l'exposition de la population
générale reste négligeable. On a constaté que l'exposition
professionnelle cutanée des opérateurs procédant à la préparation des
mélanges, au chargement des pulvérisateurs, à l'épandage de
l'insecticide ou au lavage du matériel, pouvait atteindre des valeurs
respectivement égales à 2,94 mg, 0,61 mg et 0,73 mg.
Lors d'une étude sur l'exposition à l'alpha-cyperméthrine au
cours de la préparation de formulations à base de cet insecticide, on
a évalué les niveaux d'exposition par surveillance personnelle et
statique de la concentration atmosphérique de ce composé et dosage de
ses métabolites urinaires. Au cours des deux jours pendant lesquels
les personnes exposées procédaient à la formulation de concentrés
techniques, l'exposition individuelle moyenne dans le groupe a été
respectivement de 2,8 et 4,9 mg/m3, l'exposition individuelle
moyenne du groupe au produit technique étant de 50,1 mg/m3 le
troisième jour. On n'a pas pu déceler la présence de métabolites dans
les urines (limite de détection 0,02 mg/litre). Au cours de la
préparation des diverses formulations, les ouvriers ont fait état de
sensations au niveau de l'épiderme mais en précisant qu'elles étaient
légères.
Aucune intoxication n'a été signalée.
1.5 Effets sur d'autres organismes au laboratoire et dans leur milieu
naturel
La CE50 à 48 et 96 heures (pour la croissance) chez une algue
d'eau douce, Selenastrum capricornutum, dépasse 100 µg/litre.
L'alpha-cyperméthrine est très fortement toxique pour les
invertébrés aquatiques. Les valeurs de la CE50 à 24 et 48 heures
(pour l'immobilisation de la daphnie) sont respectivement égales à 1,0
et 0,3 µg/litre, et celle de la CL50 à 24 heures (pour Gammarus
pulex) est égale à 0,05 µg/litre. L'alpha-cyperméthrine est
également très toxique pour un certain nombre d'arthropodes aquatiques
mais sa toxicité est moindre pour les mollusques. La toxicité à court
terme de ce composé peut être réduite lorsqu'il est présenté sous la
forme d'une suspension dans l'huile. Les pertes à l'épandage peuvent
provoquer des effets toxiques sur les invertébrés aquatiques, mais
comme l'alpha-cyperméthrine disparaît rapidement de l'eau, ceux-ci ont
la possibilité de récupérer. L'alpha-cyperméthrine est très fortement
toxique pour les poissons. La valeur de la CL50 à 96 heures oscille
entre 0,7 et 350 µg/litre selon le type de formulation. Les concentrés
émulsionnables sont beaucoup plus toxiques que les concentrés en
suspension, les poudres mouillables et les formulations
micro-encapsulées. Le danger de l'alpha-cyperméthrine pour les
invertébrés aquatiques et les poissons tient à sa toxicité aiguë. Rien
n'indique toutefois qu'une exposition prolongée entraîne des effets
cumulatifs.
On ne dispose d'aucune donnée concernant les effets de
l'alpha-cyperméthrine sur les microbes terricoles. Les bactéries
présentes dans les effluents n'ont pas paru affectées par une
concentration de 3 mg/litre en système fermé.
La toxicité de l'alpha-cyperméthrine pour certains carabides et
les larves de neuroptères est relativement faible et elle ne présente
guère de danger pour les stades pré-imaginaux des hyménoptères
parasitoïdes. Des études menées sur des champs de grande superficie ou
de petites parcelles ont montré que l'alpha-cyperméthrine était peu
dangereuse pour les carabides et les staphylinides mais qu'elle
présentait un risque relativement important pour les linyphiides. Les
effets sur ces populations d'insectes se sont limités à une seule
saison de croissance. En outre, l'alpha-cyperméthrine ne présente
guère de risque pour les larves de syrphides mais n'est pas dénuée
d'effets sur les coccinellides. Malgré tout, la dissipation rapide des
résidus présents sur le feuillage permet à ces animaux de reconstituer
rapidement leurs colonies.
L'épandage d'alpha-cyperméthrine n'a pas d'effets indésirables
sur l'abondance relative des arthropodes entomophages. Son utilisation
sur les céréales à petits grains n'entraînerait donc pas la
réapparition des ravageurs ou le déclenchement d'infestations
secondaires.
Des études de laboratoire ont montré que l'alpha-cyperméthrine
était peu toxique pour les lombrics. Des vers placés pendant 14 jours
dans un sol artificiel contenant ce composé à des concentrations
allant jusqu'à 100 mg/kg, n'ont présenté aucune mortalité.
Des études de toxicité aiguë également effectuées en laboratoire
ont montré que l'alpha-cyperméthrine était extrêmement toxique pour
les abeilles. Administré par voie orale, un concentré émulsionnable
d'alpha-cyperméthrine a donné une DL50 à 24 heures de 0,13
µg/abeille, la valeur correspondante pour l'administration topique
étant de 0,03 µg/abeille (de produit technique) ou 0,11 µg/abeille (de
concentré émulsionnable). La forte toxicité de l'alpha-cyperméthrine
pour les abeilles ne s'est pas manifestée au cours des épreuves de
plein champ, probablement du fait que le composé a un bref effet
répulsif qui réduit le butinage et, par voie de conséquence,
l'exposition des insectes.
On ne dispose d'aucune donnée sur la toxicité de
l'alpha-cyperméthrine pour les oiseaux.
2. Conclusions
2.1 Population générale
Lorsque l'épandage de l'alpha-cyperméthrine s'effectue
conformément aux règles de bonne pratique, son utilisation en
agriculture n'expose guère la population générale à ce composé et il
y a peu de danger pour elle.
2.2 Exposition professionnelle
Moyennant de bonnes méthodes de travail ainsi que des mesures
d'hygiène et de sécurité, l'utilisation de l'alpha-cyperméthrine ne
devrait pas présenter de danger pour les personnes qui y sont exposées
de par leur profession. L'apparition de sensations au niveau de la
face indique une contamination. Dans ces circonstances, il est bon de
revoir les méthodes de travail.
2.3 Environnement
Aux doses d'emploi recommandées, il n'est guère probable que
l'alpha-cyperméthrine puisse être libérée dans l'environnement à des
concentrations écologiquement dangereuses. Elle est fortement toxique
pour les arthropodes aquatiques, les poissons et les abeilles dans les
conditions du laboratoire. On ne peut envisager la probabilité
d'effets toxiques importants sur les invertébrés non visés et les
poissons qu'en cas de déversement accidentel, d'épandage excessif ou
d'erreur de manipulation.
3. Recommandations
* Il convient d'éviter la contamination des eaux superficielles par
l'alpha-cyperméthrine.
* L'alpha-cyperméthrine se lie fortement aux particules. D'autres
études écotoxicologiques sont à effectuer à propos des effets de
l'alpha-cyperméthrine sur les organismes qui vivent dans les
sédiments car il s'agit d'un aspect qui n'a guère retenu
l'attention jusqu'ici.
* L'absorption dans les voies digestives de l'alpha-cyperméthrine
est à étudier dans diverses conditions expérimentales.
* Il faudrait également étudier la destinée de
l'alpha-cyperméthrine après application sur l'épiderme.
* Il faudrait obtenir des données supplémentaires sur la toxicité
à long terme, la cancérogénicité et l'immunotoxicité de
l'alpha-cyperméthrine.
RESUMEN Y EVALUACION; CONCLUSIONES Y RECOMENDACIONES
1. Resumen y evaluación
1.1 Identificación, uso, destino y niveles en el medio ambiente
La alfa-cipermetrina contiene más del 90% del par de
enantió-meros con mayor actividad insecticida de los cuatro isómeros
cis de la cipermetrina en mezcla racémica.
Se trata de un insecticida piretroide sumamente activo contra una
gran variedad de plagas habituales en agricultura y ganadería. Existe
como concentrado emulsionable, formulacion de volumen ultra-bajo,
concentrado en suspensión y en mezcla con otros insecticidas.
El producto técnico es un polvo cristalino, con buena solubilidad
en acetona, ciclohexanona y xileno, y con baja solubilidad en agua. Es
estable en condiciones ácidas o neutras, pero se hidroliza a pH 12-13.
Se descompone por encima de los 220 °C.
No se dispone de información acerca de los niveles de
alfa-cipermetrina en el aire.
Es probable que la degradación de la alfa-cipermetrina en agua se
deba a procesos fotoquímicos y biológicos. El agua superficial y
subsuperficial de un estanque rociado con 15 g/ha de principio activo
contenía el 5% y el 19% de la dosis aplicada un día después del
rociamiento, y 0.1% y 2% siete días más tarde. A los 16 días de la
aplicación se encontró en el sedimento alrededor del 5% de la dosis
utilizada.
Es probable que la alfa-cipermetrina se adsorba con fuerza a las
partículas del suelo. Un año después del tratamiento con 0.5 kg de
principio activo por hectárea se encontraron en el suelo residuos
inferiores a 0.1 mg/kg.
El coeficiente de reparto n-octanol/agua de la alfa-cipermetrina
es 1.4 x 105 (log Poa = 5.16).
Las tasas de aplicación recomendadas de alfa-cipermetrina son
inferiores a las de cipermetrina, porque la primera es biológicamente
más activa. En consecuencia, los residuos en los cultivos son escasos,
y utilizando las tasas de aplicación recomendadas estos residuos
oscilan entre 0.05 y 1 mg/kg. Los residuos en peces siluroideos
marinos tratados con dosis entre el 0.001 y el 0.05% p/p de principio
activo eran de 0.3-30 mg/kg una semana después del almacenamiento, y
de 0.22 a 4.0 mg/kg tras 15 semanas de almacenamiento.
1.2 Cinética y metabolismo
La alfa-cipermetrina administrada por vía oral a ratas se elimina
por la orina como sulfato conjugado del ácido 3-(4-hidroxifenoxi)
benzoico y en parte como compuesto inalterado por las heces. De una
dosis oral única alrededor del 90% se elimina del cuerpo en un período
de cuatro días, y el 78% en el primer día. Los residuos en los tejidos
son escasos, excepto en el adiposo. La concentración en la grasa tres
días después de una dosis oral única de 2 mg/kg fue de 0.4 mg/kg. La
eliminación a partir de la grasa es bifásica: en la fase inicial tiene
una vida media de 2.5 días y en la segunda de 17 a 26 días.
La alfa-cipermetrina se metaboliza mediante la ruptura de su
enlace éster. En la rata, el alcohol fenoxibencílico de la molécula se
hidroxila y se conjuga con sulfato, y el ácido
ciclopropano-carboxílico también se conjuga (probablemente en forma de
glucurónido) antes de la excreción urinaria. En estudios con
microsomas hepáticos de ratas, conejos y seres humanos se ha
demostrado que la hidrólisis en el ester y rutas oxidativas se dan en
las tres especies, si bien la primera es la ruta predominante en las
preparaciones hepáticas de conejo y de ser humano.
En el ser humano, el 43% de una dosis oral (0.25-0.75 mg) se
excreta en la orina en un plazo de 24 h en forma de ácido cis-
ciclopropano-carboxílico libre o conjugado. La excreción urinaria no
aumentó tras la administración de 5 dosis diarias sucesivas.
En la lana de las ovejas se detectaron concentraciones altas
(hasta 1156 mg/kg) de alfa-cipermetrina 14 días después de la
aplicación por inmersión o por lavado. En la grasa subcutánea se
encontraron niveles bajos (hasta 0.04 mg/kg). Después de tratar
terneros a lo largo del dorso con 10 ml de una preparación al 1.6%, no
se detectó alfa-cipermetrina en los músculos ni en el hígado. La
máxima concentración en la grasa perirrenal durante un período de 14
días fue de 0.26 mg/kg.
Tras la aplicación de hasta 0.2 ml de principio activo a lo largo
del dorso de vacas lecheras, se encontraron en la leche de tres en 15
animales tratados residuos de alfa-cipermetrina en concentraciones que
oscilaban entre 0.003 y 0.005 mg/ml.
1.3 Efectos en mamíferos de laboratorio y en sistemas de prueba
in vitro
En roedores, la toxicidad aguda oral de la alfa-cipermetrina es
entre moderada y alta. Los valores de la DL50 en ratones y ratas son
muy variables y dependen de la concentración del compuesto y del
excipiente. A efectos prácticos, se considera representativo un valor
de la DL50 de 80 mg/kg de peso corporal. Sin embargo, se han
notificado valores más altos de DL50 aguda por vía oral. La
exposición oral aguda produce síntomas clínicos relacionados con la
actividad del sistema nervioso central.
Las aplicaciones cutáneas aisladas de alfa-cipermetrina a ratones
y ratas en concentraciones de 100 y 500 mg/kg de peso corporal,
respectivamente, no produjeron mortalidad ni síntomas de intoxicación.
La exposición de ratas por inhalación durante 4 h a una concentración
atmosférica de 400 mg/m3 tampoco ocasionó mortalidad ni signos
clínicos.
Se ha reportado que la alfa-cipermetrina de calidad técnica
produce una irritación cutánea mínima en el conejo. Algunas de las
preparaciones provocan irritación ocular grave. La alfa-cipermetrina
de calidad técnica no produce sensibilización cutanea. En cobayos
ocasionó la excitación de las terminaciones neuro-sensoriales de la
piel.
La exposición breve de ratas a concentraciones de
alfa-cipermetrina de hasta 200 mg/kg en dieta diaria durante 5 semanas
o hasta 180 mg/kg en dieta diaria durante 13 semanas no produjo
efectos tóxicos. Con dosis más elevadas, las ratas mostraron signos de
intoxicación asociados a la patología del sistema nervioso,
disminución del crecimiento o aumento del peso del hígado y los
riñones. No se pusieron de manifiesto efectos hematológicos,
bioquímicos o histopatológicos claros.
En un estudio de toxicidad oral en perros durante 13 semanas, la
dosis más alta, de 270 mg/kg causó síntomas de intoxicación, pero
todos los demás parámetros examinados (relativos a la hematología,
bioquímica clínica, análisis de orina, peso de los órganos, anatomía
patológica e histopatología) se mantuvieron inalterados. El nivel sin
efectos observados (NOEL) fue de 90 mg/kg de dieta (equivalente a 2.25
mg/kg de peso corporal al día).
En un estudio de toxicidad oral en ratas se demostró que la
alfa-cipermetrina induce neurotoxicidad a causa de alteraciones
histopatológicas de los nervios tibial y ciático, degeneración axonal
y aumento de la actividad de la beta-galactosidasa.
Se carece de datos sobre toxicidad a largo plazo, toxicidad en la
reproducción, teratogenicidad e inmunotoxicidad.
Con los datos disponibles sobre la alfa-cipermetrina, se puede
deducir que se trata de un compuesto no mutagénico en las pruebas con
Salmonella typhimurium, Escherichia coli y Saccaromyces cerevisiae,
y en las pruebas in vivo e in vitro con hepatocitos de rata, con
respecto a la inducción de aberraciones cromosómicas y producción de
lesiones en cadenas simples de ADN. No se observó aumento de las
aberraciones cromosómicas en las células de médula ósea de rata.
Se carece de datos sobre la carcinogenicidad de la
alfa-cipermetrina.
1.4 Efectos en el ser humano
La exposición de la población general a la alfa-cipermetrina es
insignificante siempre que en su utilización se apliquen buenas
prácticas agrícolas. Se comprobó que la exposición cutánea profesional
de los trabajadores durante la mezcla/carga, el rociado y el lavado
del equipo era de hasta 2.94 mg, 0.61 mg y 0.73 mg, respectivamente.
En un estudio de exposición a la alfa-cipermetrina durante la
formulación, se evaluaron los niveles de exposición mediante el
monitoreo personal y estático de las concentraciones atmosféricas y la
medición de los metabolitos en la orina. Los niveles de exposición
personal media del grupo durante los dos días de la formulación de los
concentrados de calidad técnica fueron de 2.8 y 44.9 mg/m3, mientras
que la exposición personal media del grupo al material técnico el
tercer día fue de 54.1 mg/m3. No se detectaron metabolitos en la
orina (límite de detección, 0.02 mg/litro). Durante la formulación se
informó de reacciones cutáneas ligeras.
No se han comunicado casos de envenenamiento.
1.5 Efectos en otros organismos en el laboratorio y en el medio
ambiente
El valor de la CE50 (crecimiento) en las 48 y 96 horas para el
alga de agua dulce Selenastrum capricornutum es superior a 100
µg/litro.
La alfa-cipermetrina es muy tóxica para los invertebrados
acuáticos. Los valores de la CE50 (inmovilización) a las 24 y 48 h
para Daphnia magna son de 1.0 y 0.3 µg/litro, respectivamente, y el
valor de la CL50 a las 24 h para Gammarus pulex es de 0.05
µg/litro. La alfa-cipermetrina es muy tóxica para varios grupos de
artrópodos acuáticos, pero lo es menos para los moluscos. Se puede
reducir la toxicidad a corto plazo del compuesto formulando el
producto como suspensión con mayor cantidad de aceite. Aunque el
arrastre del rociado puede producir efectos tóxicos en los
invertebrados acuáticos, la desaparición rápida de la
alfa-cipermetrina del agua facilita la recuperación.
La alfa-cipermetrina es muy tóxica para los peces. El valor de la
CL50 a las 96 h oscila entre 0.7 y 350 µg/litro, segun la
formulación. Las formulaciones de concentrados emulsionables son mucho
más tóxicas que el concentrado en suspensión, el polvo humectable y
las formulaciones microencapsuladas. El peligro del compuesto para los
invertebrados acuáticos y los peces radica en su toxicidad aguda. No
hay pruebas de que se produzcan efectos acumulativos debido a una
exposición prolongada.
No se dispone de datos acerca de los efectos de la
alfa-cipermetrina en los microorganismos del suelo. En un sistema
cerrado, no se observaron efectos en las bacterias de aguas residuales
con concentraciones de 3 mg/litro.
La toxicidad de la alfa-cipermetrina para determinados
coleopteros carábidos y larvas de neurópteros es relativamente baja,
y representa un peligro limitado en las fases preadultas de los
himenópteros parasitoides. En estudios de campo en pequeñas parcelas
y en gran escala se ha puesto de manifiesto que la alfa-cipermetrina
es poco peligrosa para los coleopteros carábidos y estafilínidos, pero
es un peligro relativamente grande para las arañas linifíidas. Los
efectos sobre las poblaciones se limitaron a una sola temporada de
crecimiento. Además, la alfa-cipermetrina es de bajo riesgo para las
larvas de sírfidos, pero tiene efectos considerables en los
coccinélidos. Sin embargo, la rápida desaparición de los residuos de
las hojas permite a estos animales recolonizar en poco tiempo las
zonas tratadas.
La utilización de alfa-cipermetrina en el campo no diminuye la
abundancia relativa de entomófagos en las comunidades de artrópodos.
Su utilización en cultivos de cereales de grano pequeño no iría
acompañada de la "reaparición" de plagas o de infestaciones de plagas
secundarias.
En las pruebas de laboratorio, la toxicidad de la
alfa-cipermetrina para las lombrices de tierra es baja. No se registró
mortalidad tras 14 días de exposición de las lombrices a
concentraciones de hasta 100 mg/kg de suelo artificial.
En pruebas de toxicidad aguda en el laboratorio se observó que la
alfa-cipermetrina es muy tóxica para las abejas. La administración
oral de una solución concentrada emulsionable dio una DL50 a las 24
h de 0.13 µg/abeja, mientras que el valor correspondiente para la
administración tópica fue de 0.03 µg/abeja (producto técnico) ó 0.11
µg/abeja (CE). La elevada toxicidad de la alfa-cipermetrina para las
abejas no se manifestó claramente en los ensayos de campo,
probablemente a causa del breve efecto repelente del producto, que
hace disminuir la actividad libadora de las abejas y, por
consiguiente, su exposición.
No se dispone de datos acerca de la toxicidad del
alfa-cipermetrina para las aves.
2. Conclusiones
2.1 Población general
Cuando la alfa-cipermetrina se aplica correctamente, la
exposición de la población general al producto es baja y probablemente
no supone riesgos.
2.2 Exposición ocupacional
Con buenas prácticas de trabajo, medidas de higiene y
precauciones de seguridad, es improbable que la alfa-cipermetrina
suponga un peligro para las personas expuestas en forma laboral. La
aparición de "sensaciones faciales" es un síntoma de exposición. En
estas circunstancias se deben re-examinar las prácticas de trabajo.
2.3 Medio ambiente
Con las cantidades recomendadas para la aplicación es improbable
que la alfa-cipermetrina alcance niveles de importancia ecológica. En
condiciones de laboratorio es muy tóxica para los artrópodos
acuáticos, los peces y las abejas. Hay cierta probabilidad de que se
produzcan efectos tóxicos importantes en invertebrados a los que no
está destinado y en peces en casos de derrame, rociado excesivo o mala
utilización del producto.
3. Recomendaciones
* Se debe evitar la contaminación de aguas superficiales.
* La alfa-cipermetrina se une fuertemente a las partículas. Se
deberían llevar a cabo nuevos estudios ecotoxicológicos sobre los
efectos del compuesto en los microorganismos de los sedimentos,
puesto que este aspecto parece haber recibido poca atención.
* Hay que investigar la absorción gastrointestinal de
alfa-cipermetrina en distintas circunstancias.
* Se debe investigar el destino final de la alfa-cipermetrina
aplicada por vía cutánea.
* Hay que obtener nueva información acerca de la
toxicidad/carcinogenicidad a largo plazo y de la inmunotoxicidad
de la alfa-cipermetrina.
6.4 Plants
The metabolism of cypermethrin in plants is described in WHO
(1989).
The degradation of alpha-cypermethrin and cypermethrin in
cabbages grown to maturity outdoors has been studied. Eighteen days
after transplanting, the cabbages were treated three times with
14C-labelled alpha-cypermethrin or cypermethrin as an EC
formulation. Each treatment consisted of 1.8 mg equivalent with a
spray concentration of 36 g/litre. This treatment was repeated after
11 and 27 days. Each box of cabbages received a total application of
5.4 mg at a dose rate equivalent to 50 g active ingredient/ha. At
harvest (3 months later) the plants were separated into old and new
outer leaves, heart, stalk and roots. No major differences between the
two compounds in distribution of radioactivity throughout the plants
or in the metabolic profile were observed. The highest radioactive
residues were present in the old outer leaves (23% for
alpha-cypermethrin and 27% for cypermethrin), lower levels being found
in new outer leaves, stalk, roots and heart. Very low levels (< 0.05
mg/kg) of both compounds were found in the soil. The major radioactive
residue at harvest was shown to be the pesticide, which was either in
the unchanged form or had undergone cis/trans-isomerization,
presumably photochemically. The profiles of the organosoluble
metabolites were similar, and the major products of alpha-cypermethrin
had chromatographic mobility similar to previously identified products
of cypermethrin metabolism, such as 3-phenoxybenzoic acid and
3-phenoxybenzyl alcohol, partly hydroxylated and/or conjugated. These
compounds were found in minor quantities (McMinn, 1983a; WHO, 1989).
Appraisal
A wide range of studies in mice, rats, dogs, sheep, cows and
humans has shown that cypermethrin is rapidly absorbed, distributed
to a variety of organs and tissues, metabolized and rapidly excreted
from the body (WHO, 1989). There are no major differences in the
absorption, distribution, retention or excretion between the species.
Differences, where they do occur, are related to the rate rather than
the nature of the metabolites formed and the conjugation reactions.
Cypermethrin, both the cis and trans isomers, and
alpha-cypermethrin are primarily metabolized by cleavage of the ester
bond. The metabolites PBA and CPA are mainly excreted as conjugates.
The type of conjugate differs in a number of animal species dosed
with cypermethrin, but humans and rats have the same pathway. Minor
quantities of hydroxylated PBA (conjugated) may also be found. The
terminal half-life of elimination of alpha-cypermethrin from the fat
of rats is 17-26 days, compared to 18.9 days for cis-cypermethrin.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Oral (technical product)
Alpha-cypermethrin is moderately to highly toxic and 3-4 times
more toxic than cypermethrin.
The clinical signs of toxicity observed in the various acute
toxicity studies on experimental animals with alpha-cypermethrin are
typical for a cyano-containing pyrethroid intoxication. They included
ataxia, abasia, gait abnormalities, choreoathetosis, "tip-toe" walk,
and increased salivation, lacrimation, piloerection, tremor and clonic
convulsions. The majority of the mortalities occurred within the first
3 h and surviving animals recovered within 7 days (Rose, 1982, 1983a).
In the study with alpha-cypermethrin administered in corn-oil
(Rose, 1983a), clonic convulsions, piloerection, salivation and
splayed hind-leg gait were found. The oral LD50 values for
alpha-cypermethrin are summarized in Table 3.
Table 3. Oral LD50 values for technical alpha-cypermethrin
Species Concentration and LD50 in mg/kg body Reference
(strain) vehicle weight (with 95%
confidence limits)
Mouse 5% in corn oil 35 (26-48) Rose (1982)
(CD) 40% in DMSO 762 (514-912) Rose (1982)
50% aqueous suspension 798 (568-1074) Rose (1982)
Rat 5% in corn oil 79 (63-98) Dewar (1981)
(Wistar) 40% in DMSO approximately 4000 Rose (1982)
50% aqueous suspension > 5000 Rose (1982)
Rat 10% in corn oil 40-80 Rose (1983a)
(Wistar) 20% in corn oil 368 (282-487) Rose (1983a)
Woollen et al. (1991) noted a higher degree of absorption of
cypermethrin when it was applied in corn oil. This could be the
explanation for the higher toxicity of alpha-cypermethrin administered
in corn oil.
7.1.2 Oral (formulations)
Formulations of alpha-cypermethrin have moderate acute oral
toxicity (Table 4). The clinical signs observed after oral
administration to rats are characteristic of cyano-containing
pyrethroid intoxication (see section 7.1.1.). The majority of the
mortalities occurred within 3 days of dosing. The degree of acute oral
toxicity of formulations containing mixtures with other active
ingredients depended on the toxicity of the latter ingredients.
7.1.3 Dermal
Alpha-cypermethrin has low dermal toxicity. No deaths or signs of
intoxication were observed in rats (Dewar, 1981; Shell, 1983a) and
mice (Rose, 1982; Shell, 1983a) receiving a single 24-h dermal
exposure of 500 mg/kg body weight (25% in DMSO) and 100 mg/kg body
weight (5% in corn oil), respectively.
The dermal LD50 values in rats of formulations of
alpha-cypermethrin and of alpha-cypermethrin mixed with another active
ingredient are summarized in Table 4. In all cases, the maximum dose
that could be applied was tested.
With the pour-on formulations, no clinical signs were observed.
Blood around the nose and eyes was the only sign seen in the case of
SC formulations. Clinical signs observed after the application of EC
or ULV formulations of alpha-cypermethrin included increased
lacrimation, chromodacryorrhoea and unkempt appearance, aggressiveness
and diarrhoea. The Fastac/BPMC formulation caused the same signs of
intoxication and also oedema at the application site. With the
Fastac/methomyl formulation, fasciculation, lethargy, salivation,
piloerection, hunched back, chromodacryorrhoea and cyanosis were
observed. Animals treated with Fastac/Azodrin formulation showed the
above-mentioned symptoms, and some additionally showed ataxia, abasia,
hypothermia, eye pallor and prostration/coma.
7.1.4 Inhalation
Groups of five male and five female albino Fischer-344 rats were
exposed for 4 h to a dust atmosphere containing 30% (m/m)
alpha-cypermethrin on silica powder at an average concentration of 1.3
g/m3 (equivalent to 0.4 g active ingredient/m3). The mass media
diameter of the dust particles was 4.2 µm (geometric standard
deviation 6.4). The animals were observed for 14 days after the
exposure but there were no signs of intoxication. Macroscopic
examination of the lungs did not reveal any effects. Thus, the acute
LC50 was > 1.3 g 30% silica powder dust/m3 or > 0.4 g active
ingredient/m3 (Blair, 1984).
Table 4. Oral and dermal LD50 values for formulated alpha-cypermethrin in rats (Fischer-344)
Formulationa LD50 in mg total formulation per kg Reference
body weight (with 95% confidence limits)
Oral Dermal
100 g/litre EC 101 (82-119) > 1800 Rose (1984d)
100 g/litre EC 136 (98-186) > 1800 Rose (1984e)
100 g/litre EC 174 (125-327) > 2000 Price (1985a)
30 g/litre EC 229 (178-292) > 2000 Rose (1984f)
30 g/litre EC 673 (597-753) > 2000 Rose (1985)
15 g/litre pour-on > 2000 > 2000 Price (1988)
10 g/litre pour-on > 2000 > 2000 Price (1988)
100 g/litre SC 1804 (1507-2168) > 2000 Price (1985b)
60 g/litre SC > 5000 > 2000 Gardner (1991)
15 g/litre SC > 5000 > 2000 Price (1986)
15 g/litre ULV 5838 (5130-6665) > 2000 Rose (1984c)
Mixtures with other active ingredients
Fastac/methomyl EC 58-97 (males) > 1900 Rose (1984h)
15/120 g/litre 73 (51-89) (females) > 1900
Fastac/BPMCb EC 310 (215-462) > 2000 Price (1987)
10/400 g/litre
Fastac/Azodrin EC 25 (18-34) > 2000 Gardner (1989)
20/400 g/litre
a EC = emulsifiable concentrate; SC = suspension concentrate; ULV = ultra-low volume
b BPMC = 2 sec-butylphenyl methylcarbamate (fenobucarb)
7.1.5 Other routes
The acute intraperitoneal LD50 for rats of a 10% solution of
alpha-cypermethrin in corn oil was 3.39 (3.03-3.83) ml/kg body weight,
or 339 mg active ingredient per kg body weight. The surviving animals
showed characteristic pyrethroid signs of intoxication, e.g., ataxia,
abasia, choreoathetosis, gait abnormalities, "tip-toe" walk and
salivation (Rose, 1984a).
7.2 Short-term exposure
7.2.1 Oral
7.2.1.1 Rat
Groups of Wistar rats (10 of each sex at each dose level and 20
of each sex as controls) were fed 0, 25, 100, 200, 400 or 800 mg
alpha-cypermethrin/kg diet (equivalent to 0, 1.25, 5, 10, 20 or 40
mg/kg body weight) for 5 weeks. At 400 and 800 mg/kg diet, signs of
intoxication, such as abnormal gait and hypersensitivity were
observed, and food intake and body weight were decreased in both sexes
compared with the control group. Changes in blood chemistry, e.g.,
decreases in protein and increases in urea levels, were observed in
both sexes of rats fed 800 mg/kg diet and in males fed 400 mg/kg. The
weights of livers and kidneys of both sexes of rats fed 800 mg/kg diet
and livers of male rats fed 400 mg/kg were increased. No
histopathological changes were observed except in the case of one
severely intoxicated male animal fed 800 mg/kg diet, which showed
sparse axonal degeneration in the sciatic nerve. No effects were seen
in the animals fed 200 mg/kg diet for five weeks (Pickering, 1982).
In a 13-week study, Wistar rats (30 males and 30 females per test
group, and a control group consisting of 60 males and 60 females),
which were initially 5 weeks old, were fed 0, 20, 60, 180 or 540 mg
alpha-cypermethrin/kg diet (equivalent to 0, 1, 3, 9 or 27 mg/kg body
weight). After six weeks of feeding, one third of the animals were
killed for interim haematological, clinical chemical and gross
post-mortem examination. The remaining animals were killed after 13
weeks. Signs of intoxication, such as abnormal gait with splayed hind
limbs, were found in 3 out of 20 males fed 540 mg/kg diet. Several
instances of transient skin sores and fur loss were observed
particularly in the rats fed 540 mg/kg. There was decreased growth,
which correlated with decreased food intake, in both sexes fed 540
mg/kg from the first week onwards. No clear effects on the
haematological and clinical chemical parameters were found. Organ
weights were comparable with those of the control animals. No
histopathological abnormalities were found except sparse axonal
degeneration in the sciatic nerve, without clinical signs of toxicity,
in two males fed 540 mg/kg. No axonopathy was observed in the three
animals with abnormal gait. There were marginal effects (decreased
growth during week one and from week 10 onwards) in the males fed 180
mg/kg diet. No effects were found in the 60-mg/kg group (Clark, 1982).
7.2.1.2 Dog
Beagle dogs (one male and one female) were fed alpha-cypermethrin
in the diet at the following concentrations: 200 mg/kg diet for 7
days, 400 mg/kg diet for 2 days, and 300 mg/kg diet for 7 days. With
200 mg/kg no signs of intoxication were observed, whereas dosing with
300 mg/kg or more caused weight loss, ataxia, subdued behaviour, head
nodding, food regurgitation, inflammation of gums and tongue, body
tremors and diminished response to stimuli. Haematological, clinical
chemical and gross pathological examination showed no effects
(Greenough & Goburdhun, 1984).
In a further study, Beagle dogs (one male and one female)
received 300 mg/kg diet for 3 days (male dog) or 4 days (female dog)
and 250 mg/kg diet for 7 days. Both animals showed the above-mentioned
signs of intoxication, the only difference being that when it was
being fed the 250-mg/kg diet the female animal showed these signs more
frequently than the male animal. There were no effects on haematology,
clinical chemical parameters, urinalysis, faecal occult blood test or
gross pathology (Greenough & Goburdhun, 1984).
In a study by Greenough et al. (1984), 36 pure bred Beagle dogs
(18 males and 18 females) received a diet containing
alpha-cypermethrin at 0, 30, 90 or 270 mg/kg diet for 13 weeks. The
group dosed at the highest concentration comprised six males and six
females while the other groups consisted of four males and four
females. All animals fed 270 mg/kg diet exhibited signs of
intoxication, such as whole body tremors, head nodding, "lip-licking",
subduedness, ataxia, agitation and a high-stepping gait. These signs
increased in both intensity and duration as the study progressed. Food
consumption, body weight gain, organ weights, ophthalmoscopy,
haematological and clinical chemical parameters, urinalysis, gross
pathology and microscopy of 18 organs and tissues of all test groups
showed no dose-related effects. In this study, the no-observed-effect
level was considered to be 90 mg/kg diet (equivalent to 2.25 mg/kg
body weight).
7.3 Skin and eye irritation; sensitization
7.3.1 Skin irritation
Undiluted technical alpha-cypermethrin was minimally irritating
when applied as a single occluded dose for 24 h to intact and abraded
rabbit skin (Dewar, 1981).
New Zealand white rabbits were used to study the primary skin
irritation of a number of alpha-cypermethrin formulations. The test
duration was 4 h, the observation period was 7-21 days, and the
formulations tested were 30 and 100 g/litre EC, 15 g/litre ULV, 10 and
15 g/litre pour-on formulation, 15 and 100 g/litre SC, and
Fastac/methomyl (15/120) EC. The EC formulations caused mild to
moderate skin irritation. Superficial necrosis was observed in one or
two animals treated with 100 g/litre EC, but there was no permanent
in-depth skin damage. The effects persisted for up to 7 days. The EC
formulations and the 100 g/litre SC formulation were classified as
mildly irritating. All other formulations tested were either
non-irritating or only slightly irritating (Rose, 1984c,d,e,f,h, 1985;
Price, 1985a,b, 1986, 1988).
7.3.2 Eye irritation
Undiluted formulations were tested for their eye irritancy
potential in groups of six rabbits using the Draize test. All the EC
formulations tested (30 or 100 g/litre) caused severe eye irritation,
including corneal opacity and damage to the iris (Rose, 1984d,e,f,
1985).
When an EC formulation (100 g/litre) and its components, both in
the undiluted form and at typical in-use dilutions (1 in 400 and 1 in
1333 aqueous dilution), were tested for eye irritancy potential, the
undiluted formulation was severely irritating, with or without
irrigation, while the undiluted blank formulations were mildly to
severely irritating. The diluted test formulations, with or without
alpha-cypermethrin or with emulsifier, were non-irritating. It was
concluded that the eye irritation resulted from the combined
formulation ingredients (especially the emulsifier) and that
alpha-cypermethrin per se gave only slight irritation, if any
(Dewar, 1981; Rose, 1984b). In-use dilutions (0.0075%) of another 100
g/litre EC formulation and its blank formulation were non-irritating
(Rose, 1984g).
Two pour-on formulations (10 and 15 g/litre) caused moderate and
slight conjunctival inflammation, respectively. The 10 g/litre
formulation was considered to be an eye irritant (Price, 1988). Two SC
formulations (15 and 100 g/litre) were mildly irritating, causing
slight conjunctival redness and chemosis (Price, 1985b, 1986). A 15
g/litre ULV formulation was mildly irritating to rabbit eyes and there
was a moderate initial pain response (Rose, 1984c). An EC formulation
containing Fastac/methomyl (15:120 g/litre) was a severe eye irritant.
The vascularization of the cornea and iritis were considered to be
irreversible (Rose, 1984h).
7.3.3 Sensitization
Technical alpha-cypermethrin was tested in the guinea-pig
maximization test of Magnusson and Kligman using groups of 10 male and
10 female guinea-pigs and a control group of 55 animals of each sex.
The following concentrations were used: intradermal injection, 0.05%
(v/v) in corn oil; topical application and challenge, 50% (m/m) in
vaseline. On the basis of the negative results it was concluded that
alpha-cypermethrin is not a skin sensitizer in guinea-pigs (Dewar,
1981).
An EC formulation (100 g/litre) and its corresponding blank were
tested, as a 50% solution in corn oil, in the Buehler guinea-pig
sensitization test. The topical challenge was carried out with a 30%
solution in corn oil. None of the animals showed positive responses at
24 or 48 h after the challenge (Rose, 1984g).
7.4 Long-term and carcinogenicity studies
No long-term or carcinogenicity studies have been conducted with
alpha-cypermethrin.
7.5 Reproduction, embryotoxicity and teratogenicity
Alpha-cypermethrin has not been tested for reproductive effects
or teratogenicity.
From the available reproduction and teratogenicity studies with
cypermethrin it is clear that no influence on reproduction performance
occurs at a level of 100 mg/kg diet, nor are there any teratogenic
effects even with dose levels high enough to cause maternal toxicity
(WHO, 1989). Furthermore, the no-observed-effect level of cypermethrin
for reproduction and teratogenicity is comparable with the
no-observed-effect levels based on other parameters of toxicity. In
consequence, there is no reason to believe that alpha-cypermethrin,
consisting of two cis isomers also present in cypermethrin, would
behave differently.
7.6 Mutagenicity and related end points
7.6.1 Mutation
The results of the various mutagenicity studies with
alpha-cypermethrin are summarized in Table 5.
Alpha-cypermethrin (in DMSO) at concentrations of 31.25, 62.5,
125, 250, 500, 1000, 2000 or 4000 µg/ml did not increase reverse gene
mutation (at the arg 4-17, trp 5-48 or hom 3-10 markers) in log- or
stationary-phase cultures or forward mutation (to cyclo-heximide
-resistance) in log-phase cultures of Saccharomyces cerevisiae XV
185-14C, either in the presence or absence of rat-liver S9 fraction.
Concentrations of 10 and 50 µg/ml 4-nitroquinoline- N-oxide and 1250
and 5000 µg/ml cyclophosphamide were used as positive controls
(Brooks, 1984).
Alpha-cypermethrin (in DMSO) at concentrations of 31.25, 62.5,
125, 250, 500, 1000, 2000 or 4000 µg/plate, both with and without
microsomal activation, did not increase reverse mutation rates in
Salmonella typhimurium TA98, TA100, TA1535, TA1537 and TA1538 or in
Escherichia coli WP2 and WP2 uvr A. Mitotic gene conversion was not
induced in liquid suspension cultures of log-phase cells of
Saccharomyces cerevisiae JD 1, dosed with solutions of
alpha-cypermethrin at concentrations of 10, 100, 500, 1000 or 5000
µg/ml, both in the presence or absence of a rat liver S9 fraction.
These studies were carried out in comparison with four positive
control compounds (Brooks, 1982).
7.6.2 Chromosomal effects
In a study by Clare & Wiggins (1984), groups of five male and
five female Wistar rats were administered a single oral dose of 2, 4
or 8 mg alpha-cypermethrin in 5% corn oil/kg body weight and killed 24
h after dosing. The control group received corn oil alone.
Cyclophosphamide was used as a positive control. Alpha-cypermethrin
caused no increase in the incidence of chromatid or chromosome
aberrations or polyploidy in bone marrow cells.
Alpha-cypermethrin in aqueous carboxymethylcellulose at
concentrations of up to 40 µg/ml did not increase the frequency of
chromatid gaps, chromatid breaks or total chromatid aberrations in rat
liver (RL4) cell cultures (Brooks, 1982).
7.6.3 DNA damage
Alpha-cypermethrin in DMSO (20%) was administered to Wistar rats
as a single oral dose of 40 mg/kg body weight. The exposure time was
6 h. Alpha-cypermethrin failed to produce any detectable DNA
single-strand damage using alkaline elution profiles of liver DNA.
Methylmethane sulfonate was used as a positive control and DMSO as the
solvent control (Wooder, 1982).
7.6.4 Conclusion
From the available data on alpha-cypermethrin, it can be
concluded that this compound is non-mutagenic in tests with
Salmonella typhimurium, Saccharomyces cerevisiae, and in vivo and
in vitro tests with rat liver cells for the induction of chromosome
aberration and production of DNA single-strand damage.
Table 5. Mutagenicity tests on microorganisms
Organism/strain Dose Type of test Metabolic Result Reference
activation
Salmonella typhimurium up to 4000 µg/plate plate with or negative Brooks (1982)
TA98, TA100, TA1535, without
TA1537, TA1538
Escherichia coli up to 4000 µg/plate plate with or negative Brooks (1982)
WP2, WP2 uvrA without
Saccharomyces cerevisiae up to 5000 µg/ml liquid suspension with or negative Brooks (1982)
JDI culture without
Saccharomyces cerevisiae up to 4000 µg/ml liquid suspension with or negative Brooks (1984)
XV 185-14C culture without
Rat liver cells (RL4) up to 40 µg/ml negative Brooks (1982)
(chromatid gaps, breaks or
aberrations)
Rat liver DNA one oral dose of negative Wooder (1982)
(DNA single strand damage) 40 mg/kg body weight
Rat bone marrow one oral dose of negative Clare & Wiggins
chromosome study up to 8 mg/kg body (1984)
weight
7.7 Special studies
7.7.1 Skin sensation
It is known that exposure to certain types of pyrethroids can
result in a transient skin sensation in humans (Le Quesne et al.,
1980).
Guinea-pigs received 0.1 ml of a 0.01, 0.1 or 1.0% solution of
alpha-cypermethrin in ethanol or a 1, 10 or 20% solution of
alpha-cypermethrin (w/v) in corn oil on the skin. Sensory stimulation
was quantified by counting the number of times each animal turned to
lick or bite its treated flank in preference to the non-treated flank.
Skin stimulation was observed during a 2-h period at all dose levels
except the lowest. In the groups of guinea-pigs treated with 10% and
20%, some animals exhibited an exaggerated hopping movement and a
repeated head shaking activity at the time of maximum skin stimulation
(40-60 min after treatment). This behaviour was not seen with the 1%
solution or more dilute ones (Hend, 1983).
7.7.2 Neurotoxicity
Large oral doses of alpha-cypermethrin and other synthetic
pyrethroids (WHO, 1989) have been shown to produce minor
histopathological lesions in the sciatic nerve of rats, described as
sparse axonopathy in peripheral nerves.
Rose (1983b) conducted a two-phase study. In the first phase, the
time-course for development and recovery from pyrethroid-induced nerve
lesions was investigated by measuring biochemical correlates of
neuropathological change (the enzymes beta-glucuronidase and
beta-galactosidase) in groups of Wistar rats (five of each sex per
group) at periods of 2-12 weeks after the start of dosing. The daily
doses of alpha-cypermethrin (96.6%), administered by stomach tube,
were 37.5 mg/kg body weight for the first 11 doses and 25.0 mg/kg body
weight for the subsequent 9 doses over a 4-week period (5 times/week).
DMSO was used as the solvent for 10 doses and then arachis oil. In
all, 21% of the animals died and more than 80% of the treated animals
showed clinical signs of intoxication. Maximum enzyme activities in
the sciatic posterior tibial nerves were found 5 weeks after the start
of the experiment and had returned to control values by 12 weeks. In
the trigeminal nerve and trigeminal ganglia, a slight but not
significant increase in enzyme activities was found.
In the second phase of the study, 10 male and 10 female Wistar
rats were given 20 oral doses of alpha-cypermethrin in DMSO (0, 10, 20
or 40 mg/kg body weight per day) over a period of 4 weeks (5
times/week). Only two animals died, one given 10 mg/kg and one given
20 mg/kg. In the 40-mg/kg group, 75% of the animals developed clinical
signs, whereas in the 20-mg/kg group only 25% of the animals showed
these clinical effects. In the 10-mg/kg group, the animals showed no
differences from the controls. No clear influence of
alpha-cypermethrin on growth was found. Five weeks after the initial
dose, which corresponded to the period of maximal enzyme changes,
biochemical changes (increases of up to 60%) indicative of a mild
axonal degeneration were found in both the distal and proximal
sections of the sciatic posterior tibial nerve in animals administered
40 mg/kg body weight. In the 20-mg/kg group, only a small (up to 20%)
increase in beta-galactosidase activity was found in the proximal
sections of the sciatic posterior nerve. The same trends were found in
the trigeminal nerve and ganglia. No changes were found in the
10-mg/kg group (Rose, 1983b).
7.7.3 Immunosuppressive action
No data on the immunosuppressive action of alpha-cypermethrin are
available.
7.8 Mechanism of toxicity - mode of action
The mechanism of toxicity and mode of action of cypermethrin (and
other pyrethroids) are extensively described in section 8.8 of the
Environmental Health Criteria 82: Cypermethrin (WHO, 1989). Recently
Vijverberg & van den Bercken (1990) and Aldridge (1990) summarized
current knowledge of the neurotoxicity and mode of action of the
different pyrethroids (see also Appendix 1).
Pyrethroids induce toxic signs that are characteristic of a
strong excitatory action on the nervous system. Toxic doses generally
cause hypersensitivity to sensory stimuli, and a number of compounds
may induce tingling sensations in the skin. Two distinct toxic
syndromes have been described in mammals. The T-syndrome is induced by
pyrethrins and non-cyano pyrethroids, and the CS-syndrome, induced by
cyano-pyrethroids such as cypermethrin and alpha-cypermethrin, is
characterized by choreoathetosis and salivation.
The available data strongly suggest that the primary target site
of pyrethroid insecticides in the vertebrate nervous system is the
sodium channel in the nerve membrane. Pyrethroids without an
alpha-cyano group cause a moderate prolongation of the transient
increase in sodium permeability of the nerve membrane during
excitation. This results in relatively short trains of repetitive
nerve impulses in sense organs, sensory (afferent) nerve fibres and,
in effect, nerve terminals. On the other hand, the alpha-cyano
pyrethroids (for instance cypermethrin and alpha-cypermethrin) cause
a long-lasting prolongation of the transient increase in sodium
permeability of the nerve membrane during excitation. This results in
long-lasting trains of repetitive impulses in sense organs and a
frequency-dependent depression of the nerve impulse in nerve fibres.
The difference in effects between permethrin (with no alpha-cyano
group) and the two insecticides cypermethrin and alpha-cypermethrin,
which have identical molecular structures except for the presence of
an alpha-cyano group on the phenoxybenzyl alcohol, indicates that it
is this alpha-cyano group that is responsible for the long-lasting
prolongation of the sodium permeability.
Since the mechanisms responsible for nerve impulse generation and
conduction are basically the same throughout the entire nervous
system, pyrethroids may also induce repetitive activity in various
parts of the brain. The difference between the symptoms of poisoning
by alpha-cyano pyrethroids and those of the classical pyrethroids is
not necessarily due to an exclusive central site of action. It may be
related to the long-lasting repetitive activity in sense organs and
possibly in other parts of the nervous system, which, in a more
advance state of poisoning, may be accompanied by a
frequency-dependent depression of the nervous impulse.
Pyrethroids also cause pronounced repetitive activity and a
prolongation of the transient increase in sodium permeability of the
nerve membrane in insects and other invertebrates. Available
information indicates that the sodium channel in the nerve membrane is
also the most important target site of pyrethroids in the invertebrate
nervous system.
Because of the universal character of the processes underlying
nerve excitability, the action of pyrethroids should not be considered
to be restricted to particular animal species or to a certain region
of the nervous system.
Although it has been established that sense organs and nerve
endings are most vulnerable to the action of pyrethroids, the ultimate
lesion that causes death will depend on the animal species,
environmental conditions, and on the chemical structure and physical
characteristics of the pyrethroid molecule.
8. EFFECTS ON HUMANS
8.1 General population exposure
No data concerning the exposure of the general population to
alpha-cypermethrin are available.
8.2 Occupational exposure
A study of alpha-cypermethrin exposures was carried out during
formulation using both technical concentrate and technical material at
Durban, South Africa. The oil-damped solid, crystalline, dry technical
concentrate contained a minimum of 90% (m/m) alpha-cypermethrin.
Exposures were assessed by personal and static monitoring of
atmospheric alpha-cypermethrin concentrations, urinary
alpha-cypermethrin metabolite concentrations and by medical
examination. Four individuals were exposed during 3 days of operation.
The group mean personal exposures for the two days whilst formulating
technical concentrate were 2.8 and 4.9 µg/m3 and the group mean
personal exposure to technical material on day 3 was 54.1 µg/m3.
Urinary alpha-cypermethrin metabolites could not be identified (limit
of detection, 0.02 mg/litre). Formulation was successfully completed,
only minor skin sensations being reported by two of the
non-operational personnel, possibly resulting from particles of
alpha-cypermethrin settling directly on the skin, face and neck. Dust
concentrations were up to 30 times greater during handling the
technical material compared with oil-damped technical concentrate, and
local exhaust dust extraction reduced dust emission by a factor of up
to 17 (Western, 1984).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
Appraisal
The acute toxicity of alpha-cypermethrin to Daphnia magna and
Gammarus pulex is similar to that of cypermethrin. However, in a
reproduction study with Daphnia magna, alpha-cypermethrin seemed to
be slightly more toxic than cypermethrin. Comparison of the results
of a reproduction study in Daphnia magna with the results of acute
tests shows that the hazard of alpha-cypermethrin lies in its acute
toxicity. There is no significant potential for cumulative effects
occurring as a result of long-term exposure to lower concentrations.
Alpha-cypermethrin is highly toxic to a number of aquatic
arthropod taxa but of low toxicity to molluscs. The short-term
toxicity of the compound can be reduced by formulating the product as
an OSC. Contamination through spray drift from commercial
applications will generally be low, and so the effects on susceptible
taxa will be limited. The rapid loss of alpha-cypermethrin from the
water gives the potential for complete recovery of affected
populations.
Laboratory studies show that values for the acute toxicity and
the toxicity to the early-life stages of fish are similar for
alpha-cypermethrin and cypermethrin. A comparison of the results from
both alpha-cypermethrin studies reveals that the hazard of the
compound results from its acute toxicity, and there is no significant
potential for additional effects occurring as a result of long-term
exposure to lower concentrations. Laboratory and field studies have
shown that the toxicity of alpha-cypermethrin to fish is greatly
influenced by the formulation, particulate formulations showing
significantly less toxicity than emulsifiable concentrates.
Field studies demonstrate that the high toxicity of
alpha-cypermethrin to fish observed in laboratory studies is not
realized under field conditions. Contamination of water bodies by
inadvertent overspraying or spray drift does not present a hazard to
fish.
9.1 Microorganisms
9.1.1 Algae
The acute toxicity of alpha-cypermethrin to a single-celled green
alga, Selenastrum capricornutum, at 24 °C has been determined. The
pesticide was dispersed using acetone, and the 2- to 4-day EC50 for
growth was above 100 µg/litre (Stephenson, 1982).
9.1.2 Bacteria
The effects of cypermethrin on microbial activity in the soil
have been investigated in a series of studies. Cypermethrin was
applied to sandy loam at concentrations of 2.5 and 250 mg/kg. No
effects on the rates of carbon dioxide evolution or oxygen uptake were
observed at the lower rate, but significant inhibition of carbon
dioxide evolution and a decrease in oxygen uptake were observed at the
higher rate of application. There were no effects at either
concentration on nitrogen fixation, ammonification, nitrification or
glucose utilization (WHO, 1989).
Sewage bacteria were unaffected by the presence of
alpha-cypermethrin (3 mg/litre) in a closed system test, while the
growth of Pseudomonas fluorescens was unaffected at 100 mg/litre
(Stone & Watkinson, 1983).
9.2 Aquatic organisms
9.2.1 Invertebrates
9.2.1.1 Laboratory studies
Acute toxicity studies with Daphnia magna (aged < 24 h) showed
that at 20 °C technical alpha-cypermethrin, dispersed in acetone under
static conditions (daily renewal), has effects at concentrations below
1 µg/litre. The 24-h and 48-h EC50 values (immobilization) were 1.1
and 0.3 µg/litre, respectively (Stephenson, 1982).
Water samples taken from field enclosures 24 h after treatment
with an EC formulation were analysed for alpha-cypermethrin and
bioassayed with Gammarus pulex. The 24-h LC50 value for this
organism was 0.05 µg/litre (Garforth, 1982a; Shires, 1982).
The effect of technical alpha-cypermethrin on survival, growth
and reproduction of Daphnia magna was studied over a period of 21
days by Garforth (1982b). The test solution was renewed daily and the
temperature ranged between 18.5 and 20.2 °C. The results are
summarized in Table 6.
Table 6. Effects of alpha-cypermethrin on the reproductive cycle of
Daphnia magnaa
Effect Nominal concentration (µg/litre)
LOEL NOEL
Survival of parent generation 0.3 0.1
Growth of parent generation 0.1 0.03
Production of young 0.1 0.03
a From: Garforth (1982b)
LOEL = Lowest-observed-effect level; NOEL = No-observed-effect
level
9.2.1.2 Field studies
Garforth (1982a) studied the effects of alpha-cypermethrin on a
range of aquatic invertebrates in metal enclosures, each containing
about 1 m3 water, placed in an outdoor experimental pond. A diluted
EC formulation was sprayed onto the water surface at concentrations of
1, 3, 10, 30 and 100 g active ingredient/ha, and samples were taken up
to 7 days after application. The concentration of alpha-cypermethrin
was around 50% of the nominal level 24 h after application and
decreased to 10 to 20% of the nominal level 7 days after application.
The lowest concentration was toxic to Asellidae. Thirteen families
of aquatic arthropods were tested; most were killed at 1 g/ha except
one species of Coenagriidae, which tolerated about 3 g/ha. Three
families of molluscs were tested and were found to be unaffected at
100 g/ha.
Studies to investigate the relative toxicity of two formulations
of alpha-cypermethrin (a 100-g/litre EC and a 100-g/litre oil-enhanced
suspension concentrate (OSC) with and without anti-evaporant agents)
to aquatic invertebrates have been carried out. Water samples were
taken from field enclosures treated with a range of doses (0.1-5 g/ha
for the EC and 0.1-10 g/ha for the two OSC formulations) and
bioassayed with Gammarus pulex. The 24-h LD50 values (in g
alpha-cypermethrin/ha equivalents) of water samples taken 24 h after
application were 0.9 for the EC, 6.3 for the OSC without
anti-evaporant, and 2.8 for the OSC plus anti-evaporant formulation.
Thus, one day after treatment, both OSC formulations were less toxic
to Gammarus pulex than the EC formulation. However, the EC
formulation lost its toxicity more rapidly than either of the OSC
formulations (see also section 4.1.2). The effects on other aquatic
invertebrate communities could not be accurately assessed, since the
results for macro-arthropods and zooplankton were inconclusive.
Coenagriidae, Chironomidae and zooplankton seemed to be relatively
tolerant. The residues in water and sediment, 22 days after treatment
with the EC at 2 g alpha-cypermethrin/ha or with both OSC formulations
at 5 g/ha, were < 0.004 µg/litre and < 0.01 mg/kg, respectively, for
all three formulations (Inglesfield, 1985b).
The hazard to aquatic invertebrates resulting from spray drift
from the aerial application of an EC (15 g alpha-cypermethrin/ha) has
been investigated by Garforth & Woodbridge (1984). Details of the
study are described in section 9.2.2.2. The sub-surface water
concentration was 0.6 µg alpha-cypermethrin/litre shortly after
application and decreased to < 0.02 µg/litre within 2 to 4 days. The
contamination initially caused a significant reduction in the
abundance of several groups of aquatic arthropods, including beetles,
chironomids, corixids, mites and zooplankton. However, within 4 to 7
weeks the affected fauna had completely recovered.
In a study by Pearson (1990), two freshwater ponds were treated
with alpha-cypermethrin as an emulsifiable concentrate in 1987. One
pond was oversprayed at 15 g active ingredient/ha, while the other was
treated with the same amount of alpha-cypermethrin but by direct
incorporation into the water (see section 4.1.2). Indigenous
populations of zooplankton nauplii and of copepods ( Cyclops spp.)
were significantly reduced in both ponds by the treatment but
recovered within 26-45 days. Other zooplankton were not plentiful but
appeared to be less severely affected in the oversprayed pond than in
the pond treated by incorporation. Populations of phantom midge larvae
( Chaoborus spp) were killed by both treatments. However, within 47
days after treatment, populations of young larvae had developed in the
oversprayed pond to almost pre-treatment numbers, and to a lesser
extent in the pond with direct incorporation.
9.2.2 Fish
9.2.2.1 Laboratory studies
The available acute 96-h LC50 values for two fish species are
summarized in Table 7.
The effects of the type of formulation on the acute toxicity to
fish are summarized in Table 8. Suspension concentrate, wettable
powder and micro-encapsulated formulations were 10 to 70 times less
acutely toxic to rainbow trout than the EC formulation (Shires,
1983b).
Table 7. Acute toxicity of technical alpha-cypermethrin in fish
Species Mean weight Vehicle Test system Temperature 96-h LC50 Reference
(g) (°C) (µg/litre) (95%
confidence limits)
Rainbow trout 3.3 dispersed via static water; 15 2.8 Stephenson (1982)
(Oncorhynchus mykiss) acetone 12 h renewal of (2.1-3.5)
test solutions
Fathead minnow 0.76 adsorbed onto continuous 23-25 0.93 Stephenson (1983)
(Pimephales promelas) pumice flow-through (0.78-1.2)
Table 8. Effect of type of formulation on the toxicity of alpha-cypermethrin to fish in laboratory studies
Species Weight Tempera- Formulation 96-h LC50 Reference
(g) ture (°C) (µg/litre)
Rainbow trout 0.8-2.5 15 100 g/litre EC 5.6 Shires (1983b)
(Oncorhynchus 250 g/litre SC 350
mykiss) 100 g/kg WP 120
50 g/kg WP 220
50 g/kg ME > 100
50 g/kg CD 65
Rainbow trout 0.11-0.25 15 15 g/litre OSC 10a Pearson (1986)
(Oncorhynchus 15 g/litre OSC 71
mykiss) 100 g/litre OSC 16a
100 g/litre OSC 56
Common carp 3.5-4 24-30 100 g/litre OSC 11 Stephenson
(Cyprinus carpio) 15 g/litre EC 0.8 (1986)
50 g/kg WP 60
Puntius 0.3-0.5 24-30 100 g/litre OSC 3.2 Stephenson
gonionotus 15 g/litre EC 0.7 (1986)
50 g/kg WP 22
a Denotes daily renewal of test solutions. All other tests were carried out without renewal of the test solutions.
EC = emulsifiable concentrate; SC = suspension concentrate; OSC = oil-enhanced suspension concentrate; WP= wettable powder;
ME = micro-encapsulated;
CD = ß-cyclodextrin.
The toxicity of alpha-cypermethrin to the early-life stages of
fish has been studied in a 34-day continuous-flow embryo-larval test
with the fathead minnow (Pimephales promelas). Eggs less than 24 h
old were exposed to nominal concentrations of 0.03 to 1.0 µg/litre.
Pre-hatch, post-hatch and overall mortality and final body weight were
recorded. On the basis of the most sensitive parameter (overall
survival) and measured exposure concentrations, the lowest
concentration of alpha-cypermethrin producing an adverse effect was
0.09 µg/litre and the highest concentration producing no effect (NOEL)
was 0.03 µg/litre (Stephenson, 1983).
9.2.2.2 Small scale field or outdoor tank studies
The acute effect of formulation on the toxicity of
alpha-cypermethrin to fish under field conditions was investigated in
studies using stainless steel enclosures placed in an experimental
pond. In the first study (Shires, 1983b), the rainbow trout (13-32 g)
was the test species and the results are summarized in Table 9. The EC
formulation was at least 30 times more toxic than any of the other
formulations. The number of fish used was not reported.
Table 9. Effect of formulation on the toxicity of alpha-cypermethrin
to rainbow trout in field studies
Formulation 14-day LD50 (g active
ingredient/ha equivalent)
100 g/litre emulsifiable concentrate 29
250 g/litre suspension concentrate > 1000
100 g/kg wettable powder > 1000
50 g/kg micro-encapsulated > 1000
In the second study, Stephenson (1987c) tested an 1.5%
oil-enriched SC formulation of alpha-cypermethrin in outdoor tanks
containing carp ( Cyprinus carpio; 6.3 g). The application rate was
approximately 4, 8 and 15-16 g active ingredient/ha applied by hand
sprayer. The water temperature ranged from 23-26 °C, the water was
aerated and the duration of the experiment was 7 days. There was 20%
mortality at all treatment rates, which was comparable with the
control group. The number of fish used was not reported.
In the third study (Shires, 1985b), rainbow trout ( Oncorhynchus
mykiss; 2-5 g) were introduced into open-ended stainless steel
enclosures placed in a mature experimental pond. Different volumes of
alpha-cypermethrin (as a diluted EC containing 100 g active
ingredient/litre) were applied with a hand-held sprayer onto the water
surface. The dose rates were equivalent to 5-500 g active
ingredient/ha. The concentrations in the water samples were dependent
on the dose applied and the time after the treatment that the samples
were taken. For instance, with a dose of 5 g alpha-cypermethrin/ha the
concentration in the water was 0.4 µg/litre or less after 96 h,
whereas with a dose of 500 g/ha residues of up to 30 µg/litre were
found. Alpha-cypermethrin was toxic to the rainbow trout at a
concentration of about 2-5 µg/litre water. In terms of nominal
application rate, the no-observed-effect level for alpha-cypermethrin
lies between 50 and 100 g alpha-cypermethrin/ha. These dose rates are
much higher than those used for crop protection purposes.
In a further study, 20 rainbow trout were placed in stainless
steel enclosures in a shallow pond. The diluted EC and SC formulations
were sprayed onto the surface of the water and the fish were monitored
for mortality over 8 days. The SC formulation did not cause mortality,
even at an application rate equivalent to 300 g active ingredient/ha.
However, high mortality (90%) occurred with the EC formulation at 30
g/ha alpha-cypermethrin, but application of 10 g active ingredient/ha
did not cause mortality. From these results it is clear that the SC
formulation has a lower toxicity than the EC formulation (Stephenson,
1987b).
The hazard to fish caused by spray drift from aerial applications
of alpha-cypermethrin to agricultural land has been investigated by
Garforth & Woodbridge (1984). The trial site was a cereal field
bordered on one side by a freshwater ditch. The water's edge was
generally less than 2 m from the crop margin. Four days prior to
application, two cages, each containing 15 common carp ( Cyprinus
carpio; 30-50 cm) were placed in the ditch. The ditch was known to
contain a good stock of different types of fish, macroinvert-ebrates
and zooplankton. An alpha-cypermethrin EC containing 100 g/litre was
applied by air at 15 g active ingredient/ha to the crop when a gentle
breeze was blowing from the crop over the ditch. The
alpha-cypermethrin concentration in the sub-surface water was 0.6
µg/litre shortly after the application and decreased to < 0.02
µg/litre within 2 to 4 days (see section 4.1.2.). None of the fish
died and no adverse effects were observed either on the carp placed in
the cages or on the indigenous species. It was concluded that
inadvertent contamination of water bodies by spray drift resulting
from the normal commercial use of alpha-cypermethrin does not present
a hazard to fish.
Several field tests in rice paddies have examined the toxicity of
alpha-cypermethrin to fish.
Stephenson (1986, 1987b) investigated the acute toxicity of 15
g/litre EC, 100 g/litre OSC and 50 g/kg WP formulations to fish in
plots of rice paddy in West Java. The application rates were 7.5, 15,
and 30 g alpha-cypermethrin/ha for the EC and OSC formulations and 30
g alpha-cypermethrin/ha for the WP formulation. Each of the
insecticide treatments was applied to the same plot on two separate
occasions; once 12 days after transplantation of the rice seedlings
and again 57 days after the transplantation. Prior to application, two
cages each containing 20 carp (Cyprinus carpio) (2.5-5 g) and two
cages each containing 20 specimens of Puntius gonionotus (1.4-5 g)
were placed in trenches cut in each plot. In addition, before the
first experiment, 30 fish of each of these two species were released
to swim freely in each plot. Survival of the fish was monitored daily
up to 7 days after each treatment. The water temperature was between
25 and 36 °C. Following the first application, only the EC formulation
at 15 and 30 g/ha resulted in significant mortality of caged and
free-swimming carp and Puntius gonionotus. The same was true for
carp following the second application, but no results were available
for Puntius gonionotus following the second application due to an
outbreak of disease among these fish.
Two other field studies were carried out in West Java to examine
the effects of an OSC formulation on the growth and survival of
free-swimming carp (6-8 g) in rice paddies. Experimental conditions
were similar to those described above for the acute studies. The water
temperature was 23-32 °C. In the first experiment, treatments with 15
g active ingredient/ha were carried out 21 and 33 days after
transplantation of the rice seedlings. In the second experiment, the
same dose rate was applied 51 and 64 days after transplanting. The
fish were monitored over periods of 3 to 4 weeks in each experiment.
No adverse effects on survival and growth of the carp were observed
(Stephenson, 1987a,b).
9.3 Terrestrial organisms
9.3.1 Earthworms
The toxicity of technical alpha-cypermethrin to the red
earthworm, Eisenia foetida, has been assessed in laboratory tests.
In the filter paper contact toxicity test, 50% mortality occurred
within 48 h at a dose of about 0.01 mg/cm2 of filter paper. However,
increases in the dose up to 1 mg/cm2 did not result in a significant
increase in mortality. In the artificial soil test no significant
mortality occurred within a period of 14 days in earthworms exposed to
up to 100 mg alpha-cypermethrin/kg soil (Inglesfield & Sherwood,
1983).
9.3.2 Invertebrates - field studies
In a study designed to investigate the effects of
alpha-cypermethrin on non-target arthropod fauna in Italian vineyards,
two formulations of alpha-cypermethrin were tested, i.e. an
emulsifiable concentrate (100 g active ingredient/litre) and a
suspension concentrate (250 g active ingredient/litre). Both were
diluted to 1.25 g active ingredient/ha and sprayed to run-off. The
effect on predators and parasites (crop foliage fauna) such as
parasitoid Hymenoptera, Heteroptera and Chrysopa carnea, soil
surface predators such as Coleoptera and Araneae, phytophagous
arthropods such as Homoptera, Thysanoptera and Acari, and other
invertebrates such as nematocerous, Diptera and epigeal fauna was
inconclusive but in most cases negative. Spiders were the only group
significantly affected by alpha-cypermethrin. The abundance of major
phytophagous insect taxa (Homoptera, Thysanoptera and Acari) was
markedly reduced by both treatments although neither treatment had any
long-term adverse effects (Inglesfield, 1984).
An emulsifiable concentrate (EC) of alpha-cypermethrin was tested
on the non-target arthropod fauna of maize in France at 20 and 30 g
active ingredient/ha and applied using tractor-mounted boom and nozzle
equipment at different stages of crop development. Organisms from the
following taxa were collected: phytophagous arthropods (Aphidoidea),
Thysanoptera (Thripidae), Cicadellidae and entomophagous arthropods;
predatory Coleoptera (Coccinellidae, Cantharidae, Carabidae and
Staphylinidae); parasitoid Hymenoptera (Braconidae, Chalcidoidea);
and predatory Diptera, Neuroptera and Aranea. In addition, the
effects on community structure were studied. The two treatments
reduced transiently the numbers of some of the entomophagous taxa,
especially Coccinellidae (Coleoptera), Carabidae (Coleoptera),
Neuroptera and Araneae. Alpha-cypermethrin at a concentration of
20 g/ha promoted some late-season resurgence of aphid populations
(Inglesfield, 1985a).
A large-scale replicated field experiment was carried out in
Indonesia to investigate the effects of an EC (10 g and 20 g
alpha-cypermethrin/ha) and an OSC (10 g and 20 g alpha-cypermethrin
per ha), applied at 17 and 62 days after transplanting using a single
fan-jet nozzle knapsack sprayer, on rice pests and their natural
enemies. Alpha-cypermethrin was applied at two crop stages, and
populations of both pests and beneficial arthropods were monitored
throughout the season. Alpha-cypermethrin produced good control of
stemborers, grasshoppers, leafhoppers and stinkbugs, the most numerous
pests found during the trial. It had a significant but short-lived
effect on spiders and, generally, no effect on either dragon-flies or
other beneficial arthropods (Shires & Inglesfield, 1986).
A study was initiated in England in 1982 to compare the effects
on entomophagous arthropods of annual applications of an EC of
alpha-cypermethrin (at a rate of 10 g active ingredient/ha in the
first year and 15 g active ingredient/ha in subsequent years) with
those resulting from the use of non-pyrethroid products. The study was
carried out on a range of crops (oilseed rape, wheat and barley) over
the duration of a 5-year arable crop rotation. A field of 8 ha was
divided into two equal areas, and each year alpha-cypermethrin was
applied to one of the areas and other products to the other area. All
treatments were carried out with standard tractor-driven boom and
nozzle spraying equipment. The crop rotation was as follows: winter
rape in 1982, winter wheat in 1983 and 1984, and winter barley in
1985. Organisms from a variety of taxa were collected during the
study, such as parasitoid Hymenoptera, predatory Diptera,
predatory Coleoptera, Araneae, phytophagous insects and other
arthropods. The results indicated that any difference between the
effects of alpha-cypermethrin and the reference compounds on
entomophagous arthropods was generally short-lived. There is no
evidence that alpha-cypermethrin treatments had any long-term effects
on any of the taxa studied. In addition, there appeared to be no
long-term effects on the relative abundance of entomophages within the
arthropod communities or on the structure and integrity of the
arthropod communities of which they form a part (Inglesfield, 1985c,
1988, 1989).
The results of these studies have been reviewed by Inglesfield
(1991). They showed that field application of alpha-cypermethrin and
cypermethrin had no adverse effects on the relative abundance of
entomophages within the arthropod communities and that the use of
these two pesticides in small grain cereals would not be associated
with pest "resurgence" or the development of secondary pest
infestations.
9.3.3 Honey-bees
9.3.3.1 Laboratory studies
The oral administration of alpha-cypermethrin in acetone produced
a 24-h LD50 of 0.06 µg/bee, whereas an EC formulation (100 g/litre)
of the pesticide yielded a 24-h LD50 of 0.13 µg formulation/bee.
After topical application, 24-h LD50 values of 0.03 µg (technical)
and 0.11 µg (EC) per bee were obtained (Murray, 1985).
The mortality of honey-bees (Apis mellifera) exposed to
Phacelia flowers 30 min after they had been treated with an EC
formulation (15 g active ingredient/ha) in a residual test was low
(15%) within 48 h. However, the foraging activity was reduced (Murray,
1985).
The residual toxicity to the honey-bee of alpha-cypermethrin as
EC, OSC, and EC/fungicide mixtures has been investigated. Bees were
exposed for 48 h to flowering Phacelia campanularia plants that had
been sprayed with each formulation at a rate of 10 or 20 g active
ingredient/ha, and the mortality was assessed 24 and 48 h after
initial exposure to the treated plants. Although there was great
variation in the results, there appeared to be no difference in the
residual toxicity of the EC and OSC formulations at a rate of 10 g
active ingredient/ha. However, at 20 g active ingredient/ha the OSC
appeared to be more toxic than the EC. Application of the EC in
admixtures did not appear to significantly increase bee mortality
(Hillaby & Inglesfield, 1986).
9.3.3.2 Field studies
Alpha-cypermethrin, applied as an EC (10 and 20 g active
ingredient/ha) by tractor-mounted boom and nozzle equipment to small
plots of flowering mustard in France, caused a sharp decline in
foraging activity of bees immediately after application, but there was
a return to normal activity within a few hours. No effect on bee
survival or hive development was observed (Shires, 1983a; Shires et
al., 1984b; Shires, 1985a).
In a further study, the same EC formulation was applied at the
same concentration on large isolated fields of flowering oilseed rape
during peak foraging activity of honey-bees in France. No increase in
bee mortality was found, but foraging activity declined for a few
hours after application at a rate of 10 g active ingredient/ha. With
a rate of 20 g active ingredient/ha a more prolonged decline in
foraging activity occurred. No effects on the overall condition of the
hives were seen at the end of the season and very low or undetectable
residues were found in dead bees, pollen, honey and wax (Shires et
al., 1984c; Shires, 1985a).
Two large and two small plots of winter wheat were enclosed
beneath large mesh-covered tunnels. A small bee-hive was placed in
each tunnel and sucrose solution was sprayed onto all of the wheat in
order to simulate aphid honey dew. Alpha-cypermethrin (an EC at 10, 15
or 30 g active ingredient/ha) was applied to the larger plots of wheat
when the bees were actively foraging the sugar deposits. No increase
in bee mortality, compared with that in the pre-treatment period, was
observed. Foraging activity in the plots declined sharply after
treatment and remained at a reduced level, probably because of its
repellent effect. Examination of the hives about 2 weeks after the
trial showed that the hives, both the control and the
alpha-cypermethrin-treated, were in excellent condition with strong
adult populations and large areas of developing brood. In
post-treatment samples, alpha-cypermethrin residues of 0.03 mg/kg of
honey and 0.01 mg/kg of wax were found. In live and dead bees
collected from the tunnel treated with 15 g/ha, a concentration of
0.026 µg/bee was found (Shires et al., 1984a; Le Blanc, 1985).
In a study by Inglesfield & Forbes (1986), alpha-cypermethrin was
applied as an OSC (10 g active ingredient/ha) and an EC (10 g active
ingredient/ha) to three fields of flowering winter-sown oilseed rape
in Germany while bees were actively foraging the crops. None of the
treatments had any significant effect on adult bee survival or on the
longer-term development of the experimental colonies. The number of
bees actively foraging in the crops declined following application.
This reduction in activity can probably be partly attributed to the
repellent effects of these treatments. Both the OSC and EC
formulations of alpha-cypermethrin had similar effects on bee
behaviour. Residues of alpha-cypermethrin in post-treatment samples of
pollen, honey and wax were below 0.01 mg/kg. In dead bees, the residue
on the day of application with the OSC was 1.8 mg/kg and in the case
of the EC was 0.12 mg/kg.
A diluted EC formulation of alpha-cypermethrin (mixed with the
fungicide vinclozolin, 100 g active ingredient/ha) was applied by a
tractor-driven boom and nozzle sprayer, at a rate of 20 g active
ingredient/ha, to a 206-ha block of flowering oilseed rape situated in
Kent, England. Shortly before spraying, which took place over a 3-day
period, five beehives were positioned at each of two sites adjacent to
the crop. At each site the hives were either fitted with pollen traps
or with traps to collect dead bees as they were removed from the hive.
The hives were observed daily before and for ten days after spraying.
Hive activity was recorded at intervals each day, and, on days when
bees were flying, pollen traps were set and samples of pollen
collected. Following the application of alpha-cypermethrin the bees
foraged normally. At least 90% of the pollen returned to the hives
during the immediate post-treatment period was found to be from
oilseed rape, showing that the bees had continued to forage on the
treated crop. The number of dead bees collected remained low
throughout the study and did not increase after application of the
alpha-cypermethrin. After the study, all hives were in good condition
and subsequently yielded a good crop of honey. A local beekeeper with
many hives adjacent to the same block of oilseed rape reported no
effects amongst his hives. It was concluded that the application of
alpha-cypermethrin at 20 g active ingredient/ha to flowering oilseed
rape had no direct effects on honey-bee survival and hive development
(Brown, 1989).
9.3.4 Leaf-cutting bees
Laboratory trials using 15 male adult alfalfa leaf-cutting bees
( Megachile rotundata F.) per group, exposed to treated filter papers
for 4 h, showed that alpha-cypermethrin EC (100 g/litre) at the rate
of 10 or 15 g active ingredient/ha caused 12 and 30% mortality,
respectively, and 42 and 100% mortality after 24 h of contact (Tasei
et al., 1987).
In a study by Tasei et al. (1987), populations of about 400
female alfalfa leaf-cutting bees were reared in three flowering
lucerne fields. One field was left untreated, while the two others
were treated with alpha-cypermethrin as an EC (10 or 15 g active
ingredient/ha), applied by a tractor-mounted fan-jet sprayer. Two days
after the treatments, counts of live females in artificial nesting
sites showed the losses due to 10 and 15 g active ingredient/ha to be
21 and 12%, respectively. After hibernation and incubation of the
progeny larvae, little effect of the treatments could be observed. The
maximum residue in leaves collected from nests was 1 mg
alpha-cypermethrin/kg. The mean values after 5, 10 and 27 days in leaf
pieces capping the nests of the bees were 0.75, 0.48, and 0.19 mg/kg
at the lower application rate and 0.59, 0.53, and 0.10 mg/kg at the
higher rate. No residues (limit of determination, 0.01 mg/kg) were
detected in live larvae but 0.07 mg/kg was found in pollen provisions
(Tasei et al., 1987).
9.3.5 Birds
Cypermethrin is practically non-toxic to birds; acute oral LD50
values are greater than 2000 mg/kg body weight. The dietary LC50
value is above 10 000 mg/kg diet (see WHO, 1989). However, studies
with alpha-cypermethrin have not been carried out.
10. COMPARISON BETWEEN ALPHA-CYPERMETHRIN AND CYPERMETHRIN
Alpha-cypermethrin comprises one quarter of the racemic mixture
cypermethrin, with which an extensive agricultural, ecological and
(eco)toxicological programme has been carried out (WHO, 1989). It
contains more than 90% of the insecticidally most active enantiomer
pair of the four cis isomers of cypermethrin, i.e. the two cis isomers
(IRcis)S and (IScis)R (see Fig. 1).
10.1 Use and residue levels
Alpha-cypermethrin is used to control the same pests in
agriculture as cypermethrin. Its rate of application to crops (5-30 g
active ingredient/ha) is lower than that of cypermethrin (10-200 g
active ingredient/ha) since alpha-cypermethrin is biologically more
active than cypermethrin.
Residue data for alpha-cypermethrin have been obtained from a
large number of supervised trials carried out worldwide. These trials
cover the most important crop groupings for which alpha-cypermethrin
is recommended, including oilseeds, pome fruits, peaches, fruiting
vegetables, berries, leafy vegetables, maize and speciality crops such
as hops and tobacco (Shell, 1984). Residues in a variety of these
crops resulting from application of alpha-cypermethrin at the
recommended rate ranged between 0.05 and 1.0 mg/kg (Shell, 1984). In
comparison, residues of cypermethrin in crops were higher and ranged
between 0.05 and 2.0 mg/kg. In comparative trials where
alpha-cypermethrin was applied at half the dose rate of cypermethrin,
alpha-cypermethrin residues in general averaged 40% (20-50%) of those
in cypermethrin-treated samples (Shell, 1984; WHO, 1989).
The residue data on cypermethrin have been evaluated by the Joint
FAO/WHO Meeting on Pesticide Residues and recommended MRLs (1979/1981)
have been published (FAO/WHO, 1980, 1982) (see Table 10). From the
available residue data obtained with good agricultural practice and
using the MRLs set for cypermethrin, suggested MRLs for
alpha-cypermethrin can be extrapolated (Table 10).
It can be concluded that alpha-cypermethrin, since it is
biologically more active, is always used at a lower application rate
than cypermethrin and, as a result, the residues on crops are
approximately half those of cypermethrin.
Table 10. Comparison of current MRLs in mg/kg product for cypermethrin
recommended by the FAO/WHO Joint Meeting on Pesticides Residues (JMPR)
with those which may be proposed for alpha-cypermethrin
Commodity Cypermethrin Alpha-cypermethrin
(JMPR)a (extrapolated)
Cotton seed 0.2 0.05
Rapeseed 0.2 0.05
Pome fruits 2 0.5
Peaches 2 0.5
Grapes 1 0.5
Citrus fruits 2 1
Tomatoes 0.5 0.1
Brassica, leafy vegetables 1 1
Lettuce 2 1
Peas, kidney beans (less pod) 0.05 0.05
Soyabeans (less pod) 0.05 0.05
Potatoes 0.05 0.05
Sugarbeet (roots) 0.05 0.05
Maize grain 0.05 0.05
a From: FAO/WHO (1980, 1982)
For analytical reasons, 0.05 mg/kg is considered the minimum practical
MRL.
10.2 Environmental impact
Because its physico-chemical properties are similar to those of
cypermethrin, alpha-cypermethrin is expected to have a similar
environmental fate to that of cypermethrin. The potential of
alpha-cypermethrin to bioaccumulate may therefore be estimated from
experimental data on the bioaccumulation of cypermethrin. This is
because the octanol/water partition coefficients of alpha-cypermethrin
(1.4 x 105; log Pow = 5.16) and cypermethrin (2 x 106; log Pow
= 6.3) are relatively similar. In fish, the bioaccumulation of
cypermethrin determined experimentally was lower than might have been
expected from its partition coefficient, presumably because it was
rapidly metabolized. This would also be expected for
alpha-cypermethrin because both the route of metabolism and its rate
are similar to those of cypermethrin (Shell, 1983b; WHO, 1989).
In the area of environmental toxicology, results available for
green algae, aquatic invertebrates, fish and bees show that the acute
toxicity of alpha-cypermethrin is slightly higher than, but broadly
similar to that of cypermethrin (Table 11). This is because the
toxicity of cypermethrin results largely from its alpha-cypermethrin
component. No toxicity data are available concerning the effects of
alpha-cypermethrin on soil microbes, but little or no effect on carbon
dioxide evolution, oxygen uptake and nitrogen fixation would be
expected if alpha-cypermethrin acts on soil microbes in a similar way
to cypermethrin (WHO, 1989). There are also no toxicity data for
alpha-cypermethrin in birds. However, cypermethrin has a low toxicity
to birds. Since alpha-cypermethrin constitutes 25% of the active
ingredients of cypermethrin and is used at lower application rates, it
is expected that alpha-cypermethrin will also have a low toxicity to
birds (Shell, 1983b).
Overall, in the natural environment, the more biologically active
alpha-cypermethrin is likely to have a similar toxicity to that of
cypermethrin. This is because alpha-cypermethrin is used at a lower
application rate than cypermethrin.
10.3 Mammalian toxicity
In acute oral toxicity studies, alpha-cypermethrin is either
equally toxic or two to three times more toxic than cypermethrin (WHO,
1989), depending on the vehicle and the concentrations used.
In Table 12, the no-observed-effect levels and
lowest-observed-effect levels of the various mouse, rat and dog
studies are compared for cypermethrin, cis-cypermethrin and
alpha-cypermethrin. Short- and long-term studies with the racemic
mixture cypermethrin (containing four cis and four trans isomers),
cis-cypermethrin (four cis isomers) and alpha-cypermethrin (two cis
isomers) have shown similar toxicological effects.
The data from the short-term toxicity studies indicate that
alpha-cypermethrin is approximately 2 to 3 times more toxic than
cypermethrin in rats and dogs. This reflects the amount of
alpha-cypermethrin present in cypermethrin.
The signs of intoxication, the effects on target organs and
tissues, and the metabolic pathway of alpha-cypermethrin are similar
to those of cis-cypermethrin. The mode of action of
alpha-cypermethrin is similar to that of cypermethrin.
Cypermethrin has been tested in a rodent multigeneration
reproduction study and for embryotoxicity and teratogenicity in two
species. There were no effects on either reproductive performance or
on fetal development, even at doses producing systemic toxicity (WHO,
1989). Alpha-cypermethrin has not been tested for reproductive
toxicity or teratogenicity, but there is no indication that it would
have effects on these parameters since it is a component of
cypermethrin.
Table 11. Comparison of environmental toxicology of cypermethrin and alpha-cypermethrin
Species Life stage/ Water Administration Measured Values for Reference
age temper- route or vehicle end-point cypermethrin alpha-
ature (°C) cypermethrin
Green alga
(Selenastrum - 24 dispersal 2- to 4-day > 100 µg/litre > 100 µg/litre Stephenson
capricornutum) via acetone EC50 (growth) (1982)
Aquatic invertebrates
Water flea up to 24 h 20 dispersal EC50 Stephenson
(Daphnia magna) old via acetone (immobilization) (1982)
24 h 1.2 µg/litre 1.1 µg/litre
48 h 0.3 µg/litre 0.3 µg/litre
Fish
Rainbow trout 3.3 g 15 dispersal 96-h LC50 2.8 µg/litre 2.8 µg/litre Stephenson
(Oncorhynchus mykiss) via acetone (1982)
Fathead minnow 0.74-0.76 g 23-25 absorbed on 96-h LC50 1.2 µg/litre 0.93 µg/litre Stephenson
(Pimephales promelas) (juvenile) to pumice (1983)
Earthworm
(Eisenia foetida) - - filter paper 48-h LD50 26.1 µg/cm2 10 µg/cm2 Rob
contact & Dorough
toxicity (1984);
Inglesfield
& Sherwood
(1983)
Table 11 (continued)
Species Life stage/ Water Administration Measured Values for Reference
age temper- route or vehicle end-point cypermethrin alpha-
ature (°C) cypermethrin
Bees
(Apis mellifera) worker - oral 24-h LD50 0.035 µg/bee 0.06 µg/bee Badmin
adminis- & Twydell
tration (1976);
Murray
(1985)
Table 12. Comparison between short- and long-term oral studies with
cypermethrin, cis-cypermethrin and alpha-cypermethrin
Animal Number of Duration of Dose level in mg/kg diet
species studies experiment No-observed- Lowest-observed-
effect level effect level
Cypermethrin (4 cis and 4 trans isomers)a
Mouse 1 2 years 400 1600
Rat 1 5 weeks 750 1500
2 13 weeks 100/150 400
1 2 years 100 1000
Dog 1 13 weeks 500 1500
1 2 years 300 750/600
cis-cypermethrin (4 cis isomers)a
Rat 1 5 weeks 100 300
Alpha-cypermethrin (2 cis isomers)
Rat 1 5 weeks 200 400
1 13 weeks 60 180
Dog 2 2-7 days 200 250
1 13 weeks 90 270
a See WHO (1989)
The WHO Task Group on Environmental Health Criteria for
Cypermethrin, which met in 1986, concluded that cypermethrin was
without mutagenic activity. Available data on alpha-cypermethrin
indicate that this compound also is non-mutagenic in tests with
Salmonella typhimurium, Saccharomyces cerevisiae, and in vivo and
in vitro tests with rat liver cells for the induction of chromosome
aberration and production of DNA single-strand damage.
Alpha-cypermethrin has not been tested for carcinogenicity, but
the long-term toxicity studies in mice and rats did not indicate any
carcinogenic potential for cypermethrin. Because cypermethrin contains
the two cis isomers present in alpha-cypermethrin, it is unlikely that
these two isomers would have a carcinogenic potential. This
supposition is supported by the fact that all the mutagenicity studies
on alpha-cypermethrin have yielded negative results.
Near lethal doses of alpha-cypermethrin and cypermethrin produce
sparse axonopathy in the peripheral nerves of rats (see section
7.7.2). Similar signs of intoxication were observed with both
compounds, and increases in beta-glucuronidase and beta-galactosidase
activities, consistent with an axonal degeneration, could only be
detected in severely intoxicated animals. The magnitude of the enzyme
changes was substantially less than for other known neurotoxic
compounds, thereby confirming the minor nature of the lesion. The
short time period needed to produce ataxia and/or abnormal gait rules
out a causal relationship between ataxia and axonopathy. The signs of
intoxication are consistent with a pharmacologically-mediated effect.
No biochemical changes indicative of axonopathy were detected in rats
given 20 oral doses of 20 mg alpha-cypermethrin/kg body weight per day
or 75 mg cypermethrin/kg body weight per day. From these comparative
studies, it is clear that the neurotoxic potential of
alpha-cypermethrin and cypermethrin is qualitatively similar, but
alpha-cypermethrin is 3 to 4 times more potent. This reflects the fact
that alpha-cypermethrin constitutes the active ingredient and 25% of
cypermethrin.
Appraisal
The effects of alpha-cypermethrin on vertebrates and
invertebrates are qualitatively and, in a number of cases, even
quantitatively similar to those of cypermethrin, reflecting the fact
that alpha-cypermethrin is composed of two of the four cis isomers
present in cypermethrin (these two being the most active components
of cypermethrin). Therefore, the toxicological information of
cypermethrin can be used to evaluate the effects of
alpha-cypermethrin where certain important toxicity studies for
alpha-cypermethrin are lacking.
Alpha-cypermethrin has a higher toxicity (lower
no-observed-effect level) than cypermethrin but its application rate
is at most only half that of cypermethrin (5-30 g active
ingredient/ha for alpha-cypermethrin and 10-200 g active
ingredient/ha for cypermethrin). Therefore, residue levels of
alpha-cypermethrin in crops are less than half those of cypermethrin.
11. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The toxicological and residue data on cypermethrin have been
evaluated by the Joint FAO/WHO Meeting on Pesticide Residues (JMPR)
(FAO/WHO, 1980, 1982), but alpha-cypermethrin has not yet been
evaluated by the JMPR.
REFERENCES
Aldridge WN (1990) An assessment of the toxicological properties of
pyrethroids and their neurotoxicity. Crit Rev Toxicol, 21(2): 89-104.
Armitage GD (1984) Fastac exposure study. Analysis of atmospheric and
surface wipe samples ex. Durban, South Africa. Sittingbourne, Shell
Research (SBRN 84.091).
Badmin JS & Twydell RS (1976) Evaluation of the insecticide WL 43467
against the honey bee Apis mellifera. Sittingbourne, Shell Research
(WKSR.0021.76).
Baldwin MK (1990) Alpha-cypermethrin (Fastac): Water solubility at
various pH values. London, Shell International Chemical Company, Ltd
(Internal report SBGR 90.158).
Blair D (1984) The toxicology of pyrethroids; the acute 4-h inhalation
toxicity of a 30% m/m Fastac on silica powder formulation.
Sittingbourne, Shell Research (SBGR 84.066).
Bosio PG (1982) Study of isomer conversion of WL85871 in various crops
from 1981 treatments. Berre, France, Shell Chimie (BEGR 82.031).
Brooks TM (1982) Toxicity studies with pyrethroids; in vitro
genotoxicity studies with WL85871. Sittingbourne, Shell Research (SBGR
81.273).
Brooks TM (1984) Genotoxicity studies with Fastac; the induction of
gene mutation in the yeast Saccharomyces cerevisiaee XV 185-14-C.
Sittingbourne, Shell Research (SBGR 84.117).
Brown K (1989) A study of the effects on honey-bees of a large scale
commercial application of "Fastac" to oilseed rape. London, Shell
International Chemical Company, Ltd (Internal report SBGR 89.198).
Clare MG & Wiggins DE (1984) Genotoxicity studies with Fastac; in
vivo cytogenetic test using rat bone marrow. Sittingbourne, Shell
Research (SBGR 84.120).
Clark DG (1982) WL85871; a 90-day feeding study in rats.
Sittingbourne, Shell Research, vol 1 and 2 (SBGR 81.293).
Coveney PC & Forbes S (1986) Analysis of soil from UK (Coates) for
residues of "Fastac" (WL85871) - soil persistence trial - third year.
Sittingbourne, Shell Research (SBGR 86.201).
a The proprietary Shell Research reports in the following list were
submitted to the IPCS by Shell.
Creedy CL & Logan CJ (1984) In vitro metabolism of cypermethrin
isomers by rat, rabbit, and human liver preparations. Sittingbourne,
Shell Research (SBGR 84.108).
Dewar AJ (1981) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity, skin and eye irritancy and skin sensitizing
potential of WL85871 including a comparison with the acute toxicity of
WL 43467 (Ripcord). Sittingbourne, Shell Research (TLGR 80.148).
Eadsforth CV, Bragt PC, & Van Sittert NJ (1988) Human dose-excretion
studies with pyrethroid insecticides cypermethrin and
alpha-cypermethrin; relevance for biological monitoring. Xenobiotica,
18(5): 603-614.
FAO (1982) Second Government Consultation on International
Harmonization of Pesticide Registration Requirements, Rome, 11-15
October 1982. Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1980) 1979 Evaluations of some pesticide residues in food.
Rome, Food and Agriculture Organization of the United Nations (FAO
Plant Production and Protection Paper 20 sup).
FAO/WHO (1982) 1981 Evaluations of some pesticide residues in food.
Rome, Food and Agriculture Organization of the United Nations (FAO
Plant Production and Protection Paper 42).
Fisher JR, Robinson J, & Debray PH (1983) A new multipurpose
insecticide. Proceedings of 10th International Congress of Plant
Protection, Brighton, England, 20-25 November 1983. Vol. 1, pp
452-459.
Forbes S (1985) Residues of Fastac (WL85871) in cured marine catfish
( Arius sp.) from Indonesia. Sittingbourne, Shell Research (SBGR
85.200).
Forbes S & Burden AN (1984) Analysis of soil from UK (Reculver) for
residues of WL85871 (Fastac) - soil persistence trial - second year.
Sittingbourne, Shell Research (SBGR 84.005).
Forbes S & Cole DA (1986) Residues of Fastac (WL85871) in sweetcorn
from Canada. Sittingbourne, Shell Research (SBGR 86.217).
Forbes S & Knight CJ (1983) Analysis of soil from UK (Reculver) for
residues of WL85871 - soil persistence trial - first year.
Sittingbourne, Shell Research (SBGR.83.162).
Forbes S & Mackay CE (1983) Analysis of soil from UK (Coates) for
residues of WL85871 (Fastac) - soil persistence trial - first year.
Sittingbourne, Shell Research (SBGR 83.418).
Forbes S & Wales GH (1985a) Analysis of soil from UK (Reculver) for
residues of Fastac (WL85871) - soil persistence trial - third year.
Sittingbourne, Shell Research (SBGR 85.070).
Forbes S & Wales GH (1985b) Analysis of soil from UK (Coates) for
residues of Fastac (WL85871) - soil persistence trial - second year.
Sittingbourne, Shell Research (SBGR 85.071).
Francis WP & Gill JP (1991) Flufenoxuron/alpha-cypermethrin - residues
in sheep tissues following treatment with PAMPASS/RENEGADE mixtures by
dip or pour-on. London, Shell International Chemical Company, Ltd
(Internal report SBGR 89.254).
Gardner JR (1989) "Fastac/Azodrin" 20/400 g/litre emulsifiable
concentrate: Acute oral and dermal toxicity. London, Shell
International Chemical Company, Ltd (Internal report SBGR 89.026).
Gardner JR (1991) Fastac 60 G/l SC (SF07396); acute oral and dermal
toxicity in rat. Sittingbourne, Shell Research (SBGR 91.020).
Garforth BM (1982a) A comparison of the toxicities of WL85871 and
Ripcord to freshwater invertebrates in small field enclosures.
Sittingbourne, Shell Research (SBGR 82.015).
Garforth BM (1982b) WL85871 and cypermethrin; chronic toxicity to
Daphnia magna. Sittingbourne, Shell Research (SBGR 82.119).
Garforth BM & Woodbridge AP (1984) Spray drift from an aerial
application of Fastac; fate and biological effects in an adjacent
freshwater ditch. Sittingbourne, Shell Research (SBGR 84.055).
Greenough RJ & Goburdhun R (1984) WL85871; Oral (dietary) maximum
tolerated dose study in dogs. Musselburgh, Inveresk Research
International (Unpublished report No. 3107, submitted to WHO by Shell
Research).
Greenough RJ, Cockrill JB, & Goburdhun R (1984) WL85871; 13-week oral
(dietary) toxicity study in dogs. Musselburgh, Inveresk Research
International (Unpublished report No. 3197, submitted to WHO by Shell
Research).
Hend RW (1983) Toxicology of pyrethroids; skin stimulation studies
using guinea-pigs. Sittingbourne, Shell Research (SBGR 83.197).
Hillaby JM (1988) Deposition of pesticide on the orchard floor
following a commercial mistblower application to apples. London, Shell
International Chemical Company, Ltd (Internal report SBGR 88.106).
Hillaby JM & Inglesfield C (1986) The residual toxicity of "Fastac"
formulations and "Fastac"/fungicide mixtures to the honey bee, Apis
mellifera L. Sittingbourne, Shell Research (SBGR 86.018).
Hutson DH (1982) WL85871; metabolism of a single oral dose in the rat.
Sittingbourne, Shell Research (SBGR 82.205).
Hutson DH & Logan CJ (1986) The metabolic fate in rats of the
pyrethroid insecticide WL85871, a mixture of two isomers of
cypermethrin. Pestic Sci, 17: 548-558.
Inglesfield C (1984) The effects of two formulations of Fastac on the
beneficial arthropod fauna of grape vines. Sittingbourne, Shell
Research (SBGR 84.039).
Inglesfield C (1985a) A field study on the effects of Fastac on the
beneficial arthropod fauna of maize in France. Sittingbourne, Shell
Research (SBGR 85.069).
Inglesfield C (1985b) A pond enclosure study of the effects of Fastac
formulations on aquatic invertebrates. Sittingbourne, Shell Research
(SBGR 85.083).
Inglesfield C (1985c) The effects of the pyrethroid insecticide
WL85871 on non-target arthropods: Field studies. Pestic Sci, 16(2):
211.
Inglesfield C (1988) Effects of "Fastac" on non-target arthropods; an
overview of a five-year arable crop rotation study. Sittingbourne,
Shell Research (SBGR 87.149).
Inglesfield C (1989) A long-term field study to investigate the
effects of alpha-cypermethrin on predatory and parasitic arthropods.
Meded Fac Landbouwwet Rijksuniv Gent, 54(3a): 895-904.
Inglesfield C (1991) Effects of alpha-cypermethrin (Fastac) on
entomophagous organisms and game-bird chick-food insects in summer
cereals. The Hague, Shell Internationale Petroleum Maatschappij B.V.
(Report Series HSE 91.008).
Inglesfield C & Forbes S (1986) A field trial to assess the effects of
"Fastac" 100 g/litre OSC on foraging honey bees in oilseed rape.
Sittingbourne, Shell Research (SBGR 86.087).
Inglesfield C & Sherwood CM (1983) Toxicity of cypermethrin and
WL85871 to the earthworm, Eisenia foetida L.
(Oligochaeta:Lumbriculidae) in laboratory tests. Sittingbourne,
Shell Research (SBGR 83.071).
Langner EJ (1980) Determination of the vapour pressure of WL 43467 and
WL 85871 at 20 °C. Sittingbourne Shell Research (Research Note No.
FCDN 80.137).
Le Blanc J (1985) Field experiments on the effects of a new pyrethroid
insecticide WL85871 on bees foraging artificial aphid honey-dew on
winter wheat. Pestic Sci, 16(2): 206.
Le Quesne PM, Maxwell IC, & Butterworth STG (1980) Transient facial
sensory symptoms following exposure to synthetic pyrethroids; a
clinical and electrophysiological assessment. Neurotoxicology, 2:
1-11.
Logan CJ (1983) WL85871; depletion from tissues of female rats after
a single oral dose. Sittingbourne, Shell Research (SBGR 83.075).
McMinn AL (1983a) The degradation of the pyrethroid insecticides
WL85871 (Fastac) and WL43481 in cabbage. Sittingbourne, Shell Research
(SBGR 83.396).
McMinn AL (1983b) The degradation of the pyrethroid insecticides
WL85871 and WL43481 in soil. Sittingbourne, Shell Research (SBGR
83.395).
Maloney SE, Maule A, & Smith ARW (1988) Microbial transformation of
the pyrethroid insecticides: Permethrin, deltamethrin, fastac,
fenvalerate and fluvalinate. Appl Environ Microbiol, 54(11):
2874-2876.
Murray A (1985) Acute and residual toxicity of a new pyrethroid
insecticide WL85871, to honey-bees. Bull Environ Contam Toxicol, 34:
560-564.
Pearson N (1986) Fastac oil-enriched suspension concentrates; acute
toxicities of two formulations to the rainbow trout, Salmo gairdneri.
Sittingbourne, Shell Research (SBGR 85.290).
Pearson N (1990) The fate of "Fastac" in experimental ponds.
Sittingbourne, Shell Research (SBGR 88.177).
Pickering RG (1982) A 5-week feeding study with WL85871 in rats.
Sittingbourne, Shell Research, vol 1 and 2 (SBGR 81.212).
Price JB (1985a) Toxicology of Fastac (WL85871); the acute oral and
percutaneous toxicity and skin irritancy of a Fastac 10% EC (SF
06510). Sittingbourne, Shell Research (SBGR 85.087).
Price JB (1985b) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity, skin and eye irritancy of a Fastac 100 g/litre
suspension concentrate (SF 06378). Sittingbourne, Shell Research (SBGR
84.298).
Price JB (1986) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity, skin and eye irritancy of SF 06615, a 15
g/litre suspension concentrate of WL85871 ("Fastac", "Fendona").
Sittingbourne, Shell Research (SBGR 85.224).
Price JB (1987) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity of Fastac/BPMC 10/400 g/litre EC (SF 06717).
Sittingbourne, Shell Research (SBGR 86.253).
Price JB (1988) Toxicology of animal health products; the acute oral
and percutaneous toxicity, skin and eye irritancy of two pour-on
formulations of Renegade, SF 06954 (10 g/litre) and SF 06977 (15
g/litre). Sittingbourne, Shell Research (SBGR 87.161).
Roberts BL & Dorough HW (1984) Relative toxicities of chemicals to the
earthworm Eisenia foedita. Environ Toxicol Chem, 3(1): 67-68.
Rose GP (1982) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity of WL85871 ( cis-2-Ripcord) comparison with
Ripcord. Sittingbourne, Shell Research (SBGR 82.130).
Rose GP (1983a) Toxicology of pyrethroids; the acute oral toxicity of
WL85871 in comparison with WL43467. Sittingbourne, Shell Research
(SBGR 83.101).
Rose GP (1983b) Neurotoxicity of WL85871 in comparison with WL43467;
the effect of twenty oral doses of WL85871 or WL43467 over a period of
4 weeks on the rat sciatic/posterior tibial nerve, trigeminal nerve
and trigeminal ganglion. Sittingbourne, Shell Research (SBGR 83.185).
Rose GP (1984a) Toxicology of pyrethroids; the acute intraperitoneal
toxicity of technical Fastac. Sittingbourne, Shell Research (SBGR
84.085).
Rose GP (1984b) Toxicology of Fastac; the eye irritancy potential of
the Fastac 100 g/litre emulsifiable concentrate formulation DF 05898
and its formulation components. Sittingbourne, Shell Research (SBGR
84.053).
Rose GP (1984c) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity, skin and eye irritancy of the Fastac 15 g/litre
ULV formulation SF 06363. Sittingbourne, Shell Research (SBGR 84.145).
Rose GP (1984d) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity, skin and eye irritancy of the Fastac 10 EC
formulation, 5835 B. Sittingbourne, Shell Research (SBGR 84.077).
Rose GP (1984e) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity, skin and eye irritancy of the Fastac 10 EC
formulation 5898 B. Sittingbourne, Shell Research (SBGR 84.078).
Rose GP (1984f) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity, skin and eye irritancy of the Fastac 3 EC
formulation, DF 06353. Sittingbourne, Shell Research (SBGR 84.084).
Rose GP (1984g) Toxicology of pyrethroids; the eye irritancy and skin
sensitizing potential of the 100 g/litre Fastac emulsifiable
concentrate formulation EF 5835. Sittingbourne, Shell Research (SBGR
84.086).
Rose GP (1984h) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity, skin and eye irritancy of the 15:120 g/litre
Fastac/methomyl emulsifiable concentrate formulation, FD 9148.
Sittingbourne, Shell Research (SBGR 84.104).
Rose GP (1985) Toxicology of insecticides; the acute oral and
percutaneous toxicity, skin and eye irritancy of the 30 g/litre Fastac
EC formulation SF 06446. Sittingbourne, Shell Research (SBGR 84.209).
Senior PL & Lavers A (1990a) Fastac - Potential dermal exposure. The
Hague, Shell Internationale Petroleum Maatschappij B.V. (Report Series
HSE 90.016).
Senior PL & Lavers A (1990b) A field study of operator exposure to
Fastac during crop spraying at Shell Research Ltd, Sittingbourne
Research Centre. The Hague, Shell Internationale Petroleum
Maatschappij B.V. (Report Series HSE 90.014).
SHELL (1983a) Review of mammalian and human toxicology; Fastac. The
Hague, Shell Internationale Petroleum Maatschappij B.V. (Review Series
MDT 83.001).
SHELL (1983b) Review of environmental toxicology; Fastac. The Hague,
Shell Internationale Petroleum Maatschappij B.V. (Review Series MDT
83.005).
SHELL (1984) Review of residue information; Fastac. London, Shell
International Chemical Company Ltd.
SHELL (1986) Determination of residues of alpha-cypermethrin in rat
blood - Gas chromatographic method. Sittingbourne, Shell Research
(Analytical Method Series, No. SAMS 436-1).
SHELL (1987a) Determination of WL 85871 and the ratio of the
enantiomer pairs in technical material and formulated products -
liquid chromatographic method. Sittingbourne, Shell Research
(Analytical Methods Series, No. SAMS 346-4).
SHELL (1987b) Intereg residues section: Ripcord residues in crops.
London, International Chemical Company, Ltd (Internal report).
SHELL (1987c) Intereg residues section: Fastac residues in crops.
London, International Chemical Company, Ltd (Internal report).
SHELL (1988a) Determination of residues of alpha-cypermethrin in
animal tissues - Gas-liquid chromatographic method. Sittingbourne,
Shell Research (Analytical Method Series, No. SAMS 461-1).
SHELL (1988b) Determination of residues of alpha-cypermethrin in milk
- Gas-liquid chromatographic method. Sittingbourne, Shell Research
(Analytical Method Series, No. SAMS 456-1).
SHELL (1989a) Determination of residues of alpha-cypermethrin in crops
- Gas chromatographic method. Sittingbourne, Shell Research
(Analytical Method Series, No. SAMS 351-2).
SHELL (1989b) Review of environmental toxicology; Fastac. The Hague,
Shell Internationale Petroleum Maatschappij B.V. (Review Series HSE
89.008).
SHELL (1990a) Determination of residues of alpha-cypermethrin in water
- Gas chromatographic method. Sittingbourne, Shell Research
(Analytical Method Series, No. SAMS 469-2).
SHELL (1990b) Determination of residues of alpha-cypermethrin in soils
- Gas chromatographic method. Sittingbourne, Shell Research
(Analytical Method Series, No. SAMS 354-2).
Sherren AJ (1988a) Residues of alpha-cypermethrin in cattle tissues
following topical treatment of calves with "Renegade" pour-on in the
UK. London, Shell International Chemical Company, Ltd (Internal report
SBGR 88.036).
Sherren AJ (1988b) Residues of alpha-cypermethrin in milk following
topical treatment of cows with "Renegade" pour-on in the UK. London,
Shell International Chemical Company, Ltd (Internal report SBGR
88.037).
Shires SW (1982) A comparison of the toxicity of WL85871 and Ripcord
to rainbow trout ( Salmo gairdneri, Richardson) in small field
enclosures. Sittingbourne, Shell Research (SBGR 82.089).
Shires SW (1983a) Pesticides and honey bees; case studies with Ripcord
and Fastac. Span, 26(3): 118-120.
Shires SW (1983b) Effect of formulation type on the toxicity of
insecticides to fish. Sittingbourne, Shell Research (SBGR 83.015).
Shires SW (1985a) A step-wise evaluation of the effects of a new
pyrethroid insecticide WL85871 on honey bees. Pestic Sci, 16(2):
205-216.
Shires SW (1985b) Toxicity of a new pyrethroid insecticide WL85871 to
rainbow trout. Bull Environ Contam Toxicol, 34: 134-137.
Shires SW & Inglesfield C (1986) A field study of the effects of
"Fastac" (WL85871) on rice pests and their natural enemies.
Sittingbourne, Shell Research (SBGR 86.003).
Shires SW, Le Blanc J, Debray P, Forbes S, & Louveaux J (1984a) Field
experiments on the effects of a new pyrethroid insecticide WL85871 on
bees foraging artificial aphid honey-dew on winter wheat. Pestic Sci,
15: 543-552.
Shires SW, Murray A, Debray P, & Le Blanc J (1984b) The effects of a
new pyrethroid insecticide WL85871 on foraging honey bees ( Apis
mellifera L.) Pestic Sci, 15: 491-499.
Shires SW, Le Blanc J, Murray A, Forbes S, & Debray P (1984c) A field
trial to assess the effects of a new pyrethroid insecticide WL85871 on
foraging honey bees in oilseed rape. J Agric Res, 23(4): 217-226.
Stephenson RR (1982) WL85871 and cypermethrin; a comparison of their
acute toxicity to Salmo gairdneri, Daphnia magna and Selenastrum
capricornutum. Sittingbourne, Shell Research (SBGR 81.277).
Stephenson RR (1983) WL85871 and cypermethrin; a comparative study of
their toxicity to the fathead minnow, Pimephales promelas
(Rafinesque). Sittingbourne, Shell Research (SBGR 82.298).
Stephenson RR (1986) "Fastac"; the acute toxicity of different
formulations to fish in rice paddies. Sittingbourne, Shell Research
(SBGR 85.201).
Stephenson RR (1987a) The effects of "Fastac" (OSC) on fish survival
and growth following its application to paddy rice. Sittingbourne,
Shell Research (SBGR 87.054).
Stephenson RR (1987b) An insecticide formulation that spares fish.
Span, 30(2): 75-77.
Stephenson RR (1987c) The effects of "Fastac" (OSC) on fish following
its application to outdoor tanks. Sittingbourne, Shell Research (SBGR
86.227).
Stone CM & Watkinson RJ (1983) WL85871; an assessment of ready
biodegradability. Sittingbourne, Shell Research (SBGR 83.206).
Tasei J-N, Carre S, Bosio PG, Debray P, & Hariot J (1987) Effects of
the pyrethroid insecticide WL85871 and Phosalone on adults and progeny
of the leaf-cutting bee, Megachile rotundata F., pollinator of
lucerne. Pestic Sci, 21: 119-128.
Van Sittert NJ, Eadsforth CV, & Bragt P (1985) Human oral
dose-excretion study with Fastac. The Hague, Shell Internationale
Petroleum Maatschappij B.V. (Report Series HSE 85.010).
Vijverberg HPM & Van Den Bercken J (1990) Neurotoxicological effects
and the mode of action of pyrethroid insecticides. Crit Rev Toxicol,
21(2): 105-126.
Western NJ (1984) Report on the assessment of Fastac exposures during
formulation of technical concentrate (TC) and technical material (TM)
at Durban, South Africa, September 1983. The Hague, Shell
Internationale Petroleum Maatschappij B.V. (Report Series HSE 84.003).
WHO (1989) Environmental Health Criteria 82: Cypermethrin. Geneva,
World Health Organization, 154 pp.
Wooder MF (1982) Studies on the effect of WL85871 on the integrity of
rat liver DNA in vivo. Sittingbourne, Shell Research (SBGR 81.225).
Woollen BH, Marsh JR, & Chester G (1991) Metabolite profiles of a
pyrethroid insecticide following oral and dermal absorption in man.
In: Proceedings of a Conference on Percutaneous Penetration,
Southampton, England, 10-12 April 1991.
Worthing CR & Hance RJ (1991) Fastac. In: The pesticides manual: a
world compendium, 9th ed. Croydon, British Crop Protection Council, pp
210-211.
APPENDIX I
Cypermethrin: Summary, Evaluation, Conclusions, and
Recommendations (Reprint from EHC 82 on Cypermethrin, WHO/IPCS, 1989)
1. Summary
1.1 General
Cypermethrin was initially synthesized in 1974 and first marketed
in 1977 as a highly active synthetic pyrethroid insecticide, effective
against a wide range of pests in agriculture, public health, and
animal husbandry. In agriculture, its main use is against foliage
pests and certain surface soil pests, such as cutworms, but because of
its rapid breakdown in soil, it is not recommended for use against
soil-borne pests below the surface.
In 1980, 92.5% of all the cypermethrin produced in the world was
used on cotton; in 1982, world production was 340 tonnes of the active
material. It is mainly used in the form of an emulsifiable
concentrate, but ultra-low volume concentrates, wettable powders, and
combined formulations with other pesticides are also available.
Chemically, cypermethrin is the alpha-cyano-3-phenoxybenzyl ester
of the dichloro analogue of chrysanthemic acid,
2,2-dimethyl-3-(2,2-dichlorovinyl) cyclopropanecarboxylic acid. The
molecule embodies three chiral centres, two in the cyclopropane ring
and one on the alpha cyano carbon. These isomers are commonly grouped
into four cis and four trans isomers, the cis group being the more
powerful insecticide. The ratio of cis to trans isomers varies from
50:50 to 40:60. Cypermethrin is the racemic mixture of all eight
isomers and, in this appraisal, cypermethrin refers exclusively to the
racemic mixture (ratio 50:50) unless otherwise stated.
Most technical grades of cypermethrin contain more than 90% of
the active material. The material varies in physical form from a
brown-yellow viscous liquid to a semi-solid.
Cypermethrin has a very low vapour pressure and solubility in
water, but it is highly soluble in a wide range of organic solvents.
Analytical methods are available for the determination of cypermethrin
in commercially available preparations. In addition, methods for the
determination of residues of cypermethrin in foods and in the
environment are well established. In most substrates, the practical
limit of determination is 0.01 mg/kg.
1.2 Environmental transport, distribution and transformation
Unlike the natural pyrethrins, cypermethrin is relatively stable
to sunlight and, though it is probable that photodegradation plays a
significant role in the degradation of the product on leaf surfaces
and in surface waters, its effects in soils are limited. The most
important photodegradation products, 2,2-dimethyl-3-
(2,2-dichlorovinyl) cyclopropane carboxylic acid (CPA),
3-phenoxy-benzoic acid (PBA) and, to some extent, the amide of the
intact ester, do not differ greatly from those resulting from
biological degradation.
Degradation in the soil occurs primarily through cleavage of the
ester linkage to give CPA, PBA, and carbon dioxide. Some of the carbon
dioxide is formed through the cleavage of both the cyclopropyl and
phenyl rings under oxidative conditions. The half-life of cypermethrin
in a typical fertile soil is between 2 and 4 weeks.
Cypermethrin is adsorbed very strongly on soil particles,
especially in soils containing large amounts of clay or organic
matter. Movement in the soil is therefore extremely limited and
downward leaching of the parent molecule through the soil does not
occur to an appreciable extent under normal conditions of use. The two
principal degradation products show, on the scale of Helling,
"intermediate mobility".
Cypermethrin is also relatively immobile in surface waters and,
when applied to the surface of a body of water at rates typical of
those used in agriculture applications, it is largely confined to the
surface film and does not reach deeper levels or the sediment in
appreciable concentrations. Cypermethrin also degrades readily in
natural waters with a typical half-life of about 2 weeks. It is
probable that both photochemical and biological processes play a part.
It has been shown that spray drift reaching surface waters adjacent to
sprayed fields does not result in long-term residues in such waters.
Accumulation studies have shown that cypermethrin is rapidly
taken up by fish (accumulation factor approximately 1000); the
half-life of residues in rainbow trout was 8 days. In view of the low
concentrations of cypermethrin that are likely to arise in water
bodies and their rapid decline, it has been concluded that, under
practical conditions, residues in fish will not reach measurable
levels.
The results of field studies have shown that, when applied at
recommended rates, the levels of cypermethrin and its degradation
products in soil and surface waters are very low. Thus, it is unlikely
that the recommended use of cypermethrin will have any effects on the
environment.
1.3 Environmental levels and human exposure
Cypermethrin is used in a wide range of crops. In general, the
maximum residue limits are low, ranging from 0.05 to 2.0 mg/kg in the
different food commodities. The residues will be further reduced
during food processing. In food of animal origin, residues may range
between 0.01 and 0.2 mg/kg product. Residues in non-food commodities
are generally higher, ranging up to 20 mg/kg product.
Total dietary intake values for man are not available, but it can
be expected that the oral exposure of the general population is low to
negligible.
1.4 Kinetics and metabolism
Absorption of cypermethrin from the gastrointestinal tract and
its elimination are quite rapid. The major metabolic reaction is
cleavage of the ester bond. Elimination of the cyclopropane moiety in
the rat, over a 7-day period, ranged from 40 to 60% in the urine and
from 30 to 50% in the faeces; elimination of the phenoxybenzyl moiety
was about 30% in the urine and 55 to 60% in the faeces. Biliary
excretion is a minor route of elimination for the cyclopropane moiety
and small amounts are exhaled as carbon dioxide. In principle, these
absorption and elimination rates and metabolic pathways hold for all
animal species studied, including domestic animals. In cows fed 100 mg
cypermethrin/day, the highest level found in milk was 0.03 mg/litre;
levels of up to 0.1 mg/kg tissue were found in subcutaneous fat. Under
practical conditions, the oral intake of cypermethrin with feed will
be much lower. Cypermethrin used as a spray or dip to combat
parasites, may give rise to maximum residues of 0.05 mg/kg tissue and
0.01 mg/litre milk.
Laying hens exposed orally to 10 mg cypermethrin/kg diet for 2
weeks, showed cypermethrin levels of up to 0.1 mg/kg in the fat, and
up to 0.09 mg/kg in the eggs (predominantly in the yolk).
Consistent with the lipophilic nature of cypermethrin, the
highest mean tissue concentrations are found in body fat, skin, liver,
kidneys, adrenals, and ovaries. Only negligible concentrations are
found in the brain. The half-life of cis-cypermethrin in the fat of
the rat ranges from 12 to 19 days and that of the trans isomer, from
3 to 4 days. In mice, these half-lives are 13 days and 1 day,
respectively.
Overall, the metabolic transformation has been similar in the
different animals studied, including man. Differences that occur have
been related to the rate of formation rather than to the nature of the
metabolites formed and to conjugation reactions. Cypermethrin (both
the cis and trans isomers) is metabolized via the cleavage of the
ester bond to phenoxybenzoic acid and cyclopropane carbolic acid. The
fact that thiocyanate has been identified in in vivo studies,
indicates that the cyanide moiety is further metabolized. The
3-phenoxybenzoic acid is mainly excreted as a conjugate. The type of
conjugate differs in a number of animal species. Phenoxybenzoic acid
is further metabolized to a hydroxy derivative and conjugated with
glucuronic acid or sulfate. The cyclopropyl moiety is mainly excreted
as a glucuronide conjugate, hydroxylation of the methyl group only
occurring to a limited extent.
Ester cleavage is much slower in certain fish species than in
other animal species, the main metabolic pathway being hydroxylation
of the phenoxybenzoic and the cyclopropyl moieties.
Ester cleavage also takes place in plants. The phenoxybenzyl and
cyclopropyl moieties are readily converted into glucoside conjugates.
In mammals, these conjugates are hydrolysed into the original acids
and metabolized.
1.5 Effects on organisms in the environment
High doses of cypermethrin may exert transient minor effects on
microflora activity in the soil. However, no influence on
ammonification and nitrification has been found.
Cypermethrin is very toxic for fish (in laboratory tests 96-h
LC50s were generally within the range of 0.4-2.8 µg/litre), and
aquatic invertebrates (LC50s in the range of 0.01 - > 5 µg/litre).
The presence of suspended solids decreases the toxicity by at least a
factor of 2, because of adsorption of cypermethrin to the solids.
Cypermethrin is not very toxic for birds. Signs of cypermethrin
intoxication were seen at dose levels of 3000 mg/kg body weight or
more. Administration of 1000 mg cypermethrin/kg body weight to laying
hens over a 5-day period did not cause signs of intoxication. However,
cypermethrin was highly toxic for honey bees in laboratory tests, the
oral LD50 ranging from 0.03 to 0.12 µg/bee. Under field conditions,
the hazard is considerably lower, because of the repellent effect of
cypermethrin on worker honey bees, which lasts for at least 6 h after
spraying.
Earthworms are not sensitive to cypermethrin. No deaths occurred
in worms exposed to levels of 100 mg/kg soil for 14 days.
In studies involving deliberate overspraying of experimental
ponds under field conditions, peak concentrations of 2.6 µg
cypermethrin/litre were measured in the water. Fish were not affected,
but populations of crustaceae, mites, and surface-breathing insects
were severely reduced. Most of these populations returned to normal
levels after 15 weeks. Free-swimming dipterous larvae and
bottom-dwelling invertebrates, snails, flatworms, etc., were not
affected. Under normal agricultural conditions (during which drifts
may reach adjacent ditches or streams), the only effects seen in
surface-breathing or surface-dwelling insects were hyperactivity or
immobilization. The relative toxicity of cypermethrin for pests and
their parasites and predators is such that the balance between
host/prey and parasites/predator may not be adversely affected in the
field. However, care should be taken where predatory mites are
important in pest management.
1.6 Effects on experimental animals and in vitro test systems
The acute oral toxicity of cypermethrin is moderate. While LD50
values differed considerably among animal species depending on the
vehicle used and the cis/trans isomeric ratios, the toxic responses in
all species were found to be very similar. The acute toxicity of the
trans isomer in the rat (LD50 > 2000 mg/kg body weight) was lower
than that of the cis isomer (LD50, 160-300 mg/kg body weight). The
onset of toxic signs of poisoning was rapid and they disappeared
within several days in survivors. The toxic signs are characterized by
salivation, tremors, increased startle response, sinuous writhing of
the whole body (choreoathetosis), and clonic seizures. Myelin and axon
degeneration were noted in the sciatic nerve at near lethal dose
levels.
Cypermethrin was moderately to severely irritating, when applied
to the skin or the eye of the rabbit. The severity was partly
dependent on the vehicle used. In guinea-pigs, a mild skin sensitizing
potential was found using the maximization test.
No toxic effects were observed in rats, fed cypermethrin at 100
mg/kg diet for 3 months. Furthermore, prolonged feeding of
cypermethrin (2 years) to dogs at a level of 300 mg/kg feed did not
produce any toxicological effects. A level of 600 mg/kg diet resulted
in reduced body weight gain, but no gross pathological or
histopathological effects were seen.
Two long-term studies on rats and one on mice were carried out.
The dose levels in the rat studies ranged up to 1500 mg/kg diet,
equivalent to 75 mg/kg body weight. No effects were seen at 150 mg/kg
diet. At the highest dose level, reduced body weight gain, increased
liver weights (accompanied by increased smooth endoplasmatic
reticulum), and some haematological and biochemical changes were
observed. No increase in tumour incidence was noted. The same type of
effects were seen in the mouse study at 1600 mg cypermethrin/kg diet.
No effects were seen in the 400 mg/kg diet group.
The effect of cypermethrin on the immune system was studied in
rats. The results showed the possibility of immune suppression by
pyrethroids. More attention should be paid to this aspect, but, at
present, no opinion can be given about its relevance in the
extrapolation of these data for man.
Repeated oral administration of cypermethrin to rats and other
animal species at levels sufficiently high to produce significant
mortality in one group of animals, produced biochemical changes in the
peripheral nerves, consistent with sparse axonal degeneration.
Histopathological changes (swelling and/or disintegration of axons of
the sciatic nerve) were observed. There was no cumulative effect. The
magnitude of the change was substantially less than that encountered
with established neurotoxic agents. The neurotoxic effects seem to be
reversible; presumably the clinical signs are not related to the
induction of neuropathological lesions.
Further evidence to support the minor nature of the nerve lesions
has been afforded by electrophysiological studies on rats.
Measurements of the maximal motor conduction velocities of the sciatic
and tail nerves of rats were made before, and at intervals of up to 5
weeks after, exposure to a single dose or repeated high doses of
cypermethrin. It was concluded from the results that, even at
near-lethal doses, cypermethrin did not cause any effects on maximal
motor conduction velocities and conduction velocities of the slower
motor fibres in rat peripheral nerves. No delayed neurotoxicity was
observed in domestic hens.
The ability of the major metabolite of cypermethrin,
3-phenoxybenzoic acid, to produce axonal changes has been investigated
and found to be negative.
In a multigeneration reproduction study on rats, dose levels up
to 500 mg/kg feed were tested. The parent animals at the highest dose
level showed decreased food intake and reduction in body weight gain.
No influence on reproductive performance or on survival of the
offspring was found. However, at the highest dose level, reductions in
litter size and total litter weights were seen. The pooled body
weights of weaning pups of the 500 mg/kg group were decreased over 3
generations. No effect was found with 100 mg cypermethrin/kg diet.
Embryotoxic and teratogenic effects were not found in rats
administered dose levels of up to 70 mg/kg body weight and clear
teratogenic effects were not observed in rabbits given dose levels of
up to 30 mg/kg body weight during days 6-18 of gestation.
Cypermethrin did not show any mutagenic activity in bacteria or
in yeast, with or without metabolic activation, or in V79 Chinese
hamster cells. Furthermore, cypermethrin gave negative results in an
in vivo chromosomal aberration test with Chinese hamsters and in
dominant lethal studies on mice. In a host-mediated assay with mice,
no increase in the rate of mitotic gene conversion in Saccharomyces
cerevisae was found. In a chromosome study using the bone marrow
cells of Chinese hamsters, cypermethrin did not increase the number of
chromosome abnormalities. However, in a micronucleus test with mouse
bone marrow cells, an increase in the frequency of polychromatic
erythrocytes with micronuclei was found after oral and dermal
applications of cypermethrin. Intraperitoneal application gave a
negative result. A sister chromatid exchange study using bone marrow
cells of mice showed a dose-response related increase in sister
chromatid exchanges of dividing cells.
In long-term/carcinogenicity studies, oral administration of
cypermethrin to rats did not induce an increase in the incidence of
tumours. In a mouse study, dose levels of up to 1600 mg
cypermethrin/kg diet did not produce any increase in tumours of types
not commonly associated with the mouse strain employed. The incidence
of tumours was similar in all groups with the exception of a slight
increase in the incidence of benign alveolar lung tumours in the
females in the 1600 mg/kg diet group. However, the increased
incidence, when compared with concurrent and historical control
incidence, was not sufficient to warrant concern. There was no
suggestion of increased malignancy and no evidence of a decrease in
the latency of the tumours. Furthermore, there was no evidence of a
carcinogenic response in the male mice in this study and, as the
results of mutagenicity studies on cypermethrin have been mainly
negative, it is concluded that there is no evidence for the
carcinogenic potential of cypermethrin.
1.7 Mechanism of toxicity
Extensive studies have been carried out to explain the mechanism
of toxicity of cypermethrin, especially with regard to the effects on
the nervous system. The results strongly suggest that the primary
target site of cypermethrin (and of pyrethroid insecticides in
general) in the vertebrate nervous system is the sodium channel in the
nerve membrane. The alpha-cyano pyrethroids, such as cypermethrin,
cause a long-lasting prolongation of the normally transient increase
in sodium permeability of the nerve membrane during excitation,
resulting in long-lasting trains of repetitive impulses in sense
organs and a frequency-dependent depression of the nerve impulse in
nerve fibres. Since the mechanisms responsible for nerve impulse
generation and conduction are basically the same throughout the entire
nervous system, pyrethroids may well act in a similar way in various
parts of the central nervous system. It is suggested that the facial
skin sensations that may be experienced by people handling
cypermethrin are brought about by repetitive firing of sensory nerve
terminals in the skin, and may be considered as an early warning
signal that exposure has occurred.
1.8 Effects on man
No cases of accidental poisoning have been reported as a result
of occupational exposure.
Skin sensations, reported by a number of authors to have occurred
during field studies, generally lasted only a few hours and did not
persist for more than one day after exposure. Neurological signs were
not observed. General medical and extensive clinical blood-chemistry
studies, and electrophysiological studies on selected motor and
sensory nerves in the legs and arms did not show any abnormalities.
2. Evaluation
Cypermethrin, an alpha-cyano pyrethroid consisting of a mixture
of 8 stereoisomers, is a highly active insecticide effective against
a wide range of pests in many food and non-food commodities.
It is stable to light and heat, it has a low vapour pressure and
is more stable in acidic than in alkaline media. Sensitive analytical
methods for the determination of residues in food and the environment
are available.
When cypermethrin is applied to crops, residues may occur in
soils and surface waters, but biological degradation is fairly rapid
and residues do not accumulate in the environment. Photo-degradation
is unlikely to play an important role. The main route of degradation
is cleavage of the ester linkage to give 2 main degradation products
containing the cyclopropane, and the phenoxybenzyl moiety. The
half-lives in the soil are determined by many factors, but are in the
range of 2-4 weeks. Cypermethrin is strongly adsorbed by soil and
downward leaching is negligible. Because of its rather fast breakdown
forming less toxic products, and the low dose rates used in good
agricultural practice, it is unlikely that cypermethrin will attain
significant levels in the environment.
Bioaccumulation in certain organisms, such as fish, took place
under laboratory conditions, but levels declined on cessation of
exposure and there are indications that, under natural conditions,
fish will not contain measurable residues.
When applied according to good agricultural practice, the levels
of cypermethrin residues in food commodities are generally low. Total
diet studies are not available, but from the available residue
information, it can be inferred that the oral intake by man is well
below the ADI.
High dose levels of cypermethrin may exert transient effects on
the soil microflora. Earthworms and other soil organisms are generally
rather resistant to cypermethrin, while fish and other aquatic
invertebrates are very sensitive. Because of its strong adsorption on
soil, only low levels of cypermethrin may leak into surface water.
These may have transient effects, mainly on surface breathing insects.
The toxicity of cypermethrin for birds is low. Bees appear to be
very sensitive in laboratory tests. Under field conditions, the effect
on bees is minimal, because cypermethrin seems to have a repellent
effect for bees. Absorption and elimination of cypermethrin has been
rapid in the different mammalian species tested. The major metabolic
reaction is cleavage of the ester bond followed by hydroxylation and
conjugation of the cyclopropane and phenoxybenzyl moiety. The highest
levels are found in body fat, which is consistent with the lipophilic
nature of cypermethrin. The half-life in the fat of the rat is about
12-19 days for the cis isomer and 3-4 days for the trans isomer.
Breakdown products in plants are bound as glucosides.
The acute toxicity of cypermethrin for mammals is of a moderate
order. The oral LD50 for the rat ranges from 200 to 4000 mg/kg body
weight. Short-term and long-term toxicity studies on rats, mice, and
dogs have shown effects on growth, on the liver and kidneys, and the
nervous system, and on haematology. A no-observed-adverse-effect level
of 7.5 mg/kg body weight has been adopted by the Task Group.
Cypermethrin was not carcinogenic for mice or rats fed diets
containing high levels of the material over a 2-year period.
Cypermethrin did not induce teratogenic effects in either rats at 70
mg/kg body weight or rabbits at 30 mg/kg body weight. It was also
shown not to have any effects on reproductive performance during a
3-generation reproduction study in rats administered 100 mg/kg diet.
In a variety of mutagenicity studies, cypermethrin was shown to be
mainly without mutagenic activity.
The mechanism of the action on the nervous system has been
extensively studied. From these studies and the occupational studies
available, it seems that the skin sensation seen in workers handling
cypermethrin, generally lasts only a few hours and does not persist
for more than one day after exposure. Other neurological signs were
not observed. These skin sensations can be considered to be an early
warning that exposure has occurred and that work practice should be
reviewed. Cypermethrin may cause eye irritation and may be a
sensitizer for certain persons.
3. Conclusions
It can be concluded that:
General population: When applied according to good agricultural
practice, exposure of the general population to cypermethrin is
negligible and is unlikely to present a hazard.
Occupational exposure: With reasonable work practices, hygiene
measures, and safety precautions, the use of cypermethrin is unlikely
to present a hazard to those occupationally exposed to it. The
occurrence of "facial sensations" is an indication of exposure. Under
these circumstances work practice should be reviewed.
Environment: With recommended application rates it is unlikely
that cypermethrin or its degradation products will attain levels of
environmental significance. Notwithstanding its high toxicity for fish
and honey bees, this is only likely to cause a problem in the case of
spillage and overspraying.
4. Recommendations
- Cypermethrin should be included among the residues looked for in
surveillance, market-basket, or total diet studies.
- Attention should be paid to the implications for the welfare of
human beings of animal studies indicating immune suppression.
- Further follow-up studies into the facial effects in human beings
should be conducted, in order to better understand this
phenomenon.
RESUME ET EVALUATION; CONCLUSIONS ET RECOMMANDATIONS
1. Résumé et évaluation
1.1 Identité, emploi, destinée et concentrations dans l'environnement
L'alpha-cyperméthrine est constituée à plus de 90% de
l'énantiomère le plus actif du point de vue insecticide parmi les
quatre isomères cis qui entrent dans la composition de la
cyperméthrine racémique.
C'est un pyréthrinoïde extrêmement actif, qui agit contre de
nombreux parasites auxquels on a affaire dans l'agriculture et
l'élevage. Il est commercialisé sous forme de concentrés
émulsionnables, de formulations à très bas volume, de concentrés pour
suspension ou en mélange avec d'autres insecticides.
Le produit technique se présente sous la forme d'une poudre
cristalline facilement soluble dans l'acétone, la cyclohexanone et le
xylène mais peu soluble dans l'eau. Il est stable en milieu acide ou
neutre mais s'hydrolyse à pH 12-13. Il se décompose au-dessus de 120
°C.
On ne dispose d'aucune donnée sur la concentration de
l'alpha-cyperméthrine dans l'air.
Dans l'eau, il est probable que l'alpha-cyperméthrine est
décomposée par voie photochimique et biologique. On a constaté que,
après avoir traité un étang à raison de 15 g de matière active par
hectare, les eaux de surface et les couches plus profondes contenaient
respectivement 5% et 19% de la dose appliquée une journée après
l'épandage et 0,1% et 2% respectivement de cette dose sept jours plus
tard. Environ 5% de la dose appliquée se retrouvaient dans les
sédiments 16 jours après l'épandage.
Il est probable que l'alpha-cyperméthrine est fortement adsorbée
aux particules du sol. Une année après un épandage à raison de 0,5 kg
de matière active par hectare, on retrouvait dans le sol des résidus
inférieurs à 0,1 mg/kg.
Le coefficient de partage de l'alpha-cyperméthrine entre le
n-octanol et l'eau est égal à 1,4 x 105 (log de Pow = 5,16).
La dose d'emploi recommandée pour l'alpha-cyperméthrine est
moindre que pour la cyperméthrine car elle est biologiquement plus
active. Il en résulte moins de résidus sur les récoltes et si l'on se
conforme aux doses recommandées, ils se situent entre 0,05 et 1 mg/kg.
Chez des poissons-chats d'eau de mer traités à raison de 0,01 à 0,05%
p/p de matière active, on a mesuré des résidus de 0,3 à 30 mg/kg une
semaine après l'entreposage et entre 0,22 et 4,0 mg/kg 15 semaines
plus tard.
1.2 Cinétique et métabolisme
Après avoir été administrée par voie orale à des rats,
l'alpha-cyperméthrine est éliminée dans les urines sous forme du
sulfo-conjugué de l'acide 3-(4-hydroxyphénoxy)benzoïque ainsi que dans
les matières fécales, en partie sans modification. Environ 90% d'une
dose orale unique sont éliminées de l'organisme en quatre jours, dont
78% au cours du premier jour. Les résidus sont faibles dans les
tissus, sauf dans les tissus lipidiques. Trois jours après
administration d'une dose orale unique de 2 mg/kg, la concentration
dans les graisses était de 0,4 mg/kg. L'élimination à partir des
graisses est biphasique; la demi-vie relative à la phase initiale est
de 2,5 jours, et de 17 à 26 jours pour la deuxième phase.
L'alpha-cyperméthrine est métabolisée par coupure de la liaison
ester. Chez le rat, la fraction alcool phénoxybenzylique est
hydroxylée et transformée en sulfo-conjugué; la fraction acide
cyclopropane-carboxylique subit également une conjugaison,
probablement sous forme de glucuronide, avant d'être excrétée par la
voie urinaire. Des études portant sur des microsomes hépatiques de
rats, de lapins et d'origine humaine ont montré que chez ces trois
espèces, il pouvait y avoir hydrolyse de l'ester et métabolisation
oxydative mais que l'hydrolyse de l'ester prédominait dans le cas des
préparations de foie provenant de lapins ou de sujets humains.
Chez l'homme, 43% d'une dose orale (0,25-0,75 mg/litre) ont été
excrétés dans les 24 heures par la voie urinaire sous forme d'acide
cis-cyclopropane-carboxylique libre ou conjugué. Après
l'administration de cinq doses quotidiennes successives, l'excrétion
urinaire n'a pas augmenté.
On a trouvé de fortes concentrations (jusqu'à 1156 mg/kg)
d'alpha-cyperméthrine dans la laine de mouton, 14 jours après avoir
traité les animaux par des bains ou des aspersions. De faibles
quantités ont été retrouvées dans la graisse sous-cutanée (jusqu'à
0,04 mg/kg). Après avoir traité des veaux le long de l'épine dorsale
avec 10 ml d'une formulation à 1,6%, on n'a pas retrouvé
d'alpha-cyperméthrine dans les muscles ni le foie. La concentration
maximale dans la graisse périrénale était de 0,25 mg/kg au bout de 14
jours.
Après avoir traité des vaches en lactation le long de l'épine
dorsale avec des formulations contenant jusqu'à 0,2 g de matière
active on a retrouvé dans le lait de 3 des 15 animaux traités, des
résidus d'alpha-cyperméthrine compris entre 0,003 et 0,005 mg/litre.
1.3 Effets sur les mammifères de laboratoire et les systèmes
d'épreuves in vitro
L'alpha-cyperméthrine présente une toxicité orale aiguë modérée
à forte pour les rongeurs. La DL50 varie beaucoup chez la souris et
le rat et dépend de la concentration du composé et du véhicule
utilisé. En pratique, on considère qu'une DL50 de 80 mg/kg de poids
corporel est représentative. Toutefois, on a fait état de valeurs plus
élevées pour la DL50 par voie orale dans des conditions
d'intoxication aiguë. Une intoxication aiguë par voie orale entraîne
des signes cliniques qui traduisent une action au niveau du système
nerveux central.
Une application cutanée d'alpha-cyperméthrine à des rats et des
souris aux doses respectives de 100 et 500 mg/kg de poids corporel,
n'a entraîné ni mortalité ni signes d'intoxication. De même, des rats
à qui l'on avait fait inhaler pendant quatre heures de
l'alpha-cyperméthrine à une concentration de 400 mg/m3, n'ont
présenté aucune mortalité ni signes cliniques d'intoxication.
L'alpha-cyperméthrine technique ne provoquerait qu'une irritation
minimale de la peau chez le lapin. En revanche, certaines formulations
de ce composé peuvent déterminer une très forte irritation oculaire.
L'alpha-cyperméthrine technique n'a pas d'effet sensibilisant cutané.
Chez le cobaye, elle provoque une stimulation des terminaisons
nerveuses sensorielles de l'épiderme.
Exposés pendant une courte période à de l'alpha-cyperméthrine à
des concentrations quotidiennes allant jusqu'à 200 mg/kg de nourriture
pendant cinq semaines ou jusqu'à 100 mg/kg de nourriture pendant 13
semaines, des rats n'ont pas présenté d'effets toxiques. Aux doses les
plus élevées, on notait des signes d'intoxication traduisant une
atteinte du système nerveux, ainsi qu'une réduction de la croissance
et une augmentation du poids du foie et des reins. Aucun effet bien
net n'a été observé en ce qui concerne les paramètres hématologiques
ou histopathologiques.
Lors d'une étude de 13 semaines au cours de laquelle des chiens
ont reçu de l'alpha-cyperméthrine par voie orale, on a observé que la
dose la plus forte (270 mg/kg de nourriture) produisait des signes
d'intoxication; en revanche tous les autres paramètres étudiés (NFS,
biochimie du sang, urines, poids des organes, anatomopathologie et
histopathologie) sont restés normaux. La dose sans effet observable
était de 90 mg/kg de nourriture (soit l'équivalent de 2,25 mg/kg de
poids corporel par jour).
Le même genre d'étude menée sur des rats a montré que
l'alpha-cyperméthrine provoquait des effets neurotoxiques imputables
à des lésions histopathologiques des nerfs tibial et sciatique, une
dégénérescence des axones et un accroissement de l'activité de la
bêta-galactosidase.
On ne dispose d'aucune donnée sur la toxicité à long terme, la
toxicité pour la fonction de reproduction, la tératogénicité ou
l'immunotoxicité.
En se basant sur les données disponibles, on peut conclure que
l'alpha-cyperméthrine n'est pas mutagène, comme le montrent les tests
effectués sur Salmonella typhimurium, Escherichia coli et
Saccharomyces cerevisiae ainsi que les épreuves in vivo et in
vitro sur des cellules de foie de rat, qui n'ont révélé ni
aberration chromosomique ni lésion de l'ADN monobrin. On n'a pas non
plus observé d'augmentation du nombre d'aberrations chromosomiques
dans des cellules de moelle osseuse de rat.
On ne dispose d'aucune donnée sur la cancérogénicité de
l'alpha-cyperméthrine.
1.4 Effets sur l'homme
Dans la mesure où l'alpha-cyperméthrine est utilisée conformément
aux règles de bonne pratique agricole, l'exposition de la population
générale reste négligeable. On a constaté que l'exposition
professionnelle cutanée des opérateurs procédant à la préparation des
mélanges, au chargement des pulvérisateurs, à l'épandage de
l'insecticide ou au lavage du matériel, pouvait atteindre des valeurs
respectivement égales à 2,94 mg, 0,61 mg et 0,73 mg.
Lors d'une étude sur l'exposition à l'alpha-cyperméthrine au
cours de la préparation de formulations à base de cet insecticide, on
a évalué les niveaux d'exposition par surveillance personnelle et
statique de la concentration atmosphérique de ce composé et dosage de
ses métabolites urinaires. Au cours des deux jours pendant lesquels
les personnes exposées procédaient à la formulation de concentrés
techniques, l'exposition individuelle moyenne dans le groupe a été
respectivement de 2,8 et 4,9 mg/m3, l'exposition individuelle
moyenne du groupe au produit technique étant de 50,1 mg/m3 le
troisième jour. On n'a pas pu déceler la présence de métabolites dans
les urines (limite de détection 0,02 mg/litre). Au cours de la
préparation des diverses formulations, les ouvriers ont fait état de
sensations au niveau de l'épiderme mais en précisant qu'elles étaient
légères.
Aucune intoxication n'a été signalée.
1.5 Effets sur d'autres organismes au laboratoire et dans leur milieu
naturel
La CE50 à 48 et 96 heures (pour la croissance) chez une algue
d'eau douce, Selenastrum capricornutum, dépasse 100 µg/litre.
L'alpha-cyperméthrine est très fortement toxique pour les
invertébrés aquatiques. Les valeurs de la CE50 à 24 et 48 heures
(pour l'immobilisation de la daphnie) sont respectivement égales à 1,0
et 0,3 µg/litre, et celle de la CL50 à 24 heures (pour Gammarus
pulex) est égale à 0,05 µg/litre. L'alpha-cyperméthrine est
également très toxique pour un certain nombre d'arthropodes aquatiques
mais sa toxicité est moindre pour les mollusques. La toxicité à court
terme de ce composé peut être réduite lorsqu'il est présenté sous la
forme d'une suspension dans l'huile. Les pertes à l'épandage peuvent
provoquer des effets toxiques sur les invertébrés aquatiques, mais
comme l'alpha-cyperméthrine disparaît rapidement de l'eau, ceux-ci ont
la possibilité de récupérer. L'alpha-cyperméthrine est très fortement
toxique pour les poissons. La valeur de la CL50 à 96 heures oscille
entre 0,7 et 350 µg/litre selon le type de formulation. Les concentrés
émulsionnables sont beaucoup plus toxiques que les concentrés en
suspension, les poudres mouillables et les formulations
micro-encapsulées. Le danger de l'alpha-cyperméthrine pour les
invertébrés aquatiques et les poissons tient à sa toxicité aiguë. Rien
n'indique toutefois qu'une exposition prolongée entraîne des effets
cumulatifs.
On ne dispose d'aucune donnée concernant les effets de
l'alpha-cyperméthrine sur les microbes terricoles. Les bactéries
présentes dans les effluents n'ont pas paru affectées par une
concentration de 3 mg/litre en système fermé.
La toxicité de l'alpha-cyperméthrine pour certains carabides et
les larves de neuroptères est relativement faible et elle ne présente
guère de danger pour les stades pré-imaginaux des hyménoptères
parasitoïdes. Des études menées sur des champs de grande superficie ou
de petites parcelles ont montré que l'alpha-cyperméthrine était peu
dangereuse pour les carabides et les staphylinides mais qu'elle
présentait un risque relativement important pour les linyphiides. Les
effets sur ces populations d'insectes se sont limités à une seule
saison de croissance. En outre, l'alpha-cyperméthrine ne présente
guère de risque pour les larves de syrphides mais n'est pas dénuée
d'effets sur les coccinellides. Malgré tout, la dissipation rapide des
résidus présents sur le feuillage permet à ces animaux de reconstituer
rapidement leurs colonies.
L'épandage d'alpha-cyperméthrine n'a pas d'effets indésirables
sur l'abondance relative des arthropodes entomophages. Son utilisation
sur les céréales à petits grains n'entraînerait donc pas la
réapparition des ravageurs ou le déclenchement d'infestations
secondaires.
Des études de laboratoire ont montré que l'alpha-cyperméthrine
était peu toxique pour les lombrics. Des vers placés pendant 14 jours
dans un sol artificiel contenant ce composé à des concentrations
allant jusqu'à 100 mg/kg, n'ont présenté aucune mortalité.
Des études de toxicité aiguë également effectuées en laboratoire
ont montré que l'alpha-cyperméthrine était extrêmement toxique pour
les abeilles. Administré par voie orale, un concentré émulsionnable
d'alpha-cyperméthrine a donné une DL50 à 24 heures de 0,13
µg/abeille, la valeur correspondante pour l'administration topique
étant de 0,03 µg/abeille (de produit technique) ou 0,11 µg/abeille (de
concentré émulsionnable). La forte toxicité de l'alpha-cyperméthrine
pour les abeilles ne s'est pas manifestée au cours des épreuves de
plein champ, probablement du fait que le composé a un bref effet
répulsif qui réduit le butinage et, par voie de conséquence,
l'exposition des insectes.
On ne dispose d'aucune donnée sur la toxicité de
l'alpha-cyperméthrine pour les oiseaux.
2. Conclusions
2.1 Population générale
Lorsque l'épandage de l'alpha-cyperméthrine s'effectue
conformément aux règles de bonne pratique, son utilisation en
agriculture n'expose guère la population générale à ce composé et il
y a peu de danger pour elle.
2.2 Exposition professionnelle
Moyennant de bonnes méthodes de travail ainsi que des mesures
d'hygiène et de sécurité, l'utilisation de l'alpha-cyperméthrine ne
devrait pas présenter de danger pour les personnes qui y sont exposées
de par leur profession. L'apparition de sensations au niveau de la
face indique une contamination. Dans ces circonstances, il est bon de
revoir les méthodes de travail.
2.3 Environnement
Aux doses d'emploi recommandées, il n'est guère probable que
l'alpha-cyperméthrine puisse être libérée dans l'environnement à des
concentrations écologiquement dangereuses. Elle est fortement toxique
pour les arthropodes aquatiques, les poissons et les abeilles dans les
conditions du laboratoire. On ne peut envisager la probabilité
d'effets toxiques importants sur les invertébrés non visés et les
poissons qu'en cas de déversement accidentel, d'épandage excessif ou
d'erreur de manipulation.
3. Recommandations
* Il convient d'éviter la contamination des eaux superficielles par
l'alpha-cyperméthrine.
* L'alpha-cyperméthrine se lie fortement aux particules. D'autres
études écotoxicologiques sont à effectuer à propos des effets de
l'alpha-cyperméthrine sur les organismes qui vivent dans les
sédiments car il s'agit d'un aspect qui n'a guère retenu
l'attention jusqu'ici.
* L'absorption dans les voies digestives de l'alpha-cyperméthrine
est à étudier dans diverses conditions expérimentales.
* Il faudrait également étudier la destinée de
l'alpha-cyperméthrine après application sur l'épiderme.
* Il faudrait obtenir des données supplémentaires sur la toxicité
à long terme, la cancérogénicité et l'immunotoxicité de
l'alpha-cyperméthrine.
RESUMEN Y EVALUACION; CONCLUSIONES Y RECOMENDACIONES
1. Resumen y evaluación
1.1 Identificación, uso, destino y niveles en el medio ambiente
La alfa-cipermetrina contiene más del 90% del par de
enantió-meros con mayor actividad insecticida de los cuatro isómeros
cis de la cipermetrina en mezcla racémica.
Se trata de un insecticida piretroide sumamente activo contra una
gran variedad de plagas habituales en agricultura y ganadería. Existe
como concentrado emulsionable, formulacion de volumen ultra-bajo,
concentrado en suspensión y en mezcla con otros insecticidas.
El producto técnico es un polvo cristalino, con buena solubilidad
en acetona, ciclohexanona y xileno, y con baja solubilidad en agua. Es
estable en condiciones ácidas o neutras, pero se hidroliza a pH 12-13.
Se descompone por encima de los 220 °C.
No se dispone de información acerca de los niveles de
alfa-cipermetrina en el aire.
Es probable que la degradación de la alfa-cipermetrina en agua se
deba a procesos fotoquímicos y biológicos. El agua superficial y
subsuperficial de un estanque rociado con 15 g/ha de principio activo
contenía el 5% y el 19% de la dosis aplicada un día después del
rociamiento, y 0.1% y 2% siete días más tarde. A los 16 días de la
aplicación se encontró en el sedimento alrededor del 5% de la dosis
utilizada.
Es probable que la alfa-cipermetrina se adsorba con fuerza a las
partículas del suelo. Un año después del tratamiento con 0.5 kg de
principio activo por hectárea se encontraron en el suelo residuos
inferiores a 0.1 mg/kg.
El coeficiente de reparto n-octanol/agua de la alfa-cipermetrina
es 1.4 x 105 (log Poa = 5.16).
Las tasas de aplicación recomendadas de alfa-cipermetrina son
inferiores a las de cipermetrina, porque la primera es biológicamente
más activa. En consecuencia, los residuos en los cultivos son escasos,
y utilizando las tasas de aplicación recomendadas estos residuos
oscilan entre 0.05 y 1 mg/kg. Los residuos en peces siluroideos
marinos tratados con dosis entre el 0.001 y el 0.05% p/p de principio
activo eran de 0.3-30 mg/kg una semana después del almacenamiento, y
de 0.22 a 4.0 mg/kg tras 15 semanas de almacenamiento.
1.2 Cinética y metabolismo
La alfa-cipermetrina administrada por vía oral a ratas se elimina
por la orina como sulfato conjugado del ácido 3-(4-hidroxifenoxi)
benzoico y en parte como compuesto inalterado por las heces. De una
dosis oral única alrededor del 90% se elimina del cuerpo en un período
de cuatro días, y el 78% en el primer día. Los residuos en los tejidos
son escasos, excepto en el adiposo. La concentración en la grasa tres
días después de una dosis oral única de 2 mg/kg fue de 0.4 mg/kg. La
eliminación a partir de la grasa es bifásica: en la fase inicial tiene
una vida media de 2.5 días y en la segunda de 17 a 26 días.
La alfa-cipermetrina se metaboliza mediante la ruptura de su
enlace éster. En la rata, el alcohol fenoxibencílico de la molécula se
hidroxila y se conjuga con sulfato, y el ácido
ciclopropano-carboxílico también se conjuga (probablemente en forma de
glucurónido) antes de la excreción urinaria. En estudios con
microsomas hepáticos de ratas, conejos y seres humanos se ha
demostrado que la hidrólisis en el ester y rutas oxidativas se dan en
las tres especies, si bien la primera es la ruta predominante en las
preparaciones hepáticas de conejo y de ser humano.
En el ser humano, el 43% de una dosis oral (0.25-0.75 mg) se
excreta en la orina en un plazo de 24 h en forma de ácido cis-
ciclopropano-carboxílico libre o conjugado. La excreción urinaria no
aumentó tras la administración de 5 dosis diarias sucesivas.
En la lana de las ovejas se detectaron concentraciones altas
(hasta 1156 mg/kg) de alfa-cipermetrina 14 días después de la
aplicación por inmersión o por lavado. En la grasa subcutánea se
encontraron niveles bajos (hasta 0.04 mg/kg). Después de tratar
terneros a lo largo del dorso con 10 ml de una preparación al 1.6%, no
se detectó alfa-cipermetrina en los músculos ni en el hígado. La
máxima concentración en la grasa perirrenal durante un período de 14
días fue de 0.26 mg/kg.
Tras la aplicación de hasta 0.2 ml de principio activo a lo largo
del dorso de vacas lecheras, se encontraron en la leche de tres en 15
animales tratados residuos de alfa-cipermetrina en concentraciones que
oscilaban entre 0.003 y 0.005 mg/ml.
1.3 Efectos en mamíferos de laboratorio y en sistemas de prueba
in vitro
En roedores, la toxicidad aguda oral de la alfa-cipermetrina es
entre moderada y alta. Los valores de la DL50 en ratones y ratas son
muy variables y dependen de la concentración del compuesto y del
excipiente. A efectos prácticos, se considera representativo un valor
de la DL50 de 80 mg/kg de peso corporal. Sin embargo, se han
notificado valores más altos de DL50 aguda por vía oral. La
exposición oral aguda produce síntomas clínicos relacionados con la
actividad del sistema nervioso central.
Las aplicaciones cutáneas aisladas de alfa-cipermetrina a ratones
y ratas en concentraciones de 100 y 500 mg/kg de peso corporal,
respectivamente, no produjeron mortalidad ni síntomas de intoxicación.
La exposición de ratas por inhalación durante 4 h a una concentración
atmosférica de 400 mg/m3 tampoco ocasionó mortalidad ni signos
clínicos.
Se ha reportado que la alfa-cipermetrina de calidad técnica
produce una irritación cutánea mínima en el conejo. Algunas de las
preparaciones provocan irritación ocular grave. La alfa-cipermetrina
de calidad técnica no produce sensibilización cutanea. En cobayos
ocasionó la excitación de las terminaciones neuro-sensoriales de la
piel.
La exposición breve de ratas a concentraciones de
alfa-cipermetrina de hasta 200 mg/kg en dieta diaria durante 5 semanas
o hasta 180 mg/kg en dieta diaria durante 13 semanas no produjo
efectos tóxicos. Con dosis más elevadas, las ratas mostraron signos de
intoxicación asociados a la patología del sistema nervioso,
disminución del crecimiento o aumento del peso del hígado y los
riñones. No se pusieron de manifiesto efectos hematológicos,
bioquímicos o histopatológicos claros.
En un estudio de toxicidad oral en perros durante 13 semanas, la
dosis más alta, de 270 mg/kg causó síntomas de intoxicación, pero
todos los demás parámetros examinados (relativos a la hematología,
bioquímica clínica, análisis de orina, peso de los órganos, anatomía
patológica e histopatología) se mantuvieron inalterados. El nivel sin
efectos observados (NOEL) fue de 90 mg/kg de dieta (equivalente a 2.25
mg/kg de peso corporal al día).
En un estudio de toxicidad oral en ratas se demostró que la
alfa-cipermetrina induce neurotoxicidad a causa de alteraciones
histopatológicas de los nervios tibial y ciático, degeneración axonal
y aumento de la actividad de la beta-galactosidasa.
Se carece de datos sobre toxicidad a largo plazo, toxicidad en la
reproducción, teratogenicidad e inmunotoxicidad.
Con los datos disponibles sobre la alfa-cipermetrina, se puede
deducir que se trata de un compuesto no mutagénico en las pruebas con
Salmonella typhimurium, Escherichia coli y Saccaromyces cerevisiae,
y en las pruebas in vivo e in vitro con hepatocitos de rata, con
respecto a la inducción de aberraciones cromosómicas y producción de
lesiones en cadenas simples de ADN. No se observó aumento de las
aberraciones cromosómicas en las células de médula ósea de rata.
Se carece de datos sobre la carcinogenicidad de la
alfa-cipermetrina.
1.4 Efectos en el ser humano
La exposición de la población general a la alfa-cipermetrina es
insignificante siempre que en su utilización se apliquen buenas
prácticas agrícolas. Se comprobó que la exposición cutánea profesional
de los trabajadores durante la mezcla/carga, el rociado y el lavado
del equipo era de hasta 2.94 mg, 0.61 mg y 0.73 mg, respectivamente.
En un estudio de exposición a la alfa-cipermetrina durante la
formulación, se evaluaron los niveles de exposición mediante el
monitoreo personal y estático de las concentraciones atmosféricas y la
medición de los metabolitos en la orina. Los niveles de exposición
personal media del grupo durante los dos días de la formulación de los
concentrados de calidad técnica fueron de 2.8 y 44.9 mg/m3, mientras
que la exposición personal media del grupo al material técnico el
tercer día fue de 54.1 mg/m3. No se detectaron metabolitos en la
orina (límite de detección, 0.02 mg/litro). Durante la formulación se
informó de reacciones cutáneas ligeras.
No se han comunicado casos de envenenamiento.
1.5 Efectos en otros organismos en el laboratorio y en el medio
ambiente
El valor de la CE50 (crecimiento) en las 48 y 96 horas para el
alga de agua dulce Selenastrum capricornutum es superior a 100
µg/litro.
La alfa-cipermetrina es muy tóxica para los invertebrados
acuáticos. Los valores de la CE50 (inmovilización) a las 24 y 48 h
para Daphnia magna son de 1.0 y 0.3 µg/litro, respectivamente, y el
valor de la CL50 a las 24 h para Gammarus pulex es de 0.05
µg/litro. La alfa-cipermetrina es muy tóxica para varios grupos de
artrópodos acuáticos, pero lo es menos para los moluscos. Se puede
reducir la toxicidad a corto plazo del compuesto formulando el
producto como suspensión con mayor cantidad de aceite. Aunque el
arrastre del rociado puede producir efectos tóxicos en los
invertebrados acuáticos, la desaparición rápida de la
alfa-cipermetrina del agua facilita la recuperación.
La alfa-cipermetrina es muy tóxica para los peces. El valor de la
CL50 a las 96 h oscila entre 0.7 y 350 µg/litro, segun la
formulación. Las formulaciones de concentrados emulsionables son mucho
más tóxicas que el concentrado en suspensión, el polvo humectable y
las formulaciones microencapsuladas. El peligro del compuesto para los
invertebrados acuáticos y los peces radica en su toxicidad aguda. No
hay pruebas de que se produzcan efectos acumulativos debido a una
exposición prolongada.
No se dispone de datos acerca de los efectos de la
alfa-cipermetrina en los microorganismos del suelo. En un sistema
cerrado, no se observaron efectos en las bacterias de aguas residuales
con concentraciones de 3 mg/litro.
La toxicidad de la alfa-cipermetrina para determinados
coleopteros carábidos y larvas de neurópteros es relativamente baja,
y representa un peligro limitado en las fases preadultas de los
himenópteros parasitoides. En estudios de campo en pequeñas parcelas
y en gran escala se ha puesto de manifiesto que la alfa-cipermetrina
es poco peligrosa para los coleopteros carábidos y estafilínidos, pero
es un peligro relativamente grande para las arañas linifíidas. Los
efectos sobre las poblaciones se limitaron a una sola temporada de
crecimiento. Además, la alfa-cipermetrina es de bajo riesgo para las
larvas de sírfidos, pero tiene efectos considerables en los
coccinélidos. Sin embargo, la rápida desaparición de los residuos de
las hojas permite a estos animales recolonizar en poco tiempo las
zonas tratadas.
La utilización de alfa-cipermetrina en el campo no diminuye la
abundancia relativa de entomófagos en las comunidades de artrópodos.
Su utilización en cultivos de cereales de grano pequeño no iría
acompañada de la "reaparición" de plagas o de infestaciones de plagas
secundarias.
En las pruebas de laboratorio, la toxicidad de la
alfa-cipermetrina para las lombrices de tierra es baja. No se registró
mortalidad tras 14 días de exposición de las lombrices a
concentraciones de hasta 100 mg/kg de suelo artificial.
En pruebas de toxicidad aguda en el laboratorio se observó que la
alfa-cipermetrina es muy tóxica para las abejas. La administración
oral de una solución concentrada emulsionable dio una DL50 a las 24
h de 0.13 µg/abeja, mientras que el valor correspondiente para la
administración tópica fue de 0.03 µg/abeja (producto técnico) ó 0.11
µg/abeja (CE). La elevada toxicidad de la alfa-cipermetrina para las
abejas no se manifestó claramente en los ensayos de campo,
probablemente a causa del breve efecto repelente del producto, que
hace disminuir la actividad libadora de las abejas y, por
consiguiente, su exposición.
No se dispone de datos acerca de la toxicidad del
alfa-cipermetrina para las aves.
2. Conclusiones
2.1 Población general
Cuando la alfa-cipermetrina se aplica correctamente, la
exposición de la población general al producto es baja y probablemente
no supone riesgos.
2.2 Exposición ocupacional
Con buenas prácticas de trabajo, medidas de higiene y
precauciones de seguridad, es improbable que la alfa-cipermetrina
suponga un peligro para las personas expuestas en forma laboral. La
aparición de "sensaciones faciales" es un síntoma de exposición. En
estas circunstancias se deben re-examinar las prácticas de trabajo.
2.3 Medio ambiente
Con las cantidades recomendadas para la aplicación es improbable
que la alfa-cipermetrina alcance niveles de importancia ecológica. En
condiciones de laboratorio es muy tóxica para los artrópodos
acuáticos, los peces y las abejas. Hay cierta probabilidad de que se
produzcan efectos tóxicos importantes en invertebrados a los que no
está destinado y en peces en casos de derrame, rociado excesivo o mala
utilización del producto.
3. Recomendaciones
* Se debe evitar la contaminación de aguas superficiales.
* La alfa-cipermetrina se une fuertemente a las partículas. Se
deberían llevar a cabo nuevos estudios ecotoxicológicos sobre los
efectos del compuesto en los microorganismos de los sedimentos,
puesto que este aspecto parece haber recibido poca atención.
* Hay que investigar la absorción gastrointestinal de
alfa-cipermetrina en distintas circunstancias.
* Se debe investigar el destino final de la alfa-cipermetrina
aplicada por vía cutánea.
* Hay que obtener nueva información acerca de la
toxicidad/carcinogenicidad a largo plazo y de la inmunotoxicidad
de la alfa-cipermetrina.
 2.1.2 Technical product
Common trade
names: Fastac, Concord, Fendona, Renegade
Purity: technical grade: > 90% pure (m/m)
Impurities: no data.
2.2 Physical and chemical properties
Alpha-cypermethrin is a racemic mixture of the two stereo-isomers
(1:1) indicated by boxes in Fig. 1 and is a crystalline powder. Some
physical and chemical properties of alpha-cypermethrin are given in
Table 1.
Table 1. Physical and chemical properties of alpha-cypermethrin (pure
enantiomeric pair; purity > 99%)
Boiling point 200 °C at 9.3 N/m2
Melting point 80.5 °C
Vapour pressure (20 °C) 170 nPa (1.7 x 107 N/m2)
Density 1.12 g/cm3 at 20 °C
1.28 g/cm3 at 22 °C
Solubility (25 °C) 0.005-0.01 mg/litre water; 620 g/litre
acetone; 515 g/litre cyclohexanone; 7 g/kg
hexane; 351 g/litre xylene
Stability It is stable under acidic or neutral
conditions (pH 3-7) but hydrolyses in
strongly alkaline media (pH 12-13). It
decomposes above 220 °C. Field data indicate
that in practice it is stable to air and
light.
Partition coefficient
n-octanol/water log Pow 5.16 (Pow = 1.4 x 105)
From: Langner (1980); Shell (1983a); Worthing & Hance (1991).
The water solubility of alpha-cypermethrin (98.0%), calculated as
the sum of the cis-1 and the cis-2 isomer (ratio 2.6:97.4)
concentrations, at 20 °C in 0.01 M buffers at pH values of
approximately 4 to 9, ranges from 4.59 to 7.87 µg/litre, as measured
by the OECD and EEC microcolumn techniques. In distilled water alone
the solubility is slightly less, i.e. 2.06 µg/litre. The solubility is
not strongly dependent on pH values within the range of 4 to 9. It is
likely that ionic strength differences account for differences in
solubility between values in pure water and in the buffer solutions
(Baldwin, 1990).
2.3 Formulations
The following formulations exist:
* "Fastac", EC (20-100 g/litre), WP (50 g/kg), SC (15-250
g/litre), ULV (6 to 15 g/litre);
* "Fendona" and "Renegade", EC (50 or 100 g/litre), SC (250
g/litre), WP (50 g/kg).
Combination with other active ingredients also exist, e.g.,
"Azofas" (alpha-cypermethrin and monocrotophos) and combinations of
alpha-cypermethrin with methomyl or Fenobucarb (Worthing & Hance,
1991).
2.4 Conversion factors
1 ppm = 17.02 mg/m3
1 mg/m3 = 0.059 ppm
2.5 Analytical methods
2.5.1 Sampling
2.5.1.1 Air
Samples are collected by drawing a measured volume of air through
a 37-mm diameter silver membrane filter with a glass fibre pre-filter.
They are analysed for total pyrethroid content ( cis- and
trans-cypermethrin isomers) by gas chromatography with electron
capture detection (ECD). The limit of determination is 0.01 µg/filter
(see Table 2) (Armitage, 1984).
2.5.1.2 Surface-wipe
Surface-wipe samples are collected using a filter paper wetted
with diethyl ether. These samples are analysed for total pyrethroid
content ( cis- and trans-cypermethrin isomers) by gas
chromatography with flame ionization detection (FID). The limit of
determination is 0.03 mg/filter (see Table 2) (Armitage, 1984).
2.5.2 Methods for determination
A method for the determination of alpha-cypermethrin in technical
material and formulated products, excluding suspension concentrates,
was described by Shell (1987a). This method is also used to determine
the ratio of the enantiomer pairs cis 1 to cis 2.
The alpha-cypermethrin content is determined by means of
high-performance liquid chromatography (HPLC), using a column packed
with Zorbax SIL, together with ultraviolet detection at 230 nm
(Shell, 1987a).
Methods have been described for the determination of
alpha-cypermethrin in water, soil, crops, and animal tissues and
fluids (see Table 2).
Table 2. Analytical methods for alpha-cypermethrin in air, soil, water and biological mediaa
Sample Extraction Clean-up Detection and Recovery Limit of References
quantification determination
Air 20% ethyl acetate column chromatography gas chromatography with - 0.01 µg/filter Armitage (1984)
in hexane chromosorb W.HP. electron capture detection
Surface 20% ethyl acetate column chromatography gas chromatography with - 30 µg/filter Armitage (1984)
wipe in hexane chromosorb W.HP. flame ionization detection
Soil anhydrous sodium liquid-solid packed column gas 95-100%b 10 µg/kg Shell (1990b)
sulfate with chromatography chromatography, electron capture
acetone/hexane using Florisil detection; confirmation by
capillary GC and packed column
GC-MS
Water solvent partition Florisil disposable capillary gas-liquid 80-100%c 0.01 µg/litre Shell (1990a)
with hexane cartridge chromatography, electron-capture
detection; confirmation by GC-MS
Crops anhydrous sodium partition between packed column gas 90-100%b 10 µg/kg Shell (1989a)
sulfate with hexane and water/ chromatography, electron capture
acetone/hexane acetonitrile; liquid- detection; confirmation by
solid chromatography capillary GC and packed
using Florisil column GC-MS
Animal acetone/hexane partition with gas-liquid chromatography, 80-100%d 10 µg/kg Shell (1988a)
tissues mixture acetonitrile or electron capture detection;
hexane-acetonitrile; confirmation by GC-MS
liquid-solid
chromatography on
Florisil
Milk diethyl ether/ cyano Bond Elut gas-liquid chromatography, 90-100%e 1 µg/litre Shell (1988b)
hexane; Extrelut cartridge electron capture detection;
extraction column confirmation by GC-MS
Table 2 (continued)
Sample Extraction Clean-up Detection and Recovery Limit of References
quantification determination
Blood acetone partition with hexane packed column gas - 10 µg/litre Shell (1986)
(rat) (washed with water); chromatography, electron capture
dried with sodium detection; confirmation by
sulfate; liquid-solid capillary GC and GC-MS
chromatography on
Florisil
a Details of the analytical methods are available from Shell International Chemical Company, London. These methods differentiate
between alpha-cypermethrin and the other isomers.
b Over the concentration range 0.05-0.5 mg/kg
c Over the concentration range 0.05-0.5 µg/litre
d Concentrations 0.1-0.2 mg/kg
e Over the concentration range 0.005-0.02 mg/litre
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Alpha-cypermethrin does not occur in nature.
3.2 Anthropogenic sources
3.2.1 Production levels and processes
Alpha-cypermethrin is manufactured from cis-2,2,dimethyl-3-
(2",2"-dichlorovinyl)-cyclopropane carboxylate ( cis-DVO), 3-phenoxy
benzaldehyde (POAL) and sodium cyanide.
After removal of the solvent, the cis-cypermethrin is
epimerized into alpha-cypermethrin. Solid alpha-cypermethrin crystals
separate and are filtered, washed and dried under vacuum before
drumming-off1.
No data are available on production levels.
3.2.2 Use
Alpha-cypermethrin has been available commercially since late
1983. It is a potent insecticide effective against a wide range of
pests, particularly Lepidoptera and Coleoptera in citrus, cotton,
forestry, fruit, rice, soybeans, tomatoes, vegetables, grapes and
other crops, at a concentration of 5-30 g active ingredient per ha.
Good control of plant-sucking Hemiptera can also be obtained if the
insecticide is applied before populations have become established. It
also controls soil-dwelling Lepidoptera.
Alpha-cypermethrin can be used in most crops for either curative
or preventive treatment. It can replace conventional insecticides in
short-interval spray programmes, or the longer residual performance
may be exploited to reduce the number of sprays per season. Either
option may be chosen since no reports of phytotoxicity have been
received even when sensitive crops have been involved in repeated
applications. It controls ectoparasites ( Boophilus microplus at a
concentration of 50 mg/litre), including strains resistant to
organophosphorus pesticides, as well as sheep lice and Melophagus
ovinus.
1 Manufacturing process of alpha-cypermethrin; Shell International
Chemical Company; letter dated 10 January 1989 (ref. CTMAR/4)
Rapid knockdown and residual control of biting flies in and
around animal housing have been obtained following direct spray
application to animals or structural surfaces. Furthermore,
alpha-cypermethrin controls Blattellidae, Culicidae, flies and other
nuisance or disease-carrying insects, at a level of 10-30 mg/m2,
with good persistence on most surfaces (Fisher et al., 1983; Worthing
& Hance, 1991).
Alpha-cypermethrin is available as an emulsifiable concentrate,
ultra-low-volume formulation and suspension concentrate (flowable
formulations). Mixtures with organophosphorus and carbamate
insecticides have also been developed. Details of formulations are
given in section 2.3.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
Data relevant to alpha-cypermethrin can be found in Environmental
Health Criteria 82: Cypermethrin (WHO, 1989).
4.1.1 Air
No information on the transport of alpha-cypermethrin in air is
available, but its volatility is very low.
4.1.2 Water
Alpha-cypermethrin as an emulsifiable concentrate (EC) was
sprayed from the air (15 g active ingredient/ha) to a field along one
side of which ran a freshwater ditch. The fate and biological effects
of spray drift in the ditch were monitored for 7 weeks after the
application (see sections 9.2.1.2 and 9.2.2.2). Deposition on the
surface of the ditch was around 5 g active ingredient/ha (30% of the
nominal application rate). Alpha-cypermethrin concentrations in the
sub-surface water were 0.6 µg/litre shortly after the application and
decreased to < 0.02 µg/litre within 2 to 4 days. No contamination of
the water was found 200 m beyond either end of the treated field
(Garforth & Woodbridge, 1984).
Two freshwater ponds were treated with an EC formulation of
alpha-cypermethrin in 1987. One pond was oversprayed with 15 g active
ingredient/ha, while the other was treated with the same amount of
alpha-cypermethrin but by direct incorporation into the water. A third
pond served as a control. One day after the treatment, 5% of the
applied substance was found in the surface film of the oversprayed
pond and 19% in the sub-surface water. Residue levels in both
compartments subsequently declined rapidly so that one week later only
0.1 and 2% were still present, respectively. In the pond that received
direct treatment, 37% of the applied alpha-cypermethrin was found in
the sub-surface water one day after treatment. The concentration
subsequently declined more rapidly than in the oversprayed pond so
that one week later only 2% was present. The concentration of alpha-
cypermethrin found in the sediment samples from both ponds 16 days
after treatment indicated that approximately 5% of the
alpha-cypermethrin applied was present at that time. Thereafter the
concentration decreased and was less than 3% in the sediment 33 days
after application. In a bioassay test, the water in both ponds was
found to be acutely toxic to Gammarus pulex for at least 4 days
after application. After a further 29 days, the water was no longer
acutely toxic. The sediment was not toxic to Gammarus pulex
(Pearson, 1990).
4.1.3 Soil
A trial in the United Kingdom (Reculver) investigated the decay
of alpha-cypermethrin in sandy-clay soil treated with a diluted EC
formulation at a dosage rate of 0.5 kg active ingredient per ha.
Samples of soil were taken from the 0-15 cm layer of each plot at
various intervals over a period of one year. Once a year a sample was
also taken from the 15-30 cm layer. The residue immediately after the
application was 0.07 mg/kg soil in the 0-15 cm layer, and within 2
weeks this had declined by 50%. The residues of alpha-cypermethrin in
samples from the 0-15 cm layer and 15-30 cm layer taken 40 weeks and
52 weeks after application were below the limit of determination, i.e.
0.01 mg/kg (Forbes & Knight, 1983).
After one year, a second application to the bare soil was made
and again a diluted EC formulation was applied at a dosage rate of 0.5
kg active ingredient/ha. Samples were taken at various intervals
during this second year. Residues of alpha-cypermethrin in the 0-15 cm
soil layer declined from 0.19 mg/kg immediately after treatment to
0.11 mg/kg after 2.1 weeks and < 0.01 mg/kg after 49 weeks. In
samples from the 15-30 cm layer no residues (< 0.01 mg/kg) were
detectable 23 and 49 weeks after application (Forbes & Burden, 1984).
In the third year of the trial, another application to the same plots
was made with the EC formulation at a dosage rate of 0.5 kg active
ingredient/ha. Residues of alpha-cypermethrin in the 0-15 cm layer
declined from 0.20 mg/kg immediately after treatment to 0.08 mg/kg
after 18 weeks and 0.01 mg/kg after 52 weeks. Residues were not
detectable in the 15-30 cm layer sampled after 32 and 52 weeks. Over
the three years of the trial there was no indication of a build-up of
alpha-cypermethrin residues in the surface soil layer or any evidence
to suggest leaching of the compound into sub-surface soil layers
(Forbes & Wales, 1985a).
A further trial was carried out in the United Kingdom (Coates) to
study the decay of alpha-cypermethrin applied to a peat type soil as
a diluted EC formulation at a dosage rate of 0.5 kg active
ingredient/ha. As in the Reculver study, residues were determined in
the 0-15 cm layer at various intervals and in the 15-30 cm layer 32
weeks after application. At the beginning of the second and third
year, one application was made as at the beginning of the first year.
The residue in the 0-15 cm layer immediately after the first
application was 0.65 mg/kg declining to 0.36 mg/kg within 2 weeks and
to 0.30 mg/kg after 8 weeks. After 16 weeks, the residue was 0.05
mg/kg or less. In the 15-30 cm layer, no residues were found after 32
weeks (Forbes & Mackay, 1983). Immediately after the second
application, the residue in the 0-15 cm layer was 0.65 mg/kg; after
two weeks the level was 0.36 mg/kg and declined to 0.07 mg/kg by 48
weeks after application. No residues were found in the 15-30 cm layer
(Forbes & Wales, 1985b).
In the third year, a residue level of 0.55 mg/kg was found in the
0-15 cm layer immediately after treatment, declining to 0.20 mg/kg
within 8 weeks and to 0.09 mg/kg after 50 weeks. In the 15-30 cm
layer, residues of 0.01 and 0.03 mg/kg were found after 40 and 50
weeks respectively. In this 3-year trial there was no indication of a
build-up of alpha-cypermethrin residues in the surface soil layers, or
any evidence to suggest significant leaching into sub-surface soil
layers (Coveney & Forbes, 1986).
4.2 Biotransformation
4.2.1 Biodegradation
Alpha-cypermethrin has been tested for "ready biodegradability"
in two tests: a) the closed bottle and modified Sturm test, and b)
growth inhibition in a Pseudomonas fluorescens growth test. In these
tests, mineralization of alpha-cypermethrin was not detected. It was
not degraded in these two tests and hence is not considered to be
readily biodegradable (Stone & Watkinson, 1983).
Maloney et al. (1988) studied the microbial transformation of
technical alpha-cypermethrin (96.3% pure) in aerobic batch enrichment
cultures. These microbial enrichments, which contained Pseudomonas
fluorescens (SM-1), Achromobacter sp. and Bacillus cereus, were
able to transform alpha-cypermethrin with a half-life of 7 to 14 days
at a concentration of 50 mg/litre in the presence of 0.05% Tween 80
(v/v). One of the major transformation products was 3-phenoxybenzoic
acid, which was further transformed to 4-hydroxy-3-phenoxybenzoic
acid.
McMinn (1983a) investigated the degradation under aerobic
conditions of alpha-cypermethrin, labelled with 14C in the benzyl
ring, in two types of soil, i.e. sandy clay loam and clay loam. The
soils were treated with 1 mg of the labelled material and gently
agitated to distribute the insecticide. Soils samples were removed for
analysis 2.5, 6, 10, 20 and 42 weeks after treatment. The initial
degradation half-lives were 27 and 13 weeks for sandy clay loam and
clay loam, respectively. However, after 42 weeks the percentage of
applied radioactivity remaining unchanged was 28.9 and 21.6%,
respectively, for the two soils. The formation of total organo-soluble
products after 42 weeks was 32.2 and 24.3% for sandy clay loam and
clay loam, respectively. Total extractable and total non-extractable
radioactivity for sandy clay loam was 32.5 and 18.0% and for clay loam
25.3 and 32.0%, respectively. Metabolites were found in both cases at
levels of 2 to 3%. Unchanged alpha-cypermethrin was present, and the
degradation products had similar chromatographic mobilities to the
previously identified major products of cypermethrin (McMinn, 1983b).
4.2.2 Bioaccumulation
The n-octanol/water partition coefficient of alpha-cypermethrin
is 1.4 x 105 (log Pow = 5.16), compared to a value for
cypermethrin of 2 x 106 (log Pow = 6.3). The actual
bioaccumulation in fish found experimentally for cypermethrin is lower
than might be expected from the partition coefficient. This should
also apply to alpha-cypermethrin, because the pathway and rate of
metabolism are comparable with those of cypermethrin (Shell, 1983b;
WHO, 1989).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
Information relevant to alpha-cypermethrin was given in
Environmental Health Criteria 82: Cypermethrin (WHO, 1989).
5.1.1 Soil
In a study on the deposition of alpha-cypermethrin on the orchard
floor following commercial application to apple trees,
alpha-cypermethrin (100 g EC/litre) was applied at a nominal dose rate
of 26 g/ha using a tractor-driven "Kinkelder" mist-blower. Following
normal practice, spray runs were made between each row of trees and
then around the perimeter of the orchard. One hour after spraying,
pesticide deposits were collected in foil-lined trays positioned on
the orchard floor and analysed. Deposition was found to be variable,
ranging from 10 to 76% of the nominal application rate (Hillaby,
1988).
5.2 Food
5.2.1 Crops
Residue data on cypermethrin have been evaluated by the Joint
FAO/WHO Meeting on Pesticide Residues (FAO/WHO, l980, 1982).
Alpha-cypermethrin application rates to crops range from 5 to 30
g active ingredient/ha. Residue data have been obtained from
supervised trials in many countries. The residue concentrations of
alpha-cypermethrin derived from recommended application rates vary
from 0.05 to 1.0 mg/kg product (Shell, 1984).
A study was carried out to determine whether there was
significant isomerization of alpha-cypermethrin after treatment of
certain crops. Grapes were treated with 10% EC applied at a rate of 18
g active ingredient/ha, and apples and lettuce with 10% EC at 15 g
active ingredient/ha. Samples were taken 3 and 7 days (grapes), 7 days
(apples) and 10 days (lettuce) after treatment. Residues of 0.17 and
0.09 mg/kg were found on grapes, 0.05 mg/kg on apples and 0.17 mg/kg
on lettuce, but none of the samples showed any significant isomer
conversion of alpha-cypermethrin (Bosio, 1982).
In 1983 two trials were carried out in Canada in which
alpha-cypermethrin was applied with a knapsack sprayer to maize
(sweetcorn). Five applications with diluted 10% EC formulations at a
dosage rate of 20 g active ingredient/ha were made, samples were
harvested 7 days after the last application, and the husks, grain and
cobs were analysed separately. Alpha-cypermethrin residues of 0.38
mg/kg were found in the husks, but no residues (limit of
determination, 0.01 mg/kg) were found in the grain or the cobs (Forbes
& Cole, 1986).
5.2.2 Fish
To reduce blow-fly infestations during the curing of marine
catfish, the fish were dipped in EC solutions (15 g/litre) at various
concentrations (0.001-0.05% active ingredient w/v) between the salting
and drying stages of the curing process. Dipping after the salting
stage in a 0.001% solution of the EC proved to be effective. The
levels of residues in treated fish were dependent on the season (wet
and dry season), storage time, concentration of the dip solution and
the size of the fish. In the wet season, the range was from 0.9 to 2.8
mg/kg, whereas in the dry season it was from 0.26 to 30.0 after one
week of storage and 0.22 to 4.0 mg/kg (wet weight of homogenized fish)
after 15 weeks of storage (Forbes, 1985).
5.2.3 Milk
A trial was carried out in 1987 in the United Kingdom where
lactating cows were treated with pour-on formulations of
alpha-cypermethrin. Two formulations were used containing either 10
g/litre or 15 g/litre (see also section 6.1.2). Either 10 ml or 20 ml
of formulation containing 0.1, 0.15 or 0.2 g active ingredient was
applied along the mid-dorsal line of five cows for each treatment.
Milk samples were taken 1, 2, 3, 4, 7, 14 and 21 days after treatment
for the analysis of alpha-cypermethrin. The residues of
alpha-cypermethrin in milk were at a maximum from 2 to 4 days after
treatment. Generally, residues were highest in the 0.2 g group, the
maximum concentration being 0.005 mg/litre (in two samples only). By
day 21 the residues in the milk from all treated cows were < 0.002
mg/litre (the limit of determination) (Sherren, 1988b).
5.3 Human exposure
In a study to quantify the maximum potential dermal exposure of
operators to crop protection products, 13 exposure pads were mounted
on each of three operators and chemicals adhering to gloves were
analysed. The operation involved three distinct stages: mixing product
and loading the tractor; spraying; and washing-up the equipment and
tractor after the exercise. The total dermal exposure for the three
operators was: mixing/loading, 2.45, 0.57 and 2.94 mg/operation;
spraying, 0.38, 0.61 and 0.40 mg/h; and washing-up, 0.12, 0.29 and
0.73 mg/operation (Senior & Lavers, 1990a,b).
6. KINETICS AND METABOLISM
Both cis and trans isomers of cypermethrin are metabolized via
cleavage of the ester bond to phenoxybenzoic acid (PBA) and
cyclopropane carboxylic acid (CPA). The PBA moiety is mainly excreted
as a conjugate. The type of conjugate differs in a number of animal
species. PBA is further metabolized to a hydroxy derivative and
conjugated as a glucuronate or sulfate. The CPA moiety is mainly
excreted as a glucuronate. Consistent with the lipophilic nature of
cypermethrin, the highest tissue concentrations are found in body fat,
skin, liver, kidneys, adrenals and ovaries. The elimination from fat
is approximately 3 to 4 times slower for the cis isomers than for the
trans isomers (WHO, 1989).
6.1 Absorption, elimination, retention and turnover
6.1.1 Rats
Alpha-cypermethrin labelled in the 14C-benzyl moiety has been
studied in Wistar rats at a concentration of approximately 2 mg/kg
body weight in corn oil. The compound, which was given by stomach
tube, was rapidly broken down and the radioactivity was mainly
eliminated in the urine as the sulfate conjugate of
3-(4-hydroxyphenoxy)benzoic acid (40-45% of the dose). Approximately
35% of the dose was eliminated in the faeces, 20% of which was
unchanged alpha-cypermethrin. The proportion of the dose excreted in
the urine and faeces within the first 24 h was approximately 78% and
within 4 days was 90%. Residues in major organs and tissues of rats 4
days after a single oral dose were in general low: liver, 0.03 and
0.05; skin, 0.04 and 0.02; adrenals, 0.03 and 0.06; and kidneys, 0.02
and 0.02 (values are expressed as mg equivalent of
alpha-cypermethrin/kg tissue for females and males, respectively).
However, in body fat, higher residues were found (0.22 and 0.42
mg/kg). The release from skin and fat was biphasic in nature. The
half-life of elimination of radioactivity from fat was approximately
2.5 days for the initial phase and 17-26 days for the slower phase
(the half-life of elimination from fat for cis-cypermethrin was 18.9
days). The half-life values for skin were 2 days for the initial phase
and 40 days for the slower phase. The radioactivity in liver and
kidneys was eliminated apparently by a monophasic process. More than
95% of the residue in fat was present as unchanged alpha-cypermethrin
(Hutson, 1982; Logan, 1983; Hutson & Logan, 1986).
6.1.2 Domestic animals
In a study by Francis & Gill (1991), a formulation containing a
mixture of flufenoxuron and alpha-cypermethrin was applied to groups
of three sheep. The formulation was applied once, either as a dip
diluted at 1:1000 to give a solution of 80 mg flufenoxuron per litre
and 60 mg alpha-cypermethrin/litre or as a pour-on solution applied
directly to the backs of the sheep giving a dose of 0.15 g active
ingredient flufenoxuron and 0.2 g alpha-cypermethrin per sheep. The
sheep were killed at 3, 7 and 14 days after application and samples of
subcutaneous fat, fleece and sheep skin were analysed. The residues of
alpha-cypermethrin in fat ranged from < 0.01 to 0.04 mg/kg and in
skin from 0.02 to 1.4 mg/kg over the three sampling periods, and they
were lower for pour-on formulations than for the dip. Highest tissue
residues were found in wool (sampled from the back); these were (for
the 3, 7 and 14 day sampling periods, respectively) 730, 1020 and 360
mg/kg for dip application and 360, 440 and 360 mg/kg for pour-on
application. Wool sampled from the side of sheep treated with pour-on
formulation were 10 to 30 times lower than wool sampled from the back
region; with the dip solution, however, wool from the side region
contained higher residues than that from the back. Pour-on application
gave lower residues than after a dip.
A trial was carried out during 1987 in the United Kingdom in
which Friesian/Hereford calves (in total 17 female animals) were
treated with an alpha-cypermethrin pour-on formulation. Ten ml of a 16
g/litre formulation was applied to calves along the middorsal line
from shoulder to tail. At 3, 7 and 14 days following treatment,
animals were sacrificed for analysis of tissues, i.e. perirenal and
subcutaneous fat, muscle, kidneys and liver. No residues were detected
in muscle and liver samples at any time (limit of determination, 0.01
mg/kg). In the kidneys a maximum of 0.03 mg/kg was found on day 7 but
by day 14 the residues had decreased to 0.01 mg/kg or less. The fat
tissues contained maximum levels on day 7, i.e. mean concentrations of
0.26 mg/kg (perirenal fat) and 0.08 mg/kg (subcutaneous fat). By day
14 these concentrations had decreased by about two and a half times
(Sherren, 1988a) (see also section 5.2.3).
6.1.3 Humans
Six volunteers (two per dose level) received a single oral dose
of 0.25, 0.5 or 0.75 mg alpha-cypermethrin and, after a period of 2-3
weeks, five successive daily doses of 0.25, 0.5 or 0.75 mg to study
the urinary excretion and bioaccumulation of alpha-cypermethrin. A
parallel study with cypermethrin itself was carried out for comparison
purposes. The metabolism and rate of excretion of a single oral dose
of alpha-cypermethrin were similar to those of cypermethrin itself.
The rate of excretion was dose-related, approximately 43% of the dose
of alpha-cypermethrin being excreted in the urine as free or
conjugated cis-cyclopropane carboxylic acid ( cis-CPA) during the
first 24 h. Urinary excretion did not increase with repeated oral
dosing; an average of 49% of alpha-cypermethrin was excreted in the
urine as free or conjugated cis-CPA within 24 h (van Sittert et al.,
1985; Eadsforth et al., 1988).
6.2 Metabolic transformation
In a study on Wistar rats using alpha-cypermethrin,
14C-labelled in the benzyl moiety, (see section 6.1.1) no evidence
was found for any racemization of the chiral centres of
alpha-cypermethrin in the residues in intestines, faeces or fat. The
major urinary metabolite was the sulfate conjugate of
3-(4-hydroxyphenoxy)benzoic acid, and smaller amounts of
3-phenoxybenzoic acid (II) and 3-(4-hydroxyphenoxy)benzoic acid (III)
were identified. In the faeces, 75% of the radioactivity in the
extract was unchanged alpha-cypermethrin; minor metabolites included
a dihydroxy metabolite (V), 3-(4-hydroxyphenoxy)benzoic acid (III),
3-phenoxybenzoic acid (II) and the 4-hydroxyphenoxy metabolite (IV).
In the adipose tissue, the 14C label was mainly associated with
unchanged alpha-cypermethrin, but a lipophilic metabolite of either
alpha-cypermethrin or 3-phenoxybenzoic acid, probably a mixture of
3-phenoxybenzoyl diacylglycerols, was also present (Hutson, 1982;
Logan, 1983; Hutson & Logan, 1986) (see Fig. 2).
6.3 In vitro metabolic transformation
Creedy & Logan (1984) studied the in vitro metabolism of
cypermethrin and alpha-cypermethrin using liver microsomal
preparations from rats, rabbits and humans. In order to obtain
information on the relative importance of the oxidative and esteric
pathways of degradation of these compounds, incubations were carried
out both in the presence and absence of an NADPH-generating system.
Both cypermethrin and alpha-cypermethrin were broken down via esteric
and oxidative pathways by the liver preparations from the three
species. For rabbit and human liver microsomes, oxidation was a minor
metabolic route compared to esteric hydrolysis in the case of both
compounds. Human liver microsomes were able to carry out the esteric
hydrolysis of alpha-cypermethrin slightly faster than cypermethrin. In
the liver preparations of all three species, cyclopropane carboxylic
acid (mainly produced via the esteric pathway) was the main metabolite
for both compounds (to the extent of approximately 90-99%). Via the
oxidative route, mono-hydroxycypermethrins, dihydroxy-cypermethrin and
small amounts of hydroxycyclopropane carboxylic acid (rat only) were
also produced.
2.1.2 Technical product
Common trade
names: Fastac, Concord, Fendona, Renegade
Purity: technical grade: > 90% pure (m/m)
Impurities: no data.
2.2 Physical and chemical properties
Alpha-cypermethrin is a racemic mixture of the two stereo-isomers
(1:1) indicated by boxes in Fig. 1 and is a crystalline powder. Some
physical and chemical properties of alpha-cypermethrin are given in
Table 1.
Table 1. Physical and chemical properties of alpha-cypermethrin (pure
enantiomeric pair; purity > 99%)
Boiling point 200 °C at 9.3 N/m2
Melting point 80.5 °C
Vapour pressure (20 °C) 170 nPa (1.7 x 107 N/m2)
Density 1.12 g/cm3 at 20 °C
1.28 g/cm3 at 22 °C
Solubility (25 °C) 0.005-0.01 mg/litre water; 620 g/litre
acetone; 515 g/litre cyclohexanone; 7 g/kg
hexane; 351 g/litre xylene
Stability It is stable under acidic or neutral
conditions (pH 3-7) but hydrolyses in
strongly alkaline media (pH 12-13). It
decomposes above 220 °C. Field data indicate
that in practice it is stable to air and
light.
Partition coefficient
n-octanol/water log Pow 5.16 (Pow = 1.4 x 105)
From: Langner (1980); Shell (1983a); Worthing & Hance (1991).
The water solubility of alpha-cypermethrin (98.0%), calculated as
the sum of the cis-1 and the cis-2 isomer (ratio 2.6:97.4)
concentrations, at 20 °C in 0.01 M buffers at pH values of
approximately 4 to 9, ranges from 4.59 to 7.87 µg/litre, as measured
by the OECD and EEC microcolumn techniques. In distilled water alone
the solubility is slightly less, i.e. 2.06 µg/litre. The solubility is
not strongly dependent on pH values within the range of 4 to 9. It is
likely that ionic strength differences account for differences in
solubility between values in pure water and in the buffer solutions
(Baldwin, 1990).
2.3 Formulations
The following formulations exist:
* "Fastac", EC (20-100 g/litre), WP (50 g/kg), SC (15-250
g/litre), ULV (6 to 15 g/litre);
* "Fendona" and "Renegade", EC (50 or 100 g/litre), SC (250
g/litre), WP (50 g/kg).
Combination with other active ingredients also exist, e.g.,
"Azofas" (alpha-cypermethrin and monocrotophos) and combinations of
alpha-cypermethrin with methomyl or Fenobucarb (Worthing & Hance,
1991).
2.4 Conversion factors
1 ppm = 17.02 mg/m3
1 mg/m3 = 0.059 ppm
2.5 Analytical methods
2.5.1 Sampling
2.5.1.1 Air
Samples are collected by drawing a measured volume of air through
a 37-mm diameter silver membrane filter with a glass fibre pre-filter.
They are analysed for total pyrethroid content ( cis- and
trans-cypermethrin isomers) by gas chromatography with electron
capture detection (ECD). The limit of determination is 0.01 µg/filter
(see Table 2) (Armitage, 1984).
2.5.1.2 Surface-wipe
Surface-wipe samples are collected using a filter paper wetted
with diethyl ether. These samples are analysed for total pyrethroid
content ( cis- and trans-cypermethrin isomers) by gas
chromatography with flame ionization detection (FID). The limit of
determination is 0.03 mg/filter (see Table 2) (Armitage, 1984).
2.5.2 Methods for determination
A method for the determination of alpha-cypermethrin in technical
material and formulated products, excluding suspension concentrates,
was described by Shell (1987a). This method is also used to determine
the ratio of the enantiomer pairs cis 1 to cis 2.
The alpha-cypermethrin content is determined by means of
high-performance liquid chromatography (HPLC), using a column packed
with Zorbax SIL, together with ultraviolet detection at 230 nm
(Shell, 1987a).
Methods have been described for the determination of
alpha-cypermethrin in water, soil, crops, and animal tissues and
fluids (see Table 2).
Table 2. Analytical methods for alpha-cypermethrin in air, soil, water and biological mediaa
Sample Extraction Clean-up Detection and Recovery Limit of References
quantification determination
Air 20% ethyl acetate column chromatography gas chromatography with - 0.01 µg/filter Armitage (1984)
in hexane chromosorb W.HP. electron capture detection
Surface 20% ethyl acetate column chromatography gas chromatography with - 30 µg/filter Armitage (1984)
wipe in hexane chromosorb W.HP. flame ionization detection
Soil anhydrous sodium liquid-solid packed column gas 95-100%b 10 µg/kg Shell (1990b)
sulfate with chromatography chromatography, electron capture
acetone/hexane using Florisil detection; confirmation by
capillary GC and packed column
GC-MS
Water solvent partition Florisil disposable capillary gas-liquid 80-100%c 0.01 µg/litre Shell (1990a)
with hexane cartridge chromatography, electron-capture
detection; confirmation by GC-MS
Crops anhydrous sodium partition between packed column gas 90-100%b 10 µg/kg Shell (1989a)
sulfate with hexane and water/ chromatography, electron capture
acetone/hexane acetonitrile; liquid- detection; confirmation by
solid chromatography capillary GC and packed
using Florisil column GC-MS
Animal acetone/hexane partition with gas-liquid chromatography, 80-100%d 10 µg/kg Shell (1988a)
tissues mixture acetonitrile or electron capture detection;
hexane-acetonitrile; confirmation by GC-MS
liquid-solid
chromatography on
Florisil
Milk diethyl ether/ cyano Bond Elut gas-liquid chromatography, 90-100%e 1 µg/litre Shell (1988b)
hexane; Extrelut cartridge electron capture detection;
extraction column confirmation by GC-MS
Table 2 (continued)
Sample Extraction Clean-up Detection and Recovery Limit of References
quantification determination
Blood acetone partition with hexane packed column gas - 10 µg/litre Shell (1986)
(rat) (washed with water); chromatography, electron capture
dried with sodium detection; confirmation by
sulfate; liquid-solid capillary GC and GC-MS
chromatography on
Florisil
a Details of the analytical methods are available from Shell International Chemical Company, London. These methods differentiate
between alpha-cypermethrin and the other isomers.
b Over the concentration range 0.05-0.5 mg/kg
c Over the concentration range 0.05-0.5 µg/litre
d Concentrations 0.1-0.2 mg/kg
e Over the concentration range 0.005-0.02 mg/litre
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Alpha-cypermethrin does not occur in nature.
3.2 Anthropogenic sources
3.2.1 Production levels and processes
Alpha-cypermethrin is manufactured from cis-2,2,dimethyl-3-
(2",2"-dichlorovinyl)-cyclopropane carboxylate ( cis-DVO), 3-phenoxy
benzaldehyde (POAL) and sodium cyanide.
After removal of the solvent, the cis-cypermethrin is
epimerized into alpha-cypermethrin. Solid alpha-cypermethrin crystals
separate and are filtered, washed and dried under vacuum before
drumming-off1.
No data are available on production levels.
3.2.2 Use
Alpha-cypermethrin has been available commercially since late
1983. It is a potent insecticide effective against a wide range of
pests, particularly Lepidoptera and Coleoptera in citrus, cotton,
forestry, fruit, rice, soybeans, tomatoes, vegetables, grapes and
other crops, at a concentration of 5-30 g active ingredient per ha.
Good control of plant-sucking Hemiptera can also be obtained if the
insecticide is applied before populations have become established. It
also controls soil-dwelling Lepidoptera.
Alpha-cypermethrin can be used in most crops for either curative
or preventive treatment. It can replace conventional insecticides in
short-interval spray programmes, or the longer residual performance
may be exploited to reduce the number of sprays per season. Either
option may be chosen since no reports of phytotoxicity have been
received even when sensitive crops have been involved in repeated
applications. It controls ectoparasites ( Boophilus microplus at a
concentration of 50 mg/litre), including strains resistant to
organophosphorus pesticides, as well as sheep lice and Melophagus
ovinus.
1 Manufacturing process of alpha-cypermethrin; Shell International
Chemical Company; letter dated 10 January 1989 (ref. CTMAR/4)
Rapid knockdown and residual control of biting flies in and
around animal housing have been obtained following direct spray
application to animals or structural surfaces. Furthermore,
alpha-cypermethrin controls Blattellidae, Culicidae, flies and other
nuisance or disease-carrying insects, at a level of 10-30 mg/m2,
with good persistence on most surfaces (Fisher et al., 1983; Worthing
& Hance, 1991).
Alpha-cypermethrin is available as an emulsifiable concentrate,
ultra-low-volume formulation and suspension concentrate (flowable
formulations). Mixtures with organophosphorus and carbamate
insecticides have also been developed. Details of formulations are
given in section 2.3.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
Data relevant to alpha-cypermethrin can be found in Environmental
Health Criteria 82: Cypermethrin (WHO, 1989).
4.1.1 Air
No information on the transport of alpha-cypermethrin in air is
available, but its volatility is very low.
4.1.2 Water
Alpha-cypermethrin as an emulsifiable concentrate (EC) was
sprayed from the air (15 g active ingredient/ha) to a field along one
side of which ran a freshwater ditch. The fate and biological effects
of spray drift in the ditch were monitored for 7 weeks after the
application (see sections 9.2.1.2 and 9.2.2.2). Deposition on the
surface of the ditch was around 5 g active ingredient/ha (30% of the
nominal application rate). Alpha-cypermethrin concentrations in the
sub-surface water were 0.6 µg/litre shortly after the application and
decreased to < 0.02 µg/litre within 2 to 4 days. No contamination of
the water was found 200 m beyond either end of the treated field
(Garforth & Woodbridge, 1984).
Two freshwater ponds were treated with an EC formulation of
alpha-cypermethrin in 1987. One pond was oversprayed with 15 g active
ingredient/ha, while the other was treated with the same amount of
alpha-cypermethrin but by direct incorporation into the water. A third
pond served as a control. One day after the treatment, 5% of the
applied substance was found in the surface film of the oversprayed
pond and 19% in the sub-surface water. Residue levels in both
compartments subsequently declined rapidly so that one week later only
0.1 and 2% were still present, respectively. In the pond that received
direct treatment, 37% of the applied alpha-cypermethrin was found in
the sub-surface water one day after treatment. The concentration
subsequently declined more rapidly than in the oversprayed pond so
that one week later only 2% was present. The concentration of alpha-
cypermethrin found in the sediment samples from both ponds 16 days
after treatment indicated that approximately 5% of the
alpha-cypermethrin applied was present at that time. Thereafter the
concentration decreased and was less than 3% in the sediment 33 days
after application. In a bioassay test, the water in both ponds was
found to be acutely toxic to Gammarus pulex for at least 4 days
after application. After a further 29 days, the water was no longer
acutely toxic. The sediment was not toxic to Gammarus pulex
(Pearson, 1990).
4.1.3 Soil
A trial in the United Kingdom (Reculver) investigated the decay
of alpha-cypermethrin in sandy-clay soil treated with a diluted EC
formulation at a dosage rate of 0.5 kg active ingredient per ha.
Samples of soil were taken from the 0-15 cm layer of each plot at
various intervals over a period of one year. Once a year a sample was
also taken from the 15-30 cm layer. The residue immediately after the
application was 0.07 mg/kg soil in the 0-15 cm layer, and within 2
weeks this had declined by 50%. The residues of alpha-cypermethrin in
samples from the 0-15 cm layer and 15-30 cm layer taken 40 weeks and
52 weeks after application were below the limit of determination, i.e.
0.01 mg/kg (Forbes & Knight, 1983).
After one year, a second application to the bare soil was made
and again a diluted EC formulation was applied at a dosage rate of 0.5
kg active ingredient/ha. Samples were taken at various intervals
during this second year. Residues of alpha-cypermethrin in the 0-15 cm
soil layer declined from 0.19 mg/kg immediately after treatment to
0.11 mg/kg after 2.1 weeks and < 0.01 mg/kg after 49 weeks. In
samples from the 15-30 cm layer no residues (< 0.01 mg/kg) were
detectable 23 and 49 weeks after application (Forbes & Burden, 1984).
In the third year of the trial, another application to the same plots
was made with the EC formulation at a dosage rate of 0.5 kg active
ingredient/ha. Residues of alpha-cypermethrin in the 0-15 cm layer
declined from 0.20 mg/kg immediately after treatment to 0.08 mg/kg
after 18 weeks and 0.01 mg/kg after 52 weeks. Residues were not
detectable in the 15-30 cm layer sampled after 32 and 52 weeks. Over
the three years of the trial there was no indication of a build-up of
alpha-cypermethrin residues in the surface soil layer or any evidence
to suggest leaching of the compound into sub-surface soil layers
(Forbes & Wales, 1985a).
A further trial was carried out in the United Kingdom (Coates) to
study the decay of alpha-cypermethrin applied to a peat type soil as
a diluted EC formulation at a dosage rate of 0.5 kg active
ingredient/ha. As in the Reculver study, residues were determined in
the 0-15 cm layer at various intervals and in the 15-30 cm layer 32
weeks after application. At the beginning of the second and third
year, one application was made as at the beginning of the first year.
The residue in the 0-15 cm layer immediately after the first
application was 0.65 mg/kg declining to 0.36 mg/kg within 2 weeks and
to 0.30 mg/kg after 8 weeks. After 16 weeks, the residue was 0.05
mg/kg or less. In the 15-30 cm layer, no residues were found after 32
weeks (Forbes & Mackay, 1983). Immediately after the second
application, the residue in the 0-15 cm layer was 0.65 mg/kg; after
two weeks the level was 0.36 mg/kg and declined to 0.07 mg/kg by 48
weeks after application. No residues were found in the 15-30 cm layer
(Forbes & Wales, 1985b).
In the third year, a residue level of 0.55 mg/kg was found in the
0-15 cm layer immediately after treatment, declining to 0.20 mg/kg
within 8 weeks and to 0.09 mg/kg after 50 weeks. In the 15-30 cm
layer, residues of 0.01 and 0.03 mg/kg were found after 40 and 50
weeks respectively. In this 3-year trial there was no indication of a
build-up of alpha-cypermethrin residues in the surface soil layers, or
any evidence to suggest significant leaching into sub-surface soil
layers (Coveney & Forbes, 1986).
4.2 Biotransformation
4.2.1 Biodegradation
Alpha-cypermethrin has been tested for "ready biodegradability"
in two tests: a) the closed bottle and modified Sturm test, and b)
growth inhibition in a Pseudomonas fluorescens growth test. In these
tests, mineralization of alpha-cypermethrin was not detected. It was
not degraded in these two tests and hence is not considered to be
readily biodegradable (Stone & Watkinson, 1983).
Maloney et al. (1988) studied the microbial transformation of
technical alpha-cypermethrin (96.3% pure) in aerobic batch enrichment
cultures. These microbial enrichments, which contained Pseudomonas
fluorescens (SM-1), Achromobacter sp. and Bacillus cereus, were
able to transform alpha-cypermethrin with a half-life of 7 to 14 days
at a concentration of 50 mg/litre in the presence of 0.05% Tween 80
(v/v). One of the major transformation products was 3-phenoxybenzoic
acid, which was further transformed to 4-hydroxy-3-phenoxybenzoic
acid.
McMinn (1983a) investigated the degradation under aerobic
conditions of alpha-cypermethrin, labelled with 14C in the benzyl
ring, in two types of soil, i.e. sandy clay loam and clay loam. The
soils were treated with 1 mg of the labelled material and gently
agitated to distribute the insecticide. Soils samples were removed for
analysis 2.5, 6, 10, 20 and 42 weeks after treatment. The initial
degradation half-lives were 27 and 13 weeks for sandy clay loam and
clay loam, respectively. However, after 42 weeks the percentage of
applied radioactivity remaining unchanged was 28.9 and 21.6%,
respectively, for the two soils. The formation of total organo-soluble
products after 42 weeks was 32.2 and 24.3% for sandy clay loam and
clay loam, respectively. Total extractable and total non-extractable
radioactivity for sandy clay loam was 32.5 and 18.0% and for clay loam
25.3 and 32.0%, respectively. Metabolites were found in both cases at
levels of 2 to 3%. Unchanged alpha-cypermethrin was present, and the
degradation products had similar chromatographic mobilities to the
previously identified major products of cypermethrin (McMinn, 1983b).
4.2.2 Bioaccumulation
The n-octanol/water partition coefficient of alpha-cypermethrin
is 1.4 x 105 (log Pow = 5.16), compared to a value for
cypermethrin of 2 x 106 (log Pow = 6.3). The actual
bioaccumulation in fish found experimentally for cypermethrin is lower
than might be expected from the partition coefficient. This should
also apply to alpha-cypermethrin, because the pathway and rate of
metabolism are comparable with those of cypermethrin (Shell, 1983b;
WHO, 1989).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
Information relevant to alpha-cypermethrin was given in
Environmental Health Criteria 82: Cypermethrin (WHO, 1989).
5.1.1 Soil
In a study on the deposition of alpha-cypermethrin on the orchard
floor following commercial application to apple trees,
alpha-cypermethrin (100 g EC/litre) was applied at a nominal dose rate
of 26 g/ha using a tractor-driven "Kinkelder" mist-blower. Following
normal practice, spray runs were made between each row of trees and
then around the perimeter of the orchard. One hour after spraying,
pesticide deposits were collected in foil-lined trays positioned on
the orchard floor and analysed. Deposition was found to be variable,
ranging from 10 to 76% of the nominal application rate (Hillaby,
1988).
5.2 Food
5.2.1 Crops
Residue data on cypermethrin have been evaluated by the Joint
FAO/WHO Meeting on Pesticide Residues (FAO/WHO, l980, 1982).
Alpha-cypermethrin application rates to crops range from 5 to 30
g active ingredient/ha. Residue data have been obtained from
supervised trials in many countries. The residue concentrations of
alpha-cypermethrin derived from recommended application rates vary
from 0.05 to 1.0 mg/kg product (Shell, 1984).
A study was carried out to determine whether there was
significant isomerization of alpha-cypermethrin after treatment of
certain crops. Grapes were treated with 10% EC applied at a rate of 18
g active ingredient/ha, and apples and lettuce with 10% EC at 15 g
active ingredient/ha. Samples were taken 3 and 7 days (grapes), 7 days
(apples) and 10 days (lettuce) after treatment. Residues of 0.17 and
0.09 mg/kg were found on grapes, 0.05 mg/kg on apples and 0.17 mg/kg
on lettuce, but none of the samples showed any significant isomer
conversion of alpha-cypermethrin (Bosio, 1982).
In 1983 two trials were carried out in Canada in which
alpha-cypermethrin was applied with a knapsack sprayer to maize
(sweetcorn). Five applications with diluted 10% EC formulations at a
dosage rate of 20 g active ingredient/ha were made, samples were
harvested 7 days after the last application, and the husks, grain and
cobs were analysed separately. Alpha-cypermethrin residues of 0.38
mg/kg were found in the husks, but no residues (limit of
determination, 0.01 mg/kg) were found in the grain or the cobs (Forbes
& Cole, 1986).
5.2.2 Fish
To reduce blow-fly infestations during the curing of marine
catfish, the fish were dipped in EC solutions (15 g/litre) at various
concentrations (0.001-0.05% active ingredient w/v) between the salting
and drying stages of the curing process. Dipping after the salting
stage in a 0.001% solution of the EC proved to be effective. The
levels of residues in treated fish were dependent on the season (wet
and dry season), storage time, concentration of the dip solution and
the size of the fish. In the wet season, the range was from 0.9 to 2.8
mg/kg, whereas in the dry season it was from 0.26 to 30.0 after one
week of storage and 0.22 to 4.0 mg/kg (wet weight of homogenized fish)
after 15 weeks of storage (Forbes, 1985).
5.2.3 Milk
A trial was carried out in 1987 in the United Kingdom where
lactating cows were treated with pour-on formulations of
alpha-cypermethrin. Two formulations were used containing either 10
g/litre or 15 g/litre (see also section 6.1.2). Either 10 ml or 20 ml
of formulation containing 0.1, 0.15 or 0.2 g active ingredient was
applied along the mid-dorsal line of five cows for each treatment.
Milk samples were taken 1, 2, 3, 4, 7, 14 and 21 days after treatment
for the analysis of alpha-cypermethrin. The residues of
alpha-cypermethrin in milk were at a maximum from 2 to 4 days after
treatment. Generally, residues were highest in the 0.2 g group, the
maximum concentration being 0.005 mg/litre (in two samples only). By
day 21 the residues in the milk from all treated cows were < 0.002
mg/litre (the limit of determination) (Sherren, 1988b).
5.3 Human exposure
In a study to quantify the maximum potential dermal exposure of
operators to crop protection products, 13 exposure pads were mounted
on each of three operators and chemicals adhering to gloves were
analysed. The operation involved three distinct stages: mixing product
and loading the tractor; spraying; and washing-up the equipment and
tractor after the exercise. The total dermal exposure for the three
operators was: mixing/loading, 2.45, 0.57 and 2.94 mg/operation;
spraying, 0.38, 0.61 and 0.40 mg/h; and washing-up, 0.12, 0.29 and
0.73 mg/operation (Senior & Lavers, 1990a,b).
6. KINETICS AND METABOLISM
Both cis and trans isomers of cypermethrin are metabolized via
cleavage of the ester bond to phenoxybenzoic acid (PBA) and
cyclopropane carboxylic acid (CPA). The PBA moiety is mainly excreted
as a conjugate. The type of conjugate differs in a number of animal
species. PBA is further metabolized to a hydroxy derivative and
conjugated as a glucuronate or sulfate. The CPA moiety is mainly
excreted as a glucuronate. Consistent with the lipophilic nature of
cypermethrin, the highest tissue concentrations are found in body fat,
skin, liver, kidneys, adrenals and ovaries. The elimination from fat
is approximately 3 to 4 times slower for the cis isomers than for the
trans isomers (WHO, 1989).
6.1 Absorption, elimination, retention and turnover
6.1.1 Rats
Alpha-cypermethrin labelled in the 14C-benzyl moiety has been
studied in Wistar rats at a concentration of approximately 2 mg/kg
body weight in corn oil. The compound, which was given by stomach
tube, was rapidly broken down and the radioactivity was mainly
eliminated in the urine as the sulfate conjugate of
3-(4-hydroxyphenoxy)benzoic acid (40-45% of the dose). Approximately
35% of the dose was eliminated in the faeces, 20% of which was
unchanged alpha-cypermethrin. The proportion of the dose excreted in
the urine and faeces within the first 24 h was approximately 78% and
within 4 days was 90%. Residues in major organs and tissues of rats 4
days after a single oral dose were in general low: liver, 0.03 and
0.05; skin, 0.04 and 0.02; adrenals, 0.03 and 0.06; and kidneys, 0.02
and 0.02 (values are expressed as mg equivalent of
alpha-cypermethrin/kg tissue for females and males, respectively).
However, in body fat, higher residues were found (0.22 and 0.42
mg/kg). The release from skin and fat was biphasic in nature. The
half-life of elimination of radioactivity from fat was approximately
2.5 days for the initial phase and 17-26 days for the slower phase
(the half-life of elimination from fat for cis-cypermethrin was 18.9
days). The half-life values for skin were 2 days for the initial phase
and 40 days for the slower phase. The radioactivity in liver and
kidneys was eliminated apparently by a monophasic process. More than
95% of the residue in fat was present as unchanged alpha-cypermethrin
(Hutson, 1982; Logan, 1983; Hutson & Logan, 1986).
6.1.2 Domestic animals
In a study by Francis & Gill (1991), a formulation containing a
mixture of flufenoxuron and alpha-cypermethrin was applied to groups
of three sheep. The formulation was applied once, either as a dip
diluted at 1:1000 to give a solution of 80 mg flufenoxuron per litre
and 60 mg alpha-cypermethrin/litre or as a pour-on solution applied
directly to the backs of the sheep giving a dose of 0.15 g active
ingredient flufenoxuron and 0.2 g alpha-cypermethrin per sheep. The
sheep were killed at 3, 7 and 14 days after application and samples of
subcutaneous fat, fleece and sheep skin were analysed. The residues of
alpha-cypermethrin in fat ranged from < 0.01 to 0.04 mg/kg and in
skin from 0.02 to 1.4 mg/kg over the three sampling periods, and they
were lower for pour-on formulations than for the dip. Highest tissue
residues were found in wool (sampled from the back); these were (for
the 3, 7 and 14 day sampling periods, respectively) 730, 1020 and 360
mg/kg for dip application and 360, 440 and 360 mg/kg for pour-on
application. Wool sampled from the side of sheep treated with pour-on
formulation were 10 to 30 times lower than wool sampled from the back
region; with the dip solution, however, wool from the side region
contained higher residues than that from the back. Pour-on application
gave lower residues than after a dip.
A trial was carried out during 1987 in the United Kingdom in
which Friesian/Hereford calves (in total 17 female animals) were
treated with an alpha-cypermethrin pour-on formulation. Ten ml of a 16
g/litre formulation was applied to calves along the middorsal line
from shoulder to tail. At 3, 7 and 14 days following treatment,
animals were sacrificed for analysis of tissues, i.e. perirenal and
subcutaneous fat, muscle, kidneys and liver. No residues were detected
in muscle and liver samples at any time (limit of determination, 0.01
mg/kg). In the kidneys a maximum of 0.03 mg/kg was found on day 7 but
by day 14 the residues had decreased to 0.01 mg/kg or less. The fat
tissues contained maximum levels on day 7, i.e. mean concentrations of
0.26 mg/kg (perirenal fat) and 0.08 mg/kg (subcutaneous fat). By day
14 these concentrations had decreased by about two and a half times
(Sherren, 1988a) (see also section 5.2.3).
6.1.3 Humans
Six volunteers (two per dose level) received a single oral dose
of 0.25, 0.5 or 0.75 mg alpha-cypermethrin and, after a period of 2-3
weeks, five successive daily doses of 0.25, 0.5 or 0.75 mg to study
the urinary excretion and bioaccumulation of alpha-cypermethrin. A
parallel study with cypermethrin itself was carried out for comparison
purposes. The metabolism and rate of excretion of a single oral dose
of alpha-cypermethrin were similar to those of cypermethrin itself.
The rate of excretion was dose-related, approximately 43% of the dose
of alpha-cypermethrin being excreted in the urine as free or
conjugated cis-cyclopropane carboxylic acid ( cis-CPA) during the
first 24 h. Urinary excretion did not increase with repeated oral
dosing; an average of 49% of alpha-cypermethrin was excreted in the
urine as free or conjugated cis-CPA within 24 h (van Sittert et al.,
1985; Eadsforth et al., 1988).
6.2 Metabolic transformation
In a study on Wistar rats using alpha-cypermethrin,
14C-labelled in the benzyl moiety, (see section 6.1.1) no evidence
was found for any racemization of the chiral centres of
alpha-cypermethrin in the residues in intestines, faeces or fat. The
major urinary metabolite was the sulfate conjugate of
3-(4-hydroxyphenoxy)benzoic acid, and smaller amounts of
3-phenoxybenzoic acid (II) and 3-(4-hydroxyphenoxy)benzoic acid (III)
were identified. In the faeces, 75% of the radioactivity in the
extract was unchanged alpha-cypermethrin; minor metabolites included
a dihydroxy metabolite (V), 3-(4-hydroxyphenoxy)benzoic acid (III),
3-phenoxybenzoic acid (II) and the 4-hydroxyphenoxy metabolite (IV).
In the adipose tissue, the 14C label was mainly associated with
unchanged alpha-cypermethrin, but a lipophilic metabolite of either
alpha-cypermethrin or 3-phenoxybenzoic acid, probably a mixture of
3-phenoxybenzoyl diacylglycerols, was also present (Hutson, 1982;
Logan, 1983; Hutson & Logan, 1986) (see Fig. 2).
6.3 In vitro metabolic transformation
Creedy & Logan (1984) studied the in vitro metabolism of
cypermethrin and alpha-cypermethrin using liver microsomal
preparations from rats, rabbits and humans. In order to obtain
information on the relative importance of the oxidative and esteric
pathways of degradation of these compounds, incubations were carried
out both in the presence and absence of an NADPH-generating system.
Both cypermethrin and alpha-cypermethrin were broken down via esteric
and oxidative pathways by the liver preparations from the three
species. For rabbit and human liver microsomes, oxidation was a minor
metabolic route compared to esteric hydrolysis in the case of both
compounds. Human liver microsomes were able to carry out the esteric
hydrolysis of alpha-cypermethrin slightly faster than cypermethrin. In
the liver preparations of all three species, cyclopropane carboxylic
acid (mainly produced via the esteric pathway) was the main metabolite
for both compounds (to the extent of approximately 90-99%). Via the
oxidative route, mono-hydroxycypermethrins, dihydroxy-cypermethrin and
small amounts of hydroxycyclopropane carboxylic acid (rat only) were
also produced.
 6.4 Plants
The metabolism of cypermethrin in plants is described in WHO
(1989).
The degradation of alpha-cypermethrin and cypermethrin in
cabbages grown to maturity outdoors has been studied. Eighteen days
after transplanting, the cabbages were treated three times with
14C-labelled alpha-cypermethrin or cypermethrin as an EC
formulation. Each treatment consisted of 1.8 mg equivalent with a
spray concentration of 36 g/litre. This treatment was repeated after
11 and 27 days. Each box of cabbages received a total application of
5.4 mg at a dose rate equivalent to 50 g active ingredient/ha. At
harvest (3 months later) the plants were separated into old and new
outer leaves, heart, stalk and roots. No major differences between the
two compounds in distribution of radioactivity throughout the plants
or in the metabolic profile were observed. The highest radioactive
residues were present in the old outer leaves (23% for
alpha-cypermethrin and 27% for cypermethrin), lower levels being found
in new outer leaves, stalk, roots and heart. Very low levels (< 0.05
mg/kg) of both compounds were found in the soil. The major radioactive
residue at harvest was shown to be the pesticide, which was either in
the unchanged form or had undergone cis/trans-isomerization,
presumably photochemically. The profiles of the organosoluble
metabolites were similar, and the major products of alpha-cypermethrin
had chromatographic mobility similar to previously identified products
of cypermethrin metabolism, such as 3-phenoxybenzoic acid and
3-phenoxybenzyl alcohol, partly hydroxylated and/or conjugated. These
compounds were found in minor quantities (McMinn, 1983a; WHO, 1989).
Appraisal
A wide range of studies in mice, rats, dogs, sheep, cows and
humans has shown that cypermethrin is rapidly absorbed, distributed
to a variety of organs and tissues, metabolized and rapidly excreted
from the body (WHO, 1989). There are no major differences in the
absorption, distribution, retention or excretion between the species.
Differences, where they do occur, are related to the rate rather than
the nature of the metabolites formed and the conjugation reactions.
Cypermethrin, both the cis and trans isomers, and
alpha-cypermethrin are primarily metabolized by cleavage of the ester
bond. The metabolites PBA and CPA are mainly excreted as conjugates.
The type of conjugate differs in a number of animal species dosed
with cypermethrin, but humans and rats have the same pathway. Minor
quantities of hydroxylated PBA (conjugated) may also be found. The
terminal half-life of elimination of alpha-cypermethrin from the fat
of rats is 17-26 days, compared to 18.9 days for cis-cypermethrin.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Oral (technical product)
Alpha-cypermethrin is moderately to highly toxic and 3-4 times
more toxic than cypermethrin.
The clinical signs of toxicity observed in the various acute
toxicity studies on experimental animals with alpha-cypermethrin are
typical for a cyano-containing pyrethroid intoxication. They included
ataxia, abasia, gait abnormalities, choreoathetosis, "tip-toe" walk,
and increased salivation, lacrimation, piloerection, tremor and clonic
convulsions. The majority of the mortalities occurred within the first
3 h and surviving animals recovered within 7 days (Rose, 1982, 1983a).
In the study with alpha-cypermethrin administered in corn-oil
(Rose, 1983a), clonic convulsions, piloerection, salivation and
splayed hind-leg gait were found. The oral LD50 values for
alpha-cypermethrin are summarized in Table 3.
Table 3. Oral LD50 values for technical alpha-cypermethrin
Species Concentration and LD50 in mg/kg body Reference
(strain) vehicle weight (with 95%
confidence limits)
Mouse 5% in corn oil 35 (26-48) Rose (1982)
(CD) 40% in DMSO 762 (514-912) Rose (1982)
50% aqueous suspension 798 (568-1074) Rose (1982)
Rat 5% in corn oil 79 (63-98) Dewar (1981)
(Wistar) 40% in DMSO approximately 4000 Rose (1982)
50% aqueous suspension > 5000 Rose (1982)
Rat 10% in corn oil 40-80 Rose (1983a)
(Wistar) 20% in corn oil 368 (282-487) Rose (1983a)
Woollen et al. (1991) noted a higher degree of absorption of
cypermethrin when it was applied in corn oil. This could be the
explanation for the higher toxicity of alpha-cypermethrin administered
in corn oil.
7.1.2 Oral (formulations)
Formulations of alpha-cypermethrin have moderate acute oral
toxicity (Table 4). The clinical signs observed after oral
administration to rats are characteristic of cyano-containing
pyrethroid intoxication (see section 7.1.1.). The majority of the
mortalities occurred within 3 days of dosing. The degree of acute oral
toxicity of formulations containing mixtures with other active
ingredients depended on the toxicity of the latter ingredients.
7.1.3 Dermal
Alpha-cypermethrin has low dermal toxicity. No deaths or signs of
intoxication were observed in rats (Dewar, 1981; Shell, 1983a) and
mice (Rose, 1982; Shell, 1983a) receiving a single 24-h dermal
exposure of 500 mg/kg body weight (25% in DMSO) and 100 mg/kg body
weight (5% in corn oil), respectively.
The dermal LD50 values in rats of formulations of
alpha-cypermethrin and of alpha-cypermethrin mixed with another active
ingredient are summarized in Table 4. In all cases, the maximum dose
that could be applied was tested.
With the pour-on formulations, no clinical signs were observed.
Blood around the nose and eyes was the only sign seen in the case of
SC formulations. Clinical signs observed after the application of EC
or ULV formulations of alpha-cypermethrin included increased
lacrimation, chromodacryorrhoea and unkempt appearance, aggressiveness
and diarrhoea. The Fastac/BPMC formulation caused the same signs of
intoxication and also oedema at the application site. With the
Fastac/methomyl formulation, fasciculation, lethargy, salivation,
piloerection, hunched back, chromodacryorrhoea and cyanosis were
observed. Animals treated with Fastac/Azodrin formulation showed the
above-mentioned symptoms, and some additionally showed ataxia, abasia,
hypothermia, eye pallor and prostration/coma.
7.1.4 Inhalation
Groups of five male and five female albino Fischer-344 rats were
exposed for 4 h to a dust atmosphere containing 30% (m/m)
alpha-cypermethrin on silica powder at an average concentration of 1.3
g/m3 (equivalent to 0.4 g active ingredient/m3). The mass media
diameter of the dust particles was 4.2 µm (geometric standard
deviation 6.4). The animals were observed for 14 days after the
exposure but there were no signs of intoxication. Macroscopic
examination of the lungs did not reveal any effects. Thus, the acute
LC50 was > 1.3 g 30% silica powder dust/m3 or > 0.4 g active
ingredient/m3 (Blair, 1984).
Table 4. Oral and dermal LD50 values for formulated alpha-cypermethrin in rats (Fischer-344)
Formulationa LD50 in mg total formulation per kg Reference
body weight (with 95% confidence limits)
Oral Dermal
100 g/litre EC 101 (82-119) > 1800 Rose (1984d)
100 g/litre EC 136 (98-186) > 1800 Rose (1984e)
100 g/litre EC 174 (125-327) > 2000 Price (1985a)
30 g/litre EC 229 (178-292) > 2000 Rose (1984f)
30 g/litre EC 673 (597-753) > 2000 Rose (1985)
15 g/litre pour-on > 2000 > 2000 Price (1988)
10 g/litre pour-on > 2000 > 2000 Price (1988)
100 g/litre SC 1804 (1507-2168) > 2000 Price (1985b)
60 g/litre SC > 5000 > 2000 Gardner (1991)
15 g/litre SC > 5000 > 2000 Price (1986)
15 g/litre ULV 5838 (5130-6665) > 2000 Rose (1984c)
Mixtures with other active ingredients
Fastac/methomyl EC 58-97 (males) > 1900 Rose (1984h)
15/120 g/litre 73 (51-89) (females) > 1900
Fastac/BPMCb EC 310 (215-462) > 2000 Price (1987)
10/400 g/litre
Fastac/Azodrin EC 25 (18-34) > 2000 Gardner (1989)
20/400 g/litre
a EC = emulsifiable concentrate; SC = suspension concentrate; ULV = ultra-low volume
b BPMC = 2 sec-butylphenyl methylcarbamate (fenobucarb)
7.1.5 Other routes
The acute intraperitoneal LD50 for rats of a 10% solution of
alpha-cypermethrin in corn oil was 3.39 (3.03-3.83) ml/kg body weight,
or 339 mg active ingredient per kg body weight. The surviving animals
showed characteristic pyrethroid signs of intoxication, e.g., ataxia,
abasia, choreoathetosis, gait abnormalities, "tip-toe" walk and
salivation (Rose, 1984a).
7.2 Short-term exposure
7.2.1 Oral
7.2.1.1 Rat
Groups of Wistar rats (10 of each sex at each dose level and 20
of each sex as controls) were fed 0, 25, 100, 200, 400 or 800 mg
alpha-cypermethrin/kg diet (equivalent to 0, 1.25, 5, 10, 20 or 40
mg/kg body weight) for 5 weeks. At 400 and 800 mg/kg diet, signs of
intoxication, such as abnormal gait and hypersensitivity were
observed, and food intake and body weight were decreased in both sexes
compared with the control group. Changes in blood chemistry, e.g.,
decreases in protein and increases in urea levels, were observed in
both sexes of rats fed 800 mg/kg diet and in males fed 400 mg/kg. The
weights of livers and kidneys of both sexes of rats fed 800 mg/kg diet
and livers of male rats fed 400 mg/kg were increased. No
histopathological changes were observed except in the case of one
severely intoxicated male animal fed 800 mg/kg diet, which showed
sparse axonal degeneration in the sciatic nerve. No effects were seen
in the animals fed 200 mg/kg diet for five weeks (Pickering, 1982).
In a 13-week study, Wistar rats (30 males and 30 females per test
group, and a control group consisting of 60 males and 60 females),
which were initially 5 weeks old, were fed 0, 20, 60, 180 or 540 mg
alpha-cypermethrin/kg diet (equivalent to 0, 1, 3, 9 or 27 mg/kg body
weight). After six weeks of feeding, one third of the animals were
killed for interim haematological, clinical chemical and gross
post-mortem examination. The remaining animals were killed after 13
weeks. Signs of intoxication, such as abnormal gait with splayed hind
limbs, were found in 3 out of 20 males fed 540 mg/kg diet. Several
instances of transient skin sores and fur loss were observed
particularly in the rats fed 540 mg/kg. There was decreased growth,
which correlated with decreased food intake, in both sexes fed 540
mg/kg from the first week onwards. No clear effects on the
haematological and clinical chemical parameters were found. Organ
weights were comparable with those of the control animals. No
histopathological abnormalities were found except sparse axonal
degeneration in the sciatic nerve, without clinical signs of toxicity,
in two males fed 540 mg/kg. No axonopathy was observed in the three
animals with abnormal gait. There were marginal effects (decreased
growth during week one and from week 10 onwards) in the males fed 180
mg/kg diet. No effects were found in the 60-mg/kg group (Clark, 1982).
7.2.1.2 Dog
Beagle dogs (one male and one female) were fed alpha-cypermethrin
in the diet at the following concentrations: 200 mg/kg diet for 7
days, 400 mg/kg diet for 2 days, and 300 mg/kg diet for 7 days. With
200 mg/kg no signs of intoxication were observed, whereas dosing with
300 mg/kg or more caused weight loss, ataxia, subdued behaviour, head
nodding, food regurgitation, inflammation of gums and tongue, body
tremors and diminished response to stimuli. Haematological, clinical
chemical and gross pathological examination showed no effects
(Greenough & Goburdhun, 1984).
In a further study, Beagle dogs (one male and one female)
received 300 mg/kg diet for 3 days (male dog) or 4 days (female dog)
and 250 mg/kg diet for 7 days. Both animals showed the above-mentioned
signs of intoxication, the only difference being that when it was
being fed the 250-mg/kg diet the female animal showed these signs more
frequently than the male animal. There were no effects on haematology,
clinical chemical parameters, urinalysis, faecal occult blood test or
gross pathology (Greenough & Goburdhun, 1984).
In a study by Greenough et al. (1984), 36 pure bred Beagle dogs
(18 males and 18 females) received a diet containing
alpha-cypermethrin at 0, 30, 90 or 270 mg/kg diet for 13 weeks. The
group dosed at the highest concentration comprised six males and six
females while the other groups consisted of four males and four
females. All animals fed 270 mg/kg diet exhibited signs of
intoxication, such as whole body tremors, head nodding, "lip-licking",
subduedness, ataxia, agitation and a high-stepping gait. These signs
increased in both intensity and duration as the study progressed. Food
consumption, body weight gain, organ weights, ophthalmoscopy,
haematological and clinical chemical parameters, urinalysis, gross
pathology and microscopy of 18 organs and tissues of all test groups
showed no dose-related effects. In this study, the no-observed-effect
level was considered to be 90 mg/kg diet (equivalent to 2.25 mg/kg
body weight).
7.3 Skin and eye irritation; sensitization
7.3.1 Skin irritation
Undiluted technical alpha-cypermethrin was minimally irritating
when applied as a single occluded dose for 24 h to intact and abraded
rabbit skin (Dewar, 1981).
New Zealand white rabbits were used to study the primary skin
irritation of a number of alpha-cypermethrin formulations. The test
duration was 4 h, the observation period was 7-21 days, and the
formulations tested were 30 and 100 g/litre EC, 15 g/litre ULV, 10 and
15 g/litre pour-on formulation, 15 and 100 g/litre SC, and
Fastac/methomyl (15/120) EC. The EC formulations caused mild to
moderate skin irritation. Superficial necrosis was observed in one or
two animals treated with 100 g/litre EC, but there was no permanent
in-depth skin damage. The effects persisted for up to 7 days. The EC
formulations and the 100 g/litre SC formulation were classified as
mildly irritating. All other formulations tested were either
non-irritating or only slightly irritating (Rose, 1984c,d,e,f,h, 1985;
Price, 1985a,b, 1986, 1988).
7.3.2 Eye irritation
Undiluted formulations were tested for their eye irritancy
potential in groups of six rabbits using the Draize test. All the EC
formulations tested (30 or 100 g/litre) caused severe eye irritation,
including corneal opacity and damage to the iris (Rose, 1984d,e,f,
1985).
When an EC formulation (100 g/litre) and its components, both in
the undiluted form and at typical in-use dilutions (1 in 400 and 1 in
1333 aqueous dilution), were tested for eye irritancy potential, the
undiluted formulation was severely irritating, with or without
irrigation, while the undiluted blank formulations were mildly to
severely irritating. The diluted test formulations, with or without
alpha-cypermethrin or with emulsifier, were non-irritating. It was
concluded that the eye irritation resulted from the combined
formulation ingredients (especially the emulsifier) and that
alpha-cypermethrin per se gave only slight irritation, if any
(Dewar, 1981; Rose, 1984b). In-use dilutions (0.0075%) of another 100
g/litre EC formulation and its blank formulation were non-irritating
(Rose, 1984g).
Two pour-on formulations (10 and 15 g/litre) caused moderate and
slight conjunctival inflammation, respectively. The 10 g/litre
formulation was considered to be an eye irritant (Price, 1988). Two SC
formulations (15 and 100 g/litre) were mildly irritating, causing
slight conjunctival redness and chemosis (Price, 1985b, 1986). A 15
g/litre ULV formulation was mildly irritating to rabbit eyes and there
was a moderate initial pain response (Rose, 1984c). An EC formulation
containing Fastac/methomyl (15:120 g/litre) was a severe eye irritant.
The vascularization of the cornea and iritis were considered to be
irreversible (Rose, 1984h).
7.3.3 Sensitization
Technical alpha-cypermethrin was tested in the guinea-pig
maximization test of Magnusson and Kligman using groups of 10 male and
10 female guinea-pigs and a control group of 55 animals of each sex.
The following concentrations were used: intradermal injection, 0.05%
(v/v) in corn oil; topical application and challenge, 50% (m/m) in
vaseline. On the basis of the negative results it was concluded that
alpha-cypermethrin is not a skin sensitizer in guinea-pigs (Dewar,
1981).
An EC formulation (100 g/litre) and its corresponding blank were
tested, as a 50% solution in corn oil, in the Buehler guinea-pig
sensitization test. The topical challenge was carried out with a 30%
solution in corn oil. None of the animals showed positive responses at
24 or 48 h after the challenge (Rose, 1984g).
7.4 Long-term and carcinogenicity studies
No long-term or carcinogenicity studies have been conducted with
alpha-cypermethrin.
7.5 Reproduction, embryotoxicity and teratogenicity
Alpha-cypermethrin has not been tested for reproductive effects
or teratogenicity.
From the available reproduction and teratogenicity studies with
cypermethrin it is clear that no influence on reproduction performance
occurs at a level of 100 mg/kg diet, nor are there any teratogenic
effects even with dose levels high enough to cause maternal toxicity
(WHO, 1989). Furthermore, the no-observed-effect level of cypermethrin
for reproduction and teratogenicity is comparable with the
no-observed-effect levels based on other parameters of toxicity. In
consequence, there is no reason to believe that alpha-cypermethrin,
consisting of two cis isomers also present in cypermethrin, would
behave differently.
7.6 Mutagenicity and related end points
7.6.1 Mutation
The results of the various mutagenicity studies with
alpha-cypermethrin are summarized in Table 5.
Alpha-cypermethrin (in DMSO) at concentrations of 31.25, 62.5,
125, 250, 500, 1000, 2000 or 4000 µg/ml did not increase reverse gene
mutation (at the arg 4-17, trp 5-48 or hom 3-10 markers) in log- or
stationary-phase cultures or forward mutation (to cyclo-heximide
-resistance) in log-phase cultures of Saccharomyces cerevisiae XV
185-14C, either in the presence or absence of rat-liver S9 fraction.
Concentrations of 10 and 50 µg/ml 4-nitroquinoline- N-oxide and 1250
and 5000 µg/ml cyclophosphamide were used as positive controls
(Brooks, 1984).
Alpha-cypermethrin (in DMSO) at concentrations of 31.25, 62.5,
125, 250, 500, 1000, 2000 or 4000 µg/plate, both with and without
microsomal activation, did not increase reverse mutation rates in
Salmonella typhimurium TA98, TA100, TA1535, TA1537 and TA1538 or in
Escherichia coli WP2 and WP2 uvr A. Mitotic gene conversion was not
induced in liquid suspension cultures of log-phase cells of
Saccharomyces cerevisiae JD 1, dosed with solutions of
alpha-cypermethrin at concentrations of 10, 100, 500, 1000 or 5000
µg/ml, both in the presence or absence of a rat liver S9 fraction.
These studies were carried out in comparison with four positive
control compounds (Brooks, 1982).
7.6.2 Chromosomal effects
In a study by Clare & Wiggins (1984), groups of five male and
five female Wistar rats were administered a single oral dose of 2, 4
or 8 mg alpha-cypermethrin in 5% corn oil/kg body weight and killed 24
h after dosing. The control group received corn oil alone.
Cyclophosphamide was used as a positive control. Alpha-cypermethrin
caused no increase in the incidence of chromatid or chromosome
aberrations or polyploidy in bone marrow cells.
Alpha-cypermethrin in aqueous carboxymethylcellulose at
concentrations of up to 40 µg/ml did not increase the frequency of
chromatid gaps, chromatid breaks or total chromatid aberrations in rat
liver (RL4) cell cultures (Brooks, 1982).
7.6.3 DNA damage
Alpha-cypermethrin in DMSO (20%) was administered to Wistar rats
as a single oral dose of 40 mg/kg body weight. The exposure time was
6 h. Alpha-cypermethrin failed to produce any detectable DNA
single-strand damage using alkaline elution profiles of liver DNA.
Methylmethane sulfonate was used as a positive control and DMSO as the
solvent control (Wooder, 1982).
7.6.4 Conclusion
From the available data on alpha-cypermethrin, it can be
concluded that this compound is non-mutagenic in tests with
Salmonella typhimurium, Saccharomyces cerevisiae, and in vivo and
in vitro tests with rat liver cells for the induction of chromosome
aberration and production of DNA single-strand damage.
Table 5. Mutagenicity tests on microorganisms
Organism/strain Dose Type of test Metabolic Result Reference
activation
Salmonella typhimurium up to 4000 µg/plate plate with or negative Brooks (1982)
TA98, TA100, TA1535, without
TA1537, TA1538
Escherichia coli up to 4000 µg/plate plate with or negative Brooks (1982)
WP2, WP2 uvrA without
Saccharomyces cerevisiae up to 5000 µg/ml liquid suspension with or negative Brooks (1982)
JDI culture without
Saccharomyces cerevisiae up to 4000 µg/ml liquid suspension with or negative Brooks (1984)
XV 185-14C culture without
Rat liver cells (RL4) up to 40 µg/ml negative Brooks (1982)
(chromatid gaps, breaks or
aberrations)
Rat liver DNA one oral dose of negative Wooder (1982)
(DNA single strand damage) 40 mg/kg body weight
Rat bone marrow one oral dose of negative Clare & Wiggins
chromosome study up to 8 mg/kg body (1984)
weight
7.7 Special studies
7.7.1 Skin sensation
It is known that exposure to certain types of pyrethroids can
result in a transient skin sensation in humans (Le Quesne et al.,
1980).
Guinea-pigs received 0.1 ml of a 0.01, 0.1 or 1.0% solution of
alpha-cypermethrin in ethanol or a 1, 10 or 20% solution of
alpha-cypermethrin (w/v) in corn oil on the skin. Sensory stimulation
was quantified by counting the number of times each animal turned to
lick or bite its treated flank in preference to the non-treated flank.
Skin stimulation was observed during a 2-h period at all dose levels
except the lowest. In the groups of guinea-pigs treated with 10% and
20%, some animals exhibited an exaggerated hopping movement and a
repeated head shaking activity at the time of maximum skin stimulation
(40-60 min after treatment). This behaviour was not seen with the 1%
solution or more dilute ones (Hend, 1983).
7.7.2 Neurotoxicity
Large oral doses of alpha-cypermethrin and other synthetic
pyrethroids (WHO, 1989) have been shown to produce minor
histopathological lesions in the sciatic nerve of rats, described as
sparse axonopathy in peripheral nerves.
Rose (1983b) conducted a two-phase study. In the first phase, the
time-course for development and recovery from pyrethroid-induced nerve
lesions was investigated by measuring biochemical correlates of
neuropathological change (the enzymes beta-glucuronidase and
beta-galactosidase) in groups of Wistar rats (five of each sex per
group) at periods of 2-12 weeks after the start of dosing. The daily
doses of alpha-cypermethrin (96.6%), administered by stomach tube,
were 37.5 mg/kg body weight for the first 11 doses and 25.0 mg/kg body
weight for the subsequent 9 doses over a 4-week period (5 times/week).
DMSO was used as the solvent for 10 doses and then arachis oil. In
all, 21% of the animals died and more than 80% of the treated animals
showed clinical signs of intoxication. Maximum enzyme activities in
the sciatic posterior tibial nerves were found 5 weeks after the start
of the experiment and had returned to control values by 12 weeks. In
the trigeminal nerve and trigeminal ganglia, a slight but not
significant increase in enzyme activities was found.
In the second phase of the study, 10 male and 10 female Wistar
rats were given 20 oral doses of alpha-cypermethrin in DMSO (0, 10, 20
or 40 mg/kg body weight per day) over a period of 4 weeks (5
times/week). Only two animals died, one given 10 mg/kg and one given
20 mg/kg. In the 40-mg/kg group, 75% of the animals developed clinical
signs, whereas in the 20-mg/kg group only 25% of the animals showed
these clinical effects. In the 10-mg/kg group, the animals showed no
differences from the controls. No clear influence of
alpha-cypermethrin on growth was found. Five weeks after the initial
dose, which corresponded to the period of maximal enzyme changes,
biochemical changes (increases of up to 60%) indicative of a mild
axonal degeneration were found in both the distal and proximal
sections of the sciatic posterior tibial nerve in animals administered
40 mg/kg body weight. In the 20-mg/kg group, only a small (up to 20%)
increase in beta-galactosidase activity was found in the proximal
sections of the sciatic posterior nerve. The same trends were found in
the trigeminal nerve and ganglia. No changes were found in the
10-mg/kg group (Rose, 1983b).
7.7.3 Immunosuppressive action
No data on the immunosuppressive action of alpha-cypermethrin are
available.
7.8 Mechanism of toxicity - mode of action
The mechanism of toxicity and mode of action of cypermethrin (and
other pyrethroids) are extensively described in section 8.8 of the
Environmental Health Criteria 82: Cypermethrin (WHO, 1989). Recently
Vijverberg & van den Bercken (1990) and Aldridge (1990) summarized
current knowledge of the neurotoxicity and mode of action of the
different pyrethroids (see also Appendix 1).
Pyrethroids induce toxic signs that are characteristic of a
strong excitatory action on the nervous system. Toxic doses generally
cause hypersensitivity to sensory stimuli, and a number of compounds
may induce tingling sensations in the skin. Two distinct toxic
syndromes have been described in mammals. The T-syndrome is induced by
pyrethrins and non-cyano pyrethroids, and the CS-syndrome, induced by
cyano-pyrethroids such as cypermethrin and alpha-cypermethrin, is
characterized by choreoathetosis and salivation.
The available data strongly suggest that the primary target site
of pyrethroid insecticides in the vertebrate nervous system is the
sodium channel in the nerve membrane. Pyrethroids without an
alpha-cyano group cause a moderate prolongation of the transient
increase in sodium permeability of the nerve membrane during
excitation. This results in relatively short trains of repetitive
nerve impulses in sense organs, sensory (afferent) nerve fibres and,
in effect, nerve terminals. On the other hand, the alpha-cyano
pyrethroids (for instance cypermethrin and alpha-cypermethrin) cause
a long-lasting prolongation of the transient increase in sodium
permeability of the nerve membrane during excitation. This results in
long-lasting trains of repetitive impulses in sense organs and a
frequency-dependent depression of the nerve impulse in nerve fibres.
The difference in effects between permethrin (with no alpha-cyano
group) and the two insecticides cypermethrin and alpha-cypermethrin,
which have identical molecular structures except for the presence of
an alpha-cyano group on the phenoxybenzyl alcohol, indicates that it
is this alpha-cyano group that is responsible for the long-lasting
prolongation of the sodium permeability.
Since the mechanisms responsible for nerve impulse generation and
conduction are basically the same throughout the entire nervous
system, pyrethroids may also induce repetitive activity in various
parts of the brain. The difference between the symptoms of poisoning
by alpha-cyano pyrethroids and those of the classical pyrethroids is
not necessarily due to an exclusive central site of action. It may be
related to the long-lasting repetitive activity in sense organs and
possibly in other parts of the nervous system, which, in a more
advance state of poisoning, may be accompanied by a
frequency-dependent depression of the nervous impulse.
Pyrethroids also cause pronounced repetitive activity and a
prolongation of the transient increase in sodium permeability of the
nerve membrane in insects and other invertebrates. Available
information indicates that the sodium channel in the nerve membrane is
also the most important target site of pyrethroids in the invertebrate
nervous system.
Because of the universal character of the processes underlying
nerve excitability, the action of pyrethroids should not be considered
to be restricted to particular animal species or to a certain region
of the nervous system.
Although it has been established that sense organs and nerve
endings are most vulnerable to the action of pyrethroids, the ultimate
lesion that causes death will depend on the animal species,
environmental conditions, and on the chemical structure and physical
characteristics of the pyrethroid molecule.
8. EFFECTS ON HUMANS
8.1 General population exposure
No data concerning the exposure of the general population to
alpha-cypermethrin are available.
8.2 Occupational exposure
A study of alpha-cypermethrin exposures was carried out during
formulation using both technical concentrate and technical material at
Durban, South Africa. The oil-damped solid, crystalline, dry technical
concentrate contained a minimum of 90% (m/m) alpha-cypermethrin.
Exposures were assessed by personal and static monitoring of
atmospheric alpha-cypermethrin concentrations, urinary
alpha-cypermethrin metabolite concentrations and by medical
examination. Four individuals were exposed during 3 days of operation.
The group mean personal exposures for the two days whilst formulating
technical concentrate were 2.8 and 4.9 µg/m3 and the group mean
personal exposure to technical material on day 3 was 54.1 µg/m3.
Urinary alpha-cypermethrin metabolites could not be identified (limit
of detection, 0.02 mg/litre). Formulation was successfully completed,
only minor skin sensations being reported by two of the
non-operational personnel, possibly resulting from particles of
alpha-cypermethrin settling directly on the skin, face and neck. Dust
concentrations were up to 30 times greater during handling the
technical material compared with oil-damped technical concentrate, and
local exhaust dust extraction reduced dust emission by a factor of up
to 17 (Western, 1984).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
Appraisal
The acute toxicity of alpha-cypermethrin to Daphnia magna and
Gammarus pulex is similar to that of cypermethrin. However, in a
reproduction study with Daphnia magna, alpha-cypermethrin seemed to
be slightly more toxic than cypermethrin. Comparison of the results
of a reproduction study in Daphnia magna with the results of acute
tests shows that the hazard of alpha-cypermethrin lies in its acute
toxicity. There is no significant potential for cumulative effects
occurring as a result of long-term exposure to lower concentrations.
Alpha-cypermethrin is highly toxic to a number of aquatic
arthropod taxa but of low toxicity to molluscs. The short-term
toxicity of the compound can be reduced by formulating the product as
an OSC. Contamination through spray drift from commercial
applications will generally be low, and so the effects on susceptible
taxa will be limited. The rapid loss of alpha-cypermethrin from the
water gives the potential for complete recovery of affected
populations.
Laboratory studies show that values for the acute toxicity and
the toxicity to the early-life stages of fish are similar for
alpha-cypermethrin and cypermethrin. A comparison of the results from
both alpha-cypermethrin studies reveals that the hazard of the
compound results from its acute toxicity, and there is no significant
potential for additional effects occurring as a result of long-term
exposure to lower concentrations. Laboratory and field studies have
shown that the toxicity of alpha-cypermethrin to fish is greatly
influenced by the formulation, particulate formulations showing
significantly less toxicity than emulsifiable concentrates.
Field studies demonstrate that the high toxicity of
alpha-cypermethrin to fish observed in laboratory studies is not
realized under field conditions. Contamination of water bodies by
inadvertent overspraying or spray drift does not present a hazard to
fish.
9.1 Microorganisms
9.1.1 Algae
The acute toxicity of alpha-cypermethrin to a single-celled green
alga, Selenastrum capricornutum, at 24 °C has been determined. The
pesticide was dispersed using acetone, and the 2- to 4-day EC50 for
growth was above 100 µg/litre (Stephenson, 1982).
9.1.2 Bacteria
The effects of cypermethrin on microbial activity in the soil
have been investigated in a series of studies. Cypermethrin was
applied to sandy loam at concentrations of 2.5 and 250 mg/kg. No
effects on the rates of carbon dioxide evolution or oxygen uptake were
observed at the lower rate, but significant inhibition of carbon
dioxide evolution and a decrease in oxygen uptake were observed at the
higher rate of application. There were no effects at either
concentration on nitrogen fixation, ammonification, nitrification or
glucose utilization (WHO, 1989).
Sewage bacteria were unaffected by the presence of
alpha-cypermethrin (3 mg/litre) in a closed system test, while the
growth of Pseudomonas fluorescens was unaffected at 100 mg/litre
(Stone & Watkinson, 1983).
9.2 Aquatic organisms
9.2.1 Invertebrates
9.2.1.1 Laboratory studies
Acute toxicity studies with Daphnia magna (aged < 24 h) showed
that at 20 °C technical alpha-cypermethrin, dispersed in acetone under
static conditions (daily renewal), has effects at concentrations below
1 µg/litre. The 24-h and 48-h EC50 values (immobilization) were 1.1
and 0.3 µg/litre, respectively (Stephenson, 1982).
Water samples taken from field enclosures 24 h after treatment
with an EC formulation were analysed for alpha-cypermethrin and
bioassayed with Gammarus pulex. The 24-h LC50 value for this
organism was 0.05 µg/litre (Garforth, 1982a; Shires, 1982).
The effect of technical alpha-cypermethrin on survival, growth
and reproduction of Daphnia magna was studied over a period of 21
days by Garforth (1982b). The test solution was renewed daily and the
temperature ranged between 18.5 and 20.2 °C. The results are
summarized in Table 6.
Table 6. Effects of alpha-cypermethrin on the reproductive cycle of
Daphnia magnaa
Effect Nominal concentration (µg/litre)
LOEL NOEL
Survival of parent generation 0.3 0.1
Growth of parent generation 0.1 0.03
Production of young 0.1 0.03
a From: Garforth (1982b)
LOEL = Lowest-observed-effect level; NOEL = No-observed-effect
level
9.2.1.2 Field studies
Garforth (1982a) studied the effects of alpha-cypermethrin on a
range of aquatic invertebrates in metal enclosures, each containing
about 1 m3 water, placed in an outdoor experimental pond. A diluted
EC formulation was sprayed onto the water surface at concentrations of
1, 3, 10, 30 and 100 g active ingredient/ha, and samples were taken up
to 7 days after application. The concentration of alpha-cypermethrin
was around 50% of the nominal level 24 h after application and
decreased to 10 to 20% of the nominal level 7 days after application.
The lowest concentration was toxic to Asellidae. Thirteen families
of aquatic arthropods were tested; most were killed at 1 g/ha except
one species of Coenagriidae, which tolerated about 3 g/ha. Three
families of molluscs were tested and were found to be unaffected at
100 g/ha.
Studies to investigate the relative toxicity of two formulations
of alpha-cypermethrin (a 100-g/litre EC and a 100-g/litre oil-enhanced
suspension concentrate (OSC) with and without anti-evaporant agents)
to aquatic invertebrates have been carried out. Water samples were
taken from field enclosures treated with a range of doses (0.1-5 g/ha
for the EC and 0.1-10 g/ha for the two OSC formulations) and
bioassayed with Gammarus pulex. The 24-h LD50 values (in g
alpha-cypermethrin/ha equivalents) of water samples taken 24 h after
application were 0.9 for the EC, 6.3 for the OSC without
anti-evaporant, and 2.8 for the OSC plus anti-evaporant formulation.
Thus, one day after treatment, both OSC formulations were less toxic
to Gammarus pulex than the EC formulation. However, the EC
formulation lost its toxicity more rapidly than either of the OSC
formulations (see also section 4.1.2). The effects on other aquatic
invertebrate communities could not be accurately assessed, since the
results for macro-arthropods and zooplankton were inconclusive.
Coenagriidae, Chironomidae and zooplankton seemed to be relatively
tolerant. The residues in water and sediment, 22 days after treatment
with the EC at 2 g alpha-cypermethrin/ha or with both OSC formulations
at 5 g/ha, were < 0.004 µg/litre and < 0.01 mg/kg, respectively, for
all three formulations (Inglesfield, 1985b).
The hazard to aquatic invertebrates resulting from spray drift
from the aerial application of an EC (15 g alpha-cypermethrin/ha) has
been investigated by Garforth & Woodbridge (1984). Details of the
study are described in section 9.2.2.2. The sub-surface water
concentration was 0.6 µg alpha-cypermethrin/litre shortly after
application and decreased to < 0.02 µg/litre within 2 to 4 days. The
contamination initially caused a significant reduction in the
abundance of several groups of aquatic arthropods, including beetles,
chironomids, corixids, mites and zooplankton. However, within 4 to 7
weeks the affected fauna had completely recovered.
In a study by Pearson (1990), two freshwater ponds were treated
with alpha-cypermethrin as an emulsifiable concentrate in 1987. One
pond was oversprayed at 15 g active ingredient/ha, while the other was
treated with the same amount of alpha-cypermethrin but by direct
incorporation into the water (see section 4.1.2). Indigenous
populations of zooplankton nauplii and of copepods ( Cyclops spp.)
were significantly reduced in both ponds by the treatment but
recovered within 26-45 days. Other zooplankton were not plentiful but
appeared to be less severely affected in the oversprayed pond than in
the pond treated by incorporation. Populations of phantom midge larvae
( Chaoborus spp) were killed by both treatments. However, within 47
days after treatment, populations of young larvae had developed in the
oversprayed pond to almost pre-treatment numbers, and to a lesser
extent in the pond with direct incorporation.
9.2.2 Fish
9.2.2.1 Laboratory studies
The available acute 96-h LC50 values for two fish species are
summarized in Table 7.
The effects of the type of formulation on the acute toxicity to
fish are summarized in Table 8. Suspension concentrate, wettable
powder and micro-encapsulated formulations were 10 to 70 times less
acutely toxic to rainbow trout than the EC formulation (Shires,
1983b).
Table 7. Acute toxicity of technical alpha-cypermethrin in fish
Species Mean weight Vehicle Test system Temperature 96-h LC50 Reference
(g) (°C) (µg/litre) (95%
confidence limits)
Rainbow trout 3.3 dispersed via static water; 15 2.8 Stephenson (1982)
(Oncorhynchus mykiss) acetone 12 h renewal of (2.1-3.5)
test solutions
Fathead minnow 0.76 adsorbed onto continuous 23-25 0.93 Stephenson (1983)
(Pimephales promelas) pumice flow-through (0.78-1.2)
Table 8. Effect of type of formulation on the toxicity of alpha-cypermethrin to fish in laboratory studies
Species Weight Tempera- Formulation 96-h LC50 Reference
(g) ture (°C) (µg/litre)
Rainbow trout 0.8-2.5 15 100 g/litre EC 5.6 Shires (1983b)
(Oncorhynchus 250 g/litre SC 350
mykiss) 100 g/kg WP 120
50 g/kg WP 220
50 g/kg ME > 100
50 g/kg CD 65
Rainbow trout 0.11-0.25 15 15 g/litre OSC 10a Pearson (1986)
(Oncorhynchus 15 g/litre OSC 71
mykiss) 100 g/litre OSC 16a
100 g/litre OSC 56
Common carp 3.5-4 24-30 100 g/litre OSC 11 Stephenson
(Cyprinus carpio) 15 g/litre EC 0.8 (1986)
50 g/kg WP 60
Puntius 0.3-0.5 24-30 100 g/litre OSC 3.2 Stephenson
gonionotus 15 g/litre EC 0.7 (1986)
50 g/kg WP 22
a Denotes daily renewal of test solutions. All other tests were carried out without renewal of the test solutions.
EC = emulsifiable concentrate; SC = suspension concentrate; OSC = oil-enhanced suspension concentrate; WP= wettable powder;
ME = micro-encapsulated;
CD = ß-cyclodextrin.
The toxicity of alpha-cypermethrin to the early-life stages of
fish has been studied in a 34-day continuous-flow embryo-larval test
with the fathead minnow (Pimephales promelas). Eggs less than 24 h
old were exposed to nominal concentrations of 0.03 to 1.0 µg/litre.
Pre-hatch, post-hatch and overall mortality and final body weight were
recorded. On the basis of the most sensitive parameter (overall
survival) and measured exposure concentrations, the lowest
concentration of alpha-cypermethrin producing an adverse effect was
0.09 µg/litre and the highest concentration producing no effect (NOEL)
was 0.03 µg/litre (Stephenson, 1983).
9.2.2.2 Small scale field or outdoor tank studies
The acute effect of formulation on the toxicity of
alpha-cypermethrin to fish under field conditions was investigated in
studies using stainless steel enclosures placed in an experimental
pond. In the first study (Shires, 1983b), the rainbow trout (13-32 g)
was the test species and the results are summarized in Table 9. The EC
formulation was at least 30 times more toxic than any of the other
formulations. The number of fish used was not reported.
Table 9. Effect of formulation on the toxicity of alpha-cypermethrin
to rainbow trout in field studies
Formulation 14-day LD50 (g active
ingredient/ha equivalent)
100 g/litre emulsifiable concentrate 29
250 g/litre suspension concentrate > 1000
100 g/kg wettable powder > 1000
50 g/kg micro-encapsulated > 1000
In the second study, Stephenson (1987c) tested an 1.5%
oil-enriched SC formulation of alpha-cypermethrin in outdoor tanks
containing carp ( Cyprinus carpio; 6.3 g). The application rate was
approximately 4, 8 and 15-16 g active ingredient/ha applied by hand
sprayer. The water temperature ranged from 23-26 °C, the water was
aerated and the duration of the experiment was 7 days. There was 20%
mortality at all treatment rates, which was comparable with the
control group. The number of fish used was not reported.
In the third study (Shires, 1985b), rainbow trout ( Oncorhynchus
mykiss; 2-5 g) were introduced into open-ended stainless steel
enclosures placed in a mature experimental pond. Different volumes of
alpha-cypermethrin (as a diluted EC containing 100 g active
ingredient/litre) were applied with a hand-held sprayer onto the water
surface. The dose rates were equivalent to 5-500 g active
ingredient/ha. The concentrations in the water samples were dependent
on the dose applied and the time after the treatment that the samples
were taken. For instance, with a dose of 5 g alpha-cypermethrin/ha the
concentration in the water was 0.4 µg/litre or less after 96 h,
whereas with a dose of 500 g/ha residues of up to 30 µg/litre were
found. Alpha-cypermethrin was toxic to the rainbow trout at a
concentration of about 2-5 µg/litre water. In terms of nominal
application rate, the no-observed-effect level for alpha-cypermethrin
lies between 50 and 100 g alpha-cypermethrin/ha. These dose rates are
much higher than those used for crop protection purposes.
In a further study, 20 rainbow trout were placed in stainless
steel enclosures in a shallow pond. The diluted EC and SC formulations
were sprayed onto the surface of the water and the fish were monitored
for mortality over 8 days. The SC formulation did not cause mortality,
even at an application rate equivalent to 300 g active ingredient/ha.
However, high mortality (90%) occurred with the EC formulation at 30
g/ha alpha-cypermethrin, but application of 10 g active ingredient/ha
did not cause mortality. From these results it is clear that the SC
formulation has a lower toxicity than the EC formulation (Stephenson,
1987b).
The hazard to fish caused by spray drift from aerial applications
of alpha-cypermethrin to agricultural land has been investigated by
Garforth & Woodbridge (1984). The trial site was a cereal field
bordered on one side by a freshwater ditch. The water's edge was
generally less than 2 m from the crop margin. Four days prior to
application, two cages, each containing 15 common carp ( Cyprinus
carpio; 30-50 cm) were placed in the ditch. The ditch was known to
contain a good stock of different types of fish, macroinvert-ebrates
and zooplankton. An alpha-cypermethrin EC containing 100 g/litre was
applied by air at 15 g active ingredient/ha to the crop when a gentle
breeze was blowing from the crop over the ditch. The
alpha-cypermethrin concentration in the sub-surface water was 0.6
µg/litre shortly after the application and decreased to < 0.02
µg/litre within 2 to 4 days (see section 4.1.2.). None of the fish
died and no adverse effects were observed either on the carp placed in
the cages or on the indigenous species. It was concluded that
inadvertent contamination of water bodies by spray drift resulting
from the normal commercial use of alpha-cypermethrin does not present
a hazard to fish.
Several field tests in rice paddies have examined the toxicity of
alpha-cypermethrin to fish.
Stephenson (1986, 1987b) investigated the acute toxicity of 15
g/litre EC, 100 g/litre OSC and 50 g/kg WP formulations to fish in
plots of rice paddy in West Java. The application rates were 7.5, 15,
and 30 g alpha-cypermethrin/ha for the EC and OSC formulations and 30
g alpha-cypermethrin/ha for the WP formulation. Each of the
insecticide treatments was applied to the same plot on two separate
occasions; once 12 days after transplantation of the rice seedlings
and again 57 days after the transplantation. Prior to application, two
cages each containing 20 carp (Cyprinus carpio) (2.5-5 g) and two
cages each containing 20 specimens of Puntius gonionotus (1.4-5 g)
were placed in trenches cut in each plot. In addition, before the
first experiment, 30 fish of each of these two species were released
to swim freely in each plot. Survival of the fish was monitored daily
up to 7 days after each treatment. The water temperature was between
25 and 36 °C. Following the first application, only the EC formulation
at 15 and 30 g/ha resulted in significant mortality of caged and
free-swimming carp and Puntius gonionotus. The same was true for
carp following the second application, but no results were available
for Puntius gonionotus following the second application due to an
outbreak of disease among these fish.
Two other field studies were carried out in West Java to examine
the effects of an OSC formulation on the growth and survival of
free-swimming carp (6-8 g) in rice paddies. Experimental conditions
were similar to those described above for the acute studies. The water
temperature was 23-32 °C. In the first experiment, treatments with 15
g active ingredient/ha were carried out 21 and 33 days after
transplantation of the rice seedlings. In the second experiment, the
same dose rate was applied 51 and 64 days after transplanting. The
fish were monitored over periods of 3 to 4 weeks in each experiment.
No adverse effects on survival and growth of the carp were observed
(Stephenson, 1987a,b).
9.3 Terrestrial organisms
9.3.1 Earthworms
The toxicity of technical alpha-cypermethrin to the red
earthworm, Eisenia foetida, has been assessed in laboratory tests.
In the filter paper contact toxicity test, 50% mortality occurred
within 48 h at a dose of about 0.01 mg/cm2 of filter paper. However,
increases in the dose up to 1 mg/cm2 did not result in a significant
increase in mortality. In the artificial soil test no significant
mortality occurred within a period of 14 days in earthworms exposed to
up to 100 mg alpha-cypermethrin/kg soil (Inglesfield & Sherwood,
1983).
9.3.2 Invertebrates - field studies
In a study designed to investigate the effects of
alpha-cypermethrin on non-target arthropod fauna in Italian vineyards,
two formulations of alpha-cypermethrin were tested, i.e. an
emulsifiable concentrate (100 g active ingredient/litre) and a
suspension concentrate (250 g active ingredient/litre). Both were
diluted to 1.25 g active ingredient/ha and sprayed to run-off. The
effect on predators and parasites (crop foliage fauna) such as
parasitoid Hymenoptera, Heteroptera and Chrysopa carnea, soil
surface predators such as Coleoptera and Araneae, phytophagous
arthropods such as Homoptera, Thysanoptera and Acari, and other
invertebrates such as nematocerous, Diptera and epigeal fauna was
inconclusive but in most cases negative. Spiders were the only group
significantly affected by alpha-cypermethrin. The abundance of major
phytophagous insect taxa (Homoptera, Thysanoptera and Acari) was
markedly reduced by both treatments although neither treatment had any
long-term adverse effects (Inglesfield, 1984).
An emulsifiable concentrate (EC) of alpha-cypermethrin was tested
on the non-target arthropod fauna of maize in France at 20 and 30 g
active ingredient/ha and applied using tractor-mounted boom and nozzle
equipment at different stages of crop development. Organisms from the
following taxa were collected: phytophagous arthropods (Aphidoidea),
Thysanoptera (Thripidae), Cicadellidae and entomophagous arthropods;
predatory Coleoptera (Coccinellidae, Cantharidae, Carabidae and
Staphylinidae); parasitoid Hymenoptera (Braconidae, Chalcidoidea);
and predatory Diptera, Neuroptera and Aranea. In addition, the
effects on community structure were studied. The two treatments
reduced transiently the numbers of some of the entomophagous taxa,
especially Coccinellidae (Coleoptera), Carabidae (Coleoptera),
Neuroptera and Araneae. Alpha-cypermethrin at a concentration of
20 g/ha promoted some late-season resurgence of aphid populations
(Inglesfield, 1985a).
A large-scale replicated field experiment was carried out in
Indonesia to investigate the effects of an EC (10 g and 20 g
alpha-cypermethrin/ha) and an OSC (10 g and 20 g alpha-cypermethrin
per ha), applied at 17 and 62 days after transplanting using a single
fan-jet nozzle knapsack sprayer, on rice pests and their natural
enemies. Alpha-cypermethrin was applied at two crop stages, and
populations of both pests and beneficial arthropods were monitored
throughout the season. Alpha-cypermethrin produced good control of
stemborers, grasshoppers, leafhoppers and stinkbugs, the most numerous
pests found during the trial. It had a significant but short-lived
effect on spiders and, generally, no effect on either dragon-flies or
other beneficial arthropods (Shires & Inglesfield, 1986).
A study was initiated in England in 1982 to compare the effects
on entomophagous arthropods of annual applications of an EC of
alpha-cypermethrin (at a rate of 10 g active ingredient/ha in the
first year and 15 g active ingredient/ha in subsequent years) with
those resulting from the use of non-pyrethroid products. The study was
carried out on a range of crops (oilseed rape, wheat and barley) over
the duration of a 5-year arable crop rotation. A field of 8 ha was
divided into two equal areas, and each year alpha-cypermethrin was
applied to one of the areas and other products to the other area. All
treatments were carried out with standard tractor-driven boom and
nozzle spraying equipment. The crop rotation was as follows: winter
rape in 1982, winter wheat in 1983 and 1984, and winter barley in
1985. Organisms from a variety of taxa were collected during the
study, such as parasitoid Hymenoptera, predatory Diptera,
predatory Coleoptera, Araneae, phytophagous insects and other
arthropods. The results indicated that any difference between the
effects of alpha-cypermethrin and the reference compounds on
entomophagous arthropods was generally short-lived. There is no
evidence that alpha-cypermethrin treatments had any long-term effects
on any of the taxa studied. In addition, there appeared to be no
long-term effects on the relative abundance of entomophages within the
arthropod communities or on the structure and integrity of the
arthropod communities of which they form a part (Inglesfield, 1985c,
1988, 1989).
The results of these studies have been reviewed by Inglesfield
(1991). They showed that field application of alpha-cypermethrin and
cypermethrin had no adverse effects on the relative abundance of
entomophages within the arthropod communities and that the use of
these two pesticides in small grain cereals would not be associated
with pest "resurgence" or the development of secondary pest
infestations.
9.3.3 Honey-bees
9.3.3.1 Laboratory studies
The oral administration of alpha-cypermethrin in acetone produced
a 24-h LD50 of 0.06 µg/bee, whereas an EC formulation (100 g/litre)
of the pesticide yielded a 24-h LD50 of 0.13 µg formulation/bee.
After topical application, 24-h LD50 values of 0.03 µg (technical)
and 0.11 µg (EC) per bee were obtained (Murray, 1985).
The mortality of honey-bees (Apis mellifera) exposed to
Phacelia flowers 30 min after they had been treated with an EC
formulation (15 g active ingredient/ha) in a residual test was low
(15%) within 48 h. However, the foraging activity was reduced (Murray,
1985).
The residual toxicity to the honey-bee of alpha-cypermethrin as
EC, OSC, and EC/fungicide mixtures has been investigated. Bees were
exposed for 48 h to flowering Phacelia campanularia plants that had
been sprayed with each formulation at a rate of 10 or 20 g active
ingredient/ha, and the mortality was assessed 24 and 48 h after
initial exposure to the treated plants. Although there was great
variation in the results, there appeared to be no difference in the
residual toxicity of the EC and OSC formulations at a rate of 10 g
active ingredient/ha. However, at 20 g active ingredient/ha the OSC
appeared to be more toxic than the EC. Application of the EC in
admixtures did not appear to significantly increase bee mortality
(Hillaby & Inglesfield, 1986).
9.3.3.2 Field studies
Alpha-cypermethrin, applied as an EC (10 and 20 g active
ingredient/ha) by tractor-mounted boom and nozzle equipment to small
plots of flowering mustard in France, caused a sharp decline in
foraging activity of bees immediately after application, but there was
a return to normal activity within a few hours. No effect on bee
survival or hive development was observed (Shires, 1983a; Shires et
al., 1984b; Shires, 1985a).
In a further study, the same EC formulation was applied at the
same concentration on large isolated fields of flowering oilseed rape
during peak foraging activity of honey-bees in France. No increase in
bee mortality was found, but foraging activity declined for a few
hours after application at a rate of 10 g active ingredient/ha. With
a rate of 20 g active ingredient/ha a more prolonged decline in
foraging activity occurred. No effects on the overall condition of the
hives were seen at the end of the season and very low or undetectable
residues were found in dead bees, pollen, honey and wax (Shires et
al., 1984c; Shires, 1985a).
Two large and two small plots of winter wheat were enclosed
beneath large mesh-covered tunnels. A small bee-hive was placed in
each tunnel and sucrose solution was sprayed onto all of the wheat in
order to simulate aphid honey dew. Alpha-cypermethrin (an EC at 10, 15
or 30 g active ingredient/ha) was applied to the larger plots of wheat
when the bees were actively foraging the sugar deposits. No increase
in bee mortality, compared with that in the pre-treatment period, was
observed. Foraging activity in the plots declined sharply after
treatment and remained at a reduced level, probably because of its
repellent effect. Examination of the hives about 2 weeks after the
trial showed that the hives, both the control and the
alpha-cypermethrin-treated, were in excellent condition with strong
adult populations and large areas of developing brood. In
post-treatment samples, alpha-cypermethrin residues of 0.03 mg/kg of
honey and 0.01 mg/kg of wax were found. In live and dead bees
collected from the tunnel treated with 15 g/ha, a concentration of
0.026 µg/bee was found (Shires et al., 1984a; Le Blanc, 1985).
In a study by Inglesfield & Forbes (1986), alpha-cypermethrin was
applied as an OSC (10 g active ingredient/ha) and an EC (10 g active
ingredient/ha) to three fields of flowering winter-sown oilseed rape
in Germany while bees were actively foraging the crops. None of the
treatments had any significant effect on adult bee survival or on the
longer-term development of the experimental colonies. The number of
bees actively foraging in the crops declined following application.
This reduction in activity can probably be partly attributed to the
repellent effects of these treatments. Both the OSC and EC
formulations of alpha-cypermethrin had similar effects on bee
behaviour. Residues of alpha-cypermethrin in post-treatment samples of
pollen, honey and wax were below 0.01 mg/kg. In dead bees, the residue
on the day of application with the OSC was 1.8 mg/kg and in the case
of the EC was 0.12 mg/kg.
A diluted EC formulation of alpha-cypermethrin (mixed with the
fungicide vinclozolin, 100 g active ingredient/ha) was applied by a
tractor-driven boom and nozzle sprayer, at a rate of 20 g active
ingredient/ha, to a 206-ha block of flowering oilseed rape situated in
Kent, England. Shortly before spraying, which took place over a 3-day
period, five beehives were positioned at each of two sites adjacent to
the crop. At each site the hives were either fitted with pollen traps
or with traps to collect dead bees as they were removed from the hive.
The hives were observed daily before and for ten days after spraying.
Hive activity was recorded at intervals each day, and, on days when
bees were flying, pollen traps were set and samples of pollen
collected. Following the application of alpha-cypermethrin the bees
foraged normally. At least 90% of the pollen returned to the hives
during the immediate post-treatment period was found to be from
oilseed rape, showing that the bees had continued to forage on the
treated crop. The number of dead bees collected remained low
throughout the study and did not increase after application of the
alpha-cypermethrin. After the study, all hives were in good condition
and subsequently yielded a good crop of honey. A local beekeeper with
many hives adjacent to the same block of oilseed rape reported no
effects amongst his hives. It was concluded that the application of
alpha-cypermethrin at 20 g active ingredient/ha to flowering oilseed
rape had no direct effects on honey-bee survival and hive development
(Brown, 1989).
9.3.4 Leaf-cutting bees
Laboratory trials using 15 male adult alfalfa leaf-cutting bees
( Megachile rotundata F.) per group, exposed to treated filter papers
for 4 h, showed that alpha-cypermethrin EC (100 g/litre) at the rate
of 10 or 15 g active ingredient/ha caused 12 and 30% mortality,
respectively, and 42 and 100% mortality after 24 h of contact (Tasei
et al., 1987).
In a study by Tasei et al. (1987), populations of about 400
female alfalfa leaf-cutting bees were reared in three flowering
lucerne fields. One field was left untreated, while the two others
were treated with alpha-cypermethrin as an EC (10 or 15 g active
ingredient/ha), applied by a tractor-mounted fan-jet sprayer. Two days
after the treatments, counts of live females in artificial nesting
sites showed the losses due to 10 and 15 g active ingredient/ha to be
21 and 12%, respectively. After hibernation and incubation of the
progeny larvae, little effect of the treatments could be observed. The
maximum residue in leaves collected from nests was 1 mg
alpha-cypermethrin/kg. The mean values after 5, 10 and 27 days in leaf
pieces capping the nests of the bees were 0.75, 0.48, and 0.19 mg/kg
at the lower application rate and 0.59, 0.53, and 0.10 mg/kg at the
higher rate. No residues (limit of determination, 0.01 mg/kg) were
detected in live larvae but 0.07 mg/kg was found in pollen provisions
(Tasei et al., 1987).
9.3.5 Birds
Cypermethrin is practically non-toxic to birds; acute oral LD50
values are greater than 2000 mg/kg body weight. The dietary LC50
value is above 10 000 mg/kg diet (see WHO, 1989). However, studies
with alpha-cypermethrin have not been carried out.
10. COMPARISON BETWEEN ALPHA-CYPERMETHRIN AND CYPERMETHRIN
Alpha-cypermethrin comprises one quarter of the racemic mixture
cypermethrin, with which an extensive agricultural, ecological and
(eco)toxicological programme has been carried out (WHO, 1989). It
contains more than 90% of the insecticidally most active enantiomer
pair of the four cis isomers of cypermethrin, i.e. the two cis isomers
(IRcis)S and (IScis)R (see Fig. 1).
10.1 Use and residue levels
Alpha-cypermethrin is used to control the same pests in
agriculture as cypermethrin. Its rate of application to crops (5-30 g
active ingredient/ha) is lower than that of cypermethrin (10-200 g
active ingredient/ha) since alpha-cypermethrin is biologically more
active than cypermethrin.
Residue data for alpha-cypermethrin have been obtained from a
large number of supervised trials carried out worldwide. These trials
cover the most important crop groupings for which alpha-cypermethrin
is recommended, including oilseeds, pome fruits, peaches, fruiting
vegetables, berries, leafy vegetables, maize and speciality crops such
as hops and tobacco (Shell, 1984). Residues in a variety of these
crops resulting from application of alpha-cypermethrin at the
recommended rate ranged between 0.05 and 1.0 mg/kg (Shell, 1984). In
comparison, residues of cypermethrin in crops were higher and ranged
between 0.05 and 2.0 mg/kg. In comparative trials where
alpha-cypermethrin was applied at half the dose rate of cypermethrin,
alpha-cypermethrin residues in general averaged 40% (20-50%) of those
in cypermethrin-treated samples (Shell, 1984; WHO, 1989).
The residue data on cypermethrin have been evaluated by the Joint
FAO/WHO Meeting on Pesticide Residues and recommended MRLs (1979/1981)
have been published (FAO/WHO, 1980, 1982) (see Table 10). From the
available residue data obtained with good agricultural practice and
using the MRLs set for cypermethrin, suggested MRLs for
alpha-cypermethrin can be extrapolated (Table 10).
It can be concluded that alpha-cypermethrin, since it is
biologically more active, is always used at a lower application rate
than cypermethrin and, as a result, the residues on crops are
approximately half those of cypermethrin.
Table 10. Comparison of current MRLs in mg/kg product for cypermethrin
recommended by the FAO/WHO Joint Meeting on Pesticides Residues (JMPR)
with those which may be proposed for alpha-cypermethrin
Commodity Cypermethrin Alpha-cypermethrin
(JMPR)a (extrapolated)
Cotton seed 0.2 0.05
Rapeseed 0.2 0.05
Pome fruits 2 0.5
Peaches 2 0.5
Grapes 1 0.5
Citrus fruits 2 1
Tomatoes 0.5 0.1
Brassica, leafy vegetables 1 1
Lettuce 2 1
Peas, kidney beans (less pod) 0.05 0.05
Soyabeans (less pod) 0.05 0.05
Potatoes 0.05 0.05
Sugarbeet (roots) 0.05 0.05
Maize grain 0.05 0.05
a From: FAO/WHO (1980, 1982)
For analytical reasons, 0.05 mg/kg is considered the minimum practical
MRL.
10.2 Environmental impact
Because its physico-chemical properties are similar to those of
cypermethrin, alpha-cypermethrin is expected to have a similar
environmental fate to that of cypermethrin. The potential of
alpha-cypermethrin to bioaccumulate may therefore be estimated from
experimental data on the bioaccumulation of cypermethrin. This is
because the octanol/water partition coefficients of alpha-cypermethrin
(1.4 x 105; log Pow = 5.16) and cypermethrin (2 x 106; log Pow
= 6.3) are relatively similar. In fish, the bioaccumulation of
cypermethrin determined experimentally was lower than might have been
expected from its partition coefficient, presumably because it was
rapidly metabolized. This would also be expected for
alpha-cypermethrin because both the route of metabolism and its rate
are similar to those of cypermethrin (Shell, 1983b; WHO, 1989).
In the area of environmental toxicology, results available for
green algae, aquatic invertebrates, fish and bees show that the acute
toxicity of alpha-cypermethrin is slightly higher than, but broadly
similar to that of cypermethrin (Table 11). This is because the
toxicity of cypermethrin results largely from its alpha-cypermethrin
component. No toxicity data are available concerning the effects of
alpha-cypermethrin on soil microbes, but little or no effect on carbon
dioxide evolution, oxygen uptake and nitrogen fixation would be
expected if alpha-cypermethrin acts on soil microbes in a similar way
to cypermethrin (WHO, 1989). There are also no toxicity data for
alpha-cypermethrin in birds. However, cypermethrin has a low toxicity
to birds. Since alpha-cypermethrin constitutes 25% of the active
ingredients of cypermethrin and is used at lower application rates, it
is expected that alpha-cypermethrin will also have a low toxicity to
birds (Shell, 1983b).
Overall, in the natural environment, the more biologically active
alpha-cypermethrin is likely to have a similar toxicity to that of
cypermethrin. This is because alpha-cypermethrin is used at a lower
application rate than cypermethrin.
10.3 Mammalian toxicity
In acute oral toxicity studies, alpha-cypermethrin is either
equally toxic or two to three times more toxic than cypermethrin (WHO,
1989), depending on the vehicle and the concentrations used.
In Table 12, the no-observed-effect levels and
lowest-observed-effect levels of the various mouse, rat and dog
studies are compared for cypermethrin, cis-cypermethrin and
alpha-cypermethrin. Short- and long-term studies with the racemic
mixture cypermethrin (containing four cis and four trans isomers),
cis-cypermethrin (four cis isomers) and alpha-cypermethrin (two cis
isomers) have shown similar toxicological effects.
The data from the short-term toxicity studies indicate that
alpha-cypermethrin is approximately 2 to 3 times more toxic than
cypermethrin in rats and dogs. This reflects the amount of
alpha-cypermethrin present in cypermethrin.
The signs of intoxication, the effects on target organs and
tissues, and the metabolic pathway of alpha-cypermethrin are similar
to those of cis-cypermethrin. The mode of action of
alpha-cypermethrin is similar to that of cypermethrin.
Cypermethrin has been tested in a rodent multigeneration
reproduction study and for embryotoxicity and teratogenicity in two
species. There were no effects on either reproductive performance or
on fetal development, even at doses producing systemic toxicity (WHO,
1989). Alpha-cypermethrin has not been tested for reproductive
toxicity or teratogenicity, but there is no indication that it would
have effects on these parameters since it is a component of
cypermethrin.
Table 11. Comparison of environmental toxicology of cypermethrin and alpha-cypermethrin
Species Life stage/ Water Administration Measured Values for Reference
age temper- route or vehicle end-point cypermethrin alpha-
ature (°C) cypermethrin
Green alga
(Selenastrum - 24 dispersal 2- to 4-day > 100 µg/litre > 100 µg/litre Stephenson
capricornutum) via acetone EC50 (growth) (1982)
Aquatic invertebrates
Water flea up to 24 h 20 dispersal EC50 Stephenson
(Daphnia magna) old via acetone (immobilization) (1982)
24 h 1.2 µg/litre 1.1 µg/litre
48 h 0.3 µg/litre 0.3 µg/litre
Fish
Rainbow trout 3.3 g 15 dispersal 96-h LC50 2.8 µg/litre 2.8 µg/litre Stephenson
(Oncorhynchus mykiss) via acetone (1982)
Fathead minnow 0.74-0.76 g 23-25 absorbed on 96-h LC50 1.2 µg/litre 0.93 µg/litre Stephenson
(Pimephales promelas) (juvenile) to pumice (1983)
Earthworm
(Eisenia foetida) - - filter paper 48-h LD50 26.1 µg/cm2 10 µg/cm2 Rob
contact & Dorough
toxicity (1984);
Inglesfield
& Sherwood
(1983)
Table 11 (continued)
Species Life stage/ Water Administration Measured Values for Reference
age temper- route or vehicle end-point cypermethrin alpha-
ature (°C) cypermethrin
Bees
(Apis mellifera) worker - oral 24-h LD50 0.035 µg/bee 0.06 µg/bee Badmin
adminis- & Twydell
tration (1976);
Murray
(1985)
Table 12. Comparison between short- and long-term oral studies with
cypermethrin, cis-cypermethrin and alpha-cypermethrin
Animal Number of Duration of Dose level in mg/kg diet
species studies experiment No-observed- Lowest-observed-
effect level effect level
Cypermethrin (4 cis and 4 trans isomers)a
Mouse 1 2 years 400 1600
Rat 1 5 weeks 750 1500
2 13 weeks 100/150 400
1 2 years 100 1000
Dog 1 13 weeks 500 1500
1 2 years 300 750/600
cis-cypermethrin (4 cis isomers)a
Rat 1 5 weeks 100 300
Alpha-cypermethrin (2 cis isomers)
Rat 1 5 weeks 200 400
1 13 weeks 60 180
Dog 2 2-7 days 200 250
1 13 weeks 90 270
a See WHO (1989)
The WHO Task Group on Environmental Health Criteria for
Cypermethrin, which met in 1986, concluded that cypermethrin was
without mutagenic activity. Available data on alpha-cypermethrin
indicate that this compound also is non-mutagenic in tests with
Salmonella typhimurium, Saccharomyces cerevisiae, and in vivo and
in vitro tests with rat liver cells for the induction of chromosome
aberration and production of DNA single-strand damage.
Alpha-cypermethrin has not been tested for carcinogenicity, but
the long-term toxicity studies in mice and rats did not indicate any
carcinogenic potential for cypermethrin. Because cypermethrin contains
the two cis isomers present in alpha-cypermethrin, it is unlikely that
these two isomers would have a carcinogenic potential. This
supposition is supported by the fact that all the mutagenicity studies
on alpha-cypermethrin have yielded negative results.
Near lethal doses of alpha-cypermethrin and cypermethrin produce
sparse axonopathy in the peripheral nerves of rats (see section
7.7.2). Similar signs of intoxication were observed with both
compounds, and increases in beta-glucuronidase and beta-galactosidase
activities, consistent with an axonal degeneration, could only be
detected in severely intoxicated animals. The magnitude of the enzyme
changes was substantially less than for other known neurotoxic
compounds, thereby confirming the minor nature of the lesion. The
short time period needed to produce ataxia and/or abnormal gait rules
out a causal relationship between ataxia and axonopathy. The signs of
intoxication are consistent with a pharmacologically-mediated effect.
No biochemical changes indicative of axonopathy were detected in rats
given 20 oral doses of 20 mg alpha-cypermethrin/kg body weight per day
or 75 mg cypermethrin/kg body weight per day. From these comparative
studies, it is clear that the neurotoxic potential of
alpha-cypermethrin and cypermethrin is qualitatively similar, but
alpha-cypermethrin is 3 to 4 times more potent. This reflects the fact
that alpha-cypermethrin constitutes the active ingredient and 25% of
cypermethrin.
Appraisal
The effects of alpha-cypermethrin on vertebrates and
invertebrates are qualitatively and, in a number of cases, even
quantitatively similar to those of cypermethrin, reflecting the fact
that alpha-cypermethrin is composed of two of the four cis isomers
present in cypermethrin (these two being the most active components
of cypermethrin). Therefore, the toxicological information of
cypermethrin can be used to evaluate the effects of
alpha-cypermethrin where certain important toxicity studies for
alpha-cypermethrin are lacking.
Alpha-cypermethrin has a higher toxicity (lower
no-observed-effect level) than cypermethrin but its application rate
is at most only half that of cypermethrin (5-30 g active
ingredient/ha for alpha-cypermethrin and 10-200 g active
ingredient/ha for cypermethrin). Therefore, residue levels of
alpha-cypermethrin in crops are less than half those of cypermethrin.
11. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The toxicological and residue data on cypermethrin have been
evaluated by the Joint FAO/WHO Meeting on Pesticide Residues (JMPR)
(FAO/WHO, 1980, 1982), but alpha-cypermethrin has not yet been
evaluated by the JMPR.
REFERENCES
Aldridge WN (1990) An assessment of the toxicological properties of
pyrethroids and their neurotoxicity. Crit Rev Toxicol, 21(2): 89-104.
Armitage GD (1984) Fastac exposure study. Analysis of atmospheric and
surface wipe samples ex. Durban, South Africa. Sittingbourne, Shell
Research (SBRN 84.091).
Badmin JS & Twydell RS (1976) Evaluation of the insecticide WL 43467
against the honey bee Apis mellifera. Sittingbourne, Shell Research
(WKSR.0021.76).
Baldwin MK (1990) Alpha-cypermethrin (Fastac): Water solubility at
various pH values. London, Shell International Chemical Company, Ltd
(Internal report SBGR 90.158).
Blair D (1984) The toxicology of pyrethroids; the acute 4-h inhalation
toxicity of a 30% m/m Fastac on silica powder formulation.
Sittingbourne, Shell Research (SBGR 84.066).
Bosio PG (1982) Study of isomer conversion of WL85871 in various crops
from 1981 treatments. Berre, France, Shell Chimie (BEGR 82.031).
Brooks TM (1982) Toxicity studies with pyrethroids; in vitro
genotoxicity studies with WL85871. Sittingbourne, Shell Research (SBGR
81.273).
Brooks TM (1984) Genotoxicity studies with Fastac; the induction of
gene mutation in the yeast Saccharomyces cerevisiaee XV 185-14-C.
Sittingbourne, Shell Research (SBGR 84.117).
Brown K (1989) A study of the effects on honey-bees of a large scale
commercial application of "Fastac" to oilseed rape. London, Shell
International Chemical Company, Ltd (Internal report SBGR 89.198).
Clare MG & Wiggins DE (1984) Genotoxicity studies with Fastac; in
vivo cytogenetic test using rat bone marrow. Sittingbourne, Shell
Research (SBGR 84.120).
Clark DG (1982) WL85871; a 90-day feeding study in rats.
Sittingbourne, Shell Research, vol 1 and 2 (SBGR 81.293).
Coveney PC & Forbes S (1986) Analysis of soil from UK (Coates) for
residues of "Fastac" (WL85871) - soil persistence trial - third year.
Sittingbourne, Shell Research (SBGR 86.201).
a The proprietary Shell Research reports in the following list were
submitted to the IPCS by Shell.
Creedy CL & Logan CJ (1984) In vitro metabolism of cypermethrin
isomers by rat, rabbit, and human liver preparations. Sittingbourne,
Shell Research (SBGR 84.108).
Dewar AJ (1981) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity, skin and eye irritancy and skin sensitizing
potential of WL85871 including a comparison with the acute toxicity of
WL 43467 (Ripcord). Sittingbourne, Shell Research (TLGR 80.148).
Eadsforth CV, Bragt PC, & Van Sittert NJ (1988) Human dose-excretion
studies with pyrethroid insecticides cypermethrin and
alpha-cypermethrin; relevance for biological monitoring. Xenobiotica,
18(5): 603-614.
FAO (1982) Second Government Consultation on International
Harmonization of Pesticide Registration Requirements, Rome, 11-15
October 1982. Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1980) 1979 Evaluations of some pesticide residues in food.
Rome, Food and Agriculture Organization of the United Nations (FAO
Plant Production and Protection Paper 20 sup).
FAO/WHO (1982) 1981 Evaluations of some pesticide residues in food.
Rome, Food and Agriculture Organization of the United Nations (FAO
Plant Production and Protection Paper 42).
Fisher JR, Robinson J, & Debray PH (1983) A new multipurpose
insecticide. Proceedings of 10th International Congress of Plant
Protection, Brighton, England, 20-25 November 1983. Vol. 1, pp
452-459.
Forbes S (1985) Residues of Fastac (WL85871) in cured marine catfish
( Arius sp.) from Indonesia. Sittingbourne, Shell Research (SBGR
85.200).
Forbes S & Burden AN (1984) Analysis of soil from UK (Reculver) for
residues of WL85871 (Fastac) - soil persistence trial - second year.
Sittingbourne, Shell Research (SBGR 84.005).
Forbes S & Cole DA (1986) Residues of Fastac (WL85871) in sweetcorn
from Canada. Sittingbourne, Shell Research (SBGR 86.217).
Forbes S & Knight CJ (1983) Analysis of soil from UK (Reculver) for
residues of WL85871 - soil persistence trial - first year.
Sittingbourne, Shell Research (SBGR.83.162).
Forbes S & Mackay CE (1983) Analysis of soil from UK (Coates) for
residues of WL85871 (Fastac) - soil persistence trial - first year.
Sittingbourne, Shell Research (SBGR 83.418).
Forbes S & Wales GH (1985a) Analysis of soil from UK (Reculver) for
residues of Fastac (WL85871) - soil persistence trial - third year.
Sittingbourne, Shell Research (SBGR 85.070).
Forbes S & Wales GH (1985b) Analysis of soil from UK (Coates) for
residues of Fastac (WL85871) - soil persistence trial - second year.
Sittingbourne, Shell Research (SBGR 85.071).
Francis WP & Gill JP (1991) Flufenoxuron/alpha-cypermethrin - residues
in sheep tissues following treatment with PAMPASS/RENEGADE mixtures by
dip or pour-on. London, Shell International Chemical Company, Ltd
(Internal report SBGR 89.254).
Gardner JR (1989) "Fastac/Azodrin" 20/400 g/litre emulsifiable
concentrate: Acute oral and dermal toxicity. London, Shell
International Chemical Company, Ltd (Internal report SBGR 89.026).
Gardner JR (1991) Fastac 60 G/l SC (SF07396); acute oral and dermal
toxicity in rat. Sittingbourne, Shell Research (SBGR 91.020).
Garforth BM (1982a) A comparison of the toxicities of WL85871 and
Ripcord to freshwater invertebrates in small field enclosures.
Sittingbourne, Shell Research (SBGR 82.015).
Garforth BM (1982b) WL85871 and cypermethrin; chronic toxicity to
Daphnia magna. Sittingbourne, Shell Research (SBGR 82.119).
Garforth BM & Woodbridge AP (1984) Spray drift from an aerial
application of Fastac; fate and biological effects in an adjacent
freshwater ditch. Sittingbourne, Shell Research (SBGR 84.055).
Greenough RJ & Goburdhun R (1984) WL85871; Oral (dietary) maximum
tolerated dose study in dogs. Musselburgh, Inveresk Research
International (Unpublished report No. 3107, submitted to WHO by Shell
Research).
Greenough RJ, Cockrill JB, & Goburdhun R (1984) WL85871; 13-week oral
(dietary) toxicity study in dogs. Musselburgh, Inveresk Research
International (Unpublished report No. 3197, submitted to WHO by Shell
Research).
Hend RW (1983) Toxicology of pyrethroids; skin stimulation studies
using guinea-pigs. Sittingbourne, Shell Research (SBGR 83.197).
Hillaby JM (1988) Deposition of pesticide on the orchard floor
following a commercial mistblower application to apples. London, Shell
International Chemical Company, Ltd (Internal report SBGR 88.106).
Hillaby JM & Inglesfield C (1986) The residual toxicity of "Fastac"
formulations and "Fastac"/fungicide mixtures to the honey bee, Apis
mellifera L. Sittingbourne, Shell Research (SBGR 86.018).
Hutson DH (1982) WL85871; metabolism of a single oral dose in the rat.
Sittingbourne, Shell Research (SBGR 82.205).
Hutson DH & Logan CJ (1986) The metabolic fate in rats of the
pyrethroid insecticide WL85871, a mixture of two isomers of
cypermethrin. Pestic Sci, 17: 548-558.
Inglesfield C (1984) The effects of two formulations of Fastac on the
beneficial arthropod fauna of grape vines. Sittingbourne, Shell
Research (SBGR 84.039).
Inglesfield C (1985a) A field study on the effects of Fastac on the
beneficial arthropod fauna of maize in France. Sittingbourne, Shell
Research (SBGR 85.069).
Inglesfield C (1985b) A pond enclosure study of the effects of Fastac
formulations on aquatic invertebrates. Sittingbourne, Shell Research
(SBGR 85.083).
Inglesfield C (1985c) The effects of the pyrethroid insecticide
WL85871 on non-target arthropods: Field studies. Pestic Sci, 16(2):
211.
Inglesfield C (1988) Effects of "Fastac" on non-target arthropods; an
overview of a five-year arable crop rotation study. Sittingbourne,
Shell Research (SBGR 87.149).
Inglesfield C (1989) A long-term field study to investigate the
effects of alpha-cypermethrin on predatory and parasitic arthropods.
Meded Fac Landbouwwet Rijksuniv Gent, 54(3a): 895-904.
Inglesfield C (1991) Effects of alpha-cypermethrin (Fastac) on
entomophagous organisms and game-bird chick-food insects in summer
cereals. The Hague, Shell Internationale Petroleum Maatschappij B.V.
(Report Series HSE 91.008).
Inglesfield C & Forbes S (1986) A field trial to assess the effects of
"Fastac" 100 g/litre OSC on foraging honey bees in oilseed rape.
Sittingbourne, Shell Research (SBGR 86.087).
Inglesfield C & Sherwood CM (1983) Toxicity of cypermethrin and
WL85871 to the earthworm, Eisenia foetida L.
(Oligochaeta:Lumbriculidae) in laboratory tests. Sittingbourne,
Shell Research (SBGR 83.071).
Langner EJ (1980) Determination of the vapour pressure of WL 43467 and
WL 85871 at 20 °C. Sittingbourne Shell Research (Research Note No.
FCDN 80.137).
Le Blanc J (1985) Field experiments on the effects of a new pyrethroid
insecticide WL85871 on bees foraging artificial aphid honey-dew on
winter wheat. Pestic Sci, 16(2): 206.
Le Quesne PM, Maxwell IC, & Butterworth STG (1980) Transient facial
sensory symptoms following exposure to synthetic pyrethroids; a
clinical and electrophysiological assessment. Neurotoxicology, 2:
1-11.
Logan CJ (1983) WL85871; depletion from tissues of female rats after
a single oral dose. Sittingbourne, Shell Research (SBGR 83.075).
McMinn AL (1983a) The degradation of the pyrethroid insecticides
WL85871 (Fastac) and WL43481 in cabbage. Sittingbourne, Shell Research
(SBGR 83.396).
McMinn AL (1983b) The degradation of the pyrethroid insecticides
WL85871 and WL43481 in soil. Sittingbourne, Shell Research (SBGR
83.395).
Maloney SE, Maule A, & Smith ARW (1988) Microbial transformation of
the pyrethroid insecticides: Permethrin, deltamethrin, fastac,
fenvalerate and fluvalinate. Appl Environ Microbiol, 54(11):
2874-2876.
Murray A (1985) Acute and residual toxicity of a new pyrethroid
insecticide WL85871, to honey-bees. Bull Environ Contam Toxicol, 34:
560-564.
Pearson N (1986) Fastac oil-enriched suspension concentrates; acute
toxicities of two formulations to the rainbow trout, Salmo gairdneri.
Sittingbourne, Shell Research (SBGR 85.290).
Pearson N (1990) The fate of "Fastac" in experimental ponds.
Sittingbourne, Shell Research (SBGR 88.177).
Pickering RG (1982) A 5-week feeding study with WL85871 in rats.
Sittingbourne, Shell Research, vol 1 and 2 (SBGR 81.212).
Price JB (1985a) Toxicology of Fastac (WL85871); the acute oral and
percutaneous toxicity and skin irritancy of a Fastac 10% EC (SF
06510). Sittingbourne, Shell Research (SBGR 85.087).
Price JB (1985b) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity, skin and eye irritancy of a Fastac 100 g/litre
suspension concentrate (SF 06378). Sittingbourne, Shell Research (SBGR
84.298).
Price JB (1986) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity, skin and eye irritancy of SF 06615, a 15
g/litre suspension concentrate of WL85871 ("Fastac", "Fendona").
Sittingbourne, Shell Research (SBGR 85.224).
Price JB (1987) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity of Fastac/BPMC 10/400 g/litre EC (SF 06717).
Sittingbourne, Shell Research (SBGR 86.253).
Price JB (1988) Toxicology of animal health products; the acute oral
and percutaneous toxicity, skin and eye irritancy of two pour-on
formulations of Renegade, SF 06954 (10 g/litre) and SF 06977 (15
g/litre). Sittingbourne, Shell Research (SBGR 87.161).
Roberts BL & Dorough HW (1984) Relative toxicities of chemicals to the
earthworm Eisenia foedita. Environ Toxicol Chem, 3(1): 67-68.
Rose GP (1982) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity of WL85871 ( cis-2-Ripcord) comparison with
Ripcord. Sittingbourne, Shell Research (SBGR 82.130).
Rose GP (1983a) Toxicology of pyrethroids; the acute oral toxicity of
WL85871 in comparison with WL43467. Sittingbourne, Shell Research
(SBGR 83.101).
Rose GP (1983b) Neurotoxicity of WL85871 in comparison with WL43467;
the effect of twenty oral doses of WL85871 or WL43467 over a period of
4 weeks on the rat sciatic/posterior tibial nerve, trigeminal nerve
and trigeminal ganglion. Sittingbourne, Shell Research (SBGR 83.185).
Rose GP (1984a) Toxicology of pyrethroids; the acute intraperitoneal
toxicity of technical Fastac. Sittingbourne, Shell Research (SBGR
84.085).
Rose GP (1984b) Toxicology of Fastac; the eye irritancy potential of
the Fastac 100 g/litre emulsifiable concentrate formulation DF 05898
and its formulation components. Sittingbourne, Shell Research (SBGR
84.053).
Rose GP (1984c) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity, skin and eye irritancy of the Fastac 15 g/litre
ULV formulation SF 06363. Sittingbourne, Shell Research (SBGR 84.145).
Rose GP (1984d) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity, skin and eye irritancy of the Fastac 10 EC
formulation, 5835 B. Sittingbourne, Shell Research (SBGR 84.077).
Rose GP (1984e) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity, skin and eye irritancy of the Fastac 10 EC
formulation 5898 B. Sittingbourne, Shell Research (SBGR 84.078).
Rose GP (1984f) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity, skin and eye irritancy of the Fastac 3 EC
formulation, DF 06353. Sittingbourne, Shell Research (SBGR 84.084).
Rose GP (1984g) Toxicology of pyrethroids; the eye irritancy and skin
sensitizing potential of the 100 g/litre Fastac emulsifiable
concentrate formulation EF 5835. Sittingbourne, Shell Research (SBGR
84.086).
Rose GP (1984h) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity, skin and eye irritancy of the 15:120 g/litre
Fastac/methomyl emulsifiable concentrate formulation, FD 9148.
Sittingbourne, Shell Research (SBGR 84.104).
Rose GP (1985) Toxicology of insecticides; the acute oral and
percutaneous toxicity, skin and eye irritancy of the 30 g/litre Fastac
EC formulation SF 06446. Sittingbourne, Shell Research (SBGR 84.209).
Senior PL & Lavers A (1990a) Fastac - Potential dermal exposure. The
Hague, Shell Internationale Petroleum Maatschappij B.V. (Report Series
HSE 90.016).
Senior PL & Lavers A (1990b) A field study of operator exposure to
Fastac during crop spraying at Shell Research Ltd, Sittingbourne
Research Centre. The Hague, Shell Internationale Petroleum
Maatschappij B.V. (Report Series HSE 90.014).
SHELL (1983a) Review of mammalian and human toxicology; Fastac. The
Hague, Shell Internationale Petroleum Maatschappij B.V. (Review Series
MDT 83.001).
SHELL (1983b) Review of environmental toxicology; Fastac. The Hague,
Shell Internationale Petroleum Maatschappij B.V. (Review Series MDT
83.005).
SHELL (1984) Review of residue information; Fastac. London, Shell
International Chemical Company Ltd.
SHELL (1986) Determination of residues of alpha-cypermethrin in rat
blood - Gas chromatographic method. Sittingbourne, Shell Research
(Analytical Method Series, No. SAMS 436-1).
SHELL (1987a) Determination of WL 85871 and the ratio of the
enantiomer pairs in technical material and formulated products -
liquid chromatographic method. Sittingbourne, Shell Research
(Analytical Methods Series, No. SAMS 346-4).
SHELL (1987b) Intereg residues section: Ripcord residues in crops.
London, International Chemical Company, Ltd (Internal report).
SHELL (1987c) Intereg residues section: Fastac residues in crops.
London, International Chemical Company, Ltd (Internal report).
SHELL (1988a) Determination of residues of alpha-cypermethrin in
animal tissues - Gas-liquid chromatographic method. Sittingbourne,
Shell Research (Analytical Method Series, No. SAMS 461-1).
SHELL (1988b) Determination of residues of alpha-cypermethrin in milk
- Gas-liquid chromatographic method. Sittingbourne, Shell Research
(Analytical Method Series, No. SAMS 456-1).
SHELL (1989a) Determination of residues of alpha-cypermethrin in crops
- Gas chromatographic method. Sittingbourne, Shell Research
(Analytical Method Series, No. SAMS 351-2).
SHELL (1989b) Review of environmental toxicology; Fastac. The Hague,
Shell Internationale Petroleum Maatschappij B.V. (Review Series HSE
89.008).
SHELL (1990a) Determination of residues of alpha-cypermethrin in water
- Gas chromatographic method. Sittingbourne, Shell Research
(Analytical Method Series, No. SAMS 469-2).
SHELL (1990b) Determination of residues of alpha-cypermethrin in soils
- Gas chromatographic method. Sittingbourne, Shell Research
(Analytical Method Series, No. SAMS 354-2).
Sherren AJ (1988a) Residues of alpha-cypermethrin in cattle tissues
following topical treatment of calves with "Renegade" pour-on in the
UK. London, Shell International Chemical Company, Ltd (Internal report
SBGR 88.036).
Sherren AJ (1988b) Residues of alpha-cypermethrin in milk following
topical treatment of cows with "Renegade" pour-on in the UK. London,
Shell International Chemical Company, Ltd (Internal report SBGR
88.037).
Shires SW (1982) A comparison of the toxicity of WL85871 and Ripcord
to rainbow trout ( Salmo gairdneri, Richardson) in small field
enclosures. Sittingbourne, Shell Research (SBGR 82.089).
Shires SW (1983a) Pesticides and honey bees; case studies with Ripcord
and Fastac. Span, 26(3): 118-120.
Shires SW (1983b) Effect of formulation type on the toxicity of
insecticides to fish. Sittingbourne, Shell Research (SBGR 83.015).
Shires SW (1985a) A step-wise evaluation of the effects of a new
pyrethroid insecticide WL85871 on honey bees. Pestic Sci, 16(2):
205-216.
Shires SW (1985b) Toxicity of a new pyrethroid insecticide WL85871 to
rainbow trout. Bull Environ Contam Toxicol, 34: 134-137.
Shires SW & Inglesfield C (1986) A field study of the effects of
"Fastac" (WL85871) on rice pests and their natural enemies.
Sittingbourne, Shell Research (SBGR 86.003).
Shires SW, Le Blanc J, Debray P, Forbes S, & Louveaux J (1984a) Field
experiments on the effects of a new pyrethroid insecticide WL85871 on
bees foraging artificial aphid honey-dew on winter wheat. Pestic Sci,
15: 543-552.
Shires SW, Murray A, Debray P, & Le Blanc J (1984b) The effects of a
new pyrethroid insecticide WL85871 on foraging honey bees ( Apis
mellifera L.) Pestic Sci, 15: 491-499.
Shires SW, Le Blanc J, Murray A, Forbes S, & Debray P (1984c) A field
trial to assess the effects of a new pyrethroid insecticide WL85871 on
foraging honey bees in oilseed rape. J Agric Res, 23(4): 217-226.
Stephenson RR (1982) WL85871 and cypermethrin; a comparison of their
acute toxicity to Salmo gairdneri, Daphnia magna and Selenastrum
capricornutum. Sittingbourne, Shell Research (SBGR 81.277).
Stephenson RR (1983) WL85871 and cypermethrin; a comparative study of
their toxicity to the fathead minnow, Pimephales promelas
(Rafinesque). Sittingbourne, Shell Research (SBGR 82.298).
Stephenson RR (1986) "Fastac"; the acute toxicity of different
formulations to fish in rice paddies. Sittingbourne, Shell Research
(SBGR 85.201).
Stephenson RR (1987a) The effects of "Fastac" (OSC) on fish survival
and growth following its application to paddy rice. Sittingbourne,
Shell Research (SBGR 87.054).
Stephenson RR (1987b) An insecticide formulation that spares fish.
Span, 30(2): 75-77.
Stephenson RR (1987c) The effects of "Fastac" (OSC) on fish following
its application to outdoor tanks. Sittingbourne, Shell Research (SBGR
86.227).
Stone CM & Watkinson RJ (1983) WL85871; an assessment of ready
biodegradability. Sittingbourne, Shell Research (SBGR 83.206).
Tasei J-N, Carre S, Bosio PG, Debray P, & Hariot J (1987) Effects of
the pyrethroid insecticide WL85871 and Phosalone on adults and progeny
of the leaf-cutting bee, Megachile rotundata F., pollinator of
lucerne. Pestic Sci, 21: 119-128.
Van Sittert NJ, Eadsforth CV, & Bragt P (1985) Human oral
dose-excretion study with Fastac. The Hague, Shell Internationale
Petroleum Maatschappij B.V. (Report Series HSE 85.010).
Vijverberg HPM & Van Den Bercken J (1990) Neurotoxicological effects
and the mode of action of pyrethroid insecticides. Crit Rev Toxicol,
21(2): 105-126.
Western NJ (1984) Report on the assessment of Fastac exposures during
formulation of technical concentrate (TC) and technical material (TM)
at Durban, South Africa, September 1983. The Hague, Shell
Internationale Petroleum Maatschappij B.V. (Report Series HSE 84.003).
WHO (1989) Environmental Health Criteria 82: Cypermethrin. Geneva,
World Health Organization, 154 pp.
Wooder MF (1982) Studies on the effect of WL85871 on the integrity of
rat liver DNA in vivo. Sittingbourne, Shell Research (SBGR 81.225).
Woollen BH, Marsh JR, & Chester G (1991) Metabolite profiles of a
pyrethroid insecticide following oral and dermal absorption in man.
In: Proceedings of a Conference on Percutaneous Penetration,
Southampton, England, 10-12 April 1991.
Worthing CR & Hance RJ (1991) Fastac. In: The pesticides manual: a
world compendium, 9th ed. Croydon, British Crop Protection Council, pp
210-211.
APPENDIX I
Cypermethrin: Summary, Evaluation, Conclusions, and
Recommendations (Reprint from EHC 82 on Cypermethrin, WHO/IPCS, 1989)
1. Summary
1.1 General
Cypermethrin was initially synthesized in 1974 and first marketed
in 1977 as a highly active synthetic pyrethroid insecticide, effective
against a wide range of pests in agriculture, public health, and
animal husbandry. In agriculture, its main use is against foliage
pests and certain surface soil pests, such as cutworms, but because of
its rapid breakdown in soil, it is not recommended for use against
soil-borne pests below the surface.
In 1980, 92.5% of all the cypermethrin produced in the world was
used on cotton; in 1982, world production was 340 tonnes of the active
material. It is mainly used in the form of an emulsifiable
concentrate, but ultra-low volume concentrates, wettable powders, and
combined formulations with other pesticides are also available.
Chemically, cypermethrin is the alpha-cyano-3-phenoxybenzyl ester
of the dichloro analogue of chrysanthemic acid,
2,2-dimethyl-3-(2,2-dichlorovinyl) cyclopropanecarboxylic acid. The
molecule embodies three chiral centres, two in the cyclopropane ring
and one on the alpha cyano carbon. These isomers are commonly grouped
into four cis and four trans isomers, the cis group being the more
powerful insecticide. The ratio of cis to trans isomers varies from
50:50 to 40:60. Cypermethrin is the racemic mixture of all eight
isomers and, in this appraisal, cypermethrin refers exclusively to the
racemic mixture (ratio 50:50) unless otherwise stated.
Most technical grades of cypermethrin contain more than 90% of
the active material. The material varies in physical form from a
brown-yellow viscous liquid to a semi-solid.
Cypermethrin has a very low vapour pressure and solubility in
water, but it is highly soluble in a wide range of organic solvents.
Analytical methods are available for the determination of cypermethrin
in commercially available preparations. In addition, methods for the
determination of residues of cypermethrin in foods and in the
environment are well established. In most substrates, the practical
limit of determination is 0.01 mg/kg.
1.2 Environmental transport, distribution and transformation
Unlike the natural pyrethrins, cypermethrin is relatively stable
to sunlight and, though it is probable that photodegradation plays a
significant role in the degradation of the product on leaf surfaces
and in surface waters, its effects in soils are limited. The most
important photodegradation products, 2,2-dimethyl-3-
(2,2-dichlorovinyl) cyclopropane carboxylic acid (CPA),
3-phenoxy-benzoic acid (PBA) and, to some extent, the amide of the
intact ester, do not differ greatly from those resulting from
biological degradation.
Degradation in the soil occurs primarily through cleavage of the
ester linkage to give CPA, PBA, and carbon dioxide. Some of the carbon
dioxide is formed through the cleavage of both the cyclopropyl and
phenyl rings under oxidative conditions. The half-life of cypermethrin
in a typical fertile soil is between 2 and 4 weeks.
Cypermethrin is adsorbed very strongly on soil particles,
especially in soils containing large amounts of clay or organic
matter. Movement in the soil is therefore extremely limited and
downward leaching of the parent molecule through the soil does not
occur to an appreciable extent under normal conditions of use. The two
principal degradation products show, on the scale of Helling,
"intermediate mobility".
Cypermethrin is also relatively immobile in surface waters and,
when applied to the surface of a body of water at rates typical of
those used in agriculture applications, it is largely confined to the
surface film and does not reach deeper levels or the sediment in
appreciable concentrations. Cypermethrin also degrades readily in
natural waters with a typical half-life of about 2 weeks. It is
probable that both photochemical and biological processes play a part.
It has been shown that spray drift reaching surface waters adjacent to
sprayed fields does not result in long-term residues in such waters.
Accumulation studies have shown that cypermethrin is rapidly
taken up by fish (accumulation factor approximately 1000); the
half-life of residues in rainbow trout was 8 days. In view of the low
concentrations of cypermethrin that are likely to arise in water
bodies and their rapid decline, it has been concluded that, under
practical conditions, residues in fish will not reach measurable
levels.
The results of field studies have shown that, when applied at
recommended rates, the levels of cypermethrin and its degradation
products in soil and surface waters are very low. Thus, it is unlikely
that the recommended use of cypermethrin will have any effects on the
environment.
1.3 Environmental levels and human exposure
Cypermethrin is used in a wide range of crops. In general, the
maximum residue limits are low, ranging from 0.05 to 2.0 mg/kg in the
different food commodities. The residues will be further reduced
during food processing. In food of animal origin, residues may range
between 0.01 and 0.2 mg/kg product. Residues in non-food commodities
are generally higher, ranging up to 20 mg/kg product.
Total dietary intake values for man are not available, but it can
be expected that the oral exposure of the general population is low to
negligible.
1.4 Kinetics and metabolism
Absorption of cypermethrin from the gastrointestinal tract and
its elimination are quite rapid. The major metabolic reaction is
cleavage of the ester bond. Elimination of the cyclopropane moiety in
the rat, over a 7-day period, ranged from 40 to 60% in the urine and
from 30 to 50% in the faeces; elimination of the phenoxybenzyl moiety
was about 30% in the urine and 55 to 60% in the faeces. Biliary
excretion is a minor route of elimination for the cyclopropane moiety
and small amounts are exhaled as carbon dioxide. In principle, these
absorption and elimination rates and metabolic pathways hold for all
animal species studied, including domestic animals. In cows fed 100 mg
cypermethrin/day, the highest level found in milk was 0.03 mg/litre;
levels of up to 0.1 mg/kg tissue were found in subcutaneous fat. Under
practical conditions, the oral intake of cypermethrin with feed will
be much lower. Cypermethrin used as a spray or dip to combat
parasites, may give rise to maximum residues of 0.05 mg/kg tissue and
0.01 mg/litre milk.
Laying hens exposed orally to 10 mg cypermethrin/kg diet for 2
weeks, showed cypermethrin levels of up to 0.1 mg/kg in the fat, and
up to 0.09 mg/kg in the eggs (predominantly in the yolk).
Consistent with the lipophilic nature of cypermethrin, the
highest mean tissue concentrations are found in body fat, skin, liver,
kidneys, adrenals, and ovaries. Only negligible concentrations are
found in the brain. The half-life of cis-cypermethrin in the fat of
the rat ranges from 12 to 19 days and that of the trans isomer, from
3 to 4 days. In mice, these half-lives are 13 days and 1 day,
respectively.
Overall, the metabolic transformation has been similar in the
different animals studied, including man. Differences that occur have
been related to the rate of formation rather than to the nature of the
metabolites formed and to conjugation reactions. Cypermethrin (both
the cis and trans isomers) is metabolized via the cleavage of the
ester bond to phenoxybenzoic acid and cyclopropane carbolic acid. The
fact that thiocyanate has been identified in in vivo studies,
indicates that the cyanide moiety is further metabolized. The
3-phenoxybenzoic acid is mainly excreted as a conjugate. The type of
conjugate differs in a number of animal species. Phenoxybenzoic acid
is further metabolized to a hydroxy derivative and conjugated with
glucuronic acid or sulfate. The cyclopropyl moiety is mainly excreted
as a glucuronide conjugate, hydroxylation of the methyl group only
occurring to a limited extent.
Ester cleavage is much slower in certain fish species than in
other animal species, the main metabolic pathway being hydroxylation
of the phenoxybenzoic and the cyclopropyl moieties.
Ester cleavage also takes place in plants. The phenoxybenzyl and
cyclopropyl moieties are readily converted into glucoside conjugates.
In mammals, these conjugates are hydrolysed into the original acids
and metabolized.
1.5 Effects on organisms in the environment
High doses of cypermethrin may exert transient minor effects on
microflora activity in the soil. However, no influence on
ammonification and nitrification has been found.
Cypermethrin is very toxic for fish (in laboratory tests 96-h
LC50s were generally within the range of 0.4-2.8 µg/litre), and
aquatic invertebrates (LC50s in the range of 0.01 - > 5 µg/litre).
The presence of suspended solids decreases the toxicity by at least a
factor of 2, because of adsorption of cypermethrin to the solids.
Cypermethrin is not very toxic for birds. Signs of cypermethrin
intoxication were seen at dose levels of 3000 mg/kg body weight or
more. Administration of 1000 mg cypermethrin/kg body weight to laying
hens over a 5-day period did not cause signs of intoxication. However,
cypermethrin was highly toxic for honey bees in laboratory tests, the
oral LD50 ranging from 0.03 to 0.12 µg/bee. Under field conditions,
the hazard is considerably lower, because of the repellent effect of
cypermethrin on worker honey bees, which lasts for at least 6 h after
spraying.
Earthworms are not sensitive to cypermethrin. No deaths occurred
in worms exposed to levels of 100 mg/kg soil for 14 days.
In studies involving deliberate overspraying of experimental
ponds under field conditions, peak concentrations of 2.6 µg
cypermethrin/litre were measured in the water. Fish were not affected,
but populations of crustaceae, mites, and surface-breathing insects
were severely reduced. Most of these populations returned to normal
levels after 15 weeks. Free-swimming dipterous larvae and
bottom-dwelling invertebrates, snails, flatworms, etc., were not
affected. Under normal agricultural conditions (during which drifts
may reach adjacent ditches or streams), the only effects seen in
surface-breathing or surface-dwelling insects were hyperactivity or
immobilization. The relative toxicity of cypermethrin for pests and
their parasites and predators is such that the balance between
host/prey and parasites/predator may not be adversely affected in the
field. However, care should be taken where predatory mites are
important in pest management.
1.6 Effects on experimental animals and in vitro test systems
The acute oral toxicity of cypermethrin is moderate. While LD50
values differed considerably among animal species depending on the
vehicle used and the cis/trans isomeric ratios, the toxic responses in
all species were found to be very similar. The acute toxicity of the
trans isomer in the rat (LD50 > 2000 mg/kg body weight) was lower
than that of the cis isomer (LD50, 160-300 mg/kg body weight). The
onset of toxic signs of poisoning was rapid and they disappeared
within several days in survivors. The toxic signs are characterized by
salivation, tremors, increased startle response, sinuous writhing of
the whole body (choreoathetosis), and clonic seizures. Myelin and axon
degeneration were noted in the sciatic nerve at near lethal dose
levels.
Cypermethrin was moderately to severely irritating, when applied
to the skin or the eye of the rabbit. The severity was partly
dependent on the vehicle used. In guinea-pigs, a mild skin sensitizing
potential was found using the maximization test.
No toxic effects were observed in rats, fed cypermethrin at 100
mg/kg diet for 3 months. Furthermore, prolonged feeding of
cypermethrin (2 years) to dogs at a level of 300 mg/kg feed did not
produce any toxicological effects. A level of 600 mg/kg diet resulted
in reduced body weight gain, but no gross pathological or
histopathological effects were seen.
Two long-term studies on rats and one on mice were carried out.
The dose levels in the rat studies ranged up to 1500 mg/kg diet,
equivalent to 75 mg/kg body weight. No effects were seen at 150 mg/kg
diet. At the highest dose level, reduced body weight gain, increased
liver weights (accompanied by increased smooth endoplasmatic
reticulum), and some haematological and biochemical changes were
observed. No increase in tumour incidence was noted. The same type of
effects were seen in the mouse study at 1600 mg cypermethrin/kg diet.
No effects were seen in the 400 mg/kg diet group.
The effect of cypermethrin on the immune system was studied in
rats. The results showed the possibility of immune suppression by
pyrethroids. More attention should be paid to this aspect, but, at
present, no opinion can be given about its relevance in the
extrapolation of these data for man.
Repeated oral administration of cypermethrin to rats and other
animal species at levels sufficiently high to produce significant
mortality in one group of animals, produced biochemical changes in the
peripheral nerves, consistent with sparse axonal degeneration.
Histopathological changes (swelling and/or disintegration of axons of
the sciatic nerve) were observed. There was no cumulative effect. The
magnitude of the change was substantially less than that encountered
with established neurotoxic agents. The neurotoxic effects seem to be
reversible; presumably the clinical signs are not related to the
induction of neuropathological lesions.
Further evidence to support the minor nature of the nerve lesions
has been afforded by electrophysiological studies on rats.
Measurements of the maximal motor conduction velocities of the sciatic
and tail nerves of rats were made before, and at intervals of up to 5
weeks after, exposure to a single dose or repeated high doses of
cypermethrin. It was concluded from the results that, even at
near-lethal doses, cypermethrin did not cause any effects on maximal
motor conduction velocities and conduction velocities of the slower
motor fibres in rat peripheral nerves. No delayed neurotoxicity was
observed in domestic hens.
The ability of the major metabolite of cypermethrin,
3-phenoxybenzoic acid, to produce axonal changes has been investigated
and found to be negative.
In a multigeneration reproduction study on rats, dose levels up
to 500 mg/kg feed were tested. The parent animals at the highest dose
level showed decreased food intake and reduction in body weight gain.
No influence on reproductive performance or on survival of the
offspring was found. However, at the highest dose level, reductions in
litter size and total litter weights were seen. The pooled body
weights of weaning pups of the 500 mg/kg group were decreased over 3
generations. No effect was found with 100 mg cypermethrin/kg diet.
Embryotoxic and teratogenic effects were not found in rats
administered dose levels of up to 70 mg/kg body weight and clear
teratogenic effects were not observed in rabbits given dose levels of
up to 30 mg/kg body weight during days 6-18 of gestation.
Cypermethrin did not show any mutagenic activity in bacteria or
in yeast, with or without metabolic activation, or in V79 Chinese
hamster cells. Furthermore, cypermethrin gave negative results in an
in vivo chromosomal aberration test with Chinese hamsters and in
dominant lethal studies on mice. In a host-mediated assay with mice,
no increase in the rate of mitotic gene conversion in Saccharomyces
cerevisae was found. In a chromosome study using the bone marrow
cells of Chinese hamsters, cypermethrin did not increase the number of
chromosome abnormalities. However, in a micronucleus test with mouse
bone marrow cells, an increase in the frequency of polychromatic
erythrocytes with micronuclei was found after oral and dermal
applications of cypermethrin. Intraperitoneal application gave a
negative result. A sister chromatid exchange study using bone marrow
cells of mice showed a dose-response related increase in sister
chromatid exchanges of dividing cells.
In long-term/carcinogenicity studies, oral administration of
cypermethrin to rats did not induce an increase in the incidence of
tumours. In a mouse study, dose levels of up to 1600 mg
cypermethrin/kg diet did not produce any increase in tumours of types
not commonly associated with the mouse strain employed. The incidence
of tumours was similar in all groups with the exception of a slight
increase in the incidence of benign alveolar lung tumours in the
females in the 1600 mg/kg diet group. However, the increased
incidence, when compared with concurrent and historical control
incidence, was not sufficient to warrant concern. There was no
suggestion of increased malignancy and no evidence of a decrease in
the latency of the tumours. Furthermore, there was no evidence of a
carcinogenic response in the male mice in this study and, as the
results of mutagenicity studies on cypermethrin have been mainly
negative, it is concluded that there is no evidence for the
carcinogenic potential of cypermethrin.
1.7 Mechanism of toxicity
Extensive studies have been carried out to explain the mechanism
of toxicity of cypermethrin, especially with regard to the effects on
the nervous system. The results strongly suggest that the primary
target site of cypermethrin (and of pyrethroid insecticides in
general) in the vertebrate nervous system is the sodium channel in the
nerve membrane. The alpha-cyano pyrethroids, such as cypermethrin,
cause a long-lasting prolongation of the normally transient increase
in sodium permeability of the nerve membrane during excitation,
resulting in long-lasting trains of repetitive impulses in sense
organs and a frequency-dependent depression of the nerve impulse in
nerve fibres. Since the mechanisms responsible for nerve impulse
generation and conduction are basically the same throughout the entire
nervous system, pyrethroids may well act in a similar way in various
parts of the central nervous system. It is suggested that the facial
skin sensations that may be experienced by people handling
cypermethrin are brought about by repetitive firing of sensory nerve
terminals in the skin, and may be considered as an early warning
signal that exposure has occurred.
1.8 Effects on man
No cases of accidental poisoning have been reported as a result
of occupational exposure.
Skin sensations, reported by a number of authors to have occurred
during field studies, generally lasted only a few hours and did not
persist for more than one day after exposure. Neurological signs were
not observed. General medical and extensive clinical blood-chemistry
studies, and electrophysiological studies on selected motor and
sensory nerves in the legs and arms did not show any abnormalities.
2. Evaluation
Cypermethrin, an alpha-cyano pyrethroid consisting of a mixture
of 8 stereoisomers, is a highly active insecticide effective against
a wide range of pests in many food and non-food commodities.
It is stable to light and heat, it has a low vapour pressure and
is more stable in acidic than in alkaline media. Sensitive analytical
methods for the determination of residues in food and the environment
are available.
When cypermethrin is applied to crops, residues may occur in
soils and surface waters, but biological degradation is fairly rapid
and residues do not accumulate in the environment. Photo-degradation
is unlikely to play an important role. The main route of degradation
is cleavage of the ester linkage to give 2 main degradation products
containing the cyclopropane, and the phenoxybenzyl moiety. The
half-lives in the soil are determined by many factors, but are in the
range of 2-4 weeks. Cypermethrin is strongly adsorbed by soil and
downward leaching is negligible. Because of its rather fast breakdown
forming less toxic products, and the low dose rates used in good
agricultural practice, it is unlikely that cypermethrin will attain
significant levels in the environment.
Bioaccumulation in certain organisms, such as fish, took place
under laboratory conditions, but levels declined on cessation of
exposure and there are indications that, under natural conditions,
fish will not contain measurable residues.
When applied according to good agricultural practice, the levels
of cypermethrin residues in food commodities are generally low. Total
diet studies are not available, but from the available residue
information, it can be inferred that the oral intake by man is well
below the ADI.
High dose levels of cypermethrin may exert transient effects on
the soil microflora. Earthworms and other soil organisms are generally
rather resistant to cypermethrin, while fish and other aquatic
invertebrates are very sensitive. Because of its strong adsorption on
soil, only low levels of cypermethrin may leak into surface water.
These may have transient effects, mainly on surface breathing insects.
The toxicity of cypermethrin for birds is low. Bees appear to be
very sensitive in laboratory tests. Under field conditions, the effect
on bees is minimal, because cypermethrin seems to have a repellent
effect for bees. Absorption and elimination of cypermethrin has been
rapid in the different mammalian species tested. The major metabolic
reaction is cleavage of the ester bond followed by hydroxylation and
conjugation of the cyclopropane and phenoxybenzyl moiety. The highest
levels are found in body fat, which is consistent with the lipophilic
nature of cypermethrin. The half-life in the fat of the rat is about
12-19 days for the cis isomer and 3-4 days for the trans isomer.
Breakdown products in plants are bound as glucosides.
The acute toxicity of cypermethrin for mammals is of a moderate
order. The oral LD50 for the rat ranges from 200 to 4000 mg/kg body
weight. Short-term and long-term toxicity studies on rats, mice, and
dogs have shown effects on growth, on the liver and kidneys, and the
nervous system, and on haematology. A no-observed-adverse-effect level
of 7.5 mg/kg body weight has been adopted by the Task Group.
Cypermethrin was not carcinogenic for mice or rats fed diets
containing high levels of the material over a 2-year period.
Cypermethrin did not induce teratogenic effects in either rats at 70
mg/kg body weight or rabbits at 30 mg/kg body weight. It was also
shown not to have any effects on reproductive performance during a
3-generation reproduction study in rats administered 100 mg/kg diet.
In a variety of mutagenicity studies, cypermethrin was shown to be
mainly without mutagenic activity.
The mechanism of the action on the nervous system has been
extensively studied. From these studies and the occupational studies
available, it seems that the skin sensation seen in workers handling
cypermethrin, generally lasts only a few hours and does not persist
for more than one day after exposure. Other neurological signs were
not observed. These skin sensations can be considered to be an early
warning that exposure has occurred and that work practice should be
reviewed. Cypermethrin may cause eye irritation and may be a
sensitizer for certain persons.
3. Conclusions
It can be concluded that:
General population: When applied according to good agricultural
practice, exposure of the general population to cypermethrin is
negligible and is unlikely to present a hazard.
Occupational exposure: With reasonable work practices, hygiene
measures, and safety precautions, the use of cypermethrin is unlikely
to present a hazard to those occupationally exposed to it. The
occurrence of "facial sensations" is an indication of exposure. Under
these circumstances work practice should be reviewed.
Environment: With recommended application rates it is unlikely
that cypermethrin or its degradation products will attain levels of
environmental significance. Notwithstanding its high toxicity for fish
and honey bees, this is only likely to cause a problem in the case of
spillage and overspraying.
4. Recommendations
- Cypermethrin should be included among the residues looked for in
surveillance, market-basket, or total diet studies.
- Attention should be paid to the implications for the welfare of
human beings of animal studies indicating immune suppression.
- Further follow-up studies into the facial effects in human beings
should be conducted, in order to better understand this
phenomenon.
RESUME ET EVALUATION; CONCLUSIONS ET RECOMMANDATIONS
1. Résumé et évaluation
1.1 Identité, emploi, destinée et concentrations dans l'environnement
L'alpha-cyperméthrine est constituée à plus de 90% de
l'énantiomère le plus actif du point de vue insecticide parmi les
quatre isomères cis qui entrent dans la composition de la
cyperméthrine racémique.
C'est un pyréthrinoïde extrêmement actif, qui agit contre de
nombreux parasites auxquels on a affaire dans l'agriculture et
l'élevage. Il est commercialisé sous forme de concentrés
émulsionnables, de formulations à très bas volume, de concentrés pour
suspension ou en mélange avec d'autres insecticides.
Le produit technique se présente sous la forme d'une poudre
cristalline facilement soluble dans l'acétone, la cyclohexanone et le
xylène mais peu soluble dans l'eau. Il est stable en milieu acide ou
neutre mais s'hydrolyse à pH 12-13. Il se décompose au-dessus de 120
°C.
On ne dispose d'aucune donnée sur la concentration de
l'alpha-cyperméthrine dans l'air.
Dans l'eau, il est probable que l'alpha-cyperméthrine est
décomposée par voie photochimique et biologique. On a constaté que,
après avoir traité un étang à raison de 15 g de matière active par
hectare, les eaux de surface et les couches plus profondes contenaient
respectivement 5% et 19% de la dose appliquée une journée après
l'épandage et 0,1% et 2% respectivement de cette dose sept jours plus
tard. Environ 5% de la dose appliquée se retrouvaient dans les
sédiments 16 jours après l'épandage.
Il est probable que l'alpha-cyperméthrine est fortement adsorbée
aux particules du sol. Une année après un épandage à raison de 0,5 kg
de matière active par hectare, on retrouvait dans le sol des résidus
inférieurs à 0,1 mg/kg.
Le coefficient de partage de l'alpha-cyperméthrine entre le
n-octanol et l'eau est égal à 1,4 x 105 (log de Pow = 5,16).
La dose d'emploi recommandée pour l'alpha-cyperméthrine est
moindre que pour la cyperméthrine car elle est biologiquement plus
active. Il en résulte moins de résidus sur les récoltes et si l'on se
conforme aux doses recommandées, ils se situent entre 0,05 et 1 mg/kg.
Chez des poissons-chats d'eau de mer traités à raison de 0,01 à 0,05%
p/p de matière active, on a mesuré des résidus de 0,3 à 30 mg/kg une
semaine après l'entreposage et entre 0,22 et 4,0 mg/kg 15 semaines
plus tard.
1.2 Cinétique et métabolisme
Après avoir été administrée par voie orale à des rats,
l'alpha-cyperméthrine est éliminée dans les urines sous forme du
sulfo-conjugué de l'acide 3-(4-hydroxyphénoxy)benzoïque ainsi que dans
les matières fécales, en partie sans modification. Environ 90% d'une
dose orale unique sont éliminées de l'organisme en quatre jours, dont
78% au cours du premier jour. Les résidus sont faibles dans les
tissus, sauf dans les tissus lipidiques. Trois jours après
administration d'une dose orale unique de 2 mg/kg, la concentration
dans les graisses était de 0,4 mg/kg. L'élimination à partir des
graisses est biphasique; la demi-vie relative à la phase initiale est
de 2,5 jours, et de 17 à 26 jours pour la deuxième phase.
L'alpha-cyperméthrine est métabolisée par coupure de la liaison
ester. Chez le rat, la fraction alcool phénoxybenzylique est
hydroxylée et transformée en sulfo-conjugué; la fraction acide
cyclopropane-carboxylique subit également une conjugaison,
probablement sous forme de glucuronide, avant d'être excrétée par la
voie urinaire. Des études portant sur des microsomes hépatiques de
rats, de lapins et d'origine humaine ont montré que chez ces trois
espèces, il pouvait y avoir hydrolyse de l'ester et métabolisation
oxydative mais que l'hydrolyse de l'ester prédominait dans le cas des
préparations de foie provenant de lapins ou de sujets humains.
Chez l'homme, 43% d'une dose orale (0,25-0,75 mg/litre) ont été
excrétés dans les 24 heures par la voie urinaire sous forme d'acide
cis-cyclopropane-carboxylique libre ou conjugué. Après
l'administration de cinq doses quotidiennes successives, l'excrétion
urinaire n'a pas augmenté.
On a trouvé de fortes concentrations (jusqu'à 1156 mg/kg)
d'alpha-cyperméthrine dans la laine de mouton, 14 jours après avoir
traité les animaux par des bains ou des aspersions. De faibles
quantités ont été retrouvées dans la graisse sous-cutanée (jusqu'à
0,04 mg/kg). Après avoir traité des veaux le long de l'épine dorsale
avec 10 ml d'une formulation à 1,6%, on n'a pas retrouvé
d'alpha-cyperméthrine dans les muscles ni le foie. La concentration
maximale dans la graisse périrénale était de 0,25 mg/kg au bout de 14
jours.
Après avoir traité des vaches en lactation le long de l'épine
dorsale avec des formulations contenant jusqu'à 0,2 g de matière
active on a retrouvé dans le lait de 3 des 15 animaux traités, des
résidus d'alpha-cyperméthrine compris entre 0,003 et 0,005 mg/litre.
1.3 Effets sur les mammifères de laboratoire et les systèmes
d'épreuves in vitro
L'alpha-cyperméthrine présente une toxicité orale aiguë modérée
à forte pour les rongeurs. La DL50 varie beaucoup chez la souris et
le rat et dépend de la concentration du composé et du véhicule
utilisé. En pratique, on considère qu'une DL50 de 80 mg/kg de poids
corporel est représentative. Toutefois, on a fait état de valeurs plus
élevées pour la DL50 par voie orale dans des conditions
d'intoxication aiguë. Une intoxication aiguë par voie orale entraîne
des signes cliniques qui traduisent une action au niveau du système
nerveux central.
Une application cutanée d'alpha-cyperméthrine à des rats et des
souris aux doses respectives de 100 et 500 mg/kg de poids corporel,
n'a entraîné ni mortalité ni signes d'intoxication. De même, des rats
à qui l'on avait fait inhaler pendant quatre heures de
l'alpha-cyperméthrine à une concentration de 400 mg/m3, n'ont
présenté aucune mortalité ni signes cliniques d'intoxication.
L'alpha-cyperméthrine technique ne provoquerait qu'une irritation
minimale de la peau chez le lapin. En revanche, certaines formulations
de ce composé peuvent déterminer une très forte irritation oculaire.
L'alpha-cyperméthrine technique n'a pas d'effet sensibilisant cutané.
Chez le cobaye, elle provoque une stimulation des terminaisons
nerveuses sensorielles de l'épiderme.
Exposés pendant une courte période à de l'alpha-cyperméthrine à
des concentrations quotidiennes allant jusqu'à 200 mg/kg de nourriture
pendant cinq semaines ou jusqu'à 100 mg/kg de nourriture pendant 13
semaines, des rats n'ont pas présenté d'effets toxiques. Aux doses les
plus élevées, on notait des signes d'intoxication traduisant une
atteinte du système nerveux, ainsi qu'une réduction de la croissance
et une augmentation du poids du foie et des reins. Aucun effet bien
net n'a été observé en ce qui concerne les paramètres hématologiques
ou histopathologiques.
Lors d'une étude de 13 semaines au cours de laquelle des chiens
ont reçu de l'alpha-cyperméthrine par voie orale, on a observé que la
dose la plus forte (270 mg/kg de nourriture) produisait des signes
d'intoxication; en revanche tous les autres paramètres étudiés (NFS,
biochimie du sang, urines, poids des organes, anatomopathologie et
histopathologie) sont restés normaux. La dose sans effet observable
était de 90 mg/kg de nourriture (soit l'équivalent de 2,25 mg/kg de
poids corporel par jour).
Le même genre d'étude menée sur des rats a montré que
l'alpha-cyperméthrine provoquait des effets neurotoxiques imputables
à des lésions histopathologiques des nerfs tibial et sciatique, une
dégénérescence des axones et un accroissement de l'activité de la
bêta-galactosidase.
On ne dispose d'aucune donnée sur la toxicité à long terme, la
toxicité pour la fonction de reproduction, la tératogénicité ou
l'immunotoxicité.
En se basant sur les données disponibles, on peut conclure que
l'alpha-cyperméthrine n'est pas mutagène, comme le montrent les tests
effectués sur Salmonella typhimurium, Escherichia coli et
Saccharomyces cerevisiae ainsi que les épreuves in vivo et in
vitro sur des cellules de foie de rat, qui n'ont révélé ni
aberration chromosomique ni lésion de l'ADN monobrin. On n'a pas non
plus observé d'augmentation du nombre d'aberrations chromosomiques
dans des cellules de moelle osseuse de rat.
On ne dispose d'aucune donnée sur la cancérogénicité de
l'alpha-cyperméthrine.
1.4 Effets sur l'homme
Dans la mesure où l'alpha-cyperméthrine est utilisée conformément
aux règles de bonne pratique agricole, l'exposition de la population
générale reste négligeable. On a constaté que l'exposition
professionnelle cutanée des opérateurs procédant à la préparation des
mélanges, au chargement des pulvérisateurs, à l'épandage de
l'insecticide ou au lavage du matériel, pouvait atteindre des valeurs
respectivement égales à 2,94 mg, 0,61 mg et 0,73 mg.
Lors d'une étude sur l'exposition à l'alpha-cyperméthrine au
cours de la préparation de formulations à base de cet insecticide, on
a évalué les niveaux d'exposition par surveillance personnelle et
statique de la concentration atmosphérique de ce composé et dosage de
ses métabolites urinaires. Au cours des deux jours pendant lesquels
les personnes exposées procédaient à la formulation de concentrés
techniques, l'exposition individuelle moyenne dans le groupe a été
respectivement de 2,8 et 4,9 mg/m3, l'exposition individuelle
moyenne du groupe au produit technique étant de 50,1 mg/m3 le
troisième jour. On n'a pas pu déceler la présence de métabolites dans
les urines (limite de détection 0,02 mg/litre). Au cours de la
préparation des diverses formulations, les ouvriers ont fait état de
sensations au niveau de l'épiderme mais en précisant qu'elles étaient
légères.
Aucune intoxication n'a été signalée.
1.5 Effets sur d'autres organismes au laboratoire et dans leur milieu
naturel
La CE50 à 48 et 96 heures (pour la croissance) chez une algue
d'eau douce, Selenastrum capricornutum, dépasse 100 µg/litre.
L'alpha-cyperméthrine est très fortement toxique pour les
invertébrés aquatiques. Les valeurs de la CE50 à 24 et 48 heures
(pour l'immobilisation de la daphnie) sont respectivement égales à 1,0
et 0,3 µg/litre, et celle de la CL50 à 24 heures (pour Gammarus
pulex) est égale à 0,05 µg/litre. L'alpha-cyperméthrine est
également très toxique pour un certain nombre d'arthropodes aquatiques
mais sa toxicité est moindre pour les mollusques. La toxicité à court
terme de ce composé peut être réduite lorsqu'il est présenté sous la
forme d'une suspension dans l'huile. Les pertes à l'épandage peuvent
provoquer des effets toxiques sur les invertébrés aquatiques, mais
comme l'alpha-cyperméthrine disparaît rapidement de l'eau, ceux-ci ont
la possibilité de récupérer. L'alpha-cyperméthrine est très fortement
toxique pour les poissons. La valeur de la CL50 à 96 heures oscille
entre 0,7 et 350 µg/litre selon le type de formulation. Les concentrés
émulsionnables sont beaucoup plus toxiques que les concentrés en
suspension, les poudres mouillables et les formulations
micro-encapsulées. Le danger de l'alpha-cyperméthrine pour les
invertébrés aquatiques et les poissons tient à sa toxicité aiguë. Rien
n'indique toutefois qu'une exposition prolongée entraîne des effets
cumulatifs.
On ne dispose d'aucune donnée concernant les effets de
l'alpha-cyperméthrine sur les microbes terricoles. Les bactéries
présentes dans les effluents n'ont pas paru affectées par une
concentration de 3 mg/litre en système fermé.
La toxicité de l'alpha-cyperméthrine pour certains carabides et
les larves de neuroptères est relativement faible et elle ne présente
guère de danger pour les stades pré-imaginaux des hyménoptères
parasitoïdes. Des études menées sur des champs de grande superficie ou
de petites parcelles ont montré que l'alpha-cyperméthrine était peu
dangereuse pour les carabides et les staphylinides mais qu'elle
présentait un risque relativement important pour les linyphiides. Les
effets sur ces populations d'insectes se sont limités à une seule
saison de croissance. En outre, l'alpha-cyperméthrine ne présente
guère de risque pour les larves de syrphides mais n'est pas dénuée
d'effets sur les coccinellides. Malgré tout, la dissipation rapide des
résidus présents sur le feuillage permet à ces animaux de reconstituer
rapidement leurs colonies.
L'épandage d'alpha-cyperméthrine n'a pas d'effets indésirables
sur l'abondance relative des arthropodes entomophages. Son utilisation
sur les céréales à petits grains n'entraînerait donc pas la
réapparition des ravageurs ou le déclenchement d'infestations
secondaires.
Des études de laboratoire ont montré que l'alpha-cyperméthrine
était peu toxique pour les lombrics. Des vers placés pendant 14 jours
dans un sol artificiel contenant ce composé à des concentrations
allant jusqu'à 100 mg/kg, n'ont présenté aucune mortalité.
Des études de toxicité aiguë également effectuées en laboratoire
ont montré que l'alpha-cyperméthrine était extrêmement toxique pour
les abeilles. Administré par voie orale, un concentré émulsionnable
d'alpha-cyperméthrine a donné une DL50 à 24 heures de 0,13
µg/abeille, la valeur correspondante pour l'administration topique
étant de 0,03 µg/abeille (de produit technique) ou 0,11 µg/abeille (de
concentré émulsionnable). La forte toxicité de l'alpha-cyperméthrine
pour les abeilles ne s'est pas manifestée au cours des épreuves de
plein champ, probablement du fait que le composé a un bref effet
répulsif qui réduit le butinage et, par voie de conséquence,
l'exposition des insectes.
On ne dispose d'aucune donnée sur la toxicité de
l'alpha-cyperméthrine pour les oiseaux.
2. Conclusions
2.1 Population générale
Lorsque l'épandage de l'alpha-cyperméthrine s'effectue
conformément aux règles de bonne pratique, son utilisation en
agriculture n'expose guère la population générale à ce composé et il
y a peu de danger pour elle.
2.2 Exposition professionnelle
Moyennant de bonnes méthodes de travail ainsi que des mesures
d'hygiène et de sécurité, l'utilisation de l'alpha-cyperméthrine ne
devrait pas présenter de danger pour les personnes qui y sont exposées
de par leur profession. L'apparition de sensations au niveau de la
face indique une contamination. Dans ces circonstances, il est bon de
revoir les méthodes de travail.
2.3 Environnement
Aux doses d'emploi recommandées, il n'est guère probable que
l'alpha-cyperméthrine puisse être libérée dans l'environnement à des
concentrations écologiquement dangereuses. Elle est fortement toxique
pour les arthropodes aquatiques, les poissons et les abeilles dans les
conditions du laboratoire. On ne peut envisager la probabilité
d'effets toxiques importants sur les invertébrés non visés et les
poissons qu'en cas de déversement accidentel, d'épandage excessif ou
d'erreur de manipulation.
3. Recommandations
* Il convient d'éviter la contamination des eaux superficielles par
l'alpha-cyperméthrine.
* L'alpha-cyperméthrine se lie fortement aux particules. D'autres
études écotoxicologiques sont à effectuer à propos des effets de
l'alpha-cyperméthrine sur les organismes qui vivent dans les
sédiments car il s'agit d'un aspect qui n'a guère retenu
l'attention jusqu'ici.
* L'absorption dans les voies digestives de l'alpha-cyperméthrine
est à étudier dans diverses conditions expérimentales.
* Il faudrait également étudier la destinée de
l'alpha-cyperméthrine après application sur l'épiderme.
* Il faudrait obtenir des données supplémentaires sur la toxicité
à long terme, la cancérogénicité et l'immunotoxicité de
l'alpha-cyperméthrine.
RESUMEN Y EVALUACION; CONCLUSIONES Y RECOMENDACIONES
1. Resumen y evaluación
1.1 Identificación, uso, destino y niveles en el medio ambiente
La alfa-cipermetrina contiene más del 90% del par de
enantió-meros con mayor actividad insecticida de los cuatro isómeros
cis de la cipermetrina en mezcla racémica.
Se trata de un insecticida piretroide sumamente activo contra una
gran variedad de plagas habituales en agricultura y ganadería. Existe
como concentrado emulsionable, formulacion de volumen ultra-bajo,
concentrado en suspensión y en mezcla con otros insecticidas.
El producto técnico es un polvo cristalino, con buena solubilidad
en acetona, ciclohexanona y xileno, y con baja solubilidad en agua. Es
estable en condiciones ácidas o neutras, pero se hidroliza a pH 12-13.
Se descompone por encima de los 220 °C.
No se dispone de información acerca de los niveles de
alfa-cipermetrina en el aire.
Es probable que la degradación de la alfa-cipermetrina en agua se
deba a procesos fotoquímicos y biológicos. El agua superficial y
subsuperficial de un estanque rociado con 15 g/ha de principio activo
contenía el 5% y el 19% de la dosis aplicada un día después del
rociamiento, y 0.1% y 2% siete días más tarde. A los 16 días de la
aplicación se encontró en el sedimento alrededor del 5% de la dosis
utilizada.
Es probable que la alfa-cipermetrina se adsorba con fuerza a las
partículas del suelo. Un año después del tratamiento con 0.5 kg de
principio activo por hectárea se encontraron en el suelo residuos
inferiores a 0.1 mg/kg.
El coeficiente de reparto n-octanol/agua de la alfa-cipermetrina
es 1.4 x 105 (log Poa = 5.16).
Las tasas de aplicación recomendadas de alfa-cipermetrina son
inferiores a las de cipermetrina, porque la primera es biológicamente
más activa. En consecuencia, los residuos en los cultivos son escasos,
y utilizando las tasas de aplicación recomendadas estos residuos
oscilan entre 0.05 y 1 mg/kg. Los residuos en peces siluroideos
marinos tratados con dosis entre el 0.001 y el 0.05% p/p de principio
activo eran de 0.3-30 mg/kg una semana después del almacenamiento, y
de 0.22 a 4.0 mg/kg tras 15 semanas de almacenamiento.
1.2 Cinética y metabolismo
La alfa-cipermetrina administrada por vía oral a ratas se elimina
por la orina como sulfato conjugado del ácido 3-(4-hidroxifenoxi)
benzoico y en parte como compuesto inalterado por las heces. De una
dosis oral única alrededor del 90% se elimina del cuerpo en un período
de cuatro días, y el 78% en el primer día. Los residuos en los tejidos
son escasos, excepto en el adiposo. La concentración en la grasa tres
días después de una dosis oral única de 2 mg/kg fue de 0.4 mg/kg. La
eliminación a partir de la grasa es bifásica: en la fase inicial tiene
una vida media de 2.5 días y en la segunda de 17 a 26 días.
La alfa-cipermetrina se metaboliza mediante la ruptura de su
enlace éster. En la rata, el alcohol fenoxibencílico de la molécula se
hidroxila y se conjuga con sulfato, y el ácido
ciclopropano-carboxílico también se conjuga (probablemente en forma de
glucurónido) antes de la excreción urinaria. En estudios con
microsomas hepáticos de ratas, conejos y seres humanos se ha
demostrado que la hidrólisis en el ester y rutas oxidativas se dan en
las tres especies, si bien la primera es la ruta predominante en las
preparaciones hepáticas de conejo y de ser humano.
En el ser humano, el 43% de una dosis oral (0.25-0.75 mg) se
excreta en la orina en un plazo de 24 h en forma de ácido cis-
ciclopropano-carboxílico libre o conjugado. La excreción urinaria no
aumentó tras la administración de 5 dosis diarias sucesivas.
En la lana de las ovejas se detectaron concentraciones altas
(hasta 1156 mg/kg) de alfa-cipermetrina 14 días después de la
aplicación por inmersión o por lavado. En la grasa subcutánea se
encontraron niveles bajos (hasta 0.04 mg/kg). Después de tratar
terneros a lo largo del dorso con 10 ml de una preparación al 1.6%, no
se detectó alfa-cipermetrina en los músculos ni en el hígado. La
máxima concentración en la grasa perirrenal durante un período de 14
días fue de 0.26 mg/kg.
Tras la aplicación de hasta 0.2 ml de principio activo a lo largo
del dorso de vacas lecheras, se encontraron en la leche de tres en 15
animales tratados residuos de alfa-cipermetrina en concentraciones que
oscilaban entre 0.003 y 0.005 mg/ml.
1.3 Efectos en mamíferos de laboratorio y en sistemas de prueba
in vitro
En roedores, la toxicidad aguda oral de la alfa-cipermetrina es
entre moderada y alta. Los valores de la DL50 en ratones y ratas son
muy variables y dependen de la concentración del compuesto y del
excipiente. A efectos prácticos, se considera representativo un valor
de la DL50 de 80 mg/kg de peso corporal. Sin embargo, se han
notificado valores más altos de DL50 aguda por vía oral. La
exposición oral aguda produce síntomas clínicos relacionados con la
actividad del sistema nervioso central.
Las aplicaciones cutáneas aisladas de alfa-cipermetrina a ratones
y ratas en concentraciones de 100 y 500 mg/kg de peso corporal,
respectivamente, no produjeron mortalidad ni síntomas de intoxicación.
La exposición de ratas por inhalación durante 4 h a una concentración
atmosférica de 400 mg/m3 tampoco ocasionó mortalidad ni signos
clínicos.
Se ha reportado que la alfa-cipermetrina de calidad técnica
produce una irritación cutánea mínima en el conejo. Algunas de las
preparaciones provocan irritación ocular grave. La alfa-cipermetrina
de calidad técnica no produce sensibilización cutanea. En cobayos
ocasionó la excitación de las terminaciones neuro-sensoriales de la
piel.
La exposición breve de ratas a concentraciones de
alfa-cipermetrina de hasta 200 mg/kg en dieta diaria durante 5 semanas
o hasta 180 mg/kg en dieta diaria durante 13 semanas no produjo
efectos tóxicos. Con dosis más elevadas, las ratas mostraron signos de
intoxicación asociados a la patología del sistema nervioso,
disminución del crecimiento o aumento del peso del hígado y los
riñones. No se pusieron de manifiesto efectos hematológicos,
bioquímicos o histopatológicos claros.
En un estudio de toxicidad oral en perros durante 13 semanas, la
dosis más alta, de 270 mg/kg causó síntomas de intoxicación, pero
todos los demás parámetros examinados (relativos a la hematología,
bioquímica clínica, análisis de orina, peso de los órganos, anatomía
patológica e histopatología) se mantuvieron inalterados. El nivel sin
efectos observados (NOEL) fue de 90 mg/kg de dieta (equivalente a 2.25
mg/kg de peso corporal al día).
En un estudio de toxicidad oral en ratas se demostró que la
alfa-cipermetrina induce neurotoxicidad a causa de alteraciones
histopatológicas de los nervios tibial y ciático, degeneración axonal
y aumento de la actividad de la beta-galactosidasa.
Se carece de datos sobre toxicidad a largo plazo, toxicidad en la
reproducción, teratogenicidad e inmunotoxicidad.
Con los datos disponibles sobre la alfa-cipermetrina, se puede
deducir que se trata de un compuesto no mutagénico en las pruebas con
Salmonella typhimurium, Escherichia coli y Saccaromyces cerevisiae,
y en las pruebas in vivo e in vitro con hepatocitos de rata, con
respecto a la inducción de aberraciones cromosómicas y producción de
lesiones en cadenas simples de ADN. No se observó aumento de las
aberraciones cromosómicas en las células de médula ósea de rata.
Se carece de datos sobre la carcinogenicidad de la
alfa-cipermetrina.
1.4 Efectos en el ser humano
La exposición de la población general a la alfa-cipermetrina es
insignificante siempre que en su utilización se apliquen buenas
prácticas agrícolas. Se comprobó que la exposición cutánea profesional
de los trabajadores durante la mezcla/carga, el rociado y el lavado
del equipo era de hasta 2.94 mg, 0.61 mg y 0.73 mg, respectivamente.
En un estudio de exposición a la alfa-cipermetrina durante la
formulación, se evaluaron los niveles de exposición mediante el
monitoreo personal y estático de las concentraciones atmosféricas y la
medición de los metabolitos en la orina. Los niveles de exposición
personal media del grupo durante los dos días de la formulación de los
concentrados de calidad técnica fueron de 2.8 y 44.9 mg/m3, mientras
que la exposición personal media del grupo al material técnico el
tercer día fue de 54.1 mg/m3. No se detectaron metabolitos en la
orina (límite de detección, 0.02 mg/litro). Durante la formulación se
informó de reacciones cutáneas ligeras.
No se han comunicado casos de envenenamiento.
1.5 Efectos en otros organismos en el laboratorio y en el medio
ambiente
El valor de la CE50 (crecimiento) en las 48 y 96 horas para el
alga de agua dulce Selenastrum capricornutum es superior a 100
µg/litro.
La alfa-cipermetrina es muy tóxica para los invertebrados
acuáticos. Los valores de la CE50 (inmovilización) a las 24 y 48 h
para Daphnia magna son de 1.0 y 0.3 µg/litro, respectivamente, y el
valor de la CL50 a las 24 h para Gammarus pulex es de 0.05
µg/litro. La alfa-cipermetrina es muy tóxica para varios grupos de
artrópodos acuáticos, pero lo es menos para los moluscos. Se puede
reducir la toxicidad a corto plazo del compuesto formulando el
producto como suspensión con mayor cantidad de aceite. Aunque el
arrastre del rociado puede producir efectos tóxicos en los
invertebrados acuáticos, la desaparición rápida de la
alfa-cipermetrina del agua facilita la recuperación.
La alfa-cipermetrina es muy tóxica para los peces. El valor de la
CL50 a las 96 h oscila entre 0.7 y 350 µg/litro, segun la
formulación. Las formulaciones de concentrados emulsionables son mucho
más tóxicas que el concentrado en suspensión, el polvo humectable y
las formulaciones microencapsuladas. El peligro del compuesto para los
invertebrados acuáticos y los peces radica en su toxicidad aguda. No
hay pruebas de que se produzcan efectos acumulativos debido a una
exposición prolongada.
No se dispone de datos acerca de los efectos de la
alfa-cipermetrina en los microorganismos del suelo. En un sistema
cerrado, no se observaron efectos en las bacterias de aguas residuales
con concentraciones de 3 mg/litro.
La toxicidad de la alfa-cipermetrina para determinados
coleopteros carábidos y larvas de neurópteros es relativamente baja,
y representa un peligro limitado en las fases preadultas de los
himenópteros parasitoides. En estudios de campo en pequeñas parcelas
y en gran escala se ha puesto de manifiesto que la alfa-cipermetrina
es poco peligrosa para los coleopteros carábidos y estafilínidos, pero
es un peligro relativamente grande para las arañas linifíidas. Los
efectos sobre las poblaciones se limitaron a una sola temporada de
crecimiento. Además, la alfa-cipermetrina es de bajo riesgo para las
larvas de sírfidos, pero tiene efectos considerables en los
coccinélidos. Sin embargo, la rápida desaparición de los residuos de
las hojas permite a estos animales recolonizar en poco tiempo las
zonas tratadas.
La utilización de alfa-cipermetrina en el campo no diminuye la
abundancia relativa de entomófagos en las comunidades de artrópodos.
Su utilización en cultivos de cereales de grano pequeño no iría
acompañada de la "reaparición" de plagas o de infestaciones de plagas
secundarias.
En las pruebas de laboratorio, la toxicidad de la
alfa-cipermetrina para las lombrices de tierra es baja. No se registró
mortalidad tras 14 días de exposición de las lombrices a
concentraciones de hasta 100 mg/kg de suelo artificial.
En pruebas de toxicidad aguda en el laboratorio se observó que la
alfa-cipermetrina es muy tóxica para las abejas. La administración
oral de una solución concentrada emulsionable dio una DL50 a las 24
h de 0.13 µg/abeja, mientras que el valor correspondiente para la
administración tópica fue de 0.03 µg/abeja (producto técnico) ó 0.11
µg/abeja (CE). La elevada toxicidad de la alfa-cipermetrina para las
abejas no se manifestó claramente en los ensayos de campo,
probablemente a causa del breve efecto repelente del producto, que
hace disminuir la actividad libadora de las abejas y, por
consiguiente, su exposición.
No se dispone de datos acerca de la toxicidad del
alfa-cipermetrina para las aves.
2. Conclusiones
2.1 Población general
Cuando la alfa-cipermetrina se aplica correctamente, la
exposición de la población general al producto es baja y probablemente
no supone riesgos.
2.2 Exposición ocupacional
Con buenas prácticas de trabajo, medidas de higiene y
precauciones de seguridad, es improbable que la alfa-cipermetrina
suponga un peligro para las personas expuestas en forma laboral. La
aparición de "sensaciones faciales" es un síntoma de exposición. En
estas circunstancias se deben re-examinar las prácticas de trabajo.
2.3 Medio ambiente
Con las cantidades recomendadas para la aplicación es improbable
que la alfa-cipermetrina alcance niveles de importancia ecológica. En
condiciones de laboratorio es muy tóxica para los artrópodos
acuáticos, los peces y las abejas. Hay cierta probabilidad de que se
produzcan efectos tóxicos importantes en invertebrados a los que no
está destinado y en peces en casos de derrame, rociado excesivo o mala
utilización del producto.
3. Recomendaciones
* Se debe evitar la contaminación de aguas superficiales.
* La alfa-cipermetrina se une fuertemente a las partículas. Se
deberían llevar a cabo nuevos estudios ecotoxicológicos sobre los
efectos del compuesto en los microorganismos de los sedimentos,
puesto que este aspecto parece haber recibido poca atención.
* Hay que investigar la absorción gastrointestinal de
alfa-cipermetrina en distintas circunstancias.
* Se debe investigar el destino final de la alfa-cipermetrina
aplicada por vía cutánea.
* Hay que obtener nueva información acerca de la
toxicidad/carcinogenicidad a largo plazo y de la inmunotoxicidad
de la alfa-cipermetrina.
6.4 Plants
The metabolism of cypermethrin in plants is described in WHO
(1989).
The degradation of alpha-cypermethrin and cypermethrin in
cabbages grown to maturity outdoors has been studied. Eighteen days
after transplanting, the cabbages were treated three times with
14C-labelled alpha-cypermethrin or cypermethrin as an EC
formulation. Each treatment consisted of 1.8 mg equivalent with a
spray concentration of 36 g/litre. This treatment was repeated after
11 and 27 days. Each box of cabbages received a total application of
5.4 mg at a dose rate equivalent to 50 g active ingredient/ha. At
harvest (3 months later) the plants were separated into old and new
outer leaves, heart, stalk and roots. No major differences between the
two compounds in distribution of radioactivity throughout the plants
or in the metabolic profile were observed. The highest radioactive
residues were present in the old outer leaves (23% for
alpha-cypermethrin and 27% for cypermethrin), lower levels being found
in new outer leaves, stalk, roots and heart. Very low levels (< 0.05
mg/kg) of both compounds were found in the soil. The major radioactive
residue at harvest was shown to be the pesticide, which was either in
the unchanged form or had undergone cis/trans-isomerization,
presumably photochemically. The profiles of the organosoluble
metabolites were similar, and the major products of alpha-cypermethrin
had chromatographic mobility similar to previously identified products
of cypermethrin metabolism, such as 3-phenoxybenzoic acid and
3-phenoxybenzyl alcohol, partly hydroxylated and/or conjugated. These
compounds were found in minor quantities (McMinn, 1983a; WHO, 1989).
Appraisal
A wide range of studies in mice, rats, dogs, sheep, cows and
humans has shown that cypermethrin is rapidly absorbed, distributed
to a variety of organs and tissues, metabolized and rapidly excreted
from the body (WHO, 1989). There are no major differences in the
absorption, distribution, retention or excretion between the species.
Differences, where they do occur, are related to the rate rather than
the nature of the metabolites formed and the conjugation reactions.
Cypermethrin, both the cis and trans isomers, and
alpha-cypermethrin are primarily metabolized by cleavage of the ester
bond. The metabolites PBA and CPA are mainly excreted as conjugates.
The type of conjugate differs in a number of animal species dosed
with cypermethrin, but humans and rats have the same pathway. Minor
quantities of hydroxylated PBA (conjugated) may also be found. The
terminal half-life of elimination of alpha-cypermethrin from the fat
of rats is 17-26 days, compared to 18.9 days for cis-cypermethrin.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Oral (technical product)
Alpha-cypermethrin is moderately to highly toxic and 3-4 times
more toxic than cypermethrin.
The clinical signs of toxicity observed in the various acute
toxicity studies on experimental animals with alpha-cypermethrin are
typical for a cyano-containing pyrethroid intoxication. They included
ataxia, abasia, gait abnormalities, choreoathetosis, "tip-toe" walk,
and increased salivation, lacrimation, piloerection, tremor and clonic
convulsions. The majority of the mortalities occurred within the first
3 h and surviving animals recovered within 7 days (Rose, 1982, 1983a).
In the study with alpha-cypermethrin administered in corn-oil
(Rose, 1983a), clonic convulsions, piloerection, salivation and
splayed hind-leg gait were found. The oral LD50 values for
alpha-cypermethrin are summarized in Table 3.
Table 3. Oral LD50 values for technical alpha-cypermethrin
Species Concentration and LD50 in mg/kg body Reference
(strain) vehicle weight (with 95%
confidence limits)
Mouse 5% in corn oil 35 (26-48) Rose (1982)
(CD) 40% in DMSO 762 (514-912) Rose (1982)
50% aqueous suspension 798 (568-1074) Rose (1982)
Rat 5% in corn oil 79 (63-98) Dewar (1981)
(Wistar) 40% in DMSO approximately 4000 Rose (1982)
50% aqueous suspension > 5000 Rose (1982)
Rat 10% in corn oil 40-80 Rose (1983a)
(Wistar) 20% in corn oil 368 (282-487) Rose (1983a)
Woollen et al. (1991) noted a higher degree of absorption of
cypermethrin when it was applied in corn oil. This could be the
explanation for the higher toxicity of alpha-cypermethrin administered
in corn oil.
7.1.2 Oral (formulations)
Formulations of alpha-cypermethrin have moderate acute oral
toxicity (Table 4). The clinical signs observed after oral
administration to rats are characteristic of cyano-containing
pyrethroid intoxication (see section 7.1.1.). The majority of the
mortalities occurred within 3 days of dosing. The degree of acute oral
toxicity of formulations containing mixtures with other active
ingredients depended on the toxicity of the latter ingredients.
7.1.3 Dermal
Alpha-cypermethrin has low dermal toxicity. No deaths or signs of
intoxication were observed in rats (Dewar, 1981; Shell, 1983a) and
mice (Rose, 1982; Shell, 1983a) receiving a single 24-h dermal
exposure of 500 mg/kg body weight (25% in DMSO) and 100 mg/kg body
weight (5% in corn oil), respectively.
The dermal LD50 values in rats of formulations of
alpha-cypermethrin and of alpha-cypermethrin mixed with another active
ingredient are summarized in Table 4. In all cases, the maximum dose
that could be applied was tested.
With the pour-on formulations, no clinical signs were observed.
Blood around the nose and eyes was the only sign seen in the case of
SC formulations. Clinical signs observed after the application of EC
or ULV formulations of alpha-cypermethrin included increased
lacrimation, chromodacryorrhoea and unkempt appearance, aggressiveness
and diarrhoea. The Fastac/BPMC formulation caused the same signs of
intoxication and also oedema at the application site. With the
Fastac/methomyl formulation, fasciculation, lethargy, salivation,
piloerection, hunched back, chromodacryorrhoea and cyanosis were
observed. Animals treated with Fastac/Azodrin formulation showed the
above-mentioned symptoms, and some additionally showed ataxia, abasia,
hypothermia, eye pallor and prostration/coma.
7.1.4 Inhalation
Groups of five male and five female albino Fischer-344 rats were
exposed for 4 h to a dust atmosphere containing 30% (m/m)
alpha-cypermethrin on silica powder at an average concentration of 1.3
g/m3 (equivalent to 0.4 g active ingredient/m3). The mass media
diameter of the dust particles was 4.2 µm (geometric standard
deviation 6.4). The animals were observed for 14 days after the
exposure but there were no signs of intoxication. Macroscopic
examination of the lungs did not reveal any effects. Thus, the acute
LC50 was > 1.3 g 30% silica powder dust/m3 or > 0.4 g active
ingredient/m3 (Blair, 1984).
Table 4. Oral and dermal LD50 values for formulated alpha-cypermethrin in rats (Fischer-344)
Formulationa LD50 in mg total formulation per kg Reference
body weight (with 95% confidence limits)
Oral Dermal
100 g/litre EC 101 (82-119) > 1800 Rose (1984d)
100 g/litre EC 136 (98-186) > 1800 Rose (1984e)
100 g/litre EC 174 (125-327) > 2000 Price (1985a)
30 g/litre EC 229 (178-292) > 2000 Rose (1984f)
30 g/litre EC 673 (597-753) > 2000 Rose (1985)
15 g/litre pour-on > 2000 > 2000 Price (1988)
10 g/litre pour-on > 2000 > 2000 Price (1988)
100 g/litre SC 1804 (1507-2168) > 2000 Price (1985b)
60 g/litre SC > 5000 > 2000 Gardner (1991)
15 g/litre SC > 5000 > 2000 Price (1986)
15 g/litre ULV 5838 (5130-6665) > 2000 Rose (1984c)
Mixtures with other active ingredients
Fastac/methomyl EC 58-97 (males) > 1900 Rose (1984h)
15/120 g/litre 73 (51-89) (females) > 1900
Fastac/BPMCb EC 310 (215-462) > 2000 Price (1987)
10/400 g/litre
Fastac/Azodrin EC 25 (18-34) > 2000 Gardner (1989)
20/400 g/litre
a EC = emulsifiable concentrate; SC = suspension concentrate; ULV = ultra-low volume
b BPMC = 2 sec-butylphenyl methylcarbamate (fenobucarb)
7.1.5 Other routes
The acute intraperitoneal LD50 for rats of a 10% solution of
alpha-cypermethrin in corn oil was 3.39 (3.03-3.83) ml/kg body weight,
or 339 mg active ingredient per kg body weight. The surviving animals
showed characteristic pyrethroid signs of intoxication, e.g., ataxia,
abasia, choreoathetosis, gait abnormalities, "tip-toe" walk and
salivation (Rose, 1984a).
7.2 Short-term exposure
7.2.1 Oral
7.2.1.1 Rat
Groups of Wistar rats (10 of each sex at each dose level and 20
of each sex as controls) were fed 0, 25, 100, 200, 400 or 800 mg
alpha-cypermethrin/kg diet (equivalent to 0, 1.25, 5, 10, 20 or 40
mg/kg body weight) for 5 weeks. At 400 and 800 mg/kg diet, signs of
intoxication, such as abnormal gait and hypersensitivity were
observed, and food intake and body weight were decreased in both sexes
compared with the control group. Changes in blood chemistry, e.g.,
decreases in protein and increases in urea levels, were observed in
both sexes of rats fed 800 mg/kg diet and in males fed 400 mg/kg. The
weights of livers and kidneys of both sexes of rats fed 800 mg/kg diet
and livers of male rats fed 400 mg/kg were increased. No
histopathological changes were observed except in the case of one
severely intoxicated male animal fed 800 mg/kg diet, which showed
sparse axonal degeneration in the sciatic nerve. No effects were seen
in the animals fed 200 mg/kg diet for five weeks (Pickering, 1982).
In a 13-week study, Wistar rats (30 males and 30 females per test
group, and a control group consisting of 60 males and 60 females),
which were initially 5 weeks old, were fed 0, 20, 60, 180 or 540 mg
alpha-cypermethrin/kg diet (equivalent to 0, 1, 3, 9 or 27 mg/kg body
weight). After six weeks of feeding, one third of the animals were
killed for interim haematological, clinical chemical and gross
post-mortem examination. The remaining animals were killed after 13
weeks. Signs of intoxication, such as abnormal gait with splayed hind
limbs, were found in 3 out of 20 males fed 540 mg/kg diet. Several
instances of transient skin sores and fur loss were observed
particularly in the rats fed 540 mg/kg. There was decreased growth,
which correlated with decreased food intake, in both sexes fed 540
mg/kg from the first week onwards. No clear effects on the
haematological and clinical chemical parameters were found. Organ
weights were comparable with those of the control animals. No
histopathological abnormalities were found except sparse axonal
degeneration in the sciatic nerve, without clinical signs of toxicity,
in two males fed 540 mg/kg. No axonopathy was observed in the three
animals with abnormal gait. There were marginal effects (decreased
growth during week one and from week 10 onwards) in the males fed 180
mg/kg diet. No effects were found in the 60-mg/kg group (Clark, 1982).
7.2.1.2 Dog
Beagle dogs (one male and one female) were fed alpha-cypermethrin
in the diet at the following concentrations: 200 mg/kg diet for 7
days, 400 mg/kg diet for 2 days, and 300 mg/kg diet for 7 days. With
200 mg/kg no signs of intoxication were observed, whereas dosing with
300 mg/kg or more caused weight loss, ataxia, subdued behaviour, head
nodding, food regurgitation, inflammation of gums and tongue, body
tremors and diminished response to stimuli. Haematological, clinical
chemical and gross pathological examination showed no effects
(Greenough & Goburdhun, 1984).
In a further study, Beagle dogs (one male and one female)
received 300 mg/kg diet for 3 days (male dog) or 4 days (female dog)
and 250 mg/kg diet for 7 days. Both animals showed the above-mentioned
signs of intoxication, the only difference being that when it was
being fed the 250-mg/kg diet the female animal showed these signs more
frequently than the male animal. There were no effects on haematology,
clinical chemical parameters, urinalysis, faecal occult blood test or
gross pathology (Greenough & Goburdhun, 1984).
In a study by Greenough et al. (1984), 36 pure bred Beagle dogs
(18 males and 18 females) received a diet containing
alpha-cypermethrin at 0, 30, 90 or 270 mg/kg diet for 13 weeks. The
group dosed at the highest concentration comprised six males and six
females while the other groups consisted of four males and four
females. All animals fed 270 mg/kg diet exhibited signs of
intoxication, such as whole body tremors, head nodding, "lip-licking",
subduedness, ataxia, agitation and a high-stepping gait. These signs
increased in both intensity and duration as the study progressed. Food
consumption, body weight gain, organ weights, ophthalmoscopy,
haematological and clinical chemical parameters, urinalysis, gross
pathology and microscopy of 18 organs and tissues of all test groups
showed no dose-related effects. In this study, the no-observed-effect
level was considered to be 90 mg/kg diet (equivalent to 2.25 mg/kg
body weight).
7.3 Skin and eye irritation; sensitization
7.3.1 Skin irritation
Undiluted technical alpha-cypermethrin was minimally irritating
when applied as a single occluded dose for 24 h to intact and abraded
rabbit skin (Dewar, 1981).
New Zealand white rabbits were used to study the primary skin
irritation of a number of alpha-cypermethrin formulations. The test
duration was 4 h, the observation period was 7-21 days, and the
formulations tested were 30 and 100 g/litre EC, 15 g/litre ULV, 10 and
15 g/litre pour-on formulation, 15 and 100 g/litre SC, and
Fastac/methomyl (15/120) EC. The EC formulations caused mild to
moderate skin irritation. Superficial necrosis was observed in one or
two animals treated with 100 g/litre EC, but there was no permanent
in-depth skin damage. The effects persisted for up to 7 days. The EC
formulations and the 100 g/litre SC formulation were classified as
mildly irritating. All other formulations tested were either
non-irritating or only slightly irritating (Rose, 1984c,d,e,f,h, 1985;
Price, 1985a,b, 1986, 1988).
7.3.2 Eye irritation
Undiluted formulations were tested for their eye irritancy
potential in groups of six rabbits using the Draize test. All the EC
formulations tested (30 or 100 g/litre) caused severe eye irritation,
including corneal opacity and damage to the iris (Rose, 1984d,e,f,
1985).
When an EC formulation (100 g/litre) and its components, both in
the undiluted form and at typical in-use dilutions (1 in 400 and 1 in
1333 aqueous dilution), were tested for eye irritancy potential, the
undiluted formulation was severely irritating, with or without
irrigation, while the undiluted blank formulations were mildly to
severely irritating. The diluted test formulations, with or without
alpha-cypermethrin or with emulsifier, were non-irritating. It was
concluded that the eye irritation resulted from the combined
formulation ingredients (especially the emulsifier) and that
alpha-cypermethrin per se gave only slight irritation, if any
(Dewar, 1981; Rose, 1984b). In-use dilutions (0.0075%) of another 100
g/litre EC formulation and its blank formulation were non-irritating
(Rose, 1984g).
Two pour-on formulations (10 and 15 g/litre) caused moderate and
slight conjunctival inflammation, respectively. The 10 g/litre
formulation was considered to be an eye irritant (Price, 1988). Two SC
formulations (15 and 100 g/litre) were mildly irritating, causing
slight conjunctival redness and chemosis (Price, 1985b, 1986). A 15
g/litre ULV formulation was mildly irritating to rabbit eyes and there
was a moderate initial pain response (Rose, 1984c). An EC formulation
containing Fastac/methomyl (15:120 g/litre) was a severe eye irritant.
The vascularization of the cornea and iritis were considered to be
irreversible (Rose, 1984h).
7.3.3 Sensitization
Technical alpha-cypermethrin was tested in the guinea-pig
maximization test of Magnusson and Kligman using groups of 10 male and
10 female guinea-pigs and a control group of 55 animals of each sex.
The following concentrations were used: intradermal injection, 0.05%
(v/v) in corn oil; topical application and challenge, 50% (m/m) in
vaseline. On the basis of the negative results it was concluded that
alpha-cypermethrin is not a skin sensitizer in guinea-pigs (Dewar,
1981).
An EC formulation (100 g/litre) and its corresponding blank were
tested, as a 50% solution in corn oil, in the Buehler guinea-pig
sensitization test. The topical challenge was carried out with a 30%
solution in corn oil. None of the animals showed positive responses at
24 or 48 h after the challenge (Rose, 1984g).
7.4 Long-term and carcinogenicity studies
No long-term or carcinogenicity studies have been conducted with
alpha-cypermethrin.
7.5 Reproduction, embryotoxicity and teratogenicity
Alpha-cypermethrin has not been tested for reproductive effects
or teratogenicity.
From the available reproduction and teratogenicity studies with
cypermethrin it is clear that no influence on reproduction performance
occurs at a level of 100 mg/kg diet, nor are there any teratogenic
effects even with dose levels high enough to cause maternal toxicity
(WHO, 1989). Furthermore, the no-observed-effect level of cypermethrin
for reproduction and teratogenicity is comparable with the
no-observed-effect levels based on other parameters of toxicity. In
consequence, there is no reason to believe that alpha-cypermethrin,
consisting of two cis isomers also present in cypermethrin, would
behave differently.
7.6 Mutagenicity and related end points
7.6.1 Mutation
The results of the various mutagenicity studies with
alpha-cypermethrin are summarized in Table 5.
Alpha-cypermethrin (in DMSO) at concentrations of 31.25, 62.5,
125, 250, 500, 1000, 2000 or 4000 µg/ml did not increase reverse gene
mutation (at the arg 4-17, trp 5-48 or hom 3-10 markers) in log- or
stationary-phase cultures or forward mutation (to cyclo-heximide
-resistance) in log-phase cultures of Saccharomyces cerevisiae XV
185-14C, either in the presence or absence of rat-liver S9 fraction.
Concentrations of 10 and 50 µg/ml 4-nitroquinoline- N-oxide and 1250
and 5000 µg/ml cyclophosphamide were used as positive controls
(Brooks, 1984).
Alpha-cypermethrin (in DMSO) at concentrations of 31.25, 62.5,
125, 250, 500, 1000, 2000 or 4000 µg/plate, both with and without
microsomal activation, did not increase reverse mutation rates in
Salmonella typhimurium TA98, TA100, TA1535, TA1537 and TA1538 or in
Escherichia coli WP2 and WP2 uvr A. Mitotic gene conversion was not
induced in liquid suspension cultures of log-phase cells of
Saccharomyces cerevisiae JD 1, dosed with solutions of
alpha-cypermethrin at concentrations of 10, 100, 500, 1000 or 5000
µg/ml, both in the presence or absence of a rat liver S9 fraction.
These studies were carried out in comparison with four positive
control compounds (Brooks, 1982).
7.6.2 Chromosomal effects
In a study by Clare & Wiggins (1984), groups of five male and
five female Wistar rats were administered a single oral dose of 2, 4
or 8 mg alpha-cypermethrin in 5% corn oil/kg body weight and killed 24
h after dosing. The control group received corn oil alone.
Cyclophosphamide was used as a positive control. Alpha-cypermethrin
caused no increase in the incidence of chromatid or chromosome
aberrations or polyploidy in bone marrow cells.
Alpha-cypermethrin in aqueous carboxymethylcellulose at
concentrations of up to 40 µg/ml did not increase the frequency of
chromatid gaps, chromatid breaks or total chromatid aberrations in rat
liver (RL4) cell cultures (Brooks, 1982).
7.6.3 DNA damage
Alpha-cypermethrin in DMSO (20%) was administered to Wistar rats
as a single oral dose of 40 mg/kg body weight. The exposure time was
6 h. Alpha-cypermethrin failed to produce any detectable DNA
single-strand damage using alkaline elution profiles of liver DNA.
Methylmethane sulfonate was used as a positive control and DMSO as the
solvent control (Wooder, 1982).
7.6.4 Conclusion
From the available data on alpha-cypermethrin, it can be
concluded that this compound is non-mutagenic in tests with
Salmonella typhimurium, Saccharomyces cerevisiae, and in vivo and
in vitro tests with rat liver cells for the induction of chromosome
aberration and production of DNA single-strand damage.
Table 5. Mutagenicity tests on microorganisms
Organism/strain Dose Type of test Metabolic Result Reference
activation
Salmonella typhimurium up to 4000 µg/plate plate with or negative Brooks (1982)
TA98, TA100, TA1535, without
TA1537, TA1538
Escherichia coli up to 4000 µg/plate plate with or negative Brooks (1982)
WP2, WP2 uvrA without
Saccharomyces cerevisiae up to 5000 µg/ml liquid suspension with or negative Brooks (1982)
JDI culture without
Saccharomyces cerevisiae up to 4000 µg/ml liquid suspension with or negative Brooks (1984)
XV 185-14C culture without
Rat liver cells (RL4) up to 40 µg/ml negative Brooks (1982)
(chromatid gaps, breaks or
aberrations)
Rat liver DNA one oral dose of negative Wooder (1982)
(DNA single strand damage) 40 mg/kg body weight
Rat bone marrow one oral dose of negative Clare & Wiggins
chromosome study up to 8 mg/kg body (1984)
weight
7.7 Special studies
7.7.1 Skin sensation
It is known that exposure to certain types of pyrethroids can
result in a transient skin sensation in humans (Le Quesne et al.,
1980).
Guinea-pigs received 0.1 ml of a 0.01, 0.1 or 1.0% solution of
alpha-cypermethrin in ethanol or a 1, 10 or 20% solution of
alpha-cypermethrin (w/v) in corn oil on the skin. Sensory stimulation
was quantified by counting the number of times each animal turned to
lick or bite its treated flank in preference to the non-treated flank.
Skin stimulation was observed during a 2-h period at all dose levels
except the lowest. In the groups of guinea-pigs treated with 10% and
20%, some animals exhibited an exaggerated hopping movement and a
repeated head shaking activity at the time of maximum skin stimulation
(40-60 min after treatment). This behaviour was not seen with the 1%
solution or more dilute ones (Hend, 1983).
7.7.2 Neurotoxicity
Large oral doses of alpha-cypermethrin and other synthetic
pyrethroids (WHO, 1989) have been shown to produce minor
histopathological lesions in the sciatic nerve of rats, described as
sparse axonopathy in peripheral nerves.
Rose (1983b) conducted a two-phase study. In the first phase, the
time-course for development and recovery from pyrethroid-induced nerve
lesions was investigated by measuring biochemical correlates of
neuropathological change (the enzymes beta-glucuronidase and
beta-galactosidase) in groups of Wistar rats (five of each sex per
group) at periods of 2-12 weeks after the start of dosing. The daily
doses of alpha-cypermethrin (96.6%), administered by stomach tube,
were 37.5 mg/kg body weight for the first 11 doses and 25.0 mg/kg body
weight for the subsequent 9 doses over a 4-week period (5 times/week).
DMSO was used as the solvent for 10 doses and then arachis oil. In
all, 21% of the animals died and more than 80% of the treated animals
showed clinical signs of intoxication. Maximum enzyme activities in
the sciatic posterior tibial nerves were found 5 weeks after the start
of the experiment and had returned to control values by 12 weeks. In
the trigeminal nerve and trigeminal ganglia, a slight but not
significant increase in enzyme activities was found.
In the second phase of the study, 10 male and 10 female Wistar
rats were given 20 oral doses of alpha-cypermethrin in DMSO (0, 10, 20
or 40 mg/kg body weight per day) over a period of 4 weeks (5
times/week). Only two animals died, one given 10 mg/kg and one given
20 mg/kg. In the 40-mg/kg group, 75% of the animals developed clinical
signs, whereas in the 20-mg/kg group only 25% of the animals showed
these clinical effects. In the 10-mg/kg group, the animals showed no
differences from the controls. No clear influence of
alpha-cypermethrin on growth was found. Five weeks after the initial
dose, which corresponded to the period of maximal enzyme changes,
biochemical changes (increases of up to 60%) indicative of a mild
axonal degeneration were found in both the distal and proximal
sections of the sciatic posterior tibial nerve in animals administered
40 mg/kg body weight. In the 20-mg/kg group, only a small (up to 20%)
increase in beta-galactosidase activity was found in the proximal
sections of the sciatic posterior nerve. The same trends were found in
the trigeminal nerve and ganglia. No changes were found in the
10-mg/kg group (Rose, 1983b).
7.7.3 Immunosuppressive action
No data on the immunosuppressive action of alpha-cypermethrin are
available.
7.8 Mechanism of toxicity - mode of action
The mechanism of toxicity and mode of action of cypermethrin (and
other pyrethroids) are extensively described in section 8.8 of the
Environmental Health Criteria 82: Cypermethrin (WHO, 1989). Recently
Vijverberg & van den Bercken (1990) and Aldridge (1990) summarized
current knowledge of the neurotoxicity and mode of action of the
different pyrethroids (see also Appendix 1).
Pyrethroids induce toxic signs that are characteristic of a
strong excitatory action on the nervous system. Toxic doses generally
cause hypersensitivity to sensory stimuli, and a number of compounds
may induce tingling sensations in the skin. Two distinct toxic
syndromes have been described in mammals. The T-syndrome is induced by
pyrethrins and non-cyano pyrethroids, and the CS-syndrome, induced by
cyano-pyrethroids such as cypermethrin and alpha-cypermethrin, is
characterized by choreoathetosis and salivation.
The available data strongly suggest that the primary target site
of pyrethroid insecticides in the vertebrate nervous system is the
sodium channel in the nerve membrane. Pyrethroids without an
alpha-cyano group cause a moderate prolongation of the transient
increase in sodium permeability of the nerve membrane during
excitation. This results in relatively short trains of repetitive
nerve impulses in sense organs, sensory (afferent) nerve fibres and,
in effect, nerve terminals. On the other hand, the alpha-cyano
pyrethroids (for instance cypermethrin and alpha-cypermethrin) cause
a long-lasting prolongation of the transient increase in sodium
permeability of the nerve membrane during excitation. This results in
long-lasting trains of repetitive impulses in sense organs and a
frequency-dependent depression of the nerve impulse in nerve fibres.
The difference in effects between permethrin (with no alpha-cyano
group) and the two insecticides cypermethrin and alpha-cypermethrin,
which have identical molecular structures except for the presence of
an alpha-cyano group on the phenoxybenzyl alcohol, indicates that it
is this alpha-cyano group that is responsible for the long-lasting
prolongation of the sodium permeability.
Since the mechanisms responsible for nerve impulse generation and
conduction are basically the same throughout the entire nervous
system, pyrethroids may also induce repetitive activity in various
parts of the brain. The difference between the symptoms of poisoning
by alpha-cyano pyrethroids and those of the classical pyrethroids is
not necessarily due to an exclusive central site of action. It may be
related to the long-lasting repetitive activity in sense organs and
possibly in other parts of the nervous system, which, in a more
advance state of poisoning, may be accompanied by a
frequency-dependent depression of the nervous impulse.
Pyrethroids also cause pronounced repetitive activity and a
prolongation of the transient increase in sodium permeability of the
nerve membrane in insects and other invertebrates. Available
information indicates that the sodium channel in the nerve membrane is
also the most important target site of pyrethroids in the invertebrate
nervous system.
Because of the universal character of the processes underlying
nerve excitability, the action of pyrethroids should not be considered
to be restricted to particular animal species or to a certain region
of the nervous system.
Although it has been established that sense organs and nerve
endings are most vulnerable to the action of pyrethroids, the ultimate
lesion that causes death will depend on the animal species,
environmental conditions, and on the chemical structure and physical
characteristics of the pyrethroid molecule.
8. EFFECTS ON HUMANS
8.1 General population exposure
No data concerning the exposure of the general population to
alpha-cypermethrin are available.
8.2 Occupational exposure
A study of alpha-cypermethrin exposures was carried out during
formulation using both technical concentrate and technical material at
Durban, South Africa. The oil-damped solid, crystalline, dry technical
concentrate contained a minimum of 90% (m/m) alpha-cypermethrin.
Exposures were assessed by personal and static monitoring of
atmospheric alpha-cypermethrin concentrations, urinary
alpha-cypermethrin metabolite concentrations and by medical
examination. Four individuals were exposed during 3 days of operation.
The group mean personal exposures for the two days whilst formulating
technical concentrate were 2.8 and 4.9 µg/m3 and the group mean
personal exposure to technical material on day 3 was 54.1 µg/m3.
Urinary alpha-cypermethrin metabolites could not be identified (limit
of detection, 0.02 mg/litre). Formulation was successfully completed,
only minor skin sensations being reported by two of the
non-operational personnel, possibly resulting from particles of
alpha-cypermethrin settling directly on the skin, face and neck. Dust
concentrations were up to 30 times greater during handling the
technical material compared with oil-damped technical concentrate, and
local exhaust dust extraction reduced dust emission by a factor of up
to 17 (Western, 1984).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
Appraisal
The acute toxicity of alpha-cypermethrin to Daphnia magna and
Gammarus pulex is similar to that of cypermethrin. However, in a
reproduction study with Daphnia magna, alpha-cypermethrin seemed to
be slightly more toxic than cypermethrin. Comparison of the results
of a reproduction study in Daphnia magna with the results of acute
tests shows that the hazard of alpha-cypermethrin lies in its acute
toxicity. There is no significant potential for cumulative effects
occurring as a result of long-term exposure to lower concentrations.
Alpha-cypermethrin is highly toxic to a number of aquatic
arthropod taxa but of low toxicity to molluscs. The short-term
toxicity of the compound can be reduced by formulating the product as
an OSC. Contamination through spray drift from commercial
applications will generally be low, and so the effects on susceptible
taxa will be limited. The rapid loss of alpha-cypermethrin from the
water gives the potential for complete recovery of affected
populations.
Laboratory studies show that values for the acute toxicity and
the toxicity to the early-life stages of fish are similar for
alpha-cypermethrin and cypermethrin. A comparison of the results from
both alpha-cypermethrin studies reveals that the hazard of the
compound results from its acute toxicity, and there is no significant
potential for additional effects occurring as a result of long-term
exposure to lower concentrations. Laboratory and field studies have
shown that the toxicity of alpha-cypermethrin to fish is greatly
influenced by the formulation, particulate formulations showing
significantly less toxicity than emulsifiable concentrates.
Field studies demonstrate that the high toxicity of
alpha-cypermethrin to fish observed in laboratory studies is not
realized under field conditions. Contamination of water bodies by
inadvertent overspraying or spray drift does not present a hazard to
fish.
9.1 Microorganisms
9.1.1 Algae
The acute toxicity of alpha-cypermethrin to a single-celled green
alga, Selenastrum capricornutum, at 24 °C has been determined. The
pesticide was dispersed using acetone, and the 2- to 4-day EC50 for
growth was above 100 µg/litre (Stephenson, 1982).
9.1.2 Bacteria
The effects of cypermethrin on microbial activity in the soil
have been investigated in a series of studies. Cypermethrin was
applied to sandy loam at concentrations of 2.5 and 250 mg/kg. No
effects on the rates of carbon dioxide evolution or oxygen uptake were
observed at the lower rate, but significant inhibition of carbon
dioxide evolution and a decrease in oxygen uptake were observed at the
higher rate of application. There were no effects at either
concentration on nitrogen fixation, ammonification, nitrification or
glucose utilization (WHO, 1989).
Sewage bacteria were unaffected by the presence of
alpha-cypermethrin (3 mg/litre) in a closed system test, while the
growth of Pseudomonas fluorescens was unaffected at 100 mg/litre
(Stone & Watkinson, 1983).
9.2 Aquatic organisms
9.2.1 Invertebrates
9.2.1.1 Laboratory studies
Acute toxicity studies with Daphnia magna (aged < 24 h) showed
that at 20 °C technical alpha-cypermethrin, dispersed in acetone under
static conditions (daily renewal), has effects at concentrations below
1 µg/litre. The 24-h and 48-h EC50 values (immobilization) were 1.1
and 0.3 µg/litre, respectively (Stephenson, 1982).
Water samples taken from field enclosures 24 h after treatment
with an EC formulation were analysed for alpha-cypermethrin and
bioassayed with Gammarus pulex. The 24-h LC50 value for this
organism was 0.05 µg/litre (Garforth, 1982a; Shires, 1982).
The effect of technical alpha-cypermethrin on survival, growth
and reproduction of Daphnia magna was studied over a period of 21
days by Garforth (1982b). The test solution was renewed daily and the
temperature ranged between 18.5 and 20.2 °C. The results are
summarized in Table 6.
Table 6. Effects of alpha-cypermethrin on the reproductive cycle of
Daphnia magnaa
Effect Nominal concentration (µg/litre)
LOEL NOEL
Survival of parent generation 0.3 0.1
Growth of parent generation 0.1 0.03
Production of young 0.1 0.03
a From: Garforth (1982b)
LOEL = Lowest-observed-effect level; NOEL = No-observed-effect
level
9.2.1.2 Field studies
Garforth (1982a) studied the effects of alpha-cypermethrin on a
range of aquatic invertebrates in metal enclosures, each containing
about 1 m3 water, placed in an outdoor experimental pond. A diluted
EC formulation was sprayed onto the water surface at concentrations of
1, 3, 10, 30 and 100 g active ingredient/ha, and samples were taken up
to 7 days after application. The concentration of alpha-cypermethrin
was around 50% of the nominal level 24 h after application and
decreased to 10 to 20% of the nominal level 7 days after application.
The lowest concentration was toxic to Asellidae. Thirteen families
of aquatic arthropods were tested; most were killed at 1 g/ha except
one species of Coenagriidae, which tolerated about 3 g/ha. Three
families of molluscs were tested and were found to be unaffected at
100 g/ha.
Studies to investigate the relative toxicity of two formulations
of alpha-cypermethrin (a 100-g/litre EC and a 100-g/litre oil-enhanced
suspension concentrate (OSC) with and without anti-evaporant agents)
to aquatic invertebrates have been carried out. Water samples were
taken from field enclosures treated with a range of doses (0.1-5 g/ha
for the EC and 0.1-10 g/ha for the two OSC formulations) and
bioassayed with Gammarus pulex. The 24-h LD50 values (in g
alpha-cypermethrin/ha equivalents) of water samples taken 24 h after
application were 0.9 for the EC, 6.3 for the OSC without
anti-evaporant, and 2.8 for the OSC plus anti-evaporant formulation.
Thus, one day after treatment, both OSC formulations were less toxic
to Gammarus pulex than the EC formulation. However, the EC
formulation lost its toxicity more rapidly than either of the OSC
formulations (see also section 4.1.2). The effects on other aquatic
invertebrate communities could not be accurately assessed, since the
results for macro-arthropods and zooplankton were inconclusive.
Coenagriidae, Chironomidae and zooplankton seemed to be relatively
tolerant. The residues in water and sediment, 22 days after treatment
with the EC at 2 g alpha-cypermethrin/ha or with both OSC formulations
at 5 g/ha, were < 0.004 µg/litre and < 0.01 mg/kg, respectively, for
all three formulations (Inglesfield, 1985b).
The hazard to aquatic invertebrates resulting from spray drift
from the aerial application of an EC (15 g alpha-cypermethrin/ha) has
been investigated by Garforth & Woodbridge (1984). Details of the
study are described in section 9.2.2.2. The sub-surface water
concentration was 0.6 µg alpha-cypermethrin/litre shortly after
application and decreased to < 0.02 µg/litre within 2 to 4 days. The
contamination initially caused a significant reduction in the
abundance of several groups of aquatic arthropods, including beetles,
chironomids, corixids, mites and zooplankton. However, within 4 to 7
weeks the affected fauna had completely recovered.
In a study by Pearson (1990), two freshwater ponds were treated
with alpha-cypermethrin as an emulsifiable concentrate in 1987. One
pond was oversprayed at 15 g active ingredient/ha, while the other was
treated with the same amount of alpha-cypermethrin but by direct
incorporation into the water (see section 4.1.2). Indigenous
populations of zooplankton nauplii and of copepods ( Cyclops spp.)
were significantly reduced in both ponds by the treatment but
recovered within 26-45 days. Other zooplankton were not plentiful but
appeared to be less severely affected in the oversprayed pond than in
the pond treated by incorporation. Populations of phantom midge larvae
( Chaoborus spp) were killed by both treatments. However, within 47
days after treatment, populations of young larvae had developed in the
oversprayed pond to almost pre-treatment numbers, and to a lesser
extent in the pond with direct incorporation.
9.2.2 Fish
9.2.2.1 Laboratory studies
The available acute 96-h LC50 values for two fish species are
summarized in Table 7.
The effects of the type of formulation on the acute toxicity to
fish are summarized in Table 8. Suspension concentrate, wettable
powder and micro-encapsulated formulations were 10 to 70 times less
acutely toxic to rainbow trout than the EC formulation (Shires,
1983b).
Table 7. Acute toxicity of technical alpha-cypermethrin in fish
Species Mean weight Vehicle Test system Temperature 96-h LC50 Reference
(g) (°C) (µg/litre) (95%
confidence limits)
Rainbow trout 3.3 dispersed via static water; 15 2.8 Stephenson (1982)
(Oncorhynchus mykiss) acetone 12 h renewal of (2.1-3.5)
test solutions
Fathead minnow 0.76 adsorbed onto continuous 23-25 0.93 Stephenson (1983)
(Pimephales promelas) pumice flow-through (0.78-1.2)
Table 8. Effect of type of formulation on the toxicity of alpha-cypermethrin to fish in laboratory studies
Species Weight Tempera- Formulation 96-h LC50 Reference
(g) ture (°C) (µg/litre)
Rainbow trout 0.8-2.5 15 100 g/litre EC 5.6 Shires (1983b)
(Oncorhynchus 250 g/litre SC 350
mykiss) 100 g/kg WP 120
50 g/kg WP 220
50 g/kg ME > 100
50 g/kg CD 65
Rainbow trout 0.11-0.25 15 15 g/litre OSC 10a Pearson (1986)
(Oncorhynchus 15 g/litre OSC 71
mykiss) 100 g/litre OSC 16a
100 g/litre OSC 56
Common carp 3.5-4 24-30 100 g/litre OSC 11 Stephenson
(Cyprinus carpio) 15 g/litre EC 0.8 (1986)
50 g/kg WP 60
Puntius 0.3-0.5 24-30 100 g/litre OSC 3.2 Stephenson
gonionotus 15 g/litre EC 0.7 (1986)
50 g/kg WP 22
a Denotes daily renewal of test solutions. All other tests were carried out without renewal of the test solutions.
EC = emulsifiable concentrate; SC = suspension concentrate; OSC = oil-enhanced suspension concentrate; WP= wettable powder;
ME = micro-encapsulated;
CD = ß-cyclodextrin.
The toxicity of alpha-cypermethrin to the early-life stages of
fish has been studied in a 34-day continuous-flow embryo-larval test
with the fathead minnow (Pimephales promelas). Eggs less than 24 h
old were exposed to nominal concentrations of 0.03 to 1.0 µg/litre.
Pre-hatch, post-hatch and overall mortality and final body weight were
recorded. On the basis of the most sensitive parameter (overall
survival) and measured exposure concentrations, the lowest
concentration of alpha-cypermethrin producing an adverse effect was
0.09 µg/litre and the highest concentration producing no effect (NOEL)
was 0.03 µg/litre (Stephenson, 1983).
9.2.2.2 Small scale field or outdoor tank studies
The acute effect of formulation on the toxicity of
alpha-cypermethrin to fish under field conditions was investigated in
studies using stainless steel enclosures placed in an experimental
pond. In the first study (Shires, 1983b), the rainbow trout (13-32 g)
was the test species and the results are summarized in Table 9. The EC
formulation was at least 30 times more toxic than any of the other
formulations. The number of fish used was not reported.
Table 9. Effect of formulation on the toxicity of alpha-cypermethrin
to rainbow trout in field studies
Formulation 14-day LD50 (g active
ingredient/ha equivalent)
100 g/litre emulsifiable concentrate 29
250 g/litre suspension concentrate > 1000
100 g/kg wettable powder > 1000
50 g/kg micro-encapsulated > 1000
In the second study, Stephenson (1987c) tested an 1.5%
oil-enriched SC formulation of alpha-cypermethrin in outdoor tanks
containing carp ( Cyprinus carpio; 6.3 g). The application rate was
approximately 4, 8 and 15-16 g active ingredient/ha applied by hand
sprayer. The water temperature ranged from 23-26 °C, the water was
aerated and the duration of the experiment was 7 days. There was 20%
mortality at all treatment rates, which was comparable with the
control group. The number of fish used was not reported.
In the third study (Shires, 1985b), rainbow trout ( Oncorhynchus
mykiss; 2-5 g) were introduced into open-ended stainless steel
enclosures placed in a mature experimental pond. Different volumes of
alpha-cypermethrin (as a diluted EC containing 100 g active
ingredient/litre) were applied with a hand-held sprayer onto the water
surface. The dose rates were equivalent to 5-500 g active
ingredient/ha. The concentrations in the water samples were dependent
on the dose applied and the time after the treatment that the samples
were taken. For instance, with a dose of 5 g alpha-cypermethrin/ha the
concentration in the water was 0.4 µg/litre or less after 96 h,
whereas with a dose of 500 g/ha residues of up to 30 µg/litre were
found. Alpha-cypermethrin was toxic to the rainbow trout at a
concentration of about 2-5 µg/litre water. In terms of nominal
application rate, the no-observed-effect level for alpha-cypermethrin
lies between 50 and 100 g alpha-cypermethrin/ha. These dose rates are
much higher than those used for crop protection purposes.
In a further study, 20 rainbow trout were placed in stainless
steel enclosures in a shallow pond. The diluted EC and SC formulations
were sprayed onto the surface of the water and the fish were monitored
for mortality over 8 days. The SC formulation did not cause mortality,
even at an application rate equivalent to 300 g active ingredient/ha.
However, high mortality (90%) occurred with the EC formulation at 30
g/ha alpha-cypermethrin, but application of 10 g active ingredient/ha
did not cause mortality. From these results it is clear that the SC
formulation has a lower toxicity than the EC formulation (Stephenson,
1987b).
The hazard to fish caused by spray drift from aerial applications
of alpha-cypermethrin to agricultural land has been investigated by
Garforth & Woodbridge (1984). The trial site was a cereal field
bordered on one side by a freshwater ditch. The water's edge was
generally less than 2 m from the crop margin. Four days prior to
application, two cages, each containing 15 common carp ( Cyprinus
carpio; 30-50 cm) were placed in the ditch. The ditch was known to
contain a good stock of different types of fish, macroinvert-ebrates
and zooplankton. An alpha-cypermethrin EC containing 100 g/litre was
applied by air at 15 g active ingredient/ha to the crop when a gentle
breeze was blowing from the crop over the ditch. The
alpha-cypermethrin concentration in the sub-surface water was 0.6
µg/litre shortly after the application and decreased to < 0.02
µg/litre within 2 to 4 days (see section 4.1.2.). None of the fish
died and no adverse effects were observed either on the carp placed in
the cages or on the indigenous species. It was concluded that
inadvertent contamination of water bodies by spray drift resulting
from the normal commercial use of alpha-cypermethrin does not present
a hazard to fish.
Several field tests in rice paddies have examined the toxicity of
alpha-cypermethrin to fish.
Stephenson (1986, 1987b) investigated the acute toxicity of 15
g/litre EC, 100 g/litre OSC and 50 g/kg WP formulations to fish in
plots of rice paddy in West Java. The application rates were 7.5, 15,
and 30 g alpha-cypermethrin/ha for the EC and OSC formulations and 30
g alpha-cypermethrin/ha for the WP formulation. Each of the
insecticide treatments was applied to the same plot on two separate
occasions; once 12 days after transplantation of the rice seedlings
and again 57 days after the transplantation. Prior to application, two
cages each containing 20 carp (Cyprinus carpio) (2.5-5 g) and two
cages each containing 20 specimens of Puntius gonionotus (1.4-5 g)
were placed in trenches cut in each plot. In addition, before the
first experiment, 30 fish of each of these two species were released
to swim freely in each plot. Survival of the fish was monitored daily
up to 7 days after each treatment. The water temperature was between
25 and 36 °C. Following the first application, only the EC formulation
at 15 and 30 g/ha resulted in significant mortality of caged and
free-swimming carp and Puntius gonionotus. The same was true for
carp following the second application, but no results were available
for Puntius gonionotus following the second application due to an
outbreak of disease among these fish.
Two other field studies were carried out in West Java to examine
the effects of an OSC formulation on the growth and survival of
free-swimming carp (6-8 g) in rice paddies. Experimental conditions
were similar to those described above for the acute studies. The water
temperature was 23-32 °C. In the first experiment, treatments with 15
g active ingredient/ha were carried out 21 and 33 days after
transplantation of the rice seedlings. In the second experiment, the
same dose rate was applied 51 and 64 days after transplanting. The
fish were monitored over periods of 3 to 4 weeks in each experiment.
No adverse effects on survival and growth of the carp were observed
(Stephenson, 1987a,b).
9.3 Terrestrial organisms
9.3.1 Earthworms
The toxicity of technical alpha-cypermethrin to the red
earthworm, Eisenia foetida, has been assessed in laboratory tests.
In the filter paper contact toxicity test, 50% mortality occurred
within 48 h at a dose of about 0.01 mg/cm2 of filter paper. However,
increases in the dose up to 1 mg/cm2 did not result in a significant
increase in mortality. In the artificial soil test no significant
mortality occurred within a period of 14 days in earthworms exposed to
up to 100 mg alpha-cypermethrin/kg soil (Inglesfield & Sherwood,
1983).
9.3.2 Invertebrates - field studies
In a study designed to investigate the effects of
alpha-cypermethrin on non-target arthropod fauna in Italian vineyards,
two formulations of alpha-cypermethrin were tested, i.e. an
emulsifiable concentrate (100 g active ingredient/litre) and a
suspension concentrate (250 g active ingredient/litre). Both were
diluted to 1.25 g active ingredient/ha and sprayed to run-off. The
effect on predators and parasites (crop foliage fauna) such as
parasitoid Hymenoptera, Heteroptera and Chrysopa carnea, soil
surface predators such as Coleoptera and Araneae, phytophagous
arthropods such as Homoptera, Thysanoptera and Acari, and other
invertebrates such as nematocerous, Diptera and epigeal fauna was
inconclusive but in most cases negative. Spiders were the only group
significantly affected by alpha-cypermethrin. The abundance of major
phytophagous insect taxa (Homoptera, Thysanoptera and Acari) was
markedly reduced by both treatments although neither treatment had any
long-term adverse effects (Inglesfield, 1984).
An emulsifiable concentrate (EC) of alpha-cypermethrin was tested
on the non-target arthropod fauna of maize in France at 20 and 30 g
active ingredient/ha and applied using tractor-mounted boom and nozzle
equipment at different stages of crop development. Organisms from the
following taxa were collected: phytophagous arthropods (Aphidoidea),
Thysanoptera (Thripidae), Cicadellidae and entomophagous arthropods;
predatory Coleoptera (Coccinellidae, Cantharidae, Carabidae and
Staphylinidae); parasitoid Hymenoptera (Braconidae, Chalcidoidea);
and predatory Diptera, Neuroptera and Aranea. In addition, the
effects on community structure were studied. The two treatments
reduced transiently the numbers of some of the entomophagous taxa,
especially Coccinellidae (Coleoptera), Carabidae (Coleoptera),
Neuroptera and Araneae. Alpha-cypermethrin at a concentration of
20 g/ha promoted some late-season resurgence of aphid populations
(Inglesfield, 1985a).
A large-scale replicated field experiment was carried out in
Indonesia to investigate the effects of an EC (10 g and 20 g
alpha-cypermethrin/ha) and an OSC (10 g and 20 g alpha-cypermethrin
per ha), applied at 17 and 62 days after transplanting using a single
fan-jet nozzle knapsack sprayer, on rice pests and their natural
enemies. Alpha-cypermethrin was applied at two crop stages, and
populations of both pests and beneficial arthropods were monitored
throughout the season. Alpha-cypermethrin produced good control of
stemborers, grasshoppers, leafhoppers and stinkbugs, the most numerous
pests found during the trial. It had a significant but short-lived
effect on spiders and, generally, no effect on either dragon-flies or
other beneficial arthropods (Shires & Inglesfield, 1986).
A study was initiated in England in 1982 to compare the effects
on entomophagous arthropods of annual applications of an EC of
alpha-cypermethrin (at a rate of 10 g active ingredient/ha in the
first year and 15 g active ingredient/ha in subsequent years) with
those resulting from the use of non-pyrethroid products. The study was
carried out on a range of crops (oilseed rape, wheat and barley) over
the duration of a 5-year arable crop rotation. A field of 8 ha was
divided into two equal areas, and each year alpha-cypermethrin was
applied to one of the areas and other products to the other area. All
treatments were carried out with standard tractor-driven boom and
nozzle spraying equipment. The crop rotation was as follows: winter
rape in 1982, winter wheat in 1983 and 1984, and winter barley in
1985. Organisms from a variety of taxa were collected during the
study, such as parasitoid Hymenoptera, predatory Diptera,
predatory Coleoptera, Araneae, phytophagous insects and other
arthropods. The results indicated that any difference between the
effects of alpha-cypermethrin and the reference compounds on
entomophagous arthropods was generally short-lived. There is no
evidence that alpha-cypermethrin treatments had any long-term effects
on any of the taxa studied. In addition, there appeared to be no
long-term effects on the relative abundance of entomophages within the
arthropod communities or on the structure and integrity of the
arthropod communities of which they form a part (Inglesfield, 1985c,
1988, 1989).
The results of these studies have been reviewed by Inglesfield
(1991). They showed that field application of alpha-cypermethrin and
cypermethrin had no adverse effects on the relative abundance of
entomophages within the arthropod communities and that the use of
these two pesticides in small grain cereals would not be associated
with pest "resurgence" or the development of secondary pest
infestations.
9.3.3 Honey-bees
9.3.3.1 Laboratory studies
The oral administration of alpha-cypermethrin in acetone produced
a 24-h LD50 of 0.06 µg/bee, whereas an EC formulation (100 g/litre)
of the pesticide yielded a 24-h LD50 of 0.13 µg formulation/bee.
After topical application, 24-h LD50 values of 0.03 µg (technical)
and 0.11 µg (EC) per bee were obtained (Murray, 1985).
The mortality of honey-bees (Apis mellifera) exposed to
Phacelia flowers 30 min after they had been treated with an EC
formulation (15 g active ingredient/ha) in a residual test was low
(15%) within 48 h. However, the foraging activity was reduced (Murray,
1985).
The residual toxicity to the honey-bee of alpha-cypermethrin as
EC, OSC, and EC/fungicide mixtures has been investigated. Bees were
exposed for 48 h to flowering Phacelia campanularia plants that had
been sprayed with each formulation at a rate of 10 or 20 g active
ingredient/ha, and the mortality was assessed 24 and 48 h after
initial exposure to the treated plants. Although there was great
variation in the results, there appeared to be no difference in the
residual toxicity of the EC and OSC formulations at a rate of 10 g
active ingredient/ha. However, at 20 g active ingredient/ha the OSC
appeared to be more toxic than the EC. Application of the EC in
admixtures did not appear to significantly increase bee mortality
(Hillaby & Inglesfield, 1986).
9.3.3.2 Field studies
Alpha-cypermethrin, applied as an EC (10 and 20 g active
ingredient/ha) by tractor-mounted boom and nozzle equipment to small
plots of flowering mustard in France, caused a sharp decline in
foraging activity of bees immediately after application, but there was
a return to normal activity within a few hours. No effect on bee
survival or hive development was observed (Shires, 1983a; Shires et
al., 1984b; Shires, 1985a).
In a further study, the same EC formulation was applied at the
same concentration on large isolated fields of flowering oilseed rape
during peak foraging activity of honey-bees in France. No increase in
bee mortality was found, but foraging activity declined for a few
hours after application at a rate of 10 g active ingredient/ha. With
a rate of 20 g active ingredient/ha a more prolonged decline in
foraging activity occurred. No effects on the overall condition of the
hives were seen at the end of the season and very low or undetectable
residues were found in dead bees, pollen, honey and wax (Shires et
al., 1984c; Shires, 1985a).
Two large and two small plots of winter wheat were enclosed
beneath large mesh-covered tunnels. A small bee-hive was placed in
each tunnel and sucrose solution was sprayed onto all of the wheat in
order to simulate aphid honey dew. Alpha-cypermethrin (an EC at 10, 15
or 30 g active ingredient/ha) was applied to the larger plots of wheat
when the bees were actively foraging the sugar deposits. No increase
in bee mortality, compared with that in the pre-treatment period, was
observed. Foraging activity in the plots declined sharply after
treatment and remained at a reduced level, probably because of its
repellent effect. Examination of the hives about 2 weeks after the
trial showed that the hives, both the control and the
alpha-cypermethrin-treated, were in excellent condition with strong
adult populations and large areas of developing brood. In
post-treatment samples, alpha-cypermethrin residues of 0.03 mg/kg of
honey and 0.01 mg/kg of wax were found. In live and dead bees
collected from the tunnel treated with 15 g/ha, a concentration of
0.026 µg/bee was found (Shires et al., 1984a; Le Blanc, 1985).
In a study by Inglesfield & Forbes (1986), alpha-cypermethrin was
applied as an OSC (10 g active ingredient/ha) and an EC (10 g active
ingredient/ha) to three fields of flowering winter-sown oilseed rape
in Germany while bees were actively foraging the crops. None of the
treatments had any significant effect on adult bee survival or on the
longer-term development of the experimental colonies. The number of
bees actively foraging in the crops declined following application.
This reduction in activity can probably be partly attributed to the
repellent effects of these treatments. Both the OSC and EC
formulations of alpha-cypermethrin had similar effects on bee
behaviour. Residues of alpha-cypermethrin in post-treatment samples of
pollen, honey and wax were below 0.01 mg/kg. In dead bees, the residue
on the day of application with the OSC was 1.8 mg/kg and in the case
of the EC was 0.12 mg/kg.
A diluted EC formulation of alpha-cypermethrin (mixed with the
fungicide vinclozolin, 100 g active ingredient/ha) was applied by a
tractor-driven boom and nozzle sprayer, at a rate of 20 g active
ingredient/ha, to a 206-ha block of flowering oilseed rape situated in
Kent, England. Shortly before spraying, which took place over a 3-day
period, five beehives were positioned at each of two sites adjacent to
the crop. At each site the hives were either fitted with pollen traps
or with traps to collect dead bees as they were removed from the hive.
The hives were observed daily before and for ten days after spraying.
Hive activity was recorded at intervals each day, and, on days when
bees were flying, pollen traps were set and samples of pollen
collected. Following the application of alpha-cypermethrin the bees
foraged normally. At least 90% of the pollen returned to the hives
during the immediate post-treatment period was found to be from
oilseed rape, showing that the bees had continued to forage on the
treated crop. The number of dead bees collected remained low
throughout the study and did not increase after application of the
alpha-cypermethrin. After the study, all hives were in good condition
and subsequently yielded a good crop of honey. A local beekeeper with
many hives adjacent to the same block of oilseed rape reported no
effects amongst his hives. It was concluded that the application of
alpha-cypermethrin at 20 g active ingredient/ha to flowering oilseed
rape had no direct effects on honey-bee survival and hive development
(Brown, 1989).
9.3.4 Leaf-cutting bees
Laboratory trials using 15 male adult alfalfa leaf-cutting bees
( Megachile rotundata F.) per group, exposed to treated filter papers
for 4 h, showed that alpha-cypermethrin EC (100 g/litre) at the rate
of 10 or 15 g active ingredient/ha caused 12 and 30% mortality,
respectively, and 42 and 100% mortality after 24 h of contact (Tasei
et al., 1987).
In a study by Tasei et al. (1987), populations of about 400
female alfalfa leaf-cutting bees were reared in three flowering
lucerne fields. One field was left untreated, while the two others
were treated with alpha-cypermethrin as an EC (10 or 15 g active
ingredient/ha), applied by a tractor-mounted fan-jet sprayer. Two days
after the treatments, counts of live females in artificial nesting
sites showed the losses due to 10 and 15 g active ingredient/ha to be
21 and 12%, respectively. After hibernation and incubation of the
progeny larvae, little effect of the treatments could be observed. The
maximum residue in leaves collected from nests was 1 mg
alpha-cypermethrin/kg. The mean values after 5, 10 and 27 days in leaf
pieces capping the nests of the bees were 0.75, 0.48, and 0.19 mg/kg
at the lower application rate and 0.59, 0.53, and 0.10 mg/kg at the
higher rate. No residues (limit of determination, 0.01 mg/kg) were
detected in live larvae but 0.07 mg/kg was found in pollen provisions
(Tasei et al., 1987).
9.3.5 Birds
Cypermethrin is practically non-toxic to birds; acute oral LD50
values are greater than 2000 mg/kg body weight. The dietary LC50
value is above 10 000 mg/kg diet (see WHO, 1989). However, studies
with alpha-cypermethrin have not been carried out.
10. COMPARISON BETWEEN ALPHA-CYPERMETHRIN AND CYPERMETHRIN
Alpha-cypermethrin comprises one quarter of the racemic mixture
cypermethrin, with which an extensive agricultural, ecological and
(eco)toxicological programme has been carried out (WHO, 1989). It
contains more than 90% of the insecticidally most active enantiomer
pair of the four cis isomers of cypermethrin, i.e. the two cis isomers
(IRcis)S and (IScis)R (see Fig. 1).
10.1 Use and residue levels
Alpha-cypermethrin is used to control the same pests in
agriculture as cypermethrin. Its rate of application to crops (5-30 g
active ingredient/ha) is lower than that of cypermethrin (10-200 g
active ingredient/ha) since alpha-cypermethrin is biologically more
active than cypermethrin.
Residue data for alpha-cypermethrin have been obtained from a
large number of supervised trials carried out worldwide. These trials
cover the most important crop groupings for which alpha-cypermethrin
is recommended, including oilseeds, pome fruits, peaches, fruiting
vegetables, berries, leafy vegetables, maize and speciality crops such
as hops and tobacco (Shell, 1984). Residues in a variety of these
crops resulting from application of alpha-cypermethrin at the
recommended rate ranged between 0.05 and 1.0 mg/kg (Shell, 1984). In
comparison, residues of cypermethrin in crops were higher and ranged
between 0.05 and 2.0 mg/kg. In comparative trials where
alpha-cypermethrin was applied at half the dose rate of cypermethrin,
alpha-cypermethrin residues in general averaged 40% (20-50%) of those
in cypermethrin-treated samples (Shell, 1984; WHO, 1989).
The residue data on cypermethrin have been evaluated by the Joint
FAO/WHO Meeting on Pesticide Residues and recommended MRLs (1979/1981)
have been published (FAO/WHO, 1980, 1982) (see Table 10). From the
available residue data obtained with good agricultural practice and
using the MRLs set for cypermethrin, suggested MRLs for
alpha-cypermethrin can be extrapolated (Table 10).
It can be concluded that alpha-cypermethrin, since it is
biologically more active, is always used at a lower application rate
than cypermethrin and, as a result, the residues on crops are
approximately half those of cypermethrin.
Table 10. Comparison of current MRLs in mg/kg product for cypermethrin
recommended by the FAO/WHO Joint Meeting on Pesticides Residues (JMPR)
with those which may be proposed for alpha-cypermethrin
Commodity Cypermethrin Alpha-cypermethrin
(JMPR)a (extrapolated)
Cotton seed 0.2 0.05
Rapeseed 0.2 0.05
Pome fruits 2 0.5
Peaches 2 0.5
Grapes 1 0.5
Citrus fruits 2 1
Tomatoes 0.5 0.1
Brassica, leafy vegetables 1 1
Lettuce 2 1
Peas, kidney beans (less pod) 0.05 0.05
Soyabeans (less pod) 0.05 0.05
Potatoes 0.05 0.05
Sugarbeet (roots) 0.05 0.05
Maize grain 0.05 0.05
a From: FAO/WHO (1980, 1982)
For analytical reasons, 0.05 mg/kg is considered the minimum practical
MRL.
10.2 Environmental impact
Because its physico-chemical properties are similar to those of
cypermethrin, alpha-cypermethrin is expected to have a similar
environmental fate to that of cypermethrin. The potential of
alpha-cypermethrin to bioaccumulate may therefore be estimated from
experimental data on the bioaccumulation of cypermethrin. This is
because the octanol/water partition coefficients of alpha-cypermethrin
(1.4 x 105; log Pow = 5.16) and cypermethrin (2 x 106; log Pow
= 6.3) are relatively similar. In fish, the bioaccumulation of
cypermethrin determined experimentally was lower than might have been
expected from its partition coefficient, presumably because it was
rapidly metabolized. This would also be expected for
alpha-cypermethrin because both the route of metabolism and its rate
are similar to those of cypermethrin (Shell, 1983b; WHO, 1989).
In the area of environmental toxicology, results available for
green algae, aquatic invertebrates, fish and bees show that the acute
toxicity of alpha-cypermethrin is slightly higher than, but broadly
similar to that of cypermethrin (Table 11). This is because the
toxicity of cypermethrin results largely from its alpha-cypermethrin
component. No toxicity data are available concerning the effects of
alpha-cypermethrin on soil microbes, but little or no effect on carbon
dioxide evolution, oxygen uptake and nitrogen fixation would be
expected if alpha-cypermethrin acts on soil microbes in a similar way
to cypermethrin (WHO, 1989). There are also no toxicity data for
alpha-cypermethrin in birds. However, cypermethrin has a low toxicity
to birds. Since alpha-cypermethrin constitutes 25% of the active
ingredients of cypermethrin and is used at lower application rates, it
is expected that alpha-cypermethrin will also have a low toxicity to
birds (Shell, 1983b).
Overall, in the natural environment, the more biologically active
alpha-cypermethrin is likely to have a similar toxicity to that of
cypermethrin. This is because alpha-cypermethrin is used at a lower
application rate than cypermethrin.
10.3 Mammalian toxicity
In acute oral toxicity studies, alpha-cypermethrin is either
equally toxic or two to three times more toxic than cypermethrin (WHO,
1989), depending on the vehicle and the concentrations used.
In Table 12, the no-observed-effect levels and
lowest-observed-effect levels of the various mouse, rat and dog
studies are compared for cypermethrin, cis-cypermethrin and
alpha-cypermethrin. Short- and long-term studies with the racemic
mixture cypermethrin (containing four cis and four trans isomers),
cis-cypermethrin (four cis isomers) and alpha-cypermethrin (two cis
isomers) have shown similar toxicological effects.
The data from the short-term toxicity studies indicate that
alpha-cypermethrin is approximately 2 to 3 times more toxic than
cypermethrin in rats and dogs. This reflects the amount of
alpha-cypermethrin present in cypermethrin.
The signs of intoxication, the effects on target organs and
tissues, and the metabolic pathway of alpha-cypermethrin are similar
to those of cis-cypermethrin. The mode of action of
alpha-cypermethrin is similar to that of cypermethrin.
Cypermethrin has been tested in a rodent multigeneration
reproduction study and for embryotoxicity and teratogenicity in two
species. There were no effects on either reproductive performance or
on fetal development, even at doses producing systemic toxicity (WHO,
1989). Alpha-cypermethrin has not been tested for reproductive
toxicity or teratogenicity, but there is no indication that it would
have effects on these parameters since it is a component of
cypermethrin.
Table 11. Comparison of environmental toxicology of cypermethrin and alpha-cypermethrin
Species Life stage/ Water Administration Measured Values for Reference
age temper- route or vehicle end-point cypermethrin alpha-
ature (°C) cypermethrin
Green alga
(Selenastrum - 24 dispersal 2- to 4-day > 100 µg/litre > 100 µg/litre Stephenson
capricornutum) via acetone EC50 (growth) (1982)
Aquatic invertebrates
Water flea up to 24 h 20 dispersal EC50 Stephenson
(Daphnia magna) old via acetone (immobilization) (1982)
24 h 1.2 µg/litre 1.1 µg/litre
48 h 0.3 µg/litre 0.3 µg/litre
Fish
Rainbow trout 3.3 g 15 dispersal 96-h LC50 2.8 µg/litre 2.8 µg/litre Stephenson
(Oncorhynchus mykiss) via acetone (1982)
Fathead minnow 0.74-0.76 g 23-25 absorbed on 96-h LC50 1.2 µg/litre 0.93 µg/litre Stephenson
(Pimephales promelas) (juvenile) to pumice (1983)
Earthworm
(Eisenia foetida) - - filter paper 48-h LD50 26.1 µg/cm2 10 µg/cm2 Rob
contact & Dorough
toxicity (1984);
Inglesfield
& Sherwood
(1983)
Table 11 (continued)
Species Life stage/ Water Administration Measured Values for Reference
age temper- route or vehicle end-point cypermethrin alpha-
ature (°C) cypermethrin
Bees
(Apis mellifera) worker - oral 24-h LD50 0.035 µg/bee 0.06 µg/bee Badmin
adminis- & Twydell
tration (1976);
Murray
(1985)
Table 12. Comparison between short- and long-term oral studies with
cypermethrin, cis-cypermethrin and alpha-cypermethrin
Animal Number of Duration of Dose level in mg/kg diet
species studies experiment No-observed- Lowest-observed-
effect level effect level
Cypermethrin (4 cis and 4 trans isomers)a
Mouse 1 2 years 400 1600
Rat 1 5 weeks 750 1500
2 13 weeks 100/150 400
1 2 years 100 1000
Dog 1 13 weeks 500 1500
1 2 years 300 750/600
cis-cypermethrin (4 cis isomers)a
Rat 1 5 weeks 100 300
Alpha-cypermethrin (2 cis isomers)
Rat 1 5 weeks 200 400
1 13 weeks 60 180
Dog 2 2-7 days 200 250
1 13 weeks 90 270
a See WHO (1989)
The WHO Task Group on Environmental Health Criteria for
Cypermethrin, which met in 1986, concluded that cypermethrin was
without mutagenic activity. Available data on alpha-cypermethrin
indicate that this compound also is non-mutagenic in tests with
Salmonella typhimurium, Saccharomyces cerevisiae, and in vivo and
in vitro tests with rat liver cells for the induction of chromosome
aberration and production of DNA single-strand damage.
Alpha-cypermethrin has not been tested for carcinogenicity, but
the long-term toxicity studies in mice and rats did not indicate any
carcinogenic potential for cypermethrin. Because cypermethrin contains
the two cis isomers present in alpha-cypermethrin, it is unlikely that
these two isomers would have a carcinogenic potential. This
supposition is supported by the fact that all the mutagenicity studies
on alpha-cypermethrin have yielded negative results.
Near lethal doses of alpha-cypermethrin and cypermethrin produce
sparse axonopathy in the peripheral nerves of rats (see section
7.7.2). Similar signs of intoxication were observed with both
compounds, and increases in beta-glucuronidase and beta-galactosidase
activities, consistent with an axonal degeneration, could only be
detected in severely intoxicated animals. The magnitude of the enzyme
changes was substantially less than for other known neurotoxic
compounds, thereby confirming the minor nature of the lesion. The
short time period needed to produce ataxia and/or abnormal gait rules
out a causal relationship between ataxia and axonopathy. The signs of
intoxication are consistent with a pharmacologically-mediated effect.
No biochemical changes indicative of axonopathy were detected in rats
given 20 oral doses of 20 mg alpha-cypermethrin/kg body weight per day
or 75 mg cypermethrin/kg body weight per day. From these comparative
studies, it is clear that the neurotoxic potential of
alpha-cypermethrin and cypermethrin is qualitatively similar, but
alpha-cypermethrin is 3 to 4 times more potent. This reflects the fact
that alpha-cypermethrin constitutes the active ingredient and 25% of
cypermethrin.
Appraisal
The effects of alpha-cypermethrin on vertebrates and
invertebrates are qualitatively and, in a number of cases, even
quantitatively similar to those of cypermethrin, reflecting the fact
that alpha-cypermethrin is composed of two of the four cis isomers
present in cypermethrin (these two being the most active components
of cypermethrin). Therefore, the toxicological information of
cypermethrin can be used to evaluate the effects of
alpha-cypermethrin where certain important toxicity studies for
alpha-cypermethrin are lacking.
Alpha-cypermethrin has a higher toxicity (lower
no-observed-effect level) than cypermethrin but its application rate
is at most only half that of cypermethrin (5-30 g active
ingredient/ha for alpha-cypermethrin and 10-200 g active
ingredient/ha for cypermethrin). Therefore, residue levels of
alpha-cypermethrin in crops are less than half those of cypermethrin.
11. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The toxicological and residue data on cypermethrin have been
evaluated by the Joint FAO/WHO Meeting on Pesticide Residues (JMPR)
(FAO/WHO, 1980, 1982), but alpha-cypermethrin has not yet been
evaluated by the JMPR.
REFERENCES
Aldridge WN (1990) An assessment of the toxicological properties of
pyrethroids and their neurotoxicity. Crit Rev Toxicol, 21(2): 89-104.
Armitage GD (1984) Fastac exposure study. Analysis of atmospheric and
surface wipe samples ex. Durban, South Africa. Sittingbourne, Shell
Research (SBRN 84.091).
Badmin JS & Twydell RS (1976) Evaluation of the insecticide WL 43467
against the honey bee Apis mellifera. Sittingbourne, Shell Research
(WKSR.0021.76).
Baldwin MK (1990) Alpha-cypermethrin (Fastac): Water solubility at
various pH values. London, Shell International Chemical Company, Ltd
(Internal report SBGR 90.158).
Blair D (1984) The toxicology of pyrethroids; the acute 4-h inhalation
toxicity of a 30% m/m Fastac on silica powder formulation.
Sittingbourne, Shell Research (SBGR 84.066).
Bosio PG (1982) Study of isomer conversion of WL85871 in various crops
from 1981 treatments. Berre, France, Shell Chimie (BEGR 82.031).
Brooks TM (1982) Toxicity studies with pyrethroids; in vitro
genotoxicity studies with WL85871. Sittingbourne, Shell Research (SBGR
81.273).
Brooks TM (1984) Genotoxicity studies with Fastac; the induction of
gene mutation in the yeast Saccharomyces cerevisiaee XV 185-14-C.
Sittingbourne, Shell Research (SBGR 84.117).
Brown K (1989) A study of the effects on honey-bees of a large scale
commercial application of "Fastac" to oilseed rape. London, Shell
International Chemical Company, Ltd (Internal report SBGR 89.198).
Clare MG & Wiggins DE (1984) Genotoxicity studies with Fastac; in
vivo cytogenetic test using rat bone marrow. Sittingbourne, Shell
Research (SBGR 84.120).
Clark DG (1982) WL85871; a 90-day feeding study in rats.
Sittingbourne, Shell Research, vol 1 and 2 (SBGR 81.293).
Coveney PC & Forbes S (1986) Analysis of soil from UK (Coates) for
residues of "Fastac" (WL85871) - soil persistence trial - third year.
Sittingbourne, Shell Research (SBGR 86.201).
a The proprietary Shell Research reports in the following list were
submitted to the IPCS by Shell.
Creedy CL & Logan CJ (1984) In vitro metabolism of cypermethrin
isomers by rat, rabbit, and human liver preparations. Sittingbourne,
Shell Research (SBGR 84.108).
Dewar AJ (1981) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity, skin and eye irritancy and skin sensitizing
potential of WL85871 including a comparison with the acute toxicity of
WL 43467 (Ripcord). Sittingbourne, Shell Research (TLGR 80.148).
Eadsforth CV, Bragt PC, & Van Sittert NJ (1988) Human dose-excretion
studies with pyrethroid insecticides cypermethrin and
alpha-cypermethrin; relevance for biological monitoring. Xenobiotica,
18(5): 603-614.
FAO (1982) Second Government Consultation on International
Harmonization of Pesticide Registration Requirements, Rome, 11-15
October 1982. Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1980) 1979 Evaluations of some pesticide residues in food.
Rome, Food and Agriculture Organization of the United Nations (FAO
Plant Production and Protection Paper 20 sup).
FAO/WHO (1982) 1981 Evaluations of some pesticide residues in food.
Rome, Food and Agriculture Organization of the United Nations (FAO
Plant Production and Protection Paper 42).
Fisher JR, Robinson J, & Debray PH (1983) A new multipurpose
insecticide. Proceedings of 10th International Congress of Plant
Protection, Brighton, England, 20-25 November 1983. Vol. 1, pp
452-459.
Forbes S (1985) Residues of Fastac (WL85871) in cured marine catfish
( Arius sp.) from Indonesia. Sittingbourne, Shell Research (SBGR
85.200).
Forbes S & Burden AN (1984) Analysis of soil from UK (Reculver) for
residues of WL85871 (Fastac) - soil persistence trial - second year.
Sittingbourne, Shell Research (SBGR 84.005).
Forbes S & Cole DA (1986) Residues of Fastac (WL85871) in sweetcorn
from Canada. Sittingbourne, Shell Research (SBGR 86.217).
Forbes S & Knight CJ (1983) Analysis of soil from UK (Reculver) for
residues of WL85871 - soil persistence trial - first year.
Sittingbourne, Shell Research (SBGR.83.162).
Forbes S & Mackay CE (1983) Analysis of soil from UK (Coates) for
residues of WL85871 (Fastac) - soil persistence trial - first year.
Sittingbourne, Shell Research (SBGR 83.418).
Forbes S & Wales GH (1985a) Analysis of soil from UK (Reculver) for
residues of Fastac (WL85871) - soil persistence trial - third year.
Sittingbourne, Shell Research (SBGR 85.070).
Forbes S & Wales GH (1985b) Analysis of soil from UK (Coates) for
residues of Fastac (WL85871) - soil persistence trial - second year.
Sittingbourne, Shell Research (SBGR 85.071).
Francis WP & Gill JP (1991) Flufenoxuron/alpha-cypermethrin - residues
in sheep tissues following treatment with PAMPASS/RENEGADE mixtures by
dip or pour-on. London, Shell International Chemical Company, Ltd
(Internal report SBGR 89.254).
Gardner JR (1989) "Fastac/Azodrin" 20/400 g/litre emulsifiable
concentrate: Acute oral and dermal toxicity. London, Shell
International Chemical Company, Ltd (Internal report SBGR 89.026).
Gardner JR (1991) Fastac 60 G/l SC (SF07396); acute oral and dermal
toxicity in rat. Sittingbourne, Shell Research (SBGR 91.020).
Garforth BM (1982a) A comparison of the toxicities of WL85871 and
Ripcord to freshwater invertebrates in small field enclosures.
Sittingbourne, Shell Research (SBGR 82.015).
Garforth BM (1982b) WL85871 and cypermethrin; chronic toxicity to
Daphnia magna. Sittingbourne, Shell Research (SBGR 82.119).
Garforth BM & Woodbridge AP (1984) Spray drift from an aerial
application of Fastac; fate and biological effects in an adjacent
freshwater ditch. Sittingbourne, Shell Research (SBGR 84.055).
Greenough RJ & Goburdhun R (1984) WL85871; Oral (dietary) maximum
tolerated dose study in dogs. Musselburgh, Inveresk Research
International (Unpublished report No. 3107, submitted to WHO by Shell
Research).
Greenough RJ, Cockrill JB, & Goburdhun R (1984) WL85871; 13-week oral
(dietary) toxicity study in dogs. Musselburgh, Inveresk Research
International (Unpublished report No. 3197, submitted to WHO by Shell
Research).
Hend RW (1983) Toxicology of pyrethroids; skin stimulation studies
using guinea-pigs. Sittingbourne, Shell Research (SBGR 83.197).
Hillaby JM (1988) Deposition of pesticide on the orchard floor
following a commercial mistblower application to apples. London, Shell
International Chemical Company, Ltd (Internal report SBGR 88.106).
Hillaby JM & Inglesfield C (1986) The residual toxicity of "Fastac"
formulations and "Fastac"/fungicide mixtures to the honey bee, Apis
mellifera L. Sittingbourne, Shell Research (SBGR 86.018).
Hutson DH (1982) WL85871; metabolism of a single oral dose in the rat.
Sittingbourne, Shell Research (SBGR 82.205).
Hutson DH & Logan CJ (1986) The metabolic fate in rats of the
pyrethroid insecticide WL85871, a mixture of two isomers of
cypermethrin. Pestic Sci, 17: 548-558.
Inglesfield C (1984) The effects of two formulations of Fastac on the
beneficial arthropod fauna of grape vines. Sittingbourne, Shell
Research (SBGR 84.039).
Inglesfield C (1985a) A field study on the effects of Fastac on the
beneficial arthropod fauna of maize in France. Sittingbourne, Shell
Research (SBGR 85.069).
Inglesfield C (1985b) A pond enclosure study of the effects of Fastac
formulations on aquatic invertebrates. Sittingbourne, Shell Research
(SBGR 85.083).
Inglesfield C (1985c) The effects of the pyrethroid insecticide
WL85871 on non-target arthropods: Field studies. Pestic Sci, 16(2):
211.
Inglesfield C (1988) Effects of "Fastac" on non-target arthropods; an
overview of a five-year arable crop rotation study. Sittingbourne,
Shell Research (SBGR 87.149).
Inglesfield C (1989) A long-term field study to investigate the
effects of alpha-cypermethrin on predatory and parasitic arthropods.
Meded Fac Landbouwwet Rijksuniv Gent, 54(3a): 895-904.
Inglesfield C (1991) Effects of alpha-cypermethrin (Fastac) on
entomophagous organisms and game-bird chick-food insects in summer
cereals. The Hague, Shell Internationale Petroleum Maatschappij B.V.
(Report Series HSE 91.008).
Inglesfield C & Forbes S (1986) A field trial to assess the effects of
"Fastac" 100 g/litre OSC on foraging honey bees in oilseed rape.
Sittingbourne, Shell Research (SBGR 86.087).
Inglesfield C & Sherwood CM (1983) Toxicity of cypermethrin and
WL85871 to the earthworm, Eisenia foetida L.
(Oligochaeta:Lumbriculidae) in laboratory tests. Sittingbourne,
Shell Research (SBGR 83.071).
Langner EJ (1980) Determination of the vapour pressure of WL 43467 and
WL 85871 at 20 °C. Sittingbourne Shell Research (Research Note No.
FCDN 80.137).
Le Blanc J (1985) Field experiments on the effects of a new pyrethroid
insecticide WL85871 on bees foraging artificial aphid honey-dew on
winter wheat. Pestic Sci, 16(2): 206.
Le Quesne PM, Maxwell IC, & Butterworth STG (1980) Transient facial
sensory symptoms following exposure to synthetic pyrethroids; a
clinical and electrophysiological assessment. Neurotoxicology, 2:
1-11.
Logan CJ (1983) WL85871; depletion from tissues of female rats after
a single oral dose. Sittingbourne, Shell Research (SBGR 83.075).
McMinn AL (1983a) The degradation of the pyrethroid insecticides
WL85871 (Fastac) and WL43481 in cabbage. Sittingbourne, Shell Research
(SBGR 83.396).
McMinn AL (1983b) The degradation of the pyrethroid insecticides
WL85871 and WL43481 in soil. Sittingbourne, Shell Research (SBGR
83.395).
Maloney SE, Maule A, & Smith ARW (1988) Microbial transformation of
the pyrethroid insecticides: Permethrin, deltamethrin, fastac,
fenvalerate and fluvalinate. Appl Environ Microbiol, 54(11):
2874-2876.
Murray A (1985) Acute and residual toxicity of a new pyrethroid
insecticide WL85871, to honey-bees. Bull Environ Contam Toxicol, 34:
560-564.
Pearson N (1986) Fastac oil-enriched suspension concentrates; acute
toxicities of two formulations to the rainbow trout, Salmo gairdneri.
Sittingbourne, Shell Research (SBGR 85.290).
Pearson N (1990) The fate of "Fastac" in experimental ponds.
Sittingbourne, Shell Research (SBGR 88.177).
Pickering RG (1982) A 5-week feeding study with WL85871 in rats.
Sittingbourne, Shell Research, vol 1 and 2 (SBGR 81.212).
Price JB (1985a) Toxicology of Fastac (WL85871); the acute oral and
percutaneous toxicity and skin irritancy of a Fastac 10% EC (SF
06510). Sittingbourne, Shell Research (SBGR 85.087).
Price JB (1985b) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity, skin and eye irritancy of a Fastac 100 g/litre
suspension concentrate (SF 06378). Sittingbourne, Shell Research (SBGR
84.298).
Price JB (1986) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity, skin and eye irritancy of SF 06615, a 15
g/litre suspension concentrate of WL85871 ("Fastac", "Fendona").
Sittingbourne, Shell Research (SBGR 85.224).
Price JB (1987) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity of Fastac/BPMC 10/400 g/litre EC (SF 06717).
Sittingbourne, Shell Research (SBGR 86.253).
Price JB (1988) Toxicology of animal health products; the acute oral
and percutaneous toxicity, skin and eye irritancy of two pour-on
formulations of Renegade, SF 06954 (10 g/litre) and SF 06977 (15
g/litre). Sittingbourne, Shell Research (SBGR 87.161).
Roberts BL & Dorough HW (1984) Relative toxicities of chemicals to the
earthworm Eisenia foedita. Environ Toxicol Chem, 3(1): 67-68.
Rose GP (1982) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity of WL85871 ( cis-2-Ripcord) comparison with
Ripcord. Sittingbourne, Shell Research (SBGR 82.130).
Rose GP (1983a) Toxicology of pyrethroids; the acute oral toxicity of
WL85871 in comparison with WL43467. Sittingbourne, Shell Research
(SBGR 83.101).
Rose GP (1983b) Neurotoxicity of WL85871 in comparison with WL43467;
the effect of twenty oral doses of WL85871 or WL43467 over a period of
4 weeks on the rat sciatic/posterior tibial nerve, trigeminal nerve
and trigeminal ganglion. Sittingbourne, Shell Research (SBGR 83.185).
Rose GP (1984a) Toxicology of pyrethroids; the acute intraperitoneal
toxicity of technical Fastac. Sittingbourne, Shell Research (SBGR
84.085).
Rose GP (1984b) Toxicology of Fastac; the eye irritancy potential of
the Fastac 100 g/litre emulsifiable concentrate formulation DF 05898
and its formulation components. Sittingbourne, Shell Research (SBGR
84.053).
Rose GP (1984c) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity, skin and eye irritancy of the Fastac 15 g/litre
ULV formulation SF 06363. Sittingbourne, Shell Research (SBGR 84.145).
Rose GP (1984d) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity, skin and eye irritancy of the Fastac 10 EC
formulation, 5835 B. Sittingbourne, Shell Research (SBGR 84.077).
Rose GP (1984e) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity, skin and eye irritancy of the Fastac 10 EC
formulation 5898 B. Sittingbourne, Shell Research (SBGR 84.078).
Rose GP (1984f) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity, skin and eye irritancy of the Fastac 3 EC
formulation, DF 06353. Sittingbourne, Shell Research (SBGR 84.084).
Rose GP (1984g) Toxicology of pyrethroids; the eye irritancy and skin
sensitizing potential of the 100 g/litre Fastac emulsifiable
concentrate formulation EF 5835. Sittingbourne, Shell Research (SBGR
84.086).
Rose GP (1984h) Toxicology of pyrethroids; the acute oral and
percutaneous toxicity, skin and eye irritancy of the 15:120 g/litre
Fastac/methomyl emulsifiable concentrate formulation, FD 9148.
Sittingbourne, Shell Research (SBGR 84.104).
Rose GP (1985) Toxicology of insecticides; the acute oral and
percutaneous toxicity, skin and eye irritancy of the 30 g/litre Fastac
EC formulation SF 06446. Sittingbourne, Shell Research (SBGR 84.209).
Senior PL & Lavers A (1990a) Fastac - Potential dermal exposure. The
Hague, Shell Internationale Petroleum Maatschappij B.V. (Report Series
HSE 90.016).
Senior PL & Lavers A (1990b) A field study of operator exposure to
Fastac during crop spraying at Shell Research Ltd, Sittingbourne
Research Centre. The Hague, Shell Internationale Petroleum
Maatschappij B.V. (Report Series HSE 90.014).
SHELL (1983a) Review of mammalian and human toxicology; Fastac. The
Hague, Shell Internationale Petroleum Maatschappij B.V. (Review Series
MDT 83.001).
SHELL (1983b) Review of environmental toxicology; Fastac. The Hague,
Shell Internationale Petroleum Maatschappij B.V. (Review Series MDT
83.005).
SHELL (1984) Review of residue information; Fastac. London, Shell
International Chemical Company Ltd.
SHELL (1986) Determination of residues of alpha-cypermethrin in rat
blood - Gas chromatographic method. Sittingbourne, Shell Research
(Analytical Method Series, No. SAMS 436-1).
SHELL (1987a) Determination of WL 85871 and the ratio of the
enantiomer pairs in technical material and formulated products -
liquid chromatographic method. Sittingbourne, Shell Research
(Analytical Methods Series, No. SAMS 346-4).
SHELL (1987b) Intereg residues section: Ripcord residues in crops.
London, International Chemical Company, Ltd (Internal report).
SHELL (1987c) Intereg residues section: Fastac residues in crops.
London, International Chemical Company, Ltd (Internal report).
SHELL (1988a) Determination of residues of alpha-cypermethrin in
animal tissues - Gas-liquid chromatographic method. Sittingbourne,
Shell Research (Analytical Method Series, No. SAMS 461-1).
SHELL (1988b) Determination of residues of alpha-cypermethrin in milk
- Gas-liquid chromatographic method. Sittingbourne, Shell Research
(Analytical Method Series, No. SAMS 456-1).
SHELL (1989a) Determination of residues of alpha-cypermethrin in crops
- Gas chromatographic method. Sittingbourne, Shell Research
(Analytical Method Series, No. SAMS 351-2).
SHELL (1989b) Review of environmental toxicology; Fastac. The Hague,
Shell Internationale Petroleum Maatschappij B.V. (Review Series HSE
89.008).
SHELL (1990a) Determination of residues of alpha-cypermethrin in water
- Gas chromatographic method. Sittingbourne, Shell Research
(Analytical Method Series, No. SAMS 469-2).
SHELL (1990b) Determination of residues of alpha-cypermethrin in soils
- Gas chromatographic method. Sittingbourne, Shell Research
(Analytical Method Series, No. SAMS 354-2).
Sherren AJ (1988a) Residues of alpha-cypermethrin in cattle tissues
following topical treatment of calves with "Renegade" pour-on in the
UK. London, Shell International Chemical Company, Ltd (Internal report
SBGR 88.036).
Sherren AJ (1988b) Residues of alpha-cypermethrin in milk following
topical treatment of cows with "Renegade" pour-on in the UK. London,
Shell International Chemical Company, Ltd (Internal report SBGR
88.037).
Shires SW (1982) A comparison of the toxicity of WL85871 and Ripcord
to rainbow trout ( Salmo gairdneri, Richardson) in small field
enclosures. Sittingbourne, Shell Research (SBGR 82.089).
Shires SW (1983a) Pesticides and honey bees; case studies with Ripcord
and Fastac. Span, 26(3): 118-120.
Shires SW (1983b) Effect of formulation type on the toxicity of
insecticides to fish. Sittingbourne, Shell Research (SBGR 83.015).
Shires SW (1985a) A step-wise evaluation of the effects of a new
pyrethroid insecticide WL85871 on honey bees. Pestic Sci, 16(2):
205-216.
Shires SW (1985b) Toxicity of a new pyrethroid insecticide WL85871 to
rainbow trout. Bull Environ Contam Toxicol, 34: 134-137.
Shires SW & Inglesfield C (1986) A field study of the effects of
"Fastac" (WL85871) on rice pests and their natural enemies.
Sittingbourne, Shell Research (SBGR 86.003).
Shires SW, Le Blanc J, Debray P, Forbes S, & Louveaux J (1984a) Field
experiments on the effects of a new pyrethroid insecticide WL85871 on
bees foraging artificial aphid honey-dew on winter wheat. Pestic Sci,
15: 543-552.
Shires SW, Murray A, Debray P, & Le Blanc J (1984b) The effects of a
new pyrethroid insecticide WL85871 on foraging honey bees ( Apis
mellifera L.) Pestic Sci, 15: 491-499.
Shires SW, Le Blanc J, Murray A, Forbes S, & Debray P (1984c) A field
trial to assess the effects of a new pyrethroid insecticide WL85871 on
foraging honey bees in oilseed rape. J Agric Res, 23(4): 217-226.
Stephenson RR (1982) WL85871 and cypermethrin; a comparison of their
acute toxicity to Salmo gairdneri, Daphnia magna and Selenastrum
capricornutum. Sittingbourne, Shell Research (SBGR 81.277).
Stephenson RR (1983) WL85871 and cypermethrin; a comparative study of
their toxicity to the fathead minnow, Pimephales promelas
(Rafinesque). Sittingbourne, Shell Research (SBGR 82.298).
Stephenson RR (1986) "Fastac"; the acute toxicity of different
formulations to fish in rice paddies. Sittingbourne, Shell Research
(SBGR 85.201).
Stephenson RR (1987a) The effects of "Fastac" (OSC) on fish survival
and growth following its application to paddy rice. Sittingbourne,
Shell Research (SBGR 87.054).
Stephenson RR (1987b) An insecticide formulation that spares fish.
Span, 30(2): 75-77.
Stephenson RR (1987c) The effects of "Fastac" (OSC) on fish following
its application to outdoor tanks. Sittingbourne, Shell Research (SBGR
86.227).
Stone CM & Watkinson RJ (1983) WL85871; an assessment of ready
biodegradability. Sittingbourne, Shell Research (SBGR 83.206).
Tasei J-N, Carre S, Bosio PG, Debray P, & Hariot J (1987) Effects of
the pyrethroid insecticide WL85871 and Phosalone on adults and progeny
of the leaf-cutting bee, Megachile rotundata F., pollinator of
lucerne. Pestic Sci, 21: 119-128.
Van Sittert NJ, Eadsforth CV, & Bragt P (1985) Human oral
dose-excretion study with Fastac. The Hague, Shell Internationale
Petroleum Maatschappij B.V. (Report Series HSE 85.010).
Vijverberg HPM & Van Den Bercken J (1990) Neurotoxicological effects
and the mode of action of pyrethroid insecticides. Crit Rev Toxicol,
21(2): 105-126.
Western NJ (1984) Report on the assessment of Fastac exposures during
formulation of technical concentrate (TC) and technical material (TM)
at Durban, South Africa, September 1983. The Hague, Shell
Internationale Petroleum Maatschappij B.V. (Report Series HSE 84.003).
WHO (1989) Environmental Health Criteria 82: Cypermethrin. Geneva,
World Health Organization, 154 pp.
Wooder MF (1982) Studies on the effect of WL85871 on the integrity of
rat liver DNA in vivo. Sittingbourne, Shell Research (SBGR 81.225).
Woollen BH, Marsh JR, & Chester G (1991) Metabolite profiles of a
pyrethroid insecticide following oral and dermal absorption in man.
In: Proceedings of a Conference on Percutaneous Penetration,
Southampton, England, 10-12 April 1991.
Worthing CR & Hance RJ (1991) Fastac. In: The pesticides manual: a
world compendium, 9th ed. Croydon, British Crop Protection Council, pp
210-211.
APPENDIX I
Cypermethrin: Summary, Evaluation, Conclusions, and
Recommendations (Reprint from EHC 82 on Cypermethrin, WHO/IPCS, 1989)
1. Summary
1.1 General
Cypermethrin was initially synthesized in 1974 and first marketed
in 1977 as a highly active synthetic pyrethroid insecticide, effective
against a wide range of pests in agriculture, public health, and
animal husbandry. In agriculture, its main use is against foliage
pests and certain surface soil pests, such as cutworms, but because of
its rapid breakdown in soil, it is not recommended for use against
soil-borne pests below the surface.
In 1980, 92.5% of all the cypermethrin produced in the world was
used on cotton; in 1982, world production was 340 tonnes of the active
material. It is mainly used in the form of an emulsifiable
concentrate, but ultra-low volume concentrates, wettable powders, and
combined formulations with other pesticides are also available.
Chemically, cypermethrin is the alpha-cyano-3-phenoxybenzyl ester
of the dichloro analogue of chrysanthemic acid,
2,2-dimethyl-3-(2,2-dichlorovinyl) cyclopropanecarboxylic acid. The
molecule embodies three chiral centres, two in the cyclopropane ring
and one on the alpha cyano carbon. These isomers are commonly grouped
into four cis and four trans isomers, the cis group being the more
powerful insecticide. The ratio of cis to trans isomers varies from
50:50 to 40:60. Cypermethrin is the racemic mixture of all eight
isomers and, in this appraisal, cypermethrin refers exclusively to the
racemic mixture (ratio 50:50) unless otherwise stated.
Most technical grades of cypermethrin contain more than 90% of
the active material. The material varies in physical form from a
brown-yellow viscous liquid to a semi-solid.
Cypermethrin has a very low vapour pressure and solubility in
water, but it is highly soluble in a wide range of organic solvents.
Analytical methods are available for the determination of cypermethrin
in commercially available preparations. In addition, methods for the
determination of residues of cypermethrin in foods and in the
environment are well established. In most substrates, the practical
limit of determination is 0.01 mg/kg.
1.2 Environmental transport, distribution and transformation
Unlike the natural pyrethrins, cypermethrin is relatively stable
to sunlight and, though it is probable that photodegradation plays a
significant role in the degradation of the product on leaf surfaces
and in surface waters, its effects in soils are limited. The most
important photodegradation products, 2,2-dimethyl-3-
(2,2-dichlorovinyl) cyclopropane carboxylic acid (CPA),
3-phenoxy-benzoic acid (PBA) and, to some extent, the amide of the
intact ester, do not differ greatly from those resulting from
biological degradation.
Degradation in the soil occurs primarily through cleavage of the
ester linkage to give CPA, PBA, and carbon dioxide. Some of the carbon
dioxide is formed through the cleavage of both the cyclopropyl and
phenyl rings under oxidative conditions. The half-life of cypermethrin
in a typical fertile soil is between 2 and 4 weeks.
Cypermethrin is adsorbed very strongly on soil particles,
especially in soils containing large amounts of clay or organic
matter. Movement in the soil is therefore extremely limited and
downward leaching of the parent molecule through the soil does not
occur to an appreciable extent under normal conditions of use. The two
principal degradation products show, on the scale of Helling,
"intermediate mobility".
Cypermethrin is also relatively immobile in surface waters and,
when applied to the surface of a body of water at rates typical of
those used in agriculture applications, it is largely confined to the
surface film and does not reach deeper levels or the sediment in
appreciable concentrations. Cypermethrin also degrades readily in
natural waters with a typical half-life of about 2 weeks. It is
probable that both photochemical and biological processes play a part.
It has been shown that spray drift reaching surface waters adjacent to
sprayed fields does not result in long-term residues in such waters.
Accumulation studies have shown that cypermethrin is rapidly
taken up by fish (accumulation factor approximately 1000); the
half-life of residues in rainbow trout was 8 days. In view of the low
concentrations of cypermethrin that are likely to arise in water
bodies and their rapid decline, it has been concluded that, under
practical conditions, residues in fish will not reach measurable
levels.
The results of field studies have shown that, when applied at
recommended rates, the levels of cypermethrin and its degradation
products in soil and surface waters are very low. Thus, it is unlikely
that the recommended use of cypermethrin will have any effects on the
environment.
1.3 Environmental levels and human exposure
Cypermethrin is used in a wide range of crops. In general, the
maximum residue limits are low, ranging from 0.05 to 2.0 mg/kg in the
different food commodities. The residues will be further reduced
during food processing. In food of animal origin, residues may range
between 0.01 and 0.2 mg/kg product. Residues in non-food commodities
are generally higher, ranging up to 20 mg/kg product.
Total dietary intake values for man are not available, but it can
be expected that the oral exposure of the general population is low to
negligible.
1.4 Kinetics and metabolism
Absorption of cypermethrin from the gastrointestinal tract and
its elimination are quite rapid. The major metabolic reaction is
cleavage of the ester bond. Elimination of the cyclopropane moiety in
the rat, over a 7-day period, ranged from 40 to 60% in the urine and
from 30 to 50% in the faeces; elimination of the phenoxybenzyl moiety
was about 30% in the urine and 55 to 60% in the faeces. Biliary
excretion is a minor route of elimination for the cyclopropane moiety
and small amounts are exhaled as carbon dioxide. In principle, these
absorption and elimination rates and metabolic pathways hold for all
animal species studied, including domestic animals. In cows fed 100 mg
cypermethrin/day, the highest level found in milk was 0.03 mg/litre;
levels of up to 0.1 mg/kg tissue were found in subcutaneous fat. Under
practical conditions, the oral intake of cypermethrin with feed will
be much lower. Cypermethrin used as a spray or dip to combat
parasites, may give rise to maximum residues of 0.05 mg/kg tissue and
0.01 mg/litre milk.
Laying hens exposed orally to 10 mg cypermethrin/kg diet for 2
weeks, showed cypermethrin levels of up to 0.1 mg/kg in the fat, and
up to 0.09 mg/kg in the eggs (predominantly in the yolk).
Consistent with the lipophilic nature of cypermethrin, the
highest mean tissue concentrations are found in body fat, skin, liver,
kidneys, adrenals, and ovaries. Only negligible concentrations are
found in the brain. The half-life of cis-cypermethrin in the fat of
the rat ranges from 12 to 19 days and that of the trans isomer, from
3 to 4 days. In mice, these half-lives are 13 days and 1 day,
respectively.
Overall, the metabolic transformation has been similar in the
different animals studied, including man. Differences that occur have
been related to the rate of formation rather than to the nature of the
metabolites formed and to conjugation reactions. Cypermethrin (both
the cis and trans isomers) is metabolized via the cleavage of the
ester bond to phenoxybenzoic acid and cyclopropane carbolic acid. The
fact that thiocyanate has been identified in in vivo studies,
indicates that the cyanide moiety is further metabolized. The
3-phenoxybenzoic acid is mainly excreted as a conjugate. The type of
conjugate differs in a number of animal species. Phenoxybenzoic acid
is further metabolized to a hydroxy derivative and conjugated with
glucuronic acid or sulfate. The cyclopropyl moiety is mainly excreted
as a glucuronide conjugate, hydroxylation of the methyl group only
occurring to a limited extent.
Ester cleavage is much slower in certain fish species than in
other animal species, the main metabolic pathway being hydroxylation
of the phenoxybenzoic and the cyclopropyl moieties.
Ester cleavage also takes place in plants. The phenoxybenzyl and
cyclopropyl moieties are readily converted into glucoside conjugates.
In mammals, these conjugates are hydrolysed into the original acids
and metabolized.
1.5 Effects on organisms in the environment
High doses of cypermethrin may exert transient minor effects on
microflora activity in the soil. However, no influence on
ammonification and nitrification has been found.
Cypermethrin is very toxic for fish (in laboratory tests 96-h
LC50s were generally within the range of 0.4-2.8 µg/litre), and
aquatic invertebrates (LC50s in the range of 0.01 - > 5 µg/litre).
The presence of suspended solids decreases the toxicity by at least a
factor of 2, because of adsorption of cypermethrin to the solids.
Cypermethrin is not very toxic for birds. Signs of cypermethrin
intoxication were seen at dose levels of 3000 mg/kg body weight or
more. Administration of 1000 mg cypermethrin/kg body weight to laying
hens over a 5-day period did not cause signs of intoxication. However,
cypermethrin was highly toxic for honey bees in laboratory tests, the
oral LD50 ranging from 0.03 to 0.12 µg/bee. Under field conditions,
the hazard is considerably lower, because of the repellent effect of
cypermethrin on worker honey bees, which lasts for at least 6 h after
spraying.
Earthworms are not sensitive to cypermethrin. No deaths occurred
in worms exposed to levels of 100 mg/kg soil for 14 days.
In studies involving deliberate overspraying of experimental
ponds under field conditions, peak concentrations of 2.6 µg
cypermethrin/litre were measured in the water. Fish were not affected,
but populations of crustaceae, mites, and surface-breathing insects
were severely reduced. Most of these populations returned to normal
levels after 15 weeks. Free-swimming dipterous larvae and
bottom-dwelling invertebrates, snails, flatworms, etc., were not
affected. Under normal agricultural conditions (during which drifts
may reach adjacent ditches or streams), the only effects seen in
surface-breathing or surface-dwelling insects were hyperactivity or
immobilization. The relative toxicity of cypermethrin for pests and
their parasites and predators is such that the balance between
host/prey and parasites/predator may not be adversely affected in the
field. However, care should be taken where predatory mites are
important in pest management.
1.6 Effects on experimental animals and in vitro test systems
The acute oral toxicity of cypermethrin is moderate. While LD50
values differed considerably among animal species depending on the
vehicle used and the cis/trans isomeric ratios, the toxic responses in
all species were found to be very similar. The acute toxicity of the
trans isomer in the rat (LD50 > 2000 mg/kg body weight) was lower
than that of the cis isomer (LD50, 160-300 mg/kg body weight). The
onset of toxic signs of poisoning was rapid and they disappeared
within several days in survivors. The toxic signs are characterized by
salivation, tremors, increased startle response, sinuous writhing of
the whole body (choreoathetosis), and clonic seizures. Myelin and axon
degeneration were noted in the sciatic nerve at near lethal dose
levels.
Cypermethrin was moderately to severely irritating, when applied
to the skin or the eye of the rabbit. The severity was partly
dependent on the vehicle used. In guinea-pigs, a mild skin sensitizing
potential was found using the maximization test.
No toxic effects were observed in rats, fed cypermethrin at 100
mg/kg diet for 3 months. Furthermore, prolonged feeding of
cypermethrin (2 years) to dogs at a level of 300 mg/kg feed did not
produce any toxicological effects. A level of 600 mg/kg diet resulted
in reduced body weight gain, but no gross pathological or
histopathological effects were seen.
Two long-term studies on rats and one on mice were carried out.
The dose levels in the rat studies ranged up to 1500 mg/kg diet,
equivalent to 75 mg/kg body weight. No effects were seen at 150 mg/kg
diet. At the highest dose level, reduced body weight gain, increased
liver weights (accompanied by increased smooth endoplasmatic
reticulum), and some haematological and biochemical changes were
observed. No increase in tumour incidence was noted. The same type of
effects were seen in the mouse study at 1600 mg cypermethrin/kg diet.
No effects were seen in the 400 mg/kg diet group.
The effect of cypermethrin on the immune system was studied in
rats. The results showed the possibility of immune suppression by
pyrethroids. More attention should be paid to this aspect, but, at
present, no opinion can be given about its relevance in the
extrapolation of these data for man.
Repeated oral administration of cypermethrin to rats and other
animal species at levels sufficiently high to produce significant
mortality in one group of animals, produced biochemical changes in the
peripheral nerves, consistent with sparse axonal degeneration.
Histopathological changes (swelling and/or disintegration of axons of
the sciatic nerve) were observed. There was no cumulative effect. The
magnitude of the change was substantially less than that encountered
with established neurotoxic agents. The neurotoxic effects seem to be
reversible; presumably the clinical signs are not related to the
induction of neuropathological lesions.
Further evidence to support the minor nature of the nerve lesions
has been afforded by electrophysiological studies on rats.
Measurements of the maximal motor conduction velocities of the sciatic
and tail nerves of rats were made before, and at intervals of up to 5
weeks after, exposure to a single dose or repeated high doses of
cypermethrin. It was concluded from the results that, even at
near-lethal doses, cypermethrin did not cause any effects on maximal
motor conduction velocities and conduction velocities of the slower
motor fibres in rat peripheral nerves. No delayed neurotoxicity was
observed in domestic hens.
The ability of the major metabolite of cypermethrin,
3-phenoxybenzoic acid, to produce axonal changes has been investigated
and found to be negative.
In a multigeneration reproduction study on rats, dose levels up
to 500 mg/kg feed were tested. The parent animals at the highest dose
level showed decreased food intake and reduction in body weight gain.
No influence on reproductive performance or on survival of the
offspring was found. However, at the highest dose level, reductions in
litter size and total litter weights were seen. The pooled body
weights of weaning pups of the 500 mg/kg group were decreased over 3
generations. No effect was found with 100 mg cypermethrin/kg diet.
Embryotoxic and teratogenic effects were not found in rats
administered dose levels of up to 70 mg/kg body weight and clear
teratogenic effects were not observed in rabbits given dose levels of
up to 30 mg/kg body weight during days 6-18 of gestation.
Cypermethrin did not show any mutagenic activity in bacteria or
in yeast, with or without metabolic activation, or in V79 Chinese
hamster cells. Furthermore, cypermethrin gave negative results in an
in vivo chromosomal aberration test with Chinese hamsters and in
dominant lethal studies on mice. In a host-mediated assay with mice,
no increase in the rate of mitotic gene conversion in Saccharomyces
cerevisae was found. In a chromosome study using the bone marrow
cells of Chinese hamsters, cypermethrin did not increase the number of
chromosome abnormalities. However, in a micronucleus test with mouse
bone marrow cells, an increase in the frequency of polychromatic
erythrocytes with micronuclei was found after oral and dermal
applications of cypermethrin. Intraperitoneal application gave a
negative result. A sister chromatid exchange study using bone marrow
cells of mice showed a dose-response related increase in sister
chromatid exchanges of dividing cells.
In long-term/carcinogenicity studies, oral administration of
cypermethrin to rats did not induce an increase in the incidence of
tumours. In a mouse study, dose levels of up to 1600 mg
cypermethrin/kg diet did not produce any increase in tumours of types
not commonly associated with the mouse strain employed. The incidence
of tumours was similar in all groups with the exception of a slight
increase in the incidence of benign alveolar lung tumours in the
females in the 1600 mg/kg diet group. However, the increased
incidence, when compared with concurrent and historical control
incidence, was not sufficient to warrant concern. There was no
suggestion of increased malignancy and no evidence of a decrease in
the latency of the tumours. Furthermore, there was no evidence of a
carcinogenic response in the male mice in this study and, as the
results of mutagenicity studies on cypermethrin have been mainly
negative, it is concluded that there is no evidence for the
carcinogenic potential of cypermethrin.
1.7 Mechanism of toxicity
Extensive studies have been carried out to explain the mechanism
of toxicity of cypermethrin, especially with regard to the effects on
the nervous system. The results strongly suggest that the primary
target site of cypermethrin (and of pyrethroid insecticides in
general) in the vertebrate nervous system is the sodium channel in the
nerve membrane. The alpha-cyano pyrethroids, such as cypermethrin,
cause a long-lasting prolongation of the normally transient increase
in sodium permeability of the nerve membrane during excitation,
resulting in long-lasting trains of repetitive impulses in sense
organs and a frequency-dependent depression of the nerve impulse in
nerve fibres. Since the mechanisms responsible for nerve impulse
generation and conduction are basically the same throughout the entire
nervous system, pyrethroids may well act in a similar way in various
parts of the central nervous system. It is suggested that the facial
skin sensations that may be experienced by people handling
cypermethrin are brought about by repetitive firing of sensory nerve
terminals in the skin, and may be considered as an early warning
signal that exposure has occurred.
1.8 Effects on man
No cases of accidental poisoning have been reported as a result
of occupational exposure.
Skin sensations, reported by a number of authors to have occurred
during field studies, generally lasted only a few hours and did not
persist for more than one day after exposure. Neurological signs were
not observed. General medical and extensive clinical blood-chemistry
studies, and electrophysiological studies on selected motor and
sensory nerves in the legs and arms did not show any abnormalities.
2. Evaluation
Cypermethrin, an alpha-cyano pyrethroid consisting of a mixture
of 8 stereoisomers, is a highly active insecticide effective against
a wide range of pests in many food and non-food commodities.
It is stable to light and heat, it has a low vapour pressure and
is more stable in acidic than in alkaline media. Sensitive analytical
methods for the determination of residues in food and the environment
are available.
When cypermethrin is applied to crops, residues may occur in
soils and surface waters, but biological degradation is fairly rapid
and residues do not accumulate in the environment. Photo-degradation
is unlikely to play an important role. The main route of degradation
is cleavage of the ester linkage to give 2 main degradation products
containing the cyclopropane, and the phenoxybenzyl moiety. The
half-lives in the soil are determined by many factors, but are in the
range of 2-4 weeks. Cypermethrin is strongly adsorbed by soil and
downward leaching is negligible. Because of its rather fast breakdown
forming less toxic products, and the low dose rates used in good
agricultural practice, it is unlikely that cypermethrin will attain
significant levels in the environment.
Bioaccumulation in certain organisms, such as fish, took place
under laboratory conditions, but levels declined on cessation of
exposure and there are indications that, under natural conditions,
fish will not contain measurable residues.
When applied according to good agricultural practice, the levels
of cypermethrin residues in food commodities are generally low. Total
diet studies are not available, but from the available residue
information, it can be inferred that the oral intake by man is well
below the ADI.
High dose levels of cypermethrin may exert transient effects on
the soil microflora. Earthworms and other soil organisms are generally
rather resistant to cypermethrin, while fish and other aquatic
invertebrates are very sensitive. Because of its strong adsorption on
soil, only low levels of cypermethrin may leak into surface water.
These may have transient effects, mainly on surface breathing insects.
The toxicity of cypermethrin for birds is low. Bees appear to be
very sensitive in laboratory tests. Under field conditions, the effect
on bees is minimal, because cypermethrin seems to have a repellent
effect for bees. Absorption and elimination of cypermethrin has been
rapid in the different mammalian species tested. The major metabolic
reaction is cleavage of the ester bond followed by hydroxylation and
conjugation of the cyclopropane and phenoxybenzyl moiety. The highest
levels are found in body fat, which is consistent with the lipophilic
nature of cypermethrin. The half-life in the fat of the rat is about
12-19 days for the cis isomer and 3-4 days for the trans isomer.
Breakdown products in plants are bound as glucosides.
The acute toxicity of cypermethrin for mammals is of a moderate
order. The oral LD50 for the rat ranges from 200 to 4000 mg/kg body
weight. Short-term and long-term toxicity studies on rats, mice, and
dogs have shown effects on growth, on the liver and kidneys, and the
nervous system, and on haematology. A no-observed-adverse-effect level
of 7.5 mg/kg body weight has been adopted by the Task Group.
Cypermethrin was not carcinogenic for mice or rats fed diets
containing high levels of the material over a 2-year period.
Cypermethrin did not induce teratogenic effects in either rats at 70
mg/kg body weight or rabbits at 30 mg/kg body weight. It was also
shown not to have any effects on reproductive performance during a
3-generation reproduction study in rats administered 100 mg/kg diet.
In a variety of mutagenicity studies, cypermethrin was shown to be
mainly without mutagenic activity.
The mechanism of the action on the nervous system has been
extensively studied. From these studies and the occupational studies
available, it seems that the skin sensation seen in workers handling
cypermethrin, generally lasts only a few hours and does not persist
for more than one day after exposure. Other neurological signs were
not observed. These skin sensations can be considered to be an early
warning that exposure has occurred and that work practice should be
reviewed. Cypermethrin may cause eye irritation and may be a
sensitizer for certain persons.
3. Conclusions
It can be concluded that:
General population: When applied according to good agricultural
practice, exposure of the general population to cypermethrin is
negligible and is unlikely to present a hazard.
Occupational exposure: With reasonable work practices, hygiene
measures, and safety precautions, the use of cypermethrin is unlikely
to present a hazard to those occupationally exposed to it. The
occurrence of "facial sensations" is an indication of exposure. Under
these circumstances work practice should be reviewed.
Environment: With recommended application rates it is unlikely
that cypermethrin or its degradation products will attain levels of
environmental significance. Notwithstanding its high toxicity for fish
and honey bees, this is only likely to cause a problem in the case of
spillage and overspraying.
4. Recommendations
- Cypermethrin should be included among the residues looked for in
surveillance, market-basket, or total diet studies.
- Attention should be paid to the implications for the welfare of
human beings of animal studies indicating immune suppression.
- Further follow-up studies into the facial effects in human beings
should be conducted, in order to better understand this
phenomenon.
RESUME ET EVALUATION; CONCLUSIONS ET RECOMMANDATIONS
1. Résumé et évaluation
1.1 Identité, emploi, destinée et concentrations dans l'environnement
L'alpha-cyperméthrine est constituée à plus de 90% de
l'énantiomère le plus actif du point de vue insecticide parmi les
quatre isomères cis qui entrent dans la composition de la
cyperméthrine racémique.
C'est un pyréthrinoïde extrêmement actif, qui agit contre de
nombreux parasites auxquels on a affaire dans l'agriculture et
l'élevage. Il est commercialisé sous forme de concentrés
émulsionnables, de formulations à très bas volume, de concentrés pour
suspension ou en mélange avec d'autres insecticides.
Le produit technique se présente sous la forme d'une poudre
cristalline facilement soluble dans l'acétone, la cyclohexanone et le
xylène mais peu soluble dans l'eau. Il est stable en milieu acide ou
neutre mais s'hydrolyse à pH 12-13. Il se décompose au-dessus de 120
°C.
On ne dispose d'aucune donnée sur la concentration de
l'alpha-cyperméthrine dans l'air.
Dans l'eau, il est probable que l'alpha-cyperméthrine est
décomposée par voie photochimique et biologique. On a constaté que,
après avoir traité un étang à raison de 15 g de matière active par
hectare, les eaux de surface et les couches plus profondes contenaient
respectivement 5% et 19% de la dose appliquée une journée après
l'épandage et 0,1% et 2% respectivement de cette dose sept jours plus
tard. Environ 5% de la dose appliquée se retrouvaient dans les
sédiments 16 jours après l'épandage.
Il est probable que l'alpha-cyperméthrine est fortement adsorbée
aux particules du sol. Une année après un épandage à raison de 0,5 kg
de matière active par hectare, on retrouvait dans le sol des résidus
inférieurs à 0,1 mg/kg.
Le coefficient de partage de l'alpha-cyperméthrine entre le
n-octanol et l'eau est égal à 1,4 x 105 (log de Pow = 5,16).
La dose d'emploi recommandée pour l'alpha-cyperméthrine est
moindre que pour la cyperméthrine car elle est biologiquement plus
active. Il en résulte moins de résidus sur les récoltes et si l'on se
conforme aux doses recommandées, ils se situent entre 0,05 et 1 mg/kg.
Chez des poissons-chats d'eau de mer traités à raison de 0,01 à 0,05%
p/p de matière active, on a mesuré des résidus de 0,3 à 30 mg/kg une
semaine après l'entreposage et entre 0,22 et 4,0 mg/kg 15 semaines
plus tard.
1.2 Cinétique et métabolisme
Après avoir été administrée par voie orale à des rats,
l'alpha-cyperméthrine est éliminée dans les urines sous forme du
sulfo-conjugué de l'acide 3-(4-hydroxyphénoxy)benzoïque ainsi que dans
les matières fécales, en partie sans modification. Environ 90% d'une
dose orale unique sont éliminées de l'organisme en quatre jours, dont
78% au cours du premier jour. Les résidus sont faibles dans les
tissus, sauf dans les tissus lipidiques. Trois jours après
administration d'une dose orale unique de 2 mg/kg, la concentration
dans les graisses était de 0,4 mg/kg. L'élimination à partir des
graisses est biphasique; la demi-vie relative à la phase initiale est
de 2,5 jours, et de 17 à 26 jours pour la deuxième phase.
L'alpha-cyperméthrine est métabolisée par coupure de la liaison
ester. Chez le rat, la fraction alcool phénoxybenzylique est
hydroxylée et transformée en sulfo-conjugué; la fraction acide
cyclopropane-carboxylique subit également une conjugaison,
probablement sous forme de glucuronide, avant d'être excrétée par la
voie urinaire. Des études portant sur des microsomes hépatiques de
rats, de lapins et d'origine humaine ont montré que chez ces trois
espèces, il pouvait y avoir hydrolyse de l'ester et métabolisation
oxydative mais que l'hydrolyse de l'ester prédominait dans le cas des
préparations de foie provenant de lapins ou de sujets humains.
Chez l'homme, 43% d'une dose orale (0,25-0,75 mg/litre) ont été
excrétés dans les 24 heures par la voie urinaire sous forme d'acide
cis-cyclopropane-carboxylique libre ou conjugué. Après
l'administration de cinq doses quotidiennes successives, l'excrétion
urinaire n'a pas augmenté.
On a trouvé de fortes concentrations (jusqu'à 1156 mg/kg)
d'alpha-cyperméthrine dans la laine de mouton, 14 jours après avoir
traité les animaux par des bains ou des aspersions. De faibles
quantités ont été retrouvées dans la graisse sous-cutanée (jusqu'à
0,04 mg/kg). Après avoir traité des veaux le long de l'épine dorsale
avec 10 ml d'une formulation à 1,6%, on n'a pas retrouvé
d'alpha-cyperméthrine dans les muscles ni le foie. La concentration
maximale dans la graisse périrénale était de 0,25 mg/kg au bout de 14
jours.
Après avoir traité des vaches en lactation le long de l'épine
dorsale avec des formulations contenant jusqu'à 0,2 g de matière
active on a retrouvé dans le lait de 3 des 15 animaux traités, des
résidus d'alpha-cyperméthrine compris entre 0,003 et 0,005 mg/litre.
1.3 Effets sur les mammifères de laboratoire et les systèmes
d'épreuves in vitro
L'alpha-cyperméthrine présente une toxicité orale aiguë modérée
à forte pour les rongeurs. La DL50 varie beaucoup chez la souris et
le rat et dépend de la concentration du composé et du véhicule
utilisé. En pratique, on considère qu'une DL50 de 80 mg/kg de poids
corporel est représentative. Toutefois, on a fait état de valeurs plus
élevées pour la DL50 par voie orale dans des conditions
d'intoxication aiguë. Une intoxication aiguë par voie orale entraîne
des signes cliniques qui traduisent une action au niveau du système
nerveux central.
Une application cutanée d'alpha-cyperméthrine à des rats et des
souris aux doses respectives de 100 et 500 mg/kg de poids corporel,
n'a entraîné ni mortalité ni signes d'intoxication. De même, des rats
à qui l'on avait fait inhaler pendant quatre heures de
l'alpha-cyperméthrine à une concentration de 400 mg/m3, n'ont
présenté aucune mortalité ni signes cliniques d'intoxication.
L'alpha-cyperméthrine technique ne provoquerait qu'une irritation
minimale de la peau chez le lapin. En revanche, certaines formulations
de ce composé peuvent déterminer une très forte irritation oculaire.
L'alpha-cyperméthrine technique n'a pas d'effet sensibilisant cutané.
Chez le cobaye, elle provoque une stimulation des terminaisons
nerveuses sensorielles de l'épiderme.
Exposés pendant une courte période à de l'alpha-cyperméthrine à
des concentrations quotidiennes allant jusqu'à 200 mg/kg de nourriture
pendant cinq semaines ou jusqu'à 100 mg/kg de nourriture pendant 13
semaines, des rats n'ont pas présenté d'effets toxiques. Aux doses les
plus élevées, on notait des signes d'intoxication traduisant une
atteinte du système nerveux, ainsi qu'une réduction de la croissance
et une augmentation du poids du foie et des reins. Aucun effet bien
net n'a été observé en ce qui concerne les paramètres hématologiques
ou histopathologiques.
Lors d'une étude de 13 semaines au cours de laquelle des chiens
ont reçu de l'alpha-cyperméthrine par voie orale, on a observé que la
dose la plus forte (270 mg/kg de nourriture) produisait des signes
d'intoxication; en revanche tous les autres paramètres étudiés (NFS,
biochimie du sang, urines, poids des organes, anatomopathologie et
histopathologie) sont restés normaux. La dose sans effet observable
était de 90 mg/kg de nourriture (soit l'équivalent de 2,25 mg/kg de
poids corporel par jour).
Le même genre d'étude menée sur des rats a montré que
l'alpha-cyperméthrine provoquait des effets neurotoxiques imputables
à des lésions histopathologiques des nerfs tibial et sciatique, une
dégénérescence des axones et un accroissement de l'activité de la
bêta-galactosidase.
On ne dispose d'aucune donnée sur la toxicité à long terme, la
toxicité pour la fonction de reproduction, la tératogénicité ou
l'immunotoxicité.
En se basant sur les données disponibles, on peut conclure que
l'alpha-cyperméthrine n'est pas mutagène, comme le montrent les tests
effectués sur Salmonella typhimurium, Escherichia coli et
Saccharomyces cerevisiae ainsi que les épreuves in vivo et in
vitro sur des cellules de foie de rat, qui n'ont révélé ni
aberration chromosomique ni lésion de l'ADN monobrin. On n'a pas non
plus observé d'augmentation du nombre d'aberrations chromosomiques
dans des cellules de moelle osseuse de rat.
On ne dispose d'aucune donnée sur la cancérogénicité de
l'alpha-cyperméthrine.
1.4 Effets sur l'homme
Dans la mesure où l'alpha-cyperméthrine est utilisée conformément
aux règles de bonne pratique agricole, l'exposition de la population
générale reste négligeable. On a constaté que l'exposition
professionnelle cutanée des opérateurs procédant à la préparation des
mélanges, au chargement des pulvérisateurs, à l'épandage de
l'insecticide ou au lavage du matériel, pouvait atteindre des valeurs
respectivement égales à 2,94 mg, 0,61 mg et 0,73 mg.
Lors d'une étude sur l'exposition à l'alpha-cyperméthrine au
cours de la préparation de formulations à base de cet insecticide, on
a évalué les niveaux d'exposition par surveillance personnelle et
statique de la concentration atmosphérique de ce composé et dosage de
ses métabolites urinaires. Au cours des deux jours pendant lesquels
les personnes exposées procédaient à la formulation de concentrés
techniques, l'exposition individuelle moyenne dans le groupe a été
respectivement de 2,8 et 4,9 mg/m3, l'exposition individuelle
moyenne du groupe au produit technique étant de 50,1 mg/m3 le
troisième jour. On n'a pas pu déceler la présence de métabolites dans
les urines (limite de détection 0,02 mg/litre). Au cours de la
préparation des diverses formulations, les ouvriers ont fait état de
sensations au niveau de l'épiderme mais en précisant qu'elles étaient
légères.
Aucune intoxication n'a été signalée.
1.5 Effets sur d'autres organismes au laboratoire et dans leur milieu
naturel
La CE50 à 48 et 96 heures (pour la croissance) chez une algue
d'eau douce, Selenastrum capricornutum, dépasse 100 µg/litre.
L'alpha-cyperméthrine est très fortement toxique pour les
invertébrés aquatiques. Les valeurs de la CE50 à 24 et 48 heures
(pour l'immobilisation de la daphnie) sont respectivement égales à 1,0
et 0,3 µg/litre, et celle de la CL50 à 24 heures (pour Gammarus
pulex) est égale à 0,05 µg/litre. L'alpha-cyperméthrine est
également très toxique pour un certain nombre d'arthropodes aquatiques
mais sa toxicité est moindre pour les mollusques. La toxicité à court
terme de ce composé peut être réduite lorsqu'il est présenté sous la
forme d'une suspension dans l'huile. Les pertes à l'épandage peuvent
provoquer des effets toxiques sur les invertébrés aquatiques, mais
comme l'alpha-cyperméthrine disparaît rapidement de l'eau, ceux-ci ont
la possibilité de récupérer. L'alpha-cyperméthrine est très fortement
toxique pour les poissons. La valeur de la CL50 à 96 heures oscille
entre 0,7 et 350 µg/litre selon le type de formulation. Les concentrés
émulsionnables sont beaucoup plus toxiques que les concentrés en
suspension, les poudres mouillables et les formulations
micro-encapsulées. Le danger de l'alpha-cyperméthrine pour les
invertébrés aquatiques et les poissons tient à sa toxicité aiguë. Rien
n'indique toutefois qu'une exposition prolongée entraîne des effets
cumulatifs.
On ne dispose d'aucune donnée concernant les effets de
l'alpha-cyperméthrine sur les microbes terricoles. Les bactéries
présentes dans les effluents n'ont pas paru affectées par une
concentration de 3 mg/litre en système fermé.
La toxicité de l'alpha-cyperméthrine pour certains carabides et
les larves de neuroptères est relativement faible et elle ne présente
guère de danger pour les stades pré-imaginaux des hyménoptères
parasitoïdes. Des études menées sur des champs de grande superficie ou
de petites parcelles ont montré que l'alpha-cyperméthrine était peu
dangereuse pour les carabides et les staphylinides mais qu'elle
présentait un risque relativement important pour les linyphiides. Les
effets sur ces populations d'insectes se sont limités à une seule
saison de croissance. En outre, l'alpha-cyperméthrine ne présente
guère de risque pour les larves de syrphides mais n'est pas dénuée
d'effets sur les coccinellides. Malgré tout, la dissipation rapide des
résidus présents sur le feuillage permet à ces animaux de reconstituer
rapidement leurs colonies.
L'épandage d'alpha-cyperméthrine n'a pas d'effets indésirables
sur l'abondance relative des arthropodes entomophages. Son utilisation
sur les céréales à petits grains n'entraînerait donc pas la
réapparition des ravageurs ou le déclenchement d'infestations
secondaires.
Des études de laboratoire ont montré que l'alpha-cyperméthrine
était peu toxique pour les lombrics. Des vers placés pendant 14 jours
dans un sol artificiel contenant ce composé à des concentrations
allant jusqu'à 100 mg/kg, n'ont présenté aucune mortalité.
Des études de toxicité aiguë également effectuées en laboratoire
ont montré que l'alpha-cyperméthrine était extrêmement toxique pour
les abeilles. Administré par voie orale, un concentré émulsionnable
d'alpha-cyperméthrine a donné une DL50 à 24 heures de 0,13
µg/abeille, la valeur correspondante pour l'administration topique
étant de 0,03 µg/abeille (de produit technique) ou 0,11 µg/abeille (de
concentré émulsionnable). La forte toxicité de l'alpha-cyperméthrine
pour les abeilles ne s'est pas manifestée au cours des épreuves de
plein champ, probablement du fait que le composé a un bref effet
répulsif qui réduit le butinage et, par voie de conséquence,
l'exposition des insectes.
On ne dispose d'aucune donnée sur la toxicité de
l'alpha-cyperméthrine pour les oiseaux.
2. Conclusions
2.1 Population générale
Lorsque l'épandage de l'alpha-cyperméthrine s'effectue
conformément aux règles de bonne pratique, son utilisation en
agriculture n'expose guère la population générale à ce composé et il
y a peu de danger pour elle.
2.2 Exposition professionnelle
Moyennant de bonnes méthodes de travail ainsi que des mesures
d'hygiène et de sécurité, l'utilisation de l'alpha-cyperméthrine ne
devrait pas présenter de danger pour les personnes qui y sont exposées
de par leur profession. L'apparition de sensations au niveau de la
face indique une contamination. Dans ces circonstances, il est bon de
revoir les méthodes de travail.
2.3 Environnement
Aux doses d'emploi recommandées, il n'est guère probable que
l'alpha-cyperméthrine puisse être libérée dans l'environnement à des
concentrations écologiquement dangereuses. Elle est fortement toxique
pour les arthropodes aquatiques, les poissons et les abeilles dans les
conditions du laboratoire. On ne peut envisager la probabilité
d'effets toxiques importants sur les invertébrés non visés et les
poissons qu'en cas de déversement accidentel, d'épandage excessif ou
d'erreur de manipulation.
3. Recommandations
* Il convient d'éviter la contamination des eaux superficielles par
l'alpha-cyperméthrine.
* L'alpha-cyperméthrine se lie fortement aux particules. D'autres
études écotoxicologiques sont à effectuer à propos des effets de
l'alpha-cyperméthrine sur les organismes qui vivent dans les
sédiments car il s'agit d'un aspect qui n'a guère retenu
l'attention jusqu'ici.
* L'absorption dans les voies digestives de l'alpha-cyperméthrine
est à étudier dans diverses conditions expérimentales.
* Il faudrait également étudier la destinée de
l'alpha-cyperméthrine après application sur l'épiderme.
* Il faudrait obtenir des données supplémentaires sur la toxicité
à long terme, la cancérogénicité et l'immunotoxicité de
l'alpha-cyperméthrine.
RESUMEN Y EVALUACION; CONCLUSIONES Y RECOMENDACIONES
1. Resumen y evaluación
1.1 Identificación, uso, destino y niveles en el medio ambiente
La alfa-cipermetrina contiene más del 90% del par de
enantió-meros con mayor actividad insecticida de los cuatro isómeros
cis de la cipermetrina en mezcla racémica.
Se trata de un insecticida piretroide sumamente activo contra una
gran variedad de plagas habituales en agricultura y ganadería. Existe
como concentrado emulsionable, formulacion de volumen ultra-bajo,
concentrado en suspensión y en mezcla con otros insecticidas.
El producto técnico es un polvo cristalino, con buena solubilidad
en acetona, ciclohexanona y xileno, y con baja solubilidad en agua. Es
estable en condiciones ácidas o neutras, pero se hidroliza a pH 12-13.
Se descompone por encima de los 220 °C.
No se dispone de información acerca de los niveles de
alfa-cipermetrina en el aire.
Es probable que la degradación de la alfa-cipermetrina en agua se
deba a procesos fotoquímicos y biológicos. El agua superficial y
subsuperficial de un estanque rociado con 15 g/ha de principio activo
contenía el 5% y el 19% de la dosis aplicada un día después del
rociamiento, y 0.1% y 2% siete días más tarde. A los 16 días de la
aplicación se encontró en el sedimento alrededor del 5% de la dosis
utilizada.
Es probable que la alfa-cipermetrina se adsorba con fuerza a las
partículas del suelo. Un año después del tratamiento con 0.5 kg de
principio activo por hectárea se encontraron en el suelo residuos
inferiores a 0.1 mg/kg.
El coeficiente de reparto n-octanol/agua de la alfa-cipermetrina
es 1.4 x 105 (log Poa = 5.16).
Las tasas de aplicación recomendadas de alfa-cipermetrina son
inferiores a las de cipermetrina, porque la primera es biológicamente
más activa. En consecuencia, los residuos en los cultivos son escasos,
y utilizando las tasas de aplicación recomendadas estos residuos
oscilan entre 0.05 y 1 mg/kg. Los residuos en peces siluroideos
marinos tratados con dosis entre el 0.001 y el 0.05% p/p de principio
activo eran de 0.3-30 mg/kg una semana después del almacenamiento, y
de 0.22 a 4.0 mg/kg tras 15 semanas de almacenamiento.
1.2 Cinética y metabolismo
La alfa-cipermetrina administrada por vía oral a ratas se elimina
por la orina como sulfato conjugado del ácido 3-(4-hidroxifenoxi)
benzoico y en parte como compuesto inalterado por las heces. De una
dosis oral única alrededor del 90% se elimina del cuerpo en un período
de cuatro días, y el 78% en el primer día. Los residuos en los tejidos
son escasos, excepto en el adiposo. La concentración en la grasa tres
días después de una dosis oral única de 2 mg/kg fue de 0.4 mg/kg. La
eliminación a partir de la grasa es bifásica: en la fase inicial tiene
una vida media de 2.5 días y en la segunda de 17 a 26 días.
La alfa-cipermetrina se metaboliza mediante la ruptura de su
enlace éster. En la rata, el alcohol fenoxibencílico de la molécula se
hidroxila y se conjuga con sulfato, y el ácido
ciclopropano-carboxílico también se conjuga (probablemente en forma de
glucurónido) antes de la excreción urinaria. En estudios con
microsomas hepáticos de ratas, conejos y seres humanos se ha
demostrado que la hidrólisis en el ester y rutas oxidativas se dan en
las tres especies, si bien la primera es la ruta predominante en las
preparaciones hepáticas de conejo y de ser humano.
En el ser humano, el 43% de una dosis oral (0.25-0.75 mg) se
excreta en la orina en un plazo de 24 h en forma de ácido cis-
ciclopropano-carboxílico libre o conjugado. La excreción urinaria no
aumentó tras la administración de 5 dosis diarias sucesivas.
En la lana de las ovejas se detectaron concentraciones altas
(hasta 1156 mg/kg) de alfa-cipermetrina 14 días después de la
aplicación por inmersión o por lavado. En la grasa subcutánea se
encontraron niveles bajos (hasta 0.04 mg/kg). Después de tratar
terneros a lo largo del dorso con 10 ml de una preparación al 1.6%, no
se detectó alfa-cipermetrina en los músculos ni en el hígado. La
máxima concentración en la grasa perirrenal durante un período de 14
días fue de 0.26 mg/kg.
Tras la aplicación de hasta 0.2 ml de principio activo a lo largo
del dorso de vacas lecheras, se encontraron en la leche de tres en 15
animales tratados residuos de alfa-cipermetrina en concentraciones que
oscilaban entre 0.003 y 0.005 mg/ml.
1.3 Efectos en mamíferos de laboratorio y en sistemas de prueba
in vitro
En roedores, la toxicidad aguda oral de la alfa-cipermetrina es
entre moderada y alta. Los valores de la DL50 en ratones y ratas son
muy variables y dependen de la concentración del compuesto y del
excipiente. A efectos prácticos, se considera representativo un valor
de la DL50 de 80 mg/kg de peso corporal. Sin embargo, se han
notificado valores más altos de DL50 aguda por vía oral. La
exposición oral aguda produce síntomas clínicos relacionados con la
actividad del sistema nervioso central.
Las aplicaciones cutáneas aisladas de alfa-cipermetrina a ratones
y ratas en concentraciones de 100 y 500 mg/kg de peso corporal,
respectivamente, no produjeron mortalidad ni síntomas de intoxicación.
La exposición de ratas por inhalación durante 4 h a una concentración
atmosférica de 400 mg/m3 tampoco ocasionó mortalidad ni signos
clínicos.
Se ha reportado que la alfa-cipermetrina de calidad técnica
produce una irritación cutánea mínima en el conejo. Algunas de las
preparaciones provocan irritación ocular grave. La alfa-cipermetrina
de calidad técnica no produce sensibilización cutanea. En cobayos
ocasionó la excitación de las terminaciones neuro-sensoriales de la
piel.
La exposición breve de ratas a concentraciones de
alfa-cipermetrina de hasta 200 mg/kg en dieta diaria durante 5 semanas
o hasta 180 mg/kg en dieta diaria durante 13 semanas no produjo
efectos tóxicos. Con dosis más elevadas, las ratas mostraron signos de
intoxicación asociados a la patología del sistema nervioso,
disminución del crecimiento o aumento del peso del hígado y los
riñones. No se pusieron de manifiesto efectos hematológicos,
bioquímicos o histopatológicos claros.
En un estudio de toxicidad oral en perros durante 13 semanas, la
dosis más alta, de 270 mg/kg causó síntomas de intoxicación, pero
todos los demás parámetros examinados (relativos a la hematología,
bioquímica clínica, análisis de orina, peso de los órganos, anatomía
patológica e histopatología) se mantuvieron inalterados. El nivel sin
efectos observados (NOEL) fue de 90 mg/kg de dieta (equivalente a 2.25
mg/kg de peso corporal al día).
En un estudio de toxicidad oral en ratas se demostró que la
alfa-cipermetrina induce neurotoxicidad a causa de alteraciones
histopatológicas de los nervios tibial y ciático, degeneración axonal
y aumento de la actividad de la beta-galactosidasa.
Se carece de datos sobre toxicidad a largo plazo, toxicidad en la
reproducción, teratogenicidad e inmunotoxicidad.
Con los datos disponibles sobre la alfa-cipermetrina, se puede
deducir que se trata de un compuesto no mutagénico en las pruebas con
Salmonella typhimurium, Escherichia coli y Saccaromyces cerevisiae,
y en las pruebas in vivo e in vitro con hepatocitos de rata, con
respecto a la inducción de aberraciones cromosómicas y producción de
lesiones en cadenas simples de ADN. No se observó aumento de las
aberraciones cromosómicas en las células de médula ósea de rata.
Se carece de datos sobre la carcinogenicidad de la
alfa-cipermetrina.
1.4 Efectos en el ser humano
La exposición de la población general a la alfa-cipermetrina es
insignificante siempre que en su utilización se apliquen buenas
prácticas agrícolas. Se comprobó que la exposición cutánea profesional
de los trabajadores durante la mezcla/carga, el rociado y el lavado
del equipo era de hasta 2.94 mg, 0.61 mg y 0.73 mg, respectivamente.
En un estudio de exposición a la alfa-cipermetrina durante la
formulación, se evaluaron los niveles de exposición mediante el
monitoreo personal y estático de las concentraciones atmosféricas y la
medición de los metabolitos en la orina. Los niveles de exposición
personal media del grupo durante los dos días de la formulación de los
concentrados de calidad técnica fueron de 2.8 y 44.9 mg/m3, mientras
que la exposición personal media del grupo al material técnico el
tercer día fue de 54.1 mg/m3. No se detectaron metabolitos en la
orina (límite de detección, 0.02 mg/litro). Durante la formulación se
informó de reacciones cutáneas ligeras.
No se han comunicado casos de envenenamiento.
1.5 Efectos en otros organismos en el laboratorio y en el medio
ambiente
El valor de la CE50 (crecimiento) en las 48 y 96 horas para el
alga de agua dulce Selenastrum capricornutum es superior a 100
µg/litro.
La alfa-cipermetrina es muy tóxica para los invertebrados
acuáticos. Los valores de la CE50 (inmovilización) a las 24 y 48 h
para Daphnia magna son de 1.0 y 0.3 µg/litro, respectivamente, y el
valor de la CL50 a las 24 h para Gammarus pulex es de 0.05
µg/litro. La alfa-cipermetrina es muy tóxica para varios grupos de
artrópodos acuáticos, pero lo es menos para los moluscos. Se puede
reducir la toxicidad a corto plazo del compuesto formulando el
producto como suspensión con mayor cantidad de aceite. Aunque el
arrastre del rociado puede producir efectos tóxicos en los
invertebrados acuáticos, la desaparición rápida de la
alfa-cipermetrina del agua facilita la recuperación.
La alfa-cipermetrina es muy tóxica para los peces. El valor de la
CL50 a las 96 h oscila entre 0.7 y 350 µg/litro, segun la
formulación. Las formulaciones de concentrados emulsionables son mucho
más tóxicas que el concentrado en suspensión, el polvo humectable y
las formulaciones microencapsuladas. El peligro del compuesto para los
invertebrados acuáticos y los peces radica en su toxicidad aguda. No
hay pruebas de que se produzcan efectos acumulativos debido a una
exposición prolongada.
No se dispone de datos acerca de los efectos de la
alfa-cipermetrina en los microorganismos del suelo. En un sistema
cerrado, no se observaron efectos en las bacterias de aguas residuales
con concentraciones de 3 mg/litro.
La toxicidad de la alfa-cipermetrina para determinados
coleopteros carábidos y larvas de neurópteros es relativamente baja,
y representa un peligro limitado en las fases preadultas de los
himenópteros parasitoides. En estudios de campo en pequeñas parcelas
y en gran escala se ha puesto de manifiesto que la alfa-cipermetrina
es poco peligrosa para los coleopteros carábidos y estafilínidos, pero
es un peligro relativamente grande para las arañas linifíidas. Los
efectos sobre las poblaciones se limitaron a una sola temporada de
crecimiento. Además, la alfa-cipermetrina es de bajo riesgo para las
larvas de sírfidos, pero tiene efectos considerables en los
coccinélidos. Sin embargo, la rápida desaparición de los residuos de
las hojas permite a estos animales recolonizar en poco tiempo las
zonas tratadas.
La utilización de alfa-cipermetrina en el campo no diminuye la
abundancia relativa de entomófagos en las comunidades de artrópodos.
Su utilización en cultivos de cereales de grano pequeño no iría
acompañada de la "reaparición" de plagas o de infestaciones de plagas
secundarias.
En las pruebas de laboratorio, la toxicidad de la
alfa-cipermetrina para las lombrices de tierra es baja. No se registró
mortalidad tras 14 días de exposición de las lombrices a
concentraciones de hasta 100 mg/kg de suelo artificial.
En pruebas de toxicidad aguda en el laboratorio se observó que la
alfa-cipermetrina es muy tóxica para las abejas. La administración
oral de una solución concentrada emulsionable dio una DL50 a las 24
h de 0.13 µg/abeja, mientras que el valor correspondiente para la
administración tópica fue de 0.03 µg/abeja (producto técnico) ó 0.11
µg/abeja (CE). La elevada toxicidad de la alfa-cipermetrina para las
abejas no se manifestó claramente en los ensayos de campo,
probablemente a causa del breve efecto repelente del producto, que
hace disminuir la actividad libadora de las abejas y, por
consiguiente, su exposición.
No se dispone de datos acerca de la toxicidad del
alfa-cipermetrina para las aves.
2. Conclusiones
2.1 Población general
Cuando la alfa-cipermetrina se aplica correctamente, la
exposición de la población general al producto es baja y probablemente
no supone riesgos.
2.2 Exposición ocupacional
Con buenas prácticas de trabajo, medidas de higiene y
precauciones de seguridad, es improbable que la alfa-cipermetrina
suponga un peligro para las personas expuestas en forma laboral. La
aparición de "sensaciones faciales" es un síntoma de exposición. En
estas circunstancias se deben re-examinar las prácticas de trabajo.
2.3 Medio ambiente
Con las cantidades recomendadas para la aplicación es improbable
que la alfa-cipermetrina alcance niveles de importancia ecológica. En
condiciones de laboratorio es muy tóxica para los artrópodos
acuáticos, los peces y las abejas. Hay cierta probabilidad de que se
produzcan efectos tóxicos importantes en invertebrados a los que no
está destinado y en peces en casos de derrame, rociado excesivo o mala
utilización del producto.
3. Recomendaciones
* Se debe evitar la contaminación de aguas superficiales.
* La alfa-cipermetrina se une fuertemente a las partículas. Se
deberían llevar a cabo nuevos estudios ecotoxicológicos sobre los
efectos del compuesto en los microorganismos de los sedimentos,
puesto que este aspecto parece haber recibido poca atención.
* Hay que investigar la absorción gastrointestinal de
alfa-cipermetrina en distintas circunstancias.
* Se debe investigar el destino final de la alfa-cipermetrina
aplicada por vía cutánea.
* Hay que obtener nueva información acerca de la
toxicidad/carcinogenicidad a largo plazo y de la inmunotoxicidad
de la alfa-cipermetrina.
