
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 144
PRINCIPLES OF EVALUATING CHEMICAL EFFECT ON THE AGED
POPULATION
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1993
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
Principles for evaluating chemical effects on the aged population.
(Environmental health criteria ; 144)
1.Aged 2.Aging 3.Environmental exposure 4.Environmental
pollutants - adverse effects 5.Hazardous substances - adverse
effects
I.Series
ISBN 92 4 157144 6 (NLM Classification: WT 104)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1993
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
PRINCIPLES FOR EVALUATING CHEMICAL EFFECTS ON THE AGED POPULATION
INTRODUCTION
1. SCOPE OF THE PROBLEM
1.1. Objectives
1.2. Definitions
1.2.1. Aging versus senescing
1.2.2. Aging of individuals and populations
1.2.3. Chemicals of concern
1.2.4. Time and dose of exposure
1.3. Chemical exposure
1.4. Aged population
1.4.1. Demographic consideration
1.4.2. Life expectancy
1.4.3. Life-style in aged populations
1.5. Theories of aging
2. STRUCTURAL AND PHYSIOLOGICAL CHANGES IN THE AGED
2.1. Changes in gene structure and function in aging
2.1.1. Chromatin structure
2.1.2. DNA repair
2.1.3. Transcription
2.1.4. Translation
2.2. Changes in tissues, organs and systems in aging
2.2.1. Nervous system
2.2.1.1 Structural changes
2.2.1.2 Biochemical changes
2.2.1.3 Functional changes
2.2.2. Sensory organs
2.2.2.1 Vision
2.2.2.2 Hearing
2.2.2.3 Olfaction
2.2.2.4 Taste
2.2.2.5 Somatic sensations
2.2.3. Endocrine system
2.2.3.1 The pituitary-thyroid axis and the
basal metabolism
2.2.3.2 The pituitary-adrenal axis
2.2.3.3 The endocrine pancreas and
carbohydrate metabolism
2.2.4. Reproductive system
2.2.4.1 Female aging
2.2.4.2 Male aging
2.2.5. Immune system
2.2.5.1 Aging of lymphoid organs
2.2.5.2 Aging of cellular constituents
2.2.5.3 Neuroendocrine-immune
2.2.6. Cardiovascular system
2.2.6.1 Heart
2.2.6.2 Blood vessels
2.2.6.3 Characteristics of atherosclerotic
lesions
2.2.6.4 Theories of atherosclerosis
2.2.7. Respiratory function
2.2.7.1 Gas-exchange organs
2.2.7.2 Erythropoietic activity
2.2.8. Kidney and body fluid distribution
2.2.8.1 Renal function
2.2.8.2 Lower urinary tract
2.2.9. Gastrointestinal function
2.2.9.1 Gastrointestinal tract
2.2.9.2 Pancreas
2.2.9.3 Liver
2.2.10. Musculo-skeletal system
2.2.10.1 Bones
2.2.10.2 Joints
2.2.10.3 Skeletal muscles
2.2.11. Skin
3. BASIS OF ALTERED SENSITIVITY TO ENVIRONMENTAL CHEMICALS
3.1. Pharmacokinetics
3.1.1. Absorption
3.1.2. Distribution
3.1.3. Metabolism
3.1.4. Excretion
3.2. Pharmacodynamics
3.2.1. Central nervous system
3.2.2. Endocrine system
3.2.2.1 Changes in hormonal availability
with age
3.2.2.2 Changes with age in the reception
of the signal by the target cells
3.2.2.3 Changes in the nature of the
hormonal message with age
3.2.3. Kidney
3.2.4. Immune system
3.2.5. Other tissues and systems
3.3. Modifying factors
3.3.1. Nutrition
3.3.2. Alcohol intake
3.3.3. Smoking
3.4. Interactions of chemicals and diseases
3.4.1. Cancer
3.4.2. Other diseases
4. APPROACHES TO EXAMINING THE EFFECTS OF CHEMICALS ON THE AGED
POPULATION
4.1. Experimental approaches
4.1.1. Principles for testing chemicals in
the aged population
4.1.2. Animal models
4.1.2.1 Animal species
4.1.2.2 Animal strain
4.1.2.3 Animal sex
4.1.2.4 Selection of age groups for
comparison
4.1.2.5 Underlying pathology of animals of
different ages
4.1.2.6 Transgenic animals
4.1.2.7 Animal husbandry
4.1.3. Chemical exposure
4.1.3.1 Dose level
4.1.3.2 Route of administration
4.1.3.3 Duration of exposure
4.1.4. Non-mammalian models
4.1.5. In vitro studies
4.1.6. Statistical considerations
4.1.7. Extrapolation of animal data to humans
4.2. Epidemiological and clinical approaches
4.2.1. Disease pattern of aged population
4.2.2. Assessment of effects of environmental
chemicals in the elderly population
4.2.3. Acute episodes
4.2.4. Concerns for the aged population
4.3. Biomarkers of aging
5. CONCLUSIONS
6. FURTHER RESEARCH
REFERENCES
APPENDIX 1
PARTICIPANTS IN THE PLANNING AND TASK GROUP MEETINGS ON PRINCIPLES
FOR EVALUATING CHEMICAL EFFECTS ON THE AGED POPULATION
Members
Dr V.N. Anisimov, N.N. Petrov Institute of Oncology, Ministry of
Health, St Petersburg, Russian Federationa,b,c,d
Dr L.S. Birnbaum, US Environmental Protection Agency, Research
Triangle Park, North Carolina, USAa,b,d
Dr G. Butenko, Institute of Gerontology, Kiev, Ukraineb
Dr R.L. Cooper, US Environmental Protection Agency, Research
Triangle Park, North Carolina, USAa,b,d
Dr V.M. Dilman, N.N. Petrov Research Institute of Oncology,
Ministry of Health, St Petersburg, Russian Federationa,c,d
Dr N. Fabris, Italian National Research Centre on Aging, Ancona,
Italyb,d
Dr N.S. Gradetskaya, Research Institute of Industrial Hygiene
and Occupational Diseases, Academy of Medical Sciences,
Moscow, Russian Federationa
Dr K. Kitani, Tokyo Metropolitan Institute of Gerontology, Tokyo,
Japanb
Dr J. Leaky, National Center for Toxicological Research,
Jefferson, Arkansas, USAb
Dr A.Y. Likhachev, N.N. Petrov Institute of Oncology, Ministry of
Health, St Petersburg, Russian Federationa,d
Dr S. Li, Chinese Academy of Preventive Medicine, Department of
Scientific Information, Beijing, Chinaa,b,d
Dr G.M. Martin, University of Washington, Department of Pathology,
Seattle, Washington, USAa,b*,c,d
Dr E. Masoro, The Texas University at San Antonio, San
Antonio, Texas, USAb
Dr N.P. Napalkov, N.N. Petrov Research Institute of Oncology,
Ministry of Health, St Petersburg, Russian Federationa
Dr G.I. Paramonova, Institute of Gerontology, Academy of Medical
Sciences, Kiev, Ukrainea
Dr J. Parizek, Czechoslovakia Academy of Sciences, Institute of
Nuclear Biology and Radiochemistry, Prague, Czechoslovakiaa
Dr P.K. Ray, Industrial Toxicology Research Centre, Council of
Scientific and Industrial Research, Lucknow, Indiaa,b*,d
Dr A. Richardson, Illinois State University, Normal, Illinois,
USAb*,d
Dr G.S. Roth, National Institute of Health, National Institute on
Aging, Baltimore, Maryland, USAb
Dr G.J.A. Speijers, National Institute for Public Health and
Environmental Protection (RIVM), Bilthoven, The
Netherlandsb,d
Dr K.T. Suzuki, National Institute for Environmental Studies,
Ibaraka, Japana,c
Dr J. Vijg, Medscand Ingeny, Leiden, The Netherlandsb
Dr J.R. Zhu, Zhong Shan Hospital, Shanghai Medical University,
Shanghai, Chinab,d
Observer
Dr E.I. Komarov, Central Research Institute of Roentgenology and
Radiology, Ministry of Health, St Petersburg, Russian
Federationa
Secretariat
Dr G.C. Becking, International Programme on Chemical Safety,
Interregional Research Unit, World Health Organization,
Research Triangle Park, North Carolina, USA (Secretary for the
Planning Meeting)a
Dr B.H. Chen, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland (Secretary
for the Task Group Meeting)b
Dr Z.P. Grigorevskaya, Centre for International Projects,
Moscow, Russian Federationa
Dr M.I. Gounar, Centre for International Projects, Moscow,
Russian Federationa
Dr H. Hermanova, Regional Office for Europe, World Health
Organization, Copenhagen, Denmarka*
Dr P.G. Jenkins, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerlandb
Dr M. Mercier, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerlandb
a Participant in Planning Meeting, St Petersburg, Russian
Federation, 5-9 September, 1988
b Participant in Task Group Meeting, Geneva, Switzerland,
9-13 December, 1991
c Submitted background information for planning meeting
d Prepared background paper for the preparation of the first
draft
* Invited but unable to attend
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the
criteria monographs as accurately as possible without unduly
delaying their publication. In the interest of all users of the
Environmental Health Criteria monographs, readers are kindly
requested to communicate any errors that may have occurred to the
Director of the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland, in order that they may be
included in corrigenda.
INTRODUCTION
The aged population and the number of chemicals in the
environment have been increasing and will undoubtedly continue to
increase. It is estimated that there will be 612 millon people aged
60 years and over by the year 2000, and of these 61% will live in
developing countries. The numerous physiological and biochemical
changes occurring during aging can modify the pharmacokinetics and
pharmacodynamics of chemicals in the elderly, resulting in either
higher or lower levels of toxicity. It is expected that the adverse
effects of chemical exposure on the elderly will increase in
importance as a health care issue. IPCS has been active in the
development and validation of methodology for the assessment of
risks from exposure to chemicals. One area of concern has been the
evaluation of methodology appropriate for the assessment of risks in
"high-risk" groups. The fifth meeting of the IPCS Programme Advisory
Committee endorsed the need for an Environmental Health Criteria
monograph dealing with the effects of chemicals on the aged
population and the aging processes. This monograph integrates
relevant studies of toxicology and gerontology; toxicology examines
the potential health effects of exposure to chemicals, while
gerontology focuses on the scientific explanations for the phenomena
and mechanism of aging.
A planning meeting was held in St Petersburg from 5 to 9
September 1988 and was organized locally by the N.N. Petrov Research
Institute of Oncology, Ministry of Health, Russian Federation.
Financial support through the UNEP Country Projects was provided by
the Centre for International Projects (CIP), State Committee for the
Protection of the Environment, Moscow, Russian Federation. Dr M.I.
Gounar, CIP, formally opened the meeting, and Dr V. Anisimov, on
behalf of Dr N.P. Napalkov, former Director of the Petrov Research
Institute of Oncology, welcomed the participants. Dr G.C. Becking
welcomed the participants on behalf of the Executive Heads of the
three IPCS cooperating organizations (UNEP/ILO/WHO). Dr V. Anisimov
and Dr L. Birnbaum were Joint Chairmen and Dr P.K. Ray and Dr A.
Likhachev were Joint Rapporteurs.
After discussing the scientific issues relevant to both the
aged population and aging processes, the committee considered that
there was sufficient epidemiological, clinical and experimental data
to support the preparation of an Environmental Health Criteria
monograph to evaluate chemical effects on the aged population.
However, the differing views on the mechanisms of aging and how
chemical exposure might alter such mechanisms preclude at present
the preparation of an evaluation of the chemical effects on the
aging process. It was decided to prepare a monograph on principles
for evaluating chemical effects on the aged population, with only a
brief discussion of the present concept of aging. An outline of the
monograph together with a list of possible authors was produced.
Drs L. Birnbaum and V. Anisimov prepared the first draft of
this monograph based on 13 background papers written by various
authors (Appendix 1). Dr V. Anisimov prepared the second draft
incorporating comments received following the circulation of the
first draft to IPCS Contact Points for Environmental Health Criteria
monographs and to IPCS Participating Institutions. Dr L. Birnbaum
made a considerable contribution to the preparation of the final
text.
A WHO Task Group Meeting met from 9 to 13 December 1991 in
Geneva. Dr B.H. Chen, IPCS, opened the meeting and welcomed the
participants on behalf of the Director, IPCS, and the three IPCS
cooperating organizations. Dr J. Vijg and Dr K. Kitani were Chairman
and Vice-Chairman, respectively, and Drs L. Birnbaum and V. Anisimov
were Joint Rapporteurs.
The Task Group considered it likely that the aged population is
more susceptible to the harmful effects of environmental chemicals.
However, very few environmental chemicals have been tested for
toxicity in the elderly. Some age-associated diseases may lead to an
increased susceptibility to the harmful action of specific
environmental chemicals. The effects of environmental chemicals on
the process of aging remain to be evaluated. It was suggested that a
special scientific workshop be devoted to this topic.
Drs B.H. Chen (IPCS Central Unit) and G.C. Becking
(Interregional Research Unit) were responsible for the overall
scientific content, and Dr P.G. Jenkins (IPCS Central Unit) was
responsible for the technical editing.
The efforts of all who helped in the preparation and
finalization of the monograph are gratefully acknowledged.
ABBREVIATIONS
ACTH adrenocorticotrophic hormone
BMAA beta- N-methylamino-1-alanine
BOAA beta- N-oxalylamino-L-alanine
cDNA complementary DNA
CNS central nervous system
GABA gamma-aminobutyric acid
GH growth hormone
GI gastrointestinal
HDL high density lipoprotein
hnRNA heterogeneous nuclear RNA
LDL low density lipoprotein
LH luteinizing hormone
mRNA messenger RNA
SDAT senile dementia of Alzheimer type
T3 triiodothyronine
T4 thyroxine
TSH thyroid-stimulating hormone
UDP uridine diphosphate
UDPGA UDP-glucuronic acid
1. SCOPE OF THE PROBLEM
1.1 Objectives
The main objective of the Group involved in the preparation of
this report was to review present knowledge concerning the effects
of environmental chemicals on the aged population and to evaluate
available models for the assessment of these effects and the
consequent risk to human health in the aged population.
About ten million natural and synthetic chemicals have been
identified by the Chemical Abstract Service Registry and some eighty
to one hundred thousand have been identified as important to
commerce. The restricted knowledge of the toxicological properties
of the natural substances makes it difficult to give clear evidence
of whether the elderly population is at risk for this category of
compounds, but the impact of these natural toxins might be even
larger than that of most man-made toxicants. As the requirements for
more toxicological data on natural toxicants become more important
internationally, the possible effects on the elderly population
should also be included in the assessment.
The following relationships need to be considered: a) the
special response of the aged as compared to that of the young
following exposure to environmental chemicals; and b) the impact of
exposure to environmental chemicals on the processes of aging. This
report focuses on the first relationship, i.e. the elderly as a
population at special risk. The elderly are heterogeneous with
respect to aging processes, life-style and diseases. Indeed, in
most instances the deficit in the majority of the elderly relates
more to life-style and diseases than to the aging processes per se.
The Group recommended that the evaluation of the effects of
environmental chemicals on the process(es) of aging should be the
focus of a separate scientific workshop. This monograph will focus
on environmental chemicals as opposed to pharmaceuticals and food
additives, although information on these latter chemicals will be
used when necessary to support the issues.
The study of the effects of chemicals on the aged population
requires the integration of two disciplines, toxicology and
gerontology. Toxicology examines the potential health effects of
exposure to chemicals, while gerontology focuses on the scientific
explanations for the phenomena and mechanisms of aging. The lack of
a unified theory of aging, together with the inability at present to
distinguish intrinsic aging from natural disease and toxic response,
creates difficulties which make the objective of the Group only
partially attainable.
1.2 Definitions
1.2.1 Aging versus senescing
Plant biologists often sharply differentiate between these
terms (Leopold, 1975). They may use the word aging to refer to all
of the changes in structure and function in an organism throughout
the life course, including the period of development. They reserve
the term senescence for the deteriorative alterations in structure
and function that are the immediate precursors of tissue and
organismal death. Mammalian gerontologists, however, typically use
the terms aging and senescence (or, more properly, senescing)
interchangeably to describe the constellation of changes that occur
after the attainment of sexual maturity and the young adult stage of
life. This is not to deny the critical importance of developmental
events in setting the stage for subsequent patterns of senescence.
For example, a specific chemical, physical or infectious agent,
acting during a crucial period of ontogeny, could conceivably
deplete, but not ablate, a subset of stem cells or their partially
differentiated progeny without any phenotypic consequences until
additional depletion, related to some normative aging process,
reaches a clinically significant threshold.
1.2.2 Aging of individuals and populations
At the organismal level, endogenously and exogenously induced
injuries are more likely to occur as the organism ages. There is a
decreasing probability, as a function of chronological time, that
the organism will survive. Thus, there is an exponential increase in
the death rate over time. Although subject to important
environmental influences, the ages at which such exponential
increments of death rates begin and the kinetics of their
progression are subject to strong genetic influences in that they
are species-specific. The basic observations have been summarized by
the following equation (Gompertz, 1825):
Rm = R0.ealpha t
where Rm indicates the mortality rate at time t, R0 is a
parameter empirically determined by extrapolating an exponential
curve back to zero time (sometimes referred to as the "initial
vulnerability"), e is the natural logarithm, t is time and alpha is
a slope constant. Better fits to empirical data are obtained if a
second constant is added to the right hand side of the above
equation (the Gompertz-Makeham equation).
The Gompertz-Makeham equation is a satisfactory approximation
to the kinetics of specific mortality in human populations in the
age range 20-80 years. Correspondingly, the value of alpha
characterizes the rate of aging only within this interval. Although
some deviations of alpha within this interval in human populations
have been noted (Pakin & Hrisanov, 1984), the analysis of the
parameters of the Gompertz-Makeham equation permit one to make
objective estimations of the changes in the mortality in populations
(Sacher, 1977; Hirsch, 1982). It is important to note that the use of
this method for measuring the rate of aging in populations of
experimental animals is especially reliable when the external
conditions (e.g., the housing of animals) remain constant throughout
the whole period of a study.
1.2.3 Chemicals of concern
The first class of agents of concern would be those with a
special potential to injure elderly subjects because of their
unusual susceptibility. This sensitivity might be the result of
intrinsic biological aging, chronic exposure to deleterious
environmental agents, a high prevalence of various age-related
diseases, or a combination of all of these. A rational approach to
this problem requires detailed knowledge of the altered physiology,
biochemistry and special pathologies of older people (those over age
65) and, especially, of the very old (those over age 85). Examples
include the special vulnerability of many elderly subjects to air
pollutants, to certain pharmaceuticals and combinations of
pharmaceuticals, and even to injury from methane gas explosions. The
latter results from a high prevalence of atrophic change in the
olfactory network, with consequent marked reduction in the ability
of many older people to detect the low concentration of sulfide
contaminants that are deliberately added to household gas to warn of
leakage. It is apparent that, with respect to such classes of
agents, public health actions can be of immediate benefit to older
people.
The second class of chemicals of concern would be those that
might modulate the processes of aging. These could either accelerate
("gerontogens") or retard ("geroprotectors") the aging processes.
1.2.4 Time and dose of exposure
As indicated above, chemical agents that have the potential to
accelerate aspects of aging could act at any time during the life
course, from before birth to death. Strictly speaking, however,
agents that are most likely to closely mimic natural aging processes
are slow, insidious and progressive. Moreover, since the phenotypic
consequences of aging are often subtle and, for the human species,
develop over a period of decades, it would be difficult indeed to
establish a minimum effective dose for such putative chemicals. The
task is somewhat less difficult in the case of agents to which the
elderly have some special vulnerability, since acute and sub-acute
end-points are often involved. For example, the prevalence of
cardiopulmonary morbidity can be related to ambient concentrations
of specific urban pollutants.
1.3 Chemical exposure
The world is irrevocably dependent on man-made chemicals,
modern technology bringing a dramatic increase in their production
and consumption. More than 750 000 chemicals are known to be in our
environment and between 1000 and 2000 new ones enter the market each
year. A major proportion of these chemicals find use as components
of various consumer products, or they enter the environment as
industrial waste, posing health risks as well as benefits.
In present-day society, we use chemicals to boost our food
production, make our lives easier and protect our health. Many of
these chemicals are hazardous and great care must be taken during
their usage, storage and disposal. Their releases into the
environment, whether intentional or not, can have severe
consequences.
Billions of tons of hazardous industrial waste materials,
produced every year, may enter the environment through complex and
interrelated pathways (air, water, food, etc.), and could affect
humans. Pesticides, fertilizers and herbicides enter the environment
as a result of direct application; nitrogen oxides, sulfur oxides
and polycyclic aromatic hydrocarbons result from combustion
processes. Many manufacturing processes liberate unwanted
by-products and waterborne and airborne wastes, which are sometimes
more toxic than the raw materials. Incidents such as the
contamination of water by mercury, the widespread distribution of
industrial oils (e.g., polychlorinated biphenyls), and the
destruction of the ozone layer in the stratosphere due to the
release of aerosol propellants (chlorofluorocarbons) have made the
public aware of the ability of some chemicals to cause unexpected
results at some point far removed from where they were originally
introduced. Chemicals undergo transformation once they enter the
environment, and a relatively harmless chemical may become a toxic
by-product. It may further enter the food chain and accumulate in
living organisms, eventually reaching humans.
In both developed and developing countries there has been a
great impact of life-styles upon the quality of life and upon the
life span of the population concerned. Of particular interest are
the aged individuals, who, having lived longer in an environment
containing toxic agents, may suffer from their cumulative effects
even when exposure levels are relatively low. The multiple
life-long, though low-level, human exposure to chemicals is
difficult to assess adequately in terms of associated health risks
(Pines et al., 1987). It has been reported that some chronic low
level exposures to chemical or physical stressors have beneficial
effects on longevity (hormesis) (Sacher, 1977; Neafsey, 1990).
Among the chronic health effects of chemicals, cancer is of
major concern. Many substances have found in recent years to be
carcinogenic in one or more species of laboratory animals (WHO,
1983). In humans, cancer is seldom manifest until 10-40 years after
exposure to the carcinogenic agent (IARC, 1990). Thus, cancers
caused by chemicals are most often observed in the aged population.
However, it is not easy to identify the hazards unless past exposure
is known. Similar comments may be made about atherosclerosis, which
may also be related to chemical exposure (Penn et al., 1981).
In many cases, especially with respect to long-term effects,
the response to a chemical may vary, quantitatively or
qualitatively, in different groups of individuals depending on
predisposing conditions, such as nutritional status, disease status,
current infection, climatic extremes, and genetic features, sex and
age of the individuals. Understanding the response of such specific
risk groups is an important area of toxicology research today.
There is no biological basis for classifying substances
according to their environmental source (e.g., industry or
agricultural use), or use patterns (e.g., food additives). Chemical
substances with neurotoxic potential, for example, are found as
natural metabolites (e.g., quinolinate), biological poisons in
plants (e.g., gossypol) and animals (e.g., batrachotoxin), natural
components of food (e.g., beta-oxalylamino-L-alanine (BOAA)) and
beverages (e.g., ethanol), food contaminants (e.g., ergot),
synthetic food additives (e.g., aspartame), flavours and fragrances
(e.g., dinitromethoxybutyl toluene), pollutants of air (e.g., lead),
water (e.g., zinc pyridinethione) and industrial processes (e.g.,
carbon disulfide), and therapeutic drugs (e.g., phenothiazines). In
addition, numerous chemicals, if they have damaging actions, may
contribute to the aging phenotype.
Chemicals influencing the processes of aging and/or affecting
the aged population may be classified into several groups according
to their chemical properties and metabolic behaviours. Chemicals
that are poorly metabolized fall into two groups. The first are
absorbed and distributed into certain tissues according to their
partitioning behaviour, based on their physical/chemical properties.
For example, organochlorine compounds concentrate in adipose tissue.
During fasting, adipose tissue is mobilized and accumulated
chemicals are liberated into body fluids. Organo-chlorine compounds
are detectable in adipose tissue, blood and breast milk long after
cessation of exposure. The second group comprises chemicals that are
poorly excreted and accumulate in the body. Some of these chemicals
are detoxified by binding to specific proteins, resulting in
long-term storage. For example, cadmium, lead and mercury induce
specific proteins such as metallothionein which help in the
detoxification of heavy metals (Oh et al., 1978; Onasaka & Cherian,
1981). The appearance of cadmium toxicity among the population over
50 years of age may be related to the decreased capacity of
metallothionein synthesis with advancing age (Hunziker & Kagi,
1985).
Chemically and biologically active chemicals are readily
metabolized. Thus, they do not accumulate in the body. Continuous
exposure to these chemicals is of concern because these may be
metabolized to reactive intermediates that can interact and damage
cellular macromolecules. Such damage may be cumulative, resulting in
the aged population being more vulnerable. Other types of chemicals
which belong to this group (e.g., NOx, SO2) can also cause more
severe adverse effects on the aged whose defensive mechanisms are
weakened. It should be stressed that several chemicals can enter the
body at the same time, causing a more complex problem.
1.4 Aged population
1.4.1 Demographic consideration
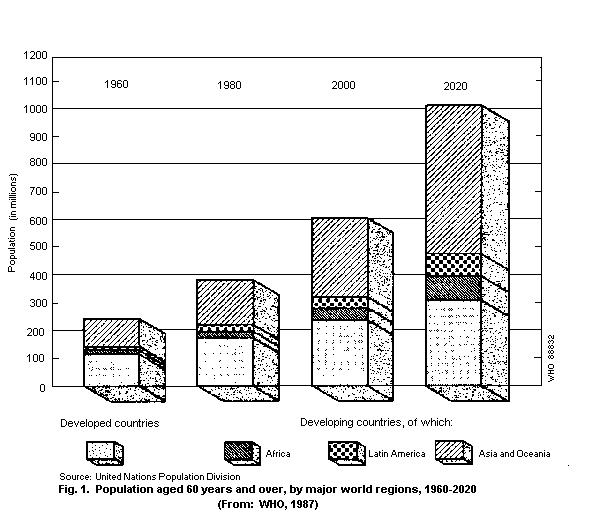
The United Nations has defined people of 60 years and over as
the aged. In 1988, it was estimated that there were about 488
million people in the world fitting this criterion. The number is
expected to rise to 612 million by the year 2000, 61% of whom (i.e.
376 million) will be living in developing countries (Fig. 1) (WHO,
1990).
Many countries are, however, using 65 years and over as the
definition of the elderly. The corresponding numbers for this age
group are 327 million in the world in 1990 and 423 million by the
year 2000, of whom 250 million will be in developing countries (WHO,
1990). The increase in the elderly population will be particularly
marked in Asia, primarily as a result of the rapid growth expected
in the numbers of the aged in China and India. This trend is
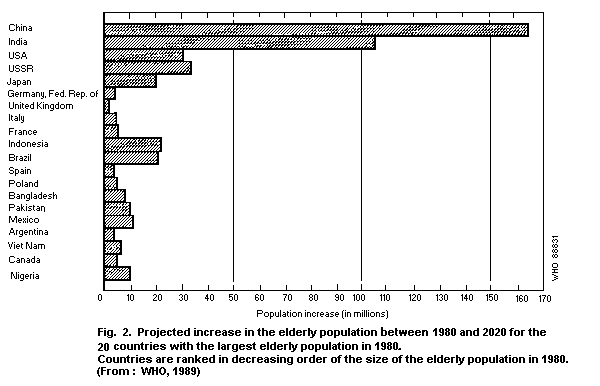
illustrated in Fig. 2, which indicates the 20 countries with the
largest aged population in 1980 and the expected growth of the aged
population. By the year 2020, there will be an increase of 270
million elderly citizens in China and India. The size of the aged
population is expected to rise by more than 20 million in both
Brazil and Indonesia, and by roughly half that number in Mexico,
Nigeria and Pakistan (WHO, 1989).
On the other hand, a much smaller absolute increase in the
elderly population is anticipated for the European countries, where
population aging began much earlier. As a result, the developing
countries will gradually account for the largest elderly population
in the world. Indonesia, for example, is expected to move from tenth
place in 1980 to fifth in 2020 (Fig. 2), and Mexico is expected to
have the eighth largest elderly population, ahead of Italy, France,
and the United Kingdom (WHO, 1989).
The elderly population of the USA is growing much more rapidly
than the population as a whole. In the 1970s, the population aged 65
and over increased by 28% and the population aged 85 and over
increased by 59%, whereas the total population increased only by
11%. The population aged 85 and over is expected to triple between
1980 and 2020 and is the fastest growing of the four older age
groups (55-64, 65-74, 75-84, and 85 and over). Census projections
for 2050 indicate that the proportion of the population aged 65 and
over (22%) will be almost twice as great as it is today (12%). In
the last two decades alone, the 65-plus population has grown by 54%
while the under-65 population has increased by only 24%. At the
beginning of this century, less than one in eight Americans was age
55 and over. The increase in the numbers of elderly people is
expected to occur in two stages. Until the year 2000, the proportion
of the population age 55 and over is expected to remain relatively
stable at 22%. By 2010, because of the maturation of the post World
War II baby boom, more than a quarter of the total population of the
USA is expected to be at least 55 years old, and one in seven of the
population will be at least 65 years old. By 2050, one in three
persons is expected to be 55 years or older and one in five will be
over 65 (US Senate Special Committee on Aging, 1986).
 It is commonly assumed that today's large percentage of elderly
people in the population is a result of increased longevity and
decreased birth rate. For example, in Japan the proportion of people
aged 65 or more had increased to 10.3% in 1985. At the same time,
the values were 15.1%, 14.5%, 12.4% and 16.9% in the United Kingdom,
Federal Republic of Germany, France and Sweden, respectively. Japan
is a new "aged-type" country with the greatest rate of increase in
the elderly in the world. By the year 2025, the proportion of the
Japanese population aged 65-plus will rise to 23.4% (Hosomi, 1990).
In China, the proportion of the population aged 60 and over was
7.3% of the total population in 1953. By 1984 it had increased to 9%
of the total population, and by the year 2000 the proportion of the
population 60 and over will be as high as 10.6%. The proportion is
predicted to increase to 26.2% by 2025 (Xiong, 1990).
At present, the aged population is growing more rapidly in
China than in countries of Europe and North America. According to
data from the US Bureau of the Census, it took 115 years (1865-1980)
for the proportion of people aged 65 or more in France to increase
from 7% to 14%, 85 years (1890-1975) in Sweden, 66 years (1944-2010)
in the USA, 45 years (1930-1975) in the United Kingdom, and 26 years
(1970-1996) in Japan. For China, the pattern of the growing number
of elderly is similar to Japan, i.e. the proportion of people aged
65 or more will be 7.4% in 2000, and by 2025 it will have increased
to 12.8% (Hosomi, 1990; Yao, 1990; Xiong, 1990).
1.4.2 Life expectancy
Life expectancy at birth is a statistical index calculated by
the use of a life table from the age-specific death rates of the
population. This illustrates the overall level of health in a
country or a region. Throughout this century, it has been evident
that, as a result of improvements in many aspects of health status,
an individual can expect to live longer. From 1960 to 1990, life
expectancy at birth for the total population had increased by 13.5
years. The life expectancy at birth for the period 1985-1990 was
estimated to be 63.9 years for the world as a whole, 74.0 years for
the more developed regions, and 61.4 years for the less developed
regions. The longest life expectancies are in Japan (78.3), Iceland
(77.5), Sweden (77.1), Switzerland (77.1), and the Netherlands
(76.9) (World Population Prospects, 1991).
The trend for life expectancy at birth in the USA showed an
increase from 1900 (46.4 for males and 49.4 for females) to 1950
(65.6 and 71, respectively) and in the year 2000 is expected to be
72.1 and 79.5 (for males and females). By 2050, it may have
increased to 73.6 for males and 81 for females (US Senate Special
Committee on Aging, 1986).
In China, the life expectancy at birth before 1949 was about 35
years of age, being one of the lowest in the world at that time. By
1957 it had increased to 57 years, from 1973 to 1975 it was 63.6
years for males and 66.3 years for females, and in 1981 it was 68
years. It is expected that the continued increase in the average
life expectancy of the people of China will be slow due to a high
death rate from cardiovascular diseases (Gu, 1986).
1.4.3 Life-style in aged populations
A basic issue in planning for the consequences of demographic
aging is whether elderly people should be considered a specific
target group for the development of services, or whether their needs
should be catered for within the context of planning for the
population as a whole. One approach to a rational policy for this
issue is to consider the nature of human aging. For this it is
necessary to view the physical, psychological and sociological
dimensions of aging as a whole.
Life-style influences the effects of chemicals on human health,
including that of the elderly, both quantitatively and
qualitatively. Environmental chemicals and their uses are diverse.
Specialized nutritional elements of the diet have become popular,
while in certain countries many people prefer predominantly
vegetarian diet. Others take supplements and additives which contain
pure preparations of vitamins, minerals, amino-acids and other
substances. Whether such substances have either adverse or
beneficial effects on the elderly and aging processes, has not
generally been fully evaluated. Another important source of human
exposure to chemicals comes from the intake of different kinds of
cosmetic agents and fragrances, such as shampoos, creams, perfumes,
oral deodorants, sunscreen and suntan lotions, and insect
repellents. These are often chemical mixtures whose components have
not been evaluated or tested beyond acute toxic potential.
Occupational status, indoor air quality, recreational
activities, exercise, eating and drinking habits, alcohol
consumption, and tobacco smoking can all affect the elderly and
aging processes to a certain degree. Elements of life-style can
strengthen or reduce the risk of developing aged-related
degenerative diseases. They can also accelerate or delay
physiological and anatomical changes. Typical examples are the
various age-related diseases caused by toxic chemicals in tobacco
smoke and the reduction of the risk of cardiovascular diseases
produced by regular exercise (Committee on Chemical Toxicity and
Aging, 1987).
As far as the possible influence of life-style factors on the
manifestations of aging is concerned, many studies have shown that
loneliness and physical and intellectual inactivity are common among
the elderly, especially widowed people. Several studies have
revealed that living conditions have an influence on health and
well-being, resulting in an increase in the demand for social care
and medical service. Marital status and living arrangements have
important significance for the unique life-style of the elderly.
There are striking differences between the proportions of elderly
males and females who are married: in many countries the proportion
of married males is twice that of married females. In general, the
proportion of widows is very high and that of widowers relatively
low (WHO, 1984).
Migration is also one of the life-style variables of the
elderly. In rural areas of Asia, many older women move to cities to
join their children after they have been widowed. Another common
type of move is the migration of the recently widowed or chronically
ill elderly from urban areas to their home towns or villages. For
many countries in Africa and Asia, the urban-rural migration is most
apparent among males, who return from urban to rural areas when they
are old. Worldwide, only a minority of elderly people live in urban
areas (WHO, 1984).
1.5 Theories of aging
During the last century, more than 100 various hypotheses
concerning the origin and mechanism of aging have been put forward.
All of them could be grouped generally into two broad categories:
those that invoke deterministic, or "programmed", alterations in
gene expression or gene structure; and those that invoke a variety
of stochastic, or "random", alterations in the structure and
function of macromolecules, cells, and organ systems. This
distinction, however, has some limitations, because stochastic
alterations in individual cells can lead to predictable phenomena in
the large populations of cells. The use of terminal differentiation
to explain the limited replicative life span of somatic cells
(Martin et al., 1974) could be an example of the blurring of the
stochastic and non-stochastic categories. For each individual cell,
differentiation is a random event; however, for a population of
cells, the process appears deterministic.
The mechanisms of aging are likely to be coupled to the
reproductive strategy of the organism. One example is the
synchronous, rapid physiological declines and mortalities that are
characteristic of species with single massive episodes of
reproduction (e.g., migrating Pacific salmon or soybean plants).
Placental mammals, however, have ample opportunity for a variety of
stochastic processes to take place during their long reproductive
and postreproductive phases. The associated patterns of structural
and functional decline can vary substantially, both qualitatively
and quantitatively, among individuals within a species and among
different related species. Evolutionary biologists in fact present
compelling arguments that aging did not evolve because of any
adaptive value to the individual or to the species, as would be
assumed by strictly programmed theories (reviewed in Rose, 1991).
Aging is thought to occur simply because of the decline in the force
of natural selection for gene action that is postreproductive. Such
gene action could be related to accumulations of late-acting
mutations in the constitutional genome or to selection for forms of
genes that have positive effects on reproductive fitness early in
the lifespan, but whose effects may be negative late in the lifespan
(the "antagonistic pleiotropy" theory of aging) (Rose, 1991).
It is beyond the scope of this monograph, however, to consider
the potentially large numbers of specific mechanisms that may be
modulated by such accumulated constitutional mutations or
pleiotropic genes. The reader is referred to recent reviews of the
many postulated theories of aging (Warner et al., 1987; Committee on
Chemical Toxicity and Aging, 1987; Finch, 1991; Cutler, 1991).
These can be classified in a variety of ways (Dilman, 1987;
Medvedev, 1990).
2. STRUCTURAL AND PHYSIOLOGICAL CHANGES IN THE AGED
2.1 Changes in gene structure and function in aging
Changes in gene expression are of critical importance to an
organism. Aging can potentially alter not only the structure of
genes, but the way in which they function. Changes in the DNA are
often thought to be integral to aging. It is clear that not only
mutations, but chromosomal rearrangements accumulate with age (Vijg,
1990). Repetitive sequence families may play a crucial role in the
processes of aging. In addition, the organization of DNA and protein
in chromatin is important structurally and functionally. Therefore,
changes in chromatin could play a major role in the age-related
change in the regulation of gene expression (Richardson et al.,
1983; Medvedev, 1984; Thakur, 1984; Richardson et al., 1985).
2.1.1 Chromatin structure
Chromatin changes may involve either proteins that interact
with DNA or the chemical structure of the DNA molecule itself.
Although no change in the stoichiometry of the major histones has
been observed with increasing age (Richardson et al., 1983;
Medvedev, 1984), several investigators have reported changes in the
subspecies of histone H1 (Medvedev, 1984; Mitsui et al., 1980;
Niedzwiecki et al., 1985). The acetylation of histones, which has
been proposed to alter histone-DNA interactions thereby making DNA
more accessible, decreases by 30% to 70% with increasing age
(O'Meara & Pochron, 1979).
With respect to age-related changes in DNA chemical structure,
there is now conclusive evidence for the "spontaneous" induction of
a variety of DNA lesions in different organs and tissues of both
humans and experimental animals (for a review, see Mullaart et al.,
1990). Most of these lesions seem to be repaired (see below), but
not all. For example, Cathcart et al. (1984) and Fraga et al. (1990)
estimated that in rats about 105 oxidative DNA lesions occur per
cell per day. Since the rate of repair does not entirely equal the
rate of induction of damage, there is a net increase of spontaneous
DNA lesions with age. Fraga et al. (1990) calculated for one
specific lesion, 8-hydroxy-deoxyguanosine, that about 80 residues
accumulate per rat cell per day.
Although some DNA lesions are repaired quickly, this is not the
case for all lesions. Indeed, after treating rats with low doses of
2-acetyl-aminofluorene (AAF), Mullaart et al. (1989) were still able
to detect about 30% of the major lesions induced as late as 21 days
after treatment. Such incomplete repair could be responsible for
accumulation of DNA lesions during continuous or frequent exposure to
genotoxic agents.
2.1.2 DNA repair
To preserve the DNA chemical structure, cells are equipped with
a battery of repair systems to remove damage. As yet the various
mechanisms of action of these DNA repair systems and their
interrelationships are incompletely understood (for a recent review,
see Lehmann et al., 1992). In general, repair systems can be divided
into three categories, i.e. direct repair, excision repair and
post-replication repair. In direct repair, the lesion itself is
removed without any further (transient) changes in the DNA
structure. Direct repair includes the enzymatic photo-reactivation
of UV-induced pyrimidine dimers and the removal of O6-alkyl
adducts by specific alkyl transferases.
DNA excision repair is brought about by a complex multi-enzyme
system, the components of which are involved in the various steps in
this repair process (Vijg & Knook, 1987). The third type of repair,
post-replication repair, does not actually remove the damage but
allows the replication system to bypass the damage. It is this
latter process especially that is considered to be associated with
nucleotide misincorporation (mutation).
Accurate assessment of an organism's capacity to repair
specific lesions is difficult and subject to error. In general, the
most reliable data can be obtained when the induction and
disappearance of the relevant lesions themselves are monitored in
the different organs and tissues of an experimental animal.
Unfortunately, in most studies on the possible existence of a
decline in DNA repair activities with age, assays were used which
measured the DNA synthesis phase of excision repair. The general
conclusion from these data, mostly obtained with cultured cells, is
that there is no age-related decline in the efficiency of DNA repair
systems (Tice & Setlow, 1985; Likhachev, 1985; Hanawalt, 1987). It
cannot be ruled out, however, that during aging DNA repair systems
become more error prone, leading to an accelerated induction of
mutations (Vijg & Knook, 1987). In any case, a certain degree of
imperfection is a general characteristic of DNA repair systems as
indicated by the actual accumulation of both DNA lesions and DNA
sequence changes (see above). The question that should be addressed
is what type of DNA alterations occur, how many exist, and at what
rate do they accumulate with age. Finally, their relevance in terms
of actual physiological decrements or the initiation of disease
should be assessed.
2.1.3 Transcription
Several review articles have been published in the past decade
that discuss the effect of age on transcription (Rothstein &
Seifert, 1981; Richardson et al., 1983; Richardson et al., 1985;
Richardson & Semsei, 1987; Slagboom & Vijg, 1989). A major problem
in this area has been the difficulty in accurately measuring the
rates of synthesis of specific RNA species and their intracellular
levels. With the major advances in recombinant DNA technology, this
problem has now been virtually eliminated and our knowledge of how
aging affects the expression of specific genes is rapidly growing.
At present, it appears that the overall transcriptional
activity of a cell declines as an organism ages. However, the level
of total RNA tends to remain constant suggesting a decline in the
rate of RNA turnover (Horbach et al., 1986).
The levels of some specific mRNA species using cDNA probes for
specific genes have been measured recently (Richardson & Semsei,
1987). In general, no consistent trend has emerged. The levels of
some mRNA species decrease with age; however, other mRNA species do
not change with age, and others actually increase (Slagboom & Vijg,
1989).
In most of the studies, a good correlation has been found
between the age-related changes in the level of an mRNA species and
the level of protein (or enzyme activity) specified by the mRNA
species. This has been demonstrated in rat liver for albumin
(Horbach et al., 1984), apha2u-globulin (Richardson et al., 1987),
and superoxide dismutase and catalase (Semsei et al., 1989), and in
rat kidney and small intestine for calbindin-D (Armbrecht et al.,
1989). The age-related decline in mitogen-induction of interleukin 2
(IL-2) (Wu et al., 1986; Nagel et al., 1988; Pahlavani et al., 1988)
and IL-3 (Li et al., 1988) mRNA in lymphocytes from rodents and
humans corresponded to the age-related decline in the biological
activities of these two interleukins. In contrast, Strong et al.
(1990) reported an uncoupling of tyrosine hydroxylase transcription
and translation in the adrenal glands of old rats.
Investigators usually assume that age-related changes in the
levels of a particular mRNA species arise from a change in
transcription. However, only a few studies have actually measured
the transcription of a specific gene as a function of age using
nuclear run-off assays. While an age-related decrease occurs in the
nuclear transcription of the alpha2u-globulin (Richardson et al.,
1987; Murty et al., 1988a), cytochrome P450(b+e) (Rath & Kanungo,
1989), and superoxide dismutase and catalase (Semsei et al., 1989)
genes, the nuclear transcription of tyrosine amino-transferase and
tryptophan oxygenase (Wellinger & Guigoz, 1986), albumin (Horbach
et al., 1988b) and the c-myc (Buckler et al., 1988) genes was
similar in young and old rodents. Studies are now underway to
explore in more detail age-changes in specified mRNA species in
terms of the transcription factors involved (Post et al. 1991).
One exciting development in the area of transcription and aging
has been the observation that dietary restriction, which enhances
the longevity of rodents, alters the expression of some genes at the
level of transcription (Richardson et al., 1987; Semsei et al.,
1989). However, the expression of all genes is not affected by
dietary restriction (Waggoner et al., 1990).
In addition to nuclear synthesis, post-transcriptional
processing of hnRNA plays an important role in the regulation of
gene expression. Müller et al. (1989) recently discussed various
views of how the post-transcriptional processing of hnRNA might
alter with age. At present, there is little evidence that major
changes occur with age in the size of the poly(A)-segment of mRNA
(Birchenall-Sparks et al., 1985). Interestingly, in the many studies
in which mRNA species have been analysed by Northern blot analysis,
there has been not a single report of a significant change in the
size of the mRNA species examined with increasing age (Richardson &
Semsei, 1987). Thus, there is very little direct evidence at present
to support the view that the processing and/or nuclear transport of
hnRNA is altered with age.
2.1.4 Translation
Increasing age generally results in a decrease in total protein
synthesis in plants, invertebrates, rodents and cultured cells
(Richardson & Birchenall-Sparks, 1983; Ward & Richardson, 1991).
Recent studies have focused on the influence of age on the
translation of mRNA into specific proteins and on the ability to
modulate age changes in protein synthesis. There is no evidence that
a decrease in the fidelity of protein synthesis occurs with
advancing age but technical limitations do not permit a definitive
conclusion (Rosenberger & Kirkwood, 1986). The influence of age on
protein synthesis differs from protein to protein and much more work
must be done in assessing the effect on key individual proteins.
Attempts to modulate protein synthesis have recently begun. The rate
of protein synthesis in the liver is higher after maturity for
dietary restricted than for ad libitum fed rats (Ward, 1988). In
an in vitro system, growth hormone increases protein synthesis in
muscles of old rats to the level found in muscles of young rats
(Sonntag et al., 1985). Much more study is required, focusing on
individual proteins, different tissues and different organisms.
2.2 Changes in tissues, organs and systems in aging
The progressive modification of body functions with age
involves alterations not only at the genetic, molecular and cellular
levels, but at the level of the tissues, organs, systems and entire
organism. It is important to attempt to differentiate between
age-related pathology and true physiological aging. This is often
difficult because the majority of age-related changes increase the
vulnerability of the aging organism to disease and ultimately death.
In the following, each organ or system will be discussed in
reference to age-related changes in its structure which might
predispose to alterations in function, not only inherently as part
of aging, but in response to environmental agents. The focus will be
on the healthy aged as opposed to the diseased.
2.2.1 Nervous system
The brain may undergo a progressive deterioration with age at
all levels of organization - structural, biochemical and functional.
CNS disorders, including Parkinson's and Alzheimer's diseases, are
common in the elderly.
2.2.1.1 Structural changes
Brain weight decreases slightly with aging. This is due to
atrophy of both grey and white matter (Creasey & Rapoport,1985). At
the cellular level, the major age-associated modification is in the
number of neurons, which are significantly diminished in discrete
areas of the brain (Brizzee, 1985), particularly in the basal
ganglia, cerebellum (probably related to decreased motor control),
locus ceruleus (associated with alterations in sleep patterns),
nucleus basalis of Meynert (associated with senile dementia of
Alzheimer type (SDAT) (Bondareff, 1986), and the spinal cord.
Neuronal loss, which is associated with an increase in the number of
glial cells, is relatively mild in the healthy aged, but is much
more severe in SDAT, Parkinson's disease and in the early aging
associated with Down's syndrome.
In addition to a reduced number of neurons, the aged brain is
characterized by a reduction in the number of dendrites and
dendritic spines, probably due to a slowing renewal process
(Scheibel & Tomiyasu, 1978). Synapse density declines in discrete
areas of the brain, but this is partially compensated by enlargement
of the remaining synapses (Bertoni-Freddari et al., 1990).
Intracellular changes include dilation and fragmentation of the
Golgi apparatus (Mervis, 1981), distortion of membranes and the
nucleus, and accumulation of lipofuscin, in both neurons and glial
cells within discrete brain areas. With advancing age, there is an
increase in neurofibrillary tangles (intracellular tangled masses of
paired helical filaments) (Terry, 1963), extracellular neuritic
plaques (a core of amyloid surrounded by material derived from
dystrophic neurites), and reactive glial and microglial cell
accumulation (Master et al., 1985). Again, these changes occur in
normal aging at a moderate level, but are much more frequent in SDAT
(Iqbal et al., 1982) and other dementias.
There are also age-related changes in the morphology of the
peripheral and autonomic nervous systems. These include reductions
in the number of sensory and motor neurons, increases in
demyelination, increases in connective tissue, and a mild loss of
myelinated fibres (Tomlinson & Irving, 1977; Spencer & Ochoa, 1981).
The central processes of dorsal root ganglion cells typically
undergo distal dystrophic and degenerative changes. Regressive
changes have been reported in the terminals of motor axons.
2.2.1.2 Biochemical changes
Besides the pathological changes, there are many age-related
alterations in brain chemistry required for cell-to-cell
communications (Rogers & Bloom, 1985; Finch, 1991). These include
changes in the concentration and/or turnover of the amines (e.g.,
acetylcholine, norepinephrine, epinephrine, dopamine, serotonin),
amino acids (e.g., glycine, glutamate and GABA) and peptides (e.g.,
enkephalin, substance P, thyrotropin-releasing hormone,
cholecystokinin, somatostatin). There are numerous studies showing
impairments of adrenergic, dopaminergic and serotonergic activity in
the senescent animal (Zhou et al., 1984; Roth & Joseph, 1988;
Telford et al., 1988). One of the underlying causes of these
alterations seems to be an overall loss of receptors (Weiss et al.,
1984; Roth & Joseph, 1988).
Synapses may utilize one or more neuromodulator (e.g.,
norepinephrine and neuropeptide). The multiple levels of control and
the regional diversification of different synapses in discrete brain
regions make it difficult to define the age-related alterations in
neurotransmitter/neuropeptide function. In fact, rather than a
uniform drop in the level of a specific neurotransmitter throughout
the nervous system, a "desynchronization" of signals may occur. For
example, while the brain content of norepinephrine and dopamine are
decreased in old age, that of serotonin is unchanged or even
increased, depending on specific brain areas. In some cases, the
greater the concentration of a neurotransmitter in a discrete brain
region, the higher the decrement with aging and vice versa (Timiras
et al., 1984). In fact, age-dependent alterations in different
neurotransmitter/neuropeptide concentrations do not always occur
simultaneously. Each neurotransmitter has its own timetable:
dopamine levels decrease in the cerebral hemispheres of rats from
the age of one year, whereas in the same areas serotonin levels
remain unaffected until three years of age (Timiras et al., 1984).
Aged-related changes in neurotransmitter receptor number and
function have also been reported (Greenberg & Weiss, 1983; Roth &
Joseph, 1988). Changes in binding affinity have not been frequently
detected. Beta-adrenergic receptor responsiveness is decreased in
the elderly (Vestal et al., 1979; Lakatta, 1980). This appears to be
due to uncoupling of the beta-receptor from the adenylate cyclase
complex which transmits the signal (Wood, 1985). In the rat pineal
gland, corpus striatum and cerebellum, a reduced responsiveness to
catecholamines is present due to a decrease in the affinity of
beta-adrenergic receptors to their ligand. However, there is no
change in receptor number (Greenberg & Weiss, 1978). This may be due
in part to a reduced ability to increase the number of
beta-adrenergic receptors after decreased noradrenergic input
(Greenberg & Weiss, 1979).
Changes in general biochemical properties of the cells occur in
the nervous system as they do elsewhere in the aging organism.
Lipid composition may change, resulting in altered membrane
viscosity. Protein synthesis decreases in discrete brain regions.
Lipofuscin accumulates, although the functional significance is
unclear, and there are alterations in electrolytes and trace
elements (Brizzee, 1985). For example, aluminium levels may increase
sharply in elderly people (Bjorksten et al., 1989). Decreases in
zinc may be important in the light of the zinc requirement of
various enzymes and growth factors, including nerve growth factor
(NGF) (Dunn et al., 1980). In addition, aging is accompanied by a
decreased brain water content (Meisami, 1988). Alterations in
vascular flow have also been reported (Katzman & Terry, 1983).
2.2.1.3 Functional changes
Despite the morphological and biochemical changes observed in
the aging brain, the functional efficiency of the nervous system
seems to be well maintained in most elderly people. However, CNS
disorders do occur in some individuals, though it may be difficult
to discriminate age-related pathology from physiological aging
phenomena. Perhaps the most ubiquitous and significant change
observed in the older organism is slowness of behaviour (Birren et
al., 1979). The slowing of behaviour with age not only appears in
motor responses and perceptual processing, but is also apparent for
the more complex processing of information associated with
short-term memory (Smith et al., 1980). Related cross-sectional
studies using global measures of intellectual function such as the
Wechsler Adult Intelligence Scale (WAIS) show evidence that some
performance abilities decline by the late 60s and early 70s, while
others (e.g., verbal abilities) appear to be maintained throughout
life in healthy individuals (Gallagher et al., 1980). The slowing of
reaction time may be associated with the age-associated slowing and
loss of coordination in motor tasks, such as those involved in
handwriting and other purposeful movements.
The age-related modification of biorhythms is exemplified by
the alterations of the sleep/wakefulness cycle, which is largely
dependent on the reticular system. Alterations of sleep patterns
with aging are qualitative rather than quantitative (Dement et al.,
1985) and affect primarily the "deep sleep" phases, as confirmed by
the alterations observed in the brain electrical activity (Müller &
Schwartz, 1978). Among neurotransmitters, serotonin seems to be
implicated.
Alterations in posture and locomotion in the elderly (Klawans &
Tanner, 1984) also depend on CNS impairment. Peripheral
modifications such as decreased nerve conduction velocity, reduced
muscle mass and increased rigidity occur. Autonomic system
dysfunction is also implicated in many pathophysiological changes
of age including hypotension, thermoregulation, gastrointestinal
function and urinary incontinence (Finch & Landfield, 1985). Other
changes in the autonomic system include changes in vascular and
cardiac reflexes, galvanic skin responses, and potency (Katzman &
Terry, 1983). Sympathetic hyperactivity is commonly present in the
aged and could interfere with cognitive functioning.
2.2.2 Sensory organs
All of the sensory organs are affected by aging, both those in
which the cells are continuously renewed (such as cutaneous sense
tissues) and those in which the cells are terminally differentiated
early in life (vision and hearing).
2.2.2.1 Vision
Both neural (retina) and optical (cornea, lens, pupil, aqueous
and vitreous humours) components of vision are affected by age. The
changes in the optical compartment are probably the primary cause of
visual impairment in the elderly (Sekuler et al., 1982). The most
common alterations are in the lens with increased hardness and
decreased transparency (Graham, 1985). The former results in reduced
refractive power (Marsh, 1980). The loss of transparency relates to
the following chemical changes in the lens: protein oxidation,
racemization, glycation, aggregation, polymerization and
precipitation (Taylor, 1989). These alterations are associated with
presbyopia and cataracts, respectively.
In the neuronal compartment, the retina undergoes progressive
loss of rods, while cones may be augmented. Morphometric analysis of
the retina demonstrates an increase in electron-dense plaques and a
decrease in the ground substance during aging. Such retinopathies
result in decreased light sensitivity and reduced colour vision
(Marsh, 1980).
Vision declines as a function of age (Weale, 1986) and can be
measured in several tests, such as the Humphrey Field Analyser
(Iwase et al., 1988), and retinal potentials (Trick, 1987). Visual
acuity is substantially decreased. The ability to detect light
gradually decreases (Sample et al., 1988) and light adaptation
declines (Katz & Robinson, 1987).
2.2.2.2 Hearing
Decrements in hearing are frequently observed in the elderly.
There is also a progressive loss of hearing in animals with age
(Willott, 1986). Both auditory structures and neuronal components
are involved. While the outer and middle ear show few modifications,
degenerative changes occur in the hair cells, which are the auditory
receptors, and in the mechano-electrical transducing organs
resulting in otosclerosis. This accounts for the preferential loss
of hearing of high frequency sounds (presbycusis) (Marsh, 1980). The
degree of hearing loss may affect the two ears differentially, thus
causing defects in sound localization. Presbycusis has a great
impact upon speech perception, since consonants, which make speech
intelligible, are generated by high frequency sounds, whereas
vowels, responsible for audibility, are produced by low frequency
sounds.
Hearing defects may also result from changes in the neural
components (Allison et al., 1984) of hearing, and in particular in
the nerves connecting the cochlea with the auditory centres in the
brain, specifically in the superior temporal gyrus.
2.2.2.3 Olfaction
The age-related alteration in the sense of smell is generally
underestimated. The reduction in olfactory sensitivity is mainly due
to the progressive loss of olfactory neurons, which protrude through
cilia from the superior nasal cavity and represent the receptor
sites for odour and the chemo-electrical transducing mechanism
(Naessen, 1971). Loss of neurons have also been demonstrated in the
olfactory bulbs of the brain (Bhatnagar et al., 1987).
2.2.2.4 Taste
Taste thresholds are known to increase with age. The taste of
salt is preferentially altered in the elderly. The loss appears due
both to a decline in the number of taste buds and papillae (Bradley,
1979) in the tongue, as well as to the loss of neurons in the
cerebral centers of the gustatory system.
2.2.2.5 Somatic sensations
The somatic sensory system (touch, pressure, vibration,
proprioception, heat, cold and pain) is variably affected by age.
Tactoperceptual ability and vibrotactile sensations are decreased in
the elderly due to the loss of Meissner end-organs and Pacinian
corpuscles present in the skin (Bruce, 1980). For more complex
somatesthetic abilities (stereognosis, body part recognition) as
well as for pain and thermal sensitivity, the biological causes of
their alterations with age involve not only the sensory end-organs,
but also affective and cognitive factors (Marsh, 1980).
2.2.3 Endocrine system
Hormones play an important, often critical, role in the
regulation of a large number of physiological and behavioural
processes, and their influence can be demonstrated throughout the
lifespan. Some hormones have a role in differentiation in that their
presence or absence during certain developmental periods will affect
the way in which physiological and behavioural processes proceed or
are expressed in adulthood. Throughout each period of the life span,
the maintenance of an appropriate endocrine milieu is essential to
the numerous homeostatic processes required for survival. With
advancing age, there are several, well-documented changes in the
ability of the organism to synthesize and secrete a number of
hormones. It is, therefore, likely that the typical age-related
change in an organism's endocrine balance would result in, or at
least contribute to, the impairment of homeostasis frequently
observed in the elderly. Such impairments can be noted in the
decreased rate of recovery of the elderly from the insults of injury
or disease.
Hormones may also play a significant role in the aging process.
For example, age-related changes in several physiological functions
appear to be closely linked to the level and pattern of hormonal
stimulation present during adulthood. As such, different patterns of
exposure to a hormonal environment may alter the "rate of aging"
within a specific neuroendocrine system and, in turn, affect the
susceptibility of the organism to environmental insults at different
segments of the life span. There are a number of different ways in
which endocrine systems and the hormonal signalling operations that
they use may undergo alterations with age and toxicant exposure.
These can be categorized as changes in: (a) the availability of
hormones for binding to the target tissues, (b) the reception of the
pertinent transmitter or hormonal signal by the target cells, and
(c) the nature of the hormonal message.
At any point in time, the concentration of a hormone in the
blood is a consequence of both its metabolism and secretion. Such
changes in the size of the available signal pool may have
corresponding effects on the magnitude of the response by the target
tissue. Other changes may reflect declines with age in the
homeostatic controls, which rely heavily on endocrine feedback
relationships within organ systems.
Serum hormonal levels, as a rule, are not maintained at
constant levels. They tend to fluctuate, sometimes markedly,
throughout a 24-h period. In the young adult man, peak morning
testosterone values can fall by one-third to an early evening nadir,
before rising again through the late evening and early morning hours
(Bremner et al., 1983). A similar circadian rhythm in circulating
levels of testosterone is prevalent in the rat (e.g., Kinon & Liu,
1973; Ellis & Desjardins, 1982). Human cortisol (Bilchert-Toft,
1978) and rat corticosterone (Moberg et al., 1975; Kato et al.,
1980) concentrations also exhibit well-known rhythmic fluctuations,
as do those of thyrotropin (Vanhaelst et al., 1972; Leppaluoto
et al., 1974) and growth hormone (Millard et al., 1985). Reported
attenuations with age in the rhythms of human and rat serum
testosterone (Bremner et al., 1983; Steiner et al., 1984),
luteinizing hormone (LH) (Vermeulen et al., 1989), and growth
hormone (Sonntag et al., 1980; Prinz et al., 1983), among other
hormones, can present differences in young-versus-old comparisons,
depending on when such sampling is performed.
While observable changes in hormonal rhythms or significant
differences in circulating hormone concentrations may reflect
disturbances in the overall functional integrity of the associated
organ system, the absence of such changes should not be necessarily
assumed to indicate a corresponding absence of a functional
alteration. The notion of a "system at risk" presupposes an increase
in the susceptibility to disruption of the homeostatic controls. An
aging system that may be undergoing a subtle erosion in its
endocrine balance could be more likely to exhibit alterations in its
response to a stressor or toxic insult. In this respect the
stimulation of growth hormone release by clonidine, L-dopa and
insulin is substantially depressed (Riegel & Miller, 1981), while
arginine-stimulated growth hormone (GH) secretion after arginine
infusion is preserved (Aschoff, 1979). Secretion stimulated by GHRH
(GH releasing hormone) is only partially reduced (Coiro et al.,
1991).
Regardless of these alterations, it remains established that
the 24 h production of GH is significantly reduced in elderly humans
(Prinz et al., 1983), whereas that of prolactin is increased
(McGinty et al., 1988; Blackman, 1987). These data have been
confirmed in animals (Ceda et al., 1986; Sonntag & Gough, 1988),
although measurements of hormonal profiles may have involved
different procedures in animals and man, thus giving rise to
slightly different interpretations.
Similar difficulties are encountered in studies of age-related
alterations in pineal hormone secretion, including melatonin, whose
circadian rhythmicity is certainly changed with age (Reiter, 1986;
Anisimov & Reiter, 1990).
In order to illustrate age-related alterations in hormone
control, it is useful to focus on the integrated systems which
involve more than one gland or hormone. Although three such systems
are reviewed below, this discussion is by no means intended to be
comprehensive. One theme common to studies of age-related changes in
endocrine function is that such alterations are often hormone and
species specific. Finally, the extent to which any of these changes
relate to potential adverse health outcomes in the older organism
remains to be demonstrated.
2.2.3.1 The pituitary-thyroid axis and the basal metabolism
Thyroid hormones are required during development for growth and
in adult life for regulating oxygen consumption. Maintenance of
thyroid function is generally assured even in old age, although
following repeated stress and demands the reserve function may
become exhausted and a dysthyroid state may follow (Ingbar, 1978).
Changes with aging in the levels of both thyroid stimulating
hormone (TSH) and thyroid hormones (thyroxine (T4) and
triiodothyronine (T3)) are controversial, because concomitant health
disturbances may cause significant fluctuations in the levels of
these hormones (Gregerman & Solomon, 1967; Utiger, 1980). Both hypo-
and hyperthyroidism are not uncommon in the elderly. In general, the
size of the thyroid decreases with age (Gambert & Tsitouras, 1985).
Older people show a normal response to decreased thyroid
function by increased secretion of TSH (Eden, 1987). TSH levels
undergo few changes (Miller, 1989), suggesting that the hypothalamic
control of TSH release has not been altered. However, structural
modifications of TSH have been reported (Klug & Adelman, 1977). T4
levels remain unchanged with age, even though the rate of synthesis
is reduced. However, the blood levels of T3 are reduced with
advanced age (Chopra et al., 1978), while levels of reverse T3 are
unchanged. It should be noted that severe and chronic illnesses, not
directly involving the thyroid, can lower the levels of T3 and
T4.
The alterations observed in thyroid hormone levels are
inadequate to explain the age-associated decline in various
functions that are dependent on thyroid hormone. One possible
explanation is that peripheral sensitivity to thyroid hormone action
is modified by aging. However, with advancing age, the basal
metabolic rate remains unchanged if based on lean body mass, but
decreases if expressed based on body surface area (Masoro, 1985).
2.2.3.2 The pituitary-adrenal axis
The major function of this axis, which is largely based on
pituitary hormones (ACTH) and adrenal hormones (corticosteroids), is
to provide an adaptive response to environmental stress (Selye,
1950; Sapolsky et al., 1986). Any harmful agent, in addition to
inducing a specific reaction in the body (anaesthesia, emotion,
fever, etc.), activates a specific and common response, the
so-called "General Adaptation Syndrome" (Selye, 1950), characterized
by increased adrenocortical secretion, thymic involution,
lymphopenia and eosinopenia. With advancing age this axis may
undergo desynchronization, thus resulting in a failure of
homeostasis and adaptation (Anisimov & Reiter, 1990).
ACTH secretion, which shows a circadian rhythm based on
melatonin fluctuations, is generally preserved in advanced age
(Halberg, 1982), although minor modifications of blood levels may
occur due to variations in renal clearance or alterations in sleep
patterns. However, there is evidence of diminished sensitivity of
the hypothalamic/pituitary axis feedback inhibition by
glucocorticoids (Greden et al., 1986; Blackman, 1987; Dilman, 1987;
Sapolsky et al., 1987). The elderly suffering from Alzheimer's
disease are extremely resistant to glucocorticoid negative feedback
(Sapolsky et al., 1986).
Finally, certain cell populations (e.g., CA3 neurons in the
hippocampus) are particularly susceptible to glucocorticoids.
Long-term stress may result in their dysfunction and death (Sapolsky
et al., 1987).
2.2.3.3 The endocrine pancreas and carbohydrate metabolism
It is well documented that with advancing age the ability to
maintain glucose homeostasis is impaired, but the underlying
mechanisms are still not well defined. Several hormones contribute
to the regulation of glucose homeostasis: above all, insulin and
glucagon, secreted by the endocrine pancreas, and somatostatins and
the pancreatic polypeptide, which modulate the secretion of insulin
and glucagon, respectively. In addition, glucose metabolism may be
affected by other hormones, including T3 and T4, growth hormone,
glucocorticoids and epinephrine (Minaker et al., 1985).
Only modest morphological alterations are observed in the
endocrine pancreas with advancing age. In spite of this fact, blood
sugar levels after fasting are elevated and glucose tolerance is
lowered in the elderly (Magal et al., 1986; Eden, 1987; Ammon
et al., 1987; Wang et al., 1988; Groop, 1989). Plasma insulin
concentration increases and insulin sensitivity decreases.
Alterations in insulin turnover are detectable in the elderly after
glucose load, such as reduced insulin secretion and increased
secretion of the inactive prohormone, proinsulin (Marx, 1987), but
these changes are too modest to account for the observed glucose
intolerance. One alternative explanation is an increase in
peripheral resistance to insulin. In fact, peripheral uptake of
glucose is indeed reduced in the elderly, due to a reduction in
insulin receptors (Pagano et al., 1981) as well as alterations in
the post-receptor signalling process (Rowe et al., 1983). No
evidence exists regarding the possible involvement of age-related
changes in glucagon affecting glucose intolerance in the elderly.
Other factors, however, may contribute to glucose intolerance.
These include: (a) reduced liver sensitivity to insulin, resulting
in reduced glycogenesis; (b) changes in diet and physical exercise;
and c) increased body fat with reduced muscle mass. This last point
seems to merit particular consideration in view of the observation
that insulin resistance is certainly increased in obese humans
(Runcie, 1985). In fact, intracellular fat accumulation leads to a
reduced concentration of insulin receptors (Bolinder et al., 1983).
2.2.4 Reproductive system
The age-related modifications of the reproductive system are
primarily based on alterations in the central nervous system,
pituitary gland and gonads. While menopause is a time-fixed event
involving cessation of ovarian function, the decline of testicular
function is a slow and gradual process, involving limited hormonal
alterations. Older persons show the normal response to deficient
gonadal function by increased synthesis of gonadotropins (Piva
et al., 1987). This occurs in both sexes. In fact, alterations in
serum levels of both luteinizing hormone (LH) and follicle
stimulating hormone (FSH) have been reported (Blackman, 1987). The
reduced presence of sex steroids in women may have an influence on
the function of other endocrine glands. Estrogens have
well-documented effects on salt and water balance and on plasma
proteins, which in turn have effects on the level of thyroid
hormones through a suppression of TSH secretion. Estrogen also
stimulates the production of growth hormone and prolactin. Thus, the
decline in gonadal function during age could have far-reaching
consequences on the individual's physiological function.
2.2.4.1 Female aging
In females, cessation of ovarian function consists of the
transfer from regular menstrual cycles to amenorrhoea, usually
preceded by a period of cycle irregularity. The initial changes have
been reported to occur in hypothalamic-pituitary control of the
ovaries. For example, the age-related decline in reproductive
function is associated with a decreased sensitivity of the
hypothalamic-pituitary complex to feed-back regulation by estrogens
(Dilman, 1971, 1987). This leads to an age-related enhancement of
pituitary gonadotropins (FSH, LH) (Chakravarty et al., 1976),
leading in turn to hyperstimulation of the ovaries. However, despite
the compensatory increase in ovarian hormone production, the level
of estrogens is insufficient to induce ovulation because of
hypothalamic insensitivity, possibly due to an age-related decrease
in the level of biogenic amines and/or peptide hormone receptors
(Dilman & Anisimov, 1979). In addition, the progressive loss of
oocytes plays an important role in the decline in reproductive
function since the reduction of maturating oocytes may induce
desynchronization of pituitary-ovary hormonal interactions
(Aschheim, 1976).
The most common consequences of menopause include imbalances of
the autonomic nervous system, psychological modifications, and
physiological alterations of target organs due to metabolic changes.
Alterations of estrogen target organs are among the most evident
effects of menopause. Vulvar skin and vaginal epithelium may undergo
atrophy. Glycogen content is generally reduced, with a consequent
decrease of lactobacilli, rise of vaginal pH, and increased growth
of pathogenic microbes. The uterus and oviducts atrophy due to the
decreases in estrogen levels. In ovaries, follicular cysts and
atresia result in response to the altered hormonal status.
Hyperplasia of the theca cells occurs. Fibrosis also occurs in these
tissues but, in addition, can affect the bladder and urethra,
resulting in an increased incidence of cystitis, dysuria and
non-infectious urethritis. The reduced thickness of the skin is also
a result of the decrease in estrogen (Schiff & Wilson, 1979).
Menopause has major health consequences for the cardiovascular
and skeletal systems. The reduction in estrogen secretion removes
the protection offered by these hormones against coronary heart
disease, development of atherosclerosis, and accompanying
alterations of lipid metabolism. Osteoporosis, resulting from
increased bone reabsorption relative to bone formation, is a common
problem in postmenopausal women (Riggs, 1987). Two types of
osteoporosis may be identified. Type I is associated with estrogen
withdrawal and may begin in middle age (Riggs & Melton, 1983). The
biological effects are linked to disruption of the complex
relationship between calcium intake and loss, and the secretion of
calcitonin, parathyroid hormone and 1,25-dihydroxy-vitamin D.
Estrogens prevent the transfer of calcium from bone to blood and its
loss through urine. This induces parathyroid hormone secretion,
which stimulates the formation of 1,25-dihydroxycholecalciferol, the
active metabolite of vitamin D. The function of the parathyroid is
affected by increasing age (Eden, 1987). The relevance of estrogen
for bone loss is further supported by the effectiveness of estrogen
therapy in delaying the osteoporotic process in post-menopausal
women (Edman, 1983). With advancing age type II osteoporosis (senile
osteoporosis) may occur, which is probably due to the poor
intestinal absorption of calcium (Riggs, 1987).
2.2.4.2 Male aging
The reproductive system is less affected by aging in males than
in females. It is generally accepted that testosterone levels are
maintained within the physiological range throughout life, although
a decrease in testosterone production in response to gonadotropin
action may occur in old age due to a reduction in Leydig cell number
and function (Harman et al., 1982). Testis and accessory sex organs
do not show substantial modifications with age, and sperm is found
in the ejaculate of very elderly men. The volume of seminal fluid is
generally decreased.
While the prostate undergoes involution in the majority of old
men, in about one-third of males it undergoes hypertrophy with
consequent obstruction of the urethra and urinary flow from the
bladder. The cause of the hypertrophy is still unclear (Mawhinney,
1985). The prostatic enlargement results in compensatory hypertrophy
of the bladder. When such compensation is no longer sufficient,
retrograde filling of the renal pelvis and ureters may occur,
resulting in hydronephrosis and eventually renal failure.
2.2.5 Immune system
With advancing age a progressive increase occurs in the
incidence of various infectious diseases, autoimmune processes and
tumours. These may be in part based on age-related defects in the
immune system. The association of so many age-related pathologies
with defects in the immune system has led to the suggestion that
aging of the immune system may be rate limiting for life span
(Walford, 1969). However, while there are numerous experimental and
clinical studies demonstrating an age-related deterioration in
immune efficiency, this decline is not sufficient to account for all
manifestations of aging.
There are several recent reviews on aging and the immune system
(Revskoy et al., 1985; Lipschitz, 1987; Segre et al., 1989; Miller,
1991). However, it is still difficult to draw a comprehensive
picture, because of the many cellular and humoral components
involved in immune reactions and the many modulating
extra-immunological factors which may also be compromised in the
elderly. The immune and haematopoietic systems are intimately
related, being derived from a common pluripotent stem cell. Both
play central roles in host defense, prevention of neoplasia, and
response to infectious agents (Lipschitz, 1987). However, basal
haematopoiesis in both animal models and man seems to be either
unchanged or minimally altered with age (Dybkder et al., 1981;
Lipschitz, 1987). The reserve capacity may be reduced resulting in a
decreased ability to respond to stress.
2.2.5.1 Aging of lymphoid organs
Peripheral lymphoid organs, such as the spleen and lymph nodes
do not show consistent modifications in size with aging. Bone marrow
is not consistently affected by age. Stem cell production is
generally well preserved in old age (Harrison et al., 1978),
although a slight change in the replication rate of stem cells has
been reported by some authors (Schneider et al., 1979). Thymic
involution has been considered to account for the major age-related
changes in the immune system, beginning at puberty. Such an
involution consists of a progressive loss of cellularity with
lymphoid cell depletion in the cortical areas and cystic changes in
the epithelial cells. These are the source of various peptides
involved in differentiating thymic lymphocytes (T-cells) from
lymphoid cells of earlier lineage. The export of newly
differentiated T-cells is reduced with advancing age (Globerson
et al., 1989). The synthesis and the secretion of polypeptide thymic
hormones, such as thymosin (McClure et al., 1982), thymopoietin
(Lewis et al., 1978) and thymulin (Bach et al., 1972), are
progressively diminished. In all cases, the reduction of thymic
endocrine activity seems to have a pathogenic role in age-related
immune dysfunctions, since replacement by exogenous administration
of the hormones is capable of restoring various immune functions in
old age (Zatz & Goldstein, 1985). The turnover of zinc, which is
essential for immunocompetence (Iwata et al., 1979; Chandra, 1985),
decreases in old age. Zinc supplementation can restore immune
functions (Fabris et al., 1990).
2.2.5.2 Aging of cellular constituents
Mature T-cells, bone marrow lymphocytes (B-cells) and natural
killer cells (NK-cells) can be detected in blood and in lymphoid
organs by specific monoclonal antibodies. With this type of
analysis, no major modifications in the proportion of the various
lymphoid cell subpopulations have been observed in humans. However,
the major alteration in the immune system appears to arise in the
functioning of T-cells (Thompson et al., 1987). While the total
number of T-cells in the peripheral blood does not change
appreciably with age, there are clear-cut differences in the
relative proportion of T-cell subtypes (Wagner et al., 1983;
Fernandes, 1984; Revskoy et al., 1985; Thompson et al., 1987;
Lipschitz, 1987).
The number of immature lymphocytes of the T-lineage increases
with age, as does the percentage of apparently activated
T-lymphocytes bearing immature thymic phenotypic markers. There is a
relative increase in cytotoxic/suppressor T-cells, and a decrease in
the number of helper/inducer T-cells (Lipschitz, 1987; Thompson et
al., 1987). Correlated with the decrease in the helper/inducer
population, is a functional defect in cell-mediated immunity
(Lipschitz, 1987; Thompson et al., 1987). Cells from aged humans or
experimental animals are less capable of responding to allogeneic
lymphocytes, phytohaemagglutinin, concanavalin A and soluble
antigens. Lymphocytes from older mice are less able to elicit
graft-versus-host reactions than those from younger mice of the same
inbred strains (Thompson et al., 1987). Fifty percent of healthy
people over age 50 have impaired cutaneous hypersensitivity
(Lipschitz, 1987; Dilman, 1987). Accompanying the decrease in
helper/inducer T-cells and cell-mediated immune functions is a rise
in autoantibodies and autoimmunity (Thompson et al., 1987).
Changes in humoral immunity (B-cell function) with aging are
more subtle (Lipschitz, 1987; Senda et al., 1989). Studies on the
effects of age on antibody production have yielded conflicting
results, perhaps because of the wide range of experimental values
generally observed in older individuals. It has, however, been well
established that aging is significantly associated with the presence
of various autoantibodies, in particular, antibodies against nuclear
antigens. There is also evidence that aging effects the rate of
antibody production by activated B-cells (Lipschitz, 1987).
From a functional point of view, defects have been observed at
various levels. Firstly, the proliferative capacity of T-cells from
old individuals is generally reduced, regardless of the stimuli used
(antigens, mitogens), and the defect consists both in a reduced
number of cells responding to stimulus and in a precocious
exhaustion of the cloning capacity (Fabris et al., 1983) of
responding cells. Secondly, the response to interleukins, which
physiologically mediate the modulation of the proliferative
reaction, is depressed and this phenomenon has been documented not
only for T-cells but also for NK cells, which are less sensitive in
old age to the boosting action of IL-2 or interferons (Provinciali &
Fabris, 1990).
With respect to accessory cells (phagocytic cells,
macrophages), their number and function are not altered by age, and,
in certain circumstances, their activity seems to be enhanced.
2.2.5.3 Neuroendocrine-immune interactions
The immune system, although regulated to a large extent by
intrinsic cellular and humoral events, is also sensitive to signals
generated from the nervous and endocrine systems. Communication
between nervous and immune networks is mediated by hormones and
neurotransmitters which reach lymphoid organs and cells via blood or
direct autonomic nervous system connections (Bullock, 1985; Felten
et al., 1985). The neuroendocrine immune interactions are mediated
by circulating humoral factors from the
pineal-hypothalamic-pituitary axis, either directly via
neuropeptides and hormones, or indirectly by the effects of this
axis on the hormonal secretion of peripheral endocrine glands, which
also exert immunomodulating actions (for review, see Fabris, 1991).
The nervous and neuroendocrine systems not only act as
modulators of the immune network, but also as targets for signals
generated within the immune system, such as those exerted by thymic
factors (Hall et al., 1989) and interleukins, (Besedovsky et al.,
1985), and by pituitary-like factors (ACTH, TSH, GH, PRL,
gonadotropins, endorphins), which are produced by mature lymphocytes
upon antigenic stimulation (Weigent et al., 1990).
The sharing of humoral signals, as well as of the specific
receptors between neuroendocrine and immune cells (for reviews, see
Fabris & Provinciali, 1989; Weigent et al., 1990), implies that
biological response modifiers of neuroendocrine-immune origin might
be developed in the near future for therapeutical purposes. On the
other hand, potentially harmful agents for one of these homeostatic
systems may also cause alterations in others.
From an experimental point of view, it has been demonstrated that
treatments of old animals with thyroid hormones (Fabris et al., 1989),
GH (Kelley et al., 1986) and analogues of LH releasing hormone
(Greenstein et al., 1987) are able to induce regrowth of the thymus
and reacquisition of its endocrine activity (for review, see Fabris
1991). Analogous treatments, such as with melatonin (Pierpaoli
et al., 1991), GH (Davila et al., 1987), TSH and thyroid hormones
(Provinciali & Fabris, 1990), are also able to recover various
age-related peripheral immune deficiencies, such as T-cell
functioning and NK cytotoxicity. In humans, little work has been
done in this area, although indirect evidence, obtained primarily
from studies on endocrinopathies in the elderly (Fabris et al.,
1989; Travaglini et al., 1990), suggest that recovery of both thymic
and peripheral immune function can be achieved by a neurohumoral
approach.
Little is known on the potential effect of thymosins,
interleukins and lymphocyte-derived pituitary-like cytokines on
age-related alterations of the nervous and of the neuroendocrine
system. Experimental information from old animals showing recovery
of the hormonal and metabolic profile following immune manipulation
(for review, see Fabris et al., 1988) is undoubtedly opening a new
research approach for human investigations.
Receptor sites for many hormones are present on the membrane of
lymphoid cells (Fabris & Provinciali, 1989). The number of
glucocorticoid receptors in spleen cells decreases in old animals
(Roth, 1979a). Hormones that modify the turnover of cyclic
nucleotides result in consequent activation or inhibition of immune
functions (Hadden, 1983). Hormones influence the production of
several lymphokines and monokines (Kelso & Munck, 1984).
The neuro-endocrine system seems to act not only as a modulator
of the immune network but also as a target for signals generated
within the immune system. Examples of such interactions are the
alterations that can be induced in the neuroendocrine balance,
either by removal of relevant lymphoid organs such as the thymus or
by dysfunction of the immune system itself as a result of reactions
to immunogenic or tolerogenic doses of antigen (Besedovsky et al.,
1975). In addition, mature lymphoid cells, when stimulated by
antigens, produce humoral factors similar, if not identical, to
classical hormones and neurotransmitters (such as ACTH, TSH, GH,
PRL, gamma-endorphins) (Blalock et al., 1985). These reciprocal
influences between the neuroendocrine and the immune systems
(Fabris, 1981; Fabris et al., 1988) occur throughout life, but have
particular relevance during aging (Fabris & Piantanelli, 1982).
2.2.6 Cardiovascular system
The frequency of cardiovascular diseases, which are the major
cause of death in industrialized countries, increases with age.
Diseases such as hypertension and atherosclerosis occur most
commonly in the elderly. In addition, degenerative changes of the
cardiovascular system, involving the myocardial cells as well as
cells of the pacing-conduction system, that arise during the aging
process lead to impaired cardiac function and arrhythmia even in
people without any clinical evidence of hypertension or coronary
artery diseases. Inadequate function of the cardiovascular system
induces effects in peripheral tissues and organs. Changes in
peripheral organs resulting in hyperlipidaemia,
hypercholesterolaemia, and hypo- and hyperglycaemia can also effect
the cardiovascular system in the elderly.
2.2.6.1 Heart
The heart itself can be considered to be made up of two parts:
a) the conduction system responsible for electrically controlling
the heart rhythm; and b) the myocardium performing the contractile
function of the heart and composed of a system of trabeculae.
The biophysical and biochemical mechanisms that govern cardiac
muscle change with age, resulting in characteristic alterations in
muscle function (Lakatta, 1987a,b). Many of the steps in the
excitation-contraction system in cardiac muscle are altered by
aging. In an isometric contraction, the transmembrane action
potential (TAP) excites the cell and the contractions that ensue are
longer in duration. The magnitude of the prolongation of
depolarization of the TAP in senescent muscle is striking (i.e.
about twofold). The action potential amplitude is also greater in
senescent than in adult muscle in both high and low calcium-loading
conditions. These deficits of the senescent muscle may be related in
part to the diminished Ca2+ pumping rate by sarcoplasmic
reticulum. The duration of the elevated myoplasmic Ca2+ level is
prolonged in senescent muscle.
Sagiv et al. (1988) found that left ventricular contractility
increases less on stimulation in elderly subjects than in younger
people. Although the aging process is associated with normal resting
contractile function, diastolic properties are altered, resulting in
reduced and delayed early left ventricular filling and enhanced
atrial contribution to diastolic volume. Exercise cardiac output is
maintained in healthy elderly individuals, but there is a shift from
reliance on an increase in heart rate and a decrease in end systolic
volume to use of the Frank-Starling mechanism to increase stroke
volume. This age difference in the cardiovascular response to
exercise is probably mediated by an age-associated decreased
responsiveness to beta-adrenergic stimulation.
In muscle tissue of the aging heart, some morphological changes
are observed both in animal and human studies (Koobs et al., 1978;
Speijers, 1983). The most common change in the aging heart is
hypertrophy (Lakatta, 1985). Other alterations consist of the
appearance of slight focal necrosis and fibrosis in the myocardium,
amyloidosis (Finch & Hayflick, 1977) and the appearance of
lipofuscin (Hendley et al., 1963; Koobs et al., 1978). Peroxidative
damage to the myocardium is cumulative and irreversible (Koobs
et al., 1978).
2.2.6.2 Blood vessels
Both physiological and morphological changes are observed in
the vascular system, especially in the small and large arteries. The
morphological changes in the arteries seen in the elderly vary
considerably both in appearance and in localization (Goyal, 1982;
Hazzard, 1985). The thickness of the aorta increases significantly
with age, while the number of nuclei in the cells of the arterial
media decreases in humans as well as in mice. The majority of these
changes in humans are categorized as atherosclerotic. These changes
can progress and result in complicated coronary atherosclerosis and
ischemic heart disease, but other factors may also cause clinical
effects such as angina pectoris, arterial spasms, and myocardial
infarcts (Speijers, 1989a).
Morphological changes in the veins are less pronounced than in
the arteries.
The physiological changes observed with aging are often a
result of changes both in heart function and in the arteries. These
changes are reflected in haemodynamic parameters such as an increase
in diastolic and systolic blood pressure, mean arterial blood
pressure and vascular resistance, and a decrease in responsiveness,
and in contraction and relaxation responses (Lakatta, 1986, 1987b;
Duckles, 1987; Mazzeo & Horvath, 1987; Zemel & Sowers, 1988;
O'Malley et al., 1988; Cleroux et al., 1988).
Aging is often accompanied by increases in the incidence and
prevalence of hypertension. Geriatric hypertension is generally of a
salt-sensitive nature with a disproportionate frequency of isolated
systolic hypertension. The age-related increase in salt sensitivity
is due to a decline in renal function (Zemel & Sowers, 1988) and
deregulation of vascular tonus. Age-associated declines in the
activity of membrane sodium/potassium-ATPase may also contribute to
geriatric hypertension because this results in increased
intracellular sodium loading, causing reduced sodium/calcium
exchange and thus increased intracellular calcium and vascular
resistance.
It is commonly accepted that atherosclerotic changes take place
to a certain extent in every individual. Multiple factors determine
the extent and velocity of the atherosclerotic process (Hazzard,
1985). The incidence of clinically observed atherosclerotic effects
is higher in elderly subjects than in younger individuals.
Atherosclerotic damages result in impaired cardiovascular function.
Atherosclerosis is defined as a multifactorial disease with
variable effects in the intima followed by changes in the media of
arteries. These changes consist of focal accumulation of lipids,
proliferation of smooth muscle cells, and accumulation of complex
carbohydrates (i.e. glycosaminoglycans, proteoglycans), blood and
blood products, collagen and calcium compounds (Campbell &
Chamley-Campbell, 1981; Velican, 1981; Speijers, 1989a). The
resultant modifications of arterial wall integrity can lead to the
following: erosion of the wall with consequent reduced resistance to
blood pressure, rupture, and haemorrhage; progressive thickening of
the wall due to reactive proliferation of tissues with consequent
reduction of blood flow; and clotting of the blood at the level of
the injured wall with consequent sudden obstruction. The basic
lesion seems to develop in the first decade of life (Lee, 1985).
In the etiology of the lesion two localized cofactors should be
taken into account: the blood supply of the arterial wall; and blood
turbulence, since lesions are more frequently found around the
orifices of arteries branching off major arteries or at bifurcations
(Patel & Vaishnaw, 1980). Hypertension, diabetes, autoimmunity and
stress are also risk factors contributing to atherosclerosis.
2.2.6.3 Characteristics of atherosclerotic lesions
The first event in the formation of the lesion is still
debated. Both an initial thickening of intima, due to an
accumulation of blood-born amorphous material (lipids, protein and
sulfated proteoglycans) and a proliferation of muscle cells (due to
still undefined stimuli), with consequent degeneration and reactive
macrophage and connective tissue accumulation, have been proposed as
major initiating phenomena (Benditt, 1977; Ross, 1981). The
subsequent phase of the lesion involves repair mechanisms that cause
further thickening of the intima. Following this, lipid increases
both in the cells and in the intercellular spaces. Lipid
accumulation is progressive, leading to an increased number of foam
(lipid-containing) cells which disintegrate and form the gruel-like
substance that has given the name of atheroma to the lesion. The
accumulation of such material acts as an irritant, inducing a
proliferative reaction (encapsulation) which leads to the
development of a plaque. Calcification follows, making the arterial
wall more rigid. Alternatively, when the capsule breaks, an ulcer
can occur, leading to loss of tissue in the arterial wall, blood
clots, haemorrhage, and consequent thrombosis and/or rupture of the
arterial wall.
2.2.6.4 Theories of atherosclerosis
Atherosclerosis is undoubtedly a multifactorial process, which
is reflected by the many theories proposed (Baker & Rogul, 1987).
The lipid accumulation theory is based on the progressive
accumulation of oxidized lipids (mainly low density lipoproteins)
not only in smooth muscle cells but also in migrating monocytes
(Avogaro et al., 1983). The link between lipid deposition and
consequent alterations remains unclear (McCaffrey et al., 1988).
Theories on monoclonal proliferation of smooth muscles cells
are based on a mutagenic event in these cells, due to a
physico-chemical or viral insult (Benditt, 1977; McCaffrey et al.,
1988), leading to the formation of a kind of benign tumour. The
thrombogenic theory is based on the early adherence of platelets to
small alterations in the endothelium, leading to thrombus formation
and release of growth factors by platelets which induce smooth
muscle cell proliferation (Ross, 1981; Bang et al., 1982). Other
risk factors that should be taken into consideration are
hypertension, cigarette smoking, increased body weight, high serum
uric acids and consumption of saturated fats.
The immune system in the elderly is weakened. Consequently
there is increased susceptibility to chronic infections,
autoimmunity, and elevation of circulating immune complexes. New
data in the literature indicate that some viral agents may cause
cardiac and arterial cell lesions and subsequent inflammation. Thus,
viruses and altered immune cells may cooperate and play a role in
arterial wall lipid accumulation, possibly acting as initiating
factors for atherosclerosis (Butenko, 1985).
2.2.7 Respiratory function
Respiratory function is based on gas exchange (oxygen
absorption and carbon dioxide elimination), gas transport (red blood
cells), and on internal metabolic processes that utilize oxygen at
the cellular level. Aging may affect all of these processes (Masoro,
1981), but it is the first that is usually most compromised in
advanced age. The decline in the gas-exchange system may involve the
lungs, the thoracic cage, the respiratory muscles and the
respiratory centres in the CNS. The deterioration of the lungs is
largely dependent on environmental factors, in particular on the
contamination of air with toxic substances, dust and microbiological
agents. Therefore, age-related lung alterations may vary according
to life styles (smoking, physical exercise), environmental
conditions (urban/rural), and intercurrent diseases (infections,
work-related diseases) (Davies, 1985).
2.2.7.1 Gas-exchange organs
The most evident lung alterations with advancing age are
represented by enlargement of alveolar ducts with flattening of
alveoli and loss of septal tissue, reduction of elastic fibres, and
increased fibrosis of the capillary system (Liebow, 1964).
Functional consequences are a reduction in the surface area for gas
exchange with an increase in the physiological dead space, reduction
of the ventilatory flow rate, and irregular distribution of blood
flow (Mauderly, 1978). Age-related alterations also occur in the
chest as a consequence of calcification of costal cartilage,
increased stiffness of costovertebral and vertebral joints, and
general rigidity of the chest. Both lung and chest alterations
contribute to the changes in lung volume and pressure: vital
capacity decreases, residual volume increases, and flow rates
(particularly expiratory flow rate) decline (Morris et al., 1971).
The alteration in the gas-exchange capacity causes reductions in
oxygen uptake and pressure and lower arterial pO2, whereas pCO2
remains constant even in very old age (Morris et al., 1971). The
control of ventilation by brain centres is also altered in old age.
It is still unknown whether such alterations are due to intrinsic
damage of the neural component or to a reduced responsiveness of
neuromuscular activity in the chest (Peterson et al., 1981).
All these alterations, while not necessarily life-threatening
for the elderly, may favour pathologies such as chronic bronchitis,
pneumonia and emphysema (Peterson et al., 1981). The concomitant
hypoxia (low oxygen levels) may cause increased production of red
blood cells with consequent polycythaemia. This may contribute to
hypertension and cardiac failure.
2.2.7.2 Erythropoietic activity
Although specific age-related alterations in the life cycle of
erythrocytes have been reported (Danon, 1969), the overall function
of the erythropoietic system seems to be well preserved. The
regenerative potential following hypoxia seems nearly inexhaustible.
In addition, haemoglobin turnover does not seem to be affected by
age (Lipschitz, 1987).
2.2.8. Kidney and body fluid distribution
The urinary tract is affected by aging both in its renal
functions of excretion and ionic control of body fluids, and in its
control of bladder and urethral activity.
2.2.8.1 Renal function
Kidney function decreases both due to anatomical and
physiological alterations with age (Wesson, 1969; Kaysen & Myers,
1985; Brown et al., 1986; Corman & Michel, 1986; Owen & Heywood,
1986; Meyer & Bellucci, 1986; Anderson & Brenner, 1986, 1987;
Goldstein et al., 1988; Euans, 1988; Rudman, 1988). These
alterations have been observed in both experimental animals and in
humans.
The weight and volume of the kidney decrease by 20 to 30%
between the ages of 30 and 90 years. The atrophy is primarily
cortical and seems to be related to intrarenal vascular changes. The
number of surviving nephrons is reduced and these remaining nephrons
tend to be enlarged (Kaysen & Myers, 1985; Brown et al., 1986;
Lindeman, 1986; Rudman, 1988). The number of glomeruli decreases by
30 to 50%, and there is an increasing percentage of sclerotic and/or
abnormal glomeruli. The glomerular filtration rate (GFR) decreases
with age resulting in an adaptive increase in glomerular perfusion
pressure (Kaysen & Myers, 1985; Lindeman, 1986; Meyer & Bellucci,
1986; Anderson & Brenner, 1986, 1987; Blum et al., 1989). This
decline in GFR is due in large part to the progressive reduction in
blood flow to the kidneys (Brown et al. 1986; Lindeman, 1986).
Glomerular mesangial volume increases by 50% and 1 out of 10
glomeruli is sclerotic at the age of 80 years compared with 1 out of
100 in the young adult (Brown et al., 1986). The renal tubules
decrease in number, proximal tubule volume and length decrease, and
distal tubules develop increased diverticula. The renal arterioles
develop intimal thickening, reduplication of the lamina elastica
interna, and mild hyalinization (Brown et al., 1986; Lindeman, 1986;
Rudman, 1988).
An age-related reduction in secretory and resorptive capacity
is seen in the tubules, which is explained by a progressive loss of
functioning nephrons (Lindeman, 1986). Tubular function, which
regulates water and salt balance, is also affected. A decrease in
the ability to concentrate urine with age has been well documented
in humans. This appears to result from a decreased medullary
tonicity caused mainly by an inability to respond normally to
antidiuretic hormones (ADH) (Kaysen & Myers, 1985; Brown et al.,
1986; Meyer & Bellucci, 1986; Lindeman, 1986; Euans, 1988). Despite
age-related decreased renal function, the blood pH, partial pressure
of carbon dioxide, and serum hydrogen carbonate concentration of the
geriatric population without renal disease do not differ
significantly from those of the young under basal conditions
(Lindeman, 1986). Both the ability to maximally dilute the urine and
to maximally concentrate it, are controlled by serum ADH and by the
action of that hormone on the collecting ducts (Kaysen & Myers,
1985; Os et al., 1987). Increased arginine-vasopressin (AVP)
secretion per unit of plasma reflects a decrease in collecting
tubule sensitivity to AVP. This change in sensitivity is not
completely offset by increased ADH release (Davis & Davis, 1987).
The suppression of ADH secretion is not maximal when serum
osmolality is reduced.
The renin-angiotensin-aldosterone system is also poorly
responsive to volume depletion in aging subjects. As a result, the
elderly cannot maximally retain sodium under conditions of plasma
volume contraction (Kaysen & Myers, 1985; Lindeman, 1986; Euans,
1988). The activity of the renin-angiotensin system is progressively
reduced with age. It has been suggested that angiotensin II does not
play an important role in the maintenance of blood pressure and
kidney hemodynamics in normal senescence (Corman & Michel, 1986).
The mean blood pressure is correlated with age and the decline in
renal function (Lindeman et al., 1987).
The kidney is also the site of vitamin D1 hydroxylation, which
is dramatically reduced during aging (Kaysen & Myers, 1985).
2.2.8.2 Lower urinary tract
The most frequent age-related alterations in the lower urinary
tract result in urine incontinence in both sexes and urine retention
in males. Incontinence occurs at high incidence although it is not
an inevitable consequence of aging. The high number of physiological
requirements for urinary continence may account for such frequent
failure (Williams & Pannill, 1982). In women, the reduction of
estrogen levels after menopause may decrease the tone of the smooth
muscle around the pelvic floor and the bladder outlet, thus
favouring urinary incontinence. In men, hypertrophy of the prostate
represents the major cause for involuntary loss of urine, because of
the associated frequent instability of the detrusor muscle. Other
causes for urinary incontinence are represented by delirium,
infections, restricted mobility and polyuria.
2.2.9 Gastrointestinal function
Changes in the gastrointestinal tract during aging consist
mainly of a reduced cell turnover, leading to mucosal hypoplasia.
Accessory organs, such as the exocrine pancreas and the liver, are
affected by aging independently.
2.2.9.1 Gastrointestinal tract
In contrast to the cardiovascular or excretory system, the
gastrointestinal (GI) tract does not exhibit marked structural or
functional changes with age (Penzes, 1984). Aging may affect all
regions of the GI tube. The first signs of aging are generally
observed in the mouth. Teeth undergo discoloration, pulp recedes
from the crown, dentine is often poorly renewed, and the gingiva
frequently recede (Walker, 1985). These alterations favour bacterial
growth which can lead to chronic periodontal inflammation and tooth
loss.
In the stomach, reduced secretion of hydrochloric acid and
pepsin is often found in the elderly. These changes result from
alterations in enzyme-secreting cells or from altered hormonal and
neural regulation. However, in some cases, increased acid secretion
may occur with advancing age, leading to gastritis, erosions and,
ultimately, ulcers (Kumpuris, 1983).
No major morphological alterations are observed in the
intestine. The relatively mild modification of villi, the increased
collagen content and the reduced mucosal cell proliferation
(Webster, 1985) cannot account for the impaired absorption of
nutrients, including minerals (calcium, iron and zinc) and vitamins
often found in the elderly. Other factors such as reduced motility
and inadequate intestinal blood supply may play a more important
role than the slightly altered anatomical integrity.
In the lower GI tract the rectal tone is generally decreased in
the elderly and the sphincter is weakened, leading to incontinence.
Neurological alterations (dementia), muscle atrophy, diarrhoea and
constipation can all contribute to anal incontinence.
2.2.9.2 Pancreas
Pancreatic function is not seriously compromised during normal
aging. Structural changes mainly involve a reduction in the number
of secreting cells, with a consequent decrease in size of the
pancreas and a moderate increase in collagen content. Reduction in
the levels of trypsin, hydrogen carbonate, amylase and lipase in the
pancreatic juice occurs commonly in the elderly (Vellas et al.,
1988). Overall, however, the functional efficiency of the pancreas
is not lost during aging.
2.2.9.3 Liver
Structural changes in the liver with age are relatively minor.
However, the most significant change in human liver is a decrease in
volume (Wynne et al., 1989). The life-span of the hepatocyte is
long, with cells only dividing once or twice during the lifetime in
the absence of a growth stimulus (Popper, 1986). Whether the
function of the aging hepatocyte is impaired is unclear. Kupffer
cell function may be impaired as demonstrated by a reduction in
phagocytic activity. Endocytosis is reduced in Kupffer, but not
endothelial, cells. An increase in collagen also characterizes aging
liver.
The function of aging liver also undergoes a number of
alterations. In humans, cholesterol synthesis is reduced in the
elderly, while biliary secretion is increased (Popper, 1986).
Hepatic blood flow is reduced by as much as 50% (Sherlock et al.,
1955). Age-related changes in liver enzyme expression have been
studied in more detail in rodents (Fishbein, 1991). The efficiency
of carbohydrate and intermediary metabolism is decreased in
senescent rats or mice of both sexes, due in part to decreased
insulin-binding and the impaired regulation of enzymes such as
pyruvate kinase. Hepatic protein synthesis also decreases with age,
and the sex-specific expression of drug and steroid-metabolizing
enzymes in male rats appears to be feminized in senescence (Kitani,
1991).
2.2.10 Musculo-skeletal system
Aging dramatically affects bone, joints and skeletal muscles.
The basic phenomena responsible for the aging of the
musculo-skeletal system are not completely understood because of the
involvement of many factors, e.g., hormones, nutrition and physical
exercise, in addition to specific age-related alterations at the
tissue level, resulting in general deterioration of the system.
2.2.10.1 Bones
Bone should not be considered as metabolically quiescent since
it is constantly remodelled according to mechanical demand and
continuous turnover due to new bone formation (by osteoblasts) and
bone resorption (by osteoclasts) (Exton-Smith, 1985).
With aging, the balance between bone formation and bone
resorption is altered in favour of resorption, resulting in a
reduction of bone mass. This starts from the medullary region with
enlargement of the cavity and moves towards the epiphysis of the
bone and then to the outer surface with a final reduction of cortex
thickness. As a consequence, the bone strength is reduced (Smith
et al., 1981). The prolonged period where bone resorption exceeds
bone formation may result in osteoporosis (Dawson-Hughes et al.,
1987; Eastell & Riggs, 1987; Riggs, 1987; Croucher et al., 1989).
The factors responsible for bone resorption and remodelling are
not well known. More knowledge is available concerning two other
factors which profoundly affect bone metabolism: calcium turnover
and hormonal profile. Calcium is absorbed through the intestinal
mucosa and is excreted from the kidney, the blood level remaining
remarkably constant throughout life. Calcium is required for many
essential functions in the body, such as cell division, cell
intermediary metabolism, and cell functions (excitability, secretion
and movement). In the case of a negative balance, calcium is
mobilized from the bone with a consequent reduction in bone mass.
Calcium metabolism is under hormonal control. Parathyroid
hormone increases the plasma calcium level by promoting bone
resorption and mobilization, whereas calcitonin lowers blood calcium
by inhibiting bone resorption. Calcitriol (the active derivative of
vitamin D) increases intestinal absorption and decreases excretion
of calcium, thus acting to increase the body burden of calcium. The
age-associated decrease in intestinal calcium absorption lowers the
plasma calcium level, leading to an increase in parathyroid hormone.
Calcitriol levels may also be deficient in the elderly, as a result,
perhaps in part, of the decrease in vitamin D synthesis in old skin
and alterations in renal metabolism.
Bone metabolism also depends on other hormonal factors.
Estrogens have a strong positive effect on bone density. After
menopause, bone resorption exceeds bone formation resulting in bone
mass loss. However, the occurrence of osteoporosis greatly depends
on the adult bone peak mass, physical activity, and calcium intake
(Eastell & Riggs, 1987; Riggs, 1987; Bornor et al., 1988; Stevenson
et al., 1988; Lindsay, 1989).
Glucocorticoids lower plasma calcium levels, which can increase
bone resorption by stimulating parathormone secretion. Thyroid
hormones may also cause bone loss, although the mechanism is still
unclear. Growth hormone, somatomedins and insulin all promote bone
formation, and therefore defective secretion (as in diabetes) may
cause osteoporosis.
2.2.10.2 Joints
Articular disorders are practically universal in elderly
people, and are associated with pain and disability. The major
alteration consists of the loss of the smooth surface of cartilage,
which becomes thicker and less elastic. The cartilage develops rough
surfaces and mechanical irregularities, which represent the early
steps in osteoarthritis (Evens & Hawkins, 1984).
2.2.10.3 Skeletal muscles
With advancing age muscles become smaller in size, due to
reduction in the number of fibres, and less elastic, due to an
increase in collagen content. The fat content of muscle tissue also
increases with age. The causes for such defects are still
controversial: alteration of sarcoplasmic reticulum, decrease of
contractile protein synthesis and a reduction in the number of
mitochondria are all found in aged muscle cells.
In addition to muscle cell alterations, the neuromuscular
junction is modified in aged muscle. The acetylcholine content of
nerve terminals is consistently reduced and acetylcholine receptors
are irregularly distributed. Motor nerve conduction velocity is also
impaired. All these defects contribute to the progressive failure of
muscular efficiency, although some muscles, such as the diaphragm,
do not seem to suffer age-related effects (Finch & Hayflick, 1977).
Thus, the additive results of the loss of bone mass, disorder of
joints, decline in skeletal muscle power and nervous system
discoordination may lead to loss of body stability, falls and bone
fracture in old people.
2.2.11 Skin
The age-related modification of the skin is the most dramatic,
common and constant event in life, so that it may constitute one of
the best external markers of aging. Only recently has it been
possible to distinguish between intrinsic aging of the skin and
photo-aging (Gilchrest, 1984). Cutaneous neoplasia is one of the
best documented interactions between aging of the organism and
environmental effects. A variety of environmental factors, the most
notable being ultraviolet radiation, has been shown to cause cancer
of the skin in humans and animal models (Rogers & Gilchrest, 1990).
The structure of skin changes throughout life (Behl et al.,
1987), although many of the alterations are due to exposure to
sunlight rather than intrinsic aging (Gilchrest, 1984). Skin
thickness increases during maturation and then gradually declines
(Vogel, 1983). However, recent studies in mice and rats by
Monteiro-Riviere et al. (1991) have not reported any significant
changes in skin thickness or blood flow from maturity throughout the
life span. Hydration, which can affect chemical solubility,
decreases while keratinization increases.
Skin aging is linked to alterations affecting both the
epidermal and the dermal components. The epidermis suffers from a
reduction in the turnover of epidermal cells, due both to a
reduction of basal cell renewal and to a slower maturation toward
the stratum corneum. The dermo-epidermal interface is flattened so
that the total external body surface area is reduced (Selmanovitz
et al., 1977). The reduction in epidermal cell turnover is
responsible for the slowing of wound healing and possibly for the
dryness and/or roughness of aged skin. The dermis is reduced in
thickness in the elderly and is characterized by a reduced collagen
content with a biochemical modification of the collagen itself,
which makes it more strong and less elastic. Vascularization in
humans is also reduced.
Aging also results in modifications in the skin appendages.
Sweat gland function is decreased resulting in impaired
thermoregulation. Sebaceous glands produce less oil and wax (dryness
of skin), and hair usually loses pigment. The numbers of skin
sensory organs (Pacinian and Meissner's corpuscles) decrease with
age and sensation is consequently modified.
Collagen aging occurs in the skin, as in other tissues, leading
to increased rigidity. Collagen is composed of several related
proteins, which are produced by fibroblasts and extruded into the
extracellular space. Here they can be chemically deposited in a
nearly pure form, as in the tendons, or immersed in an extracellular
matrix, also produced by fibroblasts, as in the skin. The collagen
fibres undergo maturation in the extracellular ground substance by
parallel arrangements of tropocollagen fibres which became assembled
together through cross-linking. This phenomenon is an index of
maturational change. With age, however, the cross-linking increases
(Houck et al., 1967), leading to a reduction of tensile strength and
plasticity (Verzar, 1968). The extracellular matrix also shows
age-related changes, since alterations in its physicochemical
composition lead to increased density, reduced permeability and
impaired transport of nutrients (Imayama & Braverman, 1989).
3. BASIS OF ALTERED SENSITIVITY TO ENVIRONMENTAL CHEMICALS
The perception of altered sensitivity of the elderly to
environmental insults is based in large part on the enhanced
incidence of adverse or idiosyncratic drug reactions in this segment
of the population (Vestal et al., 1985). The clinical pharmacology
of the aged has been extensively reviewed in the last two decades
(Triggs & Nation, 1975; Crooks et al., 1976; Reidenberg, 1980;
Vestal et al., 1985), and evidence indicates that the response to
drugs changes with age, as does the frequency of adverse drug
reactions (Krupka & Vener, 1979). This may be in part related to
issues of polypharmacy and compliance (Weber & Griffin, 1986).
Morbidity and malnutrition, which are often associated with aging,
could also contribute to altered pharmacokinetics in the elderly
(Kitani, 1988). However, it is clear that age-related differences in
drug/chemical disposition (pharmacokinetics/toxicokinetics) or
sensitivity (pharmacodynamics/toxicodynamics) also play a role in
altered responses to chemicals in the elderly. For sake of
simplicity, the terms "pharmacokinetics" and "pharmacodynamics" will
be used for both drugs and environmental chemicals.
3.1 Pharmacokinetics
Pharmacokinetics describes the processes of the absorption,
distribution, metabolism and excretion of drugs or other chemicals
in the body. The numerous physiological and biochemical changes that
occur during aging can modulate any of these processes of
disposition. Changes in chemical disposition (pharmacokinetics being
the mathematical description of these processes) can lead to an
altered dose or dose-rate to the target tissue resulting in changes
in response. This topic has been the subject of several recent
reviews (Sellers et al., 1983; Van Bezooijen, 1984; Stevenson &
Hosie, 1985; Blumberg, 1985; Birnbaum, 1987, 1989, 1991; Ritschel,
1988; Loi & Vestal, 1988; McMahon & Birnbaum, 1990a).
The highlights in this field will be covered as well as the
more recent data, especially that focusing on age-related
alterations in the pharmacokinetic behaviour of xenobiotics, as
opposed to drugs.
3.1.1 Absorption
The absorption of drugs and environmental chemicals, defined as
uptake into the blood, occurs primarily via the skin, lungs and
gastrointestinal (GI) tract. Alterations in the structure and
function of these three organs clearly occur with age. In contrast
to the skin and GI tract, little is known about the effects of aging
on pulmonary absorption of xenobiotics. Few age-related differences
have been reported in lung structure (Stiles & Tyler, 1988),
although the lung volume is greater in old rats. An age-related
decrease in respiratory function has been reported in several
species (Mauderly, 1979a,b, 1982). In addition, there appears to be
a decrease in the rate of alveolar-capillary gas exchange in older
organisms (Mauderly, 1979b).
Dermal exposure represents a major portal of entry for
environmental chemicals. Several studies have indicated a decline in
human skin permeability with aging (Christophers & Kligman, 1965).
In experimental animals, the scarce data suggest that percutaneous
absorption is decreased in senescent rodents as compared to young
adults. The chemical absorption of
2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD) and related chemicals
is greatest at weaning (Jackson et al, 1990), decreases at maturity
and then undergoes a further decline during aging (Banks et al.,
1990).
Age-related alterations in GI absorption have been studied in
more depth. A decrease in gastric acid secretion is commonly seen in
the elderly (Bender, 1968). The resulting increase in pH can alter
the ionization of compounds, enhancing or retarding their ability to
diffuse passively across cellular membranes. Decreases in gastric
motility (Lin & Hayton, 1983) can prolong the transit time of
chemicals in the gut, thus enhancing their potential for absorption.
In rats, splanchnic blood flow declines in the first year of life
but stays unchanged in the second year (Yates & Hiley, 1979; Kitani,
1988). In contrast, splanchnic blood flow declines progressively
with age in humans (Sherlock et al., 1955). An increase with age in
mucosal weight (Holt et al., 1984; Hebert & Birnbaum, 1987), as well
as intestinal epithelial cell proliferation (Holt et al., 1988), has
been reported in rats.
GI absorption can be active, passive or involve phagocytosis.
Xenobiotics are primarily absorbed by passive diffusion. Age-related
changes in the oral absorption of a variety of drugs in people have
not been observed (Castleden et al., 1977b; Stevenson et al., 1979;
Greenblatt et al., 1988). The passive absorption of TCDD in the
small intestine does not change with age in rodents (Hebert &
Birnbaum, 1987), nor does that of many small endogenous molecules,
such as glucose (Eastin & Birnbaum, 1987), vitamin A (Hollander
et al., 1986), vitamin D, vitamin B12 and niacin (Fleming &
Barrows, 1982a,b) and many amino acids (Penzes, 1974).
Active transport appears to decrease with age (Eastin &
Birnbaum, 1987). Doubek & Armbrecht (1987) have demonstrated that
the decrease in the carrier-mediated component of glucose transport
in rats occurs in the brush-border membrane of the small intestine.
Their suggestion that the decrease is due to a reduction in the
number of sodium-linked glucose carriers has been supported by
recent studies in human intestinal tissue (Vincenzini et al., 1989).
The active transport of other small molecules such as calcium and
phosphorus (Armbrecht, 1986), and galactose and iron (Reidenberg,
1980) has also been reported to decrease with age. As with the
active transport of glucose, the marked decrease in calcium active
transport of calcium occurs between young adulthood and middle age
(Mooradian & Song, 1989) and results from changes in the number of
calcium transporters in the intestinal basal lateral membranes
(Armbrecht et al., 1988).
3.1.2 Distribution
The distribution of chemicals throughout the body is governed
by the fact that the physicochemical properties of the compound
affect its transport within the body and localization to various
tissues. Lipophilic molecules readily pass across cellular membranes
and accumulate in lipid-rich tissues. Binding to proteins also
modulates distribution, since few compounds are transported free in
the blood. While there is no evidence of alterations in relative
blood volume with age, changes in body composition, blood flow and
macromolecular binding have all been documented.
The decrease in lean body mass in both animals (Lesser et al.,
1973) and humans (Novak, 1972) has been well documented. Loss of
body water with age (Edelman & Leibman, 1959) results in a decrease
in the volume of distribution for water-soluble compounds, leading
to enhanced toxicity of ethanol (York, 1982) and ethylenediamine
(Yang et al., 1984). Body fat can account for approximately 15-40%
of the total body weight in humans (Ritschel, 1983) and rats
(Bertrand et al., 1980; Birnbaum, 1983). The increased size of the
fat compartment in older, sedentary animals would be expected to
increase the body burden of lipid-soluble substances and reduce the
overall rate of elimination from older animals. Following an
inhalation exposure of old rats to methylchloroform (Schumann
et al., 1982a,b), a better fit to a physiologically based
pharmacokinetic (PbPk) model was obtained by increasing the volume
of the fat compartment from 7 to 18% of the body weight for rats and
from 4 to 18% for mice (Reitz et al., 1988). Similar improvements
were noted by Lutz et al. (1977) in their PbPk model of
polybrominated biphenyl compounds in rats.
Not only does cardiac output decrease with age (Bender, 1965),
but regional blood flow can change differentially (Yates & Hiley,
1979). Since adipose tissue volume increases but blood flow
decreases with age, lipophilic compounds tend to show greater
retention in the elderly. This has been shown for polychlorinated
biphenyls (Birnbaum, 1983) and halogenated solvents (Schumann
et al., 1982a,b). A clinical pharmacokinetic study (Klotz et al.,
1975) which demonstrated a 5-fold increase in the distribution
volume of diazepam in the elderly is in agreement with the data on
experimental animals.
Binding to blood components can also change with age. Although
the total plasma protein content does not change dramatically with
age, there is a small but significant reduction in albumin in both
animals (Rodgers & Gass, 1983) and humans (Bender et al., 1975). For
drugs or xenobiotics that can be bound, such a decrease in albumin
enables a higher concentration of free drug to reach the target
site. A decrease in binding of drugs to red blood cells has also
been reported to occur during aging (Chan et al., 1975), again
leading to a higher level of free drug.
3.1.3 Metabolism
As with absorption and distribution, there are physiological
changes that occur during aging which can influence
biotransformation reactions, both in the liver and in extrahepatic
tissues. Although the liver is the main site for the metabolism of
drugs and environmental chemicals, significant metabolic reactions
occur in all the portals of entry tissues (skin, respiratory tract,
GI tract) as well as the kidneys, gonads, adrenals, etc. Metabolism
is often considered to be divided into two types of reactions: phase
I (functionalization reactions) and phase II (conjugation
reactions). Phase I reactions tend to increase the polarity of
chemicals, and the corresponding enzymes include the
cytochrome-P450- and FAD-containing monooxygenases as well as the
alcohol and aldehyde dehydrogenases, monoamine oxidases, nitro- and
azo-reductases, esterases and amidases. Phase II reactions involve
the conjugation of functional group (either already present on the
chemical or as a result of phase I activity) with an endogenous
cofactor such as an amino acid (glycine, glutamate), peptide
(glutathione), sugar (glucuronic acid) or a small molecule such as a
sulfate, acetate or methyl group. These reactions may occur on
cellular membranes or in the cytosol, and are regulated in a
tissue-specific manner.
The only generalization that can be made concerning age-related
changes in biotransformation is that few patterns exist. Metabolic
changes with aging appear to be substrate, sex, strain and species
dependent (Schmucker & Wang, 1989; Kitani, 1988; Birnbaum, 1989;
McMahon & Birnbaum, 1990b). In addition to intrinsic changes related
to altered physiology, the study of the influence of age on
metabolism is further complicated by the effects of diet, alcohol,
drugs and pollutants, which can induce or inhibit enzyme activities
(Ritschel, 1988).
Most of the research on the effects of aging on metabolism has
focused on the microsomal mixed-function oxidases. The protein
components of this system have been reported to decline or remain
unchanged with age. In general, the decline in total cytochrome P450
levels reported in rats appears to be due to the age-related
decrease in the amount of the male-specific forms (Kamataki et al.,
1985a,b; Sun et al., 1986). This results from the age-related
decrease in circulating testosterone levels (Kitani, 1985) or to
alterations in the secretory profiles of growth hormone (Fishbein,
1991). This pattern is most pronounced in rats, few changes being
observed in other rodents or sub-human primates (Birnbaum, 1987).
However, recent human studies have suggested that some degree of
sexual dimorphism does exist in hepatic drug metabolism. Plasma
antipyrine half-lives were prolonged in elderly males, but not
elderly females, as compared to young adults (Greenblatt et al.,
1988). This was caused by an age-related reduction in clearance,
which has been interpreted as being the result of an impairment of
antipyrine metabolism in elderly men. Similar gender-specific
results were observed for the kinetics of chlordiazepoxide, another
low-clearance oxidatively metabolized drug (Loi & Vestal, 1988).
A study by Chengelis (1988a) has supported the earlier report
of Rikans (1984) that measuring components of the monooxygenase
system cannot lead to predictions of toxicity. Sex differences in
rats were only significant up to one year of age. However, Rikans
(1989a) has recently reported that metabolism of specific substrates
(aniline, benzphetamine and nitroanisole) does decline with age in
the female as well as in the male rat, but the magnitude of change
in the female is smaller. This agrees with a study by Bitar &
Shapiro (1987), who suggested that an age-related increase in haem
degradation plays a role in the decreased metabolism of hexobarbital
and aniline. This observation, that microsomal drug metabolism
activities do not have to be sexually dimorphic to be altered in old
age, implies that factors other than loss of the male-specific
cytochromes P450 contribute to the age-associated alterations in
some rat strains. Decreases in NADPH cytochrome P450 reductase in
rat liver with age have been reported by Blanco et al. (1987),
Chengelis (1988a) and Rikans (1989a).
Despite a large body of data obtained from experimental animal
studies, there is no clear evidence that monooxygenase activities
decline in the livers of healthy elderly humans. All the data
derived so far from human liver biopsy specimens have shown no
correlation between enzyme activities expressed per mg protein and
age of subjects (Boobis & Davies, 1984; Schmucker et al., 1990). The
often reported decreases in clearance values of drugs metabolized
primarily by the liver in the elderly may in large part be accounted
for by the decrease in liver volume with age (Kitani, 1988). Further
evidence is needed to determine whether hepatic drug-metabolizing
enzyme activities in humans decline with age, as is observed in some
rodents, but the extent is unlikely to be as drastic as that
observed in male rat liver.
A change in the composition of cytochrome P450 enzymes,
suggested by differential changes in enzyme activity, has been
demonstrated in male rats (Kamataki et al., 1985a; Sun & Strobel,
1986). Leakey et al. (1989a) showed that dietary restriction could
also alter the profile of cytochrome P450 isoforms, possibly by
delaying the age-related demasculinization of the liver. Friedman
et al. (1989) showed that aging affected the composition of
testosterone-binding cytochromes P450 in the liver of male rats.
Changes in P450 isoforms in old rats were also reported by
Paramonova & Dovgi (1987). Studies with humans have demonstrated
that the effect of age on metabolism even varies for different
metabolites of the same parent compound (Posner et al., 1987). This
supports the observation of changes in cytochrome P450 isoform
composition with age, as has been reported for male rat liver.
Hepatic xenobiotic metabolism can be modulated by many factors.
In humans, smoking often confounds studies of age-related changes in
pharmacokinetics. However, the age-related decrease in the
metabolism of antipyrine (Loft et al., 1988), theophylline and
cortisol (Crowley et al., 1988) may be independent of smoking
status. In fact, the inductive properties of smoking on hepatic
metabolism do not appear to diminish with age. Phenytoin also
increased theophylline metabolism to an equal extent in both young
and old healthy men (Crowley et al., 1988). In contrast, Rath &
Kanungo (1989) demonstrated that the rate of transcription of the
phenobarbital-specific isozymes was nearly two-fold higher in young
rat liver than in old. However, these differential effects may
reflect the specific isoforms involved, since smoking and
phenobarbital preferentially induce different forms. The recent
studies of Rikans (1989b), which demonstrate that induction of
hepatic microsomal drug metabolism by ethanol or acetone is
unaffected by the aging process, lend support to this concept.
Age-related changes in phase I hepatic drug-metabolizing
enzymes other than the mixed-function oxidases have been subjected
to much less examination. Rikans & Moore (1987) demonstrated an
age-related increase in liver alcohol dehydrogenase in male rats.
However, no age differences have been observed in the activity of
this enzyme in female rats. Hydrolytic reactions may decrease with
age. For example, liver esterase activity, using diethylhexyl
phthalate as a substrate, declines in old rats (Gollamudi et al.,
1983). Using aspirin as a substrate, no correlation was found
between age and esterase activity in human liver (Yelland et al.,
1991). These results provide further evidence that age is not a
major determinant of hepatic drug metabolism in the elderly
(Schmucker et al., 1990).
Alterations with age in extrahepatic phase I metabolism also
appear to be substrate, sex, strain and species specific. Pulmonary
metabolism of benzo[a]pyrene was found to increase in old rats (Sun
& Strobel, 1986) in agreement with earlier studies of Rabovsky
et al. (1984). In contrast, oxidation of 2-aminofluorene, which is
catalysed by a different isoform of cytochrome P450, decreased
(Robertson & Birnbaum, 1982). Renal metabolism of acetaminophen has
been reported to decrease with age (Beierschmitt & Weiner, 1986),
while salicylate oxidation by kidney extracts did not change in rats
(Kyle & Kocsis, 1985). Age-related alterations in intestinal Phase I
metabolism appear to be site specific. Sun & Strobel (1986) reported
that oxidation of benzo [a]pyrene in the colon increased throughout
the life of rats, while McMahon et al. (1987) observed no
age-related change in the metabolism of this substrate in the small
intestine. Newaz et al. (1983) observed higher metabolic rates of
dimethylhydrazine in colonic tissue from aging humans as compared to
younger individuals. McMahon et al. (1989) suggested that plasma
esterase hydrolysis of benzyl acetate may decline with age in both
rats and mice. Alcohol dehydrogenase activity was found to remain
unchanged in the aging colon (McMahon et al., 1987).
Changes in phase II enzymes also appear greatly variable in
rodent liver, although Loi & Vestal (1988) conclude that age has
little effect on phase II reactions in humans. The major conjugation
reactions involve sulfation, glucuronidation or reaction with
glutathione leading to mercapturic acid formation. All these
reactions are catalysed by multiple isoforms showing varying degrees
of substrate and sex specificity, as is the case for the phase I
enzymes. Iwasaki et al. (1986) demonstrated differential age effects
on two distinct sulfotransferases using male and female rats and
various alcohols and amines. Sulfation of phenolic substrates
appears to decline with age (Galinsky et al., 1986; Sweeny & Weiner,
1986) while conjugation of bile salts increases in old male rats
(Galinsky et al., 1986). In contrast, changes in female rats were
not seen (Galinsky et al., 1990). Chengelis (1988b) observed no
significant changes in sulfotransferase activity with age in either
male or female rats using beta-naphthol as the substrate. Similar
results were seen by Leakey et al. (1989b) for the conjugation of
both estrone and naphthol, whereas the sulfation of androsterone and
corticosterone increased with age in male rats.
Hepatic glucuronidation also shows substrate specificity for
alterations with age. Depending on the substrate, increases with
estrone and acetaminophen (Galinsky et al., 1986), decreases with
the rubber antioxidant 4,4'-thiobis-(6- t-butyl- m-cresol)
(Borghoff et al., 1988), or no effect with naphthol,
p-nitrophenol, morphine and testosterone have been observed
(Galinsky et al., 1986; Sweeny & Weiner, 1986) in male rats. In
contrast, Chengelis (1988b) observed a marked decrease in senescent
male and female rats using both p-nitrophenol and chloramphenicol.
To complicate matters further, Leakey et al. (1989b) also saw a
decrease in naphthol, testosterone, androsterone and
tetrahydrocortisone conjugation with glucuronic acid, but observed
no effects of aging on conjugation with 2-aminophenol,
5-hydroxytryptamine, bilirubin or estrone. Tarloff et al. (1989b)
saw no change in acetaminophen glucuronidation with advancing age.
Although the activities of the UDP-glucuronosyltransferases appear
highly variable, an age-related decrease in hepatic levels of the
cofactor, UDP-glucuronic acid (UDPGA) (Borghoff et al., 1988),
suggests that glucuronidation could be limited in older animals.
The concentration of hepatic glutathione, the cofactor involved
in the third major class of conjugation reactions, has been reported
to increase (Borghoff & Birnbaum, 1986), decrease (Stohs et al.,
1982) or remain unchanged (Chengelis, 1988b; Rikans & Moore, 1988)
in old rodents. Variability in age effects has also been reported in
the activity of the glutathione- S-transferases, which exist as a
family of dimeric proteins having broad and overlapping substrate
specificity. The isozymes are composed of two subunits from at least
six different peptides, including both homo- and heterodimers. Thus
reports of an increase (Leakey et al., 1989b), decrease (Fujita
et al., 1985; Blanco et al., 1987; Leakey et al., 1989b), or no
change (Birnbaum & Baird, 1979; Sweeny & Weiner, 1985; Borghoff &
Birnbaum, 1986; Chengelis, 1988b; Leakey et al., 1989b; Carrillo
et al., 1991) in hepatic glutathione-S-transferase activity may, as
in the cases of cytochrome P450 mixed-function oxidases,
sulfotransferases and UDP-glucuronosyltransferases, reflect
age-dependant changes in isozyme ratios (Spearman & Leibman, 1984;
Carrillo et al., 1991). Restriction in dietary protein decreases the
activity of the glutathione-S-transferases more in old than young
rodents (Carrillo et al., 1989, 1990).
Much less has been reported on the effects of aging on other
phase II metabolic reactions in the liver. Hydrolysis of expoxides
to form diols or dihydrodiols is catalysed by the epoxide
hydrolases. Epoxide hydrolase activity has been reported both to
increase (Birnbaum & Baird, 1979) and decrease (Ali et al., 1985;
Kaur & Gill, 1985; Leakey et al., 1989b) with age. Again, this may
reflect differential age effects on multiple enzymatic species.
However, some of the difference may be due to the ages of animals
used for comparison, since Chengelis (1988b) reported a gradual
increase in epoxide hydrolase activity throughout much of the life
span, followed by an abrupt decrease in senescence.
Human studies have suggested that the alterations observed in
salicylate pharmacokinetics (Cuny et al., 1979) with age might be
due to a decrease in glycine conjugation (Kyle & Kocsis, 1985).
However, elevated levels of salicyluric acid, the glycine conjugate
of salicylate, have been observed in elderly humans undergoing
chronic salicylate treatment (Montgomery & Sitar, 1981). Recent
studies in rodents have not demonstrated any age-related decline in
glycine conjugation with non-nephrotoxic doses of salicylate
(McMahon et al., l990a) or in the formation of hippuric acid from
benzoate (McMahon et al., 1989). Acetylation, however, has been
reported to decline both in man (Bauer et al., 1989) and rats
(Leakey et al., 1989b).
Changes in extrahepatic phase II reactions have been
investigated in even less depth than extrahepatic phase I reactions.
In the lung and small intestine, glucuronidation of p-nitrophenol
appeared not to change with age (Borghoff & Birnbaum, 1985), whereas
in the colon an age-related decrease was reported by McMahon et al.
(1987). In contrast, colonic glucuronidation of
4-methylumbelliferone rose significantly in older rats (McMahon
et al., l990b). A decrease in UDP-glucuronosyl transferase activity
in the kidney (Borghoff & Birnbaum, 1985; Tarloff et al., 1989a) was
accompanied by a decrease in the renal concentration of UDPGA
(Borghoff et al., 1988).
Glutathione content has been examined in a variety of tissues
(lung, kidney, brain, testes and blood) and, except for an
age-related decrease in the lens, has been found to remain constant
(Rikans & Moore, 1988). Unchanged glutathione levels in the colon
occurred although the activity of glutathione- S-transferase
decreased in this tissue (McMahon et al., 1987). Spearman & Leibman
(1983, 1984) observed differential age- and sex-related changes in
this activity in the lung depending on the substrate examined. The
renal activity of glutathione- S-transferase declines significantly
in old rats (Beierschmitt & Weiner, 1986). In contrast, elevated
gluthathione- S-transferase levels were observed in the brain of
old rats (Blanco et al., 1987), while no changes were observed in
the heart.
Epoxide hydrolase activity has been examined in the lungs,
small intestine and kidneys by Kaur & Gill (1985). They observed a
decrease in lung and small intestine activity in old rats which was
substrate dependent. In the rat kidney, epoxide hydrolase activity
decreased using trans-stilbene oxide as the substrate but did not
change with cis-stilbene oxide, supporting again the differential
effects of aging on different isozymic forms of drug-metabolizing
enzymes. In addition, deacetylation of acetaminophen in the kidney
appears to be either unchanged with age (Beierschmitt & Weiner,
1986) or slightly decreased (Tarloff et al., 1989b). Acetylation in
the kidney also decreases with age (Wabner & Chen, 1984).
One additional enzyme which can be considered to fall into the
phase II class is beta-glucuronidase. This enzyme hydrolyses
glucuronic acid from conjugated xenobiotics. The activity of this
enzyme has been reported to increase with age in rat liver
(Schmucker & Wang, 1979; Van Manen et al., 1983) and kidney
(Borghoff & Birnbaum, 1985), but remain unchanged in the colon
(McMahon et al., 1987). However, beta-glucuronidase was found to
decrease with age in fecal contents (McMahon, 1988). The balance
between glucuronidation and deglucuronidation reactions may play a
role in determining the level of reactive compounds in the organism.
Depending on the chemical, aging can result in either an
increase or a decrease in the metabolizing capacity of different
organs and tissues (liver, kidney, gastrointestinal tract, lungs and
skin). The elevation or reduction in metabolism can both lead to
higher and lower toxicity, depending on the relative reactivity of
the metabolic intermediates and end-products. Thus studies on the
effect of aging on metabolism should be considered case by case.
3.1.4 Excretion
Excretion leads to the elimination of a chemical and/or its
metabolites from the body. The kidney is the major excretory organ,
with the liver and lung also playing important roles in the
elimination process. In addition, sweat, saliva and sex-linked
processes such as lactation can serve as routes of excretion.
The effects of age on renal function appear to play a major
role in altered pharmacokinetics in the elderly (Vestal, 1978;
Koch-Weser et al., 1982). The fact that changes in the physiology of
the kidney occur has been known for many years (Schmucker, 1979).
Renal blood flow decreases with age leading to a decrease in
glomerular filtration rate. Tubular secretion and resorption are
also reduced in the elderly. The number of functional nephrons
declines to a similar extent as the decline in glomerular filtration
rate and active secretion, suggesting that the nephron loses its
function as a unit (Friedman et al., 1972). Decreases in renal
function can result in a decreased rate of renal clearance, leading
to a greater potential for elevated and/or persistent levels of
chemicals in the body which could lead to toxicity (Sellers et al.,
1983). In humans, decreased renal clearance in the elderly has been
demonstrated for many drugs, including the aminoglycosides,
tetracyclines, lithium, digoxin, procainamide, methotrexate, and
phenobarbital (Kampmann & Hansen, 1979).
Aging rodents are extremely susceptible to chronic
glomerulonephropathy (Goldstein et al., 1988), much of which may be
attributable to diet (Masoro & Yu, 1989). Altered glomerular
morphology, characterized by thickening of the basement membrane and
sclerosis, is progressive and increases in severity with advancing
age, eventually resulting in scarring and loss. Renal tubules are
also subject to degenerative changes, which are accompanied by
proteinuria, especially albuminuria (Neuhaus & Flory, 1978). This
appears to result from increases in glomerular permeability and a
loss of fixed glomerular polyanion (Baylis et al., 1988). The high
percentage of urinary protein represented by albumin in the aging
rat may be due to non-selective protein leakage into the urine
resulting from increases in glomerular permeability to large
proteins, since the protein percentage in urine approaches that in
the plasma of senescent rats (Horbach et al., 1988a). Similar
albuminuria has been observed in aging mice (Yumura et al., 1989),
and was correlated with glomerular sclerosis. Such changes, however,
should not be simply extrapolated to humans, since there is no
evidence for an increase in protein loss in the urine of the healthy
elderly.
Additional tubular changes occur in the aging kidney, resulting
in hyperplastic and degenerative changes. Some of these changes
resemble responses to specific environmental chemicals (Konishi &
Ward, 1989). A decrease in renal transport of organic acids has been
observed (Wabner & Chen, 1984). Aging appears to diminish the
turnover of sodium/potassium ATPase in the proximal tubules (Marin
et al., 1985), which could play a role in the observed age-related
decrease in tubular secretion.
Hepatic elimination may also be compromised by aging. Kitani
(1985) has suggested that the excretory capacity of the liver
decreases with age due to some functional alteration in the
hepatocytes. In contrast to rats, where blood flow does not change
after maturity (Kitani, 1988), blood flow to the liver declines in
humans (Sherlock et al., 1955). Bile flow rate has been reported to
be reduced (Borghoff et al., 1988) or remain unchanged (Kitani
et al., 1985a) in rats. Biliary transport declines, especially in
the case of polar compounds (Kitani et al., 1985a). The elimination
of sulfobromophthalein, a model compound for the study of biliary
excretion of organic anions, is also decreased in old rats (Kanai
et al., 1985). Sato et al. (1987) demonstrated that the biliary
excretion of the neutral glycoside ouabain decreases with age in
both males and females. This may be due to an age-related decrease
in hepatic uptake, resulting in less biliary elimination (Ohta
et al., 1988). In addition, the biliary canalicular transport system
declines steadily during aging (Kanai et al., 1988). The decreases
in both hepatic uptake and biliary excretion may reflect changes in
the hepatocyte plasma membrane (Zs-Nagy et al., 1986).
3.2 Pharmacodynamics
Age-related changes in chemical sensitivity cannot all be
explained on the basis of altered pharmacokinetics in the elderly.
Pharmacodynamic changes occur at the target site and may involve
changes in cell populations, cellular receptors, cellular
responsiveness or in the regulation of the amount or activity of
drugs, including the cardiac glycosides, benzodiazepines, tricyclic
anti-depressants, and the non-steroidal anti-inflammatory agents
(Bender, 1979), which have demonstrated altered receptor sensitivity
in the aged (Wilson & Hanson, 1980).
3.2.1 Central nervous system
Numerous studies have shown that, with advancing age, there is
a decrease in the ability of the nervous system to synthesize and/or
release neurotransmitters and neuropeptides (for review, see Rogers
& Bloom, 1985). This decline may reflect the fact that there are
fewer neurons present to synthesize the chemical messenger or that
the enzymes involved in their synthesis are altered. This would
imply that xenobiotics which lead to neuronal death or interfere
with neurotransmitter or neuropeptide synthesis and release could
have a greater adverse effect on the elderly than on the young
organism. An example of such an interaction has been noted within
the dopaminergic extrapyramidal circuits controlling motor movements
(nigrostriatal pathway). This pathway has been the subject of many
experimental and clinical studies and provides one of the best
functional units in which to study neurotoxicity in the aged.
Nigrostriatal neurons are lost as a normal correlate of aging, and
while these losses may not be expressed in the majority of
individuals as movement disorders, there is a clear reduction in the
functional reserve of this dopaminergic system, making it more
vulnerable to neurotoxicants. This has been demonstrated by the
observed acceleration or simulation of "age-associated" movement
disorders by neurotoxicants such as
1-methyl-4-phenyl-1,2,5,6-tetrahydro-pyridine (MPTP).
The reduced capacity to synthesize neurotransmitters that
occurs in the aged organism may also potentiate the effect of toxic
substances. Carbon disulfide is an organic solvent with a variety of
industrial applications that produces neurobehavioural dysfunctions
(Wood, 1981). The mechanism of CS2 toxicity seems to be inhibition
of dopamine-beta-hydroxylase, the enzyme that converts dopamine to
norepinephrine (McKenna & DiStefano, 1977). Since norepinephrine
metabolism declines with age, the spectrum of physiological effects
regulated by this catecholamine would be expected to suffer greater
disturbance, following exposure to CS2 or to pesticides that
reduce brain catecholamines such as methyl bromide (Honma et al.,
1987), in old rats compared to young ones.
Toxic compounds that serve as neurotransmitter receptor
blockers could have their effects on behaviour accentuated, making
neuroendocrine control of homeostasis more difficult in the elderly.
For example, the formamidine pesticides, amitraz and chlordimeform,
have been shown to block alpha-noradrenergic receptors (Costa &
Murphy, 1987; Costa et al., 1988) and induce a variety of
behavioural disorders (Boyes & Dyer, 1984; Hsu & Kakuk, 1984;
Landauer et al., 1984) in rats. Since there is an age-related
decline in noradrenergic receptors in several species (Rogers &
Bloom, 1985), exposure to these compounds may have a more pronounced
effect on the older organism.
Certain movement disorders associated with senescence may be
related to age-related impairments in the brain dopaminergic systems
(Marshall & Berrios, 1979). Waddington et al. (1985) observed a
decrease in the density of brain dopamine receptors in old rats,
with no changes in affinity. In contrast to young animals, the
receptors of old animals were not able to respond effectively to
long-term treatment. The decrease in dopamine receptor density was
selective for the D-2 receptor subtype, although the coupling
between D-1 receptors and adenylate cyclase appears to be affected
by aging. A similar loss of D-2 receptors in the elderly has also
been measured using positron tomography in the living human brain
(Wong et al., 1984). An age-related decrease in binding to the
serotonin receptor may relate to cerebral dysfunction in the elderly
(Shih & Young, 1978).
Studies with rats have indicated that the increased sensitivity
of older rats to diazepam is due to pharmacodynamic differences
(Guthrie et al., 1987). Pedigo et al. (1981) suggested this could
relate to changes in the benzodiazepine/GABA/chloride ionophore
complex. Human studies have also indicated that the site of
increased sensitivity to the benzodiazepines lies distal to the
receptor (Swift, 1985), possibly involving changes in the chloride
ionophores.
Alterations in endogenous opioid systems may play a role in
some of the behavioural changes observed in the elderly. Binding of
dihydromorphine to the opiate receptor decreases in specific areas
of the brain in aged rats as compared to young ones (Messing et al.,
1980). This reduction is due to a decrease in receptor number, with
no change in affinity. Despite a relatively large body of evidence
that some receptors and neurotransmitters are at least qualitatively
altered with aging, it is still not clear whether and how these
changes are causally related to increased sensitivity of the CNS to
certain drugs with aging, as described below.
The brain appears to exhibit increased sensitivity to
phenobarbital (Kitani et al., 1985b; Van Bezooijen et al., 1989) in
both mice and rats. Such enhanced sensitivity with age to an
anticonvulsant supports earlier studies with phenytoin (Kitani
et al., 1984). It is possible that the aging brain of both
experimental animals and humans has increased sensitivity to all CNS
depressants. Enhanced brain sensitivity has also been reported for
hexobarbital in rats (Van Bezooijen et al., 1989) and oxazepam in
mice (Kitani et al., 1986). The aging human brain appears to be more
sensitive to nitrazepam (Castleden et al., 1977a) and other
benzodiazepines (Reidenberg, 1980). Recently, zonisamide, a new drug
whose anticonvulsant properties are distinct from the other drugs,
was demonstrated to have an increased anticonvulsant effect in aging
mice (Kitani et al., 1987). Taken together, these results suggest
that these pharmacodynamic changes with age may reflect a decreased
response capability for seizures in the elderly, rather than a
specific age effect on all the distinct receptors (Kitani et al.,
1986). In fact, the lethal threshold for pentylenetetrazole in mice
has been shown to decrease with age (Nokubo & Kitani, 1988), coupled
to an increase in the threshold for maximal seizure.
The action of other environmental compounds on CNS function may
be more diffuse. Recent studies have demonstrated enhanced
sensitivity of old mice to cyanide intoxication (McMahon & Birnbaum,
1990b). Exposure to various metals has been shown to alter a variety
of CNS functions. There has been a particular interest in a
potential link between Alzheimer's disease and aluminium toxicity.
It was initially reported that the autopsied brains of Alzheimer's
patients showed elevated aluminium levels (e.g., Perl & Brody,
1980). However, others have found no such correlation. Marksberry
et al. (1981) compared the brains of Alzheimer's patients with older
adult controls and found no correlation between the density of
aluminium content and the density of neurofibrillary tangles and
neuritic plaques. However, a more recent study showed that the brain
aluminum levels were significantly increased as a function of age in
both control and Alzheimer patient populations (Bjorksten et al.,
1989).
Manganese is a metal that causes age-type neuropathy. However,
unlike aluminium, it is associated with extrapyramidal disorders,
characterized by intention tremor (Donaldson, 1987). In monkeys,
the neurological symptoms of choreo-athetoid movement, rigidity and
tremor occurred after 18 months of manganese exposure. These
clinical signs, in association with severe lesions of the globus
pallidus and subthalamic nucleus, resembled Parkinson's disease and
suggested a possible link between environmental exposure and
occurrence of the disease in aging individuals.
The neurotoxic effects of other metals have been widely
studied, particularly those of lead, which has been implicated in
reduced intellectual abilities in children (Needleman et al., 1979),
slowed reaction time (Hunter et al., 1985), and impairment of other
cognitive abilities (Winneke et al., 1982; Hansen et al., 1985).
While psychophysiological parameters appear to be relatively
insensitive to low levels of lead exposure, several studies have
demonstrated both cognitive and emotional effects due to long-term
exposure to lead (Hogstedt et al., 1983; Mantere et al., 1984).
Long-term exposure to mercury vapour has been reported to interfere
with verbal intelligence and memory performance (Piikivi et al.,
1984). Hanninen (1982) suggested that abnormalities due to mercury
exposure affect the motor system and result in intellectual
impairment, a gradual and progressive deterioration of memory
function, and emotional disability. Other metals including copper,
iron and manganese have also been reported to cause CNS dysfunction
(Grandjean, 1983). However, there are no available data on the role
of exposure to these metals in dysfunction of the CNS in the aged.
Examples of naturally occurring neurotoxic agents that
stimulate nervous system senescence have been reported in certain
island populations. For example, the Chamorro peoples of the
Marianna Island, specifically Guam and Rota, exhibited a high
incidence of amyotrophic lateral sclerosis, Parkinsonism and
Alzheimer's-like dementia that was recently linked to their diet.
The Chamorro's diet consists in part of a flour made from the seeds
of Cycas circinalis. When one of the components of these seeds,
beta- N-methylamino-l-alanine (BMAA), a compound similar in
structure to excitotoxic amino acids, was fed to macaques, they
exhibited signs of motor neuron, extrapyramidal and behavioural
dysfunction (Spencer et al., 1987).
It seems that several motor neuron disorders are associated
with the appearance of endogenous excitotoxic glutamate agonist-type
molecules. One such molecule is 2,3-pyridine dicarboxylic acid
(quinolinic acid), which has been isolated from the brains of
humans, rabbits and laboratory rodents (Moroni et al., 1984) and has
been shown to excite CNS neurons when applied iontophoretically
(Perkins & Stone, 1983). An interesting correlate is the fact that
brain concentrations of quinolinic acid increase with age (Moroni
et al., 1984), suggesting that its presence may result in the
spontaneous onset of neurode-generative conditions that are mimicked
by environmental neurotoxicants such as BMAA and
beta- N-oxalylamino-L-alanine (BOAA).
3.2.2 Endocrine system
There are several different ways in which the endocrine system
and the hormonal signalling operations involved may undergo
alterations with age and toxicant exposure. These can be categorized
as changes in: (a) the availability of hormones for binding to the
target tissues; (b) the reception of the pertinent transmitter or
hormonal signal by the target cells; and (c) the nature of the
hormonal message.
3.2.2.1 Changes in hormonal availability with age
Age-related or toxicant-induced shifts in synthesis, rate of
clearance and rate of secretion will all function to alter hormonal
concentrations. Such changes in the size of the available signal
pool may have corresponding effects on the magnitude of the response
by the target tissue. These changes may reflect a decline with age
in the homeostatic controls, which rely heavily on endocrine
feedback relationships.
Several toxicants have also been observed to cause changes in
circulating hormonal levels (Cooper et al., 1986). Significant
reductions in serum testosterone, for example, have been seen
following short-term exposure of rats to the plasticizer
dinitrobenzene (Rehnberg et al., 1988b) and the pesticide
chlordimeform, the latter also causing marked reductions in serum
LH, thyroid-stimulating hormone (TSH), T4 and T3 levels. The
effects, moreover, may be remarkably specific. Following three days
of exposure in male rats, the pesticide linuron, for instance, was
reported to decrease the serum T4 level in a dose-related manner,
while leaving T3 and the pituitary and gonadal hormones unaffected
(Rehnberg et al., 1988a).
There is also a growing body of evidence for a hormonal
influence on toxicant metabolism. A sizeable number of xenobiotics,
including both drugs and environmental toxicants, are metabolized by
the hepatic cytochrome P450 monooxygenase system (Nebert & Gonzalez,
1987). Components of this system have been found to be influenced by
glucocorticoids (Schuetz et al., 1984; Simmons et al., 1987) and
markedly affected by sex steroids (Kamataki et al., 1985b) and
growth hormone (Yamazoe et al., 1987; Zaphiropouos et al., 1989).
Consequently, persistent shifts in the circulating levels of such
hormones, as have been reported for the aging animal, could affect
the manner in which xenobiotics are metabolized following exposure.
Reported attenuations with age in the rhythms of human and rat
serum testosterone (Bremner et al., 1983; Steiner et al., 1984;
Tenover et al., 1988), LH (Vermeulen et al., 1989) and GH (Sonntag
et al., 1980), among other hormones, can present differences in
young-versus-old comparisons, depending on when such sampling is
performed. Comparable effects on hormonal rhythms have been reported
to occur in response to toxicant exposure. For example, single
injections of 2,3,7,8-tetrachlorinated dibenzo-p-dioxin (TCDD)
resulted in some evidence of alterations in prolactin and
corticosterone rhythms in rats (Jones et al., 1987). It may be that
an aging system, while still exhibiting rhythmic hormonal changes,
may be increasingly sensitive to their disruption by low toxicant
levels.
3.2.2.2 Changes with age in the reception of the signal by the
target cells
A general decline in the transmitter regulation of hormonal
function may also place an aging animal at increased risk for
toxicant exposure (Govoni et al., 1988), given that various
environmental toxicants (including, for example, the solvents
vinyltoluene, ethylbenzene and styrene, the halogenated hydrocarbon
TCDD, and certain heavy metal cations) have been reported to
interact with catecholaminergic systems (Govoni et al., 1979; Lucchi
et al., 1981; Arfini et al., 1987; Mutti et al., 1988; Russell
et al., 1988).
These changes have been observed for both neurotransmitter and
hormone receptors. There is some evidence of a decreased
responsiveness of target tissues to steroid hormones during
senescence. For example, age-related declines in the concentration
of the estrogen receptor may reflect a decline in the circulating
estrogen levels (Thakur, 1988). Impaired responsiveness could also
be due to reduced receptor translocation (Belisle & Lehoux, 1983) or
other steps in steroid action which occur after hormone binding. In
fact, age-related alterations in glucocorticoid responsiveness are
due to both receptor and post-receptor events (Kalimi, 1982).
Testicular luteinizing hormone receptor concentration and total
content also decrease with age (Amador et al., 1985). In general,
receptors for steroids, insulin, glucagon, catecholamines and
prolactin appear to decrease in concentration with increasing age in
rodents, dogs and humans (Roth, 1979b).
An additional consideration of alterations with age or toxicant
exposure in the reception of a hormonal (or transmitter) signal by
target cells concerns not only effects on the receptor itself, but
changes in the cell's membranes. In the aging rat brain, there is
evidence for a progressive decrease in membrane fluidity
(Hershkowitz, 1983; Nagy et al., 1983). This is at least partially
attributable to alterations in the lipid composition. For example,
it has been reported that aging is associated with elevations in
cholesterol, sphingolipids and saturated fatty acid chains, all
leading to increases in rigidification (Rouser et al., 1972). Since
protein activity in the membrane is influenced by the fluidity of
the lipid micro-environment, any alterations with age in membrane
viscosity may affect not only receptor functions but also enzymatic
activity.
3.2.2.3 Changes in the nature of the hormonal message with age
The antigenic site(s) on a hormone recognized by antibodies can
be quite distinct from those regions that bind to the receptors and
trigger a physiological response in the target tissue. This
distinction may have an added importance for studies in aging, since
alterations with age in peptide hormone structure have been reported
(Conn et al., 1980) that reflect changes in post-translational
processes. These effects, moreover, may be influenced by shifts in
the steroid hormonal milieu in the older animal (Ulloa-Aguirre
et al., 1988). A number of hormones are glycosylated to varying
degrees and such differences in their carbohydrate residues may
alter biological activity (Ulloa-Aguirre & Chappel, 1982; Warner
et al., 1985) and/or plasma half-life (Morell et al., 1971).
Consequently, hormonal measures based solely on immunoreactivity
per se potentially offer a somewhat inaccurate picture of
endocrine alterations with age.
3.2.3 Kidney
The aging kidney appears to be more susceptible than the young
one to drug-induced nephrotoxicity as well as to renal ischaemia. In
fact, cortical tubules of senescent rats seem more sensitive to
oxygen deprivation than do those of young rats (Miura et al., 1987).
Thus, increased susceptibility of the aging kidney can be
independent of pharmacokinetic effects.
Age-related susceptibility to nephrotoxicity has been
demonstrated for numerous drugs including salicylate, acetaminophen,
cephaloridine and doxorubicin. Kyle & Kocsis (1985) showed that
kidneys from older rats develop nephrotoxicity to a greater extent
than do those of young rats following an equal dose of salicylate on
a body weight basis. However, the dose to the kidney could be
greater in the older rats due to a decrease in the volume of
distribution. In addition, McMahon et al. (l990a) has recently
demonstrated that at toxic doses old rats produce more reactive
metabolites of salicylate than do young ones. The age-related
enhancement in cephaloridine nephrotoxicity may also be due to
pharmacokinetic changes (Goldstein et al., 1986), although renal
cortical slices from old rats demonstrated alterations in active
uptake of organic anions and cations following exposure to the
antibiotic, which were not observed in young rats. Doxorubicin
treatment resulted in greater toxicity in old rats (Colombo et al.,
1989). The onset of the delayed nephrotoxicity was noticeably faster
in the old rats; this may have been related to higher drug retention
in the kidneys of old rats.
Age-related increased susceptibility to acetaminophen
nephrotoxicity has been investigated more than that of any other
chemical in rats. Tarloff et al. (1989b) stressed that while
enhanced sensitivity to acetaminophen nephrotoxicity clearly occurs
with advancing age, great care must be taken in choosing the ages
for comparison. Beierschmitt et al. (1986a) demonstrated by both
functional and histological criteria that susceptibility to
acetaminophen-induced acute tubular nephrotoxicity increases with
age in rats. This may reflect altered susceptibility rather than an
alteration in pharmacokinetic parameters, since the generation of
reactive metabolites from acetaminophen decreases with age
(Beierschmitt & Weiner, 1986). Increasing delivery of acetaminophen
to functioning nephrons, whose numbers are decreased in the aged
kidney, does not appear to be responsible for the age-related
increase in susceptibility (Beierschmitt et al., 1986b).
Alterations with advancing age do not have to result in
increasing sensitivity. Studies by Murty et al. (1988b) indicated a
decrease in hydrocarbon-induced hyaline droplet nephropathy in male
rats during senescence. This male-specific nephropathy is associated
with the presence of alpha2u-globulin, whose synthesis is strongly
age dependent (Roy et al., 1983). Although gasoline causes extensive
nephrotoxicity in young rats, old rats are resistant. In fact,
gasoline failed to alter hyaline droplet numbers in aged male rats,
which also demonstrated an altered lysosomal response as compared to
young rats. This lack of response of aged rats to
hydrocarbon-induced hyaline droplet nephropathy suggests that these
rats would also be resistant to hydrocarbon-induced renal neoplasia.
3.2.4 Immune system
With advancing age a progressive decline in the concentration
of glucocorticoid receptors occurs in the spleen (Roth, 1979a). This
alteration may depend either on an age-related modification of the
ratio among various subsets of lymphoid cells carrying different
receptor densities or on an intrinsic failure of aged cells to
maintain an adequate turnover of receptor molecules. With advancing
age, membrane receptors for hormones of low relative molecular mass
on lymphoid cells also show alterations, although these are more
related to impaired coupling to signal transduction systems (Feldman
et al., 1984; Roth, 1988). It has been shown that the number of
receptor molecules decreases with advancing age whereas the affinity
does not seem to change. In the aged population with altered
function of the immune system, it is possible that immunomodulatory
agents present in the food potentiate immune dysfunction, making the
elderly more vulnerable to age-related diseases.
The effects of immunosuppressive compounds can be identified
from toxicological experiments with young adult rodents. These
chemicals include organotin compounds, some pesticides, halogenated
aromatic hydrocarbons (e.g., hexachlorobenzene and dioxins) and
cyclosporin (Poland & Knutson, 1982; Vos & Penninks, 1987; Vos &
Luster, 1989; Schuurman et al., 1990; Vos et al., 1990), and they
can also potentiate autoimmunity, which is found more frequently in
the elderly.
3.2.5 Other tissues and systems
There are few data on pharmacodynamic changes in other tissues
and systems during aging. An increase in the sensitivity of the
aging human myocardium to digoxin (Chavaz et al., 1974), as well as
to intravenous anaesthetics (Dundee, 1979), has been suggested. The
stomach, kidney and bone marrow are more susceptible to the adverse
effects of non-steroidal anti-inflammatory agents in the elderly
than in the young, and cerebral side effects are more common
(Huskisson, 1983). The anticoagulation properties of warfarin are
also changed in the elderly, with the aged demonstrating increased
sensitivity (Shepherd et al., 1979).
Although in most toxicological studies the cardiovascular
system is not well examined, there are several chemicals known which
can cause cardiovascular toxic or atherosclerotic effects. Such
compounds in food include erucic acid in combination with
omega-3-linolenic acid, cetolenic acid, brominated vegetable oils,
cobalt salts in combination with ethanol, lead, nitrates and high
amounts of caffeine (Speijers, 1983). On the other hand, compounds
such as saturated fatty acids, odd-numbered cyclopropenoid fatty
acids (sterculia acid), ergot alkaloids, halogenated aromatic
hydrocarbons and cadmium induce toxic effects on the vascular system
and possible potentiate atherosclerotic effect (Speijers, 1989a,b).
Conrad & Bressler (1982) investigated the cardiovascular effects of
caffeine in elderly men. Caffeine, in doses equal to those contained
in 2 to 3 cups of coffee, produces an increase in blood pressure,
but has no positive inotropic effect in healthy elderly men.
Kim & Kaminsky (1988) suggested that age plays an important
role in modifying susceptibility to the toxic metabolite of
fluroxene, 2,2,2-trifluoroethanol. They noted greater effects on the
stomach, liver, testicles, brain and kidneys of aged rats as
compared to younger animals. Enhanced sensitivity to ethanol
intoxication has also been noted in old rats (Guthrie et al., 1987),
suggesting altered tissue sensitivity. Acute ethanol hepatotoxicity
may be due to enhanced liver susceptibility to the toxin (Rikans &
Snowden, 1989).
3.3 Modifying factors
3.3.1 Nutrition
Nutrition has been shown to influence the aging processes in
rodents and the occurrence and progression of age-associated
diseases in rodents and humans. Moreover the age-associated changes
in physiological processes affect the nutrition of the mammalian
organism. However, there is little information on how aging
influences the nutritional requirements of humans, a subject that
urgently requires scientific study.
Nitrates in foods can be reduced to nitrites in the oral
cavity, GI tract and urinary bladder (Tannenbaum et al., 1978).
Nitrites react with amines in the stomach forming N-nitroso
compounds, many of which have been shown to be carcinogenic in
animal studies, and it is difficult to deny their hazard to man
(Searle, 1976; Bartsch, 1991).
During commercial processing and domestic preparation, foods
may become contaminated with toxic chemicals. For example, smoked
and grilled foods contain small amounts of polycyclic aromatic
hydrocarbons and a wide variety of phenols and other organic
compounds derived from smoke (IARC, 1990). Canned foods can become
contaminated with tin or lead.
In a study by Spagnoli et al. (1991), both 4-month-old and
4-year-old New Zealand white rabbits were fed an atherogenic diet.
Increased incidence and degree of atherosclerotic lesions were seen
in the 4-year-old rabbits. These results showed an increased
susceptibility of the older arterial wall to hypercholesterolaemia.
Although 4-year-old rabbits are still relatively young, considering
the life span of this species (10-12 years), this study suggests
that aging arteries might be vulnerable to atherogenic compounds.
Malnutrition associated with inadequate intake or uptake of
nutrients in the elderly is caused by several factors. These include
socio-economic conditions (Munro, 1984) such as a) ignorance of the
need for a balanced diet, b) poverty, c) social isolation, d)
physical dependence (disability) and physical inactivity, e) mental
disorders, and f) changes in habits, e.g., retirement (Munro, 1984;
American Dietetic Association, 1984; Ferro-Luzzi et al., 1988).
Other conditions resulting in malnutrition include malabsorption due
to a variety of intestinal conditions, alcoholism, and the use of
therapeutic drugs that interfere with nutrient utilization and
therefore with the toxicity of chemicals (Krupka & Vener, 1979;
Kohrs, 1981; Munro, 1984; Chen et al., 1985; Robertson et al, 1988).
Malnutrition in much of the aged population can enhance the
vulnerability of the elderly to the effects of the toxic chemicals
in food. It has also been reported that malnutrition alters
pharmacokinetics in different ways depending on the drugs examined
(Roe, 1983; Cusack & Denham, 1984). This can be another factor in
the altered sensitivity of the malnourished elderly to toxicants.
Major deficits or marginal nutrient status occur for proteins,
calcium, vitamins A, C and B (thiamin, riboflavin, niacin, B6 and
B12) and zinc (American Dietetic Association, 1984; Ferro-Luzzi
et al., 1988).
In aged women, a deficiency in calcium is especially associated
with osteoporosis (American Dietetic Association, 1984; Munro, 1984;
Caraceni et al., 1988; Cauley et al., 1988). The deficiency in iron
and zinc might play a role in haematological status and immune
function, while a higher intake of aluminium might have neurological
sequelae (American Dietetic Association (ADA), 1984). An iron
deficiency might make individuals more vulnerable to compounds toxic
for the haematopoietic system, e.g., hexachlorobenzene and lead.
Data showing that older individuals have a reduced need for
energy, due to slower metabolism and decreased activity, has an
important impact on the intake of both macro and micronutrients,
because the composition of the diet is based on the average caloric
or energy intake (American Dietetic Association (ADA), 1984;
Ferro-Luzzi et al., 1988). Considering this reduced need for energy,
the elderly often reduce their intake of nutrients.
Dietary manipulation can alter life span in laboratory animals
(Barrows & Kokkonen, 1978; Young 1979). Restricting food intake in
laboratory rats produces a significant extension of life span (McCay
et al., 1935; Ross, 1961). Similar effects have been observed in
mice (Weindruch & Walford, 1988; Kubos et al., 1984), as well as in
more primitive organisms including fruit flies (Harman, 1981), and
nematodes and Neurospora (Harman, 1982). Other studies have
indicated that the reduction in caloric intake that accompanies
protein restriction, and not the protein restriction per se, is
responsible for the increased longevity (Leto et al., 1976; Davis
et al., 1983; Schneider & Reed, 1985). Dietary restriction has been
shown to be effective when started as late as middle age (Cheney
et al., 1983; Kubos et al., 1984; Masoro, 1988). Food restriction
appears to act either by influencing primary aging processes or by a
general protective mechanism, rather than directly modulating
multiple specific pathogenic processes underlying specific diseases
(Masoro et al., 1991).
Chronic nephropathy in rats has been reported to be retarded by
food restriction (Saxton & Kimball, 1941). Rats fed ad libitum a
semisynthetic diet of 21% casein as the protein source exhibited a
marked age-associated progression of chronic nephropathy, whereas
rats fed 60% of the ad libitum intake developed almost no
age-related progression of this disease process (Maeda et al.,
1985). Caloric restriction appears to be more important than protein
restriction in retarding nephropathy (Maeda et al., 1985). Fat or
mineral restriction was found to have no influence on longevity
(Iwasaki et al., 1988).
Dietary restriction delays the age-dependent loss of adipocyte
responsiveness to hormones, prevents the decline in serum free fatty
acid levels, delays the increase in serum cholesterol, and reduces
the increasing triglyceride level observed in aging rats (Cooper
et al., 1977; Liepa et al., 1980; Masoro et al., 1980; Yu et al.,
1980).
Dietary restriction from weaning has been shown to delay the
onset and reduced the severity of chronic nephrosis, periarteritis,
myocardial degeneration and muscular dystrophy in very old animals
(Berg, 1976). Chronic restriction has also been shown to inhibit
certain types of tumours, decrease the incidence of neoplasms, and
increase tumour latency (Ross, 1976; Weindruch et al., 1982;
Weindruch & Walford 1988). Early work using a chemically induced
mammary carcinoma model suggested that the primary effect of caloric
restriction was on tumour promotion (Weindruch & Walford, 1988).
More recent data (Fishbein, 1991) have demonstrated that the
initiation of chemical carcinogenesis can also be decreased by
caloric restriction. For example, exposure to aflatoxin B1
resulted in less DNA-adduct formation in liver from young
calorically restricted male rats than from controls fed ad libitum.
Such changes are most probably due to changes in hepatic cytochrome
P-450 expression (Fishbein, 1991).
There is ample evidence of better maintenance of
T-cell-dependent immunological responses in aging mice chronically
restricted from weaning (Weindruch & Walford, 1988). However, it has
been reported that restriction initiated at an adult age can also
delay the age-specific decrease in immune function (Fernandes
et al., 1977; Friend et al., 1978; Weindruch & Walford, 1988).
Although caloric restriction extends life span in invertebrate
and lower vertebrate species as well as rodents (Weindruch &
Walford, 1988), as yet there is no definitive evidence whether or
not caloric restriction will increase longevity, or decrease
neoplastic and degenerative diseases, in higher mammals or in man.
There is some evidence that reduced caloric intake in man reduces
urinary output of thymidine glycol and 8-hydroxy-guanosine, which
implies reduced free-radical-mediated DNA damage (Fishbein, 1991).
However, until the mechanisms by which caloric restriction evokes
its effect are fully understood, the only way that it can be
conclusively proved whether or not caloric restriction does prolong
life in higher mammals, is to perform longevity studies in these
species. Such experiments, using non-human primates, are underway in
the USA (Fishbein, 1991), but it will be some time before definitive
data are available.
Chronic disease in the elderly is accompanied by caloric
deficit, which in turn causes breakdown of body proteins and
negative nitrogen balance as well as the utilization of fat stored
in adipose tissues. Dietary protein appears to promote
age-associated renal disease in both humans and rats (Brenner
et al., 1982).
When illness occurs, nutritional deficiencies frequently become
clinically manifest (Rudman, 1987). For example, trauma or a fall
causing fractures leads to immobilization resulting in rapid loss of
body stores of nitrogen and calcium. This slows down mending of the
fracture. Similarly, surgical procedures in the elderly often result
in delayed recovery and risks far exceeding those of younger
persons. Heart failure and malignancies can lead to cachexia with
loss of weight, muscle mass and nutrient reserves. Infection may
produce similar changes and intervene to become the terminal event.
3.3.2 Alcohol intake
Ethanol is one of the chemicals most commonly ingested by
humans. Its effects on the body are numerous and varied. Loneliness
and isolation would seem to foster consumption of alcohol beverages
among older persons. The decrease in lean body mass and body water
that occurs with aging is responsible for higher blood levels of
alcohol in elderly people than in younger adults consuming the same
quantities (Vestal et al., 1977).
Elderly individuals have a decreased tolerance for alcohol, due
to an increased sensitivity of the CNS to the depressant effect of
ethanol. The metabolic effects of ethanol are the result of either
the increase in the NADH/NAD+ ratio occurring due to ethanol
metabolism or to direct toxic effects of ethanol or its metabolite,
acetaldehyde. An increase in serum uric acid level (hyperuricaemia)
is common during heavy alcohol ingestion, thus inducing acute gouty
arthritis in patients with known gout. Hypoglycaemia and
hyperlipidaemia occur in patients who are not eating adequately or
who are ingesting a high-fat diet with ethanol, respectively.
Thrombocytopenia is caused by alcoholism in patients with advanced
alcoholic liver disease (IARC, 1988).
The use of drugs increases with aging. Acceleration or
inhibition of drug metabolism by ethanol depends on the duration of
ethanol ingestion and the presence or absence of ethanol in the body
at the time the drug is ingested (Mezey, 1981). The presence of
alcohol in the body causes a decrease in the metabolism of certain
drugs, such as antipyrine, meprobamate, pentobarbital and
benzodiazepines, resulting in increased bioavailability of the drugs
to the CNS and thereby contributing to unwanted side-effects. In
contrast, ethanol can increase the metabolism and metabolic
activation of certain xenobiotics, such as the known human
leukaemogen benzene, thus potentially leading to enhanced toxicity
(IARC, 1988).
Chronic ethanol ingestion increases tolerance to CNS
depressants in young individuals, but its effect in the elderly is
unknown. The concomitant administration of ethanol and barbiturates
results in an enhanced depressant effect of these drugs on the CNS
and can result in coma or even death. All other sedative-hypnotic
drugs tested have either synergistic or additive effects with
ethanol. Intellectual deterioration and dementia are common
complications of chronic alcoholism. Alcoholic patients show more
signs of mental aging at every chronological age (Gaitz & Baer,
1971).
Chronic excessive alcohol ingestion is associated with
increased mortality from cancer, cirrhosis, non-malignant
respiratory diseases such as emphysema, and accidents (Klatsky
et al, 1981; IARC, 1988). However, a decrease in coronary artery
disease and mortality is associated with moderate alcohol ingestion.
This may be due to increases in plasma HDL-cholesterol and decreases
in LDL-cholesterol that occur during ingestion of alcohol (Mezey,
1981). Ethanol consumption is also associated with hypertension and
an increased mortality from cerebrovascular accidents (Kozararevic
et al., 1980; Blackwelder et al., 1980).
3.3.3 Smoking
Smoking clearly plays an etiological role and produces an
acceleration of a wide spectrum of age-associated disease. Smoking
contributes to an increased mortality rate (Gupta et al., 1980). It
also provides an excellent example of problems faced in the
consideration of the environmental impact on aging.
Scientists recognize that smoking presents a complex toxic
insult through inhalation. Increased pathology in aged mice has been
reported after exposure to cigarette smoke (Matulionis, 1984). The
immune response in aged mice exposed to cigarette smoke has been
shown to be decreased at some ages but not at others (Keast & Ayre,
1981). The role of smoking in coronary heart disease has been
reviewed by Kannel (1981), who observed that the relative effect of
cigarette smoking decreases in old age and proposed that this could
be due to the selection of a more resistant population.
Estrogen-related diseases have also been associated with smoking
(Baron, 1984). Reif (1981) has examined susceptibility to lung
cancer and concluded that the shape of the susceptibility
distribution is determined by the effects of all environmental
carcinogens (both known and unknown) to which the population has
been exposed, as well as by differences in genetic susceptibility
among members of the population. Smokers and non-smokers both get
lung cancer during the same age range. Some of these studies suggest
that aged individuals are more susceptible to the effects of
smoking, whereas others suggest that duration of smoking appears to
be the critical factor (IARC, 1990). The smoker is exposed to
multiple toxic agents simultaneously, the effects of which are more
pronounced in aged individuals. Major aspects of metabolism and
pharmacokinetics are altered in aged individuals. Therefore, the
effective dose of any chemical reaching the systemic target tissues
in aged humans would be dependent on these perturbations.
3.4 Interactions of chemicals and diseases
3.4.1 Cancer
Cancer morbidity is expected to rise with age and with an
increasing percentage of elderly people living in industrialized
countries (Magnus, 1982). There is no consensus on the causes of the
age-related increase in tumour incidence. Various arguments support
the concept that an age-related accumulation of total dose of all
carcinogens accounts for tumour induction as a function of age in
sensitive individuals (Peto et al., 1975, 1985). One viewpoint is
that the sensitivity to carcinogens is stable and independent of
age, whereas another is that changes in the internal milieu of the
organism, such as the metabolic and immunological shifts of natural
aging, provide favourable conditions for tumour development with
increasing age (Burnet, 1970; Dilman, 1971).
Comparison of human epidemiological data with in vivo and
in vitro animal experimental results is difficult but does allow
some limited conclusions. It seems that environmental carcinogenic
factors as well as endogenous carcinogens are important causes of
increased tumour incidence in old people (IARC, 1990). This
conclusion is supported by the increasing incidence of occupational
cancer with increased exposure time to carcinogenic agents and by
the correlation of lung cancer incidence with the number of
cigarettes smoked (Doll & Peto, 1981; Peto, 1986). Humans, in
general, have an age-related increase in the incidence of epithelial
neoplasms (Doll, 1978), but the relationship of age to incidence of
other cancers in different organs varies (Doll, 1973; Moolgavkar &
Venzon, 1979; Anisimov, 1987; Dix, 1989). Some tumours appear most
frequently in childhood, some increase exponentially with age, and
others reach a peak at a certain age and then decline.
The data on cancer incidence among the atomic bomb survivors in
Hiroshima and Nagasaki, Japan, have been very informative concerning
radiation-related solid tumours as well as leukaemia. Age at time of
exposure appears to be a strong determinant of leukaemia risk; the
greatest absolute risk was experienced by those who were exposed at
ages 0-9 or 50 years and over (Beebe, 1979). Most of the excess
cancer deaths from solid tumours among the atomic bomb survivors
have occurred among those who were over 35 at the time of the blast.
Analysis of data on the positive correlation between the aging
rates of different species with their cancer rates and the
observation that these two processes, aging and carcinogenesis, may
be initiated and promoted by impairments of gene regulation led
Cutler & Semsei (1989) to conclude that both cancer and aging may
arise from a common set of genetic alterations. The analysis of the
interrelationship between aging and carcinogenesis should be based
on epidemiologically and experimentally confirmed data.
Epidemiological data, analysed in terms of a multi-stage model
(Kaldor & Day, 1987), can estimate the importance of age at onset,
duration of carcinogen exposure, and latency in a population. On the
level of the organism, carcinogenic agents influence not only the
cell, causing genomic damage that leads to neoplastic
transformation, but also create in the cell a microenvironment that
facilitates proliferation and clonal selection (Anisimov, 1987,
1989). Multi-stage carcinogenesis is accompanied by various
disturbances in tissue homeostasis and systems of anti-tumour
resistance that, in turn, are under the influence of systemic
(nervous, hormonal and metabolic) factors. How long it takes for
frank neoplasia to develop depends on the state of those systems at
the moment of exposure to a carcinogen or tumour promoter and the
dose.
According to the multi-stage model of carcinogenesis, the
carcinogen whose effect increases in proportion to age at exposure
affects the partially transformed cell. In this case the tumour
incidence would increase and latency would decrease, as compared to
a population exposed to the same effective dose of carcinogen at a
young age. For example, application of 7,12-
dimethylbenz [a]anthracene in small doses or
12-0-tetradecanoylphorbol-13-acetate to the skin of mice of
different ages caused neoplasms more frequently in older animals
(Stenbäck et al., 1981; Ebbesen, 1985). Exposure of mice and rats of
various ages to phenobarbital resulted in hepatocarcinogenesis only
in old animals (Ward, 1983; Ward et al., 1988). The number of events
necessary for complete malignant transformation in 15-month-old rats
under the influence of N-nitrosomethylurea is lower than in
3-month-old rats (Anisimov, 1988). In every tissue, the number of
events occurring in the stem cell before its complete transformation
is variable and depends on many factors, in particular the rate of
aging of the target tissue and of the regulatory system(s) of the
tissue (Anisimov, 1987, 1989). This model is consistent with the
analysis of age-related distribution of tumour incidence in
different sites in humans and experimental animals (Doll, 1978; Dix,
1989; Anisimov, 1987).
Epidemiological observations have shown that exposure to some
carcinogenic agents leads to a rise in cancer incidence independent
of age at the start of exposure (e.g., smoking), while other agents
induce more tumours when the exposure begins in the elderly (e.g.,
lung cancer following asbestos exposure) (Kaldor & Day, 1987; IARC,
1990).
3.4.2 Other diseases
Aging affects the functional capacity and structural integrity
of many organ systems. Environmental chemicals can also affect
several target organs. The combination of the influence of aging and
the toxic effects of chemicals in the environment might potentiate
the risk for elderly persons. An inherent problem common to all
research in chronic disease is the dissection of the respective
roles of time per se from those of primary aging. Hazzard (1985)
stated that an essential feature of human aging is the change in
physiological competence across the life span, as reflected in
homeostatic reserve. Homeostatic reserve declines at an accelerating
rate with age, normally producing death in old age from
multifunctional etiologies.
The relationship between aging and atherosclerosis is a prime
example of this conundrum. It seems most likely that the changing
picture of atherogenesis in western society has led to a large
number of people who survive into old age with not only a degree of
clinical atherosclerosis, but also with other chronic progressive
diseases, such as chronic obstructive pulmonary disease, immobility
from osteoarthritis and/or osteoporosis, and mental incompetence
from Alzheimer's disease, multi-infarct dementia or other
age-related dementing processes.
These diseases could significantly modify the response of the
organism to various environmental chemicals by decreasing or
increasing their susceptibility, followed by an acceleration of
these diseases or induction of new ones.
4. APPROACHES TO EXAMINING THE EFFECTS OF CHEMICALS ON THE AGED
POPULATION
4.1 Experimental approaches
4.1.1 Principles for testing chemicals in the aged
population
There are two principal approaches to the study of age-related
changes of any functional, morphological and/or biochemical
parameter, i.e. "longitudinal" and "cross-sectional". Longitudinal
studies consist of repeated estimations of any parameter in the same
animal in different periods of life. Cross-sectional studies involve
separate groups of animals of different ages who are examined at a
given point in time. Results obtained using the longitudinal
approach may significantly differ from the results from experiments
carried out using cross-sectional approaches. In studies on the
toxicity of chemicals, it is obligatory that identical conditions be
provided and maintained for all the animals. Problems related to the
choice of animal species, strain, sex, age, life stage and chemical
treatment (both route and dose selection) will be considered below.
4.1.2 Animal models
An extensive discussion of the choice and use of animal models
in research has been recently published (Rogers et al., 1991).
4.1.2.1 Animal species
The selection of suitable species obviously involves both
practical and economic factors. Animals with a short life span are
preferred. However, good life-table information is not available for
all species. A knowledge of species-related differences in metabolic
pathways and inherent sensitivities is also important in choosing
animal species for study. The choice of species should depend on the
experimental question as well as homology of response. For example,
while closely related isoforms of drug-metabolizing enzymes may
exist in different species, their tissue distribution and substrate
specificity may vary greatly (Nebert et al., 1991). Such differences
in isoform expression, together with reported differences in DNA
repair efficiency, could be responsible for species-related
differences in an organism's susceptibility to chemical carcinogens
(Daniel et al., 1983; Mehta et al., 1984; Anisimov, 1987; 1989).
Regardless of such metabolic differences, mice and rats are
most often used in aging research because of a short life span,
relative ease of maintenance under defined conditions, wide use in
biological research, and suitability for a variety of molecular and
genetic analyses. There are extensive life-table information for
some strains of mice and rats as well as a data base on their
spontaneous pathology. If old animals are purchased from a supplier
rather than being maintained in an investigator's own laboratory, it
is necessary to obtain information on the lifetime environment of
the animals, including housing conditions and dietary history. It
may be preferable to keep animals from weaning until the desired age
for investigation under the same controlled conditions.
Mammalian species other than rodents have only been used
sporadically in aging research, owing to their long life span, lack
of life-table information, genetic heterogeneity, restricted
availability and high cost. However, it is critical that such larger
species be used to answer certain questions. For example, studies
are currently underway to determine if dietary restriction can
prolong the lifespan of two different monkey species (Ingram et al.,
1990).
4.1.2.2 Animal strain
The choice of adequate rodent strain for experiments on aged
animals is critical. The male Fischer-344 rats is a popular model in
aging studies because of its size and growth characteristics.
However, testicular interstitial cell tumours begin to appear at the
age of 18 months in rats fed ad libitum, and by the age of 2 years
the tumour incidence approaches 100% (Fishbein, 1991). Recently, a
highly significant positive trend with time has been observed for
the increasing prevalence of leukaemia, anterior pituitary tumours
and thyroid c-cell tumours in both sexes, adrenal pheochromocytomas
in males, and mammary tumours and endometrial stromal polyps in
female F-344 rats (Rao et al., 1990). Some mouse strains suffer
from a single major disease process (e.g., tumours of the mammary
gland or liver, or chronic nephropathy), and the presence of this
disease in most animals could modify the response to chemical
exposure and complicate the interpretation of the results.
In the USA and Japan, Fischer-344 and Sprague-Dawley rats and
B6C3F1, Swiss, and CD-1 mice are most frequently used, whereas
in European countries Wistar-derived and Brown Norway rats and NMRI
and Swiss mice are the most popular strains. Inbred animals have the
advantages of greater stability and predictability of response.
However, heterogeneity of outbred animals more closely resembles the
heterogeneity of human populations. The choice of a certain animal
strain also depends on previous experience with these animals, the
final choice of an appropriate species and/or strain being dependant
on the scientific hypothesis under investigation. Each strain has
its own pattern of background pathology, which can be greatly
influenced by animal husbandry.
4.1.2.3 Animal sex
Sex-differences in response to chemicals are well known. Thus,
the use of both sexes is often necessary in toxicity testing.
However, the large gender differences in chemical toxicity and
pharmacokinetics that occur in rats become less apparent in old age
due to the decreased expression of sex-specific isoforms of the
hepatic drug-metabolizing enzymes (Kitani, 1991).
Ovarian status may significantly influence the sensitivity to
some chemical agents, modifying the biological response, as been
demonstrated for chemical carcinogens (Anisimov, 1971, 1987). It is
noteworthy that, when using females of the post-reproductive period,
the investigator must be aware that a) the ovaries in females of
some species (i.e. rats and mice) are not atrophied and may continue
to secrete steroid hormones, and b) some animals may be in
persistent estrus, while others may be in anestrus or
pseudopregnancy status (Aschheim, 1976). Furthermore, an animal's
reproductive history could influence its response to a chemical in
later life.
4.1.2.4 Selection of age groups for comparison
The problem of appropriately characterizing the animal's age
within the context of its life span is of particular importance in
experimental gerontology. Since the aging process causes significant
changes in various systems of the organism, there is a need for the
definition of some reference points for adequate comparison of the
results of different experiments. The importance of these points is
particularly significant in cross-sectional studies. One of the
confounding factors in such comparisons between experiments is the
need for frequent comparisons of results from different animal
species with different life spans (long-lived and short-lived
species and/or strains). Many authors have used two kinds of age
groups: "mature" or "adult" animals; and "old" animals. However, the
true age of "mature" rats in the reports from different
investigators fluctuates from 2 to 14 months and the age of "old"
animals from 12 to 37 months. The life cycle of experimental animals
can be divided into four periods: a) prior to weaning
(developmental); b) sexual maturation (maturational);
c) reproductive; d) pronounced age-related changes (senescent
period) (Zapadnyuk, 1971). All studies should compare animals from
multiple age periods.
4.1.2.5 Underlying pathology of animals of different ages
As mentioned previously, the background pattern of pathology
must be taken into account in the selection of animal species and
strains. A species-, strain- and sex-specific incidence of
pathology, whether neoplastic or not, is observed to increase
rapidly in the second half of the life span, even for those animals
maintained under specific pathogen-free conditions (Burek, 1978;
Anisimov, 1987; Frith & Ward, 1988; Fishbein, 1991). Among
non-neoplastic diseases in rats, the most frequent are chronic
glomerulonephropathy, cardiomyopathy, amyloidosis, and peripheral
nerve degeneration. For example, the incidence of spontaneous
leukaemia in F-344 rats frequently used in long-term studies may
exceed 30%, and the frequency of intertitial cell tumours of the
testis may reach 100% in old males. This pathology may significantly
modulate the host response to xenobiotics.
4.1.2.6 Transgenic animals
During the last decade it has become possible to add new
genetic information to the germ line of experimental animals
(Jaenisch, 1988). More recently successful attempts have even been
made to specifically alter gene sequences in the mouse germ line,
thereby ablating or correcting specific gene functions (Capecchi,
1989). In the study of the effects of chemicals on the aged
population, transgenic animal models have at least two contributions
to make. Firstly, the influence of specific genes on the age-related
susceptibility to environmental chemicals can be assessed in the
in vivo situation (Vijg & Papaconstantinou, 1990). Secondly, by
using transgenic animals harbouring a shuttle vector with one or
more mutational target genes, mutagenic effects can be studied in
different organs and tissues of animals as a function of age (Gossen
et al., 1989).
4.1.2.7 Animal husbandry
In experimental research on the sensitivity of aged animals and
the aging process, the husbandry aspects should be defined. The
quantitative and qualitative results might depend greatly on the
dietary conditions. The dietary composition should meet the minimal
requirements for nutrients, minerals, vitamins and (raw) fibre for
adult animals according to established and published standards for
laboratory animal diets. Another important dietary factor is the
quantity to which the test animals have access. They may either
receive a restricted diet containing adequate level of nutrients or
be fed ad libitum. The choice of diet depends on the questions to
be asked.
In addition to the dietary status of the animals, their
microbiological status should be defined. In some cases interaction
of the chemical with the gut microflora might modify the outcome of
the study. Specific-pathogen-free (SPF) animals are preferred. The
direct environment of the test animal, including relative humidity,
temperature, light and dark cycles, seasonal influence and stress
can all influence the final outcome of a study and therefore should
be defined carefully (Masoro, 1991).
Differences in animal husbandry may cause large variations in
biochemical and pathological effects. This is clearly illustrated by
alterations in the background data among different laboratories.
4.1.3 Chemical exposure
4.1.3.1 Dose level
The selection of dose is a difficult issue. The usual
requirement is to have a minimum number of test groups (3) plus one
control (vehicle) group. This permits the development of a
"dose-response" curve and allows for appropriate statistical
evaluation of the results. The highest dosage should induce minimal
signs of toxicity, bearing in mind that the maximum tolerated dose
for young animals could in some cases be toxic for old ones, and
that high doses may alter the toxicokinetics of the chemical.
Considering that the body weight of young and old animals could
be significantly different, a correct dosage calculation of test
substances in comparison groups is an important problem, i.e.
whether it is better to normalize per unit of body weight or body
surface, or to some other parameter such as lean body mass (Travis
et al., 1990). The available data indicate similarity of the results
when calculation of dose is performed per unit of body weight or
body surface. It should be taken into consideration that the growth
and development of various organs may have different rates, and that
relative organ weight (and consequently, the effective target dose
of the substance) may not be the same in animals of different ages.
When the substance is administered in food or water, the consumed
quantities should be taken into account, because they may differ in
animals of various ages. When the oral or dermal route of
administration is used, age-related changes in the extent and rate
of absorption may be important. Age-related changes in lung
ventilation capacity may also alter the internal dose of a chemical
when the inhalation route of administration is used. When looking
for portal-of-entry effects, a constant concentration of the agent
may be used in all age groups.
4.1.3.2 Route of administration
There is general agreement that the test substance should be
administered by the route that corresponds most closely to human
exposure. For humans, the main exposure routes are oral, dermal and
inhalation, while in animal experiments the oral route is most
frequently used. However, when pharmacokinetic studies show that
other routes of administration result in equivalent target tissue
levels, such alternative routes can be used. The use of injections
(subcutaneous, intraperitoneal, intramuscular or intravenous) may be
expedient under certain circumstances.
4.1.3.3 Duration of exposure
The question being addressed should guide the design of the
study. If the potential risk involves acute exposure of the elderly,
then an acute experimental scenario is required. If human exposure
is ongoing, long-term studies may be necessary. Exposure to the test
substance in chronic studies should start shortly after weaning and
continue for the major portion of the animal's life (at least
through the mean life span). IARC (1986) recommended 24 months of
exposure for rats and mice, and 18 to 20 months for hamsters when
studying the carcinogenic potential of chemicals. Choice of exposure
duration based on life-table characteristics would be optimal.
However, these guidelines do not apply to experiments when animals
of different ages are used at the start of the study. In this case
the dose and duration of exposure could differ in groups of young
and old animals. However, it is preferable to use identical exposure
durations whenever possible.
4.1.4 Non-mammalian models
Many species can be used as models in the study of age-related
sensitivity to environmental toxicants. These include fungi
(Neurospora crassa and Podospora anserina), protozoa
(Paramecium tetraurelia and Tetrahymena pyriformis), rotifers,
nematodes (Caenorhabditis elegans, C. briggsae and Turbatrix
aceti), and insects (Drosphila melanogaster, Musca domestica and
Tribolium confusum) (Committee on Chemical Toxicity and Aging,
1987). Non-mammalian species such as fish and salamanders have been
used in aging research (Weindruch & Walford, 1988) and are
potentially available for toxicology studies. Several of these
models have already been used to examine the effects of chemicals on
aging. Because of their short life span, ease of use and relatively
low cost, non-mammalian organisms could be important in the initial
phases of a test system to identify environmental chemicals that
might affect aging.
4.1.5 In vitro studies
For the purposes of screening xenobiotics, the use of in vitro
models can result in substantial economy and efficiency. Stock cells
and, in some cases, tissue explants can be cryopreserved in large
amounts. This permits repeated assays with comparable materials and
the sharing of common stocks by numerous laboratories. Moreover,
such stocks can be used to investigate cell-to-cell interactions,
such as metabolic cooperation and metabolic transformation. Finally,
tissue culture approaches can substantially reduce the numbers of
animals required for experimentation. Such methods cannot, however,
be expected to substitute for the intact animal experiment.
There are three general categories of in vitro methods: organ
culture, tissue explants and cell culture. Organ culture involves
the short-term maintenance of variable intact segments of tissue,
for example, the full thickness of a segment of aorta. In tissue
explants, the early migration and proliferation of epithelial and
fibroblast cell types can be observed. Cell cultures are of four
general types:
(a) primary cell cultures and mass cultures of cells taken directly
from the animal, usually after enzymatic dispersion of biopsied
tissue;
(b) established, serially passaged cultures with relatively
reproducible cycles of growth in early phases, but with limited
replicative life span, and with a genetic make-up reflecting
that of the donor age;
(c) "transformed" cell cultures with indefinite replicative
potential and generally with altered genetic makeup;
(d) established or transformed cell lines into which specific DNA
sequences ("transgenes") have been transfected.
Each of these types of models could prove useful for studies of
the effects of environmental agents on the elderly. One general
approach would be to explore the toxic effect of an agent as a
function of donor age, so as to detect unusual susceptibilities of
the cells and tissues of aged subjects. Another general approach
would be to culture the cells in vitro after in vivo treatments.
If a set of behaviours or phenotypes was observed with tissue from
young, treated subjects that proved to be comparable to that
observed with tissue from old untreated animals, an effect of the
in vivo treatments on the aging process could be inferred.
An entirely different experimental paradigm could be based on
the hypothesis that established cultures with finite replicative
life spans recapitulate the natural history of comparable cell types
in vivo - the well-known " in vitro model of cellular aging"
first developed by Hayflick & Moorhead (1961). The appropriateness
of such models for the study of aging is controversial, since it has
been proposed that the attenuation of growth observed in vitro
corresponds to terminal differentiation in vivo (Norwood & Smith,
1985; Bayreuther et al., 1988).
Of special interest would be the evaluation of agents that
exhibit unusual toxicity to putative stem cells. An excessive
depletion of stem cells could seriously compromise the regenerative
potential of tissues in aging subjects. In such studies, it would be
important to investigate a variety of cell types. Most research to
date has concentrated on in vitro aging of cultures of
fibroblastoid cells established from the fetal lung or from the
dermis of individual subjects of various ages. The precise origin of
such cells is not clear. Thus, with such cells it would be difficult
to compare age-related changes in vivo with those observed
in vitro.
A final experimental paradigm would be to use postreplicative
terminally differentiated cells in culture in order to investigate
agents for their potential to accelerate age-related alterations
observed in vivo. Such cell types might be derived through
in vitro terminal differentiation, or from normal or transformed
embryonic neuroblasts or myoblasts. A major concern, however, would
be the extent to which the experimental milieu reflected in vivo
conditions.
4.1.6 Statistical considerations
For statistical treatment of the results of short-term testing,
the statistical methods that are generally available may be used.
However, for statistical analysis of the results of long-term
testing, in particular carcinogenicity testing, the comparison of
results from treatment groups with different survival rates is a
major issue. In these cases the recommendation of IARC (Peto et al.,
1980; Gart et al., 1986) could be applied. All the tumours found at
necropsy must be evaluated as "fatal" or "incidental". This approach
permits one to conduct a comparison between young and old animals
despite the very different patterns of survival from those expected.
A crude analysis, ignoring the fact that young animals survive
longer than old ones, will overestimate the ratio of tumour
incidence in young and old animals. Conversely, a "death-rate"
analysis, treating all tumours as if they were fatal, will
over-correct for the effects of differences in survival on the
incidence of tumours discovered at the autopsy of animals that died
of unrelated causes. Bias can be eliminated only if tumours that
were discovered in an incidental context are analysed by the
prevalence method, while tumours discovered in a fatal context are
analysed by the death-rate method (Peto et al., 1980; Gart et al.,
1986).
4.1.7 Extrapolation of animal data to humans
Extrapolation of animal data to humans may be either
qualitative or quantitative. On the basis of experimental results,
the weight-of-evidence approach can help predict whether a given
substance may be considered dangerous for humans. Such information
includes data on the pharmacokinetics of a chemical, its
cytotoxicity and other toxic properties, as well as the data
obtained in in vitro and short-term tests. Of primary importance
for quantitative extrapolation, a dose-response relationship must be
detected in experimental animals. An important stage of quantitative
evaluation is extrapolation of the data obtained from exposure to
rather high doses to lower doses to which humans may be exposed in
the environment. It is necessary to take into account biological
differences, both in pharmacokinetics and pharmacodynamics, between
the test species and humans. For example, a dose taken per unit of
the body surface area, or its concentration in the daily ration,
includes a correction factor for species sensitivity and allows
extrapolation of experimental doses to man (Mantel & Schneiderman,
1975; Turusov & Parfenov, 1986; Travis et al., 1990). Of course,
species sensitivity to the action of some chemicals may also vary
significantly.
Various physiological and mathematical models have been
proposed for extrapolation of toxicological effects. Most of these
models refer to carcinogenesis (Turusov & Parfenov, 1986; Swenberg
et al., 1987; Clayson, 1988). The predictive nature of these models
for non-carcinogenic end-points remains to be determined.
4.2 Epidemiological and clinical approaches
4.2.1 Disease pattern of aged population
In order to study the pattern of disease occurring in the
elderly, the first question is "What illnesses are most common and
important among the elderly?" The answer can be most easily provided
in terms of diseases that cause death, hospitalization or visits to
a doctor's surgery.
The assessment of health status among the elderly on an
international basis is essentially limited to the use of mortality
data, since these are the only comprehensive data available. In the
developed countries, roughly 50% of all deaths occurring between the
ages of 65 and 74 years are attributable to cardiovascular diseases.
Among males in this age group, ischaemic heart disease accounts for
25% of deaths, while 11% are due to cerebrovascular diseases. For
women, 20% of deaths are attributed to ischaemic heart disease and
about 15% to stroke. Cancer accounts for another 25% of deaths among
men and women aged 65-74, lung cancer being the cause of 10% of all
deaths among elderly males. Roughly 7% of deaths are due to
respiratory diseases and 3% to external causes (WHO, 1989).
The trends in the four leading causes of death, i.e. malignant
neoplasms, heart diseases, cerebrovascular diseases and respiratory
diseases, within the age range 65-74 showed that, in selected
developed countries between 1950-1954 and 1980-1984, death rates
from all malignant neoplasms rose slightly for men but remained
essentially unchanged for women except in France. A more marked
decline in mortality is apparent for heart disease in the USA and
Australia where death rates have fallen by 25-30% since the late
1960s. Male mortality has fallen in France and Japan, but has risen
in Hungary. A similar pattern of change is also apparent for
females. Mortality from stroke has also declined in most countries,
although the timing and extent of the duration has varied from
country to country. For example, the decline in Japan occurred ten
years earlier than that in Australia. In the United Kingdom, France
and USA, rates have been falling since the 1950s. The trend in
mortality from respiratory diseases is less clear, there being
little evidence of sustained and comprehensive decline. However, it
is apparent that there has been some progress in reducing mortality
from these diseases in Australia and the United Kingdom over the
last decade (WHO, 1989).
It must be recognized from the outset that mortality data do
not always accurately reflect the underlying morbidity and are
particularly inappropriate in the case of many conditions for which
the fatality rate is low, yet which are important causes of
morbidity among the elderly. Data on causes of death are also less
reliable for the elderly than for other age groups owing to the
multiple pathological conditions often present at the time of death.
Nevertheless, with few exceptions, comprehensive morbidity data are
not available for the elderly.
Data from the USA shows that cardiovascular diseases cause the
largest proportion of hospitalization in the elderly. As a group,
diseases of the digestive system account for the second most common
diagnostic category while neoplastic diseases (90% malignant) are
the third most common cause of hospitalization. The remaining causes
are diseases of the respiratory system, injury or poisoning, and
diseases of the genitourinary or musculoskeletal systems (White,
1989). In a study from Shanghai, China, the common diseases of
hospitalized patients over 65 years of age in the 1950s, when
arranged in order of frequency, were hypertension, coronary artery
disease, chronic bronchitis, prostate hypertrophy, femur fracture,
pulmonary tuberculosis, diabetes mellitus, cholelithiasis and
tumours of various organs. Coronary artery disease, pneumonia,
hypertension and chronic bronchitis became the leading causes of
hospitalization in the 1970s (Zhu et al., 1982). In the 1980s, data
from other parts of China (Jiangxi, Liaoning, Xinjiang) showed that
chronic bronchitis or pneumonia was the most frequent cause of
hospitalization for elderly patients, followed by hypertension and
coronary artery disease (Xu et al., 1986; Shen et al., 1987; Xiong,
1989).
Data from the USA shows that diagnoses arising from visits to
the doctors surgery by people aged 75 or older, when arranged in
order of frequency, are hypertension (17.6%), chronic ischaemic
heart disease (9.5%), diabetes mellitus (6.7%), osteoarthritis (6%),
cataracts (5.1%), heart failure (4.4%), cardiac arrhythmia (3.6%),
arthropathies (3.6%), glaucoma (2.8%), hypertensive heart disease
(2.6%), angina pectoris (2.3%), chronic airway obstruction (2%) and
neoplasms (1.4%) (White, 1989).
4.2.2 Assessment of effects of environmental chemicals in the
elderly population
There are more limitations in clinical studies than in animal
toxicology studies. Firstly, the conditions in the study are not
easily controlled. Secondly, presumably harmful effects to the
subjects under study need to be avoided in designing the protocol.
The following approach is suggested.
Comprehensive, multidimensional functional assessment of the
elderly should be carried out. This involves analysis of the
physical, psychological, social, environmental and other aspects of
functioning (Zarit et al., 1985; Kane, 1987). Measurements of the
ability to perform the activities of daily life should be obtained.
Physical functioning can be measured by a combination of diagnosis,
symptom description, health reporting, days in bed or hospital
during a specific period, and reported pain or discomfort. The
subject's orientation with respect to time, place and person, as
well as short-term and long-term memory, should be assessed.
Psychological measures are used to evaluate depression, anxiety,
loneliness and sense of mental well-being, and a psychiatric history
should be recorded.
Clinical assessment involves a complete physical examination
and selective laboratory or instrumental examinations. As most
environmental chemicals affect certain parts or organ systems of the
body, laboratory or instrumental examination of these particular
parts or organ systems should be performed. The purpose of clinical
assessment is to find any structural or functional abnormalities
that may occur during the course of exposure to the environmental
chemicals.
Measures of environmental chemicals in the human body, such as
the determination of their concentration or that of their
metabolites in blood, body fluids or tissues (including nails,
hairs, etc.), faeces, urine and expired air, should be conducted.
Biomarkers of exposure, such as haemoglobin adducts, may be used
when available.
The results of the above-mentioned assessments may be
correlated and analysed to arrive at a conclusion as to whether the
environmental chemicals have harmful effects on the subjects
studied.
4.2.3 Acute episodes
There are few epidemiological reports on the effects of
exposure to environmental toxicants on the aged population. Several
acute air pollution incidents resulted in marked increases in
illness and death, mostly among the elderly. One such incident
occurred in the Meuse River Valley in Belgium in 1930 when
accumulating air contaminants trapped by an inversion caused the
death of 63 people and illness in 6000 residents. An incident in
1948 in Donora, Pennsylvania, USA, resulted from a similar inversion
that covered a wide area. Of the population of 14 000, 20 died
(compared with the expected two deaths for the same period) and 43%
fell ill. Again, the elderly were the most seriously affected. In
London, 4000 excess deaths were attributed to smoke and sulfur
dioxide in the fog of 1952, and an incident in 1962 caused 400
deaths above the normal value for the period. Similar episodes of
fog-induced mortality in various places have been described (Amdur,
1986). In all these episodes, the most vulnerable people were the
elderly, and the victims were usually suffering from acute
bronchitis and pneumonia, resulting in acute respiratory failure.
However, detailed and systematic study of the effects of
environmental chemicals on the elderly is still lacking (Amdur,
1986).
4.2.4 Concerns for the aged population
The health conditions of the elderly are quite different from
those in the early or middle age of human life. Many chronic
diseases that begin at an early age extend into advanced age. Some
pathological conditions occurring in middle age may become
symptomatic in the elderly. Certain medical problems are clearly
more prominent among the elderly, e.g., cancer of the prostate,
temporal arteritis and osteoarthritis. Illness in older people often
involves multiple organ systems that are interrelated by symptoms,
physical findings, functional capacity and treatment. The emotional
and social consequences of the physical conditions are also related.
Furthermore, the manifestations of aging processes that usually
comprise degeneration, both structural and functional, may sometimes
be difficult to differentiate from those of diseases. Aging
processes and existing diseases, as well as social and environmental
factors, act together to make the assessment and care of the elderly
complex and difficult.
As far as the effects of chemicals upon human health are
concerned, the elderly are subjected to long-term exposure to
environmental chemicals. Some exposures occur daily. The source may
be polluted air, water, food or products made of inorganic or
organic chemicals with which people frequently come into contact.
Some exposures occur during occupational activities in which people
are engaged for many years. The cumulative effect of continuous or
repeated exposure to chemicals may result in pathological changes
and clinical manifestations found in later stages of life. In
addition, the structural and functional changes of aging, usually
degenerative in nature, make the elderly more vulnerable to adverse
effects of environmental chemicals.
As mentioned above, the multiple disease status in the elderly
is usually accompanied by polypharmacy. In developed countries, the
consumption of drugs by people over 65 years of age accounts for
25-50% of total drug consumption (Vestal, 1978). The most commonly
used are neuropsychiatric and cardiovascular drugs,
anti-inflammatory analgesics and diuretics. Since these drugs are
used for the relief of symptoms rather then the cure of diseases,
repeated or sustained prescribing is the rule. Under such
conditions, interactions between environmental chemicals and drugs
should be carefully considered.
4.3 Biomarkers of aging
The term "biomarker of aging" arose in conferences sponsored by
the Fund for Integrative Biomedical Research (Regelson, 1983) and
the National Institute on Aging (Reff & Schneider, 1982). The
Committee on Chemical Toxicity and Aging (1987) defined a biomarker
of aging as "a biological event or measurement of a biological
sample that is considered to be an estimate or prediction of one or
more of the aging processes". Baker & Sprott (1988) offered the
following specific features for a biomarker of aging: (a) nonlethal;
(b) highly reproducible within and across species; (c) reflects
physiological age or some basic biological process of aging or
metabolism; (d) displays significant alterations during relatively
short time periods; (e) is crucial to the effective maintenance of
health and prevention of disease; and (f) reflects a measurable
parameter that can be predicted at a later age. Development of a
panel of biomarkers would assist in assessing the effects of
environmental chemicals that modulate aging processes in laboratory
animals and man.
There is currently a significant research effort, especially
with rodent species, to establish batteries of biological markers of
aging (Allaben et al., 1990). But how can one differentiate
alterations that are simple functions of chronological time from
those that are the results of intrinsic aging or of intrinsic aging
coupled with the effects of chronological time? One approach has
been that of comparative gerontology. Starting with a group of
taxonomically related species that vary substantially in their
maximum life-span potentials, one simply asks if the rate of change
of the putative biomarker reflects the maximum life-span potential
of the species.
From a practical point of view, various biomarkers may be used
for the measurement of aging. For different levels of integration of
an organism, different parameters may be used. At the subcellular
and cellular levels, measurements of macromolecular lesions, the
degree of collagen cross-linking, the level of lipofuscin
accumulation, specific enzyme activities, the number of particular
hormone receptors, numbers of cell divisions, etc. may be
investigated as biomarkers. Tissue and organ weights, cellularity,
growth or some functional activity (muscle strength, visual acuity,
secretion rate) may be considered. The functional activity of
motor, cardiovascular, nervous, endocrine, immune, haematopoietic
and other systems and subsystems may be considered at the systemic
level as potential biomarkers of aging. In addition, at the level of
the whole organism, the functions of the main integrative systems
should be addressed.
Test batteries that attempt to measure functional age (Webster
& Logie, 1976), physiological age (Hollingsworth et al., 1965) and
biological age (Furukawa et al., 1975; Nakamura et al., 1982;
Voitenko & Tokar, 1983; Reis & Poethig, 1984; Dubina et al., 1984;
Hochschild, 1989) have similar conceptual underpinnings in that they
utilize the great variability in performance that emerges among
adults of the same chronological age in standardized, age-sensitive
tests. Studies using this approach have been conducted on human
populations, and similar measures have been employed in a few rodent
studies (Hofecker et al., 1980; Dubina et al., 1983). This approach
can be viewed as the performance variability model of biological
age. It has considerable intuitive appeal and, in many cases,
statistical elegance. An individual may be judged biologically
younger if he is performing better than expected for his
chronological age. However, in their extensive and critical reviews,
Costa & McCrae (1980, 1985) revealed many conceptual and
methodological flaws in this approach.
Biomarkers of aging, which are markers of susceptibility, may
have their greatest utility in the study of the effects of
environmental chemicals on the processes of aging. The effects of
environmental chemicals on the elderly would be assessed most
efficiently by using biomarkers of effect (NAS, 1989).
5. CONCLUSIONS
a) With increasing age, humans become more vulnerable to
environmental challenges due to the deterioration of
physiological and psychological processes. Therefore, it is
likely that the elderly will be more susceptible to the harmful
effects of environmental chemicals.
b) The elderly are heterogeneous with respect to the extent of
deterioration of physiological and psychological processes.
c) Few, if any, of the hundreds of thousands of environmental
chemicals have been tested for increased toxicity in the
elderly.
d) Some of the many age-associated diseases may cause an increased
susceptibility to the harmful action of specific environmental
chemicals.
e) The use of animal models for aging research requires special
considerations, such as housing conditions, diets, monitoring
for infectious agents, and specifically defined pathologies. It
is also necessary to assess several age ranges based on
life-table information in order to distinguish the senescent
from the mature and developing animal.
f) The selection of the animal model should be based on the
likelihood of providing information of relevance to specific
problems in humans.
g) The characteristics of the elderly result from intrinsic aging
processes, environmental factors, and other life-span events.
Therefore, the study of the elderly may not provide
information on basic aging processes.
h) Both the size of the aged population and the number of
chemicals in the environment will undoubtedly increase over
the next few decades. It is therefore expected that the adverse
effects of chemical exposure on the elderly will increase in
importance as a health care issue.
6. FURTHER RESEARCH
a) Experimental, epidemiological and clinical data should be
obtained on:
* the toxicity, mutagenicity and carcinogenicity of
environmental chemicals in old individuals as compared to
young adults;
* the effect of age on the pharmacokinetics and
pharmaco-dynamics of environmental chemicals;
* the long-term effect of environmental chemicals on
molecular, cellular and physiological parameters as
potential biomarkers in the elderly.
b) New models should be developed for assessing the susceptibility
of the elderly to environmental chemicals as compared to young
adults. Such models should be suitable for assessment of
particular consequences and relevant to humans, and could
include:
* non-mammalian and mammalian models (e.g., Drosophila,
fish, rabbits, mini-pigs);
* transgenic animal models;
* cell lines with specific genetic characteristics.
c) For each study the appropriate applicability and use of
experimental models and techniques must be validated:
* animal models should be well-defined in terms of survival,
pathology and husbandry;
* human subjects should be examined carefully for subtle
disease status, medical history, life-style and
socio-economic status.
* standard procedures for measuring molecular, cellular and
physiological parameters should be defined (and developed
if necessary) in order to prevent misinterpretation.
d) The effects of environmental chemicals on the processes of
aging remain to be evaluated. A special scientific workshop
should be devoted to this topic.
REFERENCES
American Dietetic Association (ADA) (1984) ADA takes proactive
stance, testifies on older Americans act reauthorization. J Am Diet
Assoc, 84(7): 822-835.
Ali B, Walford RL, & Imamura T (1985) Influence of aging and poly IC
treatment on xenobiotic metabolism in mice. Life Sci, 37: 1387-1393.
Allaben WT, Chou MW, Pegram RA, Leakey J, Feues RJ, Duffy PH,
Turturro A, & Hart RW (1990) Modulation of toxicity and
carcinogenesis by caloric restriction. Korean J Toxicol, 6: 167-182.
Allison T, Hume AL, Wood CC, & Goff WR (1984) Developmental and
aging changes in somatosensory, auditory and visual evoked
potentials. Electroencephalogr Clin Neurophysiol, 58: 14-24.
Amador A, Steger RW, Bartke A, Johns A, Siler-Khodr TM, Parker R Jr,
& Shepherd AM (1985) Testicular LH receptors during aging in Fischer
344 rats. J Androl, 6: 61-64.
Amdur MO (1986) Air pollutants. In: Klassen CD, Amdur UO, & Doull J
ed. Casarett and Doull's toxicology: The basic science of poisons,
3rd ed. New York, Macmillan Publishing Co., pp 801-824.
Ammon HPT, Fahmy A, Mark M, Wahl MA, & Youssif N (1987) The effect
of glucose on insulin-release and ion movement in isolated
pancreatic islets of rats in old age. J Physiol, 384: 347-354.
Anderson S & Brenner BM (1986) The aging kidney : Structure,
function, mechanisms and therapeutic implications. J Am Geriatr Soc,
35(6): 590-593.
Anderson S & Brenner BM (1987) Effects of aging on the renal
glomerulus. Am J Med, 80(3): 435-442.
Anisimov VN (1971) [Blastomogenesis in persistent estrous rats.]
Vopr Onkol, 17(8): 67-75 (in Russian).
Anisimov VN (1987) Carcinogenesis and aging. Boca Raton, Florida,
CRC Press, vol 1 & 2.
Anisimov VN (1988) Effect of age on dose-response relationship in
carcinogenesis induced by single administration of
N-nitroso-methylurea in female rats. J Cancer Res Clin Oncol, 114:
628-635.
Anisimov VN (1989) Age-related mechanisms of susceptibility to
carcinogenesis. Semin Oncol, 16: 10-19.
Anisimov VN & Reiter RJ (1990) [Pineal function in cancer and
aging.] Vopr Onkol, 37: 259-268 (in Russian).
Arfini G, Mutti A, Vescovi P, Ferroni C, Ferrari M, Giaroli C,
Passeri M, & Franchini I (1987) Impaired dopaminergic modulation of
pituitary secretion in workers occupationally exposed to styrene:
Further evidence from PRL response to TRH stimulation. J Occup Med,
29: 826-830.
Armbrecht HJ (1986) Age-related changes in calcium and phosphorus
uptake by rat small intestines. Biochim Biophys Acta, 882: 281-286.
Armbrecht HJ, Doubek WG, & Porter SB (1988) Calcium transport by
basal lateral membrane vesicles from rat small intestine decreases
with age. Biochim Biophys Acta, 944: 367-373.
Armbrecht HJ, Boltz M, Strong R, Richardson A, Bruns MEH, &
Christakos S (1989) Expression of calbindin-D decreases with age in
intestine and kidney. Endocrinology, 125: 2950-2956.
Aschheim P (1976) Aging in the hypothalamic-hypophyseal-ovarian axis
in the rat. In: Everitt AV & Burgess JA ed. Hypothalamus, pituitary
and aging. Springfield, Illinois, Charles C. Thomas, pp 376-418.
Aschoff J (1979) Circadian rhythms: general features and
endocrinological aspects. In: Krieger DT ed. Comprehensive
endocrinology. New York, Raven Press, pp 1-61.
Avogaro P, Bittolo-Bon G, Cazzolato G, Belussi F, & Pontoglio E
(1983) Lipids and proteins of lipoproteins in human atherosclerosis.
Przegl Lek, 40: 713-718.
Bach JF, Darcenne M, Papiernik M, Barvis A, Lavasseur P, & Lebrand H
(1972) Evidence for a serum-factor secreted by the human thymus.
Lancet, ii: 1056-1058.
Baker SR & Rogul M ed. (1987) Environmental toxicity and the aging
processes: Proceedings of a workshop held in Columbia, Maryland, 1-2
October 1985. New York, Alan R. Liss, Inc., 139 pp (Progress in
Clinical and Biological Research, Volume 228).
Baker GT & Sprott RL (1988) Biomarkers of aging. Exp Gerontol, 23:
223-239.
Bang NU, Tessler SU, Heidenreich RO, Marks CA, & Mattler LE (1982)
Thrombosis and atherosclerosis. Chicago, Year Book Medical
Publishers.
Banks YB, Brewster DW, & Birnbaum LS (1990) Age-related changes in
dermal absorption of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and
2,3,4,7,8-pentachlorodibenzofuran (4-PeCDF). Fundam Appl Toxicol,
15: 163-173.
Baron JA (1984) Smoking and estrogen-related disease: A review. Am J
Epidemiol, 119: 9-22.
Barrows CH & Kokkonen GC (1978) Diet and life extension in animal
model systems. Age, 1: 131-143.
Bartsch H (1991) N-Nitrosocompounds and human cancer: where do we
stand? In: Relevance to human cancer of N-nitroso compounds, tobacco
smoke and mycotoxins. Lyon, International Agency for Research on
Cancer, pp 1-110 (IARC Scientific Publications No. 105).
Bauer LA, Black D, Gensler A, & Sprinkle J (1989) Influence of age,
renal function, and heart failure on procainamide clearance and
n-acetylprocainamide serum concentrations. Int J Clin Pharm Ther
Toxicol, 27: 213-216.
Baylis C, Fredericks M, Leypolat J, Frigon R, Wilson C, & Henderson
L (1988) The mechanisms of proteinuria in aging rats. Mech Ageing
Dev, 45: 111-126.
Bayreuther K, Rodemann KP, Francy PI, & Maier K (1988)
Differentiation of fibroblast stem cells. J Cell Sci, 10(suppl):
115-130.
Beebe GW (1979) Reflections on the work of the Atomic Bomb Casuality
Commission in Japan. Epidemiol Rev, 1: 184-210.
Behl CR, Bellantone NH, & Flynn GL (1987) Influences of age on
percutaneous absorption of drug substances. In: Kydonieuks AF &
Berrer B ed. Transdermal delivery of drugs. Boca Raton, Florida, CRC
Press, vol 2, pp 109-132.
Beierschmitt WP & Weiner M (1986) Age-related changes in renal
metabolism of acetaminophen in male Fischer 344 rats. Age, 9: 7-13.
Beierschmitt WP, Keenan KP, & Weiner M (1986a) Age-related increased
susceptibility of male Fischer 344 rats to acetaminophen
nephrotoxicity. Life Sci, 39: 2335-2342.
Beierschmitt WP, Keenan KP, & Weiner M (1986b) The development of
acetaminophen-induced nephrotoxicity in male Fischer 344 rats of
different ages. Arch Toxicol, 59: 206-210.
Belisle S & Lehoux J-G (1983) Endocrine aging in C57BL Mice. II.
Dynamics of estrogen receptors in the hypothalamic pituitary axis. J
Steroid Biochem, 18: 737-743.
Bender AD (1965) The effect of increasing age on the distribution of
peripheral blood flow in man. J Am Geriatr Soc, 13: 192-198.
Bender AD (1968) Effect of age on intestinal absorption:
Implications for drug absorption in the elderly. J Am Geriatr Soc,
16: 1331-1339.
Bender AD (1979) Drug sensitivity in the elderly. In: Crooks J &
Stevenson IH ed. Drugs and the elderly. Baltimore, Maryland,
University Park Press, pp 147-153.
Bender A, Post A, Meier J, Higson J, & Reichard G (1975) Plasma
protein binding of drugs as a function of age in adult human
subjects. J Pharm Sci, 64: 1711-1713.
Benditt EP (1977) The origin of atherosclerosis. Sci Am, 236: 74-85.
Berg BN (1976) Pathology and aging. In: Everitt AV & Burgess JA ed.
Hypothalamus, pituitary and aging. Springfield, Illinois, Charles C.
Thomas, pp 43-67.
Bertoni-Freddari C, Fattoretti P, Casoli T, Meier-Rouge W, & Ulrich
J (1990) The effect of ethanol on neuronal membrane permeability and
synaptic ultrastructure in adult and old rats. In: Bergamini E ed.
Protein metabolism and aging. New York, Wiley-Liss, Inc., p.
331-334.
Bertrand HA, Lynd FT, Masoro EJ, & Yu BP (1980) Changes in adipose
mass and cellularity through the adult life of rats fed ad libitum
or a life-prolonging restricted diet. J Gerontol, 35(6): 827-835.
Besedovsky H, Sorkin E, Keller M, & Müller J (1975) Changes in blood
hormone levels during the immune response (39057). Proc Soc Exp Biol
Med, 150: 466-470.
Besedovsky HO, Del Rey AE, & Sorkin E (1985) Immune-neuroendocrine
interactions. J Immunol, 135: 750-754.
Bhatnagar KP, Kennedy RC, Baron G, & Greenberg RA (1987) Number of
mitral cells and the bulb volume in the aging human olfactory bulb:
a quantitative morphological study. Anat Rec, 218: 73-87.
Bilchert-Toft M (1978) The adrenal glands in old age. In: Greenblatt
RB ed. Geriatric endocrinology. Vol 5: Aging. New York, Raven Press,
pp 81-102.
Birchenall-Sparks M, Richardson A, Roberts M, & Utherford M (1985)
The effect of aging on the structure and function of liver messenger
RNA. Mech Ageing Dev, 32: 99-111.
Birnbaum LS (1983) Distribution and excretion of 2,3,6,2',3',6'-and
2,4,5,2',4',5'-hexachlorobiphenyl in senescent rats. Toxicol Appl
Pharmacol, 70: 262-272.
Birnbaum LS (1987) Age-related changes in carcinogen metabolism. J
Am Geriatr Soc, 35: 51-60.
Birnbaum LS (1989) Age-related changes in drug disposition. In:
Zenser TV & Coe RM ed. Cancer and aging. Berlin, Heidelberg, New
York, Springer-Verlag, pp 25-138.
Birnbaum LS (1991) Pharmacokinetic basis of age-related changes in
sensitivity to toxicants. Annu Rev Pharmacol Toxicol, 31: 101-128.
Birnbaum LS & Baird MB (1979) Senescent changes in rodent hepatic
epoxide metabolism. Chem-Biol Interact, 26: 245-256.
Birren JE, Woods AM, & Williams MV (1979) Speed of behavior as an
indicator of age changes and the integrity of the nervous system.
In: Hoffmeister F & Muller C ed. Brain function in old age. Berlin,
Heidelberg, New York, Springer-Verlag, p 10.
Bitar MS & Shapiro BH (1987) Aberration of heme and hemoprotein in
aged female rats. Mech Ageing Dev, 38: 189-197.
Bjorksten J, Yaeger LL, & Wallace T (1989) Control of aluminium
ingestion and its relation to longevity. Int J Vitam Nutr Res, 58:
462-465.
Blackman MR (1987) Pituitary hormones and aging. Endocrinol Aging,
16(4): 981-994.
Blackwelder WC, Yano K, Rhoads GG, Kagan A, Gordon T, & Palesch Y
(1980) Alcohol and mortality: The Honolulu heart study. Am J Med,
68: 164-169.
Blalock JE, Harbour-McMenamin D, & Smith EM (1985) Peptide hormones
shared by the neuroendocrine and immunologic systems. J Immunol,
135(2): 858/5-861/5.
Blanco P, Machado A, & Satrustegui J (1987) Variations due to
hypoxia and ageing in the activities of glutathione-S-transferase
and NADPH-Cytochrome C reductase. Mech Ageing Dev, 39: 11-19.
Blum M, Averbuch M, Wolman Y, & Aviram A (1989) Protein intake and
kidney function in humans: its effect on 'normal aging'. Arch Inter
Med, 149(1): 211-212.
Blumberg JB (1985) A discussion of drug metabolism and actions in
the aged. Drug-Nutrient Interact, 4: 99-106.
Bolinder J, Kager L, Ostamn J, & Arner P (1983) Differences at the
receptor and postreceptor levels between human omental and
subcutaneous adipose tissue in the action of insulin on lipolysis.
Diabetes, 32: 117-123.
Bondareff W (1986) Neuropathology of nucleus basalis and locus
ceruleus in Alzheimer's disease. In: Scheibel AB, Wechsler AF, &
Brazier MAB ed. The biological substrates of Alzheimer's disease.
New York, London, San Francisco, Academic Press, pp 103-113.
Boobis AR & Davies DS (1984) Human cytochromes P-450. Xenobiotica,
14: 151-185.
Borghoff SJ & Birnbaum LS (1985) Age-related changes in
glucuronidation and deglucuronidation in liver, small intestine,
lung and kidney of male Fischer rats. Drug Metab Dispos, 13: 62-67.
Borghoff SJ & Birnbaum LS (1986) Age-related changes in the
metabolism and excretion of allyl isothiocyanate: A model compound
for glutathione conjugation. Drug Metab Dispos, 14: 417-422.
Borghoff SJ, Stefanski SA, & Birnbaum LS (1988) The effect of age on
the glucuronidation and toxicity of
4,4'-thiobis(6-t-butyl-m-cresol). Toxicol Appl Pharmacol, 92:
453-466.
Bornor JA, Dilworth BB, & Sullivan KM (1988) Exercise and
osteroporosis: A critique of the literature. Physiother Can, 40(3):
146-155.
Boyes WK & Dyer RS (1984) Chlordimeform produces profound,
selective, and transient changes in visual evoked potentials of
hooded rats. Exp Neurol, 86: 434-447.
Bradley RM (1979) The effect of aging on the olfactory sense. In:
Special senses in aging. Ann Arbor, Michigan, University of
Michigan, Institute of Gerontology, pp 3-8.
Bremner WJ, Vitiello MV, & Prinz PN (1983) Loss of circadian
rhythmicity in blood testosterone with aging in normal men. J Clin
Endocrinol Metab, 56: 1278-1281.
Brenner BM, Meyer TW, & Hostetter TH (1982) Dietary protein intake
and the progressive nature of kidney diseases. New Engl J Med, 307:
652-659.
Brizzee KR (1985) Neuron aging and neuron pathology. In: Johnson AH
ed. Relations between normal aging and disease. New York, Raven
Press, pp 191-224.
Brown WW, Davis BB, & Spry LA (1986) Aging and the kidney. Arch
Intern Med, 146: 1790-1796.
Bruce MF (1980) The relation of tactile thresholds to histology in
the fingers of the elderly. J Neurol Neurosurg Psychiatry, 43:
730-734.
Buckler AJ, Vie H, Sonenshein GE, & Miller RA (1988) Defective T
lymphocytes in old mice: Diminished production of mature c-myc RNA
after mitogen exposure not attributable to alterations in
transcription or RNA stability. J Immunol, 140: 2442-2446.
Bullock K (1985) Neuroanatomy of lymphoid tissue. In: Guillelmin R
ed. Neural modulation of immunity. New York, Raven Press, pp
111-141.
Burek JD (1978) Pathology of aging rats. Boca Raton, Florida, CRC
Press.
Burnet FM (1970) The concept of immunological surveillance. Prog Exp
Tumor Res, 13: 1-27.
Butenko GM (1985) Ageing of the immune system and diseases. In:
Likhachev A, Anisimov V, & Montesano R ed. Age-related factors in
carcinogenesis. Lyon, International Agency for Research on Cancer,
pp 71-83 (IARC Scientific Publications No. 58).
Campbell GR & Chamley-Campbell JH (1981) Invited review: The
cellular pathobiology of atherosclerosis. Pathology, 13: 423-440.
Capecchi MR (1989) Altering the genome by homologous recombination.
Science, 244: 1288-1292.
Caraceni MP, Corghi E, Ortolani S, Dindell M, Candotti G, & Ferrari
A (1988) Prevention of early postmenopausal bone loss. The effect of
calcitonin (Staporos). Clin Trials J, 25(6): 401-405.
Carrillo MC, Kitani K, Kanai S, Sato Y, Nokubo M, Ohta M, & Otsubo K
(1989) Differences in the influence of diet on hepatic glutathione
S-transferase activity and glutathione content between young and old
C56 black female mice. Mech Ageing Dev, 40: 1-15.
Carrillo MC, Nokubo M, Sato Y, Kanai S, Ohta M, & Kitani K (1990)
Effect of protein-free diet on activities and subunits of
glutathione S-transferase in livers of young and aged female rats.
Mech Ageing Dev, 56: 230-251.
Carrillo MC, Nokubo M, Kitani K, Kimihiko S, & Sato K (1991)
Age-related alterations of enzyme activities and subunits of hepatic
glutathione S-transferases in male and female Fischer-344 rats.
Biochim Biophys Acta, 1077: 325-331.
Castleden CM, George CF, Marcer D, & Hallet C (1977a) Increased
sensitivity to nitrazepam in old age. Br Med J, 1: 10-12.
Castleden CM, Volans CN, & Raymond K (1977b) The effect of aging on
drug absorption from the gut. Age Ageing, 6: 138-143.
Cathcart R, Schwiers E, Saul RL, & Ames BN (1984) Thymine glycol and
thymidine glycol in human and rat urine: a possible assay of
oxidative DNA damage. Proc Natl Acad Sci (USA), 81: 5633-5637.
Cauley JA, Gutai JP, Kuller LH, Ledonne D, Sandler RB, Sashin D, &
Powell JG (1988) Endogenous estrogen levels and calcium intakes in
postmenopausal women. Relationships with cortical bone measures. J
Am Med Assoc, 260(21): 3150-3155.
Ceda GP, Valenti G, Butturini U, & Hoffman AR (1986) Diminished
pituitary responsiveness to growth hormone-releasing factor in aging
male rats. Endocrinology, 118(5): 2109-2114.
Chakravarty S, Collins WP, Forecast JS, Newton JR, Oram DH, & Strudd
JWW (1976) Hormonal profiles after the menopause. Br Med J, 2:
784-787.
Chan K, Kerdall MJ, Mitchard M, Wells WOE, & Vickers MD (1975) The
effect of aging on plasma pethidine concentration. Br J Clin
Pharmacol, 2: 297-302.
Chandra RK (1985) Trace element regulation of immunity and
infection. J Am Coll Nutr, 4: 5-16.
Chavaz A, Baleurt L, Simonin P, & Fabre J (1974) Influence de l'âge
sur la digoxinémie et la digitalisation. Schweiz Med Wochenschr,
104: 1823-1825.
Chen LH, Liu S, Cook Newell ME, & Barnes K (1985) Survey of drug use
by the elderly and possible impact of drugs on nutritional status.
Drug-Nutrient Interact, 3: 73-86.
Cheney KE, Liu RK, Smith GS, Merdith PJ, Mickey MR, & Walford RL
(1983) The effect of dietary restriction of varying duration on
survival, tumour patterns, immune fraction and body temperture in
BIOC3F1 female mice. J Gerontol, 38: 420-430.
Chengelis CP (1988a) Age- and sex-related changes in the components
of the hepatic microsomal mixed function oxidase system in
Sprague-Dawley rats. Xenobiotica, 18: 1211-1224.
Chengelis CP (1988b) Age- and sex-related changes in epoxide
hydrolase, UDP-glucuronyl transferase, and PAPS-sulfotransferase in
Sprague-Dawley rats. Xenobiotica, 18: 1225-1237.
Chopra IJ, Solomon DH, Chopra U, Wu SY, Fisher DA, & Nakamura Y
(1978) Pathways or metabolism of thyroid hormones. Recent Prog Horm
Res, 34: 521-567.
Christophers E & Kligman AM (1965) Percutaneous absorption in aged
skin. In: Montagna W ed. Advances in the biology of the skin. Vol 6:
Ageing. Oxford, New York, Pergamon Press, pp 163-175.
Clayson DB (1988) Needs for biological risk assessment in
interspecies extrapolation. Environ Health Perspect, 77: 93-97.
Cleroux J, Giannattasio C, Grassi G, Seravalle G, Sampieri L,
Cuspidi C, Bolla G, Valsecchi M, Mazzola C, & Mancia G (1988)
Effects of aging on the cardiopulmonary receptor reflex in
normotensive humans. J Hypertension, 6(suppl 4): 141-144.
Coiro V, Volpi R, Cavazzini V, Bertoni P, Corradi A, Bianconi L,
Davoli C, Rossi G, & Chiodera P (1991) Restoration of normal growth
hormone responsiveness to GHRH in normal aged men by infusion of low
amounts of theophylline. J Gerontol Med Sci, 46(5): M155-M158.
Colombo T, Donelli MG, Urso R, Dallara S, Bartosek I, & Guaitani A
(1989) Doxorubicin toxicity and pharmacokinetics in old and young
rats. Exp Gerontol, 24: 159-171.
Committee on Chemical Toxicity and Aging (1987) Aging in today's
environment. Washington, DC, National Academy Press.
Conn PM, Cooper RL, McNamara MC, Rogers DC, & Shoenhardt L (1980)
Qualitative change in gonadotropin during normal aging in the male
rat. Endocrinology, 106: 1549-1553.
Conrad K & Bressler R (1982) Drug therapy for the elderly. St Louis,
Missouri, C.V. Mosby & Co.
Cooper B, Weinblatt F, & Gregerman R (1977) Enhanced activity of
hormone sensitive adenylated cyclase during dietary restriction in
the rat. J Clin Invest, 39: 467-474.
Cooper RL, Goldman JM, & Rehnberg GL (1986) Pituitary function
following treatment with reproductive toxins. Environ Health
Perspect, 70: 177-184.
Corman B & Michel JB (1986) Renin-angiotensin system,
converting-enzyme inhibition, and kidney function in aging female
rats. Am J Physiol, 251: 450-455.
Costa PT Jr & McCrae RR (1980) Functional age: A conceptual and
empirical critique. In: Haynes SG & Feinleib M ed. Epidemiology of
aging. Washington, DC, National Institutes of Health, National
Institute of Aging, National Heart, Lung, and Blood Institute, pp
23-50 (NIH Publication No. 80-969).
Costa PT & McCrae RR (1985) Concepts of functional or biological
age: A critical view. In: Andres R, Bierman EL, & Hazzard WR ed.
Principles of geriatric medicine. New York, McGraw-Hill, pp 30-37.
Costa LG & Murphy SD (1987) Interaction of the pesticide
chlordimeform with adrenergic receptors in mouse brain: An
in vitro study. Arch Toxicol, 59: 323-327.
Costa LG, Olibet G, & Murphy SD (1988) Alpha2-adrenoreceptors as a
target for formamidine pesticides: in vitro and in vivo studies
in mice. Toxicol Appl Pharmacol, 93: 319-328.
Creasey H & Rapoport SI (1985) The aging of human brain. Ann Neurol,
17(1): 2-10. Crooks J, O'Malley K, & Stevenson IH (1976)
Pharmacokinetics in the elderly. Clin Pharmacokinet, 1: 280-296.
Croucher PI, Mellish RWE, Vedi S, Garrahan NJ, & Compston JE (1989)
The relationship between resorption depth and mean interstitial bone
thickness: Age-related changes in man. Calcif Tissue Int, 45(1):
15-19.
Crowley JJ, Cusack BJ, Jue SG, Koup JR, Park BK, & Vestal RE (1988)
Aging and drug interactions. II. Effects of phenytoin and smoking on
the oxidation of theophylline and cortisol in healthy men. J
Pharmacol Exp Ther, 245: 513-523.
Cuny G, Royer RJ, Mur JM, Serot JM, Faure G, Netter P, Maillard A, &
Penin F. (1979) Pharmacokinetics of salicylates in elderly.
Gerontology, 25: 49-55.
Cusack B & Denham MJ (1984) Nutritional status and drug disposition
in the elderly. In: Roe DA ed. Drugs and nutrition in the geriatric
patients. New York, Churchill Livingstone, pp 47-70.
Cutler RG (1991) Human longevity and aging: possible role of
reactive oxygen species. Ann NY Acad Sci, 621: 1-28.
Cutler RG & Semsei I (1989) Development, cancer and aging: possible
common mechanisms of action and regulation. J Gerontol, 44(6):
25-34.
Daniel FB, Schut HA, Sandwiesh DW, Schenck KM, Hoffmann CO, Patrick
JR, & Stoner GD (1983) Interspecies comparison of benzo(a)pyrene
metabolism and DNA-adduct formation in cultured human and animal
bladder and tracheobronchial tissues. Cancer Res, 43: 4723-4729.
Danon D (1969) Biophysical aspects of red cell ageing. Bibl
Haematol, 15: 275-287.
Davies BH (1985) The respiratory system. In: Pathy MSJ ed.
Principles and practice of geriatric medicine. New York, Chichester,
Brisbane, Toronto, John Wiley and Sons.
Davila DR, Brief S, Simon J, Hammer RE, Brinster RL, & Kelley KW
(1987) Role of growth hormone in regulating T-dependent immune
events in aged, nude and transgenic rodents. J Neurosci Res, 18:
108-116.
Davis PJ & Davis FB (1987) Water excretion in the elderly.
Endocrinol Aging, 16(4): 867-875.
Davis TA, Bales CW, & Beauchene RE (1983) Differential effects of
dietary, caloric, and protein restriction in the aging rat. Exp
Gerontol, 18: 427-435.
Dawson-Hughes B, Shipp C, Sadowski L, & Dallal G (1987) Bone density
of the redius, spine, and hip in relation to percent of ideal body
weight in postmenopausal women. Calcif Tissue Int, 40(6): 310-314.
Dement W, Richardson G, Prinz P, Carskadon M, Kripke D, & Czeisler C
(1985) Changes of sleep and wakefulness with age. In: Finch CE &
Schneider EL ed. Handbook of the biology of aging, 2nd ed. New York,
Van Nostrand Reinhold Company, pp 692-717. Department of
International Economic and Social Affairs (1991) World population
prospects 1990, New York, United Nations, pp 192-215 (Population
Studies No. 120).
Dilman VM (1971) Age associated elevation of hypothalamic threshold
to feedback control and its role in development, aging and disease.
Lancet, i: 1211-1219.
Dilman VM (1987) [Four models of medicine.] Leningrad, Meditsina (in
Russian).
Dilman VM & Anisimov VN (1979) Hypothalmic mechanism of ageing and
of specific age pathology. I. Sensitivity threshold of
hypothalamo-pituitary complex to homeostatic stimuli in the
reproductive system. Exp Gerontol, 14: 161-174.
Dix D (1989) The role of aging in cancer incidence: an
epidemiological study. J Gerontol, 44(6): 10-18.
Doll R (1973) Age. In: Doll R & Vodopija I ed. Host environment
interactions in the etiology of cancer in man. Lyon, International
Agency for Research on Cancer, pp 39-48 (IARC Scientific
Publications No. 7).
Doll R (1978) An epidemiological perspective of the biology of
cancer. Cancer Res, 38: 3573-3583.
Doll R & Peto R (1981) The causes of cancer. J Natl Cancer Inst, 66:
1193-1312.
Donaldson J (1987) The physiopathologic significance of manganese in
brain: Its relation to schizophrenia and neurodegenerative
disorders. Neurotoxicology, 8: 451-462.
Doubek WG & Armbrecht HJ (1987) Changes in intestinal glucose
transport over the lifespan of the rat. Mech Ageing Dev, 39: 91-102.
Dubina TL, Dyundikova VA, & Zhuk EV (1983) Biological age and its
estimation. II. Assessment of biological age of albino rats by
multiple regression analysis. Exp Gerontol, 18: 5-18.
Dubina TL, Mints A, & Zhuk EV (1984) Biological age and its
estimation. III. Introduction of a correction to the multiple
regression model of biological age and assessment of biological age
in cross-sectional and longitudinal studies. Exp Gerontol, 19:
133-143.
Duckles SP (1987) Influence of age on vascular adrenergic
responsiveness. Blood Vessels, 24(3): 113-116.
Dundee JW (1979) Response to anaesthetic drugs in the elderly. In:
Crooks J & Stevenson IH ed. Drugs and the elderly. Baltimore,
Maryland, University Park Press, pp 179-187.
Dunn MF, Pattison SE, Storm MC, & Quiel E (1980) Comparison of the
zinc binding domains in the 7S nerve growth factor and the
zinc-insulin Hexamer. Biochemistry, 19: 718-725.
Dybkaer R, Lauritzen DM, & Krakauer R (1981) Relative reference
values for clinical chemical and haematological quantities in
"healthy" elderly people. Acta Med Scand, 209: 1-9.
Eastell R & Riggs BL (1987) Calcium homeostasis and osteoporosis.
Endocrinol Metab Clin, 16(4): 829-843.
Eastin WC Jr & Birnbaum LS (1987) Intestinal absorption of two
glucose analogues in rats of different ages. Exp Gerontol, 22:
351-357.
Ebbesen P (1985) Papilloma development on young and senescent mouse
skin treated with 12-0-tetradecanoylphorbol-13-acetate. In:
Likhachev A, Anisimov V, & Montesano R ed. Age-related factors in
carcinogenesis. Lyon, International Agency for Research on Cancer,
pp 167-170 (IARC Scientific Publications No. 58).
Edelman IS & Leibman J (1959) Anatomy of body water and
electrolytes. Am J Med, 27: 256-277.
Eden S (1987) Endocrinology in older people. Acta Obstet Gynaecol
Scand, 140(suppl): 9-22.
Edman CD (1983) Estrogen replacement therapy. In: Buchsbaum HJ ed.
The menopause. Berlin, Heidelberg, New York, Springer-Verlag.
Ellis GB & Desjardins C (1982) Male rats secrete luteinizing hormone
and testosterone episodically. Endocrinology, 110: 1618-1627.
Euans DW (1988) Renal function in the elderly. Am Fam Physician,
38(3): 147-150.
Evens RP & Hawkins DW (1984) Bone and joint disorders. In: Covington
TR & Ingram Walker J ed. Current geriatric therapy. Philadelphia,
Pennsylvania, W.B. Saunders Co., pp 277-306.
Exton-Smith AN (1985) Mineral metabolism. In: Finch CE & Schneider
EL ed. Handbook of the biology of aging. New York, Van Nostrand
Reinhold Company, pp 511-539.
Fabris N (1981) Ontogenetic and phylogenetic aspects of
neuroendocrine-immune network. Dev Comp Immunol, 5(suppl 1): 49-60.
Fabris N (1991) Neuroendocrine-immune interactions: a theoretical
approach of aging. Arch Gerontol Geriatry, 12: 219-230.
Fabris N & Piantanelli L (1982) Thymus-neuroendocrine interactions
during development and aging. In: Adelman RC & Roth GS ed. Hormones
and aging. Boca Raton, Florida, CRC Press, pp 167-181.
Fabris N & Provinciali M (1989) Hormones. In: Nelson D ed. Natural
immunity. New York, London, San Francisco, Academic Press, pp
306-347.
Fabris N, Giunta S, & Muzzioli M (1983) Decline of T-cell potential
in diabetic and aged men. J Gerontol, 38(5): 548-554.
Fabris N, Mocchegiani E, Muzzioli M, & Provinciali M (1988)
Neuroendocrine-thymus interactions: perspectives for intervention in
aging. Ann NY Acad Sci, 521: 72-87.
Fabris N, Mocchegiani E, Marriotti S, Pacini F, & Pinchera A (1989)
Thyroid-thymus interactions during development and aging. Horm Res,
31: 85-98.
Fabris N, Mocchegiani E, Muzzioli M, & Provinciali M (1990) Zinc,
immunity and aging. In: Goldstein AL ed. Biomedical advances in
aging. New York, London, Plenum Press, pp 271-281.
Fando JL, Salinas M, & Wasterlain CG (1980) Age-dependent changes in
brain protein synthesis in the rat. Neurochem Res, 5: 373-378.
Feldman RD, Limbird LE, Nadeau J, Robertson D, & Wood AJ (1984)
Alterations in leukocyte ß-receptor affinity with aging. New Engl J
Med, 310: 815-819.
Felten DL, Felten SY, Carlsan SL, Olschowka JA, & Livnat S (1985)
Neuroadrenergic and peptidergic innervation of lymphoid tissue. J
Immunol, 135: 755.
Fernandes G (1984) Nutrional factors: Modulating effects on immune
function and aging. Pharmacol Rev, 36(2): 123-129.
Fernandes G, Friend P, & Yunis EJ (1977) Influence of calorie
restriction on autoimmune disease. Fed Proc, 6: 1313.
Ferro-Luzzi A, Maiani G, Scaccini C, D'Amicis A, Borgioni G, Polito
A, Ronaldi L, Sett S, Branca F, Azzini E, Arena A, Raguzzini A,
Castata G, & Dente MG (1988) In: Lintas C & Spadoni MA ed.
Nutritional vulnerability of the Italian elderly: Facts and figures.
Rome, National Council for Research, pp 275-294 (Monografia No. 28).
Finch CE (1991) Longevity, senescence, and the genome. Chicago,
University of Chicago Press.
Finch CE & Hayflick L ed. (1977) Handbook of the biology of aging.
New York, Van Nostrand Reinhold Company.
Finch CE & Landfield PW (1985) Neuroendocrine and autonomic
functions in aging mammals. In: Finch CE & Schneider EL ed. Handbook
of the biology of aging, 2nd ed. New York, Van Nostrand Reinhold
Company, pp 645-691.
Fishbein L (1991) Biological effects of dietary restriction. Berlin,
Heidelberg, New York, Springer-Verlag.
Fleming BB & Barrows CH Jr (1982a) The influence of aging on
intestinal absorption of vitamins A + D by the rat. Exp Gerontol,
17: 115-120.
Fleming BB & Barrows CH Jr (1982b) The influence of aging on
intestinal absorption of vitamin B12 and niacin in rats. Exp
Gerontol, 17: 121-126.
Fraga CG, Shigenaga MK, Park JW, Degan P, & Ames BN (1990) Oxidative
damage to DNA during aging: 8-hydroxy-2-deoxyguanosine in rat organ
DNA and urine. Proc Natl Acad Sci (USA), 87: 4533-4537.
Friedman SA, Raizner RE, Rosen H, Solomon NA, & Wilfredo SY (1972)
Functional defects in the aging kidney. Ann Intern Med, 76: 41-45.
Friedman FK, Robinson RC, & Rifkind J (1989) Age-related changes in
the iron spin state of testosterone-binding rat liver microsomal
cytochromes P450. Biochem Biophys Res Commun, 158: 480-484.
Friend PS, Fernandes G, Good RA, Michael AF, & Yunis EJ (1978)
Dietary restrictiions early and late. Effects on nephropathy of the
NZB and NZW mouse. Lab Invest, 38: 629-632.
Frith CH & Ward JM (1988) Colour atlas of neoplastic and
non-neoplastic lesions in aging mice. Amsterdam, Oxford, New York,
Elsevier Science Publishers.
Fujita S, Katagawa H, Ishizawa H, Suzuki T, & Kitani K (1985)
Age-associated alterations in hepatic glutathione-s-transferase
activities. Biochem Pharmacol, 34: 3891-3894.
Furukawa T, Inoue M, Kajiya F, Inada H, Takasugi S, Fukui S, Takeda
H, & Abe H (1975) Assessment of biological age by multiple
regression analysis. J Gerontol, 30: 422-434.
Gaitz CM & Baer PE (1971) Characteristics of elderly patients with
alcoholism. Arch Gen Psychiatry, 24: 372.
Galinsky RE, Kane RE, & Franklin MR (1986) Effect of aging on
drug-metabolizing enzymes important in acetaminophen elimination. J
Pharmacol Exp Ther, 237: 107-113.
Galinsky RE, Johnson DH, Kane RE, & Franklin MR (1990) Effect of
aging on hepatic biotransformation in female Fischer 344 rats:
changes in sulfotransferase activities are consistent with known
gender-related changes in pituitary growth hormones secretions in
aging animals. J Pharmacol Exp Ther, 255: 577-583.
Gallagher D, Thompson LW, & Levy SM (1980) Clinical psychological
assessment of older adults. In: Poon LW ed. Aging in the 1980s:
Psychological issues. Washington, DC, American Psychological Press,
pp 19-40.
Gambert SR & Tsitouras PD (1985) Effect of age on thyroid hormone
physiology and function. J Am Geriatr Soc, 33(5): 360-365.
Gart JJ, Krewski D, Lee PN, Tarone RE, & Wahrendorf J (1986)
Statistical methods in cancer research. Vol III: The design and
analysis of long-term animal experiments. Lyon, International Agency
for Research on Cancer, 219 pp (IARC Scientific Publications No.
79).
Gilchrest BA (1984) Skin and aging processes. Boca Raton, Florida,
CRC Press, pp 1-120.
Globerson AR, Abel EI, & Ren-Menahem D (1989) Developmental aspects
of T-lymphocytes in aging. New York, London, Plenum Press, pp
363-373.
Goldstein RS, Pasino DA, & Hook JB (1986) Cephaloridine
nephrotoxicity in aging male Fischer 344 rats. Toxicology, 38:
43-63.
Goldstein RS, Tarloff JB, & Hook JB (1988) Age-related nephropathy
in laboratory rats. FASEB J, 2: 2241-2251.
Gollamudi HR, Prasanna R, Rao RH, Lawrence WH, & Autian J (1983)
Impaired metabolism of di (2-ethylhexyl)phthalate (DEHP) in old rats
- an in vitro study. J Toxicol Environ Health, 12: 623-632.
Gompertz B (1825) On the nature of the function expressive of the
law of human mortality and on a new mode of determining life
contingencies. Phil Trans R Soc London, II: 513-585.
Gossen JA, De Leeuw WJF, Tan CHT, Zwarthoff EC, Berends F, Lohman
PHM, Knook DL, & Vijg J (1989) Efficient rescue of integrated shutle
vectors from transgenic mice: a model for studying mutations in
vivo. Proc Natl Acad Sci (USA), 86: 7971-7975.
Govoni S, Memo M, Spano PF, & Trabucchi M (1979) Chronic lead
treatment differentially affects dopamine synthesis in various rat
brain areas. Toxicology, 12: 343-349.
Govoni S, Rius RA, Battaini F, Magnoni MS, Lucchi L, & Trabucchi M
(1988) The central dopaminergic system: Susceptibility to risk
factors for accelerated aging. Gerontology, 34: 29-34.
Goyal KK (1982) Changes with age in the aorta of man and mouse. Exp
Gerontol, 17: 127-132.
Graham, P (1985) The eye. In: Pathy MSJ ed. Principles and practice
of geriatric medicine. New York, Chichester, Brisbane, Toronto, John
Wiley and Sons, pp 833-844.
Grandjean P (1983) Behavioral toxicology of heavy metals. In:
Zbinden G, Cuomo V, Recagni G, & Weiss B ed. Application of
behavioral pharmacology in toxicology. New York, Raven Press, vol 2,
pp 331-339.
Greden JF, Flegel P, Haskett R, Dilsaver S, Carrol BJ, Grunhaus L, &
Genero N (1986) Age effects in serial hypothalamic-pituitary-adrenal
monitoring. Psychoneuroendocrinology, 11(2): 195-204.
Greenberg LH & Weiss B (1978) beta-Adrenergic receptors in aged rat
brain: reduced number and capacity of pineal gland to develop
supersensitivity. Science, 201: 61-63.
Greenberg LH & Weiss B (1979) Ability of aged rats to alter
beta-adrenergic receptors of brain in response to repeated
administration of reserpine and desmethylimipramine. J Pharmacol Exp
Ther, 211: 309-315.
Greenberg LH & Weiss B (1983) Neuroendocrine control of
catecholaminergic receptors in aging brain. In: Agnoli A, Crepaldi
G, Spano PF, & Trabucchi M ed. Aging brain and ergot alkaloids. New
York, Raven Press, pp 37-52.
Greenblatt DJ, Divoll MK, Harmatz JS, & Shader RI (1988) Antipyrine
absorption and disposition in the elderly. Pharmacology, 36:
125-133.
Greenstein BD, Fitzpatrick FT, Kendall MD, & Wheeler MJ (1987)
Regeneration of the thymus in old male rats treated with a stable
analogue of LHRH. J Endocrinol, 11: 345-350.
Gregerman RI & Solomon N (1967) Acceleration of thyroxine and
triiodothyronine turnover during bacterial pulmonary infections and
fever: implications for the functional state of the thyroid during
stress and in senescence. J Clin Endocrinol Metab, 27: 93-105.
Groop PH (1989) The influence of body weight, age and glucose
tolerance on the relationship between GIP secretion and beta-cell
function in man. Scand J Clin Lab Invest, 49(4): 367-379.
Gu X (1986) The life expectancy of the people of China. In: Cui Y
ed. Public health in the People's Republic of China. Beijing,
People's Medical Publishing House, pp 87-91.
Gupta SP, Mehta FS, & Irani RR (1980) Comparison of mortality rates
among bidi smokers and tobacco chewers. Ind J Cancer, 17: 149-152.
Guthrie S, Cooper RL, Thurman R, & Linnoila M (1987)
Pharmacodynamics and pharmacokinetics of ethanol, diazepam, and
pentobarbital in young and aged rats. Pharmacol Toxicol, 61:
308-312.
Hadden JW (1983) Cyclic nucleotides and related mechanisms in immune
regulation: A mini review. In: Fabris N, Garaci E, Hadden J, &
Mitchison NA ed. Immunoregulation. New York, London, Plenum Press,
pp 201-230.
Halberg F (1982) Biological rhythms, hormones and aging. In:
Vernandakis A ed. Hormones in development and aging. New York,
Spectrum Publications, pp 451-476.
Hall HRS, O'Grady MP, & Farrah JM Jr (1989) The
hypothalamic-pituitary-adrenal axis by thymic peptides. In: Hadden
JW, Massek K, & Nistico G ed. Interactions among central nervous
system, neuroendocrine and immune system. Rome, Pytagora Press, pp
112-125.
Hanawalt PC (1987) On the role of DNA damage and repair processes in
aging: evidence for and against. In: Warner HR, Butler RN, Sprott
RN, & Schneider EL ed. Modern biological theories of aging. New
York, Raven Press, pp 183-198.
Hanninen H (1982) Behavioral effects of occupational exposure to
mercury and lead. Acta Neurol Scand, 66(suppl 92): 167-175.
Hansen ON, Trillingsgaard A, Beese I, Lyngbye T, & Grandjean P
(1985) Neurobehavioral methods in assessment of children with
low-level lead exposure. In: Neurobehavioral methods in occupational
and environmental health (Document 3). Copenhagen, World Health
Organization, Regional Office for Europe, pp 183-187. Harman D
(1981) The aging process. Proc Natl Acad Sci (USA), 78: 7124-7128.
Harman D (1982) Nutritional implications of the radical theory of
aging. J Am Coll Nutr, 1: 27-34.
Harman SM, Tsitouras PD, Costa PT, & Blackman MR (1982) Reproductive
hormones in aging men. II. Basal pituitary gonadotropins and
gonadotropin responses to luteinizing hormone releasing hormone. J
Clin Endocrinol Metab, 54: 547-551.
Harrison DE, Astle CM, & Delaittre JA (1978) Loss of proliferative
capacity in immunohemopoietic stem cells caused by serial
transplantation rather than ageing. J Exp Med, 147(5): 1526-1531.
Hayflick L & Moorhead PS (1961) The serial cultivation of human
diploid cell strains. Exp Cell Res, 25: 585-621.
Hazzard WR (1985) Aging and atherosclerosis. Teasing out the
contributions of time, secondary aging, and primary aging. Clin
Geriatr Med, 1(1): 251-284.
Hebert CD & Birnbaum LS (1987) The influence of aging on intestinal
absorption of TCDD in rats. Toxicol Lett, 37: 47-55.
Hendley DD, Mildvan AS, Reporter MC, & Strehler BL (1963) The
properties of isolated human cardiac age pigment. II Chemical and
enzymatic properties. J Gerontol, 18: 250-259.
Hershkowitz M (1983) Mechanisms of brain aging - The role of
membrane fluidity. In: Gispen WH & Traber J ed. Aging of the brain.
Amsterdam, Oxford, New York, Elsevier Science Publishers, pp 85-98.
Hirsch HR (1982) Evolution of senescence: natural increase of
population dispaying Gompertz or power-law death rates and constant
of age-dependent maternity rates. J Theor Biol, 98: 321-346.
Hochschild R (1989) Improving the precision of biological age
determinations. Part I. A new approach to calculating biological
age. Exp Gerontol, 24: 289-300.
Hofecker G, Skalicky M, Kment A, & Niedermuller H (1980) Models of
the biological age of the rat. I. A factor model of age parameters.
Mech Ageing Dev, 14: 345-359.
Hogstedt C, Hane M, Agrell A, & Bodin L (1983) Neuropsychological
test results and symptoms among workers with well-defined long-term
exposure to lead. Br J Ind Med, 40: 99-105.
Hollander D, Dadufalz V, Weindruch R, & Walford RL (1986) Influence
of life-prolonging dietary restriction on intestinal vitamin A
absorption in mice. Age, 9: 57-60.
Hollingsworth JW, Hashizume A, & Jablon S (1965) Correlations
between tests of aging in Hiroshima subjects - an attempt to define
"physiological age." Yale J Biol Med, 38: 11-26.
Holt AR, Pascal RR, & Kotler DP (1984) Effect of aging upon small
intestinal structure in the Fischer rat. J Gerontol, 39: 642-647.
Holt PR, Yeh KY, & Kotler DP (1988) Altered controls of
proliferation in proxismal small intestine in the senescent rat.
Proc Natl Acad Sci (USA), 85: 2771-2775.
Honma T, Miyagawa M, & Sato M (1987) Methyl bromide alters
catecholamine and metabolite concentrations in rat brain.
Neurotoxicol Teratol, 9: 369-375.
Horbach GJM, Princen HMG, Van der Kroef M, Van Bezooijen CFA, & Yap
SH (1984) Changes in the sequence content of albumin mRNA and in
its translational activity in the rat liver with age. Biochim
Biophys Acta, 83: 60-66.
Horbach GJM, Van der Boom H, Van Bezooijen CFA, & Yap SH (1986)
Molecular aspects of age-related changes in albumin synthesis in
rats. In: Van Bezooijen CFA, Miglio F, & Knook DL ed. Liver drugs
and aging. Rijswijk, The Netherlangs, Eurage, pp 121-126 (Topics in
Aging Research in Europe, Volume 7).
Horbach GJM, Durham SK, Yap SH, & Van Bezooijen CFA (1988a) Albumin
elimination in female WAG/Rij rats with age: A longitudinal study.
Mech Ageing Dev, 43: 137-152.
Horbach GJM, Van der Boom H, Yap SH, & Van Bezooijen CFA (1988b)
Molecular aspects of age-related changes in albumin synthesis in
female WAG/Rij rats. Life Sci, 43: 1707-1714.
Hosomi T (1990) Japanese economy and society. In: Institute of
Japanese Problems, Chinese Academy of Social Sciences and Japanese
Vital Insurance Company ed. Proceeding of a Symposium on Sino-Japan
Social Countermeasure for Aging Problems. Beijing, Dong-Fang
Publishing House pp 7-30.
Houck JC, Dehesse C, & Jacob R (1967) The effect of aging upon
collagen catabolism. Symp Soc Exp Biol, 21: 403-425.
Hsu WH & Kakuk TJ (1984) Effect of amitraz and chlordimeform on
heart rate and pupil diameter in rats: Mediated by alpha
2-adrenoreceptors. Toxicol Appl Pharmacol, 73: 411-415.
Hunter J, Urbanowicz MA, Yule W, & Landsdown R (1985) Automated
testing of reaction time and its association with lead in children.
Int Arch Occup Environ Health, 57: 27-34.
Hunziker PE & Kagi JHR (1985) Metallothionein. In: Harrison PM ed.
Metalloproteins: Part II. London, MacMillan Press Ltd.
Huskisson EC (1983) NSAIDS: A topical review. Geriatr Med, 12:
867-870.
IARC (1982) Chemicals, industrial processes and industries
associated with cancer in humans. IARC Monographs, Volumes 1 to 29.
Lyon, International Agency for Research on Cancer, 292 pp (IARC
Monographs on the Evaluation of the Carcinogenic Risk of Chemicals
to Humans, Supplement 4).
IARC (1986) In: Montesano R, Bartsch H, Vainio H, Wilbourne J, &
Yamasaki H ed. Long-term and short-term assays for carcinogens: A
critical appraisal. Lyon, International Agency for Research on
Cancer, 564 pp (IARC Scientific Publications No. 83).
IARC (1988) Alcohol drinking. Lyon, International Agency for
Research on Cancer, 416 pp (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Volume 44).
IARC (1990) In: Tomatis L ed. Cancer: causes, occurrence and
control. Lyon, International Agency for Research on Cancer, 352 pp
(IARC Scientific Publications No. 100).
Imayama S & Braverman IM (1989) A hypothetical explanation for the
aging of skin. Chronologic alterations of the three-dimensional
arrangement of collagen and elastic fibres in connective tissue. To
provide a morphologic basis for a better understanding of the
"aging" of human. Am J Pathol, 134(5): 1019-1025.
Ingbar SH (1978) The influence of aging on the human thyroid hormone
economy. In: Greennblatt RB ed. Geriatric endocrinology. New York,
Raven Press, pp 13-31.
Ingram DK, Cutler RG, Weindruch R, Renquist DM, Knapka JJ, April M,
Belcher CT, Clark MA, Hatcherson CD, Marriott M, & Roth GS (1990)
Dietary restriction and aging: the initiation of a primate study. J
Gerontol, 45(5): 148-163.
Iqbal K, Grundke-Iqbal I, Merz PA, & Wisniewski HM (1982)
Age-associated neurofibrillary changes. In: Giacobini E, Filogamo G,
Giacobini G, & Vernadakis A ed. The aging brain: cellular and
molecular mechanisms of aging in the nervous system. New York, Raven
Press, pp 247-257.
Iwasaki K, Chirago T, Tada K, Noda K, & Noguchi H (1986) Age- and
sex-related changes in amine sulphoconjugations in Sprague-Dawley
strain rats. Comparison with phenol and alcohol sulphoconjugations.
Xenobiotica, 16: 717-723.
Iwasaki K, Gleiser CA, Masoro EJ, McMahan CA, Seo E, & Yu BP (1988)
Influence of the restriction of individual dietary components on
longevity and age-related disease of Fisher rats: The fat component
and the mineral component. J Gerontol, 43: B13-B21.
Iwase A, Kitazawa Y, & Ohno Y (1988) On age-releated norms of the
visual field. Jpn J Ophthalmol, 34(4): 429-437.
Iwata T, Incefy GS, Tanaka T, Fernandes G, Menendez-Botel CI, Pih K,
& Good RA (1979) Circulating thymic hormone levels in zinc
deficiency. Cell Immunol, 47: 100-105.
Jackson J, Banks YB, & Birnbaum LS (1990) Maximal dermal absorption
of TCDD occurs in weanling rats. Toxicologist, 10: 309.
Jaenisch R (1988) Transgenic animals. Science, 240: 1468-1474.
Jones MK, Weisenburger WP, Sipes IG, & Russell DH (1987) Circadian
alterations in prolactin, corticosterone, and thyroid hormone levels
and down-regulation of prolactin receptor activity by
2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol, 87:
337-350.
Kaldor JM & Day NE (1987) Interpretation of epidemiological studies
in the context of multistage model of carcinogenesis. In: Barrett JC
ed. Mechanisms of environmental carcinogenesis. Boca Raton, Florida,
CRC Press, vol 2, pp 21-57.
Kalimi M (1982) Comparison of glucocorticoid receptors in various
tissues of adult and senescent rats. Gerontology, 28: 371-376.
Kamataki T, Maeda K, Shimado M, Kitani K, Nagai T, & Kato R (1985a)
Age-related alteration in the activities of drug-metabolizing
enzymes and contents of sex-specific forms of cytochrome P450 in
liver microsomes from male and female rats. J Pharmacol Exp Ther,
233: 222-228.
Kamataki T, Shimada M, Maeda K, & Kato R (1985b) Pituitary
regulation of sex-specific forms of cytochrome P-450 in liver
microsomes of rats. Biochem Biophys Res Commun, 130: 1247-1253.
Kampmann JP & Hansen JEM (1979) Renal excretion of drugs. In: Crooks
J & Stevenson IH ed. Drugs and the elderly. Baltimore, Maryland,
University Park Press, pp 77-87.
Kanai S, Kitani K, Fujita S, & Kitagawa H (1985) The hepatic
handling of sulfobrompthalein in aging Fischer-344 rats: in vivo
and in vitro studies. Arch Gerontol Geriatr, 4: 73-85.
Kanai S, Kitani K, Sato Y, & Nokubo M (1988) The age-dependent
decline in the biliary transport maximum of conjugated
sulfobromophthalein in the rat. Arch Gerontol Geriatr, 7: 1-8.
Kane R (1987) Comprehensive assessment. In: Maddox GL ed. The
encyclopedia of aging. Berlin, Heidelberg, New York,
Springer-Verlag, pp 137-139.
Kannel WB (1981) Update on the role of cigarette smoking in coronary
artery disease (review). Am Heart J, 101: 319-328.
Kato H, Saito M, & Suda M (1980) Effect of starvation on the
circadian adrenocortical rhythm in rats. Endocrinology, 106:
918-921.
Katz ML & Robinson WG Jr (1987) Light and aging effects on vitamin E
in the retinal pigment epithelium. Vision Res, 27(11): 1875-1879.
Katzman R & Terry RD (1983) Normal aging in the nervous system. In:
Katzman R & Terry RD ed. The neurology of aging. Philadelphia,
Pennsylvania, F.A. Davis, pp 15-50.
Kaur S & Gill SS (1985) Age-related changes in the activities of
epoxide hydrolyases in different tissues of mice. Drug Metab Dispos,
13: 711-715.
Kaysen GA & Myers BD (1985) The aging kidney. Clin Geriatr Med,
1(1): 207-222.
Keast D & Ayre DJ (1981) Effects of chronic tabocco smoke exposure
on immune responses in aged mice. Arch Environ Health, 36: 201-207.
Kelley WW, Brief S, Westly HJ, Novakofski J, Bechtel PJ, Simon J, &
Walker EB (1986) GH3 pituitary adenoma cells can reverse thymic
aging in rats. Proc Natl Acad Sci (USA), 83: 5663-5667.
Kelso A & Munck A (1984) Glucocorticoid inhibition of lymphokine
secretion by alloreactive T lymphocyte clones. J Immunol, 133(2):
784-791.
Kim JCS & Kaminsky LS (1988) 2,2,2-Trifluoroethanol toxicity in aged
rats. Toxicol Pathol, 16: 35-45.
Kinon GA & Liu C (1973) Diurnal variation in plasma testosterone of
the male laboratory rat. Horm Metab Res, 5: 233-234.
Kitani K (1985) The role of the liver in pharmacokinetic and
pharmacodynamic alterations in the elderly. In: O'Malley K &
Waddington J ed. Therapeutics in the elderly. Amsterdam, Oxford, New
York, Elsevier Science Publishers, pp 19-34.
Kitani K (1988) Drugs and the ageing liver. Life Chem Rep, 6:
143-230.
Kitani K (1991) Aging of the liver: facts and theories. Arch
Gerontol Geriatr, 12: 133-154.
Kitani K, Masuda Y, Sato Y, Kanai S, & Nokubo M (1984) Increased
anti-convulsant activity in aging BDF1 mice. J Pharmacol Exp Ther,
229: 231-236.
Kitani K, Ohta M, Kanai S, & Sato Y (1985a) Sex difference in the
biliary excretion of digoxin and its metabolites in aging Wistar
rats. Arch Gerontol Geriatr, 4: 1-12.
Kitani K, Sato Y, Kanai S, Nokubo M, Ohta M, & Masuda Y (1985b)
Increased anticonvulsant effect of phenobarbital with age in mice -
a possible pharmacologic index for brain aging. Life Sci, 37:
1451-1460.
Kitani K, Sato Y, Kanai S, Nokubo M, Ohta M, Masuda Y, & Klotz U
(1986) Altered response to convulsants and anticonvulsants in aging
BDF1 mice. In: Kitani K ed. Liver and aging 1986: Liver and brain.
Amsterdam, Oxford, New York, Elsever Science Publishers, pp 293-307.
Kitani K, Sato Y, Kanai S, Nokubo M, Ohta M, & Masuda Y (1987)
Increasing anticonvulsant effect of AD-810 (Zonisamide) in aging
BDF1 mice. Life Sci, 41: 1339-1344.
Klatsky AL, Friedman GD, & Siegelaub AB (1981) Alcohol and
mortality. A ten year Kaiser-Permanente experience. Ann Intern Med,
95: 139-145.
Klawans HL & Tanner CM (1984) Movement disorders in the elderly. In:
Albert M ed. Clinical neurology of aging. Oxford, Oxford University
Press, pp 387-403.
Klotz U, Avant GR, Hoyumpa A, Schenker S, & Wilkinson GR (1975) The
effects of age and liver disease on the disposition and elimination
of diazepam in adult man. J Clin Invest, 55: 347.
Klug TL & Adelman RC (1977) Evidence for a large thyrotropin and its
accumulation during aging in rats. Biochem Biophys Res Commun, 77:
1431-1437.
Koch-Weser J, Greenblatt DJ, Seller EM, & Shoder RI (1982) Drug
disposition in old age. New Engl J Med, 306: 1081-1086.
Kohrs MB (1981) Nutritional needs of the aged. Nutrition, 7: 39-46.
Konishi N & Ward JM (1989) Increased levels of DNA synthesis in
hyperplastic renal tubules of aging nephropathy in female F344/NCR
rats. Vet Pathol, 26: 6-10
Koobs DH, Schultz RL, & Jutzy RV (1978) The origin of lipofuscin and
possible consequences to the myocardium. Arch Pathol Lab Med, 102:
66-68.
Kozararevic DJ, McGee D, Vojvodic N, Racic Z, Dawber T, Gordon T, &
Zukel W (1980) Frequency of alcohol consumption and morbidity and
mortality: The Yugoslavia cardiovascular disease study. Lancet, i:
613-616.
Krupka L & Vener A (1979) Hazards of drug use among the elderly.
Gerontologist, 19: 90-95.
Kubos C, Day NK, & Good RA (1984) Influence of early or late dietary
restirction on life span and immunological parameters in
MRL/MP-Ipr/Ipr mice. Proc Natl Acad Sci (USA), 81: 5831-5835.
Kumpuris D (1983) Gastrointestinal bleeding in the older patient.
In: Texter EC ed. The aging gut. New York, Masson Publishing Co., pp
57-63.
Kyle ME & Kocsis JJ (1985) The effect of age on salicylate-induced
nephrotoxicity in male rats. Toxicol Appl Pharmacol, 81: 337-347.
Lakatta EG (1980) Age-related alteration in the cardiovascular
response to adrenergic mediated stress. Fed Proc, 39: 3173-3177.
Lakatta EG (1985) Heart and circulation. In: Finch CE & Schneider EL
ed. Handbook of the biology of aging, 2nd ed. New York, Van Nostrand
Reinhold Company, pp 377-413.
Lakatta EG (1986) Diminished beta-adrenergic modulation of
cardiovascular function in advanced age. Cardiol Clin, 4(2):
185-200.
Lakatta EG (1987a) Cardiac muscle change in senescence. Annu Rev
Physiol, 49: 519-531.
Lakatta EG (1987b) Catecholamines and cardiovascular function in
aging. Endocrinol Metab Clin North Am, 16(4): 877-891.
Landauer MR, Tomlinson WT, Balster RL, & MacPhail RC (1984) Some
effects of the formamidine pesticide on the behavior of mice.
Neurotoxicology, 5: 91-100.
Leakey JEA, Cunny HC, Bazare J Jr, Webb PJ, Feuers RJ, Duffy PH, &
Hart RW (1989a) Effects of aging and caloric restriction on hepatic
drug metabolizing enzymes in the Fischer 344 rat. I: The cytochrome
P-450 dependent monooxygenase system. Mech Ageing Dev, 48: 145-155.
Leakey JEA, Cunny HC, Bazare J Jr, Webb PJ, Lipscomb JC, Siikkes W
Jr, Feuers RJ, Duffy PH, & Hart RW (1989b) Effects of aging and
caloric restriction on hepatic drug metabolizing enzymes in the
Fischer 344 rat. II. Effects on conjugating enzymes. Mech Ageing
Dev, 48: 157-166.
Lee KT ed. (1985) Atherosclerosis. Ann NY Acad Sci, 454: 1-327.
Lehmann AR, Hoeijmakers JHJ, Van Zealand AA, Backendorf CMP, Bridges
BA, Collins A, Fuchs RPD, Margison GP, Montesano R, Moustacchi E,
Natarajan AT, Radman M, Sarasin A, Seeberg E, Smith CA, Stefanini M,
Thompson LH, Van der Schans GP, Weber CA, & Zdzienicka MZ (1992)
Workshop on DNA repair. Mutat Res, 273: 1-28.
Leopold AC (1975) The biological significance of death in plants.
In: Benke JA, Finch CE, & Moment GB ed. The biology of aging. New
York, London, Plenum Press, pp 101-114.
Leppaluoto J, Ranta T, & Tuomisto J (1974) Diurnal variations of
serum immunoassayable thyrotropin (TSH) concentration in the rat.
Acta Physiol Scand, 90: 699-702.
Lesser GT, Deutsch S, & Markofsky J (1973) Aging in the rat:
Longitudinal and cross-sectional studies of body composition. Am J
Physiol, 225: 1472-1478.
Leto S, Kokkahen GC, & Barrows CH Jr (1976) Dietary protein, life
span and physiological variables in female mice. J Gerontol, 31:
149-154.
Levy ML (1987) Tous les pays du monde (1987). Bull Mens Inf Démogr
Econ Soc, 216:1-6.
Lewis VM, Twomey JJ, Bealmar P, Goldstein G, & Good RA (1978) Age,
thymic involution, and circulating thymic hormone activity. J Clin
Endocrinol Metab, 47: 145-150.
Li DD, Chien YK, Gu MZ, Richardson A, & Cheung HT (1988) The
age-related decline in interleukin-3 expression in mice. Life Sci,
43: 1215-1222.
Liebow AA (1964) Biochemical and structural changes in the aging
lung. Summary. In: Cander L & Moyer JH ed. Aging of the lung. New
York, London, Grune and Stratton, pp 97-104.
Liepa GU, Masoro EJ, Bertrand HA, & Yu BP (1980) Food restriction as
a modulation of age-related changes in serum lipids. Am J Physiol,
238: E253-E257.
Likhachev AJ (1985) Effect of age on DNA repair in carcinogenesis
due to alkylating agents. In: Likhachev A, Anisimov V, & Montesano R
ed. Age-related factors in carcinogenesis. Lyon, International
Agency for Research on Cancer, pp 239-246 (IARC Scientific
Publications No. 58).
Lin CF & Hayton WL (1983) GI motility and subepithelial blood flow
in mature and senescent rats. Age, 6: 46-51.
Lindeman RD (1986) The aging kidney. Compr Ther, 12(3): 43-49.
Lindeman RD, Tobin JD, & Shock NW (1987) Hypertension and the
kidney. Nephron, 47(1): 62-67
Lindsay R (1989) Osteoporosis: An updated approach to prevention and
management. Geriatrics, 44: 45-54.
Lipschitz DA (1987) Nutrition, aging and the immunohematopoietic
system. Clin Geriatr Med, 3(2): 319-328.
Loft S, Dossing M, & Poulsen HE (1988) Influence of age and
consumption of tobacco, alcohol, and caffeine on antipyrine
clearance. Hum Toxicol, 7: 277-280.
Loi CM & Vestal RE (1988) Drug metabolism in the elderly. Pharmacol
Ther, 36: 131-149.
Lucchi L, Memo M, Airaghi ML, Spano PF, & Trabucchi M (1981) Chronic
lead treatment induces in rat a specific and differential effect on
dopamine receptors in different brain areas. Brain Res, 213:
397-404.
Lutz RJ, Dedrick RL, Matthews HB, Elling TE, & Anderson MW (1977) A
preliminary pharmacokinetic model for several chlorinated biphenyls
in the rat. Drug Metab Dispos, 5: 386-396.
McCaffrey TA, Nicholson AG, Szabo PE, & Wexsler BB (1988) Aging and
atherosclerosis. The increased proliferation of arterial smooth
muscle cells isolated from old rats is associated with increased
platelet-derived growth factor-like activity. J Exp Med, 167:
163-174.
McCay CM, Crowell MM, & Mayrar LA (1935) The effect of reduced
growth upon the length of the life span and upon ultimate body size.
J Nutr, 10: 63-79.
McClure JE, Lameris N, Wara DW, & Goldstein AL (1982) Immunochemical
studies on thymosin: radioimmunoassay of thymosin alpha-1. J
Immunol, 128(1): 368-375.
McGinty D, Stern N, & Akshoomoff N (1988) Circadian and
sleep-related modulation of hormone levels changes with aging. In:
Sowers JR & Felicett JV ed. The endocrinology of aging. New York,
Raven Press, pp 75-111.
McKenna MJ & DiStefano V (1977) Carbon disulphide. II. A proposed
mechanism for the action of carbon disulfide on
dopamine-beta-hydroxylase. J Pharmacol Exp. Ther, 202: 253-266.
McMahon TF (1988) Age-related changes in colonic phase I and phase
II biotransformation: possible relationship to colon carcinogenesis.
Baltimore, University of Maryland, School of Pharmacy
(Dissertation).
McMahon TF & Birnbaum LS (1990a) Age-related changes in the
disposition and metabolism of xenobiotics. In: Cooper RL, Goldman
JM, & Harbin TJ ed. Aging and environmental toxicology. Baltimore,
Maryland, Johns Hopkins University Press, pp 7-30.
McMahon TF & Birnbaum LS (1990b) Age related change on toxicity and
biotransformation of potassium cyanide in male C57BL/6N mice.
Toxicol Appl Pharmacol, 105: 305-314.
McMahon TF, Beierschmitt WP, & Weiner M (1987) Changes in phase I
and phase II biotransformation pathways with age in male Fischer 344
rat colon: relationship to colon carcinogenesis. Cancer Lett, 36:
273-282.
McMahon TF, Diliberto JJ, & Birnbaum LS (1989) Age-related changes
in the disposition of benzyl acetate: A model compound for glycine
conjugation. Drug Metab Dispos, 17: 506-512.
McMahon TF, Diliberto JJ, & Birnbaum LS (l990a) Effects of age on
metabolism and disposition of salicylic acid (SAL) in male Fischer
344 rats. Drug Metab Dispos, 18: 494-503.
McMahon TF, Peggins JO, Centra MM, & Weiner M (l990b) Age-related
changes in biotransformation of azoxymethane and methylazoxymethanol
in vitro. Xenobiotica, 20: 501-513.
Maeda H, Gleiser CA, Masoro EJ, Murata I, McMohan CA, & Yu BP (1985)
Nutritional influences on aging of Fisher 344 rats. II. Pathology. J
Gerontol, 40: 671-688.
Magal E, Chaudhuri M, & Adelman RC (1986) The capability for
regulation of insulin secretion by somatostatin in purified
pancreatic islet B cells during aging. Mech Aging Dev, 33(2):
139-146.
Magnus K (1982) Trends in cancer incidence: Causes and practical
implications. Washington, DC, Hemisphere.
Mantel N & Schneiderman MA (1975) Estimation "safe" levels, a
hazardous undertaking. Cancer Res, 35: 1379-1386.
Mantere P, Hanninen H, Hernberg S, & Luukkonen R (1984). A
prospective follow-up study on psychological effects in workers
exposed to low levels of lead. Scand J Work Environ Health, 10:
43-50.
Marin R, Proverbio T, & Proverbio F (1985) Characterization of the
Na+, K+ -ATPase activity of basolateral membranes of kidney proximal
tubular cells from young and old rats. Biochem Pharmacol, 34:
4197-4201.
Marksberry WR, Ehmann WD, Hossain IM, Alauddin M, & Goodin DT (1981)
Instrumental neutron activation analysis of brain aluminum in
Alzheimer's disease and aging. Ann Neurol, 10: 511-516.
Marsh G (1980) Perceptual changes with aging. In: Busse EW & Blazer
DG ed. Handbook of geriatric psychiatry. New York, Van Nostrand
Reinhold Company, Chapter 7, pp 147-168.
Marshall JF & Berrios N (1979) Movement disorders of aged rats:
Reversal by dopamine receptor stimulation. Science, 206: 477-479.
Martin GM, Sprague CA, Norwood TH, & Pendergrass WR (1974) Clonal
selection, attenuation and differentiation in an in vitro model of
hyperplasia. Am J Pathol, 74: 137-154.
Marx JL (1987) A new wave of enzymes for cleaving pro-hormones.
Science, 235: 285-286.
Masoro EJ ed. (1981) CRC handbook of physiology in aging. Boca
Raton, Florida, CRC Press.
Masoro EJ (1985) Metabolism. In: Finch CE & Schneider EL ed.
Handbook of the biology of aging. New York, Van Nostrand Reinhold
Company, pp 540-563.
Masoro EJ (1988) Food restrictions in rabbits: an evolution of its
role in the study of aging. J Gerontol, 43: B59-B64.
Masoro EJ (1991) Use of rodents as models for the study of "normal
aging": Conceptual and practical issues. Neurobiol Aging, 12:
639-644.
Masoro EJ & Yu BP (1989) Diet and nephropathy. Lab Invest, 60:
165-167.
Masoro EJ, Yu BP, Bertrand HR, & Lynd FT (1980) Nutritional probe of
the aging process. Fed Proc, 39: 3178-3182.
Masoro EJ, Shimokawa I, & Yu BP (1991) Retardation of the aging
processes in rats by food restriction. Ann NY Acad Sci, 621:
337-352.
Master CL, Multhaup G, Simms G, Pottgieser J, Martins RN, &
Beyreuther K (1985) Neuronal origin of a cerebral amyloid:
Neurofibrillary tangles of Alzheimer's disease contain the same
protein as the amyloid of plaque cores and blood vessels. EMBO J, 4:
2757-2763.
Matulionis DH (1984) Chronic cigarette smoke inhalation and aging in
mice: Morphologic and functional lung abnormalities. Exp Lung Res,
7: 237-256.
Mauderly JL (1978) Effect of age on pulmonary structure and function
of immature and adult animals and man. Fed Proc, 38: 173-177.
Mauderly JL (1979a) Ventilation, lung volumes, and lung mechanics of
young adult and old Syrian hamsters. Exp Aging Res, 5: 497-508.
Mauderly JL (1979b) Effect of aging on pulmonary structure and
function of immature and adult animals and man. Fed Proc, 38:
173-177.
Mauderly JL (1982) The effect of age on respiratory function of
Fischer 344 rats. Exp Aging Res, 8: 31-36.
Mawhinney MG (1985) Etiological considerations for the growth of
stroma in benign prostatic hyperplasia. Fed Proc, 45: 2615-2617.
Mazzeo RS & Horvath SM (1987) A decline in myocardial and hepatic
norepinephrine turnover with age in Fischer 344 rats. Am J Physiol,
252: E762-E763.
Medvedev ZA (1984) Age changes of chromatin: A review. Mech Ageing
Dev, 28: 139-154.
Medvedev ZA (1990) An attempt at a rational classification of
theories of aging. Biol Rev, 65: 375-398.
Mehta T, Labuc GE, Urbanski SJ, & Archer MC (1984) Organ specificity
in the microsomal activation and toxicity of
N-nitrosomethylbenzylamine in various species. Cancer Res, 44:
4017-4022.
Meisami E (1988) Aging of the nervous system structural and
biochemical changes. In: Timiras PS ed. Physiological basis of aging
and geriatrics. New York, Macmillan Publishing Co, pp 123-143.
Mervis R (1981) Cytomorphological alterations in the aging animal
brain with emphasis on Golgi studies. In: Johnson JE ed. Aging and
cell structure. New York, London, Plenum Press, Chapter 4, pp
143-186.
Messing RB, Vasquez BJ, Spiehler VR, Martinez JL Jr, Jensen RA,
Rigter H, & McCaugh JL (1980) 3H-Dihydromorphine binding in brain
regions of young and aged rats. Life Sci, 26: 921-927.
Meyer BR & Bellucci A (1986) Renal function in the elderly. Cardiol
Clin, 4(2): 227-234.
Mezey E (1981) Alcohol and drug interactions in injury to the
digestive tract. Clin Gastroenterol, 10: 485-495.
Millard WJ, O'Sullivan DM, Fox TO, & Martin JB (1985) Sexually
dimorphic patterns of growth hormone secretion in rats. In: Crowley
WF Jr & Hofler JG ed. The episodic secretion of hormones. New York,
Chichester, Brisbane, Toronto, John Wiley and Sons, pp 287-304.
Miller SM (1989) Changes in endocrine function with aging. Clin Lab
Sci, 2(1): 19-20.
Miller R (1991) Aging and immune function. Int Rev Cytol, 124:
187-216.
Minaker KL, Meneilly GS, & Rowe JW (1985) Endocrine systems. In:
Finch CE & Schneider EL ed. Handbook of the biology of aging. New
York, Van Nostrand Reinhold Company, pp 433-456.
Mitsui Y, Sakagami H, Murota SI, & Yamada MA (1980) Age related
decline in histone H1 fraction in human diploid fibroblast cultures.
Exp Cell Res, 126: 289-298.
Miura K, Goldstein RS, Morgan DG, Pasino DA, Hewitt WR, & Hook JB
(1987) Age-related differences in susceptibility to renal ischemia
in rats. Toxicol Appl Pharmacol, 87: 284-296.
Moberg GP, Bellinger LL, & Mendel VE (1975) Effect of meal feeding
daily rhythms of plasma corticosterone and growth hormone in rat.
Neuroendocrinology, 19: 160-169.
Monteiro-Riviere NA, Banks YB, & Birnbaum LS (1991) Laser doppler
measurements of cutaneous blood flow in ageing mice and rats.
Toxicol Lett, 57: 329-338.
Montgomery PR & Sitar DS (1981) Increased serum salicylate
metabolites with age in patients receiving chronic acetylsalicylic
acid therapy. Gerontology, 27: 329-333.
Moolgavkar SH & Venzon DJ (1979) Two-event models for
carcinogenesis; incidence curves for childhood and adult tumors.
Math Biosci, 47: 55-77.
Mooradian AD & Song MK (1989) Age-related alterations in duodenal
calcium transport rate in rats. Mech Ageing Dev, 47: 221-227.
Moroni F, Lombardi G, Moneti G, & Aldinio C (1984) The excitotoxin
quinolinic acid is present in the brain of several mammals and its
cortical content increases during the aging process. Neurosci Lett,
47: 51-55.
Morell AG, Gregoriadis G, Scheinberg IH, Hickman J, & Ashwell G
(1971) The role of sialic acid in determining the survival of
glycoproteins in the circulation. J Biol Chem, 246: 1461-1467.
Morris JF, Mosk A, & Johnson LC (1971) Spirometric standards for
healthy non-smoking adults. Am Rev Respir Dis, 103: 57-67.
Mullaart E, Boerrigter METI, Lohman PHM, & Vijg J (1989) Age-related
induction and disappearance of carcinogen-DNA-adducts in livers of
rats exposed to low levels of 2-acetylaminofluorene. Chem-Biol
Interact, 69: 373-384.
Mullaart E, Lohman PHM, Berends F, & Vijk J (1990) Review DNA damage
metabolism and aging. Mutat Res, 237: 189-210.
Müller HF & Schwartz G (1978) Electroencephalograms and autopsy
findings in geropsychiatry. J Gerontol, 33: 504-513.
Muller WEG, Wenger R, Bachmann M, Ugarkovic D, Courtis NC, &
Schroder HC (1989) Poly(A) metabolism and aging: a current view.
Arch Gerontol Geriatr, 9: 31-250.
Munro HN (1984) Nutrition-related problems of middle age. Proc Nutr
Soc, 43: 281-288.
Murty CVR, Mancini MA, Chatterjee B, & Roy AK (1988a) Changes in
transcriptional activity and matrix association of alpha-2u-globulin
gene family in the rat liver during maturation and aging. Biochim
Biophys Acta, 949: 7-34.
Murty CVR, Olson MJ, Garg BD, & Roy AK (1988b) Hydrocarbon-induced
hyaline droplet nephropathy in male rats during senescence. Toxicol
Appl Pharmacol, 96: 380-392.
Mutti A, Falzoi M, Romanelli A, Bocchi MC, Ferroni C, & Franchini I
(1988) Brain dopamine as a target for solvent toxicity: Effects of
some monocyclic aromatic hydrocarbons. Toxicology, 49: 77-82.
Naessen R (1971) An enquiry on the morphological characteristics and
possible changes with age in the olfactory region of man. Acta
Otorhinol, 71: 49-62.
Nagel JE, Chopra RK, Chrest FJ, McCoy MT, Schneider EL, Holbrook NJ,
& Adler WH (1988) Decreased proliferation, interleukin 2 synthesis,
and interleukin 2 receptor expression are accompanied by decreased
mRNA expression in phytohemagglutinin-stimulated cells from elderly
donors. J Clin Invest, 81: 1096-1102.
Nagy K, ZS-Nagy V, Bertoni-Freddari C, & ZS-Nagy I (1983)
Alterations of the synaptosomal membrane 'microviscosity' in the
brain cortex of rats during aging and centrophenoxine treatment.
Arch Gerontol Geriatr, 2: 23-29.
Nakamura E, Kimura M, Nagata H, Miyao K, & Ozeki T (1982) Evaluation
of the progress of aging based on specific biological age as
estimated by various physiological functions. Jpn J Hyg, 36:
853-862.
NAS (1989) Biological markers in reproductive toxicology.
Washington, DC, National Academy of Sciences, National Academy
Press, pp 2-3.
Neafsey PJ (1990) Longevity hormesis. A review. Mech Ageing Dev, 51:
1-31.
Nebert DW & Gonzalez FJ (1987) P450 Genes - Structure, evolution and
regulation. Annu Rev Biochem, 56: 945-993.
Nebert DW, Nelson DR, Minor JC, Estabrook RW, Feyereisen R,
Fujii-Kuriyama Y, Gonzalez FJ, Guengerich FP, Gunsalus IC, Johnson
EF, Loper JC, Sato R, Waterman MR, & Waxman DR (1991) The P450
superfamily: Uptake on new sequences, gene mapping, and recommended
nomenclature. DNA Cell Biol, 10(1): 1-14.
Needleman HL, Gunnoe C, Leviton A, Reed R, Peresie H, Maher C, &
Barrett P (1979) Deficits in psychologic and classroom performance
of children with elevated dentine lead levels. New Engl J Med, 300:
689-695.
Neuhaus OW & Flory W (1978) Age-dependent changes in the excretion
of urinary proteins by the rat. Nephron, 22: 570-576.
Newaz SN, Fang W, & Strobel HW (1983) Metabolism of the carcinogen
1,2-dimethylhydrazine by isolated human colon microsomes and human
colon tumor cells in culture. Cancer, 52: 794-798.
Niedzwiecki A, Lewis PN, & Cinader B (1985) Changes of histone H1
subtypes with aging in strains of mice that possess different
immunological characteristics. J Gerontol, 40: 695-699.
Nokubo M & Kitani K (1988) Age-dependent decrease in the lethal
threshold of pentylenetetrazole in mice. Life Sci, 43: 41-47.
Norwood TH & Smith JR (1985) The cultured fibroblast-like cells as a
model for the study of aging. In: Fincht CE & Schneider EL ed. The
biology of aging, 2nd ed. New York, Van Nostrand Reinhold Company,
pp 291-321.
Novak LP (1972) Aging, total potassium, fat free mass and cell mass
in males and females between the ages of 18 and 85 years. J
Gerontol, 27: 438-443.
Oh SH, Deagen JT, Whanger PD, & Wesnig PH (1978) Biological function
of metallothionein. V. Its induction in rats by various stresses. Am
J Physiol, 234: E282-E285.
Ohta M, Kanai S, Sato Y, & Kitani K (1988) Age-dependent decrease in
the hepatic uptake and biliary excretion of ouabain in rats. Biochem
Pharmacol, 37: 935-942.
O'Malley K, Docherty JR, & Kelly GJ (1988) Adrenoceptor status and
cardiovascular function in ageing. J Hypertension, 6(suppl 10):
S59-S62.
O'Meara AR & Pochron SF (1979) Age-related effects on the
incorporation of acetate into rat liver histones. Biochim Biophys
Acta, 586: 391-401.
Onasaka S & Cherian MG (1981) The induced synthesis of
metallothionein in various tissues of rats in response to metals. I.
Effect of repeated injection of cadmium salts. Toxicology, 22:
91-101.
Os I, Kjeldsen SE, & Westheim A (1987) Aging and urinary vasopressin
excretion in healthy men. Scand J Urol Nephrol, 21(3): 235-239.
OECD (1986) Existing chemicals systematic investigation, priority
setting and chemical reviews. Paris, Organisation for Economic
Co-operation and Development.
Owen RA & Heywood R (1986) Age-related variations in renal structure
and function in Sprague-Dawley rats. Toxicol Pathol, 14(2): 158-167.
Pagano GN, Casadar N, Diana A, Psue E, Bozzo C, Ferrero F, & Lenti G
(1981) Insulin resistance in the aged: the role of peripheral
insulin receptors. Metabolism, 30: 46-49.
Pahlavani MA, Cheung HT, Cai NS, & Richardson A (1988) Influence of
dietary restriction and aging on gene expression in the immune
system of rats. In: Biomedical advances in aging. New York, London,
Plenum Press, pp 259-270.
Pakin YV & Hrisanov SM (1984) Critical analysis of the applicability
of the Compertz-Makeham law in human population. Gerontology, 30:
8-12.
Paramonova GI & Dovgi AI (1987) Induction of multiple forms of
cytochrome P450 of rat liver during aging. In: Abstracts of the All
Union Conference on Cytochrome P450 and the Environment,
Novosibirsk, 27-31 July 1987. Novosibirsk, USSR Academy of Medical
Sciences, Siberian Division, pp 100-101.
Patel DJ & Vaishnaw RN (1980) Basic hemodynamics and its role in
disease process. Baltimore, Maryland, University Park Press.
Pedigo NW, Schoemaker H, Morellia M, McDougal JN, Malisck JB, Burks
TF, & Yamamura TF (1981) Benzodiazepine receptor binding in young,
mature, and senescent rat brain and kidney. Neurobiol Aging, 2:
83-88.
Penn A, Batastini G, Soloman J, Burns F, & Albert R. (1981)
Dose-dependent size increases of aortic lesions following chronic
exposure to 7,12-dimethylbenz(a)-anthracene. Cancer Res, 71:
588-592.
Penzes L (1974) Intestinal absorption of glycine, L-alanine, and
L-leucine in the old rat. Exp Gerontol, 8: 245-252.
Penzes, L.S. (1984) Intestinal response in aging: changes in reserve
capacity. Acta Med Hung, 41(4): 263-277.
Perkins MN & Stone TW (1983) Pharmacology and regional variations of
quinolinic acid-evoked excitations in the rat central nervous
system. J Pharmacol Exp Ther, 226(2): 551-557.
Perl DP & Brody AR (1980) Alzheimer's disease: X-ray spectrometric
evidence of aluminum accumulation in neurofibrillary tangle-bearing
neurons. Science, 208: 297-299.
Peterson DD, Pack AI, Silage DA, & Fishman AP (1981) Effects of
aging on ventilatory and occlusion pressure responses to hypoxia and
hypercapnia. Am Rev Respir Dis, 124: 387-391.
Peto R (1986) Influence of dose and duration of smoking on lung
cancer rates. In: Zaridze D & Peto R ed. Tobacco: A major
international health hazard. Lyon, International Agency for Research
on Cancer, pp 23-33 (IARC Scientific Publications No. 74).
Peto R, Parish SE, & Gray RG (1975) Cancer and aging in mice and
men. Br J Cancer, 32: 411-426.
Peto R, Pike MC, Day NE, Gray RE, Lee PN, Parish S, Poto J, Richard
S, & Wahrendorf J (1980) Guidelines for simple, sensitive
significance tests for carcinogenic effects in long-term animal
experiments. In: Long-term and short-term screening assays for
carcinogens: A critical appraisal. Lyon, International Agency for
Research on Cancer, pp 311-426 (IARC Monographs on the Evaluation of
the Carcinogenic Risk of Chemicals to Humans, Supplement 2).
Peto R, Parish SE, & Gray RG (1985) There is no such thing as aging,
and cancer is not related to it. In: Likhachev AJ, Anisimov V, &
Montesano R ed. Age-related factors in carcinogenesis. Lyon,
International Agency for Research on Cancer, pp 43-53 (IARC
Scientific Publications No. 58).
Pierpaoli W, Dall'Ara A, Pedrinis E, & Regelson W (1991) The pineal
control of aging. The effects of melatonin and pineal grafting on
the survival of older mice. Ann NY Acad Sci, 621: 291-313.
Piikivi L, Hanninen H, Martelin T, & Mantere P (1984) Psychological
performance and long-term exposure to mercury vapors. Scand J Work
Environ Health, 10: 35-41.
Pines A, Cucos B, Ever-Hadani P, Ron M, & Lemesch C (1987) Changes
in pattern of organochlorine residues in blood of general Israeli
population, 1975-1986. Sci Total Environ, 66: 115-125.
Piva F, Limonta P, Maggi R, Dondi D, Messi E, Yanisi M, & Motta M
(1987) Aging and the hypothylamo-pituitery-testiculas axis in the
rat. J Steroid Biochem, 27(4-6): 707-712.
Poland A & Knutson JC (1982) 2,3,7,8,-Tetrachlorodibenzo-p-dioxin
and related halogenated aromatic hydrocarbons: examinations of the
mechanism of toxicity. Annu Rev Pharmacol Toxicol, 22: 517-554.
Popper H (1986) Aging in the liver. In: Popper H ed. Progress in
liver diseases. New York, London, Grune & Stratton, vol VIII, pp
659-683.
Posner J, Danhof M, Teunissen MWE, Breimer DD, & Whiteman PD (1987)
The disposition of antipyrine and its metabolites in young and
elderly healthy volunteers. Br J Clin Pharmacol, 24: 51-55.
Post DJ, Carter K, & Papaconstantinou J (1991) The effect of aging
on constitutive mRNA levels and lipopolysaccharide inducibility of
acute phase genes. Ann NY Acad Sci, 621: 66-77.
Prinz PN, Weitzman ED, Cunningham GR, & Karacan I (1983) Plasma
growth hormone during sleep in young and aged men. J Gerontol, 38:
519-534.
Provinciali M & Fabris H (1990) Role of pituitary-thyroid axis on
basal and lymphokine-induced NK cell activity in aging. Int J
Neurosci, 51: 373-375.
Rabovsky, J, Marion KJ, Groseclose RD, Lewis TR, & Peterson M (1984)
Low dose chronic inhalation of diesel exhaust and/or coal dust by
rats: effect of age and exposure on lung and liver cytochrome P450.
Xenobiotica, 14: 595-598.
Rao GN, Haseman JK, Grumbein S, Crawford DD, & Eustis SL (1990)
Growth, body weight, survival, and tumor trends in F344/n rats
during an eleven-year period. Toxicol Pathol, 18: 61-70.
Rath PC & Kanungo MS (1989) Age-related changes in the expression of
cytochrome p-450 (b+e) gene in the rat after phenobarbitone
administration. Biochem Biophys Res Commun, 157: 1403-1409.
Reff ME & Schneider EL ed. (1982) Biological markers of aging.
Washington, DC, US Government Printing Office, 252 pp (NIH
Publication No. 82-2221).
Regelson W (1983) Biomarkers in aging. In: Regelson W & Marott Sinex
F ed. Intervention in the aging process. New York, Alan R. Liss,
Inc., pp 3-98.
Rehnberg GL, Goldman JM, Cooper RL, Hein JF, McElroy WK, Booth KC, &
Gray LE (1988a) Effect of linuron on the brain-pituitary-testicular
reproductive axis in the rat. Toxicologist, 8: 121.
Rehnberg GL, Linder R, Goldman JM, Hein JF, McElroy WK, & Cooper RL
(1988b) Changes in testicular and serum hormone concentrations in
the male rat following treatment with m-dinitrobenzene. Toxicol Appl
Pharmacol, 95: 255-264.
Reidenberg MM (1980) Drugs in the elderly. Bull NY Acad Med, 56:
703-714. Reif AE (1981) Effect of cigarette smoking on
susceptibility to lung cancer. Oncology, 38: 76-85.
Reis W & Poethig D (1984) Chronological and biological age. Exp
Gerontol, 19: 211-216.
Reiter RJ (1986) The pineal gland: an important link to the
environment. News Physiol Sci, 1: 202-205.
Reitz RH, McDougal JN, Himmelstein MW, Nolan RJ, & Schumann AM
(1988) Physiologically based pharmacokinetics modeling with methyl
chloroform: implications for interspecies, high dose/low dose, and
dose route extrapolations. Toxicol Appl Pharmacol, 95: 185-199.
Revskoy SY, Poroshina TE, Kovaleva IG, Berstein LM, Ostroumova MN, &
Dilman VM (1985) Age-dependent metabolic immunodepression and
cancer. In: Likhachev A, Anisimov V, & Montesano R ed. Age-related
factors in carcinogenesis. Lyon, International Agency for Research
on Cancer, pp 253-259 (IARC Scientific Publications No. 58).
Richardson A & Birchenall-Sparks MC (1983) Age-related changes in
protein synthesis. Rev Biol Res Aging, 1: 255-273.
Richardson A & Semsei I (1987) Effect of aging on translation and
transcription. In: Review of biological research in aging. New York,
Alan R. Liss, Inc., vol 3, pp 467-483.
Richardson A, Birchenall-Sparks MC, & Staecker JL (1983) Aging and
transcription. In: Review of biological research in aging. New York,
Alan R. Liss, Inc., vol 2, pp 275-294.
Richardson A, Butler JA, Rutherford MS, Semsei I, Gu MZ, Fernandes
G, & Chiang WH (1987) Effect of age and dietary restriction on the
expression of alpha-2u-globulin. J Biol Chem, 262: 12821-12825.
Richardson A, Roberts M, & Rutherford M (1985) Aging and gene
expression. In: Review of biological research in aging. New York,
Alan R. Liss, Inc., vol 2, pp 395-419.
Riegel GD & Miller AE (1981) Aging effect on hormone concentrations
and actions. In: Florini JR ed. Handbook of biochemistry in aging.
Boca Raton, Florida, CRC Press, pp 231-256.
Riggs BL (1987) Pathogenesis of osteoporosis. Am J Obstet Gynecol,
156: 1342-1346.
Riggs BL & Melton LJ III (1983) Evidence for two distinct syndromes
of involutional osteoporosis. Am J Med, 75: 899-901.
Rikans LE (1984) Influence of aging on the susceptibility of rats to
hepatotoxic injury. Toxicol Appl Pharmacol, 73: 243-249.
Rikans LE (1989a) Hepatic drug metabolism in female Fischer rats as
a function of age. Drug Metab Dispos, 17: 114-116.
Rikans L (1989b) Effects of ethanol on microsomal drug metabolism in
aging female rats. I. Induction. Mech Ageing Dev, 48: 267-280.
Rikans LE & Moore DR (1987) Effect of age and sex on allyl alcohol
hepatotoxicity in rats: role of liver alcohol and aldehyde
dehydrogenase activities. J Pharmacol Exp Ther, 243: 20-26.
Rikans LE & Moore DR (1988) Effect of aging on aqueous phase
antioxidents in tissues of male Fischer rats. Biochem Biophys Acta,
966: 269-275.
Rikans LE & Snowden CD (1989) Influence of aging on acute ethanol
hepatotoxicity in female rats. Pharmacologist, 31: 177.
Ritschel WA (1983) Pharmacokinetics in the aged. In: Pagliaro LA &
Pagliaro AM ed. Pharmacologic aspects of aging. St. Louis, Missouri,
C.V. Mosby & Co., pp 219-256.
Ritschel WA (1988) Gerontokinetics: The pharmacokinetics of drugs in
the elderly. Caldwell, New Jersey, Telford Press, 114 pp.
Robertson IGC & Birnbaum LS (1982) Age-related changes in mutagen
activation by rat tissues. Chem-Biol Interact, 38: 243-252.
Robertson DRC, Waller DG, Renwick AG, & George CF (1988) Age-related
changes in the pharmacokinetics and pharmacodynamic of nifedipine.
Br J Clin Pharmacol, 25: 297-305.
Rodgers J & Gass G (1983) The effect of age on serum proteins in
mice. Exp Gerontol, 14: 169-173.
Roe DA (1983) Drugs and nutrition in the elderly. In: Geriatric
nutrition, 2nd ed. Englewood, New Jersey, Prentice-Hall, Inc., pp
176-200.
Rogers J & Bloom FE (1985) Neurotransmitter metabolism and function
in the aging central nervous system. In: Finch CE & Schneider EL ed.
Handbook of the biology of aging, 2nd ed. New York, Van Nostrand
Reinhold Company, pp 645-691.
Rogers GS & Gilchrest BA (1990) The senile epidermis: environmental
influences on skin ageing and cutaneous carcinogenesis. Br J
Dermatol, 122(suppl 35): 55-60.
Rogers J, Magistretti PJ, & Bolis LC (1991) Animal models for aging
research. Neurobiol Aging, 12(spec issue): 625-701.
Rose MR (1991) Evolutionary biology of aging. New York, London,
Oxford University Press.
Rosenberger RF & Kirkwood TBL (1986) Errors and the integrity of
genetic information transfer. In: Kirkwood TBL, Rosenberger RF, &
Galas DJ, ed. Accuracy in molecular processes. London, Chapman and
Hall, pp 17-35.
Ross MH (1961) Length of life and nutrition in rat. J Nutr, 75:
197-210.
Ross MH (1976) Nutrition and longevity in experimental animals. In:
Winick M ed. Nutrition and aging. New York, Chichester, Brisbane,
Toronto, John Wiley and Sons, pp 23-41.
Ross R (1981) Atherosclerosis: A problem of the biology of arterial
wall cells and their interactions with blood components.
Arteriosclerosis, 1: 293-311.
Roth GS (1979a) Hormone action during aging: alterations and
mechanisms. Mech Ageing Dev, 9: 497-514.
Roth GS (1979b) Hormone receptor changes during adulthood and
senscence: significance for aging research. Fed Proc, 38: 1910-1914.
Roth GS (1988) Receptor and postreceptor changes during lymphocyte
aging. In: Platt D ed. Blood cells, rheology, and aging. Berlin,
Heidelberg, New York, Springer-Verlag, pp 150-154.
Roth GS & Joseph JA (1988) Peculiarities of the effect of hormones
and transmitters during aging: Modulation of changes in dopaminergic
action. Gerontology, 34: 22-28.
Rothstein M & Seifert SC (1981) RNA synthesis. In: Handbook of
biochemistry in aging. Boca Raton, Florida, CRC Press, pp 51-63.
Rouser G, Kritchevsky G, & Yamamoto A (1972) Lipids in the nervous
system of different species as a function of age: Brain, spinal
cord, peripheral nerve, purified whole cell preparations and
sub-cellular particulates: Regulatory mechanisms and membrane
structure. Adv Lipid Res, 10: 261-360.
Rowe JW, Minaker KL, Paollotta J, & Fliers JS (1983)
Characterization of the insulin resistance of aging. J Clin Invest,
71: 1581-1587.
Roy AK, Nath TS, Motwani NM, & Chatterjee B (1983) Age dependent
regulation of the polymorphic forms of alpha2u-globulin. J Biol
Chem, 258: 10123-10127.
Rudman D (1987) Assessment of nutritional status. In: Braunwald E,
Isselbacher KJ, Petersdorf RG, Wilson JD, Martin JB, & Fauci AS ed.
Harrison's principles of internal medicine, 11th ed. New York,
McGraw-Hill, Chapter 71, pp 309-392.
Rudman D (1988) Kidney senescence: A model for aging. Nutr Rev,
46(6): 209-214.
Runcie J (1985) Obesity. In: Pathy MWSJ ed. Principles and practice
of geriatric medicine. New York, Chichester, Brisbane, Toronto, John
Wiley and Sons, Chapter 22.1, pp 277-284.
Russell DH, Buckley AR, Shah GN, Sipes IG, Blask DE, & Benson B
(1988) Hypothalamic site of action of
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Toxicol Appl Pharmacol,
94: 496-502.
Sacher GA (1977) Life table modification and life prolongation. In:
Finch CE & Hayflick L ed. Handbook of the biology of aging. New
York, Van Nostrand Reinhold Company, pp 582-638.
Sagiv M, Goldhammer E, Abinader EG, & Rudoy J (1988) Aging and the
effect of increased after-load on left ventricular contractile
state. Med Sci Sports Exercise, 20(3): 281-284.
Sample PA, Esterson FD, Weinreb RN, & Boynton RM (1988) The aging
lens: in vivo assessement of light absorption in 84 human eyes.
Invest Ophtalmol Visual Sci, 29(8): 1306-1311.
Sapolsky R, Armanini M, Packan D, & Tornbaugn G (1987) Stress and
glucocorticoids in aging. Endocrinol Metab Clin, 16(4): 965-979.
Sapolsky RM, Krey LC, & McEwen BS (1986) The adrenocortical axis in
the aged rat: Impaired sensitivity to both fast and delayed feedback
inhibition. Neurobiol Aging, 7: 331-335.
Sato Y, Kanai S, & Kitani K (1987) Biliary excretion of ouabain in
aging male and female F344 rats. Arch Gerontol Geriatr, 6: 141-152.
Saxton JA Jr & Kimball GC (1941) Relation to nephrosis and other
diseases of albino rat to age and to modifications of diet. Arch
Pathol, 32: 951-965.
Scheibel AB & Tomiyasu U (1978) Dendritic sprouting in Alzheimer
presenile dementia. Exp Neurol, 60: 1-8.
Schiff I & Wilson E (1979) Clinical aspects of aging of the female
reproductive system. Aging, 4: 10-28.
Schmucker D (1979) Age-related changes in drug disposition. Pharm
Rev, 30: 445-456.
Schmucker DL & Wang RK (1979) Rat lysosomal enzymes: effects of
animal age and phenobarbital. Age Aging, 2: 93-96.
Schmucker DL & Wang RK (1989) Dietary restriction postpones the
age-dependent compromise of male rat liver microsomal
monooxygenases. Prog Clin Biol Res, 287: 283-288.
Schmucker DL, Woodhouse KW, Wang RK, Wynne H, James QF, McManus M, &
Kremers P (1990) Effects of age and gender on in vitro properties
of human liver microsomal monoxygenases. Clin Pharmacol Ther, 48:
365-377.
Schneider EL (1978) The aging reproductive system. New York, Raven
Press, vol 4.
Schneider EL & Reed JD (1985) Life extension. New Engl J Med, 312:
1139-1168.
Schneider EL, Sternberg H, Tice RR, Senula GC, Kra D, Smith JR, &
Bynum G (1979) Cellular replication and aging. Mech Ageing Dev, 9:
313-324.
Schuetz EG, Wrighton SA, Barwick JL, & Guzelian PS (1984) Induction
of cytochrome P-450 by glucocorticoids in rat liver. I. Evidence
that glucocorticoids and pregnenolone 16 alpha-carbonitrile regulate
de novo synthesis of a common form of cytochrome P-450 in cultures
of adult rat hepatocytes and in the liver in vivo. J Biol Chem, 259:
1999-2006.
Schumann AM, Fox TR, & Watanabe PG (1982a) A comparison of the fate
of inhaled methyl chloroform (1,1,1-trichloroethane) following
single or repeated exposure in rats and mice. Fundam Appl Toxicol,
2: 27-32.
Schumann AM, Fox TR, & Watanabe PG (1982b) Methylchloroform
(1,1,1-trichloroethane): Pharmacokinetics in rats and mice following
inhalation exposure. Toxicol Appl Pharmacol, 62: 390-401.
Schuurman HJ, Krajnc-Franken MAM, Kuper CF, Van Loveren H, & Vos JG
(1990) Immune system. In: Haschek-Hoch WM & Rousseaux CG ed.
Fundamentals of toxicologic pathology. New York, London, San
Francisco, Academic Press, pp 1-103.
Searle CE (1976) Chemical carcinogens. Washington, DC, American
Chemical Society (ACS Monograph No. 173)
Segre D, Miller RA, Abraham GN, Weigle WO, & Warner HR (1989) Aging
and the immune system. J Gerontol, 44(6): 8164-8168.
Sekuler R, Kline D, & Dismuskes K (1982) Aging and human visual
functions. New York, Alan R. Liss, Inc.
Sellers EM, Frecker RC, & Romach MK (1983) Drug metabolism in the
elderly: confounding of age, smoking, and ethanol effects. Drug
Metab Rev, 14: 225-250.
Selmanowitz VJ, Rizer RL, & Orentreich N (1977) Aging of the skin
and its appendages. In: Finch CE & Hayflick L ed. Handbook of the
biology of aging. New York, Van Nostrand Reinhold Company, pp
496-509.
Selye H (1950) Stress: The physiology and pathology of exposure to
stress. Montreal, Acta Inc.
Semsei I, Rao G, & Richardson A (1989) Changes in the expression of
superoxide dismutase and catalase as a function of age and dietary
restriction. Biochem Biophys Res Commun, 164: 620-625.
Senda S, Cheng E, & Kawanishi H (1989) IgG in murine intestinal
secretions. Aging effect and possible physiological role. Scand J
Immunol, 29(1): 41-47.
Shen DX, Ren R, Zhao YR, & Ma XZ (1987) [Disease pattern analysis in
hospitalized elderly patients in Jingzhou.] Chin J Geriatr, 6: 44
(in Chinese).
Shepherd AMM, Wilson N, & Stevenson IH (1979) Warfarin sensitivity
in the elderly. In: Crooks J & Stevenson IH ed. Drugs and the
elderly. Baltimore, Maryland, University Park Press, pp 199-209.
Sherlock S, Bearn AG, Billing BH, & Paterson JCS (1955) Splanchnic
blood flow of peripheral plasma bromosulphthalein method: The
relation of peripheral bromosulphthalein level to the calculated
flow. J Lab Clin Med, 35: 923-932.
Shih JC & Young H (1978) The alteration of serotonin binding sites
in aged human brain. Life Sci, 23: 1441-1448.
Simmons DL, McQuiddy P, & Kasper CB (1987) Induction of the hepatic
mixed-function oxidase system by synthetic glucocorticoids -
Transcriptional and post-transcriptional regulation. J Biol Chem,
262: 326-332.
Slagboom PE & Vijg J (1989) Genetic instability and aging: theories,
facts, and future perspectives. Genome, 31: 373-385.
Smith DBD, Thompson LW, & Michalewski HJ (1980) Average evoked
potential research in adult aging - status and prospects. In: Aging
in the 1980s. Washington, DC, American Psychological Association, pp
135-151.
Smith EL, Sempos CT, & Purvis RW (1981) Bone mass and strengh
decline with age. In: Smith EL & Serfass RC ed. Exercise and aging:
the scientific basis. Hillside, New Jersey, Enslow Publishers, pp.
Sonntag WE & Gough ME (1988) Growth hormone releasing hormone
induced release on growth hormone in aging male rats: dependence on
pharmacological manipulation and endogenous somatostatin release.
Neuroendocrinology, 47: 482-488.
Sonntag WE, Steger RW, Forman LJ, & Meites J (1980) Decreased
pulsatile release of growth hormone in old male rats. Endocrinology,
107: 1875-1879.
Sonntag WE, Hylka V, & Meites J (1985) Growth hormone restores
protein synthesis in skeletal muscle of old rats. J Gerontol, 40:
689-694.
Spagnoli LG, Orlandi A, Manuello A, Santeusanio G, Angelis C,
Lucreziotti R, & Ramacci MT (1991) Aging and atherosclerosis in the
rabbit 1. Distribution, prevalence and morphology of atherosclerotic
lesions. Atherosclerosis, 89: 11-24.
Spearman ME & Leibman KC (1983) Hepatic and pulmonary cytosolic
metabolism of epoxides: effects of aging on conjugation with
glutathione. Life Sci, 33: 2615-2625.
Spearman ME & Leibman KC (1984) Aging selectivity alters
glutathione-S-transferase isozyme concentrations in liver and lung
cytosol. Drug Metab Dispos, 12: 661-671.
Speijers GJA (1983) [Toxic effects of rapeseed oil, erucic acid and
linolenic acid in the rat.] Utrecht, The Netherlands, University of
Utrecht, pp 1-143 (in Dutch).
Speijers GJA (1989a) [Literature review on experimental
atherosclerosis research.] Bilthoven, The Netherlands, National
Institute Public Health and Environmental Protection (in Dutch).
Speijers GJA (1989b) [Toxicological studies of ergot alkaloids.] De
Ware(n) Chem, 19(3): 189-194 (in Dutch).
Spencer PS & Ochoa J (1981) The mammalian peripheral nervous system
in old age. In: Johnson GJ Jr ed. Aging and cell structure. New
York, London, Plenum Press, pp 35-103.
Spencer PS, Nunn PB, Hugon J, Ludolph AC, Ross SM, Roy DN, &
Robertson RC (1987). Guam amyotrophic lateral
sclerosis-Parkinsonism-Dementia linked to a plant excitant
neurotoxin. Science, 237: 465-466.
Steiner RA, Bremner WJ, Clifton DK, & Dorsa DM (1984) Reduced
pulsatile luteinizing hormone and testosterone secretion in the male
rat. Biol Reprod, 31: 251-258.
Stenbäck F, Peto R, & Shubik P (1981) Initiation and promotion at
different age and doses in 2200 mice: III. Linear extrapolation from
high doses may underestimate low-dose tumor risk. Br J Cancer, 44:
23-34.
Stevenson IH & Hosie J (1985) Pharmacokinetics in the elderly. In:
O'Malley K & Waddington JL ed. Therapeutics in the elderly.
Amsterdam, Oxford, New York, Elsevier Science Publishers, pp 35-41.
Stevenson IH, Salem SAM, & Shepherd AMM (1979) Studies on drug
absorption and metabolism in the elderly. In: Crooks J & Stevenson
IH ed. Drugs and the elderly. Baltimore, Maryland, University Park
Press, pp 51-63.
Stevenson JC, Whitehead MI, Padwick M, Endacott JA, Suttonm C, Banks
LM, Freemantle C, Spinks TJ, & Hesp R (1988) Dietary intake of
calcium and postmenopausal bone loss. Br Med J, 297: 15-17.
Stiles J & Tyler WS (1988) Age-related morphometric differences in
responses of rat lungs to ozone. Toxicol Appl Pharmacol, 92:
274-285.
Stohs SJ, Al-Turk WA, & Angle CR (1982) Glutathione-S-transferase
and glutathione reductase activities in hepatic and extra-hepatic
tissues of female mice as a function of age. Biochem Pharmacol, 31:
2113-2116.
Strong R, Moore MA, Hale C, Wessels-Reiker M, Armbrecht HJ, &
Richardson A (1990) Modulation of tyrosine hydroxylase gene
expression in the rat adrenal gland by age and reserpine. Brain Res,
525(1): 126-132.
Sun J & Strobel HW (1986) Aging affects the drug metabolism systems
of rat liver, kidney, colon, and lung in a differential fashion. Exp
Gerontol, 21: 523-534.
Sun J, Lau PO, & Strobel HW (1986) Aging modifies the expression of
hepatic microsomal cytochrome P450 after pretreatment of rats with
b-naphoflavone or phenobarbital. Exp Gerontol, 21: 65-73.
Sweeny DJ & Weiner M (1985) Metabolism of acetaminophen in
hepatocytes isolated from mice and rats of various ages. Drug Metab
Dispos, 13: 377-379.
Sweeny DJ & Weiner M (1986) Effect of aging on the metabolism of
p-nitroanisole and p-nitrophenyl in isolated rat hepatocytes. Age,
9: 95-98.
Swenberg GJA, Richardson FC, Boucheron JA, Deal FH, Belinsky SA,
Charbonneau M, & Short BG (1987) High- to low-dose extrapolation:
critical determinants involved in the dose response of carcinogenic
substances. Environ Health Perspect, 76: 57-63.
Swift CG (1985) Benzodiazepine pharmacodynamics in the elderly. In:
O'Malley K & Stevenson IL ed. Therapeutics in the elderly.
Amsterdam, Oxford, New York, Elsevier Science Publishers, pp 77-90.
Tannenbaum SR, Fett D, Young VR, Land PD, & Bruce WR (1978) Nitrite
and nitrate are formed by endogenous synthesis in the human
intestine. Science, 200: 1478-1489.
Tarloff JB, Goldstein RS, Milo BA, & Hook JB (1989a) Role of
pharmacokinetics and metabolism in the enhanced susceptibility of
middle-aged Sprague-Dawley rats to acetaminophen nephrotoxicity.
Drug Metab Dispos, 17: 139-146.
Tarloff JB, Goldstein RS, Morgan DG, & Hook JB (1989b) Acetaminophen
and p-aminophenol nephrotoxicity in aging male Sprague-Dawley and
Fischer 344 rats. Fundam Appl Toxicol, 12: 78-91.
Taylor A (1989) Associations between nutrition and cataract. Nutr
Res, 47: 225-234.
Telford N, Mobbs CV, Osterburg HH, & Finch CE (1988) Alterations in
hypothalamic serotonergic-catecholaminergic relationships in aging
C57BL/6J female mice. Exp Gerontol, 23: 481-489.
Tenover JS, Matsumoto AM, Clifton DK, & Bremner WJ (1988)
Age-related alterations in the circadian rhythms of pulsatile
luteinizing hormone and testosterone secretion in healthy men. J
Gerontol, 43: M163-M169.
Terry RD (1963) The fine structure of neurofibrillary tangles in
Alzheimer's disease. J Neuropathol Exp Neurol, 22: 269-292.
Thakur MD (1984) Age-related changes in the structure and function
of chromatin: A review. Mech Ageing Dev, 27: 263-286.
Thakur MK (1988) Molecular mechanism of steroid hormone action
during aging. A review. Mech Ageing Dev, 45: 93-110.
Thompson JS, Robbins J, & Cooper JK (1987) Nutrition and immune
function in the geriatric population. Clin Geriatr Med, 3(2):
309-317.
Tice RR & Setlow RB (1985) DNA repair and replication in aging
organisms and cells. In: Finch CE & Schneider EL ed. Handbook of the
biology of aging, 2nd ed. New York, Van Nostrand Reinhold Company,
pp 173-224.
Timiras PS, Hudson DB, & Segall PE (1984) Lifetime brain serotonin:
Regional effects of age and precursor availability. Neurobiol Aging,
5: 235-242.
Tomlinson BE & Irving D (1977) The numbers of limb motor neurons in
the human lumbosacral cord throughout life. J Neurol Sci, 34:
213-219.
Travaglini P, Mocchegiani E, Togni E, Muratori M, Re T, Bazzani N, &
Fabris N (1990) Thymulin and zinc circulating level in patient with
GH and PRL secreting pituitary adenomas. Int J Neurosci, 51:
269-271.
Travis CC, White RC, & Ward RC (1990) Interspecies extrapolation of
pharmacokinetics. J Theor Biol, 142: 285-307.
Trick LR (1987) Age-related alterations in retinal function. Doc
Ophthalmol, 65(1): 35-43.
Triggs EJ & Nation RL (1975) Pharmacokinetics in the aged: A review.
J Pharmacokinet Biopharm, 3: 387-418.
Turusov VS & Parfenov YuD (1986) [Methods of revealing and risk
assessment of chemical carcinogens.] Moscow, Meditsina, pp 134-136
(in Russian).
Ulloa-Aguirre A & Chappel SC (1982) Multiple species of
follicle-stimulating hormone exist within the anterior pituitary of
male golden hamsters. J Endocrinol, 95: 257-266.
Ulloa-Aguirre A, Espinoza R, Damian-Matsumura P, & Chappel SC (1988)
Immunological and biological potencies of the different molecular
species of gonadotrophins. Hum Reprod, 3: 491-501.
US Senate Special Committee on Aging (1986) Aging America: Trends
and projections, 1985-86 edition. Washington, DC, US Government
Printing Office, pp 14-17.
Utiger RD (1980) Decreased extrathyroidal triiodothyronine
production in monthyroidal ilness: benefit or harm? Am J Med, 69:
807-810.
Van Bezooijen CFA (1984) Influence of age-related changes in rodent
liver morphology and physiology on drug metabolism - A review. Mech
Ageing Dev, 25: 1-22.
Van Bezooijen CFA, Groen C, Stijnen AM, & Danhof M (1989) The effect
of age on the pharmacokinetics and pharmacodynamics of drugs in
rats. Age, 12: 112.
Vanhaelst L, Van Cauter E, Degaute JP, & Goldstein J (1972)
Circadian variations of serum thyrotropin levels in man. J Clin
Endocrinol Metab, 35: 489-492.
Van Manen R, De Priester W, & Knook DL (1983) Lysosomal activities
in aging rat liver. I. Variation in enzyme activity within the liver
lobule. Mech Ageing Dev, 22: 159-165.
Velican C (1981) Are animal models suitable for the study of
ischaemic heart disease. Rev Roum Méd Méd Interne, 19(1): 5-19.
Vellas B, Balas D, Guidet M, Moreau J, Senegas F, Bouisson M,
Albarede JL, & Ribet A (1988) Vieillissement du pancréas. Son
implication dans les états de dénutrition chez les personnes agées.
Presse Méd, 17: 2067-2069.
Vermeulen A, Deslypere JP, & Kaufman JM (1989) Influence of
antiopioids on luteinizing hormone pulsatility in aging men. J Clin
Endocrinol Metab, 68: 68-72.
Verzar F (1968) Intrinsic and extrinsic factors of molecular aging.
Exp Gerontol, 3: 69-75.
Vestal RE (1978) Drug use in the elderly: a review of problems and
special considerations. Drugs, 16: 358-382.
Vestal RE, McGuire EA, Tobin JD, Andres R, Norris AH, & Ulzey E
(1977) Aging and ethanol metabolism. Clin Pharmacol Ther, 21:
343-354.
Vestal RE, Wood AJJ, & Shand DG (1979) Reduced b-adrenoceptor
sensitivity in the elderly. Clin Pharmacol Ther, 26: 181-186.
Vestal RE, Jue SG, & Cusack BJ (1985) Increased risk of adverse drug
reactions in the elderly: Fact or myth. In: O'Malley K & Waddington
JL ed. Therapeutics in the elderly. Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp 97-104.
Vijg J (1990) DNA sequence changes in aging: How frequent, how
important? Aging, 2: 105-123.
Vijg J & Knook DL (1987) DNA Repair in relation to the aging
process. J Am Geriatr Soc, 35(6): 532-541.
Vijg J & Papaconstantinou J (1990) Aging and longevity genes:
strategies for identifying DNA sequences controlling life span. J
Gerontol, 45(5): B179-B182.
Vincenzini MT, Iantomasi T, Stio M, Favelli F, Vanni P, Tonelli F, &
Treves C (1989) Glucose transport during ageing by human intestinal
brush-border membrane vesicles. Mech Ageing Dev, 48: 33-41.
Vogel HB (1983) Effects of age on the biomechanical and biochemical
properties of rat and human skin. J Soc Cosmet Chem, 34: 453-463.
Voitenko VP & Tokar AV (1983) The assessment of biological age and
sex differences of human aging. Exp Aging Res, 9: 239-244.
Vos JG & Luster MI (1989) Immune alterations. In: Kimbrough RD &
Jensen AA ed. Halogenated biphenyls, terphenyls, naphtalenes,
dibenzodioxins and related products. Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp 295-322.
Vos JG & Penninks AH (1987) Dioxin and organotin compounds as model
immunotoxic chemicals. In: De Matteis F & Lock EA ed. Selectively
and molecular mechanism of toxicity. London, MacMillan Press Ltd, pp
85-102.
Vos JG, De Klerk A, Krajnc EI, Van Loveren H, & Rosing J (1990)
Immunotoxicity of Bis (tri-n-butylin) oxide in the rat; effects on
thymus-dependent immunity and on non specific resistance following
long-term exposure in young versus aged rats (1990). Toxicol Appl
Pharmacol, 105: 144-155.
Wabner CL & Chen TS (1984) Renal transport of organic acids in the
aging. Pharmacologist, 26: 206.
Waddington JL, O'Boyle KM, Molloy AG, Youssef HA, King DJ, & Cooper
SJ (1985) Neurotransmitter receptors and ageing:
Dopamine/neuroleptic receptors, involuntary movements and the
disease process of schizophrenia. In: O'Malley K & Stevenson IL ed.
Therapeutics in the elderly. Amsterdam, Oxford, New York, Elsevier
Science Publishers, pp 63-76.
Waggoner S, Gu MZ, Chiang WH, & Richardson A (1990) The effect of
dietary restriction on the expression of genes. In: Genetic effects
of aging, II. Caldwell, New Jersey, Telford Press, pp 255-273.
Wagner PA, Jernigan JA, Bailey LB, Nickens C, & Brazzi GA (1983)
Zinc nutriture and cell-mediated immunity in the aged. Int J Vitam
Nutr Res, 53: 94-101. Walford RL (1969) The immunological theory of
aging. Copenhagen, Munksgaard.
Walker DM (1985) Oral disease. In: Pathy MSJ ed. Principles and
practice of geriatric medicine. New York, Chichester, Brisbane,
Toronto, John Wiley and Sons.
Wang SV, Halban PA, & Rowe JW (1988) Effects of aging on insulin
synthesis and secretion. Differential effects on preproinsulin
messenger RNA levels, proinsulin biosynthesis, and secretion of
newly made and preformed insulin in the rat. J Clin Invest, 81(1):
176-184.
Ward JM (1983) Increased susceptibility of livers of aged F344/NCr
rats to the effects of phenobarbital on the incidence, morphology,
and histochemistry of hepatocellular foci and neoplasms. J Natl
Cancer Inst, 71: 815-823.
Ward WF (1988) Enhancement by food restriction of liver protein
synthesis in the aging Fischer 344 rat. J Gerontol, 43: B50-B53.
Ward W & Richardson A (1991) Effect of age on liver protein
synthesis and degradation. Hepatology, 14: 935-948.
Ward JM, Lynch P, & Riggs C (1988) Rapid development of
hepatocellular neoplasms in aging male C3H/HeNCr mice given
phenobarbital. Cancer Lett, 39: 9-18.
Warner BA, Dufau ML, & Santen RJ (1985) Effects of aging and illness
on the pituitary testicular axis in men: Qualitative as well as
quantitative changes in luteinizing hormone. J Clin Endocrinol
Metab, 60: 263-268.
Warner HR, Butler RN, Spratt RW, & Schneider EL (1987) Modern
biological theories of aging. New York, Raven Press.
Weale RA (1986) Aging and vision. Vision Res, 26(9): 1507-1512.
Weber JCP & Griffin JP (1986) Prescriptions, adverse reactions, and
the elderly. Lancet, i: 1220.
Webster SGP (1985) Absorption of nutrients in old age. In: Pathy MSJ
ed. Principles and practice of geriatric medicine. New York,
Chichester, Brisbane, Toronto, John Wiley and Sons, Chapter 11.2, pp
265-290.
Webster IW & Logie AR (1976) A relationship between function and
health status in female subjects. J Gerontol, 31: 546-550.
Weigent DA, Carr DJ, & Blalock JE (1990) Bidirectional communication
between the neuroendocrine and immune system. Common hormones and
hormone receptors. Ann NY Acad Sci, 579: 17-27.
Weindruch R & Walford RL (1988). The retardation of aging and
dietary restriction. Springfield, Illinois, Charles C. Thomas.
Weindruch R, Gottesman SR, & Walford RL (1982) Modification of
age-related immune decline in mice dietarily restricted from or
after mid-adulthood. Proc Natl Acad Sci (USA), 79: 898-902.
Weiss B, Clark MB, & Greenberg LH (1984) Modulation of
catecholaminergic receptors during development and aging. In: Lajtha
A ed. Handbook of neurochemistry. Vol 6:- Receptors in the nervous
system. New York, London, Plenum Press, pp 595-627.
Wellinger R & Guigoz Y (1986) The effect of age on the induction of
tyrosine aminotransferase and tryptophan oxygenase genes by
physiological stress. Mech Ageing Dev, 34: 203-217.
Wesson LG Jr (1969) Renal hemodynamics in physiological states. In:
Physiology of the human kidney. New York, London, Grune and
Stratton, pp 98-100.
White L (1989) Epidemiology. In: Maddox GL ed. The encyclopedia of
aging. Berlin, Heidelberg, New York, Springer-Verlag, pp 216-223.
WHO (1983) Environmental Health Criteria 27: Guidelines on studies
in environmental epidemiology. Geneva, World Health Organization,
351 pp.
WHO (1984) The uses of epidemiology in the study of the elderly.
Report of a WHO Scientific Group on the Epidemiology of Aging.
Geneva, World Health Organization, pp. 23-25 (WHO Technical Report
Series 706).
WHO (1987) World health statistics annual. Geneva, World Health
Organization.
WHO (1989) Health of the elderly. Report of a WHO Expert Committee.
Geneva, World Health Organization, pp 14-30 (WHO Technical Report
Series 779).
WHO (1990) Global estimates for health situation assessment and
projections, 1990. Geneva, World Health Organization, Division of
Epidemiological Surveillance and Health Situation and Trend
Assessment, p 3.
Williams ME & Pannill FC (1982) Urinary incontinence in the elderly.
Ann Intern Med, 97: 895-907.
Willott JF (1986) Effects of aging, hearing loss, and anatomical
location on thresholds of inferior colliculus neurons in C57BL/6 and
CBA mice. J Neurophysiol, 56(2): 391-408.
Wilson K & Hanson J (1980) The effects of extremes of age on drug
action. Methods Findings Exp Clin Pharmacol, 2: 303-312.
Winneke G, Hrdina KG, & Brockhouse A (1982) Neuropsychological
studies in children with elevated tooth-lead concentrations. A pilot
study. Int Arch Occup Environ Health, 51: 169-183.
Wong DF, Wagner HN, & Dannals RF (1984) Effects of age on dopamine
and seratonin receptors pressured by positron tomography in the
living human brain. Science, 226: 1393-1396.
Wood RW (1981) Neurobehavioral toxicity of carbon disulfide.
Neurobehav Toxicol Teratol, 3: 397-405.
Wood AJJ (1985) Beta adrenoceptors and aging. In: O'Malley K &
Stevenson IL ed. Therapeutics in the elderly. Amsterdam, Oxford, New
York, Elsevier Science Publishers, pp 43-49.
Wu W, Pahlavani M, Cheung HT, & Richardson A (1986) The effect of
aging on the expression of interleukin 2 messenger ribonucleic acid.
Cell Immunol, 100: 224-231.
Wynne HA, Cope LH, Mutch E, Rawlins MD, Woodhouse KW, & James OF
(1989) The effect of age upon liver volume and apparent liver blood
flow on healthy man. Hepatology, 9: 927-301.
Xiong ZM (1989) [Common diseases and major causes of death in
elderly patients in Jiangxi.] Chin J Geriatr, 8: 135 (in Chinese).
Xiong BZ (1990) Technology progress, aging process and reappoint to
a position.] In: Institute of Japanese Problems, Chinese Academy of
Social Sciences ed. [Proceeding of Symposium on Sino-Japan Social
Countermeasure for Aging Problem.] Beijing, East Publishing House,
pp 186-196 (in Chinese).
Xu XF, You K, Liu D, He L, Cheng S, & Wu JS (1986) [Disease pattern
analysis in hospitalized elderly patients in Uromqi.] Chin J
Geriatr, 5: 15 (in Chinese).
Yamazoe Y, Shimada M, Murayama N, & Kato R (1987) Suppression of
levels of phenobarbital-inducible rat-liver cytochrome P-450 by
pituitary hormone. J Biol Chem, 262: 7423-7428.
Yang RSH, Tallant MJ, & McKelvey JA (1984) Age-dependent
pharmacokinetic changes of ethylenediamine in Fischer 344 rats
parallel to a two-year chronic toxicity study. Fundam Appl Toxicol,
4: 663-670.
Yao SB (1990) [The trend and characters of Chinese aged population.]
In: Institute of Japanese Problems, Chinese Academy of Social
Sciences ed. [Proceedings of Symposium on Sino-Japan Social
Countermeasure for Aging Problems.] Beijing, East Publishing House,
pp 64-74 (in Chinese).
Yates MS & Hiley CR (1979) The effect of age and cardiac output and
its distribution in the rat. Experientia (Basel), 35: 78-79.
Yelland C, Summerbell J, Nicholson E, Herd B, Wynne H, & Woodhouse
KW, (1991) The association of age with aspirin esterase activity in
human liver. Age Ageing, 20(1): 16-8.
York JL (1982) Body water content, ethanol pharmacokinetics, and the
responsiveness to ethanol in young and old rats. Dev Pharmacol Ther,
4: 106-116.
Young VR (1979) Diet as modulator of aging and longevity. Fed Proc,
38: 1994-2000.
Yu BP, Bertrand HR, & Masoro EJ (1980) Nutrition-aging influence of
catecholamine-promoted lipolysis. Metabolism, 29: 438-444.
Yumura W, Sugino N, Nagasawa R, Kubo S, Hirokawa K, & Maruyama N
(1989) Age-associated changes in renal glomeruli of mice. Exp
Gerontol, 24: 237-249.
Zapadnyuk VI (1971) [On an age-periodisation of laboratory animals.]
In: [Gerontology and geriatrics. 1970-1971 Annual book: Aging of the
cell.] Kiev, Research Institute of Gerontology, pp 433-438 (in
Russian).
Zarit SH, Eiler J, & Hassinger M (1985) Clinical assessment. In:
Birren JE & Schaie KW ed. Handbook of the psychology of aging, 2nd
ed. New York, Van Nostrand Reinhold Company, pp 725-754.
Zaphiropouos PG, Mode A, Norstedt G, & Gustavsson JA (1989)
Regulation of sexual differentiation in drug and steroid metabolism.
Trends Pharmacol Sci, 10: 149-153.
Zatz MM & Goldstein AL (1985) Thymosins, lymphokines and the
immunology of aging. Gerontology, 31: 263-277.
Zemel MB & Sowers JR (1988) Salt sensitivity and systemic
hypertension in the elderly. Am J Cardiol, 61: 7H-12H.
Zhou L-W, Weiss B, Freilich JS, & Greenberg LH (1984) Impaired
recovery of alpha - and alpha2-adrenergic receptors in brain tissue
of aged rats. J Gerontol, 39: 538-546.
Zhu JR, Li WX, & Wang YY (1982) [Common diseases and major causes of
death in the elderly with analysis of 8947 cases over 65 years of
age.] Chin J Geriatr, 1: 110-118 (in Chinese).
Zs-Nagy I, Kitani K, Ohta M, & Imahori K (1986) Age-dependent
decrease in lateral diffusion constant of proteins in the plasma
membrane of hepatocytes as revealed by fluorescence recovery after
photobleaching in tissue smears. Arch Gerontol Geriatr, 5: 131-146.
APPENDIX 1. Background Papers
Anisimov, V.N., Approaches to examine the effects of chemicals on
the aged population: experimental approaches.
Birnbaum, L.S., Basis of altered sensitivity of the elderly to
chemicals - pharmacokinetics and pharmacodynamics.
Cooper, R.L., & Goldman, J.M., Alterations in susceptibility to
toxic compounds in the aged central nervous system and endocrine
system.
Dilman, V.M., Theories and mechanics of aging.
Fabris, N., Systemic biology of aging.
Ingram, D.K., Evaluating effects of chemical exposure on aging:
development of conceptual models.
Li, S., Aged population: demographic, life expectancy, and
lifestyle.
Likhachev, A.J., Age related peculiarities of repair of DNA damage
with carcinogens.
Martin, G.M., Definitions on aging.
Ray, P.K., Jaffery, F.N., & Viswathan, P.N.,
1. Chemical exposure of the elderly
2. Modifying factors: nutrition, state of health, life style,
alcohol intake, smoking and ionizing radiation.
Richardson, A., The molecular biology of aging: translation,
transcription and chromatin structure.
Speijers, G.J.A., Groups at risk and their chemical exposure as well
as nutrition as a source of chemicals and as a confounding factor.
Zhu, J.R., Approaches to examine the effects of chemicals on the
aged population: epidemiological and clinical approaches.
It is commonly assumed that today's large percentage of elderly
people in the population is a result of increased longevity and
decreased birth rate. For example, in Japan the proportion of people
aged 65 or more had increased to 10.3% in 1985. At the same time,
the values were 15.1%, 14.5%, 12.4% and 16.9% in the United Kingdom,
Federal Republic of Germany, France and Sweden, respectively. Japan
is a new "aged-type" country with the greatest rate of increase in
the elderly in the world. By the year 2025, the proportion of the
Japanese population aged 65-plus will rise to 23.4% (Hosomi, 1990).
In China, the proportion of the population aged 60 and over was
7.3% of the total population in 1953. By 1984 it had increased to 9%
of the total population, and by the year 2000 the proportion of the
population 60 and over will be as high as 10.6%. The proportion is
predicted to increase to 26.2% by 2025 (Xiong, 1990).
At present, the aged population is growing more rapidly in
China than in countries of Europe and North America. According to
data from the US Bureau of the Census, it took 115 years (1865-1980)
for the proportion of people aged 65 or more in France to increase
from 7% to 14%, 85 years (1890-1975) in Sweden, 66 years (1944-2010)
in the USA, 45 years (1930-1975) in the United Kingdom, and 26 years
(1970-1996) in Japan. For China, the pattern of the growing number
of elderly is similar to Japan, i.e. the proportion of people aged
65 or more will be 7.4% in 2000, and by 2025 it will have increased
to 12.8% (Hosomi, 1990; Yao, 1990; Xiong, 1990).
1.4.2 Life expectancy
Life expectancy at birth is a statistical index calculated by
the use of a life table from the age-specific death rates of the
population. This illustrates the overall level of health in a
country or a region. Throughout this century, it has been evident
that, as a result of improvements in many aspects of health status,
an individual can expect to live longer. From 1960 to 1990, life
expectancy at birth for the total population had increased by 13.5
years. The life expectancy at birth for the period 1985-1990 was
estimated to be 63.9 years for the world as a whole, 74.0 years for
the more developed regions, and 61.4 years for the less developed
regions. The longest life expectancies are in Japan (78.3), Iceland
(77.5), Sweden (77.1), Switzerland (77.1), and the Netherlands
(76.9) (World Population Prospects, 1991).
The trend for life expectancy at birth in the USA showed an
increase from 1900 (46.4 for males and 49.4 for females) to 1950
(65.6 and 71, respectively) and in the year 2000 is expected to be
72.1 and 79.5 (for males and females). By 2050, it may have
increased to 73.6 for males and 81 for females (US Senate Special
Committee on Aging, 1986).
In China, the life expectancy at birth before 1949 was about 35
years of age, being one of the lowest in the world at that time. By
1957 it had increased to 57 years, from 1973 to 1975 it was 63.6
years for males and 66.3 years for females, and in 1981 it was 68
years. It is expected that the continued increase in the average
life expectancy of the people of China will be slow due to a high
death rate from cardiovascular diseases (Gu, 1986).
1.4.3 Life-style in aged populations
A basic issue in planning for the consequences of demographic
aging is whether elderly people should be considered a specific
target group for the development of services, or whether their needs
should be catered for within the context of planning for the
population as a whole. One approach to a rational policy for this
issue is to consider the nature of human aging. For this it is
necessary to view the physical, psychological and sociological
dimensions of aging as a whole.
Life-style influences the effects of chemicals on human health,
including that of the elderly, both quantitatively and
qualitatively. Environmental chemicals and their uses are diverse.
Specialized nutritional elements of the diet have become popular,
while in certain countries many people prefer predominantly
vegetarian diet. Others take supplements and additives which contain
pure preparations of vitamins, minerals, amino-acids and other
substances. Whether such substances have either adverse or
beneficial effects on the elderly and aging processes, has not
generally been fully evaluated. Another important source of human
exposure to chemicals comes from the intake of different kinds of
cosmetic agents and fragrances, such as shampoos, creams, perfumes,
oral deodorants, sunscreen and suntan lotions, and insect
repellents. These are often chemical mixtures whose components have
not been evaluated or tested beyond acute toxic potential.
Occupational status, indoor air quality, recreational
activities, exercise, eating and drinking habits, alcohol
consumption, and tobacco smoking can all affect the elderly and
aging processes to a certain degree. Elements of life-style can
strengthen or reduce the risk of developing aged-related
degenerative diseases. They can also accelerate or delay
physiological and anatomical changes. Typical examples are the
various age-related diseases caused by toxic chemicals in tobacco
smoke and the reduction of the risk of cardiovascular diseases
produced by regular exercise (Committee on Chemical Toxicity and
Aging, 1987).
As far as the possible influence of life-style factors on the
manifestations of aging is concerned, many studies have shown that
loneliness and physical and intellectual inactivity are common among
the elderly, especially widowed people. Several studies have
revealed that living conditions have an influence on health and
well-being, resulting in an increase in the demand for social care
and medical service. Marital status and living arrangements have
important significance for the unique life-style of the elderly.
There are striking differences between the proportions of elderly
males and females who are married: in many countries the proportion
of married males is twice that of married females. In general, the
proportion of widows is very high and that of widowers relatively
low (WHO, 1984).
Migration is also one of the life-style variables of the
elderly. In rural areas of Asia, many older women move to cities to
join their children after they have been widowed. Another common
type of move is the migration of the recently widowed or chronically
ill elderly from urban areas to their home towns or villages. For
many countries in Africa and Asia, the urban-rural migration is most
apparent among males, who return from urban to rural areas when they
are old. Worldwide, only a minority of elderly people live in urban
areas (WHO, 1984).
1.5 Theories of aging
During the last century, more than 100 various hypotheses
concerning the origin and mechanism of aging have been put forward.
All of them could be grouped generally into two broad categories:
those that invoke deterministic, or "programmed", alterations in
gene expression or gene structure; and those that invoke a variety
of stochastic, or "random", alterations in the structure and
function of macromolecules, cells, and organ systems. This
distinction, however, has some limitations, because stochastic
alterations in individual cells can lead to predictable phenomena in
the large populations of cells. The use of terminal differentiation
to explain the limited replicative life span of somatic cells
(Martin et al., 1974) could be an example of the blurring of the
stochastic and non-stochastic categories. For each individual cell,
differentiation is a random event; however, for a population of
cells, the process appears deterministic.
The mechanisms of aging are likely to be coupled to the
reproductive strategy of the organism. One example is the
synchronous, rapid physiological declines and mortalities that are
characteristic of species with single massive episodes of
reproduction (e.g., migrating Pacific salmon or soybean plants).
Placental mammals, however, have ample opportunity for a variety of
stochastic processes to take place during their long reproductive
and postreproductive phases. The associated patterns of structural
and functional decline can vary substantially, both qualitatively
and quantitatively, among individuals within a species and among
different related species. Evolutionary biologists in fact present
compelling arguments that aging did not evolve because of any
adaptive value to the individual or to the species, as would be
assumed by strictly programmed theories (reviewed in Rose, 1991).
Aging is thought to occur simply because of the decline in the force
of natural selection for gene action that is postreproductive. Such
gene action could be related to accumulations of late-acting
mutations in the constitutional genome or to selection for forms of
genes that have positive effects on reproductive fitness early in
the lifespan, but whose effects may be negative late in the lifespan
(the "antagonistic pleiotropy" theory of aging) (Rose, 1991).
It is beyond the scope of this monograph, however, to consider
the potentially large numbers of specific mechanisms that may be
modulated by such accumulated constitutional mutations or
pleiotropic genes. The reader is referred to recent reviews of the
many postulated theories of aging (Warner et al., 1987; Committee on
Chemical Toxicity and Aging, 1987; Finch, 1991; Cutler, 1991).
These can be classified in a variety of ways (Dilman, 1987;
Medvedev, 1990).
2. STRUCTURAL AND PHYSIOLOGICAL CHANGES IN THE AGED
2.1 Changes in gene structure and function in aging
Changes in gene expression are of critical importance to an
organism. Aging can potentially alter not only the structure of
genes, but the way in which they function. Changes in the DNA are
often thought to be integral to aging. It is clear that not only
mutations, but chromosomal rearrangements accumulate with age (Vijg,
1990). Repetitive sequence families may play a crucial role in the
processes of aging. In addition, the organization of DNA and protein
in chromatin is important structurally and functionally. Therefore,
changes in chromatin could play a major role in the age-related
change in the regulation of gene expression (Richardson et al.,
1983; Medvedev, 1984; Thakur, 1984; Richardson et al., 1985).
2.1.1 Chromatin structure
Chromatin changes may involve either proteins that interact
with DNA or the chemical structure of the DNA molecule itself.
Although no change in the stoichiometry of the major histones has
been observed with increasing age (Richardson et al., 1983;
Medvedev, 1984), several investigators have reported changes in the
subspecies of histone H1 (Medvedev, 1984; Mitsui et al., 1980;
Niedzwiecki et al., 1985). The acetylation of histones, which has
been proposed to alter histone-DNA interactions thereby making DNA
more accessible, decreases by 30% to 70% with increasing age
(O'Meara & Pochron, 1979).
With respect to age-related changes in DNA chemical structure,
there is now conclusive evidence for the "spontaneous" induction of
a variety of DNA lesions in different organs and tissues of both
humans and experimental animals (for a review, see Mullaart et al.,
1990). Most of these lesions seem to be repaired (see below), but
not all. For example, Cathcart et al. (1984) and Fraga et al. (1990)
estimated that in rats about 105 oxidative DNA lesions occur per
cell per day. Since the rate of repair does not entirely equal the
rate of induction of damage, there is a net increase of spontaneous
DNA lesions with age. Fraga et al. (1990) calculated for one
specific lesion, 8-hydroxy-deoxyguanosine, that about 80 residues
accumulate per rat cell per day.
Although some DNA lesions are repaired quickly, this is not the
case for all lesions. Indeed, after treating rats with low doses of
2-acetyl-aminofluorene (AAF), Mullaart et al. (1989) were still able
to detect about 30% of the major lesions induced as late as 21 days
after treatment. Such incomplete repair could be responsible for
accumulation of DNA lesions during continuous or frequent exposure to
genotoxic agents.
2.1.2 DNA repair
To preserve the DNA chemical structure, cells are equipped with
a battery of repair systems to remove damage. As yet the various
mechanisms of action of these DNA repair systems and their
interrelationships are incompletely understood (for a recent review,
see Lehmann et al., 1992). In general, repair systems can be divided
into three categories, i.e. direct repair, excision repair and
post-replication repair. In direct repair, the lesion itself is
removed without any further (transient) changes in the DNA
structure. Direct repair includes the enzymatic photo-reactivation
of UV-induced pyrimidine dimers and the removal of O6-alkyl
adducts by specific alkyl transferases.
DNA excision repair is brought about by a complex multi-enzyme
system, the components of which are involved in the various steps in
this repair process (Vijg & Knook, 1987). The third type of repair,
post-replication repair, does not actually remove the damage but
allows the replication system to bypass the damage. It is this
latter process especially that is considered to be associated with
nucleotide misincorporation (mutation).
Accurate assessment of an organism's capacity to repair
specific lesions is difficult and subject to error. In general, the
most reliable data can be obtained when the induction and
disappearance of the relevant lesions themselves are monitored in
the different organs and tissues of an experimental animal.
Unfortunately, in most studies on the possible existence of a
decline in DNA repair activities with age, assays were used which
measured the DNA synthesis phase of excision repair. The general
conclusion from these data, mostly obtained with cultured cells, is
that there is no age-related decline in the efficiency of DNA repair
systems (Tice & Setlow, 1985; Likhachev, 1985; Hanawalt, 1987). It
cannot be ruled out, however, that during aging DNA repair systems
become more error prone, leading to an accelerated induction of
mutations (Vijg & Knook, 1987). In any case, a certain degree of
imperfection is a general characteristic of DNA repair systems as
indicated by the actual accumulation of both DNA lesions and DNA
sequence changes (see above). The question that should be addressed
is what type of DNA alterations occur, how many exist, and at what
rate do they accumulate with age. Finally, their relevance in terms
of actual physiological decrements or the initiation of disease
should be assessed.
2.1.3 Transcription
Several review articles have been published in the past decade
that discuss the effect of age on transcription (Rothstein &
Seifert, 1981; Richardson et al., 1983; Richardson et al., 1985;
Richardson & Semsei, 1987; Slagboom & Vijg, 1989). A major problem
in this area has been the difficulty in accurately measuring the
rates of synthesis of specific RNA species and their intracellular
levels. With the major advances in recombinant DNA technology, this
problem has now been virtually eliminated and our knowledge of how
aging affects the expression of specific genes is rapidly growing.
At present, it appears that the overall transcriptional
activity of a cell declines as an organism ages. However, the level
of total RNA tends to remain constant suggesting a decline in the
rate of RNA turnover (Horbach et al., 1986).
The levels of some specific mRNA species using cDNA probes for
specific genes have been measured recently (Richardson & Semsei,
1987). In general, no consistent trend has emerged. The levels of
some mRNA species decrease with age; however, other mRNA species do
not change with age, and others actually increase (Slagboom & Vijg,
1989).
In most of the studies, a good correlation has been found
between the age-related changes in the level of an mRNA species and
the level of protein (or enzyme activity) specified by the mRNA
species. This has been demonstrated in rat liver for albumin
(Horbach et al., 1984), apha2u-globulin (Richardson et al., 1987),
and superoxide dismutase and catalase (Semsei et al., 1989), and in
rat kidney and small intestine for calbindin-D (Armbrecht et al.,
1989). The age-related decline in mitogen-induction of interleukin 2
(IL-2) (Wu et al., 1986; Nagel et al., 1988; Pahlavani et al., 1988)
and IL-3 (Li et al., 1988) mRNA in lymphocytes from rodents and
humans corresponded to the age-related decline in the biological
activities of these two interleukins. In contrast, Strong et al.
(1990) reported an uncoupling of tyrosine hydroxylase transcription
and translation in the adrenal glands of old rats.
Investigators usually assume that age-related changes in the
levels of a particular mRNA species arise from a change in
transcription. However, only a few studies have actually measured
the transcription of a specific gene as a function of age using
nuclear run-off assays. While an age-related decrease occurs in the
nuclear transcription of the alpha2u-globulin (Richardson et al.,
1987; Murty et al., 1988a), cytochrome P450(b+e) (Rath & Kanungo,
1989), and superoxide dismutase and catalase (Semsei et al., 1989)
genes, the nuclear transcription of tyrosine amino-transferase and
tryptophan oxygenase (Wellinger & Guigoz, 1986), albumin (Horbach
et al., 1988b) and the c-myc (Buckler et al., 1988) genes was
similar in young and old rodents. Studies are now underway to
explore in more detail age-changes in specified mRNA species in
terms of the transcription factors involved (Post et al. 1991).
One exciting development in the area of transcription and aging
has been the observation that dietary restriction, which enhances
the longevity of rodents, alters the expression of some genes at the
level of transcription (Richardson et al., 1987; Semsei et al.,
1989). However, the expression of all genes is not affected by
dietary restriction (Waggoner et al., 1990).
In addition to nuclear synthesis, post-transcriptional
processing of hnRNA plays an important role in the regulation of
gene expression. Müller et al. (1989) recently discussed various
views of how the post-transcriptional processing of hnRNA might
alter with age. At present, there is little evidence that major
changes occur with age in the size of the poly(A)-segment of mRNA
(Birchenall-Sparks et al., 1985). Interestingly, in the many studies
in which mRNA species have been analysed by Northern blot analysis,
there has been not a single report of a significant change in the
size of the mRNA species examined with increasing age (Richardson &
Semsei, 1987). Thus, there is very little direct evidence at present
to support the view that the processing and/or nuclear transport of
hnRNA is altered with age.
2.1.4 Translation
Increasing age generally results in a decrease in total protein
synthesis in plants, invertebrates, rodents and cultured cells
(Richardson & Birchenall-Sparks, 1983; Ward & Richardson, 1991).
Recent studies have focused on the influence of age on the
translation of mRNA into specific proteins and on the ability to
modulate age changes in protein synthesis. There is no evidence that
a decrease in the fidelity of protein synthesis occurs with
advancing age but technical limitations do not permit a definitive
conclusion (Rosenberger & Kirkwood, 1986). The influence of age on
protein synthesis differs from protein to protein and much more work
must be done in assessing the effect on key individual proteins.
Attempts to modulate protein synthesis have recently begun. The rate
of protein synthesis in the liver is higher after maturity for
dietary restricted than for ad libitum fed rats (Ward, 1988). In
an in vitro system, growth hormone increases protein synthesis in
muscles of old rats to the level found in muscles of young rats
(Sonntag et al., 1985). Much more study is required, focusing on
individual proteins, different tissues and different organisms.
2.2 Changes in tissues, organs and systems in aging
The progressive modification of body functions with age
involves alterations not only at the genetic, molecular and cellular
levels, but at the level of the tissues, organs, systems and entire
organism. It is important to attempt to differentiate between
age-related pathology and true physiological aging. This is often
difficult because the majority of age-related changes increase the
vulnerability of the aging organism to disease and ultimately death.
In the following, each organ or system will be discussed in
reference to age-related changes in its structure which might
predispose to alterations in function, not only inherently as part
of aging, but in response to environmental agents. The focus will be
on the healthy aged as opposed to the diseased.
2.2.1 Nervous system
The brain may undergo a progressive deterioration with age at
all levels of organization - structural, biochemical and functional.
CNS disorders, including Parkinson's and Alzheimer's diseases, are
common in the elderly.
2.2.1.1 Structural changes
Brain weight decreases slightly with aging. This is due to
atrophy of both grey and white matter (Creasey & Rapoport,1985). At
the cellular level, the major age-associated modification is in the
number of neurons, which are significantly diminished in discrete
areas of the brain (Brizzee, 1985), particularly in the basal
ganglia, cerebellum (probably related to decreased motor control),
locus ceruleus (associated with alterations in sleep patterns),
nucleus basalis of Meynert (associated with senile dementia of
Alzheimer type (SDAT) (Bondareff, 1986), and the spinal cord.
Neuronal loss, which is associated with an increase in the number of
glial cells, is relatively mild in the healthy aged, but is much
more severe in SDAT, Parkinson's disease and in the early aging
associated with Down's syndrome.
In addition to a reduced number of neurons, the aged brain is
characterized by a reduction in the number of dendrites and
dendritic spines, probably due to a slowing renewal process
(Scheibel & Tomiyasu, 1978). Synapse density declines in discrete
areas of the brain, but this is partially compensated by enlargement
of the remaining synapses (Bertoni-Freddari et al., 1990).
Intracellular changes include dilation and fragmentation of the
Golgi apparatus (Mervis, 1981), distortion of membranes and the
nucleus, and accumulation of lipofuscin, in both neurons and glial
cells within discrete brain areas. With advancing age, there is an
increase in neurofibrillary tangles (intracellular tangled masses of
paired helical filaments) (Terry, 1963), extracellular neuritic
plaques (a core of amyloid surrounded by material derived from
dystrophic neurites), and reactive glial and microglial cell
accumulation (Master et al., 1985). Again, these changes occur in
normal aging at a moderate level, but are much more frequent in SDAT
(Iqbal et al., 1982) and other dementias.
There are also age-related changes in the morphology of the
peripheral and autonomic nervous systems. These include reductions
in the number of sensory and motor neurons, increases in
demyelination, increases in connective tissue, and a mild loss of
myelinated fibres (Tomlinson & Irving, 1977; Spencer & Ochoa, 1981).
The central processes of dorsal root ganglion cells typically
undergo distal dystrophic and degenerative changes. Regressive
changes have been reported in the terminals of motor axons.
2.2.1.2 Biochemical changes
Besides the pathological changes, there are many age-related
alterations in brain chemistry required for cell-to-cell
communications (Rogers & Bloom, 1985; Finch, 1991). These include
changes in the concentration and/or turnover of the amines (e.g.,
acetylcholine, norepinephrine, epinephrine, dopamine, serotonin),
amino acids (e.g., glycine, glutamate and GABA) and peptides (e.g.,
enkephalin, substance P, thyrotropin-releasing hormone,
cholecystokinin, somatostatin). There are numerous studies showing
impairments of adrenergic, dopaminergic and serotonergic activity in
the senescent animal (Zhou et al., 1984; Roth & Joseph, 1988;
Telford et al., 1988). One of the underlying causes of these
alterations seems to be an overall loss of receptors (Weiss et al.,
1984; Roth & Joseph, 1988).
Synapses may utilize one or more neuromodulator (e.g.,
norepinephrine and neuropeptide). The multiple levels of control and
the regional diversification of different synapses in discrete brain
regions make it difficult to define the age-related alterations in
neurotransmitter/neuropeptide function. In fact, rather than a
uniform drop in the level of a specific neurotransmitter throughout
the nervous system, a "desynchronization" of signals may occur. For
example, while the brain content of norepinephrine and dopamine are
decreased in old age, that of serotonin is unchanged or even
increased, depending on specific brain areas. In some cases, the
greater the concentration of a neurotransmitter in a discrete brain
region, the higher the decrement with aging and vice versa (Timiras
et al., 1984). In fact, age-dependent alterations in different
neurotransmitter/neuropeptide concentrations do not always occur
simultaneously. Each neurotransmitter has its own timetable:
dopamine levels decrease in the cerebral hemispheres of rats from
the age of one year, whereas in the same areas serotonin levels
remain unaffected until three years of age (Timiras et al., 1984).
Aged-related changes in neurotransmitter receptor number and
function have also been reported (Greenberg & Weiss, 1983; Roth &
Joseph, 1988). Changes in binding affinity have not been frequently
detected. Beta-adrenergic receptor responsiveness is decreased in
the elderly (Vestal et al., 1979; Lakatta, 1980). This appears to be
due to uncoupling of the beta-receptor from the adenylate cyclase
complex which transmits the signal (Wood, 1985). In the rat pineal
gland, corpus striatum and cerebellum, a reduced responsiveness to
catecholamines is present due to a decrease in the affinity of
beta-adrenergic receptors to their ligand. However, there is no
change in receptor number (Greenberg & Weiss, 1978). This may be due
in part to a reduced ability to increase the number of
beta-adrenergic receptors after decreased noradrenergic input
(Greenberg & Weiss, 1979).
Changes in general biochemical properties of the cells occur in
the nervous system as they do elsewhere in the aging organism.
Lipid composition may change, resulting in altered membrane
viscosity. Protein synthesis decreases in discrete brain regions.
Lipofuscin accumulates, although the functional significance is
unclear, and there are alterations in electrolytes and trace
elements (Brizzee, 1985). For example, aluminium levels may increase
sharply in elderly people (Bjorksten et al., 1989). Decreases in
zinc may be important in the light of the zinc requirement of
various enzymes and growth factors, including nerve growth factor
(NGF) (Dunn et al., 1980). In addition, aging is accompanied by a
decreased brain water content (Meisami, 1988). Alterations in
vascular flow have also been reported (Katzman & Terry, 1983).
2.2.1.3 Functional changes
Despite the morphological and biochemical changes observed in
the aging brain, the functional efficiency of the nervous system
seems to be well maintained in most elderly people. However, CNS
disorders do occur in some individuals, though it may be difficult
to discriminate age-related pathology from physiological aging
phenomena. Perhaps the most ubiquitous and significant change
observed in the older organism is slowness of behaviour (Birren et
al., 1979). The slowing of behaviour with age not only appears in
motor responses and perceptual processing, but is also apparent for
the more complex processing of information associated with
short-term memory (Smith et al., 1980). Related cross-sectional
studies using global measures of intellectual function such as the
Wechsler Adult Intelligence Scale (WAIS) show evidence that some
performance abilities decline by the late 60s and early 70s, while
others (e.g., verbal abilities) appear to be maintained throughout
life in healthy individuals (Gallagher et al., 1980). The slowing of
reaction time may be associated with the age-associated slowing and
loss of coordination in motor tasks, such as those involved in
handwriting and other purposeful movements.
The age-related modification of biorhythms is exemplified by
the alterations of the sleep/wakefulness cycle, which is largely
dependent on the reticular system. Alterations of sleep patterns
with aging are qualitative rather than quantitative (Dement et al.,
1985) and affect primarily the "deep sleep" phases, as confirmed by
the alterations observed in the brain electrical activity (Müller &
Schwartz, 1978). Among neurotransmitters, serotonin seems to be
implicated.
Alterations in posture and locomotion in the elderly (Klawans &
Tanner, 1984) also depend on CNS impairment. Peripheral
modifications such as decreased nerve conduction velocity, reduced
muscle mass and increased rigidity occur. Autonomic system
dysfunction is also implicated in many pathophysiological changes
of age including hypotension, thermoregulation, gastrointestinal
function and urinary incontinence (Finch & Landfield, 1985). Other
changes in the autonomic system include changes in vascular and
cardiac reflexes, galvanic skin responses, and potency (Katzman &
Terry, 1983). Sympathetic hyperactivity is commonly present in the
aged and could interfere with cognitive functioning.
2.2.2 Sensory organs
All of the sensory organs are affected by aging, both those in
which the cells are continuously renewed (such as cutaneous sense
tissues) and those in which the cells are terminally differentiated
early in life (vision and hearing).
2.2.2.1 Vision
Both neural (retina) and optical (cornea, lens, pupil, aqueous
and vitreous humours) components of vision are affected by age. The
changes in the optical compartment are probably the primary cause of
visual impairment in the elderly (Sekuler et al., 1982). The most
common alterations are in the lens with increased hardness and
decreased transparency (Graham, 1985). The former results in reduced
refractive power (Marsh, 1980). The loss of transparency relates to
the following chemical changes in the lens: protein oxidation,
racemization, glycation, aggregation, polymerization and
precipitation (Taylor, 1989). These alterations are associated with
presbyopia and cataracts, respectively.
In the neuronal compartment, the retina undergoes progressive
loss of rods, while cones may be augmented. Morphometric analysis of
the retina demonstrates an increase in electron-dense plaques and a
decrease in the ground substance during aging. Such retinopathies
result in decreased light sensitivity and reduced colour vision
(Marsh, 1980).
Vision declines as a function of age (Weale, 1986) and can be
measured in several tests, such as the Humphrey Field Analyser
(Iwase et al., 1988), and retinal potentials (Trick, 1987). Visual
acuity is substantially decreased. The ability to detect light
gradually decreases (Sample et al., 1988) and light adaptation
declines (Katz & Robinson, 1987).
2.2.2.2 Hearing
Decrements in hearing are frequently observed in the elderly.
There is also a progressive loss of hearing in animals with age
(Willott, 1986). Both auditory structures and neuronal components
are involved. While the outer and middle ear show few modifications,
degenerative changes occur in the hair cells, which are the auditory
receptors, and in the mechano-electrical transducing organs
resulting in otosclerosis. This accounts for the preferential loss
of hearing of high frequency sounds (presbycusis) (Marsh, 1980). The
degree of hearing loss may affect the two ears differentially, thus
causing defects in sound localization. Presbycusis has a great
impact upon speech perception, since consonants, which make speech
intelligible, are generated by high frequency sounds, whereas
vowels, responsible for audibility, are produced by low frequency
sounds.
Hearing defects may also result from changes in the neural
components (Allison et al., 1984) of hearing, and in particular in
the nerves connecting the cochlea with the auditory centres in the
brain, specifically in the superior temporal gyrus.
2.2.2.3 Olfaction
The age-related alteration in the sense of smell is generally
underestimated. The reduction in olfactory sensitivity is mainly due
to the progressive loss of olfactory neurons, which protrude through
cilia from the superior nasal cavity and represent the receptor
sites for odour and the chemo-electrical transducing mechanism
(Naessen, 1971). Loss of neurons have also been demonstrated in the
olfactory bulbs of the brain (Bhatnagar et al., 1987).
2.2.2.4 Taste
Taste thresholds are known to increase with age. The taste of
salt is preferentially altered in the elderly. The loss appears due
both to a decline in the number of taste buds and papillae (Bradley,
1979) in the tongue, as well as to the loss of neurons in the
cerebral centers of the gustatory system.
2.2.2.5 Somatic sensations
The somatic sensory system (touch, pressure, vibration,
proprioception, heat, cold and pain) is variably affected by age.
Tactoperceptual ability and vibrotactile sensations are decreased in
the elderly due to the loss of Meissner end-organs and Pacinian
corpuscles present in the skin (Bruce, 1980). For more complex
somatesthetic abilities (stereognosis, body part recognition) as
well as for pain and thermal sensitivity, the biological causes of
their alterations with age involve not only the sensory end-organs,
but also affective and cognitive factors (Marsh, 1980).
2.2.3 Endocrine system
Hormones play an important, often critical, role in the
regulation of a large number of physiological and behavioural
processes, and their influence can be demonstrated throughout the
lifespan. Some hormones have a role in differentiation in that their
presence or absence during certain developmental periods will affect
the way in which physiological and behavioural processes proceed or
are expressed in adulthood. Throughout each period of the life span,
the maintenance of an appropriate endocrine milieu is essential to
the numerous homeostatic processes required for survival. With
advancing age, there are several, well-documented changes in the
ability of the organism to synthesize and secrete a number of
hormones. It is, therefore, likely that the typical age-related
change in an organism's endocrine balance would result in, or at
least contribute to, the impairment of homeostasis frequently
observed in the elderly. Such impairments can be noted in the
decreased rate of recovery of the elderly from the insults of injury
or disease.
Hormones may also play a significant role in the aging process.
For example, age-related changes in several physiological functions
appear to be closely linked to the level and pattern of hormonal
stimulation present during adulthood. As such, different patterns of
exposure to a hormonal environment may alter the "rate of aging"
within a specific neuroendocrine system and, in turn, affect the
susceptibility of the organism to environmental insults at different
segments of the life span. There are a number of different ways in
which endocrine systems and the hormonal signalling operations that
they use may undergo alterations with age and toxicant exposure.
These can be categorized as changes in: (a) the availability of
hormones for binding to the target tissues, (b) the reception of the
pertinent transmitter or hormonal signal by the target cells, and
(c) the nature of the hormonal message.
At any point in time, the concentration of a hormone in the
blood is a consequence of both its metabolism and secretion. Such
changes in the size of the available signal pool may have
corresponding effects on the magnitude of the response by the target
tissue. Other changes may reflect declines with age in the
homeostatic controls, which rely heavily on endocrine feedback
relationships within organ systems.
Serum hormonal levels, as a rule, are not maintained at
constant levels. They tend to fluctuate, sometimes markedly,
throughout a 24-h period. In the young adult man, peak morning
testosterone values can fall by one-third to an early evening nadir,
before rising again through the late evening and early morning hours
(Bremner et al., 1983). A similar circadian rhythm in circulating
levels of testosterone is prevalent in the rat (e.g., Kinon & Liu,
1973; Ellis & Desjardins, 1982). Human cortisol (Bilchert-Toft,
1978) and rat corticosterone (Moberg et al., 1975; Kato et al.,
1980) concentrations also exhibit well-known rhythmic fluctuations,
as do those of thyrotropin (Vanhaelst et al., 1972; Leppaluoto
et al., 1974) and growth hormone (Millard et al., 1985). Reported
attenuations with age in the rhythms of human and rat serum
testosterone (Bremner et al., 1983; Steiner et al., 1984),
luteinizing hormone (LH) (Vermeulen et al., 1989), and growth
hormone (Sonntag et al., 1980; Prinz et al., 1983), among other
hormones, can present differences in young-versus-old comparisons,
depending on when such sampling is performed.
While observable changes in hormonal rhythms or significant
differences in circulating hormone concentrations may reflect
disturbances in the overall functional integrity of the associated
organ system, the absence of such changes should not be necessarily
assumed to indicate a corresponding absence of a functional
alteration. The notion of a "system at risk" presupposes an increase
in the susceptibility to disruption of the homeostatic controls. An
aging system that may be undergoing a subtle erosion in its
endocrine balance could be more likely to exhibit alterations in its
response to a stressor or toxic insult. In this respect the
stimulation of growth hormone release by clonidine, L-dopa and
insulin is substantially depressed (Riegel & Miller, 1981), while
arginine-stimulated growth hormone (GH) secretion after arginine
infusion is preserved (Aschoff, 1979). Secretion stimulated by GHRH
(GH releasing hormone) is only partially reduced (Coiro et al.,
1991).
Regardless of these alterations, it remains established that
the 24 h production of GH is significantly reduced in elderly humans
(Prinz et al., 1983), whereas that of prolactin is increased
(McGinty et al., 1988; Blackman, 1987). These data have been
confirmed in animals (Ceda et al., 1986; Sonntag & Gough, 1988),
although measurements of hormonal profiles may have involved
different procedures in animals and man, thus giving rise to
slightly different interpretations.
Similar difficulties are encountered in studies of age-related
alterations in pineal hormone secretion, including melatonin, whose
circadian rhythmicity is certainly changed with age (Reiter, 1986;
Anisimov & Reiter, 1990).
In order to illustrate age-related alterations in hormone
control, it is useful to focus on the integrated systems which
involve more than one gland or hormone. Although three such systems
are reviewed below, this discussion is by no means intended to be
comprehensive. One theme common to studies of age-related changes in
endocrine function is that such alterations are often hormone and
species specific. Finally, the extent to which any of these changes
relate to potential adverse health outcomes in the older organism
remains to be demonstrated.
2.2.3.1 The pituitary-thyroid axis and the basal metabolism
Thyroid hormones are required during development for growth and
in adult life for regulating oxygen consumption. Maintenance of
thyroid function is generally assured even in old age, although
following repeated stress and demands the reserve function may
become exhausted and a dysthyroid state may follow (Ingbar, 1978).
Changes with aging in the levels of both thyroid stimulating
hormone (TSH) and thyroid hormones (thyroxine (T4) and
triiodothyronine (T3)) are controversial, because concomitant health
disturbances may cause significant fluctuations in the levels of
these hormones (Gregerman & Solomon, 1967; Utiger, 1980). Both hypo-
and hyperthyroidism are not uncommon in the elderly. In general, the
size of the thyroid decreases with age (Gambert & Tsitouras, 1985).
Older people show a normal response to decreased thyroid
function by increased secretion of TSH (Eden, 1987). TSH levels
undergo few changes (Miller, 1989), suggesting that the hypothalamic
control of TSH release has not been altered. However, structural
modifications of TSH have been reported (Klug & Adelman, 1977). T4
levels remain unchanged with age, even though the rate of synthesis
is reduced. However, the blood levels of T3 are reduced with
advanced age (Chopra et al., 1978), while levels of reverse T3 are
unchanged. It should be noted that severe and chronic illnesses, not
directly involving the thyroid, can lower the levels of T3 and
T4.
The alterations observed in thyroid hormone levels are
inadequate to explain the age-associated decline in various
functions that are dependent on thyroid hormone. One possible
explanation is that peripheral sensitivity to thyroid hormone action
is modified by aging. However, with advancing age, the basal
metabolic rate remains unchanged if based on lean body mass, but
decreases if expressed based on body surface area (Masoro, 1985).
2.2.3.2 The pituitary-adrenal axis
The major function of this axis, which is largely based on
pituitary hormones (ACTH) and adrenal hormones (corticosteroids), is
to provide an adaptive response to environmental stress (Selye,
1950; Sapolsky et al., 1986). Any harmful agent, in addition to
inducing a specific reaction in the body (anaesthesia, emotion,
fever, etc.), activates a specific and common response, the
so-called "General Adaptation Syndrome" (Selye, 1950), characterized
by increased adrenocortical secretion, thymic involution,
lymphopenia and eosinopenia. With advancing age this axis may
undergo desynchronization, thus resulting in a failure of
homeostasis and adaptation (Anisimov & Reiter, 1990).
ACTH secretion, which shows a circadian rhythm based on
melatonin fluctuations, is generally preserved in advanced age
(Halberg, 1982), although minor modifications of blood levels may
occur due to variations in renal clearance or alterations in sleep
patterns. However, there is evidence of diminished sensitivity of
the hypothalamic/pituitary axis feedback inhibition by
glucocorticoids (Greden et al., 1986; Blackman, 1987; Dilman, 1987;
Sapolsky et al., 1987). The elderly suffering from Alzheimer's
disease are extremely resistant to glucocorticoid negative feedback
(Sapolsky et al., 1986).
Finally, certain cell populations (e.g., CA3 neurons in the
hippocampus) are particularly susceptible to glucocorticoids.
Long-term stress may result in their dysfunction and death (Sapolsky
et al., 1987).
2.2.3.3 The endocrine pancreas and carbohydrate metabolism
It is well documented that with advancing age the ability to
maintain glucose homeostasis is impaired, but the underlying
mechanisms are still not well defined. Several hormones contribute
to the regulation of glucose homeostasis: above all, insulin and
glucagon, secreted by the endocrine pancreas, and somatostatins and
the pancreatic polypeptide, which modulate the secretion of insulin
and glucagon, respectively. In addition, glucose metabolism may be
affected by other hormones, including T3 and T4, growth hormone,
glucocorticoids and epinephrine (Minaker et al., 1985).
Only modest morphological alterations are observed in the
endocrine pancreas with advancing age. In spite of this fact, blood
sugar levels after fasting are elevated and glucose tolerance is
lowered in the elderly (Magal et al., 1986; Eden, 1987; Ammon
et al., 1987; Wang et al., 1988; Groop, 1989). Plasma insulin
concentration increases and insulin sensitivity decreases.
Alterations in insulin turnover are detectable in the elderly after
glucose load, such as reduced insulin secretion and increased
secretion of the inactive prohormone, proinsulin (Marx, 1987), but
these changes are too modest to account for the observed glucose
intolerance. One alternative explanation is an increase in
peripheral resistance to insulin. In fact, peripheral uptake of
glucose is indeed reduced in the elderly, due to a reduction in
insulin receptors (Pagano et al., 1981) as well as alterations in
the post-receptor signalling process (Rowe et al., 1983). No
evidence exists regarding the possible involvement of age-related
changes in glucagon affecting glucose intolerance in the elderly.
Other factors, however, may contribute to glucose intolerance.
These include: (a) reduced liver sensitivity to insulin, resulting
in reduced glycogenesis; (b) changes in diet and physical exercise;
and c) increased body fat with reduced muscle mass. This last point
seems to merit particular consideration in view of the observation
that insulin resistance is certainly increased in obese humans
(Runcie, 1985). In fact, intracellular fat accumulation leads to a
reduced concentration of insulin receptors (Bolinder et al., 1983).
2.2.4 Reproductive system
The age-related modifications of the reproductive system are
primarily based on alterations in the central nervous system,
pituitary gland and gonads. While menopause is a time-fixed event
involving cessation of ovarian function, the decline of testicular
function is a slow and gradual process, involving limited hormonal
alterations. Older persons show the normal response to deficient
gonadal function by increased synthesis of gonadotropins (Piva
et al., 1987). This occurs in both sexes. In fact, alterations in
serum levels of both luteinizing hormone (LH) and follicle
stimulating hormone (FSH) have been reported (Blackman, 1987). The
reduced presence of sex steroids in women may have an influence on
the function of other endocrine glands. Estrogens have
well-documented effects on salt and water balance and on plasma
proteins, which in turn have effects on the level of thyroid
hormones through a suppression of TSH secretion. Estrogen also
stimulates the production of growth hormone and prolactin. Thus, the
decline in gonadal function during age could have far-reaching
consequences on the individual's physiological function.
2.2.4.1 Female aging
In females, cessation of ovarian function consists of the
transfer from regular menstrual cycles to amenorrhoea, usually
preceded by a period of cycle irregularity. The initial changes have
been reported to occur in hypothalamic-pituitary control of the
ovaries. For example, the age-related decline in reproductive
function is associated with a decreased sensitivity of the
hypothalamic-pituitary complex to feed-back regulation by estrogens
(Dilman, 1971, 1987). This leads to an age-related enhancement of
pituitary gonadotropins (FSH, LH) (Chakravarty et al., 1976),
leading in turn to hyperstimulation of the ovaries. However, despite
the compensatory increase in ovarian hormone production, the level
of estrogens is insufficient to induce ovulation because of
hypothalamic insensitivity, possibly due to an age-related decrease
in the level of biogenic amines and/or peptide hormone receptors
(Dilman & Anisimov, 1979). In addition, the progressive loss of
oocytes plays an important role in the decline in reproductive
function since the reduction of maturating oocytes may induce
desynchronization of pituitary-ovary hormonal interactions
(Aschheim, 1976).
The most common consequences of menopause include imbalances of
the autonomic nervous system, psychological modifications, and
physiological alterations of target organs due to metabolic changes.
Alterations of estrogen target organs are among the most evident
effects of menopause. Vulvar skin and vaginal epithelium may undergo
atrophy. Glycogen content is generally reduced, with a consequent
decrease of lactobacilli, rise of vaginal pH, and increased growth
of pathogenic microbes. The uterus and oviducts atrophy due to the
decreases in estrogen levels. In ovaries, follicular cysts and
atresia result in response to the altered hormonal status.
Hyperplasia of the theca cells occurs. Fibrosis also occurs in these
tissues but, in addition, can affect the bladder and urethra,
resulting in an increased incidence of cystitis, dysuria and
non-infectious urethritis. The reduced thickness of the skin is also
a result of the decrease in estrogen (Schiff & Wilson, 1979).
Menopause has major health consequences for the cardiovascular
and skeletal systems. The reduction in estrogen secretion removes
the protection offered by these hormones against coronary heart
disease, development of atherosclerosis, and accompanying
alterations of lipid metabolism. Osteoporosis, resulting from
increased bone reabsorption relative to bone formation, is a common
problem in postmenopausal women (Riggs, 1987). Two types of
osteoporosis may be identified. Type I is associated with estrogen
withdrawal and may begin in middle age (Riggs & Melton, 1983). The
biological effects are linked to disruption of the complex
relationship between calcium intake and loss, and the secretion of
calcitonin, parathyroid hormone and 1,25-dihydroxy-vitamin D.
Estrogens prevent the transfer of calcium from bone to blood and its
loss through urine. This induces parathyroid hormone secretion,
which stimulates the formation of 1,25-dihydroxycholecalciferol, the
active metabolite of vitamin D. The function of the parathyroid is
affected by increasing age (Eden, 1987). The relevance of estrogen
for bone loss is further supported by the effectiveness of estrogen
therapy in delaying the osteoporotic process in post-menopausal
women (Edman, 1983). With advancing age type II osteoporosis (senile
osteoporosis) may occur, which is probably due to the poor
intestinal absorption of calcium (Riggs, 1987).
2.2.4.2 Male aging
The reproductive system is less affected by aging in males than
in females. It is generally accepted that testosterone levels are
maintained within the physiological range throughout life, although
a decrease in testosterone production in response to gonadotropin
action may occur in old age due to a reduction in Leydig cell number
and function (Harman et al., 1982). Testis and accessory sex organs
do not show substantial modifications with age, and sperm is found
in the ejaculate of very elderly men. The volume of seminal fluid is
generally decreased.
While the prostate undergoes involution in the majority of old
men, in about one-third of males it undergoes hypertrophy with
consequent obstruction of the urethra and urinary flow from the
bladder. The cause of the hypertrophy is still unclear (Mawhinney,
1985). The prostatic enlargement results in compensatory hypertrophy
of the bladder. When such compensation is no longer sufficient,
retrograde filling of the renal pelvis and ureters may occur,
resulting in hydronephrosis and eventually renal failure.
2.2.5 Immune system
With advancing age a progressive increase occurs in the
incidence of various infectious diseases, autoimmune processes and
tumours. These may be in part based on age-related defects in the
immune system. The association of so many age-related pathologies
with defects in the immune system has led to the suggestion that
aging of the immune system may be rate limiting for life span
(Walford, 1969). However, while there are numerous experimental and
clinical studies demonstrating an age-related deterioration in
immune efficiency, this decline is not sufficient to account for all
manifestations of aging.
There are several recent reviews on aging and the immune system
(Revskoy et al., 1985; Lipschitz, 1987; Segre et al., 1989; Miller,
1991). However, it is still difficult to draw a comprehensive
picture, because of the many cellular and humoral components
involved in immune reactions and the many modulating
extra-immunological factors which may also be compromised in the
elderly. The immune and haematopoietic systems are intimately
related, being derived from a common pluripotent stem cell. Both
play central roles in host defense, prevention of neoplasia, and
response to infectious agents (Lipschitz, 1987). However, basal
haematopoiesis in both animal models and man seems to be either
unchanged or minimally altered with age (Dybkder et al., 1981;
Lipschitz, 1987). The reserve capacity may be reduced resulting in a
decreased ability to respond to stress.
2.2.5.1 Aging of lymphoid organs
Peripheral lymphoid organs, such as the spleen and lymph nodes
do not show consistent modifications in size with aging. Bone marrow
is not consistently affected by age. Stem cell production is
generally well preserved in old age (Harrison et al., 1978),
although a slight change in the replication rate of stem cells has
been reported by some authors (Schneider et al., 1979). Thymic
involution has been considered to account for the major age-related
changes in the immune system, beginning at puberty. Such an
involution consists of a progressive loss of cellularity with
lymphoid cell depletion in the cortical areas and cystic changes in
the epithelial cells. These are the source of various peptides
involved in differentiating thymic lymphocytes (T-cells) from
lymphoid cells of earlier lineage. The export of newly
differentiated T-cells is reduced with advancing age (Globerson
et al., 1989). The synthesis and the secretion of polypeptide thymic
hormones, such as thymosin (McClure et al., 1982), thymopoietin
(Lewis et al., 1978) and thymulin (Bach et al., 1972), are
progressively diminished. In all cases, the reduction of thymic
endocrine activity seems to have a pathogenic role in age-related
immune dysfunctions, since replacement by exogenous administration
of the hormones is capable of restoring various immune functions in
old age (Zatz & Goldstein, 1985). The turnover of zinc, which is
essential for immunocompetence (Iwata et al., 1979; Chandra, 1985),
decreases in old age. Zinc supplementation can restore immune
functions (Fabris et al., 1990).
2.2.5.2 Aging of cellular constituents
Mature T-cells, bone marrow lymphocytes (B-cells) and natural
killer cells (NK-cells) can be detected in blood and in lymphoid
organs by specific monoclonal antibodies. With this type of
analysis, no major modifications in the proportion of the various
lymphoid cell subpopulations have been observed in humans. However,
the major alteration in the immune system appears to arise in the
functioning of T-cells (Thompson et al., 1987). While the total
number of T-cells in the peripheral blood does not change
appreciably with age, there are clear-cut differences in the
relative proportion of T-cell subtypes (Wagner et al., 1983;
Fernandes, 1984; Revskoy et al., 1985; Thompson et al., 1987;
Lipschitz, 1987).
The number of immature lymphocytes of the T-lineage increases
with age, as does the percentage of apparently activated
T-lymphocytes bearing immature thymic phenotypic markers. There is a
relative increase in cytotoxic/suppressor T-cells, and a decrease in
the number of helper/inducer T-cells (Lipschitz, 1987; Thompson et
al., 1987). Correlated with the decrease in the helper/inducer
population, is a functional defect in cell-mediated immunity
(Lipschitz, 1987; Thompson et al., 1987). Cells from aged humans or
experimental animals are less capable of responding to allogeneic
lymphocytes, phytohaemagglutinin, concanavalin A and soluble
antigens. Lymphocytes from older mice are less able to elicit
graft-versus-host reactions than those from younger mice of the same
inbred strains (Thompson et al., 1987). Fifty percent of healthy
people over age 50 have impaired cutaneous hypersensitivity
(Lipschitz, 1987; Dilman, 1987). Accompanying the decrease in
helper/inducer T-cells and cell-mediated immune functions is a rise
in autoantibodies and autoimmunity (Thompson et al., 1987).
Changes in humoral immunity (B-cell function) with aging are
more subtle (Lipschitz, 1987; Senda et al., 1989). Studies on the
effects of age on antibody production have yielded conflicting
results, perhaps because of the wide range of experimental values
generally observed in older individuals. It has, however, been well
established that aging is significantly associated with the presence
of various autoantibodies, in particular, antibodies against nuclear
antigens. There is also evidence that aging effects the rate of
antibody production by activated B-cells (Lipschitz, 1987).
From a functional point of view, defects have been observed at
various levels. Firstly, the proliferative capacity of T-cells from
old individuals is generally reduced, regardless of the stimuli used
(antigens, mitogens), and the defect consists both in a reduced
number of cells responding to stimulus and in a precocious
exhaustion of the cloning capacity (Fabris et al., 1983) of
responding cells. Secondly, the response to interleukins, which
physiologically mediate the modulation of the proliferative
reaction, is depressed and this phenomenon has been documented not
only for T-cells but also for NK cells, which are less sensitive in
old age to the boosting action of IL-2 or interferons (Provinciali &
Fabris, 1990).
With respect to accessory cells (phagocytic cells,
macrophages), their number and function are not altered by age, and,
in certain circumstances, their activity seems to be enhanced.
2.2.5.3 Neuroendocrine-immune interactions
The immune system, although regulated to a large extent by
intrinsic cellular and humoral events, is also sensitive to signals
generated from the nervous and endocrine systems. Communication
between nervous and immune networks is mediated by hormones and
neurotransmitters which reach lymphoid organs and cells via blood or
direct autonomic nervous system connections (Bullock, 1985; Felten
et al., 1985). The neuroendocrine immune interactions are mediated
by circulating humoral factors from the
pineal-hypothalamic-pituitary axis, either directly via
neuropeptides and hormones, or indirectly by the effects of this
axis on the hormonal secretion of peripheral endocrine glands, which
also exert immunomodulating actions (for review, see Fabris, 1991).
The nervous and neuroendocrine systems not only act as
modulators of the immune network, but also as targets for signals
generated within the immune system, such as those exerted by thymic
factors (Hall et al., 1989) and interleukins, (Besedovsky et al.,
1985), and by pituitary-like factors (ACTH, TSH, GH, PRL,
gonadotropins, endorphins), which are produced by mature lymphocytes
upon antigenic stimulation (Weigent et al., 1990).
The sharing of humoral signals, as well as of the specific
receptors between neuroendocrine and immune cells (for reviews, see
Fabris & Provinciali, 1989; Weigent et al., 1990), implies that
biological response modifiers of neuroendocrine-immune origin might
be developed in the near future for therapeutical purposes. On the
other hand, potentially harmful agents for one of these homeostatic
systems may also cause alterations in others.
From an experimental point of view, it has been demonstrated that
treatments of old animals with thyroid hormones (Fabris et al., 1989),
GH (Kelley et al., 1986) and analogues of LH releasing hormone
(Greenstein et al., 1987) are able to induce regrowth of the thymus
and reacquisition of its endocrine activity (for review, see Fabris
1991). Analogous treatments, such as with melatonin (Pierpaoli
et al., 1991), GH (Davila et al., 1987), TSH and thyroid hormones
(Provinciali & Fabris, 1990), are also able to recover various
age-related peripheral immune deficiencies, such as T-cell
functioning and NK cytotoxicity. In humans, little work has been
done in this area, although indirect evidence, obtained primarily
from studies on endocrinopathies in the elderly (Fabris et al.,
1989; Travaglini et al., 1990), suggest that recovery of both thymic
and peripheral immune function can be achieved by a neurohumoral
approach.
Little is known on the potential effect of thymosins,
interleukins and lymphocyte-derived pituitary-like cytokines on
age-related alterations of the nervous and of the neuroendocrine
system. Experimental information from old animals showing recovery
of the hormonal and metabolic profile following immune manipulation
(for review, see Fabris et al., 1988) is undoubtedly opening a new
research approach for human investigations.
Receptor sites for many hormones are present on the membrane of
lymphoid cells (Fabris & Provinciali, 1989). The number of
glucocorticoid receptors in spleen cells decreases in old animals
(Roth, 1979a). Hormones that modify the turnover of cyclic
nucleotides result in consequent activation or inhibition of immune
functions (Hadden, 1983). Hormones influence the production of
several lymphokines and monokines (Kelso & Munck, 1984).
The neuro-endocrine system seems to act not only as a modulator
of the immune network but also as a target for signals generated
within the immune system. Examples of such interactions are the
alterations that can be induced in the neuroendocrine balance,
either by removal of relevant lymphoid organs such as the thymus or
by dysfunction of the immune system itself as a result of reactions
to immunogenic or tolerogenic doses of antigen (Besedovsky et al.,
1975). In addition, mature lymphoid cells, when stimulated by
antigens, produce humoral factors similar, if not identical, to
classical hormones and neurotransmitters (such as ACTH, TSH, GH,
PRL, gamma-endorphins) (Blalock et al., 1985). These reciprocal
influences between the neuroendocrine and the immune systems
(Fabris, 1981; Fabris et al., 1988) occur throughout life, but have
particular relevance during aging (Fabris & Piantanelli, 1982).
2.2.6 Cardiovascular system
The frequency of cardiovascular diseases, which are the major
cause of death in industrialized countries, increases with age.
Diseases such as hypertension and atherosclerosis occur most
commonly in the elderly. In addition, degenerative changes of the
cardiovascular system, involving the myocardial cells as well as
cells of the pacing-conduction system, that arise during the aging
process lead to impaired cardiac function and arrhythmia even in
people without any clinical evidence of hypertension or coronary
artery diseases. Inadequate function of the cardiovascular system
induces effects in peripheral tissues and organs. Changes in
peripheral organs resulting in hyperlipidaemia,
hypercholesterolaemia, and hypo- and hyperglycaemia can also effect
the cardiovascular system in the elderly.
2.2.6.1 Heart
The heart itself can be considered to be made up of two parts:
a) the conduction system responsible for electrically controlling
the heart rhythm; and b) the myocardium performing the contractile
function of the heart and composed of a system of trabeculae.
The biophysical and biochemical mechanisms that govern cardiac
muscle change with age, resulting in characteristic alterations in
muscle function (Lakatta, 1987a,b). Many of the steps in the
excitation-contraction system in cardiac muscle are altered by
aging. In an isometric contraction, the transmembrane action
potential (TAP) excites the cell and the contractions that ensue are
longer in duration. The magnitude of the prolongation of
depolarization of the TAP in senescent muscle is striking (i.e.
about twofold). The action potential amplitude is also greater in
senescent than in adult muscle in both high and low calcium-loading
conditions. These deficits of the senescent muscle may be related in
part to the diminished Ca2+ pumping rate by sarcoplasmic
reticulum. The duration of the elevated myoplasmic Ca2+ level is
prolonged in senescent muscle.
Sagiv et al. (1988) found that left ventricular contractility
increases less on stimulation in elderly subjects than in younger
people. Although the aging process is associated with normal resting
contractile function, diastolic properties are altered, resulting in
reduced and delayed early left ventricular filling and enhanced
atrial contribution to diastolic volume. Exercise cardiac output is
maintained in healthy elderly individuals, but there is a shift from
reliance on an increase in heart rate and a decrease in end systolic
volume to use of the Frank-Starling mechanism to increase stroke
volume. This age difference in the cardiovascular response to
exercise is probably mediated by an age-associated decreased
responsiveness to beta-adrenergic stimulation.
In muscle tissue of the aging heart, some morphological changes
are observed both in animal and human studies (Koobs et al., 1978;
Speijers, 1983). The most common change in the aging heart is
hypertrophy (Lakatta, 1985). Other alterations consist of the
appearance of slight focal necrosis and fibrosis in the myocardium,
amyloidosis (Finch & Hayflick, 1977) and the appearance of
lipofuscin (Hendley et al., 1963; Koobs et al., 1978). Peroxidative
damage to the myocardium is cumulative and irreversible (Koobs
et al., 1978).
2.2.6.2 Blood vessels
Both physiological and morphological changes are observed in
the vascular system, especially in the small and large arteries. The
morphological changes in the arteries seen in the elderly vary
considerably both in appearance and in localization (Goyal, 1982;
Hazzard, 1985). The thickness of the aorta increases significantly
with age, while the number of nuclei in the cells of the arterial
media decreases in humans as well as in mice. The majority of these
changes in humans are categorized as atherosclerotic. These changes
can progress and result in complicated coronary atherosclerosis and
ischemic heart disease, but other factors may also cause clinical
effects such as angina pectoris, arterial spasms, and myocardial
infarcts (Speijers, 1989a).
Morphological changes in the veins are less pronounced than in
the arteries.
The physiological changes observed with aging are often a
result of changes both in heart function and in the arteries. These
changes are reflected in haemodynamic parameters such as an increase
in diastolic and systolic blood pressure, mean arterial blood
pressure and vascular resistance, and a decrease in responsiveness,
and in contraction and relaxation responses (Lakatta, 1986, 1987b;
Duckles, 1987; Mazzeo & Horvath, 1987; Zemel & Sowers, 1988;
O'Malley et al., 1988; Cleroux et al., 1988).
Aging is often accompanied by increases in the incidence and
prevalence of hypertension. Geriatric hypertension is generally of a
salt-sensitive nature with a disproportionate frequency of isolated
systolic hypertension. The age-related increase in salt sensitivity
is due to a decline in renal function (Zemel & Sowers, 1988) and
deregulation of vascular tonus. Age-associated declines in the
activity of membrane sodium/potassium-ATPase may also contribute to
geriatric hypertension because this results in increased
intracellular sodium loading, causing reduced sodium/calcium
exchange and thus increased intracellular calcium and vascular
resistance.
It is commonly accepted that atherosclerotic changes take place
to a certain extent in every individual. Multiple factors determine
the extent and velocity of the atherosclerotic process (Hazzard,
1985). The incidence of clinically observed atherosclerotic effects
is higher in elderly subjects than in younger individuals.
Atherosclerotic damages result in impaired cardiovascular function.
Atherosclerosis is defined as a multifactorial disease with
variable effects in the intima followed by changes in the media of
arteries. These changes consist of focal accumulation of lipids,
proliferation of smooth muscle cells, and accumulation of complex
carbohydrates (i.e. glycosaminoglycans, proteoglycans), blood and
blood products, collagen and calcium compounds (Campbell &
Chamley-Campbell, 1981; Velican, 1981; Speijers, 1989a). The
resultant modifications of arterial wall integrity can lead to the
following: erosion of the wall with consequent reduced resistance to
blood pressure, rupture, and haemorrhage; progressive thickening of
the wall due to reactive proliferation of tissues with consequent
reduction of blood flow; and clotting of the blood at the level of
the injured wall with consequent sudden obstruction. The basic
lesion seems to develop in the first decade of life (Lee, 1985).
In the etiology of the lesion two localized cofactors should be
taken into account: the blood supply of the arterial wall; and blood
turbulence, since lesions are more frequently found around the
orifices of arteries branching off major arteries or at bifurcations
(Patel & Vaishnaw, 1980). Hypertension, diabetes, autoimmunity and
stress are also risk factors contributing to atherosclerosis.
2.2.6.3 Characteristics of atherosclerotic lesions
The first event in the formation of the lesion is still
debated. Both an initial thickening of intima, due to an
accumulation of blood-born amorphous material (lipids, protein and
sulfated proteoglycans) and a proliferation of muscle cells (due to
still undefined stimuli), with consequent degeneration and reactive
macrophage and connective tissue accumulation, have been proposed as
major initiating phenomena (Benditt, 1977; Ross, 1981). The
subsequent phase of the lesion involves repair mechanisms that cause
further thickening of the intima. Following this, lipid increases
both in the cells and in the intercellular spaces. Lipid
accumulation is progressive, leading to an increased number of foam
(lipid-containing) cells which disintegrate and form the gruel-like
substance that has given the name of atheroma to the lesion. The
accumulation of such material acts as an irritant, inducing a
proliferative reaction (encapsulation) which leads to the
development of a plaque. Calcification follows, making the arterial
wall more rigid. Alternatively, when the capsule breaks, an ulcer
can occur, leading to loss of tissue in the arterial wall, blood
clots, haemorrhage, and consequent thrombosis and/or rupture of the
arterial wall.
2.2.6.4 Theories of atherosclerosis
Atherosclerosis is undoubtedly a multifactorial process, which
is reflected by the many theories proposed (Baker & Rogul, 1987).
The lipid accumulation theory is based on the progressive
accumulation of oxidized lipids (mainly low density lipoproteins)
not only in smooth muscle cells but also in migrating monocytes
(Avogaro et al., 1983). The link between lipid deposition and
consequent alterations remains unclear (McCaffrey et al., 1988).
Theories on monoclonal proliferation of smooth muscles cells
are based on a mutagenic event in these cells, due to a
physico-chemical or viral insult (Benditt, 1977; McCaffrey et al.,
1988), leading to the formation of a kind of benign tumour. The
thrombogenic theory is based on the early adherence of platelets to
small alterations in the endothelium, leading to thrombus formation
and release of growth factors by platelets which induce smooth
muscle cell proliferation (Ross, 1981; Bang et al., 1982). Other
risk factors that should be taken into consideration are
hypertension, cigarette smoking, increased body weight, high serum
uric acids and consumption of saturated fats.
The immune system in the elderly is weakened. Consequently
there is increased susceptibility to chronic infections,
autoimmunity, and elevation of circulating immune complexes. New
data in the literature indicate that some viral agents may cause
cardiac and arterial cell lesions and subsequent inflammation. Thus,
viruses and altered immune cells may cooperate and play a role in
arterial wall lipid accumulation, possibly acting as initiating
factors for atherosclerosis (Butenko, 1985).
2.2.7 Respiratory function
Respiratory function is based on gas exchange (oxygen
absorption and carbon dioxide elimination), gas transport (red blood
cells), and on internal metabolic processes that utilize oxygen at
the cellular level. Aging may affect all of these processes (Masoro,
1981), but it is the first that is usually most compromised in
advanced age. The decline in the gas-exchange system may involve the
lungs, the thoracic cage, the respiratory muscles and the
respiratory centres in the CNS. The deterioration of the lungs is
largely dependent on environmental factors, in particular on the
contamination of air with toxic substances, dust and microbiological
agents. Therefore, age-related lung alterations may vary according
to life styles (smoking, physical exercise), environmental
conditions (urban/rural), and intercurrent diseases (infections,
work-related diseases) (Davies, 1985).
2.2.7.1 Gas-exchange organs
The most evident lung alterations with advancing age are
represented by enlargement of alveolar ducts with flattening of
alveoli and loss of septal tissue, reduction of elastic fibres, and
increased fibrosis of the capillary system (Liebow, 1964).
Functional consequences are a reduction in the surface area for gas
exchange with an increase in the physiological dead space, reduction
of the ventilatory flow rate, and irregular distribution of blood
flow (Mauderly, 1978). Age-related alterations also occur in the
chest as a consequence of calcification of costal cartilage,
increased stiffness of costovertebral and vertebral joints, and
general rigidity of the chest. Both lung and chest alterations
contribute to the changes in lung volume and pressure: vital
capacity decreases, residual volume increases, and flow rates
(particularly expiratory flow rate) decline (Morris et al., 1971).
The alteration in the gas-exchange capacity causes reductions in
oxygen uptake and pressure and lower arterial pO2, whereas pCO2
remains constant even in very old age (Morris et al., 1971). The
control of ventilation by brain centres is also altered in old age.
It is still unknown whether such alterations are due to intrinsic
damage of the neural component or to a reduced responsiveness of
neuromuscular activity in the chest (Peterson et al., 1981).
All these alterations, while not necessarily life-threatening
for the elderly, may favour pathologies such as chronic bronchitis,
pneumonia and emphysema (Peterson et al., 1981). The concomitant
hypoxia (low oxygen levels) may cause increased production of red
blood cells with consequent polycythaemia. This may contribute to
hypertension and cardiac failure.
2.2.7.2 Erythropoietic activity
Although specific age-related alterations in the life cycle of
erythrocytes have been reported (Danon, 1969), the overall function
of the erythropoietic system seems to be well preserved. The
regenerative potential following hypoxia seems nearly inexhaustible.
In addition, haemoglobin turnover does not seem to be affected by
age (Lipschitz, 1987).
2.2.8. Kidney and body fluid distribution
The urinary tract is affected by aging both in its renal
functions of excretion and ionic control of body fluids, and in its
control of bladder and urethral activity.
2.2.8.1 Renal function
Kidney function decreases both due to anatomical and
physiological alterations with age (Wesson, 1969; Kaysen & Myers,
1985; Brown et al., 1986; Corman & Michel, 1986; Owen & Heywood,
1986; Meyer & Bellucci, 1986; Anderson & Brenner, 1986, 1987;
Goldstein et al., 1988; Euans, 1988; Rudman, 1988). These
alterations have been observed in both experimental animals and in
humans.
The weight and volume of the kidney decrease by 20 to 30%
between the ages of 30 and 90 years. The atrophy is primarily
cortical and seems to be related to intrarenal vascular changes. The
number of surviving nephrons is reduced and these remaining nephrons
tend to be enlarged (Kaysen & Myers, 1985; Brown et al., 1986;
Lindeman, 1986; Rudman, 1988). The number of glomeruli decreases by
30 to 50%, and there is an increasing percentage of sclerotic and/or
abnormal glomeruli. The glomerular filtration rate (GFR) decreases
with age resulting in an adaptive increase in glomerular perfusion
pressure (Kaysen & Myers, 1985; Lindeman, 1986; Meyer & Bellucci,
1986; Anderson & Brenner, 1986, 1987; Blum et al., 1989). This
decline in GFR is due in large part to the progressive reduction in
blood flow to the kidneys (Brown et al. 1986; Lindeman, 1986).
Glomerular mesangial volume increases by 50% and 1 out of 10
glomeruli is sclerotic at the age of 80 years compared with 1 out of
100 in the young adult (Brown et al., 1986). The renal tubules
decrease in number, proximal tubule volume and length decrease, and
distal tubules develop increased diverticula. The renal arterioles
develop intimal thickening, reduplication of the lamina elastica
interna, and mild hyalinization (Brown et al., 1986; Lindeman, 1986;
Rudman, 1988).
An age-related reduction in secretory and resorptive capacity
is seen in the tubules, which is explained by a progressive loss of
functioning nephrons (Lindeman, 1986). Tubular function, which
regulates water and salt balance, is also affected. A decrease in
the ability to concentrate urine with age has been well documented
in humans. This appears to result from a decreased medullary
tonicity caused mainly by an inability to respond normally to
antidiuretic hormones (ADH) (Kaysen & Myers, 1985; Brown et al.,
1986; Meyer & Bellucci, 1986; Lindeman, 1986; Euans, 1988). Despite
age-related decreased renal function, the blood pH, partial pressure
of carbon dioxide, and serum hydrogen carbonate concentration of the
geriatric population without renal disease do not differ
significantly from those of the young under basal conditions
(Lindeman, 1986). Both the ability to maximally dilute the urine and
to maximally concentrate it, are controlled by serum ADH and by the
action of that hormone on the collecting ducts (Kaysen & Myers,
1985; Os et al., 1987). Increased arginine-vasopressin (AVP)
secretion per unit of plasma reflects a decrease in collecting
tubule sensitivity to AVP. This change in sensitivity is not
completely offset by increased ADH release (Davis & Davis, 1987).
The suppression of ADH secretion is not maximal when serum
osmolality is reduced.
The renin-angiotensin-aldosterone system is also poorly
responsive to volume depletion in aging subjects. As a result, the
elderly cannot maximally retain sodium under conditions of plasma
volume contraction (Kaysen & Myers, 1985; Lindeman, 1986; Euans,
1988). The activity of the renin-angiotensin system is progressively
reduced with age. It has been suggested that angiotensin II does not
play an important role in the maintenance of blood pressure and
kidney hemodynamics in normal senescence (Corman & Michel, 1986).
The mean blood pressure is correlated with age and the decline in
renal function (Lindeman et al., 1987).
The kidney is also the site of vitamin D1 hydroxylation, which
is dramatically reduced during aging (Kaysen & Myers, 1985).
2.2.8.2 Lower urinary tract
The most frequent age-related alterations in the lower urinary
tract result in urine incontinence in both sexes and urine retention
in males. Incontinence occurs at high incidence although it is not
an inevitable consequence of aging. The high number of physiological
requirements for urinary continence may account for such frequent
failure (Williams & Pannill, 1982). In women, the reduction of
estrogen levels after menopause may decrease the tone of the smooth
muscle around the pelvic floor and the bladder outlet, thus
favouring urinary incontinence. In men, hypertrophy of the prostate
represents the major cause for involuntary loss of urine, because of
the associated frequent instability of the detrusor muscle. Other
causes for urinary incontinence are represented by delirium,
infections, restricted mobility and polyuria.
2.2.9 Gastrointestinal function
Changes in the gastrointestinal tract during aging consist
mainly of a reduced cell turnover, leading to mucosal hypoplasia.
Accessory organs, such as the exocrine pancreas and the liver, are
affected by aging independently.
2.2.9.1 Gastrointestinal tract
In contrast to the cardiovascular or excretory system, the
gastrointestinal (GI) tract does not exhibit marked structural or
functional changes with age (Penzes, 1984). Aging may affect all
regions of the GI tube. The first signs of aging are generally
observed in the mouth. Teeth undergo discoloration, pulp recedes
from the crown, dentine is often poorly renewed, and the gingiva
frequently recede (Walker, 1985). These alterations favour bacterial
growth which can lead to chronic periodontal inflammation and tooth
loss.
In the stomach, reduced secretion of hydrochloric acid and
pepsin is often found in the elderly. These changes result from
alterations in enzyme-secreting cells or from altered hormonal and
neural regulation. However, in some cases, increased acid secretion
may occur with advancing age, leading to gastritis, erosions and,
ultimately, ulcers (Kumpuris, 1983).
No major morphological alterations are observed in the
intestine. The relatively mild modification of villi, the increased
collagen content and the reduced mucosal cell proliferation
(Webster, 1985) cannot account for the impaired absorption of
nutrients, including minerals (calcium, iron and zinc) and vitamins
often found in the elderly. Other factors such as reduced motility
and inadequate intestinal blood supply may play a more important
role than the slightly altered anatomical integrity.
In the lower GI tract the rectal tone is generally decreased in
the elderly and the sphincter is weakened, leading to incontinence.
Neurological alterations (dementia), muscle atrophy, diarrhoea and
constipation can all contribute to anal incontinence.
2.2.9.2 Pancreas
Pancreatic function is not seriously compromised during normal
aging. Structural changes mainly involve a reduction in the number
of secreting cells, with a consequent decrease in size of the
pancreas and a moderate increase in collagen content. Reduction in
the levels of trypsin, hydrogen carbonate, amylase and lipase in the
pancreatic juice occurs commonly in the elderly (Vellas et al.,
1988). Overall, however, the functional efficiency of the pancreas
is not lost during aging.
2.2.9.3 Liver
Structural changes in the liver with age are relatively minor.
However, the most significant change in human liver is a decrease in
volume (Wynne et al., 1989). The life-span of the hepatocyte is
long, with cells only dividing once or twice during the lifetime in
the absence of a growth stimulus (Popper, 1986). Whether the
function of the aging hepatocyte is impaired is unclear. Kupffer
cell function may be impaired as demonstrated by a reduction in
phagocytic activity. Endocytosis is reduced in Kupffer, but not
endothelial, cells. An increase in collagen also characterizes aging
liver.
The function of aging liver also undergoes a number of
alterations. In humans, cholesterol synthesis is reduced in the
elderly, while biliary secretion is increased (Popper, 1986).
Hepatic blood flow is reduced by as much as 50% (Sherlock et al.,
1955). Age-related changes in liver enzyme expression have been
studied in more detail in rodents (Fishbein, 1991). The efficiency
of carbohydrate and intermediary metabolism is decreased in
senescent rats or mice of both sexes, due in part to decreased
insulin-binding and the impaired regulation of enzymes such as
pyruvate kinase. Hepatic protein synthesis also decreases with age,
and the sex-specific expression of drug and steroid-metabolizing
enzymes in male rats appears to be feminized in senescence (Kitani,
1991).
2.2.10 Musculo-skeletal system
Aging dramatically affects bone, joints and skeletal muscles.
The basic phenomena responsible for the aging of the
musculo-skeletal system are not completely understood because of the
involvement of many factors, e.g., hormones, nutrition and physical
exercise, in addition to specific age-related alterations at the
tissue level, resulting in general deterioration of the system.
2.2.10.1 Bones
Bone should not be considered as metabolically quiescent since
it is constantly remodelled according to mechanical demand and
continuous turnover due to new bone formation (by osteoblasts) and
bone resorption (by osteoclasts) (Exton-Smith, 1985).
With aging, the balance between bone formation and bone
resorption is altered in favour of resorption, resulting in a
reduction of bone mass. This starts from the medullary region with
enlargement of the cavity and moves towards the epiphysis of the
bone and then to the outer surface with a final reduction of cortex
thickness. As a consequence, the bone strength is reduced (Smith
et al., 1981). The prolonged period where bone resorption exceeds
bone formation may result in osteoporosis (Dawson-Hughes et al.,
1987; Eastell & Riggs, 1987; Riggs, 1987; Croucher et al., 1989).
The factors responsible for bone resorption and remodelling are
not well known. More knowledge is available concerning two other
factors which profoundly affect bone metabolism: calcium turnover
and hormonal profile. Calcium is absorbed through the intestinal
mucosa and is excreted from the kidney, the blood level remaining
remarkably constant throughout life. Calcium is required for many
essential functions in the body, such as cell division, cell
intermediary metabolism, and cell functions (excitability, secretion
and movement). In the case of a negative balance, calcium is
mobilized from the bone with a consequent reduction in bone mass.
Calcium metabolism is under hormonal control. Parathyroid
hormone increases the plasma calcium level by promoting bone
resorption and mobilization, whereas calcitonin lowers blood calcium
by inhibiting bone resorption. Calcitriol (the active derivative of
vitamin D) increases intestinal absorption and decreases excretion
of calcium, thus acting to increase the body burden of calcium. The
age-associated decrease in intestinal calcium absorption lowers the
plasma calcium level, leading to an increase in parathyroid hormone.
Calcitriol levels may also be deficient in the elderly, as a result,
perhaps in part, of the decrease in vitamin D synthesis in old skin
and alterations in renal metabolism.
Bone metabolism also depends on other hormonal factors.
Estrogens have a strong positive effect on bone density. After
menopause, bone resorption exceeds bone formation resulting in bone
mass loss. However, the occurrence of osteoporosis greatly depends
on the adult bone peak mass, physical activity, and calcium intake
(Eastell & Riggs, 1987; Riggs, 1987; Bornor et al., 1988; Stevenson
et al., 1988; Lindsay, 1989).
Glucocorticoids lower plasma calcium levels, which can increase
bone resorption by stimulating parathormone secretion. Thyroid
hormones may also cause bone loss, although the mechanism is still
unclear. Growth hormone, somatomedins and insulin all promote bone
formation, and therefore defective secretion (as in diabetes) may
cause osteoporosis.
2.2.10.2 Joints
Articular disorders are practically universal in elderly
people, and are associated with pain and disability. The major
alteration consists of the loss of the smooth surface of cartilage,
which becomes thicker and less elastic. The cartilage develops rough
surfaces and mechanical irregularities, which represent the early
steps in osteoarthritis (Evens & Hawkins, 1984).
2.2.10.3 Skeletal muscles
With advancing age muscles become smaller in size, due to
reduction in the number of fibres, and less elastic, due to an
increase in collagen content. The fat content of muscle tissue also
increases with age. The causes for such defects are still
controversial: alteration of sarcoplasmic reticulum, decrease of
contractile protein synthesis and a reduction in the number of
mitochondria are all found in aged muscle cells.
In addition to muscle cell alterations, the neuromuscular
junction is modified in aged muscle. The acetylcholine content of
nerve terminals is consistently reduced and acetylcholine receptors
are irregularly distributed. Motor nerve conduction velocity is also
impaired. All these defects contribute to the progressive failure of
muscular efficiency, although some muscles, such as the diaphragm,
do not seem to suffer age-related effects (Finch & Hayflick, 1977).
Thus, the additive results of the loss of bone mass, disorder of
joints, decline in skeletal muscle power and nervous system
discoordination may lead to loss of body stability, falls and bone
fracture in old people.
2.2.11 Skin
The age-related modification of the skin is the most dramatic,
common and constant event in life, so that it may constitute one of
the best external markers of aging. Only recently has it been
possible to distinguish between intrinsic aging of the skin and
photo-aging (Gilchrest, 1984). Cutaneous neoplasia is one of the
best documented interactions between aging of the organism and
environmental effects. A variety of environmental factors, the most
notable being ultraviolet radiation, has been shown to cause cancer
of the skin in humans and animal models (Rogers & Gilchrest, 1990).
The structure of skin changes throughout life (Behl et al.,
1987), although many of the alterations are due to exposure to
sunlight rather than intrinsic aging (Gilchrest, 1984). Skin
thickness increases during maturation and then gradually declines
(Vogel, 1983). However, recent studies in mice and rats by
Monteiro-Riviere et al. (1991) have not reported any significant
changes in skin thickness or blood flow from maturity throughout the
life span. Hydration, which can affect chemical solubility,
decreases while keratinization increases.
Skin aging is linked to alterations affecting both the
epidermal and the dermal components. The epidermis suffers from a
reduction in the turnover of epidermal cells, due both to a
reduction of basal cell renewal and to a slower maturation toward
the stratum corneum. The dermo-epidermal interface is flattened so
that the total external body surface area is reduced (Selmanovitz
et al., 1977). The reduction in epidermal cell turnover is
responsible for the slowing of wound healing and possibly for the
dryness and/or roughness of aged skin. The dermis is reduced in
thickness in the elderly and is characterized by a reduced collagen
content with a biochemical modification of the collagen itself,
which makes it more strong and less elastic. Vascularization in
humans is also reduced.
Aging also results in modifications in the skin appendages.
Sweat gland function is decreased resulting in impaired
thermoregulation. Sebaceous glands produce less oil and wax (dryness
of skin), and hair usually loses pigment. The numbers of skin
sensory organs (Pacinian and Meissner's corpuscles) decrease with
age and sensation is consequently modified.
Collagen aging occurs in the skin, as in other tissues, leading
to increased rigidity. Collagen is composed of several related
proteins, which are produced by fibroblasts and extruded into the
extracellular space. Here they can be chemically deposited in a
nearly pure form, as in the tendons, or immersed in an extracellular
matrix, also produced by fibroblasts, as in the skin. The collagen
fibres undergo maturation in the extracellular ground substance by
parallel arrangements of tropocollagen fibres which became assembled
together through cross-linking. This phenomenon is an index of
maturational change. With age, however, the cross-linking increases
(Houck et al., 1967), leading to a reduction of tensile strength and
plasticity (Verzar, 1968). The extracellular matrix also shows
age-related changes, since alterations in its physicochemical
composition lead to increased density, reduced permeability and
impaired transport of nutrients (Imayama & Braverman, 1989).
3. BASIS OF ALTERED SENSITIVITY TO ENVIRONMENTAL CHEMICALS
The perception of altered sensitivity of the elderly to
environmental insults is based in large part on the enhanced
incidence of adverse or idiosyncratic drug reactions in this segment
of the population (Vestal et al., 1985). The clinical pharmacology
of the aged has been extensively reviewed in the last two decades
(Triggs & Nation, 1975; Crooks et al., 1976; Reidenberg, 1980;
Vestal et al., 1985), and evidence indicates that the response to
drugs changes with age, as does the frequency of adverse drug
reactions (Krupka & Vener, 1979). This may be in part related to
issues of polypharmacy and compliance (Weber & Griffin, 1986).
Morbidity and malnutrition, which are often associated with aging,
could also contribute to altered pharmacokinetics in the elderly
(Kitani, 1988). However, it is clear that age-related differences in
drug/chemical disposition (pharmacokinetics/toxicokinetics) or
sensitivity (pharmacodynamics/toxicodynamics) also play a role in
altered responses to chemicals in the elderly. For sake of
simplicity, the terms "pharmacokinetics" and "pharmacodynamics" will
be used for both drugs and environmental chemicals.
3.1 Pharmacokinetics
Pharmacokinetics describes the processes of the absorption,
distribution, metabolism and excretion of drugs or other chemicals
in the body. The numerous physiological and biochemical changes that
occur during aging can modulate any of these processes of
disposition. Changes in chemical disposition (pharmacokinetics being
the mathematical description of these processes) can lead to an
altered dose or dose-rate to the target tissue resulting in changes
in response. This topic has been the subject of several recent
reviews (Sellers et al., 1983; Van Bezooijen, 1984; Stevenson &
Hosie, 1985; Blumberg, 1985; Birnbaum, 1987, 1989, 1991; Ritschel,
1988; Loi & Vestal, 1988; McMahon & Birnbaum, 1990a).
The highlights in this field will be covered as well as the
more recent data, especially that focusing on age-related
alterations in the pharmacokinetic behaviour of xenobiotics, as
opposed to drugs.
3.1.1 Absorption
The absorption of drugs and environmental chemicals, defined as
uptake into the blood, occurs primarily via the skin, lungs and
gastrointestinal (GI) tract. Alterations in the structure and
function of these three organs clearly occur with age. In contrast
to the skin and GI tract, little is known about the effects of aging
on pulmonary absorption of xenobiotics. Few age-related differences
have been reported in lung structure (Stiles & Tyler, 1988),
although the lung volume is greater in old rats. An age-related
decrease in respiratory function has been reported in several
species (Mauderly, 1979a,b, 1982). In addition, there appears to be
a decrease in the rate of alveolar-capillary gas exchange in older
organisms (Mauderly, 1979b).
Dermal exposure represents a major portal of entry for
environmental chemicals. Several studies have indicated a decline in
human skin permeability with aging (Christophers & Kligman, 1965).
In experimental animals, the scarce data suggest that percutaneous
absorption is decreased in senescent rodents as compared to young
adults. The chemical absorption of
2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD) and related chemicals
is greatest at weaning (Jackson et al, 1990), decreases at maturity
and then undergoes a further decline during aging (Banks et al.,
1990).
Age-related alterations in GI absorption have been studied in
more depth. A decrease in gastric acid secretion is commonly seen in
the elderly (Bender, 1968). The resulting increase in pH can alter
the ionization of compounds, enhancing or retarding their ability to
diffuse passively across cellular membranes. Decreases in gastric
motility (Lin & Hayton, 1983) can prolong the transit time of
chemicals in the gut, thus enhancing their potential for absorption.
In rats, splanchnic blood flow declines in the first year of life
but stays unchanged in the second year (Yates & Hiley, 1979; Kitani,
1988). In contrast, splanchnic blood flow declines progressively
with age in humans (Sherlock et al., 1955). An increase with age in
mucosal weight (Holt et al., 1984; Hebert & Birnbaum, 1987), as well
as intestinal epithelial cell proliferation (Holt et al., 1988), has
been reported in rats.
GI absorption can be active, passive or involve phagocytosis.
Xenobiotics are primarily absorbed by passive diffusion. Age-related
changes in the oral absorption of a variety of drugs in people have
not been observed (Castleden et al., 1977b; Stevenson et al., 1979;
Greenblatt et al., 1988). The passive absorption of TCDD in the
small intestine does not change with age in rodents (Hebert &
Birnbaum, 1987), nor does that of many small endogenous molecules,
such as glucose (Eastin & Birnbaum, 1987), vitamin A (Hollander
et al., 1986), vitamin D, vitamin B12 and niacin (Fleming &
Barrows, 1982a,b) and many amino acids (Penzes, 1974).
Active transport appears to decrease with age (Eastin &
Birnbaum, 1987). Doubek & Armbrecht (1987) have demonstrated that
the decrease in the carrier-mediated component of glucose transport
in rats occurs in the brush-border membrane of the small intestine.
Their suggestion that the decrease is due to a reduction in the
number of sodium-linked glucose carriers has been supported by
recent studies in human intestinal tissue (Vincenzini et al., 1989).
The active transport of other small molecules such as calcium and
phosphorus (Armbrecht, 1986), and galactose and iron (Reidenberg,
1980) has also been reported to decrease with age. As with the
active transport of glucose, the marked decrease in calcium active
transport of calcium occurs between young adulthood and middle age
(Mooradian & Song, 1989) and results from changes in the number of
calcium transporters in the intestinal basal lateral membranes
(Armbrecht et al., 1988).
3.1.2 Distribution
The distribution of chemicals throughout the body is governed
by the fact that the physicochemical properties of the compound
affect its transport within the body and localization to various
tissues. Lipophilic molecules readily pass across cellular membranes
and accumulate in lipid-rich tissues. Binding to proteins also
modulates distribution, since few compounds are transported free in
the blood. While there is no evidence of alterations in relative
blood volume with age, changes in body composition, blood flow and
macromolecular binding have all been documented.
The decrease in lean body mass in both animals (Lesser et al.,
1973) and humans (Novak, 1972) has been well documented. Loss of
body water with age (Edelman & Leibman, 1959) results in a decrease
in the volume of distribution for water-soluble compounds, leading
to enhanced toxicity of ethanol (York, 1982) and ethylenediamine
(Yang et al., 1984). Body fat can account for approximately 15-40%
of the total body weight in humans (Ritschel, 1983) and rats
(Bertrand et al., 1980; Birnbaum, 1983). The increased size of the
fat compartment in older, sedentary animals would be expected to
increase the body burden of lipid-soluble substances and reduce the
overall rate of elimination from older animals. Following an
inhalation exposure of old rats to methylchloroform (Schumann
et al., 1982a,b), a better fit to a physiologically based
pharmacokinetic (PbPk) model was obtained by increasing the volume
of the fat compartment from 7 to 18% of the body weight for rats and
from 4 to 18% for mice (Reitz et al., 1988). Similar improvements
were noted by Lutz et al. (1977) in their PbPk model of
polybrominated biphenyl compounds in rats.
Not only does cardiac output decrease with age (Bender, 1965),
but regional blood flow can change differentially (Yates & Hiley,
1979). Since adipose tissue volume increases but blood flow
decreases with age, lipophilic compounds tend to show greater
retention in the elderly. This has been shown for polychlorinated
biphenyls (Birnbaum, 1983) and halogenated solvents (Schumann
et al., 1982a,b). A clinical pharmacokinetic study (Klotz et al.,
1975) which demonstrated a 5-fold increase in the distribution
volume of diazepam in the elderly is in agreement with the data on
experimental animals.
Binding to blood components can also change with age. Although
the total plasma protein content does not change dramatically with
age, there is a small but significant reduction in albumin in both
animals (Rodgers & Gass, 1983) and humans (Bender et al., 1975). For
drugs or xenobiotics that can be bound, such a decrease in albumin
enables a higher concentration of free drug to reach the target
site. A decrease in binding of drugs to red blood cells has also
been reported to occur during aging (Chan et al., 1975), again
leading to a higher level of free drug.
3.1.3 Metabolism
As with absorption and distribution, there are physiological
changes that occur during aging which can influence
biotransformation reactions, both in the liver and in extrahepatic
tissues. Although the liver is the main site for the metabolism of
drugs and environmental chemicals, significant metabolic reactions
occur in all the portals of entry tissues (skin, respiratory tract,
GI tract) as well as the kidneys, gonads, adrenals, etc. Metabolism
is often considered to be divided into two types of reactions: phase
I (functionalization reactions) and phase II (conjugation
reactions). Phase I reactions tend to increase the polarity of
chemicals, and the corresponding enzymes include the
cytochrome-P450- and FAD-containing monooxygenases as well as the
alcohol and aldehyde dehydrogenases, monoamine oxidases, nitro- and
azo-reductases, esterases and amidases. Phase II reactions involve
the conjugation of functional group (either already present on the
chemical or as a result of phase I activity) with an endogenous
cofactor such as an amino acid (glycine, glutamate), peptide
(glutathione), sugar (glucuronic acid) or a small molecule such as a
sulfate, acetate or methyl group. These reactions may occur on
cellular membranes or in the cytosol, and are regulated in a
tissue-specific manner.
The only generalization that can be made concerning age-related
changes in biotransformation is that few patterns exist. Metabolic
changes with aging appear to be substrate, sex, strain and species
dependent (Schmucker & Wang, 1989; Kitani, 1988; Birnbaum, 1989;
McMahon & Birnbaum, 1990b). In addition to intrinsic changes related
to altered physiology, the study of the influence of age on
metabolism is further complicated by the effects of diet, alcohol,
drugs and pollutants, which can induce or inhibit enzyme activities
(Ritschel, 1988).
Most of the research on the effects of aging on metabolism has
focused on the microsomal mixed-function oxidases. The protein
components of this system have been reported to decline or remain
unchanged with age. In general, the decline in total cytochrome P450
levels reported in rats appears to be due to the age-related
decrease in the amount of the male-specific forms (Kamataki et al.,
1985a,b; Sun et al., 1986). This results from the age-related
decrease in circulating testosterone levels (Kitani, 1985) or to
alterations in the secretory profiles of growth hormone (Fishbein,
1991). This pattern is most pronounced in rats, few changes being
observed in other rodents or sub-human primates (Birnbaum, 1987).
However, recent human studies have suggested that some degree of
sexual dimorphism does exist in hepatic drug metabolism. Plasma
antipyrine half-lives were prolonged in elderly males, but not
elderly females, as compared to young adults (Greenblatt et al.,
1988). This was caused by an age-related reduction in clearance,
which has been interpreted as being the result of an impairment of
antipyrine metabolism in elderly men. Similar gender-specific
results were observed for the kinetics of chlordiazepoxide, another
low-clearance oxidatively metabolized drug (Loi & Vestal, 1988).
A study by Chengelis (1988a) has supported the earlier report
of Rikans (1984) that measuring components of the monooxygenase
system cannot lead to predictions of toxicity. Sex differences in
rats were only significant up to one year of age. However, Rikans
(1989a) has recently reported that metabolism of specific substrates
(aniline, benzphetamine and nitroanisole) does decline with age in
the female as well as in the male rat, but the magnitude of change
in the female is smaller. This agrees with a study by Bitar &
Shapiro (1987), who suggested that an age-related increase in haem
degradation plays a role in the decreased metabolism of hexobarbital
and aniline. This observation, that microsomal drug metabolism
activities do not have to be sexually dimorphic to be altered in old
age, implies that factors other than loss of the male-specific
cytochromes P450 contribute to the age-associated alterations in
some rat strains. Decreases in NADPH cytochrome P450 reductase in
rat liver with age have been reported by Blanco et al. (1987),
Chengelis (1988a) and Rikans (1989a).
Despite a large body of data obtained from experimental animal
studies, there is no clear evidence that monooxygenase activities
decline in the livers of healthy elderly humans. All the data
derived so far from human liver biopsy specimens have shown no
correlation between enzyme activities expressed per mg protein and
age of subjects (Boobis & Davies, 1984; Schmucker et al., 1990). The
often reported decreases in clearance values of drugs metabolized
primarily by the liver in the elderly may in large part be accounted
for by the decrease in liver volume with age (Kitani, 1988). Further
evidence is needed to determine whether hepatic drug-metabolizing
enzyme activities in humans decline with age, as is observed in some
rodents, but the extent is unlikely to be as drastic as that
observed in male rat liver.
A change in the composition of cytochrome P450 enzymes,
suggested by differential changes in enzyme activity, has been
demonstrated in male rats (Kamataki et al., 1985a; Sun & Strobel,
1986). Leakey et al. (1989a) showed that dietary restriction could
also alter the profile of cytochrome P450 isoforms, possibly by
delaying the age-related demasculinization of the liver. Friedman
et al. (1989) showed that aging affected the composition of
testosterone-binding cytochromes P450 in the liver of male rats.
Changes in P450 isoforms in old rats were also reported by
Paramonova & Dovgi (1987). Studies with humans have demonstrated
that the effect of age on metabolism even varies for different
metabolites of the same parent compound (Posner et al., 1987). This
supports the observation of changes in cytochrome P450 isoform
composition with age, as has been reported for male rat liver.
Hepatic xenobiotic metabolism can be modulated by many factors.
In humans, smoking often confounds studies of age-related changes in
pharmacokinetics. However, the age-related decrease in the
metabolism of antipyrine (Loft et al., 1988), theophylline and
cortisol (Crowley et al., 1988) may be independent of smoking
status. In fact, the inductive properties of smoking on hepatic
metabolism do not appear to diminish with age. Phenytoin also
increased theophylline metabolism to an equal extent in both young
and old healthy men (Crowley et al., 1988). In contrast, Rath &
Kanungo (1989) demonstrated that the rate of transcription of the
phenobarbital-specific isozymes was nearly two-fold higher in young
rat liver than in old. However, these differential effects may
reflect the specific isoforms involved, since smoking and
phenobarbital preferentially induce different forms. The recent
studies of Rikans (1989b), which demonstrate that induction of
hepatic microsomal drug metabolism by ethanol or acetone is
unaffected by the aging process, lend support to this concept.
Age-related changes in phase I hepatic drug-metabolizing
enzymes other than the mixed-function oxidases have been subjected
to much less examination. Rikans & Moore (1987) demonstrated an
age-related increase in liver alcohol dehydrogenase in male rats.
However, no age differences have been observed in the activity of
this enzyme in female rats. Hydrolytic reactions may decrease with
age. For example, liver esterase activity, using diethylhexyl
phthalate as a substrate, declines in old rats (Gollamudi et al.,
1983). Using aspirin as a substrate, no correlation was found
between age and esterase activity in human liver (Yelland et al.,
1991). These results provide further evidence that age is not a
major determinant of hepatic drug metabolism in the elderly
(Schmucker et al., 1990).
Alterations with age in extrahepatic phase I metabolism also
appear to be substrate, sex, strain and species specific. Pulmonary
metabolism of benzo[a]pyrene was found to increase in old rats (Sun
& Strobel, 1986) in agreement with earlier studies of Rabovsky
et al. (1984). In contrast, oxidation of 2-aminofluorene, which is
catalysed by a different isoform of cytochrome P450, decreased
(Robertson & Birnbaum, 1982). Renal metabolism of acetaminophen has
been reported to decrease with age (Beierschmitt & Weiner, 1986),
while salicylate oxidation by kidney extracts did not change in rats
(Kyle & Kocsis, 1985). Age-related alterations in intestinal Phase I
metabolism appear to be site specific. Sun & Strobel (1986) reported
that oxidation of benzo [a]pyrene in the colon increased throughout
the life of rats, while McMahon et al. (1987) observed no
age-related change in the metabolism of this substrate in the small
intestine. Newaz et al. (1983) observed higher metabolic rates of
dimethylhydrazine in colonic tissue from aging humans as compared to
younger individuals. McMahon et al. (1989) suggested that plasma
esterase hydrolysis of benzyl acetate may decline with age in both
rats and mice. Alcohol dehydrogenase activity was found to remain
unchanged in the aging colon (McMahon et al., 1987).
Changes in phase II enzymes also appear greatly variable in
rodent liver, although Loi & Vestal (1988) conclude that age has
little effect on phase II reactions in humans. The major conjugation
reactions involve sulfation, glucuronidation or reaction with
glutathione leading to mercapturic acid formation. All these
reactions are catalysed by multiple isoforms showing varying degrees
of substrate and sex specificity, as is the case for the phase I
enzymes. Iwasaki et al. (1986) demonstrated differential age effects
on two distinct sulfotransferases using male and female rats and
various alcohols and amines. Sulfation of phenolic substrates
appears to decline with age (Galinsky et al., 1986; Sweeny & Weiner,
1986) while conjugation of bile salts increases in old male rats
(Galinsky et al., 1986). In contrast, changes in female rats were
not seen (Galinsky et al., 1990). Chengelis (1988b) observed no
significant changes in sulfotransferase activity with age in either
male or female rats using beta-naphthol as the substrate. Similar
results were seen by Leakey et al. (1989b) for the conjugation of
both estrone and naphthol, whereas the sulfation of androsterone and
corticosterone increased with age in male rats.
Hepatic glucuronidation also shows substrate specificity for
alterations with age. Depending on the substrate, increases with
estrone and acetaminophen (Galinsky et al., 1986), decreases with
the rubber antioxidant 4,4'-thiobis-(6- t-butyl- m-cresol)
(Borghoff et al., 1988), or no effect with naphthol,
p-nitrophenol, morphine and testosterone have been observed
(Galinsky et al., 1986; Sweeny & Weiner, 1986) in male rats. In
contrast, Chengelis (1988b) observed a marked decrease in senescent
male and female rats using both p-nitrophenol and chloramphenicol.
To complicate matters further, Leakey et al. (1989b) also saw a
decrease in naphthol, testosterone, androsterone and
tetrahydrocortisone conjugation with glucuronic acid, but observed
no effects of aging on conjugation with 2-aminophenol,
5-hydroxytryptamine, bilirubin or estrone. Tarloff et al. (1989b)
saw no change in acetaminophen glucuronidation with advancing age.
Although the activities of the UDP-glucuronosyltransferases appear
highly variable, an age-related decrease in hepatic levels of the
cofactor, UDP-glucuronic acid (UDPGA) (Borghoff et al., 1988),
suggests that glucuronidation could be limited in older animals.
The concentration of hepatic glutathione, the cofactor involved
in the third major class of conjugation reactions, has been reported
to increase (Borghoff & Birnbaum, 1986), decrease (Stohs et al.,
1982) or remain unchanged (Chengelis, 1988b; Rikans & Moore, 1988)
in old rodents. Variability in age effects has also been reported in
the activity of the glutathione- S-transferases, which exist as a
family of dimeric proteins having broad and overlapping substrate
specificity. The isozymes are composed of two subunits from at least
six different peptides, including both homo- and heterodimers. Thus
reports of an increase (Leakey et al., 1989b), decrease (Fujita
et al., 1985; Blanco et al., 1987; Leakey et al., 1989b), or no
change (Birnbaum & Baird, 1979; Sweeny & Weiner, 1985; Borghoff &
Birnbaum, 1986; Chengelis, 1988b; Leakey et al., 1989b; Carrillo
et al., 1991) in hepatic glutathione-S-transferase activity may, as
in the cases of cytochrome P450 mixed-function oxidases,
sulfotransferases and UDP-glucuronosyltransferases, reflect
age-dependant changes in isozyme ratios (Spearman & Leibman, 1984;
Carrillo et al., 1991). Restriction in dietary protein decreases the
activity of the glutathione-S-transferases more in old than young
rodents (Carrillo et al., 1989, 1990).
Much less has been reported on the effects of aging on other
phase II metabolic reactions in the liver. Hydrolysis of expoxides
to form diols or dihydrodiols is catalysed by the epoxide
hydrolases. Epoxide hydrolase activity has been reported both to
increase (Birnbaum & Baird, 1979) and decrease (Ali et al., 1985;
Kaur & Gill, 1985; Leakey et al., 1989b) with age. Again, this may
reflect differential age effects on multiple enzymatic species.
However, some of the difference may be due to the ages of animals
used for comparison, since Chengelis (1988b) reported a gradual
increase in epoxide hydrolase activity throughout much of the life
span, followed by an abrupt decrease in senescence.
Human studies have suggested that the alterations observed in
salicylate pharmacokinetics (Cuny et al., 1979) with age might be
due to a decrease in glycine conjugation (Kyle & Kocsis, 1985).
However, elevated levels of salicyluric acid, the glycine conjugate
of salicylate, have been observed in elderly humans undergoing
chronic salicylate treatment (Montgomery & Sitar, 1981). Recent
studies in rodents have not demonstrated any age-related decline in
glycine conjugation with non-nephrotoxic doses of salicylate
(McMahon et al., l990a) or in the formation of hippuric acid from
benzoate (McMahon et al., 1989). Acetylation, however, has been
reported to decline both in man (Bauer et al., 1989) and rats
(Leakey et al., 1989b).
Changes in extrahepatic phase II reactions have been
investigated in even less depth than extrahepatic phase I reactions.
In the lung and small intestine, glucuronidation of p-nitrophenol
appeared not to change with age (Borghoff & Birnbaum, 1985), whereas
in the colon an age-related decrease was reported by McMahon et al.
(1987). In contrast, colonic glucuronidation of
4-methylumbelliferone rose significantly in older rats (McMahon
et al., l990b). A decrease in UDP-glucuronosyl transferase activity
in the kidney (Borghoff & Birnbaum, 1985; Tarloff et al., 1989a) was
accompanied by a decrease in the renal concentration of UDPGA
(Borghoff et al., 1988).
Glutathione content has been examined in a variety of tissues
(lung, kidney, brain, testes and blood) and, except for an
age-related decrease in the lens, has been found to remain constant
(Rikans & Moore, 1988). Unchanged glutathione levels in the colon
occurred although the activity of glutathione- S-transferase
decreased in this tissue (McMahon et al., 1987). Spearman & Leibman
(1983, 1984) observed differential age- and sex-related changes in
this activity in the lung depending on the substrate examined. The
renal activity of glutathione- S-transferase declines significantly
in old rats (Beierschmitt & Weiner, 1986). In contrast, elevated
gluthathione- S-transferase levels were observed in the brain of
old rats (Blanco et al., 1987), while no changes were observed in
the heart.
Epoxide hydrolase activity has been examined in the lungs,
small intestine and kidneys by Kaur & Gill (1985). They observed a
decrease in lung and small intestine activity in old rats which was
substrate dependent. In the rat kidney, epoxide hydrolase activity
decreased using trans-stilbene oxide as the substrate but did not
change with cis-stilbene oxide, supporting again the differential
effects of aging on different isozymic forms of drug-metabolizing
enzymes. In addition, deacetylation of acetaminophen in the kidney
appears to be either unchanged with age (Beierschmitt & Weiner,
1986) or slightly decreased (Tarloff et al., 1989b). Acetylation in
the kidney also decreases with age (Wabner & Chen, 1984).
One additional enzyme which can be considered to fall into the
phase II class is beta-glucuronidase. This enzyme hydrolyses
glucuronic acid from conjugated xenobiotics. The activity of this
enzyme has been reported to increase with age in rat liver
(Schmucker & Wang, 1979; Van Manen et al., 1983) and kidney
(Borghoff & Birnbaum, 1985), but remain unchanged in the colon
(McMahon et al., 1987). However, beta-glucuronidase was found to
decrease with age in fecal contents (McMahon, 1988). The balance
between glucuronidation and deglucuronidation reactions may play a
role in determining the level of reactive compounds in the organism.
Depending on the chemical, aging can result in either an
increase or a decrease in the metabolizing capacity of different
organs and tissues (liver, kidney, gastrointestinal tract, lungs and
skin). The elevation or reduction in metabolism can both lead to
higher and lower toxicity, depending on the relative reactivity of
the metabolic intermediates and end-products. Thus studies on the
effect of aging on metabolism should be considered case by case.
3.1.4 Excretion
Excretion leads to the elimination of a chemical and/or its
metabolites from the body. The kidney is the major excretory organ,
with the liver and lung also playing important roles in the
elimination process. In addition, sweat, saliva and sex-linked
processes such as lactation can serve as routes of excretion.
The effects of age on renal function appear to play a major
role in altered pharmacokinetics in the elderly (Vestal, 1978;
Koch-Weser et al., 1982). The fact that changes in the physiology of
the kidney occur has been known for many years (Schmucker, 1979).
Renal blood flow decreases with age leading to a decrease in
glomerular filtration rate. Tubular secretion and resorption are
also reduced in the elderly. The number of functional nephrons
declines to a similar extent as the decline in glomerular filtration
rate and active secretion, suggesting that the nephron loses its
function as a unit (Friedman et al., 1972). Decreases in renal
function can result in a decreased rate of renal clearance, leading
to a greater potential for elevated and/or persistent levels of
chemicals in the body which could lead to toxicity (Sellers et al.,
1983). In humans, decreased renal clearance in the elderly has been
demonstrated for many drugs, including the aminoglycosides,
tetracyclines, lithium, digoxin, procainamide, methotrexate, and
phenobarbital (Kampmann & Hansen, 1979).
Aging rodents are extremely susceptible to chronic
glomerulonephropathy (Goldstein et al., 1988), much of which may be
attributable to diet (Masoro & Yu, 1989). Altered glomerular
morphology, characterized by thickening of the basement membrane and
sclerosis, is progressive and increases in severity with advancing
age, eventually resulting in scarring and loss. Renal tubules are
also subject to degenerative changes, which are accompanied by
proteinuria, especially albuminuria (Neuhaus & Flory, 1978). This
appears to result from increases in glomerular permeability and a
loss of fixed glomerular polyanion (Baylis et al., 1988). The high
percentage of urinary protein represented by albumin in the aging
rat may be due to non-selective protein leakage into the urine
resulting from increases in glomerular permeability to large
proteins, since the protein percentage in urine approaches that in
the plasma of senescent rats (Horbach et al., 1988a). Similar
albuminuria has been observed in aging mice (Yumura et al., 1989),
and was correlated with glomerular sclerosis. Such changes, however,
should not be simply extrapolated to humans, since there is no
evidence for an increase in protein loss in the urine of the healthy
elderly.
Additional tubular changes occur in the aging kidney, resulting
in hyperplastic and degenerative changes. Some of these changes
resemble responses to specific environmental chemicals (Konishi &
Ward, 1989). A decrease in renal transport of organic acids has been
observed (Wabner & Chen, 1984). Aging appears to diminish the
turnover of sodium/potassium ATPase in the proximal tubules (Marin
et al., 1985), which could play a role in the observed age-related
decrease in tubular secretion.
Hepatic elimination may also be compromised by aging. Kitani
(1985) has suggested that the excretory capacity of the liver
decreases with age due to some functional alteration in the
hepatocytes. In contrast to rats, where blood flow does not change
after maturity (Kitani, 1988), blood flow to the liver declines in
humans (Sherlock et al., 1955). Bile flow rate has been reported to
be reduced (Borghoff et al., 1988) or remain unchanged (Kitani
et al., 1985a) in rats. Biliary transport declines, especially in
the case of polar compounds (Kitani et al., 1985a). The elimination
of sulfobromophthalein, a model compound for the study of biliary
excretion of organic anions, is also decreased in old rats (Kanai
et al., 1985). Sato et al. (1987) demonstrated that the biliary
excretion of the neutral glycoside ouabain decreases with age in
both males and females. This may be due to an age-related decrease
in hepatic uptake, resulting in less biliary elimination (Ohta
et al., 1988). In addition, the biliary canalicular transport system
declines steadily during aging (Kanai et al., 1988). The decreases
in both hepatic uptake and biliary excretion may reflect changes in
the hepatocyte plasma membrane (Zs-Nagy et al., 1986).
3.2 Pharmacodynamics
Age-related changes in chemical sensitivity cannot all be
explained on the basis of altered pharmacokinetics in the elderly.
Pharmacodynamic changes occur at the target site and may involve
changes in cell populations, cellular receptors, cellular
responsiveness or in the regulation of the amount or activity of
drugs, including the cardiac glycosides, benzodiazepines, tricyclic
anti-depressants, and the non-steroidal anti-inflammatory agents
(Bender, 1979), which have demonstrated altered receptor sensitivity
in the aged (Wilson & Hanson, 1980).
3.2.1 Central nervous system
Numerous studies have shown that, with advancing age, there is
a decrease in the ability of the nervous system to synthesize and/or
release neurotransmitters and neuropeptides (for review, see Rogers
& Bloom, 1985). This decline may reflect the fact that there are
fewer neurons present to synthesize the chemical messenger or that
the enzymes involved in their synthesis are altered. This would
imply that xenobiotics which lead to neuronal death or interfere
with neurotransmitter or neuropeptide synthesis and release could
have a greater adverse effect on the elderly than on the young
organism. An example of such an interaction has been noted within
the dopaminergic extrapyramidal circuits controlling motor movements
(nigrostriatal pathway). This pathway has been the subject of many
experimental and clinical studies and provides one of the best
functional units in which to study neurotoxicity in the aged.
Nigrostriatal neurons are lost as a normal correlate of aging, and
while these losses may not be expressed in the majority of
individuals as movement disorders, there is a clear reduction in the
functional reserve of this dopaminergic system, making it more
vulnerable to neurotoxicants. This has been demonstrated by the
observed acceleration or simulation of "age-associated" movement
disorders by neurotoxicants such as
1-methyl-4-phenyl-1,2,5,6-tetrahydro-pyridine (MPTP).
The reduced capacity to synthesize neurotransmitters that
occurs in the aged organism may also potentiate the effect of toxic
substances. Carbon disulfide is an organic solvent with a variety of
industrial applications that produces neurobehavioural dysfunctions
(Wood, 1981). The mechanism of CS2 toxicity seems to be inhibition
of dopamine-beta-hydroxylase, the enzyme that converts dopamine to
norepinephrine (McKenna & DiStefano, 1977). Since norepinephrine
metabolism declines with age, the spectrum of physiological effects
regulated by this catecholamine would be expected to suffer greater
disturbance, following exposure to CS2 or to pesticides that
reduce brain catecholamines such as methyl bromide (Honma et al.,
1987), in old rats compared to young ones.
Toxic compounds that serve as neurotransmitter receptor
blockers could have their effects on behaviour accentuated, making
neuroendocrine control of homeostasis more difficult in the elderly.
For example, the formamidine pesticides, amitraz and chlordimeform,
have been shown to block alpha-noradrenergic receptors (Costa &
Murphy, 1987; Costa et al., 1988) and induce a variety of
behavioural disorders (Boyes & Dyer, 1984; Hsu & Kakuk, 1984;
Landauer et al., 1984) in rats. Since there is an age-related
decline in noradrenergic receptors in several species (Rogers &
Bloom, 1985), exposure to these compounds may have a more pronounced
effect on the older organism.
Certain movement disorders associated with senescence may be
related to age-related impairments in the brain dopaminergic systems
(Marshall & Berrios, 1979). Waddington et al. (1985) observed a
decrease in the density of brain dopamine receptors in old rats,
with no changes in affinity. In contrast to young animals, the
receptors of old animals were not able to respond effectively to
long-term treatment. The decrease in dopamine receptor density was
selective for the D-2 receptor subtype, although the coupling
between D-1 receptors and adenylate cyclase appears to be affected
by aging. A similar loss of D-2 receptors in the elderly has also
been measured using positron tomography in the living human brain
(Wong et al., 1984). An age-related decrease in binding to the
serotonin receptor may relate to cerebral dysfunction in the elderly
(Shih & Young, 1978).
Studies with rats have indicated that the increased sensitivity
of older rats to diazepam is due to pharmacodynamic differences
(Guthrie et al., 1987). Pedigo et al. (1981) suggested this could
relate to changes in the benzodiazepine/GABA/chloride ionophore
complex. Human studies have also indicated that the site of
increased sensitivity to the benzodiazepines lies distal to the
receptor (Swift, 1985), possibly involving changes in the chloride
ionophores.
Alterations in endogenous opioid systems may play a role in
some of the behavioural changes observed in the elderly. Binding of
dihydromorphine to the opiate receptor decreases in specific areas
of the brain in aged rats as compared to young ones (Messing et al.,
1980). This reduction is due to a decrease in receptor number, with
no change in affinity. Despite a relatively large body of evidence
that some receptors and neurotransmitters are at least qualitatively
altered with aging, it is still not clear whether and how these
changes are causally related to increased sensitivity of the CNS to
certain drugs with aging, as described below.
The brain appears to exhibit increased sensitivity to
phenobarbital (Kitani et al., 1985b; Van Bezooijen et al., 1989) in
both mice and rats. Such enhanced sensitivity with age to an
anticonvulsant supports earlier studies with phenytoin (Kitani
et al., 1984). It is possible that the aging brain of both
experimental animals and humans has increased sensitivity to all CNS
depressants. Enhanced brain sensitivity has also been reported for
hexobarbital in rats (Van Bezooijen et al., 1989) and oxazepam in
mice (Kitani et al., 1986). The aging human brain appears to be more
sensitive to nitrazepam (Castleden et al., 1977a) and other
benzodiazepines (Reidenberg, 1980). Recently, zonisamide, a new drug
whose anticonvulsant properties are distinct from the other drugs,
was demonstrated to have an increased anticonvulsant effect in aging
mice (Kitani et al., 1987). Taken together, these results suggest
that these pharmacodynamic changes with age may reflect a decreased
response capability for seizures in the elderly, rather than a
specific age effect on all the distinct receptors (Kitani et al.,
1986). In fact, the lethal threshold for pentylenetetrazole in mice
has been shown to decrease with age (Nokubo & Kitani, 1988), coupled
to an increase in the threshold for maximal seizure.
The action of other environmental compounds on CNS function may
be more diffuse. Recent studies have demonstrated enhanced
sensitivity of old mice to cyanide intoxication (McMahon & Birnbaum,
1990b). Exposure to various metals has been shown to alter a variety
of CNS functions. There has been a particular interest in a
potential link between Alzheimer's disease and aluminium toxicity.
It was initially reported that the autopsied brains of Alzheimer's
patients showed elevated aluminium levels (e.g., Perl & Brody,
1980). However, others have found no such correlation. Marksberry
et al. (1981) compared the brains of Alzheimer's patients with older
adult controls and found no correlation between the density of
aluminium content and the density of neurofibrillary tangles and
neuritic plaques. However, a more recent study showed that the brain
aluminum levels were significantly increased as a function of age in
both control and Alzheimer patient populations (Bjorksten et al.,
1989).
Manganese is a metal that causes age-type neuropathy. However,
unlike aluminium, it is associated with extrapyramidal disorders,
characterized by intention tremor (Donaldson, 1987). In monkeys,
the neurological symptoms of choreo-athetoid movement, rigidity and
tremor occurred after 18 months of manganese exposure. These
clinical signs, in association with severe lesions of the globus
pallidus and subthalamic nucleus, resembled Parkinson's disease and
suggested a possible link between environmental exposure and
occurrence of the disease in aging individuals.
The neurotoxic effects of other metals have been widely
studied, particularly those of lead, which has been implicated in
reduced intellectual abilities in children (Needleman et al., 1979),
slowed reaction time (Hunter et al., 1985), and impairment of other
cognitive abilities (Winneke et al., 1982; Hansen et al., 1985).
While psychophysiological parameters appear to be relatively
insensitive to low levels of lead exposure, several studies have
demonstrated both cognitive and emotional effects due to long-term
exposure to lead (Hogstedt et al., 1983; Mantere et al., 1984).
Long-term exposure to mercury vapour has been reported to interfere
with verbal intelligence and memory performance (Piikivi et al.,
1984). Hanninen (1982) suggested that abnormalities due to mercury
exposure affect the motor system and result in intellectual
impairment, a gradual and progressive deterioration of memory
function, and emotional disability. Other metals including copper,
iron and manganese have also been reported to cause CNS dysfunction
(Grandjean, 1983). However, there are no available data on the role
of exposure to these metals in dysfunction of the CNS in the aged.
Examples of naturally occurring neurotoxic agents that
stimulate nervous system senescence have been reported in certain
island populations. For example, the Chamorro peoples of the
Marianna Island, specifically Guam and Rota, exhibited a high
incidence of amyotrophic lateral sclerosis, Parkinsonism and
Alzheimer's-like dementia that was recently linked to their diet.
The Chamorro's diet consists in part of a flour made from the seeds
of Cycas circinalis. When one of the components of these seeds,
beta- N-methylamino-l-alanine (BMAA), a compound similar in
structure to excitotoxic amino acids, was fed to macaques, they
exhibited signs of motor neuron, extrapyramidal and behavioural
dysfunction (Spencer et al., 1987).
It seems that several motor neuron disorders are associated
with the appearance of endogenous excitotoxic glutamate agonist-type
molecules. One such molecule is 2,3-pyridine dicarboxylic acid
(quinolinic acid), which has been isolated from the brains of
humans, rabbits and laboratory rodents (Moroni et al., 1984) and has
been shown to excite CNS neurons when applied iontophoretically
(Perkins & Stone, 1983). An interesting correlate is the fact that
brain concentrations of quinolinic acid increase with age (Moroni
et al., 1984), suggesting that its presence may result in the
spontaneous onset of neurode-generative conditions that are mimicked
by environmental neurotoxicants such as BMAA and
beta- N-oxalylamino-L-alanine (BOAA).
3.2.2 Endocrine system
There are several different ways in which the endocrine system
and the hormonal signalling operations involved may undergo
alterations with age and toxicant exposure. These can be categorized
as changes in: (a) the availability of hormones for binding to the
target tissues; (b) the reception of the pertinent transmitter or
hormonal signal by the target cells; and (c) the nature of the
hormonal message.
3.2.2.1 Changes in hormonal availability with age
Age-related or toxicant-induced shifts in synthesis, rate of
clearance and rate of secretion will all function to alter hormonal
concentrations. Such changes in the size of the available signal
pool may have corresponding effects on the magnitude of the response
by the target tissue. These changes may reflect a decline with age
in the homeostatic controls, which rely heavily on endocrine
feedback relationships.
Several toxicants have also been observed to cause changes in
circulating hormonal levels (Cooper et al., 1986). Significant
reductions in serum testosterone, for example, have been seen
following short-term exposure of rats to the plasticizer
dinitrobenzene (Rehnberg et al., 1988b) and the pesticide
chlordimeform, the latter also causing marked reductions in serum
LH, thyroid-stimulating hormone (TSH), T4 and T3 levels. The
effects, moreover, may be remarkably specific. Following three days
of exposure in male rats, the pesticide linuron, for instance, was
reported to decrease the serum T4 level in a dose-related manner,
while leaving T3 and the pituitary and gonadal hormones unaffected
(Rehnberg et al., 1988a).
There is also a growing body of evidence for a hormonal
influence on toxicant metabolism. A sizeable number of xenobiotics,
including both drugs and environmental toxicants, are metabolized by
the hepatic cytochrome P450 monooxygenase system (Nebert & Gonzalez,
1987). Components of this system have been found to be influenced by
glucocorticoids (Schuetz et al., 1984; Simmons et al., 1987) and
markedly affected by sex steroids (Kamataki et al., 1985b) and
growth hormone (Yamazoe et al., 1987; Zaphiropouos et al., 1989).
Consequently, persistent shifts in the circulating levels of such
hormones, as have been reported for the aging animal, could affect
the manner in which xenobiotics are metabolized following exposure.
Reported attenuations with age in the rhythms of human and rat
serum testosterone (Bremner et al., 1983; Steiner et al., 1984;
Tenover et al., 1988), LH (Vermeulen et al., 1989) and GH (Sonntag
et al., 1980), among other hormones, can present differences in
young-versus-old comparisons, depending on when such sampling is
performed. Comparable effects on hormonal rhythms have been reported
to occur in response to toxicant exposure. For example, single
injections of 2,3,7,8-tetrachlorinated dibenzo-p-dioxin (TCDD)
resulted in some evidence of alterations in prolactin and
corticosterone rhythms in rats (Jones et al., 1987). It may be that
an aging system, while still exhibiting rhythmic hormonal changes,
may be increasingly sensitive to their disruption by low toxicant
levels.
3.2.2.2 Changes with age in the reception of the signal by the
target cells
A general decline in the transmitter regulation of hormonal
function may also place an aging animal at increased risk for
toxicant exposure (Govoni et al., 1988), given that various
environmental toxicants (including, for example, the solvents
vinyltoluene, ethylbenzene and styrene, the halogenated hydrocarbon
TCDD, and certain heavy metal cations) have been reported to
interact with catecholaminergic systems (Govoni et al., 1979; Lucchi
et al., 1981; Arfini et al., 1987; Mutti et al., 1988; Russell
et al., 1988).
These changes have been observed for both neurotransmitter and
hormone receptors. There is some evidence of a decreased
responsiveness of target tissues to steroid hormones during
senescence. For example, age-related declines in the concentration
of the estrogen receptor may reflect a decline in the circulating
estrogen levels (Thakur, 1988). Impaired responsiveness could also
be due to reduced receptor translocation (Belisle & Lehoux, 1983) or
other steps in steroid action which occur after hormone binding. In
fact, age-related alterations in glucocorticoid responsiveness are
due to both receptor and post-receptor events (Kalimi, 1982).
Testicular luteinizing hormone receptor concentration and total
content also decrease with age (Amador et al., 1985). In general,
receptors for steroids, insulin, glucagon, catecholamines and
prolactin appear to decrease in concentration with increasing age in
rodents, dogs and humans (Roth, 1979b).
An additional consideration of alterations with age or toxicant
exposure in the reception of a hormonal (or transmitter) signal by
target cells concerns not only effects on the receptor itself, but
changes in the cell's membranes. In the aging rat brain, there is
evidence for a progressive decrease in membrane fluidity
(Hershkowitz, 1983; Nagy et al., 1983). This is at least partially
attributable to alterations in the lipid composition. For example,
it has been reported that aging is associated with elevations in
cholesterol, sphingolipids and saturated fatty acid chains, all
leading to increases in rigidification (Rouser et al., 1972). Since
protein activity in the membrane is influenced by the fluidity of
the lipid micro-environment, any alterations with age in membrane
viscosity may affect not only receptor functions but also enzymatic
activity.
3.2.2.3 Changes in the nature of the hormonal message with age
The antigenic site(s) on a hormone recognized by antibodies can
be quite distinct from those regions that bind to the receptors and
trigger a physiological response in the target tissue. This
distinction may have an added importance for studies in aging, since
alterations with age in peptide hormone structure have been reported
(Conn et al., 1980) that reflect changes in post-translational
processes. These effects, moreover, may be influenced by shifts in
the steroid hormonal milieu in the older animal (Ulloa-Aguirre
et al., 1988). A number of hormones are glycosylated to varying
degrees and such differences in their carbohydrate residues may
alter biological activity (Ulloa-Aguirre & Chappel, 1982; Warner
et al., 1985) and/or plasma half-life (Morell et al., 1971).
Consequently, hormonal measures based solely on immunoreactivity
per se potentially offer a somewhat inaccurate picture of
endocrine alterations with age.
3.2.3 Kidney
The aging kidney appears to be more susceptible than the young
one to drug-induced nephrotoxicity as well as to renal ischaemia. In
fact, cortical tubules of senescent rats seem more sensitive to
oxygen deprivation than do those of young rats (Miura et al., 1987).
Thus, increased susceptibility of the aging kidney can be
independent of pharmacokinetic effects.
Age-related susceptibility to nephrotoxicity has been
demonstrated for numerous drugs including salicylate, acetaminophen,
cephaloridine and doxorubicin. Kyle & Kocsis (1985) showed that
kidneys from older rats develop nephrotoxicity to a greater extent
than do those of young rats following an equal dose of salicylate on
a body weight basis. However, the dose to the kidney could be
greater in the older rats due to a decrease in the volume of
distribution. In addition, McMahon et al. (l990a) has recently
demonstrated that at toxic doses old rats produce more reactive
metabolites of salicylate than do young ones. The age-related
enhancement in cephaloridine nephrotoxicity may also be due to
pharmacokinetic changes (Goldstein et al., 1986), although renal
cortical slices from old rats demonstrated alterations in active
uptake of organic anions and cations following exposure to the
antibiotic, which were not observed in young rats. Doxorubicin
treatment resulted in greater toxicity in old rats (Colombo et al.,
1989). The onset of the delayed nephrotoxicity was noticeably faster
in the old rats; this may have been related to higher drug retention
in the kidneys of old rats.
Age-related increased susceptibility to acetaminophen
nephrotoxicity has been investigated more than that of any other
chemical in rats. Tarloff et al. (1989b) stressed that while
enhanced sensitivity to acetaminophen nephrotoxicity clearly occurs
with advancing age, great care must be taken in choosing the ages
for comparison. Beierschmitt et al. (1986a) demonstrated by both
functional and histological criteria that susceptibility to
acetaminophen-induced acute tubular nephrotoxicity increases with
age in rats. This may reflect altered susceptibility rather than an
alteration in pharmacokinetic parameters, since the generation of
reactive metabolites from acetaminophen decreases with age
(Beierschmitt & Weiner, 1986). Increasing delivery of acetaminophen
to functioning nephrons, whose numbers are decreased in the aged
kidney, does not appear to be responsible for the age-related
increase in susceptibility (Beierschmitt et al., 1986b).
Alterations with advancing age do not have to result in
increasing sensitivity. Studies by Murty et al. (1988b) indicated a
decrease in hydrocarbon-induced hyaline droplet nephropathy in male
rats during senescence. This male-specific nephropathy is associated
with the presence of alpha2u-globulin, whose synthesis is strongly
age dependent (Roy et al., 1983). Although gasoline causes extensive
nephrotoxicity in young rats, old rats are resistant. In fact,
gasoline failed to alter hyaline droplet numbers in aged male rats,
which also demonstrated an altered lysosomal response as compared to
young rats. This lack of response of aged rats to
hydrocarbon-induced hyaline droplet nephropathy suggests that these
rats would also be resistant to hydrocarbon-induced renal neoplasia.
3.2.4 Immune system
With advancing age a progressive decline in the concentration
of glucocorticoid receptors occurs in the spleen (Roth, 1979a). This
alteration may depend either on an age-related modification of the
ratio among various subsets of lymphoid cells carrying different
receptor densities or on an intrinsic failure of aged cells to
maintain an adequate turnover of receptor molecules. With advancing
age, membrane receptors for hormones of low relative molecular mass
on lymphoid cells also show alterations, although these are more
related to impaired coupling to signal transduction systems (Feldman
et al., 1984; Roth, 1988). It has been shown that the number of
receptor molecules decreases with advancing age whereas the affinity
does not seem to change. In the aged population with altered
function of the immune system, it is possible that immunomodulatory
agents present in the food potentiate immune dysfunction, making the
elderly more vulnerable to age-related diseases.
The effects of immunosuppressive compounds can be identified
from toxicological experiments with young adult rodents. These
chemicals include organotin compounds, some pesticides, halogenated
aromatic hydrocarbons (e.g., hexachlorobenzene and dioxins) and
cyclosporin (Poland & Knutson, 1982; Vos & Penninks, 1987; Vos &
Luster, 1989; Schuurman et al., 1990; Vos et al., 1990), and they
can also potentiate autoimmunity, which is found more frequently in
the elderly.
3.2.5 Other tissues and systems
There are few data on pharmacodynamic changes in other tissues
and systems during aging. An increase in the sensitivity of the
aging human myocardium to digoxin (Chavaz et al., 1974), as well as
to intravenous anaesthetics (Dundee, 1979), has been suggested. The
stomach, kidney and bone marrow are more susceptible to the adverse
effects of non-steroidal anti-inflammatory agents in the elderly
than in the young, and cerebral side effects are more common
(Huskisson, 1983). The anticoagulation properties of warfarin are
also changed in the elderly, with the aged demonstrating increased
sensitivity (Shepherd et al., 1979).
Although in most toxicological studies the cardiovascular
system is not well examined, there are several chemicals known which
can cause cardiovascular toxic or atherosclerotic effects. Such
compounds in food include erucic acid in combination with
omega-3-linolenic acid, cetolenic acid, brominated vegetable oils,
cobalt salts in combination with ethanol, lead, nitrates and high
amounts of caffeine (Speijers, 1983). On the other hand, compounds
such as saturated fatty acids, odd-numbered cyclopropenoid fatty
acids (sterculia acid), ergot alkaloids, halogenated aromatic
hydrocarbons and cadmium induce toxic effects on the vascular system
and possible potentiate atherosclerotic effect (Speijers, 1989a,b).
Conrad & Bressler (1982) investigated the cardiovascular effects of
caffeine in elderly men. Caffeine, in doses equal to those contained
in 2 to 3 cups of coffee, produces an increase in blood pressure,
but has no positive inotropic effect in healthy elderly men.
Kim & Kaminsky (1988) suggested that age plays an important
role in modifying susceptibility to the toxic metabolite of
fluroxene, 2,2,2-trifluoroethanol. They noted greater effects on the
stomach, liver, testicles, brain and kidneys of aged rats as
compared to younger animals. Enhanced sensitivity to ethanol
intoxication has also been noted in old rats (Guthrie et al., 1987),
suggesting altered tissue sensitivity. Acute ethanol hepatotoxicity
may be due to enhanced liver susceptibility to the toxin (Rikans &
Snowden, 1989).
3.3 Modifying factors
3.3.1 Nutrition
Nutrition has been shown to influence the aging processes in
rodents and the occurrence and progression of age-associated
diseases in rodents and humans. Moreover the age-associated changes
in physiological processes affect the nutrition of the mammalian
organism. However, there is little information on how aging
influences the nutritional requirements of humans, a subject that
urgently requires scientific study.
Nitrates in foods can be reduced to nitrites in the oral
cavity, GI tract and urinary bladder (Tannenbaum et al., 1978).
Nitrites react with amines in the stomach forming N-nitroso
compounds, many of which have been shown to be carcinogenic in
animal studies, and it is difficult to deny their hazard to man
(Searle, 1976; Bartsch, 1991).
During commercial processing and domestic preparation, foods
may become contaminated with toxic chemicals. For example, smoked
and grilled foods contain small amounts of polycyclic aromatic
hydrocarbons and a wide variety of phenols and other organic
compounds derived from smoke (IARC, 1990). Canned foods can become
contaminated with tin or lead.
In a study by Spagnoli et al. (1991), both 4-month-old and
4-year-old New Zealand white rabbits were fed an atherogenic diet.
Increased incidence and degree of atherosclerotic lesions were seen
in the 4-year-old rabbits. These results showed an increased
susceptibility of the older arterial wall to hypercholesterolaemia.
Although 4-year-old rabbits are still relatively young, considering
the life span of this species (10-12 years), this study suggests
that aging arteries might be vulnerable to atherogenic compounds.
Malnutrition associated with inadequate intake or uptake of
nutrients in the elderly is caused by several factors. These include
socio-economic conditions (Munro, 1984) such as a) ignorance of the
need for a balanced diet, b) poverty, c) social isolation, d)
physical dependence (disability) and physical inactivity, e) mental
disorders, and f) changes in habits, e.g., retirement (Munro, 1984;
American Dietetic Association, 1984; Ferro-Luzzi et al., 1988).
Other conditions resulting in malnutrition include malabsorption due
to a variety of intestinal conditions, alcoholism, and the use of
therapeutic drugs that interfere with nutrient utilization and
therefore with the toxicity of chemicals (Krupka & Vener, 1979;
Kohrs, 1981; Munro, 1984; Chen et al., 1985; Robertson et al, 1988).
Malnutrition in much of the aged population can enhance the
vulnerability of the elderly to the effects of the toxic chemicals
in food. It has also been reported that malnutrition alters
pharmacokinetics in different ways depending on the drugs examined
(Roe, 1983; Cusack & Denham, 1984). This can be another factor in
the altered sensitivity of the malnourished elderly to toxicants.
Major deficits or marginal nutrient status occur for proteins,
calcium, vitamins A, C and B (thiamin, riboflavin, niacin, B6 and
B12) and zinc (American Dietetic Association, 1984; Ferro-Luzzi
et al., 1988).
In aged women, a deficiency in calcium is especially associated
with osteoporosis (American Dietetic Association, 1984; Munro, 1984;
Caraceni et al., 1988; Cauley et al., 1988). The deficiency in iron
and zinc might play a role in haematological status and immune
function, while a higher intake of aluminium might have neurological
sequelae (American Dietetic Association (ADA), 1984). An iron
deficiency might make individuals more vulnerable to compounds toxic
for the haematopoietic system, e.g., hexachlorobenzene and lead.
Data showing that older individuals have a reduced need for
energy, due to slower metabolism and decreased activity, has an
important impact on the intake of both macro and micronutrients,
because the composition of the diet is based on the average caloric
or energy intake (American Dietetic Association (ADA), 1984;
Ferro-Luzzi et al., 1988). Considering this reduced need for energy,
the elderly often reduce their intake of nutrients.
Dietary manipulation can alter life span in laboratory animals
(Barrows & Kokkonen, 1978; Young 1979). Restricting food intake in
laboratory rats produces a significant extension of life span (McCay
et al., 1935; Ross, 1961). Similar effects have been observed in
mice (Weindruch & Walford, 1988; Kubos et al., 1984), as well as in
more primitive organisms including fruit flies (Harman, 1981), and
nematodes and Neurospora (Harman, 1982). Other studies have
indicated that the reduction in caloric intake that accompanies
protein restriction, and not the protein restriction per se, is
responsible for the increased longevity (Leto et al., 1976; Davis
et al., 1983; Schneider & Reed, 1985). Dietary restriction has been
shown to be effective when started as late as middle age (Cheney
et al., 1983; Kubos et al., 1984; Masoro, 1988). Food restriction
appears to act either by influencing primary aging processes or by a
general protective mechanism, rather than directly modulating
multiple specific pathogenic processes underlying specific diseases
(Masoro et al., 1991).
Chronic nephropathy in rats has been reported to be retarded by
food restriction (Saxton & Kimball, 1941). Rats fed ad libitum a
semisynthetic diet of 21% casein as the protein source exhibited a
marked age-associated progression of chronic nephropathy, whereas
rats fed 60% of the ad libitum intake developed almost no
age-related progression of this disease process (Maeda et al.,
1985). Caloric restriction appears to be more important than protein
restriction in retarding nephropathy (Maeda et al., 1985). Fat or
mineral restriction was found to have no influence on longevity
(Iwasaki et al., 1988).
Dietary restriction delays the age-dependent loss of adipocyte
responsiveness to hormones, prevents the decline in serum free fatty
acid levels, delays the increase in serum cholesterol, and reduces
the increasing triglyceride level observed in aging rats (Cooper
et al., 1977; Liepa et al., 1980; Masoro et al., 1980; Yu et al.,
1980).
Dietary restriction from weaning has been shown to delay the
onset and reduced the severity of chronic nephrosis, periarteritis,
myocardial degeneration and muscular dystrophy in very old animals
(Berg, 1976). Chronic restriction has also been shown to inhibit
certain types of tumours, decrease the incidence of neoplasms, and
increase tumour latency (Ross, 1976; Weindruch et al., 1982;
Weindruch & Walford 1988). Early work using a chemically induced
mammary carcinoma model suggested that the primary effect of caloric
restriction was on tumour promotion (Weindruch & Walford, 1988).
More recent data (Fishbein, 1991) have demonstrated that the
initiation of chemical carcinogenesis can also be decreased by
caloric restriction. For example, exposure to aflatoxin B1
resulted in less DNA-adduct formation in liver from young
calorically restricted male rats than from controls fed ad libitum.
Such changes are most probably due to changes in hepatic cytochrome
P-450 expression (Fishbein, 1991).
There is ample evidence of better maintenance of
T-cell-dependent immunological responses in aging mice chronically
restricted from weaning (Weindruch & Walford, 1988). However, it has
been reported that restriction initiated at an adult age can also
delay the age-specific decrease in immune function (Fernandes
et al., 1977; Friend et al., 1978; Weindruch & Walford, 1988).
Although caloric restriction extends life span in invertebrate
and lower vertebrate species as well as rodents (Weindruch &
Walford, 1988), as yet there is no definitive evidence whether or
not caloric restriction will increase longevity, or decrease
neoplastic and degenerative diseases, in higher mammals or in man.
There is some evidence that reduced caloric intake in man reduces
urinary output of thymidine glycol and 8-hydroxy-guanosine, which
implies reduced free-radical-mediated DNA damage (Fishbein, 1991).
However, until the mechanisms by which caloric restriction evokes
its effect are fully understood, the only way that it can be
conclusively proved whether or not caloric restriction does prolong
life in higher mammals, is to perform longevity studies in these
species. Such experiments, using non-human primates, are underway in
the USA (Fishbein, 1991), but it will be some time before definitive
data are available.
Chronic disease in the elderly is accompanied by caloric
deficit, which in turn causes breakdown of body proteins and
negative nitrogen balance as well as the utilization of fat stored
in adipose tissues. Dietary protein appears to promote
age-associated renal disease in both humans and rats (Brenner
et al., 1982).
When illness occurs, nutritional deficiencies frequently become
clinically manifest (Rudman, 1987). For example, trauma or a fall
causing fractures leads to immobilization resulting in rapid loss of
body stores of nitrogen and calcium. This slows down mending of the
fracture. Similarly, surgical procedures in the elderly often result
in delayed recovery and risks far exceeding those of younger
persons. Heart failure and malignancies can lead to cachexia with
loss of weight, muscle mass and nutrient reserves. Infection may
produce similar changes and intervene to become the terminal event.
3.3.2 Alcohol intake
Ethanol is one of the chemicals most commonly ingested by
humans. Its effects on the body are numerous and varied. Loneliness
and isolation would seem to foster consumption of alcohol beverages
among older persons. The decrease in lean body mass and body water
that occurs with aging is responsible for higher blood levels of
alcohol in elderly people than in younger adults consuming the same
quantities (Vestal et al., 1977).
Elderly individuals have a decreased tolerance for alcohol, due
to an increased sensitivity of the CNS to the depressant effect of
ethanol. The metabolic effects of ethanol are the result of either
the increase in the NADH/NAD+ ratio occurring due to ethanol
metabolism or to direct toxic effects of ethanol or its metabolite,
acetaldehyde. An increase in serum uric acid level (hyperuricaemia)
is common during heavy alcohol ingestion, thus inducing acute gouty
arthritis in patients with known gout. Hypoglycaemia and
hyperlipidaemia occur in patients who are not eating adequately or
who are ingesting a high-fat diet with ethanol, respectively.
Thrombocytopenia is caused by alcoholism in patients with advanced
alcoholic liver disease (IARC, 1988).
The use of drugs increases with aging. Acceleration or
inhibition of drug metabolism by ethanol depends on the duration of
ethanol ingestion and the presence or absence of ethanol in the body
at the time the drug is ingested (Mezey, 1981). The presence of
alcohol in the body causes a decrease in the metabolism of certain
drugs, such as antipyrine, meprobamate, pentobarbital and
benzodiazepines, resulting in increased bioavailability of the drugs
to the CNS and thereby contributing to unwanted side-effects. In
contrast, ethanol can increase the metabolism and metabolic
activation of certain xenobiotics, such as the known human
leukaemogen benzene, thus potentially leading to enhanced toxicity
(IARC, 1988).
Chronic ethanol ingestion increases tolerance to CNS
depressants in young individuals, but its effect in the elderly is
unknown. The concomitant administration of ethanol and barbiturates
results in an enhanced depressant effect of these drugs on the CNS
and can result in coma or even death. All other sedative-hypnotic
drugs tested have either synergistic or additive effects with
ethanol. Intellectual deterioration and dementia are common
complications of chronic alcoholism. Alcoholic patients show more
signs of mental aging at every chronological age (Gaitz & Baer,
1971).
Chronic excessive alcohol ingestion is associated with
increased mortality from cancer, cirrhosis, non-malignant
respiratory diseases such as emphysema, and accidents (Klatsky
et al, 1981; IARC, 1988). However, a decrease in coronary artery
disease and mortality is associated with moderate alcohol ingestion.
This may be due to increases in plasma HDL-cholesterol and decreases
in LDL-cholesterol that occur during ingestion of alcohol (Mezey,
1981). Ethanol consumption is also associated with hypertension and
an increased mortality from cerebrovascular accidents (Kozararevic
et al., 1980; Blackwelder et al., 1980).
3.3.3 Smoking
Smoking clearly plays an etiological role and produces an
acceleration of a wide spectrum of age-associated disease. Smoking
contributes to an increased mortality rate (Gupta et al., 1980). It
also provides an excellent example of problems faced in the
consideration of the environmental impact on aging.
Scientists recognize that smoking presents a complex toxic
insult through inhalation. Increased pathology in aged mice has been
reported after exposure to cigarette smoke (Matulionis, 1984). The
immune response in aged mice exposed to cigarette smoke has been
shown to be decreased at some ages but not at others (Keast & Ayre,
1981). The role of smoking in coronary heart disease has been
reviewed by Kannel (1981), who observed that the relative effect of
cigarette smoking decreases in old age and proposed that this could
be due to the selection of a more resistant population.
Estrogen-related diseases have also been associated with smoking
(Baron, 1984). Reif (1981) has examined susceptibility to lung
cancer and concluded that the shape of the susceptibility
distribution is determined by the effects of all environmental
carcinogens (both known and unknown) to which the population has
been exposed, as well as by differences in genetic susceptibility
among members of the population. Smokers and non-smokers both get
lung cancer during the same age range. Some of these studies suggest
that aged individuals are more susceptible to the effects of
smoking, whereas others suggest that duration of smoking appears to
be the critical factor (IARC, 1990). The smoker is exposed to
multiple toxic agents simultaneously, the effects of which are more
pronounced in aged individuals. Major aspects of metabolism and
pharmacokinetics are altered in aged individuals. Therefore, the
effective dose of any chemical reaching the systemic target tissues
in aged humans would be dependent on these perturbations.
3.4 Interactions of chemicals and diseases
3.4.1 Cancer
Cancer morbidity is expected to rise with age and with an
increasing percentage of elderly people living in industrialized
countries (Magnus, 1982). There is no consensus on the causes of the
age-related increase in tumour incidence. Various arguments support
the concept that an age-related accumulation of total dose of all
carcinogens accounts for tumour induction as a function of age in
sensitive individuals (Peto et al., 1975, 1985). One viewpoint is
that the sensitivity to carcinogens is stable and independent of
age, whereas another is that changes in the internal milieu of the
organism, such as the metabolic and immunological shifts of natural
aging, provide favourable conditions for tumour development with
increasing age (Burnet, 1970; Dilman, 1971).
Comparison of human epidemiological data with in vivo and
in vitro animal experimental results is difficult but does allow
some limited conclusions. It seems that environmental carcinogenic
factors as well as endogenous carcinogens are important causes of
increased tumour incidence in old people (IARC, 1990). This
conclusion is supported by the increasing incidence of occupational
cancer with increased exposure time to carcinogenic agents and by
the correlation of lung cancer incidence with the number of
cigarettes smoked (Doll & Peto, 1981; Peto, 1986). Humans, in
general, have an age-related increase in the incidence of epithelial
neoplasms (Doll, 1978), but the relationship of age to incidence of
other cancers in different organs varies (Doll, 1973; Moolgavkar &
Venzon, 1979; Anisimov, 1987; Dix, 1989). Some tumours appear most
frequently in childhood, some increase exponentially with age, and
others reach a peak at a certain age and then decline.
The data on cancer incidence among the atomic bomb survivors in
Hiroshima and Nagasaki, Japan, have been very informative concerning
radiation-related solid tumours as well as leukaemia. Age at time of
exposure appears to be a strong determinant of leukaemia risk; the
greatest absolute risk was experienced by those who were exposed at
ages 0-9 or 50 years and over (Beebe, 1979). Most of the excess
cancer deaths from solid tumours among the atomic bomb survivors
have occurred among those who were over 35 at the time of the blast.
Analysis of data on the positive correlation between the aging
rates of different species with their cancer rates and the
observation that these two processes, aging and carcinogenesis, may
be initiated and promoted by impairments of gene regulation led
Cutler & Semsei (1989) to conclude that both cancer and aging may
arise from a common set of genetic alterations. The analysis of the
interrelationship between aging and carcinogenesis should be based
on epidemiologically and experimentally confirmed data.
Epidemiological data, analysed in terms of a multi-stage model
(Kaldor & Day, 1987), can estimate the importance of age at onset,
duration of carcinogen exposure, and latency in a population. On the
level of the organism, carcinogenic agents influence not only the
cell, causing genomic damage that leads to neoplastic
transformation, but also create in the cell a microenvironment that
facilitates proliferation and clonal selection (Anisimov, 1987,
1989). Multi-stage carcinogenesis is accompanied by various
disturbances in tissue homeostasis and systems of anti-tumour
resistance that, in turn, are under the influence of systemic
(nervous, hormonal and metabolic) factors. How long it takes for
frank neoplasia to develop depends on the state of those systems at
the moment of exposure to a carcinogen or tumour promoter and the
dose.
According to the multi-stage model of carcinogenesis, the
carcinogen whose effect increases in proportion to age at exposure
affects the partially transformed cell. In this case the tumour
incidence would increase and latency would decrease, as compared to
a population exposed to the same effective dose of carcinogen at a
young age. For example, application of 7,12-
dimethylbenz [a]anthracene in small doses or
12-0-tetradecanoylphorbol-13-acetate to the skin of mice of
different ages caused neoplasms more frequently in older animals
(Stenbäck et al., 1981; Ebbesen, 1985). Exposure of mice and rats of
various ages to phenobarbital resulted in hepatocarcinogenesis only
in old animals (Ward, 1983; Ward et al., 1988). The number of events
necessary for complete malignant transformation in 15-month-old rats
under the influence of N-nitrosomethylurea is lower than in
3-month-old rats (Anisimov, 1988). In every tissue, the number of
events occurring in the stem cell before its complete transformation
is variable and depends on many factors, in particular the rate of
aging of the target tissue and of the regulatory system(s) of the
tissue (Anisimov, 1987, 1989). This model is consistent with the
analysis of age-related distribution of tumour incidence in
different sites in humans and experimental animals (Doll, 1978; Dix,
1989; Anisimov, 1987).
Epidemiological observations have shown that exposure to some
carcinogenic agents leads to a rise in cancer incidence independent
of age at the start of exposure (e.g., smoking), while other agents
induce more tumours when the exposure begins in the elderly (e.g.,
lung cancer following asbestos exposure) (Kaldor & Day, 1987; IARC,
1990).
3.4.2 Other diseases
Aging affects the functional capacity and structural integrity
of many organ systems. Environmental chemicals can also affect
several target organs. The combination of the influence of aging and
the toxic effects of chemicals in the environment might potentiate
the risk for elderly persons. An inherent problem common to all
research in chronic disease is the dissection of the respective
roles of time per se from those of primary aging. Hazzard (1985)
stated that an essential feature of human aging is the change in
physiological competence across the life span, as reflected in
homeostatic reserve. Homeostatic reserve declines at an accelerating
rate with age, normally producing death in old age from
multifunctional etiologies.
The relationship between aging and atherosclerosis is a prime
example of this conundrum. It seems most likely that the changing
picture of atherogenesis in western society has led to a large
number of people who survive into old age with not only a degree of
clinical atherosclerosis, but also with other chronic progressive
diseases, such as chronic obstructive pulmonary disease, immobility
from osteoarthritis and/or osteoporosis, and mental incompetence
from Alzheimer's disease, multi-infarct dementia or other
age-related dementing processes.
These diseases could significantly modify the response of the
organism to various environmental chemicals by decreasing or
increasing their susceptibility, followed by an acceleration of
these diseases or induction of new ones.
4. APPROACHES TO EXAMINING THE EFFECTS OF CHEMICALS ON THE AGED
POPULATION
4.1 Experimental approaches
4.1.1 Principles for testing chemicals in the aged
population
There are two principal approaches to the study of age-related
changes of any functional, morphological and/or biochemical
parameter, i.e. "longitudinal" and "cross-sectional". Longitudinal
studies consist of repeated estimations of any parameter in the same
animal in different periods of life. Cross-sectional studies involve
separate groups of animals of different ages who are examined at a
given point in time. Results obtained using the longitudinal
approach may significantly differ from the results from experiments
carried out using cross-sectional approaches. In studies on the
toxicity of chemicals, it is obligatory that identical conditions be
provided and maintained for all the animals. Problems related to the
choice of animal species, strain, sex, age, life stage and chemical
treatment (both route and dose selection) will be considered below.
4.1.2 Animal models
An extensive discussion of the choice and use of animal models
in research has been recently published (Rogers et al., 1991).
4.1.2.1 Animal species
The selection of suitable species obviously involves both
practical and economic factors. Animals with a short life span are
preferred. However, good life-table information is not available for
all species. A knowledge of species-related differences in metabolic
pathways and inherent sensitivities is also important in choosing
animal species for study. The choice of species should depend on the
experimental question as well as homology of response. For example,
while closely related isoforms of drug-metabolizing enzymes may
exist in different species, their tissue distribution and substrate
specificity may vary greatly (Nebert et al., 1991). Such differences
in isoform expression, together with reported differences in DNA
repair efficiency, could be responsible for species-related
differences in an organism's susceptibility to chemical carcinogens
(Daniel et al., 1983; Mehta et al., 1984; Anisimov, 1987; 1989).
Regardless of such metabolic differences, mice and rats are
most often used in aging research because of a short life span,
relative ease of maintenance under defined conditions, wide use in
biological research, and suitability for a variety of molecular and
genetic analyses. There are extensive life-table information for
some strains of mice and rats as well as a data base on their
spontaneous pathology. If old animals are purchased from a supplier
rather than being maintained in an investigator's own laboratory, it
is necessary to obtain information on the lifetime environment of
the animals, including housing conditions and dietary history. It
may be preferable to keep animals from weaning until the desired age
for investigation under the same controlled conditions.
Mammalian species other than rodents have only been used
sporadically in aging research, owing to their long life span, lack
of life-table information, genetic heterogeneity, restricted
availability and high cost. However, it is critical that such larger
species be used to answer certain questions. For example, studies
are currently underway to determine if dietary restriction can
prolong the lifespan of two different monkey species (Ingram et al.,
1990).
4.1.2.2 Animal strain
The choice of adequate rodent strain for experiments on aged
animals is critical. The male Fischer-344 rats is a popular model in
aging studies because of its size and growth characteristics.
However, testicular interstitial cell tumours begin to appear at the
age of 18 months in rats fed ad libitum, and by the age of 2 years
the tumour incidence approaches 100% (Fishbein, 1991). Recently, a
highly significant positive trend with time has been observed for
the increasing prevalence of leukaemia, anterior pituitary tumours
and thyroid c-cell tumours in both sexes, adrenal pheochromocytomas
in males, and mammary tumours and endometrial stromal polyps in
female F-344 rats (Rao et al., 1990). Some mouse strains suffer
from a single major disease process (e.g., tumours of the mammary
gland or liver, or chronic nephropathy), and the presence of this
disease in most animals could modify the response to chemical
exposure and complicate the interpretation of the results.
In the USA and Japan, Fischer-344 and Sprague-Dawley rats and
B6C3F1, Swiss, and CD-1 mice are most frequently used, whereas
in European countries Wistar-derived and Brown Norway rats and NMRI
and Swiss mice are the most popular strains. Inbred animals have the
advantages of greater stability and predictability of response.
However, heterogeneity of outbred animals more closely resembles the
heterogeneity of human populations. The choice of a certain animal
strain also depends on previous experience with these animals, the
final choice of an appropriate species and/or strain being dependant
on the scientific hypothesis under investigation. Each strain has
its own pattern of background pathology, which can be greatly
influenced by animal husbandry.
4.1.2.3 Animal sex
Sex-differences in response to chemicals are well known. Thus,
the use of both sexes is often necessary in toxicity testing.
However, the large gender differences in chemical toxicity and
pharmacokinetics that occur in rats become less apparent in old age
due to the decreased expression of sex-specific isoforms of the
hepatic drug-metabolizing enzymes (Kitani, 1991).
Ovarian status may significantly influence the sensitivity to
some chemical agents, modifying the biological response, as been
demonstrated for chemical carcinogens (Anisimov, 1971, 1987). It is
noteworthy that, when using females of the post-reproductive period,
the investigator must be aware that a) the ovaries in females of
some species (i.e. rats and mice) are not atrophied and may continue
to secrete steroid hormones, and b) some animals may be in
persistent estrus, while others may be in anestrus or
pseudopregnancy status (Aschheim, 1976). Furthermore, an animal's
reproductive history could influence its response to a chemical in
later life.
4.1.2.4 Selection of age groups for comparison
The problem of appropriately characterizing the animal's age
within the context of its life span is of particular importance in
experimental gerontology. Since the aging process causes significant
changes in various systems of the organism, there is a need for the
definition of some reference points for adequate comparison of the
results of different experiments. The importance of these points is
particularly significant in cross-sectional studies. One of the
confounding factors in such comparisons between experiments is the
need for frequent comparisons of results from different animal
species with different life spans (long-lived and short-lived
species and/or strains). Many authors have used two kinds of age
groups: "mature" or "adult" animals; and "old" animals. However, the
true age of "mature" rats in the reports from different
investigators fluctuates from 2 to 14 months and the age of "old"
animals from 12 to 37 months. The life cycle of experimental animals
can be divided into four periods: a) prior to weaning
(developmental); b) sexual maturation (maturational);
c) reproductive; d) pronounced age-related changes (senescent
period) (Zapadnyuk, 1971). All studies should compare animals from
multiple age periods.
4.1.2.5 Underlying pathology of animals of different ages
As mentioned previously, the background pattern of pathology
must be taken into account in the selection of animal species and
strains. A species-, strain- and sex-specific incidence of
pathology, whether neoplastic or not, is observed to increase
rapidly in the second half of the life span, even for those animals
maintained under specific pathogen-free conditions (Burek, 1978;
Anisimov, 1987; Frith & Ward, 1988; Fishbein, 1991). Among
non-neoplastic diseases in rats, the most frequent are chronic
glomerulonephropathy, cardiomyopathy, amyloidosis, and peripheral
nerve degeneration. For example, the incidence of spontaneous
leukaemia in F-344 rats frequently used in long-term studies may
exceed 30%, and the frequency of intertitial cell tumours of the
testis may reach 100% in old males. This pathology may significantly
modulate the host response to xenobiotics.
4.1.2.6 Transgenic animals
During the last decade it has become possible to add new
genetic information to the germ line of experimental animals
(Jaenisch, 1988). More recently successful attempts have even been
made to specifically alter gene sequences in the mouse germ line,
thereby ablating or correcting specific gene functions (Capecchi,
1989). In the study of the effects of chemicals on the aged
population, transgenic animal models have at least two contributions
to make. Firstly, the influence of specific genes on the age-related
susceptibility to environmental chemicals can be assessed in the
in vivo situation (Vijg & Papaconstantinou, 1990). Secondly, by
using transgenic animals harbouring a shuttle vector with one or
more mutational target genes, mutagenic effects can be studied in
different organs and tissues of animals as a function of age (Gossen
et al., 1989).
4.1.2.7 Animal husbandry
In experimental research on the sensitivity of aged animals and
the aging process, the husbandry aspects should be defined. The
quantitative and qualitative results might depend greatly on the
dietary conditions. The dietary composition should meet the minimal
requirements for nutrients, minerals, vitamins and (raw) fibre for
adult animals according to established and published standards for
laboratory animal diets. Another important dietary factor is the
quantity to which the test animals have access. They may either
receive a restricted diet containing adequate level of nutrients or
be fed ad libitum. The choice of diet depends on the questions to
be asked.
In addition to the dietary status of the animals, their
microbiological status should be defined. In some cases interaction
of the chemical with the gut microflora might modify the outcome of
the study. Specific-pathogen-free (SPF) animals are preferred. The
direct environment of the test animal, including relative humidity,
temperature, light and dark cycles, seasonal influence and stress
can all influence the final outcome of a study and therefore should
be defined carefully (Masoro, 1991).
Differences in animal husbandry may cause large variations in
biochemical and pathological effects. This is clearly illustrated by
alterations in the background data among different laboratories.
4.1.3 Chemical exposure
4.1.3.1 Dose level
The selection of dose is a difficult issue. The usual
requirement is to have a minimum number of test groups (3) plus one
control (vehicle) group. This permits the development of a
"dose-response" curve and allows for appropriate statistical
evaluation of the results. The highest dosage should induce minimal
signs of toxicity, bearing in mind that the maximum tolerated dose
for young animals could in some cases be toxic for old ones, and
that high doses may alter the toxicokinetics of the chemical.
Considering that the body weight of young and old animals could
be significantly different, a correct dosage calculation of test
substances in comparison groups is an important problem, i.e.
whether it is better to normalize per unit of body weight or body
surface, or to some other parameter such as lean body mass (Travis
et al., 1990). The available data indicate similarity of the results
when calculation of dose is performed per unit of body weight or
body surface. It should be taken into consideration that the growth
and development of various organs may have different rates, and that
relative organ weight (and consequently, the effective target dose
of the substance) may not be the same in animals of different ages.
When the substance is administered in food or water, the consumed
quantities should be taken into account, because they may differ in
animals of various ages. When the oral or dermal route of
administration is used, age-related changes in the extent and rate
of absorption may be important. Age-related changes in lung
ventilation capacity may also alter the internal dose of a chemical
when the inhalation route of administration is used. When looking
for portal-of-entry effects, a constant concentration of the agent
may be used in all age groups.
4.1.3.2 Route of administration
There is general agreement that the test substance should be
administered by the route that corresponds most closely to human
exposure. For humans, the main exposure routes are oral, dermal and
inhalation, while in animal experiments the oral route is most
frequently used. However, when pharmacokinetic studies show that
other routes of administration result in equivalent target tissue
levels, such alternative routes can be used. The use of injections
(subcutaneous, intraperitoneal, intramuscular or intravenous) may be
expedient under certain circumstances.
4.1.3.3 Duration of exposure
The question being addressed should guide the design of the
study. If the potential risk involves acute exposure of the elderly,
then an acute experimental scenario is required. If human exposure
is ongoing, long-term studies may be necessary. Exposure to the test
substance in chronic studies should start shortly after weaning and
continue for the major portion of the animal's life (at least
through the mean life span). IARC (1986) recommended 24 months of
exposure for rats and mice, and 18 to 20 months for hamsters when
studying the carcinogenic potential of chemicals. Choice of exposure
duration based on life-table characteristics would be optimal.
However, these guidelines do not apply to experiments when animals
of different ages are used at the start of the study. In this case
the dose and duration of exposure could differ in groups of young
and old animals. However, it is preferable to use identical exposure
durations whenever possible.
4.1.4 Non-mammalian models
Many species can be used as models in the study of age-related
sensitivity to environmental toxicants. These include fungi
(Neurospora crassa and Podospora anserina), protozoa
(Paramecium tetraurelia and Tetrahymena pyriformis), rotifers,
nematodes (Caenorhabditis elegans, C. briggsae and Turbatrix
aceti), and insects (Drosphila melanogaster, Musca domestica and
Tribolium confusum) (Committee on Chemical Toxicity and Aging,
1987). Non-mammalian species such as fish and salamanders have been
used in aging research (Weindruch & Walford, 1988) and are
potentially available for toxicology studies. Several of these
models have already been used to examine the effects of chemicals on
aging. Because of their short life span, ease of use and relatively
low cost, non-mammalian organisms could be important in the initial
phases of a test system to identify environmental chemicals that
might affect aging.
4.1.5 In vitro studies
For the purposes of screening xenobiotics, the use of in vitro
models can result in substantial economy and efficiency. Stock cells
and, in some cases, tissue explants can be cryopreserved in large
amounts. This permits repeated assays with comparable materials and
the sharing of common stocks by numerous laboratories. Moreover,
such stocks can be used to investigate cell-to-cell interactions,
such as metabolic cooperation and metabolic transformation. Finally,
tissue culture approaches can substantially reduce the numbers of
animals required for experimentation. Such methods cannot, however,
be expected to substitute for the intact animal experiment.
There are three general categories of in vitro methods: organ
culture, tissue explants and cell culture. Organ culture involves
the short-term maintenance of variable intact segments of tissue,
for example, the full thickness of a segment of aorta. In tissue
explants, the early migration and proliferation of epithelial and
fibroblast cell types can be observed. Cell cultures are of four
general types:
(a) primary cell cultures and mass cultures of cells taken directly
from the animal, usually after enzymatic dispersion of biopsied
tissue;
(b) established, serially passaged cultures with relatively
reproducible cycles of growth in early phases, but with limited
replicative life span, and with a genetic make-up reflecting
that of the donor age;
(c) "transformed" cell cultures with indefinite replicative
potential and generally with altered genetic makeup;
(d) established or transformed cell lines into which specific DNA
sequences ("transgenes") have been transfected.
Each of these types of models could prove useful for studies of
the effects of environmental agents on the elderly. One general
approach would be to explore the toxic effect of an agent as a
function of donor age, so as to detect unusual susceptibilities of
the cells and tissues of aged subjects. Another general approach
would be to culture the cells in vitro after in vivo treatments.
If a set of behaviours or phenotypes was observed with tissue from
young, treated subjects that proved to be comparable to that
observed with tissue from old untreated animals, an effect of the
in vivo treatments on the aging process could be inferred.
An entirely different experimental paradigm could be based on
the hypothesis that established cultures with finite replicative
life spans recapitulate the natural history of comparable cell types
in vivo - the well-known " in vitro model of cellular aging"
first developed by Hayflick & Moorhead (1961). The appropriateness
of such models for the study of aging is controversial, since it has
been proposed that the attenuation of growth observed in vitro
corresponds to terminal differentiation in vivo (Norwood & Smith,
1985; Bayreuther et al., 1988).
Of special interest would be the evaluation of agents that
exhibit unusual toxicity to putative stem cells. An excessive
depletion of stem cells could seriously compromise the regenerative
potential of tissues in aging subjects. In such studies, it would be
important to investigate a variety of cell types. Most research to
date has concentrated on in vitro aging of cultures of
fibroblastoid cells established from the fetal lung or from the
dermis of individual subjects of various ages. The precise origin of
such cells is not clear. Thus, with such cells it would be difficult
to compare age-related changes in vivo with those observed
in vitro.
A final experimental paradigm would be to use postreplicative
terminally differentiated cells in culture in order to investigate
agents for their potential to accelerate age-related alterations
observed in vivo. Such cell types might be derived through
in vitro terminal differentiation, or from normal or transformed
embryonic neuroblasts or myoblasts. A major concern, however, would
be the extent to which the experimental milieu reflected in vivo
conditions.
4.1.6 Statistical considerations
For statistical treatment of the results of short-term testing,
the statistical methods that are generally available may be used.
However, for statistical analysis of the results of long-term
testing, in particular carcinogenicity testing, the comparison of
results from treatment groups with different survival rates is a
major issue. In these cases the recommendation of IARC (Peto et al.,
1980; Gart et al., 1986) could be applied. All the tumours found at
necropsy must be evaluated as "fatal" or "incidental". This approach
permits one to conduct a comparison between young and old animals
despite the very different patterns of survival from those expected.
A crude analysis, ignoring the fact that young animals survive
longer than old ones, will overestimate the ratio of tumour
incidence in young and old animals. Conversely, a "death-rate"
analysis, treating all tumours as if they were fatal, will
over-correct for the effects of differences in survival on the
incidence of tumours discovered at the autopsy of animals that died
of unrelated causes. Bias can be eliminated only if tumours that
were discovered in an incidental context are analysed by the
prevalence method, while tumours discovered in a fatal context are
analysed by the death-rate method (Peto et al., 1980; Gart et al.,
1986).
4.1.7 Extrapolation of animal data to humans
Extrapolation of animal data to humans may be either
qualitative or quantitative. On the basis of experimental results,
the weight-of-evidence approach can help predict whether a given
substance may be considered dangerous for humans. Such information
includes data on the pharmacokinetics of a chemical, its
cytotoxicity and other toxic properties, as well as the data
obtained in in vitro and short-term tests. Of primary importance
for quantitative extrapolation, a dose-response relationship must be
detected in experimental animals. An important stage of quantitative
evaluation is extrapolation of the data obtained from exposure to
rather high doses to lower doses to which humans may be exposed in
the environment. It is necessary to take into account biological
differences, both in pharmacokinetics and pharmacodynamics, between
the test species and humans. For example, a dose taken per unit of
the body surface area, or its concentration in the daily ration,
includes a correction factor for species sensitivity and allows
extrapolation of experimental doses to man (Mantel & Schneiderman,
1975; Turusov & Parfenov, 1986; Travis et al., 1990). Of course,
species sensitivity to the action of some chemicals may also vary
significantly.
Various physiological and mathematical models have been
proposed for extrapolation of toxicological effects. Most of these
models refer to carcinogenesis (Turusov & Parfenov, 1986; Swenberg
et al., 1987; Clayson, 1988). The predictive nature of these models
for non-carcinogenic end-points remains to be determined.
4.2 Epidemiological and clinical approaches
4.2.1 Disease pattern of aged population
In order to study the pattern of disease occurring in the
elderly, the first question is "What illnesses are most common and
important among the elderly?" The answer can be most easily provided
in terms of diseases that cause death, hospitalization or visits to
a doctor's surgery.
The assessment of health status among the elderly on an
international basis is essentially limited to the use of mortality
data, since these are the only comprehensive data available. In the
developed countries, roughly 50% of all deaths occurring between the
ages of 65 and 74 years are attributable to cardiovascular diseases.
Among males in this age group, ischaemic heart disease accounts for
25% of deaths, while 11% are due to cerebrovascular diseases. For
women, 20% of deaths are attributed to ischaemic heart disease and
about 15% to stroke. Cancer accounts for another 25% of deaths among
men and women aged 65-74, lung cancer being the cause of 10% of all
deaths among elderly males. Roughly 7% of deaths are due to
respiratory diseases and 3% to external causes (WHO, 1989).
The trends in the four leading causes of death, i.e. malignant
neoplasms, heart diseases, cerebrovascular diseases and respiratory
diseases, within the age range 65-74 showed that, in selected
developed countries between 1950-1954 and 1980-1984, death rates
from all malignant neoplasms rose slightly for men but remained
essentially unchanged for women except in France. A more marked
decline in mortality is apparent for heart disease in the USA and
Australia where death rates have fallen by 25-30% since the late
1960s. Male mortality has fallen in France and Japan, but has risen
in Hungary. A similar pattern of change is also apparent for
females. Mortality from stroke has also declined in most countries,
although the timing and extent of the duration has varied from
country to country. For example, the decline in Japan occurred ten
years earlier than that in Australia. In the United Kingdom, France
and USA, rates have been falling since the 1950s. The trend in
mortality from respiratory diseases is less clear, there being
little evidence of sustained and comprehensive decline. However, it
is apparent that there has been some progress in reducing mortality
from these diseases in Australia and the United Kingdom over the
last decade (WHO, 1989).
It must be recognized from the outset that mortality data do
not always accurately reflect the underlying morbidity and are
particularly inappropriate in the case of many conditions for which
the fatality rate is low, yet which are important causes of
morbidity among the elderly. Data on causes of death are also less
reliable for the elderly than for other age groups owing to the
multiple pathological conditions often present at the time of death.
Nevertheless, with few exceptions, comprehensive morbidity data are
not available for the elderly.
Data from the USA shows that cardiovascular diseases cause the
largest proportion of hospitalization in the elderly. As a group,
diseases of the digestive system account for the second most common
diagnostic category while neoplastic diseases (90% malignant) are
the third most common cause of hospitalization. The remaining causes
are diseases of the respiratory system, injury or poisoning, and
diseases of the genitourinary or musculoskeletal systems (White,
1989). In a study from Shanghai, China, the common diseases of
hospitalized patients over 65 years of age in the 1950s, when
arranged in order of frequency, were hypertension, coronary artery
disease, chronic bronchitis, prostate hypertrophy, femur fracture,
pulmonary tuberculosis, diabetes mellitus, cholelithiasis and
tumours of various organs. Coronary artery disease, pneumonia,
hypertension and chronic bronchitis became the leading causes of
hospitalization in the 1970s (Zhu et al., 1982). In the 1980s, data
from other parts of China (Jiangxi, Liaoning, Xinjiang) showed that
chronic bronchitis or pneumonia was the most frequent cause of
hospitalization for elderly patients, followed by hypertension and
coronary artery disease (Xu et al., 1986; Shen et al., 1987; Xiong,
1989).
Data from the USA shows that diagnoses arising from visits to
the doctors surgery by people aged 75 or older, when arranged in
order of frequency, are hypertension (17.6%), chronic ischaemic
heart disease (9.5%), diabetes mellitus (6.7%), osteoarthritis (6%),
cataracts (5.1%), heart failure (4.4%), cardiac arrhythmia (3.6%),
arthropathies (3.6%), glaucoma (2.8%), hypertensive heart disease
(2.6%), angina pectoris (2.3%), chronic airway obstruction (2%) and
neoplasms (1.4%) (White, 1989).
4.2.2 Assessment of effects of environmental chemicals in the
elderly population
There are more limitations in clinical studies than in animal
toxicology studies. Firstly, the conditions in the study are not
easily controlled. Secondly, presumably harmful effects to the
subjects under study need to be avoided in designing the protocol.
The following approach is suggested.
Comprehensive, multidimensional functional assessment of the
elderly should be carried out. This involves analysis of the
physical, psychological, social, environmental and other aspects of
functioning (Zarit et al., 1985; Kane, 1987). Measurements of the
ability to perform the activities of daily life should be obtained.
Physical functioning can be measured by a combination of diagnosis,
symptom description, health reporting, days in bed or hospital
during a specific period, and reported pain or discomfort. The
subject's orientation with respect to time, place and person, as
well as short-term and long-term memory, should be assessed.
Psychological measures are used to evaluate depression, anxiety,
loneliness and sense of mental well-being, and a psychiatric history
should be recorded.
Clinical assessment involves a complete physical examination
and selective laboratory or instrumental examinations. As most
environmental chemicals affect certain parts or organ systems of the
body, laboratory or instrumental examination of these particular
parts or organ systems should be performed. The purpose of clinical
assessment is to find any structural or functional abnormalities
that may occur during the course of exposure to the environmental
chemicals.
Measures of environmental chemicals in the human body, such as
the determination of their concentration or that of their
metabolites in blood, body fluids or tissues (including nails,
hairs, etc.), faeces, urine and expired air, should be conducted.
Biomarkers of exposure, such as haemoglobin adducts, may be used
when available.
The results of the above-mentioned assessments may be
correlated and analysed to arrive at a conclusion as to whether the
environmental chemicals have harmful effects on the subjects
studied.
4.2.3 Acute episodes
There are few epidemiological reports on the effects of
exposure to environmental toxicants on the aged population. Several
acute air pollution incidents resulted in marked increases in
illness and death, mostly among the elderly. One such incident
occurred in the Meuse River Valley in Belgium in 1930 when
accumulating air contaminants trapped by an inversion caused the
death of 63 people and illness in 6000 residents. An incident in
1948 in Donora, Pennsylvania, USA, resulted from a similar inversion
that covered a wide area. Of the population of 14 000, 20 died
(compared with the expected two deaths for the same period) and 43%
fell ill. Again, the elderly were the most seriously affected. In
London, 4000 excess deaths were attributed to smoke and sulfur
dioxide in the fog of 1952, and an incident in 1962 caused 400
deaths above the normal value for the period. Similar episodes of
fog-induced mortality in various places have been described (Amdur,
1986). In all these episodes, the most vulnerable people were the
elderly, and the victims were usually suffering from acute
bronchitis and pneumonia, resulting in acute respiratory failure.
However, detailed and systematic study of the effects of
environmental chemicals on the elderly is still lacking (Amdur,
1986).
4.2.4 Concerns for the aged population
The health conditions of the elderly are quite different from
those in the early or middle age of human life. Many chronic
diseases that begin at an early age extend into advanced age. Some
pathological conditions occurring in middle age may become
symptomatic in the elderly. Certain medical problems are clearly
more prominent among the elderly, e.g., cancer of the prostate,
temporal arteritis and osteoarthritis. Illness in older people often
involves multiple organ systems that are interrelated by symptoms,
physical findings, functional capacity and treatment. The emotional
and social consequences of the physical conditions are also related.
Furthermore, the manifestations of aging processes that usually
comprise degeneration, both structural and functional, may sometimes
be difficult to differentiate from those of diseases. Aging
processes and existing diseases, as well as social and environmental
factors, act together to make the assessment and care of the elderly
complex and difficult.
As far as the effects of chemicals upon human health are
concerned, the elderly are subjected to long-term exposure to
environmental chemicals. Some exposures occur daily. The source may
be polluted air, water, food or products made of inorganic or
organic chemicals with which people frequently come into contact.
Some exposures occur during occupational activities in which people
are engaged for many years. The cumulative effect of continuous or
repeated exposure to chemicals may result in pathological changes
and clinical manifestations found in later stages of life. In
addition, the structural and functional changes of aging, usually
degenerative in nature, make the elderly more vulnerable to adverse
effects of environmental chemicals.
As mentioned above, the multiple disease status in the elderly
is usually accompanied by polypharmacy. In developed countries, the
consumption of drugs by people over 65 years of age accounts for
25-50% of total drug consumption (Vestal, 1978). The most commonly
used are neuropsychiatric and cardiovascular drugs,
anti-inflammatory analgesics and diuretics. Since these drugs are
used for the relief of symptoms rather then the cure of diseases,
repeated or sustained prescribing is the rule. Under such
conditions, interactions between environmental chemicals and drugs
should be carefully considered.
4.3 Biomarkers of aging
The term "biomarker of aging" arose in conferences sponsored by
the Fund for Integrative Biomedical Research (Regelson, 1983) and
the National Institute on Aging (Reff & Schneider, 1982). The
Committee on Chemical Toxicity and Aging (1987) defined a biomarker
of aging as "a biological event or measurement of a biological
sample that is considered to be an estimate or prediction of one or
more of the aging processes". Baker & Sprott (1988) offered the
following specific features for a biomarker of aging: (a) nonlethal;
(b) highly reproducible within and across species; (c) reflects
physiological age or some basic biological process of aging or
metabolism; (d) displays significant alterations during relatively
short time periods; (e) is crucial to the effective maintenance of
health and prevention of disease; and (f) reflects a measurable
parameter that can be predicted at a later age. Development of a
panel of biomarkers would assist in assessing the effects of
environmental chemicals that modulate aging processes in laboratory
animals and man.
There is currently a significant research effort, especially
with rodent species, to establish batteries of biological markers of
aging (Allaben et al., 1990). But how can one differentiate
alterations that are simple functions of chronological time from
those that are the results of intrinsic aging or of intrinsic aging
coupled with the effects of chronological time? One approach has
been that of comparative gerontology. Starting with a group of
taxonomically related species that vary substantially in their
maximum life-span potentials, one simply asks if the rate of change
of the putative biomarker reflects the maximum life-span potential
of the species.
From a practical point of view, various biomarkers may be used
for the measurement of aging. For different levels of integration of
an organism, different parameters may be used. At the subcellular
and cellular levels, measurements of macromolecular lesions, the
degree of collagen cross-linking, the level of lipofuscin
accumulation, specific enzyme activities, the number of particular
hormone receptors, numbers of cell divisions, etc. may be
investigated as biomarkers. Tissue and organ weights, cellularity,
growth or some functional activity (muscle strength, visual acuity,
secretion rate) may be considered. The functional activity of
motor, cardiovascular, nervous, endocrine, immune, haematopoietic
and other systems and subsystems may be considered at the systemic
level as potential biomarkers of aging. In addition, at the level of
the whole organism, the functions of the main integrative systems
should be addressed.
Test batteries that attempt to measure functional age (Webster
& Logie, 1976), physiological age (Hollingsworth et al., 1965) and
biological age (Furukawa et al., 1975; Nakamura et al., 1982;
Voitenko & Tokar, 1983; Reis & Poethig, 1984; Dubina et al., 1984;
Hochschild, 1989) have similar conceptual underpinnings in that they
utilize the great variability in performance that emerges among
adults of the same chronological age in standardized, age-sensitive
tests. Studies using this approach have been conducted on human
populations, and similar measures have been employed in a few rodent
studies (Hofecker et al., 1980; Dubina et al., 1983). This approach
can be viewed as the performance variability model of biological
age. It has considerable intuitive appeal and, in many cases,
statistical elegance. An individual may be judged biologically
younger if he is performing better than expected for his
chronological age. However, in their extensive and critical reviews,
Costa & McCrae (1980, 1985) revealed many conceptual and
methodological flaws in this approach.
Biomarkers of aging, which are markers of susceptibility, may
have their greatest utility in the study of the effects of
environmental chemicals on the processes of aging. The effects of
environmental chemicals on the elderly would be assessed most
efficiently by using biomarkers of effect (NAS, 1989).
5. CONCLUSIONS
a) With increasing age, humans become more vulnerable to
environmental challenges due to the deterioration of
physiological and psychological processes. Therefore, it is
likely that the elderly will be more susceptible to the harmful
effects of environmental chemicals.
b) The elderly are heterogeneous with respect to the extent of
deterioration of physiological and psychological processes.
c) Few, if any, of the hundreds of thousands of environmental
chemicals have been tested for increased toxicity in the
elderly.
d) Some of the many age-associated diseases may cause an increased
susceptibility to the harmful action of specific environmental
chemicals.
e) The use of animal models for aging research requires special
considerations, such as housing conditions, diets, monitoring
for infectious agents, and specifically defined pathologies. It
is also necessary to assess several age ranges based on
life-table information in order to distinguish the senescent
from the mature and developing animal.
f) The selection of the animal model should be based on the
likelihood of providing information of relevance to specific
problems in humans.
g) The characteristics of the elderly result from intrinsic aging
processes, environmental factors, and other life-span events.
Therefore, the study of the elderly may not provide
information on basic aging processes.
h) Both the size of the aged population and the number of
chemicals in the environment will undoubtedly increase over
the next few decades. It is therefore expected that the adverse
effects of chemical exposure on the elderly will increase in
importance as a health care issue.
6. FURTHER RESEARCH
a) Experimental, epidemiological and clinical data should be
obtained on:
* the toxicity, mutagenicity and carcinogenicity of
environmental chemicals in old individuals as compared to
young adults;
* the effect of age on the pharmacokinetics and
pharmaco-dynamics of environmental chemicals;
* the long-term effect of environmental chemicals on
molecular, cellular and physiological parameters as
potential biomarkers in the elderly.
b) New models should be developed for assessing the susceptibility
of the elderly to environmental chemicals as compared to young
adults. Such models should be suitable for assessment of
particular consequences and relevant to humans, and could
include:
* non-mammalian and mammalian models (e.g., Drosophila,
fish, rabbits, mini-pigs);
* transgenic animal models;
* cell lines with specific genetic characteristics.
c) For each study the appropriate applicability and use of
experimental models and techniques must be validated:
* animal models should be well-defined in terms of survival,
pathology and husbandry;
* human subjects should be examined carefully for subtle
disease status, medical history, life-style and
socio-economic status.
* standard procedures for measuring molecular, cellular and
physiological parameters should be defined (and developed
if necessary) in order to prevent misinterpretation.
d) The effects of environmental chemicals on the processes of
aging remain to be evaluated. A special scientific workshop
should be devoted to this topic.
REFERENCES
American Dietetic Association (ADA) (1984) ADA takes proactive
stance, testifies on older Americans act reauthorization. J Am Diet
Assoc, 84(7): 822-835.
Ali B, Walford RL, & Imamura T (1985) Influence of aging and poly IC
treatment on xenobiotic metabolism in mice. Life Sci, 37: 1387-1393.
Allaben WT, Chou MW, Pegram RA, Leakey J, Feues RJ, Duffy PH,
Turturro A, & Hart RW (1990) Modulation of toxicity and
carcinogenesis by caloric restriction. Korean J Toxicol, 6: 167-182.
Allison T, Hume AL, Wood CC, & Goff WR (1984) Developmental and
aging changes in somatosensory, auditory and visual evoked
potentials. Electroencephalogr Clin Neurophysiol, 58: 14-24.
Amador A, Steger RW, Bartke A, Johns A, Siler-Khodr TM, Parker R Jr,
& Shepherd AM (1985) Testicular LH receptors during aging in Fischer
344 rats. J Androl, 6: 61-64.
Amdur MO (1986) Air pollutants. In: Klassen CD, Amdur UO, & Doull J
ed. Casarett and Doull's toxicology: The basic science of poisons,
3rd ed. New York, Macmillan Publishing Co., pp 801-824.
Ammon HPT, Fahmy A, Mark M, Wahl MA, & Youssif N (1987) The effect
of glucose on insulin-release and ion movement in isolated
pancreatic islets of rats in old age. J Physiol, 384: 347-354.
Anderson S & Brenner BM (1986) The aging kidney : Structure,
function, mechanisms and therapeutic implications. J Am Geriatr Soc,
35(6): 590-593.
Anderson S & Brenner BM (1987) Effects of aging on the renal
glomerulus. Am J Med, 80(3): 435-442.
Anisimov VN (1971) [Blastomogenesis in persistent estrous rats.]
Vopr Onkol, 17(8): 67-75 (in Russian).
Anisimov VN (1987) Carcinogenesis and aging. Boca Raton, Florida,
CRC Press, vol 1 & 2.
Anisimov VN (1988) Effect of age on dose-response relationship in
carcinogenesis induced by single administration of
N-nitroso-methylurea in female rats. J Cancer Res Clin Oncol, 114:
628-635.
Anisimov VN (1989) Age-related mechanisms of susceptibility to
carcinogenesis. Semin Oncol, 16: 10-19.
Anisimov VN & Reiter RJ (1990) [Pineal function in cancer and
aging.] Vopr Onkol, 37: 259-268 (in Russian).
Arfini G, Mutti A, Vescovi P, Ferroni C, Ferrari M, Giaroli C,
Passeri M, & Franchini I (1987) Impaired dopaminergic modulation of
pituitary secretion in workers occupationally exposed to styrene:
Further evidence from PRL response to TRH stimulation. J Occup Med,
29: 826-830.
Armbrecht HJ (1986) Age-related changes in calcium and phosphorus
uptake by rat small intestines. Biochim Biophys Acta, 882: 281-286.
Armbrecht HJ, Doubek WG, & Porter SB (1988) Calcium transport by
basal lateral membrane vesicles from rat small intestine decreases
with age. Biochim Biophys Acta, 944: 367-373.
Armbrecht HJ, Boltz M, Strong R, Richardson A, Bruns MEH, &
Christakos S (1989) Expression of calbindin-D decreases with age in
intestine and kidney. Endocrinology, 125: 2950-2956.
Aschheim P (1976) Aging in the hypothalamic-hypophyseal-ovarian axis
in the rat. In: Everitt AV & Burgess JA ed. Hypothalamus, pituitary
and aging. Springfield, Illinois, Charles C. Thomas, pp 376-418.
Aschoff J (1979) Circadian rhythms: general features and
endocrinological aspects. In: Krieger DT ed. Comprehensive
endocrinology. New York, Raven Press, pp 1-61.
Avogaro P, Bittolo-Bon G, Cazzolato G, Belussi F, & Pontoglio E
(1983) Lipids and proteins of lipoproteins in human atherosclerosis.
Przegl Lek, 40: 713-718.
Bach JF, Darcenne M, Papiernik M, Barvis A, Lavasseur P, & Lebrand H
(1972) Evidence for a serum-factor secreted by the human thymus.
Lancet, ii: 1056-1058.
Baker SR & Rogul M ed. (1987) Environmental toxicity and the aging
processes: Proceedings of a workshop held in Columbia, Maryland, 1-2
October 1985. New York, Alan R. Liss, Inc., 139 pp (Progress in
Clinical and Biological Research, Volume 228).
Baker GT & Sprott RL (1988) Biomarkers of aging. Exp Gerontol, 23:
223-239.
Bang NU, Tessler SU, Heidenreich RO, Marks CA, & Mattler LE (1982)
Thrombosis and atherosclerosis. Chicago, Year Book Medical
Publishers.
Banks YB, Brewster DW, & Birnbaum LS (1990) Age-related changes in
dermal absorption of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and
2,3,4,7,8-pentachlorodibenzofuran (4-PeCDF). Fundam Appl Toxicol,
15: 163-173.
Baron JA (1984) Smoking and estrogen-related disease: A review. Am J
Epidemiol, 119: 9-22.
Barrows CH & Kokkonen GC (1978) Diet and life extension in animal
model systems. Age, 1: 131-143.
Bartsch H (1991) N-Nitrosocompounds and human cancer: where do we
stand? In: Relevance to human cancer of N-nitroso compounds, tobacco
smoke and mycotoxins. Lyon, International Agency for Research on
Cancer, pp 1-110 (IARC Scientific Publications No. 105).
Bauer LA, Black D, Gensler A, & Sprinkle J (1989) Influence of age,
renal function, and heart failure on procainamide clearance and
n-acetylprocainamide serum concentrations. Int J Clin Pharm Ther
Toxicol, 27: 213-216.
Baylis C, Fredericks M, Leypolat J, Frigon R, Wilson C, & Henderson
L (1988) The mechanisms of proteinuria in aging rats. Mech Ageing
Dev, 45: 111-126.
Bayreuther K, Rodemann KP, Francy PI, & Maier K (1988)
Differentiation of fibroblast stem cells. J Cell Sci, 10(suppl):
115-130.
Beebe GW (1979) Reflections on the work of the Atomic Bomb Casuality
Commission in Japan. Epidemiol Rev, 1: 184-210.
Behl CR, Bellantone NH, & Flynn GL (1987) Influences of age on
percutaneous absorption of drug substances. In: Kydonieuks AF &
Berrer B ed. Transdermal delivery of drugs. Boca Raton, Florida, CRC
Press, vol 2, pp 109-132.
Beierschmitt WP & Weiner M (1986) Age-related changes in renal
metabolism of acetaminophen in male Fischer 344 rats. Age, 9: 7-13.
Beierschmitt WP, Keenan KP, & Weiner M (1986a) Age-related increased
susceptibility of male Fischer 344 rats to acetaminophen
nephrotoxicity. Life Sci, 39: 2335-2342.
Beierschmitt WP, Keenan KP, & Weiner M (1986b) The development of
acetaminophen-induced nephrotoxicity in male Fischer 344 rats of
different ages. Arch Toxicol, 59: 206-210.
Belisle S & Lehoux J-G (1983) Endocrine aging in C57BL Mice. II.
Dynamics of estrogen receptors in the hypothalamic pituitary axis. J
Steroid Biochem, 18: 737-743.
Bender AD (1965) The effect of increasing age on the distribution of
peripheral blood flow in man. J Am Geriatr Soc, 13: 192-198.
Bender AD (1968) Effect of age on intestinal absorption:
Implications for drug absorption in the elderly. J Am Geriatr Soc,
16: 1331-1339.
Bender AD (1979) Drug sensitivity in the elderly. In: Crooks J &
Stevenson IH ed. Drugs and the elderly. Baltimore, Maryland,
University Park Press, pp 147-153.
Bender A, Post A, Meier J, Higson J, & Reichard G (1975) Plasma
protein binding of drugs as a function of age in adult human
subjects. J Pharm Sci, 64: 1711-1713.
Benditt EP (1977) The origin of atherosclerosis. Sci Am, 236: 74-85.
Berg BN (1976) Pathology and aging. In: Everitt AV & Burgess JA ed.
Hypothalamus, pituitary and aging. Springfield, Illinois, Charles C.
Thomas, pp 43-67.
Bertoni-Freddari C, Fattoretti P, Casoli T, Meier-Rouge W, & Ulrich
J (1990) The effect of ethanol on neuronal membrane permeability and
synaptic ultrastructure in adult and old rats. In: Bergamini E ed.
Protein metabolism and aging. New York, Wiley-Liss, Inc., p.
331-334.
Bertrand HA, Lynd FT, Masoro EJ, & Yu BP (1980) Changes in adipose
mass and cellularity through the adult life of rats fed ad libitum
or a life-prolonging restricted diet. J Gerontol, 35(6): 827-835.
Besedovsky H, Sorkin E, Keller M, & Müller J (1975) Changes in blood
hormone levels during the immune response (39057). Proc Soc Exp Biol
Med, 150: 466-470.
Besedovsky HO, Del Rey AE, & Sorkin E (1985) Immune-neuroendocrine
interactions. J Immunol, 135: 750-754.
Bhatnagar KP, Kennedy RC, Baron G, & Greenberg RA (1987) Number of
mitral cells and the bulb volume in the aging human olfactory bulb:
a quantitative morphological study. Anat Rec, 218: 73-87.
Bilchert-Toft M (1978) The adrenal glands in old age. In: Greenblatt
RB ed. Geriatric endocrinology. Vol 5: Aging. New York, Raven Press,
pp 81-102.
Birchenall-Sparks M, Richardson A, Roberts M, & Utherford M (1985)
The effect of aging on the structure and function of liver messenger
RNA. Mech Ageing Dev, 32: 99-111.
Birnbaum LS (1983) Distribution and excretion of 2,3,6,2',3',6'-and
2,4,5,2',4',5'-hexachlorobiphenyl in senescent rats. Toxicol Appl
Pharmacol, 70: 262-272.
Birnbaum LS (1987) Age-related changes in carcinogen metabolism. J
Am Geriatr Soc, 35: 51-60.
Birnbaum LS (1989) Age-related changes in drug disposition. In:
Zenser TV & Coe RM ed. Cancer and aging. Berlin, Heidelberg, New
York, Springer-Verlag, pp 25-138.
Birnbaum LS (1991) Pharmacokinetic basis of age-related changes in
sensitivity to toxicants. Annu Rev Pharmacol Toxicol, 31: 101-128.
Birnbaum LS & Baird MB (1979) Senescent changes in rodent hepatic
epoxide metabolism. Chem-Biol Interact, 26: 245-256.
Birren JE, Woods AM, & Williams MV (1979) Speed of behavior as an
indicator of age changes and the integrity of the nervous system.
In: Hoffmeister F & Muller C ed. Brain function in old age. Berlin,
Heidelberg, New York, Springer-Verlag, p 10.
Bitar MS & Shapiro BH (1987) Aberration of heme and hemoprotein in
aged female rats. Mech Ageing Dev, 38: 189-197.
Bjorksten J, Yaeger LL, & Wallace T (1989) Control of aluminium
ingestion and its relation to longevity. Int J Vitam Nutr Res, 58:
462-465.
Blackman MR (1987) Pituitary hormones and aging. Endocrinol Aging,
16(4): 981-994.
Blackwelder WC, Yano K, Rhoads GG, Kagan A, Gordon T, & Palesch Y
(1980) Alcohol and mortality: The Honolulu heart study. Am J Med,
68: 164-169.
Blalock JE, Harbour-McMenamin D, & Smith EM (1985) Peptide hormones
shared by the neuroendocrine and immunologic systems. J Immunol,
135(2): 858/5-861/5.
Blanco P, Machado A, & Satrustegui J (1987) Variations due to
hypoxia and ageing in the activities of glutathione-S-transferase
and NADPH-Cytochrome C reductase. Mech Ageing Dev, 39: 11-19.
Blum M, Averbuch M, Wolman Y, & Aviram A (1989) Protein intake and
kidney function in humans: its effect on 'normal aging'. Arch Inter
Med, 149(1): 211-212.
Blumberg JB (1985) A discussion of drug metabolism and actions in
the aged. Drug-Nutrient Interact, 4: 99-106.
Bolinder J, Kager L, Ostamn J, & Arner P (1983) Differences at the
receptor and postreceptor levels between human omental and
subcutaneous adipose tissue in the action of insulin on lipolysis.
Diabetes, 32: 117-123.
Bondareff W (1986) Neuropathology of nucleus basalis and locus
ceruleus in Alzheimer's disease. In: Scheibel AB, Wechsler AF, &
Brazier MAB ed. The biological substrates of Alzheimer's disease.
New York, London, San Francisco, Academic Press, pp 103-113.
Boobis AR & Davies DS (1984) Human cytochromes P-450. Xenobiotica,
14: 151-185.
Borghoff SJ & Birnbaum LS (1985) Age-related changes in
glucuronidation and deglucuronidation in liver, small intestine,
lung and kidney of male Fischer rats. Drug Metab Dispos, 13: 62-67.
Borghoff SJ & Birnbaum LS (1986) Age-related changes in the
metabolism and excretion of allyl isothiocyanate: A model compound
for glutathione conjugation. Drug Metab Dispos, 14: 417-422.
Borghoff SJ, Stefanski SA, & Birnbaum LS (1988) The effect of age on
the glucuronidation and toxicity of
4,4'-thiobis(6-t-butyl-m-cresol). Toxicol Appl Pharmacol, 92:
453-466.
Bornor JA, Dilworth BB, & Sullivan KM (1988) Exercise and
osteroporosis: A critique of the literature. Physiother Can, 40(3):
146-155.
Boyes WK & Dyer RS (1984) Chlordimeform produces profound,
selective, and transient changes in visual evoked potentials of
hooded rats. Exp Neurol, 86: 434-447.
Bradley RM (1979) The effect of aging on the olfactory sense. In:
Special senses in aging. Ann Arbor, Michigan, University of
Michigan, Institute of Gerontology, pp 3-8.
Bremner WJ, Vitiello MV, & Prinz PN (1983) Loss of circadian
rhythmicity in blood testosterone with aging in normal men. J Clin
Endocrinol Metab, 56: 1278-1281.
Brenner BM, Meyer TW, & Hostetter TH (1982) Dietary protein intake
and the progressive nature of kidney diseases. New Engl J Med, 307:
652-659.
Brizzee KR (1985) Neuron aging and neuron pathology. In: Johnson AH
ed. Relations between normal aging and disease. New York, Raven
Press, pp 191-224.
Brown WW, Davis BB, & Spry LA (1986) Aging and the kidney. Arch
Intern Med, 146: 1790-1796.
Bruce MF (1980) The relation of tactile thresholds to histology in
the fingers of the elderly. J Neurol Neurosurg Psychiatry, 43:
730-734.
Buckler AJ, Vie H, Sonenshein GE, & Miller RA (1988) Defective T
lymphocytes in old mice: Diminished production of mature c-myc RNA
after mitogen exposure not attributable to alterations in
transcription or RNA stability. J Immunol, 140: 2442-2446.
Bullock K (1985) Neuroanatomy of lymphoid tissue. In: Guillelmin R
ed. Neural modulation of immunity. New York, Raven Press, pp
111-141.
Burek JD (1978) Pathology of aging rats. Boca Raton, Florida, CRC
Press.
Burnet FM (1970) The concept of immunological surveillance. Prog Exp
Tumor Res, 13: 1-27.
Butenko GM (1985) Ageing of the immune system and diseases. In:
Likhachev A, Anisimov V, & Montesano R ed. Age-related factors in
carcinogenesis. Lyon, International Agency for Research on Cancer,
pp 71-83 (IARC Scientific Publications No. 58).
Campbell GR & Chamley-Campbell JH (1981) Invited review: The
cellular pathobiology of atherosclerosis. Pathology, 13: 423-440.
Capecchi MR (1989) Altering the genome by homologous recombination.
Science, 244: 1288-1292.
Caraceni MP, Corghi E, Ortolani S, Dindell M, Candotti G, & Ferrari
A (1988) Prevention of early postmenopausal bone loss. The effect of
calcitonin (Staporos). Clin Trials J, 25(6): 401-405.
Carrillo MC, Kitani K, Kanai S, Sato Y, Nokubo M, Ohta M, & Otsubo K
(1989) Differences in the influence of diet on hepatic glutathione
S-transferase activity and glutathione content between young and old
C56 black female mice. Mech Ageing Dev, 40: 1-15.
Carrillo MC, Nokubo M, Sato Y, Kanai S, Ohta M, & Kitani K (1990)
Effect of protein-free diet on activities and subunits of
glutathione S-transferase in livers of young and aged female rats.
Mech Ageing Dev, 56: 230-251.
Carrillo MC, Nokubo M, Kitani K, Kimihiko S, & Sato K (1991)
Age-related alterations of enzyme activities and subunits of hepatic
glutathione S-transferases in male and female Fischer-344 rats.
Biochim Biophys Acta, 1077: 325-331.
Castleden CM, George CF, Marcer D, & Hallet C (1977a) Increased
sensitivity to nitrazepam in old age. Br Med J, 1: 10-12.
Castleden CM, Volans CN, & Raymond K (1977b) The effect of aging on
drug absorption from the gut. Age Ageing, 6: 138-143.
Cathcart R, Schwiers E, Saul RL, & Ames BN (1984) Thymine glycol and
thymidine glycol in human and rat urine: a possible assay of
oxidative DNA damage. Proc Natl Acad Sci (USA), 81: 5633-5637.
Cauley JA, Gutai JP, Kuller LH, Ledonne D, Sandler RB, Sashin D, &
Powell JG (1988) Endogenous estrogen levels and calcium intakes in
postmenopausal women. Relationships with cortical bone measures. J
Am Med Assoc, 260(21): 3150-3155.
Ceda GP, Valenti G, Butturini U, & Hoffman AR (1986) Diminished
pituitary responsiveness to growth hormone-releasing factor in aging
male rats. Endocrinology, 118(5): 2109-2114.
Chakravarty S, Collins WP, Forecast JS, Newton JR, Oram DH, & Strudd
JWW (1976) Hormonal profiles after the menopause. Br Med J, 2:
784-787.
Chan K, Kerdall MJ, Mitchard M, Wells WOE, & Vickers MD (1975) The
effect of aging on plasma pethidine concentration. Br J Clin
Pharmacol, 2: 297-302.
Chandra RK (1985) Trace element regulation of immunity and
infection. J Am Coll Nutr, 4: 5-16.
Chavaz A, Baleurt L, Simonin P, & Fabre J (1974) Influence de l'âge
sur la digoxinémie et la digitalisation. Schweiz Med Wochenschr,
104: 1823-1825.
Chen LH, Liu S, Cook Newell ME, & Barnes K (1985) Survey of drug use
by the elderly and possible impact of drugs on nutritional status.
Drug-Nutrient Interact, 3: 73-86.
Cheney KE, Liu RK, Smith GS, Merdith PJ, Mickey MR, & Walford RL
(1983) The effect of dietary restriction of varying duration on
survival, tumour patterns, immune fraction and body temperture in
BIOC3F1 female mice. J Gerontol, 38: 420-430.
Chengelis CP (1988a) Age- and sex-related changes in the components
of the hepatic microsomal mixed function oxidase system in
Sprague-Dawley rats. Xenobiotica, 18: 1211-1224.
Chengelis CP (1988b) Age- and sex-related changes in epoxide
hydrolase, UDP-glucuronyl transferase, and PAPS-sulfotransferase in
Sprague-Dawley rats. Xenobiotica, 18: 1225-1237.
Chopra IJ, Solomon DH, Chopra U, Wu SY, Fisher DA, & Nakamura Y
(1978) Pathways or metabolism of thyroid hormones. Recent Prog Horm
Res, 34: 521-567.
Christophers E & Kligman AM (1965) Percutaneous absorption in aged
skin. In: Montagna W ed. Advances in the biology of the skin. Vol 6:
Ageing. Oxford, New York, Pergamon Press, pp 163-175.
Clayson DB (1988) Needs for biological risk assessment in
interspecies extrapolation. Environ Health Perspect, 77: 93-97.
Cleroux J, Giannattasio C, Grassi G, Seravalle G, Sampieri L,
Cuspidi C, Bolla G, Valsecchi M, Mazzola C, & Mancia G (1988)
Effects of aging on the cardiopulmonary receptor reflex in
normotensive humans. J Hypertension, 6(suppl 4): 141-144.
Coiro V, Volpi R, Cavazzini V, Bertoni P, Corradi A, Bianconi L,
Davoli C, Rossi G, & Chiodera P (1991) Restoration of normal growth
hormone responsiveness to GHRH in normal aged men by infusion of low
amounts of theophylline. J Gerontol Med Sci, 46(5): M155-M158.
Colombo T, Donelli MG, Urso R, Dallara S, Bartosek I, & Guaitani A
(1989) Doxorubicin toxicity and pharmacokinetics in old and young
rats. Exp Gerontol, 24: 159-171.
Committee on Chemical Toxicity and Aging (1987) Aging in today's
environment. Washington, DC, National Academy Press.
Conn PM, Cooper RL, McNamara MC, Rogers DC, & Shoenhardt L (1980)
Qualitative change in gonadotropin during normal aging in the male
rat. Endocrinology, 106: 1549-1553.
Conrad K & Bressler R (1982) Drug therapy for the elderly. St Louis,
Missouri, C.V. Mosby & Co.
Cooper B, Weinblatt F, & Gregerman R (1977) Enhanced activity of
hormone sensitive adenylated cyclase during dietary restriction in
the rat. J Clin Invest, 39: 467-474.
Cooper RL, Goldman JM, & Rehnberg GL (1986) Pituitary function
following treatment with reproductive toxins. Environ Health
Perspect, 70: 177-184.
Corman B & Michel JB (1986) Renin-angiotensin system,
converting-enzyme inhibition, and kidney function in aging female
rats. Am J Physiol, 251: 450-455.
Costa PT Jr & McCrae RR (1980) Functional age: A conceptual and
empirical critique. In: Haynes SG & Feinleib M ed. Epidemiology of
aging. Washington, DC, National Institutes of Health, National
Institute of Aging, National Heart, Lung, and Blood Institute, pp
23-50 (NIH Publication No. 80-969).
Costa PT & McCrae RR (1985) Concepts of functional or biological
age: A critical view. In: Andres R, Bierman EL, & Hazzard WR ed.
Principles of geriatric medicine. New York, McGraw-Hill, pp 30-37.
Costa LG & Murphy SD (1987) Interaction of the pesticide
chlordimeform with adrenergic receptors in mouse brain: An
in vitro study. Arch Toxicol, 59: 323-327.
Costa LG, Olibet G, & Murphy SD (1988) Alpha2-adrenoreceptors as a
target for formamidine pesticides: in vitro and in vivo studies
in mice. Toxicol Appl Pharmacol, 93: 319-328.
Creasey H & Rapoport SI (1985) The aging of human brain. Ann Neurol,
17(1): 2-10. Crooks J, O'Malley K, & Stevenson IH (1976)
Pharmacokinetics in the elderly. Clin Pharmacokinet, 1: 280-296.
Croucher PI, Mellish RWE, Vedi S, Garrahan NJ, & Compston JE (1989)
The relationship between resorption depth and mean interstitial bone
thickness: Age-related changes in man. Calcif Tissue Int, 45(1):
15-19.
Crowley JJ, Cusack BJ, Jue SG, Koup JR, Park BK, & Vestal RE (1988)
Aging and drug interactions. II. Effects of phenytoin and smoking on
the oxidation of theophylline and cortisol in healthy men. J
Pharmacol Exp Ther, 245: 513-523.
Cuny G, Royer RJ, Mur JM, Serot JM, Faure G, Netter P, Maillard A, &
Penin F. (1979) Pharmacokinetics of salicylates in elderly.
Gerontology, 25: 49-55.
Cusack B & Denham MJ (1984) Nutritional status and drug disposition
in the elderly. In: Roe DA ed. Drugs and nutrition in the geriatric
patients. New York, Churchill Livingstone, pp 47-70.
Cutler RG (1991) Human longevity and aging: possible role of
reactive oxygen species. Ann NY Acad Sci, 621: 1-28.
Cutler RG & Semsei I (1989) Development, cancer and aging: possible
common mechanisms of action and regulation. J Gerontol, 44(6):
25-34.
Daniel FB, Schut HA, Sandwiesh DW, Schenck KM, Hoffmann CO, Patrick
JR, & Stoner GD (1983) Interspecies comparison of benzo(a)pyrene
metabolism and DNA-adduct formation in cultured human and animal
bladder and tracheobronchial tissues. Cancer Res, 43: 4723-4729.
Danon D (1969) Biophysical aspects of red cell ageing. Bibl
Haematol, 15: 275-287.
Davies BH (1985) The respiratory system. In: Pathy MSJ ed.
Principles and practice of geriatric medicine. New York, Chichester,
Brisbane, Toronto, John Wiley and Sons.
Davila DR, Brief S, Simon J, Hammer RE, Brinster RL, & Kelley KW
(1987) Role of growth hormone in regulating T-dependent immune
events in aged, nude and transgenic rodents. J Neurosci Res, 18:
108-116.
Davis PJ & Davis FB (1987) Water excretion in the elderly.
Endocrinol Aging, 16(4): 867-875.
Davis TA, Bales CW, & Beauchene RE (1983) Differential effects of
dietary, caloric, and protein restriction in the aging rat. Exp
Gerontol, 18: 427-435.
Dawson-Hughes B, Shipp C, Sadowski L, & Dallal G (1987) Bone density
of the redius, spine, and hip in relation to percent of ideal body
weight in postmenopausal women. Calcif Tissue Int, 40(6): 310-314.
Dement W, Richardson G, Prinz P, Carskadon M, Kripke D, & Czeisler C
(1985) Changes of sleep and wakefulness with age. In: Finch CE &
Schneider EL ed. Handbook of the biology of aging, 2nd ed. New York,
Van Nostrand Reinhold Company, pp 692-717. Department of
International Economic and Social Affairs (1991) World population
prospects 1990, New York, United Nations, pp 192-215 (Population
Studies No. 120).
Dilman VM (1971) Age associated elevation of hypothalamic threshold
to feedback control and its role in development, aging and disease.
Lancet, i: 1211-1219.
Dilman VM (1987) [Four models of medicine.] Leningrad, Meditsina (in
Russian).
Dilman VM & Anisimov VN (1979) Hypothalmic mechanism of ageing and
of specific age pathology. I. Sensitivity threshold of
hypothalamo-pituitary complex to homeostatic stimuli in the
reproductive system. Exp Gerontol, 14: 161-174.
Dix D (1989) The role of aging in cancer incidence: an
epidemiological study. J Gerontol, 44(6): 10-18.
Doll R (1973) Age. In: Doll R & Vodopija I ed. Host environment
interactions in the etiology of cancer in man. Lyon, International
Agency for Research on Cancer, pp 39-48 (IARC Scientific
Publications No. 7).
Doll R (1978) An epidemiological perspective of the biology of
cancer. Cancer Res, 38: 3573-3583.
Doll R & Peto R (1981) The causes of cancer. J Natl Cancer Inst, 66:
1193-1312.
Donaldson J (1987) The physiopathologic significance of manganese in
brain: Its relation to schizophrenia and neurodegenerative
disorders. Neurotoxicology, 8: 451-462.
Doubek WG & Armbrecht HJ (1987) Changes in intestinal glucose
transport over the lifespan of the rat. Mech Ageing Dev, 39: 91-102.
Dubina TL, Dyundikova VA, & Zhuk EV (1983) Biological age and its
estimation. II. Assessment of biological age of albino rats by
multiple regression analysis. Exp Gerontol, 18: 5-18.
Dubina TL, Mints A, & Zhuk EV (1984) Biological age and its
estimation. III. Introduction of a correction to the multiple
regression model of biological age and assessment of biological age
in cross-sectional and longitudinal studies. Exp Gerontol, 19:
133-143.
Duckles SP (1987) Influence of age on vascular adrenergic
responsiveness. Blood Vessels, 24(3): 113-116.
Dundee JW (1979) Response to anaesthetic drugs in the elderly. In:
Crooks J & Stevenson IH ed. Drugs and the elderly. Baltimore,
Maryland, University Park Press, pp 179-187.
Dunn MF, Pattison SE, Storm MC, & Quiel E (1980) Comparison of the
zinc binding domains in the 7S nerve growth factor and the
zinc-insulin Hexamer. Biochemistry, 19: 718-725.
Dybkaer R, Lauritzen DM, & Krakauer R (1981) Relative reference
values for clinical chemical and haematological quantities in
"healthy" elderly people. Acta Med Scand, 209: 1-9.
Eastell R & Riggs BL (1987) Calcium homeostasis and osteoporosis.
Endocrinol Metab Clin, 16(4): 829-843.
Eastin WC Jr & Birnbaum LS (1987) Intestinal absorption of two
glucose analogues in rats of different ages. Exp Gerontol, 22:
351-357.
Ebbesen P (1985) Papilloma development on young and senescent mouse
skin treated with 12-0-tetradecanoylphorbol-13-acetate. In:
Likhachev A, Anisimov V, & Montesano R ed. Age-related factors in
carcinogenesis. Lyon, International Agency for Research on Cancer,
pp 167-170 (IARC Scientific Publications No. 58).
Edelman IS & Leibman J (1959) Anatomy of body water and
electrolytes. Am J Med, 27: 256-277.
Eden S (1987) Endocrinology in older people. Acta Obstet Gynaecol
Scand, 140(suppl): 9-22.
Edman CD (1983) Estrogen replacement therapy. In: Buchsbaum HJ ed.
The menopause. Berlin, Heidelberg, New York, Springer-Verlag.
Ellis GB & Desjardins C (1982) Male rats secrete luteinizing hormone
and testosterone episodically. Endocrinology, 110: 1618-1627.
Euans DW (1988) Renal function in the elderly. Am Fam Physician,
38(3): 147-150.
Evens RP & Hawkins DW (1984) Bone and joint disorders. In: Covington
TR & Ingram Walker J ed. Current geriatric therapy. Philadelphia,
Pennsylvania, W.B. Saunders Co., pp 277-306.
Exton-Smith AN (1985) Mineral metabolism. In: Finch CE & Schneider
EL ed. Handbook of the biology of aging. New York, Van Nostrand
Reinhold Company, pp 511-539.
Fabris N (1981) Ontogenetic and phylogenetic aspects of
neuroendocrine-immune network. Dev Comp Immunol, 5(suppl 1): 49-60.
Fabris N (1991) Neuroendocrine-immune interactions: a theoretical
approach of aging. Arch Gerontol Geriatry, 12: 219-230.
Fabris N & Piantanelli L (1982) Thymus-neuroendocrine interactions
during development and aging. In: Adelman RC & Roth GS ed. Hormones
and aging. Boca Raton, Florida, CRC Press, pp 167-181.
Fabris N & Provinciali M (1989) Hormones. In: Nelson D ed. Natural
immunity. New York, London, San Francisco, Academic Press, pp
306-347.
Fabris N, Giunta S, & Muzzioli M (1983) Decline of T-cell potential
in diabetic and aged men. J Gerontol, 38(5): 548-554.
Fabris N, Mocchegiani E, Muzzioli M, & Provinciali M (1988)
Neuroendocrine-thymus interactions: perspectives for intervention in
aging. Ann NY Acad Sci, 521: 72-87.
Fabris N, Mocchegiani E, Marriotti S, Pacini F, & Pinchera A (1989)
Thyroid-thymus interactions during development and aging. Horm Res,
31: 85-98.
Fabris N, Mocchegiani E, Muzzioli M, & Provinciali M (1990) Zinc,
immunity and aging. In: Goldstein AL ed. Biomedical advances in
aging. New York, London, Plenum Press, pp 271-281.
Fando JL, Salinas M, & Wasterlain CG (1980) Age-dependent changes in
brain protein synthesis in the rat. Neurochem Res, 5: 373-378.
Feldman RD, Limbird LE, Nadeau J, Robertson D, & Wood AJ (1984)
Alterations in leukocyte ß-receptor affinity with aging. New Engl J
Med, 310: 815-819.
Felten DL, Felten SY, Carlsan SL, Olschowka JA, & Livnat S (1985)
Neuroadrenergic and peptidergic innervation of lymphoid tissue. J
Immunol, 135: 755.
Fernandes G (1984) Nutrional factors: Modulating effects on immune
function and aging. Pharmacol Rev, 36(2): 123-129.
Fernandes G, Friend P, & Yunis EJ (1977) Influence of calorie
restriction on autoimmune disease. Fed Proc, 6: 1313.
Ferro-Luzzi A, Maiani G, Scaccini C, D'Amicis A, Borgioni G, Polito
A, Ronaldi L, Sett S, Branca F, Azzini E, Arena A, Raguzzini A,
Castata G, & Dente MG (1988) In: Lintas C & Spadoni MA ed.
Nutritional vulnerability of the Italian elderly: Facts and figures.
Rome, National Council for Research, pp 275-294 (Monografia No. 28).
Finch CE (1991) Longevity, senescence, and the genome. Chicago,
University of Chicago Press.
Finch CE & Hayflick L ed. (1977) Handbook of the biology of aging.
New York, Van Nostrand Reinhold Company.
Finch CE & Landfield PW (1985) Neuroendocrine and autonomic
functions in aging mammals. In: Finch CE & Schneider EL ed. Handbook
of the biology of aging, 2nd ed. New York, Van Nostrand Reinhold
Company, pp 645-691.
Fishbein L (1991) Biological effects of dietary restriction. Berlin,
Heidelberg, New York, Springer-Verlag.
Fleming BB & Barrows CH Jr (1982a) The influence of aging on
intestinal absorption of vitamins A + D by the rat. Exp Gerontol,
17: 115-120.
Fleming BB & Barrows CH Jr (1982b) The influence of aging on
intestinal absorption of vitamin B12 and niacin in rats. Exp
Gerontol, 17: 121-126.
Fraga CG, Shigenaga MK, Park JW, Degan P, & Ames BN (1990) Oxidative
damage to DNA during aging: 8-hydroxy-2-deoxyguanosine in rat organ
DNA and urine. Proc Natl Acad Sci (USA), 87: 4533-4537.
Friedman SA, Raizner RE, Rosen H, Solomon NA, & Wilfredo SY (1972)
Functional defects in the aging kidney. Ann Intern Med, 76: 41-45.
Friedman FK, Robinson RC, & Rifkind J (1989) Age-related changes in
the iron spin state of testosterone-binding rat liver microsomal
cytochromes P450. Biochem Biophys Res Commun, 158: 480-484.
Friend PS, Fernandes G, Good RA, Michael AF, & Yunis EJ (1978)
Dietary restrictiions early and late. Effects on nephropathy of the
NZB and NZW mouse. Lab Invest, 38: 629-632.
Frith CH & Ward JM (1988) Colour atlas of neoplastic and
non-neoplastic lesions in aging mice. Amsterdam, Oxford, New York,
Elsevier Science Publishers.
Fujita S, Katagawa H, Ishizawa H, Suzuki T, & Kitani K (1985)
Age-associated alterations in hepatic glutathione-s-transferase
activities. Biochem Pharmacol, 34: 3891-3894.
Furukawa T, Inoue M, Kajiya F, Inada H, Takasugi S, Fukui S, Takeda
H, & Abe H (1975) Assessment of biological age by multiple
regression analysis. J Gerontol, 30: 422-434.
Gaitz CM & Baer PE (1971) Characteristics of elderly patients with
alcoholism. Arch Gen Psychiatry, 24: 372.
Galinsky RE, Kane RE, & Franklin MR (1986) Effect of aging on
drug-metabolizing enzymes important in acetaminophen elimination. J
Pharmacol Exp Ther, 237: 107-113.
Galinsky RE, Johnson DH, Kane RE, & Franklin MR (1990) Effect of
aging on hepatic biotransformation in female Fischer 344 rats:
changes in sulfotransferase activities are consistent with known
gender-related changes in pituitary growth hormones secretions in
aging animals. J Pharmacol Exp Ther, 255: 577-583.
Gallagher D, Thompson LW, & Levy SM (1980) Clinical psychological
assessment of older adults. In: Poon LW ed. Aging in the 1980s:
Psychological issues. Washington, DC, American Psychological Press,
pp 19-40.
Gambert SR & Tsitouras PD (1985) Effect of age on thyroid hormone
physiology and function. J Am Geriatr Soc, 33(5): 360-365.
Gart JJ, Krewski D, Lee PN, Tarone RE, & Wahrendorf J (1986)
Statistical methods in cancer research. Vol III: The design and
analysis of long-term animal experiments. Lyon, International Agency
for Research on Cancer, 219 pp (IARC Scientific Publications No.
79).
Gilchrest BA (1984) Skin and aging processes. Boca Raton, Florida,
CRC Press, pp 1-120.
Globerson AR, Abel EI, & Ren-Menahem D (1989) Developmental aspects
of T-lymphocytes in aging. New York, London, Plenum Press, pp
363-373.
Goldstein RS, Pasino DA, & Hook JB (1986) Cephaloridine
nephrotoxicity in aging male Fischer 344 rats. Toxicology, 38:
43-63.
Goldstein RS, Tarloff JB, & Hook JB (1988) Age-related nephropathy
in laboratory rats. FASEB J, 2: 2241-2251.
Gollamudi HR, Prasanna R, Rao RH, Lawrence WH, & Autian J (1983)
Impaired metabolism of di (2-ethylhexyl)phthalate (DEHP) in old rats
- an in vitro study. J Toxicol Environ Health, 12: 623-632.
Gompertz B (1825) On the nature of the function expressive of the
law of human mortality and on a new mode of determining life
contingencies. Phil Trans R Soc London, II: 513-585.
Gossen JA, De Leeuw WJF, Tan CHT, Zwarthoff EC, Berends F, Lohman
PHM, Knook DL, & Vijg J (1989) Efficient rescue of integrated shutle
vectors from transgenic mice: a model for studying mutations in
vivo. Proc Natl Acad Sci (USA), 86: 7971-7975.
Govoni S, Memo M, Spano PF, & Trabucchi M (1979) Chronic lead
treatment differentially affects dopamine synthesis in various rat
brain areas. Toxicology, 12: 343-349.
Govoni S, Rius RA, Battaini F, Magnoni MS, Lucchi L, & Trabucchi M
(1988) The central dopaminergic system: Susceptibility to risk
factors for accelerated aging. Gerontology, 34: 29-34.
Goyal KK (1982) Changes with age in the aorta of man and mouse. Exp
Gerontol, 17: 127-132.
Graham, P (1985) The eye. In: Pathy MSJ ed. Principles and practice
of geriatric medicine. New York, Chichester, Brisbane, Toronto, John
Wiley and Sons, pp 833-844.
Grandjean P (1983) Behavioral toxicology of heavy metals. In:
Zbinden G, Cuomo V, Recagni G, & Weiss B ed. Application of
behavioral pharmacology in toxicology. New York, Raven Press, vol 2,
pp 331-339.
Greden JF, Flegel P, Haskett R, Dilsaver S, Carrol BJ, Grunhaus L, &
Genero N (1986) Age effects in serial hypothalamic-pituitary-adrenal
monitoring. Psychoneuroendocrinology, 11(2): 195-204.
Greenberg LH & Weiss B (1978) beta-Adrenergic receptors in aged rat
brain: reduced number and capacity of pineal gland to develop
supersensitivity. Science, 201: 61-63.
Greenberg LH & Weiss B (1979) Ability of aged rats to alter
beta-adrenergic receptors of brain in response to repeated
administration of reserpine and desmethylimipramine. J Pharmacol Exp
Ther, 211: 309-315.
Greenberg LH & Weiss B (1983) Neuroendocrine control of
catecholaminergic receptors in aging brain. In: Agnoli A, Crepaldi
G, Spano PF, & Trabucchi M ed. Aging brain and ergot alkaloids. New
York, Raven Press, pp 37-52.
Greenblatt DJ, Divoll MK, Harmatz JS, & Shader RI (1988) Antipyrine
absorption and disposition in the elderly. Pharmacology, 36:
125-133.
Greenstein BD, Fitzpatrick FT, Kendall MD, & Wheeler MJ (1987)
Regeneration of the thymus in old male rats treated with a stable
analogue of LHRH. J Endocrinol, 11: 345-350.
Gregerman RI & Solomon N (1967) Acceleration of thyroxine and
triiodothyronine turnover during bacterial pulmonary infections and
fever: implications for the functional state of the thyroid during
stress and in senescence. J Clin Endocrinol Metab, 27: 93-105.
Groop PH (1989) The influence of body weight, age and glucose
tolerance on the relationship between GIP secretion and beta-cell
function in man. Scand J Clin Lab Invest, 49(4): 367-379.
Gu X (1986) The life expectancy of the people of China. In: Cui Y
ed. Public health in the People's Republic of China. Beijing,
People's Medical Publishing House, pp 87-91.
Gupta SP, Mehta FS, & Irani RR (1980) Comparison of mortality rates
among bidi smokers and tobacco chewers. Ind J Cancer, 17: 149-152.
Guthrie S, Cooper RL, Thurman R, & Linnoila M (1987)
Pharmacodynamics and pharmacokinetics of ethanol, diazepam, and
pentobarbital in young and aged rats. Pharmacol Toxicol, 61:
308-312.
Hadden JW (1983) Cyclic nucleotides and related mechanisms in immune
regulation: A mini review. In: Fabris N, Garaci E, Hadden J, &
Mitchison NA ed. Immunoregulation. New York, London, Plenum Press,
pp 201-230.
Halberg F (1982) Biological rhythms, hormones and aging. In:
Vernandakis A ed. Hormones in development and aging. New York,
Spectrum Publications, pp 451-476.
Hall HRS, O'Grady MP, & Farrah JM Jr (1989) The
hypothalamic-pituitary-adrenal axis by thymic peptides. In: Hadden
JW, Massek K, & Nistico G ed. Interactions among central nervous
system, neuroendocrine and immune system. Rome, Pytagora Press, pp
112-125.
Hanawalt PC (1987) On the role of DNA damage and repair processes in
aging: evidence for and against. In: Warner HR, Butler RN, Sprott
RN, & Schneider EL ed. Modern biological theories of aging. New
York, Raven Press, pp 183-198.
Hanninen H (1982) Behavioral effects of occupational exposure to
mercury and lead. Acta Neurol Scand, 66(suppl 92): 167-175.
Hansen ON, Trillingsgaard A, Beese I, Lyngbye T, & Grandjean P
(1985) Neurobehavioral methods in assessment of children with
low-level lead exposure. In: Neurobehavioral methods in occupational
and environmental health (Document 3). Copenhagen, World Health
Organization, Regional Office for Europe, pp 183-187. Harman D
(1981) The aging process. Proc Natl Acad Sci (USA), 78: 7124-7128.
Harman D (1982) Nutritional implications of the radical theory of
aging. J Am Coll Nutr, 1: 27-34.
Harman SM, Tsitouras PD, Costa PT, & Blackman MR (1982) Reproductive
hormones in aging men. II. Basal pituitary gonadotropins and
gonadotropin responses to luteinizing hormone releasing hormone. J
Clin Endocrinol Metab, 54: 547-551.
Harrison DE, Astle CM, & Delaittre JA (1978) Loss of proliferative
capacity in immunohemopoietic stem cells caused by serial
transplantation rather than ageing. J Exp Med, 147(5): 1526-1531.
Hayflick L & Moorhead PS (1961) The serial cultivation of human
diploid cell strains. Exp Cell Res, 25: 585-621.
Hazzard WR (1985) Aging and atherosclerosis. Teasing out the
contributions of time, secondary aging, and primary aging. Clin
Geriatr Med, 1(1): 251-284.
Hebert CD & Birnbaum LS (1987) The influence of aging on intestinal
absorption of TCDD in rats. Toxicol Lett, 37: 47-55.
Hendley DD, Mildvan AS, Reporter MC, & Strehler BL (1963) The
properties of isolated human cardiac age pigment. II Chemical and
enzymatic properties. J Gerontol, 18: 250-259.
Hershkowitz M (1983) Mechanisms of brain aging - The role of
membrane fluidity. In: Gispen WH & Traber J ed. Aging of the brain.
Amsterdam, Oxford, New York, Elsevier Science Publishers, pp 85-98.
Hirsch HR (1982) Evolution of senescence: natural increase of
population dispaying Gompertz or power-law death rates and constant
of age-dependent maternity rates. J Theor Biol, 98: 321-346.
Hochschild R (1989) Improving the precision of biological age
determinations. Part I. A new approach to calculating biological
age. Exp Gerontol, 24: 289-300.
Hofecker G, Skalicky M, Kment A, & Niedermuller H (1980) Models of
the biological age of the rat. I. A factor model of age parameters.
Mech Ageing Dev, 14: 345-359.
Hogstedt C, Hane M, Agrell A, & Bodin L (1983) Neuropsychological
test results and symptoms among workers with well-defined long-term
exposure to lead. Br J Ind Med, 40: 99-105.
Hollander D, Dadufalz V, Weindruch R, & Walford RL (1986) Influence
of life-prolonging dietary restriction on intestinal vitamin A
absorption in mice. Age, 9: 57-60.
Hollingsworth JW, Hashizume A, & Jablon S (1965) Correlations
between tests of aging in Hiroshima subjects - an attempt to define
"physiological age." Yale J Biol Med, 38: 11-26.
Holt AR, Pascal RR, & Kotler DP (1984) Effect of aging upon small
intestinal structure in the Fischer rat. J Gerontol, 39: 642-647.
Holt PR, Yeh KY, & Kotler DP (1988) Altered controls of
proliferation in proxismal small intestine in the senescent rat.
Proc Natl Acad Sci (USA), 85: 2771-2775.
Honma T, Miyagawa M, & Sato M (1987) Methyl bromide alters
catecholamine and metabolite concentrations in rat brain.
Neurotoxicol Teratol, 9: 369-375.
Horbach GJM, Princen HMG, Van der Kroef M, Van Bezooijen CFA, & Yap
SH (1984) Changes in the sequence content of albumin mRNA and in
its translational activity in the rat liver with age. Biochim
Biophys Acta, 83: 60-66.
Horbach GJM, Van der Boom H, Van Bezooijen CFA, & Yap SH (1986)
Molecular aspects of age-related changes in albumin synthesis in
rats. In: Van Bezooijen CFA, Miglio F, & Knook DL ed. Liver drugs
and aging. Rijswijk, The Netherlangs, Eurage, pp 121-126 (Topics in
Aging Research in Europe, Volume 7).
Horbach GJM, Durham SK, Yap SH, & Van Bezooijen CFA (1988a) Albumin
elimination in female WAG/Rij rats with age: A longitudinal study.
Mech Ageing Dev, 43: 137-152.
Horbach GJM, Van der Boom H, Yap SH, & Van Bezooijen CFA (1988b)
Molecular aspects of age-related changes in albumin synthesis in
female WAG/Rij rats. Life Sci, 43: 1707-1714.
Hosomi T (1990) Japanese economy and society. In: Institute of
Japanese Problems, Chinese Academy of Social Sciences and Japanese
Vital Insurance Company ed. Proceeding of a Symposium on Sino-Japan
Social Countermeasure for Aging Problems. Beijing, Dong-Fang
Publishing House pp 7-30.
Houck JC, Dehesse C, & Jacob R (1967) The effect of aging upon
collagen catabolism. Symp Soc Exp Biol, 21: 403-425.
Hsu WH & Kakuk TJ (1984) Effect of amitraz and chlordimeform on
heart rate and pupil diameter in rats: Mediated by alpha
2-adrenoreceptors. Toxicol Appl Pharmacol, 73: 411-415.
Hunter J, Urbanowicz MA, Yule W, & Landsdown R (1985) Automated
testing of reaction time and its association with lead in children.
Int Arch Occup Environ Health, 57: 27-34.
Hunziker PE & Kagi JHR (1985) Metallothionein. In: Harrison PM ed.
Metalloproteins: Part II. London, MacMillan Press Ltd.
Huskisson EC (1983) NSAIDS: A topical review. Geriatr Med, 12:
867-870.
IARC (1982) Chemicals, industrial processes and industries
associated with cancer in humans. IARC Monographs, Volumes 1 to 29.
Lyon, International Agency for Research on Cancer, 292 pp (IARC
Monographs on the Evaluation of the Carcinogenic Risk of Chemicals
to Humans, Supplement 4).
IARC (1986) In: Montesano R, Bartsch H, Vainio H, Wilbourne J, &
Yamasaki H ed. Long-term and short-term assays for carcinogens: A
critical appraisal. Lyon, International Agency for Research on
Cancer, 564 pp (IARC Scientific Publications No. 83).
IARC (1988) Alcohol drinking. Lyon, International Agency for
Research on Cancer, 416 pp (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Volume 44).
IARC (1990) In: Tomatis L ed. Cancer: causes, occurrence and
control. Lyon, International Agency for Research on Cancer, 352 pp
(IARC Scientific Publications No. 100).
Imayama S & Braverman IM (1989) A hypothetical explanation for the
aging of skin. Chronologic alterations of the three-dimensional
arrangement of collagen and elastic fibres in connective tissue. To
provide a morphologic basis for a better understanding of the
"aging" of human. Am J Pathol, 134(5): 1019-1025.
Ingbar SH (1978) The influence of aging on the human thyroid hormone
economy. In: Greennblatt RB ed. Geriatric endocrinology. New York,
Raven Press, pp 13-31.
Ingram DK, Cutler RG, Weindruch R, Renquist DM, Knapka JJ, April M,
Belcher CT, Clark MA, Hatcherson CD, Marriott M, & Roth GS (1990)
Dietary restriction and aging: the initiation of a primate study. J
Gerontol, 45(5): 148-163.
Iqbal K, Grundke-Iqbal I, Merz PA, & Wisniewski HM (1982)
Age-associated neurofibrillary changes. In: Giacobini E, Filogamo G,
Giacobini G, & Vernadakis A ed. The aging brain: cellular and
molecular mechanisms of aging in the nervous system. New York, Raven
Press, pp 247-257.
Iwasaki K, Chirago T, Tada K, Noda K, & Noguchi H (1986) Age- and
sex-related changes in amine sulphoconjugations in Sprague-Dawley
strain rats. Comparison with phenol and alcohol sulphoconjugations.
Xenobiotica, 16: 717-723.
Iwasaki K, Gleiser CA, Masoro EJ, McMahan CA, Seo E, & Yu BP (1988)
Influence of the restriction of individual dietary components on
longevity and age-related disease of Fisher rats: The fat component
and the mineral component. J Gerontol, 43: B13-B21.
Iwase A, Kitazawa Y, & Ohno Y (1988) On age-releated norms of the
visual field. Jpn J Ophthalmol, 34(4): 429-437.
Iwata T, Incefy GS, Tanaka T, Fernandes G, Menendez-Botel CI, Pih K,
& Good RA (1979) Circulating thymic hormone levels in zinc
deficiency. Cell Immunol, 47: 100-105.
Jackson J, Banks YB, & Birnbaum LS (1990) Maximal dermal absorption
of TCDD occurs in weanling rats. Toxicologist, 10: 309.
Jaenisch R (1988) Transgenic animals. Science, 240: 1468-1474.
Jones MK, Weisenburger WP, Sipes IG, & Russell DH (1987) Circadian
alterations in prolactin, corticosterone, and thyroid hormone levels
and down-regulation of prolactin receptor activity by
2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol, 87:
337-350.
Kaldor JM & Day NE (1987) Interpretation of epidemiological studies
in the context of multistage model of carcinogenesis. In: Barrett JC
ed. Mechanisms of environmental carcinogenesis. Boca Raton, Florida,
CRC Press, vol 2, pp 21-57.
Kalimi M (1982) Comparison of glucocorticoid receptors in various
tissues of adult and senescent rats. Gerontology, 28: 371-376.
Kamataki T, Maeda K, Shimado M, Kitani K, Nagai T, & Kato R (1985a)
Age-related alteration in the activities of drug-metabolizing
enzymes and contents of sex-specific forms of cytochrome P450 in
liver microsomes from male and female rats. J Pharmacol Exp Ther,
233: 222-228.
Kamataki T, Shimada M, Maeda K, & Kato R (1985b) Pituitary
regulation of sex-specific forms of cytochrome P-450 in liver
microsomes of rats. Biochem Biophys Res Commun, 130: 1247-1253.
Kampmann JP & Hansen JEM (1979) Renal excretion of drugs. In: Crooks
J & Stevenson IH ed. Drugs and the elderly. Baltimore, Maryland,
University Park Press, pp 77-87.
Kanai S, Kitani K, Fujita S, & Kitagawa H (1985) The hepatic
handling of sulfobrompthalein in aging Fischer-344 rats: in vivo
and in vitro studies. Arch Gerontol Geriatr, 4: 73-85.
Kanai S, Kitani K, Sato Y, & Nokubo M (1988) The age-dependent
decline in the biliary transport maximum of conjugated
sulfobromophthalein in the rat. Arch Gerontol Geriatr, 7: 1-8.
Kane R (1987) Comprehensive assessment. In: Maddox GL ed. The
encyclopedia of aging. Berlin, Heidelberg, New York,
Springer-Verlag, pp 137-139.
Kannel WB (1981) Update on the role of cigarette smoking in coronary
artery disease (review). Am Heart J, 101: 319-328.
Kato H, Saito M, & Suda M (1980) Effect of starvation on the
circadian adrenocortical rhythm in rats. Endocrinology, 106:
918-921.
Katz ML & Robinson WG Jr (1987) Light and aging effects on vitamin E
in the retinal pigment epithelium. Vision Res, 27(11): 1875-1879.
Katzman R & Terry RD (1983) Normal aging in the nervous system. In:
Katzman R & Terry RD ed. The neurology of aging. Philadelphia,
Pennsylvania, F.A. Davis, pp 15-50.
Kaur S & Gill SS (1985) Age-related changes in the activities of
epoxide hydrolyases in different tissues of mice. Drug Metab Dispos,
13: 711-715.
Kaysen GA & Myers BD (1985) The aging kidney. Clin Geriatr Med,
1(1): 207-222.
Keast D & Ayre DJ (1981) Effects of chronic tabocco smoke exposure
on immune responses in aged mice. Arch Environ Health, 36: 201-207.
Kelley WW, Brief S, Westly HJ, Novakofski J, Bechtel PJ, Simon J, &
Walker EB (1986) GH3 pituitary adenoma cells can reverse thymic
aging in rats. Proc Natl Acad Sci (USA), 83: 5663-5667.
Kelso A & Munck A (1984) Glucocorticoid inhibition of lymphokine
secretion by alloreactive T lymphocyte clones. J Immunol, 133(2):
784-791.
Kim JCS & Kaminsky LS (1988) 2,2,2-Trifluoroethanol toxicity in aged
rats. Toxicol Pathol, 16: 35-45.
Kinon GA & Liu C (1973) Diurnal variation in plasma testosterone of
the male laboratory rat. Horm Metab Res, 5: 233-234.
Kitani K (1985) The role of the liver in pharmacokinetic and
pharmacodynamic alterations in the elderly. In: O'Malley K &
Waddington J ed. Therapeutics in the elderly. Amsterdam, Oxford, New
York, Elsevier Science Publishers, pp 19-34.
Kitani K (1988) Drugs and the ageing liver. Life Chem Rep, 6:
143-230.
Kitani K (1991) Aging of the liver: facts and theories. Arch
Gerontol Geriatr, 12: 133-154.
Kitani K, Masuda Y, Sato Y, Kanai S, & Nokubo M (1984) Increased
anti-convulsant activity in aging BDF1 mice. J Pharmacol Exp Ther,
229: 231-236.
Kitani K, Ohta M, Kanai S, & Sato Y (1985a) Sex difference in the
biliary excretion of digoxin and its metabolites in aging Wistar
rats. Arch Gerontol Geriatr, 4: 1-12.
Kitani K, Sato Y, Kanai S, Nokubo M, Ohta M, & Masuda Y (1985b)
Increased anticonvulsant effect of phenobarbital with age in mice -
a possible pharmacologic index for brain aging. Life Sci, 37:
1451-1460.
Kitani K, Sato Y, Kanai S, Nokubo M, Ohta M, Masuda Y, & Klotz U
(1986) Altered response to convulsants and anticonvulsants in aging
BDF1 mice. In: Kitani K ed. Liver and aging 1986: Liver and brain.
Amsterdam, Oxford, New York, Elsever Science Publishers, pp 293-307.
Kitani K, Sato Y, Kanai S, Nokubo M, Ohta M, & Masuda Y (1987)
Increasing anticonvulsant effect of AD-810 (Zonisamide) in aging
BDF1 mice. Life Sci, 41: 1339-1344.
Klatsky AL, Friedman GD, & Siegelaub AB (1981) Alcohol and
mortality. A ten year Kaiser-Permanente experience. Ann Intern Med,
95: 139-145.
Klawans HL & Tanner CM (1984) Movement disorders in the elderly. In:
Albert M ed. Clinical neurology of aging. Oxford, Oxford University
Press, pp 387-403.
Klotz U, Avant GR, Hoyumpa A, Schenker S, & Wilkinson GR (1975) The
effects of age and liver disease on the disposition and elimination
of diazepam in adult man. J Clin Invest, 55: 347.
Klug TL & Adelman RC (1977) Evidence for a large thyrotropin and its
accumulation during aging in rats. Biochem Biophys Res Commun, 77:
1431-1437.
Koch-Weser J, Greenblatt DJ, Seller EM, & Shoder RI (1982) Drug
disposition in old age. New Engl J Med, 306: 1081-1086.
Kohrs MB (1981) Nutritional needs of the aged. Nutrition, 7: 39-46.
Konishi N & Ward JM (1989) Increased levels of DNA synthesis in
hyperplastic renal tubules of aging nephropathy in female F344/NCR
rats. Vet Pathol, 26: 6-10
Koobs DH, Schultz RL, & Jutzy RV (1978) The origin of lipofuscin and
possible consequences to the myocardium. Arch Pathol Lab Med, 102:
66-68.
Kozararevic DJ, McGee D, Vojvodic N, Racic Z, Dawber T, Gordon T, &
Zukel W (1980) Frequency of alcohol consumption and morbidity and
mortality: The Yugoslavia cardiovascular disease study. Lancet, i:
613-616.
Krupka L & Vener A (1979) Hazards of drug use among the elderly.
Gerontologist, 19: 90-95.
Kubos C, Day NK, & Good RA (1984) Influence of early or late dietary
restirction on life span and immunological parameters in
MRL/MP-Ipr/Ipr mice. Proc Natl Acad Sci (USA), 81: 5831-5835.
Kumpuris D (1983) Gastrointestinal bleeding in the older patient.
In: Texter EC ed. The aging gut. New York, Masson Publishing Co., pp
57-63.
Kyle ME & Kocsis JJ (1985) The effect of age on salicylate-induced
nephrotoxicity in male rats. Toxicol Appl Pharmacol, 81: 337-347.
Lakatta EG (1980) Age-related alteration in the cardiovascular
response to adrenergic mediated stress. Fed Proc, 39: 3173-3177.
Lakatta EG (1985) Heart and circulation. In: Finch CE & Schneider EL
ed. Handbook of the biology of aging, 2nd ed. New York, Van Nostrand
Reinhold Company, pp 377-413.
Lakatta EG (1986) Diminished beta-adrenergic modulation of
cardiovascular function in advanced age. Cardiol Clin, 4(2):
185-200.
Lakatta EG (1987a) Cardiac muscle change in senescence. Annu Rev
Physiol, 49: 519-531.
Lakatta EG (1987b) Catecholamines and cardiovascular function in
aging. Endocrinol Metab Clin North Am, 16(4): 877-891.
Landauer MR, Tomlinson WT, Balster RL, & MacPhail RC (1984) Some
effects of the formamidine pesticide on the behavior of mice.
Neurotoxicology, 5: 91-100.
Leakey JEA, Cunny HC, Bazare J Jr, Webb PJ, Feuers RJ, Duffy PH, &
Hart RW (1989a) Effects of aging and caloric restriction on hepatic
drug metabolizing enzymes in the Fischer 344 rat. I: The cytochrome
P-450 dependent monooxygenase system. Mech Ageing Dev, 48: 145-155.
Leakey JEA, Cunny HC, Bazare J Jr, Webb PJ, Lipscomb JC, Siikkes W
Jr, Feuers RJ, Duffy PH, & Hart RW (1989b) Effects of aging and
caloric restriction on hepatic drug metabolizing enzymes in the
Fischer 344 rat. II. Effects on conjugating enzymes. Mech Ageing
Dev, 48: 157-166.
Lee KT ed. (1985) Atherosclerosis. Ann NY Acad Sci, 454: 1-327.
Lehmann AR, Hoeijmakers JHJ, Van Zealand AA, Backendorf CMP, Bridges
BA, Collins A, Fuchs RPD, Margison GP, Montesano R, Moustacchi E,
Natarajan AT, Radman M, Sarasin A, Seeberg E, Smith CA, Stefanini M,
Thompson LH, Van der Schans GP, Weber CA, & Zdzienicka MZ (1992)
Workshop on DNA repair. Mutat Res, 273: 1-28.
Leopold AC (1975) The biological significance of death in plants.
In: Benke JA, Finch CE, & Moment GB ed. The biology of aging. New
York, London, Plenum Press, pp 101-114.
Leppaluoto J, Ranta T, & Tuomisto J (1974) Diurnal variations of
serum immunoassayable thyrotropin (TSH) concentration in the rat.
Acta Physiol Scand, 90: 699-702.
Lesser GT, Deutsch S, & Markofsky J (1973) Aging in the rat:
Longitudinal and cross-sectional studies of body composition. Am J
Physiol, 225: 1472-1478.
Leto S, Kokkahen GC, & Barrows CH Jr (1976) Dietary protein, life
span and physiological variables in female mice. J Gerontol, 31:
149-154.
Levy ML (1987) Tous les pays du monde (1987). Bull Mens Inf Démogr
Econ Soc, 216:1-6.
Lewis VM, Twomey JJ, Bealmar P, Goldstein G, & Good RA (1978) Age,
thymic involution, and circulating thymic hormone activity. J Clin
Endocrinol Metab, 47: 145-150.
Li DD, Chien YK, Gu MZ, Richardson A, & Cheung HT (1988) The
age-related decline in interleukin-3 expression in mice. Life Sci,
43: 1215-1222.
Liebow AA (1964) Biochemical and structural changes in the aging
lung. Summary. In: Cander L & Moyer JH ed. Aging of the lung. New
York, London, Grune and Stratton, pp 97-104.
Liepa GU, Masoro EJ, Bertrand HA, & Yu BP (1980) Food restriction as
a modulation of age-related changes in serum lipids. Am J Physiol,
238: E253-E257.
Likhachev AJ (1985) Effect of age on DNA repair in carcinogenesis
due to alkylating agents. In: Likhachev A, Anisimov V, & Montesano R
ed. Age-related factors in carcinogenesis. Lyon, International
Agency for Research on Cancer, pp 239-246 (IARC Scientific
Publications No. 58).
Lin CF & Hayton WL (1983) GI motility and subepithelial blood flow
in mature and senescent rats. Age, 6: 46-51.
Lindeman RD (1986) The aging kidney. Compr Ther, 12(3): 43-49.
Lindeman RD, Tobin JD, & Shock NW (1987) Hypertension and the
kidney. Nephron, 47(1): 62-67
Lindsay R (1989) Osteoporosis: An updated approach to prevention and
management. Geriatrics, 44: 45-54.
Lipschitz DA (1987) Nutrition, aging and the immunohematopoietic
system. Clin Geriatr Med, 3(2): 319-328.
Loft S, Dossing M, & Poulsen HE (1988) Influence of age and
consumption of tobacco, alcohol, and caffeine on antipyrine
clearance. Hum Toxicol, 7: 277-280.
Loi CM & Vestal RE (1988) Drug metabolism in the elderly. Pharmacol
Ther, 36: 131-149.
Lucchi L, Memo M, Airaghi ML, Spano PF, & Trabucchi M (1981) Chronic
lead treatment induces in rat a specific and differential effect on
dopamine receptors in different brain areas. Brain Res, 213:
397-404.
Lutz RJ, Dedrick RL, Matthews HB, Elling TE, & Anderson MW (1977) A
preliminary pharmacokinetic model for several chlorinated biphenyls
in the rat. Drug Metab Dispos, 5: 386-396.
McCaffrey TA, Nicholson AG, Szabo PE, & Wexsler BB (1988) Aging and
atherosclerosis. The increased proliferation of arterial smooth
muscle cells isolated from old rats is associated with increased
platelet-derived growth factor-like activity. J Exp Med, 167:
163-174.
McCay CM, Crowell MM, & Mayrar LA (1935) The effect of reduced
growth upon the length of the life span and upon ultimate body size.
J Nutr, 10: 63-79.
McClure JE, Lameris N, Wara DW, & Goldstein AL (1982) Immunochemical
studies on thymosin: radioimmunoassay of thymosin alpha-1. J
Immunol, 128(1): 368-375.
McGinty D, Stern N, & Akshoomoff N (1988) Circadian and
sleep-related modulation of hormone levels changes with aging. In:
Sowers JR & Felicett JV ed. The endocrinology of aging. New York,
Raven Press, pp 75-111.
McKenna MJ & DiStefano V (1977) Carbon disulphide. II. A proposed
mechanism for the action of carbon disulfide on
dopamine-beta-hydroxylase. J Pharmacol Exp. Ther, 202: 253-266.
McMahon TF (1988) Age-related changes in colonic phase I and phase
II biotransformation: possible relationship to colon carcinogenesis.
Baltimore, University of Maryland, School of Pharmacy
(Dissertation).
McMahon TF & Birnbaum LS (1990a) Age-related changes in the
disposition and metabolism of xenobiotics. In: Cooper RL, Goldman
JM, & Harbin TJ ed. Aging and environmental toxicology. Baltimore,
Maryland, Johns Hopkins University Press, pp 7-30.
McMahon TF & Birnbaum LS (1990b) Age related change on toxicity and
biotransformation of potassium cyanide in male C57BL/6N mice.
Toxicol Appl Pharmacol, 105: 305-314.
McMahon TF, Beierschmitt WP, & Weiner M (1987) Changes in phase I
and phase II biotransformation pathways with age in male Fischer 344
rat colon: relationship to colon carcinogenesis. Cancer Lett, 36:
273-282.
McMahon TF, Diliberto JJ, & Birnbaum LS (1989) Age-related changes
in the disposition of benzyl acetate: A model compound for glycine
conjugation. Drug Metab Dispos, 17: 506-512.
McMahon TF, Diliberto JJ, & Birnbaum LS (l990a) Effects of age on
metabolism and disposition of salicylic acid (SAL) in male Fischer
344 rats. Drug Metab Dispos, 18: 494-503.
McMahon TF, Peggins JO, Centra MM, & Weiner M (l990b) Age-related
changes in biotransformation of azoxymethane and methylazoxymethanol
in vitro. Xenobiotica, 20: 501-513.
Maeda H, Gleiser CA, Masoro EJ, Murata I, McMohan CA, & Yu BP (1985)
Nutritional influences on aging of Fisher 344 rats. II. Pathology. J
Gerontol, 40: 671-688.
Magal E, Chaudhuri M, & Adelman RC (1986) The capability for
regulation of insulin secretion by somatostatin in purified
pancreatic islet B cells during aging. Mech Aging Dev, 33(2):
139-146.
Magnus K (1982) Trends in cancer incidence: Causes and practical
implications. Washington, DC, Hemisphere.
Mantel N & Schneiderman MA (1975) Estimation "safe" levels, a
hazardous undertaking. Cancer Res, 35: 1379-1386.
Mantere P, Hanninen H, Hernberg S, & Luukkonen R (1984). A
prospective follow-up study on psychological effects in workers
exposed to low levels of lead. Scand J Work Environ Health, 10:
43-50.
Marin R, Proverbio T, & Proverbio F (1985) Characterization of the
Na+, K+ -ATPase activity of basolateral membranes of kidney proximal
tubular cells from young and old rats. Biochem Pharmacol, 34:
4197-4201.
Marksberry WR, Ehmann WD, Hossain IM, Alauddin M, & Goodin DT (1981)
Instrumental neutron activation analysis of brain aluminum in
Alzheimer's disease and aging. Ann Neurol, 10: 511-516.
Marsh G (1980) Perceptual changes with aging. In: Busse EW & Blazer
DG ed. Handbook of geriatric psychiatry. New York, Van Nostrand
Reinhold Company, Chapter 7, pp 147-168.
Marshall JF & Berrios N (1979) Movement disorders of aged rats:
Reversal by dopamine receptor stimulation. Science, 206: 477-479.
Martin GM, Sprague CA, Norwood TH, & Pendergrass WR (1974) Clonal
selection, attenuation and differentiation in an in vitro model of
hyperplasia. Am J Pathol, 74: 137-154.
Marx JL (1987) A new wave of enzymes for cleaving pro-hormones.
Science, 235: 285-286.
Masoro EJ ed. (1981) CRC handbook of physiology in aging. Boca
Raton, Florida, CRC Press.
Masoro EJ (1985) Metabolism. In: Finch CE & Schneider EL ed.
Handbook of the biology of aging. New York, Van Nostrand Reinhold
Company, pp 540-563.
Masoro EJ (1988) Food restrictions in rabbits: an evolution of its
role in the study of aging. J Gerontol, 43: B59-B64.
Masoro EJ (1991) Use of rodents as models for the study of "normal
aging": Conceptual and practical issues. Neurobiol Aging, 12:
639-644.
Masoro EJ & Yu BP (1989) Diet and nephropathy. Lab Invest, 60:
165-167.
Masoro EJ, Yu BP, Bertrand HR, & Lynd FT (1980) Nutritional probe of
the aging process. Fed Proc, 39: 3178-3182.
Masoro EJ, Shimokawa I, & Yu BP (1991) Retardation of the aging
processes in rats by food restriction. Ann NY Acad Sci, 621:
337-352.
Master CL, Multhaup G, Simms G, Pottgieser J, Martins RN, &
Beyreuther K (1985) Neuronal origin of a cerebral amyloid:
Neurofibrillary tangles of Alzheimer's disease contain the same
protein as the amyloid of plaque cores and blood vessels. EMBO J, 4:
2757-2763.
Matulionis DH (1984) Chronic cigarette smoke inhalation and aging in
mice: Morphologic and functional lung abnormalities. Exp Lung Res,
7: 237-256.
Mauderly JL (1978) Effect of age on pulmonary structure and function
of immature and adult animals and man. Fed Proc, 38: 173-177.
Mauderly JL (1979a) Ventilation, lung volumes, and lung mechanics of
young adult and old Syrian hamsters. Exp Aging Res, 5: 497-508.
Mauderly JL (1979b) Effect of aging on pulmonary structure and
function of immature and adult animals and man. Fed Proc, 38:
173-177.
Mauderly JL (1982) The effect of age on respiratory function of
Fischer 344 rats. Exp Aging Res, 8: 31-36.
Mawhinney MG (1985) Etiological considerations for the growth of
stroma in benign prostatic hyperplasia. Fed Proc, 45: 2615-2617.
Mazzeo RS & Horvath SM (1987) A decline in myocardial and hepatic
norepinephrine turnover with age in Fischer 344 rats. Am J Physiol,
252: E762-E763.
Medvedev ZA (1984) Age changes of chromatin: A review. Mech Ageing
Dev, 28: 139-154.
Medvedev ZA (1990) An attempt at a rational classification of
theories of aging. Biol Rev, 65: 375-398.
Mehta T, Labuc GE, Urbanski SJ, & Archer MC (1984) Organ specificity
in the microsomal activation and toxicity of
N-nitrosomethylbenzylamine in various species. Cancer Res, 44:
4017-4022.
Meisami E (1988) Aging of the nervous system structural and
biochemical changes. In: Timiras PS ed. Physiological basis of aging
and geriatrics. New York, Macmillan Publishing Co, pp 123-143.
Mervis R (1981) Cytomorphological alterations in the aging animal
brain with emphasis on Golgi studies. In: Johnson JE ed. Aging and
cell structure. New York, London, Plenum Press, Chapter 4, pp
143-186.
Messing RB, Vasquez BJ, Spiehler VR, Martinez JL Jr, Jensen RA,
Rigter H, & McCaugh JL (1980) 3H-Dihydromorphine binding in brain
regions of young and aged rats. Life Sci, 26: 921-927.
Meyer BR & Bellucci A (1986) Renal function in the elderly. Cardiol
Clin, 4(2): 227-234.
Mezey E (1981) Alcohol and drug interactions in injury to the
digestive tract. Clin Gastroenterol, 10: 485-495.
Millard WJ, O'Sullivan DM, Fox TO, & Martin JB (1985) Sexually
dimorphic patterns of growth hormone secretion in rats. In: Crowley
WF Jr & Hofler JG ed. The episodic secretion of hormones. New York,
Chichester, Brisbane, Toronto, John Wiley and Sons, pp 287-304.
Miller SM (1989) Changes in endocrine function with aging. Clin Lab
Sci, 2(1): 19-20.
Miller R (1991) Aging and immune function. Int Rev Cytol, 124:
187-216.
Minaker KL, Meneilly GS, & Rowe JW (1985) Endocrine systems. In:
Finch CE & Schneider EL ed. Handbook of the biology of aging. New
York, Van Nostrand Reinhold Company, pp 433-456.
Mitsui Y, Sakagami H, Murota SI, & Yamada MA (1980) Age related
decline in histone H1 fraction in human diploid fibroblast cultures.
Exp Cell Res, 126: 289-298.
Miura K, Goldstein RS, Morgan DG, Pasino DA, Hewitt WR, & Hook JB
(1987) Age-related differences in susceptibility to renal ischemia
in rats. Toxicol Appl Pharmacol, 87: 284-296.
Moberg GP, Bellinger LL, & Mendel VE (1975) Effect of meal feeding
daily rhythms of plasma corticosterone and growth hormone in rat.
Neuroendocrinology, 19: 160-169.
Monteiro-Riviere NA, Banks YB, & Birnbaum LS (1991) Laser doppler
measurements of cutaneous blood flow in ageing mice and rats.
Toxicol Lett, 57: 329-338.
Montgomery PR & Sitar DS (1981) Increased serum salicylate
metabolites with age in patients receiving chronic acetylsalicylic
acid therapy. Gerontology, 27: 329-333.
Moolgavkar SH & Venzon DJ (1979) Two-event models for
carcinogenesis; incidence curves for childhood and adult tumors.
Math Biosci, 47: 55-77.
Mooradian AD & Song MK (1989) Age-related alterations in duodenal
calcium transport rate in rats. Mech Ageing Dev, 47: 221-227.
Moroni F, Lombardi G, Moneti G, & Aldinio C (1984) The excitotoxin
quinolinic acid is present in the brain of several mammals and its
cortical content increases during the aging process. Neurosci Lett,
47: 51-55.
Morell AG, Gregoriadis G, Scheinberg IH, Hickman J, & Ashwell G
(1971) The role of sialic acid in determining the survival of
glycoproteins in the circulation. J Biol Chem, 246: 1461-1467.
Morris JF, Mosk A, & Johnson LC (1971) Spirometric standards for
healthy non-smoking adults. Am Rev Respir Dis, 103: 57-67.
Mullaart E, Boerrigter METI, Lohman PHM, & Vijg J (1989) Age-related
induction and disappearance of carcinogen-DNA-adducts in livers of
rats exposed to low levels of 2-acetylaminofluorene. Chem-Biol
Interact, 69: 373-384.
Mullaart E, Lohman PHM, Berends F, & Vijk J (1990) Review DNA damage
metabolism and aging. Mutat Res, 237: 189-210.
Müller HF & Schwartz G (1978) Electroencephalograms and autopsy
findings in geropsychiatry. J Gerontol, 33: 504-513.
Muller WEG, Wenger R, Bachmann M, Ugarkovic D, Courtis NC, &
Schroder HC (1989) Poly(A) metabolism and aging: a current view.
Arch Gerontol Geriatr, 9: 31-250.
Munro HN (1984) Nutrition-related problems of middle age. Proc Nutr
Soc, 43: 281-288.
Murty CVR, Mancini MA, Chatterjee B, & Roy AK (1988a) Changes in
transcriptional activity and matrix association of alpha-2u-globulin
gene family in the rat liver during maturation and aging. Biochim
Biophys Acta, 949: 7-34.
Murty CVR, Olson MJ, Garg BD, & Roy AK (1988b) Hydrocarbon-induced
hyaline droplet nephropathy in male rats during senescence. Toxicol
Appl Pharmacol, 96: 380-392.
Mutti A, Falzoi M, Romanelli A, Bocchi MC, Ferroni C, & Franchini I
(1988) Brain dopamine as a target for solvent toxicity: Effects of
some monocyclic aromatic hydrocarbons. Toxicology, 49: 77-82.
Naessen R (1971) An enquiry on the morphological characteristics and
possible changes with age in the olfactory region of man. Acta
Otorhinol, 71: 49-62.
Nagel JE, Chopra RK, Chrest FJ, McCoy MT, Schneider EL, Holbrook NJ,
& Adler WH (1988) Decreased proliferation, interleukin 2 synthesis,
and interleukin 2 receptor expression are accompanied by decreased
mRNA expression in phytohemagglutinin-stimulated cells from elderly
donors. J Clin Invest, 81: 1096-1102.
Nagy K, ZS-Nagy V, Bertoni-Freddari C, & ZS-Nagy I (1983)
Alterations of the synaptosomal membrane 'microviscosity' in the
brain cortex of rats during aging and centrophenoxine treatment.
Arch Gerontol Geriatr, 2: 23-29.
Nakamura E, Kimura M, Nagata H, Miyao K, & Ozeki T (1982) Evaluation
of the progress of aging based on specific biological age as
estimated by various physiological functions. Jpn J Hyg, 36:
853-862.
NAS (1989) Biological markers in reproductive toxicology.
Washington, DC, National Academy of Sciences, National Academy
Press, pp 2-3.
Neafsey PJ (1990) Longevity hormesis. A review. Mech Ageing Dev, 51:
1-31.
Nebert DW & Gonzalez FJ (1987) P450 Genes - Structure, evolution and
regulation. Annu Rev Biochem, 56: 945-993.
Nebert DW, Nelson DR, Minor JC, Estabrook RW, Feyereisen R,
Fujii-Kuriyama Y, Gonzalez FJ, Guengerich FP, Gunsalus IC, Johnson
EF, Loper JC, Sato R, Waterman MR, & Waxman DR (1991) The P450
superfamily: Uptake on new sequences, gene mapping, and recommended
nomenclature. DNA Cell Biol, 10(1): 1-14.
Needleman HL, Gunnoe C, Leviton A, Reed R, Peresie H, Maher C, &
Barrett P (1979) Deficits in psychologic and classroom performance
of children with elevated dentine lead levels. New Engl J Med, 300:
689-695.
Neuhaus OW & Flory W (1978) Age-dependent changes in the excretion
of urinary proteins by the rat. Nephron, 22: 570-576.
Newaz SN, Fang W, & Strobel HW (1983) Metabolism of the carcinogen
1,2-dimethylhydrazine by isolated human colon microsomes and human
colon tumor cells in culture. Cancer, 52: 794-798.
Niedzwiecki A, Lewis PN, & Cinader B (1985) Changes of histone H1
subtypes with aging in strains of mice that possess different
immunological characteristics. J Gerontol, 40: 695-699.
Nokubo M & Kitani K (1988) Age-dependent decrease in the lethal
threshold of pentylenetetrazole in mice. Life Sci, 43: 41-47.
Norwood TH & Smith JR (1985) The cultured fibroblast-like cells as a
model for the study of aging. In: Fincht CE & Schneider EL ed. The
biology of aging, 2nd ed. New York, Van Nostrand Reinhold Company,
pp 291-321.
Novak LP (1972) Aging, total potassium, fat free mass and cell mass
in males and females between the ages of 18 and 85 years. J
Gerontol, 27: 438-443.
Oh SH, Deagen JT, Whanger PD, & Wesnig PH (1978) Biological function
of metallothionein. V. Its induction in rats by various stresses. Am
J Physiol, 234: E282-E285.
Ohta M, Kanai S, Sato Y, & Kitani K (1988) Age-dependent decrease in
the hepatic uptake and biliary excretion of ouabain in rats. Biochem
Pharmacol, 37: 935-942.
O'Malley K, Docherty JR, & Kelly GJ (1988) Adrenoceptor status and
cardiovascular function in ageing. J Hypertension, 6(suppl 10):
S59-S62.
O'Meara AR & Pochron SF (1979) Age-related effects on the
incorporation of acetate into rat liver histones. Biochim Biophys
Acta, 586: 391-401.
Onasaka S & Cherian MG (1981) The induced synthesis of
metallothionein in various tissues of rats in response to metals. I.
Effect of repeated injection of cadmium salts. Toxicology, 22:
91-101.
Os I, Kjeldsen SE, & Westheim A (1987) Aging and urinary vasopressin
excretion in healthy men. Scand J Urol Nephrol, 21(3): 235-239.
OECD (1986) Existing chemicals systematic investigation, priority
setting and chemical reviews. Paris, Organisation for Economic
Co-operation and Development.
Owen RA & Heywood R (1986) Age-related variations in renal structure
and function in Sprague-Dawley rats. Toxicol Pathol, 14(2): 158-167.
Pagano GN, Casadar N, Diana A, Psue E, Bozzo C, Ferrero F, & Lenti G
(1981) Insulin resistance in the aged: the role of peripheral
insulin receptors. Metabolism, 30: 46-49.
Pahlavani MA, Cheung HT, Cai NS, & Richardson A (1988) Influence of
dietary restriction and aging on gene expression in the immune
system of rats. In: Biomedical advances in aging. New York, London,
Plenum Press, pp 259-270.
Pakin YV & Hrisanov SM (1984) Critical analysis of the applicability
of the Compertz-Makeham law in human population. Gerontology, 30:
8-12.
Paramonova GI & Dovgi AI (1987) Induction of multiple forms of
cytochrome P450 of rat liver during aging. In: Abstracts of the All
Union Conference on Cytochrome P450 and the Environment,
Novosibirsk, 27-31 July 1987. Novosibirsk, USSR Academy of Medical
Sciences, Siberian Division, pp 100-101.
Patel DJ & Vaishnaw RN (1980) Basic hemodynamics and its role in
disease process. Baltimore, Maryland, University Park Press.
Pedigo NW, Schoemaker H, Morellia M, McDougal JN, Malisck JB, Burks
TF, & Yamamura TF (1981) Benzodiazepine receptor binding in young,
mature, and senescent rat brain and kidney. Neurobiol Aging, 2:
83-88.
Penn A, Batastini G, Soloman J, Burns F, & Albert R. (1981)
Dose-dependent size increases of aortic lesions following chronic
exposure to 7,12-dimethylbenz(a)-anthracene. Cancer Res, 71:
588-592.
Penzes L (1974) Intestinal absorption of glycine, L-alanine, and
L-leucine in the old rat. Exp Gerontol, 8: 245-252.
Penzes, L.S. (1984) Intestinal response in aging: changes in reserve
capacity. Acta Med Hung, 41(4): 263-277.
Perkins MN & Stone TW (1983) Pharmacology and regional variations of
quinolinic acid-evoked excitations in the rat central nervous
system. J Pharmacol Exp Ther, 226(2): 551-557.
Perl DP & Brody AR (1980) Alzheimer's disease: X-ray spectrometric
evidence of aluminum accumulation in neurofibrillary tangle-bearing
neurons. Science, 208: 297-299.
Peterson DD, Pack AI, Silage DA, & Fishman AP (1981) Effects of
aging on ventilatory and occlusion pressure responses to hypoxia and
hypercapnia. Am Rev Respir Dis, 124: 387-391.
Peto R (1986) Influence of dose and duration of smoking on lung
cancer rates. In: Zaridze D & Peto R ed. Tobacco: A major
international health hazard. Lyon, International Agency for Research
on Cancer, pp 23-33 (IARC Scientific Publications No. 74).
Peto R, Parish SE, & Gray RG (1975) Cancer and aging in mice and
men. Br J Cancer, 32: 411-426.
Peto R, Pike MC, Day NE, Gray RE, Lee PN, Parish S, Poto J, Richard
S, & Wahrendorf J (1980) Guidelines for simple, sensitive
significance tests for carcinogenic effects in long-term animal
experiments. In: Long-term and short-term screening assays for
carcinogens: A critical appraisal. Lyon, International Agency for
Research on Cancer, pp 311-426 (IARC Monographs on the Evaluation of
the Carcinogenic Risk of Chemicals to Humans, Supplement 2).
Peto R, Parish SE, & Gray RG (1985) There is no such thing as aging,
and cancer is not related to it. In: Likhachev AJ, Anisimov V, &
Montesano R ed. Age-related factors in carcinogenesis. Lyon,
International Agency for Research on Cancer, pp 43-53 (IARC
Scientific Publications No. 58).
Pierpaoli W, Dall'Ara A, Pedrinis E, & Regelson W (1991) The pineal
control of aging. The effects of melatonin and pineal grafting on
the survival of older mice. Ann NY Acad Sci, 621: 291-313.
Piikivi L, Hanninen H, Martelin T, & Mantere P (1984) Psychological
performance and long-term exposure to mercury vapors. Scand J Work
Environ Health, 10: 35-41.
Pines A, Cucos B, Ever-Hadani P, Ron M, & Lemesch C (1987) Changes
in pattern of organochlorine residues in blood of general Israeli
population, 1975-1986. Sci Total Environ, 66: 115-125.
Piva F, Limonta P, Maggi R, Dondi D, Messi E, Yanisi M, & Motta M
(1987) Aging and the hypothylamo-pituitery-testiculas axis in the
rat. J Steroid Biochem, 27(4-6): 707-712.
Poland A & Knutson JC (1982) 2,3,7,8,-Tetrachlorodibenzo-p-dioxin
and related halogenated aromatic hydrocarbons: examinations of the
mechanism of toxicity. Annu Rev Pharmacol Toxicol, 22: 517-554.
Popper H (1986) Aging in the liver. In: Popper H ed. Progress in
liver diseases. New York, London, Grune & Stratton, vol VIII, pp
659-683.
Posner J, Danhof M, Teunissen MWE, Breimer DD, & Whiteman PD (1987)
The disposition of antipyrine and its metabolites in young and
elderly healthy volunteers. Br J Clin Pharmacol, 24: 51-55.
Post DJ, Carter K, & Papaconstantinou J (1991) The effect of aging
on constitutive mRNA levels and lipopolysaccharide inducibility of
acute phase genes. Ann NY Acad Sci, 621: 66-77.
Prinz PN, Weitzman ED, Cunningham GR, & Karacan I (1983) Plasma
growth hormone during sleep in young and aged men. J Gerontol, 38:
519-534.
Provinciali M & Fabris H (1990) Role of pituitary-thyroid axis on
basal and lymphokine-induced NK cell activity in aging. Int J
Neurosci, 51: 373-375.
Rabovsky, J, Marion KJ, Groseclose RD, Lewis TR, & Peterson M (1984)
Low dose chronic inhalation of diesel exhaust and/or coal dust by
rats: effect of age and exposure on lung and liver cytochrome P450.
Xenobiotica, 14: 595-598.
Rao GN, Haseman JK, Grumbein S, Crawford DD, & Eustis SL (1990)
Growth, body weight, survival, and tumor trends in F344/n rats
during an eleven-year period. Toxicol Pathol, 18: 61-70.
Rath PC & Kanungo MS (1989) Age-related changes in the expression of
cytochrome p-450 (b+e) gene in the rat after phenobarbitone
administration. Biochem Biophys Res Commun, 157: 1403-1409.
Reff ME & Schneider EL ed. (1982) Biological markers of aging.
Washington, DC, US Government Printing Office, 252 pp (NIH
Publication No. 82-2221).
Regelson W (1983) Biomarkers in aging. In: Regelson W & Marott Sinex
F ed. Intervention in the aging process. New York, Alan R. Liss,
Inc., pp 3-98.
Rehnberg GL, Goldman JM, Cooper RL, Hein JF, McElroy WK, Booth KC, &
Gray LE (1988a) Effect of linuron on the brain-pituitary-testicular
reproductive axis in the rat. Toxicologist, 8: 121.
Rehnberg GL, Linder R, Goldman JM, Hein JF, McElroy WK, & Cooper RL
(1988b) Changes in testicular and serum hormone concentrations in
the male rat following treatment with m-dinitrobenzene. Toxicol Appl
Pharmacol, 95: 255-264.
Reidenberg MM (1980) Drugs in the elderly. Bull NY Acad Med, 56:
703-714. Reif AE (1981) Effect of cigarette smoking on
susceptibility to lung cancer. Oncology, 38: 76-85.
Reis W & Poethig D (1984) Chronological and biological age. Exp
Gerontol, 19: 211-216.
Reiter RJ (1986) The pineal gland: an important link to the
environment. News Physiol Sci, 1: 202-205.
Reitz RH, McDougal JN, Himmelstein MW, Nolan RJ, & Schumann AM
(1988) Physiologically based pharmacokinetics modeling with methyl
chloroform: implications for interspecies, high dose/low dose, and
dose route extrapolations. Toxicol Appl Pharmacol, 95: 185-199.
Revskoy SY, Poroshina TE, Kovaleva IG, Berstein LM, Ostroumova MN, &
Dilman VM (1985) Age-dependent metabolic immunodepression and
cancer. In: Likhachev A, Anisimov V, & Montesano R ed. Age-related
factors in carcinogenesis. Lyon, International Agency for Research
on Cancer, pp 253-259 (IARC Scientific Publications No. 58).
Richardson A & Birchenall-Sparks MC (1983) Age-related changes in
protein synthesis. Rev Biol Res Aging, 1: 255-273.
Richardson A & Semsei I (1987) Effect of aging on translation and
transcription. In: Review of biological research in aging. New York,
Alan R. Liss, Inc., vol 3, pp 467-483.
Richardson A, Birchenall-Sparks MC, & Staecker JL (1983) Aging and
transcription. In: Review of biological research in aging. New York,
Alan R. Liss, Inc., vol 2, pp 275-294.
Richardson A, Butler JA, Rutherford MS, Semsei I, Gu MZ, Fernandes
G, & Chiang WH (1987) Effect of age and dietary restriction on the
expression of alpha-2u-globulin. J Biol Chem, 262: 12821-12825.
Richardson A, Roberts M, & Rutherford M (1985) Aging and gene
expression. In: Review of biological research in aging. New York,
Alan R. Liss, Inc., vol 2, pp 395-419.
Riegel GD & Miller AE (1981) Aging effect on hormone concentrations
and actions. In: Florini JR ed. Handbook of biochemistry in aging.
Boca Raton, Florida, CRC Press, pp 231-256.
Riggs BL (1987) Pathogenesis of osteoporosis. Am J Obstet Gynecol,
156: 1342-1346.
Riggs BL & Melton LJ III (1983) Evidence for two distinct syndromes
of involutional osteoporosis. Am J Med, 75: 899-901.
Rikans LE (1984) Influence of aging on the susceptibility of rats to
hepatotoxic injury. Toxicol Appl Pharmacol, 73: 243-249.
Rikans LE (1989a) Hepatic drug metabolism in female Fischer rats as
a function of age. Drug Metab Dispos, 17: 114-116.
Rikans L (1989b) Effects of ethanol on microsomal drug metabolism in
aging female rats. I. Induction. Mech Ageing Dev, 48: 267-280.
Rikans LE & Moore DR (1987) Effect of age and sex on allyl alcohol
hepatotoxicity in rats: role of liver alcohol and aldehyde
dehydrogenase activities. J Pharmacol Exp Ther, 243: 20-26.
Rikans LE & Moore DR (1988) Effect of aging on aqueous phase
antioxidents in tissues of male Fischer rats. Biochem Biophys Acta,
966: 269-275.
Rikans LE & Snowden CD (1989) Influence of aging on acute ethanol
hepatotoxicity in female rats. Pharmacologist, 31: 177.
Ritschel WA (1983) Pharmacokinetics in the aged. In: Pagliaro LA &
Pagliaro AM ed. Pharmacologic aspects of aging. St. Louis, Missouri,
C.V. Mosby & Co., pp 219-256.
Ritschel WA (1988) Gerontokinetics: The pharmacokinetics of drugs in
the elderly. Caldwell, New Jersey, Telford Press, 114 pp.
Robertson IGC & Birnbaum LS (1982) Age-related changes in mutagen
activation by rat tissues. Chem-Biol Interact, 38: 243-252.
Robertson DRC, Waller DG, Renwick AG, & George CF (1988) Age-related
changes in the pharmacokinetics and pharmacodynamic of nifedipine.
Br J Clin Pharmacol, 25: 297-305.
Rodgers J & Gass G (1983) The effect of age on serum proteins in
mice. Exp Gerontol, 14: 169-173.
Roe DA (1983) Drugs and nutrition in the elderly. In: Geriatric
nutrition, 2nd ed. Englewood, New Jersey, Prentice-Hall, Inc., pp
176-200.
Rogers J & Bloom FE (1985) Neurotransmitter metabolism and function
in the aging central nervous system. In: Finch CE & Schneider EL ed.
Handbook of the biology of aging, 2nd ed. New York, Van Nostrand
Reinhold Company, pp 645-691.
Rogers GS & Gilchrest BA (1990) The senile epidermis: environmental
influences on skin ageing and cutaneous carcinogenesis. Br J
Dermatol, 122(suppl 35): 55-60.
Rogers J, Magistretti PJ, & Bolis LC (1991) Animal models for aging
research. Neurobiol Aging, 12(spec issue): 625-701.
Rose MR (1991) Evolutionary biology of aging. New York, London,
Oxford University Press.
Rosenberger RF & Kirkwood TBL (1986) Errors and the integrity of
genetic information transfer. In: Kirkwood TBL, Rosenberger RF, &
Galas DJ, ed. Accuracy in molecular processes. London, Chapman and
Hall, pp 17-35.
Ross MH (1961) Length of life and nutrition in rat. J Nutr, 75:
197-210.
Ross MH (1976) Nutrition and longevity in experimental animals. In:
Winick M ed. Nutrition and aging. New York, Chichester, Brisbane,
Toronto, John Wiley and Sons, pp 23-41.
Ross R (1981) Atherosclerosis: A problem of the biology of arterial
wall cells and their interactions with blood components.
Arteriosclerosis, 1: 293-311.
Roth GS (1979a) Hormone action during aging: alterations and
mechanisms. Mech Ageing Dev, 9: 497-514.
Roth GS (1979b) Hormone receptor changes during adulthood and
senscence: significance for aging research. Fed Proc, 38: 1910-1914.
Roth GS (1988) Receptor and postreceptor changes during lymphocyte
aging. In: Platt D ed. Blood cells, rheology, and aging. Berlin,
Heidelberg, New York, Springer-Verlag, pp 150-154.
Roth GS & Joseph JA (1988) Peculiarities of the effect of hormones
and transmitters during aging: Modulation of changes in dopaminergic
action. Gerontology, 34: 22-28.
Rothstein M & Seifert SC (1981) RNA synthesis. In: Handbook of
biochemistry in aging. Boca Raton, Florida, CRC Press, pp 51-63.
Rouser G, Kritchevsky G, & Yamamoto A (1972) Lipids in the nervous
system of different species as a function of age: Brain, spinal
cord, peripheral nerve, purified whole cell preparations and
sub-cellular particulates: Regulatory mechanisms and membrane
structure. Adv Lipid Res, 10: 261-360.
Rowe JW, Minaker KL, Paollotta J, & Fliers JS (1983)
Characterization of the insulin resistance of aging. J Clin Invest,
71: 1581-1587.
Roy AK, Nath TS, Motwani NM, & Chatterjee B (1983) Age dependent
regulation of the polymorphic forms of alpha2u-globulin. J Biol
Chem, 258: 10123-10127.
Rudman D (1987) Assessment of nutritional status. In: Braunwald E,
Isselbacher KJ, Petersdorf RG, Wilson JD, Martin JB, & Fauci AS ed.
Harrison's principles of internal medicine, 11th ed. New York,
McGraw-Hill, Chapter 71, pp 309-392.
Rudman D (1988) Kidney senescence: A model for aging. Nutr Rev,
46(6): 209-214.
Runcie J (1985) Obesity. In: Pathy MWSJ ed. Principles and practice
of geriatric medicine. New York, Chichester, Brisbane, Toronto, John
Wiley and Sons, Chapter 22.1, pp 277-284.
Russell DH, Buckley AR, Shah GN, Sipes IG, Blask DE, & Benson B
(1988) Hypothalamic site of action of
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Toxicol Appl Pharmacol,
94: 496-502.
Sacher GA (1977) Life table modification and life prolongation. In:
Finch CE & Hayflick L ed. Handbook of the biology of aging. New
York, Van Nostrand Reinhold Company, pp 582-638.
Sagiv M, Goldhammer E, Abinader EG, & Rudoy J (1988) Aging and the
effect of increased after-load on left ventricular contractile
state. Med Sci Sports Exercise, 20(3): 281-284.
Sample PA, Esterson FD, Weinreb RN, & Boynton RM (1988) The aging
lens: in vivo assessement of light absorption in 84 human eyes.
Invest Ophtalmol Visual Sci, 29(8): 1306-1311.
Sapolsky R, Armanini M, Packan D, & Tornbaugn G (1987) Stress and
glucocorticoids in aging. Endocrinol Metab Clin, 16(4): 965-979.
Sapolsky RM, Krey LC, & McEwen BS (1986) The adrenocortical axis in
the aged rat: Impaired sensitivity to both fast and delayed feedback
inhibition. Neurobiol Aging, 7: 331-335.
Sato Y, Kanai S, & Kitani K (1987) Biliary excretion of ouabain in
aging male and female F344 rats. Arch Gerontol Geriatr, 6: 141-152.
Saxton JA Jr & Kimball GC (1941) Relation to nephrosis and other
diseases of albino rat to age and to modifications of diet. Arch
Pathol, 32: 951-965.
Scheibel AB & Tomiyasu U (1978) Dendritic sprouting in Alzheimer
presenile dementia. Exp Neurol, 60: 1-8.
Schiff I & Wilson E (1979) Clinical aspects of aging of the female
reproductive system. Aging, 4: 10-28.
Schmucker D (1979) Age-related changes in drug disposition. Pharm
Rev, 30: 445-456.
Schmucker DL & Wang RK (1979) Rat lysosomal enzymes: effects of
animal age and phenobarbital. Age Aging, 2: 93-96.
Schmucker DL & Wang RK (1989) Dietary restriction postpones the
age-dependent compromise of male rat liver microsomal
monooxygenases. Prog Clin Biol Res, 287: 283-288.
Schmucker DL, Woodhouse KW, Wang RK, Wynne H, James QF, McManus M, &
Kremers P (1990) Effects of age and gender on in vitro properties
of human liver microsomal monoxygenases. Clin Pharmacol Ther, 48:
365-377.
Schneider EL (1978) The aging reproductive system. New York, Raven
Press, vol 4.
Schneider EL & Reed JD (1985) Life extension. New Engl J Med, 312:
1139-1168.
Schneider EL, Sternberg H, Tice RR, Senula GC, Kra D, Smith JR, &
Bynum G (1979) Cellular replication and aging. Mech Ageing Dev, 9:
313-324.
Schuetz EG, Wrighton SA, Barwick JL, & Guzelian PS (1984) Induction
of cytochrome P-450 by glucocorticoids in rat liver. I. Evidence
that glucocorticoids and pregnenolone 16 alpha-carbonitrile regulate
de novo synthesis of a common form of cytochrome P-450 in cultures
of adult rat hepatocytes and in the liver in vivo. J Biol Chem, 259:
1999-2006.
Schumann AM, Fox TR, & Watanabe PG (1982a) A comparison of the fate
of inhaled methyl chloroform (1,1,1-trichloroethane) following
single or repeated exposure in rats and mice. Fundam Appl Toxicol,
2: 27-32.
Schumann AM, Fox TR, & Watanabe PG (1982b) Methylchloroform
(1,1,1-trichloroethane): Pharmacokinetics in rats and mice following
inhalation exposure. Toxicol Appl Pharmacol, 62: 390-401.
Schuurman HJ, Krajnc-Franken MAM, Kuper CF, Van Loveren H, & Vos JG
(1990) Immune system. In: Haschek-Hoch WM & Rousseaux CG ed.
Fundamentals of toxicologic pathology. New York, London, San
Francisco, Academic Press, pp 1-103.
Searle CE (1976) Chemical carcinogens. Washington, DC, American
Chemical Society (ACS Monograph No. 173)
Segre D, Miller RA, Abraham GN, Weigle WO, & Warner HR (1989) Aging
and the immune system. J Gerontol, 44(6): 8164-8168.
Sekuler R, Kline D, & Dismuskes K (1982) Aging and human visual
functions. New York, Alan R. Liss, Inc.
Sellers EM, Frecker RC, & Romach MK (1983) Drug metabolism in the
elderly: confounding of age, smoking, and ethanol effects. Drug
Metab Rev, 14: 225-250.
Selmanowitz VJ, Rizer RL, & Orentreich N (1977) Aging of the skin
and its appendages. In: Finch CE & Hayflick L ed. Handbook of the
biology of aging. New York, Van Nostrand Reinhold Company, pp
496-509.
Selye H (1950) Stress: The physiology and pathology of exposure to
stress. Montreal, Acta Inc.
Semsei I, Rao G, & Richardson A (1989) Changes in the expression of
superoxide dismutase and catalase as a function of age and dietary
restriction. Biochem Biophys Res Commun, 164: 620-625.
Senda S, Cheng E, & Kawanishi H (1989) IgG in murine intestinal
secretions. Aging effect and possible physiological role. Scand J
Immunol, 29(1): 41-47.
Shen DX, Ren R, Zhao YR, & Ma XZ (1987) [Disease pattern analysis in
hospitalized elderly patients in Jingzhou.] Chin J Geriatr, 6: 44
(in Chinese).
Shepherd AMM, Wilson N, & Stevenson IH (1979) Warfarin sensitivity
in the elderly. In: Crooks J & Stevenson IH ed. Drugs and the
elderly. Baltimore, Maryland, University Park Press, pp 199-209.
Sherlock S, Bearn AG, Billing BH, & Paterson JCS (1955) Splanchnic
blood flow of peripheral plasma bromosulphthalein method: The
relation of peripheral bromosulphthalein level to the calculated
flow. J Lab Clin Med, 35: 923-932.
Shih JC & Young H (1978) The alteration of serotonin binding sites
in aged human brain. Life Sci, 23: 1441-1448.
Simmons DL, McQuiddy P, & Kasper CB (1987) Induction of the hepatic
mixed-function oxidase system by synthetic glucocorticoids -
Transcriptional and post-transcriptional regulation. J Biol Chem,
262: 326-332.
Slagboom PE & Vijg J (1989) Genetic instability and aging: theories,
facts, and future perspectives. Genome, 31: 373-385.
Smith DBD, Thompson LW, & Michalewski HJ (1980) Average evoked
potential research in adult aging - status and prospects. In: Aging
in the 1980s. Washington, DC, American Psychological Association, pp
135-151.
Smith EL, Sempos CT, & Purvis RW (1981) Bone mass and strengh
decline with age. In: Smith EL & Serfass RC ed. Exercise and aging:
the scientific basis. Hillside, New Jersey, Enslow Publishers, pp.
Sonntag WE & Gough ME (1988) Growth hormone releasing hormone
induced release on growth hormone in aging male rats: dependence on
pharmacological manipulation and endogenous somatostatin release.
Neuroendocrinology, 47: 482-488.
Sonntag WE, Steger RW, Forman LJ, & Meites J (1980) Decreased
pulsatile release of growth hormone in old male rats. Endocrinology,
107: 1875-1879.
Sonntag WE, Hylka V, & Meites J (1985) Growth hormone restores
protein synthesis in skeletal muscle of old rats. J Gerontol, 40:
689-694.
Spagnoli LG, Orlandi A, Manuello A, Santeusanio G, Angelis C,
Lucreziotti R, & Ramacci MT (1991) Aging and atherosclerosis in the
rabbit 1. Distribution, prevalence and morphology of atherosclerotic
lesions. Atherosclerosis, 89: 11-24.
Spearman ME & Leibman KC (1983) Hepatic and pulmonary cytosolic
metabolism of epoxides: effects of aging on conjugation with
glutathione. Life Sci, 33: 2615-2625.
Spearman ME & Leibman KC (1984) Aging selectivity alters
glutathione-S-transferase isozyme concentrations in liver and lung
cytosol. Drug Metab Dispos, 12: 661-671.
Speijers GJA (1983) [Toxic effects of rapeseed oil, erucic acid and
linolenic acid in the rat.] Utrecht, The Netherlands, University of
Utrecht, pp 1-143 (in Dutch).
Speijers GJA (1989a) [Literature review on experimental
atherosclerosis research.] Bilthoven, The Netherlands, National
Institute Public Health and Environmental Protection (in Dutch).
Speijers GJA (1989b) [Toxicological studies of ergot alkaloids.] De
Ware(n) Chem, 19(3): 189-194 (in Dutch).
Spencer PS & Ochoa J (1981) The mammalian peripheral nervous system
in old age. In: Johnson GJ Jr ed. Aging and cell structure. New
York, London, Plenum Press, pp 35-103.
Spencer PS, Nunn PB, Hugon J, Ludolph AC, Ross SM, Roy DN, &
Robertson RC (1987). Guam amyotrophic lateral
sclerosis-Parkinsonism-Dementia linked to a plant excitant
neurotoxin. Science, 237: 465-466.
Steiner RA, Bremner WJ, Clifton DK, & Dorsa DM (1984) Reduced
pulsatile luteinizing hormone and testosterone secretion in the male
rat. Biol Reprod, 31: 251-258.
Stenbäck F, Peto R, & Shubik P (1981) Initiation and promotion at
different age and doses in 2200 mice: III. Linear extrapolation from
high doses may underestimate low-dose tumor risk. Br J Cancer, 44:
23-34.
Stevenson IH & Hosie J (1985) Pharmacokinetics in the elderly. In:
O'Malley K & Waddington JL ed. Therapeutics in the elderly.
Amsterdam, Oxford, New York, Elsevier Science Publishers, pp 35-41.
Stevenson IH, Salem SAM, & Shepherd AMM (1979) Studies on drug
absorption and metabolism in the elderly. In: Crooks J & Stevenson
IH ed. Drugs and the elderly. Baltimore, Maryland, University Park
Press, pp 51-63.
Stevenson JC, Whitehead MI, Padwick M, Endacott JA, Suttonm C, Banks
LM, Freemantle C, Spinks TJ, & Hesp R (1988) Dietary intake of
calcium and postmenopausal bone loss. Br Med J, 297: 15-17.
Stiles J & Tyler WS (1988) Age-related morphometric differences in
responses of rat lungs to ozone. Toxicol Appl Pharmacol, 92:
274-285.
Stohs SJ, Al-Turk WA, & Angle CR (1982) Glutathione-S-transferase
and glutathione reductase activities in hepatic and extra-hepatic
tissues of female mice as a function of age. Biochem Pharmacol, 31:
2113-2116.
Strong R, Moore MA, Hale C, Wessels-Reiker M, Armbrecht HJ, &
Richardson A (1990) Modulation of tyrosine hydroxylase gene
expression in the rat adrenal gland by age and reserpine. Brain Res,
525(1): 126-132.
Sun J & Strobel HW (1986) Aging affects the drug metabolism systems
of rat liver, kidney, colon, and lung in a differential fashion. Exp
Gerontol, 21: 523-534.
Sun J, Lau PO, & Strobel HW (1986) Aging modifies the expression of
hepatic microsomal cytochrome P450 after pretreatment of rats with
b-naphoflavone or phenobarbital. Exp Gerontol, 21: 65-73.
Sweeny DJ & Weiner M (1985) Metabolism of acetaminophen in
hepatocytes isolated from mice and rats of various ages. Drug Metab
Dispos, 13: 377-379.
Sweeny DJ & Weiner M (1986) Effect of aging on the metabolism of
p-nitroanisole and p-nitrophenyl in isolated rat hepatocytes. Age,
9: 95-98.
Swenberg GJA, Richardson FC, Boucheron JA, Deal FH, Belinsky SA,
Charbonneau M, & Short BG (1987) High- to low-dose extrapolation:
critical determinants involved in the dose response of carcinogenic
substances. Environ Health Perspect, 76: 57-63.
Swift CG (1985) Benzodiazepine pharmacodynamics in the elderly. In:
O'Malley K & Stevenson IL ed. Therapeutics in the elderly.
Amsterdam, Oxford, New York, Elsevier Science Publishers, pp 77-90.
Tannenbaum SR, Fett D, Young VR, Land PD, & Bruce WR (1978) Nitrite
and nitrate are formed by endogenous synthesis in the human
intestine. Science, 200: 1478-1489.
Tarloff JB, Goldstein RS, Milo BA, & Hook JB (1989a) Role of
pharmacokinetics and metabolism in the enhanced susceptibility of
middle-aged Sprague-Dawley rats to acetaminophen nephrotoxicity.
Drug Metab Dispos, 17: 139-146.
Tarloff JB, Goldstein RS, Morgan DG, & Hook JB (1989b) Acetaminophen
and p-aminophenol nephrotoxicity in aging male Sprague-Dawley and
Fischer 344 rats. Fundam Appl Toxicol, 12: 78-91.
Taylor A (1989) Associations between nutrition and cataract. Nutr
Res, 47: 225-234.
Telford N, Mobbs CV, Osterburg HH, & Finch CE (1988) Alterations in
hypothalamic serotonergic-catecholaminergic relationships in aging
C57BL/6J female mice. Exp Gerontol, 23: 481-489.
Tenover JS, Matsumoto AM, Clifton DK, & Bremner WJ (1988)
Age-related alterations in the circadian rhythms of pulsatile
luteinizing hormone and testosterone secretion in healthy men. J
Gerontol, 43: M163-M169.
Terry RD (1963) The fine structure of neurofibrillary tangles in
Alzheimer's disease. J Neuropathol Exp Neurol, 22: 269-292.
Thakur MD (1984) Age-related changes in the structure and function
of chromatin: A review. Mech Ageing Dev, 27: 263-286.
Thakur MK (1988) Molecular mechanism of steroid hormone action
during aging. A review. Mech Ageing Dev, 45: 93-110.
Thompson JS, Robbins J, & Cooper JK (1987) Nutrition and immune
function in the geriatric population. Clin Geriatr Med, 3(2):
309-317.
Tice RR & Setlow RB (1985) DNA repair and replication in aging
organisms and cells. In: Finch CE & Schneider EL ed. Handbook of the
biology of aging, 2nd ed. New York, Van Nostrand Reinhold Company,
pp 173-224.
Timiras PS, Hudson DB, & Segall PE (1984) Lifetime brain serotonin:
Regional effects of age and precursor availability. Neurobiol Aging,
5: 235-242.
Tomlinson BE & Irving D (1977) The numbers of limb motor neurons in
the human lumbosacral cord throughout life. J Neurol Sci, 34:
213-219.
Travaglini P, Mocchegiani E, Togni E, Muratori M, Re T, Bazzani N, &
Fabris N (1990) Thymulin and zinc circulating level in patient with
GH and PRL secreting pituitary adenomas. Int J Neurosci, 51:
269-271.
Travis CC, White RC, & Ward RC (1990) Interspecies extrapolation of
pharmacokinetics. J Theor Biol, 142: 285-307.
Trick LR (1987) Age-related alterations in retinal function. Doc
Ophthalmol, 65(1): 35-43.
Triggs EJ & Nation RL (1975) Pharmacokinetics in the aged: A review.
J Pharmacokinet Biopharm, 3: 387-418.
Turusov VS & Parfenov YuD (1986) [Methods of revealing and risk
assessment of chemical carcinogens.] Moscow, Meditsina, pp 134-136
(in Russian).
Ulloa-Aguirre A & Chappel SC (1982) Multiple species of
follicle-stimulating hormone exist within the anterior pituitary of
male golden hamsters. J Endocrinol, 95: 257-266.
Ulloa-Aguirre A, Espinoza R, Damian-Matsumura P, & Chappel SC (1988)
Immunological and biological potencies of the different molecular
species of gonadotrophins. Hum Reprod, 3: 491-501.
US Senate Special Committee on Aging (1986) Aging America: Trends
and projections, 1985-86 edition. Washington, DC, US Government
Printing Office, pp 14-17.
Utiger RD (1980) Decreased extrathyroidal triiodothyronine
production in monthyroidal ilness: benefit or harm? Am J Med, 69:
807-810.
Van Bezooijen CFA (1984) Influence of age-related changes in rodent
liver morphology and physiology on drug metabolism - A review. Mech
Ageing Dev, 25: 1-22.
Van Bezooijen CFA, Groen C, Stijnen AM, & Danhof M (1989) The effect
of age on the pharmacokinetics and pharmacodynamics of drugs in
rats. Age, 12: 112.
Vanhaelst L, Van Cauter E, Degaute JP, & Goldstein J (1972)
Circadian variations of serum thyrotropin levels in man. J Clin
Endocrinol Metab, 35: 489-492.
Van Manen R, De Priester W, & Knook DL (1983) Lysosomal activities
in aging rat liver. I. Variation in enzyme activity within the liver
lobule. Mech Ageing Dev, 22: 159-165.
Velican C (1981) Are animal models suitable for the study of
ischaemic heart disease. Rev Roum Méd Méd Interne, 19(1): 5-19.
Vellas B, Balas D, Guidet M, Moreau J, Senegas F, Bouisson M,
Albarede JL, & Ribet A (1988) Vieillissement du pancréas. Son
implication dans les états de dénutrition chez les personnes agées.
Presse Méd, 17: 2067-2069.
Vermeulen A, Deslypere JP, & Kaufman JM (1989) Influence of
antiopioids on luteinizing hormone pulsatility in aging men. J Clin
Endocrinol Metab, 68: 68-72.
Verzar F (1968) Intrinsic and extrinsic factors of molecular aging.
Exp Gerontol, 3: 69-75.
Vestal RE (1978) Drug use in the elderly: a review of problems and
special considerations. Drugs, 16: 358-382.
Vestal RE, McGuire EA, Tobin JD, Andres R, Norris AH, & Ulzey E
(1977) Aging and ethanol metabolism. Clin Pharmacol Ther, 21:
343-354.
Vestal RE, Wood AJJ, & Shand DG (1979) Reduced b-adrenoceptor
sensitivity in the elderly. Clin Pharmacol Ther, 26: 181-186.
Vestal RE, Jue SG, & Cusack BJ (1985) Increased risk of adverse drug
reactions in the elderly: Fact or myth. In: O'Malley K & Waddington
JL ed. Therapeutics in the elderly. Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp 97-104.
Vijg J (1990) DNA sequence changes in aging: How frequent, how
important? Aging, 2: 105-123.
Vijg J & Knook DL (1987) DNA Repair in relation to the aging
process. J Am Geriatr Soc, 35(6): 532-541.
Vijg J & Papaconstantinou J (1990) Aging and longevity genes:
strategies for identifying DNA sequences controlling life span. J
Gerontol, 45(5): B179-B182.
Vincenzini MT, Iantomasi T, Stio M, Favelli F, Vanni P, Tonelli F, &
Treves C (1989) Glucose transport during ageing by human intestinal
brush-border membrane vesicles. Mech Ageing Dev, 48: 33-41.
Vogel HB (1983) Effects of age on the biomechanical and biochemical
properties of rat and human skin. J Soc Cosmet Chem, 34: 453-463.
Voitenko VP & Tokar AV (1983) The assessment of biological age and
sex differences of human aging. Exp Aging Res, 9: 239-244.
Vos JG & Luster MI (1989) Immune alterations. In: Kimbrough RD &
Jensen AA ed. Halogenated biphenyls, terphenyls, naphtalenes,
dibenzodioxins and related products. Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp 295-322.
Vos JG & Penninks AH (1987) Dioxin and organotin compounds as model
immunotoxic chemicals. In: De Matteis F & Lock EA ed. Selectively
and molecular mechanism of toxicity. London, MacMillan Press Ltd, pp
85-102.
Vos JG, De Klerk A, Krajnc EI, Van Loveren H, & Rosing J (1990)
Immunotoxicity of Bis (tri-n-butylin) oxide in the rat; effects on
thymus-dependent immunity and on non specific resistance following
long-term exposure in young versus aged rats (1990). Toxicol Appl
Pharmacol, 105: 144-155.
Wabner CL & Chen TS (1984) Renal transport of organic acids in the
aging. Pharmacologist, 26: 206.
Waddington JL, O'Boyle KM, Molloy AG, Youssef HA, King DJ, & Cooper
SJ (1985) Neurotransmitter receptors and ageing:
Dopamine/neuroleptic receptors, involuntary movements and the
disease process of schizophrenia. In: O'Malley K & Stevenson IL ed.
Therapeutics in the elderly. Amsterdam, Oxford, New York, Elsevier
Science Publishers, pp 63-76.
Waggoner S, Gu MZ, Chiang WH, & Richardson A (1990) The effect of
dietary restriction on the expression of genes. In: Genetic effects
of aging, II. Caldwell, New Jersey, Telford Press, pp 255-273.
Wagner PA, Jernigan JA, Bailey LB, Nickens C, & Brazzi GA (1983)
Zinc nutriture and cell-mediated immunity in the aged. Int J Vitam
Nutr Res, 53: 94-101. Walford RL (1969) The immunological theory of
aging. Copenhagen, Munksgaard.
Walker DM (1985) Oral disease. In: Pathy MSJ ed. Principles and
practice of geriatric medicine. New York, Chichester, Brisbane,
Toronto, John Wiley and Sons.
Wang SV, Halban PA, & Rowe JW (1988) Effects of aging on insulin
synthesis and secretion. Differential effects on preproinsulin
messenger RNA levels, proinsulin biosynthesis, and secretion of
newly made and preformed insulin in the rat. J Clin Invest, 81(1):
176-184.
Ward JM (1983) Increased susceptibility of livers of aged F344/NCr
rats to the effects of phenobarbital on the incidence, morphology,
and histochemistry of hepatocellular foci and neoplasms. J Natl
Cancer Inst, 71: 815-823.
Ward WF (1988) Enhancement by food restriction of liver protein
synthesis in the aging Fischer 344 rat. J Gerontol, 43: B50-B53.
Ward W & Richardson A (1991) Effect of age on liver protein
synthesis and degradation. Hepatology, 14: 935-948.
Ward JM, Lynch P, & Riggs C (1988) Rapid development of
hepatocellular neoplasms in aging male C3H/HeNCr mice given
phenobarbital. Cancer Lett, 39: 9-18.
Warner BA, Dufau ML, & Santen RJ (1985) Effects of aging and illness
on the pituitary testicular axis in men: Qualitative as well as
quantitative changes in luteinizing hormone. J Clin Endocrinol
Metab, 60: 263-268.
Warner HR, Butler RN, Spratt RW, & Schneider EL (1987) Modern
biological theories of aging. New York, Raven Press.
Weale RA (1986) Aging and vision. Vision Res, 26(9): 1507-1512.
Weber JCP & Griffin JP (1986) Prescriptions, adverse reactions, and
the elderly. Lancet, i: 1220.
Webster SGP (1985) Absorption of nutrients in old age. In: Pathy MSJ
ed. Principles and practice of geriatric medicine. New York,
Chichester, Brisbane, Toronto, John Wiley and Sons, Chapter 11.2, pp
265-290.
Webster IW & Logie AR (1976) A relationship between function and
health status in female subjects. J Gerontol, 31: 546-550.
Weigent DA, Carr DJ, & Blalock JE (1990) Bidirectional communication
between the neuroendocrine and immune system. Common hormones and
hormone receptors. Ann NY Acad Sci, 579: 17-27.
Weindruch R & Walford RL (1988). The retardation of aging and
dietary restriction. Springfield, Illinois, Charles C. Thomas.
Weindruch R, Gottesman SR, & Walford RL (1982) Modification of
age-related immune decline in mice dietarily restricted from or
after mid-adulthood. Proc Natl Acad Sci (USA), 79: 898-902.
Weiss B, Clark MB, & Greenberg LH (1984) Modulation of
catecholaminergic receptors during development and aging. In: Lajtha
A ed. Handbook of neurochemistry. Vol 6:- Receptors in the nervous
system. New York, London, Plenum Press, pp 595-627.
Wellinger R & Guigoz Y (1986) The effect of age on the induction of
tyrosine aminotransferase and tryptophan oxygenase genes by
physiological stress. Mech Ageing Dev, 34: 203-217.
Wesson LG Jr (1969) Renal hemodynamics in physiological states. In:
Physiology of the human kidney. New York, London, Grune and
Stratton, pp 98-100.
White L (1989) Epidemiology. In: Maddox GL ed. The encyclopedia of
aging. Berlin, Heidelberg, New York, Springer-Verlag, pp 216-223.
WHO (1983) Environmental Health Criteria 27: Guidelines on studies
in environmental epidemiology. Geneva, World Health Organization,
351 pp.
WHO (1984) The uses of epidemiology in the study of the elderly.
Report of a WHO Scientific Group on the Epidemiology of Aging.
Geneva, World Health Organization, pp. 23-25 (WHO Technical Report
Series 706).
WHO (1987) World health statistics annual. Geneva, World Health
Organization.
WHO (1989) Health of the elderly. Report of a WHO Expert Committee.
Geneva, World Health Organization, pp 14-30 (WHO Technical Report
Series 779).
WHO (1990) Global estimates for health situation assessment and
projections, 1990. Geneva, World Health Organization, Division of
Epidemiological Surveillance and Health Situation and Trend
Assessment, p 3.
Williams ME & Pannill FC (1982) Urinary incontinence in the elderly.
Ann Intern Med, 97: 895-907.
Willott JF (1986) Effects of aging, hearing loss, and anatomical
location on thresholds of inferior colliculus neurons in C57BL/6 and
CBA mice. J Neurophysiol, 56(2): 391-408.
Wilson K & Hanson J (1980) The effects of extremes of age on drug
action. Methods Findings Exp Clin Pharmacol, 2: 303-312.
Winneke G, Hrdina KG, & Brockhouse A (1982) Neuropsychological
studies in children with elevated tooth-lead concentrations. A pilot
study. Int Arch Occup Environ Health, 51: 169-183.
Wong DF, Wagner HN, & Dannals RF (1984) Effects of age on dopamine
and seratonin receptors pressured by positron tomography in the
living human brain. Science, 226: 1393-1396.
Wood RW (1981) Neurobehavioral toxicity of carbon disulfide.
Neurobehav Toxicol Teratol, 3: 397-405.
Wood AJJ (1985) Beta adrenoceptors and aging. In: O'Malley K &
Stevenson IL ed. Therapeutics in the elderly. Amsterdam, Oxford, New
York, Elsevier Science Publishers, pp 43-49.
Wu W, Pahlavani M, Cheung HT, & Richardson A (1986) The effect of
aging on the expression of interleukin 2 messenger ribonucleic acid.
Cell Immunol, 100: 224-231.
Wynne HA, Cope LH, Mutch E, Rawlins MD, Woodhouse KW, & James OF
(1989) The effect of age upon liver volume and apparent liver blood
flow on healthy man. Hepatology, 9: 927-301.
Xiong ZM (1989) [Common diseases and major causes of death in
elderly patients in Jiangxi.] Chin J Geriatr, 8: 135 (in Chinese).
Xiong BZ (1990) Technology progress, aging process and reappoint to
a position.] In: Institute of Japanese Problems, Chinese Academy of
Social Sciences ed. [Proceeding of Symposium on Sino-Japan Social
Countermeasure for Aging Problem.] Beijing, East Publishing House,
pp 186-196 (in Chinese).
Xu XF, You K, Liu D, He L, Cheng S, & Wu JS (1986) [Disease pattern
analysis in hospitalized elderly patients in Uromqi.] Chin J
Geriatr, 5: 15 (in Chinese).
Yamazoe Y, Shimada M, Murayama N, & Kato R (1987) Suppression of
levels of phenobarbital-inducible rat-liver cytochrome P-450 by
pituitary hormone. J Biol Chem, 262: 7423-7428.
Yang RSH, Tallant MJ, & McKelvey JA (1984) Age-dependent
pharmacokinetic changes of ethylenediamine in Fischer 344 rats
parallel to a two-year chronic toxicity study. Fundam Appl Toxicol,
4: 663-670.
Yao SB (1990) [The trend and characters of Chinese aged population.]
In: Institute of Japanese Problems, Chinese Academy of Social
Sciences ed. [Proceedings of Symposium on Sino-Japan Social
Countermeasure for Aging Problems.] Beijing, East Publishing House,
pp 64-74 (in Chinese).
Yates MS & Hiley CR (1979) The effect of age and cardiac output and
its distribution in the rat. Experientia (Basel), 35: 78-79.
Yelland C, Summerbell J, Nicholson E, Herd B, Wynne H, & Woodhouse
KW, (1991) The association of age with aspirin esterase activity in
human liver. Age Ageing, 20(1): 16-8.
York JL (1982) Body water content, ethanol pharmacokinetics, and the
responsiveness to ethanol in young and old rats. Dev Pharmacol Ther,
4: 106-116.
Young VR (1979) Diet as modulator of aging and longevity. Fed Proc,
38: 1994-2000.
Yu BP, Bertrand HR, & Masoro EJ (1980) Nutrition-aging influence of
catecholamine-promoted lipolysis. Metabolism, 29: 438-444.
Yumura W, Sugino N, Nagasawa R, Kubo S, Hirokawa K, & Maruyama N
(1989) Age-associated changes in renal glomeruli of mice. Exp
Gerontol, 24: 237-249.
Zapadnyuk VI (1971) [On an age-periodisation of laboratory animals.]
In: [Gerontology and geriatrics. 1970-1971 Annual book: Aging of the
cell.] Kiev, Research Institute of Gerontology, pp 433-438 (in
Russian).
Zarit SH, Eiler J, & Hassinger M (1985) Clinical assessment. In:
Birren JE & Schaie KW ed. Handbook of the psychology of aging, 2nd
ed. New York, Van Nostrand Reinhold Company, pp 725-754.
Zaphiropouos PG, Mode A, Norstedt G, & Gustavsson JA (1989)
Regulation of sexual differentiation in drug and steroid metabolism.
Trends Pharmacol Sci, 10: 149-153.
Zatz MM & Goldstein AL (1985) Thymosins, lymphokines and the
immunology of aging. Gerontology, 31: 263-277.
Zemel MB & Sowers JR (1988) Salt sensitivity and systemic
hypertension in the elderly. Am J Cardiol, 61: 7H-12H.
Zhou L-W, Weiss B, Freilich JS, & Greenberg LH (1984) Impaired
recovery of alpha - and alpha2-adrenergic receptors in brain tissue
of aged rats. J Gerontol, 39: 538-546.
Zhu JR, Li WX, & Wang YY (1982) [Common diseases and major causes of
death in the elderly with analysis of 8947 cases over 65 years of
age.] Chin J Geriatr, 1: 110-118 (in Chinese).
Zs-Nagy I, Kitani K, Ohta M, & Imahori K (1986) Age-dependent
decrease in lateral diffusion constant of proteins in the plasma
membrane of hepatocytes as revealed by fluorescence recovery after
photobleaching in tissue smears. Arch Gerontol Geriatr, 5: 131-146.
APPENDIX 1. Background Papers
Anisimov, V.N., Approaches to examine the effects of chemicals on
the aged population: experimental approaches.
Birnbaum, L.S., Basis of altered sensitivity of the elderly to
chemicals - pharmacokinetics and pharmacodynamics.
Cooper, R.L., & Goldman, J.M., Alterations in susceptibility to
toxic compounds in the aged central nervous system and endocrine
system.
Dilman, V.M., Theories and mechanics of aging.
Fabris, N., Systemic biology of aging.
Ingram, D.K., Evaluating effects of chemical exposure on aging:
development of conceptual models.
Li, S., Aged population: demographic, life expectancy, and
lifestyle.
Likhachev, A.J., Age related peculiarities of repair of DNA damage
with carcinogens.
Martin, G.M., Definitions on aging.
Ray, P.K., Jaffery, F.N., & Viswathan, P.N.,
1. Chemical exposure of the elderly
2. Modifying factors: nutrition, state of health, life style,
alcohol intake, smoking and ionizing radiation.
Richardson, A., The molecular biology of aging: translation,
transcription and chromatin structure.
Speijers, G.J.A., Groups at risk and their chemical exposure as well
as nutrition as a source of chemicals and as a confounding factor.
Zhu, J.R., Approaches to examine the effects of chemicals on the
aged population: epidemiological and clinical approaches.

 It is commonly assumed that today's large percentage of elderly
people in the population is a result of increased longevity and
decreased birth rate. For example, in Japan the proportion of people
aged 65 or more had increased to 10.3% in 1985. At the same time,
the values were 15.1%, 14.5%, 12.4% and 16.9% in the United Kingdom,
Federal Republic of Germany, France and Sweden, respectively. Japan
is a new "aged-type" country with the greatest rate of increase in
the elderly in the world. By the year 2025, the proportion of the
Japanese population aged 65-plus will rise to 23.4% (Hosomi, 1990).
In China, the proportion of the population aged 60 and over was
7.3% of the total population in 1953. By 1984 it had increased to 9%
of the total population, and by the year 2000 the proportion of the
population 60 and over will be as high as 10.6%. The proportion is
predicted to increase to 26.2% by 2025 (Xiong, 1990).
At present, the aged population is growing more rapidly in
China than in countries of Europe and North America. According to
data from the US Bureau of the Census, it took 115 years (1865-1980)
for the proportion of people aged 65 or more in France to increase
from 7% to 14%, 85 years (1890-1975) in Sweden, 66 years (1944-2010)
in the USA, 45 years (1930-1975) in the United Kingdom, and 26 years
(1970-1996) in Japan. For China, the pattern of the growing number
of elderly is similar to Japan, i.e. the proportion of people aged
65 or more will be 7.4% in 2000, and by 2025 it will have increased
to 12.8% (Hosomi, 1990; Yao, 1990; Xiong, 1990).
1.4.2 Life expectancy
Life expectancy at birth is a statistical index calculated by
the use of a life table from the age-specific death rates of the
population. This illustrates the overall level of health in a
country or a region. Throughout this century, it has been evident
that, as a result of improvements in many aspects of health status,
an individual can expect to live longer. From 1960 to 1990, life
expectancy at birth for the total population had increased by 13.5
years. The life expectancy at birth for the period 1985-1990 was
estimated to be 63.9 years for the world as a whole, 74.0 years for
the more developed regions, and 61.4 years for the less developed
regions. The longest life expectancies are in Japan (78.3), Iceland
(77.5), Sweden (77.1), Switzerland (77.1), and the Netherlands
(76.9) (World Population Prospects, 1991).
The trend for life expectancy at birth in the USA showed an
increase from 1900 (46.4 for males and 49.4 for females) to 1950
(65.6 and 71, respectively) and in the year 2000 is expected to be
72.1 and 79.5 (for males and females). By 2050, it may have
increased to 73.6 for males and 81 for females (US Senate Special
Committee on Aging, 1986).
In China, the life expectancy at birth before 1949 was about 35
years of age, being one of the lowest in the world at that time. By
1957 it had increased to 57 years, from 1973 to 1975 it was 63.6
years for males and 66.3 years for females, and in 1981 it was 68
years. It is expected that the continued increase in the average
life expectancy of the people of China will be slow due to a high
death rate from cardiovascular diseases (Gu, 1986).
1.4.3 Life-style in aged populations
A basic issue in planning for the consequences of demographic
aging is whether elderly people should be considered a specific
target group for the development of services, or whether their needs
should be catered for within the context of planning for the
population as a whole. One approach to a rational policy for this
issue is to consider the nature of human aging. For this it is
necessary to view the physical, psychological and sociological
dimensions of aging as a whole.
Life-style influences the effects of chemicals on human health,
including that of the elderly, both quantitatively and
qualitatively. Environmental chemicals and their uses are diverse.
Specialized nutritional elements of the diet have become popular,
while in certain countries many people prefer predominantly
vegetarian diet. Others take supplements and additives which contain
pure preparations of vitamins, minerals, amino-acids and other
substances. Whether such substances have either adverse or
beneficial effects on the elderly and aging processes, has not
generally been fully evaluated. Another important source of human
exposure to chemicals comes from the intake of different kinds of
cosmetic agents and fragrances, such as shampoos, creams, perfumes,
oral deodorants, sunscreen and suntan lotions, and insect
repellents. These are often chemical mixtures whose components have
not been evaluated or tested beyond acute toxic potential.
Occupational status, indoor air quality, recreational
activities, exercise, eating and drinking habits, alcohol
consumption, and tobacco smoking can all affect the elderly and
aging processes to a certain degree. Elements of life-style can
strengthen or reduce the risk of developing aged-related
degenerative diseases. They can also accelerate or delay
physiological and anatomical changes. Typical examples are the
various age-related diseases caused by toxic chemicals in tobacco
smoke and the reduction of the risk of cardiovascular diseases
produced by regular exercise (Committee on Chemical Toxicity and
Aging, 1987).
As far as the possible influence of life-style factors on the
manifestations of aging is concerned, many studies have shown that
loneliness and physical and intellectual inactivity are common among
the elderly, especially widowed people. Several studies have
revealed that living conditions have an influence on health and
well-being, resulting in an increase in the demand for social care
and medical service. Marital status and living arrangements have
important significance for the unique life-style of the elderly.
There are striking differences between the proportions of elderly
males and females who are married: in many countries the proportion
of married males is twice that of married females. In general, the
proportion of widows is very high and that of widowers relatively
low (WHO, 1984).
Migration is also one of the life-style variables of the
elderly. In rural areas of Asia, many older women move to cities to
join their children after they have been widowed. Another common
type of move is the migration of the recently widowed or chronically
ill elderly from urban areas to their home towns or villages. For
many countries in Africa and Asia, the urban-rural migration is most
apparent among males, who return from urban to rural areas when they
are old. Worldwide, only a minority of elderly people live in urban
areas (WHO, 1984).
1.5 Theories of aging
During the last century, more than 100 various hypotheses
concerning the origin and mechanism of aging have been put forward.
All of them could be grouped generally into two broad categories:
those that invoke deterministic, or "programmed", alterations in
gene expression or gene structure; and those that invoke a variety
of stochastic, or "random", alterations in the structure and
function of macromolecules, cells, and organ systems. This
distinction, however, has some limitations, because stochastic
alterations in individual cells can lead to predictable phenomena in
the large populations of cells. The use of terminal differentiation
to explain the limited replicative life span of somatic cells
(Martin et al., 1974) could be an example of the blurring of the
stochastic and non-stochastic categories. For each individual cell,
differentiation is a random event; however, for a population of
cells, the process appears deterministic.
The mechanisms of aging are likely to be coupled to the
reproductive strategy of the organism. One example is the
synchronous, rapid physiological declines and mortalities that are
characteristic of species with single massive episodes of
reproduction (e.g., migrating Pacific salmon or soybean plants).
Placental mammals, however, have ample opportunity for a variety of
stochastic processes to take place during their long reproductive
and postreproductive phases. The associated patterns of structural
and functional decline can vary substantially, both qualitatively
and quantitatively, among individuals within a species and among
different related species. Evolutionary biologists in fact present
compelling arguments that aging did not evolve because of any
adaptive value to the individual or to the species, as would be
assumed by strictly programmed theories (reviewed in Rose, 1991).
Aging is thought to occur simply because of the decline in the force
of natural selection for gene action that is postreproductive. Such
gene action could be related to accumulations of late-acting
mutations in the constitutional genome or to selection for forms of
genes that have positive effects on reproductive fitness early in
the lifespan, but whose effects may be negative late in the lifespan
(the "antagonistic pleiotropy" theory of aging) (Rose, 1991).
It is beyond the scope of this monograph, however, to consider
the potentially large numbers of specific mechanisms that may be
modulated by such accumulated constitutional mutations or
pleiotropic genes. The reader is referred to recent reviews of the
many postulated theories of aging (Warner et al., 1987; Committee on
Chemical Toxicity and Aging, 1987; Finch, 1991; Cutler, 1991).
These can be classified in a variety of ways (Dilman, 1987;
Medvedev, 1990).
2. STRUCTURAL AND PHYSIOLOGICAL CHANGES IN THE AGED
2.1 Changes in gene structure and function in aging
Changes in gene expression are of critical importance to an
organism. Aging can potentially alter not only the structure of
genes, but the way in which they function. Changes in the DNA are
often thought to be integral to aging. It is clear that not only
mutations, but chromosomal rearrangements accumulate with age (Vijg,
1990). Repetitive sequence families may play a crucial role in the
processes of aging. In addition, the organization of DNA and protein
in chromatin is important structurally and functionally. Therefore,
changes in chromatin could play a major role in the age-related
change in the regulation of gene expression (Richardson et al.,
1983; Medvedev, 1984; Thakur, 1984; Richardson et al., 1985).
2.1.1 Chromatin structure
Chromatin changes may involve either proteins that interact
with DNA or the chemical structure of the DNA molecule itself.
Although no change in the stoichiometry of the major histones has
been observed with increasing age (Richardson et al., 1983;
Medvedev, 1984), several investigators have reported changes in the
subspecies of histone H1 (Medvedev, 1984; Mitsui et al., 1980;
Niedzwiecki et al., 1985). The acetylation of histones, which has
been proposed to alter histone-DNA interactions thereby making DNA
more accessible, decreases by 30% to 70% with increasing age
(O'Meara & Pochron, 1979).
With respect to age-related changes in DNA chemical structure,
there is now conclusive evidence for the "spontaneous" induction of
a variety of DNA lesions in different organs and tissues of both
humans and experimental animals (for a review, see Mullaart et al.,
1990). Most of these lesions seem to be repaired (see below), but
not all. For example, Cathcart et al. (1984) and Fraga et al. (1990)
estimated that in rats about 105 oxidative DNA lesions occur per
cell per day. Since the rate of repair does not entirely equal the
rate of induction of damage, there is a net increase of spontaneous
DNA lesions with age. Fraga et al. (1990) calculated for one
specific lesion, 8-hydroxy-deoxyguanosine, that about 80 residues
accumulate per rat cell per day.
Although some DNA lesions are repaired quickly, this is not the
case for all lesions. Indeed, after treating rats with low doses of
2-acetyl-aminofluorene (AAF), Mullaart et al. (1989) were still able
to detect about 30% of the major lesions induced as late as 21 days
after treatment. Such incomplete repair could be responsible for
accumulation of DNA lesions during continuous or frequent exposure to
genotoxic agents.
2.1.2 DNA repair
To preserve the DNA chemical structure, cells are equipped with
a battery of repair systems to remove damage. As yet the various
mechanisms of action of these DNA repair systems and their
interrelationships are incompletely understood (for a recent review,
see Lehmann et al., 1992). In general, repair systems can be divided
into three categories, i.e. direct repair, excision repair and
post-replication repair. In direct repair, the lesion itself is
removed without any further (transient) changes in the DNA
structure. Direct repair includes the enzymatic photo-reactivation
of UV-induced pyrimidine dimers and the removal of O6-alkyl
adducts by specific alkyl transferases.
DNA excision repair is brought about by a complex multi-enzyme
system, the components of which are involved in the various steps in
this repair process (Vijg & Knook, 1987). The third type of repair,
post-replication repair, does not actually remove the damage but
allows the replication system to bypass the damage. It is this
latter process especially that is considered to be associated with
nucleotide misincorporation (mutation).
Accurate assessment of an organism's capacity to repair
specific lesions is difficult and subject to error. In general, the
most reliable data can be obtained when the induction and
disappearance of the relevant lesions themselves are monitored in
the different organs and tissues of an experimental animal.
Unfortunately, in most studies on the possible existence of a
decline in DNA repair activities with age, assays were used which
measured the DNA synthesis phase of excision repair. The general
conclusion from these data, mostly obtained with cultured cells, is
that there is no age-related decline in the efficiency of DNA repair
systems (Tice & Setlow, 1985; Likhachev, 1985; Hanawalt, 1987). It
cannot be ruled out, however, that during aging DNA repair systems
become more error prone, leading to an accelerated induction of
mutations (Vijg & Knook, 1987). In any case, a certain degree of
imperfection is a general characteristic of DNA repair systems as
indicated by the actual accumulation of both DNA lesions and DNA
sequence changes (see above). The question that should be addressed
is what type of DNA alterations occur, how many exist, and at what
rate do they accumulate with age. Finally, their relevance in terms
of actual physiological decrements or the initiation of disease
should be assessed.
2.1.3 Transcription
Several review articles have been published in the past decade
that discuss the effect of age on transcription (Rothstein &
Seifert, 1981; Richardson et al., 1983; Richardson et al., 1985;
Richardson & Semsei, 1987; Slagboom & Vijg, 1989). A major problem
in this area has been the difficulty in accurately measuring the
rates of synthesis of specific RNA species and their intracellular
levels. With the major advances in recombinant DNA technology, this
problem has now been virtually eliminated and our knowledge of how
aging affects the expression of specific genes is rapidly growing.
At present, it appears that the overall transcriptional
activity of a cell declines as an organism ages. However, the level
of total RNA tends to remain constant suggesting a decline in the
rate of RNA turnover (Horbach et al., 1986).
The levels of some specific mRNA species using cDNA probes for
specific genes have been measured recently (Richardson & Semsei,
1987). In general, no consistent trend has emerged. The levels of
some mRNA species decrease with age; however, other mRNA species do
not change with age, and others actually increase (Slagboom & Vijg,
1989).
In most of the studies, a good correlation has been found
between the age-related changes in the level of an mRNA species and
the level of protein (or enzyme activity) specified by the mRNA
species. This has been demonstrated in rat liver for albumin
(Horbach et al., 1984), apha2u-globulin (Richardson et al., 1987),
and superoxide dismutase and catalase (Semsei et al., 1989), and in
rat kidney and small intestine for calbindin-D (Armbrecht et al.,
1989). The age-related decline in mitogen-induction of interleukin 2
(IL-2) (Wu et al., 1986; Nagel et al., 1988; Pahlavani et al., 1988)
and IL-3 (Li et al., 1988) mRNA in lymphocytes from rodents and
humans corresponded to the age-related decline in the biological
activities of these two interleukins. In contrast, Strong et al.
(1990) reported an uncoupling of tyrosine hydroxylase transcription
and translation in the adrenal glands of old rats.
Investigators usually assume that age-related changes in the
levels of a particular mRNA species arise from a change in
transcription. However, only a few studies have actually measured
the transcription of a specific gene as a function of age using
nuclear run-off assays. While an age-related decrease occurs in the
nuclear transcription of the alpha2u-globulin (Richardson et al.,
1987; Murty et al., 1988a), cytochrome P450(b+e) (Rath & Kanungo,
1989), and superoxide dismutase and catalase (Semsei et al., 1989)
genes, the nuclear transcription of tyrosine amino-transferase and
tryptophan oxygenase (Wellinger & Guigoz, 1986), albumin (Horbach
et al., 1988b) and the c-myc (Buckler et al., 1988) genes was
similar in young and old rodents. Studies are now underway to
explore in more detail age-changes in specified mRNA species in
terms of the transcription factors involved (Post et al. 1991).
One exciting development in the area of transcription and aging
has been the observation that dietary restriction, which enhances
the longevity of rodents, alters the expression of some genes at the
level of transcription (Richardson et al., 1987; Semsei et al.,
1989). However, the expression of all genes is not affected by
dietary restriction (Waggoner et al., 1990).
In addition to nuclear synthesis, post-transcriptional
processing of hnRNA plays an important role in the regulation of
gene expression. Müller et al. (1989) recently discussed various
views of how the post-transcriptional processing of hnRNA might
alter with age. At present, there is little evidence that major
changes occur with age in the size of the poly(A)-segment of mRNA
(Birchenall-Sparks et al., 1985). Interestingly, in the many studies
in which mRNA species have been analysed by Northern blot analysis,
there has been not a single report of a significant change in the
size of the mRNA species examined with increasing age (Richardson &
Semsei, 1987). Thus, there is very little direct evidence at present
to support the view that the processing and/or nuclear transport of
hnRNA is altered with age.
2.1.4 Translation
Increasing age generally results in a decrease in total protein
synthesis in plants, invertebrates, rodents and cultured cells
(Richardson & Birchenall-Sparks, 1983; Ward & Richardson, 1991).
Recent studies have focused on the influence of age on the
translation of mRNA into specific proteins and on the ability to
modulate age changes in protein synthesis. There is no evidence that
a decrease in the fidelity of protein synthesis occurs with
advancing age but technical limitations do not permit a definitive
conclusion (Rosenberger & Kirkwood, 1986). The influence of age on
protein synthesis differs from protein to protein and much more work
must be done in assessing the effect on key individual proteins.
Attempts to modulate protein synthesis have recently begun. The rate
of protein synthesis in the liver is higher after maturity for
dietary restricted than for ad libitum fed rats (Ward, 1988). In
an in vitro system, growth hormone increases protein synthesis in
muscles of old rats to the level found in muscles of young rats
(Sonntag et al., 1985). Much more study is required, focusing on
individual proteins, different tissues and different organisms.
2.2 Changes in tissues, organs and systems in aging
The progressive modification of body functions with age
involves alterations not only at the genetic, molecular and cellular
levels, but at the level of the tissues, organs, systems and entire
organism. It is important to attempt to differentiate between
age-related pathology and true physiological aging. This is often
difficult because the majority of age-related changes increase the
vulnerability of the aging organism to disease and ultimately death.
In the following, each organ or system will be discussed in
reference to age-related changes in its structure which might
predispose to alterations in function, not only inherently as part
of aging, but in response to environmental agents. The focus will be
on the healthy aged as opposed to the diseased.
2.2.1 Nervous system
The brain may undergo a progressive deterioration with age at
all levels of organization - structural, biochemical and functional.
CNS disorders, including Parkinson's and Alzheimer's diseases, are
common in the elderly.
2.2.1.1 Structural changes
Brain weight decreases slightly with aging. This is due to
atrophy of both grey and white matter (Creasey & Rapoport,1985). At
the cellular level, the major age-associated modification is in the
number of neurons, which are significantly diminished in discrete
areas of the brain (Brizzee, 1985), particularly in the basal
ganglia, cerebellum (probably related to decreased motor control),
locus ceruleus (associated with alterations in sleep patterns),
nucleus basalis of Meynert (associated with senile dementia of
Alzheimer type (SDAT) (Bondareff, 1986), and the spinal cord.
Neuronal loss, which is associated with an increase in the number of
glial cells, is relatively mild in the healthy aged, but is much
more severe in SDAT, Parkinson's disease and in the early aging
associated with Down's syndrome.
In addition to a reduced number of neurons, the aged brain is
characterized by a reduction in the number of dendrites and
dendritic spines, probably due to a slowing renewal process
(Scheibel & Tomiyasu, 1978). Synapse density declines in discrete
areas of the brain, but this is partially compensated by enlargement
of the remaining synapses (Bertoni-Freddari et al., 1990).
Intracellular changes include dilation and fragmentation of the
Golgi apparatus (Mervis, 1981), distortion of membranes and the
nucleus, and accumulation of lipofuscin, in both neurons and glial
cells within discrete brain areas. With advancing age, there is an
increase in neurofibrillary tangles (intracellular tangled masses of
paired helical filaments) (Terry, 1963), extracellular neuritic
plaques (a core of amyloid surrounded by material derived from
dystrophic neurites), and reactive glial and microglial cell
accumulation (Master et al., 1985). Again, these changes occur in
normal aging at a moderate level, but are much more frequent in SDAT
(Iqbal et al., 1982) and other dementias.
There are also age-related changes in the morphology of the
peripheral and autonomic nervous systems. These include reductions
in the number of sensory and motor neurons, increases in
demyelination, increases in connective tissue, and a mild loss of
myelinated fibres (Tomlinson & Irving, 1977; Spencer & Ochoa, 1981).
The central processes of dorsal root ganglion cells typically
undergo distal dystrophic and degenerative changes. Regressive
changes have been reported in the terminals of motor axons.
2.2.1.2 Biochemical changes
Besides the pathological changes, there are many age-related
alterations in brain chemistry required for cell-to-cell
communications (Rogers & Bloom, 1985; Finch, 1991). These include
changes in the concentration and/or turnover of the amines (e.g.,
acetylcholine, norepinephrine, epinephrine, dopamine, serotonin),
amino acids (e.g., glycine, glutamate and GABA) and peptides (e.g.,
enkephalin, substance P, thyrotropin-releasing hormone,
cholecystokinin, somatostatin). There are numerous studies showing
impairments of adrenergic, dopaminergic and serotonergic activity in
the senescent animal (Zhou et al., 1984; Roth & Joseph, 1988;
Telford et al., 1988). One of the underlying causes of these
alterations seems to be an overall loss of receptors (Weiss et al.,
1984; Roth & Joseph, 1988).
Synapses may utilize one or more neuromodulator (e.g.,
norepinephrine and neuropeptide). The multiple levels of control and
the regional diversification of different synapses in discrete brain
regions make it difficult to define the age-related alterations in
neurotransmitter/neuropeptide function. In fact, rather than a
uniform drop in the level of a specific neurotransmitter throughout
the nervous system, a "desynchronization" of signals may occur. For
example, while the brain content of norepinephrine and dopamine are
decreased in old age, that of serotonin is unchanged or even
increased, depending on specific brain areas. In some cases, the
greater the concentration of a neurotransmitter in a discrete brain
region, the higher the decrement with aging and vice versa (Timiras
et al., 1984). In fact, age-dependent alterations in different
neurotransmitter/neuropeptide concentrations do not always occur
simultaneously. Each neurotransmitter has its own timetable:
dopamine levels decrease in the cerebral hemispheres of rats from
the age of one year, whereas in the same areas serotonin levels
remain unaffected until three years of age (Timiras et al., 1984).
Aged-related changes in neurotransmitter receptor number and
function have also been reported (Greenberg & Weiss, 1983; Roth &
Joseph, 1988). Changes in binding affinity have not been frequently
detected. Beta-adrenergic receptor responsiveness is decreased in
the elderly (Vestal et al., 1979; Lakatta, 1980). This appears to be
due to uncoupling of the beta-receptor from the adenylate cyclase
complex which transmits the signal (Wood, 1985). In the rat pineal
gland, corpus striatum and cerebellum, a reduced responsiveness to
catecholamines is present due to a decrease in the affinity of
beta-adrenergic receptors to their ligand. However, there is no
change in receptor number (Greenberg & Weiss, 1978). This may be due
in part to a reduced ability to increase the number of
beta-adrenergic receptors after decreased noradrenergic input
(Greenberg & Weiss, 1979).
Changes in general biochemical properties of the cells occur in
the nervous system as they do elsewhere in the aging organism.
Lipid composition may change, resulting in altered membrane
viscosity. Protein synthesis decreases in discrete brain regions.
Lipofuscin accumulates, although the functional significance is
unclear, and there are alterations in electrolytes and trace
elements (Brizzee, 1985). For example, aluminium levels may increase
sharply in elderly people (Bjorksten et al., 1989). Decreases in
zinc may be important in the light of the zinc requirement of
various enzymes and growth factors, including nerve growth factor
(NGF) (Dunn et al., 1980). In addition, aging is accompanied by a
decreased brain water content (Meisami, 1988). Alterations in
vascular flow have also been reported (Katzman & Terry, 1983).
2.2.1.3 Functional changes
Despite the morphological and biochemical changes observed in
the aging brain, the functional efficiency of the nervous system
seems to be well maintained in most elderly people. However, CNS
disorders do occur in some individuals, though it may be difficult
to discriminate age-related pathology from physiological aging
phenomena. Perhaps the most ubiquitous and significant change
observed in the older organism is slowness of behaviour (Birren et
al., 1979). The slowing of behaviour with age not only appears in
motor responses and perceptual processing, but is also apparent for
the more complex processing of information associated with
short-term memory (Smith et al., 1980). Related cross-sectional
studies using global measures of intellectual function such as the
Wechsler Adult Intelligence Scale (WAIS) show evidence that some
performance abilities decline by the late 60s and early 70s, while
others (e.g., verbal abilities) appear to be maintained throughout
life in healthy individuals (Gallagher et al., 1980). The slowing of
reaction time may be associated with the age-associated slowing and
loss of coordination in motor tasks, such as those involved in
handwriting and other purposeful movements.
The age-related modification of biorhythms is exemplified by
the alterations of the sleep/wakefulness cycle, which is largely
dependent on the reticular system. Alterations of sleep patterns
with aging are qualitative rather than quantitative (Dement et al.,
1985) and affect primarily the "deep sleep" phases, as confirmed by
the alterations observed in the brain electrical activity (Müller &
Schwartz, 1978). Among neurotransmitters, serotonin seems to be
implicated.
Alterations in posture and locomotion in the elderly (Klawans &
Tanner, 1984) also depend on CNS impairment. Peripheral
modifications such as decreased nerve conduction velocity, reduced
muscle mass and increased rigidity occur. Autonomic system
dysfunction is also implicated in many pathophysiological changes
of age including hypotension, thermoregulation, gastrointestinal
function and urinary incontinence (Finch & Landfield, 1985). Other
changes in the autonomic system include changes in vascular and
cardiac reflexes, galvanic skin responses, and potency (Katzman &
Terry, 1983). Sympathetic hyperactivity is commonly present in the
aged and could interfere with cognitive functioning.
2.2.2 Sensory organs
All of the sensory organs are affected by aging, both those in
which the cells are continuously renewed (such as cutaneous sense
tissues) and those in which the cells are terminally differentiated
early in life (vision and hearing).
2.2.2.1 Vision
Both neural (retina) and optical (cornea, lens, pupil, aqueous
and vitreous humours) components of vision are affected by age. The
changes in the optical compartment are probably the primary cause of
visual impairment in the elderly (Sekuler et al., 1982). The most
common alterations are in the lens with increased hardness and
decreased transparency (Graham, 1985). The former results in reduced
refractive power (Marsh, 1980). The loss of transparency relates to
the following chemical changes in the lens: protein oxidation,
racemization, glycation, aggregation, polymerization and
precipitation (Taylor, 1989). These alterations are associated with
presbyopia and cataracts, respectively.
In the neuronal compartment, the retina undergoes progressive
loss of rods, while cones may be augmented. Morphometric analysis of
the retina demonstrates an increase in electron-dense plaques and a
decrease in the ground substance during aging. Such retinopathies
result in decreased light sensitivity and reduced colour vision
(Marsh, 1980).
Vision declines as a function of age (Weale, 1986) and can be
measured in several tests, such as the Humphrey Field Analyser
(Iwase et al., 1988), and retinal potentials (Trick, 1987). Visual
acuity is substantially decreased. The ability to detect light
gradually decreases (Sample et al., 1988) and light adaptation
declines (Katz & Robinson, 1987).
2.2.2.2 Hearing
Decrements in hearing are frequently observed in the elderly.
There is also a progressive loss of hearing in animals with age
(Willott, 1986). Both auditory structures and neuronal components
are involved. While the outer and middle ear show few modifications,
degenerative changes occur in the hair cells, which are the auditory
receptors, and in the mechano-electrical transducing organs
resulting in otosclerosis. This accounts for the preferential loss
of hearing of high frequency sounds (presbycusis) (Marsh, 1980). The
degree of hearing loss may affect the two ears differentially, thus
causing defects in sound localization. Presbycusis has a great
impact upon speech perception, since consonants, which make speech
intelligible, are generated by high frequency sounds, whereas
vowels, responsible for audibility, are produced by low frequency
sounds.
Hearing defects may also result from changes in the neural
components (Allison et al., 1984) of hearing, and in particular in
the nerves connecting the cochlea with the auditory centres in the
brain, specifically in the superior temporal gyrus.
2.2.2.3 Olfaction
The age-related alteration in the sense of smell is generally
underestimated. The reduction in olfactory sensitivity is mainly due
to the progressive loss of olfactory neurons, which protrude through
cilia from the superior nasal cavity and represent the receptor
sites for odour and the chemo-electrical transducing mechanism
(Naessen, 1971). Loss of neurons have also been demonstrated in the
olfactory bulbs of the brain (Bhatnagar et al., 1987).
2.2.2.4 Taste
Taste thresholds are known to increase with age. The taste of
salt is preferentially altered in the elderly. The loss appears due
both to a decline in the number of taste buds and papillae (Bradley,
1979) in the tongue, as well as to the loss of neurons in the
cerebral centers of the gustatory system.
2.2.2.5 Somatic sensations
The somatic sensory system (touch, pressure, vibration,
proprioception, heat, cold and pain) is variably affected by age.
Tactoperceptual ability and vibrotactile sensations are decreased in
the elderly due to the loss of Meissner end-organs and Pacinian
corpuscles present in the skin (Bruce, 1980). For more complex
somatesthetic abilities (stereognosis, body part recognition) as
well as for pain and thermal sensitivity, the biological causes of
their alterations with age involve not only the sensory end-organs,
but also affective and cognitive factors (Marsh, 1980).
2.2.3 Endocrine system
Hormones play an important, often critical, role in the
regulation of a large number of physiological and behavioural
processes, and their influence can be demonstrated throughout the
lifespan. Some hormones have a role in differentiation in that their
presence or absence during certain developmental periods will affect
the way in which physiological and behavioural processes proceed or
are expressed in adulthood. Throughout each period of the life span,
the maintenance of an appropriate endocrine milieu is essential to
the numerous homeostatic processes required for survival. With
advancing age, there are several, well-documented changes in the
ability of the organism to synthesize and secrete a number of
hormones. It is, therefore, likely that the typical age-related
change in an organism's endocrine balance would result in, or at
least contribute to, the impairment of homeostasis frequently
observed in the elderly. Such impairments can be noted in the
decreased rate of recovery of the elderly from the insults of injury
or disease.
Hormones may also play a significant role in the aging process.
For example, age-related changes in several physiological functions
appear to be closely linked to the level and pattern of hormonal
stimulation present during adulthood. As such, different patterns of
exposure to a hormonal environment may alter the "rate of aging"
within a specific neuroendocrine system and, in turn, affect the
susceptibility of the organism to environmental insults at different
segments of the life span. There are a number of different ways in
which endocrine systems and the hormonal signalling operations that
they use may undergo alterations with age and toxicant exposure.
These can be categorized as changes in: (a) the availability of
hormones for binding to the target tissues, (b) the reception of the
pertinent transmitter or hormonal signal by the target cells, and
(c) the nature of the hormonal message.
At any point in time, the concentration of a hormone in the
blood is a consequence of both its metabolism and secretion. Such
changes in the size of the available signal pool may have
corresponding effects on the magnitude of the response by the target
tissue. Other changes may reflect declines with age in the
homeostatic controls, which rely heavily on endocrine feedback
relationships within organ systems.
Serum hormonal levels, as a rule, are not maintained at
constant levels. They tend to fluctuate, sometimes markedly,
throughout a 24-h period. In the young adult man, peak morning
testosterone values can fall by one-third to an early evening nadir,
before rising again through the late evening and early morning hours
(Bremner et al., 1983). A similar circadian rhythm in circulating
levels of testosterone is prevalent in the rat (e.g., Kinon & Liu,
1973; Ellis & Desjardins, 1982). Human cortisol (Bilchert-Toft,
1978) and rat corticosterone (Moberg et al., 1975; Kato et al.,
1980) concentrations also exhibit well-known rhythmic fluctuations,
as do those of thyrotropin (Vanhaelst et al., 1972; Leppaluoto
et al., 1974) and growth hormone (Millard et al., 1985). Reported
attenuations with age in the rhythms of human and rat serum
testosterone (Bremner et al., 1983; Steiner et al., 1984),
luteinizing hormone (LH) (Vermeulen et al., 1989), and growth
hormone (Sonntag et al., 1980; Prinz et al., 1983), among other
hormones, can present differences in young-versus-old comparisons,
depending on when such sampling is performed.
While observable changes in hormonal rhythms or significant
differences in circulating hormone concentrations may reflect
disturbances in the overall functional integrity of the associated
organ system, the absence of such changes should not be necessarily
assumed to indicate a corresponding absence of a functional
alteration. The notion of a "system at risk" presupposes an increase
in the susceptibility to disruption of the homeostatic controls. An
aging system that may be undergoing a subtle erosion in its
endocrine balance could be more likely to exhibit alterations in its
response to a stressor or toxic insult. In this respect the
stimulation of growth hormone release by clonidine, L-dopa and
insulin is substantially depressed (Riegel & Miller, 1981), while
arginine-stimulated growth hormone (GH) secretion after arginine
infusion is preserved (Aschoff, 1979). Secretion stimulated by GHRH
(GH releasing hormone) is only partially reduced (Coiro et al.,
1991).
Regardless of these alterations, it remains established that
the 24 h production of GH is significantly reduced in elderly humans
(Prinz et al., 1983), whereas that of prolactin is increased
(McGinty et al., 1988; Blackman, 1987). These data have been
confirmed in animals (Ceda et al., 1986; Sonntag & Gough, 1988),
although measurements of hormonal profiles may have involved
different procedures in animals and man, thus giving rise to
slightly different interpretations.
Similar difficulties are encountered in studies of age-related
alterations in pineal hormone secretion, including melatonin, whose
circadian rhythmicity is certainly changed with age (Reiter, 1986;
Anisimov & Reiter, 1990).
In order to illustrate age-related alterations in hormone
control, it is useful to focus on the integrated systems which
involve more than one gland or hormone. Although three such systems
are reviewed below, this discussion is by no means intended to be
comprehensive. One theme common to studies of age-related changes in
endocrine function is that such alterations are often hormone and
species specific. Finally, the extent to which any of these changes
relate to potential adverse health outcomes in the older organism
remains to be demonstrated.
2.2.3.1 The pituitary-thyroid axis and the basal metabolism
Thyroid hormones are required during development for growth and
in adult life for regulating oxygen consumption. Maintenance of
thyroid function is generally assured even in old age, although
following repeated stress and demands the reserve function may
become exhausted and a dysthyroid state may follow (Ingbar, 1978).
Changes with aging in the levels of both thyroid stimulating
hormone (TSH) and thyroid hormones (thyroxine (T4) and
triiodothyronine (T3)) are controversial, because concomitant health
disturbances may cause significant fluctuations in the levels of
these hormones (Gregerman & Solomon, 1967; Utiger, 1980). Both hypo-
and hyperthyroidism are not uncommon in the elderly. In general, the
size of the thyroid decreases with age (Gambert & Tsitouras, 1985).
Older people show a normal response to decreased thyroid
function by increased secretion of TSH (Eden, 1987). TSH levels
undergo few changes (Miller, 1989), suggesting that the hypothalamic
control of TSH release has not been altered. However, structural
modifications of TSH have been reported (Klug & Adelman, 1977). T4
levels remain unchanged with age, even though the rate of synthesis
is reduced. However, the blood levels of T3 are reduced with
advanced age (Chopra et al., 1978), while levels of reverse T3 are
unchanged. It should be noted that severe and chronic illnesses, not
directly involving the thyroid, can lower the levels of T3 and
T4.
The alterations observed in thyroid hormone levels are
inadequate to explain the age-associated decline in various
functions that are dependent on thyroid hormone. One possible
explanation is that peripheral sensitivity to thyroid hormone action
is modified by aging. However, with advancing age, the basal
metabolic rate remains unchanged if based on lean body mass, but
decreases if expressed based on body surface area (Masoro, 1985).
2.2.3.2 The pituitary-adrenal axis
The major function of this axis, which is largely based on
pituitary hormones (ACTH) and adrenal hormones (corticosteroids), is
to provide an adaptive response to environmental stress (Selye,
1950; Sapolsky et al., 1986). Any harmful agent, in addition to
inducing a specific reaction in the body (anaesthesia, emotion,
fever, etc.), activates a specific and common response, the
so-called "General Adaptation Syndrome" (Selye, 1950), characterized
by increased adrenocortical secretion, thymic involution,
lymphopenia and eosinopenia. With advancing age this axis may
undergo desynchronization, thus resulting in a failure of
homeostasis and adaptation (Anisimov & Reiter, 1990).
ACTH secretion, which shows a circadian rhythm based on
melatonin fluctuations, is generally preserved in advanced age
(Halberg, 1982), although minor modifications of blood levels may
occur due to variations in renal clearance or alterations in sleep
patterns. However, there is evidence of diminished sensitivity of
the hypothalamic/pituitary axis feedback inhibition by
glucocorticoids (Greden et al., 1986; Blackman, 1987; Dilman, 1987;
Sapolsky et al., 1987). The elderly suffering from Alzheimer's
disease are extremely resistant to glucocorticoid negative feedback
(Sapolsky et al., 1986).
Finally, certain cell populations (e.g., CA3 neurons in the
hippocampus) are particularly susceptible to glucocorticoids.
Long-term stress may result in their dysfunction and death (Sapolsky
et al., 1987).
2.2.3.3 The endocrine pancreas and carbohydrate metabolism
It is well documented that with advancing age the ability to
maintain glucose homeostasis is impaired, but the underlying
mechanisms are still not well defined. Several hormones contribute
to the regulation of glucose homeostasis: above all, insulin and
glucagon, secreted by the endocrine pancreas, and somatostatins and
the pancreatic polypeptide, which modulate the secretion of insulin
and glucagon, respectively. In addition, glucose metabolism may be
affected by other hormones, including T3 and T4, growth hormone,
glucocorticoids and epinephrine (Minaker et al., 1985).
Only modest morphological alterations are observed in the
endocrine pancreas with advancing age. In spite of this fact, blood
sugar levels after fasting are elevated and glucose tolerance is
lowered in the elderly (Magal et al., 1986; Eden, 1987; Ammon
et al., 1987; Wang et al., 1988; Groop, 1989). Plasma insulin
concentration increases and insulin sensitivity decreases.
Alterations in insulin turnover are detectable in the elderly after
glucose load, such as reduced insulin secretion and increased
secretion of the inactive prohormone, proinsulin (Marx, 1987), but
these changes are too modest to account for the observed glucose
intolerance. One alternative explanation is an increase in
peripheral resistance to insulin. In fact, peripheral uptake of
glucose is indeed reduced in the elderly, due to a reduction in
insulin receptors (Pagano et al., 1981) as well as alterations in
the post-receptor signalling process (Rowe et al., 1983). No
evidence exists regarding the possible involvement of age-related
changes in glucagon affecting glucose intolerance in the elderly.
Other factors, however, may contribute to glucose intolerance.
These include: (a) reduced liver sensitivity to insulin, resulting
in reduced glycogenesis; (b) changes in diet and physical exercise;
and c) increased body fat with reduced muscle mass. This last point
seems to merit particular consideration in view of the observation
that insulin resistance is certainly increased in obese humans
(Runcie, 1985). In fact, intracellular fat accumulation leads to a
reduced concentration of insulin receptors (Bolinder et al., 1983).
2.2.4 Reproductive system
The age-related modifications of the reproductive system are
primarily based on alterations in the central nervous system,
pituitary gland and gonads. While menopause is a time-fixed event
involving cessation of ovarian function, the decline of testicular
function is a slow and gradual process, involving limited hormonal
alterations. Older persons show the normal response to deficient
gonadal function by increased synthesis of gonadotropins (Piva
et al., 1987). This occurs in both sexes. In fact, alterations in
serum levels of both luteinizing hormone (LH) and follicle
stimulating hormone (FSH) have been reported (Blackman, 1987). The
reduced presence of sex steroids in women may have an influence on
the function of other endocrine glands. Estrogens have
well-documented effects on salt and water balance and on plasma
proteins, which in turn have effects on the level of thyroid
hormones through a suppression of TSH secretion. Estrogen also
stimulates the production of growth hormone and prolactin. Thus, the
decline in gonadal function during age could have far-reaching
consequences on the individual's physiological function.
2.2.4.1 Female aging
In females, cessation of ovarian function consists of the
transfer from regular menstrual cycles to amenorrhoea, usually
preceded by a period of cycle irregularity. The initial changes have
been reported to occur in hypothalamic-pituitary control of the
ovaries. For example, the age-related decline in reproductive
function is associated with a decreased sensitivity of the
hypothalamic-pituitary complex to feed-back regulation by estrogens
(Dilman, 1971, 1987). This leads to an age-related enhancement of
pituitary gonadotropins (FSH, LH) (Chakravarty et al., 1976),
leading in turn to hyperstimulation of the ovaries. However, despite
the compensatory increase in ovarian hormone production, the level
of estrogens is insufficient to induce ovulation because of
hypothalamic insensitivity, possibly due to an age-related decrease
in the level of biogenic amines and/or peptide hormone receptors
(Dilman & Anisimov, 1979). In addition, the progressive loss of
oocytes plays an important role in the decline in reproductive
function since the reduction of maturating oocytes may induce
desynchronization of pituitary-ovary hormonal interactions
(Aschheim, 1976).
The most common consequences of menopause include imbalances of
the autonomic nervous system, psychological modifications, and
physiological alterations of target organs due to metabolic changes.
Alterations of estrogen target organs are among the most evident
effects of menopause. Vulvar skin and vaginal epithelium may undergo
atrophy. Glycogen content is generally reduced, with a consequent
decrease of lactobacilli, rise of vaginal pH, and increased growth
of pathogenic microbes. The uterus and oviducts atrophy due to the
decreases in estrogen levels. In ovaries, follicular cysts and
atresia result in response to the altered hormonal status.
Hyperplasia of the theca cells occurs. Fibrosis also occurs in these
tissues but, in addition, can affect the bladder and urethra,
resulting in an increased incidence of cystitis, dysuria and
non-infectious urethritis. The reduced thickness of the skin is also
a result of the decrease in estrogen (Schiff & Wilson, 1979).
Menopause has major health consequences for the cardiovascular
and skeletal systems. The reduction in estrogen secretion removes
the protection offered by these hormones against coronary heart
disease, development of atherosclerosis, and accompanying
alterations of lipid metabolism. Osteoporosis, resulting from
increased bone reabsorption relative to bone formation, is a common
problem in postmenopausal women (Riggs, 1987). Two types of
osteoporosis may be identified. Type I is associated with estrogen
withdrawal and may begin in middle age (Riggs & Melton, 1983). The
biological effects are linked to disruption of the complex
relationship between calcium intake and loss, and the secretion of
calcitonin, parathyroid hormone and 1,25-dihydroxy-vitamin D.
Estrogens prevent the transfer of calcium from bone to blood and its
loss through urine. This induces parathyroid hormone secretion,
which stimulates the formation of 1,25-dihydroxycholecalciferol, the
active metabolite of vitamin D. The function of the parathyroid is
affected by increasing age (Eden, 1987). The relevance of estrogen
for bone loss is further supported by the effectiveness of estrogen
therapy in delaying the osteoporotic process in post-menopausal
women (Edman, 1983). With advancing age type II osteoporosis (senile
osteoporosis) may occur, which is probably due to the poor
intestinal absorption of calcium (Riggs, 1987).
2.2.4.2 Male aging
The reproductive system is less affected by aging in males than
in females. It is generally accepted that testosterone levels are
maintained within the physiological range throughout life, although
a decrease in testosterone production in response to gonadotropin
action may occur in old age due to a reduction in Leydig cell number
and function (Harman et al., 1982). Testis and accessory sex organs
do not show substantial modifications with age, and sperm is found
in the ejaculate of very elderly men. The volume of seminal fluid is
generally decreased.
While the prostate undergoes involution in the majority of old
men, in about one-third of males it undergoes hypertrophy with
consequent obstruction of the urethra and urinary flow from the
bladder. The cause of the hypertrophy is still unclear (Mawhinney,
1985). The prostatic enlargement results in compensatory hypertrophy
of the bladder. When such compensation is no longer sufficient,
retrograde filling of the renal pelvis and ureters may occur,
resulting in hydronephrosis and eventually renal failure.
2.2.5 Immune system
With advancing age a progressive increase occurs in the
incidence of various infectious diseases, autoimmune processes and
tumours. These may be in part based on age-related defects in the
immune system. The association of so many age-related pathologies
with defects in the immune system has led to the suggestion that
aging of the immune system may be rate limiting for life span
(Walford, 1969). However, while there are numerous experimental and
clinical studies demonstrating an age-related deterioration in
immune efficiency, this decline is not sufficient to account for all
manifestations of aging.
There are several recent reviews on aging and the immune system
(Revskoy et al., 1985; Lipschitz, 1987; Segre et al., 1989; Miller,
1991). However, it is still difficult to draw a comprehensive
picture, because of the many cellular and humoral components
involved in immune reactions and the many modulating
extra-immunological factors which may also be compromised in the
elderly. The immune and haematopoietic systems are intimately
related, being derived from a common pluripotent stem cell. Both
play central roles in host defense, prevention of neoplasia, and
response to infectious agents (Lipschitz, 1987). However, basal
haematopoiesis in both animal models and man seems to be either
unchanged or minimally altered with age (Dybkder et al., 1981;
Lipschitz, 1987). The reserve capacity may be reduced resulting in a
decreased ability to respond to stress.
2.2.5.1 Aging of lymphoid organs
Peripheral lymphoid organs, such as the spleen and lymph nodes
do not show consistent modifications in size with aging. Bone marrow
is not consistently affected by age. Stem cell production is
generally well preserved in old age (Harrison et al., 1978),
although a slight change in the replication rate of stem cells has
been reported by some authors (Schneider et al., 1979). Thymic
involution has been considered to account for the major age-related
changes in the immune system, beginning at puberty. Such an
involution consists of a progressive loss of cellularity with
lymphoid cell depletion in the cortical areas and cystic changes in
the epithelial cells. These are the source of various peptides
involved in differentiating thymic lymphocytes (T-cells) from
lymphoid cells of earlier lineage. The export of newly
differentiated T-cells is reduced with advancing age (Globerson
et al., 1989). The synthesis and the secretion of polypeptide thymic
hormones, such as thymosin (McClure et al., 1982), thymopoietin
(Lewis et al., 1978) and thymulin (Bach et al., 1972), are
progressively diminished. In all cases, the reduction of thymic
endocrine activity seems to have a pathogenic role in age-related
immune dysfunctions, since replacement by exogenous administration
of the hormones is capable of restoring various immune functions in
old age (Zatz & Goldstein, 1985). The turnover of zinc, which is
essential for immunocompetence (Iwata et al., 1979; Chandra, 1985),
decreases in old age. Zinc supplementation can restore immune
functions (Fabris et al., 1990).
2.2.5.2 Aging of cellular constituents
Mature T-cells, bone marrow lymphocytes (B-cells) and natural
killer cells (NK-cells) can be detected in blood and in lymphoid
organs by specific monoclonal antibodies. With this type of
analysis, no major modifications in the proportion of the various
lymphoid cell subpopulations have been observed in humans. However,
the major alteration in the immune system appears to arise in the
functioning of T-cells (Thompson et al., 1987). While the total
number of T-cells in the peripheral blood does not change
appreciably with age, there are clear-cut differences in the
relative proportion of T-cell subtypes (Wagner et al., 1983;
Fernandes, 1984; Revskoy et al., 1985; Thompson et al., 1987;
Lipschitz, 1987).
The number of immature lymphocytes of the T-lineage increases
with age, as does the percentage of apparently activated
T-lymphocytes bearing immature thymic phenotypic markers. There is a
relative increase in cytotoxic/suppressor T-cells, and a decrease in
the number of helper/inducer T-cells (Lipschitz, 1987; Thompson et
al., 1987). Correlated with the decrease in the helper/inducer
population, is a functional defect in cell-mediated immunity
(Lipschitz, 1987; Thompson et al., 1987). Cells from aged humans or
experimental animals are less capable of responding to allogeneic
lymphocytes, phytohaemagglutinin, concanavalin A and soluble
antigens. Lymphocytes from older mice are less able to elicit
graft-versus-host reactions than those from younger mice of the same
inbred strains (Thompson et al., 1987). Fifty percent of healthy
people over age 50 have impaired cutaneous hypersensitivity
(Lipschitz, 1987; Dilman, 1987). Accompanying the decrease in
helper/inducer T-cells and cell-mediated immune functions is a rise
in autoantibodies and autoimmunity (Thompson et al., 1987).
Changes in humoral immunity (B-cell function) with aging are
more subtle (Lipschitz, 1987; Senda et al., 1989). Studies on the
effects of age on antibody production have yielded conflicting
results, perhaps because of the wide range of experimental values
generally observed in older individuals. It has, however, been well
established that aging is significantly associated with the presence
of various autoantibodies, in particular, antibodies against nuclear
antigens. There is also evidence that aging effects the rate of
antibody production by activated B-cells (Lipschitz, 1987).
From a functional point of view, defects have been observed at
various levels. Firstly, the proliferative capacity of T-cells from
old individuals is generally reduced, regardless of the stimuli used
(antigens, mitogens), and the defect consists both in a reduced
number of cells responding to stimulus and in a precocious
exhaustion of the cloning capacity (Fabris et al., 1983) of
responding cells. Secondly, the response to interleukins, which
physiologically mediate the modulation of the proliferative
reaction, is depressed and this phenomenon has been documented not
only for T-cells but also for NK cells, which are less sensitive in
old age to the boosting action of IL-2 or interferons (Provinciali &
Fabris, 1990).
With respect to accessory cells (phagocytic cells,
macrophages), their number and function are not altered by age, and,
in certain circumstances, their activity seems to be enhanced.
2.2.5.3 Neuroendocrine-immune interactions
The immune system, although regulated to a large extent by
intrinsic cellular and humoral events, is also sensitive to signals
generated from the nervous and endocrine systems. Communication
between nervous and immune networks is mediated by hormones and
neurotransmitters which reach lymphoid organs and cells via blood or
direct autonomic nervous system connections (Bullock, 1985; Felten
et al., 1985). The neuroendocrine immune interactions are mediated
by circulating humoral factors from the
pineal-hypothalamic-pituitary axis, either directly via
neuropeptides and hormones, or indirectly by the effects of this
axis on the hormonal secretion of peripheral endocrine glands, which
also exert immunomodulating actions (for review, see Fabris, 1991).
The nervous and neuroendocrine systems not only act as
modulators of the immune network, but also as targets for signals
generated within the immune system, such as those exerted by thymic
factors (Hall et al., 1989) and interleukins, (Besedovsky et al.,
1985), and by pituitary-like factors (ACTH, TSH, GH, PRL,
gonadotropins, endorphins), which are produced by mature lymphocytes
upon antigenic stimulation (Weigent et al., 1990).
The sharing of humoral signals, as well as of the specific
receptors between neuroendocrine and immune cells (for reviews, see
Fabris & Provinciali, 1989; Weigent et al., 1990), implies that
biological response modifiers of neuroendocrine-immune origin might
be developed in the near future for therapeutical purposes. On the
other hand, potentially harmful agents for one of these homeostatic
systems may also cause alterations in others.
From an experimental point of view, it has been demonstrated that
treatments of old animals with thyroid hormones (Fabris et al., 1989),
GH (Kelley et al., 1986) and analogues of LH releasing hormone
(Greenstein et al., 1987) are able to induce regrowth of the thymus
and reacquisition of its endocrine activity (for review, see Fabris
1991). Analogous treatments, such as with melatonin (Pierpaoli
et al., 1991), GH (Davila et al., 1987), TSH and thyroid hormones
(Provinciali & Fabris, 1990), are also able to recover various
age-related peripheral immune deficiencies, such as T-cell
functioning and NK cytotoxicity. In humans, little work has been
done in this area, although indirect evidence, obtained primarily
from studies on endocrinopathies in the elderly (Fabris et al.,
1989; Travaglini et al., 1990), suggest that recovery of both thymic
and peripheral immune function can be achieved by a neurohumoral
approach.
Little is known on the potential effect of thymosins,
interleukins and lymphocyte-derived pituitary-like cytokines on
age-related alterations of the nervous and of the neuroendocrine
system. Experimental information from old animals showing recovery
of the hormonal and metabolic profile following immune manipulation
(for review, see Fabris et al., 1988) is undoubtedly opening a new
research approach for human investigations.
Receptor sites for many hormones are present on the membrane of
lymphoid cells (Fabris & Provinciali, 1989). The number of
glucocorticoid receptors in spleen cells decreases in old animals
(Roth, 1979a). Hormones that modify the turnover of cyclic
nucleotides result in consequent activation or inhibition of immune
functions (Hadden, 1983). Hormones influence the production of
several lymphokines and monokines (Kelso & Munck, 1984).
The neuro-endocrine system seems to act not only as a modulator
of the immune network but also as a target for signals generated
within the immune system. Examples of such interactions are the
alterations that can be induced in the neuroendocrine balance,
either by removal of relevant lymphoid organs such as the thymus or
by dysfunction of the immune system itself as a result of reactions
to immunogenic or tolerogenic doses of antigen (Besedovsky et al.,
1975). In addition, mature lymphoid cells, when stimulated by
antigens, produce humoral factors similar, if not identical, to
classical hormones and neurotransmitters (such as ACTH, TSH, GH,
PRL, gamma-endorphins) (Blalock et al., 1985). These reciprocal
influences between the neuroendocrine and the immune systems
(Fabris, 1981; Fabris et al., 1988) occur throughout life, but have
particular relevance during aging (Fabris & Piantanelli, 1982).
2.2.6 Cardiovascular system
The frequency of cardiovascular diseases, which are the major
cause of death in industrialized countries, increases with age.
Diseases such as hypertension and atherosclerosis occur most
commonly in the elderly. In addition, degenerative changes of the
cardiovascular system, involving the myocardial cells as well as
cells of the pacing-conduction system, that arise during the aging
process lead to impaired cardiac function and arrhythmia even in
people without any clinical evidence of hypertension or coronary
artery diseases. Inadequate function of the cardiovascular system
induces effects in peripheral tissues and organs. Changes in
peripheral organs resulting in hyperlipidaemia,
hypercholesterolaemia, and hypo- and hyperglycaemia can also effect
the cardiovascular system in the elderly.
2.2.6.1 Heart
The heart itself can be considered to be made up of two parts:
a) the conduction system responsible for electrically controlling
the heart rhythm; and b) the myocardium performing the contractile
function of the heart and composed of a system of trabeculae.
The biophysical and biochemical mechanisms that govern cardiac
muscle change with age, resulting in characteristic alterations in
muscle function (Lakatta, 1987a,b). Many of the steps in the
excitation-contraction system in cardiac muscle are altered by
aging. In an isometric contraction, the transmembrane action
potential (TAP) excites the cell and the contractions that ensue are
longer in duration. The magnitude of the prolongation of
depolarization of the TAP in senescent muscle is striking (i.e.
about twofold). The action potential amplitude is also greater in
senescent than in adult muscle in both high and low calcium-loading
conditions. These deficits of the senescent muscle may be related in
part to the diminished Ca2+ pumping rate by sarcoplasmic
reticulum. The duration of the elevated myoplasmic Ca2+ level is
prolonged in senescent muscle.
Sagiv et al. (1988) found that left ventricular contractility
increases less on stimulation in elderly subjects than in younger
people. Although the aging process is associated with normal resting
contractile function, diastolic properties are altered, resulting in
reduced and delayed early left ventricular filling and enhanced
atrial contribution to diastolic volume. Exercise cardiac output is
maintained in healthy elderly individuals, but there is a shift from
reliance on an increase in heart rate and a decrease in end systolic
volume to use of the Frank-Starling mechanism to increase stroke
volume. This age difference in the cardiovascular response to
exercise is probably mediated by an age-associated decreased
responsiveness to beta-adrenergic stimulation.
In muscle tissue of the aging heart, some morphological changes
are observed both in animal and human studies (Koobs et al., 1978;
Speijers, 1983). The most common change in the aging heart is
hypertrophy (Lakatta, 1985). Other alterations consist of the
appearance of slight focal necrosis and fibrosis in the myocardium,
amyloidosis (Finch & Hayflick, 1977) and the appearance of
lipofuscin (Hendley et al., 1963; Koobs et al., 1978). Peroxidative
damage to the myocardium is cumulative and irreversible (Koobs
et al., 1978).
2.2.6.2 Blood vessels
Both physiological and morphological changes are observed in
the vascular system, especially in the small and large arteries. The
morphological changes in the arteries seen in the elderly vary
considerably both in appearance and in localization (Goyal, 1982;
Hazzard, 1985). The thickness of the aorta increases significantly
with age, while the number of nuclei in the cells of the arterial
media decreases in humans as well as in mice. The majority of these
changes in humans are categorized as atherosclerotic. These changes
can progress and result in complicated coronary atherosclerosis and
ischemic heart disease, but other factors may also cause clinical
effects such as angina pectoris, arterial spasms, and myocardial
infarcts (Speijers, 1989a).
Morphological changes in the veins are less pronounced than in
the arteries.
The physiological changes observed with aging are often a
result of changes both in heart function and in the arteries. These
changes are reflected in haemodynamic parameters such as an increase
in diastolic and systolic blood pressure, mean arterial blood
pressure and vascular resistance, and a decrease in responsiveness,
and in contraction and relaxation responses (Lakatta, 1986, 1987b;
Duckles, 1987; Mazzeo & Horvath, 1987; Zemel & Sowers, 1988;
O'Malley et al., 1988; Cleroux et al., 1988).
Aging is often accompanied by increases in the incidence and
prevalence of hypertension. Geriatric hypertension is generally of a
salt-sensitive nature with a disproportionate frequency of isolated
systolic hypertension. The age-related increase in salt sensitivity
is due to a decline in renal function (Zemel & Sowers, 1988) and
deregulation of vascular tonus. Age-associated declines in the
activity of membrane sodium/potassium-ATPase may also contribute to
geriatric hypertension because this results in increased
intracellular sodium loading, causing reduced sodium/calcium
exchange and thus increased intracellular calcium and vascular
resistance.
It is commonly accepted that atherosclerotic changes take place
to a certain extent in every individual. Multiple factors determine
the extent and velocity of the atherosclerotic process (Hazzard,
1985). The incidence of clinically observed atherosclerotic effects
is higher in elderly subjects than in younger individuals.
Atherosclerotic damages result in impaired cardiovascular function.
Atherosclerosis is defined as a multifactorial disease with
variable effects in the intima followed by changes in the media of
arteries. These changes consist of focal accumulation of lipids,
proliferation of smooth muscle cells, and accumulation of complex
carbohydrates (i.e. glycosaminoglycans, proteoglycans), blood and
blood products, collagen and calcium compounds (Campbell &
Chamley-Campbell, 1981; Velican, 1981; Speijers, 1989a). The
resultant modifications of arterial wall integrity can lead to the
following: erosion of the wall with consequent reduced resistance to
blood pressure, rupture, and haemorrhage; progressive thickening of
the wall due to reactive proliferation of tissues with consequent
reduction of blood flow; and clotting of the blood at the level of
the injured wall with consequent sudden obstruction. The basic
lesion seems to develop in the first decade of life (Lee, 1985).
In the etiology of the lesion two localized cofactors should be
taken into account: the blood supply of the arterial wall; and blood
turbulence, since lesions are more frequently found around the
orifices of arteries branching off major arteries or at bifurcations
(Patel & Vaishnaw, 1980). Hypertension, diabetes, autoimmunity and
stress are also risk factors contributing to atherosclerosis.
2.2.6.3 Characteristics of atherosclerotic lesions
The first event in the formation of the lesion is still
debated. Both an initial thickening of intima, due to an
accumulation of blood-born amorphous material (lipids, protein and
sulfated proteoglycans) and a proliferation of muscle cells (due to
still undefined stimuli), with consequent degeneration and reactive
macrophage and connective tissue accumulation, have been proposed as
major initiating phenomena (Benditt, 1977; Ross, 1981). The
subsequent phase of the lesion involves repair mechanisms that cause
further thickening of the intima. Following this, lipid increases
both in the cells and in the intercellular spaces. Lipid
accumulation is progressive, leading to an increased number of foam
(lipid-containing) cells which disintegrate and form the gruel-like
substance that has given the name of atheroma to the lesion. The
accumulation of such material acts as an irritant, inducing a
proliferative reaction (encapsulation) which leads to the
development of a plaque. Calcification follows, making the arterial
wall more rigid. Alternatively, when the capsule breaks, an ulcer
can occur, leading to loss of tissue in the arterial wall, blood
clots, haemorrhage, and consequent thrombosis and/or rupture of the
arterial wall.
2.2.6.4 Theories of atherosclerosis
Atherosclerosis is undoubtedly a multifactorial process, which
is reflected by the many theories proposed (Baker & Rogul, 1987).
The lipid accumulation theory is based on the progressive
accumulation of oxidized lipids (mainly low density lipoproteins)
not only in smooth muscle cells but also in migrating monocytes
(Avogaro et al., 1983). The link between lipid deposition and
consequent alterations remains unclear (McCaffrey et al., 1988).
Theories on monoclonal proliferation of smooth muscles cells
are based on a mutagenic event in these cells, due to a
physico-chemical or viral insult (Benditt, 1977; McCaffrey et al.,
1988), leading to the formation of a kind of benign tumour. The
thrombogenic theory is based on the early adherence of platelets to
small alterations in the endothelium, leading to thrombus formation
and release of growth factors by platelets which induce smooth
muscle cell proliferation (Ross, 1981; Bang et al., 1982). Other
risk factors that should be taken into consideration are
hypertension, cigarette smoking, increased body weight, high serum
uric acids and consumption of saturated fats.
The immune system in the elderly is weakened. Consequently
there is increased susceptibility to chronic infections,
autoimmunity, and elevation of circulating immune complexes. New
data in the literature indicate that some viral agents may cause
cardiac and arterial cell lesions and subsequent inflammation. Thus,
viruses and altered immune cells may cooperate and play a role in
arterial wall lipid accumulation, possibly acting as initiating
factors for atherosclerosis (Butenko, 1985).
2.2.7 Respiratory function
Respiratory function is based on gas exchange (oxygen
absorption and carbon dioxide elimination), gas transport (red blood
cells), and on internal metabolic processes that utilize oxygen at
the cellular level. Aging may affect all of these processes (Masoro,
1981), but it is the first that is usually most compromised in
advanced age. The decline in the gas-exchange system may involve the
lungs, the thoracic cage, the respiratory muscles and the
respiratory centres in the CNS. The deterioration of the lungs is
largely dependent on environmental factors, in particular on the
contamination of air with toxic substances, dust and microbiological
agents. Therefore, age-related lung alterations may vary according
to life styles (smoking, physical exercise), environmental
conditions (urban/rural), and intercurrent diseases (infections,
work-related diseases) (Davies, 1985).
2.2.7.1 Gas-exchange organs
The most evident lung alterations with advancing age are
represented by enlargement of alveolar ducts with flattening of
alveoli and loss of septal tissue, reduction of elastic fibres, and
increased fibrosis of the capillary system (Liebow, 1964).
Functional consequences are a reduction in the surface area for gas
exchange with an increase in the physiological dead space, reduction
of the ventilatory flow rate, and irregular distribution of blood
flow (Mauderly, 1978). Age-related alterations also occur in the
chest as a consequence of calcification of costal cartilage,
increased stiffness of costovertebral and vertebral joints, and
general rigidity of the chest. Both lung and chest alterations
contribute to the changes in lung volume and pressure: vital
capacity decreases, residual volume increases, and flow rates
(particularly expiratory flow rate) decline (Morris et al., 1971).
The alteration in the gas-exchange capacity causes reductions in
oxygen uptake and pressure and lower arterial pO2, whereas pCO2
remains constant even in very old age (Morris et al., 1971). The
control of ventilation by brain centres is also altered in old age.
It is still unknown whether such alterations are due to intrinsic
damage of the neural component or to a reduced responsiveness of
neuromuscular activity in the chest (Peterson et al., 1981).
All these alterations, while not necessarily life-threatening
for the elderly, may favour pathologies such as chronic bronchitis,
pneumonia and emphysema (Peterson et al., 1981). The concomitant
hypoxia (low oxygen levels) may cause increased production of red
blood cells with consequent polycythaemia. This may contribute to
hypertension and cardiac failure.
2.2.7.2 Erythropoietic activity
Although specific age-related alterations in the life cycle of
erythrocytes have been reported (Danon, 1969), the overall function
of the erythropoietic system seems to be well preserved. The
regenerative potential following hypoxia seems nearly inexhaustible.
In addition, haemoglobin turnover does not seem to be affected by
age (Lipschitz, 1987).
2.2.8. Kidney and body fluid distribution
The urinary tract is affected by aging both in its renal
functions of excretion and ionic control of body fluids, and in its
control of bladder and urethral activity.
2.2.8.1 Renal function
Kidney function decreases both due to anatomical and
physiological alterations with age (Wesson, 1969; Kaysen & Myers,
1985; Brown et al., 1986; Corman & Michel, 1986; Owen & Heywood,
1986; Meyer & Bellucci, 1986; Anderson & Brenner, 1986, 1987;
Goldstein et al., 1988; Euans, 1988; Rudman, 1988). These
alterations have been observed in both experimental animals and in
humans.
The weight and volume of the kidney decrease by 20 to 30%
between the ages of 30 and 90 years. The atrophy is primarily
cortical and seems to be related to intrarenal vascular changes. The
number of surviving nephrons is reduced and these remaining nephrons
tend to be enlarged (Kaysen & Myers, 1985; Brown et al., 1986;
Lindeman, 1986; Rudman, 1988). The number of glomeruli decreases by
30 to 50%, and there is an increasing percentage of sclerotic and/or
abnormal glomeruli. The glomerular filtration rate (GFR) decreases
with age resulting in an adaptive increase in glomerular perfusion
pressure (Kaysen & Myers, 1985; Lindeman, 1986; Meyer & Bellucci,
1986; Anderson & Brenner, 1986, 1987; Blum et al., 1989). This
decline in GFR is due in large part to the progressive reduction in
blood flow to the kidneys (Brown et al. 1986; Lindeman, 1986).
Glomerular mesangial volume increases by 50% and 1 out of 10
glomeruli is sclerotic at the age of 80 years compared with 1 out of
100 in the young adult (Brown et al., 1986). The renal tubules
decrease in number, proximal tubule volume and length decrease, and
distal tubules develop increased diverticula. The renal arterioles
develop intimal thickening, reduplication of the lamina elastica
interna, and mild hyalinization (Brown et al., 1986; Lindeman, 1986;
Rudman, 1988).
An age-related reduction in secretory and resorptive capacity
is seen in the tubules, which is explained by a progressive loss of
functioning nephrons (Lindeman, 1986). Tubular function, which
regulates water and salt balance, is also affected. A decrease in
the ability to concentrate urine with age has been well documented
in humans. This appears to result from a decreased medullary
tonicity caused mainly by an inability to respond normally to
antidiuretic hormones (ADH) (Kaysen & Myers, 1985; Brown et al.,
1986; Meyer & Bellucci, 1986; Lindeman, 1986; Euans, 1988). Despite
age-related decreased renal function, the blood pH, partial pressure
of carbon dioxide, and serum hydrogen carbonate concentration of the
geriatric population without renal disease do not differ
significantly from those of the young under basal conditions
(Lindeman, 1986). Both the ability to maximally dilute the urine and
to maximally concentrate it, are controlled by serum ADH and by the
action of that hormone on the collecting ducts (Kaysen & Myers,
1985; Os et al., 1987). Increased arginine-vasopressin (AVP)
secretion per unit of plasma reflects a decrease in collecting
tubule sensitivity to AVP. This change in sensitivity is not
completely offset by increased ADH release (Davis & Davis, 1987).
The suppression of ADH secretion is not maximal when serum
osmolality is reduced.
The renin-angiotensin-aldosterone system is also poorly
responsive to volume depletion in aging subjects. As a result, the
elderly cannot maximally retain sodium under conditions of plasma
volume contraction (Kaysen & Myers, 1985; Lindeman, 1986; Euans,
1988). The activity of the renin-angiotensin system is progressively
reduced with age. It has been suggested that angiotensin II does not
play an important role in the maintenance of blood pressure and
kidney hemodynamics in normal senescence (Corman & Michel, 1986).
The mean blood pressure is correlated with age and the decline in
renal function (Lindeman et al., 1987).
The kidney is also the site of vitamin D1 hydroxylation, which
is dramatically reduced during aging (Kaysen & Myers, 1985).
2.2.8.2 Lower urinary tract
The most frequent age-related alterations in the lower urinary
tract result in urine incontinence in both sexes and urine retention
in males. Incontinence occurs at high incidence although it is not
an inevitable consequence of aging. The high number of physiological
requirements for urinary continence may account for such frequent
failure (Williams & Pannill, 1982). In women, the reduction of
estrogen levels after menopause may decrease the tone of the smooth
muscle around the pelvic floor and the bladder outlet, thus
favouring urinary incontinence. In men, hypertrophy of the prostate
represents the major cause for involuntary loss of urine, because of
the associated frequent instability of the detrusor muscle. Other
causes for urinary incontinence are represented by delirium,
infections, restricted mobility and polyuria.
2.2.9 Gastrointestinal function
Changes in the gastrointestinal tract during aging consist
mainly of a reduced cell turnover, leading to mucosal hypoplasia.
Accessory organs, such as the exocrine pancreas and the liver, are
affected by aging independently.
2.2.9.1 Gastrointestinal tract
In contrast to the cardiovascular or excretory system, the
gastrointestinal (GI) tract does not exhibit marked structural or
functional changes with age (Penzes, 1984). Aging may affect all
regions of the GI tube. The first signs of aging are generally
observed in the mouth. Teeth undergo discoloration, pulp recedes
from the crown, dentine is often poorly renewed, and the gingiva
frequently recede (Walker, 1985). These alterations favour bacterial
growth which can lead to chronic periodontal inflammation and tooth
loss.
In the stomach, reduced secretion of hydrochloric acid and
pepsin is often found in the elderly. These changes result from
alterations in enzyme-secreting cells or from altered hormonal and
neural regulation. However, in some cases, increased acid secretion
may occur with advancing age, leading to gastritis, erosions and,
ultimately, ulcers (Kumpuris, 1983).
No major morphological alterations are observed in the
intestine. The relatively mild modification of villi, the increased
collagen content and the reduced mucosal cell proliferation
(Webster, 1985) cannot account for the impaired absorption of
nutrients, including minerals (calcium, iron and zinc) and vitamins
often found in the elderly. Other factors such as reduced motility
and inadequate intestinal blood supply may play a more important
role than the slightly altered anatomical integrity.
In the lower GI tract the rectal tone is generally decreased in
the elderly and the sphincter is weakened, leading to incontinence.
Neurological alterations (dementia), muscle atrophy, diarrhoea and
constipation can all contribute to anal incontinence.
2.2.9.2 Pancreas
Pancreatic function is not seriously compromised during normal
aging. Structural changes mainly involve a reduction in the number
of secreting cells, with a consequent decrease in size of the
pancreas and a moderate increase in collagen content. Reduction in
the levels of trypsin, hydrogen carbonate, amylase and lipase in the
pancreatic juice occurs commonly in the elderly (Vellas et al.,
1988). Overall, however, the functional efficiency of the pancreas
is not lost during aging.
2.2.9.3 Liver
Structural changes in the liver with age are relatively minor.
However, the most significant change in human liver is a decrease in
volume (Wynne et al., 1989). The life-span of the hepatocyte is
long, with cells only dividing once or twice during the lifetime in
the absence of a growth stimulus (Popper, 1986). Whether the
function of the aging hepatocyte is impaired is unclear. Kupffer
cell function may be impaired as demonstrated by a reduction in
phagocytic activity. Endocytosis is reduced in Kupffer, but not
endothelial, cells. An increase in collagen also characterizes aging
liver.
The function of aging liver also undergoes a number of
alterations. In humans, cholesterol synthesis is reduced in the
elderly, while biliary secretion is increased (Popper, 1986).
Hepatic blood flow is reduced by as much as 50% (Sherlock et al.,
1955). Age-related changes in liver enzyme expression have been
studied in more detail in rodents (Fishbein, 1991). The efficiency
of carbohydrate and intermediary metabolism is decreased in
senescent rats or mice of both sexes, due in part to decreased
insulin-binding and the impaired regulation of enzymes such as
pyruvate kinase. Hepatic protein synthesis also decreases with age,
and the sex-specific expression of drug and steroid-metabolizing
enzymes in male rats appears to be feminized in senescence (Kitani,
1991).
2.2.10 Musculo-skeletal system
Aging dramatically affects bone, joints and skeletal muscles.
The basic phenomena responsible for the aging of the
musculo-skeletal system are not completely understood because of the
involvement of many factors, e.g., hormones, nutrition and physical
exercise, in addition to specific age-related alterations at the
tissue level, resulting in general deterioration of the system.
2.2.10.1 Bones
Bone should not be considered as metabolically quiescent since
it is constantly remodelled according to mechanical demand and
continuous turnover due to new bone formation (by osteoblasts) and
bone resorption (by osteoclasts) (Exton-Smith, 1985).
With aging, the balance between bone formation and bone
resorption is altered in favour of resorption, resulting in a
reduction of bone mass. This starts from the medullary region with
enlargement of the cavity and moves towards the epiphysis of the
bone and then to the outer surface with a final reduction of cortex
thickness. As a consequence, the bone strength is reduced (Smith
et al., 1981). The prolonged period where bone resorption exceeds
bone formation may result in osteoporosis (Dawson-Hughes et al.,
1987; Eastell & Riggs, 1987; Riggs, 1987; Croucher et al., 1989).
The factors responsible for bone resorption and remodelling are
not well known. More knowledge is available concerning two other
factors which profoundly affect bone metabolism: calcium turnover
and hormonal profile. Calcium is absorbed through the intestinal
mucosa and is excreted from the kidney, the blood level remaining
remarkably constant throughout life. Calcium is required for many
essential functions in the body, such as cell division, cell
intermediary metabolism, and cell functions (excitability, secretion
and movement). In the case of a negative balance, calcium is
mobilized from the bone with a consequent reduction in bone mass.
Calcium metabolism is under hormonal control. Parathyroid
hormone increases the plasma calcium level by promoting bone
resorption and mobilization, whereas calcitonin lowers blood calcium
by inhibiting bone resorption. Calcitriol (the active derivative of
vitamin D) increases intestinal absorption and decreases excretion
of calcium, thus acting to increase the body burden of calcium. The
age-associated decrease in intestinal calcium absorption lowers the
plasma calcium level, leading to an increase in parathyroid hormone.
Calcitriol levels may also be deficient in the elderly, as a result,
perhaps in part, of the decrease in vitamin D synthesis in old skin
and alterations in renal metabolism.
Bone metabolism also depends on other hormonal factors.
Estrogens have a strong positive effect on bone density. After
menopause, bone resorption exceeds bone formation resulting in bone
mass loss. However, the occurrence of osteoporosis greatly depends
on the adult bone peak mass, physical activity, and calcium intake
(Eastell & Riggs, 1987; Riggs, 1987; Bornor et al., 1988; Stevenson
et al., 1988; Lindsay, 1989).
Glucocorticoids lower plasma calcium levels, which can increase
bone resorption by stimulating parathormone secretion. Thyroid
hormones may also cause bone loss, although the mechanism is still
unclear. Growth hormone, somatomedins and insulin all promote bone
formation, and therefore defective secretion (as in diabetes) may
cause osteoporosis.
2.2.10.2 Joints
Articular disorders are practically universal in elderly
people, and are associated with pain and disability. The major
alteration consists of the loss of the smooth surface of cartilage,
which becomes thicker and less elastic. The cartilage develops rough
surfaces and mechanical irregularities, which represent the early
steps in osteoarthritis (Evens & Hawkins, 1984).
2.2.10.3 Skeletal muscles
With advancing age muscles become smaller in size, due to
reduction in the number of fibres, and less elastic, due to an
increase in collagen content. The fat content of muscle tissue also
increases with age. The causes for such defects are still
controversial: alteration of sarcoplasmic reticulum, decrease of
contractile protein synthesis and a reduction in the number of
mitochondria are all found in aged muscle cells.
In addition to muscle cell alterations, the neuromuscular
junction is modified in aged muscle. The acetylcholine content of
nerve terminals is consistently reduced and acetylcholine receptors
are irregularly distributed. Motor nerve conduction velocity is also
impaired. All these defects contribute to the progressive failure of
muscular efficiency, although some muscles, such as the diaphragm,
do not seem to suffer age-related effects (Finch & Hayflick, 1977).
Thus, the additive results of the loss of bone mass, disorder of
joints, decline in skeletal muscle power and nervous system
discoordination may lead to loss of body stability, falls and bone
fracture in old people.
2.2.11 Skin
The age-related modification of the skin is the most dramatic,
common and constant event in life, so that it may constitute one of
the best external markers of aging. Only recently has it been
possible to distinguish between intrinsic aging of the skin and
photo-aging (Gilchrest, 1984). Cutaneous neoplasia is one of the
best documented interactions between aging of the organism and
environmental effects. A variety of environmental factors, the most
notable being ultraviolet radiation, has been shown to cause cancer
of the skin in humans and animal models (Rogers & Gilchrest, 1990).
The structure of skin changes throughout life (Behl et al.,
1987), although many of the alterations are due to exposure to
sunlight rather than intrinsic aging (Gilchrest, 1984). Skin
thickness increases during maturation and then gradually declines
(Vogel, 1983). However, recent studies in mice and rats by
Monteiro-Riviere et al. (1991) have not reported any significant
changes in skin thickness or blood flow from maturity throughout the
life span. Hydration, which can affect chemical solubility,
decreases while keratinization increases.
Skin aging is linked to alterations affecting both the
epidermal and the dermal components. The epidermis suffers from a
reduction in the turnover of epidermal cells, due both to a
reduction of basal cell renewal and to a slower maturation toward
the stratum corneum. The dermo-epidermal interface is flattened so
that the total external body surface area is reduced (Selmanovitz
et al., 1977). The reduction in epidermal cell turnover is
responsible for the slowing of wound healing and possibly for the
dryness and/or roughness of aged skin. The dermis is reduced in
thickness in the elderly and is characterized by a reduced collagen
content with a biochemical modification of the collagen itself,
which makes it more strong and less elastic. Vascularization in
humans is also reduced.
Aging also results in modifications in the skin appendages.
Sweat gland function is decreased resulting in impaired
thermoregulation. Sebaceous glands produce less oil and wax (dryness
of skin), and hair usually loses pigment. The numbers of skin
sensory organs (Pacinian and Meissner's corpuscles) decrease with
age and sensation is consequently modified.
Collagen aging occurs in the skin, as in other tissues, leading
to increased rigidity. Collagen is composed of several related
proteins, which are produced by fibroblasts and extruded into the
extracellular space. Here they can be chemically deposited in a
nearly pure form, as in the tendons, or immersed in an extracellular
matrix, also produced by fibroblasts, as in the skin. The collagen
fibres undergo maturation in the extracellular ground substance by
parallel arrangements of tropocollagen fibres which became assembled
together through cross-linking. This phenomenon is an index of
maturational change. With age, however, the cross-linking increases
(Houck et al., 1967), leading to a reduction of tensile strength and
plasticity (Verzar, 1968). The extracellular matrix also shows
age-related changes, since alterations in its physicochemical
composition lead to increased density, reduced permeability and
impaired transport of nutrients (Imayama & Braverman, 1989).
3. BASIS OF ALTERED SENSITIVITY TO ENVIRONMENTAL CHEMICALS
The perception of altered sensitivity of the elderly to
environmental insults is based in large part on the enhanced
incidence of adverse or idiosyncratic drug reactions in this segment
of the population (Vestal et al., 1985). The clinical pharmacology
of the aged has been extensively reviewed in the last two decades
(Triggs & Nation, 1975; Crooks et al., 1976; Reidenberg, 1980;
Vestal et al., 1985), and evidence indicates that the response to
drugs changes with age, as does the frequency of adverse drug
reactions (Krupka & Vener, 1979). This may be in part related to
issues of polypharmacy and compliance (Weber & Griffin, 1986).
Morbidity and malnutrition, which are often associated with aging,
could also contribute to altered pharmacokinetics in the elderly
(Kitani, 1988). However, it is clear that age-related differences in
drug/chemical disposition (pharmacokinetics/toxicokinetics) or
sensitivity (pharmacodynamics/toxicodynamics) also play a role in
altered responses to chemicals in the elderly. For sake of
simplicity, the terms "pharmacokinetics" and "pharmacodynamics" will
be used for both drugs and environmental chemicals.
3.1 Pharmacokinetics
Pharmacokinetics describes the processes of the absorption,
distribution, metabolism and excretion of drugs or other chemicals
in the body. The numerous physiological and biochemical changes that
occur during aging can modulate any of these processes of
disposition. Changes in chemical disposition (pharmacokinetics being
the mathematical description of these processes) can lead to an
altered dose or dose-rate to the target tissue resulting in changes
in response. This topic has been the subject of several recent
reviews (Sellers et al., 1983; Van Bezooijen, 1984; Stevenson &
Hosie, 1985; Blumberg, 1985; Birnbaum, 1987, 1989, 1991; Ritschel,
1988; Loi & Vestal, 1988; McMahon & Birnbaum, 1990a).
The highlights in this field will be covered as well as the
more recent data, especially that focusing on age-related
alterations in the pharmacokinetic behaviour of xenobiotics, as
opposed to drugs.
3.1.1 Absorption
The absorption of drugs and environmental chemicals, defined as
uptake into the blood, occurs primarily via the skin, lungs and
gastrointestinal (GI) tract. Alterations in the structure and
function of these three organs clearly occur with age. In contrast
to the skin and GI tract, little is known about the effects of aging
on pulmonary absorption of xenobiotics. Few age-related differences
have been reported in lung structure (Stiles & Tyler, 1988),
although the lung volume is greater in old rats. An age-related
decrease in respiratory function has been reported in several
species (Mauderly, 1979a,b, 1982). In addition, there appears to be
a decrease in the rate of alveolar-capillary gas exchange in older
organisms (Mauderly, 1979b).
Dermal exposure represents a major portal of entry for
environmental chemicals. Several studies have indicated a decline in
human skin permeability with aging (Christophers & Kligman, 1965).
In experimental animals, the scarce data suggest that percutaneous
absorption is decreased in senescent rodents as compared to young
adults. The chemical absorption of
2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD) and related chemicals
is greatest at weaning (Jackson et al, 1990), decreases at maturity
and then undergoes a further decline during aging (Banks et al.,
1990).
Age-related alterations in GI absorption have been studied in
more depth. A decrease in gastric acid secretion is commonly seen in
the elderly (Bender, 1968). The resulting increase in pH can alter
the ionization of compounds, enhancing or retarding their ability to
diffuse passively across cellular membranes. Decreases in gastric
motility (Lin & Hayton, 1983) can prolong the transit time of
chemicals in the gut, thus enhancing their potential for absorption.
In rats, splanchnic blood flow declines in the first year of life
but stays unchanged in the second year (Yates & Hiley, 1979; Kitani,
1988). In contrast, splanchnic blood flow declines progressively
with age in humans (Sherlock et al., 1955). An increase with age in
mucosal weight (Holt et al., 1984; Hebert & Birnbaum, 1987), as well
as intestinal epithelial cell proliferation (Holt et al., 1988), has
been reported in rats.
GI absorption can be active, passive or involve phagocytosis.
Xenobiotics are primarily absorbed by passive diffusion. Age-related
changes in the oral absorption of a variety of drugs in people have
not been observed (Castleden et al., 1977b; Stevenson et al., 1979;
Greenblatt et al., 1988). The passive absorption of TCDD in the
small intestine does not change with age in rodents (Hebert &
Birnbaum, 1987), nor does that of many small endogenous molecules,
such as glucose (Eastin & Birnbaum, 1987), vitamin A (Hollander
et al., 1986), vitamin D, vitamin B12 and niacin (Fleming &
Barrows, 1982a,b) and many amino acids (Penzes, 1974).
Active transport appears to decrease with age (Eastin &
Birnbaum, 1987). Doubek & Armbrecht (1987) have demonstrated that
the decrease in the carrier-mediated component of glucose transport
in rats occurs in the brush-border membrane of the small intestine.
Their suggestion that the decrease is due to a reduction in the
number of sodium-linked glucose carriers has been supported by
recent studies in human intestinal tissue (Vincenzini et al., 1989).
The active transport of other small molecules such as calcium and
phosphorus (Armbrecht, 1986), and galactose and iron (Reidenberg,
1980) has also been reported to decrease with age. As with the
active transport of glucose, the marked decrease in calcium active
transport of calcium occurs between young adulthood and middle age
(Mooradian & Song, 1989) and results from changes in the number of
calcium transporters in the intestinal basal lateral membranes
(Armbrecht et al., 1988).
3.1.2 Distribution
The distribution of chemicals throughout the body is governed
by the fact that the physicochemical properties of the compound
affect its transport within the body and localization to various
tissues. Lipophilic molecules readily pass across cellular membranes
and accumulate in lipid-rich tissues. Binding to proteins also
modulates distribution, since few compounds are transported free in
the blood. While there is no evidence of alterations in relative
blood volume with age, changes in body composition, blood flow and
macromolecular binding have all been documented.
The decrease in lean body mass in both animals (Lesser et al.,
1973) and humans (Novak, 1972) has been well documented. Loss of
body water with age (Edelman & Leibman, 1959) results in a decrease
in the volume of distribution for water-soluble compounds, leading
to enhanced toxicity of ethanol (York, 1982) and ethylenediamine
(Yang et al., 1984). Body fat can account for approximately 15-40%
of the total body weight in humans (Ritschel, 1983) and rats
(Bertrand et al., 1980; Birnbaum, 1983). The increased size of the
fat compartment in older, sedentary animals would be expected to
increase the body burden of lipid-soluble substances and reduce the
overall rate of elimination from older animals. Following an
inhalation exposure of old rats to methylchloroform (Schumann
et al., 1982a,b), a better fit to a physiologically based
pharmacokinetic (PbPk) model was obtained by increasing the volume
of the fat compartment from 7 to 18% of the body weight for rats and
from 4 to 18% for mice (Reitz et al., 1988). Similar improvements
were noted by Lutz et al. (1977) in their PbPk model of
polybrominated biphenyl compounds in rats.
Not only does cardiac output decrease with age (Bender, 1965),
but regional blood flow can change differentially (Yates & Hiley,
1979). Since adipose tissue volume increases but blood flow
decreases with age, lipophilic compounds tend to show greater
retention in the elderly. This has been shown for polychlorinated
biphenyls (Birnbaum, 1983) and halogenated solvents (Schumann
et al., 1982a,b). A clinical pharmacokinetic study (Klotz et al.,
1975) which demonstrated a 5-fold increase in the distribution
volume of diazepam in the elderly is in agreement with the data on
experimental animals.
Binding to blood components can also change with age. Although
the total plasma protein content does not change dramatically with
age, there is a small but significant reduction in albumin in both
animals (Rodgers & Gass, 1983) and humans (Bender et al., 1975). For
drugs or xenobiotics that can be bound, such a decrease in albumin
enables a higher concentration of free drug to reach the target
site. A decrease in binding of drugs to red blood cells has also
been reported to occur during aging (Chan et al., 1975), again
leading to a higher level of free drug.
3.1.3 Metabolism
As with absorption and distribution, there are physiological
changes that occur during aging which can influence
biotransformation reactions, both in the liver and in extrahepatic
tissues. Although the liver is the main site for the metabolism of
drugs and environmental chemicals, significant metabolic reactions
occur in all the portals of entry tissues (skin, respiratory tract,
GI tract) as well as the kidneys, gonads, adrenals, etc. Metabolism
is often considered to be divided into two types of reactions: phase
I (functionalization reactions) and phase II (conjugation
reactions). Phase I reactions tend to increase the polarity of
chemicals, and the corresponding enzymes include the
cytochrome-P450- and FAD-containing monooxygenases as well as the
alcohol and aldehyde dehydrogenases, monoamine oxidases, nitro- and
azo-reductases, esterases and amidases. Phase II reactions involve
the conjugation of functional group (either already present on the
chemical or as a result of phase I activity) with an endogenous
cofactor such as an amino acid (glycine, glutamate), peptide
(glutathione), sugar (glucuronic acid) or a small molecule such as a
sulfate, acetate or methyl group. These reactions may occur on
cellular membranes or in the cytosol, and are regulated in a
tissue-specific manner.
The only generalization that can be made concerning age-related
changes in biotransformation is that few patterns exist. Metabolic
changes with aging appear to be substrate, sex, strain and species
dependent (Schmucker & Wang, 1989; Kitani, 1988; Birnbaum, 1989;
McMahon & Birnbaum, 1990b). In addition to intrinsic changes related
to altered physiology, the study of the influence of age on
metabolism is further complicated by the effects of diet, alcohol,
drugs and pollutants, which can induce or inhibit enzyme activities
(Ritschel, 1988).
Most of the research on the effects of aging on metabolism has
focused on the microsomal mixed-function oxidases. The protein
components of this system have been reported to decline or remain
unchanged with age. In general, the decline in total cytochrome P450
levels reported in rats appears to be due to the age-related
decrease in the amount of the male-specific forms (Kamataki et al.,
1985a,b; Sun et al., 1986). This results from the age-related
decrease in circulating testosterone levels (Kitani, 1985) or to
alterations in the secretory profiles of growth hormone (Fishbein,
1991). This pattern is most pronounced in rats, few changes being
observed in other rodents or sub-human primates (Birnbaum, 1987).
However, recent human studies have suggested that some degree of
sexual dimorphism does exist in hepatic drug metabolism. Plasma
antipyrine half-lives were prolonged in elderly males, but not
elderly females, as compared to young adults (Greenblatt et al.,
1988). This was caused by an age-related reduction in clearance,
which has been interpreted as being the result of an impairment of
antipyrine metabolism in elderly men. Similar gender-specific
results were observed for the kinetics of chlordiazepoxide, another
low-clearance oxidatively metabolized drug (Loi & Vestal, 1988).
A study by Chengelis (1988a) has supported the earlier report
of Rikans (1984) that measuring components of the monooxygenase
system cannot lead to predictions of toxicity. Sex differences in
rats were only significant up to one year of age. However, Rikans
(1989a) has recently reported that metabolism of specific substrates
(aniline, benzphetamine and nitroanisole) does decline with age in
the female as well as in the male rat, but the magnitude of change
in the female is smaller. This agrees with a study by Bitar &
Shapiro (1987), who suggested that an age-related increase in haem
degradation plays a role in the decreased metabolism of hexobarbital
and aniline. This observation, that microsomal drug metabolism
activities do not have to be sexually dimorphic to be altered in old
age, implies that factors other than loss of the male-specific
cytochromes P450 contribute to the age-associated alterations in
some rat strains. Decreases in NADPH cytochrome P450 reductase in
rat liver with age have been reported by Blanco et al. (1987),
Chengelis (1988a) and Rikans (1989a).
Despite a large body of data obtained from experimental animal
studies, there is no clear evidence that monooxygenase activities
decline in the livers of healthy elderly humans. All the data
derived so far from human liver biopsy specimens have shown no
correlation between enzyme activities expressed per mg protein and
age of subjects (Boobis & Davies, 1984; Schmucker et al., 1990). The
often reported decreases in clearance values of drugs metabolized
primarily by the liver in the elderly may in large part be accounted
for by the decrease in liver volume with age (Kitani, 1988). Further
evidence is needed to determine whether hepatic drug-metabolizing
enzyme activities in humans decline with age, as is observed in some
rodents, but the extent is unlikely to be as drastic as that
observed in male rat liver.
A change in the composition of cytochrome P450 enzymes,
suggested by differential changes in enzyme activity, has been
demonstrated in male rats (Kamataki et al., 1985a; Sun & Strobel,
1986). Leakey et al. (1989a) showed that dietary restriction could
also alter the profile of cytochrome P450 isoforms, possibly by
delaying the age-related demasculinization of the liver. Friedman
et al. (1989) showed that aging affected the composition of
testosterone-binding cytochromes P450 in the liver of male rats.
Changes in P450 isoforms in old rats were also reported by
Paramonova & Dovgi (1987). Studies with humans have demonstrated
that the effect of age on metabolism even varies for different
metabolites of the same parent compound (Posner et al., 1987). This
supports the observation of changes in cytochrome P450 isoform
composition with age, as has been reported for male rat liver.
Hepatic xenobiotic metabolism can be modulated by many factors.
In humans, smoking often confounds studies of age-related changes in
pharmacokinetics. However, the age-related decrease in the
metabolism of antipyrine (Loft et al., 1988), theophylline and
cortisol (Crowley et al., 1988) may be independent of smoking
status. In fact, the inductive properties of smoking on hepatic
metabolism do not appear to diminish with age. Phenytoin also
increased theophylline metabolism to an equal extent in both young
and old healthy men (Crowley et al., 1988). In contrast, Rath &
Kanungo (1989) demonstrated that the rate of transcription of the
phenobarbital-specific isozymes was nearly two-fold higher in young
rat liver than in old. However, these differential effects may
reflect the specific isoforms involved, since smoking and
phenobarbital preferentially induce different forms. The recent
studies of Rikans (1989b), which demonstrate that induction of
hepatic microsomal drug metabolism by ethanol or acetone is
unaffected by the aging process, lend support to this concept.
Age-related changes in phase I hepatic drug-metabolizing
enzymes other than the mixed-function oxidases have been subjected
to much less examination. Rikans & Moore (1987) demonstrated an
age-related increase in liver alcohol dehydrogenase in male rats.
However, no age differences have been observed in the activity of
this enzyme in female rats. Hydrolytic reactions may decrease with
age. For example, liver esterase activity, using diethylhexyl
phthalate as a substrate, declines in old rats (Gollamudi et al.,
1983). Using aspirin as a substrate, no correlation was found
between age and esterase activity in human liver (Yelland et al.,
1991). These results provide further evidence that age is not a
major determinant of hepatic drug metabolism in the elderly
(Schmucker et al., 1990).
Alterations with age in extrahepatic phase I metabolism also
appear to be substrate, sex, strain and species specific. Pulmonary
metabolism of benzo[a]pyrene was found to increase in old rats (Sun
& Strobel, 1986) in agreement with earlier studies of Rabovsky
et al. (1984). In contrast, oxidation of 2-aminofluorene, which is
catalysed by a different isoform of cytochrome P450, decreased
(Robertson & Birnbaum, 1982). Renal metabolism of acetaminophen has
been reported to decrease with age (Beierschmitt & Weiner, 1986),
while salicylate oxidation by kidney extracts did not change in rats
(Kyle & Kocsis, 1985). Age-related alterations in intestinal Phase I
metabolism appear to be site specific. Sun & Strobel (1986) reported
that oxidation of benzo [a]pyrene in the colon increased throughout
the life of rats, while McMahon et al. (1987) observed no
age-related change in the metabolism of this substrate in the small
intestine. Newaz et al. (1983) observed higher metabolic rates of
dimethylhydrazine in colonic tissue from aging humans as compared to
younger individuals. McMahon et al. (1989) suggested that plasma
esterase hydrolysis of benzyl acetate may decline with age in both
rats and mice. Alcohol dehydrogenase activity was found to remain
unchanged in the aging colon (McMahon et al., 1987).
Changes in phase II enzymes also appear greatly variable in
rodent liver, although Loi & Vestal (1988) conclude that age has
little effect on phase II reactions in humans. The major conjugation
reactions involve sulfation, glucuronidation or reaction with
glutathione leading to mercapturic acid formation. All these
reactions are catalysed by multiple isoforms showing varying degrees
of substrate and sex specificity, as is the case for the phase I
enzymes. Iwasaki et al. (1986) demonstrated differential age effects
on two distinct sulfotransferases using male and female rats and
various alcohols and amines. Sulfation of phenolic substrates
appears to decline with age (Galinsky et al., 1986; Sweeny & Weiner,
1986) while conjugation of bile salts increases in old male rats
(Galinsky et al., 1986). In contrast, changes in female rats were
not seen (Galinsky et al., 1990). Chengelis (1988b) observed no
significant changes in sulfotransferase activity with age in either
male or female rats using beta-naphthol as the substrate. Similar
results were seen by Leakey et al. (1989b) for the conjugation of
both estrone and naphthol, whereas the sulfation of androsterone and
corticosterone increased with age in male rats.
Hepatic glucuronidation also shows substrate specificity for
alterations with age. Depending on the substrate, increases with
estrone and acetaminophen (Galinsky et al., 1986), decreases with
the rubber antioxidant 4,4'-thiobis-(6- t-butyl- m-cresol)
(Borghoff et al., 1988), or no effect with naphthol,
p-nitrophenol, morphine and testosterone have been observed
(Galinsky et al., 1986; Sweeny & Weiner, 1986) in male rats. In
contrast, Chengelis (1988b) observed a marked decrease in senescent
male and female rats using both p-nitrophenol and chloramphenicol.
To complicate matters further, Leakey et al. (1989b) also saw a
decrease in naphthol, testosterone, androsterone and
tetrahydrocortisone conjugation with glucuronic acid, but observed
no effects of aging on conjugation with 2-aminophenol,
5-hydroxytryptamine, bilirubin or estrone. Tarloff et al. (1989b)
saw no change in acetaminophen glucuronidation with advancing age.
Although the activities of the UDP-glucuronosyltransferases appear
highly variable, an age-related decrease in hepatic levels of the
cofactor, UDP-glucuronic acid (UDPGA) (Borghoff et al., 1988),
suggests that glucuronidation could be limited in older animals.
The concentration of hepatic glutathione, the cofactor involved
in the third major class of conjugation reactions, has been reported
to increase (Borghoff & Birnbaum, 1986), decrease (Stohs et al.,
1982) or remain unchanged (Chengelis, 1988b; Rikans & Moore, 1988)
in old rodents. Variability in age effects has also been reported in
the activity of the glutathione- S-transferases, which exist as a
family of dimeric proteins having broad and overlapping substrate
specificity. The isozymes are composed of two subunits from at least
six different peptides, including both homo- and heterodimers. Thus
reports of an increase (Leakey et al., 1989b), decrease (Fujita
et al., 1985; Blanco et al., 1987; Leakey et al., 1989b), or no
change (Birnbaum & Baird, 1979; Sweeny & Weiner, 1985; Borghoff &
Birnbaum, 1986; Chengelis, 1988b; Leakey et al., 1989b; Carrillo
et al., 1991) in hepatic glutathione-S-transferase activity may, as
in the cases of cytochrome P450 mixed-function oxidases,
sulfotransferases and UDP-glucuronosyltransferases, reflect
age-dependant changes in isozyme ratios (Spearman & Leibman, 1984;
Carrillo et al., 1991). Restriction in dietary protein decreases the
activity of the glutathione-S-transferases more in old than young
rodents (Carrillo et al., 1989, 1990).
Much less has been reported on the effects of aging on other
phase II metabolic reactions in the liver. Hydrolysis of expoxides
to form diols or dihydrodiols is catalysed by the epoxide
hydrolases. Epoxide hydrolase activity has been reported both to
increase (Birnbaum & Baird, 1979) and decrease (Ali et al., 1985;
Kaur & Gill, 1985; Leakey et al., 1989b) with age. Again, this may
reflect differential age effects on multiple enzymatic species.
However, some of the difference may be due to the ages of animals
used for comparison, since Chengelis (1988b) reported a gradual
increase in epoxide hydrolase activity throughout much of the life
span, followed by an abrupt decrease in senescence.
Human studies have suggested that the alterations observed in
salicylate pharmacokinetics (Cuny et al., 1979) with age might be
due to a decrease in glycine conjugation (Kyle & Kocsis, 1985).
However, elevated levels of salicyluric acid, the glycine conjugate
of salicylate, have been observed in elderly humans undergoing
chronic salicylate treatment (Montgomery & Sitar, 1981). Recent
studies in rodents have not demonstrated any age-related decline in
glycine conjugation with non-nephrotoxic doses of salicylate
(McMahon et al., l990a) or in the formation of hippuric acid from
benzoate (McMahon et al., 1989). Acetylation, however, has been
reported to decline both in man (Bauer et al., 1989) and rats
(Leakey et al., 1989b).
Changes in extrahepatic phase II reactions have been
investigated in even less depth than extrahepatic phase I reactions.
In the lung and small intestine, glucuronidation of p-nitrophenol
appeared not to change with age (Borghoff & Birnbaum, 1985), whereas
in the colon an age-related decrease was reported by McMahon et al.
(1987). In contrast, colonic glucuronidation of
4-methylumbelliferone rose significantly in older rats (McMahon
et al., l990b). A decrease in UDP-glucuronosyl transferase activity
in the kidney (Borghoff & Birnbaum, 1985; Tarloff et al., 1989a) was
accompanied by a decrease in the renal concentration of UDPGA
(Borghoff et al., 1988).
Glutathione content has been examined in a variety of tissues
(lung, kidney, brain, testes and blood) and, except for an
age-related decrease in the lens, has been found to remain constant
(Rikans & Moore, 1988). Unchanged glutathione levels in the colon
occurred although the activity of glutathione- S-transferase
decreased in this tissue (McMahon et al., 1987). Spearman & Leibman
(1983, 1984) observed differential age- and sex-related changes in
this activity in the lung depending on the substrate examined. The
renal activity of glutathione- S-transferase declines significantly
in old rats (Beierschmitt & Weiner, 1986). In contrast, elevated
gluthathione- S-transferase levels were observed in the brain of
old rats (Blanco et al., 1987), while no changes were observed in
the heart.
Epoxide hydrolase activity has been examined in the lungs,
small intestine and kidneys by Kaur & Gill (1985). They observed a
decrease in lung and small intestine activity in old rats which was
substrate dependent. In the rat kidney, epoxide hydrolase activity
decreased using trans-stilbene oxide as the substrate but did not
change with cis-stilbene oxide, supporting again the differential
effects of aging on different isozymic forms of drug-metabolizing
enzymes. In addition, deacetylation of acetaminophen in the kidney
appears to be either unchanged with age (Beierschmitt & Weiner,
1986) or slightly decreased (Tarloff et al., 1989b). Acetylation in
the kidney also decreases with age (Wabner & Chen, 1984).
One additional enzyme which can be considered to fall into the
phase II class is beta-glucuronidase. This enzyme hydrolyses
glucuronic acid from conjugated xenobiotics. The activity of this
enzyme has been reported to increase with age in rat liver
(Schmucker & Wang, 1979; Van Manen et al., 1983) and kidney
(Borghoff & Birnbaum, 1985), but remain unchanged in the colon
(McMahon et al., 1987). However, beta-glucuronidase was found to
decrease with age in fecal contents (McMahon, 1988). The balance
between glucuronidation and deglucuronidation reactions may play a
role in determining the level of reactive compounds in the organism.
Depending on the chemical, aging can result in either an
increase or a decrease in the metabolizing capacity of different
organs and tissues (liver, kidney, gastrointestinal tract, lungs and
skin). The elevation or reduction in metabolism can both lead to
higher and lower toxicity, depending on the relative reactivity of
the metabolic intermediates and end-products. Thus studies on the
effect of aging on metabolism should be considered case by case.
3.1.4 Excretion
Excretion leads to the elimination of a chemical and/or its
metabolites from the body. The kidney is the major excretory organ,
with the liver and lung also playing important roles in the
elimination process. In addition, sweat, saliva and sex-linked
processes such as lactation can serve as routes of excretion.
The effects of age on renal function appear to play a major
role in altered pharmacokinetics in the elderly (Vestal, 1978;
Koch-Weser et al., 1982). The fact that changes in the physiology of
the kidney occur has been known for many years (Schmucker, 1979).
Renal blood flow decreases with age leading to a decrease in
glomerular filtration rate. Tubular secretion and resorption are
also reduced in the elderly. The number of functional nephrons
declines to a similar extent as the decline in glomerular filtration
rate and active secretion, suggesting that the nephron loses its
function as a unit (Friedman et al., 1972). Decreases in renal
function can result in a decreased rate of renal clearance, leading
to a greater potential for elevated and/or persistent levels of
chemicals in the body which could lead to toxicity (Sellers et al.,
1983). In humans, decreased renal clearance in the elderly has been
demonstrated for many drugs, including the aminoglycosides,
tetracyclines, lithium, digoxin, procainamide, methotrexate, and
phenobarbital (Kampmann & Hansen, 1979).
Aging rodents are extremely susceptible to chronic
glomerulonephropathy (Goldstein et al., 1988), much of which may be
attributable to diet (Masoro & Yu, 1989). Altered glomerular
morphology, characterized by thickening of the basement membrane and
sclerosis, is progressive and increases in severity with advancing
age, eventually resulting in scarring and loss. Renal tubules are
also subject to degenerative changes, which are accompanied by
proteinuria, especially albuminuria (Neuhaus & Flory, 1978). This
appears to result from increases in glomerular permeability and a
loss of fixed glomerular polyanion (Baylis et al., 1988). The high
percentage of urinary protein represented by albumin in the aging
rat may be due to non-selective protein leakage into the urine
resulting from increases in glomerular permeability to large
proteins, since the protein percentage in urine approaches that in
the plasma of senescent rats (Horbach et al., 1988a). Similar
albuminuria has been observed in aging mice (Yumura et al., 1989),
and was correlated with glomerular sclerosis. Such changes, however,
should not be simply extrapolated to humans, since there is no
evidence for an increase in protein loss in the urine of the healthy
elderly.
Additional tubular changes occur in the aging kidney, resulting
in hyperplastic and degenerative changes. Some of these changes
resemble responses to specific environmental chemicals (Konishi &
Ward, 1989). A decrease in renal transport of organic acids has been
observed (Wabner & Chen, 1984). Aging appears to diminish the
turnover of sodium/potassium ATPase in the proximal tubules (Marin
et al., 1985), which could play a role in the observed age-related
decrease in tubular secretion.
Hepatic elimination may also be compromised by aging. Kitani
(1985) has suggested that the excretory capacity of the liver
decreases with age due to some functional alteration in the
hepatocytes. In contrast to rats, where blood flow does not change
after maturity (Kitani, 1988), blood flow to the liver declines in
humans (Sherlock et al., 1955). Bile flow rate has been reported to
be reduced (Borghoff et al., 1988) or remain unchanged (Kitani
et al., 1985a) in rats. Biliary transport declines, especially in
the case of polar compounds (Kitani et al., 1985a). The elimination
of sulfobromophthalein, a model compound for the study of biliary
excretion of organic anions, is also decreased in old rats (Kanai
et al., 1985). Sato et al. (1987) demonstrated that the biliary
excretion of the neutral glycoside ouabain decreases with age in
both males and females. This may be due to an age-related decrease
in hepatic uptake, resulting in less biliary elimination (Ohta
et al., 1988). In addition, the biliary canalicular transport system
declines steadily during aging (Kanai et al., 1988). The decreases
in both hepatic uptake and biliary excretion may reflect changes in
the hepatocyte plasma membrane (Zs-Nagy et al., 1986).
3.2 Pharmacodynamics
Age-related changes in chemical sensitivity cannot all be
explained on the basis of altered pharmacokinetics in the elderly.
Pharmacodynamic changes occur at the target site and may involve
changes in cell populations, cellular receptors, cellular
responsiveness or in the regulation of the amount or activity of
drugs, including the cardiac glycosides, benzodiazepines, tricyclic
anti-depressants, and the non-steroidal anti-inflammatory agents
(Bender, 1979), which have demonstrated altered receptor sensitivity
in the aged (Wilson & Hanson, 1980).
3.2.1 Central nervous system
Numerous studies have shown that, with advancing age, there is
a decrease in the ability of the nervous system to synthesize and/or
release neurotransmitters and neuropeptides (for review, see Rogers
& Bloom, 1985). This decline may reflect the fact that there are
fewer neurons present to synthesize the chemical messenger or that
the enzymes involved in their synthesis are altered. This would
imply that xenobiotics which lead to neuronal death or interfere
with neurotransmitter or neuropeptide synthesis and release could
have a greater adverse effect on the elderly than on the young
organism. An example of such an interaction has been noted within
the dopaminergic extrapyramidal circuits controlling motor movements
(nigrostriatal pathway). This pathway has been the subject of many
experimental and clinical studies and provides one of the best
functional units in which to study neurotoxicity in the aged.
Nigrostriatal neurons are lost as a normal correlate of aging, and
while these losses may not be expressed in the majority of
individuals as movement disorders, there is a clear reduction in the
functional reserve of this dopaminergic system, making it more
vulnerable to neurotoxicants. This has been demonstrated by the
observed acceleration or simulation of "age-associated" movement
disorders by neurotoxicants such as
1-methyl-4-phenyl-1,2,5,6-tetrahydro-pyridine (MPTP).
The reduced capacity to synthesize neurotransmitters that
occurs in the aged organism may also potentiate the effect of toxic
substances. Carbon disulfide is an organic solvent with a variety of
industrial applications that produces neurobehavioural dysfunctions
(Wood, 1981). The mechanism of CS2 toxicity seems to be inhibition
of dopamine-beta-hydroxylase, the enzyme that converts dopamine to
norepinephrine (McKenna & DiStefano, 1977). Since norepinephrine
metabolism declines with age, the spectrum of physiological effects
regulated by this catecholamine would be expected to suffer greater
disturbance, following exposure to CS2 or to pesticides that
reduce brain catecholamines such as methyl bromide (Honma et al.,
1987), in old rats compared to young ones.
Toxic compounds that serve as neurotransmitter receptor
blockers could have their effects on behaviour accentuated, making
neuroendocrine control of homeostasis more difficult in the elderly.
For example, the formamidine pesticides, amitraz and chlordimeform,
have been shown to block alpha-noradrenergic receptors (Costa &
Murphy, 1987; Costa et al., 1988) and induce a variety of
behavioural disorders (Boyes & Dyer, 1984; Hsu & Kakuk, 1984;
Landauer et al., 1984) in rats. Since there is an age-related
decline in noradrenergic receptors in several species (Rogers &
Bloom, 1985), exposure to these compounds may have a more pronounced
effect on the older organism.
Certain movement disorders associated with senescence may be
related to age-related impairments in the brain dopaminergic systems
(Marshall & Berrios, 1979). Waddington et al. (1985) observed a
decrease in the density of brain dopamine receptors in old rats,
with no changes in affinity. In contrast to young animals, the
receptors of old animals were not able to respond effectively to
long-term treatment. The decrease in dopamine receptor density was
selective for the D-2 receptor subtype, although the coupling
between D-1 receptors and adenylate cyclase appears to be affected
by aging. A similar loss of D-2 receptors in the elderly has also
been measured using positron tomography in the living human brain
(Wong et al., 1984). An age-related decrease in binding to the
serotonin receptor may relate to cerebral dysfunction in the elderly
(Shih & Young, 1978).
Studies with rats have indicated that the increased sensitivity
of older rats to diazepam is due to pharmacodynamic differences
(Guthrie et al., 1987). Pedigo et al. (1981) suggested this could
relate to changes in the benzodiazepine/GABA/chloride ionophore
complex. Human studies have also indicated that the site of
increased sensitivity to the benzodiazepines lies distal to the
receptor (Swift, 1985), possibly involving changes in the chloride
ionophores.
Alterations in endogenous opioid systems may play a role in
some of the behavioural changes observed in the elderly. Binding of
dihydromorphine to the opiate receptor decreases in specific areas
of the brain in aged rats as compared to young ones (Messing et al.,
1980). This reduction is due to a decrease in receptor number, with
no change in affinity. Despite a relatively large body of evidence
that some receptors and neurotransmitters are at least qualitatively
altered with aging, it is still not clear whether and how these
changes are causally related to increased sensitivity of the CNS to
certain drugs with aging, as described below.
The brain appears to exhibit increased sensitivity to
phenobarbital (Kitani et al., 1985b; Van Bezooijen et al., 1989) in
both mice and rats. Such enhanced sensitivity with age to an
anticonvulsant supports earlier studies with phenytoin (Kitani
et al., 1984). It is possible that the aging brain of both
experimental animals and humans has increased sensitivity to all CNS
depressants. Enhanced brain sensitivity has also been reported for
hexobarbital in rats (Van Bezooijen et al., 1989) and oxazepam in
mice (Kitani et al., 1986). The aging human brain appears to be more
sensitive to nitrazepam (Castleden et al., 1977a) and other
benzodiazepines (Reidenberg, 1980). Recently, zonisamide, a new drug
whose anticonvulsant properties are distinct from the other drugs,
was demonstrated to have an increased anticonvulsant effect in aging
mice (Kitani et al., 1987). Taken together, these results suggest
that these pharmacodynamic changes with age may reflect a decreased
response capability for seizures in the elderly, rather than a
specific age effect on all the distinct receptors (Kitani et al.,
1986). In fact, the lethal threshold for pentylenetetrazole in mice
has been shown to decrease with age (Nokubo & Kitani, 1988), coupled
to an increase in the threshold for maximal seizure.
The action of other environmental compounds on CNS function may
be more diffuse. Recent studies have demonstrated enhanced
sensitivity of old mice to cyanide intoxication (McMahon & Birnbaum,
1990b). Exposure to various metals has been shown to alter a variety
of CNS functions. There has been a particular interest in a
potential link between Alzheimer's disease and aluminium toxicity.
It was initially reported that the autopsied brains of Alzheimer's
patients showed elevated aluminium levels (e.g., Perl & Brody,
1980). However, others have found no such correlation. Marksberry
et al. (1981) compared the brains of Alzheimer's patients with older
adult controls and found no correlation between the density of
aluminium content and the density of neurofibrillary tangles and
neuritic plaques. However, a more recent study showed that the brain
aluminum levels were significantly increased as a function of age in
both control and Alzheimer patient populations (Bjorksten et al.,
1989).
Manganese is a metal that causes age-type neuropathy. However,
unlike aluminium, it is associated with extrapyramidal disorders,
characterized by intention tremor (Donaldson, 1987). In monkeys,
the neurological symptoms of choreo-athetoid movement, rigidity and
tremor occurred after 18 months of manganese exposure. These
clinical signs, in association with severe lesions of the globus
pallidus and subthalamic nucleus, resembled Parkinson's disease and
suggested a possible link between environmental exposure and
occurrence of the disease in aging individuals.
The neurotoxic effects of other metals have been widely
studied, particularly those of lead, which has been implicated in
reduced intellectual abilities in children (Needleman et al., 1979),
slowed reaction time (Hunter et al., 1985), and impairment of other
cognitive abilities (Winneke et al., 1982; Hansen et al., 1985).
While psychophysiological parameters appear to be relatively
insensitive to low levels of lead exposure, several studies have
demonstrated both cognitive and emotional effects due to long-term
exposure to lead (Hogstedt et al., 1983; Mantere et al., 1984).
Long-term exposure to mercury vapour has been reported to interfere
with verbal intelligence and memory performance (Piikivi et al.,
1984). Hanninen (1982) suggested that abnormalities due to mercury
exposure affect the motor system and result in intellectual
impairment, a gradual and progressive deterioration of memory
function, and emotional disability. Other metals including copper,
iron and manganese have also been reported to cause CNS dysfunction
(Grandjean, 1983). However, there are no available data on the role
of exposure to these metals in dysfunction of the CNS in the aged.
Examples of naturally occurring neurotoxic agents that
stimulate nervous system senescence have been reported in certain
island populations. For example, the Chamorro peoples of the
Marianna Island, specifically Guam and Rota, exhibited a high
incidence of amyotrophic lateral sclerosis, Parkinsonism and
Alzheimer's-like dementia that was recently linked to their diet.
The Chamorro's diet consists in part of a flour made from the seeds
of Cycas circinalis. When one of the components of these seeds,
beta- N-methylamino-l-alanine (BMAA), a compound similar in
structure to excitotoxic amino acids, was fed to macaques, they
exhibited signs of motor neuron, extrapyramidal and behavioural
dysfunction (Spencer et al., 1987).
It seems that several motor neuron disorders are associated
with the appearance of endogenous excitotoxic glutamate agonist-type
molecules. One such molecule is 2,3-pyridine dicarboxylic acid
(quinolinic acid), which has been isolated from the brains of
humans, rabbits and laboratory rodents (Moroni et al., 1984) and has
been shown to excite CNS neurons when applied iontophoretically
(Perkins & Stone, 1983). An interesting correlate is the fact that
brain concentrations of quinolinic acid increase with age (Moroni
et al., 1984), suggesting that its presence may result in the
spontaneous onset of neurode-generative conditions that are mimicked
by environmental neurotoxicants such as BMAA and
beta- N-oxalylamino-L-alanine (BOAA).
3.2.2 Endocrine system
There are several different ways in which the endocrine system
and the hormonal signalling operations involved may undergo
alterations with age and toxicant exposure. These can be categorized
as changes in: (a) the availability of hormones for binding to the
target tissues; (b) the reception of the pertinent transmitter or
hormonal signal by the target cells; and (c) the nature of the
hormonal message.
3.2.2.1 Changes in hormonal availability with age
Age-related or toxicant-induced shifts in synthesis, rate of
clearance and rate of secretion will all function to alter hormonal
concentrations. Such changes in the size of the available signal
pool may have corresponding effects on the magnitude of the response
by the target tissue. These changes may reflect a decline with age
in the homeostatic controls, which rely heavily on endocrine
feedback relationships.
Several toxicants have also been observed to cause changes in
circulating hormonal levels (Cooper et al., 1986). Significant
reductions in serum testosterone, for example, have been seen
following short-term exposure of rats to the plasticizer
dinitrobenzene (Rehnberg et al., 1988b) and the pesticide
chlordimeform, the latter also causing marked reductions in serum
LH, thyroid-stimulating hormone (TSH), T4 and T3 levels. The
effects, moreover, may be remarkably specific. Following three days
of exposure in male rats, the pesticide linuron, for instance, was
reported to decrease the serum T4 level in a dose-related manner,
while leaving T3 and the pituitary and gonadal hormones unaffected
(Rehnberg et al., 1988a).
There is also a growing body of evidence for a hormonal
influence on toxicant metabolism. A sizeable number of xenobiotics,
including both drugs and environmental toxicants, are metabolized by
the hepatic cytochrome P450 monooxygenase system (Nebert & Gonzalez,
1987). Components of this system have been found to be influenced by
glucocorticoids (Schuetz et al., 1984; Simmons et al., 1987) and
markedly affected by sex steroids (Kamataki et al., 1985b) and
growth hormone (Yamazoe et al., 1987; Zaphiropouos et al., 1989).
Consequently, persistent shifts in the circulating levels of such
hormones, as have been reported for the aging animal, could affect
the manner in which xenobiotics are metabolized following exposure.
Reported attenuations with age in the rhythms of human and rat
serum testosterone (Bremner et al., 1983; Steiner et al., 1984;
Tenover et al., 1988), LH (Vermeulen et al., 1989) and GH (Sonntag
et al., 1980), among other hormones, can present differences in
young-versus-old comparisons, depending on when such sampling is
performed. Comparable effects on hormonal rhythms have been reported
to occur in response to toxicant exposure. For example, single
injections of 2,3,7,8-tetrachlorinated dibenzo-p-dioxin (TCDD)
resulted in some evidence of alterations in prolactin and
corticosterone rhythms in rats (Jones et al., 1987). It may be that
an aging system, while still exhibiting rhythmic hormonal changes,
may be increasingly sensitive to their disruption by low toxicant
levels.
3.2.2.2 Changes with age in the reception of the signal by the
target cells
A general decline in the transmitter regulation of hormonal
function may also place an aging animal at increased risk for
toxicant exposure (Govoni et al., 1988), given that various
environmental toxicants (including, for example, the solvents
vinyltoluene, ethylbenzene and styrene, the halogenated hydrocarbon
TCDD, and certain heavy metal cations) have been reported to
interact with catecholaminergic systems (Govoni et al., 1979; Lucchi
et al., 1981; Arfini et al., 1987; Mutti et al., 1988; Russell
et al., 1988).
These changes have been observed for both neurotransmitter and
hormone receptors. There is some evidence of a decreased
responsiveness of target tissues to steroid hormones during
senescence. For example, age-related declines in the concentration
of the estrogen receptor may reflect a decline in the circulating
estrogen levels (Thakur, 1988). Impaired responsiveness could also
be due to reduced receptor translocation (Belisle & Lehoux, 1983) or
other steps in steroid action which occur after hormone binding. In
fact, age-related alterations in glucocorticoid responsiveness are
due to both receptor and post-receptor events (Kalimi, 1982).
Testicular luteinizing hormone receptor concentration and total
content also decrease with age (Amador et al., 1985). In general,
receptors for steroids, insulin, glucagon, catecholamines and
prolactin appear to decrease in concentration with increasing age in
rodents, dogs and humans (Roth, 1979b).
An additional consideration of alterations with age or toxicant
exposure in the reception of a hormonal (or transmitter) signal by
target cells concerns not only effects on the receptor itself, but
changes in the cell's membranes. In the aging rat brain, there is
evidence for a progressive decrease in membrane fluidity
(Hershkowitz, 1983; Nagy et al., 1983). This is at least partially
attributable to alterations in the lipid composition. For example,
it has been reported that aging is associated with elevations in
cholesterol, sphingolipids and saturated fatty acid chains, all
leading to increases in rigidification (Rouser et al., 1972). Since
protein activity in the membrane is influenced by the fluidity of
the lipid micro-environment, any alterations with age in membrane
viscosity may affect not only receptor functions but also enzymatic
activity.
3.2.2.3 Changes in the nature of the hormonal message with age
The antigenic site(s) on a hormone recognized by antibodies can
be quite distinct from those regions that bind to the receptors and
trigger a physiological response in the target tissue. This
distinction may have an added importance for studies in aging, since
alterations with age in peptide hormone structure have been reported
(Conn et al., 1980) that reflect changes in post-translational
processes. These effects, moreover, may be influenced by shifts in
the steroid hormonal milieu in the older animal (Ulloa-Aguirre
et al., 1988). A number of hormones are glycosylated to varying
degrees and such differences in their carbohydrate residues may
alter biological activity (Ulloa-Aguirre & Chappel, 1982; Warner
et al., 1985) and/or plasma half-life (Morell et al., 1971).
Consequently, hormonal measures based solely on immunoreactivity
per se potentially offer a somewhat inaccurate picture of
endocrine alterations with age.
3.2.3 Kidney
The aging kidney appears to be more susceptible than the young
one to drug-induced nephrotoxicity as well as to renal ischaemia. In
fact, cortical tubules of senescent rats seem more sensitive to
oxygen deprivation than do those of young rats (Miura et al., 1987).
Thus, increased susceptibility of the aging kidney can be
independent of pharmacokinetic effects.
Age-related susceptibility to nephrotoxicity has been
demonstrated for numerous drugs including salicylate, acetaminophen,
cephaloridine and doxorubicin. Kyle & Kocsis (1985) showed that
kidneys from older rats develop nephrotoxicity to a greater extent
than do those of young rats following an equal dose of salicylate on
a body weight basis. However, the dose to the kidney could be
greater in the older rats due to a decrease in the volume of
distribution. In addition, McMahon et al. (l990a) has recently
demonstrated that at toxic doses old rats produce more reactive
metabolites of salicylate than do young ones. The age-related
enhancement in cephaloridine nephrotoxicity may also be due to
pharmacokinetic changes (Goldstein et al., 1986), although renal
cortical slices from old rats demonstrated alterations in active
uptake of organic anions and cations following exposure to the
antibiotic, which were not observed in young rats. Doxorubicin
treatment resulted in greater toxicity in old rats (Colombo et al.,
1989). The onset of the delayed nephrotoxicity was noticeably faster
in the old rats; this may have been related to higher drug retention
in the kidneys of old rats.
Age-related increased susceptibility to acetaminophen
nephrotoxicity has been investigated more than that of any other
chemical in rats. Tarloff et al. (1989b) stressed that while
enhanced sensitivity to acetaminophen nephrotoxicity clearly occurs
with advancing age, great care must be taken in choosing the ages
for comparison. Beierschmitt et al. (1986a) demonstrated by both
functional and histological criteria that susceptibility to
acetaminophen-induced acute tubular nephrotoxicity increases with
age in rats. This may reflect altered susceptibility rather than an
alteration in pharmacokinetic parameters, since the generation of
reactive metabolites from acetaminophen decreases with age
(Beierschmitt & Weiner, 1986). Increasing delivery of acetaminophen
to functioning nephrons, whose numbers are decreased in the aged
kidney, does not appear to be responsible for the age-related
increase in susceptibility (Beierschmitt et al., 1986b).
Alterations with advancing age do not have to result in
increasing sensitivity. Studies by Murty et al. (1988b) indicated a
decrease in hydrocarbon-induced hyaline droplet nephropathy in male
rats during senescence. This male-specific nephropathy is associated
with the presence of alpha2u-globulin, whose synthesis is strongly
age dependent (Roy et al., 1983). Although gasoline causes extensive
nephrotoxicity in young rats, old rats are resistant. In fact,
gasoline failed to alter hyaline droplet numbers in aged male rats,
which also demonstrated an altered lysosomal response as compared to
young rats. This lack of response of aged rats to
hydrocarbon-induced hyaline droplet nephropathy suggests that these
rats would also be resistant to hydrocarbon-induced renal neoplasia.
3.2.4 Immune system
With advancing age a progressive decline in the concentration
of glucocorticoid receptors occurs in the spleen (Roth, 1979a). This
alteration may depend either on an age-related modification of the
ratio among various subsets of lymphoid cells carrying different
receptor densities or on an intrinsic failure of aged cells to
maintain an adequate turnover of receptor molecules. With advancing
age, membrane receptors for hormones of low relative molecular mass
on lymphoid cells also show alterations, although these are more
related to impaired coupling to signal transduction systems (Feldman
et al., 1984; Roth, 1988). It has been shown that the number of
receptor molecules decreases with advancing age whereas the affinity
does not seem to change. In the aged population with altered
function of the immune system, it is possible that immunomodulatory
agents present in the food potentiate immune dysfunction, making the
elderly more vulnerable to age-related diseases.
The effects of immunosuppressive compounds can be identified
from toxicological experiments with young adult rodents. These
chemicals include organotin compounds, some pesticides, halogenated
aromatic hydrocarbons (e.g., hexachlorobenzene and dioxins) and
cyclosporin (Poland & Knutson, 1982; Vos & Penninks, 1987; Vos &
Luster, 1989; Schuurman et al., 1990; Vos et al., 1990), and they
can also potentiate autoimmunity, which is found more frequently in
the elderly.
3.2.5 Other tissues and systems
There are few data on pharmacodynamic changes in other tissues
and systems during aging. An increase in the sensitivity of the
aging human myocardium to digoxin (Chavaz et al., 1974), as well as
to intravenous anaesthetics (Dundee, 1979), has been suggested. The
stomach, kidney and bone marrow are more susceptible to the adverse
effects of non-steroidal anti-inflammatory agents in the elderly
than in the young, and cerebral side effects are more common
(Huskisson, 1983). The anticoagulation properties of warfarin are
also changed in the elderly, with the aged demonstrating increased
sensitivity (Shepherd et al., 1979).
Although in most toxicological studies the cardiovascular
system is not well examined, there are several chemicals known which
can cause cardiovascular toxic or atherosclerotic effects. Such
compounds in food include erucic acid in combination with
omega-3-linolenic acid, cetolenic acid, brominated vegetable oils,
cobalt salts in combination with ethanol, lead, nitrates and high
amounts of caffeine (Speijers, 1983). On the other hand, compounds
such as saturated fatty acids, odd-numbered cyclopropenoid fatty
acids (sterculia acid), ergot alkaloids, halogenated aromatic
hydrocarbons and cadmium induce toxic effects on the vascular system
and possible potentiate atherosclerotic effect (Speijers, 1989a,b).
Conrad & Bressler (1982) investigated the cardiovascular effects of
caffeine in elderly men. Caffeine, in doses equal to those contained
in 2 to 3 cups of coffee, produces an increase in blood pressure,
but has no positive inotropic effect in healthy elderly men.
Kim & Kaminsky (1988) suggested that age plays an important
role in modifying susceptibility to the toxic metabolite of
fluroxene, 2,2,2-trifluoroethanol. They noted greater effects on the
stomach, liver, testicles, brain and kidneys of aged rats as
compared to younger animals. Enhanced sensitivity to ethanol
intoxication has also been noted in old rats (Guthrie et al., 1987),
suggesting altered tissue sensitivity. Acute ethanol hepatotoxicity
may be due to enhanced liver susceptibility to the toxin (Rikans &
Snowden, 1989).
3.3 Modifying factors
3.3.1 Nutrition
Nutrition has been shown to influence the aging processes in
rodents and the occurrence and progression of age-associated
diseases in rodents and humans. Moreover the age-associated changes
in physiological processes affect the nutrition of the mammalian
organism. However, there is little information on how aging
influences the nutritional requirements of humans, a subject that
urgently requires scientific study.
Nitrates in foods can be reduced to nitrites in the oral
cavity, GI tract and urinary bladder (Tannenbaum et al., 1978).
Nitrites react with amines in the stomach forming N-nitroso
compounds, many of which have been shown to be carcinogenic in
animal studies, and it is difficult to deny their hazard to man
(Searle, 1976; Bartsch, 1991).
During commercial processing and domestic preparation, foods
may become contaminated with toxic chemicals. For example, smoked
and grilled foods contain small amounts of polycyclic aromatic
hydrocarbons and a wide variety of phenols and other organic
compounds derived from smoke (IARC, 1990). Canned foods can become
contaminated with tin or lead.
In a study by Spagnoli et al. (1991), both 4-month-old and
4-year-old New Zealand white rabbits were fed an atherogenic diet.
Increased incidence and degree of atherosclerotic lesions were seen
in the 4-year-old rabbits. These results showed an increased
susceptibility of the older arterial wall to hypercholesterolaemia.
Although 4-year-old rabbits are still relatively young, considering
the life span of this species (10-12 years), this study suggests
that aging arteries might be vulnerable to atherogenic compounds.
Malnutrition associated with inadequate intake or uptake of
nutrients in the elderly is caused by several factors. These include
socio-economic conditions (Munro, 1984) such as a) ignorance of the
need for a balanced diet, b) poverty, c) social isolation, d)
physical dependence (disability) and physical inactivity, e) mental
disorders, and f) changes in habits, e.g., retirement (Munro, 1984;
American Dietetic Association, 1984; Ferro-Luzzi et al., 1988).
Other conditions resulting in malnutrition include malabsorption due
to a variety of intestinal conditions, alcoholism, and the use of
therapeutic drugs that interfere with nutrient utilization and
therefore with the toxicity of chemicals (Krupka & Vener, 1979;
Kohrs, 1981; Munro, 1984; Chen et al., 1985; Robertson et al, 1988).
Malnutrition in much of the aged population can enhance the
vulnerability of the elderly to the effects of the toxic chemicals
in food. It has also been reported that malnutrition alters
pharmacokinetics in different ways depending on the drugs examined
(Roe, 1983; Cusack & Denham, 1984). This can be another factor in
the altered sensitivity of the malnourished elderly to toxicants.
Major deficits or marginal nutrient status occur for proteins,
calcium, vitamins A, C and B (thiamin, riboflavin, niacin, B6 and
B12) and zinc (American Dietetic Association, 1984; Ferro-Luzzi
et al., 1988).
In aged women, a deficiency in calcium is especially associated
with osteoporosis (American Dietetic Association, 1984; Munro, 1984;
Caraceni et al., 1988; Cauley et al., 1988). The deficiency in iron
and zinc might play a role in haematological status and immune
function, while a higher intake of aluminium might have neurological
sequelae (American Dietetic Association (ADA), 1984). An iron
deficiency might make individuals more vulnerable to compounds toxic
for the haematopoietic system, e.g., hexachlorobenzene and lead.
Data showing that older individuals have a reduced need for
energy, due to slower metabolism and decreased activity, has an
important impact on the intake of both macro and micronutrients,
because the composition of the diet is based on the average caloric
or energy intake (American Dietetic Association (ADA), 1984;
Ferro-Luzzi et al., 1988). Considering this reduced need for energy,
the elderly often reduce their intake of nutrients.
Dietary manipulation can alter life span in laboratory animals
(Barrows & Kokkonen, 1978; Young 1979). Restricting food intake in
laboratory rats produces a significant extension of life span (McCay
et al., 1935; Ross, 1961). Similar effects have been observed in
mice (Weindruch & Walford, 1988; Kubos et al., 1984), as well as in
more primitive organisms including fruit flies (Harman, 1981), and
nematodes and Neurospora (Harman, 1982). Other studies have
indicated that the reduction in caloric intake that accompanies
protein restriction, and not the protein restriction per se, is
responsible for the increased longevity (Leto et al., 1976; Davis
et al., 1983; Schneider & Reed, 1985). Dietary restriction has been
shown to be effective when started as late as middle age (Cheney
et al., 1983; Kubos et al., 1984; Masoro, 1988). Food restriction
appears to act either by influencing primary aging processes or by a
general protective mechanism, rather than directly modulating
multiple specific pathogenic processes underlying specific diseases
(Masoro et al., 1991).
Chronic nephropathy in rats has been reported to be retarded by
food restriction (Saxton & Kimball, 1941). Rats fed ad libitum a
semisynthetic diet of 21% casein as the protein source exhibited a
marked age-associated progression of chronic nephropathy, whereas
rats fed 60% of the ad libitum intake developed almost no
age-related progression of this disease process (Maeda et al.,
1985). Caloric restriction appears to be more important than protein
restriction in retarding nephropathy (Maeda et al., 1985). Fat or
mineral restriction was found to have no influence on longevity
(Iwasaki et al., 1988).
Dietary restriction delays the age-dependent loss of adipocyte
responsiveness to hormones, prevents the decline in serum free fatty
acid levels, delays the increase in serum cholesterol, and reduces
the increasing triglyceride level observed in aging rats (Cooper
et al., 1977; Liepa et al., 1980; Masoro et al., 1980; Yu et al.,
1980).
Dietary restriction from weaning has been shown to delay the
onset and reduced the severity of chronic nephrosis, periarteritis,
myocardial degeneration and muscular dystrophy in very old animals
(Berg, 1976). Chronic restriction has also been shown to inhibit
certain types of tumours, decrease the incidence of neoplasms, and
increase tumour latency (Ross, 1976; Weindruch et al., 1982;
Weindruch & Walford 1988). Early work using a chemically induced
mammary carcinoma model suggested that the primary effect of caloric
restriction was on tumour promotion (Weindruch & Walford, 1988).
More recent data (Fishbein, 1991) have demonstrated that the
initiation of chemical carcinogenesis can also be decreased by
caloric restriction. For example, exposure to aflatoxin B1
resulted in less DNA-adduct formation in liver from young
calorically restricted male rats than from controls fed ad libitum.
Such changes are most probably due to changes in hepatic cytochrome
P-450 expression (Fishbein, 1991).
There is ample evidence of better maintenance of
T-cell-dependent immunological responses in aging mice chronically
restricted from weaning (Weindruch & Walford, 1988). However, it has
been reported that restriction initiated at an adult age can also
delay the age-specific decrease in immune function (Fernandes
et al., 1977; Friend et al., 1978; Weindruch & Walford, 1988).
Although caloric restriction extends life span in invertebrate
and lower vertebrate species as well as rodents (Weindruch &
Walford, 1988), as yet there is no definitive evidence whether or
not caloric restriction will increase longevity, or decrease
neoplastic and degenerative diseases, in higher mammals or in man.
There is some evidence that reduced caloric intake in man reduces
urinary output of thymidine glycol and 8-hydroxy-guanosine, which
implies reduced free-radical-mediated DNA damage (Fishbein, 1991).
However, until the mechanisms by which caloric restriction evokes
its effect are fully understood, the only way that it can be
conclusively proved whether or not caloric restriction does prolong
life in higher mammals, is to perform longevity studies in these
species. Such experiments, using non-human primates, are underway in
the USA (Fishbein, 1991), but it will be some time before definitive
data are available.
Chronic disease in the elderly is accompanied by caloric
deficit, which in turn causes breakdown of body proteins and
negative nitrogen balance as well as the utilization of fat stored
in adipose tissues. Dietary protein appears to promote
age-associated renal disease in both humans and rats (Brenner
et al., 1982).
When illness occurs, nutritional deficiencies frequently become
clinically manifest (Rudman, 1987). For example, trauma or a fall
causing fractures leads to immobilization resulting in rapid loss of
body stores of nitrogen and calcium. This slows down mending of the
fracture. Similarly, surgical procedures in the elderly often result
in delayed recovery and risks far exceeding those of younger
persons. Heart failure and malignancies can lead to cachexia with
loss of weight, muscle mass and nutrient reserves. Infection may
produce similar changes and intervene to become the terminal event.
3.3.2 Alcohol intake
Ethanol is one of the chemicals most commonly ingested by
humans. Its effects on the body are numerous and varied. Loneliness
and isolation would seem to foster consumption of alcohol beverages
among older persons. The decrease in lean body mass and body water
that occurs with aging is responsible for higher blood levels of
alcohol in elderly people than in younger adults consuming the same
quantities (Vestal et al., 1977).
Elderly individuals have a decreased tolerance for alcohol, due
to an increased sensitivity of the CNS to the depressant effect of
ethanol. The metabolic effects of ethanol are the result of either
the increase in the NADH/NAD+ ratio occurring due to ethanol
metabolism or to direct toxic effects of ethanol or its metabolite,
acetaldehyde. An increase in serum uric acid level (hyperuricaemia)
is common during heavy alcohol ingestion, thus inducing acute gouty
arthritis in patients with known gout. Hypoglycaemia and
hyperlipidaemia occur in patients who are not eating adequately or
who are ingesting a high-fat diet with ethanol, respectively.
Thrombocytopenia is caused by alcoholism in patients with advanced
alcoholic liver disease (IARC, 1988).
The use of drugs increases with aging. Acceleration or
inhibition of drug metabolism by ethanol depends on the duration of
ethanol ingestion and the presence or absence of ethanol in the body
at the time the drug is ingested (Mezey, 1981). The presence of
alcohol in the body causes a decrease in the metabolism of certain
drugs, such as antipyrine, meprobamate, pentobarbital and
benzodiazepines, resulting in increased bioavailability of the drugs
to the CNS and thereby contributing to unwanted side-effects. In
contrast, ethanol can increase the metabolism and metabolic
activation of certain xenobiotics, such as the known human
leukaemogen benzene, thus potentially leading to enhanced toxicity
(IARC, 1988).
Chronic ethanol ingestion increases tolerance to CNS
depressants in young individuals, but its effect in the elderly is
unknown. The concomitant administration of ethanol and barbiturates
results in an enhanced depressant effect of these drugs on the CNS
and can result in coma or even death. All other sedative-hypnotic
drugs tested have either synergistic or additive effects with
ethanol. Intellectual deterioration and dementia are common
complications of chronic alcoholism. Alcoholic patients show more
signs of mental aging at every chronological age (Gaitz & Baer,
1971).
Chronic excessive alcohol ingestion is associated with
increased mortality from cancer, cirrhosis, non-malignant
respiratory diseases such as emphysema, and accidents (Klatsky
et al, 1981; IARC, 1988). However, a decrease in coronary artery
disease and mortality is associated with moderate alcohol ingestion.
This may be due to increases in plasma HDL-cholesterol and decreases
in LDL-cholesterol that occur during ingestion of alcohol (Mezey,
1981). Ethanol consumption is also associated with hypertension and
an increased mortality from cerebrovascular accidents (Kozararevic
et al., 1980; Blackwelder et al., 1980).
3.3.3 Smoking
Smoking clearly plays an etiological role and produces an
acceleration of a wide spectrum of age-associated disease. Smoking
contributes to an increased mortality rate (Gupta et al., 1980). It
also provides an excellent example of problems faced in the
consideration of the environmental impact on aging.
Scientists recognize that smoking presents a complex toxic
insult through inhalation. Increased pathology in aged mice has been
reported after exposure to cigarette smoke (Matulionis, 1984). The
immune response in aged mice exposed to cigarette smoke has been
shown to be decreased at some ages but not at others (Keast & Ayre,
1981). The role of smoking in coronary heart disease has been
reviewed by Kannel (1981), who observed that the relative effect of
cigarette smoking decreases in old age and proposed that this could
be due to the selection of a more resistant population.
Estrogen-related diseases have also been associated with smoking
(Baron, 1984). Reif (1981) has examined susceptibility to lung
cancer and concluded that the shape of the susceptibility
distribution is determined by the effects of all environmental
carcinogens (both known and unknown) to which the population has
been exposed, as well as by differences in genetic susceptibility
among members of the population. Smokers and non-smokers both get
lung cancer during the same age range. Some of these studies suggest
that aged individuals are more susceptible to the effects of
smoking, whereas others suggest that duration of smoking appears to
be the critical factor (IARC, 1990). The smoker is exposed to
multiple toxic agents simultaneously, the effects of which are more
pronounced in aged individuals. Major aspects of metabolism and
pharmacokinetics are altered in aged individuals. Therefore, the
effective dose of any chemical reaching the systemic target tissues
in aged humans would be dependent on these perturbations.
3.4 Interactions of chemicals and diseases
3.4.1 Cancer
Cancer morbidity is expected to rise with age and with an
increasing percentage of elderly people living in industrialized
countries (Magnus, 1982). There is no consensus on the causes of the
age-related increase in tumour incidence. Various arguments support
the concept that an age-related accumulation of total dose of all
carcinogens accounts for tumour induction as a function of age in
sensitive individuals (Peto et al., 1975, 1985). One viewpoint is
that the sensitivity to carcinogens is stable and independent of
age, whereas another is that changes in the internal milieu of the
organism, such as the metabolic and immunological shifts of natural
aging, provide favourable conditions for tumour development with
increasing age (Burnet, 1970; Dilman, 1971).
Comparison of human epidemiological data with in vivo and
in vitro animal experimental results is difficult but does allow
some limited conclusions. It seems that environmental carcinogenic
factors as well as endogenous carcinogens are important causes of
increased tumour incidence in old people (IARC, 1990). This
conclusion is supported by the increasing incidence of occupational
cancer with increased exposure time to carcinogenic agents and by
the correlation of lung cancer incidence with the number of
cigarettes smoked (Doll & Peto, 1981; Peto, 1986). Humans, in
general, have an age-related increase in the incidence of epithelial
neoplasms (Doll, 1978), but the relationship of age to incidence of
other cancers in different organs varies (Doll, 1973; Moolgavkar &
Venzon, 1979; Anisimov, 1987; Dix, 1989). Some tumours appear most
frequently in childhood, some increase exponentially with age, and
others reach a peak at a certain age and then decline.
The data on cancer incidence among the atomic bomb survivors in
Hiroshima and Nagasaki, Japan, have been very informative concerning
radiation-related solid tumours as well as leukaemia. Age at time of
exposure appears to be a strong determinant of leukaemia risk; the
greatest absolute risk was experienced by those who were exposed at
ages 0-9 or 50 years and over (Beebe, 1979). Most of the excess
cancer deaths from solid tumours among the atomic bomb survivors
have occurred among those who were over 35 at the time of the blast.
Analysis of data on the positive correlation between the aging
rates of different species with their cancer rates and the
observation that these two processes, aging and carcinogenesis, may
be initiated and promoted by impairments of gene regulation led
Cutler & Semsei (1989) to conclude that both cancer and aging may
arise from a common set of genetic alterations. The analysis of the
interrelationship between aging and carcinogenesis should be based
on epidemiologically and experimentally confirmed data.
Epidemiological data, analysed in terms of a multi-stage model
(Kaldor & Day, 1987), can estimate the importance of age at onset,
duration of carcinogen exposure, and latency in a population. On the
level of the organism, carcinogenic agents influence not only the
cell, causing genomic damage that leads to neoplastic
transformation, but also create in the cell a microenvironment that
facilitates proliferation and clonal selection (Anisimov, 1987,
1989). Multi-stage carcinogenesis is accompanied by various
disturbances in tissue homeostasis and systems of anti-tumour
resistance that, in turn, are under the influence of systemic
(nervous, hormonal and metabolic) factors. How long it takes for
frank neoplasia to develop depends on the state of those systems at
the moment of exposure to a carcinogen or tumour promoter and the
dose.
According to the multi-stage model of carcinogenesis, the
carcinogen whose effect increases in proportion to age at exposure
affects the partially transformed cell. In this case the tumour
incidence would increase and latency would decrease, as compared to
a population exposed to the same effective dose of carcinogen at a
young age. For example, application of 7,12-
dimethylbenz [a]anthracene in small doses or
12-0-tetradecanoylphorbol-13-acetate to the skin of mice of
different ages caused neoplasms more frequently in older animals
(Stenbäck et al., 1981; Ebbesen, 1985). Exposure of mice and rats of
various ages to phenobarbital resulted in hepatocarcinogenesis only
in old animals (Ward, 1983; Ward et al., 1988). The number of events
necessary for complete malignant transformation in 15-month-old rats
under the influence of N-nitrosomethylurea is lower than in
3-month-old rats (Anisimov, 1988). In every tissue, the number of
events occurring in the stem cell before its complete transformation
is variable and depends on many factors, in particular the rate of
aging of the target tissue and of the regulatory system(s) of the
tissue (Anisimov, 1987, 1989). This model is consistent with the
analysis of age-related distribution of tumour incidence in
different sites in humans and experimental animals (Doll, 1978; Dix,
1989; Anisimov, 1987).
Epidemiological observations have shown that exposure to some
carcinogenic agents leads to a rise in cancer incidence independent
of age at the start of exposure (e.g., smoking), while other agents
induce more tumours when the exposure begins in the elderly (e.g.,
lung cancer following asbestos exposure) (Kaldor & Day, 1987; IARC,
1990).
3.4.2 Other diseases
Aging affects the functional capacity and structural integrity
of many organ systems. Environmental chemicals can also affect
several target organs. The combination of the influence of aging and
the toxic effects of chemicals in the environment might potentiate
the risk for elderly persons. An inherent problem common to all
research in chronic disease is the dissection of the respective
roles of time per se from those of primary aging. Hazzard (1985)
stated that an essential feature of human aging is the change in
physiological competence across the life span, as reflected in
homeostatic reserve. Homeostatic reserve declines at an accelerating
rate with age, normally producing death in old age from
multifunctional etiologies.
The relationship between aging and atherosclerosis is a prime
example of this conundrum. It seems most likely that the changing
picture of atherogenesis in western society has led to a large
number of people who survive into old age with not only a degree of
clinical atherosclerosis, but also with other chronic progressive
diseases, such as chronic obstructive pulmonary disease, immobility
from osteoarthritis and/or osteoporosis, and mental incompetence
from Alzheimer's disease, multi-infarct dementia or other
age-related dementing processes.
These diseases could significantly modify the response of the
organism to various environmental chemicals by decreasing or
increasing their susceptibility, followed by an acceleration of
these diseases or induction of new ones.
4. APPROACHES TO EXAMINING THE EFFECTS OF CHEMICALS ON THE AGED
POPULATION
4.1 Experimental approaches
4.1.1 Principles for testing chemicals in the aged
population
There are two principal approaches to the study of age-related
changes of any functional, morphological and/or biochemical
parameter, i.e. "longitudinal" and "cross-sectional". Longitudinal
studies consist of repeated estimations of any parameter in the same
animal in different periods of life. Cross-sectional studies involve
separate groups of animals of different ages who are examined at a
given point in time. Results obtained using the longitudinal
approach may significantly differ from the results from experiments
carried out using cross-sectional approaches. In studies on the
toxicity of chemicals, it is obligatory that identical conditions be
provided and maintained for all the animals. Problems related to the
choice of animal species, strain, sex, age, life stage and chemical
treatment (both route and dose selection) will be considered below.
4.1.2 Animal models
An extensive discussion of the choice and use of animal models
in research has been recently published (Rogers et al., 1991).
4.1.2.1 Animal species
The selection of suitable species obviously involves both
practical and economic factors. Animals with a short life span are
preferred. However, good life-table information is not available for
all species. A knowledge of species-related differences in metabolic
pathways and inherent sensitivities is also important in choosing
animal species for study. The choice of species should depend on the
experimental question as well as homology of response. For example,
while closely related isoforms of drug-metabolizing enzymes may
exist in different species, their tissue distribution and substrate
specificity may vary greatly (Nebert et al., 1991). Such differences
in isoform expression, together with reported differences in DNA
repair efficiency, could be responsible for species-related
differences in an organism's susceptibility to chemical carcinogens
(Daniel et al., 1983; Mehta et al., 1984; Anisimov, 1987; 1989).
Regardless of such metabolic differences, mice and rats are
most often used in aging research because of a short life span,
relative ease of maintenance under defined conditions, wide use in
biological research, and suitability for a variety of molecular and
genetic analyses. There are extensive life-table information for
some strains of mice and rats as well as a data base on their
spontaneous pathology. If old animals are purchased from a supplier
rather than being maintained in an investigator's own laboratory, it
is necessary to obtain information on the lifetime environment of
the animals, including housing conditions and dietary history. It
may be preferable to keep animals from weaning until the desired age
for investigation under the same controlled conditions.
Mammalian species other than rodents have only been used
sporadically in aging research, owing to their long life span, lack
of life-table information, genetic heterogeneity, restricted
availability and high cost. However, it is critical that such larger
species be used to answer certain questions. For example, studies
are currently underway to determine if dietary restriction can
prolong the lifespan of two different monkey species (Ingram et al.,
1990).
4.1.2.2 Animal strain
The choice of adequate rodent strain for experiments on aged
animals is critical. The male Fischer-344 rats is a popular model in
aging studies because of its size and growth characteristics.
However, testicular interstitial cell tumours begin to appear at the
age of 18 months in rats fed ad libitum, and by the age of 2 years
the tumour incidence approaches 100% (Fishbein, 1991). Recently, a
highly significant positive trend with time has been observed for
the increasing prevalence of leukaemia, anterior pituitary tumours
and thyroid c-cell tumours in both sexes, adrenal pheochromocytomas
in males, and mammary tumours and endometrial stromal polyps in
female F-344 rats (Rao et al., 1990). Some mouse strains suffer
from a single major disease process (e.g., tumours of the mammary
gland or liver, or chronic nephropathy), and the presence of this
disease in most animals could modify the response to chemical
exposure and complicate the interpretation of the results.
In the USA and Japan, Fischer-344 and Sprague-Dawley rats and
B6C3F1, Swiss, and CD-1 mice are most frequently used, whereas
in European countries Wistar-derived and Brown Norway rats and NMRI
and Swiss mice are the most popular strains. Inbred animals have the
advantages of greater stability and predictability of response.
However, heterogeneity of outbred animals more closely resembles the
heterogeneity of human populations. The choice of a certain animal
strain also depends on previous experience with these animals, the
final choice of an appropriate species and/or strain being dependant
on the scientific hypothesis under investigation. Each strain has
its own pattern of background pathology, which can be greatly
influenced by animal husbandry.
4.1.2.3 Animal sex
Sex-differences in response to chemicals are well known. Thus,
the use of both sexes is often necessary in toxicity testing.
However, the large gender differences in chemical toxicity and
pharmacokinetics that occur in rats become less apparent in old age
due to the decreased expression of sex-specific isoforms of the
hepatic drug-metabolizing enzymes (Kitani, 1991).
Ovarian status may significantly influence the sensitivity to
some chemical agents, modifying the biological response, as been
demonstrated for chemical carcinogens (Anisimov, 1971, 1987). It is
noteworthy that, when using females of the post-reproductive period,
the investigator must be aware that a) the ovaries in females of
some species (i.e. rats and mice) are not atrophied and may continue
to secrete steroid hormones, and b) some animals may be in
persistent estrus, while others may be in anestrus or
pseudopregnancy status (Aschheim, 1976). Furthermore, an animal's
reproductive history could influence its response to a chemical in
later life.
4.1.2.4 Selection of age groups for comparison
The problem of appropriately characterizing the animal's age
within the context of its life span is of particular importance in
experimental gerontology. Since the aging process causes significant
changes in various systems of the organism, there is a need for the
definition of some reference points for adequate comparison of the
results of different experiments. The importance of these points is
particularly significant in cross-sectional studies. One of the
confounding factors in such comparisons between experiments is the
need for frequent comparisons of results from different animal
species with different life spans (long-lived and short-lived
species and/or strains). Many authors have used two kinds of age
groups: "mature" or "adult" animals; and "old" animals. However, the
true age of "mature" rats in the reports from different
investigators fluctuates from 2 to 14 months and the age of "old"
animals from 12 to 37 months. The life cycle of experimental animals
can be divided into four periods: a) prior to weaning
(developmental); b) sexual maturation (maturational);
c) reproductive; d) pronounced age-related changes (senescent
period) (Zapadnyuk, 1971). All studies should compare animals from
multiple age periods.
4.1.2.5 Underlying pathology of animals of different ages
As mentioned previously, the background pattern of pathology
must be taken into account in the selection of animal species and
strains. A species-, strain- and sex-specific incidence of
pathology, whether neoplastic or not, is observed to increase
rapidly in the second half of the life span, even for those animals
maintained under specific pathogen-free conditions (Burek, 1978;
Anisimov, 1987; Frith & Ward, 1988; Fishbein, 1991). Among
non-neoplastic diseases in rats, the most frequent are chronic
glomerulonephropathy, cardiomyopathy, amyloidosis, and peripheral
nerve degeneration. For example, the incidence of spontaneous
leukaemia in F-344 rats frequently used in long-term studies may
exceed 30%, and the frequency of intertitial cell tumours of the
testis may reach 100% in old males. This pathology may significantly
modulate the host response to xenobiotics.
4.1.2.6 Transgenic animals
During the last decade it has become possible to add new
genetic information to the germ line of experimental animals
(Jaenisch, 1988). More recently successful attempts have even been
made to specifically alter gene sequences in the mouse germ line,
thereby ablating or correcting specific gene functions (Capecchi,
1989). In the study of the effects of chemicals on the aged
population, transgenic animal models have at least two contributions
to make. Firstly, the influence of specific genes on the age-related
susceptibility to environmental chemicals can be assessed in the
in vivo situation (Vijg & Papaconstantinou, 1990). Secondly, by
using transgenic animals harbouring a shuttle vector with one or
more mutational target genes, mutagenic effects can be studied in
different organs and tissues of animals as a function of age (Gossen
et al., 1989).
4.1.2.7 Animal husbandry
In experimental research on the sensitivity of aged animals and
the aging process, the husbandry aspects should be defined. The
quantitative and qualitative results might depend greatly on the
dietary conditions. The dietary composition should meet the minimal
requirements for nutrients, minerals, vitamins and (raw) fibre for
adult animals according to established and published standards for
laboratory animal diets. Another important dietary factor is the
quantity to which the test animals have access. They may either
receive a restricted diet containing adequate level of nutrients or
be fed ad libitum. The choice of diet depends on the questions to
be asked.
In addition to the dietary status of the animals, their
microbiological status should be defined. In some cases interaction
of the chemical with the gut microflora might modify the outcome of
the study. Specific-pathogen-free (SPF) animals are preferred. The
direct environment of the test animal, including relative humidity,
temperature, light and dark cycles, seasonal influence and stress
can all influence the final outcome of a study and therefore should
be defined carefully (Masoro, 1991).
Differences in animal husbandry may cause large variations in
biochemical and pathological effects. This is clearly illustrated by
alterations in the background data among different laboratories.
4.1.3 Chemical exposure
4.1.3.1 Dose level
The selection of dose is a difficult issue. The usual
requirement is to have a minimum number of test groups (3) plus one
control (vehicle) group. This permits the development of a
"dose-response" curve and allows for appropriate statistical
evaluation of the results. The highest dosage should induce minimal
signs of toxicity, bearing in mind that the maximum tolerated dose
for young animals could in some cases be toxic for old ones, and
that high doses may alter the toxicokinetics of the chemical.
Considering that the body weight of young and old animals could
be significantly different, a correct dosage calculation of test
substances in comparison groups is an important problem, i.e.
whether it is better to normalize per unit of body weight or body
surface, or to some other parameter such as lean body mass (Travis
et al., 1990). The available data indicate similarity of the results
when calculation of dose is performed per unit of body weight or
body surface. It should be taken into consideration that the growth
and development of various organs may have different rates, and that
relative organ weight (and consequently, the effective target dose
of the substance) may not be the same in animals of different ages.
When the substance is administered in food or water, the consumed
quantities should be taken into account, because they may differ in
animals of various ages. When the oral or dermal route of
administration is used, age-related changes in the extent and rate
of absorption may be important. Age-related changes in lung
ventilation capacity may also alter the internal dose of a chemical
when the inhalation route of administration is used. When looking
for portal-of-entry effects, a constant concentration of the agent
may be used in all age groups.
4.1.3.2 Route of administration
There is general agreement that the test substance should be
administered by the route that corresponds most closely to human
exposure. For humans, the main exposure routes are oral, dermal and
inhalation, while in animal experiments the oral route is most
frequently used. However, when pharmacokinetic studies show that
other routes of administration result in equivalent target tissue
levels, such alternative routes can be used. The use of injections
(subcutaneous, intraperitoneal, intramuscular or intravenous) may be
expedient under certain circumstances.
4.1.3.3 Duration of exposure
The question being addressed should guide the design of the
study. If the potential risk involves acute exposure of the elderly,
then an acute experimental scenario is required. If human exposure
is ongoing, long-term studies may be necessary. Exposure to the test
substance in chronic studies should start shortly after weaning and
continue for the major portion of the animal's life (at least
through the mean life span). IARC (1986) recommended 24 months of
exposure for rats and mice, and 18 to 20 months for hamsters when
studying the carcinogenic potential of chemicals. Choice of exposure
duration based on life-table characteristics would be optimal.
However, these guidelines do not apply to experiments when animals
of different ages are used at the start of the study. In this case
the dose and duration of exposure could differ in groups of young
and old animals. However, it is preferable to use identical exposure
durations whenever possible.
4.1.4 Non-mammalian models
Many species can be used as models in the study of age-related
sensitivity to environmental toxicants. These include fungi
(Neurospora crassa and Podospora anserina), protozoa
(Paramecium tetraurelia and Tetrahymena pyriformis), rotifers,
nematodes (Caenorhabditis elegans, C. briggsae and Turbatrix
aceti), and insects (Drosphila melanogaster, Musca domestica and
Tribolium confusum) (Committee on Chemical Toxicity and Aging,
1987). Non-mammalian species such as fish and salamanders have been
used in aging research (Weindruch & Walford, 1988) and are
potentially available for toxicology studies. Several of these
models have already been used to examine the effects of chemicals on
aging. Because of their short life span, ease of use and relatively
low cost, non-mammalian organisms could be important in the initial
phases of a test system to identify environmental chemicals that
might affect aging.
4.1.5 In vitro studies
For the purposes of screening xenobiotics, the use of in vitro
models can result in substantial economy and efficiency. Stock cells
and, in some cases, tissue explants can be cryopreserved in large
amounts. This permits repeated assays with comparable materials and
the sharing of common stocks by numerous laboratories. Moreover,
such stocks can be used to investigate cell-to-cell interactions,
such as metabolic cooperation and metabolic transformation. Finally,
tissue culture approaches can substantially reduce the numbers of
animals required for experimentation. Such methods cannot, however,
be expected to substitute for the intact animal experiment.
There are three general categories of in vitro methods: organ
culture, tissue explants and cell culture. Organ culture involves
the short-term maintenance of variable intact segments of tissue,
for example, the full thickness of a segment of aorta. In tissue
explants, the early migration and proliferation of epithelial and
fibroblast cell types can be observed. Cell cultures are of four
general types:
(a) primary cell cultures and mass cultures of cells taken directly
from the animal, usually after enzymatic dispersion of biopsied
tissue;
(b) established, serially passaged cultures with relatively
reproducible cycles of growth in early phases, but with limited
replicative life span, and with a genetic make-up reflecting
that of the donor age;
(c) "transformed" cell cultures with indefinite replicative
potential and generally with altered genetic makeup;
(d) established or transformed cell lines into which specific DNA
sequences ("transgenes") have been transfected.
Each of these types of models could prove useful for studies of
the effects of environmental agents on the elderly. One general
approach would be to explore the toxic effect of an agent as a
function of donor age, so as to detect unusual susceptibilities of
the cells and tissues of aged subjects. Another general approach
would be to culture the cells in vitro after in vivo treatments.
If a set of behaviours or phenotypes was observed with tissue from
young, treated subjects that proved to be comparable to that
observed with tissue from old untreated animals, an effect of the
in vivo treatments on the aging process could be inferred.
An entirely different experimental paradigm could be based on
the hypothesis that established cultures with finite replicative
life spans recapitulate the natural history of comparable cell types
in vivo - the well-known " in vitro model of cellular aging"
first developed by Hayflick & Moorhead (1961). The appropriateness
of such models for the study of aging is controversial, since it has
been proposed that the attenuation of growth observed in vitro
corresponds to terminal differentiation in vivo (Norwood & Smith,
1985; Bayreuther et al., 1988).
Of special interest would be the evaluation of agents that
exhibit unusual toxicity to putative stem cells. An excessive
depletion of stem cells could seriously compromise the regenerative
potential of tissues in aging subjects. In such studies, it would be
important to investigate a variety of cell types. Most research to
date has concentrated on in vitro aging of cultures of
fibroblastoid cells established from the fetal lung or from the
dermis of individual subjects of various ages. The precise origin of
such cells is not clear. Thus, with such cells it would be difficult
to compare age-related changes in vivo with those observed
in vitro.
A final experimental paradigm would be to use postreplicative
terminally differentiated cells in culture in order to investigate
agents for their potential to accelerate age-related alterations
observed in vivo. Such cell types might be derived through
in vitro terminal differentiation, or from normal or transformed
embryonic neuroblasts or myoblasts. A major concern, however, would
be the extent to which the experimental milieu reflected in vivo
conditions.
4.1.6 Statistical considerations
For statistical treatment of the results of short-term testing,
the statistical methods that are generally available may be used.
However, for statistical analysis of the results of long-term
testing, in particular carcinogenicity testing, the comparison of
results from treatment groups with different survival rates is a
major issue. In these cases the recommendation of IARC (Peto et al.,
1980; Gart et al., 1986) could be applied. All the tumours found at
necropsy must be evaluated as "fatal" or "incidental". This approach
permits one to conduct a comparison between young and old animals
despite the very different patterns of survival from those expected.
A crude analysis, ignoring the fact that young animals survive
longer than old ones, will overestimate the ratio of tumour
incidence in young and old animals. Conversely, a "death-rate"
analysis, treating all tumours as if they were fatal, will
over-correct for the effects of differences in survival on the
incidence of tumours discovered at the autopsy of animals that died
of unrelated causes. Bias can be eliminated only if tumours that
were discovered in an incidental context are analysed by the
prevalence method, while tumours discovered in a fatal context are
analysed by the death-rate method (Peto et al., 1980; Gart et al.,
1986).
4.1.7 Extrapolation of animal data to humans
Extrapolation of animal data to humans may be either
qualitative or quantitative. On the basis of experimental results,
the weight-of-evidence approach can help predict whether a given
substance may be considered dangerous for humans. Such information
includes data on the pharmacokinetics of a chemical, its
cytotoxicity and other toxic properties, as well as the data
obtained in in vitro and short-term tests. Of primary importance
for quantitative extrapolation, a dose-response relationship must be
detected in experimental animals. An important stage of quantitative
evaluation is extrapolation of the data obtained from exposure to
rather high doses to lower doses to which humans may be exposed in
the environment. It is necessary to take into account biological
differences, both in pharmacokinetics and pharmacodynamics, between
the test species and humans. For example, a dose taken per unit of
the body surface area, or its concentration in the daily ration,
includes a correction factor for species sensitivity and allows
extrapolation of experimental doses to man (Mantel & Schneiderman,
1975; Turusov & Parfenov, 1986; Travis et al., 1990). Of course,
species sensitivity to the action of some chemicals may also vary
significantly.
Various physiological and mathematical models have been
proposed for extrapolation of toxicological effects. Most of these
models refer to carcinogenesis (Turusov & Parfenov, 1986; Swenberg
et al., 1987; Clayson, 1988). The predictive nature of these models
for non-carcinogenic end-points remains to be determined.
4.2 Epidemiological and clinical approaches
4.2.1 Disease pattern of aged population
In order to study the pattern of disease occurring in the
elderly, the first question is "What illnesses are most common and
important among the elderly?" The answer can be most easily provided
in terms of diseases that cause death, hospitalization or visits to
a doctor's surgery.
The assessment of health status among the elderly on an
international basis is essentially limited to the use of mortality
data, since these are the only comprehensive data available. In the
developed countries, roughly 50% of all deaths occurring between the
ages of 65 and 74 years are attributable to cardiovascular diseases.
Among males in this age group, ischaemic heart disease accounts for
25% of deaths, while 11% are due to cerebrovascular diseases. For
women, 20% of deaths are attributed to ischaemic heart disease and
about 15% to stroke. Cancer accounts for another 25% of deaths among
men and women aged 65-74, lung cancer being the cause of 10% of all
deaths among elderly males. Roughly 7% of deaths are due to
respiratory diseases and 3% to external causes (WHO, 1989).
The trends in the four leading causes of death, i.e. malignant
neoplasms, heart diseases, cerebrovascular diseases and respiratory
diseases, within the age range 65-74 showed that, in selected
developed countries between 1950-1954 and 1980-1984, death rates
from all malignant neoplasms rose slightly for men but remained
essentially unchanged for women except in France. A more marked
decline in mortality is apparent for heart disease in the USA and
Australia where death rates have fallen by 25-30% since the late
1960s. Male mortality has fallen in France and Japan, but has risen
in Hungary. A similar pattern of change is also apparent for
females. Mortality from stroke has also declined in most countries,
although the timing and extent of the duration has varied from
country to country. For example, the decline in Japan occurred ten
years earlier than that in Australia. In the United Kingdom, France
and USA, rates have been falling since the 1950s. The trend in
mortality from respiratory diseases is less clear, there being
little evidence of sustained and comprehensive decline. However, it
is apparent that there has been some progress in reducing mortality
from these diseases in Australia and the United Kingdom over the
last decade (WHO, 1989).
It must be recognized from the outset that mortality data do
not always accurately reflect the underlying morbidity and are
particularly inappropriate in the case of many conditions for which
the fatality rate is low, yet which are important causes of
morbidity among the elderly. Data on causes of death are also less
reliable for the elderly than for other age groups owing to the
multiple pathological conditions often present at the time of death.
Nevertheless, with few exceptions, comprehensive morbidity data are
not available for the elderly.
Data from the USA shows that cardiovascular diseases cause the
largest proportion of hospitalization in the elderly. As a group,
diseases of the digestive system account for the second most common
diagnostic category while neoplastic diseases (90% malignant) are
the third most common cause of hospitalization. The remaining causes
are diseases of the respiratory system, injury or poisoning, and
diseases of the genitourinary or musculoskeletal systems (White,
1989). In a study from Shanghai, China, the common diseases of
hospitalized patients over 65 years of age in the 1950s, when
arranged in order of frequency, were hypertension, coronary artery
disease, chronic bronchitis, prostate hypertrophy, femur fracture,
pulmonary tuberculosis, diabetes mellitus, cholelithiasis and
tumours of various organs. Coronary artery disease, pneumonia,
hypertension and chronic bronchitis became the leading causes of
hospitalization in the 1970s (Zhu et al., 1982). In the 1980s, data
from other parts of China (Jiangxi, Liaoning, Xinjiang) showed that
chronic bronchitis or pneumonia was the most frequent cause of
hospitalization for elderly patients, followed by hypertension and
coronary artery disease (Xu et al., 1986; Shen et al., 1987; Xiong,
1989).
Data from the USA shows that diagnoses arising from visits to
the doctors surgery by people aged 75 or older, when arranged in
order of frequency, are hypertension (17.6%), chronic ischaemic
heart disease (9.5%), diabetes mellitus (6.7%), osteoarthritis (6%),
cataracts (5.1%), heart failure (4.4%), cardiac arrhythmia (3.6%),
arthropathies (3.6%), glaucoma (2.8%), hypertensive heart disease
(2.6%), angina pectoris (2.3%), chronic airway obstruction (2%) and
neoplasms (1.4%) (White, 1989).
4.2.2 Assessment of effects of environmental chemicals in the
elderly population
There are more limitations in clinical studies than in animal
toxicology studies. Firstly, the conditions in the study are not
easily controlled. Secondly, presumably harmful effects to the
subjects under study need to be avoided in designing the protocol.
The following approach is suggested.
Comprehensive, multidimensional functional assessment of the
elderly should be carried out. This involves analysis of the
physical, psychological, social, environmental and other aspects of
functioning (Zarit et al., 1985; Kane, 1987). Measurements of the
ability to perform the activities of daily life should be obtained.
Physical functioning can be measured by a combination of diagnosis,
symptom description, health reporting, days in bed or hospital
during a specific period, and reported pain or discomfort. The
subject's orientation with respect to time, place and person, as
well as short-term and long-term memory, should be assessed.
Psychological measures are used to evaluate depression, anxiety,
loneliness and sense of mental well-being, and a psychiatric history
should be recorded.
Clinical assessment involves a complete physical examination
and selective laboratory or instrumental examinations. As most
environmental chemicals affect certain parts or organ systems of the
body, laboratory or instrumental examination of these particular
parts or organ systems should be performed. The purpose of clinical
assessment is to find any structural or functional abnormalities
that may occur during the course of exposure to the environmental
chemicals.
Measures of environmental chemicals in the human body, such as
the determination of their concentration or that of their
metabolites in blood, body fluids or tissues (including nails,
hairs, etc.), faeces, urine and expired air, should be conducted.
Biomarkers of exposure, such as haemoglobin adducts, may be used
when available.
The results of the above-mentioned assessments may be
correlated and analysed to arrive at a conclusion as to whether the
environmental chemicals have harmful effects on the subjects
studied.
4.2.3 Acute episodes
There are few epidemiological reports on the effects of
exposure to environmental toxicants on the aged population. Several
acute air pollution incidents resulted in marked increases in
illness and death, mostly among the elderly. One such incident
occurred in the Meuse River Valley in Belgium in 1930 when
accumulating air contaminants trapped by an inversion caused the
death of 63 people and illness in 6000 residents. An incident in
1948 in Donora, Pennsylvania, USA, resulted from a similar inversion
that covered a wide area. Of the population of 14 000, 20 died
(compared with the expected two deaths for the same period) and 43%
fell ill. Again, the elderly were the most seriously affected. In
London, 4000 excess deaths were attributed to smoke and sulfur
dioxide in the fog of 1952, and an incident in 1962 caused 400
deaths above the normal value for the period. Similar episodes of
fog-induced mortality in various places have been described (Amdur,
1986). In all these episodes, the most vulnerable people were the
elderly, and the victims were usually suffering from acute
bronchitis and pneumonia, resulting in acute respiratory failure.
However, detailed and systematic study of the effects of
environmental chemicals on the elderly is still lacking (Amdur,
1986).
4.2.4 Concerns for the aged population
The health conditions of the elderly are quite different from
those in the early or middle age of human life. Many chronic
diseases that begin at an early age extend into advanced age. Some
pathological conditions occurring in middle age may become
symptomatic in the elderly. Certain medical problems are clearly
more prominent among the elderly, e.g., cancer of the prostate,
temporal arteritis and osteoarthritis. Illness in older people often
involves multiple organ systems that are interrelated by symptoms,
physical findings, functional capacity and treatment. The emotional
and social consequences of the physical conditions are also related.
Furthermore, the manifestations of aging processes that usually
comprise degeneration, both structural and functional, may sometimes
be difficult to differentiate from those of diseases. Aging
processes and existing diseases, as well as social and environmental
factors, act together to make the assessment and care of the elderly
complex and difficult.
As far as the effects of chemicals upon human health are
concerned, the elderly are subjected to long-term exposure to
environmental chemicals. Some exposures occur daily. The source may
be polluted air, water, food or products made of inorganic or
organic chemicals with which people frequently come into contact.
Some exposures occur during occupational activities in which people
are engaged for many years. The cumulative effect of continuous or
repeated exposure to chemicals may result in pathological changes
and clinical manifestations found in later stages of life. In
addition, the structural and functional changes of aging, usually
degenerative in nature, make the elderly more vulnerable to adverse
effects of environmental chemicals.
As mentioned above, the multiple disease status in the elderly
is usually accompanied by polypharmacy. In developed countries, the
consumption of drugs by people over 65 years of age accounts for
25-50% of total drug consumption (Vestal, 1978). The most commonly
used are neuropsychiatric and cardiovascular drugs,
anti-inflammatory analgesics and diuretics. Since these drugs are
used for the relief of symptoms rather then the cure of diseases,
repeated or sustained prescribing is the rule. Under such
conditions, interactions between environmental chemicals and drugs
should be carefully considered.
4.3 Biomarkers of aging
The term "biomarker of aging" arose in conferences sponsored by
the Fund for Integrative Biomedical Research (Regelson, 1983) and
the National Institute on Aging (Reff & Schneider, 1982). The
Committee on Chemical Toxicity and Aging (1987) defined a biomarker
of aging as "a biological event or measurement of a biological
sample that is considered to be an estimate or prediction of one or
more of the aging processes". Baker & Sprott (1988) offered the
following specific features for a biomarker of aging: (a) nonlethal;
(b) highly reproducible within and across species; (c) reflects
physiological age or some basic biological process of aging or
metabolism; (d) displays significant alterations during relatively
short time periods; (e) is crucial to the effective maintenance of
health and prevention of disease; and (f) reflects a measurable
parameter that can be predicted at a later age. Development of a
panel of biomarkers would assist in assessing the effects of
environmental chemicals that modulate aging processes in laboratory
animals and man.
There is currently a significant research effort, especially
with rodent species, to establish batteries of biological markers of
aging (Allaben et al., 1990). But how can one differentiate
alterations that are simple functions of chronological time from
those that are the results of intrinsic aging or of intrinsic aging
coupled with the effects of chronological time? One approach has
been that of comparative gerontology. Starting with a group of
taxonomically related species that vary substantially in their
maximum life-span potentials, one simply asks if the rate of change
of the putative biomarker reflects the maximum life-span potential
of the species.
From a practical point of view, various biomarkers may be used
for the measurement of aging. For different levels of integration of
an organism, different parameters may be used. At the subcellular
and cellular levels, measurements of macromolecular lesions, the
degree of collagen cross-linking, the level of lipofuscin
accumulation, specific enzyme activities, the number of particular
hormone receptors, numbers of cell divisions, etc. may be
investigated as biomarkers. Tissue and organ weights, cellularity,
growth or some functional activity (muscle strength, visual acuity,
secretion rate) may be considered. The functional activity of
motor, cardiovascular, nervous, endocrine, immune, haematopoietic
and other systems and subsystems may be considered at the systemic
level as potential biomarkers of aging. In addition, at the level of
the whole organism, the functions of the main integrative systems
should be addressed.
Test batteries that attempt to measure functional age (Webster
& Logie, 1976), physiological age (Hollingsworth et al., 1965) and
biological age (Furukawa et al., 1975; Nakamura et al., 1982;
Voitenko & Tokar, 1983; Reis & Poethig, 1984; Dubina et al., 1984;
Hochschild, 1989) have similar conceptual underpinnings in that they
utilize the great variability in performance that emerges among
adults of the same chronological age in standardized, age-sensitive
tests. Studies using this approach have been conducted on human
populations, and similar measures have been employed in a few rodent
studies (Hofecker et al., 1980; Dubina et al., 1983). This approach
can be viewed as the performance variability model of biological
age. It has considerable intuitive appeal and, in many cases,
statistical elegance. An individual may be judged biologically
younger if he is performing better than expected for his
chronological age. However, in their extensive and critical reviews,
Costa & McCrae (1980, 1985) revealed many conceptual and
methodological flaws in this approach.
Biomarkers of aging, which are markers of susceptibility, may
have their greatest utility in the study of the effects of
environmental chemicals on the processes of aging. The effects of
environmental chemicals on the elderly would be assessed most
efficiently by using biomarkers of effect (NAS, 1989).
5. CONCLUSIONS
a) With increasing age, humans become more vulnerable to
environmental challenges due to the deterioration of
physiological and psychological processes. Therefore, it is
likely that the elderly will be more susceptible to the harmful
effects of environmental chemicals.
b) The elderly are heterogeneous with respect to the extent of
deterioration of physiological and psychological processes.
c) Few, if any, of the hundreds of thousands of environmental
chemicals have been tested for increased toxicity in the
elderly.
d) Some of the many age-associated diseases may cause an increased
susceptibility to the harmful action of specific environmental
chemicals.
e) The use of animal models for aging research requires special
considerations, such as housing conditions, diets, monitoring
for infectious agents, and specifically defined pathologies. It
is also necessary to assess several age ranges based on
life-table information in order to distinguish the senescent
from the mature and developing animal.
f) The selection of the animal model should be based on the
likelihood of providing information of relevance to specific
problems in humans.
g) The characteristics of the elderly result from intrinsic aging
processes, environmental factors, and other life-span events.
Therefore, the study of the elderly may not provide
information on basic aging processes.
h) Both the size of the aged population and the number of
chemicals in the environment will undoubtedly increase over
the next few decades. It is therefore expected that the adverse
effects of chemical exposure on the elderly will increase in
importance as a health care issue.
6. FURTHER RESEARCH
a) Experimental, epidemiological and clinical data should be
obtained on:
* the toxicity, mutagenicity and carcinogenicity of
environmental chemicals in old individuals as compared to
young adults;
* the effect of age on the pharmacokinetics and
pharmaco-dynamics of environmental chemicals;
* the long-term effect of environmental chemicals on
molecular, cellular and physiological parameters as
potential biomarkers in the elderly.
b) New models should be developed for assessing the susceptibility
of the elderly to environmental chemicals as compared to young
adults. Such models should be suitable for assessment of
particular consequences and relevant to humans, and could
include:
* non-mammalian and mammalian models (e.g., Drosophila,
fish, rabbits, mini-pigs);
* transgenic animal models;
* cell lines with specific genetic characteristics.
c) For each study the appropriate applicability and use of
experimental models and techniques must be validated:
* animal models should be well-defined in terms of survival,
pathology and husbandry;
* human subjects should be examined carefully for subtle
disease status, medical history, life-style and
socio-economic status.
* standard procedures for measuring molecular, cellular and
physiological parameters should be defined (and developed
if necessary) in order to prevent misinterpretation.
d) The effects of environmental chemicals on the processes of
aging remain to be evaluated. A special scientific workshop
should be devoted to this topic.
REFERENCES
American Dietetic Association (ADA) (1984) ADA takes proactive
stance, testifies on older Americans act reauthorization. J Am Diet
Assoc, 84(7): 822-835.
Ali B, Walford RL, & Imamura T (1985) Influence of aging and poly IC
treatment on xenobiotic metabolism in mice. Life Sci, 37: 1387-1393.
Allaben WT, Chou MW, Pegram RA, Leakey J, Feues RJ, Duffy PH,
Turturro A, & Hart RW (1990) Modulation of toxicity and
carcinogenesis by caloric restriction. Korean J Toxicol, 6: 167-182.
Allison T, Hume AL, Wood CC, & Goff WR (1984) Developmental and
aging changes in somatosensory, auditory and visual evoked
potentials. Electroencephalogr Clin Neurophysiol, 58: 14-24.
Amador A, Steger RW, Bartke A, Johns A, Siler-Khodr TM, Parker R Jr,
& Shepherd AM (1985) Testicular LH receptors during aging in Fischer
344 rats. J Androl, 6: 61-64.
Amdur MO (1986) Air pollutants. In: Klassen CD, Amdur UO, & Doull J
ed. Casarett and Doull's toxicology: The basic science of poisons,
3rd ed. New York, Macmillan Publishing Co., pp 801-824.
Ammon HPT, Fahmy A, Mark M, Wahl MA, & Youssif N (1987) The effect
of glucose on insulin-release and ion movement in isolated
pancreatic islets of rats in old age. J Physiol, 384: 347-354.
Anderson S & Brenner BM (1986) The aging kidney : Structure,
function, mechanisms and therapeutic implications. J Am Geriatr Soc,
35(6): 590-593.
Anderson S & Brenner BM (1987) Effects of aging on the renal
glomerulus. Am J Med, 80(3): 435-442.
Anisimov VN (1971) [Blastomogenesis in persistent estrous rats.]
Vopr Onkol, 17(8): 67-75 (in Russian).
Anisimov VN (1987) Carcinogenesis and aging. Boca Raton, Florida,
CRC Press, vol 1 & 2.
Anisimov VN (1988) Effect of age on dose-response relationship in
carcinogenesis induced by single administration of
N-nitroso-methylurea in female rats. J Cancer Res Clin Oncol, 114:
628-635.
Anisimov VN (1989) Age-related mechanisms of susceptibility to
carcinogenesis. Semin Oncol, 16: 10-19.
Anisimov VN & Reiter RJ (1990) [Pineal function in cancer and
aging.] Vopr Onkol, 37: 259-268 (in Russian).
Arfini G, Mutti A, Vescovi P, Ferroni C, Ferrari M, Giaroli C,
Passeri M, & Franchini I (1987) Impaired dopaminergic modulation of
pituitary secretion in workers occupationally exposed to styrene:
Further evidence from PRL response to TRH stimulation. J Occup Med,
29: 826-830.
Armbrecht HJ (1986) Age-related changes in calcium and phosphorus
uptake by rat small intestines. Biochim Biophys Acta, 882: 281-286.
Armbrecht HJ, Doubek WG, & Porter SB (1988) Calcium transport by
basal lateral membrane vesicles from rat small intestine decreases
with age. Biochim Biophys Acta, 944: 367-373.
Armbrecht HJ, Boltz M, Strong R, Richardson A, Bruns MEH, &
Christakos S (1989) Expression of calbindin-D decreases with age in
intestine and kidney. Endocrinology, 125: 2950-2956.
Aschheim P (1976) Aging in the hypothalamic-hypophyseal-ovarian axis
in the rat. In: Everitt AV & Burgess JA ed. Hypothalamus, pituitary
and aging. Springfield, Illinois, Charles C. Thomas, pp 376-418.
Aschoff J (1979) Circadian rhythms: general features and
endocrinological aspects. In: Krieger DT ed. Comprehensive
endocrinology. New York, Raven Press, pp 1-61.
Avogaro P, Bittolo-Bon G, Cazzolato G, Belussi F, & Pontoglio E
(1983) Lipids and proteins of lipoproteins in human atherosclerosis.
Przegl Lek, 40: 713-718.
Bach JF, Darcenne M, Papiernik M, Barvis A, Lavasseur P, & Lebrand H
(1972) Evidence for a serum-factor secreted by the human thymus.
Lancet, ii: 1056-1058.
Baker SR & Rogul M ed. (1987) Environmental toxicity and the aging
processes: Proceedings of a workshop held in Columbia, Maryland, 1-2
October 1985. New York, Alan R. Liss, Inc., 139 pp (Progress in
Clinical and Biological Research, Volume 228).
Baker GT & Sprott RL (1988) Biomarkers of aging. Exp Gerontol, 23:
223-239.
Bang NU, Tessler SU, Heidenreich RO, Marks CA, & Mattler LE (1982)
Thrombosis and atherosclerosis. Chicago, Year Book Medical
Publishers.
Banks YB, Brewster DW, & Birnbaum LS (1990) Age-related changes in
dermal absorption of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and
2,3,4,7,8-pentachlorodibenzofuran (4-PeCDF). Fundam Appl Toxicol,
15: 163-173.
Baron JA (1984) Smoking and estrogen-related disease: A review. Am J
Epidemiol, 119: 9-22.
Barrows CH & Kokkonen GC (1978) Diet and life extension in animal
model systems. Age, 1: 131-143.
Bartsch H (1991) N-Nitrosocompounds and human cancer: where do we
stand? In: Relevance to human cancer of N-nitroso compounds, tobacco
smoke and mycotoxins. Lyon, International Agency for Research on
Cancer, pp 1-110 (IARC Scientific Publications No. 105).
Bauer LA, Black D, Gensler A, & Sprinkle J (1989) Influence of age,
renal function, and heart failure on procainamide clearance and
n-acetylprocainamide serum concentrations. Int J Clin Pharm Ther
Toxicol, 27: 213-216.
Baylis C, Fredericks M, Leypolat J, Frigon R, Wilson C, & Henderson
L (1988) The mechanisms of proteinuria in aging rats. Mech Ageing
Dev, 45: 111-126.
Bayreuther K, Rodemann KP, Francy PI, & Maier K (1988)
Differentiation of fibroblast stem cells. J Cell Sci, 10(suppl):
115-130.
Beebe GW (1979) Reflections on the work of the Atomic Bomb Casuality
Commission in Japan. Epidemiol Rev, 1: 184-210.
Behl CR, Bellantone NH, & Flynn GL (1987) Influences of age on
percutaneous absorption of drug substances. In: Kydonieuks AF &
Berrer B ed. Transdermal delivery of drugs. Boca Raton, Florida, CRC
Press, vol 2, pp 109-132.
Beierschmitt WP & Weiner M (1986) Age-related changes in renal
metabolism of acetaminophen in male Fischer 344 rats. Age, 9: 7-13.
Beierschmitt WP, Keenan KP, & Weiner M (1986a) Age-related increased
susceptibility of male Fischer 344 rats to acetaminophen
nephrotoxicity. Life Sci, 39: 2335-2342.
Beierschmitt WP, Keenan KP, & Weiner M (1986b) The development of
acetaminophen-induced nephrotoxicity in male Fischer 344 rats of
different ages. Arch Toxicol, 59: 206-210.
Belisle S & Lehoux J-G (1983) Endocrine aging in C57BL Mice. II.
Dynamics of estrogen receptors in the hypothalamic pituitary axis. J
Steroid Biochem, 18: 737-743.
Bender AD (1965) The effect of increasing age on the distribution of
peripheral blood flow in man. J Am Geriatr Soc, 13: 192-198.
Bender AD (1968) Effect of age on intestinal absorption:
Implications for drug absorption in the elderly. J Am Geriatr Soc,
16: 1331-1339.
Bender AD (1979) Drug sensitivity in the elderly. In: Crooks J &
Stevenson IH ed. Drugs and the elderly. Baltimore, Maryland,
University Park Press, pp 147-153.
Bender A, Post A, Meier J, Higson J, & Reichard G (1975) Plasma
protein binding of drugs as a function of age in adult human
subjects. J Pharm Sci, 64: 1711-1713.
Benditt EP (1977) The origin of atherosclerosis. Sci Am, 236: 74-85.
Berg BN (1976) Pathology and aging. In: Everitt AV & Burgess JA ed.
Hypothalamus, pituitary and aging. Springfield, Illinois, Charles C.
Thomas, pp 43-67.
Bertoni-Freddari C, Fattoretti P, Casoli T, Meier-Rouge W, & Ulrich
J (1990) The effect of ethanol on neuronal membrane permeability and
synaptic ultrastructure in adult and old rats. In: Bergamini E ed.
Protein metabolism and aging. New York, Wiley-Liss, Inc., p.
331-334.
Bertrand HA, Lynd FT, Masoro EJ, & Yu BP (1980) Changes in adipose
mass and cellularity through the adult life of rats fed ad libitum
or a life-prolonging restricted diet. J Gerontol, 35(6): 827-835.
Besedovsky H, Sorkin E, Keller M, & Müller J (1975) Changes in blood
hormone levels during the immune response (39057). Proc Soc Exp Biol
Med, 150: 466-470.
Besedovsky HO, Del Rey AE, & Sorkin E (1985) Immune-neuroendocrine
interactions. J Immunol, 135: 750-754.
Bhatnagar KP, Kennedy RC, Baron G, & Greenberg RA (1987) Number of
mitral cells and the bulb volume in the aging human olfactory bulb:
a quantitative morphological study. Anat Rec, 218: 73-87.
Bilchert-Toft M (1978) The adrenal glands in old age. In: Greenblatt
RB ed. Geriatric endocrinology. Vol 5: Aging. New York, Raven Press,
pp 81-102.
Birchenall-Sparks M, Richardson A, Roberts M, & Utherford M (1985)
The effect of aging on the structure and function of liver messenger
RNA. Mech Ageing Dev, 32: 99-111.
Birnbaum LS (1983) Distribution and excretion of 2,3,6,2',3',6'-and
2,4,5,2',4',5'-hexachlorobiphenyl in senescent rats. Toxicol Appl
Pharmacol, 70: 262-272.
Birnbaum LS (1987) Age-related changes in carcinogen metabolism. J
Am Geriatr Soc, 35: 51-60.
Birnbaum LS (1989) Age-related changes in drug disposition. In:
Zenser TV & Coe RM ed. Cancer and aging. Berlin, Heidelberg, New
York, Springer-Verlag, pp 25-138.
Birnbaum LS (1991) Pharmacokinetic basis of age-related changes in
sensitivity to toxicants. Annu Rev Pharmacol Toxicol, 31: 101-128.
Birnbaum LS & Baird MB (1979) Senescent changes in rodent hepatic
epoxide metabolism. Chem-Biol Interact, 26: 245-256.
Birren JE, Woods AM, & Williams MV (1979) Speed of behavior as an
indicator of age changes and the integrity of the nervous system.
In: Hoffmeister F & Muller C ed. Brain function in old age. Berlin,
Heidelberg, New York, Springer-Verlag, p 10.
Bitar MS & Shapiro BH (1987) Aberration of heme and hemoprotein in
aged female rats. Mech Ageing Dev, 38: 189-197.
Bjorksten J, Yaeger LL, & Wallace T (1989) Control of aluminium
ingestion and its relation to longevity. Int J Vitam Nutr Res, 58:
462-465.
Blackman MR (1987) Pituitary hormones and aging. Endocrinol Aging,
16(4): 981-994.
Blackwelder WC, Yano K, Rhoads GG, Kagan A, Gordon T, & Palesch Y
(1980) Alcohol and mortality: The Honolulu heart study. Am J Med,
68: 164-169.
Blalock JE, Harbour-McMenamin D, & Smith EM (1985) Peptide hormones
shared by the neuroendocrine and immunologic systems. J Immunol,
135(2): 858/5-861/5.
Blanco P, Machado A, & Satrustegui J (1987) Variations due to
hypoxia and ageing in the activities of glutathione-S-transferase
and NADPH-Cytochrome C reductase. Mech Ageing Dev, 39: 11-19.
Blum M, Averbuch M, Wolman Y, & Aviram A (1989) Protein intake and
kidney function in humans: its effect on 'normal aging'. Arch Inter
Med, 149(1): 211-212.
Blumberg JB (1985) A discussion of drug metabolism and actions in
the aged. Drug-Nutrient Interact, 4: 99-106.
Bolinder J, Kager L, Ostamn J, & Arner P (1983) Differences at the
receptor and postreceptor levels between human omental and
subcutaneous adipose tissue in the action of insulin on lipolysis.
Diabetes, 32: 117-123.
Bondareff W (1986) Neuropathology of nucleus basalis and locus
ceruleus in Alzheimer's disease. In: Scheibel AB, Wechsler AF, &
Brazier MAB ed. The biological substrates of Alzheimer's disease.
New York, London, San Francisco, Academic Press, pp 103-113.
Boobis AR & Davies DS (1984) Human cytochromes P-450. Xenobiotica,
14: 151-185.
Borghoff SJ & Birnbaum LS (1985) Age-related changes in
glucuronidation and deglucuronidation in liver, small intestine,
lung and kidney of male Fischer rats. Drug Metab Dispos, 13: 62-67.
Borghoff SJ & Birnbaum LS (1986) Age-related changes in the
metabolism and excretion of allyl isothiocyanate: A model compound
for glutathione conjugation. Drug Metab Dispos, 14: 417-422.
Borghoff SJ, Stefanski SA, & Birnbaum LS (1988) The effect of age on
the glucuronidation and toxicity of
4,4'-thiobis(6-t-butyl-m-cresol). Toxicol Appl Pharmacol, 92:
453-466.
Bornor JA, Dilworth BB, & Sullivan KM (1988) Exercise and
osteroporosis: A critique of the literature. Physiother Can, 40(3):
146-155.
Boyes WK & Dyer RS (1984) Chlordimeform produces profound,
selective, and transient changes in visual evoked potentials of
hooded rats. Exp Neurol, 86: 434-447.
Bradley RM (1979) The effect of aging on the olfactory sense. In:
Special senses in aging. Ann Arbor, Michigan, University of
Michigan, Institute of Gerontology, pp 3-8.
Bremner WJ, Vitiello MV, & Prinz PN (1983) Loss of circadian
rhythmicity in blood testosterone with aging in normal men. J Clin
Endocrinol Metab, 56: 1278-1281.
Brenner BM, Meyer TW, & Hostetter TH (1982) Dietary protein intake
and the progressive nature of kidney diseases. New Engl J Med, 307:
652-659.
Brizzee KR (1985) Neuron aging and neuron pathology. In: Johnson AH
ed. Relations between normal aging and disease. New York, Raven
Press, pp 191-224.
Brown WW, Davis BB, & Spry LA (1986) Aging and the kidney. Arch
Intern Med, 146: 1790-1796.
Bruce MF (1980) The relation of tactile thresholds to histology in
the fingers of the elderly. J Neurol Neurosurg Psychiatry, 43:
730-734.
Buckler AJ, Vie H, Sonenshein GE, & Miller RA (1988) Defective T
lymphocytes in old mice: Diminished production of mature c-myc RNA
after mitogen exposure not attributable to alterations in
transcription or RNA stability. J Immunol, 140: 2442-2446.
Bullock K (1985) Neuroanatomy of lymphoid tissue. In: Guillelmin R
ed. Neural modulation of immunity. New York, Raven Press, pp
111-141.
Burek JD (1978) Pathology of aging rats. Boca Raton, Florida, CRC
Press.
Burnet FM (1970) The concept of immunological surveillance. Prog Exp
Tumor Res, 13: 1-27.
Butenko GM (1985) Ageing of the immune system and diseases. In:
Likhachev A, Anisimov V, & Montesano R ed. Age-related factors in
carcinogenesis. Lyon, International Agency for Research on Cancer,
pp 71-83 (IARC Scientific Publications No. 58).
Campbell GR & Chamley-Campbell JH (1981) Invited review: The
cellular pathobiology of atherosclerosis. Pathology, 13: 423-440.
Capecchi MR (1989) Altering the genome by homologous recombination.
Science, 244: 1288-1292.
Caraceni MP, Corghi E, Ortolani S, Dindell M, Candotti G, & Ferrari
A (1988) Prevention of early postmenopausal bone loss. The effect of
calcitonin (Staporos). Clin Trials J, 25(6): 401-405.
Carrillo MC, Kitani K, Kanai S, Sato Y, Nokubo M, Ohta M, & Otsubo K
(1989) Differences in the influence of diet on hepatic glutathione
S-transferase activity and glutathione content between young and old
C56 black female mice. Mech Ageing Dev, 40: 1-15.
Carrillo MC, Nokubo M, Sato Y, Kanai S, Ohta M, & Kitani K (1990)
Effect of protein-free diet on activities and subunits of
glutathione S-transferase in livers of young and aged female rats.
Mech Ageing Dev, 56: 230-251.
Carrillo MC, Nokubo M, Kitani K, Kimihiko S, & Sato K (1991)
Age-related alterations of enzyme activities and subunits of hepatic
glutathione S-transferases in male and female Fischer-344 rats.
Biochim Biophys Acta, 1077: 325-331.
Castleden CM, George CF, Marcer D, & Hallet C (1977a) Increased
sensitivity to nitrazepam in old age. Br Med J, 1: 10-12.
Castleden CM, Volans CN, & Raymond K (1977b) The effect of aging on
drug absorption from the gut. Age Ageing, 6: 138-143.
Cathcart R, Schwiers E, Saul RL, & Ames BN (1984) Thymine glycol and
thymidine glycol in human and rat urine: a possible assay of
oxidative DNA damage. Proc Natl Acad Sci (USA), 81: 5633-5637.
Cauley JA, Gutai JP, Kuller LH, Ledonne D, Sandler RB, Sashin D, &
Powell JG (1988) Endogenous estrogen levels and calcium intakes in
postmenopausal women. Relationships with cortical bone measures. J
Am Med Assoc, 260(21): 3150-3155.
Ceda GP, Valenti G, Butturini U, & Hoffman AR (1986) Diminished
pituitary responsiveness to growth hormone-releasing factor in aging
male rats. Endocrinology, 118(5): 2109-2114.
Chakravarty S, Collins WP, Forecast JS, Newton JR, Oram DH, & Strudd
JWW (1976) Hormonal profiles after the menopause. Br Med J, 2:
784-787.
Chan K, Kerdall MJ, Mitchard M, Wells WOE, & Vickers MD (1975) The
effect of aging on plasma pethidine concentration. Br J Clin
Pharmacol, 2: 297-302.
Chandra RK (1985) Trace element regulation of immunity and
infection. J Am Coll Nutr, 4: 5-16.
Chavaz A, Baleurt L, Simonin P, & Fabre J (1974) Influence de l'âge
sur la digoxinémie et la digitalisation. Schweiz Med Wochenschr,
104: 1823-1825.
Chen LH, Liu S, Cook Newell ME, & Barnes K (1985) Survey of drug use
by the elderly and possible impact of drugs on nutritional status.
Drug-Nutrient Interact, 3: 73-86.
Cheney KE, Liu RK, Smith GS, Merdith PJ, Mickey MR, & Walford RL
(1983) The effect of dietary restriction of varying duration on
survival, tumour patterns, immune fraction and body temperture in
BIOC3F1 female mice. J Gerontol, 38: 420-430.
Chengelis CP (1988a) Age- and sex-related changes in the components
of the hepatic microsomal mixed function oxidase system in
Sprague-Dawley rats. Xenobiotica, 18: 1211-1224.
Chengelis CP (1988b) Age- and sex-related changes in epoxide
hydrolase, UDP-glucuronyl transferase, and PAPS-sulfotransferase in
Sprague-Dawley rats. Xenobiotica, 18: 1225-1237.
Chopra IJ, Solomon DH, Chopra U, Wu SY, Fisher DA, & Nakamura Y
(1978) Pathways or metabolism of thyroid hormones. Recent Prog Horm
Res, 34: 521-567.
Christophers E & Kligman AM (1965) Percutaneous absorption in aged
skin. In: Montagna W ed. Advances in the biology of the skin. Vol 6:
Ageing. Oxford, New York, Pergamon Press, pp 163-175.
Clayson DB (1988) Needs for biological risk assessment in
interspecies extrapolation. Environ Health Perspect, 77: 93-97.
Cleroux J, Giannattasio C, Grassi G, Seravalle G, Sampieri L,
Cuspidi C, Bolla G, Valsecchi M, Mazzola C, & Mancia G (1988)
Effects of aging on the cardiopulmonary receptor reflex in
normotensive humans. J Hypertension, 6(suppl 4): 141-144.
Coiro V, Volpi R, Cavazzini V, Bertoni P, Corradi A, Bianconi L,
Davoli C, Rossi G, & Chiodera P (1991) Restoration of normal growth
hormone responsiveness to GHRH in normal aged men by infusion of low
amounts of theophylline. J Gerontol Med Sci, 46(5): M155-M158.
Colombo T, Donelli MG, Urso R, Dallara S, Bartosek I, & Guaitani A
(1989) Doxorubicin toxicity and pharmacokinetics in old and young
rats. Exp Gerontol, 24: 159-171.
Committee on Chemical Toxicity and Aging (1987) Aging in today's
environment. Washington, DC, National Academy Press.
Conn PM, Cooper RL, McNamara MC, Rogers DC, & Shoenhardt L (1980)
Qualitative change in gonadotropin during normal aging in the male
rat. Endocrinology, 106: 1549-1553.
Conrad K & Bressler R (1982) Drug therapy for the elderly. St Louis,
Missouri, C.V. Mosby & Co.
Cooper B, Weinblatt F, & Gregerman R (1977) Enhanced activity of
hormone sensitive adenylated cyclase during dietary restriction in
the rat. J Clin Invest, 39: 467-474.
Cooper RL, Goldman JM, & Rehnberg GL (1986) Pituitary function
following treatment with reproductive toxins. Environ Health
Perspect, 70: 177-184.
Corman B & Michel JB (1986) Renin-angiotensin system,
converting-enzyme inhibition, and kidney function in aging female
rats. Am J Physiol, 251: 450-455.
Costa PT Jr & McCrae RR (1980) Functional age: A conceptual and
empirical critique. In: Haynes SG & Feinleib M ed. Epidemiology of
aging. Washington, DC, National Institutes of Health, National
Institute of Aging, National Heart, Lung, and Blood Institute, pp
23-50 (NIH Publication No. 80-969).
Costa PT & McCrae RR (1985) Concepts of functional or biological
age: A critical view. In: Andres R, Bierman EL, & Hazzard WR ed.
Principles of geriatric medicine. New York, McGraw-Hill, pp 30-37.
Costa LG & Murphy SD (1987) Interaction of the pesticide
chlordimeform with adrenergic receptors in mouse brain: An
in vitro study. Arch Toxicol, 59: 323-327.
Costa LG, Olibet G, & Murphy SD (1988) Alpha2-adrenoreceptors as a
target for formamidine pesticides: in vitro and in vivo studies
in mice. Toxicol Appl Pharmacol, 93: 319-328.
Creasey H & Rapoport SI (1985) The aging of human brain. Ann Neurol,
17(1): 2-10. Crooks J, O'Malley K, & Stevenson IH (1976)
Pharmacokinetics in the elderly. Clin Pharmacokinet, 1: 280-296.
Croucher PI, Mellish RWE, Vedi S, Garrahan NJ, & Compston JE (1989)
The relationship between resorption depth and mean interstitial bone
thickness: Age-related changes in man. Calcif Tissue Int, 45(1):
15-19.
Crowley JJ, Cusack BJ, Jue SG, Koup JR, Park BK, & Vestal RE (1988)
Aging and drug interactions. II. Effects of phenytoin and smoking on
the oxidation of theophylline and cortisol in healthy men. J
Pharmacol Exp Ther, 245: 513-523.
Cuny G, Royer RJ, Mur JM, Serot JM, Faure G, Netter P, Maillard A, &
Penin F. (1979) Pharmacokinetics of salicylates in elderly.
Gerontology, 25: 49-55.
Cusack B & Denham MJ (1984) Nutritional status and drug disposition
in the elderly. In: Roe DA ed. Drugs and nutrition in the geriatric
patients. New York, Churchill Livingstone, pp 47-70.
Cutler RG (1991) Human longevity and aging: possible role of
reactive oxygen species. Ann NY Acad Sci, 621: 1-28.
Cutler RG & Semsei I (1989) Development, cancer and aging: possible
common mechanisms of action and regulation. J Gerontol, 44(6):
25-34.
Daniel FB, Schut HA, Sandwiesh DW, Schenck KM, Hoffmann CO, Patrick
JR, & Stoner GD (1983) Interspecies comparison of benzo(a)pyrene
metabolism and DNA-adduct formation in cultured human and animal
bladder and tracheobronchial tissues. Cancer Res, 43: 4723-4729.
Danon D (1969) Biophysical aspects of red cell ageing. Bibl
Haematol, 15: 275-287.
Davies BH (1985) The respiratory system. In: Pathy MSJ ed.
Principles and practice of geriatric medicine. New York, Chichester,
Brisbane, Toronto, John Wiley and Sons.
Davila DR, Brief S, Simon J, Hammer RE, Brinster RL, & Kelley KW
(1987) Role of growth hormone in regulating T-dependent immune
events in aged, nude and transgenic rodents. J Neurosci Res, 18:
108-116.
Davis PJ & Davis FB (1987) Water excretion in the elderly.
Endocrinol Aging, 16(4): 867-875.
Davis TA, Bales CW, & Beauchene RE (1983) Differential effects of
dietary, caloric, and protein restriction in the aging rat. Exp
Gerontol, 18: 427-435.
Dawson-Hughes B, Shipp C, Sadowski L, & Dallal G (1987) Bone density
of the redius, spine, and hip in relation to percent of ideal body
weight in postmenopausal women. Calcif Tissue Int, 40(6): 310-314.
Dement W, Richardson G, Prinz P, Carskadon M, Kripke D, & Czeisler C
(1985) Changes of sleep and wakefulness with age. In: Finch CE &
Schneider EL ed. Handbook of the biology of aging, 2nd ed. New York,
Van Nostrand Reinhold Company, pp 692-717. Department of
International Economic and Social Affairs (1991) World population
prospects 1990, New York, United Nations, pp 192-215 (Population
Studies No. 120).
Dilman VM (1971) Age associated elevation of hypothalamic threshold
to feedback control and its role in development, aging and disease.
Lancet, i: 1211-1219.
Dilman VM (1987) [Four models of medicine.] Leningrad, Meditsina (in
Russian).
Dilman VM & Anisimov VN (1979) Hypothalmic mechanism of ageing and
of specific age pathology. I. Sensitivity threshold of
hypothalamo-pituitary complex to homeostatic stimuli in the
reproductive system. Exp Gerontol, 14: 161-174.
Dix D (1989) The role of aging in cancer incidence: an
epidemiological study. J Gerontol, 44(6): 10-18.
Doll R (1973) Age. In: Doll R & Vodopija I ed. Host environment
interactions in the etiology of cancer in man. Lyon, International
Agency for Research on Cancer, pp 39-48 (IARC Scientific
Publications No. 7).
Doll R (1978) An epidemiological perspective of the biology of
cancer. Cancer Res, 38: 3573-3583.
Doll R & Peto R (1981) The causes of cancer. J Natl Cancer Inst, 66:
1193-1312.
Donaldson J (1987) The physiopathologic significance of manganese in
brain: Its relation to schizophrenia and neurodegenerative
disorders. Neurotoxicology, 8: 451-462.
Doubek WG & Armbrecht HJ (1987) Changes in intestinal glucose
transport over the lifespan of the rat. Mech Ageing Dev, 39: 91-102.
Dubina TL, Dyundikova VA, & Zhuk EV (1983) Biological age and its
estimation. II. Assessment of biological age of albino rats by
multiple regression analysis. Exp Gerontol, 18: 5-18.
Dubina TL, Mints A, & Zhuk EV (1984) Biological age and its
estimation. III. Introduction of a correction to the multiple
regression model of biological age and assessment of biological age
in cross-sectional and longitudinal studies. Exp Gerontol, 19:
133-143.
Duckles SP (1987) Influence of age on vascular adrenergic
responsiveness. Blood Vessels, 24(3): 113-116.
Dundee JW (1979) Response to anaesthetic drugs in the elderly. In:
Crooks J & Stevenson IH ed. Drugs and the elderly. Baltimore,
Maryland, University Park Press, pp 179-187.
Dunn MF, Pattison SE, Storm MC, & Quiel E (1980) Comparison of the
zinc binding domains in the 7S nerve growth factor and the
zinc-insulin Hexamer. Biochemistry, 19: 718-725.
Dybkaer R, Lauritzen DM, & Krakauer R (1981) Relative reference
values for clinical chemical and haematological quantities in
"healthy" elderly people. Acta Med Scand, 209: 1-9.
Eastell R & Riggs BL (1987) Calcium homeostasis and osteoporosis.
Endocrinol Metab Clin, 16(4): 829-843.
Eastin WC Jr & Birnbaum LS (1987) Intestinal absorption of two
glucose analogues in rats of different ages. Exp Gerontol, 22:
351-357.
Ebbesen P (1985) Papilloma development on young and senescent mouse
skin treated with 12-0-tetradecanoylphorbol-13-acetate. In:
Likhachev A, Anisimov V, & Montesano R ed. Age-related factors in
carcinogenesis. Lyon, International Agency for Research on Cancer,
pp 167-170 (IARC Scientific Publications No. 58).
Edelman IS & Leibman J (1959) Anatomy of body water and
electrolytes. Am J Med, 27: 256-277.
Eden S (1987) Endocrinology in older people. Acta Obstet Gynaecol
Scand, 140(suppl): 9-22.
Edman CD (1983) Estrogen replacement therapy. In: Buchsbaum HJ ed.
The menopause. Berlin, Heidelberg, New York, Springer-Verlag.
Ellis GB & Desjardins C (1982) Male rats secrete luteinizing hormone
and testosterone episodically. Endocrinology, 110: 1618-1627.
Euans DW (1988) Renal function in the elderly. Am Fam Physician,
38(3): 147-150.
Evens RP & Hawkins DW (1984) Bone and joint disorders. In: Covington
TR & Ingram Walker J ed. Current geriatric therapy. Philadelphia,
Pennsylvania, W.B. Saunders Co., pp 277-306.
Exton-Smith AN (1985) Mineral metabolism. In: Finch CE & Schneider
EL ed. Handbook of the biology of aging. New York, Van Nostrand
Reinhold Company, pp 511-539.
Fabris N (1981) Ontogenetic and phylogenetic aspects of
neuroendocrine-immune network. Dev Comp Immunol, 5(suppl 1): 49-60.
Fabris N (1991) Neuroendocrine-immune interactions: a theoretical
approach of aging. Arch Gerontol Geriatry, 12: 219-230.
Fabris N & Piantanelli L (1982) Thymus-neuroendocrine interactions
during development and aging. In: Adelman RC & Roth GS ed. Hormones
and aging. Boca Raton, Florida, CRC Press, pp 167-181.
Fabris N & Provinciali M (1989) Hormones. In: Nelson D ed. Natural
immunity. New York, London, San Francisco, Academic Press, pp
306-347.
Fabris N, Giunta S, & Muzzioli M (1983) Decline of T-cell potential
in diabetic and aged men. J Gerontol, 38(5): 548-554.
Fabris N, Mocchegiani E, Muzzioli M, & Provinciali M (1988)
Neuroendocrine-thymus interactions: perspectives for intervention in
aging. Ann NY Acad Sci, 521: 72-87.
Fabris N, Mocchegiani E, Marriotti S, Pacini F, & Pinchera A (1989)
Thyroid-thymus interactions during development and aging. Horm Res,
31: 85-98.
Fabris N, Mocchegiani E, Muzzioli M, & Provinciali M (1990) Zinc,
immunity and aging. In: Goldstein AL ed. Biomedical advances in
aging. New York, London, Plenum Press, pp 271-281.
Fando JL, Salinas M, & Wasterlain CG (1980) Age-dependent changes in
brain protein synthesis in the rat. Neurochem Res, 5: 373-378.
Feldman RD, Limbird LE, Nadeau J, Robertson D, & Wood AJ (1984)
Alterations in leukocyte ß-receptor affinity with aging. New Engl J
Med, 310: 815-819.
Felten DL, Felten SY, Carlsan SL, Olschowka JA, & Livnat S (1985)
Neuroadrenergic and peptidergic innervation of lymphoid tissue. J
Immunol, 135: 755.
Fernandes G (1984) Nutrional factors: Modulating effects on immune
function and aging. Pharmacol Rev, 36(2): 123-129.
Fernandes G, Friend P, & Yunis EJ (1977) Influence of calorie
restriction on autoimmune disease. Fed Proc, 6: 1313.
Ferro-Luzzi A, Maiani G, Scaccini C, D'Amicis A, Borgioni G, Polito
A, Ronaldi L, Sett S, Branca F, Azzini E, Arena A, Raguzzini A,
Castata G, & Dente MG (1988) In: Lintas C & Spadoni MA ed.
Nutritional vulnerability of the Italian elderly: Facts and figures.
Rome, National Council for Research, pp 275-294 (Monografia No. 28).
Finch CE (1991) Longevity, senescence, and the genome. Chicago,
University of Chicago Press.
Finch CE & Hayflick L ed. (1977) Handbook of the biology of aging.
New York, Van Nostrand Reinhold Company.
Finch CE & Landfield PW (1985) Neuroendocrine and autonomic
functions in aging mammals. In: Finch CE & Schneider EL ed. Handbook
of the biology of aging, 2nd ed. New York, Van Nostrand Reinhold
Company, pp 645-691.
Fishbein L (1991) Biological effects of dietary restriction. Berlin,
Heidelberg, New York, Springer-Verlag.
Fleming BB & Barrows CH Jr (1982a) The influence of aging on
intestinal absorption of vitamins A + D by the rat. Exp Gerontol,
17: 115-120.
Fleming BB & Barrows CH Jr (1982b) The influence of aging on
intestinal absorption of vitamin B12 and niacin in rats. Exp
Gerontol, 17: 121-126.
Fraga CG, Shigenaga MK, Park JW, Degan P, & Ames BN (1990) Oxidative
damage to DNA during aging: 8-hydroxy-2-deoxyguanosine in rat organ
DNA and urine. Proc Natl Acad Sci (USA), 87: 4533-4537.
Friedman SA, Raizner RE, Rosen H, Solomon NA, & Wilfredo SY (1972)
Functional defects in the aging kidney. Ann Intern Med, 76: 41-45.
Friedman FK, Robinson RC, & Rifkind J (1989) Age-related changes in
the iron spin state of testosterone-binding rat liver microsomal
cytochromes P450. Biochem Biophys Res Commun, 158: 480-484.
Friend PS, Fernandes G, Good RA, Michael AF, & Yunis EJ (1978)
Dietary restrictiions early and late. Effects on nephropathy of the
NZB and NZW mouse. Lab Invest, 38: 629-632.
Frith CH & Ward JM (1988) Colour atlas of neoplastic and
non-neoplastic lesions in aging mice. Amsterdam, Oxford, New York,
Elsevier Science Publishers.
Fujita S, Katagawa H, Ishizawa H, Suzuki T, & Kitani K (1985)
Age-associated alterations in hepatic glutathione-s-transferase
activities. Biochem Pharmacol, 34: 3891-3894.
Furukawa T, Inoue M, Kajiya F, Inada H, Takasugi S, Fukui S, Takeda
H, & Abe H (1975) Assessment of biological age by multiple
regression analysis. J Gerontol, 30: 422-434.
Gaitz CM & Baer PE (1971) Characteristics of elderly patients with
alcoholism. Arch Gen Psychiatry, 24: 372.
Galinsky RE, Kane RE, & Franklin MR (1986) Effect of aging on
drug-metabolizing enzymes important in acetaminophen elimination. J
Pharmacol Exp Ther, 237: 107-113.
Galinsky RE, Johnson DH, Kane RE, & Franklin MR (1990) Effect of
aging on hepatic biotransformation in female Fischer 344 rats:
changes in sulfotransferase activities are consistent with known
gender-related changes in pituitary growth hormones secretions in
aging animals. J Pharmacol Exp Ther, 255: 577-583.
Gallagher D, Thompson LW, & Levy SM (1980) Clinical psychological
assessment of older adults. In: Poon LW ed. Aging in the 1980s:
Psychological issues. Washington, DC, American Psychological Press,
pp 19-40.
Gambert SR & Tsitouras PD (1985) Effect of age on thyroid hormone
physiology and function. J Am Geriatr Soc, 33(5): 360-365.
Gart JJ, Krewski D, Lee PN, Tarone RE, & Wahrendorf J (1986)
Statistical methods in cancer research. Vol III: The design and
analysis of long-term animal experiments. Lyon, International Agency
for Research on Cancer, 219 pp (IARC Scientific Publications No.
79).
Gilchrest BA (1984) Skin and aging processes. Boca Raton, Florida,
CRC Press, pp 1-120.
Globerson AR, Abel EI, & Ren-Menahem D (1989) Developmental aspects
of T-lymphocytes in aging. New York, London, Plenum Press, pp
363-373.
Goldstein RS, Pasino DA, & Hook JB (1986) Cephaloridine
nephrotoxicity in aging male Fischer 344 rats. Toxicology, 38:
43-63.
Goldstein RS, Tarloff JB, & Hook JB (1988) Age-related nephropathy
in laboratory rats. FASEB J, 2: 2241-2251.
Gollamudi HR, Prasanna R, Rao RH, Lawrence WH, & Autian J (1983)
Impaired metabolism of di (2-ethylhexyl)phthalate (DEHP) in old rats
- an in vitro study. J Toxicol Environ Health, 12: 623-632.
Gompertz B (1825) On the nature of the function expressive of the
law of human mortality and on a new mode of determining life
contingencies. Phil Trans R Soc London, II: 513-585.
Gossen JA, De Leeuw WJF, Tan CHT, Zwarthoff EC, Berends F, Lohman
PHM, Knook DL, & Vijg J (1989) Efficient rescue of integrated shutle
vectors from transgenic mice: a model for studying mutations in
vivo. Proc Natl Acad Sci (USA), 86: 7971-7975.
Govoni S, Memo M, Spano PF, & Trabucchi M (1979) Chronic lead
treatment differentially affects dopamine synthesis in various rat
brain areas. Toxicology, 12: 343-349.
Govoni S, Rius RA, Battaini F, Magnoni MS, Lucchi L, & Trabucchi M
(1988) The central dopaminergic system: Susceptibility to risk
factors for accelerated aging. Gerontology, 34: 29-34.
Goyal KK (1982) Changes with age in the aorta of man and mouse. Exp
Gerontol, 17: 127-132.
Graham, P (1985) The eye. In: Pathy MSJ ed. Principles and practice
of geriatric medicine. New York, Chichester, Brisbane, Toronto, John
Wiley and Sons, pp 833-844.
Grandjean P (1983) Behavioral toxicology of heavy metals. In:
Zbinden G, Cuomo V, Recagni G, & Weiss B ed. Application of
behavioral pharmacology in toxicology. New York, Raven Press, vol 2,
pp 331-339.
Greden JF, Flegel P, Haskett R, Dilsaver S, Carrol BJ, Grunhaus L, &
Genero N (1986) Age effects in serial hypothalamic-pituitary-adrenal
monitoring. Psychoneuroendocrinology, 11(2): 195-204.
Greenberg LH & Weiss B (1978) beta-Adrenergic receptors in aged rat
brain: reduced number and capacity of pineal gland to develop
supersensitivity. Science, 201: 61-63.
Greenberg LH & Weiss B (1979) Ability of aged rats to alter
beta-adrenergic receptors of brain in response to repeated
administration of reserpine and desmethylimipramine. J Pharmacol Exp
Ther, 211: 309-315.
Greenberg LH & Weiss B (1983) Neuroendocrine control of
catecholaminergic receptors in aging brain. In: Agnoli A, Crepaldi
G, Spano PF, & Trabucchi M ed. Aging brain and ergot alkaloids. New
York, Raven Press, pp 37-52.
Greenblatt DJ, Divoll MK, Harmatz JS, & Shader RI (1988) Antipyrine
absorption and disposition in the elderly. Pharmacology, 36:
125-133.
Greenstein BD, Fitzpatrick FT, Kendall MD, & Wheeler MJ (1987)
Regeneration of the thymus in old male rats treated with a stable
analogue of LHRH. J Endocrinol, 11: 345-350.
Gregerman RI & Solomon N (1967) Acceleration of thyroxine and
triiodothyronine turnover during bacterial pulmonary infections and
fever: implications for the functional state of the thyroid during
stress and in senescence. J Clin Endocrinol Metab, 27: 93-105.
Groop PH (1989) The influence of body weight, age and glucose
tolerance on the relationship between GIP secretion and beta-cell
function in man. Scand J Clin Lab Invest, 49(4): 367-379.
Gu X (1986) The life expectancy of the people of China. In: Cui Y
ed. Public health in the People's Republic of China. Beijing,
People's Medical Publishing House, pp 87-91.
Gupta SP, Mehta FS, & Irani RR (1980) Comparison of mortality rates
among bidi smokers and tobacco chewers. Ind J Cancer, 17: 149-152.
Guthrie S, Cooper RL, Thurman R, & Linnoila M (1987)
Pharmacodynamics and pharmacokinetics of ethanol, diazepam, and
pentobarbital in young and aged rats. Pharmacol Toxicol, 61:
308-312.
Hadden JW (1983) Cyclic nucleotides and related mechanisms in immune
regulation: A mini review. In: Fabris N, Garaci E, Hadden J, &
Mitchison NA ed. Immunoregulation. New York, London, Plenum Press,
pp 201-230.
Halberg F (1982) Biological rhythms, hormones and aging. In:
Vernandakis A ed. Hormones in development and aging. New York,
Spectrum Publications, pp 451-476.
Hall HRS, O'Grady MP, & Farrah JM Jr (1989) The
hypothalamic-pituitary-adrenal axis by thymic peptides. In: Hadden
JW, Massek K, & Nistico G ed. Interactions among central nervous
system, neuroendocrine and immune system. Rome, Pytagora Press, pp
112-125.
Hanawalt PC (1987) On the role of DNA damage and repair processes in
aging: evidence for and against. In: Warner HR, Butler RN, Sprott
RN, & Schneider EL ed. Modern biological theories of aging. New
York, Raven Press, pp 183-198.
Hanninen H (1982) Behavioral effects of occupational exposure to
mercury and lead. Acta Neurol Scand, 66(suppl 92): 167-175.
Hansen ON, Trillingsgaard A, Beese I, Lyngbye T, & Grandjean P
(1985) Neurobehavioral methods in assessment of children with
low-level lead exposure. In: Neurobehavioral methods in occupational
and environmental health (Document 3). Copenhagen, World Health
Organization, Regional Office for Europe, pp 183-187. Harman D
(1981) The aging process. Proc Natl Acad Sci (USA), 78: 7124-7128.
Harman D (1982) Nutritional implications of the radical theory of
aging. J Am Coll Nutr, 1: 27-34.
Harman SM, Tsitouras PD, Costa PT, & Blackman MR (1982) Reproductive
hormones in aging men. II. Basal pituitary gonadotropins and
gonadotropin responses to luteinizing hormone releasing hormone. J
Clin Endocrinol Metab, 54: 547-551.
Harrison DE, Astle CM, & Delaittre JA (1978) Loss of proliferative
capacity in immunohemopoietic stem cells caused by serial
transplantation rather than ageing. J Exp Med, 147(5): 1526-1531.
Hayflick L & Moorhead PS (1961) The serial cultivation of human
diploid cell strains. Exp Cell Res, 25: 585-621.
Hazzard WR (1985) Aging and atherosclerosis. Teasing out the
contributions of time, secondary aging, and primary aging. Clin
Geriatr Med, 1(1): 251-284.
Hebert CD & Birnbaum LS (1987) The influence of aging on intestinal
absorption of TCDD in rats. Toxicol Lett, 37: 47-55.
Hendley DD, Mildvan AS, Reporter MC, & Strehler BL (1963) The
properties of isolated human cardiac age pigment. II Chemical and
enzymatic properties. J Gerontol, 18: 250-259.
Hershkowitz M (1983) Mechanisms of brain aging - The role of
membrane fluidity. In: Gispen WH & Traber J ed. Aging of the brain.
Amsterdam, Oxford, New York, Elsevier Science Publishers, pp 85-98.
Hirsch HR (1982) Evolution of senescence: natural increase of
population dispaying Gompertz or power-law death rates and constant
of age-dependent maternity rates. J Theor Biol, 98: 321-346.
Hochschild R (1989) Improving the precision of biological age
determinations. Part I. A new approach to calculating biological
age. Exp Gerontol, 24: 289-300.
Hofecker G, Skalicky M, Kment A, & Niedermuller H (1980) Models of
the biological age of the rat. I. A factor model of age parameters.
Mech Ageing Dev, 14: 345-359.
Hogstedt C, Hane M, Agrell A, & Bodin L (1983) Neuropsychological
test results and symptoms among workers with well-defined long-term
exposure to lead. Br J Ind Med, 40: 99-105.
Hollander D, Dadufalz V, Weindruch R, & Walford RL (1986) Influence
of life-prolonging dietary restriction on intestinal vitamin A
absorption in mice. Age, 9: 57-60.
Hollingsworth JW, Hashizume A, & Jablon S (1965) Correlations
between tests of aging in Hiroshima subjects - an attempt to define
"physiological age." Yale J Biol Med, 38: 11-26.
Holt AR, Pascal RR, & Kotler DP (1984) Effect of aging upon small
intestinal structure in the Fischer rat. J Gerontol, 39: 642-647.
Holt PR, Yeh KY, & Kotler DP (1988) Altered controls of
proliferation in proxismal small intestine in the senescent rat.
Proc Natl Acad Sci (USA), 85: 2771-2775.
Honma T, Miyagawa M, & Sato M (1987) Methyl bromide alters
catecholamine and metabolite concentrations in rat brain.
Neurotoxicol Teratol, 9: 369-375.
Horbach GJM, Princen HMG, Van der Kroef M, Van Bezooijen CFA, & Yap
SH (1984) Changes in the sequence content of albumin mRNA and in
its translational activity in the rat liver with age. Biochim
Biophys Acta, 83: 60-66.
Horbach GJM, Van der Boom H, Van Bezooijen CFA, & Yap SH (1986)
Molecular aspects of age-related changes in albumin synthesis in
rats. In: Van Bezooijen CFA, Miglio F, & Knook DL ed. Liver drugs
and aging. Rijswijk, The Netherlangs, Eurage, pp 121-126 (Topics in
Aging Research in Europe, Volume 7).
Horbach GJM, Durham SK, Yap SH, & Van Bezooijen CFA (1988a) Albumin
elimination in female WAG/Rij rats with age: A longitudinal study.
Mech Ageing Dev, 43: 137-152.
Horbach GJM, Van der Boom H, Yap SH, & Van Bezooijen CFA (1988b)
Molecular aspects of age-related changes in albumin synthesis in
female WAG/Rij rats. Life Sci, 43: 1707-1714.
Hosomi T (1990) Japanese economy and society. In: Institute of
Japanese Problems, Chinese Academy of Social Sciences and Japanese
Vital Insurance Company ed. Proceeding of a Symposium on Sino-Japan
Social Countermeasure for Aging Problems. Beijing, Dong-Fang
Publishing House pp 7-30.
Houck JC, Dehesse C, & Jacob R (1967) The effect of aging upon
collagen catabolism. Symp Soc Exp Biol, 21: 403-425.
Hsu WH & Kakuk TJ (1984) Effect of amitraz and chlordimeform on
heart rate and pupil diameter in rats: Mediated by alpha
2-adrenoreceptors. Toxicol Appl Pharmacol, 73: 411-415.
Hunter J, Urbanowicz MA, Yule W, & Landsdown R (1985) Automated
testing of reaction time and its association with lead in children.
Int Arch Occup Environ Health, 57: 27-34.
Hunziker PE & Kagi JHR (1985) Metallothionein. In: Harrison PM ed.
Metalloproteins: Part II. London, MacMillan Press Ltd.
Huskisson EC (1983) NSAIDS: A topical review. Geriatr Med, 12:
867-870.
IARC (1982) Chemicals, industrial processes and industries
associated with cancer in humans. IARC Monographs, Volumes 1 to 29.
Lyon, International Agency for Research on Cancer, 292 pp (IARC
Monographs on the Evaluation of the Carcinogenic Risk of Chemicals
to Humans, Supplement 4).
IARC (1986) In: Montesano R, Bartsch H, Vainio H, Wilbourne J, &
Yamasaki H ed. Long-term and short-term assays for carcinogens: A
critical appraisal. Lyon, International Agency for Research on
Cancer, 564 pp (IARC Scientific Publications No. 83).
IARC (1988) Alcohol drinking. Lyon, International Agency for
Research on Cancer, 416 pp (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Volume 44).
IARC (1990) In: Tomatis L ed. Cancer: causes, occurrence and
control. Lyon, International Agency for Research on Cancer, 352 pp
(IARC Scientific Publications No. 100).
Imayama S & Braverman IM (1989) A hypothetical explanation for the
aging of skin. Chronologic alterations of the three-dimensional
arrangement of collagen and elastic fibres in connective tissue. To
provide a morphologic basis for a better understanding of the
"aging" of human. Am J Pathol, 134(5): 1019-1025.
Ingbar SH (1978) The influence of aging on the human thyroid hormone
economy. In: Greennblatt RB ed. Geriatric endocrinology. New York,
Raven Press, pp 13-31.
Ingram DK, Cutler RG, Weindruch R, Renquist DM, Knapka JJ, April M,
Belcher CT, Clark MA, Hatcherson CD, Marriott M, & Roth GS (1990)
Dietary restriction and aging: the initiation of a primate study. J
Gerontol, 45(5): 148-163.
Iqbal K, Grundke-Iqbal I, Merz PA, & Wisniewski HM (1982)
Age-associated neurofibrillary changes. In: Giacobini E, Filogamo G,
Giacobini G, & Vernadakis A ed. The aging brain: cellular and
molecular mechanisms of aging in the nervous system. New York, Raven
Press, pp 247-257.
Iwasaki K, Chirago T, Tada K, Noda K, & Noguchi H (1986) Age- and
sex-related changes in amine sulphoconjugations in Sprague-Dawley
strain rats. Comparison with phenol and alcohol sulphoconjugations.
Xenobiotica, 16: 717-723.
Iwasaki K, Gleiser CA, Masoro EJ, McMahan CA, Seo E, & Yu BP (1988)
Influence of the restriction of individual dietary components on
longevity and age-related disease of Fisher rats: The fat component
and the mineral component. J Gerontol, 43: B13-B21.
Iwase A, Kitazawa Y, & Ohno Y (1988) On age-releated norms of the
visual field. Jpn J Ophthalmol, 34(4): 429-437.
Iwata T, Incefy GS, Tanaka T, Fernandes G, Menendez-Botel CI, Pih K,
& Good RA (1979) Circulating thymic hormone levels in zinc
deficiency. Cell Immunol, 47: 100-105.
Jackson J, Banks YB, & Birnbaum LS (1990) Maximal dermal absorption
of TCDD occurs in weanling rats. Toxicologist, 10: 309.
Jaenisch R (1988) Transgenic animals. Science, 240: 1468-1474.
Jones MK, Weisenburger WP, Sipes IG, & Russell DH (1987) Circadian
alterations in prolactin, corticosterone, and thyroid hormone levels
and down-regulation of prolactin receptor activity by
2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol, 87:
337-350.
Kaldor JM & Day NE (1987) Interpretation of epidemiological studies
in the context of multistage model of carcinogenesis. In: Barrett JC
ed. Mechanisms of environmental carcinogenesis. Boca Raton, Florida,
CRC Press, vol 2, pp 21-57.
Kalimi M (1982) Comparison of glucocorticoid receptors in various
tissues of adult and senescent rats. Gerontology, 28: 371-376.
Kamataki T, Maeda K, Shimado M, Kitani K, Nagai T, & Kato R (1985a)
Age-related alteration in the activities of drug-metabolizing
enzymes and contents of sex-specific forms of cytochrome P450 in
liver microsomes from male and female rats. J Pharmacol Exp Ther,
233: 222-228.
Kamataki T, Shimada M, Maeda K, & Kato R (1985b) Pituitary
regulation of sex-specific forms of cytochrome P-450 in liver
microsomes of rats. Biochem Biophys Res Commun, 130: 1247-1253.
Kampmann JP & Hansen JEM (1979) Renal excretion of drugs. In: Crooks
J & Stevenson IH ed. Drugs and the elderly. Baltimore, Maryland,
University Park Press, pp 77-87.
Kanai S, Kitani K, Fujita S, & Kitagawa H (1985) The hepatic
handling of sulfobrompthalein in aging Fischer-344 rats: in vivo
and in vitro studies. Arch Gerontol Geriatr, 4: 73-85.
Kanai S, Kitani K, Sato Y, & Nokubo M (1988) The age-dependent
decline in the biliary transport maximum of conjugated
sulfobromophthalein in the rat. Arch Gerontol Geriatr, 7: 1-8.
Kane R (1987) Comprehensive assessment. In: Maddox GL ed. The
encyclopedia of aging. Berlin, Heidelberg, New York,
Springer-Verlag, pp 137-139.
Kannel WB (1981) Update on the role of cigarette smoking in coronary
artery disease (review). Am Heart J, 101: 319-328.
Kato H, Saito M, & Suda M (1980) Effect of starvation on the
circadian adrenocortical rhythm in rats. Endocrinology, 106:
918-921.
Katz ML & Robinson WG Jr (1987) Light and aging effects on vitamin E
in the retinal pigment epithelium. Vision Res, 27(11): 1875-1879.
Katzman R & Terry RD (1983) Normal aging in the nervous system. In:
Katzman R & Terry RD ed. The neurology of aging. Philadelphia,
Pennsylvania, F.A. Davis, pp 15-50.
Kaur S & Gill SS (1985) Age-related changes in the activities of
epoxide hydrolyases in different tissues of mice. Drug Metab Dispos,
13: 711-715.
Kaysen GA & Myers BD (1985) The aging kidney. Clin Geriatr Med,
1(1): 207-222.
Keast D & Ayre DJ (1981) Effects of chronic tabocco smoke exposure
on immune responses in aged mice. Arch Environ Health, 36: 201-207.
Kelley WW, Brief S, Westly HJ, Novakofski J, Bechtel PJ, Simon J, &
Walker EB (1986) GH3 pituitary adenoma cells can reverse thymic
aging in rats. Proc Natl Acad Sci (USA), 83: 5663-5667.
Kelso A & Munck A (1984) Glucocorticoid inhibition of lymphokine
secretion by alloreactive T lymphocyte clones. J Immunol, 133(2):
784-791.
Kim JCS & Kaminsky LS (1988) 2,2,2-Trifluoroethanol toxicity in aged
rats. Toxicol Pathol, 16: 35-45.
Kinon GA & Liu C (1973) Diurnal variation in plasma testosterone of
the male laboratory rat. Horm Metab Res, 5: 233-234.
Kitani K (1985) The role of the liver in pharmacokinetic and
pharmacodynamic alterations in the elderly. In: O'Malley K &
Waddington J ed. Therapeutics in the elderly. Amsterdam, Oxford, New
York, Elsevier Science Publishers, pp 19-34.
Kitani K (1988) Drugs and the ageing liver. Life Chem Rep, 6:
143-230.
Kitani K (1991) Aging of the liver: facts and theories. Arch
Gerontol Geriatr, 12: 133-154.
Kitani K, Masuda Y, Sato Y, Kanai S, & Nokubo M (1984) Increased
anti-convulsant activity in aging BDF1 mice. J Pharmacol Exp Ther,
229: 231-236.
Kitani K, Ohta M, Kanai S, & Sato Y (1985a) Sex difference in the
biliary excretion of digoxin and its metabolites in aging Wistar
rats. Arch Gerontol Geriatr, 4: 1-12.
Kitani K, Sato Y, Kanai S, Nokubo M, Ohta M, & Masuda Y (1985b)
Increased anticonvulsant effect of phenobarbital with age in mice -
a possible pharmacologic index for brain aging. Life Sci, 37:
1451-1460.
Kitani K, Sato Y, Kanai S, Nokubo M, Ohta M, Masuda Y, & Klotz U
(1986) Altered response to convulsants and anticonvulsants in aging
BDF1 mice. In: Kitani K ed. Liver and aging 1986: Liver and brain.
Amsterdam, Oxford, New York, Elsever Science Publishers, pp 293-307.
Kitani K, Sato Y, Kanai S, Nokubo M, Ohta M, & Masuda Y (1987)
Increasing anticonvulsant effect of AD-810 (Zonisamide) in aging
BDF1 mice. Life Sci, 41: 1339-1344.
Klatsky AL, Friedman GD, & Siegelaub AB (1981) Alcohol and
mortality. A ten year Kaiser-Permanente experience. Ann Intern Med,
95: 139-145.
Klawans HL & Tanner CM (1984) Movement disorders in the elderly. In:
Albert M ed. Clinical neurology of aging. Oxford, Oxford University
Press, pp 387-403.
Klotz U, Avant GR, Hoyumpa A, Schenker S, & Wilkinson GR (1975) The
effects of age and liver disease on the disposition and elimination
of diazepam in adult man. J Clin Invest, 55: 347.
Klug TL & Adelman RC (1977) Evidence for a large thyrotropin and its
accumulation during aging in rats. Biochem Biophys Res Commun, 77:
1431-1437.
Koch-Weser J, Greenblatt DJ, Seller EM, & Shoder RI (1982) Drug
disposition in old age. New Engl J Med, 306: 1081-1086.
Kohrs MB (1981) Nutritional needs of the aged. Nutrition, 7: 39-46.
Konishi N & Ward JM (1989) Increased levels of DNA synthesis in
hyperplastic renal tubules of aging nephropathy in female F344/NCR
rats. Vet Pathol, 26: 6-10
Koobs DH, Schultz RL, & Jutzy RV (1978) The origin of lipofuscin and
possible consequences to the myocardium. Arch Pathol Lab Med, 102:
66-68.
Kozararevic DJ, McGee D, Vojvodic N, Racic Z, Dawber T, Gordon T, &
Zukel W (1980) Frequency of alcohol consumption and morbidity and
mortality: The Yugoslavia cardiovascular disease study. Lancet, i:
613-616.
Krupka L & Vener A (1979) Hazards of drug use among the elderly.
Gerontologist, 19: 90-95.
Kubos C, Day NK, & Good RA (1984) Influence of early or late dietary
restirction on life span and immunological parameters in
MRL/MP-Ipr/Ipr mice. Proc Natl Acad Sci (USA), 81: 5831-5835.
Kumpuris D (1983) Gastrointestinal bleeding in the older patient.
In: Texter EC ed. The aging gut. New York, Masson Publishing Co., pp
57-63.
Kyle ME & Kocsis JJ (1985) The effect of age on salicylate-induced
nephrotoxicity in male rats. Toxicol Appl Pharmacol, 81: 337-347.
Lakatta EG (1980) Age-related alteration in the cardiovascular
response to adrenergic mediated stress. Fed Proc, 39: 3173-3177.
Lakatta EG (1985) Heart and circulation. In: Finch CE & Schneider EL
ed. Handbook of the biology of aging, 2nd ed. New York, Van Nostrand
Reinhold Company, pp 377-413.
Lakatta EG (1986) Diminished beta-adrenergic modulation of
cardiovascular function in advanced age. Cardiol Clin, 4(2):
185-200.
Lakatta EG (1987a) Cardiac muscle change in senescence. Annu Rev
Physiol, 49: 519-531.
Lakatta EG (1987b) Catecholamines and cardiovascular function in
aging. Endocrinol Metab Clin North Am, 16(4): 877-891.
Landauer MR, Tomlinson WT, Balster RL, & MacPhail RC (1984) Some
effects of the formamidine pesticide on the behavior of mice.
Neurotoxicology, 5: 91-100.
Leakey JEA, Cunny HC, Bazare J Jr, Webb PJ, Feuers RJ, Duffy PH, &
Hart RW (1989a) Effects of aging and caloric restriction on hepatic
drug metabolizing enzymes in the Fischer 344 rat. I: The cytochrome
P-450 dependent monooxygenase system. Mech Ageing Dev, 48: 145-155.
Leakey JEA, Cunny HC, Bazare J Jr, Webb PJ, Lipscomb JC, Siikkes W
Jr, Feuers RJ, Duffy PH, & Hart RW (1989b) Effects of aging and
caloric restriction on hepatic drug metabolizing enzymes in the
Fischer 344 rat. II. Effects on conjugating enzymes. Mech Ageing
Dev, 48: 157-166.
Lee KT ed. (1985) Atherosclerosis. Ann NY Acad Sci, 454: 1-327.
Lehmann AR, Hoeijmakers JHJ, Van Zealand AA, Backendorf CMP, Bridges
BA, Collins A, Fuchs RPD, Margison GP, Montesano R, Moustacchi E,
Natarajan AT, Radman M, Sarasin A, Seeberg E, Smith CA, Stefanini M,
Thompson LH, Van der Schans GP, Weber CA, & Zdzienicka MZ (1992)
Workshop on DNA repair. Mutat Res, 273: 1-28.
Leopold AC (1975) The biological significance of death in plants.
In: Benke JA, Finch CE, & Moment GB ed. The biology of aging. New
York, London, Plenum Press, pp 101-114.
Leppaluoto J, Ranta T, & Tuomisto J (1974) Diurnal variations of
serum immunoassayable thyrotropin (TSH) concentration in the rat.
Acta Physiol Scand, 90: 699-702.
Lesser GT, Deutsch S, & Markofsky J (1973) Aging in the rat:
Longitudinal and cross-sectional studies of body composition. Am J
Physiol, 225: 1472-1478.
Leto S, Kokkahen GC, & Barrows CH Jr (1976) Dietary protein, life
span and physiological variables in female mice. J Gerontol, 31:
149-154.
Levy ML (1987) Tous les pays du monde (1987). Bull Mens Inf Démogr
Econ Soc, 216:1-6.
Lewis VM, Twomey JJ, Bealmar P, Goldstein G, & Good RA (1978) Age,
thymic involution, and circulating thymic hormone activity. J Clin
Endocrinol Metab, 47: 145-150.
Li DD, Chien YK, Gu MZ, Richardson A, & Cheung HT (1988) The
age-related decline in interleukin-3 expression in mice. Life Sci,
43: 1215-1222.
Liebow AA (1964) Biochemical and structural changes in the aging
lung. Summary. In: Cander L & Moyer JH ed. Aging of the lung. New
York, London, Grune and Stratton, pp 97-104.
Liepa GU, Masoro EJ, Bertrand HA, & Yu BP (1980) Food restriction as
a modulation of age-related changes in serum lipids. Am J Physiol,
238: E253-E257.
Likhachev AJ (1985) Effect of age on DNA repair in carcinogenesis
due to alkylating agents. In: Likhachev A, Anisimov V, & Montesano R
ed. Age-related factors in carcinogenesis. Lyon, International
Agency for Research on Cancer, pp 239-246 (IARC Scientific
Publications No. 58).
Lin CF & Hayton WL (1983) GI motility and subepithelial blood flow
in mature and senescent rats. Age, 6: 46-51.
Lindeman RD (1986) The aging kidney. Compr Ther, 12(3): 43-49.
Lindeman RD, Tobin JD, & Shock NW (1987) Hypertension and the
kidney. Nephron, 47(1): 62-67
Lindsay R (1989) Osteoporosis: An updated approach to prevention and
management. Geriatrics, 44: 45-54.
Lipschitz DA (1987) Nutrition, aging and the immunohematopoietic
system. Clin Geriatr Med, 3(2): 319-328.
Loft S, Dossing M, & Poulsen HE (1988) Influence of age and
consumption of tobacco, alcohol, and caffeine on antipyrine
clearance. Hum Toxicol, 7: 277-280.
Loi CM & Vestal RE (1988) Drug metabolism in the elderly. Pharmacol
Ther, 36: 131-149.
Lucchi L, Memo M, Airaghi ML, Spano PF, & Trabucchi M (1981) Chronic
lead treatment induces in rat a specific and differential effect on
dopamine receptors in different brain areas. Brain Res, 213:
397-404.
Lutz RJ, Dedrick RL, Matthews HB, Elling TE, & Anderson MW (1977) A
preliminary pharmacokinetic model for several chlorinated biphenyls
in the rat. Drug Metab Dispos, 5: 386-396.
McCaffrey TA, Nicholson AG, Szabo PE, & Wexsler BB (1988) Aging and
atherosclerosis. The increased proliferation of arterial smooth
muscle cells isolated from old rats is associated with increased
platelet-derived growth factor-like activity. J Exp Med, 167:
163-174.
McCay CM, Crowell MM, & Mayrar LA (1935) The effect of reduced
growth upon the length of the life span and upon ultimate body size.
J Nutr, 10: 63-79.
McClure JE, Lameris N, Wara DW, & Goldstein AL (1982) Immunochemical
studies on thymosin: radioimmunoassay of thymosin alpha-1. J
Immunol, 128(1): 368-375.
McGinty D, Stern N, & Akshoomoff N (1988) Circadian and
sleep-related modulation of hormone levels changes with aging. In:
Sowers JR & Felicett JV ed. The endocrinology of aging. New York,
Raven Press, pp 75-111.
McKenna MJ & DiStefano V (1977) Carbon disulphide. II. A proposed
mechanism for the action of carbon disulfide on
dopamine-beta-hydroxylase. J Pharmacol Exp. Ther, 202: 253-266.
McMahon TF (1988) Age-related changes in colonic phase I and phase
II biotransformation: possible relationship to colon carcinogenesis.
Baltimore, University of Maryland, School of Pharmacy
(Dissertation).
McMahon TF & Birnbaum LS (1990a) Age-related changes in the
disposition and metabolism of xenobiotics. In: Cooper RL, Goldman
JM, & Harbin TJ ed. Aging and environmental toxicology. Baltimore,
Maryland, Johns Hopkins University Press, pp 7-30.
McMahon TF & Birnbaum LS (1990b) Age related change on toxicity and
biotransformation of potassium cyanide in male C57BL/6N mice.
Toxicol Appl Pharmacol, 105: 305-314.
McMahon TF, Beierschmitt WP, & Weiner M (1987) Changes in phase I
and phase II biotransformation pathways with age in male Fischer 344
rat colon: relationship to colon carcinogenesis. Cancer Lett, 36:
273-282.
McMahon TF, Diliberto JJ, & Birnbaum LS (1989) Age-related changes
in the disposition of benzyl acetate: A model compound for glycine
conjugation. Drug Metab Dispos, 17: 506-512.
McMahon TF, Diliberto JJ, & Birnbaum LS (l990a) Effects of age on
metabolism and disposition of salicylic acid (SAL) in male Fischer
344 rats. Drug Metab Dispos, 18: 494-503.
McMahon TF, Peggins JO, Centra MM, & Weiner M (l990b) Age-related
changes in biotransformation of azoxymethane and methylazoxymethanol
in vitro. Xenobiotica, 20: 501-513.
Maeda H, Gleiser CA, Masoro EJ, Murata I, McMohan CA, & Yu BP (1985)
Nutritional influences on aging of Fisher 344 rats. II. Pathology. J
Gerontol, 40: 671-688.
Magal E, Chaudhuri M, & Adelman RC (1986) The capability for
regulation of insulin secretion by somatostatin in purified
pancreatic islet B cells during aging. Mech Aging Dev, 33(2):
139-146.
Magnus K (1982) Trends in cancer incidence: Causes and practical
implications. Washington, DC, Hemisphere.
Mantel N & Schneiderman MA (1975) Estimation "safe" levels, a
hazardous undertaking. Cancer Res, 35: 1379-1386.
Mantere P, Hanninen H, Hernberg S, & Luukkonen R (1984). A
prospective follow-up study on psychological effects in workers
exposed to low levels of lead. Scand J Work Environ Health, 10:
43-50.
Marin R, Proverbio T, & Proverbio F (1985) Characterization of the
Na+, K+ -ATPase activity of basolateral membranes of kidney proximal
tubular cells from young and old rats. Biochem Pharmacol, 34:
4197-4201.
Marksberry WR, Ehmann WD, Hossain IM, Alauddin M, & Goodin DT (1981)
Instrumental neutron activation analysis of brain aluminum in
Alzheimer's disease and aging. Ann Neurol, 10: 511-516.
Marsh G (1980) Perceptual changes with aging. In: Busse EW & Blazer
DG ed. Handbook of geriatric psychiatry. New York, Van Nostrand
Reinhold Company, Chapter 7, pp 147-168.
Marshall JF & Berrios N (1979) Movement disorders of aged rats:
Reversal by dopamine receptor stimulation. Science, 206: 477-479.
Martin GM, Sprague CA, Norwood TH, & Pendergrass WR (1974) Clonal
selection, attenuation and differentiation in an in vitro model of
hyperplasia. Am J Pathol, 74: 137-154.
Marx JL (1987) A new wave of enzymes for cleaving pro-hormones.
Science, 235: 285-286.
Masoro EJ ed. (1981) CRC handbook of physiology in aging. Boca
Raton, Florida, CRC Press.
Masoro EJ (1985) Metabolism. In: Finch CE & Schneider EL ed.
Handbook of the biology of aging. New York, Van Nostrand Reinhold
Company, pp 540-563.
Masoro EJ (1988) Food restrictions in rabbits: an evolution of its
role in the study of aging. J Gerontol, 43: B59-B64.
Masoro EJ (1991) Use of rodents as models for the study of "normal
aging": Conceptual and practical issues. Neurobiol Aging, 12:
639-644.
Masoro EJ & Yu BP (1989) Diet and nephropathy. Lab Invest, 60:
165-167.
Masoro EJ, Yu BP, Bertrand HR, & Lynd FT (1980) Nutritional probe of
the aging process. Fed Proc, 39: 3178-3182.
Masoro EJ, Shimokawa I, & Yu BP (1991) Retardation of the aging
processes in rats by food restriction. Ann NY Acad Sci, 621:
337-352.
Master CL, Multhaup G, Simms G, Pottgieser J, Martins RN, &
Beyreuther K (1985) Neuronal origin of a cerebral amyloid:
Neurofibrillary tangles of Alzheimer's disease contain the same
protein as the amyloid of plaque cores and blood vessels. EMBO J, 4:
2757-2763.
Matulionis DH (1984) Chronic cigarette smoke inhalation and aging in
mice: Morphologic and functional lung abnormalities. Exp Lung Res,
7: 237-256.
Mauderly JL (1978) Effect of age on pulmonary structure and function
of immature and adult animals and man. Fed Proc, 38: 173-177.
Mauderly JL (1979a) Ventilation, lung volumes, and lung mechanics of
young adult and old Syrian hamsters. Exp Aging Res, 5: 497-508.
Mauderly JL (1979b) Effect of aging on pulmonary structure and
function of immature and adult animals and man. Fed Proc, 38:
173-177.
Mauderly JL (1982) The effect of age on respiratory function of
Fischer 344 rats. Exp Aging Res, 8: 31-36.
Mawhinney MG (1985) Etiological considerations for the growth of
stroma in benign prostatic hyperplasia. Fed Proc, 45: 2615-2617.
Mazzeo RS & Horvath SM (1987) A decline in myocardial and hepatic
norepinephrine turnover with age in Fischer 344 rats. Am J Physiol,
252: E762-E763.
Medvedev ZA (1984) Age changes of chromatin: A review. Mech Ageing
Dev, 28: 139-154.
Medvedev ZA (1990) An attempt at a rational classification of
theories of aging. Biol Rev, 65: 375-398.
Mehta T, Labuc GE, Urbanski SJ, & Archer MC (1984) Organ specificity
in the microsomal activation and toxicity of
N-nitrosomethylbenzylamine in various species. Cancer Res, 44:
4017-4022.
Meisami E (1988) Aging of the nervous system structural and
biochemical changes. In: Timiras PS ed. Physiological basis of aging
and geriatrics. New York, Macmillan Publishing Co, pp 123-143.
Mervis R (1981) Cytomorphological alterations in the aging animal
brain with emphasis on Golgi studies. In: Johnson JE ed. Aging and
cell structure. New York, London, Plenum Press, Chapter 4, pp
143-186.
Messing RB, Vasquez BJ, Spiehler VR, Martinez JL Jr, Jensen RA,
Rigter H, & McCaugh JL (1980) 3H-Dihydromorphine binding in brain
regions of young and aged rats. Life Sci, 26: 921-927.
Meyer BR & Bellucci A (1986) Renal function in the elderly. Cardiol
Clin, 4(2): 227-234.
Mezey E (1981) Alcohol and drug interactions in injury to the
digestive tract. Clin Gastroenterol, 10: 485-495.
Millard WJ, O'Sullivan DM, Fox TO, & Martin JB (1985) Sexually
dimorphic patterns of growth hormone secretion in rats. In: Crowley
WF Jr & Hofler JG ed. The episodic secretion of hormones. New York,
Chichester, Brisbane, Toronto, John Wiley and Sons, pp 287-304.
Miller SM (1989) Changes in endocrine function with aging. Clin Lab
Sci, 2(1): 19-20.
Miller R (1991) Aging and immune function. Int Rev Cytol, 124:
187-216.
Minaker KL, Meneilly GS, & Rowe JW (1985) Endocrine systems. In:
Finch CE & Schneider EL ed. Handbook of the biology of aging. New
York, Van Nostrand Reinhold Company, pp 433-456.
Mitsui Y, Sakagami H, Murota SI, & Yamada MA (1980) Age related
decline in histone H1 fraction in human diploid fibroblast cultures.
Exp Cell Res, 126: 289-298.
Miura K, Goldstein RS, Morgan DG, Pasino DA, Hewitt WR, & Hook JB
(1987) Age-related differences in susceptibility to renal ischemia
in rats. Toxicol Appl Pharmacol, 87: 284-296.
Moberg GP, Bellinger LL, & Mendel VE (1975) Effect of meal feeding
daily rhythms of plasma corticosterone and growth hormone in rat.
Neuroendocrinology, 19: 160-169.
Monteiro-Riviere NA, Banks YB, & Birnbaum LS (1991) Laser doppler
measurements of cutaneous blood flow in ageing mice and rats.
Toxicol Lett, 57: 329-338.
Montgomery PR & Sitar DS (1981) Increased serum salicylate
metabolites with age in patients receiving chronic acetylsalicylic
acid therapy. Gerontology, 27: 329-333.
Moolgavkar SH & Venzon DJ (1979) Two-event models for
carcinogenesis; incidence curves for childhood and adult tumors.
Math Biosci, 47: 55-77.
Mooradian AD & Song MK (1989) Age-related alterations in duodenal
calcium transport rate in rats. Mech Ageing Dev, 47: 221-227.
Moroni F, Lombardi G, Moneti G, & Aldinio C (1984) The excitotoxin
quinolinic acid is present in the brain of several mammals and its
cortical content increases during the aging process. Neurosci Lett,
47: 51-55.
Morell AG, Gregoriadis G, Scheinberg IH, Hickman J, & Ashwell G
(1971) The role of sialic acid in determining the survival of
glycoproteins in the circulation. J Biol Chem, 246: 1461-1467.
Morris JF, Mosk A, & Johnson LC (1971) Spirometric standards for
healthy non-smoking adults. Am Rev Respir Dis, 103: 57-67.
Mullaart E, Boerrigter METI, Lohman PHM, & Vijg J (1989) Age-related
induction and disappearance of carcinogen-DNA-adducts in livers of
rats exposed to low levels of 2-acetylaminofluorene. Chem-Biol
Interact, 69: 373-384.
Mullaart E, Lohman PHM, Berends F, & Vijk J (1990) Review DNA damage
metabolism and aging. Mutat Res, 237: 189-210.
Müller HF & Schwartz G (1978) Electroencephalograms and autopsy
findings in geropsychiatry. J Gerontol, 33: 504-513.
Muller WEG, Wenger R, Bachmann M, Ugarkovic D, Courtis NC, &
Schroder HC (1989) Poly(A) metabolism and aging: a current view.
Arch Gerontol Geriatr, 9: 31-250.
Munro HN (1984) Nutrition-related problems of middle age. Proc Nutr
Soc, 43: 281-288.
Murty CVR, Mancini MA, Chatterjee B, & Roy AK (1988a) Changes in
transcriptional activity and matrix association of alpha-2u-globulin
gene family in the rat liver during maturation and aging. Biochim
Biophys Acta, 949: 7-34.
Murty CVR, Olson MJ, Garg BD, & Roy AK (1988b) Hydrocarbon-induced
hyaline droplet nephropathy in male rats during senescence. Toxicol
Appl Pharmacol, 96: 380-392.
Mutti A, Falzoi M, Romanelli A, Bocchi MC, Ferroni C, & Franchini I
(1988) Brain dopamine as a target for solvent toxicity: Effects of
some monocyclic aromatic hydrocarbons. Toxicology, 49: 77-82.
Naessen R (1971) An enquiry on the morphological characteristics and
possible changes with age in the olfactory region of man. Acta
Otorhinol, 71: 49-62.
Nagel JE, Chopra RK, Chrest FJ, McCoy MT, Schneider EL, Holbrook NJ,
& Adler WH (1988) Decreased proliferation, interleukin 2 synthesis,
and interleukin 2 receptor expression are accompanied by decreased
mRNA expression in phytohemagglutinin-stimulated cells from elderly
donors. J Clin Invest, 81: 1096-1102.
Nagy K, ZS-Nagy V, Bertoni-Freddari C, & ZS-Nagy I (1983)
Alterations of the synaptosomal membrane 'microviscosity' in the
brain cortex of rats during aging and centrophenoxine treatment.
Arch Gerontol Geriatr, 2: 23-29.
Nakamura E, Kimura M, Nagata H, Miyao K, & Ozeki T (1982) Evaluation
of the progress of aging based on specific biological age as
estimated by various physiological functions. Jpn J Hyg, 36:
853-862.
NAS (1989) Biological markers in reproductive toxicology.
Washington, DC, National Academy of Sciences, National Academy
Press, pp 2-3.
Neafsey PJ (1990) Longevity hormesis. A review. Mech Ageing Dev, 51:
1-31.
Nebert DW & Gonzalez FJ (1987) P450 Genes - Structure, evolution and
regulation. Annu Rev Biochem, 56: 945-993.
Nebert DW, Nelson DR, Minor JC, Estabrook RW, Feyereisen R,
Fujii-Kuriyama Y, Gonzalez FJ, Guengerich FP, Gunsalus IC, Johnson
EF, Loper JC, Sato R, Waterman MR, & Waxman DR (1991) The P450
superfamily: Uptake on new sequences, gene mapping, and recommended
nomenclature. DNA Cell Biol, 10(1): 1-14.
Needleman HL, Gunnoe C, Leviton A, Reed R, Peresie H, Maher C, &
Barrett P (1979) Deficits in psychologic and classroom performance
of children with elevated dentine lead levels. New Engl J Med, 300:
689-695.
Neuhaus OW & Flory W (1978) Age-dependent changes in the excretion
of urinary proteins by the rat. Nephron, 22: 570-576.
Newaz SN, Fang W, & Strobel HW (1983) Metabolism of the carcinogen
1,2-dimethylhydrazine by isolated human colon microsomes and human
colon tumor cells in culture. Cancer, 52: 794-798.
Niedzwiecki A, Lewis PN, & Cinader B (1985) Changes of histone H1
subtypes with aging in strains of mice that possess different
immunological characteristics. J Gerontol, 40: 695-699.
Nokubo M & Kitani K (1988) Age-dependent decrease in the lethal
threshold of pentylenetetrazole in mice. Life Sci, 43: 41-47.
Norwood TH & Smith JR (1985) The cultured fibroblast-like cells as a
model for the study of aging. In: Fincht CE & Schneider EL ed. The
biology of aging, 2nd ed. New York, Van Nostrand Reinhold Company,
pp 291-321.
Novak LP (1972) Aging, total potassium, fat free mass and cell mass
in males and females between the ages of 18 and 85 years. J
Gerontol, 27: 438-443.
Oh SH, Deagen JT, Whanger PD, & Wesnig PH (1978) Biological function
of metallothionein. V. Its induction in rats by various stresses. Am
J Physiol, 234: E282-E285.
Ohta M, Kanai S, Sato Y, & Kitani K (1988) Age-dependent decrease in
the hepatic uptake and biliary excretion of ouabain in rats. Biochem
Pharmacol, 37: 935-942.
O'Malley K, Docherty JR, & Kelly GJ (1988) Adrenoceptor status and
cardiovascular function in ageing. J Hypertension, 6(suppl 10):
S59-S62.
O'Meara AR & Pochron SF (1979) Age-related effects on the
incorporation of acetate into rat liver histones. Biochim Biophys
Acta, 586: 391-401.
Onasaka S & Cherian MG (1981) The induced synthesis of
metallothionein in various tissues of rats in response to metals. I.
Effect of repeated injection of cadmium salts. Toxicology, 22:
91-101.
Os I, Kjeldsen SE, & Westheim A (1987) Aging and urinary vasopressin
excretion in healthy men. Scand J Urol Nephrol, 21(3): 235-239.
OECD (1986) Existing chemicals systematic investigation, priority
setting and chemical reviews. Paris, Organisation for Economic
Co-operation and Development.
Owen RA & Heywood R (1986) Age-related variations in renal structure
and function in Sprague-Dawley rats. Toxicol Pathol, 14(2): 158-167.
Pagano GN, Casadar N, Diana A, Psue E, Bozzo C, Ferrero F, & Lenti G
(1981) Insulin resistance in the aged: the role of peripheral
insulin receptors. Metabolism, 30: 46-49.
Pahlavani MA, Cheung HT, Cai NS, & Richardson A (1988) Influence of
dietary restriction and aging on gene expression in the immune
system of rats. In: Biomedical advances in aging. New York, London,
Plenum Press, pp 259-270.
Pakin YV & Hrisanov SM (1984) Critical analysis of the applicability
of the Compertz-Makeham law in human population. Gerontology, 30:
8-12.
Paramonova GI & Dovgi AI (1987) Induction of multiple forms of
cytochrome P450 of rat liver during aging. In: Abstracts of the All
Union Conference on Cytochrome P450 and the Environment,
Novosibirsk, 27-31 July 1987. Novosibirsk, USSR Academy of Medical
Sciences, Siberian Division, pp 100-101.
Patel DJ & Vaishnaw RN (1980) Basic hemodynamics and its role in
disease process. Baltimore, Maryland, University Park Press.
Pedigo NW, Schoemaker H, Morellia M, McDougal JN, Malisck JB, Burks
TF, & Yamamura TF (1981) Benzodiazepine receptor binding in young,
mature, and senescent rat brain and kidney. Neurobiol Aging, 2:
83-88.
Penn A, Batastini G, Soloman J, Burns F, & Albert R. (1981)
Dose-dependent size increases of aortic lesions following chronic
exposure to 7,12-dimethylbenz(a)-anthracene. Cancer Res, 71:
588-592.
Penzes L (1974) Intestinal absorption of glycine, L-alanine, and
L-leucine in the old rat. Exp Gerontol, 8: 245-252.
Penzes, L.S. (1984) Intestinal response in aging: changes in reserve
capacity. Acta Med Hung, 41(4): 263-277.
Perkins MN & Stone TW (1983) Pharmacology and regional variations of
quinolinic acid-evoked excitations in the rat central nervous
system. J Pharmacol Exp Ther, 226(2): 551-557.
Perl DP & Brody AR (1980) Alzheimer's disease: X-ray spectrometric
evidence of aluminum accumulation in neurofibrillary tangle-bearing
neurons. Science, 208: 297-299.
Peterson DD, Pack AI, Silage DA, & Fishman AP (1981) Effects of
aging on ventilatory and occlusion pressure responses to hypoxia and
hypercapnia. Am Rev Respir Dis, 124: 387-391.
Peto R (1986) Influence of dose and duration of smoking on lung
cancer rates. In: Zaridze D & Peto R ed. Tobacco: A major
international health hazard. Lyon, International Agency for Research
on Cancer, pp 23-33 (IARC Scientific Publications No. 74).
Peto R, Parish SE, & Gray RG (1975) Cancer and aging in mice and
men. Br J Cancer, 32: 411-426.
Peto R, Pike MC, Day NE, Gray RE, Lee PN, Parish S, Poto J, Richard
S, & Wahrendorf J (1980) Guidelines for simple, sensitive
significance tests for carcinogenic effects in long-term animal
experiments. In: Long-term and short-term screening assays for
carcinogens: A critical appraisal. Lyon, International Agency for
Research on Cancer, pp 311-426 (IARC Monographs on the Evaluation of
the Carcinogenic Risk of Chemicals to Humans, Supplement 2).
Peto R, Parish SE, & Gray RG (1985) There is no such thing as aging,
and cancer is not related to it. In: Likhachev AJ, Anisimov V, &
Montesano R ed. Age-related factors in carcinogenesis. Lyon,
International Agency for Research on Cancer, pp 43-53 (IARC
Scientific Publications No. 58).
Pierpaoli W, Dall'Ara A, Pedrinis E, & Regelson W (1991) The pineal
control of aging. The effects of melatonin and pineal grafting on
the survival of older mice. Ann NY Acad Sci, 621: 291-313.
Piikivi L, Hanninen H, Martelin T, & Mantere P (1984) Psychological
performance and long-term exposure to mercury vapors. Scand J Work
Environ Health, 10: 35-41.
Pines A, Cucos B, Ever-Hadani P, Ron M, & Lemesch C (1987) Changes
in pattern of organochlorine residues in blood of general Israeli
population, 1975-1986. Sci Total Environ, 66: 115-125.
Piva F, Limonta P, Maggi R, Dondi D, Messi E, Yanisi M, & Motta M
(1987) Aging and the hypothylamo-pituitery-testiculas axis in the
rat. J Steroid Biochem, 27(4-6): 707-712.
Poland A & Knutson JC (1982) 2,3,7,8,-Tetrachlorodibenzo-p-dioxin
and related halogenated aromatic hydrocarbons: examinations of the
mechanism of toxicity. Annu Rev Pharmacol Toxicol, 22: 517-554.
Popper H (1986) Aging in the liver. In: Popper H ed. Progress in
liver diseases. New York, London, Grune & Stratton, vol VIII, pp
659-683.
Posner J, Danhof M, Teunissen MWE, Breimer DD, & Whiteman PD (1987)
The disposition of antipyrine and its metabolites in young and
elderly healthy volunteers. Br J Clin Pharmacol, 24: 51-55.
Post DJ, Carter K, & Papaconstantinou J (1991) The effect of aging
on constitutive mRNA levels and lipopolysaccharide inducibility of
acute phase genes. Ann NY Acad Sci, 621: 66-77.
Prinz PN, Weitzman ED, Cunningham GR, & Karacan I (1983) Plasma
growth hormone during sleep in young and aged men. J Gerontol, 38:
519-534.
Provinciali M & Fabris H (1990) Role of pituitary-thyroid axis on
basal and lymphokine-induced NK cell activity in aging. Int J
Neurosci, 51: 373-375.
Rabovsky, J, Marion KJ, Groseclose RD, Lewis TR, & Peterson M (1984)
Low dose chronic inhalation of diesel exhaust and/or coal dust by
rats: effect of age and exposure on lung and liver cytochrome P450.
Xenobiotica, 14: 595-598.
Rao GN, Haseman JK, Grumbein S, Crawford DD, & Eustis SL (1990)
Growth, body weight, survival, and tumor trends in F344/n rats
during an eleven-year period. Toxicol Pathol, 18: 61-70.
Rath PC & Kanungo MS (1989) Age-related changes in the expression of
cytochrome p-450 (b+e) gene in the rat after phenobarbitone
administration. Biochem Biophys Res Commun, 157: 1403-1409.
Reff ME & Schneider EL ed. (1982) Biological markers of aging.
Washington, DC, US Government Printing Office, 252 pp (NIH
Publication No. 82-2221).
Regelson W (1983) Biomarkers in aging. In: Regelson W & Marott Sinex
F ed. Intervention in the aging process. New York, Alan R. Liss,
Inc., pp 3-98.
Rehnberg GL, Goldman JM, Cooper RL, Hein JF, McElroy WK, Booth KC, &
Gray LE (1988a) Effect of linuron on the brain-pituitary-testicular
reproductive axis in the rat. Toxicologist, 8: 121.
Rehnberg GL, Linder R, Goldman JM, Hein JF, McElroy WK, & Cooper RL
(1988b) Changes in testicular and serum hormone concentrations in
the male rat following treatment with m-dinitrobenzene. Toxicol Appl
Pharmacol, 95: 255-264.
Reidenberg MM (1980) Drugs in the elderly. Bull NY Acad Med, 56:
703-714. Reif AE (1981) Effect of cigarette smoking on
susceptibility to lung cancer. Oncology, 38: 76-85.
Reis W & Poethig D (1984) Chronological and biological age. Exp
Gerontol, 19: 211-216.
Reiter RJ (1986) The pineal gland: an important link to the
environment. News Physiol Sci, 1: 202-205.
Reitz RH, McDougal JN, Himmelstein MW, Nolan RJ, & Schumann AM
(1988) Physiologically based pharmacokinetics modeling with methyl
chloroform: implications for interspecies, high dose/low dose, and
dose route extrapolations. Toxicol Appl Pharmacol, 95: 185-199.
Revskoy SY, Poroshina TE, Kovaleva IG, Berstein LM, Ostroumova MN, &
Dilman VM (1985) Age-dependent metabolic immunodepression and
cancer. In: Likhachev A, Anisimov V, & Montesano R ed. Age-related
factors in carcinogenesis. Lyon, International Agency for Research
on Cancer, pp 253-259 (IARC Scientific Publications No. 58).
Richardson A & Birchenall-Sparks MC (1983) Age-related changes in
protein synthesis. Rev Biol Res Aging, 1: 255-273.
Richardson A & Semsei I (1987) Effect of aging on translation and
transcription. In: Review of biological research in aging. New York,
Alan R. Liss, Inc., vol 3, pp 467-483.
Richardson A, Birchenall-Sparks MC, & Staecker JL (1983) Aging and
transcription. In: Review of biological research in aging. New York,
Alan R. Liss, Inc., vol 2, pp 275-294.
Richardson A, Butler JA, Rutherford MS, Semsei I, Gu MZ, Fernandes
G, & Chiang WH (1987) Effect of age and dietary restriction on the
expression of alpha-2u-globulin. J Biol Chem, 262: 12821-12825.
Richardson A, Roberts M, & Rutherford M (1985) Aging and gene
expression. In: Review of biological research in aging. New York,
Alan R. Liss, Inc., vol 2, pp 395-419.
Riegel GD & Miller AE (1981) Aging effect on hormone concentrations
and actions. In: Florini JR ed. Handbook of biochemistry in aging.
Boca Raton, Florida, CRC Press, pp 231-256.
Riggs BL (1987) Pathogenesis of osteoporosis. Am J Obstet Gynecol,
156: 1342-1346.
Riggs BL & Melton LJ III (1983) Evidence for two distinct syndromes
of involutional osteoporosis. Am J Med, 75: 899-901.
Rikans LE (1984) Influence of aging on the susceptibility of rats to
hepatotoxic injury. Toxicol Appl Pharmacol, 73: 243-249.
Rikans LE (1989a) Hepatic drug metabolism in female Fischer rats as
a function of age. Drug Metab Dispos, 17: 114-116.
Rikans L (1989b) Effects of ethanol on microsomal drug metabolism in
aging female rats. I. Induction. Mech Ageing Dev, 48: 267-280.
Rikans LE & Moore DR (1987) Effect of age and sex on allyl alcohol
hepatotoxicity in rats: role of liver alcohol and aldehyde
dehydrogenase activities. J Pharmacol Exp Ther, 243: 20-26.
Rikans LE & Moore DR (1988) Effect of aging on aqueous phase
antioxidents in tissues of male Fischer rats. Biochem Biophys Acta,
966: 269-275.
Rikans LE & Snowden CD (1989) Influence of aging on acute ethanol
hepatotoxicity in female rats. Pharmacologist, 31: 177.
Ritschel WA (1983) Pharmacokinetics in the aged. In: Pagliaro LA &
Pagliaro AM ed. Pharmacologic aspects of aging. St. Louis, Missouri,
C.V. Mosby & Co., pp 219-256.
Ritschel WA (1988) Gerontokinetics: The pharmacokinetics of drugs in
the elderly. Caldwell, New Jersey, Telford Press, 114 pp.
Robertson IGC & Birnbaum LS (1982) Age-related changes in mutagen
activation by rat tissues. Chem-Biol Interact, 38: 243-252.
Robertson DRC, Waller DG, Renwick AG, & George CF (1988) Age-related
changes in the pharmacokinetics and pharmacodynamic of nifedipine.
Br J Clin Pharmacol, 25: 297-305.
Rodgers J & Gass G (1983) The effect of age on serum proteins in
mice. Exp Gerontol, 14: 169-173.
Roe DA (1983) Drugs and nutrition in the elderly. In: Geriatric
nutrition, 2nd ed. Englewood, New Jersey, Prentice-Hall, Inc., pp
176-200.
Rogers J & Bloom FE (1985) Neurotransmitter metabolism and function
in the aging central nervous system. In: Finch CE & Schneider EL ed.
Handbook of the biology of aging, 2nd ed. New York, Van Nostrand
Reinhold Company, pp 645-691.
Rogers GS & Gilchrest BA (1990) The senile epidermis: environmental
influences on skin ageing and cutaneous carcinogenesis. Br J
Dermatol, 122(suppl 35): 55-60.
Rogers J, Magistretti PJ, & Bolis LC (1991) Animal models for aging
research. Neurobiol Aging, 12(spec issue): 625-701.
Rose MR (1991) Evolutionary biology of aging. New York, London,
Oxford University Press.
Rosenberger RF & Kirkwood TBL (1986) Errors and the integrity of
genetic information transfer. In: Kirkwood TBL, Rosenberger RF, &
Galas DJ, ed. Accuracy in molecular processes. London, Chapman and
Hall, pp 17-35.
Ross MH (1961) Length of life and nutrition in rat. J Nutr, 75:
197-210.
Ross MH (1976) Nutrition and longevity in experimental animals. In:
Winick M ed. Nutrition and aging. New York, Chichester, Brisbane,
Toronto, John Wiley and Sons, pp 23-41.
Ross R (1981) Atherosclerosis: A problem of the biology of arterial
wall cells and their interactions with blood components.
Arteriosclerosis, 1: 293-311.
Roth GS (1979a) Hormone action during aging: alterations and
mechanisms. Mech Ageing Dev, 9: 497-514.
Roth GS (1979b) Hormone receptor changes during adulthood and
senscence: significance for aging research. Fed Proc, 38: 1910-1914.
Roth GS (1988) Receptor and postreceptor changes during lymphocyte
aging. In: Platt D ed. Blood cells, rheology, and aging. Berlin,
Heidelberg, New York, Springer-Verlag, pp 150-154.
Roth GS & Joseph JA (1988) Peculiarities of the effect of hormones
and transmitters during aging: Modulation of changes in dopaminergic
action. Gerontology, 34: 22-28.
Rothstein M & Seifert SC (1981) RNA synthesis. In: Handbook of
biochemistry in aging. Boca Raton, Florida, CRC Press, pp 51-63.
Rouser G, Kritchevsky G, & Yamamoto A (1972) Lipids in the nervous
system of different species as a function of age: Brain, spinal
cord, peripheral nerve, purified whole cell preparations and
sub-cellular particulates: Regulatory mechanisms and membrane
structure. Adv Lipid Res, 10: 261-360.
Rowe JW, Minaker KL, Paollotta J, & Fliers JS (1983)
Characterization of the insulin resistance of aging. J Clin Invest,
71: 1581-1587.
Roy AK, Nath TS, Motwani NM, & Chatterjee B (1983) Age dependent
regulation of the polymorphic forms of alpha2u-globulin. J Biol
Chem, 258: 10123-10127.
Rudman D (1987) Assessment of nutritional status. In: Braunwald E,
Isselbacher KJ, Petersdorf RG, Wilson JD, Martin JB, & Fauci AS ed.
Harrison's principles of internal medicine, 11th ed. New York,
McGraw-Hill, Chapter 71, pp 309-392.
Rudman D (1988) Kidney senescence: A model for aging. Nutr Rev,
46(6): 209-214.
Runcie J (1985) Obesity. In: Pathy MWSJ ed. Principles and practice
of geriatric medicine. New York, Chichester, Brisbane, Toronto, John
Wiley and Sons, Chapter 22.1, pp 277-284.
Russell DH, Buckley AR, Shah GN, Sipes IG, Blask DE, & Benson B
(1988) Hypothalamic site of action of
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Toxicol Appl Pharmacol,
94: 496-502.
Sacher GA (1977) Life table modification and life prolongation. In:
Finch CE & Hayflick L ed. Handbook of the biology of aging. New
York, Van Nostrand Reinhold Company, pp 582-638.
Sagiv M, Goldhammer E, Abinader EG, & Rudoy J (1988) Aging and the
effect of increased after-load on left ventricular contractile
state. Med Sci Sports Exercise, 20(3): 281-284.
Sample PA, Esterson FD, Weinreb RN, & Boynton RM (1988) The aging
lens: in vivo assessement of light absorption in 84 human eyes.
Invest Ophtalmol Visual Sci, 29(8): 1306-1311.
Sapolsky R, Armanini M, Packan D, & Tornbaugn G (1987) Stress and
glucocorticoids in aging. Endocrinol Metab Clin, 16(4): 965-979.
Sapolsky RM, Krey LC, & McEwen BS (1986) The adrenocortical axis in
the aged rat: Impaired sensitivity to both fast and delayed feedback
inhibition. Neurobiol Aging, 7: 331-335.
Sato Y, Kanai S, & Kitani K (1987) Biliary excretion of ouabain in
aging male and female F344 rats. Arch Gerontol Geriatr, 6: 141-152.
Saxton JA Jr & Kimball GC (1941) Relation to nephrosis and other
diseases of albino rat to age and to modifications of diet. Arch
Pathol, 32: 951-965.
Scheibel AB & Tomiyasu U (1978) Dendritic sprouting in Alzheimer
presenile dementia. Exp Neurol, 60: 1-8.
Schiff I & Wilson E (1979) Clinical aspects of aging of the female
reproductive system. Aging, 4: 10-28.
Schmucker D (1979) Age-related changes in drug disposition. Pharm
Rev, 30: 445-456.
Schmucker DL & Wang RK (1979) Rat lysosomal enzymes: effects of
animal age and phenobarbital. Age Aging, 2: 93-96.
Schmucker DL & Wang RK (1989) Dietary restriction postpones the
age-dependent compromise of male rat liver microsomal
monooxygenases. Prog Clin Biol Res, 287: 283-288.
Schmucker DL, Woodhouse KW, Wang RK, Wynne H, James QF, McManus M, &
Kremers P (1990) Effects of age and gender on in vitro properties
of human liver microsomal monoxygenases. Clin Pharmacol Ther, 48:
365-377.
Schneider EL (1978) The aging reproductive system. New York, Raven
Press, vol 4.
Schneider EL & Reed JD (1985) Life extension. New Engl J Med, 312:
1139-1168.
Schneider EL, Sternberg H, Tice RR, Senula GC, Kra D, Smith JR, &
Bynum G (1979) Cellular replication and aging. Mech Ageing Dev, 9:
313-324.
Schuetz EG, Wrighton SA, Barwick JL, & Guzelian PS (1984) Induction
of cytochrome P-450 by glucocorticoids in rat liver. I. Evidence
that glucocorticoids and pregnenolone 16 alpha-carbonitrile regulate
de novo synthesis of a common form of cytochrome P-450 in cultures
of adult rat hepatocytes and in the liver in vivo. J Biol Chem, 259:
1999-2006.
Schumann AM, Fox TR, & Watanabe PG (1982a) A comparison of the fate
of inhaled methyl chloroform (1,1,1-trichloroethane) following
single or repeated exposure in rats and mice. Fundam Appl Toxicol,
2: 27-32.
Schumann AM, Fox TR, & Watanabe PG (1982b) Methylchloroform
(1,1,1-trichloroethane): Pharmacokinetics in rats and mice following
inhalation exposure. Toxicol Appl Pharmacol, 62: 390-401.
Schuurman HJ, Krajnc-Franken MAM, Kuper CF, Van Loveren H, & Vos JG
(1990) Immune system. In: Haschek-Hoch WM & Rousseaux CG ed.
Fundamentals of toxicologic pathology. New York, London, San
Francisco, Academic Press, pp 1-103.
Searle CE (1976) Chemical carcinogens. Washington, DC, American
Chemical Society (ACS Monograph No. 173)
Segre D, Miller RA, Abraham GN, Weigle WO, & Warner HR (1989) Aging
and the immune system. J Gerontol, 44(6): 8164-8168.
Sekuler R, Kline D, & Dismuskes K (1982) Aging and human visual
functions. New York, Alan R. Liss, Inc.
Sellers EM, Frecker RC, & Romach MK (1983) Drug metabolism in the
elderly: confounding of age, smoking, and ethanol effects. Drug
Metab Rev, 14: 225-250.
Selmanowitz VJ, Rizer RL, & Orentreich N (1977) Aging of the skin
and its appendages. In: Finch CE & Hayflick L ed. Handbook of the
biology of aging. New York, Van Nostrand Reinhold Company, pp
496-509.
Selye H (1950) Stress: The physiology and pathology of exposure to
stress. Montreal, Acta Inc.
Semsei I, Rao G, & Richardson A (1989) Changes in the expression of
superoxide dismutase and catalase as a function of age and dietary
restriction. Biochem Biophys Res Commun, 164: 620-625.
Senda S, Cheng E, & Kawanishi H (1989) IgG in murine intestinal
secretions. Aging effect and possible physiological role. Scand J
Immunol, 29(1): 41-47.
Shen DX, Ren R, Zhao YR, & Ma XZ (1987) [Disease pattern analysis in
hospitalized elderly patients in Jingzhou.] Chin J Geriatr, 6: 44
(in Chinese).
Shepherd AMM, Wilson N, & Stevenson IH (1979) Warfarin sensitivity
in the elderly. In: Crooks J & Stevenson IH ed. Drugs and the
elderly. Baltimore, Maryland, University Park Press, pp 199-209.
Sherlock S, Bearn AG, Billing BH, & Paterson JCS (1955) Splanchnic
blood flow of peripheral plasma bromosulphthalein method: The
relation of peripheral bromosulphthalein level to the calculated
flow. J Lab Clin Med, 35: 923-932.
Shih JC & Young H (1978) The alteration of serotonin binding sites
in aged human brain. Life Sci, 23: 1441-1448.
Simmons DL, McQuiddy P, & Kasper CB (1987) Induction of the hepatic
mixed-function oxidase system by synthetic glucocorticoids -
Transcriptional and post-transcriptional regulation. J Biol Chem,
262: 326-332.
Slagboom PE & Vijg J (1989) Genetic instability and aging: theories,
facts, and future perspectives. Genome, 31: 373-385.
Smith DBD, Thompson LW, & Michalewski HJ (1980) Average evoked
potential research in adult aging - status and prospects. In: Aging
in the 1980s. Washington, DC, American Psychological Association, pp
135-151.
Smith EL, Sempos CT, & Purvis RW (1981) Bone mass and strengh
decline with age. In: Smith EL & Serfass RC ed. Exercise and aging:
the scientific basis. Hillside, New Jersey, Enslow Publishers, pp.
Sonntag WE & Gough ME (1988) Growth hormone releasing hormone
induced release on growth hormone in aging male rats: dependence on
pharmacological manipulation and endogenous somatostatin release.
Neuroendocrinology, 47: 482-488.
Sonntag WE, Steger RW, Forman LJ, & Meites J (1980) Decreased
pulsatile release of growth hormone in old male rats. Endocrinology,
107: 1875-1879.
Sonntag WE, Hylka V, & Meites J (1985) Growth hormone restores
protein synthesis in skeletal muscle of old rats. J Gerontol, 40:
689-694.
Spagnoli LG, Orlandi A, Manuello A, Santeusanio G, Angelis C,
Lucreziotti R, & Ramacci MT (1991) Aging and atherosclerosis in the
rabbit 1. Distribution, prevalence and morphology of atherosclerotic
lesions. Atherosclerosis, 89: 11-24.
Spearman ME & Leibman KC (1983) Hepatic and pulmonary cytosolic
metabolism of epoxides: effects of aging on conjugation with
glutathione. Life Sci, 33: 2615-2625.
Spearman ME & Leibman KC (1984) Aging selectivity alters
glutathione-S-transferase isozyme concentrations in liver and lung
cytosol. Drug Metab Dispos, 12: 661-671.
Speijers GJA (1983) [Toxic effects of rapeseed oil, erucic acid and
linolenic acid in the rat.] Utrecht, The Netherlands, University of
Utrecht, pp 1-143 (in Dutch).
Speijers GJA (1989a) [Literature review on experimental
atherosclerosis research.] Bilthoven, The Netherlands, National
Institute Public Health and Environmental Protection (in Dutch).
Speijers GJA (1989b) [Toxicological studies of ergot alkaloids.] De
Ware(n) Chem, 19(3): 189-194 (in Dutch).
Spencer PS & Ochoa J (1981) The mammalian peripheral nervous system
in old age. In: Johnson GJ Jr ed. Aging and cell structure. New
York, London, Plenum Press, pp 35-103.
Spencer PS, Nunn PB, Hugon J, Ludolph AC, Ross SM, Roy DN, &
Robertson RC (1987). Guam amyotrophic lateral
sclerosis-Parkinsonism-Dementia linked to a plant excitant
neurotoxin. Science, 237: 465-466.
Steiner RA, Bremner WJ, Clifton DK, & Dorsa DM (1984) Reduced
pulsatile luteinizing hormone and testosterone secretion in the male
rat. Biol Reprod, 31: 251-258.
Stenbäck F, Peto R, & Shubik P (1981) Initiation and promotion at
different age and doses in 2200 mice: III. Linear extrapolation from
high doses may underestimate low-dose tumor risk. Br J Cancer, 44:
23-34.
Stevenson IH & Hosie J (1985) Pharmacokinetics in the elderly. In:
O'Malley K & Waddington JL ed. Therapeutics in the elderly.
Amsterdam, Oxford, New York, Elsevier Science Publishers, pp 35-41.
Stevenson IH, Salem SAM, & Shepherd AMM (1979) Studies on drug
absorption and metabolism in the elderly. In: Crooks J & Stevenson
IH ed. Drugs and the elderly. Baltimore, Maryland, University Park
Press, pp 51-63.
Stevenson JC, Whitehead MI, Padwick M, Endacott JA, Suttonm C, Banks
LM, Freemantle C, Spinks TJ, & Hesp R (1988) Dietary intake of
calcium and postmenopausal bone loss. Br Med J, 297: 15-17.
Stiles J & Tyler WS (1988) Age-related morphometric differences in
responses of rat lungs to ozone. Toxicol Appl Pharmacol, 92:
274-285.
Stohs SJ, Al-Turk WA, & Angle CR (1982) Glutathione-S-transferase
and glutathione reductase activities in hepatic and extra-hepatic
tissues of female mice as a function of age. Biochem Pharmacol, 31:
2113-2116.
Strong R, Moore MA, Hale C, Wessels-Reiker M, Armbrecht HJ, &
Richardson A (1990) Modulation of tyrosine hydroxylase gene
expression in the rat adrenal gland by age and reserpine. Brain Res,
525(1): 126-132.
Sun J & Strobel HW (1986) Aging affects the drug metabolism systems
of rat liver, kidney, colon, and lung in a differential fashion. Exp
Gerontol, 21: 523-534.
Sun J, Lau PO, & Strobel HW (1986) Aging modifies the expression of
hepatic microsomal cytochrome P450 after pretreatment of rats with
b-naphoflavone or phenobarbital. Exp Gerontol, 21: 65-73.
Sweeny DJ & Weiner M (1985) Metabolism of acetaminophen in
hepatocytes isolated from mice and rats of various ages. Drug Metab
Dispos, 13: 377-379.
Sweeny DJ & Weiner M (1986) Effect of aging on the metabolism of
p-nitroanisole and p-nitrophenyl in isolated rat hepatocytes. Age,
9: 95-98.
Swenberg GJA, Richardson FC, Boucheron JA, Deal FH, Belinsky SA,
Charbonneau M, & Short BG (1987) High- to low-dose extrapolation:
critical determinants involved in the dose response of carcinogenic
substances. Environ Health Perspect, 76: 57-63.
Swift CG (1985) Benzodiazepine pharmacodynamics in the elderly. In:
O'Malley K & Stevenson IL ed. Therapeutics in the elderly.
Amsterdam, Oxford, New York, Elsevier Science Publishers, pp 77-90.
Tannenbaum SR, Fett D, Young VR, Land PD, & Bruce WR (1978) Nitrite
and nitrate are formed by endogenous synthesis in the human
intestine. Science, 200: 1478-1489.
Tarloff JB, Goldstein RS, Milo BA, & Hook JB (1989a) Role of
pharmacokinetics and metabolism in the enhanced susceptibility of
middle-aged Sprague-Dawley rats to acetaminophen nephrotoxicity.
Drug Metab Dispos, 17: 139-146.
Tarloff JB, Goldstein RS, Morgan DG, & Hook JB (1989b) Acetaminophen
and p-aminophenol nephrotoxicity in aging male Sprague-Dawley and
Fischer 344 rats. Fundam Appl Toxicol, 12: 78-91.
Taylor A (1989) Associations between nutrition and cataract. Nutr
Res, 47: 225-234.
Telford N, Mobbs CV, Osterburg HH, & Finch CE (1988) Alterations in
hypothalamic serotonergic-catecholaminergic relationships in aging
C57BL/6J female mice. Exp Gerontol, 23: 481-489.
Tenover JS, Matsumoto AM, Clifton DK, & Bremner WJ (1988)
Age-related alterations in the circadian rhythms of pulsatile
luteinizing hormone and testosterone secretion in healthy men. J
Gerontol, 43: M163-M169.
Terry RD (1963) The fine structure of neurofibrillary tangles in
Alzheimer's disease. J Neuropathol Exp Neurol, 22: 269-292.
Thakur MD (1984) Age-related changes in the structure and function
of chromatin: A review. Mech Ageing Dev, 27: 263-286.
Thakur MK (1988) Molecular mechanism of steroid hormone action
during aging. A review. Mech Ageing Dev, 45: 93-110.
Thompson JS, Robbins J, & Cooper JK (1987) Nutrition and immune
function in the geriatric population. Clin Geriatr Med, 3(2):
309-317.
Tice RR & Setlow RB (1985) DNA repair and replication in aging
organisms and cells. In: Finch CE & Schneider EL ed. Handbook of the
biology of aging, 2nd ed. New York, Van Nostrand Reinhold Company,
pp 173-224.
Timiras PS, Hudson DB, & Segall PE (1984) Lifetime brain serotonin:
Regional effects of age and precursor availability. Neurobiol Aging,
5: 235-242.
Tomlinson BE & Irving D (1977) The numbers of limb motor neurons in
the human lumbosacral cord throughout life. J Neurol Sci, 34:
213-219.
Travaglini P, Mocchegiani E, Togni E, Muratori M, Re T, Bazzani N, &
Fabris N (1990) Thymulin and zinc circulating level in patient with
GH and PRL secreting pituitary adenomas. Int J Neurosci, 51:
269-271.
Travis CC, White RC, & Ward RC (1990) Interspecies extrapolation of
pharmacokinetics. J Theor Biol, 142: 285-307.
Trick LR (1987) Age-related alterations in retinal function. Doc
Ophthalmol, 65(1): 35-43.
Triggs EJ & Nation RL (1975) Pharmacokinetics in the aged: A review.
J Pharmacokinet Biopharm, 3: 387-418.
Turusov VS & Parfenov YuD (1986) [Methods of revealing and risk
assessment of chemical carcinogens.] Moscow, Meditsina, pp 134-136
(in Russian).
Ulloa-Aguirre A & Chappel SC (1982) Multiple species of
follicle-stimulating hormone exist within the anterior pituitary of
male golden hamsters. J Endocrinol, 95: 257-266.
Ulloa-Aguirre A, Espinoza R, Damian-Matsumura P, & Chappel SC (1988)
Immunological and biological potencies of the different molecular
species of gonadotrophins. Hum Reprod, 3: 491-501.
US Senate Special Committee on Aging (1986) Aging America: Trends
and projections, 1985-86 edition. Washington, DC, US Government
Printing Office, pp 14-17.
Utiger RD (1980) Decreased extrathyroidal triiodothyronine
production in monthyroidal ilness: benefit or harm? Am J Med, 69:
807-810.
Van Bezooijen CFA (1984) Influence of age-related changes in rodent
liver morphology and physiology on drug metabolism - A review. Mech
Ageing Dev, 25: 1-22.
Van Bezooijen CFA, Groen C, Stijnen AM, & Danhof M (1989) The effect
of age on the pharmacokinetics and pharmacodynamics of drugs in
rats. Age, 12: 112.
Vanhaelst L, Van Cauter E, Degaute JP, & Goldstein J (1972)
Circadian variations of serum thyrotropin levels in man. J Clin
Endocrinol Metab, 35: 489-492.
Van Manen R, De Priester W, & Knook DL (1983) Lysosomal activities
in aging rat liver. I. Variation in enzyme activity within the liver
lobule. Mech Ageing Dev, 22: 159-165.
Velican C (1981) Are animal models suitable for the study of
ischaemic heart disease. Rev Roum Méd Méd Interne, 19(1): 5-19.
Vellas B, Balas D, Guidet M, Moreau J, Senegas F, Bouisson M,
Albarede JL, & Ribet A (1988) Vieillissement du pancréas. Son
implication dans les états de dénutrition chez les personnes agées.
Presse Méd, 17: 2067-2069.
Vermeulen A, Deslypere JP, & Kaufman JM (1989) Influence of
antiopioids on luteinizing hormone pulsatility in aging men. J Clin
Endocrinol Metab, 68: 68-72.
Verzar F (1968) Intrinsic and extrinsic factors of molecular aging.
Exp Gerontol, 3: 69-75.
Vestal RE (1978) Drug use in the elderly: a review of problems and
special considerations. Drugs, 16: 358-382.
Vestal RE, McGuire EA, Tobin JD, Andres R, Norris AH, & Ulzey E
(1977) Aging and ethanol metabolism. Clin Pharmacol Ther, 21:
343-354.
Vestal RE, Wood AJJ, & Shand DG (1979) Reduced b-adrenoceptor
sensitivity in the elderly. Clin Pharmacol Ther, 26: 181-186.
Vestal RE, Jue SG, & Cusack BJ (1985) Increased risk of adverse drug
reactions in the elderly: Fact or myth. In: O'Malley K & Waddington
JL ed. Therapeutics in the elderly. Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp 97-104.
Vijg J (1990) DNA sequence changes in aging: How frequent, how
important? Aging, 2: 105-123.
Vijg J & Knook DL (1987) DNA Repair in relation to the aging
process. J Am Geriatr Soc, 35(6): 532-541.
Vijg J & Papaconstantinou J (1990) Aging and longevity genes:
strategies for identifying DNA sequences controlling life span. J
Gerontol, 45(5): B179-B182.
Vincenzini MT, Iantomasi T, Stio M, Favelli F, Vanni P, Tonelli F, &
Treves C (1989) Glucose transport during ageing by human intestinal
brush-border membrane vesicles. Mech Ageing Dev, 48: 33-41.
Vogel HB (1983) Effects of age on the biomechanical and biochemical
properties of rat and human skin. J Soc Cosmet Chem, 34: 453-463.
Voitenko VP & Tokar AV (1983) The assessment of biological age and
sex differences of human aging. Exp Aging Res, 9: 239-244.
Vos JG & Luster MI (1989) Immune alterations. In: Kimbrough RD &
Jensen AA ed. Halogenated biphenyls, terphenyls, naphtalenes,
dibenzodioxins and related products. Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp 295-322.
Vos JG & Penninks AH (1987) Dioxin and organotin compounds as model
immunotoxic chemicals. In: De Matteis F & Lock EA ed. Selectively
and molecular mechanism of toxicity. London, MacMillan Press Ltd, pp
85-102.
Vos JG, De Klerk A, Krajnc EI, Van Loveren H, & Rosing J (1990)
Immunotoxicity of Bis (tri-n-butylin) oxide in the rat; effects on
thymus-dependent immunity and on non specific resistance following
long-term exposure in young versus aged rats (1990). Toxicol Appl
Pharmacol, 105: 144-155.
Wabner CL & Chen TS (1984) Renal transport of organic acids in the
aging. Pharmacologist, 26: 206.
Waddington JL, O'Boyle KM, Molloy AG, Youssef HA, King DJ, & Cooper
SJ (1985) Neurotransmitter receptors and ageing:
Dopamine/neuroleptic receptors, involuntary movements and the
disease process of schizophrenia. In: O'Malley K & Stevenson IL ed.
Therapeutics in the elderly. Amsterdam, Oxford, New York, Elsevier
Science Publishers, pp 63-76.
Waggoner S, Gu MZ, Chiang WH, & Richardson A (1990) The effect of
dietary restriction on the expression of genes. In: Genetic effects
of aging, II. Caldwell, New Jersey, Telford Press, pp 255-273.
Wagner PA, Jernigan JA, Bailey LB, Nickens C, & Brazzi GA (1983)
Zinc nutriture and cell-mediated immunity in the aged. Int J Vitam
Nutr Res, 53: 94-101. Walford RL (1969) The immunological theory of
aging. Copenhagen, Munksgaard.
Walker DM (1985) Oral disease. In: Pathy MSJ ed. Principles and
practice of geriatric medicine. New York, Chichester, Brisbane,
Toronto, John Wiley and Sons.
Wang SV, Halban PA, & Rowe JW (1988) Effects of aging on insulin
synthesis and secretion. Differential effects on preproinsulin
messenger RNA levels, proinsulin biosynthesis, and secretion of
newly made and preformed insulin in the rat. J Clin Invest, 81(1):
176-184.
Ward JM (1983) Increased susceptibility of livers of aged F344/NCr
rats to the effects of phenobarbital on the incidence, morphology,
and histochemistry of hepatocellular foci and neoplasms. J Natl
Cancer Inst, 71: 815-823.
Ward WF (1988) Enhancement by food restriction of liver protein
synthesis in the aging Fischer 344 rat. J Gerontol, 43: B50-B53.
Ward W & Richardson A (1991) Effect of age on liver protein
synthesis and degradation. Hepatology, 14: 935-948.
Ward JM, Lynch P, & Riggs C (1988) Rapid development of
hepatocellular neoplasms in aging male C3H/HeNCr mice given
phenobarbital. Cancer Lett, 39: 9-18.
Warner BA, Dufau ML, & Santen RJ (1985) Effects of aging and illness
on the pituitary testicular axis in men: Qualitative as well as
quantitative changes in luteinizing hormone. J Clin Endocrinol
Metab, 60: 263-268.
Warner HR, Butler RN, Spratt RW, & Schneider EL (1987) Modern
biological theories of aging. New York, Raven Press.
Weale RA (1986) Aging and vision. Vision Res, 26(9): 1507-1512.
Weber JCP & Griffin JP (1986) Prescriptions, adverse reactions, and
the elderly. Lancet, i: 1220.
Webster SGP (1985) Absorption of nutrients in old age. In: Pathy MSJ
ed. Principles and practice of geriatric medicine. New York,
Chichester, Brisbane, Toronto, John Wiley and Sons, Chapter 11.2, pp
265-290.
Webster IW & Logie AR (1976) A relationship between function and
health status in female subjects. J Gerontol, 31: 546-550.
Weigent DA, Carr DJ, & Blalock JE (1990) Bidirectional communication
between the neuroendocrine and immune system. Common hormones and
hormone receptors. Ann NY Acad Sci, 579: 17-27.
Weindruch R & Walford RL (1988). The retardation of aging and
dietary restriction. Springfield, Illinois, Charles C. Thomas.
Weindruch R, Gottesman SR, & Walford RL (1982) Modification of
age-related immune decline in mice dietarily restricted from or
after mid-adulthood. Proc Natl Acad Sci (USA), 79: 898-902.
Weiss B, Clark MB, & Greenberg LH (1984) Modulation of
catecholaminergic receptors during development and aging. In: Lajtha
A ed. Handbook of neurochemistry. Vol 6:- Receptors in the nervous
system. New York, London, Plenum Press, pp 595-627.
Wellinger R & Guigoz Y (1986) The effect of age on the induction of
tyrosine aminotransferase and tryptophan oxygenase genes by
physiological stress. Mech Ageing Dev, 34: 203-217.
Wesson LG Jr (1969) Renal hemodynamics in physiological states. In:
Physiology of the human kidney. New York, London, Grune and
Stratton, pp 98-100.
White L (1989) Epidemiology. In: Maddox GL ed. The encyclopedia of
aging. Berlin, Heidelberg, New York, Springer-Verlag, pp 216-223.
WHO (1983) Environmental Health Criteria 27: Guidelines on studies
in environmental epidemiology. Geneva, World Health Organization,
351 pp.
WHO (1984) The uses of epidemiology in the study of the elderly.
Report of a WHO Scientific Group on the Epidemiology of Aging.
Geneva, World Health Organization, pp. 23-25 (WHO Technical Report
Series 706).
WHO (1987) World health statistics annual. Geneva, World Health
Organization.
WHO (1989) Health of the elderly. Report of a WHO Expert Committee.
Geneva, World Health Organization, pp 14-30 (WHO Technical Report
Series 779).
WHO (1990) Global estimates for health situation assessment and
projections, 1990. Geneva, World Health Organization, Division of
Epidemiological Surveillance and Health Situation and Trend
Assessment, p 3.
Williams ME & Pannill FC (1982) Urinary incontinence in the elderly.
Ann Intern Med, 97: 895-907.
Willott JF (1986) Effects of aging, hearing loss, and anatomical
location on thresholds of inferior colliculus neurons in C57BL/6 and
CBA mice. J Neurophysiol, 56(2): 391-408.
Wilson K & Hanson J (1980) The effects of extremes of age on drug
action. Methods Findings Exp Clin Pharmacol, 2: 303-312.
Winneke G, Hrdina KG, & Brockhouse A (1982) Neuropsychological
studies in children with elevated tooth-lead concentrations. A pilot
study. Int Arch Occup Environ Health, 51: 169-183.
Wong DF, Wagner HN, & Dannals RF (1984) Effects of age on dopamine
and seratonin receptors pressured by positron tomography in the
living human brain. Science, 226: 1393-1396.
Wood RW (1981) Neurobehavioral toxicity of carbon disulfide.
Neurobehav Toxicol Teratol, 3: 397-405.
Wood AJJ (1985) Beta adrenoceptors and aging. In: O'Malley K &
Stevenson IL ed. Therapeutics in the elderly. Amsterdam, Oxford, New
York, Elsevier Science Publishers, pp 43-49.
Wu W, Pahlavani M, Cheung HT, & Richardson A (1986) The effect of
aging on the expression of interleukin 2 messenger ribonucleic acid.
Cell Immunol, 100: 224-231.
Wynne HA, Cope LH, Mutch E, Rawlins MD, Woodhouse KW, & James OF
(1989) The effect of age upon liver volume and apparent liver blood
flow on healthy man. Hepatology, 9: 927-301.
Xiong ZM (1989) [Common diseases and major causes of death in
elderly patients in Jiangxi.] Chin J Geriatr, 8: 135 (in Chinese).
Xiong BZ (1990) Technology progress, aging process and reappoint to
a position.] In: Institute of Japanese Problems, Chinese Academy of
Social Sciences ed. [Proceeding of Symposium on Sino-Japan Social
Countermeasure for Aging Problem.] Beijing, East Publishing House,
pp 186-196 (in Chinese).
Xu XF, You K, Liu D, He L, Cheng S, & Wu JS (1986) [Disease pattern
analysis in hospitalized elderly patients in Uromqi.] Chin J
Geriatr, 5: 15 (in Chinese).
Yamazoe Y, Shimada M, Murayama N, & Kato R (1987) Suppression of
levels of phenobarbital-inducible rat-liver cytochrome P-450 by
pituitary hormone. J Biol Chem, 262: 7423-7428.
Yang RSH, Tallant MJ, & McKelvey JA (1984) Age-dependent
pharmacokinetic changes of ethylenediamine in Fischer 344 rats
parallel to a two-year chronic toxicity study. Fundam Appl Toxicol,
4: 663-670.
Yao SB (1990) [The trend and characters of Chinese aged population.]
In: Institute of Japanese Problems, Chinese Academy of Social
Sciences ed. [Proceedings of Symposium on Sino-Japan Social
Countermeasure for Aging Problems.] Beijing, East Publishing House,
pp 64-74 (in Chinese).
Yates MS & Hiley CR (1979) The effect of age and cardiac output and
its distribution in the rat. Experientia (Basel), 35: 78-79.
Yelland C, Summerbell J, Nicholson E, Herd B, Wynne H, & Woodhouse
KW, (1991) The association of age with aspirin esterase activity in
human liver. Age Ageing, 20(1): 16-8.
York JL (1982) Body water content, ethanol pharmacokinetics, and the
responsiveness to ethanol in young and old rats. Dev Pharmacol Ther,
4: 106-116.
Young VR (1979) Diet as modulator of aging and longevity. Fed Proc,
38: 1994-2000.
Yu BP, Bertrand HR, & Masoro EJ (1980) Nutrition-aging influence of
catecholamine-promoted lipolysis. Metabolism, 29: 438-444.
Yumura W, Sugino N, Nagasawa R, Kubo S, Hirokawa K, & Maruyama N
(1989) Age-associated changes in renal glomeruli of mice. Exp
Gerontol, 24: 237-249.
Zapadnyuk VI (1971) [On an age-periodisation of laboratory animals.]
In: [Gerontology and geriatrics. 1970-1971 Annual book: Aging of the
cell.] Kiev, Research Institute of Gerontology, pp 433-438 (in
Russian).
Zarit SH, Eiler J, & Hassinger M (1985) Clinical assessment. In:
Birren JE & Schaie KW ed. Handbook of the psychology of aging, 2nd
ed. New York, Van Nostrand Reinhold Company, pp 725-754.
Zaphiropouos PG, Mode A, Norstedt G, & Gustavsson JA (1989)
Regulation of sexual differentiation in drug and steroid metabolism.
Trends Pharmacol Sci, 10: 149-153.
Zatz MM & Goldstein AL (1985) Thymosins, lymphokines and the
immunology of aging. Gerontology, 31: 263-277.
Zemel MB & Sowers JR (1988) Salt sensitivity and systemic
hypertension in the elderly. Am J Cardiol, 61: 7H-12H.
Zhou L-W, Weiss B, Freilich JS, & Greenberg LH (1984) Impaired
recovery of alpha - and alpha2-adrenergic receptors in brain tissue
of aged rats. J Gerontol, 39: 538-546.
Zhu JR, Li WX, & Wang YY (1982) [Common diseases and major causes of
death in the elderly with analysis of 8947 cases over 65 years of
age.] Chin J Geriatr, 1: 110-118 (in Chinese).
Zs-Nagy I, Kitani K, Ohta M, & Imahori K (1986) Age-dependent
decrease in lateral diffusion constant of proteins in the plasma
membrane of hepatocytes as revealed by fluorescence recovery after
photobleaching in tissue smears. Arch Gerontol Geriatr, 5: 131-146.
APPENDIX 1. Background Papers
Anisimov, V.N., Approaches to examine the effects of chemicals on
the aged population: experimental approaches.
Birnbaum, L.S., Basis of altered sensitivity of the elderly to
chemicals - pharmacokinetics and pharmacodynamics.
Cooper, R.L., & Goldman, J.M., Alterations in susceptibility to
toxic compounds in the aged central nervous system and endocrine
system.
Dilman, V.M., Theories and mechanics of aging.
Fabris, N., Systemic biology of aging.
Ingram, D.K., Evaluating effects of chemical exposure on aging:
development of conceptual models.
Li, S., Aged population: demographic, life expectancy, and
lifestyle.
Likhachev, A.J., Age related peculiarities of repair of DNA damage
with carcinogens.
Martin, G.M., Definitions on aging.
Ray, P.K., Jaffery, F.N., & Viswathan, P.N.,
1. Chemical exposure of the elderly
2. Modifying factors: nutrition, state of health, life style,
alcohol intake, smoking and ionizing radiation.
Richardson, A., The molecular biology of aging: translation,
transcription and chromatin structure.
Speijers, G.J.A., Groups at risk and their chemical exposure as well
as nutrition as a source of chemicals and as a confounding factor.
Zhu, J.R., Approaches to examine the effects of chemicals on the
aged population: epidemiological and clinical approaches.
It is commonly assumed that today's large percentage of elderly
people in the population is a result of increased longevity and
decreased birth rate. For example, in Japan the proportion of people
aged 65 or more had increased to 10.3% in 1985. At the same time,
the values were 15.1%, 14.5%, 12.4% and 16.9% in the United Kingdom,
Federal Republic of Germany, France and Sweden, respectively. Japan
is a new "aged-type" country with the greatest rate of increase in
the elderly in the world. By the year 2025, the proportion of the
Japanese population aged 65-plus will rise to 23.4% (Hosomi, 1990).
In China, the proportion of the population aged 60 and over was
7.3% of the total population in 1953. By 1984 it had increased to 9%
of the total population, and by the year 2000 the proportion of the
population 60 and over will be as high as 10.6%. The proportion is
predicted to increase to 26.2% by 2025 (Xiong, 1990).
At present, the aged population is growing more rapidly in
China than in countries of Europe and North America. According to
data from the US Bureau of the Census, it took 115 years (1865-1980)
for the proportion of people aged 65 or more in France to increase
from 7% to 14%, 85 years (1890-1975) in Sweden, 66 years (1944-2010)
in the USA, 45 years (1930-1975) in the United Kingdom, and 26 years
(1970-1996) in Japan. For China, the pattern of the growing number
of elderly is similar to Japan, i.e. the proportion of people aged
65 or more will be 7.4% in 2000, and by 2025 it will have increased
to 12.8% (Hosomi, 1990; Yao, 1990; Xiong, 1990).
1.4.2 Life expectancy
Life expectancy at birth is a statistical index calculated by
the use of a life table from the age-specific death rates of the
population. This illustrates the overall level of health in a
country or a region. Throughout this century, it has been evident
that, as a result of improvements in many aspects of health status,
an individual can expect to live longer. From 1960 to 1990, life
expectancy at birth for the total population had increased by 13.5
years. The life expectancy at birth for the period 1985-1990 was
estimated to be 63.9 years for the world as a whole, 74.0 years for
the more developed regions, and 61.4 years for the less developed
regions. The longest life expectancies are in Japan (78.3), Iceland
(77.5), Sweden (77.1), Switzerland (77.1), and the Netherlands
(76.9) (World Population Prospects, 1991).
The trend for life expectancy at birth in the USA showed an
increase from 1900 (46.4 for males and 49.4 for females) to 1950
(65.6 and 71, respectively) and in the year 2000 is expected to be
72.1 and 79.5 (for males and females). By 2050, it may have
increased to 73.6 for males and 81 for females (US Senate Special
Committee on Aging, 1986).
In China, the life expectancy at birth before 1949 was about 35
years of age, being one of the lowest in the world at that time. By
1957 it had increased to 57 years, from 1973 to 1975 it was 63.6
years for males and 66.3 years for females, and in 1981 it was 68
years. It is expected that the continued increase in the average
life expectancy of the people of China will be slow due to a high
death rate from cardiovascular diseases (Gu, 1986).
1.4.3 Life-style in aged populations
A basic issue in planning for the consequences of demographic
aging is whether elderly people should be considered a specific
target group for the development of services, or whether their needs
should be catered for within the context of planning for the
population as a whole. One approach to a rational policy for this
issue is to consider the nature of human aging. For this it is
necessary to view the physical, psychological and sociological
dimensions of aging as a whole.
Life-style influences the effects of chemicals on human health,
including that of the elderly, both quantitatively and
qualitatively. Environmental chemicals and their uses are diverse.
Specialized nutritional elements of the diet have become popular,
while in certain countries many people prefer predominantly
vegetarian diet. Others take supplements and additives which contain
pure preparations of vitamins, minerals, amino-acids and other
substances. Whether such substances have either adverse or
beneficial effects on the elderly and aging processes, has not
generally been fully evaluated. Another important source of human
exposure to chemicals comes from the intake of different kinds of
cosmetic agents and fragrances, such as shampoos, creams, perfumes,
oral deodorants, sunscreen and suntan lotions, and insect
repellents. These are often chemical mixtures whose components have
not been evaluated or tested beyond acute toxic potential.
Occupational status, indoor air quality, recreational
activities, exercise, eating and drinking habits, alcohol
consumption, and tobacco smoking can all affect the elderly and
aging processes to a certain degree. Elements of life-style can
strengthen or reduce the risk of developing aged-related
degenerative diseases. They can also accelerate or delay
physiological and anatomical changes. Typical examples are the
various age-related diseases caused by toxic chemicals in tobacco
smoke and the reduction of the risk of cardiovascular diseases
produced by regular exercise (Committee on Chemical Toxicity and
Aging, 1987).
As far as the possible influence of life-style factors on the
manifestations of aging is concerned, many studies have shown that
loneliness and physical and intellectual inactivity are common among
the elderly, especially widowed people. Several studies have
revealed that living conditions have an influence on health and
well-being, resulting in an increase in the demand for social care
and medical service. Marital status and living arrangements have
important significance for the unique life-style of the elderly.
There are striking differences between the proportions of elderly
males and females who are married: in many countries the proportion
of married males is twice that of married females. In general, the
proportion of widows is very high and that of widowers relatively
low (WHO, 1984).
Migration is also one of the life-style variables of the
elderly. In rural areas of Asia, many older women move to cities to
join their children after they have been widowed. Another common
type of move is the migration of the recently widowed or chronically
ill elderly from urban areas to their home towns or villages. For
many countries in Africa and Asia, the urban-rural migration is most
apparent among males, who return from urban to rural areas when they
are old. Worldwide, only a minority of elderly people live in urban
areas (WHO, 1984).
1.5 Theories of aging
During the last century, more than 100 various hypotheses
concerning the origin and mechanism of aging have been put forward.
All of them could be grouped generally into two broad categories:
those that invoke deterministic, or "programmed", alterations in
gene expression or gene structure; and those that invoke a variety
of stochastic, or "random", alterations in the structure and
function of macromolecules, cells, and organ systems. This
distinction, however, has some limitations, because stochastic
alterations in individual cells can lead to predictable phenomena in
the large populations of cells. The use of terminal differentiation
to explain the limited replicative life span of somatic cells
(Martin et al., 1974) could be an example of the blurring of the
stochastic and non-stochastic categories. For each individual cell,
differentiation is a random event; however, for a population of
cells, the process appears deterministic.
The mechanisms of aging are likely to be coupled to the
reproductive strategy of the organism. One example is the
synchronous, rapid physiological declines and mortalities that are
characteristic of species with single massive episodes of
reproduction (e.g., migrating Pacific salmon or soybean plants).
Placental mammals, however, have ample opportunity for a variety of
stochastic processes to take place during their long reproductive
and postreproductive phases. The associated patterns of structural
and functional decline can vary substantially, both qualitatively
and quantitatively, among individuals within a species and among
different related species. Evolutionary biologists in fact present
compelling arguments that aging did not evolve because of any
adaptive value to the individual or to the species, as would be
assumed by strictly programmed theories (reviewed in Rose, 1991).
Aging is thought to occur simply because of the decline in the force
of natural selection for gene action that is postreproductive. Such
gene action could be related to accumulations of late-acting
mutations in the constitutional genome or to selection for forms of
genes that have positive effects on reproductive fitness early in
the lifespan, but whose effects may be negative late in the lifespan
(the "antagonistic pleiotropy" theory of aging) (Rose, 1991).
It is beyond the scope of this monograph, however, to consider
the potentially large numbers of specific mechanisms that may be
modulated by such accumulated constitutional mutations or
pleiotropic genes. The reader is referred to recent reviews of the
many postulated theories of aging (Warner et al., 1987; Committee on
Chemical Toxicity and Aging, 1987; Finch, 1991; Cutler, 1991).
These can be classified in a variety of ways (Dilman, 1987;
Medvedev, 1990).
2. STRUCTURAL AND PHYSIOLOGICAL CHANGES IN THE AGED
2.1 Changes in gene structure and function in aging
Changes in gene expression are of critical importance to an
organism. Aging can potentially alter not only the structure of
genes, but the way in which they function. Changes in the DNA are
often thought to be integral to aging. It is clear that not only
mutations, but chromosomal rearrangements accumulate with age (Vijg,
1990). Repetitive sequence families may play a crucial role in the
processes of aging. In addition, the organization of DNA and protein
in chromatin is important structurally and functionally. Therefore,
changes in chromatin could play a major role in the age-related
change in the regulation of gene expression (Richardson et al.,
1983; Medvedev, 1984; Thakur, 1984; Richardson et al., 1985).
2.1.1 Chromatin structure
Chromatin changes may involve either proteins that interact
with DNA or the chemical structure of the DNA molecule itself.
Although no change in the stoichiometry of the major histones has
been observed with increasing age (Richardson et al., 1983;
Medvedev, 1984), several investigators have reported changes in the
subspecies of histone H1 (Medvedev, 1984; Mitsui et al., 1980;
Niedzwiecki et al., 1985). The acetylation of histones, which has
been proposed to alter histone-DNA interactions thereby making DNA
more accessible, decreases by 30% to 70% with increasing age
(O'Meara & Pochron, 1979).
With respect to age-related changes in DNA chemical structure,
there is now conclusive evidence for the "spontaneous" induction of
a variety of DNA lesions in different organs and tissues of both
humans and experimental animals (for a review, see Mullaart et al.,
1990). Most of these lesions seem to be repaired (see below), but
not all. For example, Cathcart et al. (1984) and Fraga et al. (1990)
estimated that in rats about 105 oxidative DNA lesions occur per
cell per day. Since the rate of repair does not entirely equal the
rate of induction of damage, there is a net increase of spontaneous
DNA lesions with age. Fraga et al. (1990) calculated for one
specific lesion, 8-hydroxy-deoxyguanosine, that about 80 residues
accumulate per rat cell per day.
Although some DNA lesions are repaired quickly, this is not the
case for all lesions. Indeed, after treating rats with low doses of
2-acetyl-aminofluorene (AAF), Mullaart et al. (1989) were still able
to detect about 30% of the major lesions induced as late as 21 days
after treatment. Such incomplete repair could be responsible for
accumulation of DNA lesions during continuous or frequent exposure to
genotoxic agents.
2.1.2 DNA repair
To preserve the DNA chemical structure, cells are equipped with
a battery of repair systems to remove damage. As yet the various
mechanisms of action of these DNA repair systems and their
interrelationships are incompletely understood (for a recent review,
see Lehmann et al., 1992). In general, repair systems can be divided
into three categories, i.e. direct repair, excision repair and
post-replication repair. In direct repair, the lesion itself is
removed without any further (transient) changes in the DNA
structure. Direct repair includes the enzymatic photo-reactivation
of UV-induced pyrimidine dimers and the removal of O6-alkyl
adducts by specific alkyl transferases.
DNA excision repair is brought about by a complex multi-enzyme
system, the components of which are involved in the various steps in
this repair process (Vijg & Knook, 1987). The third type of repair,
post-replication repair, does not actually remove the damage but
allows the replication system to bypass the damage. It is this
latter process especially that is considered to be associated with
nucleotide misincorporation (mutation).
Accurate assessment of an organism's capacity to repair
specific lesions is difficult and subject to error. In general, the
most reliable data can be obtained when the induction and
disappearance of the relevant lesions themselves are monitored in
the different organs and tissues of an experimental animal.
Unfortunately, in most studies on the possible existence of a
decline in DNA repair activities with age, assays were used which
measured the DNA synthesis phase of excision repair. The general
conclusion from these data, mostly obtained with cultured cells, is
that there is no age-related decline in the efficiency of DNA repair
systems (Tice & Setlow, 1985; Likhachev, 1985; Hanawalt, 1987). It
cannot be ruled out, however, that during aging DNA repair systems
become more error prone, leading to an accelerated induction of
mutations (Vijg & Knook, 1987). In any case, a certain degree of
imperfection is a general characteristic of DNA repair systems as
indicated by the actual accumulation of both DNA lesions and DNA
sequence changes (see above). The question that should be addressed
is what type of DNA alterations occur, how many exist, and at what
rate do they accumulate with age. Finally, their relevance in terms
of actual physiological decrements or the initiation of disease
should be assessed.
2.1.3 Transcription
Several review articles have been published in the past decade
that discuss the effect of age on transcription (Rothstein &
Seifert, 1981; Richardson et al., 1983; Richardson et al., 1985;
Richardson & Semsei, 1987; Slagboom & Vijg, 1989). A major problem
in this area has been the difficulty in accurately measuring the
rates of synthesis of specific RNA species and their intracellular
levels. With the major advances in recombinant DNA technology, this
problem has now been virtually eliminated and our knowledge of how
aging affects the expression of specific genes is rapidly growing.
At present, it appears that the overall transcriptional
activity of a cell declines as an organism ages. However, the level
of total RNA tends to remain constant suggesting a decline in the
rate of RNA turnover (Horbach et al., 1986).
The levels of some specific mRNA species using cDNA probes for
specific genes have been measured recently (Richardson & Semsei,
1987). In general, no consistent trend has emerged. The levels of
some mRNA species decrease with age; however, other mRNA species do
not change with age, and others actually increase (Slagboom & Vijg,
1989).
In most of the studies, a good correlation has been found
between the age-related changes in the level of an mRNA species and
the level of protein (or enzyme activity) specified by the mRNA
species. This has been demonstrated in rat liver for albumin
(Horbach et al., 1984), apha2u-globulin (Richardson et al., 1987),
and superoxide dismutase and catalase (Semsei et al., 1989), and in
rat kidney and small intestine for calbindin-D (Armbrecht et al.,
1989). The age-related decline in mitogen-induction of interleukin 2
(IL-2) (Wu et al., 1986; Nagel et al., 1988; Pahlavani et al., 1988)
and IL-3 (Li et al., 1988) mRNA in lymphocytes from rodents and
humans corresponded to the age-related decline in the biological
activities of these two interleukins. In contrast, Strong et al.
(1990) reported an uncoupling of tyrosine hydroxylase transcription
and translation in the adrenal glands of old rats.
Investigators usually assume that age-related changes in the
levels of a particular mRNA species arise from a change in
transcription. However, only a few studies have actually measured
the transcription of a specific gene as a function of age using
nuclear run-off assays. While an age-related decrease occurs in the
nuclear transcription of the alpha2u-globulin (Richardson et al.,
1987; Murty et al., 1988a), cytochrome P450(b+e) (Rath & Kanungo,
1989), and superoxide dismutase and catalase (Semsei et al., 1989)
genes, the nuclear transcription of tyrosine amino-transferase and
tryptophan oxygenase (Wellinger & Guigoz, 1986), albumin (Horbach
et al., 1988b) and the c-myc (Buckler et al., 1988) genes was
similar in young and old rodents. Studies are now underway to
explore in more detail age-changes in specified mRNA species in
terms of the transcription factors involved (Post et al. 1991).
One exciting development in the area of transcription and aging
has been the observation that dietary restriction, which enhances
the longevity of rodents, alters the expression of some genes at the
level of transcription (Richardson et al., 1987; Semsei et al.,
1989). However, the expression of all genes is not affected by
dietary restriction (Waggoner et al., 1990).
In addition to nuclear synthesis, post-transcriptional
processing of hnRNA plays an important role in the regulation of
gene expression. Müller et al. (1989) recently discussed various
views of how the post-transcriptional processing of hnRNA might
alter with age. At present, there is little evidence that major
changes occur with age in the size of the poly(A)-segment of mRNA
(Birchenall-Sparks et al., 1985). Interestingly, in the many studies
in which mRNA species have been analysed by Northern blot analysis,
there has been not a single report of a significant change in the
size of the mRNA species examined with increasing age (Richardson &
Semsei, 1987). Thus, there is very little direct evidence at present
to support the view that the processing and/or nuclear transport of
hnRNA is altered with age.
2.1.4 Translation
Increasing age generally results in a decrease in total protein
synthesis in plants, invertebrates, rodents and cultured cells
(Richardson & Birchenall-Sparks, 1983; Ward & Richardson, 1991).
Recent studies have focused on the influence of age on the
translation of mRNA into specific proteins and on the ability to
modulate age changes in protein synthesis. There is no evidence that
a decrease in the fidelity of protein synthesis occurs with
advancing age but technical limitations do not permit a definitive
conclusion (Rosenberger & Kirkwood, 1986). The influence of age on
protein synthesis differs from protein to protein and much more work
must be done in assessing the effect on key individual proteins.
Attempts to modulate protein synthesis have recently begun. The rate
of protein synthesis in the liver is higher after maturity for
dietary restricted than for ad libitum fed rats (Ward, 1988). In
an in vitro system, growth hormone increases protein synthesis in
muscles of old rats to the level found in muscles of young rats
(Sonntag et al., 1985). Much more study is required, focusing on
individual proteins, different tissues and different organisms.
2.2 Changes in tissues, organs and systems in aging
The progressive modification of body functions with age
involves alterations not only at the genetic, molecular and cellular
levels, but at the level of the tissues, organs, systems and entire
organism. It is important to attempt to differentiate between
age-related pathology and true physiological aging. This is often
difficult because the majority of age-related changes increase the
vulnerability of the aging organism to disease and ultimately death.
In the following, each organ or system will be discussed in
reference to age-related changes in its structure which might
predispose to alterations in function, not only inherently as part
of aging, but in response to environmental agents. The focus will be
on the healthy aged as opposed to the diseased.
2.2.1 Nervous system
The brain may undergo a progressive deterioration with age at
all levels of organization - structural, biochemical and functional.
CNS disorders, including Parkinson's and Alzheimer's diseases, are
common in the elderly.
2.2.1.1 Structural changes
Brain weight decreases slightly with aging. This is due to
atrophy of both grey and white matter (Creasey & Rapoport,1985). At
the cellular level, the major age-associated modification is in the
number of neurons, which are significantly diminished in discrete
areas of the brain (Brizzee, 1985), particularly in the basal
ganglia, cerebellum (probably related to decreased motor control),
locus ceruleus (associated with alterations in sleep patterns),
nucleus basalis of Meynert (associated with senile dementia of
Alzheimer type (SDAT) (Bondareff, 1986), and the spinal cord.
Neuronal loss, which is associated with an increase in the number of
glial cells, is relatively mild in the healthy aged, but is much
more severe in SDAT, Parkinson's disease and in the early aging
associated with Down's syndrome.
In addition to a reduced number of neurons, the aged brain is
characterized by a reduction in the number of dendrites and
dendritic spines, probably due to a slowing renewal process
(Scheibel & Tomiyasu, 1978). Synapse density declines in discrete
areas of the brain, but this is partially compensated by enlargement
of the remaining synapses (Bertoni-Freddari et al., 1990).
Intracellular changes include dilation and fragmentation of the
Golgi apparatus (Mervis, 1981), distortion of membranes and the
nucleus, and accumulation of lipofuscin, in both neurons and glial
cells within discrete brain areas. With advancing age, there is an
increase in neurofibrillary tangles (intracellular tangled masses of
paired helical filaments) (Terry, 1963), extracellular neuritic
plaques (a core of amyloid surrounded by material derived from
dystrophic neurites), and reactive glial and microglial cell
accumulation (Master et al., 1985). Again, these changes occur in
normal aging at a moderate level, but are much more frequent in SDAT
(Iqbal et al., 1982) and other dementias.
There are also age-related changes in the morphology of the
peripheral and autonomic nervous systems. These include reductions
in the number of sensory and motor neurons, increases in
demyelination, increases in connective tissue, and a mild loss of
myelinated fibres (Tomlinson & Irving, 1977; Spencer & Ochoa, 1981).
The central processes of dorsal root ganglion cells typically
undergo distal dystrophic and degenerative changes. Regressive
changes have been reported in the terminals of motor axons.
2.2.1.2 Biochemical changes
Besides the pathological changes, there are many age-related
alterations in brain chemistry required for cell-to-cell
communications (Rogers & Bloom, 1985; Finch, 1991). These include
changes in the concentration and/or turnover of the amines (e.g.,
acetylcholine, norepinephrine, epinephrine, dopamine, serotonin),
amino acids (e.g., glycine, glutamate and GABA) and peptides (e.g.,
enkephalin, substance P, thyrotropin-releasing hormone,
cholecystokinin, somatostatin). There are numerous studies showing
impairments of adrenergic, dopaminergic and serotonergic activity in
the senescent animal (Zhou et al., 1984; Roth & Joseph, 1988;
Telford et al., 1988). One of the underlying causes of these
alterations seems to be an overall loss of receptors (Weiss et al.,
1984; Roth & Joseph, 1988).
Synapses may utilize one or more neuromodulator (e.g.,
norepinephrine and neuropeptide). The multiple levels of control and
the regional diversification of different synapses in discrete brain
regions make it difficult to define the age-related alterations in
neurotransmitter/neuropeptide function. In fact, rather than a
uniform drop in the level of a specific neurotransmitter throughout
the nervous system, a "desynchronization" of signals may occur. For
example, while the brain content of norepinephrine and dopamine are
decreased in old age, that of serotonin is unchanged or even
increased, depending on specific brain areas. In some cases, the
greater the concentration of a neurotransmitter in a discrete brain
region, the higher the decrement with aging and vice versa (Timiras
et al., 1984). In fact, age-dependent alterations in different
neurotransmitter/neuropeptide concentrations do not always occur
simultaneously. Each neurotransmitter has its own timetable:
dopamine levels decrease in the cerebral hemispheres of rats from
the age of one year, whereas in the same areas serotonin levels
remain unaffected until three years of age (Timiras et al., 1984).
Aged-related changes in neurotransmitter receptor number and
function have also been reported (Greenberg & Weiss, 1983; Roth &
Joseph, 1988). Changes in binding affinity have not been frequently
detected. Beta-adrenergic receptor responsiveness is decreased in
the elderly (Vestal et al., 1979; Lakatta, 1980). This appears to be
due to uncoupling of the beta-receptor from the adenylate cyclase
complex which transmits the signal (Wood, 1985). In the rat pineal
gland, corpus striatum and cerebellum, a reduced responsiveness to
catecholamines is present due to a decrease in the affinity of
beta-adrenergic receptors to their ligand. However, there is no
change in receptor number (Greenberg & Weiss, 1978). This may be due
in part to a reduced ability to increase the number of
beta-adrenergic receptors after decreased noradrenergic input
(Greenberg & Weiss, 1979).
Changes in general biochemical properties of the cells occur in
the nervous system as they do elsewhere in the aging organism.
Lipid composition may change, resulting in altered membrane
viscosity. Protein synthesis decreases in discrete brain regions.
Lipofuscin accumulates, although the functional significance is
unclear, and there are alterations in electrolytes and trace
elements (Brizzee, 1985). For example, aluminium levels may increase
sharply in elderly people (Bjorksten et al., 1989). Decreases in
zinc may be important in the light of the zinc requirement of
various enzymes and growth factors, including nerve growth factor
(NGF) (Dunn et al., 1980). In addition, aging is accompanied by a
decreased brain water content (Meisami, 1988). Alterations in
vascular flow have also been reported (Katzman & Terry, 1983).
2.2.1.3 Functional changes
Despite the morphological and biochemical changes observed in
the aging brain, the functional efficiency of the nervous system
seems to be well maintained in most elderly people. However, CNS
disorders do occur in some individuals, though it may be difficult
to discriminate age-related pathology from physiological aging
phenomena. Perhaps the most ubiquitous and significant change
observed in the older organism is slowness of behaviour (Birren et
al., 1979). The slowing of behaviour with age not only appears in
motor responses and perceptual processing, but is also apparent for
the more complex processing of information associated with
short-term memory (Smith et al., 1980). Related cross-sectional
studies using global measures of intellectual function such as the
Wechsler Adult Intelligence Scale (WAIS) show evidence that some
performance abilities decline by the late 60s and early 70s, while
others (e.g., verbal abilities) appear to be maintained throughout
life in healthy individuals (Gallagher et al., 1980). The slowing of
reaction time may be associated with the age-associated slowing and
loss of coordination in motor tasks, such as those involved in
handwriting and other purposeful movements.
The age-related modification of biorhythms is exemplified by
the alterations of the sleep/wakefulness cycle, which is largely
dependent on the reticular system. Alterations of sleep patterns
with aging are qualitative rather than quantitative (Dement et al.,
1985) and affect primarily the "deep sleep" phases, as confirmed by
the alterations observed in the brain electrical activity (Müller &
Schwartz, 1978). Among neurotransmitters, serotonin seems to be
implicated.
Alterations in posture and locomotion in the elderly (Klawans &
Tanner, 1984) also depend on CNS impairment. Peripheral
modifications such as decreased nerve conduction velocity, reduced
muscle mass and increased rigidity occur. Autonomic system
dysfunction is also implicated in many pathophysiological changes
of age including hypotension, thermoregulation, gastrointestinal
function and urinary incontinence (Finch & Landfield, 1985). Other
changes in the autonomic system include changes in vascular and
cardiac reflexes, galvanic skin responses, and potency (Katzman &
Terry, 1983). Sympathetic hyperactivity is commonly present in the
aged and could interfere with cognitive functioning.
2.2.2 Sensory organs
All of the sensory organs are affected by aging, both those in
which the cells are continuously renewed (such as cutaneous sense
tissues) and those in which the cells are terminally differentiated
early in life (vision and hearing).
2.2.2.1 Vision
Both neural (retina) and optical (cornea, lens, pupil, aqueous
and vitreous humours) components of vision are affected by age. The
changes in the optical compartment are probably the primary cause of
visual impairment in the elderly (Sekuler et al., 1982). The most
common alterations are in the lens with increased hardness and
decreased transparency (Graham, 1985). The former results in reduced
refractive power (Marsh, 1980). The loss of transparency relates to
the following chemical changes in the lens: protein oxidation,
racemization, glycation, aggregation, polymerization and
precipitation (Taylor, 1989). These alterations are associated with
presbyopia and cataracts, respectively.
In the neuronal compartment, the retina undergoes progressive
loss of rods, while cones may be augmented. Morphometric analysis of
the retina demonstrates an increase in electron-dense plaques and a
decrease in the ground substance during aging. Such retinopathies
result in decreased light sensitivity and reduced colour vision
(Marsh, 1980).
Vision declines as a function of age (Weale, 1986) and can be
measured in several tests, such as the Humphrey Field Analyser
(Iwase et al., 1988), and retinal potentials (Trick, 1987). Visual
acuity is substantially decreased. The ability to detect light
gradually decreases (Sample et al., 1988) and light adaptation
declines (Katz & Robinson, 1987).
2.2.2.2 Hearing
Decrements in hearing are frequently observed in the elderly.
There is also a progressive loss of hearing in animals with age
(Willott, 1986). Both auditory structures and neuronal components
are involved. While the outer and middle ear show few modifications,
degenerative changes occur in the hair cells, which are the auditory
receptors, and in the mechano-electrical transducing organs
resulting in otosclerosis. This accounts for the preferential loss
of hearing of high frequency sounds (presbycusis) (Marsh, 1980). The
degree of hearing loss may affect the two ears differentially, thus
causing defects in sound localization. Presbycusis has a great
impact upon speech perception, since consonants, which make speech
intelligible, are generated by high frequency sounds, whereas
vowels, responsible for audibility, are produced by low frequency
sounds.
Hearing defects may also result from changes in the neural
components (Allison et al., 1984) of hearing, and in particular in
the nerves connecting the cochlea with the auditory centres in the
brain, specifically in the superior temporal gyrus.
2.2.2.3 Olfaction
The age-related alteration in the sense of smell is generally
underestimated. The reduction in olfactory sensitivity is mainly due
to the progressive loss of olfactory neurons, which protrude through
cilia from the superior nasal cavity and represent the receptor
sites for odour and the chemo-electrical transducing mechanism
(Naessen, 1971). Loss of neurons have also been demonstrated in the
olfactory bulbs of the brain (Bhatnagar et al., 1987).
2.2.2.4 Taste
Taste thresholds are known to increase with age. The taste of
salt is preferentially altered in the elderly. The loss appears due
both to a decline in the number of taste buds and papillae (Bradley,
1979) in the tongue, as well as to the loss of neurons in the
cerebral centers of the gustatory system.
2.2.2.5 Somatic sensations
The somatic sensory system (touch, pressure, vibration,
proprioception, heat, cold and pain) is variably affected by age.
Tactoperceptual ability and vibrotactile sensations are decreased in
the elderly due to the loss of Meissner end-organs and Pacinian
corpuscles present in the skin (Bruce, 1980). For more complex
somatesthetic abilities (stereognosis, body part recognition) as
well as for pain and thermal sensitivity, the biological causes of
their alterations with age involve not only the sensory end-organs,
but also affective and cognitive factors (Marsh, 1980).
2.2.3 Endocrine system
Hormones play an important, often critical, role in the
regulation of a large number of physiological and behavioural
processes, and their influence can be demonstrated throughout the
lifespan. Some hormones have a role in differentiation in that their
presence or absence during certain developmental periods will affect
the way in which physiological and behavioural processes proceed or
are expressed in adulthood. Throughout each period of the life span,
the maintenance of an appropriate endocrine milieu is essential to
the numerous homeostatic processes required for survival. With
advancing age, there are several, well-documented changes in the
ability of the organism to synthesize and secrete a number of
hormones. It is, therefore, likely that the typical age-related
change in an organism's endocrine balance would result in, or at
least contribute to, the impairment of homeostasis frequently
observed in the elderly. Such impairments can be noted in the
decreased rate of recovery of the elderly from the insults of injury
or disease.
Hormones may also play a significant role in the aging process.
For example, age-related changes in several physiological functions
appear to be closely linked to the level and pattern of hormonal
stimulation present during adulthood. As such, different patterns of
exposure to a hormonal environment may alter the "rate of aging"
within a specific neuroendocrine system and, in turn, affect the
susceptibility of the organism to environmental insults at different
segments of the life span. There are a number of different ways in
which endocrine systems and the hormonal signalling operations that
they use may undergo alterations with age and toxicant exposure.
These can be categorized as changes in: (a) the availability of
hormones for binding to the target tissues, (b) the reception of the
pertinent transmitter or hormonal signal by the target cells, and
(c) the nature of the hormonal message.
At any point in time, the concentration of a hormone in the
blood is a consequence of both its metabolism and secretion. Such
changes in the size of the available signal pool may have
corresponding effects on the magnitude of the response by the target
tissue. Other changes may reflect declines with age in the
homeostatic controls, which rely heavily on endocrine feedback
relationships within organ systems.
Serum hormonal levels, as a rule, are not maintained at
constant levels. They tend to fluctuate, sometimes markedly,
throughout a 24-h period. In the young adult man, peak morning
testosterone values can fall by one-third to an early evening nadir,
before rising again through the late evening and early morning hours
(Bremner et al., 1983). A similar circadian rhythm in circulating
levels of testosterone is prevalent in the rat (e.g., Kinon & Liu,
1973; Ellis & Desjardins, 1982). Human cortisol (Bilchert-Toft,
1978) and rat corticosterone (Moberg et al., 1975; Kato et al.,
1980) concentrations also exhibit well-known rhythmic fluctuations,
as do those of thyrotropin (Vanhaelst et al., 1972; Leppaluoto
et al., 1974) and growth hormone (Millard et al., 1985). Reported
attenuations with age in the rhythms of human and rat serum
testosterone (Bremner et al., 1983; Steiner et al., 1984),
luteinizing hormone (LH) (Vermeulen et al., 1989), and growth
hormone (Sonntag et al., 1980; Prinz et al., 1983), among other
hormones, can present differences in young-versus-old comparisons,
depending on when such sampling is performed.
While observable changes in hormonal rhythms or significant
differences in circulating hormone concentrations may reflect
disturbances in the overall functional integrity of the associated
organ system, the absence of such changes should not be necessarily
assumed to indicate a corresponding absence of a functional
alteration. The notion of a "system at risk" presupposes an increase
in the susceptibility to disruption of the homeostatic controls. An
aging system that may be undergoing a subtle erosion in its
endocrine balance could be more likely to exhibit alterations in its
response to a stressor or toxic insult. In this respect the
stimulation of growth hormone release by clonidine, L-dopa and
insulin is substantially depressed (Riegel & Miller, 1981), while
arginine-stimulated growth hormone (GH) secretion after arginine
infusion is preserved (Aschoff, 1979). Secretion stimulated by GHRH
(GH releasing hormone) is only partially reduced (Coiro et al.,
1991).
Regardless of these alterations, it remains established that
the 24 h production of GH is significantly reduced in elderly humans
(Prinz et al., 1983), whereas that of prolactin is increased
(McGinty et al., 1988; Blackman, 1987). These data have been
confirmed in animals (Ceda et al., 1986; Sonntag & Gough, 1988),
although measurements of hormonal profiles may have involved
different procedures in animals and man, thus giving rise to
slightly different interpretations.
Similar difficulties are encountered in studies of age-related
alterations in pineal hormone secretion, including melatonin, whose
circadian rhythmicity is certainly changed with age (Reiter, 1986;
Anisimov & Reiter, 1990).
In order to illustrate age-related alterations in hormone
control, it is useful to focus on the integrated systems which
involve more than one gland or hormone. Although three such systems
are reviewed below, this discussion is by no means intended to be
comprehensive. One theme common to studies of age-related changes in
endocrine function is that such alterations are often hormone and
species specific. Finally, the extent to which any of these changes
relate to potential adverse health outcomes in the older organism
remains to be demonstrated.
2.2.3.1 The pituitary-thyroid axis and the basal metabolism
Thyroid hormones are required during development for growth and
in adult life for regulating oxygen consumption. Maintenance of
thyroid function is generally assured even in old age, although
following repeated stress and demands the reserve function may
become exhausted and a dysthyroid state may follow (Ingbar, 1978).
Changes with aging in the levels of both thyroid stimulating
hormone (TSH) and thyroid hormones (thyroxine (T4) and
triiodothyronine (T3)) are controversial, because concomitant health
disturbances may cause significant fluctuations in the levels of
these hormones (Gregerman & Solomon, 1967; Utiger, 1980). Both hypo-
and hyperthyroidism are not uncommon in the elderly. In general, the
size of the thyroid decreases with age (Gambert & Tsitouras, 1985).
Older people show a normal response to decreased thyroid
function by increased secretion of TSH (Eden, 1987). TSH levels
undergo few changes (Miller, 1989), suggesting that the hypothalamic
control of TSH release has not been altered. However, structural
modifications of TSH have been reported (Klug & Adelman, 1977). T4
levels remain unchanged with age, even though the rate of synthesis
is reduced. However, the blood levels of T3 are reduced with
advanced age (Chopra et al., 1978), while levels of reverse T3 are
unchanged. It should be noted that severe and chronic illnesses, not
directly involving the thyroid, can lower the levels of T3 and
T4.
The alterations observed in thyroid hormone levels are
inadequate to explain the age-associated decline in various
functions that are dependent on thyroid hormone. One possible
explanation is that peripheral sensitivity to thyroid hormone action
is modified by aging. However, with advancing age, the basal
metabolic rate remains unchanged if based on lean body mass, but
decreases if expressed based on body surface area (Masoro, 1985).
2.2.3.2 The pituitary-adrenal axis
The major function of this axis, which is largely based on
pituitary hormones (ACTH) and adrenal hormones (corticosteroids), is
to provide an adaptive response to environmental stress (Selye,
1950; Sapolsky et al., 1986). Any harmful agent, in addition to
inducing a specific reaction in the body (anaesthesia, emotion,
fever, etc.), activates a specific and common response, the
so-called "General Adaptation Syndrome" (Selye, 1950), characterized
by increased adrenocortical secretion, thymic involution,
lymphopenia and eosinopenia. With advancing age this axis may
undergo desynchronization, thus resulting in a failure of
homeostasis and adaptation (Anisimov & Reiter, 1990).
ACTH secretion, which shows a circadian rhythm based on
melatonin fluctuations, is generally preserved in advanced age
(Halberg, 1982), although minor modifications of blood levels may
occur due to variations in renal clearance or alterations in sleep
patterns. However, there is evidence of diminished sensitivity of
the hypothalamic/pituitary axis feedback inhibition by
glucocorticoids (Greden et al., 1986; Blackman, 1987; Dilman, 1987;
Sapolsky et al., 1987). The elderly suffering from Alzheimer's
disease are extremely resistant to glucocorticoid negative feedback
(Sapolsky et al., 1986).
Finally, certain cell populations (e.g., CA3 neurons in the
hippocampus) are particularly susceptible to glucocorticoids.
Long-term stress may result in their dysfunction and death (Sapolsky
et al., 1987).
2.2.3.3 The endocrine pancreas and carbohydrate metabolism
It is well documented that with advancing age the ability to
maintain glucose homeostasis is impaired, but the underlying
mechanisms are still not well defined. Several hormones contribute
to the regulation of glucose homeostasis: above all, insulin and
glucagon, secreted by the endocrine pancreas, and somatostatins and
the pancreatic polypeptide, which modulate the secretion of insulin
and glucagon, respectively. In addition, glucose metabolism may be
affected by other hormones, including T3 and T4, growth hormone,
glucocorticoids and epinephrine (Minaker et al., 1985).
Only modest morphological alterations are observed in the
endocrine pancreas with advancing age. In spite of this fact, blood
sugar levels after fasting are elevated and glucose tolerance is
lowered in the elderly (Magal et al., 1986; Eden, 1987; Ammon
et al., 1987; Wang et al., 1988; Groop, 1989). Plasma insulin
concentration increases and insulin sensitivity decreases.
Alterations in insulin turnover are detectable in the elderly after
glucose load, such as reduced insulin secretion and increased
secretion of the inactive prohormone, proinsulin (Marx, 1987), but
these changes are too modest to account for the observed glucose
intolerance. One alternative explanation is an increase in
peripheral resistance to insulin. In fact, peripheral uptake of
glucose is indeed reduced in the elderly, due to a reduction in
insulin receptors (Pagano et al., 1981) as well as alterations in
the post-receptor signalling process (Rowe et al., 1983). No
evidence exists regarding the possible involvement of age-related
changes in glucagon affecting glucose intolerance in the elderly.
Other factors, however, may contribute to glucose intolerance.
These include: (a) reduced liver sensitivity to insulin, resulting
in reduced glycogenesis; (b) changes in diet and physical exercise;
and c) increased body fat with reduced muscle mass. This last point
seems to merit particular consideration in view of the observation
that insulin resistance is certainly increased in obese humans
(Runcie, 1985). In fact, intracellular fat accumulation leads to a
reduced concentration of insulin receptors (Bolinder et al., 1983).
2.2.4 Reproductive system
The age-related modifications of the reproductive system are
primarily based on alterations in the central nervous system,
pituitary gland and gonads. While menopause is a time-fixed event
involving cessation of ovarian function, the decline of testicular
function is a slow and gradual process, involving limited hormonal
alterations. Older persons show the normal response to deficient
gonadal function by increased synthesis of gonadotropins (Piva
et al., 1987). This occurs in both sexes. In fact, alterations in
serum levels of both luteinizing hormone (LH) and follicle
stimulating hormone (FSH) have been reported (Blackman, 1987). The
reduced presence of sex steroids in women may have an influence on
the function of other endocrine glands. Estrogens have
well-documented effects on salt and water balance and on plasma
proteins, which in turn have effects on the level of thyroid
hormones through a suppression of TSH secretion. Estrogen also
stimulates the production of growth hormone and prolactin. Thus, the
decline in gonadal function during age could have far-reaching
consequences on the individual's physiological function.
2.2.4.1 Female aging
In females, cessation of ovarian function consists of the
transfer from regular menstrual cycles to amenorrhoea, usually
preceded by a period of cycle irregularity. The initial changes have
been reported to occur in hypothalamic-pituitary control of the
ovaries. For example, the age-related decline in reproductive
function is associated with a decreased sensitivity of the
hypothalamic-pituitary complex to feed-back regulation by estrogens
(Dilman, 1971, 1987). This leads to an age-related enhancement of
pituitary gonadotropins (FSH, LH) (Chakravarty et al., 1976),
leading in turn to hyperstimulation of the ovaries. However, despite
the compensatory increase in ovarian hormone production, the level
of estrogens is insufficient to induce ovulation because of
hypothalamic insensitivity, possibly due to an age-related decrease
in the level of biogenic amines and/or peptide hormone receptors
(Dilman & Anisimov, 1979). In addition, the progressive loss of
oocytes plays an important role in the decline in reproductive
function since the reduction of maturating oocytes may induce
desynchronization of pituitary-ovary hormonal interactions
(Aschheim, 1976).
The most common consequences of menopause include imbalances of
the autonomic nervous system, psychological modifications, and
physiological alterations of target organs due to metabolic changes.
Alterations of estrogen target organs are among the most evident
effects of menopause. Vulvar skin and vaginal epithelium may undergo
atrophy. Glycogen content is generally reduced, with a consequent
decrease of lactobacilli, rise of vaginal pH, and increased growth
of pathogenic microbes. The uterus and oviducts atrophy due to the
decreases in estrogen levels. In ovaries, follicular cysts and
atresia result in response to the altered hormonal status.
Hyperplasia of the theca cells occurs. Fibrosis also occurs in these
tissues but, in addition, can affect the bladder and urethra,
resulting in an increased incidence of cystitis, dysuria and
non-infectious urethritis. The reduced thickness of the skin is also
a result of the decrease in estrogen (Schiff & Wilson, 1979).
Menopause has major health consequences for the cardiovascular
and skeletal systems. The reduction in estrogen secretion removes
the protection offered by these hormones against coronary heart
disease, development of atherosclerosis, and accompanying
alterations of lipid metabolism. Osteoporosis, resulting from
increased bone reabsorption relative to bone formation, is a common
problem in postmenopausal women (Riggs, 1987). Two types of
osteoporosis may be identified. Type I is associated with estrogen
withdrawal and may begin in middle age (Riggs & Melton, 1983). The
biological effects are linked to disruption of the complex
relationship between calcium intake and loss, and the secretion of
calcitonin, parathyroid hormone and 1,25-dihydroxy-vitamin D.
Estrogens prevent the transfer of calcium from bone to blood and its
loss through urine. This induces parathyroid hormone secretion,
which stimulates the formation of 1,25-dihydroxycholecalciferol, the
active metabolite of vitamin D. The function of the parathyroid is
affected by increasing age (Eden, 1987). The relevance of estrogen
for bone loss is further supported by the effectiveness of estrogen
therapy in delaying the osteoporotic process in post-menopausal
women (Edman, 1983). With advancing age type II osteoporosis (senile
osteoporosis) may occur, which is probably due to the poor
intestinal absorption of calcium (Riggs, 1987).
2.2.4.2 Male aging
The reproductive system is less affected by aging in males than
in females. It is generally accepted that testosterone levels are
maintained within the physiological range throughout life, although
a decrease in testosterone production in response to gonadotropin
action may occur in old age due to a reduction in Leydig cell number
and function (Harman et al., 1982). Testis and accessory sex organs
do not show substantial modifications with age, and sperm is found
in the ejaculate of very elderly men. The volume of seminal fluid is
generally decreased.
While the prostate undergoes involution in the majority of old
men, in about one-third of males it undergoes hypertrophy with
consequent obstruction of the urethra and urinary flow from the
bladder. The cause of the hypertrophy is still unclear (Mawhinney,
1985). The prostatic enlargement results in compensatory hypertrophy
of the bladder. When such compensation is no longer sufficient,
retrograde filling of the renal pelvis and ureters may occur,
resulting in hydronephrosis and eventually renal failure.
2.2.5 Immune system
With advancing age a progressive increase occurs in the
incidence of various infectious diseases, autoimmune processes and
tumours. These may be in part based on age-related defects in the
immune system. The association of so many age-related pathologies
with defects in the immune system has led to the suggestion that
aging of the immune system may be rate limiting for life span
(Walford, 1969). However, while there are numerous experimental and
clinical studies demonstrating an age-related deterioration in
immune efficiency, this decline is not sufficient to account for all
manifestations of aging.
There are several recent reviews on aging and the immune system
(Revskoy et al., 1985; Lipschitz, 1987; Segre et al., 1989; Miller,
1991). However, it is still difficult to draw a comprehensive
picture, because of the many cellular and humoral components
involved in immune reactions and the many modulating
extra-immunological factors which may also be compromised in the
elderly. The immune and haematopoietic systems are intimately
related, being derived from a common pluripotent stem cell. Both
play central roles in host defense, prevention of neoplasia, and
response to infectious agents (Lipschitz, 1987). However, basal
haematopoiesis in both animal models and man seems to be either
unchanged or minimally altered with age (Dybkder et al., 1981;
Lipschitz, 1987). The reserve capacity may be reduced resulting in a
decreased ability to respond to stress.
2.2.5.1 Aging of lymphoid organs
Peripheral lymphoid organs, such as the spleen and lymph nodes
do not show consistent modifications in size with aging. Bone marrow
is not consistently affected by age. Stem cell production is
generally well preserved in old age (Harrison et al., 1978),
although a slight change in the replication rate of stem cells has
been reported by some authors (Schneider et al., 1979). Thymic
involution has been considered to account for the major age-related
changes in the immune system, beginning at puberty. Such an
involution consists of a progressive loss of cellularity with
lymphoid cell depletion in the cortical areas and cystic changes in
the epithelial cells. These are the source of various peptides
involved in differentiating thymic lymphocytes (T-cells) from
lymphoid cells of earlier lineage. The export of newly
differentiated T-cells is reduced with advancing age (Globerson
et al., 1989). The synthesis and the secretion of polypeptide thymic
hormones, such as thymosin (McClure et al., 1982), thymopoietin
(Lewis et al., 1978) and thymulin (Bach et al., 1972), are
progressively diminished. In all cases, the reduction of thymic
endocrine activity seems to have a pathogenic role in age-related
immune dysfunctions, since replacement by exogenous administration
of the hormones is capable of restoring various immune functions in
old age (Zatz & Goldstein, 1985). The turnover of zinc, which is
essential for immunocompetence (Iwata et al., 1979; Chandra, 1985),
decreases in old age. Zinc supplementation can restore immune
functions (Fabris et al., 1990).
2.2.5.2 Aging of cellular constituents
Mature T-cells, bone marrow lymphocytes (B-cells) and natural
killer cells (NK-cells) can be detected in blood and in lymphoid
organs by specific monoclonal antibodies. With this type of
analysis, no major modifications in the proportion of the various
lymphoid cell subpopulations have been observed in humans. However,
the major alteration in the immune system appears to arise in the
functioning of T-cells (Thompson et al., 1987). While the total
number of T-cells in the peripheral blood does not change
appreciably with age, there are clear-cut differences in the
relative proportion of T-cell subtypes (Wagner et al., 1983;
Fernandes, 1984; Revskoy et al., 1985; Thompson et al., 1987;
Lipschitz, 1987).
The number of immature lymphocytes of the T-lineage increases
with age, as does the percentage of apparently activated
T-lymphocytes bearing immature thymic phenotypic markers. There is a
relative increase in cytotoxic/suppressor T-cells, and a decrease in
the number of helper/inducer T-cells (Lipschitz, 1987; Thompson et
al., 1987). Correlated with the decrease in the helper/inducer
population, is a functional defect in cell-mediated immunity
(Lipschitz, 1987; Thompson et al., 1987). Cells from aged humans or
experimental animals are less capable of responding to allogeneic
lymphocytes, phytohaemagglutinin, concanavalin A and soluble
antigens. Lymphocytes from older mice are less able to elicit
graft-versus-host reactions than those from younger mice of the same
inbred strains (Thompson et al., 1987). Fifty percent of healthy
people over age 50 have impaired cutaneous hypersensitivity
(Lipschitz, 1987; Dilman, 1987). Accompanying the decrease in
helper/inducer T-cells and cell-mediated immune functions is a rise
in autoantibodies and autoimmunity (Thompson et al., 1987).
Changes in humoral immunity (B-cell function) with aging are
more subtle (Lipschitz, 1987; Senda et al., 1989). Studies on the
effects of age on antibody production have yielded conflicting
results, perhaps because of the wide range of experimental values
generally observed in older individuals. It has, however, been well
established that aging is significantly associated with the presence
of various autoantibodies, in particular, antibodies against nuclear
antigens. There is also evidence that aging effects the rate of
antibody production by activated B-cells (Lipschitz, 1987).
From a functional point of view, defects have been observed at
various levels. Firstly, the proliferative capacity of T-cells from
old individuals is generally reduced, regardless of the stimuli used
(antigens, mitogens), and the defect consists both in a reduced
number of cells responding to stimulus and in a precocious
exhaustion of the cloning capacity (Fabris et al., 1983) of
responding cells. Secondly, the response to interleukins, which
physiologically mediate the modulation of the proliferative
reaction, is depressed and this phenomenon has been documented not
only for T-cells but also for NK cells, which are less sensitive in
old age to the boosting action of IL-2 or interferons (Provinciali &
Fabris, 1990).
With respect to accessory cells (phagocytic cells,
macrophages), their number and function are not altered by age, and,
in certain circumstances, their activity seems to be enhanced.
2.2.5.3 Neuroendocrine-immune interactions
The immune system, although regulated to a large extent by
intrinsic cellular and humoral events, is also sensitive to signals
generated from the nervous and endocrine systems. Communication
between nervous and immune networks is mediated by hormones and
neurotransmitters which reach lymphoid organs and cells via blood or
direct autonomic nervous system connections (Bullock, 1985; Felten
et al., 1985). The neuroendocrine immune interactions are mediated
by circulating humoral factors from the
pineal-hypothalamic-pituitary axis, either directly via
neuropeptides and hormones, or indirectly by the effects of this
axis on the hormonal secretion of peripheral endocrine glands, which
also exert immunomodulating actions (for review, see Fabris, 1991).
The nervous and neuroendocrine systems not only act as
modulators of the immune network, but also as targets for signals
generated within the immune system, such as those exerted by thymic
factors (Hall et al., 1989) and interleukins, (Besedovsky et al.,
1985), and by pituitary-like factors (ACTH, TSH, GH, PRL,
gonadotropins, endorphins), which are produced by mature lymphocytes
upon antigenic stimulation (Weigent et al., 1990).
The sharing of humoral signals, as well as of the specific
receptors between neuroendocrine and immune cells (for reviews, see
Fabris & Provinciali, 1989; Weigent et al., 1990), implies that
biological response modifiers of neuroendocrine-immune origin might
be developed in the near future for therapeutical purposes. On the
other hand, potentially harmful agents for one of these homeostatic
systems may also cause alterations in others.
From an experimental point of view, it has been demonstrated that
treatments of old animals with thyroid hormones (Fabris et al., 1989),
GH (Kelley et al., 1986) and analogues of LH releasing hormone
(Greenstein et al., 1987) are able to induce regrowth of the thymus
and reacquisition of its endocrine activity (for review, see Fabris
1991). Analogous treatments, such as with melatonin (Pierpaoli
et al., 1991), GH (Davila et al., 1987), TSH and thyroid hormones
(Provinciali & Fabris, 1990), are also able to recover various
age-related peripheral immune deficiencies, such as T-cell
functioning and NK cytotoxicity. In humans, little work has been
done in this area, although indirect evidence, obtained primarily
from studies on endocrinopathies in the elderly (Fabris et al.,
1989; Travaglini et al., 1990), suggest that recovery of both thymic
and peripheral immune function can be achieved by a neurohumoral
approach.
Little is known on the potential effect of thymosins,
interleukins and lymphocyte-derived pituitary-like cytokines on
age-related alterations of the nervous and of the neuroendocrine
system. Experimental information from old animals showing recovery
of the hormonal and metabolic profile following immune manipulation
(for review, see Fabris et al., 1988) is undoubtedly opening a new
research approach for human investigations.
Receptor sites for many hormones are present on the membrane of
lymphoid cells (Fabris & Provinciali, 1989). The number of
glucocorticoid receptors in spleen cells decreases in old animals
(Roth, 1979a). Hormones that modify the turnover of cyclic
nucleotides result in consequent activation or inhibition of immune
functions (Hadden, 1983). Hormones influence the production of
several lymphokines and monokines (Kelso & Munck, 1984).
The neuro-endocrine system seems to act not only as a modulator
of the immune network but also as a target for signals generated
within the immune system. Examples of such interactions are the
alterations that can be induced in the neuroendocrine balance,
either by removal of relevant lymphoid organs such as the thymus or
by dysfunction of the immune system itself as a result of reactions
to immunogenic or tolerogenic doses of antigen (Besedovsky et al.,
1975). In addition, mature lymphoid cells, when stimulated by
antigens, produce humoral factors similar, if not identical, to
classical hormones and neurotransmitters (such as ACTH, TSH, GH,
PRL, gamma-endorphins) (Blalock et al., 1985). These reciprocal
influences between the neuroendocrine and the immune systems
(Fabris, 1981; Fabris et al., 1988) occur throughout life, but have
particular relevance during aging (Fabris & Piantanelli, 1982).
2.2.6 Cardiovascular system
The frequency of cardiovascular diseases, which are the major
cause of death in industrialized countries, increases with age.
Diseases such as hypertension and atherosclerosis occur most
commonly in the elderly. In addition, degenerative changes of the
cardiovascular system, involving the myocardial cells as well as
cells of the pacing-conduction system, that arise during the aging
process lead to impaired cardiac function and arrhythmia even in
people without any clinical evidence of hypertension or coronary
artery diseases. Inadequate function of the cardiovascular system
induces effects in peripheral tissues and organs. Changes in
peripheral organs resulting in hyperlipidaemia,
hypercholesterolaemia, and hypo- and hyperglycaemia can also effect
the cardiovascular system in the elderly.
2.2.6.1 Heart
The heart itself can be considered to be made up of two parts:
a) the conduction system responsible for electrically controlling
the heart rhythm; and b) the myocardium performing the contractile
function of the heart and composed of a system of trabeculae.
The biophysical and biochemical mechanisms that govern cardiac
muscle change with age, resulting in characteristic alterations in
muscle function (Lakatta, 1987a,b). Many of the steps in the
excitation-contraction system in cardiac muscle are altered by
aging. In an isometric contraction, the transmembrane action
potential (TAP) excites the cell and the contractions that ensue are
longer in duration. The magnitude of the prolongation of
depolarization of the TAP in senescent muscle is striking (i.e.
about twofold). The action potential amplitude is also greater in
senescent than in adult muscle in both high and low calcium-loading
conditions. These deficits of the senescent muscle may be related in
part to the diminished Ca2+ pumping rate by sarcoplasmic
reticulum. The duration of the elevated myoplasmic Ca2+ level is
prolonged in senescent muscle.
Sagiv et al. (1988) found that left ventricular contractility
increases less on stimulation in elderly subjects than in younger
people. Although the aging process is associated with normal resting
contractile function, diastolic properties are altered, resulting in
reduced and delayed early left ventricular filling and enhanced
atrial contribution to diastolic volume. Exercise cardiac output is
maintained in healthy elderly individuals, but there is a shift from
reliance on an increase in heart rate and a decrease in end systolic
volume to use of the Frank-Starling mechanism to increase stroke
volume. This age difference in the cardiovascular response to
exercise is probably mediated by an age-associated decreased
responsiveness to beta-adrenergic stimulation.
In muscle tissue of the aging heart, some morphological changes
are observed both in animal and human studies (Koobs et al., 1978;
Speijers, 1983). The most common change in the aging heart is
hypertrophy (Lakatta, 1985). Other alterations consist of the
appearance of slight focal necrosis and fibrosis in the myocardium,
amyloidosis (Finch & Hayflick, 1977) and the appearance of
lipofuscin (Hendley et al., 1963; Koobs et al., 1978). Peroxidative
damage to the myocardium is cumulative and irreversible (Koobs
et al., 1978).
2.2.6.2 Blood vessels
Both physiological and morphological changes are observed in
the vascular system, especially in the small and large arteries. The
morphological changes in the arteries seen in the elderly vary
considerably both in appearance and in localization (Goyal, 1982;
Hazzard, 1985). The thickness of the aorta increases significantly
with age, while the number of nuclei in the cells of the arterial
media decreases in humans as well as in mice. The majority of these
changes in humans are categorized as atherosclerotic. These changes
can progress and result in complicated coronary atherosclerosis and
ischemic heart disease, but other factors may also cause clinical
effects such as angina pectoris, arterial spasms, and myocardial
infarcts (Speijers, 1989a).
Morphological changes in the veins are less pronounced than in
the arteries.
The physiological changes observed with aging are often a
result of changes both in heart function and in the arteries. These
changes are reflected in haemodynamic parameters such as an increase
in diastolic and systolic blood pressure, mean arterial blood
pressure and vascular resistance, and a decrease in responsiveness,
and in contraction and relaxation responses (Lakatta, 1986, 1987b;
Duckles, 1987; Mazzeo & Horvath, 1987; Zemel & Sowers, 1988;
O'Malley et al., 1988; Cleroux et al., 1988).
Aging is often accompanied by increases in the incidence and
prevalence of hypertension. Geriatric hypertension is generally of a
salt-sensitive nature with a disproportionate frequency of isolated
systolic hypertension. The age-related increase in salt sensitivity
is due to a decline in renal function (Zemel & Sowers, 1988) and
deregulation of vascular tonus. Age-associated declines in the
activity of membrane sodium/potassium-ATPase may also contribute to
geriatric hypertension because this results in increased
intracellular sodium loading, causing reduced sodium/calcium
exchange and thus increased intracellular calcium and vascular
resistance.
It is commonly accepted that atherosclerotic changes take place
to a certain extent in every individual. Multiple factors determine
the extent and velocity of the atherosclerotic process (Hazzard,
1985). The incidence of clinically observed atherosclerotic effects
is higher in elderly subjects than in younger individuals.
Atherosclerotic damages result in impaired cardiovascular function.
Atherosclerosis is defined as a multifactorial disease with
variable effects in the intima followed by changes in the media of
arteries. These changes consist of focal accumulation of lipids,
proliferation of smooth muscle cells, and accumulation of complex
carbohydrates (i.e. glycosaminoglycans, proteoglycans), blood and
blood products, collagen and calcium compounds (Campbell &
Chamley-Campbell, 1981; Velican, 1981; Speijers, 1989a). The
resultant modifications of arterial wall integrity can lead to the
following: erosion of the wall with consequent reduced resistance to
blood pressure, rupture, and haemorrhage; progressive thickening of
the wall due to reactive proliferation of tissues with consequent
reduction of blood flow; and clotting of the blood at the level of
the injured wall with consequent sudden obstruction. The basic
lesion seems to develop in the first decade of life (Lee, 1985).
In the etiology of the lesion two localized cofactors should be
taken into account: the blood supply of the arterial wall; and blood
turbulence, since lesions are more frequently found around the
orifices of arteries branching off major arteries or at bifurcations
(Patel & Vaishnaw, 1980). Hypertension, diabetes, autoimmunity and
stress are also risk factors contributing to atherosclerosis.
2.2.6.3 Characteristics of atherosclerotic lesions
The first event in the formation of the lesion is still
debated. Both an initial thickening of intima, due to an
accumulation of blood-born amorphous material (lipids, protein and
sulfated proteoglycans) and a proliferation of muscle cells (due to
still undefined stimuli), with consequent degeneration and reactive
macrophage and connective tissue accumulation, have been proposed as
major initiating phenomena (Benditt, 1977; Ross, 1981). The
subsequent phase of the lesion involves repair mechanisms that cause
further thickening of the intima. Following this, lipid increases
both in the cells and in the intercellular spaces. Lipid
accumulation is progressive, leading to an increased number of foam
(lipid-containing) cells which disintegrate and form the gruel-like
substance that has given the name of atheroma to the lesion. The
accumulation of such material acts as an irritant, inducing a
proliferative reaction (encapsulation) which leads to the
development of a plaque. Calcification follows, making the arterial
wall more rigid. Alternatively, when the capsule breaks, an ulcer
can occur, leading to loss of tissue in the arterial wall, blood
clots, haemorrhage, and consequent thrombosis and/or rupture of the
arterial wall.
2.2.6.4 Theories of atherosclerosis
Atherosclerosis is undoubtedly a multifactorial process, which
is reflected by the many theories proposed (Baker & Rogul, 1987).
The lipid accumulation theory is based on the progressive
accumulation of oxidized lipids (mainly low density lipoproteins)
not only in smooth muscle cells but also in migrating monocytes
(Avogaro et al., 1983). The link between lipid deposition and
consequent alterations remains unclear (McCaffrey et al., 1988).
Theories on monoclonal proliferation of smooth muscles cells
are based on a mutagenic event in these cells, due to a
physico-chemical or viral insult (Benditt, 1977; McCaffrey et al.,
1988), leading to the formation of a kind of benign tumour. The
thrombogenic theory is based on the early adherence of platelets to
small alterations in the endothelium, leading to thrombus formation
and release of growth factors by platelets which induce smooth
muscle cell proliferation (Ross, 1981; Bang et al., 1982). Other
risk factors that should be taken into consideration are
hypertension, cigarette smoking, increased body weight, high serum
uric acids and consumption of saturated fats.
The immune system in the elderly is weakened. Consequently
there is increased susceptibility to chronic infections,
autoimmunity, and elevation of circulating immune complexes. New
data in the literature indicate that some viral agents may cause
cardiac and arterial cell lesions and subsequent inflammation. Thus,
viruses and altered immune cells may cooperate and play a role in
arterial wall lipid accumulation, possibly acting as initiating
factors for atherosclerosis (Butenko, 1985).
2.2.7 Respiratory function
Respiratory function is based on gas exchange (oxygen
absorption and carbon dioxide elimination), gas transport (red blood
cells), and on internal metabolic processes that utilize oxygen at
the cellular level. Aging may affect all of these processes (Masoro,
1981), but it is the first that is usually most compromised in
advanced age. The decline in the gas-exchange system may involve the
lungs, the thoracic cage, the respiratory muscles and the
respiratory centres in the CNS. The deterioration of the lungs is
largely dependent on environmental factors, in particular on the
contamination of air with toxic substances, dust and microbiological
agents. Therefore, age-related lung alterations may vary according
to life styles (smoking, physical exercise), environmental
conditions (urban/rural), and intercurrent diseases (infections,
work-related diseases) (Davies, 1985).
2.2.7.1 Gas-exchange organs
The most evident lung alterations with advancing age are
represented by enlargement of alveolar ducts with flattening of
alveoli and loss of septal tissue, reduction of elastic fibres, and
increased fibrosis of the capillary system (Liebow, 1964).
Functional consequences are a reduction in the surface area for gas
exchange with an increase in the physiological dead space, reduction
of the ventilatory flow rate, and irregular distribution of blood
flow (Mauderly, 1978). Age-related alterations also occur in the
chest as a consequence of calcification of costal cartilage,
increased stiffness of costovertebral and vertebral joints, and
general rigidity of the chest. Both lung and chest alterations
contribute to the changes in lung volume and pressure: vital
capacity decreases, residual volume increases, and flow rates
(particularly expiratory flow rate) decline (Morris et al., 1971).
The alteration in the gas-exchange capacity causes reductions in
oxygen uptake and pressure and lower arterial pO2, whereas pCO2
remains constant even in very old age (Morris et al., 1971). The
control of ventilation by brain centres is also altered in old age.
It is still unknown whether such alterations are due to intrinsic
damage of the neural component or to a reduced responsiveness of
neuromuscular activity in the chest (Peterson et al., 1981).
All these alterations, while not necessarily life-threatening
for the elderly, may favour pathologies such as chronic bronchitis,
pneumonia and emphysema (Peterson et al., 1981). The concomitant
hypoxia (low oxygen levels) may cause increased production of red
blood cells with consequent polycythaemia. This may contribute to
hypertension and cardiac failure.
2.2.7.2 Erythropoietic activity
Although specific age-related alterations in the life cycle of
erythrocytes have been reported (Danon, 1969), the overall function
of the erythropoietic system seems to be well preserved. The
regenerative potential following hypoxia seems nearly inexhaustible.
In addition, haemoglobin turnover does not seem to be affected by
age (Lipschitz, 1987).
2.2.8. Kidney and body fluid distribution
The urinary tract is affected by aging both in its renal
functions of excretion and ionic control of body fluids, and in its
control of bladder and urethral activity.
2.2.8.1 Renal function
Kidney function decreases both due to anatomical and
physiological alterations with age (Wesson, 1969; Kaysen & Myers,
1985; Brown et al., 1986; Corman & Michel, 1986; Owen & Heywood,
1986; Meyer & Bellucci, 1986; Anderson & Brenner, 1986, 1987;
Goldstein et al., 1988; Euans, 1988; Rudman, 1988). These
alterations have been observed in both experimental animals and in
humans.
The weight and volume of the kidney decrease by 20 to 30%
between the ages of 30 and 90 years. The atrophy is primarily
cortical and seems to be related to intrarenal vascular changes. The
number of surviving nephrons is reduced and these remaining nephrons
tend to be enlarged (Kaysen & Myers, 1985; Brown et al., 1986;
Lindeman, 1986; Rudman, 1988). The number of glomeruli decreases by
30 to 50%, and there is an increasing percentage of sclerotic and/or
abnormal glomeruli. The glomerular filtration rate (GFR) decreases
with age resulting in an adaptive increase in glomerular perfusion
pressure (Kaysen & Myers, 1985; Lindeman, 1986; Meyer & Bellucci,
1986; Anderson & Brenner, 1986, 1987; Blum et al., 1989). This
decline in GFR is due in large part to the progressive reduction in
blood flow to the kidneys (Brown et al. 1986; Lindeman, 1986).
Glomerular mesangial volume increases by 50% and 1 out of 10
glomeruli is sclerotic at the age of 80 years compared with 1 out of
100 in the young adult (Brown et al., 1986). The renal tubules
decrease in number, proximal tubule volume and length decrease, and
distal tubules develop increased diverticula. The renal arterioles
develop intimal thickening, reduplication of the lamina elastica
interna, and mild hyalinization (Brown et al., 1986; Lindeman, 1986;
Rudman, 1988).
An age-related reduction in secretory and resorptive capacity
is seen in the tubules, which is explained by a progressive loss of
functioning nephrons (Lindeman, 1986). Tubular function, which
regulates water and salt balance, is also affected. A decrease in
the ability to concentrate urine with age has been well documented
in humans. This appears to result from a decreased medullary
tonicity caused mainly by an inability to respond normally to
antidiuretic hormones (ADH) (Kaysen & Myers, 1985; Brown et al.,
1986; Meyer & Bellucci, 1986; Lindeman, 1986; Euans, 1988). Despite
age-related decreased renal function, the blood pH, partial pressure
of carbon dioxide, and serum hydrogen carbonate concentration of the
geriatric population without renal disease do not differ
significantly from those of the young under basal conditions
(Lindeman, 1986). Both the ability to maximally dilute the urine and
to maximally concentrate it, are controlled by serum ADH and by the
action of that hormone on the collecting ducts (Kaysen & Myers,
1985; Os et al., 1987). Increased arginine-vasopressin (AVP)
secretion per unit of plasma reflects a decrease in collecting
tubule sensitivity to AVP. This change in sensitivity is not
completely offset by increased ADH release (Davis & Davis, 1987).
The suppression of ADH secretion is not maximal when serum
osmolality is reduced.
The renin-angiotensin-aldosterone system is also poorly
responsive to volume depletion in aging subjects. As a result, the
elderly cannot maximally retain sodium under conditions of plasma
volume contraction (Kaysen & Myers, 1985; Lindeman, 1986; Euans,
1988). The activity of the renin-angiotensin system is progressively
reduced with age. It has been suggested that angiotensin II does not
play an important role in the maintenance of blood pressure and
kidney hemodynamics in normal senescence (Corman & Michel, 1986).
The mean blood pressure is correlated with age and the decline in
renal function (Lindeman et al., 1987).
The kidney is also the site of vitamin D1 hydroxylation, which
is dramatically reduced during aging (Kaysen & Myers, 1985).
2.2.8.2 Lower urinary tract
The most frequent age-related alterations in the lower urinary
tract result in urine incontinence in both sexes and urine retention
in males. Incontinence occurs at high incidence although it is not
an inevitable consequence of aging. The high number of physiological
requirements for urinary continence may account for such frequent
failure (Williams & Pannill, 1982). In women, the reduction of
estrogen levels after menopause may decrease the tone of the smooth
muscle around the pelvic floor and the bladder outlet, thus
favouring urinary incontinence. In men, hypertrophy of the prostate
represents the major cause for involuntary loss of urine, because of
the associated frequent instability of the detrusor muscle. Other
causes for urinary incontinence are represented by delirium,
infections, restricted mobility and polyuria.
2.2.9 Gastrointestinal function
Changes in the gastrointestinal tract during aging consist
mainly of a reduced cell turnover, leading to mucosal hypoplasia.
Accessory organs, such as the exocrine pancreas and the liver, are
affected by aging independently.
2.2.9.1 Gastrointestinal tract
In contrast to the cardiovascular or excretory system, the
gastrointestinal (GI) tract does not exhibit marked structural or
functional changes with age (Penzes, 1984). Aging may affect all
regions of the GI tube. The first signs of aging are generally
observed in the mouth. Teeth undergo discoloration, pulp recedes
from the crown, dentine is often poorly renewed, and the gingiva
frequently recede (Walker, 1985). These alterations favour bacterial
growth which can lead to chronic periodontal inflammation and tooth
loss.
In the stomach, reduced secretion of hydrochloric acid and
pepsin is often found in the elderly. These changes result from
alterations in enzyme-secreting cells or from altered hormonal and
neural regulation. However, in some cases, increased acid secretion
may occur with advancing age, leading to gastritis, erosions and,
ultimately, ulcers (Kumpuris, 1983).
No major morphological alterations are observed in the
intestine. The relatively mild modification of villi, the increased
collagen content and the reduced mucosal cell proliferation
(Webster, 1985) cannot account for the impaired absorption of
nutrients, including minerals (calcium, iron and zinc) and vitamins
often found in the elderly. Other factors such as reduced motility
and inadequate intestinal blood supply may play a more important
role than the slightly altered anatomical integrity.
In the lower GI tract the rectal tone is generally decreased in
the elderly and the sphincter is weakened, leading to incontinence.
Neurological alterations (dementia), muscle atrophy, diarrhoea and
constipation can all contribute to anal incontinence.
2.2.9.2 Pancreas
Pancreatic function is not seriously compromised during normal
aging. Structural changes mainly involve a reduction in the number
of secreting cells, with a consequent decrease in size of the
pancreas and a moderate increase in collagen content. Reduction in
the levels of trypsin, hydrogen carbonate, amylase and lipase in the
pancreatic juice occurs commonly in the elderly (Vellas et al.,
1988). Overall, however, the functional efficiency of the pancreas
is not lost during aging.
2.2.9.3 Liver
Structural changes in the liver with age are relatively minor.
However, the most significant change in human liver is a decrease in
volume (Wynne et al., 1989). The life-span of the hepatocyte is
long, with cells only dividing once or twice during the lifetime in
the absence of a growth stimulus (Popper, 1986). Whether the
function of the aging hepatocyte is impaired is unclear. Kupffer
cell function may be impaired as demonstrated by a reduction in
phagocytic activity. Endocytosis is reduced in Kupffer, but not
endothelial, cells. An increase in collagen also characterizes aging
liver.
The function of aging liver also undergoes a number of
alterations. In humans, cholesterol synthesis is reduced in the
elderly, while biliary secretion is increased (Popper, 1986).
Hepatic blood flow is reduced by as much as 50% (Sherlock et al.,
1955). Age-related changes in liver enzyme expression have been
studied in more detail in rodents (Fishbein, 1991). The efficiency
of carbohydrate and intermediary metabolism is decreased in
senescent rats or mice of both sexes, due in part to decreased
insulin-binding and the impaired regulation of enzymes such as
pyruvate kinase. Hepatic protein synthesis also decreases with age,
and the sex-specific expression of drug and steroid-metabolizing
enzymes in male rats appears to be feminized in senescence (Kitani,
1991).
2.2.10 Musculo-skeletal system
Aging dramatically affects bone, joints and skeletal muscles.
The basic phenomena responsible for the aging of the
musculo-skeletal system are not completely understood because of the
involvement of many factors, e.g., hormones, nutrition and physical
exercise, in addition to specific age-related alterations at the
tissue level, resulting in general deterioration of the system.
2.2.10.1 Bones
Bone should not be considered as metabolically quiescent since
it is constantly remodelled according to mechanical demand and
continuous turnover due to new bone formation (by osteoblasts) and
bone resorption (by osteoclasts) (Exton-Smith, 1985).
With aging, the balance between bone formation and bone
resorption is altered in favour of resorption, resulting in a
reduction of bone mass. This starts from the medullary region with
enlargement of the cavity and moves towards the epiphysis of the
bone and then to the outer surface with a final reduction of cortex
thickness. As a consequence, the bone strength is reduced (Smith
et al., 1981). The prolonged period where bone resorption exceeds
bone formation may result in osteoporosis (Dawson-Hughes et al.,
1987; Eastell & Riggs, 1987; Riggs, 1987; Croucher et al., 1989).
The factors responsible for bone resorption and remodelling are
not well known. More knowledge is available concerning two other
factors which profoundly affect bone metabolism: calcium turnover
and hormonal profile. Calcium is absorbed through the intestinal
mucosa and is excreted from the kidney, the blood level remaining
remarkably constant throughout life. Calcium is required for many
essential functions in the body, such as cell division, cell
intermediary metabolism, and cell functions (excitability, secretion
and movement). In the case of a negative balance, calcium is
mobilized from the bone with a consequent reduction in bone mass.
Calcium metabolism is under hormonal control. Parathyroid
hormone increases the plasma calcium level by promoting bone
resorption and mobilization, whereas calcitonin lowers blood calcium
by inhibiting bone resorption. Calcitriol (the active derivative of
vitamin D) increases intestinal absorption and decreases excretion
of calcium, thus acting to increase the body burden of calcium. The
age-associated decrease in intestinal calcium absorption lowers the
plasma calcium level, leading to an increase in parathyroid hormone.
Calcitriol levels may also be deficient in the elderly, as a result,
perhaps in part, of the decrease in vitamin D synthesis in old skin
and alterations in renal metabolism.
Bone metabolism also depends on other hormonal factors.
Estrogens have a strong positive effect on bone density. After
menopause, bone resorption exceeds bone formation resulting in bone
mass loss. However, the occurrence of osteoporosis greatly depends
on the adult bone peak mass, physical activity, and calcium intake
(Eastell & Riggs, 1987; Riggs, 1987; Bornor et al., 1988; Stevenson
et al., 1988; Lindsay, 1989).
Glucocorticoids lower plasma calcium levels, which can increase
bone resorption by stimulating parathormone secretion. Thyroid
hormones may also cause bone loss, although the mechanism is still
unclear. Growth hormone, somatomedins and insulin all promote bone
formation, and therefore defective secretion (as in diabetes) may
cause osteoporosis.
2.2.10.2 Joints
Articular disorders are practically universal in elderly
people, and are associated with pain and disability. The major
alteration consists of the loss of the smooth surface of cartilage,
which becomes thicker and less elastic. The cartilage develops rough
surfaces and mechanical irregularities, which represent the early
steps in osteoarthritis (Evens & Hawkins, 1984).
2.2.10.3 Skeletal muscles
With advancing age muscles become smaller in size, due to
reduction in the number of fibres, and less elastic, due to an
increase in collagen content. The fat content of muscle tissue also
increases with age. The causes for such defects are still
controversial: alteration of sarcoplasmic reticulum, decrease of
contractile protein synthesis and a reduction in the number of
mitochondria are all found in aged muscle cells.
In addition to muscle cell alterations, the neuromuscular
junction is modified in aged muscle. The acetylcholine content of
nerve terminals is consistently reduced and acetylcholine receptors
are irregularly distributed. Motor nerve conduction velocity is also
impaired. All these defects contribute to the progressive failure of
muscular efficiency, although some muscles, such as the diaphragm,
do not seem to suffer age-related effects (Finch & Hayflick, 1977).
Thus, the additive results of the loss of bone mass, disorder of
joints, decline in skeletal muscle power and nervous system
discoordination may lead to loss of body stability, falls and bone
fracture in old people.
2.2.11 Skin
The age-related modification of the skin is the most dramatic,
common and constant event in life, so that it may constitute one of
the best external markers of aging. Only recently has it been
possible to distinguish between intrinsic aging of the skin and
photo-aging (Gilchrest, 1984). Cutaneous neoplasia is one of the
best documented interactions between aging of the organism and
environmental effects. A variety of environmental factors, the most
notable being ultraviolet radiation, has been shown to cause cancer
of the skin in humans and animal models (Rogers & Gilchrest, 1990).
The structure of skin changes throughout life (Behl et al.,
1987), although many of the alterations are due to exposure to
sunlight rather than intrinsic aging (Gilchrest, 1984). Skin
thickness increases during maturation and then gradually declines
(Vogel, 1983). However, recent studies in mice and rats by
Monteiro-Riviere et al. (1991) have not reported any significant
changes in skin thickness or blood flow from maturity throughout the
life span. Hydration, which can affect chemical solubility,
decreases while keratinization increases.
Skin aging is linked to alterations affecting both the
epidermal and the dermal components. The epidermis suffers from a
reduction in the turnover of epidermal cells, due both to a
reduction of basal cell renewal and to a slower maturation toward
the stratum corneum. The dermo-epidermal interface is flattened so
that the total external body surface area is reduced (Selmanovitz
et al., 1977). The reduction in epidermal cell turnover is
responsible for the slowing of wound healing and possibly for the
dryness and/or roughness of aged skin. The dermis is reduced in
thickness in the elderly and is characterized by a reduced collagen
content with a biochemical modification of the collagen itself,
which makes it more strong and less elastic. Vascularization in
humans is also reduced.
Aging also results in modifications in the skin appendages.
Sweat gland function is decreased resulting in impaired
thermoregulation. Sebaceous glands produce less oil and wax (dryness
of skin), and hair usually loses pigment. The numbers of skin
sensory organs (Pacinian and Meissner's corpuscles) decrease with
age and sensation is consequently modified.
Collagen aging occurs in the skin, as in other tissues, leading
to increased rigidity. Collagen is composed of several related
proteins, which are produced by fibroblasts and extruded into the
extracellular space. Here they can be chemically deposited in a
nearly pure form, as in the tendons, or immersed in an extracellular
matrix, also produced by fibroblasts, as in the skin. The collagen
fibres undergo maturation in the extracellular ground substance by
parallel arrangements of tropocollagen fibres which became assembled
together through cross-linking. This phenomenon is an index of
maturational change. With age, however, the cross-linking increases
(Houck et al., 1967), leading to a reduction of tensile strength and
plasticity (Verzar, 1968). The extracellular matrix also shows
age-related changes, since alterations in its physicochemical
composition lead to increased density, reduced permeability and
impaired transport of nutrients (Imayama & Braverman, 1989).
3. BASIS OF ALTERED SENSITIVITY TO ENVIRONMENTAL CHEMICALS
The perception of altered sensitivity of the elderly to
environmental insults is based in large part on the enhanced
incidence of adverse or idiosyncratic drug reactions in this segment
of the population (Vestal et al., 1985). The clinical pharmacology
of the aged has been extensively reviewed in the last two decades
(Triggs & Nation, 1975; Crooks et al., 1976; Reidenberg, 1980;
Vestal et al., 1985), and evidence indicates that the response to
drugs changes with age, as does the frequency of adverse drug
reactions (Krupka & Vener, 1979). This may be in part related to
issues of polypharmacy and compliance (Weber & Griffin, 1986).
Morbidity and malnutrition, which are often associated with aging,
could also contribute to altered pharmacokinetics in the elderly
(Kitani, 1988). However, it is clear that age-related differences in
drug/chemical disposition (pharmacokinetics/toxicokinetics) or
sensitivity (pharmacodynamics/toxicodynamics) also play a role in
altered responses to chemicals in the elderly. For sake of
simplicity, the terms "pharmacokinetics" and "pharmacodynamics" will
be used for both drugs and environmental chemicals.
3.1 Pharmacokinetics
Pharmacokinetics describes the processes of the absorption,
distribution, metabolism and excretion of drugs or other chemicals
in the body. The numerous physiological and biochemical changes that
occur during aging can modulate any of these processes of
disposition. Changes in chemical disposition (pharmacokinetics being
the mathematical description of these processes) can lead to an
altered dose or dose-rate to the target tissue resulting in changes
in response. This topic has been the subject of several recent
reviews (Sellers et al., 1983; Van Bezooijen, 1984; Stevenson &
Hosie, 1985; Blumberg, 1985; Birnbaum, 1987, 1989, 1991; Ritschel,
1988; Loi & Vestal, 1988; McMahon & Birnbaum, 1990a).
The highlights in this field will be covered as well as the
more recent data, especially that focusing on age-related
alterations in the pharmacokinetic behaviour of xenobiotics, as
opposed to drugs.
3.1.1 Absorption
The absorption of drugs and environmental chemicals, defined as
uptake into the blood, occurs primarily via the skin, lungs and
gastrointestinal (GI) tract. Alterations in the structure and
function of these three organs clearly occur with age. In contrast
to the skin and GI tract, little is known about the effects of aging
on pulmonary absorption of xenobiotics. Few age-related differences
have been reported in lung structure (Stiles & Tyler, 1988),
although the lung volume is greater in old rats. An age-related
decrease in respiratory function has been reported in several
species (Mauderly, 1979a,b, 1982). In addition, there appears to be
a decrease in the rate of alveolar-capillary gas exchange in older
organisms (Mauderly, 1979b).
Dermal exposure represents a major portal of entry for
environmental chemicals. Several studies have indicated a decline in
human skin permeability with aging (Christophers & Kligman, 1965).
In experimental animals, the scarce data suggest that percutaneous
absorption is decreased in senescent rodents as compared to young
adults. The chemical absorption of
2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD) and related chemicals
is greatest at weaning (Jackson et al, 1990), decreases at maturity
and then undergoes a further decline during aging (Banks et al.,
1990).
Age-related alterations in GI absorption have been studied in
more depth. A decrease in gastric acid secretion is commonly seen in
the elderly (Bender, 1968). The resulting increase in pH can alter
the ionization of compounds, enhancing or retarding their ability to
diffuse passively across cellular membranes. Decreases in gastric
motility (Lin & Hayton, 1983) can prolong the transit time of
chemicals in the gut, thus enhancing their potential for absorption.
In rats, splanchnic blood flow declines in the first year of life
but stays unchanged in the second year (Yates & Hiley, 1979; Kitani,
1988). In contrast, splanchnic blood flow declines progressively
with age in humans (Sherlock et al., 1955). An increase with age in
mucosal weight (Holt et al., 1984; Hebert & Birnbaum, 1987), as well
as intestinal epithelial cell proliferation (Holt et al., 1988), has
been reported in rats.
GI absorption can be active, passive or involve phagocytosis.
Xenobiotics are primarily absorbed by passive diffusion. Age-related
changes in the oral absorption of a variety of drugs in people have
not been observed (Castleden et al., 1977b; Stevenson et al., 1979;
Greenblatt et al., 1988). The passive absorption of TCDD in the
small intestine does not change with age in rodents (Hebert &
Birnbaum, 1987), nor does that of many small endogenous molecules,
such as glucose (Eastin & Birnbaum, 1987), vitamin A (Hollander
et al., 1986), vitamin D, vitamin B12 and niacin (Fleming &
Barrows, 1982a,b) and many amino acids (Penzes, 1974).
Active transport appears to decrease with age (Eastin &
Birnbaum, 1987). Doubek & Armbrecht (1987) have demonstrated that
the decrease in the carrier-mediated component of glucose transport
in rats occurs in the brush-border membrane of the small intestine.
Their suggestion that the decrease is due to a reduction in the
number of sodium-linked glucose carriers has been supported by
recent studies in human intestinal tissue (Vincenzini et al., 1989).
The active transport of other small molecules such as calcium and
phosphorus (Armbrecht, 1986), and galactose and iron (Reidenberg,
1980) has also been reported to decrease with age. As with the
active transport of glucose, the marked decrease in calcium active
transport of calcium occurs between young adulthood and middle age
(Mooradian & Song, 1989) and results from changes in the number of
calcium transporters in the intestinal basal lateral membranes
(Armbrecht et al., 1988).
3.1.2 Distribution
The distribution of chemicals throughout the body is governed
by the fact that the physicochemical properties of the compound
affect its transport within the body and localization to various
tissues. Lipophilic molecules readily pass across cellular membranes
and accumulate in lipid-rich tissues. Binding to proteins also
modulates distribution, since few compounds are transported free in
the blood. While there is no evidence of alterations in relative
blood volume with age, changes in body composition, blood flow and
macromolecular binding have all been documented.
The decrease in lean body mass in both animals (Lesser et al.,
1973) and humans (Novak, 1972) has been well documented. Loss of
body water with age (Edelman & Leibman, 1959) results in a decrease
in the volume of distribution for water-soluble compounds, leading
to enhanced toxicity of ethanol (York, 1982) and ethylenediamine
(Yang et al., 1984). Body fat can account for approximately 15-40%
of the total body weight in humans (Ritschel, 1983) and rats
(Bertrand et al., 1980; Birnbaum, 1983). The increased size of the
fat compartment in older, sedentary animals would be expected to
increase the body burden of lipid-soluble substances and reduce the
overall rate of elimination from older animals. Following an
inhalation exposure of old rats to methylchloroform (Schumann
et al., 1982a,b), a better fit to a physiologically based
pharmacokinetic (PbPk) model was obtained by increasing the volume
of the fat compartment from 7 to 18% of the body weight for rats and
from 4 to 18% for mice (Reitz et al., 1988). Similar improvements
were noted by Lutz et al. (1977) in their PbPk model of
polybrominated biphenyl compounds in rats.
Not only does cardiac output decrease with age (Bender, 1965),
but regional blood flow can change differentially (Yates & Hiley,
1979). Since adipose tissue volume increases but blood flow
decreases with age, lipophilic compounds tend to show greater
retention in the elderly. This has been shown for polychlorinated
biphenyls (Birnbaum, 1983) and halogenated solvents (Schumann
et al., 1982a,b). A clinical pharmacokinetic study (Klotz et al.,
1975) which demonstrated a 5-fold increase in the distribution
volume of diazepam in the elderly is in agreement with the data on
experimental animals.
Binding to blood components can also change with age. Although
the total plasma protein content does not change dramatically with
age, there is a small but significant reduction in albumin in both
animals (Rodgers & Gass, 1983) and humans (Bender et al., 1975). For
drugs or xenobiotics that can be bound, such a decrease in albumin
enables a higher concentration of free drug to reach the target
site. A decrease in binding of drugs to red blood cells has also
been reported to occur during aging (Chan et al., 1975), again
leading to a higher level of free drug.
3.1.3 Metabolism
As with absorption and distribution, there are physiological
changes that occur during aging which can influence
biotransformation reactions, both in the liver and in extrahepatic
tissues. Although the liver is the main site for the metabolism of
drugs and environmental chemicals, significant metabolic reactions
occur in all the portals of entry tissues (skin, respiratory tract,
GI tract) as well as the kidneys, gonads, adrenals, etc. Metabolism
is often considered to be divided into two types of reactions: phase
I (functionalization reactions) and phase II (conjugation
reactions). Phase I reactions tend to increase the polarity of
chemicals, and the corresponding enzymes include the
cytochrome-P450- and FAD-containing monooxygenases as well as the
alcohol and aldehyde dehydrogenases, monoamine oxidases, nitro- and
azo-reductases, esterases and amidases. Phase II reactions involve
the conjugation of functional group (either already present on the
chemical or as a result of phase I activity) with an endogenous
cofactor such as an amino acid (glycine, glutamate), peptide
(glutathione), sugar (glucuronic acid) or a small molecule such as a
sulfate, acetate or methyl group. These reactions may occur on
cellular membranes or in the cytosol, and are regulated in a
tissue-specific manner.
The only generalization that can be made concerning age-related
changes in biotransformation is that few patterns exist. Metabolic
changes with aging appear to be substrate, sex, strain and species
dependent (Schmucker & Wang, 1989; Kitani, 1988; Birnbaum, 1989;
McMahon & Birnbaum, 1990b). In addition to intrinsic changes related
to altered physiology, the study of the influence of age on
metabolism is further complicated by the effects of diet, alcohol,
drugs and pollutants, which can induce or inhibit enzyme activities
(Ritschel, 1988).
Most of the research on the effects of aging on metabolism has
focused on the microsomal mixed-function oxidases. The protein
components of this system have been reported to decline or remain
unchanged with age. In general, the decline in total cytochrome P450
levels reported in rats appears to be due to the age-related
decrease in the amount of the male-specific forms (Kamataki et al.,
1985a,b; Sun et al., 1986). This results from the age-related
decrease in circulating testosterone levels (Kitani, 1985) or to
alterations in the secretory profiles of growth hormone (Fishbein,
1991). This pattern is most pronounced in rats, few changes being
observed in other rodents or sub-human primates (Birnbaum, 1987).
However, recent human studies have suggested that some degree of
sexual dimorphism does exist in hepatic drug metabolism. Plasma
antipyrine half-lives were prolonged in elderly males, but not
elderly females, as compared to young adults (Greenblatt et al.,
1988). This was caused by an age-related reduction in clearance,
which has been interpreted as being the result of an impairment of
antipyrine metabolism in elderly men. Similar gender-specific
results were observed for the kinetics of chlordiazepoxide, another
low-clearance oxidatively metabolized drug (Loi & Vestal, 1988).
A study by Chengelis (1988a) has supported the earlier report
of Rikans (1984) that measuring components of the monooxygenase
system cannot lead to predictions of toxicity. Sex differences in
rats were only significant up to one year of age. However, Rikans
(1989a) has recently reported that metabolism of specific substrates
(aniline, benzphetamine and nitroanisole) does decline with age in
the female as well as in the male rat, but the magnitude of change
in the female is smaller. This agrees with a study by Bitar &
Shapiro (1987), who suggested that an age-related increase in haem
degradation plays a role in the decreased metabolism of hexobarbital
and aniline. This observation, that microsomal drug metabolism
activities do not have to be sexually dimorphic to be altered in old
age, implies that factors other than loss of the male-specific
cytochromes P450 contribute to the age-associated alterations in
some rat strains. Decreases in NADPH cytochrome P450 reductase in
rat liver with age have been reported by Blanco et al. (1987),
Chengelis (1988a) and Rikans (1989a).
Despite a large body of data obtained from experimental animal
studies, there is no clear evidence that monooxygenase activities
decline in the livers of healthy elderly humans. All the data
derived so far from human liver biopsy specimens have shown no
correlation between enzyme activities expressed per mg protein and
age of subjects (Boobis & Davies, 1984; Schmucker et al., 1990). The
often reported decreases in clearance values of drugs metabolized
primarily by the liver in the elderly may in large part be accounted
for by the decrease in liver volume with age (Kitani, 1988). Further
evidence is needed to determine whether hepatic drug-metabolizing
enzyme activities in humans decline with age, as is observed in some
rodents, but the extent is unlikely to be as drastic as that
observed in male rat liver.
A change in the composition of cytochrome P450 enzymes,
suggested by differential changes in enzyme activity, has been
demonstrated in male rats (Kamataki et al., 1985a; Sun & Strobel,
1986). Leakey et al. (1989a) showed that dietary restriction could
also alter the profile of cytochrome P450 isoforms, possibly by
delaying the age-related demasculinization of the liver. Friedman
et al. (1989) showed that aging affected the composition of
testosterone-binding cytochromes P450 in the liver of male rats.
Changes in P450 isoforms in old rats were also reported by
Paramonova & Dovgi (1987). Studies with humans have demonstrated
that the effect of age on metabolism even varies for different
metabolites of the same parent compound (Posner et al., 1987). This
supports the observation of changes in cytochrome P450 isoform
composition with age, as has been reported for male rat liver.
Hepatic xenobiotic metabolism can be modulated by many factors.
In humans, smoking often confounds studies of age-related changes in
pharmacokinetics. However, the age-related decrease in the
metabolism of antipyrine (Loft et al., 1988), theophylline and
cortisol (Crowley et al., 1988) may be independent of smoking
status. In fact, the inductive properties of smoking on hepatic
metabolism do not appear to diminish with age. Phenytoin also
increased theophylline metabolism to an equal extent in both young
and old healthy men (Crowley et al., 1988). In contrast, Rath &
Kanungo (1989) demonstrated that the rate of transcription of the
phenobarbital-specific isozymes was nearly two-fold higher in young
rat liver than in old. However, these differential effects may
reflect the specific isoforms involved, since smoking and
phenobarbital preferentially induce different forms. The recent
studies of Rikans (1989b), which demonstrate that induction of
hepatic microsomal drug metabolism by ethanol or acetone is
unaffected by the aging process, lend support to this concept.
Age-related changes in phase I hepatic drug-metabolizing
enzymes other than the mixed-function oxidases have been subjected
to much less examination. Rikans & Moore (1987) demonstrated an
age-related increase in liver alcohol dehydrogenase in male rats.
However, no age differences have been observed in the activity of
this enzyme in female rats. Hydrolytic reactions may decrease with
age. For example, liver esterase activity, using diethylhexyl
phthalate as a substrate, declines in old rats (Gollamudi et al.,
1983). Using aspirin as a substrate, no correlation was found
between age and esterase activity in human liver (Yelland et al.,
1991). These results provide further evidence that age is not a
major determinant of hepatic drug metabolism in the elderly
(Schmucker et al., 1990).
Alterations with age in extrahepatic phase I metabolism also
appear to be substrate, sex, strain and species specific. Pulmonary
metabolism of benzo[a]pyrene was found to increase in old rats (Sun
& Strobel, 1986) in agreement with earlier studies of Rabovsky
et al. (1984). In contrast, oxidation of 2-aminofluorene, which is
catalysed by a different isoform of cytochrome P450, decreased
(Robertson & Birnbaum, 1982). Renal metabolism of acetaminophen has
been reported to decrease with age (Beierschmitt & Weiner, 1986),
while salicylate oxidation by kidney extracts did not change in rats
(Kyle & Kocsis, 1985). Age-related alterations in intestinal Phase I
metabolism appear to be site specific. Sun & Strobel (1986) reported
that oxidation of benzo [a]pyrene in the colon increased throughout
the life of rats, while McMahon et al. (1987) observed no
age-related change in the metabolism of this substrate in the small
intestine. Newaz et al. (1983) observed higher metabolic rates of
dimethylhydrazine in colonic tissue from aging humans as compared to
younger individuals. McMahon et al. (1989) suggested that plasma
esterase hydrolysis of benzyl acetate may decline with age in both
rats and mice. Alcohol dehydrogenase activity was found to remain
unchanged in the aging colon (McMahon et al., 1987).
Changes in phase II enzymes also appear greatly variable in
rodent liver, although Loi & Vestal (1988) conclude that age has
little effect on phase II reactions in humans. The major conjugation
reactions involve sulfation, glucuronidation or reaction with
glutathione leading to mercapturic acid formation. All these
reactions are catalysed by multiple isoforms showing varying degrees
of substrate and sex specificity, as is the case for the phase I
enzymes. Iwasaki et al. (1986) demonstrated differential age effects
on two distinct sulfotransferases using male and female rats and
various alcohols and amines. Sulfation of phenolic substrates
appears to decline with age (Galinsky et al., 1986; Sweeny & Weiner,
1986) while conjugation of bile salts increases in old male rats
(Galinsky et al., 1986). In contrast, changes in female rats were
not seen (Galinsky et al., 1990). Chengelis (1988b) observed no
significant changes in sulfotransferase activity with age in either
male or female rats using beta-naphthol as the substrate. Similar
results were seen by Leakey et al. (1989b) for the conjugation of
both estrone and naphthol, whereas the sulfation of androsterone and
corticosterone increased with age in male rats.
Hepatic glucuronidation also shows substrate specificity for
alterations with age. Depending on the substrate, increases with
estrone and acetaminophen (Galinsky et al., 1986), decreases with
the rubber antioxidant 4,4'-thiobis-(6- t-butyl- m-cresol)
(Borghoff et al., 1988), or no effect with naphthol,
p-nitrophenol, morphine and testosterone have been observed
(Galinsky et al., 1986; Sweeny & Weiner, 1986) in male rats. In
contrast, Chengelis (1988b) observed a marked decrease in senescent
male and female rats using both p-nitrophenol and chloramphenicol.
To complicate matters further, Leakey et al. (1989b) also saw a
decrease in naphthol, testosterone, androsterone and
tetrahydrocortisone conjugation with glucuronic acid, but observed
no effects of aging on conjugation with 2-aminophenol,
5-hydroxytryptamine, bilirubin or estrone. Tarloff et al. (1989b)
saw no change in acetaminophen glucuronidation with advancing age.
Although the activities of the UDP-glucuronosyltransferases appear
highly variable, an age-related decrease in hepatic levels of the
cofactor, UDP-glucuronic acid (UDPGA) (Borghoff et al., 1988),
suggests that glucuronidation could be limited in older animals.
The concentration of hepatic glutathione, the cofactor involved
in the third major class of conjugation reactions, has been reported
to increase (Borghoff & Birnbaum, 1986), decrease (Stohs et al.,
1982) or remain unchanged (Chengelis, 1988b; Rikans & Moore, 1988)
in old rodents. Variability in age effects has also been reported in
the activity of the glutathione- S-transferases, which exist as a
family of dimeric proteins having broad and overlapping substrate
specificity. The isozymes are composed of two subunits from at least
six different peptides, including both homo- and heterodimers. Thus
reports of an increase (Leakey et al., 1989b), decrease (Fujita
et al., 1985; Blanco et al., 1987; Leakey et al., 1989b), or no
change (Birnbaum & Baird, 1979; Sweeny & Weiner, 1985; Borghoff &
Birnbaum, 1986; Chengelis, 1988b; Leakey et al., 1989b; Carrillo
et al., 1991) in hepatic glutathione-S-transferase activity may, as
in the cases of cytochrome P450 mixed-function oxidases,
sulfotransferases and UDP-glucuronosyltransferases, reflect
age-dependant changes in isozyme ratios (Spearman & Leibman, 1984;
Carrillo et al., 1991). Restriction in dietary protein decreases the
activity of the glutathione-S-transferases more in old than young
rodents (Carrillo et al., 1989, 1990).
Much less has been reported on the effects of aging on other
phase II metabolic reactions in the liver. Hydrolysis of expoxides
to form diols or dihydrodiols is catalysed by the epoxide
hydrolases. Epoxide hydrolase activity has been reported both to
increase (Birnbaum & Baird, 1979) and decrease (Ali et al., 1985;
Kaur & Gill, 1985; Leakey et al., 1989b) with age. Again, this may
reflect differential age effects on multiple enzymatic species.
However, some of the difference may be due to the ages of animals
used for comparison, since Chengelis (1988b) reported a gradual
increase in epoxide hydrolase activity throughout much of the life
span, followed by an abrupt decrease in senescence.
Human studies have suggested that the alterations observed in
salicylate pharmacokinetics (Cuny et al., 1979) with age might be
due to a decrease in glycine conjugation (Kyle & Kocsis, 1985).
However, elevated levels of salicyluric acid, the glycine conjugate
of salicylate, have been observed in elderly humans undergoing
chronic salicylate treatment (Montgomery & Sitar, 1981). Recent
studies in rodents have not demonstrated any age-related decline in
glycine conjugation with non-nephrotoxic doses of salicylate
(McMahon et al., l990a) or in the formation of hippuric acid from
benzoate (McMahon et al., 1989). Acetylation, however, has been
reported to decline both in man (Bauer et al., 1989) and rats
(Leakey et al., 1989b).
Changes in extrahepatic phase II reactions have been
investigated in even less depth than extrahepatic phase I reactions.
In the lung and small intestine, glucuronidation of p-nitrophenol
appeared not to change with age (Borghoff & Birnbaum, 1985), whereas
in the colon an age-related decrease was reported by McMahon et al.
(1987). In contrast, colonic glucuronidation of
4-methylumbelliferone rose significantly in older rats (McMahon
et al., l990b). A decrease in UDP-glucuronosyl transferase activity
in the kidney (Borghoff & Birnbaum, 1985; Tarloff et al., 1989a) was
accompanied by a decrease in the renal concentration of UDPGA
(Borghoff et al., 1988).
Glutathione content has been examined in a variety of tissues
(lung, kidney, brain, testes and blood) and, except for an
age-related decrease in the lens, has been found to remain constant
(Rikans & Moore, 1988). Unchanged glutathione levels in the colon
occurred although the activity of glutathione- S-transferase
decreased in this tissue (McMahon et al., 1987). Spearman & Leibman
(1983, 1984) observed differential age- and sex-related changes in
this activity in the lung depending on the substrate examined. The
renal activity of glutathione- S-transferase declines significantly
in old rats (Beierschmitt & Weiner, 1986). In contrast, elevated
gluthathione- S-transferase levels were observed in the brain of
old rats (Blanco et al., 1987), while no changes were observed in
the heart.
Epoxide hydrolase activity has been examined in the lungs,
small intestine and kidneys by Kaur & Gill (1985). They observed a
decrease in lung and small intestine activity in old rats which was
substrate dependent. In the rat kidney, epoxide hydrolase activity
decreased using trans-stilbene oxide as the substrate but did not
change with cis-stilbene oxide, supporting again the differential
effects of aging on different isozymic forms of drug-metabolizing
enzymes. In addition, deacetylation of acetaminophen in the kidney
appears to be either unchanged with age (Beierschmitt & Weiner,
1986) or slightly decreased (Tarloff et al., 1989b). Acetylation in
the kidney also decreases with age (Wabner & Chen, 1984).
One additional enzyme which can be considered to fall into the
phase II class is beta-glucuronidase. This enzyme hydrolyses
glucuronic acid from conjugated xenobiotics. The activity of this
enzyme has been reported to increase with age in rat liver
(Schmucker & Wang, 1979; Van Manen et al., 1983) and kidney
(Borghoff & Birnbaum, 1985), but remain unchanged in the colon
(McMahon et al., 1987). However, beta-glucuronidase was found to
decrease with age in fecal contents (McMahon, 1988). The balance
between glucuronidation and deglucuronidation reactions may play a
role in determining the level of reactive compounds in the organism.
Depending on the chemical, aging can result in either an
increase or a decrease in the metabolizing capacity of different
organs and tissues (liver, kidney, gastrointestinal tract, lungs and
skin). The elevation or reduction in metabolism can both lead to
higher and lower toxicity, depending on the relative reactivity of
the metabolic intermediates and end-products. Thus studies on the
effect of aging on metabolism should be considered case by case.
3.1.4 Excretion
Excretion leads to the elimination of a chemical and/or its
metabolites from the body. The kidney is the major excretory organ,
with the liver and lung also playing important roles in the
elimination process. In addition, sweat, saliva and sex-linked
processes such as lactation can serve as routes of excretion.
The effects of age on renal function appear to play a major
role in altered pharmacokinetics in the elderly (Vestal, 1978;
Koch-Weser et al., 1982). The fact that changes in the physiology of
the kidney occur has been known for many years (Schmucker, 1979).
Renal blood flow decreases with age leading to a decrease in
glomerular filtration rate. Tubular secretion and resorption are
also reduced in the elderly. The number of functional nephrons
declines to a similar extent as the decline in glomerular filtration
rate and active secretion, suggesting that the nephron loses its
function as a unit (Friedman et al., 1972). Decreases in renal
function can result in a decreased rate of renal clearance, leading
to a greater potential for elevated and/or persistent levels of
chemicals in the body which could lead to toxicity (Sellers et al.,
1983). In humans, decreased renal clearance in the elderly has been
demonstrated for many drugs, including the aminoglycosides,
tetracyclines, lithium, digoxin, procainamide, methotrexate, and
phenobarbital (Kampmann & Hansen, 1979).
Aging rodents are extremely susceptible to chronic
glomerulonephropathy (Goldstein et al., 1988), much of which may be
attributable to diet (Masoro & Yu, 1989). Altered glomerular
morphology, characterized by thickening of the basement membrane and
sclerosis, is progressive and increases in severity with advancing
age, eventually resulting in scarring and loss. Renal tubules are
also subject to degenerative changes, which are accompanied by
proteinuria, especially albuminuria (Neuhaus & Flory, 1978). This
appears to result from increases in glomerular permeability and a
loss of fixed glomerular polyanion (Baylis et al., 1988). The high
percentage of urinary protein represented by albumin in the aging
rat may be due to non-selective protein leakage into the urine
resulting from increases in glomerular permeability to large
proteins, since the protein percentage in urine approaches that in
the plasma of senescent rats (Horbach et al., 1988a). Similar
albuminuria has been observed in aging mice (Yumura et al., 1989),
and was correlated with glomerular sclerosis. Such changes, however,
should not be simply extrapolated to humans, since there is no
evidence for an increase in protein loss in the urine of the healthy
elderly.
Additional tubular changes occur in the aging kidney, resulting
in hyperplastic and degenerative changes. Some of these changes
resemble responses to specific environmental chemicals (Konishi &
Ward, 1989). A decrease in renal transport of organic acids has been
observed (Wabner & Chen, 1984). Aging appears to diminish the
turnover of sodium/potassium ATPase in the proximal tubules (Marin
et al., 1985), which could play a role in the observed age-related
decrease in tubular secretion.
Hepatic elimination may also be compromised by aging. Kitani
(1985) has suggested that the excretory capacity of the liver
decreases with age due to some functional alteration in the
hepatocytes. In contrast to rats, where blood flow does not change
after maturity (Kitani, 1988), blood flow to the liver declines in
humans (Sherlock et al., 1955). Bile flow rate has been reported to
be reduced (Borghoff et al., 1988) or remain unchanged (Kitani
et al., 1985a) in rats. Biliary transport declines, especially in
the case of polar compounds (Kitani et al., 1985a). The elimination
of sulfobromophthalein, a model compound for the study of biliary
excretion of organic anions, is also decreased in old rats (Kanai
et al., 1985). Sato et al. (1987) demonstrated that the biliary
excretion of the neutral glycoside ouabain decreases with age in
both males and females. This may be due to an age-related decrease
in hepatic uptake, resulting in less biliary elimination (Ohta
et al., 1988). In addition, the biliary canalicular transport system
declines steadily during aging (Kanai et al., 1988). The decreases
in both hepatic uptake and biliary excretion may reflect changes in
the hepatocyte plasma membrane (Zs-Nagy et al., 1986).
3.2 Pharmacodynamics
Age-related changes in chemical sensitivity cannot all be
explained on the basis of altered pharmacokinetics in the elderly.
Pharmacodynamic changes occur at the target site and may involve
changes in cell populations, cellular receptors, cellular
responsiveness or in the regulation of the amount or activity of
drugs, including the cardiac glycosides, benzodiazepines, tricyclic
anti-depressants, and the non-steroidal anti-inflammatory agents
(Bender, 1979), which have demonstrated altered receptor sensitivity
in the aged (Wilson & Hanson, 1980).
3.2.1 Central nervous system
Numerous studies have shown that, with advancing age, there is
a decrease in the ability of the nervous system to synthesize and/or
release neurotransmitters and neuropeptides (for review, see Rogers
& Bloom, 1985). This decline may reflect the fact that there are
fewer neurons present to synthesize the chemical messenger or that
the enzymes involved in their synthesis are altered. This would
imply that xenobiotics which lead to neuronal death or interfere
with neurotransmitter or neuropeptide synthesis and release could
have a greater adverse effect on the elderly than on the young
organism. An example of such an interaction has been noted within
the dopaminergic extrapyramidal circuits controlling motor movements
(nigrostriatal pathway). This pathway has been the subject of many
experimental and clinical studies and provides one of the best
functional units in which to study neurotoxicity in the aged.
Nigrostriatal neurons are lost as a normal correlate of aging, and
while these losses may not be expressed in the majority of
individuals as movement disorders, there is a clear reduction in the
functional reserve of this dopaminergic system, making it more
vulnerable to neurotoxicants. This has been demonstrated by the
observed acceleration or simulation of "age-associated" movement
disorders by neurotoxicants such as
1-methyl-4-phenyl-1,2,5,6-tetrahydro-pyridine (MPTP).
The reduced capacity to synthesize neurotransmitters that
occurs in the aged organism may also potentiate the effect of toxic
substances. Carbon disulfide is an organic solvent with a variety of
industrial applications that produces neurobehavioural dysfunctions
(Wood, 1981). The mechanism of CS2 toxicity seems to be inhibition
of dopamine-beta-hydroxylase, the enzyme that converts dopamine to
norepinephrine (McKenna & DiStefano, 1977). Since norepinephrine
metabolism declines with age, the spectrum of physiological effects
regulated by this catecholamine would be expected to suffer greater
disturbance, following exposure to CS2 or to pesticides that
reduce brain catecholamines such as methyl bromide (Honma et al.,
1987), in old rats compared to young ones.
Toxic compounds that serve as neurotransmitter receptor
blockers could have their effects on behaviour accentuated, making
neuroendocrine control of homeostasis more difficult in the elderly.
For example, the formamidine pesticides, amitraz and chlordimeform,
have been shown to block alpha-noradrenergic receptors (Costa &
Murphy, 1987; Costa et al., 1988) and induce a variety of
behavioural disorders (Boyes & Dyer, 1984; Hsu & Kakuk, 1984;
Landauer et al., 1984) in rats. Since there is an age-related
decline in noradrenergic receptors in several species (Rogers &
Bloom, 1985), exposure to these compounds may have a more pronounced
effect on the older organism.
Certain movement disorders associated with senescence may be
related to age-related impairments in the brain dopaminergic systems
(Marshall & Berrios, 1979). Waddington et al. (1985) observed a
decrease in the density of brain dopamine receptors in old rats,
with no changes in affinity. In contrast to young animals, the
receptors of old animals were not able to respond effectively to
long-term treatment. The decrease in dopamine receptor density was
selective for the D-2 receptor subtype, although the coupling
between D-1 receptors and adenylate cyclase appears to be affected
by aging. A similar loss of D-2 receptors in the elderly has also
been measured using positron tomography in the living human brain
(Wong et al., 1984). An age-related decrease in binding to the
serotonin receptor may relate to cerebral dysfunction in the elderly
(Shih & Young, 1978).
Studies with rats have indicated that the increased sensitivity
of older rats to diazepam is due to pharmacodynamic differences
(Guthrie et al., 1987). Pedigo et al. (1981) suggested this could
relate to changes in the benzodiazepine/GABA/chloride ionophore
complex. Human studies have also indicated that the site of
increased sensitivity to the benzodiazepines lies distal to the
receptor (Swift, 1985), possibly involving changes in the chloride
ionophores.
Alterations in endogenous opioid systems may play a role in
some of the behavioural changes observed in the elderly. Binding of
dihydromorphine to the opiate receptor decreases in specific areas
of the brain in aged rats as compared to young ones (Messing et al.,
1980). This reduction is due to a decrease in receptor number, with
no change in affinity. Despite a relatively large body of evidence
that some receptors and neurotransmitters are at least qualitatively
altered with aging, it is still not clear whether and how these
changes are causally related to increased sensitivity of the CNS to
certain drugs with aging, as described below.
The brain appears to exhibit increased sensitivity to
phenobarbital (Kitani et al., 1985b; Van Bezooijen et al., 1989) in
both mice and rats. Such enhanced sensitivity with age to an
anticonvulsant supports earlier studies with phenytoin (Kitani
et al., 1984). It is possible that the aging brain of both
experimental animals and humans has increased sensitivity to all CNS
depressants. Enhanced brain sensitivity has also been reported for
hexobarbital in rats (Van Bezooijen et al., 1989) and oxazepam in
mice (Kitani et al., 1986). The aging human brain appears to be more
sensitive to nitrazepam (Castleden et al., 1977a) and other
benzodiazepines (Reidenberg, 1980). Recently, zonisamide, a new drug
whose anticonvulsant properties are distinct from the other drugs,
was demonstrated to have an increased anticonvulsant effect in aging
mice (Kitani et al., 1987). Taken together, these results suggest
that these pharmacodynamic changes with age may reflect a decreased
response capability for seizures in the elderly, rather than a
specific age effect on all the distinct receptors (Kitani et al.,
1986). In fact, the lethal threshold for pentylenetetrazole in mice
has been shown to decrease with age (Nokubo & Kitani, 1988), coupled
to an increase in the threshold for maximal seizure.
The action of other environmental compounds on CNS function may
be more diffuse. Recent studies have demonstrated enhanced
sensitivity of old mice to cyanide intoxication (McMahon & Birnbaum,
1990b). Exposure to various metals has been shown to alter a variety
of CNS functions. There has been a particular interest in a
potential link between Alzheimer's disease and aluminium toxicity.
It was initially reported that the autopsied brains of Alzheimer's
patients showed elevated aluminium levels (e.g., Perl & Brody,
1980). However, others have found no such correlation. Marksberry
et al. (1981) compared the brains of Alzheimer's patients with older
adult controls and found no correlation between the density of
aluminium content and the density of neurofibrillary tangles and
neuritic plaques. However, a more recent study showed that the brain
aluminum levels were significantly increased as a function of age in
both control and Alzheimer patient populations (Bjorksten et al.,
1989).
Manganese is a metal that causes age-type neuropathy. However,
unlike aluminium, it is associated with extrapyramidal disorders,
characterized by intention tremor (Donaldson, 1987). In monkeys,
the neurological symptoms of choreo-athetoid movement, rigidity and
tremor occurred after 18 months of manganese exposure. These
clinical signs, in association with severe lesions of the globus
pallidus and subthalamic nucleus, resembled Parkinson's disease and
suggested a possible link between environmental exposure and
occurrence of the disease in aging individuals.
The neurotoxic effects of other metals have been widely
studied, particularly those of lead, which has been implicated in
reduced intellectual abilities in children (Needleman et al., 1979),
slowed reaction time (Hunter et al., 1985), and impairment of other
cognitive abilities (Winneke et al., 1982; Hansen et al., 1985).
While psychophysiological parameters appear to be relatively
insensitive to low levels of lead exposure, several studies have
demonstrated both cognitive and emotional effects due to long-term
exposure to lead (Hogstedt et al., 1983; Mantere et al., 1984).
Long-term exposure to mercury vapour has been reported to interfere
with verbal intelligence and memory performance (Piikivi et al.,
1984). Hanninen (1982) suggested that abnormalities due to mercury
exposure affect the motor system and result in intellectual
impairment, a gradual and progressive deterioration of memory
function, and emotional disability. Other metals including copper,
iron and manganese have also been reported to cause CNS dysfunction
(Grandjean, 1983). However, there are no available data on the role
of exposure to these metals in dysfunction of the CNS in the aged.
Examples of naturally occurring neurotoxic agents that
stimulate nervous system senescence have been reported in certain
island populations. For example, the Chamorro peoples of the
Marianna Island, specifically Guam and Rota, exhibited a high
incidence of amyotrophic lateral sclerosis, Parkinsonism and
Alzheimer's-like dementia that was recently linked to their diet.
The Chamorro's diet consists in part of a flour made from the seeds
of Cycas circinalis. When one of the components of these seeds,
beta- N-methylamino-l-alanine (BMAA), a compound similar in
structure to excitotoxic amino acids, was fed to macaques, they
exhibited signs of motor neuron, extrapyramidal and behavioural
dysfunction (Spencer et al., 1987).
It seems that several motor neuron disorders are associated
with the appearance of endogenous excitotoxic glutamate agonist-type
molecules. One such molecule is 2,3-pyridine dicarboxylic acid
(quinolinic acid), which has been isolated from the brains of
humans, rabbits and laboratory rodents (Moroni et al., 1984) and has
been shown to excite CNS neurons when applied iontophoretically
(Perkins & Stone, 1983). An interesting correlate is the fact that
brain concentrations of quinolinic acid increase with age (Moroni
et al., 1984), suggesting that its presence may result in the
spontaneous onset of neurode-generative conditions that are mimicked
by environmental neurotoxicants such as BMAA and
beta- N-oxalylamino-L-alanine (BOAA).
3.2.2 Endocrine system
There are several different ways in which the endocrine system
and the hormonal signalling operations involved may undergo
alterations with age and toxicant exposure. These can be categorized
as changes in: (a) the availability of hormones for binding to the
target tissues; (b) the reception of the pertinent transmitter or
hormonal signal by the target cells; and (c) the nature of the
hormonal message.
3.2.2.1 Changes in hormonal availability with age
Age-related or toxicant-induced shifts in synthesis, rate of
clearance and rate of secretion will all function to alter hormonal
concentrations. Such changes in the size of the available signal
pool may have corresponding effects on the magnitude of the response
by the target tissue. These changes may reflect a decline with age
in the homeostatic controls, which rely heavily on endocrine
feedback relationships.
Several toxicants have also been observed to cause changes in
circulating hormonal levels (Cooper et al., 1986). Significant
reductions in serum testosterone, for example, have been seen
following short-term exposure of rats to the plasticizer
dinitrobenzene (Rehnberg et al., 1988b) and the pesticide
chlordimeform, the latter also causing marked reductions in serum
LH, thyroid-stimulating hormone (TSH), T4 and T3 levels. The
effects, moreover, may be remarkably specific. Following three days
of exposure in male rats, the pesticide linuron, for instance, was
reported to decrease the serum T4 level in a dose-related manner,
while leaving T3 and the pituitary and gonadal hormones unaffected
(Rehnberg et al., 1988a).
There is also a growing body of evidence for a hormonal
influence on toxicant metabolism. A sizeable number of xenobiotics,
including both drugs and environmental toxicants, are metabolized by
the hepatic cytochrome P450 monooxygenase system (Nebert & Gonzalez,
1987). Components of this system have been found to be influenced by
glucocorticoids (Schuetz et al., 1984; Simmons et al., 1987) and
markedly affected by sex steroids (Kamataki et al., 1985b) and
growth hormone (Yamazoe et al., 1987; Zaphiropouos et al., 1989).
Consequently, persistent shifts in the circulating levels of such
hormones, as have been reported for the aging animal, could affect
the manner in which xenobiotics are metabolized following exposure.
Reported attenuations with age in the rhythms of human and rat
serum testosterone (Bremner et al., 1983; Steiner et al., 1984;
Tenover et al., 1988), LH (Vermeulen et al., 1989) and GH (Sonntag
et al., 1980), among other hormones, can present differences in
young-versus-old comparisons, depending on when such sampling is
performed. Comparable effects on hormonal rhythms have been reported
to occur in response to toxicant exposure. For example, single
injections of 2,3,7,8-tetrachlorinated dibenzo-p-dioxin (TCDD)
resulted in some evidence of alterations in prolactin and
corticosterone rhythms in rats (Jones et al., 1987). It may be that
an aging system, while still exhibiting rhythmic hormonal changes,
may be increasingly sensitive to their disruption by low toxicant
levels.
3.2.2.2 Changes with age in the reception of the signal by the
target cells
A general decline in the transmitter regulation of hormonal
function may also place an aging animal at increased risk for
toxicant exposure (Govoni et al., 1988), given that various
environmental toxicants (including, for example, the solvents
vinyltoluene, ethylbenzene and styrene, the halogenated hydrocarbon
TCDD, and certain heavy metal cations) have been reported to
interact with catecholaminergic systems (Govoni et al., 1979; Lucchi
et al., 1981; Arfini et al., 1987; Mutti et al., 1988; Russell
et al., 1988).
These changes have been observed for both neurotransmitter and
hormone receptors. There is some evidence of a decreased
responsiveness of target tissues to steroid hormones during
senescence. For example, age-related declines in the concentration
of the estrogen receptor may reflect a decline in the circulating
estrogen levels (Thakur, 1988). Impaired responsiveness could also
be due to reduced receptor translocation (Belisle & Lehoux, 1983) or
other steps in steroid action which occur after hormone binding. In
fact, age-related alterations in glucocorticoid responsiveness are
due to both receptor and post-receptor events (Kalimi, 1982).
Testicular luteinizing hormone receptor concentration and total
content also decrease with age (Amador et al., 1985). In general,
receptors for steroids, insulin, glucagon, catecholamines and
prolactin appear to decrease in concentration with increasing age in
rodents, dogs and humans (Roth, 1979b).
An additional consideration of alterations with age or toxicant
exposure in the reception of a hormonal (or transmitter) signal by
target cells concerns not only effects on the receptor itself, but
changes in the cell's membranes. In the aging rat brain, there is
evidence for a progressive decrease in membrane fluidity
(Hershkowitz, 1983; Nagy et al., 1983). This is at least partially
attributable to alterations in the lipid composition. For example,
it has been reported that aging is associated with elevations in
cholesterol, sphingolipids and saturated fatty acid chains, all
leading to increases in rigidification (Rouser et al., 1972). Since
protein activity in the membrane is influenced by the fluidity of
the lipid micro-environment, any alterations with age in membrane
viscosity may affect not only receptor functions but also enzymatic
activity.
3.2.2.3 Changes in the nature of the hormonal message with age
The antigenic site(s) on a hormone recognized by antibodies can
be quite distinct from those regions that bind to the receptors and
trigger a physiological response in the target tissue. This
distinction may have an added importance for studies in aging, since
alterations with age in peptide hormone structure have been reported
(Conn et al., 1980) that reflect changes in post-translational
processes. These effects, moreover, may be influenced by shifts in
the steroid hormonal milieu in the older animal (Ulloa-Aguirre
et al., 1988). A number of hormones are glycosylated to varying
degrees and such differences in their carbohydrate residues may
alter biological activity (Ulloa-Aguirre & Chappel, 1982; Warner
et al., 1985) and/or plasma half-life (Morell et al., 1971).
Consequently, hormonal measures based solely on immunoreactivity
per se potentially offer a somewhat inaccurate picture of
endocrine alterations with age.
3.2.3 Kidney
The aging kidney appears to be more susceptible than the young
one to drug-induced nephrotoxicity as well as to renal ischaemia. In
fact, cortical tubules of senescent rats seem more sensitive to
oxygen deprivation than do those of young rats (Miura et al., 1987).
Thus, increased susceptibility of the aging kidney can be
independent of pharmacokinetic effects.
Age-related susceptibility to nephrotoxicity has been
demonstrated for numerous drugs including salicylate, acetaminophen,
cephaloridine and doxorubicin. Kyle & Kocsis (1985) showed that
kidneys from older rats develop nephrotoxicity to a greater extent
than do those of young rats following an equal dose of salicylate on
a body weight basis. However, the dose to the kidney could be
greater in the older rats due to a decrease in the volume of
distribution. In addition, McMahon et al. (l990a) has recently
demonstrated that at toxic doses old rats produce more reactive
metabolites of salicylate than do young ones. The age-related
enhancement in cephaloridine nephrotoxicity may also be due to
pharmacokinetic changes (Goldstein et al., 1986), although renal
cortical slices from old rats demonstrated alterations in active
uptake of organic anions and cations following exposure to the
antibiotic, which were not observed in young rats. Doxorubicin
treatment resulted in greater toxicity in old rats (Colombo et al.,
1989). The onset of the delayed nephrotoxicity was noticeably faster
in the old rats; this may have been related to higher drug retention
in the kidneys of old rats.
Age-related increased susceptibility to acetaminophen
nephrotoxicity has been investigated more than that of any other
chemical in rats. Tarloff et al. (1989b) stressed that while
enhanced sensitivity to acetaminophen nephrotoxicity clearly occurs
with advancing age, great care must be taken in choosing the ages
for comparison. Beierschmitt et al. (1986a) demonstrated by both
functional and histological criteria that susceptibility to
acetaminophen-induced acute tubular nephrotoxicity increases with
age in rats. This may reflect altered susceptibility rather than an
alteration in pharmacokinetic parameters, since the generation of
reactive metabolites from acetaminophen decreases with age
(Beierschmitt & Weiner, 1986). Increasing delivery of acetaminophen
to functioning nephrons, whose numbers are decreased in the aged
kidney, does not appear to be responsible for the age-related
increase in susceptibility (Beierschmitt et al., 1986b).
Alterations with advancing age do not have to result in
increasing sensitivity. Studies by Murty et al. (1988b) indicated a
decrease in hydrocarbon-induced hyaline droplet nephropathy in male
rats during senescence. This male-specific nephropathy is associated
with the presence of alpha2u-globulin, whose synthesis is strongly
age dependent (Roy et al., 1983). Although gasoline causes extensive
nephrotoxicity in young rats, old rats are resistant. In fact,
gasoline failed to alter hyaline droplet numbers in aged male rats,
which also demonstrated an altered lysosomal response as compared to
young rats. This lack of response of aged rats to
hydrocarbon-induced hyaline droplet nephropathy suggests that these
rats would also be resistant to hydrocarbon-induced renal neoplasia.
3.2.4 Immune system
With advancing age a progressive decline in the concentration
of glucocorticoid receptors occurs in the spleen (Roth, 1979a). This
alteration may depend either on an age-related modification of the
ratio among various subsets of lymphoid cells carrying different
receptor densities or on an intrinsic failure of aged cells to
maintain an adequate turnover of receptor molecules. With advancing
age, membrane receptors for hormones of low relative molecular mass
on lymphoid cells also show alterations, although these are more
related to impaired coupling to signal transduction systems (Feldman
et al., 1984; Roth, 1988). It has been shown that the number of
receptor molecules decreases with advancing age whereas the affinity
does not seem to change. In the aged population with altered
function of the immune system, it is possible that immunomodulatory
agents present in the food potentiate immune dysfunction, making the
elderly more vulnerable to age-related diseases.
The effects of immunosuppressive compounds can be identified
from toxicological experiments with young adult rodents. These
chemicals include organotin compounds, some pesticides, halogenated
aromatic hydrocarbons (e.g., hexachlorobenzene and dioxins) and
cyclosporin (Poland & Knutson, 1982; Vos & Penninks, 1987; Vos &
Luster, 1989; Schuurman et al., 1990; Vos et al., 1990), and they
can also potentiate autoimmunity, which is found more frequently in
the elderly.
3.2.5 Other tissues and systems
There are few data on pharmacodynamic changes in other tissues
and systems during aging. An increase in the sensitivity of the
aging human myocardium to digoxin (Chavaz et al., 1974), as well as
to intravenous anaesthetics (Dundee, 1979), has been suggested. The
stomach, kidney and bone marrow are more susceptible to the adverse
effects of non-steroidal anti-inflammatory agents in the elderly
than in the young, and cerebral side effects are more common
(Huskisson, 1983). The anticoagulation properties of warfarin are
also changed in the elderly, with the aged demonstrating increased
sensitivity (Shepherd et al., 1979).
Although in most toxicological studies the cardiovascular
system is not well examined, there are several chemicals known which
can cause cardiovascular toxic or atherosclerotic effects. Such
compounds in food include erucic acid in combination with
omega-3-linolenic acid, cetolenic acid, brominated vegetable oils,
cobalt salts in combination with ethanol, lead, nitrates and high
amounts of caffeine (Speijers, 1983). On the other hand, compounds
such as saturated fatty acids, odd-numbered cyclopropenoid fatty
acids (sterculia acid), ergot alkaloids, halogenated aromatic
hydrocarbons and cadmium induce toxic effects on the vascular system
and possible potentiate atherosclerotic effect (Speijers, 1989a,b).
Conrad & Bressler (1982) investigated the cardiovascular effects of
caffeine in elderly men. Caffeine, in doses equal to those contained
in 2 to 3 cups of coffee, produces an increase in blood pressure,
but has no positive inotropic effect in healthy elderly men.
Kim & Kaminsky (1988) suggested that age plays an important
role in modifying susceptibility to the toxic metabolite of
fluroxene, 2,2,2-trifluoroethanol. They noted greater effects on the
stomach, liver, testicles, brain and kidneys of aged rats as
compared to younger animals. Enhanced sensitivity to ethanol
intoxication has also been noted in old rats (Guthrie et al., 1987),
suggesting altered tissue sensitivity. Acute ethanol hepatotoxicity
may be due to enhanced liver susceptibility to the toxin (Rikans &
Snowden, 1989).
3.3 Modifying factors
3.3.1 Nutrition
Nutrition has been shown to influence the aging processes in
rodents and the occurrence and progression of age-associated
diseases in rodents and humans. Moreover the age-associated changes
in physiological processes affect the nutrition of the mammalian
organism. However, there is little information on how aging
influences the nutritional requirements of humans, a subject that
urgently requires scientific study.
Nitrates in foods can be reduced to nitrites in the oral
cavity, GI tract and urinary bladder (Tannenbaum et al., 1978).
Nitrites react with amines in the stomach forming N-nitroso
compounds, many of which have been shown to be carcinogenic in
animal studies, and it is difficult to deny their hazard to man
(Searle, 1976; Bartsch, 1991).
During commercial processing and domestic preparation, foods
may become contaminated with toxic chemicals. For example, smoked
and grilled foods contain small amounts of polycyclic aromatic
hydrocarbons and a wide variety of phenols and other organic
compounds derived from smoke (IARC, 1990). Canned foods can become
contaminated with tin or lead.
In a study by Spagnoli et al. (1991), both 4-month-old and
4-year-old New Zealand white rabbits were fed an atherogenic diet.
Increased incidence and degree of atherosclerotic lesions were seen
in the 4-year-old rabbits. These results showed an increased
susceptibility of the older arterial wall to hypercholesterolaemia.
Although 4-year-old rabbits are still relatively young, considering
the life span of this species (10-12 years), this study suggests
that aging arteries might be vulnerable to atherogenic compounds.
Malnutrition associated with inadequate intake or uptake of
nutrients in the elderly is caused by several factors. These include
socio-economic conditions (Munro, 1984) such as a) ignorance of the
need for a balanced diet, b) poverty, c) social isolation, d)
physical dependence (disability) and physical inactivity, e) mental
disorders, and f) changes in habits, e.g., retirement (Munro, 1984;
American Dietetic Association, 1984; Ferro-Luzzi et al., 1988).
Other conditions resulting in malnutrition include malabsorption due
to a variety of intestinal conditions, alcoholism, and the use of
therapeutic drugs that interfere with nutrient utilization and
therefore with the toxicity of chemicals (Krupka & Vener, 1979;
Kohrs, 1981; Munro, 1984; Chen et al., 1985; Robertson et al, 1988).
Malnutrition in much of the aged population can enhance the
vulnerability of the elderly to the effects of the toxic chemicals
in food. It has also been reported that malnutrition alters
pharmacokinetics in different ways depending on the drugs examined
(Roe, 1983; Cusack & Denham, 1984). This can be another factor in
the altered sensitivity of the malnourished elderly to toxicants.
Major deficits or marginal nutrient status occur for proteins,
calcium, vitamins A, C and B (thiamin, riboflavin, niacin, B6 and
B12) and zinc (American Dietetic Association, 1984; Ferro-Luzzi
et al., 1988).
In aged women, a deficiency in calcium is especially associated
with osteoporosis (American Dietetic Association, 1984; Munro, 1984;
Caraceni et al., 1988; Cauley et al., 1988). The deficiency in iron
and zinc might play a role in haematological status and immune
function, while a higher intake of aluminium might have neurological
sequelae (American Dietetic Association (ADA), 1984). An iron
deficiency might make individuals more vulnerable to compounds toxic
for the haematopoietic system, e.g., hexachlorobenzene and lead.
Data showing that older individuals have a reduced need for
energy, due to slower metabolism and decreased activity, has an
important impact on the intake of both macro and micronutrients,
because the composition of the diet is based on the average caloric
or energy intake (American Dietetic Association (ADA), 1984;
Ferro-Luzzi et al., 1988). Considering this reduced need for energy,
the elderly often reduce their intake of nutrients.
Dietary manipulation can alter life span in laboratory animals
(Barrows & Kokkonen, 1978; Young 1979). Restricting food intake in
laboratory rats produces a significant extension of life span (McCay
et al., 1935; Ross, 1961). Similar effects have been observed in
mice (Weindruch & Walford, 1988; Kubos et al., 1984), as well as in
more primitive organisms including fruit flies (Harman, 1981), and
nematodes and Neurospora (Harman, 1982). Other studies have
indicated that the reduction in caloric intake that accompanies
protein restriction, and not the protein restriction per se, is
responsible for the increased longevity (Leto et al., 1976; Davis
et al., 1983; Schneider & Reed, 1985). Dietary restriction has been
shown to be effective when started as late as middle age (Cheney
et al., 1983; Kubos et al., 1984; Masoro, 1988). Food restriction
appears to act either by influencing primary aging processes or by a
general protective mechanism, rather than directly modulating
multiple specific pathogenic processes underlying specific diseases
(Masoro et al., 1991).
Chronic nephropathy in rats has been reported to be retarded by
food restriction (Saxton & Kimball, 1941). Rats fed ad libitum a
semisynthetic diet of 21% casein as the protein source exhibited a
marked age-associated progression of chronic nephropathy, whereas
rats fed 60% of the ad libitum intake developed almost no
age-related progression of this disease process (Maeda et al.,
1985). Caloric restriction appears to be more important than protein
restriction in retarding nephropathy (Maeda et al., 1985). Fat or
mineral restriction was found to have no influence on longevity
(Iwasaki et al., 1988).
Dietary restriction delays the age-dependent loss of adipocyte
responsiveness to hormones, prevents the decline in serum free fatty
acid levels, delays the increase in serum cholesterol, and reduces
the increasing triglyceride level observed in aging rats (Cooper
et al., 1977; Liepa et al., 1980; Masoro et al., 1980; Yu et al.,
1980).
Dietary restriction from weaning has been shown to delay the
onset and reduced the severity of chronic nephrosis, periarteritis,
myocardial degeneration and muscular dystrophy in very old animals
(Berg, 1976). Chronic restriction has also been shown to inhibit
certain types of tumours, decrease the incidence of neoplasms, and
increase tumour latency (Ross, 1976; Weindruch et al., 1982;
Weindruch & Walford 1988). Early work using a chemically induced
mammary carcinoma model suggested that the primary effect of caloric
restriction was on tumour promotion (Weindruch & Walford, 1988).
More recent data (Fishbein, 1991) have demonstrated that the
initiation of chemical carcinogenesis can also be decreased by
caloric restriction. For example, exposure to aflatoxin B1
resulted in less DNA-adduct formation in liver from young
calorically restricted male rats than from controls fed ad libitum.
Such changes are most probably due to changes in hepatic cytochrome
P-450 expression (Fishbein, 1991).
There is ample evidence of better maintenance of
T-cell-dependent immunological responses in aging mice chronically
restricted from weaning (Weindruch & Walford, 1988). However, it has
been reported that restriction initiated at an adult age can also
delay the age-specific decrease in immune function (Fernandes
et al., 1977; Friend et al., 1978; Weindruch & Walford, 1988).
Although caloric restriction extends life span in invertebrate
and lower vertebrate species as well as rodents (Weindruch &
Walford, 1988), as yet there is no definitive evidence whether or
not caloric restriction will increase longevity, or decrease
neoplastic and degenerative diseases, in higher mammals or in man.
There is some evidence that reduced caloric intake in man reduces
urinary output of thymidine glycol and 8-hydroxy-guanosine, which
implies reduced free-radical-mediated DNA damage (Fishbein, 1991).
However, until the mechanisms by which caloric restriction evokes
its effect are fully understood, the only way that it can be
conclusively proved whether or not caloric restriction does prolong
life in higher mammals, is to perform longevity studies in these
species. Such experiments, using non-human primates, are underway in
the USA (Fishbein, 1991), but it will be some time before definitive
data are available.
Chronic disease in the elderly is accompanied by caloric
deficit, which in turn causes breakdown of body proteins and
negative nitrogen balance as well as the utilization of fat stored
in adipose tissues. Dietary protein appears to promote
age-associated renal disease in both humans and rats (Brenner
et al., 1982).
When illness occurs, nutritional deficiencies frequently become
clinically manifest (Rudman, 1987). For example, trauma or a fall
causing fractures leads to immobilization resulting in rapid loss of
body stores of nitrogen and calcium. This slows down mending of the
fracture. Similarly, surgical procedures in the elderly often result
in delayed recovery and risks far exceeding those of younger
persons. Heart failure and malignancies can lead to cachexia with
loss of weight, muscle mass and nutrient reserves. Infection may
produce similar changes and intervene to become the terminal event.
3.3.2 Alcohol intake
Ethanol is one of the chemicals most commonly ingested by
humans. Its effects on the body are numerous and varied. Loneliness
and isolation would seem to foster consumption of alcohol beverages
among older persons. The decrease in lean body mass and body water
that occurs with aging is responsible for higher blood levels of
alcohol in elderly people than in younger adults consuming the same
quantities (Vestal et al., 1977).
Elderly individuals have a decreased tolerance for alcohol, due
to an increased sensitivity of the CNS to the depressant effect of
ethanol. The metabolic effects of ethanol are the result of either
the increase in the NADH/NAD+ ratio occurring due to ethanol
metabolism or to direct toxic effects of ethanol or its metabolite,
acetaldehyde. An increase in serum uric acid level (hyperuricaemia)
is common during heavy alcohol ingestion, thus inducing acute gouty
arthritis in patients with known gout. Hypoglycaemia and
hyperlipidaemia occur in patients who are not eating adequately or
who are ingesting a high-fat diet with ethanol, respectively.
Thrombocytopenia is caused by alcoholism in patients with advanced
alcoholic liver disease (IARC, 1988).
The use of drugs increases with aging. Acceleration or
inhibition of drug metabolism by ethanol depends on the duration of
ethanol ingestion and the presence or absence of ethanol in the body
at the time the drug is ingested (Mezey, 1981). The presence of
alcohol in the body causes a decrease in the metabolism of certain
drugs, such as antipyrine, meprobamate, pentobarbital and
benzodiazepines, resulting in increased bioavailability of the drugs
to the CNS and thereby contributing to unwanted side-effects. In
contrast, ethanol can increase the metabolism and metabolic
activation of certain xenobiotics, such as the known human
leukaemogen benzene, thus potentially leading to enhanced toxicity
(IARC, 1988).
Chronic ethanol ingestion increases tolerance to CNS
depressants in young individuals, but its effect in the elderly is
unknown. The concomitant administration of ethanol and barbiturates
results in an enhanced depressant effect of these drugs on the CNS
and can result in coma or even death. All other sedative-hypnotic
drugs tested have either synergistic or additive effects with
ethanol. Intellectual deterioration and dementia are common
complications of chronic alcoholism. Alcoholic patients show more
signs of mental aging at every chronological age (Gaitz & Baer,
1971).
Chronic excessive alcohol ingestion is associated with
increased mortality from cancer, cirrhosis, non-malignant
respiratory diseases such as emphysema, and accidents (Klatsky
et al, 1981; IARC, 1988). However, a decrease in coronary artery
disease and mortality is associated with moderate alcohol ingestion.
This may be due to increases in plasma HDL-cholesterol and decreases
in LDL-cholesterol that occur during ingestion of alcohol (Mezey,
1981). Ethanol consumption is also associated with hypertension and
an increased mortality from cerebrovascular accidents (Kozararevic
et al., 1980; Blackwelder et al., 1980).
3.3.3 Smoking
Smoking clearly plays an etiological role and produces an
acceleration of a wide spectrum of age-associated disease. Smoking
contributes to an increased mortality rate (Gupta et al., 1980). It
also provides an excellent example of problems faced in the
consideration of the environmental impact on aging.
Scientists recognize that smoking presents a complex toxic
insult through inhalation. Increased pathology in aged mice has been
reported after exposure to cigarette smoke (Matulionis, 1984). The
immune response in aged mice exposed to cigarette smoke has been
shown to be decreased at some ages but not at others (Keast & Ayre,
1981). The role of smoking in coronary heart disease has been
reviewed by Kannel (1981), who observed that the relative effect of
cigarette smoking decreases in old age and proposed that this could
be due to the selection of a more resistant population.
Estrogen-related diseases have also been associated with smoking
(Baron, 1984). Reif (1981) has examined susceptibility to lung
cancer and concluded that the shape of the susceptibility
distribution is determined by the effects of all environmental
carcinogens (both known and unknown) to which the population has
been exposed, as well as by differences in genetic susceptibility
among members of the population. Smokers and non-smokers both get
lung cancer during the same age range. Some of these studies suggest
that aged individuals are more susceptible to the effects of
smoking, whereas others suggest that duration of smoking appears to
be the critical factor (IARC, 1990). The smoker is exposed to
multiple toxic agents simultaneously, the effects of which are more
pronounced in aged individuals. Major aspects of metabolism and
pharmacokinetics are altered in aged individuals. Therefore, the
effective dose of any chemical reaching the systemic target tissues
in aged humans would be dependent on these perturbations.
3.4 Interactions of chemicals and diseases
3.4.1 Cancer
Cancer morbidity is expected to rise with age and with an
increasing percentage of elderly people living in industrialized
countries (Magnus, 1982). There is no consensus on the causes of the
age-related increase in tumour incidence. Various arguments support
the concept that an age-related accumulation of total dose of all
carcinogens accounts for tumour induction as a function of age in
sensitive individuals (Peto et al., 1975, 1985). One viewpoint is
that the sensitivity to carcinogens is stable and independent of
age, whereas another is that changes in the internal milieu of the
organism, such as the metabolic and immunological shifts of natural
aging, provide favourable conditions for tumour development with
increasing age (Burnet, 1970; Dilman, 1971).
Comparison of human epidemiological data with in vivo and
in vitro animal experimental results is difficult but does allow
some limited conclusions. It seems that environmental carcinogenic
factors as well as endogenous carcinogens are important causes of
increased tumour incidence in old people (IARC, 1990). This
conclusion is supported by the increasing incidence of occupational
cancer with increased exposure time to carcinogenic agents and by
the correlation of lung cancer incidence with the number of
cigarettes smoked (Doll & Peto, 1981; Peto, 1986). Humans, in
general, have an age-related increase in the incidence of epithelial
neoplasms (Doll, 1978), but the relationship of age to incidence of
other cancers in different organs varies (Doll, 1973; Moolgavkar &
Venzon, 1979; Anisimov, 1987; Dix, 1989). Some tumours appear most
frequently in childhood, some increase exponentially with age, and
others reach a peak at a certain age and then decline.
The data on cancer incidence among the atomic bomb survivors in
Hiroshima and Nagasaki, Japan, have been very informative concerning
radiation-related solid tumours as well as leukaemia. Age at time of
exposure appears to be a strong determinant of leukaemia risk; the
greatest absolute risk was experienced by those who were exposed at
ages 0-9 or 50 years and over (Beebe, 1979). Most of the excess
cancer deaths from solid tumours among the atomic bomb survivors
have occurred among those who were over 35 at the time of the blast.
Analysis of data on the positive correlation between the aging
rates of different species with their cancer rates and the
observation that these two processes, aging and carcinogenesis, may
be initiated and promoted by impairments of gene regulation led
Cutler & Semsei (1989) to conclude that both cancer and aging may
arise from a common set of genetic alterations. The analysis of the
interrelationship between aging and carcinogenesis should be based
on epidemiologically and experimentally confirmed data.
Epidemiological data, analysed in terms of a multi-stage model
(Kaldor & Day, 1987), can estimate the importance of age at onset,
duration of carcinogen exposure, and latency in a population. On the
level of the organism, carcinogenic agents influence not only the
cell, causing genomic damage that leads to neoplastic
transformation, but also create in the cell a microenvironment that
facilitates proliferation and clonal selection (Anisimov, 1987,
1989). Multi-stage carcinogenesis is accompanied by various
disturbances in tissue homeostasis and systems of anti-tumour
resistance that, in turn, are under the influence of systemic
(nervous, hormonal and metabolic) factors. How long it takes for
frank neoplasia to develop depends on the state of those systems at
the moment of exposure to a carcinogen or tumour promoter and the
dose.
According to the multi-stage model of carcinogenesis, the
carcinogen whose effect increases in proportion to age at exposure
affects the partially transformed cell. In this case the tumour
incidence would increase and latency would decrease, as compared to
a population exposed to the same effective dose of carcinogen at a
young age. For example, application of 7,12-
dimethylbenz [a]anthracene in small doses or
12-0-tetradecanoylphorbol-13-acetate to the skin of mice of
different ages caused neoplasms more frequently in older animals
(Stenbäck et al., 1981; Ebbesen, 1985). Exposure of mice and rats of
various ages to phenobarbital resulted in hepatocarcinogenesis only
in old animals (Ward, 1983; Ward et al., 1988). The number of events
necessary for complete malignant transformation in 15-month-old rats
under the influence of N-nitrosomethylurea is lower than in
3-month-old rats (Anisimov, 1988). In every tissue, the number of
events occurring in the stem cell before its complete transformation
is variable and depends on many factors, in particular the rate of
aging of the target tissue and of the regulatory system(s) of the
tissue (Anisimov, 1987, 1989). This model is consistent with the
analysis of age-related distribution of tumour incidence in
different sites in humans and experimental animals (Doll, 1978; Dix,
1989; Anisimov, 1987).
Epidemiological observations have shown that exposure to some
carcinogenic agents leads to a rise in cancer incidence independent
of age at the start of exposure (e.g., smoking), while other agents
induce more tumours when the exposure begins in the elderly (e.g.,
lung cancer following asbestos exposure) (Kaldor & Day, 1987; IARC,
1990).
3.4.2 Other diseases
Aging affects the functional capacity and structural integrity
of many organ systems. Environmental chemicals can also affect
several target organs. The combination of the influence of aging and
the toxic effects of chemicals in the environment might potentiate
the risk for elderly persons. An inherent problem common to all
research in chronic disease is the dissection of the respective
roles of time per se from those of primary aging. Hazzard (1985)
stated that an essential feature of human aging is the change in
physiological competence across the life span, as reflected in
homeostatic reserve. Homeostatic reserve declines at an accelerating
rate with age, normally producing death in old age from
multifunctional etiologies.
The relationship between aging and atherosclerosis is a prime
example of this conundrum. It seems most likely that the changing
picture of atherogenesis in western society has led to a large
number of people who survive into old age with not only a degree of
clinical atherosclerosis, but also with other chronic progressive
diseases, such as chronic obstructive pulmonary disease, immobility
from osteoarthritis and/or osteoporosis, and mental incompetence
from Alzheimer's disease, multi-infarct dementia or other
age-related dementing processes.
These diseases could significantly modify the response of the
organism to various environmental chemicals by decreasing or
increasing their susceptibility, followed by an acceleration of
these diseases or induction of new ones.
4. APPROACHES TO EXAMINING THE EFFECTS OF CHEMICALS ON THE AGED
POPULATION
4.1 Experimental approaches
4.1.1 Principles for testing chemicals in the aged
population
There are two principal approaches to the study of age-related
changes of any functional, morphological and/or biochemical
parameter, i.e. "longitudinal" and "cross-sectional". Longitudinal
studies consist of repeated estimations of any parameter in the same
animal in different periods of life. Cross-sectional studies involve
separate groups of animals of different ages who are examined at a
given point in time. Results obtained using the longitudinal
approach may significantly differ from the results from experiments
carried out using cross-sectional approaches. In studies on the
toxicity of chemicals, it is obligatory that identical conditions be
provided and maintained for all the animals. Problems related to the
choice of animal species, strain, sex, age, life stage and chemical
treatment (both route and dose selection) will be considered below.
4.1.2 Animal models
An extensive discussion of the choice and use of animal models
in research has been recently published (Rogers et al., 1991).
4.1.2.1 Animal species
The selection of suitable species obviously involves both
practical and economic factors. Animals with a short life span are
preferred. However, good life-table information is not available for
all species. A knowledge of species-related differences in metabolic
pathways and inherent sensitivities is also important in choosing
animal species for study. The choice of species should depend on the
experimental question as well as homology of response. For example,
while closely related isoforms of drug-metabolizing enzymes may
exist in different species, their tissue distribution and substrate
specificity may vary greatly (Nebert et al., 1991). Such differences
in isoform expression, together with reported differences in DNA
repair efficiency, could be responsible for species-related
differences in an organism's susceptibility to chemical carcinogens
(Daniel et al., 1983; Mehta et al., 1984; Anisimov, 1987; 1989).
Regardless of such metabolic differences, mice and rats are
most often used in aging research because of a short life span,
relative ease of maintenance under defined conditions, wide use in
biological research, and suitability for a variety of molecular and
genetic analyses. There are extensive life-table information for
some strains of mice and rats as well as a data base on their
spontaneous pathology. If old animals are purchased from a supplier
rather than being maintained in an investigator's own laboratory, it
is necessary to obtain information on the lifetime environment of
the animals, including housing conditions and dietary history. It
may be preferable to keep animals from weaning until the desired age
for investigation under the same controlled conditions.
Mammalian species other than rodents have only been used
sporadically in aging research, owing to their long life span, lack
of life-table information, genetic heterogeneity, restricted
availability and high cost. However, it is critical that such larger
species be used to answer certain questions. For example, studies
are currently underway to determine if dietary restriction can
prolong the lifespan of two different monkey species (Ingram et al.,
1990).
4.1.2.2 Animal strain
The choice of adequate rodent strain for experiments on aged
animals is critical. The male Fischer-344 rats is a popular model in
aging studies because of its size and growth characteristics.
However, testicular interstitial cell tumours begin to appear at the
age of 18 months in rats fed ad libitum, and by the age of 2 years
the tumour incidence approaches 100% (Fishbein, 1991). Recently, a
highly significant positive trend with time has been observed for
the increasing prevalence of leukaemia, anterior pituitary tumours
and thyroid c-cell tumours in both sexes, adrenal pheochromocytomas
in males, and mammary tumours and endometrial stromal polyps in
female F-344 rats (Rao et al., 1990). Some mouse strains suffer
from a single major disease process (e.g., tumours of the mammary
gland or liver, or chronic nephropathy), and the presence of this
disease in most animals could modify the response to chemical
exposure and complicate the interpretation of the results.
In the USA and Japan, Fischer-344 and Sprague-Dawley rats and
B6C3F1, Swiss, and CD-1 mice are most frequently used, whereas
in European countries Wistar-derived and Brown Norway rats and NMRI
and Swiss mice are the most popular strains. Inbred animals have the
advantages of greater stability and predictability of response.
However, heterogeneity of outbred animals more closely resembles the
heterogeneity of human populations. The choice of a certain animal
strain also depends on previous experience with these animals, the
final choice of an appropriate species and/or strain being dependant
on the scientific hypothesis under investigation. Each strain has
its own pattern of background pathology, which can be greatly
influenced by animal husbandry.
4.1.2.3 Animal sex
Sex-differences in response to chemicals are well known. Thus,
the use of both sexes is often necessary in toxicity testing.
However, the large gender differences in chemical toxicity and
pharmacokinetics that occur in rats become less apparent in old age
due to the decreased expression of sex-specific isoforms of the
hepatic drug-metabolizing enzymes (Kitani, 1991).
Ovarian status may significantly influence the sensitivity to
some chemical agents, modifying the biological response, as been
demonstrated for chemical carcinogens (Anisimov, 1971, 1987). It is
noteworthy that, when using females of the post-reproductive period,
the investigator must be aware that a) the ovaries in females of
some species (i.e. rats and mice) are not atrophied and may continue
to secrete steroid hormones, and b) some animals may be in
persistent estrus, while others may be in anestrus or
pseudopregnancy status (Aschheim, 1976). Furthermore, an animal's
reproductive history could influence its response to a chemical in
later life.
4.1.2.4 Selection of age groups for comparison
The problem of appropriately characterizing the animal's age
within the context of its life span is of particular importance in
experimental gerontology. Since the aging process causes significant
changes in various systems of the organism, there is a need for the
definition of some reference points for adequate comparison of the
results of different experiments. The importance of these points is
particularly significant in cross-sectional studies. One of the
confounding factors in such comparisons between experiments is the
need for frequent comparisons of results from different animal
species with different life spans (long-lived and short-lived
species and/or strains). Many authors have used two kinds of age
groups: "mature" or "adult" animals; and "old" animals. However, the
true age of "mature" rats in the reports from different
investigators fluctuates from 2 to 14 months and the age of "old"
animals from 12 to 37 months. The life cycle of experimental animals
can be divided into four periods: a) prior to weaning
(developmental); b) sexual maturation (maturational);
c) reproductive; d) pronounced age-related changes (senescent
period) (Zapadnyuk, 1971). All studies should compare animals from
multiple age periods.
4.1.2.5 Underlying pathology of animals of different ages
As mentioned previously, the background pattern of pathology
must be taken into account in the selection of animal species and
strains. A species-, strain- and sex-specific incidence of
pathology, whether neoplastic or not, is observed to increase
rapidly in the second half of the life span, even for those animals
maintained under specific pathogen-free conditions (Burek, 1978;
Anisimov, 1987; Frith & Ward, 1988; Fishbein, 1991). Among
non-neoplastic diseases in rats, the most frequent are chronic
glomerulonephropathy, cardiomyopathy, amyloidosis, and peripheral
nerve degeneration. For example, the incidence of spontaneous
leukaemia in F-344 rats frequently used in long-term studies may
exceed 30%, and the frequency of intertitial cell tumours of the
testis may reach 100% in old males. This pathology may significantly
modulate the host response to xenobiotics.
4.1.2.6 Transgenic animals
During the last decade it has become possible to add new
genetic information to the germ line of experimental animals
(Jaenisch, 1988). More recently successful attempts have even been
made to specifically alter gene sequences in the mouse germ line,
thereby ablating or correcting specific gene functions (Capecchi,
1989). In the study of the effects of chemicals on the aged
population, transgenic animal models have at least two contributions
to make. Firstly, the influence of specific genes on the age-related
susceptibility to environmental chemicals can be assessed in the
in vivo situation (Vijg & Papaconstantinou, 1990). Secondly, by
using transgenic animals harbouring a shuttle vector with one or
more mutational target genes, mutagenic effects can be studied in
different organs and tissues of animals as a function of age (Gossen
et al., 1989).
4.1.2.7 Animal husbandry
In experimental research on the sensitivity of aged animals and
the aging process, the husbandry aspects should be defined. The
quantitative and qualitative results might depend greatly on the
dietary conditions. The dietary composition should meet the minimal
requirements for nutrients, minerals, vitamins and (raw) fibre for
adult animals according to established and published standards for
laboratory animal diets. Another important dietary factor is the
quantity to which the test animals have access. They may either
receive a restricted diet containing adequate level of nutrients or
be fed ad libitum. The choice of diet depends on the questions to
be asked.
In addition to the dietary status of the animals, their
microbiological status should be defined. In some cases interaction
of the chemical with the gut microflora might modify the outcome of
the study. Specific-pathogen-free (SPF) animals are preferred. The
direct environment of the test animal, including relative humidity,
temperature, light and dark cycles, seasonal influence and stress
can all influence the final outcome of a study and therefore should
be defined carefully (Masoro, 1991).
Differences in animal husbandry may cause large variations in
biochemical and pathological effects. This is clearly illustrated by
alterations in the background data among different laboratories.
4.1.3 Chemical exposure
4.1.3.1 Dose level
The selection of dose is a difficult issue. The usual
requirement is to have a minimum number of test groups (3) plus one
control (vehicle) group. This permits the development of a
"dose-response" curve and allows for appropriate statistical
evaluation of the results. The highest dosage should induce minimal
signs of toxicity, bearing in mind that the maximum tolerated dose
for young animals could in some cases be toxic for old ones, and
that high doses may alter the toxicokinetics of the chemical.
Considering that the body weight of young and old animals could
be significantly different, a correct dosage calculation of test
substances in comparison groups is an important problem, i.e.
whether it is better to normalize per unit of body weight or body
surface, or to some other parameter such as lean body mass (Travis
et al., 1990). The available data indicate similarity of the results
when calculation of dose is performed per unit of body weight or
body surface. It should be taken into consideration that the growth
and development of various organs may have different rates, and that
relative organ weight (and consequently, the effective target dose
of the substance) may not be the same in animals of different ages.
When the substance is administered in food or water, the consumed
quantities should be taken into account, because they may differ in
animals of various ages. When the oral or dermal route of
administration is used, age-related changes in the extent and rate
of absorption may be important. Age-related changes in lung
ventilation capacity may also alter the internal dose of a chemical
when the inhalation route of administration is used. When looking
for portal-of-entry effects, a constant concentration of the agent
may be used in all age groups.
4.1.3.2 Route of administration
There is general agreement that the test substance should be
administered by the route that corresponds most closely to human
exposure. For humans, the main exposure routes are oral, dermal and
inhalation, while in animal experiments the oral route is most
frequently used. However, when pharmacokinetic studies show that
other routes of administration result in equivalent target tissue
levels, such alternative routes can be used. The use of injections
(subcutaneous, intraperitoneal, intramuscular or intravenous) may be
expedient under certain circumstances.
4.1.3.3 Duration of exposure
The question being addressed should guide the design of the
study. If the potential risk involves acute exposure of the elderly,
then an acute experimental scenario is required. If human exposure
is ongoing, long-term studies may be necessary. Exposure to the test
substance in chronic studies should start shortly after weaning and
continue for the major portion of the animal's life (at least
through the mean life span). IARC (1986) recommended 24 months of
exposure for rats and mice, and 18 to 20 months for hamsters when
studying the carcinogenic potential of chemicals. Choice of exposure
duration based on life-table characteristics would be optimal.
However, these guidelines do not apply to experiments when animals
of different ages are used at the start of the study. In this case
the dose and duration of exposure could differ in groups of young
and old animals. However, it is preferable to use identical exposure
durations whenever possible.
4.1.4 Non-mammalian models
Many species can be used as models in the study of age-related
sensitivity to environmental toxicants. These include fungi
(Neurospora crassa and Podospora anserina), protozoa
(Paramecium tetraurelia and Tetrahymena pyriformis), rotifers,
nematodes (Caenorhabditis elegans, C. briggsae and Turbatrix
aceti), and insects (Drosphila melanogaster, Musca domestica and
Tribolium confusum) (Committee on Chemical Toxicity and Aging,
1987). Non-mammalian species such as fish and salamanders have been
used in aging research (Weindruch & Walford, 1988) and are
potentially available for toxicology studies. Several of these
models have already been used to examine the effects of chemicals on
aging. Because of their short life span, ease of use and relatively
low cost, non-mammalian organisms could be important in the initial
phases of a test system to identify environmental chemicals that
might affect aging.
4.1.5 In vitro studies
For the purposes of screening xenobiotics, the use of in vitro
models can result in substantial economy and efficiency. Stock cells
and, in some cases, tissue explants can be cryopreserved in large
amounts. This permits repeated assays with comparable materials and
the sharing of common stocks by numerous laboratories. Moreover,
such stocks can be used to investigate cell-to-cell interactions,
such as metabolic cooperation and metabolic transformation. Finally,
tissue culture approaches can substantially reduce the numbers of
animals required for experimentation. Such methods cannot, however,
be expected to substitute for the intact animal experiment.
There are three general categories of in vitro methods: organ
culture, tissue explants and cell culture. Organ culture involves
the short-term maintenance of variable intact segments of tissue,
for example, the full thickness of a segment of aorta. In tissue
explants, the early migration and proliferation of epithelial and
fibroblast cell types can be observed. Cell cultures are of four
general types:
(a) primary cell cultures and mass cultures of cells taken directly
from the animal, usually after enzymatic dispersion of biopsied
tissue;
(b) established, serially passaged cultures with relatively
reproducible cycles of growth in early phases, but with limited
replicative life span, and with a genetic make-up reflecting
that of the donor age;
(c) "transformed" cell cultures with indefinite replicative
potential and generally with altered genetic makeup;
(d) established or transformed cell lines into which specific DNA
sequences ("transgenes") have been transfected.
Each of these types of models could prove useful for studies of
the effects of environmental agents on the elderly. One general
approach would be to explore the toxic effect of an agent as a
function of donor age, so as to detect unusual susceptibilities of
the cells and tissues of aged subjects. Another general approach
would be to culture the cells in vitro after in vivo treatments.
If a set of behaviours or phenotypes was observed with tissue from
young, treated subjects that proved to be comparable to that
observed with tissue from old untreated animals, an effect of the
in vivo treatments on the aging process could be inferred.
An entirely different experimental paradigm could be based on
the hypothesis that established cultures with finite replicative
life spans recapitulate the natural history of comparable cell types
in vivo - the well-known " in vitro model of cellular aging"
first developed by Hayflick & Moorhead (1961). The appropriateness
of such models for the study of aging is controversial, since it has
been proposed that the attenuation of growth observed in vitro
corresponds to terminal differentiation in vivo (Norwood & Smith,
1985; Bayreuther et al., 1988).
Of special interest would be the evaluation of agents that
exhibit unusual toxicity to putative stem cells. An excessive
depletion of stem cells could seriously compromise the regenerative
potential of tissues in aging subjects. In such studies, it would be
important to investigate a variety of cell types. Most research to
date has concentrated on in vitro aging of cultures of
fibroblastoid cells established from the fetal lung or from the
dermis of individual subjects of various ages. The precise origin of
such cells is not clear. Thus, with such cells it would be difficult
to compare age-related changes in vivo with those observed
in vitro.
A final experimental paradigm would be to use postreplicative
terminally differentiated cells in culture in order to investigate
agents for their potential to accelerate age-related alterations
observed in vivo. Such cell types might be derived through
in vitro terminal differentiation, or from normal or transformed
embryonic neuroblasts or myoblasts. A major concern, however, would
be the extent to which the experimental milieu reflected in vivo
conditions.
4.1.6 Statistical considerations
For statistical treatment of the results of short-term testing,
the statistical methods that are generally available may be used.
However, for statistical analysis of the results of long-term
testing, in particular carcinogenicity testing, the comparison of
results from treatment groups with different survival rates is a
major issue. In these cases the recommendation of IARC (Peto et al.,
1980; Gart et al., 1986) could be applied. All the tumours found at
necropsy must be evaluated as "fatal" or "incidental". This approach
permits one to conduct a comparison between young and old animals
despite the very different patterns of survival from those expected.
A crude analysis, ignoring the fact that young animals survive
longer than old ones, will overestimate the ratio of tumour
incidence in young and old animals. Conversely, a "death-rate"
analysis, treating all tumours as if they were fatal, will
over-correct for the effects of differences in survival on the
incidence of tumours discovered at the autopsy of animals that died
of unrelated causes. Bias can be eliminated only if tumours that
were discovered in an incidental context are analysed by the
prevalence method, while tumours discovered in a fatal context are
analysed by the death-rate method (Peto et al., 1980; Gart et al.,
1986).
4.1.7 Extrapolation of animal data to humans
Extrapolation of animal data to humans may be either
qualitative or quantitative. On the basis of experimental results,
the weight-of-evidence approach can help predict whether a given
substance may be considered dangerous for humans. Such information
includes data on the pharmacokinetics of a chemical, its
cytotoxicity and other toxic properties, as well as the data
obtained in in vitro and short-term tests. Of primary importance
for quantitative extrapolation, a dose-response relationship must be
detected in experimental animals. An important stage of quantitative
evaluation is extrapolation of the data obtained from exposure to
rather high doses to lower doses to which humans may be exposed in
the environment. It is necessary to take into account biological
differences, both in pharmacokinetics and pharmacodynamics, between
the test species and humans. For example, a dose taken per unit of
the body surface area, or its concentration in the daily ration,
includes a correction factor for species sensitivity and allows
extrapolation of experimental doses to man (Mantel & Schneiderman,
1975; Turusov & Parfenov, 1986; Travis et al., 1990). Of course,
species sensitivity to the action of some chemicals may also vary
significantly.
Various physiological and mathematical models have been
proposed for extrapolation of toxicological effects. Most of these
models refer to carcinogenesis (Turusov & Parfenov, 1986; Swenberg
et al., 1987; Clayson, 1988). The predictive nature of these models
for non-carcinogenic end-points remains to be determined.
4.2 Epidemiological and clinical approaches
4.2.1 Disease pattern of aged population
In order to study the pattern of disease occurring in the
elderly, the first question is "What illnesses are most common and
important among the elderly?" The answer can be most easily provided
in terms of diseases that cause death, hospitalization or visits to
a doctor's surgery.
The assessment of health status among the elderly on an
international basis is essentially limited to the use of mortality
data, since these are the only comprehensive data available. In the
developed countries, roughly 50% of all deaths occurring between the
ages of 65 and 74 years are attributable to cardiovascular diseases.
Among males in this age group, ischaemic heart disease accounts for
25% of deaths, while 11% are due to cerebrovascular diseases. For
women, 20% of deaths are attributed to ischaemic heart disease and
about 15% to stroke. Cancer accounts for another 25% of deaths among
men and women aged 65-74, lung cancer being the cause of 10% of all
deaths among elderly males. Roughly 7% of deaths are due to
respiratory diseases and 3% to external causes (WHO, 1989).
The trends in the four leading causes of death, i.e. malignant
neoplasms, heart diseases, cerebrovascular diseases and respiratory
diseases, within the age range 65-74 showed that, in selected
developed countries between 1950-1954 and 1980-1984, death rates
from all malignant neoplasms rose slightly for men but remained
essentially unchanged for women except in France. A more marked
decline in mortality is apparent for heart disease in the USA and
Australia where death rates have fallen by 25-30% since the late
1960s. Male mortality has fallen in France and Japan, but has risen
in Hungary. A similar pattern of change is also apparent for
females. Mortality from stroke has also declined in most countries,
although the timing and extent of the duration has varied from
country to country. For example, the decline in Japan occurred ten
years earlier than that in Australia. In the United Kingdom, France
and USA, rates have been falling since the 1950s. The trend in
mortality from respiratory diseases is less clear, there being
little evidence of sustained and comprehensive decline. However, it
is apparent that there has been some progress in reducing mortality
from these diseases in Australia and the United Kingdom over the
last decade (WHO, 1989).
It must be recognized from the outset that mortality data do
not always accurately reflect the underlying morbidity and are
particularly inappropriate in the case of many conditions for which
the fatality rate is low, yet which are important causes of
morbidity among the elderly. Data on causes of death are also less
reliable for the elderly than for other age groups owing to the
multiple pathological conditions often present at the time of death.
Nevertheless, with few exceptions, comprehensive morbidity data are
not available for the elderly.
Data from the USA shows that cardiovascular diseases cause the
largest proportion of hospitalization in the elderly. As a group,
diseases of the digestive system account for the second most common
diagnostic category while neoplastic diseases (90% malignant) are
the third most common cause of hospitalization. The remaining causes
are diseases of the respiratory system, injury or poisoning, and
diseases of the genitourinary or musculoskeletal systems (White,
1989). In a study from Shanghai, China, the common diseases of
hospitalized patients over 65 years of age in the 1950s, when
arranged in order of frequency, were hypertension, coronary artery
disease, chronic bronchitis, prostate hypertrophy, femur fracture,
pulmonary tuberculosis, diabetes mellitus, cholelithiasis and
tumours of various organs. Coronary artery disease, pneumonia,
hypertension and chronic bronchitis became the leading causes of
hospitalization in the 1970s (Zhu et al., 1982). In the 1980s, data
from other parts of China (Jiangxi, Liaoning, Xinjiang) showed that
chronic bronchitis or pneumonia was the most frequent cause of
hospitalization for elderly patients, followed by hypertension and
coronary artery disease (Xu et al., 1986; Shen et al., 1987; Xiong,
1989).
Data from the USA shows that diagnoses arising from visits to
the doctors surgery by people aged 75 or older, when arranged in
order of frequency, are hypertension (17.6%), chronic ischaemic
heart disease (9.5%), diabetes mellitus (6.7%), osteoarthritis (6%),
cataracts (5.1%), heart failure (4.4%), cardiac arrhythmia (3.6%),
arthropathies (3.6%), glaucoma (2.8%), hypertensive heart disease
(2.6%), angina pectoris (2.3%), chronic airway obstruction (2%) and
neoplasms (1.4%) (White, 1989).
4.2.2 Assessment of effects of environmental chemicals in the
elderly population
There are more limitations in clinical studies than in animal
toxicology studies. Firstly, the conditions in the study are not
easily controlled. Secondly, presumably harmful effects to the
subjects under study need to be avoided in designing the protocol.
The following approach is suggested.
Comprehensive, multidimensional functional assessment of the
elderly should be carried out. This involves analysis of the
physical, psychological, social, environmental and other aspects of
functioning (Zarit et al., 1985; Kane, 1987). Measurements of the
ability to perform the activities of daily life should be obtained.
Physical functioning can be measured by a combination of diagnosis,
symptom description, health reporting, days in bed or hospital
during a specific period, and reported pain or discomfort. The
subject's orientation with respect to time, place and person, as
well as short-term and long-term memory, should be assessed.
Psychological measures are used to evaluate depression, anxiety,
loneliness and sense of mental well-being, and a psychiatric history
should be recorded.
Clinical assessment involves a complete physical examination
and selective laboratory or instrumental examinations. As most
environmental chemicals affect certain parts or organ systems of the
body, laboratory or instrumental examination of these particular
parts or organ systems should be performed. The purpose of clinical
assessment is to find any structural or functional abnormalities
that may occur during the course of exposure to the environmental
chemicals.
Measures of environmental chemicals in the human body, such as
the determination of their concentration or that of their
metabolites in blood, body fluids or tissues (including nails,
hairs, etc.), faeces, urine and expired air, should be conducted.
Biomarkers of exposure, such as haemoglobin adducts, may be used
when available.
The results of the above-mentioned assessments may be
correlated and analysed to arrive at a conclusion as to whether the
environmental chemicals have harmful effects on the subjects
studied.
4.2.3 Acute episodes
There are few epidemiological reports on the effects of
exposure to environmental toxicants on the aged population. Several
acute air pollution incidents resulted in marked increases in
illness and death, mostly among the elderly. One such incident
occurred in the Meuse River Valley in Belgium in 1930 when
accumulating air contaminants trapped by an inversion caused the
death of 63 people and illness in 6000 residents. An incident in
1948 in Donora, Pennsylvania, USA, resulted from a similar inversion
that covered a wide area. Of the population of 14 000, 20 died
(compared with the expected two deaths for the same period) and 43%
fell ill. Again, the elderly were the most seriously affected. In
London, 4000 excess deaths were attributed to smoke and sulfur
dioxide in the fog of 1952, and an incident in 1962 caused 400
deaths above the normal value for the period. Similar episodes of
fog-induced mortality in various places have been described (Amdur,
1986). In all these episodes, the most vulnerable people were the
elderly, and the victims were usually suffering from acute
bronchitis and pneumonia, resulting in acute respiratory failure.
However, detailed and systematic study of the effects of
environmental chemicals on the elderly is still lacking (Amdur,
1986).
4.2.4 Concerns for the aged population
The health conditions of the elderly are quite different from
those in the early or middle age of human life. Many chronic
diseases that begin at an early age extend into advanced age. Some
pathological conditions occurring in middle age may become
symptomatic in the elderly. Certain medical problems are clearly
more prominent among the elderly, e.g., cancer of the prostate,
temporal arteritis and osteoarthritis. Illness in older people often
involves multiple organ systems that are interrelated by symptoms,
physical findings, functional capacity and treatment. The emotional
and social consequences of the physical conditions are also related.
Furthermore, the manifestations of aging processes that usually
comprise degeneration, both structural and functional, may sometimes
be difficult to differentiate from those of diseases. Aging
processes and existing diseases, as well as social and environmental
factors, act together to make the assessment and care of the elderly
complex and difficult.
As far as the effects of chemicals upon human health are
concerned, the elderly are subjected to long-term exposure to
environmental chemicals. Some exposures occur daily. The source may
be polluted air, water, food or products made of inorganic or
organic chemicals with which people frequently come into contact.
Some exposures occur during occupational activities in which people
are engaged for many years. The cumulative effect of continuous or
repeated exposure to chemicals may result in pathological changes
and clinical manifestations found in later stages of life. In
addition, the structural and functional changes of aging, usually
degenerative in nature, make the elderly more vulnerable to adverse
effects of environmental chemicals.
As mentioned above, the multiple disease status in the elderly
is usually accompanied by polypharmacy. In developed countries, the
consumption of drugs by people over 65 years of age accounts for
25-50% of total drug consumption (Vestal, 1978). The most commonly
used are neuropsychiatric and cardiovascular drugs,
anti-inflammatory analgesics and diuretics. Since these drugs are
used for the relief of symptoms rather then the cure of diseases,
repeated or sustained prescribing is the rule. Under such
conditions, interactions between environmental chemicals and drugs
should be carefully considered.
4.3 Biomarkers of aging
The term "biomarker of aging" arose in conferences sponsored by
the Fund for Integrative Biomedical Research (Regelson, 1983) and
the National Institute on Aging (Reff & Schneider, 1982). The
Committee on Chemical Toxicity and Aging (1987) defined a biomarker
of aging as "a biological event or measurement of a biological
sample that is considered to be an estimate or prediction of one or
more of the aging processes". Baker & Sprott (1988) offered the
following specific features for a biomarker of aging: (a) nonlethal;
(b) highly reproducible within and across species; (c) reflects
physiological age or some basic biological process of aging or
metabolism; (d) displays significant alterations during relatively
short time periods; (e) is crucial to the effective maintenance of
health and prevention of disease; and (f) reflects a measurable
parameter that can be predicted at a later age. Development of a
panel of biomarkers would assist in assessing the effects of
environmental chemicals that modulate aging processes in laboratory
animals and man.
There is currently a significant research effort, especially
with rodent species, to establish batteries of biological markers of
aging (Allaben et al., 1990). But how can one differentiate
alterations that are simple functions of chronological time from
those that are the results of intrinsic aging or of intrinsic aging
coupled with the effects of chronological time? One approach has
been that of comparative gerontology. Starting with a group of
taxonomically related species that vary substantially in their
maximum life-span potentials, one simply asks if the rate of change
of the putative biomarker reflects the maximum life-span potential
of the species.
From a practical point of view, various biomarkers may be used
for the measurement of aging. For different levels of integration of
an organism, different parameters may be used. At the subcellular
and cellular levels, measurements of macromolecular lesions, the
degree of collagen cross-linking, the level of lipofuscin
accumulation, specific enzyme activities, the number of particular
hormone receptors, numbers of cell divisions, etc. may be
investigated as biomarkers. Tissue and organ weights, cellularity,
growth or some functional activity (muscle strength, visual acuity,
secretion rate) may be considered. The functional activity of
motor, cardiovascular, nervous, endocrine, immune, haematopoietic
and other systems and subsystems may be considered at the systemic
level as potential biomarkers of aging. In addition, at the level of
the whole organism, the functions of the main integrative systems
should be addressed.
Test batteries that attempt to measure functional age (Webster
& Logie, 1976), physiological age (Hollingsworth et al., 1965) and
biological age (Furukawa et al., 1975; Nakamura et al., 1982;
Voitenko & Tokar, 1983; Reis & Poethig, 1984; Dubina et al., 1984;
Hochschild, 1989) have similar conceptual underpinnings in that they
utilize the great variability in performance that emerges among
adults of the same chronological age in standardized, age-sensitive
tests. Studies using this approach have been conducted on human
populations, and similar measures have been employed in a few rodent
studies (Hofecker et al., 1980; Dubina et al., 1983). This approach
can be viewed as the performance variability model of biological
age. It has considerable intuitive appeal and, in many cases,
statistical elegance. An individual may be judged biologically
younger if he is performing better than expected for his
chronological age. However, in their extensive and critical reviews,
Costa & McCrae (1980, 1985) revealed many conceptual and
methodological flaws in this approach.
Biomarkers of aging, which are markers of susceptibility, may
have their greatest utility in the study of the effects of
environmental chemicals on the processes of aging. The effects of
environmental chemicals on the elderly would be assessed most
efficiently by using biomarkers of effect (NAS, 1989).
5. CONCLUSIONS
a) With increasing age, humans become more vulnerable to
environmental challenges due to the deterioration of
physiological and psychological processes. Therefore, it is
likely that the elderly will be more susceptible to the harmful
effects of environmental chemicals.
b) The elderly are heterogeneous with respect to the extent of
deterioration of physiological and psychological processes.
c) Few, if any, of the hundreds of thousands of environmental
chemicals have been tested for increased toxicity in the
elderly.
d) Some of the many age-associated diseases may cause an increased
susceptibility to the harmful action of specific environmental
chemicals.
e) The use of animal models for aging research requires special
considerations, such as housing conditions, diets, monitoring
for infectious agents, and specifically defined pathologies. It
is also necessary to assess several age ranges based on
life-table information in order to distinguish the senescent
from the mature and developing animal.
f) The selection of the animal model should be based on the
likelihood of providing information of relevance to specific
problems in humans.
g) The characteristics of the elderly result from intrinsic aging
processes, environmental factors, and other life-span events.
Therefore, the study of the elderly may not provide
information on basic aging processes.
h) Both the size of the aged population and the number of
chemicals in the environment will undoubtedly increase over
the next few decades. It is therefore expected that the adverse
effects of chemical exposure on the elderly will increase in
importance as a health care issue.
6. FURTHER RESEARCH
a) Experimental, epidemiological and clinical data should be
obtained on:
* the toxicity, mutagenicity and carcinogenicity of
environmental chemicals in old individuals as compared to
young adults;
* the effect of age on the pharmacokinetics and
pharmaco-dynamics of environmental chemicals;
* the long-term effect of environmental chemicals on
molecular, cellular and physiological parameters as
potential biomarkers in the elderly.
b) New models should be developed for assessing the susceptibility
of the elderly to environmental chemicals as compared to young
adults. Such models should be suitable for assessment of
particular consequences and relevant to humans, and could
include:
* non-mammalian and mammalian models (e.g., Drosophila,
fish, rabbits, mini-pigs);
* transgenic animal models;
* cell lines with specific genetic characteristics.
c) For each study the appropriate applicability and use of
experimental models and techniques must be validated:
* animal models should be well-defined in terms of survival,
pathology and husbandry;
* human subjects should be examined carefully for subtle
disease status, medical history, life-style and
socio-economic status.
* standard procedures for measuring molecular, cellular and
physiological parameters should be defined (and developed
if necessary) in order to prevent misinterpretation.
d) The effects of environmental chemicals on the processes of
aging remain to be evaluated. A special scientific workshop
should be devoted to this topic.
REFERENCES
American Dietetic Association (ADA) (1984) ADA takes proactive
stance, testifies on older Americans act reauthorization. J Am Diet
Assoc, 84(7): 822-835.
Ali B, Walford RL, & Imamura T (1985) Influence of aging and poly IC
treatment on xenobiotic metabolism in mice. Life Sci, 37: 1387-1393.
Allaben WT, Chou MW, Pegram RA, Leakey J, Feues RJ, Duffy PH,
Turturro A, & Hart RW (1990) Modulation of toxicity and
carcinogenesis by caloric restriction. Korean J Toxicol, 6: 167-182.
Allison T, Hume AL, Wood CC, & Goff WR (1984) Developmental and
aging changes in somatosensory, auditory and visual evoked
potentials. Electroencephalogr Clin Neurophysiol, 58: 14-24.
Amador A, Steger RW, Bartke A, Johns A, Siler-Khodr TM, Parker R Jr,
& Shepherd AM (1985) Testicular LH receptors during aging in Fischer
344 rats. J Androl, 6: 61-64.
Amdur MO (1986) Air pollutants. In: Klassen CD, Amdur UO, & Doull J
ed. Casarett and Doull's toxicology: The basic science of poisons,
3rd ed. New York, Macmillan Publishing Co., pp 801-824.
Ammon HPT, Fahmy A, Mark M, Wahl MA, & Youssif N (1987) The effect
of glucose on insulin-release and ion movement in isolated
pancreatic islets of rats in old age. J Physiol, 384: 347-354.
Anderson S & Brenner BM (1986) The aging kidney : Structure,
function, mechanisms and therapeutic implications. J Am Geriatr Soc,
35(6): 590-593.
Anderson S & Brenner BM (1987) Effects of aging on the renal
glomerulus. Am J Med, 80(3): 435-442.
Anisimov VN (1971) [Blastomogenesis in persistent estrous rats.]
Vopr Onkol, 17(8): 67-75 (in Russian).
Anisimov VN (1987) Carcinogenesis and aging. Boca Raton, Florida,
CRC Press, vol 1 & 2.
Anisimov VN (1988) Effect of age on dose-response relationship in
carcinogenesis induced by single administration of
N-nitroso-methylurea in female rats. J Cancer Res Clin Oncol, 114:
628-635.
Anisimov VN (1989) Age-related mechanisms of susceptibility to
carcinogenesis. Semin Oncol, 16: 10-19.
Anisimov VN & Reiter RJ (1990) [Pineal function in cancer and
aging.] Vopr Onkol, 37: 259-268 (in Russian).
Arfini G, Mutti A, Vescovi P, Ferroni C, Ferrari M, Giaroli C,
Passeri M, & Franchini I (1987) Impaired dopaminergic modulation of
pituitary secretion in workers occupationally exposed to styrene:
Further evidence from PRL response to TRH stimulation. J Occup Med,
29: 826-830.
Armbrecht HJ (1986) Age-related changes in calcium and phosphorus
uptake by rat small intestines. Biochim Biophys Acta, 882: 281-286.
Armbrecht HJ, Doubek WG, & Porter SB (1988) Calcium transport by
basal lateral membrane vesicles from rat small intestine decreases
with age. Biochim Biophys Acta, 944: 367-373.
Armbrecht HJ, Boltz M, Strong R, Richardson A, Bruns MEH, &
Christakos S (1989) Expression of calbindin-D decreases with age in
intestine and kidney. Endocrinology, 125: 2950-2956.
Aschheim P (1976) Aging in the hypothalamic-hypophyseal-ovarian axis
in the rat. In: Everitt AV & Burgess JA ed. Hypothalamus, pituitary
and aging. Springfield, Illinois, Charles C. Thomas, pp 376-418.
Aschoff J (1979) Circadian rhythms: general features and
endocrinological aspects. In: Krieger DT ed. Comprehensive
endocrinology. New York, Raven Press, pp 1-61.
Avogaro P, Bittolo-Bon G, Cazzolato G, Belussi F, & Pontoglio E
(1983) Lipids and proteins of lipoproteins in human atherosclerosis.
Przegl Lek, 40: 713-718.
Bach JF, Darcenne M, Papiernik M, Barvis A, Lavasseur P, & Lebrand H
(1972) Evidence for a serum-factor secreted by the human thymus.
Lancet, ii: 1056-1058.
Baker SR & Rogul M ed. (1987) Environmental toxicity and the aging
processes: Proceedings of a workshop held in Columbia, Maryland, 1-2
October 1985. New York, Alan R. Liss, Inc., 139 pp (Progress in
Clinical and Biological Research, Volume 228).
Baker GT & Sprott RL (1988) Biomarkers of aging. Exp Gerontol, 23:
223-239.
Bang NU, Tessler SU, Heidenreich RO, Marks CA, & Mattler LE (1982)
Thrombosis and atherosclerosis. Chicago, Year Book Medical
Publishers.
Banks YB, Brewster DW, & Birnbaum LS (1990) Age-related changes in
dermal absorption of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and
2,3,4,7,8-pentachlorodibenzofuran (4-PeCDF). Fundam Appl Toxicol,
15: 163-173.
Baron JA (1984) Smoking and estrogen-related disease: A review. Am J
Epidemiol, 119: 9-22.
Barrows CH & Kokkonen GC (1978) Diet and life extension in animal
model systems. Age, 1: 131-143.
Bartsch H (1991) N-Nitrosocompounds and human cancer: where do we
stand? In: Relevance to human cancer of N-nitroso compounds, tobacco
smoke and mycotoxins. Lyon, International Agency for Research on
Cancer, pp 1-110 (IARC Scientific Publications No. 105).
Bauer LA, Black D, Gensler A, & Sprinkle J (1989) Influence of age,
renal function, and heart failure on procainamide clearance and
n-acetylprocainamide serum concentrations. Int J Clin Pharm Ther
Toxicol, 27: 213-216.
Baylis C, Fredericks M, Leypolat J, Frigon R, Wilson C, & Henderson
L (1988) The mechanisms of proteinuria in aging rats. Mech Ageing
Dev, 45: 111-126.
Bayreuther K, Rodemann KP, Francy PI, & Maier K (1988)
Differentiation of fibroblast stem cells. J Cell Sci, 10(suppl):
115-130.
Beebe GW (1979) Reflections on the work of the Atomic Bomb Casuality
Commission in Japan. Epidemiol Rev, 1: 184-210.
Behl CR, Bellantone NH, & Flynn GL (1987) Influences of age on
percutaneous absorption of drug substances. In: Kydonieuks AF &
Berrer B ed. Transdermal delivery of drugs. Boca Raton, Florida, CRC
Press, vol 2, pp 109-132.
Beierschmitt WP & Weiner M (1986) Age-related changes in renal
metabolism of acetaminophen in male Fischer 344 rats. Age, 9: 7-13.
Beierschmitt WP, Keenan KP, & Weiner M (1986a) Age-related increased
susceptibility of male Fischer 344 rats to acetaminophen
nephrotoxicity. Life Sci, 39: 2335-2342.
Beierschmitt WP, Keenan KP, & Weiner M (1986b) The development of
acetaminophen-induced nephrotoxicity in male Fischer 344 rats of
different ages. Arch Toxicol, 59: 206-210.
Belisle S & Lehoux J-G (1983) Endocrine aging in C57BL Mice. II.
Dynamics of estrogen receptors in the hypothalamic pituitary axis. J
Steroid Biochem, 18: 737-743.
Bender AD (1965) The effect of increasing age on the distribution of
peripheral blood flow in man. J Am Geriatr Soc, 13: 192-198.
Bender AD (1968) Effect of age on intestinal absorption:
Implications for drug absorption in the elderly. J Am Geriatr Soc,
16: 1331-1339.
Bender AD (1979) Drug sensitivity in the elderly. In: Crooks J &
Stevenson IH ed. Drugs and the elderly. Baltimore, Maryland,
University Park Press, pp 147-153.
Bender A, Post A, Meier J, Higson J, & Reichard G (1975) Plasma
protein binding of drugs as a function of age in adult human
subjects. J Pharm Sci, 64: 1711-1713.
Benditt EP (1977) The origin of atherosclerosis. Sci Am, 236: 74-85.
Berg BN (1976) Pathology and aging. In: Everitt AV & Burgess JA ed.
Hypothalamus, pituitary and aging. Springfield, Illinois, Charles C.
Thomas, pp 43-67.
Bertoni-Freddari C, Fattoretti P, Casoli T, Meier-Rouge W, & Ulrich
J (1990) The effect of ethanol on neuronal membrane permeability and
synaptic ultrastructure in adult and old rats. In: Bergamini E ed.
Protein metabolism and aging. New York, Wiley-Liss, Inc., p.
331-334.
Bertrand HA, Lynd FT, Masoro EJ, & Yu BP (1980) Changes in adipose
mass and cellularity through the adult life of rats fed ad libitum
or a life-prolonging restricted diet. J Gerontol, 35(6): 827-835.
Besedovsky H, Sorkin E, Keller M, & Müller J (1975) Changes in blood
hormone levels during the immune response (39057). Proc Soc Exp Biol
Med, 150: 466-470.
Besedovsky HO, Del Rey AE, & Sorkin E (1985) Immune-neuroendocrine
interactions. J Immunol, 135: 750-754.
Bhatnagar KP, Kennedy RC, Baron G, & Greenberg RA (1987) Number of
mitral cells and the bulb volume in the aging human olfactory bulb:
a quantitative morphological study. Anat Rec, 218: 73-87.
Bilchert-Toft M (1978) The adrenal glands in old age. In: Greenblatt
RB ed. Geriatric endocrinology. Vol 5: Aging. New York, Raven Press,
pp 81-102.
Birchenall-Sparks M, Richardson A, Roberts M, & Utherford M (1985)
The effect of aging on the structure and function of liver messenger
RNA. Mech Ageing Dev, 32: 99-111.
Birnbaum LS (1983) Distribution and excretion of 2,3,6,2',3',6'-and
2,4,5,2',4',5'-hexachlorobiphenyl in senescent rats. Toxicol Appl
Pharmacol, 70: 262-272.
Birnbaum LS (1987) Age-related changes in carcinogen metabolism. J
Am Geriatr Soc, 35: 51-60.
Birnbaum LS (1989) Age-related changes in drug disposition. In:
Zenser TV & Coe RM ed. Cancer and aging. Berlin, Heidelberg, New
York, Springer-Verlag, pp 25-138.
Birnbaum LS (1991) Pharmacokinetic basis of age-related changes in
sensitivity to toxicants. Annu Rev Pharmacol Toxicol, 31: 101-128.
Birnbaum LS & Baird MB (1979) Senescent changes in rodent hepatic
epoxide metabolism. Chem-Biol Interact, 26: 245-256.
Birren JE, Woods AM, & Williams MV (1979) Speed of behavior as an
indicator of age changes and the integrity of the nervous system.
In: Hoffmeister F & Muller C ed. Brain function in old age. Berlin,
Heidelberg, New York, Springer-Verlag, p 10.
Bitar MS & Shapiro BH (1987) Aberration of heme and hemoprotein in
aged female rats. Mech Ageing Dev, 38: 189-197.
Bjorksten J, Yaeger LL, & Wallace T (1989) Control of aluminium
ingestion and its relation to longevity. Int J Vitam Nutr Res, 58:
462-465.
Blackman MR (1987) Pituitary hormones and aging. Endocrinol Aging,
16(4): 981-994.
Blackwelder WC, Yano K, Rhoads GG, Kagan A, Gordon T, & Palesch Y
(1980) Alcohol and mortality: The Honolulu heart study. Am J Med,
68: 164-169.
Blalock JE, Harbour-McMenamin D, & Smith EM (1985) Peptide hormones
shared by the neuroendocrine and immunologic systems. J Immunol,
135(2): 858/5-861/5.
Blanco P, Machado A, & Satrustegui J (1987) Variations due to
hypoxia and ageing in the activities of glutathione-S-transferase
and NADPH-Cytochrome C reductase. Mech Ageing Dev, 39: 11-19.
Blum M, Averbuch M, Wolman Y, & Aviram A (1989) Protein intake and
kidney function in humans: its effect on 'normal aging'. Arch Inter
Med, 149(1): 211-212.
Blumberg JB (1985) A discussion of drug metabolism and actions in
the aged. Drug-Nutrient Interact, 4: 99-106.
Bolinder J, Kager L, Ostamn J, & Arner P (1983) Differences at the
receptor and postreceptor levels between human omental and
subcutaneous adipose tissue in the action of insulin on lipolysis.
Diabetes, 32: 117-123.
Bondareff W (1986) Neuropathology of nucleus basalis and locus
ceruleus in Alzheimer's disease. In: Scheibel AB, Wechsler AF, &
Brazier MAB ed. The biological substrates of Alzheimer's disease.
New York, London, San Francisco, Academic Press, pp 103-113.
Boobis AR & Davies DS (1984) Human cytochromes P-450. Xenobiotica,
14: 151-185.
Borghoff SJ & Birnbaum LS (1985) Age-related changes in
glucuronidation and deglucuronidation in liver, small intestine,
lung and kidney of male Fischer rats. Drug Metab Dispos, 13: 62-67.
Borghoff SJ & Birnbaum LS (1986) Age-related changes in the
metabolism and excretion of allyl isothiocyanate: A model compound
for glutathione conjugation. Drug Metab Dispos, 14: 417-422.
Borghoff SJ, Stefanski SA, & Birnbaum LS (1988) The effect of age on
the glucuronidation and toxicity of
4,4'-thiobis(6-t-butyl-m-cresol). Toxicol Appl Pharmacol, 92:
453-466.
Bornor JA, Dilworth BB, & Sullivan KM (1988) Exercise and
osteroporosis: A critique of the literature. Physiother Can, 40(3):
146-155.
Boyes WK & Dyer RS (1984) Chlordimeform produces profound,
selective, and transient changes in visual evoked potentials of
hooded rats. Exp Neurol, 86: 434-447.
Bradley RM (1979) The effect of aging on the olfactory sense. In:
Special senses in aging. Ann Arbor, Michigan, University of
Michigan, Institute of Gerontology, pp 3-8.
Bremner WJ, Vitiello MV, & Prinz PN (1983) Loss of circadian
rhythmicity in blood testosterone with aging in normal men. J Clin
Endocrinol Metab, 56: 1278-1281.
Brenner BM, Meyer TW, & Hostetter TH (1982) Dietary protein intake
and the progressive nature of kidney diseases. New Engl J Med, 307:
652-659.
Brizzee KR (1985) Neuron aging and neuron pathology. In: Johnson AH
ed. Relations between normal aging and disease. New York, Raven
Press, pp 191-224.
Brown WW, Davis BB, & Spry LA (1986) Aging and the kidney. Arch
Intern Med, 146: 1790-1796.
Bruce MF (1980) The relation of tactile thresholds to histology in
the fingers of the elderly. J Neurol Neurosurg Psychiatry, 43:
730-734.
Buckler AJ, Vie H, Sonenshein GE, & Miller RA (1988) Defective T
lymphocytes in old mice: Diminished production of mature c-myc RNA
after mitogen exposure not attributable to alterations in
transcription or RNA stability. J Immunol, 140: 2442-2446.
Bullock K (1985) Neuroanatomy of lymphoid tissue. In: Guillelmin R
ed. Neural modulation of immunity. New York, Raven Press, pp
111-141.
Burek JD (1978) Pathology of aging rats. Boca Raton, Florida, CRC
Press.
Burnet FM (1970) The concept of immunological surveillance. Prog Exp
Tumor Res, 13: 1-27.
Butenko GM (1985) Ageing of the immune system and diseases. In:
Likhachev A, Anisimov V, & Montesano R ed. Age-related factors in
carcinogenesis. Lyon, International Agency for Research on Cancer,
pp 71-83 (IARC Scientific Publications No. 58).
Campbell GR & Chamley-Campbell JH (1981) Invited review: The
cellular pathobiology of atherosclerosis. Pathology, 13: 423-440.
Capecchi MR (1989) Altering the genome by homologous recombination.
Science, 244: 1288-1292.
Caraceni MP, Corghi E, Ortolani S, Dindell M, Candotti G, & Ferrari
A (1988) Prevention of early postmenopausal bone loss. The effect of
calcitonin (Staporos). Clin Trials J, 25(6): 401-405.
Carrillo MC, Kitani K, Kanai S, Sato Y, Nokubo M, Ohta M, & Otsubo K
(1989) Differences in the influence of diet on hepatic glutathione
S-transferase activity and glutathione content between young and old
C56 black female mice. Mech Ageing Dev, 40: 1-15.
Carrillo MC, Nokubo M, Sato Y, Kanai S, Ohta M, & Kitani K (1990)
Effect of protein-free diet on activities and subunits of
glutathione S-transferase in livers of young and aged female rats.
Mech Ageing Dev, 56: 230-251.
Carrillo MC, Nokubo M, Kitani K, Kimihiko S, & Sato K (1991)
Age-related alterations of enzyme activities and subunits of hepatic
glutathione S-transferases in male and female Fischer-344 rats.
Biochim Biophys Acta, 1077: 325-331.
Castleden CM, George CF, Marcer D, & Hallet C (1977a) Increased
sensitivity to nitrazepam in old age. Br Med J, 1: 10-12.
Castleden CM, Volans CN, & Raymond K (1977b) The effect of aging on
drug absorption from the gut. Age Ageing, 6: 138-143.
Cathcart R, Schwiers E, Saul RL, & Ames BN (1984) Thymine glycol and
thymidine glycol in human and rat urine: a possible assay of
oxidative DNA damage. Proc Natl Acad Sci (USA), 81: 5633-5637.
Cauley JA, Gutai JP, Kuller LH, Ledonne D, Sandler RB, Sashin D, &
Powell JG (1988) Endogenous estrogen levels and calcium intakes in
postmenopausal women. Relationships with cortical bone measures. J
Am Med Assoc, 260(21): 3150-3155.
Ceda GP, Valenti G, Butturini U, & Hoffman AR (1986) Diminished
pituitary responsiveness to growth hormone-releasing factor in aging
male rats. Endocrinology, 118(5): 2109-2114.
Chakravarty S, Collins WP, Forecast JS, Newton JR, Oram DH, & Strudd
JWW (1976) Hormonal profiles after the menopause. Br Med J, 2:
784-787.
Chan K, Kerdall MJ, Mitchard M, Wells WOE, & Vickers MD (1975) The
effect of aging on plasma pethidine concentration. Br J Clin
Pharmacol, 2: 297-302.
Chandra RK (1985) Trace element regulation of immunity and
infection. J Am Coll Nutr, 4: 5-16.
Chavaz A, Baleurt L, Simonin P, & Fabre J (1974) Influence de l'âge
sur la digoxinémie et la digitalisation. Schweiz Med Wochenschr,
104: 1823-1825.
Chen LH, Liu S, Cook Newell ME, & Barnes K (1985) Survey of drug use
by the elderly and possible impact of drugs on nutritional status.
Drug-Nutrient Interact, 3: 73-86.
Cheney KE, Liu RK, Smith GS, Merdith PJ, Mickey MR, & Walford RL
(1983) The effect of dietary restriction of varying duration on
survival, tumour patterns, immune fraction and body temperture in
BIOC3F1 female mice. J Gerontol, 38: 420-430.
Chengelis CP (1988a) Age- and sex-related changes in the components
of the hepatic microsomal mixed function oxidase system in
Sprague-Dawley rats. Xenobiotica, 18: 1211-1224.
Chengelis CP (1988b) Age- and sex-related changes in epoxide
hydrolase, UDP-glucuronyl transferase, and PAPS-sulfotransferase in
Sprague-Dawley rats. Xenobiotica, 18: 1225-1237.
Chopra IJ, Solomon DH, Chopra U, Wu SY, Fisher DA, & Nakamura Y
(1978) Pathways or metabolism of thyroid hormones. Recent Prog Horm
Res, 34: 521-567.
Christophers E & Kligman AM (1965) Percutaneous absorption in aged
skin. In: Montagna W ed. Advances in the biology of the skin. Vol 6:
Ageing. Oxford, New York, Pergamon Press, pp 163-175.
Clayson DB (1988) Needs for biological risk assessment in
interspecies extrapolation. Environ Health Perspect, 77: 93-97.
Cleroux J, Giannattasio C, Grassi G, Seravalle G, Sampieri L,
Cuspidi C, Bolla G, Valsecchi M, Mazzola C, & Mancia G (1988)
Effects of aging on the cardiopulmonary receptor reflex in
normotensive humans. J Hypertension, 6(suppl 4): 141-144.
Coiro V, Volpi R, Cavazzini V, Bertoni P, Corradi A, Bianconi L,
Davoli C, Rossi G, & Chiodera P (1991) Restoration of normal growth
hormone responsiveness to GHRH in normal aged men by infusion of low
amounts of theophylline. J Gerontol Med Sci, 46(5): M155-M158.
Colombo T, Donelli MG, Urso R, Dallara S, Bartosek I, & Guaitani A
(1989) Doxorubicin toxicity and pharmacokinetics in old and young
rats. Exp Gerontol, 24: 159-171.
Committee on Chemical Toxicity and Aging (1987) Aging in today's
environment. Washington, DC, National Academy Press.
Conn PM, Cooper RL, McNamara MC, Rogers DC, & Shoenhardt L (1980)
Qualitative change in gonadotropin during normal aging in the male
rat. Endocrinology, 106: 1549-1553.
Conrad K & Bressler R (1982) Drug therapy for the elderly. St Louis,
Missouri, C.V. Mosby & Co.
Cooper B, Weinblatt F, & Gregerman R (1977) Enhanced activity of
hormone sensitive adenylated cyclase during dietary restriction in
the rat. J Clin Invest, 39: 467-474.
Cooper RL, Goldman JM, & Rehnberg GL (1986) Pituitary function
following treatment with reproductive toxins. Environ Health
Perspect, 70: 177-184.
Corman B & Michel JB (1986) Renin-angiotensin system,
converting-enzyme inhibition, and kidney function in aging female
rats. Am J Physiol, 251: 450-455.
Costa PT Jr & McCrae RR (1980) Functional age: A conceptual and
empirical critique. In: Haynes SG & Feinleib M ed. Epidemiology of
aging. Washington, DC, National Institutes of Health, National
Institute of Aging, National Heart, Lung, and Blood Institute, pp
23-50 (NIH Publication No. 80-969).
Costa PT & McCrae RR (1985) Concepts of functional or biological
age: A critical view. In: Andres R, Bierman EL, & Hazzard WR ed.
Principles of geriatric medicine. New York, McGraw-Hill, pp 30-37.
Costa LG & Murphy SD (1987) Interaction of the pesticide
chlordimeform with adrenergic receptors in mouse brain: An
in vitro study. Arch Toxicol, 59: 323-327.
Costa LG, Olibet G, & Murphy SD (1988) Alpha2-adrenoreceptors as a
target for formamidine pesticides: in vitro and in vivo studies
in mice. Toxicol Appl Pharmacol, 93: 319-328.
Creasey H & Rapoport SI (1985) The aging of human brain. Ann Neurol,
17(1): 2-10. Crooks J, O'Malley K, & Stevenson IH (1976)
Pharmacokinetics in the elderly. Clin Pharmacokinet, 1: 280-296.
Croucher PI, Mellish RWE, Vedi S, Garrahan NJ, & Compston JE (1989)
The relationship between resorption depth and mean interstitial bone
thickness: Age-related changes in man. Calcif Tissue Int, 45(1):
15-19.
Crowley JJ, Cusack BJ, Jue SG, Koup JR, Park BK, & Vestal RE (1988)
Aging and drug interactions. II. Effects of phenytoin and smoking on
the oxidation of theophylline and cortisol in healthy men. J
Pharmacol Exp Ther, 245: 513-523.
Cuny G, Royer RJ, Mur JM, Serot JM, Faure G, Netter P, Maillard A, &
Penin F. (1979) Pharmacokinetics of salicylates in elderly.
Gerontology, 25: 49-55.
Cusack B & Denham MJ (1984) Nutritional status and drug disposition
in the elderly. In: Roe DA ed. Drugs and nutrition in the geriatric
patients. New York, Churchill Livingstone, pp 47-70.
Cutler RG (1991) Human longevity and aging: possible role of
reactive oxygen species. Ann NY Acad Sci, 621: 1-28.
Cutler RG & Semsei I (1989) Development, cancer and aging: possible
common mechanisms of action and regulation. J Gerontol, 44(6):
25-34.
Daniel FB, Schut HA, Sandwiesh DW, Schenck KM, Hoffmann CO, Patrick
JR, & Stoner GD (1983) Interspecies comparison of benzo(a)pyrene
metabolism and DNA-adduct formation in cultured human and animal
bladder and tracheobronchial tissues. Cancer Res, 43: 4723-4729.
Danon D (1969) Biophysical aspects of red cell ageing. Bibl
Haematol, 15: 275-287.
Davies BH (1985) The respiratory system. In: Pathy MSJ ed.
Principles and practice of geriatric medicine. New York, Chichester,
Brisbane, Toronto, John Wiley and Sons.
Davila DR, Brief S, Simon J, Hammer RE, Brinster RL, & Kelley KW
(1987) Role of growth hormone in regulating T-dependent immune
events in aged, nude and transgenic rodents. J Neurosci Res, 18:
108-116.
Davis PJ & Davis FB (1987) Water excretion in the elderly.
Endocrinol Aging, 16(4): 867-875.
Davis TA, Bales CW, & Beauchene RE (1983) Differential effects of
dietary, caloric, and protein restriction in the aging rat. Exp
Gerontol, 18: 427-435.
Dawson-Hughes B, Shipp C, Sadowski L, & Dallal G (1987) Bone density
of the redius, spine, and hip in relation to percent of ideal body
weight in postmenopausal women. Calcif Tissue Int, 40(6): 310-314.
Dement W, Richardson G, Prinz P, Carskadon M, Kripke D, & Czeisler C
(1985) Changes of sleep and wakefulness with age. In: Finch CE &
Schneider EL ed. Handbook of the biology of aging, 2nd ed. New York,
Van Nostrand Reinhold Company, pp 692-717. Department of
International Economic and Social Affairs (1991) World population
prospects 1990, New York, United Nations, pp 192-215 (Population
Studies No. 120).
Dilman VM (1971) Age associated elevation of hypothalamic threshold
to feedback control and its role in development, aging and disease.
Lancet, i: 1211-1219.
Dilman VM (1987) [Four models of medicine.] Leningrad, Meditsina (in
Russian).
Dilman VM & Anisimov VN (1979) Hypothalmic mechanism of ageing and
of specific age pathology. I. Sensitivity threshold of
hypothalamo-pituitary complex to homeostatic stimuli in the
reproductive system. Exp Gerontol, 14: 161-174.
Dix D (1989) The role of aging in cancer incidence: an
epidemiological study. J Gerontol, 44(6): 10-18.
Doll R (1973) Age. In: Doll R & Vodopija I ed. Host environment
interactions in the etiology of cancer in man. Lyon, International
Agency for Research on Cancer, pp 39-48 (IARC Scientific
Publications No. 7).
Doll R (1978) An epidemiological perspective of the biology of
cancer. Cancer Res, 38: 3573-3583.
Doll R & Peto R (1981) The causes of cancer. J Natl Cancer Inst, 66:
1193-1312.
Donaldson J (1987) The physiopathologic significance of manganese in
brain: Its relation to schizophrenia and neurodegenerative
disorders. Neurotoxicology, 8: 451-462.
Doubek WG & Armbrecht HJ (1987) Changes in intestinal glucose
transport over the lifespan of the rat. Mech Ageing Dev, 39: 91-102.
Dubina TL, Dyundikova VA, & Zhuk EV (1983) Biological age and its
estimation. II. Assessment of biological age of albino rats by
multiple regression analysis. Exp Gerontol, 18: 5-18.
Dubina TL, Mints A, & Zhuk EV (1984) Biological age and its
estimation. III. Introduction of a correction to the multiple
regression model of biological age and assessment of biological age
in cross-sectional and longitudinal studies. Exp Gerontol, 19:
133-143.
Duckles SP (1987) Influence of age on vascular adrenergic
responsiveness. Blood Vessels, 24(3): 113-116.
Dundee JW (1979) Response to anaesthetic drugs in the elderly. In:
Crooks J & Stevenson IH ed. Drugs and the elderly. Baltimore,
Maryland, University Park Press, pp 179-187.
Dunn MF, Pattison SE, Storm MC, & Quiel E (1980) Comparison of the
zinc binding domains in the 7S nerve growth factor and the
zinc-insulin Hexamer. Biochemistry, 19: 718-725.
Dybkaer R, Lauritzen DM, & Krakauer R (1981) Relative reference
values for clinical chemical and haematological quantities in
"healthy" elderly people. Acta Med Scand, 209: 1-9.
Eastell R & Riggs BL (1987) Calcium homeostasis and osteoporosis.
Endocrinol Metab Clin, 16(4): 829-843.
Eastin WC Jr & Birnbaum LS (1987) Intestinal absorption of two
glucose analogues in rats of different ages. Exp Gerontol, 22:
351-357.
Ebbesen P (1985) Papilloma development on young and senescent mouse
skin treated with 12-0-tetradecanoylphorbol-13-acetate. In:
Likhachev A, Anisimov V, & Montesano R ed. Age-related factors in
carcinogenesis. Lyon, International Agency for Research on Cancer,
pp 167-170 (IARC Scientific Publications No. 58).
Edelman IS & Leibman J (1959) Anatomy of body water and
electrolytes. Am J Med, 27: 256-277.
Eden S (1987) Endocrinology in older people. Acta Obstet Gynaecol
Scand, 140(suppl): 9-22.
Edman CD (1983) Estrogen replacement therapy. In: Buchsbaum HJ ed.
The menopause. Berlin, Heidelberg, New York, Springer-Verlag.
Ellis GB & Desjardins C (1982) Male rats secrete luteinizing hormone
and testosterone episodically. Endocrinology, 110: 1618-1627.
Euans DW (1988) Renal function in the elderly. Am Fam Physician,
38(3): 147-150.
Evens RP & Hawkins DW (1984) Bone and joint disorders. In: Covington
TR & Ingram Walker J ed. Current geriatric therapy. Philadelphia,
Pennsylvania, W.B. Saunders Co., pp 277-306.
Exton-Smith AN (1985) Mineral metabolism. In: Finch CE & Schneider
EL ed. Handbook of the biology of aging. New York, Van Nostrand
Reinhold Company, pp 511-539.
Fabris N (1981) Ontogenetic and phylogenetic aspects of
neuroendocrine-immune network. Dev Comp Immunol, 5(suppl 1): 49-60.
Fabris N (1991) Neuroendocrine-immune interactions: a theoretical
approach of aging. Arch Gerontol Geriatry, 12: 219-230.
Fabris N & Piantanelli L (1982) Thymus-neuroendocrine interactions
during development and aging. In: Adelman RC & Roth GS ed. Hormones
and aging. Boca Raton, Florida, CRC Press, pp 167-181.
Fabris N & Provinciali M (1989) Hormones. In: Nelson D ed. Natural
immunity. New York, London, San Francisco, Academic Press, pp
306-347.
Fabris N, Giunta S, & Muzzioli M (1983) Decline of T-cell potential
in diabetic and aged men. J Gerontol, 38(5): 548-554.
Fabris N, Mocchegiani E, Muzzioli M, & Provinciali M (1988)
Neuroendocrine-thymus interactions: perspectives for intervention in
aging. Ann NY Acad Sci, 521: 72-87.
Fabris N, Mocchegiani E, Marriotti S, Pacini F, & Pinchera A (1989)
Thyroid-thymus interactions during development and aging. Horm Res,
31: 85-98.
Fabris N, Mocchegiani E, Muzzioli M, & Provinciali M (1990) Zinc,
immunity and aging. In: Goldstein AL ed. Biomedical advances in
aging. New York, London, Plenum Press, pp 271-281.
Fando JL, Salinas M, & Wasterlain CG (1980) Age-dependent changes in
brain protein synthesis in the rat. Neurochem Res, 5: 373-378.
Feldman RD, Limbird LE, Nadeau J, Robertson D, & Wood AJ (1984)
Alterations in leukocyte ß-receptor affinity with aging. New Engl J
Med, 310: 815-819.
Felten DL, Felten SY, Carlsan SL, Olschowka JA, & Livnat S (1985)
Neuroadrenergic and peptidergic innervation of lymphoid tissue. J
Immunol, 135: 755.
Fernandes G (1984) Nutrional factors: Modulating effects on immune
function and aging. Pharmacol Rev, 36(2): 123-129.
Fernandes G, Friend P, & Yunis EJ (1977) Influence of calorie
restriction on autoimmune disease. Fed Proc, 6: 1313.
Ferro-Luzzi A, Maiani G, Scaccini C, D'Amicis A, Borgioni G, Polito
A, Ronaldi L, Sett S, Branca F, Azzini E, Arena A, Raguzzini A,
Castata G, & Dente MG (1988) In: Lintas C & Spadoni MA ed.
Nutritional vulnerability of the Italian elderly: Facts and figures.
Rome, National Council for Research, pp 275-294 (Monografia No. 28).
Finch CE (1991) Longevity, senescence, and the genome. Chicago,
University of Chicago Press.
Finch CE & Hayflick L ed. (1977) Handbook of the biology of aging.
New York, Van Nostrand Reinhold Company.
Finch CE & Landfield PW (1985) Neuroendocrine and autonomic
functions in aging mammals. In: Finch CE & Schneider EL ed. Handbook
of the biology of aging, 2nd ed. New York, Van Nostrand Reinhold
Company, pp 645-691.
Fishbein L (1991) Biological effects of dietary restriction. Berlin,
Heidelberg, New York, Springer-Verlag.
Fleming BB & Barrows CH Jr (1982a) The influence of aging on
intestinal absorption of vitamins A + D by the rat. Exp Gerontol,
17: 115-120.
Fleming BB & Barrows CH Jr (1982b) The influence of aging on
intestinal absorption of vitamin B12 and niacin in rats. Exp
Gerontol, 17: 121-126.
Fraga CG, Shigenaga MK, Park JW, Degan P, & Ames BN (1990) Oxidative
damage to DNA during aging: 8-hydroxy-2-deoxyguanosine in rat organ
DNA and urine. Proc Natl Acad Sci (USA), 87: 4533-4537.
Friedman SA, Raizner RE, Rosen H, Solomon NA, & Wilfredo SY (1972)
Functional defects in the aging kidney. Ann Intern Med, 76: 41-45.
Friedman FK, Robinson RC, & Rifkind J (1989) Age-related changes in
the iron spin state of testosterone-binding rat liver microsomal
cytochromes P450. Biochem Biophys Res Commun, 158: 480-484.
Friend PS, Fernandes G, Good RA, Michael AF, & Yunis EJ (1978)
Dietary restrictiions early and late. Effects on nephropathy of the
NZB and NZW mouse. Lab Invest, 38: 629-632.
Frith CH & Ward JM (1988) Colour atlas of neoplastic and
non-neoplastic lesions in aging mice. Amsterdam, Oxford, New York,
Elsevier Science Publishers.
Fujita S, Katagawa H, Ishizawa H, Suzuki T, & Kitani K (1985)
Age-associated alterations in hepatic glutathione-s-transferase
activities. Biochem Pharmacol, 34: 3891-3894.
Furukawa T, Inoue M, Kajiya F, Inada H, Takasugi S, Fukui S, Takeda
H, & Abe H (1975) Assessment of biological age by multiple
regression analysis. J Gerontol, 30: 422-434.
Gaitz CM & Baer PE (1971) Characteristics of elderly patients with
alcoholism. Arch Gen Psychiatry, 24: 372.
Galinsky RE, Kane RE, & Franklin MR (1986) Effect of aging on
drug-metabolizing enzymes important in acetaminophen elimination. J
Pharmacol Exp Ther, 237: 107-113.
Galinsky RE, Johnson DH, Kane RE, & Franklin MR (1990) Effect of
aging on hepatic biotransformation in female Fischer 344 rats:
changes in sulfotransferase activities are consistent with known
gender-related changes in pituitary growth hormones secretions in
aging animals. J Pharmacol Exp Ther, 255: 577-583.
Gallagher D, Thompson LW, & Levy SM (1980) Clinical psychological
assessment of older adults. In: Poon LW ed. Aging in the 1980s:
Psychological issues. Washington, DC, American Psychological Press,
pp 19-40.
Gambert SR & Tsitouras PD (1985) Effect of age on thyroid hormone
physiology and function. J Am Geriatr Soc, 33(5): 360-365.
Gart JJ, Krewski D, Lee PN, Tarone RE, & Wahrendorf J (1986)
Statistical methods in cancer research. Vol III: The design and
analysis of long-term animal experiments. Lyon, International Agency
for Research on Cancer, 219 pp (IARC Scientific Publications No.
79).
Gilchrest BA (1984) Skin and aging processes. Boca Raton, Florida,
CRC Press, pp 1-120.
Globerson AR, Abel EI, & Ren-Menahem D (1989) Developmental aspects
of T-lymphocytes in aging. New York, London, Plenum Press, pp
363-373.
Goldstein RS, Pasino DA, & Hook JB (1986) Cephaloridine
nephrotoxicity in aging male Fischer 344 rats. Toxicology, 38:
43-63.
Goldstein RS, Tarloff JB, & Hook JB (1988) Age-related nephropathy
in laboratory rats. FASEB J, 2: 2241-2251.
Gollamudi HR, Prasanna R, Rao RH, Lawrence WH, & Autian J (1983)
Impaired metabolism of di (2-ethylhexyl)phthalate (DEHP) in old rats
- an in vitro study. J Toxicol Environ Health, 12: 623-632.
Gompertz B (1825) On the nature of the function expressive of the
law of human mortality and on a new mode of determining life
contingencies. Phil Trans R Soc London, II: 513-585.
Gossen JA, De Leeuw WJF, Tan CHT, Zwarthoff EC, Berends F, Lohman
PHM, Knook DL, & Vijg J (1989) Efficient rescue of integrated shutle
vectors from transgenic mice: a model for studying mutations in
vivo. Proc Natl Acad Sci (USA), 86: 7971-7975.
Govoni S, Memo M, Spano PF, & Trabucchi M (1979) Chronic lead
treatment differentially affects dopamine synthesis in various rat
brain areas. Toxicology, 12: 343-349.
Govoni S, Rius RA, Battaini F, Magnoni MS, Lucchi L, & Trabucchi M
(1988) The central dopaminergic system: Susceptibility to risk
factors for accelerated aging. Gerontology, 34: 29-34.
Goyal KK (1982) Changes with age in the aorta of man and mouse. Exp
Gerontol, 17: 127-132.
Graham, P (1985) The eye. In: Pathy MSJ ed. Principles and practice
of geriatric medicine. New York, Chichester, Brisbane, Toronto, John
Wiley and Sons, pp 833-844.
Grandjean P (1983) Behavioral toxicology of heavy metals. In:
Zbinden G, Cuomo V, Recagni G, & Weiss B ed. Application of
behavioral pharmacology in toxicology. New York, Raven Press, vol 2,
pp 331-339.
Greden JF, Flegel P, Haskett R, Dilsaver S, Carrol BJ, Grunhaus L, &
Genero N (1986) Age effects in serial hypothalamic-pituitary-adrenal
monitoring. Psychoneuroendocrinology, 11(2): 195-204.
Greenberg LH & Weiss B (1978) beta-Adrenergic receptors in aged rat
brain: reduced number and capacity of pineal gland to develop
supersensitivity. Science, 201: 61-63.
Greenberg LH & Weiss B (1979) Ability of aged rats to alter
beta-adrenergic receptors of brain in response to repeated
administration of reserpine and desmethylimipramine. J Pharmacol Exp
Ther, 211: 309-315.
Greenberg LH & Weiss B (1983) Neuroendocrine control of
catecholaminergic receptors in aging brain. In: Agnoli A, Crepaldi
G, Spano PF, & Trabucchi M ed. Aging brain and ergot alkaloids. New
York, Raven Press, pp 37-52.
Greenblatt DJ, Divoll MK, Harmatz JS, & Shader RI (1988) Antipyrine
absorption and disposition in the elderly. Pharmacology, 36:
125-133.
Greenstein BD, Fitzpatrick FT, Kendall MD, & Wheeler MJ (1987)
Regeneration of the thymus in old male rats treated with a stable
analogue of LHRH. J Endocrinol, 11: 345-350.
Gregerman RI & Solomon N (1967) Acceleration of thyroxine and
triiodothyronine turnover during bacterial pulmonary infections and
fever: implications for the functional state of the thyroid during
stress and in senescence. J Clin Endocrinol Metab, 27: 93-105.
Groop PH (1989) The influence of body weight, age and glucose
tolerance on the relationship between GIP secretion and beta-cell
function in man. Scand J Clin Lab Invest, 49(4): 367-379.
Gu X (1986) The life expectancy of the people of China. In: Cui Y
ed. Public health in the People's Republic of China. Beijing,
People's Medical Publishing House, pp 87-91.
Gupta SP, Mehta FS, & Irani RR (1980) Comparison of mortality rates
among bidi smokers and tobacco chewers. Ind J Cancer, 17: 149-152.
Guthrie S, Cooper RL, Thurman R, & Linnoila M (1987)
Pharmacodynamics and pharmacokinetics of ethanol, diazepam, and
pentobarbital in young and aged rats. Pharmacol Toxicol, 61:
308-312.
Hadden JW (1983) Cyclic nucleotides and related mechanisms in immune
regulation: A mini review. In: Fabris N, Garaci E, Hadden J, &
Mitchison NA ed. Immunoregulation. New York, London, Plenum Press,
pp 201-230.
Halberg F (1982) Biological rhythms, hormones and aging. In:
Vernandakis A ed. Hormones in development and aging. New York,
Spectrum Publications, pp 451-476.
Hall HRS, O'Grady MP, & Farrah JM Jr (1989) The
hypothalamic-pituitary-adrenal axis by thymic peptides. In: Hadden
JW, Massek K, & Nistico G ed. Interactions among central nervous
system, neuroendocrine and immune system. Rome, Pytagora Press, pp
112-125.
Hanawalt PC (1987) On the role of DNA damage and repair processes in
aging: evidence for and against. In: Warner HR, Butler RN, Sprott
RN, & Schneider EL ed. Modern biological theories of aging. New
York, Raven Press, pp 183-198.
Hanninen H (1982) Behavioral effects of occupational exposure to
mercury and lead. Acta Neurol Scand, 66(suppl 92): 167-175.
Hansen ON, Trillingsgaard A, Beese I, Lyngbye T, & Grandjean P
(1985) Neurobehavioral methods in assessment of children with
low-level lead exposure. In: Neurobehavioral methods in occupational
and environmental health (Document 3). Copenhagen, World Health
Organization, Regional Office for Europe, pp 183-187. Harman D
(1981) The aging process. Proc Natl Acad Sci (USA), 78: 7124-7128.
Harman D (1982) Nutritional implications of the radical theory of
aging. J Am Coll Nutr, 1: 27-34.
Harman SM, Tsitouras PD, Costa PT, & Blackman MR (1982) Reproductive
hormones in aging men. II. Basal pituitary gonadotropins and
gonadotropin responses to luteinizing hormone releasing hormone. J
Clin Endocrinol Metab, 54: 547-551.
Harrison DE, Astle CM, & Delaittre JA (1978) Loss of proliferative
capacity in immunohemopoietic stem cells caused by serial
transplantation rather than ageing. J Exp Med, 147(5): 1526-1531.
Hayflick L & Moorhead PS (1961) The serial cultivation of human
diploid cell strains. Exp Cell Res, 25: 585-621.
Hazzard WR (1985) Aging and atherosclerosis. Teasing out the
contributions of time, secondary aging, and primary aging. Clin
Geriatr Med, 1(1): 251-284.
Hebert CD & Birnbaum LS (1987) The influence of aging on intestinal
absorption of TCDD in rats. Toxicol Lett, 37: 47-55.
Hendley DD, Mildvan AS, Reporter MC, & Strehler BL (1963) The
properties of isolated human cardiac age pigment. II Chemical and
enzymatic properties. J Gerontol, 18: 250-259.
Hershkowitz M (1983) Mechanisms of brain aging - The role of
membrane fluidity. In: Gispen WH & Traber J ed. Aging of the brain.
Amsterdam, Oxford, New York, Elsevier Science Publishers, pp 85-98.
Hirsch HR (1982) Evolution of senescence: natural increase of
population dispaying Gompertz or power-law death rates and constant
of age-dependent maternity rates. J Theor Biol, 98: 321-346.
Hochschild R (1989) Improving the precision of biological age
determinations. Part I. A new approach to calculating biological
age. Exp Gerontol, 24: 289-300.
Hofecker G, Skalicky M, Kment A, & Niedermuller H (1980) Models of
the biological age of the rat. I. A factor model of age parameters.
Mech Ageing Dev, 14: 345-359.
Hogstedt C, Hane M, Agrell A, & Bodin L (1983) Neuropsychological
test results and symptoms among workers with well-defined long-term
exposure to lead. Br J Ind Med, 40: 99-105.
Hollander D, Dadufalz V, Weindruch R, & Walford RL (1986) Influence
of life-prolonging dietary restriction on intestinal vitamin A
absorption in mice. Age, 9: 57-60.
Hollingsworth JW, Hashizume A, & Jablon S (1965) Correlations
between tests of aging in Hiroshima subjects - an attempt to define
"physiological age." Yale J Biol Med, 38: 11-26.
Holt AR, Pascal RR, & Kotler DP (1984) Effect of aging upon small
intestinal structure in the Fischer rat. J Gerontol, 39: 642-647.
Holt PR, Yeh KY, & Kotler DP (1988) Altered controls of
proliferation in proxismal small intestine in the senescent rat.
Proc Natl Acad Sci (USA), 85: 2771-2775.
Honma T, Miyagawa M, & Sato M (1987) Methyl bromide alters
catecholamine and metabolite concentrations in rat brain.
Neurotoxicol Teratol, 9: 369-375.
Horbach GJM, Princen HMG, Van der Kroef M, Van Bezooijen CFA, & Yap
SH (1984) Changes in the sequence content of albumin mRNA and in
its translational activity in the rat liver with age. Biochim
Biophys Acta, 83: 60-66.
Horbach GJM, Van der Boom H, Van Bezooijen CFA, & Yap SH (1986)
Molecular aspects of age-related changes in albumin synthesis in
rats. In: Van Bezooijen CFA, Miglio F, & Knook DL ed. Liver drugs
and aging. Rijswijk, The Netherlangs, Eurage, pp 121-126 (Topics in
Aging Research in Europe, Volume 7).
Horbach GJM, Durham SK, Yap SH, & Van Bezooijen CFA (1988a) Albumin
elimination in female WAG/Rij rats with age: A longitudinal study.
Mech Ageing Dev, 43: 137-152.
Horbach GJM, Van der Boom H, Yap SH, & Van Bezooijen CFA (1988b)
Molecular aspects of age-related changes in albumin synthesis in
female WAG/Rij rats. Life Sci, 43: 1707-1714.
Hosomi T (1990) Japanese economy and society. In: Institute of
Japanese Problems, Chinese Academy of Social Sciences and Japanese
Vital Insurance Company ed. Proceeding of a Symposium on Sino-Japan
Social Countermeasure for Aging Problems. Beijing, Dong-Fang
Publishing House pp 7-30.
Houck JC, Dehesse C, & Jacob R (1967) The effect of aging upon
collagen catabolism. Symp Soc Exp Biol, 21: 403-425.
Hsu WH & Kakuk TJ (1984) Effect of amitraz and chlordimeform on
heart rate and pupil diameter in rats: Mediated by alpha
2-adrenoreceptors. Toxicol Appl Pharmacol, 73: 411-415.
Hunter J, Urbanowicz MA, Yule W, & Landsdown R (1985) Automated
testing of reaction time and its association with lead in children.
Int Arch Occup Environ Health, 57: 27-34.
Hunziker PE & Kagi JHR (1985) Metallothionein. In: Harrison PM ed.
Metalloproteins: Part II. London, MacMillan Press Ltd.
Huskisson EC (1983) NSAIDS: A topical review. Geriatr Med, 12:
867-870.
IARC (1982) Chemicals, industrial processes and industries
associated with cancer in humans. IARC Monographs, Volumes 1 to 29.
Lyon, International Agency for Research on Cancer, 292 pp (IARC
Monographs on the Evaluation of the Carcinogenic Risk of Chemicals
to Humans, Supplement 4).
IARC (1986) In: Montesano R, Bartsch H, Vainio H, Wilbourne J, &
Yamasaki H ed. Long-term and short-term assays for carcinogens: A
critical appraisal. Lyon, International Agency for Research on
Cancer, 564 pp (IARC Scientific Publications No. 83).
IARC (1988) Alcohol drinking. Lyon, International Agency for
Research on Cancer, 416 pp (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Volume 44).
IARC (1990) In: Tomatis L ed. Cancer: causes, occurrence and
control. Lyon, International Agency for Research on Cancer, 352 pp
(IARC Scientific Publications No. 100).
Imayama S & Braverman IM (1989) A hypothetical explanation for the
aging of skin. Chronologic alterations of the three-dimensional
arrangement of collagen and elastic fibres in connective tissue. To
provide a morphologic basis for a better understanding of the
"aging" of human. Am J Pathol, 134(5): 1019-1025.
Ingbar SH (1978) The influence of aging on the human thyroid hormone
economy. In: Greennblatt RB ed. Geriatric endocrinology. New York,
Raven Press, pp 13-31.
Ingram DK, Cutler RG, Weindruch R, Renquist DM, Knapka JJ, April M,
Belcher CT, Clark MA, Hatcherson CD, Marriott M, & Roth GS (1990)
Dietary restriction and aging: the initiation of a primate study. J
Gerontol, 45(5): 148-163.
Iqbal K, Grundke-Iqbal I, Merz PA, & Wisniewski HM (1982)
Age-associated neurofibrillary changes. In: Giacobini E, Filogamo G,
Giacobini G, & Vernadakis A ed. The aging brain: cellular and
molecular mechanisms of aging in the nervous system. New York, Raven
Press, pp 247-257.
Iwasaki K, Chirago T, Tada K, Noda K, & Noguchi H (1986) Age- and
sex-related changes in amine sulphoconjugations in Sprague-Dawley
strain rats. Comparison with phenol and alcohol sulphoconjugations.
Xenobiotica, 16: 717-723.
Iwasaki K, Gleiser CA, Masoro EJ, McMahan CA, Seo E, & Yu BP (1988)
Influence of the restriction of individual dietary components on
longevity and age-related disease of Fisher rats: The fat component
and the mineral component. J Gerontol, 43: B13-B21.
Iwase A, Kitazawa Y, & Ohno Y (1988) On age-releated norms of the
visual field. Jpn J Ophthalmol, 34(4): 429-437.
Iwata T, Incefy GS, Tanaka T, Fernandes G, Menendez-Botel CI, Pih K,
& Good RA (1979) Circulating thymic hormone levels in zinc
deficiency. Cell Immunol, 47: 100-105.
Jackson J, Banks YB, & Birnbaum LS (1990) Maximal dermal absorption
of TCDD occurs in weanling rats. Toxicologist, 10: 309.
Jaenisch R (1988) Transgenic animals. Science, 240: 1468-1474.
Jones MK, Weisenburger WP, Sipes IG, & Russell DH (1987) Circadian
alterations in prolactin, corticosterone, and thyroid hormone levels
and down-regulation of prolactin receptor activity by
2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol, 87:
337-350.
Kaldor JM & Day NE (1987) Interpretation of epidemiological studies
in the context of multistage model of carcinogenesis. In: Barrett JC
ed. Mechanisms of environmental carcinogenesis. Boca Raton, Florida,
CRC Press, vol 2, pp 21-57.
Kalimi M (1982) Comparison of glucocorticoid receptors in various
tissues of adult and senescent rats. Gerontology, 28: 371-376.
Kamataki T, Maeda K, Shimado M, Kitani K, Nagai T, & Kato R (1985a)
Age-related alteration in the activities of drug-metabolizing
enzymes and contents of sex-specific forms of cytochrome P450 in
liver microsomes from male and female rats. J Pharmacol Exp Ther,
233: 222-228.
Kamataki T, Shimada M, Maeda K, & Kato R (1985b) Pituitary
regulation of sex-specific forms of cytochrome P-450 in liver
microsomes of rats. Biochem Biophys Res Commun, 130: 1247-1253.
Kampmann JP & Hansen JEM (1979) Renal excretion of drugs. In: Crooks
J & Stevenson IH ed. Drugs and the elderly. Baltimore, Maryland,
University Park Press, pp 77-87.
Kanai S, Kitani K, Fujita S, & Kitagawa H (1985) The hepatic
handling of sulfobrompthalein in aging Fischer-344 rats: in vivo
and in vitro studies. Arch Gerontol Geriatr, 4: 73-85.
Kanai S, Kitani K, Sato Y, & Nokubo M (1988) The age-dependent
decline in the biliary transport maximum of conjugated
sulfobromophthalein in the rat. Arch Gerontol Geriatr, 7: 1-8.
Kane R (1987) Comprehensive assessment. In: Maddox GL ed. The
encyclopedia of aging. Berlin, Heidelberg, New York,
Springer-Verlag, pp 137-139.
Kannel WB (1981) Update on the role of cigarette smoking in coronary
artery disease (review). Am Heart J, 101: 319-328.
Kato H, Saito M, & Suda M (1980) Effect of starvation on the
circadian adrenocortical rhythm in rats. Endocrinology, 106:
918-921.
Katz ML & Robinson WG Jr (1987) Light and aging effects on vitamin E
in the retinal pigment epithelium. Vision Res, 27(11): 1875-1879.
Katzman R & Terry RD (1983) Normal aging in the nervous system. In:
Katzman R & Terry RD ed. The neurology of aging. Philadelphia,
Pennsylvania, F.A. Davis, pp 15-50.
Kaur S & Gill SS (1985) Age-related changes in the activities of
epoxide hydrolyases in different tissues of mice. Drug Metab Dispos,
13: 711-715.
Kaysen GA & Myers BD (1985) The aging kidney. Clin Geriatr Med,
1(1): 207-222.
Keast D & Ayre DJ (1981) Effects of chronic tabocco smoke exposure
on immune responses in aged mice. Arch Environ Health, 36: 201-207.
Kelley WW, Brief S, Westly HJ, Novakofski J, Bechtel PJ, Simon J, &
Walker EB (1986) GH3 pituitary adenoma cells can reverse thymic
aging in rats. Proc Natl Acad Sci (USA), 83: 5663-5667.
Kelso A & Munck A (1984) Glucocorticoid inhibition of lymphokine
secretion by alloreactive T lymphocyte clones. J Immunol, 133(2):
784-791.
Kim JCS & Kaminsky LS (1988) 2,2,2-Trifluoroethanol toxicity in aged
rats. Toxicol Pathol, 16: 35-45.
Kinon GA & Liu C (1973) Diurnal variation in plasma testosterone of
the male laboratory rat. Horm Metab Res, 5: 233-234.
Kitani K (1985) The role of the liver in pharmacokinetic and
pharmacodynamic alterations in the elderly. In: O'Malley K &
Waddington J ed. Therapeutics in the elderly. Amsterdam, Oxford, New
York, Elsevier Science Publishers, pp 19-34.
Kitani K (1988) Drugs and the ageing liver. Life Chem Rep, 6:
143-230.
Kitani K (1991) Aging of the liver: facts and theories. Arch
Gerontol Geriatr, 12: 133-154.
Kitani K, Masuda Y, Sato Y, Kanai S, & Nokubo M (1984) Increased
anti-convulsant activity in aging BDF1 mice. J Pharmacol Exp Ther,
229: 231-236.
Kitani K, Ohta M, Kanai S, & Sato Y (1985a) Sex difference in the
biliary excretion of digoxin and its metabolites in aging Wistar
rats. Arch Gerontol Geriatr, 4: 1-12.
Kitani K, Sato Y, Kanai S, Nokubo M, Ohta M, & Masuda Y (1985b)
Increased anticonvulsant effect of phenobarbital with age in mice -
a possible pharmacologic index for brain aging. Life Sci, 37:
1451-1460.
Kitani K, Sato Y, Kanai S, Nokubo M, Ohta M, Masuda Y, & Klotz U
(1986) Altered response to convulsants and anticonvulsants in aging
BDF1 mice. In: Kitani K ed. Liver and aging 1986: Liver and brain.
Amsterdam, Oxford, New York, Elsever Science Publishers, pp 293-307.
Kitani K, Sato Y, Kanai S, Nokubo M, Ohta M, & Masuda Y (1987)
Increasing anticonvulsant effect of AD-810 (Zonisamide) in aging
BDF1 mice. Life Sci, 41: 1339-1344.
Klatsky AL, Friedman GD, & Siegelaub AB (1981) Alcohol and
mortality. A ten year Kaiser-Permanente experience. Ann Intern Med,
95: 139-145.
Klawans HL & Tanner CM (1984) Movement disorders in the elderly. In:
Albert M ed. Clinical neurology of aging. Oxford, Oxford University
Press, pp 387-403.
Klotz U, Avant GR, Hoyumpa A, Schenker S, & Wilkinson GR (1975) The
effects of age and liver disease on the disposition and elimination
of diazepam in adult man. J Clin Invest, 55: 347.
Klug TL & Adelman RC (1977) Evidence for a large thyrotropin and its
accumulation during aging in rats. Biochem Biophys Res Commun, 77:
1431-1437.
Koch-Weser J, Greenblatt DJ, Seller EM, & Shoder RI (1982) Drug
disposition in old age. New Engl J Med, 306: 1081-1086.
Kohrs MB (1981) Nutritional needs of the aged. Nutrition, 7: 39-46.
Konishi N & Ward JM (1989) Increased levels of DNA synthesis in
hyperplastic renal tubules of aging nephropathy in female F344/NCR
rats. Vet Pathol, 26: 6-10
Koobs DH, Schultz RL, & Jutzy RV (1978) The origin of lipofuscin and
possible consequences to the myocardium. Arch Pathol Lab Med, 102:
66-68.
Kozararevic DJ, McGee D, Vojvodic N, Racic Z, Dawber T, Gordon T, &
Zukel W (1980) Frequency of alcohol consumption and morbidity and
mortality: The Yugoslavia cardiovascular disease study. Lancet, i:
613-616.
Krupka L & Vener A (1979) Hazards of drug use among the elderly.
Gerontologist, 19: 90-95.
Kubos C, Day NK, & Good RA (1984) Influence of early or late dietary
restirction on life span and immunological parameters in
MRL/MP-Ipr/Ipr mice. Proc Natl Acad Sci (USA), 81: 5831-5835.
Kumpuris D (1983) Gastrointestinal bleeding in the older patient.
In: Texter EC ed. The aging gut. New York, Masson Publishing Co., pp
57-63.
Kyle ME & Kocsis JJ (1985) The effect of age on salicylate-induced
nephrotoxicity in male rats. Toxicol Appl Pharmacol, 81: 337-347.
Lakatta EG (1980) Age-related alteration in the cardiovascular
response to adrenergic mediated stress. Fed Proc, 39: 3173-3177.
Lakatta EG (1985) Heart and circulation. In: Finch CE & Schneider EL
ed. Handbook of the biology of aging, 2nd ed. New York, Van Nostrand
Reinhold Company, pp 377-413.
Lakatta EG (1986) Diminished beta-adrenergic modulation of
cardiovascular function in advanced age. Cardiol Clin, 4(2):
185-200.
Lakatta EG (1987a) Cardiac muscle change in senescence. Annu Rev
Physiol, 49: 519-531.
Lakatta EG (1987b) Catecholamines and cardiovascular function in
aging. Endocrinol Metab Clin North Am, 16(4): 877-891.
Landauer MR, Tomlinson WT, Balster RL, & MacPhail RC (1984) Some
effects of the formamidine pesticide on the behavior of mice.
Neurotoxicology, 5: 91-100.
Leakey JEA, Cunny HC, Bazare J Jr, Webb PJ, Feuers RJ, Duffy PH, &
Hart RW (1989a) Effects of aging and caloric restriction on hepatic
drug metabolizing enzymes in the Fischer 344 rat. I: The cytochrome
P-450 dependent monooxygenase system. Mech Ageing Dev, 48: 145-155.
Leakey JEA, Cunny HC, Bazare J Jr, Webb PJ, Lipscomb JC, Siikkes W
Jr, Feuers RJ, Duffy PH, & Hart RW (1989b) Effects of aging and
caloric restriction on hepatic drug metabolizing enzymes in the
Fischer 344 rat. II. Effects on conjugating enzymes. Mech Ageing
Dev, 48: 157-166.
Lee KT ed. (1985) Atherosclerosis. Ann NY Acad Sci, 454: 1-327.
Lehmann AR, Hoeijmakers JHJ, Van Zealand AA, Backendorf CMP, Bridges
BA, Collins A, Fuchs RPD, Margison GP, Montesano R, Moustacchi E,
Natarajan AT, Radman M, Sarasin A, Seeberg E, Smith CA, Stefanini M,
Thompson LH, Van der Schans GP, Weber CA, & Zdzienicka MZ (1992)
Workshop on DNA repair. Mutat Res, 273: 1-28.
Leopold AC (1975) The biological significance of death in plants.
In: Benke JA, Finch CE, & Moment GB ed. The biology of aging. New
York, London, Plenum Press, pp 101-114.
Leppaluoto J, Ranta T, & Tuomisto J (1974) Diurnal variations of
serum immunoassayable thyrotropin (TSH) concentration in the rat.
Acta Physiol Scand, 90: 699-702.
Lesser GT, Deutsch S, & Markofsky J (1973) Aging in the rat:
Longitudinal and cross-sectional studies of body composition. Am J
Physiol, 225: 1472-1478.
Leto S, Kokkahen GC, & Barrows CH Jr (1976) Dietary protein, life
span and physiological variables in female mice. J Gerontol, 31:
149-154.
Levy ML (1987) Tous les pays du monde (1987). Bull Mens Inf Démogr
Econ Soc, 216:1-6.
Lewis VM, Twomey JJ, Bealmar P, Goldstein G, & Good RA (1978) Age,
thymic involution, and circulating thymic hormone activity. J Clin
Endocrinol Metab, 47: 145-150.
Li DD, Chien YK, Gu MZ, Richardson A, & Cheung HT (1988) The
age-related decline in interleukin-3 expression in mice. Life Sci,
43: 1215-1222.
Liebow AA (1964) Biochemical and structural changes in the aging
lung. Summary. In: Cander L & Moyer JH ed. Aging of the lung. New
York, London, Grune and Stratton, pp 97-104.
Liepa GU, Masoro EJ, Bertrand HA, & Yu BP (1980) Food restriction as
a modulation of age-related changes in serum lipids. Am J Physiol,
238: E253-E257.
Likhachev AJ (1985) Effect of age on DNA repair in carcinogenesis
due to alkylating agents. In: Likhachev A, Anisimov V, & Montesano R
ed. Age-related factors in carcinogenesis. Lyon, International
Agency for Research on Cancer, pp 239-246 (IARC Scientific
Publications No. 58).
Lin CF & Hayton WL (1983) GI motility and subepithelial blood flow
in mature and senescent rats. Age, 6: 46-51.
Lindeman RD (1986) The aging kidney. Compr Ther, 12(3): 43-49.
Lindeman RD, Tobin JD, & Shock NW (1987) Hypertension and the
kidney. Nephron, 47(1): 62-67
Lindsay R (1989) Osteoporosis: An updated approach to prevention and
management. Geriatrics, 44: 45-54.
Lipschitz DA (1987) Nutrition, aging and the immunohematopoietic
system. Clin Geriatr Med, 3(2): 319-328.
Loft S, Dossing M, & Poulsen HE (1988) Influence of age and
consumption of tobacco, alcohol, and caffeine on antipyrine
clearance. Hum Toxicol, 7: 277-280.
Loi CM & Vestal RE (1988) Drug metabolism in the elderly. Pharmacol
Ther, 36: 131-149.
Lucchi L, Memo M, Airaghi ML, Spano PF, & Trabucchi M (1981) Chronic
lead treatment induces in rat a specific and differential effect on
dopamine receptors in different brain areas. Brain Res, 213:
397-404.
Lutz RJ, Dedrick RL, Matthews HB, Elling TE, & Anderson MW (1977) A
preliminary pharmacokinetic model for several chlorinated biphenyls
in the rat. Drug Metab Dispos, 5: 386-396.
McCaffrey TA, Nicholson AG, Szabo PE, & Wexsler BB (1988) Aging and
atherosclerosis. The increased proliferation of arterial smooth
muscle cells isolated from old rats is associated with increased
platelet-derived growth factor-like activity. J Exp Med, 167:
163-174.
McCay CM, Crowell MM, & Mayrar LA (1935) The effect of reduced
growth upon the length of the life span and upon ultimate body size.
J Nutr, 10: 63-79.
McClure JE, Lameris N, Wara DW, & Goldstein AL (1982) Immunochemical
studies on thymosin: radioimmunoassay of thymosin alpha-1. J
Immunol, 128(1): 368-375.
McGinty D, Stern N, & Akshoomoff N (1988) Circadian and
sleep-related modulation of hormone levels changes with aging. In:
Sowers JR & Felicett JV ed. The endocrinology of aging. New York,
Raven Press, pp 75-111.
McKenna MJ & DiStefano V (1977) Carbon disulphide. II. A proposed
mechanism for the action of carbon disulfide on
dopamine-beta-hydroxylase. J Pharmacol Exp. Ther, 202: 253-266.
McMahon TF (1988) Age-related changes in colonic phase I and phase
II biotransformation: possible relationship to colon carcinogenesis.
Baltimore, University of Maryland, School of Pharmacy
(Dissertation).
McMahon TF & Birnbaum LS (1990a) Age-related changes in the
disposition and metabolism of xenobiotics. In: Cooper RL, Goldman
JM, & Harbin TJ ed. Aging and environmental toxicology. Baltimore,
Maryland, Johns Hopkins University Press, pp 7-30.
McMahon TF & Birnbaum LS (1990b) Age related change on toxicity and
biotransformation of potassium cyanide in male C57BL/6N mice.
Toxicol Appl Pharmacol, 105: 305-314.
McMahon TF, Beierschmitt WP, & Weiner M (1987) Changes in phase I
and phase II biotransformation pathways with age in male Fischer 344
rat colon: relationship to colon carcinogenesis. Cancer Lett, 36:
273-282.
McMahon TF, Diliberto JJ, & Birnbaum LS (1989) Age-related changes
in the disposition of benzyl acetate: A model compound for glycine
conjugation. Drug Metab Dispos, 17: 506-512.
McMahon TF, Diliberto JJ, & Birnbaum LS (l990a) Effects of age on
metabolism and disposition of salicylic acid (SAL) in male Fischer
344 rats. Drug Metab Dispos, 18: 494-503.
McMahon TF, Peggins JO, Centra MM, & Weiner M (l990b) Age-related
changes in biotransformation of azoxymethane and methylazoxymethanol
in vitro. Xenobiotica, 20: 501-513.
Maeda H, Gleiser CA, Masoro EJ, Murata I, McMohan CA, & Yu BP (1985)
Nutritional influences on aging of Fisher 344 rats. II. Pathology. J
Gerontol, 40: 671-688.
Magal E, Chaudhuri M, & Adelman RC (1986) The capability for
regulation of insulin secretion by somatostatin in purified
pancreatic islet B cells during aging. Mech Aging Dev, 33(2):
139-146.
Magnus K (1982) Trends in cancer incidence: Causes and practical
implications. Washington, DC, Hemisphere.
Mantel N & Schneiderman MA (1975) Estimation "safe" levels, a
hazardous undertaking. Cancer Res, 35: 1379-1386.
Mantere P, Hanninen H, Hernberg S, & Luukkonen R (1984). A
prospective follow-up study on psychological effects in workers
exposed to low levels of lead. Scand J Work Environ Health, 10:
43-50.
Marin R, Proverbio T, & Proverbio F (1985) Characterization of the
Na+, K+ -ATPase activity of basolateral membranes of kidney proximal
tubular cells from young and old rats. Biochem Pharmacol, 34:
4197-4201.
Marksberry WR, Ehmann WD, Hossain IM, Alauddin M, & Goodin DT (1981)
Instrumental neutron activation analysis of brain aluminum in
Alzheimer's disease and aging. Ann Neurol, 10: 511-516.
Marsh G (1980) Perceptual changes with aging. In: Busse EW & Blazer
DG ed. Handbook of geriatric psychiatry. New York, Van Nostrand
Reinhold Company, Chapter 7, pp 147-168.
Marshall JF & Berrios N (1979) Movement disorders of aged rats:
Reversal by dopamine receptor stimulation. Science, 206: 477-479.
Martin GM, Sprague CA, Norwood TH, & Pendergrass WR (1974) Clonal
selection, attenuation and differentiation in an in vitro model of
hyperplasia. Am J Pathol, 74: 137-154.
Marx JL (1987) A new wave of enzymes for cleaving pro-hormones.
Science, 235: 285-286.
Masoro EJ ed. (1981) CRC handbook of physiology in aging. Boca
Raton, Florida, CRC Press.
Masoro EJ (1985) Metabolism. In: Finch CE & Schneider EL ed.
Handbook of the biology of aging. New York, Van Nostrand Reinhold
Company, pp 540-563.
Masoro EJ (1988) Food restrictions in rabbits: an evolution of its
role in the study of aging. J Gerontol, 43: B59-B64.
Masoro EJ (1991) Use of rodents as models for the study of "normal
aging": Conceptual and practical issues. Neurobiol Aging, 12:
639-644.
Masoro EJ & Yu BP (1989) Diet and nephropathy. Lab Invest, 60:
165-167.
Masoro EJ, Yu BP, Bertrand HR, & Lynd FT (1980) Nutritional probe of
the aging process. Fed Proc, 39: 3178-3182.
Masoro EJ, Shimokawa I, & Yu BP (1991) Retardation of the aging
processes in rats by food restriction. Ann NY Acad Sci, 621:
337-352.
Master CL, Multhaup G, Simms G, Pottgieser J, Martins RN, &
Beyreuther K (1985) Neuronal origin of a cerebral amyloid:
Neurofibrillary tangles of Alzheimer's disease contain the same
protein as the amyloid of plaque cores and blood vessels. EMBO J, 4:
2757-2763.
Matulionis DH (1984) Chronic cigarette smoke inhalation and aging in
mice: Morphologic and functional lung abnormalities. Exp Lung Res,
7: 237-256.
Mauderly JL (1978) Effect of age on pulmonary structure and function
of immature and adult animals and man. Fed Proc, 38: 173-177.
Mauderly JL (1979a) Ventilation, lung volumes, and lung mechanics of
young adult and old Syrian hamsters. Exp Aging Res, 5: 497-508.
Mauderly JL (1979b) Effect of aging on pulmonary structure and
function of immature and adult animals and man. Fed Proc, 38:
173-177.
Mauderly JL (1982) The effect of age on respiratory function of
Fischer 344 rats. Exp Aging Res, 8: 31-36.
Mawhinney MG (1985) Etiological considerations for the growth of
stroma in benign prostatic hyperplasia. Fed Proc, 45: 2615-2617.
Mazzeo RS & Horvath SM (1987) A decline in myocardial and hepatic
norepinephrine turnover with age in Fischer 344 rats. Am J Physiol,
252: E762-E763.
Medvedev ZA (1984) Age changes of chromatin: A review. Mech Ageing
Dev, 28: 139-154.
Medvedev ZA (1990) An attempt at a rational classification of
theories of aging. Biol Rev, 65: 375-398.
Mehta T, Labuc GE, Urbanski SJ, & Archer MC (1984) Organ specificity
in the microsomal activation and toxicity of
N-nitrosomethylbenzylamine in various species. Cancer Res, 44:
4017-4022.
Meisami E (1988) Aging of the nervous system structural and
biochemical changes. In: Timiras PS ed. Physiological basis of aging
and geriatrics. New York, Macmillan Publishing Co, pp 123-143.
Mervis R (1981) Cytomorphological alterations in the aging animal
brain with emphasis on Golgi studies. In: Johnson JE ed. Aging and
cell structure. New York, London, Plenum Press, Chapter 4, pp
143-186.
Messing RB, Vasquez BJ, Spiehler VR, Martinez JL Jr, Jensen RA,
Rigter H, & McCaugh JL (1980) 3H-Dihydromorphine binding in brain
regions of young and aged rats. Life Sci, 26: 921-927.
Meyer BR & Bellucci A (1986) Renal function in the elderly. Cardiol
Clin, 4(2): 227-234.
Mezey E (1981) Alcohol and drug interactions in injury to the
digestive tract. Clin Gastroenterol, 10: 485-495.
Millard WJ, O'Sullivan DM, Fox TO, & Martin JB (1985) Sexually
dimorphic patterns of growth hormone secretion in rats. In: Crowley
WF Jr & Hofler JG ed. The episodic secretion of hormones. New York,
Chichester, Brisbane, Toronto, John Wiley and Sons, pp 287-304.
Miller SM (1989) Changes in endocrine function with aging. Clin Lab
Sci, 2(1): 19-20.
Miller R (1991) Aging and immune function. Int Rev Cytol, 124:
187-216.
Minaker KL, Meneilly GS, & Rowe JW (1985) Endocrine systems. In:
Finch CE & Schneider EL ed. Handbook of the biology of aging. New
York, Van Nostrand Reinhold Company, pp 433-456.
Mitsui Y, Sakagami H, Murota SI, & Yamada MA (1980) Age related
decline in histone H1 fraction in human diploid fibroblast cultures.
Exp Cell Res, 126: 289-298.
Miura K, Goldstein RS, Morgan DG, Pasino DA, Hewitt WR, & Hook JB
(1987) Age-related differences in susceptibility to renal ischemia
in rats. Toxicol Appl Pharmacol, 87: 284-296.
Moberg GP, Bellinger LL, & Mendel VE (1975) Effect of meal feeding
daily rhythms of plasma corticosterone and growth hormone in rat.
Neuroendocrinology, 19: 160-169.
Monteiro-Riviere NA, Banks YB, & Birnbaum LS (1991) Laser doppler
measurements of cutaneous blood flow in ageing mice and rats.
Toxicol Lett, 57: 329-338.
Montgomery PR & Sitar DS (1981) Increased serum salicylate
metabolites with age in patients receiving chronic acetylsalicylic
acid therapy. Gerontology, 27: 329-333.
Moolgavkar SH & Venzon DJ (1979) Two-event models for
carcinogenesis; incidence curves for childhood and adult tumors.
Math Biosci, 47: 55-77.
Mooradian AD & Song MK (1989) Age-related alterations in duodenal
calcium transport rate in rats. Mech Ageing Dev, 47: 221-227.
Moroni F, Lombardi G, Moneti G, & Aldinio C (1984) The excitotoxin
quinolinic acid is present in the brain of several mammals and its
cortical content increases during the aging process. Neurosci Lett,
47: 51-55.
Morell AG, Gregoriadis G, Scheinberg IH, Hickman J, & Ashwell G
(1971) The role of sialic acid in determining the survival of
glycoproteins in the circulation. J Biol Chem, 246: 1461-1467.
Morris JF, Mosk A, & Johnson LC (1971) Spirometric standards for
healthy non-smoking adults. Am Rev Respir Dis, 103: 57-67.
Mullaart E, Boerrigter METI, Lohman PHM, & Vijg J (1989) Age-related
induction and disappearance of carcinogen-DNA-adducts in livers of
rats exposed to low levels of 2-acetylaminofluorene. Chem-Biol
Interact, 69: 373-384.
Mullaart E, Lohman PHM, Berends F, & Vijk J (1990) Review DNA damage
metabolism and aging. Mutat Res, 237: 189-210.
Müller HF & Schwartz G (1978) Electroencephalograms and autopsy
findings in geropsychiatry. J Gerontol, 33: 504-513.
Muller WEG, Wenger R, Bachmann M, Ugarkovic D, Courtis NC, &
Schroder HC (1989) Poly(A) metabolism and aging: a current view.
Arch Gerontol Geriatr, 9: 31-250.
Munro HN (1984) Nutrition-related problems of middle age. Proc Nutr
Soc, 43: 281-288.
Murty CVR, Mancini MA, Chatterjee B, & Roy AK (1988a) Changes in
transcriptional activity and matrix association of alpha-2u-globulin
gene family in the rat liver during maturation and aging. Biochim
Biophys Acta, 949: 7-34.
Murty CVR, Olson MJ, Garg BD, & Roy AK (1988b) Hydrocarbon-induced
hyaline droplet nephropathy in male rats during senescence. Toxicol
Appl Pharmacol, 96: 380-392.
Mutti A, Falzoi M, Romanelli A, Bocchi MC, Ferroni C, & Franchini I
(1988) Brain dopamine as a target for solvent toxicity: Effects of
some monocyclic aromatic hydrocarbons. Toxicology, 49: 77-82.
Naessen R (1971) An enquiry on the morphological characteristics and
possible changes with age in the olfactory region of man. Acta
Otorhinol, 71: 49-62.
Nagel JE, Chopra RK, Chrest FJ, McCoy MT, Schneider EL, Holbrook NJ,
& Adler WH (1988) Decreased proliferation, interleukin 2 synthesis,
and interleukin 2 receptor expression are accompanied by decreased
mRNA expression in phytohemagglutinin-stimulated cells from elderly
donors. J Clin Invest, 81: 1096-1102.
Nagy K, ZS-Nagy V, Bertoni-Freddari C, & ZS-Nagy I (1983)
Alterations of the synaptosomal membrane 'microviscosity' in the
brain cortex of rats during aging and centrophenoxine treatment.
Arch Gerontol Geriatr, 2: 23-29.
Nakamura E, Kimura M, Nagata H, Miyao K, & Ozeki T (1982) Evaluation
of the progress of aging based on specific biological age as
estimated by various physiological functions. Jpn J Hyg, 36:
853-862.
NAS (1989) Biological markers in reproductive toxicology.
Washington, DC, National Academy of Sciences, National Academy
Press, pp 2-3.
Neafsey PJ (1990) Longevity hormesis. A review. Mech Ageing Dev, 51:
1-31.
Nebert DW & Gonzalez FJ (1987) P450 Genes - Structure, evolution and
regulation. Annu Rev Biochem, 56: 945-993.
Nebert DW, Nelson DR, Minor JC, Estabrook RW, Feyereisen R,
Fujii-Kuriyama Y, Gonzalez FJ, Guengerich FP, Gunsalus IC, Johnson
EF, Loper JC, Sato R, Waterman MR, & Waxman DR (1991) The P450
superfamily: Uptake on new sequences, gene mapping, and recommended
nomenclature. DNA Cell Biol, 10(1): 1-14.
Needleman HL, Gunnoe C, Leviton A, Reed R, Peresie H, Maher C, &
Barrett P (1979) Deficits in psychologic and classroom performance
of children with elevated dentine lead levels. New Engl J Med, 300:
689-695.
Neuhaus OW & Flory W (1978) Age-dependent changes in the excretion
of urinary proteins by the rat. Nephron, 22: 570-576.
Newaz SN, Fang W, & Strobel HW (1983) Metabolism of the carcinogen
1,2-dimethylhydrazine by isolated human colon microsomes and human
colon tumor cells in culture. Cancer, 52: 794-798.
Niedzwiecki A, Lewis PN, & Cinader B (1985) Changes of histone H1
subtypes with aging in strains of mice that possess different
immunological characteristics. J Gerontol, 40: 695-699.
Nokubo M & Kitani K (1988) Age-dependent decrease in the lethal
threshold of pentylenetetrazole in mice. Life Sci, 43: 41-47.
Norwood TH & Smith JR (1985) The cultured fibroblast-like cells as a
model for the study of aging. In: Fincht CE & Schneider EL ed. The
biology of aging, 2nd ed. New York, Van Nostrand Reinhold Company,
pp 291-321.
Novak LP (1972) Aging, total potassium, fat free mass and cell mass
in males and females between the ages of 18 and 85 years. J
Gerontol, 27: 438-443.
Oh SH, Deagen JT, Whanger PD, & Wesnig PH (1978) Biological function
of metallothionein. V. Its induction in rats by various stresses. Am
J Physiol, 234: E282-E285.
Ohta M, Kanai S, Sato Y, & Kitani K (1988) Age-dependent decrease in
the hepatic uptake and biliary excretion of ouabain in rats. Biochem
Pharmacol, 37: 935-942.
O'Malley K, Docherty JR, & Kelly GJ (1988) Adrenoceptor status and
cardiovascular function in ageing. J Hypertension, 6(suppl 10):
S59-S62.
O'Meara AR & Pochron SF (1979) Age-related effects on the
incorporation of acetate into rat liver histones. Biochim Biophys
Acta, 586: 391-401.
Onasaka S & Cherian MG (1981) The induced synthesis of
metallothionein in various tissues of rats in response to metals. I.
Effect of repeated injection of cadmium salts. Toxicology, 22:
91-101.
Os I, Kjeldsen SE, & Westheim A (1987) Aging and urinary vasopressin
excretion in healthy men. Scand J Urol Nephrol, 21(3): 235-239.
OECD (1986) Existing chemicals systematic investigation, priority
setting and chemical reviews. Paris, Organisation for Economic
Co-operation and Development.
Owen RA & Heywood R (1986) Age-related variations in renal structure
and function in Sprague-Dawley rats. Toxicol Pathol, 14(2): 158-167.
Pagano GN, Casadar N, Diana A, Psue E, Bozzo C, Ferrero F, & Lenti G
(1981) Insulin resistance in the aged: the role of peripheral
insulin receptors. Metabolism, 30: 46-49.
Pahlavani MA, Cheung HT, Cai NS, & Richardson A (1988) Influence of
dietary restriction and aging on gene expression in the immune
system of rats. In: Biomedical advances in aging. New York, London,
Plenum Press, pp 259-270.
Pakin YV & Hrisanov SM (1984) Critical analysis of the applicability
of the Compertz-Makeham law in human population. Gerontology, 30:
8-12.
Paramonova GI & Dovgi AI (1987) Induction of multiple forms of
cytochrome P450 of rat liver during aging. In: Abstracts of the All
Union Conference on Cytochrome P450 and the Environment,
Novosibirsk, 27-31 July 1987. Novosibirsk, USSR Academy of Medical
Sciences, Siberian Division, pp 100-101.
Patel DJ & Vaishnaw RN (1980) Basic hemodynamics and its role in
disease process. Baltimore, Maryland, University Park Press.
Pedigo NW, Schoemaker H, Morellia M, McDougal JN, Malisck JB, Burks
TF, & Yamamura TF (1981) Benzodiazepine receptor binding in young,
mature, and senescent rat brain and kidney. Neurobiol Aging, 2:
83-88.
Penn A, Batastini G, Soloman J, Burns F, & Albert R. (1981)
Dose-dependent size increases of aortic lesions following chronic
exposure to 7,12-dimethylbenz(a)-anthracene. Cancer Res, 71:
588-592.
Penzes L (1974) Intestinal absorption of glycine, L-alanine, and
L-leucine in the old rat. Exp Gerontol, 8: 245-252.
Penzes, L.S. (1984) Intestinal response in aging: changes in reserve
capacity. Acta Med Hung, 41(4): 263-277.
Perkins MN & Stone TW (1983) Pharmacology and regional variations of
quinolinic acid-evoked excitations in the rat central nervous
system. J Pharmacol Exp Ther, 226(2): 551-557.
Perl DP & Brody AR (1980) Alzheimer's disease: X-ray spectrometric
evidence of aluminum accumulation in neurofibrillary tangle-bearing
neurons. Science, 208: 297-299.
Peterson DD, Pack AI, Silage DA, & Fishman AP (1981) Effects of
aging on ventilatory and occlusion pressure responses to hypoxia and
hypercapnia. Am Rev Respir Dis, 124: 387-391.
Peto R (1986) Influence of dose and duration of smoking on lung
cancer rates. In: Zaridze D & Peto R ed. Tobacco: A major
international health hazard. Lyon, International Agency for Research
on Cancer, pp 23-33 (IARC Scientific Publications No. 74).
Peto R, Parish SE, & Gray RG (1975) Cancer and aging in mice and
men. Br J Cancer, 32: 411-426.
Peto R, Pike MC, Day NE, Gray RE, Lee PN, Parish S, Poto J, Richard
S, & Wahrendorf J (1980) Guidelines for simple, sensitive
significance tests for carcinogenic effects in long-term animal
experiments. In: Long-term and short-term screening assays for
carcinogens: A critical appraisal. Lyon, International Agency for
Research on Cancer, pp 311-426 (IARC Monographs on the Evaluation of
the Carcinogenic Risk of Chemicals to Humans, Supplement 2).
Peto R, Parish SE, & Gray RG (1985) There is no such thing as aging,
and cancer is not related to it. In: Likhachev AJ, Anisimov V, &
Montesano R ed. Age-related factors in carcinogenesis. Lyon,
International Agency for Research on Cancer, pp 43-53 (IARC
Scientific Publications No. 58).
Pierpaoli W, Dall'Ara A, Pedrinis E, & Regelson W (1991) The pineal
control of aging. The effects of melatonin and pineal grafting on
the survival of older mice. Ann NY Acad Sci, 621: 291-313.
Piikivi L, Hanninen H, Martelin T, & Mantere P (1984) Psychological
performance and long-term exposure to mercury vapors. Scand J Work
Environ Health, 10: 35-41.
Pines A, Cucos B, Ever-Hadani P, Ron M, & Lemesch C (1987) Changes
in pattern of organochlorine residues in blood of general Israeli
population, 1975-1986. Sci Total Environ, 66: 115-125.
Piva F, Limonta P, Maggi R, Dondi D, Messi E, Yanisi M, & Motta M
(1987) Aging and the hypothylamo-pituitery-testiculas axis in the
rat. J Steroid Biochem, 27(4-6): 707-712.
Poland A & Knutson JC (1982) 2,3,7,8,-Tetrachlorodibenzo-p-dioxin
and related halogenated aromatic hydrocarbons: examinations of the
mechanism of toxicity. Annu Rev Pharmacol Toxicol, 22: 517-554.
Popper H (1986) Aging in the liver. In: Popper H ed. Progress in
liver diseases. New York, London, Grune & Stratton, vol VIII, pp
659-683.
Posner J, Danhof M, Teunissen MWE, Breimer DD, & Whiteman PD (1987)
The disposition of antipyrine and its metabolites in young and
elderly healthy volunteers. Br J Clin Pharmacol, 24: 51-55.
Post DJ, Carter K, & Papaconstantinou J (1991) The effect of aging
on constitutive mRNA levels and lipopolysaccharide inducibility of
acute phase genes. Ann NY Acad Sci, 621: 66-77.
Prinz PN, Weitzman ED, Cunningham GR, & Karacan I (1983) Plasma
growth hormone during sleep in young and aged men. J Gerontol, 38:
519-534.
Provinciali M & Fabris H (1990) Role of pituitary-thyroid axis on
basal and lymphokine-induced NK cell activity in aging. Int J
Neurosci, 51: 373-375.
Rabovsky, J, Marion KJ, Groseclose RD, Lewis TR, & Peterson M (1984)
Low dose chronic inhalation of diesel exhaust and/or coal dust by
rats: effect of age and exposure on lung and liver cytochrome P450.
Xenobiotica, 14: 595-598.
Rao GN, Haseman JK, Grumbein S, Crawford DD, & Eustis SL (1990)
Growth, body weight, survival, and tumor trends in F344/n rats
during an eleven-year period. Toxicol Pathol, 18: 61-70.
Rath PC & Kanungo MS (1989) Age-related changes in the expression of
cytochrome p-450 (b+e) gene in the rat after phenobarbitone
administration. Biochem Biophys Res Commun, 157: 1403-1409.
Reff ME & Schneider EL ed. (1982) Biological markers of aging.
Washington, DC, US Government Printing Office, 252 pp (NIH
Publication No. 82-2221).
Regelson W (1983) Biomarkers in aging. In: Regelson W & Marott Sinex
F ed. Intervention in the aging process. New York, Alan R. Liss,
Inc., pp 3-98.
Rehnberg GL, Goldman JM, Cooper RL, Hein JF, McElroy WK, Booth KC, &
Gray LE (1988a) Effect of linuron on the brain-pituitary-testicular
reproductive axis in the rat. Toxicologist, 8: 121.
Rehnberg GL, Linder R, Goldman JM, Hein JF, McElroy WK, & Cooper RL
(1988b) Changes in testicular and serum hormone concentrations in
the male rat following treatment with m-dinitrobenzene. Toxicol Appl
Pharmacol, 95: 255-264.
Reidenberg MM (1980) Drugs in the elderly. Bull NY Acad Med, 56:
703-714. Reif AE (1981) Effect of cigarette smoking on
susceptibility to lung cancer. Oncology, 38: 76-85.
Reis W & Poethig D (1984) Chronological and biological age. Exp
Gerontol, 19: 211-216.
Reiter RJ (1986) The pineal gland: an important link to the
environment. News Physiol Sci, 1: 202-205.
Reitz RH, McDougal JN, Himmelstein MW, Nolan RJ, & Schumann AM
(1988) Physiologically based pharmacokinetics modeling with methyl
chloroform: implications for interspecies, high dose/low dose, and
dose route extrapolations. Toxicol Appl Pharmacol, 95: 185-199.
Revskoy SY, Poroshina TE, Kovaleva IG, Berstein LM, Ostroumova MN, &
Dilman VM (1985) Age-dependent metabolic immunodepression and
cancer. In: Likhachev A, Anisimov V, & Montesano R ed. Age-related
factors in carcinogenesis. Lyon, International Agency for Research
on Cancer, pp 253-259 (IARC Scientific Publications No. 58).
Richardson A & Birchenall-Sparks MC (1983) Age-related changes in
protein synthesis. Rev Biol Res Aging, 1: 255-273.
Richardson A & Semsei I (1987) Effect of aging on translation and
transcription. In: Review of biological research in aging. New York,
Alan R. Liss, Inc., vol 3, pp 467-483.
Richardson A, Birchenall-Sparks MC, & Staecker JL (1983) Aging and
transcription. In: Review of biological research in aging. New York,
Alan R. Liss, Inc., vol 2, pp 275-294.
Richardson A, Butler JA, Rutherford MS, Semsei I, Gu MZ, Fernandes
G, & Chiang WH (1987) Effect of age and dietary restriction on the
expression of alpha-2u-globulin. J Biol Chem, 262: 12821-12825.
Richardson A, Roberts M, & Rutherford M (1985) Aging and gene
expression. In: Review of biological research in aging. New York,
Alan R. Liss, Inc., vol 2, pp 395-419.
Riegel GD & Miller AE (1981) Aging effect on hormone concentrations
and actions. In: Florini JR ed. Handbook of biochemistry in aging.
Boca Raton, Florida, CRC Press, pp 231-256.
Riggs BL (1987) Pathogenesis of osteoporosis. Am J Obstet Gynecol,
156: 1342-1346.
Riggs BL & Melton LJ III (1983) Evidence for two distinct syndromes
of involutional osteoporosis. Am J Med, 75: 899-901.
Rikans LE (1984) Influence of aging on the susceptibility of rats to
hepatotoxic injury. Toxicol Appl Pharmacol, 73: 243-249.
Rikans LE (1989a) Hepatic drug metabolism in female Fischer rats as
a function of age. Drug Metab Dispos, 17: 114-116.
Rikans L (1989b) Effects of ethanol on microsomal drug metabolism in
aging female rats. I. Induction. Mech Ageing Dev, 48: 267-280.
Rikans LE & Moore DR (1987) Effect of age and sex on allyl alcohol
hepatotoxicity in rats: role of liver alcohol and aldehyde
dehydrogenase activities. J Pharmacol Exp Ther, 243: 20-26.
Rikans LE & Moore DR (1988) Effect of aging on aqueous phase
antioxidents in tissues of male Fischer rats. Biochem Biophys Acta,
966: 269-275.
Rikans LE & Snowden CD (1989) Influence of aging on acute ethanol
hepatotoxicity in female rats. Pharmacologist, 31: 177.
Ritschel WA (1983) Pharmacokinetics in the aged. In: Pagliaro LA &
Pagliaro AM ed. Pharmacologic aspects of aging. St. Louis, Missouri,
C.V. Mosby & Co., pp 219-256.
Ritschel WA (1988) Gerontokinetics: The pharmacokinetics of drugs in
the elderly. Caldwell, New Jersey, Telford Press, 114 pp.
Robertson IGC & Birnbaum LS (1982) Age-related changes in mutagen
activation by rat tissues. Chem-Biol Interact, 38: 243-252.
Robertson DRC, Waller DG, Renwick AG, & George CF (1988) Age-related
changes in the pharmacokinetics and pharmacodynamic of nifedipine.
Br J Clin Pharmacol, 25: 297-305.
Rodgers J & Gass G (1983) The effect of age on serum proteins in
mice. Exp Gerontol, 14: 169-173.
Roe DA (1983) Drugs and nutrition in the elderly. In: Geriatric
nutrition, 2nd ed. Englewood, New Jersey, Prentice-Hall, Inc., pp
176-200.
Rogers J & Bloom FE (1985) Neurotransmitter metabolism and function
in the aging central nervous system. In: Finch CE & Schneider EL ed.
Handbook of the biology of aging, 2nd ed. New York, Van Nostrand
Reinhold Company, pp 645-691.
Rogers GS & Gilchrest BA (1990) The senile epidermis: environmental
influences on skin ageing and cutaneous carcinogenesis. Br J
Dermatol, 122(suppl 35): 55-60.
Rogers J, Magistretti PJ, & Bolis LC (1991) Animal models for aging
research. Neurobiol Aging, 12(spec issue): 625-701.
Rose MR (1991) Evolutionary biology of aging. New York, London,
Oxford University Press.
Rosenberger RF & Kirkwood TBL (1986) Errors and the integrity of
genetic information transfer. In: Kirkwood TBL, Rosenberger RF, &
Galas DJ, ed. Accuracy in molecular processes. London, Chapman and
Hall, pp 17-35.
Ross MH (1961) Length of life and nutrition in rat. J Nutr, 75:
197-210.
Ross MH (1976) Nutrition and longevity in experimental animals. In:
Winick M ed. Nutrition and aging. New York, Chichester, Brisbane,
Toronto, John Wiley and Sons, pp 23-41.
Ross R (1981) Atherosclerosis: A problem of the biology of arterial
wall cells and their interactions with blood components.
Arteriosclerosis, 1: 293-311.
Roth GS (1979a) Hormone action during aging: alterations and
mechanisms. Mech Ageing Dev, 9: 497-514.
Roth GS (1979b) Hormone receptor changes during adulthood and
senscence: significance for aging research. Fed Proc, 38: 1910-1914.
Roth GS (1988) Receptor and postreceptor changes during lymphocyte
aging. In: Platt D ed. Blood cells, rheology, and aging. Berlin,
Heidelberg, New York, Springer-Verlag, pp 150-154.
Roth GS & Joseph JA (1988) Peculiarities of the effect of hormones
and transmitters during aging: Modulation of changes in dopaminergic
action. Gerontology, 34: 22-28.
Rothstein M & Seifert SC (1981) RNA synthesis. In: Handbook of
biochemistry in aging. Boca Raton, Florida, CRC Press, pp 51-63.
Rouser G, Kritchevsky G, & Yamamoto A (1972) Lipids in the nervous
system of different species as a function of age: Brain, spinal
cord, peripheral nerve, purified whole cell preparations and
sub-cellular particulates: Regulatory mechanisms and membrane
structure. Adv Lipid Res, 10: 261-360.
Rowe JW, Minaker KL, Paollotta J, & Fliers JS (1983)
Characterization of the insulin resistance of aging. J Clin Invest,
71: 1581-1587.
Roy AK, Nath TS, Motwani NM, & Chatterjee B (1983) Age dependent
regulation of the polymorphic forms of alpha2u-globulin. J Biol
Chem, 258: 10123-10127.
Rudman D (1987) Assessment of nutritional status. In: Braunwald E,
Isselbacher KJ, Petersdorf RG, Wilson JD, Martin JB, & Fauci AS ed.
Harrison's principles of internal medicine, 11th ed. New York,
McGraw-Hill, Chapter 71, pp 309-392.
Rudman D (1988) Kidney senescence: A model for aging. Nutr Rev,
46(6): 209-214.
Runcie J (1985) Obesity. In: Pathy MWSJ ed. Principles and practice
of geriatric medicine. New York, Chichester, Brisbane, Toronto, John
Wiley and Sons, Chapter 22.1, pp 277-284.
Russell DH, Buckley AR, Shah GN, Sipes IG, Blask DE, & Benson B
(1988) Hypothalamic site of action of
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Toxicol Appl Pharmacol,
94: 496-502.
Sacher GA (1977) Life table modification and life prolongation. In:
Finch CE & Hayflick L ed. Handbook of the biology of aging. New
York, Van Nostrand Reinhold Company, pp 582-638.
Sagiv M, Goldhammer E, Abinader EG, & Rudoy J (1988) Aging and the
effect of increased after-load on left ventricular contractile
state. Med Sci Sports Exercise, 20(3): 281-284.
Sample PA, Esterson FD, Weinreb RN, & Boynton RM (1988) The aging
lens: in vivo assessement of light absorption in 84 human eyes.
Invest Ophtalmol Visual Sci, 29(8): 1306-1311.
Sapolsky R, Armanini M, Packan D, & Tornbaugn G (1987) Stress and
glucocorticoids in aging. Endocrinol Metab Clin, 16(4): 965-979.
Sapolsky RM, Krey LC, & McEwen BS (1986) The adrenocortical axis in
the aged rat: Impaired sensitivity to both fast and delayed feedback
inhibition. Neurobiol Aging, 7: 331-335.
Sato Y, Kanai S, & Kitani K (1987) Biliary excretion of ouabain in
aging male and female F344 rats. Arch Gerontol Geriatr, 6: 141-152.
Saxton JA Jr & Kimball GC (1941) Relation to nephrosis and other
diseases of albino rat to age and to modifications of diet. Arch
Pathol, 32: 951-965.
Scheibel AB & Tomiyasu U (1978) Dendritic sprouting in Alzheimer
presenile dementia. Exp Neurol, 60: 1-8.
Schiff I & Wilson E (1979) Clinical aspects of aging of the female
reproductive system. Aging, 4: 10-28.
Schmucker D (1979) Age-related changes in drug disposition. Pharm
Rev, 30: 445-456.
Schmucker DL & Wang RK (1979) Rat lysosomal enzymes: effects of
animal age and phenobarbital. Age Aging, 2: 93-96.
Schmucker DL & Wang RK (1989) Dietary restriction postpones the
age-dependent compromise of male rat liver microsomal
monooxygenases. Prog Clin Biol Res, 287: 283-288.
Schmucker DL, Woodhouse KW, Wang RK, Wynne H, James QF, McManus M, &
Kremers P (1990) Effects of age and gender on in vitro properties
of human liver microsomal monoxygenases. Clin Pharmacol Ther, 48:
365-377.
Schneider EL (1978) The aging reproductive system. New York, Raven
Press, vol 4.
Schneider EL & Reed JD (1985) Life extension. New Engl J Med, 312:
1139-1168.
Schneider EL, Sternberg H, Tice RR, Senula GC, Kra D, Smith JR, &
Bynum G (1979) Cellular replication and aging. Mech Ageing Dev, 9:
313-324.
Schuetz EG, Wrighton SA, Barwick JL, & Guzelian PS (1984) Induction
of cytochrome P-450 by glucocorticoids in rat liver. I. Evidence
that glucocorticoids and pregnenolone 16 alpha-carbonitrile regulate
de novo synthesis of a common form of cytochrome P-450 in cultures
of adult rat hepatocytes and in the liver in vivo. J Biol Chem, 259:
1999-2006.
Schumann AM, Fox TR, & Watanabe PG (1982a) A comparison of the fate
of inhaled methyl chloroform (1,1,1-trichloroethane) following
single or repeated exposure in rats and mice. Fundam Appl Toxicol,
2: 27-32.
Schumann AM, Fox TR, & Watanabe PG (1982b) Methylchloroform
(1,1,1-trichloroethane): Pharmacokinetics in rats and mice following
inhalation exposure. Toxicol Appl Pharmacol, 62: 390-401.
Schuurman HJ, Krajnc-Franken MAM, Kuper CF, Van Loveren H, & Vos JG
(1990) Immune system. In: Haschek-Hoch WM & Rousseaux CG ed.
Fundamentals of toxicologic pathology. New York, London, San
Francisco, Academic Press, pp 1-103.
Searle CE (1976) Chemical carcinogens. Washington, DC, American
Chemical Society (ACS Monograph No. 173)
Segre D, Miller RA, Abraham GN, Weigle WO, & Warner HR (1989) Aging
and the immune system. J Gerontol, 44(6): 8164-8168.
Sekuler R, Kline D, & Dismuskes K (1982) Aging and human visual
functions. New York, Alan R. Liss, Inc.
Sellers EM, Frecker RC, & Romach MK (1983) Drug metabolism in the
elderly: confounding of age, smoking, and ethanol effects. Drug
Metab Rev, 14: 225-250.
Selmanowitz VJ, Rizer RL, & Orentreich N (1977) Aging of the skin
and its appendages. In: Finch CE & Hayflick L ed. Handbook of the
biology of aging. New York, Van Nostrand Reinhold Company, pp
496-509.
Selye H (1950) Stress: The physiology and pathology of exposure to
stress. Montreal, Acta Inc.
Semsei I, Rao G, & Richardson A (1989) Changes in the expression of
superoxide dismutase and catalase as a function of age and dietary
restriction. Biochem Biophys Res Commun, 164: 620-625.
Senda S, Cheng E, & Kawanishi H (1989) IgG in murine intestinal
secretions. Aging effect and possible physiological role. Scand J
Immunol, 29(1): 41-47.
Shen DX, Ren R, Zhao YR, & Ma XZ (1987) [Disease pattern analysis in
hospitalized elderly patients in Jingzhou.] Chin J Geriatr, 6: 44
(in Chinese).
Shepherd AMM, Wilson N, & Stevenson IH (1979) Warfarin sensitivity
in the elderly. In: Crooks J & Stevenson IH ed. Drugs and the
elderly. Baltimore, Maryland, University Park Press, pp 199-209.
Sherlock S, Bearn AG, Billing BH, & Paterson JCS (1955) Splanchnic
blood flow of peripheral plasma bromosulphthalein method: The
relation of peripheral bromosulphthalein level to the calculated
flow. J Lab Clin Med, 35: 923-932.
Shih JC & Young H (1978) The alteration of serotonin binding sites
in aged human brain. Life Sci, 23: 1441-1448.
Simmons DL, McQuiddy P, & Kasper CB (1987) Induction of the hepatic
mixed-function oxidase system by synthetic glucocorticoids -
Transcriptional and post-transcriptional regulation. J Biol Chem,
262: 326-332.
Slagboom PE & Vijg J (1989) Genetic instability and aging: theories,
facts, and future perspectives. Genome, 31: 373-385.
Smith DBD, Thompson LW, & Michalewski HJ (1980) Average evoked
potential research in adult aging - status and prospects. In: Aging
in the 1980s. Washington, DC, American Psychological Association, pp
135-151.
Smith EL, Sempos CT, & Purvis RW (1981) Bone mass and strengh
decline with age. In: Smith EL & Serfass RC ed. Exercise and aging:
the scientific basis. Hillside, New Jersey, Enslow Publishers, pp.
Sonntag WE & Gough ME (1988) Growth hormone releasing hormone
induced release on growth hormone in aging male rats: dependence on
pharmacological manipulation and endogenous somatostatin release.
Neuroendocrinology, 47: 482-488.
Sonntag WE, Steger RW, Forman LJ, & Meites J (1980) Decreased
pulsatile release of growth hormone in old male rats. Endocrinology,
107: 1875-1879.
Sonntag WE, Hylka V, & Meites J (1985) Growth hormone restores
protein synthesis in skeletal muscle of old rats. J Gerontol, 40:
689-694.
Spagnoli LG, Orlandi A, Manuello A, Santeusanio G, Angelis C,
Lucreziotti R, & Ramacci MT (1991) Aging and atherosclerosis in the
rabbit 1. Distribution, prevalence and morphology of atherosclerotic
lesions. Atherosclerosis, 89: 11-24.
Spearman ME & Leibman KC (1983) Hepatic and pulmonary cytosolic
metabolism of epoxides: effects of aging on conjugation with
glutathione. Life Sci, 33: 2615-2625.
Spearman ME & Leibman KC (1984) Aging selectivity alters
glutathione-S-transferase isozyme concentrations in liver and lung
cytosol. Drug Metab Dispos, 12: 661-671.
Speijers GJA (1983) [Toxic effects of rapeseed oil, erucic acid and
linolenic acid in the rat.] Utrecht, The Netherlands, University of
Utrecht, pp 1-143 (in Dutch).
Speijers GJA (1989a) [Literature review on experimental
atherosclerosis research.] Bilthoven, The Netherlands, National
Institute Public Health and Environmental Protection (in Dutch).
Speijers GJA (1989b) [Toxicological studies of ergot alkaloids.] De
Ware(n) Chem, 19(3): 189-194 (in Dutch).
Spencer PS & Ochoa J (1981) The mammalian peripheral nervous system
in old age. In: Johnson GJ Jr ed. Aging and cell structure. New
York, London, Plenum Press, pp 35-103.
Spencer PS, Nunn PB, Hugon J, Ludolph AC, Ross SM, Roy DN, &
Robertson RC (1987). Guam amyotrophic lateral
sclerosis-Parkinsonism-Dementia linked to a plant excitant
neurotoxin. Science, 237: 465-466.
Steiner RA, Bremner WJ, Clifton DK, & Dorsa DM (1984) Reduced
pulsatile luteinizing hormone and testosterone secretion in the male
rat. Biol Reprod, 31: 251-258.
Stenbäck F, Peto R, & Shubik P (1981) Initiation and promotion at
different age and doses in 2200 mice: III. Linear extrapolation from
high doses may underestimate low-dose tumor risk. Br J Cancer, 44:
23-34.
Stevenson IH & Hosie J (1985) Pharmacokinetics in the elderly. In:
O'Malley K & Waddington JL ed. Therapeutics in the elderly.
Amsterdam, Oxford, New York, Elsevier Science Publishers, pp 35-41.
Stevenson IH, Salem SAM, & Shepherd AMM (1979) Studies on drug
absorption and metabolism in the elderly. In: Crooks J & Stevenson
IH ed. Drugs and the elderly. Baltimore, Maryland, University Park
Press, pp 51-63.
Stevenson JC, Whitehead MI, Padwick M, Endacott JA, Suttonm C, Banks
LM, Freemantle C, Spinks TJ, & Hesp R (1988) Dietary intake of
calcium and postmenopausal bone loss. Br Med J, 297: 15-17.
Stiles J & Tyler WS (1988) Age-related morphometric differences in
responses of rat lungs to ozone. Toxicol Appl Pharmacol, 92:
274-285.
Stohs SJ, Al-Turk WA, & Angle CR (1982) Glutathione-S-transferase
and glutathione reductase activities in hepatic and extra-hepatic
tissues of female mice as a function of age. Biochem Pharmacol, 31:
2113-2116.
Strong R, Moore MA, Hale C, Wessels-Reiker M, Armbrecht HJ, &
Richardson A (1990) Modulation of tyrosine hydroxylase gene
expression in the rat adrenal gland by age and reserpine. Brain Res,
525(1): 126-132.
Sun J & Strobel HW (1986) Aging affects the drug metabolism systems
of rat liver, kidney, colon, and lung in a differential fashion. Exp
Gerontol, 21: 523-534.
Sun J, Lau PO, & Strobel HW (1986) Aging modifies the expression of
hepatic microsomal cytochrome P450 after pretreatment of rats with
b-naphoflavone or phenobarbital. Exp Gerontol, 21: 65-73.
Sweeny DJ & Weiner M (1985) Metabolism of acetaminophen in
hepatocytes isolated from mice and rats of various ages. Drug Metab
Dispos, 13: 377-379.
Sweeny DJ & Weiner M (1986) Effect of aging on the metabolism of
p-nitroanisole and p-nitrophenyl in isolated rat hepatocytes. Age,
9: 95-98.
Swenberg GJA, Richardson FC, Boucheron JA, Deal FH, Belinsky SA,
Charbonneau M, & Short BG (1987) High- to low-dose extrapolation:
critical determinants involved in the dose response of carcinogenic
substances. Environ Health Perspect, 76: 57-63.
Swift CG (1985) Benzodiazepine pharmacodynamics in the elderly. In:
O'Malley K & Stevenson IL ed. Therapeutics in the elderly.
Amsterdam, Oxford, New York, Elsevier Science Publishers, pp 77-90.
Tannenbaum SR, Fett D, Young VR, Land PD, & Bruce WR (1978) Nitrite
and nitrate are formed by endogenous synthesis in the human
intestine. Science, 200: 1478-1489.
Tarloff JB, Goldstein RS, Milo BA, & Hook JB (1989a) Role of
pharmacokinetics and metabolism in the enhanced susceptibility of
middle-aged Sprague-Dawley rats to acetaminophen nephrotoxicity.
Drug Metab Dispos, 17: 139-146.
Tarloff JB, Goldstein RS, Morgan DG, & Hook JB (1989b) Acetaminophen
and p-aminophenol nephrotoxicity in aging male Sprague-Dawley and
Fischer 344 rats. Fundam Appl Toxicol, 12: 78-91.
Taylor A (1989) Associations between nutrition and cataract. Nutr
Res, 47: 225-234.
Telford N, Mobbs CV, Osterburg HH, & Finch CE (1988) Alterations in
hypothalamic serotonergic-catecholaminergic relationships in aging
C57BL/6J female mice. Exp Gerontol, 23: 481-489.
Tenover JS, Matsumoto AM, Clifton DK, & Bremner WJ (1988)
Age-related alterations in the circadian rhythms of pulsatile
luteinizing hormone and testosterone secretion in healthy men. J
Gerontol, 43: M163-M169.
Terry RD (1963) The fine structure of neurofibrillary tangles in
Alzheimer's disease. J Neuropathol Exp Neurol, 22: 269-292.
Thakur MD (1984) Age-related changes in the structure and function
of chromatin: A review. Mech Ageing Dev, 27: 263-286.
Thakur MK (1988) Molecular mechanism of steroid hormone action
during aging. A review. Mech Ageing Dev, 45: 93-110.
Thompson JS, Robbins J, & Cooper JK (1987) Nutrition and immune
function in the geriatric population. Clin Geriatr Med, 3(2):
309-317.
Tice RR & Setlow RB (1985) DNA repair and replication in aging
organisms and cells. In: Finch CE & Schneider EL ed. Handbook of the
biology of aging, 2nd ed. New York, Van Nostrand Reinhold Company,
pp 173-224.
Timiras PS, Hudson DB, & Segall PE (1984) Lifetime brain serotonin:
Regional effects of age and precursor availability. Neurobiol Aging,
5: 235-242.
Tomlinson BE & Irving D (1977) The numbers of limb motor neurons in
the human lumbosacral cord throughout life. J Neurol Sci, 34:
213-219.
Travaglini P, Mocchegiani E, Togni E, Muratori M, Re T, Bazzani N, &
Fabris N (1990) Thymulin and zinc circulating level in patient with
GH and PRL secreting pituitary adenomas. Int J Neurosci, 51:
269-271.
Travis CC, White RC, & Ward RC (1990) Interspecies extrapolation of
pharmacokinetics. J Theor Biol, 142: 285-307.
Trick LR (1987) Age-related alterations in retinal function. Doc
Ophthalmol, 65(1): 35-43.
Triggs EJ & Nation RL (1975) Pharmacokinetics in the aged: A review.
J Pharmacokinet Biopharm, 3: 387-418.
Turusov VS & Parfenov YuD (1986) [Methods of revealing and risk
assessment of chemical carcinogens.] Moscow, Meditsina, pp 134-136
(in Russian).
Ulloa-Aguirre A & Chappel SC (1982) Multiple species of
follicle-stimulating hormone exist within the anterior pituitary of
male golden hamsters. J Endocrinol, 95: 257-266.
Ulloa-Aguirre A, Espinoza R, Damian-Matsumura P, & Chappel SC (1988)
Immunological and biological potencies of the different molecular
species of gonadotrophins. Hum Reprod, 3: 491-501.
US Senate Special Committee on Aging (1986) Aging America: Trends
and projections, 1985-86 edition. Washington, DC, US Government
Printing Office, pp 14-17.
Utiger RD (1980) Decreased extrathyroidal triiodothyronine
production in monthyroidal ilness: benefit or harm? Am J Med, 69:
807-810.
Van Bezooijen CFA (1984) Influence of age-related changes in rodent
liver morphology and physiology on drug metabolism - A review. Mech
Ageing Dev, 25: 1-22.
Van Bezooijen CFA, Groen C, Stijnen AM, & Danhof M (1989) The effect
of age on the pharmacokinetics and pharmacodynamics of drugs in
rats. Age, 12: 112.
Vanhaelst L, Van Cauter E, Degaute JP, & Goldstein J (1972)
Circadian variations of serum thyrotropin levels in man. J Clin
Endocrinol Metab, 35: 489-492.
Van Manen R, De Priester W, & Knook DL (1983) Lysosomal activities
in aging rat liver. I. Variation in enzyme activity within the liver
lobule. Mech Ageing Dev, 22: 159-165.
Velican C (1981) Are animal models suitable for the study of
ischaemic heart disease. Rev Roum Méd Méd Interne, 19(1): 5-19.
Vellas B, Balas D, Guidet M, Moreau J, Senegas F, Bouisson M,
Albarede JL, & Ribet A (1988) Vieillissement du pancréas. Son
implication dans les états de dénutrition chez les personnes agées.
Presse Méd, 17: 2067-2069.
Vermeulen A, Deslypere JP, & Kaufman JM (1989) Influence of
antiopioids on luteinizing hormone pulsatility in aging men. J Clin
Endocrinol Metab, 68: 68-72.
Verzar F (1968) Intrinsic and extrinsic factors of molecular aging.
Exp Gerontol, 3: 69-75.
Vestal RE (1978) Drug use in the elderly: a review of problems and
special considerations. Drugs, 16: 358-382.
Vestal RE, McGuire EA, Tobin JD, Andres R, Norris AH, & Ulzey E
(1977) Aging and ethanol metabolism. Clin Pharmacol Ther, 21:
343-354.
Vestal RE, Wood AJJ, & Shand DG (1979) Reduced b-adrenoceptor
sensitivity in the elderly. Clin Pharmacol Ther, 26: 181-186.
Vestal RE, Jue SG, & Cusack BJ (1985) Increased risk of adverse drug
reactions in the elderly: Fact or myth. In: O'Malley K & Waddington
JL ed. Therapeutics in the elderly. Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp 97-104.
Vijg J (1990) DNA sequence changes in aging: How frequent, how
important? Aging, 2: 105-123.
Vijg J & Knook DL (1987) DNA Repair in relation to the aging
process. J Am Geriatr Soc, 35(6): 532-541.
Vijg J & Papaconstantinou J (1990) Aging and longevity genes:
strategies for identifying DNA sequences controlling life span. J
Gerontol, 45(5): B179-B182.
Vincenzini MT, Iantomasi T, Stio M, Favelli F, Vanni P, Tonelli F, &
Treves C (1989) Glucose transport during ageing by human intestinal
brush-border membrane vesicles. Mech Ageing Dev, 48: 33-41.
Vogel HB (1983) Effects of age on the biomechanical and biochemical
properties of rat and human skin. J Soc Cosmet Chem, 34: 453-463.
Voitenko VP & Tokar AV (1983) The assessment of biological age and
sex differences of human aging. Exp Aging Res, 9: 239-244.
Vos JG & Luster MI (1989) Immune alterations. In: Kimbrough RD &
Jensen AA ed. Halogenated biphenyls, terphenyls, naphtalenes,
dibenzodioxins and related products. Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp 295-322.
Vos JG & Penninks AH (1987) Dioxin and organotin compounds as model
immunotoxic chemicals. In: De Matteis F & Lock EA ed. Selectively
and molecular mechanism of toxicity. London, MacMillan Press Ltd, pp
85-102.
Vos JG, De Klerk A, Krajnc EI, Van Loveren H, & Rosing J (1990)
Immunotoxicity of Bis (tri-n-butylin) oxide in the rat; effects on
thymus-dependent immunity and on non specific resistance following
long-term exposure in young versus aged rats (1990). Toxicol Appl
Pharmacol, 105: 144-155.
Wabner CL & Chen TS (1984) Renal transport of organic acids in the
aging. Pharmacologist, 26: 206.
Waddington JL, O'Boyle KM, Molloy AG, Youssef HA, King DJ, & Cooper
SJ (1985) Neurotransmitter receptors and ageing:
Dopamine/neuroleptic receptors, involuntary movements and the
disease process of schizophrenia. In: O'Malley K & Stevenson IL ed.
Therapeutics in the elderly. Amsterdam, Oxford, New York, Elsevier
Science Publishers, pp 63-76.
Waggoner S, Gu MZ, Chiang WH, & Richardson A (1990) The effect of
dietary restriction on the expression of genes. In: Genetic effects
of aging, II. Caldwell, New Jersey, Telford Press, pp 255-273.
Wagner PA, Jernigan JA, Bailey LB, Nickens C, & Brazzi GA (1983)
Zinc nutriture and cell-mediated immunity in the aged. Int J Vitam
Nutr Res, 53: 94-101. Walford RL (1969) The immunological theory of
aging. Copenhagen, Munksgaard.
Walker DM (1985) Oral disease. In: Pathy MSJ ed. Principles and
practice of geriatric medicine. New York, Chichester, Brisbane,
Toronto, John Wiley and Sons.
Wang SV, Halban PA, & Rowe JW (1988) Effects of aging on insulin
synthesis and secretion. Differential effects on preproinsulin
messenger RNA levels, proinsulin biosynthesis, and secretion of
newly made and preformed insulin in the rat. J Clin Invest, 81(1):
176-184.
Ward JM (1983) Increased susceptibility of livers of aged F344/NCr
rats to the effects of phenobarbital on the incidence, morphology,
and histochemistry of hepatocellular foci and neoplasms. J Natl
Cancer Inst, 71: 815-823.
Ward WF (1988) Enhancement by food restriction of liver protein
synthesis in the aging Fischer 344 rat. J Gerontol, 43: B50-B53.
Ward W & Richardson A (1991) Effect of age on liver protein
synthesis and degradation. Hepatology, 14: 935-948.
Ward JM, Lynch P, & Riggs C (1988) Rapid development of
hepatocellular neoplasms in aging male C3H/HeNCr mice given
phenobarbital. Cancer Lett, 39: 9-18.
Warner BA, Dufau ML, & Santen RJ (1985) Effects of aging and illness
on the pituitary testicular axis in men: Qualitative as well as
quantitative changes in luteinizing hormone. J Clin Endocrinol
Metab, 60: 263-268.
Warner HR, Butler RN, Spratt RW, & Schneider EL (1987) Modern
biological theories of aging. New York, Raven Press.
Weale RA (1986) Aging and vision. Vision Res, 26(9): 1507-1512.
Weber JCP & Griffin JP (1986) Prescriptions, adverse reactions, and
the elderly. Lancet, i: 1220.
Webster SGP (1985) Absorption of nutrients in old age. In: Pathy MSJ
ed. Principles and practice of geriatric medicine. New York,
Chichester, Brisbane, Toronto, John Wiley and Sons, Chapter 11.2, pp
265-290.
Webster IW & Logie AR (1976) A relationship between function and
health status in female subjects. J Gerontol, 31: 546-550.
Weigent DA, Carr DJ, & Blalock JE (1990) Bidirectional communication
between the neuroendocrine and immune system. Common hormones and
hormone receptors. Ann NY Acad Sci, 579: 17-27.
Weindruch R & Walford RL (1988). The retardation of aging and
dietary restriction. Springfield, Illinois, Charles C. Thomas.
Weindruch R, Gottesman SR, & Walford RL (1982) Modification of
age-related immune decline in mice dietarily restricted from or
after mid-adulthood. Proc Natl Acad Sci (USA), 79: 898-902.
Weiss B, Clark MB, & Greenberg LH (1984) Modulation of
catecholaminergic receptors during development and aging. In: Lajtha
A ed. Handbook of neurochemistry. Vol 6:- Receptors in the nervous
system. New York, London, Plenum Press, pp 595-627.
Wellinger R & Guigoz Y (1986) The effect of age on the induction of
tyrosine aminotransferase and tryptophan oxygenase genes by
physiological stress. Mech Ageing Dev, 34: 203-217.
Wesson LG Jr (1969) Renal hemodynamics in physiological states. In:
Physiology of the human kidney. New York, London, Grune and
Stratton, pp 98-100.
White L (1989) Epidemiology. In: Maddox GL ed. The encyclopedia of
aging. Berlin, Heidelberg, New York, Springer-Verlag, pp 216-223.
WHO (1983) Environmental Health Criteria 27: Guidelines on studies
in environmental epidemiology. Geneva, World Health Organization,
351 pp.
WHO (1984) The uses of epidemiology in the study of the elderly.
Report of a WHO Scientific Group on the Epidemiology of Aging.
Geneva, World Health Organization, pp. 23-25 (WHO Technical Report
Series 706).
WHO (1987) World health statistics annual. Geneva, World Health
Organization.
WHO (1989) Health of the elderly. Report of a WHO Expert Committee.
Geneva, World Health Organization, pp 14-30 (WHO Technical Report
Series 779).
WHO (1990) Global estimates for health situation assessment and
projections, 1990. Geneva, World Health Organization, Division of
Epidemiological Surveillance and Health Situation and Trend
Assessment, p 3.
Williams ME & Pannill FC (1982) Urinary incontinence in the elderly.
Ann Intern Med, 97: 895-907.
Willott JF (1986) Effects of aging, hearing loss, and anatomical
location on thresholds of inferior colliculus neurons in C57BL/6 and
CBA mice. J Neurophysiol, 56(2): 391-408.
Wilson K & Hanson J (1980) The effects of extremes of age on drug
action. Methods Findings Exp Clin Pharmacol, 2: 303-312.
Winneke G, Hrdina KG, & Brockhouse A (1982) Neuropsychological
studies in children with elevated tooth-lead concentrations. A pilot
study. Int Arch Occup Environ Health, 51: 169-183.
Wong DF, Wagner HN, & Dannals RF (1984) Effects of age on dopamine
and seratonin receptors pressured by positron tomography in the
living human brain. Science, 226: 1393-1396.
Wood RW (1981) Neurobehavioral toxicity of carbon disulfide.
Neurobehav Toxicol Teratol, 3: 397-405.
Wood AJJ (1985) Beta adrenoceptors and aging. In: O'Malley K &
Stevenson IL ed. Therapeutics in the elderly. Amsterdam, Oxford, New
York, Elsevier Science Publishers, pp 43-49.
Wu W, Pahlavani M, Cheung HT, & Richardson A (1986) The effect of
aging on the expression of interleukin 2 messenger ribonucleic acid.
Cell Immunol, 100: 224-231.
Wynne HA, Cope LH, Mutch E, Rawlins MD, Woodhouse KW, & James OF
(1989) The effect of age upon liver volume and apparent liver blood
flow on healthy man. Hepatology, 9: 927-301.
Xiong ZM (1989) [Common diseases and major causes of death in
elderly patients in Jiangxi.] Chin J Geriatr, 8: 135 (in Chinese).
Xiong BZ (1990) Technology progress, aging process and reappoint to
a position.] In: Institute of Japanese Problems, Chinese Academy of
Social Sciences ed. [Proceeding of Symposium on Sino-Japan Social
Countermeasure for Aging Problem.] Beijing, East Publishing House,
pp 186-196 (in Chinese).
Xu XF, You K, Liu D, He L, Cheng S, & Wu JS (1986) [Disease pattern
analysis in hospitalized elderly patients in Uromqi.] Chin J
Geriatr, 5: 15 (in Chinese).
Yamazoe Y, Shimada M, Murayama N, & Kato R (1987) Suppression of
levels of phenobarbital-inducible rat-liver cytochrome P-450 by
pituitary hormone. J Biol Chem, 262: 7423-7428.
Yang RSH, Tallant MJ, & McKelvey JA (1984) Age-dependent
pharmacokinetic changes of ethylenediamine in Fischer 344 rats
parallel to a two-year chronic toxicity study. Fundam Appl Toxicol,
4: 663-670.
Yao SB (1990) [The trend and characters of Chinese aged population.]
In: Institute of Japanese Problems, Chinese Academy of Social
Sciences ed. [Proceedings of Symposium on Sino-Japan Social
Countermeasure for Aging Problems.] Beijing, East Publishing House,
pp 64-74 (in Chinese).
Yates MS & Hiley CR (1979) The effect of age and cardiac output and
its distribution in the rat. Experientia (Basel), 35: 78-79.
Yelland C, Summerbell J, Nicholson E, Herd B, Wynne H, & Woodhouse
KW, (1991) The association of age with aspirin esterase activity in
human liver. Age Ageing, 20(1): 16-8.
York JL (1982) Body water content, ethanol pharmacokinetics, and the
responsiveness to ethanol in young and old rats. Dev Pharmacol Ther,
4: 106-116.
Young VR (1979) Diet as modulator of aging and longevity. Fed Proc,
38: 1994-2000.
Yu BP, Bertrand HR, & Masoro EJ (1980) Nutrition-aging influence of
catecholamine-promoted lipolysis. Metabolism, 29: 438-444.
Yumura W, Sugino N, Nagasawa R, Kubo S, Hirokawa K, & Maruyama N
(1989) Age-associated changes in renal glomeruli of mice. Exp
Gerontol, 24: 237-249.
Zapadnyuk VI (1971) [On an age-periodisation of laboratory animals.]
In: [Gerontology and geriatrics. 1970-1971 Annual book: Aging of the
cell.] Kiev, Research Institute of Gerontology, pp 433-438 (in
Russian).
Zarit SH, Eiler J, & Hassinger M (1985) Clinical assessment. In:
Birren JE & Schaie KW ed. Handbook of the psychology of aging, 2nd
ed. New York, Van Nostrand Reinhold Company, pp 725-754.
Zaphiropouos PG, Mode A, Norstedt G, & Gustavsson JA (1989)
Regulation of sexual differentiation in drug and steroid metabolism.
Trends Pharmacol Sci, 10: 149-153.
Zatz MM & Goldstein AL (1985) Thymosins, lymphokines and the
immunology of aging. Gerontology, 31: 263-277.
Zemel MB & Sowers JR (1988) Salt sensitivity and systemic
hypertension in the elderly. Am J Cardiol, 61: 7H-12H.
Zhou L-W, Weiss B, Freilich JS, & Greenberg LH (1984) Impaired
recovery of alpha - and alpha2-adrenergic receptors in brain tissue
of aged rats. J Gerontol, 39: 538-546.
Zhu JR, Li WX, & Wang YY (1982) [Common diseases and major causes of
death in the elderly with analysis of 8947 cases over 65 years of
age.] Chin J Geriatr, 1: 110-118 (in Chinese).
Zs-Nagy I, Kitani K, Ohta M, & Imahori K (1986) Age-dependent
decrease in lateral diffusion constant of proteins in the plasma
membrane of hepatocytes as revealed by fluorescence recovery after
photobleaching in tissue smears. Arch Gerontol Geriatr, 5: 131-146.
APPENDIX 1. Background Papers
Anisimov, V.N., Approaches to examine the effects of chemicals on
the aged population: experimental approaches.
Birnbaum, L.S., Basis of altered sensitivity of the elderly to
chemicals - pharmacokinetics and pharmacodynamics.
Cooper, R.L., & Goldman, J.M., Alterations in susceptibility to
toxic compounds in the aged central nervous system and endocrine
system.
Dilman, V.M., Theories and mechanics of aging.
Fabris, N., Systemic biology of aging.
Ingram, D.K., Evaluating effects of chemical exposure on aging:
development of conceptual models.
Li, S., Aged population: demographic, life expectancy, and
lifestyle.
Likhachev, A.J., Age related peculiarities of repair of DNA damage
with carcinogens.
Martin, G.M., Definitions on aging.
Ray, P.K., Jaffery, F.N., & Viswathan, P.N.,
1. Chemical exposure of the elderly
2. Modifying factors: nutrition, state of health, life style,
alcohol intake, smoking and ionizing radiation.
Richardson, A., The molecular biology of aging: translation,
transcription and chromatin structure.
Speijers, G.J.A., Groups at risk and their chemical exposure as well
as nutrition as a source of chemicals and as a confounding factor.
Zhu, J.R., Approaches to examine the effects of chemicals on the
aged population: epidemiological and clinical approaches.
